User login
Difficult patient, or something else? A review of personality disorders
Specific behaviors or expressed thoughts may signal a need for screening. Take into account an individual’s strengths and limitations when designing a Tx approach.
THE CASES
Winston S* is a 23-year-old man referred by a psychiatrist colleague for primary care. He works delivering papers in the early morning hours and spends his day alone in his apartment mainly eating frozen pizza. He has worked solitary jobs his entire life and says he prefers it that way. His answers to questions lack emotion. He doesn’t seem to have any friends or regular contact with family. He follows the medical advice he receives but can’t seem to get out of the house to exercise or socialize. His psychiatrist was treating him with a selective serotonin reuptake inhibitor for depression when he was referred.
Denise L* is a 37-year-old woman who transferred to your practice because she says the previous practice’s office manager was disrespectful and the doctor did not listen to her. She has been “very appreciative” of you and your “well-run office.” You have addressed her fibromyalgia and she has shared several personal details about her life. In the following weeks, you receive several phone calls and messages from her. At a follow-up visit, she asks questions about your family and seems agitated when you hesitate to answer. She questions whether you remember details of her history. She pushes, “Did you remember that, doctor?” She also mentions that your front desk staff seems rude to her.
Ruth B* is an 82-year-old woman whose blood pressure measured in your office is 176/94 mm Hg. When you recommend starting a medication and getting blood tests, she responds with a litany of fearful questions. She seems immobilized by worries about treatment and equally so about the risks of nontreatment. You can’t seem to get past the anxiety to decide on a satisfactory plan. She has to write everything down on a notepad and worries if she does not get every detail.
●
* This patient’s name has been changed to protect his identity. The other 2 patients are an amalgam of patients for whom the authors have provided care.
According to a survey of practicing primary care physicians, as many as 15% of patient encounters can be difficult.1 Demanding, intrusive, or angry patients who reject health care interventions are often-cited sources of these difficulties.2,3 While it is true that patient, physician, and environmental factors may contribute to challenging interactions, some patients who are “difficult” may actually have a personality disorder that requires a distinctive approach to care. Recognizing these patients can help empower physicians to provide compassionate and effective care, reduce team angst, and minimize burnout.
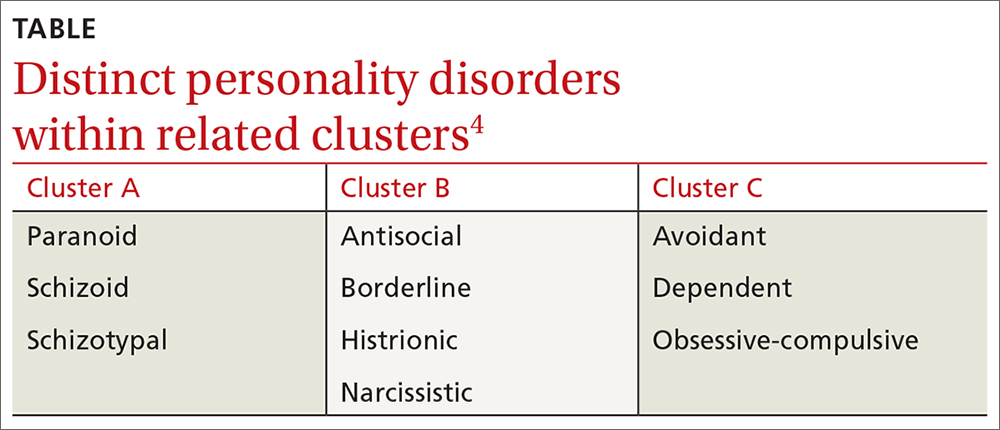
❚ What qualifies as a personality disorder? A personality disorder is an enduring pattern of inner experience and behavior that deviates markedly from the expectations of the individual’s culture, is pervasive and inflexible, has an onset in adolescence or early adulthood, is unchanging over time, and leads to distress or impairment in social or occupational functioning.4 The prevalence of any personality disorder seems to have increased over the past decade from 9.1%4 to 12.16%.5 The Diagnostic and Statistical Manual of Mental Disorders (DSM-5) classifies personality disorders in 3 clusters—A, B, and C (TABLE4)—with prevalence rates at 7.23%, 5.53%, and 6.7%, respectively.5 The review below will focus on the distinct personality disorders exhibited by the patients described in the opening cases.
Continue to: A closer look at the clusters...
A closer look at the clusters
Cluster A disorders
Paranoid, schizoid, and schizotypal disorders are part of this cluster. These patients exhibit odd or eccentric thinking and behavior. Individuals with schizoid personality disorder, for instance, usually lack relationships and lack the desire to acquire and maintain relationships.4 They often organize their lives to remain isolated and will choose occupations that require little social interaction. They sometimes view themselves as observers rather than participants in their own lives.6
Cluster B disorders
Dramatic, overly emotional, or unpredictable thinking and behavior are characteristic of individuals who have antisocial, borderline, histrionic, or narcissistic disorders. Patients with borderline personality disorder (BPD), for example, demonstrate a longstanding pattern of instability in affect, self-image, and relationships.4 Patients with BPD often display extreme interpersonal hypersensitivity and make frantic efforts to avoid real or imagined abandonment. Identity disturbance, feelings of emptiness, and efforts to avoid abandonment have all been associated with increased suicide risk.7
In a primary care setting, such a patient may display extremely strong reactions to minor disappointments. When the physician is unavailable for a last-minute appointment or to authorize an unscheduled medication refill or to receive an after-hours phone call, the patient may become irate. The physician, who previously was idealized by the patient as “the only person who understands me,” is now devalued as “the worst doctor I’ve ever had.”8
Cluster C disorders
With these individuals, anxious or fearful thinking and behavior predominate. Avoidant, dependent, and obsessive-compulsive disorders are included in this cluster.
Dependent personality disorder (DPD) is characterized by a pervasive and extreme need to be taken care of. Submissive and clingy behavior and fear of separation are excessive. This patient may have difficulty making everyday decisions, being assertive, or expressing disagreement with others.4
Obsessive-compulsive personality disorder falls in this cluster and is typified by a pervasive preoccupation with orderliness, perfectionism, and control, at the price of flexibility and efficiency. This individual may be reluctant to get rid of sentimental objects, have rigid moral beliefs, and have significant difficulty working with others who do not follow their rules.4
Continue to: These clues may suggest...
These clues may suggest a personality disorder
If you find that encounters with a particular patient are growing increasingly difficult, consider whether the following behaviors, attitudes, and patterns of thinking are coming into play. If they are, you may want to consider using a screening tool, which we’ll discuss in a moment.
❚ Clues to cluster A disorders
- The patient has no peer relationships outside immediate family.
- The patient almost always chooses solitary activities for work and personal enjoyment.
❚ Cluster B clues
- Hypersensitivity to treatment disagreements or cancelled appointments are common (and likely experienced as rejection).
- Mood changes occur very quickly, even during a single visit.
- There is a history of many failed relationships with providers and others.
- The patient will describe an individual as both “wonderful” and “terrible” (ie, splitting) and may do so during the course of one visit.
- The patient may also split groups (eg, medical staff) by affective extremes (eg, adoration and hatred).
- The patient may hint at suicide or acts of self-harm.7
❚ Cluster C clues
- There is an excessive dependency on family, friends, or providers.
- Significant anxiety is experienced when the patient has to make an independent decision.
- There is a fear of relationship loss and resultant vulnerability to exploitation or abuse.
- Pervasive perfectionism makes treatment planning or course changes difficult.
- Anxiety and fear are unrelieved despite support and ample information.
Consider these screening tools
Several screening tools for personality disorders can be used to follow up on your initial clinical impressions. We also highly recommend you consider concurrent screening for substance abuse, as addiction is a common comorbidity with personality disorders.
❚
❚ A sampling of screening tools. The Standardised Assessment of Personality Abbreviated Scale (SAPAS)9 is an 8-item measure that correlates well with disorders in clusters A and C.
BPD (cluster B) has many brief scale options, including the McLean Screening Instrument for Borderline Personality Disorder (MSI-BPD).10 This 10-item questionnaire demonstrates sensitivity and specificity for BPD.
The International Personality Disorder Examination (IPDE) includes a 15-minute screening tool to help identify patients who may have any personality disorder, regardless of cluster.11
Improve patient encounters with these Tx pearls
In the family medicine clinic, a collaborative primary care and behavioral health team can be extremely helpful in the diagnosis and management of patients with personality disorders.12 First-line treatment of these disorders is psychotherapy, whereas medications are mainly used for symptom management. See Black and colleagues’ work for a thorough discussion on psychopharmacology considerations with personality disorders. 13
The following tips can help you to improve your interactions with patients who have personality disorders.
❚ Cluster A approaches
- Recommend treatment that respects the patient’s need for relative isolation.14
- Don’t be personally offended by your patient’s flat or disinterested affect or concrete thinking; don’t let it diminish the emotional support you provide.6
- Consult with a health psychologist (who has expertise in physical health conditions, brief treatments, and the medical system) to connect the patient with a long-term therapist. It is better to focus on fundamental changes, rather than employing brief behavioral techniques, for symptom relief. Patients with personality disorders tend to have better outcomes with long-term psychological care.15
❚ Cluster B approaches
- Set boundaries—eg, specific time limits for visits—and keep them.8
- Schedule brief, more frequent, appointments to reduce perceived feelings of abandonment.
- Coordinate plans with the entire clinic team to avoid splitting and blaming.16
- Avoid providing patients with personal information, as it may provide fodder for splitting behavior. 8
- Do not take things personally. Let patients “own” their own distress. These patients often take an emotional toll on the provider.16
- Engage the help of a health psychologist to reduce burnout and for more long-term continuity of care. A health psychologist who specializes in dialectical behavioral therapy to work on emotion regulation, distress tolerance, and interpersonal effectiveness would be ideal.17
Continue to: Cluster C approaches...
❚
❚ Cluster C approaches
- Engage the help of family and other trusted individuals in supporting treatment plans.18,19
- Try to provide just 2 treatment choices to the patient and reinforce his or her responsibility to help make the decision collaboratively. This step is important since it is difficult to enhance autonomy in these patients.20
- Engage the help of a cognitive behavioral therapist who can work on assertiveness and problem-solving skills.19
- Be empathetic with the patient and patiently build a trusting relationship, rather than “arguing” with the patient about each specific worry.20
- Make only one change at a time. Give small assignments to the patient, such as monitoring symptoms or reading up on their condition. These can help the patient feel more in control.21
- Present information in brief, clear terms. Avoid “grey areas” to reduce anxiety.21
- Engage a behavioral health provider to reduce rigid expectations and ideally increase feelings of self-esteem; this has been shown to predict better treatment outcomes.22
CASES
Mr. S displays cluster-A characteristics of schizoid personality disorder in addition to the depression he is being treated for. His physician was not put off by his flat affect and respected his limitations with social activities. Use of a stationary bike was recommended for exercise rather than walks outdoors. He also preferred phone calls to in-person encounters, so his follow-up visits were conducted by phone.
Ms. L exhibits cluster-B characteristics of BPD. You begin the tricky dance of setting limits, keeping communication clear, and not blaming yourself or others on your team for Ms. L’s feelings. You schedule regular visits with explicit time limits and discuss with your entire team how to avoid splitting. You involve a psychologist, familiar with treating BPD, who helps the patient learn positive interpersonal coping skills.
Ms. B displays cluster-C characteristics of dependent and obsessive-compulsive personality disorders. At her follow-up visit, you provide a great deal of empathy and try not to argue her out of each worry that she brings up. You make one change at a time and enlist the help of her daughter in giving her pills at home and offering reassurance. You collaborate with a cognitive behavioral therapist who works on exposing her to moderately anxiety-provoking situations/decisions.
1. Hull SK, Broquet K. How to manage difficult patient encounters. Fam Pract Manag. 2007;14:30-34.
2. Groves JE. Taking care of the hateful patient. N Engl J Med.1978;298: 883-887.
3. O’Dowd TC. Five years of heartsink patients in primary care. BMJ. 1988;297:528-530.
4. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th edition (DSM-5). American Psychiatric Publishing; 2013.
5. Volkert J, Gablonski TC, Rabung S. Prevalence of personality disorders in the general adult population in Western countries: systematic review and meta-analysis. Br J Psychiatry. 2018;213:709-715.
6. Esterberg ML, Goulding SM, Walker EF. Cluster A personality disorders: schizotypal, schizoid and paranoid personality disorders in childhood and adolescence. J Psychopathol Behav Assess. 2010;32:515-528.
7. Yen S, Peters JR, Nishar S, et al. Association of borderline personality disorder criteria with suicide attempts: findings from the collaborative longitudinal study of personality disorders over 10 years of follow-up. JAMA Psychiatry. 2021;78:187-194.
8. Dubovsky AN, Kiefer MM. Borderline personality disorder in the primary care setting. Med Clin North Am. 2014;98:1049-1064.
9. Hesse M, Moran P. (2010). Screening for personality disorder with the Standardised Assessment of Personality: Abbreviated Scale (SAPAS): further evidence of concurrent validity. BMC Psychiatry. 2010;10:10.
10. Zanarini MC, Vujanovic AA, Parachini EA, et al. A screening measure for BPD: the McLean screening instrument for borderline personality disorder (MSI-BPD). J Pers Disord. 2003;17:568-573.
11. Loranger AW, Sartorius N, Andreoli A, et al. The International Personality Disorder Examination. The World Health Organization/Alcohol, Drug Abuse, and Mental Health Administration international pilot study of personality disorders. Arch Gen Psychiatry. 1994;51:215-224.12. Nelson KJ, Skodol A, Friedman M. Pharmacotherapy for personality disorders. UpToDate. Accessed April 22, 2021. www.uptodate.com/contents/pharmacotherapy-for-personality-disorders
13. Black D, Paris J, Schulz C. Evidence-based integrated biopsychosocial treatment of borderline personality disorder. In: Muse M (ed). Cognitive Behavioral Psychopharmacology: the Clinical Practice of Evidence-Based Biopsychosocial Integration. Wiley; 2017:137-166.
14. Beck AT, Davis DD, Freeman A. Cognitive Therapy of Personality Disorders. 3rd ed. The Guilford Press; 2015.
15. Thylstrup B, Hesse M. “I am not complaining”–ambivalence construct in schizoid personality disorder. Am J Psychother. 2009;63:147-167.
16. Ricke AK, Lee MJ, Chambers JE. The difficult patient: borderline personality disorder in the obstetrical and gynecological patient. Obstet Gynecol Surv. 2012;67:495-502.
17. Seow LLY, Page AC, Hooke GR. Severity of borderline personality disorder symptoms as a moderator of the association between the use of dialectical behaviour therapy skills and treatment outcomes. Psychother Res. 2020;30:920-933.
18. Nichols WC. Integrative marital and family treatment of dependent personality disorders. In: MacFarlane MM (Ed.) Family Treatment of Personality Disorders: Advances in Clinical Practice. Haworth Clinical Practice Press; 2004:173-204.
19. Disney KL. Dependent personality disorder: a critical review. Clin Psychol Rev. 2013;33:1184-1196.
20. Bender DS. The therapeutic alliance in the treatment of personality disorders. J Psychiatr Pract. 2005;11:73-87.
21. Ward RK. Assessment and management of personality disorders. Am Fam Physician. 2004;70:1505-1512.
22. Cummings JA, Hayes AM, Cardaciotto L, et al. The dynamics of self-esteem in cognitive therapy for avoidant and obsessive-compulsive personality disorders: an adaptive role of self-esteem variability? Cognit Ther Res. 2012;36:272-281.
Specific behaviors or expressed thoughts may signal a need for screening. Take into account an individual’s strengths and limitations when designing a Tx approach.
Specific behaviors or expressed thoughts may signal a need for screening. Take into account an individual’s strengths and limitations when designing a Tx approach.
THE CASES
Winston S* is a 23-year-old man referred by a psychiatrist colleague for primary care. He works delivering papers in the early morning hours and spends his day alone in his apartment mainly eating frozen pizza. He has worked solitary jobs his entire life and says he prefers it that way. His answers to questions lack emotion. He doesn’t seem to have any friends or regular contact with family. He follows the medical advice he receives but can’t seem to get out of the house to exercise or socialize. His psychiatrist was treating him with a selective serotonin reuptake inhibitor for depression when he was referred.
Denise L* is a 37-year-old woman who transferred to your practice because she says the previous practice’s office manager was disrespectful and the doctor did not listen to her. She has been “very appreciative” of you and your “well-run office.” You have addressed her fibromyalgia and she has shared several personal details about her life. In the following weeks, you receive several phone calls and messages from her. At a follow-up visit, she asks questions about your family and seems agitated when you hesitate to answer. She questions whether you remember details of her history. She pushes, “Did you remember that, doctor?” She also mentions that your front desk staff seems rude to her.
Ruth B* is an 82-year-old woman whose blood pressure measured in your office is 176/94 mm Hg. When you recommend starting a medication and getting blood tests, she responds with a litany of fearful questions. She seems immobilized by worries about treatment and equally so about the risks of nontreatment. You can’t seem to get past the anxiety to decide on a satisfactory plan. She has to write everything down on a notepad and worries if she does not get every detail.
●
* This patient’s name has been changed to protect his identity. The other 2 patients are an amalgam of patients for whom the authors have provided care.
According to a survey of practicing primary care physicians, as many as 15% of patient encounters can be difficult.1 Demanding, intrusive, or angry patients who reject health care interventions are often-cited sources of these difficulties.2,3 While it is true that patient, physician, and environmental factors may contribute to challenging interactions, some patients who are “difficult” may actually have a personality disorder that requires a distinctive approach to care. Recognizing these patients can help empower physicians to provide compassionate and effective care, reduce team angst, and minimize burnout.
❚ What qualifies as a personality disorder? A personality disorder is an enduring pattern of inner experience and behavior that deviates markedly from the expectations of the individual’s culture, is pervasive and inflexible, has an onset in adolescence or early adulthood, is unchanging over time, and leads to distress or impairment in social or occupational functioning.4 The prevalence of any personality disorder seems to have increased over the past decade from 9.1%4 to 12.16%.5 The Diagnostic and Statistical Manual of Mental Disorders (DSM-5) classifies personality disorders in 3 clusters—A, B, and C (TABLE4)—with prevalence rates at 7.23%, 5.53%, and 6.7%, respectively.5 The review below will focus on the distinct personality disorders exhibited by the patients described in the opening cases.

Continue to: A closer look at the clusters...
A closer look at the clusters
Cluster A disorders
Paranoid, schizoid, and schizotypal disorders are part of this cluster. These patients exhibit odd or eccentric thinking and behavior. Individuals with schizoid personality disorder, for instance, usually lack relationships and lack the desire to acquire and maintain relationships.4 They often organize their lives to remain isolated and will choose occupations that require little social interaction. They sometimes view themselves as observers rather than participants in their own lives.6
Cluster B disorders
Dramatic, overly emotional, or unpredictable thinking and behavior are characteristic of individuals who have antisocial, borderline, histrionic, or narcissistic disorders. Patients with borderline personality disorder (BPD), for example, demonstrate a longstanding pattern of instability in affect, self-image, and relationships.4 Patients with BPD often display extreme interpersonal hypersensitivity and make frantic efforts to avoid real or imagined abandonment. Identity disturbance, feelings of emptiness, and efforts to avoid abandonment have all been associated with increased suicide risk.7
In a primary care setting, such a patient may display extremely strong reactions to minor disappointments. When the physician is unavailable for a last-minute appointment or to authorize an unscheduled medication refill or to receive an after-hours phone call, the patient may become irate. The physician, who previously was idealized by the patient as “the only person who understands me,” is now devalued as “the worst doctor I’ve ever had.”8
Cluster C disorders
With these individuals, anxious or fearful thinking and behavior predominate. Avoidant, dependent, and obsessive-compulsive disorders are included in this cluster.
Dependent personality disorder (DPD) is characterized by a pervasive and extreme need to be taken care of. Submissive and clingy behavior and fear of separation are excessive. This patient may have difficulty making everyday decisions, being assertive, or expressing disagreement with others.4
Obsessive-compulsive personality disorder falls in this cluster and is typified by a pervasive preoccupation with orderliness, perfectionism, and control, at the price of flexibility and efficiency. This individual may be reluctant to get rid of sentimental objects, have rigid moral beliefs, and have significant difficulty working with others who do not follow their rules.4
Continue to: These clues may suggest...
These clues may suggest a personality disorder
If you find that encounters with a particular patient are growing increasingly difficult, consider whether the following behaviors, attitudes, and patterns of thinking are coming into play. If they are, you may want to consider using a screening tool, which we’ll discuss in a moment.
❚ Clues to cluster A disorders
- The patient has no peer relationships outside immediate family.
- The patient almost always chooses solitary activities for work and personal enjoyment.
❚ Cluster B clues
- Hypersensitivity to treatment disagreements or cancelled appointments are common (and likely experienced as rejection).
- Mood changes occur very quickly, even during a single visit.
- There is a history of many failed relationships with providers and others.
- The patient will describe an individual as both “wonderful” and “terrible” (ie, splitting) and may do so during the course of one visit.
- The patient may also split groups (eg, medical staff) by affective extremes (eg, adoration and hatred).
- The patient may hint at suicide or acts of self-harm.7
❚ Cluster C clues
- There is an excessive dependency on family, friends, or providers.
- Significant anxiety is experienced when the patient has to make an independent decision.
- There is a fear of relationship loss and resultant vulnerability to exploitation or abuse.
- Pervasive perfectionism makes treatment planning or course changes difficult.
- Anxiety and fear are unrelieved despite support and ample information.
Consider these screening tools
Several screening tools for personality disorders can be used to follow up on your initial clinical impressions. We also highly recommend you consider concurrent screening for substance abuse, as addiction is a common comorbidity with personality disorders.
❚
❚ A sampling of screening tools. The Standardised Assessment of Personality Abbreviated Scale (SAPAS)9 is an 8-item measure that correlates well with disorders in clusters A and C.
BPD (cluster B) has many brief scale options, including the McLean Screening Instrument for Borderline Personality Disorder (MSI-BPD).10 This 10-item questionnaire demonstrates sensitivity and specificity for BPD.
The International Personality Disorder Examination (IPDE) includes a 15-minute screening tool to help identify patients who may have any personality disorder, regardless of cluster.11
Improve patient encounters with these Tx pearls
In the family medicine clinic, a collaborative primary care and behavioral health team can be extremely helpful in the diagnosis and management of patients with personality disorders.12 First-line treatment of these disorders is psychotherapy, whereas medications are mainly used for symptom management. See Black and colleagues’ work for a thorough discussion on psychopharmacology considerations with personality disorders. 13
The following tips can help you to improve your interactions with patients who have personality disorders.
❚ Cluster A approaches
- Recommend treatment that respects the patient’s need for relative isolation.14
- Don’t be personally offended by your patient’s flat or disinterested affect or concrete thinking; don’t let it diminish the emotional support you provide.6
- Consult with a health psychologist (who has expertise in physical health conditions, brief treatments, and the medical system) to connect the patient with a long-term therapist. It is better to focus on fundamental changes, rather than employing brief behavioral techniques, for symptom relief. Patients with personality disorders tend to have better outcomes with long-term psychological care.15
❚ Cluster B approaches
- Set boundaries—eg, specific time limits for visits—and keep them.8
- Schedule brief, more frequent, appointments to reduce perceived feelings of abandonment.
- Coordinate plans with the entire clinic team to avoid splitting and blaming.16
- Avoid providing patients with personal information, as it may provide fodder for splitting behavior. 8
- Do not take things personally. Let patients “own” their own distress. These patients often take an emotional toll on the provider.16
- Engage the help of a health psychologist to reduce burnout and for more long-term continuity of care. A health psychologist who specializes in dialectical behavioral therapy to work on emotion regulation, distress tolerance, and interpersonal effectiveness would be ideal.17
Continue to: Cluster C approaches...
❚
❚ Cluster C approaches
- Engage the help of family and other trusted individuals in supporting treatment plans.18,19
- Try to provide just 2 treatment choices to the patient and reinforce his or her responsibility to help make the decision collaboratively. This step is important since it is difficult to enhance autonomy in these patients.20
- Engage the help of a cognitive behavioral therapist who can work on assertiveness and problem-solving skills.19
- Be empathetic with the patient and patiently build a trusting relationship, rather than “arguing” with the patient about each specific worry.20
- Make only one change at a time. Give small assignments to the patient, such as monitoring symptoms or reading up on their condition. These can help the patient feel more in control.21
- Present information in brief, clear terms. Avoid “grey areas” to reduce anxiety.21
- Engage a behavioral health provider to reduce rigid expectations and ideally increase feelings of self-esteem; this has been shown to predict better treatment outcomes.22
CASES
Mr. S displays cluster-A characteristics of schizoid personality disorder in addition to the depression he is being treated for. His physician was not put off by his flat affect and respected his limitations with social activities. Use of a stationary bike was recommended for exercise rather than walks outdoors. He also preferred phone calls to in-person encounters, so his follow-up visits were conducted by phone.
Ms. L exhibits cluster-B characteristics of BPD. You begin the tricky dance of setting limits, keeping communication clear, and not blaming yourself or others on your team for Ms. L’s feelings. You schedule regular visits with explicit time limits and discuss with your entire team how to avoid splitting. You involve a psychologist, familiar with treating BPD, who helps the patient learn positive interpersonal coping skills.
Ms. B displays cluster-C characteristics of dependent and obsessive-compulsive personality disorders. At her follow-up visit, you provide a great deal of empathy and try not to argue her out of each worry that she brings up. You make one change at a time and enlist the help of her daughter in giving her pills at home and offering reassurance. You collaborate with a cognitive behavioral therapist who works on exposing her to moderately anxiety-provoking situations/decisions.
THE CASES
Winston S* is a 23-year-old man referred by a psychiatrist colleague for primary care. He works delivering papers in the early morning hours and spends his day alone in his apartment mainly eating frozen pizza. He has worked solitary jobs his entire life and says he prefers it that way. His answers to questions lack emotion. He doesn’t seem to have any friends or regular contact with family. He follows the medical advice he receives but can’t seem to get out of the house to exercise or socialize. His psychiatrist was treating him with a selective serotonin reuptake inhibitor for depression when he was referred.
Denise L* is a 37-year-old woman who transferred to your practice because she says the previous practice’s office manager was disrespectful and the doctor did not listen to her. She has been “very appreciative” of you and your “well-run office.” You have addressed her fibromyalgia and she has shared several personal details about her life. In the following weeks, you receive several phone calls and messages from her. At a follow-up visit, she asks questions about your family and seems agitated when you hesitate to answer. She questions whether you remember details of her history. She pushes, “Did you remember that, doctor?” She also mentions that your front desk staff seems rude to her.
Ruth B* is an 82-year-old woman whose blood pressure measured in your office is 176/94 mm Hg. When you recommend starting a medication and getting blood tests, she responds with a litany of fearful questions. She seems immobilized by worries about treatment and equally so about the risks of nontreatment. You can’t seem to get past the anxiety to decide on a satisfactory plan. She has to write everything down on a notepad and worries if she does not get every detail.
●
* This patient’s name has been changed to protect his identity. The other 2 patients are an amalgam of patients for whom the authors have provided care.
According to a survey of practicing primary care physicians, as many as 15% of patient encounters can be difficult.1 Demanding, intrusive, or angry patients who reject health care interventions are often-cited sources of these difficulties.2,3 While it is true that patient, physician, and environmental factors may contribute to challenging interactions, some patients who are “difficult” may actually have a personality disorder that requires a distinctive approach to care. Recognizing these patients can help empower physicians to provide compassionate and effective care, reduce team angst, and minimize burnout.
❚ What qualifies as a personality disorder? A personality disorder is an enduring pattern of inner experience and behavior that deviates markedly from the expectations of the individual’s culture, is pervasive and inflexible, has an onset in adolescence or early adulthood, is unchanging over time, and leads to distress or impairment in social or occupational functioning.4 The prevalence of any personality disorder seems to have increased over the past decade from 9.1%4 to 12.16%.5 The Diagnostic and Statistical Manual of Mental Disorders (DSM-5) classifies personality disorders in 3 clusters—A, B, and C (TABLE4)—with prevalence rates at 7.23%, 5.53%, and 6.7%, respectively.5 The review below will focus on the distinct personality disorders exhibited by the patients described in the opening cases.

