User login
Achieve better glucose control for your hospitalized patients
- Use the basal/bolus insulin regimen for inpatients with diabetes. It follows normal physiological insulin rhythm and is associated with significantly better glycemic control than the sliding-scale regimen. (B)
- If a patient on a basal/bolus regimen consistently requires supplemental insulin, reevaluate baseline dosing and make adjustments as needed. (B)
- Whenever possible, switch hospitalized patients to their outpatient diabetes control regimen ≥24 hours prior to discharge. (C)
Strength of recommendation (SOR)
- Good-quality patient-oriented evidence
- Inconsistent or limited-quality patient-oriented evidence
- Consensus, usual practice, opinion, disease-oriented evidence, case series
Mr. H, a 62-year-old with type 2 diabetes, hypertension, and hypercholesterolemia, arrives at the emergency department complaining of acute onset chest pain. An EKG shows no ischemic changes and his initial cardiac enzymes are normal, but Mr. H is admitted to telemetry for further monitoring and to rule out myocardial infarction. Mr. H normally takes metformin and glipizide to manage his diabetes; his most recent glycosylated hemoglobin (HbA1c) was 8.2. After admission, he is placed on a diabetic diet and switched to insulin.
As primary care physicians, we all care for patients like Mr. H, who are hospitalized because of cardiovascular or other symptoms and have diabetes—a comorbidity that affects an estimated 12% to 25% of inpatients.1 We are also well aware of the elevated risks such patients face—for bacterial infection, impaired wound healing, and reduced tissue and organ perfusion,2 among others. In one study, a single blood glucose reading >220 mg/dL was associated with a nearly 6-fold increase in nosocomial infection.2 A number of recent studies have also found hyperglycemia to be an independent marker of overall inpatient mortality.1,3-5
American Diabetes Association goals. In 2008, the ADA issued new glycemic control goals for inpatients with diabetes. For critically ill patients, the association recommends that blood glucose levels be maintained at <140 mg/dL—and as close to 110 mg/dL as possible. For patients who are hospitalized but are not critically ill, the ADA recommends fasting blood glucose levels of 90 to 130 mg/dL and postprandial levels <180 mg/dL.6
As the ADA recommendations make clear, it is imperative that we do everything possible to lower the blood glucose levels of our hospitalized patients. Ironically, though, fear of hypoglycemia has prevented many physicians from putting patients with diabetes on a basal/bolus insulin protocol1—a dosing regimen that, according to at least one recent report, is more effective than the traditional sliding-scale insulin regimen.7 (See “No heightened hypoglycemia risk with basal/bolus regimen”) To help you achieve glycemic targets safely and confidently using the basal/bolus regimen, we’ve assembled this review of the latest evidence, complete with strategies for success.
Oral agents are no match for the hospital routine
The hospital environment interferes with the patterns and schedules that people with diabetes rely on to manage their condition. Thus, it is not unusual even for patients whose glucose levels were very well-controlled at home to have poor glycemic control as inpatients. Dietary change is one of the primary reasons.
Mealtimes typically deviate from the patient’s at-home schedule. In addition, patients are often put on a calorie-restricted, carefully enforced diabetic diet, which is quite different from their usual eating pattern. NPO orders are also common in preparation for diagnostic testing or other procedures. And some medications—particularly high doses of steroids—affect glucose levels. It is difficult to adjust oral hypoglycemic agents to accommodate such variations.
A look at Mr. H’s regimen. Mr. H’s physician knew that continuation of his oral medications—particularly glipizide—in combination with the hospital’s strict diabetic diet could result in hypoglycemia. Continuing to take metformin was also a concern, given that Mr. H was at risk for new cardiac symptoms—a contraindication to metformin use. So his physician switched him over to insulin, a safer alternative.
Finding the right insulin regimen
For years, a sliding-scale regimen was the most common approach to glycemic management of inpatients with diabetes. This concept, developed in 1934, originally used urine glucose testing to determine dosing, and its convenience and ease of treatment initiation led to widespread use. Although many variations have been introduced over the years, traditional sliding-scale regimens use short-acting analog or regular insulin in predetermined doses based on blood glucose readings at mealtimes and bedtime.
Despite the popularity of this method, however, there is little evidence to support it. Sliding-scale insulin as monotherapy has not been associated with effective glycemic control or improved outcomes.8,9 By design, this traditional regimen makes hyperglycemia the threshold for action, rather than taking action to prevent it. The result: wide fluctuations in blood sugar levels and the potential for prolonged periods of hyperglycemia.
Basal/bolus: A better approach
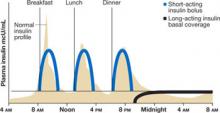
Mr. H’s physician started him on a basal/ bolus insulin regimen, which is more aggressive than a sliding-scale protocol and, as such, has prompted some physicians to view it warily. This strategy, in which a basal dose of long-acting insulin—typically given at bedtime—is accompanied by boluses of short-acting insulin at mealtimes,1,10-12 follows the normal physiological release of insulin (FIGURE 1). Basic metabolic insulin is required to cover endogenous hepatic glucose production, even among diabetes patients who are NPO, and prandial insulin requirements are determined by exogenous glucose intake, whether in the form of a meal, intravenous (IV) fluids, tube feeding, or total parenteral nutrition (TPN).
Dosing guidelines. For most patients with type 2 diabetes, the correct daily insulin dose is 0.5 to 0.7 U/kg,1,13 but factors other than weight also need to be considered:
- Previous insulin use. A lower initial dose (0.4 U/kg/d) may be preferable for insulin-naive patients, whereas a higher dose (0.7 U/kg/d) may be necessary for those with a history of insulin resistance.1,3
- Risk of hypoglycemia. To be on the safe side, start patients who are at high risk of hypoglycemia (eg, because they are very lean, have hepatic or renal failure, or are undergoing hemodialysis) with a very low dose (0.3 U/kg/d).
- Other drugs or TPN regimen. A higher dose (0.7 U/kg/d) is appropriate for patients on high doses of steroids.1,3 Even smaller doses of oral or IV glucocorticoids increase the risk of hyperglycemia, particularly after a meal. Patients receiving TPN or other enteral feedings may also require higher doses of insulin.
Doing the math. To determine specific insulin requirements, calculate the total daily dose and divide it in half. The patient should receive half of the total as long-acting insulin for basal coverage, usually at bedtime. Divide the remaining half into 3 equal portions; administer each portion as a short-acting insulin bolus with each meal.1,3,10,11
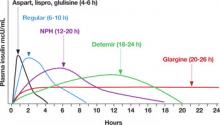
Mr. H’s insulin requirements. Mr. H weighs 100 kg (220 pounds), so he needs 60 units (0.6 U/kg) of insulin per day. His physician writes an order for 30 units (one half of 60 units) of a long-acting insulin (glargine or detemir) at bedtime, and 10 units (one third of the remaining 30 units) of a short-acting insulin (as-part, lispro, or glulisine) with each meal (FIGURE 2).14
FIGURE 1
Basal/bolus regimen mimics normal insulin profile
Source: Polonsky KS, et al. J Clin Invest.12
FIGURE 2
Insulin types and duration
NPH, neutral protamine Hagedorn.
Source: Hirsch B. N Engl J Med.14 Copyright 2005 Massachusetts Medical Society.
More insulin needed? Figuring out how much
It’s not unusual for patients on a basal/bolus regimen—particularly those like Mr. H, who have never been on insulin—to need supplemental insulin.15 Short-acting insulin is always used for this purpose, whether it is administered before a meal or at bedtime.
If a patient is hyperglycemic (>150 mg/dL) before a meal, a correction scale (TABLE) can be used to determine how much additional insulin to give. The supplemental insulin should be given at the same time as the mealtime bolus.
If hyperglycemia is detected at bedtime, a more conservative approach is needed to prevent overnight hypoglycemia. Thus, additional insulin is recommended at bedtime only if the blood glucose reading is >200 mg/dL, and approximately half of the recommended mealtime correction dose should be given.16
TABLE
Insulin correction scale: Calculating the supplemental dose
| BLOOD GLUCOSE mg/dL | EXTRA INSULIN | |
|---|---|---|
| PREMEAL (NO. OF UNITS) | BEDTIME (NO. OF UNITS) | |
| 150-199 | 1 | None |
| 200-249 | 2 | 1 |
| 250-299 | 3 | 2 |
| 300-349 | 4 | 2 |
| ≥350 | 5 | 3 |
| Source: Walsh J, et al. Torrey Pines Press.16 | ||
Is it time to revise the dosing regimen?
Consistent use of a correction scale to adjust the dosage generally indicates that the patient’s baseline dosing regimen needs to be revised. Dynamic insulin coverage requires careful monitoring, with blood glucose levels recorded and reviewed for trends that suggest a change is needed.
Poor subcutaneous perfusion, for example, may lead to a decreased or erratic uptake of injected insulin. Also, stress-related hyperglycemia may decrease or increase over the course of a hospital stay. And changes in medication, such as a decreasing steroid taper, may change overall insulin demand.15,17
With vigilant monitoring, the basal/bolus regimen may be adjusted upward to 110% of current dosing for a patient with frequent elevated blood glucose readings—provided the patient’s glucose levels have not fallen below 80 mg/dL. Conversely, the regimen may be adjusted downward to 80% for a patient who continues to be hypoglycemic. Unlike the supplemental dosing based on the correction scale, these revised regimens affect both the basal (long-acting) and bolus (short-acting) doses.
To ensure timely adjustments to your patient’s regimen, make sure that all your orders for insulin administration are accompanied by provisions for revising the dosing regimen when changes in patient status occur. (Protocols for managing both hypo- and hyperglycemia should, of course, be part of your orders as well.)
A basal/bolus insulin regimen is more aggressive than a sliding-scale protocol, and fear of hypoglycemia has historically kept physicians from using it.1 The Randomized Study of Basal/Bolus Insulin Therapy in the inpatient management of patients with type 2 diabetes (RABBIT 2), published in 2007, addressed this concern. The researchers compared blood glucose levels for inpatients on a sliding-scale insulin regimen with those of patients on a basal/bolus regimen and found no difference in the frequency of hypoglycemia.7 None of the participants were critically ill.
The study did show, however, that those on the sliding-scale regimen had higher mean fasting and random blood glucose levels than those on the basal/bolus regimen. Of patients on the basal/bolus regimen, 66% reached the target—a mean blood glucose <140 mg/dL—vs 38% of those on the sliding-scale regimen. What’s more, 14% of those on the traditional regimen never achieved levels <240 mg/dL, whereas all of those in the basal/bolus group did. The mean daily insulin dose was significantly higher for those on the basal/bolus plan vs the sliding scale regimen (42 vs 12.5 units, respectively).7
RABBIT 2 provides clear evidence of significant improvement in glycemic control among inpatients on a basal/bolus insulin regimen, but patient-oriented outcomes have yet to be measured. However, emerging evidence of the impact of hyperglycemia on morbidity and mortality among diabetes patients in intensive care18,19 has led the American College of Endocrinology5 and the Society of Hospital Medicine, among others, to recommend using basal/bolus insulin in the management of inpatients with diabetes.
IV insulin’s role—and is it expanding?
IV insulin is the treatment of choice for patients in diabetic ketoacidosis, but recent research suggests that it may also be the preferred approach to diabetes management in other critically ill patients, as well as in those undergoing surgery.18-20
In a study comparing outcomes of surgical ICU patients managed with IV insulin during the perioperative and postoperative periods with surgical patients on conventional diabetes management, Van den Berghe found a 45% reduction in mortality rates among those receiving insulin infusions (4.6% of those on IV insulin died, compared with 8% of those receiving subcutaneous insulin). The use of IV insulin therapy also decreased the time spent in intensive care, although it did not shorten the overall length of stay.19
Regular insulin is used most often for insulin infusions. Some trials with ultra–short-acting insulin have been done, but the findings were inconclusive.
Your patient is leaving: Ease the transition
For inpatients with diabetes, discharge planning includes a transition, from insulin to oral agents, perhaps, and from maintaining glucose control based on a hospital schedule to adjusting to the patterns of daily life at home. Particular care is required for patients who will be transitioned from IV to subcutaneous insulin. IV insulin has a half-life of only 10 minutes, so the initial subcutaneous dose should be administered about 1 hour prior to discontinuation of the infusion. Failure to plan accordingly may result in significant hyperglycemia and associated complications.17,21
Research suggests that patients be switched to their outpatient diabetes management plan at least 24 hours before discharge, a protocol that was followed in Mr. H’s case. He remained in the hospital for 5 days. After myocardial infarction was ruled out, Mr. H underwent a nuclear medicine cardiac stress test for which he needed to be NPO. When testing was completed, Mr. H resumed a diabetic diet, and discharge planning began. Since his diabetes was not well controlled on admission and he required >20 units of insulin per day in the hospital, Mr. H’s physician opted to include long-acting insulin at bedtime in his outpatient regimen. On the day before Mr. H was scheduled to leave the hospital, the physician discontinued the short-acting mealtime insulin and restarted oral metformin twice daily, closely monitoring the patient’s glucose levels until discharge. The physician told Mr. H to schedule a follow-up visit within a week so that his new outpatient regimen could be reviewed.
Ideally, a diabetes nurse specialist will be available, not only to get involved in discharge planning, but also to provide patient education, care, and advice. Researchers found that hospital stays for patients with diabetes were shortened (8 days vs 11 days) when a diabetes nurse specialist was involved in their care. The patients were also more knowledgeable and satisfied.22
Although severity of illness, planned or unplanned procedures, and changes from usual dietary patterns may limit the utility of some oral agents, no large studies have investigated the impact of oral diabetes medications on inpatient outcomes.1 For a patient who has excellent outpatient glycemic control and is not critically ill, continuation of some or all oral agents may be appropriate. Consider the following:
Metformin. This agent has the benefit of not causing hypoglycemia and of facilitating weight loss. Metformin is, however, contraindicated in patients with renal insufficiency, congestive heart failure, cardiovascular collapse, acute myocardial infarction, and septicemia.24
Despite the warning, metformin is often used in patients with these contraindications. A recent systematic review of more than 17,000 patients taking the drug did not uncover a single case of lactic acidosis.25 With appropriate monitoring, metformin may be a useful inpatient treatment for some patients.
Sulfonylureas. These agents should be limited in the inpatient setting because of their long action and propensity to cause hypoglycemia. In addition, some questions have arisen about the safety of these medications in patients with vascular disease and acute cardiac events.23,26 Despite this, there is no rigorous data to specifically advise against keeping inpatients with diabetes on sulfonylureas.
Thiazolidinediones. These agents should be used with caution in the inpatient setting. Although they have relatively few acute adverse effects, they have been shown to increase intravascular volume and have the potential to exacerbate congestive heart failure.27
Take advantage of bedside conversations. An inpatient stay offers physicians and patients the opportunity to work together to fine-tune components of the diabetic regimen.23 Make the most of these opportunities. In addition, once the patient goes home, you’ll need to ensure close follow-up to reconcile the differences between home self-management and the controlled hospital environment.
Correspondence
Donald R. Woolever, MD, Family Medicine Residency Program, Central Maine Medical Center, 76 High Street, Lewiston, ME 04240; [email protected].
1. Clement S, Braithwaite SS, Magee MF, et al. Management of diabetes and hyperglycemia in hospitals. Diabetes Care. 2004;27:553-591.
2. Pomposelli JJ, Baxter JK, III, Babineau TJ, et al. Early postoperative glucose control predict nosocomial infection rate in diabetic patients. J Parenter Enteral Nutr. 1998;22:77-81.
3. Umpierrez GE, Isaacs SD, Bazargan N, You X, Thaler LM, Kitabchi AE. Hyperglycemia: an independent marker of in-hospital mortality in patients with undiagnosed diabetes. J Clin Endocrinol Metab. 2002;87:978-982.
4. Deedwania P, Kosiborod M, Barrett E, et al. Hyperglycemia and acute coronary syndrome: a scientific statement from the American Heart Association Diabetes Committee of the Council on Nutrition, Physical Activity, and Metabolism. Circulation. 2008;117:1610-1619.
5. Garber AJ, Moghissi ES, Bransome ED, Jr, et al. American College of Endocrinology Task Force on Inpatient Diabetes Metabolic Control. American College of Endocrinology position statement on inpatient diabetes and metabolic control. Endocr Pract. 2004;10 (suppl 2):S4-S9.
6. American Diabetes Association: Standards of Medical Care in Diabetes—2008. Diabetes Care. 2008;31(suppl 1):S12-S54.
7. Umpierrez GE, Smiley D, Zisman A, et al. Randmonized study of basal-bolus insulin therapy in the inpatient management of patients with type 2 diabetes. Diabetes Care. 2007;30:2181-2186.
8. Browning LA, Dumo P. Sliding scale insulin: an antiquated approach to glycemic control in hospitalized patients. Am J Health Syst Pharm. 2004;61:1611-1614.
9. Robbins L. Let’s get the sliding scale out of medicine. Med Rec Ann. 1963;56:201.-
10. Inzucchi SE. Management of hyperglycemia in the hospital setting. N Engl J Med. 2006;355:1903-1911.
11. Abourizk N, Vora CK, Verna PK. Inpatient diabetology: the new frontier. J Gen Intern Med. 2004;19 (5 pt 1):479-480.
12. Polonsky KS, Given BD, van Cauter E. Twenty-four-hour profiles and pulsatile patterns of insulin secretion in normal and obese subjects. J Clin Invest. 1988;81:442-448.
13. Leahy JL. Insulin management of diabetic patients on general medicine and surgical floors. Endocr Pract. 2006;12(suppl 3):S86-S90.
14. Hirsch IB. Insulin analogues. N Engl J Med. 2005;352:174-183.
15. Donner TW, Flammer KM. Diabetes management in the hospital. Med Clin North Am. 2008;92:407-425.
16. Walsh J, Roberts R, Bailey T, Varma CB. Using Insulin: Everything You Need to Know for Success With Insulin. San Diego, Calif: Torrey Pines Press; 2003.
17. Lien LF, Angelyn Bethel M, Feinglos M. In-hospital management of type 2 diabetes mellitus. Med Clin North Am. 2004;88:1085-1105.
18. Funary AP, Wu Y. Effect of hyperglycemia and continuous intravenous insulin infusions on outcome of cardiac surgical procedures: the Portland Diabetic Project. Endocr Pract. 2004;10(suppl 2):S21-S33.
19. Van den Berghe G, Wouters P, Weekers F, et al. Intensive insulin therapy in the critically ill patient. N Engl J Med. 2001;345:1359-1367.
20. Malmberg K. for the DIGAMI group. Prospective randomised study of intensive insulin treatment on long term survival after acute myocardial infarction in patients with diabetes mellitus. BMJ. 1997;314:1512-1515.
21. Lien LF, Spratt SE, Woods Z, Osborne KK, Feinglos MN. Optimizing hospital use of intravenous insulin therapy: improved management of hyperglycemia and error reduction with a new nomogram. Endocr Pract. 2005;11:240-253.
22. Davies M, Dixon S, Currie CI, Davis RE, Peters JP. Evaluation of a hospital diabetes specialist nursing service: a randomized controlled trial. Diabet Med. 2001;18:301-307.
23. O’Rourke B. Myocardial K-ATP channels in preconditioning. Circ Res. 2000;87:845-855.
24. Glucophage [package insert]. Princeton, NJ: Bristol-Myers-Squibb; 1998.
25. Salpeter S, Greyber E, Pasternak GA, Salpeter EE. Risk of fatal and nonfatal lactic acidosis with metformin use in type 2 diabetes mellitus. Cochrane Database Syst Rev. 2003(2);CD002967.-
26. Brady P, Terzic A. The sulfonylurea controversy: more questions from the heart. J Am Coll Cardiol. 1998;31:950-956.
27. Gillies P, Dunn C. Pioglitazone. Drugs. 2000;60:333-343.
- Use the basal/bolus insulin regimen for inpatients with diabetes. It follows normal physiological insulin rhythm and is associated with significantly better glycemic control than the sliding-scale regimen. (B)
- If a patient on a basal/bolus regimen consistently requires supplemental insulin, reevaluate baseline dosing and make adjustments as needed. (B)
- Whenever possible, switch hospitalized patients to their outpatient diabetes control regimen ≥24 hours prior to discharge. (C)
Strength of recommendation (SOR)
- Good-quality patient-oriented evidence
- Inconsistent or limited-quality patient-oriented evidence
- Consensus, usual practice, opinion, disease-oriented evidence, case series
Mr. H, a 62-year-old with type 2 diabetes, hypertension, and hypercholesterolemia, arrives at the emergency department complaining of acute onset chest pain. An EKG shows no ischemic changes and his initial cardiac enzymes are normal, but Mr. H is admitted to telemetry for further monitoring and to rule out myocardial infarction. Mr. H normally takes metformin and glipizide to manage his diabetes; his most recent glycosylated hemoglobin (HbA1c) was 8.2. After admission, he is placed on a diabetic diet and switched to insulin.
As primary care physicians, we all care for patients like Mr. H, who are hospitalized because of cardiovascular or other symptoms and have diabetes—a comorbidity that affects an estimated 12% to 25% of inpatients.1 We are also well aware of the elevated risks such patients face—for bacterial infection, impaired wound healing, and reduced tissue and organ perfusion,2 among others. In one study, a single blood glucose reading >220 mg/dL was associated with a nearly 6-fold increase in nosocomial infection.2 A number of recent studies have also found hyperglycemia to be an independent marker of overall inpatient mortality.1,3-5
American Diabetes Association goals. In 2008, the ADA issued new glycemic control goals for inpatients with diabetes. For critically ill patients, the association recommends that blood glucose levels be maintained at <140 mg/dL—and as close to 110 mg/dL as possible. For patients who are hospitalized but are not critically ill, the ADA recommends fasting blood glucose levels of 90 to 130 mg/dL and postprandial levels <180 mg/dL.6
As the ADA recommendations make clear, it is imperative that we do everything possible to lower the blood glucose levels of our hospitalized patients. Ironically, though, fear of hypoglycemia has prevented many physicians from putting patients with diabetes on a basal/bolus insulin protocol1—a dosing regimen that, according to at least one recent report, is more effective than the traditional sliding-scale insulin regimen.7 (See “No heightened hypoglycemia risk with basal/bolus regimen”) To help you achieve glycemic targets safely and confidently using the basal/bolus regimen, we’ve assembled this review of the latest evidence, complete with strategies for success.
Oral agents are no match for the hospital routine
The hospital environment interferes with the patterns and schedules that people with diabetes rely on to manage their condition. Thus, it is not unusual even for patients whose glucose levels were very well-controlled at home to have poor glycemic control as inpatients. Dietary change is one of the primary reasons.
Mealtimes typically deviate from the patient’s at-home schedule. In addition, patients are often put on a calorie-restricted, carefully enforced diabetic diet, which is quite different from their usual eating pattern. NPO orders are also common in preparation for diagnostic testing or other procedures. And some medications—particularly high doses of steroids—affect glucose levels. It is difficult to adjust oral hypoglycemic agents to accommodate such variations.
A look at Mr. H’s regimen. Mr. H’s physician knew that continuation of his oral medications—particularly glipizide—in combination with the hospital’s strict diabetic diet could result in hypoglycemia. Continuing to take metformin was also a concern, given that Mr. H was at risk for new cardiac symptoms—a contraindication to metformin use. So his physician switched him over to insulin, a safer alternative.
Finding the right insulin regimen
For years, a sliding-scale regimen was the most common approach to glycemic management of inpatients with diabetes. This concept, developed in 1934, originally used urine glucose testing to determine dosing, and its convenience and ease of treatment initiation led to widespread use. Although many variations have been introduced over the years, traditional sliding-scale regimens use short-acting analog or regular insulin in predetermined doses based on blood glucose readings at mealtimes and bedtime.
Despite the popularity of this method, however, there is little evidence to support it. Sliding-scale insulin as monotherapy has not been associated with effective glycemic control or improved outcomes.8,9 By design, this traditional regimen makes hyperglycemia the threshold for action, rather than taking action to prevent it. The result: wide fluctuations in blood sugar levels and the potential for prolonged periods of hyperglycemia.
Basal/bolus: A better approach
Mr. H’s physician started him on a basal/ bolus insulin regimen, which is more aggressive than a sliding-scale protocol and, as such, has prompted some physicians to view it warily. This strategy, in which a basal dose of long-acting insulin—typically given at bedtime—is accompanied by boluses of short-acting insulin at mealtimes,1,10-12 follows the normal physiological release of insulin (FIGURE 1). Basic metabolic insulin is required to cover endogenous hepatic glucose production, even among diabetes patients who are NPO, and prandial insulin requirements are determined by exogenous glucose intake, whether in the form of a meal, intravenous (IV) fluids, tube feeding, or total parenteral nutrition (TPN).
Dosing guidelines. For most patients with type 2 diabetes, the correct daily insulin dose is 0.5 to 0.7 U/kg,1,13 but factors other than weight also need to be considered:
- Previous insulin use. A lower initial dose (0.4 U/kg/d) may be preferable for insulin-naive patients, whereas a higher dose (0.7 U/kg/d) may be necessary for those with a history of insulin resistance.1,3
- Risk of hypoglycemia. To be on the safe side, start patients who are at high risk of hypoglycemia (eg, because they are very lean, have hepatic or renal failure, or are undergoing hemodialysis) with a very low dose (0.3 U/kg/d).
- Other drugs or TPN regimen. A higher dose (0.7 U/kg/d) is appropriate for patients on high doses of steroids.1,3 Even smaller doses of oral or IV glucocorticoids increase the risk of hyperglycemia, particularly after a meal. Patients receiving TPN or other enteral feedings may also require higher doses of insulin.
Doing the math. To determine specific insulin requirements, calculate the total daily dose and divide it in half. The patient should receive half of the total as long-acting insulin for basal coverage, usually at bedtime. Divide the remaining half into 3 equal portions; administer each portion as a short-acting insulin bolus with each meal.1,3,10,11
Mr. H’s insulin requirements. Mr. H weighs 100 kg (220 pounds), so he needs 60 units (0.6 U/kg) of insulin per day. His physician writes an order for 30 units (one half of 60 units) of a long-acting insulin (glargine or detemir) at bedtime, and 10 units (one third of the remaining 30 units) of a short-acting insulin (as-part, lispro, or glulisine) with each meal (FIGURE 2).14
FIGURE 1
Basal/bolus regimen mimics normal insulin profile
Source: Polonsky KS, et al. J Clin Invest.12
FIGURE 2
Insulin types and duration
NPH, neutral protamine Hagedorn.
Source: Hirsch B. N Engl J Med.14 Copyright 2005 Massachusetts Medical Society.
More insulin needed? Figuring out how much
It’s not unusual for patients on a basal/bolus regimen—particularly those like Mr. H, who have never been on insulin—to need supplemental insulin.15 Short-acting insulin is always used for this purpose, whether it is administered before a meal or at bedtime.
If a patient is hyperglycemic (>150 mg/dL) before a meal, a correction scale (TABLE) can be used to determine how much additional insulin to give. The supplemental insulin should be given at the same time as the mealtime bolus.
If hyperglycemia is detected at bedtime, a more conservative approach is needed to prevent overnight hypoglycemia. Thus, additional insulin is recommended at bedtime only if the blood glucose reading is >200 mg/dL, and approximately half of the recommended mealtime correction dose should be given.16
TABLE
Insulin correction scale: Calculating the supplemental dose
| BLOOD GLUCOSE mg/dL | EXTRA INSULIN | |
|---|---|---|
| PREMEAL (NO. OF UNITS) | BEDTIME (NO. OF UNITS) | |
| 150-199 | 1 | None |
| 200-249 | 2 | 1 |
| 250-299 | 3 | 2 |
| 300-349 | 4 | 2 |
| ≥350 | 5 | 3 |
| Source: Walsh J, et al. Torrey Pines Press.16 | ||
Is it time to revise the dosing regimen?
Consistent use of a correction scale to adjust the dosage generally indicates that the patient’s baseline dosing regimen needs to be revised. Dynamic insulin coverage requires careful monitoring, with blood glucose levels recorded and reviewed for trends that suggest a change is needed.
Poor subcutaneous perfusion, for example, may lead to a decreased or erratic uptake of injected insulin. Also, stress-related hyperglycemia may decrease or increase over the course of a hospital stay. And changes in medication, such as a decreasing steroid taper, may change overall insulin demand.15,17
With vigilant monitoring, the basal/bolus regimen may be adjusted upward to 110% of current dosing for a patient with frequent elevated blood glucose readings—provided the patient’s glucose levels have not fallen below 80 mg/dL. Conversely, the regimen may be adjusted downward to 80% for a patient who continues to be hypoglycemic. Unlike the supplemental dosing based on the correction scale, these revised regimens affect both the basal (long-acting) and bolus (short-acting) doses.
To ensure timely adjustments to your patient’s regimen, make sure that all your orders for insulin administration are accompanied by provisions for revising the dosing regimen when changes in patient status occur. (Protocols for managing both hypo- and hyperglycemia should, of course, be part of your orders as well.)
A basal/bolus insulin regimen is more aggressive than a sliding-scale protocol, and fear of hypoglycemia has historically kept physicians from using it.1 The Randomized Study of Basal/Bolus Insulin Therapy in the inpatient management of patients with type 2 diabetes (RABBIT 2), published in 2007, addressed this concern. The researchers compared blood glucose levels for inpatients on a sliding-scale insulin regimen with those of patients on a basal/bolus regimen and found no difference in the frequency of hypoglycemia.7 None of the participants were critically ill.
The study did show, however, that those on the sliding-scale regimen had higher mean fasting and random blood glucose levels than those on the basal/bolus regimen. Of patients on the basal/bolus regimen, 66% reached the target—a mean blood glucose <140 mg/dL—vs 38% of those on the sliding-scale regimen. What’s more, 14% of those on the traditional regimen never achieved levels <240 mg/dL, whereas all of those in the basal/bolus group did. The mean daily insulin dose was significantly higher for those on the basal/bolus plan vs the sliding scale regimen (42 vs 12.5 units, respectively).7
RABBIT 2 provides clear evidence of significant improvement in glycemic control among inpatients on a basal/bolus insulin regimen, but patient-oriented outcomes have yet to be measured. However, emerging evidence of the impact of hyperglycemia on morbidity and mortality among diabetes patients in intensive care18,19 has led the American College of Endocrinology5 and the Society of Hospital Medicine, among others, to recommend using basal/bolus insulin in the management of inpatients with diabetes.
IV insulin’s role—and is it expanding?
IV insulin is the treatment of choice for patients in diabetic ketoacidosis, but recent research suggests that it may also be the preferred approach to diabetes management in other critically ill patients, as well as in those undergoing surgery.18-20
In a study comparing outcomes of surgical ICU patients managed with IV insulin during the perioperative and postoperative periods with surgical patients on conventional diabetes management, Van den Berghe found a 45% reduction in mortality rates among those receiving insulin infusions (4.6% of those on IV insulin died, compared with 8% of those receiving subcutaneous insulin). The use of IV insulin therapy also decreased the time spent in intensive care, although it did not shorten the overall length of stay.19
Regular insulin is used most often for insulin infusions. Some trials with ultra–short-acting insulin have been done, but the findings were inconclusive.
Your patient is leaving: Ease the transition
For inpatients with diabetes, discharge planning includes a transition, from insulin to oral agents, perhaps, and from maintaining glucose control based on a hospital schedule to adjusting to the patterns of daily life at home. Particular care is required for patients who will be transitioned from IV to subcutaneous insulin. IV insulin has a half-life of only 10 minutes, so the initial subcutaneous dose should be administered about 1 hour prior to discontinuation of the infusion. Failure to plan accordingly may result in significant hyperglycemia and associated complications.17,21
Research suggests that patients be switched to their outpatient diabetes management plan at least 24 hours before discharge, a protocol that was followed in Mr. H’s case. He remained in the hospital for 5 days. After myocardial infarction was ruled out, Mr. H underwent a nuclear medicine cardiac stress test for which he needed to be NPO. When testing was completed, Mr. H resumed a diabetic diet, and discharge planning began. Since his diabetes was not well controlled on admission and he required >20 units of insulin per day in the hospital, Mr. H’s physician opted to include long-acting insulin at bedtime in his outpatient regimen. On the day before Mr. H was scheduled to leave the hospital, the physician discontinued the short-acting mealtime insulin and restarted oral metformin twice daily, closely monitoring the patient’s glucose levels until discharge. The physician told Mr. H to schedule a follow-up visit within a week so that his new outpatient regimen could be reviewed.
Ideally, a diabetes nurse specialist will be available, not only to get involved in discharge planning, but also to provide patient education, care, and advice. Researchers found that hospital stays for patients with diabetes were shortened (8 days vs 11 days) when a diabetes nurse specialist was involved in their care. The patients were also more knowledgeable and satisfied.22
Although severity of illness, planned or unplanned procedures, and changes from usual dietary patterns may limit the utility of some oral agents, no large studies have investigated the impact of oral diabetes medications on inpatient outcomes.1 For a patient who has excellent outpatient glycemic control and is not critically ill, continuation of some or all oral agents may be appropriate. Consider the following:
Metformin. This agent has the benefit of not causing hypoglycemia and of facilitating weight loss. Metformin is, however, contraindicated in patients with renal insufficiency, congestive heart failure, cardiovascular collapse, acute myocardial infarction, and septicemia.24
Despite the warning, metformin is often used in patients with these contraindications. A recent systematic review of more than 17,000 patients taking the drug did not uncover a single case of lactic acidosis.25 With appropriate monitoring, metformin may be a useful inpatient treatment for some patients.
Sulfonylureas. These agents should be limited in the inpatient setting because of their long action and propensity to cause hypoglycemia. In addition, some questions have arisen about the safety of these medications in patients with vascular disease and acute cardiac events.23,26 Despite this, there is no rigorous data to specifically advise against keeping inpatients with diabetes on sulfonylureas.
Thiazolidinediones. These agents should be used with caution in the inpatient setting. Although they have relatively few acute adverse effects, they have been shown to increase intravascular volume and have the potential to exacerbate congestive heart failure.27
Take advantage of bedside conversations. An inpatient stay offers physicians and patients the opportunity to work together to fine-tune components of the diabetic regimen.23 Make the most of these opportunities. In addition, once the patient goes home, you’ll need to ensure close follow-up to reconcile the differences between home self-management and the controlled hospital environment.
Correspondence
Donald R. Woolever, MD, Family Medicine Residency Program, Central Maine Medical Center, 76 High Street, Lewiston, ME 04240; [email protected].
- Use the basal/bolus insulin regimen for inpatients with diabetes. It follows normal physiological insulin rhythm and is associated with significantly better glycemic control than the sliding-scale regimen. (B)
- If a patient on a basal/bolus regimen consistently requires supplemental insulin, reevaluate baseline dosing and make adjustments as needed. (B)
- Whenever possible, switch hospitalized patients to their outpatient diabetes control regimen ≥24 hours prior to discharge. (C)
Strength of recommendation (SOR)
- Good-quality patient-oriented evidence
- Inconsistent or limited-quality patient-oriented evidence
- Consensus, usual practice, opinion, disease-oriented evidence, case series
Mr. H, a 62-year-old with type 2 diabetes, hypertension, and hypercholesterolemia, arrives at the emergency department complaining of acute onset chest pain. An EKG shows no ischemic changes and his initial cardiac enzymes are normal, but Mr. H is admitted to telemetry for further monitoring and to rule out myocardial infarction. Mr. H normally takes metformin and glipizide to manage his diabetes; his most recent glycosylated hemoglobin (HbA1c) was 8.2. After admission, he is placed on a diabetic diet and switched to insulin.
As primary care physicians, we all care for patients like Mr. H, who are hospitalized because of cardiovascular or other symptoms and have diabetes—a comorbidity that affects an estimated 12% to 25% of inpatients.1 We are also well aware of the elevated risks such patients face—for bacterial infection, impaired wound healing, and reduced tissue and organ perfusion,2 among others. In one study, a single blood glucose reading >220 mg/dL was associated with a nearly 6-fold increase in nosocomial infection.2 A number of recent studies have also found hyperglycemia to be an independent marker of overall inpatient mortality.1,3-5
American Diabetes Association goals. In 2008, the ADA issued new glycemic control goals for inpatients with diabetes. For critically ill patients, the association recommends that blood glucose levels be maintained at <140 mg/dL—and as close to 110 mg/dL as possible. For patients who are hospitalized but are not critically ill, the ADA recommends fasting blood glucose levels of 90 to 130 mg/dL and postprandial levels <180 mg/dL.6
As the ADA recommendations make clear, it is imperative that we do everything possible to lower the blood glucose levels of our hospitalized patients. Ironically, though, fear of hypoglycemia has prevented many physicians from putting patients with diabetes on a basal/bolus insulin protocol1—a dosing regimen that, according to at least one recent report, is more effective than the traditional sliding-scale insulin regimen.7 (See “No heightened hypoglycemia risk with basal/bolus regimen”) To help you achieve glycemic targets safely and confidently using the basal/bolus regimen, we’ve assembled this review of the latest evidence, complete with strategies for success.
Oral agents are no match for the hospital routine
The hospital environment interferes with the patterns and schedules that people with diabetes rely on to manage their condition. Thus, it is not unusual even for patients whose glucose levels were very well-controlled at home to have poor glycemic control as inpatients. Dietary change is one of the primary reasons.
Mealtimes typically deviate from the patient’s at-home schedule. In addition, patients are often put on a calorie-restricted, carefully enforced diabetic diet, which is quite different from their usual eating pattern. NPO orders are also common in preparation for diagnostic testing or other procedures. And some medications—particularly high doses of steroids—affect glucose levels. It is difficult to adjust oral hypoglycemic agents to accommodate such variations.
A look at Mr. H’s regimen. Mr. H’s physician knew that continuation of his oral medications—particularly glipizide—in combination with the hospital’s strict diabetic diet could result in hypoglycemia. Continuing to take metformin was also a concern, given that Mr. H was at risk for new cardiac symptoms—a contraindication to metformin use. So his physician switched him over to insulin, a safer alternative.
Finding the right insulin regimen
For years, a sliding-scale regimen was the most common approach to glycemic management of inpatients with diabetes. This concept, developed in 1934, originally used urine glucose testing to determine dosing, and its convenience and ease of treatment initiation led to widespread use. Although many variations have been introduced over the years, traditional sliding-scale regimens use short-acting analog or regular insulin in predetermined doses based on blood glucose readings at mealtimes and bedtime.
Despite the popularity of this method, however, there is little evidence to support it. Sliding-scale insulin as monotherapy has not been associated with effective glycemic control or improved outcomes.8,9 By design, this traditional regimen makes hyperglycemia the threshold for action, rather than taking action to prevent it. The result: wide fluctuations in blood sugar levels and the potential for prolonged periods of hyperglycemia.
Basal/bolus: A better approach
Mr. H’s physician started him on a basal/ bolus insulin regimen, which is more aggressive than a sliding-scale protocol and, as such, has prompted some physicians to view it warily. This strategy, in which a basal dose of long-acting insulin—typically given at bedtime—is accompanied by boluses of short-acting insulin at mealtimes,1,10-12 follows the normal physiological release of insulin (FIGURE 1). Basic metabolic insulin is required to cover endogenous hepatic glucose production, even among diabetes patients who are NPO, and prandial insulin requirements are determined by exogenous glucose intake, whether in the form of a meal, intravenous (IV) fluids, tube feeding, or total parenteral nutrition (TPN).
Dosing guidelines. For most patients with type 2 diabetes, the correct daily insulin dose is 0.5 to 0.7 U/kg,1,13 but factors other than weight also need to be considered:
- Previous insulin use. A lower initial dose (0.4 U/kg/d) may be preferable for insulin-naive patients, whereas a higher dose (0.7 U/kg/d) may be necessary for those with a history of insulin resistance.1,3
- Risk of hypoglycemia. To be on the safe side, start patients who are at high risk of hypoglycemia (eg, because they are very lean, have hepatic or renal failure, or are undergoing hemodialysis) with a very low dose (0.3 U/kg/d).
- Other drugs or TPN regimen. A higher dose (0.7 U/kg/d) is appropriate for patients on high doses of steroids.1,3 Even smaller doses of oral or IV glucocorticoids increase the risk of hyperglycemia, particularly after a meal. Patients receiving TPN or other enteral feedings may also require higher doses of insulin.
Doing the math. To determine specific insulin requirements, calculate the total daily dose and divide it in half. The patient should receive half of the total as long-acting insulin for basal coverage, usually at bedtime. Divide the remaining half into 3 equal portions; administer each portion as a short-acting insulin bolus with each meal.1,3,10,11
Mr. H’s insulin requirements. Mr. H weighs 100 kg (220 pounds), so he needs 60 units (0.6 U/kg) of insulin per day. His physician writes an order for 30 units (one half of 60 units) of a long-acting insulin (glargine or detemir) at bedtime, and 10 units (one third of the remaining 30 units) of a short-acting insulin (as-part, lispro, or glulisine) with each meal (FIGURE 2).14
FIGURE 1
Basal/bolus regimen mimics normal insulin profile
Source: Polonsky KS, et al. J Clin Invest.12
FIGURE 2
Insulin types and duration
NPH, neutral protamine Hagedorn.
Source: Hirsch B. N Engl J Med.14 Copyright 2005 Massachusetts Medical Society.
More insulin needed? Figuring out how much
It’s not unusual for patients on a basal/bolus regimen—particularly those like Mr. H, who have never been on insulin—to need supplemental insulin.15 Short-acting insulin is always used for this purpose, whether it is administered before a meal or at bedtime.
If a patient is hyperglycemic (>150 mg/dL) before a meal, a correction scale (TABLE) can be used to determine how much additional insulin to give. The supplemental insulin should be given at the same time as the mealtime bolus.
If hyperglycemia is detected at bedtime, a more conservative approach is needed to prevent overnight hypoglycemia. Thus, additional insulin is recommended at bedtime only if the blood glucose reading is >200 mg/dL, and approximately half of the recommended mealtime correction dose should be given.16
TABLE
Insulin correction scale: Calculating the supplemental dose
| BLOOD GLUCOSE mg/dL | EXTRA INSULIN | |
|---|---|---|
| PREMEAL (NO. OF UNITS) | BEDTIME (NO. OF UNITS) | |
| 150-199 | 1 | None |
| 200-249 | 2 | 1 |
| 250-299 | 3 | 2 |
| 300-349 | 4 | 2 |
| ≥350 | 5 | 3 |
| Source: Walsh J, et al. Torrey Pines Press.16 | ||
Is it time to revise the dosing regimen?
Consistent use of a correction scale to adjust the dosage generally indicates that the patient’s baseline dosing regimen needs to be revised. Dynamic insulin coverage requires careful monitoring, with blood glucose levels recorded and reviewed for trends that suggest a change is needed.
Poor subcutaneous perfusion, for example, may lead to a decreased or erratic uptake of injected insulin. Also, stress-related hyperglycemia may decrease or increase over the course of a hospital stay. And changes in medication, such as a decreasing steroid taper, may change overall insulin demand.15,17
With vigilant monitoring, the basal/bolus regimen may be adjusted upward to 110% of current dosing for a patient with frequent elevated blood glucose readings—provided the patient’s glucose levels have not fallen below 80 mg/dL. Conversely, the regimen may be adjusted downward to 80% for a patient who continues to be hypoglycemic. Unlike the supplemental dosing based on the correction scale, these revised regimens affect both the basal (long-acting) and bolus (short-acting) doses.
To ensure timely adjustments to your patient’s regimen, make sure that all your orders for insulin administration are accompanied by provisions for revising the dosing regimen when changes in patient status occur. (Protocols for managing both hypo- and hyperglycemia should, of course, be part of your orders as well.)
A basal/bolus insulin regimen is more aggressive than a sliding-scale protocol, and fear of hypoglycemia has historically kept physicians from using it.1 The Randomized Study of Basal/Bolus Insulin Therapy in the inpatient management of patients with type 2 diabetes (RABBIT 2), published in 2007, addressed this concern. The researchers compared blood glucose levels for inpatients on a sliding-scale insulin regimen with those of patients on a basal/bolus regimen and found no difference in the frequency of hypoglycemia.7 None of the participants were critically ill.
The study did show, however, that those on the sliding-scale regimen had higher mean fasting and random blood glucose levels than those on the basal/bolus regimen. Of patients on the basal/bolus regimen, 66% reached the target—a mean blood glucose <140 mg/dL—vs 38% of those on the sliding-scale regimen. What’s more, 14% of those on the traditional regimen never achieved levels <240 mg/dL, whereas all of those in the basal/bolus group did. The mean daily insulin dose was significantly higher for those on the basal/bolus plan vs the sliding scale regimen (42 vs 12.5 units, respectively).7
RABBIT 2 provides clear evidence of significant improvement in glycemic control among inpatients on a basal/bolus insulin regimen, but patient-oriented outcomes have yet to be measured. However, emerging evidence of the impact of hyperglycemia on morbidity and mortality among diabetes patients in intensive care18,19 has led the American College of Endocrinology5 and the Society of Hospital Medicine, among others, to recommend using basal/bolus insulin in the management of inpatients with diabetes.
IV insulin’s role—and is it expanding?
IV insulin is the treatment of choice for patients in diabetic ketoacidosis, but recent research suggests that it may also be the preferred approach to diabetes management in other critically ill patients, as well as in those undergoing surgery.18-20
In a study comparing outcomes of surgical ICU patients managed with IV insulin during the perioperative and postoperative periods with surgical patients on conventional diabetes management, Van den Berghe found a 45% reduction in mortality rates among those receiving insulin infusions (4.6% of those on IV insulin died, compared with 8% of those receiving subcutaneous insulin). The use of IV insulin therapy also decreased the time spent in intensive care, although it did not shorten the overall length of stay.19
Regular insulin is used most often for insulin infusions. Some trials with ultra–short-acting insulin have been done, but the findings were inconclusive.
Your patient is leaving: Ease the transition
For inpatients with diabetes, discharge planning includes a transition, from insulin to oral agents, perhaps, and from maintaining glucose control based on a hospital schedule to adjusting to the patterns of daily life at home. Particular care is required for patients who will be transitioned from IV to subcutaneous insulin. IV insulin has a half-life of only 10 minutes, so the initial subcutaneous dose should be administered about 1 hour prior to discontinuation of the infusion. Failure to plan accordingly may result in significant hyperglycemia and associated complications.17,21
Research suggests that patients be switched to their outpatient diabetes management plan at least 24 hours before discharge, a protocol that was followed in Mr. H’s case. He remained in the hospital for 5 days. After myocardial infarction was ruled out, Mr. H underwent a nuclear medicine cardiac stress test for which he needed to be NPO. When testing was completed, Mr. H resumed a diabetic diet, and discharge planning began. Since his diabetes was not well controlled on admission and he required >20 units of insulin per day in the hospital, Mr. H’s physician opted to include long-acting insulin at bedtime in his outpatient regimen. On the day before Mr. H was scheduled to leave the hospital, the physician discontinued the short-acting mealtime insulin and restarted oral metformin twice daily, closely monitoring the patient’s glucose levels until discharge. The physician told Mr. H to schedule a follow-up visit within a week so that his new outpatient regimen could be reviewed.
Ideally, a diabetes nurse specialist will be available, not only to get involved in discharge planning, but also to provide patient education, care, and advice. Researchers found that hospital stays for patients with diabetes were shortened (8 days vs 11 days) when a diabetes nurse specialist was involved in their care. The patients were also more knowledgeable and satisfied.22
Although severity of illness, planned or unplanned procedures, and changes from usual dietary patterns may limit the utility of some oral agents, no large studies have investigated the impact of oral diabetes medications on inpatient outcomes.1 For a patient who has excellent outpatient glycemic control and is not critically ill, continuation of some or all oral agents may be appropriate. Consider the following:
Metformin. This agent has the benefit of not causing hypoglycemia and of facilitating weight loss. Metformin is, however, contraindicated in patients with renal insufficiency, congestive heart failure, cardiovascular collapse, acute myocardial infarction, and septicemia.24
Despite the warning, metformin is often used in patients with these contraindications. A recent systematic review of more than 17,000 patients taking the drug did not uncover a single case of lactic acidosis.25 With appropriate monitoring, metformin may be a useful inpatient treatment for some patients.
Sulfonylureas. These agents should be limited in the inpatient setting because of their long action and propensity to cause hypoglycemia. In addition, some questions have arisen about the safety of these medications in patients with vascular disease and acute cardiac events.23,26 Despite this, there is no rigorous data to specifically advise against keeping inpatients with diabetes on sulfonylureas.
Thiazolidinediones. These agents should be used with caution in the inpatient setting. Although they have relatively few acute adverse effects, they have been shown to increase intravascular volume and have the potential to exacerbate congestive heart failure.27
Take advantage of bedside conversations. An inpatient stay offers physicians and patients the opportunity to work together to fine-tune components of the diabetic regimen.23 Make the most of these opportunities. In addition, once the patient goes home, you’ll need to ensure close follow-up to reconcile the differences between home self-management and the controlled hospital environment.
Correspondence
Donald R. Woolever, MD, Family Medicine Residency Program, Central Maine Medical Center, 76 High Street, Lewiston, ME 04240; [email protected].
1. Clement S, Braithwaite SS, Magee MF, et al. Management of diabetes and hyperglycemia in hospitals. Diabetes Care. 2004;27:553-591.
2. Pomposelli JJ, Baxter JK, III, Babineau TJ, et al. Early postoperative glucose control predict nosocomial infection rate in diabetic patients. J Parenter Enteral Nutr. 1998;22:77-81.
3. Umpierrez GE, Isaacs SD, Bazargan N, You X, Thaler LM, Kitabchi AE. Hyperglycemia: an independent marker of in-hospital mortality in patients with undiagnosed diabetes. J Clin Endocrinol Metab. 2002;87:978-982.
4. Deedwania P, Kosiborod M, Barrett E, et al. Hyperglycemia and acute coronary syndrome: a scientific statement from the American Heart Association Diabetes Committee of the Council on Nutrition, Physical Activity, and Metabolism. Circulation. 2008;117:1610-1619.
5. Garber AJ, Moghissi ES, Bransome ED, Jr, et al. American College of Endocrinology Task Force on Inpatient Diabetes Metabolic Control. American College of Endocrinology position statement on inpatient diabetes and metabolic control. Endocr Pract. 2004;10 (suppl 2):S4-S9.
6. American Diabetes Association: Standards of Medical Care in Diabetes—2008. Diabetes Care. 2008;31(suppl 1):S12-S54.
7. Umpierrez GE, Smiley D, Zisman A, et al. Randmonized study of basal-bolus insulin therapy in the inpatient management of patients with type 2 diabetes. Diabetes Care. 2007;30:2181-2186.
8. Browning LA, Dumo P. Sliding scale insulin: an antiquated approach to glycemic control in hospitalized patients. Am J Health Syst Pharm. 2004;61:1611-1614.
9. Robbins L. Let’s get the sliding scale out of medicine. Med Rec Ann. 1963;56:201.-
10. Inzucchi SE. Management of hyperglycemia in the hospital setting. N Engl J Med. 2006;355:1903-1911.
11. Abourizk N, Vora CK, Verna PK. Inpatient diabetology: the new frontier. J Gen Intern Med. 2004;19 (5 pt 1):479-480.
12. Polonsky KS, Given BD, van Cauter E. Twenty-four-hour profiles and pulsatile patterns of insulin secretion in normal and obese subjects. J Clin Invest. 1988;81:442-448.
13. Leahy JL. Insulin management of diabetic patients on general medicine and surgical floors. Endocr Pract. 2006;12(suppl 3):S86-S90.
14. Hirsch IB. Insulin analogues. N Engl J Med. 2005;352:174-183.
15. Donner TW, Flammer KM. Diabetes management in the hospital. Med Clin North Am. 2008;92:407-425.
16. Walsh J, Roberts R, Bailey T, Varma CB. Using Insulin: Everything You Need to Know for Success With Insulin. San Diego, Calif: Torrey Pines Press; 2003.
17. Lien LF, Angelyn Bethel M, Feinglos M. In-hospital management of type 2 diabetes mellitus. Med Clin North Am. 2004;88:1085-1105.
18. Funary AP, Wu Y. Effect of hyperglycemia and continuous intravenous insulin infusions on outcome of cardiac surgical procedures: the Portland Diabetic Project. Endocr Pract. 2004;10(suppl 2):S21-S33.
19. Van den Berghe G, Wouters P, Weekers F, et al. Intensive insulin therapy in the critically ill patient. N Engl J Med. 2001;345:1359-1367.
20. Malmberg K. for the DIGAMI group. Prospective randomised study of intensive insulin treatment on long term survival after acute myocardial infarction in patients with diabetes mellitus. BMJ. 1997;314:1512-1515.
21. Lien LF, Spratt SE, Woods Z, Osborne KK, Feinglos MN. Optimizing hospital use of intravenous insulin therapy: improved management of hyperglycemia and error reduction with a new nomogram. Endocr Pract. 2005;11:240-253.
22. Davies M, Dixon S, Currie CI, Davis RE, Peters JP. Evaluation of a hospital diabetes specialist nursing service: a randomized controlled trial. Diabet Med. 2001;18:301-307.
23. O’Rourke B. Myocardial K-ATP channels in preconditioning. Circ Res. 2000;87:845-855.
24. Glucophage [package insert]. Princeton, NJ: Bristol-Myers-Squibb; 1998.
25. Salpeter S, Greyber E, Pasternak GA, Salpeter EE. Risk of fatal and nonfatal lactic acidosis with metformin use in type 2 diabetes mellitus. Cochrane Database Syst Rev. 2003(2);CD002967.-
26. Brady P, Terzic A. The sulfonylurea controversy: more questions from the heart. J Am Coll Cardiol. 1998;31:950-956.
27. Gillies P, Dunn C. Pioglitazone. Drugs. 2000;60:333-343.
1. Clement S, Braithwaite SS, Magee MF, et al. Management of diabetes and hyperglycemia in hospitals. Diabetes Care. 2004;27:553-591.
2. Pomposelli JJ, Baxter JK, III, Babineau TJ, et al. Early postoperative glucose control predict nosocomial infection rate in diabetic patients. J Parenter Enteral Nutr. 1998;22:77-81.
3. Umpierrez GE, Isaacs SD, Bazargan N, You X, Thaler LM, Kitabchi AE. Hyperglycemia: an independent marker of in-hospital mortality in patients with undiagnosed diabetes. J Clin Endocrinol Metab. 2002;87:978-982.
4. Deedwania P, Kosiborod M, Barrett E, et al. Hyperglycemia and acute coronary syndrome: a scientific statement from the American Heart Association Diabetes Committee of the Council on Nutrition, Physical Activity, and Metabolism. Circulation. 2008;117:1610-1619.
5. Garber AJ, Moghissi ES, Bransome ED, Jr, et al. American College of Endocrinology Task Force on Inpatient Diabetes Metabolic Control. American College of Endocrinology position statement on inpatient diabetes and metabolic control. Endocr Pract. 2004;10 (suppl 2):S4-S9.
6. American Diabetes Association: Standards of Medical Care in Diabetes—2008. Diabetes Care. 2008;31(suppl 1):S12-S54.
7. Umpierrez GE, Smiley D, Zisman A, et al. Randmonized study of basal-bolus insulin therapy in the inpatient management of patients with type 2 diabetes. Diabetes Care. 2007;30:2181-2186.
8. Browning LA, Dumo P. Sliding scale insulin: an antiquated approach to glycemic control in hospitalized patients. Am J Health Syst Pharm. 2004;61:1611-1614.
9. Robbins L. Let’s get the sliding scale out of medicine. Med Rec Ann. 1963;56:201.-
10. Inzucchi SE. Management of hyperglycemia in the hospital setting. N Engl J Med. 2006;355:1903-1911.
11. Abourizk N, Vora CK, Verna PK. Inpatient diabetology: the new frontier. J Gen Intern Med. 2004;19 (5 pt 1):479-480.
12. Polonsky KS, Given BD, van Cauter E. Twenty-four-hour profiles and pulsatile patterns of insulin secretion in normal and obese subjects. J Clin Invest. 1988;81:442-448.
13. Leahy JL. Insulin management of diabetic patients on general medicine and surgical floors. Endocr Pract. 2006;12(suppl 3):S86-S90.
14. Hirsch IB. Insulin analogues. N Engl J Med. 2005;352:174-183.
15. Donner TW, Flammer KM. Diabetes management in the hospital. Med Clin North Am. 2008;92:407-425.
16. Walsh J, Roberts R, Bailey T, Varma CB. Using Insulin: Everything You Need to Know for Success With Insulin. San Diego, Calif: Torrey Pines Press; 2003.
17. Lien LF, Angelyn Bethel M, Feinglos M. In-hospital management of type 2 diabetes mellitus. Med Clin North Am. 2004;88:1085-1105.
18. Funary AP, Wu Y. Effect of hyperglycemia and continuous intravenous insulin infusions on outcome of cardiac surgical procedures: the Portland Diabetic Project. Endocr Pract. 2004;10(suppl 2):S21-S33.
19. Van den Berghe G, Wouters P, Weekers F, et al. Intensive insulin therapy in the critically ill patient. N Engl J Med. 2001;345:1359-1367.
20. Malmberg K. for the DIGAMI group. Prospective randomised study of intensive insulin treatment on long term survival after acute myocardial infarction in patients with diabetes mellitus. BMJ. 1997;314:1512-1515.
21. Lien LF, Spratt SE, Woods Z, Osborne KK, Feinglos MN. Optimizing hospital use of intravenous insulin therapy: improved management of hyperglycemia and error reduction with a new nomogram. Endocr Pract. 2005;11:240-253.
22. Davies M, Dixon S, Currie CI, Davis RE, Peters JP. Evaluation of a hospital diabetes specialist nursing service: a randomized controlled trial. Diabet Med. 2001;18:301-307.
23. O’Rourke B. Myocardial K-ATP channels in preconditioning. Circ Res. 2000;87:845-855.
24. Glucophage [package insert]. Princeton, NJ: Bristol-Myers-Squibb; 1998.
25. Salpeter S, Greyber E, Pasternak GA, Salpeter EE. Risk of fatal and nonfatal lactic acidosis with metformin use in type 2 diabetes mellitus. Cochrane Database Syst Rev. 2003(2);CD002967.-
26. Brady P, Terzic A. The sulfonylurea controversy: more questions from the heart. J Am Coll Cardiol. 1998;31:950-956.
27. Gillies P, Dunn C. Pioglitazone. Drugs. 2000;60:333-343.
10 billing & coding tips to boost your reimbursement
Times are tough for primary care physicians—so tough that American Academy of Family Physicians’ President Jim King, MD, recently called for health care reform to ensure that coverage is affordable and that “physicians can continue to care for [patients] without fear of bankruptcy.”1 Yet in virtually every family practice, opportunities to maximize reimbursements are missed. Undercoding, omitting modifiers, and submitting claims without the documentation needed to support them are everyday events.
The lost revenue is no small change. At the current Medicare reimbursement rate of $96.01 for a 99214 visit and $63.73 for a 99213 visit, a physician who undercodes just one level 4 visit per day could lose as much as $8,393 over the course of a year.2
Some family physicians undercode simply because they underestimate the value of the services they provide. Others deliberately take a conservative approach in hopes of avoiding a government audit—a misguided tactic that some coders believe is as likely as habitual overcoding to arouse suspicion.3 For still other physicians, the time it takes to document a level 4 visit is not worth the trouble. Brushing up on the requirements for higher-level visits (TABLES 1 AND 2)4 and using encounter templates to guide you through a review of systems, symptoms, and severity can help lighten the documentation load.
To provide additional help, we’ve developed 10 coding and billing tips based on our experiences in family practice. Each of these can help you to maximize reimbursement.
TABLE 1
Established patient visits: CPT codes and documentation requirements
| E/M CODE | |||||
|---|---|---|---|---|---|
| 99211 | 99212 | 99213 | 99214 | 99215 | |
| History | |||||
| Chief complaint | Required | Required | Required | Required | Required |
| History of present illness | NR | 1-3 elements | 1-3 elements | ≥4 elements or ≥3 chronic diseases | ≥4 elements or ≥3 chronic diseases |
| Review of systems | NR | NR | 1 system | 2-9 systems | ≥10 systems |
| Past history/family history/social history | NR | NR | NR | 1 element | ≥2 elements |
| Examination | NR | 1 system (1-5 elements) | 2 brief systems (6-11 elements) | 1 detailed system + 1 brief system (≥12 elements) | 8 systems or 1 complete single system (comprehensive) |
| Medical decision making | |||||
| Risk | NR | Minimal | Low | Moderate | High |
| Diagnosis or treatment options | Minimal | Minimal | Low | Moderate | High |
| Data | NR | Minimal | Low/Moderate | Moderate | High |
| Time* | 5 minutes | 10 minutes | 15 minutes | 25 minutes | 40 minutes |
| CPT, current procedural terminology; E/M, evaluation and management; HPI, history of present illness; NR, not required. | |||||
| *At least one half of total face-to-face time must involve counseling or coordination of care. | |||||
| Adapted from: American Medical Association.4 | |||||
TABLE 2
New patient visits: CPT codes and documentation requirements
| E/M CODE | |||||
|---|---|---|---|---|---|
| 99201 | 99202 | 99203 | 99204 | 99205 | |
| History | |||||
| Chief complaint | Required | Required | Required | Required | Required |
| History of present illness | 1-3 elements | 1-3 elements | ≥4 elements or ≥3 chronic diseases | ≥4 elements or ≥3 chronic diseases | ≥4 elements or ≥3 chronic diseases |
| Review of systems | NR | 1 system | 2 systems | ≥10 systems | ≥10 systems |
| Past history/family history/social history | NR | NR | 1 element | ≥3 elements | ≥3 elements |
| Examination | 1 system (1-5 elements) | 2 brief systems (6-11 elements) | 1 detailed system + 1 brief system (≥12 elements) | 8 systems or 1 complete single system (comprehensive) | 8 systems or 1 complete single system (comprehensive) |
| Medical decision making | |||||
| Risk | Minimal | Minimal | Low | Moderate | High |
| Diagnosis or treatment options | Minimal | Minimal | Low | Moderate | High |
| Data | Minimal | Minimal | Low | Moderate | High |
| Time* | 10 minutes | 20 minutes | 30 minutes | 45 minutes | 60 minutes |
| CPT, current procedural terminology; E/M, evaluation and management; HPI, history of present illness; NR, not required. | |||||
| *At least one half of total face-to-face time must involve counseling or coordination of care. | |||||
| Adapted from: American Medical Association.4 | |||||
1. Document and bill more 99214s
Centers for Medicare & Medicaid Services (CMS) data show that in 2006, family physicians billed 55.2% of their established outpatient visits as level 3s (99213) and 31.6% as level 4s (99214).2 Evidence suggests that the percentage of 99214s could legitimately be higher. A study comparing family physicians’ choice of codes with those selected by expert coders revealed that the physicians undercoded one third of their established patient visits. In most cases, visits that warranted 99214 codes were instead coded as 99213s.5
To bill for a level 4 established patient visit, CPT (Current Procedural Terminology) guidelines require you to fulfill 2 out of 3 of the following components:
- a detailed history
- a detailed physical examination
- medical decision making of moderate complexity.4
When the history and medical decision making indicate a higher level of complexity, you can bill for a 99214 visit without having to count or document individual body systems or detailed exam elements. A new diagnosis with a prescription, an order for laboratory tests or X-rays, or a request for a specialty consult are all examples of moderately complex decision making. When it is necessary to show that you performed a comprehensive system review to justify a 99214 claim, history forms, filled out in the waiting room and subsequently reviewed with the patient, can be a valuable time-saver.
2. Avoid the 99203/99204 “complexity” pitfall
In 2006, CMS data showed that family physicians billed 43.9% of new patient visits as level 3s (99203) and just 28.5% as level 4s (99204).2 In many cases, opportunities to bill for 99204s are missed.
Unlike a level 4 visit for an established patient, a 99204 code requires all 3 components—a detailed history, detailed physical examination, and moderately complex decision making (TABLE 2).4 Thorough data collection is crucial to justify the higher level code, which is appropriate whenever a new patient presents with a complex medical history warranting a new diagnosis, new medication, and tests or a specialty evaluation.
Beware of the tendency to code the visit based on the complexity of the diagnosis, rather than the extent of decision making involved. A new patient visit from a woman, age 57, who presents with congestion and a persistent cough occasionally accompanied by chest pain might warrant a 99204 if her medical history (eg, obesity, hypertension, and gastroesophageal reflux disease) and review of systems made it necessary to rule out acute myocardial infarction and congestive heart failure, among other serious conditions, before arriving at a diagnosis of bronchitis. If you’re unsure of whether you can use the higher code, review the coding and documentation requirements in TABLE 2.
3. Remember to use modifier -25 with the proper documentation
The Office of Inspector General notes that you can bill for an office procedure performed on the same day as you evaluate the patient, if the procedure “is significant, separately identifiable, and above and beyond the usual preoperative and postoperative care associated with the procedure….” To do so, though, it is necessary to attach modifier-25 to the evaluation and management (E/M) code, and to provide evidence that you performed 2 separate services.
Proper documentation is critical here. In 2002, Medicare approved some 29 million claims using modifier -25, then disallowed nearly 35% of them for failing to meet the documentation requirements.6 How can you avoid a similar fate?
While most third-party payers do not require physicians who bill for an E/M service and a procedural service for the same patient on the same day to submit 2 separate progress notes, the work performed for each must be clearly defined. If you saw a patient with diabetes for a medication check and she asked you to remove a wart, you would need to document the dimensions, depth, and location of the wart, along with details of your targeted evaluation and management.
4. Know when to bill for preventive and E/M services
We’re all familiar with the patient who comes in for a yearly health maintenance examination, then wants to discuss her depression or chronic back pain. In such a case, you may be justified in billing for both preventive services and an office visit—again, using modifier -25 to indicate that you provided significant, separate services.
The distinction can be harder to establish than when separating an E/M service and a procedure, however. If the acute or chronic problem that you evaluate is stable and closely related to the preventive examination—well-controlled asthma not requiring a change in medication, for example—submitting an E/M code is not warranted. But a new problem or an exacerbation of an existing problem requiring a significant history, physical examination, and treatment beyond what would typically be performed during a routine preventive visit would be a valid reason to bill for E/M services.
Management of 2 or more medically significant chronic problems requiring prescription refills and either laboratory or radiographic tests also justifies concomitant billing of an E/M code.
The best laid plans…. Even when billing for preventive and problem-oriented care is appropriate and the proper codes and documentation are submitted, you may not be reimbursed for both. Some third-party payers will pay a portion of each; others will deny the additional claim entirely. There are also some health plans that will require any patient who generates 2 charges on the same day to pay 2 separate copays.
5. Charge for patient counseling
When more than half the time you spend with a patient is devoted to counseling or coordination of care, “time may be considered the key or controlling factor to qualify for a particular level of E/M service,” according to CPT guidelines.4 That is, you may be able to justify the use of a higher level E/M code based solely on time, regardless of the complexity and detail of the medical history, physical examination, or medical decision making (TABLES 1 AND 2).
Medicare’s Documentation Guidelines for E/M Services direct physicians to document the total time of the patient encounter and to describe in detail the nature of the counseling or activities to coordinate care.7 That said, time spent before and after the face-to-face encounter—retrieving and reviewing records or test results in preparation for the visit and arranging referrals or communicating with other health care providers afterwards, for example—cannot be counted toward the total time of the patient encounter.
6. Watch your words when billing for derm procedures
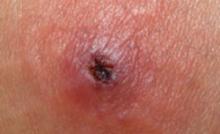
To maximize your reimbursement of dermatologic procedures, you need to be especially mindful of the terminology you use and the descriptive details you record.
Start with terminology. A biopsy generally indicates that only a portion of a lesion was removed to obtain a histologic diagnosis, as in the case of a punch biopsy. When you remove an entire lesion, you use either a shave (horizontal partial-thickness cut that does not include the entire dermal layer) or an excision (a full-thickness removal of the lesion through the dermis to the adipose tissue). Using the correct terminology will ensure that you are properly reimbursed for the procedure you performed.
Focus on measurements. Size matters, too: The larger the lesion, the greater the reimbursement.
To bill for an excision, the size of the lesion must be documented and the excised area calculated by adding the lesion’s maximum diameter plus the sum of the narrowest margin.4 While margins are counted for excisions, that’s not the case with shaved lesions. The margins of a shaved lesion are not factored into the reimbursement formula, so document only the measurement of the lesion itself.
Location also dictates the scale of reimbursement, which is typically lower for procedures involving the trunk, arms, or legs than for those on the face or in the anogenital area. Malignant lesions also generate higher charges.
File multiple claims for multiple lesions. When multiple lesions are biopsied or removed during a single visit, file multiple claims, using modifier -59 for distinct (separate) procedural services.8 Be aware, however, that third-party payers may not provide full reimbursement for each lesion. For Medicare enrollees, routine excision of skin lesions is considered cosmetic and is not covered unless the lesions have malignant or potentially malignant, symptomatic, or functionally impairing features.
7. Use a template for the “Welcome to Medicare” exam
All new Medicare Part B beneficiaries are entitled to a “Welcome to Medicare” exam within their first 6 months of enrollment. It has 7 elements, all of which are required for full reimbursement. To appropriately conduct and bill for this exam, create a template listing all the requisite elements:
- A comprehensive review of the patient’s medical, social, and family history
- A review of risk factors for depression
- A review of functional ability and level of safety
- A focused physical exam (weight, height, blood pressure, and visual acuity are the only requirements)
- An electrocardiogram, with interpretation
- Brief education, counseling, and referral to address any issues discovered in the first 5 elements
- Brief education, counseling, and referral, with a written plan for the patient regarding preventive services covered by Part B.9
To be reimbursed for the Welcome to Medicare exam, it is necessary to use 2 separate billing codes: G0344 for the physical examination (paid at a rate equal to a 99203 visit) and G0366 for the electrocardiogram.9 Although CMS does not provide coverage for a general preventive examination other than this initial “Welcome to Medicare” visit, many recommended preventive health services are covered by Medicare at specified intervals (TABLE 3).9
TABLE 3
Preventive services covered by Medicare
|
|
| Adapted from: Centers for Medicare and Medicaid Services.9 | |
8. Code injections with care
Whether you are administering vaccines or analgesics, coding for injections presents multiple opportunities for error. Physicians often include the code for a vaccine, but forget the procedure code for its administration.
Omitting the dose indication is another common occurrence. (If you inject a 30-mg dose of ketorolac and submit a J1885 code, which covers a 15-mg dose, for example, it is necessary to indicate that you administered a double dose.) It’s also not unusual for physicians to fail to include all the required codes for patients who receive multiple vaccinations at a single visit.
If a 68-year-old man, an established patient, comes in for an annual flu shot and is given the pneumococcal vaccine during the same visit, the correct codes would be:
- 90658 (flu vaccine)
- G0008 (flu vaccine administration)
- 90732 (pneumococcal vaccine)
- G0009 (pneumococcal vaccine administration).
Omitting both procedure codes could cost you nearly $20—and could run into thousands of dollars a year if the error is a daily occurrence.
9. Prioritize diagnoses
Many patients present with multiple diagnoses to be addressed during a single routine office visit, each of which may be applicable for billing for services rendered. ICD-9 coding guidelines state that physicians should “list first the ICD-9-CM code for the diagnosis, condition, problem or other reason for the encounter/visit shown in the medical record to be chiefly responsible for the services provided, then list additional codes that describe any coexisting conditions.”10
Selecting the primary diagnosis for billing and coding, then listing the others in order of importance lets third-party payers know how you prioritized patient care—and helps ensure that you are reimbursed accordingly. (Be sure to list active and acute medical conditions discussed during the visit on the encounter form [eg, type 2 diabetes, hypertension] rather than those that are stable and not addressed that day—eg, seasonal allergies or migraine headaches.)
10. Bill extra for emergency services
From time to time, an unexpected office emergency arises that takes you away from the patient you are currently evaluating. In such cases, you can use CPT code 99058 to bill for services “provided on an emergency basis in the office, which disrupts other scheduled office services”—and bill for basic services provided to the patient, as well.4 Documentation, of course, must include the chief complaint, evaluation, diagnosis, and therapeutic plan and fully describe the emergent nature of the service to justify billing for the “emergency encounter.”
