User login
Caring for patients with co-occurring mental health & substance use disorders
THE CASE
Janice J* visits her family physician with complaints of chest pain, shortness of breath, and heart palpitations that are usually worse at night. Her medical history is significant for deep vein thrombosis secondary to an underlying hypercoagulability condition (rheumatoid arthritis) diagnosed 2 months earlier. She also has a history of opioid use disorder and has been on buprenorphine/naloxone therapy for 3 years. Her family medical history is unremarkable. She works full-time and lives with her 8-year-old son. On physical exam, she appears anxious; her cardiac and pulmonary exams are normal. A completed workup rules out cardiac or pulmonary problems.
- What is your diagnosis?
- How would you treat this patient?
* The patient’s name has been changed to protect her identity.
CO-OCCURRING DISORDERS: SCOPE OF THE PROBLEM
Co-occurring disorders, previously called “dual diagnosis,” refers to the coexistence of a mental health disorder and a substance use disorder. The obsolete term, dual diagnosis, specified the presence of 2 co-occurring Axis I diagnoses or the presence of an Axis I diagnosis and an Axis II diagnosis (such as mental disability). The change in nomenclature more precisely describes the co-existing mental health and substance use disorders.
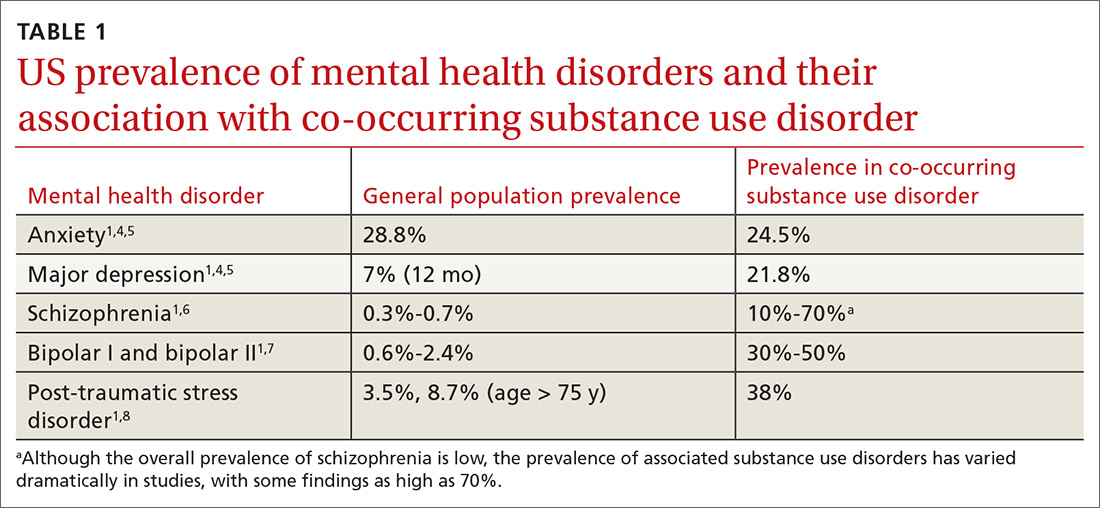
Currently the Diagnostic and Statistical Manual of Mental Disorders, 5th edition, (DSM-5) includes no diagnostic criteria for this dual condition.1 The criteria for mental health disorders and for substance use disorders comprise separate lists. Criteria for substance use disorder fall broadly into categories of “impaired [self] control, social impairment, risky behaviors, increased tolerance, and withdrawal symptoms.”1 It is estimated that 8.5 million US adults have co-occurring disorders, per the 2017 National Survey on Drug Use and Health conducted by the Substance Abuse and Mental Health Services Administration.2 Distinguishing which of the 2 conditions occurred first can be challenging. It has been suggested that the lifetime prevalence of a mental health disorder with a coexisting substance use disorder is greater than 40%3,4 (TABLE 11,4-8). For patients with schizophrenia and bipolar disorder, these numbers may be higher.
The consequences of undiagnosed and untreated co-occurring disorders include poor medication adherence, physical comorbidities (and decreased overall health), diminished self-care, increased suicide risk or aggression, increased risky sexual behavior, and possible incarceration.9
WHEN SHOULD YOU SUSPECT CO-OCCURRING DISORDERS?
Diagnosing a second condition can also be difficult when a patient’s symptoms are actually adverse effects of substances or prescribed medications. For example, a patient with worsening anxiety may also exhibit increasing blood pressure resistant to treatment. The cause of the patient’s fluctuating blood pressures may actually be the result of his or her use of alcohol to self-treat the anxiety. In addition to self-medication, other underlying factors may be at play, including genetic vulnerability, environment, and lifestyle.14 In the case we present, the patient’s conditions arose independently.
Anxiety disorders, with a lifetime risk of 28.8% in the US population,4 may be the primary mental health issue in many patients with co-occurring disorders, but this cannot be assumed in lieu of a complete workup.2,8,9,15 Substance use disorders in the general population have a past-year and lifetime prevalence of 14.6%.1,4,16,17 Because the causal and temporal association between anxiety and substance abuse is not always clear, it’s important to separate the diagnoses of the mental health and substance use disorders.
Continue to: MAKING THE DIAGNOSIS
MAKING THE DIAGNOSIS
To make an accurate diagnosis of co-occurring disorder, it is essential to take a complete history focusing on the timeline of symptoms, previous diagnoses and treatments, if any, and substance-free periods. Details gathered from these inquiries will help to separate symptoms of a primary mental health disorder from adverse effects of medication, withdrawal symptoms, or symptoms related to an underlying chronic medical condition.
Optimally, the diagnosis of a mental health disorder should be considered following a substance-free period. If this is not possible, a chart review may reveal a time when the patient did not have a substance use disorder.18
A diagnosis of substance use disorder requires that the patient manifest at least 2 of 11 behaviors listed in the DSM-5 over a 12-month period.1 The criteria focus on the amount of substance used, the time spent securing the substance, risky behaviors associated with the substance, and tolerance to the substance.
DON'T DEFER MENTAL HEALTH Tx
It is necessary to treat co-occurring disorders simultaneously. The old idea of deferring treatment of a mental health issue until the substance use disorder is resolved no longer applies.19,20 Treating substance use problems without addressing comorbid mental health issues can negatively impact treatment progress and increase risk for relapse. In a similar way, leaving substance use problems untreated is associated with nonadherence in mental health treatment, poor engagement, and dropout.21,22
Integrated services. Due to this condition’s level of clinical complexity, the optimal treatment approach is an interdisciplinary one in which integrated services are offered at a single location by a team of medical, mental health, and substance use providers (see “The case for behavioral health integration into primary care” in the June issue). An evidence-based example of such an approach is the Integrated Dual Disorder Treatment (IDDT) model—a comprehensive, integrated method of treating severe mental health disorders, including substance use disorders.21,22 IDDT combines coordinated services such as pharmacologic, psychological, educational, and social interventions to address the needs of patients and their family members. The IDDT model conceptualizes and treats co-occurring disorders within a biopsychosocial framework. Specific services may include medical detoxification, pharmacotherapy, patient and family education, behavioral and cognitive therapies, contingency management, self-help support groups, supported employment, residential/housing assistance, and case management services.23,24
Continue to: Medications for the mental health component
Medications for the mental health component. For patients who prefer medication treatment to cognitive behavioral therapy (CBT), or for whom CBT is unavailable, treat the mental health disorder per customary practice for the diagnosis (TABLE 225-30). For psychotic disorders, use an antipsychotic, adding a selective serotonin reuptake inhibitor (SSRI) or serotonin-norepinephrine reuptake inhibitor (SNRI) as needed depending on the presence of negative symptoms.25,31 For bipolar spectrum disorder, start a mood stabilizer32; for depressive disorders initiate an SSRI or SNRI.27 Anxiety disorders respond optimally when treated with SSRIs or SNRIs. Buspirone may be prescribed alone or as an adjunct for anxiety, and it does not cause mood-altering or withdrawal effects. Benzodiazepines in a controlled and monitored setting are an option in some antianxiety treatment plans. Consultation with a psychiatrist will help to determine the best treatment in these situations.
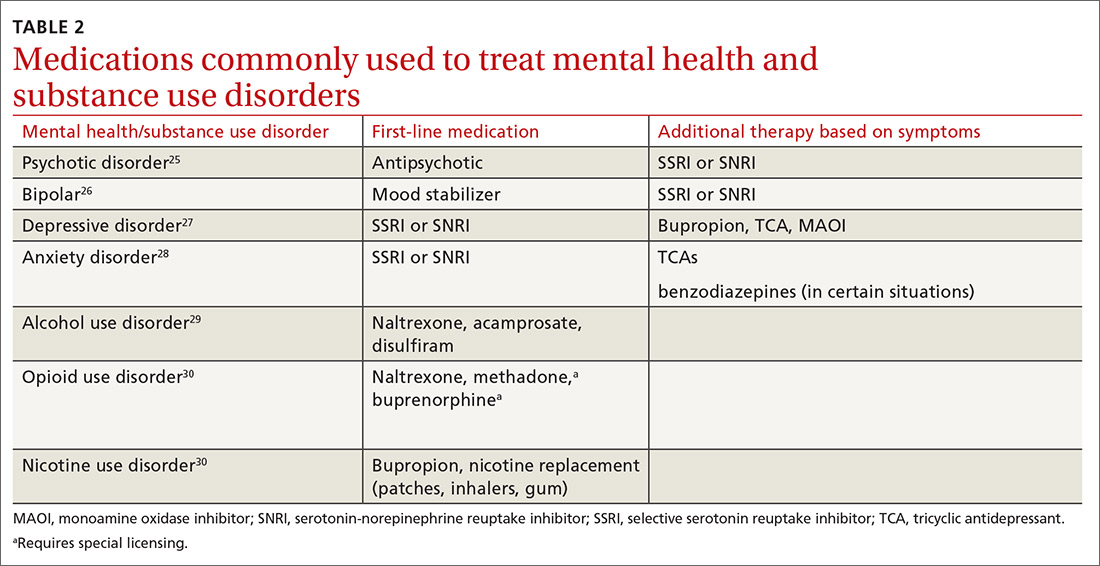
In all cases, treat the substance use disorder concurrently. Treatment options vary depending on the substance of choice. Although often overlooked, there can be simultaneous nicotine abuse. Oral or inhaled medications for nicotine abuse treatment are limited. The range of pharmacologic options for alcohol use disorder includes naltrexone, acamprosate, and disulfiram.29,33 Pharmacologic treatment options for opioid use disorder include naltrexone, methadone, and a combination of naloxone and buprenorphine.34
Physicians who wish to prescribe buprenorphine must qualify for and complete a certified 8 hour waiver-training course, which is then approved by the Drug Enforcement Agency (under the DATA 2000 – Drug and Alcohol Act 2000). The physician obtains the designation of a data-waived physician and is assigned a special identification number to prescribe these medications.35,36 Methadone may be provided only in a licensed methadone maintenance program. Regular and random drug urine screen requirements apply to all treatment programs.
Psychosocial and behavioral interventions are essential to the successful treatment of co-occurring disorders. Evidence-based behavioral and cognitive therapies are recommended for promoting adaptive coping skills and healthy lifestyle behaviors in co-occurring disorder populations.23,24,37-40 Motivational interviewing enhances motivation and adherence when patients demonstrate resistance or ambivalence.41,42 Mindfulness-based interventions have been shown to be effective and may be particularly beneficial for treating cravings/urges and promoting relapse prevention.37,39,40,43-46
Psychotropic medications, as with other treatment components, are most effective when used in combination with services that simultaneously address the patient’s biological, psychological, and social needs.
Continue to: The grassroots organization...
The grassroots organization National Alliance on Mental Illness (www.nami.org) recommends self-help and support groups, which include 12-step, faith-based and non-faith–based programs.20
For any treatment method to be successful, there needs to be a level of customization and individualization. Some patients may respond to medication or nonmedication treatments only, and others may need a combination of treatments.
CASE
The physician recalls a past diagnosis of anxiety and asks Ms. J if there are any new stressors or changes causing concern. The patient expresses concern about an opioid use relapse secondary to her recent diagnosis of rheumatoid arthritis, which may be life altering or limiting.
Even though she has been doing well and has been adherent to her daily buprenorphine treatment, she worries for the well-being of her family and what would happen if she cannot work, becomes incapacitated, or dies at a young age. She has never considered herself an anxious person and is surprised that anxiety could cause such pronounced physical symptoms.
The physician discusses different modalities of treatment, including counseling with an onsite psychologist, a trial of an anti-anxiety medication such as sertraline, or return office visits with the physician. They decide first to schedule an appointment with the psychologist, and Ms. J promises to find more time for self-wellness activities, such as exercise.
After 3 months of therapy, the patient decides to space out treatment to every 2 to 3 months and does not report any more episodes of chest pain or shortness of breath.
CORRESPONDENCE
Kristen Rundell, MD, Northwood-High Building, 2231 N. High Street, Suite 211, Columbus, OH 43201; [email protected].
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Arlington, VA: APA; 2013.
2. SAMHSA. Key substance use and mental health indicators in the United States: results from the 2017 National Survey on Drug Use and Health. 2017. https://www.samhsa.gov/data/sites/default/files/cbhsq-reports/NSDUHFFR2017/NSDUHFFR2017.htm#cooccur2. Accessed August 16, 2019.
3. Conway KP, Compton W, Stinson FS, et al. Lifetime comorbidity of DSM-IV mood and anxiety disorders and specific drug use disorders: results from the National Epidemiologic Survey on Alcohol and Related Conditions. J Clin Psychiatry. 2006;67:247-257.
4. Kessler RC, Berglund P, Demler O, et al. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:593-602.
5. Grant BF, Stinson FS, Dawson DA, et al. Prevalence and co-occurrence of substance use disorders and independent mood and anxiety disorders: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Arch Gen Psychiatry. 2004;61:807-816.
6. Dixon L. Dual diagnosis of substance abuse in schizophrenia: prevalence and impact on outcomes. Schizophr Res. 1999;35(suppl):S93-S100.
7. Merikangas KR, Jin R, He JP, et al. Prevalence and correlates of bipolar spectrum disorder in the World Mental Health Survey Initiative. Arch Gen Psychiatry. 2011;68:241-251.
8. Cottler LB, Compton WM 3rd, Mager D, et al. Posttraumatic stress disorder among substance users from the general population. Am J Psychiatry. 1992;149:664-670.
9. Kessler RC, Angermeyer M, Anthony JC, et al. Lifetime prevalence and age-of-onset distributions of mental disorders in the World Health Organization’s World Mental Health Survey Initiative. World Psychiatry. 2007;6:168-176.
10. Burns L, Teesson M, O’Neill K. The impact of comorbid anxiety and depression on alcohol treatment outcomes. Addiction. 2005;100:787-796.
11. Magidson JF, Liu SM, Lejuez CW, et al. Comparison of the course of substance use disorders among individuals with and without generalized anxiety disorder in a nationally representative sample. J Psychiatr Res. 2012;46:659666.
12. Boschloo L, Vogelzangs N, van den Brink W, et al. Alcohol use disorders and the course of depressive and anxiety disorders. Br J Psychiatry. 2012;200:476-484.
13. Schuckit MA. Comorbidity between substance use disorders and psychiatric conditions. Addiction. 2006;101(suppl 1):76-88.
14. Buckley PF. Prevalence and consequences of the dual diagnosis of substance abuse and severe mental illness. J Clin Psychiatry. 2006;67(suppl 7):5-9.
15. Salo R, Flower K, Kielstein A, et al. Psychiatric comorbidity in methamphetamine dependence. Psychiatry Res. 2011;186:356-361.
16. Torrens M, Gilchrist G, Domingo-Salvany A. Psychiatric comorbidity in illicit drug users: substance-induced versus independent disorders. Drug Alcohol Depend. 2011;113:147-156.
17. Buckner JD, Timpano KR, Zvolensky MJ, et al. Implications of comorbid alcohol dependence among individuals with social anxiety disorder. Depress Anxiety. 2008;25:1028-1037.
18. Kushner MG, Abrams K, Borchardt C. The relationship between anxiety disorders and alcohol use disorders: a review of major perspectives and findings. Clin Psychol Rev. 2000;20:149-171.
19. McHugh RK. Treatment of co-occurring anxiety disorders and substance use disorders. Harv Rev Psychiatry. 2015;23:99-111.
20. National Alliance on Mental Illness. Dual diagnosis. NAMI Web site. www.nami.org/Learn-More/Mental-Health-Conditions/related-conditions/dual-diagnosis. Reviewed August 2017. Accessed July 23, 2019.
21. SAMSHA. Substance Abuse Treatment for Persons with Co-Occurring Disorders. Treatment Improvement Protocol (TIP) series No. 42. HHS Publication No. (SMA) 13-3992. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2013.
22. SAMHSA. Treatment of co-occurring disorders. In: Medication-Assisted Treatment for Opioid Addiction in Opioid Treatment Programs. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2005.
23. Drake RE, Mueser KT, Brunette MF, et al. A review of treatments for people with severe mental illnesses and co-occurring substance use disorders. Psychiatr Rehabil J. 2004;27:360-374.
24. Kola LA, Kruszynski R. Adapting the integrated dual-disorder treatment model for addiction services. Alcohol Treat Q. 2010;28:437-450.
25. American Psychiatric Association. Practice guideline for the treatment of patients with schizophrenia, 2nd ed. https://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/schizophrenia.pdf. Published 2010. Accessed August 2, 2019.
26. American Psychiatric Association. Practice guideline for the treatment of patients with bipolar disorder, 2nd ed. https://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/bipolar.pdf. Published 2010. Accessed August 2, 2019.
27. American Psychiatric Association. Practice Guideline for the Treatment of Patients with Major Depressive Disorder. https://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/mdd.pdf. Published October 2010. Accessed July 23, 2019.
28. American Psychiatric Association. Practice guideline for the treatment of patients with panic disorder, 2nd ed. https://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/panicdisorder.pdf. Published January 2009. Accessed August 2, 2019.
29. American Psychiatric Association. Practice guideline for the pharmacological treatment of patients with alcohol use disorder. https://psychiatryonline.org/doi/pdf/10.1176/appi.books.9781615371969. Accessed August 2, 2019.
30. American Psychiatric Association. Practice guideline for the treatment of patients with substance use disorders, 2nd ed. https://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/substanceuse.pdf. Published 2010. Accessed August 2, 2019.
31. Petrakis IL, Nich C, Ralevski E. Psychotic spectrum disorders and alcohol abuse: a review of pharmacotherapeutic strategies and a report on the effectiveness of naltrexone and disulfiram. Schizophr Bull. 2006;32:644-654.
32. McIntyre RS, Yoon J. Efficacy of antimanic treatments in mixed states. Bipolar Disord. 2012;14(suppl 2):22-36.
33. Volpicelli JR, Alterman AI, Hayashida M, et al. Naltrexone in the treatment of alcohol dependence. Arch Gen Psychiatry. 1992;49:876-880.
34. Lee JD, Nunes EV Jr, Novo P, et al. Comparative effectiveness of extended-release naltrexone versus buprenorphine-naloxone for opioid relapse prevention (X:BOT): a multicentre, open-label, randomized controlled trial. Lancet. 2018;391:309-318.
35. US Department of Justice. DEA requirements for DATA waived physicians (DWPs). Drug Enforcement Administration, Diversion Control Division Web site. www.deadiversion.usdoj.gov/pubs/docs/dwp_buprenorphine.htm. Accessed August 2, 2019.
36. SAMHSA. Buprenorphine waiver management. https://www.samhsa.gov/medication-assisted-treatment/buprenorphine-waiver-management. SAMHSA Web site. Updated May 7, 2019. Accessed August 2, 2019.
37. Bowen S, Chawla N, Witkiewitz K. Mindfulness-based relapse prevention for addictive behaviors. In: Baer RA, ed. Mindfulness-Based Treatment Approaches: A Clinician’s Guide to Evidence Base and Applications. London, UK: Elsevier; 2014.
38. Dixon L, McFarlane W, Lefley H, et al. Evidence-based practices for services to families of people with psychiatric disabilities. Psychiatr Serv. 2001;52:903-910.
39. Hayes SC, Levin M, Plumb-Vilardaga J, et al. Acceptance and commitment therapy and contextual behavioral science: examining the progress of a distinctive model of behavioral and cognitive therapy. Behav Ther. 2013;44:180-198.
40. Osilla KC, Hepner KA, Muñoz RF, et al. Developing an integrated treatment for substance use and depression using cognitive behavioral therapy. J Subst Abuse Treat. 2009;37:412-420.
41. Martino S, Carroll K, Kostas D, et al. Dual diagnosis motivational interviewing: a modification of motivational interviewing for substance-abusing patients with psychotic disorders. J Subst Abuse Treat. 2002;23:297-308.
42. Rollnick S, Miller WR. What is motivational interviewing? Behav Cogn Psychother. 1995;23:325-334.
43. Garland EL. Disrupting the downward spiral of chronic pain and opioid addiction with mindfulness-oriented recovery enhancement: a review of clinical outcomes and neurocognitive targets. J Pain Palliat Care Pharmacother. 2014;28:122-129.
44. Garland EL, Manusov EG, Froeliger B, et al. Mindfulness-oriented recovery enhancement for chronic pain and prescription opioid misuse: results from an early-stage randomized controlled trial. J Consult Clin Psychol. 2014;82:448-459.
45. Marlatt GA, Donovan DM. Relapse Prevention: Maintenance Strategies in the Treatment of Addictive Behaviors, 2nd ed. New York, NY: Guilford Press; 2007.
46. Zgierska A, Rabago D, Chawla N, et al. Mindfulness meditation for substance use disorders: a systematic review. Subst Abus. 2009;30:266-294.
THE CASE
Janice J* visits her family physician with complaints of chest pain, shortness of breath, and heart palpitations that are usually worse at night. Her medical history is significant for deep vein thrombosis secondary to an underlying hypercoagulability condition (rheumatoid arthritis) diagnosed 2 months earlier. She also has a history of opioid use disorder and has been on buprenorphine/naloxone therapy for 3 years. Her family medical history is unremarkable. She works full-time and lives with her 8-year-old son. On physical exam, she appears anxious; her cardiac and pulmonary exams are normal. A completed workup rules out cardiac or pulmonary problems.
- What is your diagnosis?
- How would you treat this patient?
* The patient’s name has been changed to protect her identity.
CO-OCCURRING DISORDERS: SCOPE OF THE PROBLEM
Co-occurring disorders, previously called “dual diagnosis,” refers to the coexistence of a mental health disorder and a substance use disorder. The obsolete term, dual diagnosis, specified the presence of 2 co-occurring Axis I diagnoses or the presence of an Axis I diagnosis and an Axis II diagnosis (such as mental disability). The change in nomenclature more precisely describes the co-existing mental health and substance use disorders.
Currently the Diagnostic and Statistical Manual of Mental Disorders, 5th edition, (DSM-5) includes no diagnostic criteria for this dual condition.1 The criteria for mental health disorders and for substance use disorders comprise separate lists. Criteria for substance use disorder fall broadly into categories of “impaired [self] control, social impairment, risky behaviors, increased tolerance, and withdrawal symptoms.”1 It is estimated that 8.5 million US adults have co-occurring disorders, per the 2017 National Survey on Drug Use and Health conducted by the Substance Abuse and Mental Health Services Administration.2 Distinguishing which of the 2 conditions occurred first can be challenging. It has been suggested that the lifetime prevalence of a mental health disorder with a coexisting substance use disorder is greater than 40%3,4 (TABLE 11,4-8). For patients with schizophrenia and bipolar disorder, these numbers may be higher.

The consequences of undiagnosed and untreated co-occurring disorders include poor medication adherence, physical comorbidities (and decreased overall health), diminished self-care, increased suicide risk or aggression, increased risky sexual behavior, and possible incarceration.9
WHEN SHOULD YOU SUSPECT CO-OCCURRING DISORDERS?
Diagnosing a second condition can also be difficult when a patient’s symptoms are actually adverse effects of substances or prescribed medications. For example, a patient with worsening anxiety may also exhibit increasing blood pressure resistant to treatment. The cause of the patient’s fluctuating blood pressures may actually be the result of his or her use of alcohol to self-treat the anxiety. In addition to self-medication, other underlying factors may be at play, including genetic vulnerability, environment, and lifestyle.14 In the case we present, the patient’s conditions arose independently.
Anxiety disorders, with a lifetime risk of 28.8% in the US population,4 may be the primary mental health issue in many patients with co-occurring disorders, but this cannot be assumed in lieu of a complete workup.2,8,9,15 Substance use disorders in the general population have a past-year and lifetime prevalence of 14.6%.1,4,16,17 Because the causal and temporal association between anxiety and substance abuse is not always clear, it’s important to separate the diagnoses of the mental health and substance use disorders.
Continue to: MAKING THE DIAGNOSIS
MAKING THE DIAGNOSIS
To make an accurate diagnosis of co-occurring disorder, it is essential to take a complete history focusing on the timeline of symptoms, previous diagnoses and treatments, if any, and substance-free periods. Details gathered from these inquiries will help to separate symptoms of a primary mental health disorder from adverse effects of medication, withdrawal symptoms, or symptoms related to an underlying chronic medical condition.
Optimally, the diagnosis of a mental health disorder should be considered following a substance-free period. If this is not possible, a chart review may reveal a time when the patient did not have a substance use disorder.18
A diagnosis of substance use disorder requires that the patient manifest at least 2 of 11 behaviors listed in the DSM-5 over a 12-month period.1 The criteria focus on the amount of substance used, the time spent securing the substance, risky behaviors associated with the substance, and tolerance to the substance.
DON'T DEFER MENTAL HEALTH Tx
It is necessary to treat co-occurring disorders simultaneously. The old idea of deferring treatment of a mental health issue until the substance use disorder is resolved no longer applies.19,20 Treating substance use problems without addressing comorbid mental health issues can negatively impact treatment progress and increase risk for relapse. In a similar way, leaving substance use problems untreated is associated with nonadherence in mental health treatment, poor engagement, and dropout.21,22
Integrated services. Due to this condition’s level of clinical complexity, the optimal treatment approach is an interdisciplinary one in which integrated services are offered at a single location by a team of medical, mental health, and substance use providers (see “The case for behavioral health integration into primary care” in the June issue). An evidence-based example of such an approach is the Integrated Dual Disorder Treatment (IDDT) model—a comprehensive, integrated method of treating severe mental health disorders, including substance use disorders.21,22 IDDT combines coordinated services such as pharmacologic, psychological, educational, and social interventions to address the needs of patients and their family members. The IDDT model conceptualizes and treats co-occurring disorders within a biopsychosocial framework. Specific services may include medical detoxification, pharmacotherapy, patient and family education, behavioral and cognitive therapies, contingency management, self-help support groups, supported employment, residential/housing assistance, and case management services.23,24
Continue to: Medications for the mental health component
Medications for the mental health component. For patients who prefer medication treatment to cognitive behavioral therapy (CBT), or for whom CBT is unavailable, treat the mental health disorder per customary practice for the diagnosis (TABLE 225-30). For psychotic disorders, use an antipsychotic, adding a selective serotonin reuptake inhibitor (SSRI) or serotonin-norepinephrine reuptake inhibitor (SNRI) as needed depending on the presence of negative symptoms.25,31 For bipolar spectrum disorder, start a mood stabilizer32; for depressive disorders initiate an SSRI or SNRI.27 Anxiety disorders respond optimally when treated with SSRIs or SNRIs. Buspirone may be prescribed alone or as an adjunct for anxiety, and it does not cause mood-altering or withdrawal effects. Benzodiazepines in a controlled and monitored setting are an option in some antianxiety treatment plans. Consultation with a psychiatrist will help to determine the best treatment in these situations.

In all cases, treat the substance use disorder concurrently. Treatment options vary depending on the substance of choice. Although often overlooked, there can be simultaneous nicotine abuse. Oral or inhaled medications for nicotine abuse treatment are limited. The range of pharmacologic options for alcohol use disorder includes naltrexone, acamprosate, and disulfiram.29,33 Pharmacologic treatment options for opioid use disorder include naltrexone, methadone, and a combination of naloxone and buprenorphine.34
Physicians who wish to prescribe buprenorphine must qualify for and complete a certified 8 hour waiver-training course, which is then approved by the Drug Enforcement Agency (under the DATA 2000 – Drug and Alcohol Act 2000). The physician obtains the designation of a data-waived physician and is assigned a special identification number to prescribe these medications.35,36 Methadone may be provided only in a licensed methadone maintenance program. Regular and random drug urine screen requirements apply to all treatment programs.
Psychosocial and behavioral interventions are essential to the successful treatment of co-occurring disorders. Evidence-based behavioral and cognitive therapies are recommended for promoting adaptive coping skills and healthy lifestyle behaviors in co-occurring disorder populations.23,24,37-40 Motivational interviewing enhances motivation and adherence when patients demonstrate resistance or ambivalence.41,42 Mindfulness-based interventions have been shown to be effective and may be particularly beneficial for treating cravings/urges and promoting relapse prevention.37,39,40,43-46
Psychotropic medications, as with other treatment components, are most effective when used in combination with services that simultaneously address the patient’s biological, psychological, and social needs.
Continue to: The grassroots organization...
The grassroots organization National Alliance on Mental Illness (www.nami.org) recommends self-help and support groups, which include 12-step, faith-based and non-faith–based programs.20
For any treatment method to be successful, there needs to be a level of customization and individualization. Some patients may respond to medication or nonmedication treatments only, and others may need a combination of treatments.
CASE
The physician recalls a past diagnosis of anxiety and asks Ms. J if there are any new stressors or changes causing concern. The patient expresses concern about an opioid use relapse secondary to her recent diagnosis of rheumatoid arthritis, which may be life altering or limiting.
Even though she has been doing well and has been adherent to her daily buprenorphine treatment, she worries for the well-being of her family and what would happen if she cannot work, becomes incapacitated, or dies at a young age. She has never considered herself an anxious person and is surprised that anxiety could cause such pronounced physical symptoms.
The physician discusses different modalities of treatment, including counseling with an onsite psychologist, a trial of an anti-anxiety medication such as sertraline, or return office visits with the physician. They decide first to schedule an appointment with the psychologist, and Ms. J promises to find more time for self-wellness activities, such as exercise.
After 3 months of therapy, the patient decides to space out treatment to every 2 to 3 months and does not report any more episodes of chest pain or shortness of breath.
CORRESPONDENCE
Kristen Rundell, MD, Northwood-High Building, 2231 N. High Street, Suite 211, Columbus, OH 43201; [email protected].
THE CASE
Janice J* visits her family physician with complaints of chest pain, shortness of breath, and heart palpitations that are usually worse at night. Her medical history is significant for deep vein thrombosis secondary to an underlying hypercoagulability condition (rheumatoid arthritis) diagnosed 2 months earlier. She also has a history of opioid use disorder and has been on buprenorphine/naloxone therapy for 3 years. Her family medical history is unremarkable. She works full-time and lives with her 8-year-old son. On physical exam, she appears anxious; her cardiac and pulmonary exams are normal. A completed workup rules out cardiac or pulmonary problems.
- What is your diagnosis?
- How would you treat this patient?
* The patient’s name has been changed to protect her identity.
CO-OCCURRING DISORDERS: SCOPE OF THE PROBLEM
Co-occurring disorders, previously called “dual diagnosis,” refers to the coexistence of a mental health disorder and a substance use disorder. The obsolete term, dual diagnosis, specified the presence of 2 co-occurring Axis I diagnoses or the presence of an Axis I diagnosis and an Axis II diagnosis (such as mental disability). The change in nomenclature more precisely describes the co-existing mental health and substance use disorders.
Currently the Diagnostic and Statistical Manual of Mental Disorders, 5th edition, (DSM-5) includes no diagnostic criteria for this dual condition.1 The criteria for mental health disorders and for substance use disorders comprise separate lists. Criteria for substance use disorder fall broadly into categories of “impaired [self] control, social impairment, risky behaviors, increased tolerance, and withdrawal symptoms.”1 It is estimated that 8.5 million US adults have co-occurring disorders, per the 2017 National Survey on Drug Use and Health conducted by the Substance Abuse and Mental Health Services Administration.2 Distinguishing which of the 2 conditions occurred first can be challenging. It has been suggested that the lifetime prevalence of a mental health disorder with a coexisting substance use disorder is greater than 40%3,4 (TABLE 11,4-8). For patients with schizophrenia and bipolar disorder, these numbers may be higher.