Continue to: A closer look at the clusters...
A closer look at the clusters
Cluster A disorders
Paranoid, schizoid, and schizotypal disorders are part of this cluster. These patients exhibit odd or eccentric thinking and behavior. Individuals with schizoid personality disorder, for instance, usually lack relationships and lack the desire to acquire and maintain relationships.4 They often organize their lives to remain isolated and will choose occupations that require little social interaction. They sometimes view themselves as observers rather than participants in their own lives.6
Cluster B disorders
Dramatic, overly emotional, or unpredictable thinking and behavior are characteristic of individuals who have antisocial, borderline, histrionic, or narcissistic disorders. Patients with borderline personality disorder (BPD), for example, demonstrate a longstanding pattern of instability in affect, self-image, and relationships.4 Patients with BPD often display extreme interpersonal hypersensitivity and make frantic efforts to avoid real or imagined abandonment. Identity disturbance, feelings of emptiness, and efforts to avoid abandonment have all been associated with increased suicide risk.7
In a primary care setting, such a patient may display extremely strong reactions to minor disappointments. When the physician is unavailable for a last-minute appointment or to authorize an unscheduled medication refill or to receive an after-hours phone call, the patient may become irate. The physician, who previously was idealized by the patient as “the only person who understands me,” is now devalued as “the worst doctor I’ve ever had.”8
Cluster C disorders
With these individuals, anxious or fearful thinking and behavior predominate. Avoidant, dependent, and obsessive-compulsive disorders are included in this cluster.
Dependent personality disorder (DPD) is characterized by a pervasive and extreme need to be taken care of. Submissive and clingy behavior and fear of separation are excessive. This patient may have difficulty making everyday decisions, being assertive, or expressing disagreement with others.4
Obsessive-compulsive personality disorder falls in this cluster and is typified by a pervasive preoccupation with orderliness, perfectionism, and control, at the price of flexibility and efficiency. This individual may be reluctant to get rid of sentimental objects, have rigid moral beliefs, and have significant difficulty working with others who do not follow their rules.4
Continue to: These clues may suggest...
These clues may suggest a personality disorder
If you find that encounters with a particular patient are growing increasingly difficult, consider whether the following behaviors, attitudes, and patterns of thinking are coming into play. If they are, you may want to consider using a screening tool, which we’ll discuss in a moment.
❚ Clues to cluster A disorders
- The patient has no peer relationships outside immediate family.
- The patient almost always chooses solitary activities for work and personal enjoyment.
❚ Cluster B clues
- Hypersensitivity to treatment disagreements or cancelled appointments are common (and likely experienced as rejection).
- Mood changes occur very quickly, even during a single visit.
- There is a history of many failed relationships with providers and others.
- The patient will describe an individual as both “wonderful” and “terrible” (ie, splitting) and may do so during the course of one visit.
- The patient may also split groups (eg, medical staff) by affective extremes (eg, adoration and hatred).
- The patient may hint at suicide or acts of self-harm.7
❚ Cluster C clues
- There is an excessive dependency on family, friends, or providers.
- Significant anxiety is experienced when the patient has to make an independent decision.
- There is a fear of relationship loss and resultant vulnerability to exploitation or abuse.
- Pervasive perfectionism makes treatment planning or course changes difficult.
- Anxiety and fear are unrelieved despite support and ample information.
Consider these screening tools
Several screening tools for personality disorders can be used to follow up on your initial clinical impressions. We also highly recommend you consider concurrent screening for substance abuse, as addiction is a common comorbidity with personality disorders.
❚
❚ A sampling of screening tools. The Standardised Assessment of Personality Abbreviated Scale (SAPAS)9 is an 8-item measure that correlates well with disorders in clusters A and C.
BPD (cluster B) has many brief scale options, including the McLean Screening Instrument for Borderline Personality Disorder (MSI-BPD).10 This 10-item questionnaire demonstrates sensitivity and specificity for BPD.
The International Personality Disorder Examination (IPDE) includes a 15-minute screening tool to help identify patients who may have any personality disorder, regardless of cluster.11
Improve patient encounters with these Tx pearls
In the family medicine clinic, a collaborative primary care and behavioral health team can be extremely helpful in the diagnosis and management of patients with personality disorders.12 First-line treatment of these disorders is psychotherapy, whereas medications are mainly used for symptom management. See Black and colleagues’ work for a thorough discussion on psychopharmacology considerations with personality disorders. 13
The following tips can help you to improve your interactions with patients who have personality disorders.
❚ Cluster A approaches
- Recommend treatment that respects the patient’s need for relative isolation.14
- Don’t be personally offended by your patient’s flat or disinterested affect or concrete thinking; don’t let it diminish the emotional support you provide.6
- Consult with a health psychologist (who has expertise in physical health conditions, brief treatments, and the medical system) to connect the patient with a long-term therapist. It is better to focus on fundamental changes, rather than employing brief behavioral techniques, for symptom relief. Patients with personality disorders tend to have better outcomes with long-term psychological care.15
❚ Cluster B approaches
- Set boundaries—eg, specific time limits for visits—and keep them.8
- Schedule brief, more frequent, appointments to reduce perceived feelings of abandonment.
- Coordinate plans with the entire clinic team to avoid splitting and blaming.16
- Avoid providing patients with personal information, as it may provide fodder for splitting behavior. 8
- Do not take things personally. Let patients “own” their own distress. These patients often take an emotional toll on the provider.16
- Engage the help of a health psychologist to reduce burnout and for more long-term continuity of care. A health psychologist who specializes in dialectical behavioral therapy to work on emotion regulation, distress tolerance, and interpersonal effectiveness would be ideal.17
Continue to: Cluster C approaches...
❚
❚ Cluster C approaches
- Engage the help of family and other trusted individuals in supporting treatment plans.18,19
- Try to provide just 2 treatment choices to the patient and reinforce his or her responsibility to help make the decision collaboratively. This step is important since it is difficult to enhance autonomy in these patients.20
- Engage the help of a cognitive behavioral therapist who can work on assertiveness and problem-solving skills.19
- Be empathetic with the patient and patiently build a trusting relationship, rather than “arguing” with the patient about each specific worry.20
- Make only one change at a time. Give small assignments to the patient, such as monitoring symptoms or reading up on their condition. These can help the patient feel more in control.21
- Present information in brief, clear terms. Avoid “grey areas” to reduce anxiety.21
- Engage a behavioral health provider to reduce rigid expectations and ideally increase feelings of self-esteem; this has been shown to predict better treatment outcomes.22
CASES
Mr. S displays cluster-A characteristics of schizoid personality disorder in addition to the depression he is being treated for. His physician was not put off by his flat affect and respected his limitations with social activities. Use of a stationary bike was recommended for exercise rather than walks outdoors. He also preferred phone calls to in-person encounters, so his follow-up visits were conducted by phone.
Ms. L exhibits cluster-B characteristics of BPD. You begin the tricky dance of setting limits, keeping communication clear, and not blaming yourself or others on your team for Ms. L’s feelings. You schedule regular visits with explicit time limits and discuss with your entire team how to avoid splitting. You involve a psychologist, familiar with treating BPD, who helps the patient learn positive interpersonal coping skills.
Ms. B displays cluster-C characteristics of dependent and obsessive-compulsive personality disorders. At her follow-up visit, you provide a great deal of empathy and try not to argue her out of each worry that she brings up. You make one change at a time and enlist the help of her daughter in giving her pills at home and offering reassurance. You collaborate with a cognitive behavioral therapist who works on exposing her to moderately anxiety-provoking situations/decisions.
1. Hull SK, Broquet K. How to manage difficult patient encounters. Fam Pract Manag. 2007;14:30-34.
2. Groves JE. Taking care of the hateful patient. N Engl J Med.1978;298: 883-887.
3. O’Dowd TC. Five years of heartsink patients in primary care. BMJ. 1988;297:528-530.
4. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th edition (DSM-5). American Psychiatric Publishing; 2013.
5. Volkert J, Gablonski TC, Rabung S. Prevalence of personality disorders in the general adult population in Western countries: systematic review and meta-analysis. Br J Psychiatry. 2018;213:709-715.
6. Esterberg ML, Goulding SM, Walker EF. Cluster A personality disorders: schizotypal, schizoid and paranoid personality disorders in childhood and adolescence. J Psychopathol Behav Assess. 2010;32:515-528.
7. Yen S, Peters JR, Nishar S, et al. Association of borderline personality disorder criteria with suicide attempts: findings from the collaborative longitudinal study of personality disorders over 10 years of follow-up. JAMA Psychiatry. 2021;78:187-194.
8. Dubovsky AN, Kiefer MM. Borderline personality disorder in the primary care setting. Med Clin North Am. 2014;98:1049-1064.
9. Hesse M, Moran P. (2010). Screening for personality disorder with the Standardised Assessment of Personality: Abbreviated Scale (SAPAS): further evidence of concurrent validity. BMC Psychiatry. 2010;10:10.
10. Zanarini MC, Vujanovic AA, Parachini EA, et al. A screening measure for BPD: the McLean screening instrument for borderline personality disorder (MSI-BPD). J Pers Disord. 2003;17:568-573.
11. Loranger AW, Sartorius N, Andreoli A, et al. The International Personality Disorder Examination. The World Health Organization/Alcohol, Drug Abuse, and Mental Health Administration international pilot study of personality disorders. Arch Gen Psychiatry. 1994;51:215-224.12. Nelson KJ, Skodol A, Friedman M. Pharmacotherapy for personality disorders. UpToDate. Accessed April 22, 2021. www.uptodate.com/contents/pharmacotherapy-for-personality-disorders
13. Black D, Paris J, Schulz C. Evidence-based integrated biopsychosocial treatment of borderline personality disorder. In: Muse M (ed). Cognitive Behavioral Psychopharmacology: the Clinical Practice of Evidence-Based Biopsychosocial Integration. Wiley; 2017:137-166.
14. Beck AT, Davis DD, Freeman A. Cognitive Therapy of Personality Disorders. 3rd ed. The Guilford Press; 2015.
15. Thylstrup B, Hesse M. “I am not complaining”–ambivalence construct in schizoid personality disorder. Am J Psychother. 2009;63:147-167.
16. Ricke AK, Lee MJ, Chambers JE. The difficult patient: borderline personality disorder in the obstetrical and gynecological patient. Obstet Gynecol Surv. 2012;67:495-502.
17. Seow LLY, Page AC, Hooke GR. Severity of borderline personality disorder symptoms as a moderator of the association between the use of dialectical behaviour therapy skills and treatment outcomes. Psychother Res. 2020;30:920-933.
18. Nichols WC. Integrative marital and family treatment of dependent personality disorders. In: MacFarlane MM (Ed.) Family Treatment of Personality Disorders: Advances in Clinical Practice. Haworth Clinical Practice Press; 2004:173-204.
19. Disney KL. Dependent personality disorder: a critical review. Clin Psychol Rev. 2013;33:1184-1196.
20. Bender DS. The therapeutic alliance in the treatment of personality disorders. J Psychiatr Pract. 2005;11:73-87.
21. Ward RK. Assessment and management of personality disorders. Am Fam Physician. 2004;70:1505-1512.
22. Cummings JA, Hayes AM, Cardaciotto L, et al. The dynamics of self-esteem in cognitive therapy for avoidant and obsessive-compulsive personality disorders: an adaptive role of self-esteem variability? Cognit Ther Res. 2012;36:272-281.
1. Hull SK, Broquet K. How to manage difficult patient encounters. Fam Pract Manag. 2007;14:30-34.
2. Groves JE. Taking care of the hateful patient. N Engl J Med.1978;298: 883-887.
3. O’Dowd TC. Five years of heartsink patients in primary care. BMJ. 1988;297:528-530.
4. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th edition (DSM-5). American Psychiatric Publishing; 2013.
5. Volkert J, Gablonski TC, Rabung S. Prevalence of personality disorders in the general adult population in Western countries: systematic review and meta-analysis. Br J Psychiatry. 2018;213:709-715.
6. Esterberg ML, Goulding SM, Walker EF. Cluster A personality disorders: schizotypal, schizoid and paranoid personality disorders in childhood and adolescence. J Psychopathol Behav Assess. 2010;32:515-528.
7. Yen S, Peters JR, Nishar S, et al. Association of borderline personality disorder criteria with suicide attempts: findings from the collaborative longitudinal study of personality disorders over 10 years of follow-up. JAMA Psychiatry. 2021;78:187-194.
8. Dubovsky AN, Kiefer MM. Borderline personality disorder in the primary care setting. Med Clin North Am. 2014;98:1049-1064.
9. Hesse M, Moran P. (2010). Screening for personality disorder with the Standardised Assessment of Personality: Abbreviated Scale (SAPAS): further evidence of concurrent validity. BMC Psychiatry. 2010;10:10.
10. Zanarini MC, Vujanovic AA, Parachini EA, et al. A screening measure for BPD: the McLean screening instrument for borderline personality disorder (MSI-BPD). J Pers Disord. 2003;17:568-573.
11. Loranger AW, Sartorius N, Andreoli A, et al. The International Personality Disorder Examination. The World Health Organization/Alcohol, Drug Abuse, and Mental Health Administration international pilot study of personality disorders. Arch Gen Psychiatry. 1994;51:215-224.12. Nelson KJ, Skodol A, Friedman M. Pharmacotherapy for personality disorders. UpToDate. Accessed April 22, 2021. www.uptodate.com/contents/pharmacotherapy-for-personality-disorders
13. Black D, Paris J, Schulz C. Evidence-based integrated biopsychosocial treatment of borderline personality disorder. In: Muse M (ed). Cognitive Behavioral Psychopharmacology: the Clinical Practice of Evidence-Based Biopsychosocial Integration. Wiley; 2017:137-166.
14. Beck AT, Davis DD, Freeman A. Cognitive Therapy of Personality Disorders. 3rd ed. The Guilford Press; 2015.
15. Thylstrup B, Hesse M. “I am not complaining”–ambivalence construct in schizoid personality disorder. Am J Psychother. 2009;63:147-167.
16. Ricke AK, Lee MJ, Chambers JE. The difficult patient: borderline personality disorder in the obstetrical and gynecological patient. Obstet Gynecol Surv. 2012;67:495-502.
17. Seow LLY, Page AC, Hooke GR. Severity of borderline personality disorder symptoms as a moderator of the association between the use of dialectical behaviour therapy skills and treatment outcomes. Psychother Res. 2020;30:920-933.
18. Nichols WC. Integrative marital and family treatment of dependent personality disorders. In: MacFarlane MM (Ed.) Family Treatment of Personality Disorders: Advances in Clinical Practice. Haworth Clinical Practice Press; 2004:173-204.
19. Disney KL. Dependent personality disorder: a critical review. Clin Psychol Rev. 2013;33:1184-1196.
20. Bender DS. The therapeutic alliance in the treatment of personality disorders. J Psychiatr Pract. 2005;11:73-87.
21. Ward RK. Assessment and management of personality disorders. Am Fam Physician. 2004;70:1505-1512.
22. Cummings JA, Hayes AM, Cardaciotto L, et al. The dynamics of self-esteem in cognitive therapy for avoidant and obsessive-compulsive personality disorders: an adaptive role of self-esteem variability? Cognit Ther Res. 2012;36:272-281.
Alcohol use disorder: How best to screen and intervene
THE CASE
Ms. E, a 42-year-old woman, visited her new physician for a physical exam. When asked about alcohol intake, she reported that she drank 3 to 4 beers after work and sometimes 5 to 8 beers a day on the weekends. Occasionally, she exceeded those amounts, but she didn’t feel guilty about her drinking. She was often late to work and said her relationship with her boyfriend was strained. A review of systems was positive for fatigue, poor concentration, abdominal pain, and weight gain. Her body mass index was 41, pulse 100 beats/min, blood pressure 125/75 mm Hg, and she was afebrile. Her physical exam was otherwise within normal limits.
How would you proceed with this patient?
Alcohol use disorder (AUD) is a common and often untreated condition that is increasingly prevalent in the United States.1 The Diagnostic and Statistical Manual of Mental Disorders, 5th Edition (DSM-5) characterizes AUD as a combination of signs and symptoms typifying alcohol abuse and dependence (discussed in a bit).2
Data from the 2015 National Survey on Drug Use and Health (NSDUH) showed 15.7 million Americans with AUD, affecting 6.2% of the population ages 18 years or older and 2.5% of adolescents ages 12 to 17 years.3
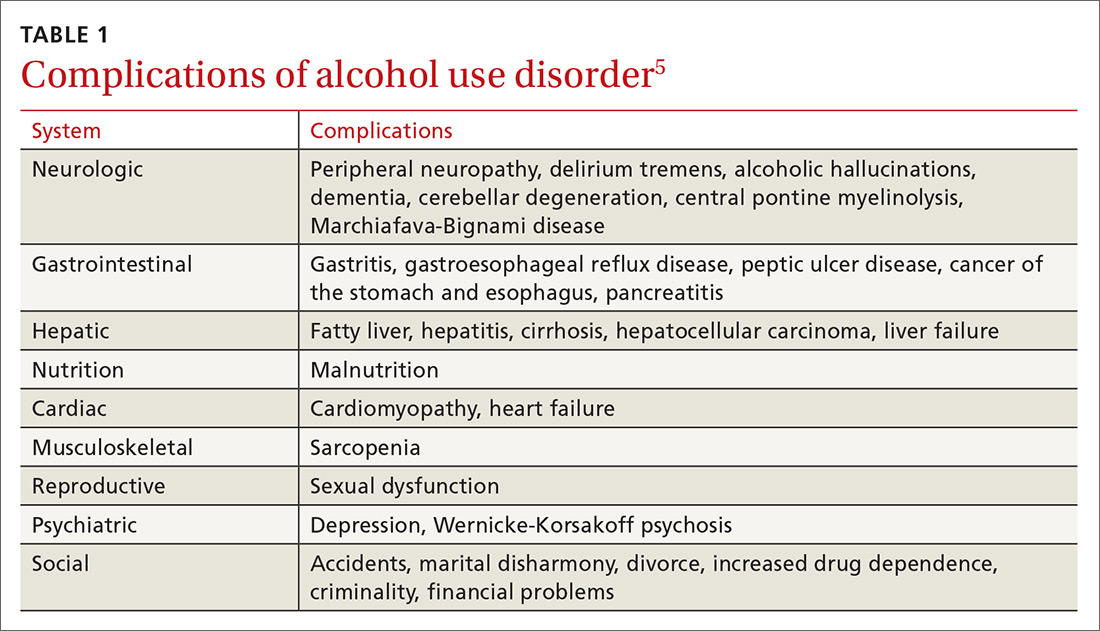
Alcohol use and AUD account for an estimated 3.8% of all global deaths and 4.6% of global disability-adjusted life years.4 AUD adversely affects several systems (TABLE 15), and patients with AUD are sicker and more likely to die younger than those without AUD.4 In the United States, prevalence of AUD has increased in recent years among women, older adults, racial minorities, and individuals with a low education level.6
Screening for AUD is reasonable and straightforward, although diagnosis and treatment of AUD in primary care settings may be challenging due to competing clinical priorities; lack of training, resources, and support; and skepticism about the efficacy of behavioral and pharmacologic treatments.7,8 However, family physicians are in an excellent position to diagnose and help address the complex biopsychosocial needs of patients with AUD, often in collaboration with colleagues and community organizations.
Signs and symptoms of AUD
In clinical practice, at least 2 of the following 11 behaviors or symptoms are required to diagnose AUD2:
- consuming larger amounts of alcohol over a longer period than intended
- persistent desire or unsuccessful efforts to cut down or control alcohol use
- making a significant effort to obtain, use, or recover from alcohol
In moderate-to-severe cases:
- cravings or urges to use alcohol
- recurrent failure to fulfill major work, school, or social obligations
- continued alcohol use despite recurrent social and interpersonal problems
- giving up social, occupational, and recreational activities due to alcohol
- using alcohol in physically dangerous situations
- continued alcohol use despite having physical or psychological problems
- tolerance to alcohol’s effects
- withdrawal symptoms.
Continue to: Patients meet criteria for mild AUD severity if...
Patients meet criteria for mild AUD severity if they exhibit 2 or 3 symptoms, moderate AUD with 4 or 5 symptoms, and severe AUD if there are 6 or more symptoms.2
Those who meet criteria for AUD and are able to stop using alcohol are deemed to be in early remission if the criteria have gone unfulfilled for at least 3 months and less than 12 months. Patients are considered to be in sustained remission if they have not met criteria for AUD at any time during a period of 12 months or longer.
How to detect AUD
Several clues in a patient’s history can suggest AUD (TABLE 29,10). Most imbibers are unaware of the dangers and may consider themselves merely “social drinkers.” Binge drinking may be an early indicator of vulnerability to AUD and should be assessed as part of a thorough clinical evaluation.11 The US Preventive Services Task Force (USPSTF) recommends (Grade B) that clinicians screen adults ages 18 years or older for alcohol misuse.12

