User login
Surgical Comanagement by Hospitalists: Continued Improvement Over 5 Years
In surgical comanagement (SCM), surgeons and hospitalists share responsibility of care for surgical patients. While SCM has been increasingly utilized, many of the reported models are a modification of the consultation model, in which a group of rotating hospitalists, internists, or geriatricians care for the surgical patients, often after medical complications may have occured.1-4
In August 2012, we implemented SCM in Orthopedic and Neurosurgery services at our institution.5 This model is unique because the same Internal Medicine hospitalists are dedicated year round to the same surgical service. SCM hospitalists see patients on their assigned surgical service only; they do not see patients on the Internal Medicine service. After the first year of implementing SCM, we conducted a propensity score–weighted study with 17,057 discharges in the pre-SCM group (January 2009 to July 2012) and 5,533 discharges in the post-SCM group (September 2012 to September 2013).5 In this study, SCM was associated with a decrease in medical complications, length of stay (LOS), medical consultations, 30-day readmissions, and cost.5
Since SCM requires ongoing investment by institutions, we now report a follow-up study to explore if there were continued improvements in patient outcomes with SCM. In this study, we evaluate if there was a decrease in medical complications, LOS, number of medical consultations, rapid response team calls, and code blues and an increase in patient satisfaction with SCM in Orthopedic and Neurosurgery services between 2012 and 2018.
METHODS
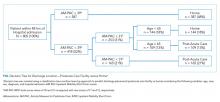
We included 26,380 discharges from Orthopedic and Neurosurgery services between September 1, 2012, and June 30, 2018, at our academic medical center. We excluded patients discharged in August 2012 as we transitioned to the SCM model. Our Institutional Review Board exempted this study from further review.
SCM Structure
SCM structure was detailed in a prior article.5 We have 3.0 clinical full-time equivalents on the Orthopedic surgery SCM service and 1.2 on the Neurosurgery SCM service. On weekdays, during the day (8
During the day, SCM hospitalists receive the first call for medical issues. After 5
SCM hospitalists screen the entire patient list on their assigned surgery service each day. After screening the patient list, SCM hospitalists formally see select patients with preventable or active medical conditions and write notes on the patient’s chart. There are no set criteria to determine which patients would be seen by SCM. This is because surgeries can decompensate stable medical conditions or new unexpected medical complications may occur. Additionally, in our prior study, we reported that SCM reduced medical complications and LOS regardless of age or patient acuity.5
Outcomes
Our primary outcome was proportion of patients with ≥1 medical complication (sepsis, pneumonia, urinary tract infection, delirium, acute kidney injury, atrial fibrillation, or ileus). Our secondary outcomes included mean LOS, proportion of patients with ≥2 medical consultations, rapid response team calls, code blues, and top-box patient satisfaction score. Though cost is an important consideration in implementing SCM, limited financial data were available. However, since LOS is a key component in calculating direct costs,6 we estimated the cost savings per discharge using mean direct cost per day and the difference in mean LOS between pre- and post-SCM groups.5
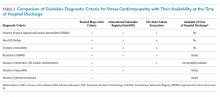
We defined medical complications using International Classification of Disease (ICD) Codes 9 or 10 that were coded as “not present on admission” (Appendix 1). We used Hospital Consumer Assessment of Healthcare Providers and Systems (HCAHPS) survey for three questions for patient satisfaction: Did doctors treat with courtesy and respect, listen carefully, and explain things in a way you could understand?
Statistical Analysis
We used regression analysis to assess trends in patient characteristics by year (Appendix 2). Logistic regression with logit link was used to assess the yearly change in our binary outcomes (proportion of patients with ≥1 medical complication, those with ≥2 medical consultations, rapid response team calls, code blue, and top-box patient satisfaction score) and reported odds ratios. Gamma regression with identity link was performed for our continuous outcome (LOS). Beta coefficient was reported to estimate the yearly change in LOS under their original scales. Age, primary insurance, race, Charlson comorbidity score, general or regional anesthesia, surgical service, and duration of surgery were adjusted in the regression analyses for outcomes. SAS 9.4 was used for analysis.
RESULTS
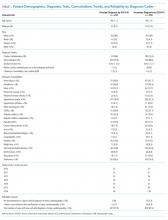
Patient characteristics are shown in Table 1. Overall, 62.8% patients were discharged from Orthopedic surgery service, 72.5% patients underwent elective surgery, and 88.8% received general anesthesia. Between 2012 and 2018, there was a significant increase in the median age of patients (from 60 years to 63 years), mean Charlson comorbidity score increased from 1.07 to 1.46, and median case mix index, a measure of patient acuity, increased from 2.10 to 2.36 (Appendix 2).
Comparing pre-SCM unadjusted rates reported in our prior study (January 2009 to July 2012) to post-SCM (September 2012 to June 2018; Appendix 3), patients with ≥1 medical complication decreased from 10.1% to 6.1%, LOS (mean ± standard deviation) changed from 5.4 ± 2.2 days to 4.6 ± 5.8 days, patients with ≥2 medical consultations decreased from 19.4% to 9.2%, rapid response team calls changed from 1% to 0.9%, code blues changed from 0.3% to 0.2%, and patients with top-box patient satisfaction score increased from 86.4% to 94.2%.5
In the adjusted analysis from 2012 to 2018, the odds of patients with ≥1 medical complication decreased by 3.8% per year (P = .01), estimated LOS decreased by 0.3 days per year (P < .0001), and the odds of rapid response team calls decreased by 12.2% per year (P = .001; Table 2). Changes over time in the odds of patients with ≥2 medical consultations, code blues, or top-box patient satisfaction score were not statistically significant (Table 2). Based on the LOS reduction pre- to post-SCM, there were estimated average direct cost savings of $3,424 per discharge between 2012 and 2018.
DISCUSSION
Since the implementation of SCM on Orthopedic and Neurosurgery services at our institution, there was a decrease in medical complications, LOS, and rapid response team calls. To our knowledge, this is one of the largest studies evaluating the benefits of SCM over 5.8 years. Similar to our prior studies on this SCM model of care,5,7 other studies have reported a decrease in medical complications,8-10 LOS,11-13 and cost of care14 with SCM.
While the changes in the unadjusted rates of outcomes over the years appeared to be small, while our patient population became older and sicker, there were significant changes in several of our outcomes in the adjusted analysis. We believe that SCM hospitalists have developed a skill set and understanding of these surgical patients over time and can manage more medically complex patients without an increase in medical complications or LOS. We attribute this to our unique SCM model in which the same hospitalists stay year round on the same surgical service. SCM hospitalists have built trusting relationships with the surgical team with greater involvement in decision making, care planning, and patient selection. With minimal turnover in the SCM group and with ongoing learning, SCM hospitalists can anticipate fluid or pain medication requirements after specific surgeries and the surgery-specific medical complications. SCM hospitalists are available on the patient units to provide timely intervention in case of medical deterioration; answer any questions from patients, families, or nursing while the surgical teams may be in the operating room; and coordinate with other medical consultants or outpatient providers as needed.
This study has several limitations. This is a single-center study at an academic institution, limited to two surgical services. We did not have a control group and multiple hospital-wide interventions may have affected these outcomes. This is an observational study in which unobserved variables may bias the results. We used ICD codes to identify medical complications, which relies on the quality of physician documentation. While our response rate of 21.1% for HCAHPS was comparable to the national average of 26.7%, it may not reliably represent our patient population.15 Lastly, we had limited financial data.
CONCLUSION
With the move toward value-based payment and increasing medical complexity of surgical patients, SCM by hospitalists may deliver high-quality care.
1. Auerbach AD, Wachter RM, Cheng HQ, et al. Comanagement of surgical patients between neurosurgeons and hospitalists. Arch Intern Med. 2010;170(22):2004-2010. https://doi.org/10.1001/archinternmed.2010.432
2. Ruiz ME, Merino RÁ, Rodríguez R, Sánchez GM, Alonso A, Barbero M. Effect of comanagement with internal medicine on hospital stay of patients admitted to the service of otolaryngology. Acta Otorrinolaringol Esp. 2015;66(5):264-268. https://doi.org/10.1016/j.otorri.2014.09.010.
3. Tadros RO, Faries PL, Malik R, et al. The effect of a hospitalist comanagement service on vascular surgery inpatients. J Vasc Surg. 2015;61(6):1550-1555. https://doi.org/10.1016/j.jvs.2015.01.006
4. Gregersen M, Mørch MM, Hougaard K, Damsgaard EM. Geriatric intervention in elderly patients with hip fracture in an orthopedic ward. J Inj Violence Res. 2012;4(2):45-51. https://doi.org/10.5249/jivr.v4i2.96
5. Rohatgi N, Loftus P, Grujic O, Cullen M, Hopkins J, Ahuja N. Surgical comanagement by hospitalists improves patient outcomes: A propensity score analysis. Ann Surg. 2016;264(2):275-282. https://doi.org/10.1097/SLA.0000000000001629
6. Polverejan E, Gardiner JC, Bradley CJ, Holmes-Rovner M, Rovner D. Estimating mean hospital cost as a function of length of stay and patient characteristics. Health Econ. 2003;12(11):935-947. https://doi.org/10.1002/hec.774
7. Rohatgi N, Wei PH, Grujic O, Ahuja N. Surgical Comanagement by hospitalists in colorectal surgery. J Am Coll Surg. 2018;227(4):404-410. https://doi.org/10.1016/j.jamcollsurg.2018.06.011
8. Huddleston JM, Long KH, Naessens JM, et al. Medical and surgical comanagement after elective hip and knee arthroplasty: A randomized, controlled trial. Ann Intern Med. 2004;141(1):28-38. https://doi.org/10.7326/0003-4819-141-1-200407060-00012.
9. Swart E, Vasudeva E, Makhni EC, Macaulay W, Bozic KJ. Dedicated perioperative hip fracture comanagement programs are cost-effective in high-volume centers: An economic analysis. Clin Orthop Relat Res. 2016;474(1):222-233. https://doi.org/10.1007/s11999-015-4494-4.
10. Iberti CT, Briones A, Gabriel E, Dunn AS. Hospitalist-vascular surgery comanagement: Effects on complications and mortality. Hosp Pract. 2016;44(5):233-236. https://doi.org/10.1080/21548331.2016.1259543.
11. Kammerlander C, Roth T, Friedman SM, et al. Ortho-geriatric service--A literature review comparing different models. Osteoporos Int. 2010;21(Suppl 4):S637-S646. https://doi.org/10.1007/s00198-010-1396-x.
12. Bracey DN, Kiymaz TC, Holst DC, et al. An orthopedic-hospitalist comanaged hip fracture service reduces inpatient length of stay. Geriatr Orthop Surg Rehabil. 2016;7(4):171-177. https://doi.org/10.1177/2151458516661383.
13. Duplantier NL, Briski DC, Luce LT, Meyer MS, Ochsner JL, Chimento GF. The effects of a hospitalist comanagement model for joint arthroplasty patients in a teaching facility. J Arthroplasty. 2016;31(3):567-572. https://doi.org/10.1016/j.arth.2015.10.010.
14. Roy A, Heckman MG, Roy V. Associations between the hospitalist model of care and quality-of-care-related outcomes in patients undergoing hip fracture surgery. Mayo Clin Proc. 2006;81(1):28-31. https://doi.org/10.4065/81.1.28.
15. Godden E, Paseka A, Gnida J, Inguanzo J. The impact of response rate on Hospital Consumer Assessment of Healthcare Providers and System (HCAHPS) dimension scores. Patient Exp J. 2019;6(1):105-114. https://doi.org/10.35680/2372-0247.1357.
In surgical comanagement (SCM), surgeons and hospitalists share responsibility of care for surgical patients. While SCM has been increasingly utilized, many of the reported models are a modification of the consultation model, in which a group of rotating hospitalists, internists, or geriatricians care for the surgical patients, often after medical complications may have occured.1-4
In August 2012, we implemented SCM in Orthopedic and Neurosurgery services at our institution.5 This model is unique because the same Internal Medicine hospitalists are dedicated year round to the same surgical service. SCM hospitalists see patients on their assigned surgical service only; they do not see patients on the Internal Medicine service. After the first year of implementing SCM, we conducted a propensity score–weighted study with 17,057 discharges in the pre-SCM group (January 2009 to July 2012) and 5,533 discharges in the post-SCM group (September 2012 to September 2013).5 In this study, SCM was associated with a decrease in medical complications, length of stay (LOS), medical consultations, 30-day readmissions, and cost.5
Since SCM requires ongoing investment by institutions, we now report a follow-up study to explore if there were continued improvements in patient outcomes with SCM. In this study, we evaluate if there was a decrease in medical complications, LOS, number of medical consultations, rapid response team calls, and code blues and an increase in patient satisfaction with SCM in Orthopedic and Neurosurgery services between 2012 and 2018.
METHODS
We included 26,380 discharges from Orthopedic and Neurosurgery services between September 1, 2012, and June 30, 2018, at our academic medical center. We excluded patients discharged in August 2012 as we transitioned to the SCM model. Our Institutional Review Board exempted this study from further review.
SCM Structure
SCM structure was detailed in a prior article.5 We have 3.0 clinical full-time equivalents on the Orthopedic surgery SCM service and 1.2 on the Neurosurgery SCM service. On weekdays, during the day (8
During the day, SCM hospitalists receive the first call for medical issues. After 5
SCM hospitalists screen the entire patient list on their assigned surgery service each day. After screening the patient list, SCM hospitalists formally see select patients with preventable or active medical conditions and write notes on the patient’s chart. There are no set criteria to determine which patients would be seen by SCM. This is because surgeries can decompensate stable medical conditions or new unexpected medical complications may occur. Additionally, in our prior study, we reported that SCM reduced medical complications and LOS regardless of age or patient acuity.5
Outcomes
Our primary outcome was proportion of patients with ≥1 medical complication (sepsis, pneumonia, urinary tract infection, delirium, acute kidney injury, atrial fibrillation, or ileus). Our secondary outcomes included mean LOS, proportion of patients with ≥2 medical consultations, rapid response team calls, code blues, and top-box patient satisfaction score. Though cost is an important consideration in implementing SCM, limited financial data were available. However, since LOS is a key component in calculating direct costs,6 we estimated the cost savings per discharge using mean direct cost per day and the difference in mean LOS between pre- and post-SCM groups.5
We defined medical complications using International Classification of Disease (ICD) Codes 9 or 10 that were coded as “not present on admission” (Appendix 1). We used Hospital Consumer Assessment of Healthcare Providers and Systems (HCAHPS) survey for three questions for patient satisfaction: Did doctors treat with courtesy and respect, listen carefully, and explain things in a way you could understand?
Statistical Analysis
We used regression analysis to assess trends in patient characteristics by year (Appendix 2). Logistic regression with logit link was used to assess the yearly change in our binary outcomes (proportion of patients with ≥1 medical complication, those with ≥2 medical consultations, rapid response team calls, code blue, and top-box patient satisfaction score) and reported odds ratios. Gamma regression with identity link was performed for our continuous outcome (LOS). Beta coefficient was reported to estimate the yearly change in LOS under their original scales. Age, primary insurance, race, Charlson comorbidity score, general or regional anesthesia, surgical service, and duration of surgery were adjusted in the regression analyses for outcomes. SAS 9.4 was used for analysis.
RESULTS
Patient characteristics are shown in Table 1. Overall, 62.8% patients were discharged from Orthopedic surgery service, 72.5% patients underwent elective surgery, and 88.8% received general anesthesia. Between 2012 and 2018, there was a significant increase in the median age of patients (from 60 years to 63 years), mean Charlson comorbidity score increased from 1.07 to 1.46, and median case mix index, a measure of patient acuity, increased from 2.10 to 2.36 (Appendix 2).
Comparing pre-SCM unadjusted rates reported in our prior study (January 2009 to July 2012) to post-SCM (September 2012 to June 2018; Appendix 3), patients with ≥1 medical complication decreased from 10.1% to 6.1%, LOS (mean ± standard deviation) changed from 5.4 ± 2.2 days to 4.6 ± 5.8 days, patients with ≥2 medical consultations decreased from 19.4% to 9.2%, rapid response team calls changed from 1% to 0.9%, code blues changed from 0.3% to 0.2%, and patients with top-box patient satisfaction score increased from 86.4% to 94.2%.5
In the adjusted analysis from 2012 to 2018, the odds of patients with ≥1 medical complication decreased by 3.8% per year (P = .01), estimated LOS decreased by 0.3 days per year (P < .0001), and the odds of rapid response team calls decreased by 12.2% per year (P = .001; Table 2). Changes over time in the odds of patients with ≥2 medical consultations, code blues, or top-box patient satisfaction score were not statistically significant (Table 2). Based on the LOS reduction pre- to post-SCM, there were estimated average direct cost savings of $3,424 per discharge between 2012 and 2018.
DISCUSSION
Since the implementation of SCM on Orthopedic and Neurosurgery services at our institution, there was a decrease in medical complications, LOS, and rapid response team calls. To our knowledge, this is one of the largest studies evaluating the benefits of SCM over 5.8 years. Similar to our prior studies on this SCM model of care,5,7 other studies have reported a decrease in medical complications,8-10 LOS,11-13 and cost of care14 with SCM.
While the changes in the unadjusted rates of outcomes over the years appeared to be small, while our patient population became older and sicker, there were significant changes in several of our outcomes in the adjusted analysis. We believe that SCM hospitalists have developed a skill set and understanding of these surgical patients over time and can manage more medically complex patients without an increase in medical complications or LOS. We attribute this to our unique SCM model in which the same hospitalists stay year round on the same surgical service. SCM hospitalists have built trusting relationships with the surgical team with greater involvement in decision making, care planning, and patient selection. With minimal turnover in the SCM group and with ongoing learning, SCM hospitalists can anticipate fluid or pain medication requirements after specific surgeries and the surgery-specific medical complications. SCM hospitalists are available on the patient units to provide timely intervention in case of medical deterioration; answer any questions from patients, families, or nursing while the surgical teams may be in the operating room; and coordinate with other medical consultants or outpatient providers as needed.
This study has several limitations. This is a single-center study at an academic institution, limited to two surgical services. We did not have a control group and multiple hospital-wide interventions may have affected these outcomes. This is an observational study in which unobserved variables may bias the results. We used ICD codes to identify medical complications, which relies on the quality of physician documentation. While our response rate of 21.1% for HCAHPS was comparable to the national average of 26.7%, it may not reliably represent our patient population.15 Lastly, we had limited financial data.
CONCLUSION
With the move toward value-based payment and increasing medical complexity of surgical patients, SCM by hospitalists may deliver high-quality care.
In surgical comanagement (SCM), surgeons and hospitalists share responsibility of care for surgical patients. While SCM has been increasingly utilized, many of the reported models are a modification of the consultation model, in which a group of rotating hospitalists, internists, or geriatricians care for the surgical patients, often after medical complications may have occured.1-4
In August 2012, we implemented SCM in Orthopedic and Neurosurgery services at our institution.5 This model is unique because the same Internal Medicine hospitalists are dedicated year round to the same surgical service. SCM hospitalists see patients on their assigned surgical service only; they do not see patients on the Internal Medicine service. After the first year of implementing SCM, we conducted a propensity score–weighted study with 17,057 discharges in the pre-SCM group (January 2009 to July 2012) and 5,533 discharges in the post-SCM group (September 2012 to September 2013).5 In this study, SCM was associated with a decrease in medical complications, length of stay (LOS), medical consultations, 30-day readmissions, and cost.5
Since SCM requires ongoing investment by institutions, we now report a follow-up study to explore if there were continued improvements in patient outcomes with SCM. In this study, we evaluate if there was a decrease in medical complications, LOS, number of medical consultations, rapid response team calls, and code blues and an increase in patient satisfaction with SCM in Orthopedic and Neurosurgery services between 2012 and 2018.
METHODS
We included 26,380 discharges from Orthopedic and Neurosurgery services between September 1, 2012, and June 30, 2018, at our academic medical center. We excluded patients discharged in August 2012 as we transitioned to the SCM model. Our Institutional Review Board exempted this study from further review.
SCM Structure
SCM structure was detailed in a prior article.5 We have 3.0 clinical full-time equivalents on the Orthopedic surgery SCM service and 1.2 on the Neurosurgery SCM service. On weekdays, during the day (8
During the day, SCM hospitalists receive the first call for medical issues. After 5
SCM hospitalists screen the entire patient list on their assigned surgery service each day. After screening the patient list, SCM hospitalists formally see select patients with preventable or active medical conditions and write notes on the patient’s chart. There are no set criteria to determine which patients would be seen by SCM. This is because surgeries can decompensate stable medical conditions or new unexpected medical complications may occur. Additionally, in our prior study, we reported that SCM reduced medical complications and LOS regardless of age or patient acuity.5
Outcomes
Our primary outcome was proportion of patients with ≥1 medical complication (sepsis, pneumonia, urinary tract infection, delirium, acute kidney injury, atrial fibrillation, or ileus). Our secondary outcomes included mean LOS, proportion of patients with ≥2 medical consultations, rapid response team calls, code blues, and top-box patient satisfaction score. Though cost is an important consideration in implementing SCM, limited financial data were available. However, since LOS is a key component in calculating direct costs,6 we estimated the cost savings per discharge using mean direct cost per day and the difference in mean LOS between pre- and post-SCM groups.5
We defined medical complications using International Classification of Disease (ICD) Codes 9 or 10 that were coded as “not present on admission” (Appendix 1). We used Hospital Consumer Assessment of Healthcare Providers and Systems (HCAHPS) survey for three questions for patient satisfaction: Did doctors treat with courtesy and respect, listen carefully, and explain things in a way you could understand?
Statistical Analysis
We used regression analysis to assess trends in patient characteristics by year (Appendix 2). Logistic regression with logit link was used to assess the yearly change in our binary outcomes (proportion of patients with ≥1 medical complication, those with ≥2 medical consultations, rapid response team calls, code blue, and top-box patient satisfaction score) and reported odds ratios. Gamma regression with identity link was performed for our continuous outcome (LOS). Beta coefficient was reported to estimate the yearly change in LOS under their original scales. Age, primary insurance, race, Charlson comorbidity score, general or regional anesthesia, surgical service, and duration of surgery were adjusted in the regression analyses for outcomes. SAS 9.4 was used for analysis.
