User login
Prazosin for PTSD: Sorting out the evidence
Mr. H, age 43, presents to your clinic for management of posttraumatic stress disorder (PTSD). At his last appointment 8 weeks ago, he was continued on fluoxetine, 60 mg/d; he had been stable on this medication for 6 months. Today, Mr. H reports an increase in the frequency and severity of nightmares. He states that he wakes at least 3 times every week with “disturbing dreams” about his time in the military and does not feel rested even when he sleeps through the night. His Clinician-Administered PTSD Scale (CAPS) score is 95 on this visit, suggesting extreme PTSD symptomatology. Mr. H asks if anything can be done to reduce the frequency and intensity of his nightmares.
PTSD is the development of characteristic symptoms following exposure to ≥1 traumatic events. According to DSM-5, PTSD symptoms include the presence of ≥1 intrusion symptoms (recurrent, intrusive memories of the traumatic event; recurrent distressing dreams; dissociative reactions), persistent avoidance of stimuli, negative alterations in cognition and mood, and marked alterations in arousal and reactivity associated with the traumatic event(s).1 The symptoms must be present for >1 month, cause clinically significant distress or impairment in functioning, and not be attributable to the psychologic effects of a substance or medical conditions.1 This article focuses specifically on the hyperarousal symptoms, and the clinical controversies surrounding the use of prazosin for PTSD.
Prazosin for PTSD treatment
Sleep disorders are extremely common in patients with PTSD. Up to 90% of patients report sleep disturbances, and up to 70% report nightmares.2 Prazosin has been widely used in the treatment of PTSD-related sleep disorders and nightmares.The American Psychiatric Association3 and the British Association of Psychopharmacology4 guidelines in-clude prazosin as a first-line recommendation for treatment of PTSD. However, updated 2017 guidelines from the Veterans Affairs/Department of Defense (VA/DoD)5 and data from the 2018 Prazosin and Combat Trauma PTSD (PACT) trial6 contradict these original recommendations. Previously, the 2010 VA/DoD guideline said prazosin had insufficient evidence for monotherapy, but recommended it as adjunctive treatment for sleep and nightmares.7 The updated 2017 VA/DoD guideline recommends “weak against” prazosin use for global symptoms of PTSD, and says there is insufficient evidence for its use in nightmares.5 Below we summarize the findings of studies that contributed to those original recommendations, along with results of the PACT trial.
Raskind et al8,9 conducted 2 studies of prazosin use in combat veterans with PTSD. In both studies, prazosin had significant positive effects on the Clinician-Administered PTSD Scale (CAPS) and Clinical Global Impression of Change (CGIC) scores.8,9 The 2007 study also found significant effects of prazosin on Pittsburgh Sleep Quality Index (PSQI) scores.9
Raskind et al10 conducted another study in 2013 of prazosin use for active-duty soldiers who had combat trauma PTSD with nightmares. Prazosin had positive effects for nightmares, sleep quality, and CAPS scores.10
Germain et al11 reviewed prazosin for treating sleep disturbances in US military veterans. Prazosin was associated with significant improvements in insomnia and daytime PTSD symptom severity as demonstrated by changes in PSQI and CAPS scores.11
Taylor et al12 examined the effects of prazosin on sleep measures and clinical symptoms in civilians with PTSD. Prazosin significantly increased total sleep time, rapid eye movement sleep time, and CGIC scores while significantly decreasing trauma-related nightmares.12
Continue to: Overall, these trials...
Overall, these trials found efficacy for the use of prazosin for patients diagnosed with PTSD; however, the population size in each of these studies was small.
Results of the PACT trial
The PACT trial was a 26-week, multicenter, double-blind, randomized, placebo-controlled trial conducted across 12 VA medical centers.6 During the first 5 weeks, participants were randomized to receive placebo or prazosin, which could be titrated up to 20 mg/d in men and 12 mg/d in women. Participants remained on that dose from the end of Week 5 through Week 10. At that time, other pharmacologic therapies and psychotherapy could be added, discontinued, or adjusted. The mean maintenance total daily dose of prazosin was 14.8 mg.
A total of 413 patients were screened, 304 were randomized (152 per group), and 271 completed the 10-week primary outcome assessment. The population was almost entirely male (96.1% in the prazosin group and 99.3% in the placebo group), and most participants were White (64.5% in the prazosin group and 69.1% in the placebo group), with an average age of approximately 50 years. Primary outcomes included change from baseline to Week 10 in both CAPS item B2 (“recurrent distressing dreams”) and PSQI scores. CGIC score was evaluated at Week 10.
At Week 10, none of the primary outcomes were found to be statistically significant. The mean difference in change from baseline to Week 10 in CAPS item B2 score and PSQI score were 0.2 (P = .38) and 0.1 (P = .80), respectively. There was no significant difference in mean CGIC scores (P = .96). Repeated measures of CAPS item B2, PSQI, and CGIC scores were conducted through Week 26 as secondary outcomes. No significant differences were found. This study concluded that prazosin did not alleviate distressing dreams, improve sleep quality, or improve overall clinical symptoms.6
The PACT trial: Strengths and weaknesses
The PACT trial is the largest placebo-controlled trial for prazosin use in PTSD to date. It failed to show efficacy of prazosin for PTSD-associated nightmares, which contradicts previous studies. Although the mean total daily dose of prazosin was adequate and primary outcomes were measured with appropriate scales, the study failed to enroll the desired number of patients, which increased the possibility of false-negative results. Furthermore, participant recruitment may have led to selection bias because all participants were clinically stable, which could explain the lack of efficacy. However, the average CAPS scores were 80.7 in the prazosin group and 81.9 in the placebo group, which indicates that these patients had significant symptomatology at baseline and before entering the study.
Continue to: A major theme...
A major theme of studies evaluating prazosin treatment for PTSD is a focus on a military population and military-related trauma. Other than Taylor et al12 (N=13), none of these trials included patients who were diagnosed with PTSD due to other traumas, such as sexual trauma, which limits the generalizability of the results. Furthermore, apart from the PACT trial, none of these studies had >100 participants, which further reduces external validity. Current guidelines have not been updated to include the results of the PACT trial, and it is unclear if the results of this trial are strong enough to change clinical practice.
CASE CONTINUED
To ensure patient-centered care, the treating clinicians conduct a risk/benefit discussion with the patient regarding starting prazosin. Mr. H opts to try prazosin, so the clinicians initiate a low dose (1 mg/d) to mitigate adverse effects, and plan to titrate to clinical effect or intolerability. Per evidence from the trials discussed, it is likely Mr. H will need to be titrated to at least 5 to 6 mg/d to see a clinical effect.
Related Resource
North CS, Hong BA, Downs DL. PTSD: A systematic approach to diagnosis and treatment. Current Psychiatry 2018;17(4):35-43.
Drug Brand Names
Fluoxetine • Prozac
Prazosin • Minipress
1. Diagnostic and statistical manual of mental disorders, 5th ed. American Psychiatric Association; 2013.
2. Maher MJ, Rego SA, Asnis, GM. Sleep disturbances in patients with post-traumatic stress disorder: epidemiology, impact and approaches to management. CNS Drugs. 2006;20(7):567-590.
3. Benedek DM, Friedman MJ, Zatzick D, et al. Guideline watch (March 2009): Practice guideline for the treatment of patients with acute stress disorder and posttraumatic stress disorder. APA Practice Guidelines. Published 2010. Accessed March 14, 2021. https://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/acutestressdisorderptsd-watch.pdf
4. Baldwin DS, Anderson IM, Nutt DJ, et al. Evidence-based pharmacological treatment of anxiety disorders, post-traumatic stress disorder and obsessive-compulsive disorder: a revision of the 2005 guidelines from the British Association for Psychopharmacology. J Psychopharmacol. 2014;28(5):403-439. doi: 10.1177/0269881114525674
5. Department of Veterans Affairs, Department of Defense. VA/DoD clinical practice guideline for the management of posttraumatic stress disorder and acute stress disorder. Version 3.0. Published 2017. Accessed February 5, 2021. https://www.healthquality.va.gov/guidelines/MH/ptsd/VADoDPTSDCPGFinal012418.pdf
6. Raskind MA, Peskind ER, Chow B, et al. Trial of prazosin for post-traumatic stress disorder in military veterans. N Engl J Med. 2018;378(6):507-517.
7. Department of Veterans Affairs, Department of Defense. VA/DoD clinical practice guideline: management of post-traumatic stress. Version 2.0. Published 2010. Accessed February 5, 2021. https://www.healthquality.va.gov/guidelines/MH/ptsd/cpg_PTSD-full-201011612.PDF
8. Raskind MA, Peskind ER, Katner ED, et al. Reduction of nightmares and other PTSD symptoms in combat veterans by prazosin: a placebo-controlled study. Am J Psychiatry. 2003;160(2):371-373.
9. Raskind MA, Peskind ER, Hoff DJ, et al. A parallel group placebo-controlled study of prazosin for trauma nightmares and sleep disturbance in combat veterans with post-traumatic stress disorder. Biol Psychiatry. 2007;61(8):928-934.
10. Raskind MA, Peterson K, Williams T, et al. A trial of prazosin for combat trauma PTSD with nightmares in active-duty soldiers returned from Iraq and Afghanistan. Am J Psychiatry. 2013;170(9):1003-1010.
11. Germain A, Richardson R, Moul DE, et al. Placebo-controlled comparison of prazosin and cognitive-behavioral treatments for sleep disturbances in US military veterans. J Psychosom Res. 2012;72(2):89-96.
12. Taylor FB, Martin P, Thompson C, et al. Prazosin effects on objective sleep measures and clinical symptoms in civilian trauma posttraumatic stress disorder: a placebo-controlled study. Biol Psychiatry. 2008;63(6):629-632.
Mr. H, age 43, presents to your clinic for management of posttraumatic stress disorder (PTSD). At his last appointment 8 weeks ago, he was continued on fluoxetine, 60 mg/d; he had been stable on this medication for 6 months. Today, Mr. H reports an increase in the frequency and severity of nightmares. He states that he wakes at least 3 times every week with “disturbing dreams” about his time in the military and does not feel rested even when he sleeps through the night. His Clinician-Administered PTSD Scale (CAPS) score is 95 on this visit, suggesting extreme PTSD symptomatology. Mr. H asks if anything can be done to reduce the frequency and intensity of his nightmares.
PTSD is the development of characteristic symptoms following exposure to ≥1 traumatic events. According to DSM-5, PTSD symptoms include the presence of ≥1 intrusion symptoms (recurrent, intrusive memories of the traumatic event; recurrent distressing dreams; dissociative reactions), persistent avoidance of stimuli, negative alterations in cognition and mood, and marked alterations in arousal and reactivity associated with the traumatic event(s).1 The symptoms must be present for >1 month, cause clinically significant distress or impairment in functioning, and not be attributable to the psychologic effects of a substance or medical conditions.1 This article focuses specifically on the hyperarousal symptoms, and the clinical controversies surrounding the use of prazosin for PTSD.
Prazosin for PTSD treatment
Sleep disorders are extremely common in patients with PTSD. Up to 90% of patients report sleep disturbances, and up to 70% report nightmares.2 Prazosin has been widely used in the treatment of PTSD-related sleep disorders and nightmares.The American Psychiatric Association3 and the British Association of Psychopharmacology4 guidelines in-clude prazosin as a first-line recommendation for treatment of PTSD. However, updated 2017 guidelines from the Veterans Affairs/Department of Defense (VA/DoD)5 and data from the 2018 Prazosin and Combat Trauma PTSD (PACT) trial6 contradict these original recommendations. Previously, the 2010 VA/DoD guideline said prazosin had insufficient evidence for monotherapy, but recommended it as adjunctive treatment for sleep and nightmares.7 The updated 2017 VA/DoD guideline recommends “weak against” prazosin use for global symptoms of PTSD, and says there is insufficient evidence for its use in nightmares.5 Below we summarize the findings of studies that contributed to those original recommendations, along with results of the PACT trial.
Raskind et al8,9 conducted 2 studies of prazosin use in combat veterans with PTSD. In both studies, prazosin had significant positive effects on the Clinician-Administered PTSD Scale (CAPS) and Clinical Global Impression of Change (CGIC) scores.8,9 The 2007 study also found significant effects of prazosin on Pittsburgh Sleep Quality Index (PSQI) scores.9
Raskind et al10 conducted another study in 2013 of prazosin use for active-duty soldiers who had combat trauma PTSD with nightmares. Prazosin had positive effects for nightmares, sleep quality, and CAPS scores.10
Germain et al11 reviewed prazosin for treating sleep disturbances in US military veterans. Prazosin was associated with significant improvements in insomnia and daytime PTSD symptom severity as demonstrated by changes in PSQI and CAPS scores.11
Taylor et al12 examined the effects of prazosin on sleep measures and clinical symptoms in civilians with PTSD. Prazosin significantly increased total sleep time, rapid eye movement sleep time, and CGIC scores while significantly decreasing trauma-related nightmares.12
Continue to: Overall, these trials...
Overall, these trials found efficacy for the use of prazosin for patients diagnosed with PTSD; however, the population size in each of these studies was small.
Results of the PACT trial
The PACT trial was a 26-week, multicenter, double-blind, randomized, placebo-controlled trial conducted across 12 VA medical centers.6 During the first 5 weeks, participants were randomized to receive placebo or prazosin, which could be titrated up to 20 mg/d in men and 12 mg/d in women. Participants remained on that dose from the end of Week 5 through Week 10. At that time, other pharmacologic therapies and psychotherapy could be added, discontinued, or adjusted. The mean maintenance total daily dose of prazosin was 14.8 mg.
A total of 413 patients were screened, 304 were randomized (152 per group), and 271 completed the 10-week primary outcome assessment. The population was almost entirely male (96.1% in the prazosin group and 99.3% in the placebo group), and most participants were White (64.5% in the prazosin group and 69.1% in the placebo group), with an average age of approximately 50 years. Primary outcomes included change from baseline to Week 10 in both CAPS item B2 (“recurrent distressing dreams”) and PSQI scores. CGIC score was evaluated at Week 10.
At Week 10, none of the primary outcomes were found to be statistically significant. The mean difference in change from baseline to Week 10 in CAPS item B2 score and PSQI score were 0.2 (P = .38) and 0.1 (P = .80), respectively. There was no significant difference in mean CGIC scores (P = .96). Repeated measures of CAPS item B2, PSQI, and CGIC scores were conducted through Week 26 as secondary outcomes. No significant differences were found. This study concluded that prazosin did not alleviate distressing dreams, improve sleep quality, or improve overall clinical symptoms.6
The PACT trial: Strengths and weaknesses
The PACT trial is the largest placebo-controlled trial for prazosin use in PTSD to date. It failed to show efficacy of prazosin for PTSD-associated nightmares, which contradicts previous studies. Although the mean total daily dose of prazosin was adequate and primary outcomes were measured with appropriate scales, the study failed to enroll the desired number of patients, which increased the possibility of false-negative results. Furthermore, participant recruitment may have led to selection bias because all participants were clinically stable, which could explain the lack of efficacy. However, the average CAPS scores were 80.7 in the prazosin group and 81.9 in the placebo group, which indicates that these patients had significant symptomatology at baseline and before entering the study.
Continue to: A major theme...
A major theme of studies evaluating prazosin treatment for PTSD is a focus on a military population and military-related trauma. Other than Taylor et al12 (N=13), none of these trials included patients who were diagnosed with PTSD due to other traumas, such as sexual trauma, which limits the generalizability of the results. Furthermore, apart from the PACT trial, none of these studies had >100 participants, which further reduces external validity. Current guidelines have not been updated to include the results of the PACT trial, and it is unclear if the results of this trial are strong enough to change clinical practice.
CASE CONTINUED
To ensure patient-centered care, the treating clinicians conduct a risk/benefit discussion with the patient regarding starting prazosin. Mr. H opts to try prazosin, so the clinicians initiate a low dose (1 mg/d) to mitigate adverse effects, and plan to titrate to clinical effect or intolerability. Per evidence from the trials discussed, it is likely Mr. H will need to be titrated to at least 5 to 6 mg/d to see a clinical effect.
Related Resource
North CS, Hong BA, Downs DL. PTSD: A systematic approach to diagnosis and treatment. Current Psychiatry 2018;17(4):35-43.
Drug Brand Names
Fluoxetine • Prozac
Prazosin • Minipress
Mr. H, age 43, presents to your clinic for management of posttraumatic stress disorder (PTSD). At his last appointment 8 weeks ago, he was continued on fluoxetine, 60 mg/d; he had been stable on this medication for 6 months. Today, Mr. H reports an increase in the frequency and severity of nightmares. He states that he wakes at least 3 times every week with “disturbing dreams” about his time in the military and does not feel rested even when he sleeps through the night. His Clinician-Administered PTSD Scale (CAPS) score is 95 on this visit, suggesting extreme PTSD symptomatology. Mr. H asks if anything can be done to reduce the frequency and intensity of his nightmares.
PTSD is the development of characteristic symptoms following exposure to ≥1 traumatic events. According to DSM-5, PTSD symptoms include the presence of ≥1 intrusion symptoms (recurrent, intrusive memories of the traumatic event; recurrent distressing dreams; dissociative reactions), persistent avoidance of stimuli, negative alterations in cognition and mood, and marked alterations in arousal and reactivity associated with the traumatic event(s).1 The symptoms must be present for >1 month, cause clinically significant distress or impairment in functioning, and not be attributable to the psychologic effects of a substance or medical conditions.1 This article focuses specifically on the hyperarousal symptoms, and the clinical controversies surrounding the use of prazosin for PTSD.
Prazosin for PTSD treatment
Sleep disorders are extremely common in patients with PTSD. Up to 90% of patients report sleep disturbances, and up to 70% report nightmares.2 Prazosin has been widely used in the treatment of PTSD-related sleep disorders and nightmares.The American Psychiatric Association3 and the British Association of Psychopharmacology4 guidelines in-clude prazosin as a first-line recommendation for treatment of PTSD. However, updated 2017 guidelines from the Veterans Affairs/Department of Defense (VA/DoD)5 and data from the 2018 Prazosin and Combat Trauma PTSD (PACT) trial6 contradict these original recommendations. Previously, the 2010 VA/DoD guideline said prazosin had insufficient evidence for monotherapy, but recommended it as adjunctive treatment for sleep and nightmares.7 The updated 2017 VA/DoD guideline recommends “weak against” prazosin use for global symptoms of PTSD, and says there is insufficient evidence for its use in nightmares.5 Below we summarize the findings of studies that contributed to those original recommendations, along with results of the PACT trial.
Raskind et al8,9 conducted 2 studies of prazosin use in combat veterans with PTSD. In both studies, prazosin had significant positive effects on the Clinician-Administered PTSD Scale (CAPS) and Clinical Global Impression of Change (CGIC) scores.8,9 The 2007 study also found significant effects of prazosin on Pittsburgh Sleep Quality Index (PSQI) scores.9
Raskind et al10 conducted another study in 2013 of prazosin use for active-duty soldiers who had combat trauma PTSD with nightmares. Prazosin had positive effects for nightmares, sleep quality, and CAPS scores.10
Germain et al11 reviewed prazosin for treating sleep disturbances in US military veterans. Prazosin was associated with significant improvements in insomnia and daytime PTSD symptom severity as demonstrated by changes in PSQI and CAPS scores.11
Taylor et al12 examined the effects of prazosin on sleep measures and clinical symptoms in civilians with PTSD. Prazosin significantly increased total sleep time, rapid eye movement sleep time, and CGIC scores while significantly decreasing trauma-related nightmares.12
Continue to: Overall, these trials...
Overall, these trials found efficacy for the use of prazosin for patients diagnosed with PTSD; however, the population size in each of these studies was small.
Results of the PACT trial
The PACT trial was a 26-week, multicenter, double-blind, randomized, placebo-controlled trial conducted across 12 VA medical centers.6 During the first 5 weeks, participants were randomized to receive placebo or prazosin, which could be titrated up to 20 mg/d in men and 12 mg/d in women. Participants remained on that dose from the end of Week 5 through Week 10. At that time, other pharmacologic therapies and psychotherapy could be added, discontinued, or adjusted. The mean maintenance total daily dose of prazosin was 14.8 mg.
A total of 413 patients were screened, 304 were randomized (152 per group), and 271 completed the 10-week primary outcome assessment. The population was almost entirely male (96.1% in the prazosin group and 99.3% in the placebo group), and most participants were White (64.5% in the prazosin group and 69.1% in the placebo group), with an average age of approximately 50 years. Primary outcomes included change from baseline to Week 10 in both CAPS item B2 (“recurrent distressing dreams”) and PSQI scores. CGIC score was evaluated at Week 10.
At Week 10, none of the primary outcomes were found to be statistically significant. The mean difference in change from baseline to Week 10 in CAPS item B2 score and PSQI score were 0.2 (P = .38) and 0.1 (P = .80), respectively. There was no significant difference in mean CGIC scores (P = .96). Repeated measures of CAPS item B2, PSQI, and CGIC scores were conducted through Week 26 as secondary outcomes. No significant differences were found. This study concluded that prazosin did not alleviate distressing dreams, improve sleep quality, or improve overall clinical symptoms.6
The PACT trial: Strengths and weaknesses
The PACT trial is the largest placebo-controlled trial for prazosin use in PTSD to date. It failed to show efficacy of prazosin for PTSD-associated nightmares, which contradicts previous studies. Although the mean total daily dose of prazosin was adequate and primary outcomes were measured with appropriate scales, the study failed to enroll the desired number of patients, which increased the possibility of false-negative results. Furthermore, participant recruitment may have led to selection bias because all participants were clinically stable, which could explain the lack of efficacy. However, the average CAPS scores were 80.7 in the prazosin group and 81.9 in the placebo group, which indicates that these patients had significant symptomatology at baseline and before entering the study.
Continue to: A major theme...
A major theme of studies evaluating prazosin treatment for PTSD is a focus on a military population and military-related trauma. Other than Taylor et al12 (N=13), none of these trials included patients who were diagnosed with PTSD due to other traumas, such as sexual trauma, which limits the generalizability of the results. Furthermore, apart from the PACT trial, none of these studies had >100 participants, which further reduces external validity. Current guidelines have not been updated to include the results of the PACT trial, and it is unclear if the results of this trial are strong enough to change clinical practice.
CASE CONTINUED
To ensure patient-centered care, the treating clinicians conduct a risk/benefit discussion with the patient regarding starting prazosin. Mr. H opts to try prazosin, so the clinicians initiate a low dose (1 mg/d) to mitigate adverse effects, and plan to titrate to clinical effect or intolerability. Per evidence from the trials discussed, it is likely Mr. H will need to be titrated to at least 5 to 6 mg/d to see a clinical effect.