Studies demonstrate that both genetic and environmental factors play important roles in the development of AUD.13 A family history of excessive alcohol use increases the risk of AUD. Comorbidity of AUD and other mental health conditions is extremely common. For example, high rates of association between major depressive disorder and AUD have been observed.14
Tools to use in screening and diagnosing AUD
Screening for AUD during an office visit can be done fairly quickly. While 96% of primary care physicians screen for alcohol misuse in some way, only 38% use 1 of the 3 tools recommended by the USPSTF15—the Alcohol Use Disorders Identification Test (AUDIT), the abbreviated AUDIT-C, or the National Institute on Alcohol Abuse and Alcoholism (NIAAA) single question screen—which detect the full spectrum of alcohol misuse in adults.12 Although the commonly used CAGE questionnaire is one of the most studied self-report tools, it has lower sensitivity at a lower level of alcohol intake.16
Continue to: The NIAAA single-question screen asks...
The NIAAA single-question screen asks how many times in the past year the patient had ≥4 drinks (women) or ≥5 drinks (men) in a day.15 The sensitivity and specificity of single-question screening are 82% to 87% and 61% to 79%, respectively, and the test has been validated in several different settings.12 The AUDIT screening tool, freely available from the World Health Organization, is a 10-item questionnaire that probes an individual’s alcohol intake, alcohol dependence, and adverse consequences of alcohol use. Administration of the AUDIT typically requires only 2 minutes. AUDIT-C17 is an abbreviated version of the AUDIT questionnaire that asks 3 consumption questions to screen for AUD.
It was found that AUDIT scores in the range of 8 to 15 indicate a medium-level alcohol problem, whereas a score of ≥16 indicates a high-level alcohol problem. The AUDIT-C is scored from 0 to 12, with ≥4 indicating a problem in men and ≥3
THE CASE
The physician had used the NIAAA single- question screen to determine that Ms. E drank more than 4 beers per day during social events and weekends, which occurred 2 to 3 times per month over the past year. She lives alone and said that she’d been seeing less and less of her boyfriend lately. Her score on the Patient Health Questionnaire (PHQ), which screens for depression, was 11, indicating moderate impairment. Her response on the CAGE questionnaire was negative for a problem with alcohol. However, her AUDIT score was 17, indicating a high-level alcohol problem. Based on these findings, her physician expressed concern that her alcohol use might be contributing to her symptoms and difficulties.
Although she did not have a history of increasing usage per day, a persistent desire to cut down, significant effort to obtain alcohol, or cravings, she was having work troubles and continued to drink even though it was straining relationships, promoting weight gain, and causing abdominal pain.
The physician asked her to schedule a return visit and ordered several blood studies. He also offered to connect her with a colleague with whom he collaborated who could speak with her about possible alcohol use disorders and depression.
Continue to: Selecting blood work in screening for AUD
Selecting blood work in screening for AUD
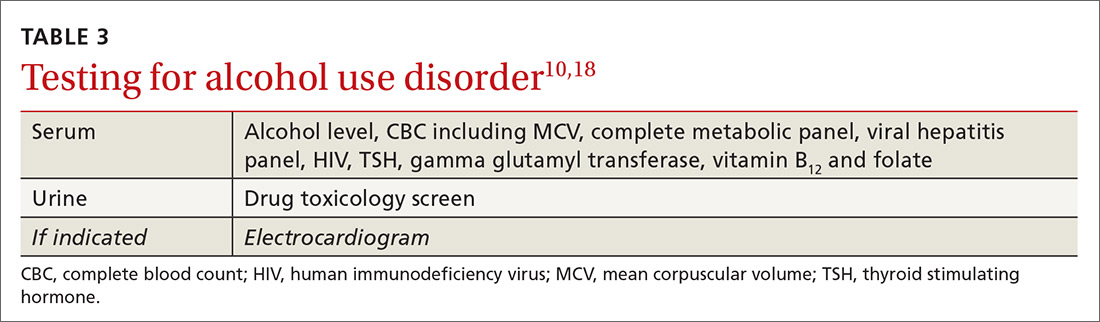
Lab tests used to measure hepatic injury due to alcohol include gamma-glutamyl-transferase, aspartate aminotransferase (AST), alanine aminotransferase (ALT), and macrocytic volume, although the indices of hepatic damage have low specificity. Elevated serum ethanol levels can reveal recent alcohol use, and vitamin deficiencies and other abnormalities can be used to differentiate other causes of hepatic inflammation and co-existing health issues (TABLE 310,18). A number of as-yet-unvalidated biomarkers are being studied to assist in screening, diagnosing, and treating AUD.18
What treatment approaches work for AUD?
Family physicians can efficiently and productively address AUD by using alcohol screening and brief intervention, which have been shown to reduce risky drinking. Reimbursement for this service is covered by such CPT codes as 99408, 99409, or H0049, or with other evaluation and management (E/M) codes by using modifier 25.
Treatment of AUD varies and should be customized to each patient’s needs, readiness, preferences, and resources. Individual and group counseling approaches can be effective, and medications are available for inpatient and outpatient settings. Psychotherapy options include brief interventions, 12-step programs (eg, Alcoholics Anonymous—https://www.aa.org/pages/en_US/find-aa-resources),motivational enhancement therapy, and cognitive behavioral therapy. Although it is beyond the scope of this article to describe these options in detail, resources are available for those who wish to learn more.19-21
Psychopharmacologic management includes US Food and Drug Administration (FDA)-approved medications such as disulfiram, naltrexone, and acamprosate, and off-label uses of other medications (TABLE 49). Not enough empiric evidence is available to judge the effectiveness of these medications in adolescents, and the FDA has not approved them for such use. Evidence from meta-analyses comparing naltrexone and acamprosate have shown naltrexone to be more efficacious in reducing heavy drinking and cravings, while acamprosate is effective in promoting abstinence.22,23 Naltrexone combined with behavioral intervention reduces the heavy drinking days and percentage of abstinence days.24
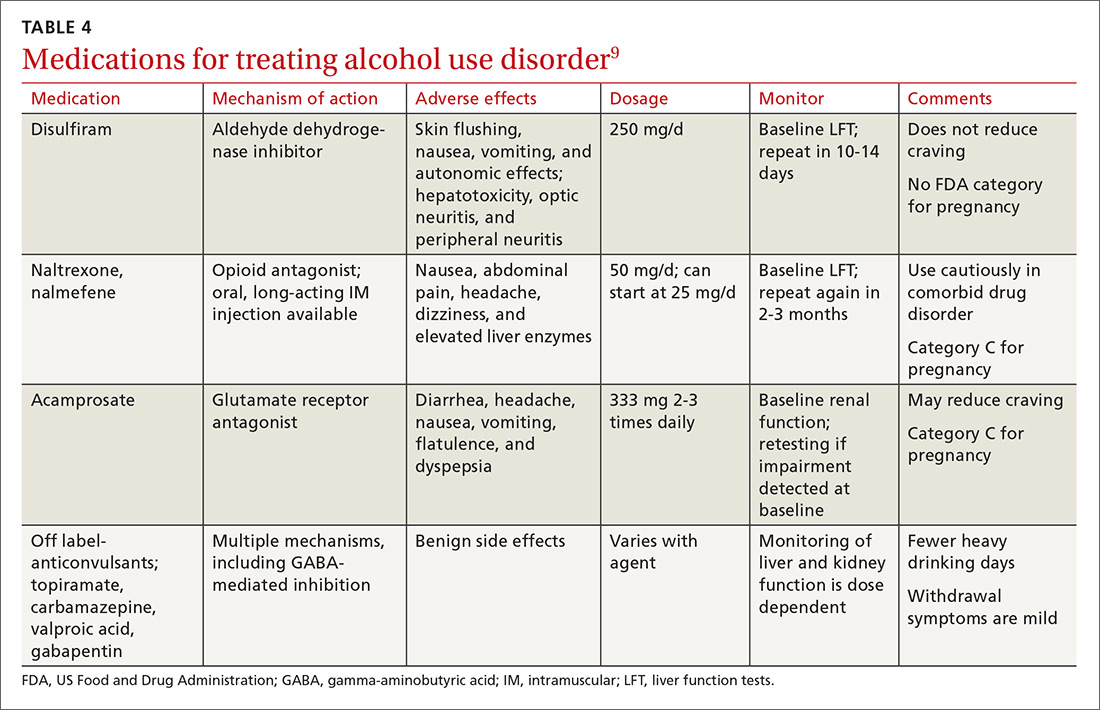
Current guideline recommendations from the American Psychiatric Association25 include:
- Naltrexone and acamprosate are recommended to treat patients with moderate-to-severe AUD in specific circumstances (eg, when nonpharmacologic approaches have failed to produce an effect or when patients prefer to use one of these medications).
- Topiramate and gabapentin are also suggested as medications for patients with moderate-to-severe AUD, but typically after first trying naltrexone and acamprosate.
- Disulfiram generally should not be used as first-line treatment. It produces physical reactions (eg, flushing) if alcohol is consumed within 12 to 24 hours of medication use.
Continue to: THE CASE
THE CASE
Ms. E was open to the idea of decreasing her alcohol use and agreed that she was depressed. Her lab tests at follow-up were normal other than an elevated AST/ALT of 90/80 U/L. S
She continued to get counseling for her AUD and for her comorbid depression in addition to taking a selective serotonin reuptake inhibitor. She is now in early remission for her alcohol use.
CORRESPONDENCE
Jaividhya Dasarathy, MD, Department of Family Medicine, Metro Health Medical Center, 2500 MetroHealth Drive, Cleveland, OH 44109; [email protected].
1. Grant BF, Goldstein RB, Saha TD, et al. Epidemiology of DSM-5 alcohol use disorder: results from the National Epidemiologic Survey on Alcohol and Related Conditions III. JAMA Psychiatry. 2015;72:757-766.
2. APA. Diagnostic and Statistical Manual of Mental Disorders, 5th ed. Washington DC; 2013.
3. HHS. Results from the 2015 National Survey on Drug Use and Health: summary of national findings. https://www.samhsa.gov/data/sites/default/files/NSDUH-DetTabs-2015/NSDUH-DetTabs-2015/NSDUH-DetTabs-2015.pdf. Accessed November 27, 2018.
4. Rehm J, Mathers C, Popova S, et al. Global burden of disease and injury and economic cost attributable to alcohol use and alcohol-use disorders. Lancet. 2009;373:2223-2233.
5. Chase V, Neild R, Sadler CW, et al. The medical complications of alcohol use: understanding mechanisms to improve management. Drug Alcohol Rev. 2005;24:253-265.
6. Grant BF, Chou SP, Saha TD, et al. Prevalence of 12-month alcohol use, high-risk drinking, and DSM-IV alcohol use disorder in the United States, 2001-2002 to 2012-2013: results from the National Epidemiologic Survey on Alcohol and Related Conditions. JAMA Psychiatry. 2017;74:911-923.
7. Williams EC, Achtmeyer CE, Young JP, et al. Barriers to and facilitators of alcohol use disorder pharmacotherapy in primary care: a qualitative study in five VA clinics. J Gen Intern Med. 2018;33:258-267.
8. Zhang DX, Li ST, Lee QK, et al. Systematic review of guidelines on managing patients with harmful use of alcohol in primary healthcare settings. Alcohol Alcohol. 2017;52:595-609.
9. Wackernah RC, Minnick MJ, Clapp P. Alcohol use disorder: pathophysiology, effects, and pharmacologic options for treatment. Subst Abuse Rehabil. 2014;5:1-12.
10. Kattimani S, Bharadwaj B. Clinical management of alcohol withdrawal: a systematic review. Ind Psychiatry J. 2013;22:100-108.
11. Gowin JL, Sloan ME, Stangl BL, et al. Vulnerability for alcohol use disorder and rate of alcohol consumption. Am J Psychiatry. 2017;174:1094-1101.
12. Moyer VA; Preventive Services Task Force. Screening and behavioral counseling interventions in primary care to reduce alcohol misuse: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2013;159:210-218.
13. Tarter RE, Alterman AI, Edwards KL. Vulnerability to alcoholism in men: a behavior-genetic perspective. J Stud Alcohol. 1985;46:329-356.
14. Brière FN, Rohde P, Seeley JR, et al. Comorbidity between major depression and alcohol use disorder from adolescence to adulthood [published online ahead of print, October 22, 2013]. Compr Psychiatry. 2014;55:526-533. doi: 10.1016/j.comppsych.2013.10.007.
15. Tan CH, Hungerford DW, Denny CH, et al. Screening for alcohol misuse: practices among U.S. primary care providers, DocStyles 2016. Am J Prev Med. 2018;54:173-180.
16. Aertgeerts B, Buntinx F, Kester A. The value of the CAGE in screening for alcohol abuse and alcohol dependence in general clinical populations: a diagnostic meta-analysis. J Clin Epidemiol. 2004;57:30-39.
17. Bush K, Kivlahan DR, McDonell MB, et al. The AUDIT alcohol consumption questions (AUDIT-C): an effective brief screening test for problem drinking. Ambulatory Care Quality Improvement Project (ACQUIP). Alcohol Use Disorders Identification Test. Arch Intern Med. 1998;158:1789-1795.
18. Nanau RM, Neuman MG. Biomolecules and biomarkers used in diagnosis of alcohol drinking and in monitoring therapeutic interventions. Biomolecules. 2015;5:1339-1385.
19. Raddock M, Martukovich R, Berko E, et al. 7 tools to help patients adopt healthier behaviors. J Fam Pract. 2015;64:97-103.
20. AHRQ. Whitlock EP, Green CA, Polen MR, et al. Behavioral Counseling Interventions in Primary Care to Reduce Risky/Harmful Alcohol Use. 2004. https://www.ncbi.nlm.nih.gov/books/NBK42863/. Accessed November 17, 2018.
21. Miller WR, Baca C, Compton WM, et al. Addressing substance abuse in health care settings. Alcohol Clin Exp Res. 2006;30:292-302.
22. Maisel NC, Blodgett JC, Wilbourne PL, et al. Meta-analysis of naltrexone and acamprosate for treating alcohol use disorders: when are these medications most helpful? Addiction. 2013;108:275-293.
23. Rosner S, Leucht S, Lehert P, et al. Acamprosate supports abstinence, naltrexone prevents excessive drinking: evidence from a meta-analysis with unreported outcomes. J Psychopharmacol. 2008;22:11-23.
24. Anton RF, O’Malley SS, Ciraulo DA, et al. Combined pharmacotherapies and behavioral interventions for alcohol dependence: the COMBINE study: a randomized controlled trial. JAMA. 2006;295:2003-2017.
25. Reus VI, Fochtmann LJ, Bukstein O, et al. The American Psychiatric Association Practice Guideline for the Pharmacological Treatment of Patients With Alcohol Use Disorder. Am J Psychiatry. 2018;175:86-90.
THE CASE
Ms. E, a 42-year-old woman, visited her new physician for a physical exam. When asked about alcohol intake, she reported that she drank 3 to 4 beers after work and sometimes 5 to 8 beers a day on the weekends. Occasionally, she exceeded those amounts, but she didn’t feel guilty about her drinking. She was often late to work and said her relationship with her boyfriend was strained. A review of systems was positive for fatigue, poor concentration, abdominal pain, and weight gain. Her body mass index was 41, pulse 100 beats/min, blood pressure 125/75 mm Hg, and she was afebrile. Her physical exam was otherwise within normal limits.
How would you proceed with this patient?
Alcohol use disorder (AUD) is a common and often untreated condition that is increasingly prevalent in the United States.1 The Diagnostic and Statistical Manual of Mental Disorders, 5th Edition (DSM-5) characterizes AUD as a combination of signs and symptoms typifying alcohol abuse and dependence (discussed in a bit).2
Data from the 2015 National Survey on Drug Use and Health (NSDUH) showed 15.7 million Americans with AUD, affecting 6.2% of the population ages 18 years or older and 2.5% of adolescents ages 12 to 17 years.3
Alcohol use and AUD account for an estimated 3.8% of all global deaths and 4.6% of global disability-adjusted life years.4 AUD adversely affects several systems (TABLE 15), and patients with AUD are sicker and more likely to die younger than those without AUD.4 In the United States, prevalence of AUD has increased in recent years among women, older adults, racial minorities, and individuals with a low education level.6

Screening for AUD is reasonable and straightforward, although diagnosis and treatment of AUD in primary care settings may be challenging due to competing clinical priorities; lack of training, resources, and support; and skepticism about the efficacy of behavioral and pharmacologic treatments.7,8 However, family physicians are in an excellent position to diagnose and help address the complex biopsychosocial needs of patients with AUD, often in collaboration with colleagues and community organizations.
Signs and symptoms of AUD
In clinical practice, at least 2 of the following 11 behaviors or symptoms are required to diagnose AUD2:
- consuming larger amounts of alcohol over a longer period than intended
- persistent desire or unsuccessful efforts to cut down or control alcohol use
- making a significant effort to obtain, use, or recover from alcohol
In moderate-to-severe cases:
- cravings or urges to use alcohol
- recurrent failure to fulfill major work, school, or social obligations
- continued alcohol use despite recurrent social and interpersonal problems
- giving up social, occupational, and recreational activities due to alcohol
- using alcohol in physically dangerous situations
- continued alcohol use despite having physical or psychological problems
- tolerance to alcohol’s effects
- withdrawal symptoms.
Continue to: Patients meet criteria for mild AUD severity if...
Patients meet criteria for mild AUD severity if they exhibit 2 or 3 symptoms, moderate AUD with 4 or 5 symptoms, and severe AUD if there are 6 or more symptoms.2
Those who meet criteria for AUD and are able to stop using alcohol are deemed to be in early remission if the criteria have gone unfulfilled for at least 3 months and less than 12 months. Patients are considered to be in sustained remission if they have not met criteria for AUD at any time during a period of 12 months or longer.
How to detect AUD
Several clues in a patient’s history can suggest AUD (TABLE 29,10). Most imbibers are unaware of the dangers and may consider themselves merely “social drinkers.” Binge drinking may be an early indicator of vulnerability to AUD and should be assessed as part of a thorough clinical evaluation.11 The US Preventive Services Task Force (USPSTF) recommends (Grade B) that clinicians screen adults ages 18 years or older for alcohol misuse.12

Studies demonstrate that both genetic and environmental factors play important roles in the development of AUD.13 A family history of excessive alcohol use increases the risk of AUD. Comorbidity of AUD and other mental health conditions is extremely common. For example, high rates of association between major depressive disorder and AUD have been observed.14
Tools to use in screening and diagnosing AUD
Screening for AUD during an office visit can be done fairly quickly. While 96% of primary care physicians screen for alcohol misuse in some way, only 38% use 1 of the 3 tools recommended by the USPSTF15—the Alcohol Use Disorders Identification Test (AUDIT), the abbreviated AUDIT-C, or the National Institute on Alcohol Abuse and Alcoholism (NIAAA) single question screen—which detect the full spectrum of alcohol misuse in adults.12 Although the commonly used CAGE questionnaire is one of the most studied self-report tools, it has lower sensitivity at a lower level of alcohol intake.16
Continue to: The NIAAA single-question screen asks...
The NIAAA single-question screen asks how many times in the past year the patient had ≥4 drinks (women) or ≥5 drinks (men) in a day.15 The sensitivity and specificity of single-question screening are 82% to 87% and 61% to 79%, respectively, and the test has been validated in several different settings.12 The AUDIT screening tool, freely available from the World Health Organization, is a 10-item questionnaire that probes an individual’s alcohol intake, alcohol dependence, and adverse consequences of alcohol use. Administration of the AUDIT typically requires only 2 minutes. AUDIT-C17 is an abbreviated version of the AUDIT questionnaire that asks 3 consumption questions to screen for AUD.
It was found that AUDIT scores in the range of 8 to 15 indicate a medium-level alcohol problem, whereas a score of ≥16 indicates a high-level alcohol problem. The AUDIT-C is scored from 0 to 12, with ≥4 indicating a problem in men and ≥3
THE CASE
The physician had used the NIAAA single- question screen to determine that Ms. E drank more than 4 beers per day during social events and weekends, which occurred 2 to 3 times per month over the past year. She lives alone and said that she’d been seeing less and less of her boyfriend lately. Her score on the Patient Health Questionnaire (PHQ), which screens for depression, was 11, indicating moderate impairment. Her response on the CAGE questionnaire was negative for a problem with alcohol. However, her AUDIT score was 17, indicating a high-level alcohol problem. Based on these findings, her physician expressed concern that her alcohol use might be contributing to her symptoms and difficulties.
Although she did not have a history of increasing usage per day, a persistent desire to cut down, significant effort to obtain alcohol, or cravings, she was having work troubles and continued to drink even though it was straining relationships, promoting weight gain, and causing abdominal pain.
The physician asked her to schedule a return visit and ordered several blood studies. He also offered to connect her with a colleague with whom he collaborated who could speak with her about possible alcohol use disorders and depression.
Continue to: Selecting blood work in screening for AUD
Selecting blood work in screening for AUD
Lab tests used to measure hepatic injury due to alcohol include gamma-glutamyl-transferase, aspartate aminotransferase (AST), alanine aminotransferase (ALT), and macrocytic volume, although the indices of hepatic damage have low specificity. Elevated serum ethanol levels can reveal recent alcohol use, and vitamin deficiencies and other abnormalities can be used to differentiate other causes of hepatic inflammation and co-existing health issues (TABLE 310,18). A number of as-yet-unvalidated biomarkers are being studied to assist in screening, diagnosing, and treating AUD.18

What treatment approaches work for AUD?
Family physicians can efficiently and productively address AUD by using alcohol screening and brief intervention, which have been shown to reduce risky drinking. Reimbursement for this service is covered by such CPT codes as 99408, 99409, or H0049, or with other evaluation and management (E/M) codes by using modifier 25.
Treatment of AUD varies and should be customized to each patient’s needs, readiness, preferences, and resources. Individual and group counseling approaches can be effective, and medications are available for inpatient and outpatient settings. Psychotherapy options include brief interventions, 12-step programs (eg, Alcoholics Anonymous—https://www.aa.org/pages/en_US/find-aa-resources),motivational enhancement therapy, and cognitive behavioral therapy. Although it is beyond the scope of this article to describe these options in detail, resources are available for those who wish to learn more.19-21
Psychopharmacologic management includes US Food and Drug Administration (FDA)-approved medications such as disulfiram, naltrexone, and acamprosate, and off-label uses of other medications (TABLE 49). Not enough empiric evidence is available to judge the effectiveness of these medications in adolescents, and the FDA has not approved them for such use. Evidence from meta-analyses comparing naltrexone and acamprosate have shown naltrexone to be more efficacious in reducing heavy drinking and cravings, while acamprosate is effective in promoting abstinence.22,23 Naltrexone combined with behavioral intervention reduces the heavy drinking days and percentage of abstinence days.24

Current guideline recommendations from the American Psychiatric Association25 include:
- Naltrexone and acamprosate are recommended to treat patients with moderate-to-severe AUD in specific circumstances (eg, when nonpharmacologic approaches have failed to produce an effect or when patients prefer to use one of these medications).
- Topiramate and gabapentin are also suggested as medications for patients with moderate-to-severe AUD, but typically after first trying naltrexone and acamprosate.
- Disulfiram generally should not be used as first-line treatment. It produces physical reactions (eg, flushing) if alcohol is consumed within 12 to 24 hours of medication use.
Continue to: THE CASE
THE CASE
Ms. E was open to the idea of decreasing her alcohol use and agreed that she was depressed. Her lab tests at follow-up were normal other than an elevated AST/ALT of 90/80 U/L. S
She continued to get counseling for her AUD and for her comorbid depression in addition to taking a selective serotonin reuptake inhibitor. She is now in early remission for her alcohol use.
CORRESPONDENCE
Jaividhya Dasarathy, MD, Department of Family Medicine, Metro Health Medical Center, 2500 MetroHealth Drive, Cleveland, OH 44109; [email protected].
THE CASE
Ms. E, a 42-year-old woman, visited her new physician for a physical exam. When asked about alcohol intake, she reported that she drank 3 to 4 beers after work and sometimes 5 to 8 beers a day on the weekends. Occasionally, she exceeded those amounts, but she didn’t feel guilty about her drinking. She was often late to work and said her relationship with her boyfriend was strained. A review of systems was positive for fatigue, poor concentration, abdominal pain, and weight gain. Her body mass index was 41, pulse 100 beats/min, blood pressure 125/75 mm Hg, and she was afebrile. Her physical exam was otherwise within normal limits.
How would you proceed with this patient?
Alcohol use disorder (AUD) is a common and often untreated condition that is increasingly prevalent in the United States.1 The Diagnostic and Statistical Manual of Mental Disorders, 5th Edition (DSM-5) characterizes AUD as a combination of signs and symptoms typifying alcohol abuse and dependence (discussed in a bit).2
Data from the 2015 National Survey on Drug Use and Health (NSDUH) showed 15.7 million Americans with AUD, affecting 6.2% of the population ages 18 years or older and 2.5% of adolescents ages 12 to 17 years.3
Alcohol use and AUD account for an estimated 3.8% of all global deaths and 4.6% of global disability-adjusted life years.4 AUD adversely affects several systems (TABLE 15), and patients with AUD are sicker and more likely to die younger than those without AUD.4 In the United States, prevalence of AUD has increased in recent years among women, older adults, racial minorities, and individuals with a low education level.6

Screening for AUD is reasonable and straightforward, although diagnosis and treatment of AUD in primary care settings may be challenging due to competing clinical priorities; lack of training, resources, and support; and skepticism about the efficacy of behavioral and pharmacologic treatments.7,8 However, family physicians are in an excellent position to diagnose and help address the complex biopsychosocial needs of patients with AUD, often in collaboration with colleagues and community organizations.
Signs and symptoms of AUD
In clinical practice, at least 2 of the following 11 behaviors or symptoms are required to diagnose AUD2:
- consuming larger amounts of alcohol over a longer period than intended
- persistent desire or unsuccessful efforts to cut down or control alcohol use
- making a significant effort to obtain, use, or recover from alcohol
In moderate-to-severe cases:
- cravings or urges to use alcohol
- recurrent failure to fulfill major work, school, or social obligations
- continued alcohol use despite recurrent social and interpersonal problems
- giving up social, occupational, and recreational activities due to alcohol
- using alcohol in physically dangerous situations
- continued alcohol use despite having physical or psychological problems
- tolerance to alcohol’s effects
- withdrawal symptoms.
Continue to: Patients meet criteria for mild AUD severity if...
Patients meet criteria for mild AUD severity if they exhibit 2 or 3 symptoms, moderate AUD with 4 or 5 symptoms, and severe AUD if there are 6 or more symptoms.2
Those who meet criteria for AUD and are able to stop using alcohol are deemed to be in early remission if the criteria have gone unfulfilled for at least 3 months and less than 12 months. Patients are considered to be in sustained remission if they have not met criteria for AUD at any time during a period of 12 months or longer.
How to detect AUD
Several clues in a patient’s history can suggest AUD (TABLE 29,10). Most imbibers are unaware of the dangers and may consider themselves merely “social drinkers.” Binge drinking may be an early indicator of vulnerability to AUD and should be assessed as part of a thorough clinical evaluation.11 The US Preventive Services Task Force (USPSTF) recommends (Grade B) that clinicians screen adults ages 18 years or older for alcohol misuse.12