The consequences of undiagnosed and untreated co-occurring disorders include poor medication adherence, physical comorbidities (and decreased overall health), diminished self-care, increased suicide risk or aggression, increased risky sexual behavior, and possible incarceration.9
WHEN SHOULD YOU SUSPECT CO-OCCURRING DISORDERS?
Diagnosing a second condition can also be difficult when a patient’s symptoms are actually adverse effects of substances or prescribed medications. For example, a patient with worsening anxiety may also exhibit increasing blood pressure resistant to treatment. The cause of the patient’s fluctuating blood pressures may actually be the result of his or her use of alcohol to self-treat the anxiety. In addition to self-medication, other underlying factors may be at play, including genetic vulnerability, environment, and lifestyle.14 In the case we present, the patient’s conditions arose independently.
Anxiety disorders, with a lifetime risk of 28.8% in the US population,4 may be the primary mental health issue in many patients with co-occurring disorders, but this cannot be assumed in lieu of a complete workup.2,8,9,15 Substance use disorders in the general population have a past-year and lifetime prevalence of 14.6%.1,4,16,17 Because the causal and temporal association between anxiety and substance abuse is not always clear, it’s important to separate the diagnoses of the mental health and substance use disorders.
Continue to: MAKING THE DIAGNOSIS
MAKING THE DIAGNOSIS
To make an accurate diagnosis of co-occurring disorder, it is essential to take a complete history focusing on the timeline of symptoms, previous diagnoses and treatments, if any, and substance-free periods. Details gathered from these inquiries will help to separate symptoms of a primary mental health disorder from adverse effects of medication, withdrawal symptoms, or symptoms related to an underlying chronic medical condition.
Optimally, the diagnosis of a mental health disorder should be considered following a substance-free period. If this is not possible, a chart review may reveal a time when the patient did not have a substance use disorder.18
A diagnosis of substance use disorder requires that the patient manifest at least 2 of 11 behaviors listed in the DSM-5 over a 12-month period.1 The criteria focus on the amount of substance used, the time spent securing the substance, risky behaviors associated with the substance, and tolerance to the substance.
DON'T DEFER MENTAL HEALTH Tx
It is necessary to treat co-occurring disorders simultaneously. The old idea of deferring treatment of a mental health issue until the substance use disorder is resolved no longer applies.19,20 Treating substance use problems without addressing comorbid mental health issues can negatively impact treatment progress and increase risk for relapse. In a similar way, leaving substance use problems untreated is associated with nonadherence in mental health treatment, poor engagement, and dropout.21,22
Integrated services. Due to this condition’s level of clinical complexity, the optimal treatment approach is an interdisciplinary one in which integrated services are offered at a single location by a team of medical, mental health, and substance use providers (see “The case for behavioral health integration into primary care” in the June issue). An evidence-based example of such an approach is the Integrated Dual Disorder Treatment (IDDT) model—a comprehensive, integrated method of treating severe mental health disorders, including substance use disorders.21,22 IDDT combines coordinated services such as pharmacologic, psychological, educational, and social interventions to address the needs of patients and their family members. The IDDT model conceptualizes and treats co-occurring disorders within a biopsychosocial framework. Specific services may include medical detoxification, pharmacotherapy, patient and family education, behavioral and cognitive therapies, contingency management, self-help support groups, supported employment, residential/housing assistance, and case management services.23,24
Continue to: Medications for the mental health component
Medications for the mental health component. For patients who prefer medication treatment to cognitive behavioral therapy (CBT), or for whom CBT is unavailable, treat the mental health disorder per customary practice for the diagnosis (TABLE 225-30). For psychotic disorders, use an antipsychotic, adding a selective serotonin reuptake inhibitor (SSRI) or serotonin-norepinephrine reuptake inhibitor (SNRI) as needed depending on the presence of negative symptoms.25,31 For bipolar spectrum disorder, start a mood stabilizer32; for depressive disorders initiate an SSRI or SNRI.27 Anxiety disorders respond optimally when treated with SSRIs or SNRIs. Buspirone may be prescribed alone or as an adjunct for anxiety, and it does not cause mood-altering or withdrawal effects. Benzodiazepines in a controlled and monitored setting are an option in some antianxiety treatment plans. Consultation with a psychiatrist will help to determine the best treatment in these situations.

In all cases, treat the substance use disorder concurrently. Treatment options vary depending on the substance of choice. Although often overlooked, there can be simultaneous nicotine abuse. Oral or inhaled medications for nicotine abuse treatment are limited. The range of pharmacologic options for alcohol use disorder includes naltrexone, acamprosate, and disulfiram.29,33 Pharmacologic treatment options for opioid use disorder include naltrexone, methadone, and a combination of naloxone and buprenorphine.34
Physicians who wish to prescribe buprenorphine must qualify for and complete a certified 8 hour waiver-training course, which is then approved by the Drug Enforcement Agency (under the DATA 2000 – Drug and Alcohol Act 2000). The physician obtains the designation of a data-waived physician and is assigned a special identification number to prescribe these medications.35,36 Methadone may be provided only in a licensed methadone maintenance program. Regular and random drug urine screen requirements apply to all treatment programs.
Psychosocial and behavioral interventions are essential to the successful treatment of co-occurring disorders. Evidence-based behavioral and cognitive therapies are recommended for promoting adaptive coping skills and healthy lifestyle behaviors in co-occurring disorder populations.23,24,37-40 Motivational interviewing enhances motivation and adherence when patients demonstrate resistance or ambivalence.41,42 Mindfulness-based interventions have been shown to be effective and may be particularly beneficial for treating cravings/urges and promoting relapse prevention.37,39,40,43-46
Psychotropic medications, as with other treatment components, are most effective when used in combination with services that simultaneously address the patient’s biological, psychological, and social needs.
Continue to: The grassroots organization...
The grassroots organization National Alliance on Mental Illness (www.nami.org) recommends self-help and support groups, which include 12-step, faith-based and non-faith–based programs.20
For any treatment method to be successful, there needs to be a level of customization and individualization. Some patients may respond to medication or nonmedication treatments only, and others may need a combination of treatments.
CASE
The physician recalls a past diagnosis of anxiety and asks Ms. J if there are any new stressors or changes causing concern. The patient expresses concern about an opioid use relapse secondary to her recent diagnosis of rheumatoid arthritis, which may be life altering or limiting.
Even though she has been doing well and has been adherent to her daily buprenorphine treatment, she worries for the well-being of her family and what would happen if she cannot work, becomes incapacitated, or dies at a young age. She has never considered herself an anxious person and is surprised that anxiety could cause such pronounced physical symptoms.
The physician discusses different modalities of treatment, including counseling with an onsite psychologist, a trial of an anti-anxiety medication such as sertraline, or return office visits with the physician. They decide first to schedule an appointment with the psychologist, and Ms. J promises to find more time for self-wellness activities, such as exercise.
After 3 months of therapy, the patient decides to space out treatment to every 2 to 3 months and does not report any more episodes of chest pain or shortness of breath.
CORRESPONDENCE
Kristen Rundell, MD, Northwood-High Building, 2231 N. High Street, Suite 211, Columbus, OH 43201; [email protected].
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Arlington, VA: APA; 2013.
2. SAMHSA. Key substance use and mental health indicators in the United States: results from the 2017 National Survey on Drug Use and Health. 2017. https://www.samhsa.gov/data/sites/default/files/cbhsq-reports/NSDUHFFR2017/NSDUHFFR2017.htm#cooccur2. Accessed August 16, 2019.
3. Conway KP, Compton W, Stinson FS, et al. Lifetime comorbidity of DSM-IV mood and anxiety disorders and specific drug use disorders: results from the National Epidemiologic Survey on Alcohol and Related Conditions. J Clin Psychiatry. 2006;67:247-257.
4. Kessler RC, Berglund P, Demler O, et al. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:593-602.
5. Grant BF, Stinson FS, Dawson DA, et al. Prevalence and co-occurrence of substance use disorders and independent mood and anxiety disorders: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Arch Gen Psychiatry. 2004;61:807-816.
6. Dixon L. Dual diagnosis of substance abuse in schizophrenia: prevalence and impact on outcomes. Schizophr Res. 1999;35(suppl):S93-S100.
7. Merikangas KR, Jin R, He JP, et al. Prevalence and correlates of bipolar spectrum disorder in the World Mental Health Survey Initiative. Arch Gen Psychiatry. 2011;68:241-251.
8. Cottler LB, Compton WM 3rd, Mager D, et al. Posttraumatic stress disorder among substance users from the general population. Am J Psychiatry. 1992;149:664-670.
9. Kessler RC, Angermeyer M, Anthony JC, et al. Lifetime prevalence and age-of-onset distributions of mental disorders in the World Health Organization’s World Mental Health Survey Initiative. World Psychiatry. 2007;6:168-176.
10. Burns L, Teesson M, O’Neill K. The impact of comorbid anxiety and depression on alcohol treatment outcomes. Addiction. 2005;100:787-796.
11. Magidson JF, Liu SM, Lejuez CW, et al. Comparison of the course of substance use disorders among individuals with and without generalized anxiety disorder in a nationally representative sample. J Psychiatr Res. 2012;46:659666.
12. Boschloo L, Vogelzangs N, van den Brink W, et al. Alcohol use disorders and the course of depressive and anxiety disorders. Br J Psychiatry. 2012;200:476-484.
13. Schuckit MA. Comorbidity between substance use disorders and psychiatric conditions. Addiction. 2006;101(suppl 1):76-88.
14. Buckley PF. Prevalence and consequences of the dual diagnosis of substance abuse and severe mental illness. J Clin Psychiatry. 2006;67(suppl 7):5-9.
15. Salo R, Flower K, Kielstein A, et al. Psychiatric comorbidity in methamphetamine dependence. Psychiatry Res. 2011;186:356-361.
16. Torrens M, Gilchrist G, Domingo-Salvany A. Psychiatric comorbidity in illicit drug users: substance-induced versus independent disorders. Drug Alcohol Depend. 2011;113:147-156.
17. Buckner JD, Timpano KR, Zvolensky MJ, et al. Implications of comorbid alcohol dependence among individuals with social anxiety disorder. Depress Anxiety. 2008;25:1028-1037.
18. Kushner MG, Abrams K, Borchardt C. The relationship between anxiety disorders and alcohol use disorders: a review of major perspectives and findings. Clin Psychol Rev. 2000;20:149-171.
19. McHugh RK. Treatment of co-occurring anxiety disorders and substance use disorders. Harv Rev Psychiatry. 2015;23:99-111.
20. National Alliance on Mental Illness. Dual diagnosis. NAMI Web site. www.nami.org/Learn-More/Mental-Health-Conditions/related-conditions/dual-diagnosis. Reviewed August 2017. Accessed July 23, 2019.
21. SAMSHA. Substance Abuse Treatment for Persons with Co-Occurring Disorders. Treatment Improvement Protocol (TIP) series No. 42. HHS Publication No. (SMA) 13-3992. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2013.
22. SAMHSA. Treatment of co-occurring disorders. In: Medication-Assisted Treatment for Opioid Addiction in Opioid Treatment Programs. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2005.
23. Drake RE, Mueser KT, Brunette MF, et al. A review of treatments for people with severe mental illnesses and co-occurring substance use disorders. Psychiatr Rehabil J. 2004;27:360-374.
24. Kola LA, Kruszynski R. Adapting the integrated dual-disorder treatment model for addiction services. Alcohol Treat Q. 2010;28:437-450.
25. American Psychiatric Association. Practice guideline for the treatment of patients with schizophrenia, 2nd ed. https://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/schizophrenia.pdf. Published 2010. Accessed August 2, 2019.
26. American Psychiatric Association. Practice guideline for the treatment of patients with bipolar disorder, 2nd ed. https://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/bipolar.pdf. Published 2010. Accessed August 2, 2019.
27. American Psychiatric Association. Practice Guideline for the Treatment of Patients with Major Depressive Disorder. https://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/mdd.pdf. Published October 2010. Accessed July 23, 2019.
28. American Psychiatric Association. Practice guideline for the treatment of patients with panic disorder, 2nd ed. https://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/panicdisorder.pdf. Published January 2009. Accessed August 2, 2019.
29. American Psychiatric Association. Practice guideline for the pharmacological treatment of patients with alcohol use disorder. https://psychiatryonline.org/doi/pdf/10.1176/appi.books.9781615371969. Accessed August 2, 2019.
30. American Psychiatric Association. Practice guideline for the treatment of patients with substance use disorders, 2nd ed. https://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/substanceuse.pdf. Published 2010. Accessed August 2, 2019.
31. Petrakis IL, Nich C, Ralevski E. Psychotic spectrum disorders and alcohol abuse: a review of pharmacotherapeutic strategies and a report on the effectiveness of naltrexone and disulfiram. Schizophr Bull. 2006;32:644-654.
32. McIntyre RS, Yoon J. Efficacy of antimanic treatments in mixed states. Bipolar Disord. 2012;14(suppl 2):22-36.
33. Volpicelli JR, Alterman AI, Hayashida M, et al. Naltrexone in the treatment of alcohol dependence. Arch Gen Psychiatry. 1992;49:876-880.
34. Lee JD, Nunes EV Jr, Novo P, et al. Comparative effectiveness of extended-release naltrexone versus buprenorphine-naloxone for opioid relapse prevention (X:BOT): a multicentre, open-label, randomized controlled trial. Lancet. 2018;391:309-318.
35. US Department of Justice. DEA requirements for DATA waived physicians (DWPs). Drug Enforcement Administration, Diversion Control Division Web site. www.deadiversion.usdoj.gov/pubs/docs/dwp_buprenorphine.htm. Accessed August 2, 2019.
36. SAMHSA. Buprenorphine waiver management. https://www.samhsa.gov/medication-assisted-treatment/buprenorphine-waiver-management. SAMHSA Web site. Updated May 7, 2019. Accessed August 2, 2019.
37. Bowen S, Chawla N, Witkiewitz K. Mindfulness-based relapse prevention for addictive behaviors. In: Baer RA, ed. Mindfulness-Based Treatment Approaches: A Clinician’s Guide to Evidence Base and Applications. London, UK: Elsevier; 2014.
38. Dixon L, McFarlane W, Lefley H, et al. Evidence-based practices for services to families of people with psychiatric disabilities. Psychiatr Serv. 2001;52:903-910.
39. Hayes SC, Levin M, Plumb-Vilardaga J, et al. Acceptance and commitment therapy and contextual behavioral science: examining the progress of a distinctive model of behavioral and cognitive therapy. Behav Ther. 2013;44:180-198.
40. Osilla KC, Hepner KA, Muñoz RF, et al. Developing an integrated treatment for substance use and depression using cognitive behavioral therapy. J Subst Abuse Treat. 2009;37:412-420.
41. Martino S, Carroll K, Kostas D, et al. Dual diagnosis motivational interviewing: a modification of motivational interviewing for substance-abusing patients with psychotic disorders. J Subst Abuse Treat. 2002;23:297-308.
42. Rollnick S, Miller WR. What is motivational interviewing? Behav Cogn Psychother. 1995;23:325-334.
43. Garland EL. Disrupting the downward spiral of chronic pain and opioid addiction with mindfulness-oriented recovery enhancement: a review of clinical outcomes and neurocognitive targets. J Pain Palliat Care Pharmacother. 2014;28:122-129.
44. Garland EL, Manusov EG, Froeliger B, et al. Mindfulness-oriented recovery enhancement for chronic pain and prescription opioid misuse: results from an early-stage randomized controlled trial. J Consult Clin Psychol. 2014;82:448-459.
45. Marlatt GA, Donovan DM. Relapse Prevention: Maintenance Strategies in the Treatment of Addictive Behaviors, 2nd ed. New York, NY: Guilford Press; 2007.
46. Zgierska A, Rabago D, Chawla N, et al. Mindfulness meditation for substance use disorders: a systematic review. Subst Abus. 2009;30:266-294.
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Arlington, VA: APA; 2013.
2. SAMHSA. Key substance use and mental health indicators in the United States: results from the 2017 National Survey on Drug Use and Health. 2017. https://www.samhsa.gov/data/sites/default/files/cbhsq-reports/NSDUHFFR2017/NSDUHFFR2017.htm#cooccur2. Accessed August 16, 2019.
3. Conway KP, Compton W, Stinson FS, et al. Lifetime comorbidity of DSM-IV mood and anxiety disorders and specific drug use disorders: results from the National Epidemiologic Survey on Alcohol and Related Conditions. J Clin Psychiatry. 2006;67:247-257.
4. Kessler RC, Berglund P, Demler O, et al. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:593-602.
5. Grant BF, Stinson FS, Dawson DA, et al. Prevalence and co-occurrence of substance use disorders and independent mood and anxiety disorders: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Arch Gen Psychiatry. 2004;61:807-816.
6. Dixon L. Dual diagnosis of substance abuse in schizophrenia: prevalence and impact on outcomes. Schizophr Res. 1999;35(suppl):S93-S100.
7. Merikangas KR, Jin R, He JP, et al. Prevalence and correlates of bipolar spectrum disorder in the World Mental Health Survey Initiative. Arch Gen Psychiatry. 2011;68:241-251.
8. Cottler LB, Compton WM 3rd, Mager D, et al. Posttraumatic stress disorder among substance users from the general population. Am J Psychiatry. 1992;149:664-670.
9. Kessler RC, Angermeyer M, Anthony JC, et al. Lifetime prevalence and age-of-onset distributions of mental disorders in the World Health Organization’s World Mental Health Survey Initiative. World Psychiatry. 2007;6:168-176.
10. Burns L, Teesson M, O’Neill K. The impact of comorbid anxiety and depression on alcohol treatment outcomes. Addiction. 2005;100:787-796.
11. Magidson JF, Liu SM, Lejuez CW, et al. Comparison of the course of substance use disorders among individuals with and without generalized anxiety disorder in a nationally representative sample. J Psychiatr Res. 2012;46:659666.
12. Boschloo L, Vogelzangs N, van den Brink W, et al. Alcohol use disorders and the course of depressive and anxiety disorders. Br J Psychiatry. 2012;200:476-484.
13. Schuckit MA. Comorbidity between substance use disorders and psychiatric conditions. Addiction. 2006;101(suppl 1):76-88.
14. Buckley PF. Prevalence and consequences of the dual diagnosis of substance abuse and severe mental illness. J Clin Psychiatry. 2006;67(suppl 7):5-9.
15. Salo R, Flower K, Kielstein A, et al. Psychiatric comorbidity in methamphetamine dependence. Psychiatry Res. 2011;186:356-361.
16. Torrens M, Gilchrist G, Domingo-Salvany A. Psychiatric comorbidity in illicit drug users: substance-induced versus independent disorders. Drug Alcohol Depend. 2011;113:147-156.
17. Buckner JD, Timpano KR, Zvolensky MJ, et al. Implications of comorbid alcohol dependence among individuals with social anxiety disorder. Depress Anxiety. 2008;25:1028-1037.
18. Kushner MG, Abrams K, Borchardt C. The relationship between anxiety disorders and alcohol use disorders: a review of major perspectives and findings. Clin Psychol Rev. 2000;20:149-171.
19. McHugh RK. Treatment of co-occurring anxiety disorders and substance use disorders. Harv Rev Psychiatry. 2015;23:99-111.
20. National Alliance on Mental Illness. Dual diagnosis. NAMI Web site. www.nami.org/Learn-More/Mental-Health-Conditions/related-conditions/dual-diagnosis. Reviewed August 2017. Accessed July 23, 2019.
21. SAMSHA. Substance Abuse Treatment for Persons with Co-Occurring Disorders. Treatment Improvement Protocol (TIP) series No. 42. HHS Publication No. (SMA) 13-3992. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2013.
22. SAMHSA. Treatment of co-occurring disorders. In: Medication-Assisted Treatment for Opioid Addiction in Opioid Treatment Programs. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2005.
23. Drake RE, Mueser KT, Brunette MF, et al. A review of treatments for people with severe mental illnesses and co-occurring substance use disorders. Psychiatr Rehabil J. 2004;27:360-374.
24. Kola LA, Kruszynski R. Adapting the integrated dual-disorder treatment model for addiction services. Alcohol Treat Q. 2010;28:437-450.
25. American Psychiatric Association. Practice guideline for the treatment of patients with schizophrenia, 2nd ed. https://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/schizophrenia.pdf. Published 2010. Accessed August 2, 2019.
26. American Psychiatric Association. Practice guideline for the treatment of patients with bipolar disorder, 2nd ed. https://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/bipolar.pdf. Published 2010. Accessed August 2, 2019.
27. American Psychiatric Association. Practice Guideline for the Treatment of Patients with Major Depressive Disorder. https://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/mdd.pdf. Published October 2010. Accessed July 23, 2019.
28. American Psychiatric Association. Practice guideline for the treatment of patients with panic disorder, 2nd ed. https://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/panicdisorder.pdf. Published January 2009. Accessed August 2, 2019.
29. American Psychiatric Association. Practice guideline for the pharmacological treatment of patients with alcohol use disorder. https://psychiatryonline.org/doi/pdf/10.1176/appi.books.9781615371969. Accessed August 2, 2019.
30. American Psychiatric Association. Practice guideline for the treatment of patients with substance use disorders, 2nd ed. https://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/substanceuse.pdf. Published 2010. Accessed August 2, 2019.
31. Petrakis IL, Nich C, Ralevski E. Psychotic spectrum disorders and alcohol abuse: a review of pharmacotherapeutic strategies and a report on the effectiveness of naltrexone and disulfiram. Schizophr Bull. 2006;32:644-654.
32. McIntyre RS, Yoon J. Efficacy of antimanic treatments in mixed states. Bipolar Disord. 2012;14(suppl 2):22-36.
33. Volpicelli JR, Alterman AI, Hayashida M, et al. Naltrexone in the treatment of alcohol dependence. Arch Gen Psychiatry. 1992;49:876-880.
34. Lee JD, Nunes EV Jr, Novo P, et al. Comparative effectiveness of extended-release naltrexone versus buprenorphine-naloxone for opioid relapse prevention (X:BOT): a multicentre, open-label, randomized controlled trial. Lancet. 2018;391:309-318.
35. US Department of Justice. DEA requirements for DATA waived physicians (DWPs). Drug Enforcement Administration, Diversion Control Division Web site. www.deadiversion.usdoj.gov/pubs/docs/dwp_buprenorphine.htm. Accessed August 2, 2019.
36. SAMHSA. Buprenorphine waiver management. https://www.samhsa.gov/medication-assisted-treatment/buprenorphine-waiver-management. SAMHSA Web site. Updated May 7, 2019. Accessed August 2, 2019.
37. Bowen S, Chawla N, Witkiewitz K. Mindfulness-based relapse prevention for addictive behaviors. In: Baer RA, ed. Mindfulness-Based Treatment Approaches: A Clinician’s Guide to Evidence Base and Applications. London, UK: Elsevier; 2014.
38. Dixon L, McFarlane W, Lefley H, et al. Evidence-based practices for services to families of people with psychiatric disabilities. Psychiatr Serv. 2001;52:903-910.
39. Hayes SC, Levin M, Plumb-Vilardaga J, et al. Acceptance and commitment therapy and contextual behavioral science: examining the progress of a distinctive model of behavioral and cognitive therapy. Behav Ther. 2013;44:180-198.
40. Osilla KC, Hepner KA, Muñoz RF, et al. Developing an integrated treatment for substance use and depression using cognitive behavioral therapy. J Subst Abuse Treat. 2009;37:412-420.
41. Martino S, Carroll K, Kostas D, et al. Dual diagnosis motivational interviewing: a modification of motivational interviewing for substance-abusing patients with psychotic disorders. J Subst Abuse Treat. 2002;23:297-308.
42. Rollnick S, Miller WR. What is motivational interviewing? Behav Cogn Psychother. 1995;23:325-334.
43. Garland EL. Disrupting the downward spiral of chronic pain and opioid addiction with mindfulness-oriented recovery enhancement: a review of clinical outcomes and neurocognitive targets. J Pain Palliat Care Pharmacother. 2014;28:122-129.
44. Garland EL, Manusov EG, Froeliger B, et al. Mindfulness-oriented recovery enhancement for chronic pain and prescription opioid misuse: results from an early-stage randomized controlled trial. J Consult Clin Psychol. 2014;82:448-459.
45. Marlatt GA, Donovan DM. Relapse Prevention: Maintenance Strategies in the Treatment of Addictive Behaviors, 2nd ed. New York, NY: Guilford Press; 2007.
46. Zgierska A, Rabago D, Chawla N, et al. Mindfulness meditation for substance use disorders: a systematic review. Subst Abus. 2009;30:266-294.
The case for behavioral health integration into primary care
In a typical primary care practice, detecting and managing mental health problems competes with other priorities such as treating acute physical illness, monitoring chronic disease, providing preventive health services, and assessing compliance with standards of care.1 These competing demands for a primary care provider’s time, paired with limited mental health resources in the community, may result in suboptimal behavioral health care.1-3 Even when referrals are made to
Approximately 30% of adults with physical disorders also have one or more behavioral health conditions, such as anxiety, panic, mood, or substance use disorders.6 Although physical and behavioral health conditions are inextricably linked, their assessment and treatment get separated into different silos.7 Given that fewer than 20% of depressed patients are seen by a psychiatrist or psychologist,8 the responsibility of providing mental health care often falls on the primary care physician.8,9
Efforts to improve the treatment of common mental disorders in primary care have traditionally focused on screening for these disorders, educating primary care providers, developing treatment guidelines, and referring patients to mental health specialty care.10 However, behavioral health integration offers another way forward.
WHAT IS BEHAVIORAL HEALTH INTEGRATION?
Behavioral health integration (BHI) in primary care refers to primary care physicians and behavioral health clinicians working in concert with patients to address their primary care and behavioral health needs.11
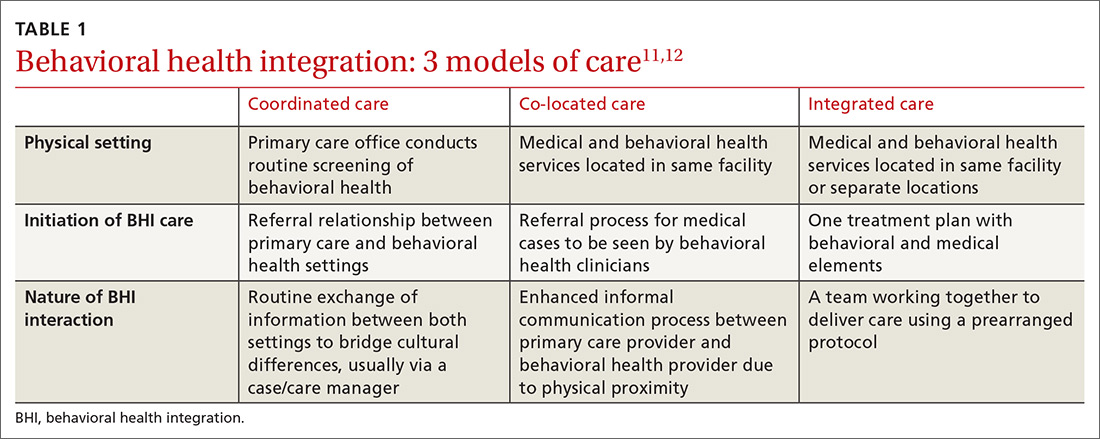
Numerous overlapping terms have been used to describe BHI, and this has caused some confusion. In 2013, the Agency for Healthcare Research and Quality (AHRQ) issued a lexicon standardizing the terminology used in BHI.11 The commonly used terms are
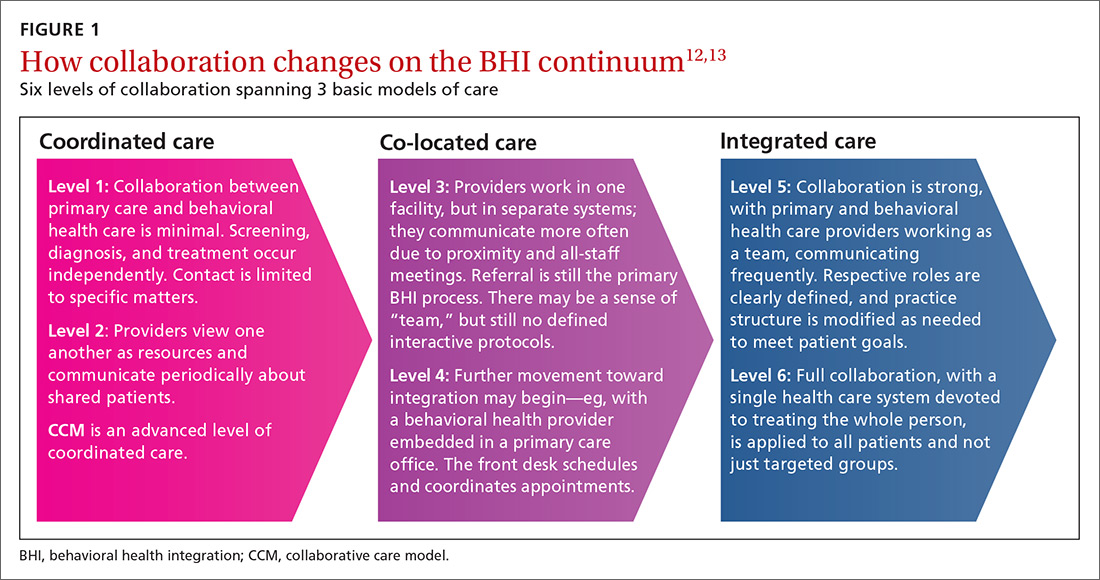
COORDINATED CARE AND THE COLLABORATIVE CARE MODEL
BHI at the level of coordinated care has almost exclusively been studied and practiced along the lines of the collaborative care model (CCM).14-16 This model represents an advanced level of coordinated care in the BHI continuum. The most substantial evidence for CCM lies in the management of depression and anxiety.14-16
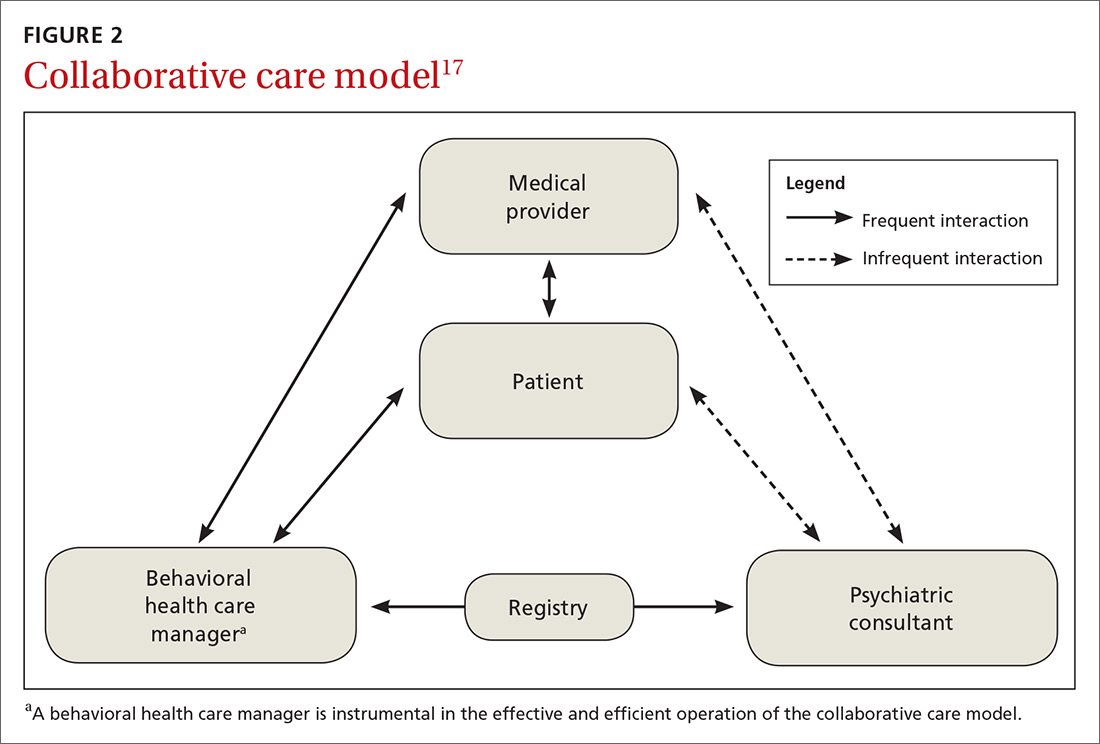
Usual care involves the primary care physician and the patient. CCM adds 2 vital roles—a behavioral health care manager and a psychiatric consultant. A behavioral health care manager is typically a counselor, clinical social worker, psychologist, or psychiatric nurse who performs all care-management tasks including offering psychotherapy when that is part of the treatment plan.
Continue to: The care manager's functions include...
The care manager’s functions include systematic follow-up with structured monitoring of symptoms and treatment adherence, coordination and communication among care providers, patient education, and self-management support, including the use of motivational interviewing. The behavioral health care manager performs this systematic follow up by maintaining a patient “registry”—case-management software used in conjunction with, or embedded in, the practice electronic health record to track patients’ data and clinical outcomes, as well as to facilitate decision-making.
The care manager communicates with the psychiatrist, who offers suggestions for drug therapy, which is prescribed by the primary care physician. The care manager also regularly evaluates the patient’s status using a standardized scale, communicates these scores to the psychiatrist, and transmits any recommendations to the primary care physician (FIGURE 2).17
EVIDENCE FOR CCM
Collaborative and routine care were compared in a 2012 Cochrane review that included 79 randomized controlled trials (RCTs) involving 24,308 patients worldwide.16 Seventy-two of the 79 RCTs focused on patients with depression or depression with anxiety, while 6 studies included participants with only anxiety disorders.16 One additional study focused on mental health quality of life. (To learn about CCM and severe mental illness and substance use disorder, see “Less well studied: CCM and severe mental illness, alcohol dependence.”18-20)
SIDEBAR
Less well studied: CCM and severe mental illness, alcohol dependence
Evidence for collaborative care in severe mental illness (SMI) is very limited. SMI is defined as schizophrenia or other schizophrenia-like psychoses (eg, schizophreniform and schizoaffective disorders), bipolar affective disorder, or other psychosis.
A 2013 Cochrane review identified only 1 RCT involving 306 veterans with bipolar disease.18 The review concluded that there was low-quality evidence that collaborative care led to a relative risk reduction of 25% for psychiatric admissions at Year 2 compared with standard care (RR = 0.75; 95% CI, 0.57-0.99).18
One 2017 RCT involving 245 veterans that looked at a collaborative care model for patients with severe mental illness found a modest benefit for physical health-related quality of life, but did not find any benefit in mental health outcomes.19
Alcohol dependence. There is very limited, but high-quality, evidence for the utility of CCM in alcohol dependence. In one RCT, 163 veterans were assigned to either CCM or referral to standard treatment in a specialty outpatient addiction treatment program. The CCM group had a significantly higher proportion of participants engaged in treatment over the study’s 26 weeks (odds ratio [OR] = 5.36; 95% CI, 2.99-9.59). The percentage of heavy drinking days was significantly lower in the CCM group (OR = 2.16; 95% CI, 1.27-3.66), while overall abstinence did not differ between groups.20
For adults with depression treated with the CCM, significantly greater improvement in depression outcome measures was seen in the short-term (standardized mean difference [SMD] = -0.34; 95% confidence interval [CI], -0.41 to -0.27; risk ratio [RR] = 1.32; 95% CI, 1.22-1.43), in the medium term (SMD = -0.28; 95% CI, -0.41 to -0.15; RR = 1.31; 95% CI, 1.17–1.48), and in the long term (SMD = -0.35; 95% CI, -0.46 to -0.24; RR = 1.29; 95% CI, 1.18–1.41).16
Comparisons of mental health quality of life over the short term (0-6 months), medium term (7-12 months), and long term (13-24 months) did not show any significant difference between CCM and routine care.16 Comparisons of physical health quality of life over the short term and medium term did not show any significant difference between CCM and routine care.16
Continue to: Significantly greater improvement...
Significantly greater improvement in anxiety outcomes was seen for adults treated with CCM in the short term (SMD = -0.30; 95% CI, -0.44 to -0.17; RR = 1.50; 95% CI, 1.21–1.87), in the medium term (SMD = -0.33; 95% CI, -0.47 to -0.19; RR = 1.41; 95% CI, 1.18-1.69), and in the long term (SMD = -0.20; 95% CI, -0.34 to -0.06; RR = 1.26; 95% CI, 1.11–1.42).16
A 2016 systematic review of 94 RCTs involving more than 25,000 patients also provided high-quality evidence that collaborative care yields small-to-moderate improvements in symptoms from mood disorders and mental health-related quality of life.15 A 2006 meta-analysis of 37 RCTs comprising 12,355 patients showed that collaborative care involving a case manager is more effective than standard care in improving depression outcomes at 6 months (SMD = 0.25; 95% CI, 0.18-0.32) and up to 5 years (SMD = 0.15; 95% CI, 0.001-0.31).21
Better care of mental health disorders also improves medical outcomes
Several trials have focused on jointly managing depression and a chronic physical condition such as chronic pain, diabetes, and coronary heart disease,22 demonstrating improved outcomes for both depression and the comanaged conditions.
- Chronic pain. When compared with usual care, collaborative care resulted in moderate reductions in both pain severity and associated disability (41.5% vs 17.3%; RR = 2.4; 95% CI, 1.6-3.2).23
- Diabetes. Patients managed collaboratively were more likely to have a decrease of ≥ 1% in the glycated hemoglobin level from baseline (36% vs 19%; P = .006).24
- Cardiovascular disease. Significant real-world risk reduction was achieved by improving blood pressure control (58% achieved blood pressure control compared with a projected target of 20%).22
IS THERE A COMMON THREAD AMONG SUCCESSFUL CCMs?
Attempts to identify commonalities between the many iterations of successful CCMs have produced varying results due to differing selections of relevant RCTs.25-29 However, a few common features have been identified:
- care managers assess symptoms at baseline and at follow-up using a standardized measure such as the Patient Health Questionnaire (PHQ-9);
- care managers monitor treatment adherence;
- follow-up is active for at least 16 weeks;
- primary care and mental health providers actively engage in patient management; and
- mental health specialists regularly supervise care managers.
The one feature that is consistent with improved outcomes is the presence of the care manager.25-29
Continue to: The improvement associated...
The improvement associated with collaborative care is clinically meaningful to patients and physicians. In one RCT, collaborative care doubled response rates of depression treatment compared with usual care.3 Quality improvement data from real-world implementation of collaborative care programs suggests that similar outcomes can be achieved in a variety of settings.30
COST BENEFITS OF CCM
Collaborative care for depression is associated with lower health care costs.29,31
A meta-analysis of 57 RCTs in 2012 showed that CCM improves depression outcomes across populations, settings, and outcome domains, and that these results are achieved at little to no increase in treatment costs compared with usual care (Cohen’s d = 0.05; 95% CI, –0.02–0.12).26
When collaborative care was compared with routine care in an RCT involving 1801 primary care patients ≥ 60 years who were suffering from depression, a cost saving of $3363 per patient over 4 years was demonstrated in the intervention arm.31
A technical analysis of 94 RCTs in 2015 concluded that CCM is cost effective compared with usual care, with a range of $15,000 to $80,000 per quality-adjusted life year gained. These studies also indicated that organizations’ costs to implement CCM increase in the short term. Based on this analysis, organizations would need to invest between $3 to $22 per patient per month to implement and sustain CCMs, depending on the prevalence of depression in the population.29
Continue to: OTHER MODELS OF BHI
OTHER MODELS OF BHI
Higher levels of BHI such as co-location and integration do not have the same quality of evidence as CCM.
A 2009 Cochrane review of 42 studies involving 3880 patients found that mental health workers delivering psychological therapy and psychosocial interventions in primary care settings brought about significant reductions in primary care physician consultations (SMD = ‐0.17; 95% CI, ‐0.30 to ‐0.05); a relative risk reduction of 23% in psychotropic prescribing (RR = 0.67; 95% CI, 0.56–0.79); a decrease in prescribing costs (SMD = ‐0.22; 95% CI, ‐0.38 to ‐0.07); and a relative risk reduction in mental health referral of 87% (RR = 0.13; 95% CI, 0.09–0.20) for the patients they were seeing.32 The authors concluded the changes were modest in magnitude and inconsistent across different studies.32
Embedding medical providers in behavior health centers—ie, the reverse co-location model—also has very limited evidence. An RCT involving 120 veterans found that patients enrolled in a reverse co-location clinic did significantly better than controls seen in a general care clinic in terms of continuity of care and preventive care such as screening for hypertension (84.7% vs 65.6%; X 2 = 5.9, P = .01), diabetes (71.2% vs 45.9%; X 2 = 7.9, P < .005), hepatitis (39% vs 14.8%; X 2 = 9, P = .003), and cholesterol (79.7% vs 57.4%; X 2 = 6.9, P = .009).33
HOW TO IMPLEMENT A SUCCESSFUL BHI PROGRAM
A demonstration and evaluation project involving 11 diverse practices in Colorado explored ways to integrate behavioral health in primary care. Five main themes emerged34,35:
- Frame integrated care as a necessary paradigm shift to patient-centered, whole-person health care.
- Define relationships and protocols up front, understanding that they will evolve.
- Build inclusive, empowered teams to provide the foundation for integration.
- Develop a change management strategy of continuous evaluation and course correction.
- Use targeted data collection pertinent to integrated care to drive improvement and impart accountability.
The Institute for Clinical and Economic Review has organized an extensive list of resources36 for implementing BHI models, a sampling of which is shown in TABLE 2.