RESULTS
Patient characteristics are shown in Table 1. Overall, 62.8% patients were discharged from Orthopedic surgery service, 72.5% patients underwent elective surgery, and 88.8% received general anesthesia. Between 2012 and 2018, there was a significant increase in the median age of patients (from 60 years to 63 years), mean Charlson comorbidity score increased from 1.07 to 1.46, and median case mix index, a measure of patient acuity, increased from 2.10 to 2.36 (Appendix 2).
Comparing pre-SCM unadjusted rates reported in our prior study (January 2009 to July 2012) to post-SCM (September 2012 to June 2018; Appendix 3), patients with ≥1 medical complication decreased from 10.1% to 6.1%, LOS (mean ± standard deviation) changed from 5.4 ± 2.2 days to 4.6 ± 5.8 days, patients with ≥2 medical consultations decreased from 19.4% to 9.2%, rapid response team calls changed from 1% to 0.9%, code blues changed from 0.3% to 0.2%, and patients with top-box patient satisfaction score increased from 86.4% to 94.2%.5
In the adjusted analysis from 2012 to 2018, the odds of patients with ≥1 medical complication decreased by 3.8% per year (P = .01), estimated LOS decreased by 0.3 days per year (P < .0001), and the odds of rapid response team calls decreased by 12.2% per year (P = .001; Table 2). Changes over time in the odds of patients with ≥2 medical consultations, code blues, or top-box patient satisfaction score were not statistically significant (Table 2). Based on the LOS reduction pre- to post-SCM, there were estimated average direct cost savings of $3,424 per discharge between 2012 and 2018.
DISCUSSION
Since the implementation of SCM on Orthopedic and Neurosurgery services at our institution, there was a decrease in medical complications, LOS, and rapid response team calls. To our knowledge, this is one of the largest studies evaluating the benefits of SCM over 5.8 years. Similar to our prior studies on this SCM model of care,5,7 other studies have reported a decrease in medical complications,8-10 LOS,11-13 and cost of care14 with SCM.
While the changes in the unadjusted rates of outcomes over the years appeared to be small, while our patient population became older and sicker, there were significant changes in several of our outcomes in the adjusted analysis. We believe that SCM hospitalists have developed a skill set and understanding of these surgical patients over time and can manage more medically complex patients without an increase in medical complications or LOS. We attribute this to our unique SCM model in which the same hospitalists stay year round on the same surgical service. SCM hospitalists have built trusting relationships with the surgical team with greater involvement in decision making, care planning, and patient selection. With minimal turnover in the SCM group and with ongoing learning, SCM hospitalists can anticipate fluid or pain medication requirements after specific surgeries and the surgery-specific medical complications. SCM hospitalists are available on the patient units to provide timely intervention in case of medical deterioration; answer any questions from patients, families, or nursing while the surgical teams may be in the operating room; and coordinate with other medical consultants or outpatient providers as needed.
This study has several limitations. This is a single-center study at an academic institution, limited to two surgical services. We did not have a control group and multiple hospital-wide interventions may have affected these outcomes. This is an observational study in which unobserved variables may bias the results. We used ICD codes to identify medical complications, which relies on the quality of physician documentation. While our response rate of 21.1% for HCAHPS was comparable to the national average of 26.7%, it may not reliably represent our patient population.15 Lastly, we had limited financial data.
CONCLUSION
With the move toward value-based payment and increasing medical complexity of surgical patients, SCM by hospitalists may deliver high-quality care.
1. Auerbach AD, Wachter RM, Cheng HQ, et al. Comanagement of surgical patients between neurosurgeons and hospitalists. Arch Intern Med. 2010;170(22):2004-2010. https://doi.org/10.1001/archinternmed.2010.432
2. Ruiz ME, Merino RÁ, Rodríguez R, Sánchez GM, Alonso A, Barbero M. Effect of comanagement with internal medicine on hospital stay of patients admitted to the service of otolaryngology. Acta Otorrinolaringol Esp. 2015;66(5):264-268. https://doi.org/10.1016/j.otorri.2014.09.010.
3. Tadros RO, Faries PL, Malik R, et al. The effect of a hospitalist comanagement service on vascular surgery inpatients. J Vasc Surg. 2015;61(6):1550-1555. https://doi.org/10.1016/j.jvs.2015.01.006
4. Gregersen M, Mørch MM, Hougaard K, Damsgaard EM. Geriatric intervention in elderly patients with hip fracture in an orthopedic ward. J Inj Violence Res. 2012;4(2):45-51. https://doi.org/10.5249/jivr.v4i2.96
5. Rohatgi N, Loftus P, Grujic O, Cullen M, Hopkins J, Ahuja N. Surgical comanagement by hospitalists improves patient outcomes: A propensity score analysis. Ann Surg. 2016;264(2):275-282. https://doi.org/10.1097/SLA.0000000000001629
6. Polverejan E, Gardiner JC, Bradley CJ, Holmes-Rovner M, Rovner D. Estimating mean hospital cost as a function of length of stay and patient characteristics. Health Econ. 2003;12(11):935-947. https://doi.org/10.1002/hec.774
7. Rohatgi N, Wei PH, Grujic O, Ahuja N. Surgical Comanagement by hospitalists in colorectal surgery. J Am Coll Surg. 2018;227(4):404-410. https://doi.org/10.1016/j.jamcollsurg.2018.06.011
8. Huddleston JM, Long KH, Naessens JM, et al. Medical and surgical comanagement after elective hip and knee arthroplasty: A randomized, controlled trial. Ann Intern Med. 2004;141(1):28-38. https://doi.org/10.7326/0003-4819-141-1-200407060-00012.
9. Swart E, Vasudeva E, Makhni EC, Macaulay W, Bozic KJ. Dedicated perioperative hip fracture comanagement programs are cost-effective in high-volume centers: An economic analysis. Clin Orthop Relat Res. 2016;474(1):222-233. https://doi.org/10.1007/s11999-015-4494-4.
10. Iberti CT, Briones A, Gabriel E, Dunn AS. Hospitalist-vascular surgery comanagement: Effects on complications and mortality. Hosp Pract. 2016;44(5):233-236. https://doi.org/10.1080/21548331.2016.1259543.
11. Kammerlander C, Roth T, Friedman SM, et al. Ortho-geriatric service--A literature review comparing different models. Osteoporos Int. 2010;21(Suppl 4):S637-S646. https://doi.org/10.1007/s00198-010-1396-x.
12. Bracey DN, Kiymaz TC, Holst DC, et al. An orthopedic-hospitalist comanaged hip fracture service reduces inpatient length of stay. Geriatr Orthop Surg Rehabil. 2016;7(4):171-177. https://doi.org/10.1177/2151458516661383.
13. Duplantier NL, Briski DC, Luce LT, Meyer MS, Ochsner JL, Chimento GF. The effects of a hospitalist comanagement model for joint arthroplasty patients in a teaching facility. J Arthroplasty. 2016;31(3):567-572. https://doi.org/10.1016/j.arth.2015.10.010.
14. Roy A, Heckman MG, Roy V. Associations between the hospitalist model of care and quality-of-care-related outcomes in patients undergoing hip fracture surgery. Mayo Clin Proc. 2006;81(1):28-31. https://doi.org/10.4065/81.1.28.
15. Godden E, Paseka A, Gnida J, Inguanzo J. The impact of response rate on Hospital Consumer Assessment of Healthcare Providers and System (HCAHPS) dimension scores. Patient Exp J. 2019;6(1):105-114. https://doi.org/10.35680/2372-0247.1357.
1. Auerbach AD, Wachter RM, Cheng HQ, et al. Comanagement of surgical patients between neurosurgeons and hospitalists. Arch Intern Med. 2010;170(22):2004-2010. https://doi.org/10.1001/archinternmed.2010.432
2. Ruiz ME, Merino RÁ, Rodríguez R, Sánchez GM, Alonso A, Barbero M. Effect of comanagement with internal medicine on hospital stay of patients admitted to the service of otolaryngology. Acta Otorrinolaringol Esp. 2015;66(5):264-268. https://doi.org/10.1016/j.otorri.2014.09.010.
3. Tadros RO, Faries PL, Malik R, et al. The effect of a hospitalist comanagement service on vascular surgery inpatients. J Vasc Surg. 2015;61(6):1550-1555. https://doi.org/10.1016/j.jvs.2015.01.006
4. Gregersen M, Mørch MM, Hougaard K, Damsgaard EM. Geriatric intervention in elderly patients with hip fracture in an orthopedic ward. J Inj Violence Res. 2012;4(2):45-51. https://doi.org/10.5249/jivr.v4i2.96
5. Rohatgi N, Loftus P, Grujic O, Cullen M, Hopkins J, Ahuja N. Surgical comanagement by hospitalists improves patient outcomes: A propensity score analysis. Ann Surg. 2016;264(2):275-282. https://doi.org/10.1097/SLA.0000000000001629
6. Polverejan E, Gardiner JC, Bradley CJ, Holmes-Rovner M, Rovner D. Estimating mean hospital cost as a function of length of stay and patient characteristics. Health Econ. 2003;12(11):935-947. https://doi.org/10.1002/hec.774
7. Rohatgi N, Wei PH, Grujic O, Ahuja N. Surgical Comanagement by hospitalists in colorectal surgery. J Am Coll Surg. 2018;227(4):404-410. https://doi.org/10.1016/j.jamcollsurg.2018.06.011
8. Huddleston JM, Long KH, Naessens JM, et al. Medical and surgical comanagement after elective hip and knee arthroplasty: A randomized, controlled trial. Ann Intern Med. 2004;141(1):28-38. https://doi.org/10.7326/0003-4819-141-1-200407060-00012.
9. Swart E, Vasudeva E, Makhni EC, Macaulay W, Bozic KJ. Dedicated perioperative hip fracture comanagement programs are cost-effective in high-volume centers: An economic analysis. Clin Orthop Relat Res. 2016;474(1):222-233. https://doi.org/10.1007/s11999-015-4494-4.
10. Iberti CT, Briones A, Gabriel E, Dunn AS. Hospitalist-vascular surgery comanagement: Effects on complications and mortality. Hosp Pract. 2016;44(5):233-236. https://doi.org/10.1080/21548331.2016.1259543.
11. Kammerlander C, Roth T, Friedman SM, et al. Ortho-geriatric service--A literature review comparing different models. Osteoporos Int. 2010;21(Suppl 4):S637-S646. https://doi.org/10.1007/s00198-010-1396-x.
12. Bracey DN, Kiymaz TC, Holst DC, et al. An orthopedic-hospitalist comanaged hip fracture service reduces inpatient length of stay. Geriatr Orthop Surg Rehabil. 2016;7(4):171-177. https://doi.org/10.1177/2151458516661383.
13. Duplantier NL, Briski DC, Luce LT, Meyer MS, Ochsner JL, Chimento GF. The effects of a hospitalist comanagement model for joint arthroplasty patients in a teaching facility. J Arthroplasty. 2016;31(3):567-572. https://doi.org/10.1016/j.arth.2015.10.010.
14. Roy A, Heckman MG, Roy V. Associations between the hospitalist model of care and quality-of-care-related outcomes in patients undergoing hip fracture surgery. Mayo Clin Proc. 2006;81(1):28-31. https://doi.org/10.4065/81.1.28.
15. Godden E, Paseka A, Gnida J, Inguanzo J. The impact of response rate on Hospital Consumer Assessment of Healthcare Providers and System (HCAHPS) dimension scores. Patient Exp J. 2019;6(1):105-114. https://doi.org/10.35680/2372-0247.1357.
© 2020 Society of Hospital Medicine
Describing Variability of Inpatient Consultation Practices: Physician, Patient, and Admission Factors
Inpatient consultation is an extremely common practice with the potential to improve patient outcomes significantly.1-3 However, variability in consultation practices may be risky for patients. In addition to underuse when the benefit is clear, the overuse of consultation may lead to additional testing and therapies, increased length of stay (LOS) and costs, conflicting recommendations, and opportunities for communication breakdown.
Consultation use is often at the discretion of individual providers. While this decision is frequently driven by patient needs, significant variation in consultation practices not fully explained by patient factors exists.1 Prior work has described hospital-level variation1 and that primary care physicians use more consultation than hospitalists.4 However, other factors affecting consultation remain unknown. We sought to explore physician-, patient-, and admission-level factors associated with consultation use on inpatient general medicine services.
METHODS
Study Design
We conducted a retrospective analysis of data from the University of Chicago Hospitalist Project (UCHP). UCHP is a longstanding study of the care of hospitalized patients admitted to the University of Chicago general medicine services, involving both patient data collection and physician experience surveys.5 Data were obtained for enrolled UCHP patients between 2011-2016 from the Center for Research Informatics (CRI). The University of Chicago Institutional Review Board approved this study.
Data Collection
Attendings and patients consented to UCHP participation. Data collection details are described elsewhere.5,6 Data from EpicCare (EpicSystems Corp, Wisconsin) and Centricity Billing (GE Healthcare, Illinois) were obtained via CRI for all encounters of enrolled UCHP patients during the study period (N = 218,591).
Attending Attribution
We determined attending attribution for admissions as follows: the attending author of the first history and physical (H&P) was assigned. If this was unavailable, the attending author of the first progress note (PN) was assigned. For patients admitted by hospitalists on admitting shifts to nonteaching services (ie, service without residents/students), the author of the first PN was assigned if different from H&P. Where available, attribution was corroborated with call schedules.
Sample and Variables
All encounters containing inpatient admissions to the University of Chicago from May 10, 2011 (Electronic Health Record activation date), through December 31, 2016, were considered for inclusion (N = 51,171, Appendix 1). Admissions including only documentation from ancillary services were excluded (eg, encounters for hemodialysis or physical therapy). Admissions were limited to a length of stay (LOS) ≤ 5 days, corresponding to the average US inpatient LOS of 4.6 days,7 to minimize the likelihood of attending handoffs (N = 31,592). If attending attribution was not possible via the above-described methods, the admission was eliminated (N = 3,103; 10.9% of admissions with LOS ≤ 5 days). Finally, the sample was restricted to general medicine service admissions under attendings enrolled in UCHP who completed surveys. After the application of all criteria, 6,153 admissions remained for analysis.
The outcome variable was the number of consultations per admission, determined by counting the unique number of services creating clinical documentation, and subtracting one for the primary team. If the Medical/Surgical intensive care unit (ICU) was a service, then two were subtracted to account for the ICU transfer.
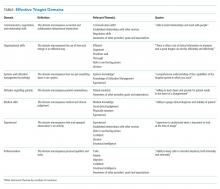
Attending years in practice (ie, years since medical school graduation) and gender were determined from public resources. Practice characteristics were determined from UCHP attending surveys, which address perceptions of workload and satisfaction (Appendix 2).
Patient characteristics (gender, age, Elixhauser Indices) and admission characteristics (LOS, season of admission, payor) were determined from UCHP and CRI data. The Elixhauser Index uses a well-validated system combining the presence/absence of 31 comorbidities to predict mortality and 30-day readmission.8 Elixhauser Indices were calculated using the “Creation of Elixhauser Comorbidity Index Scores 1.0” software.9 For admissions under hospitalist attendings, teaching/nonteaching team was ascertained via internal teaching service calendars.
Analysis
We used descriptive statistics to examine demographic characteristics. The difference between the lowest and highest quartile consultation use was determined via a two-sample t test. Given the multilevel nature of our count data, we used a mixed-effects Poisson model accounting for within-group variation by clustering on attending and patient (3-level random-effects model). The analysis was done using Stata 15 (StataCorp, Texas).
RESULTS
From 2011 to 2016, 14,848 patients and 88 attendings were enrolled in UCHP; 4,772 patients (32%) and 69 attendings (59.4%) had data available and were included. Mean LOS was 3.0 days (SD = 1.3). Table 1 describes the characteristics of attendings, patients, and admissions.
Seventy-six percent of admissions included at least one consultation. Consultation use varied widely, ranging from 0 to 10 per admission (mean = 1.39, median = 1; standard deviation [SD] = 1.17). The number of consultations per admission in the highest quartile of consultation frequency (mean = 3.47, median = 3) was 5.7-fold that of the lowest quartile (mean = 0.613, median = 1; P <.001).
In multivariable regression, physician-, patient-, and admission-level characteristics were associated with the differential use of consultation (Table 2). On teaching services, consultations called by hospitalist vs nonhospitalist generalists did not differ (P =.361). However, hospitalists on nonteaching services called 8.6% more consultations than hospitalists on teaching services (P =.02). Attending agreement with survey item “The interruption of my personal life by work is a problem” was associated with 8.2% fewer consultations per admission (P =.002).
Patients older than 75 years received 19% fewer consultations compared with patients younger than 49 years (P <.001). Compared with Medicare, Medicaid admissions had 12.2% fewer consultations (P <.001), whereas privately insured admissions had 10.7% more (P =.001). The number of consultations per admission decreased every year, with 45.3% fewer consultations in 2015 than 2011 (P <.001). Consultations increased by each 22% per day increase in LOS (P <.001).
DISCUSSION
Our analysis described several physician-, patient-, and admission-level characteristics associated with the use of inpatient consultation. Our results strengthen prior work demonstrating that patient-level factors alone are insufficient to explain consultation variability.1
Hospitalists on nonteaching services called more consultations, which may reflect a higher workload on these services. Busy hospitalists on nonteaching teams may lack time to delve deeply into clinical problems and require more consultations, especially for work with heavy cognitive loads such as diagnosis. “Outsourcing” tasks when workload increases occurs in other cognitive activities such as teaching.10 The association between work interrupting personal life and fewer consultations may also implicate the effects of time. Attendings who are experiencing work encroaching on their personal lives may be those spending more time with patients and consulting less. This finding merits further study, especially with increasing concern about balancing time spent in meaningful patient care activities with risk of physician burnout.
This finding could also indicate that trainee participation modifies consultation use for hospitalists. Teaching service teams with more individual members may allow a greater pool of collective knowledge, decreasing the need for consultation to answer clinical questions.11 Interestingly, there was no difference in consultation use between generalists or subspecialists and hospitalists on teaching services, possibly suggesting a unique effect in hospitalists who vary clinical practice depending on team structure. These differences deserve further investigation, with implications for education and resource utilization.
We were surprised by the finding that consultations decreased each year, despite increasing patient complexity and availability of consultation services. This could be explained by a growing emphasis on shortening LOS in our institution, thus shifting consultative care to outpatient settings. Understanding these effects is critically important with growing evidence that consultation improves patient outcomes because these external pressures could lead to unintended consequences for quality or access to care.
Several findings related to patient factors additionally emerged, including age and insurance status. Although related to medical complexity, these effects persist despite adjustment, which raises the question of whether they contribute to the decision to seek consultation. Older patients received fewer consultations, which could reflect the use of more conservative practice models in the elderly,12 or ageism, which is associated with undertreatment.13 With respect to insurance status, Medicaid patients were associated with fewer consultations. This finding is consistent with previous work showing the decreased intensity of hospital services used for Medicaid patients.14Our study has limitations. Our data were from one large urban academic center that limits generalizability. Although systematic and redundant, attending attribution may have been flawed: incomplete or erroneous documentation could have led to attribution error, and we cannot rule out the possibility of service handoffs. We used a LOS ≤ 5 days to minimize this possibility, but this limits the applicability of our findings to longer admissions. Unsurprisingly, longer LOS correlated with the increased use of consultation even within our restricted sample, and future work should examine the effects of prolonged LOS. As a retrospective analysis, unmeasured confounders due to our limited adjustment will likely explain some findings, although we took steps to address this in our statistical design. Finally, we could not measure patient outcomes and, therefore, cannot determine the value of more or fewer consultations for specific patients or illnesses. Positive and negative outcomes of increased consultation are described, and understanding the impact of consultation is critical for further study.2,3
CONCLUSION
We found that the use of consultation on general medicine services varies widely between admissions, with large differences between the highest and lowest frequencies of use. This variation can be partially explained by several physician-, patient-, and admission-level characteristics. Our work may help identify patient and attending groups at high risk for under- or overuse of consultation and guide the subsequent development of interventions to improve value in consultation. One additional consultation over the average LOS of 4.6 days adds $420 per admission or $4.8 billion to the 11.5 million annual Medicare admissions.15 Increasing research, guidelines, and education on the judicious use of inpatient consultation will be key in maximizing high-value care and improving patient outcomes.
Acknowledgments
The authors would like to acknowledge the invaluable support and assistance of the University of Chicago Hospitalist Project, the Pritzker School of Medicine Summer Research Program, the University of Chicago Center for Quality, and the University of Chicago Center for Health and the Social Sciences (CHeSS). The authors would additionally like to thank John Cursio, PhD, for his support and guidance in statistical analysis for this project.
Disclaimer
The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. The funders had no role in the design of the study; the collection, analysis, and interpretation of the data; or the decision to approve publication of the finished manuscript. Preliminary results of this analysis were presented at the 2018 Society of Hospital Medicine Annual Meeting in Orlando, Florida. All coauthors have seen and agree with the contents of the manuscript. The submission is not under review by any other publication.
1. Stevens JP, Nyweide D, Maresh S, et al. Variation in inpatient consultation among older adults in the United States. J Gen Intern Med. 2015;30(7):992-999. https://doi.org/10.1007/s11606-015-3216-7.
2. Lahey T, Shah R, Gittzus J, Schwartzman J, Kirkland K. Infectious diseases consultation lowers mortality from Staphylococcus aureus bacteremia. Medicine (Baltimore). 2009;88(5):263-267. https://doi.org/10.1097/MD.0b013e3181b8fccb.
3. Morrison RS, Dietrich J, Ladwig S, et al. Palliative care consultation teams cut hospital costs for Medicaid beneficiaries. Health Aff Proj Hope. 2011;30(3):454-463. https://doi.org/10.1377/hlthaff.2010.0929.