Related Resource
North CS, Hong BA, Downs DL. PTSD: A systematic approach to diagnosis and treatment. Current Psychiatry 2018;17(4):35-43.
Drug Brand Names
Fluoxetine • Prozac
Prazosin • Minipress
1. Diagnostic and statistical manual of mental disorders, 5th ed. American Psychiatric Association; 2013.
2. Maher MJ, Rego SA, Asnis, GM. Sleep disturbances in patients with post-traumatic stress disorder: epidemiology, impact and approaches to management. CNS Drugs. 2006;20(7):567-590.
3. Benedek DM, Friedman MJ, Zatzick D, et al. Guideline watch (March 2009): Practice guideline for the treatment of patients with acute stress disorder and posttraumatic stress disorder. APA Practice Guidelines. Published 2010. Accessed March 14, 2021. https://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/acutestressdisorderptsd-watch.pdf
4. Baldwin DS, Anderson IM, Nutt DJ, et al. Evidence-based pharmacological treatment of anxiety disorders, post-traumatic stress disorder and obsessive-compulsive disorder: a revision of the 2005 guidelines from the British Association for Psychopharmacology. J Psychopharmacol. 2014;28(5):403-439. doi: 10.1177/0269881114525674
5. Department of Veterans Affairs, Department of Defense. VA/DoD clinical practice guideline for the management of posttraumatic stress disorder and acute stress disorder. Version 3.0. Published 2017. Accessed February 5, 2021. https://www.healthquality.va.gov/guidelines/MH/ptsd/VADoDPTSDCPGFinal012418.pdf
6. Raskind MA, Peskind ER, Chow B, et al. Trial of prazosin for post-traumatic stress disorder in military veterans. N Engl J Med. 2018;378(6):507-517.
7. Department of Veterans Affairs, Department of Defense. VA/DoD clinical practice guideline: management of post-traumatic stress. Version 2.0. Published 2010. Accessed February 5, 2021. https://www.healthquality.va.gov/guidelines/MH/ptsd/cpg_PTSD-full-201011612.PDF
8. Raskind MA, Peskind ER, Katner ED, et al. Reduction of nightmares and other PTSD symptoms in combat veterans by prazosin: a placebo-controlled study. Am J Psychiatry. 2003;160(2):371-373.
9. Raskind MA, Peskind ER, Hoff DJ, et al. A parallel group placebo-controlled study of prazosin for trauma nightmares and sleep disturbance in combat veterans with post-traumatic stress disorder. Biol Psychiatry. 2007;61(8):928-934.
10. Raskind MA, Peterson K, Williams T, et al. A trial of prazosin for combat trauma PTSD with nightmares in active-duty soldiers returned from Iraq and Afghanistan. Am J Psychiatry. 2013;170(9):1003-1010.
11. Germain A, Richardson R, Moul DE, et al. Placebo-controlled comparison of prazosin and cognitive-behavioral treatments for sleep disturbances in US military veterans. J Psychosom Res. 2012;72(2):89-96.
12. Taylor FB, Martin P, Thompson C, et al. Prazosin effects on objective sleep measures and clinical symptoms in civilian trauma posttraumatic stress disorder: a placebo-controlled study. Biol Psychiatry. 2008;63(6):629-632.
1. Diagnostic and statistical manual of mental disorders, 5th ed. American Psychiatric Association; 2013.
2. Maher MJ, Rego SA, Asnis, GM. Sleep disturbances in patients with post-traumatic stress disorder: epidemiology, impact and approaches to management. CNS Drugs. 2006;20(7):567-590.
3. Benedek DM, Friedman MJ, Zatzick D, et al. Guideline watch (March 2009): Practice guideline for the treatment of patients with acute stress disorder and posttraumatic stress disorder. APA Practice Guidelines. Published 2010. Accessed March 14, 2021. https://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/acutestressdisorderptsd-watch.pdf
4. Baldwin DS, Anderson IM, Nutt DJ, et al. Evidence-based pharmacological treatment of anxiety disorders, post-traumatic stress disorder and obsessive-compulsive disorder: a revision of the 2005 guidelines from the British Association for Psychopharmacology. J Psychopharmacol. 2014;28(5):403-439. doi: 10.1177/0269881114525674
5. Department of Veterans Affairs, Department of Defense. VA/DoD clinical practice guideline for the management of posttraumatic stress disorder and acute stress disorder. Version 3.0. Published 2017. Accessed February 5, 2021. https://www.healthquality.va.gov/guidelines/MH/ptsd/VADoDPTSDCPGFinal012418.pdf
6. Raskind MA, Peskind ER, Chow B, et al. Trial of prazosin for post-traumatic stress disorder in military veterans. N Engl J Med. 2018;378(6):507-517.
7. Department of Veterans Affairs, Department of Defense. VA/DoD clinical practice guideline: management of post-traumatic stress. Version 2.0. Published 2010. Accessed February 5, 2021. https://www.healthquality.va.gov/guidelines/MH/ptsd/cpg_PTSD-full-201011612.PDF
8. Raskind MA, Peskind ER, Katner ED, et al. Reduction of nightmares and other PTSD symptoms in combat veterans by prazosin: a placebo-controlled study. Am J Psychiatry. 2003;160(2):371-373.
9. Raskind MA, Peskind ER, Hoff DJ, et al. A parallel group placebo-controlled study of prazosin for trauma nightmares and sleep disturbance in combat veterans with post-traumatic stress disorder. Biol Psychiatry. 2007;61(8):928-934.
10. Raskind MA, Peterson K, Williams T, et al. A trial of prazosin for combat trauma PTSD with nightmares in active-duty soldiers returned from Iraq and Afghanistan. Am J Psychiatry. 2013;170(9):1003-1010.
11. Germain A, Richardson R, Moul DE, et al. Placebo-controlled comparison of prazosin and cognitive-behavioral treatments for sleep disturbances in US military veterans. J Psychosom Res. 2012;72(2):89-96.
12. Taylor FB, Martin P, Thompson C, et al. Prazosin effects on objective sleep measures and clinical symptoms in civilian trauma posttraumatic stress disorder: a placebo-controlled study. Biol Psychiatry. 2008;63(6):629-632.
Antidepressants: Is a higher dose always better?
Mr. E, age 39, presents to the mental health (MH) intake clinic, reporting he has had depressed mood almost every day, lack of interests, poor appetite, difficulty sleeping, inability to concentrate on daily activities, low energy and motivation, and feelings of guilt. He is diagnosed with major depressive disorder and agrees to a trial of sertraline, which is titrated up to 100 mg/d. He is also referred to the MH pharmacy clinic for interim visits.
Four weeks later during a follow-up visit, Mr. E reports tolerating sertraline, 100 mg/d, with a slight improvement in his mood. He reports that he has started working on his previous hobbies again and tries to consistently eat 2 meals a day. He feels that his sleep remains unchanged. He would like to enroll in school again, but is concerned about his poor concentration. He asks whether a further increase in his sertraline dose would improve his symptoms. What would you advise?
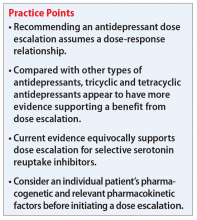
Escalating antidepressant doses up to, or even above, the FDA-approved maximum dose is a strategy for clinicians to consider for patients who are nonresponders or partial responders to treatment. This practice assumes that the effectiveness of an antidepressant is dependent on the dosage. However, based on our review of available literature, this recommendation is equivocally supported for general practice.
Selective serotonin reuptake inhibitors
The Table1-3 summarizes the results of 3 studies of high-dose selective serotonin reuptake inhibitors (SSRIs).
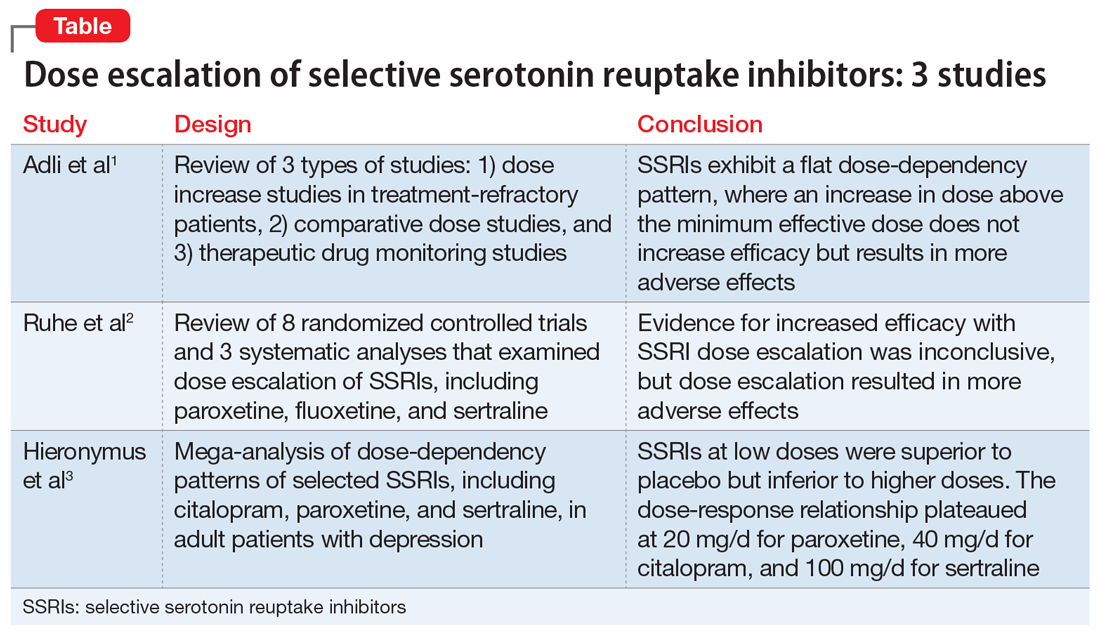
Adli et al1 evaluated 3 types of studies—studies of patients with treatment-resistant depression receiving high-dose treatment, comparative dose studies, and studies of therapeutic drug-monitoring (TDM) of antidepressants—to assess the effectiveness of high-dose antidepressants after a treatment failure with a medium dose. They concluded that SSRIs exhibit a flat dose-dependency pattern, where increasing a dose above the minimum effective dose (MED) does not increase efficacy but results in more adverse effects. Because treatment at the MED inhibits 70% of serotonin reuptake and is only marginally less effective than medium therapeutic doses, the authors recommended reserving treatment at higher doses for patients who have failed other standard treatment options, such as augmentation.
Ruhe et al2 evaluated 8 randomized controlled trials and 3 systematic analyses that investigated dose escalation of SSRIs, including paroxetine, fluoxetine, and sertraline. The authors noted that all included studies had methodological limitations and discussed 1 study that showed potential benefit from dose escalation when dropouts due to adverse effects were excluded from analysis. They determined that the evidence for increased efficacy with dose escalation was inconclusive; however, dose escalation un-doubtedly resulted in more adverse effects.
Hieronymus et al3 found a dose-dependency pattern with selected SSRIs—citalopram, paroxetine, and sertraline—in a mega-analysis of studies of adult patients with depression. All company-funded, acute-phase, placebo-controlled fixed-dose trials of these agents were included in this analysis. It included a total of 2,859 patients: 600 patients received citalopram (10 to 60 mg/d); 1,043 patients received paroxetine (10 to 40 mg/d); 481 patients received sertraline (50 to 400 mg/d); and 735 patients received placebo. They further divided the SSRIs into “low” vs “optimal” doses based on the dose curves of these agents. For citalopram, 10 to 20 mg/d was considered low vs 40 to 60 mg/d, which was considered optimal. For paroxetine, 10 mg/d was considered low vs other doses as optimal (20, 30, and 40 mg/d). For sertraline, 50 mg was considered low vs other doses as optimal (100, 200, and 400 mg/d). The authors concluded that at low doses, these antidepressants were superior to placebo but inferior to higher doses. Interestingly, they suggested that the dose-response relationship plateaued at 20 mg/d for paroxetine, 40 mg/d for citalopram, and 100 mg/d for sertraline. One of the limitations of the study was a lack of information on the tolerability of higher vs lower doses.
Continue to: Other antidepressants
Other antidepressants
Adli et al1 found a high-dose study and several comparative studies that supported a dose-response relationship with a reasonable degree of tolerability for venlafaxine, but there were no pertinent studies that evaluated mirtazapine. The only fixed-dose study found for bupropion did not support a dose-response relationship.1
The authors also concluded that there may be evidence supporting high-dose prescribing of tricyclic and tetracyclic antidepressants (TCAs and TeCAs, respectively). Despite the lack of clinical data that directly addressed the dose-dependency of TCAs and TeCAs, the authors supported dose escalation with amitriptyline, clomipramine, imipramine, desipramine, nortriptyline, and maprotiline, based on the data from comparative dose and TDM studies.1 The authors urged caution in interpreting and applying the results of TDM studies because the pharmacodynamic of each medication—such as being linear, curvilinear, or uncorrelated— may vary, which suggests there is a targeted therapeutic dose range.1
Important considerations
Differences in the pharmacokinetic and pharmacogenetic properties of individual medications may account for the mixed outcomes found when evaluating antidepressant dose-response relationships. Genetic polymorphisms of cytochrome (CYP) P450 enzymes, mainly CYP2D6 and CYP2D19, have been shown to directly affect antidepressants’ serum levels. Depending on the patient’s phenotype expression, such as poor, intermediate, extensive (ie, normal), or ultra-metabolizers, use of a specific antidepressant at a similar dose may result in therapeutic effectiveness, ineffectiveness, or toxicity. For antidepressants such as TCAs, which have a narrow therapeutic index compared with SSRIs, the differences in pharmacokinetic and pharmacogenetic properties becomes more impactful.1,4
Escalation within approved dose ranges
Few quality studies have conclusively found a relationship between antidepressant dose escalation within the FDA-approved dose ranges and efficacy, and there are few to no recommendations for prescribing doses above FDA-approved ranges. However, in clinical practice, clinicians may consider a dose escalation within the allowable dose ranges based on anecdotal evidence from previous patient cases. Consideration of relevant pharmacokinetic parameters and the patient’s individual pharmacogenetic factors may further guide clinicians and patients in making an informed decision on dose escalation to and beyond the FDA-approved doses.
CASE CONTINUED
After reviewing the evidence of antidepressant dose escalation and Mr. E’s progress, the MH pharmacist recommends that the psychiatrist increase Mr. E’s sertraline to 150 mg/d with close monitoring.
Related Resources
- Berney P. Dose-response relationship of recent antidepressants in the short-term treatment of depression. Dialogues Clin Neurosci. 2005;7:249.
- Jakubovski E, Varigonda AL, Freemantle N, et al. Systematic review and meta-analysis: dose-response relationship of selective serotonin reuptake inhibitors in major depressive disorder. Am J Psychiatry. 2016;173:174-183.
Drug Brand Names
Amitriptyline • Elavil
Bupropion • Wellbutrin
Citalopram • Celexa
Clomipramine • Anafranil
Desipramine • Norpramin
Fluoxetine • Prozac
Imipramine • Tofranil
Maprotiline • Ludiomil
Mirtazapine • Remeron
Nortriptyline • Pamelor
Paroxetine • Paxil
Sertraline • Zoloft
Venlafaxine • Effexor
1. Adli M, Baethge C, Heinz A, et al. Is dose escalation of antidepressants a rational strategy after a medium-dose treatment has failed? A systematic review. Eur Arch Psychiatry Clin Neurosci. 2005;255(6):387-400.
2. Ruhe HG, Huyser J, Swinkels JA, et al. Dose escalation for insufficient response to standard-dose selective serotonin reuptake inhibitors in major depressive disorder. Bri J Psychiatry. 2006;189:309-316.
3. Hieronymus F, Nilsson S, Eriksson E. A mega-analysis of fixed-dose trials reveals dose dependency and a rapid onset of action for the antidepressant effect of three selective serotonin reuptake inhibitors. Transl Psychiatry. 2016;6(6):e834. doi: 10.1038/tp.2016.104
4. Nassan M, Nicholson WY, Elliott MA, et al. Pharmacokinetic pharmacogenetic prescribing guidelines for antidepressants: a template for psychiatric precision medicine. Mayo Clin Proc. 2016;91(7):897-907.
Mr. E, age 39, presents to the mental health (MH) intake clinic, reporting he has had depressed mood almost every day, lack of interests, poor appetite, difficulty sleeping, inability to concentrate on daily activities, low energy and motivation, and feelings of guilt. He is diagnosed with major depressive disorder and agrees to a trial of sertraline, which is titrated up to 100 mg/d. He is also referred to the MH pharmacy clinic for interim visits.
Four weeks later during a follow-up visit, Mr. E reports tolerating sertraline, 100 mg/d, with a slight improvement in his mood. He reports that he has started working on his previous hobbies again and tries to consistently eat 2 meals a day. He feels that his sleep remains unchanged. He would like to enroll in school again, but is concerned about his poor concentration. He asks whether a further increase in his sertraline dose would improve his symptoms. What would you advise?
Escalating antidepressant doses up to, or even above, the FDA-approved maximum dose is a strategy for clinicians to consider for patients who are nonresponders or partial responders to treatment. This practice assumes that the effectiveness of an antidepressant is dependent on the dosage. However, based on our review of available literature, this recommendation is equivocally supported for general practice.
Selective serotonin reuptake inhibitors
The Table1-3 summarizes the results of 3 studies of high-dose selective serotonin reuptake inhibitors (SSRIs).

Adli et al1 evaluated 3 types of studies—studies of patients with treatment-resistant depression receiving high-dose treatment, comparative dose studies, and studies of therapeutic drug-monitoring (TDM) of antidepressants—to assess the effectiveness of high-dose antidepressants after a treatment failure with a medium dose. They concluded that SSRIs exhibit a flat dose-dependency pattern, where increasing a dose above the minimum effective dose (MED) does not increase efficacy but results in more adverse effects. Because treatment at the MED inhibits 70% of serotonin reuptake and is only marginally less effective than medium therapeutic doses, the authors recommended reserving treatment at higher doses for patients who have failed other standard treatment options, such as augmentation.
Ruhe et al2 evaluated 8 randomized controlled trials and 3 systematic analyses that investigated dose escalation of SSRIs, including paroxetine, fluoxetine, and sertraline. The authors noted that all included studies had methodological limitations and discussed 1 study that showed potential benefit from dose escalation when dropouts due to adverse effects were excluded from analysis. They determined that the evidence for increased efficacy with dose escalation was inconclusive; however, dose escalation un-doubtedly resulted in more adverse effects.
Hieronymus et al3 found a dose-dependency pattern with selected SSRIs—citalopram, paroxetine, and sertraline—in a mega-analysis of studies of adult patients with depression. All company-funded, acute-phase, placebo-controlled fixed-dose trials of these agents were included in this analysis. It included a total of 2,859 patients: 600 patients received citalopram (10 to 60 mg/d); 1,043 patients received paroxetine (10 to 40 mg/d); 481 patients received sertraline (50 to 400 mg/d); and 735 patients received placebo. They further divided the SSRIs into “low” vs “optimal” doses based on the dose curves of these agents. For citalopram, 10 to 20 mg/d was considered low vs 40 to 60 mg/d, which was considered optimal. For paroxetine, 10 mg/d was considered low vs other doses as optimal (20, 30, and 40 mg/d). For sertraline, 50 mg was considered low vs other doses as optimal (100, 200, and 400 mg/d). The authors concluded that at low doses, these antidepressants were superior to placebo but inferior to higher doses. Interestingly, they suggested that the dose-response relationship plateaued at 20 mg/d for paroxetine, 40 mg/d for citalopram, and 100 mg/d for sertraline. One of the limitations of the study was a lack of information on the tolerability of higher vs lower doses.
Continue to: Other antidepressants
Other antidepressants
Adli et al1 found a high-dose study and several comparative studies that supported a dose-response relationship with a reasonable degree of tolerability for venlafaxine, but there were no pertinent studies that evaluated mirtazapine. The only fixed-dose study found for bupropion did not support a dose-response relationship.1
The authors also concluded that there may be evidence supporting high-dose prescribing of tricyclic and tetracyclic antidepressants (TCAs and TeCAs, respectively). Despite the lack of clinical data that directly addressed the dose-dependency of TCAs and TeCAs, the authors supported dose escalation with amitriptyline, clomipramine, imipramine, desipramine, nortriptyline, and maprotiline, based on the data from comparative dose and TDM studies.1 The authors urged caution in interpreting and applying the results of TDM studies because the pharmacodynamic of each medication—such as being linear, curvilinear, or uncorrelated— may vary, which suggests there is a targeted therapeutic dose range.1
Important considerations
Differences in the pharmacokinetic and pharmacogenetic properties of individual medications may account for the mixed outcomes found when evaluating antidepressant dose-response relationships. Genetic polymorphisms of cytochrome (CYP) P450 enzymes, mainly CYP2D6 and CYP2D19, have been shown to directly affect antidepressants’ serum levels. Depending on the patient’s phenotype expression, such as poor, intermediate, extensive (ie, normal), or ultra-metabolizers, use of a specific antidepressant at a similar dose may result in therapeutic effectiveness, ineffectiveness, or toxicity. For antidepressants such as TCAs, which have a narrow therapeutic index compared with SSRIs, the differences in pharmacokinetic and pharmacogenetic properties becomes more impactful.1,4
Escalation within approved dose ranges
Few quality studies have conclusively found a relationship between antidepressant dose escalation within the FDA-approved dose ranges and efficacy, and there are few to no recommendations for prescribing doses above FDA-approved ranges. However, in clinical practice, clinicians may consider a dose escalation within the allowable dose ranges based on anecdotal evidence from previous patient cases. Consideration of relevant pharmacokinetic parameters and the patient’s individual pharmacogenetic factors may further guide clinicians and patients in making an informed decision on dose escalation to and beyond the FDA-approved doses.
CASE CONTINUED
After reviewing the evidence of antidepressant dose escalation and Mr. E’s progress, the MH pharmacist recommends that the psychiatrist increase Mr. E’s sertraline to 150 mg/d with close monitoring.
Related Resources
- Berney P. Dose-response relationship of recent antidepressants in the short-term treatment of depression. Dialogues Clin Neurosci. 2005;7:249.
- Jakubovski E, Varigonda AL, Freemantle N, et al. Systematic review and meta-analysis: dose-response relationship of selective serotonin reuptake inhibitors in major depressive disorder. Am J Psychiatry. 2016;173:174-183.