Continue to: TAKE-AWAY POINTS
TAKE-AWAY POINTS
There is high quality evidence that collaborative care works for the management of depression and anxiety disorder in primary care, and this is associated with significant cost savings.
CORRESPONDENCE
Rajesh (FNU) Rajesh, MD, Main Campus Family Medicine Clinic, MetroHealth, 2500 MetroHealth Drive, Cleveland, OH 44109; [email protected]
1. Rost K, Nutting P, Smith J, et al. The role of competing demands in the treatment provided primary care patients with major depression. Arch Fam Med. 2000;9:150-154.
2. Rush A, Trivedi M, Carmody T, et al. One-year clinical outcomes of depressed public sector outpatients: a benchmark for subsequent studies. Biol Psychiatry. 2004;56:46-53.
3. Unützer J, Katon W, Callahan CM, et al. Collaborative care management of late-life depression in the primary care setting. JAMA. 2002;288:2836-2845.
4. Department of Veterans Affairs. Bradford DW, Slubicki MN, McDuffie J, et al. Effects of care models to improve general medical outcomes for individuals with serious mental illness. 2011. https://www.hsrd.research.va.gov/publications/esp/smi-REPORT.pdf. Accessed August 22, 2018.
5. Druss BG, von Esenwein S. Improving general medical care for persons with mental and addictive disorders: systematic review. Gen Hosp Psychiatry. 2006;28:145-153.
6. Druss BG, Walker ER. Mental Disorders and Medical Comorbidity. Research Synthesis Report No. 21. Princeton, NJ: The Robert Wood Johnson Foundation; February 2011.
7. Reed SJ, Shore KK, Tice JA. Effectiveness and value of integrating behavioral health into primary care. JAMA Intern Med. 2016;176:691-692.
8. Young AS, Klap R, Sherbourne CD, et al. The quality of care for depressive and anxiety disorders in the United States. Arch Gen Psychiatry. 2001;58:55-61.
9. Butler M, Kane RL, McAlpine D, et al. Integration of mental health/substance abuse and primary care. Rockville, MD: Agency for Healthcare Research and Quality; 2008. http://www.ncbi.nlm.nih.gov/books/NBK38632/. Accessed March 2, 2019.
10. Unützer J, Schoenbaum M, Druss B, et al. Transforming mental health care at the interface with general medicine: report for the presidents commission. Psychiatr Serv. 2006;57:37-47. doi: 10.1176/appi.ps.57.1.37.
11. Peek CJ; the National Integration Academy Council. Lexicon for behavioral health and primary care integration: concepts and definitions developed by expert consensus. AHRQ. https://integrationacademy.ahrq.gov/sites/default/files/Lexicon.pdf. Published April 2013. Accessed May 29, 2019.
12. Heath B, Wise Romero P, Reynolds K. A standard framework for levels of integrated healthcare and update throughout the document. SAMHSA-HRSA. https://www.integration.samhsa.gov/integrated-care-models/A_Standard_Framework_for_Levels_of_Integrated_Healthcare.pdf. Published March 2013. Accessed May 29, 2019.
13. Integrating physical and behavioral health care: promising Medicaid models. The Henry J. Kaiser Family Foundation. https://www.kff.org/wp-content/uploads/2014/02/8553-integrating-physical-and-behavioral-health-care-promising-medicaid-models.pdf. Published February 2014. Accessed May 29, 2019.
14. Vanderlip ER, Rundell J, Avery M, et al. Dissemination of integrated care within adult primary care settings: the collaborative care model. SAMHSA-HRSA. https://www.integration.samhsa.gov/integrated-care-models/APA-APM-Dissemination-Integrated-Care-Report.pdf. Published 2016. Accessed May 29, 2019.
15. Gerrity M. Evolving models of behavioral health integration: evidence update 2010-2015. Milbank Memorial Fund. https://www.milbank.org/wp-content/uploads/2016/05/Evolving-Models-of-BHI.pdf. Published May 2016. Accessed May 29, 2019.
16. Archer J, Bower P, Gilbody S, et al. Collaborative care for depression and anxiety problems. Cochrane Database Syst Rev. 2012;10.1002/14651858.cd006525.pub2.
17. Team Structure. University of Washington AIMs Center. https://aims.uw.edu/collaborative-care/team-structure. Published 2017.Accessed May 29, 2019.
18. Reilly S, Planner C, Gask L, et al. Collaborative care approaches for people with severe mental illness. Cochrane Database Syst Rev. 2013;(11):CD009531.
19. Kilbourne AM, Barbaresso MM, Lai Z, et al. Improving physical health in patients with chronic mental disorders. J Clin Psychiatry. 2017;78:129-137.
20. Oslin DW, Lynch KG, Maisto HSA, et al. A randomized clinical trial of alcohol care management delivered in Department of Veterans Affairs primary care clinics versus specialty addiction treatment. J Gen Intern Med. 2013;29:162-168.
21. Gilbody S, Bower P, Fletcher J, et al. Collaborative care for depression: a cumulative meta-analysis and review of longer-term outcomes. Arch Intern Med. 2006;166:2314-2321.
22. Rossom RC, Solberg LI, Magnan S, et al. Impact of a national collaborative care initiative for patients with depression and diabetes or cardiovascular disease. Gen Hosp Psychiatry. 2017;15:77-85.
23. Kroenke K, Bair MJ, Damush TM, et al. Optimized antidepressant therapy and pain self-management in primary care patients with depression and musculoskeletal pain. JAMA. 2009;301:2009-2110.
24. Katon WJ, Lin EH, Von Korff M, et al. Collaborative care for patients with depression and chronic illnesses. N Engl J Med. 2010;363:2611-2620.
25. Miller CJ, Grogan-Kaylor A, Perron BE, et al. Collaborative chronic care models for mental health conditions. Med Care. 2013;51:922-930.
26. Woltmann E, Grogan-Kaylor A, Perron B, et al. Comparative effectiveness of collaborative chronic care models for mental health conditions across primary, specialty, and behavioral health care settings: systematic review and meta-analysis. Am J Psychiatry. 2012;11:790-804.
27. U.S. Department of Veterans Affairs. Rubenstein LV, Williams JW Jr, Danz M, et al. Determining key features of effective depression interventions. 2009. http://www.hsrd.research.va.gov/publications/esp/depinter.cfm. Accessed August 22, 2018.
28. Coventry PA, Hudson JL, Kontopantelis E, et al. Characteristics of effective collaborative care for treatment of depression: a systematic review and meta-regression of 74 randomised controlled trials. PLoS One. 2014;9:e108114.
29. Institute for Clinical and Economic Review. Tice JA, Ollendorf DA, Reed SJ, et al. Integrating behavioral health into primary care. 2015. https://icer-review.org/wp-content/uploads/2016/01/BHI_Final_Report_0602151.pdf. Accessed August 27, 2018.
30. Rubenstein LV, Chaney EF, Ober S, et al. Using evidence-based quality improvement methods for translating depression collaborative care research into practice. Fam Syst Health. 2010;28:91-113.
31. Unützer J, Katon WJ, Fan MY, et al. Long-term cost effects of collaborative care for late-life depression. Am J Manag Care. 2008;14:95-100.
32. Harkness EF, Bower PJ. On-site mental health workers delivering psychological therapy and psychosocial interventions to patients in primary care: effects on the professional practice of primary care providers. Cochrane Database Syst Rev. 2009;(1):CD000532.
33. Druss BG, Rohrbaugh RM, Levinson CM, et al. Integrated medical care for patients with serious psychiatric illness. Arch Gen Psychiatry. 2001;58:861-868.
34. Davis M, Balasubramanian BA, Waller E, et al. Integrating behavioral and physical health care in the real world: early lessons from advancing care together. J Am Board Fam Med. 2013;26:588-602.
35. Gold SB, Green LA, Peek CJ. From our practices to yours: key messages for the journey to integrated behavioral health. J Am Board Fam Med. 2017;30:25-34.
36. Institute for Clinical and Economic Review. Integrating behavioral health into primary care. 2015. https://icer-review.org/wp-content/uploads/2016/02/CTAF_BHI_Action_Guide_060215.pdf. Accessed April 25, 2019.
In a typical primary care practice, detecting and managing mental health problems competes with other priorities such as treating acute physical illness, monitoring chronic disease, providing preventive health services, and assessing compliance with standards of care.1 These competing demands for a primary care provider’s time, paired with limited mental health resources in the community, may result in suboptimal behavioral health care.1-3 Even when referrals are made to
Approximately 30% of adults with physical disorders also have one or more behavioral health conditions, such as anxiety, panic, mood, or substance use disorders.6 Although physical and behavioral health conditions are inextricably linked, their assessment and treatment get separated into different silos.7 Given that fewer than 20% of depressed patients are seen by a psychiatrist or psychologist,8 the responsibility of providing mental health care often falls on the primary care physician.8,9
Efforts to improve the treatment of common mental disorders in primary care have traditionally focused on screening for these disorders, educating primary care providers, developing treatment guidelines, and referring patients to mental health specialty care.10 However, behavioral health integration offers another way forward.
WHAT IS BEHAVIORAL HEALTH INTEGRATION?
Behavioral health integration (BHI) in primary care refers to primary care physicians and behavioral health clinicians working in concert with patients to address their primary care and behavioral health needs.11

Numerous overlapping terms have been used to describe BHI, and this has caused some confusion. In 2013, the Agency for Healthcare Research and Quality (AHRQ) issued a lexicon standardizing the terminology used in BHI.11 The commonly used terms are

COORDINATED CARE AND THE COLLABORATIVE CARE MODEL
BHI at the level of coordinated care has almost exclusively been studied and practiced along the lines of the collaborative care model (CCM).14-16 This model represents an advanced level of coordinated care in the BHI continuum. The most substantial evidence for CCM lies in the management of depression and anxiety.14-16
Usual care involves the primary care physician and the patient. CCM adds 2 vital roles—a behavioral health care manager and a psychiatric consultant. A behavioral health care manager is typically a counselor, clinical social worker, psychologist, or psychiatric nurse who performs all care-management tasks including offering psychotherapy when that is part of the treatment plan.
Continue to: The care manager's functions include...
The care manager’s functions include systematic follow-up with structured monitoring of symptoms and treatment adherence, coordination and communication among care providers, patient education, and self-management support, including the use of motivational interviewing. The behavioral health care manager performs this systematic follow up by maintaining a patient “registry”—case-management software used in conjunction with, or embedded in, the practice electronic health record to track patients’ data and clinical outcomes, as well as to facilitate decision-making.
The care manager communicates with the psychiatrist, who offers suggestions for drug therapy, which is prescribed by the primary care physician. The care manager also regularly evaluates the patient’s status using a standardized scale, communicates these scores to the psychiatrist, and transmits any recommendations to the primary care physician (FIGURE 2).17

EVIDENCE FOR CCM
Collaborative and routine care were compared in a 2012 Cochrane review that included 79 randomized controlled trials (RCTs) involving 24,308 patients worldwide.16 Seventy-two of the 79 RCTs focused on patients with depression or depression with anxiety, while 6 studies included participants with only anxiety disorders.16 One additional study focused on mental health quality of life. (To learn about CCM and severe mental illness and substance use disorder, see “Less well studied: CCM and severe mental illness, alcohol dependence.”18-20)
SIDEBAR
Less well studied: CCM and severe mental illness, alcohol dependence
Evidence for collaborative care in severe mental illness (SMI) is very limited. SMI is defined as schizophrenia or other schizophrenia-like psychoses (eg, schizophreniform and schizoaffective disorders), bipolar affective disorder, or other psychosis.
A 2013 Cochrane review identified only 1 RCT involving 306 veterans with bipolar disease.18 The review concluded that there was low-quality evidence that collaborative care led to a relative risk reduction of 25% for psychiatric admissions at Year 2 compared with standard care (RR = 0.75; 95% CI, 0.57-0.99).18
One 2017 RCT involving 245 veterans that looked at a collaborative care model for patients with severe mental illness found a modest benefit for physical health-related quality of life, but did not find any benefit in mental health outcomes.19
Alcohol dependence. There is very limited, but high-quality, evidence for the utility of CCM in alcohol dependence. In one RCT, 163 veterans were assigned to either CCM or referral to standard treatment in a specialty outpatient addiction treatment program. The CCM group had a significantly higher proportion of participants engaged in treatment over the study’s 26 weeks (odds ratio [OR] = 5.36; 95% CI, 2.99-9.59). The percentage of heavy drinking days was significantly lower in the CCM group (OR = 2.16; 95% CI, 1.27-3.66), while overall abstinence did not differ between groups.20
For adults with depression treated with the CCM, significantly greater improvement in depression outcome measures was seen in the short-term (standardized mean difference [SMD] = -0.34; 95% confidence interval [CI], -0.41 to -0.27; risk ratio [RR] = 1.32; 95% CI, 1.22-1.43), in the medium term (SMD = -0.28; 95% CI, -0.41 to -0.15; RR = 1.31; 95% CI, 1.17–1.48), and in the long term (SMD = -0.35; 95% CI, -0.46 to -0.24; RR = 1.29; 95% CI, 1.18–1.41).16
Comparisons of mental health quality of life over the short term (0-6 months), medium term (7-12 months), and long term (13-24 months) did not show any significant difference between CCM and routine care.16 Comparisons of physical health quality of life over the short term and medium term did not show any significant difference between CCM and routine care.16
Continue to: Significantly greater improvement...
Significantly greater improvement in anxiety outcomes was seen for adults treated with CCM in the short term (SMD = -0.30; 95% CI, -0.44 to -0.17; RR = 1.50; 95% CI, 1.21–1.87), in the medium term (SMD = -0.33; 95% CI, -0.47 to -0.19; RR = 1.41; 95% CI, 1.18-1.69), and in the long term (SMD = -0.20; 95% CI, -0.34 to -0.06; RR = 1.26; 95% CI, 1.11–1.42).16
A 2016 systematic review of 94 RCTs involving more than 25,000 patients also provided high-quality evidence that collaborative care yields small-to-moderate improvements in symptoms from mood disorders and mental health-related quality of life.15 A 2006 meta-analysis of 37 RCTs comprising 12,355 patients showed that collaborative care involving a case manager is more effective than standard care in improving depression outcomes at 6 months (SMD = 0.25; 95% CI, 0.18-0.32) and up to 5 years (SMD = 0.15; 95% CI, 0.001-0.31).21
Better care of mental health disorders also improves medical outcomes
Several trials have focused on jointly managing depression and a chronic physical condition such as chronic pain, diabetes, and coronary heart disease,22 demonstrating improved outcomes for both depression and the comanaged conditions.
- Chronic pain. When compared with usual care, collaborative care resulted in moderate reductions in both pain severity and associated disability (41.5% vs 17.3%; RR = 2.4; 95% CI, 1.6-3.2).23
- Diabetes. Patients managed collaboratively were more likely to have a decrease of ≥ 1% in the glycated hemoglobin level from baseline (36% vs 19%; P = .006).24
- Cardiovascular disease. Significant real-world risk reduction was achieved by improving blood pressure control (58% achieved blood pressure control compared with a projected target of 20%).22
IS THERE A COMMON THREAD AMONG SUCCESSFUL CCMs?
Attempts to identify commonalities between the many iterations of successful CCMs have produced varying results due to differing selections of relevant RCTs.25-29 However, a few common features have been identified:
- care managers assess symptoms at baseline and at follow-up using a standardized measure such as the Patient Health Questionnaire (PHQ-9);
- care managers monitor treatment adherence;
- follow-up is active for at least 16 weeks;
- primary care and mental health providers actively engage in patient management; and
- mental health specialists regularly supervise care managers.
The one feature that is consistent with improved outcomes is the presence of the care manager.25-29
Continue to: The improvement associated...
The improvement associated with collaborative care is clinically meaningful to patients and physicians. In one RCT, collaborative care doubled response rates of depression treatment compared with usual care.3 Quality improvement data from real-world implementation of collaborative care programs suggests that similar outcomes can be achieved in a variety of settings.30
COST BENEFITS OF CCM
Collaborative care for depression is associated with lower health care costs.29,31
A meta-analysis of 57 RCTs in 2012 showed that CCM improves depression outcomes across populations, settings, and outcome domains, and that these results are achieved at little to no increase in treatment costs compared with usual care (Cohen’s d = 0.05; 95% CI, –0.02–0.12).26
When collaborative care was compared with routine care in an RCT involving 1801 primary care patients ≥ 60 years who were suffering from depression, a cost saving of $3363 per patient over 4 years was demonstrated in the intervention arm.31
A technical analysis of 94 RCTs in 2015 concluded that CCM is cost effective compared with usual care, with a range of $15,000 to $80,000 per quality-adjusted life year gained. These studies also indicated that organizations’ costs to implement CCM increase in the short term. Based on this analysis, organizations would need to invest between $3 to $22 per patient per month to implement and sustain CCMs, depending on the prevalence of depression in the population.29
Continue to: OTHER MODELS OF BHI
OTHER MODELS OF BHI
Higher levels of BHI such as co-location and integration do not have the same quality of evidence as CCM.
A 2009 Cochrane review of 42 studies involving 3880 patients found that mental health workers delivering psychological therapy and psychosocial interventions in primary care settings brought about significant reductions in primary care physician consultations (SMD = ‐0.17; 95% CI, ‐0.30 to ‐0.05); a relative risk reduction of 23% in psychotropic prescribing (RR = 0.67; 95% CI, 0.56–0.79); a decrease in prescribing costs (SMD = ‐0.22; 95% CI, ‐0.38 to ‐0.07); and a relative risk reduction in mental health referral of 87% (RR = 0.13; 95% CI, 0.09–0.20) for the patients they were seeing.32 The authors concluded the changes were modest in magnitude and inconsistent across different studies.32
Embedding medical providers in behavior health centers—ie, the reverse co-location model—also has very limited evidence. An RCT involving 120 veterans found that patients enrolled in a reverse co-location clinic did significantly better than controls seen in a general care clinic in terms of continuity of care and preventive care such as screening for hypertension (84.7% vs 65.6%; X 2 = 5.9, P = .01), diabetes (71.2% vs 45.9%; X 2 = 7.9, P < .005), hepatitis (39% vs 14.8%; X 2 = 9, P = .003), and cholesterol (79.7% vs 57.4%; X 2 = 6.9, P = .009).33
HOW TO IMPLEMENT A SUCCESSFUL BHI PROGRAM
A demonstration and evaluation project involving 11 diverse practices in Colorado explored ways to integrate behavioral health in primary care. Five main themes emerged34,35:
- Frame integrated care as a necessary paradigm shift to patient-centered, whole-person health care.
- Define relationships and protocols up front, understanding that they will evolve.
- Build inclusive, empowered teams to provide the foundation for integration.
- Develop a change management strategy of continuous evaluation and course correction.
- Use targeted data collection pertinent to integrated care to drive improvement and impart accountability.
The Institute for Clinical and Economic Review has organized an extensive list of resources36 for implementing BHI models, a sampling of which is shown in TABLE 2.