Be aware, however, that even when you code and document appropriately, you may not receive full reimbursement. Medicare and Medicaid often bundle emergency services with other services provided on the same day. Other third-party payers respond in different ways: Some pay the full fee; others pay only a small percentage. Depending on the payer’s policy, billing for a level 4 or 5 E/M visit may be preferable.
Disclosures
Dr. Heidelbaugh is a consultant for Takeda Pharmaceuticals North America, Inc. Dr. Riley and Ms. Habetler reported no potential conflict of interest relevant to this article.
Correspondence
Joel J. Heidelbaugh, MD, Ypsilanti Health Center, 200 Arnet, Suite 200 Ypsilanti, MI 48198; [email protected]
1. Arvantes J. Senate prepares to debate two Medicare payment proposals. June 11, 2008. http://www.aafp.org/online/en/home/publications/news/news-now/government-medicine/20080611bauc-grass-bills.html. Accessed June 17, 2008.
2. Centers for Medicare & Medicaid Services (CMS). Medicare Utilization for Part B. Available at: http://www.cms.hhs.gov/MedicareFeeforSvcPartsAB/04_MedicareUtilizationforPartB.asp. Accessed June 25, 2008.
3. Lowes R. Code your way to better reimbursement. Med Econ. 2007 Oct 19;84:48-54.
4. American Medical Association. Current Procedural Terminology 2008. Chicago: American Medical Association; 2008.
5. King MS, Sharp L, Lipsky M. Accuracy of CPT evaluation and management coding by family physicians. J Am Board Fam Pract. 2001;14:184-192.
6. Department of Health and Human Services Office of the Inspector General. Use of Modifier -25. November 2005. OEI-07-03-00470. Available at: http://www.oig.hhs.gov/oei/reports/oei-07-03-00470.pdf. Accessed June 16, 2008.
7. CMS. 1997 Documentation Guidelines for Evaluation and Management Services. http://www.cms.hhs.gov/MLNProducts/Downloads/MASTER1.pdf. Accessed June 13, 2008.
8. Department of Health and Human Services Office of the Inspector General Use of Modifier -59 to Bypass Medicare’s National Correct Coding Initiative Edits. November 2005. OEI-03-02-00771. http://www.oig.hhs.gov/oei/reports/oei-03-02-00771.pdf. Accessed June 22, 2008.
9. CMS Your Guide to Medicare’s Preventive Services. http://www.medicare.gov/publications/pubs/pdf/10110.pdf. Accessed July 31, 2008.
10. ICD-9-CM Official Guidelines for Coding and Reporting. Effective April 1, 2005. http://www.cdc.gov/nchs/data/icd9/icdguide.pdf. Accessed June 21, 2008.
Times are tough for primary care physicians—so tough that American Academy of Family Physicians’ President Jim King, MD, recently called for health care reform to ensure that coverage is affordable and that “physicians can continue to care for [patients] without fear of bankruptcy.”1 Yet in virtually every family practice, opportunities to maximize reimbursements are missed. Undercoding, omitting modifiers, and submitting claims without the documentation needed to support them are everyday events.
The lost revenue is no small change. At the current Medicare reimbursement rate of $96.01 for a 99214 visit and $63.73 for a 99213 visit, a physician who undercodes just one level 4 visit per day could lose as much as $8,393 over the course of a year.2
Some family physicians undercode simply because they underestimate the value of the services they provide. Others deliberately take a conservative approach in hopes of avoiding a government audit—a misguided tactic that some coders believe is as likely as habitual overcoding to arouse suspicion.3 For still other physicians, the time it takes to document a level 4 visit is not worth the trouble. Brushing up on the requirements for higher-level visits (TABLES 1 AND 2)4 and using encounter templates to guide you through a review of systems, symptoms, and severity can help lighten the documentation load.
To provide additional help, we’ve developed 10 coding and billing tips based on our experiences in family practice. Each of these can help you to maximize reimbursement.
TABLE 1
Established patient visits: CPT codes and documentation requirements
| E/M CODE | |||||
|---|---|---|---|---|---|
| 99211 | 99212 | 99213 | 99214 | 99215 | |
| History | |||||
| Chief complaint | Required | Required | Required | Required | Required |
| History of present illness | NR | 1-3 elements | 1-3 elements | ≥4 elements or ≥3 chronic diseases | ≥4 elements or ≥3 chronic diseases |
| Review of systems | NR | NR | 1 system | 2-9 systems | ≥10 systems |
| Past history/family history/social history | NR | NR | NR | 1 element | ≥2 elements |
| Examination | NR | 1 system (1-5 elements) | 2 brief systems (6-11 elements) | 1 detailed system + 1 brief system (≥12 elements) | 8 systems or 1 complete single system (comprehensive) |
| Medical decision making | |||||
| Risk | NR | Minimal | Low | Moderate | High |
| Diagnosis or treatment options | Minimal | Minimal | Low | Moderate | High |
| Data | NR | Minimal | Low/Moderate | Moderate | High |
| Time* | 5 minutes | 10 minutes | 15 minutes | 25 minutes | 40 minutes |
| CPT, current procedural terminology; E/M, evaluation and management; HPI, history of present illness; NR, not required. | |||||
| *At least one half of total face-to-face time must involve counseling or coordination of care. | |||||
| Adapted from: American Medical Association.4 | |||||
TABLE 2
New patient visits: CPT codes and documentation requirements
| E/M CODE | |||||
|---|---|---|---|---|---|
| 99201 | 99202 | 99203 | 99204 | 99205 | |
| History | |||||
| Chief complaint | Required | Required | Required | Required | Required |
| History of present illness | 1-3 elements | 1-3 elements | ≥4 elements or ≥3 chronic diseases | ≥4 elements or ≥3 chronic diseases | ≥4 elements or ≥3 chronic diseases |
| Review of systems | NR | 1 system | 2 systems | ≥10 systems | ≥10 systems |
| Past history/family history/social history | NR | NR | 1 element | ≥3 elements | ≥3 elements |
| Examination | 1 system (1-5 elements) | 2 brief systems (6-11 elements) | 1 detailed system + 1 brief system (≥12 elements) | 8 systems or 1 complete single system (comprehensive) | 8 systems or 1 complete single system (comprehensive) |
| Medical decision making | |||||
| Risk | Minimal | Minimal | Low | Moderate | High |
| Diagnosis or treatment options | Minimal | Minimal | Low | Moderate | High |
| Data | Minimal | Minimal | Low | Moderate | High |
| Time* | 10 minutes | 20 minutes | 30 minutes | 45 minutes | 60 minutes |
| CPT, current procedural terminology; E/M, evaluation and management; HPI, history of present illness; NR, not required. | |||||
| *At least one half of total face-to-face time must involve counseling or coordination of care. | |||||
| Adapted from: American Medical Association.4 | |||||
1. Document and bill more 99214s
Centers for Medicare & Medicaid Services (CMS) data show that in 2006, family physicians billed 55.2% of their established outpatient visits as level 3s (99213) and 31.6% as level 4s (99214).2 Evidence suggests that the percentage of 99214s could legitimately be higher. A study comparing family physicians’ choice of codes with those selected by expert coders revealed that the physicians undercoded one third of their established patient visits. In most cases, visits that warranted 99214 codes were instead coded as 99213s.5
To bill for a level 4 established patient visit, CPT (Current Procedural Terminology) guidelines require you to fulfill 2 out of 3 of the following components:
- a detailed history
- a detailed physical examination
- medical decision making of moderate complexity.4
When the history and medical decision making indicate a higher level of complexity, you can bill for a 99214 visit without having to count or document individual body systems or detailed exam elements. A new diagnosis with a prescription, an order for laboratory tests or X-rays, or a request for a specialty consult are all examples of moderately complex decision making. When it is necessary to show that you performed a comprehensive system review to justify a 99214 claim, history forms, filled out in the waiting room and subsequently reviewed with the patient, can be a valuable time-saver.
2. Avoid the 99203/99204 “complexity” pitfall
In 2006, CMS data showed that family physicians billed 43.9% of new patient visits as level 3s (99203) and just 28.5% as level 4s (99204).2 In many cases, opportunities to bill for 99204s are missed.
Unlike a level 4 visit for an established patient, a 99204 code requires all 3 components—a detailed history, detailed physical examination, and moderately complex decision making (TABLE 2).4 Thorough data collection is crucial to justify the higher level code, which is appropriate whenever a new patient presents with a complex medical history warranting a new diagnosis, new medication, and tests or a specialty evaluation.
Beware of the tendency to code the visit based on the complexity of the diagnosis, rather than the extent of decision making involved. A new patient visit from a woman, age 57, who presents with congestion and a persistent cough occasionally accompanied by chest pain might warrant a 99204 if her medical history (eg, obesity, hypertension, and gastroesophageal reflux disease) and review of systems made it necessary to rule out acute myocardial infarction and congestive heart failure, among other serious conditions, before arriving at a diagnosis of bronchitis. If you’re unsure of whether you can use the higher code, review the coding and documentation requirements in TABLE 2.
3. Remember to use modifier -25 with the proper documentation
The Office of Inspector General notes that you can bill for an office procedure performed on the same day as you evaluate the patient, if the procedure “is significant, separately identifiable, and above and beyond the usual preoperative and postoperative care associated with the procedure….” To do so, though, it is necessary to attach modifier-25 to the evaluation and management (E/M) code, and to provide evidence that you performed 2 separate services.
Proper documentation is critical here. In 2002, Medicare approved some 29 million claims using modifier -25, then disallowed nearly 35% of them for failing to meet the documentation requirements.6 How can you avoid a similar fate?
While most third-party payers do not require physicians who bill for an E/M service and a procedural service for the same patient on the same day to submit 2 separate progress notes, the work performed for each must be clearly defined. If you saw a patient with diabetes for a medication check and she asked you to remove a wart, you would need to document the dimensions, depth, and location of the wart, along with details of your targeted evaluation and management.
4. Know when to bill for preventive and E/M services
We’re all familiar with the patient who comes in for a yearly health maintenance examination, then wants to discuss her depression or chronic back pain. In such a case, you may be justified in billing for both preventive services and an office visit—again, using modifier -25 to indicate that you provided significant, separate services.
The distinction can be harder to establish than when separating an E/M service and a procedure, however. If the acute or chronic problem that you evaluate is stable and closely related to the preventive examination—well-controlled asthma not requiring a change in medication, for example—submitting an E/M code is not warranted. But a new problem or an exacerbation of an existing problem requiring a significant history, physical examination, and treatment beyond what would typically be performed during a routine preventive visit would be a valid reason to bill for E/M services.
Management of 2 or more medically significant chronic problems requiring prescription refills and either laboratory or radiographic tests also justifies concomitant billing of an E/M code.
The best laid plans…. Even when billing for preventive and problem-oriented care is appropriate and the proper codes and documentation are submitted, you may not be reimbursed for both. Some third-party payers will pay a portion of each; others will deny the additional claim entirely. There are also some health plans that will require any patient who generates 2 charges on the same day to pay 2 separate copays.
5. Charge for patient counseling
When more than half the time you spend with a patient is devoted to counseling or coordination of care, “time may be considered the key or controlling factor to qualify for a particular level of E/M service,” according to CPT guidelines.4 That is, you may be able to justify the use of a higher level E/M code based solely on time, regardless of the complexity and detail of the medical history, physical examination, or medical decision making (TABLES 1 AND 2).
Medicare’s Documentation Guidelines for E/M Services direct physicians to document the total time of the patient encounter and to describe in detail the nature of the counseling or activities to coordinate care.7 That said, time spent before and after the face-to-face encounter—retrieving and reviewing records or test results in preparation for the visit and arranging referrals or communicating with other health care providers afterwards, for example—cannot be counted toward the total time of the patient encounter.
6. Watch your words when billing for derm procedures
To maximize your reimbursement of dermatologic procedures, you need to be especially mindful of the terminology you use and the descriptive details you record.
Start with terminology. A biopsy generally indicates that only a portion of a lesion was removed to obtain a histologic diagnosis, as in the case of a punch biopsy. When you remove an entire lesion, you use either a shave (horizontal partial-thickness cut that does not include the entire dermal layer) or an excision (a full-thickness removal of the lesion through the dermis to the adipose tissue). Using the correct terminology will ensure that you are properly reimbursed for the procedure you performed.
Focus on measurements. Size matters, too: The larger the lesion, the greater the reimbursement.
To bill for an excision, the size of the lesion must be documented and the excised area calculated by adding the lesion’s maximum diameter plus the sum of the narrowest margin.4 While margins are counted for excisions, that’s not the case with shaved lesions. The margins of a shaved lesion are not factored into the reimbursement formula, so document only the measurement of the lesion itself.
Location also dictates the scale of reimbursement, which is typically lower for procedures involving the trunk, arms, or legs than for those on the face or in the anogenital area. Malignant lesions also generate higher charges.
File multiple claims for multiple lesions. When multiple lesions are biopsied or removed during a single visit, file multiple claims, using modifier -59 for distinct (separate) procedural services.8 Be aware, however, that third-party payers may not provide full reimbursement for each lesion. For Medicare enrollees, routine excision of skin lesions is considered cosmetic and is not covered unless the lesions have malignant or potentially malignant, symptomatic, or functionally impairing features.
7. Use a template for the “Welcome to Medicare” exam
All new Medicare Part B beneficiaries are entitled to a “Welcome to Medicare” exam within their first 6 months of enrollment. It has 7 elements, all of which are required for full reimbursement. To appropriately conduct and bill for this exam, create a template listing all the requisite elements:
- A comprehensive review of the patient’s medical, social, and family history
- A review of risk factors for depression
- A review of functional ability and level of safety
- A focused physical exam (weight, height, blood pressure, and visual acuity are the only requirements)
- An electrocardiogram, with interpretation
- Brief education, counseling, and referral to address any issues discovered in the first 5 elements
- Brief education, counseling, and referral, with a written plan for the patient regarding preventive services covered by Part B.9
To be reimbursed for the Welcome to Medicare exam, it is necessary to use 2 separate billing codes: G0344 for the physical examination (paid at a rate equal to a 99203 visit) and G0366 for the electrocardiogram.9 Although CMS does not provide coverage for a general preventive examination other than this initial “Welcome to Medicare” visit, many recommended preventive health services are covered by Medicare at specified intervals (TABLE 3).9
TABLE 3
Preventive services covered by Medicare
|
|
| Adapted from: Centers for Medicare and Medicaid Services.9 | |
8. Code injections with care
Whether you are administering vaccines or analgesics, coding for injections presents multiple opportunities for error. Physicians often include the code for a vaccine, but forget the procedure code for its administration.
Omitting the dose indication is another common occurrence. (If you inject a 30-mg dose of ketorolac and submit a J1885 code, which covers a 15-mg dose, for example, it is necessary to indicate that you administered a double dose.) It’s also not unusual for physicians to fail to include all the required codes for patients who receive multiple vaccinations at a single visit.
If a 68-year-old man, an established patient, comes in for an annual flu shot and is given the pneumococcal vaccine during the same visit, the correct codes would be:
- 90658 (flu vaccine)
- G0008 (flu vaccine administration)
- 90732 (pneumococcal vaccine)
- G0009 (pneumococcal vaccine administration).
Omitting both procedure codes could cost you nearly $20—and could run into thousands of dollars a year if the error is a daily occurrence.
9. Prioritize diagnoses
Many patients present with multiple diagnoses to be addressed during a single routine office visit, each of which may be applicable for billing for services rendered. ICD-9 coding guidelines state that physicians should “list first the ICD-9-CM code for the diagnosis, condition, problem or other reason for the encounter/visit shown in the medical record to be chiefly responsible for the services provided, then list additional codes that describe any coexisting conditions.”10
Selecting the primary diagnosis for billing and coding, then listing the others in order of importance lets third-party payers know how you prioritized patient care—and helps ensure that you are reimbursed accordingly. (Be sure to list active and acute medical conditions discussed during the visit on the encounter form [eg, type 2 diabetes, hypertension] rather than those that are stable and not addressed that day—eg, seasonal allergies or migraine headaches.)
10. Bill extra for emergency services
From time to time, an unexpected office emergency arises that takes you away from the patient you are currently evaluating. In such cases, you can use CPT code 99058 to bill for services “provided on an emergency basis in the office, which disrupts other scheduled office services”—and bill for basic services provided to the patient, as well.4 Documentation, of course, must include the chief complaint, evaluation, diagnosis, and therapeutic plan and fully describe the emergent nature of the service to justify billing for the “emergency encounter.”
Be aware, however, that even when you code and document appropriately, you may not receive full reimbursement. Medicare and Medicaid often bundle emergency services with other services provided on the same day. Other third-party payers respond in different ways: Some pay the full fee; others pay only a small percentage. Depending on the payer’s policy, billing for a level 4 or 5 E/M visit may be preferable.
Disclosures
Dr. Heidelbaugh is a consultant for Takeda Pharmaceuticals North America, Inc. Dr. Riley and Ms. Habetler reported no potential conflict of interest relevant to this article.
Correspondence
Joel J. Heidelbaugh, MD, Ypsilanti Health Center, 200 Arnet, Suite 200 Ypsilanti, MI 48198; [email protected]
Times are tough for primary care physicians—so tough that American Academy of Family Physicians’ President Jim King, MD, recently called for health care reform to ensure that coverage is affordable and that “physicians can continue to care for [patients] without fear of bankruptcy.”1 Yet in virtually every family practice, opportunities to maximize reimbursements are missed. Undercoding, omitting modifiers, and submitting claims without the documentation needed to support them are everyday events.
The lost revenue is no small change. At the current Medicare reimbursement rate of $96.01 for a 99214 visit and $63.73 for a 99213 visit, a physician who undercodes just one level 4 visit per day could lose as much as $8,393 over the course of a year.2
Some family physicians undercode simply because they underestimate the value of the services they provide. Others deliberately take a conservative approach in hopes of avoiding a government audit—a misguided tactic that some coders believe is as likely as habitual overcoding to arouse suspicion.3 For still other physicians, the time it takes to document a level 4 visit is not worth the trouble. Brushing up on the requirements for higher-level visits (TABLES 1 AND 2)4 and using encounter templates to guide you through a review of systems, symptoms, and severity can help lighten the documentation load.
To provide additional help, we’ve developed 10 coding and billing tips based on our experiences in family practice. Each of these can help you to maximize reimbursement.
TABLE 1
Established patient visits: CPT codes and documentation requirements
| E/M CODE | |||||
|---|---|---|---|---|---|
| 99211 | 99212 | 99213 | 99214 | 99215 | |
| History | |||||
| Chief complaint | Required | Required | Required | Required | Required |
| History of present illness | NR | 1-3 elements | 1-3 elements | ≥4 elements or ≥3 chronic diseases | ≥4 elements or ≥3 chronic diseases |
| Review of systems | NR | NR | 1 system | 2-9 systems | ≥10 systems |
| Past history/family history/social history | NR | NR | NR | 1 element | ≥2 elements |
| Examination | NR | 1 system (1-5 elements) | 2 brief systems (6-11 elements) | 1 detailed system + 1 brief system (≥12 elements) | 8 systems or 1 complete single system (comprehensive) |
| Medical decision making | |||||
| Risk | NR | Minimal | Low | Moderate | High |
| Diagnosis or treatment options | Minimal | Minimal | Low | Moderate | High |
| Data | NR | Minimal | Low/Moderate | Moderate | High |
| Time* | 5 minutes | 10 minutes | 15 minutes | 25 minutes | 40 minutes |
| CPT, current procedural terminology; E/M, evaluation and management; HPI, history of present illness; NR, not required. | |||||
| *At least one half of total face-to-face time must involve counseling or coordination of care. | |||||
| Adapted from: American Medical Association.4 | |||||
TABLE 2
New patient visits: CPT codes and documentation requirements
| E/M CODE | |||||
|---|---|---|---|---|---|
| 99201 | 99202 | 99203 | 99204 | 99205 | |
| History | |||||
| Chief complaint | Required | Required | Required | Required | Required |
| History of present illness | 1-3 elements | 1-3 elements | ≥4 elements or ≥3 chronic diseases | ≥4 elements or ≥3 chronic diseases | ≥4 elements or ≥3 chronic diseases |
| Review of systems | NR | 1 system | 2 systems | ≥10 systems | ≥10 systems |
| Past history/family history/social history | NR | NR | 1 element | ≥3 elements | ≥3 elements |
| Examination | 1 system (1-5 elements) | 2 brief systems (6-11 elements) | 1 detailed system + 1 brief system (≥12 elements) | 8 systems or 1 complete single system (comprehensive) | 8 systems or 1 complete single system (comprehensive) |
| Medical decision making | |||||
| Risk | Minimal | Minimal | Low | Moderate | High |
| Diagnosis or treatment options | Minimal | Minimal | Low | Moderate | High |
| Data | Minimal | Minimal | Low | Moderate | High |
| Time* | 10 minutes | 20 minutes | 30 minutes | 45 minutes | 60 minutes |
| CPT, current procedural terminology; E/M, evaluation and management; HPI, history of present illness; NR, not required. | |||||
| *At least one half of total face-to-face time must involve counseling or coordination of care. | |||||
| Adapted from: American Medical Association.4 | |||||
1. Document and bill more 99214s
Centers for Medicare & Medicaid Services (CMS) data show that in 2006, family physicians billed 55.2% of their established outpatient visits as level 3s (99213) and 31.6% as level 4s (99214).2 Evidence suggests that the percentage of 99214s could legitimately be higher. A study comparing family physicians’ choice of codes with those selected by expert coders revealed that the physicians undercoded one third of their established patient visits. In most cases, visits that warranted 99214 codes were instead coded as 99213s.5
To bill for a level 4 established patient visit, CPT (Current Procedural Terminology) guidelines require you to fulfill 2 out of 3 of the following components:
- a detailed history
- a detailed physical examination
- medical decision making of moderate complexity.4
When the history and medical decision making indicate a higher level of complexity, you can bill for a 99214 visit without having to count or document individual body systems or detailed exam elements. A new diagnosis with a prescription, an order for laboratory tests or X-rays, or a request for a specialty consult are all examples of moderately complex decision making. When it is necessary to show that you performed a comprehensive system review to justify a 99214 claim, history forms, filled out in the waiting room and subsequently reviewed with the patient, can be a valuable time-saver.
2. Avoid the 99203/99204 “complexity” pitfall
In 2006, CMS data showed that family physicians billed 43.9% of new patient visits as level 3s (99203) and just 28.5% as level 4s (99204).2 In many cases, opportunities to bill for 99204s are missed.
Unlike a level 4 visit for an established patient, a 99204 code requires all 3 components—a detailed history, detailed physical examination, and moderately complex decision making (TABLE 2).4 Thorough data collection is crucial to justify the higher level code, which is appropriate whenever a new patient presents with a complex medical history warranting a new diagnosis, new medication, and tests or a specialty evaluation.
Beware of the tendency to code the visit based on the complexity of the diagnosis, rather than the extent of decision making involved. A new patient visit from a woman, age 57, who presents with congestion and a persistent cough occasionally accompanied by chest pain might warrant a 99204 if her medical history (eg, obesity, hypertension, and gastroesophageal reflux disease) and review of systems made it necessary to rule out acute myocardial infarction and congestive heart failure, among other serious conditions, before arriving at a diagnosis of bronchitis. If you’re unsure of whether you can use the higher code, review the coding and documentation requirements in TABLE 2.
3. Remember to use modifier -25 with the proper documentation
The Office of Inspector General notes that you can bill for an office procedure performed on the same day as you evaluate the patient, if the procedure “is significant, separately identifiable, and above and beyond the usual preoperative and postoperative care associated with the procedure….” To do so, though, it is necessary to attach modifier-25 to the evaluation and management (E/M) code, and to provide evidence that you performed 2 separate services.
Proper documentation is critical here. In 2002, Medicare approved some 29 million claims using modifier -25, then disallowed nearly 35% of them for failing to meet the documentation requirements.6 How can you avoid a similar fate?
While most third-party payers do not require physicians who bill for an E/M service and a procedural service for the same patient on the same day to submit 2 separate progress notes, the work performed for each must be clearly defined. If you saw a patient with diabetes for a medication check and she asked you to remove a wart, you would need to document the dimensions, depth, and location of the wart, along with details of your targeted evaluation and management.
4. Know when to bill for preventive and E/M services
We’re all familiar with the patient who comes in for a yearly health maintenance examination, then wants to discuss her depression or chronic back pain. In such a case, you may be justified in billing for both preventive services and an office visit—again, using modifier -25 to indicate that you provided significant, separate services.
The distinction can be harder to establish than when separating an E/M service and a procedure, however. If the acute or chronic problem that you evaluate is stable and closely related to the preventive examination—well-controlled asthma not requiring a change in medication, for example—submitting an E/M code is not warranted. But a new problem or an exacerbation of an existing problem requiring a significant history, physical examination, and treatment beyond what would typically be performed during a routine preventive visit would be a valid reason to bill for E/M services.
Management of 2 or more medically significant chronic problems requiring prescription refills and either laboratory or radiographic tests also justifies concomitant billing of an E/M code.
The best laid plans…. Even when billing for preventive and problem-oriented care is appropriate and the proper codes and documentation are submitted, you may not be reimbursed for both. Some third-party payers will pay a portion of each; others will deny the additional claim entirely. There are also some health plans that will require any patient who generates 2 charges on the same day to pay 2 separate copays.
5. Charge for patient counseling
When more than half the time you spend with a patient is devoted to counseling or coordination of care, “time may be considered the key or controlling factor to qualify for a particular level of E/M service,” according to CPT guidelines.4 That is, you may be able to justify the use of a higher level E/M code based solely on time, regardless of the complexity and detail of the medical history, physical examination, or medical decision making (TABLES 1 AND 2).
Medicare’s Documentation Guidelines for E/M Services direct physicians to document the total time of the patient encounter and to describe in detail the nature of the counseling or activities to coordinate care.7 That said, time spent before and after the face-to-face encounter—retrieving and reviewing records or test results in preparation for the visit and arranging referrals or communicating with other health care providers afterwards, for example—cannot be counted toward the total time of the patient encounter.
6. Watch your words when billing for derm procedures
To maximize your reimbursement of dermatologic procedures, you need to be especially mindful of the terminology you use and the descriptive details you record.
Start with terminology. A biopsy generally indicates that only a portion of a lesion was removed to obtain a histologic diagnosis, as in the case of a punch biopsy. When you remove an entire lesion, you use either a shave (horizontal partial-thickness cut that does not include the entire dermal layer) or an excision (a full-thickness removal of the lesion through the dermis to the adipose tissue). Using the correct terminology will ensure that you are properly reimbursed for the procedure you performed.
Focus on measurements. Size matters, too: The larger the lesion, the greater the reimbursement.
To bill for an excision, the size of the lesion must be documented and the excised area calculated by adding the lesion’s maximum diameter plus the sum of the narrowest margin.4 While margins are counted for excisions, that’s not the case with shaved lesions. The margins of a shaved lesion are not factored into the reimbursement formula, so document only the measurement of the lesion itself.
Location also dictates the scale of reimbursement, which is typically lower for procedures involving the trunk, arms, or legs than for those on the face or in the anogenital area. Malignant lesions also generate higher charges.
File multiple claims for multiple lesions. When multiple lesions are biopsied or removed during a single visit, file multiple claims, using modifier -59 for distinct (separate) procedural services.8 Be aware, however, that third-party payers may not provide full reimbursement for each lesion. For Medicare enrollees, routine excision of skin lesions is considered cosmetic and is not covered unless the lesions have malignant or potentially malignant, symptomatic, or functionally impairing features.
7. Use a template for the “Welcome to Medicare” exam
All new Medicare Part B beneficiaries are entitled to a “Welcome to Medicare” exam within their first 6 months of enrollment. It has 7 elements, all of which are required for full reimbursement. To appropriately conduct and bill for this exam, create a template listing all the requisite elements:
- A comprehensive review of the patient’s medical, social, and family history
- A review of risk factors for depression
- A review of functional ability and level of safety
- A focused physical exam (weight, height, blood pressure, and visual acuity are the only requirements)
- An electrocardiogram, with interpretation
- Brief education, counseling, and referral to address any issues discovered in the first 5 elements
- Brief education, counseling, and referral, with a written plan for the patient regarding preventive services covered by Part B.9
To be reimbursed for the Welcome to Medicare exam, it is necessary to use 2 separate billing codes: G0344 for the physical examination (paid at a rate equal to a 99203 visit) and G0366 for the electrocardiogram.9 Although CMS does not provide coverage for a general preventive examination other than this initial “Welcome to Medicare” visit, many recommended preventive health services are covered by Medicare at specified intervals (TABLE 3).9
TABLE 3
Preventive services covered by Medicare
|
|
| Adapted from: Centers for Medicare and Medicaid Services.9 | |
8. Code injections with care
Whether you are administering vaccines or analgesics, coding for injections presents multiple opportunities for error. Physicians often include the code for a vaccine, but forget the procedure code for its administration.
Omitting the dose indication is another common occurrence. (If you inject a 30-mg dose of ketorolac and submit a J1885 code, which covers a 15-mg dose, for example, it is necessary to indicate that you administered a double dose.) It’s also not unusual for physicians to fail to include all the required codes for patients who receive multiple vaccinations at a single visit.
If a 68-year-old man, an established patient, comes in for an annual flu shot and is given the pneumococcal vaccine during the same visit, the correct codes would be:
- 90658 (flu vaccine)
- G0008 (flu vaccine administration)
- 90732 (pneumococcal vaccine)
- G0009 (pneumococcal vaccine administration).
Omitting both procedure codes could cost you nearly $20—and could run into thousands of dollars a year if the error is a daily occurrence.
9. Prioritize diagnoses
Many patients present with multiple diagnoses to be addressed during a single routine office visit, each of which may be applicable for billing for services rendered. ICD-9 coding guidelines state that physicians should “list first the ICD-9-CM code for the diagnosis, condition, problem or other reason for the encounter/visit shown in the medical record to be chiefly responsible for the services provided, then list additional codes that describe any coexisting conditions.”10
Selecting the primary diagnosis for billing and coding, then listing the others in order of importance lets third-party payers know how you prioritized patient care—and helps ensure that you are reimbursed accordingly. (Be sure to list active and acute medical conditions discussed during the visit on the encounter form [eg, type 2 diabetes, hypertension] rather than those that are stable and not addressed that day—eg, seasonal allergies or migraine headaches.)
10. Bill extra for emergency services
From time to time, an unexpected office emergency arises that takes you away from the patient you are currently evaluating. In such cases, you can use CPT code 99058 to bill for services “provided on an emergency basis in the office, which disrupts other scheduled office services”—and bill for basic services provided to the patient, as well.4 Documentation, of course, must include the chief complaint, evaluation, diagnosis, and therapeutic plan and fully describe the emergent nature of the service to justify billing for the “emergency encounter.”
Be aware, however, that even when you code and document appropriately, you may not receive full reimbursement. Medicare and Medicaid often bundle emergency services with other services provided on the same day. Other third-party payers respond in different ways: Some pay the full fee; others pay only a small percentage. Depending on the payer’s policy, billing for a level 4 or 5 E/M visit may be preferable.
Disclosures
Dr. Heidelbaugh is a consultant for Takeda Pharmaceuticals North America, Inc. Dr. Riley and Ms. Habetler reported no potential conflict of interest relevant to this article.
Correspondence
Joel J. Heidelbaugh, MD, Ypsilanti Health Center, 200 Arnet, Suite 200 Ypsilanti, MI 48198; [email protected]
1. Arvantes J. Senate prepares to debate two Medicare payment proposals. June 11, 2008. http://www.aafp.org/online/en/home/publications/news/news-now/government-medicine/20080611bauc-grass-bills.html. Accessed June 17, 2008.
2. Centers for Medicare & Medicaid Services (CMS). Medicare Utilization for Part B. Available at: http://www.cms.hhs.gov/MedicareFeeforSvcPartsAB/04_MedicareUtilizationforPartB.asp. Accessed June 25, 2008.
3. Lowes R. Code your way to better reimbursement. Med Econ. 2007 Oct 19;84:48-54.
4. American Medical Association. Current Procedural Terminology 2008. Chicago: American Medical Association; 2008.
5. King MS, Sharp L, Lipsky M. Accuracy of CPT evaluation and management coding by family physicians. J Am Board Fam Pract. 2001;14:184-192.
6. Department of Health and Human Services Office of the Inspector General. Use of Modifier -25. November 2005. OEI-07-03-00470. Available at: http://www.oig.hhs.gov/oei/reports/oei-07-03-00470.pdf. Accessed June 16, 2008.
7. CMS. 1997 Documentation Guidelines for Evaluation and Management Services. http://www.cms.hhs.gov/MLNProducts/Downloads/MASTER1.pdf. Accessed June 13, 2008.
8. Department of Health and Human Services Office of the Inspector General Use of Modifier -59 to Bypass Medicare’s National Correct Coding Initiative Edits. November 2005. OEI-03-02-00771. http://www.oig.hhs.gov/oei/reports/oei-03-02-00771.pdf. Accessed June 22, 2008.
9. CMS Your Guide to Medicare’s Preventive Services. http://www.medicare.gov/publications/pubs/pdf/10110.pdf. Accessed July 31, 2008.
10. ICD-9-CM Official Guidelines for Coding and Reporting. Effective April 1, 2005. http://www.cdc.gov/nchs/data/icd9/icdguide.pdf. Accessed June 21, 2008.
1. Arvantes J. Senate prepares to debate two Medicare payment proposals. June 11, 2008. http://www.aafp.org/online/en/home/publications/news/news-now/government-medicine/20080611bauc-grass-bills.html. Accessed June 17, 2008.
2. Centers for Medicare & Medicaid Services (CMS). Medicare Utilization for Part B. Available at: http://www.cms.hhs.gov/MedicareFeeforSvcPartsAB/04_MedicareUtilizationforPartB.asp. Accessed June 25, 2008.
3. Lowes R. Code your way to better reimbursement. Med Econ. 2007 Oct 19;84:48-54.
4. American Medical Association. Current Procedural Terminology 2008. Chicago: American Medical Association; 2008.
5. King MS, Sharp L, Lipsky M. Accuracy of CPT evaluation and management coding by family physicians. J Am Board Fam Pract. 2001;14:184-192.
6. Department of Health and Human Services Office of the Inspector General. Use of Modifier -25. November 2005. OEI-07-03-00470. Available at: http://www.oig.hhs.gov/oei/reports/oei-07-03-00470.pdf. Accessed June 16, 2008.
7. CMS. 1997 Documentation Guidelines for Evaluation and Management Services. http://www.cms.hhs.gov/MLNProducts/Downloads/MASTER1.pdf. Accessed June 13, 2008.
8. Department of Health and Human Services Office of the Inspector General Use of Modifier -59 to Bypass Medicare’s National Correct Coding Initiative Edits. November 2005. OEI-03-02-00771. http://www.oig.hhs.gov/oei/reports/oei-03-02-00771.pdf. Accessed June 22, 2008.
9. CMS Your Guide to Medicare’s Preventive Services. http://www.medicare.gov/publications/pubs/pdf/10110.pdf. Accessed July 31, 2008.
10. ICD-9-CM Official Guidelines for Coding and Reporting. Effective April 1, 2005. http://www.cdc.gov/nchs/data/icd9/icdguide.pdf. Accessed June 21, 2008.
Tools to speed your heel pain diagnosis
- Advise patients with tendinopathy to decrease physical activity, do stretching exercises (C), undergo eccentric calf muscle training (B), use heel lifts (C), modify shoe fit, and take nonsteroidal anti-inflammatory drugs (NSAIDs) regularly for a few days, then as needed (B).
- The mainstay of treatment for calcaneal apophysitis in children is rest (C). Other options include heel lifts, stretching programs, icing, gel heel cups, and anti-inflammatory agents (C).
- Treatment options for plantar fasciitis include NSAIDs, stretching exercises, gel cups, arch supports, night splints, steroid injections, extracorporeal shock wave therapy, and surgery (B).
Strength of recommendation (SOR)
- Good-quality patient-oriented evidence
- Inconsistent or limited-quality patient-oriented evidence
- Consensus, usual practice, opinion, disease-oriented
One of your patients, a 40-year-old woman, recently began an exercise program, and she now says she has persistent heel pain. Your first suspicion is “another plantar fasciitis case.” However, after asking a few questions and performing a brief examination, you realize the problem is not what you expected. The pain is in the wrong place for plantar fasciitis and the patient’s history is atypical. How should you proceed?
Knowing the precise location of maximum pain or tenderness (FIGURES 1A–1C) and pairing that with key findings from the exam and history (TABLE 1) can help you reach an accurate diagnosis and formulate proper treatment (TABLE 2).
Each of the 3 general areas of heel pain—posterior, plantar, and medial—introduces a unique differential. Bilateral symptoms or multiple joint involvement, of course, raises the possibility of associated systemic disease.
FIGURE 1
Common causes of heel pain by location
TABLE 1
A quick guide to narrowing your heel pain diagnosis
| AFFECTED AREA | ONSET OF PAIN | HISTORY AND KEY FINDINGS | LIKELY DIAGNOSIS |
|---|---|---|---|
| Posterior heel | Acute |
| Achilles rupture |
| Achilles strain | ||
| Chronic |
| Achilles tendinopathy | |
| Retrocalcaneal bursitis | ||
| Calcaneal apophysitis | ||
| Posterior impingement | ||
| Plantar surface | Acute |
| Calcaneal fracture |
| Plantar fascial rupture | ||
| Chronic |
| Plantar fasciitis | |
| Calcaneal stress fracture | ||
| Fat pad syndrome | ||
| Medial heel | Subacute |
| Posterior tibial tendonitis |
| Chronic |
| Posterior tibial tendon dysfunction | |
| Tarsal tunnel syndrome |
Posterior heel pain
The common causes of posterior heel pain are Achilles tendinopathy, retrocalcaneal bursitis, calcaneal apophysitis, posterior impingement (FIGURE 1A), and Achilles tendon strain or rupture. Rarer causes are sciatica, peroneal tendonitis, Haglund’s deformity, pump bump, and systemic disorders. The patient’s history and precise location of maximal tenderness1 differentiates these problems.
Achilles tendinopathy (tendonitis): Is the patient an athlete?
Insertional and noninsertional Achilles tendinopathy are the most common causes of persistent posterior heel pain.2,3 The inflammatory process occurs in the fatty tissue surrounding the Achilles tendon (the paratenon) rather than in the tendon itself. Patients tend to be highly active (often athletes) and may have recently increased their activity. Ask patients, too, whether they have recently taken a fluoroquinolone antibiotic. This drug class is known to increase the risk of both tendonitis and tendon rupture,4 and in July of this year the FDA directed drug manufacturers to add a black-box warning to that effect.5
Evaluation of noninsertional tendinopathy. Tenderness is usually located 2 to 6 cm above the Achilles insertion. Nodularity, swelling, or fluctuance of the tendon may be evident. Diagnosis generally can be made clinically. If confirmation is needed, consider ultrasonography or magnetic resonance imaging.
Treatment. Advise patients to decrease physical activity and do stretching exercises, undergo eccentric calf muscle training, use heel lifts, modify shoe fit, and use systemic or topical nonsteroidal anti-inflammatory drugs (NSAIDs) regularly for a few days, then as needed. Refractory cases may require surgery.6 New therapies that have proven effective include extracorporeal shock wave therapy (ESWT), prolotherapy (dextrose injections), and local application of nitroglycerin patches or gel.7-18 ESWT can be expensive and is not widely available. Prolotherapy can be performed with minimal training, but is still relatively new. Topical nitroglycerin is affordable, but beware of such side effects as headache and hypotension.
Evaluation of insertional tendinopathy. Inflammation occurs at the tendon’s insertion to bone (enthesitis). Pain typically is at the midline and is reproduced by palpating the tendon insertion or by passively stretching the heel. The presentation may be difficult to distinguish from retrocalcaneal bursitis (discussed below).
Treatment is similar to that used for noninsertional tendinopathy. However, if insertional tendinopathy occurs in conjunction with a Haglund’s deformity (bony overgrowth of the calcaneus), surgery may be indicated, because noninvasive measures tend to fail.19
Use steroid injections with extreme caution due to the theoretical risk of tendon rupture.20 Injections are effective when directed at concomitant inflammation of the retrocalcaneal bursa, but accurate positioning and careful postinjection care are paramount. After an injection, a patient may need absolute rest or even immobilization to protect from tendon rupture. Emphasize a careful return to activity or athletic training.
Retrocalcaneal bursitis: Look for subtle swelling
The retrocalcaneal bursa lies between the Achilles tendon and the calcaneus near the tendon’s insertion. This bursa may become inflamed with repetitive stress or with insertional Achilles tendinopathy.
Evaluation. Swelling is usually present but may be subtle. Pain is located just lateral to the midline of the posterior heel at the superior angle of the calcaneus, and it may also be medial to the tendon opposite the lateral location.
Treatment. The bursitis often responds to icing and ice massage, shoe-fit adjustments, heel lifts, Achilles stretching programs, and systemic or topical NSAIDs.2 Steroid injections are likely beneficial, but use them with caution and take care to avoid the Achilles tendon insertion.
Calcaneal apophysitis affects highly active kids
Calcaneal apophysitis (Sever’s disease) is a painful inflammation in the heels of skeletally immature children where the Achilles tendon inserts in the calcaneus apophysis.
Evaluation. Associated with peak growth rate and high activity level, this inflammatory process usually occurs in boys between the ages of 10 and 12 years, and in girls between the ages of 8 and 10 years.21 The process is similar to that occurring at other sites of traction apophysitis, such as Osgood-Schlatter disease at the tibial tuberosity. Children most susceptible are highly active, wear poorly fitting footwear, run frequently on hard surfaces, and have tight Achilles tendons. Clinical diagnosis usually suffices, although plain x-ray films can verify an active apophysis and rule out other sources of pain, such as tarsal coalition, calcaneal stress fractures, or infection.22
Treatment. Calcaneal apophysitis is typically self-limiting, and the mainstay of treatment is rest. Heel lifts, stretching programs, icing, gel heel cups, and anti-inflammatory agents may also be used.23
Posterior impingement: Pain with full plantar flexion
Posterior impingement at the ankle joint may be self-originating or arise as a consequence of an os trigonum, a posterior sesamoid bone of the talus that exists as a normal variant. In some cases, this bone creates a barrier to full plantar flexion at the ankle joint and creates pain at the posterior heel.
Evaluation. Pain with full plantar flexion is a critical distinguishing feature, because most other pathologies in the posterior heel cause pain with dorsiflexion at the ankle.24,25 Patients often are involved in activities that require forced plantar flexion, such as gymnastics or dancing. Diagnosis is clinical for the most part, but plain x-ray films may confirm the presence of an os trigonum. Magnetic resonance imaging (MRI) is warranted for patients with persistent symptoms; it may reveal a hypertrophied synovial lining or other pathology (such as osteochondritis). MRI is also indicated before more invasive therapies, such as steroid injections or surgery.
Treatment. Advise rest with or without immobilization, NSAIDs, or local steroid injections. Severe impingement or recalcitrant cases may require surgical release of the posterior synovium or removal of an os trigonum.24,25
Achilles strain and rupture: Middle-aged men are susceptible
The Achilles tendon is most susceptible to injury in middle-aged men who are active in sports requiring loading and sudden contraction of the calf muscles, such as basketball or football, although injuries may occur in a variety of other settings. A strain of the Achilles tendon should be carefully differentiated from a complete rupture. While strains can be treated similarly to Achilles tendinopathy, complete rupture is a much larger concern.
Evaluation. When the Achilles tendon ruptures, patients describe sudden pain and a pop that is often audible. Poor plantar flexion of the foot ensues.26 Telltale signs on examination are a positive Thompson’s test (little or no plantar flexion with a calf squeeze) and a visible defect in the tendon. The rupture site is usually 1 to 2 inches proximal to its insertion on the calcaneus.
Treatment. Most of these patients should be seen by an orthopedic surgeon as soon as possible. For active and younger adults, treatment is almost always early surgical repair.27 For some older individuals who are less active, nonsurgical management includes graduated casting, which progressively lessens plantar flexion over 6 to 10 weeks, followed by physical therapy.
3 less common causes of posterior heel pain
Haglund’s deformity is an overgrowth of the calcaneus at the insertion of the Achilles tendon.3 Caused by overuse and poorly fitted shoes, this condition commonly requires surgical intervention.
Pump bump is an inflamed superficial bursa commonly associated with a Haglund’s deformity, and it may respond to NSAIDs, shoe-fit modification, ice massage, or steroid injection.
Peroneal tendonitis is a tendinopathy of evertors and external rotators of the foot. The pain will follow the tendons posterior to the lateral malleolus and extend to the lateral midfoot. It is also treated with rest, NSAIDs, icing, and physical therapy.
Plantar-surface heel pain
The problems most likely to cause plantar-surface pain (FIGURE 1B) are plantar fasciitis, stress fracture of the calcaneus, and fat pad syndrome.
Plantar fasciitis: Pain is worst in the morning
This is by far the most common cause of heel pain primary care physicians will see. Rarely, infection and neoplasia will cause unilateral plantar heel pain.4
Evaluation. Tenderness localized to the plantar surface of the heel in adults usually indicates plantar fasciitis.
Pain is worst with the first step of the morning, and lessens with activity. The tender spot is the medial calcaneal tubercle, with pain radiating through the arch.1,28-30
Treatment. The many therapeutic modalities—NSAIDs, stretching exercises, gel cups, arch supports, night splints, steroid injections, ESWT, and surgery—have been extensively reviewed elsewhere, including in a Cochrane review from 2005.31-33
Calcaneal stress fracture: Suspect it in runners
Calcaneal stress fractures are relatively rare, but may occur in those who put significant stressors on their feet, such as avid runners or military recruits.
Evaluation. Most patients report a recent increase in frequency or intensity of activity, and runners can tell you when it is during their run that the pain begins. As the stress fracture worsens, the pain begins earlier in the activity and eventually is present with even minimal activity. A key distinction from plantar fasciitis, in which pain lessens with activity, is that the pain of a stress fracture typically worsens with activity and diminishes with rest.34
Physical exam provides few clues except for the “squeeze test” (FIGURE 1A) Putting pressure on both the medial and lateral calcaneal tuberosities will cause discomfort. Pain will be absent in the posterior structures of the heel. Placing a vibrating 128-cps tuning fork on the calcaneus should also increase discomfort.
Plain x-ray films may be falsely negative, especially during the first 2 to 3 weeks of pain. Three-phase bone scans are nearly 100% sensitive for detecting stress fractures, with changes evident in as little as 1 to 2 days after injury. The specificity of MRI scans is superior to that of bone scans and can reveal alternate problems.35
Treatment. Activity modification reduces trauma to the heel. Encourage patients to walk if they are pain free and to increase activity as comfort allows. Tell patients to stop activity if the fracture becomes symptomatic. Advanced fractures demand an absolute absence of weight bearing.
Pain can be controlled with NSAIDs TABLE 2 and ice. Lab and animal data have suggested that NSAIDs may impede fracture healing rates, but no similar data exist regarding their effect on stress fractures.36 Symptoms abate within 2 or 3 weeks. Advise athletes to resume activity slowly in a stepwise progression, letting them know that a return to full activity is likely within 6 to 8 weeks. Have runners restart their routine at half their customary distance, increasing it by no more than 10% to 15% per week.
Any medical condition that weakens the bone may predispose a patient to stress fracture. To prevent primary and secondary stress fractures, correct the patient’s underlying medical problems. Evaluate young, thin women with a stress fracture for the “female athlete triad” (osteopenia, disordered eating, menstrual irregularity). The elderly are also at risk for stress fractures due to osteopenia or osteoporosis.
TABLE 2
Heel pain treatment options: A look at the evidence
| DIAGNOSIS | TREATMENTS | SOR | REFERENCE |
|---|---|---|---|
| Achilles tendinopathy | NSAIDs Topical NSAIDs Eccentric calf muscle training Stretching Heel lifts Ice Topical nitrates Prolotherapy (dextrose injections) ESWT Surgery | B B B C C C B B B C | 18 17 12,18 16,19 16,19 16,19 7,10,18 15,18 9,18 19 |
| Retrocalcaneal bursitis | NSAIDs Heel lifts Steroid Injections (with caution) | C C C | 2 2 2 |
| Calcaneal apophysitis | Rest NSAIDs Heel lifts Stretching Icing Gel heel cups | C C C C C C | 21,22 21,22 21,22 21,22 21,22 21,22 |
| Posterior impingement | Rest NSAIDs Steroid injections Surgery | C C C C | 24 24 24 24 |
| Plantar fasciitis | NSAIDs Stretches Gel cups Steroid iontophoresis Arch supports ESWT Night splints Steroid injections Surgery | B B B B B B B B B | 28,31 28,31 28,31 28,31 28,31 28,31,33 28,33 28,31 28,31 |
| Calcaneal stress fracture | NSAIDs Activity moderation Icing | C C C | 34,36 34 34 |
| Fat pad syndrome | NSAIDs Rest Gel heel cups Icing | C C C C | 37 37 37 37 |
| Posterior tibial tendon dysfunction | Weight loss Icing Physical therapy Arch supports/bracing NSAIDs Surgery | C C C C C B | 40 40 40 41 38 38,39 |
| Tarsal tunnel syndrome | Arch supports NSAIDs Activity modification Physical therapy Neuromodulators Steroid injections Surgery | C C C C C C B | 1,43 42,43 42,43 42,43 42 44 46,47 |
| ESWT, extracorporeal shock wave therapy; NSAIDs, nonsteroidal anti-inflammatory drugs. | |||
| Strength of recommendation (SOR) A Good-quality patient-oriented evidence B Inconsistent or limited-quality patient-oriented evidence C Consensus, usual practice, opinion, disease-oriented | |||
Fat pad syndrome: More diffuse pain than plantar fasciitis
The plantar surface of the heel is protected by a thick fat pad. Those at risk of a thinned fat pad include the elderly (the pad thins with age), the obese (increased stress to the pad), and those who have previously received a corticosteroid injection in the pad. Cumulative or acute trauma to the heel can also cause contusion to the heel pad.
Evaluation. Pain typically is located more posteriorly than classic plantar fasciitis pain and is more diffuse. Pain from the fat pad should not radiate toward the arch and is not exacerbated by dorsiflexion of the foot.1
Treatment. Recommend relative rest, gel heel cups, NSAIDs, and ice.37
Less common causes of plantar-surface pain
Lateral plantar nerve entrapment may also cause neuropathic pain on the plantar surface. Patients who experience a painful pop in their heel associated with trauma may have ruptured their plantar fascia. A fallen arch may also be noted on exam. Treatment of both of these conditions is similar to that of plantar fasciitis.
Acute calcaneal fracture results from trauma, such as a fall from a height onto the soles of the feet. Look for localized pain and swelling around the calcaneus and evaluate the neurovascular status of the foot. Initial treatment includes elevating the foot, avoiding weight bearing, applying ice, controlling pain, and using a posterior splint. Many of these fractures require surgical fixation.
Medial heel pain
Posterior tibial tendonitis/dysfunction and tarsal tunnel syndrome are best classified as medial in location (FIGURE 1C). However, the pain is often more diffuse and may radiate to either the posterior or plantar heel.
Posterior tibial tendonitis/dysfunction are linked to obesity
Posterior tibial tendonitis (PTT) and posterior tibial tendon dysfunction (PTTD) are related diagnoses. PTTD refers to increased laxity of the tendon resulting in flat foot and increased heel varus. It is the most common cause of acquired flat foot in adults. PTT may exist separately or as part of PTTD.
Evaluation. Patients complain of pain at the posterior edge of the medial malleolus that may extend toward the arch of the foot.38,39 Patients may also experience swelling or redness in the area. Both PTT and PTTD seem related to overuse and obesity. Young or nonobese patients with PTT or PTTD often have underlying systemic arthropathies.35
Treatment. Early treatment is necessary to prevent progression of tendon incompetence. Interventions include weight loss, NSAIDs, icing, physical therapy,40 and orthotics or bracing for arch and ankle support. You may also try immobilization in a short leg cast for 6 weeks.41 If conservative measures fail, surgery may be necessary for tendon repair, tendon transfer, calcaneal osteotomy, or tarsal bone fusion.38,39
Tarsal tunnel syndrome: Pain can occur at night
Tarsal tunnel syndrome (TTS) is the most common compression neuropathy of the lower extremity. The tarsal tunnel is a fibro-osseous structure along the medial ankle that contains the tibial nerve, the posterior tibial artery, and the tendons of the tibialis posterior, flexor digitorum longus, and flexor hallucis longus. The posterior tibial nerve can become irritated as it runs through the tunnel. The inciting incident can be either a severe stretch to the nerve (from a medial ankle sprain) or from an anatomic compression. Pes planus foot or posterior tibial dysfunction have also been implicated as common causes.1
Evaluation. Patients describe poorly localized pain with numbness and burning along the medial ankle, arch, or heel, with radiation proximally.42,43 Symptoms are aggravated by exercise, and night pain is not uncommon. The tenderness of TTS is more diffuse than that from plantar fasciitis, and symptoms are evident directly over the tarsal tunnel itself.
The classic finding is a positive Tinel’s sign (reproduction of symptoms by tapping over the posterior tibial nerve as it passes through the tarsal tunnel). Placing the foot in dorsiflexion and eversion may also reproduce symptoms.1
Imaging results are not always definitive, but can be helpful in determining the cause of the compression. Plain films and CT can detect fracture or bony deformity, while MRI is more helpful in evaluating soft-tissue structures, such as ganglions or varicosities. Abnormal nerve conduction studies can be suggestive of TTS, but a normal result does not rule out the diagnosis.
Treatment follows a stepped progression. Initially try activity modification, orthotics, and physical therapy. Physical therapy concentrates on medial arch strengthening, Achilles stretching, and ankle proprioception exercises.
NSAIDs and neuromodulatory drugs (tricyclic antidepressants and antiseizure medications) have shown some success. Steroid injections have been effective when given at the site of entrapment,44 but care must be taken to avoid the posterior tibial tendon. If patients do not improve following these measures, they may require cast immobilization.45
Surgery is a possibility when other options fail. The cause of the neural compression is identified in 60% to 80% of cases.46,47 Success rates for various procedures of tarsal tunnel release and tibial nerve decompression range from 75% to 91%. If neural compression is absent, investigate other systemic causes of peripheral neuropathy, such as diabetes or alcoholism.4
Systemic diagnoses
Bilateral heel pain, multiple joint involvement, or fever suggests systemic disease. Common diseases affecting the heel include rheumatoid arthritis, ankylosing spondylitis, psoriatic arthritis, reactive arthritis, and inflammatory bowel disease.1 Successful treatment of these disorders should relieve associated heel pain.
Correspondence
H. E. Woodall, MD, AnMed Health Family Medicine Residency, 2000 E Greenville Street, Suite 3600, Anderson, SC 29621; [email protected]
1. Aldridge T. Diagnosing heel pain in adults. Am Fam Physician. 2004;70:332-338.
2. Aronow MS. Posterior heel pain (retrocalcaneal bursitis, insertional and noninsertional Achilles tendinopathy). Clin Podiatr Med Surg. 2005;22:19-43.
3. Alvarez-Nemegyei J, Canoso J. Heel pain: diagnosis and treatment, step by step. Clev Clin J Med. 2006;73:465-471.
4. Thomas J, Christensen J, Kravitz S, et al. The diagnosis and treatment of heel pain. J Foot Ankle Surg. 2001;40:329-337.
5. US Food and Drug Administration. Fluoroquinolone antimicrobial drugs. Available at: http://www.fda.gov/cder/drug/infopage/fluoroquinolones/default.htm. Accessed October 7, 2008.
6. Lebrun CM. Management of Achilles tendinopathy. Clin J Sport Med. 2008;18:106-107.
7. Kane TP, Ismail M, Calder JD. Topical glyceryl trinitrate and noninsertional Achilles tendinopathy: a clinical and cellular investigation. Am J Sports Med. 2008;36:1160-1163.
8. Stergioulas A, Stergioula M, Aarskog R, et al. Effects of low-level laser therapy and eccentric exercises in the treatment of recreational athletes with chronic Achilles tendinopathy. Am J Sports Med. 2008;36:881-887.
9. Rompe JD, Furia J, Maffulli N. Eccentric loading compared with shock wave treatment for chronic insertional Achilles tendinopathy. J Bone Joint Surg Am. 2008;90:52-61.
10. Paoloni JA, Murrell Ga. Three-year follow up study of topical glyceryl trinitrate treatment of chronic noninsertional Achilles tendinopathy. Foot Ankle Int. 2007;28:1064-1068.
11. McLaughlan G, Handoll H. Interventions for treating acute and chronic Achilles tendonitis. Cochrane Database Syst Rev. 2001;(2):CD000232.-
12. Fahlstrom M, Jonsson P, Lorentzon R, et al. Chronic Achilles tendon pain treated with eccentric calfmuscle training. Knee Surg Sports Traumatol Arthrosc. 2003;11:327-333.
13. Costa M, Shepstone L, Donnell S, et al. Shock wave therapy for chronic Achilles tendon pain. Clin Orthop. 2005;44:199-204.
14. Furia J. High-energy extracorporeal shock wave therapy as a treatment for insertional Achilles tendinopathy. Am J Sports Med. 2006;34:733-740.
15. Maxwell NJ, Ryan MB, Taunton JE, et al. Sonographically guided intratendinous injection of hyperosmolar dextrose to treat chronic tendinosis of the Achilles tendon. Am J Roentgenol. 2007;189:W215-W220.Available at: http://www.ajronline.org/content/vol189/issue4/. Accessed May 15, 2008.
16. McShane JM, Ostick B, McCabe F. Noninsertional Achilles tendinopathy: pathology and management. Curr Sports Med Rep. 2007;6:288-292.
17. Russell AL. Peroxicam 0.5% topical gel compared to placebo in the treatment of acute soft tissue injuries: a double-blind study comparing efficacy and safety. Clin Invest Med. 1991;1:35-43.
18. Glaser T, Poddar S, Tweed B. What’s the best way to treat Achilles tendinopathy? J Fam Pract. 2008;57:261-263.
19. Solan M, Davies M. Management of insertional tendinopathy of the Achilles tendon. Foot Ankle Clin N Am. 2007;12:597-615.
20. Hugate R, Pennypacker, Saunders M, et al. The effects of intratendinous and retrocalcaneal intrabursal injections of corticosteroid on the biomechanical properties of rabbit Achilles tendons. J Bone Joint Surg Am. 2004;86:794-801.
21. Ishikawa SN. Conditions of the calcaneus in skeletally immature patients. Foot Ankle Clin N Am. 2005;10:503-512.
22. Hendrix CL. Calcaneal apophysitis (Sever’s disease). Clin Podiatr Med Surg. 2005;22:55-62.
23. Kassas KJ, Cassettari-Wayhs A. Childhood and adolescent sports-related overuse injuries. Am Fam Phys. 2006;73:1014-1021.
24. Van Dijk CN. Anterior and Posterior Ankle Impingement. Foot Ankle Clin. 2006;11:663-683.
25. Maquirriain J. Posterior ankle impingement syndrome. J Am Acad Orthop Surg. 2005;13:365-371.
26. Maffulli N. Chronic rupture of tendo Achillis. Foot Ankle Clin. 2007;12:583-596.
27. Bhandari M, Guyatt GH, Siddiqui F, et al. Treatment of acute Achilles tendon ruptures: a systematic overview and meta-analysis. Clin Orthop Relat Res. 2002;400:190-200.
28. Cole C, Seto C, Gazewood J. Plantar Fasciitis: Evidence based review of diagnosis and therapy. Am Fam Phys. 2005;72:2237-2242.
29. Barrett SL, O’Malley R. Plantar fasciitis and other causes of heel pain. Am Fam Physician. 1999;59:2200-2206.
30. Schroeder B. American College of Foot and Ankle Surgeons: diagnosis and treatment of heel pain. Am Fam Physician. 2002;65:1686-1687.
31. Crawford F, Thomson C. Interventions for treating plantar heel pain. Cochrane Database Syst Rev. 2003;(3):CD000416.-
32. Pribut SM. Current approaches to the management of plantar heel pain syndrome, including the role of injectable corticosteroids. J Am Podiatr Med. 2007;97:68-74.
33. Porter MD, Shadbolt B. Intralesional corticosteroid injection versus extracoporeal shock wave therapy for plantar fasciopathy. Clin J Sport Med. 2005;15:119-124.
34. Weber JM, Vidt LG, Gehl RS, et al. Calcaneal stress fracture. Clin Podiatr Med Surg. 2005;22:45-54.
35. Dodson NB, Dodson EE, Shromoff PJ. Imaging strategies for diagnosing calcaneal and cuboid stress fractures. Clin Podiatr Med Surg. 2008;25:183-201.
36. Wheeler P, Batt ME. Do nonsteroidal anti-inflammatory drugs adversely affect stress fracture healing? A short review. Br J Sports Med. 2005;39:65-69.
37. O’Connor FG, Sallis R, Wilder R, St. Pierre P. Sports Medicine: Just the Facts. 1st ed. New York: McGraw-Hill Professional; 2004:386,504.
38. Lake C, Trexler G, Barringer W. Posterior tibial tendon dysfunction: a review of pain and activity levels of twenty-one patients. J Prosth Ortho. 1999;11:2-5.
39. Bulstra G, Olsthoorn G, Niek van Dijk C. Tendonoscopy of the posterior tibial tendon. Foot Ankle Clin. 2006;11:421-427.
40. American College of Foot and Ankle Surgeons. Posterior tibial tendon dysfunction (PTTD). 2005. Available at: http://www.footphysicians.com/footankleinfo/pttd.htm. Accessed October 1, 2008.
41. Thordarson D. Orthopaedic Surgery Essentials: Foot and Ankle. Philadelphia: Lippincott Williams & Wilkins; 2004:176.
42. Stroud CC. Heel pain, plantar fasciitis, and tarsal tunnel syndrome. Curr Opin Ortho. 2002;13:89-92.
43. Franson J, Baravarian B. Tarsal tunnel syndrome: a compression neuropathy involving four distinct tunnels. Clin Podiatr Med Surg. 2006;23:597-609.
44. Jolly GP, Zgonis T, Hendrix CL. Neurogenic heel pain. Clin Podiatr Med Surg. 2005;22:101-103.
45. Juliano PJ, Harris TG. Plantar fasciitis, entrapment neuropathies, and tarsal tunnel syndrome: current up to date treatment. Curr Opin Orth. 2004;15:49-54.
46. Gondring WH, Shields B, Wenger S. An outcomes analysis of surgical treatment of tarsal tunnel syndrome. Foot Ankle Int. 2003;24:545-550.
47. Sammarco GJ, Chang L. Outcome of surgical treatment of tarsal tunnel syndrome. Foot Ankle Int. 2003;24:125-131.
- Advise patients with tendinopathy to decrease physical activity, do stretching exercises (C), undergo eccentric calf muscle training (B), use heel lifts (C), modify shoe fit, and take nonsteroidal anti-inflammatory drugs (NSAIDs) regularly for a few days, then as needed (B).
- The mainstay of treatment for calcaneal apophysitis in children is rest (C). Other options include heel lifts, stretching programs, icing, gel heel cups, and anti-inflammatory agents (C).
- Treatment options for plantar fasciitis include NSAIDs, stretching exercises, gel cups, arch supports, night splints, steroid injections, extracorporeal shock wave therapy, and surgery (B).
Strength of recommendation (SOR)
- Good-quality patient-oriented evidence
- Inconsistent or limited-quality patient-oriented evidence
- Consensus, usual practice, opinion, disease-oriented
One of your patients, a 40-year-old woman, recently began an exercise program, and she now says she has persistent heel pain. Your first suspicion is “another plantar fasciitis case.” However, after asking a few questions and performing a brief examination, you realize the problem is not what you expected. The pain is in the wrong place for plantar fasciitis and the patient’s history is atypical. How should you proceed?
Knowing the precise location of maximum pain or tenderness (FIGURES 1A–1C) and pairing that with key findings from the exam and history (TABLE 1) can help you reach an accurate diagnosis and formulate proper treatment (TABLE 2).
Each of the 3 general areas of heel pain—posterior, plantar, and medial—introduces a unique differential. Bilateral symptoms or multiple joint involvement, of course, raises the possibility of associated systemic disease.
FIGURE 1
Common causes of heel pain by location
TABLE 1
A quick guide to narrowing your heel pain diagnosis
| AFFECTED AREA | ONSET OF PAIN | HISTORY AND KEY FINDINGS | LIKELY DIAGNOSIS |
|---|---|---|---|
| Posterior heel | Acute |
| Achilles rupture |
| Achilles strain | ||
| Chronic |
| Achilles tendinopathy | |
| Retrocalcaneal bursitis | ||
| Calcaneal apophysitis | ||
| Posterior impingement | ||
| Plantar surface | Acute |
| Calcaneal fracture |
| Plantar fascial rupture | ||
| Chronic |
| Plantar fasciitis | |
| Calcaneal stress fracture | ||
| Fat pad syndrome | ||
| Medial heel | Subacute |
| Posterior tibial tendonitis |
| Chronic |
| Posterior tibial tendon dysfunction | |
| Tarsal tunnel syndrome |
Posterior heel pain
The common causes of posterior heel pain are Achilles tendinopathy, retrocalcaneal bursitis, calcaneal apophysitis, posterior impingement (FIGURE 1A), and Achilles tendon strain or rupture. Rarer causes are sciatica, peroneal tendonitis, Haglund’s deformity, pump bump, and systemic disorders. The patient’s history and precise location of maximal tenderness1 differentiates these problems.
Achilles tendinopathy (tendonitis): Is the patient an athlete?
Insertional and noninsertional Achilles tendinopathy are the most common causes of persistent posterior heel pain.2,3 The inflammatory process occurs in the fatty tissue surrounding the Achilles tendon (the paratenon) rather than in the tendon itself. Patients tend to be highly active (often athletes) and may have recently increased their activity. Ask patients, too, whether they have recently taken a fluoroquinolone antibiotic. This drug class is known to increase the risk of both tendonitis and tendon rupture,4 and in July of this year the FDA directed drug manufacturers to add a black-box warning to that effect.5
Evaluation of noninsertional tendinopathy. Tenderness is usually located 2 to 6 cm above the Achilles insertion. Nodularity, swelling, or fluctuance of the tendon may be evident. Diagnosis generally can be made clinically. If confirmation is needed, consider ultrasonography or magnetic resonance imaging.
Treatment. Advise patients to decrease physical activity and do stretching exercises, undergo eccentric calf muscle training, use heel lifts, modify shoe fit, and use systemic or topical nonsteroidal anti-inflammatory drugs (NSAIDs) regularly for a few days, then as needed. Refractory cases may require surgery.6 New therapies that have proven effective include extracorporeal shock wave therapy (ESWT), prolotherapy (dextrose injections), and local application of nitroglycerin patches or gel.7-18 ESWT can be expensive and is not widely available. Prolotherapy can be performed with minimal training, but is still relatively new. Topical nitroglycerin is affordable, but beware of such side effects as headache and hypotension.
Evaluation of insertional tendinopathy. Inflammation occurs at the tendon’s insertion to bone (enthesitis). Pain typically is at the midline and is reproduced by palpating the tendon insertion or by passively stretching the heel. The presentation may be difficult to distinguish from retrocalcaneal bursitis (discussed below).
Treatment is similar to that used for noninsertional tendinopathy. However, if insertional tendinopathy occurs in conjunction with a Haglund’s deformity (bony overgrowth of the calcaneus), surgery may be indicated, because noninvasive measures tend to fail.19
Use steroid injections with extreme caution due to the theoretical risk of tendon rupture.20 Injections are effective when directed at concomitant inflammation of the retrocalcaneal bursa, but accurate positioning and careful postinjection care are paramount. After an injection, a patient may need absolute rest or even immobilization to protect from tendon rupture. Emphasize a careful return to activity or athletic training.
Retrocalcaneal bursitis: Look for subtle swelling
The retrocalcaneal bursa lies between the Achilles tendon and the calcaneus near the tendon’s insertion. This bursa may become inflamed with repetitive stress or with insertional Achilles tendinopathy.
Evaluation. Swelling is usually present but may be subtle. Pain is located just lateral to the midline of the posterior heel at the superior angle of the calcaneus, and it may also be medial to the tendon opposite the lateral location.
Treatment. The bursitis often responds to icing and ice massage, shoe-fit adjustments, heel lifts, Achilles stretching programs, and systemic or topical NSAIDs.2 Steroid injections are likely beneficial, but use them with caution and take care to avoid the Achilles tendon insertion.
Calcaneal apophysitis affects highly active kids
Calcaneal apophysitis (Sever’s disease) is a painful inflammation in the heels of skeletally immature children where the Achilles tendon inserts in the calcaneus apophysis.
Evaluation. Associated with peak growth rate and high activity level, this inflammatory process usually occurs in boys between the ages of 10 and 12 years, and in girls between the ages of 8 and 10 years.21 The process is similar to that occurring at other sites of traction apophysitis, such as Osgood-Schlatter disease at the tibial tuberosity. Children most susceptible are highly active, wear poorly fitting footwear, run frequently on hard surfaces, and have tight Achilles tendons. Clinical diagnosis usually suffices, although plain x-ray films can verify an active apophysis and rule out other sources of pain, such as tarsal coalition, calcaneal stress fractures, or infection.22
Treatment. Calcaneal apophysitis is typically self-limiting, and the mainstay of treatment is rest. Heel lifts, stretching programs, icing, gel heel cups, and anti-inflammatory agents may also be used.23
Posterior impingement: Pain with full plantar flexion
Posterior impingement at the ankle joint may be self-originating or arise as a consequence of an os trigonum, a posterior sesamoid bone of the talus that exists as a normal variant. In some cases, this bone creates a barrier to full plantar flexion at the ankle joint and creates pain at the posterior heel.
Evaluation. Pain with full plantar flexion is a critical distinguishing feature, because most other pathologies in the posterior heel cause pain with dorsiflexion at the ankle.24,25 Patients often are involved in activities that require forced plantar flexion, such as gymnastics or dancing. Diagnosis is clinical for the most part, but plain x-ray films may confirm the presence of an os trigonum. Magnetic resonance imaging (MRI) is warranted for patients with persistent symptoms; it may reveal a hypertrophied synovial lining or other pathology (such as osteochondritis). MRI is also indicated before more invasive therapies, such as steroid injections or surgery.
Treatment. Advise rest with or without immobilization, NSAIDs, or local steroid injections. Severe impingement or recalcitrant cases may require surgical release of the posterior synovium or removal of an os trigonum.24,25
Achilles strain and rupture: Middle-aged men are susceptible
The Achilles tendon is most susceptible to injury in middle-aged men who are active in sports requiring loading and sudden contraction of the calf muscles, such as basketball or football, although injuries may occur in a variety of other settings. A strain of the Achilles tendon should be carefully differentiated from a complete rupture. While strains can be treated similarly to Achilles tendinopathy, complete rupture is a much larger concern.
Evaluation. When the Achilles tendon ruptures, patients describe sudden pain and a pop that is often audible. Poor plantar flexion of the foot ensues.26 Telltale signs on examination are a positive Thompson’s test (little or no plantar flexion with a calf squeeze) and a visible defect in the tendon. The rupture site is usually 1 to 2 inches proximal to its insertion on the calcaneus.
Treatment. Most of these patients should be seen by an orthopedic surgeon as soon as possible. For active and younger adults, treatment is almost always early surgical repair.27 For some older individuals who are less active, nonsurgical management includes graduated casting, which progressively lessens plantar flexion over 6 to 10 weeks, followed by physical therapy.
3 less common causes of posterior heel pain
Haglund’s deformity is an overgrowth of the calcaneus at the insertion of the Achilles tendon.3 Caused by overuse and poorly fitted shoes, this condition commonly requires surgical intervention.
Pump bump is an inflamed superficial bursa commonly associated with a Haglund’s deformity, and it may respond to NSAIDs, shoe-fit modification, ice massage, or steroid injection.
Peroneal tendonitis is a tendinopathy of evertors and external rotators of the foot. The pain will follow the tendons posterior to the lateral malleolus and extend to the lateral midfoot. It is also treated with rest, NSAIDs, icing, and physical therapy.
Plantar-surface heel pain
The problems most likely to cause plantar-surface pain (FIGURE 1B) are plantar fasciitis, stress fracture of the calcaneus, and fat pad syndrome.