Studies demonstrate that both genetic and environmental factors play important roles in the development of AUD.13 A family history of excessive alcohol use increases the risk of AUD. Comorbidity of AUD and other mental health conditions is extremely common. For example, high rates of association between major depressive disorder and AUD have been observed.14
Tools to use in screening and diagnosing AUD
Screening for AUD during an office visit can be done fairly quickly. While 96% of primary care physicians screen for alcohol misuse in some way, only 38% use 1 of the 3 tools recommended by the USPSTF15—the Alcohol Use Disorders Identification Test (AUDIT), the abbreviated AUDIT-C, or the National Institute on Alcohol Abuse and Alcoholism (NIAAA) single question screen—which detect the full spectrum of alcohol misuse in adults.12 Although the commonly used CAGE questionnaire is one of the most studied self-report tools, it has lower sensitivity at a lower level of alcohol intake.16
Continue to: The NIAAA single-question screen asks...
The NIAAA single-question screen asks how many times in the past year the patient had ≥4 drinks (women) or ≥5 drinks (men) in a day.15 The sensitivity and specificity of single-question screening are 82% to 87% and 61% to 79%, respectively, and the test has been validated in several different settings.12 The AUDIT screening tool, freely available from the World Health Organization, is a 10-item questionnaire that probes an individual’s alcohol intake, alcohol dependence, and adverse consequences of alcohol use. Administration of the AUDIT typically requires only 2 minutes. AUDIT-C17 is an abbreviated version of the AUDIT questionnaire that asks 3 consumption questions to screen for AUD.
It was found that AUDIT scores in the range of 8 to 15 indicate a medium-level alcohol problem, whereas a score of ≥16 indicates a high-level alcohol problem. The AUDIT-C is scored from 0 to 12, with ≥4 indicating a problem in men and ≥3
THE CASE
The physician had used the NIAAA single- question screen to determine that Ms. E drank more than 4 beers per day during social events and weekends, which occurred 2 to 3 times per month over the past year. She lives alone and said that she’d been seeing less and less of her boyfriend lately. Her score on the Patient Health Questionnaire (PHQ), which screens for depression, was 11, indicating moderate impairment. Her response on the CAGE questionnaire was negative for a problem with alcohol. However, her AUDIT score was 17, indicating a high-level alcohol problem. Based on these findings, her physician expressed concern that her alcohol use might be contributing to her symptoms and difficulties.
Although she did not have a history of increasing usage per day, a persistent desire to cut down, significant effort to obtain alcohol, or cravings, she was having work troubles and continued to drink even though it was straining relationships, promoting weight gain, and causing abdominal pain.
The physician asked her to schedule a return visit and ordered several blood studies. He also offered to connect her with a colleague with whom he collaborated who could speak with her about possible alcohol use disorders and depression.
Continue to: Selecting blood work in screening for AUD
Selecting blood work in screening for AUD
Lab tests used to measure hepatic injury due to alcohol include gamma-glutamyl-transferase, aspartate aminotransferase (AST), alanine aminotransferase (ALT), and macrocytic volume, although the indices of hepatic damage have low specificity. Elevated serum ethanol levels can reveal recent alcohol use, and vitamin deficiencies and other abnormalities can be used to differentiate other causes of hepatic inflammation and co-existing health issues (TABLE 310,18). A number of as-yet-unvalidated biomarkers are being studied to assist in screening, diagnosing, and treating AUD.18

What treatment approaches work for AUD?
Family physicians can efficiently and productively address AUD by using alcohol screening and brief intervention, which have been shown to reduce risky drinking. Reimbursement for this service is covered by such CPT codes as 99408, 99409, or H0049, or with other evaluation and management (E/M) codes by using modifier 25.
Treatment of AUD varies and should be customized to each patient’s needs, readiness, preferences, and resources. Individual and group counseling approaches can be effective, and medications are available for inpatient and outpatient settings. Psychotherapy options include brief interventions, 12-step programs (eg, Alcoholics Anonymous—https://www.aa.org/pages/en_US/find-aa-resources),motivational enhancement therapy, and cognitive behavioral therapy. Although it is beyond the scope of this article to describe these options in detail, resources are available for those who wish to learn more.19-21
Psychopharmacologic management includes US Food and Drug Administration (FDA)-approved medications such as disulfiram, naltrexone, and acamprosate, and off-label uses of other medications (TABLE 49). Not enough empiric evidence is available to judge the effectiveness of these medications in adolescents, and the FDA has not approved them for such use. Evidence from meta-analyses comparing naltrexone and acamprosate have shown naltrexone to be more efficacious in reducing heavy drinking and cravings, while acamprosate is effective in promoting abstinence.22,23 Naltrexone combined with behavioral intervention reduces the heavy drinking days and percentage of abstinence days.24

Current guideline recommendations from the American Psychiatric Association25 include:
- Naltrexone and acamprosate are recommended to treat patients with moderate-to-severe AUD in specific circumstances (eg, when nonpharmacologic approaches have failed to produce an effect or when patients prefer to use one of these medications).
- Topiramate and gabapentin are also suggested as medications for patients with moderate-to-severe AUD, but typically after first trying naltrexone and acamprosate.
- Disulfiram generally should not be used as first-line treatment. It produces physical reactions (eg, flushing) if alcohol is consumed within 12 to 24 hours of medication use.
Continue to: THE CASE
THE CASE
Ms. E was open to the idea of decreasing her alcohol use and agreed that she was depressed. Her lab tests at follow-up were normal other than an elevated AST/ALT of 90/80 U/L. S
She continued to get counseling for her AUD and for her comorbid depression in addition to taking a selective serotonin reuptake inhibitor. She is now in early remission for her alcohol use.
CORRESPONDENCE
Jaividhya Dasarathy, MD, Department of Family Medicine, Metro Health Medical Center, 2500 MetroHealth Drive, Cleveland, OH 44109; [email protected].
1. Grant BF, Goldstein RB, Saha TD, et al. Epidemiology of DSM-5 alcohol use disorder: results from the National Epidemiologic Survey on Alcohol and Related Conditions III. JAMA Psychiatry. 2015;72:757-766.
2. APA. Diagnostic and Statistical Manual of Mental Disorders, 5th ed. Washington DC; 2013.
3. HHS. Results from the 2015 National Survey on Drug Use and Health: summary of national findings. https://www.samhsa.gov/data/sites/default/files/NSDUH-DetTabs-2015/NSDUH-DetTabs-2015/NSDUH-DetTabs-2015.pdf. Accessed November 27, 2018.
4. Rehm J, Mathers C, Popova S, et al. Global burden of disease and injury and economic cost attributable to alcohol use and alcohol-use disorders. Lancet. 2009;373:2223-2233.
5. Chase V, Neild R, Sadler CW, et al. The medical complications of alcohol use: understanding mechanisms to improve management. Drug Alcohol Rev. 2005;24:253-265.
6. Grant BF, Chou SP, Saha TD, et al. Prevalence of 12-month alcohol use, high-risk drinking, and DSM-IV alcohol use disorder in the United States, 2001-2002 to 2012-2013: results from the National Epidemiologic Survey on Alcohol and Related Conditions. JAMA Psychiatry. 2017;74:911-923.
7. Williams EC, Achtmeyer CE, Young JP, et al. Barriers to and facilitators of alcohol use disorder pharmacotherapy in primary care: a qualitative study in five VA clinics. J Gen Intern Med. 2018;33:258-267.
8. Zhang DX, Li ST, Lee QK, et al. Systematic review of guidelines on managing patients with harmful use of alcohol in primary healthcare settings. Alcohol Alcohol. 2017;52:595-609.
9. Wackernah RC, Minnick MJ, Clapp P. Alcohol use disorder: pathophysiology, effects, and pharmacologic options for treatment. Subst Abuse Rehabil. 2014;5:1-12.
10. Kattimani S, Bharadwaj B. Clinical management of alcohol withdrawal: a systematic review. Ind Psychiatry J. 2013;22:100-108.
11. Gowin JL, Sloan ME, Stangl BL, et al. Vulnerability for alcohol use disorder and rate of alcohol consumption. Am J Psychiatry. 2017;174:1094-1101.
12. Moyer VA; Preventive Services Task Force. Screening and behavioral counseling interventions in primary care to reduce alcohol misuse: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2013;159:210-218.
13. Tarter RE, Alterman AI, Edwards KL. Vulnerability to alcoholism in men: a behavior-genetic perspective. J Stud Alcohol. 1985;46:329-356.
14. Brière FN, Rohde P, Seeley JR, et al. Comorbidity between major depression and alcohol use disorder from adolescence to adulthood [published online ahead of print, October 22, 2013]. Compr Psychiatry. 2014;55:526-533. doi: 10.1016/j.comppsych.2013.10.007.
15. Tan CH, Hungerford DW, Denny CH, et al. Screening for alcohol misuse: practices among U.S. primary care providers, DocStyles 2016. Am J Prev Med. 2018;54:173-180.
16. Aertgeerts B, Buntinx F, Kester A. The value of the CAGE in screening for alcohol abuse and alcohol dependence in general clinical populations: a diagnostic meta-analysis. J Clin Epidemiol. 2004;57:30-39.
17. Bush K, Kivlahan DR, McDonell MB, et al. The AUDIT alcohol consumption questions (AUDIT-C): an effective brief screening test for problem drinking. Ambulatory Care Quality Improvement Project (ACQUIP). Alcohol Use Disorders Identification Test. Arch Intern Med. 1998;158:1789-1795.
18. Nanau RM, Neuman MG. Biomolecules and biomarkers used in diagnosis of alcohol drinking and in monitoring therapeutic interventions. Biomolecules. 2015;5:1339-1385.
19. Raddock M, Martukovich R, Berko E, et al. 7 tools to help patients adopt healthier behaviors. J Fam Pract. 2015;64:97-103.
20. AHRQ. Whitlock EP, Green CA, Polen MR, et al. Behavioral Counseling Interventions in Primary Care to Reduce Risky/Harmful Alcohol Use. 2004. https://www.ncbi.nlm.nih.gov/books/NBK42863/. Accessed November 17, 2018.
21. Miller WR, Baca C, Compton WM, et al. Addressing substance abuse in health care settings. Alcohol Clin Exp Res. 2006;30:292-302.
22. Maisel NC, Blodgett JC, Wilbourne PL, et al. Meta-analysis of naltrexone and acamprosate for treating alcohol use disorders: when are these medications most helpful? Addiction. 2013;108:275-293.
23. Rosner S, Leucht S, Lehert P, et al. Acamprosate supports abstinence, naltrexone prevents excessive drinking: evidence from a meta-analysis with unreported outcomes. J Psychopharmacol. 2008;22:11-23.
24. Anton RF, O’Malley SS, Ciraulo DA, et al. Combined pharmacotherapies and behavioral interventions for alcohol dependence: the COMBINE study: a randomized controlled trial. JAMA. 2006;295:2003-2017.
25. Reus VI, Fochtmann LJ, Bukstein O, et al. The American Psychiatric Association Practice Guideline for the Pharmacological Treatment of Patients With Alcohol Use Disorder. Am J Psychiatry. 2018;175:86-90.
1. Grant BF, Goldstein RB, Saha TD, et al. Epidemiology of DSM-5 alcohol use disorder: results from the National Epidemiologic Survey on Alcohol and Related Conditions III. JAMA Psychiatry. 2015;72:757-766.
2. APA. Diagnostic and Statistical Manual of Mental Disorders, 5th ed. Washington DC; 2013.
3. HHS. Results from the 2015 National Survey on Drug Use and Health: summary of national findings. https://www.samhsa.gov/data/sites/default/files/NSDUH-DetTabs-2015/NSDUH-DetTabs-2015/NSDUH-DetTabs-2015.pdf. Accessed November 27, 2018.
4. Rehm J, Mathers C, Popova S, et al. Global burden of disease and injury and economic cost attributable to alcohol use and alcohol-use disorders. Lancet. 2009;373:2223-2233.
5. Chase V, Neild R, Sadler CW, et al. The medical complications of alcohol use: understanding mechanisms to improve management. Drug Alcohol Rev. 2005;24:253-265.
6. Grant BF, Chou SP, Saha TD, et al. Prevalence of 12-month alcohol use, high-risk drinking, and DSM-IV alcohol use disorder in the United States, 2001-2002 to 2012-2013: results from the National Epidemiologic Survey on Alcohol and Related Conditions. JAMA Psychiatry. 2017;74:911-923.
7. Williams EC, Achtmeyer CE, Young JP, et al. Barriers to and facilitators of alcohol use disorder pharmacotherapy in primary care: a qualitative study in five VA clinics. J Gen Intern Med. 2018;33:258-267.
8. Zhang DX, Li ST, Lee QK, et al. Systematic review of guidelines on managing patients with harmful use of alcohol in primary healthcare settings. Alcohol Alcohol. 2017;52:595-609.
9. Wackernah RC, Minnick MJ, Clapp P. Alcohol use disorder: pathophysiology, effects, and pharmacologic options for treatment. Subst Abuse Rehabil. 2014;5:1-12.
10. Kattimani S, Bharadwaj B. Clinical management of alcohol withdrawal: a systematic review. Ind Psychiatry J. 2013;22:100-108.
11. Gowin JL, Sloan ME, Stangl BL, et al. Vulnerability for alcohol use disorder and rate of alcohol consumption. Am J Psychiatry. 2017;174:1094-1101.
12. Moyer VA; Preventive Services Task Force. Screening and behavioral counseling interventions in primary care to reduce alcohol misuse: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2013;159:210-218.
13. Tarter RE, Alterman AI, Edwards KL. Vulnerability to alcoholism in men: a behavior-genetic perspective. J Stud Alcohol. 1985;46:329-356.
14. Brière FN, Rohde P, Seeley JR, et al. Comorbidity between major depression and alcohol use disorder from adolescence to adulthood [published online ahead of print, October 22, 2013]. Compr Psychiatry. 2014;55:526-533. doi: 10.1016/j.comppsych.2013.10.007.
15. Tan CH, Hungerford DW, Denny CH, et al. Screening for alcohol misuse: practices among U.S. primary care providers, DocStyles 2016. Am J Prev Med. 2018;54:173-180.
16. Aertgeerts B, Buntinx F, Kester A. The value of the CAGE in screening for alcohol abuse and alcohol dependence in general clinical populations: a diagnostic meta-analysis. J Clin Epidemiol. 2004;57:30-39.
17. Bush K, Kivlahan DR, McDonell MB, et al. The AUDIT alcohol consumption questions (AUDIT-C): an effective brief screening test for problem drinking. Ambulatory Care Quality Improvement Project (ACQUIP). Alcohol Use Disorders Identification Test. Arch Intern Med. 1998;158:1789-1795.
18. Nanau RM, Neuman MG. Biomolecules and biomarkers used in diagnosis of alcohol drinking and in monitoring therapeutic interventions. Biomolecules. 2015;5:1339-1385.
19. Raddock M, Martukovich R, Berko E, et al. 7 tools to help patients adopt healthier behaviors. J Fam Pract. 2015;64:97-103.
20. AHRQ. Whitlock EP, Green CA, Polen MR, et al. Behavioral Counseling Interventions in Primary Care to Reduce Risky/Harmful Alcohol Use. 2004. https://www.ncbi.nlm.nih.gov/books/NBK42863/. Accessed November 17, 2018.
21. Miller WR, Baca C, Compton WM, et al. Addressing substance abuse in health care settings. Alcohol Clin Exp Res. 2006;30:292-302.
22. Maisel NC, Blodgett JC, Wilbourne PL, et al. Meta-analysis of naltrexone and acamprosate for treating alcohol use disorders: when are these medications most helpful? Addiction. 2013;108:275-293.
23. Rosner S, Leucht S, Lehert P, et al. Acamprosate supports abstinence, naltrexone prevents excessive drinking: evidence from a meta-analysis with unreported outcomes. J Psychopharmacol. 2008;22:11-23.
24. Anton RF, O’Malley SS, Ciraulo DA, et al. Combined pharmacotherapies and behavioral interventions for alcohol dependence: the COMBINE study: a randomized controlled trial. JAMA. 2006;295:2003-2017.
25. Reus VI, Fochtmann LJ, Bukstein O, et al. The American Psychiatric Association Practice Guideline for the Pharmacological Treatment of Patients With Alcohol Use Disorder. Am J Psychiatry. 2018;175:86-90.
Targeting depression: Primary care tips and tools
THE CASE
As you get ready to see your next patient, 52-year-old Jim M, you see in his chart that during an annual routine nurse screening (per office protocol), he scored positive for depressed mood/anhedonia on the Patient Health Questionnaire-2 (PHQ-2) and scored a 21 out of 27 on the full version (PHQ-9), suggesting that he has severe major depressive disorder and that antidepressants should be considered.
When you enter the exam room, you notice his sad expression, poor eye contact, and stooped posture. Mr. M says his wife “made him” come to see you. He reports low energy and not wanting to leave his house, which started about a year earlier after he lost his job. When you discuss his job loss and the impact it has had on him, he sheepishly admits to sometimes thinking that things would be better if he were dead. Upon further questioning, you learn that he does not have suicidal intentions or plans.
HOW WOULD YOU PROCEED WITH THIS PATIENT?
Depression is the most common mental health complaint in primary care settings; in 2015, an estimated 16.1 million (6.7%) adults in the United States ages 18 or older had at least one depressive episode in the past year.1 Depression results in significant health, work, and social life impairments,2 and comorbid anxiety is highly prevalent in patients with depression.
Primary care physicians see almost twice as many mental health patients as psychiatrists3 due to barriers in behavioral health treatment (such as wait times, cost, and stigma) and the fact that primary care physicians often provide first-line access to behavioral health resources. Depression is caused by biological, psychological, and social factors, and primary care physicians are ideally positioned to develop therapeutic, healing relationships with patients that coincide with the biopsychosocial model of the disease.4
This review will provide some useful tips and tools to ensure that these patients get the care they need.
Depression? Or are other factors at play?
Major depressive disorder (MDD) is defined as a clinically significant change in mood that lasts at least 2 weeks.5 The main symptoms of MDD include depressed mood and markedly diminished interest or pleasure; additional symptoms may include reduced self-esteem, weight/appetite changes, fatigue or reduced energy, guilt/worthlessness, decreased activity, poor concentration, and suicidal thinking.5 To meet the criteria for a diagnosis of MDD, patients must experience symptoms for most of the day, nearly every day. (Dysthymia or persistent depressive disorder is a type of depression that is milder and more chronic than MDD, but does not have as many symptoms as MDD.) The focus of this article will be on MDD.
Shared symptoms with other disorders
Depression often displays some of the same symptoms as bereavement disorder and adjustment disorder, as well as other conditions.
Grief over loss and depressive symptoms circumscribed to a stressor are considered bereavement disorder and adjustment disorder, respectively. These disorders are usually limited to weeks or months as the patient adapts to his/her particular situation.
Organic problems such as nutritional deficiencies and sleep apnea can cause, exacerbate, or mimic depression (TABLE 16). Pain and depression are often associated, in that chronic pain can precipitate or perpetuate depression.7