Continue to: TAKE-AWAY POINTS
TAKE-AWAY POINTS
There is high quality evidence that collaborative care works for the management of depression and anxiety disorder in primary care, and this is associated with significant cost savings.
CORRESPONDENCE
Rajesh (FNU) Rajesh, MD, Main Campus Family Medicine Clinic, MetroHealth, 2500 MetroHealth Drive, Cleveland, OH 44109; [email protected]
In a typical primary care practice, detecting and managing mental health problems competes with other priorities such as treating acute physical illness, monitoring chronic disease, providing preventive health services, and assessing compliance with standards of care.1 These competing demands for a primary care provider’s time, paired with limited mental health resources in the community, may result in suboptimal behavioral health care.1-3 Even when referrals are made to
Approximately 30% of adults with physical disorders also have one or more behavioral health conditions, such as anxiety, panic, mood, or substance use disorders.6 Although physical and behavioral health conditions are inextricably linked, their assessment and treatment get separated into different silos.7 Given that fewer than 20% of depressed patients are seen by a psychiatrist or psychologist,8 the responsibility of providing mental health care often falls on the primary care physician.8,9
Efforts to improve the treatment of common mental disorders in primary care have traditionally focused on screening for these disorders, educating primary care providers, developing treatment guidelines, and referring patients to mental health specialty care.10 However, behavioral health integration offers another way forward.
WHAT IS BEHAVIORAL HEALTH INTEGRATION?
Behavioral health integration (BHI) in primary care refers to primary care physicians and behavioral health clinicians working in concert with patients to address their primary care and behavioral health needs.11

Numerous overlapping terms have been used to describe BHI, and this has caused some confusion. In 2013, the Agency for Healthcare Research and Quality (AHRQ) issued a lexicon standardizing the terminology used in BHI.11 The commonly used terms are

COORDINATED CARE AND THE COLLABORATIVE CARE MODEL
BHI at the level of coordinated care has almost exclusively been studied and practiced along the lines of the collaborative care model (CCM).14-16 This model represents an advanced level of coordinated care in the BHI continuum. The most substantial evidence for CCM lies in the management of depression and anxiety.14-16
Usual care involves the primary care physician and the patient. CCM adds 2 vital roles—a behavioral health care manager and a psychiatric consultant. A behavioral health care manager is typically a counselor, clinical social worker, psychologist, or psychiatric nurse who performs all care-management tasks including offering psychotherapy when that is part of the treatment plan.
Continue to: The care manager's functions include...
The care manager’s functions include systematic follow-up with structured monitoring of symptoms and treatment adherence, coordination and communication among care providers, patient education, and self-management support, including the use of motivational interviewing. The behavioral health care manager performs this systematic follow up by maintaining a patient “registry”—case-management software used in conjunction with, or embedded in, the practice electronic health record to track patients’ data and clinical outcomes, as well as to facilitate decision-making.
The care manager communicates with the psychiatrist, who offers suggestions for drug therapy, which is prescribed by the primary care physician. The care manager also regularly evaluates the patient’s status using a standardized scale, communicates these scores to the psychiatrist, and transmits any recommendations to the primary care physician (FIGURE 2).17

EVIDENCE FOR CCM
Collaborative and routine care were compared in a 2012 Cochrane review that included 79 randomized controlled trials (RCTs) involving 24,308 patients worldwide.16 Seventy-two of the 79 RCTs focused on patients with depression or depression with anxiety, while 6 studies included participants with only anxiety disorders.16 One additional study focused on mental health quality of life. (To learn about CCM and severe mental illness and substance use disorder, see “Less well studied: CCM and severe mental illness, alcohol dependence.”18-20)
SIDEBAR
Less well studied: CCM and severe mental illness, alcohol dependence
Evidence for collaborative care in severe mental illness (SMI) is very limited. SMI is defined as schizophrenia or other schizophrenia-like psychoses (eg, schizophreniform and schizoaffective disorders), bipolar affective disorder, or other psychosis.
A 2013 Cochrane review identified only 1 RCT involving 306 veterans with bipolar disease.18 The review concluded that there was low-quality evidence that collaborative care led to a relative risk reduction of 25% for psychiatric admissions at Year 2 compared with standard care (RR = 0.75; 95% CI, 0.57-0.99).18
One 2017 RCT involving 245 veterans that looked at a collaborative care model for patients with severe mental illness found a modest benefit for physical health-related quality of life, but did not find any benefit in mental health outcomes.19
Alcohol dependence. There is very limited, but high-quality, evidence for the utility of CCM in alcohol dependence. In one RCT, 163 veterans were assigned to either CCM or referral to standard treatment in a specialty outpatient addiction treatment program. The CCM group had a significantly higher proportion of participants engaged in treatment over the study’s 26 weeks (odds ratio [OR] = 5.36; 95% CI, 2.99-9.59). The percentage of heavy drinking days was significantly lower in the CCM group (OR = 2.16; 95% CI, 1.27-3.66), while overall abstinence did not differ between groups.20
For adults with depression treated with the CCM, significantly greater improvement in depression outcome measures was seen in the short-term (standardized mean difference [SMD] = -0.34; 95% confidence interval [CI], -0.41 to -0.27; risk ratio [RR] = 1.32; 95% CI, 1.22-1.43), in the medium term (SMD = -0.28; 95% CI, -0.41 to -0.15; RR = 1.31; 95% CI, 1.17–1.48), and in the long term (SMD = -0.35; 95% CI, -0.46 to -0.24; RR = 1.29; 95% CI, 1.18–1.41).16
Comparisons of mental health quality of life over the short term (0-6 months), medium term (7-12 months), and long term (13-24 months) did not show any significant difference between CCM and routine care.16 Comparisons of physical health quality of life over the short term and medium term did not show any significant difference between CCM and routine care.16
Continue to: Significantly greater improvement...
Significantly greater improvement in anxiety outcomes was seen for adults treated with CCM in the short term (SMD = -0.30; 95% CI, -0.44 to -0.17; RR = 1.50; 95% CI, 1.21–1.87), in the medium term (SMD = -0.33; 95% CI, -0.47 to -0.19; RR = 1.41; 95% CI, 1.18-1.69), and in the long term (SMD = -0.20; 95% CI, -0.34 to -0.06; RR = 1.26; 95% CI, 1.11–1.42).16
A 2016 systematic review of 94 RCTs involving more than 25,000 patients also provided high-quality evidence that collaborative care yields small-to-moderate improvements in symptoms from mood disorders and mental health-related quality of life.15 A 2006 meta-analysis of 37 RCTs comprising 12,355 patients showed that collaborative care involving a case manager is more effective than standard care in improving depression outcomes at 6 months (SMD = 0.25; 95% CI, 0.18-0.32) and up to 5 years (SMD = 0.15; 95% CI, 0.001-0.31).21
Better care of mental health disorders also improves medical outcomes
Several trials have focused on jointly managing depression and a chronic physical condition such as chronic pain, diabetes, and coronary heart disease,22 demonstrating improved outcomes for both depression and the comanaged conditions.
- Chronic pain. When compared with usual care, collaborative care resulted in moderate reductions in both pain severity and associated disability (41.5% vs 17.3%; RR = 2.4; 95% CI, 1.6-3.2).23
- Diabetes. Patients managed collaboratively were more likely to have a decrease of ≥ 1% in the glycated hemoglobin level from baseline (36% vs 19%; P = .006).24
- Cardiovascular disease. Significant real-world risk reduction was achieved by improving blood pressure control (58% achieved blood pressure control compared with a projected target of 20%).22
IS THERE A COMMON THREAD AMONG SUCCESSFUL CCMs?
Attempts to identify commonalities between the many iterations of successful CCMs have produced varying results due to differing selections of relevant RCTs.25-29 However, a few common features have been identified:
- care managers assess symptoms at baseline and at follow-up using a standardized measure such as the Patient Health Questionnaire (PHQ-9);
- care managers monitor treatment adherence;
- follow-up is active for at least 16 weeks;
- primary care and mental health providers actively engage in patient management; and
- mental health specialists regularly supervise care managers.
The one feature that is consistent with improved outcomes is the presence of the care manager.25-29
Continue to: The improvement associated...
The improvement associated with collaborative care is clinically meaningful to patients and physicians. In one RCT, collaborative care doubled response rates of depression treatment compared with usual care.3 Quality improvement data from real-world implementation of collaborative care programs suggests that similar outcomes can be achieved in a variety of settings.30
COST BENEFITS OF CCM
Collaborative care for depression is associated with lower health care costs.29,31
A meta-analysis of 57 RCTs in 2012 showed that CCM improves depression outcomes across populations, settings, and outcome domains, and that these results are achieved at little to no increase in treatment costs compared with usual care (Cohen’s d = 0.05; 95% CI, –0.02–0.12).26
When collaborative care was compared with routine care in an RCT involving 1801 primary care patients ≥ 60 years who were suffering from depression, a cost saving of $3363 per patient over 4 years was demonstrated in the intervention arm.31
A technical analysis of 94 RCTs in 2015 concluded that CCM is cost effective compared with usual care, with a range of $15,000 to $80,000 per quality-adjusted life year gained. These studies also indicated that organizations’ costs to implement CCM increase in the short term. Based on this analysis, organizations would need to invest between $3 to $22 per patient per month to implement and sustain CCMs, depending on the prevalence of depression in the population.29
Continue to: OTHER MODELS OF BHI
OTHER MODELS OF BHI
Higher levels of BHI such as co-location and integration do not have the same quality of evidence as CCM.
A 2009 Cochrane review of 42 studies involving 3880 patients found that mental health workers delivering psychological therapy and psychosocial interventions in primary care settings brought about significant reductions in primary care physician consultations (SMD = ‐0.17; 95% CI, ‐0.30 to ‐0.05); a relative risk reduction of 23% in psychotropic prescribing (RR = 0.67; 95% CI, 0.56–0.79); a decrease in prescribing costs (SMD = ‐0.22; 95% CI, ‐0.38 to ‐0.07); and a relative risk reduction in mental health referral of 87% (RR = 0.13; 95% CI, 0.09–0.20) for the patients they were seeing.32 The authors concluded the changes were modest in magnitude and inconsistent across different studies.32
Embedding medical providers in behavior health centers—ie, the reverse co-location model—also has very limited evidence. An RCT involving 120 veterans found that patients enrolled in a reverse co-location clinic did significantly better than controls seen in a general care clinic in terms of continuity of care and preventive care such as screening for hypertension (84.7% vs 65.6%; X 2 = 5.9, P = .01), diabetes (71.2% vs 45.9%; X 2 = 7.9, P < .005), hepatitis (39% vs 14.8%; X 2 = 9, P = .003), and cholesterol (79.7% vs 57.4%; X 2 = 6.9, P = .009).33
HOW TO IMPLEMENT A SUCCESSFUL BHI PROGRAM
A demonstration and evaluation project involving 11 diverse practices in Colorado explored ways to integrate behavioral health in primary care. Five main themes emerged34,35:
- Frame integrated care as a necessary paradigm shift to patient-centered, whole-person health care.
- Define relationships and protocols up front, understanding that they will evolve.
- Build inclusive, empowered teams to provide the foundation for integration.
- Develop a change management strategy of continuous evaluation and course correction.
- Use targeted data collection pertinent to integrated care to drive improvement and impart accountability.
The Institute for Clinical and Economic Review has organized an extensive list of resources36 for implementing BHI models, a sampling of which is shown in TABLE 2.

Continue to: TAKE-AWAY POINTS
TAKE-AWAY POINTS
There is high quality evidence that collaborative care works for the management of depression and anxiety disorder in primary care, and this is associated with significant cost savings.
CORRESPONDENCE
Rajesh (FNU) Rajesh, MD, Main Campus Family Medicine Clinic, MetroHealth, 2500 MetroHealth Drive, Cleveland, OH 44109; [email protected]
1. Rost K, Nutting P, Smith J, et al. The role of competing demands in the treatment provided primary care patients with major depression. Arch Fam Med. 2000;9:150-154.
2. Rush A, Trivedi M, Carmody T, et al. One-year clinical outcomes of depressed public sector outpatients: a benchmark for subsequent studies. Biol Psychiatry. 2004;56:46-53.
3. Unützer J, Katon W, Callahan CM, et al. Collaborative care management of late-life depression in the primary care setting. JAMA. 2002;288:2836-2845.
4. Department of Veterans Affairs. Bradford DW, Slubicki MN, McDuffie J, et al. Effects of care models to improve general medical outcomes for individuals with serious mental illness. 2011. https://www.hsrd.research.va.gov/publications/esp/smi-REPORT.pdf. Accessed August 22, 2018.
5. Druss BG, von Esenwein S. Improving general medical care for persons with mental and addictive disorders: systematic review. Gen Hosp Psychiatry. 2006;28:145-153.
6. Druss BG, Walker ER. Mental Disorders and Medical Comorbidity. Research Synthesis Report No. 21. Princeton, NJ: The Robert Wood Johnson Foundation; February 2011.
7. Reed SJ, Shore KK, Tice JA. Effectiveness and value of integrating behavioral health into primary care. JAMA Intern Med. 2016;176:691-692.
8. Young AS, Klap R, Sherbourne CD, et al. The quality of care for depressive and anxiety disorders in the United States. Arch Gen Psychiatry. 2001;58:55-61.
9. Butler M, Kane RL, McAlpine D, et al. Integration of mental health/substance abuse and primary care. Rockville, MD: Agency for Healthcare Research and Quality; 2008. http://www.ncbi.nlm.nih.gov/books/NBK38632/. Accessed March 2, 2019.
10. Unützer J, Schoenbaum M, Druss B, et al. Transforming mental health care at the interface with general medicine: report for the presidents commission. Psychiatr Serv. 2006;57:37-47. doi: 10.1176/appi.ps.57.1.37.
11. Peek CJ; the National Integration Academy Council. Lexicon for behavioral health and primary care integration: concepts and definitions developed by expert consensus. AHRQ. https://integrationacademy.ahrq.gov/sites/default/files/Lexicon.pdf. Published April 2013. Accessed May 29, 2019.
12. Heath B, Wise Romero P, Reynolds K. A standard framework for levels of integrated healthcare and update throughout the document. SAMHSA-HRSA. https://www.integration.samhsa.gov/integrated-care-models/A_Standard_Framework_for_Levels_of_Integrated_Healthcare.pdf. Published March 2013. Accessed May 29, 2019.
13. Integrating physical and behavioral health care: promising Medicaid models. The Henry J. Kaiser Family Foundation. https://www.kff.org/wp-content/uploads/2014/02/8553-integrating-physical-and-behavioral-health-care-promising-medicaid-models.pdf. Published February 2014. Accessed May 29, 2019.
14. Vanderlip ER, Rundell J, Avery M, et al. Dissemination of integrated care within adult primary care settings: the collaborative care model. SAMHSA-HRSA. https://www.integration.samhsa.gov/integrated-care-models/APA-APM-Dissemination-Integrated-Care-Report.pdf. Published 2016. Accessed May 29, 2019.
15. Gerrity M. Evolving models of behavioral health integration: evidence update 2010-2015. Milbank Memorial Fund. https://www.milbank.org/wp-content/uploads/2016/05/Evolving-Models-of-BHI.pdf. Published May 2016. Accessed May 29, 2019.
16. Archer J, Bower P, Gilbody S, et al. Collaborative care for depression and anxiety problems. Cochrane Database Syst Rev. 2012;10.1002/14651858.cd006525.pub2.
17. Team Structure. University of Washington AIMs Center. https://aims.uw.edu/collaborative-care/team-structure. Published 2017.Accessed May 29, 2019.
18. Reilly S, Planner C, Gask L, et al. Collaborative care approaches for people with severe mental illness. Cochrane Database Syst Rev. 2013;(11):CD009531.
19. Kilbourne AM, Barbaresso MM, Lai Z, et al. Improving physical health in patients with chronic mental disorders. J Clin Psychiatry. 2017;78:129-137.
20. Oslin DW, Lynch KG, Maisto HSA, et al. A randomized clinical trial of alcohol care management delivered in Department of Veterans Affairs primary care clinics versus specialty addiction treatment. J Gen Intern Med. 2013;29:162-168.
21. Gilbody S, Bower P, Fletcher J, et al. Collaborative care for depression: a cumulative meta-analysis and review of longer-term outcomes. Arch Intern Med. 2006;166:2314-2321.
22. Rossom RC, Solberg LI, Magnan S, et al. Impact of a national collaborative care initiative for patients with depression and diabetes or cardiovascular disease. Gen Hosp Psychiatry. 2017;15:77-85.
23. Kroenke K, Bair MJ, Damush TM, et al. Optimized antidepressant therapy and pain self-management in primary care patients with depression and musculoskeletal pain. JAMA. 2009;301:2009-2110.
24. Katon WJ, Lin EH, Von Korff M, et al. Collaborative care for patients with depression and chronic illnesses. N Engl J Med. 2010;363:2611-2620.
25. Miller CJ, Grogan-Kaylor A, Perron BE, et al. Collaborative chronic care models for mental health conditions. Med Care. 2013;51:922-930.
26. Woltmann E, Grogan-Kaylor A, Perron B, et al. Comparative effectiveness of collaborative chronic care models for mental health conditions across primary, specialty, and behavioral health care settings: systematic review and meta-analysis. Am J Psychiatry. 2012;11:790-804.
27. U.S. Department of Veterans Affairs. Rubenstein LV, Williams JW Jr, Danz M, et al. Determining key features of effective depression interventions. 2009. http://www.hsrd.research.va.gov/publications/esp/depinter.cfm. Accessed August 22, 2018.
28. Coventry PA, Hudson JL, Kontopantelis E, et al. Characteristics of effective collaborative care for treatment of depression: a systematic review and meta-regression of 74 randomised controlled trials. PLoS One. 2014;9:e108114.
29. Institute for Clinical and Economic Review. Tice JA, Ollendorf DA, Reed SJ, et al. Integrating behavioral health into primary care. 2015. https://icer-review.org/wp-content/uploads/2016/01/BHI_Final_Report_0602151.pdf. Accessed August 27, 2018.
30. Rubenstein LV, Chaney EF, Ober S, et al. Using evidence-based quality improvement methods for translating depression collaborative care research into practice. Fam Syst Health. 2010;28:91-113.
31. Unützer J, Katon WJ, Fan MY, et al. Long-term cost effects of collaborative care for late-life depression. Am J Manag Care. 2008;14:95-100.
32. Harkness EF, Bower PJ. On-site mental health workers delivering psychological therapy and psychosocial interventions to patients in primary care: effects on the professional practice of primary care providers. Cochrane Database Syst Rev. 2009;(1):CD000532.
33. Druss BG, Rohrbaugh RM, Levinson CM, et al. Integrated medical care for patients with serious psychiatric illness. Arch Gen Psychiatry. 2001;58:861-868.
34. Davis M, Balasubramanian BA, Waller E, et al. Integrating behavioral and physical health care in the real world: early lessons from advancing care together. J Am Board Fam Med. 2013;26:588-602.
35. Gold SB, Green LA, Peek CJ. From our practices to yours: key messages for the journey to integrated behavioral health. J Am Board Fam Med. 2017;30:25-34.
36. Institute for Clinical and Economic Review. Integrating behavioral health into primary care. 2015. https://icer-review.org/wp-content/uploads/2016/02/CTAF_BHI_Action_Guide_060215.pdf. Accessed April 25, 2019.
1. Rost K, Nutting P, Smith J, et al. The role of competing demands in the treatment provided primary care patients with major depression. Arch Fam Med. 2000;9:150-154.
2. Rush A, Trivedi M, Carmody T, et al. One-year clinical outcomes of depressed public sector outpatients: a benchmark for subsequent studies. Biol Psychiatry. 2004;56:46-53.
3. Unützer J, Katon W, Callahan CM, et al. Collaborative care management of late-life depression in the primary care setting. JAMA. 2002;288:2836-2845.
4. Department of Veterans Affairs. Bradford DW, Slubicki MN, McDuffie J, et al. Effects of care models to improve general medical outcomes for individuals with serious mental illness. 2011. https://www.hsrd.research.va.gov/publications/esp/smi-REPORT.pdf. Accessed August 22, 2018.
5. Druss BG, von Esenwein S. Improving general medical care for persons with mental and addictive disorders: systematic review. Gen Hosp Psychiatry. 2006;28:145-153.
6. Druss BG, Walker ER. Mental Disorders and Medical Comorbidity. Research Synthesis Report No. 21. Princeton, NJ: The Robert Wood Johnson Foundation; February 2011.
7. Reed SJ, Shore KK, Tice JA. Effectiveness and value of integrating behavioral health into primary care. JAMA Intern Med. 2016;176:691-692.
8. Young AS, Klap R, Sherbourne CD, et al. The quality of care for depressive and anxiety disorders in the United States. Arch Gen Psychiatry. 2001;58:55-61.
9. Butler M, Kane RL, McAlpine D, et al. Integration of mental health/substance abuse and primary care. Rockville, MD: Agency for Healthcare Research and Quality; 2008. http://www.ncbi.nlm.nih.gov/books/NBK38632/. Accessed March 2, 2019.
10. Unützer J, Schoenbaum M, Druss B, et al. Transforming mental health care at the interface with general medicine: report for the presidents commission. Psychiatr Serv. 2006;57:37-47. doi: 10.1176/appi.ps.57.1.37.
11. Peek CJ; the National Integration Academy Council. Lexicon for behavioral health and primary care integration: concepts and definitions developed by expert consensus. AHRQ. https://integrationacademy.ahrq.gov/sites/default/files/Lexicon.pdf. Published April 2013. Accessed May 29, 2019.
12. Heath B, Wise Romero P, Reynolds K. A standard framework for levels of integrated healthcare and update throughout the document. SAMHSA-HRSA. https://www.integration.samhsa.gov/integrated-care-models/A_Standard_Framework_for_Levels_of_Integrated_Healthcare.pdf. Published March 2013. Accessed May 29, 2019.
13. Integrating physical and behavioral health care: promising Medicaid models. The Henry J. Kaiser Family Foundation. https://www.kff.org/wp-content/uploads/2014/02/8553-integrating-physical-and-behavioral-health-care-promising-medicaid-models.pdf. Published February 2014. Accessed May 29, 2019.
14. Vanderlip ER, Rundell J, Avery M, et al. Dissemination of integrated care within adult primary care settings: the collaborative care model. SAMHSA-HRSA. https://www.integration.samhsa.gov/integrated-care-models/APA-APM-Dissemination-Integrated-Care-Report.pdf. Published 2016. Accessed May 29, 2019.
15. Gerrity M. Evolving models of behavioral health integration: evidence update 2010-2015. Milbank Memorial Fund. https://www.milbank.org/wp-content/uploads/2016/05/Evolving-Models-of-BHI.pdf. Published May 2016. Accessed May 29, 2019.
16. Archer J, Bower P, Gilbody S, et al. Collaborative care for depression and anxiety problems. Cochrane Database Syst Rev. 2012;10.1002/14651858.cd006525.pub2.
17. Team Structure. University of Washington AIMs Center. https://aims.uw.edu/collaborative-care/team-structure. Published 2017.Accessed May 29, 2019.
18. Reilly S, Planner C, Gask L, et al. Collaborative care approaches for people with severe mental illness. Cochrane Database Syst Rev. 2013;(11):CD009531.
19. Kilbourne AM, Barbaresso MM, Lai Z, et al. Improving physical health in patients with chronic mental disorders. J Clin Psychiatry. 2017;78:129-137.
20. Oslin DW, Lynch KG, Maisto HSA, et al. A randomized clinical trial of alcohol care management delivered in Department of Veterans Affairs primary care clinics versus specialty addiction treatment. J Gen Intern Med. 2013;29:162-168.
21. Gilbody S, Bower P, Fletcher J, et al. Collaborative care for depression: a cumulative meta-analysis and review of longer-term outcomes. Arch Intern Med. 2006;166:2314-2321.
22. Rossom RC, Solberg LI, Magnan S, et al. Impact of a national collaborative care initiative for patients with depression and diabetes or cardiovascular disease. Gen Hosp Psychiatry. 2017;15:77-85.
23. Kroenke K, Bair MJ, Damush TM, et al. Optimized antidepressant therapy and pain self-management in primary care patients with depression and musculoskeletal pain. JAMA. 2009;301:2009-2110.
24. Katon WJ, Lin EH, Von Korff M, et al. Collaborative care for patients with depression and chronic illnesses. N Engl J Med. 2010;363:2611-2620.
25. Miller CJ, Grogan-Kaylor A, Perron BE, et al. Collaborative chronic care models for mental health conditions. Med Care. 2013;51:922-930.
26. Woltmann E, Grogan-Kaylor A, Perron B, et al. Comparative effectiveness of collaborative chronic care models for mental health conditions across primary, specialty, and behavioral health care settings: systematic review and meta-analysis. Am J Psychiatry. 2012;11:790-804.
27. U.S. Department of Veterans Affairs. Rubenstein LV, Williams JW Jr, Danz M, et al. Determining key features of effective depression interventions. 2009. http://www.hsrd.research.va.gov/publications/esp/depinter.cfm. Accessed August 22, 2018.
28. Coventry PA, Hudson JL, Kontopantelis E, et al. Characteristics of effective collaborative care for treatment of depression: a systematic review and meta-regression of 74 randomised controlled trials. PLoS One. 2014;9:e108114.
29. Institute for Clinical and Economic Review. Tice JA, Ollendorf DA, Reed SJ, et al. Integrating behavioral health into primary care. 2015. https://icer-review.org/wp-content/uploads/2016/01/BHI_Final_Report_0602151.pdf. Accessed August 27, 2018.
30. Rubenstein LV, Chaney EF, Ober S, et al. Using evidence-based quality improvement methods for translating depression collaborative care research into practice. Fam Syst Health. 2010;28:91-113.
31. Unützer J, Katon WJ, Fan MY, et al. Long-term cost effects of collaborative care for late-life depression. Am J Manag Care. 2008;14:95-100.
32. Harkness EF, Bower PJ. On-site mental health workers delivering psychological therapy and psychosocial interventions to patients in primary care: effects on the professional practice of primary care providers. Cochrane Database Syst Rev. 2009;(1):CD000532.
33. Druss BG, Rohrbaugh RM, Levinson CM, et al. Integrated medical care for patients with serious psychiatric illness. Arch Gen Psychiatry. 2001;58:861-868.
34. Davis M, Balasubramanian BA, Waller E, et al. Integrating behavioral and physical health care in the real world: early lessons from advancing care together. J Am Board Fam Med. 2013;26:588-602.
35. Gold SB, Green LA, Peek CJ. From our practices to yours: key messages for the journey to integrated behavioral health. J Am Board Fam Med. 2017;30:25-34.
36. Institute for Clinical and Economic Review. Integrating behavioral health into primary care. 2015. https://icer-review.org/wp-content/uploads/2016/02/CTAF_BHI_Action_Guide_060215.pdf. Accessed April 25, 2019.
Postpartum anxiety: More common than you think
THE CASE
Julia* is a 31-year-old woman, gravida 3 para 3, who presents to your office for evaluation after a recent emergency department (ED) visit. Her husband and children are with her. She is 4 months postpartum after an uncomplicated normal spontaneous vaginal delivery. She is breastfeeding her healthy baby boy and is using an intrauterine device for birth control. She went to the ED last week after “choking on a chip” while having lunch with her children. It felt like she “couldn’t breathe.” She called 911 herself. The ED evaluation was unremarkable. Her discharge diagnosis was “panic attack,” and she was sent home with a prescription for lorazepam.
Since the incident, she has been unable to eat any solid foods and has lost 7 pounds. She also reports a globus sensation, extreme fear of swallowing, insomnia, and pervasive thoughts that she could die at any moment and leave her children motherless. She has not taken the lorazepam.
She has a history of self-reported anxiety dating back to high school but no history of panic attacks. She has never been diagnosed with an anxiety disorder and has never before been prescribed anti-anxiety medication. She doesn’t have a history of postpartum depression in prior pregnancies, and a depression screening at her postpartum visit 2 months ago was negative.
●
*The patient’s name has been changed to protect her identity.
During the perinatal period, women are particularly vulnerable to affective disorders, and primary care physicians are encouraged to routinely screen for and treat depression in pregnant and postpartum women.1 However, anxiety disorders have a higher incidence than mood disorders in the general population,2 and perinatal anxiety may be more widely underrecognized and undertreated than depression.3 In addition, higher depression scores early in pregnancy have been shown to predict higher anxiety later in pregnancy.4
As family physicians, we are well-trained to recognize and treat anxiety disorders in the general patient population; however, we may lack the awareness and tools to identify these conditions in the perinatal period. Given our frequent encounters with both mom and baby in a child’s first year of life, we are uniquely positioned to promptly recognize, diagnose, and treat postpartum anxiety and thereby improve health outcomes for families.
DEFINING PERINATAL ANXIETY
Anxiety disorders (including generalized anxiety disorder, panic, phobia, and social anxiety) are the most common mental health disorders evaluated and treated in the primary care setting, with a lifetime prevalence of close to 30%.2
Continue to: A recent report from...
A recent report from the Centers for Disease Control and Prevention (CDC) estimates that 1 in 9 women experience symptoms of postpartum depression.5 The prevalence of anxiety disorders during pregnancy and the early postpartum period is not as well-known, but studies suggest that perinatal anxiety is much more prevalent than depression. In one study, generalized anxiety disorder (GAD) in the pre- and postnatal periods was 15.8% and 17.1%, respectively; an incidence far exceeding that of perinatal depression (3.9% and 4.8%, for the same periods).6 Additional evidence suggests that even more women in the postnatal period experience clinically significant levels of anxiety but do not meet full diagnostic criteria for an anxiety disorder.7
In another study, 9.5% of women met criteria for GAD at some point during pregnancy, with highest anxiety levels in the first trimester.8 Women with a history of GAD, lower education, lack of social support, and personal history of child abuse have the highest risk for postpartum anxiety. Women with a history of posttraumatic stress disorder (PTSD) may be twice as likely to develop postpartum anxiety as healthy women.9
It has been well-documented that sleep disruption—which is very common in new mothers in the postnatal period—contributes to mood and anxiety disorders.10,11
Clarifying a diagnosis of postpartum anxiety
The Diagnostic and Statistical Manual of Mental Disorders, 5th edition (DSM-5)12 specifies no diagnosis of postpartum anxiety disorder. And no standardized diagnostic criteria exist. It is likely that in some cases, postpartum anxiety represents an exacerbation of underlying GAD, and in other cases it is a situational disorder brought about by specific circumstances of the peripartum period.
The DSM-5 does, however, provide a helpful diagnostic approach. It defines a diagnosis of postpartum depression as being a variant of major depressive disorder (MDD) in which a woman must 1) meet criteria for a major depressive episode; and 2) occur during pregnancy or within 4 weeks of delivery. In practice, many clinicians extend the second requirement to include the first year postpartum.13 There is a “with anxious distress” specifier for major depression in the DSM-5, but the 2 disorders are otherwise unlinked.
Continue to: To apply the...
To apply the DSM-5 principles for postpartum depression to postpartum anxiety, a patient would need to 1) meet the diagnostic criteria for an anxiety disorder that 2) have their onset within a specified perinatal period. Variant presentations of anxiety in the postpartum period might include panic disorder and phobias, which could also interfere with a woman’s ability to care for her child.
The DSM-5 offers the following criteria for GAD12:
- excessive worry about a variety of topics
- worry that is experienced as hard to control
- worry associated with at least 3 physical or cognitive symptoms: edginess/restlessness, tiring easily, impaired concentration, irritability
- anxiety, worry, or associated symptoms that make it hard to carry out day-to-day activities and responsibilities
- symptoms that are unrelated to any other medical conditions and cannot be explained by the effect of substances including a prescription medication, alcohol, or recreational drugs
- symptoms that are not better explained by a different mental disorder.
Debilitating effects of postpartum anxiety
Many women experience some level of anxiety during pregnancy and early postpartum—anxiety that may range from normal and adaptive to debilitating.14 While the challenges of caring for a newborn are likely to bring some level of anxiety, these symptoms should be transient and not interfere with a woman’s capacity to care for her infant, herself, or her family.
Postpartum anxiety has been associated with a prior fear of giving birth, fear of death (of both mother and baby), lack of control, lack of self-confidence, and lack of confidence in the medical system.9 The experience of such ongoing disturbing thoughts or feelings of worry and tension that affect a woman’s ability to manage from day to day should indicate an illness state that deserves medical attention.
Mothers with postpartum anxiety disorders report significantly less bonding with their infants than do mothers without anxiety.15 A recent narrative review describes numerous studies that illustrate the negative effects of postpartum anxiety on bonding, breastfeeding, infant temperament, early childhood development, and conduct disorders.16 Anxious women may be less likely to initiate breastfeeding, have more challenges with breastfeeding, and even have a different milk composition.17 Women with prenatal anxiety are also more likely to stop breastfeeding prematurely.18 Children of anxious mothers may be more likely to have a difficult temperament and to display more distress.19 There are small studies demonstrating deficits in early infant development and increases in conduct disorder in the male offspring of anxious women.20
Continue to: SCREENING FOR POSTPARTUM ANXIETY
SCREENING FOR POSTPARTUM ANXIETY
Screening for perinatal depression has become standard of care, and the Edinburgh Postnatal Depression Scale (EPDS) is a widely used instrument.1 The EPDS, a 10-question self-report scale, was created and validated to screen for perinatal depression, with a cutoff of > 10/30 usually considered a positive result.
Researchers have investigated the utility of the EPDS as a screening tool for perinatal anxiety as well.21-23 These studies show some promise, but there are questions as to whether a total score or a subscale score of the EPDS is most accurate in detecting anxiety. Women with perinatal anxiety may score low on the total EPDS, yet score higher on 3 anxiety-specific questions (TABLE 123). For this reason, several studies propose an EPDS anxiety subscore or subscale (referred to as EPDS-3A).