Plantar fasciitis: Pain is worst in the morning
This is by far the most common cause of heel pain primary care physicians will see. Rarely, infection and neoplasia will cause unilateral plantar heel pain.4
Evaluation. Tenderness localized to the plantar surface of the heel in adults usually indicates plantar fasciitis.
Pain is worst with the first step of the morning, and lessens with activity. The tender spot is the medial calcaneal tubercle, with pain radiating through the arch.1,28-30
Treatment. The many therapeutic modalities—NSAIDs, stretching exercises, gel cups, arch supports, night splints, steroid injections, ESWT, and surgery—have been extensively reviewed elsewhere, including in a Cochrane review from 2005.31-33
Calcaneal stress fracture: Suspect it in runners
Calcaneal stress fractures are relatively rare, but may occur in those who put significant stressors on their feet, such as avid runners or military recruits.
Evaluation. Most patients report a recent increase in frequency or intensity of activity, and runners can tell you when it is during their run that the pain begins. As the stress fracture worsens, the pain begins earlier in the activity and eventually is present with even minimal activity. A key distinction from plantar fasciitis, in which pain lessens with activity, is that the pain of a stress fracture typically worsens with activity and diminishes with rest.34
Physical exam provides few clues except for the “squeeze test” (FIGURE 1A) Putting pressure on both the medial and lateral calcaneal tuberosities will cause discomfort. Pain will be absent in the posterior structures of the heel. Placing a vibrating 128-cps tuning fork on the calcaneus should also increase discomfort.
Plain x-ray films may be falsely negative, especially during the first 2 to 3 weeks of pain. Three-phase bone scans are nearly 100% sensitive for detecting stress fractures, with changes evident in as little as 1 to 2 days after injury. The specificity of MRI scans is superior to that of bone scans and can reveal alternate problems.35
Treatment. Activity modification reduces trauma to the heel. Encourage patients to walk if they are pain free and to increase activity as comfort allows. Tell patients to stop activity if the fracture becomes symptomatic. Advanced fractures demand an absolute absence of weight bearing.
Pain can be controlled with NSAIDs TABLE 2 and ice. Lab and animal data have suggested that NSAIDs may impede fracture healing rates, but no similar data exist regarding their effect on stress fractures.36 Symptoms abate within 2 or 3 weeks. Advise athletes to resume activity slowly in a stepwise progression, letting them know that a return to full activity is likely within 6 to 8 weeks. Have runners restart their routine at half their customary distance, increasing it by no more than 10% to 15% per week.
Any medical condition that weakens the bone may predispose a patient to stress fracture. To prevent primary and secondary stress fractures, correct the patient’s underlying medical problems. Evaluate young, thin women with a stress fracture for the “female athlete triad” (osteopenia, disordered eating, menstrual irregularity). The elderly are also at risk for stress fractures due to osteopenia or osteoporosis.
TABLE 2
Heel pain treatment options: A look at the evidence
| DIAGNOSIS | TREATMENTS | SOR | REFERENCE |
|---|---|---|---|
| Achilles tendinopathy | NSAIDs Topical NSAIDs Eccentric calf muscle training Stretching Heel lifts Ice Topical nitrates Prolotherapy (dextrose injections) ESWT Surgery | B B B C C C B B B C | 18 17 12,18 16,19 16,19 16,19 7,10,18 15,18 9,18 19 |
| Retrocalcaneal bursitis | NSAIDs Heel lifts Steroid Injections (with caution) | C C C | 2 2 2 |
| Calcaneal apophysitis | Rest NSAIDs Heel lifts Stretching Icing Gel heel cups | C C C C C C | 21,22 21,22 21,22 21,22 21,22 21,22 |
| Posterior impingement | Rest NSAIDs Steroid injections Surgery | C C C C | 24 24 24 24 |
| Plantar fasciitis | NSAIDs Stretches Gel cups Steroid iontophoresis Arch supports ESWT Night splints Steroid injections Surgery | B B B B B B B B B | 28,31 28,31 28,31 28,31 28,31 28,31,33 28,33 28,31 28,31 |
| Calcaneal stress fracture | NSAIDs Activity moderation Icing | C C C | 34,36 34 34 |
| Fat pad syndrome | NSAIDs Rest Gel heel cups Icing | C C C C | 37 37 37 37 |
| Posterior tibial tendon dysfunction | Weight loss Icing Physical therapy Arch supports/bracing NSAIDs Surgery | C C C C C B | 40 40 40 41 38 38,39 |
| Tarsal tunnel syndrome | Arch supports NSAIDs Activity modification Physical therapy Neuromodulators Steroid injections Surgery | C C C C C C B | 1,43 42,43 42,43 42,43 42 44 46,47 |
| ESWT, extracorporeal shock wave therapy; NSAIDs, nonsteroidal anti-inflammatory drugs. | |||
| Strength of recommendation (SOR) A Good-quality patient-oriented evidence B Inconsistent or limited-quality patient-oriented evidence C Consensus, usual practice, opinion, disease-oriented | |||
Fat pad syndrome: More diffuse pain than plantar fasciitis
The plantar surface of the heel is protected by a thick fat pad. Those at risk of a thinned fat pad include the elderly (the pad thins with age), the obese (increased stress to the pad), and those who have previously received a corticosteroid injection in the pad. Cumulative or acute trauma to the heel can also cause contusion to the heel pad.
Evaluation. Pain typically is located more posteriorly than classic plantar fasciitis pain and is more diffuse. Pain from the fat pad should not radiate toward the arch and is not exacerbated by dorsiflexion of the foot.1
Treatment. Recommend relative rest, gel heel cups, NSAIDs, and ice.37
Less common causes of plantar-surface pain
Lateral plantar nerve entrapment may also cause neuropathic pain on the plantar surface. Patients who experience a painful pop in their heel associated with trauma may have ruptured their plantar fascia. A fallen arch may also be noted on exam. Treatment of both of these conditions is similar to that of plantar fasciitis.
Acute calcaneal fracture results from trauma, such as a fall from a height onto the soles of the feet. Look for localized pain and swelling around the calcaneus and evaluate the neurovascular status of the foot. Initial treatment includes elevating the foot, avoiding weight bearing, applying ice, controlling pain, and using a posterior splint. Many of these fractures require surgical fixation.
Medial heel pain
Posterior tibial tendonitis/dysfunction and tarsal tunnel syndrome are best classified as medial in location (FIGURE 1C). However, the pain is often more diffuse and may radiate to either the posterior or plantar heel.
Posterior tibial tendonitis/dysfunction are linked to obesity
Posterior tibial tendonitis (PTT) and posterior tibial tendon dysfunction (PTTD) are related diagnoses. PTTD refers to increased laxity of the tendon resulting in flat foot and increased heel varus. It is the most common cause of acquired flat foot in adults. PTT may exist separately or as part of PTTD.
Evaluation. Patients complain of pain at the posterior edge of the medial malleolus that may extend toward the arch of the foot.38,39 Patients may also experience swelling or redness in the area. Both PTT and PTTD seem related to overuse and obesity. Young or nonobese patients with PTT or PTTD often have underlying systemic arthropathies.35
Treatment. Early treatment is necessary to prevent progression of tendon incompetence. Interventions include weight loss, NSAIDs, icing, physical therapy,40 and orthotics or bracing for arch and ankle support. You may also try immobilization in a short leg cast for 6 weeks.41 If conservative measures fail, surgery may be necessary for tendon repair, tendon transfer, calcaneal osteotomy, or tarsal bone fusion.38,39
Tarsal tunnel syndrome: Pain can occur at night
Tarsal tunnel syndrome (TTS) is the most common compression neuropathy of the lower extremity. The tarsal tunnel is a fibro-osseous structure along the medial ankle that contains the tibial nerve, the posterior tibial artery, and the tendons of the tibialis posterior, flexor digitorum longus, and flexor hallucis longus. The posterior tibial nerve can become irritated as it runs through the tunnel. The inciting incident can be either a severe stretch to the nerve (from a medial ankle sprain) or from an anatomic compression. Pes planus foot or posterior tibial dysfunction have also been implicated as common causes.1
Evaluation. Patients describe poorly localized pain with numbness and burning along the medial ankle, arch, or heel, with radiation proximally.42,43 Symptoms are aggravated by exercise, and night pain is not uncommon. The tenderness of TTS is more diffuse than that from plantar fasciitis, and symptoms are evident directly over the tarsal tunnel itself.
The classic finding is a positive Tinel’s sign (reproduction of symptoms by tapping over the posterior tibial nerve as it passes through the tarsal tunnel). Placing the foot in dorsiflexion and eversion may also reproduce symptoms.1
Imaging results are not always definitive, but can be helpful in determining the cause of the compression. Plain films and CT can detect fracture or bony deformity, while MRI is more helpful in evaluating soft-tissue structures, such as ganglions or varicosities. Abnormal nerve conduction studies can be suggestive of TTS, but a normal result does not rule out the diagnosis.
Treatment follows a stepped progression. Initially try activity modification, orthotics, and physical therapy. Physical therapy concentrates on medial arch strengthening, Achilles stretching, and ankle proprioception exercises.
NSAIDs and neuromodulatory drugs (tricyclic antidepressants and antiseizure medications) have shown some success. Steroid injections have been effective when given at the site of entrapment,44 but care must be taken to avoid the posterior tibial tendon. If patients do not improve following these measures, they may require cast immobilization.45
Surgery is a possibility when other options fail. The cause of the neural compression is identified in 60% to 80% of cases.46,47 Success rates for various procedures of tarsal tunnel release and tibial nerve decompression range from 75% to 91%. If neural compression is absent, investigate other systemic causes of peripheral neuropathy, such as diabetes or alcoholism.4
Systemic diagnoses
Bilateral heel pain, multiple joint involvement, or fever suggests systemic disease. Common diseases affecting the heel include rheumatoid arthritis, ankylosing spondylitis, psoriatic arthritis, reactive arthritis, and inflammatory bowel disease.1 Successful treatment of these disorders should relieve associated heel pain.
Correspondence
H. E. Woodall, MD, AnMed Health Family Medicine Residency, 2000 E Greenville Street, Suite 3600, Anderson, SC 29621; [email protected]
- Advise patients with tendinopathy to decrease physical activity, do stretching exercises (C), undergo eccentric calf muscle training (B), use heel lifts (C), modify shoe fit, and take nonsteroidal anti-inflammatory drugs (NSAIDs) regularly for a few days, then as needed (B).
- The mainstay of treatment for calcaneal apophysitis in children is rest (C). Other options include heel lifts, stretching programs, icing, gel heel cups, and anti-inflammatory agents (C).
- Treatment options for plantar fasciitis include NSAIDs, stretching exercises, gel cups, arch supports, night splints, steroid injections, extracorporeal shock wave therapy, and surgery (B).
Strength of recommendation (SOR)
- Good-quality patient-oriented evidence
- Inconsistent or limited-quality patient-oriented evidence
- Consensus, usual practice, opinion, disease-oriented
One of your patients, a 40-year-old woman, recently began an exercise program, and she now says she has persistent heel pain. Your first suspicion is “another plantar fasciitis case.” However, after asking a few questions and performing a brief examination, you realize the problem is not what you expected. The pain is in the wrong place for plantar fasciitis and the patient’s history is atypical. How should you proceed?
Knowing the precise location of maximum pain or tenderness (FIGURES 1A–1C) and pairing that with key findings from the exam and history (TABLE 1) can help you reach an accurate diagnosis and formulate proper treatment (TABLE 2).
Each of the 3 general areas of heel pain—posterior, plantar, and medial—introduces a unique differential. Bilateral symptoms or multiple joint involvement, of course, raises the possibility of associated systemic disease.
FIGURE 1
Common causes of heel pain by location
TABLE 1
A quick guide to narrowing your heel pain diagnosis
| AFFECTED AREA | ONSET OF PAIN | HISTORY AND KEY FINDINGS | LIKELY DIAGNOSIS |
|---|---|---|---|
| Posterior heel | Acute |
| Achilles rupture |
| Achilles strain | ||
| Chronic |
| Achilles tendinopathy | |
| Retrocalcaneal bursitis | ||
| Calcaneal apophysitis | ||
| Posterior impingement | ||
| Plantar surface | Acute |
| Calcaneal fracture |
| Plantar fascial rupture | ||
| Chronic |
| Plantar fasciitis | |
| Calcaneal stress fracture | ||
| Fat pad syndrome | ||
| Medial heel | Subacute |
| Posterior tibial tendonitis |
| Chronic |
| Posterior tibial tendon dysfunction | |
| Tarsal tunnel syndrome |
Posterior heel pain
The common causes of posterior heel pain are Achilles tendinopathy, retrocalcaneal bursitis, calcaneal apophysitis, posterior impingement (FIGURE 1A), and Achilles tendon strain or rupture. Rarer causes are sciatica, peroneal tendonitis, Haglund’s deformity, pump bump, and systemic disorders. The patient’s history and precise location of maximal tenderness1 differentiates these problems.
Achilles tendinopathy (tendonitis): Is the patient an athlete?
Insertional and noninsertional Achilles tendinopathy are the most common causes of persistent posterior heel pain.2,3 The inflammatory process occurs in the fatty tissue surrounding the Achilles tendon (the paratenon) rather than in the tendon itself. Patients tend to be highly active (often athletes) and may have recently increased their activity. Ask patients, too, whether they have recently taken a fluoroquinolone antibiotic. This drug class is known to increase the risk of both tendonitis and tendon rupture,4 and in July of this year the FDA directed drug manufacturers to add a black-box warning to that effect.5
Evaluation of noninsertional tendinopathy. Tenderness is usually located 2 to 6 cm above the Achilles insertion. Nodularity, swelling, or fluctuance of the tendon may be evident. Diagnosis generally can be made clinically. If confirmation is needed, consider ultrasonography or magnetic resonance imaging.
Treatment. Advise patients to decrease physical activity and do stretching exercises, undergo eccentric calf muscle training, use heel lifts, modify shoe fit, and use systemic or topical nonsteroidal anti-inflammatory drugs (NSAIDs) regularly for a few days, then as needed. Refractory cases may require surgery.6 New therapies that have proven effective include extracorporeal shock wave therapy (ESWT), prolotherapy (dextrose injections), and local application of nitroglycerin patches or gel.7-18 ESWT can be expensive and is not widely available. Prolotherapy can be performed with minimal training, but is still relatively new. Topical nitroglycerin is affordable, but beware of such side effects as headache and hypotension.
Evaluation of insertional tendinopathy. Inflammation occurs at the tendon’s insertion to bone (enthesitis). Pain typically is at the midline and is reproduced by palpating the tendon insertion or by passively stretching the heel. The presentation may be difficult to distinguish from retrocalcaneal bursitis (discussed below).
Treatment is similar to that used for noninsertional tendinopathy. However, if insertional tendinopathy occurs in conjunction with a Haglund’s deformity (bony overgrowth of the calcaneus), surgery may be indicated, because noninvasive measures tend to fail.19
Use steroid injections with extreme caution due to the theoretical risk of tendon rupture.20 Injections are effective when directed at concomitant inflammation of the retrocalcaneal bursa, but accurate positioning and careful postinjection care are paramount. After an injection, a patient may need absolute rest or even immobilization to protect from tendon rupture. Emphasize a careful return to activity or athletic training.
Retrocalcaneal bursitis: Look for subtle swelling
The retrocalcaneal bursa lies between the Achilles tendon and the calcaneus near the tendon’s insertion. This bursa may become inflamed with repetitive stress or with insertional Achilles tendinopathy.
Evaluation. Swelling is usually present but may be subtle. Pain is located just lateral to the midline of the posterior heel at the superior angle of the calcaneus, and it may also be medial to the tendon opposite the lateral location.
Treatment. The bursitis often responds to icing and ice massage, shoe-fit adjustments, heel lifts, Achilles stretching programs, and systemic or topical NSAIDs.2 Steroid injections are likely beneficial, but use them with caution and take care to avoid the Achilles tendon insertion.
Calcaneal apophysitis affects highly active kids
Calcaneal apophysitis (Sever’s disease) is a painful inflammation in the heels of skeletally immature children where the Achilles tendon inserts in the calcaneus apophysis.
Evaluation. Associated with peak growth rate and high activity level, this inflammatory process usually occurs in boys between the ages of 10 and 12 years, and in girls between the ages of 8 and 10 years.21 The process is similar to that occurring at other sites of traction apophysitis, such as Osgood-Schlatter disease at the tibial tuberosity. Children most susceptible are highly active, wear poorly fitting footwear, run frequently on hard surfaces, and have tight Achilles tendons. Clinical diagnosis usually suffices, although plain x-ray films can verify an active apophysis and rule out other sources of pain, such as tarsal coalition, calcaneal stress fractures, or infection.22
Treatment. Calcaneal apophysitis is typically self-limiting, and the mainstay of treatment is rest. Heel lifts, stretching programs, icing, gel heel cups, and anti-inflammatory agents may also be used.23
Posterior impingement: Pain with full plantar flexion
Posterior impingement at the ankle joint may be self-originating or arise as a consequence of an os trigonum, a posterior sesamoid bone of the talus that exists as a normal variant. In some cases, this bone creates a barrier to full plantar flexion at the ankle joint and creates pain at the posterior heel.
Evaluation. Pain with full plantar flexion is a critical distinguishing feature, because most other pathologies in the posterior heel cause pain with dorsiflexion at the ankle.24,25 Patients often are involved in activities that require forced plantar flexion, such as gymnastics or dancing. Diagnosis is clinical for the most part, but plain x-ray films may confirm the presence of an os trigonum. Magnetic resonance imaging (MRI) is warranted for patients with persistent symptoms; it may reveal a hypertrophied synovial lining or other pathology (such as osteochondritis). MRI is also indicated before more invasive therapies, such as steroid injections or surgery.
Treatment. Advise rest with or without immobilization, NSAIDs, or local steroid injections. Severe impingement or recalcitrant cases may require surgical release of the posterior synovium or removal of an os trigonum.24,25
Achilles strain and rupture: Middle-aged men are susceptible
The Achilles tendon is most susceptible to injury in middle-aged men who are active in sports requiring loading and sudden contraction of the calf muscles, such as basketball or football, although injuries may occur in a variety of other settings. A strain of the Achilles tendon should be carefully differentiated from a complete rupture. While strains can be treated similarly to Achilles tendinopathy, complete rupture is a much larger concern.
Evaluation. When the Achilles tendon ruptures, patients describe sudden pain and a pop that is often audible. Poor plantar flexion of the foot ensues.26 Telltale signs on examination are a positive Thompson’s test (little or no plantar flexion with a calf squeeze) and a visible defect in the tendon. The rupture site is usually 1 to 2 inches proximal to its insertion on the calcaneus.
Treatment. Most of these patients should be seen by an orthopedic surgeon as soon as possible. For active and younger adults, treatment is almost always early surgical repair.27 For some older individuals who are less active, nonsurgical management includes graduated casting, which progressively lessens plantar flexion over 6 to 10 weeks, followed by physical therapy.
3 less common causes of posterior heel pain
Haglund’s deformity is an overgrowth of the calcaneus at the insertion of the Achilles tendon.3 Caused by overuse and poorly fitted shoes, this condition commonly requires surgical intervention.
Pump bump is an inflamed superficial bursa commonly associated with a Haglund’s deformity, and it may respond to NSAIDs, shoe-fit modification, ice massage, or steroid injection.
Peroneal tendonitis is a tendinopathy of evertors and external rotators of the foot. The pain will follow the tendons posterior to the lateral malleolus and extend to the lateral midfoot. It is also treated with rest, NSAIDs, icing, and physical therapy.
Plantar-surface heel pain
The problems most likely to cause plantar-surface pain (FIGURE 1B) are plantar fasciitis, stress fracture of the calcaneus, and fat pad syndrome.
Plantar fasciitis: Pain is worst in the morning
This is by far the most common cause of heel pain primary care physicians will see. Rarely, infection and neoplasia will cause unilateral plantar heel pain.4
Evaluation. Tenderness localized to the plantar surface of the heel in adults usually indicates plantar fasciitis.
Pain is worst with the first step of the morning, and lessens with activity. The tender spot is the medial calcaneal tubercle, with pain radiating through the arch.1,28-30
Treatment. The many therapeutic modalities—NSAIDs, stretching exercises, gel cups, arch supports, night splints, steroid injections, ESWT, and surgery—have been extensively reviewed elsewhere, including in a Cochrane review from 2005.31-33
Calcaneal stress fracture: Suspect it in runners
Calcaneal stress fractures are relatively rare, but may occur in those who put significant stressors on their feet, such as avid runners or military recruits.
Evaluation. Most patients report a recent increase in frequency or intensity of activity, and runners can tell you when it is during their run that the pain begins. As the stress fracture worsens, the pain begins earlier in the activity and eventually is present with even minimal activity. A key distinction from plantar fasciitis, in which pain lessens with activity, is that the pain of a stress fracture typically worsens with activity and diminishes with rest.34
Physical exam provides few clues except for the “squeeze test” (FIGURE 1A) Putting pressure on both the medial and lateral calcaneal tuberosities will cause discomfort. Pain will be absent in the posterior structures of the heel. Placing a vibrating 128-cps tuning fork on the calcaneus should also increase discomfort.
Plain x-ray films may be falsely negative, especially during the first 2 to 3 weeks of pain. Three-phase bone scans are nearly 100% sensitive for detecting stress fractures, with changes evident in as little as 1 to 2 days after injury. The specificity of MRI scans is superior to that of bone scans and can reveal alternate problems.35
Treatment. Activity modification reduces trauma to the heel. Encourage patients to walk if they are pain free and to increase activity as comfort allows. Tell patients to stop activity if the fracture becomes symptomatic. Advanced fractures demand an absolute absence of weight bearing.
Pain can be controlled with NSAIDs TABLE 2 and ice. Lab and animal data have suggested that NSAIDs may impede fracture healing rates, but no similar data exist regarding their effect on stress fractures.36 Symptoms abate within 2 or 3 weeks. Advise athletes to resume activity slowly in a stepwise progression, letting them know that a return to full activity is likely within 6 to 8 weeks. Have runners restart their routine at half their customary distance, increasing it by no more than 10% to 15% per week.
Any medical condition that weakens the bone may predispose a patient to stress fracture. To prevent primary and secondary stress fractures, correct the patient’s underlying medical problems. Evaluate young, thin women with a stress fracture for the “female athlete triad” (osteopenia, disordered eating, menstrual irregularity). The elderly are also at risk for stress fractures due to osteopenia or osteoporosis.
TABLE 2
Heel pain treatment options: A look at the evidence
| DIAGNOSIS | TREATMENTS | SOR | REFERENCE |
|---|---|---|---|
| Achilles tendinopathy | NSAIDs Topical NSAIDs Eccentric calf muscle training Stretching Heel lifts Ice Topical nitrates Prolotherapy (dextrose injections) ESWT Surgery | B B B C C C B B B C | 18 17 12,18 16,19 16,19 16,19 7,10,18 15,18 9,18 19 |
| Retrocalcaneal bursitis | NSAIDs Heel lifts Steroid Injections (with caution) | C C C | 2 2 2 |
| Calcaneal apophysitis | Rest NSAIDs Heel lifts Stretching Icing Gel heel cups | C C C C C C | 21,22 21,22 21,22 21,22 21,22 21,22 |
| Posterior impingement | Rest NSAIDs Steroid injections Surgery | C C C C | 24 24 24 24 |
| Plantar fasciitis | NSAIDs Stretches Gel cups Steroid iontophoresis Arch supports ESWT Night splints Steroid injections Surgery | B B B B B B B B B | 28,31 28,31 28,31 28,31 28,31 28,31,33 28,33 28,31 28,31 |
| Calcaneal stress fracture | NSAIDs Activity moderation Icing | C C C | 34,36 34 34 |
| Fat pad syndrome | NSAIDs Rest Gel heel cups Icing | C C C C | 37 37 37 37 |
| Posterior tibial tendon dysfunction | Weight loss Icing Physical therapy Arch supports/bracing NSAIDs Surgery | C C C C C B | 40 40 40 41 38 38,39 |
| Tarsal tunnel syndrome | Arch supports NSAIDs Activity modification Physical therapy Neuromodulators Steroid injections Surgery | C C C C C C B | 1,43 42,43 42,43 42,43 42 44 46,47 |
| ESWT, extracorporeal shock wave therapy; NSAIDs, nonsteroidal anti-inflammatory drugs. | |||
| Strength of recommendation (SOR) A Good-quality patient-oriented evidence B Inconsistent or limited-quality patient-oriented evidence C Consensus, usual practice, opinion, disease-oriented | |||
Fat pad syndrome: More diffuse pain than plantar fasciitis
The plantar surface of the heel is protected by a thick fat pad. Those at risk of a thinned fat pad include the elderly (the pad thins with age), the obese (increased stress to the pad), and those who have previously received a corticosteroid injection in the pad. Cumulative or acute trauma to the heel can also cause contusion to the heel pad.
Evaluation. Pain typically is located more posteriorly than classic plantar fasciitis pain and is more diffuse. Pain from the fat pad should not radiate toward the arch and is not exacerbated by dorsiflexion of the foot.1
Treatment. Recommend relative rest, gel heel cups, NSAIDs, and ice.37
Less common causes of plantar-surface pain
Lateral plantar nerve entrapment may also cause neuropathic pain on the plantar surface. Patients who experience a painful pop in their heel associated with trauma may have ruptured their plantar fascia. A fallen arch may also be noted on exam. Treatment of both of these conditions is similar to that of plantar fasciitis.
Acute calcaneal fracture results from trauma, such as a fall from a height onto the soles of the feet. Look for localized pain and swelling around the calcaneus and evaluate the neurovascular status of the foot. Initial treatment includes elevating the foot, avoiding weight bearing, applying ice, controlling pain, and using a posterior splint. Many of these fractures require surgical fixation.
Medial heel pain
Posterior tibial tendonitis/dysfunction and tarsal tunnel syndrome are best classified as medial in location (FIGURE 1C). However, the pain is often more diffuse and may radiate to either the posterior or plantar heel.
Posterior tibial tendonitis/dysfunction are linked to obesity
Posterior tibial tendonitis (PTT) and posterior tibial tendon dysfunction (PTTD) are related diagnoses. PTTD refers to increased laxity of the tendon resulting in flat foot and increased heel varus. It is the most common cause of acquired flat foot in adults. PTT may exist separately or as part of PTTD.
Evaluation. Patients complain of pain at the posterior edge of the medial malleolus that may extend toward the arch of the foot.38,39 Patients may also experience swelling or redness in the area. Both PTT and PTTD seem related to overuse and obesity. Young or nonobese patients with PTT or PTTD often have underlying systemic arthropathies.35
Treatment. Early treatment is necessary to prevent progression of tendon incompetence. Interventions include weight loss, NSAIDs, icing, physical therapy,40 and orthotics or bracing for arch and ankle support. You may also try immobilization in a short leg cast for 6 weeks.41 If conservative measures fail, surgery may be necessary for tendon repair, tendon transfer, calcaneal osteotomy, or tarsal bone fusion.38,39
Tarsal tunnel syndrome: Pain can occur at night
Tarsal tunnel syndrome (TTS) is the most common compression neuropathy of the lower extremity. The tarsal tunnel is a fibro-osseous structure along the medial ankle that contains the tibial nerve, the posterior tibial artery, and the tendons of the tibialis posterior, flexor digitorum longus, and flexor hallucis longus. The posterior tibial nerve can become irritated as it runs through the tunnel. The inciting incident can be either a severe stretch to the nerve (from a medial ankle sprain) or from an anatomic compression. Pes planus foot or posterior tibial dysfunction have also been implicated as common causes.1
Evaluation. Patients describe poorly localized pain with numbness and burning along the medial ankle, arch, or heel, with radiation proximally.42,43 Symptoms are aggravated by exercise, and night pain is not uncommon. The tenderness of TTS is more diffuse than that from plantar fasciitis, and symptoms are evident directly over the tarsal tunnel itself.
The classic finding is a positive Tinel’s sign (reproduction of symptoms by tapping over the posterior tibial nerve as it passes through the tarsal tunnel). Placing the foot in dorsiflexion and eversion may also reproduce symptoms.1
Imaging results are not always definitive, but can be helpful in determining the cause of the compression. Plain films and CT can detect fracture or bony deformity, while MRI is more helpful in evaluating soft-tissue structures, such as ganglions or varicosities. Abnormal nerve conduction studies can be suggestive of TTS, but a normal result does not rule out the diagnosis.
Treatment follows a stepped progression. Initially try activity modification, orthotics, and physical therapy. Physical therapy concentrates on medial arch strengthening, Achilles stretching, and ankle proprioception exercises.
NSAIDs and neuromodulatory drugs (tricyclic antidepressants and antiseizure medications) have shown some success. Steroid injections have been effective when given at the site of entrapment,44 but care must be taken to avoid the posterior tibial tendon. If patients do not improve following these measures, they may require cast immobilization.45
Surgery is a possibility when other options fail. The cause of the neural compression is identified in 60% to 80% of cases.46,47 Success rates for various procedures of tarsal tunnel release and tibial nerve decompression range from 75% to 91%. If neural compression is absent, investigate other systemic causes of peripheral neuropathy, such as diabetes or alcoholism.4
Systemic diagnoses
Bilateral heel pain, multiple joint involvement, or fever suggests systemic disease. Common diseases affecting the heel include rheumatoid arthritis, ankylosing spondylitis, psoriatic arthritis, reactive arthritis, and inflammatory bowel disease.1 Successful treatment of these disorders should relieve associated heel pain.
Correspondence
H. E. Woodall, MD, AnMed Health Family Medicine Residency, 2000 E Greenville Street, Suite 3600, Anderson, SC 29621; [email protected]
1. Aldridge T. Diagnosing heel pain in adults. Am Fam Physician. 2004;70:332-338.
2. Aronow MS. Posterior heel pain (retrocalcaneal bursitis, insertional and noninsertional Achilles tendinopathy). Clin Podiatr Med Surg. 2005;22:19-43.
3. Alvarez-Nemegyei J, Canoso J. Heel pain: diagnosis and treatment, step by step. Clev Clin J Med. 2006;73:465-471.
4. Thomas J, Christensen J, Kravitz S, et al. The diagnosis and treatment of heel pain. J Foot Ankle Surg. 2001;40:329-337.
5. US Food and Drug Administration. Fluoroquinolone antimicrobial drugs. Available at: http://www.fda.gov/cder/drug/infopage/fluoroquinolones/default.htm. Accessed October 7, 2008.
6. Lebrun CM. Management of Achilles tendinopathy. Clin J Sport Med. 2008;18:106-107.
7. Kane TP, Ismail M, Calder JD. Topical glyceryl trinitrate and noninsertional Achilles tendinopathy: a clinical and cellular investigation. Am J Sports Med. 2008;36:1160-1163.
8. Stergioulas A, Stergioula M, Aarskog R, et al. Effects of low-level laser therapy and eccentric exercises in the treatment of recreational athletes with chronic Achilles tendinopathy. Am J Sports Med. 2008;36:881-887.
9. Rompe JD, Furia J, Maffulli N. Eccentric loading compared with shock wave treatment for chronic insertional Achilles tendinopathy. J Bone Joint Surg Am. 2008;90:52-61.
10. Paoloni JA, Murrell Ga. Three-year follow up study of topical glyceryl trinitrate treatment of chronic noninsertional Achilles tendinopathy. Foot Ankle Int. 2007;28:1064-1068.
11. McLaughlan G, Handoll H. Interventions for treating acute and chronic Achilles tendonitis. Cochrane Database Syst Rev. 2001;(2):CD000232.-
12. Fahlstrom M, Jonsson P, Lorentzon R, et al. Chronic Achilles tendon pain treated with eccentric calfmuscle training. Knee Surg Sports Traumatol Arthrosc. 2003;11:327-333.
13. Costa M, Shepstone L, Donnell S, et al. Shock wave therapy for chronic Achilles tendon pain. Clin Orthop. 2005;44:199-204.
14. Furia J. High-energy extracorporeal shock wave therapy as a treatment for insertional Achilles tendinopathy. Am J Sports Med. 2006;34:733-740.
15. Maxwell NJ, Ryan MB, Taunton JE, et al. Sonographically guided intratendinous injection of hyperosmolar dextrose to treat chronic tendinosis of the Achilles tendon. Am J Roentgenol. 2007;189:W215-W220.Available at: http://www.ajronline.org/content/vol189/issue4/. Accessed May 15, 2008.
16. McShane JM, Ostick B, McCabe F. Noninsertional Achilles tendinopathy: pathology and management. Curr Sports Med Rep. 2007;6:288-292.
17. Russell AL. Peroxicam 0.5% topical gel compared to placebo in the treatment of acute soft tissue injuries: a double-blind study comparing efficacy and safety. Clin Invest Med. 1991;1:35-43.
18. Glaser T, Poddar S, Tweed B. What’s the best way to treat Achilles tendinopathy? J Fam Pract. 2008;57:261-263.
19. Solan M, Davies M. Management of insertional tendinopathy of the Achilles tendon. Foot Ankle Clin N Am. 2007;12:597-615.
20. Hugate R, Pennypacker, Saunders M, et al. The effects of intratendinous and retrocalcaneal intrabursal injections of corticosteroid on the biomechanical properties of rabbit Achilles tendons. J Bone Joint Surg Am. 2004;86:794-801.
21. Ishikawa SN. Conditions of the calcaneus in skeletally immature patients. Foot Ankle Clin N Am. 2005;10:503-512.
22. Hendrix CL. Calcaneal apophysitis (Sever’s disease). Clin Podiatr Med Surg. 2005;22:55-62.
23. Kassas KJ, Cassettari-Wayhs A. Childhood and adolescent sports-related overuse injuries. Am Fam Phys. 2006;73:1014-1021.
24. Van Dijk CN. Anterior and Posterior Ankle Impingement. Foot Ankle Clin. 2006;11:663-683.
25. Maquirriain J. Posterior ankle impingement syndrome. J Am Acad Orthop Surg. 2005;13:365-371.
26. Maffulli N. Chronic rupture of tendo Achillis. Foot Ankle Clin. 2007;12:583-596.
27. Bhandari M, Guyatt GH, Siddiqui F, et al. Treatment of acute Achilles tendon ruptures: a systematic overview and meta-analysis. Clin Orthop Relat Res. 2002;400:190-200.
28. Cole C, Seto C, Gazewood J. Plantar Fasciitis: Evidence based review of diagnosis and therapy. Am Fam Phys. 2005;72:2237-2242.
29. Barrett SL, O’Malley R. Plantar fasciitis and other causes of heel pain. Am Fam Physician. 1999;59:2200-2206.
30. Schroeder B. American College of Foot and Ankle Surgeons: diagnosis and treatment of heel pain. Am Fam Physician. 2002;65:1686-1687.
31. Crawford F, Thomson C. Interventions for treating plantar heel pain. Cochrane Database Syst Rev. 2003;(3):CD000416.-
32. Pribut SM. Current approaches to the management of plantar heel pain syndrome, including the role of injectable corticosteroids. J Am Podiatr Med. 2007;97:68-74.
33. Porter MD, Shadbolt B. Intralesional corticosteroid injection versus extracoporeal shock wave therapy for plantar fasciopathy. Clin J Sport Med. 2005;15:119-124.
34. Weber JM, Vidt LG, Gehl RS, et al. Calcaneal stress fracture. Clin Podiatr Med Surg. 2005;22:45-54.
35. Dodson NB, Dodson EE, Shromoff PJ. Imaging strategies for diagnosing calcaneal and cuboid stress fractures. Clin Podiatr Med Surg. 2008;25:183-201.
36. Wheeler P, Batt ME. Do nonsteroidal anti-inflammatory drugs adversely affect stress fracture healing? A short review. Br J Sports Med. 2005;39:65-69.
37. O’Connor FG, Sallis R, Wilder R, St. Pierre P. Sports Medicine: Just the Facts. 1st ed. New York: McGraw-Hill Professional; 2004:386,504.
38. Lake C, Trexler G, Barringer W. Posterior tibial tendon dysfunction: a review of pain and activity levels of twenty-one patients. J Prosth Ortho. 1999;11:2-5.
39. Bulstra G, Olsthoorn G, Niek van Dijk C. Tendonoscopy of the posterior tibial tendon. Foot Ankle Clin. 2006;11:421-427.
40. American College of Foot and Ankle Surgeons. Posterior tibial tendon dysfunction (PTTD). 2005. Available at: http://www.footphysicians.com/footankleinfo/pttd.htm. Accessed October 1, 2008.
41. Thordarson D. Orthopaedic Surgery Essentials: Foot and Ankle. Philadelphia: Lippincott Williams & Wilkins; 2004:176.
42. Stroud CC. Heel pain, plantar fasciitis, and tarsal tunnel syndrome. Curr Opin Ortho. 2002;13:89-92.
43. Franson J, Baravarian B. Tarsal tunnel syndrome: a compression neuropathy involving four distinct tunnels. Clin Podiatr Med Surg. 2006;23:597-609.
44. Jolly GP, Zgonis T, Hendrix CL. Neurogenic heel pain. Clin Podiatr Med Surg. 2005;22:101-103.
45. Juliano PJ, Harris TG. Plantar fasciitis, entrapment neuropathies, and tarsal tunnel syndrome: current up to date treatment. Curr Opin Orth. 2004;15:49-54.
46. Gondring WH, Shields B, Wenger S. An outcomes analysis of surgical treatment of tarsal tunnel syndrome. Foot Ankle Int. 2003;24:545-550.
47. Sammarco GJ, Chang L. Outcome of surgical treatment of tarsal tunnel syndrome. Foot Ankle Int. 2003;24:125-131.
1. Aldridge T. Diagnosing heel pain in adults. Am Fam Physician. 2004;70:332-338.
2. Aronow MS. Posterior heel pain (retrocalcaneal bursitis, insertional and noninsertional Achilles tendinopathy). Clin Podiatr Med Surg. 2005;22:19-43.
3. Alvarez-Nemegyei J, Canoso J. Heel pain: diagnosis and treatment, step by step. Clev Clin J Med. 2006;73:465-471.
4. Thomas J, Christensen J, Kravitz S, et al. The diagnosis and treatment of heel pain. J Foot Ankle Surg. 2001;40:329-337.
5. US Food and Drug Administration. Fluoroquinolone antimicrobial drugs. Available at: http://www.fda.gov/cder/drug/infopage/fluoroquinolones/default.htm. Accessed October 7, 2008.
6. Lebrun CM. Management of Achilles tendinopathy. Clin J Sport Med. 2008;18:106-107.
7. Kane TP, Ismail M, Calder JD. Topical glyceryl trinitrate and noninsertional Achilles tendinopathy: a clinical and cellular investigation. Am J Sports Med. 2008;36:1160-1163.
8. Stergioulas A, Stergioula M, Aarskog R, et al. Effects of low-level laser therapy and eccentric exercises in the treatment of recreational athletes with chronic Achilles tendinopathy. Am J Sports Med. 2008;36:881-887.
9. Rompe JD, Furia J, Maffulli N. Eccentric loading compared with shock wave treatment for chronic insertional Achilles tendinopathy. J Bone Joint Surg Am. 2008;90:52-61.
10. Paoloni JA, Murrell Ga. Three-year follow up study of topical glyceryl trinitrate treatment of chronic noninsertional Achilles tendinopathy. Foot Ankle Int. 2007;28:1064-1068.
11. McLaughlan G, Handoll H. Interventions for treating acute and chronic Achilles tendonitis. Cochrane Database Syst Rev. 2001;(2):CD000232.-
12. Fahlstrom M, Jonsson P, Lorentzon R, et al. Chronic Achilles tendon pain treated with eccentric calfmuscle training. Knee Surg Sports Traumatol Arthrosc. 2003;11:327-333.
13. Costa M, Shepstone L, Donnell S, et al. Shock wave therapy for chronic Achilles tendon pain. Clin Orthop. 2005;44:199-204.
14. Furia J. High-energy extracorporeal shock wave therapy as a treatment for insertional Achilles tendinopathy. Am J Sports Med. 2006;34:733-740.
15. Maxwell NJ, Ryan MB, Taunton JE, et al. Sonographically guided intratendinous injection of hyperosmolar dextrose to treat chronic tendinosis of the Achilles tendon. Am J Roentgenol. 2007;189:W215-W220.Available at: http://www.ajronline.org/content/vol189/issue4/. Accessed May 15, 2008.
16. McShane JM, Ostick B, McCabe F. Noninsertional Achilles tendinopathy: pathology and management. Curr Sports Med Rep. 2007;6:288-292.
17. Russell AL. Peroxicam 0.5% topical gel compared to placebo in the treatment of acute soft tissue injuries: a double-blind study comparing efficacy and safety. Clin Invest Med. 1991;1:35-43.
18. Glaser T, Poddar S, Tweed B. What’s the best way to treat Achilles tendinopathy? J Fam Pract. 2008;57:261-263.
19. Solan M, Davies M. Management of insertional tendinopathy of the Achilles tendon. Foot Ankle Clin N Am. 2007;12:597-615.
20. Hugate R, Pennypacker, Saunders M, et al. The effects of intratendinous and retrocalcaneal intrabursal injections of corticosteroid on the biomechanical properties of rabbit Achilles tendons. J Bone Joint Surg Am. 2004;86:794-801.
21. Ishikawa SN. Conditions of the calcaneus in skeletally immature patients. Foot Ankle Clin N Am. 2005;10:503-512.
22. Hendrix CL. Calcaneal apophysitis (Sever’s disease). Clin Podiatr Med Surg. 2005;22:55-62.
23. Kassas KJ, Cassettari-Wayhs A. Childhood and adolescent sports-related overuse injuries. Am Fam Phys. 2006;73:1014-1021.
24. Van Dijk CN. Anterior and Posterior Ankle Impingement. Foot Ankle Clin. 2006;11:663-683.
25. Maquirriain J. Posterior ankle impingement syndrome. J Am Acad Orthop Surg. 2005;13:365-371.
26. Maffulli N. Chronic rupture of tendo Achillis. Foot Ankle Clin. 2007;12:583-596.
27. Bhandari M, Guyatt GH, Siddiqui F, et al. Treatment of acute Achilles tendon ruptures: a systematic overview and meta-analysis. Clin Orthop Relat Res. 2002;400:190-200.
28. Cole C, Seto C, Gazewood J. Plantar Fasciitis: Evidence based review of diagnosis and therapy. Am Fam Phys. 2005;72:2237-2242.
29. Barrett SL, O’Malley R. Plantar fasciitis and other causes of heel pain. Am Fam Physician. 1999;59:2200-2206.
30. Schroeder B. American College of Foot and Ankle Surgeons: diagnosis and treatment of heel pain. Am Fam Physician. 2002;65:1686-1687.
31. Crawford F, Thomson C. Interventions for treating plantar heel pain. Cochrane Database Syst Rev. 2003;(3):CD000416.-
32. Pribut SM. Current approaches to the management of plantar heel pain syndrome, including the role of injectable corticosteroids. J Am Podiatr Med. 2007;97:68-74.
33. Porter MD, Shadbolt B. Intralesional corticosteroid injection versus extracoporeal shock wave therapy for plantar fasciopathy. Clin J Sport Med. 2005;15:119-124.
34. Weber JM, Vidt LG, Gehl RS, et al. Calcaneal stress fracture. Clin Podiatr Med Surg. 2005;22:45-54.
35. Dodson NB, Dodson EE, Shromoff PJ. Imaging strategies for diagnosing calcaneal and cuboid stress fractures. Clin Podiatr Med Surg. 2008;25:183-201.
36. Wheeler P, Batt ME. Do nonsteroidal anti-inflammatory drugs adversely affect stress fracture healing? A short review. Br J Sports Med. 2005;39:65-69.
37. O’Connor FG, Sallis R, Wilder R, St. Pierre P. Sports Medicine: Just the Facts. 1st ed. New York: McGraw-Hill Professional; 2004:386,504.
38. Lake C, Trexler G, Barringer W. Posterior tibial tendon dysfunction: a review of pain and activity levels of twenty-one patients. J Prosth Ortho. 1999;11:2-5.
39. Bulstra G, Olsthoorn G, Niek van Dijk C. Tendonoscopy of the posterior tibial tendon. Foot Ankle Clin. 2006;11:421-427.
40. American College of Foot and Ankle Surgeons. Posterior tibial tendon dysfunction (PTTD). 2005. Available at: http://www.footphysicians.com/footankleinfo/pttd.htm. Accessed October 1, 2008.
41. Thordarson D. Orthopaedic Surgery Essentials: Foot and Ankle. Philadelphia: Lippincott Williams & Wilkins; 2004:176.
42. Stroud CC. Heel pain, plantar fasciitis, and tarsal tunnel syndrome. Curr Opin Ortho. 2002;13:89-92.
43. Franson J, Baravarian B. Tarsal tunnel syndrome: a compression neuropathy involving four distinct tunnels. Clin Podiatr Med Surg. 2006;23:597-609.
44. Jolly GP, Zgonis T, Hendrix CL. Neurogenic heel pain. Clin Podiatr Med Surg. 2005;22:101-103.
45. Juliano PJ, Harris TG. Plantar fasciitis, entrapment neuropathies, and tarsal tunnel syndrome: current up to date treatment. Curr Opin Orth. 2004;15:49-54.
46. Gondring WH, Shields B, Wenger S. An outcomes analysis of surgical treatment of tarsal tunnel syndrome. Foot Ankle Int. 2003;24:545-550.
47. Sammarco GJ, Chang L. Outcome of surgical treatment of tarsal tunnel syndrome. Foot Ankle Int. 2003;24:125-131.
Managing osteoarthritis: What’s best for your patient?
- Teach patients that self-care is key to successful management of osteoarthritis (Osteoarthritis Research Society International [OARSI] Evidence 1a
- Encourage patients to regularly engage in aerobic, muscle-strengthening, and range-of-motion exercise (Ia: knee; IV: hip).
- Recommend that patients try acetaminophen (≤4 g/d) before considering other analgesics for mild to moderate joint pain (Ia: knee; IV: hip).
- Prescribe the lowest effective dose of nonsteroidal anti-inflammatory drugs (NSAIDs) and avoid using them for long-term therapy (Ia).
OARSI level of evidence:
Ia: Meta-analysis of randomized controlled trials (RCTs)
Ib: RCT
IIa: Controlled study without randomization
IIb: Quasi-experimental study
III: Nonexperimental, descriptive studies
IV: Expert committee reports/opinion/experience
Osteoarthritis (OA) and other rheumatic conditions account for as many office visits as cardiovascular disease or essential hypertension, according to national data, and most involve primary care physicians.1 As the population ages, the prevalence of OA—estimated at 46.4 million in 2005 in the United States alone—will continue to rise.2,3 So, too, will the number of patients needing treatment for pain and functional limitations related to OA of the hips and knees.
Physicians who treat these patients have a new tool at their disposal: the Osteoarthritis Research Society International (OARSI)’s evidence-based, expert consensus guidelines for the management of hip and knee OA. These recommendations, published in February 2008, are the first “internationally agreed and universally applicable guidelines for the management of these global disorders.”4
In caring for patients with OA of the hips or knees, family physicians should keep in mind 2 guiding principles at the heart of the OARSI recommendations:
- the importance of lifestyle modification, including regular exercise, in coping with this degenerative, potentially debilitating disease; and
- the need to incorporate both nonpharmacologic interventions and drug therapy to achieve optimal care.4
International team sifts through the evidence
To develop the guidelines, OARSI convened a committee of 16 physicians from 6 countries and 2 continents, with expertise in 4 disciplines: rheumatology, orthopedics, evidence-based medicine, and primary care. The team reviewed national and regional guidelines and studied systematic reviews; meta-analyses; randomized controlled trials (RCTs); controlled and uncontrolled trials; cohort, case-control, and cross-sectional studies; and economic evaluations from 1945 through 2001. The team also conducted a systematic review of evidence from January 2002 through January 2006.4,5 To ensure the quality of evidence hierarchy, the team used internationally accepted research tools.
AP and tunnel images are key to OA diagnosis
A diagnosis of knee or hip osteoarthritis (OA) requires a medical history; physical examination; radiologic assessment, with standing X-rays of the lower extremities, including anterior-posterior and tunnel views for knee OA; and the exclusion of other conditions.30 The tunnel view shown here reveals bone-on-bone articulation in the medial compartment of the left knee, and demonstrates the importance of standing X-rays.
Differential diagnosis includes gout, pseudogout, rheumatoid arthritis, patella-femoral pain, pes anserine (knee) bursitis, iliotibial band pathology, meniscal tear, cruciate tears, and tumors. No blood tests are indicated unless an inflammatory process is suspected. Synovial fluid in an osteoarthritic knee has a white cell count of <2000/uL.31
The team used several criteria to rate the recommended strategies, including level of evidence, effect size for pain relief, level of consensus, and strength of recommendation (SOR). All of these criteria are included (and defined) in an at-a-glance summary of the OARSI recommendations ( TABLE ).
In particular, the SOR, which is used throughout this article, is an overall rating that reflects the opinions of the team members after consideration of the research evidence for efficacy, safety, and cost-effectiveness. It is based on a visual analog scale of 0 to 100 mm and is expressed as a percentage.
TABLE
OARSI guidelines rate the evidence for osteoarthritis treatment options
| RECOMMENDATION | SOR, % (95% CI)*/ LEVEL OF CONSENSUS, %† | LEVEL OF EVIDENCE‡ | ES (95% CI)§ |
|---|---|---|---|
| Nonpharmacologic | |||
| Education, self-help, patient-driven treatment | 97 (95 to 99)/NA | Ia: education | NA |
| Aerobic, muscle-strengthening, and range-of-motion exercises | 96 (93 to 99)/85 | Ia: knee IV: hip Ib: hip, water-based | 0.52 (0.34 to 0.70): aerobic 0.32 (0.23 to 0.42): strength 0.25 (0.02 to 0.47): water-based |
| Weight loss | 96 (92 to 100)/100 | Ia | 0.13 (-0.12 to 0.38) |
| Walking aids | 90 (84 to 96)/100 | IV | NA |
| Physical therapy | 89 (82 to 96)/100 | IV | NA |
| Appropriate footwear/insoles | 77 (66 to 88)/92 | IV: footwear Ia: insoles | NA |
| Knee braces | 76 (69 to 83)/92 | Ia | NA |
| Telephone contact | 66 (57 to 75)/77 | Ia: knee; IV: hip | 0.12 (0 to 0.24) |
| Thermal modalities | 64 (60 to 68)/77 | Ia | 0.69 (-0.07 to 1.45) |
| Acupuncture | 59 (47 to 71)/69 | Ia | 0.51 (0.23 to 0.79) |
| TENS | 58 (45 to 72)/69 | Ia | NA |
| Pharmacologic | |||
| Oral NSAIDs | 93 (88 to 99)/100 | Ia | 0.32 (0.24 to 0.39) |
| Acetaminophen ≤4 g/d | 92 (88 to 99)/77 | Ia: knee; IV: hip | 0.21 (0.02 to 0.41) |
| Topical NSAIDs/capsaicin | 85 (75 to 95)/100 | Ia | 0.41 (0.22 to 0.59) |
| Weak opioids/narcotics | 82 (74 to 90)/92 | Ia | NA |
| IA corticosteroid injections | 78 (61 to 95)/69 | Ia: knee; Ib: hip | 0.72 (0.42 to 1.02) |
| IA hyaluronate injections | 64 (43 to 85)/85 | Ia | 0.32 (0.17 to 0.47) |
| Glucosamine and/or chondroitin | 63 (44 to 82)/92 | Ia: glucosamine | 0.45 (0.04 to 0.86) |
| Surgical treatments | |||
| Joint replacement | 96 (94 to 98)/92 | III | NA |
| Unicompartmental knee replacement | 76 (64 to 88)/100 | IIIb | NA |
| Osteotomy/joint preservation | 75 (64 to 86)/100 | IIb | NA |
| Joint fusion | 69 (57 to 82)/100 | IV | NA |
| Joint lavage/arthroscopic debridement | 60 (47 to 82)/100 | Ib | 0.09 (-0.27 to 0.44): lavage -0.01 (-0.37 to 0.35): debridement |
| CI, confidence interval; ES, effect size for pain relief; IA, intraarticular; NA, not available; NSAIDs, nonsteroidal anti-inflammatory drugs; OARSI, Osteoarthritis Research Society International; SOR, strength of recommendation; TENS, transcutaneous electrical nerve stimulation | |||
| * SOR (strength of recommendation) is an overall rating that reflects the opinions of OARSI team members after consideration of the research evidence for efficacy, safety, and cost-effectiveness. SOR is based on a visual analog scale of 0 to 100 mm and is expressed as a percentage. | |||
| †Level of consensus is the estimated extent of agreement among committee members, expressed as a percentage. | |||
| ‡Level of evidence is broken into 6 categories: Ia: meta-analysis of randomized controlled trials (RCTs); Ib: RCT; IIa: controlled study without randomization; IIb: quasi-experimental study; III: nonexperimental, descriptive studies; and IV: expert committee reports/opinion/experience. | |||
| §ES (effect size for pain relief) is a measure of the standard mean difference between interventions (eg, treatment vs placebo): 0.2 (small); 0.5 (moderate); and >0.8 (large). The ES refers to the knee and hip unless otherwise specified. | |||
| Adapted from: Zhang et al.4 | |||
OARSI emphasizes patient education
Patient education about self-care and lifestyle modifications, such as weight loss, exercise, and pacing of activities to reduce the load on the affected joints, is OARSI’s strongest nonpharmacologic recommendation (SOR: 97%). The guidelines also call for the following interventions:
- correcting mechanical abnormalities of the skeleton;
- helping patients lose weight;
- assisting patients with smoking cessation efforts;6
- directing the use of nonprescription medications;
- prescribing assistive devices; and
- prescribing appropriate prescription drugs.
Nondrug options: Exercise that achy joint
To many patients, being told to exercise a joint in which movement is associated with stiffness and bone-on-bone pain seems counterintuitive. Referring to the findings of the OARSI panel may be helpful in explaining the importance of regular aerobic, muscle-strengthening, and range-of-motion exercises, all of which are strongly recommended (SOR: 96%). Exercise can be as simple as “regular aerobic walking” and home-based strengthening of the quadriceps.4 For patients with arthritic hips, water-based exercises are recommended.
Obesity can increase the risk of developing OA of the hips and knees, and excess weight puts extra stress on joints that are already arthritic. Thus, weight loss is both a risk modification factor (see “Is your patient at risk of OA? Take steps now” ) and a key OA management strategy (SOR: 96%). In a meta-regression analysis conducted by the committee, a reduction of >5% of body weight or a loss at a rate of >0.24% per week was associated with significant improvement in disability. One RCT had a number needed to treat (NNT) of 3 (95% confidence interval [CI], 2-9) to achieve improved pain and function scores after a 2-month low-energy diet.7
Risk factors for osteoarthritis (OA) include:14-19
- mechanical abnormalities, such as varus (bowlegged) and valgus (knock-kneed) angulations;
- flat feet, and heel pronation and supination;
- a history of joint surgery or acute injuries, particularly to the anterior cruciate ligament (ACL) or meniscus;
- obesity;
- manual labor (any job that involves heavy lifting, together with kneeling and squatting);
- participation in competitive or high-intensity sports; and
- a family history of OA (based on mounting evidence of a genetic link).20-22
Lack of neuromuscular control (proprioception) of the knee is another risk factor, since it can expose the internal joint to forces that would otherwise be absorbed by muscle. Exposure of the joint to excess forces can occur if the impact is rapid, leaving the muscle without adequate time to contract to absorb the force, or the muscle is fatigued and weak from prolonged exercise.23,24
Work with patients to modify risk. In discussing risk modification with patients, emphasize that high-intensity running, especially when practiced for years, increases the risk of OA of the knees.25 Indeed, high-impact activity of any kind subjects knee cartilage to significant single and repetitive impact loads and torsional loads.17,26 Point out, however, that some physical activity is needed to maintain normal metabolic activity of cartilage in a healthy joint and that recreational, mild-intensity running or jogging does not appear to increase the risk for OA.27
Be aggressive with knee injuries. As noted earlier, a history of acute ACL or meniscus injury is a risk factor for OA. Knee trauma with effusions that develop rapidly (within 2-12 hours) is associated with high risk of significant intraarticular damage to the ACL, meniscus, and articular cartilage.28 A study of pediatric and adolescent patients who underwent magnetic resonance imaging for possible internal knee injury found cartilage injuries to be the most common.29
To avoid additional damage, manage knee trauma with effusions as a significant injury. Treatment includes bracing, physical therapy, low-impact exercise, and possibly even cross-training or job modification. Advise patients to continue physical therapy until strength and proprioception are fully recovered and no pain or effusion remains, which generally takes about 6 to 8 weeks, and not to return to normal activity prematurely.
Don’t underestimate the power of a phone call
Other nonpharmacologic recommendations include referral to a physical therapist for evaluation and exercise instruction (SOR: 89%); instruction in the use of walking aids, such as a cane or crutch in the contralateral hand, to improve biomechanics (SOR: 90%); and the use of braces to support unstable knees, an unproven intervention that may increase proprioception and stability (SOR: 76%). Physicians should also recommend footwear with insoles or lateral wedges to decrease lateral thrust of the knee and medial compartment forces (SOR: 77%).
Regular telephone contact, possibly on a monthly basis, is a suggested strategy for promoting self-care, tested in patients with OA of the knee but recommended for those with arthritic hips solely on the basis of expert opinion. A number of other modalities, including thermal therapy (heat treatments with warm water or wax, or cold therapy with a 20-minute ice massage), transcutaneous electrical nerve stimulation (TENS), and acupuncture, are recommended for symptom relief.
Drug therapy: Start with acetaminophen
The OARSI guidelines cite acetaminophen as an “effective initial oral analgesic” for mild to moderate pain in patients with OA of the hips or knees (SOR: 92%).4 In analyses conducted by the committee, the NNT to achieve an improvement in pain ranged from 1 to 2 in an earlier systematic review8 to 4 to 16 in a subsequent meta-analysis.9
Prescribe NSAIDs for short-term relief. While acetaminophen is considered the preferred long-term oral treatment, the strongest pharmacologic recommendation for alleviating the pain and stiffness associated with OA of the hip or knee is for nonsteroidal anti-inflammatory drugs (NSAIDs) (SOR: 93%). The caveat, however, is that NSAIDs should be used in the lowest effective dose and are not considered a long-term option. Patients with increased gastrointestinal (GI) risk should use either a cyclooxygenase-2 (COX-2) agent or an NSAID with a proton pump inhibitor or misoprostol for GI protection.
For those with cardiovascular risks, both nonselective NSAIDs and COX-2 agents require caution; here, too, the lowest dose for the shortest possible duration is recommended.
The guidelines also call for the use of topical agents, such as topical NSAIDs and capsaicin, for relief of symptoms (SOR: 85%). The NNT for topical NSAIDs was 3 (95% CI, 2-4);4 capsaicin had an NNT of 4 (95% CI, 3-5) after 4 weeks of therapy.4 The recommendations also note that glucosamine and/or chondroitin sulfate may alleviate some symptoms of osteoarthritis of the knee, but should be discontinued if no benefit is observed after 6 months.
When something stronger is needed. For moderate to severe pain that has not responded to oral agents, intraarticular (IA) injections with corticosteroids are recommended, as are IA hyaluronate injections (SOR: 78% and 64%, respectively). Weak opioids/low-dose narcotics round out the recommendations for treating moderate pain, with stronger opioids reserved for patients whose pain is severe.
When to consider surgery
Joint replacement surgery is recommended for patients who do not achieve adequate pain relief and functional improvement from nonpharmacologic and pharmacologic modalities (SOR: 96%). A meta-analysis of 74 studies assessing quality of life 1 to 7 years after total hip and total knee replacement (THR and TKR) found substantial improvement in pain and function, but variable effects on mental health and social functioning. Risk factors for poor outcomes include older age; more (or more severe) preoperative pain; medical comorbidities; musculoskeletal comorbidities such as low back pain, with functional limitations; low mental health scores; and OA in the hip that was not replaced.10,11
Unicompartmental knee replacement (UKR) had an SOR of 76%. Reviews that compared TKR to UKR found similar 5-year outcomes in knee pain and function. Those who underwent UKR had better range of motion, but prosthesis survival at 10 years was better in those with TKR (>90% vs 85% to 90%).12
In young adults, osteotomy and jointpreserving procedures are recommended for hip OA, especially when dysplasia is present. In young, active patients with unicompartment OA, high tibial osteotomies may delay TKR by as long as 10 years.13
Joint lavage and arthroscopic debridement in knee OA remain controversial, although they may provide short-term symptom relief (SOR: 60%). Joint fusion as a salvage procedure after failed TKR had an SOR of 69%.
Work as a team to improve outcomes
The inevitable increase in the number of patients with OA of the hips and knees underscores the importance of having a range of treatment strategies, often best delivered by a multidisciplinary team with the family physician at the helm. The OARSI guidelines, which are backed by both a thorough review of research findings and expert consensus, can help you convince patients to take an active role in managing this potentially debilitating condition. Patients’ commitment to lifestyle modifications and self-management, bolstered by your guidance and support, is the most effective way to keep patients with OA on the move.
Correspondence
Greg P. Gutierrez, MD, Associate Professor, University of Colorado Denver Health Sciences Center, Department of Family Medicine, Denver Health and Hospital, 660 Bannock St., Denver, CO 80218; [email protected].
1. Hootman JM. Magnitude and characteristics of arthritis and other rheumatic conditions on ambulatory medical care visits, United States, 1997. Arthritis Rheum. 2002;47:571-581.
2. Lawrence R, Helmick CG, Arnett FC, et al. Estimates of the prevalence of arthritis and selected musculoskeletal disorders in the United States. Arthritis Rheum. 1998;41:778-799.
3. US Department of Health and Human Services. CDC: prevalence of doctor-diagnosed arthritis and arthritis-attributable activity limitation - United States, 2003–2005. MMWR. 2006;55:1089-1092.
4. Zhang W, Moskowitz RW, Nuki G, et al. OARSI recommendations for the management of hip and knee osteoarthritis, Part II: OARSI evidence-based, expert consensus guidelines. Osteoarthritis Cartilage. 2008;16:137-162.
5. Zhang W, Moskowitz RW, Nuki G, et al. OARSI recommendations for the management of hip and knee osteoarthritis, Part I: critical appraisal of existing treatment guidelines and systematic review of current research evidence. Osteoarthritis Cartilage. 2007;15:981-1000.
6. Amin S, Niu J, Guermazi A, et al. Cigarette smoking and the risk for cartilage loss and knee pain in men with knee osteoarthritis. Ann Rheum Dis. 2007;66:18-22.
7. Christensen R, Astrup A, Bliddal H. Weight loss: the treatment of choice for knee osteoarthritis? A randomized trial. Osteoarthritis Cartilage. 2005;13:20-27.
8. Towheed TE, Hochberg MC, Judd MG, Wells G. Acetaminophen for osteoarthritis. Cochrane Database Syst Rev. 2003;(2):CD004257.-
9. Towheed TE, Maxwell L, Judd MG, Catton M, Wells G. Acetaminophen for osteoarthritis. Cochrane Database Syst Rev. 2006;(1):CD004257.-
10. Ethgen O, Bruyere O, Richy F, Dardennes C, Reginster JY. Health-related quality of life in total hip and total knee arthroplasty. A qualitative and systematic review of the literature. J Bone Joint Surg Am. 2004;86-A:963-974.
11. Lingard EA, Katz JN, Wright EA, Sledge CB. Kinemax Outcomes Group. Predicting the outcome of total knee arthroplasty. J Bone Joint Surg Am. 2004;86-A:2179-2186.
12. Griffin T, Rowden L, Morgan D, Atkinson R, Woodruff P, Madden G. Unicompartmental knee arthroplasty for the treatment of unicompartmental osteoarthritis: a systematic study. ANZ J Surg. 2007;77:214-221.
13. Virolainen P, Arc HT. High tibial osteotomy for the treatment of osteoarthritis of the knee: a review of the literature and a meta-analysis of follow-up studies. Arch Orthop Trauma Surg. 2004;124:258-261.
14. Felson DT. Relation of obesity and of vocational and avocational risk factors to osteoarthritis. J Rheumatol. 2005;32:1133-1135.
15. Gelber AC, Hochberg MC, Mead LA, Wang NY, Wigley FM, Klag MJ. Joint injury in young adults and risk for subsequent knee and hip osteoarthritis. Ann Intern Med. 2000;133:321-328.
16. Sharma L, Song J, Felson DT, Cahue S, Shamiyeh E, Dunlop DD. The role of knee alignment in disease progression and functional decline in knee osteoarthritis. JAMA. 2001;286:188-195.
17. Lequesne MG, Dang N, Lane NE. Sport practice and osteoarthritis of the limbs. Osteoarthritis Cartilage. 1997;5:75-86.
18. Griffin TM, Guilak F. The role of mechanical loading in the onset and progression of osteoarthritis. Exerc Sport Sci Rev. 2005;33:195-200.
19. Maetzel A, Makela M, Hawker G, Bombardier C. Osteoarthritis of the hip and knee and mechanical occupational exposure: a systematic overview of the evidence. J Rheumatol. 1997;24:1599-1607.
20. Zhai G, Ding C, Stankovich J, Cicuttini F, Jones G. The genetic contribution to longitudinal changes in knee structure and muscle strength: a sibpair study. Arthritis Rheum. 2005;52:2830-2834.
21. Hirsch R, Lethbridge-Cejku M, Hanson R, et al. Familial aggregation of osteoarthritis: data from the Baltimore Longitudinal Study on Aging. Arthritis Rheum. 1998;41:1227-1232.
22. Felson DT, Couropmitree NN, Chaisson CE, et al. Evidence for a Mendelian gene in a segregation analysis of generalized radiographic osteoarthritis: the Framingham Study. Arthritis Rheum. 1998;41:1064-1071.
23. Christina KA, White SC, Gilchrist LA. Effect of localized muscle fatigue on vertical ground reaction forces and ankle joint motion during running. Hum Mov Sci. 2001;20:257-276.
24. Mizrahi J, Verbitsky O, Isakov E. Fatigue-related loading imbalance on the shank in running: a possible factor in stress fractures. Ann Biomed Eng. 2000;28:463-469.
25. McAlindon TE, Wilson PW, Aliabadi P, Weissman B, Felson DT. Level of physical activity and the risk of radiographic and symptomatic knee osteoarthritis in the elderly: the Framingham study. Am J Med. 1999;106:151-157.
26. Buckwalter JA. Sports, joint injury, and posttraumatic osteoarthritis. J Orthop Sports Phys Ther. 2003;33:578-588.
27. Conaghan PG. Update on osteoarthritis part 1: current concepts and the relation to exercise. British J Sports Med. 2002;36:330-333.
28. Maffulli N, Binfield PM, King JB, Good CJ. Acute haemarthrosis of the knee in athletes. A prospective study of 106 cases. J Bone Joint Surg Br. 1993;75:945-949.
29. Oeppen RS, Connolly SA, Bencardino JT, Jaramillo D. Acute injury of the articular cartilage and subchondral bone: a common but unrecognized lesion in the immature knee. Am J Roentgenol. 2004;182:111-117.
30. Felson DT. Osteoarthritis of the knee. N Engl J Med. 2006;354:841-848.
31. Hassebacher B. Arthrocentesis, synovial fluid analysis and synovial biopsy. In: Schumacher HR, Klippel JH, Koopman WJ, eds. Primer on the Rheumatic Diseases. Atlanta, GA: Arthritis Foundation; 1993:67-72.
- Teach patients that self-care is key to successful management of osteoarthritis (Osteoarthritis Research Society International [OARSI] Evidence 1a
- Encourage patients to regularly engage in aerobic, muscle-strengthening, and range-of-motion exercise (Ia: knee; IV: hip).
- Recommend that patients try acetaminophen (≤4 g/d) before considering other analgesics for mild to moderate joint pain (Ia: knee; IV: hip).
- Prescribe the lowest effective dose of nonsteroidal anti-inflammatory drugs (NSAIDs) and avoid using them for long-term therapy (Ia).
OARSI level of evidence:
Ia: Meta-analysis of randomized controlled trials (RCTs)
Ib: RCT
IIa: Controlled study without randomization
IIb: Quasi-experimental study
III: Nonexperimental, descriptive studies
IV: Expert committee reports/opinion/experience
Osteoarthritis (OA) and other rheumatic conditions account for as many office visits as cardiovascular disease or essential hypertension, according to national data, and most involve primary care physicians.1 As the population ages, the prevalence of OA—estimated at 46.4 million in 2005 in the United States alone—will continue to rise.2,3 So, too, will the number of patients needing treatment for pain and functional limitations related to OA of the hips and knees.
Physicians who treat these patients have a new tool at their disposal: the Osteoarthritis Research Society International (OARSI)’s evidence-based, expert consensus guidelines for the management of hip and knee OA. These recommendations, published in February 2008, are the first “internationally agreed and universally applicable guidelines for the management of these global disorders.”4
In caring for patients with OA of the hips or knees, family physicians should keep in mind 2 guiding principles at the heart of the OARSI recommendations:
- the importance of lifestyle modification, including regular exercise, in coping with this degenerative, potentially debilitating disease; and
- the need to incorporate both nonpharmacologic interventions and drug therapy to achieve optimal care.4
International team sifts through the evidence
To develop the guidelines, OARSI convened a committee of 16 physicians from 6 countries and 2 continents, with expertise in 4 disciplines: rheumatology, orthopedics, evidence-based medicine, and primary care. The team reviewed national and regional guidelines and studied systematic reviews; meta-analyses; randomized controlled trials (RCTs); controlled and uncontrolled trials; cohort, case-control, and cross-sectional studies; and economic evaluations from 1945 through 2001. The team also conducted a systematic review of evidence from January 2002 through January 2006.4,5 To ensure the quality of evidence hierarchy, the team used internationally accepted research tools.
AP and tunnel images are key to OA diagnosis
A diagnosis of knee or hip osteoarthritis (OA) requires a medical history; physical examination; radiologic assessment, with standing X-rays of the lower extremities, including anterior-posterior and tunnel views for knee OA; and the exclusion of other conditions.30 The tunnel view shown here reveals bone-on-bone articulation in the medial compartment of the left knee, and demonstrates the importance of standing X-rays.
Differential diagnosis includes gout, pseudogout, rheumatoid arthritis, patella-femoral pain, pes anserine (knee) bursitis, iliotibial band pathology, meniscal tear, cruciate tears, and tumors. No blood tests are indicated unless an inflammatory process is suspected. Synovial fluid in an osteoarthritic knee has a white cell count of <2000/uL.31
The team used several criteria to rate the recommended strategies, including level of evidence, effect size for pain relief, level of consensus, and strength of recommendation (SOR). All of these criteria are included (and defined) in an at-a-glance summary of the OARSI recommendations ( TABLE ).
In particular, the SOR, which is used throughout this article, is an overall rating that reflects the opinions of the team members after consideration of the research evidence for efficacy, safety, and cost-effectiveness. It is based on a visual analog scale of 0 to 100 mm and is expressed as a percentage.