4. Stevens JP, Nyweide DJ, Maresh S, Hatfield LA, Howell MD, Landon BE. Comparison of hospital resource use and outcomes among hospitalists, primary care physicians, and other generalists. JAMA Intern Med. 2017;177(12):1781. https://doi.org/10.1001/jamainternmed.2017.5824.
5. Meltzer D. Effects of physician experience on costs and outcomes on an academic general medicine service: Results of a trial of hospitalists. Ann Intern Med. 2002;137(11):866. https://doi.org/10.7326/0003-4819-137-11-200212030-00007.
6. Martin SK, Farnan JM, Flores A, Kurina LM, Meltzer DO, Arora VM. Exploring entrustment: Housestaff autonomy and patient readmission. Am J Med. 2014;127(8):791-797. https://doi.org/10.1016/j.amjmed.2014.04.013.
7. HCUP-US NIS Overview. https://www.hcup-us.ahrq.gov/nisoverview.jsp. Accessed July 7, 2017.
8. Austin SR, Wong Y-N, Uzzo RG, Beck JR, Egleston BL. Why summary comorbidity measures such as the Charlson Comorbidity Index and Elixhauser Score work. Med Care. 2015;53(9):e65-e72. https://doi.org/10.1097/MLR.0b013e318297429c.
9. Elixhauser Comorbidity Software. Elixhauser Comorbidity Software. https://www.hcup-us.ahrq.gov/toolssoftware/comorbidity/comorbidity.jsp#references. Accessed May 13, 2019.
10. Roshetsky LM, Coltri A, Flores A, et al. No time for teaching? Inpatient attending physicians’ workload and teaching before and after the implementation of the 2003 duty hours regulations. Acad Med J Assoc Am Med Coll. 2013;88(9):1293-1298. https://doi.org/10.1097/ACM.0b013e31829eb795.
11. Barnett ML, Boddupalli D, Nundy S, Bates DW. Comparative accuracy of diagnosis by collective intelligence of multiple physicians vs individual physicians. JAMA Netw Open. 2019;2(3):e190096. https://doi.org/10.1001/jamanetworkopen.2019.0096.
12. Aoyama T, Kunisawa S, Fushimi K, Sawa T, Imanaka Y. Comparison of surgical and conservative treatment outcomes for type A aortic dissection in elderly patients. J Cardiothorac Surg. 2018;13(1):129. https://doi.org/10.1186/s13019-018-0814-6.
13. Lindau ST, Schumm LP, Laumann EO, Levinson W, O’Muircheartaigh CA, Waite LJ. A study of sexuality and health among older adults in the United States. N Engl J Med. 2007;357(8):762-774. https://doi.org/10.1056/NEJMoa067423.
14. Yergan J, Flood AB, Diehr P, LoGerfo JP. Relationship between patient source of payment and the intensity of hospital services. Med Care. 1988;26(11):1111-1114. https://doi.org/10.1097/00005650-198811000-00009.
15. Center for Medicare and Medicaid Services. MDCR INPT HOSP 1.; 2008. https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/CMSProgramStatistics/2013/Downloads/MDCR_UTIL/CPS_MDCR_INPT_HOSP_1.pdf. Accessed April 15, 2018.
Inpatient consultation is an extremely common practice with the potential to improve patient outcomes significantly.1-3 However, variability in consultation practices may be risky for patients. In addition to underuse when the benefit is clear, the overuse of consultation may lead to additional testing and therapies, increased length of stay (LOS) and costs, conflicting recommendations, and opportunities for communication breakdown.
Consultation use is often at the discretion of individual providers. While this decision is frequently driven by patient needs, significant variation in consultation practices not fully explained by patient factors exists.1 Prior work has described hospital-level variation1 and that primary care physicians use more consultation than hospitalists.4 However, other factors affecting consultation remain unknown. We sought to explore physician-, patient-, and admission-level factors associated with consultation use on inpatient general medicine services.
METHODS
Study Design
We conducted a retrospective analysis of data from the University of Chicago Hospitalist Project (UCHP). UCHP is a longstanding study of the care of hospitalized patients admitted to the University of Chicago general medicine services, involving both patient data collection and physician experience surveys.5 Data were obtained for enrolled UCHP patients between 2011-2016 from the Center for Research Informatics (CRI). The University of Chicago Institutional Review Board approved this study.
Data Collection
Attendings and patients consented to UCHP participation. Data collection details are described elsewhere.5,6 Data from EpicCare (EpicSystems Corp, Wisconsin) and Centricity Billing (GE Healthcare, Illinois) were obtained via CRI for all encounters of enrolled UCHP patients during the study period (N = 218,591).
Attending Attribution
We determined attending attribution for admissions as follows: the attending author of the first history and physical (H&P) was assigned. If this was unavailable, the attending author of the first progress note (PN) was assigned. For patients admitted by hospitalists on admitting shifts to nonteaching services (ie, service without residents/students), the author of the first PN was assigned if different from H&P. Where available, attribution was corroborated with call schedules.
Sample and Variables
All encounters containing inpatient admissions to the University of Chicago from May 10, 2011 (Electronic Health Record activation date), through December 31, 2016, were considered for inclusion (N = 51,171, Appendix 1). Admissions including only documentation from ancillary services were excluded (eg, encounters for hemodialysis or physical therapy). Admissions were limited to a length of stay (LOS) ≤ 5 days, corresponding to the average US inpatient LOS of 4.6 days,7 to minimize the likelihood of attending handoffs (N = 31,592). If attending attribution was not possible via the above-described methods, the admission was eliminated (N = 3,103; 10.9% of admissions with LOS ≤ 5 days). Finally, the sample was restricted to general medicine service admissions under attendings enrolled in UCHP who completed surveys. After the application of all criteria, 6,153 admissions remained for analysis.
The outcome variable was the number of consultations per admission, determined by counting the unique number of services creating clinical documentation, and subtracting one for the primary team. If the Medical/Surgical intensive care unit (ICU) was a service, then two were subtracted to account for the ICU transfer.
Attending years in practice (ie, years since medical school graduation) and gender were determined from public resources. Practice characteristics were determined from UCHP attending surveys, which address perceptions of workload and satisfaction (Appendix 2).
Patient characteristics (gender, age, Elixhauser Indices) and admission characteristics (LOS, season of admission, payor) were determined from UCHP and CRI data. The Elixhauser Index uses a well-validated system combining the presence/absence of 31 comorbidities to predict mortality and 30-day readmission.8 Elixhauser Indices were calculated using the “Creation of Elixhauser Comorbidity Index Scores 1.0” software.9 For admissions under hospitalist attendings, teaching/nonteaching team was ascertained via internal teaching service calendars.
Analysis
We used descriptive statistics to examine demographic characteristics. The difference between the lowest and highest quartile consultation use was determined via a two-sample t test. Given the multilevel nature of our count data, we used a mixed-effects Poisson model accounting for within-group variation by clustering on attending and patient (3-level random-effects model). The analysis was done using Stata 15 (StataCorp, Texas).
RESULTS
From 2011 to 2016, 14,848 patients and 88 attendings were enrolled in UCHP; 4,772 patients (32%) and 69 attendings (59.4%) had data available and were included. Mean LOS was 3.0 days (SD = 1.3). Table 1 describes the characteristics of attendings, patients, and admissions.
Seventy-six percent of admissions included at least one consultation. Consultation use varied widely, ranging from 0 to 10 per admission (mean = 1.39, median = 1; standard deviation [SD] = 1.17). The number of consultations per admission in the highest quartile of consultation frequency (mean = 3.47, median = 3) was 5.7-fold that of the lowest quartile (mean = 0.613, median = 1; P <.001).
In multivariable regression, physician-, patient-, and admission-level characteristics were associated with the differential use of consultation (Table 2). On teaching services, consultations called by hospitalist vs nonhospitalist generalists did not differ (P =.361). However, hospitalists on nonteaching services called 8.6% more consultations than hospitalists on teaching services (P =.02). Attending agreement with survey item “The interruption of my personal life by work is a problem” was associated with 8.2% fewer consultations per admission (P =.002).
Patients older than 75 years received 19% fewer consultations compared with patients younger than 49 years (P <.001). Compared with Medicare, Medicaid admissions had 12.2% fewer consultations (P <.001), whereas privately insured admissions had 10.7% more (P =.001). The number of consultations per admission decreased every year, with 45.3% fewer consultations in 2015 than 2011 (P <.001). Consultations increased by each 22% per day increase in LOS (P <.001).
DISCUSSION
Our analysis described several physician-, patient-, and admission-level characteristics associated with the use of inpatient consultation. Our results strengthen prior work demonstrating that patient-level factors alone are insufficient to explain consultation variability.1
Hospitalists on nonteaching services called more consultations, which may reflect a higher workload on these services. Busy hospitalists on nonteaching teams may lack time to delve deeply into clinical problems and require more consultations, especially for work with heavy cognitive loads such as diagnosis. “Outsourcing” tasks when workload increases occurs in other cognitive activities such as teaching.10 The association between work interrupting personal life and fewer consultations may also implicate the effects of time. Attendings who are experiencing work encroaching on their personal lives may be those spending more time with patients and consulting less. This finding merits further study, especially with increasing concern about balancing time spent in meaningful patient care activities with risk of physician burnout.
This finding could also indicate that trainee participation modifies consultation use for hospitalists. Teaching service teams with more individual members may allow a greater pool of collective knowledge, decreasing the need for consultation to answer clinical questions.11 Interestingly, there was no difference in consultation use between generalists or subspecialists and hospitalists on teaching services, possibly suggesting a unique effect in hospitalists who vary clinical practice depending on team structure. These differences deserve further investigation, with implications for education and resource utilization.
We were surprised by the finding that consultations decreased each year, despite increasing patient complexity and availability of consultation services. This could be explained by a growing emphasis on shortening LOS in our institution, thus shifting consultative care to outpatient settings. Understanding these effects is critically important with growing evidence that consultation improves patient outcomes because these external pressures could lead to unintended consequences for quality or access to care.
Several findings related to patient factors additionally emerged, including age and insurance status. Although related to medical complexity, these effects persist despite adjustment, which raises the question of whether they contribute to the decision to seek consultation. Older patients received fewer consultations, which could reflect the use of more conservative practice models in the elderly,12 or ageism, which is associated with undertreatment.13 With respect to insurance status, Medicaid patients were associated with fewer consultations. This finding is consistent with previous work showing the decreased intensity of hospital services used for Medicaid patients.14Our study has limitations. Our data were from one large urban academic center that limits generalizability. Although systematic and redundant, attending attribution may have been flawed: incomplete or erroneous documentation could have led to attribution error, and we cannot rule out the possibility of service handoffs. We used a LOS ≤ 5 days to minimize this possibility, but this limits the applicability of our findings to longer admissions. Unsurprisingly, longer LOS correlated with the increased use of consultation even within our restricted sample, and future work should examine the effects of prolonged LOS. As a retrospective analysis, unmeasured confounders due to our limited adjustment will likely explain some findings, although we took steps to address this in our statistical design. Finally, we could not measure patient outcomes and, therefore, cannot determine the value of more or fewer consultations for specific patients or illnesses. Positive and negative outcomes of increased consultation are described, and understanding the impact of consultation is critical for further study.2,3
CONCLUSION
We found that the use of consultation on general medicine services varies widely between admissions, with large differences between the highest and lowest frequencies of use. This variation can be partially explained by several physician-, patient-, and admission-level characteristics. Our work may help identify patient and attending groups at high risk for under- or overuse of consultation and guide the subsequent development of interventions to improve value in consultation. One additional consultation over the average LOS of 4.6 days adds $420 per admission or $4.8 billion to the 11.5 million annual Medicare admissions.15 Increasing research, guidelines, and education on the judicious use of inpatient consultation will be key in maximizing high-value care and improving patient outcomes.
Acknowledgments
The authors would like to acknowledge the invaluable support and assistance of the University of Chicago Hospitalist Project, the Pritzker School of Medicine Summer Research Program, the University of Chicago Center for Quality, and the University of Chicago Center for Health and the Social Sciences (CHeSS). The authors would additionally like to thank John Cursio, PhD, for his support and guidance in statistical analysis for this project.
Disclaimer
The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. The funders had no role in the design of the study; the collection, analysis, and interpretation of the data; or the decision to approve publication of the finished manuscript. Preliminary results of this analysis were presented at the 2018 Society of Hospital Medicine Annual Meeting in Orlando, Florida. All coauthors have seen and agree with the contents of the manuscript. The submission is not under review by any other publication.
Inpatient consultation is an extremely common practice with the potential to improve patient outcomes significantly.1-3 However, variability in consultation practices may be risky for patients. In addition to underuse when the benefit is clear, the overuse of consultation may lead to additional testing and therapies, increased length of stay (LOS) and costs, conflicting recommendations, and opportunities for communication breakdown.
Consultation use is often at the discretion of individual providers. While this decision is frequently driven by patient needs, significant variation in consultation practices not fully explained by patient factors exists.1 Prior work has described hospital-level variation1 and that primary care physicians use more consultation than hospitalists.4 However, other factors affecting consultation remain unknown. We sought to explore physician-, patient-, and admission-level factors associated with consultation use on inpatient general medicine services.
METHODS
Study Design
We conducted a retrospective analysis of data from the University of Chicago Hospitalist Project (UCHP). UCHP is a longstanding study of the care of hospitalized patients admitted to the University of Chicago general medicine services, involving both patient data collection and physician experience surveys.5 Data were obtained for enrolled UCHP patients between 2011-2016 from the Center for Research Informatics (CRI). The University of Chicago Institutional Review Board approved this study.
Data Collection
Attendings and patients consented to UCHP participation. Data collection details are described elsewhere.5,6 Data from EpicCare (EpicSystems Corp, Wisconsin) and Centricity Billing (GE Healthcare, Illinois) were obtained via CRI for all encounters of enrolled UCHP patients during the study period (N = 218,591).
Attending Attribution
We determined attending attribution for admissions as follows: the attending author of the first history and physical (H&P) was assigned. If this was unavailable, the attending author of the first progress note (PN) was assigned. For patients admitted by hospitalists on admitting shifts to nonteaching services (ie, service without residents/students), the author of the first PN was assigned if different from H&P. Where available, attribution was corroborated with call schedules.
Sample and Variables
All encounters containing inpatient admissions to the University of Chicago from May 10, 2011 (Electronic Health Record activation date), through December 31, 2016, were considered for inclusion (N = 51,171, Appendix 1). Admissions including only documentation from ancillary services were excluded (eg, encounters for hemodialysis or physical therapy). Admissions were limited to a length of stay (LOS) ≤ 5 days, corresponding to the average US inpatient LOS of 4.6 days,7 to minimize the likelihood of attending handoffs (N = 31,592). If attending attribution was not possible via the above-described methods, the admission was eliminated (N = 3,103; 10.9% of admissions with LOS ≤ 5 days). Finally, the sample was restricted to general medicine service admissions under attendings enrolled in UCHP who completed surveys. After the application of all criteria, 6,153 admissions remained for analysis.
The outcome variable was the number of consultations per admission, determined by counting the unique number of services creating clinical documentation, and subtracting one for the primary team. If the Medical/Surgical intensive care unit (ICU) was a service, then two were subtracted to account for the ICU transfer.
Attending years in practice (ie, years since medical school graduation) and gender were determined from public resources. Practice characteristics were determined from UCHP attending surveys, which address perceptions of workload and satisfaction (Appendix 2).
Patient characteristics (gender, age, Elixhauser Indices) and admission characteristics (LOS, season of admission, payor) were determined from UCHP and CRI data. The Elixhauser Index uses a well-validated system combining the presence/absence of 31 comorbidities to predict mortality and 30-day readmission.8 Elixhauser Indices were calculated using the “Creation of Elixhauser Comorbidity Index Scores 1.0” software.9 For admissions under hospitalist attendings, teaching/nonteaching team was ascertained via internal teaching service calendars.
Analysis
We used descriptive statistics to examine demographic characteristics. The difference between the lowest and highest quartile consultation use was determined via a two-sample t test. Given the multilevel nature of our count data, we used a mixed-effects Poisson model accounting for within-group variation by clustering on attending and patient (3-level random-effects model). The analysis was done using Stata 15 (StataCorp, Texas).
RESULTS
From 2011 to 2016, 14,848 patients and 88 attendings were enrolled in UCHP; 4,772 patients (32%) and 69 attendings (59.4%) had data available and were included. Mean LOS was 3.0 days (SD = 1.3). Table 1 describes the characteristics of attendings, patients, and admissions.
Seventy-six percent of admissions included at least one consultation. Consultation use varied widely, ranging from 0 to 10 per admission (mean = 1.39, median = 1; standard deviation [SD] = 1.17). The number of consultations per admission in the highest quartile of consultation frequency (mean = 3.47, median = 3) was 5.7-fold that of the lowest quartile (mean = 0.613, median = 1; P <.001).
In multivariable regression, physician-, patient-, and admission-level characteristics were associated with the differential use of consultation (Table 2). On teaching services, consultations called by hospitalist vs nonhospitalist generalists did not differ (P =.361). However, hospitalists on nonteaching services called 8.6% more consultations than hospitalists on teaching services (P =.02). Attending agreement with survey item “The interruption of my personal life by work is a problem” was associated with 8.2% fewer consultations per admission (P =.002).
Patients older than 75 years received 19% fewer consultations compared with patients younger than 49 years (P <.001). Compared with Medicare, Medicaid admissions had 12.2% fewer consultations (P <.001), whereas privately insured admissions had 10.7% more (P =.001). The number of consultations per admission decreased every year, with 45.3% fewer consultations in 2015 than 2011 (P <.001). Consultations increased by each 22% per day increase in LOS (P <.001).
DISCUSSION
Our analysis described several physician-, patient-, and admission-level characteristics associated with the use of inpatient consultation. Our results strengthen prior work demonstrating that patient-level factors alone are insufficient to explain consultation variability.1
Hospitalists on nonteaching services called more consultations, which may reflect a higher workload on these services. Busy hospitalists on nonteaching teams may lack time to delve deeply into clinical problems and require more consultations, especially for work with heavy cognitive loads such as diagnosis. “Outsourcing” tasks when workload increases occurs in other cognitive activities such as teaching.10 The association between work interrupting personal life and fewer consultations may also implicate the effects of time. Attendings who are experiencing work encroaching on their personal lives may be those spending more time with patients and consulting less. This finding merits further study, especially with increasing concern about balancing time spent in meaningful patient care activities with risk of physician burnout.
This finding could also indicate that trainee participation modifies consultation use for hospitalists. Teaching service teams with more individual members may allow a greater pool of collective knowledge, decreasing the need for consultation to answer clinical questions.11 Interestingly, there was no difference in consultation use between generalists or subspecialists and hospitalists on teaching services, possibly suggesting a unique effect in hospitalists who vary clinical practice depending on team structure. These differences deserve further investigation, with implications for education and resource utilization.
We were surprised by the finding that consultations decreased each year, despite increasing patient complexity and availability of consultation services. This could be explained by a growing emphasis on shortening LOS in our institution, thus shifting consultative care to outpatient settings. Understanding these effects is critically important with growing evidence that consultation improves patient outcomes because these external pressures could lead to unintended consequences for quality or access to care.
Several findings related to patient factors additionally emerged, including age and insurance status. Although related to medical complexity, these effects persist despite adjustment, which raises the question of whether they contribute to the decision to seek consultation. Older patients received fewer consultations, which could reflect the use of more conservative practice models in the elderly,12 or ageism, which is associated with undertreatment.13 With respect to insurance status, Medicaid patients were associated with fewer consultations. This finding is consistent with previous work showing the decreased intensity of hospital services used for Medicaid patients.14Our study has limitations. Our data were from one large urban academic center that limits generalizability. Although systematic and redundant, attending attribution may have been flawed: incomplete or erroneous documentation could have led to attribution error, and we cannot rule out the possibility of service handoffs. We used a LOS ≤ 5 days to minimize this possibility, but this limits the applicability of our findings to longer admissions. Unsurprisingly, longer LOS correlated with the increased use of consultation even within our restricted sample, and future work should examine the effects of prolonged LOS. As a retrospective analysis, unmeasured confounders due to our limited adjustment will likely explain some findings, although we took steps to address this in our statistical design. Finally, we could not measure patient outcomes and, therefore, cannot determine the value of more or fewer consultations for specific patients or illnesses. Positive and negative outcomes of increased consultation are described, and understanding the impact of consultation is critical for further study.2,3
CONCLUSION
We found that the use of consultation on general medicine services varies widely between admissions, with large differences between the highest and lowest frequencies of use. This variation can be partially explained by several physician-, patient-, and admission-level characteristics. Our work may help identify patient and attending groups at high risk for under- or overuse of consultation and guide the subsequent development of interventions to improve value in consultation. One additional consultation over the average LOS of 4.6 days adds $420 per admission or $4.8 billion to the 11.5 million annual Medicare admissions.15 Increasing research, guidelines, and education on the judicious use of inpatient consultation will be key in maximizing high-value care and improving patient outcomes.
Acknowledgments
The authors would like to acknowledge the invaluable support and assistance of the University of Chicago Hospitalist Project, the Pritzker School of Medicine Summer Research Program, the University of Chicago Center for Quality, and the University of Chicago Center for Health and the Social Sciences (CHeSS). The authors would additionally like to thank John Cursio, PhD, for his support and guidance in statistical analysis for this project.
Disclaimer
The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. The funders had no role in the design of the study; the collection, analysis, and interpretation of the data; or the decision to approve publication of the finished manuscript. Preliminary results of this analysis were presented at the 2018 Society of Hospital Medicine Annual Meeting in Orlando, Florida. All coauthors have seen and agree with the contents of the manuscript. The submission is not under review by any other publication.
1. Stevens JP, Nyweide D, Maresh S, et al. Variation in inpatient consultation among older adults in the United States. J Gen Intern Med. 2015;30(7):992-999. https://doi.org/10.1007/s11606-015-3216-7.