Of note, there are some women who will score high on the subscale who do not ultimately meet the criteria for an anxiety disorder diagnosis. Clinicians should not over-interpret these scores and should always use sound clinical judgment when making a diagnosis.
Research has also focused on using the GAD 7-item (GAD-7) scale (TABLE 224),25 and on the
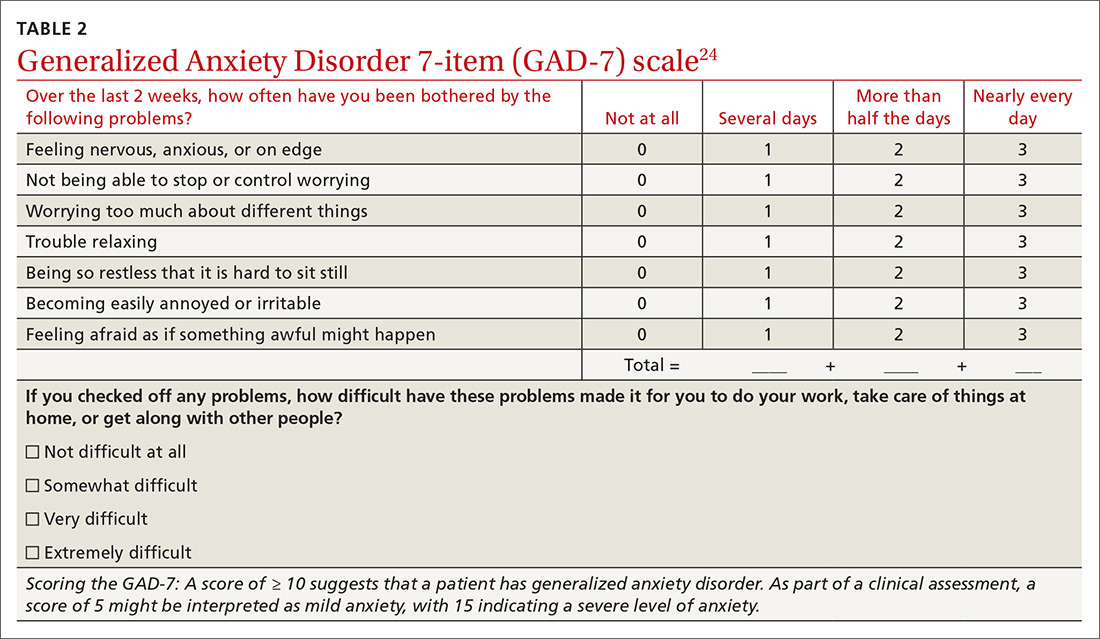
Family physicians may consider using the EPDS subscale if they are already using the EPDS, or adding the GAD-7 as a separate screening instrument during a postpartum visit. To date there is no one standard recommendation or screening tool.
Continue to: NONPHARMACOLOGIC TREATMENT
NONPHARMACOLOGIC TREATMENT
As one would with any patient who has situational anxiety, help new mothers find ways to increase their coping skills, reduce stress, and mobilize social supports and family resources. Given the association between sleep disruption and perinatal anxiety, counsel new mothers, especially those at high risk for postpartum anxiety, to prioritize sleep during this vulnerable time. To that end, consider recommending that they ask partners, family members, or friends to help them take care of the infant at night (or during the day). Such nonmedical interventions may be sufficient for women with mild anxiety.
Very few studies have addressed nonpharmacologic management of postpartum anxiety, but cognitive behavioral therapy (CBT) has been shown to help in managing and treating anxiety disorders outside of pregnancy.28 A few small studies indicate promise for CBT and for mindfulness-based interventions (MBIs) during pregnancy.29
A 2016 systematic review of pharmacologic and nonpharmacologic treatment of anxiety in the perinatal period found support for the use of CBT for panic disorder and specific phobias both in pregnancy and postpartum.30 A very small study found that teaching mothers to massage their preterm infants decreased maternal anxiety.31
If the patient is amenable, it is reasonable to start with behavioral interventions like CBT or MBI before pharmacologic treatment—particularly when physicians have mental health professionals embedded in their primary care team.
PHARMACOLOGIC TREATMENT
Selective serotonin reuptake inhibitors (SSRIs) and serotonin norepinephrine reuptake inhibitors (SNRIs) are considered first-line treatment for moderate to severe anxiety disorders in the perinatal and postnatal period.
Continue to: SSRIs in pregnancy
SSRIs in pregnancy. Lacking support of randomized controlled trials, most recommendations regarding SSRIs in pregnancy come from expert consensus or cohort and case control studies. Studies have raised concerns for an increased rate of congenital heart defects among fetuses exposed to paroxetine32 and primary pulmonary hypertension with all SSRIs.33 But the absolute risks are quite small. There have also been concerns regarding low birth weight and preterm birth, but it is possible that these outcomes result from the depression itself rather than the medication.34
Unfortunately, there are very few studies evaluating the efficacy of SSRIs in treating postpartum depression35 and even fewer that specifically evaluate their effect on perinatal anxiety. Many experts believe that not treating anxiety/depression is actually more harmful than the fetal effects of SSRIs, and that SSRIs are largely safe in both pregnancy and while breastfeeding, with benefits outweighing the risks.
SSRIs while breastfeeding. SSRIs have been found to be present in varying levels in breastmilk but may or may not be present in the serum of nursing infants.36 A 2008 guideline from the American College of Obstetricians and Gynecologists lists paroxetine, sertraline, and fluvoxamine as slightly safer than fluoxetine, escitalopram, and citalopram.37 A 2015 systematic review similarly concluded that sertraline and paroxetine have the most safety data on lactation.38 Lowest effective dose is always recommended to minimize exposure.
Benzodiazepines. As in the general population, benzodiazepines should be reserved for short-term use in acute anxiety and panic because they are associated with such adverse effects as worsening of depression/anxiety and risk of dependence and overdose. Longer-acting benzodiazepines (eg, clonazepam) are generally not recommended in lactation because of reported effects on infants, including sedation. Shorter-acting benzodiazepines (eg, lorazepam) are considered safer in lactation.39
THE CASE
Julia saw her family physician 4 more times, was evaluated by an ear-nose-and-throat specialist for her throat complaints, saw a therapist for CBT and a psychiatrist for medication, had 3 more ED visits, and lost 23 pounds before she finally agreed to start an SSRI for postpartum anxiety. She screened high on the EPDS-3A (9/9) despite scoring low on the full EPDS for perinatal depression (total, 9/30).
Continue to: Because of her swallowing impediments...
Because of her swallowing impediments and because she was breastfeeding, sertraline solution was started at very small doses. It was titrated weekly to obtain therapeutic levels. By 4 weeks, her weight stabilized. By 8 weeks, she started gaining weight and sleeping better. She saw the therapist regularly to continue CBT techniques. Over the next several months she started eating a normal diet. She is currently maintained on her SSRI, is still breastfeeding, and has achieved insight into her perinatal anxiety disorder.
CORRESPONDENCE
Veronica Jordan, MD, 3569 Round Barn Cir #200, Santa Rosa, CA 95403; [email protected].
1. O’Connor E, Rossom RC, Henninger M, et al. Primary care screening for and treatment of depression in pregnant and postpartum women: evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2016;315:388-406.
2. Kessler RC, Berglund P, Demler O, et al. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:593-602.
3. Giardinelli L, Innocenti A, Benni L, et al. Depression and anxiety in perinatal period: prevalence and risk factors in an Italian sample. Arch Womens Ment Health. 2012;15:21-30.
4. Rallis S, Skouteris H, McCabe M, et al. A prospective examination of depression, anxiety and stress throughout pregnancy. Women Birth. 2014;27:e36-e42.
5. Ko JY, Rockhill KM, Tong VT, et al. Trends in postpartum depressive symptoms — 27 States, 2004, 2008, and 2012. MMWR Morb Mortal Wkly Rep. 2017;66:153-158.
6. Fairbrother N, Janssen P, Antony MM, et al. Perinatal anxiety disorder prevalence and incidence. J Affect Disord. 2016;200:148-155.
7. Phillips J, Sharpe L, Matthey S, et al. Maternally focused worry. Arch Womens Ment Health. 2009;12:409-418.
8. Buist A, Gotman N, Yonkers KA. Generalized anxiety disorder: course and risk factors in pregnancy. J Affect Disord. 2011;131:277-283.
9. Schlomi Polachek I, Huller Harari L, Baum M, et al. Postpartum anxiety in a cohort of women from the general population: risk factors and association with depression during last week of pregnancy, postpartum depression and postpartum PTSD. Isr J Psychiatry Relat Sci. 2014;51:128-134.
10. Bei B, Coo S, Trinder J. Sleep and mood during pregnancy and the postpartum period. Sleep Med Clin. 2015;10:25-33.
11. Lawson A, Murphy KE, Sloan E, et al. The relationship between sleep and postpartum mental disorders: a systematic review. J Affect Disord. 2015;176:65-77.
12. APA. Diagnostic and Statistical Manual of Mental Disorders, 5th ed. Washington, DC: American Psychiatric Association Publishing; 2013.
13. Langan R, Goodbred AJ. Identification and management of peripartum depression. Am Fam Physician. 2016;93:852-858.
14. Ali E. Women’s experiences with postpartum anxiety disorders: a narrative literature review. Int J Womens Health. 2018;10:237-249.
15. Tietz A, Zietlow AL, Reck C. Maternal bonding in mothers with postpartum anxiety disorder: the crucial role of subclinical depressive symptoms and maternal avoidance behaviour. Arch Womens Ment Health. 2014;17:433-442.
16. Field T. Postnatal anxiety prevalence, predictors and effects on development: a narrative review. Infant Behav Dev. 2018;51:24-32.
17. Serim Demirgoren B, Ozbek A, Ormen M, et al. Do mothers with high sodium levels in their breast milk have high depression and anxiety scores? J Int Med Res. 2017;45:843-848.
18. Ystrom E. Breastfeeding cessation and symptoms of anxiety and depression: a longitudinal cohort study. BMC Pregnancy Childbirth. 2012;12:36.
19. Britton JR. Infant temperament and maternal anxiety and depressed mood in the early postpartum period. Women Health. 2011;51:55-71.
20. Glasheen C, Richardson GA, Kim KH, et al. Exposure to maternal pre- and postnatal depression and anxiety symptoms: risk for major depression, anxiety disorders, and conduct disorder in adolescent offspring. Dev Psychopathol. 2013;26:1045-1063.
21. Petrozzi A, Gagliardi L. Anxious and depressive components of Edinburgh Postnatal Depression Scale in maternal postpartum psychological problems. J Perinat Med. 2013;41:343-348.
22. Bina R, Harrington D. The Edinburgh Postnatal Depression Scale: screening tool for postpartum anxiety as well? Findings from a confirmatory factor analysis of the Hebrew version. Matern Child Health J. 2016;20:904-914.
23. Matthey S, Fisher J, Rowe H. Using the Edinburgh postnatal depression scale to screen for anxiety disorders: conceptual and methodological considerations J Affect Disord. 2013;146:224-230.
24. Spitzer RL, Kroenke K, Williams JB, et al. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166:1092-1097.
25. Simpson W, Glazer M, Michalski N, et al. Comparative efficacy of the Generalized Anxiety Disorder 7-Item Scale and the Edinburgh Postnatal Depression Scale as screening tools for generalized anxiety disorder in pregnancy and the postpartum period. Can J Psychiatry. 2014;59:434-440.
26. Moran TE, Polanin JR, Wenzel A. The Postpartum Worry Scale-Revised: an initial validation of a measure of postpartum worry. Arch Womens Ment Health. 2014;17:41-48.
27. Fallon V, Halford JCG, Bennett KM, et al. The Postpartum Specific Anxiety Scale: development and preliminary validation. Arch Womens Ment Health. 2016;19:1079-1090.
28. Hofmann SG, Smits JA. Cognitive-behavioral therapy for adult anxiety disorders: a meta-analysis of randomized placebo-controlled trials. J Clin Psychiatry. 2008;69:621-632.
29. Goodman JH, Guarino A, Chenausky K, et al. CALM Pregnancy: results of a pilot study of mindfulness-based cognitive therapy for perinatal anxiety. Arch Womens Ment Health. 2014;17:373-387.
30. Marchesi C, Ossola P, Amerio A, et al. Clinical management of perinatal anxiety disorders: a systematic review. J Affect Disord. 2016;190:543-550.
31. Feijó L, Hernandez-Reif M, Field T, et al. Mothers’ depressed mood and anxiety levels are reduced after massaging their preterm infants. Infant Behav Devel. 2006;29:476-480.
32. Bérard A, Iessa N, Chaabane S, et al. The risk of major cardiac malformations associated with paroxetine use during the first trimester of pregnancy: a systematic review and meta-analysis. Br J Clin Pharmacol. 2016;81:589-604.
33. Huybrechts KF, Bateman BT, Palmsten K, et al. Antidepressant use late in pregnancy and risk of persistent pulmonary hypertension of the newborn. JAMA. 2015;313:2142-2151.
34. Cantarutti A, Merlino L, Monzani E, et al. Is the risk of preterm birth and low birth weight affected by the use of antidepressant agents during pregnancy? A population-based investigation. PLoS One. 2016;11:e0168115.
35. Molyneaux E, Howard LM, McGeown HR, et al. Antidepressant treatment for postnatal depression. Cochrane Database Syst Rev. 2014;11:CD002018.
36. Freeman MP. Postpartum depression treatment and breastfeeding. J Clin Psychiatry. 2009;70:e35.
37. ACOG Committee on Practice Bulletins—number 92. Use of psychiatric medications during pregnancy and lactation. Obstet Gynecol. 2008;111:1001-1020.
38. Orsolini L, Bellantuono C. Serotonin reuptake inhibitors and breastfeeding: a systematic review. Hum Psychopharmacol. 2015;30:4-20.
39. NIH. Drugs and Lactation Database. https://toxnet.nlm.nih.gov/newtoxnet/lactmed.htm. Accessed February 26, 2019.
THE CASE
Julia* is a 31-year-old woman, gravida 3 para 3, who presents to your office for evaluation after a recent emergency department (ED) visit. Her husband and children are with her. She is 4 months postpartum after an uncomplicated normal spontaneous vaginal delivery. She is breastfeeding her healthy baby boy and is using an intrauterine device for birth control. She went to the ED last week after “choking on a chip” while having lunch with her children. It felt like she “couldn’t breathe.” She called 911 herself. The ED evaluation was unremarkable. Her discharge diagnosis was “panic attack,” and she was sent home with a prescription for lorazepam.
Since the incident, she has been unable to eat any solid foods and has lost 7 pounds. She also reports a globus sensation, extreme fear of swallowing, insomnia, and pervasive thoughts that she could die at any moment and leave her children motherless. She has not taken the lorazepam.
She has a history of self-reported anxiety dating back to high school but no history of panic attacks. She has never been diagnosed with an anxiety disorder and has never before been prescribed anti-anxiety medication. She doesn’t have a history of postpartum depression in prior pregnancies, and a depression screening at her postpartum visit 2 months ago was negative.
●
*The patient’s name has been changed to protect her identity.
During the perinatal period, women are particularly vulnerable to affective disorders, and primary care physicians are encouraged to routinely screen for and treat depression in pregnant and postpartum women.1 However, anxiety disorders have a higher incidence than mood disorders in the general population,2 and perinatal anxiety may be more widely underrecognized and undertreated than depression.3 In addition, higher depression scores early in pregnancy have been shown to predict higher anxiety later in pregnancy.4
As family physicians, we are well-trained to recognize and treat anxiety disorders in the general patient population; however, we may lack the awareness and tools to identify these conditions in the perinatal period. Given our frequent encounters with both mom and baby in a child’s first year of life, we are uniquely positioned to promptly recognize, diagnose, and treat postpartum anxiety and thereby improve health outcomes for families.
DEFINING PERINATAL ANXIETY
Anxiety disorders (including generalized anxiety disorder, panic, phobia, and social anxiety) are the most common mental health disorders evaluated and treated in the primary care setting, with a lifetime prevalence of close to 30%.2
Continue to: A recent report from...
A recent report from the Centers for Disease Control and Prevention (CDC) estimates that 1 in 9 women experience symptoms of postpartum depression.5 The prevalence of anxiety disorders during pregnancy and the early postpartum period is not as well-known, but studies suggest that perinatal anxiety is much more prevalent than depression. In one study, generalized anxiety disorder (GAD) in the pre- and postnatal periods was 15.8% and 17.1%, respectively; an incidence far exceeding that of perinatal depression (3.9% and 4.8%, for the same periods).6 Additional evidence suggests that even more women in the postnatal period experience clinically significant levels of anxiety but do not meet full diagnostic criteria for an anxiety disorder.7
In another study, 9.5% of women met criteria for GAD at some point during pregnancy, with highest anxiety levels in the first trimester.8 Women with a history of GAD, lower education, lack of social support, and personal history of child abuse have the highest risk for postpartum anxiety. Women with a history of posttraumatic stress disorder (PTSD) may be twice as likely to develop postpartum anxiety as healthy women.9
It has been well-documented that sleep disruption—which is very common in new mothers in the postnatal period—contributes to mood and anxiety disorders.10,11
Clarifying a diagnosis of postpartum anxiety
The Diagnostic and Statistical Manual of Mental Disorders, 5th edition (DSM-5)12 specifies no diagnosis of postpartum anxiety disorder. And no standardized diagnostic criteria exist. It is likely that in some cases, postpartum anxiety represents an exacerbation of underlying GAD, and in other cases it is a situational disorder brought about by specific circumstances of the peripartum period.
The DSM-5 does, however, provide a helpful diagnostic approach. It defines a diagnosis of postpartum depression as being a variant of major depressive disorder (MDD) in which a woman must 1) meet criteria for a major depressive episode; and 2) occur during pregnancy or within 4 weeks of delivery. In practice, many clinicians extend the second requirement to include the first year postpartum.13 There is a “with anxious distress” specifier for major depression in the DSM-5, but the 2 disorders are otherwise unlinked.
Continue to: To apply the...
To apply the DSM-5 principles for postpartum depression to postpartum anxiety, a patient would need to 1) meet the diagnostic criteria for an anxiety disorder that 2) have their onset within a specified perinatal period. Variant presentations of anxiety in the postpartum period might include panic disorder and phobias, which could also interfere with a woman’s ability to care for her child.
The DSM-5 offers the following criteria for GAD12:
- excessive worry about a variety of topics
- worry that is experienced as hard to control
- worry associated with at least 3 physical or cognitive symptoms: edginess/restlessness, tiring easily, impaired concentration, irritability
- anxiety, worry, or associated symptoms that make it hard to carry out day-to-day activities and responsibilities
- symptoms that are unrelated to any other medical conditions and cannot be explained by the effect of substances including a prescription medication, alcohol, or recreational drugs
- symptoms that are not better explained by a different mental disorder.
Debilitating effects of postpartum anxiety
Many women experience some level of anxiety during pregnancy and early postpartum—anxiety that may range from normal and adaptive to debilitating.14 While the challenges of caring for a newborn are likely to bring some level of anxiety, these symptoms should be transient and not interfere with a woman’s capacity to care for her infant, herself, or her family.
Postpartum anxiety has been associated with a prior fear of giving birth, fear of death (of both mother and baby), lack of control, lack of self-confidence, and lack of confidence in the medical system.9 The experience of such ongoing disturbing thoughts or feelings of worry and tension that affect a woman’s ability to manage from day to day should indicate an illness state that deserves medical attention.
Mothers with postpartum anxiety disorders report significantly less bonding with their infants than do mothers without anxiety.15 A recent narrative review describes numerous studies that illustrate the negative effects of postpartum anxiety on bonding, breastfeeding, infant temperament, early childhood development, and conduct disorders.16 Anxious women may be less likely to initiate breastfeeding, have more challenges with breastfeeding, and even have a different milk composition.17 Women with prenatal anxiety are also more likely to stop breastfeeding prematurely.18 Children of anxious mothers may be more likely to have a difficult temperament and to display more distress.19 There are small studies demonstrating deficits in early infant development and increases in conduct disorder in the male offspring of anxious women.20
Continue to: SCREENING FOR POSTPARTUM ANXIETY
SCREENING FOR POSTPARTUM ANXIETY
Screening for perinatal depression has become standard of care, and the Edinburgh Postnatal Depression Scale (EPDS) is a widely used instrument.1 The EPDS, a 10-question self-report scale, was created and validated to screen for perinatal depression, with a cutoff of > 10/30 usually considered a positive result.
Researchers have investigated the utility of the EPDS as a screening tool for perinatal anxiety as well.21-23 These studies show some promise, but there are questions as to whether a total score or a subscale score of the EPDS is most accurate in detecting anxiety. Women with perinatal anxiety may score low on the total EPDS, yet score higher on 3 anxiety-specific questions (TABLE 123). For this reason, several studies propose an EPDS anxiety subscore or subscale (referred to as EPDS-3A).

Of note, there are some women who will score high on the subscale who do not ultimately meet the criteria for an anxiety disorder diagnosis. Clinicians should not over-interpret these scores and should always use sound clinical judgment when making a diagnosis.
Research has also focused on using the GAD 7-item (GAD-7) scale (TABLE 224),25 and on the

Family physicians may consider using the EPDS subscale if they are already using the EPDS, or adding the GAD-7 as a separate screening instrument during a postpartum visit. To date there is no one standard recommendation or screening tool.
Continue to: NONPHARMACOLOGIC TREATMENT
NONPHARMACOLOGIC TREATMENT
As one would with any patient who has situational anxiety, help new mothers find ways to increase their coping skills, reduce stress, and mobilize social supports and family resources. Given the association between sleep disruption and perinatal anxiety, counsel new mothers, especially those at high risk for postpartum anxiety, to prioritize sleep during this vulnerable time. To that end, consider recommending that they ask partners, family members, or friends to help them take care of the infant at night (or during the day). Such nonmedical interventions may be sufficient for women with mild anxiety.
Very few studies have addressed nonpharmacologic management of postpartum anxiety, but cognitive behavioral therapy (CBT) has been shown to help in managing and treating anxiety disorders outside of pregnancy.28 A few small studies indicate promise for CBT and for mindfulness-based interventions (MBIs) during pregnancy.29
A 2016 systematic review of pharmacologic and nonpharmacologic treatment of anxiety in the perinatal period found support for the use of CBT for panic disorder and specific phobias both in pregnancy and postpartum.30 A very small study found that teaching mothers to massage their preterm infants decreased maternal anxiety.31
If the patient is amenable, it is reasonable to start with behavioral interventions like CBT or MBI before pharmacologic treatment—particularly when physicians have mental health professionals embedded in their primary care team.
PHARMACOLOGIC TREATMENT
Selective serotonin reuptake inhibitors (SSRIs) and serotonin norepinephrine reuptake inhibitors (SNRIs) are considered first-line treatment for moderate to severe anxiety disorders in the perinatal and postnatal period.
Continue to: SSRIs in pregnancy
SSRIs in pregnancy. Lacking support of randomized controlled trials, most recommendations regarding SSRIs in pregnancy come from expert consensus or cohort and case control studies. Studies have raised concerns for an increased rate of congenital heart defects among fetuses exposed to paroxetine32 and primary pulmonary hypertension with all SSRIs.33 But the absolute risks are quite small. There have also been concerns regarding low birth weight and preterm birth, but it is possible that these outcomes result from the depression itself rather than the medication.34
Unfortunately, there are very few studies evaluating the efficacy of SSRIs in treating postpartum depression35 and even fewer that specifically evaluate their effect on perinatal anxiety. Many experts believe that not treating anxiety/depression is actually more harmful than the fetal effects of SSRIs, and that SSRIs are largely safe in both pregnancy and while breastfeeding, with benefits outweighing the risks.
SSRIs while breastfeeding. SSRIs have been found to be present in varying levels in breastmilk but may or may not be present in the serum of nursing infants.36 A 2008 guideline from the American College of Obstetricians and Gynecologists lists paroxetine, sertraline, and fluvoxamine as slightly safer than fluoxetine, escitalopram, and citalopram.37 A 2015 systematic review similarly concluded that sertraline and paroxetine have the most safety data on lactation.38 Lowest effective dose is always recommended to minimize exposure.
Benzodiazepines. As in the general population, benzodiazepines should be reserved for short-term use in acute anxiety and panic because they are associated with such adverse effects as worsening of depression/anxiety and risk of dependence and overdose. Longer-acting benzodiazepines (eg, clonazepam) are generally not recommended in lactation because of reported effects on infants, including sedation. Shorter-acting benzodiazepines (eg, lorazepam) are considered safer in lactation.39
THE CASE
Julia saw her family physician 4 more times, was evaluated by an ear-nose-and-throat specialist for her throat complaints, saw a therapist for CBT and a psychiatrist for medication, had 3 more ED visits, and lost 23 pounds before she finally agreed to start an SSRI for postpartum anxiety. She screened high on the EPDS-3A (9/9) despite scoring low on the full EPDS for perinatal depression (total, 9/30).
Continue to: Because of her swallowing impediments...
Because of her swallowing impediments and because she was breastfeeding, sertraline solution was started at very small doses. It was titrated weekly to obtain therapeutic levels. By 4 weeks, her weight stabilized. By 8 weeks, she started gaining weight and sleeping better. She saw the therapist regularly to continue CBT techniques. Over the next several months she started eating a normal diet. She is currently maintained on her SSRI, is still breastfeeding, and has achieved insight into her perinatal anxiety disorder.
CORRESPONDENCE
Veronica Jordan, MD, 3569 Round Barn Cir #200, Santa Rosa, CA 95403; [email protected].
THE CASE
Julia* is a 31-year-old woman, gravida 3 para 3, who presents to your office for evaluation after a recent emergency department (ED) visit. Her husband and children are with her. She is 4 months postpartum after an uncomplicated normal spontaneous vaginal delivery. She is breastfeeding her healthy baby boy and is using an intrauterine device for birth control. She went to the ED last week after “choking on a chip” while having lunch with her children. It felt like she “couldn’t breathe.” She called 911 herself. The ED evaluation was unremarkable. Her discharge diagnosis was “panic attack,” and she was sent home with a prescription for lorazepam.
Since the incident, she has been unable to eat any solid foods and has lost 7 pounds. She also reports a globus sensation, extreme fear of swallowing, insomnia, and pervasive thoughts that she could die at any moment and leave her children motherless. She has not taken the lorazepam.
She has a history of self-reported anxiety dating back to high school but no history of panic attacks. She has never been diagnosed with an anxiety disorder and has never before been prescribed anti-anxiety medication. She doesn’t have a history of postpartum depression in prior pregnancies, and a depression screening at her postpartum visit 2 months ago was negative.
●
*The patient’s name has been changed to protect her identity.
During the perinatal period, women are particularly vulnerable to affective disorders, and primary care physicians are encouraged to routinely screen for and treat depression in pregnant and postpartum women.1 However, anxiety disorders have a higher incidence than mood disorders in the general population,2 and perinatal anxiety may be more widely underrecognized and undertreated than depression.3 In addition, higher depression scores early in pregnancy have been shown to predict higher anxiety later in pregnancy.4
As family physicians, we are well-trained to recognize and treat anxiety disorders in the general patient population; however, we may lack the awareness and tools to identify these conditions in the perinatal period. Given our frequent encounters with both mom and baby in a child’s first year of life, we are uniquely positioned to promptly recognize, diagnose, and treat postpartum anxiety and thereby improve health outcomes for families.
DEFINING PERINATAL ANXIETY
Anxiety disorders (including generalized anxiety disorder, panic, phobia, and social anxiety) are the most common mental health disorders evaluated and treated in the primary care setting, with a lifetime prevalence of close to 30%.2
Continue to: A recent report from...
A recent report from the Centers for Disease Control and Prevention (CDC) estimates that 1 in 9 women experience symptoms of postpartum depression.5 The prevalence of anxiety disorders during pregnancy and the early postpartum period is not as well-known, but studies suggest that perinatal anxiety is much more prevalent than depression. In one study, generalized anxiety disorder (GAD) in the pre- and postnatal periods was 15.8% and 17.1%, respectively; an incidence far exceeding that of perinatal depression (3.9% and 4.8%, for the same periods).6 Additional evidence suggests that even more women in the postnatal period experience clinically significant levels of anxiety but do not meet full diagnostic criteria for an anxiety disorder.7
In another study, 9.5% of women met criteria for GAD at some point during pregnancy, with highest anxiety levels in the first trimester.8 Women with a history of GAD, lower education, lack of social support, and personal history of child abuse have the highest risk for postpartum anxiety. Women with a history of posttraumatic stress disorder (PTSD) may be twice as likely to develop postpartum anxiety as healthy women.9
It has been well-documented that sleep disruption—which is very common in new mothers in the postnatal period—contributes to mood and anxiety disorders.10,11
Clarifying a diagnosis of postpartum anxiety
The Diagnostic and Statistical Manual of Mental Disorders, 5th edition (DSM-5)12 specifies no diagnosis of postpartum anxiety disorder. And no standardized diagnostic criteria exist. It is likely that in some cases, postpartum anxiety represents an exacerbation of underlying GAD, and in other cases it is a situational disorder brought about by specific circumstances of the peripartum period.
The DSM-5 does, however, provide a helpful diagnostic approach. It defines a diagnosis of postpartum depression as being a variant of major depressive disorder (MDD) in which a woman must 1) meet criteria for a major depressive episode; and 2) occur during pregnancy or within 4 weeks of delivery. In practice, many clinicians extend the second requirement to include the first year postpartum.13 There is a “with anxious distress” specifier for major depression in the DSM-5, but the 2 disorders are otherwise unlinked.
Continue to: To apply the...
To apply the DSM-5 principles for postpartum depression to postpartum anxiety, a patient would need to 1) meet the diagnostic criteria for an anxiety disorder that 2) have their onset within a specified perinatal period. Variant presentations of anxiety in the postpartum period might include panic disorder and phobias, which could also interfere with a woman’s ability to care for her child.
The DSM-5 offers the following criteria for GAD12:
- excessive worry about a variety of topics
- worry that is experienced as hard to control
- worry associated with at least 3 physical or cognitive symptoms: edginess/restlessness, tiring easily, impaired concentration, irritability
- anxiety, worry, or associated symptoms that make it hard to carry out day-to-day activities and responsibilities
- symptoms that are unrelated to any other medical conditions and cannot be explained by the effect of substances including a prescription medication, alcohol, or recreational drugs
- symptoms that are not better explained by a different mental disorder.
Debilitating effects of postpartum anxiety
Many women experience some level of anxiety during pregnancy and early postpartum—anxiety that may range from normal and adaptive to debilitating.14 While the challenges of caring for a newborn are likely to bring some level of anxiety, these symptoms should be transient and not interfere with a woman’s capacity to care for her infant, herself, or her family.
Postpartum anxiety has been associated with a prior fear of giving birth, fear of death (of both mother and baby), lack of control, lack of self-confidence, and lack of confidence in the medical system.9 The experience of such ongoing disturbing thoughts or feelings of worry and tension that affect a woman’s ability to manage from day to day should indicate an illness state that deserves medical attention.
Mothers with postpartum anxiety disorders report significantly less bonding with their infants than do mothers without anxiety.15 A recent narrative review describes numerous studies that illustrate the negative effects of postpartum anxiety on bonding, breastfeeding, infant temperament, early childhood development, and conduct disorders.16 Anxious women may be less likely to initiate breastfeeding, have more challenges with breastfeeding, and even have a different milk composition.17 Women with prenatal anxiety are also more likely to stop breastfeeding prematurely.18 Children of anxious mothers may be more likely to have a difficult temperament and to display more distress.19 There are small studies demonstrating deficits in early infant development and increases in conduct disorder in the male offspring of anxious women.20
Continue to: SCREENING FOR POSTPARTUM ANXIETY
SCREENING FOR POSTPARTUM ANXIETY
Screening for perinatal depression has become standard of care, and the Edinburgh Postnatal Depression Scale (EPDS) is a widely used instrument.1 The EPDS, a 10-question self-report scale, was created and validated to screen for perinatal depression, with a cutoff of > 10/30 usually considered a positive result.
Researchers have investigated the utility of the EPDS as a screening tool for perinatal anxiety as well.21-23 These studies show some promise, but there are questions as to whether a total score or a subscale score of the EPDS is most accurate in detecting anxiety. Women with perinatal anxiety may score low on the total EPDS, yet score higher on 3 anxiety-specific questions (TABLE 123). For this reason, several studies propose an EPDS anxiety subscore or subscale (referred to as EPDS-3A).