TABLE
OARSI guidelines rate the evidence for osteoarthritis treatment options
| RECOMMENDATION | SOR, % (95% CI)*/ LEVEL OF CONSENSUS, %† | LEVEL OF EVIDENCE‡ | ES (95% CI)§ |
|---|---|---|---|
| Nonpharmacologic | |||
| Education, self-help, patient-driven treatment | 97 (95 to 99)/NA | Ia: education | NA |
| Aerobic, muscle-strengthening, and range-of-motion exercises | 96 (93 to 99)/85 | Ia: knee IV: hip Ib: hip, water-based | 0.52 (0.34 to 0.70): aerobic 0.32 (0.23 to 0.42): strength 0.25 (0.02 to 0.47): water-based |
| Weight loss | 96 (92 to 100)/100 | Ia | 0.13 (-0.12 to 0.38) |
| Walking aids | 90 (84 to 96)/100 | IV | NA |
| Physical therapy | 89 (82 to 96)/100 | IV | NA |
| Appropriate footwear/insoles | 77 (66 to 88)/92 | IV: footwear Ia: insoles | NA |
| Knee braces | 76 (69 to 83)/92 | Ia | NA |
| Telephone contact | 66 (57 to 75)/77 | Ia: knee; IV: hip | 0.12 (0 to 0.24) |
| Thermal modalities | 64 (60 to 68)/77 | Ia | 0.69 (-0.07 to 1.45) |
| Acupuncture | 59 (47 to 71)/69 | Ia | 0.51 (0.23 to 0.79) |
| TENS | 58 (45 to 72)/69 | Ia | NA |
| Pharmacologic | |||
| Oral NSAIDs | 93 (88 to 99)/100 | Ia | 0.32 (0.24 to 0.39) |
| Acetaminophen ≤4 g/d | 92 (88 to 99)/77 | Ia: knee; IV: hip | 0.21 (0.02 to 0.41) |
| Topical NSAIDs/capsaicin | 85 (75 to 95)/100 | Ia | 0.41 (0.22 to 0.59) |
| Weak opioids/narcotics | 82 (74 to 90)/92 | Ia | NA |
| IA corticosteroid injections | 78 (61 to 95)/69 | Ia: knee; Ib: hip | 0.72 (0.42 to 1.02) |
| IA hyaluronate injections | 64 (43 to 85)/85 | Ia | 0.32 (0.17 to 0.47) |
| Glucosamine and/or chondroitin | 63 (44 to 82)/92 | Ia: glucosamine | 0.45 (0.04 to 0.86) |
| Surgical treatments | |||
| Joint replacement | 96 (94 to 98)/92 | III | NA |
| Unicompartmental knee replacement | 76 (64 to 88)/100 | IIIb | NA |
| Osteotomy/joint preservation | 75 (64 to 86)/100 | IIb | NA |
| Joint fusion | 69 (57 to 82)/100 | IV | NA |
| Joint lavage/arthroscopic debridement | 60 (47 to 82)/100 | Ib | 0.09 (-0.27 to 0.44): lavage -0.01 (-0.37 to 0.35): debridement |
| CI, confidence interval; ES, effect size for pain relief; IA, intraarticular; NA, not available; NSAIDs, nonsteroidal anti-inflammatory drugs; OARSI, Osteoarthritis Research Society International; SOR, strength of recommendation; TENS, transcutaneous electrical nerve stimulation | |||
| * SOR (strength of recommendation) is an overall rating that reflects the opinions of OARSI team members after consideration of the research evidence for efficacy, safety, and cost-effectiveness. SOR is based on a visual analog scale of 0 to 100 mm and is expressed as a percentage. | |||
| †Level of consensus is the estimated extent of agreement among committee members, expressed as a percentage. | |||
| ‡Level of evidence is broken into 6 categories: Ia: meta-analysis of randomized controlled trials (RCTs); Ib: RCT; IIa: controlled study without randomization; IIb: quasi-experimental study; III: nonexperimental, descriptive studies; and IV: expert committee reports/opinion/experience. | |||
| §ES (effect size for pain relief) is a measure of the standard mean difference between interventions (eg, treatment vs placebo): 0.2 (small); 0.5 (moderate); and >0.8 (large). The ES refers to the knee and hip unless otherwise specified. | |||
| Adapted from: Zhang et al.4 | |||
OARSI emphasizes patient education
Patient education about self-care and lifestyle modifications, such as weight loss, exercise, and pacing of activities to reduce the load on the affected joints, is OARSI’s strongest nonpharmacologic recommendation (SOR: 97%). The guidelines also call for the following interventions:
- correcting mechanical abnormalities of the skeleton;
- helping patients lose weight;
- assisting patients with smoking cessation efforts;6
- directing the use of nonprescription medications;
- prescribing assistive devices; and
- prescribing appropriate prescription drugs.
Nondrug options: Exercise that achy joint
To many patients, being told to exercise a joint in which movement is associated with stiffness and bone-on-bone pain seems counterintuitive. Referring to the findings of the OARSI panel may be helpful in explaining the importance of regular aerobic, muscle-strengthening, and range-of-motion exercises, all of which are strongly recommended (SOR: 96%). Exercise can be as simple as “regular aerobic walking” and home-based strengthening of the quadriceps.4 For patients with arthritic hips, water-based exercises are recommended.
Obesity can increase the risk of developing OA of the hips and knees, and excess weight puts extra stress on joints that are already arthritic. Thus, weight loss is both a risk modification factor (see “Is your patient at risk of OA? Take steps now” ) and a key OA management strategy (SOR: 96%). In a meta-regression analysis conducted by the committee, a reduction of >5% of body weight or a loss at a rate of >0.24% per week was associated with significant improvement in disability. One RCT had a number needed to treat (NNT) of 3 (95% confidence interval [CI], 2-9) to achieve improved pain and function scores after a 2-month low-energy diet.7
Risk factors for osteoarthritis (OA) include:14-19
- mechanical abnormalities, such as varus (bowlegged) and valgus (knock-kneed) angulations;
- flat feet, and heel pronation and supination;
- a history of joint surgery or acute injuries, particularly to the anterior cruciate ligament (ACL) or meniscus;
- obesity;
- manual labor (any job that involves heavy lifting, together with kneeling and squatting);
- participation in competitive or high-intensity sports; and
- a family history of OA (based on mounting evidence of a genetic link).20-22
Lack of neuromuscular control (proprioception) of the knee is another risk factor, since it can expose the internal joint to forces that would otherwise be absorbed by muscle. Exposure of the joint to excess forces can occur if the impact is rapid, leaving the muscle without adequate time to contract to absorb the force, or the muscle is fatigued and weak from prolonged exercise.23,24
Work with patients to modify risk. In discussing risk modification with patients, emphasize that high-intensity running, especially when practiced for years, increases the risk of OA of the knees.25 Indeed, high-impact activity of any kind subjects knee cartilage to significant single and repetitive impact loads and torsional loads.17,26 Point out, however, that some physical activity is needed to maintain normal metabolic activity of cartilage in a healthy joint and that recreational, mild-intensity running or jogging does not appear to increase the risk for OA.27
Be aggressive with knee injuries. As noted earlier, a history of acute ACL or meniscus injury is a risk factor for OA. Knee trauma with effusions that develop rapidly (within 2-12 hours) is associated with high risk of significant intraarticular damage to the ACL, meniscus, and articular cartilage.28 A study of pediatric and adolescent patients who underwent magnetic resonance imaging for possible internal knee injury found cartilage injuries to be the most common.29
To avoid additional damage, manage knee trauma with effusions as a significant injury. Treatment includes bracing, physical therapy, low-impact exercise, and possibly even cross-training or job modification. Advise patients to continue physical therapy until strength and proprioception are fully recovered and no pain or effusion remains, which generally takes about 6 to 8 weeks, and not to return to normal activity prematurely.
Don’t underestimate the power of a phone call
Other nonpharmacologic recommendations include referral to a physical therapist for evaluation and exercise instruction (SOR: 89%); instruction in the use of walking aids, such as a cane or crutch in the contralateral hand, to improve biomechanics (SOR: 90%); and the use of braces to support unstable knees, an unproven intervention that may increase proprioception and stability (SOR: 76%). Physicians should also recommend footwear with insoles or lateral wedges to decrease lateral thrust of the knee and medial compartment forces (SOR: 77%).
Regular telephone contact, possibly on a monthly basis, is a suggested strategy for promoting self-care, tested in patients with OA of the knee but recommended for those with arthritic hips solely on the basis of expert opinion. A number of other modalities, including thermal therapy (heat treatments with warm water or wax, or cold therapy with a 20-minute ice massage), transcutaneous electrical nerve stimulation (TENS), and acupuncture, are recommended for symptom relief.
Drug therapy: Start with acetaminophen
The OARSI guidelines cite acetaminophen as an “effective initial oral analgesic” for mild to moderate pain in patients with OA of the hips or knees (SOR: 92%).4 In analyses conducted by the committee, the NNT to achieve an improvement in pain ranged from 1 to 2 in an earlier systematic review8 to 4 to 16 in a subsequent meta-analysis.9
Prescribe NSAIDs for short-term relief. While acetaminophen is considered the preferred long-term oral treatment, the strongest pharmacologic recommendation for alleviating the pain and stiffness associated with OA of the hip or knee is for nonsteroidal anti-inflammatory drugs (NSAIDs) (SOR: 93%). The caveat, however, is that NSAIDs should be used in the lowest effective dose and are not considered a long-term option. Patients with increased gastrointestinal (GI) risk should use either a cyclooxygenase-2 (COX-2) agent or an NSAID with a proton pump inhibitor or misoprostol for GI protection.
For those with cardiovascular risks, both nonselective NSAIDs and COX-2 agents require caution; here, too, the lowest dose for the shortest possible duration is recommended.
The guidelines also call for the use of topical agents, such as topical NSAIDs and capsaicin, for relief of symptoms (SOR: 85%). The NNT for topical NSAIDs was 3 (95% CI, 2-4);4 capsaicin had an NNT of 4 (95% CI, 3-5) after 4 weeks of therapy.4 The recommendations also note that glucosamine and/or chondroitin sulfate may alleviate some symptoms of osteoarthritis of the knee, but should be discontinued if no benefit is observed after 6 months.
When something stronger is needed. For moderate to severe pain that has not responded to oral agents, intraarticular (IA) injections with corticosteroids are recommended, as are IA hyaluronate injections (SOR: 78% and 64%, respectively). Weak opioids/low-dose narcotics round out the recommendations for treating moderate pain, with stronger opioids reserved for patients whose pain is severe.
When to consider surgery
Joint replacement surgery is recommended for patients who do not achieve adequate pain relief and functional improvement from nonpharmacologic and pharmacologic modalities (SOR: 96%). A meta-analysis of 74 studies assessing quality of life 1 to 7 years after total hip and total knee replacement (THR and TKR) found substantial improvement in pain and function, but variable effects on mental health and social functioning. Risk factors for poor outcomes include older age; more (or more severe) preoperative pain; medical comorbidities; musculoskeletal comorbidities such as low back pain, with functional limitations; low mental health scores; and OA in the hip that was not replaced.10,11
Unicompartmental knee replacement (UKR) had an SOR of 76%. Reviews that compared TKR to UKR found similar 5-year outcomes in knee pain and function. Those who underwent UKR had better range of motion, but prosthesis survival at 10 years was better in those with TKR (>90% vs 85% to 90%).12
In young adults, osteotomy and jointpreserving procedures are recommended for hip OA, especially when dysplasia is present. In young, active patients with unicompartment OA, high tibial osteotomies may delay TKR by as long as 10 years.13
Joint lavage and arthroscopic debridement in knee OA remain controversial, although they may provide short-term symptom relief (SOR: 60%). Joint fusion as a salvage procedure after failed TKR had an SOR of 69%.
Work as a team to improve outcomes
The inevitable increase in the number of patients with OA of the hips and knees underscores the importance of having a range of treatment strategies, often best delivered by a multidisciplinary team with the family physician at the helm. The OARSI guidelines, which are backed by both a thorough review of research findings and expert consensus, can help you convince patients to take an active role in managing this potentially debilitating condition. Patients’ commitment to lifestyle modifications and self-management, bolstered by your guidance and support, is the most effective way to keep patients with OA on the move.
Correspondence
Greg P. Gutierrez, MD, Associate Professor, University of Colorado Denver Health Sciences Center, Department of Family Medicine, Denver Health and Hospital, 660 Bannock St., Denver, CO 80218; [email protected].
- Teach patients that self-care is key to successful management of osteoarthritis (Osteoarthritis Research Society International [OARSI] Evidence 1a
- Encourage patients to regularly engage in aerobic, muscle-strengthening, and range-of-motion exercise (Ia: knee; IV: hip).
- Recommend that patients try acetaminophen (≤4 g/d) before considering other analgesics for mild to moderate joint pain (Ia: knee; IV: hip).
- Prescribe the lowest effective dose of nonsteroidal anti-inflammatory drugs (NSAIDs) and avoid using them for long-term therapy (Ia).
OARSI level of evidence:
Ia: Meta-analysis of randomized controlled trials (RCTs)
Ib: RCT
IIa: Controlled study without randomization
IIb: Quasi-experimental study
III: Nonexperimental, descriptive studies
IV: Expert committee reports/opinion/experience
Osteoarthritis (OA) and other rheumatic conditions account for as many office visits as cardiovascular disease or essential hypertension, according to national data, and most involve primary care physicians.1 As the population ages, the prevalence of OA—estimated at 46.4 million in 2005 in the United States alone—will continue to rise.2,3 So, too, will the number of patients needing treatment for pain and functional limitations related to OA of the hips and knees.
Physicians who treat these patients have a new tool at their disposal: the Osteoarthritis Research Society International (OARSI)’s evidence-based, expert consensus guidelines for the management of hip and knee OA. These recommendations, published in February 2008, are the first “internationally agreed and universally applicable guidelines for the management of these global disorders.”4
In caring for patients with OA of the hips or knees, family physicians should keep in mind 2 guiding principles at the heart of the OARSI recommendations:
- the importance of lifestyle modification, including regular exercise, in coping with this degenerative, potentially debilitating disease; and
- the need to incorporate both nonpharmacologic interventions and drug therapy to achieve optimal care.4
International team sifts through the evidence
To develop the guidelines, OARSI convened a committee of 16 physicians from 6 countries and 2 continents, with expertise in 4 disciplines: rheumatology, orthopedics, evidence-based medicine, and primary care. The team reviewed national and regional guidelines and studied systematic reviews; meta-analyses; randomized controlled trials (RCTs); controlled and uncontrolled trials; cohort, case-control, and cross-sectional studies; and economic evaluations from 1945 through 2001. The team also conducted a systematic review of evidence from January 2002 through January 2006.4,5 To ensure the quality of evidence hierarchy, the team used internationally accepted research tools.
AP and tunnel images are key to OA diagnosis
A diagnosis of knee or hip osteoarthritis (OA) requires a medical history; physical examination; radiologic assessment, with standing X-rays of the lower extremities, including anterior-posterior and tunnel views for knee OA; and the exclusion of other conditions.30 The tunnel view shown here reveals bone-on-bone articulation in the medial compartment of the left knee, and demonstrates the importance of standing X-rays.
Differential diagnosis includes gout, pseudogout, rheumatoid arthritis, patella-femoral pain, pes anserine (knee) bursitis, iliotibial band pathology, meniscal tear, cruciate tears, and tumors. No blood tests are indicated unless an inflammatory process is suspected. Synovial fluid in an osteoarthritic knee has a white cell count of <2000/uL.31
The team used several criteria to rate the recommended strategies, including level of evidence, effect size for pain relief, level of consensus, and strength of recommendation (SOR). All of these criteria are included (and defined) in an at-a-glance summary of the OARSI recommendations ( TABLE ).
In particular, the SOR, which is used throughout this article, is an overall rating that reflects the opinions of the team members after consideration of the research evidence for efficacy, safety, and cost-effectiveness. It is based on a visual analog scale of 0 to 100 mm and is expressed as a percentage.
TABLE
OARSI guidelines rate the evidence for osteoarthritis treatment options
| RECOMMENDATION | SOR, % (95% CI)*/ LEVEL OF CONSENSUS, %† | LEVEL OF EVIDENCE‡ | ES (95% CI)§ |
|---|---|---|---|
| Nonpharmacologic | |||
| Education, self-help, patient-driven treatment | 97 (95 to 99)/NA | Ia: education | NA |
| Aerobic, muscle-strengthening, and range-of-motion exercises | 96 (93 to 99)/85 | Ia: knee IV: hip Ib: hip, water-based | 0.52 (0.34 to 0.70): aerobic 0.32 (0.23 to 0.42): strength 0.25 (0.02 to 0.47): water-based |
| Weight loss | 96 (92 to 100)/100 | Ia | 0.13 (-0.12 to 0.38) |
| Walking aids | 90 (84 to 96)/100 | IV | NA |
| Physical therapy | 89 (82 to 96)/100 | IV | NA |
| Appropriate footwear/insoles | 77 (66 to 88)/92 | IV: footwear Ia: insoles | NA |
| Knee braces | 76 (69 to 83)/92 | Ia | NA |
| Telephone contact | 66 (57 to 75)/77 | Ia: knee; IV: hip | 0.12 (0 to 0.24) |
| Thermal modalities | 64 (60 to 68)/77 | Ia | 0.69 (-0.07 to 1.45) |
| Acupuncture | 59 (47 to 71)/69 | Ia | 0.51 (0.23 to 0.79) |
| TENS | 58 (45 to 72)/69 | Ia | NA |
| Pharmacologic | |||
| Oral NSAIDs | 93 (88 to 99)/100 | Ia | 0.32 (0.24 to 0.39) |
| Acetaminophen ≤4 g/d | 92 (88 to 99)/77 | Ia: knee; IV: hip | 0.21 (0.02 to 0.41) |
| Topical NSAIDs/capsaicin | 85 (75 to 95)/100 | Ia | 0.41 (0.22 to 0.59) |
| Weak opioids/narcotics | 82 (74 to 90)/92 | Ia | NA |
| IA corticosteroid injections | 78 (61 to 95)/69 | Ia: knee; Ib: hip | 0.72 (0.42 to 1.02) |
| IA hyaluronate injections | 64 (43 to 85)/85 | Ia | 0.32 (0.17 to 0.47) |
| Glucosamine and/or chondroitin | 63 (44 to 82)/92 | Ia: glucosamine | 0.45 (0.04 to 0.86) |
| Surgical treatments | |||
| Joint replacement | 96 (94 to 98)/92 | III | NA |
| Unicompartmental knee replacement | 76 (64 to 88)/100 | IIIb | NA |
| Osteotomy/joint preservation | 75 (64 to 86)/100 | IIb | NA |
| Joint fusion | 69 (57 to 82)/100 | IV | NA |
| Joint lavage/arthroscopic debridement | 60 (47 to 82)/100 | Ib | 0.09 (-0.27 to 0.44): lavage -0.01 (-0.37 to 0.35): debridement |
| CI, confidence interval; ES, effect size for pain relief; IA, intraarticular; NA, not available; NSAIDs, nonsteroidal anti-inflammatory drugs; OARSI, Osteoarthritis Research Society International; SOR, strength of recommendation; TENS, transcutaneous electrical nerve stimulation | |||
| * SOR (strength of recommendation) is an overall rating that reflects the opinions of OARSI team members after consideration of the research evidence for efficacy, safety, and cost-effectiveness. SOR is based on a visual analog scale of 0 to 100 mm and is expressed as a percentage. | |||
| †Level of consensus is the estimated extent of agreement among committee members, expressed as a percentage. | |||
| ‡Level of evidence is broken into 6 categories: Ia: meta-analysis of randomized controlled trials (RCTs); Ib: RCT; IIa: controlled study without randomization; IIb: quasi-experimental study; III: nonexperimental, descriptive studies; and IV: expert committee reports/opinion/experience. | |||
| §ES (effect size for pain relief) is a measure of the standard mean difference between interventions (eg, treatment vs placebo): 0.2 (small); 0.5 (moderate); and >0.8 (large). The ES refers to the knee and hip unless otherwise specified. | |||
| Adapted from: Zhang et al.4 | |||
OARSI emphasizes patient education
Patient education about self-care and lifestyle modifications, such as weight loss, exercise, and pacing of activities to reduce the load on the affected joints, is OARSI’s strongest nonpharmacologic recommendation (SOR: 97%). The guidelines also call for the following interventions:
- correcting mechanical abnormalities of the skeleton;
- helping patients lose weight;
- assisting patients with smoking cessation efforts;6
- directing the use of nonprescription medications;
- prescribing assistive devices; and
- prescribing appropriate prescription drugs.
Nondrug options: Exercise that achy joint
To many patients, being told to exercise a joint in which movement is associated with stiffness and bone-on-bone pain seems counterintuitive. Referring to the findings of the OARSI panel may be helpful in explaining the importance of regular aerobic, muscle-strengthening, and range-of-motion exercises, all of which are strongly recommended (SOR: 96%). Exercise can be as simple as “regular aerobic walking” and home-based strengthening of the quadriceps.4 For patients with arthritic hips, water-based exercises are recommended.
Obesity can increase the risk of developing OA of the hips and knees, and excess weight puts extra stress on joints that are already arthritic. Thus, weight loss is both a risk modification factor (see “Is your patient at risk of OA? Take steps now” ) and a key OA management strategy (SOR: 96%). In a meta-regression analysis conducted by the committee, a reduction of >5% of body weight or a loss at a rate of >0.24% per week was associated with significant improvement in disability. One RCT had a number needed to treat (NNT) of 3 (95% confidence interval [CI], 2-9) to achieve improved pain and function scores after a 2-month low-energy diet.7
Risk factors for osteoarthritis (OA) include:14-19
- mechanical abnormalities, such as varus (bowlegged) and valgus (knock-kneed) angulations;
- flat feet, and heel pronation and supination;
- a history of joint surgery or acute injuries, particularly to the anterior cruciate ligament (ACL) or meniscus;
- obesity;
- manual labor (any job that involves heavy lifting, together with kneeling and squatting);
- participation in competitive or high-intensity sports; and
- a family history of OA (based on mounting evidence of a genetic link).20-22
Lack of neuromuscular control (proprioception) of the knee is another risk factor, since it can expose the internal joint to forces that would otherwise be absorbed by muscle. Exposure of the joint to excess forces can occur if the impact is rapid, leaving the muscle without adequate time to contract to absorb the force, or the muscle is fatigued and weak from prolonged exercise.23,24
Work with patients to modify risk. In discussing risk modification with patients, emphasize that high-intensity running, especially when practiced for years, increases the risk of OA of the knees.25 Indeed, high-impact activity of any kind subjects knee cartilage to significant single and repetitive impact loads and torsional loads.17,26 Point out, however, that some physical activity is needed to maintain normal metabolic activity of cartilage in a healthy joint and that recreational, mild-intensity running or jogging does not appear to increase the risk for OA.27
Be aggressive with knee injuries. As noted earlier, a history of acute ACL or meniscus injury is a risk factor for OA. Knee trauma with effusions that develop rapidly (within 2-12 hours) is associated with high risk of significant intraarticular damage to the ACL, meniscus, and articular cartilage.28 A study of pediatric and adolescent patients who underwent magnetic resonance imaging for possible internal knee injury found cartilage injuries to be the most common.29
To avoid additional damage, manage knee trauma with effusions as a significant injury. Treatment includes bracing, physical therapy, low-impact exercise, and possibly even cross-training or job modification. Advise patients to continue physical therapy until strength and proprioception are fully recovered and no pain or effusion remains, which generally takes about 6 to 8 weeks, and not to return to normal activity prematurely.
Don’t underestimate the power of a phone call
Other nonpharmacologic recommendations include referral to a physical therapist for evaluation and exercise instruction (SOR: 89%); instruction in the use of walking aids, such as a cane or crutch in the contralateral hand, to improve biomechanics (SOR: 90%); and the use of braces to support unstable knees, an unproven intervention that may increase proprioception and stability (SOR: 76%). Physicians should also recommend footwear with insoles or lateral wedges to decrease lateral thrust of the knee and medial compartment forces (SOR: 77%).
Regular telephone contact, possibly on a monthly basis, is a suggested strategy for promoting self-care, tested in patients with OA of the knee but recommended for those with arthritic hips solely on the basis of expert opinion. A number of other modalities, including thermal therapy (heat treatments with warm water or wax, or cold therapy with a 20-minute ice massage), transcutaneous electrical nerve stimulation (TENS), and acupuncture, are recommended for symptom relief.
Drug therapy: Start with acetaminophen
The OARSI guidelines cite acetaminophen as an “effective initial oral analgesic” for mild to moderate pain in patients with OA of the hips or knees (SOR: 92%).4 In analyses conducted by the committee, the NNT to achieve an improvement in pain ranged from 1 to 2 in an earlier systematic review8 to 4 to 16 in a subsequent meta-analysis.9
Prescribe NSAIDs for short-term relief. While acetaminophen is considered the preferred long-term oral treatment, the strongest pharmacologic recommendation for alleviating the pain and stiffness associated with OA of the hip or knee is for nonsteroidal anti-inflammatory drugs (NSAIDs) (SOR: 93%). The caveat, however, is that NSAIDs should be used in the lowest effective dose and are not considered a long-term option. Patients with increased gastrointestinal (GI) risk should use either a cyclooxygenase-2 (COX-2) agent or an NSAID with a proton pump inhibitor or misoprostol for GI protection.
For those with cardiovascular risks, both nonselective NSAIDs and COX-2 agents require caution; here, too, the lowest dose for the shortest possible duration is recommended.
The guidelines also call for the use of topical agents, such as topical NSAIDs and capsaicin, for relief of symptoms (SOR: 85%). The NNT for topical NSAIDs was 3 (95% CI, 2-4);4 capsaicin had an NNT of 4 (95% CI, 3-5) after 4 weeks of therapy.4 The recommendations also note that glucosamine and/or chondroitin sulfate may alleviate some symptoms of osteoarthritis of the knee, but should be discontinued if no benefit is observed after 6 months.
When something stronger is needed. For moderate to severe pain that has not responded to oral agents, intraarticular (IA) injections with corticosteroids are recommended, as are IA hyaluronate injections (SOR: 78% and 64%, respectively). Weak opioids/low-dose narcotics round out the recommendations for treating moderate pain, with stronger opioids reserved for patients whose pain is severe.
When to consider surgery
Joint replacement surgery is recommended for patients who do not achieve adequate pain relief and functional improvement from nonpharmacologic and pharmacologic modalities (SOR: 96%). A meta-analysis of 74 studies assessing quality of life 1 to 7 years after total hip and total knee replacement (THR and TKR) found substantial improvement in pain and function, but variable effects on mental health and social functioning. Risk factors for poor outcomes include older age; more (or more severe) preoperative pain; medical comorbidities; musculoskeletal comorbidities such as low back pain, with functional limitations; low mental health scores; and OA in the hip that was not replaced.10,11
Unicompartmental knee replacement (UKR) had an SOR of 76%. Reviews that compared TKR to UKR found similar 5-year outcomes in knee pain and function. Those who underwent UKR had better range of motion, but prosthesis survival at 10 years was better in those with TKR (>90% vs 85% to 90%).12
In young adults, osteotomy and jointpreserving procedures are recommended for hip OA, especially when dysplasia is present. In young, active patients with unicompartment OA, high tibial osteotomies may delay TKR by as long as 10 years.13
Joint lavage and arthroscopic debridement in knee OA remain controversial, although they may provide short-term symptom relief (SOR: 60%). Joint fusion as a salvage procedure after failed TKR had an SOR of 69%.
Work as a team to improve outcomes
The inevitable increase in the number of patients with OA of the hips and knees underscores the importance of having a range of treatment strategies, often best delivered by a multidisciplinary team with the family physician at the helm. The OARSI guidelines, which are backed by both a thorough review of research findings and expert consensus, can help you convince patients to take an active role in managing this potentially debilitating condition. Patients’ commitment to lifestyle modifications and self-management, bolstered by your guidance and support, is the most effective way to keep patients with OA on the move.
Correspondence
Greg P. Gutierrez, MD, Associate Professor, University of Colorado Denver Health Sciences Center, Department of Family Medicine, Denver Health and Hospital, 660 Bannock St., Denver, CO 80218; [email protected].
1. Hootman JM. Magnitude and characteristics of arthritis and other rheumatic conditions on ambulatory medical care visits, United States, 1997. Arthritis Rheum. 2002;47:571-581.
2. Lawrence R, Helmick CG, Arnett FC, et al. Estimates of the prevalence of arthritis and selected musculoskeletal disorders in the United States. Arthritis Rheum. 1998;41:778-799.
3. US Department of Health and Human Services. CDC: prevalence of doctor-diagnosed arthritis and arthritis-attributable activity limitation - United States, 2003–2005. MMWR. 2006;55:1089-1092.
4. Zhang W, Moskowitz RW, Nuki G, et al. OARSI recommendations for the management of hip and knee osteoarthritis, Part II: OARSI evidence-based, expert consensus guidelines. Osteoarthritis Cartilage. 2008;16:137-162.
5. Zhang W, Moskowitz RW, Nuki G, et al. OARSI recommendations for the management of hip and knee osteoarthritis, Part I: critical appraisal of existing treatment guidelines and systematic review of current research evidence. Osteoarthritis Cartilage. 2007;15:981-1000.
6. Amin S, Niu J, Guermazi A, et al. Cigarette smoking and the risk for cartilage loss and knee pain in men with knee osteoarthritis. Ann Rheum Dis. 2007;66:18-22.
7. Christensen R, Astrup A, Bliddal H. Weight loss: the treatment of choice for knee osteoarthritis? A randomized trial. Osteoarthritis Cartilage. 2005;13:20-27.
8. Towheed TE, Hochberg MC, Judd MG, Wells G. Acetaminophen for osteoarthritis. Cochrane Database Syst Rev. 2003;(2):CD004257.-
9. Towheed TE, Maxwell L, Judd MG, Catton M, Wells G. Acetaminophen for osteoarthritis. Cochrane Database Syst Rev. 2006;(1):CD004257.-
10. Ethgen O, Bruyere O, Richy F, Dardennes C, Reginster JY. Health-related quality of life in total hip and total knee arthroplasty. A qualitative and systematic review of the literature. J Bone Joint Surg Am. 2004;86-A:963-974.
11. Lingard EA, Katz JN, Wright EA, Sledge CB. Kinemax Outcomes Group. Predicting the outcome of total knee arthroplasty. J Bone Joint Surg Am. 2004;86-A:2179-2186.
12. Griffin T, Rowden L, Morgan D, Atkinson R, Woodruff P, Madden G. Unicompartmental knee arthroplasty for the treatment of unicompartmental osteoarthritis: a systematic study. ANZ J Surg. 2007;77:214-221.
13. Virolainen P, Arc HT. High tibial osteotomy for the treatment of osteoarthritis of the knee: a review of the literature and a meta-analysis of follow-up studies. Arch Orthop Trauma Surg. 2004;124:258-261.
14. Felson DT. Relation of obesity and of vocational and avocational risk factors to osteoarthritis. J Rheumatol. 2005;32:1133-1135.
15. Gelber AC, Hochberg MC, Mead LA, Wang NY, Wigley FM, Klag MJ. Joint injury in young adults and risk for subsequent knee and hip osteoarthritis. Ann Intern Med. 2000;133:321-328.
16. Sharma L, Song J, Felson DT, Cahue S, Shamiyeh E, Dunlop DD. The role of knee alignment in disease progression and functional decline in knee osteoarthritis. JAMA. 2001;286:188-195.
17. Lequesne MG, Dang N, Lane NE. Sport practice and osteoarthritis of the limbs. Osteoarthritis Cartilage. 1997;5:75-86.
18. Griffin TM, Guilak F. The role of mechanical loading in the onset and progression of osteoarthritis. Exerc Sport Sci Rev. 2005;33:195-200.
19. Maetzel A, Makela M, Hawker G, Bombardier C. Osteoarthritis of the hip and knee and mechanical occupational exposure: a systematic overview of the evidence. J Rheumatol. 1997;24:1599-1607.
20. Zhai G, Ding C, Stankovich J, Cicuttini F, Jones G. The genetic contribution to longitudinal changes in knee structure and muscle strength: a sibpair study. Arthritis Rheum. 2005;52:2830-2834.
21. Hirsch R, Lethbridge-Cejku M, Hanson R, et al. Familial aggregation of osteoarthritis: data from the Baltimore Longitudinal Study on Aging. Arthritis Rheum. 1998;41:1227-1232.
22. Felson DT, Couropmitree NN, Chaisson CE, et al. Evidence for a Mendelian gene in a segregation analysis of generalized radiographic osteoarthritis: the Framingham Study. Arthritis Rheum. 1998;41:1064-1071.
23. Christina KA, White SC, Gilchrist LA. Effect of localized muscle fatigue on vertical ground reaction forces and ankle joint motion during running. Hum Mov Sci. 2001;20:257-276.
24. Mizrahi J, Verbitsky O, Isakov E. Fatigue-related loading imbalance on the shank in running: a possible factor in stress fractures. Ann Biomed Eng. 2000;28:463-469.
25. McAlindon TE, Wilson PW, Aliabadi P, Weissman B, Felson DT. Level of physical activity and the risk of radiographic and symptomatic knee osteoarthritis in the elderly: the Framingham study. Am J Med. 1999;106:151-157.
26. Buckwalter JA. Sports, joint injury, and posttraumatic osteoarthritis. J Orthop Sports Phys Ther. 2003;33:578-588.
27. Conaghan PG. Update on osteoarthritis part 1: current concepts and the relation to exercise. British J Sports Med. 2002;36:330-333.
28. Maffulli N, Binfield PM, King JB, Good CJ. Acute haemarthrosis of the knee in athletes. A prospective study of 106 cases. J Bone Joint Surg Br. 1993;75:945-949.
29. Oeppen RS, Connolly SA, Bencardino JT, Jaramillo D. Acute injury of the articular cartilage and subchondral bone: a common but unrecognized lesion in the immature knee. Am J Roentgenol. 2004;182:111-117.
30. Felson DT. Osteoarthritis of the knee. N Engl J Med. 2006;354:841-848.
31. Hassebacher B. Arthrocentesis, synovial fluid analysis and synovial biopsy. In: Schumacher HR, Klippel JH, Koopman WJ, eds. Primer on the Rheumatic Diseases. Atlanta, GA: Arthritis Foundation; 1993:67-72.
1. Hootman JM. Magnitude and characteristics of arthritis and other rheumatic conditions on ambulatory medical care visits, United States, 1997. Arthritis Rheum. 2002;47:571-581.
2. Lawrence R, Helmick CG, Arnett FC, et al. Estimates of the prevalence of arthritis and selected musculoskeletal disorders in the United States. Arthritis Rheum. 1998;41:778-799.
3. US Department of Health and Human Services. CDC: prevalence of doctor-diagnosed arthritis and arthritis-attributable activity limitation - United States, 2003–2005. MMWR. 2006;55:1089-1092.
4. Zhang W, Moskowitz RW, Nuki G, et al. OARSI recommendations for the management of hip and knee osteoarthritis, Part II: OARSI evidence-based, expert consensus guidelines. Osteoarthritis Cartilage. 2008;16:137-162.
5. Zhang W, Moskowitz RW, Nuki G, et al. OARSI recommendations for the management of hip and knee osteoarthritis, Part I: critical appraisal of existing treatment guidelines and systematic review of current research evidence. Osteoarthritis Cartilage. 2007;15:981-1000.
6. Amin S, Niu J, Guermazi A, et al. Cigarette smoking and the risk for cartilage loss and knee pain in men with knee osteoarthritis. Ann Rheum Dis. 2007;66:18-22.
7. Christensen R, Astrup A, Bliddal H. Weight loss: the treatment of choice for knee osteoarthritis? A randomized trial. Osteoarthritis Cartilage. 2005;13:20-27.
8. Towheed TE, Hochberg MC, Judd MG, Wells G. Acetaminophen for osteoarthritis. Cochrane Database Syst Rev. 2003;(2):CD004257.-
9. Towheed TE, Maxwell L, Judd MG, Catton M, Wells G. Acetaminophen for osteoarthritis. Cochrane Database Syst Rev. 2006;(1):CD004257.-
10. Ethgen O, Bruyere O, Richy F, Dardennes C, Reginster JY. Health-related quality of life in total hip and total knee arthroplasty. A qualitative and systematic review of the literature. J Bone Joint Surg Am. 2004;86-A:963-974.
11. Lingard EA, Katz JN, Wright EA, Sledge CB. Kinemax Outcomes Group. Predicting the outcome of total knee arthroplasty. J Bone Joint Surg Am. 2004;86-A:2179-2186.
12. Griffin T, Rowden L, Morgan D, Atkinson R, Woodruff P, Madden G. Unicompartmental knee arthroplasty for the treatment of unicompartmental osteoarthritis: a systematic study. ANZ J Surg. 2007;77:214-221.
13. Virolainen P, Arc HT. High tibial osteotomy for the treatment of osteoarthritis of the knee: a review of the literature and a meta-analysis of follow-up studies. Arch Orthop Trauma Surg. 2004;124:258-261.
14. Felson DT. Relation of obesity and of vocational and avocational risk factors to osteoarthritis. J Rheumatol. 2005;32:1133-1135.
15. Gelber AC, Hochberg MC, Mead LA, Wang NY, Wigley FM, Klag MJ. Joint injury in young adults and risk for subsequent knee and hip osteoarthritis. Ann Intern Med. 2000;133:321-328.
16. Sharma L, Song J, Felson DT, Cahue S, Shamiyeh E, Dunlop DD. The role of knee alignment in disease progression and functional decline in knee osteoarthritis. JAMA. 2001;286:188-195.
17. Lequesne MG, Dang N, Lane NE. Sport practice and osteoarthritis of the limbs. Osteoarthritis Cartilage. 1997;5:75-86.
18. Griffin TM, Guilak F. The role of mechanical loading in the onset and progression of osteoarthritis. Exerc Sport Sci Rev. 2005;33:195-200.
19. Maetzel A, Makela M, Hawker G, Bombardier C. Osteoarthritis of the hip and knee and mechanical occupational exposure: a systematic overview of the evidence. J Rheumatol. 1997;24:1599-1607.
20. Zhai G, Ding C, Stankovich J, Cicuttini F, Jones G. The genetic contribution to longitudinal changes in knee structure and muscle strength: a sibpair study. Arthritis Rheum. 2005;52:2830-2834.
21. Hirsch R, Lethbridge-Cejku M, Hanson R, et al. Familial aggregation of osteoarthritis: data from the Baltimore Longitudinal Study on Aging. Arthritis Rheum. 1998;41:1227-1232.
22. Felson DT, Couropmitree NN, Chaisson CE, et al. Evidence for a Mendelian gene in a segregation analysis of generalized radiographic osteoarthritis: the Framingham Study. Arthritis Rheum. 1998;41:1064-1071.
23. Christina KA, White SC, Gilchrist LA. Effect of localized muscle fatigue on vertical ground reaction forces and ankle joint motion during running. Hum Mov Sci. 2001;20:257-276.
24. Mizrahi J, Verbitsky O, Isakov E. Fatigue-related loading imbalance on the shank in running: a possible factor in stress fractures. Ann Biomed Eng. 2000;28:463-469.
25. McAlindon TE, Wilson PW, Aliabadi P, Weissman B, Felson DT. Level of physical activity and the risk of radiographic and symptomatic knee osteoarthritis in the elderly: the Framingham study. Am J Med. 1999;106:151-157.
26. Buckwalter JA. Sports, joint injury, and posttraumatic osteoarthritis. J Orthop Sports Phys Ther. 2003;33:578-588.
27. Conaghan PG. Update on osteoarthritis part 1: current concepts and the relation to exercise. British J Sports Med. 2002;36:330-333.
28. Maffulli N, Binfield PM, King JB, Good CJ. Acute haemarthrosis of the knee in athletes. A prospective study of 106 cases. J Bone Joint Surg Br. 1993;75:945-949.
29. Oeppen RS, Connolly SA, Bencardino JT, Jaramillo D. Acute injury of the articular cartilage and subchondral bone: a common but unrecognized lesion in the immature knee. Am J Roentgenol. 2004;182:111-117.
30. Felson DT. Osteoarthritis of the knee. N Engl J Med. 2006;354:841-848.
31. Hassebacher B. Arthrocentesis, synovial fluid analysis and synovial biopsy. In: Schumacher HR, Klippel JH, Koopman WJ, eds. Primer on the Rheumatic Diseases. Atlanta, GA: Arthritis Foundation; 1993:67-72.
Help patients gain better asthma control
- Assess asthma severity before initiating treatment; monitor asthma control to guide adjustments in therapy using measures of impairment (B) and risk (C) (National Heart, Lung, and Blood Institute [NHLBI] and National Asthma Education and Prevention Program [NAEPP] third expert panel report [EPR-3]).
- Base treatment decisions on recommendations specific to each age group (0-4 years, 5-11 years, and ≥12 years) (A).
- Use spirometry in patients ≥5 years of age to diagnose asthma, classify severity, and assess control (C).
- Provide each patient with a written asthma action plan with instructions for daily disease management, as well as identification of, and response to, worsening symptoms (B).
EPR-3 evidence categories:
- Randomized, controlled trials (RCTs), rich body of data
- RCTs, limited body of data
- Nonrandomized trials and observational studies
- Panel consensus judgment
JJ, a 4-year-old boy, was taken to an urgent care clinic 3 times last winter for “recurrent bronchitis” and given a 7-day course of prednisone and antibiotics at each visit. His mother reports that “his colds always seem to go to his chest” and his skin is always dry. She was given a nebulizer and albuterol for use when JJ begins wheezing, which often happens when he has a cold, plays vigorously, or visits a friend who has cats.
JJ is one of approximately 6.7 million children—and 22.9 million US residents—who have asthma.1 To help guide the care of patients like JJ, the National Heart, Lung, and Blood Institute (NHLBI) and National Asthma Education and Prevention Program (NAEPP) released the third expert panel report (EPR-3) in 2007. Available at http://www.nhlbi.nih.gov/guidelines/asthma/asthgdln.htm, the EPR-3 provides the most comprehensive evidence-based guidance for the diagnosis and management of asthma to date.2
The guidelines were an invaluable resource for JJ’s family physician, who referred to them to categorize the severity of JJ’s asthma as “mild persistent.” In initiating treatment, JJ’s physician relied on specific recommendations for children 0 to 4 years of age to prescribe low-dose inhaled corticosteroids (ICS). Without the new guidelines, which underscore the safety of controller medication for young children, JJ’s physician would likely have been reluctant to place a 4-year-old on ICS.
This review highlights the EPR-3’s key recommendations to encourage widespread implementation by family physicians.
The EPR-3: What’s changed
The 2007 guidelines:
Recommend assessing asthma severity before starting treatment and assessing asthma control to guide adjustments in treatment.
Address both severity and control in terms of impairment and risk.
Feature 3 age breakdowns (0-4 years, 5-11 years, and ≥12 years) and a 6-step approach to asthma management.
Make it easier to individualize and adjust treatment.
What’s changed?
There’s a new paradigm
The 2007 update to guidelines released in 1997 and 2002 reflects a paradigm shift in the overall approach to asthma management. The change in focus addresses the highly variable nature of asthma2 and the recognition that asthma severity and asthma control are distinct concepts serving different functions in clinical practice.
Severity and control in 2 domains. Asthma severity—a measure of the intrinsic intensity of the disease process—is ideally assessed before initiating treatment. In contrast, asthma control is monitored over time to guide adjustments to therapy. The guidelines call for assessing severity and control within the domains of:
- impairment, based on asthma symptoms (identified by patient or caregiver recall of the past 2-4 weeks), quality of life, and functional limitations; and
- risk, of asthma exacerbations, progressive decline in pulmonary function (or reduced lung growth in children), or adverse events. Predictors of increased risk for exacerbations or death include persistent and/or severe airflow obstruction; at least 2 visits to the emergency department or hospitalizations for asthma within the past year; and a history of intubation or admission to intensive care, especially within the past 5 years.
The specific criteria for determining asthma severity and assessing asthma control are detailed in FIGURES 1 AND 2, respectively. Because treatment affects impairment and risk differently, this dual assessment helps ensure that therapeutic interventions minimize all manifestations of asthma as much as possible.
More steps and age-specific interventions. The EPR-3’s stepwise approach to asthma therapy has gone from 4 steps to 6, and the recommended treatments, as well as the levels of severity and criteria for assessing control that guide them, are now divided into 3 age groups: 0 to 4 years, 5 to 11 years, and ≥12 years (FIGURE 3). The previous guidelines, issued in 2002, divided treatment recommendations into 2 age groups: ≤5 years and >5 years. The EPR-3’s expansion makes it easier for physicians to initiate, individualize, and adjust treatment.
FIGURE 1
Classifying asthma severity and initiating therapy in children, adolescents, and adults
EIB, exercise-induced bronchospasm; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; ICS, inhaled corticosteroids; NA, not applicable; OCS, oral corticosteroids; SABA, short-acting β2-adrenergic agonist.
*Normal FEV1/FVC values are defined according to age: 8–9 years (85%), 20–39 years (80%), 40–59 years (75%), 60–80 years (70%).
†For treatment purposes, children with at least 2 exacerbations (eg, requiring urgent, unscheduled care; hospitalization; or intensive care unit admission) or adolescents/adults with at least 2 exacerbations requiring OCS in the past year may be considered the same as patients who have persistent asthma, even in the absence of impairment levels consistent with persistent asthma.
Adapted from: National Heart, Lung, and Blood Institute (NHLBI).2
FIGURE 2
Assessing asthma control and adjusting therapy
ACQ, Asthma Control Questionnaire; ACT, Asthma Control Test; ATAQ, Asthma Therapy Assessment Questionnaire; EIB, exercise-induced bronchospasm; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; N/A, not applicable; OCS, oral corticosteroids; SABA, short-acting β2-adrenergic agonist.
*ACQ values of 0.76 to 1.4 are indeterminate regarding well-controlled asthma.
†For treatment purposes, children with at least 2 exacerbations (eg, requiring urgent, unscheduled care; hospitalization; or intensive care unit admission) or adolescents/adults with at least 2 exacerbations requiring OCS in the past year may be considered the same as patients who have asthma that is not well controlled, even in the absence of impairment levels consistent with that classification.
Adapted from: National Heart, Lung, and Blood Institute (NHLBI).2
FIGURE 3
Stepwise approach for managing asthma
EIB, exercise-induced bronchospasm; ICS, inhaled corticosteroid; LABA, long-acting β2-adrenergic agonist; LTRA, leukotriene receptor antagonist; OCS, oral corticosteroid; PRN, as needed; SABA, short-acting β2-adrenergic agonist.
Adapted from: National Heart, Lung, and Blood Institute (NHLBI).2
Putting guidelines into practice begins with the history
A detailed medical history and a physical examination focusing on the upper respiratory tract, chest, and skin are needed to arrive at an asthma diagnosis. JJ’s physician asked his mother to describe recent symptoms and inquired about comorbid conditions that can aggravate asthma. He also identified viral respiratory infections, environmental causes, and activity as precipitating factors.
In considering an asthma diagnosis, try to determine the presence of episodic symptoms of airflow obstruction or bronchial hyperresponsiveness, as well as airflow obstruction that is at least partly reversible (an increase in forced expiratory volume in 1 second [FEV1] of >200 mL and ≥12% from baseline or an increase of ≥10% of predicted FEV1), and to exclude alternative diagnoses.
EPR-3 emphasizes spirometry
Recognizing that patients’ perception of airflow obstruction is highly variable and that pulmonary function measures do not always correlate directly with symptoms,3,4 the EPR-3 recommends spirometry for patients ≥5 years of age, both before and after bronchodilation. In addition to helping to confirm an asthma diagnosis, spirometry is the preferred measure of pulmonary function in classifying severity, because peak expiratory flow (PEF) testing has not proven reliable.5,6
Objective measurement of pulmonary function is difficult to obtain in children <5 years of age. If diagnosis remains uncertain for patients in this age group, a therapeutic trial of medication is recommended. In JJ’s case, however, 3 courses of oral corticosteroids (OCS) in less than 6 months were indicative of persistent asthma.
Spirometry is often underutilized. For patients ≥5 years of age, spirometry is a vital tool, but often underutilized in family practice. A recent study by Yawn and colleagues found that family physicians made changes in the management of approximately half of the asthma patients who underwent spirometry.7 (Information about spirometry training is available through the National Institute for Occupational Safety and Health at http://www.cdc.gov/niosh.) Referral to a specialist is recommended if the physician has difficulty making a differential diagnosis or is unable to perform spirometry on a patient who presents with atypical signs and symptoms of asthma.
What is the patient’s level of severity?
In patients who are not yet receiving long-term controller therapy, severity level is based on an assessment of impairment and risk (FIGURE 1). For patients who are already receiving treatment, severity is determined by the minimum pharmacologic therapy needed to maintain asthma control.
The severity classification—intermittent asthma or persistent asthma that is mild, moderate, or severe—is determined by the most severe category in which any feature occurs. (In children, FEV1/FVC [forced vital capacity] ratio has been shown to be a more sensitive determinant of severity than FEV1,4 which may be more useful in predicting exacerbations.8)
Asthma management: Preferred and alternative Tx
The recommended stepwise interventions include both preferred therapies (evidence-based) and alternative treatments (listed alphabetically in FIGURE 3 because there is insufficient evidence to rank them). The additional steps and age categories support the goal of using the least possible medication needed to maintain good control and minimize the potential for adverse events.
In initiating treatment, select the step that corresponds to the level of severity in the bottom row of FIGURE 1; to adjust medications, determine the patient’s level of asthma control and follow the corresponding guidance in the bottom row of FIGURE 2.
Inhaled corticosteroids remain the bedrock of therapy
ICS, the most potent and consistently effective long-term controller therapy, remain the foundation of therapy for patients of all ages who have persistent asthma. (Evidence: A).
Several of the age-based recommendations follow, with a focus on preferred treatments:
Children 0 to 4 years of age
- The guidelines recommend low-dose ICS at Step 2 (Evidence: A) and medium-dose ICS at Step 3 (Evidence: D), as inhaled corticosteroids have been shown to reduce impairment and risk in this age group.9-16 The potential risk is generally limited to a small reduction in growth velocity during the first year of treatment, and offset by the benefits of therapy.15,16
- Add a long-acting β2-adrenergic agonist (LABA) or montelukast to medium-dose ICS therapy at Step 4 rather than increasing the ICS dose (Evidence: D) to avoid the risk of side effects associated with high-dose ICS. Montelukast has demonstrated efficacy in children 2 to 5 years of age with persistent asthma.17
- Recommendations for preferred therapy at Steps 5 (high-dose ICS + LABA or montelukast) and 6 (Step 5 therapy + OCS) are based on expert panel judgment (Evidence: D). When severe persistent asthma warrants Step 6 therapy, start with a 2-week course of the lowest possible dose of OCS to confirm reversibility.
- In this age group, a therapeutic trial with close monitoring is recommended for patients whose asthma is not well controlled. If there is no response in 4 to 6 weeks, consider alternative therapies or diagnoses (Evidence: D).
Children 5 to 11 years of age
- For Step 3 therapy, the guidelines recommend either low-dose ICS plus a LABA, leukotriene receptor antagonist (LTRA), or theophylline; or medium-dose ICS (Evidence: B). Treatment decisions at Step 3 depend on whether impairment or risk is the chief concern, as well as on safety considerations.
- For Steps 4 and 5, ICS (medium dose for Step 5 and high dose for Step 6) plus a LABA is preferred, based on studies of patients ≥12 years of age (Evidence: B). Step 6 builds on Step 5, adding an OCS to the LABA/ICS combination (Evidence: D).
- If theophylline is prescribed—a viable option if cost and adherence to inhaled medications are key concerns—serum levels must be closely monitored because of the risk of toxicity.
- Closely monitor and be prepared to identify and respond to anaphylaxis in a child at Step 2, 3, or 4 who is receiving allergen immunotherapy.
Adolescents ≥12 years of age and adults
- There are 2 preferred Step 3 treatments: Low-dose ICS plus a LABA, or medium-dose ICS. The combination therapy has shown greater improvement in impairment24,25 and risk24-26 compared with the higher dose of ICS.
- Preferred treatments at Steps 4, 5, and 6 are the same as those for children ages 5 to 11 years, with one exception: Subcutaneous anti-IgE therapy (omalizumab) may be added to the regimen at Steps 5 and 6 for adolescents and adults with severe persistent allergic asthma to reduce the risk of exacerbations.27
Weigh the benefits and risks of therapy
Safety is a key consideration for all asthma patients. Carefully weigh the benefits and risks of therapy, including the rare but potential risk of life-threatening or fatal exacerbations with daily LABA therapy28 and systemic effects with higher doses of ICS.23 Patients who begin receiving oral corticosteroids require close monitoring, regardless of age.
Regular reassessment and monitoring are critical
Schedule visits at 2- to 6-week intervals for those who are starting therapy or require a step up to achieve or regain asthma control. After control is achieved, reassess at least every 1 to 6 months. Measures of asthma control are the same as those used to assess severity, with the addition of validated multidimensional questionnaires (eg, Asthma Control Test [ACT])29 to gauge impairment.
JJ’s physician scheduled a follow-up visit in 4 weeks, at which time he did a reassessment based on a physical exam and symptom recall. Finding JJ’s asthma to be well controlled, the physician asked the boy’s mother to bring him back to the office in 2 months, or earlier if symptoms recurred.
TABLE W1
Asthma education resources
| Allergy & Asthma Network Mothers of Asthmatics 2751 Prosperity Avenue, Suite 150 Fairfax, VA 22030 www.breatherville.org (800) 878-4403 or (703) 641-9595 | Asthma and Allergy Foundation of America 1233 20th Street, NW, Suite 402 Washington, DC 20036 www.aafa.org (800) 727-8462 |
| American Academy of Allergy, Asthma, and Immunology 555 East Wells Street, Suite 1100 Milwaukee, WI 53202-3823 www.aaaai.org (414) 272-6071 | Centers for Disease Control and Prevention 1600 Clifton Road Atlanta, GA 30333 www.cdc.gov (800) 311-3435 |
| American Association for Respiratory Care 9125 North macArthur boulevard, Suite 100 Irving, TX 75063 www.aarc.org (972) 243-2272 | Food Allergy & Anaphylaxis Network 11781 lee Jackson Highway, Suite 160 Fairfax, VA 22033 www.foodallergy.org (800) 929-4040 |
| American College of Allergy, Asthma, and Immunology 85 West Algonquin road, Suite 550 Arlington Heights, IL 60005 www.acaai.org (800) 842-7777 or (847) 427-1200 | National Heart, Lung, and Blood Institute Information Center P.O. Box 30105 Bethesda, MD 20824-0105 www.nhlbi.nih.gov (301) 592-8573 |
| American Lung Association 61 Broadway New York, NY 10006 www.lungusa.org (800) 586-4872 | National Jewish Medical and Research Center (Lung Line) 1400 Jackson Street Denver, CO 80206 www.njc.org (800) 222-lUNG |
| Association of Asthma Educators 1215 Anthony Avenue Columbia, SC 29201 www.asthmaeducators.org (888) 988-7747 | US Environmental Protection Agency National Center for Environmental Publications P.O. Box 42419 Cincinnati, OH 45242-0419 www.airnow.gov (800) 490-9198 |
Does your patient require a step down or step up?
A step down is recommended for patients whose asthma is well controlled for 3 months or more. Reduce the dose of ICS gradually, about 25% to 50% every 3 months, because deterioration in asthma control is highly variable. Review adherence and medication administration technique with patients whose asthma is not well controlled, and consider a step up in treatment. If an alternative treatment is used but does not result in an adequate response, it should be discontinued and the preferred treatment used before stepping up. Refer patients to an asthma specialist if their asthma does not respond to these adjustments.
Partner with patients for optimal care
The EPR-3 recommends the integration of patient education into all aspects of asthma care. To forge an active partnership, identify and address concerns about the condition and its treatment and involve the patient and family in developing treatment goals and making treatment decisions. If the patient is old enough, encourage self-monitoring and management.
The EPR-3 recommends that physicians give every patient a written asthma action plan that addresses individual symptoms and/or PEF measurements and includes instructions for self-management. Daily PEF monitoring can be useful in identifying early changes in the disease state and evaluating response to changes in therapy. It is ideal for those who have moderate to severe persistent asthma, difficulty recognizing signs of exacerbations, or a history of severe exacerbations.
Correspondence
Stuart W. Stoloff, MD, Clinical Professor, Department of Family and Community Medicine, University of Nevada–Reno, 1200 Mountain Street, Suite 220, Carson City, NV 89703; [email protected].
1. National Center for Health Statistics. Fast stats A to Z. Available at: www.cdc.gov/nchs/fastats/asthma.htm. Accessed August 1, 2008.
2. National Heart, Lung, and Blood Institute (NHLBI). National Asthma Education and Prevention Program Expert Panel Report 3: Guidelines for the Diagnosis and Management of Asthma. Full Report 2007. Bethesda, MD: NHLBI; August 2007. NIH publication no. 07-4051. Available at: http://www.nhlbi.nih.gov/guidelines/asthma/asthgdln.htm. Accessed July 17, 2008.
3. Stout JW, Visness CM, Enright P, et al. Classification of asthma severity in children: the contribution of pulmonary function testing. Arch Pediatr Adolesc Med. 2006;160:844-850.
4. Bacharier LB, Strunk RC, Mauger D, et al. Classifying asthma severity in children: mismatch between symptoms, medication use, and lung function. Am J Respir Crit Care Med. 2004;170:426-432.
5. Eid N, Yandell B, Howell L, Eddy M, Sheikh S. Can children with asthma? Pediatrics. 2000;105:354-358.
6. Llewellin P, Sawyer G, Lewis S, et al. The relationship between FEV1 and PEF in the assessment of the severity of airways obstruction. Respirology. 2002;7:333-337.
7. Yawn BP, Enright PL, Lemanske RF, Jr, et al. Spirometry can be done in family physicians’ offices and alters clinical decisions in management of asthma and COPD. Chest. 2007;132:1162-1168.
8. Fuhlbrigge AL, Kitch BT, Paltiel AD, et al. FEV1 is associated with risk of asthma attacks in a pediatric population. J Allergy Clin Immunol. 2001;107:61-67.
9. Roorda RJ, Mezei G, Bisgaard H, Maden C. Response of preschool children with asthma symptoms to fluticasone propionate. J Allergy Clin Immunol. 2001;108:540-546.
10. Baker JW, Mellon M, Wald J, Welch M, Cruz-Rivera M, Walton-Bowen K. A multiple-dosing, placebo-controlled study of budesonide inhalation suspension given once or twice daily for treatment of persistent asthma in young children and infants. Pediatrics. 1999;103:414-421.
11. Kemp JP, Skoner DP, Szefler SJ, Walton-Bowen K, Cruz-Rivera M, Smith JA. Once-daily budesonide inhalation suspension for the treatment of persistent asthma in infants and young children. Ann Allergy Asthma Immunol. 1999;83:231-239.
12. Shapiro G, Mendelson L, Kraemer MJ, Cruz-Rivera M, Walton-Bowen K, Smith JA. Efficacy and safety of budesonide inhalation suspension (Pulmicort Respules) in young children with inhaled steroid-dependent, persistent asthma. J Allergy Clin Immunol. 1998;102:789-796.
13. Bisgaard H, Gillies J, Groenewald M, Maden C. The effect of inhaled fluticasone propionate in the treatment of young asthmatic children: a dose comparison study. Am J Respir Crit Care Med. 1999;160:126-131.
14. Szefler SJ, Eigen H. Budesonide inhalation suspension: a nebulized corticosteroid for persistent asthma. J Allergy Clin Immunol. 2002;109:730-742.
15. Guilbert TW, Morgan WJ, Zeiger RS, et al. Long-term inhaled corticosteroids in preschool children at high risk for asthma. N Engl J Med. 2006;354:1985-1997.
16. Bisgaard H, Allen D, Milanowski J, Kalev I, Willits L, Davies P. Twelve-month safety and efficacy of inhaled fluticasone propionate in children aged 1 to 3 years with recurrent wheezing. Pediatrics. 2004;113:e87-e94.
17. Knorr B, Franchi LM, Bisgaard H, et al. Montelukast, a leukotriene receptor antagonist, for the treatment of persistent asthma in children aged 2 to 5 years. Pediatrics 2001;108:e48.-
18. Russell G, Williams DA, Weller P, Price JF. Salmeterol xinafoate in children on high dose inhaled steroids. Ann Allergy Asthma Immunol. 1995;75:423-428.
19. Zimmerman B, D’Urzo A, Bérubé D. Efficacy and safety of formoterol Turbuhaler when added to inhaled corticosteroid treatment in children with asthma. Pediatr Pulmonol. 2004;37:122-127.
20. Simons FE, Villa JR, Lee BW, et al. Montelukast added to budesonide in children with persistent asthma: a randomized, double-blind, crossover study. J Pediatr. 2001;138:694-698.
21. Shapiro G, Bronsky EA, LaForce CF, et al. Dose-related efficacy of budesonide administered via a dry powder inhaler in the treatment of children with moderate to severe persistent asthma. J Pediatr. 1998;132:976-982.
22. Pauwels RA, Lofdahl C-G, Postma DS, et al. for the Formoterol and Corticosteroids Establishing Therapy (FACET) International Study Group. Effect of inhaled formoterol and budesonide on exacerbations of asthma. N Engl J Med. 1997;337:1405-1411.
23. Tattersfield AE, Harrison TW, Hubbard RB, Mortimer K. Safety of inhaled corticosteroids. Proc Am Thorac Soc. 2004;1:171-175.
24. Bateman ED, Boushey HA, Bousquet J, et al. For the GOAL Investigators Group. Can guideline-defined asthma control be achieved? The Gaining Optimal Asthma ControL study. Am J Respir Crit Care Med. 2004;170:836-844.
25. O’Byrne PM, Barnes PJ, Rodriguez-Roisin R, et al. Low dose inhaled budesonide and formoterol in mild persistent asthma: the OPTIMA randomized trial. Am J Respir Crit Care Med. 2001;164:1392-1397.
26. Masoli M, Weatherall M, Holt S, Beasley R. Moderate dose inhaled corticosteroids plus salmeterol versus higher doses of inhaled corticosteroids in symptomatic asthma. Thorax. 2005;60:730-734.
27. Bousquet J, Wenzel S, Holgate S, Lumry W, Freeman P, Fox H. Predicting response to omalizumab, an anti-IgE antibody, in patients with allergic asthma. Chest. 2004;125:1378-1386.
28. Nelson HS, Weiss ST, Bleecker ER, Yancey SW, Dorinsky PM. For the SMART Study Group. The Salmeterol Multicenter Asthma Research Trial: a comparison of usual pharmacotherapy for asthma or usual pharmacotherapy plus salmeterol. Chest. 2006;129:15-26.
29. Nathan RA, Sorkness CA, Kosinski M, et al. Development of the asthma control test: a survey for assessing asthma control. J Allergy Clin Immunol. 2004;113:59-65.
- Assess asthma severity before initiating treatment; monitor asthma control to guide adjustments in therapy using measures of impairment (B) and risk (C) (National Heart, Lung, and Blood Institute [NHLBI] and National Asthma Education and Prevention Program [NAEPP] third expert panel report [EPR-3]).
- Base treatment decisions on recommendations specific to each age group (0-4 years, 5-11 years, and ≥12 years) (A).
- Use spirometry in patients ≥5 years of age to diagnose asthma, classify severity, and assess control (C).
- Provide each patient with a written asthma action plan with instructions for daily disease management, as well as identification of, and response to, worsening symptoms (B).
EPR-3 evidence categories:
- Randomized, controlled trials (RCTs), rich body of data
- RCTs, limited body of data
- Nonrandomized trials and observational studies
- Panel consensus judgment
JJ, a 4-year-old boy, was taken to an urgent care clinic 3 times last winter for “recurrent bronchitis” and given a 7-day course of prednisone and antibiotics at each visit. His mother reports that “his colds always seem to go to his chest” and his skin is always dry. She was given a nebulizer and albuterol for use when JJ begins wheezing, which often happens when he has a cold, plays vigorously, or visits a friend who has cats.
JJ is one of approximately 6.7 million children—and 22.9 million US residents—who have asthma.1 To help guide the care of patients like JJ, the National Heart, Lung, and Blood Institute (NHLBI) and National Asthma Education and Prevention Program (NAEPP) released the third expert panel report (EPR-3) in 2007. Available at http://www.nhlbi.nih.gov/guidelines/asthma/asthgdln.htm, the EPR-3 provides the most comprehensive evidence-based guidance for the diagnosis and management of asthma to date.2
The guidelines were an invaluable resource for JJ’s family physician, who referred to them to categorize the severity of JJ’s asthma as “mild persistent.” In initiating treatment, JJ’s physician relied on specific recommendations for children 0 to 4 years of age to prescribe low-dose inhaled corticosteroids (ICS). Without the new guidelines, which underscore the safety of controller medication for young children, JJ’s physician would likely have been reluctant to place a 4-year-old on ICS.
This review highlights the EPR-3’s key recommendations to encourage widespread implementation by family physicians.
The EPR-3: What’s changed
The 2007 guidelines:
Recommend assessing asthma severity before starting treatment and assessing asthma control to guide adjustments in treatment.
Address both severity and control in terms of impairment and risk.
Feature 3 age breakdowns (0-4 years, 5-11 years, and ≥12 years) and a 6-step approach to asthma management.
Make it easier to individualize and adjust treatment.
What’s changed?
There’s a new paradigm
The 2007 update to guidelines released in 1997 and 2002 reflects a paradigm shift in the overall approach to asthma management. The change in focus addresses the highly variable nature of asthma2 and the recognition that asthma severity and asthma control are distinct concepts serving different functions in clinical practice.
Severity and control in 2 domains. Asthma severity—a measure of the intrinsic intensity of the disease process—is ideally assessed before initiating treatment. In contrast, asthma control is monitored over time to guide adjustments to therapy. The guidelines call for assessing severity and control within the domains of:
- impairment, based on asthma symptoms (identified by patient or caregiver recall of the past 2-4 weeks), quality of life, and functional limitations; and
- risk, of asthma exacerbations, progressive decline in pulmonary function (or reduced lung growth in children), or adverse events. Predictors of increased risk for exacerbations or death include persistent and/or severe airflow obstruction; at least 2 visits to the emergency department or hospitalizations for asthma within the past year; and a history of intubation or admission to intensive care, especially within the past 5 years.
The specific criteria for determining asthma severity and assessing asthma control are detailed in FIGURES 1 AND 2, respectively. Because treatment affects impairment and risk differently, this dual assessment helps ensure that therapeutic interventions minimize all manifestations of asthma as much as possible.
More steps and age-specific interventions. The EPR-3’s stepwise approach to asthma therapy has gone from 4 steps to 6, and the recommended treatments, as well as the levels of severity and criteria for assessing control that guide them, are now divided into 3 age groups: 0 to 4 years, 5 to 11 years, and ≥12 years (FIGURE 3). The previous guidelines, issued in 2002, divided treatment recommendations into 2 age groups: ≤5 years and >5 years. The EPR-3’s expansion makes it easier for physicians to initiate, individualize, and adjust treatment.
FIGURE 1
Classifying asthma severity and initiating therapy in children, adolescents, and adults
EIB, exercise-induced bronchospasm; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; ICS, inhaled corticosteroids; NA, not applicable; OCS, oral corticosteroids; SABA, short-acting β2-adrenergic agonist.
*Normal FEV1/FVC values are defined according to age: 8–9 years (85%), 20–39 years (80%), 40–59 years (75%), 60–80 years (70%).
†For treatment purposes, children with at least 2 exacerbations (eg, requiring urgent, unscheduled care; hospitalization; or intensive care unit admission) or adolescents/adults with at least 2 exacerbations requiring OCS in the past year may be considered the same as patients who have persistent asthma, even in the absence of impairment levels consistent with persistent asthma.
Adapted from: National Heart, Lung, and Blood Institute (NHLBI).2
FIGURE 2
Assessing asthma control and adjusting therapy
ACQ, Asthma Control Questionnaire; ACT, Asthma Control Test; ATAQ, Asthma Therapy Assessment Questionnaire; EIB, exercise-induced bronchospasm; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; N/A, not applicable; OCS, oral corticosteroids; SABA, short-acting β2-adrenergic agonist.
*ACQ values of 0.76 to 1.4 are indeterminate regarding well-controlled asthma.
†For treatment purposes, children with at least 2 exacerbations (eg, requiring urgent, unscheduled care; hospitalization; or intensive care unit admission) or adolescents/adults with at least 2 exacerbations requiring OCS in the past year may be considered the same as patients who have asthma that is not well controlled, even in the absence of impairment levels consistent with that classification.
Adapted from: National Heart, Lung, and Blood Institute (NHLBI).2
FIGURE 3
Stepwise approach for managing asthma
EIB, exercise-induced bronchospasm; ICS, inhaled corticosteroid; LABA, long-acting β2-adrenergic agonist; LTRA, leukotriene receptor antagonist; OCS, oral corticosteroid; PRN, as needed; SABA, short-acting β2-adrenergic agonist.
Adapted from: National Heart, Lung, and Blood Institute (NHLBI).2
Putting guidelines into practice begins with the history
A detailed medical history and a physical examination focusing on the upper respiratory tract, chest, and skin are needed to arrive at an asthma diagnosis. JJ’s physician asked his mother to describe recent symptoms and inquired about comorbid conditions that can aggravate asthma. He also identified viral respiratory infections, environmental causes, and activity as precipitating factors.
In considering an asthma diagnosis, try to determine the presence of episodic symptoms of airflow obstruction or bronchial hyperresponsiveness, as well as airflow obstruction that is at least partly reversible (an increase in forced expiratory volume in 1 second [FEV1] of >200 mL and ≥12% from baseline or an increase of ≥10% of predicted FEV1), and to exclude alternative diagnoses.
EPR-3 emphasizes spirometry
Recognizing that patients’ perception of airflow obstruction is highly variable and that pulmonary function measures do not always correlate directly with symptoms,3,4 the EPR-3 recommends spirometry for patients ≥5 years of age, both before and after bronchodilation. In addition to helping to confirm an asthma diagnosis, spirometry is the preferred measure of pulmonary function in classifying severity, because peak expiratory flow (PEF) testing has not proven reliable.5,6
Objective measurement of pulmonary function is difficult to obtain in children <5 years of age. If diagnosis remains uncertain for patients in this age group, a therapeutic trial of medication is recommended. In JJ’s case, however, 3 courses of oral corticosteroids (OCS) in less than 6 months were indicative of persistent asthma.
Spirometry is often underutilized. For patients ≥5 years of age, spirometry is a vital tool, but often underutilized in family practice. A recent study by Yawn and colleagues found that family physicians made changes in the management of approximately half of the asthma patients who underwent spirometry.7 (Information about spirometry training is available through the National Institute for Occupational Safety and Health at http://www.cdc.gov/niosh.) Referral to a specialist is recommended if the physician has difficulty making a differential diagnosis or is unable to perform spirometry on a patient who presents with atypical signs and symptoms of asthma.
What is the patient’s level of severity?
In patients who are not yet receiving long-term controller therapy, severity level is based on an assessment of impairment and risk (FIGURE 1). For patients who are already receiving treatment, severity is determined by the minimum pharmacologic therapy needed to maintain asthma control.
The severity classification—intermittent asthma or persistent asthma that is mild, moderate, or severe—is determined by the most severe category in which any feature occurs. (In children, FEV1/FVC [forced vital capacity] ratio has been shown to be a more sensitive determinant of severity than FEV1,4 which may be more useful in predicting exacerbations.8)
Asthma management: Preferred and alternative Tx
The recommended stepwise interventions include both preferred therapies (evidence-based) and alternative treatments (listed alphabetically in FIGURE 3 because there is insufficient evidence to rank them). The additional steps and age categories support the goal of using the least possible medication needed to maintain good control and minimize the potential for adverse events.
In initiating treatment, select the step that corresponds to the level of severity in the bottom row of FIGURE 1; to adjust medications, determine the patient’s level of asthma control and follow the corresponding guidance in the bottom row of FIGURE 2.
Inhaled corticosteroids remain the bedrock of therapy
ICS, the most potent and consistently effective long-term controller therapy, remain the foundation of therapy for patients of all ages who have persistent asthma. (Evidence: A).
Several of the age-based recommendations follow, with a focus on preferred treatments:
Children 0 to 4 years of age
- The guidelines recommend low-dose ICS at Step 2 (Evidence: A) and medium-dose ICS at Step 3 (Evidence: D), as inhaled corticosteroids have been shown to reduce impairment and risk in this age group.9-16 The potential risk is generally limited to a small reduction in growth velocity during the first year of treatment, and offset by the benefits of therapy.15,16
- Add a long-acting β2-adrenergic agonist (LABA) or montelukast to medium-dose ICS therapy at Step 4 rather than increasing the ICS dose (Evidence: D) to avoid the risk of side effects associated with high-dose ICS. Montelukast has demonstrated efficacy in children 2 to 5 years of age with persistent asthma.17
- Recommendations for preferred therapy at Steps 5 (high-dose ICS + LABA or montelukast) and 6 (Step 5 therapy + OCS) are based on expert panel judgment (Evidence: D). When severe persistent asthma warrants Step 6 therapy, start with a 2-week course of the lowest possible dose of OCS to confirm reversibility.
- In this age group, a therapeutic trial with close monitoring is recommended for patients whose asthma is not well controlled. If there is no response in 4 to 6 weeks, consider alternative therapies or diagnoses (Evidence: D).
Children 5 to 11 years of age
- For Step 3 therapy, the guidelines recommend either low-dose ICS plus a LABA, leukotriene receptor antagonist (LTRA), or theophylline; or medium-dose ICS (Evidence: B). Treatment decisions at Step 3 depend on whether impairment or risk is the chief concern, as well as on safety considerations.