Bipolar disorder consists of both depressive and manic episodes; patients may be misdiagnosed and treated for depression alone.
Substance intoxication or withdrawal can precipitate or perpetuate depression. A period of abstinence of at least one month may be necessary to see if depressive symptoms persist or resolve.
Premenstrual dysphoric disorder is defined as a period of depressed mood that is limited to the final week before the onset of menses and resolves in the week post-menses.
How to make the diagnosis
Inquiring about prolonged feelings of sadness and/or lack of enjoyment in activities is an effective way to begin the screening process for depression.8 Screening tools such as the PHQ-9 (TABLE 29), Beck Depression Inventory, Hamilton Rating Scale for Depression, and Geriatric Depression Scale are useful when combined with a clinical interview. Another useful tool is the Mood Disorder Questionnaire, which can help one determine if a patient is suffering from depression or bipolar disorder. It’s available at: http://www.dbsalliance.org/pdfs/MDQ.pdf. (Asking about a history of consecutive days of elevated, expansive, or irritable mood accompanied by increased activity or energy can also provide valuable insight.)
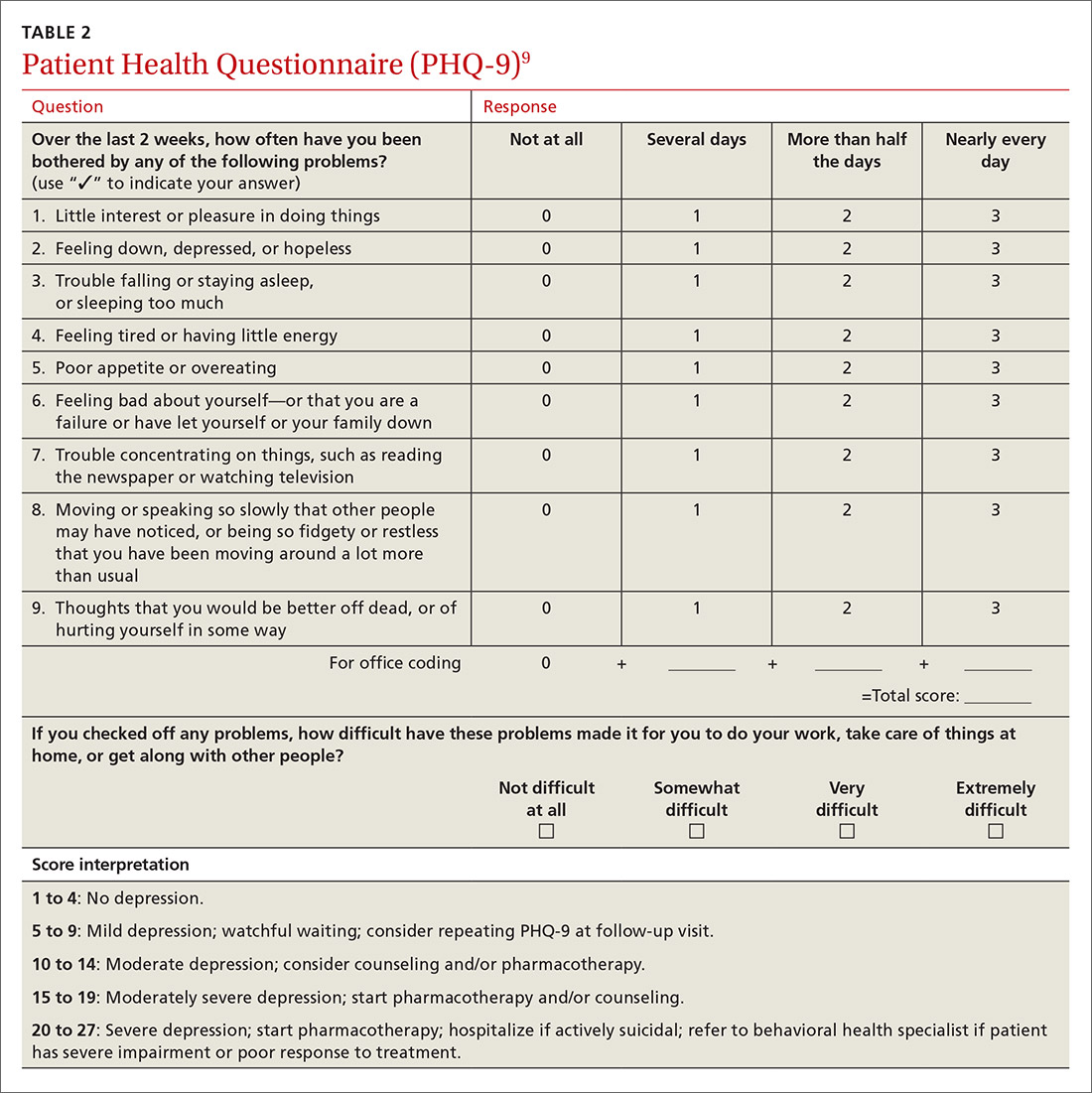
For its part, the US Preventive Services Task Force recommends screening adults for depression when adequate systems are in place (eg, referrals to settings that can provide necessary care) so as “to assure accurate diagnosis, effective treatment, and follow-up.”10-12
Assessing severity. Asking about functional impairments at work and at home and with academics and relationships will help determine severity, as will inquiring about a patient’s past or current suicidal thoughts. About two-thirds of all patients with depression contemplate suicide and 10% to 15% will attempt suicide.13
There is no evidence that inquiring about thoughts of death or suicide exacerbates suicidal risk.14,15 Confirming a diagnosis of MDD may require multiple visits, but should not delay treatment.
Making the most of the tools at your disposal
As a family physician (FP), you are especially well positioned to help patients suffering from MDD by offering education, counseling, and support; prescribing antidepressants; and coordinating care. Collaboration with behavioral health teams may be beneficial, especially in complex and treatment-resistant cases.
Counseling, alone or combined with pharmacotherapy, may improve patient outcomes.16,17 A first step may be recommending behavior modifications (such as adequate sleep, exercise, and a healthy diet). FPs can learn to utilize several counseling techniques, such as motivational interviewing, solution-focused therapy, and supportive therapy, for a variety of clinical situations in which behavioral change would be helpful.18 Establishing a therapeutic alliance through empathy and creating treatment expectations are key to helping patients overcome depression.19,20 Referral to a therapist can help identify and manage psychosocial factors that are often inherent in depression. Explaining to the patient that depression is best improved with a combination of medication and therapy is often helpful in motivating the patient to see a therapist.
Selecting an antidepressant. There is insufficient evidence to show differences in remission rates or times to remission among antidepressants,21 so medication choice involves balancing factors such as cost, previous treatments, adverse effects, and comorbid conditions (TABLE 322). A recent systematic review and meta-analysis involving 66 studies and more than 15,000 patients found tricyclic/tetracyclic antidepressants and selective serotonin reuptake inhibitors (SSRIs) to have the best evidence for treatment of depression in the primary care setting.23 Ask the patient about previous antidepressant prescriptions they were given, if any, and weigh the benefits and adverse effects with the patient.
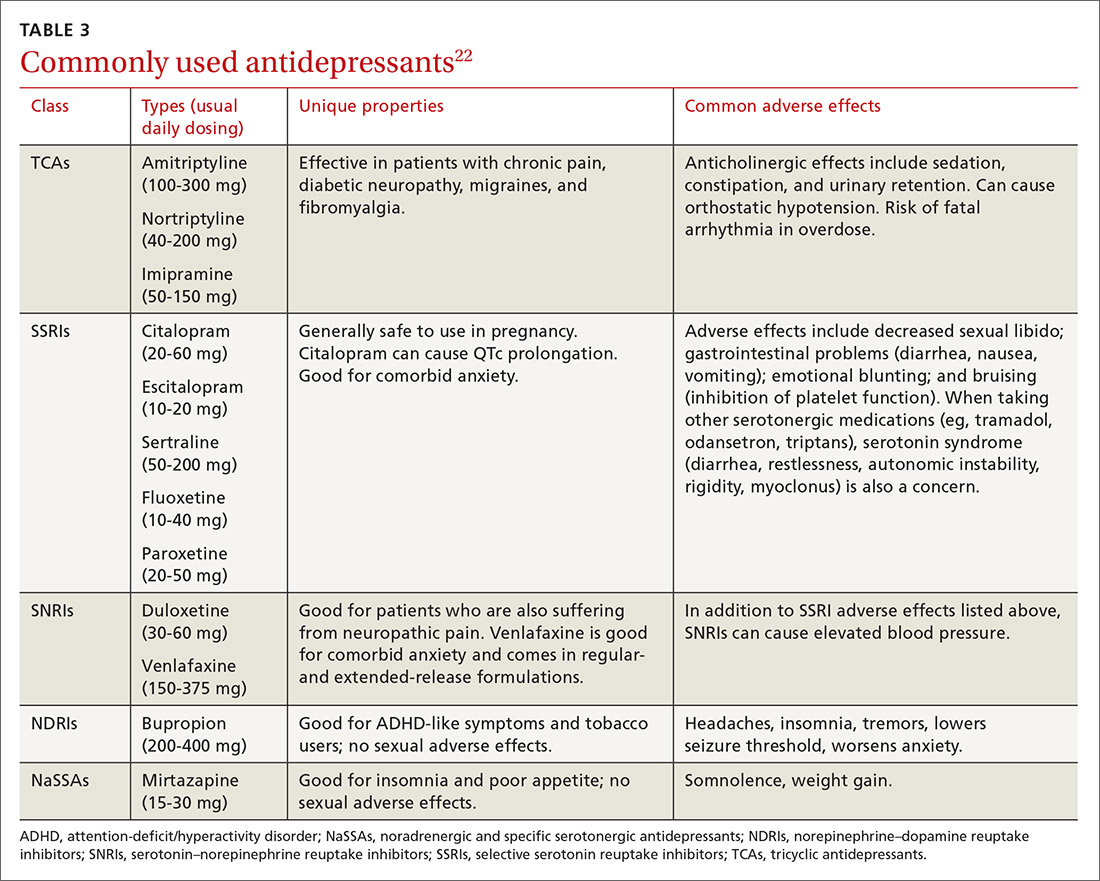
Patients may notice a partial response as early as one to 2 weeks after starting treatment with antidepressants, but it’s important to tell them that a full response can take up to 4 to 6 weeks. The goal of treatment is remission of depressive symptoms, which is defined as scoring below the cutoff point on a validated depression scale, such as less than 5 on the PHQ-9.24 It’s advisable to increase the antidepressant dose if the patient has a partial response and switch to a new class if the patient has no response or severe adverse effects.
Antidepressants should be maintained for at least 6 months or the length of a previous episode, whichever is greater.24 Prophylactic treatment should be considered for patients who have had severe episodes in the past (eg, a history of suicidal ideations and/or past hospitalizations). If an antidepressant is discontinued, it should be tapered over one to 2 weeks to minimize the risk of discontinuation syndrome (flu-like symptoms, nausea, insomnia, and hyperarousal). There is a lack of consistent evidence for the use of St. John’s wort, and as such, it is not recommended.24
Adjunct medications can also be used when remission does not occur after 8 to 12 weeks of maximum antidepressant doses. Insomnia, which is a common complaint in patients with MDD, can be treated with trazodone (an off-label indication), diphenhydramine, or melatonin. (See “Insomnia: Getting to the cause, facilitating relief.”) Benzodiazepines and other hypnotics (eg, zolpidem) can be used initially until antidepressants have had time to become effective. Antipsychotics such as aripiprazole, risperidone, quetiapine, and ziprasidone can be used to treat psychotic symptoms of depression or boost antidepressant effectiveness.25 Lithium and thyroxine are effective for treatment-resistant depression.26 Nutraceuticals such as S-Adenosyl-L-methionine, methylfolate, omega-3, and vitamin D can reduce depressive symptoms when combined with an antidepressant.27
There is some evidence to support combining 2 antidepressants from different classes (eg, an SSRI plus a serotonin–norepinephrine reuptake inhibitor [SNRI] or norepinephrine–dopamine reuptake inhibitor, or an SNRI plus a noradrenergic and specific serotonergic antidepressant) when adjunct therapy has proven ineffective.28
Inpatient psychiatric admission is warranted in severe cases, such as when a patient has active suicidal intentions/plans or poor self-care.
Your critical role, even when depression is co-managed
Collaborative care for depression (patient contact with both primary and behavioral health care providers in the same clinic) significantly improves clinical outcomes at 6 months compared to primary care treatment alone.29 Patients who have failed 2 therapeutic trials (at least 6-8 weeks of separate antidepressant treatments without response) are considered treatment-resistant.30 Referral to a psychiatrist is appropriate in this setting to determine alternative treatment options.
› CASE
Based on further conversation with Mr. M, you learn that he actually began exhibiting symptoms of depression (anhedonia, poor concentration, insomnia) years before he lost his job, but that he had considered the symptoms “normal” for his age. He reports that he didn’t want to socialize with others anymore and harbors feelings of worthlessness. You tell him that you believe he is suffering from MDD and talk to him about some options for treatment. You decide together to begin a trial of escitalopram 10 mg/d, as it was covered by his insurance, has minimal adverse effects, and was a good match for his symptoms. You also educate and instruct Mr. M on self-management goals such as limiting alcohol intake, eating at least 2 meals a day, walking with his wife each evening, and following a regular sleep schedule. You discuss a safety plan with Mr. M, should his depressive symptoms worsen. Specifically, you tell him that if he begins to have suicidal intentions or plans, he should call 911 or go to the nearest emergency department.
Mr. M returns 4 weeks later and reports that his mood has slightly improved, as evidenced by a brighter affect and increased energy, so you increase the dose of escitalopram to 20 mg/d. At his third visit 4 weeks later, Mr. M discloses a remote history of trauma and current intermittent heavy drinking. After offering support and education and discussing his options, you refer Mr. M to a counselor in your clinic through a “warm handoff” (the counselor is brought briefly into the current session with the patient to meet and set up an appointment). During this time, he is given information about an outpatient substance abuse treatment group.
Mr. M’s PHQ-9 improves by 8 points by his fourth visit 4 weeks later. He reports that he is still taking the escitalopram and you recommend he continue to take it. Mr. M tells you he’s been seeing the counselor at your clinic every other week and that he has begun attending meetings with the substance abuse group. He also says that he and his wife go out for walks now and then. Mr. M says he feels as though he is a failure, prompting you to recommend that he explore the cognitive distortions (ie, inaccurate thoughts that reinforce negative feelings) with his therapist.
You schedule another appointment with Mr. M in 3 months to keep track of his progress. Fortunately, Mr. M’s therapist works in the same clinic as you, so you can contact her to discuss his progress with therapy.
CORRESPONDENCE
Michael Raddock, MD, 2500 MetroHealth Drive, Cleveland, OH 44109; [email protected].
1. National Institute of Mental Health. Major depression among adults. National Institute of Mental Health Web site. Available at: http://www.nimh.nih.gov/health/statistics/prevalence/major-depression-among-adults.shtml. 2014. Accessed June 22, 2016.
2. Cameron C, Habert J, Anand L, et al. Optimizing the management of depression: primary care experience. Psychiatry Res. 2014;220:S45-S57.
3. Wang PS, Lane M, Olfson M, et al. Twelve-month use of mental health services in the United States: results from the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:629-640.
4. Schotte CK, Van Den Bossche B, De Doncker D, et al. A biopsychosocial model as a guide for psychoeducation and treatment of depression. Depress Anxiety. 2006;23:312-324.
5. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Washington, DC: American Psychiatric Association, 2013:160-161.
6. Sadock BJ, Sadock VA. Kaplan and Sadock’s Synopsis of Psychiatry. 9th ed. Philadelphia, PA: Lippincott Williams & Wilkins, 2003:830-834.
7. Fishbain DA, Cutler R, Rosomoff HL, et al. Chronic pain-associated depression: antecedent or consequence of chronic pain? A review. Clin J Pain. 1997;13:116-137.
8. Arroll B, Khin N, Kerse N. Screening for depression in primary care with two verbally asked questions: cross sectional study. BMJ. 2003;327:1144-1146.
9. Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606-613.
10. US Preventive Services Task Force. Depression in adults: Screening. US Preventive Services Task Force Web site. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/depression-in-adults-screening. Accessed March 13, 2017.
11. Thombs BD, Ziegelstein RC. Does depression screening improve depression outcomes in primary care? BMJ. 2014;348:g1253.
12. Siu AL, Bibbins-Domingo K, Grossman DC, et al. Screening for depression in adults: US Preventive Services Task Force recommendation statement. JAMA. 2016;315:380-387.
13. Sadock BJ, Sadock VA. Kaplan and Sadock’s Synopsis of Psychiatry. 9th ed. Philadelphia, PA: Lippincott Williams & Wilkins, 2003:543.
14. Gould MS, Marrocco FA, Kleinman M, et al. Evaluating iatrogenic risk of youth suicide screening programs: a randomized controlled trial. JAMA. 2005;293:1635-1643.
15. Eynan R, Bergmans Y, Antony J, et al. The effects of suicide ideation assessments on urges to self-harm and suicide. Crisis. 2014;35:123-131.
16. Pampallona S, Bollini P, Tibaldi G, et al. Combined pharmacotherapy and psychological treatment for depression: a systematic review. Arch Gen Psychiatry. 2004;61:714-719.
17. Ishak WW, Ha K, Kapitanski N, et al. The impact of psychotherapy, pharmacotherapy, and their combination on quality of life in depression. Harv Rev Psychiatry. 2011;19:277-289.
18. Raddock M, Martukovich R, Berko E, et al. 7 tools to help patients adopt healthier behaviors. J Fam Pract. 2015;64:97-103.
19. Castonguay LG, Constantino MJ, Holtforth MG. The working alliance: Where are we and where should we go? Psychotherapy (Chic). 2006;43:271-279.
20. Greenberg RP, Constantino MJ, Bruce N. Are patient expectations still relevant for psychotherapy process and outcome? Clin Psychol Rev. 2006;26:657-678.
21. Warden D, Rush AJ, Trivedi MH, et al. The STAR*D Project results: a comprehensive review of findings. Curr Psychiatry Rep. 2007;9:449-459.
22. Sadock BJ, Sadock VA. Kaplan and Sadock’s Synopsis of Psychiatry. 9th ed. Philadelphia, PA: Lippincott Williams & Wilkins, 2003:558.
23. Linde K, Kriston L, Rücker G, et al. Efficacy and acceptability of pharmacological treatments for depressive disorders in primary care: systematic review and network meta-analysis. Ann Fam Med. 2015;13:69-79.
24. American Psychiatric Association. Practice guideline for the treatment of patients with major depressive disorder. 3rd ed. 2010. Available at: http://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/mdd.pdf. Accessed December 23, 2016.
25. Zhou X, Keitner GI, Qin B, et al. Atypical antipsychotic treatment for treatment-resistant depression: A systematic review and network meta-analysis. Int J Neuropsychopharmacol. 2015;18:pyv060.
26. Nierenberg AA, Fava M, Trivedi MH, et al. A comparison of lithium and T(3) augmentation following two failed medication treatments for depression: a STAR*D report. Am J Psychiatry. 2006;163:1519-1530; quiz 1665.
27. Sarris J, Murphy J, Mischoulon D, et al. Adjunctive nutraceuticals for depression: A systematic review and meta-analyses. Am J Psychiatry. 2016;173:575-587.
28. Dodd S, Horgan D, Malhi GS, et al. To combine or not to combine? A literature review of antidepressant combination therapy. J Affect Disord. 2005;89:1-11.
29. Gilbody S, Bower P, Fletcher J, et al. Collaborative care for depression: a cumulative meta-analysis and review of longer-term outcomes. Arch Intern Med. 2006;166:2314-2321.
30. Papakostas GI, Fava M. Pharmacotherapy for Depression and Treatment-Resistant Depression. Hackensack, NJ: World Scientific. 2010:4.
THE CASE
As you get ready to see your next patient, 52-year-old Jim M, you see in his chart that during an annual routine nurse screening (per office protocol), he scored positive for depressed mood/anhedonia on the Patient Health Questionnaire-2 (PHQ-2) and scored a 21 out of 27 on the full version (PHQ-9), suggesting that he has severe major depressive disorder and that antidepressants should be considered.
When you enter the exam room, you notice his sad expression, poor eye contact, and stooped posture. Mr. M says his wife “made him” come to see you. He reports low energy and not wanting to leave his house, which started about a year earlier after he lost his job. When you discuss his job loss and the impact it has had on him, he sheepishly admits to sometimes thinking that things would be better if he were dead. Upon further questioning, you learn that he does not have suicidal intentions or plans.
HOW WOULD YOU PROCEED WITH THIS PATIENT?
Depression is the most common mental health complaint in primary care settings; in 2015, an estimated 16.1 million (6.7%) adults in the United States ages 18 or older had at least one depressive episode in the past year.1 Depression results in significant health, work, and social life impairments,2 and comorbid anxiety is highly prevalent in patients with depression.
Primary care physicians see almost twice as many mental health patients as psychiatrists3 due to barriers in behavioral health treatment (such as wait times, cost, and stigma) and the fact that primary care physicians often provide first-line access to behavioral health resources. Depression is caused by biological, psychological, and social factors, and primary care physicians are ideally positioned to develop therapeutic, healing relationships with patients that coincide with the biopsychosocial model of the disease.4
This review will provide some useful tips and tools to ensure that these patients get the care they need.
Depression? Or are other factors at play?
Major depressive disorder (MDD) is defined as a clinically significant change in mood that lasts at least 2 weeks.5 The main symptoms of MDD include depressed mood and markedly diminished interest or pleasure; additional symptoms may include reduced self-esteem, weight/appetite changes, fatigue or reduced energy, guilt/worthlessness, decreased activity, poor concentration, and suicidal thinking.5 To meet the criteria for a diagnosis of MDD, patients must experience symptoms for most of the day, nearly every day. (Dysthymia or persistent depressive disorder is a type of depression that is milder and more chronic than MDD, but does not have as many symptoms as MDD.) The focus of this article will be on MDD.
Shared symptoms with other disorders
Depression often displays some of the same symptoms as bereavement disorder and adjustment disorder, as well as other conditions.
Grief over loss and depressive symptoms circumscribed to a stressor are considered bereavement disorder and adjustment disorder, respectively. These disorders are usually limited to weeks or months as the patient adapts to his/her particular situation.
Organic problems such as nutritional deficiencies and sleep apnea can cause, exacerbate, or mimic depression (TABLE 16). Pain and depression are often associated, in that chronic pain can precipitate or perpetuate depression.7

Bipolar disorder consists of both depressive and manic episodes; patients may be misdiagnosed and treated for depression alone.
Substance intoxication or withdrawal can precipitate or perpetuate depression. A period of abstinence of at least one month may be necessary to see if depressive symptoms persist or resolve.
Premenstrual dysphoric disorder is defined as a period of depressed mood that is limited to the final week before the onset of menses and resolves in the week post-menses.
How to make the diagnosis
Inquiring about prolonged feelings of sadness and/or lack of enjoyment in activities is an effective way to begin the screening process for depression.8 Screening tools such as the PHQ-9 (TABLE 29), Beck Depression Inventory, Hamilton Rating Scale for Depression, and Geriatric Depression Scale are useful when combined with a clinical interview. Another useful tool is the Mood Disorder Questionnaire, which can help one determine if a patient is suffering from depression or bipolar disorder. It’s available at: http://www.dbsalliance.org/pdfs/MDQ.pdf. (Asking about a history of consecutive days of elevated, expansive, or irritable mood accompanied by increased activity or energy can also provide valuable insight.)

For its part, the US Preventive Services Task Force recommends screening adults for depression when adequate systems are in place (eg, referrals to settings that can provide necessary care) so as “to assure accurate diagnosis, effective treatment, and follow-up.”10-12
Assessing severity. Asking about functional impairments at work and at home and with academics and relationships will help determine severity, as will inquiring about a patient’s past or current suicidal thoughts. About two-thirds of all patients with depression contemplate suicide and 10% to 15% will attempt suicide.13
There is no evidence that inquiring about thoughts of death or suicide exacerbates suicidal risk.14,15 Confirming a diagnosis of MDD may require multiple visits, but should not delay treatment.
Making the most of the tools at your disposal
As a family physician (FP), you are especially well positioned to help patients suffering from MDD by offering education, counseling, and support; prescribing antidepressants; and coordinating care. Collaboration with behavioral health teams may be beneficial, especially in complex and treatment-resistant cases.
Counseling, alone or combined with pharmacotherapy, may improve patient outcomes.16,17 A first step may be recommending behavior modifications (such as adequate sleep, exercise, and a healthy diet). FPs can learn to utilize several counseling techniques, such as motivational interviewing, solution-focused therapy, and supportive therapy, for a variety of clinical situations in which behavioral change would be helpful.18 Establishing a therapeutic alliance through empathy and creating treatment expectations are key to helping patients overcome depression.19,20 Referral to a therapist can help identify and manage psychosocial factors that are often inherent in depression. Explaining to the patient that depression is best improved with a combination of medication and therapy is often helpful in motivating the patient to see a therapist.
Selecting an antidepressant. There is insufficient evidence to show differences in remission rates or times to remission among antidepressants,21 so medication choice involves balancing factors such as cost, previous treatments, adverse effects, and comorbid conditions (TABLE 322). A recent systematic review and meta-analysis involving 66 studies and more than 15,000 patients found tricyclic/tetracyclic antidepressants and selective serotonin reuptake inhibitors (SSRIs) to have the best evidence for treatment of depression in the primary care setting.23 Ask the patient about previous antidepressant prescriptions they were given, if any, and weigh the benefits and adverse effects with the patient.

Patients may notice a partial response as early as one to 2 weeks after starting treatment with antidepressants, but it’s important to tell them that a full response can take up to 4 to 6 weeks. The goal of treatment is remission of depressive symptoms, which is defined as scoring below the cutoff point on a validated depression scale, such as less than 5 on the PHQ-9.24 It’s advisable to increase the antidepressant dose if the patient has a partial response and switch to a new class if the patient has no response or severe adverse effects.
Antidepressants should be maintained for at least 6 months or the length of a previous episode, whichever is greater.24 Prophylactic treatment should be considered for patients who have had severe episodes in the past (eg, a history of suicidal ideations and/or past hospitalizations). If an antidepressant is discontinued, it should be tapered over one to 2 weeks to minimize the risk of discontinuation syndrome (flu-like symptoms, nausea, insomnia, and hyperarousal). There is a lack of consistent evidence for the use of St. John’s wort, and as such, it is not recommended.24
Adjunct medications can also be used when remission does not occur after 8 to 12 weeks of maximum antidepressant doses. Insomnia, which is a common complaint in patients with MDD, can be treated with trazodone (an off-label indication), diphenhydramine, or melatonin. (See “Insomnia: Getting to the cause, facilitating relief.”) Benzodiazepines and other hypnotics (eg, zolpidem) can be used initially until antidepressants have had time to become effective. Antipsychotics such as aripiprazole, risperidone, quetiapine, and ziprasidone can be used to treat psychotic symptoms of depression or boost antidepressant effectiveness.25 Lithium and thyroxine are effective for treatment-resistant depression.26 Nutraceuticals such as S-Adenosyl-L-methionine, methylfolate, omega-3, and vitamin D can reduce depressive symptoms when combined with an antidepressant.27
There is some evidence to support combining 2 antidepressants from different classes (eg, an SSRI plus a serotonin–norepinephrine reuptake inhibitor [SNRI] or norepinephrine–dopamine reuptake inhibitor, or an SNRI plus a noradrenergic and specific serotonergic antidepressant) when adjunct therapy has proven ineffective.28
Inpatient psychiatric admission is warranted in severe cases, such as when a patient has active suicidal intentions/plans or poor self-care.
Your critical role, even when depression is co-managed
Collaborative care for depression (patient contact with both primary and behavioral health care providers in the same clinic) significantly improves clinical outcomes at 6 months compared to primary care treatment alone.29 Patients who have failed 2 therapeutic trials (at least 6-8 weeks of separate antidepressant treatments without response) are considered treatment-resistant.30 Referral to a psychiatrist is appropriate in this setting to determine alternative treatment options.
› CASE
Based on further conversation with Mr. M, you learn that he actually began exhibiting symptoms of depression (anhedonia, poor concentration, insomnia) years before he lost his job, but that he had considered the symptoms “normal” for his age. He reports that he didn’t want to socialize with others anymore and harbors feelings of worthlessness. You tell him that you believe he is suffering from MDD and talk to him about some options for treatment. You decide together to begin a trial of escitalopram 10 mg/d, as it was covered by his insurance, has minimal adverse effects, and was a good match for his symptoms. You also educate and instruct Mr. M on self-management goals such as limiting alcohol intake, eating at least 2 meals a day, walking with his wife each evening, and following a regular sleep schedule. You discuss a safety plan with Mr. M, should his depressive symptoms worsen. Specifically, you tell him that if he begins to have suicidal intentions or plans, he should call 911 or go to the nearest emergency department.
Mr. M returns 4 weeks later and reports that his mood has slightly improved, as evidenced by a brighter affect and increased energy, so you increase the dose of escitalopram to 20 mg/d. At his third visit 4 weeks later, Mr. M discloses a remote history of trauma and current intermittent heavy drinking. After offering support and education and discussing his options, you refer Mr. M to a counselor in your clinic through a “warm handoff” (the counselor is brought briefly into the current session with the patient to meet and set up an appointment). During this time, he is given information about an outpatient substance abuse treatment group.
Mr. M’s PHQ-9 improves by 8 points by his fourth visit 4 weeks later. He reports that he is still taking the escitalopram and you recommend he continue to take it. Mr. M tells you he’s been seeing the counselor at your clinic every other week and that he has begun attending meetings with the substance abuse group. He also says that he and his wife go out for walks now and then. Mr. M says he feels as though he is a failure, prompting you to recommend that he explore the cognitive distortions (ie, inaccurate thoughts that reinforce negative feelings) with his therapist.
You schedule another appointment with Mr. M in 3 months to keep track of his progress. Fortunately, Mr. M’s therapist works in the same clinic as you, so you can contact her to discuss his progress with therapy.
CORRESPONDENCE
Michael Raddock, MD, 2500 MetroHealth Drive, Cleveland, OH 44109; [email protected].
THE CASE
As you get ready to see your next patient, 52-year-old Jim M, you see in his chart that during an annual routine nurse screening (per office protocol), he scored positive for depressed mood/anhedonia on the Patient Health Questionnaire-2 (PHQ-2) and scored a 21 out of 27 on the full version (PHQ-9), suggesting that he has severe major depressive disorder and that antidepressants should be considered.
When you enter the exam room, you notice his sad expression, poor eye contact, and stooped posture. Mr. M says his wife “made him” come to see you. He reports low energy and not wanting to leave his house, which started about a year earlier after he lost his job. When you discuss his job loss and the impact it has had on him, he sheepishly admits to sometimes thinking that things would be better if he were dead. Upon further questioning, you learn that he does not have suicidal intentions or plans.
HOW WOULD YOU PROCEED WITH THIS PATIENT?
Depression is the most common mental health complaint in primary care settings; in 2015, an estimated 16.1 million (6.7%) adults in the United States ages 18 or older had at least one depressive episode in the past year.1 Depression results in significant health, work, and social life impairments,2 and comorbid anxiety is highly prevalent in patients with depression.
Primary care physicians see almost twice as many mental health patients as psychiatrists3 due to barriers in behavioral health treatment (such as wait times, cost, and stigma) and the fact that primary care physicians often provide first-line access to behavioral health resources. Depression is caused by biological, psychological, and social factors, and primary care physicians are ideally positioned to develop therapeutic, healing relationships with patients that coincide with the biopsychosocial model of the disease.4
This review will provide some useful tips and tools to ensure that these patients get the care they need.
Depression? Or are other factors at play?
Major depressive disorder (MDD) is defined as a clinically significant change in mood that lasts at least 2 weeks.5 The main symptoms of MDD include depressed mood and markedly diminished interest or pleasure; additional symptoms may include reduced self-esteem, weight/appetite changes, fatigue or reduced energy, guilt/worthlessness, decreased activity, poor concentration, and suicidal thinking.5 To meet the criteria for a diagnosis of MDD, patients must experience symptoms for most of the day, nearly every day. (Dysthymia or persistent depressive disorder is a type of depression that is milder and more chronic than MDD, but does not have as many symptoms as MDD.) The focus of this article will be on MDD.
Shared symptoms with other disorders
Depression often displays some of the same symptoms as bereavement disorder and adjustment disorder, as well as other conditions.
Grief over loss and depressive symptoms circumscribed to a stressor are considered bereavement disorder and adjustment disorder, respectively. These disorders are usually limited to weeks or months as the patient adapts to his/her particular situation.
Organic problems such as nutritional deficiencies and sleep apnea can cause, exacerbate, or mimic depression (TABLE 16). Pain and depression are often associated, in that chronic pain can precipitate or perpetuate depression.7

Bipolar disorder consists of both depressive and manic episodes; patients may be misdiagnosed and treated for depression alone.
Substance intoxication or withdrawal can precipitate or perpetuate depression. A period of abstinence of at least one month may be necessary to see if depressive symptoms persist or resolve.
Premenstrual dysphoric disorder is defined as a period of depressed mood that is limited to the final week before the onset of menses and resolves in the week post-menses.
How to make the diagnosis
Inquiring about prolonged feelings of sadness and/or lack of enjoyment in activities is an effective way to begin the screening process for depression.8 Screening tools such as the PHQ-9 (TABLE 29), Beck Depression Inventory, Hamilton Rating Scale for Depression, and Geriatric Depression Scale are useful when combined with a clinical interview. Another useful tool is the Mood Disorder Questionnaire, which can help one determine if a patient is suffering from depression or bipolar disorder. It’s available at: http://www.dbsalliance.org/pdfs/MDQ.pdf. (Asking about a history of consecutive days of elevated, expansive, or irritable mood accompanied by increased activity or energy can also provide valuable insight.)