Of note, there are some women who will score high on the subscale who do not ultimately meet the criteria for an anxiety disorder diagnosis. Clinicians should not over-interpret these scores and should always use sound clinical judgment when making a diagnosis.
Research has also focused on using the GAD 7-item (GAD-7) scale (TABLE 224),25 and on the

Family physicians may consider using the EPDS subscale if they are already using the EPDS, or adding the GAD-7 as a separate screening instrument during a postpartum visit. To date there is no one standard recommendation or screening tool.
Continue to: NONPHARMACOLOGIC TREATMENT
NONPHARMACOLOGIC TREATMENT
As one would with any patient who has situational anxiety, help new mothers find ways to increase their coping skills, reduce stress, and mobilize social supports and family resources. Given the association between sleep disruption and perinatal anxiety, counsel new mothers, especially those at high risk for postpartum anxiety, to prioritize sleep during this vulnerable time. To that end, consider recommending that they ask partners, family members, or friends to help them take care of the infant at night (or during the day). Such nonmedical interventions may be sufficient for women with mild anxiety.
Very few studies have addressed nonpharmacologic management of postpartum anxiety, but cognitive behavioral therapy (CBT) has been shown to help in managing and treating anxiety disorders outside of pregnancy.28 A few small studies indicate promise for CBT and for mindfulness-based interventions (MBIs) during pregnancy.29
A 2016 systematic review of pharmacologic and nonpharmacologic treatment of anxiety in the perinatal period found support for the use of CBT for panic disorder and specific phobias both in pregnancy and postpartum.30 A very small study found that teaching mothers to massage their preterm infants decreased maternal anxiety.31
If the patient is amenable, it is reasonable to start with behavioral interventions like CBT or MBI before pharmacologic treatment—particularly when physicians have mental health professionals embedded in their primary care team.
PHARMACOLOGIC TREATMENT
Selective serotonin reuptake inhibitors (SSRIs) and serotonin norepinephrine reuptake inhibitors (SNRIs) are considered first-line treatment for moderate to severe anxiety disorders in the perinatal and postnatal period.
Continue to: SSRIs in pregnancy
SSRIs in pregnancy. Lacking support of randomized controlled trials, most recommendations regarding SSRIs in pregnancy come from expert consensus or cohort and case control studies. Studies have raised concerns for an increased rate of congenital heart defects among fetuses exposed to paroxetine32 and primary pulmonary hypertension with all SSRIs.33 But the absolute risks are quite small. There have also been concerns regarding low birth weight and preterm birth, but it is possible that these outcomes result from the depression itself rather than the medication.34
Unfortunately, there are very few studies evaluating the efficacy of SSRIs in treating postpartum depression35 and even fewer that specifically evaluate their effect on perinatal anxiety. Many experts believe that not treating anxiety/depression is actually more harmful than the fetal effects of SSRIs, and that SSRIs are largely safe in both pregnancy and while breastfeeding, with benefits outweighing the risks.
SSRIs while breastfeeding. SSRIs have been found to be present in varying levels in breastmilk but may or may not be present in the serum of nursing infants.36 A 2008 guideline from the American College of Obstetricians and Gynecologists lists paroxetine, sertraline, and fluvoxamine as slightly safer than fluoxetine, escitalopram, and citalopram.37 A 2015 systematic review similarly concluded that sertraline and paroxetine have the most safety data on lactation.38 Lowest effective dose is always recommended to minimize exposure.
Benzodiazepines. As in the general population, benzodiazepines should be reserved for short-term use in acute anxiety and panic because they are associated with such adverse effects as worsening of depression/anxiety and risk of dependence and overdose. Longer-acting benzodiazepines (eg, clonazepam) are generally not recommended in lactation because of reported effects on infants, including sedation. Shorter-acting benzodiazepines (eg, lorazepam) are considered safer in lactation.39
THE CASE
Julia saw her family physician 4 more times, was evaluated by an ear-nose-and-throat specialist for her throat complaints, saw a therapist for CBT and a psychiatrist for medication, had 3 more ED visits, and lost 23 pounds before she finally agreed to start an SSRI for postpartum anxiety. She screened high on the EPDS-3A (9/9) despite scoring low on the full EPDS for perinatal depression (total, 9/30).
Continue to: Because of her swallowing impediments...
Because of her swallowing impediments and because she was breastfeeding, sertraline solution was started at very small doses. It was titrated weekly to obtain therapeutic levels. By 4 weeks, her weight stabilized. By 8 weeks, she started gaining weight and sleeping better. She saw the therapist regularly to continue CBT techniques. Over the next several months she started eating a normal diet. She is currently maintained on her SSRI, is still breastfeeding, and has achieved insight into her perinatal anxiety disorder.
CORRESPONDENCE
Veronica Jordan, MD, 3569 Round Barn Cir #200, Santa Rosa, CA 95403; [email protected].
1. O’Connor E, Rossom RC, Henninger M, et al. Primary care screening for and treatment of depression in pregnant and postpartum women: evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2016;315:388-406.
2. Kessler RC, Berglund P, Demler O, et al. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:593-602.
3. Giardinelli L, Innocenti A, Benni L, et al. Depression and anxiety in perinatal period: prevalence and risk factors in an Italian sample. Arch Womens Ment Health. 2012;15:21-30.
4. Rallis S, Skouteris H, McCabe M, et al. A prospective examination of depression, anxiety and stress throughout pregnancy. Women Birth. 2014;27:e36-e42.
5. Ko JY, Rockhill KM, Tong VT, et al. Trends in postpartum depressive symptoms — 27 States, 2004, 2008, and 2012. MMWR Morb Mortal Wkly Rep. 2017;66:153-158.
6. Fairbrother N, Janssen P, Antony MM, et al. Perinatal anxiety disorder prevalence and incidence. J Affect Disord. 2016;200:148-155.
7. Phillips J, Sharpe L, Matthey S, et al. Maternally focused worry. Arch Womens Ment Health. 2009;12:409-418.
8. Buist A, Gotman N, Yonkers KA. Generalized anxiety disorder: course and risk factors in pregnancy. J Affect Disord. 2011;131:277-283.
9. Schlomi Polachek I, Huller Harari L, Baum M, et al. Postpartum anxiety in a cohort of women from the general population: risk factors and association with depression during last week of pregnancy, postpartum depression and postpartum PTSD. Isr J Psychiatry Relat Sci. 2014;51:128-134.
10. Bei B, Coo S, Trinder J. Sleep and mood during pregnancy and the postpartum period. Sleep Med Clin. 2015;10:25-33.
11. Lawson A, Murphy KE, Sloan E, et al. The relationship between sleep and postpartum mental disorders: a systematic review. J Affect Disord. 2015;176:65-77.
12. APA. Diagnostic and Statistical Manual of Mental Disorders, 5th ed. Washington, DC: American Psychiatric Association Publishing; 2013.
13. Langan R, Goodbred AJ. Identification and management of peripartum depression. Am Fam Physician. 2016;93:852-858.
14. Ali E. Women’s experiences with postpartum anxiety disorders: a narrative literature review. Int J Womens Health. 2018;10:237-249.
15. Tietz A, Zietlow AL, Reck C. Maternal bonding in mothers with postpartum anxiety disorder: the crucial role of subclinical depressive symptoms and maternal avoidance behaviour. Arch Womens Ment Health. 2014;17:433-442.
16. Field T. Postnatal anxiety prevalence, predictors and effects on development: a narrative review. Infant Behav Dev. 2018;51:24-32.
17. Serim Demirgoren B, Ozbek A, Ormen M, et al. Do mothers with high sodium levels in their breast milk have high depression and anxiety scores? J Int Med Res. 2017;45:843-848.
18. Ystrom E. Breastfeeding cessation and symptoms of anxiety and depression: a longitudinal cohort study. BMC Pregnancy Childbirth. 2012;12:36.
19. Britton JR. Infant temperament and maternal anxiety and depressed mood in the early postpartum period. Women Health. 2011;51:55-71.
20. Glasheen C, Richardson GA, Kim KH, et al. Exposure to maternal pre- and postnatal depression and anxiety symptoms: risk for major depression, anxiety disorders, and conduct disorder in adolescent offspring. Dev Psychopathol. 2013;26:1045-1063.
21. Petrozzi A, Gagliardi L. Anxious and depressive components of Edinburgh Postnatal Depression Scale in maternal postpartum psychological problems. J Perinat Med. 2013;41:343-348.
22. Bina R, Harrington D. The Edinburgh Postnatal Depression Scale: screening tool for postpartum anxiety as well? Findings from a confirmatory factor analysis of the Hebrew version. Matern Child Health J. 2016;20:904-914.
23. Matthey S, Fisher J, Rowe H. Using the Edinburgh postnatal depression scale to screen for anxiety disorders: conceptual and methodological considerations J Affect Disord. 2013;146:224-230.
24. Spitzer RL, Kroenke K, Williams JB, et al. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166:1092-1097.
25. Simpson W, Glazer M, Michalski N, et al. Comparative efficacy of the Generalized Anxiety Disorder 7-Item Scale and the Edinburgh Postnatal Depression Scale as screening tools for generalized anxiety disorder in pregnancy and the postpartum period. Can J Psychiatry. 2014;59:434-440.
26. Moran TE, Polanin JR, Wenzel A. The Postpartum Worry Scale-Revised: an initial validation of a measure of postpartum worry. Arch Womens Ment Health. 2014;17:41-48.
27. Fallon V, Halford JCG, Bennett KM, et al. The Postpartum Specific Anxiety Scale: development and preliminary validation. Arch Womens Ment Health. 2016;19:1079-1090.
28. Hofmann SG, Smits JA. Cognitive-behavioral therapy for adult anxiety disorders: a meta-analysis of randomized placebo-controlled trials. J Clin Psychiatry. 2008;69:621-632.
29. Goodman JH, Guarino A, Chenausky K, et al. CALM Pregnancy: results of a pilot study of mindfulness-based cognitive therapy for perinatal anxiety. Arch Womens Ment Health. 2014;17:373-387.
30. Marchesi C, Ossola P, Amerio A, et al. Clinical management of perinatal anxiety disorders: a systematic review. J Affect Disord. 2016;190:543-550.
31. Feijó L, Hernandez-Reif M, Field T, et al. Mothers’ depressed mood and anxiety levels are reduced after massaging their preterm infants. Infant Behav Devel. 2006;29:476-480.
32. Bérard A, Iessa N, Chaabane S, et al. The risk of major cardiac malformations associated with paroxetine use during the first trimester of pregnancy: a systematic review and meta-analysis. Br J Clin Pharmacol. 2016;81:589-604.
33. Huybrechts KF, Bateman BT, Palmsten K, et al. Antidepressant use late in pregnancy and risk of persistent pulmonary hypertension of the newborn. JAMA. 2015;313:2142-2151.
34. Cantarutti A, Merlino L, Monzani E, et al. Is the risk of preterm birth and low birth weight affected by the use of antidepressant agents during pregnancy? A population-based investigation. PLoS One. 2016;11:e0168115.
35. Molyneaux E, Howard LM, McGeown HR, et al. Antidepressant treatment for postnatal depression. Cochrane Database Syst Rev. 2014;11:CD002018.
36. Freeman MP. Postpartum depression treatment and breastfeeding. J Clin Psychiatry. 2009;70:e35.
37. ACOG Committee on Practice Bulletins—number 92. Use of psychiatric medications during pregnancy and lactation. Obstet Gynecol. 2008;111:1001-1020.
38. Orsolini L, Bellantuono C. Serotonin reuptake inhibitors and breastfeeding: a systematic review. Hum Psychopharmacol. 2015;30:4-20.
39. NIH. Drugs and Lactation Database. https://toxnet.nlm.nih.gov/newtoxnet/lactmed.htm. Accessed February 26, 2019.
1. O’Connor E, Rossom RC, Henninger M, et al. Primary care screening for and treatment of depression in pregnant and postpartum women: evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2016;315:388-406.
2. Kessler RC, Berglund P, Demler O, et al. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:593-602.
3. Giardinelli L, Innocenti A, Benni L, et al. Depression and anxiety in perinatal period: prevalence and risk factors in an Italian sample. Arch Womens Ment Health. 2012;15:21-30.
4. Rallis S, Skouteris H, McCabe M, et al. A prospective examination of depression, anxiety and stress throughout pregnancy. Women Birth. 2014;27:e36-e42.
5. Ko JY, Rockhill KM, Tong VT, et al. Trends in postpartum depressive symptoms — 27 States, 2004, 2008, and 2012. MMWR Morb Mortal Wkly Rep. 2017;66:153-158.
6. Fairbrother N, Janssen P, Antony MM, et al. Perinatal anxiety disorder prevalence and incidence. J Affect Disord. 2016;200:148-155.
7. Phillips J, Sharpe L, Matthey S, et al. Maternally focused worry. Arch Womens Ment Health. 2009;12:409-418.
8. Buist A, Gotman N, Yonkers KA. Generalized anxiety disorder: course and risk factors in pregnancy. J Affect Disord. 2011;131:277-283.
9. Schlomi Polachek I, Huller Harari L, Baum M, et al. Postpartum anxiety in a cohort of women from the general population: risk factors and association with depression during last week of pregnancy, postpartum depression and postpartum PTSD. Isr J Psychiatry Relat Sci. 2014;51:128-134.
10. Bei B, Coo S, Trinder J. Sleep and mood during pregnancy and the postpartum period. Sleep Med Clin. 2015;10:25-33.
11. Lawson A, Murphy KE, Sloan E, et al. The relationship between sleep and postpartum mental disorders: a systematic review. J Affect Disord. 2015;176:65-77.
12. APA. Diagnostic and Statistical Manual of Mental Disorders, 5th ed. Washington, DC: American Psychiatric Association Publishing; 2013.
13. Langan R, Goodbred AJ. Identification and management of peripartum depression. Am Fam Physician. 2016;93:852-858.
14. Ali E. Women’s experiences with postpartum anxiety disorders: a narrative literature review. Int J Womens Health. 2018;10:237-249.
15. Tietz A, Zietlow AL, Reck C. Maternal bonding in mothers with postpartum anxiety disorder: the crucial role of subclinical depressive symptoms and maternal avoidance behaviour. Arch Womens Ment Health. 2014;17:433-442.
16. Field T. Postnatal anxiety prevalence, predictors and effects on development: a narrative review. Infant Behav Dev. 2018;51:24-32.
17. Serim Demirgoren B, Ozbek A, Ormen M, et al. Do mothers with high sodium levels in their breast milk have high depression and anxiety scores? J Int Med Res. 2017;45:843-848.
18. Ystrom E. Breastfeeding cessation and symptoms of anxiety and depression: a longitudinal cohort study. BMC Pregnancy Childbirth. 2012;12:36.
19. Britton JR. Infant temperament and maternal anxiety and depressed mood in the early postpartum period. Women Health. 2011;51:55-71.
20. Glasheen C, Richardson GA, Kim KH, et al. Exposure to maternal pre- and postnatal depression and anxiety symptoms: risk for major depression, anxiety disorders, and conduct disorder in adolescent offspring. Dev Psychopathol. 2013;26:1045-1063.
21. Petrozzi A, Gagliardi L. Anxious and depressive components of Edinburgh Postnatal Depression Scale in maternal postpartum psychological problems. J Perinat Med. 2013;41:343-348.
22. Bina R, Harrington D. The Edinburgh Postnatal Depression Scale: screening tool for postpartum anxiety as well? Findings from a confirmatory factor analysis of the Hebrew version. Matern Child Health J. 2016;20:904-914.
23. Matthey S, Fisher J, Rowe H. Using the Edinburgh postnatal depression scale to screen for anxiety disorders: conceptual and methodological considerations J Affect Disord. 2013;146:224-230.
24. Spitzer RL, Kroenke K, Williams JB, et al. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166:1092-1097.
25. Simpson W, Glazer M, Michalski N, et al. Comparative efficacy of the Generalized Anxiety Disorder 7-Item Scale and the Edinburgh Postnatal Depression Scale as screening tools for generalized anxiety disorder in pregnancy and the postpartum period. Can J Psychiatry. 2014;59:434-440.
26. Moran TE, Polanin JR, Wenzel A. The Postpartum Worry Scale-Revised: an initial validation of a measure of postpartum worry. Arch Womens Ment Health. 2014;17:41-48.
27. Fallon V, Halford JCG, Bennett KM, et al. The Postpartum Specific Anxiety Scale: development and preliminary validation. Arch Womens Ment Health. 2016;19:1079-1090.
28. Hofmann SG, Smits JA. Cognitive-behavioral therapy for adult anxiety disorders: a meta-analysis of randomized placebo-controlled trials. J Clin Psychiatry. 2008;69:621-632.
29. Goodman JH, Guarino A, Chenausky K, et al. CALM Pregnancy: results of a pilot study of mindfulness-based cognitive therapy for perinatal anxiety. Arch Womens Ment Health. 2014;17:373-387.
30. Marchesi C, Ossola P, Amerio A, et al. Clinical management of perinatal anxiety disorders: a systematic review. J Affect Disord. 2016;190:543-550.
31. Feijó L, Hernandez-Reif M, Field T, et al. Mothers’ depressed mood and anxiety levels are reduced after massaging their preterm infants. Infant Behav Devel. 2006;29:476-480.
32. Bérard A, Iessa N, Chaabane S, et al. The risk of major cardiac malformations associated with paroxetine use during the first trimester of pregnancy: a systematic review and meta-analysis. Br J Clin Pharmacol. 2016;81:589-604.
33. Huybrechts KF, Bateman BT, Palmsten K, et al. Antidepressant use late in pregnancy and risk of persistent pulmonary hypertension of the newborn. JAMA. 2015;313:2142-2151.
34. Cantarutti A, Merlino L, Monzani E, et al. Is the risk of preterm birth and low birth weight affected by the use of antidepressant agents during pregnancy? A population-based investigation. PLoS One. 2016;11:e0168115.
35. Molyneaux E, Howard LM, McGeown HR, et al. Antidepressant treatment for postnatal depression. Cochrane Database Syst Rev. 2014;11:CD002018.
36. Freeman MP. Postpartum depression treatment and breastfeeding. J Clin Psychiatry. 2009;70:e35.
37. ACOG Committee on Practice Bulletins—number 92. Use of psychiatric medications during pregnancy and lactation. Obstet Gynecol. 2008;111:1001-1020.
38. Orsolini L, Bellantuono C. Serotonin reuptake inhibitors and breastfeeding: a systematic review. Hum Psychopharmacol. 2015;30:4-20.
39. NIH. Drugs and Lactation Database. https://toxnet.nlm.nih.gov/newtoxnet/lactmed.htm. Accessed February 26, 2019.
Alcohol use disorder: How best to screen and intervene
THE CASE
Ms. E, a 42-year-old woman, visited her new physician for a physical exam. When asked about alcohol intake, she reported that she drank 3 to 4 beers after work and sometimes 5 to 8 beers a day on the weekends. Occasionally, she exceeded those amounts, but she didn’t feel guilty about her drinking. She was often late to work and said her relationship with her boyfriend was strained. A review of systems was positive for fatigue, poor concentration, abdominal pain, and weight gain. Her body mass index was 41, pulse 100 beats/min, blood pressure 125/75 mm Hg, and she was afebrile. Her physical exam was otherwise within normal limits.
How would you proceed with this patient?
Alcohol use disorder (AUD) is a common and often untreated condition that is increasingly prevalent in the United States.1 The Diagnostic and Statistical Manual of Mental Disorders, 5th Edition (DSM-5) characterizes AUD as a combination of signs and symptoms typifying alcohol abuse and dependence (discussed in a bit).2
Data from the 2015 National Survey on Drug Use and Health (NSDUH) showed 15.7 million Americans with AUD, affecting 6.2% of the population ages 18 years or older and 2.5% of adolescents ages 12 to 17 years.3
Alcohol use and AUD account for an estimated 3.8% of all global deaths and 4.6% of global disability-adjusted life years.4 AUD adversely affects several systems (TABLE 15), and patients with AUD are sicker and more likely to die younger than those without AUD.4 In the United States, prevalence of AUD has increased in recent years among women, older adults, racial minorities, and individuals with a low education level.6
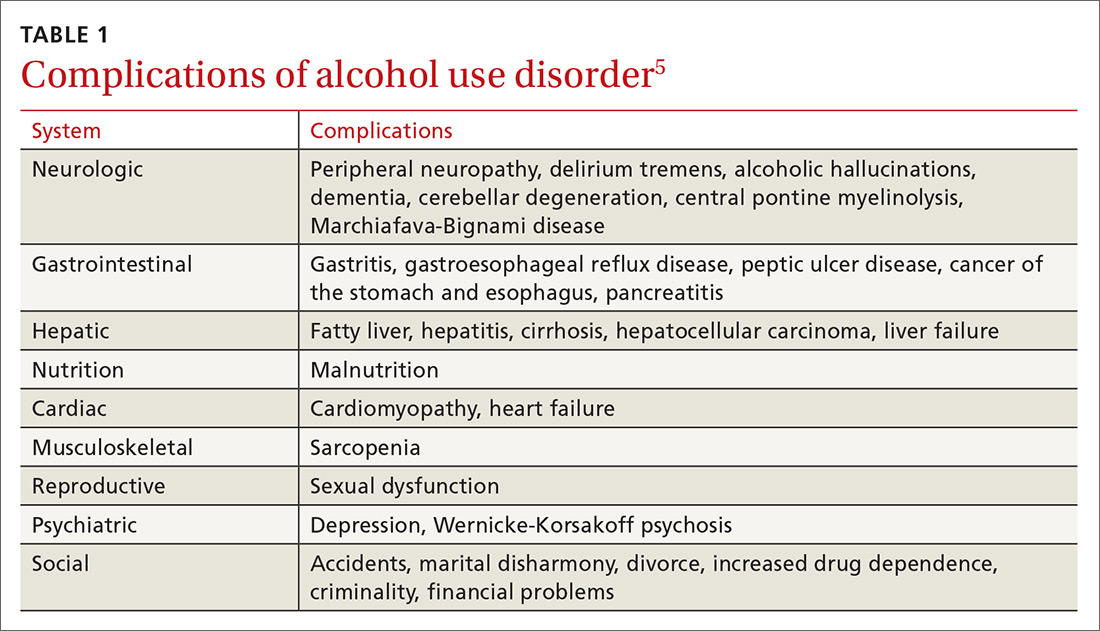
Screening for AUD is reasonable and straightforward, although diagnosis and treatment of AUD in primary care settings may be challenging due to competing clinical priorities; lack of training, resources, and support; and skepticism about the efficacy of behavioral and pharmacologic treatments.7,8 However, family physicians are in an excellent position to diagnose and help address the complex biopsychosocial needs of patients with AUD, often in collaboration with colleagues and community organizations.
Signs and symptoms of AUD
In clinical practice, at least 2 of the following 11 behaviors or symptoms are required to diagnose AUD2:
- consuming larger amounts of alcohol over a longer period than intended
- persistent desire or unsuccessful efforts to cut down or control alcohol use
- making a significant effort to obtain, use, or recover from alcohol
In moderate-to-severe cases:
- cravings or urges to use alcohol
- recurrent failure to fulfill major work, school, or social obligations
- continued alcohol use despite recurrent social and interpersonal problems
- giving up social, occupational, and recreational activities due to alcohol
- using alcohol in physically dangerous situations
- continued alcohol use despite having physical or psychological problems
- tolerance to alcohol’s effects
- withdrawal symptoms.
Continue to: Patients meet criteria for mild AUD severity if...
Patients meet criteria for mild AUD severity if they exhibit 2 or 3 symptoms, moderate AUD with 4 or 5 symptoms, and severe AUD if there are 6 or more symptoms.2
Those who meet criteria for AUD and are able to stop using alcohol are deemed to be in early remission if the criteria have gone unfulfilled for at least 3 months and less than 12 months. Patients are considered to be in sustained remission if they have not met criteria for AUD at any time during a period of 12 months or longer.
How to detect AUD
Several clues in a patient’s history can suggest AUD (TABLE 29,10). Most imbibers are unaware of the dangers and may consider themselves merely “social drinkers.” Binge drinking may be an early indicator of vulnerability to AUD and should be assessed as part of a thorough clinical evaluation.11 The US Preventive Services Task Force (USPSTF) recommends (Grade B) that clinicians screen adults ages 18 years or older for alcohol misuse.12

Studies demonstrate that both genetic and environmental factors play important roles in the development of AUD.13 A family history of excessive alcohol use increases the risk of AUD. Comorbidity of AUD and other mental health conditions is extremely common. For example, high rates of association between major depressive disorder and AUD have been observed.14
Tools to use in screening and diagnosing AUD
Screening for AUD during an office visit can be done fairly quickly. While 96% of primary care physicians screen for alcohol misuse in some way, only 38% use 1 of the 3 tools recommended by the USPSTF15—the Alcohol Use Disorders Identification Test (AUDIT), the abbreviated AUDIT-C, or the National Institute on Alcohol Abuse and Alcoholism (NIAAA) single question screen—which detect the full spectrum of alcohol misuse in adults.12 Although the commonly used CAGE questionnaire is one of the most studied self-report tools, it has lower sensitivity at a lower level of alcohol intake.16
Continue to: The NIAAA single-question screen asks...
The NIAAA single-question screen asks how many times in the past year the patient had ≥4 drinks (women) or ≥5 drinks (men) in a day.15 The sensitivity and specificity of single-question screening are 82% to 87% and 61% to 79%, respectively, and the test has been validated in several different settings.12 The AUDIT screening tool, freely available from the World Health Organization, is a 10-item questionnaire that probes an individual’s alcohol intake, alcohol dependence, and adverse consequences of alcohol use. Administration of the AUDIT typically requires only 2 minutes. AUDIT-C17 is an abbreviated version of the AUDIT questionnaire that asks 3 consumption questions to screen for AUD.
It was found that AUDIT scores in the range of 8 to 15 indicate a medium-level alcohol problem, whereas a score of ≥16 indicates a high-level alcohol problem. The AUDIT-C is scored from 0 to 12, with ≥4 indicating a problem in men and ≥3
THE CASE
The physician had used the NIAAA single- question screen to determine that Ms. E drank more than 4 beers per day during social events and weekends, which occurred 2 to 3 times per month over the past year. She lives alone and said that she’d been seeing less and less of her boyfriend lately. Her score on the Patient Health Questionnaire (PHQ), which screens for depression, was 11, indicating moderate impairment. Her response on the CAGE questionnaire was negative for a problem with alcohol. However, her AUDIT score was 17, indicating a high-level alcohol problem. Based on these findings, her physician expressed concern that her alcohol use might be contributing to her symptoms and difficulties.
Although she did not have a history of increasing usage per day, a persistent desire to cut down, significant effort to obtain alcohol, or cravings, she was having work troubles and continued to drink even though it was straining relationships, promoting weight gain, and causing abdominal pain.
The physician asked her to schedule a return visit and ordered several blood studies. He also offered to connect her with a colleague with whom he collaborated who could speak with her about possible alcohol use disorders and depression.
Continue to: Selecting blood work in screening for AUD
Selecting blood work in screening for AUD
Lab tests used to measure hepatic injury due to alcohol include gamma-glutamyl-transferase, aspartate aminotransferase (AST), alanine aminotransferase (ALT), and macrocytic volume, although the indices of hepatic damage have low specificity. Elevated serum ethanol levels can reveal recent alcohol use, and vitamin deficiencies and other abnormalities can be used to differentiate other causes of hepatic inflammation and co-existing health issues (TABLE 310,18). A number of as-yet-unvalidated biomarkers are being studied to assist in screening, diagnosing, and treating AUD.18
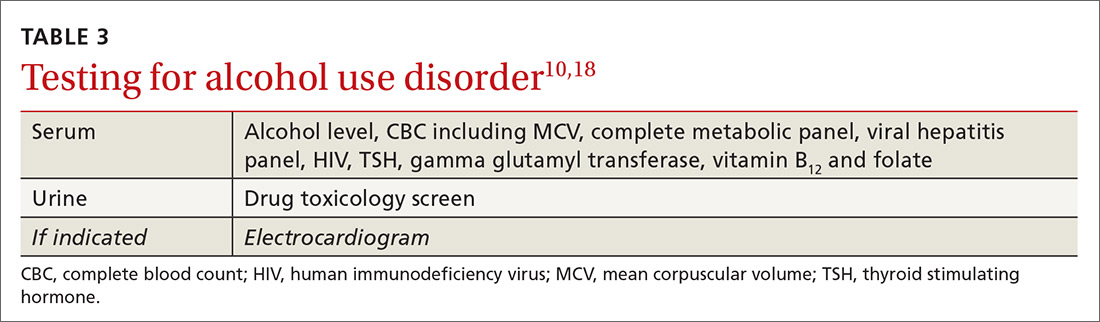
What treatment approaches work for AUD?
Family physicians can efficiently and productively address AUD by using alcohol screening and brief intervention, which have been shown to reduce risky drinking. Reimbursement for this service is covered by such CPT codes as 99408, 99409, or H0049, or with other evaluation and management (E/M) codes by using modifier 25.
Treatment of AUD varies and should be customized to each patient’s needs, readiness, preferences, and resources. Individual and group counseling approaches can be effective, and medications are available for inpatient and outpatient settings. Psychotherapy options include brief interventions, 12-step programs (eg, Alcoholics Anonymous—https://www.aa.org/pages/en_US/find-aa-resources),motivational enhancement therapy, and cognitive behavioral therapy. Although it is beyond the scope of this article to describe these options in detail, resources are available for those who wish to learn more.19-21
Psychopharmacologic management includes US Food and Drug Administration (FDA)-approved medications such as disulfiram, naltrexone, and acamprosate, and off-label uses of other medications (TABLE 49). Not enough empiric evidence is available to judge the effectiveness of these medications in adolescents, and the FDA has not approved them for such use. Evidence from meta-analyses comparing naltrexone and acamprosate have shown naltrexone to be more efficacious in reducing heavy drinking and cravings, while acamprosate is effective in promoting abstinence.22,23 Naltrexone combined with behavioral intervention reduces the heavy drinking days and percentage of abstinence days.24
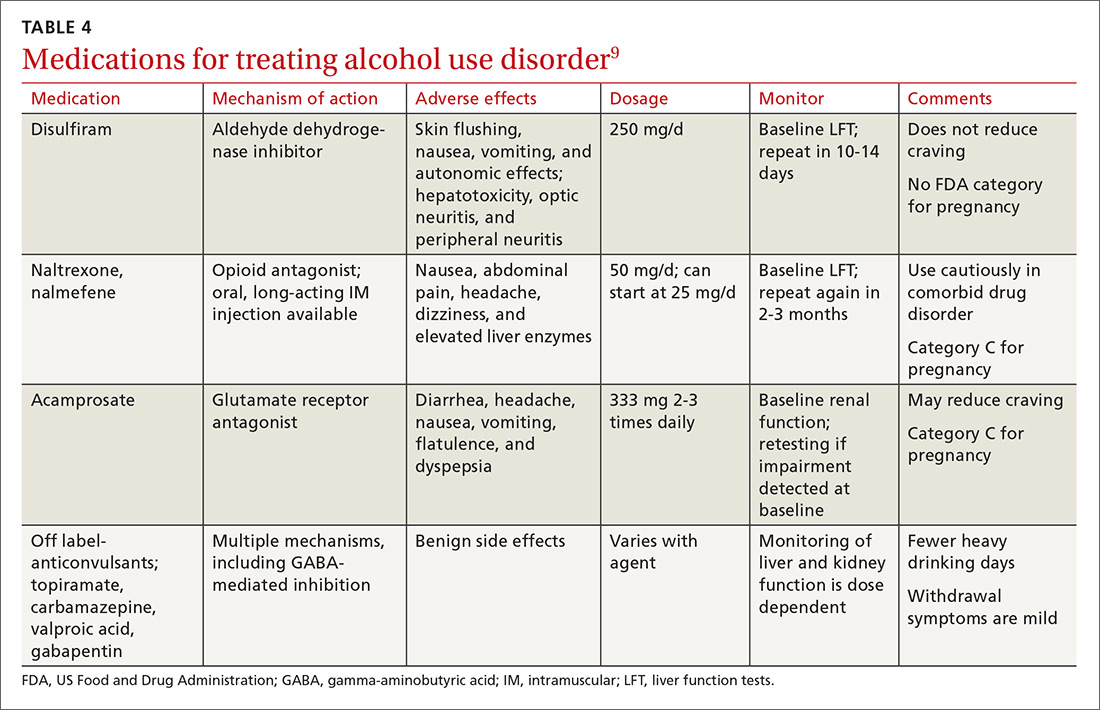
Current guideline recommendations from the American Psychiatric Association25 include:
- Naltrexone and acamprosate are recommended to treat patients with moderate-to-severe AUD in specific circumstances (eg, when nonpharmacologic approaches have failed to produce an effect or when patients prefer to use one of these medications).
- Topiramate and gabapentin are also suggested as medications for patients with moderate-to-severe AUD, but typically after first trying naltrexone and acamprosate.
- Disulfiram generally should not be used as first-line treatment. It produces physical reactions (eg, flushing) if alcohol is consumed within 12 to 24 hours of medication use.
Continue to: THE CASE
THE CASE
Ms. E was open to the idea of decreasing her alcohol use and agreed that she was depressed. Her lab tests at follow-up were normal other than an elevated AST/ALT of 90/80 U/L. S
She continued to get counseling for her AUD and for her comorbid depression in addition to taking a selective serotonin reuptake inhibitor. She is now in early remission for her alcohol use.
CORRESPONDENCE
Jaividhya Dasarathy, MD, Department of Family Medicine, Metro Health Medical Center, 2500 MetroHealth Drive, Cleveland, OH 44109; [email protected].
1. Grant BF, Goldstein RB, Saha TD, et al. Epidemiology of DSM-5 alcohol use disorder: results from the National Epidemiologic Survey on Alcohol and Related Conditions III. JAMA Psychiatry. 2015;72:757-766.
2. APA. Diagnostic and Statistical Manual of Mental Disorders, 5th ed. Washington DC; 2013.
3. HHS. Results from the 2015 National Survey on Drug Use and Health: summary of national findings. https://www.samhsa.gov/data/sites/default/files/NSDUH-DetTabs-2015/NSDUH-DetTabs-2015/NSDUH-DetTabs-2015.pdf. Accessed November 27, 2018.
4. Rehm J, Mathers C, Popova S, et al. Global burden of disease and injury and economic cost attributable to alcohol use and alcohol-use disorders. Lancet. 2009;373:2223-2233.
5. Chase V, Neild R, Sadler CW, et al. The medical complications of alcohol use: understanding mechanisms to improve management. Drug Alcohol Rev. 2005;24:253-265.
6. Grant BF, Chou SP, Saha TD, et al. Prevalence of 12-month alcohol use, high-risk drinking, and DSM-IV alcohol use disorder in the United States, 2001-2002 to 2012-2013: results from the National Epidemiologic Survey on Alcohol and Related Conditions. JAMA Psychiatry. 2017;74:911-923.
7. Williams EC, Achtmeyer CE, Young JP, et al. Barriers to and facilitators of alcohol use disorder pharmacotherapy in primary care: a qualitative study in five VA clinics. J Gen Intern Med. 2018;33:258-267.
8. Zhang DX, Li ST, Lee QK, et al. Systematic review of guidelines on managing patients with harmful use of alcohol in primary healthcare settings. Alcohol Alcohol. 2017;52:595-609.
9. Wackernah RC, Minnick MJ, Clapp P. Alcohol use disorder: pathophysiology, effects, and pharmacologic options for treatment. Subst Abuse Rehabil. 2014;5:1-12.
10. Kattimani S, Bharadwaj B. Clinical management of alcohol withdrawal: a systematic review. Ind Psychiatry J. 2013;22:100-108.
11. Gowin JL, Sloan ME, Stangl BL, et al. Vulnerability for alcohol use disorder and rate of alcohol consumption. Am J Psychiatry. 2017;174:1094-1101.
12. Moyer VA; Preventive Services Task Force. Screening and behavioral counseling interventions in primary care to reduce alcohol misuse: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2013;159:210-218.
13. Tarter RE, Alterman AI, Edwards KL. Vulnerability to alcoholism in men: a behavior-genetic perspective. J Stud Alcohol. 1985;46:329-356.
14. Brière FN, Rohde P, Seeley JR, et al. Comorbidity between major depression and alcohol use disorder from adolescence to adulthood [published online ahead of print, October 22, 2013]. Compr Psychiatry. 2014;55:526-533. doi: 10.1016/j.comppsych.2013.10.007.
15. Tan CH, Hungerford DW, Denny CH, et al. Screening for alcohol misuse: practices among U.S. primary care providers, DocStyles 2016. Am J Prev Med. 2018;54:173-180.
16. Aertgeerts B, Buntinx F, Kester A. The value of the CAGE in screening for alcohol abuse and alcohol dependence in general clinical populations: a diagnostic meta-analysis. J Clin Epidemiol. 2004;57:30-39.
17. Bush K, Kivlahan DR, McDonell MB, et al. The AUDIT alcohol consumption questions (AUDIT-C): an effective brief screening test for problem drinking. Ambulatory Care Quality Improvement Project (ACQUIP). Alcohol Use Disorders Identification Test. Arch Intern Med. 1998;158:1789-1795.
18. Nanau RM, Neuman MG. Biomolecules and biomarkers used in diagnosis of alcohol drinking and in monitoring therapeutic interventions. Biomolecules. 2015;5:1339-1385.
19. Raddock M, Martukovich R, Berko E, et al. 7 tools to help patients adopt healthier behaviors. J Fam Pract. 2015;64:97-103.
20. AHRQ. Whitlock EP, Green CA, Polen MR, et al. Behavioral Counseling Interventions in Primary Care to Reduce Risky/Harmful Alcohol Use. 2004. https://www.ncbi.nlm.nih.gov/books/NBK42863/. Accessed November 17, 2018.
21. Miller WR, Baca C, Compton WM, et al. Addressing substance abuse in health care settings. Alcohol Clin Exp Res. 2006;30:292-302.
22. Maisel NC, Blodgett JC, Wilbourne PL, et al. Meta-analysis of naltrexone and acamprosate for treating alcohol use disorders: when are these medications most helpful? Addiction. 2013;108:275-293.
23. Rosner S, Leucht S, Lehert P, et al. Acamprosate supports abstinence, naltrexone prevents excessive drinking: evidence from a meta-analysis with unreported outcomes. J Psychopharmacol. 2008;22:11-23.
24. Anton RF, O’Malley SS, Ciraulo DA, et al. Combined pharmacotherapies and behavioral interventions for alcohol dependence: the COMBINE study: a randomized controlled trial. JAMA. 2006;295:2003-2017.
25. Reus VI, Fochtmann LJ, Bukstein O, et al. The American Psychiatric Association Practice Guideline for the Pharmacological Treatment of Patients With Alcohol Use Disorder. Am J Psychiatry. 2018;175:86-90.
THE CASE
Ms. E, a 42-year-old woman, visited her new physician for a physical exam. When asked about alcohol intake, she reported that she drank 3 to 4 beers after work and sometimes 5 to 8 beers a day on the weekends. Occasionally, she exceeded those amounts, but she didn’t feel guilty about her drinking. She was often late to work and said her relationship with her boyfriend was strained. A review of systems was positive for fatigue, poor concentration, abdominal pain, and weight gain. Her body mass index was 41, pulse 100 beats/min, blood pressure 125/75 mm Hg, and she was afebrile. Her physical exam was otherwise within normal limits.
How would you proceed with this patient?
Alcohol use disorder (AUD) is a common and often untreated condition that is increasingly prevalent in the United States.1 The Diagnostic and Statistical Manual of Mental Disorders, 5th Edition (DSM-5) characterizes AUD as a combination of signs and symptoms typifying alcohol abuse and dependence (discussed in a bit).2
Data from the 2015 National Survey on Drug Use and Health (NSDUH) showed 15.7 million Americans with AUD, affecting 6.2% of the population ages 18 years or older and 2.5% of adolescents ages 12 to 17 years.3
Alcohol use and AUD account for an estimated 3.8% of all global deaths and 4.6% of global disability-adjusted life years.4 AUD adversely affects several systems (TABLE 15), and patients with AUD are sicker and more likely to die younger than those without AUD.4 In the United States, prevalence of AUD has increased in recent years among women, older adults, racial minorities, and individuals with a low education level.6