Drug Brand Names
Amitriptyline • Elavil
Bupropion • Wellbutrin
Citalopram • Celexa
Clomipramine • Anafranil
Desipramine • Norpramin
Fluoxetine • Prozac
Imipramine • Tofranil
Maprotiline • Ludiomil
Mirtazapine • Remeron
Nortriptyline • Pamelor
Paroxetine • Paxil
Sertraline • Zoloft
Venlafaxine • Effexor
Mr. E, age 39, presents to the mental health (MH) intake clinic, reporting he has had depressed mood almost every day, lack of interests, poor appetite, difficulty sleeping, inability to concentrate on daily activities, low energy and motivation, and feelings of guilt. He is diagnosed with major depressive disorder and agrees to a trial of sertraline, which is titrated up to 100 mg/d. He is also referred to the MH pharmacy clinic for interim visits.
Four weeks later during a follow-up visit, Mr. E reports tolerating sertraline, 100 mg/d, with a slight improvement in his mood. He reports that he has started working on his previous hobbies again and tries to consistently eat 2 meals a day. He feels that his sleep remains unchanged. He would like to enroll in school again, but is concerned about his poor concentration. He asks whether a further increase in his sertraline dose would improve his symptoms. What would you advise?
Escalating antidepressant doses up to, or even above, the FDA-approved maximum dose is a strategy for clinicians to consider for patients who are nonresponders or partial responders to treatment. This practice assumes that the effectiveness of an antidepressant is dependent on the dosage. However, based on our review of available literature, this recommendation is equivocally supported for general practice.
Selective serotonin reuptake inhibitors
The Table1-3 summarizes the results of 3 studies of high-dose selective serotonin reuptake inhibitors (SSRIs).

Adli et al1 evaluated 3 types of studies—studies of patients with treatment-resistant depression receiving high-dose treatment, comparative dose studies, and studies of therapeutic drug-monitoring (TDM) of antidepressants—to assess the effectiveness of high-dose antidepressants after a treatment failure with a medium dose. They concluded that SSRIs exhibit a flat dose-dependency pattern, where increasing a dose above the minimum effective dose (MED) does not increase efficacy but results in more adverse effects. Because treatment at the MED inhibits 70% of serotonin reuptake and is only marginally less effective than medium therapeutic doses, the authors recommended reserving treatment at higher doses for patients who have failed other standard treatment options, such as augmentation.
Ruhe et al2 evaluated 8 randomized controlled trials and 3 systematic analyses that investigated dose escalation of SSRIs, including paroxetine, fluoxetine, and sertraline. The authors noted that all included studies had methodological limitations and discussed 1 study that showed potential benefit from dose escalation when dropouts due to adverse effects were excluded from analysis. They determined that the evidence for increased efficacy with dose escalation was inconclusive; however, dose escalation un-doubtedly resulted in more adverse effects.
Hieronymus et al3 found a dose-dependency pattern with selected SSRIs—citalopram, paroxetine, and sertraline—in a mega-analysis of studies of adult patients with depression. All company-funded, acute-phase, placebo-controlled fixed-dose trials of these agents were included in this analysis. It included a total of 2,859 patients: 600 patients received citalopram (10 to 60 mg/d); 1,043 patients received paroxetine (10 to 40 mg/d); 481 patients received sertraline (50 to 400 mg/d); and 735 patients received placebo. They further divided the SSRIs into “low” vs “optimal” doses based on the dose curves of these agents. For citalopram, 10 to 20 mg/d was considered low vs 40 to 60 mg/d, which was considered optimal. For paroxetine, 10 mg/d was considered low vs other doses as optimal (20, 30, and 40 mg/d). For sertraline, 50 mg was considered low vs other doses as optimal (100, 200, and 400 mg/d). The authors concluded that at low doses, these antidepressants were superior to placebo but inferior to higher doses. Interestingly, they suggested that the dose-response relationship plateaued at 20 mg/d for paroxetine, 40 mg/d for citalopram, and 100 mg/d for sertraline. One of the limitations of the study was a lack of information on the tolerability of higher vs lower doses.
Continue to: Other antidepressants
Other antidepressants
Adli et al1 found a high-dose study and several comparative studies that supported a dose-response relationship with a reasonable degree of tolerability for venlafaxine, but there were no pertinent studies that evaluated mirtazapine. The only fixed-dose study found for bupropion did not support a dose-response relationship.1
The authors also concluded that there may be evidence supporting high-dose prescribing of tricyclic and tetracyclic antidepressants (TCAs and TeCAs, respectively). Despite the lack of clinical data that directly addressed the dose-dependency of TCAs and TeCAs, the authors supported dose escalation with amitriptyline, clomipramine, imipramine, desipramine, nortriptyline, and maprotiline, based on the data from comparative dose and TDM studies.1 The authors urged caution in interpreting and applying the results of TDM studies because the pharmacodynamic of each medication—such as being linear, curvilinear, or uncorrelated— may vary, which suggests there is a targeted therapeutic dose range.1
Important considerations
Differences in the pharmacokinetic and pharmacogenetic properties of individual medications may account for the mixed outcomes found when evaluating antidepressant dose-response relationships. Genetic polymorphisms of cytochrome (CYP) P450 enzymes, mainly CYP2D6 and CYP2D19, have been shown to directly affect antidepressants’ serum levels. Depending on the patient’s phenotype expression, such as poor, intermediate, extensive (ie, normal), or ultra-metabolizers, use of a specific antidepressant at a similar dose may result in therapeutic effectiveness, ineffectiveness, or toxicity. For antidepressants such as TCAs, which have a narrow therapeutic index compared with SSRIs, the differences in pharmacokinetic and pharmacogenetic properties becomes more impactful.1,4
Escalation within approved dose ranges
Few quality studies have conclusively found a relationship between antidepressant dose escalation within the FDA-approved dose ranges and efficacy, and there are few to no recommendations for prescribing doses above FDA-approved ranges. However, in clinical practice, clinicians may consider a dose escalation within the allowable dose ranges based on anecdotal evidence from previous patient cases. Consideration of relevant pharmacokinetic parameters and the patient’s individual pharmacogenetic factors may further guide clinicians and patients in making an informed decision on dose escalation to and beyond the FDA-approved doses.
CASE CONTINUED
After reviewing the evidence of antidepressant dose escalation and Mr. E’s progress, the MH pharmacist recommends that the psychiatrist increase Mr. E’s sertraline to 150 mg/d with close monitoring.
Related Resources
- Berney P. Dose-response relationship of recent antidepressants in the short-term treatment of depression. Dialogues Clin Neurosci. 2005;7:249.
- Jakubovski E, Varigonda AL, Freemantle N, et al. Systematic review and meta-analysis: dose-response relationship of selective serotonin reuptake inhibitors in major depressive disorder. Am J Psychiatry. 2016;173:174-183.
Drug Brand Names
Amitriptyline • Elavil
Bupropion • Wellbutrin
Citalopram • Celexa
Clomipramine • Anafranil
Desipramine • Norpramin
Fluoxetine • Prozac
Imipramine • Tofranil
Maprotiline • Ludiomil
Mirtazapine • Remeron
Nortriptyline • Pamelor
Paroxetine • Paxil
Sertraline • Zoloft
Venlafaxine • Effexor
1. Adli M, Baethge C, Heinz A, et al. Is dose escalation of antidepressants a rational strategy after a medium-dose treatment has failed? A systematic review. Eur Arch Psychiatry Clin Neurosci. 2005;255(6):387-400.
2. Ruhe HG, Huyser J, Swinkels JA, et al. Dose escalation for insufficient response to standard-dose selective serotonin reuptake inhibitors in major depressive disorder. Bri J Psychiatry. 2006;189:309-316.
3. Hieronymus F, Nilsson S, Eriksson E. A mega-analysis of fixed-dose trials reveals dose dependency and a rapid onset of action for the antidepressant effect of three selective serotonin reuptake inhibitors. Transl Psychiatry. 2016;6(6):e834. doi: 10.1038/tp.2016.104
4. Nassan M, Nicholson WY, Elliott MA, et al. Pharmacokinetic pharmacogenetic prescribing guidelines for antidepressants: a template for psychiatric precision medicine. Mayo Clin Proc. 2016;91(7):897-907.
1. Adli M, Baethge C, Heinz A, et al. Is dose escalation of antidepressants a rational strategy after a medium-dose treatment has failed? A systematic review. Eur Arch Psychiatry Clin Neurosci. 2005;255(6):387-400.
2. Ruhe HG, Huyser J, Swinkels JA, et al. Dose escalation for insufficient response to standard-dose selective serotonin reuptake inhibitors in major depressive disorder. Bri J Psychiatry. 2006;189:309-316.
3. Hieronymus F, Nilsson S, Eriksson E. A mega-analysis of fixed-dose trials reveals dose dependency and a rapid onset of action for the antidepressant effect of three selective serotonin reuptake inhibitors. Transl Psychiatry. 2016;6(6):e834. doi: 10.1038/tp.2016.104
4. Nassan M, Nicholson WY, Elliott MA, et al. Pharmacokinetic pharmacogenetic prescribing guidelines for antidepressants: a template for psychiatric precision medicine. Mayo Clin Proc. 2016;91(7):897-907.
Management of major depressive disorder with psychotic features
Mrs. C, age 56, has a history of major depressive disorder (MDD). She has been stable for 5 years without medication. Six months ago, she presented to you, along with her son, seeking help. She reported that she had been experiencing insomnia, fatigue, and was not engaging in hobbies. Her son told you that his mother had lost weight and had been avoiding family dinners. Mrs. C reported recurrent thoughts of dying and heard voices vividly telling her that she was a burden and that her family would be better off without her. However, there was no imminent danger of self-harm. At that appointment, you initiated
Since that time, Mrs. C has followed up with you monthly with good response to the medications. Currently, she states her depression is much improved, and she denies hearing voices for approximately 5 months.
Based on her presentation and response, what do the data suggest about her length of treatment, and when should you consider tapering the antipsychotic medication?
In DSM-5, MDD with psychotic features is a severe subtype of MDD that is defined as a major depressive episode characterized by delusions and/or hallucinations.1 In the general population, the lifetime prevalence of this disorder varies from 0.35% to 1%, and the rate is higher in older patients.2 Risk factors include female gender, family history, and concomitant bipolar disorder.2
Epidemiologic studies have shown that psychotic features can occur in 15% to 20% of patients with MDD. The psychotic features that occur during these episodes are delusions and hallucinations.1 These features can be either mood-congruent (related to the depressive themes of worthlessness or guilt) or mood-incongruent (ie, unrelated to depressive themes).1
Treatment options: ECT or pharmacotherapy
Guidelines from the American Psychiatric Association3 and the National Institute for Clinical Excellence4 recommend treating depression with psychosis with electroconvulsive therapy (ECT) or with combined antidepressant and antipsychotic medications as first-line options. The Texas Medication Algorithm Project (TMAP) Algorithm for MDD,5 which closely focuses on treatment of MDD with psychotic features, can be used for treatment decisions (see Related Resources).
Electroconvulsive therapy is known to be efficacious in treating patients with MDD with psychotic features and should be considered as a treatment option. However, medication therapy is often chosen as the initial treatment due to the limitations of ECT, including accessibility, cost, and patient preference. However, in certain cases, ECT is the preferred option because it can provide rapid and significant improvement in patients with severe psychosis, suicidality, or risk of imminent harm.
Continue to: Pharmacotherapy
Pharmacotherapy for the treatment of MDD with psychotic features should consist of a combination of an antidepressant and antipsychotic medication. This combination has been shown to be more effective than either agent alone. Some combinations have been studied specifically for MDD with psychosis. The Study of the Pharmacotherapy of Psychotic Depression (STOP-PD), a 12-week, double-blind, randomized controlled trial, found that the combination of sertraline and olanzapine was efficacious and superior to monotherapy with olanzapine in an acute setting.6 In another study, the combination of olanzapine and
How long should treatment last?
The optimal timeline for treating patients with MDD with psychotic features is unknown. According to the TMAP algorithm and expert opinion, the continuation phase of pharmacotherapy should include treatment for at least 4 months with an antipsychotic medication and at least 2 years to lifetime treatment with an antidepressant.5 The STOP-PD II study, which was a continuation of the 12-week STOP-PD study, examined antipsychotic duration to determine the effects of continuing olanzapine once an episode of psychotic depression had responded to olanzapine and sertraline.11 Patients who had achieved remission after receiving olanzapine and sertraline were randomized to continue to receive this combination or to receive sertraline plus placebo for 36 weeks. The primary outcome was relapse, which was broadly defined as 1 of the following11:
- a Structured Clinical Interview for the DSM (SCID)-rated assessment that revealed the patient had enough symptoms to meet criteria for a DSM-IV major depressive episode
- a 17-item HAM-D scoren of ≥18
- SCID-rated psychosis
- other significant clinical worsening, defined as having a suicide plan or attempting suicide, developing SCID-rated symptoms of mania or hypomania, or being hospitalized in a psychiatric unit.
Compared with sertraline plus placebo, continuing sertraline plus olanzapine reduced the risk of relapse over 36 weeks (hazard ratio, 0.25; 95% confidence interval, 0.13 to 0.48; P < .001).11 However, as expected, the incidence of adverse effects such as weight gain and parkinsonism was higher in the olanzapine group. Therefore, it is important to consider the potential long-term adverse effects of continuing antipsychotic medications.
Weighing the evidence
Electroconvulsive therapy is considered a first-line treatment option for MDD with psychotic features; however, because of limitations associated with this approach, antidepressants plus antipsychotics are often utilized as an initial treatment.
Continue to: CASE
CASE CONTINUED
Based on the results of the STOP-PD and STOP-PD II trials, Mrs. C should be continued on sertraline plus olanzapine for at least another 3 to 6 months before an olanzapine taper should be considered. At that time, the risks and benefits of a taper vs continuing therapy should be considered. Given her history of MDD and the severity of this most recent episode, sertraline therapy should be continued for at least 2 years, and possibly indefinitely.
Related Resources
- Texas Medication Algorithm Project. Algorithm for the treatment of major depressive disorder with psychotic features. https://chsciowa.org/sites/chsciowa.org/files/resource/files/9_-_depression_med_algorithm_supplement.pdf
- Dold M, Bartova L, Kautzky A, et al. Psychotic features in patients with major depressive disorder: a report from the European Group for the Study of Resistant Depression. J Clin Psychiatry. 2019;80(1):17m12090. doi: 10.4088/ JCP.17m12090
- Flint AJ, Meyers BS, Rothschild AJ, et al. Effect of continuing olanzapine vs placebo on relapse among patients with psychotic depression in remission: the STOP-PD II randomized clinical trial. JAMA. 2019;322(7): 622-631.
Drug Brand Names
Amitriptyline • Elavil, Endep
Fluoxetine • Prozac
Haloperidol • Haldol
Olanzapine • Zyprexa
Quetiapine • Seroquel
Sertraline • Zoloft
Venlafaxine • Effexor
1. Diagnostic and statistical manual of mental disorders, 5th ed. American Psychiatric Association; 2013.
2. Jääskeläinen E, Juola T, Korpela H, et al. Epidemiology of psychotic depression - systematic review and meta-analysis. Psychol Med. 2018;48(6):905-918.
3. American Psychiatric Association. Practice guideline for the treatment of patients with major depressive disorder (revision). Am J Psychiatry. 2000;157(4)(suppl):1-45.
4. National Institute for Clinical Excellence. Depression in adults: recognition and management: clinical guideline [CG90]. National Institute for Health and Clinical Excellence. Published October 28, 2009. Accessed January 12, 2021. https://www.nice.org.uk/guidance/cg90
5. Crimson ML, Trivedi M, Pigott TA, et al. The Texas Medication Algorithm Project: report of the Texas Consensus Conference Panel on medication treatment of major depressive disorder. J Clin Psychiatry. 1999;60(3):142-156.
6. Meyers BS, Flint AJ, Rothschild AJ, et al. A double-blind randomized controlled trial of olanzapine plus sertraline vs olanzapine plus placebo for psychotic depression: the study of pharmacotherapy for psychotic depression -- the STOP-PD study. Arch Gen Psychiatry. 2009;66(8):838-847.
7. Rothschild AJ, Williamson DJ, Tohen MF, et al. A double-blind, randomized study of olanzapine and olanzapine/fluoxetine combination for major depression with psychotic features. J Clin Psychopharmacol. 2004;24(4):365-373.
8. Wijkstra J, Burger H, van den Broek WW, et al. Treatment of unipolar psychotic depression: a randomized, doubleblind study comparing imipramine, venlafaxine, and venlafaxine plus quetiapine. Acta Psychiatr Scand. 2010;21(3):190-200.
9. Muller-Siecheneder F, Muller M, Hillert A, et al. Risperidone versus haloperidol and amitriptyline in the treatment of patients with a combined psychotic and depressive syndrome. J Clin Psychopharm. 1998;18(2):111-120.
10. Spiker DG, Weiss JC, Dealy RS, et al. The pharmacological treatment of delusional depression. Am J Psychiatry. 1985;142(4):430-436.
11. Flint AJ, Meyers BS, Rothschild AJ, et al. Effect of continuing olanzapine vs placebo on relapse among patients with psychotic depression in remission: the STOP-PD II randomized clinical trial. JAMA. 2019;322(7):622-631.
Mrs. C, age 56, has a history of major depressive disorder (MDD). She has been stable for 5 years without medication. Six months ago, she presented to you, along with her son, seeking help. She reported that she had been experiencing insomnia, fatigue, and was not engaging in hobbies. Her son told you that his mother had lost weight and had been avoiding family dinners. Mrs. C reported recurrent thoughts of dying and heard voices vividly telling her that she was a burden and that her family would be better off without her. However, there was no imminent danger of self-harm. At that appointment, you initiated
Since that time, Mrs. C has followed up with you monthly with good response to the medications. Currently, she states her depression is much improved, and she denies hearing voices for approximately 5 months.
Based on her presentation and response, what do the data suggest about her length of treatment, and when should you consider tapering the antipsychotic medication?
In DSM-5, MDD with psychotic features is a severe subtype of MDD that is defined as a major depressive episode characterized by delusions and/or hallucinations.1 In the general population, the lifetime prevalence of this disorder varies from 0.35% to 1%, and the rate is higher in older patients.2 Risk factors include female gender, family history, and concomitant bipolar disorder.2
Epidemiologic studies have shown that psychotic features can occur in 15% to 20% of patients with MDD. The psychotic features that occur during these episodes are delusions and hallucinations.1 These features can be either mood-congruent (related to the depressive themes of worthlessness or guilt) or mood-incongruent (ie, unrelated to depressive themes).1
Treatment options: ECT or pharmacotherapy
Guidelines from the American Psychiatric Association3 and the National Institute for Clinical Excellence4 recommend treating depression with psychosis with electroconvulsive therapy (ECT) or with combined antidepressant and antipsychotic medications as first-line options. The Texas Medication Algorithm Project (TMAP) Algorithm for MDD,5 which closely focuses on treatment of MDD with psychotic features, can be used for treatment decisions (see Related Resources).
Electroconvulsive therapy is known to be efficacious in treating patients with MDD with psychotic features and should be considered as a treatment option. However, medication therapy is often chosen as the initial treatment due to the limitations of ECT, including accessibility, cost, and patient preference. However, in certain cases, ECT is the preferred option because it can provide rapid and significant improvement in patients with severe psychosis, suicidality, or risk of imminent harm.
Continue to: Pharmacotherapy
Pharmacotherapy for the treatment of MDD with psychotic features should consist of a combination of an antidepressant and antipsychotic medication. This combination has been shown to be more effective than either agent alone. Some combinations have been studied specifically for MDD with psychosis. The Study of the Pharmacotherapy of Psychotic Depression (STOP-PD), a 12-week, double-blind, randomized controlled trial, found that the combination of sertraline and olanzapine was efficacious and superior to monotherapy with olanzapine in an acute setting.6 In another study, the combination of olanzapine and
How long should treatment last?
The optimal timeline for treating patients with MDD with psychotic features is unknown. According to the TMAP algorithm and expert opinion, the continuation phase of pharmacotherapy should include treatment for at least 4 months with an antipsychotic medication and at least 2 years to lifetime treatment with an antidepressant.5 The STOP-PD II study, which was a continuation of the 12-week STOP-PD study, examined antipsychotic duration to determine the effects of continuing olanzapine once an episode of psychotic depression had responded to olanzapine and sertraline.11 Patients who had achieved remission after receiving olanzapine and sertraline were randomized to continue to receive this combination or to receive sertraline plus placebo for 36 weeks. The primary outcome was relapse, which was broadly defined as 1 of the following11:
- a Structured Clinical Interview for the DSM (SCID)-rated assessment that revealed the patient had enough symptoms to meet criteria for a DSM-IV major depressive episode
- a 17-item HAM-D scoren of ≥18
- SCID-rated psychosis
- other significant clinical worsening, defined as having a suicide plan or attempting suicide, developing SCID-rated symptoms of mania or hypomania, or being hospitalized in a psychiatric unit.
Compared with sertraline plus placebo, continuing sertraline plus olanzapine reduced the risk of relapse over 36 weeks (hazard ratio, 0.25; 95% confidence interval, 0.13 to 0.48; P < .001).11 However, as expected, the incidence of adverse effects such as weight gain and parkinsonism was higher in the olanzapine group. Therefore, it is important to consider the potential long-term adverse effects of continuing antipsychotic medications.
Weighing the evidence
Electroconvulsive therapy is considered a first-line treatment option for MDD with psychotic features; however, because of limitations associated with this approach, antidepressants plus antipsychotics are often utilized as an initial treatment.
Continue to: CASE
CASE CONTINUED
Based on the results of the STOP-PD and STOP-PD II trials, Mrs. C should be continued on sertraline plus olanzapine for at least another 3 to 6 months before an olanzapine taper should be considered. At that time, the risks and benefits of a taper vs continuing therapy should be considered. Given her history of MDD and the severity of this most recent episode, sertraline therapy should be continued for at least 2 years, and possibly indefinitely.
Related Resources
- Texas Medication Algorithm Project. Algorithm for the treatment of major depressive disorder with psychotic features. https://chsciowa.org/sites/chsciowa.org/files/resource/files/9_-_depression_med_algorithm_supplement.pdf
- Dold M, Bartova L, Kautzky A, et al. Psychotic features in patients with major depressive disorder: a report from the European Group for the Study of Resistant Depression. J Clin Psychiatry. 2019;80(1):17m12090. doi: 10.4088/ JCP.17m12090
- Flint AJ, Meyers BS, Rothschild AJ, et al. Effect of continuing olanzapine vs placebo on relapse among patients with psychotic depression in remission: the STOP-PD II randomized clinical trial. JAMA. 2019;322(7): 622-631.