For its part, the US Preventive Services Task Force recommends screening adults for depression when adequate systems are in place (eg, referrals to settings that can provide necessary care) so as “to assure accurate diagnosis, effective treatment, and follow-up.”10-12
Assessing severity. Asking about functional impairments at work and at home and with academics and relationships will help determine severity, as will inquiring about a patient’s past or current suicidal thoughts. About two-thirds of all patients with depression contemplate suicide and 10% to 15% will attempt suicide.13
There is no evidence that inquiring about thoughts of death or suicide exacerbates suicidal risk.14,15 Confirming a diagnosis of MDD may require multiple visits, but should not delay treatment.
Making the most of the tools at your disposal
As a family physician (FP), you are especially well positioned to help patients suffering from MDD by offering education, counseling, and support; prescribing antidepressants; and coordinating care. Collaboration with behavioral health teams may be beneficial, especially in complex and treatment-resistant cases.
Counseling, alone or combined with pharmacotherapy, may improve patient outcomes.16,17 A first step may be recommending behavior modifications (such as adequate sleep, exercise, and a healthy diet). FPs can learn to utilize several counseling techniques, such as motivational interviewing, solution-focused therapy, and supportive therapy, for a variety of clinical situations in which behavioral change would be helpful.18 Establishing a therapeutic alliance through empathy and creating treatment expectations are key to helping patients overcome depression.19,20 Referral to a therapist can help identify and manage psychosocial factors that are often inherent in depression. Explaining to the patient that depression is best improved with a combination of medication and therapy is often helpful in motivating the patient to see a therapist.
Selecting an antidepressant. There is insufficient evidence to show differences in remission rates or times to remission among antidepressants,21 so medication choice involves balancing factors such as cost, previous treatments, adverse effects, and comorbid conditions (TABLE 322). A recent systematic review and meta-analysis involving 66 studies and more than 15,000 patients found tricyclic/tetracyclic antidepressants and selective serotonin reuptake inhibitors (SSRIs) to have the best evidence for treatment of depression in the primary care setting.23 Ask the patient about previous antidepressant prescriptions they were given, if any, and weigh the benefits and adverse effects with the patient.