Screening for AUD is reasonable and straightforward, although diagnosis and treatment of AUD in primary care settings may be challenging due to competing clinical priorities; lack of training, resources, and support; and skepticism about the efficacy of behavioral and pharmacologic treatments.7,8 However, family physicians are in an excellent position to diagnose and help address the complex biopsychosocial needs of patients with AUD, often in collaboration with colleagues and community organizations.
Signs and symptoms of AUD
In clinical practice, at least 2 of the following 11 behaviors or symptoms are required to diagnose AUD2:
- consuming larger amounts of alcohol over a longer period than intended
- persistent desire or unsuccessful efforts to cut down or control alcohol use
- making a significant effort to obtain, use, or recover from alcohol
In moderate-to-severe cases:
- cravings or urges to use alcohol
- recurrent failure to fulfill major work, school, or social obligations
- continued alcohol use despite recurrent social and interpersonal problems
- giving up social, occupational, and recreational activities due to alcohol
- using alcohol in physically dangerous situations
- continued alcohol use despite having physical or psychological problems
- tolerance to alcohol’s effects
- withdrawal symptoms.
Continue to: Patients meet criteria for mild AUD severity if...
Patients meet criteria for mild AUD severity if they exhibit 2 or 3 symptoms, moderate AUD with 4 or 5 symptoms, and severe AUD if there are 6 or more symptoms.2
Those who meet criteria for AUD and are able to stop using alcohol are deemed to be in early remission if the criteria have gone unfulfilled for at least 3 months and less than 12 months. Patients are considered to be in sustained remission if they have not met criteria for AUD at any time during a period of 12 months or longer.
How to detect AUD
Several clues in a patient’s history can suggest AUD (TABLE 29,10). Most imbibers are unaware of the dangers and may consider themselves merely “social drinkers.” Binge drinking may be an early indicator of vulnerability to AUD and should be assessed as part of a thorough clinical evaluation.11 The US Preventive Services Task Force (USPSTF) recommends (Grade B) that clinicians screen adults ages 18 years or older for alcohol misuse.12

Studies demonstrate that both genetic and environmental factors play important roles in the development of AUD.13 A family history of excessive alcohol use increases the risk of AUD. Comorbidity of AUD and other mental health conditions is extremely common. For example, high rates of association between major depressive disorder and AUD have been observed.14
Tools to use in screening and diagnosing AUD
Screening for AUD during an office visit can be done fairly quickly. While 96% of primary care physicians screen for alcohol misuse in some way, only 38% use 1 of the 3 tools recommended by the USPSTF15—the Alcohol Use Disorders Identification Test (AUDIT), the abbreviated AUDIT-C, or the National Institute on Alcohol Abuse and Alcoholism (NIAAA) single question screen—which detect the full spectrum of alcohol misuse in adults.12 Although the commonly used CAGE questionnaire is one of the most studied self-report tools, it has lower sensitivity at a lower level of alcohol intake.16
Continue to: The NIAAA single-question screen asks...
The NIAAA single-question screen asks how many times in the past year the patient had ≥4 drinks (women) or ≥5 drinks (men) in a day.15 The sensitivity and specificity of single-question screening are 82% to 87% and 61% to 79%, respectively, and the test has been validated in several different settings.12 The AUDIT screening tool, freely available from the World Health Organization, is a 10-item questionnaire that probes an individual’s alcohol intake, alcohol dependence, and adverse consequences of alcohol use. Administration of the AUDIT typically requires only 2 minutes. AUDIT-C17 is an abbreviated version of the AUDIT questionnaire that asks 3 consumption questions to screen for AUD.
It was found that AUDIT scores in the range of 8 to 15 indicate a medium-level alcohol problem, whereas a score of ≥16 indicates a high-level alcohol problem. The AUDIT-C is scored from 0 to 12, with ≥4 indicating a problem in men and ≥3
THE CASE
The physician had used the NIAAA single- question screen to determine that Ms. E drank more than 4 beers per day during social events and weekends, which occurred 2 to 3 times per month over the past year. She lives alone and said that she’d been seeing less and less of her boyfriend lately. Her score on the Patient Health Questionnaire (PHQ), which screens for depression, was 11, indicating moderate impairment. Her response on the CAGE questionnaire was negative for a problem with alcohol. However, her AUDIT score was 17, indicating a high-level alcohol problem. Based on these findings, her physician expressed concern that her alcohol use might be contributing to her symptoms and difficulties.
Although she did not have a history of increasing usage per day, a persistent desire to cut down, significant effort to obtain alcohol, or cravings, she was having work troubles and continued to drink even though it was straining relationships, promoting weight gain, and causing abdominal pain.
The physician asked her to schedule a return visit and ordered several blood studies. He also offered to connect her with a colleague with whom he collaborated who could speak with her about possible alcohol use disorders and depression.
Continue to: Selecting blood work in screening for AUD
Selecting blood work in screening for AUD
Lab tests used to measure hepatic injury due to alcohol include gamma-glutamyl-transferase, aspartate aminotransferase (AST), alanine aminotransferase (ALT), and macrocytic volume, although the indices of hepatic damage have low specificity. Elevated serum ethanol levels can reveal recent alcohol use, and vitamin deficiencies and other abnormalities can be used to differentiate other causes of hepatic inflammation and co-existing health issues (TABLE 310,18). A number of as-yet-unvalidated biomarkers are being studied to assist in screening, diagnosing, and treating AUD.18

What treatment approaches work for AUD?
Family physicians can efficiently and productively address AUD by using alcohol screening and brief intervention, which have been shown to reduce risky drinking. Reimbursement for this service is covered by such CPT codes as 99408, 99409, or H0049, or with other evaluation and management (E/M) codes by using modifier 25.
Treatment of AUD varies and should be customized to each patient’s needs, readiness, preferences, and resources. Individual and group counseling approaches can be effective, and medications are available for inpatient and outpatient settings. Psychotherapy options include brief interventions, 12-step programs (eg, Alcoholics Anonymous—https://www.aa.org/pages/en_US/find-aa-resources),motivational enhancement therapy, and cognitive behavioral therapy. Although it is beyond the scope of this article to describe these options in detail, resources are available for those who wish to learn more.19-21
Psychopharmacologic management includes US Food and Drug Administration (FDA)-approved medications such as disulfiram, naltrexone, and acamprosate, and off-label uses of other medications (TABLE 49). Not enough empiric evidence is available to judge the effectiveness of these medications in adolescents, and the FDA has not approved them for such use. Evidence from meta-analyses comparing naltrexone and acamprosate have shown naltrexone to be more efficacious in reducing heavy drinking and cravings, while acamprosate is effective in promoting abstinence.22,23 Naltrexone combined with behavioral intervention reduces the heavy drinking days and percentage of abstinence days.24

Current guideline recommendations from the American Psychiatric Association25 include:
- Naltrexone and acamprosate are recommended to treat patients with moderate-to-severe AUD in specific circumstances (eg, when nonpharmacologic approaches have failed to produce an effect or when patients prefer to use one of these medications).
- Topiramate and gabapentin are also suggested as medications for patients with moderate-to-severe AUD, but typically after first trying naltrexone and acamprosate.
- Disulfiram generally should not be used as first-line treatment. It produces physical reactions (eg, flushing) if alcohol is consumed within 12 to 24 hours of medication use.
Continue to: THE CASE
THE CASE
Ms. E was open to the idea of decreasing her alcohol use and agreed that she was depressed. Her lab tests at follow-up were normal other than an elevated AST/ALT of 90/80 U/L. S
She continued to get counseling for her AUD and for her comorbid depression in addition to taking a selective serotonin reuptake inhibitor. She is now in early remission for her alcohol use.
CORRESPONDENCE
Jaividhya Dasarathy, MD, Department of Family Medicine, Metro Health Medical Center, 2500 MetroHealth Drive, Cleveland, OH 44109; [email protected].
THE CASE
Ms. E, a 42-year-old woman, visited her new physician for a physical exam. When asked about alcohol intake, she reported that she drank 3 to 4 beers after work and sometimes 5 to 8 beers a day on the weekends. Occasionally, she exceeded those amounts, but she didn’t feel guilty about her drinking. She was often late to work and said her relationship with her boyfriend was strained. A review of systems was positive for fatigue, poor concentration, abdominal pain, and weight gain. Her body mass index was 41, pulse 100 beats/min, blood pressure 125/75 mm Hg, and she was afebrile. Her physical exam was otherwise within normal limits.
How would you proceed with this patient?
Alcohol use disorder (AUD) is a common and often untreated condition that is increasingly prevalent in the United States.1 The Diagnostic and Statistical Manual of Mental Disorders, 5th Edition (DSM-5) characterizes AUD as a combination of signs and symptoms typifying alcohol abuse and dependence (discussed in a bit).2
Data from the 2015 National Survey on Drug Use and Health (NSDUH) showed 15.7 million Americans with AUD, affecting 6.2% of the population ages 18 years or older and 2.5% of adolescents ages 12 to 17 years.3
Alcohol use and AUD account for an estimated 3.8% of all global deaths and 4.6% of global disability-adjusted life years.4 AUD adversely affects several systems (TABLE 15), and patients with AUD are sicker and more likely to die younger than those without AUD.4 In the United States, prevalence of AUD has increased in recent years among women, older adults, racial minorities, and individuals with a low education level.6

Screening for AUD is reasonable and straightforward, although diagnosis and treatment of AUD in primary care settings may be challenging due to competing clinical priorities; lack of training, resources, and support; and skepticism about the efficacy of behavioral and pharmacologic treatments.7,8 However, family physicians are in an excellent position to diagnose and help address the complex biopsychosocial needs of patients with AUD, often in collaboration with colleagues and community organizations.
Signs and symptoms of AUD
In clinical practice, at least 2 of the following 11 behaviors or symptoms are required to diagnose AUD2:
- consuming larger amounts of alcohol over a longer period than intended
- persistent desire or unsuccessful efforts to cut down or control alcohol use
- making a significant effort to obtain, use, or recover from alcohol
In moderate-to-severe cases:
- cravings or urges to use alcohol
- recurrent failure to fulfill major work, school, or social obligations
- continued alcohol use despite recurrent social and interpersonal problems
- giving up social, occupational, and recreational activities due to alcohol
- using alcohol in physically dangerous situations
- continued alcohol use despite having physical or psychological problems
- tolerance to alcohol’s effects
- withdrawal symptoms.
Continue to: Patients meet criteria for mild AUD severity if...
Patients meet criteria for mild AUD severity if they exhibit 2 or 3 symptoms, moderate AUD with 4 or 5 symptoms, and severe AUD if there are 6 or more symptoms.2
Those who meet criteria for AUD and are able to stop using alcohol are deemed to be in early remission if the criteria have gone unfulfilled for at least 3 months and less than 12 months. Patients are considered to be in sustained remission if they have not met criteria for AUD at any time during a period of 12 months or longer.
How to detect AUD
Several clues in a patient’s history can suggest AUD (TABLE 29,10). Most imbibers are unaware of the dangers and may consider themselves merely “social drinkers.” Binge drinking may be an early indicator of vulnerability to AUD and should be assessed as part of a thorough clinical evaluation.11 The US Preventive Services Task Force (USPSTF) recommends (Grade B) that clinicians screen adults ages 18 years or older for alcohol misuse.12

Studies demonstrate that both genetic and environmental factors play important roles in the development of AUD.13 A family history of excessive alcohol use increases the risk of AUD. Comorbidity of AUD and other mental health conditions is extremely common. For example, high rates of association between major depressive disorder and AUD have been observed.14
Tools to use in screening and diagnosing AUD
Screening for AUD during an office visit can be done fairly quickly. While 96% of primary care physicians screen for alcohol misuse in some way, only 38% use 1 of the 3 tools recommended by the USPSTF15—the Alcohol Use Disorders Identification Test (AUDIT), the abbreviated AUDIT-C, or the National Institute on Alcohol Abuse and Alcoholism (NIAAA) single question screen—which detect the full spectrum of alcohol misuse in adults.12 Although the commonly used CAGE questionnaire is one of the most studied self-report tools, it has lower sensitivity at a lower level of alcohol intake.16
Continue to: The NIAAA single-question screen asks...
The NIAAA single-question screen asks how many times in the past year the patient had ≥4 drinks (women) or ≥5 drinks (men) in a day.15 The sensitivity and specificity of single-question screening are 82% to 87% and 61% to 79%, respectively, and the test has been validated in several different settings.12 The AUDIT screening tool, freely available from the World Health Organization, is a 10-item questionnaire that probes an individual’s alcohol intake, alcohol dependence, and adverse consequences of alcohol use. Administration of the AUDIT typically requires only 2 minutes. AUDIT-C17 is an abbreviated version of the AUDIT questionnaire that asks 3 consumption questions to screen for AUD.
It was found that AUDIT scores in the range of 8 to 15 indicate a medium-level alcohol problem, whereas a score of ≥16 indicates a high-level alcohol problem. The AUDIT-C is scored from 0 to 12, with ≥4 indicating a problem in men and ≥3
THE CASE
The physician had used the NIAAA single- question screen to determine that Ms. E drank more than 4 beers per day during social events and weekends, which occurred 2 to 3 times per month over the past year. She lives alone and said that she’d been seeing less and less of her boyfriend lately. Her score on the Patient Health Questionnaire (PHQ), which screens for depression, was 11, indicating moderate impairment. Her response on the CAGE questionnaire was negative for a problem with alcohol. However, her AUDIT score was 17, indicating a high-level alcohol problem. Based on these findings, her physician expressed concern that her alcohol use might be contributing to her symptoms and difficulties.
Although she did not have a history of increasing usage per day, a persistent desire to cut down, significant effort to obtain alcohol, or cravings, she was having work troubles and continued to drink even though it was straining relationships, promoting weight gain, and causing abdominal pain.
The physician asked her to schedule a return visit and ordered several blood studies. He also offered to connect her with a colleague with whom he collaborated who could speak with her about possible alcohol use disorders and depression.
Continue to: Selecting blood work in screening for AUD
Selecting blood work in screening for AUD
Lab tests used to measure hepatic injury due to alcohol include gamma-glutamyl-transferase, aspartate aminotransferase (AST), alanine aminotransferase (ALT), and macrocytic volume, although the indices of hepatic damage have low specificity. Elevated serum ethanol levels can reveal recent alcohol use, and vitamin deficiencies and other abnormalities can be used to differentiate other causes of hepatic inflammation and co-existing health issues (TABLE 310,18). A number of as-yet-unvalidated biomarkers are being studied to assist in screening, diagnosing, and treating AUD.18

What treatment approaches work for AUD?
Family physicians can efficiently and productively address AUD by using alcohol screening and brief intervention, which have been shown to reduce risky drinking. Reimbursement for this service is covered by such CPT codes as 99408, 99409, or H0049, or with other evaluation and management (E/M) codes by using modifier 25.
Treatment of AUD varies and should be customized to each patient’s needs, readiness, preferences, and resources. Individual and group counseling approaches can be effective, and medications are available for inpatient and outpatient settings. Psychotherapy options include brief interventions, 12-step programs (eg, Alcoholics Anonymous—https://www.aa.org/pages/en_US/find-aa-resources),motivational enhancement therapy, and cognitive behavioral therapy. Although it is beyond the scope of this article to describe these options in detail, resources are available for those who wish to learn more.19-21
Psychopharmacologic management includes US Food and Drug Administration (FDA)-approved medications such as disulfiram, naltrexone, and acamprosate, and off-label uses of other medications (TABLE 49). Not enough empiric evidence is available to judge the effectiveness of these medications in adolescents, and the FDA has not approved them for such use. Evidence from meta-analyses comparing naltrexone and acamprosate have shown naltrexone to be more efficacious in reducing heavy drinking and cravings, while acamprosate is effective in promoting abstinence.22,23 Naltrexone combined with behavioral intervention reduces the heavy drinking days and percentage of abstinence days.24