Drug Brand Names
Amitriptyline • Elavil, Endep
Fluoxetine • Prozac
Haloperidol • Haldol
Olanzapine • Zyprexa
Quetiapine • Seroquel
Sertraline • Zoloft
Venlafaxine • Effexor
Mrs. C, age 56, has a history of major depressive disorder (MDD). She has been stable for 5 years without medication. Six months ago, she presented to you, along with her son, seeking help. She reported that she had been experiencing insomnia, fatigue, and was not engaging in hobbies. Her son told you that his mother had lost weight and had been avoiding family dinners. Mrs. C reported recurrent thoughts of dying and heard voices vividly telling her that she was a burden and that her family would be better off without her. However, there was no imminent danger of self-harm. At that appointment, you initiated
Since that time, Mrs. C has followed up with you monthly with good response to the medications. Currently, she states her depression is much improved, and she denies hearing voices for approximately 5 months.
Based on her presentation and response, what do the data suggest about her length of treatment, and when should you consider tapering the antipsychotic medication?
In DSM-5, MDD with psychotic features is a severe subtype of MDD that is defined as a major depressive episode characterized by delusions and/or hallucinations.1 In the general population, the lifetime prevalence of this disorder varies from 0.35% to 1%, and the rate is higher in older patients.2 Risk factors include female gender, family history, and concomitant bipolar disorder.2
Epidemiologic studies have shown that psychotic features can occur in 15% to 20% of patients with MDD. The psychotic features that occur during these episodes are delusions and hallucinations.1 These features can be either mood-congruent (related to the depressive themes of worthlessness or guilt) or mood-incongruent (ie, unrelated to depressive themes).1
Treatment options: ECT or pharmacotherapy
Guidelines from the American Psychiatric Association3 and the National Institute for Clinical Excellence4 recommend treating depression with psychosis with electroconvulsive therapy (ECT) or with combined antidepressant and antipsychotic medications as first-line options. The Texas Medication Algorithm Project (TMAP) Algorithm for MDD,5 which closely focuses on treatment of MDD with psychotic features, can be used for treatment decisions (see Related Resources).
Electroconvulsive therapy is known to be efficacious in treating patients with MDD with psychotic features and should be considered as a treatment option. However, medication therapy is often chosen as the initial treatment due to the limitations of ECT, including accessibility, cost, and patient preference. However, in certain cases, ECT is the preferred option because it can provide rapid and significant improvement in patients with severe psychosis, suicidality, or risk of imminent harm.
Continue to: Pharmacotherapy
Pharmacotherapy for the treatment of MDD with psychotic features should consist of a combination of an antidepressant and antipsychotic medication. This combination has been shown to be more effective than either agent alone. Some combinations have been studied specifically for MDD with psychosis. The Study of the Pharmacotherapy of Psychotic Depression (STOP-PD), a 12-week, double-blind, randomized controlled trial, found that the combination of sertraline and olanzapine was efficacious and superior to monotherapy with olanzapine in an acute setting.6 In another study, the combination of olanzapine and
How long should treatment last?
The optimal timeline for treating patients with MDD with psychotic features is unknown. According to the TMAP algorithm and expert opinion, the continuation phase of pharmacotherapy should include treatment for at least 4 months with an antipsychotic medication and at least 2 years to lifetime treatment with an antidepressant.5 The STOP-PD II study, which was a continuation of the 12-week STOP-PD study, examined antipsychotic duration to determine the effects of continuing olanzapine once an episode of psychotic depression had responded to olanzapine and sertraline.11 Patients who had achieved remission after receiving olanzapine and sertraline were randomized to continue to receive this combination or to receive sertraline plus placebo for 36 weeks. The primary outcome was relapse, which was broadly defined as 1 of the following11:
- a Structured Clinical Interview for the DSM (SCID)-rated assessment that revealed the patient had enough symptoms to meet criteria for a DSM-IV major depressive episode
- a 17-item HAM-D scoren of ≥18
- SCID-rated psychosis
- other significant clinical worsening, defined as having a suicide plan or attempting suicide, developing SCID-rated symptoms of mania or hypomania, or being hospitalized in a psychiatric unit.
Compared with sertraline plus placebo, continuing sertraline plus olanzapine reduced the risk of relapse over 36 weeks (hazard ratio, 0.25; 95% confidence interval, 0.13 to 0.48; P < .001).11 However, as expected, the incidence of adverse effects such as weight gain and parkinsonism was higher in the olanzapine group. Therefore, it is important to consider the potential long-term adverse effects of continuing antipsychotic medications.
Weighing the evidence
Electroconvulsive therapy is considered a first-line treatment option for MDD with psychotic features; however, because of limitations associated with this approach, antidepressants plus antipsychotics are often utilized as an initial treatment.
Continue to: CASE
CASE CONTINUED
Based on the results of the STOP-PD and STOP-PD II trials, Mrs. C should be continued on sertraline plus olanzapine for at least another 3 to 6 months before an olanzapine taper should be considered. At that time, the risks and benefits of a taper vs continuing therapy should be considered. Given her history of MDD and the severity of this most recent episode, sertraline therapy should be continued for at least 2 years, and possibly indefinitely.
Related Resources
- Texas Medication Algorithm Project. Algorithm for the treatment of major depressive disorder with psychotic features. https://chsciowa.org/sites/chsciowa.org/files/resource/files/9_-_depression_med_algorithm_supplement.pdf
- Dold M, Bartova L, Kautzky A, et al. Psychotic features in patients with major depressive disorder: a report from the European Group for the Study of Resistant Depression. J Clin Psychiatry. 2019;80(1):17m12090. doi: 10.4088/ JCP.17m12090
- Flint AJ, Meyers BS, Rothschild AJ, et al. Effect of continuing olanzapine vs placebo on relapse among patients with psychotic depression in remission: the STOP-PD II randomized clinical trial. JAMA. 2019;322(7): 622-631.
Drug Brand Names
Amitriptyline • Elavil, Endep
Fluoxetine • Prozac
Haloperidol • Haldol
Olanzapine • Zyprexa
Quetiapine • Seroquel
Sertraline • Zoloft
Venlafaxine • Effexor
1. Diagnostic and statistical manual of mental disorders, 5th ed. American Psychiatric Association; 2013.
2. Jääskeläinen E, Juola T, Korpela H, et al. Epidemiology of psychotic depression - systematic review and meta-analysis. Psychol Med. 2018;48(6):905-918.
3. American Psychiatric Association. Practice guideline for the treatment of patients with major depressive disorder (revision). Am J Psychiatry. 2000;157(4)(suppl):1-45.
4. National Institute for Clinical Excellence. Depression in adults: recognition and management: clinical guideline [CG90]. National Institute for Health and Clinical Excellence. Published October 28, 2009. Accessed January 12, 2021. https://www.nice.org.uk/guidance/cg90
5. Crimson ML, Trivedi M, Pigott TA, et al. The Texas Medication Algorithm Project: report of the Texas Consensus Conference Panel on medication treatment of major depressive disorder. J Clin Psychiatry. 1999;60(3):142-156.
6. Meyers BS, Flint AJ, Rothschild AJ, et al. A double-blind randomized controlled trial of olanzapine plus sertraline vs olanzapine plus placebo for psychotic depression: the study of pharmacotherapy for psychotic depression -- the STOP-PD study. Arch Gen Psychiatry. 2009;66(8):838-847.
7. Rothschild AJ, Williamson DJ, Tohen MF, et al. A double-blind, randomized study of olanzapine and olanzapine/fluoxetine combination for major depression with psychotic features. J Clin Psychopharmacol. 2004;24(4):365-373.
8. Wijkstra J, Burger H, van den Broek WW, et al. Treatment of unipolar psychotic depression: a randomized, doubleblind study comparing imipramine, venlafaxine, and venlafaxine plus quetiapine. Acta Psychiatr Scand. 2010;21(3):190-200.
9. Muller-Siecheneder F, Muller M, Hillert A, et al. Risperidone versus haloperidol and amitriptyline in the treatment of patients with a combined psychotic and depressive syndrome. J Clin Psychopharm. 1998;18(2):111-120.
10. Spiker DG, Weiss JC, Dealy RS, et al. The pharmacological treatment of delusional depression. Am J Psychiatry. 1985;142(4):430-436.
11. Flint AJ, Meyers BS, Rothschild AJ, et al. Effect of continuing olanzapine vs placebo on relapse among patients with psychotic depression in remission: the STOP-PD II randomized clinical trial. JAMA. 2019;322(7):622-631.
1. Diagnostic and statistical manual of mental disorders, 5th ed. American Psychiatric Association; 2013.
2. Jääskeläinen E, Juola T, Korpela H, et al. Epidemiology of psychotic depression - systematic review and meta-analysis. Psychol Med. 2018;48(6):905-918.
3. American Psychiatric Association. Practice guideline for the treatment of patients with major depressive disorder (revision). Am J Psychiatry. 2000;157(4)(suppl):1-45.
4. National Institute for Clinical Excellence. Depression in adults: recognition and management: clinical guideline [CG90]. National Institute for Health and Clinical Excellence. Published October 28, 2009. Accessed January 12, 2021. https://www.nice.org.uk/guidance/cg90
5. Crimson ML, Trivedi M, Pigott TA, et al. The Texas Medication Algorithm Project: report of the Texas Consensus Conference Panel on medication treatment of major depressive disorder. J Clin Psychiatry. 1999;60(3):142-156.
6. Meyers BS, Flint AJ, Rothschild AJ, et al. A double-blind randomized controlled trial of olanzapine plus sertraline vs olanzapine plus placebo for psychotic depression: the study of pharmacotherapy for psychotic depression -- the STOP-PD study. Arch Gen Psychiatry. 2009;66(8):838-847.
7. Rothschild AJ, Williamson DJ, Tohen MF, et al. A double-blind, randomized study of olanzapine and olanzapine/fluoxetine combination for major depression with psychotic features. J Clin Psychopharmacol. 2004;24(4):365-373.
8. Wijkstra J, Burger H, van den Broek WW, et al. Treatment of unipolar psychotic depression: a randomized, doubleblind study comparing imipramine, venlafaxine, and venlafaxine plus quetiapine. Acta Psychiatr Scand. 2010;21(3):190-200.
9. Muller-Siecheneder F, Muller M, Hillert A, et al. Risperidone versus haloperidol and amitriptyline in the treatment of patients with a combined psychotic and depressive syndrome. J Clin Psychopharm. 1998;18(2):111-120.
10. Spiker DG, Weiss JC, Dealy RS, et al. The pharmacological treatment of delusional depression. Am J Psychiatry. 1985;142(4):430-436.
11. Flint AJ, Meyers BS, Rothschild AJ, et al. Effect of continuing olanzapine vs placebo on relapse among patients with psychotic depression in remission: the STOP-PD II randomized clinical trial. JAMA. 2019;322(7):622-631.
Elaborate hallucinations, but is it a psychotic disorder?
CASE Visual, auditory, and tactile hallucinations
Mr. B, age 93, is brought to the emergency department by his son after experiencing hallucinations where he reportedly saw and heard individuals in his home. In frustration, Mr. B wielded a knife because he “wanted them to go away.”
Mr. B and his son report that the hallucinations had begun 2 years ago, without prior trauma, medication changes, changes in social situation, or other apparent precipitating events. The hallucinations “come and go,” without preceding symptoms, but have recurring content involving a friendly man named “Harry,” people coming out of the television, 2 children playing, and water covering the floor. Mr. B acknowledges these are hallucinations and had not felt threatened by them until recently, when he wielded the knife. He often tries to talk to them, but they do not reply.
Mr. B also reports intermittent auditory hallucinations including voices at home (non-command) and papers rustling. He also describes tactile hallucinations, where he says he can feel Harry and others prodding him, knocking things out of his hands, or splashing him with water.
Mr. B is admitted to the hospital because he is a danger to himself and others. While on the inpatient unit, Mr. B is pleasant with staff, and eats and sleeps normally; however, he continues to have hallucinations of Harry. Mr. B reports seeing Harry in the hall, and says that Harry pulls out Mr. B’s earpiece and steals his fork. Mr. B also reports hearing a sound “like a bee buzzing.” Mr. B is started on risperidone, 1 mg nightly, for a presumed psychotic disorder.
HISTORY Independent and in good health
Mr. B lives alone and is independent in his activities of daily living. He spends his days at home, often visited by his children, who bring him groceries and other necessities.
Mr. B takes no medications, and has no history of psychiatric treatment; psychotic, manic, or depressive episodes; posttraumatic stress disorder; obsessive-compulsive disorder; or recent emotional stress. His medical history includes chronic progressive hearing loss, which is managed with hearing aids; macular degeneration; and prior bilateral cataract surgeries.
EVALUATION Mental status exam and objective findings
During his evaluation, Mr. B appears well-nourished, and wears glasses and hearing aids. During the interview, he is euthymic with appropriately reactive affect. He is talkative but redirectable, with a goal-directed thought process. Mr. B does not appear to be internally preoccupied. His hearing is impaired, and he often requires questions to be repeated loudly. He is oriented to person, place, and time. There are no signs of delusions, paranoia, thought blocking, thought broadcasting/insertion, or referential thinking. He denies depressed mood, anhedonia, fatigue, sleep changes, or manic symptoms. He denies the occurrence of auditory or visual hallucinations during the evaluation.
Continue to: A neurologic exam shows...
A neurologic exam shows impaired hearing bilaterally and impaired visual acuity. Even with glasses, both eyes have acuity only to finger counting. All other cranial nerves are normal, and Mr. B’s strength, sensation, and cerebellar function are all intact, without rigidity, numbness, or tingling. His gait is steady without a walker, with symmetric arm swing and slight dragging of his feet. His vitals are stable, with normal orthostatic pressures.
Other objective data include a score of 24/30 on the Mini-Mental State Examination, notable for deficits in visuospatial orientation, attention, and calculation, with language and copying limited by poor vision. Mr. B scores 16/22 on the Montreal Cognitive Assessment (MoCA)-Blind (adapted version of MoCA), which is equivalent to a 22/30 on the MoCA, indicating some mild cognitive impairment; however, this modified test is still limited by his poor hearing. His serum and urine laboratory workup show no liver, kidney, metabolic, or electrolyte abnormalities, no sign of infection, negative urine drug screen, and normal B12 and thyroid-stimulating hormone levels. He undergoes a brain MRI, which shows chronic microvascular ischemic change, without mass lesions, infarction, or other pathology.
[polldaddy:10729178]
The authors’ observations
Given Mr. B’s presentation, we ruled out a primary psychotic disorder. He had no psychiatric history, with organized thought, a reactive affect, and no delusions, paranoia, or other psychotic symptoms, all pointing against psychosis. His brain MRI showed no malignancy or other lesions. He had no substance use history to suggest intoxication/withdrawal. His intact attention and orientation did not suggest delirium, and his serum and urine studies were all negative. Although his blaming Harry for knocking things out of his hands could suggest confabulation, Mr. B had no other signs of Korsakoff syndrome, such as ataxia, general confusion, or malnourishment.
We also considered early dementia. There was suspicion for Lewy body dementia given Mr. B’s prominent fluctuating visual hallucinations; however, he displayed no other signs of the disorder, such as parkinsonism, dysautonomia, or sensitivity to the antipsychotic (risperidone 1 mg nightly) started on admission. The presence of 1 core feature of Lewy body dementia—visual hallucinations—indicated a possible, but not probable, diagnosis. Additionally, Mr. B did not have the characteristic features of other types of dementia, such as the stepwise progression of vascular dementia, the behavioral disinhibition of frontotemporal dementia, or the insidious forgetfulness, confusion, language problems, or paranoia that may appear in Alzheimer’s disease. Remarkably, he had a relatively normal brain MRI for his age, given chronic microvascular ischemic changes, and cognitive testing that indicated only mild impairment further pointed against a dementia process.
Charles Bonnet syndrome
Based on Mr. B’s severe vision loss and history of ocular surgeries, we diagnosed him with CBS, described as visual hallucinations in the presence of impaired vision. Charles Bonnet syndrome has been observed in several disorders that affect vision, most commonly macular degeneration, diabetic retinopathy, and glaucoma, with an estimated prevalence of 11% to 39% in older patients with ocular disease.1,2 Visual hallucinations in CBS occur due to ocular disease, likely resulting from changes in afferent sensory input to visual cortical regions of the brain. Table 13 outlines the features of visual hallucinations in patients with CBS. The subsequent disinhibition and spontaneous firing of the visual association cortices leads to the “release hallucinations” of the syndrome.4 The disorder is thought to be significantly underdiagnosed—in a survey of patients with CBS, only 15% had reported their visual hallucinations to a physician.5

Continue to: Mr. B's symptoms...
Mr. B’s symptoms are atypical for CBS, but they fit the diagnosis when considering the entire clinical picture. While hallucinations in CBS are more often simple shapes, complex hallucinations including people and scenes have been noted in several instances.6
Similar to Mr. B’s case, patients with CBS can have recurring figures in their hallucinations, and the images may even move across the visual field.1 Patients with CBS also frequently recognize that their hallucinations are not real, and may or may not be distressed by them.4 Patients with CBS often have hallucinations multiple times daily, lasting from a few seconds to many minutes,7 consistent with Mr. B’s temporary symptoms.
Although auditory and tactile hallucinations are typically not included in CBS, they can also be explained by Mr. B’s significant sensory impairment. Severe hearing impairment in geriatric adults has been associated with auditory hallucinations8; in 1 survey, half of these hallucinations consisted of voices.9 In contrast, tactile hallucinations are not described in sensory deprivation literature. However, in the context of Mr. B’s severe comorbid hearing and vision loss, we propose that these hallucinations reflect his interpretation of sensory events around him, and their integration into his extensive hallucination framework. In other words, Harry poking him and causing him to drop things may be Mr. B’s way of rationalizing events that he has trouble perceiving entirely, or his mild forgetfulness. Mr. B’s social isolation is another factor that may worsen his sensory deprivation and contribute to his extensive hallucinations.10 Additionally, his mild cognitive deficits on testing with chronic microvascular changes on the MRI may suggest a mild vascular-related dementia process, which could also exacerbate his hallucinations. While classic CBS occurs without cognitive impairment, dementia can often co-occur with CBS.11
TREATMENT No significant improvement with medications
During his inpatient stay, Mr. B is treated with risperidone, 1 mg nightly, and is also started on donepezil, 5 mg/d, to treat a possible comorbid dementia. However, he continues to hallucinate without significant improvement.
[polldaddy:10729181]
The authors’ observations
There is no definitive treatment for CBS, and while the hallucinations may spontaneously resolve, per case reports, this typically occurs only as visual loss progresses to total blindness.12 However, many patients can have the hallucinations remit after the underlying ocular etiology is corrected, such as through ocular surgery.13 Other optical interventions, such as special glasses or contact lenses, may help maximize remaining vision.8 In patients without this option, such as Mr. B, there are limited data on beneficial medications for CBS.
Continue to: Evidence for treatment of CBS...
Evidence for treatment of CBS with antipsychotic medications is mixed. Some case studies have found them to be ineffective, while others have found agents such as olanzapine or risperidone to be partially helpful in reducing symptoms.14 There are also data from case reports that may support the use of cholinesterase inhibitors such as donepezil, antiepileptics (carbamazepine, valproate, gabapentin, and clonazepam), and certain antidepressants (escitalopram, venlafaxine) (Table 28,11).3
Addressing loneliness and social isolation
With minimal definitive evidence for pharmacologic management, the most important intervention for treating CBS may be changing the patient’s sensory environment. Specifically, loneliness and social isolation are major exacerbating factors of CBS, and many clinicians advocate for the consistent presence of a sympathetic professional. Reassurance that hallucinations are from ocular disease rather than a primary mental disorder may be extremely relieving for patients.11 A psychoeducation or support group may also be beneficial, not only for giving patients more social contact, but also for teaching them coping skills or strategies to reduce hallucinations, such as distraction, turning on more lights, or even certain eye/blinking movements.11 Table 28,11 (page 49) outlines behavioral interventions for CBS.
Regardless of etiology, Mr. B’s hallucinations significantly affected his quality of life. During his inpatient stay, he was treated with
OUTCOME Home care and family involvement
After discussion with Mr. B and his family about the risks and benefits of medication, the risperidone and donepezil are discontinued. Ultimately, it is determined that Mr. B requires a higher level of home care, both for his safety and to improve his social contact. Mr. B returns home with a combination of a professional home health aide and increased family involvement.
Bottom Line
When evaluating visual hallucinations in older adults, Charles Bonnet syndrome (CBS) should be considered. Sensory deprivation and social isolation are significant risk factors for CBS. While evidence is inconclusive for medical treatment, reassurance and behavioral interventions can often improve symptoms.
Continue to: Related Resources
Related Resources
- Charles Bonnet Syndrome Foundation. http://www.charlesbonnetsyndrome.org
- Schultz G, Melzack R. The Charles Bonnet syndrome: ‘phantom visual images’. Perception. 1991;20:809-825.
- Menon GJ, Rahman I, Menon SJ, et al. Complex visual hallucinations in the visually impaired: the Charles Bonnet syndrome. Surv Ophthalmol. 2003;48(1):58-72.
Drug Brand Names
Carbamazepine • Tegretol
Clonazepam • Klonopin
Donepezil • Aricept
Escitalopram • Lexapro
Gabapentin • Neurontin
Olanzapine • Zyprexa
Risperidone • Risperdal
Valproate • Depakote
Venlafaxine • Effexor
1. Menon GJ, Rahman I, Menon SJ, et al. Complex visual hallucinations in the visually impaired: the Charles Bonnet syndrome. Surv Ophthalmol. 2003;48(1):58-72.
2. Cox TM, Ffytche DH. Negative outcome Charles Bonnet syndrome. Br J Ophthalmol. 2014;98(9):1236-1239.
3. Pelak VS. Visual release hallucinations (Charles Bonnet syndrome). UpToDate. Updated February 5, 2019. Accessed September 17, 2020. https://www.uptodate.com/contents/visual-release-hallucinations-charles-bonnet-syndrome
4. Burke W. The neural basis of Charles Bonnet hallucinations: a hypothesis. J Neurol Neurosurg Psychiatry. 2002;73(5):535-541.
5. Scott IU, Schein OD, Feuer WJ, et al. Visual hallucinations in patients with retinal disease. Am J Ophthalmol. 2001;131(5):590-598.
6. Lepore FE. Spontaneous visual phenomena with visual loss: 104 patients with lesions of retinal and neural afferent pathways. Neurology. 1990;40(3 Pt 1):444-447.