Patients may notice a partial response as early as one to 2 weeks after starting treatment with antidepressants, but it’s important to tell them that a full response can take up to 4 to 6 weeks. The goal of treatment is remission of depressive symptoms, which is defined as scoring below the cutoff point on a validated depression scale, such as less than 5 on the PHQ-9.24 It’s advisable to increase the antidepressant dose if the patient has a partial response and switch to a new class if the patient has no response or severe adverse effects.
Antidepressants should be maintained for at least 6 months or the length of a previous episode, whichever is greater.24 Prophylactic treatment should be considered for patients who have had severe episodes in the past (eg, a history of suicidal ideations and/or past hospitalizations). If an antidepressant is discontinued, it should be tapered over one to 2 weeks to minimize the risk of discontinuation syndrome (flu-like symptoms, nausea, insomnia, and hyperarousal). There is a lack of consistent evidence for the use of St. John’s wort, and as such, it is not recommended.24
Adjunct medications can also be used when remission does not occur after 8 to 12 weeks of maximum antidepressant doses. Insomnia, which is a common complaint in patients with MDD, can be treated with trazodone (an off-label indication), diphenhydramine, or melatonin. (See “Insomnia: Getting to the cause, facilitating relief.”) Benzodiazepines and other hypnotics (eg, zolpidem) can be used initially until antidepressants have had time to become effective. Antipsychotics such as aripiprazole, risperidone, quetiapine, and ziprasidone can be used to treat psychotic symptoms of depression or boost antidepressant effectiveness.25 Lithium and thyroxine are effective for treatment-resistant depression.26 Nutraceuticals such as S-Adenosyl-L-methionine, methylfolate, omega-3, and vitamin D can reduce depressive symptoms when combined with an antidepressant.27
There is some evidence to support combining 2 antidepressants from different classes (eg, an SSRI plus a serotonin–norepinephrine reuptake inhibitor [SNRI] or norepinephrine–dopamine reuptake inhibitor, or an SNRI plus a noradrenergic and specific serotonergic antidepressant) when adjunct therapy has proven ineffective.28
Inpatient psychiatric admission is warranted in severe cases, such as when a patient has active suicidal intentions/plans or poor self-care.
Your critical role, even when depression is co-managed
Collaborative care for depression (patient contact with both primary and behavioral health care providers in the same clinic) significantly improves clinical outcomes at 6 months compared to primary care treatment alone.29 Patients who have failed 2 therapeutic trials (at least 6-8 weeks of separate antidepressant treatments without response) are considered treatment-resistant.30 Referral to a psychiatrist is appropriate in this setting to determine alternative treatment options.
› CASE
Based on further conversation with Mr. M, you learn that he actually began exhibiting symptoms of depression (anhedonia, poor concentration, insomnia) years before he lost his job, but that he had considered the symptoms “normal” for his age. He reports that he didn’t want to socialize with others anymore and harbors feelings of worthlessness. You tell him that you believe he is suffering from MDD and talk to him about some options for treatment. You decide together to begin a trial of escitalopram 10 mg/d, as it was covered by his insurance, has minimal adverse effects, and was a good match for his symptoms. You also educate and instruct Mr. M on self-management goals such as limiting alcohol intake, eating at least 2 meals a day, walking with his wife each evening, and following a regular sleep schedule. You discuss a safety plan with Mr. M, should his depressive symptoms worsen. Specifically, you tell him that if he begins to have suicidal intentions or plans, he should call 911 or go to the nearest emergency department.
Mr. M returns 4 weeks later and reports that his mood has slightly improved, as evidenced by a brighter affect and increased energy, so you increase the dose of escitalopram to 20 mg/d. At his third visit 4 weeks later, Mr. M discloses a remote history of trauma and current intermittent heavy drinking. After offering support and education and discussing his options, you refer Mr. M to a counselor in your clinic through a “warm handoff” (the counselor is brought briefly into the current session with the patient to meet and set up an appointment). During this time, he is given information about an outpatient substance abuse treatment group.
Mr. M’s PHQ-9 improves by 8 points by his fourth visit 4 weeks later. He reports that he is still taking the escitalopram and you recommend he continue to take it. Mr. M tells you he’s been seeing the counselor at your clinic every other week and that he has begun attending meetings with the substance abuse group. He also says that he and his wife go out for walks now and then. Mr. M says he feels as though he is a failure, prompting you to recommend that he explore the cognitive distortions (ie, inaccurate thoughts that reinforce negative feelings) with his therapist.
You schedule another appointment with Mr. M in 3 months to keep track of his progress. Fortunately, Mr. M’s therapist works in the same clinic as you, so you can contact her to discuss his progress with therapy.
CORRESPONDENCE
Michael Raddock, MD, 2500 MetroHealth Drive, Cleveland, OH 44109; [email protected].
1. National Institute of Mental Health. Major depression among adults. National Institute of Mental Health Web site. Available at: http://www.nimh.nih.gov/health/statistics/prevalence/major-depression-among-adults.shtml. 2014. Accessed June 22, 2016.
2. Cameron C, Habert J, Anand L, et al. Optimizing the management of depression: primary care experience. Psychiatry Res. 2014;220:S45-S57.
3. Wang PS, Lane M, Olfson M, et al. Twelve-month use of mental health services in the United States: results from the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:629-640.
4. Schotte CK, Van Den Bossche B, De Doncker D, et al. A biopsychosocial model as a guide for psychoeducation and treatment of depression. Depress Anxiety. 2006;23:312-324.
5. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Washington, DC: American Psychiatric Association, 2013:160-161.
6. Sadock BJ, Sadock VA. Kaplan and Sadock’s Synopsis of Psychiatry. 9th ed. Philadelphia, PA: Lippincott Williams & Wilkins, 2003:830-834.
7. Fishbain DA, Cutler R, Rosomoff HL, et al. Chronic pain-associated depression: antecedent or consequence of chronic pain? A review. Clin J Pain. 1997;13:116-137.
8. Arroll B, Khin N, Kerse N. Screening for depression in primary care with two verbally asked questions: cross sectional study. BMJ. 2003;327:1144-1146.
9. Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606-613.
10. US Preventive Services Task Force. Depression in adults: Screening. US Preventive Services Task Force Web site. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/depression-in-adults-screening. Accessed March 13, 2017.
11. Thombs BD, Ziegelstein RC. Does depression screening improve depression outcomes in primary care? BMJ. 2014;348:g1253.
12. Siu AL, Bibbins-Domingo K, Grossman DC, et al. Screening for depression in adults: US Preventive Services Task Force recommendation statement. JAMA. 2016;315:380-387.
13. Sadock BJ, Sadock VA. Kaplan and Sadock’s Synopsis of Psychiatry. 9th ed. Philadelphia, PA: Lippincott Williams & Wilkins, 2003:543.
14. Gould MS, Marrocco FA, Kleinman M, et al. Evaluating iatrogenic risk of youth suicide screening programs: a randomized controlled trial. JAMA. 2005;293:1635-1643.
15. Eynan R, Bergmans Y, Antony J, et al. The effects of suicide ideation assessments on urges to self-harm and suicide. Crisis. 2014;35:123-131.
16. Pampallona S, Bollini P, Tibaldi G, et al. Combined pharmacotherapy and psychological treatment for depression: a systematic review. Arch Gen Psychiatry. 2004;61:714-719.
17. Ishak WW, Ha K, Kapitanski N, et al. The impact of psychotherapy, pharmacotherapy, and their combination on quality of life in depression. Harv Rev Psychiatry. 2011;19:277-289.
18. Raddock M, Martukovich R, Berko E, et al. 7 tools to help patients adopt healthier behaviors. J Fam Pract. 2015;64:97-103.
19. Castonguay LG, Constantino MJ, Holtforth MG. The working alliance: Where are we and where should we go? Psychotherapy (Chic). 2006;43:271-279.
20. Greenberg RP, Constantino MJ, Bruce N. Are patient expectations still relevant for psychotherapy process and outcome? Clin Psychol Rev. 2006;26:657-678.
21. Warden D, Rush AJ, Trivedi MH, et al. The STAR*D Project results: a comprehensive review of findings. Curr Psychiatry Rep. 2007;9:449-459.
22. Sadock BJ, Sadock VA. Kaplan and Sadock’s Synopsis of Psychiatry. 9th ed. Philadelphia, PA: Lippincott Williams & Wilkins, 2003:558.
23. Linde K, Kriston L, Rücker G, et al. Efficacy and acceptability of pharmacological treatments for depressive disorders in primary care: systematic review and network meta-analysis. Ann Fam Med. 2015;13:69-79.
24. American Psychiatric Association. Practice guideline for the treatment of patients with major depressive disorder. 3rd ed. 2010. Available at: http://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/mdd.pdf. Accessed December 23, 2016.
25. Zhou X, Keitner GI, Qin B, et al. Atypical antipsychotic treatment for treatment-resistant depression: A systematic review and network meta-analysis. Int J Neuropsychopharmacol. 2015;18:pyv060.
26. Nierenberg AA, Fava M, Trivedi MH, et al. A comparison of lithium and T(3) augmentation following two failed medication treatments for depression: a STAR*D report. Am J Psychiatry. 2006;163:1519-1530; quiz 1665.
27. Sarris J, Murphy J, Mischoulon D, et al. Adjunctive nutraceuticals for depression: A systematic review and meta-analyses. Am J Psychiatry. 2016;173:575-587.
28. Dodd S, Horgan D, Malhi GS, et al. To combine or not to combine? A literature review of antidepressant combination therapy. J Affect Disord. 2005;89:1-11.
29. Gilbody S, Bower P, Fletcher J, et al. Collaborative care for depression: a cumulative meta-analysis and review of longer-term outcomes. Arch Intern Med. 2006;166:2314-2321.
30. Papakostas GI, Fava M. Pharmacotherapy for Depression and Treatment-Resistant Depression. Hackensack, NJ: World Scientific. 2010:4.
1. National Institute of Mental Health. Major depression among adults. National Institute of Mental Health Web site. Available at: http://www.nimh.nih.gov/health/statistics/prevalence/major-depression-among-adults.shtml. 2014. Accessed June 22, 2016.
2. Cameron C, Habert J, Anand L, et al. Optimizing the management of depression: primary care experience. Psychiatry Res. 2014;220:S45-S57.
3. Wang PS, Lane M, Olfson M, et al. Twelve-month use of mental health services in the United States: results from the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:629-640.
4. Schotte CK, Van Den Bossche B, De Doncker D, et al. A biopsychosocial model as a guide for psychoeducation and treatment of depression. Depress Anxiety. 2006;23:312-324.
5. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Washington, DC: American Psychiatric Association, 2013:160-161.
6. Sadock BJ, Sadock VA. Kaplan and Sadock’s Synopsis of Psychiatry. 9th ed. Philadelphia, PA: Lippincott Williams & Wilkins, 2003:830-834.
7. Fishbain DA, Cutler R, Rosomoff HL, et al. Chronic pain-associated depression: antecedent or consequence of chronic pain? A review. Clin J Pain. 1997;13:116-137.
8. Arroll B, Khin N, Kerse N. Screening for depression in primary care with two verbally asked questions: cross sectional study. BMJ. 2003;327:1144-1146.
9. Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606-613.
10. US Preventive Services Task Force. Depression in adults: Screening. US Preventive Services Task Force Web site. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/depression-in-adults-screening. Accessed March 13, 2017.
11. Thombs BD, Ziegelstein RC. Does depression screening improve depression outcomes in primary care? BMJ. 2014;348:g1253.
12. Siu AL, Bibbins-Domingo K, Grossman DC, et al. Screening for depression in adults: US Preventive Services Task Force recommendation statement. JAMA. 2016;315:380-387.
13. Sadock BJ, Sadock VA. Kaplan and Sadock’s Synopsis of Psychiatry. 9th ed. Philadelphia, PA: Lippincott Williams & Wilkins, 2003:543.
14. Gould MS, Marrocco FA, Kleinman M, et al. Evaluating iatrogenic risk of youth suicide screening programs: a randomized controlled trial. JAMA. 2005;293:1635-1643.
15. Eynan R, Bergmans Y, Antony J, et al. The effects of suicide ideation assessments on urges to self-harm and suicide. Crisis. 2014;35:123-131.
16. Pampallona S, Bollini P, Tibaldi G, et al. Combined pharmacotherapy and psychological treatment for depression: a systematic review. Arch Gen Psychiatry. 2004;61:714-719.
17. Ishak WW, Ha K, Kapitanski N, et al. The impact of psychotherapy, pharmacotherapy, and their combination on quality of life in depression. Harv Rev Psychiatry. 2011;19:277-289.
18. Raddock M, Martukovich R, Berko E, et al. 7 tools to help patients adopt healthier behaviors. J Fam Pract. 2015;64:97-103.
19. Castonguay LG, Constantino MJ, Holtforth MG. The working alliance: Where are we and where should we go? Psychotherapy (Chic). 2006;43:271-279.
20. Greenberg RP, Constantino MJ, Bruce N. Are patient expectations still relevant for psychotherapy process and outcome? Clin Psychol Rev. 2006;26:657-678.
21. Warden D, Rush AJ, Trivedi MH, et al. The STAR*D Project results: a comprehensive review of findings. Curr Psychiatry Rep. 2007;9:449-459.
22. Sadock BJ, Sadock VA. Kaplan and Sadock’s Synopsis of Psychiatry. 9th ed. Philadelphia, PA: Lippincott Williams & Wilkins, 2003:558.
23. Linde K, Kriston L, Rücker G, et al. Efficacy and acceptability of pharmacological treatments for depressive disorders in primary care: systematic review and network meta-analysis. Ann Fam Med. 2015;13:69-79.
24. American Psychiatric Association. Practice guideline for the treatment of patients with major depressive disorder. 3rd ed. 2010. Available at: http://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/mdd.pdf. Accessed December 23, 2016.
25. Zhou X, Keitner GI, Qin B, et al. Atypical antipsychotic treatment for treatment-resistant depression: A systematic review and network meta-analysis. Int J Neuropsychopharmacol. 2015;18:pyv060.
26. Nierenberg AA, Fava M, Trivedi MH, et al. A comparison of lithium and T(3) augmentation following two failed medication treatments for depression: a STAR*D report. Am J Psychiatry. 2006;163:1519-1530; quiz 1665.
27. Sarris J, Murphy J, Mischoulon D, et al. Adjunctive nutraceuticals for depression: A systematic review and meta-analyses. Am J Psychiatry. 2016;173:575-587.
28. Dodd S, Horgan D, Malhi GS, et al. To combine or not to combine? A literature review of antidepressant combination therapy. J Affect Disord. 2005;89:1-11.
29. Gilbody S, Bower P, Fletcher J, et al. Collaborative care for depression: a cumulative meta-analysis and review of longer-term outcomes. Arch Intern Med. 2006;166:2314-2321.
30. Papakostas GI, Fava M. Pharmacotherapy for Depression and Treatment-Resistant Depression. Hackensack, NJ: World Scientific. 2010:4.
7 tools to help patients adopt healthier behaviors
› Determine the patient’s stage of change (Precontemplation, Contemplation, Preparation, Action, Maintenance, or Relapse) before selecting an intervention to help him or her change health-related behaviors. B
› Consider using motivational interviewing or narrative techniques to help patients who aren’t yet ready to change their health-related behaviors or who plan to do so within 6 months. C
› Be aware that patients seldom become motivated to change behaviors by being given information about health risks and benefits; to overcome ambivalence, they need to focus on their core values and goals. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE › Your patient, Bob G, age 47, has a body mass index of 33, hypertension (blood pressure 150/85 mm Hg), and elevated cholesterol (low-density lipoprotein level, 187 mg/dL) and glucose levels (fasting glucose 122 mg/dL, with an HbA1c of 6.1%). He gets out of breath when he plays with his 2 children. His father has diabetes and had a myocardial infarction (MI) at age 55; Mr. G tells you he is concerned he will develop similar health problems. Mr. G frequents fast food restaurants and eats high-calorie snacks after work, especially when he feels stressed. During a recent office visit, he expresses his desire to “be there” for his children and says he is motivated to lose weight to prevent diabetes and/or an MI.
How would you proceed?
Most health conditions in the United States are directly or indirectly the result of patients’ health-related behaviors.1 Fortunately, family physicians (FPs) and primary care teams are in an excellent position to help their patients make healthy behavior changes by using brief, evidence-based interventions that can be implemented during the typical office visit.
Specifically, the use of the following 7 techniques can build on patients’ own motivations, successes, and life circumstances to improve their satisfaction and self-efficacy:
- the 5 As (Ask, Advise, Assess, Assist, Arrange)
- the FRAMES protocol (Feedback, Responsibility, Advice, Menu, Empathy, and Self-efficacy)
- teachable moments (TM)
- solution-focused brief therapy (SFBT)
- cognitive behavioral therapy (CBT)
- narrative techniques (NT)
- motivational interviewing (MI).
But before we describe the practical application of these 7 techniques, we’ll begin by explaining a few underlying concepts for helping patients change their health-related behaviors.
Understanding what does—and doesn’t—help patients change
Research from the field of psychology and other social sciences has described several important concepts that affect how FPs can best help their patients change to healthier behaviors.2-9 First, several “common factors” have been found to reliably predict behavior change. The likelihood of change is strongly tied to the patient’s strengths, the environment, and the quality of the physician-patient relationship. The patient’s expectations and the techniques a physician uses also predict behavior change, but to a lesser extent.10
Second, patients seldom become motivated to change ingrained behaviors solely by being provided with information about the risks and benefits associated with those behaviors. People overcome ambivalence and develop motivation for change when they align their behaviors with their core values and goals. FPs can help patients link their motivation to change to specific plans and environments. This can then facilitate small changes that can yield large returns by increasing a patient’s self-efficacy and sense of control.2-9
Third, willpower is a finite but renewable resource that increases or decreases based on an individual’s internal and external environments. Reliance on willpower alone to make changes is unlikely to be successful without shaping the environment to support the new behavior.11
A patient’s readiness to change affects choice of technique
Knowing how ready a patient is to change is important for determining which approaches are likely to be effective at a given visit. Prochaska and DiClemente developed a model that defines 6 stages of change: Precontemplation (patient does not intend to change in the next 6 months), Contemplation (patient intends to change within the next 6 months), Preparation (patient intends to change within the next month), Action (patient has made specific changes within the past 6 months), Maintenance (patient works to prevent relapse), and Relapse (patient returns to an earlier stage) (TABLE).12
Patients who identify change as important and are ready to make changes benefit from collaborative work with an FP or other clinicians on the how, when, where, and who (eg, the patient, his or her significant other, family, and friends) of the new behaviors. These individuals are in the Preparation or Action stages of change, which comprise roughly 20% of patients.13 In these circumstances, techniques such as the 5 As, FRAMES, TM, SFBT, and CBT can be effective.
For the estimated 80% of patients who are in the Precontemplation or Contemplation stages and are unsure about the relative importance of changing behaviors and/or lack confidence to make changes, these directive techniques can cause defensiveness, which can make both the patient and the FP uncomfortable. For such patients, approaches that build on the patient’s own motivations and stories, such as MI and NT, may be preferred.
3 techniques that overlap
The FRAMES protocol and TM are based on behavior change theories, and each mixes directive techniques with relationship building to facilitate health behavior change. There is overlap in concepts across the 5 As, FRAMES, and TM, and some evidence suggests these approaches can be adapted for use in primary care settings.14-16
The 5 As is a brief intervention in which the FP sets an agenda and provides advice at the outset. This technique has been shown to improve smoking cessation rates in pregnant women compared with physician recommendations alone.17 It may also help with weight loss for patients who are ready to change and are given support for their
efforts.18
Putting the 5 As into action
CASE › An FP who wants to use the 5 As technique to assist Mr. G might proceed as follows: Ask: “How often do you exercise and follow a diet?” Advise: “I recommend that you start exercising 30 minutes each day and start following a healthier diet. It is one of the most important things you can do for your health.” Assess: “Are you willing to start exercising and trying a diet in the next month?” Assist: “Here is a list of local recreation centers and some information about a healthy diet.” Arrange: “I’d like to have one of the nurses call you in a week to see how things are going and have you return in a month for a follow-up appointment.”
The 6 components of the FRAMES protocol overlap with the 5 As.14 FRAMES utilizes relationship-building by explicitly reinforcing patient autonomy, offering a menu of choices, and acknowledging patient strengths.14
Putting the FRAMES protocol into action
CASE › Using the FRAMES protocol for Mr. G might consist of the following: Feedback: “Your eating habits and lack of exercise have contributed to your weight, high glucose and cholesterol levels, and shortness of breath.” Responsibility: “The decision to lose weight is a choice only you can make.” Advice: “I recommend that you start regularly exercising and eating healthily.” Menu: “Here are some options that many people find helpful when they try to lose weight.” Empathy: “It is challenging to change the way we eat and exercise.” Self-efficacy: “You have been able to overcome a lot of difficult things in your life already and it seems very important to you to make these changes.”
TM begins with the FP linking a patient concern, such as shortness of breath, to a physician concern, such as obesity.15 The FP then provides advice, assesses readiness, and responds based on the patient’s stage of change.16
Putting TM into action
CASE › Using the TM approach to help Mr. G might work as follows: Link a patient concern with specific behavioral change: “I think that your shortness of breath is caused by your weight.” Recommend change, offer support, and ask for commitment: “I recommend that you lose 15 pounds. I’m confident that you can do this, and am here to help you. Are you ready to talk about some specific ways you can do this?” Respond based on the patient’s readiness to change: “All right, let’s talk about healthy food choices and exercise.” (This statement would be appropriate if Mr. G was in the Preparation or Action stage of change.)
Solution-focused brief therapy
SFBT highlights a patient’s previous successes and strengths, as opposed to exploring problems and past failures.19,20 The FP fosters behavior change by using strategic questions to develop an intervention with the patient.21 SFBT involves encouraging patients to find exceptions to current problems and increasing the occurrence of current beneficial behaviors.19 This approach begins with the patient identifying a problem for which he or she would like help. The FP helps the patient explore solutions and/or exceptions to this problem that have worked for the patient previously (or solutions/exceptions that the patient can imagine). The FP does not offer suggestions to solve the patient’s problem. Instead, the patient and FP collaboratively identify and support the patient’s strengths, and they develop a behavioral task to try based on these patient-derived solutions.22
Putting SFBT into action
CASE › A physician who wants to use SFBT to help Mr. G might start by asking an “exception” question (“When have you been able to eat in a more healthy way?”) and following up with a “difference” question (“What was different about those times?”). Perhaps Mr. G remembers that previously he had improved his diet by buying and keeping a bag of apples in the car to snack on. He additionally recalls that he ate less at night if he brushed his teeth right after dinner.
Mr. G decides to revisit the apple and brushing strategies. Mr. G’s physician commends him for wanting to be there for his kids and identifying the apple and brushing strategies. She helps him design a small “experiment” in which he would use these strategies and observe the outcomes. They arrange to speak in one month to discuss how things are going.
Cognitive behavioral therapy
CBT is a practical, goal-directed, action-oriented treatment that focuses on helping patients make changes in their thinking and behavior.23-28 A basic premise of CBT is that emotions are difficult to change directly, so CBT targets distressing emotions by focusing on changing thoughts and/or behaviors that contribute to those emotions. CBT can be useful when the patient and FP can find a link between the patient’s thoughts and a troubling behavior. After thoroughly assessing situations that bother the patient, the FP provides the patient with an empathic summary that captures the essence of the problem. CBT practitioners typically conceptualize problems and plan treatment by working with the patient to gather information on the patient’s thoughts, feelings, and behaviors.
By exploring patterns of thinking that lead to self-destructive behaviors, an FP can help the patient understand and challenge strongly held but often limited patterns of thinking. For example, a patient with depression-related overeating might think, “I am worthless. Nothing ever goes right for me.” A patient with anxiety-related smoking may believe, “I am in danger.” Through a collaborative, respectful relationship, patients learn to test their “hypotheses,” challenge their thoughts, and experiment with alternate ways of thinking and behaving. Patients are given homework assignments, such as tracking their thoughts and behaviors, practicing relaxation techniques, and challenging automatic ways of viewing themselves and the world around them.
Putting CBT into action
CASE › An FP who wants to implement CBT to help Mr. G would begin by trying to understand his patient’s view: “Tell me how your weight is a part of your life.” Next, he would offer Mr. G an empathic summary: “You’re worried that your weight could cause some of the same health problems your dad has and that will prevent you from being the kind of active father you want to be. You sometimes eat when you are feeling stressed and tired, but then you feel worse afterwards.” He would assign Mr. G homework: “Notice and write down your thoughts when you are eating due to stress rather than hunger. Bring this in and we can look at it together.” The FP might also teach Mr. G relaxation breathing, and encourage him to try doing 5 relaxation breaths when he feels stressed and wants to eat.
Narrative techniques
NT can be effective for patients who are in the Precontemplation or Contemplation stages of change.29-33 NT focuses on the patient’s story, context, and language. FPs explore connections, discuss hypotheses, strategize, share power with the patient, and offer reflections in order to understand the patient’s illness experience. This approach fits well with the complex way that many behaviors are woven into the concerns that patients bring to their FPs. As a patient’s story unfolds, the diagnosis and treatment can occur simultaneously. The FP involves the patient in choices about how to proceed and what to focus on together.
NT avoids unsolicited advice and interpretations and rarely imposes the FP’s agenda on the patient. This approach invites the FP and patient to co-create an understanding and narrative of what the symptoms mean, why they are there, and what can be done about them. Ultimately, this approach can result in a new narrative that puts the patient on the path to healing.29-33
Putting NT into action
CASE › A physician might implement an NT approach with Mr. G to co-create a narrative about health and life goals by asking him: “Tell me about how losing weight fits into your goals for being there for your children. Tell me about how you see yourself avoiding some of the health problems your dad has faced.”
Motivational interviewing
For patients who are in the Precontemplation or Contemplation stages of change, MI might be a helpful approach. MI is a person-centered counseling style that addresses ambivalence about change while strengthening internal motivation for, and commitment to, change. It originally was used in addiction treatment, but has since been studied for and applied to a wide variety of medical and psychological conditions.34
MI has an underlying perspective (often called the “spirit” of MI) that includes partnership, acceptance, compassion, and evocation.34 Partnership implies a respectful collaboration between equals—while the FP may be an expert on a particular diagnosis, the patient is the expert on herself. Acceptance is unconditional positive regard and involves a nonjudgmental and person-centered recognition of an individual’s absolute worth and potential that supports autonomy and affirms strengths. Compassion is the sense of actively promoting a patient’s well being and prioritizing his or her needs over your own. Evocation refers to calling forth the patient’s own wisdom based on a realization that the patient has motivation and resources that can be elicited.
In contrast to a deficit model (“You are lacking something; I have it, and I will install it in you”), MI focuses on strengths (“You have what you need, and together we will find it”).
The skills of MI are practiced in a series of 4 sequential and overlapping processes known as Engaging, Focusing, Evoking, and Planning.34 Engaging is establishing a helpful connection and working relationship with a patient. Focusing is developing and maintaining a specific direction toward a goal (or goals). Evoking is eliciting the patient’s own motivations for change. Planning is developing commitment to change and formulating a specific action plan. Five core communication skills are used flexibly and strategically during these 4 processes: asking open questions, affirming, reflective listening, summarizing, and informing and advising with permission.34
Putting MI into action
CASE › An FP who wants to use MI with Mr. G would begin the Engaging and Focusing processes by asking permission: “May I ask you a question about weight loss?” If Mr. G says Yes, the FP would start the process of Evoking using scaling questions, such as: “On a scale of one to 10, where one means it’s not at all important, and 10 means that it’s very important, how important to you is losing weight?" (Mr. G: “I’d say 9, it is very important to me.”) “On a scale of one to 10, where one means that you are not at all confident, and 10 means that you are extremely confident, how confident are you that you can lose weight?” (Mr. G: “I’m a 6.”)
“Why are you at 6 rather than 1?” (Mr. G: “I have lost a few pounds in the past, so I know a little bit about losing weight.”) “What would have to happen for you to get to 7, that is, for you to become just a little bit more confident?” (Mr. G: “I would need to get my family’s support.”)
The FP would implement the Planning process by suggesting that Mr. G talk to his wife about taking a walk with him after dinner and buying skim milk instead of 2%.
CORRESPONDENCE
Michael Raddock, MD, Department of Family Medicine, MetroHealth Medical Center; 2500 MetroHealth Drive, Cleveland, Ohio 44109; [email protected]
1. Mokdad AH, Marks JS, Stroup DF, et al. Actual causes of death in the United States, 2000. JAMA. 2004;291:1238-1245.
2. Heath C, Heath D. Switch: How to Change Things When Change is Hard. New York, NY: Crown Business; 2010.
3. Achor S. The Happiness Advantage: The Seven Principles of Positive Psychology That Fuel Success and Performance at Work. New York, NY: Crown Business; 2010.
4. Dweck CS. Mindset: The New Psychology of Success. New York, NY: Ballantine Books; 2006.
5. Kotter JP, Cohen DS. The Heart of Change: Real-Life Stories of How People Change Their Organizations. Boston, MA: Harvard Business School Publishing; 2002.
6. Wansink B. Mindless Eating: Why We Eat More Than We Think. New York, NY: Bantam; 2006.
7. Thaler RH, Sunstein CS. Nudge: Improving Decisions About Health, Wealth, and Happiness. New York, NY: Penguin Books; 2009.
8. Maurer R. One Small Step Can Change Your Life: The Kaizen Way. New York, NY: Workman Publishing Company; 2004.
9. Patterson K, Grenny J, Maxfield D, et al. Influencer: The New Science of Leading Change. New York, NY: McGraw-Hill; 2007.
10. Hubble MA, Duncan BL, Miller SD, eds. The Heart and Soul of Change: What Works in Therapy. Washington, DC: American Psychological Association; 1999.
11. Baumeister RF, Tierney J. Willpower: Rediscovering the Greatest Human Strength. New York, NY: Penguin Books; 2011.
12. Prochaska JO, DiClemente CC. The Transtheoretical Approach: Crossing Traditional Boundaries of Therapy. Homewood, IL: Dow Jones/Irwin; 1984.
13. Prochaska JO, Norcross JC. Stages of change. Psychother: Theory, Res, Pract, Training. 2001;38:443-448.
14. Searight HR. Realistic approaches to counseling in the office setting. Am Fam Physician. 2009;79:277-284.
15. Cohen DJ, Clark EC, Lawson PJ, et al. Identifying teachable moments for health behavior counseling in primary care. Patient Educ Couns. 2011;85:e8-e15.
16. Flocke SA, Antognoli E, Step MM, et al. A teachable moment communication process for smoking cessation talk: description of a group randomized clinician-focused intervention. BMC Health Serv Res. 2012;12:109.
17. Fiore MC, Bailey WC, Cohen SJ, et al. Treating Tobacco Use and Dependence. Clinical Practice Guideline. Rockville, MD: U.S. Department of Health and Human Services; 2000.
18. Alexander SC, Cox ME, Boling Turer CL, et al. Do the Five A’s work when physicians counsel about weight loss? Fam Med. 2011;43:179-184.
19. Trepper TS, McCollum E, De Jong P, et al. Solution focused therapy treatment manual for working with individuals. Solution Focused Brief Therapy Association Web site. Available at: http://www.sfbta.org/research.pdf. Accessed December 23, 2014.
20. Molnar A, de Shazer S. Solution-focused therapy: Towards the identification of therapeutic tasks. J Marital Fam Ther. 1987;13:349-358.
21. Greenberg G, Ganshorn K, Danilkewich A. Solution-focused therapy. Counseling model for busy family physicians. Can Fam Physician. 2001;47:2289-2295.
22. Giorlando ME, Schilling RJ. On becoming a solution-focused physician: The MED-STAT acronym. Families Syst Health. 1997;15:361-373.
23. Beck AT. Thinking and depression. I. Idiosyncratic content and cognitive distortions. Arch Gen Psychiatry. 1963;9:324-333.
24. Beck AT. Thinking and depression. II. Theory and therapy. Arch Gen Psychiatry. 1964;10:561-571.
25. Beck AT. The current state of cognitive therapy: a 40-year retrospective. Arch Gen Psychiatry. 2005;62:953-959.
26. Wright JH, Beck AT, Thase ME. Cognitive therapy. In: Hales RE, Yudofsky SC, Talbott JA, eds. Textbook of Clinical Psychiatry. 4th ed. Washington, DC: American Psychiatric Publishing; 2003:1245-1284.
27. Clark DA, Beck AT, Alford BA. Scientific Foundations of Cognitive Theory and Therapy of Depression. New York, NY: John Wiley & Sons; 1999.
28. Wright JH, Basco MR, Thase ME. Learning Cognitive-Behavior Therapy: An Illustrated Guide. Arlington, VA: American Psychiatric Publishing; 2006.
29. Launer J. Narrative-based Primary Care: A Practical Guide. Abingdon, United Kingdom: Radcliffe Medical Press; 2002.
30. Engel JD, Zarconi J, Pethtel L, et al. Narrative in Health Care: Healing Patients, Practitioners, Profession, and Community. Abingdon, United Kingdom: Radcliffe Publishing; 2008.
31. Charon R. Narrative Medicine: Honoring the Stories of Illness. New York, NY: Oxford University Press; 2006.
32. Kleinman A. The Illness Narratives: Suffering, Healing & the Human Condition. New York, NY: Basic Books; 1988.
33. Kleinman A, Eisenberg L, Good B. Culture, illness, and care: clinical lessons from anthropologic and cross-cultural research. Ann Intern Med. 1978;88:251-258.
34. Miller WR, Rollnick S. Motivational Interviewing: Helping People Change. 3rd ed. New York, NY: The Guilford Press; 2013.
› Determine the patient’s stage of change (Precontemplation, Contemplation, Preparation, Action, Maintenance, or Relapse) before selecting an intervention to help him or her change health-related behaviors. B
› Consider using motivational interviewing or narrative techniques to help patients who aren’t yet ready to change their health-related behaviors or who plan to do so within 6 months. C
› Be aware that patients seldom become motivated to change behaviors by being given information about health risks and benefits; to overcome ambivalence, they need to focus on their core values and goals. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE › Your patient, Bob G, age 47, has a body mass index of 33, hypertension (blood pressure 150/85 mm Hg), and elevated cholesterol (low-density lipoprotein level, 187 mg/dL) and glucose levels (fasting glucose 122 mg/dL, with an HbA1c of 6.1%). He gets out of breath when he plays with his 2 children. His father has diabetes and had a myocardial infarction (MI) at age 55; Mr. G tells you he is concerned he will develop similar health problems. Mr. G frequents fast food restaurants and eats high-calorie snacks after work, especially when he feels stressed. During a recent office visit, he expresses his desire to “be there” for his children and says he is motivated to lose weight to prevent diabetes and/or an MI.
How would you proceed?
Most health conditions in the United States are directly or indirectly the result of patients’ health-related behaviors.1 Fortunately, family physicians (FPs) and primary care teams are in an excellent position to help their patients make healthy behavior changes by using brief, evidence-based interventions that can be implemented during the typical office visit.
Specifically, the use of the following 7 techniques can build on patients’ own motivations, successes, and life circumstances to improve their satisfaction and self-efficacy:
- the 5 As (Ask, Advise, Assess, Assist, Arrange)
- the FRAMES protocol (Feedback, Responsibility, Advice, Menu, Empathy, and Self-efficacy)
- teachable moments (TM)
- solution-focused brief therapy (SFBT)
- cognitive behavioral therapy (CBT)
- narrative techniques (NT)
- motivational interviewing (MI).
But before we describe the practical application of these 7 techniques, we’ll begin by explaining a few underlying concepts for helping patients change their health-related behaviors.
Understanding what does—and doesn’t—help patients change
Research from the field of psychology and other social sciences has described several important concepts that affect how FPs can best help their patients change to healthier behaviors.2-9 First, several “common factors” have been found to reliably predict behavior change. The likelihood of change is strongly tied to the patient’s strengths, the environment, and the quality of the physician-patient relationship. The patient’s expectations and the techniques a physician uses also predict behavior change, but to a lesser extent.10
Second, patients seldom become motivated to change ingrained behaviors solely by being provided with information about the risks and benefits associated with those behaviors. People overcome ambivalence and develop motivation for change when they align their behaviors with their core values and goals. FPs can help patients link their motivation to change to specific plans and environments. This can then facilitate small changes that can yield large returns by increasing a patient’s self-efficacy and sense of control.2-9