- For Steps 4 and 5, ICS (medium dose for Step 5 and high dose for Step 6) plus a LABA is preferred, based on studies of patients ≥12 years of age (Evidence: B). Step 6 builds on Step 5, adding an OCS to the LABA/ICS combination (Evidence: D).
- If theophylline is prescribed—a viable option if cost and adherence to inhaled medications are key concerns—serum levels must be closely monitored because of the risk of toxicity.
- Closely monitor and be prepared to identify and respond to anaphylaxis in a child at Step 2, 3, or 4 who is receiving allergen immunotherapy.
Adolescents ≥12 years of age and adults
- There are 2 preferred Step 3 treatments: Low-dose ICS plus a LABA, or medium-dose ICS. The combination therapy has shown greater improvement in impairment24,25 and risk24-26 compared with the higher dose of ICS.
- Preferred treatments at Steps 4, 5, and 6 are the same as those for children ages 5 to 11 years, with one exception: Subcutaneous anti-IgE therapy (omalizumab) may be added to the regimen at Steps 5 and 6 for adolescents and adults with severe persistent allergic asthma to reduce the risk of exacerbations.27
Weigh the benefits and risks of therapy
Safety is a key consideration for all asthma patients. Carefully weigh the benefits and risks of therapy, including the rare but potential risk of life-threatening or fatal exacerbations with daily LABA therapy28 and systemic effects with higher doses of ICS.23 Patients who begin receiving oral corticosteroids require close monitoring, regardless of age.
Regular reassessment and monitoring are critical
Schedule visits at 2- to 6-week intervals for those who are starting therapy or require a step up to achieve or regain asthma control. After control is achieved, reassess at least every 1 to 6 months. Measures of asthma control are the same as those used to assess severity, with the addition of validated multidimensional questionnaires (eg, Asthma Control Test [ACT])29 to gauge impairment.
JJ’s physician scheduled a follow-up visit in 4 weeks, at which time he did a reassessment based on a physical exam and symptom recall. Finding JJ’s asthma to be well controlled, the physician asked the boy’s mother to bring him back to the office in 2 months, or earlier if symptoms recurred.
TABLE W1
Asthma education resources
| Allergy & Asthma Network Mothers of Asthmatics 2751 Prosperity Avenue, Suite 150 Fairfax, VA 22030 www.breatherville.org (800) 878-4403 or (703) 641-9595 | Asthma and Allergy Foundation of America 1233 20th Street, NW, Suite 402 Washington, DC 20036 www.aafa.org (800) 727-8462 |
| American Academy of Allergy, Asthma, and Immunology 555 East Wells Street, Suite 1100 Milwaukee, WI 53202-3823 www.aaaai.org (414) 272-6071 | Centers for Disease Control and Prevention 1600 Clifton Road Atlanta, GA 30333 www.cdc.gov (800) 311-3435 |
| American Association for Respiratory Care 9125 North macArthur boulevard, Suite 100 Irving, TX 75063 www.aarc.org (972) 243-2272 | Food Allergy & Anaphylaxis Network 11781 lee Jackson Highway, Suite 160 Fairfax, VA 22033 www.foodallergy.org (800) 929-4040 |
| American College of Allergy, Asthma, and Immunology 85 West Algonquin road, Suite 550 Arlington Heights, IL 60005 www.acaai.org (800) 842-7777 or (847) 427-1200 | National Heart, Lung, and Blood Institute Information Center P.O. Box 30105 Bethesda, MD 20824-0105 www.nhlbi.nih.gov (301) 592-8573 |
| American Lung Association 61 Broadway New York, NY 10006 www.lungusa.org (800) 586-4872 | National Jewish Medical and Research Center (Lung Line) 1400 Jackson Street Denver, CO 80206 www.njc.org (800) 222-lUNG |
| Association of Asthma Educators 1215 Anthony Avenue Columbia, SC 29201 www.asthmaeducators.org (888) 988-7747 | US Environmental Protection Agency National Center for Environmental Publications P.O. Box 42419 Cincinnati, OH 45242-0419 www.airnow.gov (800) 490-9198 |
Does your patient require a step down or step up?
A step down is recommended for patients whose asthma is well controlled for 3 months or more. Reduce the dose of ICS gradually, about 25% to 50% every 3 months, because deterioration in asthma control is highly variable. Review adherence and medication administration technique with patients whose asthma is not well controlled, and consider a step up in treatment. If an alternative treatment is used but does not result in an adequate response, it should be discontinued and the preferred treatment used before stepping up. Refer patients to an asthma specialist if their asthma does not respond to these adjustments.
Partner with patients for optimal care
The EPR-3 recommends the integration of patient education into all aspects of asthma care. To forge an active partnership, identify and address concerns about the condition and its treatment and involve the patient and family in developing treatment goals and making treatment decisions. If the patient is old enough, encourage self-monitoring and management.
The EPR-3 recommends that physicians give every patient a written asthma action plan that addresses individual symptoms and/or PEF measurements and includes instructions for self-management. Daily PEF monitoring can be useful in identifying early changes in the disease state and evaluating response to changes in therapy. It is ideal for those who have moderate to severe persistent asthma, difficulty recognizing signs of exacerbations, or a history of severe exacerbations.
Correspondence
Stuart W. Stoloff, MD, Clinical Professor, Department of Family and Community Medicine, University of Nevada–Reno, 1200 Mountain Street, Suite 220, Carson City, NV 89703; [email protected].
- Assess asthma severity before initiating treatment; monitor asthma control to guide adjustments in therapy using measures of impairment (B) and risk (C) (National Heart, Lung, and Blood Institute [NHLBI] and National Asthma Education and Prevention Program [NAEPP] third expert panel report [EPR-3]).
- Base treatment decisions on recommendations specific to each age group (0-4 years, 5-11 years, and ≥12 years) (A).
- Use spirometry in patients ≥5 years of age to diagnose asthma, classify severity, and assess control (C).
- Provide each patient with a written asthma action plan with instructions for daily disease management, as well as identification of, and response to, worsening symptoms (B).
EPR-3 evidence categories:
- Randomized, controlled trials (RCTs), rich body of data
- RCTs, limited body of data
- Nonrandomized trials and observational studies
- Panel consensus judgment
JJ, a 4-year-old boy, was taken to an urgent care clinic 3 times last winter for “recurrent bronchitis” and given a 7-day course of prednisone and antibiotics at each visit. His mother reports that “his colds always seem to go to his chest” and his skin is always dry. She was given a nebulizer and albuterol for use when JJ begins wheezing, which often happens when he has a cold, plays vigorously, or visits a friend who has cats.
JJ is one of approximately 6.7 million children—and 22.9 million US residents—who have asthma.1 To help guide the care of patients like JJ, the National Heart, Lung, and Blood Institute (NHLBI) and National Asthma Education and Prevention Program (NAEPP) released the third expert panel report (EPR-3) in 2007. Available at http://www.nhlbi.nih.gov/guidelines/asthma/asthgdln.htm, the EPR-3 provides the most comprehensive evidence-based guidance for the diagnosis and management of asthma to date.2
The guidelines were an invaluable resource for JJ’s family physician, who referred to them to categorize the severity of JJ’s asthma as “mild persistent.” In initiating treatment, JJ’s physician relied on specific recommendations for children 0 to 4 years of age to prescribe low-dose inhaled corticosteroids (ICS). Without the new guidelines, which underscore the safety of controller medication for young children, JJ’s physician would likely have been reluctant to place a 4-year-old on ICS.
This review highlights the EPR-3’s key recommendations to encourage widespread implementation by family physicians.
The EPR-3: What’s changed
The 2007 guidelines:
Recommend assessing asthma severity before starting treatment and assessing asthma control to guide adjustments in treatment.
Address both severity and control in terms of impairment and risk.
Feature 3 age breakdowns (0-4 years, 5-11 years, and ≥12 years) and a 6-step approach to asthma management.
Make it easier to individualize and adjust treatment.
What’s changed?
There’s a new paradigm
The 2007 update to guidelines released in 1997 and 2002 reflects a paradigm shift in the overall approach to asthma management. The change in focus addresses the highly variable nature of asthma2 and the recognition that asthma severity and asthma control are distinct concepts serving different functions in clinical practice.
Severity and control in 2 domains. Asthma severity—a measure of the intrinsic intensity of the disease process—is ideally assessed before initiating treatment. In contrast, asthma control is monitored over time to guide adjustments to therapy. The guidelines call for assessing severity and control within the domains of:
- impairment, based on asthma symptoms (identified by patient or caregiver recall of the past 2-4 weeks), quality of life, and functional limitations; and
- risk, of asthma exacerbations, progressive decline in pulmonary function (or reduced lung growth in children), or adverse events. Predictors of increased risk for exacerbations or death include persistent and/or severe airflow obstruction; at least 2 visits to the emergency department or hospitalizations for asthma within the past year; and a history of intubation or admission to intensive care, especially within the past 5 years.
The specific criteria for determining asthma severity and assessing asthma control are detailed in FIGURES 1 AND 2, respectively. Because treatment affects impairment and risk differently, this dual assessment helps ensure that therapeutic interventions minimize all manifestations of asthma as much as possible.
More steps and age-specific interventions. The EPR-3’s stepwise approach to asthma therapy has gone from 4 steps to 6, and the recommended treatments, as well as the levels of severity and criteria for assessing control that guide them, are now divided into 3 age groups: 0 to 4 years, 5 to 11 years, and ≥12 years (FIGURE 3). The previous guidelines, issued in 2002, divided treatment recommendations into 2 age groups: ≤5 years and >5 years. The EPR-3’s expansion makes it easier for physicians to initiate, individualize, and adjust treatment.
FIGURE 1
Classifying asthma severity and initiating therapy in children, adolescents, and adults
EIB, exercise-induced bronchospasm; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; ICS, inhaled corticosteroids; NA, not applicable; OCS, oral corticosteroids; SABA, short-acting β2-adrenergic agonist.
*Normal FEV1/FVC values are defined according to age: 8–9 years (85%), 20–39 years (80%), 40–59 years (75%), 60–80 years (70%).
†For treatment purposes, children with at least 2 exacerbations (eg, requiring urgent, unscheduled care; hospitalization; or intensive care unit admission) or adolescents/adults with at least 2 exacerbations requiring OCS in the past year may be considered the same as patients who have persistent asthma, even in the absence of impairment levels consistent with persistent asthma.
Adapted from: National Heart, Lung, and Blood Institute (NHLBI).2
FIGURE 2
Assessing asthma control and adjusting therapy
ACQ, Asthma Control Questionnaire; ACT, Asthma Control Test; ATAQ, Asthma Therapy Assessment Questionnaire; EIB, exercise-induced bronchospasm; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; N/A, not applicable; OCS, oral corticosteroids; SABA, short-acting β2-adrenergic agonist.
*ACQ values of 0.76 to 1.4 are indeterminate regarding well-controlled asthma.
†For treatment purposes, children with at least 2 exacerbations (eg, requiring urgent, unscheduled care; hospitalization; or intensive care unit admission) or adolescents/adults with at least 2 exacerbations requiring OCS in the past year may be considered the same as patients who have asthma that is not well controlled, even in the absence of impairment levels consistent with that classification.
Adapted from: National Heart, Lung, and Blood Institute (NHLBI).2
FIGURE 3
Stepwise approach for managing asthma
EIB, exercise-induced bronchospasm; ICS, inhaled corticosteroid; LABA, long-acting β2-adrenergic agonist; LTRA, leukotriene receptor antagonist; OCS, oral corticosteroid; PRN, as needed; SABA, short-acting β2-adrenergic agonist.
Adapted from: National Heart, Lung, and Blood Institute (NHLBI).2
Putting guidelines into practice begins with the history
A detailed medical history and a physical examination focusing on the upper respiratory tract, chest, and skin are needed to arrive at an asthma diagnosis. JJ’s physician asked his mother to describe recent symptoms and inquired about comorbid conditions that can aggravate asthma. He also identified viral respiratory infections, environmental causes, and activity as precipitating factors.
In considering an asthma diagnosis, try to determine the presence of episodic symptoms of airflow obstruction or bronchial hyperresponsiveness, as well as airflow obstruction that is at least partly reversible (an increase in forced expiratory volume in 1 second [FEV1] of >200 mL and ≥12% from baseline or an increase of ≥10% of predicted FEV1), and to exclude alternative diagnoses.
EPR-3 emphasizes spirometry
Recognizing that patients’ perception of airflow obstruction is highly variable and that pulmonary function measures do not always correlate directly with symptoms,3,4 the EPR-3 recommends spirometry for patients ≥5 years of age, both before and after bronchodilation. In addition to helping to confirm an asthma diagnosis, spirometry is the preferred measure of pulmonary function in classifying severity, because peak expiratory flow (PEF) testing has not proven reliable.5,6
Objective measurement of pulmonary function is difficult to obtain in children <5 years of age. If diagnosis remains uncertain for patients in this age group, a therapeutic trial of medication is recommended. In JJ’s case, however, 3 courses of oral corticosteroids (OCS) in less than 6 months were indicative of persistent asthma.
Spirometry is often underutilized. For patients ≥5 years of age, spirometry is a vital tool, but often underutilized in family practice. A recent study by Yawn and colleagues found that family physicians made changes in the management of approximately half of the asthma patients who underwent spirometry.7 (Information about spirometry training is available through the National Institute for Occupational Safety and Health at http://www.cdc.gov/niosh.) Referral to a specialist is recommended if the physician has difficulty making a differential diagnosis or is unable to perform spirometry on a patient who presents with atypical signs and symptoms of asthma.
What is the patient’s level of severity?
In patients who are not yet receiving long-term controller therapy, severity level is based on an assessment of impairment and risk (FIGURE 1). For patients who are already receiving treatment, severity is determined by the minimum pharmacologic therapy needed to maintain asthma control.
The severity classification—intermittent asthma or persistent asthma that is mild, moderate, or severe—is determined by the most severe category in which any feature occurs. (In children, FEV1/FVC [forced vital capacity] ratio has been shown to be a more sensitive determinant of severity than FEV1,4 which may be more useful in predicting exacerbations.8)
Asthma management: Preferred and alternative Tx
The recommended stepwise interventions include both preferred therapies (evidence-based) and alternative treatments (listed alphabetically in FIGURE 3 because there is insufficient evidence to rank them). The additional steps and age categories support the goal of using the least possible medication needed to maintain good control and minimize the potential for adverse events.
In initiating treatment, select the step that corresponds to the level of severity in the bottom row of FIGURE 1; to adjust medications, determine the patient’s level of asthma control and follow the corresponding guidance in the bottom row of FIGURE 2.
Inhaled corticosteroids remain the bedrock of therapy
ICS, the most potent and consistently effective long-term controller therapy, remain the foundation of therapy for patients of all ages who have persistent asthma. (Evidence: A).
Several of the age-based recommendations follow, with a focus on preferred treatments:
Children 0 to 4 years of age
- The guidelines recommend low-dose ICS at Step 2 (Evidence: A) and medium-dose ICS at Step 3 (Evidence: D), as inhaled corticosteroids have been shown to reduce impairment and risk in this age group.9-16 The potential risk is generally limited to a small reduction in growth velocity during the first year of treatment, and offset by the benefits of therapy.15,16
- Add a long-acting β2-adrenergic agonist (LABA) or montelukast to medium-dose ICS therapy at Step 4 rather than increasing the ICS dose (Evidence: D) to avoid the risk of side effects associated with high-dose ICS. Montelukast has demonstrated efficacy in children 2 to 5 years of age with persistent asthma.17
- Recommendations for preferred therapy at Steps 5 (high-dose ICS + LABA or montelukast) and 6 (Step 5 therapy + OCS) are based on expert panel judgment (Evidence: D). When severe persistent asthma warrants Step 6 therapy, start with a 2-week course of the lowest possible dose of OCS to confirm reversibility.
- In this age group, a therapeutic trial with close monitoring is recommended for patients whose asthma is not well controlled. If there is no response in 4 to 6 weeks, consider alternative therapies or diagnoses (Evidence: D).
Children 5 to 11 years of age
- For Step 3 therapy, the guidelines recommend either low-dose ICS plus a LABA, leukotriene receptor antagonist (LTRA), or theophylline; or medium-dose ICS (Evidence: B). Treatment decisions at Step 3 depend on whether impairment or risk is the chief concern, as well as on safety considerations.
- For Steps 4 and 5, ICS (medium dose for Step 5 and high dose for Step 6) plus a LABA is preferred, based on studies of patients ≥12 years of age (Evidence: B). Step 6 builds on Step 5, adding an OCS to the LABA/ICS combination (Evidence: D).
- If theophylline is prescribed—a viable option if cost and adherence to inhaled medications are key concerns—serum levels must be closely monitored because of the risk of toxicity.
- Closely monitor and be prepared to identify and respond to anaphylaxis in a child at Step 2, 3, or 4 who is receiving allergen immunotherapy.
Adolescents ≥12 years of age and adults
- There are 2 preferred Step 3 treatments: Low-dose ICS plus a LABA, or medium-dose ICS. The combination therapy has shown greater improvement in impairment24,25 and risk24-26 compared with the higher dose of ICS.
- Preferred treatments at Steps 4, 5, and 6 are the same as those for children ages 5 to 11 years, with one exception: Subcutaneous anti-IgE therapy (omalizumab) may be added to the regimen at Steps 5 and 6 for adolescents and adults with severe persistent allergic asthma to reduce the risk of exacerbations.27
Weigh the benefits and risks of therapy
Safety is a key consideration for all asthma patients. Carefully weigh the benefits and risks of therapy, including the rare but potential risk of life-threatening or fatal exacerbations with daily LABA therapy28 and systemic effects with higher doses of ICS.23 Patients who begin receiving oral corticosteroids require close monitoring, regardless of age.
Regular reassessment and monitoring are critical
Schedule visits at 2- to 6-week intervals for those who are starting therapy or require a step up to achieve or regain asthma control. After control is achieved, reassess at least every 1 to 6 months. Measures of asthma control are the same as those used to assess severity, with the addition of validated multidimensional questionnaires (eg, Asthma Control Test [ACT])29 to gauge impairment.
JJ’s physician scheduled a follow-up visit in 4 weeks, at which time he did a reassessment based on a physical exam and symptom recall. Finding JJ’s asthma to be well controlled, the physician asked the boy’s mother to bring him back to the office in 2 months, or earlier if symptoms recurred.
TABLE W1
Asthma education resources
| Allergy & Asthma Network Mothers of Asthmatics 2751 Prosperity Avenue, Suite 150 Fairfax, VA 22030 www.breatherville.org (800) 878-4403 or (703) 641-9595 | Asthma and Allergy Foundation of America 1233 20th Street, NW, Suite 402 Washington, DC 20036 www.aafa.org (800) 727-8462 |
| American Academy of Allergy, Asthma, and Immunology 555 East Wells Street, Suite 1100 Milwaukee, WI 53202-3823 www.aaaai.org (414) 272-6071 | Centers for Disease Control and Prevention 1600 Clifton Road Atlanta, GA 30333 www.cdc.gov (800) 311-3435 |
| American Association for Respiratory Care 9125 North macArthur boulevard, Suite 100 Irving, TX 75063 www.aarc.org (972) 243-2272 | Food Allergy & Anaphylaxis Network 11781 lee Jackson Highway, Suite 160 Fairfax, VA 22033 www.foodallergy.org (800) 929-4040 |
| American College of Allergy, Asthma, and Immunology 85 West Algonquin road, Suite 550 Arlington Heights, IL 60005 www.acaai.org (800) 842-7777 or (847) 427-1200 | National Heart, Lung, and Blood Institute Information Center P.O. Box 30105 Bethesda, MD 20824-0105 www.nhlbi.nih.gov (301) 592-8573 |
| American Lung Association 61 Broadway New York, NY 10006 www.lungusa.org (800) 586-4872 | National Jewish Medical and Research Center (Lung Line) 1400 Jackson Street Denver, CO 80206 www.njc.org (800) 222-lUNG |
| Association of Asthma Educators 1215 Anthony Avenue Columbia, SC 29201 www.asthmaeducators.org (888) 988-7747 | US Environmental Protection Agency National Center for Environmental Publications P.O. Box 42419 Cincinnati, OH 45242-0419 www.airnow.gov (800) 490-9198 |
Does your patient require a step down or step up?
A step down is recommended for patients whose asthma is well controlled for 3 months or more. Reduce the dose of ICS gradually, about 25% to 50% every 3 months, because deterioration in asthma control is highly variable. Review adherence and medication administration technique with patients whose asthma is not well controlled, and consider a step up in treatment. If an alternative treatment is used but does not result in an adequate response, it should be discontinued and the preferred treatment used before stepping up. Refer patients to an asthma specialist if their asthma does not respond to these adjustments.
Partner with patients for optimal care
The EPR-3 recommends the integration of patient education into all aspects of asthma care. To forge an active partnership, identify and address concerns about the condition and its treatment and involve the patient and family in developing treatment goals and making treatment decisions. If the patient is old enough, encourage self-monitoring and management.
The EPR-3 recommends that physicians give every patient a written asthma action plan that addresses individual symptoms and/or PEF measurements and includes instructions for self-management. Daily PEF monitoring can be useful in identifying early changes in the disease state and evaluating response to changes in therapy. It is ideal for those who have moderate to severe persistent asthma, difficulty recognizing signs of exacerbations, or a history of severe exacerbations.
Correspondence
Stuart W. Stoloff, MD, Clinical Professor, Department of Family and Community Medicine, University of Nevada–Reno, 1200 Mountain Street, Suite 220, Carson City, NV 89703; [email protected].
1. National Center for Health Statistics. Fast stats A to Z. Available at: www.cdc.gov/nchs/fastats/asthma.htm. Accessed August 1, 2008.
2. National Heart, Lung, and Blood Institute (NHLBI). National Asthma Education and Prevention Program Expert Panel Report 3: Guidelines for the Diagnosis and Management of Asthma. Full Report 2007. Bethesda, MD: NHLBI; August 2007. NIH publication no. 07-4051. Available at: http://www.nhlbi.nih.gov/guidelines/asthma/asthgdln.htm. Accessed July 17, 2008.
3. Stout JW, Visness CM, Enright P, et al. Classification of asthma severity in children: the contribution of pulmonary function testing. Arch Pediatr Adolesc Med. 2006;160:844-850.
4. Bacharier LB, Strunk RC, Mauger D, et al. Classifying asthma severity in children: mismatch between symptoms, medication use, and lung function. Am J Respir Crit Care Med. 2004;170:426-432.
5. Eid N, Yandell B, Howell L, Eddy M, Sheikh S. Can children with asthma? Pediatrics. 2000;105:354-358.
6. Llewellin P, Sawyer G, Lewis S, et al. The relationship between FEV1 and PEF in the assessment of the severity of airways obstruction. Respirology. 2002;7:333-337.
7. Yawn BP, Enright PL, Lemanske RF, Jr, et al. Spirometry can be done in family physicians’ offices and alters clinical decisions in management of asthma and COPD. Chest. 2007;132:1162-1168.
8. Fuhlbrigge AL, Kitch BT, Paltiel AD, et al. FEV1 is associated with risk of asthma attacks in a pediatric population. J Allergy Clin Immunol. 2001;107:61-67.
9. Roorda RJ, Mezei G, Bisgaard H, Maden C. Response of preschool children with asthma symptoms to fluticasone propionate. J Allergy Clin Immunol. 2001;108:540-546.
10. Baker JW, Mellon M, Wald J, Welch M, Cruz-Rivera M, Walton-Bowen K. A multiple-dosing, placebo-controlled study of budesonide inhalation suspension given once or twice daily for treatment of persistent asthma in young children and infants. Pediatrics. 1999;103:414-421.
11. Kemp JP, Skoner DP, Szefler SJ, Walton-Bowen K, Cruz-Rivera M, Smith JA. Once-daily budesonide inhalation suspension for the treatment of persistent asthma in infants and young children. Ann Allergy Asthma Immunol. 1999;83:231-239.
12. Shapiro G, Mendelson L, Kraemer MJ, Cruz-Rivera M, Walton-Bowen K, Smith JA. Efficacy and safety of budesonide inhalation suspension (Pulmicort Respules) in young children with inhaled steroid-dependent, persistent asthma. J Allergy Clin Immunol. 1998;102:789-796.
13. Bisgaard H, Gillies J, Groenewald M, Maden C. The effect of inhaled fluticasone propionate in the treatment of young asthmatic children: a dose comparison study. Am J Respir Crit Care Med. 1999;160:126-131.
14. Szefler SJ, Eigen H. Budesonide inhalation suspension: a nebulized corticosteroid for persistent asthma. J Allergy Clin Immunol. 2002;109:730-742.
15. Guilbert TW, Morgan WJ, Zeiger RS, et al. Long-term inhaled corticosteroids in preschool children at high risk for asthma. N Engl J Med. 2006;354:1985-1997.
16. Bisgaard H, Allen D, Milanowski J, Kalev I, Willits L, Davies P. Twelve-month safety and efficacy of inhaled fluticasone propionate in children aged 1 to 3 years with recurrent wheezing. Pediatrics. 2004;113:e87-e94.
17. Knorr B, Franchi LM, Bisgaard H, et al. Montelukast, a leukotriene receptor antagonist, for the treatment of persistent asthma in children aged 2 to 5 years. Pediatrics 2001;108:e48.-
18. Russell G, Williams DA, Weller P, Price JF. Salmeterol xinafoate in children on high dose inhaled steroids. Ann Allergy Asthma Immunol. 1995;75:423-428.
19. Zimmerman B, D’Urzo A, Bérubé D. Efficacy and safety of formoterol Turbuhaler when added to inhaled corticosteroid treatment in children with asthma. Pediatr Pulmonol. 2004;37:122-127.
20. Simons FE, Villa JR, Lee BW, et al. Montelukast added to budesonide in children with persistent asthma: a randomized, double-blind, crossover study. J Pediatr. 2001;138:694-698.
21. Shapiro G, Bronsky EA, LaForce CF, et al. Dose-related efficacy of budesonide administered via a dry powder inhaler in the treatment of children with moderate to severe persistent asthma. J Pediatr. 1998;132:976-982.
22. Pauwels RA, Lofdahl C-G, Postma DS, et al. for the Formoterol and Corticosteroids Establishing Therapy (FACET) International Study Group. Effect of inhaled formoterol and budesonide on exacerbations of asthma. N Engl J Med. 1997;337:1405-1411.
23. Tattersfield AE, Harrison TW, Hubbard RB, Mortimer K. Safety of inhaled corticosteroids. Proc Am Thorac Soc. 2004;1:171-175.
24. Bateman ED, Boushey HA, Bousquet J, et al. For the GOAL Investigators Group. Can guideline-defined asthma control be achieved? The Gaining Optimal Asthma ControL study. Am J Respir Crit Care Med. 2004;170:836-844.
25. O’Byrne PM, Barnes PJ, Rodriguez-Roisin R, et al. Low dose inhaled budesonide and formoterol in mild persistent asthma: the OPTIMA randomized trial. Am J Respir Crit Care Med. 2001;164:1392-1397.
26. Masoli M, Weatherall M, Holt S, Beasley R. Moderate dose inhaled corticosteroids plus salmeterol versus higher doses of inhaled corticosteroids in symptomatic asthma. Thorax. 2005;60:730-734.
27. Bousquet J, Wenzel S, Holgate S, Lumry W, Freeman P, Fox H. Predicting response to omalizumab, an anti-IgE antibody, in patients with allergic asthma. Chest. 2004;125:1378-1386.
28. Nelson HS, Weiss ST, Bleecker ER, Yancey SW, Dorinsky PM. For the SMART Study Group. The Salmeterol Multicenter Asthma Research Trial: a comparison of usual pharmacotherapy for asthma or usual pharmacotherapy plus salmeterol. Chest. 2006;129:15-26.
29. Nathan RA, Sorkness CA, Kosinski M, et al. Development of the asthma control test: a survey for assessing asthma control. J Allergy Clin Immunol. 2004;113:59-65.
1. National Center for Health Statistics. Fast stats A to Z. Available at: www.cdc.gov/nchs/fastats/asthma.htm. Accessed August 1, 2008.
2. National Heart, Lung, and Blood Institute (NHLBI). National Asthma Education and Prevention Program Expert Panel Report 3: Guidelines for the Diagnosis and Management of Asthma. Full Report 2007. Bethesda, MD: NHLBI; August 2007. NIH publication no. 07-4051. Available at: http://www.nhlbi.nih.gov/guidelines/asthma/asthgdln.htm. Accessed July 17, 2008.
3. Stout JW, Visness CM, Enright P, et al. Classification of asthma severity in children: the contribution of pulmonary function testing. Arch Pediatr Adolesc Med. 2006;160:844-850.
4. Bacharier LB, Strunk RC, Mauger D, et al. Classifying asthma severity in children: mismatch between symptoms, medication use, and lung function. Am J Respir Crit Care Med. 2004;170:426-432.
5. Eid N, Yandell B, Howell L, Eddy M, Sheikh S. Can children with asthma? Pediatrics. 2000;105:354-358.
6. Llewellin P, Sawyer G, Lewis S, et al. The relationship between FEV1 and PEF in the assessment of the severity of airways obstruction. Respirology. 2002;7:333-337.
7. Yawn BP, Enright PL, Lemanske RF, Jr, et al. Spirometry can be done in family physicians’ offices and alters clinical decisions in management of asthma and COPD. Chest. 2007;132:1162-1168.
8. Fuhlbrigge AL, Kitch BT, Paltiel AD, et al. FEV1 is associated with risk of asthma attacks in a pediatric population. J Allergy Clin Immunol. 2001;107:61-67.
9. Roorda RJ, Mezei G, Bisgaard H, Maden C. Response of preschool children with asthma symptoms to fluticasone propionate. J Allergy Clin Immunol. 2001;108:540-546.
10. Baker JW, Mellon M, Wald J, Welch M, Cruz-Rivera M, Walton-Bowen K. A multiple-dosing, placebo-controlled study of budesonide inhalation suspension given once or twice daily for treatment of persistent asthma in young children and infants. Pediatrics. 1999;103:414-421.
11. Kemp JP, Skoner DP, Szefler SJ, Walton-Bowen K, Cruz-Rivera M, Smith JA. Once-daily budesonide inhalation suspension for the treatment of persistent asthma in infants and young children. Ann Allergy Asthma Immunol. 1999;83:231-239.
12. Shapiro G, Mendelson L, Kraemer MJ, Cruz-Rivera M, Walton-Bowen K, Smith JA. Efficacy and safety of budesonide inhalation suspension (Pulmicort Respules) in young children with inhaled steroid-dependent, persistent asthma. J Allergy Clin Immunol. 1998;102:789-796.
13. Bisgaard H, Gillies J, Groenewald M, Maden C. The effect of inhaled fluticasone propionate in the treatment of young asthmatic children: a dose comparison study. Am J Respir Crit Care Med. 1999;160:126-131.
14. Szefler SJ, Eigen H. Budesonide inhalation suspension: a nebulized corticosteroid for persistent asthma. J Allergy Clin Immunol. 2002;109:730-742.
15. Guilbert TW, Morgan WJ, Zeiger RS, et al. Long-term inhaled corticosteroids in preschool children at high risk for asthma. N Engl J Med. 2006;354:1985-1997.
16. Bisgaard H, Allen D, Milanowski J, Kalev I, Willits L, Davies P. Twelve-month safety and efficacy of inhaled fluticasone propionate in children aged 1 to 3 years with recurrent wheezing. Pediatrics. 2004;113:e87-e94.
17. Knorr B, Franchi LM, Bisgaard H, et al. Montelukast, a leukotriene receptor antagonist, for the treatment of persistent asthma in children aged 2 to 5 years. Pediatrics 2001;108:e48.-
18. Russell G, Williams DA, Weller P, Price JF. Salmeterol xinafoate in children on high dose inhaled steroids. Ann Allergy Asthma Immunol. 1995;75:423-428.
19. Zimmerman B, D’Urzo A, Bérubé D. Efficacy and safety of formoterol Turbuhaler when added to inhaled corticosteroid treatment in children with asthma. Pediatr Pulmonol. 2004;37:122-127.
20. Simons FE, Villa JR, Lee BW, et al. Montelukast added to budesonide in children with persistent asthma: a randomized, double-blind, crossover study. J Pediatr. 2001;138:694-698.
21. Shapiro G, Bronsky EA, LaForce CF, et al. Dose-related efficacy of budesonide administered via a dry powder inhaler in the treatment of children with moderate to severe persistent asthma. J Pediatr. 1998;132:976-982.
22. Pauwels RA, Lofdahl C-G, Postma DS, et al. for the Formoterol and Corticosteroids Establishing Therapy (FACET) International Study Group. Effect of inhaled formoterol and budesonide on exacerbations of asthma. N Engl J Med. 1997;337:1405-1411.
23. Tattersfield AE, Harrison TW, Hubbard RB, Mortimer K. Safety of inhaled corticosteroids. Proc Am Thorac Soc. 2004;1:171-175.
24. Bateman ED, Boushey HA, Bousquet J, et al. For the GOAL Investigators Group. Can guideline-defined asthma control be achieved? The Gaining Optimal Asthma ControL study. Am J Respir Crit Care Med. 2004;170:836-844.
25. O’Byrne PM, Barnes PJ, Rodriguez-Roisin R, et al. Low dose inhaled budesonide and formoterol in mild persistent asthma: the OPTIMA randomized trial. Am J Respir Crit Care Med. 2001;164:1392-1397.
26. Masoli M, Weatherall M, Holt S, Beasley R. Moderate dose inhaled corticosteroids plus salmeterol versus higher doses of inhaled corticosteroids in symptomatic asthma. Thorax. 2005;60:730-734.
27. Bousquet J, Wenzel S, Holgate S, Lumry W, Freeman P, Fox H. Predicting response to omalizumab, an anti-IgE antibody, in patients with allergic asthma. Chest. 2004;125:1378-1386.
28. Nelson HS, Weiss ST, Bleecker ER, Yancey SW, Dorinsky PM. For the SMART Study Group. The Salmeterol Multicenter Asthma Research Trial: a comparison of usual pharmacotherapy for asthma or usual pharmacotherapy plus salmeterol. Chest. 2006;129:15-26.
29. Nathan RA, Sorkness CA, Kosinski M, et al. Development of the asthma control test: a survey for assessing asthma control. J Allergy Clin Immunol. 2004;113:59-65.
CA-MRSA lesions: What works, what doesn’t
- Community-acquired methicillin-resistant Staphylococcus aureus (CA-MRSA) abscesses are best managed surgically; postprocedure antibiotics do not substantially improve outcomes. Cure rates with incision and drainage alone are at least 90% (A).
- If incision and drainage fail to promote healing within 7 days, the oral antibiotics of choice are trimethoprim-sulfamethoxazole and tetracycline (C).
- Eradication of nasal carriage of CA-MRSA is generally not useful in preventing spread of clinical MRSA infections in communities (B).
Strength of recommendation (SOR)
- Good quality patient-oriented evidence
- Inconsistent or limited-quality patient-oriented evidence
- Consensus, usual practice, opinion, disease-oriented evidence, case series
A previously healthy law student arrives at your office complaining of “abdominal pain.” You discover on examination that she has an erythematous, indurated, and tender 3-cm lesion on her suprapubic region. The lesion has no point, but its center is boggy. The patient’s temperature is normal. Would you give her an antibiotic? Would you cover immediately for community-acquired methicillin-resistant Staphylococcus aureus (CA-MRSA)? What other factors might influence your decision?
The incidence of MRSA is increasing in communities across the United States, challenging our assumptions about evaluation and management of skin and soft-tissue infections. In this article, I outline a rational approach to managing patients who have lesions likely to have been caused by CA-MRSA (TABLE).
TABLE
Suspect CA-MRSA? Consider this treatment approach6,7
| CLASS | PATIENT CRITERIA | MANAGEMENT | ANTIBIOTIC CHOICES |
|---|---|---|---|
| 1 | Afebrile and healthy; lesion nonfluctuant | If no drainable abscess, give common first-line antibiotic for SSTI; reassess for response | Semisynthetic penicillin, oral first- or second-generation cephalosporin, macrolide, clindamycin |
| 2 | Fluctuant or pustular lesion <5 cm; with or without fever | Surgical drainage of abscess if possible. Use I&D presumptively for MRSA and monitor closely for response; inpatient management may be indicated | Trimethoprim-sulfamethoxazole, tetracycline, clindamycin |
| 3 | Toxic appearance or at least 1 unstable comorbidity or a limb-threatening infection; lesion >5 cm | Hospital admission with broadspectrum antibiotics for MRSA coverage; consider infectious disease consultation | Broad-spectrum, including vancomycin |
| 4 | Sepsis syndrome or life-threatening infection (necrotizing fasciitis) | Above plus aggressive surgical debridement | Above with infectious disease guidance |
| CA-MRSA, community-acquired methicillin-resistant Staphylococcus aureus; I&D, incision and drainage; MRSA, methicillin-resistant Staphylococcus aureus; SSTI, skin and soft-tissue infection. | |||
When to suspect MRSA skin infection
Patients with CA-MRSA skin infection often report a “spider bite,” as lesions appear suddenly and unexpectedly in areas without a history of trauma.1 The lesions very often are pustular with central necrosis, and there may be purulent drainage, redness, tenderness, and palpable fluctuance. CA-MRSA can cause impetigo, but the often benign nature of this clinical infection makes management decisions less crucial. CA-MRSA skin lesions can occur anywhere on the body, though most often they appear in the axillae or the groin and buttocks. Patients may or may not have a fever.
Individuals who are at increased risk for CA-MRSA disease include users of health clubs or participants in contact sports, men who have sex with men, children younger than 2 years of age, users of intravenous drugs, military personnel, and prisoners.2,3 However, the absence of these factors in a patient with a skin or soft-tissue infection does not rule out MRSA.4
Regardless of the lesion’s appearance or the patient’s epidemiologic history, consider CA-MRSA if its prevalence in your community reaches 10% to 15%.
- Community-acquired methicillin-resistant Staphylococcus aureus (CA-MRSA) causes up to 74% of purulent skin and soft-tissue infections in communities throughout the United States.1 By definition, this infection occurs in patients who have not been hospitalized and have not undergone medical procedures within the prior year.12
- The annual incidence of CA-MRSA was reported to be 18.0-25.7 cases per 100,000 population between 2001 and 2002.13 Clusters of CA-MRSA have been identified among Alaskan natives, Native American Indians, and Pacific Islanders.12
- Most often this organism causes skin and soft-tissue infections, though cardiac, respiratory, blood, and bone infections can also occur.14
- CA-MRSA species are genotypically distinct from hospital-acquired MRSA. One marker for CA-MRSA, Panton-Valentine leucocidin (PVL), is most often detected in cases of severe and systemic infection, and it may be a virulence factor.15 However, the presence of PVL does not necessarily correlate directly with antibiotic resistance.
- Historically, CA-MRSA was primarily resistant to beta-lactams and erythromycin. More recent strains have also demonstrated resistance to tetracycline and clindamycin.
- Retrospective analyses show that patients with CA-MRSA tend to receive inadequate initial antibiotic coverage, and, independent of this, they tend to have worse clinical outcomes than those infected by methicillin-sensitive strains.16
Hospitalize any patient who exhibits fever or hypothermia, tachycardia greater than 100 beats per minute, or hypotension with a systolic blood pressure <90 mm Hg or 20 mm Hg lower than baseline. A skin lesion >5 cm is also likely to require hospitalization and parenteral antibiotics.5
Treatment: Incision and drainage most important
Several management schemes have been proposed to guide the appropriate level of therapy based on presenting patient characteristics.6,7 If a lesion is clearly fluctuant, incise it and drain the fluid, or refer the patient for surgical consultation. If the lesion is not clearly fluctuant, needle aspiration may help to determine the need for more extensive incision and drainage or to collect a specimen for culture. Although culture of skin lesions may not have been routine in the past, the advent of CA-MRSA has made it so, particularly given that MRSA lesions may not be clinically distinguishable from those caused by nonresistant S aureus.
Periodic postprocedure follow-up is indicated to ensure resolution of the infection. At our health center, patients return every few days for an appointment with nursing staff for wound irrigation and packing change until the lesion visibly improves. Systemic effects from the infection are monitored, as well.
Adult patients in 1 study were treated with incision and drainage by a surgeon.8 The technique described in the article used an 11 blade and a “sawing motion,” creating a wide opening. The wound cavity was explored for loculations and packed. This technique is identical to that used in the office. There is one caveat, though: This study included abscesses larger than 5 cm and patients with compromised immune systems—situations not routinely managed in the primary care office.
Are antibiotics indicated after incision and drainage for MRSA? In this same study, cure rates with incision and drainage alone were just over 90%.8 The cure rate in the treatment arm also receiving an antibiotic was 84% (difference was not statistically significant), and coverage was inadequate for MRSA. Treatment with cephalexin after incision and drainage resulted in 1 patient harmed for every 14 treated (NNH=14). A pediatric study also showed that antibiotics do not affect the outcome of skin lesions following incision and drainage.5 When deciding whether to prescribe postprocedure antibiotics, keep in mind the need to avoid contributing further to bacterial resistance.
Generally if incision and drainage fail to promote healing of the MRSA lesion within 7 days, start the patient on trimethoprim-sulfamethoxazole or tetracycline. Clindamycin is an option, though resistance to it is becoming more common. Adjust the antibiotic choice as needed when culture and sensitivity results become available.
Trimethoprim-sulfamethoxazole is generally well tolerated at the recommended dose of 1 to 2 double-strength tablets (160 mg TMP, 800 mg SMX) twice daily for adults. If a patient’s creatinine clearance is 15 to 30 mL/min, reduce the dose by half. The rate of sulfa allergy is similar to other antibiotics, at 3%.
Tetracycline’s dosing schedule—for adults, 250 or 500 mg 4 times daily—makes it difficult to use. Gastrointestinal upset, phototoxicity, and hepatotoxicity can occur. The possibility of tooth discoloration precludes tetracycline’s use in children.
Clindamycin carries a high rate of gastrointestinal-related problems, Clostridium difficile infection in particular (10% incidence administered in any route). Inducible resistance to clindamycin is 50% in MRSA infections.9 Recent use of antibiotics may increase the likelihood of clindamycin resistance, with erythromycin in particular inducing this resistance. Its dosage typically is 150 to 300 mg every 6 hours.
Doxycycline and minocycline are not recommended, as they carry a 21% failure rate.9
Linezolid is costly and has many drug interactions. In particular, linezolid has the potential to cause serotonin syndrome with agents that affect the serotonergic system. Linezolid may also interact with medications that affect the adrenergic system (pressors). Its routine use in the community without infectious disease consultation is not advised.
For lesions that are not fluctuant or purulent, appropriate first-line antibiotics are semisynthetic penicillins (dicloxacillin), first- or second-generation oral cephalosporins, macrolides, and clindamycin.9 These antibiotics are preferable for group A streptococcal infections, erysipelas (which can be quite aggressive), and impetigo. Adjustments can be made as culture results become available or if the clinical response is inadequate. There is no particular utility in waiting to administer oral antibiotics in cases of erysipelas or impetigo, though topical antibiotics can often be used for limited cases of impetigo.
Prevention: Simple precautions are the rule
Most CA-MRSA infections result from direct contact with a patient’s wound or from wound drainage on environmental surfaces.
In the medical office. In addition to using sterile technique during incision and drainage, be sure that all staff members wash their hands with soap and water or with an alcohol-based sanitizer. For the most part, MRSA remains susceptible to triclosan, a topical antiseptic in commercially available hand soaps.
Clean equipment as needed with 10% sodium hypochlorite solution or another agent effective against MRSA. Surgical instruments should be disposable or sterilized after each use.
At the patient’s home. Instruct patients to clean wounds wearing fresh disposable gloves each time and to cover wounds with new, dry dressings. Tell families to avoid sharing linens and clothing unless they have been washed in hot soap and water and dried in a heated dryer. MRSA can live for weeks to months on surfaces exposed to infected wounds,10 and these surfaces can be disinfected with a 10% bleach solution.
Sports environments. Athletes with CA-MRSA infections should not compete unless the wound can be completely covered with a dry dressing. Recommend to those in charge of school or commercial facilities that, in cases of confirmed MRSA infection, they routinely clean locker rooms and sports equipment with either a 10% bleach solution or commercial disinfectant. There is no evidence, however, that more widespread or vigorous cleaning—such as dismantling a training room and all its cardio-fitness equipment for disinfecting—prevents the spread of MRSA.
Encourage athletes to wash their hands properly. Communal towels should be washed in hot water (>140°F) with bleach before reuse. Personal equipment should be cleaned per the instructions of the manufacturer. Athletes should use a clean towel to provide a barrier between their skin and the surfaces of weight-room or cardio-fitness equipment. They should also clean equipment before and after use with an appropriate cleanser, such as a disinfectant hand-wipe.
Unproductive efforts you can avoid. Screening household contacts for MRSA is not useful, and attempts to eliminate colonization are generally ineffective. In a large military study, use of intranasal mupirocin failed to decrease nasal carriage of MRSA and the incidence of MRSA infections.11 The MRSA nasal colonization rate was 3.9%; 121 individuals with MRSA colonization needed to be treated with nasal mupirocin to prevent 1 MRSA infection in the total study population.
More complex antibiotic regimens are sometimes used in an attempt to eradicate MRSA carriage, though they also have limited effectiveness and carry the general risks of antibiotic use (gastrointestinal disturbance, allergic reaction, etc). If your office is considering an eradication attempt, consult with an infectious disease clinician first.
1. Moran GJ, Krishnadasan A, Gorwitz RJ, et al. Methicillin-resistant S aureus infections among patients in the emergency department. N Engl J Med. 2006;355:666-674.
2. Cohen PR. The skin in the gym: a comprehensive review of the cutaneous manifestations of community-acquired methicillin-resistant Staphylococcus aureus infection in athletes. Clin Dermatol. 2008;26:16-26.
3. Cohen PR. Community-acquired methicillin-resistant Staphylococcus aureus skin infections: implications for patients and practitioners. Am J Clin Dermatol. 2007;8:259-270.
4. Miller LG, Perdreau-Remington F, Bayer AS, et al. Clinical and epidemiologic characteristics cannot distinguish community associated methicillin resistant Staph aureus infection from methicillin susceptible S aureus infection: a prospective investigation. Clin Infect Dis. 2007;44:471-482.
5. Lee MC, Rios AM, Aten MF, et al. Management and outcome of children with skin and soft tissue abscesses caused by community-acquired methicillin-resistant Staphylococcus aureus. Pediatr Infect Dis J. 2004;23:123-127.
6. Eron LJ, Lipsky BA, Low DE, et al. Managing skin and soft tissue infections: expert panel recommendations on key decision points. J Antimicrob Chemother. 2003;52(suppl S1):i3-i17.
7. CDC, AMA, IDSA, Outpatient management of skin and soft tissue infections in the era of community-associated MRSA. Available at: http://www.ama-assn.org/ama1/pub/upload/mm/36/ca_mrsa_desk_102007.pdf. Accessed June 8, 2008.
8. Rajendran PM, Young D, Maurer T, et al. Randomized, double-blind, placebo-controlled trial of cephalexin for treatment of uncomplicated skin abscesses in a population at risk for community-acquired methicillin-resistant Staphylococcus aureus infection. Antimicrob Agents Chemother. 2007;51:4044-4048.
9. Stevens DL, Bisno AL, Chambers HF, et al. Practice guidelines for the diagnosis and management of skin and soft-tissue infections. Clin Infect Dis. 2005;41:1373-1406.
10. Dellit TH, Ducin J. Guidelines for Evaluation and Management of Community-Associated Methicillin Resistant Staphylococcus aureus Skin and Soft Tissue Infections in Outpatient Settings. Available at: http://www.metrokc.gov/health/providers/epidemiology/MRSA-guidelines.pdf. Accessed August 6, 2008.
11. Ellis MW, Griffith ME, Dooley DP, et al. Targeted intranasal mupirocin to prevent colonization and infection by community-associated methicillin-resistant Staphylococcus aureus strains in soldiers: a cluster randomized controlled trial. Antimicrob Agents Chemother. 2007;51:3591-3598.
12. Centers for Disease Control and Prevention. Healthcare-associated methicillin resistant Staphylococcus aureus (MRSA). Available at: http://www.cdc.gov/ncidod/dhqp/ar_mrsa.html. Accessed April 14, 2008.
13. Fridkin SK, Hageman JC, Morrison M, et al. Methicillin-reistant Staphylococcus aureus disease in three communities. N Engl J Med. 2005;352:2362-a.
14. Active Bacterial Core Surveillance (ABCs) Report, Emerging Infections Program Network methicillin-resistant Staphylococcus aureus, 2006. Available at: http://www.cdc.gov/ncidod/dbmd/abcs/survreports/mrsa06.pdf. Accessed May 5, 2008.
15. Holmes A, Ganner M, McGuane S, et al. Staphylococcus aureus isolates carrying panton-valentine leucocidin genes in England and Wales: frequency, characterization, and association with clinical disease. J Clin Microbiol. 2005;43:2384-2390.
16. Davis SL, Perri MB, Donabedian SM, et al. Epidemiology and outcomes of community-associated methicillin-resistant Staphylococcus aureus infection. J Clin Microbiol. 2007;45:1705-1711.
- Community-acquired methicillin-resistant Staphylococcus aureus (CA-MRSA) abscesses are best managed surgically; postprocedure antibiotics do not substantially improve outcomes. Cure rates with incision and drainage alone are at least 90% (A).
- If incision and drainage fail to promote healing within 7 days, the oral antibiotics of choice are trimethoprim-sulfamethoxazole and tetracycline (C).
- Eradication of nasal carriage of CA-MRSA is generally not useful in preventing spread of clinical MRSA infections in communities (B).
Strength of recommendation (SOR)
- Good quality patient-oriented evidence
- Inconsistent or limited-quality patient-oriented evidence
- Consensus, usual practice, opinion, disease-oriented evidence, case series
A previously healthy law student arrives at your office complaining of “abdominal pain.” You discover on examination that she has an erythematous, indurated, and tender 3-cm lesion on her suprapubic region. The lesion has no point, but its center is boggy. The patient’s temperature is normal. Would you give her an antibiotic? Would you cover immediately for community-acquired methicillin-resistant Staphylococcus aureus (CA-MRSA)? What other factors might influence your decision?
The incidence of MRSA is increasing in communities across the United States, challenging our assumptions about evaluation and management of skin and soft-tissue infections. In this article, I outline a rational approach to managing patients who have lesions likely to have been caused by CA-MRSA (TABLE).
TABLE
Suspect CA-MRSA? Consider this treatment approach6,7
| CLASS | PATIENT CRITERIA | MANAGEMENT | ANTIBIOTIC CHOICES |
|---|---|---|---|
| 1 | Afebrile and healthy; lesion nonfluctuant | If no drainable abscess, give common first-line antibiotic for SSTI; reassess for response | Semisynthetic penicillin, oral first- or second-generation cephalosporin, macrolide, clindamycin |
| 2 | Fluctuant or pustular lesion <5 cm; with or without fever | Surgical drainage of abscess if possible. Use I&D presumptively for MRSA and monitor closely for response; inpatient management may be indicated | Trimethoprim-sulfamethoxazole, tetracycline, clindamycin |
| 3 | Toxic appearance or at least 1 unstable comorbidity or a limb-threatening infection; lesion >5 cm | Hospital admission with broadspectrum antibiotics for MRSA coverage; consider infectious disease consultation | Broad-spectrum, including vancomycin |
| 4 | Sepsis syndrome or life-threatening infection (necrotizing fasciitis) | Above plus aggressive surgical debridement | Above with infectious disease guidance |
| CA-MRSA, community-acquired methicillin-resistant Staphylococcus aureus; I&D, incision and drainage; MRSA, methicillin-resistant Staphylococcus aureus; SSTI, skin and soft-tissue infection. | |||
When to suspect MRSA skin infection
Patients with CA-MRSA skin infection often report a “spider bite,” as lesions appear suddenly and unexpectedly in areas without a history of trauma.1 The lesions very often are pustular with central necrosis, and there may be purulent drainage, redness, tenderness, and palpable fluctuance. CA-MRSA can cause impetigo, but the often benign nature of this clinical infection makes management decisions less crucial. CA-MRSA skin lesions can occur anywhere on the body, though most often they appear in the axillae or the groin and buttocks. Patients may or may not have a fever.
Individuals who are at increased risk for CA-MRSA disease include users of health clubs or participants in contact sports, men who have sex with men, children younger than 2 years of age, users of intravenous drugs, military personnel, and prisoners.2,3 However, the absence of these factors in a patient with a skin or soft-tissue infection does not rule out MRSA.4
Regardless of the lesion’s appearance or the patient’s epidemiologic history, consider CA-MRSA if its prevalence in your community reaches 10% to 15%.
- Community-acquired methicillin-resistant Staphylococcus aureus (CA-MRSA) causes up to 74% of purulent skin and soft-tissue infections in communities throughout the United States.1 By definition, this infection occurs in patients who have not been hospitalized and have not undergone medical procedures within the prior year.12
- The annual incidence of CA-MRSA was reported to be 18.0-25.7 cases per 100,000 population between 2001 and 2002.13 Clusters of CA-MRSA have been identified among Alaskan natives, Native American Indians, and Pacific Islanders.12
- Most often this organism causes skin and soft-tissue infections, though cardiac, respiratory, blood, and bone infections can also occur.14
- CA-MRSA species are genotypically distinct from hospital-acquired MRSA. One marker for CA-MRSA, Panton-Valentine leucocidin (PVL), is most often detected in cases of severe and systemic infection, and it may be a virulence factor.15 However, the presence of PVL does not necessarily correlate directly with antibiotic resistance.
- Historically, CA-MRSA was primarily resistant to beta-lactams and erythromycin. More recent strains have also demonstrated resistance to tetracycline and clindamycin.
- Retrospective analyses show that patients with CA-MRSA tend to receive inadequate initial antibiotic coverage, and, independent of this, they tend to have worse clinical outcomes than those infected by methicillin-sensitive strains.16
Hospitalize any patient who exhibits fever or hypothermia, tachycardia greater than 100 beats per minute, or hypotension with a systolic blood pressure <90 mm Hg or 20 mm Hg lower than baseline. A skin lesion >5 cm is also likely to require hospitalization and parenteral antibiotics.5
Treatment: Incision and drainage most important
Several management schemes have been proposed to guide the appropriate level of therapy based on presenting patient characteristics.6,7 If a lesion is clearly fluctuant, incise it and drain the fluid, or refer the patient for surgical consultation. If the lesion is not clearly fluctuant, needle aspiration may help to determine the need for more extensive incision and drainage or to collect a specimen for culture. Although culture of skin lesions may not have been routine in the past, the advent of CA-MRSA has made it so, particularly given that MRSA lesions may not be clinically distinguishable from those caused by nonresistant S aureus.
Periodic postprocedure follow-up is indicated to ensure resolution of the infection. At our health center, patients return every few days for an appointment with nursing staff for wound irrigation and packing change until the lesion visibly improves. Systemic effects from the infection are monitored, as well.
Adult patients in 1 study were treated with incision and drainage by a surgeon.8 The technique described in the article used an 11 blade and a “sawing motion,” creating a wide opening. The wound cavity was explored for loculations and packed. This technique is identical to that used in the office. There is one caveat, though: This study included abscesses larger than 5 cm and patients with compromised immune systems—situations not routinely managed in the primary care office.
Are antibiotics indicated after incision and drainage for MRSA? In this same study, cure rates with incision and drainage alone were just over 90%.8 The cure rate in the treatment arm also receiving an antibiotic was 84% (difference was not statistically significant), and coverage was inadequate for MRSA. Treatment with cephalexin after incision and drainage resulted in 1 patient harmed for every 14 treated (NNH=14). A pediatric study also showed that antibiotics do not affect the outcome of skin lesions following incision and drainage.5 When deciding whether to prescribe postprocedure antibiotics, keep in mind the need to avoid contributing further to bacterial resistance.
Generally if incision and drainage fail to promote healing of the MRSA lesion within 7 days, start the patient on trimethoprim-sulfamethoxazole or tetracycline. Clindamycin is an option, though resistance to it is becoming more common. Adjust the antibiotic choice as needed when culture and sensitivity results become available.
Trimethoprim-sulfamethoxazole is generally well tolerated at the recommended dose of 1 to 2 double-strength tablets (160 mg TMP, 800 mg SMX) twice daily for adults. If a patient’s creatinine clearance is 15 to 30 mL/min, reduce the dose by half. The rate of sulfa allergy is similar to other antibiotics, at 3%.
Tetracycline’s dosing schedule—for adults, 250 or 500 mg 4 times daily—makes it difficult to use. Gastrointestinal upset, phototoxicity, and hepatotoxicity can occur. The possibility of tooth discoloration precludes tetracycline’s use in children.
Clindamycin carries a high rate of gastrointestinal-related problems, Clostridium difficile infection in particular (10% incidence administered in any route). Inducible resistance to clindamycin is 50% in MRSA infections.9 Recent use of antibiotics may increase the likelihood of clindamycin resistance, with erythromycin in particular inducing this resistance. Its dosage typically is 150 to 300 mg every 6 hours.
Doxycycline and minocycline are not recommended, as they carry a 21% failure rate.9
Linezolid is costly and has many drug interactions. In particular, linezolid has the potential to cause serotonin syndrome with agents that affect the serotonergic system. Linezolid may also interact with medications that affect the adrenergic system (pressors). Its routine use in the community without infectious disease consultation is not advised.
For lesions that are not fluctuant or purulent, appropriate first-line antibiotics are semisynthetic penicillins (dicloxacillin), first- or second-generation oral cephalosporins, macrolides, and clindamycin.9 These antibiotics are preferable for group A streptococcal infections, erysipelas (which can be quite aggressive), and impetigo. Adjustments can be made as culture results become available or if the clinical response is inadequate. There is no particular utility in waiting to administer oral antibiotics in cases of erysipelas or impetigo, though topical antibiotics can often be used for limited cases of impetigo.
Prevention: Simple precautions are the rule
Most CA-MRSA infections result from direct contact with a patient’s wound or from wound drainage on environmental surfaces.
In the medical office. In addition to using sterile technique during incision and drainage, be sure that all staff members wash their hands with soap and water or with an alcohol-based sanitizer. For the most part, MRSA remains susceptible to triclosan, a topical antiseptic in commercially available hand soaps.
Clean equipment as needed with 10% sodium hypochlorite solution or another agent effective against MRSA. Surgical instruments should be disposable or sterilized after each use.
At the patient’s home. Instruct patients to clean wounds wearing fresh disposable gloves each time and to cover wounds with new, dry dressings. Tell families to avoid sharing linens and clothing unless they have been washed in hot soap and water and dried in a heated dryer. MRSA can live for weeks to months on surfaces exposed to infected wounds,10 and these surfaces can be disinfected with a 10% bleach solution.
Sports environments. Athletes with CA-MRSA infections should not compete unless the wound can be completely covered with a dry dressing. Recommend to those in charge of school or commercial facilities that, in cases of confirmed MRSA infection, they routinely clean locker rooms and sports equipment with either a 10% bleach solution or commercial disinfectant. There is no evidence, however, that more widespread or vigorous cleaning—such as dismantling a training room and all its cardio-fitness equipment for disinfecting—prevents the spread of MRSA.
Encourage athletes to wash their hands properly. Communal towels should be washed in hot water (>140°F) with bleach before reuse. Personal equipment should be cleaned per the instructions of the manufacturer. Athletes should use a clean towel to provide a barrier between their skin and the surfaces of weight-room or cardio-fitness equipment. They should also clean equipment before and after use with an appropriate cleanser, such as a disinfectant hand-wipe.
Unproductive efforts you can avoid. Screening household contacts for MRSA is not useful, and attempts to eliminate colonization are generally ineffective. In a large military study, use of intranasal mupirocin failed to decrease nasal carriage of MRSA and the incidence of MRSA infections.11 The MRSA nasal colonization rate was 3.9%; 121 individuals with MRSA colonization needed to be treated with nasal mupirocin to prevent 1 MRSA infection in the total study population.
More complex antibiotic regimens are sometimes used in an attempt to eradicate MRSA carriage, though they also have limited effectiveness and carry the general risks of antibiotic use (gastrointestinal disturbance, allergic reaction, etc). If your office is considering an eradication attempt, consult with an infectious disease clinician first.
- Community-acquired methicillin-resistant Staphylococcus aureus (CA-MRSA) abscesses are best managed surgically; postprocedure antibiotics do not substantially improve outcomes. Cure rates with incision and drainage alone are at least 90% (A).
- If incision and drainage fail to promote healing within 7 days, the oral antibiotics of choice are trimethoprim-sulfamethoxazole and tetracycline (C).
- Eradication of nasal carriage of CA-MRSA is generally not useful in preventing spread of clinical MRSA infections in communities (B).
Strength of recommendation (SOR)
- Good quality patient-oriented evidence
- Inconsistent or limited-quality patient-oriented evidence
- Consensus, usual practice, opinion, disease-oriented evidence, case series
A previously healthy law student arrives at your office complaining of “abdominal pain.” You discover on examination that she has an erythematous, indurated, and tender 3-cm lesion on her suprapubic region. The lesion has no point, but its center is boggy. The patient’s temperature is normal. Would you give her an antibiotic? Would you cover immediately for community-acquired methicillin-resistant Staphylococcus aureus (CA-MRSA)? What other factors might influence your decision?
The incidence of MRSA is increasing in communities across the United States, challenging our assumptions about evaluation and management of skin and soft-tissue infections. In this article, I outline a rational approach to managing patients who have lesions likely to have been caused by CA-MRSA (TABLE).
TABLE
Suspect CA-MRSA? Consider this treatment approach6,7
| CLASS | PATIENT CRITERIA | MANAGEMENT | ANTIBIOTIC CHOICES |
|---|---|---|---|
| 1 | Afebrile and healthy; lesion nonfluctuant | If no drainable abscess, give common first-line antibiotic for SSTI; reassess for response | Semisynthetic penicillin, oral first- or second-generation cephalosporin, macrolide, clindamycin |
| 2 | Fluctuant or pustular lesion <5 cm; with or without fever | Surgical drainage of abscess if possible. Use I&D presumptively for MRSA and monitor closely for response; inpatient management may be indicated | Trimethoprim-sulfamethoxazole, tetracycline, clindamycin |
| 3 | Toxic appearance or at least 1 unstable comorbidity or a limb-threatening infection; lesion >5 cm | Hospital admission with broadspectrum antibiotics for MRSA coverage; consider infectious disease consultation | Broad-spectrum, including vancomycin |
| 4 | Sepsis syndrome or life-threatening infection (necrotizing fasciitis) | Above plus aggressive surgical debridement | Above with infectious disease guidance |
| CA-MRSA, community-acquired methicillin-resistant Staphylococcus aureus; I&D, incision and drainage; MRSA, methicillin-resistant Staphylococcus aureus; SSTI, skin and soft-tissue infection. | |||
When to suspect MRSA skin infection
Patients with CA-MRSA skin infection often report a “spider bite,” as lesions appear suddenly and unexpectedly in areas without a history of trauma.1 The lesions very often are pustular with central necrosis, and there may be purulent drainage, redness, tenderness, and palpable fluctuance. CA-MRSA can cause impetigo, but the often benign nature of this clinical infection makes management decisions less crucial. CA-MRSA skin lesions can occur anywhere on the body, though most often they appear in the axillae or the groin and buttocks. Patients may or may not have a fever.
Individuals who are at increased risk for CA-MRSA disease include users of health clubs or participants in contact sports, men who have sex with men, children younger than 2 years of age, users of intravenous drugs, military personnel, and prisoners.2,3 However, the absence of these factors in a patient with a skin or soft-tissue infection does not rule out MRSA.4
Regardless of the lesion’s appearance or the patient’s epidemiologic history, consider CA-MRSA if its prevalence in your community reaches 10% to 15%.
- Community-acquired methicillin-resistant Staphylococcus aureus (CA-MRSA) causes up to 74% of purulent skin and soft-tissue infections in communities throughout the United States.1 By definition, this infection occurs in patients who have not been hospitalized and have not undergone medical procedures within the prior year.12
- The annual incidence of CA-MRSA was reported to be 18.0-25.7 cases per 100,000 population between 2001 and 2002.13 Clusters of CA-MRSA have been identified among Alaskan natives, Native American Indians, and Pacific Islanders.12
- Most often this organism causes skin and soft-tissue infections, though cardiac, respiratory, blood, and bone infections can also occur.14
- CA-MRSA species are genotypically distinct from hospital-acquired MRSA. One marker for CA-MRSA, Panton-Valentine leucocidin (PVL), is most often detected in cases of severe and systemic infection, and it may be a virulence factor.15 However, the presence of PVL does not necessarily correlate directly with antibiotic resistance.
- Historically, CA-MRSA was primarily resistant to beta-lactams and erythromycin. More recent strains have also demonstrated resistance to tetracycline and clindamycin.
- Retrospective analyses show that patients with CA-MRSA tend to receive inadequate initial antibiotic coverage, and, independent of this, they tend to have worse clinical outcomes than those infected by methicillin-sensitive strains.16
Hospitalize any patient who exhibits fever or hypothermia, tachycardia greater than 100 beats per minute, or hypotension with a systolic blood pressure <90 mm Hg or 20 mm Hg lower than baseline. A skin lesion >5 cm is also likely to require hospitalization and parenteral antibiotics.5
Treatment: Incision and drainage most important
Several management schemes have been proposed to guide the appropriate level of therapy based on presenting patient characteristics.6,7 If a lesion is clearly fluctuant, incise it and drain the fluid, or refer the patient for surgical consultation. If the lesion is not clearly fluctuant, needle aspiration may help to determine the need for more extensive incision and drainage or to collect a specimen for culture. Although culture of skin lesions may not have been routine in the past, the advent of CA-MRSA has made it so, particularly given that MRSA lesions may not be clinically distinguishable from those caused by nonresistant S aureus.
Periodic postprocedure follow-up is indicated to ensure resolution of the infection. At our health center, patients return every few days for an appointment with nursing staff for wound irrigation and packing change until the lesion visibly improves. Systemic effects from the infection are monitored, as well.
Adult patients in 1 study were treated with incision and drainage by a surgeon.8 The technique described in the article used an 11 blade and a “sawing motion,” creating a wide opening. The wound cavity was explored for loculations and packed. This technique is identical to that used in the office. There is one caveat, though: This study included abscesses larger than 5 cm and patients with compromised immune systems—situations not routinely managed in the primary care office.
Are antibiotics indicated after incision and drainage for MRSA? In this same study, cure rates with incision and drainage alone were just over 90%.8 The cure rate in the treatment arm also receiving an antibiotic was 84% (difference was not statistically significant), and coverage was inadequate for MRSA. Treatment with cephalexin after incision and drainage resulted in 1 patient harmed for every 14 treated (NNH=14). A pediatric study also showed that antibiotics do not affect the outcome of skin lesions following incision and drainage.5 When deciding whether to prescribe postprocedure antibiotics, keep in mind the need to avoid contributing further to bacterial resistance.
Generally if incision and drainage fail to promote healing of the MRSA lesion within 7 days, start the patient on trimethoprim-sulfamethoxazole or tetracycline. Clindamycin is an option, though resistance to it is becoming more common. Adjust the antibiotic choice as needed when culture and sensitivity results become available.
Trimethoprim-sulfamethoxazole is generally well tolerated at the recommended dose of 1 to 2 double-strength tablets (160 mg TMP, 800 mg SMX) twice daily for adults. If a patient’s creatinine clearance is 15 to 30 mL/min, reduce the dose by half. The rate of sulfa allergy is similar to other antibiotics, at 3%.
Tetracycline’s dosing schedule—for adults, 250 or 500 mg 4 times daily—makes it difficult to use. Gastrointestinal upset, phototoxicity, and hepatotoxicity can occur. The possibility of tooth discoloration precludes tetracycline’s use in children.
Clindamycin carries a high rate of gastrointestinal-related problems, Clostridium difficile infection in particular (10% incidence administered in any route). Inducible resistance to clindamycin is 50% in MRSA infections.9 Recent use of antibiotics may increase the likelihood of clindamycin resistance, with erythromycin in particular inducing this resistance. Its dosage typically is 150 to 300 mg every 6 hours.
Doxycycline and minocycline are not recommended, as they carry a 21% failure rate.9
Linezolid is costly and has many drug interactions. In particular, linezolid has the potential to cause serotonin syndrome with agents that affect the serotonergic system. Linezolid may also interact with medications that affect the adrenergic system (pressors). Its routine use in the community without infectious disease consultation is not advised.
For lesions that are not fluctuant or purulent, appropriate first-line antibiotics are semisynthetic penicillins (dicloxacillin), first- or second-generation oral cephalosporins, macrolides, and clindamycin.9 These antibiotics are preferable for group A streptococcal infections, erysipelas (which can be quite aggressive), and impetigo. Adjustments can be made as culture results become available or if the clinical response is inadequate. There is no particular utility in waiting to administer oral antibiotics in cases of erysipelas or impetigo, though topical antibiotics can often be used for limited cases of impetigo.
Prevention: Simple precautions are the rule
Most CA-MRSA infections result from direct contact with a patient’s wound or from wound drainage on environmental surfaces.
In the medical office. In addition to using sterile technique during incision and drainage, be sure that all staff members wash their hands with soap and water or with an alcohol-based sanitizer. For the most part, MRSA remains susceptible to triclosan, a topical antiseptic in commercially available hand soaps.
Clean equipment as needed with 10% sodium hypochlorite solution or another agent effective against MRSA. Surgical instruments should be disposable or sterilized after each use.
At the patient’s home. Instruct patients to clean wounds wearing fresh disposable gloves each time and to cover wounds with new, dry dressings. Tell families to avoid sharing linens and clothing unless they have been washed in hot soap and water and dried in a heated dryer. MRSA can live for weeks to months on surfaces exposed to infected wounds,10 and these surfaces can be disinfected with a 10% bleach solution.
Sports environments. Athletes with CA-MRSA infections should not compete unless the wound can be completely covered with a dry dressing. Recommend to those in charge of school or commercial facilities that, in cases of confirmed MRSA infection, they routinely clean locker rooms and sports equipment with either a 10% bleach solution or commercial disinfectant. There is no evidence, however, that more widespread or vigorous cleaning—such as dismantling a training room and all its cardio-fitness equipment for disinfecting—prevents the spread of MRSA.
Encourage athletes to wash their hands properly. Communal towels should be washed in hot water (>140°F) with bleach before reuse. Personal equipment should be cleaned per the instructions of the manufacturer. Athletes should use a clean towel to provide a barrier between their skin and the surfaces of weight-room or cardio-fitness equipment. They should also clean equipment before and after use with an appropriate cleanser, such as a disinfectant hand-wipe.
Unproductive efforts you can avoid. Screening household contacts for MRSA is not useful, and attempts to eliminate colonization are generally ineffective. In a large military study, use of intranasal mupirocin failed to decrease nasal carriage of MRSA and the incidence of MRSA infections.11 The MRSA nasal colonization rate was 3.9%; 121 individuals with MRSA colonization needed to be treated with nasal mupirocin to prevent 1 MRSA infection in the total study population.
More complex antibiotic regimens are sometimes used in an attempt to eradicate MRSA carriage, though they also have limited effectiveness and carry the general risks of antibiotic use (gastrointestinal disturbance, allergic reaction, etc). If your office is considering an eradication attempt, consult with an infectious disease clinician first.
1. Moran GJ, Krishnadasan A, Gorwitz RJ, et al. Methicillin-resistant S aureus infections among patients in the emergency department. N Engl J Med. 2006;355:666-674.
2. Cohen PR. The skin in the gym: a comprehensive review of the cutaneous manifestations of community-acquired methicillin-resistant Staphylococcus aureus infection in athletes. Clin Dermatol. 2008;26:16-26.
3. Cohen PR. Community-acquired methicillin-resistant Staphylococcus aureus skin infections: implications for patients and practitioners. Am J Clin Dermatol. 2007;8:259-270.
4. Miller LG, Perdreau-Remington F, Bayer AS, et al. Clinical and epidemiologic characteristics cannot distinguish community associated methicillin resistant Staph aureus infection from methicillin susceptible S aureus infection: a prospective investigation. Clin Infect Dis. 2007;44:471-482.