7. Nesher R, Nesher G, Epstein E, et al. Charles Bonnet syndrome in glaucoma patients with low vision. J Glaucoma. 2001;10(5):396-400.
8. Pang L. Hallucinations experienced by visually impaired: Charles Bonnet syndrome. Optom Vis Sci. 2016;93(12):1466-1478.
9. Linszen M, Van Zanten G, Teunisse R, et al. Auditory hallucinations in adults with hearing impairment: a large prevalence study. Psychological Medicine. 2019;49(1):132-139.
10. Teunisse RJ, Cruysberg JR, Hoefnagels WH, et al. Social and psychological characteristics of elderly visually handicapped patients with the Charles Bonnet syndrome. Compr Psychiatry. 1999;40(4):315-319.
11. Eperjesi F, Akbarali A. Rehabilitation in Charles Bonnet syndrome: a review of treatment options. Clin Exp Optom. 2004;87(3):149-152.
12. Fernandez A, Lichtshein G, Vieweg WVR. The Charles Bonnet syndrome: a review. J Nen Ment Dis. 1997;185(3):195-200.
13. Rosenbaum F, Harati Y, Rolak L, et al. Visual hallucinations in sane people: Charles Bonnet syndrome. J Am Geriatr Soc. 1987;35(1):66-68.
14. Coletti Moja M, Milano E, Gasverde S, et al. Olanzapine therapy in hallucinatory visions related to Bonnet syndrome. Neurol Sci. 2005;26(3):168-170.
CASE Visual, auditory, and tactile hallucinations
Mr. B, age 93, is brought to the emergency department by his son after experiencing hallucinations where he reportedly saw and heard individuals in his home. In frustration, Mr. B wielded a knife because he “wanted them to go away.”
Mr. B and his son report that the hallucinations had begun 2 years ago, without prior trauma, medication changes, changes in social situation, or other apparent precipitating events. The hallucinations “come and go,” without preceding symptoms, but have recurring content involving a friendly man named “Harry,” people coming out of the television, 2 children playing, and water covering the floor. Mr. B acknowledges these are hallucinations and had not felt threatened by them until recently, when he wielded the knife. He often tries to talk to them, but they do not reply.
Mr. B also reports intermittent auditory hallucinations including voices at home (non-command) and papers rustling. He also describes tactile hallucinations, where he says he can feel Harry and others prodding him, knocking things out of his hands, or splashing him with water.
Mr. B is admitted to the hospital because he is a danger to himself and others. While on the inpatient unit, Mr. B is pleasant with staff, and eats and sleeps normally; however, he continues to have hallucinations of Harry. Mr. B reports seeing Harry in the hall, and says that Harry pulls out Mr. B’s earpiece and steals his fork. Mr. B also reports hearing a sound “like a bee buzzing.” Mr. B is started on risperidone, 1 mg nightly, for a presumed psychotic disorder.
HISTORY Independent and in good health
Mr. B lives alone and is independent in his activities of daily living. He spends his days at home, often visited by his children, who bring him groceries and other necessities.
Mr. B takes no medications, and has no history of psychiatric treatment; psychotic, manic, or depressive episodes; posttraumatic stress disorder; obsessive-compulsive disorder; or recent emotional stress. His medical history includes chronic progressive hearing loss, which is managed with hearing aids; macular degeneration; and prior bilateral cataract surgeries.
EVALUATION Mental status exam and objective findings
During his evaluation, Mr. B appears well-nourished, and wears glasses and hearing aids. During the interview, he is euthymic with appropriately reactive affect. He is talkative but redirectable, with a goal-directed thought process. Mr. B does not appear to be internally preoccupied. His hearing is impaired, and he often requires questions to be repeated loudly. He is oriented to person, place, and time. There are no signs of delusions, paranoia, thought blocking, thought broadcasting/insertion, or referential thinking. He denies depressed mood, anhedonia, fatigue, sleep changes, or manic symptoms. He denies the occurrence of auditory or visual hallucinations during the evaluation.
Continue to: A neurologic exam shows...
A neurologic exam shows impaired hearing bilaterally and impaired visual acuity. Even with glasses, both eyes have acuity only to finger counting. All other cranial nerves are normal, and Mr. B’s strength, sensation, and cerebellar function are all intact, without rigidity, numbness, or tingling. His gait is steady without a walker, with symmetric arm swing and slight dragging of his feet. His vitals are stable, with normal orthostatic pressures.
Other objective data include a score of 24/30 on the Mini-Mental State Examination, notable for deficits in visuospatial orientation, attention, and calculation, with language and copying limited by poor vision. Mr. B scores 16/22 on the Montreal Cognitive Assessment (MoCA)-Blind (adapted version of MoCA), which is equivalent to a 22/30 on the MoCA, indicating some mild cognitive impairment; however, this modified test is still limited by his poor hearing. His serum and urine laboratory workup show no liver, kidney, metabolic, or electrolyte abnormalities, no sign of infection, negative urine drug screen, and normal B12 and thyroid-stimulating hormone levels. He undergoes a brain MRI, which shows chronic microvascular ischemic change, without mass lesions, infarction, or other pathology.
[polldaddy:10729178]
The authors’ observations
Given Mr. B’s presentation, we ruled out a primary psychotic disorder. He had no psychiatric history, with organized thought, a reactive affect, and no delusions, paranoia, or other psychotic symptoms, all pointing against psychosis. His brain MRI showed no malignancy or other lesions. He had no substance use history to suggest intoxication/withdrawal. His intact attention and orientation did not suggest delirium, and his serum and urine studies were all negative. Although his blaming Harry for knocking things out of his hands could suggest confabulation, Mr. B had no other signs of Korsakoff syndrome, such as ataxia, general confusion, or malnourishment.
We also considered early dementia. There was suspicion for Lewy body dementia given Mr. B’s prominent fluctuating visual hallucinations; however, he displayed no other signs of the disorder, such as parkinsonism, dysautonomia, or sensitivity to the antipsychotic (risperidone 1 mg nightly) started on admission. The presence of 1 core feature of Lewy body dementia—visual hallucinations—indicated a possible, but not probable, diagnosis. Additionally, Mr. B did not have the characteristic features of other types of dementia, such as the stepwise progression of vascular dementia, the behavioral disinhibition of frontotemporal dementia, or the insidious forgetfulness, confusion, language problems, or paranoia that may appear in Alzheimer’s disease. Remarkably, he had a relatively normal brain MRI for his age, given chronic microvascular ischemic changes, and cognitive testing that indicated only mild impairment further pointed against a dementia process.
Charles Bonnet syndrome
Based on Mr. B’s severe vision loss and history of ocular surgeries, we diagnosed him with CBS, described as visual hallucinations in the presence of impaired vision. Charles Bonnet syndrome has been observed in several disorders that affect vision, most commonly macular degeneration, diabetic retinopathy, and glaucoma, with an estimated prevalence of 11% to 39% in older patients with ocular disease.1,2 Visual hallucinations in CBS occur due to ocular disease, likely resulting from changes in afferent sensory input to visual cortical regions of the brain. Table 13 outlines the features of visual hallucinations in patients with CBS. The subsequent disinhibition and spontaneous firing of the visual association cortices leads to the “release hallucinations” of the syndrome.4 The disorder is thought to be significantly underdiagnosed—in a survey of patients with CBS, only 15% had reported their visual hallucinations to a physician.5

Continue to: Mr. B's symptoms...
Mr. B’s symptoms are atypical for CBS, but they fit the diagnosis when considering the entire clinical picture. While hallucinations in CBS are more often simple shapes, complex hallucinations including people and scenes have been noted in several instances.6
Similar to Mr. B’s case, patients with CBS can have recurring figures in their hallucinations, and the images may even move across the visual field.1 Patients with CBS also frequently recognize that their hallucinations are not real, and may or may not be distressed by them.4 Patients with CBS often have hallucinations multiple times daily, lasting from a few seconds to many minutes,7 consistent with Mr. B’s temporary symptoms.
Although auditory and tactile hallucinations are typically not included in CBS, they can also be explained by Mr. B’s significant sensory impairment. Severe hearing impairment in geriatric adults has been associated with auditory hallucinations8; in 1 survey, half of these hallucinations consisted of voices.9 In contrast, tactile hallucinations are not described in sensory deprivation literature. However, in the context of Mr. B’s severe comorbid hearing and vision loss, we propose that these hallucinations reflect his interpretation of sensory events around him, and their integration into his extensive hallucination framework. In other words, Harry poking him and causing him to drop things may be Mr. B’s way of rationalizing events that he has trouble perceiving entirely, or his mild forgetfulness. Mr. B’s social isolation is another factor that may worsen his sensory deprivation and contribute to his extensive hallucinations.10 Additionally, his mild cognitive deficits on testing with chronic microvascular changes on the MRI may suggest a mild vascular-related dementia process, which could also exacerbate his hallucinations. While classic CBS occurs without cognitive impairment, dementia can often co-occur with CBS.11
TREATMENT No significant improvement with medications
During his inpatient stay, Mr. B is treated with risperidone, 1 mg nightly, and is also started on donepezil, 5 mg/d, to treat a possible comorbid dementia. However, he continues to hallucinate without significant improvement.
[polldaddy:10729181]
The authors’ observations
There is no definitive treatment for CBS, and while the hallucinations may spontaneously resolve, per case reports, this typically occurs only as visual loss progresses to total blindness.12 However, many patients can have the hallucinations remit after the underlying ocular etiology is corrected, such as through ocular surgery.13 Other optical interventions, such as special glasses or contact lenses, may help maximize remaining vision.8 In patients without this option, such as Mr. B, there are limited data on beneficial medications for CBS.
Continue to: Evidence for treatment of CBS...
Evidence for treatment of CBS with antipsychotic medications is mixed. Some case studies have found them to be ineffective, while others have found agents such as olanzapine or risperidone to be partially helpful in reducing symptoms.14 There are also data from case reports that may support the use of cholinesterase inhibitors such as donepezil, antiepileptics (carbamazepine, valproate, gabapentin, and clonazepam), and certain antidepressants (escitalopram, venlafaxine) (Table 28,11).3
Addressing loneliness and social isolation
With minimal definitive evidence for pharmacologic management, the most important intervention for treating CBS may be changing the patient’s sensory environment. Specifically, loneliness and social isolation are major exacerbating factors of CBS, and many clinicians advocate for the consistent presence of a sympathetic professional. Reassurance that hallucinations are from ocular disease rather than a primary mental disorder may be extremely relieving for patients.11 A psychoeducation or support group may also be beneficial, not only for giving patients more social contact, but also for teaching them coping skills or strategies to reduce hallucinations, such as distraction, turning on more lights, or even certain eye/blinking movements.11 Table 28,11 (page 49) outlines behavioral interventions for CBS.
Regardless of etiology, Mr. B’s hallucinations significantly affected his quality of life. During his inpatient stay, he was treated with
OUTCOME Home care and family involvement
After discussion with Mr. B and his family about the risks and benefits of medication, the risperidone and donepezil are discontinued. Ultimately, it is determined that Mr. B requires a higher level of home care, both for his safety and to improve his social contact. Mr. B returns home with a combination of a professional home health aide and increased family involvement.
Bottom Line
When evaluating visual hallucinations in older adults, Charles Bonnet syndrome (CBS) should be considered. Sensory deprivation and social isolation are significant risk factors for CBS. While evidence is inconclusive for medical treatment, reassurance and behavioral interventions can often improve symptoms.
Continue to: Related Resources
Related Resources
- Charles Bonnet Syndrome Foundation. http://www.charlesbonnetsyndrome.org
- Schultz G, Melzack R. The Charles Bonnet syndrome: ‘phantom visual images’. Perception. 1991;20:809-825.
- Menon GJ, Rahman I, Menon SJ, et al. Complex visual hallucinations in the visually impaired: the Charles Bonnet syndrome. Surv Ophthalmol. 2003;48(1):58-72.
Drug Brand Names
Carbamazepine • Tegretol
Clonazepam • Klonopin
Donepezil • Aricept
Escitalopram • Lexapro
Gabapentin • Neurontin
Olanzapine • Zyprexa
Risperidone • Risperdal
Valproate • Depakote
Venlafaxine • Effexor
CASE Visual, auditory, and tactile hallucinations
Mr. B, age 93, is brought to the emergency department by his son after experiencing hallucinations where he reportedly saw and heard individuals in his home. In frustration, Mr. B wielded a knife because he “wanted them to go away.”
Mr. B and his son report that the hallucinations had begun 2 years ago, without prior trauma, medication changes, changes in social situation, or other apparent precipitating events. The hallucinations “come and go,” without preceding symptoms, but have recurring content involving a friendly man named “Harry,” people coming out of the television, 2 children playing, and water covering the floor. Mr. B acknowledges these are hallucinations and had not felt threatened by them until recently, when he wielded the knife. He often tries to talk to them, but they do not reply.
Mr. B also reports intermittent auditory hallucinations including voices at home (non-command) and papers rustling. He also describes tactile hallucinations, where he says he can feel Harry and others prodding him, knocking things out of his hands, or splashing him with water.
Mr. B is admitted to the hospital because he is a danger to himself and others. While on the inpatient unit, Mr. B is pleasant with staff, and eats and sleeps normally; however, he continues to have hallucinations of Harry. Mr. B reports seeing Harry in the hall, and says that Harry pulls out Mr. B’s earpiece and steals his fork. Mr. B also reports hearing a sound “like a bee buzzing.” Mr. B is started on risperidone, 1 mg nightly, for a presumed psychotic disorder.
HISTORY Independent and in good health
Mr. B lives alone and is independent in his activities of daily living. He spends his days at home, often visited by his children, who bring him groceries and other necessities.
Mr. B takes no medications, and has no history of psychiatric treatment; psychotic, manic, or depressive episodes; posttraumatic stress disorder; obsessive-compulsive disorder; or recent emotional stress. His medical history includes chronic progressive hearing loss, which is managed with hearing aids; macular degeneration; and prior bilateral cataract surgeries.
EVALUATION Mental status exam and objective findings
During his evaluation, Mr. B appears well-nourished, and wears glasses and hearing aids. During the interview, he is euthymic with appropriately reactive affect. He is talkative but redirectable, with a goal-directed thought process. Mr. B does not appear to be internally preoccupied. His hearing is impaired, and he often requires questions to be repeated loudly. He is oriented to person, place, and time. There are no signs of delusions, paranoia, thought blocking, thought broadcasting/insertion, or referential thinking. He denies depressed mood, anhedonia, fatigue, sleep changes, or manic symptoms. He denies the occurrence of auditory or visual hallucinations during the evaluation.
Continue to: A neurologic exam shows...
A neurologic exam shows impaired hearing bilaterally and impaired visual acuity. Even with glasses, both eyes have acuity only to finger counting. All other cranial nerves are normal, and Mr. B’s strength, sensation, and cerebellar function are all intact, without rigidity, numbness, or tingling. His gait is steady without a walker, with symmetric arm swing and slight dragging of his feet. His vitals are stable, with normal orthostatic pressures.
Other objective data include a score of 24/30 on the Mini-Mental State Examination, notable for deficits in visuospatial orientation, attention, and calculation, with language and copying limited by poor vision. Mr. B scores 16/22 on the Montreal Cognitive Assessment (MoCA)-Blind (adapted version of MoCA), which is equivalent to a 22/30 on the MoCA, indicating some mild cognitive impairment; however, this modified test is still limited by his poor hearing. His serum and urine laboratory workup show no liver, kidney, metabolic, or electrolyte abnormalities, no sign of infection, negative urine drug screen, and normal B12 and thyroid-stimulating hormone levels. He undergoes a brain MRI, which shows chronic microvascular ischemic change, without mass lesions, infarction, or other pathology.
[polldaddy:10729178]
The authors’ observations
Given Mr. B’s presentation, we ruled out a primary psychotic disorder. He had no psychiatric history, with organized thought, a reactive affect, and no delusions, paranoia, or other psychotic symptoms, all pointing against psychosis. His brain MRI showed no malignancy or other lesions. He had no substance use history to suggest intoxication/withdrawal. His intact attention and orientation did not suggest delirium, and his serum and urine studies were all negative. Although his blaming Harry for knocking things out of his hands could suggest confabulation, Mr. B had no other signs of Korsakoff syndrome, such as ataxia, general confusion, or malnourishment.
We also considered early dementia. There was suspicion for Lewy body dementia given Mr. B’s prominent fluctuating visual hallucinations; however, he displayed no other signs of the disorder, such as parkinsonism, dysautonomia, or sensitivity to the antipsychotic (risperidone 1 mg nightly) started on admission. The presence of 1 core feature of Lewy body dementia—visual hallucinations—indicated a possible, but not probable, diagnosis. Additionally, Mr. B did not have the characteristic features of other types of dementia, such as the stepwise progression of vascular dementia, the behavioral disinhibition of frontotemporal dementia, or the insidious forgetfulness, confusion, language problems, or paranoia that may appear in Alzheimer’s disease. Remarkably, he had a relatively normal brain MRI for his age, given chronic microvascular ischemic changes, and cognitive testing that indicated only mild impairment further pointed against a dementia process.
Charles Bonnet syndrome
Based on Mr. B’s severe vision loss and history of ocular surgeries, we diagnosed him with CBS, described as visual hallucinations in the presence of impaired vision. Charles Bonnet syndrome has been observed in several disorders that affect vision, most commonly macular degeneration, diabetic retinopathy, and glaucoma, with an estimated prevalence of 11% to 39% in older patients with ocular disease.1,2 Visual hallucinations in CBS occur due to ocular disease, likely resulting from changes in afferent sensory input to visual cortical regions of the brain. Table 13 outlines the features of visual hallucinations in patients with CBS. The subsequent disinhibition and spontaneous firing of the visual association cortices leads to the “release hallucinations” of the syndrome.4 The disorder is thought to be significantly underdiagnosed—in a survey of patients with CBS, only 15% had reported their visual hallucinations to a physician.5

Continue to: Mr. B's symptoms...
Mr. B’s symptoms are atypical for CBS, but they fit the diagnosis when considering the entire clinical picture. While hallucinations in CBS are more often simple shapes, complex hallucinations including people and scenes have been noted in several instances.6
Similar to Mr. B’s case, patients with CBS can have recurring figures in their hallucinations, and the images may even move across the visual field.1 Patients with CBS also frequently recognize that their hallucinations are not real, and may or may not be distressed by them.4 Patients with CBS often have hallucinations multiple times daily, lasting from a few seconds to many minutes,7 consistent with Mr. B’s temporary symptoms.
Although auditory and tactile hallucinations are typically not included in CBS, they can also be explained by Mr. B’s significant sensory impairment. Severe hearing impairment in geriatric adults has been associated with auditory hallucinations8; in 1 survey, half of these hallucinations consisted of voices.9 In contrast, tactile hallucinations are not described in sensory deprivation literature. However, in the context of Mr. B’s severe comorbid hearing and vision loss, we propose that these hallucinations reflect his interpretation of sensory events around him, and their integration into his extensive hallucination framework. In other words, Harry poking him and causing him to drop things may be Mr. B’s way of rationalizing events that he has trouble perceiving entirely, or his mild forgetfulness. Mr. B’s social isolation is another factor that may worsen his sensory deprivation and contribute to his extensive hallucinations.10 Additionally, his mild cognitive deficits on testing with chronic microvascular changes on the MRI may suggest a mild vascular-related dementia process, which could also exacerbate his hallucinations. While classic CBS occurs without cognitive impairment, dementia can often co-occur with CBS.11
TREATMENT No significant improvement with medications
During his inpatient stay, Mr. B is treated with risperidone, 1 mg nightly, and is also started on donepezil, 5 mg/d, to treat a possible comorbid dementia. However, he continues to hallucinate without significant improvement.
[polldaddy:10729181]
The authors’ observations
There is no definitive treatment for CBS, and while the hallucinations may spontaneously resolve, per case reports, this typically occurs only as visual loss progresses to total blindness.12 However, many patients can have the hallucinations remit after the underlying ocular etiology is corrected, such as through ocular surgery.13 Other optical interventions, such as special glasses or contact lenses, may help maximize remaining vision.8 In patients without this option, such as Mr. B, there are limited data on beneficial medications for CBS.
Continue to: Evidence for treatment of CBS...
Evidence for treatment of CBS with antipsychotic medications is mixed. Some case studies have found them to be ineffective, while others have found agents such as olanzapine or risperidone to be partially helpful in reducing symptoms.14 There are also data from case reports that may support the use of cholinesterase inhibitors such as donepezil, antiepileptics (carbamazepine, valproate, gabapentin, and clonazepam), and certain antidepressants (escitalopram, venlafaxine) (Table 28,11).3
Addressing loneliness and social isolation
With minimal definitive evidence for pharmacologic management, the most important intervention for treating CBS may be changing the patient’s sensory environment. Specifically, loneliness and social isolation are major exacerbating factors of CBS, and many clinicians advocate for the consistent presence of a sympathetic professional. Reassurance that hallucinations are from ocular disease rather than a primary mental disorder may be extremely relieving for patients.11 A psychoeducation or support group may also be beneficial, not only for giving patients more social contact, but also for teaching them coping skills or strategies to reduce hallucinations, such as distraction, turning on more lights, or even certain eye/blinking movements.11 Table 28,11 (page 49) outlines behavioral interventions for CBS.
Regardless of etiology, Mr. B’s hallucinations significantly affected his quality of life. During his inpatient stay, he was treated with
OUTCOME Home care and family involvement
After discussion with Mr. B and his family about the risks and benefits of medication, the risperidone and donepezil are discontinued. Ultimately, it is determined that Mr. B requires a higher level of home care, both for his safety and to improve his social contact. Mr. B returns home with a combination of a professional home health aide and increased family involvement.
Bottom Line
When evaluating visual hallucinations in older adults, Charles Bonnet syndrome (CBS) should be considered. Sensory deprivation and social isolation are significant risk factors for CBS. While evidence is inconclusive for medical treatment, reassurance and behavioral interventions can often improve symptoms.
Continue to: Related Resources
Related Resources
- Charles Bonnet Syndrome Foundation. http://www.charlesbonnetsyndrome.org
- Schultz G, Melzack R. The Charles Bonnet syndrome: ‘phantom visual images’. Perception. 1991;20:809-825.
- Menon GJ, Rahman I, Menon SJ, et al. Complex visual hallucinations in the visually impaired: the Charles Bonnet syndrome. Surv Ophthalmol. 2003;48(1):58-72.