2. Lahey T, Shah R, Gittzus J, Schwartzman J, Kirkland K. Infectious diseases consultation lowers mortality from Staphylococcus aureus bacteremia. Medicine (Baltimore). 2009;88(5):263-267. https://doi.org/10.1097/MD.0b013e3181b8fccb.
3. Morrison RS, Dietrich J, Ladwig S, et al. Palliative care consultation teams cut hospital costs for Medicaid beneficiaries. Health Aff Proj Hope. 2011;30(3):454-463. https://doi.org/10.1377/hlthaff.2010.0929.
4. Stevens JP, Nyweide DJ, Maresh S, Hatfield LA, Howell MD, Landon BE. Comparison of hospital resource use and outcomes among hospitalists, primary care physicians, and other generalists. JAMA Intern Med. 2017;177(12):1781. https://doi.org/10.1001/jamainternmed.2017.5824.
5. Meltzer D. Effects of physician experience on costs and outcomes on an academic general medicine service: Results of a trial of hospitalists. Ann Intern Med. 2002;137(11):866. https://doi.org/10.7326/0003-4819-137-11-200212030-00007.
6. Martin SK, Farnan JM, Flores A, Kurina LM, Meltzer DO, Arora VM. Exploring entrustment: Housestaff autonomy and patient readmission. Am J Med. 2014;127(8):791-797. https://doi.org/10.1016/j.amjmed.2014.04.013.
7. HCUP-US NIS Overview. https://www.hcup-us.ahrq.gov/nisoverview.jsp. Accessed July 7, 2017.
8. Austin SR, Wong Y-N, Uzzo RG, Beck JR, Egleston BL. Why summary comorbidity measures such as the Charlson Comorbidity Index and Elixhauser Score work. Med Care. 2015;53(9):e65-e72. https://doi.org/10.1097/MLR.0b013e318297429c.
9. Elixhauser Comorbidity Software. Elixhauser Comorbidity Software. https://www.hcup-us.ahrq.gov/toolssoftware/comorbidity/comorbidity.jsp#references. Accessed May 13, 2019.
10. Roshetsky LM, Coltri A, Flores A, et al. No time for teaching? Inpatient attending physicians’ workload and teaching before and after the implementation of the 2003 duty hours regulations. Acad Med J Assoc Am Med Coll. 2013;88(9):1293-1298. https://doi.org/10.1097/ACM.0b013e31829eb795.
11. Barnett ML, Boddupalli D, Nundy S, Bates DW. Comparative accuracy of diagnosis by collective intelligence of multiple physicians vs individual physicians. JAMA Netw Open. 2019;2(3):e190096. https://doi.org/10.1001/jamanetworkopen.2019.0096.
12. Aoyama T, Kunisawa S, Fushimi K, Sawa T, Imanaka Y. Comparison of surgical and conservative treatment outcomes for type A aortic dissection in elderly patients. J Cardiothorac Surg. 2018;13(1):129. https://doi.org/10.1186/s13019-018-0814-6.
13. Lindau ST, Schumm LP, Laumann EO, Levinson W, O’Muircheartaigh CA, Waite LJ. A study of sexuality and health among older adults in the United States. N Engl J Med. 2007;357(8):762-774. https://doi.org/10.1056/NEJMoa067423.
14. Yergan J, Flood AB, Diehr P, LoGerfo JP. Relationship between patient source of payment and the intensity of hospital services. Med Care. 1988;26(11):1111-1114. https://doi.org/10.1097/00005650-198811000-00009.
15. Center for Medicare and Medicaid Services. MDCR INPT HOSP 1.; 2008. https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/CMSProgramStatistics/2013/Downloads/MDCR_UTIL/CPS_MDCR_INPT_HOSP_1.pdf. Accessed April 15, 2018.
1. Stevens JP, Nyweide D, Maresh S, et al. Variation in inpatient consultation among older adults in the United States. J Gen Intern Med. 2015;30(7):992-999. https://doi.org/10.1007/s11606-015-3216-7.
2. Lahey T, Shah R, Gittzus J, Schwartzman J, Kirkland K. Infectious diseases consultation lowers mortality from Staphylococcus aureus bacteremia. Medicine (Baltimore). 2009;88(5):263-267. https://doi.org/10.1097/MD.0b013e3181b8fccb.
3. Morrison RS, Dietrich J, Ladwig S, et al. Palliative care consultation teams cut hospital costs for Medicaid beneficiaries. Health Aff Proj Hope. 2011;30(3):454-463. https://doi.org/10.1377/hlthaff.2010.0929.
4. Stevens JP, Nyweide DJ, Maresh S, Hatfield LA, Howell MD, Landon BE. Comparison of hospital resource use and outcomes among hospitalists, primary care physicians, and other generalists. JAMA Intern Med. 2017;177(12):1781. https://doi.org/10.1001/jamainternmed.2017.5824.
5. Meltzer D. Effects of physician experience on costs and outcomes on an academic general medicine service: Results of a trial of hospitalists. Ann Intern Med. 2002;137(11):866. https://doi.org/10.7326/0003-4819-137-11-200212030-00007.
6. Martin SK, Farnan JM, Flores A, Kurina LM, Meltzer DO, Arora VM. Exploring entrustment: Housestaff autonomy and patient readmission. Am J Med. 2014;127(8):791-797. https://doi.org/10.1016/j.amjmed.2014.04.013.
7. HCUP-US NIS Overview. https://www.hcup-us.ahrq.gov/nisoverview.jsp. Accessed July 7, 2017.
8. Austin SR, Wong Y-N, Uzzo RG, Beck JR, Egleston BL. Why summary comorbidity measures such as the Charlson Comorbidity Index and Elixhauser Score work. Med Care. 2015;53(9):e65-e72. https://doi.org/10.1097/MLR.0b013e318297429c.
9. Elixhauser Comorbidity Software. Elixhauser Comorbidity Software. https://www.hcup-us.ahrq.gov/toolssoftware/comorbidity/comorbidity.jsp#references. Accessed May 13, 2019.
10. Roshetsky LM, Coltri A, Flores A, et al. No time for teaching? Inpatient attending physicians’ workload and teaching before and after the implementation of the 2003 duty hours regulations. Acad Med J Assoc Am Med Coll. 2013;88(9):1293-1298. https://doi.org/10.1097/ACM.0b013e31829eb795.
11. Barnett ML, Boddupalli D, Nundy S, Bates DW. Comparative accuracy of diagnosis by collective intelligence of multiple physicians vs individual physicians. JAMA Netw Open. 2019;2(3):e190096. https://doi.org/10.1001/jamanetworkopen.2019.0096.
12. Aoyama T, Kunisawa S, Fushimi K, Sawa T, Imanaka Y. Comparison of surgical and conservative treatment outcomes for type A aortic dissection in elderly patients. J Cardiothorac Surg. 2018;13(1):129. https://doi.org/10.1186/s13019-018-0814-6.
13. Lindau ST, Schumm LP, Laumann EO, Levinson W, O’Muircheartaigh CA, Waite LJ. A study of sexuality and health among older adults in the United States. N Engl J Med. 2007;357(8):762-774. https://doi.org/10.1056/NEJMoa067423.
14. Yergan J, Flood AB, Diehr P, LoGerfo JP. Relationship between patient source of payment and the intensity of hospital services. Med Care. 1988;26(11):1111-1114. https://doi.org/10.1097/00005650-198811000-00009.
15. Center for Medicare and Medicaid Services. MDCR INPT HOSP 1.; 2008. https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/CMSProgramStatistics/2013/Downloads/MDCR_UTIL/CPS_MDCR_INPT_HOSP_1.pdf. Accessed April 15, 2018.
© 2020 Society of Hospital Medicine
Utility of ICD Codes for Stress Cardiomyopathy in Hospital Administrative Databases: What Do They Signify?
Stress cardiomyopathy (SCM), also known as takotsubo cardiomyopathy, is a nonischemic cardiomyopathy initially identified in Japan in 1990. In 2006, SCM gained an International Classification of Diseases code at the 9th Clinical Modification (ICD-9 CM). Subsequently, several epidemiological studies have used ICD codes to evaluate trends in the diagnosis of SCM;1-8 however, to our knowledge, no previous studies have validated ICD-9 or -10 codes using chart review. We aimed to determine the positive predictive value (PPV) and the limitations of these ICD codes among hospitalized patients.
METHODS
We performed a retrospective cohort study at a single tertiary care center, identifying all adults aged ≥18 years from 2010 to 2016 who were hospitalized with a first known diagnosis of SCM in our Electronic Health Records (EHR) system (Cerner, Stoltenberg Consulting, Inc., Bethel Park, Pennsylvania), which includes both inpatient and outpatient records. We included patients hospitalized with a principal or secondary ICD-9 discharge diagnosis code of 429.83 (for those hospitalized before October 2015) or an ICD-10 discharge diagnosis code of I51.81 (for those hospitalized from October 1, 2015 through December 2016). We excluded hospital readmissions and patients with recurrent SCM, but we could not administratively remove patients who carried a prior diagnosis of SCM made previously at other institutions. One investigator (KW) then reviewed our EHR for a documentation of SCM anywhere in the chart by performing a systematic review of discharge, admission, consultation, daily progress notes, as well as biomarkers, electrocardiograms, echocardiograms, and coronary angiograms. If the first reviewer did not find documentation of SCM anywhere in the EHR, this finding was confirmed by a second chart review by a cardiologist (QP).
Principal and secondary discharge diagnoses were entered into our administrative database by hospital coders using standard coding practices. Because ICD codes also record comorbidities that were present prior to admission, we determined whether each patient had a new diagnosis of SCM during the hospitalization. If not, we considered their ICD code as a preexisting comorbidity and labeled these as chronic cases.
We recorded age, sex, race, ethnicity, and frequency of echocardiogram and cardiac catheterization among all patients. To determine the burden of other comorbidities, we used the Charlson Comorbidity Index and the Elixhauser Comorbidity Index,9,10 but limited our reporting to comorbidities with >5% prevalence.
Our primary aim was to measure the PPV of these ICD codes to determine a diagnosis of SCM. This was done by dividing the total number of cases with a clinical documentation of SCM by the total number of patients with an ICD diagnosis of SCM. As secondary aims, we noted the percentage of new and chronic SCM, the proportion of patients who underwent echocardiography and/or cardiac catheterization and recorded the annual number of total cases of confirmed SCM from 2010 to 2016. Trends were evaluated using the Cochran-Armitage test. To better understand the difference between patients given a principal and secondary code for SCM, we compared these two groups using summary statistics using t tests and chi-squared tests as appropriate, noted the PPV, and determined the 95% confidence intervals of ICD codes in these subgroups. This study was approved by the institutional review board of Baystate Medical Center (#1109756-4). Statistical analysis was done using JMP version12.0.1 (SAS Institute, Cary, North Carolina, 2015).
RESULTS
During 2010-2016, a total of 592 patients with a first known ICD code in our EHR for SCM were hospitalized, comprising 242 (41.0%) with a principal diagnosis code. Upon chart review, we were unable to confirm a clinical diagnosis of SCM among 12 (2.0%) patients. In addition, 38 (6.4%) were chronic cases of SCM, without evidence of active disease at the time of hospitalization. In general, chronic cases typically carried an SCM diagnosis from a hospitalization at a non-Baystate hospital (outside our EHR), or from an outpatient setting. Occasionally, we also found cases where the diagnosis of SCM was mentioned but testing was not pursued, and the patient had no symptoms that were attributed to SCM. Overall use of echocardiogram and cardiac angiography was 91.5% and 66.8%, respectively, and was lower in chronic than in new cases of SCM.
Compared with patients with a secondary diagnosis code, patients with a principal diagnosis of SCM underwent more cardiac angiography and echocardiography (Table 1). When comparing the difference between those with principal and secondary ICD codes, we found that 237 (98%) vs 305 (87%) were new cases of SCM, respectively, and all 12 patients without any clinical diagnosis of SCM had been given a secondary ICD code. Between 2010 and 2016, we noted a significant increase in the number of cases of SCM (Cochrane–Armitage, P < .0001).
The overall PPV (95% CI) of either principal or secondary ICD codes for any form or presentation of SCM was 98.0% (96.4-98.8) with no difference in PPV between the coding systems (ICD-9, 66% of cases, PPV 98% [96.0-99.0] vs ICD-10, PPV 98% [94.9-99.2; P = .98]). Because all patients without a diagnosis of SCM were given secondary ICD codes, this changed the PPV (95% CI) for principal and secondary SCM to 100% (98.4-100.0) and 96.6% (94.1-98.0), respectively. When chronic cases were included as noncases, the PPV (95% CI) to detect a new case of SCM decreased to 97.9% (95.2-99.1) and 87.1% (83.0-90.2) for principal and secondary SCM, respectively (Table 1).
DISCUSSION
In this study, we found a strong relationship between the receipt of an ICD code for SCM and the clinical documentation of a diagnosis of SCM, with an overall PPV of 98%. The PPV was higher when the sample was limited to those assigned a principal ICD code for SCM, but it was lower when considering that some ICD codes represented chronic SCM from prior hospitalizations, despite our attempts to exclude these cases administratively prior to chart review. Furthermore, cardiac catheterization and echocardiography were used inconsistently and were less frequent among secondary compared with a principal diagnosis of SCM. Thus, although a principal ICD diagnosis code for SCM appears to accurately reflect a diagnosis of SCM, a secondary code for SCM appears less reliable. These findings suggest that future epidemiological studies can rely on principal diagnosis codes for use in research studies, but that they should use caution when including patients with secondary codes for SCM.
Our study makes an important contribution to the literature because it quantitates the reliability of ICD codes to identify patients with SCM. This finding is important because multiple studies have used this code to study trends in the incidence of this disease,1-8 and futures studies will almost certainly continue to do so. Our results also showed similar demographics and trends in the incidence of SCM compared with those of prior studies1-3,11 but additionally revealed that these codes also have some important limitations.
A key factor to remember is that neither a clinical diagnosis nor an ICD code at the time of hospital discharge is based upon formal diagnostic criteria for SCM. Importantly, all currently proposed diagnostic criteria require resolution of typical regional wall motion abnormalities before finalizing a research-grade diagnosis of SCM (Table 2).12,13 However, because the median time to recovery of ejection fraction in SCM is between three and four weeks after hospital discharge (with some recovery extending much longer),6 it is almost impossible to make a research-grade diagnosis of SCM after a three- to four-day hospitalization. Moreover, 33% of our patients did not undergo cardiac catheterization, 8.5% did not undergo echocardiography, and it is our experience that testing for pheochromocytoma and myocarditis is rarely done. Thus, we emphasize that ICD codes for SCM assigned at the time of hospital discharge represent a clinical diagnosis of SCM and not research-grade criteria for this disease. This is a significant limitation of prior epidemiologic studies that consider only the short time frame of hospitalization.
A limitation of our study is that we did not attempt to measure sensitivity, specificity, or the negative predictive value of these codes. This is because measurement of these diagnostic features would require sampling some of our hospital’s 53,000 annual hospital admissions to find cases where SCM was present but not recognized. This did not seem practical, particularly because it might also require directly overreading imaging studies. Moreover, we believe that for the purposes of future epidemiology research, the PPV is the most important feature of these codes because a high PPV indicates that when a principal ICD code is present, it almost always represents a new case of SCM. Other limitations include this being a single-center study; the rates of echocardiograms, cardiac angiography, clinical diagnosis, and coding may differ at other institutions.
In conclusion, we found a high PPV of ICD codes for SCM, particularly among patients with a principal discharge diagnosis of SCM. However, we also found that approximately 8% of cases were either wrongly coded or were chronic cases. Moreover, because of the need to document resolution of wall motion abnormalities, essentially no patients met the research-grade diagnostic criteria at the time of hospital discharge. Although this increases our confidence in the results of past studies, it also provides some caution to researchers who may use these codes in the future.
1. Khera R, Light-McGroary K, Zahr F, Horwitz PA, Girotra S. Trends in hospitalization for takotsubo cardiomyopathy in the United States. Am Heart J. 2016;172:53-63. https://doi.org/10.1016/j.ahj.2015.10.022.
2. Murugiah K, Wang Y, Desai NR, et al. Trends in short- and long-term outcomes for takotsubo cardiomyopathy among medicare fee-for-service beneficiaries, 2007 to 2012. JACC Heart Fail. 2016;4(3):197-205. https://doi.org/10.1016/j.jchf.2015.09.013.
3. Brinjikji W, El-Sayed AM, Salka S. In-hospital mortality among patients with takotsubo cardiomyopathy: a study of the National Inpatient Sample 2008 to 2009. Am Heart J. 2012;164(2):215-221. https://doi.org/10.1016/j.ahj.2012.04.010.
4. Smilowitz NR, Hausvater A, Reynolds HR. Hospital readmission following takotsubo syndrome. Eur Heart J Qual Care Clin Outcomes. 2018;5(2):114-120. https://doi.org/10.1093/ehjqcco/qcy045.
5. Vallabhajosyula S, Deshmukh AJ, Kashani K, Prasad A, Sakhuja A. Tako-Tsubo cardiomyopathy in severe sepsis: nationwide trends, predictors, and outcomes. J Am Heart Assoc. 2018;7(18):e009160. https://doi.org/10.1161/JAHA.118.009160.
6. Shaikh N, Sardar M, Jacob A, et al. Possible predictive factors for recovery of left ventricular systolic function in takotsubo cardiomyopathy. Intractable Rare Dis Res. 2018;7(2):100-105. https://doi.org/10.5582/irdr.2018.01042.
7. Shah M, Ram P, Lo KBU, et al. Etiologies, predictors, and economic impact of readmission within 1 month among patients with takotsubo cardiomyopathy. Clin Cardiol. 2018;41(7):916-923. https://doi.org/10.1002/clc.22974.
8. Misumida N, Ogunbayo GO, Kim SM, Abdel-Latif A, Ziada KM, Sorrell VL. Clinical outcome of takotsubo cardiomyopathy diagnosed with or without coronary angiography. Angiology. 2019;70(1):56-61. https://doi.org/10.1177/0003319718782049.
9. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373-383. https://doi.org/10.1016/0021-9681(87)90171-8.
10. Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8-27. https://doi.org/10.1097/00005650-199801000-00004.
11. Templin C, Ghadri JR, Diekmann J, et al. Clinical features and outcomes of takotsubo (stress) cardiomyopathy. N Engl J Med. Sep 3 2015;373(10):929-938. https://doi.org/10.1056/NEJMoa1406761.
12. Medina de Chazal H, Del Buono MG, Keyser-Marcus L, et al. Stress cardiomyopathy diagnosis and treatment: JACC state-of-the-art review. J Am Coll Cardiol. 2018;72(16):1955-1971. https://doi.org/10.1016/j.jacc.2018.07.072.
13. Ghadri JR, Wittstein IS, Prasad A, et al. international expert consensus document on takotsubo syndrome (part I): clinical characteristics, diagnostic criteria, and pathophysiology. Eur Heart J. 2018;39(22):2032-2046. https://doi.org/10.1093/eurheartj/ehy076.
Stress cardiomyopathy (SCM), also known as takotsubo cardiomyopathy, is a nonischemic cardiomyopathy initially identified in Japan in 1990. In 2006, SCM gained an International Classification of Diseases code at the 9th Clinical Modification (ICD-9 CM). Subsequently, several epidemiological studies have used ICD codes to evaluate trends in the diagnosis of SCM;1-8 however, to our knowledge, no previous studies have validated ICD-9 or -10 codes using chart review. We aimed to determine the positive predictive value (PPV) and the limitations of these ICD codes among hospitalized patients.
METHODS
We performed a retrospective cohort study at a single tertiary care center, identifying all adults aged ≥18 years from 2010 to 2016 who were hospitalized with a first known diagnosis of SCM in our Electronic Health Records (EHR) system (Cerner, Stoltenberg Consulting, Inc., Bethel Park, Pennsylvania), which includes both inpatient and outpatient records. We included patients hospitalized with a principal or secondary ICD-9 discharge diagnosis code of 429.83 (for those hospitalized before October 2015) or an ICD-10 discharge diagnosis code of I51.81 (for those hospitalized from October 1, 2015 through December 2016). We excluded hospital readmissions and patients with recurrent SCM, but we could not administratively remove patients who carried a prior diagnosis of SCM made previously at other institutions. One investigator (KW) then reviewed our EHR for a documentation of SCM anywhere in the chart by performing a systematic review of discharge, admission, consultation, daily progress notes, as well as biomarkers, electrocardiograms, echocardiograms, and coronary angiograms. If the first reviewer did not find documentation of SCM anywhere in the EHR, this finding was confirmed by a second chart review by a cardiologist (QP).
Principal and secondary discharge diagnoses were entered into our administrative database by hospital coders using standard coding practices. Because ICD codes also record comorbidities that were present prior to admission, we determined whether each patient had a new diagnosis of SCM during the hospitalization. If not, we considered their ICD code as a preexisting comorbidity and labeled these as chronic cases.
We recorded age, sex, race, ethnicity, and frequency of echocardiogram and cardiac catheterization among all patients. To determine the burden of other comorbidities, we used the Charlson Comorbidity Index and the Elixhauser Comorbidity Index,9,10 but limited our reporting to comorbidities with >5% prevalence.
Our primary aim was to measure the PPV of these ICD codes to determine a diagnosis of SCM. This was done by dividing the total number of cases with a clinical documentation of SCM by the total number of patients with an ICD diagnosis of SCM. As secondary aims, we noted the percentage of new and chronic SCM, the proportion of patients who underwent echocardiography and/or cardiac catheterization and recorded the annual number of total cases of confirmed SCM from 2010 to 2016. Trends were evaluated using the Cochran-Armitage test. To better understand the difference between patients given a principal and secondary code for SCM, we compared these two groups using summary statistics using t tests and chi-squared tests as appropriate, noted the PPV, and determined the 95% confidence intervals of ICD codes in these subgroups. This study was approved by the institutional review board of Baystate Medical Center (#1109756-4). Statistical analysis was done using JMP version12.0.1 (SAS Institute, Cary, North Carolina, 2015).