5. Lee MC, Rios AM, Aten MF, et al. Management and outcome of children with skin and soft tissue abscesses caused by community-acquired methicillin-resistant Staphylococcus aureus. Pediatr Infect Dis J. 2004;23:123-127.
6. Eron LJ, Lipsky BA, Low DE, et al. Managing skin and soft tissue infections: expert panel recommendations on key decision points. J Antimicrob Chemother. 2003;52(suppl S1):i3-i17.
7. CDC, AMA, IDSA, Outpatient management of skin and soft tissue infections in the era of community-associated MRSA. Available at: http://www.ama-assn.org/ama1/pub/upload/mm/36/ca_mrsa_desk_102007.pdf. Accessed June 8, 2008.
8. Rajendran PM, Young D, Maurer T, et al. Randomized, double-blind, placebo-controlled trial of cephalexin for treatment of uncomplicated skin abscesses in a population at risk for community-acquired methicillin-resistant Staphylococcus aureus infection. Antimicrob Agents Chemother. 2007;51:4044-4048.
9. Stevens DL, Bisno AL, Chambers HF, et al. Practice guidelines for the diagnosis and management of skin and soft-tissue infections. Clin Infect Dis. 2005;41:1373-1406.
10. Dellit TH, Ducin J. Guidelines for Evaluation and Management of Community-Associated Methicillin Resistant Staphylococcus aureus Skin and Soft Tissue Infections in Outpatient Settings. Available at: http://www.metrokc.gov/health/providers/epidemiology/MRSA-guidelines.pdf. Accessed August 6, 2008.
11. Ellis MW, Griffith ME, Dooley DP, et al. Targeted intranasal mupirocin to prevent colonization and infection by community-associated methicillin-resistant Staphylococcus aureus strains in soldiers: a cluster randomized controlled trial. Antimicrob Agents Chemother. 2007;51:3591-3598.
12. Centers for Disease Control and Prevention. Healthcare-associated methicillin resistant Staphylococcus aureus (MRSA). Available at: http://www.cdc.gov/ncidod/dhqp/ar_mrsa.html. Accessed April 14, 2008.
13. Fridkin SK, Hageman JC, Morrison M, et al. Methicillin-reistant Staphylococcus aureus disease in three communities. N Engl J Med. 2005;352:2362-a.
14. Active Bacterial Core Surveillance (ABCs) Report, Emerging Infections Program Network methicillin-resistant Staphylococcus aureus, 2006. Available at: http://www.cdc.gov/ncidod/dbmd/abcs/survreports/mrsa06.pdf. Accessed May 5, 2008.
15. Holmes A, Ganner M, McGuane S, et al. Staphylococcus aureus isolates carrying panton-valentine leucocidin genes in England and Wales: frequency, characterization, and association with clinical disease. J Clin Microbiol. 2005;43:2384-2390.
16. Davis SL, Perri MB, Donabedian SM, et al. Epidemiology and outcomes of community-associated methicillin-resistant Staphylococcus aureus infection. J Clin Microbiol. 2007;45:1705-1711.
1. Moran GJ, Krishnadasan A, Gorwitz RJ, et al. Methicillin-resistant S aureus infections among patients in the emergency department. N Engl J Med. 2006;355:666-674.
2. Cohen PR. The skin in the gym: a comprehensive review of the cutaneous manifestations of community-acquired methicillin-resistant Staphylococcus aureus infection in athletes. Clin Dermatol. 2008;26:16-26.
3. Cohen PR. Community-acquired methicillin-resistant Staphylococcus aureus skin infections: implications for patients and practitioners. Am J Clin Dermatol. 2007;8:259-270.
4. Miller LG, Perdreau-Remington F, Bayer AS, et al. Clinical and epidemiologic characteristics cannot distinguish community associated methicillin resistant Staph aureus infection from methicillin susceptible S aureus infection: a prospective investigation. Clin Infect Dis. 2007;44:471-482.
5. Lee MC, Rios AM, Aten MF, et al. Management and outcome of children with skin and soft tissue abscesses caused by community-acquired methicillin-resistant Staphylococcus aureus. Pediatr Infect Dis J. 2004;23:123-127.
6. Eron LJ, Lipsky BA, Low DE, et al. Managing skin and soft tissue infections: expert panel recommendations on key decision points. J Antimicrob Chemother. 2003;52(suppl S1):i3-i17.
7. CDC, AMA, IDSA, Outpatient management of skin and soft tissue infections in the era of community-associated MRSA. Available at: http://www.ama-assn.org/ama1/pub/upload/mm/36/ca_mrsa_desk_102007.pdf. Accessed June 8, 2008.
8. Rajendran PM, Young D, Maurer T, et al. Randomized, double-blind, placebo-controlled trial of cephalexin for treatment of uncomplicated skin abscesses in a population at risk for community-acquired methicillin-resistant Staphylococcus aureus infection. Antimicrob Agents Chemother. 2007;51:4044-4048.
9. Stevens DL, Bisno AL, Chambers HF, et al. Practice guidelines for the diagnosis and management of skin and soft-tissue infections. Clin Infect Dis. 2005;41:1373-1406.
10. Dellit TH, Ducin J. Guidelines for Evaluation and Management of Community-Associated Methicillin Resistant Staphylococcus aureus Skin and Soft Tissue Infections in Outpatient Settings. Available at: http://www.metrokc.gov/health/providers/epidemiology/MRSA-guidelines.pdf. Accessed August 6, 2008.
11. Ellis MW, Griffith ME, Dooley DP, et al. Targeted intranasal mupirocin to prevent colonization and infection by community-associated methicillin-resistant Staphylococcus aureus strains in soldiers: a cluster randomized controlled trial. Antimicrob Agents Chemother. 2007;51:3591-3598.
12. Centers for Disease Control and Prevention. Healthcare-associated methicillin resistant Staphylococcus aureus (MRSA). Available at: http://www.cdc.gov/ncidod/dhqp/ar_mrsa.html. Accessed April 14, 2008.
13. Fridkin SK, Hageman JC, Morrison M, et al. Methicillin-reistant Staphylococcus aureus disease in three communities. N Engl J Med. 2005;352:2362-a.
14. Active Bacterial Core Surveillance (ABCs) Report, Emerging Infections Program Network methicillin-resistant Staphylococcus aureus, 2006. Available at: http://www.cdc.gov/ncidod/dbmd/abcs/survreports/mrsa06.pdf. Accessed May 5, 2008.
15. Holmes A, Ganner M, McGuane S, et al. Staphylococcus aureus isolates carrying panton-valentine leucocidin genes in England and Wales: frequency, characterization, and association with clinical disease. J Clin Microbiol. 2005;43:2384-2390.
16. Davis SL, Perri MB, Donabedian SM, et al. Epidemiology and outcomes of community-associated methicillin-resistant Staphylococcus aureus infection. J Clin Microbiol. 2007;45:1705-1711.
Are your COPD patients benefiting from best practices?
- Perform spirometric testing on any patient who complains of difficulty breathing and has a history of smoking or risk factors for chronic obstructive pulmonary disease (COPD) (American College of Physicians grade: Strong recommendation, moderate-quality evidence)
- Use inhaled bronchodilators and oral glucocorticosteroids for COPD exacerbations (Global Initiative for Chronic Obstructive Lung Disease [GOLD] Evidence A)
- Use antibiotics for COPD exacerbations (GOLD Evidence B)
- Use long-acting beta-agonists, long-acting anticholinergics, or inhaled steroids for chronic, stable COPD (American College of Physicians grade: Strong recommendation, high-quality evidence)
- Smoking cessation is the most effective way to decrease the risk of COPD progression (GOLD Evidence A)
GOLD Evidence categories
- Randomized controlled trials (RCTs); rich body of data
- RCTs; limited body of data
- Nonrandomized trials; observational studies
- Panel consensus judgment
A new patient comes into your office and tells you he experiences labored breathing on exertion, smokes a pack of cigarettes a day, and has a smoker’s cough.
- Would you perform spirometry to gauge airway obstruction?
- How do you think your decision would compare with those of your colleagues?
In this article, we put your answer into context by revealing just how underutilized spirometry is.
We also use a progressive case example to illustrate evidence-based recommendations and management tips for chronic obstructive pulmonary disease (COPD) and address often overlooked gaps in care.
Many of the recommendations in this article come from the Global Initiative for Chronic Obstructive Lung Disease (GOLD), published in the American Journal of Respiratory and Critical Care Medicine1 and updated online at www.goldcopd.org. (This initiative, begun in 1998, provides specific, evidence-based guidelines on the prevention, assessment, and management of COPD patients.) We also refer to newly published American College of Physicians (ACP) evidence-based guidelines for managing chronic, stable COPD.2,3
CASE: Shortness of breath, smoker’s cough
Mr. Jones, a 57-year-old patient in our practice, says that for the past 3 months he has increasingly experienced shortness of breath when walking up a flight of stairs. He has smoked cigarettes for many years and also acknowledges having a smoker’s cough. He brings up clear phlegm on most days.
Dyspnea is the most common symptom reported by patients with COPD. In a study of 2678 patients, the first and most troublesome symptom noted was dyspnea (71%), followed by cough (19%).4 Patients typically say their dyspnea has worsened over time. It tends to occur daily, particularly with exercise. Cough may be intermittent and nonproductive.
Consider the diagnosis whenever a patient with dyspnea has a risk factor for COPD, such as smoking (~80% of cases); extended second-hand smoke exposure; contact with occupational dust, home cooking and heating fuels, or other potentially toxic chemicals; or has a history of recurrent lung infections.5 With patients in their 30s or 40s exhibiting signs and symptoms suggestive of COPD, consider a work-up for alpha-1 antitrypsin deficiency. (See “Does your patient have alpha-1 antitrypsin deficiency?”.)
Physical examination has limited usefulness. It exhibits poor sensitivity for detecting mild-to-moderate COPD, unless wheezing is present. Wheezing in smokers (more than 40 pack-years) has a positive likelihood ratio of 8.3 for obstructive airway disease.6
Physical diagnosis is easier with more severe disease, especially if patients show classic signs of COPD, such as pursed-lip breathing, decreased breath sounds, and prolonged expiratory wheezes.
Spirometry is key, and underused. Demonstrating airflow obstruction on spirometry is essential to a COPD diagnosis. An FEV1/FVC ratio <0.70 or FEV1 <80% in patients who have received a test-bronchodilator confirms airflow obstruction.
Amazingly, a COPD diagnosis is assigned to less than half of the estimated 24 million patients with airflow obstruction in United States,7 despite the fact that COPD is the 4th leading cause of death, and the 12th leading cause of morbidity.1 Most of those who are identified have advanced disease.8 This dramatic underdiagnosis is attributable to the underuse of office spirometry as a diagnostic tool.9
A Canadian study revealed that only 21% of physicians ordered spirometry when managing a middle-aged smoker with cough.10,11 Another study showed that only 22% of North American physicians would order spirometry for a smoker with cough.10,12 Only a third of patients had undergone spirometry within 2 years of a new diagnosis of COPD. The lowest frequency of testing was among elderly patients, especially among those older than 75 years.10 (Caveat: as patients age, FEV1 naturally declines, making it easy to overdiagnose airflow obstruction in elderly patients.8,13)
The above data regarding underuse of spirometry apply to symptomatic patients. A recent US Preventive Services Task Force analysis found that screening asymptomatic smokers does not improve health outcomes; the number needed to test with spirometry would be in the “hundreds” to defer a single exacerbation.9 (ACP grade: strong recommendation, moderate-quality evidence.)
Reserve chest radiographs and CT scans to rule out other disorders. Patients with COPD usually have elements of both chronic bronchitis (productive cough for 3 months in 2 consecutive years) and emphysema (defined anatomically as abnormal enlargement of airways distal to terminal bronchioles and destruction of alveolar walls). Radiographic tests may reveal the telltale signs of emphysema (flattened diaphragms, blebs, and bullous changes), but they are not necessary to make the diagnosis. They may be used, however, to exclude other causes of dyspnea, including congestive heart failure, pulmonary emboli, and interstitial lung disease (TABLE).
Recommendation 1 With patients who have respiratory symptoms, particularly dyspnea, perform spirometry to diagnose airflow obstruction. Spirometry should not be used to screen for airflow obstruction in asymptomatic individuals. (Grade: strong recommendation, moderate-quality evidence.)
Recommendation 2 Reserve treatment for patients who have respiratory symptoms and an FEV1 <60% predicted, as documented by spirometry. (Grade: strong recommendation, moderate-quality evidence.)
Recommendation 3 Prescribe 1 of the following maintenance monotherapies for symptomatic patients with COPD and an FEV1 <60% predicted: long-acting inhaled beta-agonists, long-acting inhaled anticholinergics, or inhaled corticosteroids. (Grade: strong recommendation, high-quality evidence.)
Recommendation 4 You may want to consider combination inhaled therapies for symptomatic patients with COPD and an FEV1 <60% predicted. (Grade: weak recommendation, moderate-quality evidence.)
Recommendation 5 Prescribe oxygen therapy for patients with COPD and resting hypoxemia (PaO2 ≤55 mm Hg). (Grade: strong recommendation, moderate-quality evidence.)
Recommendation 6 Consider prescribing pulmonary rehabilitation for symptomatic individuals with COPD who have an FEV1 <50% predicted. (Grade: weak recommendation, moderate-quality evidence.)
* Modified from Qaseem A et al. Diagnosis and management of stable chronic obstructive pulmonary disease: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2007;147:633-638.
TABLE
Suspect COPD? Rule out these disorders
| DISORDER | NOTABLE CHARACTERISTICS |
|---|---|
| Asthma | Usually begins in childhood. Can be associated with cough only. Airflow obstruction is usually reversible with bronchodilator (may coexist with COPD) |
| Cystic fibrosis | Symptoms usually begin in early childhood. Associated with sinus disease, GI disturbances, and infertility. Bronchiectasis noted on chest x-ray. Order sweat chloride test if suspected. Genetic testing is also available |
| Interstitial lung disease | Interstitial pattern on chest x-ray and thin-cut CT scan of lungs |
| Pneumonia | Fever, chills, cough, and infiltrate on chest x-ray |
| Congestive heart failure | Orthopnea, paroxysmal nocturnal dyspnea, and characteristic chest x-ray findings |
| Pulmonary embolism | Breathing difficulty and chest pain usually of sudden onset. CT angiography is diagnostic |
| Anxiety | Hyperventilation, panic attacks, increased stress |
CASE: Spirometry reveals airflow obstruction, FEV1 <50%
Mr. Jones underwent spirometry, which revealed airflow obstruction and an FEV1 <50%. We gave him a short-acting betaagonist to be used as needed. Two weeks later, he returned to the office with increasing cough and purulent sputum production, as well as worsening dyspnea.
The patient’s condition is consistent with an acute exacerbation of baseline COPD symptoms. Worsening dyspnea, cough, and sputum production—sometimes with purulence—are often accompanied by fever, fatigue, and anorexia.7
Match antibiotic therapy to sputum culture results or disease severity. Exacerbations are usually triggered by infection. Although an offending organism cannot be identified in one third of cases, common bacterial pathogens include Hemophilus influenza, Streptococcus pneumoniae, and Moraxella catarrhalis. Antibiotics have been shown to decrease mortality in patients with COPD exacerbations1,5,7,14,15 (GOLD Evidence B). For mild-to-moderate exacerbations, older antibiotics such as trimethoprim/sulfamethoxazole or doxycycline are often appropriate. For more severe exacerbations, and for patients with chronic, comorbid conditions such as diabetes mellitus, a second- or third-generation cephalosporin or fluoroquinolone may be preferable.
Use steroids and beta-agonists. Oral steroids are also effective in treating exacerbations (GOLD Evidence A), although the dose of steroids required has not been adequately studied. Prednisone, 40 mg/d for 7 to 10 days, is reasonable and safe.1 Also prescribe an inhaled short-acting beta-agonist for symptom control (GOLD Evidence A).1
CASE: Doxycycline 100 mg bid, and prednisone 40 mg/d for 7 days
Mr. Jones returned to the office 2 weeks after the acute exacerbation, feeling much better after receiving doxycycline 100 mg bid and prednisone 40 mg/d for 7 days. He was no longer coughing up purulent sputum, but he still felt short of breath walking to his mailbox and while doing household chores. He wondered what else could be done to improve his quality of life.
The airflow obstruction associated with COPD, unlike that of asthma, is irreversible and varies little,16 and its progression is persistent. That is why prevention is an important goal for physicians and their patients. However, treatment can lessen the frequency of exacerbations and severity of symptoms, particularly dyspnea on exertion.
Alpha-1 antitrypsin deficiency is an autosomal recessive disorder that causes COPD and liver cirrhosis.17 Alpha-1 antitrypsin protects the lungs from proteases released from inflammatory processes such as pneumonia and from inhaled toxic particles. When this glycoprotein is absent, proteases destroy airways and alveoli. Consider the diagnosis with younger patients. The disease is easily confused with asthma or smoking-induced COPD. It predominantly affects the lower lobes. Diagnosis is made by testing blood levels for the enzyme or genetic analysis.
Treatment is the same as for other causes of COPD. Although no evidence-based recommendations are available at this time, replacement of alpha-1 antitrypsin is indicated for certain patients. Smoking cessation is critical.
In our initial assessment of the patient, his FEV1 was <50%. There was no need to repeat spirometry, as the evidence does not support ongoing spirometric evaluation.2,3 Symptomatic patients with significant airflow obstruction (FEV1 <60% predicted) are the ones most likely to benefit from therapy (ACP grade: strong recommendation, moderate-quality evidence).2,3 Conversely, there is little evidence to justify treating asymptomatic patients who have airflow obstruction.
Monotherapy with long-acting inhaled beta-agonists, inhaled corticosteroids, or long-acting inhaled anticholinergics has been shown to reduce exacerbations and is preferable to short-acting, inhaled beta-agonists or short-acting anticholinergics (ACP grade: strong recommendation, high-quality evidence).2,3 At this time, evidence is insufficient to support the use of combined therapies—eg, inhaled steroids plus long-acting beta-agonists.2,3
For patients with a PaO2 ≤55 mm Hg, survival is improved by using supplemental oxygen therapy for 15 or more hours a day. (ACP grade: strong recommendation, moderate quality evidence).2,3
Finally, for symptomatic patients with an FEV1 <50%, pulmonary rehabilitation may reduce hospitalizations and increase exercise capacity (ACP grade: weak recommendation, moderate-quality evidence).2,3
For Mr. Jones, we prescribed 1 inhalation daily of the long-acting anticholinergic inhaler, tiotropium.
Smoking cessation critical
COPD progresses with aging and with continued smoking, and smoking cessation is critical to any management strategy.
CASE: Tiotropium, 1 inhalation daily, and a smoking cessation plan
We referred Mr. Jones to an outpatient smoking cessation program and gave him American Academy of Family Physicians patient education materials to review. His exercise tolerance improved with 1 inhalation daily of the long-acting anticholinergic inhaler, tiotropium, and he is making progress in his efforts to quit smoking.
Correspondence
Dean Gianakos, MD, Lynchburg Family Medicine Residency, 2097 Langhorne Road, Lynchburg, VA 24501; [email protected]
1. Rabe KF, Hurd S, Anzueto A, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease (GOLD). Am J Respir Crit Care Med. 2007;176:532-555.
2. Qaseem A, Snow V, Shekelle P, et al. Diagnosis and management of stable chronic obstructive pulmonary disease: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2007;147:633-638.
3. Wilt TJ, Niewoehner D, MacDonald R, et al. Management of stable chronic obstructive pulmonary disease: a systematic review for a clinical practice guideline. Ann Intern Med. 2007;147:639-653.
4. Kesten S, Menjoge S. Patient-reported symptoms of chronic obstructive pulmonary disease in clinical trials. Chest. 2005;128(4):249S.-
5. Pauwels RA, Buist AS, Ma P, et al. GOLD Scientific Committee. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. Respir Care. 2001;46:798-825.
6. Straus SE, McAlister FA, Sackett DL, et al. The accuracy of patient history, wheezing, and laryngeal measurements in diagnosing obstructive airway disease. JAMA. 2000;283:1853-1857.
7. Wise RA, Tashkin DP. Optimizing treatment of chronic obstructive pulmonary disease: an assessment of current therapies. Am J Med. 2007;120(8A):S4-S13.
8. Lin K, Watkins B, Johnson T, et al. Screening for chronic obstructive pulmonary disease using spirometry: summary of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med. 2008;148:535-543.
9. Sundblad BM, Larsson K, Nathell L. Low awareness of COPD among physicians. Clin Respir J. 2007;(1):11-16.
10. Han M, Kim MG, Mardon R, et al. Spirometry utilization for COPD: how do we measure up? Chest. 2007;132:403-409.
11. Kesten S, Chapman K. Physician perceptions and management of COPD. Chest. 1993;104:254-258.
12. Chapman K, Tashkin D, Pye D. Gender bias in the diagnosis of COPD. Chest. 2001;119:1691-1695.
13. Nazir SA, Al-Hamed MM, Erbland ML. Chronic obstructive pulmonary disease in the older patient. Clin Chest Med. 2007;28:703-715.
14. Ram FS, Rodriguez-Roisin R, Granados-Navarrete A, et al. Antibiotics for exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2006;(2):CD004403.
15. Littner M. In the clinic. Chronic obstructive pulmonary disease. Ann Intern Med. 2008;148(5):ITC3-1-ITC3-16.
16. Dewar M, Curry W. Chronic obstructive pulmonary disease: diagnostic considerations. Am Fam Physician. 2006;73:669-678.
17. Kohnlein T, Welte T. Alpha-1 antitrypsin deficiency: pathogenesis, clinical presentation, diagnosis, and treatment. Am J Med. 2008;121:3-9.
- Perform spirometric testing on any patient who complains of difficulty breathing and has a history of smoking or risk factors for chronic obstructive pulmonary disease (COPD) (American College of Physicians grade: Strong recommendation, moderate-quality evidence)
- Use inhaled bronchodilators and oral glucocorticosteroids for COPD exacerbations (Global Initiative for Chronic Obstructive Lung Disease [GOLD] Evidence A)
- Use antibiotics for COPD exacerbations (GOLD Evidence B)
- Use long-acting beta-agonists, long-acting anticholinergics, or inhaled steroids for chronic, stable COPD (American College of Physicians grade: Strong recommendation, high-quality evidence)
- Smoking cessation is the most effective way to decrease the risk of COPD progression (GOLD Evidence A)
GOLD Evidence categories
- Randomized controlled trials (RCTs); rich body of data
- RCTs; limited body of data
- Nonrandomized trials; observational studies
- Panel consensus judgment
A new patient comes into your office and tells you he experiences labored breathing on exertion, smokes a pack of cigarettes a day, and has a smoker’s cough.
- Would you perform spirometry to gauge airway obstruction?
- How do you think your decision would compare with those of your colleagues?
In this article, we put your answer into context by revealing just how underutilized spirometry is.
We also use a progressive case example to illustrate evidence-based recommendations and management tips for chronic obstructive pulmonary disease (COPD) and address often overlooked gaps in care.
Many of the recommendations in this article come from the Global Initiative for Chronic Obstructive Lung Disease (GOLD), published in the American Journal of Respiratory and Critical Care Medicine1 and updated online at www.goldcopd.org. (This initiative, begun in 1998, provides specific, evidence-based guidelines on the prevention, assessment, and management of COPD patients.) We also refer to newly published American College of Physicians (ACP) evidence-based guidelines for managing chronic, stable COPD.2,3
CASE: Shortness of breath, smoker’s cough
Mr. Jones, a 57-year-old patient in our practice, says that for the past 3 months he has increasingly experienced shortness of breath when walking up a flight of stairs. He has smoked cigarettes for many years and also acknowledges having a smoker’s cough. He brings up clear phlegm on most days.
Dyspnea is the most common symptom reported by patients with COPD. In a study of 2678 patients, the first and most troublesome symptom noted was dyspnea (71%), followed by cough (19%).4 Patients typically say their dyspnea has worsened over time. It tends to occur daily, particularly with exercise. Cough may be intermittent and nonproductive.
Consider the diagnosis whenever a patient with dyspnea has a risk factor for COPD, such as smoking (~80% of cases); extended second-hand smoke exposure; contact with occupational dust, home cooking and heating fuels, or other potentially toxic chemicals; or has a history of recurrent lung infections.5 With patients in their 30s or 40s exhibiting signs and symptoms suggestive of COPD, consider a work-up for alpha-1 antitrypsin deficiency. (See “Does your patient have alpha-1 antitrypsin deficiency?”.)
Physical examination has limited usefulness. It exhibits poor sensitivity for detecting mild-to-moderate COPD, unless wheezing is present. Wheezing in smokers (more than 40 pack-years) has a positive likelihood ratio of 8.3 for obstructive airway disease.6
Physical diagnosis is easier with more severe disease, especially if patients show classic signs of COPD, such as pursed-lip breathing, decreased breath sounds, and prolonged expiratory wheezes.
Spirometry is key, and underused. Demonstrating airflow obstruction on spirometry is essential to a COPD diagnosis. An FEV1/FVC ratio <0.70 or FEV1 <80% in patients who have received a test-bronchodilator confirms airflow obstruction.
Amazingly, a COPD diagnosis is assigned to less than half of the estimated 24 million patients with airflow obstruction in United States,7 despite the fact that COPD is the 4th leading cause of death, and the 12th leading cause of morbidity.1 Most of those who are identified have advanced disease.8 This dramatic underdiagnosis is attributable to the underuse of office spirometry as a diagnostic tool.9
A Canadian study revealed that only 21% of physicians ordered spirometry when managing a middle-aged smoker with cough.10,11 Another study showed that only 22% of North American physicians would order spirometry for a smoker with cough.10,12 Only a third of patients had undergone spirometry within 2 years of a new diagnosis of COPD. The lowest frequency of testing was among elderly patients, especially among those older than 75 years.10 (Caveat: as patients age, FEV1 naturally declines, making it easy to overdiagnose airflow obstruction in elderly patients.8,13)
The above data regarding underuse of spirometry apply to symptomatic patients. A recent US Preventive Services Task Force analysis found that screening asymptomatic smokers does not improve health outcomes; the number needed to test with spirometry would be in the “hundreds” to defer a single exacerbation.9 (ACP grade: strong recommendation, moderate-quality evidence.)
Reserve chest radiographs and CT scans to rule out other disorders. Patients with COPD usually have elements of both chronic bronchitis (productive cough for 3 months in 2 consecutive years) and emphysema (defined anatomically as abnormal enlargement of airways distal to terminal bronchioles and destruction of alveolar walls). Radiographic tests may reveal the telltale signs of emphysema (flattened diaphragms, blebs, and bullous changes), but they are not necessary to make the diagnosis. They may be used, however, to exclude other causes of dyspnea, including congestive heart failure, pulmonary emboli, and interstitial lung disease (TABLE).
Recommendation 1 With patients who have respiratory symptoms, particularly dyspnea, perform spirometry to diagnose airflow obstruction. Spirometry should not be used to screen for airflow obstruction in asymptomatic individuals. (Grade: strong recommendation, moderate-quality evidence.)
Recommendation 2 Reserve treatment for patients who have respiratory symptoms and an FEV1 <60% predicted, as documented by spirometry. (Grade: strong recommendation, moderate-quality evidence.)
Recommendation 3 Prescribe 1 of the following maintenance monotherapies for symptomatic patients with COPD and an FEV1 <60% predicted: long-acting inhaled beta-agonists, long-acting inhaled anticholinergics, or inhaled corticosteroids. (Grade: strong recommendation, high-quality evidence.)
Recommendation 4 You may want to consider combination inhaled therapies for symptomatic patients with COPD and an FEV1 <60% predicted. (Grade: weak recommendation, moderate-quality evidence.)
Recommendation 5 Prescribe oxygen therapy for patients with COPD and resting hypoxemia (PaO2 ≤55 mm Hg). (Grade: strong recommendation, moderate-quality evidence.)
Recommendation 6 Consider prescribing pulmonary rehabilitation for symptomatic individuals with COPD who have an FEV1 <50% predicted. (Grade: weak recommendation, moderate-quality evidence.)
* Modified from Qaseem A et al. Diagnosis and management of stable chronic obstructive pulmonary disease: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2007;147:633-638.
TABLE
Suspect COPD? Rule out these disorders
| DISORDER | NOTABLE CHARACTERISTICS |
|---|---|
| Asthma | Usually begins in childhood. Can be associated with cough only. Airflow obstruction is usually reversible with bronchodilator (may coexist with COPD) |
| Cystic fibrosis | Symptoms usually begin in early childhood. Associated with sinus disease, GI disturbances, and infertility. Bronchiectasis noted on chest x-ray. Order sweat chloride test if suspected. Genetic testing is also available |
| Interstitial lung disease | Interstitial pattern on chest x-ray and thin-cut CT scan of lungs |
| Pneumonia | Fever, chills, cough, and infiltrate on chest x-ray |
| Congestive heart failure | Orthopnea, paroxysmal nocturnal dyspnea, and characteristic chest x-ray findings |
| Pulmonary embolism | Breathing difficulty and chest pain usually of sudden onset. CT angiography is diagnostic |
| Anxiety | Hyperventilation, panic attacks, increased stress |
CASE: Spirometry reveals airflow obstruction, FEV1 <50%
Mr. Jones underwent spirometry, which revealed airflow obstruction and an FEV1 <50%. We gave him a short-acting betaagonist to be used as needed. Two weeks later, he returned to the office with increasing cough and purulent sputum production, as well as worsening dyspnea.
The patient’s condition is consistent with an acute exacerbation of baseline COPD symptoms. Worsening dyspnea, cough, and sputum production—sometimes with purulence—are often accompanied by fever, fatigue, and anorexia.7
Match antibiotic therapy to sputum culture results or disease severity. Exacerbations are usually triggered by infection. Although an offending organism cannot be identified in one third of cases, common bacterial pathogens include Hemophilus influenza, Streptococcus pneumoniae, and Moraxella catarrhalis. Antibiotics have been shown to decrease mortality in patients with COPD exacerbations1,5,7,14,15 (GOLD Evidence B). For mild-to-moderate exacerbations, older antibiotics such as trimethoprim/sulfamethoxazole or doxycycline are often appropriate. For more severe exacerbations, and for patients with chronic, comorbid conditions such as diabetes mellitus, a second- or third-generation cephalosporin or fluoroquinolone may be preferable.
Use steroids and beta-agonists. Oral steroids are also effective in treating exacerbations (GOLD Evidence A), although the dose of steroids required has not been adequately studied. Prednisone, 40 mg/d for 7 to 10 days, is reasonable and safe.1 Also prescribe an inhaled short-acting beta-agonist for symptom control (GOLD Evidence A).1
CASE: Doxycycline 100 mg bid, and prednisone 40 mg/d for 7 days
Mr. Jones returned to the office 2 weeks after the acute exacerbation, feeling much better after receiving doxycycline 100 mg bid and prednisone 40 mg/d for 7 days. He was no longer coughing up purulent sputum, but he still felt short of breath walking to his mailbox and while doing household chores. He wondered what else could be done to improve his quality of life.
The airflow obstruction associated with COPD, unlike that of asthma, is irreversible and varies little,16 and its progression is persistent. That is why prevention is an important goal for physicians and their patients. However, treatment can lessen the frequency of exacerbations and severity of symptoms, particularly dyspnea on exertion.
Alpha-1 antitrypsin deficiency is an autosomal recessive disorder that causes COPD and liver cirrhosis.17 Alpha-1 antitrypsin protects the lungs from proteases released from inflammatory processes such as pneumonia and from inhaled toxic particles. When this glycoprotein is absent, proteases destroy airways and alveoli. Consider the diagnosis with younger patients. The disease is easily confused with asthma or smoking-induced COPD. It predominantly affects the lower lobes. Diagnosis is made by testing blood levels for the enzyme or genetic analysis.
Treatment is the same as for other causes of COPD. Although no evidence-based recommendations are available at this time, replacement of alpha-1 antitrypsin is indicated for certain patients. Smoking cessation is critical.
In our initial assessment of the patient, his FEV1 was <50%. There was no need to repeat spirometry, as the evidence does not support ongoing spirometric evaluation.2,3 Symptomatic patients with significant airflow obstruction (FEV1 <60% predicted) are the ones most likely to benefit from therapy (ACP grade: strong recommendation, moderate-quality evidence).2,3 Conversely, there is little evidence to justify treating asymptomatic patients who have airflow obstruction.
Monotherapy with long-acting inhaled beta-agonists, inhaled corticosteroids, or long-acting inhaled anticholinergics has been shown to reduce exacerbations and is preferable to short-acting, inhaled beta-agonists or short-acting anticholinergics (ACP grade: strong recommendation, high-quality evidence).2,3 At this time, evidence is insufficient to support the use of combined therapies—eg, inhaled steroids plus long-acting beta-agonists.2,3
For patients with a PaO2 ≤55 mm Hg, survival is improved by using supplemental oxygen therapy for 15 or more hours a day. (ACP grade: strong recommendation, moderate quality evidence).2,3
Finally, for symptomatic patients with an FEV1 <50%, pulmonary rehabilitation may reduce hospitalizations and increase exercise capacity (ACP grade: weak recommendation, moderate-quality evidence).2,3
For Mr. Jones, we prescribed 1 inhalation daily of the long-acting anticholinergic inhaler, tiotropium.
Smoking cessation critical
COPD progresses with aging and with continued smoking, and smoking cessation is critical to any management strategy.
CASE: Tiotropium, 1 inhalation daily, and a smoking cessation plan
We referred Mr. Jones to an outpatient smoking cessation program and gave him American Academy of Family Physicians patient education materials to review. His exercise tolerance improved with 1 inhalation daily of the long-acting anticholinergic inhaler, tiotropium, and he is making progress in his efforts to quit smoking.
Correspondence
Dean Gianakos, MD, Lynchburg Family Medicine Residency, 2097 Langhorne Road, Lynchburg, VA 24501; [email protected]
- Perform spirometric testing on any patient who complains of difficulty breathing and has a history of smoking or risk factors for chronic obstructive pulmonary disease (COPD) (American College of Physicians grade: Strong recommendation, moderate-quality evidence)
- Use inhaled bronchodilators and oral glucocorticosteroids for COPD exacerbations (Global Initiative for Chronic Obstructive Lung Disease [GOLD] Evidence A)
- Use antibiotics for COPD exacerbations (GOLD Evidence B)
- Use long-acting beta-agonists, long-acting anticholinergics, or inhaled steroids for chronic, stable COPD (American College of Physicians grade: Strong recommendation, high-quality evidence)
- Smoking cessation is the most effective way to decrease the risk of COPD progression (GOLD Evidence A)
GOLD Evidence categories
- Randomized controlled trials (RCTs); rich body of data
- RCTs; limited body of data
- Nonrandomized trials; observational studies
- Panel consensus judgment
A new patient comes into your office and tells you he experiences labored breathing on exertion, smokes a pack of cigarettes a day, and has a smoker’s cough.
- Would you perform spirometry to gauge airway obstruction?
- How do you think your decision would compare with those of your colleagues?
In this article, we put your answer into context by revealing just how underutilized spirometry is.
We also use a progressive case example to illustrate evidence-based recommendations and management tips for chronic obstructive pulmonary disease (COPD) and address often overlooked gaps in care.
Many of the recommendations in this article come from the Global Initiative for Chronic Obstructive Lung Disease (GOLD), published in the American Journal of Respiratory and Critical Care Medicine1 and updated online at www.goldcopd.org. (This initiative, begun in 1998, provides specific, evidence-based guidelines on the prevention, assessment, and management of COPD patients.) We also refer to newly published American College of Physicians (ACP) evidence-based guidelines for managing chronic, stable COPD.2,3
CASE: Shortness of breath, smoker’s cough
Mr. Jones, a 57-year-old patient in our practice, says that for the past 3 months he has increasingly experienced shortness of breath when walking up a flight of stairs. He has smoked cigarettes for many years and also acknowledges having a smoker’s cough. He brings up clear phlegm on most days.
Dyspnea is the most common symptom reported by patients with COPD. In a study of 2678 patients, the first and most troublesome symptom noted was dyspnea (71%), followed by cough (19%).4 Patients typically say their dyspnea has worsened over time. It tends to occur daily, particularly with exercise. Cough may be intermittent and nonproductive.
Consider the diagnosis whenever a patient with dyspnea has a risk factor for COPD, such as smoking (~80% of cases); extended second-hand smoke exposure; contact with occupational dust, home cooking and heating fuels, or other potentially toxic chemicals; or has a history of recurrent lung infections.5 With patients in their 30s or 40s exhibiting signs and symptoms suggestive of COPD, consider a work-up for alpha-1 antitrypsin deficiency. (See “Does your patient have alpha-1 antitrypsin deficiency?”.)
Physical examination has limited usefulness. It exhibits poor sensitivity for detecting mild-to-moderate COPD, unless wheezing is present. Wheezing in smokers (more than 40 pack-years) has a positive likelihood ratio of 8.3 for obstructive airway disease.6
Physical diagnosis is easier with more severe disease, especially if patients show classic signs of COPD, such as pursed-lip breathing, decreased breath sounds, and prolonged expiratory wheezes.
Spirometry is key, and underused. Demonstrating airflow obstruction on spirometry is essential to a COPD diagnosis. An FEV1/FVC ratio <0.70 or FEV1 <80% in patients who have received a test-bronchodilator confirms airflow obstruction.
Amazingly, a COPD diagnosis is assigned to less than half of the estimated 24 million patients with airflow obstruction in United States,7 despite the fact that COPD is the 4th leading cause of death, and the 12th leading cause of morbidity.1 Most of those who are identified have advanced disease.8 This dramatic underdiagnosis is attributable to the underuse of office spirometry as a diagnostic tool.9
A Canadian study revealed that only 21% of physicians ordered spirometry when managing a middle-aged smoker with cough.10,11 Another study showed that only 22% of North American physicians would order spirometry for a smoker with cough.10,12 Only a third of patients had undergone spirometry within 2 years of a new diagnosis of COPD. The lowest frequency of testing was among elderly patients, especially among those older than 75 years.10 (Caveat: as patients age, FEV1 naturally declines, making it easy to overdiagnose airflow obstruction in elderly patients.8,13)
The above data regarding underuse of spirometry apply to symptomatic patients. A recent US Preventive Services Task Force analysis found that screening asymptomatic smokers does not improve health outcomes; the number needed to test with spirometry would be in the “hundreds” to defer a single exacerbation.9 (ACP grade: strong recommendation, moderate-quality evidence.)
Reserve chest radiographs and CT scans to rule out other disorders. Patients with COPD usually have elements of both chronic bronchitis (productive cough for 3 months in 2 consecutive years) and emphysema (defined anatomically as abnormal enlargement of airways distal to terminal bronchioles and destruction of alveolar walls). Radiographic tests may reveal the telltale signs of emphysema (flattened diaphragms, blebs, and bullous changes), but they are not necessary to make the diagnosis. They may be used, however, to exclude other causes of dyspnea, including congestive heart failure, pulmonary emboli, and interstitial lung disease (TABLE).
Recommendation 1 With patients who have respiratory symptoms, particularly dyspnea, perform spirometry to diagnose airflow obstruction. Spirometry should not be used to screen for airflow obstruction in asymptomatic individuals. (Grade: strong recommendation, moderate-quality evidence.)
Recommendation 2 Reserve treatment for patients who have respiratory symptoms and an FEV1 <60% predicted, as documented by spirometry. (Grade: strong recommendation, moderate-quality evidence.)
Recommendation 3 Prescribe 1 of the following maintenance monotherapies for symptomatic patients with COPD and an FEV1 <60% predicted: long-acting inhaled beta-agonists, long-acting inhaled anticholinergics, or inhaled corticosteroids. (Grade: strong recommendation, high-quality evidence.)
Recommendation 4 You may want to consider combination inhaled therapies for symptomatic patients with COPD and an FEV1 <60% predicted. (Grade: weak recommendation, moderate-quality evidence.)
Recommendation 5 Prescribe oxygen therapy for patients with COPD and resting hypoxemia (PaO2 ≤55 mm Hg). (Grade: strong recommendation, moderate-quality evidence.)
Recommendation 6 Consider prescribing pulmonary rehabilitation for symptomatic individuals with COPD who have an FEV1 <50% predicted. (Grade: weak recommendation, moderate-quality evidence.)
* Modified from Qaseem A et al. Diagnosis and management of stable chronic obstructive pulmonary disease: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2007;147:633-638.
TABLE
Suspect COPD? Rule out these disorders
| DISORDER | NOTABLE CHARACTERISTICS |
|---|---|
| Asthma | Usually begins in childhood. Can be associated with cough only. Airflow obstruction is usually reversible with bronchodilator (may coexist with COPD) |
| Cystic fibrosis | Symptoms usually begin in early childhood. Associated with sinus disease, GI disturbances, and infertility. Bronchiectasis noted on chest x-ray. Order sweat chloride test if suspected. Genetic testing is also available |
| Interstitial lung disease | Interstitial pattern on chest x-ray and thin-cut CT scan of lungs |
| Pneumonia | Fever, chills, cough, and infiltrate on chest x-ray |
| Congestive heart failure | Orthopnea, paroxysmal nocturnal dyspnea, and characteristic chest x-ray findings |
| Pulmonary embolism | Breathing difficulty and chest pain usually of sudden onset. CT angiography is diagnostic |
| Anxiety | Hyperventilation, panic attacks, increased stress |
CASE: Spirometry reveals airflow obstruction, FEV1 <50%
Mr. Jones underwent spirometry, which revealed airflow obstruction and an FEV1 <50%. We gave him a short-acting betaagonist to be used as needed. Two weeks later, he returned to the office with increasing cough and purulent sputum production, as well as worsening dyspnea.
The patient’s condition is consistent with an acute exacerbation of baseline COPD symptoms. Worsening dyspnea, cough, and sputum production—sometimes with purulence—are often accompanied by fever, fatigue, and anorexia.7
Match antibiotic therapy to sputum culture results or disease severity. Exacerbations are usually triggered by infection. Although an offending organism cannot be identified in one third of cases, common bacterial pathogens include Hemophilus influenza, Streptococcus pneumoniae, and Moraxella catarrhalis. Antibiotics have been shown to decrease mortality in patients with COPD exacerbations1,5,7,14,15 (GOLD Evidence B). For mild-to-moderate exacerbations, older antibiotics such as trimethoprim/sulfamethoxazole or doxycycline are often appropriate. For more severe exacerbations, and for patients with chronic, comorbid conditions such as diabetes mellitus, a second- or third-generation cephalosporin or fluoroquinolone may be preferable.
Use steroids and beta-agonists. Oral steroids are also effective in treating exacerbations (GOLD Evidence A), although the dose of steroids required has not been adequately studied. Prednisone, 40 mg/d for 7 to 10 days, is reasonable and safe.1 Also prescribe an inhaled short-acting beta-agonist for symptom control (GOLD Evidence A).1
CASE: Doxycycline 100 mg bid, and prednisone 40 mg/d for 7 days
Mr. Jones returned to the office 2 weeks after the acute exacerbation, feeling much better after receiving doxycycline 100 mg bid and prednisone 40 mg/d for 7 days. He was no longer coughing up purulent sputum, but he still felt short of breath walking to his mailbox and while doing household chores. He wondered what else could be done to improve his quality of life.
The airflow obstruction associated with COPD, unlike that of asthma, is irreversible and varies little,16 and its progression is persistent. That is why prevention is an important goal for physicians and their patients. However, treatment can lessen the frequency of exacerbations and severity of symptoms, particularly dyspnea on exertion.
Alpha-1 antitrypsin deficiency is an autosomal recessive disorder that causes COPD and liver cirrhosis.17 Alpha-1 antitrypsin protects the lungs from proteases released from inflammatory processes such as pneumonia and from inhaled toxic particles. When this glycoprotein is absent, proteases destroy airways and alveoli. Consider the diagnosis with younger patients. The disease is easily confused with asthma or smoking-induced COPD. It predominantly affects the lower lobes. Diagnosis is made by testing blood levels for the enzyme or genetic analysis.
Treatment is the same as for other causes of COPD. Although no evidence-based recommendations are available at this time, replacement of alpha-1 antitrypsin is indicated for certain patients. Smoking cessation is critical.
In our initial assessment of the patient, his FEV1 was <50%. There was no need to repeat spirometry, as the evidence does not support ongoing spirometric evaluation.2,3 Symptomatic patients with significant airflow obstruction (FEV1 <60% predicted) are the ones most likely to benefit from therapy (ACP grade: strong recommendation, moderate-quality evidence).2,3 Conversely, there is little evidence to justify treating asymptomatic patients who have airflow obstruction.
Monotherapy with long-acting inhaled beta-agonists, inhaled corticosteroids, or long-acting inhaled anticholinergics has been shown to reduce exacerbations and is preferable to short-acting, inhaled beta-agonists or short-acting anticholinergics (ACP grade: strong recommendation, high-quality evidence).2,3 At this time, evidence is insufficient to support the use of combined therapies—eg, inhaled steroids plus long-acting beta-agonists.2,3
For patients with a PaO2 ≤55 mm Hg, survival is improved by using supplemental oxygen therapy for 15 or more hours a day. (ACP grade: strong recommendation, moderate quality evidence).2,3
Finally, for symptomatic patients with an FEV1 <50%, pulmonary rehabilitation may reduce hospitalizations and increase exercise capacity (ACP grade: weak recommendation, moderate-quality evidence).2,3
For Mr. Jones, we prescribed 1 inhalation daily of the long-acting anticholinergic inhaler, tiotropium.
Smoking cessation critical
COPD progresses with aging and with continued smoking, and smoking cessation is critical to any management strategy.
CASE: Tiotropium, 1 inhalation daily, and a smoking cessation plan
We referred Mr. Jones to an outpatient smoking cessation program and gave him American Academy of Family Physicians patient education materials to review. His exercise tolerance improved with 1 inhalation daily of the long-acting anticholinergic inhaler, tiotropium, and he is making progress in his efforts to quit smoking.
Correspondence
Dean Gianakos, MD, Lynchburg Family Medicine Residency, 2097 Langhorne Road, Lynchburg, VA 24501; [email protected]
1. Rabe KF, Hurd S, Anzueto A, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease (GOLD). Am J Respir Crit Care Med. 2007;176:532-555.
2. Qaseem A, Snow V, Shekelle P, et al. Diagnosis and management of stable chronic obstructive pulmonary disease: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2007;147:633-638.
3. Wilt TJ, Niewoehner D, MacDonald R, et al. Management of stable chronic obstructive pulmonary disease: a systematic review for a clinical practice guideline. Ann Intern Med. 2007;147:639-653.
4. Kesten S, Menjoge S. Patient-reported symptoms of chronic obstructive pulmonary disease in clinical trials. Chest. 2005;128(4):249S.-
5. Pauwels RA, Buist AS, Ma P, et al. GOLD Scientific Committee. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. Respir Care. 2001;46:798-825.
6. Straus SE, McAlister FA, Sackett DL, et al. The accuracy of patient history, wheezing, and laryngeal measurements in diagnosing obstructive airway disease. JAMA. 2000;283:1853-1857.
7. Wise RA, Tashkin DP. Optimizing treatment of chronic obstructive pulmonary disease: an assessment of current therapies. Am J Med. 2007;120(8A):S4-S13.
8. Lin K, Watkins B, Johnson T, et al. Screening for chronic obstructive pulmonary disease using spirometry: summary of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med. 2008;148:535-543.
9. Sundblad BM, Larsson K, Nathell L. Low awareness of COPD among physicians. Clin Respir J. 2007;(1):11-16.
10. Han M, Kim MG, Mardon R, et al. Spirometry utilization for COPD: how do we measure up? Chest. 2007;132:403-409.
11. Kesten S, Chapman K. Physician perceptions and management of COPD. Chest. 1993;104:254-258.
12. Chapman K, Tashkin D, Pye D. Gender bias in the diagnosis of COPD. Chest. 2001;119:1691-1695.
13. Nazir SA, Al-Hamed MM, Erbland ML. Chronic obstructive pulmonary disease in the older patient. Clin Chest Med. 2007;28:703-715.
14. Ram FS, Rodriguez-Roisin R, Granados-Navarrete A, et al. Antibiotics for exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2006;(2):CD004403.
15. Littner M. In the clinic. Chronic obstructive pulmonary disease. Ann Intern Med. 2008;148(5):ITC3-1-ITC3-16.
16. Dewar M, Curry W. Chronic obstructive pulmonary disease: diagnostic considerations. Am Fam Physician. 2006;73:669-678.
17. Kohnlein T, Welte T. Alpha-1 antitrypsin deficiency: pathogenesis, clinical presentation, diagnosis, and treatment. Am J Med. 2008;121:3-9.
1. Rabe KF, Hurd S, Anzueto A, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease (GOLD). Am J Respir Crit Care Med. 2007;176:532-555.
2. Qaseem A, Snow V, Shekelle P, et al. Diagnosis and management of stable chronic obstructive pulmonary disease: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2007;147:633-638.
3. Wilt TJ, Niewoehner D, MacDonald R, et al. Management of stable chronic obstructive pulmonary disease: a systematic review for a clinical practice guideline. Ann Intern Med. 2007;147:639-653.
4. Kesten S, Menjoge S. Patient-reported symptoms of chronic obstructive pulmonary disease in clinical trials. Chest. 2005;128(4):249S.-
5. Pauwels RA, Buist AS, Ma P, et al. GOLD Scientific Committee. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. Respir Care. 2001;46:798-825.
6. Straus SE, McAlister FA, Sackett DL, et al. The accuracy of patient history, wheezing, and laryngeal measurements in diagnosing obstructive airway disease. JAMA. 2000;283:1853-1857.
7. Wise RA, Tashkin DP. Optimizing treatment of chronic obstructive pulmonary disease: an assessment of current therapies. Am J Med. 2007;120(8A):S4-S13.
8. Lin K, Watkins B, Johnson T, et al. Screening for chronic obstructive pulmonary disease using spirometry: summary of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med. 2008;148:535-543.
9. Sundblad BM, Larsson K, Nathell L. Low awareness of COPD among physicians. Clin Respir J. 2007;(1):11-16.
10. Han M, Kim MG, Mardon R, et al. Spirometry utilization for COPD: how do we measure up? Chest. 2007;132:403-409.
11. Kesten S, Chapman K. Physician perceptions and management of COPD. Chest. 1993;104:254-258.
12. Chapman K, Tashkin D, Pye D. Gender bias in the diagnosis of COPD. Chest. 2001;119:1691-1695.
13. Nazir SA, Al-Hamed MM, Erbland ML. Chronic obstructive pulmonary disease in the older patient. Clin Chest Med. 2007;28:703-715.
14. Ram FS, Rodriguez-Roisin R, Granados-Navarrete A, et al. Antibiotics for exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2006;(2):CD004403.
15. Littner M. In the clinic. Chronic obstructive pulmonary disease. Ann Intern Med. 2008;148(5):ITC3-1-ITC3-16.
16. Dewar M, Curry W. Chronic obstructive pulmonary disease: diagnostic considerations. Am Fam Physician. 2006;73:669-678.
17. Kohnlein T, Welte T. Alpha-1 antitrypsin deficiency: pathogenesis, clinical presentation, diagnosis, and treatment. Am J Med. 2008;121:3-9.
Nail disorders and systemic disease: What the nails tell us
Can you name these 2 nail conditions?
What underlying diseases do you suspect are behind these conditions?
If you said onycholysis (left) and red lunula (right), you are correct. As for the underlying diseases: The patient with onycholysis has hyperthyroidism and the patient with red lunula has chronic obstructive pulmonary disease (COPD). Onycholysis and red lunula are among the more common changes to the morphology (shape) and color of the nail—the 2 ways by which nail changes are classified.
Nail abnormalities can be a revealing sign of underlying disease, and because the nails are readily examined, a convenient diagnostic tool, as well.
This review of common—and not so common—nail disorders shows which changes to the nail are more likely to occur with which underlying internal diseases.
Nail anatomy
Nail changes are classified according to whether they occur in the morphology (shape) or color of the nail. Onycholysis, clubbing, and koilonychia are some of the most common changes in the morphology of the nail. Red lunula is one of the most common changes in the color of the nail.
- Amyloid and multiple myeloma
- Anemia
- Bronchiectasis
- Carcinoma (lung)
- Erythropoietic porphyria
- Histiocytosis X
- Ischemia (peripheral)
- Leprosy
- Lupus erythematosus
- Neuritis
- Pellagra
- Pemphigus vulgaris
- Pleural effusion
- Porphyria cutanea tarda
- Psoriatic arthritis
- Reiter’s syndrome
- Scleroderma
- Syphilis (secondary and tertiary)
- Thyroid disease
What you’ll see: Distal separation of the nail plate from the underlying nail bed. Nails with onycholysis are usually smooth, firm, and without nail bed inflammation. It is not a disease of the nail matrix, though nail discoloration may appear underneath the nail as a result of secondary infection.
What to suspect: Onycholysis is associated with many systemic conditions, including thyroid disease—especially hyperthyroidism. (See list at left.) The nail changes seen with hyperthyroidism usually consist of onycholysis beginning in the fourth or fifth nail, the so-called Plummer’s nails.1 Nakatsui and Lin2 have suggested that patients with unexplained onycholysis be screened for asymptomatic thyroid disease.
What you’ll see: Increased transverse and longitudinal nail curvature with fibrovascular hyperplasia of the soft tissue proximal to the cuticle. With clubbing, the Lovibond’s angle, formed between the dorsal surface of the distal phalanx and the nail plate, is greater than 180 degrees. Schamroth’s sign—the disappearance of the normal window between the back surfaces of opposite terminal phalanges—may also be present.3
What to suspect: Clubbing may be hereditary, idiopathic, or acquired in association with a variety of disorders. It may also be unilateral or bilateral. Unilateral clubbing has been associated with hemiplegia and vascular lesions, while bilateral clubbing has been linked to neoplastic, pulmonary, cardiac, gastrointestinal, infectious, endocrine, vascular, and multisystem diseases.
Cribier et al4 studied the frequency of nail disorders in HIV-infected patients and found that clubbing affects 5.8% of these patients. Moreover, Cribier’s data reinforced the notion that clubbing could be an early sign of AIDS in pediatric patients, and thus play a role in diagnosis.
What you’ll see: Concave thin nails with everted edges shaped like a spoon and capable of retaining a drop of water. It is more common in fingernails, but is occasionally seen in toenails.
What to suspect: This nail sign may result from trauma, constant exposure of hands to petroleum-based solvents, or nail-patella syndrome. Koilonychia is most commonly associated with iron deficiency anemia and occasionally occurs in patients with hemochromatosis. Other frequent systemic causes of koilonychia include coronary disease and hypothyroidism.5 In addition, koilonychia is sometimes a normal variant in infants; it usually disappears in the first few years of life.
What you’ll see: Proximal separation of the nail plate from the nail bed. This typically results in shedding of the nail.
What to suspect: Trauma is the usual cause. Less common causes include poor nutritional status, febrile illness, or drug sensitivity.
Wester et al6 observed the development of onychomadesis in a critically ill patient with a large pulmonary abscess. Onychomadesis is often a clinical manifestation of pemphigus vulgaris.7 It has also been associated with Kawasaki disease8 and hand, foot, and mouth disease.9
What you’ll see: Transverse depressions in the nail plate that occur as a result of a temporary cessation in nail growth.
What to suspect: The causes are similar to those of onychomadesis and include trauma, poor nutritional status, febrile illness, and drug sensitivity.
What you’ll see: Pinpoint (or larger) depressions in an otherwise normal nail.
What to suspect: Pitting is usually associated with psoriasis and affects 10% to 15% of patients with the disorder.10 Pitting has also been reported in patients with Reiter’s syndrome (and other connective tissue disorders), sarcoidosis, pemphigus, alopecia areata, and incontinentia pigmenti.5
What you’ll see: Transverse white bands parallel to the lunula. These bands usually occur in pairs and extend all the way across the nail.
This nail disorder is uncommon, and is 1 of 3 forms of leukonychia caused by abnormalities in nail bed vascularization. (The other 2 forms—Terry’s nails and half-and-half nails—are described on page 513.)
What to suspect: Muehrcke’s nails appear in patients with hypoalbuminemia and can improve if serum albumin levels return to normal. They may also occur in patients with:11,12
- nephrotic syndrome,
- glomerulonephritis,
- liver disease,
- malnutrition, and
- those who have undergone chemotherapy.
Muehrcke’s lines have also been described in a patient with Peutz-Jeghers syndrome,13 as well as in a heart transplant recipient.14
What you’ll see: Most of the nail plate is white, with a narrow pink distal band. All nails tend to be uniformly affected, with an appearance of ground glass.15 Terry’s nails have been found in 80% of patients with cirrhosis of the liver.15
What to suspect: One study found Terry’s nails in 25% of 512 consecutive hospital inpatients, with researchers linking the disorder with cirrhosis, chronic CHF, and adult-onset diabetes mellitus.16 On rare occasions, Terry’s nails have been reported in hemodialysis patients and renal transplant recipients.17 Terry’s nails have also been observed in HIV patients.4
Half-and-half nails (Lindsay’s nails)
What you’ll see: The proximal portion on the nail bed is white because of edema of the nail bed and capillary network; the distal portion is pink or reddish brown. The nail plate is unaffected.
What to suspect: This nail disorder has occurred in patients with renal disease associated with azotemia.18 Half-and-half nails have also been detected in hemodialysis patients, renal transplant recipients,17 and in HIV patients.4
What you’ll see: The lunula is red. In addition to the red lunula pictured here, there is also the absence of lunula and azure lunula.
What to suspect: Red lunula has been associated with alopecia areata, and collagen vascular disease. It has also occurred in patients on oral prednisone for rheumatoid arthritis. Red lunulae are seen in cardiac failure, COPD, cirrhosis, chronic urticaria, psoriasis, and carbon monoxide poisoning.19
Absence of lunula was the most common nail disorder in a group of hemodialysis patients (31.9%) and has also been reported in renal transplant recipients (17.1%).17 Azure lunula occurs in patients with Wilson disease. It has also occurred in argyria and in patients taking medications like 5-fluorouracil and azidothymidine.20
What you’ll see: Extravasations of blood from the longitudinally oriented vessels of the nail bed. These hemorrhages do not blanch. They form as a result of the nail plate-dermis structural relationship and tend to be seen in older patients.
What to suspect: While trauma is the most common cause, they may also occur with psoriasis and fungal infection.
Bacterial endocarditis is the most common systemic disease associated with splinter hemorrhages. These hemorrhages are more common in subacute, rather than acute, infection. Although splinter hemorrhages in subacute bacterial endocarditis have been described as proximally located,21 there are no sufficient data to confirm this—mainly because splinter lesions migrate distally as the nail grows.22
Splinter hemorrhages may also be associated with mitral stenosis, vasculitis, cirrhosis, trichinosis, scurvy, chronic glomerulonephritis, and Darier’s disease. However, due to the diverse and common causes of splinter hemorrhages, they cannot be used as an isolated sign of illness, except when they are accompanied by things like fever, Roth’s spots, Osler’s nodes, Janeway’s lesions, or a heart murmur, since any of the above would greatly increase their significance.
- Systemic disease typically affects more than 1 nail.5,23
- Fingernails usually provide more accurate information than toenails because clinical signs on toenails are often modified by trauma.23
- Fingernails grow at a rate of 0.1 mm/day and toenails grow at a rate of 0.03 mm/day.5,23 Thus, you can estimate the time at which an initial insult occurred by measuring the distance between the cuticle and the leading edge of any pigmentation change.
Correspondence
Dimitris Rigopoulos, assistant professor of dermatology, University of Athens Medical School, 5 Ionos Dragoumi Street, 16121 Athens, Greece; [email protected].
1. Jabbour SA. Cutaneous manifestations of endocrine disorders: a guide for dermatologists. Am J Clin Dermatol. 2003;4:315-331.
2. Nakatsui T, Lin AN. Onycholysis and thyroid disease: report of three cases. J Cutan Med Surg. 1998;3:40-42.
3. Spicknall KE, Zirwas MJ, English JC, III. Clubbing: an update on diagnosis, differential diagnosis, pathophysiology, and clinical relevance. J Am Acad Dermatol. 2005;52:1020-1028.
4. Cribier B, Mena ML, Rey D, et al. Nail changes in patients infected with human immunodeficiency virus. A prospective controlled study. Arch Dermatol. 1998;134:1216-1220.
5. Zaiac MN, Daniel CR, III. Nails in systemic disease. Dermatol Ther. 2002;5:99-106.
6. Wester JP, van Eps RS, Stouthamer A, Girbes AR. Critical illness onychomadesis. Intensive Care Med. 2000;26:1698-700.
7. Engineer L, Norton LA, Ahmed AR. Nail involvement in pemphigus vulgaris. J Am Acad Dermatol. 2000;43:529-535.
8. Ciastko AR. Onychomadesis and Kawasaki disease. CMAJ. 2002;166:1069.-
9. Clementz GC, Mancini AJ. Nail matrix arrest following hand-foot-mouth disease: a report of five children. Pediatr Dermatol. 2000;17(1):7-11.
10. Mayeaux EJ, Jr. Nail disorders. Prim Care. 2000;27:333-351.
11. Muehrcke RC. The fingernails in chronic hypoalbuminemia. BMJ. 1956;1:1327.-
12. D’Alessandro A, Muzi G, Monaco A, Filiberto S, Barboni A, Abbritti G. Yellow nail syndrome: does protein leakage play a role? Eur Respir J. 2001;87:5435-5441.
13. Skoog S, Boardman L. Muehrcke’s nails in Peutz-Jeghers syndrome with hepatic adenoma. Clin Gastroenterol Hepatol. 2004;2:XXIV.-
14. Nabai H. Nail changes before and after heart transplantation: personal observation by a physician. Cutis. 1998;61:31-32.
15. Dupont AS, Magy N, Humbert P, Dupond JL. Nail manifestations of systemic diseases. Rev Prat. 2000;50:2236-2240.
16. Holzberg M, Walker HK. Terry’s nails: revised definition and new correlations. Lancet. 1984;2:896.-
17. Saray Y, Seckin D, Gulec AT, Akgun S, Haberal M. Nail disorders in hemodialysis patients and renal transplant recipients: a case-control study. J Am Acad Dermatol. 2004;50:197-202.
18. Dyachenko P, Monselise A, Shustak A, et al. Nail disorders in patients with chronic renal failure and undergoing haemodialysis treatment: a case control study. J Eur Acad Dermatol Venereol. 2007;21:340-344.
19. Cohen PR. Red lunulae: case report and literature review. J AM Acad Dermatol. 1992;26:292.-
20. Tanner LS, Gross DJ. Generalized argyria. Cutis. 1990;45:237.-
21. Saccente M, Cobbs CG. Clinical approach to infective endocarditis. Cardio Clin. 1996;14:351-362.
22. Swartz MN, Weiburg AN. Infections due to gram-positive bacteria. In: Fitzpatrick TB, Elsen AZ, Wolff K, Freedberg IM, Austen KF, eds. Dermatology in General Medicine. 4th ed. New York: McGraw-Hill;1993:2309-2334.
23. Lawry M, Daniel CR. Nails in systemic disease. In: Scher RK, Daniel CR III, eds. Nails: Diagnosis, Therapy, Surgery. 3rd ed. Philadelphia: Elsevier Saunders; 2005:147-176.
Can you name these 2 nail conditions?
What underlying diseases do you suspect are behind these conditions?
If you said onycholysis (left) and red lunula (right), you are correct. As for the underlying diseases: The patient with onycholysis has hyperthyroidism and the patient with red lunula has chronic obstructive pulmonary disease (COPD). Onycholysis and red lunula are among the more common changes to the morphology (shape) and color of the nail—the 2 ways by which nail changes are classified.
Nail abnormalities can be a revealing sign of underlying disease, and because the nails are readily examined, a convenient diagnostic tool, as well.
This review of common—and not so common—nail disorders shows which changes to the nail are more likely to occur with which underlying internal diseases.
Nail anatomy
Nail changes are classified according to whether they occur in the morphology (shape) or color of the nail. Onycholysis, clubbing, and koilonychia are some of the most common changes in the morphology of the nail. Red lunula is one of the most common changes in the color of the nail.
- Amyloid and multiple myeloma
- Anemia
- Bronchiectasis
- Carcinoma (lung)
- Erythropoietic porphyria
- Histiocytosis X
- Ischemia (peripheral)
- Leprosy
- Lupus erythematosus
- Neuritis
- Pellagra
- Pemphigus vulgaris
- Pleural effusion
- Porphyria cutanea tarda
- Psoriatic arthritis
- Reiter’s syndrome
- Scleroderma
- Syphilis (secondary and tertiary)
- Thyroid disease
What you’ll see: Distal separation of the nail plate from the underlying nail bed. Nails with onycholysis are usually smooth, firm, and without nail bed inflammation. It is not a disease of the nail matrix, though nail discoloration may appear underneath the nail as a result of secondary infection.
What to suspect: Onycholysis is associated with many systemic conditions, including thyroid disease—especially hyperthyroidism. (See list at left.) The nail changes seen with hyperthyroidism usually consist of onycholysis beginning in the fourth or fifth nail, the so-called Plummer’s nails.1 Nakatsui and Lin2 have suggested that patients with unexplained onycholysis be screened for asymptomatic thyroid disease.
What you’ll see: Increased transverse and longitudinal nail curvature with fibrovascular hyperplasia of the soft tissue proximal to the cuticle. With clubbing, the Lovibond’s angle, formed between the dorsal surface of the distal phalanx and the nail plate, is greater than 180 degrees. Schamroth’s sign—the disappearance of the normal window between the back surfaces of opposite terminal phalanges—may also be present.3
What to suspect: Clubbing may be hereditary, idiopathic, or acquired in association with a variety of disorders. It may also be unilateral or bilateral. Unilateral clubbing has been associated with hemiplegia and vascular lesions, while bilateral clubbing has been linked to neoplastic, pulmonary, cardiac, gastrointestinal, infectious, endocrine, vascular, and multisystem diseases.
Cribier et al4 studied the frequency of nail disorders in HIV-infected patients and found that clubbing affects 5.8% of these patients. Moreover, Cribier’s data reinforced the notion that clubbing could be an early sign of AIDS in pediatric patients, and thus play a role in diagnosis.
What you’ll see: Concave thin nails with everted edges shaped like a spoon and capable of retaining a drop of water. It is more common in fingernails, but is occasionally seen in toenails.
What to suspect: This nail sign may result from trauma, constant exposure of hands to petroleum-based solvents, or nail-patella syndrome. Koilonychia is most commonly associated with iron deficiency anemia and occasionally occurs in patients with hemochromatosis. Other frequent systemic causes of koilonychia include coronary disease and hypothyroidism.5 In addition, koilonychia is sometimes a normal variant in infants; it usually disappears in the first few years of life.
What you’ll see: Proximal separation of the nail plate from the nail bed. This typically results in shedding of the nail.
What to suspect: Trauma is the usual cause. Less common causes include poor nutritional status, febrile illness, or drug sensitivity.
Wester et al6 observed the development of onychomadesis in a critically ill patient with a large pulmonary abscess. Onychomadesis is often a clinical manifestation of pemphigus vulgaris.7 It has also been associated with Kawasaki disease8 and hand, foot, and mouth disease.9
What you’ll see: Transverse depressions in the nail plate that occur as a result of a temporary cessation in nail growth.
What to suspect: The causes are similar to those of onychomadesis and include trauma, poor nutritional status, febrile illness, and drug sensitivity.
What you’ll see: Pinpoint (or larger) depressions in an otherwise normal nail.
What to suspect: Pitting is usually associated with psoriasis and affects 10% to 15% of patients with the disorder.10 Pitting has also been reported in patients with Reiter’s syndrome (and other connective tissue disorders), sarcoidosis, pemphigus, alopecia areata, and incontinentia pigmenti.5
What you’ll see: Transverse white bands parallel to the lunula. These bands usually occur in pairs and extend all the way across the nail.
This nail disorder is uncommon, and is 1 of 3 forms of leukonychia caused by abnormalities in nail bed vascularization. (The other 2 forms—Terry’s nails and half-and-half nails—are described on page 513.)
What to suspect: Muehrcke’s nails appear in patients with hypoalbuminemia and can improve if serum albumin levels return to normal. They may also occur in patients with:11,12
- nephrotic syndrome,
- glomerulonephritis,
- liver disease,
- malnutrition, and
- those who have undergone chemotherapy.
Muehrcke’s lines have also been described in a patient with Peutz-Jeghers syndrome,13 as well as in a heart transplant recipient.14
What you’ll see: Most of the nail plate is white, with a narrow pink distal band. All nails tend to be uniformly affected, with an appearance of ground glass.15 Terry’s nails have been found in 80% of patients with cirrhosis of the liver.15
What to suspect: One study found Terry’s nails in 25% of 512 consecutive hospital inpatients, with researchers linking the disorder with cirrhosis, chronic CHF, and adult-onset diabetes mellitus.16 On rare occasions, Terry’s nails have been reported in hemodialysis patients and renal transplant recipients.17 Terry’s nails have also been observed in HIV patients.4
Half-and-half nails (Lindsay’s nails)
What you’ll see: The proximal portion on the nail bed is white because of edema of the nail bed and capillary network; the distal portion is pink or reddish brown. The nail plate is unaffected.
What to suspect: This nail disorder has occurred in patients with renal disease associated with azotemia.18 Half-and-half nails have also been detected in hemodialysis patients, renal transplant recipients,17 and in HIV patients.4
What you’ll see: The lunula is red. In addition to the red lunula pictured here, there is also the absence of lunula and azure lunula.
What to suspect: Red lunula has been associated with alopecia areata, and collagen vascular disease. It has also occurred in patients on oral prednisone for rheumatoid arthritis. Red lunulae are seen in cardiac failure, COPD, cirrhosis, chronic urticaria, psoriasis, and carbon monoxide poisoning.19
Absence of lunula was the most common nail disorder in a group of hemodialysis patients (31.9%) and has also been reported in renal transplant recipients (17.1%).17 Azure lunula occurs in patients with Wilson disease. It has also occurred in argyria and in patients taking medications like 5-fluorouracil and azidothymidine.20
What you’ll see: Extravasations of blood from the longitudinally oriented vessels of the nail bed. These hemorrhages do not blanch. They form as a result of the nail plate-dermis structural relationship and tend to be seen in older patients.
What to suspect: While trauma is the most common cause, they may also occur with psoriasis and fungal infection.
Bacterial endocarditis is the most common systemic disease associated with splinter hemorrhages. These hemorrhages are more common in subacute, rather than acute, infection. Although splinter hemorrhages in subacute bacterial endocarditis have been described as proximally located,21 there are no sufficient data to confirm this—mainly because splinter lesions migrate distally as the nail grows.22
Splinter hemorrhages may also be associated with mitral stenosis, vasculitis, cirrhosis, trichinosis, scurvy, chronic glomerulonephritis, and Darier’s disease. However, due to the diverse and common causes of splinter hemorrhages, they cannot be used as an isolated sign of illness, except when they are accompanied by things like fever, Roth’s spots, Osler’s nodes, Janeway’s lesions, or a heart murmur, since any of the above would greatly increase their significance.
- Systemic disease typically affects more than 1 nail.5,23
- Fingernails usually provide more accurate information than toenails because clinical signs on toenails are often modified by trauma.23
- Fingernails grow at a rate of 0.1 mm/day and toenails grow at a rate of 0.03 mm/day.5,23 Thus, you can estimate the time at which an initial insult occurred by measuring the distance between the cuticle and the leading edge of any pigmentation change.
Correspondence
Dimitris Rigopoulos, assistant professor of dermatology, University of Athens Medical School, 5 Ionos Dragoumi Street, 16121 Athens, Greece; [email protected].
Can you name these 2 nail conditions?
What underlying diseases do you suspect are behind these conditions?
If you said onycholysis (left) and red lunula (right), you are correct. As for the underlying diseases: The patient with onycholysis has hyperthyroidism and the patient with red lunula has chronic obstructive pulmonary disease (COPD). Onycholysis and red lunula are among the more common changes to the morphology (shape) and color of the nail—the 2 ways by which nail changes are classified.
Nail abnormalities can be a revealing sign of underlying disease, and because the nails are readily examined, a convenient diagnostic tool, as well.
This review of common—and not so common—nail disorders shows which changes to the nail are more likely to occur with which underlying internal diseases.