Drug Brand Names
Carbamazepine • Tegretol
Clonazepam • Klonopin
Donepezil • Aricept
Escitalopram • Lexapro
Gabapentin • Neurontin
Olanzapine • Zyprexa
Risperidone • Risperdal
Valproate • Depakote
Venlafaxine • Effexor
1. Menon GJ, Rahman I, Menon SJ, et al. Complex visual hallucinations in the visually impaired: the Charles Bonnet syndrome. Surv Ophthalmol. 2003;48(1):58-72.
2. Cox TM, Ffytche DH. Negative outcome Charles Bonnet syndrome. Br J Ophthalmol. 2014;98(9):1236-1239.
3. Pelak VS. Visual release hallucinations (Charles Bonnet syndrome). UpToDate. Updated February 5, 2019. Accessed September 17, 2020. https://www.uptodate.com/contents/visual-release-hallucinations-charles-bonnet-syndrome
4. Burke W. The neural basis of Charles Bonnet hallucinations: a hypothesis. J Neurol Neurosurg Psychiatry. 2002;73(5):535-541.
5. Scott IU, Schein OD, Feuer WJ, et al. Visual hallucinations in patients with retinal disease. Am J Ophthalmol. 2001;131(5):590-598.
6. Lepore FE. Spontaneous visual phenomena with visual loss: 104 patients with lesions of retinal and neural afferent pathways. Neurology. 1990;40(3 Pt 1):444-447.
7. Nesher R, Nesher G, Epstein E, et al. Charles Bonnet syndrome in glaucoma patients with low vision. J Glaucoma. 2001;10(5):396-400.
8. Pang L. Hallucinations experienced by visually impaired: Charles Bonnet syndrome. Optom Vis Sci. 2016;93(12):1466-1478.
9. Linszen M, Van Zanten G, Teunisse R, et al. Auditory hallucinations in adults with hearing impairment: a large prevalence study. Psychological Medicine. 2019;49(1):132-139.
10. Teunisse RJ, Cruysberg JR, Hoefnagels WH, et al. Social and psychological characteristics of elderly visually handicapped patients with the Charles Bonnet syndrome. Compr Psychiatry. 1999;40(4):315-319.
11. Eperjesi F, Akbarali A. Rehabilitation in Charles Bonnet syndrome: a review of treatment options. Clin Exp Optom. 2004;87(3):149-152.
12. Fernandez A, Lichtshein G, Vieweg WVR. The Charles Bonnet syndrome: a review. J Nen Ment Dis. 1997;185(3):195-200.
13. Rosenbaum F, Harati Y, Rolak L, et al. Visual hallucinations in sane people: Charles Bonnet syndrome. J Am Geriatr Soc. 1987;35(1):66-68.
14. Coletti Moja M, Milano E, Gasverde S, et al. Olanzapine therapy in hallucinatory visions related to Bonnet syndrome. Neurol Sci. 2005;26(3):168-170.
1. Menon GJ, Rahman I, Menon SJ, et al. Complex visual hallucinations in the visually impaired: the Charles Bonnet syndrome. Surv Ophthalmol. 2003;48(1):58-72.
2. Cox TM, Ffytche DH. Negative outcome Charles Bonnet syndrome. Br J Ophthalmol. 2014;98(9):1236-1239.
3. Pelak VS. Visual release hallucinations (Charles Bonnet syndrome). UpToDate. Updated February 5, 2019. Accessed September 17, 2020. https://www.uptodate.com/contents/visual-release-hallucinations-charles-bonnet-syndrome
4. Burke W. The neural basis of Charles Bonnet hallucinations: a hypothesis. J Neurol Neurosurg Psychiatry. 2002;73(5):535-541.
5. Scott IU, Schein OD, Feuer WJ, et al. Visual hallucinations in patients with retinal disease. Am J Ophthalmol. 2001;131(5):590-598.
6. Lepore FE. Spontaneous visual phenomena with visual loss: 104 patients with lesions of retinal and neural afferent pathways. Neurology. 1990;40(3 Pt 1):444-447.
7. Nesher R, Nesher G, Epstein E, et al. Charles Bonnet syndrome in glaucoma patients with low vision. J Glaucoma. 2001;10(5):396-400.
8. Pang L. Hallucinations experienced by visually impaired: Charles Bonnet syndrome. Optom Vis Sci. 2016;93(12):1466-1478.
9. Linszen M, Van Zanten G, Teunisse R, et al. Auditory hallucinations in adults with hearing impairment: a large prevalence study. Psychological Medicine. 2019;49(1):132-139.
10. Teunisse RJ, Cruysberg JR, Hoefnagels WH, et al. Social and psychological characteristics of elderly visually handicapped patients with the Charles Bonnet syndrome. Compr Psychiatry. 1999;40(4):315-319.
11. Eperjesi F, Akbarali A. Rehabilitation in Charles Bonnet syndrome: a review of treatment options. Clin Exp Optom. 2004;87(3):149-152.
12. Fernandez A, Lichtshein G, Vieweg WVR. The Charles Bonnet syndrome: a review. J Nen Ment Dis. 1997;185(3):195-200.
13. Rosenbaum F, Harati Y, Rolak L, et al. Visual hallucinations in sane people: Charles Bonnet syndrome. J Am Geriatr Soc. 1987;35(1):66-68.
14. Coletti Moja M, Milano E, Gasverde S, et al. Olanzapine therapy in hallucinatory visions related to Bonnet syndrome. Neurol Sci. 2005;26(3):168-170.
Pharmacotherapy for alcohol use disorder in patients with hepatic impairment
Mr. S, age 64, presents for an outpatient follow-up after a recent hospital discharge for alcohol detoxification. He reports a long history of alcohol use, which has resulted in numerous hospital admissions. He has recently been receiving care from a gastroenterologist because the results of laboratory testing suggested hepatic impairment (Table 1). Mr. S says that a friend of his was able to stop drinking by taking a medication, and he wonders if he can be prescribed a medication to help him as well.
A chart review shows that Mr. S recently underwent paracentesis, during which 6 liters of fluid were removed. Additionally, an abdominal ultrasound confirmed hepatic cirrhosis.
According to the World Health Organization, alcohol consumption contributes to 3 million deaths annually.2 The highest proportion of these deaths (21.3%) is due to alcohol-associated gastrointestinal complications, including alcoholic and infectious hepatitis, pancreatitis, and cirrhosis. Because the liver is the primary site of ethanol metabolism, it sustains the greatest degree of tissue injury with heavy alcohol consumption. Additionally, the association of harmful use of alcohol with risky sexual behavior may partially explain the higher prevalence of viral hepatitis among persons with alcohol use disorder (AUD) compared with the general population. Alcoholic liver disease (ALD) progresses through several stages, beginning with hepatic steatosis and progressing through alcohol-related hepatitis, fibrosis, cirrhosis, and potentially hepatocellular carcinoma.3
Liver markers of alcohol use
Although biological markers can be used in clinical practice to screen and monitor for alcohol abuse, making a diagnosis of ALD can be challenging. Typically, a history of heavy alcohol consumption in addition to certain physical signs and laboratory tests for liver disease are the best indicators of ALD. However, the clinical assessment can be confounded by patients who deny or minimize how much alcohol they have consumed. Furthermore, physical and laboratory findings may not be specific to ALD.
Liver enzymes, including aspartate aminotransferase (AST), alanine aminotransferase (ALT), and gamma-glutamyltransferase (GGT), have historically been used as the basis of diagnosing ALD. In addition to elevated bilirubin and evidence of macrocytic anemia, elevations in these enzymes may suggest heavy alcohol use, but these values alone are inadequate to establish ALD. Gamma-glutamyltransferase is found in cell membranes of several body tissues, including the liver and spleen, and therefore is not specific to liver damage. However, elevated GGT is the best indicator of excessive alcohol consumption because it has greater sensitivity than AST and ALT.1,3,4
Although these biomarkers are helpful in diagnosing ALD, they lose some of their utility in patients with advanced liver disease. Patients with severe liver dysfunction may not have elevated serum aminotransferase levels because the degree of liver enzyme elevation does not correlate well with the severity of ALD. For example, patients with advanced cirrhosis may have liver enzyme levels that appear normal. However, the pattern of elevation in transaminases can be helpful in making a diagnosis of liver dysfunction; using the ratio of AST to ALT may aid in diagnosing ALD, because AST is elevated more than twice that of ALT in >80% of patients with ALD.1,3,4
Table 21,3,4 shows the progression of ALD from steatohepatitis to alcoholic hepatitis to cirrhosis. In steatohepatitis, transaminitis is present but all other biomarkers normal. In alcoholic hepatitis, transaminitis is present along with elevated alkaline phosphatase, elevated bilirubin, and elevated international normalized ratio (INR). In alcoholic cirrhosis, the AST-to-ALT ratio is >2, and hypoalbuminemia, hyperbilirubinemia, and coagulopathy (evidenced by elevated INR) are present, consistent with long-term liver damage.1,3,4
Continue to: FDA-approved medications
FDA-approved medications
Three medications—acamprosate, naltrexone, and disulfiram—currently are FDA-approved for treating AUD.5,6 Additionally, several other medications have shown varying levels of efficacy in treating patients with AUD but are not FDA-approved for this indication (Table 3).5-8
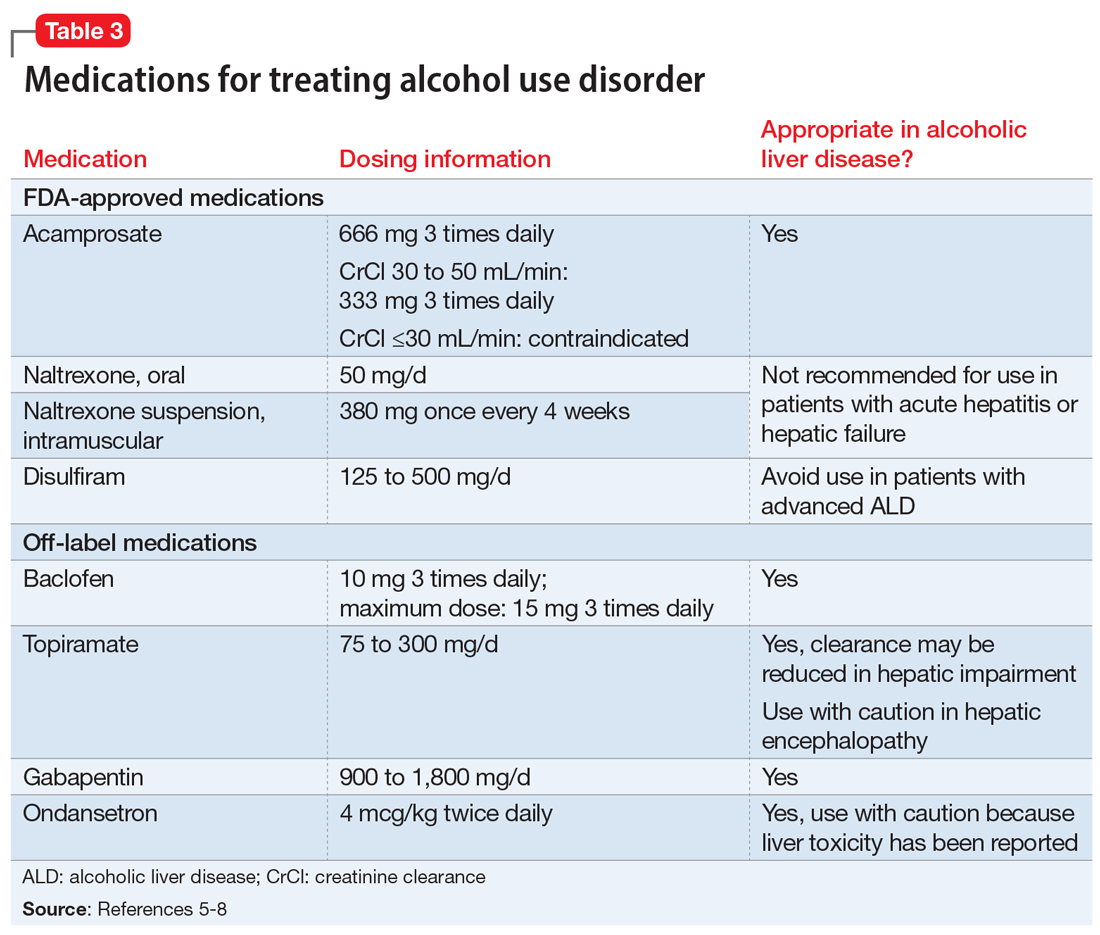
Acamprosate is thought to create a balance of inhibitor and excitatory neurotransmitters by functioning as a glutamate antagonist and gamma-aminobutyric acid (GABA) agonist. This is speculated to aid in abstinence from alcohol. Data suggests that acamprosate may be more effective for maintaining abstinence than for inducing remission in individuals who have not yet detoxified from alcohol. Because of its renal excretion, acamprosate is the only FDA-approved medication for AUD that is not associated with liver toxicity. The most commonly reported adverse effect with acamprosate use is diarrhea.
Naltrexone, a mu-opioid receptor antagonist, is available in both tablet and long-acting IM injection formulations. Naltrexone blocks the binding of endorphins created by alcohol consumption to opioid receptors. This results in diminished dopamine release and is speculated to decrease reward and positive reinforcement with alcohol consumption, leading to fewer heavy drinking days. Due to hepatic metabolism, naltrexone use carries a risk of liver injury. Cases of hepatitis and clinically significant liver dysfunction as well as transient, asymptomatic, hepatic transaminase elevations have been observed in patients who receive naltrexone. Because of the absence of first-pass metabolism, long-acting IM naltrexone may produce less hepatotoxicity than the oral formulation. When the FDA approved both formulations of naltrexone, a “black-box” warning was issued concerning the risk of liver damage; however, these warnings have since been removed from their respective prescribing information.
Disulfiram inhibits acetaldehyde dehydrogenase, resulting in elevated acetaldehyde concentrations after consuming alcohol. In theory, this medication reduces a person’s desire to drink due to the negative physiological and physical effects associated with increased acetaldehyde, including hypotension, flushing, nausea, and vomiting. Although most of these reactions are short-lived, disulfiram can induce hepatotoxicity and liver failure that may prove fatal. Disulfiram should be avoided in patients with advanced ALD.
Off-label medications for AUD
Additional pharmacotherapeutic agents have been evaluated in patients with AUD. Baclofen, topiramate, gabapentin, and ondansetron have shown varying levels of efficacy and pose minimal concern in patients with ALD.
Continue to: Baclofen
Baclofen. Although findings are conflicting, baclofen is the only agent that has been specifically studied for treating AUD in patients with ALD. A GABA B receptor antagonist, baclofen is currently FDA-approved for treating spasticity. In a series of open-label and double-blind studies, baclofen has been shown to effectively reduce alcohol intake, promote abstinence, and prevent relapse.5,6 Further studies identified a possible dose-related response, noting that 20 mg taken 3 times daily may confer additional response over 10 mg taken 3 times daily.5,6 Conversely, the ALPADIR study failed to demonstrate superiority of baclofen vs placebo in the maintenance of abstinence from alcohol despite dosing at 180 mg/d.9 This study did, however, find a significant reduction in alcohol craving in favor of baclofen.9 Further, in a randomized controlled trial (RCT) conducted in veterans with chronic hepatitis C, baclofen 30 mg/d failed to show superiority over placebo with regard to increasing abstinence or reducing alcohol use
Topiramate. A recent meta-analysis found that topiramate use may result in fewer drinking days, heavy drinking days, and number of drinks per drinking day.7 Additionally, topiramate has demonstrated a statistically significant reduction in alcohol craving as well as the ability to decrease all liver function test values.5 This agent should be used with caution in patients with hepatic encephalopathy because the adverse cognitive effects associated with topiramate may confound the clinical course and treatment of such.
Gabapentin. The use of gabapentin to treat patients with AUD is supported by multiple RCTs. In studies that evaluated dose-related response, higher doses of gabapentin (up to 1,800 mg/d) showed greater efficacy than lower doses (ie, 900 mg/d).8 Because gabapentin does not undergo hepatic metabolism, its use in patients with ALD is considered safe. Although the abuse potential of gabapentin is less defined in patients with AUD, there have been reports of abuse in other high-risk populations (ie, those with opioid use disorder, incarcerated persons, and those who misuse prescriptions recreationally).8
Ondansetron is speculated to decrease the reward from alcohol via the down-regulation of dopaminergic neurons. Studies examining ondansetron for patients with AUD have found that it decreases alcohol cravings in those with early-onset alcoholism (initial onset at age ≤25), but not in late-onset alcoholism (initial onset at age >25).5 However, the ondansetron doses used in these trials were very low (4 mcg/kg), and those doses are not available commercially.5
CASE CONTINUED
Following a discussion of available pharmacotherapeutic options for AUD, Mr. S is started on baclofen, 10 mg 3 times daily, with plans for dose titration. At a 2-week follow-up appointment, Mr. S reports that he had not been taking baclofen as often as instructed; however, he denies further alcohol consumption and re-commits to baclofen treatment. Unfortunately, Mr. S is soon admitted to hospice care due to continued decompensation and is unable to attend any additional outpatient follow-up appointments. Three months after his initial outpatient contact, Mr. S dies due to alcoholic cirrhosis.
Related Resources
• Crabb DW, Im GY, Szabo G, et al. Diagnosis and treatment of alcohol-related liver diseases: 2019 practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2020;71(1):306-333.
• Murail AR, Carey WD. Disease management. Liver test interpretation - approach to the patient with liver disease: a guide to commonly used liver tests. Cleveland Clinic Center for Continuing Education. Updated August 2017. www.clevelandclinicmeded. com/medicalpubs/diseasemanagement/hepatology/ guide-to-common-liver-tests/
Drug Brand Names
Acamprosate • Campral
Baclofen • Lioresal
Disulfiram • Antabuse
Gabapentin • Neurontin
Naltrexone • Revia, Vivitrol
Ondansetron • Zofran
Topiramate • Topamax
1. Agrawal S, Dhiman RK, Limdi JK. Evaluation of abnormal liver function tests. Postgrad Med J. 2016;92(1086):223-234.
2. World Health Organization. Global status report on alcohol and health 2018. Published 2018. Accessed November 5, 2020. https://www.who.int/substance_abuse/publications/global_alcohol_report/gsr_2018/en/
3. Osna NA, Donohue TM, Kharbanda KK. Alcoholic liver disease: pathogenesis and current management. Alcohol Res. 2017;38(2):147-161.
4. Leggio L, Lee MR. Treatment of alcohol use disorder in patients with alcoholic liver disease. Am J Med. 2017;130(2):124-134.
5. Addolorato G, Mirijello A, Leggio L, et al. Management of alcohol dependence in patients with liver disease. CNS Drugs. 2013;27(4):287-299.
6. Vuittonet CL, Halse M, Leggio L, et al. Pharmacotherapy for alcoholic patients with alcoholic liver disease. Am J Health Syst Pharm. 2014;71(15):1265-1276.
7. Jonas DE, Amick HR, Feltner C, et al. Pharmacotherapy for adults with alcohol use disorders in outpatient settings. JAMA. 2014;311(18):1889-1900.
8. Mason BJ, Quello S, Shadan F. Gabapentin for the treatment of alcohol use disorder. Expert Opin Investig Drugs. 2018;27(1):113-124.
9. Reynaud M, Aubin HJ, Trinquet F, et al. A randomized, placebo-controlled study of high-dose baclofen in alcohol-dependent patients-the ALPADIR study. Alcohol Alcohol. 2017;52(4):439-446.
10. Hauser P, Fuller B, Ho S, et al. The safety and efficacy of baclofen to reduce alcohol use in veterans with chronic hepatitis C: a randomized controlled trial. Addiction. 2017;112(7):1173-1183.
Mr. S, age 64, presents for an outpatient follow-up after a recent hospital discharge for alcohol detoxification. He reports a long history of alcohol use, which has resulted in numerous hospital admissions. He has recently been receiving care from a gastroenterologist because the results of laboratory testing suggested hepatic impairment (Table 1). Mr. S says that a friend of his was able to stop drinking by taking a medication, and he wonders if he can be prescribed a medication to help him as well.

A chart review shows that Mr. S recently underwent paracentesis, during which 6 liters of fluid were removed. Additionally, an abdominal ultrasound confirmed hepatic cirrhosis.
According to the World Health Organization, alcohol consumption contributes to 3 million deaths annually.2 The highest proportion of these deaths (21.3%) is due to alcohol-associated gastrointestinal complications, including alcoholic and infectious hepatitis, pancreatitis, and cirrhosis. Because the liver is the primary site of ethanol metabolism, it sustains the greatest degree of tissue injury with heavy alcohol consumption. Additionally, the association of harmful use of alcohol with risky sexual behavior may partially explain the higher prevalence of viral hepatitis among persons with alcohol use disorder (AUD) compared with the general population. Alcoholic liver disease (ALD) progresses through several stages, beginning with hepatic steatosis and progressing through alcohol-related hepatitis, fibrosis, cirrhosis, and potentially hepatocellular carcinoma.3
Liver markers of alcohol use
Although biological markers can be used in clinical practice to screen and monitor for alcohol abuse, making a diagnosis of ALD can be challenging. Typically, a history of heavy alcohol consumption in addition to certain physical signs and laboratory tests for liver disease are the best indicators of ALD. However, the clinical assessment can be confounded by patients who deny or minimize how much alcohol they have consumed. Furthermore, physical and laboratory findings may not be specific to ALD.
Liver enzymes, including aspartate aminotransferase (AST), alanine aminotransferase (ALT), and gamma-glutamyltransferase (GGT), have historically been used as the basis of diagnosing ALD. In addition to elevated bilirubin and evidence of macrocytic anemia, elevations in these enzymes may suggest heavy alcohol use, but these values alone are inadequate to establish ALD. Gamma-glutamyltransferase is found in cell membranes of several body tissues, including the liver and spleen, and therefore is not specific to liver damage. However, elevated GGT is the best indicator of excessive alcohol consumption because it has greater sensitivity than AST and ALT.1,3,4
Although these biomarkers are helpful in diagnosing ALD, they lose some of their utility in patients with advanced liver disease. Patients with severe liver dysfunction may not have elevated serum aminotransferase levels because the degree of liver enzyme elevation does not correlate well with the severity of ALD. For example, patients with advanced cirrhosis may have liver enzyme levels that appear normal. However, the pattern of elevation in transaminases can be helpful in making a diagnosis of liver dysfunction; using the ratio of AST to ALT may aid in diagnosing ALD, because AST is elevated more than twice that of ALT in >80% of patients with ALD.1,3,4
Table 21,3,4 shows the progression of ALD from steatohepatitis to alcoholic hepatitis to cirrhosis. In steatohepatitis, transaminitis is present but all other biomarkers normal. In alcoholic hepatitis, transaminitis is present along with elevated alkaline phosphatase, elevated bilirubin, and elevated international normalized ratio (INR). In alcoholic cirrhosis, the AST-to-ALT ratio is >2, and hypoalbuminemia, hyperbilirubinemia, and coagulopathy (evidenced by elevated INR) are present, consistent with long-term liver damage.1,3,4

Continue to: FDA-approved medications
FDA-approved medications
Three medications—acamprosate, naltrexone, and disulfiram—currently are FDA-approved for treating AUD.5,6 Additionally, several other medications have shown varying levels of efficacy in treating patients with AUD but are not FDA-approved for this indication (Table 3).5-8

Acamprosate is thought to create a balance of inhibitor and excitatory neurotransmitters by functioning as a glutamate antagonist and gamma-aminobutyric acid (GABA) agonist. This is speculated to aid in abstinence from alcohol. Data suggests that acamprosate may be more effective for maintaining abstinence than for inducing remission in individuals who have not yet detoxified from alcohol. Because of its renal excretion, acamprosate is the only FDA-approved medication for AUD that is not associated with liver toxicity. The most commonly reported adverse effect with acamprosate use is diarrhea.