Third, willpower is a finite but renewable resource that increases or decreases based on an individual’s internal and external environments. Reliance on willpower alone to make changes is unlikely to be successful without shaping the environment to support the new behavior.11
A patient’s readiness to change affects choice of technique
Knowing how ready a patient is to change is important for determining which approaches are likely to be effective at a given visit. Prochaska and DiClemente developed a model that defines 6 stages of change: Precontemplation (patient does not intend to change in the next 6 months), Contemplation (patient intends to change within the next 6 months), Preparation (patient intends to change within the next month), Action (patient has made specific changes within the past 6 months), Maintenance (patient works to prevent relapse), and Relapse (patient returns to an earlier stage) (TABLE).12
Patients who identify change as important and are ready to make changes benefit from collaborative work with an FP or other clinicians on the how, when, where, and who (eg, the patient, his or her significant other, family, and friends) of the new behaviors. These individuals are in the Preparation or Action stages of change, which comprise roughly 20% of patients.13 In these circumstances, techniques such as the 5 As, FRAMES, TM, SFBT, and CBT can be effective.
For the estimated 80% of patients who are in the Precontemplation or Contemplation stages and are unsure about the relative importance of changing behaviors and/or lack confidence to make changes, these directive techniques can cause defensiveness, which can make both the patient and the FP uncomfortable. For such patients, approaches that build on the patient’s own motivations and stories, such as MI and NT, may be preferred.
3 techniques that overlap
The FRAMES protocol and TM are based on behavior change theories, and each mixes directive techniques with relationship building to facilitate health behavior change. There is overlap in concepts across the 5 As, FRAMES, and TM, and some evidence suggests these approaches can be adapted for use in primary care settings.14-16
The 5 As is a brief intervention in which the FP sets an agenda and provides advice at the outset. This technique has been shown to improve smoking cessation rates in pregnant women compared with physician recommendations alone.17 It may also help with weight loss for patients who are ready to change and are given support for their
efforts.18
Putting the 5 As into action
CASE › An FP who wants to use the 5 As technique to assist Mr. G might proceed as follows: Ask: “How often do you exercise and follow a diet?” Advise: “I recommend that you start exercising 30 minutes each day and start following a healthier diet. It is one of the most important things you can do for your health.” Assess: “Are you willing to start exercising and trying a diet in the next month?” Assist: “Here is a list of local recreation centers and some information about a healthy diet.” Arrange: “I’d like to have one of the nurses call you in a week to see how things are going and have you return in a month for a follow-up appointment.”
The 6 components of the FRAMES protocol overlap with the 5 As.14 FRAMES utilizes relationship-building by explicitly reinforcing patient autonomy, offering a menu of choices, and acknowledging patient strengths.14
Putting the FRAMES protocol into action
CASE › Using the FRAMES protocol for Mr. G might consist of the following: Feedback: “Your eating habits and lack of exercise have contributed to your weight, high glucose and cholesterol levels, and shortness of breath.” Responsibility: “The decision to lose weight is a choice only you can make.” Advice: “I recommend that you start regularly exercising and eating healthily.” Menu: “Here are some options that many people find helpful when they try to lose weight.” Empathy: “It is challenging to change the way we eat and exercise.” Self-efficacy: “You have been able to overcome a lot of difficult things in your life already and it seems very important to you to make these changes.”
TM begins with the FP linking a patient concern, such as shortness of breath, to a physician concern, such as obesity.15 The FP then provides advice, assesses readiness, and responds based on the patient’s stage of change.16
Putting TM into action
CASE › Using the TM approach to help Mr. G might work as follows: Link a patient concern with specific behavioral change: “I think that your shortness of breath is caused by your weight.” Recommend change, offer support, and ask for commitment: “I recommend that you lose 15 pounds. I’m confident that you can do this, and am here to help you. Are you ready to talk about some specific ways you can do this?” Respond based on the patient’s readiness to change: “All right, let’s talk about healthy food choices and exercise.” (This statement would be appropriate if Mr. G was in the Preparation or Action stage of change.)
Solution-focused brief therapy
SFBT highlights a patient’s previous successes and strengths, as opposed to exploring problems and past failures.19,20 The FP fosters behavior change by using strategic questions to develop an intervention with the patient.21 SFBT involves encouraging patients to find exceptions to current problems and increasing the occurrence of current beneficial behaviors.19 This approach begins with the patient identifying a problem for which he or she would like help. The FP helps the patient explore solutions and/or exceptions to this problem that have worked for the patient previously (or solutions/exceptions that the patient can imagine). The FP does not offer suggestions to solve the patient’s problem. Instead, the patient and FP collaboratively identify and support the patient’s strengths, and they develop a behavioral task to try based on these patient-derived solutions.22
Putting SFBT into action
CASE › A physician who wants to use SFBT to help Mr. G might start by asking an “exception” question (“When have you been able to eat in a more healthy way?”) and following up with a “difference” question (“What was different about those times?”). Perhaps Mr. G remembers that previously he had improved his diet by buying and keeping a bag of apples in the car to snack on. He additionally recalls that he ate less at night if he brushed his teeth right after dinner.
Mr. G decides to revisit the apple and brushing strategies. Mr. G’s physician commends him for wanting to be there for his kids and identifying the apple and brushing strategies. She helps him design a small “experiment” in which he would use these strategies and observe the outcomes. They arrange to speak in one month to discuss how things are going.
Cognitive behavioral therapy
CBT is a practical, goal-directed, action-oriented treatment that focuses on helping patients make changes in their thinking and behavior.23-28 A basic premise of CBT is that emotions are difficult to change directly, so CBT targets distressing emotions by focusing on changing thoughts and/or behaviors that contribute to those emotions. CBT can be useful when the patient and FP can find a link between the patient’s thoughts and a troubling behavior. After thoroughly assessing situations that bother the patient, the FP provides the patient with an empathic summary that captures the essence of the problem. CBT practitioners typically conceptualize problems and plan treatment by working with the patient to gather information on the patient’s thoughts, feelings, and behaviors.
By exploring patterns of thinking that lead to self-destructive behaviors, an FP can help the patient understand and challenge strongly held but often limited patterns of thinking. For example, a patient with depression-related overeating might think, “I am worthless. Nothing ever goes right for me.” A patient with anxiety-related smoking may believe, “I am in danger.” Through a collaborative, respectful relationship, patients learn to test their “hypotheses,” challenge their thoughts, and experiment with alternate ways of thinking and behaving. Patients are given homework assignments, such as tracking their thoughts and behaviors, practicing relaxation techniques, and challenging automatic ways of viewing themselves and the world around them.
Putting CBT into action
CASE › An FP who wants to implement CBT to help Mr. G would begin by trying to understand his patient’s view: “Tell me how your weight is a part of your life.” Next, he would offer Mr. G an empathic summary: “You’re worried that your weight could cause some of the same health problems your dad has and that will prevent you from being the kind of active father you want to be. You sometimes eat when you are feeling stressed and tired, but then you feel worse afterwards.” He would assign Mr. G homework: “Notice and write down your thoughts when you are eating due to stress rather than hunger. Bring this in and we can look at it together.” The FP might also teach Mr. G relaxation breathing, and encourage him to try doing 5 relaxation breaths when he feels stressed and wants to eat.
Narrative techniques
NT can be effective for patients who are in the Precontemplation or Contemplation stages of change.29-33 NT focuses on the patient’s story, context, and language. FPs explore connections, discuss hypotheses, strategize, share power with the patient, and offer reflections in order to understand the patient’s illness experience. This approach fits well with the complex way that many behaviors are woven into the concerns that patients bring to their FPs. As a patient’s story unfolds, the diagnosis and treatment can occur simultaneously. The FP involves the patient in choices about how to proceed and what to focus on together.
NT avoids unsolicited advice and interpretations and rarely imposes the FP’s agenda on the patient. This approach invites the FP and patient to co-create an understanding and narrative of what the symptoms mean, why they are there, and what can be done about them. Ultimately, this approach can result in a new narrative that puts the patient on the path to healing.29-33
Putting NT into action
CASE › A physician might implement an NT approach with Mr. G to co-create a narrative about health and life goals by asking him: “Tell me about how losing weight fits into your goals for being there for your children. Tell me about how you see yourself avoiding some of the health problems your dad has faced.”
Motivational interviewing
For patients who are in the Precontemplation or Contemplation stages of change, MI might be a helpful approach. MI is a person-centered counseling style that addresses ambivalence about change while strengthening internal motivation for, and commitment to, change. It originally was used in addiction treatment, but has since been studied for and applied to a wide variety of medical and psychological conditions.34
MI has an underlying perspective (often called the “spirit” of MI) that includes partnership, acceptance, compassion, and evocation.34 Partnership implies a respectful collaboration between equals—while the FP may be an expert on a particular diagnosis, the patient is the expert on herself. Acceptance is unconditional positive regard and involves a nonjudgmental and person-centered recognition of an individual’s absolute worth and potential that supports autonomy and affirms strengths. Compassion is the sense of actively promoting a patient’s well being and prioritizing his or her needs over your own. Evocation refers to calling forth the patient’s own wisdom based on a realization that the patient has motivation and resources that can be elicited.
In contrast to a deficit model (“You are lacking something; I have it, and I will install it in you”), MI focuses on strengths (“You have what you need, and together we will find it”).
The skills of MI are practiced in a series of 4 sequential and overlapping processes known as Engaging, Focusing, Evoking, and Planning.34 Engaging is establishing a helpful connection and working relationship with a patient. Focusing is developing and maintaining a specific direction toward a goal (or goals). Evoking is eliciting the patient’s own motivations for change. Planning is developing commitment to change and formulating a specific action plan. Five core communication skills are used flexibly and strategically during these 4 processes: asking open questions, affirming, reflective listening, summarizing, and informing and advising with permission.34
Putting MI into action
CASE › An FP who wants to use MI with Mr. G would begin the Engaging and Focusing processes by asking permission: “May I ask you a question about weight loss?” If Mr. G says Yes, the FP would start the process of Evoking using scaling questions, such as: “On a scale of one to 10, where one means it’s not at all important, and 10 means that it’s very important, how important to you is losing weight?" (Mr. G: “I’d say 9, it is very important to me.”) “On a scale of one to 10, where one means that you are not at all confident, and 10 means that you are extremely confident, how confident are you that you can lose weight?” (Mr. G: “I’m a 6.”)
“Why are you at 6 rather than 1?” (Mr. G: “I have lost a few pounds in the past, so I know a little bit about losing weight.”) “What would have to happen for you to get to 7, that is, for you to become just a little bit more confident?” (Mr. G: “I would need to get my family’s support.”)
The FP would implement the Planning process by suggesting that Mr. G talk to his wife about taking a walk with him after dinner and buying skim milk instead of 2%.
CORRESPONDENCE
Michael Raddock, MD, Department of Family Medicine, MetroHealth Medical Center; 2500 MetroHealth Drive, Cleveland, Ohio 44109; [email protected]
› Determine the patient’s stage of change (Precontemplation, Contemplation, Preparation, Action, Maintenance, or Relapse) before selecting an intervention to help him or her change health-related behaviors. B
› Consider using motivational interviewing or narrative techniques to help patients who aren’t yet ready to change their health-related behaviors or who plan to do so within 6 months. C
› Be aware that patients seldom become motivated to change behaviors by being given information about health risks and benefits; to overcome ambivalence, they need to focus on their core values and goals. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE › Your patient, Bob G, age 47, has a body mass index of 33, hypertension (blood pressure 150/85 mm Hg), and elevated cholesterol (low-density lipoprotein level, 187 mg/dL) and glucose levels (fasting glucose 122 mg/dL, with an HbA1c of 6.1%). He gets out of breath when he plays with his 2 children. His father has diabetes and had a myocardial infarction (MI) at age 55; Mr. G tells you he is concerned he will develop similar health problems. Mr. G frequents fast food restaurants and eats high-calorie snacks after work, especially when he feels stressed. During a recent office visit, he expresses his desire to “be there” for his children and says he is motivated to lose weight to prevent diabetes and/or an MI.
How would you proceed?
Most health conditions in the United States are directly or indirectly the result of patients’ health-related behaviors.1 Fortunately, family physicians (FPs) and primary care teams are in an excellent position to help their patients make healthy behavior changes by using brief, evidence-based interventions that can be implemented during the typical office visit.
Specifically, the use of the following 7 techniques can build on patients’ own motivations, successes, and life circumstances to improve their satisfaction and self-efficacy:
- the 5 As (Ask, Advise, Assess, Assist, Arrange)
- the FRAMES protocol (Feedback, Responsibility, Advice, Menu, Empathy, and Self-efficacy)
- teachable moments (TM)
- solution-focused brief therapy (SFBT)
- cognitive behavioral therapy (CBT)
- narrative techniques (NT)
- motivational interviewing (MI).
But before we describe the practical application of these 7 techniques, we’ll begin by explaining a few underlying concepts for helping patients change their health-related behaviors.
Understanding what does—and doesn’t—help patients change
Research from the field of psychology and other social sciences has described several important concepts that affect how FPs can best help their patients change to healthier behaviors.2-9 First, several “common factors” have been found to reliably predict behavior change. The likelihood of change is strongly tied to the patient’s strengths, the environment, and the quality of the physician-patient relationship. The patient’s expectations and the techniques a physician uses also predict behavior change, but to a lesser extent.10
Second, patients seldom become motivated to change ingrained behaviors solely by being provided with information about the risks and benefits associated with those behaviors. People overcome ambivalence and develop motivation for change when they align their behaviors with their core values and goals. FPs can help patients link their motivation to change to specific plans and environments. This can then facilitate small changes that can yield large returns by increasing a patient’s self-efficacy and sense of control.2-9
Third, willpower is a finite but renewable resource that increases or decreases based on an individual’s internal and external environments. Reliance on willpower alone to make changes is unlikely to be successful without shaping the environment to support the new behavior.11
A patient’s readiness to change affects choice of technique
Knowing how ready a patient is to change is important for determining which approaches are likely to be effective at a given visit. Prochaska and DiClemente developed a model that defines 6 stages of change: Precontemplation (patient does not intend to change in the next 6 months), Contemplation (patient intends to change within the next 6 months), Preparation (patient intends to change within the next month), Action (patient has made specific changes within the past 6 months), Maintenance (patient works to prevent relapse), and Relapse (patient returns to an earlier stage) (TABLE).12
Patients who identify change as important and are ready to make changes benefit from collaborative work with an FP or other clinicians on the how, when, where, and who (eg, the patient, his or her significant other, family, and friends) of the new behaviors. These individuals are in the Preparation or Action stages of change, which comprise roughly 20% of patients.13 In these circumstances, techniques such as the 5 As, FRAMES, TM, SFBT, and CBT can be effective.
For the estimated 80% of patients who are in the Precontemplation or Contemplation stages and are unsure about the relative importance of changing behaviors and/or lack confidence to make changes, these directive techniques can cause defensiveness, which can make both the patient and the FP uncomfortable. For such patients, approaches that build on the patient’s own motivations and stories, such as MI and NT, may be preferred.
3 techniques that overlap
The FRAMES protocol and TM are based on behavior change theories, and each mixes directive techniques with relationship building to facilitate health behavior change. There is overlap in concepts across the 5 As, FRAMES, and TM, and some evidence suggests these approaches can be adapted for use in primary care settings.14-16
The 5 As is a brief intervention in which the FP sets an agenda and provides advice at the outset. This technique has been shown to improve smoking cessation rates in pregnant women compared with physician recommendations alone.17 It may also help with weight loss for patients who are ready to change and are given support for their
efforts.18
Putting the 5 As into action
CASE › An FP who wants to use the 5 As technique to assist Mr. G might proceed as follows: Ask: “How often do you exercise and follow a diet?” Advise: “I recommend that you start exercising 30 minutes each day and start following a healthier diet. It is one of the most important things you can do for your health.” Assess: “Are you willing to start exercising and trying a diet in the next month?” Assist: “Here is a list of local recreation centers and some information about a healthy diet.” Arrange: “I’d like to have one of the nurses call you in a week to see how things are going and have you return in a month for a follow-up appointment.”
The 6 components of the FRAMES protocol overlap with the 5 As.14 FRAMES utilizes relationship-building by explicitly reinforcing patient autonomy, offering a menu of choices, and acknowledging patient strengths.14
Putting the FRAMES protocol into action
CASE › Using the FRAMES protocol for Mr. G might consist of the following: Feedback: “Your eating habits and lack of exercise have contributed to your weight, high glucose and cholesterol levels, and shortness of breath.” Responsibility: “The decision to lose weight is a choice only you can make.” Advice: “I recommend that you start regularly exercising and eating healthily.” Menu: “Here are some options that many people find helpful when they try to lose weight.” Empathy: “It is challenging to change the way we eat and exercise.” Self-efficacy: “You have been able to overcome a lot of difficult things in your life already and it seems very important to you to make these changes.”
TM begins with the FP linking a patient concern, such as shortness of breath, to a physician concern, such as obesity.15 The FP then provides advice, assesses readiness, and responds based on the patient’s stage of change.16
Putting TM into action
CASE › Using the TM approach to help Mr. G might work as follows: Link a patient concern with specific behavioral change: “I think that your shortness of breath is caused by your weight.” Recommend change, offer support, and ask for commitment: “I recommend that you lose 15 pounds. I’m confident that you can do this, and am here to help you. Are you ready to talk about some specific ways you can do this?” Respond based on the patient’s readiness to change: “All right, let’s talk about healthy food choices and exercise.” (This statement would be appropriate if Mr. G was in the Preparation or Action stage of change.)
Solution-focused brief therapy
SFBT highlights a patient’s previous successes and strengths, as opposed to exploring problems and past failures.19,20 The FP fosters behavior change by using strategic questions to develop an intervention with the patient.21 SFBT involves encouraging patients to find exceptions to current problems and increasing the occurrence of current beneficial behaviors.19 This approach begins with the patient identifying a problem for which he or she would like help. The FP helps the patient explore solutions and/or exceptions to this problem that have worked for the patient previously (or solutions/exceptions that the patient can imagine). The FP does not offer suggestions to solve the patient’s problem. Instead, the patient and FP collaboratively identify and support the patient’s strengths, and they develop a behavioral task to try based on these patient-derived solutions.22
Putting SFBT into action
CASE › A physician who wants to use SFBT to help Mr. G might start by asking an “exception” question (“When have you been able to eat in a more healthy way?”) and following up with a “difference” question (“What was different about those times?”). Perhaps Mr. G remembers that previously he had improved his diet by buying and keeping a bag of apples in the car to snack on. He additionally recalls that he ate less at night if he brushed his teeth right after dinner.
Mr. G decides to revisit the apple and brushing strategies. Mr. G’s physician commends him for wanting to be there for his kids and identifying the apple and brushing strategies. She helps him design a small “experiment” in which he would use these strategies and observe the outcomes. They arrange to speak in one month to discuss how things are going.
Cognitive behavioral therapy
CBT is a practical, goal-directed, action-oriented treatment that focuses on helping patients make changes in their thinking and behavior.23-28 A basic premise of CBT is that emotions are difficult to change directly, so CBT targets distressing emotions by focusing on changing thoughts and/or behaviors that contribute to those emotions. CBT can be useful when the patient and FP can find a link between the patient’s thoughts and a troubling behavior. After thoroughly assessing situations that bother the patient, the FP provides the patient with an empathic summary that captures the essence of the problem. CBT practitioners typically conceptualize problems and plan treatment by working with the patient to gather information on the patient’s thoughts, feelings, and behaviors.
By exploring patterns of thinking that lead to self-destructive behaviors, an FP can help the patient understand and challenge strongly held but often limited patterns of thinking. For example, a patient with depression-related overeating might think, “I am worthless. Nothing ever goes right for me.” A patient with anxiety-related smoking may believe, “I am in danger.” Through a collaborative, respectful relationship, patients learn to test their “hypotheses,” challenge their thoughts, and experiment with alternate ways of thinking and behaving. Patients are given homework assignments, such as tracking their thoughts and behaviors, practicing relaxation techniques, and challenging automatic ways of viewing themselves and the world around them.
Putting CBT into action
CASE › An FP who wants to implement CBT to help Mr. G would begin by trying to understand his patient’s view: “Tell me how your weight is a part of your life.” Next, he would offer Mr. G an empathic summary: “You’re worried that your weight could cause some of the same health problems your dad has and that will prevent you from being the kind of active father you want to be. You sometimes eat when you are feeling stressed and tired, but then you feel worse afterwards.” He would assign Mr. G homework: “Notice and write down your thoughts when you are eating due to stress rather than hunger. Bring this in and we can look at it together.” The FP might also teach Mr. G relaxation breathing, and encourage him to try doing 5 relaxation breaths when he feels stressed and wants to eat.
Narrative techniques
NT can be effective for patients who are in the Precontemplation or Contemplation stages of change.29-33 NT focuses on the patient’s story, context, and language. FPs explore connections, discuss hypotheses, strategize, share power with the patient, and offer reflections in order to understand the patient’s illness experience. This approach fits well with the complex way that many behaviors are woven into the concerns that patients bring to their FPs. As a patient’s story unfolds, the diagnosis and treatment can occur simultaneously. The FP involves the patient in choices about how to proceed and what to focus on together.
NT avoids unsolicited advice and interpretations and rarely imposes the FP’s agenda on the patient. This approach invites the FP and patient to co-create an understanding and narrative of what the symptoms mean, why they are there, and what can be done about them. Ultimately, this approach can result in a new narrative that puts the patient on the path to healing.29-33
Putting NT into action
CASE › A physician might implement an NT approach with Mr. G to co-create a narrative about health and life goals by asking him: “Tell me about how losing weight fits into your goals for being there for your children. Tell me about how you see yourself avoiding some of the health problems your dad has faced.”
Motivational interviewing
For patients who are in the Precontemplation or Contemplation stages of change, MI might be a helpful approach. MI is a person-centered counseling style that addresses ambivalence about change while strengthening internal motivation for, and commitment to, change. It originally was used in addiction treatment, but has since been studied for and applied to a wide variety of medical and psychological conditions.34
MI has an underlying perspective (often called the “spirit” of MI) that includes partnership, acceptance, compassion, and evocation.34 Partnership implies a respectful collaboration between equals—while the FP may be an expert on a particular diagnosis, the patient is the expert on herself. Acceptance is unconditional positive regard and involves a nonjudgmental and person-centered recognition of an individual’s absolute worth and potential that supports autonomy and affirms strengths. Compassion is the sense of actively promoting a patient’s well being and prioritizing his or her needs over your own. Evocation refers to calling forth the patient’s own wisdom based on a realization that the patient has motivation and resources that can be elicited.
In contrast to a deficit model (“You are lacking something; I have it, and I will install it in you”), MI focuses on strengths (“You have what you need, and together we will find it”).
The skills of MI are practiced in a series of 4 sequential and overlapping processes known as Engaging, Focusing, Evoking, and Planning.34 Engaging is establishing a helpful connection and working relationship with a patient. Focusing is developing and maintaining a specific direction toward a goal (or goals). Evoking is eliciting the patient’s own motivations for change. Planning is developing commitment to change and formulating a specific action plan. Five core communication skills are used flexibly and strategically during these 4 processes: asking open questions, affirming, reflective listening, summarizing, and informing and advising with permission.34
Putting MI into action
CASE › An FP who wants to use MI with Mr. G would begin the Engaging and Focusing processes by asking permission: “May I ask you a question about weight loss?” If Mr. G says Yes, the FP would start the process of Evoking using scaling questions, such as: “On a scale of one to 10, where one means it’s not at all important, and 10 means that it’s very important, how important to you is losing weight?" (Mr. G: “I’d say 9, it is very important to me.”) “On a scale of one to 10, where one means that you are not at all confident, and 10 means that you are extremely confident, how confident are you that you can lose weight?” (Mr. G: “I’m a 6.”)
“Why are you at 6 rather than 1?” (Mr. G: “I have lost a few pounds in the past, so I know a little bit about losing weight.”) “What would have to happen for you to get to 7, that is, for you to become just a little bit more confident?” (Mr. G: “I would need to get my family’s support.”)
The FP would implement the Planning process by suggesting that Mr. G talk to his wife about taking a walk with him after dinner and buying skim milk instead of 2%.
CORRESPONDENCE
Michael Raddock, MD, Department of Family Medicine, MetroHealth Medical Center; 2500 MetroHealth Drive, Cleveland, Ohio 44109; [email protected]
1. Mokdad AH, Marks JS, Stroup DF, et al. Actual causes of death in the United States, 2000. JAMA. 2004;291:1238-1245.
2. Heath C, Heath D. Switch: How to Change Things When Change is Hard. New York, NY: Crown Business; 2010.
3. Achor S. The Happiness Advantage: The Seven Principles of Positive Psychology That Fuel Success and Performance at Work. New York, NY: Crown Business; 2010.
4. Dweck CS. Mindset: The New Psychology of Success. New York, NY: Ballantine Books; 2006.
5. Kotter JP, Cohen DS. The Heart of Change: Real-Life Stories of How People Change Their Organizations. Boston, MA: Harvard Business School Publishing; 2002.
6. Wansink B. Mindless Eating: Why We Eat More Than We Think. New York, NY: Bantam; 2006.
7. Thaler RH, Sunstein CS. Nudge: Improving Decisions About Health, Wealth, and Happiness. New York, NY: Penguin Books; 2009.
8. Maurer R. One Small Step Can Change Your Life: The Kaizen Way. New York, NY: Workman Publishing Company; 2004.
9. Patterson K, Grenny J, Maxfield D, et al. Influencer: The New Science of Leading Change. New York, NY: McGraw-Hill; 2007.
10. Hubble MA, Duncan BL, Miller SD, eds. The Heart and Soul of Change: What Works in Therapy. Washington, DC: American Psychological Association; 1999.
11. Baumeister RF, Tierney J. Willpower: Rediscovering the Greatest Human Strength. New York, NY: Penguin Books; 2011.
12. Prochaska JO, DiClemente CC. The Transtheoretical Approach: Crossing Traditional Boundaries of Therapy. Homewood, IL: Dow Jones/Irwin; 1984.
13. Prochaska JO, Norcross JC. Stages of change. Psychother: Theory, Res, Pract, Training. 2001;38:443-448.
14. Searight HR. Realistic approaches to counseling in the office setting. Am Fam Physician. 2009;79:277-284.
15. Cohen DJ, Clark EC, Lawson PJ, et al. Identifying teachable moments for health behavior counseling in primary care. Patient Educ Couns. 2011;85:e8-e15.
16. Flocke SA, Antognoli E, Step MM, et al. A teachable moment communication process for smoking cessation talk: description of a group randomized clinician-focused intervention. BMC Health Serv Res. 2012;12:109.
17. Fiore MC, Bailey WC, Cohen SJ, et al. Treating Tobacco Use and Dependence. Clinical Practice Guideline. Rockville, MD: U.S. Department of Health and Human Services; 2000.
18. Alexander SC, Cox ME, Boling Turer CL, et al. Do the Five A’s work when physicians counsel about weight loss? Fam Med. 2011;43:179-184.
19. Trepper TS, McCollum E, De Jong P, et al. Solution focused therapy treatment manual for working with individuals. Solution Focused Brief Therapy Association Web site. Available at: http://www.sfbta.org/research.pdf. Accessed December 23, 2014.
20. Molnar A, de Shazer S. Solution-focused therapy: Towards the identification of therapeutic tasks. J Marital Fam Ther. 1987;13:349-358.
21. Greenberg G, Ganshorn K, Danilkewich A. Solution-focused therapy. Counseling model for busy family physicians. Can Fam Physician. 2001;47:2289-2295.
22. Giorlando ME, Schilling RJ. On becoming a solution-focused physician: The MED-STAT acronym. Families Syst Health. 1997;15:361-373.
23. Beck AT. Thinking and depression. I. Idiosyncratic content and cognitive distortions. Arch Gen Psychiatry. 1963;9:324-333.
24. Beck AT. Thinking and depression. II. Theory and therapy. Arch Gen Psychiatry. 1964;10:561-571.
25. Beck AT. The current state of cognitive therapy: a 40-year retrospective. Arch Gen Psychiatry. 2005;62:953-959.
26. Wright JH, Beck AT, Thase ME. Cognitive therapy. In: Hales RE, Yudofsky SC, Talbott JA, eds. Textbook of Clinical Psychiatry. 4th ed. Washington, DC: American Psychiatric Publishing; 2003:1245-1284.
27. Clark DA, Beck AT, Alford BA. Scientific Foundations of Cognitive Theory and Therapy of Depression. New York, NY: John Wiley & Sons; 1999.
28. Wright JH, Basco MR, Thase ME. Learning Cognitive-Behavior Therapy: An Illustrated Guide. Arlington, VA: American Psychiatric Publishing; 2006.
29. Launer J. Narrative-based Primary Care: A Practical Guide. Abingdon, United Kingdom: Radcliffe Medical Press; 2002.
30. Engel JD, Zarconi J, Pethtel L, et al. Narrative in Health Care: Healing Patients, Practitioners, Profession, and Community. Abingdon, United Kingdom: Radcliffe Publishing; 2008.
31. Charon R. Narrative Medicine: Honoring the Stories of Illness. New York, NY: Oxford University Press; 2006.
32. Kleinman A. The Illness Narratives: Suffering, Healing & the Human Condition. New York, NY: Basic Books; 1988.
33. Kleinman A, Eisenberg L, Good B. Culture, illness, and care: clinical lessons from anthropologic and cross-cultural research. Ann Intern Med. 1978;88:251-258.
34. Miller WR, Rollnick S. Motivational Interviewing: Helping People Change. 3rd ed. New York, NY: The Guilford Press; 2013.
1. Mokdad AH, Marks JS, Stroup DF, et al. Actual causes of death in the United States, 2000. JAMA. 2004;291:1238-1245.
2. Heath C, Heath D. Switch: How to Change Things When Change is Hard. New York, NY: Crown Business; 2010.
3. Achor S. The Happiness Advantage: The Seven Principles of Positive Psychology That Fuel Success and Performance at Work. New York, NY: Crown Business; 2010.
4. Dweck CS. Mindset: The New Psychology of Success. New York, NY: Ballantine Books; 2006.
5. Kotter JP, Cohen DS. The Heart of Change: Real-Life Stories of How People Change Their Organizations. Boston, MA: Harvard Business School Publishing; 2002.
6. Wansink B. Mindless Eating: Why We Eat More Than We Think. New York, NY: Bantam; 2006.
7. Thaler RH, Sunstein CS. Nudge: Improving Decisions About Health, Wealth, and Happiness. New York, NY: Penguin Books; 2009.
8. Maurer R. One Small Step Can Change Your Life: The Kaizen Way. New York, NY: Workman Publishing Company; 2004.
9. Patterson K, Grenny J, Maxfield D, et al. Influencer: The New Science of Leading Change. New York, NY: McGraw-Hill; 2007.
10. Hubble MA, Duncan BL, Miller SD, eds. The Heart and Soul of Change: What Works in Therapy. Washington, DC: American Psychological Association; 1999.
11. Baumeister RF, Tierney J. Willpower: Rediscovering the Greatest Human Strength. New York, NY: Penguin Books; 2011.
12. Prochaska JO, DiClemente CC. The Transtheoretical Approach: Crossing Traditional Boundaries of Therapy. Homewood, IL: Dow Jones/Irwin; 1984.
13. Prochaska JO, Norcross JC. Stages of change. Psychother: Theory, Res, Pract, Training. 2001;38:443-448.
14. Searight HR. Realistic approaches to counseling in the office setting. Am Fam Physician. 2009;79:277-284.
15. Cohen DJ, Clark EC, Lawson PJ, et al. Identifying teachable moments for health behavior counseling in primary care. Patient Educ Couns. 2011;85:e8-e15.
16. Flocke SA, Antognoli E, Step MM, et al. A teachable moment communication process for smoking cessation talk: description of a group randomized clinician-focused intervention. BMC Health Serv Res. 2012;12:109.
17. Fiore MC, Bailey WC, Cohen SJ, et al. Treating Tobacco Use and Dependence. Clinical Practice Guideline. Rockville, MD: U.S. Department of Health and Human Services; 2000.
18. Alexander SC, Cox ME, Boling Turer CL, et al. Do the Five A’s work when physicians counsel about weight loss? Fam Med. 2011;43:179-184.
19. Trepper TS, McCollum E, De Jong P, et al. Solution focused therapy treatment manual for working with individuals. Solution Focused Brief Therapy Association Web site. Available at: http://www.sfbta.org/research.pdf. Accessed December 23, 2014.
20. Molnar A, de Shazer S. Solution-focused therapy: Towards the identification of therapeutic tasks. J Marital Fam Ther. 1987;13:349-358.
21. Greenberg G, Ganshorn K, Danilkewich A. Solution-focused therapy. Counseling model for busy family physicians. Can Fam Physician. 2001;47:2289-2295.
22. Giorlando ME, Schilling RJ. On becoming a solution-focused physician: The MED-STAT acronym. Families Syst Health. 1997;15:361-373.
23. Beck AT. Thinking and depression. I. Idiosyncratic content and cognitive distortions. Arch Gen Psychiatry. 1963;9:324-333.
24. Beck AT. Thinking and depression. II. Theory and therapy. Arch Gen Psychiatry. 1964;10:561-571.
25. Beck AT. The current state of cognitive therapy: a 40-year retrospective. Arch Gen Psychiatry. 2005;62:953-959.
26. Wright JH, Beck AT, Thase ME. Cognitive therapy. In: Hales RE, Yudofsky SC, Talbott JA, eds. Textbook of Clinical Psychiatry. 4th ed. Washington, DC: American Psychiatric Publishing; 2003:1245-1284.
27. Clark DA, Beck AT, Alford BA. Scientific Foundations of Cognitive Theory and Therapy of Depression. New York, NY: John Wiley & Sons; 1999.
28. Wright JH, Basco MR, Thase ME. Learning Cognitive-Behavior Therapy: An Illustrated Guide. Arlington, VA: American Psychiatric Publishing; 2006.
29. Launer J. Narrative-based Primary Care: A Practical Guide. Abingdon, United Kingdom: Radcliffe Medical Press; 2002.
30. Engel JD, Zarconi J, Pethtel L, et al. Narrative in Health Care: Healing Patients, Practitioners, Profession, and Community. Abingdon, United Kingdom: Radcliffe Publishing; 2008.
31. Charon R. Narrative Medicine: Honoring the Stories of Illness. New York, NY: Oxford University Press; 2006.
32. Kleinman A. The Illness Narratives: Suffering, Healing & the Human Condition. New York, NY: Basic Books; 1988.
33. Kleinman A, Eisenberg L, Good B. Culture, illness, and care: clinical lessons from anthropologic and cross-cultural research. Ann Intern Med. 1978;88:251-258.
34. Miller WR, Rollnick S. Motivational Interviewing: Helping People Change. 3rd ed. New York, NY: The Guilford Press; 2013.