Nail anatomy
Nail changes are classified according to whether they occur in the morphology (shape) or color of the nail. Onycholysis, clubbing, and koilonychia are some of the most common changes in the morphology of the nail. Red lunula is one of the most common changes in the color of the nail.
- Amyloid and multiple myeloma
- Anemia
- Bronchiectasis
- Carcinoma (lung)
- Erythropoietic porphyria
- Histiocytosis X
- Ischemia (peripheral)
- Leprosy
- Lupus erythematosus
- Neuritis
- Pellagra
- Pemphigus vulgaris
- Pleural effusion
- Porphyria cutanea tarda
- Psoriatic arthritis
- Reiter’s syndrome
- Scleroderma
- Syphilis (secondary and tertiary)
- Thyroid disease
What you’ll see: Distal separation of the nail plate from the underlying nail bed. Nails with onycholysis are usually smooth, firm, and without nail bed inflammation. It is not a disease of the nail matrix, though nail discoloration may appear underneath the nail as a result of secondary infection.
What to suspect: Onycholysis is associated with many systemic conditions, including thyroid disease—especially hyperthyroidism. (See list at left.) The nail changes seen with hyperthyroidism usually consist of onycholysis beginning in the fourth or fifth nail, the so-called Plummer’s nails.1 Nakatsui and Lin2 have suggested that patients with unexplained onycholysis be screened for asymptomatic thyroid disease.
What you’ll see: Increased transverse and longitudinal nail curvature with fibrovascular hyperplasia of the soft tissue proximal to the cuticle. With clubbing, the Lovibond’s angle, formed between the dorsal surface of the distal phalanx and the nail plate, is greater than 180 degrees. Schamroth’s sign—the disappearance of the normal window between the back surfaces of opposite terminal phalanges—may also be present.3
What to suspect: Clubbing may be hereditary, idiopathic, or acquired in association with a variety of disorders. It may also be unilateral or bilateral. Unilateral clubbing has been associated with hemiplegia and vascular lesions, while bilateral clubbing has been linked to neoplastic, pulmonary, cardiac, gastrointestinal, infectious, endocrine, vascular, and multisystem diseases.
Cribier et al4 studied the frequency of nail disorders in HIV-infected patients and found that clubbing affects 5.8% of these patients. Moreover, Cribier’s data reinforced the notion that clubbing could be an early sign of AIDS in pediatric patients, and thus play a role in diagnosis.
What you’ll see: Concave thin nails with everted edges shaped like a spoon and capable of retaining a drop of water. It is more common in fingernails, but is occasionally seen in toenails.
What to suspect: This nail sign may result from trauma, constant exposure of hands to petroleum-based solvents, or nail-patella syndrome. Koilonychia is most commonly associated with iron deficiency anemia and occasionally occurs in patients with hemochromatosis. Other frequent systemic causes of koilonychia include coronary disease and hypothyroidism.5 In addition, koilonychia is sometimes a normal variant in infants; it usually disappears in the first few years of life.
What you’ll see: Proximal separation of the nail plate from the nail bed. This typically results in shedding of the nail.
What to suspect: Trauma is the usual cause. Less common causes include poor nutritional status, febrile illness, or drug sensitivity.
Wester et al6 observed the development of onychomadesis in a critically ill patient with a large pulmonary abscess. Onychomadesis is often a clinical manifestation of pemphigus vulgaris.7 It has also been associated with Kawasaki disease8 and hand, foot, and mouth disease.9
What you’ll see: Transverse depressions in the nail plate that occur as a result of a temporary cessation in nail growth.
What to suspect: The causes are similar to those of onychomadesis and include trauma, poor nutritional status, febrile illness, and drug sensitivity.
What you’ll see: Pinpoint (or larger) depressions in an otherwise normal nail.
What to suspect: Pitting is usually associated with psoriasis and affects 10% to 15% of patients with the disorder.10 Pitting has also been reported in patients with Reiter’s syndrome (and other connective tissue disorders), sarcoidosis, pemphigus, alopecia areata, and incontinentia pigmenti.5
What you’ll see: Transverse white bands parallel to the lunula. These bands usually occur in pairs and extend all the way across the nail.
This nail disorder is uncommon, and is 1 of 3 forms of leukonychia caused by abnormalities in nail bed vascularization. (The other 2 forms—Terry’s nails and half-and-half nails—are described on page 513.)
What to suspect: Muehrcke’s nails appear in patients with hypoalbuminemia and can improve if serum albumin levels return to normal. They may also occur in patients with:11,12
- nephrotic syndrome,
- glomerulonephritis,
- liver disease,
- malnutrition, and
- those who have undergone chemotherapy.
Muehrcke’s lines have also been described in a patient with Peutz-Jeghers syndrome,13 as well as in a heart transplant recipient.14
What you’ll see: Most of the nail plate is white, with a narrow pink distal band. All nails tend to be uniformly affected, with an appearance of ground glass.15 Terry’s nails have been found in 80% of patients with cirrhosis of the liver.15
What to suspect: One study found Terry’s nails in 25% of 512 consecutive hospital inpatients, with researchers linking the disorder with cirrhosis, chronic CHF, and adult-onset diabetes mellitus.16 On rare occasions, Terry’s nails have been reported in hemodialysis patients and renal transplant recipients.17 Terry’s nails have also been observed in HIV patients.4
Half-and-half nails (Lindsay’s nails)
What you’ll see: The proximal portion on the nail bed is white because of edema of the nail bed and capillary network; the distal portion is pink or reddish brown. The nail plate is unaffected.
What to suspect: This nail disorder has occurred in patients with renal disease associated with azotemia.18 Half-and-half nails have also been detected in hemodialysis patients, renal transplant recipients,17 and in HIV patients.4
What you’ll see: The lunula is red. In addition to the red lunula pictured here, there is also the absence of lunula and azure lunula.
What to suspect: Red lunula has been associated with alopecia areata, and collagen vascular disease. It has also occurred in patients on oral prednisone for rheumatoid arthritis. Red lunulae are seen in cardiac failure, COPD, cirrhosis, chronic urticaria, psoriasis, and carbon monoxide poisoning.19
Absence of lunula was the most common nail disorder in a group of hemodialysis patients (31.9%) and has also been reported in renal transplant recipients (17.1%).17 Azure lunula occurs in patients with Wilson disease. It has also occurred in argyria and in patients taking medications like 5-fluorouracil and azidothymidine.20
What you’ll see: Extravasations of blood from the longitudinally oriented vessels of the nail bed. These hemorrhages do not blanch. They form as a result of the nail plate-dermis structural relationship and tend to be seen in older patients.
What to suspect: While trauma is the most common cause, they may also occur with psoriasis and fungal infection.
Bacterial endocarditis is the most common systemic disease associated with splinter hemorrhages. These hemorrhages are more common in subacute, rather than acute, infection. Although splinter hemorrhages in subacute bacterial endocarditis have been described as proximally located,21 there are no sufficient data to confirm this—mainly because splinter lesions migrate distally as the nail grows.22
Splinter hemorrhages may also be associated with mitral stenosis, vasculitis, cirrhosis, trichinosis, scurvy, chronic glomerulonephritis, and Darier’s disease. However, due to the diverse and common causes of splinter hemorrhages, they cannot be used as an isolated sign of illness, except when they are accompanied by things like fever, Roth’s spots, Osler’s nodes, Janeway’s lesions, or a heart murmur, since any of the above would greatly increase their significance.
- Systemic disease typically affects more than 1 nail.5,23
- Fingernails usually provide more accurate information than toenails because clinical signs on toenails are often modified by trauma.23
- Fingernails grow at a rate of 0.1 mm/day and toenails grow at a rate of 0.03 mm/day.5,23 Thus, you can estimate the time at which an initial insult occurred by measuring the distance between the cuticle and the leading edge of any pigmentation change.
Correspondence
Dimitris Rigopoulos, assistant professor of dermatology, University of Athens Medical School, 5 Ionos Dragoumi Street, 16121 Athens, Greece; [email protected].
1. Jabbour SA. Cutaneous manifestations of endocrine disorders: a guide for dermatologists. Am J Clin Dermatol. 2003;4:315-331.
2. Nakatsui T, Lin AN. Onycholysis and thyroid disease: report of three cases. J Cutan Med Surg. 1998;3:40-42.
3. Spicknall KE, Zirwas MJ, English JC, III. Clubbing: an update on diagnosis, differential diagnosis, pathophysiology, and clinical relevance. J Am Acad Dermatol. 2005;52:1020-1028.
4. Cribier B, Mena ML, Rey D, et al. Nail changes in patients infected with human immunodeficiency virus. A prospective controlled study. Arch Dermatol. 1998;134:1216-1220.
5. Zaiac MN, Daniel CR, III. Nails in systemic disease. Dermatol Ther. 2002;5:99-106.
6. Wester JP, van Eps RS, Stouthamer A, Girbes AR. Critical illness onychomadesis. Intensive Care Med. 2000;26:1698-700.
7. Engineer L, Norton LA, Ahmed AR. Nail involvement in pemphigus vulgaris. J Am Acad Dermatol. 2000;43:529-535.
8. Ciastko AR. Onychomadesis and Kawasaki disease. CMAJ. 2002;166:1069.-
9. Clementz GC, Mancini AJ. Nail matrix arrest following hand-foot-mouth disease: a report of five children. Pediatr Dermatol. 2000;17(1):7-11.
10. Mayeaux EJ, Jr. Nail disorders. Prim Care. 2000;27:333-351.
11. Muehrcke RC. The fingernails in chronic hypoalbuminemia. BMJ. 1956;1:1327.-
12. D’Alessandro A, Muzi G, Monaco A, Filiberto S, Barboni A, Abbritti G. Yellow nail syndrome: does protein leakage play a role? Eur Respir J. 2001;87:5435-5441.
13. Skoog S, Boardman L. Muehrcke’s nails in Peutz-Jeghers syndrome with hepatic adenoma. Clin Gastroenterol Hepatol. 2004;2:XXIV.-
14. Nabai H. Nail changes before and after heart transplantation: personal observation by a physician. Cutis. 1998;61:31-32.
15. Dupont AS, Magy N, Humbert P, Dupond JL. Nail manifestations of systemic diseases. Rev Prat. 2000;50:2236-2240.
16. Holzberg M, Walker HK. Terry’s nails: revised definition and new correlations. Lancet. 1984;2:896.-
17. Saray Y, Seckin D, Gulec AT, Akgun S, Haberal M. Nail disorders in hemodialysis patients and renal transplant recipients: a case-control study. J Am Acad Dermatol. 2004;50:197-202.
18. Dyachenko P, Monselise A, Shustak A, et al. Nail disorders in patients with chronic renal failure and undergoing haemodialysis treatment: a case control study. J Eur Acad Dermatol Venereol. 2007;21:340-344.
19. Cohen PR. Red lunulae: case report and literature review. J AM Acad Dermatol. 1992;26:292.-
20. Tanner LS, Gross DJ. Generalized argyria. Cutis. 1990;45:237.-
21. Saccente M, Cobbs CG. Clinical approach to infective endocarditis. Cardio Clin. 1996;14:351-362.
22. Swartz MN, Weiburg AN. Infections due to gram-positive bacteria. In: Fitzpatrick TB, Elsen AZ, Wolff K, Freedberg IM, Austen KF, eds. Dermatology in General Medicine. 4th ed. New York: McGraw-Hill;1993:2309-2334.
23. Lawry M, Daniel CR. Nails in systemic disease. In: Scher RK, Daniel CR III, eds. Nails: Diagnosis, Therapy, Surgery. 3rd ed. Philadelphia: Elsevier Saunders; 2005:147-176.
1. Jabbour SA. Cutaneous manifestations of endocrine disorders: a guide for dermatologists. Am J Clin Dermatol. 2003;4:315-331.
2. Nakatsui T, Lin AN. Onycholysis and thyroid disease: report of three cases. J Cutan Med Surg. 1998;3:40-42.
3. Spicknall KE, Zirwas MJ, English JC, III. Clubbing: an update on diagnosis, differential diagnosis, pathophysiology, and clinical relevance. J Am Acad Dermatol. 2005;52:1020-1028.
4. Cribier B, Mena ML, Rey D, et al. Nail changes in patients infected with human immunodeficiency virus. A prospective controlled study. Arch Dermatol. 1998;134:1216-1220.
5. Zaiac MN, Daniel CR, III. Nails in systemic disease. Dermatol Ther. 2002;5:99-106.
6. Wester JP, van Eps RS, Stouthamer A, Girbes AR. Critical illness onychomadesis. Intensive Care Med. 2000;26:1698-700.
7. Engineer L, Norton LA, Ahmed AR. Nail involvement in pemphigus vulgaris. J Am Acad Dermatol. 2000;43:529-535.
8. Ciastko AR. Onychomadesis and Kawasaki disease. CMAJ. 2002;166:1069.-
9. Clementz GC, Mancini AJ. Nail matrix arrest following hand-foot-mouth disease: a report of five children. Pediatr Dermatol. 2000;17(1):7-11.
10. Mayeaux EJ, Jr. Nail disorders. Prim Care. 2000;27:333-351.
11. Muehrcke RC. The fingernails in chronic hypoalbuminemia. BMJ. 1956;1:1327.-
12. D’Alessandro A, Muzi G, Monaco A, Filiberto S, Barboni A, Abbritti G. Yellow nail syndrome: does protein leakage play a role? Eur Respir J. 2001;87:5435-5441.
13. Skoog S, Boardman L. Muehrcke’s nails in Peutz-Jeghers syndrome with hepatic adenoma. Clin Gastroenterol Hepatol. 2004;2:XXIV.-
14. Nabai H. Nail changes before and after heart transplantation: personal observation by a physician. Cutis. 1998;61:31-32.
15. Dupont AS, Magy N, Humbert P, Dupond JL. Nail manifestations of systemic diseases. Rev Prat. 2000;50:2236-2240.
16. Holzberg M, Walker HK. Terry’s nails: revised definition and new correlations. Lancet. 1984;2:896.-
17. Saray Y, Seckin D, Gulec AT, Akgun S, Haberal M. Nail disorders in hemodialysis patients and renal transplant recipients: a case-control study. J Am Acad Dermatol. 2004;50:197-202.
18. Dyachenko P, Monselise A, Shustak A, et al. Nail disorders in patients with chronic renal failure and undergoing haemodialysis treatment: a case control study. J Eur Acad Dermatol Venereol. 2007;21:340-344.
19. Cohen PR. Red lunulae: case report and literature review. J AM Acad Dermatol. 1992;26:292.-
20. Tanner LS, Gross DJ. Generalized argyria. Cutis. 1990;45:237.-
21. Saccente M, Cobbs CG. Clinical approach to infective endocarditis. Cardio Clin. 1996;14:351-362.
22. Swartz MN, Weiburg AN. Infections due to gram-positive bacteria. In: Fitzpatrick TB, Elsen AZ, Wolff K, Freedberg IM, Austen KF, eds. Dermatology in General Medicine. 4th ed. New York: McGraw-Hill;1993:2309-2334.
23. Lawry M, Daniel CR. Nails in systemic disease. In: Scher RK, Daniel CR III, eds. Nails: Diagnosis, Therapy, Surgery. 3rd ed. Philadelphia: Elsevier Saunders; 2005:147-176.
Benign prostatic hyperplasia: Treat or wait?
- Talk to every male patient over the age of 50 about urinary function (C).
- Utilize questionnaires, such as the International Prostate Symptom Score to evaluate the patient's perception of symptom severity and quality of life (A).
- Rule out potential causes of lower urinary tract symptoms with a thorough medical history, focused physical exam (including digital rectal examination and neurological assessments), and appropriate laboratory evaluations (C).
- When choosing treatment for benign prostatic hyperplasia, remember that quality of life is generally more important than symptom severity (A).
Strength of recommendation (SOR)
- Good quality patient-oriented evidence
- Inconsistent or limited-quality patient-oriented evidence
- Consensus, usual practice, opinion, disease-oriented evidence, case series
"My wife is mad at me—and she's worried, too," says Dan, a 65-year-old patient of yours. "She's been telling me to come see you, but I've been putting it off.
"I've been getting up 4 and 5 times a night to urinate, and we can't drive an hour without me having to stop at least once to use a restroom."
With a deep sigh, Dan says: "My wife is worried that I have cancer or something."
"And I'm worried, too," he admits.
Benign prostatic hyperplasia (BPH), and its clinical expression as lower urinary tract symptoms—urinary frequency, urgency, nocturia, decreased force of stream, and incomplete bladder emptying—comprise a major health concern for many older men. Approximately 50% of men over age 60 have at least microscopic BPH, while 90% over age 90 have evidence of the abnormality.1
Many men fail to seek help for lower urinary tract symptoms associated with BPH,2-4 even though these often moderate to severe symptoms are associated with decreased quality of life, anxiety, and depression.5 Your patient may be uncomfortable broaching the subject, as Dan was, for fear that he may have cancer. He may dismiss the symptoms as a natural consequence of aging,6 or he may believe that there are no effective treatments or that treatment will cause unwanted side effects.
Bring up the subject with all men over 50
To dispel these misconceptions and ensure that there are no current or ensuing serious complications,4 you should routinely talk about urinary function with every male patient over age 50. Because the incidence of BPH increases not only with age but also with other comorbid conditions such as diabetes7 and erectile dysfunction (ED),8 you should discuss the symptoms and potential complications of BPH with patients who present with these comorbidities. You can reassure them that BPH is not cancer, nor is it a precursor to prostate cancer; rather it is a fairly common, treatable disorder.
What's right for your patient? Watchful waiting? a-Blocker therapy? Surgery?
Questionnaire can help, addresses quality of life
Questionnaires such as the International Prostate Symptom Score (IPSS) (PATIENT HANDOUT)9 and the similar American Urological Association symptom index (AUA-SI) (available on page 44 of http://www.auanet.org/guidelines/main_reports/bph_management/chapt_1_appendix.pdf) can help you evaluate your patient's symptom severity.2,6
The IPSS, with 3 categories of symptom severity (mild 0 to 7, moderate 8 to 19, severe 20 to 35) and a global quality-of-life question also referred to as the "Bother Score," is a validated tool for monitoring disease distress and clinical change.10,11 The quality-of-life question is a good indicator for assessing whether watchful waiting might be preferred to active treatment.9,12
Further categorizing the symptoms is not helpful. Lower urinary tract symptoms have traditionally been divided into irritative symptoms such as nocturia, urgency, and frequency, attributed to bladder and prostatic smooth muscle contractions, and obstructive symptoms such as hesitancy, decreased force of stream, and incomplete emptying, attributed to increased glandular mass.1 This distinction, however, is not helpful inasmuch as irritative symptoms can result from increased tissue mass alone and obstructive symptoms from muscle hypertonicity alone; additionally, most BPH patients have a combination of both.13,14
Consider comorbidities and overactive bladder
Common comorbidities for a patient with BPH include obesity, diabetes mellitus, and low high-density lipoprotein levels. Both irritative and obstructive symptoms are likely, without prior lower urinary tract disorders or ongoing neurological disease.13-15 Multiple epidemiological studies have established clear, clinically relevant associations between BPH-related lower urinary tract symptoms and ED and ejaculatory dysfunction.8
It is also important to note that lower urinary tract symptoms may often arise due to overactive bladder; in fact, symptoms of overactive bladder and BPH overlap to a large degree.16 The diagnostic challenge is only increased by the fact that while overactive bladder is an additional cause of lower urinary tract symptoms, it may also coexist with BPH-related bladder outlet obstruction.17 The similarity in clinical presentation of the 2 conditions may make them hard to distinguish.
Rule out infection, urinary tract stones
There is general agreement about the need to exclude other potential etiologies of lower urinary tract symptoms in older men.4,15 Thus, you need to consider such causes as urinary tract stones, infections, or cancer; comorbid conditions that may affect bladder function or lead to polyuria; drug side effects; or sleep disturbances associated with chronic insomnia, depression, ethanol abuse, or sleep apnea.13,14
Digital rectal exam (DRE). The physical exam should include both a DRE and a search for neurological deficits to look for evidence that lower urinary tract symptoms are not BPH-related. The DRE should assess for stool impaction and prostate symmetry, nodularity, and consistency. Prostate volume estimates by DRE are not reliable and generally underestimate actual values while correlating poorly with BPH symptoms.14,18
Urinalysis. If you suspect BPH, you'll need to order a urinalysis to screen for infection, cancer, or stones and additional lab studies based on the patient's history, including measurements of serum creatinine, calcium, glucose, and prostate-specific antigen (PSA), among others.4,15
PSA values. These values should be checked if the patient's life expectancy is greater than a decade and a diagnosis of prostate cancer would influence treatment decisions. Adjustments of accepted norms should account for increasing age (40 to 50 years, 0-2.5 ng/mL; 51 to 60 years, 2.5-3.5 ng/mL; 61 to 70 years, 3.5-4.5 ng/mL; 71 to 80 years, 4.5-6.5 ng/mL), and urologic referral should be made as indicated.18
PSA determination is a more accurate reflection of prostate volume than a DRE and helps establish a pretreatment reference point before 5-a reductase inhibitor therapy.19 These drugs lower PSA concentrations approximately 50% and may complicate subsequent cancer screening.15
The US Preventive Services Task Force (USPSTF) clinical guidelines for prostate cancer screening notes that among patients with enlarged prostates, the specificity of PSA testing is lower, and thus PSA is a less accurate means of detecting cancer in BPH patients.20 Indeed, the USPSTF guidelines are ambivalent on the utility of PSA, in part because of the heterogeneity of prostate tumors, although they do confirm the greater accuracy of PSA testing over DRE.20
The guidelines state that screening is most effective at determining patients with a particularly good or poor long-term prognosis, which constitutes a fairly small minority of patients, but is less effective in the larger middle group.20 Regarding the particular means of testing PSA, the USPSTF guidelines note that free or complex testing is primarily useful to distinguish whether a patient should undergo a biopsy among those with a PSA level of 4.0 to 9.9 ng/mL.20 A more recent perspective from Cleveland Clinic clinicians indicates that the "PSA cutoff era" is now past and that decisions for further prostate cancer screening should be made with a patient's DRE and family history data in mind.21
Diagnostic studies. Noninvasive urine flow rates, postvoid residual measures, pressure-flow studies, cystoscopy, and renal or transrectal ultrasound are optional unless dictated by specific circumstances, including recurrent hematuria, pelvic pain, or urinary retention, in which case urologic consultation is indicated.4,15
Weigh patient preference against symptom severity
The treatment goals for a patient with BPH-related lower urinary tract symptoms must focus on improving and maintaining quality of life, achieving and sustaining symptom control, and avoiding disease progression.22 In choosing a specific treatment, weigh the patient's preferences against symptom severity and specific physiologic variables; even individuals with moderate IPSS ratings may improve (40%) or show no change (45%) with watchful waiting.23 (The AUA outlines treatment options for patients with moderate to severe symptoms in its BPH practice guidelines. They can be accessed on page 16 of http://www.auanet.org/guidelines/main_reports/bph_management/chapt_1_appendix.pdf.
Quality-of-life issues—how much lower urinary tract symptoms interfere with work, social life, sleep, sexual function, and travel—are generally more important than the symptoms per se.14 The AUA has published a diagnosis and treatment algorithm for BPH that is very helpful for practitioners.4 It is available on page 7 of http://www.auanet.org/guidelines/main_reports/bph_management/chapt_1_appendix.pdf.
Watchful waiting—even with high IPSS ratings
Watchful waiting is an option for patients experiencing minimal bother—even with high IPSS ratings—because the risk for progression is relatively small.4,14,15 If you choose this route, encourage the patient to minimize alcohol and caffeine use and the intake of fluids in the evening, and minimize the use of a-agonist, anticholinergic, antihistaminic, and calcium-channel blocker medications.
Where nocturia is a particular problem, diuretics timed to minimize night-time urine production, daytime naps, and use of antidiuretic hormones (although contraindicated in patients with congestive heart failure) may be appropriate.24,25 Notably, in the context of combined bladder outlet obstruction and detrusor overactivity validated by urodynamic studies, there are recent studies identifying a role for anticholinergics.26,27
Medical therapy before surgery
Medical therapy has supplanted surgery as the primary therapeutic tool for BPH-related lower urinary tract symptoms.4 a-Adrenergic antagonists decrease prostatic and urethral smooth muscle tone, induce tissue apoptosis through tumor growth factor-beta signaling, and increase detrusor muscle vascular supply, while 5-a reductase inhibitors block conversion of testosterone to dihydrotestosterone and reduce prostate volume ( TABLE ).4,14,15,28-40
a-Adrenergic blockers. Nonselective a-adrenergic blockers include terazosin, doxazosin, and alfuzosin. Their greater selectivity for nonprostatic peripheral vasculature a-1B receptors than for prostatic a-1A receptors account for their potential to cause orthostatic hypotension. A fourth agent, tamsulosin, is mostly selective for the prostatic a-1A receptor and does not have a clinically significant effect on blood pressure.30
At therapeutic doses, these drugs have comparable efficacy in lowering IPSS scores, increasing urine flow rates, and improving symptoms.4 Potential side effects include asthenia, headache, dizziness, and peripheral edema. Early postural hypotension and later rebound hypertension on withdrawal are primarily seen with terazosin and doxazosin, which require titration and tapering over 2 to 3 weeks when being introduced or eliminated. The uroselectivity of alfuzosin, as well as new dosing formulations, have helped reduce hypotensive side effects.28,29 Like tamsulosin, it can be started and stopped directly.
TABLE
Medical therapies for BPH at a glance4,14,15,28-40
| TYPE OF THERAPY | ACTIVITY | EFFICACY IN CLINICAL TRIALS | SIDE EFFECTS | INDICATIONS | NUMBER NEEDED TO TREAT* |
|---|---|---|---|---|---|
| a-Adrenergic blockers Nonselective Terazosin Doxazosin Alfuzosin Selective Tamsulosin |
|
|
|
| Terazosin 4.0 (to achieve >10% improvement in Boyarsky score, an older measure comparable to the IPSS)31 Doxazosin 13.7 (for the prevention of clinical progression)32 Alfuzosin 5.8 (to achieve =3 points improvement in IPSS)33 Tamsulosin 4.5 (to achieve =25% increase in AUA score)34 |
| 5- a Reductase inhibitors Dutasteride Finasteride |
|
| Dutasteride mild-to-moderate symptoms : 10 (to achieve 2-point improvement in AUA-SS) severe symptoms: 6.3 (to achieve 2-point improvement in AUA-SS)37,38 Finasteride 15.0 (for the prevention of clinical progression)32 | ||
| Combination therapy with a-Adrenergic blockers and 5-a reductase inhibitors |
|
|
| Combination of doxazosin + finasteride 8.4 (for the prevention of clinical progression)32 | |
| Phytotherapy Saw palmetto |
|
|
|
| N/A |
| *Number needed to treat (NNT) values should not be regarded as points of efficacy comparison since they are not consistently based on head-to-head trials, are derived from different patient populations, and may refer to different efficacy end points as well as different lengths of follow-up. | |||||
| AUA-SS, American Urological Association symptom score; BPH, benign prostatic hyperplasia; EAU, European Association of Urology; IPSS, International Prostate Symptom Score; LUTS, lower urinary tract symptoms; N/A, not available; PSA, prostate-specific antigen; QOL, quality of life. | |||||
Although infrequently reported in clinical trials, rhinitis and ejaculatory dysfunction are known side effects of tamsulosin and alfuzosin.30,41 a1-Blockers have recently been reported as possibly having an association with intraoperative floppy iris syndrome (IFIS), a surgical condition that has been observed during phacoemulsification cataract surgery.42 The etiology of this syndrome is unknown. Patients undergoing cataract surgery who are taking a1-blockers should inform their surgeons, who should be prepared for possible modifications to the surgical technique. The benefit of stopping a1-blocker therapy prior to cataract surgery has not been established.43-45
5- a Reductase inhibitors. Finasteride and dutasteride are comparable in efficacy and have been shown to decrease prostate volume (20%-30%), lower IPSS ratings 3 to 4 points, increase urine flow rates, and decrease urinary retention and the need for surgery (50%) when compared with placebo.15 Their clinical effect appears gradually over 3 to 6 months, and they are most beneficial when prostate volume exceeds 40 mL.35
Decreased libido (6%), ED (8%), and ejaculatory disorders (4%) are the main side effects of these drugs, as is their lowering of PSA levels by as much as half.15 This latter effect may prompt checking PSA velocities and free:total PSA ratios as a part of prostate cancer screening. Additionally, finasteride may reduce the prevalence of prostate cancer almost 25% compared with placebo, but more high-grade tumors may be associated with its use.36 The reason for this difference and its clinical importance require further study.36,46,47
Combination therapy. Combination therapy with a-adrenergic blockers and 5-a reductase inhibitors has increased due to results from the long-term (4.5 years) Medical Therapy of Prostatic Symptoms (MTOPS) study.32 It compared the efficacy of placebo, doxazosin, finasteride, and combination therapy on clinical progression measures of BPH. These were defined as an increase of 4 points on the IPSS, acute urinary retention, urinary incontinence, renal insufficiency, or recurrent urinary tract infections. All drug treatments significantly improved symptom scores, but the combination was clearly superior.32 Additionally, combination therapy and finasteride significantly reduced urinary retention and the need for surgery, whereas doxazosin did not.
The number needed to treat (NNT) for the prevention of a single instance of clinical progression over a 4-year period was 8.4 for combination therapy, compared with 13.7 for doxazosin monotherapy and 15.0 for finasteride monotherapy ( TABLE ).32
A secondary analysis, conducted to establish the NNT for disease progression in patients with larger baseline prostates or higher serum PSA, found that among patients with a PSA level >4.0 ng/mL, the NNT was 4.7 (vs 7.2 for finasteride), and for patients with a prostate volume >40 mL, the NNT was 4.9 (vs 7.2 for finasteride).32 These results suggest that patients with larger glands and higher PSA values, who are at greatest risk for progression, would benefit from combination approaches, although absolute threshold values are not yet clear.4
A combination of an a-adrenergic blocker and an anticholinergic medication may also be used in the treatment of comorbid lower urinary tract symptoms and overactive bladder. A 12-week placebo-controlled trial of a combination of tamsulosin and the anticholinergic tolterodine found significant benefits in terms of IPSS scores, urgency episodes, frequency of micturitions, quality-of-life scores, and patient perception of treatment benefit.48
Phytotherapy. Saw palmetto is derived from the ripe berries of the American dwarf palm (Serenoa repens or Sabal serrulata); retail sales in the United States totaled over $20 million in 2004.49 The mechanism of action is uncertain, but may involve antiandrogen activity. Short-term improvement of nocturia and peak urinary flow comparable with that of finasteride has been suggested by meta-analyses involving almost 3000 patients in trials ranging from 1 month to 1 year.39 However, neither American nor European guidelines recommend its use.4,15,40
A 6-month, double-blind, placebo-controlled trial of urtica dioica (stinging nettle) in 620 BPH patients found a significant improvement in IPSS scores, peak flow rates, and a small but significant reduction in prostate size among patients taking urtica dioica compared with baseline.50
Bothersome symptoms? Consider surgery
Transurethral resection of the prostate (TURP) was the primary surgical approach during most of the 20th century and remains the benchmark. TURP involves removing a portion of the prostate through the urethra.4 When compared with watchful waiting, TURP achieved better outcomes with men most bothered by symptoms at the outset. Watchful waiting was also considered safe, but 24% of this group underwent surgery during the 3 years. There were no increases in urinary incontinence or ED among surgically treated patients.51
TURP is indicated for patients with refractory urinary retention due to severe bladder outlet obstruction, recurrent urinary tract infections, progressive renal insufficiency, hematuria unresponsive to 5-a reductase inhibitors, bladder stones, and BPH-related hydronephrosis. Lower urinary tract symptom improvement is expected in 80% to 90% of cases, with a retained efficacy of 75% for at least 7 years and a risk of repeating surgery of only 1% annually.4,14,15 IPSS scores may decrease 15 to 20 points, but quality of life is enhanced only with severe lower urinary tract symptoms, and postoperative ejaculatory dysfunction (65%-70%) is expected, along with 1% to 2% perioperative mortality.15
It's important to note that TURP is a procedure that requires a hospital stay and is associated with a variety of potential side effects, including sexual dysfunction, bladder neck contracture, urinary tract infection, hematuria, and irritative voiding symptoms, while patients may also require blood transfusions.4,52
Other surgical therapies include open prostatectomies for patients with glands =80 mL and transurethral incision of the prostate (TUIP) for glands <30 mL.1,4,15 TUIP is a simpler outpatient operation than TURP. It offers equivalent symptomatic relief and less associated ejaculatory dysfunction or bleeding, but has a higher rate of reoperation.1,4,15 High-risk surgical candidates with severe urinary retention may also receive prostatic stents, but significant complications of pain, infection, and encrustation are common.4
Holmium laser enucleation of the prostate (HOLEP) is another alternative to TURP that has demonstrated equivalent efficacy in terms of AUA-SI scores, peak flow rates, and quality-of-life scores in studies of up to 3 years in length. Longer-term studies are required to determine its efficacy beyond that time frame.53-56
Minimally invasive route for high-risk patients
Transurethral needle ablation (TUNA) and transurethral microwave thermotherapy (TUMT) offer AUA-approved alternative treatment choices based on the severity of symptoms and the presence of complications.4 TUNA uses radiofrequency waves administered through 2 18-gauge needles to heat prostatic tissue. An outpatient procedure, TUNA is effective over the long term, demonstrating a low failure rate (25% after 5 years); however, temporary side effects such as irritative urinary symptoms and urinary retention can occur.4,57
With >100,000 procedures performed, TUMT is the most frequent minimally invasive treatment utilized worldwide. Heat destroys targeted prostatic tissue, while a cooling system protects the prostatic urethra. Its efficacy has been demonstrated by randomized trials, and its failure rate documented at 10% to 16% annually.4 Morbidity is related mainly to required indwelling catheterization for 4 to 6 weeks following intervention. However, it is an outpatient, low-risk procedure well-suited to high-risk patients or those who oppose surgery.
Correspondence
Darryl Chutka, MD, Division of Preventive and Occupational Medicine and Internal Medicine, Mayo Clinic College of Medicine, Rochester, MN 55905; [email protected].
1. Granville LJ. Prostate disease. In: Cobbs EL, Duthie EH, Murphy JB, eds. Geriatrics review syllabus: a core curriculum in geriatric medicine. 5th ed. Malden, Mass: Blackwell Publishing; 2002:384-385.
2. Collins MFM, Friedman RH, Ash A, Hall R, Moskowitz M. Underdetection of clinical benign prostatic hyperplasia in a general medical practice. J Gen Intern Med. 1996;11:513-518.
3. Hegarty NJ, Fitzpatrick JM. High intensity focused ultrasound in benign prostatic hyperplasia. Eur J Ultrasound. 1999;9:55-60.
4. AUA Practice Guidelines Committee. AUA guideline on management of benign prostatic hyperplasia (2003). Chapter 1: Diagnosis and treatment recommendations. J Urol. 2003;170:530-547.
5. Welch G, Weinger K, Barry MJ. Quality-of-life impact of lower urinary tract symptom severity: results from the health professionals follow-up study. Urology. 2002;59:245-250.
6. Cunningham-Burley S, Allbutt, Garraway WM, Lee AJ, Russell EBAW. Perceptions of urinary symptoms and health-care-seeking behavior amongst men aged 40-79 years. Br J Gen Pract. 1996;46:349-352.
7. Wei JT, Calhoun E, Jacobsen SJ. Urologic diseases in America project: benign prostatic hyperplasia. J Urol. 2005;173:1256-1261.
8. Rosen RC, Giuliano F, Carson CC. Sexual dysfunction and lower urinary tract symptoms associated with benign prostatic hyperplasia. Eur Urol. 2005;47:824-837.
9. Assessing prostate symptoms. Patient UK. EMIS and Patient Information Publications 1997-2007. Available at: http://www.patient.co.uk/showdoc/23069171/. Accessed January 3, 2007.
10. O'Leary MP. Validity of the "bother score" in the evaluation and treatment of symptomatic benign prostatic hyperplasia. Rev Urol. 2005;7:1-10.
11. Barry MJ, Fowler FJ, Jr, O'Leary MP, Bruskewitz RC, Holtgrewe HL, Mebust WK, Cockett AT. The American Urological Association symptom index for benign prostatic hyperplasia. The Measurement Committee of the American Urological Association. J Urol. 1992;148:1549-1557.
12. Barry MJ. Benign prostatic hyperplasia. In: Dale DC, Federman DD, eds. ACP Medicine. New York: WebMD; 2005.
13. Barry M, Roehrborn C. Management of benign prostate hyperplasia. Annu Rev Med. 1997;48:177-189.
14. Dubeau CE. Benign prostate disorders. In: Hazzard WR, Blass JP, Halter JB, Ouslander JG, Tinetti ME, eds. Principles of Geriatric Medicine and Gerontology. 5th ed. New York: McGraw-Hill; 2003:1303-1310.
15. Madersbacher S, Alivizatos G, Nordling J, Sanz CR, Emberton M, de la Rosette JJ. EAU 2004 Guidelines on assessment, therapy and follow-up of men with lower urinary symptoms suggestive of benign prostatic obstruction (BPH Guidelines). Eur Urol. 2004;46:547-554.
16. Chapple CR, Roehrborn CG. A shifted paradigm for the further understanding, evaluation, and treatment of lower urinary tract symptoms in men: focus on the bladder. Eur Urol. 2006;49:651-659.
17. Knutson T, Edlund C, Fall M, Dahlstrand C. BPH with coexisting overactive bladder dysfunction—an everyday urological dilemma. Neurourol Urodyn. 2001;20:237-247.
18. Hellerstedt BA, Pienta KJ. In: Hazzard WR, Blass JP, Ouslander JG, Tinetti ME, eds. Principles of Geriatric Medicine and Gerontology. 5th ed. New York: McGraw-Hill; 2003:1303-10.
19. Roehrborn CG, McConnell JD, Bonilla J, Rosenblatt S, et al. Serum prostate-specific antigen is a strong predictor of future prostate growth in men with benign prostatic hyperplasia: PROSCAR long-term efficacy and safety study. J Urol. 2000;163:13-20.
20. Harris R, Lohr KN. Screening for prostate cancer: an update of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med. 2002;137:917-929.
21. Jones JS, Klein E. Four no more: the 'PSA cutoff era' is over. Cleve Clin J Med. 2008;75:30-32.
22. O'Leary MP. Lower urinary tract symptoms/benign prostatic hyperplasia: Maintaining symptom control and reducing complications. Urology. 2003;62(Suppl 3A):15-23.
23. Oesterling JE. Benign prostatic hyperplasia: medical and minimally invasive treatment options. N Engl J Med. 1995;332:99-109.
24. Weiss JP, Blaivas JG. Nocturia. J Urol. 2000;163:5-12.
25. Reynard JM, Cannon A, Yang Q, Abrams P. A novel therapy for nocturnal polyuria: a double-blind randomized trial of furosemide against placebo. Br J Urol. 1998;81:215-218.
26. Abrams P, Kaplan S, De Konig Gans HJ, Millard R. Safety and tolerability of tolterodine for the treatment of overactive bladder in men with bladder outlet obstruction. J Urol. 2006;175:999-1004.
27. Athanasopoulos A, Gyftopoulos K, Giannitsas K, Fisfis J, Perimenis P, Barbalias G. Combination treatment with an alpha-blocker plus an anticholinergic for bladder outlet obstruction: a prospective, randomized controlled study. J Urol. 2003;169:2253-2256
28. van Kerrebroeck P, Jardin A, Laval KU, van Cangh P. Efficacy and safety of a new prolonged release formulation of alfuzosin 10 mg once daily versus alfuzosin 2.5 mg thrice daily and placebo in patients with symptomatic benign prostatic hyperplasia. Eur Urol. 2000;7:306-313.
29. Mottet N, Bressolle F, Delmas V, Robert M, Costa P. Prostatic tissual distribution of alfuzosin in patients with benign prostatic hyperplasia following repeated oral administration. Eur Urol. 2003;44:101-105.
30. Beduschi MC, Beduschi R, Oesterling JE. Alpha-blockade therapy for benign prostatic hyperplasia: from a nonselective to a more selective alpha1A-adrenergic antagonist. Urology. 1998;51:861-872.
31. Lepor H, Auerbach S, Puras-Baez A, et al. A randomized, placebo-controlled multicenter study of the efficacy and safety of terazosin in the treatment of benign prostatic hyperplasia. J Urol. 1992;148:1467-1474.
32. McConnell JD, Roehrborn CG, Bautista OM, et at. The long-term effect of doxazosin, finasteride, and combination therapy on the clinical progression of benign prostatic hyperplasia. N Engl J Med. 2003;349:2387-2398.
33. Roehrborn CG. Efficacy and safety of once-daily alfuzosin in the treatment of lower urinary tract symptoms and clinical benign prostatic hyperplasia: a randomized, placebo-controlled trial. Urology. 2001;58:953-959.
34. Lepor H. Long-term evaluation of tamsulosin in benign prostatic hyperplasia: placebo-controlled, double-blind extension of phase III trial. Tamsulosin Investigator Group. Urology. 1998;51:901-906.
35. Clifford GM, Farmer RDT. Medical therapy for benign prostatic hyperplasia: a review of the literature. Eur Urol. 2000;38:2-19.
36. Thompson IM, Goodman PJ, Tangen CM, et al. The influence of finasteride on the development of prostate cancer. N Engl J Med. 2003;349:215-224.
37. Roehrborn CG. The clinical benefits of dutasteride treatment for LUTS and BPH. Rev Urol. 2004;6(Suppl 9):S22-SS30.
38. Roehrborn CG, Lukkarinen O, Mark S, Siami P, Ramsdell J, Zinner N. Long-term sustained improvement in symptoms of benign prostatic hyperplasia with the dual 5alpha-reductase inhibitor dutasteride: results of 4-year studies. BJU Int. 2005;96:572-577.
39. Edzard E. The risk-benefit profile of commonly used herbal therapies: ginkgo, St John's wort, ginseng, Echinacea, saw palmetto, and kava. Ann Intern Med. 2002;136:42-53.
40. Bent S, Kane C, Shinohara K, et al. Saw palmetto for benign prostatic hyperplasia. N Engl J Med. 2006;354:557-566.
41. Food and Drug Administration. ALFOTAM trial. Center for Drug Administration and Research. Available at: www.pbm.va.gov/criteria/Alpha-Blocker%20CFU%207-2005.pdf. Accessed November 16, 2006.
42. Chang DF, Campbell JR. Intraoperative floppy iris syndrome associated with tamsulosin. J Cataract Refract Surg. 2005;31:664-673.
43. Uroxatral [package insert]. Bridgewater, NJ: Sanofi-Aventis U.S.; 2007.
44. Cardura XL [package insert]. New York, NY: Pfizer Roerig; 2006.
45. Flomax [package insert]. Ridgefield, CT: Boehringer Ingelheim Pharmaceuticals; 2008.
46. Finasteride [package insert]. Whitehouse Station, NJ: Merck & Co. Inc.; 2007.
47. Dutasteride [package insert]. Research Triangle Park, NC: GlaxoSmithKline; 2005.
48. Kaplan SA, Roehrborn CG, Rovner ES, Carlsson M, Bavendam T, Guan Z. Tolterodine and tamsulosin for treatment of men with lower urinary tract symptoms and overactive bladder: a randomized controlled trial. JAMA. 2006;296:2319-2328.Erratum in: JAMA 2007; 297:1195.
49. Healing Herbs and Natural Remedies. Available at: http://www.herbsandnaturalremedies.com/topsellers.htm. Accessed January 3, 2007.
50. Safarinejad MR. Urtica dioica for treatment of benign prostatic hyperplasia: a prospective, randomized, double-blind, placebo-controlled, crossover study. J Herb Pharmacother. 2005;5:1-11.
51. Wasson JH, Reda DJ, Bruskewitz RC, Elinson J, Keller AM, Henderson WG. A comparison of transurethral surgery with watchful waiting for moderate symptoms of benign prostatic hyperplasia. N Engl J Med. 1995;332:75-79.
52. Hammadeh MY, Madaan S, Hines J, Philp T. 5-year outcome of a prospective randomized trial to compare transurethral electrovaporization of the prostate and standard transurethral resection. Urology. 2003;61:1166-1171.
53. Ahyai SA, Lehrich K, Kuntz RM. Holmium laser enucleation versus transurethral resection of the prostate: 3-year follow-up results of a randomized clinical trial. Eur Urol. 2007;52:1456-1464.
54. Kuntz RM. Laser treatment of benign prostatic hyperplasia. World J Urol. 2007;25:241-247.
55. Gupta N, Sivaramakrishna, Kumar R, Dogra PN, Seth A. Comparison of standard transurethral resection, transurethral vapour resection and holmium laser enucleation of the prostate for managing benign prostatic hyperplasia of >40 g. BJU Int. 2006;97:85-89.
56. Tan AH, Gilling PJ, Kennett KM, Frampton C, Westenberg AM, Fraundorfer MR. A randomized trial comparing holmium laser enucleation of the prostate with transurethral resection of the prostate for the treatment of bladder outlet obstruction secondary to benign prostatic hyperplasia in large glands (40 to 200 grams). J Urol. 2003;170(4 Pt 1):1270-1274.
57. Zlotta AR, Giannakopoulos X, Maehlum O, Ostrem T, Schulman CC. Long-term evaluation of transurethral needle ablation of the prostate (TUNA) for treatment of symptomatic benign prostatic hyperplasia: clinical outcome up to five years from three centers. Eur Urol. 2003;44:89-93.
- Talk to every male patient over the age of 50 about urinary function (C).
- Utilize questionnaires, such as the International Prostate Symptom Score to evaluate the patient's perception of symptom severity and quality of life (A).
- Rule out potential causes of lower urinary tract symptoms with a thorough medical history, focused physical exam (including digital rectal examination and neurological assessments), and appropriate laboratory evaluations (C).
- When choosing treatment for benign prostatic hyperplasia, remember that quality of life is generally more important than symptom severity (A).
Strength of recommendation (SOR)
- Good quality patient-oriented evidence
- Inconsistent or limited-quality patient-oriented evidence
- Consensus, usual practice, opinion, disease-oriented evidence, case series
"My wife is mad at me—and she's worried, too," says Dan, a 65-year-old patient of yours. "She's been telling me to come see you, but I've been putting it off.
"I've been getting up 4 and 5 times a night to urinate, and we can't drive an hour without me having to stop at least once to use a restroom."
With a deep sigh, Dan says: "My wife is worried that I have cancer or something."
"And I'm worried, too," he admits.
Benign prostatic hyperplasia (BPH), and its clinical expression as lower urinary tract symptoms—urinary frequency, urgency, nocturia, decreased force of stream, and incomplete bladder emptying—comprise a major health concern for many older men. Approximately 50% of men over age 60 have at least microscopic BPH, while 90% over age 90 have evidence of the abnormality.1
Many men fail to seek help for lower urinary tract symptoms associated with BPH,2-4 even though these often moderate to severe symptoms are associated with decreased quality of life, anxiety, and depression.5 Your patient may be uncomfortable broaching the subject, as Dan was, for fear that he may have cancer. He may dismiss the symptoms as a natural consequence of aging,6 or he may believe that there are no effective treatments or that treatment will cause unwanted side effects.
Bring up the subject with all men over 50
To dispel these misconceptions and ensure that there are no current or ensuing serious complications,4 you should routinely talk about urinary function with every male patient over age 50. Because the incidence of BPH increases not only with age but also with other comorbid conditions such as diabetes7 and erectile dysfunction (ED),8 you should discuss the symptoms and potential complications of BPH with patients who present with these comorbidities. You can reassure them that BPH is not cancer, nor is it a precursor to prostate cancer; rather it is a fairly common, treatable disorder.
What's right for your patient? Watchful waiting? a-Blocker therapy? Surgery?
Questionnaire can help, addresses quality of life
Questionnaires such as the International Prostate Symptom Score (IPSS) (PATIENT HANDOUT)9 and the similar American Urological Association symptom index (AUA-SI) (available on page 44 of http://www.auanet.org/guidelines/main_reports/bph_management/chapt_1_appendix.pdf) can help you evaluate your patient's symptom severity.2,6
The IPSS, with 3 categories of symptom severity (mild 0 to 7, moderate 8 to 19, severe 20 to 35) and a global quality-of-life question also referred to as the "Bother Score," is a validated tool for monitoring disease distress and clinical change.10,11 The quality-of-life question is a good indicator for assessing whether watchful waiting might be preferred to active treatment.9,12
Further categorizing the symptoms is not helpful. Lower urinary tract symptoms have traditionally been divided into irritative symptoms such as nocturia, urgency, and frequency, attributed to bladder and prostatic smooth muscle contractions, and obstructive symptoms such as hesitancy, decreased force of stream, and incomplete emptying, attributed to increased glandular mass.1 This distinction, however, is not helpful inasmuch as irritative symptoms can result from increased tissue mass alone and obstructive symptoms from muscle hypertonicity alone; additionally, most BPH patients have a combination of both.13,14
Consider comorbidities and overactive bladder
Common comorbidities for a patient with BPH include obesity, diabetes mellitus, and low high-density lipoprotein levels. Both irritative and obstructive symptoms are likely, without prior lower urinary tract disorders or ongoing neurological disease.13-15 Multiple epidemiological studies have established clear, clinically relevant associations between BPH-related lower urinary tract symptoms and ED and ejaculatory dysfunction.8
It is also important to note that lower urinary tract symptoms may often arise due to overactive bladder; in fact, symptoms of overactive bladder and BPH overlap to a large degree.16 The diagnostic challenge is only increased by the fact that while overactive bladder is an additional cause of lower urinary tract symptoms, it may also coexist with BPH-related bladder outlet obstruction.17 The similarity in clinical presentation of the 2 conditions may make them hard to distinguish.
Rule out infection, urinary tract stones
There is general agreement about the need to exclude other potential etiologies of lower urinary tract symptoms in older men.4,15 Thus, you need to consider such causes as urinary tract stones, infections, or cancer; comorbid conditions that may affect bladder function or lead to polyuria; drug side effects; or sleep disturbances associated with chronic insomnia, depression, ethanol abuse, or sleep apnea.13,14
Digital rectal exam (DRE). The physical exam should include both a DRE and a search for neurological deficits to look for evidence that lower urinary tract symptoms are not BPH-related. The DRE should assess for stool impaction and prostate symmetry, nodularity, and consistency. Prostate volume estimates by DRE are not reliable and generally underestimate actual values while correlating poorly with BPH symptoms.14,18
Urinalysis. If you suspect BPH, you'll need to order a urinalysis to screen for infection, cancer, or stones and additional lab studies based on the patient's history, including measurements of serum creatinine, calcium, glucose, and prostate-specific antigen (PSA), among others.4,15
PSA values. These values should be checked if the patient's life expectancy is greater than a decade and a diagnosis of prostate cancer would influence treatment decisions. Adjustments of accepted norms should account for increasing age (40 to 50 years, 0-2.5 ng/mL; 51 to 60 years, 2.5-3.5 ng/mL; 61 to 70 years, 3.5-4.5 ng/mL; 71 to 80 years, 4.5-6.5 ng/mL), and urologic referral should be made as indicated.18
PSA determination is a more accurate reflection of prostate volume than a DRE and helps establish a pretreatment reference point before 5-a reductase inhibitor therapy.19 These drugs lower PSA concentrations approximately 50% and may complicate subsequent cancer screening.15
The US Preventive Services Task Force (USPSTF) clinical guidelines for prostate cancer screening notes that among patients with enlarged prostates, the specificity of PSA testing is lower, and thus PSA is a less accurate means of detecting cancer in BPH patients.20 Indeed, the USPSTF guidelines are ambivalent on the utility of PSA, in part because of the heterogeneity of prostate tumors, although they do confirm the greater accuracy of PSA testing over DRE.20
The guidelines state that screening is most effective at determining patients with a particularly good or poor long-term prognosis, which constitutes a fairly small minority of patients, but is less effective in the larger middle group.20 Regarding the particular means of testing PSA, the USPSTF guidelines note that free or complex testing is primarily useful to distinguish whether a patient should undergo a biopsy among those with a PSA level of 4.0 to 9.9 ng/mL.20 A more recent perspective from Cleveland Clinic clinicians indicates that the "PSA cutoff era" is now past and that decisions for further prostate cancer screening should be made with a patient's DRE and family history data in mind.21
Diagnostic studies. Noninvasive urine flow rates, postvoid residual measures, pressure-flow studies, cystoscopy, and renal or transrectal ultrasound are optional unless dictated by specific circumstances, including recurrent hematuria, pelvic pain, or urinary retention, in which case urologic consultation is indicated.4,15
Weigh patient preference against symptom severity
The treatment goals for a patient with BPH-related lower urinary tract symptoms must focus on improving and maintaining quality of life, achieving and sustaining symptom control, and avoiding disease progression.22 In choosing a specific treatment, weigh the patient's preferences against symptom severity and specific physiologic variables; even individuals with moderate IPSS ratings may improve (40%) or show no change (45%) with watchful waiting.23 (The AUA outlines treatment options for patients with moderate to severe symptoms in its BPH practice guidelines. They can be accessed on page 16 of http://www.auanet.org/guidelines/main_reports/bph_management/chapt_1_appendix.pdf.
Quality-of-life issues—how much lower urinary tract symptoms interfere with work, social life, sleep, sexual function, and travel—are generally more important than the symptoms per se.14 The AUA has published a diagnosis and treatment algorithm for BPH that is very helpful for practitioners.4 It is available on page 7 of http://www.auanet.org/guidelines/main_reports/bph_management/chapt_1_appendix.pdf.
Watchful waiting—even with high IPSS ratings
Watchful waiting is an option for patients experiencing minimal bother—even with high IPSS ratings—because the risk for progression is relatively small.4,14,15 If you choose this route, encourage the patient to minimize alcohol and caffeine use and the intake of fluids in the evening, and minimize the use of a-agonist, anticholinergic, antihistaminic, and calcium-channel blocker medications.
Where nocturia is a particular problem, diuretics timed to minimize night-time urine production, daytime naps, and use of antidiuretic hormones (although contraindicated in patients with congestive heart failure) may be appropriate.24,25 Notably, in the context of combined bladder outlet obstruction and detrusor overactivity validated by urodynamic studies, there are recent studies identifying a role for anticholinergics.26,27
Medical therapy before surgery
Medical therapy has supplanted surgery as the primary therapeutic tool for BPH-related lower urinary tract symptoms.4 a-Adrenergic antagonists decrease prostatic and urethral smooth muscle tone, induce tissue apoptosis through tumor growth factor-beta signaling, and increase detrusor muscle vascular supply, while 5-a reductase inhibitors block conversion of testosterone to dihydrotestosterone and reduce prostate volume ( TABLE ).4,14,15,28-40
a-Adrenergic blockers. Nonselective a-adrenergic blockers include terazosin, doxazosin, and alfuzosin. Their greater selectivity for nonprostatic peripheral vasculature a-1B receptors than for prostatic a-1A receptors account for their potential to cause orthostatic hypotension. A fourth agent, tamsulosin, is mostly selective for the prostatic a-1A receptor and does not have a clinically significant effect on blood pressure.30
At therapeutic doses, these drugs have comparable efficacy in lowering IPSS scores, increasing urine flow rates, and improving symptoms.4 Potential side effects include asthenia, headache, dizziness, and peripheral edema. Early postural hypotension and later rebound hypertension on withdrawal are primarily seen with terazosin and doxazosin, which require titration and tapering over 2 to 3 weeks when being introduced or eliminated. The uroselectivity of alfuzosin, as well as new dosing formulations, have helped reduce hypotensive side effects.28,29 Like tamsulosin, it can be started and stopped directly.
TABLE
Medical therapies for BPH at a glance4,14,15,28-40
| TYPE OF THERAPY | ACTIVITY | EFFICACY IN CLINICAL TRIALS | SIDE EFFECTS | INDICATIONS | NUMBER NEEDED TO TREAT* |
|---|---|---|---|---|---|
| a-Adrenergic blockers Nonselective Terazosin Doxazosin Alfuzosin Selective Tamsulosin |
|
|
|
| Terazosin 4.0 (to achieve >10% improvement in Boyarsky score, an older measure comparable to the IPSS)31 Doxazosin 13.7 (for the prevention of clinical progression)32 Alfuzosin 5.8 (to achieve =3 points improvement in IPSS)33 Tamsulosin 4.5 (to achieve =25% increase in AUA score)34 |
| 5- a Reductase inhibitors Dutasteride Finasteride |
|
| Dutasteride mild-to-moderate symptoms : 10 (to achieve 2-point improvement in AUA-SS) severe symptoms: 6.3 (to achieve 2-point improvement in AUA-SS)37,38 Finasteride 15.0 (for the prevention of clinical progression)32 | ||
| Combination therapy with a-Adrenergic blockers and 5-a reductase inhibitors |
|
|
| Combination of doxazosin + finasteride 8.4 (for the prevention of clinical progression)32 | |
| Phytotherapy Saw palmetto |
|
|
|
| N/A |
| *Number needed to treat (NNT) values should not be regarded as points of efficacy comparison since they are not consistently based on head-to-head trials, are derived from different patient populations, and may refer to different efficacy end points as well as different lengths of follow-up. | |||||
| AUA-SS, American Urological Association symptom score; BPH, benign prostatic hyperplasia; EAU, European Association of Urology; IPSS, International Prostate Symptom Score; LUTS, lower urinary tract symptoms; N/A, not available; PSA, prostate-specific antigen; QOL, quality of life. | |||||
Although infrequently reported in clinical trials, rhinitis and ejaculatory dysfunction are known side effects of tamsulosin and alfuzosin.30,41 a1-Blockers have recently been reported as possibly having an association with intraoperative floppy iris syndrome (IFIS), a surgical condition that has been observed during phacoemulsification cataract surgery.42 The etiology of this syndrome is unknown. Patients undergoing cataract surgery who are taking a1-blockers should inform their surgeons, who should be prepared for possible modifications to the surgical technique. The benefit of stopping a1-blocker therapy prior to cataract surgery has not been established.43-45
5- a Reductase inhibitors. Finasteride and dutasteride are comparable in efficacy and have been shown to decrease prostate volume (20%-30%), lower IPSS ratings 3 to 4 points, increase urine flow rates, and decrease urinary retention and the need for surgery (50%) when compared with placebo.15 Their clinical effect appears gradually over 3 to 6 months, and they are most beneficial when prostate volume exceeds 40 mL.35
Decreased libido (6%), ED (8%), and ejaculatory disorders (4%) are the main side effects of these drugs, as is their lowering of PSA levels by as much as half.15 This latter effect may prompt checking PSA velocities and free:total PSA ratios as a part of prostate cancer screening. Additionally, finasteride may reduce the prevalence of prostate cancer almost 25% compared with placebo, but more high-grade tumors may be associated with its use.36 The reason for this difference and its clinical importance require further study.36,46,47
Combination therapy. Combination therapy with a-adrenergic blockers and 5-a reductase inhibitors has increased due to results from the long-term (4.5 years) Medical Therapy of Prostatic Symptoms (MTOPS) study.32 It compared the efficacy of placebo, doxazosin, finasteride, and combination therapy on clinical progression measures of BPH. These were defined as an increase of 4 points on the IPSS, acute urinary retention, urinary incontinence, renal insufficiency, or recurrent urinary tract infections. All drug treatments significantly improved symptom scores, but the combination was clearly superior.32 Additionally, combination therapy and finasteride significantly reduced urinary retention and the need for surgery, whereas doxazosin did not.
The number needed to treat (NNT) for the prevention of a single instance of clinical progression over a 4-year period was 8.4 for combination therapy, compared with 13.7 for doxazosin monotherapy and 15.0 for finasteride monotherapy ( TABLE ).32
A secondary analysis, conducted to establish the NNT for disease progression in patients with larger baseline prostates or higher serum PSA, found that among patients with a PSA level >4.0 ng/mL, the NNT was 4.7 (vs 7.2 for finasteride), and for patients with a prostate volume >40 mL, the NNT was 4.9 (vs 7.2 for finasteride).32 These results suggest that patients with larger glands and higher PSA values, who are at greatest risk for progression, would benefit from combination approaches, although absolute threshold values are not yet clear.4
A combination of an a-adrenergic blocker and an anticholinergic medication may also be used in the treatment of comorbid lower urinary tract symptoms and overactive bladder. A 12-week placebo-controlled trial of a combination of tamsulosin and the anticholinergic tolterodine found significant benefits in terms of IPSS scores, urgency episodes, frequency of micturitions, quality-of-life scores, and patient perception of treatment benefit.48
Phytotherapy. Saw palmetto is derived from the ripe berries of the American dwarf palm (Serenoa repens or Sabal serrulata); retail sales in the United States totaled over $20 million in 2004.49 The mechanism of action is uncertain, but may involve antiandrogen activity. Short-term improvement of nocturia and peak urinary flow comparable with that of finasteride has been suggested by meta-analyses involving almost 3000 patients in trials ranging from 1 month to 1 year.39 However, neither American nor European guidelines recommend its use.4,15,40
A 6-month, double-blind, placebo-controlled trial of urtica dioica (stinging nettle) in 620 BPH patients found a significant improvement in IPSS scores, peak flow rates, and a small but significant reduction in prostate size among patients taking urtica dioica compared with baseline.50
Bothersome symptoms? Consider surgery
Transurethral resection of the prostate (TURP) was the primary surgical approach during most of the 20th century and remains the benchmark. TURP involves removing a portion of the prostate through the urethra.4 When compared with watchful waiting, TURP achieved better outcomes with men most bothered by symptoms at the outset. Watchful waiting was also considered safe, but 24% of this group underwent surgery during the 3 years. There were no increases in urinary incontinence or ED among surgically treated patients.51
TURP is indicated for patients with refractory urinary retention due to severe bladder outlet obstruction, recurrent urinary tract infections, progressive renal insufficiency, hematuria unresponsive to 5-a reductase inhibitors, bladder stones, and BPH-related hydronephrosis. Lower urinary tract symptom improvement is expected in 80% to 90% of cases, with a retained efficacy of 75% for at least 7 years and a risk of repeating surgery of only 1% annually.4,14,15 IPSS scores may decrease 15 to 20 points, but quality of life is enhanced only with severe lower urinary tract symptoms, and postoperative ejaculatory dysfunction (65%-70%) is expected, along with 1% to 2% perioperative mortality.15
It's important to note that TURP is a procedure that requires a hospital stay and is associated with a variety of potential side effects, including sexual dysfunction, bladder neck contracture, urinary tract infection, hematuria, and irritative voiding symptoms, while patients may also require blood transfusions.4,52
Other surgical therapies include open prostatectomies for patients with glands =80 mL and transurethral incision of the prostate (TUIP) for glands <30 mL.1,4,15 TUIP is a simpler outpatient operation than TURP. It offers equivalent symptomatic relief and less associated ejaculatory dysfunction or bleeding, but has a higher rate of reoperation.1,4,15 High-risk surgical candidates with severe urinary retention may also receive prostatic stents, but significant complications of pain, infection, and encrustation are common.4
Holmium laser enucleation of the prostate (HOLEP) is another alternative to TURP that has demonstrated equivalent efficacy in terms of AUA-SI scores, peak flow rates, and quality-of-life scores in studies of up to 3 years in length. Longer-term studies are required to determine its efficacy beyond that time frame.53-56
Minimally invasive route for high-risk patients
Transurethral needle ablation (TUNA) and transurethral microwave thermotherapy (TUMT) offer AUA-approved alternative treatment choices based on the severity of symptoms and the presence of complications.4 TUNA uses radiofrequency waves administered through 2 18-gauge needles to heat prostatic tissue. An outpatient procedure, TUNA is effective over the long term, demonstrating a low failure rate (25% after 5 years); however, temporary side effects such as irritative urinary symptoms and urinary retention can occur.4,57
With >100,000 procedures performed, TUMT is the most frequent minimally invasive treatment utilized worldwide. Heat destroys targeted prostatic tissue, while a cooling system protects the prostatic urethra. Its efficacy has been demonstrated by randomized trials, and its failure rate documented at 10% to 16% annually.4 Morbidity is related mainly to required indwelling catheterization for 4 to 6 weeks following intervention. However, it is an outpatient, low-risk procedure well-suited to high-risk patients or those who oppose surgery.
Correspondence
Darryl Chutka, MD, Division of Preventive and Occupational Medicine and Internal Medicine, Mayo Clinic College of Medicine, Rochester, MN 55905; [email protected].
- Talk to every male patient over the age of 50 about urinary function (C).
- Utilize questionnaires, such as the International Prostate Symptom Score to evaluate the patient's perception of symptom severity and quality of life (A).
- Rule out potential causes of lower urinary tract symptoms with a thorough medical history, focused physical exam (including digital rectal examination and neurological assessments), and appropriate laboratory evaluations (C).
- When choosing treatment for benign prostatic hyperplasia, remember that quality of life is generally more important than symptom severity (A).
Strength of recommendation (SOR)
- Good quality patient-oriented evidence
- Inconsistent or limited-quality patient-oriented evidence
- Consensus, usual practice, opinion, disease-oriented evidence, case series
"My wife is mad at me—and she's worried, too," says Dan, a 65-year-old patient of yours. "She's been telling me to come see you, but I've been putting it off.
"I've been getting up 4 and 5 times a night to urinate, and we can't drive an hour without me having to stop at least once to use a restroom."
With a deep sigh, Dan says: "My wife is worried that I have cancer or something."
"And I'm worried, too," he admits.
Benign prostatic hyperplasia (BPH), and its clinical expression as lower urinary tract symptoms—urinary frequency, urgency, nocturia, decreased force of stream, and incomplete bladder emptying—comprise a major health concern for many older men. Approximately 50% of men over age 60 have at least microscopic BPH, while 90% over age 90 have evidence of the abnormality.1
Many men fail to seek help for lower urinary tract symptoms associated with BPH,2-4 even though these often moderate to severe symptoms are associated with decreased quality of life, anxiety, and depression.5 Your patient may be uncomfortable broaching the subject, as Dan was, for fear that he may have cancer. He may dismiss the symptoms as a natural consequence of aging,6 or he may believe that there are no effective treatments or that treatment will cause unwanted side effects.
Bring up the subject with all men over 50
To dispel these misconceptions and ensure that there are no current or ensuing serious complications,4 you should routinely talk about urinary function with every male patient over age 50. Because the incidence of BPH increases not only with age but also with other comorbid conditions such as diabetes7 and erectile dysfunction (ED),8 you should discuss the symptoms and potential complications of BPH with patients who present with these comorbidities. You can reassure them that BPH is not cancer, nor is it a precursor to prostate cancer; rather it is a fairly common, treatable disorder.
What's right for your patient? Watchful waiting? a-Blocker therapy? Surgery?
Questionnaire can help, addresses quality of life
Questionnaires such as the International Prostate Symptom Score (IPSS) (PATIENT HANDOUT)9 and the similar American Urological Association symptom index (AUA-SI) (available on page 44 of http://www.auanet.org/guidelines/main_reports/bph_management/chapt_1_appendix.pdf) can help you evaluate your patient's symptom severity.2,6
The IPSS, with 3 categories of symptom severity (mild 0 to 7, moderate 8 to 19, severe 20 to 35) and a global quality-of-life question also referred to as the "Bother Score," is a validated tool for monitoring disease distress and clinical change.10,11 The quality-of-life question is a good indicator for assessing whether watchful waiting might be preferred to active treatment.9,12
Further categorizing the symptoms is not helpful. Lower urinary tract symptoms have traditionally been divided into irritative symptoms such as nocturia, urgency, and frequency, attributed to bladder and prostatic smooth muscle contractions, and obstructive symptoms such as hesitancy, decreased force of stream, and incomplete emptying, attributed to increased glandular mass.1 This distinction, however, is not helpful inasmuch as irritative symptoms can result from increased tissue mass alone and obstructive symptoms from muscle hypertonicity alone; additionally, most BPH patients have a combination of both.13,14
Consider comorbidities and overactive bladder
Common comorbidities for a patient with BPH include obesity, diabetes mellitus, and low high-density lipoprotein levels. Both irritative and obstructive symptoms are likely, without prior lower urinary tract disorders or ongoing neurological disease.13-15 Multiple epidemiological studies have established clear, clinically relevant associations between BPH-related lower urinary tract symptoms and ED and ejaculatory dysfunction.8
It is also important to note that lower urinary tract symptoms may often arise due to overactive bladder; in fact, symptoms of overactive bladder and BPH overlap to a large degree.16 The diagnostic challenge is only increased by the fact that while overactive bladder is an additional cause of lower urinary tract symptoms, it may also coexist with BPH-related bladder outlet obstruction.17 The similarity in clinical presentation of the 2 conditions may make them hard to distinguish.
Rule out infection, urinary tract stones
There is general agreement about the need to exclude other potential etiologies of lower urinary tract symptoms in older men.4,15 Thus, you need to consider such causes as urinary tract stones, infections, or cancer; comorbid conditions that may affect bladder function or lead to polyuria; drug side effects; or sleep disturbances associated with chronic insomnia, depression, ethanol abuse, or sleep apnea.13,14
Digital rectal exam (DRE). The physical exam should include both a DRE and a search for neurological deficits to look for evidence that lower urinary tract symptoms are not BPH-related. The DRE should assess for stool impaction and prostate symmetry, nodularity, and consistency. Prostate volume estimates by DRE are not reliable and generally underestimate actual values while correlating poorly with BPH symptoms.14,18
Urinalysis. If you suspect BPH, you'll need to order a urinalysis to screen for infection, cancer, or stones and additional lab studies based on the patient's history, including measurements of serum creatinine, calcium, glucose, and prostate-specific antigen (PSA), among others.4,15
PSA values. These values should be checked if the patient's life expectancy is greater than a decade and a diagnosis of prostate cancer would influence treatment decisions. Adjustments of accepted norms should account for increasing age (40 to 50 years, 0-2.5 ng/mL; 51 to 60 years, 2.5-3.5 ng/mL; 61 to 70 years, 3.5-4.5 ng/mL; 71 to 80 years, 4.5-6.5 ng/mL), and urologic referral should be made as indicated.18
PSA determination is a more accurate reflection of prostate volume than a DRE and helps establish a pretreatment reference point before 5-a reductase inhibitor therapy.19 These drugs lower PSA concentrations approximately 50% and may complicate subsequent cancer screening.15
The US Preventive Services Task Force (USPSTF) clinical guidelines for prostate cancer screening notes that among patients with enlarged prostates, the specificity of PSA testing is lower, and thus PSA is a less accurate means of detecting cancer in BPH patients.20 Indeed, the USPSTF guidelines are ambivalent on the utility of PSA, in part because of the heterogeneity of prostate tumors, although they do confirm the greater accuracy of PSA testing over DRE.20
The guidelines state that screening is most effective at determining patients with a particularly good or poor long-term prognosis, which constitutes a fairly small minority of patients, but is less effective in the larger middle group.20 Regarding the particular means of testing PSA, the USPSTF guidelines note that free or complex testing is primarily useful to distinguish whether a patient should undergo a biopsy among those with a PSA level of 4.0 to 9.9 ng/mL.20 A more recent perspective from Cleveland Clinic clinicians indicates that the "PSA cutoff era" is now past and that decisions for further prostate cancer screening should be made with a patient's DRE and family history data in mind.21
Diagnostic studies. Noninvasive urine flow rates, postvoid residual measures, pressure-flow studies, cystoscopy, and renal or transrectal ultrasound are optional unless dictated by specific circumstances, including recurrent hematuria, pelvic pain, or urinary retention, in which case urologic consultation is indicated.4,15
Weigh patient preference against symptom severity
The treatment goals for a patient with BPH-related lower urinary tract symptoms must focus on improving and maintaining quality of life, achieving and sustaining symptom control, and avoiding disease progression.22 In choosing a specific treatment, weigh the patient's preferences against symptom severity and specific physiologic variables; even individuals with moderate IPSS ratings may improve (40%) or show no change (45%) with watchful waiting.23 (The AUA outlines treatment options for patients with moderate to severe symptoms in its BPH practice guidelines. They can be accessed on page 16 of http://www.auanet.org/guidelines/main_reports/bph_management/chapt_1_appendix.pdf.