Naltrexone, a mu-opioid receptor antagonist, is available in both tablet and long-acting IM injection formulations. Naltrexone blocks the binding of endorphins created by alcohol consumption to opioid receptors. This results in diminished dopamine release and is speculated to decrease reward and positive reinforcement with alcohol consumption, leading to fewer heavy drinking days. Due to hepatic metabolism, naltrexone use carries a risk of liver injury. Cases of hepatitis and clinically significant liver dysfunction as well as transient, asymptomatic, hepatic transaminase elevations have been observed in patients who receive naltrexone. Because of the absence of first-pass metabolism, long-acting IM naltrexone may produce less hepatotoxicity than the oral formulation. When the FDA approved both formulations of naltrexone, a “black-box” warning was issued concerning the risk of liver damage; however, these warnings have since been removed from their respective prescribing information.
Disulfiram inhibits acetaldehyde dehydrogenase, resulting in elevated acetaldehyde concentrations after consuming alcohol. In theory, this medication reduces a person’s desire to drink due to the negative physiological and physical effects associated with increased acetaldehyde, including hypotension, flushing, nausea, and vomiting. Although most of these reactions are short-lived, disulfiram can induce hepatotoxicity and liver failure that may prove fatal. Disulfiram should be avoided in patients with advanced ALD.
Off-label medications for AUD
Additional pharmacotherapeutic agents have been evaluated in patients with AUD. Baclofen, topiramate, gabapentin, and ondansetron have shown varying levels of efficacy and pose minimal concern in patients with ALD.
Continue to: Baclofen
Baclofen. Although findings are conflicting, baclofen is the only agent that has been specifically studied for treating AUD in patients with ALD. A GABA B receptor antagonist, baclofen is currently FDA-approved for treating spasticity. In a series of open-label and double-blind studies, baclofen has been shown to effectively reduce alcohol intake, promote abstinence, and prevent relapse.5,6 Further studies identified a possible dose-related response, noting that 20 mg taken 3 times daily may confer additional response over 10 mg taken 3 times daily.5,6 Conversely, the ALPADIR study failed to demonstrate superiority of baclofen vs placebo in the maintenance of abstinence from alcohol despite dosing at 180 mg/d.9 This study did, however, find a significant reduction in alcohol craving in favor of baclofen.9 Further, in a randomized controlled trial (RCT) conducted in veterans with chronic hepatitis C, baclofen 30 mg/d failed to show superiority over placebo with regard to increasing abstinence or reducing alcohol use
Topiramate. A recent meta-analysis found that topiramate use may result in fewer drinking days, heavy drinking days, and number of drinks per drinking day.7 Additionally, topiramate has demonstrated a statistically significant reduction in alcohol craving as well as the ability to decrease all liver function test values.5 This agent should be used with caution in patients with hepatic encephalopathy because the adverse cognitive effects associated with topiramate may confound the clinical course and treatment of such.
Gabapentin. The use of gabapentin to treat patients with AUD is supported by multiple RCTs. In studies that evaluated dose-related response, higher doses of gabapentin (up to 1,800 mg/d) showed greater efficacy than lower doses (ie, 900 mg/d).8 Because gabapentin does not undergo hepatic metabolism, its use in patients with ALD is considered safe. Although the abuse potential of gabapentin is less defined in patients with AUD, there have been reports of abuse in other high-risk populations (ie, those with opioid use disorder, incarcerated persons, and those who misuse prescriptions recreationally).8
Ondansetron is speculated to decrease the reward from alcohol via the down-regulation of dopaminergic neurons. Studies examining ondansetron for patients with AUD have found that it decreases alcohol cravings in those with early-onset alcoholism (initial onset at age ≤25), but not in late-onset alcoholism (initial onset at age >25).5 However, the ondansetron doses used in these trials were very low (4 mcg/kg), and those doses are not available commercially.5
CASE CONTINUED
Following a discussion of available pharmacotherapeutic options for AUD, Mr. S is started on baclofen, 10 mg 3 times daily, with plans for dose titration. At a 2-week follow-up appointment, Mr. S reports that he had not been taking baclofen as often as instructed; however, he denies further alcohol consumption and re-commits to baclofen treatment. Unfortunately, Mr. S is soon admitted to hospice care due to continued decompensation and is unable to attend any additional outpatient follow-up appointments. Three months after his initial outpatient contact, Mr. S dies due to alcoholic cirrhosis.
Related Resources
• Crabb DW, Im GY, Szabo G, et al. Diagnosis and treatment of alcohol-related liver diseases: 2019 practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2020;71(1):306-333.
• Murail AR, Carey WD. Disease management. Liver test interpretation - approach to the patient with liver disease: a guide to commonly used liver tests. Cleveland Clinic Center for Continuing Education. Updated August 2017. www.clevelandclinicmeded. com/medicalpubs/diseasemanagement/hepatology/ guide-to-common-liver-tests/
Drug Brand Names
Acamprosate • Campral
Baclofen • Lioresal
Disulfiram • Antabuse
Gabapentin • Neurontin
Naltrexone • Revia, Vivitrol
Ondansetron • Zofran
Topiramate • Topamax
Mr. S, age 64, presents for an outpatient follow-up after a recent hospital discharge for alcohol detoxification. He reports a long history of alcohol use, which has resulted in numerous hospital admissions. He has recently been receiving care from a gastroenterologist because the results of laboratory testing suggested hepatic impairment (Table 1). Mr. S says that a friend of his was able to stop drinking by taking a medication, and he wonders if he can be prescribed a medication to help him as well.

A chart review shows that Mr. S recently underwent paracentesis, during which 6 liters of fluid were removed. Additionally, an abdominal ultrasound confirmed hepatic cirrhosis.
According to the World Health Organization, alcohol consumption contributes to 3 million deaths annually.2 The highest proportion of these deaths (21.3%) is due to alcohol-associated gastrointestinal complications, including alcoholic and infectious hepatitis, pancreatitis, and cirrhosis. Because the liver is the primary site of ethanol metabolism, it sustains the greatest degree of tissue injury with heavy alcohol consumption. Additionally, the association of harmful use of alcohol with risky sexual behavior may partially explain the higher prevalence of viral hepatitis among persons with alcohol use disorder (AUD) compared with the general population. Alcoholic liver disease (ALD) progresses through several stages, beginning with hepatic steatosis and progressing through alcohol-related hepatitis, fibrosis, cirrhosis, and potentially hepatocellular carcinoma.3
Liver markers of alcohol use
Although biological markers can be used in clinical practice to screen and monitor for alcohol abuse, making a diagnosis of ALD can be challenging. Typically, a history of heavy alcohol consumption in addition to certain physical signs and laboratory tests for liver disease are the best indicators of ALD. However, the clinical assessment can be confounded by patients who deny or minimize how much alcohol they have consumed. Furthermore, physical and laboratory findings may not be specific to ALD.
Liver enzymes, including aspartate aminotransferase (AST), alanine aminotransferase (ALT), and gamma-glutamyltransferase (GGT), have historically been used as the basis of diagnosing ALD. In addition to elevated bilirubin and evidence of macrocytic anemia, elevations in these enzymes may suggest heavy alcohol use, but these values alone are inadequate to establish ALD. Gamma-glutamyltransferase is found in cell membranes of several body tissues, including the liver and spleen, and therefore is not specific to liver damage. However, elevated GGT is the best indicator of excessive alcohol consumption because it has greater sensitivity than AST and ALT.1,3,4
Although these biomarkers are helpful in diagnosing ALD, they lose some of their utility in patients with advanced liver disease. Patients with severe liver dysfunction may not have elevated serum aminotransferase levels because the degree of liver enzyme elevation does not correlate well with the severity of ALD. For example, patients with advanced cirrhosis may have liver enzyme levels that appear normal. However, the pattern of elevation in transaminases can be helpful in making a diagnosis of liver dysfunction; using the ratio of AST to ALT may aid in diagnosing ALD, because AST is elevated more than twice that of ALT in >80% of patients with ALD.1,3,4
Table 21,3,4 shows the progression of ALD from steatohepatitis to alcoholic hepatitis to cirrhosis. In steatohepatitis, transaminitis is present but all other biomarkers normal. In alcoholic hepatitis, transaminitis is present along with elevated alkaline phosphatase, elevated bilirubin, and elevated international normalized ratio (INR). In alcoholic cirrhosis, the AST-to-ALT ratio is >2, and hypoalbuminemia, hyperbilirubinemia, and coagulopathy (evidenced by elevated INR) are present, consistent with long-term liver damage.1,3,4

Continue to: FDA-approved medications
FDA-approved medications
Three medications—acamprosate, naltrexone, and disulfiram—currently are FDA-approved for treating AUD.5,6 Additionally, several other medications have shown varying levels of efficacy in treating patients with AUD but are not FDA-approved for this indication (Table 3).5-8

Acamprosate is thought to create a balance of inhibitor and excitatory neurotransmitters by functioning as a glutamate antagonist and gamma-aminobutyric acid (GABA) agonist. This is speculated to aid in abstinence from alcohol. Data suggests that acamprosate may be more effective for maintaining abstinence than for inducing remission in individuals who have not yet detoxified from alcohol. Because of its renal excretion, acamprosate is the only FDA-approved medication for AUD that is not associated with liver toxicity. The most commonly reported adverse effect with acamprosate use is diarrhea.
Naltrexone, a mu-opioid receptor antagonist, is available in both tablet and long-acting IM injection formulations. Naltrexone blocks the binding of endorphins created by alcohol consumption to opioid receptors. This results in diminished dopamine release and is speculated to decrease reward and positive reinforcement with alcohol consumption, leading to fewer heavy drinking days. Due to hepatic metabolism, naltrexone use carries a risk of liver injury. Cases of hepatitis and clinically significant liver dysfunction as well as transient, asymptomatic, hepatic transaminase elevations have been observed in patients who receive naltrexone. Because of the absence of first-pass metabolism, long-acting IM naltrexone may produce less hepatotoxicity than the oral formulation. When the FDA approved both formulations of naltrexone, a “black-box” warning was issued concerning the risk of liver damage; however, these warnings have since been removed from their respective prescribing information.
Disulfiram inhibits acetaldehyde dehydrogenase, resulting in elevated acetaldehyde concentrations after consuming alcohol. In theory, this medication reduces a person’s desire to drink due to the negative physiological and physical effects associated with increased acetaldehyde, including hypotension, flushing, nausea, and vomiting. Although most of these reactions are short-lived, disulfiram can induce hepatotoxicity and liver failure that may prove fatal. Disulfiram should be avoided in patients with advanced ALD.
Off-label medications for AUD
Additional pharmacotherapeutic agents have been evaluated in patients with AUD. Baclofen, topiramate, gabapentin, and ondansetron have shown varying levels of efficacy and pose minimal concern in patients with ALD.
Continue to: Baclofen
Baclofen. Although findings are conflicting, baclofen is the only agent that has been specifically studied for treating AUD in patients with ALD. A GABA B receptor antagonist, baclofen is currently FDA-approved for treating spasticity. In a series of open-label and double-blind studies, baclofen has been shown to effectively reduce alcohol intake, promote abstinence, and prevent relapse.5,6 Further studies identified a possible dose-related response, noting that 20 mg taken 3 times daily may confer additional response over 10 mg taken 3 times daily.5,6 Conversely, the ALPADIR study failed to demonstrate superiority of baclofen vs placebo in the maintenance of abstinence from alcohol despite dosing at 180 mg/d.9 This study did, however, find a significant reduction in alcohol craving in favor of baclofen.9 Further, in a randomized controlled trial (RCT) conducted in veterans with chronic hepatitis C, baclofen 30 mg/d failed to show superiority over placebo with regard to increasing abstinence or reducing alcohol use
Topiramate. A recent meta-analysis found that topiramate use may result in fewer drinking days, heavy drinking days, and number of drinks per drinking day.7 Additionally, topiramate has demonstrated a statistically significant reduction in alcohol craving as well as the ability to decrease all liver function test values.5 This agent should be used with caution in patients with hepatic encephalopathy because the adverse cognitive effects associated with topiramate may confound the clinical course and treatment of such.
Gabapentin. The use of gabapentin to treat patients with AUD is supported by multiple RCTs. In studies that evaluated dose-related response, higher doses of gabapentin (up to 1,800 mg/d) showed greater efficacy than lower doses (ie, 900 mg/d).8 Because gabapentin does not undergo hepatic metabolism, its use in patients with ALD is considered safe. Although the abuse potential of gabapentin is less defined in patients with AUD, there have been reports of abuse in other high-risk populations (ie, those with opioid use disorder, incarcerated persons, and those who misuse prescriptions recreationally).8
Ondansetron is speculated to decrease the reward from alcohol via the down-regulation of dopaminergic neurons. Studies examining ondansetron for patients with AUD have found that it decreases alcohol cravings in those with early-onset alcoholism (initial onset at age ≤25), but not in late-onset alcoholism (initial onset at age >25).5 However, the ondansetron doses used in these trials were very low (4 mcg/kg), and those doses are not available commercially.5
CASE CONTINUED
Following a discussion of available pharmacotherapeutic options for AUD, Mr. S is started on baclofen, 10 mg 3 times daily, with plans for dose titration. At a 2-week follow-up appointment, Mr. S reports that he had not been taking baclofen as often as instructed; however, he denies further alcohol consumption and re-commits to baclofen treatment. Unfortunately, Mr. S is soon admitted to hospice care due to continued decompensation and is unable to attend any additional outpatient follow-up appointments. Three months after his initial outpatient contact, Mr. S dies due to alcoholic cirrhosis.
Related Resources
• Crabb DW, Im GY, Szabo G, et al. Diagnosis and treatment of alcohol-related liver diseases: 2019 practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2020;71(1):306-333.
• Murail AR, Carey WD. Disease management. Liver test interpretation - approach to the patient with liver disease: a guide to commonly used liver tests. Cleveland Clinic Center for Continuing Education. Updated August 2017. www.clevelandclinicmeded. com/medicalpubs/diseasemanagement/hepatology/ guide-to-common-liver-tests/
Drug Brand Names
Acamprosate • Campral
Baclofen • Lioresal
Disulfiram • Antabuse
Gabapentin • Neurontin
Naltrexone • Revia, Vivitrol
Ondansetron • Zofran
Topiramate • Topamax
1. Agrawal S, Dhiman RK, Limdi JK. Evaluation of abnormal liver function tests. Postgrad Med J. 2016;92(1086):223-234.
2. World Health Organization. Global status report on alcohol and health 2018. Published 2018. Accessed November 5, 2020. https://www.who.int/substance_abuse/publications/global_alcohol_report/gsr_2018/en/
3. Osna NA, Donohue TM, Kharbanda KK. Alcoholic liver disease: pathogenesis and current management. Alcohol Res. 2017;38(2):147-161.
4. Leggio L, Lee MR. Treatment of alcohol use disorder in patients with alcoholic liver disease. Am J Med. 2017;130(2):124-134.
5. Addolorato G, Mirijello A, Leggio L, et al. Management of alcohol dependence in patients with liver disease. CNS Drugs. 2013;27(4):287-299.
6. Vuittonet CL, Halse M, Leggio L, et al. Pharmacotherapy for alcoholic patients with alcoholic liver disease. Am J Health Syst Pharm. 2014;71(15):1265-1276.
7. Jonas DE, Amick HR, Feltner C, et al. Pharmacotherapy for adults with alcohol use disorders in outpatient settings. JAMA. 2014;311(18):1889-1900.
8. Mason BJ, Quello S, Shadan F. Gabapentin for the treatment of alcohol use disorder. Expert Opin Investig Drugs. 2018;27(1):113-124.
9. Reynaud M, Aubin HJ, Trinquet F, et al. A randomized, placebo-controlled study of high-dose baclofen in alcohol-dependent patients-the ALPADIR study. Alcohol Alcohol. 2017;52(4):439-446.
10. Hauser P, Fuller B, Ho S, et al. The safety and efficacy of baclofen to reduce alcohol use in veterans with chronic hepatitis C: a randomized controlled trial. Addiction. 2017;112(7):1173-1183.
1. Agrawal S, Dhiman RK, Limdi JK. Evaluation of abnormal liver function tests. Postgrad Med J. 2016;92(1086):223-234.
2. World Health Organization. Global status report on alcohol and health 2018. Published 2018. Accessed November 5, 2020. https://www.who.int/substance_abuse/publications/global_alcohol_report/gsr_2018/en/
3. Osna NA, Donohue TM, Kharbanda KK. Alcoholic liver disease: pathogenesis and current management. Alcohol Res. 2017;38(2):147-161.
4. Leggio L, Lee MR. Treatment of alcohol use disorder in patients with alcoholic liver disease. Am J Med. 2017;130(2):124-134.
5. Addolorato G, Mirijello A, Leggio L, et al. Management of alcohol dependence in patients with liver disease. CNS Drugs. 2013;27(4):287-299.
6. Vuittonet CL, Halse M, Leggio L, et al. Pharmacotherapy for alcoholic patients with alcoholic liver disease. Am J Health Syst Pharm. 2014;71(15):1265-1276.
7. Jonas DE, Amick HR, Feltner C, et al. Pharmacotherapy for adults with alcohol use disorders in outpatient settings. JAMA. 2014;311(18):1889-1900.
8. Mason BJ, Quello S, Shadan F. Gabapentin for the treatment of alcohol use disorder. Expert Opin Investig Drugs. 2018;27(1):113-124.
9. Reynaud M, Aubin HJ, Trinquet F, et al. A randomized, placebo-controlled study of high-dose baclofen in alcohol-dependent patients-the ALPADIR study. Alcohol Alcohol. 2017;52(4):439-446.
10. Hauser P, Fuller B, Ho S, et al. The safety and efficacy of baclofen to reduce alcohol use in veterans with chronic hepatitis C: a randomized controlled trial. Addiction. 2017;112(7):1173-1183.
The COPD patient who couldn’t stop worrying
CASE A passive wish to die
Ms. M, age 76, has a history of major depressive disorder, unspecified anxiety disorder, and severe chronic obstructive pulmonary disease (COPD), for which she requires supplemental oxygen. She is admitted to a psychiatric hospital after several months of increased dysphoria, rumination, anhedonia, and a passive wish to die. She also has a decreased appetite and has lost 10 lb, experiences frequent daily episodes of shortness of breath and associated racing thoughts, and has a rapid heart rate.
HISTORY Past medication trials
In addition to COPD, Ms. M’s medical history includes hypertension. Past psychotropic medication trials used to treat her depression and anxiety have included aripiprazole, 5 mg/d; duloxetine, 60 mg/d; fluoxetine, 40 mg/d; mirtazapine, 30 mg nightly; buspirone, 10 mg twice daily; and clonazepam, 0.5 mg twice daily. She has no history of psychotherapy, and because of her uncontrolled anxiety and depression, she has never completed a pulmonary rehabilitation program.
Her current medications include salmeterol, 50 mcg inhaled twice daily, for COPD; amlodipine, 10 mg/d, for hypertension; buspirone, 10 mg twice daily, for anxiety; and duloxetine, 60 mg/d, for depression.
EXAMINATION No evidence of dementia
On examination, Ms. M is alert and oriented to person, place, date, and situation. Overall, she has mild difficulty with attention and short-term recall, which appears to be due to poor effort; intact long-term memory; and is able to abstract appropriately. There is no evidence of dementia.
A mental status exam reveals a frail, elderly woman with fair-to-poor hygiene, cooperative behavior, slowed motor activity, slowed speech with low volume, low mood, and depressed affect with constricted range. Her thought process is linear, her thought content includes passive death wishes, and she does not have hallucinations.
Bitemporal electroconvulsive therapy (ECT), 1.0 ms pulse width at 1.5 times Ms. M’s seizure threshold 3 times weekly, is initiated to treat her depression, with seizure duration averaging 45 seconds for each session. She receives a total of 8 treatments over the course of admission. Buspirone, 10 mg twice daily, is stopped shortly after admission, but she continues to receive duloxetine, 60 mg/d. Ms. M continues to have shortness of breath, palpitations, fearful ruminations about the future, and difficulty falling asleep.
[polldaddy:10673878]
The authors’ observations
The treatment team explores other options, such as benzodiazepines, psychotherapy modalities, and mindfulness exercises, to treat Ms. M’s anxiety and comorbid COPD. Lorazepam, 0.5 mg twice daily, was chosen to treat her acute anxiety. Due to Ms. M’s need for supplemental oxygen, the treatment team attempted to mitigate the risk of using a benzodiazepine by limiting its use to the minimum effective dose. The teams also looked for alternative therapies.
Continue to: Evalution of anxiety...
Evaluation of anxiety and depression in a patient with COPD is complicated by a high degree of symptom overlap. Patients with COPD may experience anxiety symptoms such as shortness of breath, rapid heart rate, numbness/tingling, and racing thoughts, and/or depressive symptoms such as decreased energy, impaired sleep, and impaired concentration. It can therefore be difficult to discern if a symptom is attributable to the physical diagnosis, the psychiatric diagnosis, or a combination of both. Catastrophic thinking about mild physical symptoms is common in patients with COPD. This can lead to hyperventilation and hypocapnia (manifested by lightheadedness, dizziness, paresthesia, and altered consciousness), with a reciprocally escalating cascade of anxiety and somatic symptoms.1
First-line therapy for anxiety disorders with comorbid COPD is CBT and other nonpharmacologic interventions.2,3
Although there is little evidence that traditional pharmacologic treatments (eg, antidepressants, benzodiazepines) have a statistically significant effect on anxiety and depression in COPD, studies have found that they have some clinical benefit.3 Risks, however, limit the utility of certain agents. Sedative-hypnotics potentially decrease respiratory drive and, particularly in older patients, antidepressants’ sedating effects can increase the risk of falls3 leading to increased morbidity, hospitalization, and mortality.