RESULTS
During 2010-2016, a total of 592 patients with a first known ICD code in our EHR for SCM were hospitalized, comprising 242 (41.0%) with a principal diagnosis code. Upon chart review, we were unable to confirm a clinical diagnosis of SCM among 12 (2.0%) patients. In addition, 38 (6.4%) were chronic cases of SCM, without evidence of active disease at the time of hospitalization. In general, chronic cases typically carried an SCM diagnosis from a hospitalization at a non-Baystate hospital (outside our EHR), or from an outpatient setting. Occasionally, we also found cases where the diagnosis of SCM was mentioned but testing was not pursued, and the patient had no symptoms that were attributed to SCM. Overall use of echocardiogram and cardiac angiography was 91.5% and 66.8%, respectively, and was lower in chronic than in new cases of SCM.
Compared with patients with a secondary diagnosis code, patients with a principal diagnosis of SCM underwent more cardiac angiography and echocardiography (Table 1). When comparing the difference between those with principal and secondary ICD codes, we found that 237 (98%) vs 305 (87%) were new cases of SCM, respectively, and all 12 patients without any clinical diagnosis of SCM had been given a secondary ICD code. Between 2010 and 2016, we noted a significant increase in the number of cases of SCM (Cochrane–Armitage, P < .0001).
The overall PPV (95% CI) of either principal or secondary ICD codes for any form or presentation of SCM was 98.0% (96.4-98.8) with no difference in PPV between the coding systems (ICD-9, 66% of cases, PPV 98% [96.0-99.0] vs ICD-10, PPV 98% [94.9-99.2; P = .98]). Because all patients without a diagnosis of SCM were given secondary ICD codes, this changed the PPV (95% CI) for principal and secondary SCM to 100% (98.4-100.0) and 96.6% (94.1-98.0), respectively. When chronic cases were included as noncases, the PPV (95% CI) to detect a new case of SCM decreased to 97.9% (95.2-99.1) and 87.1% (83.0-90.2) for principal and secondary SCM, respectively (Table 1).
DISCUSSION
In this study, we found a strong relationship between the receipt of an ICD code for SCM and the clinical documentation of a diagnosis of SCM, with an overall PPV of 98%. The PPV was higher when the sample was limited to those assigned a principal ICD code for SCM, but it was lower when considering that some ICD codes represented chronic SCM from prior hospitalizations, despite our attempts to exclude these cases administratively prior to chart review. Furthermore, cardiac catheterization and echocardiography were used inconsistently and were less frequent among secondary compared with a principal diagnosis of SCM. Thus, although a principal ICD diagnosis code for SCM appears to accurately reflect a diagnosis of SCM, a secondary code for SCM appears less reliable. These findings suggest that future epidemiological studies can rely on principal diagnosis codes for use in research studies, but that they should use caution when including patients with secondary codes for SCM.
Our study makes an important contribution to the literature because it quantitates the reliability of ICD codes to identify patients with SCM. This finding is important because multiple studies have used this code to study trends in the incidence of this disease,1-8 and futures studies will almost certainly continue to do so. Our results also showed similar demographics and trends in the incidence of SCM compared with those of prior studies1-3,11 but additionally revealed that these codes also have some important limitations.
A key factor to remember is that neither a clinical diagnosis nor an ICD code at the time of hospital discharge is based upon formal diagnostic criteria for SCM. Importantly, all currently proposed diagnostic criteria require resolution of typical regional wall motion abnormalities before finalizing a research-grade diagnosis of SCM (Table 2).12,13 However, because the median time to recovery of ejection fraction in SCM is between three and four weeks after hospital discharge (with some recovery extending much longer),6 it is almost impossible to make a research-grade diagnosis of SCM after a three- to four-day hospitalization. Moreover, 33% of our patients did not undergo cardiac catheterization, 8.5% did not undergo echocardiography, and it is our experience that testing for pheochromocytoma and myocarditis is rarely done. Thus, we emphasize that ICD codes for SCM assigned at the time of hospital discharge represent a clinical diagnosis of SCM and not research-grade criteria for this disease. This is a significant limitation of prior epidemiologic studies that consider only the short time frame of hospitalization.
A limitation of our study is that we did not attempt to measure sensitivity, specificity, or the negative predictive value of these codes. This is because measurement of these diagnostic features would require sampling some of our hospital’s 53,000 annual hospital admissions to find cases where SCM was present but not recognized. This did not seem practical, particularly because it might also require directly overreading imaging studies. Moreover, we believe that for the purposes of future epidemiology research, the PPV is the most important feature of these codes because a high PPV indicates that when a principal ICD code is present, it almost always represents a new case of SCM. Other limitations include this being a single-center study; the rates of echocardiograms, cardiac angiography, clinical diagnosis, and coding may differ at other institutions.
In conclusion, we found a high PPV of ICD codes for SCM, particularly among patients with a principal discharge diagnosis of SCM. However, we also found that approximately 8% of cases were either wrongly coded or were chronic cases. Moreover, because of the need to document resolution of wall motion abnormalities, essentially no patients met the research-grade diagnostic criteria at the time of hospital discharge. Although this increases our confidence in the results of past studies, it also provides some caution to researchers who may use these codes in the future.
Stress cardiomyopathy (SCM), also known as takotsubo cardiomyopathy, is a nonischemic cardiomyopathy initially identified in Japan in 1990. In 2006, SCM gained an International Classification of Diseases code at the 9th Clinical Modification (ICD-9 CM). Subsequently, several epidemiological studies have used ICD codes to evaluate trends in the diagnosis of SCM;1-8 however, to our knowledge, no previous studies have validated ICD-9 or -10 codes using chart review. We aimed to determine the positive predictive value (PPV) and the limitations of these ICD codes among hospitalized patients.
METHODS
We performed a retrospective cohort study at a single tertiary care center, identifying all adults aged ≥18 years from 2010 to 2016 who were hospitalized with a first known diagnosis of SCM in our Electronic Health Records (EHR) system (Cerner, Stoltenberg Consulting, Inc., Bethel Park, Pennsylvania), which includes both inpatient and outpatient records. We included patients hospitalized with a principal or secondary ICD-9 discharge diagnosis code of 429.83 (for those hospitalized before October 2015) or an ICD-10 discharge diagnosis code of I51.81 (for those hospitalized from October 1, 2015 through December 2016). We excluded hospital readmissions and patients with recurrent SCM, but we could not administratively remove patients who carried a prior diagnosis of SCM made previously at other institutions. One investigator (KW) then reviewed our EHR for a documentation of SCM anywhere in the chart by performing a systematic review of discharge, admission, consultation, daily progress notes, as well as biomarkers, electrocardiograms, echocardiograms, and coronary angiograms. If the first reviewer did not find documentation of SCM anywhere in the EHR, this finding was confirmed by a second chart review by a cardiologist (QP).
Principal and secondary discharge diagnoses were entered into our administrative database by hospital coders using standard coding practices. Because ICD codes also record comorbidities that were present prior to admission, we determined whether each patient had a new diagnosis of SCM during the hospitalization. If not, we considered their ICD code as a preexisting comorbidity and labeled these as chronic cases.
We recorded age, sex, race, ethnicity, and frequency of echocardiogram and cardiac catheterization among all patients. To determine the burden of other comorbidities, we used the Charlson Comorbidity Index and the Elixhauser Comorbidity Index,9,10 but limited our reporting to comorbidities with >5% prevalence.
Our primary aim was to measure the PPV of these ICD codes to determine a diagnosis of SCM. This was done by dividing the total number of cases with a clinical documentation of SCM by the total number of patients with an ICD diagnosis of SCM. As secondary aims, we noted the percentage of new and chronic SCM, the proportion of patients who underwent echocardiography and/or cardiac catheterization and recorded the annual number of total cases of confirmed SCM from 2010 to 2016. Trends were evaluated using the Cochran-Armitage test. To better understand the difference between patients given a principal and secondary code for SCM, we compared these two groups using summary statistics using t tests and chi-squared tests as appropriate, noted the PPV, and determined the 95% confidence intervals of ICD codes in these subgroups. This study was approved by the institutional review board of Baystate Medical Center (#1109756-4). Statistical analysis was done using JMP version12.0.1 (SAS Institute, Cary, North Carolina, 2015).
RESULTS
During 2010-2016, a total of 592 patients with a first known ICD code in our EHR for SCM were hospitalized, comprising 242 (41.0%) with a principal diagnosis code. Upon chart review, we were unable to confirm a clinical diagnosis of SCM among 12 (2.0%) patients. In addition, 38 (6.4%) were chronic cases of SCM, without evidence of active disease at the time of hospitalization. In general, chronic cases typically carried an SCM diagnosis from a hospitalization at a non-Baystate hospital (outside our EHR), or from an outpatient setting. Occasionally, we also found cases where the diagnosis of SCM was mentioned but testing was not pursued, and the patient had no symptoms that were attributed to SCM. Overall use of echocardiogram and cardiac angiography was 91.5% and 66.8%, respectively, and was lower in chronic than in new cases of SCM.
Compared with patients with a secondary diagnosis code, patients with a principal diagnosis of SCM underwent more cardiac angiography and echocardiography (Table 1). When comparing the difference between those with principal and secondary ICD codes, we found that 237 (98%) vs 305 (87%) were new cases of SCM, respectively, and all 12 patients without any clinical diagnosis of SCM had been given a secondary ICD code. Between 2010 and 2016, we noted a significant increase in the number of cases of SCM (Cochrane–Armitage, P < .0001).
The overall PPV (95% CI) of either principal or secondary ICD codes for any form or presentation of SCM was 98.0% (96.4-98.8) with no difference in PPV between the coding systems (ICD-9, 66% of cases, PPV 98% [96.0-99.0] vs ICD-10, PPV 98% [94.9-99.2; P = .98]). Because all patients without a diagnosis of SCM were given secondary ICD codes, this changed the PPV (95% CI) for principal and secondary SCM to 100% (98.4-100.0) and 96.6% (94.1-98.0), respectively. When chronic cases were included as noncases, the PPV (95% CI) to detect a new case of SCM decreased to 97.9% (95.2-99.1) and 87.1% (83.0-90.2) for principal and secondary SCM, respectively (Table 1).
DISCUSSION
In this study, we found a strong relationship between the receipt of an ICD code for SCM and the clinical documentation of a diagnosis of SCM, with an overall PPV of 98%. The PPV was higher when the sample was limited to those assigned a principal ICD code for SCM, but it was lower when considering that some ICD codes represented chronic SCM from prior hospitalizations, despite our attempts to exclude these cases administratively prior to chart review. Furthermore, cardiac catheterization and echocardiography were used inconsistently and were less frequent among secondary compared with a principal diagnosis of SCM. Thus, although a principal ICD diagnosis code for SCM appears to accurately reflect a diagnosis of SCM, a secondary code for SCM appears less reliable. These findings suggest that future epidemiological studies can rely on principal diagnosis codes for use in research studies, but that they should use caution when including patients with secondary codes for SCM.
Our study makes an important contribution to the literature because it quantitates the reliability of ICD codes to identify patients with SCM. This finding is important because multiple studies have used this code to study trends in the incidence of this disease,1-8 and futures studies will almost certainly continue to do so. Our results also showed similar demographics and trends in the incidence of SCM compared with those of prior studies1-3,11 but additionally revealed that these codes also have some important limitations.
A key factor to remember is that neither a clinical diagnosis nor an ICD code at the time of hospital discharge is based upon formal diagnostic criteria for SCM. Importantly, all currently proposed diagnostic criteria require resolution of typical regional wall motion abnormalities before finalizing a research-grade diagnosis of SCM (Table 2).12,13 However, because the median time to recovery of ejection fraction in SCM is between three and four weeks after hospital discharge (with some recovery extending much longer),6 it is almost impossible to make a research-grade diagnosis of SCM after a three- to four-day hospitalization. Moreover, 33% of our patients did not undergo cardiac catheterization, 8.5% did not undergo echocardiography, and it is our experience that testing for pheochromocytoma and myocarditis is rarely done. Thus, we emphasize that ICD codes for SCM assigned at the time of hospital discharge represent a clinical diagnosis of SCM and not research-grade criteria for this disease. This is a significant limitation of prior epidemiologic studies that consider only the short time frame of hospitalization.
A limitation of our study is that we did not attempt to measure sensitivity, specificity, or the negative predictive value of these codes. This is because measurement of these diagnostic features would require sampling some of our hospital’s 53,000 annual hospital admissions to find cases where SCM was present but not recognized. This did not seem practical, particularly because it might also require directly overreading imaging studies. Moreover, we believe that for the purposes of future epidemiology research, the PPV is the most important feature of these codes because a high PPV indicates that when a principal ICD code is present, it almost always represents a new case of SCM. Other limitations include this being a single-center study; the rates of echocardiograms, cardiac angiography, clinical diagnosis, and coding may differ at other institutions.
In conclusion, we found a high PPV of ICD codes for SCM, particularly among patients with a principal discharge diagnosis of SCM. However, we also found that approximately 8% of cases were either wrongly coded or were chronic cases. Moreover, because of the need to document resolution of wall motion abnormalities, essentially no patients met the research-grade diagnostic criteria at the time of hospital discharge. Although this increases our confidence in the results of past studies, it also provides some caution to researchers who may use these codes in the future.
1. Khera R, Light-McGroary K, Zahr F, Horwitz PA, Girotra S. Trends in hospitalization for takotsubo cardiomyopathy in the United States. Am Heart J. 2016;172:53-63. https://doi.org/10.1016/j.ahj.2015.10.022.
2. Murugiah K, Wang Y, Desai NR, et al. Trends in short- and long-term outcomes for takotsubo cardiomyopathy among medicare fee-for-service beneficiaries, 2007 to 2012. JACC Heart Fail. 2016;4(3):197-205. https://doi.org/10.1016/j.jchf.2015.09.013.
3. Brinjikji W, El-Sayed AM, Salka S. In-hospital mortality among patients with takotsubo cardiomyopathy: a study of the National Inpatient Sample 2008 to 2009. Am Heart J. 2012;164(2):215-221. https://doi.org/10.1016/j.ahj.2012.04.010.
4. Smilowitz NR, Hausvater A, Reynolds HR. Hospital readmission following takotsubo syndrome. Eur Heart J Qual Care Clin Outcomes. 2018;5(2):114-120. https://doi.org/10.1093/ehjqcco/qcy045.
5. Vallabhajosyula S, Deshmukh AJ, Kashani K, Prasad A, Sakhuja A. Tako-Tsubo cardiomyopathy in severe sepsis: nationwide trends, predictors, and outcomes. J Am Heart Assoc. 2018;7(18):e009160. https://doi.org/10.1161/JAHA.118.009160.
6. Shaikh N, Sardar M, Jacob A, et al. Possible predictive factors for recovery of left ventricular systolic function in takotsubo cardiomyopathy. Intractable Rare Dis Res. 2018;7(2):100-105. https://doi.org/10.5582/irdr.2018.01042.
7. Shah M, Ram P, Lo KBU, et al. Etiologies, predictors, and economic impact of readmission within 1 month among patients with takotsubo cardiomyopathy. Clin Cardiol. 2018;41(7):916-923. https://doi.org/10.1002/clc.22974.
8. Misumida N, Ogunbayo GO, Kim SM, Abdel-Latif A, Ziada KM, Sorrell VL. Clinical outcome of takotsubo cardiomyopathy diagnosed with or without coronary angiography. Angiology. 2019;70(1):56-61. https://doi.org/10.1177/0003319718782049.
9. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373-383. https://doi.org/10.1016/0021-9681(87)90171-8.
10. Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8-27. https://doi.org/10.1097/00005650-199801000-00004.
11. Templin C, Ghadri JR, Diekmann J, et al. Clinical features and outcomes of takotsubo (stress) cardiomyopathy. N Engl J Med. Sep 3 2015;373(10):929-938. https://doi.org/10.1056/NEJMoa1406761.
12. Medina de Chazal H, Del Buono MG, Keyser-Marcus L, et al. Stress cardiomyopathy diagnosis and treatment: JACC state-of-the-art review. J Am Coll Cardiol. 2018;72(16):1955-1971. https://doi.org/10.1016/j.jacc.2018.07.072.
13. Ghadri JR, Wittstein IS, Prasad A, et al. international expert consensus document on takotsubo syndrome (part I): clinical characteristics, diagnostic criteria, and pathophysiology. Eur Heart J. 2018;39(22):2032-2046. https://doi.org/10.1093/eurheartj/ehy076.
1. Khera R, Light-McGroary K, Zahr F, Horwitz PA, Girotra S. Trends in hospitalization for takotsubo cardiomyopathy in the United States. Am Heart J. 2016;172:53-63. https://doi.org/10.1016/j.ahj.2015.10.022.
2. Murugiah K, Wang Y, Desai NR, et al. Trends in short- and long-term outcomes for takotsubo cardiomyopathy among medicare fee-for-service beneficiaries, 2007 to 2012. JACC Heart Fail. 2016;4(3):197-205. https://doi.org/10.1016/j.jchf.2015.09.013.
3. Brinjikji W, El-Sayed AM, Salka S. In-hospital mortality among patients with takotsubo cardiomyopathy: a study of the National Inpatient Sample 2008 to 2009. Am Heart J. 2012;164(2):215-221. https://doi.org/10.1016/j.ahj.2012.04.010.
4. Smilowitz NR, Hausvater A, Reynolds HR. Hospital readmission following takotsubo syndrome. Eur Heart J Qual Care Clin Outcomes. 2018;5(2):114-120. https://doi.org/10.1093/ehjqcco/qcy045.
5. Vallabhajosyula S, Deshmukh AJ, Kashani K, Prasad A, Sakhuja A. Tako-Tsubo cardiomyopathy in severe sepsis: nationwide trends, predictors, and outcomes. J Am Heart Assoc. 2018;7(18):e009160. https://doi.org/10.1161/JAHA.118.009160.
6. Shaikh N, Sardar M, Jacob A, et al. Possible predictive factors for recovery of left ventricular systolic function in takotsubo cardiomyopathy. Intractable Rare Dis Res. 2018;7(2):100-105. https://doi.org/10.5582/irdr.2018.01042.
7. Shah M, Ram P, Lo KBU, et al. Etiologies, predictors, and economic impact of readmission within 1 month among patients with takotsubo cardiomyopathy. Clin Cardiol. 2018;41(7):916-923. https://doi.org/10.1002/clc.22974.
8. Misumida N, Ogunbayo GO, Kim SM, Abdel-Latif A, Ziada KM, Sorrell VL. Clinical outcome of takotsubo cardiomyopathy diagnosed with or without coronary angiography. Angiology. 2019;70(1):56-61. https://doi.org/10.1177/0003319718782049.
9. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373-383. https://doi.org/10.1016/0021-9681(87)90171-8.
10. Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8-27. https://doi.org/10.1097/00005650-199801000-00004.
11. Templin C, Ghadri JR, Diekmann J, et al. Clinical features and outcomes of takotsubo (stress) cardiomyopathy. N Engl J Med. Sep 3 2015;373(10):929-938. https://doi.org/10.1056/NEJMoa1406761.
12. Medina de Chazal H, Del Buono MG, Keyser-Marcus L, et al. Stress cardiomyopathy diagnosis and treatment: JACC state-of-the-art review. J Am Coll Cardiol. 2018;72(16):1955-1971. https://doi.org/10.1016/j.jacc.2018.07.072.
13. Ghadri JR, Wittstein IS, Prasad A, et al. international expert consensus document on takotsubo syndrome (part I): clinical characteristics, diagnostic criteria, and pathophysiology. Eur Heart J. 2018;39(22):2032-2046. https://doi.org/10.1093/eurheartj/ehy076.
© 2020 Society of Hospital Medicine
Prediction of Disposition Within 48 Hours of Hospital Admission Using Patient Mobility Scores
The loss of mobility during hospitalization is common and is an important reason why more than 40% of hospitalized Medicare patients require placement in a postacute facility.1,2 Discharge planning may be delayed when the medical team focuses on managing acute medical issues without recognizing a patient’s rehabilitation needs until near the time of discharge.3 For patients who require rehabilitation in a postacute facility, delays in discharge can exacerbate hospital-acquired mobility loss and prolong functional recovery.2,4 In addition, even small increases in length of stay have substantial financial impact.5 Increased efficiency in the discharge process has the potential to reduce healthcare costs, facilitate patient recovery, and reduce delays for new admissions awaiting beds.6 For effective discharge planning, a proactive, patient-centered, interdisciplinary approach that considers patient mobility status is needed.3
Systematic measurement of patient mobility that extends beyond evaluations by physical therapists is not common practice, but has the potential to facilitate early discharge planning.7,8 At our hospital, mobility assessment is performed routinely using a reliable and valid interdisciplinary assessment of mobility throughout the patient’s entire hospitalization.9 We recently showed that nurse-recorded mobility status within the first 24 hours of hospitalization was associated with discharge disposition,7 but a prediction tool to help aid clinicians in the discharge planning process would be more useful. In this study, we evaluated the predictive ability of a patient’s mobility score, obtained within 48 hours of hospital admission, to identify the need for postacute care in a diverse patient population.
METHODS
After receiving approval from the Johns Hopkins Institutional Review Board, we conducted analyses on a retrospective cohort of 821 admissions (777 unique patients admitted between January 1, 2017 and August 25, 2017) who were hospitalized for ≥72 hours on two inpatient units (medical and neurological/neurosurgical) at The Johns Hopkins Hospital (JHH). These units were chosen to reduce the potential for both selection and measurement bias. First, these units manage a diverse patient population that is likely to generalize to a general hospital population. Second, the nursing staff on these units has the most accurate and consistent documentation compliance for our predictor variable.