Quality-of-life issues—how much lower urinary tract symptoms interfere with work, social life, sleep, sexual function, and travel—are generally more important than the symptoms per se.14 The AUA has published a diagnosis and treatment algorithm for BPH that is very helpful for practitioners.4 It is available on page 7 of http://www.auanet.org/guidelines/main_reports/bph_management/chapt_1_appendix.pdf.
Watchful waiting—even with high IPSS ratings
Watchful waiting is an option for patients experiencing minimal bother—even with high IPSS ratings—because the risk for progression is relatively small.4,14,15 If you choose this route, encourage the patient to minimize alcohol and caffeine use and the intake of fluids in the evening, and minimize the use of a-agonist, anticholinergic, antihistaminic, and calcium-channel blocker medications.
Where nocturia is a particular problem, diuretics timed to minimize night-time urine production, daytime naps, and use of antidiuretic hormones (although contraindicated in patients with congestive heart failure) may be appropriate.24,25 Notably, in the context of combined bladder outlet obstruction and detrusor overactivity validated by urodynamic studies, there are recent studies identifying a role for anticholinergics.26,27
Medical therapy before surgery
Medical therapy has supplanted surgery as the primary therapeutic tool for BPH-related lower urinary tract symptoms.4 a-Adrenergic antagonists decrease prostatic and urethral smooth muscle tone, induce tissue apoptosis through tumor growth factor-beta signaling, and increase detrusor muscle vascular supply, while 5-a reductase inhibitors block conversion of testosterone to dihydrotestosterone and reduce prostate volume ( TABLE ).4,14,15,28-40
a-Adrenergic blockers. Nonselective a-adrenergic blockers include terazosin, doxazosin, and alfuzosin. Their greater selectivity for nonprostatic peripheral vasculature a-1B receptors than for prostatic a-1A receptors account for their potential to cause orthostatic hypotension. A fourth agent, tamsulosin, is mostly selective for the prostatic a-1A receptor and does not have a clinically significant effect on blood pressure.30
At therapeutic doses, these drugs have comparable efficacy in lowering IPSS scores, increasing urine flow rates, and improving symptoms.4 Potential side effects include asthenia, headache, dizziness, and peripheral edema. Early postural hypotension and later rebound hypertension on withdrawal are primarily seen with terazosin and doxazosin, which require titration and tapering over 2 to 3 weeks when being introduced or eliminated. The uroselectivity of alfuzosin, as well as new dosing formulations, have helped reduce hypotensive side effects.28,29 Like tamsulosin, it can be started and stopped directly.
TABLE
Medical therapies for BPH at a glance4,14,15,28-40
| TYPE OF THERAPY | ACTIVITY | EFFICACY IN CLINICAL TRIALS | SIDE EFFECTS | INDICATIONS | NUMBER NEEDED TO TREAT* |
|---|---|---|---|---|---|
| a-Adrenergic blockers Nonselective Terazosin Doxazosin Alfuzosin Selective Tamsulosin |
|
|
|
| Terazosin 4.0 (to achieve >10% improvement in Boyarsky score, an older measure comparable to the IPSS)31 Doxazosin 13.7 (for the prevention of clinical progression)32 Alfuzosin 5.8 (to achieve =3 points improvement in IPSS)33 Tamsulosin 4.5 (to achieve =25% increase in AUA score)34 |
| 5- a Reductase inhibitors Dutasteride Finasteride |
|
| Dutasteride mild-to-moderate symptoms : 10 (to achieve 2-point improvement in AUA-SS) severe symptoms: 6.3 (to achieve 2-point improvement in AUA-SS)37,38 Finasteride 15.0 (for the prevention of clinical progression)32 | ||
| Combination therapy with a-Adrenergic blockers and 5-a reductase inhibitors |
|
|
| Combination of doxazosin + finasteride 8.4 (for the prevention of clinical progression)32 | |
| Phytotherapy Saw palmetto |
|
|
|
| N/A |
| *Number needed to treat (NNT) values should not be regarded as points of efficacy comparison since they are not consistently based on head-to-head trials, are derived from different patient populations, and may refer to different efficacy end points as well as different lengths of follow-up. | |||||
| AUA-SS, American Urological Association symptom score; BPH, benign prostatic hyperplasia; EAU, European Association of Urology; IPSS, International Prostate Symptom Score; LUTS, lower urinary tract symptoms; N/A, not available; PSA, prostate-specific antigen; QOL, quality of life. | |||||
Although infrequently reported in clinical trials, rhinitis and ejaculatory dysfunction are known side effects of tamsulosin and alfuzosin.30,41 a1-Blockers have recently been reported as possibly having an association with intraoperative floppy iris syndrome (IFIS), a surgical condition that has been observed during phacoemulsification cataract surgery.42 The etiology of this syndrome is unknown. Patients undergoing cataract surgery who are taking a1-blockers should inform their surgeons, who should be prepared for possible modifications to the surgical technique. The benefit of stopping a1-blocker therapy prior to cataract surgery has not been established.43-45
5- a Reductase inhibitors. Finasteride and dutasteride are comparable in efficacy and have been shown to decrease prostate volume (20%-30%), lower IPSS ratings 3 to 4 points, increase urine flow rates, and decrease urinary retention and the need for surgery (50%) when compared with placebo.15 Their clinical effect appears gradually over 3 to 6 months, and they are most beneficial when prostate volume exceeds 40 mL.35
Decreased libido (6%), ED (8%), and ejaculatory disorders (4%) are the main side effects of these drugs, as is their lowering of PSA levels by as much as half.15 This latter effect may prompt checking PSA velocities and free:total PSA ratios as a part of prostate cancer screening. Additionally, finasteride may reduce the prevalence of prostate cancer almost 25% compared with placebo, but more high-grade tumors may be associated with its use.36 The reason for this difference and its clinical importance require further study.36,46,47
Combination therapy. Combination therapy with a-adrenergic blockers and 5-a reductase inhibitors has increased due to results from the long-term (4.5 years) Medical Therapy of Prostatic Symptoms (MTOPS) study.32 It compared the efficacy of placebo, doxazosin, finasteride, and combination therapy on clinical progression measures of BPH. These were defined as an increase of 4 points on the IPSS, acute urinary retention, urinary incontinence, renal insufficiency, or recurrent urinary tract infections. All drug treatments significantly improved symptom scores, but the combination was clearly superior.32 Additionally, combination therapy and finasteride significantly reduced urinary retention and the need for surgery, whereas doxazosin did not.
The number needed to treat (NNT) for the prevention of a single instance of clinical progression over a 4-year period was 8.4 for combination therapy, compared with 13.7 for doxazosin monotherapy and 15.0 for finasteride monotherapy ( TABLE ).32
A secondary analysis, conducted to establish the NNT for disease progression in patients with larger baseline prostates or higher serum PSA, found that among patients with a PSA level >4.0 ng/mL, the NNT was 4.7 (vs 7.2 for finasteride), and for patients with a prostate volume >40 mL, the NNT was 4.9 (vs 7.2 for finasteride).32 These results suggest that patients with larger glands and higher PSA values, who are at greatest risk for progression, would benefit from combination approaches, although absolute threshold values are not yet clear.4
A combination of an a-adrenergic blocker and an anticholinergic medication may also be used in the treatment of comorbid lower urinary tract symptoms and overactive bladder. A 12-week placebo-controlled trial of a combination of tamsulosin and the anticholinergic tolterodine found significant benefits in terms of IPSS scores, urgency episodes, frequency of micturitions, quality-of-life scores, and patient perception of treatment benefit.48
Phytotherapy. Saw palmetto is derived from the ripe berries of the American dwarf palm (Serenoa repens or Sabal serrulata); retail sales in the United States totaled over $20 million in 2004.49 The mechanism of action is uncertain, but may involve antiandrogen activity. Short-term improvement of nocturia and peak urinary flow comparable with that of finasteride has been suggested by meta-analyses involving almost 3000 patients in trials ranging from 1 month to 1 year.39 However, neither American nor European guidelines recommend its use.4,15,40
A 6-month, double-blind, placebo-controlled trial of urtica dioica (stinging nettle) in 620 BPH patients found a significant improvement in IPSS scores, peak flow rates, and a small but significant reduction in prostate size among patients taking urtica dioica compared with baseline.50
Bothersome symptoms? Consider surgery
Transurethral resection of the prostate (TURP) was the primary surgical approach during most of the 20th century and remains the benchmark. TURP involves removing a portion of the prostate through the urethra.4 When compared with watchful waiting, TURP achieved better outcomes with men most bothered by symptoms at the outset. Watchful waiting was also considered safe, but 24% of this group underwent surgery during the 3 years. There were no increases in urinary incontinence or ED among surgically treated patients.51
TURP is indicated for patients with refractory urinary retention due to severe bladder outlet obstruction, recurrent urinary tract infections, progressive renal insufficiency, hematuria unresponsive to 5-a reductase inhibitors, bladder stones, and BPH-related hydronephrosis. Lower urinary tract symptom improvement is expected in 80% to 90% of cases, with a retained efficacy of 75% for at least 7 years and a risk of repeating surgery of only 1% annually.4,14,15 IPSS scores may decrease 15 to 20 points, but quality of life is enhanced only with severe lower urinary tract symptoms, and postoperative ejaculatory dysfunction (65%-70%) is expected, along with 1% to 2% perioperative mortality.15
It's important to note that TURP is a procedure that requires a hospital stay and is associated with a variety of potential side effects, including sexual dysfunction, bladder neck contracture, urinary tract infection, hematuria, and irritative voiding symptoms, while patients may also require blood transfusions.4,52
Other surgical therapies include open prostatectomies for patients with glands =80 mL and transurethral incision of the prostate (TUIP) for glands <30 mL.1,4,15 TUIP is a simpler outpatient operation than TURP. It offers equivalent symptomatic relief and less associated ejaculatory dysfunction or bleeding, but has a higher rate of reoperation.1,4,15 High-risk surgical candidates with severe urinary retention may also receive prostatic stents, but significant complications of pain, infection, and encrustation are common.4
Holmium laser enucleation of the prostate (HOLEP) is another alternative to TURP that has demonstrated equivalent efficacy in terms of AUA-SI scores, peak flow rates, and quality-of-life scores in studies of up to 3 years in length. Longer-term studies are required to determine its efficacy beyond that time frame.53-56
Minimally invasive route for high-risk patients
Transurethral needle ablation (TUNA) and transurethral microwave thermotherapy (TUMT) offer AUA-approved alternative treatment choices based on the severity of symptoms and the presence of complications.4 TUNA uses radiofrequency waves administered through 2 18-gauge needles to heat prostatic tissue. An outpatient procedure, TUNA is effective over the long term, demonstrating a low failure rate (25% after 5 years); however, temporary side effects such as irritative urinary symptoms and urinary retention can occur.4,57
With >100,000 procedures performed, TUMT is the most frequent minimally invasive treatment utilized worldwide. Heat destroys targeted prostatic tissue, while a cooling system protects the prostatic urethra. Its efficacy has been demonstrated by randomized trials, and its failure rate documented at 10% to 16% annually.4 Morbidity is related mainly to required indwelling catheterization for 4 to 6 weeks following intervention. However, it is an outpatient, low-risk procedure well-suited to high-risk patients or those who oppose surgery.
Correspondence
Darryl Chutka, MD, Division of Preventive and Occupational Medicine and Internal Medicine, Mayo Clinic College of Medicine, Rochester, MN 55905; [email protected].
1. Granville LJ. Prostate disease. In: Cobbs EL, Duthie EH, Murphy JB, eds. Geriatrics review syllabus: a core curriculum in geriatric medicine. 5th ed. Malden, Mass: Blackwell Publishing; 2002:384-385.
2. Collins MFM, Friedman RH, Ash A, Hall R, Moskowitz M. Underdetection of clinical benign prostatic hyperplasia in a general medical practice. J Gen Intern Med. 1996;11:513-518.
3. Hegarty NJ, Fitzpatrick JM. High intensity focused ultrasound in benign prostatic hyperplasia. Eur J Ultrasound. 1999;9:55-60.
4. AUA Practice Guidelines Committee. AUA guideline on management of benign prostatic hyperplasia (2003). Chapter 1: Diagnosis and treatment recommendations. J Urol. 2003;170:530-547.
5. Welch G, Weinger K, Barry MJ. Quality-of-life impact of lower urinary tract symptom severity: results from the health professionals follow-up study. Urology. 2002;59:245-250.
6. Cunningham-Burley S, Allbutt, Garraway WM, Lee AJ, Russell EBAW. Perceptions of urinary symptoms and health-care-seeking behavior amongst men aged 40-79 years. Br J Gen Pract. 1996;46:349-352.
7. Wei JT, Calhoun E, Jacobsen SJ. Urologic diseases in America project: benign prostatic hyperplasia. J Urol. 2005;173:1256-1261.
8. Rosen RC, Giuliano F, Carson CC. Sexual dysfunction and lower urinary tract symptoms associated with benign prostatic hyperplasia. Eur Urol. 2005;47:824-837.
9. Assessing prostate symptoms. Patient UK. EMIS and Patient Information Publications 1997-2007. Available at: http://www.patient.co.uk/showdoc/23069171/. Accessed January 3, 2007.
10. O'Leary MP. Validity of the "bother score" in the evaluation and treatment of symptomatic benign prostatic hyperplasia. Rev Urol. 2005;7:1-10.
11. Barry MJ, Fowler FJ, Jr, O'Leary MP, Bruskewitz RC, Holtgrewe HL, Mebust WK, Cockett AT. The American Urological Association symptom index for benign prostatic hyperplasia. The Measurement Committee of the American Urological Association. J Urol. 1992;148:1549-1557.
12. Barry MJ. Benign prostatic hyperplasia. In: Dale DC, Federman DD, eds. ACP Medicine. New York: WebMD; 2005.
13. Barry M, Roehrborn C. Management of benign prostate hyperplasia. Annu Rev Med. 1997;48:177-189.
14. Dubeau CE. Benign prostate disorders. In: Hazzard WR, Blass JP, Halter JB, Ouslander JG, Tinetti ME, eds. Principles of Geriatric Medicine and Gerontology. 5th ed. New York: McGraw-Hill; 2003:1303-1310.
15. Madersbacher S, Alivizatos G, Nordling J, Sanz CR, Emberton M, de la Rosette JJ. EAU 2004 Guidelines on assessment, therapy and follow-up of men with lower urinary symptoms suggestive of benign prostatic obstruction (BPH Guidelines). Eur Urol. 2004;46:547-554.
16. Chapple CR, Roehrborn CG. A shifted paradigm for the further understanding, evaluation, and treatment of lower urinary tract symptoms in men: focus on the bladder. Eur Urol. 2006;49:651-659.
17. Knutson T, Edlund C, Fall M, Dahlstrand C. BPH with coexisting overactive bladder dysfunction—an everyday urological dilemma. Neurourol Urodyn. 2001;20:237-247.
18. Hellerstedt BA, Pienta KJ. In: Hazzard WR, Blass JP, Ouslander JG, Tinetti ME, eds. Principles of Geriatric Medicine and Gerontology. 5th ed. New York: McGraw-Hill; 2003:1303-10.
19. Roehrborn CG, McConnell JD, Bonilla J, Rosenblatt S, et al. Serum prostate-specific antigen is a strong predictor of future prostate growth in men with benign prostatic hyperplasia: PROSCAR long-term efficacy and safety study. J Urol. 2000;163:13-20.
20. Harris R, Lohr KN. Screening for prostate cancer: an update of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med. 2002;137:917-929.
21. Jones JS, Klein E. Four no more: the 'PSA cutoff era' is over. Cleve Clin J Med. 2008;75:30-32.
22. O'Leary MP. Lower urinary tract symptoms/benign prostatic hyperplasia: Maintaining symptom control and reducing complications. Urology. 2003;62(Suppl 3A):15-23.
23. Oesterling JE. Benign prostatic hyperplasia: medical and minimally invasive treatment options. N Engl J Med. 1995;332:99-109.
24. Weiss JP, Blaivas JG. Nocturia. J Urol. 2000;163:5-12.
25. Reynard JM, Cannon A, Yang Q, Abrams P. A novel therapy for nocturnal polyuria: a double-blind randomized trial of furosemide against placebo. Br J Urol. 1998;81:215-218.
26. Abrams P, Kaplan S, De Konig Gans HJ, Millard R. Safety and tolerability of tolterodine for the treatment of overactive bladder in men with bladder outlet obstruction. J Urol. 2006;175:999-1004.
27. Athanasopoulos A, Gyftopoulos K, Giannitsas K, Fisfis J, Perimenis P, Barbalias G. Combination treatment with an alpha-blocker plus an anticholinergic for bladder outlet obstruction: a prospective, randomized controlled study. J Urol. 2003;169:2253-2256
28. van Kerrebroeck P, Jardin A, Laval KU, van Cangh P. Efficacy and safety of a new prolonged release formulation of alfuzosin 10 mg once daily versus alfuzosin 2.5 mg thrice daily and placebo in patients with symptomatic benign prostatic hyperplasia. Eur Urol. 2000;7:306-313.
29. Mottet N, Bressolle F, Delmas V, Robert M, Costa P. Prostatic tissual distribution of alfuzosin in patients with benign prostatic hyperplasia following repeated oral administration. Eur Urol. 2003;44:101-105.
30. Beduschi MC, Beduschi R, Oesterling JE. Alpha-blockade therapy for benign prostatic hyperplasia: from a nonselective to a more selective alpha1A-adrenergic antagonist. Urology. 1998;51:861-872.
31. Lepor H, Auerbach S, Puras-Baez A, et al. A randomized, placebo-controlled multicenter study of the efficacy and safety of terazosin in the treatment of benign prostatic hyperplasia. J Urol. 1992;148:1467-1474.
32. McConnell JD, Roehrborn CG, Bautista OM, et at. The long-term effect of doxazosin, finasteride, and combination therapy on the clinical progression of benign prostatic hyperplasia. N Engl J Med. 2003;349:2387-2398.
33. Roehrborn CG. Efficacy and safety of once-daily alfuzosin in the treatment of lower urinary tract symptoms and clinical benign prostatic hyperplasia: a randomized, placebo-controlled trial. Urology. 2001;58:953-959.
34. Lepor H. Long-term evaluation of tamsulosin in benign prostatic hyperplasia: placebo-controlled, double-blind extension of phase III trial. Tamsulosin Investigator Group. Urology. 1998;51:901-906.
35. Clifford GM, Farmer RDT. Medical therapy for benign prostatic hyperplasia: a review of the literature. Eur Urol. 2000;38:2-19.
36. Thompson IM, Goodman PJ, Tangen CM, et al. The influence of finasteride on the development of prostate cancer. N Engl J Med. 2003;349:215-224.
37. Roehrborn CG. The clinical benefits of dutasteride treatment for LUTS and BPH. Rev Urol. 2004;6(Suppl 9):S22-SS30.
38. Roehrborn CG, Lukkarinen O, Mark S, Siami P, Ramsdell J, Zinner N. Long-term sustained improvement in symptoms of benign prostatic hyperplasia with the dual 5alpha-reductase inhibitor dutasteride: results of 4-year studies. BJU Int. 2005;96:572-577.
39. Edzard E. The risk-benefit profile of commonly used herbal therapies: ginkgo, St John's wort, ginseng, Echinacea, saw palmetto, and kava. Ann Intern Med. 2002;136:42-53.
40. Bent S, Kane C, Shinohara K, et al. Saw palmetto for benign prostatic hyperplasia. N Engl J Med. 2006;354:557-566.
41. Food and Drug Administration. ALFOTAM trial. Center for Drug Administration and Research. Available at: www.pbm.va.gov/criteria/Alpha-Blocker%20CFU%207-2005.pdf. Accessed November 16, 2006.
42. Chang DF, Campbell JR. Intraoperative floppy iris syndrome associated with tamsulosin. J Cataract Refract Surg. 2005;31:664-673.
43. Uroxatral [package insert]. Bridgewater, NJ: Sanofi-Aventis U.S.; 2007.
44. Cardura XL [package insert]. New York, NY: Pfizer Roerig; 2006.
45. Flomax [package insert]. Ridgefield, CT: Boehringer Ingelheim Pharmaceuticals; 2008.
46. Finasteride [package insert]. Whitehouse Station, NJ: Merck & Co. Inc.; 2007.
47. Dutasteride [package insert]. Research Triangle Park, NC: GlaxoSmithKline; 2005.
48. Kaplan SA, Roehrborn CG, Rovner ES, Carlsson M, Bavendam T, Guan Z. Tolterodine and tamsulosin for treatment of men with lower urinary tract symptoms and overactive bladder: a randomized controlled trial. JAMA. 2006;296:2319-2328.Erratum in: JAMA 2007; 297:1195.
49. Healing Herbs and Natural Remedies. Available at: http://www.herbsandnaturalremedies.com/topsellers.htm. Accessed January 3, 2007.
50. Safarinejad MR. Urtica dioica for treatment of benign prostatic hyperplasia: a prospective, randomized, double-blind, placebo-controlled, crossover study. J Herb Pharmacother. 2005;5:1-11.
51. Wasson JH, Reda DJ, Bruskewitz RC, Elinson J, Keller AM, Henderson WG. A comparison of transurethral surgery with watchful waiting for moderate symptoms of benign prostatic hyperplasia. N Engl J Med. 1995;332:75-79.
52. Hammadeh MY, Madaan S, Hines J, Philp T. 5-year outcome of a prospective randomized trial to compare transurethral electrovaporization of the prostate and standard transurethral resection. Urology. 2003;61:1166-1171.
53. Ahyai SA, Lehrich K, Kuntz RM. Holmium laser enucleation versus transurethral resection of the prostate: 3-year follow-up results of a randomized clinical trial. Eur Urol. 2007;52:1456-1464.
54. Kuntz RM. Laser treatment of benign prostatic hyperplasia. World J Urol. 2007;25:241-247.
55. Gupta N, Sivaramakrishna, Kumar R, Dogra PN, Seth A. Comparison of standard transurethral resection, transurethral vapour resection and holmium laser enucleation of the prostate for managing benign prostatic hyperplasia of >40 g. BJU Int. 2006;97:85-89.
56. Tan AH, Gilling PJ, Kennett KM, Frampton C, Westenberg AM, Fraundorfer MR. A randomized trial comparing holmium laser enucleation of the prostate with transurethral resection of the prostate for the treatment of bladder outlet obstruction secondary to benign prostatic hyperplasia in large glands (40 to 200 grams). J Urol. 2003;170(4 Pt 1):1270-1274.
57. Zlotta AR, Giannakopoulos X, Maehlum O, Ostrem T, Schulman CC. Long-term evaluation of transurethral needle ablation of the prostate (TUNA) for treatment of symptomatic benign prostatic hyperplasia: clinical outcome up to five years from three centers. Eur Urol. 2003;44:89-93.
1. Granville LJ. Prostate disease. In: Cobbs EL, Duthie EH, Murphy JB, eds. Geriatrics review syllabus: a core curriculum in geriatric medicine. 5th ed. Malden, Mass: Blackwell Publishing; 2002:384-385.
2. Collins MFM, Friedman RH, Ash A, Hall R, Moskowitz M. Underdetection of clinical benign prostatic hyperplasia in a general medical practice. J Gen Intern Med. 1996;11:513-518.
3. Hegarty NJ, Fitzpatrick JM. High intensity focused ultrasound in benign prostatic hyperplasia. Eur J Ultrasound. 1999;9:55-60.
4. AUA Practice Guidelines Committee. AUA guideline on management of benign prostatic hyperplasia (2003). Chapter 1: Diagnosis and treatment recommendations. J Urol. 2003;170:530-547.
5. Welch G, Weinger K, Barry MJ. Quality-of-life impact of lower urinary tract symptom severity: results from the health professionals follow-up study. Urology. 2002;59:245-250.
6. Cunningham-Burley S, Allbutt, Garraway WM, Lee AJ, Russell EBAW. Perceptions of urinary symptoms and health-care-seeking behavior amongst men aged 40-79 years. Br J Gen Pract. 1996;46:349-352.
7. Wei JT, Calhoun E, Jacobsen SJ. Urologic diseases in America project: benign prostatic hyperplasia. J Urol. 2005;173:1256-1261.
8. Rosen RC, Giuliano F, Carson CC. Sexual dysfunction and lower urinary tract symptoms associated with benign prostatic hyperplasia. Eur Urol. 2005;47:824-837.
9. Assessing prostate symptoms. Patient UK. EMIS and Patient Information Publications 1997-2007. Available at: http://www.patient.co.uk/showdoc/23069171/. Accessed January 3, 2007.
10. O'Leary MP. Validity of the "bother score" in the evaluation and treatment of symptomatic benign prostatic hyperplasia. Rev Urol. 2005;7:1-10.
11. Barry MJ, Fowler FJ, Jr, O'Leary MP, Bruskewitz RC, Holtgrewe HL, Mebust WK, Cockett AT. The American Urological Association symptom index for benign prostatic hyperplasia. The Measurement Committee of the American Urological Association. J Urol. 1992;148:1549-1557.
12. Barry MJ. Benign prostatic hyperplasia. In: Dale DC, Federman DD, eds. ACP Medicine. New York: WebMD; 2005.
13. Barry M, Roehrborn C. Management of benign prostate hyperplasia. Annu Rev Med. 1997;48:177-189.
14. Dubeau CE. Benign prostate disorders. In: Hazzard WR, Blass JP, Halter JB, Ouslander JG, Tinetti ME, eds. Principles of Geriatric Medicine and Gerontology. 5th ed. New York: McGraw-Hill; 2003:1303-1310.
15. Madersbacher S, Alivizatos G, Nordling J, Sanz CR, Emberton M, de la Rosette JJ. EAU 2004 Guidelines on assessment, therapy and follow-up of men with lower urinary symptoms suggestive of benign prostatic obstruction (BPH Guidelines). Eur Urol. 2004;46:547-554.
16. Chapple CR, Roehrborn CG. A shifted paradigm for the further understanding, evaluation, and treatment of lower urinary tract symptoms in men: focus on the bladder. Eur Urol. 2006;49:651-659.
17. Knutson T, Edlund C, Fall M, Dahlstrand C. BPH with coexisting overactive bladder dysfunction—an everyday urological dilemma. Neurourol Urodyn. 2001;20:237-247.
18. Hellerstedt BA, Pienta KJ. In: Hazzard WR, Blass JP, Ouslander JG, Tinetti ME, eds. Principles of Geriatric Medicine and Gerontology. 5th ed. New York: McGraw-Hill; 2003:1303-10.
19. Roehrborn CG, McConnell JD, Bonilla J, Rosenblatt S, et al. Serum prostate-specific antigen is a strong predictor of future prostate growth in men with benign prostatic hyperplasia: PROSCAR long-term efficacy and safety study. J Urol. 2000;163:13-20.
20. Harris R, Lohr KN. Screening for prostate cancer: an update of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med. 2002;137:917-929.
21. Jones JS, Klein E. Four no more: the 'PSA cutoff era' is over. Cleve Clin J Med. 2008;75:30-32.
22. O'Leary MP. Lower urinary tract symptoms/benign prostatic hyperplasia: Maintaining symptom control and reducing complications. Urology. 2003;62(Suppl 3A):15-23.
23. Oesterling JE. Benign prostatic hyperplasia: medical and minimally invasive treatment options. N Engl J Med. 1995;332:99-109.
24. Weiss JP, Blaivas JG. Nocturia. J Urol. 2000;163:5-12.
25. Reynard JM, Cannon A, Yang Q, Abrams P. A novel therapy for nocturnal polyuria: a double-blind randomized trial of furosemide against placebo. Br J Urol. 1998;81:215-218.
26. Abrams P, Kaplan S, De Konig Gans HJ, Millard R. Safety and tolerability of tolterodine for the treatment of overactive bladder in men with bladder outlet obstruction. J Urol. 2006;175:999-1004.
27. Athanasopoulos A, Gyftopoulos K, Giannitsas K, Fisfis J, Perimenis P, Barbalias G. Combination treatment with an alpha-blocker plus an anticholinergic for bladder outlet obstruction: a prospective, randomized controlled study. J Urol. 2003;169:2253-2256
28. van Kerrebroeck P, Jardin A, Laval KU, van Cangh P. Efficacy and safety of a new prolonged release formulation of alfuzosin 10 mg once daily versus alfuzosin 2.5 mg thrice daily and placebo in patients with symptomatic benign prostatic hyperplasia. Eur Urol. 2000;7:306-313.
29. Mottet N, Bressolle F, Delmas V, Robert M, Costa P. Prostatic tissual distribution of alfuzosin in patients with benign prostatic hyperplasia following repeated oral administration. Eur Urol. 2003;44:101-105.
30. Beduschi MC, Beduschi R, Oesterling JE. Alpha-blockade therapy for benign prostatic hyperplasia: from a nonselective to a more selective alpha1A-adrenergic antagonist. Urology. 1998;51:861-872.
31. Lepor H, Auerbach S, Puras-Baez A, et al. A randomized, placebo-controlled multicenter study of the efficacy and safety of terazosin in the treatment of benign prostatic hyperplasia. J Urol. 1992;148:1467-1474.
32. McConnell JD, Roehrborn CG, Bautista OM, et at. The long-term effect of doxazosin, finasteride, and combination therapy on the clinical progression of benign prostatic hyperplasia. N Engl J Med. 2003;349:2387-2398.
33. Roehrborn CG. Efficacy and safety of once-daily alfuzosin in the treatment of lower urinary tract symptoms and clinical benign prostatic hyperplasia: a randomized, placebo-controlled trial. Urology. 2001;58:953-959.
34. Lepor H. Long-term evaluation of tamsulosin in benign prostatic hyperplasia: placebo-controlled, double-blind extension of phase III trial. Tamsulosin Investigator Group. Urology. 1998;51:901-906.
35. Clifford GM, Farmer RDT. Medical therapy for benign prostatic hyperplasia: a review of the literature. Eur Urol. 2000;38:2-19.
36. Thompson IM, Goodman PJ, Tangen CM, et al. The influence of finasteride on the development of prostate cancer. N Engl J Med. 2003;349:215-224.
37. Roehrborn CG. The clinical benefits of dutasteride treatment for LUTS and BPH. Rev Urol. 2004;6(Suppl 9):S22-SS30.
38. Roehrborn CG, Lukkarinen O, Mark S, Siami P, Ramsdell J, Zinner N. Long-term sustained improvement in symptoms of benign prostatic hyperplasia with the dual 5alpha-reductase inhibitor dutasteride: results of 4-year studies. BJU Int. 2005;96:572-577.
39. Edzard E. The risk-benefit profile of commonly used herbal therapies: ginkgo, St John's wort, ginseng, Echinacea, saw palmetto, and kava. Ann Intern Med. 2002;136:42-53.
40. Bent S, Kane C, Shinohara K, et al. Saw palmetto for benign prostatic hyperplasia. N Engl J Med. 2006;354:557-566.
41. Food and Drug Administration. ALFOTAM trial. Center for Drug Administration and Research. Available at: www.pbm.va.gov/criteria/Alpha-Blocker%20CFU%207-2005.pdf. Accessed November 16, 2006.
42. Chang DF, Campbell JR. Intraoperative floppy iris syndrome associated with tamsulosin. J Cataract Refract Surg. 2005;31:664-673.
43. Uroxatral [package insert]. Bridgewater, NJ: Sanofi-Aventis U.S.; 2007.
44. Cardura XL [package insert]. New York, NY: Pfizer Roerig; 2006.
45. Flomax [package insert]. Ridgefield, CT: Boehringer Ingelheim Pharmaceuticals; 2008.
46. Finasteride [package insert]. Whitehouse Station, NJ: Merck & Co. Inc.; 2007.
47. Dutasteride [package insert]. Research Triangle Park, NC: GlaxoSmithKline; 2005.
48. Kaplan SA, Roehrborn CG, Rovner ES, Carlsson M, Bavendam T, Guan Z. Tolterodine and tamsulosin for treatment of men with lower urinary tract symptoms and overactive bladder: a randomized controlled trial. JAMA. 2006;296:2319-2328.Erratum in: JAMA 2007; 297:1195.
49. Healing Herbs and Natural Remedies. Available at: http://www.herbsandnaturalremedies.com/topsellers.htm. Accessed January 3, 2007.
50. Safarinejad MR. Urtica dioica for treatment of benign prostatic hyperplasia: a prospective, randomized, double-blind, placebo-controlled, crossover study. J Herb Pharmacother. 2005;5:1-11.
51. Wasson JH, Reda DJ, Bruskewitz RC, Elinson J, Keller AM, Henderson WG. A comparison of transurethral surgery with watchful waiting for moderate symptoms of benign prostatic hyperplasia. N Engl J Med. 1995;332:75-79.
52. Hammadeh MY, Madaan S, Hines J, Philp T. 5-year outcome of a prospective randomized trial to compare transurethral electrovaporization of the prostate and standard transurethral resection. Urology. 2003;61:1166-1171.
53. Ahyai SA, Lehrich K, Kuntz RM. Holmium laser enucleation versus transurethral resection of the prostate: 3-year follow-up results of a randomized clinical trial. Eur Urol. 2007;52:1456-1464.
54. Kuntz RM. Laser treatment of benign prostatic hyperplasia. World J Urol. 2007;25:241-247.
55. Gupta N, Sivaramakrishna, Kumar R, Dogra PN, Seth A. Comparison of standard transurethral resection, transurethral vapour resection and holmium laser enucleation of the prostate for managing benign prostatic hyperplasia of >40 g. BJU Int. 2006;97:85-89.
56. Tan AH, Gilling PJ, Kennett KM, Frampton C, Westenberg AM, Fraundorfer MR. A randomized trial comparing holmium laser enucleation of the prostate with transurethral resection of the prostate for the treatment of bladder outlet obstruction secondary to benign prostatic hyperplasia in large glands (40 to 200 grams). J Urol. 2003;170(4 Pt 1):1270-1274.
57. Zlotta AR, Giannakopoulos X, Maehlum O, Ostrem T, Schulman CC. Long-term evaluation of transurethral needle ablation of the prostate (TUNA) for treatment of symptomatic benign prostatic hyperplasia: clinical outcome up to five years from three centers. Eur Urol. 2003;44:89-93.
Statins and elevated liver tests: What’s the fuss?
- Order liver function tests before starting statin therapy, 12 weeks after initiation, with any dose increase, and periodically for long-term maintenance therapy (C).
- Mild elevations of alanine aminotransferase (ALT) or aspartate aminotransferase (AST) (<3 times the upper limit of normal [ULN]) following statin therapy do not appear to lead to significant liver toxicity over time (C).
- Other medications that lower low-density lipoprotein (LDL), and might be substituted for statins, may not improve morbidity and mortality (C).
Strength of recommendation (SOR)
- Good-quality patient-oriented evidence
- Inconsistent or limited-quality patient-oriented evidence
- Consensus, usual practice, opinion, disease-oriented evidence, case series
Are we more aggressive than ever when it comes to our use of statins? You bet.
Should this prompt a heightened attention to hepatic safety? In a word, no. The more detailed, evidence-based answer (which follows) makes 2 things clear:
1. Clinically significant hepatic injury following statin use is very rare.
2. While US Food and Drug Administration (FDA) labeling recommends routine monitoring of serum transaminase levels prior to and during statin therapy, the evidence suggests that such routine monitoring is not clinically necessary.
More potent statins, more combination therapy
Our prescribing has become more aggressive to keep pace with National Cholesterol Education Program (NCEP) recommendations. In 2001, the NCEP Adult Treatment Panel III indicated:
- LDL cholesterol should be the primary target of therapy.
- The LDL cholesterol goal should be based on the patient’s risk of cardiovascular disease.
- Statins are the most effective agents to achieve treatment goals.1
Three years later, the NCEP advised that in light of more recent clinical trials, even more aggressive (ie, lower) LDL goals should be considered for patients at very high risk, high risk, and moderately high risk for cardiovascular disease.2
As a result, we are prescribing higher doses of statins, more potent statins, and more combination therapies of statins with other lipid-altering agents. Not surprisingly, this trend has prompted concerns about the potential increase in toxicities/side effects of statins.
An interesting clinical question is whether statins are appropriate when the cause of hepatic enzyme elevation appears to be excess fat in the liver. There is some evidence that treatment of fatty infiltration of the liver may lower transaminase levels and improve histological findings.14 In general, though, no medications have been demonstrated to improve patient-oriented outcomes such as mortality or need for liver transplant.15
This review examines the hepatic safety profile of statins and details why there’s no need to stop treatment based on moderate elevations in liver function tests. The most common serious side effect of statins—muscle damage/rhabdomyolysis—is rare, and is not extensively discussed here.
Clinical trials: Risk is small
A review of 35 randomized clinical statin trials reported from 1966 to 2005, involving 74,102 patients, reported an absolute risk of transaminase (also referred to as aminotransferase) elevations from statin therapy of only about 4 per 1000 patients (risk difference [RD]=4.2; 95% confidence interval [CI], 1.5-6.9).3 The same researchers’ analysis of 28 clinical trials involving 62,184 patients showed a relative risk of increased transaminase of 1.3 (95% CI, 1.06-1.59), achieving statistical significance only for the fluvastatin and lovastatin trials.3
High-dose statin therapy. A review of clinical trials involving high-dose statin therapy found rates of hepatic enzyme elevation (defined as ALT or AST >3 times the ULN on 2 or more consecutive occasions) to be quite low (<1.3%).4 Higher statin doses were more likely to increase enzyme levels, though reduction in the dose or withdrawal of the statin resulted in normalization of the liver enzymes.
A study of patients ages 65 to 85 years who were treated with high-dose atorvastatin (80 mg per day) vs moderate dose pravastatin (40 mg per day) resulted in only 11 of 893 (1.23%) patients discontinuing the drug following abnormal liver function tests; most of these were in the high-dose treatment arm.5
Small risk in clinical practice, too
Clinical trials often have lower rates of adverse effects from medications than are seen in usual clinical practice.6 This may be because the stringent application of patient selection and exclusion criteria used in the administration of clinical trials does not occur in the “real world.”
However, the FDA database reported only 0.69 cases of hepatitis/liver failure per million statin prescriptions through 2004.4 A retrospective review of 1194 patients treated with a statin showed that 85% (1014) of patients had at least 1 monitoring test of transaminases performed during the year of the study. Of these, 10 (1.0%) had a significant elevation and 5 (0.5%) had a moderate elevation of transaminases. A review of the patient records demonstrated that none of these abnormalities appeared to be related to the use of statins, suggesting that routine monitoring of transaminases with statin therapy is not clinically necessary.7
A retrospective review over a 5-year period of 23,000 patients receiving statins in a large health maintenance organization found that only 17 (0.1%) patients had severe elevations of ALT (defined as >10 times the ULN). Of those 17 patients, 13 cases were associated with drug-drug interactions, and all but 1 resolved with discontinuation of the statin.8
What to monitor, how often
Product labeling for all statins advises measurement of transaminases (AST as well as ALT), although some liver experts would recommend ALT alone. ALT is found primarily in the liver, while AST is also found in muscle (cardiac and skeletal), kidneys, brain, pancreas, lungs, leukocytes, and erythrocytes. AST is, therefore, less specific for hepatic damage than ALT.
Routine monitoring of other liver function tests that measure the liver’s transport ability (eg, bilirubin, alkaline phosphate) or synthetic ability (eg, albumin, prothrombin time) will increase the likelihood of false-positive results and increase expense; they should not be done.
The 2002 American Gastroenterological Association guidelines recommend that for any hepatotoxic drug, if the ALT and/or AST elevations are <5 times the ULN, the drug should be stopped and the enzymes rechecked after an appropriate interval before pursuing a more extensive evaluation for liver disease.9
The FDA labeling information for all statins recommends liver function testing before putting a patient on a statin, 12 weeks after initiation, at any dose increase, and “periodically” for long-term maintenance therapy (TABLE 1).10 These recommendations are based on expert opinion only, because most data suggest that significant liver damage from statins is very rare and that routine monitoring of liver enzymes is not necessary.
The ACC/AHA/NHLBI Clinical Advisory on the Use and Safety of Statins agrees with the FDA, although it specifies “periodically” to mean annually.11
TABLE 1
When to monitor liver function in patients taking statins10,11
| WHEN TO CHECK ALT/AST | WHAT TO DO |
|---|---|
| Initiation of treatment or increase in dose | Begin/increase dose of statin if ALT and AST are <3 times the ULN |
| 12 weeks after initiation of statin therapy | Discontinue the statin (or lower the dose) if ALT or AST are >3 times the ULN |
| Long-term (annually or “periodically”) | |
| ALT, alanine aminotransferase; AST, aspartate aminotransferase; ULN, upper limit of normal. | |
It’s difficult to predict hepatic effects
Individual statins vary as to potency, efficacy, metabolism, and drug interactions. However, the exact mechanism of how statins cause elevations of ALT and AST is unknown, making it difficult to predict the hepatic effects of an individual statin based on its characteristics.
One analysis of multiple clinical trials concluded that overall statin toxicity (muscle, liver, etc.) was not directly related to the degree of lowering LDL cholesterol; instead, it correlated with the dose of the statin.12 As seen in TABLE 2, statins have variable drug-drug interactions based on their metabolism by the cytochrome P450 system. Drugs that increase the level of a statin in the blood may potentially increase the risk for toxicity and may warrant more cautious monitoring of liver enzymes, but are not necessarily contraindications to statin therapy.
Cyclosporine, macrolide antibiotics, azole antifungal agents, and other cytochrome P450 inhibitors (TABLE 2) are among the relative contraindications to the use of statins, more for concerns about myopathy than hepatoxicity.1 If these medications are used with a statin, consider more frequent monitoring of transaminases.
TABLE 2
Statin snapshot: LDL reductions to expect, interactions to avoid
| LOVASTATIN (MEVACOR, GENERICS) | PRAVASTATIN (PRAVACHOL, GENERICS) | SIMVASTATIN (ZOCOR, GENERICS) | FLUVASTATIN (LESCOL, GENERICS) | ATORVASTATIN (LIPITOR) | ROSUVASTATIN (CRESTOR) | |
|---|---|---|---|---|---|---|
| Usual daily dose | 20 to 80 mg | 40 to 80 mg | 10 to 80 mg | 20 to 80 mg | 10 to 80 mg | 10 to 40 mg |
| LDL reduction | 27% to 42% | 34% to 37% | 30% to 47% | 22% to 35% | 39% to 60% | 52% to 63% |
| % protein binding | >95% | 50% | 95% | 98% | >98% | 88% |
| Cytochrome P450 metabolism | 3A4* | None | 3A4* | 2C9 (75%)† 2C8 (5%) 3A4 (20%) | 3A4* | Limited 2C9 (10%) |
| * Drugs that may significantly increase statin levels via competitive metabolism or inhibition of CYP3A4 enzymes include macrolide antibiotics, HIV protease inhibitors, azole antifungal agents, calcium channel blockers, fluoxetine, cimetidine, cyclosporine, and omeprazole. Grapefruit juice may also have this effect. | ||||||
| † Drugs that may significantly increase statin levels via interference with CYP2C9 enzymes include phenytoin, glyburide, cimetidine, omeprazole, diclofenac, and cyclosporine. | ||||||
| Source: Physicians’ Desk Reference. 2008. 62nd ed. Montvale, NJ: Thomson PDR; 2008. | ||||||
Discontinue the statin?
ACC/AHA/NHLBI recommendations indicate that you should discontinue (or lower the dose of) statin therapy if the ALT or AST are above 3 times the ULN on 2 consecutive occasions.11 When elevations of ALT or AST are <3 times the ULN, consider the following:
- Statins have rigorously proven benefits for preventing morbidity and mortality due to atherosclerotic cardiovascular disease. A meta-analysis of more than 70,000 patients concluded that the number needed to treat to prevent 1 cardiovascular event was 27 and the number needed to harm (NNH) was 197. For more serious events such as creatine kinase >10 times the ULN, the NNH was 3400. Rhabdomyolysis alone was rare with a NNH of 7428.4
- Other medications that lower LDL and might be substituted for statins may not improve morbidity and mortality. For example, a recent clinical trial of ezetimibe (Zetia) reminds clinicians to be cautious in assuming that treatments that improve biochemical parameters such as LDL will necessarily result in improved clinical outcomes.13
- Mild elevations of ALT or AST (<3 times the ULN) following statin therapy are not known to lead to any significant liver toxicity over time.
- To date, there are no randomized controlled trials evaluating the optimal management of liver enzyme elevations with statin therapy.
Correspondence
Edward Onusko, MD, Family Health Center, 825 W. Locust, Wilmington, OH 45177; [email protected].
1. Expert panel on detection evaluation and treatment of high blood cholesterol in adults. Executive summary of the third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III). JAMA. 2001;285:2486-2497.
2. Grundy SM, Cleeman JI, Bairey Merz CN, et al. Implications of recent clinical trials for the National Cholesterol Education Program Adult treatment Panel III guidelines. Circulation. 2004;110:227-239.
3. Kashani MS, Phillips CO, Foody JM, et al. Risks associated with statin therapy. Circulation. 2006;114:2788-2797.
4. Davidson MH, Robinson JG. Safety of aggressive lipid management. J Am Coll Cardiol. 2007;49:1753-1762.
5. Deedwania P, Stone PH, Bairey Merz CN, et al. Effects of intensive versus moderate lipid-lowering therapy on myocardial ischemia in older patients with coronary heart disease. Circulation. 2007;115:700-707.
6. Navarro VJ, Senior JR. Drug-related hepatotoxicity. N Engl J Med. 2006;354:731-739.
7. Smith CC, Bernstein LI, Davis RB, et al. Screening for statin-related toxicity. Arch Intern Med. 2003;163:688-692.
8. Charles EC, Olson KL, Sandhoff BG, et al. Evaluation of cases of severe statin-related transaminitis within a large health maintenance organization. Am J Med. 2005;118:618-624.
9. American Gastroenterological Association. American Gastroenterological Association medical position statement: evaluation of liver chemistry tests. Gastroenterology. 2002;123:1364.-
10. Weismantel D. What laboratory monitoring is appropriate to detect adverse drug reactions in patients on cholesterol-lowering agents? J Fam Pract 2001;50:927-928.
11. Pasternak RC, Smith SC, Bairey-Merz CN, Grundy SM, Cleeman JI, Lenfant C. ACC/AHA/NHLBI Advisory on the use and safety of statins. Circulation. 2002;106:1024-1028.
12. Alsheikh-Ali AA, Maddurkuri PV, Han H, Karas RH. Effect of lipid lowering on risk of elevated liver enzymes, rhabdomyolysis, and cancer: insights from large randomized statin trials. J Am Coll Cardiol. 2007;50:409-418.
13. In brief: Zetia and Vytorin: The ENHANCE study. Med Lett Drugs Ther. 2008;50(1278):5.-
14. Bayard M, Holt J, Boroughs E. Nonalcoholic fatty liver disease. Am Fam Physician. 2006;73:1961-1968.
15. Angulo P. Nonalcoholic fatty liver disease. N Engl J Med. 2002;346:1221-1231.
- Order liver function tests before starting statin therapy, 12 weeks after initiation, with any dose increase, and periodically for long-term maintenance therapy (C).
- Mild elevations of alanine aminotransferase (ALT) or aspartate aminotransferase (AST) (<3 times the upper limit of normal [ULN]) following statin therapy do not appear to lead to significant liver toxicity over time (C).
- Other medications that lower low-density lipoprotein (LDL), and might be substituted for statins, may not improve morbidity and mortality (C).
Strength of recommendation (SOR)
- Good-quality patient-oriented evidence
- Inconsistent or limited-quality patient-oriented evidence
- Consensus, usual practice, opinion, disease-oriented evidence, case series
Are we more aggressive than ever when it comes to our use of statins? You bet.
Should this prompt a heightened attention to hepatic safety? In a word, no. The more detailed, evidence-based answer (which follows) makes 2 things clear:
1. Clinically significant hepatic injury following statin use is very rare.
2. While US Food and Drug Administration (FDA) labeling recommends routine monitoring of serum transaminase levels prior to and during statin therapy, the evidence suggests that such routine monitoring is not clinically necessary.
More potent statins, more combination therapy
Our prescribing has become more aggressive to keep pace with National Cholesterol Education Program (NCEP) recommendations. In 2001, the NCEP Adult Treatment Panel III indicated:
- LDL cholesterol should be the primary target of therapy.
- The LDL cholesterol goal should be based on the patient’s risk of cardiovascular disease.
- Statins are the most effective agents to achieve treatment goals.1
Three years later, the NCEP advised that in light of more recent clinical trials, even more aggressive (ie, lower) LDL goals should be considered for patients at very high risk, high risk, and moderately high risk for cardiovascular disease.2
As a result, we are prescribing higher doses of statins, more potent statins, and more combination therapies of statins with other lipid-altering agents. Not surprisingly, this trend has prompted concerns about the potential increase in toxicities/side effects of statins.
An interesting clinical question is whether statins are appropriate when the cause of hepatic enzyme elevation appears to be excess fat in the liver. There is some evidence that treatment of fatty infiltration of the liver may lower transaminase levels and improve histological findings.14 In general, though, no medications have been demonstrated to improve patient-oriented outcomes such as mortality or need for liver transplant.15
This review examines the hepatic safety profile of statins and details why there’s no need to stop treatment based on moderate elevations in liver function tests. The most common serious side effect of statins—muscle damage/rhabdomyolysis—is rare, and is not extensively discussed here.
Clinical trials: Risk is small
A review of 35 randomized clinical statin trials reported from 1966 to 2005, involving 74,102 patients, reported an absolute risk of transaminase (also referred to as aminotransferase) elevations from statin therapy of only about 4 per 1000 patients (risk difference [RD]=4.2; 95% confidence interval [CI], 1.5-6.9).3 The same researchers’ analysis of 28 clinical trials involving 62,184 patients showed a relative risk of increased transaminase of 1.3 (95% CI, 1.06-1.59), achieving statistical significance only for the fluvastatin and lovastatin trials.3
High-dose statin therapy. A review of clinical trials involving high-dose statin therapy found rates of hepatic enzyme elevation (defined as ALT or AST >3 times the ULN on 2 or more consecutive occasions) to be quite low (<1.3%).4 Higher statin doses were more likely to increase enzyme levels, though reduction in the dose or withdrawal of the statin resulted in normalization of the liver enzymes.
A study of patients ages 65 to 85 years who were treated with high-dose atorvastatin (80 mg per day) vs moderate dose pravastatin (40 mg per day) resulted in only 11 of 893 (1.23%) patients discontinuing the drug following abnormal liver function tests; most of these were in the high-dose treatment arm.5
Small risk in clinical practice, too
Clinical trials often have lower rates of adverse effects from medications than are seen in usual clinical practice.6 This may be because the stringent application of patient selection and exclusion criteria used in the administration of clinical trials does not occur in the “real world.”
However, the FDA database reported only 0.69 cases of hepatitis/liver failure per million statin prescriptions through 2004.4 A retrospective review of 1194 patients treated with a statin showed that 85% (1014) of patients had at least 1 monitoring test of transaminases performed during the year of the study. Of these, 10 (1.0%) had a significant elevation and 5 (0.5%) had a moderate elevation of transaminases. A review of the patient records demonstrated that none of these abnormalities appeared to be related to the use of statins, suggesting that routine monitoring of transaminases with statin therapy is not clinically necessary.7
A retrospective review over a 5-year period of 23,000 patients receiving statins in a large health maintenance organization found that only 17 (0.1%) patients had severe elevations of ALT (defined as >10 times the ULN). Of those 17 patients, 13 cases were associated with drug-drug interactions, and all but 1 resolved with discontinuation of the statin.8
What to monitor, how often
Product labeling for all statins advises measurement of transaminases (AST as well as ALT), although some liver experts would recommend ALT alone. ALT is found primarily in the liver, while AST is also found in muscle (cardiac and skeletal), kidneys, brain, pancreas, lungs, leukocytes, and erythrocytes. AST is, therefore, less specific for hepatic damage than ALT.
Routine monitoring of other liver function tests that measure the liver’s transport ability (eg, bilirubin, alkaline phosphate) or synthetic ability (eg, albumin, prothrombin time) will increase the likelihood of false-positive results and increase expense; they should not be done.
The 2002 American Gastroenterological Association guidelines recommend that for any hepatotoxic drug, if the ALT and/or AST elevations are <5 times the ULN, the drug should be stopped and the enzymes rechecked after an appropriate interval before pursuing a more extensive evaluation for liver disease.9
The FDA labeling information for all statins recommends liver function testing before putting a patient on a statin, 12 weeks after initiation, at any dose increase, and “periodically” for long-term maintenance therapy (TABLE 1).10 These recommendations are based on expert opinion only, because most data suggest that significant liver damage from statins is very rare and that routine monitoring of liver enzymes is not necessary.
The ACC/AHA/NHLBI Clinical Advisory on the Use and Safety of Statins agrees with the FDA, although it specifies “periodically” to mean annually.11
TABLE 1
When to monitor liver function in patients taking statins10,11
| WHEN TO CHECK ALT/AST | WHAT TO DO |
|---|---|
| Initiation of treatment or increase in dose | Begin/increase dose of statin if ALT and AST are <3 times the ULN |
| 12 weeks after initiation of statin therapy | Discontinue the statin (or lower the dose) if ALT or AST are >3 times the ULN |
| Long-term (annually or “periodically”) | |
| ALT, alanine aminotransferase; AST, aspartate aminotransferase; ULN, upper limit of normal. | |
It’s difficult to predict hepatic effects
Individual statins vary as to potency, efficacy, metabolism, and drug interactions. However, the exact mechanism of how statins cause elevations of ALT and AST is unknown, making it difficult to predict the hepatic effects of an individual statin based on its characteristics.
One analysis of multiple clinical trials concluded that overall statin toxicity (muscle, liver, etc.) was not directly related to the degree of lowering LDL cholesterol; instead, it correlated with the dose of the statin.12 As seen in TABLE 2, statins have variable drug-drug interactions based on their metabolism by the cytochrome P450 system. Drugs that increase the level of a statin in the blood may potentially increase the risk for toxicity and may warrant more cautious monitoring of liver enzymes, but are not necessarily contraindications to statin therapy.
Cyclosporine, macrolide antibiotics, azole antifungal agents, and other cytochrome P450 inhibitors (TABLE 2) are among the relative contraindications to the use of statins, more for concerns about myopathy than hepatoxicity.1 If these medications are used with a statin, consider more frequent monitoring of transaminases.
TABLE 2
Statin snapshot: LDL reductions to expect, interactions to avoid
| LOVASTATIN (MEVACOR, GENERICS) | PRAVASTATIN (PRAVACHOL, GENERICS) | SIMVASTATIN (ZOCOR, GENERICS) | FLUVASTATIN (LESCOL, GENERICS) | ATORVASTATIN (LIPITOR) | ROSUVASTATIN (CRESTOR) | |
|---|---|---|---|---|---|---|
| Usual daily dose | 20 to 80 mg | 40 to 80 mg | 10 to 80 mg | 20 to 80 mg | 10 to 80 mg | 10 to 40 mg |
| LDL reduction | 27% to 42% | 34% to 37% | 30% to 47% | 22% to 35% | 39% to 60% | 52% to 63% |
| % protein binding | >95% | 50% | 95% | 98% | >98% | 88% |
| Cytochrome P450 metabolism | 3A4* | None | 3A4* | 2C9 (75%)† 2C8 (5%) 3A4 (20%) | 3A4* | Limited 2C9 (10%) |
| * Drugs that may significantly increase statin levels via competitive metabolism or inhibition of CYP3A4 enzymes include macrolide antibiotics, HIV protease inhibitors, azole antifungal agents, calcium channel blockers, fluoxetine, cimetidine, cyclosporine, and omeprazole. Grapefruit juice may also have this effect. | ||||||
| † Drugs that may significantly increase statin levels via interference with CYP2C9 enzymes include phenytoin, glyburide, cimetidine, omeprazole, diclofenac, and cyclosporine. | ||||||
| Source: Physicians’ Desk Reference. 2008. 62nd ed. Montvale, NJ: Thomson PDR; 2008. | ||||||
Discontinue the statin?
ACC/AHA/NHLBI recommendations indicate that you should discontinue (or lower the dose of) statin therapy if the ALT or AST are above 3 times the ULN on 2 consecutive occasions.11 When elevations of ALT or AST are <3 times the ULN, consider the following:
- Statins have rigorously proven benefits for preventing morbidity and mortality due to atherosclerotic cardiovascular disease. A meta-analysis of more than 70,000 patients concluded that the number needed to treat to prevent 1 cardiovascular event was 27 and the number needed to harm (NNH) was 197. For more serious events such as creatine kinase >10 times the ULN, the NNH was 3400. Rhabdomyolysis alone was rare with a NNH of 7428.4
- Other medications that lower LDL and might be substituted for statins may not improve morbidity and mortality. For example, a recent clinical trial of ezetimibe (Zetia) reminds clinicians to be cautious in assuming that treatments that improve biochemical parameters such as LDL will necessarily result in improved clinical outcomes.13
- Mild elevations of ALT or AST (<3 times the ULN) following statin therapy are not known to lead to any significant liver toxicity over time.
- To date, there are no randomized controlled trials evaluating the optimal management of liver enzyme elevations with statin therapy.
Correspondence
Edward Onusko, MD, Family Health Center, 825 W. Locust, Wilmington, OH 45177; [email protected].
- Order liver function tests before starting statin therapy, 12 weeks after initiation, with any dose increase, and periodically for long-term maintenance therapy (C).
- Mild elevations of alanine aminotransferase (ALT) or aspartate aminotransferase (AST) (<3 times the upper limit of normal [ULN]) following statin therapy do not appear to lead to significant liver toxicity over time (C).
- Other medications that lower low-density lipoprotein (LDL), and might be substituted for statins, may not improve morbidity and mortality (C).
Strength of recommendation (SOR)
- Good-quality patient-oriented evidence
- Inconsistent or limited-quality patient-oriented evidence
- Consensus, usual practice, opinion, disease-oriented evidence, case series
Are we more aggressive than ever when it comes to our use of statins? You bet.
Should this prompt a heightened attention to hepatic safety? In a word, no. The more detailed, evidence-based answer (which follows) makes 2 things clear:
1. Clinically significant hepatic injury following statin use is very rare.
2. While US Food and Drug Administration (FDA) labeling recommends routine monitoring of serum transaminase levels prior to and during statin therapy, the evidence suggests that such routine monitoring is not clinically necessary.
More potent statins, more combination therapy
Our prescribing has become more aggressive to keep pace with National Cholesterol Education Program (NCEP) recommendations. In 2001, the NCEP Adult Treatment Panel III indicated:
- LDL cholesterol should be the primary target of therapy.
- The LDL cholesterol goal should be based on the patient’s risk of cardiovascular disease.
- Statins are the most effective agents to achieve treatment goals.1
Three years later, the NCEP advised that in light of more recent clinical trials, even more aggressive (ie, lower) LDL goals should be considered for patients at very high risk, high risk, and moderately high risk for cardiovascular disease.2
As a result, we are prescribing higher doses of statins, more potent statins, and more combination therapies of statins with other lipid-altering agents. Not surprisingly, this trend has prompted concerns about the potential increase in toxicities/side effects of statins.
An interesting clinical question is whether statins are appropriate when the cause of hepatic enzyme elevation appears to be excess fat in the liver. There is some evidence that treatment of fatty infiltration of the liver may lower transaminase levels and improve histological findings.14 In general, though, no medications have been demonstrated to improve patient-oriented outcomes such as mortality or need for liver transplant.15
This review examines the hepatic safety profile of statins and details why there’s no need to stop treatment based on moderate elevations in liver function tests. The most common serious side effect of statins—muscle damage/rhabdomyolysis—is rare, and is not extensively discussed here.
Clinical trials: Risk is small
A review of 35 randomized clinical statin trials reported from 1966 to 2005, involving 74,102 patients, reported an absolute risk of transaminase (also referred to as aminotransferase) elevations from statin therapy of only about 4 per 1000 patients (risk difference [RD]=4.2; 95% confidence interval [CI], 1.5-6.9).3 The same researchers’ analysis of 28 clinical trials involving 62,184 patients showed a relative risk of increased transaminase of 1.3 (95% CI, 1.06-1.59), achieving statistical significance only for the fluvastatin and lovastatin trials.3
High-dose statin therapy. A review of clinical trials involving high-dose statin therapy found rates of hepatic enzyme elevation (defined as ALT or AST >3 times the ULN on 2 or more consecutive occasions) to be quite low (<1.3%).4 Higher statin doses were more likely to increase enzyme levels, though reduction in the dose or withdrawal of the statin resulted in normalization of the liver enzymes.
A study of patients ages 65 to 85 years who were treated with high-dose atorvastatin (80 mg per day) vs moderate dose pravastatin (40 mg per day) resulted in only 11 of 893 (1.23%) patients discontinuing the drug following abnormal liver function tests; most of these were in the high-dose treatment arm.5
Small risk in clinical practice, too
Clinical trials often have lower rates of adverse effects from medications than are seen in usual clinical practice.6 This may be because the stringent application of patient selection and exclusion criteria used in the administration of clinical trials does not occur in the “real world.”
However, the FDA database reported only 0.69 cases of hepatitis/liver failure per million statin prescriptions through 2004.4 A retrospective review of 1194 patients treated with a statin showed that 85% (1014) of patients had at least 1 monitoring test of transaminases performed during the year of the study. Of these, 10 (1.0%) had a significant elevation and 5 (0.5%) had a moderate elevation of transaminases. A review of the patient records demonstrated that none of these abnormalities appeared to be related to the use of statins, suggesting that routine monitoring of transaminases with statin therapy is not clinically necessary.7
A retrospective review over a 5-year period of 23,000 patients receiving statins in a large health maintenance organization found that only 17 (0.1%) patients had severe elevations of ALT (defined as >10 times the ULN). Of those 17 patients, 13 cases were associated with drug-drug interactions, and all but 1 resolved with discontinuation of the statin.8
What to monitor, how often
Product labeling for all statins advises measurement of transaminases (AST as well as ALT), although some liver experts would recommend ALT alone. ALT is found primarily in the liver, while AST is also found in muscle (cardiac and skeletal), kidneys, brain, pancreas, lungs, leukocytes, and erythrocytes. AST is, therefore, less specific for hepatic damage than ALT.
Routine monitoring of other liver function tests that measure the liver’s transport ability (eg, bilirubin, alkaline phosphate) or synthetic ability (eg, albumin, prothrombin time) will increase the likelihood of false-positive results and increase expense; they should not be done.
The 2002 American Gastroenterological Association guidelines recommend that for any hepatotoxic drug, if the ALT and/or AST elevations are <5 times the ULN, the drug should be stopped and the enzymes rechecked after an appropriate interval before pursuing a more extensive evaluation for liver disease.9
The FDA labeling information for all statins recommends liver function testing before putting a patient on a statin, 12 weeks after initiation, at any dose increase, and “periodically” for long-term maintenance therapy (TABLE 1).10 These recommendations are based on expert opinion only, because most data suggest that significant liver damage from statins is very rare and that routine monitoring of liver enzymes is not necessary.
The ACC/AHA/NHLBI Clinical Advisory on the Use and Safety of Statins agrees with the FDA, although it specifies “periodically” to mean annually.11
TABLE 1
When to monitor liver function in patients taking statins10,11
| WHEN TO CHECK ALT/AST | WHAT TO DO |
|---|---|
| Initiation of treatment or increase in dose | Begin/increase dose of statin if ALT and AST are <3 times the ULN |
| 12 weeks after initiation of statin therapy | Discontinue the statin (or lower the dose) if ALT or AST are >3 times the ULN |
| Long-term (annually or “periodically”) | |
| ALT, alanine aminotransferase; AST, aspartate aminotransferase; ULN, upper limit of normal. | |
It’s difficult to predict hepatic effects
Individual statins vary as to potency, efficacy, metabolism, and drug interactions. However, the exact mechanism of how statins cause elevations of ALT and AST is unknown, making it difficult to predict the hepatic effects of an individual statin based on its characteristics.
One analysis of multiple clinical trials concluded that overall statin toxicity (muscle, liver, etc.) was not directly related to the degree of lowering LDL cholesterol; instead, it correlated with the dose of the statin.12 As seen in TABLE 2, statins have variable drug-drug interactions based on their metabolism by the cytochrome P450 system. Drugs that increase the level of a statin in the blood may potentially increase the risk for toxicity and may warrant more cautious monitoring of liver enzymes, but are not necessarily contraindications to statin therapy.
Cyclosporine, macrolide antibiotics, azole antifungal agents, and other cytochrome P450 inhibitors (TABLE 2) are among the relative contraindications to the use of statins, more for concerns about myopathy than hepatoxicity.1 If these medications are used with a statin, consider more frequent monitoring of transaminases.
TABLE 2
Statin snapshot: LDL reductions to expect, interactions to avoid
| LOVASTATIN (MEVACOR, GENERICS) | PRAVASTATIN (PRAVACHOL, GENERICS) | SIMVASTATIN (ZOCOR, GENERICS) | FLUVASTATIN (LESCOL, GENERICS) | ATORVASTATIN (LIPITOR) | ROSUVASTATIN (CRESTOR) | |
|---|---|---|---|---|---|---|
| Usual daily dose | 20 to 80 mg | 40 to 80 mg | 10 to 80 mg | 20 to 80 mg | 10 to 80 mg | 10 to 40 mg |
| LDL reduction | 27% to 42% | 34% to 37% | 30% to 47% | 22% to 35% | 39% to 60% | 52% to 63% |
| % protein binding | >95% | 50% | 95% | 98% | >98% | 88% |
| Cytochrome P450 metabolism | 3A4* | None | 3A4* | 2C9 (75%)† 2C8 (5%) 3A4 (20%) | 3A4* | Limited 2C9 (10%) |
| * Drugs that may significantly increase statin levels via competitive metabolism or inhibition of CYP3A4 enzymes include macrolide antibiotics, HIV protease inhibitors, azole antifungal agents, calcium channel blockers, fluoxetine, cimetidine, cyclosporine, and omeprazole. Grapefruit juice may also have this effect. | ||||||
| † Drugs that may significantly increase statin levels via interference with CYP2C9 enzymes include phenytoin, glyburide, cimetidine, omeprazole, diclofenac, and cyclosporine. | ||||||
| Source: Physicians’ Desk Reference. 2008. 62nd ed. Montvale, NJ: Thomson PDR; 2008. | ||||||
Discontinue the statin?
ACC/AHA/NHLBI recommendations indicate that you should discontinue (or lower the dose of) statin therapy if the ALT or AST are above 3 times the ULN on 2 consecutive occasions.11 When elevations of ALT or AST are <3 times the ULN, consider the following:
- Statins have rigorously proven benefits for preventing morbidity and mortality due to atherosclerotic cardiovascular disease. A meta-analysis of more than 70,000 patients concluded that the number needed to treat to prevent 1 cardiovascular event was 27 and the number needed to harm (NNH) was 197. For more serious events such as creatine kinase >10 times the ULN, the NNH was 3400. Rhabdomyolysis alone was rare with a NNH of 7428.4
- Other medications that lower LDL and might be substituted for statins may not improve morbidity and mortality. For example, a recent clinical trial of ezetimibe (Zetia) reminds clinicians to be cautious in assuming that treatments that improve biochemical parameters such as LDL will necessarily result in improved clinical outcomes.13
- Mild elevations of ALT or AST (<3 times the ULN) following statin therapy are not known to lead to any significant liver toxicity over time.
- To date, there are no randomized controlled trials evaluating the optimal management of liver enzyme elevations with statin therapy.
Correspondence
Edward Onusko, MD, Family Health Center, 825 W. Locust, Wilmington, OH 45177; [email protected].
1. Expert panel on detection evaluation and treatment of high blood cholesterol in adults. Executive summary of the third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III). JAMA. 2001;285:2486-2497.
2. Grundy SM, Cleeman JI, Bairey Merz CN, et al. Implications of recent clinical trials for the National Cholesterol Education Program Adult treatment Panel III guidelines. Circulation. 2004;110:227-239.
3. Kashani MS, Phillips CO, Foody JM, et al. Risks associated with statin therapy. Circulation. 2006;114:2788-2797.
4. Davidson MH, Robinson JG. Safety of aggressive lipid management. J Am Coll Cardiol. 2007;49:1753-1762.
5. Deedwania P, Stone PH, Bairey Merz CN, et al. Effects of intensive versus moderate lipid-lowering therapy on myocardial ischemia in older patients with coronary heart disease. Circulation. 2007;115:700-707.
6. Navarro VJ, Senior JR. Drug-related hepatotoxicity. N Engl J Med. 2006;354:731-739.
7. Smith CC, Bernstein LI, Davis RB, et al. Screening for statin-related toxicity. Arch Intern Med. 2003;163:688-692.
8. Charles EC, Olson KL, Sandhoff BG, et al. Evaluation of cases of severe statin-related transaminitis within a large health maintenance organization. Am J Med. 2005;118:618-624.
9. American Gastroenterological Association. American Gastroenterological Association medical position statement: evaluation of liver chemistry tests. Gastroenterology. 2002;123:1364.-
10. Weismantel D. What laboratory monitoring is appropriate to detect adverse drug reactions in patients on cholesterol-lowering agents? J Fam Pract 2001;50:927-928.
11. Pasternak RC, Smith SC, Bairey-Merz CN, Grundy SM, Cleeman JI, Lenfant C. ACC/AHA/NHLBI Advisory on the use and safety of statins. Circulation. 2002;106:1024-1028.
12. Alsheikh-Ali AA, Maddurkuri PV, Han H, Karas RH. Effect of lipid lowering on risk of elevated liver enzymes, rhabdomyolysis, and cancer: insights from large randomized statin trials. J Am Coll Cardiol. 2007;50:409-418.
13. In brief: Zetia and Vytorin: The ENHANCE study. Med Lett Drugs Ther. 2008;50(1278):5.-
14. Bayard M, Holt J, Boroughs E. Nonalcoholic fatty liver disease. Am Fam Physician. 2006;73:1961-1968.
15. Angulo P. Nonalcoholic fatty liver disease. N Engl J Med. 2002;346:1221-1231.
1. Expert panel on detection evaluation and treatment of high blood cholesterol in adults. Executive summary of the third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III). JAMA. 2001;285:2486-2497.
2. Grundy SM, Cleeman JI, Bairey Merz CN, et al. Implications of recent clinical trials for the National Cholesterol Education Program Adult treatment Panel III guidelines. Circulation. 2004;110:227-239.
3. Kashani MS, Phillips CO, Foody JM, et al. Risks associated with statin therapy. Circulation. 2006;114:2788-2797.
4. Davidson MH, Robinson JG. Safety of aggressive lipid management. J Am Coll Cardiol. 2007;49:1753-1762.
5. Deedwania P, Stone PH, Bairey Merz CN, et al. Effects of intensive versus moderate lipid-lowering therapy on myocardial ischemia in older patients with coronary heart disease. Circulation. 2007;115:700-707.
6. Navarro VJ, Senior JR. Drug-related hepatotoxicity. N Engl J Med. 2006;354:731-739.
7. Smith CC, Bernstein LI, Davis RB, et al. Screening for statin-related toxicity. Arch Intern Med. 2003;163:688-692.
8. Charles EC, Olson KL, Sandhoff BG, et al. Evaluation of cases of severe statin-related transaminitis within a large health maintenance organization. Am J Med. 2005;118:618-624.
9. American Gastroenterological Association. American Gastroenterological Association medical position statement: evaluation of liver chemistry tests. Gastroenterology. 2002;123:1364.-
10. Weismantel D. What laboratory monitoring is appropriate to detect adverse drug reactions in patients on cholesterol-lowering agents? J Fam Pract 2001;50:927-928.
11. Pasternak RC, Smith SC, Bairey-Merz CN, Grundy SM, Cleeman JI, Lenfant C. ACC/AHA/NHLBI Advisory on the use and safety of statins. Circulation. 2002;106:1024-1028.
12. Alsheikh-Ali AA, Maddurkuri PV, Han H, Karas RH. Effect of lipid lowering on risk of elevated liver enzymes, rhabdomyolysis, and cancer: insights from large randomized statin trials. J Am Coll Cardiol. 2007;50:409-418.
13. In brief: Zetia and Vytorin: The ENHANCE study. Med Lett Drugs Ther. 2008;50(1278):5.-
14. Bayard M, Holt J, Boroughs E. Nonalcoholic fatty liver disease. Am Fam Physician. 2006;73:1961-1968.
15. Angulo P. Nonalcoholic fatty liver disease. N Engl J Med. 2002;346:1221-1231.