Current guideline recommendations from the American Psychiatric Association25 include:
- Naltrexone and acamprosate are recommended to treat patients with moderate-to-severe AUD in specific circumstances (eg, when nonpharmacologic approaches have failed to produce an effect or when patients prefer to use one of these medications).
- Topiramate and gabapentin are also suggested as medications for patients with moderate-to-severe AUD, but typically after first trying naltrexone and acamprosate.
- Disulfiram generally should not be used as first-line treatment. It produces physical reactions (eg, flushing) if alcohol is consumed within 12 to 24 hours of medication use.
Continue to: THE CASE
THE CASE
Ms. E was open to the idea of decreasing her alcohol use and agreed that she was depressed. Her lab tests at follow-up were normal other than an elevated AST/ALT of 90/80 U/L. S
She continued to get counseling for her AUD and for her comorbid depression in addition to taking a selective serotonin reuptake inhibitor. She is now in early remission for her alcohol use.
CORRESPONDENCE
Jaividhya Dasarathy, MD, Department of Family Medicine, Metro Health Medical Center, 2500 MetroHealth Drive, Cleveland, OH 44109; [email protected].
1. Grant BF, Goldstein RB, Saha TD, et al. Epidemiology of DSM-5 alcohol use disorder: results from the National Epidemiologic Survey on Alcohol and Related Conditions III. JAMA Psychiatry. 2015;72:757-766.
2. APA. Diagnostic and Statistical Manual of Mental Disorders, 5th ed. Washington DC; 2013.
3. HHS. Results from the 2015 National Survey on Drug Use and Health: summary of national findings. https://www.samhsa.gov/data/sites/default/files/NSDUH-DetTabs-2015/NSDUH-DetTabs-2015/NSDUH-DetTabs-2015.pdf. Accessed November 27, 2018.
4. Rehm J, Mathers C, Popova S, et al. Global burden of disease and injury and economic cost attributable to alcohol use and alcohol-use disorders. Lancet. 2009;373:2223-2233.
5. Chase V, Neild R, Sadler CW, et al. The medical complications of alcohol use: understanding mechanisms to improve management. Drug Alcohol Rev. 2005;24:253-265.
6. Grant BF, Chou SP, Saha TD, et al. Prevalence of 12-month alcohol use, high-risk drinking, and DSM-IV alcohol use disorder in the United States, 2001-2002 to 2012-2013: results from the National Epidemiologic Survey on Alcohol and Related Conditions. JAMA Psychiatry. 2017;74:911-923.
7. Williams EC, Achtmeyer CE, Young JP, et al. Barriers to and facilitators of alcohol use disorder pharmacotherapy in primary care: a qualitative study in five VA clinics. J Gen Intern Med. 2018;33:258-267.
8. Zhang DX, Li ST, Lee QK, et al. Systematic review of guidelines on managing patients with harmful use of alcohol in primary healthcare settings. Alcohol Alcohol. 2017;52:595-609.
9. Wackernah RC, Minnick MJ, Clapp P. Alcohol use disorder: pathophysiology, effects, and pharmacologic options for treatment. Subst Abuse Rehabil. 2014;5:1-12.
10. Kattimani S, Bharadwaj B. Clinical management of alcohol withdrawal: a systematic review. Ind Psychiatry J. 2013;22:100-108.
11. Gowin JL, Sloan ME, Stangl BL, et al. Vulnerability for alcohol use disorder and rate of alcohol consumption. Am J Psychiatry. 2017;174:1094-1101.
12. Moyer VA; Preventive Services Task Force. Screening and behavioral counseling interventions in primary care to reduce alcohol misuse: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2013;159:210-218.
13. Tarter RE, Alterman AI, Edwards KL. Vulnerability to alcoholism in men: a behavior-genetic perspective. J Stud Alcohol. 1985;46:329-356.
14. Brière FN, Rohde P, Seeley JR, et al. Comorbidity between major depression and alcohol use disorder from adolescence to adulthood [published online ahead of print, October 22, 2013]. Compr Psychiatry. 2014;55:526-533. doi: 10.1016/j.comppsych.2013.10.007.
15. Tan CH, Hungerford DW, Denny CH, et al. Screening for alcohol misuse: practices among U.S. primary care providers, DocStyles 2016. Am J Prev Med. 2018;54:173-180.
16. Aertgeerts B, Buntinx F, Kester A. The value of the CAGE in screening for alcohol abuse and alcohol dependence in general clinical populations: a diagnostic meta-analysis. J Clin Epidemiol. 2004;57:30-39.
17. Bush K, Kivlahan DR, McDonell MB, et al. The AUDIT alcohol consumption questions (AUDIT-C): an effective brief screening test for problem drinking. Ambulatory Care Quality Improvement Project (ACQUIP). Alcohol Use Disorders Identification Test. Arch Intern Med. 1998;158:1789-1795.
18. Nanau RM, Neuman MG. Biomolecules and biomarkers used in diagnosis of alcohol drinking and in monitoring therapeutic interventions. Biomolecules. 2015;5:1339-1385.
19. Raddock M, Martukovich R, Berko E, et al. 7 tools to help patients adopt healthier behaviors. J Fam Pract. 2015;64:97-103.
20. AHRQ. Whitlock EP, Green CA, Polen MR, et al. Behavioral Counseling Interventions in Primary Care to Reduce Risky/Harmful Alcohol Use. 2004. https://www.ncbi.nlm.nih.gov/books/NBK42863/. Accessed November 17, 2018.
21. Miller WR, Baca C, Compton WM, et al. Addressing substance abuse in health care settings. Alcohol Clin Exp Res. 2006;30:292-302.
22. Maisel NC, Blodgett JC, Wilbourne PL, et al. Meta-analysis of naltrexone and acamprosate for treating alcohol use disorders: when are these medications most helpful? Addiction. 2013;108:275-293.
23. Rosner S, Leucht S, Lehert P, et al. Acamprosate supports abstinence, naltrexone prevents excessive drinking: evidence from a meta-analysis with unreported outcomes. J Psychopharmacol. 2008;22:11-23.
24. Anton RF, O’Malley SS, Ciraulo DA, et al. Combined pharmacotherapies and behavioral interventions for alcohol dependence: the COMBINE study: a randomized controlled trial. JAMA. 2006;295:2003-2017.
25. Reus VI, Fochtmann LJ, Bukstein O, et al. The American Psychiatric Association Practice Guideline for the Pharmacological Treatment of Patients With Alcohol Use Disorder. Am J Psychiatry. 2018;175:86-90.
1. Grant BF, Goldstein RB, Saha TD, et al. Epidemiology of DSM-5 alcohol use disorder: results from the National Epidemiologic Survey on Alcohol and Related Conditions III. JAMA Psychiatry. 2015;72:757-766.
2. APA. Diagnostic and Statistical Manual of Mental Disorders, 5th ed. Washington DC; 2013.
3. HHS. Results from the 2015 National Survey on Drug Use and Health: summary of national findings. https://www.samhsa.gov/data/sites/default/files/NSDUH-DetTabs-2015/NSDUH-DetTabs-2015/NSDUH-DetTabs-2015.pdf. Accessed November 27, 2018.
4. Rehm J, Mathers C, Popova S, et al. Global burden of disease and injury and economic cost attributable to alcohol use and alcohol-use disorders. Lancet. 2009;373:2223-2233.
5. Chase V, Neild R, Sadler CW, et al. The medical complications of alcohol use: understanding mechanisms to improve management. Drug Alcohol Rev. 2005;24:253-265.
6. Grant BF, Chou SP, Saha TD, et al. Prevalence of 12-month alcohol use, high-risk drinking, and DSM-IV alcohol use disorder in the United States, 2001-2002 to 2012-2013: results from the National Epidemiologic Survey on Alcohol and Related Conditions. JAMA Psychiatry. 2017;74:911-923.
7. Williams EC, Achtmeyer CE, Young JP, et al. Barriers to and facilitators of alcohol use disorder pharmacotherapy in primary care: a qualitative study in five VA clinics. J Gen Intern Med. 2018;33:258-267.
8. Zhang DX, Li ST, Lee QK, et al. Systematic review of guidelines on managing patients with harmful use of alcohol in primary healthcare settings. Alcohol Alcohol. 2017;52:595-609.
9. Wackernah RC, Minnick MJ, Clapp P. Alcohol use disorder: pathophysiology, effects, and pharmacologic options for treatment. Subst Abuse Rehabil. 2014;5:1-12.
10. Kattimani S, Bharadwaj B. Clinical management of alcohol withdrawal: a systematic review. Ind Psychiatry J. 2013;22:100-108.
11. Gowin JL, Sloan ME, Stangl BL, et al. Vulnerability for alcohol use disorder and rate of alcohol consumption. Am J Psychiatry. 2017;174:1094-1101.
12. Moyer VA; Preventive Services Task Force. Screening and behavioral counseling interventions in primary care to reduce alcohol misuse: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2013;159:210-218.
13. Tarter RE, Alterman AI, Edwards KL. Vulnerability to alcoholism in men: a behavior-genetic perspective. J Stud Alcohol. 1985;46:329-356.
14. Brière FN, Rohde P, Seeley JR, et al. Comorbidity between major depression and alcohol use disorder from adolescence to adulthood [published online ahead of print, October 22, 2013]. Compr Psychiatry. 2014;55:526-533. doi: 10.1016/j.comppsych.2013.10.007.
15. Tan CH, Hungerford DW, Denny CH, et al. Screening for alcohol misuse: practices among U.S. primary care providers, DocStyles 2016. Am J Prev Med. 2018;54:173-180.
16. Aertgeerts B, Buntinx F, Kester A. The value of the CAGE in screening for alcohol abuse and alcohol dependence in general clinical populations: a diagnostic meta-analysis. J Clin Epidemiol. 2004;57:30-39.
17. Bush K, Kivlahan DR, McDonell MB, et al. The AUDIT alcohol consumption questions (AUDIT-C): an effective brief screening test for problem drinking. Ambulatory Care Quality Improvement Project (ACQUIP). Alcohol Use Disorders Identification Test. Arch Intern Med. 1998;158:1789-1795.
18. Nanau RM, Neuman MG. Biomolecules and biomarkers used in diagnosis of alcohol drinking and in monitoring therapeutic interventions. Biomolecules. 2015;5:1339-1385.
19. Raddock M, Martukovich R, Berko E, et al. 7 tools to help patients adopt healthier behaviors. J Fam Pract. 2015;64:97-103.
20. AHRQ. Whitlock EP, Green CA, Polen MR, et al. Behavioral Counseling Interventions in Primary Care to Reduce Risky/Harmful Alcohol Use. 2004. https://www.ncbi.nlm.nih.gov/books/NBK42863/. Accessed November 17, 2018.
21. Miller WR, Baca C, Compton WM, et al. Addressing substance abuse in health care settings. Alcohol Clin Exp Res. 2006;30:292-302.
22. Maisel NC, Blodgett JC, Wilbourne PL, et al. Meta-analysis of naltrexone and acamprosate for treating alcohol use disorders: when are these medications most helpful? Addiction. 2013;108:275-293.
23. Rosner S, Leucht S, Lehert P, et al. Acamprosate supports abstinence, naltrexone prevents excessive drinking: evidence from a meta-analysis with unreported outcomes. J Psychopharmacol. 2008;22:11-23.
24. Anton RF, O’Malley SS, Ciraulo DA, et al. Combined pharmacotherapies and behavioral interventions for alcohol dependence: the COMBINE study: a randomized controlled trial. JAMA. 2006;295:2003-2017.
25. Reus VI, Fochtmann LJ, Bukstein O, et al. The American Psychiatric Association Practice Guideline for the Pharmacological Treatment of Patients With Alcohol Use Disorder. Am J Psychiatry. 2018;175:86-90.
How to treat complicated grief
THE CASE
Al* is a 48-year-old patient whose wife, Vera, died of complications from chronic illness 14 months ago. Al thinks about Vera constantly and says he still has difficulty accepting that she is gone. He does not leave the house much anymore and continues to set a place for her at the kitchen table on special occasions. He says, “Some nights in bed, I swear I can hear her in the living room.”
How would you proceed with this patient?
* The names of the patient and his spouse have been changed to protect their identities.
After the loss of a loved one, grief is a natural response to the separation and stress that go along with the death. Most people, after suffering a loss, experience distress that varies in intensity and gradually decreases over time. Thus, the grieving individual does not act as they would normally if they were not bereaved. However, gains are generally made month by month, and most people adjust to the grief and adapt their lives after some time dealing with the absence of the loved one.1
There’s grief, and then there’s complicated grief
For about 2% to 4% of the population who have experienced a significant loss, complicated grief is an issue.2 As its hallmark, complicated grief exceeds the typical amount of time (6-12 months) that people need to recover from a loss. Prevalence has been estimated at 10% to 20% among grieving individuals for whom the death being grieved was that of a romantic partner or child.2 At increased risk for this disorder are women older than 60 years, patients diagnosed with depression or substance abuse, individuals under financial strain, and those who have experienced a violent or sudden loss.3
The Diagnostic and Statistical Manual of Mental Disorders, 5th Edition (DSM-5) has conceptualized complicated grief with the name, persistent complex bereavement disorder (PCBD).4 While the guidelines for the definition are still in progress, several specified symptoms must have been present for at least 6 months to a year or more (TABLE 14). For instance, the patient has been ruminating about the death, has been unable to accept the death, or has felt shocked or numb. They may also experience anger, have difficulty trusting others, and be preoccupied with the deceased (eg, sense they can hear their lost loved one, feel the loved one’s pain for them). Symptoms of PCBD may also include experiencing vivid reminders of the loss and avoiding situations that bring up thoughts about the death.4 (Of note: A grief diagnosis in ICD-10 is captured by the code F43.21; however, there is no specific code for complicated grief or PCBD.)
PCBD is a “condition for further study” in DSM-5; it was omitted from DSM-IV only after much debate. One reason for its omission was concern that clinicians might “pathologize” grief more than it needs to be.5 Grief is regarded as a natural process that might be stymied by a formal diagnosis leading to medical treatment.
Shifting the grief diagnosis paradigm
One new development is that recently bereaved patients can be diagnosed with depression if they meet the criteria for that diagnosis. In the past, someone who met criteria for major depression would be excluded from that diagnosis if the depression ensued from grief. DSM-5 no longer makes that distinction.4 Given this diagnostic shift, one might wonder about the difference between PCBD and depression, particularly if the patient is a grieving individual with a current diagnosis of depression.5
Continue to: Differences between PCBD and major depression
Differences between PCBD and major depression. While antidepressant medication is helpful for patients with moderate-to-severe depression, it has thus far been less helpful for those solely experiencing complicated grief.6 The same holds true for traditional psychotherapy. While family physicians can confidently refer people to psychotherapy for depression, it is not as efficacious as focused therapy designed for those with PCBD.6
Other differences between PCBD and major depression involve the constructs of guilt and yearning. Depressed patients typically feel guilty about a number of things, while those with complicated grief have specific death-focused guilt.7 Depressed people generally do not yearn, while those with grief yearn for their loved one. Finally, and most concerning to clinicians, some patients with PCBD have suicidal thoughts.8 While such thoughts in depression are often linked to hopelessness, suicidal thoughts for grieving individuals are generally driven by a desire to be reunited with the deceased loved one.
While these differences may help in making treatment decisions, there can be overlap between depression and complicated grief. As with many mental health diagnoses, major depression and PCBD are not mutually exclusive.4
The role of hospice. Another factor sometimes associated with complicated grief is any hindrance to the survivor’s ability to communicate or say goodbye to the loved one at the end of life.9 This may be avoided if the loved one is in hospice care and is not subjected to procedures that impair communication (ie, ventilator use, sedation). Medicare requires that certified hospice programs offer bereavement services for 1 year following patient death.10 Some hospice providers even offer bereavement services to those not enrolled in hospice. However, evidence indicates that only about 30% of bereaved caregivers take advantage of hospice bereavement services.11 Family physicians may help patients during this process by providing an early referral to hospice services and recommending bereavement counseling. Referral to hospice care can also facilitate discussions that the patient may need to have with the physician or others regarding spirituality. Hospital chaplains can also be referred or get involved with patients and family upon request.
Assessment focal points and tools
As is the case with most mental health concerns, primary care is at the forefront of early assessment. Evaluation of grief is an ongoing process and is multifactorial. One focus is the intensity of the grief. Is the patient reacting to the loss in a way that is disproportionately severe when compared with others who grieve? Another factor is the time elapsed since the loss. If the loss was more than 6 months ago, the patient should have made some progress. Assess grieving patients at around 6 months post-loss to determine how they are handling grief. As mentioned, DSM-5 has criteria for PCBD that providers can use in determining a patient’s grief status. Also needed are assessments for the other DSM-5 issues often associated with loss: depression and post-traumatic stress disorder.
Continue to: While no clinical measure is perfect...
While no clinical measure is perfect, there are tools that can help in assessing patients for the possibility of complicated grief (TABLE 2). Also keep in mind that no measure can make a diagnosis of PCBD, as it is a clinical judgment, not a score on a scale. Furthermore, there is no measure that can accurately predict future complicated grief.6 In most busy practices, the Brief Grief Questionnaire (http://www.massgeneral.org/psychiatry/assets/Brief_Grief_Questionnaire.pdf ) would be the easiest tool to administer, but a case could be made for any of the measures.
Treatment
The literature base emphasizes that PCBD treatment requires a different focus than that applied to uncomplicated grief. And while most people with major depression will respond to medication and psychotherapy, there are provisos to keep in mind when depression is associated with complicated grief.
Complicated grief treatment (CGT) has been studied extensively.6 This treatment combines some of the tenets of evidence-based PTSD treatments, interpersonal therapy for grief, and cognitive behavioral therapy. CGT is generally an individual treatment, although group therapy using some of its tenets can also be effective. According to complicated grief researchers, tasks to accomplish in CGT include establishing a “new normal” following the loss, promoting self-regulation in the grieving, building social connections, and setting aspirational goals for the future.6 Other goals are to revisit the world, tell stories of the past, and relive old memories in a more positive light. Common suggestions in CGT that run parallel to conventional thoughts on dealing with grief include increasing time outside the home, getting more involved interpersonally, and increasing mindfulness-based practices.
A second-line evidence-based treatment for PCBD is the use of selective serotonin-reuptake inhibitors (SSRIs).6 Some studies have found benefit from SSRI treatment, although the findings are preliminary and modest.12 One observational study examined patients who had recently experienced loss and were receiving CGT with or without medication. Researchers found that CGT with medication (citalopram) led to a 61% positive response rate while CGT alone led to a 41% response rate.13 Thus, findings revealed some benefit to combining an antidepressant with CGT, indicating that SSRIs may be helpful as an adjunct treatment.
THE CASE
Al was treated for complicated grief by his family physician and a psychologist for approximately a year. He responded well to an SSRI and received psychotherapy that focused on the tenets of CGT. Prior to his last psychotherapy visit, he reported leaving the house regularly to dine at restaurants and meet up with co-workers after hours. He said, “I still miss Vera quite a bit, but I know that I feel better.”
CORRESPONDENCE
Scott A. Fields, PhD, 3200 MacCorkle Avenue Southeast, 5th Floor, Robert C. Byrd Clinical Teaching Center, Department of Family Medicine, Charleston, WV 25304; [email protected]
1. Cozza SJ, Fisher JE, Mauro C, et al. Performance of DSM-5 persistent complex bereavement disorder criteria in a community sample of bereaved military family members. Am J Psychiatry. 2016;173:919-929.
2. Kersting A, Brähler E, Glaesmer H, et al. Prevalence of complicated grief in a representative population-based sample. J Affect Disord. 2011;131:339-343.
3. Fujisawa D, Miyashita M, Nakajima S, et al. Prevalence and determinants of complicated grief in general population. J Affect Disord. 2010;127:352-358.
4. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed. Washington, DC: APA Press; 2013.
5. Shear MK, Simon N, Wall M, et al. Complicated grief and related issues for DSM-5. Depress Anxiety. 2011;28:103-117.
6. Shear MK. Clinical Practice. Complicated grief. N Engl J Med. 2015;372:153-160.
7. Wolfelt AD. Counseling Skills for Companioning the Mourner: The Fundamentals of Effective Grief Counseling. Fort Collins, CO: Companion Press; 2016.
8. Szanto K, Shear MK, Houck PR, et al. Indirect self-destructive behavior and overt suicidality in patients with complicated grief. J Clin Psychiatry. 2006;67:233-239.
9. Otani H, Yoshida S, Morita T, et al. Meaningful communication before death, but not present at the time of death itself, is associated with better outcomes on measures of depression and complicated grief among bereaved family members of cancer patients. J Pain Symptom Manage. 2017;54:273-279.
10. CMS. Medicare benefit policy manual: coverage of hospice services under hospital insurance. www.cms.gov/Regulations-and-guidance/Guidance/Manuals/downloads/bp102c09.pdf. Accessed February 25, 2018.
11. Cherlin E, Barry LC, Prigerson H, et al. Bereavement services for family caregivers: how often used, why, and why not. J Palliat Med. 2007;10:148–158.
12. Bui E, Nidal-Vicens M, Simon NM. Pharmacologic approaches to the treatment of complicated grief: rationale and a brief review of the literature. Dialogues Clin Neurosci. 2012;14:149-157.
13. Shear MK, Reynolds CF 3rd, Simon NM, et al. Optimizing treatment of complicated grief: a randomized clinical trial. JAMA Psychiatry. 2016;73:685-694.
THE CASE
Al* is a 48-year-old patient whose wife, Vera, died of complications from chronic illness 14 months ago. Al thinks about Vera constantly and says he still has difficulty accepting that she is gone. He does not leave the house much anymore and continues to set a place for her at the kitchen table on special occasions. He says, “Some nights in bed, I swear I can hear her in the living room.”
How would you proceed with this patient?
* The names of the patient and his spouse have been changed to protect their identities.
After the loss of a loved one, grief is a natural response to the separation and stress that go along with the death. Most people, after suffering a loss, experience distress that varies in intensity and gradually decreases over time. Thus, the grieving individual does not act as they would normally if they were not bereaved. However, gains are generally made month by month, and most people adjust to the grief and adapt their lives after some time dealing with the absence of the loved one.1
There’s grief, and then there’s complicated grief
For about 2% to 4% of the population who have experienced a significant loss, complicated grief is an issue.2 As its hallmark, complicated grief exceeds the typical amount of time (6-12 months) that people need to recover from a loss. Prevalence has been estimated at 10% to 20% among grieving individuals for whom the death being grieved was that of a romantic partner or child.2 At increased risk for this disorder are women older than 60 years, patients diagnosed with depression or substance abuse, individuals under financial strain, and those who have experienced a violent or sudden loss.3
The Diagnostic and Statistical Manual of Mental Disorders, 5th Edition (DSM-5) has conceptualized complicated grief with the name, persistent complex bereavement disorder (PCBD).4 While the guidelines for the definition are still in progress, several specified symptoms must have been present for at least 6 months to a year or more (TABLE 14). For instance, the patient has been ruminating about the death, has been unable to accept the death, or has felt shocked or numb. They may also experience anger, have difficulty trusting others, and be preoccupied with the deceased (eg, sense they can hear their lost loved one, feel the loved one’s pain for them). Symptoms of PCBD may also include experiencing vivid reminders of the loss and avoiding situations that bring up thoughts about the death.4 (Of note: A grief diagnosis in ICD-10 is captured by the code F43.21; however, there is no specific code for complicated grief or PCBD.)

PCBD is a “condition for further study” in DSM-5; it was omitted from DSM-IV only after much debate. One reason for its omission was concern that clinicians might “pathologize” grief more than it needs to be.5 Grief is regarded as a natural process that might be stymied by a formal diagnosis leading to medical treatment.
Shifting the grief diagnosis paradigm
One new development is that recently bereaved patients can be diagnosed with depression if they meet the criteria for that diagnosis. In the past, someone who met criteria for major depression would be excluded from that diagnosis if the depression ensued from grief. DSM-5 no longer makes that distinction.4 Given this diagnostic shift, one might wonder about the difference between PCBD and depression, particularly if the patient is a grieving individual with a current diagnosis of depression.5
Continue to: Differences between PCBD and major depression
Differences between PCBD and major depression. While antidepressant medication is helpful for patients with moderate-to-severe depression, it has thus far been less helpful for those solely experiencing complicated grief.6 The same holds true for traditional psychotherapy. While family physicians can confidently refer people to psychotherapy for depression, it is not as efficacious as focused therapy designed for those with PCBD.6
Other differences between PCBD and major depression involve the constructs of guilt and yearning. Depressed patients typically feel guilty about a number of things, while those with complicated grief have specific death-focused guilt.7 Depressed people generally do not yearn, while those with grief yearn for their loved one. Finally, and most concerning to clinicians, some patients with PCBD have suicidal thoughts.8 While such thoughts in depression are often linked to hopelessness, suicidal thoughts for grieving individuals are generally driven by a desire to be reunited with the deceased loved one.
While these differences may help in making treatment decisions, there can be overlap between depression and complicated grief. As with many mental health diagnoses, major depression and PCBD are not mutually exclusive.4
The role of hospice. Another factor sometimes associated with complicated grief is any hindrance to the survivor’s ability to communicate or say goodbye to the loved one at the end of life.9 This may be avoided if the loved one is in hospice care and is not subjected to procedures that impair communication (ie, ventilator use, sedation). Medicare requires that certified hospice programs offer bereavement services for 1 year following patient death.10 Some hospice providers even offer bereavement services to those not enrolled in hospice. However, evidence indicates that only about 30% of bereaved caregivers take advantage of hospice bereavement services.11 Family physicians may help patients during this process by providing an early referral to hospice services and recommending bereavement counseling. Referral to hospice care can also facilitate discussions that the patient may need to have with the physician or others regarding spirituality. Hospital chaplains can also be referred or get involved with patients and family upon request.
Assessment focal points and tools
As is the case with most mental health concerns, primary care is at the forefront of early assessment. Evaluation of grief is an ongoing process and is multifactorial. One focus is the intensity of the grief. Is the patient reacting to the loss in a way that is disproportionately severe when compared with others who grieve? Another factor is the time elapsed since the loss. If the loss was more than 6 months ago, the patient should have made some progress. Assess grieving patients at around 6 months post-loss to determine how they are handling grief. As mentioned, DSM-5 has criteria for PCBD that providers can use in determining a patient’s grief status. Also needed are assessments for the other DSM-5 issues often associated with loss: depression and post-traumatic stress disorder.
Continue to: While no clinical measure is perfect...
While no clinical measure is perfect, there are tools that can help in assessing patients for the possibility of complicated grief (TABLE 2). Also keep in mind that no measure can make a diagnosis of PCBD, as it is a clinical judgment, not a score on a scale. Furthermore, there is no measure that can accurately predict future complicated grief.6 In most busy practices, the Brief Grief Questionnaire (http://www.massgeneral.org/psychiatry/assets/Brief_Grief_Questionnaire.pdf ) would be the easiest tool to administer, but a case could be made for any of the measures.

Treatment
The literature base emphasizes that PCBD treatment requires a different focus than that applied to uncomplicated grief. And while most people with major depression will respond to medication and psychotherapy, there are provisos to keep in mind when depression is associated with complicated grief.
Complicated grief treatment (CGT) has been studied extensively.6 This treatment combines some of the tenets of evidence-based PTSD treatments, interpersonal therapy for grief, and cognitive behavioral therapy. CGT is generally an individual treatment, although group therapy using some of its tenets can also be effective. According to complicated grief researchers, tasks to accomplish in CGT include establishing a “new normal” following the loss, promoting self-regulation in the grieving, building social connections, and setting aspirational goals for the future.6 Other goals are to revisit the world, tell stories of the past, and relive old memories in a more positive light. Common suggestions in CGT that run parallel to conventional thoughts on dealing with grief include increasing time outside the home, getting more involved interpersonally, and increasing mindfulness-based practices.
A second-line evidence-based treatment for PCBD is the use of selective serotonin-reuptake inhibitors (SSRIs).6 Some studies have found benefit from SSRI treatment, although the findings are preliminary and modest.12 One observational study examined patients who had recently experienced loss and were receiving CGT with or without medication. Researchers found that CGT with medication (citalopram) led to a 61% positive response rate while CGT alone led to a 41% response rate.13 Thus, findings revealed some benefit to combining an antidepressant with CGT, indicating that SSRIs may be helpful as an adjunct treatment.
THE CASE
Al was treated for complicated grief by his family physician and a psychologist for approximately a year. He responded well to an SSRI and received psychotherapy that focused on the tenets of CGT. Prior to his last psychotherapy visit, he reported leaving the house regularly to dine at restaurants and meet up with co-workers after hours. He said, “I still miss Vera quite a bit, but I know that I feel better.”
CORRESPONDENCE
Scott A. Fields, PhD, 3200 MacCorkle Avenue Southeast, 5th Floor, Robert C. Byrd Clinical Teaching Center, Department of Family Medicine, Charleston, WV 25304; [email protected]
THE CASE
Al* is a 48-year-old patient whose wife, Vera, died of complications from chronic illness 14 months ago. Al thinks about Vera constantly and says he still has difficulty accepting that she is gone. He does not leave the house much anymore and continues to set a place for her at the kitchen table on special occasions. He says, “Some nights in bed, I swear I can hear her in the living room.”
How would you proceed with this patient?
* The names of the patient and his spouse have been changed to protect their identities.
After the loss of a loved one, grief is a natural response to the separation and stress that go along with the death. Most people, after suffering a loss, experience distress that varies in intensity and gradually decreases over time. Thus, the grieving individual does not act as they would normally if they were not bereaved. However, gains are generally made month by month, and most people adjust to the grief and adapt their lives after some time dealing with the absence of the loved one.1
There’s grief, and then there’s complicated grief
For about 2% to 4% of the population who have experienced a significant loss, complicated grief is an issue.2 As its hallmark, complicated grief exceeds the typical amount of time (6-12 months) that people need to recover from a loss. Prevalence has been estimated at 10% to 20% among grieving individuals for whom the death being grieved was that of a romantic partner or child.2 At increased risk for this disorder are women older than 60 years, patients diagnosed with depression or substance abuse, individuals under financial strain, and those who have experienced a violent or sudden loss.3
The Diagnostic and Statistical Manual of Mental Disorders, 5th Edition (DSM-5) has conceptualized complicated grief with the name, persistent complex bereavement disorder (PCBD).4 While the guidelines for the definition are still in progress, several specified symptoms must have been present for at least 6 months to a year or more (TABLE 14). For instance, the patient has been ruminating about the death, has been unable to accept the death, or has felt shocked or numb. They may also experience anger, have difficulty trusting others, and be preoccupied with the deceased (eg, sense they can hear their lost loved one, feel the loved one’s pain for them). Symptoms of PCBD may also include experiencing vivid reminders of the loss and avoiding situations that bring up thoughts about the death.4 (Of note: A grief diagnosis in ICD-10 is captured by the code F43.21; however, there is no specific code for complicated grief or PCBD.)

PCBD is a “condition for further study” in DSM-5; it was omitted from DSM-IV only after much debate. One reason for its omission was concern that clinicians might “pathologize” grief more than it needs to be.5 Grief is regarded as a natural process that might be stymied by a formal diagnosis leading to medical treatment.
Shifting the grief diagnosis paradigm
One new development is that recently bereaved patients can be diagnosed with depression if they meet the criteria for that diagnosis. In the past, someone who met criteria for major depression would be excluded from that diagnosis if the depression ensued from grief. DSM-5 no longer makes that distinction.4 Given this diagnostic shift, one might wonder about the difference between PCBD and depression, particularly if the patient is a grieving individual with a current diagnosis of depression.5
Continue to: Differences between PCBD and major depression
Differences between PCBD and major depression. While antidepressant medication is helpful for patients with moderate-to-severe depression, it has thus far been less helpful for those solely experiencing complicated grief.6 The same holds true for traditional psychotherapy. While family physicians can confidently refer people to psychotherapy for depression, it is not as efficacious as focused therapy designed for those with PCBD.6
Other differences between PCBD and major depression involve the constructs of guilt and yearning. Depressed patients typically feel guilty about a number of things, while those with complicated grief have specific death-focused guilt.7 Depressed people generally do not yearn, while those with grief yearn for their loved one. Finally, and most concerning to clinicians, some patients with PCBD have suicidal thoughts.8 While such thoughts in depression are often linked to hopelessness, suicidal thoughts for grieving individuals are generally driven by a desire to be reunited with the deceased loved one.
While these differences may help in making treatment decisions, there can be overlap between depression and complicated grief. As with many mental health diagnoses, major depression and PCBD are not mutually exclusive.4
The role of hospice. Another factor sometimes associated with complicated grief is any hindrance to the survivor’s ability to communicate or say goodbye to the loved one at the end of life.9 This may be avoided if the loved one is in hospice care and is not subjected to procedures that impair communication (ie, ventilator use, sedation). Medicare requires that certified hospice programs offer bereavement services for 1 year following patient death.10 Some hospice providers even offer bereavement services to those not enrolled in hospice. However, evidence indicates that only about 30% of bereaved caregivers take advantage of hospice bereavement services.11 Family physicians may help patients during this process by providing an early referral to hospice services and recommending bereavement counseling. Referral to hospice care can also facilitate discussions that the patient may need to have with the physician or others regarding spirituality. Hospital chaplains can also be referred or get involved with patients and family upon request.
Assessment focal points and tools
As is the case with most mental health concerns, primary care is at the forefront of early assessment. Evaluation of grief is an ongoing process and is multifactorial. One focus is the intensity of the grief. Is the patient reacting to the loss in a way that is disproportionately severe when compared with others who grieve? Another factor is the time elapsed since the loss. If the loss was more than 6 months ago, the patient should have made some progress. Assess grieving patients at around 6 months post-loss to determine how they are handling grief. As mentioned, DSM-5 has criteria for PCBD that providers can use in determining a patient’s grief status. Also needed are assessments for the other DSM-5 issues often associated with loss: depression and post-traumatic stress disorder.
Continue to: While no clinical measure is perfect...
While no clinical measure is perfect, there are tools that can help in assessing patients for the possibility of complicated grief (TABLE 2). Also keep in mind that no measure can make a diagnosis of PCBD, as it is a clinical judgment, not a score on a scale. Furthermore, there is no measure that can accurately predict future complicated grief.6 In most busy practices, the Brief Grief Questionnaire (http://www.massgeneral.org/psychiatry/assets/Brief_Grief_Questionnaire.pdf ) would be the easiest tool to administer, but a case could be made for any of the measures.

Treatment
The literature base emphasizes that PCBD treatment requires a different focus than that applied to uncomplicated grief. And while most people with major depression will respond to medication and psychotherapy, there are provisos to keep in mind when depression is associated with complicated grief.
Complicated grief treatment (CGT) has been studied extensively.6 This treatment combines some of the tenets of evidence-based PTSD treatments, interpersonal therapy for grief, and cognitive behavioral therapy. CGT is generally an individual treatment, although group therapy using some of its tenets can also be effective. According to complicated grief researchers, tasks to accomplish in CGT include establishing a “new normal” following the loss, promoting self-regulation in the grieving, building social connections, and setting aspirational goals for the future.6 Other goals are to revisit the world, tell stories of the past, and relive old memories in a more positive light. Common suggestions in CGT that run parallel to conventional thoughts on dealing with grief include increasing time outside the home, getting more involved interpersonally, and increasing mindfulness-based practices.
A second-line evidence-based treatment for PCBD is the use of selective serotonin-reuptake inhibitors (SSRIs).6 Some studies have found benefit from SSRI treatment, although the findings are preliminary and modest.12 One observational study examined patients who had recently experienced loss and were receiving CGT with or without medication. Researchers found that CGT with medication (citalopram) led to a 61% positive response rate while CGT alone led to a 41% response rate.13 Thus, findings revealed some benefit to combining an antidepressant with CGT, indicating that SSRIs may be helpful as an adjunct treatment.
THE CASE
Al was treated for complicated grief by his family physician and a psychologist for approximately a year. He responded well to an SSRI and received psychotherapy that focused on the tenets of CGT. Prior to his last psychotherapy visit, he reported leaving the house regularly to dine at restaurants and meet up with co-workers after hours. He said, “I still miss Vera quite a bit, but I know that I feel better.”
CORRESPONDENCE
Scott A. Fields, PhD, 3200 MacCorkle Avenue Southeast, 5th Floor, Robert C. Byrd Clinical Teaching Center, Department of Family Medicine, Charleston, WV 25304; [email protected]
1. Cozza SJ, Fisher JE, Mauro C, et al. Performance of DSM-5 persistent complex bereavement disorder criteria in a community sample of bereaved military family members. Am J Psychiatry. 2016;173:919-929.
2. Kersting A, Brähler E, Glaesmer H, et al. Prevalence of complicated grief in a representative population-based sample. J Affect Disord. 2011;131:339-343.
3. Fujisawa D, Miyashita M, Nakajima S, et al. Prevalence and determinants of complicated grief in general population. J Affect Disord. 2010;127:352-358.
4. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed. Washington, DC: APA Press; 2013.
5. Shear MK, Simon N, Wall M, et al. Complicated grief and related issues for DSM-5. Depress Anxiety. 2011;28:103-117.
6. Shear MK. Clinical Practice. Complicated grief. N Engl J Med. 2015;372:153-160.
7. Wolfelt AD. Counseling Skills for Companioning the Mourner: The Fundamentals of Effective Grief Counseling. Fort Collins, CO: Companion Press; 2016.
8. Szanto K, Shear MK, Houck PR, et al. Indirect self-destructive behavior and overt suicidality in patients with complicated grief. J Clin Psychiatry. 2006;67:233-239.
9. Otani H, Yoshida S, Morita T, et al. Meaningful communication before death, but not present at the time of death itself, is associated with better outcomes on measures of depression and complicated grief among bereaved family members of cancer patients. J Pain Symptom Manage. 2017;54:273-279.
10. CMS. Medicare benefit policy manual: coverage of hospice services under hospital insurance. www.cms.gov/Regulations-and-guidance/Guidance/Manuals/downloads/bp102c09.pdf. Accessed February 25, 2018.
11. Cherlin E, Barry LC, Prigerson H, et al. Bereavement services for family caregivers: how often used, why, and why not. J Palliat Med. 2007;10:148–158.
12. Bui E, Nidal-Vicens M, Simon NM. Pharmacologic approaches to the treatment of complicated grief: rationale and a brief review of the literature. Dialogues Clin Neurosci. 2012;14:149-157.
13. Shear MK, Reynolds CF 3rd, Simon NM, et al. Optimizing treatment of complicated grief: a randomized clinical trial. JAMA Psychiatry. 2016;73:685-694.
1. Cozza SJ, Fisher JE, Mauro C, et al. Performance of DSM-5 persistent complex bereavement disorder criteria in a community sample of bereaved military family members. Am J Psychiatry. 2016;173:919-929.
2. Kersting A, Brähler E, Glaesmer H, et al. Prevalence of complicated grief in a representative population-based sample. J Affect Disord. 2011;131:339-343.
3. Fujisawa D, Miyashita M, Nakajima S, et al. Prevalence and determinants of complicated grief in general population. J Affect Disord. 2010;127:352-358.
4. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed. Washington, DC: APA Press; 2013.
5. Shear MK, Simon N, Wall M, et al. Complicated grief and related issues for DSM-5. Depress Anxiety. 2011;28:103-117.
6. Shear MK. Clinical Practice. Complicated grief. N Engl J Med. 2015;372:153-160.
7. Wolfelt AD. Counseling Skills for Companioning the Mourner: The Fundamentals of Effective Grief Counseling. Fort Collins, CO: Companion Press; 2016.
8. Szanto K, Shear MK, Houck PR, et al. Indirect self-destructive behavior and overt suicidality in patients with complicated grief. J Clin Psychiatry. 2006;67:233-239.
9. Otani H, Yoshida S, Morita T, et al. Meaningful communication before death, but not present at the time of death itself, is associated with better outcomes on measures of depression and complicated grief among bereaved family members of cancer patients. J Pain Symptom Manage. 2017;54:273-279.
10. CMS. Medicare benefit policy manual: coverage of hospice services under hospital insurance. www.cms.gov/Regulations-and-guidance/Guidance/Manuals/downloads/bp102c09.pdf. Accessed February 25, 2018.
11. Cherlin E, Barry LC, Prigerson H, et al. Bereavement services for family caregivers: how often used, why, and why not. J Palliat Med. 2007;10:148–158.
12. Bui E, Nidal-Vicens M, Simon NM. Pharmacologic approaches to the treatment of complicated grief: rationale and a brief review of the literature. Dialogues Clin Neurosci. 2012;14:149-157.
13. Shear MK, Reynolds CF 3rd, Simon NM, et al. Optimizing treatment of complicated grief: a randomized clinical trial. JAMA Psychiatry. 2016;73:685-694.