Mobility Measure
The Activity Measure for Post Acute Care Inpatient Mobility Short Form (AM-PAC IMSF) is a measure of functional capacity. This short form is widely used and is nicknamed “6 clicks.” It has questions for six mobility tasks, and each question is scored on a four-point Likert scale.9 Patients do not have to attempt the tasks to be scored. Clinicians can score items using clinical judgement based on observation or discussion with the patient, family, or other clinicians. The interrater reliability is very good (Intraclass Correlation Coefficient = .85-.99)9 and construct validity has been demonstrated for the inpatient hospital population (AM-PAC IMSF correlations with: functional independence measure [FIM] = .65; Katz activities of daily living [ADL] = .80; 2-minute walk = .73; 5-times sit-to-stand = −.69).9 At JHH, the AM-PAC IMSF is scored at admission by nursing staff (>90% documentation compliance on the units in this study); these admission scores were used.
Outcome and Predictors
Discharge location (postacute care facility vs home) was the primary outcome in this study, as recorded in a discrete field in the electronic medical record (EMR). To ensure the validity of this measure, we performed manual chart audits on a sample of patients (n = 300). It was confirmed that the measure entered in the discrete field in the EMR correctly identified the disposition (home vs postacute care facility) in all cases. The primary predictor was the lowest AM-PAC IMSF score obtained within 48 hours after hospital admission, reflecting the patient’s capability to mobilize after hospital admission. Raw scores were converted to scale scores (0-100) for analysis.9 Additional predictors considered included: age, sex, race, and primary diagnosis, all of which were readily available from the EMR at the time of hospital admission. We then grouped the primary diagnosis into the following categories using ICD-10 codes upon admission: Oncologic, Progressive Neurological, Sudden Onset Neurological, and Medical/Other.
Statistical Analysis
We constructed a classification tree, a machine learning approach,10 to predict discharge placement (postacute facility vs home) based on the patients’ hospital admission characteristics and AM-PAC IMSF score. The prediction model was developed using the classification tree approach, as opposed to a logistic regression model. This approach allows for the inclusion of higher-order interactions (ie, interactions of more than two predictors) which would need to be explicitly specified otherwise and a priori we did not have strong evidence from prior studies to guide the model construction. The classification tree was constructed and evaluated by dividing our sample into a 70% training set and a 30% validation set using random sampling within key strata defined by age (<65 vs ≥65 years), gender, and quartile of the AM-PAC IMSF score. The classification tree was developed using the training set. Next, measures of predictive accuracy (ie, the proportion of correctly classified patients with placement in a postacute facility [sensitivity]) and the proportion of correctly classified patients not discharged to postacute care (ie, to home, specificity), were estimated by applying the validation set to the classification tree. The R statistical package rpart11 with procedure rpart was used to construct the classification tree using standard criteria for growing (Gini index10) and pruning (misclassification error estimated by leave-1-out cross-validation12) the tree.
RESULTS
DISCUSSION
Improving the efficiency of hospital discharge planning is of great interest to hospital-based clinicians and administrators. Identifying patients early who are likely to need placement in a postacute facility is an important first step. Using a reliable and valid nursing assessment tool of patient mobility to help with discharge planning is an attractive and feasible approach. The literature on predicting disposition is very limited and has focused primarily on patients with stroke or joint replacement.13,14 Previously, we used the same measure of mobility within 24 hours of admission to show an association with discharge disposition.7 Here, we expanded upon that prior research to include mobility assessment within a 48-hour window from admission in a diverse patient population. Using a machine learning approach, we were able to predict 73% of hospital discharges correctly using only the patient’s mobility score and age. Having tools such as this simple decision tree to identify discharge locations early in a patient’s hospitalization has the potential to increase efficiency in the discharge planning process.
Despite being able to classify the discharge disposition correctly for most patients, our sensitivity for predicting postacute care need was low. There are likely other patient and system factors that could be collected near the time of hospital admission, such as the patient’s prior level of function, the difference between function at baseline and admission, their prior living situation (eg, long term care, home environment), social support, and hospital relationships with postacute care facilities that may help to improve the prediction of postacute care placement.15 We recommend that future research consider these and other potentially important predictors. However, the specificity was high enough that all patients who score positive merit evaluation for possible postacute care. While our patient sample was diverse, it did not focus on some patients who may be more likely to be discharged to a postacute facility, such as the geriatric population. This may be a potential limitation to our study and will require this tool to be tested in more patient groups. A final limitation is the grouping of all potential types of postacute care into one category since important differences exist between the care provided at skilled nursing facilities with or without rehabilitation and inpatient acute rehabilitation. Despite these limitations, this study emphasizes the value of a systematic mobility assessment and provides a simple decision tree to help providers begin early discharge planning by anticipating patient rehabilitation needs.
Acknowledgments
The authors thank Christina Lin, MD and Sophia Andrews, PT, DPT for their assistance with data validation.
1. Greysen SR, Patel MS. Annals for hospitalists inpatient notes-bedrest is toxic—why mobility matters in the hospital. Ann Intern Med. 2018;169(2):HO2-HO3. https://doi.org/10.7326/M18-1427.
2. Greysen SR, Stijacic Cenzer I, Boscardin WJ, Covinsky KE. Functional impairment: an unmeasured marker of Medicare costs for postacute care of older adults. J Am Geriatr Soc. 2017;65(9):1996-2002. https://doi.org/10.1111/jgs.14955.
3. Wong EL, Yam CH, Cheung AW, et al. Barriers to effective discharge planning: a qualitative study investigating the perspectives of frontline healthcare professionals. BMC Health Serv Res. 2011;11(1):242. https://doi.org/10.1186/1472-6963-11-242.
4. Greysen HM, Greysen SR. Mobility assessment in the hospital: what are the “next steps”? J Hosp Med. 2017;12(6):477-478. https://doi.org/10.12788/jhm.2759.
5. Lord RK, Mayhew CR, Korupolu R, et al. ICU early physical rehabilitation programs: financial modeling of cost savings. Crit Care Med. 2013;41(3):717-724. https://doi.org/10.1097/CCM.0b013e3182711de2.
6. McDonagh MS, Smith DH, Goddard M. Measuring appropriate use of acute beds: a systematic review of methods and results. Health Policy. 2000;53(3):157-184. https://doi.org/10.1016/S0168-8510(00)00092-0.
7. Hoyer EH, Young DL, Friedman LA, et al. Routine inpatient mobility assessment and hospital discharge planning. JAMA Intern Med. 2019;179(1):118-120. https://doi.org/10.1001/jamainternmed.2018.5145.
8. Brown CJ, Redden DT, Flood KL, Allman RM. The underrecognized epidemic of low mobility during hospitalization of older adults. J Am Geriatr Soc. 2009;57(9):1660-1665. https://doi.org/10.1111/j.1532-5415.2009.02393.x.
9. Hoyer EH, Young DL, Klein LM, et al. Toward a common language for measuring patient mobility in the hospital: reliability and construct validity of interprofessional mobility measures. Phys Ther. 2018;98(2):133-142. https://doi.org/10.1093/ptj/pzx110.
10. Breiman L, Friedman J, Olshen R, Stone C. Classification and Regression Trees. Belmont, CA: Wadsworth; 1984.
11. Therneau T, Atkinson B. rpart: recursive partitioning and regression trees. R package version. 2018;4:1-13. https://CRAN.R-project.org/package=rpart.
12. Friedman J, Hastie T, Tibshirani R. The Elements of Statistical Learning. New York, NY: Springer; 2001.
13. Stein J, Bettger JP, Sicklick A, Hedeman R, Magdon-Ismail Z, Schwamm LH. Use of a standardized assessment to predict rehabilitation care after acute stroke. Arch Phys Med Rehabil. 2015;96(2):210-217. https://doi.org/10.1016/j.apmr.2014.07.403.
14. Gholson JJ, Pugely AJ, Bedard NA, Duchman KR, Anthony CA, Callaghan JJ. Can we predict discharge status after total joint arthroplasty? A calculator to predict home discharge. J Arthroplasty. 2016;31(12):2705-2709. https://doi.org/10.1016/j.arth.2016.08.010.
15. Zimmermann BM, Koné I, Rost M, Leu A, Wangmo T, Elger BS. Factors associated with post-acute discharge location after hospital stay: a cross-sectional study from a Swiss hospital. BMC Health Serv Res. 2019;19(1):289. https://doi.org/10.1186/s12913-019-4101-6.
The loss of mobility during hospitalization is common and is an important reason why more than 40% of hospitalized Medicare patients require placement in a postacute facility.1,2 Discharge planning may be delayed when the medical team focuses on managing acute medical issues without recognizing a patient’s rehabilitation needs until near the time of discharge.3 For patients who require rehabilitation in a postacute facility, delays in discharge can exacerbate hospital-acquired mobility loss and prolong functional recovery.2,4 In addition, even small increases in length of stay have substantial financial impact.5 Increased efficiency in the discharge process has the potential to reduce healthcare costs, facilitate patient recovery, and reduce delays for new admissions awaiting beds.6 For effective discharge planning, a proactive, patient-centered, interdisciplinary approach that considers patient mobility status is needed.3
Systematic measurement of patient mobility that extends beyond evaluations by physical therapists is not common practice, but has the potential to facilitate early discharge planning.7,8 At our hospital, mobility assessment is performed routinely using a reliable and valid interdisciplinary assessment of mobility throughout the patient’s entire hospitalization.9 We recently showed that nurse-recorded mobility status within the first 24 hours of hospitalization was associated with discharge disposition,7 but a prediction tool to help aid clinicians in the discharge planning process would be more useful. In this study, we evaluated the predictive ability of a patient’s mobility score, obtained within 48 hours of hospital admission, to identify the need for postacute care in a diverse patient population.
METHODS
After receiving approval from the Johns Hopkins Institutional Review Board, we conducted analyses on a retrospective cohort of 821 admissions (777 unique patients admitted between January 1, 2017 and August 25, 2017) who were hospitalized for ≥72 hours on two inpatient units (medical and neurological/neurosurgical) at The Johns Hopkins Hospital (JHH). These units were chosen to reduce the potential for both selection and measurement bias. First, these units manage a diverse patient population that is likely to generalize to a general hospital population. Second, the nursing staff on these units has the most accurate and consistent documentation compliance for our predictor variable.
Mobility Measure
The Activity Measure for Post Acute Care Inpatient Mobility Short Form (AM-PAC IMSF) is a measure of functional capacity. This short form is widely used and is nicknamed “6 clicks.” It has questions for six mobility tasks, and each question is scored on a four-point Likert scale.9 Patients do not have to attempt the tasks to be scored. Clinicians can score items using clinical judgement based on observation or discussion with the patient, family, or other clinicians. The interrater reliability is very good (Intraclass Correlation Coefficient = .85-.99)9 and construct validity has been demonstrated for the inpatient hospital population (AM-PAC IMSF correlations with: functional independence measure [FIM] = .65; Katz activities of daily living [ADL] = .80; 2-minute walk = .73; 5-times sit-to-stand = −.69).9 At JHH, the AM-PAC IMSF is scored at admission by nursing staff (>90% documentation compliance on the units in this study); these admission scores were used.
Outcome and Predictors
Discharge location (postacute care facility vs home) was the primary outcome in this study, as recorded in a discrete field in the electronic medical record (EMR). To ensure the validity of this measure, we performed manual chart audits on a sample of patients (n = 300). It was confirmed that the measure entered in the discrete field in the EMR correctly identified the disposition (home vs postacute care facility) in all cases. The primary predictor was the lowest AM-PAC IMSF score obtained within 48 hours after hospital admission, reflecting the patient’s capability to mobilize after hospital admission. Raw scores were converted to scale scores (0-100) for analysis.9 Additional predictors considered included: age, sex, race, and primary diagnosis, all of which were readily available from the EMR at the time of hospital admission. We then grouped the primary diagnosis into the following categories using ICD-10 codes upon admission: Oncologic, Progressive Neurological, Sudden Onset Neurological, and Medical/Other.
Statistical Analysis
We constructed a classification tree, a machine learning approach,10 to predict discharge placement (postacute facility vs home) based on the patients’ hospital admission characteristics and AM-PAC IMSF score. The prediction model was developed using the classification tree approach, as opposed to a logistic regression model. This approach allows for the inclusion of higher-order interactions (ie, interactions of more than two predictors) which would need to be explicitly specified otherwise and a priori we did not have strong evidence from prior studies to guide the model construction. The classification tree was constructed and evaluated by dividing our sample into a 70% training set and a 30% validation set using random sampling within key strata defined by age (<65 vs ≥65 years), gender, and quartile of the AM-PAC IMSF score. The classification tree was developed using the training set. Next, measures of predictive accuracy (ie, the proportion of correctly classified patients with placement in a postacute facility [sensitivity]) and the proportion of correctly classified patients not discharged to postacute care (ie, to home, specificity), were estimated by applying the validation set to the classification tree. The R statistical package rpart11 with procedure rpart was used to construct the classification tree using standard criteria for growing (Gini index10) and pruning (misclassification error estimated by leave-1-out cross-validation12) the tree.
RESULTS
DISCUSSION
Improving the efficiency of hospital discharge planning is of great interest to hospital-based clinicians and administrators. Identifying patients early who are likely to need placement in a postacute facility is an important first step. Using a reliable and valid nursing assessment tool of patient mobility to help with discharge planning is an attractive and feasible approach. The literature on predicting disposition is very limited and has focused primarily on patients with stroke or joint replacement.13,14 Previously, we used the same measure of mobility within 24 hours of admission to show an association with discharge disposition.7 Here, we expanded upon that prior research to include mobility assessment within a 48-hour window from admission in a diverse patient population. Using a machine learning approach, we were able to predict 73% of hospital discharges correctly using only the patient’s mobility score and age. Having tools such as this simple decision tree to identify discharge locations early in a patient’s hospitalization has the potential to increase efficiency in the discharge planning process.
Despite being able to classify the discharge disposition correctly for most patients, our sensitivity for predicting postacute care need was low. There are likely other patient and system factors that could be collected near the time of hospital admission, such as the patient’s prior level of function, the difference between function at baseline and admission, their prior living situation (eg, long term care, home environment), social support, and hospital relationships with postacute care facilities that may help to improve the prediction of postacute care placement.15 We recommend that future research consider these and other potentially important predictors. However, the specificity was high enough that all patients who score positive merit evaluation for possible postacute care. While our patient sample was diverse, it did not focus on some patients who may be more likely to be discharged to a postacute facility, such as the geriatric population. This may be a potential limitation to our study and will require this tool to be tested in more patient groups. A final limitation is the grouping of all potential types of postacute care into one category since important differences exist between the care provided at skilled nursing facilities with or without rehabilitation and inpatient acute rehabilitation. Despite these limitations, this study emphasizes the value of a systematic mobility assessment and provides a simple decision tree to help providers begin early discharge planning by anticipating patient rehabilitation needs.
Acknowledgments
The authors thank Christina Lin, MD and Sophia Andrews, PT, DPT for their assistance with data validation.
The loss of mobility during hospitalization is common and is an important reason why more than 40% of hospitalized Medicare patients require placement in a postacute facility.1,2 Discharge planning may be delayed when the medical team focuses on managing acute medical issues without recognizing a patient’s rehabilitation needs until near the time of discharge.3 For patients who require rehabilitation in a postacute facility, delays in discharge can exacerbate hospital-acquired mobility loss and prolong functional recovery.2,4 In addition, even small increases in length of stay have substantial financial impact.5 Increased efficiency in the discharge process has the potential to reduce healthcare costs, facilitate patient recovery, and reduce delays for new admissions awaiting beds.6 For effective discharge planning, a proactive, patient-centered, interdisciplinary approach that considers patient mobility status is needed.3
Systematic measurement of patient mobility that extends beyond evaluations by physical therapists is not common practice, but has the potential to facilitate early discharge planning.7,8 At our hospital, mobility assessment is performed routinely using a reliable and valid interdisciplinary assessment of mobility throughout the patient’s entire hospitalization.9 We recently showed that nurse-recorded mobility status within the first 24 hours of hospitalization was associated with discharge disposition,7 but a prediction tool to help aid clinicians in the discharge planning process would be more useful. In this study, we evaluated the predictive ability of a patient’s mobility score, obtained within 48 hours of hospital admission, to identify the need for postacute care in a diverse patient population.
METHODS
After receiving approval from the Johns Hopkins Institutional Review Board, we conducted analyses on a retrospective cohort of 821 admissions (777 unique patients admitted between January 1, 2017 and August 25, 2017) who were hospitalized for ≥72 hours on two inpatient units (medical and neurological/neurosurgical) at The Johns Hopkins Hospital (JHH). These units were chosen to reduce the potential for both selection and measurement bias. First, these units manage a diverse patient population that is likely to generalize to a general hospital population. Second, the nursing staff on these units has the most accurate and consistent documentation compliance for our predictor variable.
Mobility Measure
The Activity Measure for Post Acute Care Inpatient Mobility Short Form (AM-PAC IMSF) is a measure of functional capacity. This short form is widely used and is nicknamed “6 clicks.” It has questions for six mobility tasks, and each question is scored on a four-point Likert scale.9 Patients do not have to attempt the tasks to be scored. Clinicians can score items using clinical judgement based on observation or discussion with the patient, family, or other clinicians. The interrater reliability is very good (Intraclass Correlation Coefficient = .85-.99)9 and construct validity has been demonstrated for the inpatient hospital population (AM-PAC IMSF correlations with: functional independence measure [FIM] = .65; Katz activities of daily living [ADL] = .80; 2-minute walk = .73; 5-times sit-to-stand = −.69).9 At JHH, the AM-PAC IMSF is scored at admission by nursing staff (>90% documentation compliance on the units in this study); these admission scores were used.
Outcome and Predictors
Discharge location (postacute care facility vs home) was the primary outcome in this study, as recorded in a discrete field in the electronic medical record (EMR). To ensure the validity of this measure, we performed manual chart audits on a sample of patients (n = 300). It was confirmed that the measure entered in the discrete field in the EMR correctly identified the disposition (home vs postacute care facility) in all cases. The primary predictor was the lowest AM-PAC IMSF score obtained within 48 hours after hospital admission, reflecting the patient’s capability to mobilize after hospital admission. Raw scores were converted to scale scores (0-100) for analysis.9 Additional predictors considered included: age, sex, race, and primary diagnosis, all of which were readily available from the EMR at the time of hospital admission. We then grouped the primary diagnosis into the following categories using ICD-10 codes upon admission: Oncologic, Progressive Neurological, Sudden Onset Neurological, and Medical/Other.
Statistical Analysis
We constructed a classification tree, a machine learning approach,10 to predict discharge placement (postacute facility vs home) based on the patients’ hospital admission characteristics and AM-PAC IMSF score. The prediction model was developed using the classification tree approach, as opposed to a logistic regression model. This approach allows for the inclusion of higher-order interactions (ie, interactions of more than two predictors) which would need to be explicitly specified otherwise and a priori we did not have strong evidence from prior studies to guide the model construction. The classification tree was constructed and evaluated by dividing our sample into a 70% training set and a 30% validation set using random sampling within key strata defined by age (<65 vs ≥65 years), gender, and quartile of the AM-PAC IMSF score. The classification tree was developed using the training set. Next, measures of predictive accuracy (ie, the proportion of correctly classified patients with placement in a postacute facility [sensitivity]) and the proportion of correctly classified patients not discharged to postacute care (ie, to home, specificity), were estimated by applying the validation set to the classification tree. The R statistical package rpart11 with procedure rpart was used to construct the classification tree using standard criteria for growing (Gini index10) and pruning (misclassification error estimated by leave-1-out cross-validation12) the tree.
RESULTS
DISCUSSION
Improving the efficiency of hospital discharge planning is of great interest to hospital-based clinicians and administrators. Identifying patients early who are likely to need placement in a postacute facility is an important first step. Using a reliable and valid nursing assessment tool of patient mobility to help with discharge planning is an attractive and feasible approach. The literature on predicting disposition is very limited and has focused primarily on patients with stroke or joint replacement.13,14 Previously, we used the same measure of mobility within 24 hours of admission to show an association with discharge disposition.7 Here, we expanded upon that prior research to include mobility assessment within a 48-hour window from admission in a diverse patient population. Using a machine learning approach, we were able to predict 73% of hospital discharges correctly using only the patient’s mobility score and age. Having tools such as this simple decision tree to identify discharge locations early in a patient’s hospitalization has the potential to increase efficiency in the discharge planning process.
Despite being able to classify the discharge disposition correctly for most patients, our sensitivity for predicting postacute care need was low. There are likely other patient and system factors that could be collected near the time of hospital admission, such as the patient’s prior level of function, the difference between function at baseline and admission, their prior living situation (eg, long term care, home environment), social support, and hospital relationships with postacute care facilities that may help to improve the prediction of postacute care placement.15 We recommend that future research consider these and other potentially important predictors. However, the specificity was high enough that all patients who score positive merit evaluation for possible postacute care. While our patient sample was diverse, it did not focus on some patients who may be more likely to be discharged to a postacute facility, such as the geriatric population. This may be a potential limitation to our study and will require this tool to be tested in more patient groups. A final limitation is the grouping of all potential types of postacute care into one category since important differences exist between the care provided at skilled nursing facilities with or without rehabilitation and inpatient acute rehabilitation. Despite these limitations, this study emphasizes the value of a systematic mobility assessment and provides a simple decision tree to help providers begin early discharge planning by anticipating patient rehabilitation needs.
Acknowledgments
The authors thank Christina Lin, MD and Sophia Andrews, PT, DPT for their assistance with data validation.
1. Greysen SR, Patel MS. Annals for hospitalists inpatient notes-bedrest is toxic—why mobility matters in the hospital. Ann Intern Med. 2018;169(2):HO2-HO3. https://doi.org/10.7326/M18-1427.
2. Greysen SR, Stijacic Cenzer I, Boscardin WJ, Covinsky KE. Functional impairment: an unmeasured marker of Medicare costs for postacute care of older adults. J Am Geriatr Soc. 2017;65(9):1996-2002. https://doi.org/10.1111/jgs.14955.
3. Wong EL, Yam CH, Cheung AW, et al. Barriers to effective discharge planning: a qualitative study investigating the perspectives of frontline healthcare professionals. BMC Health Serv Res. 2011;11(1):242. https://doi.org/10.1186/1472-6963-11-242.
4. Greysen HM, Greysen SR. Mobility assessment in the hospital: what are the “next steps”? J Hosp Med. 2017;12(6):477-478. https://doi.org/10.12788/jhm.2759.
5. Lord RK, Mayhew CR, Korupolu R, et al. ICU early physical rehabilitation programs: financial modeling of cost savings. Crit Care Med. 2013;41(3):717-724. https://doi.org/10.1097/CCM.0b013e3182711de2.
6. McDonagh MS, Smith DH, Goddard M. Measuring appropriate use of acute beds: a systematic review of methods and results. Health Policy. 2000;53(3):157-184. https://doi.org/10.1016/S0168-8510(00)00092-0.
7. Hoyer EH, Young DL, Friedman LA, et al. Routine inpatient mobility assessment and hospital discharge planning. JAMA Intern Med. 2019;179(1):118-120. https://doi.org/10.1001/jamainternmed.2018.5145.
8. Brown CJ, Redden DT, Flood KL, Allman RM. The underrecognized epidemic of low mobility during hospitalization of older adults. J Am Geriatr Soc. 2009;57(9):1660-1665. https://doi.org/10.1111/j.1532-5415.2009.02393.x.
9. Hoyer EH, Young DL, Klein LM, et al. Toward a common language for measuring patient mobility in the hospital: reliability and construct validity of interprofessional mobility measures. Phys Ther. 2018;98(2):133-142. https://doi.org/10.1093/ptj/pzx110.
10. Breiman L, Friedman J, Olshen R, Stone C. Classification and Regression Trees. Belmont, CA: Wadsworth; 1984.
11. Therneau T, Atkinson B. rpart: recursive partitioning and regression trees. R package version. 2018;4:1-13. https://CRAN.R-project.org/package=rpart.
12. Friedman J, Hastie T, Tibshirani R. The Elements of Statistical Learning. New York, NY: Springer; 2001.
13. Stein J, Bettger JP, Sicklick A, Hedeman R, Magdon-Ismail Z, Schwamm LH. Use of a standardized assessment to predict rehabilitation care after acute stroke. Arch Phys Med Rehabil. 2015;96(2):210-217. https://doi.org/10.1016/j.apmr.2014.07.403.
14. Gholson JJ, Pugely AJ, Bedard NA, Duchman KR, Anthony CA, Callaghan JJ. Can we predict discharge status after total joint arthroplasty? A calculator to predict home discharge. J Arthroplasty. 2016;31(12):2705-2709. https://doi.org/10.1016/j.arth.2016.08.010.
15. Zimmermann BM, Koné I, Rost M, Leu A, Wangmo T, Elger BS. Factors associated with post-acute discharge location after hospital stay: a cross-sectional study from a Swiss hospital. BMC Health Serv Res. 2019;19(1):289. https://doi.org/10.1186/s12913-019-4101-6.
1. Greysen SR, Patel MS. Annals for hospitalists inpatient notes-bedrest is toxic—why mobility matters in the hospital. Ann Intern Med. 2018;169(2):HO2-HO3. https://doi.org/10.7326/M18-1427.
2. Greysen SR, Stijacic Cenzer I, Boscardin WJ, Covinsky KE. Functional impairment: an unmeasured marker of Medicare costs for postacute care of older adults. J Am Geriatr Soc. 2017;65(9):1996-2002. https://doi.org/10.1111/jgs.14955.
3. Wong EL, Yam CH, Cheung AW, et al. Barriers to effective discharge planning: a qualitative study investigating the perspectives of frontline healthcare professionals. BMC Health Serv Res. 2011;11(1):242. https://doi.org/10.1186/1472-6963-11-242.
4. Greysen HM, Greysen SR. Mobility assessment in the hospital: what are the “next steps”? J Hosp Med. 2017;12(6):477-478. https://doi.org/10.12788/jhm.2759.
5. Lord RK, Mayhew CR, Korupolu R, et al. ICU early physical rehabilitation programs: financial modeling of cost savings. Crit Care Med. 2013;41(3):717-724. https://doi.org/10.1097/CCM.0b013e3182711de2.
6. McDonagh MS, Smith DH, Goddard M. Measuring appropriate use of acute beds: a systematic review of methods and results. Health Policy. 2000;53(3):157-184. https://doi.org/10.1016/S0168-8510(00)00092-0.
7. Hoyer EH, Young DL, Friedman LA, et al. Routine inpatient mobility assessment and hospital discharge planning. JAMA Intern Med. 2019;179(1):118-120. https://doi.org/10.1001/jamainternmed.2018.5145.
8. Brown CJ, Redden DT, Flood KL, Allman RM. The underrecognized epidemic of low mobility during hospitalization of older adults. J Am Geriatr Soc. 2009;57(9):1660-1665. https://doi.org/10.1111/j.1532-5415.2009.02393.x.
9. Hoyer EH, Young DL, Klein LM, et al. Toward a common language for measuring patient mobility in the hospital: reliability and construct validity of interprofessional mobility measures. Phys Ther. 2018;98(2):133-142. https://doi.org/10.1093/ptj/pzx110.
10. Breiman L, Friedman J, Olshen R, Stone C. Classification and Regression Trees. Belmont, CA: Wadsworth; 1984.
11. Therneau T, Atkinson B. rpart: recursive partitioning and regression trees. R package version. 2018;4:1-13. https://CRAN.R-project.org/package=rpart.
12. Friedman J, Hastie T, Tibshirani R. The Elements of Statistical Learning. New York, NY: Springer; 2001.
13. Stein J, Bettger JP, Sicklick A, Hedeman R, Magdon-Ismail Z, Schwamm LH. Use of a standardized assessment to predict rehabilitation care after acute stroke. Arch Phys Med Rehabil. 2015;96(2):210-217. https://doi.org/10.1016/j.apmr.2014.07.403.
14. Gholson JJ, Pugely AJ, Bedard NA, Duchman KR, Anthony CA, Callaghan JJ. Can we predict discharge status after total joint arthroplasty? A calculator to predict home discharge. J Arthroplasty. 2016;31(12):2705-2709. https://doi.org/10.1016/j.arth.2016.08.010.
15. Zimmermann BM, Koné I, Rost M, Leu A, Wangmo T, Elger BS. Factors associated with post-acute discharge location after hospital stay: a cross-sectional study from a Swiss hospital. BMC Health Serv Res. 2019;19(1):289. https://doi.org/10.1186/s12913-019-4101-6.
© 2019 Society of Hospital Medicine
Hospitalists as Triagists: Description of the Triagist Role across Academic Medical Centers
Hospital medicine has grown dramatically over the past 20 years.1,2 A recent survey regarding hospitalists’ clinical roles showed an expansion to triaging emergency department (ED) medical admissions and transfers from outside hospitals.3 From the hospitalist perspective, triaging involves the evaluation of patients for potential admission.4 With scrutiny on ED metrics, such as wait times (https://www.medicare.gov/hospitalcompare/search.html), health system administrators have heightened expectations for efficient patient flow, which increasingly falls to hospitalists.5-7
Despite the growth in hospitalists’ triagist activities, there has been little formal assessment of their role. We hypothesized that this role differs from inpatient care in significant ways.6-8 We sought to describe the triagist role in adult academic inpatient medicine settings to understand the responsibilities and skill set required.
METHODS
Ten academic medical center (AMC) sites were recruited from Research Committee session attendees at the 2014 Society of Hospital Medicine national meeting and the 2014 Society of General Internal Medicine southern regional meeting. The AMCs were geographically diverse: three Western, two Midwestern, two Southern, one Northeastern, and two Southeastern. Site representatives were identified and completed a web-based questionnaire about their AMC (see Appendix 1 for the information collected). Clarifications regarding survey responses were performed via conference calls between the authors (STV, ESW) and site representatives.
Hospitalist Survey
In January 2018, surveys were sent to 583 physicians who worked as triagists. Participants received an anonymous 28-item RedCap survey by e-mail and were sent up to five reminder e-mails over six weeks (see Appendix 2 for the questions analyzed in this paper). Respondents were given the option to be entered in a gift card drawing.
Demographic information and individual workflow/practices were obtained. A 5-point Likert scale (strongly disagree – strongly agree) was used to assess hospitalists’ concurrence with current providers (eg, ED, clinic providers) regarding the management and whether patients must meet the utilization management (UM) criteria for admission. Time estimates used 5% increments and were categorized into four frequency categories based on the local modes provided in responses: Seldom (0%-10%), Occasional (15%-35%), Half-the-Time (40%-60%), and Frequently (65%-100%). Free text responses on effective/ineffective triagist qualities were elicited. Responses were included for analysis if at least 70% of questions were completed.
Data Analysis
Quantitative
Descriptive statistics were calculated for each variable. The Kruskal-Wallis test was used to evaluate differences across AMCs in the time spent on in-person evaluation and communication. Weighting, based on the ratio of hospitalists to survey respondents at each AMC, was used to calculate the average institutional percentages across the study sample.
Qualitative
Responses to open-ended questions were analyzed using thematic analysis.9 Three independent reviewers (STV, JC, ESW) read, analyzed, and grouped the responses by codes. Codes were then assessed for overlap and grouped into themes by one reviewer (STV). A table of themes with supporting quotes and the number of mentions was subsequently developed by all three reviewers. Similar themes were combined to create domains. The domains were reviewed by the steering committee members to create a consensus description (Appendix 3).
The University of Texas Health San Antonio’s Institutional Review Board and participating institutions approved the study as exempt.
RESULTS
Site Characteristics
Representatives from 10 AMCs reported data on a range of one to four hospitals for a total of 22 hospitals. The median reported that the number of medical patients admitted in a 24-hour period was 31-40 (range, 11-20 to >50). The median group size of hospitalists was 41-50 (range, 0-10 to >70).
The survey response rate was 40% (n = 235), ranging from 9%-70% between institutions. Self-identified female hospitalists accounted for 52% of respondents. Four percent were 25-29 years old, 66% were 30-39 years old, 24% were 40-49 years old, and 6% were ≥50 years old. The average clinical time spent as a triagist was 16%.
Description of Triagist Activities
The activities identified by the majority of respondents across all sites included transferring patients within the hospital (73%), and assessing/approving patient transfers from outside hospitals and clinics (82%). Internal transfer activities reported by >50% of respondents included allocating patients within the hospital or bed capacity coordination, assessing intensive care unit transfers, assigning ED admissions, and consulting other services. The ED accounted for an average of 55% of calls received. Respondents also reported being involved with the documentation related to these activities.
Similarities and Differences across AMCs
Two AMCs did not have a dedicated triagist; instead, physicians supervised residents and advanced practice providers. Among the eight sites with triagists, triaging was predominantly done by faculty physicians contacted via pagers. At seven of these sites, 100% of hospitalists worked as triagists. The triage service was covered by faculty physicians from 8-24 hours per day.
Bed boards and transfer centers staffed by registered nurses, nurse coordinators, house supervisors, or physicians were common support systems, though this infrastructure was organized differently across institutions. A UM review before admission was performed at three institutions 24 hours/day. The remaining institutions reviewed patients retrospectively.
Twenty-eight percent of hospitalists across all sites “Disagreed” or “Strongly disagreed” that a patient must meet UM criteria for admission. Forty-two percent had “Frequent” different opinions regarding patient management than the consulting provider.
Triagist and current provider communication practices varied widely across AMCs (Figure). There was significant variability in verbal communication (P = .02), with >70% of respondents at two AMCs reporting verbal communication at least half the time, but <30% reporting this frequency at two other AMCs. Respondents reported variable use of electronic communication (ie, notes/orders in the electronic health record) across AMCs (
The practice of evaluating patients in person also varied significantly across AMCs (P < .0001, Figure). Across hospitalists, only 28% see patients in person about “Half-the-Time” or more.
Differences within AMCs
Variability within AMCs was greatest for the rate of verbal communication practices, with a typical interquartile range (IQR) of 20% to 90% among the hospitalists within a given AMC and for the rate of electronic communication with a typical IQR of 0% to 50%. For other survey questions, the IQR was typically 15 to 20 percentage points.
Thematic Analysis
We received 207 and 203 responses (88% and 86%, respectively) to the open-ended questions “What qualities does an effective triagist have?’ and ‘What qualities make a triagist ineffective?” We identified 22 themes for effective and ineffective qualities, which were grouped into seven domains (Table). All themes had at least three mentions by respondents. The three most frequently mentioned themes, communication skills, efficiency, and systems knowledge, had greater than 60 mentions.
DISCUSSION
Our study of the triagist role at 10 AMCs describes critical triagist functions and identifies key findings across and within AMCs. Twenty-eight percent of hospitalists reported admitting patients even when the patient did not meet the admission criteria, consistent with previous research demonstrating the influence of factors other than clinical disease severity on triage decisions.10 However, preventable admissions remain a hospital-level quality metric.11,12 Triagists must often balance each patient’s circumstances with the complexities of the system. Juggling the competing demands of the system while providing patient-centered care can be challenging and may explain why attending physicians are more frequently filling this role.13
Local context/culture is likely to play a role in the variation across sites; however, compensation for the time spent may also be a factor. If triage activities are not reimbursable, this could lead to less documentation and a lower likelihood that patients are evaluated in person.14 This reason may also explain why all hospitalists were required to serve as a triagist at most sites.
Currently, no consensus definition of the triagist role has been developed. Our results demonstrate that this role is heterogeneous and grounded in the local healthcare system practices. We propose the following working definition of the triagist: a physician who assesses patients for admission, actively supporting the transition of the patient from the outpatient to the inpatient setting. A triagist should be equipped with a skill set that includes not only clinical knowledge but also emphasizes systems knowledge, awareness of others’ goals, efficiency, an ability to communicate effectively, and the knowledge of UM. We recommend that medical directors of hospitalist programs focus their attention on locally specific, systems-based skills development when orienting new hospitalists. The financial aspects of cost should be considered and delineated as well.
Our analysis is limited in several respects. Participant AMCs were not randomly chosen, but do represent a broad array of facility types, group size, and geographic regions. The low response rates at some AMCs may result in an inaccurate representation of those sites. Data was not obtained on hospitalists that did not respond to the survey; therefore, nonresponse bias may affect outcomes. This research used self-report rather than direct observation, which could be subject to recall and social desirability bias. Finally, our results may not be generalizable to nonacademic institutions.
CONCLUSION
The hospitalist role as triagist at AMCs emphasizes communication, organizational skills, efficiency, systems-based practice, and UM knowledge. Although we found significant variation across and within AMCs, internal transfer activities were common across programs. Hospitalist programs should focus on systems-based skills development to prepare hospitalists for the role. The skill set necessary for triagist responsibilities also has implications for internal medicine resident education.4 With increasing emphasis on value and system effectiveness in care delivery, further studies of the triagist role should be undertaken.
Acknowledgments
The TRIAGIST Collaborative Group consists of: Maralyssa Bann, MD, Andrew White, MD (University of Washington); Jagriti Chadha, MD (University of Kentucky); Joel Boggan, MD (Duke University); Sherwin Hsu, MD (UCLA); Jeff Liao, MD (Harvard Medical School); Tabatha Matthias, DO (University of Nebraska Medical Center); Tresa McNeal, MD (Scott and White Texas A&M); Roxana Naderi, MD, Khooshbu Shah, MD (University of Colorado); David Schmit, MD (University of Texas Health San Antonio); Manivannan Veerasamy, MD (Michigan State University).
Disclaimer
The views expressed in this article are those of the authors and do not necessarily reflect the po
1. Kisuule F, Howell EE. Hospitalists and their impact on quality, patient safety, and satisfaction. Obstet Gynecol Clin North Am. 2015; 42(3):433-446. https://doi.org/10.1016/j.ogc.2015.05.003.
2. Wachter, RM, Goldman, L. Zero to 50,000-The 20th anniversary of the hospitalist. N Engl J Med. 2016;375(11): 1009-1011. https://doi.org/10.1056/NEJMp1607958.
3. Vasilevskis EE, Knebel RJ, Wachter RM, Auerbach AD. California hospital leaders’ views of hospitalists: meeting needs of the present and future. J Hosp Med. 2009;4:528-534. https://doi.org/10.1002/jhm.529.
4. Wang ES, Velásquez ST, Smith CJ, et al. Triaging inpatient admissions: an opportunity for resident education. J Gen Intern Med. 2019; 34(5):754-757. https://doi.org/10.1007/s11606-019-04882-2.
5. Briones A, Markoff B, Kathuria N, et al. A model of a hospitalist role in the care of admitted patients in the emergency department. J Hosp Med. 2010;5(6):360-364. https://doi.org/10.1002/jhm.636.
6. Howell EE, Bessman ES, Rubin HR. Hospitalists and an innovative emergency department admission process. J Gen Intern Med. 2004;19:266-268. https://doi.org/10.1111/j.1525-1497.2004.30431.x.
7. Howell E, Bessman E, Marshall R, Wright S. Hospitalist bed management effecting throughput from the emergency department to the intensive care unit. J Crit Care. 2010;25:184-189. https://doi.org/10.1016/j.jcrc.2009.08.004.
8. Chadaga SR, Shockley L, Keniston A, et al. Hospitalist-led medicine emergency department team: associations with throughput, timeliness of patient care, and satisfaction. J Hosp Med. 2012;7:562-566. https://doi.org/10.1002/jhm.1957.
9. Braun, V. Clarke, V. Using thematic analysis in psychology. Qualitative Research in Psychology. 2006;77-101. https://doi.org/10.1191/1478088706qp063oa.
10. Lewis Hunter AE, Spatz ES, Bernstein SL, Rosenthal MS. Factors influencing hospital admission of non-critically ill patients presenting to the emergency department: a cross-sectional study. J Gen Intern Med. 2016;31(1):37-44. https://doi.org/10.1007/s11606-015-3438-8.
11. Patel KK, Vakharia N, Pile J, Howell EH, Rothberg MB. Preventable admissions on a general medicine service: prevalence, causes and comparison with AHRQ prevention quality indicators-a cross-sectional analysis. J Gen Intern Med. 2016;31(6):597-601. https://doi.org/10.1007/s11606-016-3615-4.
12. Daniels LM1, Sorita A2, Kashiwagi DT, et al. Characterizing potentially preventable admissions: a mixed methods study of rates, associated factors, outcomes, and physician decision-making. J Gen Intern Med. 2018;33(5):737-744. https://doi.org/10.1007/s11606-017-4285-6.
13. Howard-Anderson J, Lonowski S, Vangala S, Tseng CH, Busuttil A, Afsar-Manesh N. Readmissions in the era of patient engagement. JAMA Intern Med. 2014;174(11):1870-1872. https://doi.org/10.1001/jamainternmed.2014.4782.
14. Hinami K, Whelan CT, Miller JA, Wolosin RJ, Wetterneck TB, Society of Hospital Medicine Career Satisfaction Task Force. Job characteristics, satisfaction, and burnout across hospitalist practice models. J Hosp Med. 2012;7(5):402-410. https://doi.org/10.1002/jhm.1907
Hospital medicine has grown dramatically over the past 20 years.1,2 A recent survey regarding hospitalists’ clinical roles showed an expansion to triaging emergency department (ED) medical admissions and transfers from outside hospitals.3 From the hospitalist perspective, triaging involves the evaluation of patients for potential admission.4 With scrutiny on ED metrics, such as wait times (https://www.medicare.gov/hospitalcompare/search.html), health system administrators have heightened expectations for efficient patient flow, which increasingly falls to hospitalists.5-7
Despite the growth in hospitalists’ triagist activities, there has been little formal assessment of their role. We hypothesized that this role differs from inpatient care in significant ways.6-8 We sought to describe the triagist role in adult academic inpatient medicine settings to understand the responsibilities and skill set required.
METHODS
Ten academic medical center (AMC) sites were recruited from Research Committee session attendees at the 2014 Society of Hospital Medicine national meeting and the 2014 Society of General Internal Medicine southern regional meeting. The AMCs were geographically diverse: three Western, two Midwestern, two Southern, one Northeastern, and two Southeastern. Site representatives were identified and completed a web-based questionnaire about their AMC (see Appendix 1 for the information collected). Clarifications regarding survey responses were performed via conference calls between the authors (STV, ESW) and site representatives.
Hospitalist Survey
In January 2018, surveys were sent to 583 physicians who worked as triagists. Participants received an anonymous 28-item RedCap survey by e-mail and were sent up to five reminder e-mails over six weeks (see Appendix 2 for the questions analyzed in this paper). Respondents were given the option to be entered in a gift card drawing.
Demographic information and individual workflow/practices were obtained. A 5-point Likert scale (strongly disagree – strongly agree) was used to assess hospitalists’ concurrence with current providers (eg, ED, clinic providers) regarding the management and whether patients must meet the utilization management (UM) criteria for admission. Time estimates used 5% increments and were categorized into four frequency categories based on the local modes provided in responses: Seldom (0%-10%), Occasional (15%-35%), Half-the-Time (40%-60%), and Frequently (65%-100%). Free text responses on effective/ineffective triagist qualities were elicited. Responses were included for analysis if at least 70% of questions were completed.
Data Analysis
Quantitative
Descriptive statistics were calculated for each variable. The Kruskal-Wallis test was used to evaluate differences across AMCs in the time spent on in-person evaluation and communication. Weighting, based on the ratio of hospitalists to survey respondents at each AMC, was used to calculate the average institutional percentages across the study sample.
Qualitative
Responses to open-ended questions were analyzed using thematic analysis.9 Three independent reviewers (STV, JC, ESW) read, analyzed, and grouped the responses by codes. Codes were then assessed for overlap and grouped into themes by one reviewer (STV). A table of themes with supporting quotes and the number of mentions was subsequently developed by all three reviewers. Similar themes were combined to create domains. The domains were reviewed by the steering committee members to create a consensus description (Appendix 3).
The University of Texas Health San Antonio’s Institutional Review Board and participating institutions approved the study as exempt.
RESULTS
Site Characteristics
Representatives from 10 AMCs reported data on a range of one to four hospitals for a total of 22 hospitals. The median reported that the number of medical patients admitted in a 24-hour period was 31-40 (range, 11-20 to >50). The median group size of hospitalists was 41-50 (range, 0-10 to >70).
The survey response rate was 40% (n = 235), ranging from 9%-70% between institutions. Self-identified female hospitalists accounted for 52% of respondents. Four percent were 25-29 years old, 66% were 30-39 years old, 24% were 40-49 years old, and 6% were ≥50 years old. The average clinical time spent as a triagist was 16%.
Description of Triagist Activities
The activities identified by the majority of respondents across all sites included transferring patients within the hospital (73%), and assessing/approving patient transfers from outside hospitals and clinics (82%). Internal transfer activities reported by >50% of respondents included allocating patients within the hospital or bed capacity coordination, assessing intensive care unit transfers, assigning ED admissions, and consulting other services. The ED accounted for an average of 55% of calls received. Respondents also reported being involved with the documentation related to these activities.
Similarities and Differences across AMCs
Two AMCs did not have a dedicated triagist; instead, physicians supervised residents and advanced practice providers. Among the eight sites with triagists, triaging was predominantly done by faculty physicians contacted via pagers. At seven of these sites, 100% of hospitalists worked as triagists. The triage service was covered by faculty physicians from 8-24 hours per day.
Bed boards and transfer centers staffed by registered nurses, nurse coordinators, house supervisors, or physicians were common support systems, though this infrastructure was organized differently across institutions. A UM review before admission was performed at three institutions 24 hours/day. The remaining institutions reviewed patients retrospectively.
Twenty-eight percent of hospitalists across all sites “Disagreed” or “Strongly disagreed” that a patient must meet UM criteria for admission. Forty-two percent had “Frequent” different opinions regarding patient management than the consulting provider.
Triagist and current provider communication practices varied widely across AMCs (Figure). There was significant variability in verbal communication (P = .02), with >70% of respondents at two AMCs reporting verbal communication at least half the time, but <30% reporting this frequency at two other AMCs. Respondents reported variable use of electronic communication (ie, notes/orders in the electronic health record) across AMCs (
The practice of evaluating patients in person also varied significantly across AMCs (P < .0001, Figure). Across hospitalists, only 28% see patients in person about “Half-the-Time” or more.
Differences within AMCs
Variability within AMCs was greatest for the rate of verbal communication practices, with a typical interquartile range (IQR) of 20% to 90% among the hospitalists within a given AMC and for the rate of electronic communication with a typical IQR of 0% to 50%. For other survey questions, the IQR was typically 15 to 20 percentage points.
Thematic Analysis
We received 207 and 203 responses (88% and 86%, respectively) to the open-ended questions “What qualities does an effective triagist have?’ and ‘What qualities make a triagist ineffective?” We identified 22 themes for effective and ineffective qualities, which were grouped into seven domains (Table). All themes had at least three mentions by respondents. The three most frequently mentioned themes, communication skills, efficiency, and systems knowledge, had greater than 60 mentions.
DISCUSSION
Our study of the triagist role at 10 AMCs describes critical triagist functions and identifies key findings across and within AMCs. Twenty-eight percent of hospitalists reported admitting patients even when the patient did not meet the admission criteria, consistent with previous research demonstrating the influence of factors other than clinical disease severity on triage decisions.10 However, preventable admissions remain a hospital-level quality metric.11,12 Triagists must often balance each patient’s circumstances with the complexities of the system. Juggling the competing demands of the system while providing patient-centered care can be challenging and may explain why attending physicians are more frequently filling this role.13
Local context/culture is likely to play a role in the variation across sites; however, compensation for the time spent may also be a factor. If triage activities are not reimbursable, this could lead to less documentation and a lower likelihood that patients are evaluated in person.14 This reason may also explain why all hospitalists were required to serve as a triagist at most sites.
Currently, no consensus definition of the triagist role has been developed. Our results demonstrate that this role is heterogeneous and grounded in the local healthcare system practices. We propose the following working definition of the triagist: a physician who assesses patients for admission, actively supporting the transition of the patient from the outpatient to the inpatient setting. A triagist should be equipped with a skill set that includes not only clinical knowledge but also emphasizes systems knowledge, awareness of others’ goals, efficiency, an ability to communicate effectively, and the knowledge of UM. We recommend that medical directors of hospitalist programs focus their attention on locally specific, systems-based skills development when orienting new hospitalists. The financial aspects of cost should be considered and delineated as well.
Our analysis is limited in several respects. Participant AMCs were not randomly chosen, but do represent a broad array of facility types, group size, and geographic regions. The low response rates at some AMCs may result in an inaccurate representation of those sites. Data was not obtained on hospitalists that did not respond to the survey; therefore, nonresponse bias may affect outcomes. This research used self-report rather than direct observation, which could be subject to recall and social desirability bias. Finally, our results may not be generalizable to nonacademic institutions.
CONCLUSION
The hospitalist role as triagist at AMCs emphasizes communication, organizational skills, efficiency, systems-based practice, and UM knowledge. Although we found significant variation across and within AMCs, internal transfer activities were common across programs. Hospitalist programs should focus on systems-based skills development to prepare hospitalists for the role. The skill set necessary for triagist responsibilities also has implications for internal medicine resident education.4 With increasing emphasis on value and system effectiveness in care delivery, further studies of the triagist role should be undertaken.
Acknowledgments
The TRIAGIST Collaborative Group consists of: Maralyssa Bann, MD, Andrew White, MD (University of Washington); Jagriti Chadha, MD (University of Kentucky); Joel Boggan, MD (Duke University); Sherwin Hsu, MD (UCLA); Jeff Liao, MD (Harvard Medical School); Tabatha Matthias, DO (University of Nebraska Medical Center); Tresa McNeal, MD (Scott and White Texas A&M); Roxana Naderi, MD, Khooshbu Shah, MD (University of Colorado); David Schmit, MD (University of Texas Health San Antonio); Manivannan Veerasamy, MD (Michigan State University).
Disclaimer
The views expressed in this article are those of the authors and do not necessarily reflect the po
Hospital medicine has grown dramatically over the past 20 years.1,2 A recent survey regarding hospitalists’ clinical roles showed an expansion to triaging emergency department (ED) medical admissions and transfers from outside hospitals.3 From the hospitalist perspective, triaging involves the evaluation of patients for potential admission.4 With scrutiny on ED metrics, such as wait times (https://www.medicare.gov/hospitalcompare/search.html), health system administrators have heightened expectations for efficient patient flow, which increasingly falls to hospitalists.5-7
Despite the growth in hospitalists’ triagist activities, there has been little formal assessment of their role. We hypothesized that this role differs from inpatient care in significant ways.6-8 We sought to describe the triagist role in adult academic inpatient medicine settings to understand the responsibilities and skill set required.
METHODS
Ten academic medical center (AMC) sites were recruited from Research Committee session attendees at the 2014 Society of Hospital Medicine national meeting and the 2014 Society of General Internal Medicine southern regional meeting. The AMCs were geographically diverse: three Western, two Midwestern, two Southern, one Northeastern, and two Southeastern. Site representatives were identified and completed a web-based questionnaire about their AMC (see Appendix 1 for the information collected). Clarifications regarding survey responses were performed via conference calls between the authors (STV, ESW) and site representatives.
Hospitalist Survey
In January 2018, surveys were sent to 583 physicians who worked as triagists. Participants received an anonymous 28-item RedCap survey by e-mail and were sent up to five reminder e-mails over six weeks (see Appendix 2 for the questions analyzed in this paper). Respondents were given the option to be entered in a gift card drawing.
Demographic information and individual workflow/practices were obtained. A 5-point Likert scale (strongly disagree – strongly agree) was used to assess hospitalists’ concurrence with current providers (eg, ED, clinic providers) regarding the management and whether patients must meet the utilization management (UM) criteria for admission. Time estimates used 5% increments and were categorized into four frequency categories based on the local modes provided in responses: Seldom (0%-10%), Occasional (15%-35%), Half-the-Time (40%-60%), and Frequently (65%-100%). Free text responses on effective/ineffective triagist qualities were elicited. Responses were included for analysis if at least 70% of questions were completed.
Data Analysis
Quantitative
Descriptive statistics were calculated for each variable. The Kruskal-Wallis test was used to evaluate differences across AMCs in the time spent on in-person evaluation and communication. Weighting, based on the ratio of hospitalists to survey respondents at each AMC, was used to calculate the average institutional percentages across the study sample.
Qualitative
Responses to open-ended questions were analyzed using thematic analysis.9 Three independent reviewers (STV, JC, ESW) read, analyzed, and grouped the responses by codes. Codes were then assessed for overlap and grouped into themes by one reviewer (STV). A table of themes with supporting quotes and the number of mentions was subsequently developed by all three reviewers. Similar themes were combined to create domains. The domains were reviewed by the steering committee members to create a consensus description (Appendix 3).
The University of Texas Health San Antonio’s Institutional Review Board and participating institutions approved the study as exempt.
RESULTS
Site Characteristics
Representatives from 10 AMCs reported data on a range of one to four hospitals for a total of 22 hospitals. The median reported that the number of medical patients admitted in a 24-hour period was 31-40 (range, 11-20 to >50). The median group size of hospitalists was 41-50 (range, 0-10 to >70).
The survey response rate was 40% (n = 235), ranging from 9%-70% between institutions. Self-identified female hospitalists accounted for 52% of respondents. Four percent were 25-29 years old, 66% were 30-39 years old, 24% were 40-49 years old, and 6% were ≥50 years old. The average clinical time spent as a triagist was 16%.
Description of Triagist Activities
The activities identified by the majority of respondents across all sites included transferring patients within the hospital (73%), and assessing/approving patient transfers from outside hospitals and clinics (82%). Internal transfer activities reported by >50% of respondents included allocating patients within the hospital or bed capacity coordination, assessing intensive care unit transfers, assigning ED admissions, and consulting other services. The ED accounted for an average of 55% of calls received. Respondents also reported being involved with the documentation related to these activities.
Similarities and Differences across AMCs
Two AMCs did not have a dedicated triagist; instead, physicians supervised residents and advanced practice providers. Among the eight sites with triagists, triaging was predominantly done by faculty physicians contacted via pagers. At seven of these sites, 100% of hospitalists worked as triagists. The triage service was covered by faculty physicians from 8-24 hours per day.
Bed boards and transfer centers staffed by registered nurses, nurse coordinators, house supervisors, or physicians were common support systems, though this infrastructure was organized differently across institutions. A UM review before admission was performed at three institutions 24 hours/day. The remaining institutions reviewed patients retrospectively.
Twenty-eight percent of hospitalists across all sites “Disagreed” or “Strongly disagreed” that a patient must meet UM criteria for admission. Forty-two percent had “Frequent” different opinions regarding patient management than the consulting provider.
Triagist and current provider communication practices varied widely across AMCs (Figure). There was significant variability in verbal communication (P = .02), with >70% of respondents at two AMCs reporting verbal communication at least half the time, but <30% reporting this frequency at two other AMCs. Respondents reported variable use of electronic communication (ie, notes/orders in the electronic health record) across AMCs (
The practice of evaluating patients in person also varied significantly across AMCs (P < .0001, Figure). Across hospitalists, only 28% see patients in person about “Half-the-Time” or more.
Differences within AMCs
Variability within AMCs was greatest for the rate of verbal communication practices, with a typical interquartile range (IQR) of 20% to 90% among the hospitalists within a given AMC and for the rate of electronic communication with a typical IQR of 0% to 50%. For other survey questions, the IQR was typically 15 to 20 percentage points.
Thematic Analysis
We received 207 and 203 responses (88% and 86%, respectively) to the open-ended questions “What qualities does an effective triagist have?’ and ‘What qualities make a triagist ineffective?” We identified 22 themes for effective and ineffective qualities, which were grouped into seven domains (Table). All themes had at least three mentions by respondents. The three most frequently mentioned themes, communication skills, efficiency, and systems knowledge, had greater than 60 mentions.
DISCUSSION
Our study of the triagist role at 10 AMCs describes critical triagist functions and identifies key findings across and within AMCs. Twenty-eight percent of hospitalists reported admitting patients even when the patient did not meet the admission criteria, consistent with previous research demonstrating the influence of factors other than clinical disease severity on triage decisions.10 However, preventable admissions remain a hospital-level quality metric.11,12 Triagists must often balance each patient’s circumstances with the complexities of the system. Juggling the competing demands of the system while providing patient-centered care can be challenging and may explain why attending physicians are more frequently filling this role.13
Local context/culture is likely to play a role in the variation across sites; however, compensation for the time spent may also be a factor. If triage activities are not reimbursable, this could lead to less documentation and a lower likelihood that patients are evaluated in person.14 This reason may also explain why all hospitalists were required to serve as a triagist at most sites.
Currently, no consensus definition of the triagist role has been developed. Our results demonstrate that this role is heterogeneous and grounded in the local healthcare system practices. We propose the following working definition of the triagist: a physician who assesses patients for admission, actively supporting the transition of the patient from the outpatient to the inpatient setting. A triagist should be equipped with a skill set that includes not only clinical knowledge but also emphasizes systems knowledge, awareness of others’ goals, efficiency, an ability to communicate effectively, and the knowledge of UM. We recommend that medical directors of hospitalist programs focus their attention on locally specific, systems-based skills development when orienting new hospitalists. The financial aspects of cost should be considered and delineated as well.
Our analysis is limited in several respects. Participant AMCs were not randomly chosen, but do represent a broad array of facility types, group size, and geographic regions. The low response rates at some AMCs may result in an inaccurate representation of those sites. Data was not obtained on hospitalists that did not respond to the survey; therefore, nonresponse bias may affect outcomes. This research used self-report rather than direct observation, which could be subject to recall and social desirability bias. Finally, our results may not be generalizable to nonacademic institutions.
CONCLUSION
The hospitalist role as triagist at AMCs emphasizes communication, organizational skills, efficiency, systems-based practice, and UM knowledge. Although we found significant variation across and within AMCs, internal transfer activities were common across programs. Hospitalist programs should focus on systems-based skills development to prepare hospitalists for the role. The skill set necessary for triagist responsibilities also has implications for internal medicine resident education.4 With increasing emphasis on value and system effectiveness in care delivery, further studies of the triagist role should be undertaken.
Acknowledgments
The TRIAGIST Collaborative Group consists of: Maralyssa Bann, MD, Andrew White, MD (University of Washington); Jagriti Chadha, MD (University of Kentucky); Joel Boggan, MD (Duke University); Sherwin Hsu, MD (UCLA); Jeff Liao, MD (Harvard Medical School); Tabatha Matthias, DO (University of Nebraska Medical Center); Tresa McNeal, MD (Scott and White Texas A&M); Roxana Naderi, MD, Khooshbu Shah, MD (University of Colorado); David Schmit, MD (University of Texas Health San Antonio); Manivannan Veerasamy, MD (Michigan State University).
Disclaimer
The views expressed in this article are those of the authors and do not necessarily reflect the po
1. Kisuule F, Howell EE. Hospitalists and their impact on quality, patient safety, and satisfaction. Obstet Gynecol Clin North Am. 2015; 42(3):433-446. https://doi.org/10.1016/j.ogc.2015.05.003.
2. Wachter, RM, Goldman, L. Zero to 50,000-The 20th anniversary of the hospitalist. N Engl J Med. 2016;375(11): 1009-1011. https://doi.org/10.1056/NEJMp1607958.
3. Vasilevskis EE, Knebel RJ, Wachter RM, Auerbach AD. California hospital leaders’ views of hospitalists: meeting needs of the present and future. J Hosp Med. 2009;4:528-534. https://doi.org/10.1002/jhm.529.
4. Wang ES, Velásquez ST, Smith CJ, et al. Triaging inpatient admissions: an opportunity for resident education. J Gen Intern Med. 2019; 34(5):754-757. https://doi.org/10.1007/s11606-019-04882-2.
5. Briones A, Markoff B, Kathuria N, et al. A model of a hospitalist role in the care of admitted patients in the emergency department. J Hosp Med. 2010;5(6):360-364. https://doi.org/10.1002/jhm.636.
6. Howell EE, Bessman ES, Rubin HR. Hospitalists and an innovative emergency department admission process. J Gen Intern Med. 2004;19:266-268. https://doi.org/10.1111/j.1525-1497.2004.30431.x.
7. Howell E, Bessman E, Marshall R, Wright S. Hospitalist bed management effecting throughput from the emergency department to the intensive care unit. J Crit Care. 2010;25:184-189. https://doi.org/10.1016/j.jcrc.2009.08.004.
8. Chadaga SR, Shockley L, Keniston A, et al. Hospitalist-led medicine emergency department team: associations with throughput, timeliness of patient care, and satisfaction. J Hosp Med. 2012;7:562-566. https://doi.org/10.1002/jhm.1957.
9. Braun, V. Clarke, V. Using thematic analysis in psychology. Qualitative Research in Psychology. 2006;77-101. https://doi.org/10.1191/1478088706qp063oa.
10. Lewis Hunter AE, Spatz ES, Bernstein SL, Rosenthal MS. Factors influencing hospital admission of non-critically ill patients presenting to the emergency department: a cross-sectional study. J Gen Intern Med. 2016;31(1):37-44. https://doi.org/10.1007/s11606-015-3438-8.
11. Patel KK, Vakharia N, Pile J, Howell EH, Rothberg MB. Preventable admissions on a general medicine service: prevalence, causes and comparison with AHRQ prevention quality indicators-a cross-sectional analysis. J Gen Intern Med. 2016;31(6):597-601. https://doi.org/10.1007/s11606-016-3615-4.
12. Daniels LM1, Sorita A2, Kashiwagi DT, et al. Characterizing potentially preventable admissions: a mixed methods study of rates, associated factors, outcomes, and physician decision-making. J Gen Intern Med. 2018;33(5):737-744. https://doi.org/10.1007/s11606-017-4285-6.
13. Howard-Anderson J, Lonowski S, Vangala S, Tseng CH, Busuttil A, Afsar-Manesh N. Readmissions in the era of patient engagement. JAMA Intern Med. 2014;174(11):1870-1872. https://doi.org/10.1001/jamainternmed.2014.4782.
14. Hinami K, Whelan CT, Miller JA, Wolosin RJ, Wetterneck TB, Society of Hospital Medicine Career Satisfaction Task Force. Job characteristics, satisfaction, and burnout across hospitalist practice models. J Hosp Med. 2012;7(5):402-410. https://doi.org/10.1002/jhm.1907
1. Kisuule F, Howell EE. Hospitalists and their impact on quality, patient safety, and satisfaction. Obstet Gynecol Clin North Am. 2015; 42(3):433-446. https://doi.org/10.1016/j.ogc.2015.05.003.
2. Wachter, RM, Goldman, L. Zero to 50,000-The 20th anniversary of the hospitalist. N Engl J Med. 2016;375(11): 1009-1011. https://doi.org/10.1056/NEJMp1607958.
3. Vasilevskis EE, Knebel RJ, Wachter RM, Auerbach AD. California hospital leaders’ views of hospitalists: meeting needs of the present and future. J Hosp Med. 2009;4:528-534. https://doi.org/10.1002/jhm.529.
4. Wang ES, Velásquez ST, Smith CJ, et al. Triaging inpatient admissions: an opportunity for resident education. J Gen Intern Med. 2019; 34(5):754-757. https://doi.org/10.1007/s11606-019-04882-2.
5. Briones A, Markoff B, Kathuria N, et al. A model of a hospitalist role in the care of admitted patients in the emergency department. J Hosp Med. 2010;5(6):360-364. https://doi.org/10.1002/jhm.636.
6. Howell EE, Bessman ES, Rubin HR. Hospitalists and an innovative emergency department admission process. J Gen Intern Med. 2004;19:266-268. https://doi.org/10.1111/j.1525-1497.2004.30431.x.
7. Howell E, Bessman E, Marshall R, Wright S. Hospitalist bed management effecting throughput from the emergency department to the intensive care unit. J Crit Care. 2010;25:184-189. https://doi.org/10.1016/j.jcrc.2009.08.004.
8. Chadaga SR, Shockley L, Keniston A, et al. Hospitalist-led medicine emergency department team: associations with throughput, timeliness of patient care, and satisfaction. J Hosp Med. 2012;7:562-566. https://doi.org/10.1002/jhm.1957.
9. Braun, V. Clarke, V. Using thematic analysis in psychology. Qualitative Research in Psychology. 2006;77-101. https://doi.org/10.1191/1478088706qp063oa.
10. Lewis Hunter AE, Spatz ES, Bernstein SL, Rosenthal MS. Factors influencing hospital admission of non-critically ill patients presenting to the emergency department: a cross-sectional study. J Gen Intern Med. 2016;31(1):37-44. https://doi.org/10.1007/s11606-015-3438-8.
11. Patel KK, Vakharia N, Pile J, Howell EH, Rothberg MB. Preventable admissions on a general medicine service: prevalence, causes and comparison with AHRQ prevention quality indicators-a cross-sectional analysis. J Gen Intern Med. 2016;31(6):597-601. https://doi.org/10.1007/s11606-016-3615-4.
12. Daniels LM1, Sorita A2, Kashiwagi DT, et al. Characterizing potentially preventable admissions: a mixed methods study of rates, associated factors, outcomes, and physician decision-making. J Gen Intern Med. 2018;33(5):737-744. https://doi.org/10.1007/s11606-017-4285-6.
13. Howard-Anderson J, Lonowski S, Vangala S, Tseng CH, Busuttil A, Afsar-Manesh N. Readmissions in the era of patient engagement. JAMA Intern Med. 2014;174(11):1870-1872. https://doi.org/10.1001/jamainternmed.2014.4782.
14. Hinami K, Whelan CT, Miller JA, Wolosin RJ, Wetterneck TB, Society of Hospital Medicine Career Satisfaction Task Force. Job characteristics, satisfaction, and burnout across hospitalist practice models. J Hosp Med. 2012;7(5):402-410. https://doi.org/10.1002/jhm.1907
© 2019 Society of Hospital Medicine