TREATMENT Mindfulness techniques and meditation
Ms. M’s symptoms show no improvement with the addition of lorazepam, 0.5 mg twice daily. A clinician teaches Ms. M mindfulness techniques, and she begins a trial of daily, individual, guided meditation using a meditation app. Respiratory therapists also instruct her on controlled breathing techniques such as pursed-lips breathing, diaphragmatic breathing, and deep breathing. They also encourage Ms. M to participate in the daily exercise group while on the unit.
[polldaddy:10673881]
The authors’ observations
Research indicates that low doses of opioids are safe and effective for refractory breathlessness in patients with severe COPD(those with an arterial partial pressure of oxygen ≤55 mm Hg or arterial oxygen saturation ≤88%).6,7
Continue to: The current opioid crisis...
The current opioid crisis prompts additional caution in prescribing, especially when considering using short-acting, immediate-release opioids such as morphine, which have a greater potential for abuse and dependence.
Many patients with COPD in the end-of-life phase and in severe pain or discomfort due to the advanced stages of their illness receive opioids as part of palliative care. Patients with COPD whose medical care is predominantly palliative may benefit greatly from being prescribed opioids. Most patients with COPD who find relief from low-dose opioids usually have 6 to 12 months to live, and low-dose opioids may help them obtain the best possible quality of life.
Choosing opioids as a treatment involves the risk of physiologic dependence and opioid use disorder. For Ms. M, the potential benefits were thought to outweigh such risks.
OUTCOME Breathlessness improves, anxiety decreases
Ms. M’s lorazepam is discontinued, and immediate-release morphine is prescribed at a low dose of 1 mg/d on an as-needed basis for anxiety with good effect. Ms. M’s breathlessness improves, leading to an overall decrease in anxiety. She does not experience sedation, confusion, or adverse respiratory effects.
Ms. M’s anxiety and depression improve over the course of the hospitalization with this regimen. On hospital Day 25, she is discharged with a plan to continue duloxetine, 60 mg/d, ECT twice weekly, and low-dose morphine, 1 mg/d, as needed for anxiety. She is referred for pulmonary rehabilitation and CBT to maintain remission.
[polldaddy:10673882]
Continue to: The authors' observations
The authors’ observations
Ms. M’s case highlights several challenges associated with treating psychiatric illness in a patient with a chronic medical illness. The relationship between COPD, anxiety, and depression is complex, and is associated with reduced quality of life, increasing severity of pulmonary disease, increased dyspnea, a sense of loss and inability to cope, and decreased self-efficacy and adherence to treatment.9-11
Bottom Line
When traditional antidepressant and anxiolytic therapies have not sufficiently helped, consider low-dose, once-daily opioids to address refractory breathlessness in a patient with COPD with comorbid anxiety and depression. This treatment can lead patients to participate in rehabilitation therapies and improve their quality of life.
Related Resources
- Alexopoulos G, Kiosses D, Sirey J, et al. Untangling therapeutic ingredients of a personalized intervention for patients with depression and severe COPD. Am J Geriatr Psychiatry. 2014;22(11):1316-1324.
- Jackson D, Banerjee S, Sirey J, et al. Two interventions for patients with major depression and severe chronic obstructive pulmonary disease: impact on quality of life. Am J Geriatr Psychiatry. 2018;27(5):502-511.
Drug Brand Names
Amlodipine • Norvasc
Aripiprazole • Abilify
Buspirone • Buspar
Clonazepam • Klonopin
Duloxetine • Cymbalta
Fluoxetine • Prozac
Hydromorphone • Dilaudid
Levodopa • Sinemet
Lorazepam • Ativan
Mirtazapine • Remeron
Morphine • MS Contin
Naloxone • Narcan
Oxycodone • Oxycontin
Salmeterol • Serevent Diskus
1. Harnett D. The difficult-to-treat psychiatric patient with comorbid medical illness. In: Dewan M, Pies R, eds. The difficult-to-treat psychiatric patient. Washington, DC: American Psychiatric Association Publishing; 2001:325-357.
2. Panagioti M, Scott C, Blakemore A, et al. Overview of the prevalence, impact, and management of depression and anxiety in chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2014;9:1289-1306.
3. Cafarella P, Effing T, Usmani ZA, et al. Treatments for anxiety and depression in patients with chronic obstructive pulmonary disease: a literature review. Respirology. 2012;17(4):627-638.
4. Heslop-Marshall K, Baker C, Carrick-Sen D, et al. Randomised controlled trial of cognitive behavioural therapy in COPD. ERJ Open Res. 2018;4:00094-2018. doi: 10.1183/23120541.00094-2018.
5. de Godoy DV, de Godoy RF. A randomized controlled trial of the effect of psychotherapy on anxiety and depression in chronic obstructive pulmonary disease. Arch Phys Med Rehabil. 2003;84(8):1154-1157.
6. Abernethy A, Currow D, Frith P, et al. Randomised, double blind, placebo controlled crossover trial of sustained release morphine for the management of refractory dyspnoea. BMJ. 2003;327(7414):523-528.
7. Janowiak P, Krajnik M, Podolec Z, et al. Dosimetrically administered nebulized morphine for breathlessness in very severe chronic obstructive pulmonary disease: a randomized, controlled trial. BMC Pulm Med. 2017;17:186.
8. Rocker G, Horton R, Currow D, et al. Palliation of dyspnoea in advanced COPD: revisiting a role for opioids. Thorax. 2009;64(10):910-915.
9. Pooler A, Beech R. Examining the relationship between anxiety and depression and exacerbations of COPD which result in hospital admission: a systematic review. Int J Chron Obstruct Pulmon Dis. 2014;9:315-330.
10. Carmen Valenza M, Valenza-Peña G, Torres-Sánchez I, et al. Effectiveness of controlled breathing techniques on anxiety and depression in hospitalized patients with COPD: a randomized clinical trial. Respir Care. 2014;59(2):209-215.
11. Pollok J, van Agteren J, Esterman A, et al. Psychological therapies for the treatment of depression in chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2019;3:CD012347. doi: 10.1002/14651858.CD012347.pub2.
12. Roberts N, Kidd L, Kirkwood K, et al. A systematic review of the content and delivery of education in pulmonary rehabilitation programmes. Respiratory Medicine. 2018;145:161-181.
13. Pumar M, Gray C, Walsh J, et al. Anxiety and depression-important psychological comorbidities of COPD. J Thorac Dis. 2014;6(11):1615-1631.
14. Alexopoulos G, Kiosses D, Sirey J, et al. Untangling therapeutic ingredients of a personalized intervention for patients with depression and severe COPD. Am J Geriatr Psychiatry. 2014;22(11):1316-1324.
15. Jackson D, Banerjee S, Sirey J, et al. Two interventions for patients with major depression and severe chronic obstructive pulmonary disease: impact on quality of life. Am J Geriatr Psychiatry. 2018;27(5):502-511.
CASE A passive wish to die
Ms. M, age 76, has a history of major depressive disorder, unspecified anxiety disorder, and severe chronic obstructive pulmonary disease (COPD), for which she requires supplemental oxygen. She is admitted to a psychiatric hospital after several months of increased dysphoria, rumination, anhedonia, and a passive wish to die. She also has a decreased appetite and has lost 10 lb, experiences frequent daily episodes of shortness of breath and associated racing thoughts, and has a rapid heart rate.
HISTORY Past medication trials
In addition to COPD, Ms. M’s medical history includes hypertension. Past psychotropic medication trials used to treat her depression and anxiety have included aripiprazole, 5 mg/d; duloxetine, 60 mg/d; fluoxetine, 40 mg/d; mirtazapine, 30 mg nightly; buspirone, 10 mg twice daily; and clonazepam, 0.5 mg twice daily. She has no history of psychotherapy, and because of her uncontrolled anxiety and depression, she has never completed a pulmonary rehabilitation program.
Her current medications include salmeterol, 50 mcg inhaled twice daily, for COPD; amlodipine, 10 mg/d, for hypertension; buspirone, 10 mg twice daily, for anxiety; and duloxetine, 60 mg/d, for depression.
EXAMINATION No evidence of dementia
On examination, Ms. M is alert and oriented to person, place, date, and situation. Overall, she has mild difficulty with attention and short-term recall, which appears to be due to poor effort; intact long-term memory; and is able to abstract appropriately. There is no evidence of dementia.
A mental status exam reveals a frail, elderly woman with fair-to-poor hygiene, cooperative behavior, slowed motor activity, slowed speech with low volume, low mood, and depressed affect with constricted range. Her thought process is linear, her thought content includes passive death wishes, and she does not have hallucinations.
Bitemporal electroconvulsive therapy (ECT), 1.0 ms pulse width at 1.5 times Ms. M’s seizure threshold 3 times weekly, is initiated to treat her depression, with seizure duration averaging 45 seconds for each session. She receives a total of 8 treatments over the course of admission. Buspirone, 10 mg twice daily, is stopped shortly after admission, but she continues to receive duloxetine, 60 mg/d. Ms. M continues to have shortness of breath, palpitations, fearful ruminations about the future, and difficulty falling asleep.
[polldaddy:10673878]
The authors’ observations
The treatment team explores other options, such as benzodiazepines, psychotherapy modalities, and mindfulness exercises, to treat Ms. M’s anxiety and comorbid COPD. Lorazepam, 0.5 mg twice daily, was chosen to treat her acute anxiety. Due to Ms. M’s need for supplemental oxygen, the treatment team attempted to mitigate the risk of using a benzodiazepine by limiting its use to the minimum effective dose. The teams also looked for alternative therapies.
Continue to: Evalution of anxiety...
Evaluation of anxiety and depression in a patient with COPD is complicated by a high degree of symptom overlap. Patients with COPD may experience anxiety symptoms such as shortness of breath, rapid heart rate, numbness/tingling, and racing thoughts, and/or depressive symptoms such as decreased energy, impaired sleep, and impaired concentration. It can therefore be difficult to discern if a symptom is attributable to the physical diagnosis, the psychiatric diagnosis, or a combination of both. Catastrophic thinking about mild physical symptoms is common in patients with COPD. This can lead to hyperventilation and hypocapnia (manifested by lightheadedness, dizziness, paresthesia, and altered consciousness), with a reciprocally escalating cascade of anxiety and somatic symptoms.1
First-line therapy for anxiety disorders with comorbid COPD is CBT and other nonpharmacologic interventions.2,3
Although there is little evidence that traditional pharmacologic treatments (eg, antidepressants, benzodiazepines) have a statistically significant effect on anxiety and depression in COPD, studies have found that they have some clinical benefit.3 Risks, however, limit the utility of certain agents. Sedative-hypnotics potentially decrease respiratory drive and, particularly in older patients, antidepressants’ sedating effects can increase the risk of falls3 leading to increased morbidity, hospitalization, and mortality.
TREATMENT Mindfulness techniques and meditation
Ms. M’s symptoms show no improvement with the addition of lorazepam, 0.5 mg twice daily. A clinician teaches Ms. M mindfulness techniques, and she begins a trial of daily, individual, guided meditation using a meditation app. Respiratory therapists also instruct her on controlled breathing techniques such as pursed-lips breathing, diaphragmatic breathing, and deep breathing. They also encourage Ms. M to participate in the daily exercise group while on the unit.
[polldaddy:10673881]
The authors’ observations
Research indicates that low doses of opioids are safe and effective for refractory breathlessness in patients with severe COPD(those with an arterial partial pressure of oxygen ≤55 mm Hg or arterial oxygen saturation ≤88%).6,7
Continue to: The current opioid crisis...
The current opioid crisis prompts additional caution in prescribing, especially when considering using short-acting, immediate-release opioids such as morphine, which have a greater potential for abuse and dependence.
Many patients with COPD in the end-of-life phase and in severe pain or discomfort due to the advanced stages of their illness receive opioids as part of palliative care. Patients with COPD whose medical care is predominantly palliative may benefit greatly from being prescribed opioids. Most patients with COPD who find relief from low-dose opioids usually have 6 to 12 months to live, and low-dose opioids may help them obtain the best possible quality of life.
Choosing opioids as a treatment involves the risk of physiologic dependence and opioid use disorder. For Ms. M, the potential benefits were thought to outweigh such risks.
OUTCOME Breathlessness improves, anxiety decreases
Ms. M’s lorazepam is discontinued, and immediate-release morphine is prescribed at a low dose of 1 mg/d on an as-needed basis for anxiety with good effect. Ms. M’s breathlessness improves, leading to an overall decrease in anxiety. She does not experience sedation, confusion, or adverse respiratory effects.
Ms. M’s anxiety and depression improve over the course of the hospitalization with this regimen. On hospital Day 25, she is discharged with a plan to continue duloxetine, 60 mg/d, ECT twice weekly, and low-dose morphine, 1 mg/d, as needed for anxiety. She is referred for pulmonary rehabilitation and CBT to maintain remission.
[polldaddy:10673882]
Continue to: The authors' observations
The authors’ observations
Ms. M’s case highlights several challenges associated with treating psychiatric illness in a patient with a chronic medical illness. The relationship between COPD, anxiety, and depression is complex, and is associated with reduced quality of life, increasing severity of pulmonary disease, increased dyspnea, a sense of loss and inability to cope, and decreased self-efficacy and adherence to treatment.9-11
Bottom Line
When traditional antidepressant and anxiolytic therapies have not sufficiently helped, consider low-dose, once-daily opioids to address refractory breathlessness in a patient with COPD with comorbid anxiety and depression. This treatment can lead patients to participate in rehabilitation therapies and improve their quality of life.
Related Resources
- Alexopoulos G, Kiosses D, Sirey J, et al. Untangling therapeutic ingredients of a personalized intervention for patients with depression and severe COPD. Am J Geriatr Psychiatry. 2014;22(11):1316-1324.
- Jackson D, Banerjee S, Sirey J, et al. Two interventions for patients with major depression and severe chronic obstructive pulmonary disease: impact on quality of life. Am J Geriatr Psychiatry. 2018;27(5):502-511.
Drug Brand Names
Amlodipine • Norvasc
Aripiprazole • Abilify
Buspirone • Buspar
Clonazepam • Klonopin
Duloxetine • Cymbalta
Fluoxetine • Prozac
Hydromorphone • Dilaudid
Levodopa • Sinemet
Lorazepam • Ativan
Mirtazapine • Remeron
Morphine • MS Contin
Naloxone • Narcan
Oxycodone • Oxycontin
Salmeterol • Serevent Diskus
CASE A passive wish to die
Ms. M, age 76, has a history of major depressive disorder, unspecified anxiety disorder, and severe chronic obstructive pulmonary disease (COPD), for which she requires supplemental oxygen. She is admitted to a psychiatric hospital after several months of increased dysphoria, rumination, anhedonia, and a passive wish to die. She also has a decreased appetite and has lost 10 lb, experiences frequent daily episodes of shortness of breath and associated racing thoughts, and has a rapid heart rate.
HISTORY Past medication trials
In addition to COPD, Ms. M’s medical history includes hypertension. Past psychotropic medication trials used to treat her depression and anxiety have included aripiprazole, 5 mg/d; duloxetine, 60 mg/d; fluoxetine, 40 mg/d; mirtazapine, 30 mg nightly; buspirone, 10 mg twice daily; and clonazepam, 0.5 mg twice daily. She has no history of psychotherapy, and because of her uncontrolled anxiety and depression, she has never completed a pulmonary rehabilitation program.
Her current medications include salmeterol, 50 mcg inhaled twice daily, for COPD; amlodipine, 10 mg/d, for hypertension; buspirone, 10 mg twice daily, for anxiety; and duloxetine, 60 mg/d, for depression.
EXAMINATION No evidence of dementia
On examination, Ms. M is alert and oriented to person, place, date, and situation. Overall, she has mild difficulty with attention and short-term recall, which appears to be due to poor effort; intact long-term memory; and is able to abstract appropriately. There is no evidence of dementia.
A mental status exam reveals a frail, elderly woman with fair-to-poor hygiene, cooperative behavior, slowed motor activity, slowed speech with low volume, low mood, and depressed affect with constricted range. Her thought process is linear, her thought content includes passive death wishes, and she does not have hallucinations.
Bitemporal electroconvulsive therapy (ECT), 1.0 ms pulse width at 1.5 times Ms. M’s seizure threshold 3 times weekly, is initiated to treat her depression, with seizure duration averaging 45 seconds for each session. She receives a total of 8 treatments over the course of admission. Buspirone, 10 mg twice daily, is stopped shortly after admission, but she continues to receive duloxetine, 60 mg/d. Ms. M continues to have shortness of breath, palpitations, fearful ruminations about the future, and difficulty falling asleep.
[polldaddy:10673878]
The authors’ observations
The treatment team explores other options, such as benzodiazepines, psychotherapy modalities, and mindfulness exercises, to treat Ms. M’s anxiety and comorbid COPD. Lorazepam, 0.5 mg twice daily, was chosen to treat her acute anxiety. Due to Ms. M’s need for supplemental oxygen, the treatment team attempted to mitigate the risk of using a benzodiazepine by limiting its use to the minimum effective dose. The teams also looked for alternative therapies.
Continue to: Evalution of anxiety...
Evaluation of anxiety and depression in a patient with COPD is complicated by a high degree of symptom overlap. Patients with COPD may experience anxiety symptoms such as shortness of breath, rapid heart rate, numbness/tingling, and racing thoughts, and/or depressive symptoms such as decreased energy, impaired sleep, and impaired concentration. It can therefore be difficult to discern if a symptom is attributable to the physical diagnosis, the psychiatric diagnosis, or a combination of both. Catastrophic thinking about mild physical symptoms is common in patients with COPD. This can lead to hyperventilation and hypocapnia (manifested by lightheadedness, dizziness, paresthesia, and altered consciousness), with a reciprocally escalating cascade of anxiety and somatic symptoms.1
First-line therapy for anxiety disorders with comorbid COPD is CBT and other nonpharmacologic interventions.2,3
Although there is little evidence that traditional pharmacologic treatments (eg, antidepressants, benzodiazepines) have a statistically significant effect on anxiety and depression in COPD, studies have found that they have some clinical benefit.3 Risks, however, limit the utility of certain agents. Sedative-hypnotics potentially decrease respiratory drive and, particularly in older patients, antidepressants’ sedating effects can increase the risk of falls3 leading to increased morbidity, hospitalization, and mortality.
TREATMENT Mindfulness techniques and meditation
Ms. M’s symptoms show no improvement with the addition of lorazepam, 0.5 mg twice daily. A clinician teaches Ms. M mindfulness techniques, and she begins a trial of daily, individual, guided meditation using a meditation app. Respiratory therapists also instruct her on controlled breathing techniques such as pursed-lips breathing, diaphragmatic breathing, and deep breathing. They also encourage Ms. M to participate in the daily exercise group while on the unit.
[polldaddy:10673881]
The authors’ observations
Research indicates that low doses of opioids are safe and effective for refractory breathlessness in patients with severe COPD(those with an arterial partial pressure of oxygen ≤55 mm Hg or arterial oxygen saturation ≤88%).6,7
Continue to: The current opioid crisis...
The current opioid crisis prompts additional caution in prescribing, especially when considering using short-acting, immediate-release opioids such as morphine, which have a greater potential for abuse and dependence.
Many patients with COPD in the end-of-life phase and in severe pain or discomfort due to the advanced stages of their illness receive opioids as part of palliative care. Patients with COPD whose medical care is predominantly palliative may benefit greatly from being prescribed opioids. Most patients with COPD who find relief from low-dose opioids usually have 6 to 12 months to live, and low-dose opioids may help them obtain the best possible quality of life.
Choosing opioids as a treatment involves the risk of physiologic dependence and opioid use disorder. For Ms. M, the potential benefits were thought to outweigh such risks.
OUTCOME Breathlessness improves, anxiety decreases
Ms. M’s lorazepam is discontinued, and immediate-release morphine is prescribed at a low dose of 1 mg/d on an as-needed basis for anxiety with good effect. Ms. M’s breathlessness improves, leading to an overall decrease in anxiety. She does not experience sedation, confusion, or adverse respiratory effects.
Ms. M’s anxiety and depression improve over the course of the hospitalization with this regimen. On hospital Day 25, she is discharged with a plan to continue duloxetine, 60 mg/d, ECT twice weekly, and low-dose morphine, 1 mg/d, as needed for anxiety. She is referred for pulmonary rehabilitation and CBT to maintain remission.
[polldaddy:10673882]
Continue to: The authors' observations
The authors’ observations
Ms. M’s case highlights several challenges associated with treating psychiatric illness in a patient with a chronic medical illness. The relationship between COPD, anxiety, and depression is complex, and is associated with reduced quality of life, increasing severity of pulmonary disease, increased dyspnea, a sense of loss and inability to cope, and decreased self-efficacy and adherence to treatment.9-11
Bottom Line
When traditional antidepressant and anxiolytic therapies have not sufficiently helped, consider low-dose, once-daily opioids to address refractory breathlessness in a patient with COPD with comorbid anxiety and depression. This treatment can lead patients to participate in rehabilitation therapies and improve their quality of life.
Related Resources
- Alexopoulos G, Kiosses D, Sirey J, et al. Untangling therapeutic ingredients of a personalized intervention for patients with depression and severe COPD. Am J Geriatr Psychiatry. 2014;22(11):1316-1324.
- Jackson D, Banerjee S, Sirey J, et al. Two interventions for patients with major depression and severe chronic obstructive pulmonary disease: impact on quality of life. Am J Geriatr Psychiatry. 2018;27(5):502-511.
Drug Brand Names
Amlodipine • Norvasc
Aripiprazole • Abilify
Buspirone • Buspar
Clonazepam • Klonopin
Duloxetine • Cymbalta
Fluoxetine • Prozac
Hydromorphone • Dilaudid
Levodopa • Sinemet
Lorazepam • Ativan
Mirtazapine • Remeron
Morphine • MS Contin
Naloxone • Narcan
Oxycodone • Oxycontin
Salmeterol • Serevent Diskus
1. Harnett D. The difficult-to-treat psychiatric patient with comorbid medical illness. In: Dewan M, Pies R, eds. The difficult-to-treat psychiatric patient. Washington, DC: American Psychiatric Association Publishing; 2001:325-357.
2. Panagioti M, Scott C, Blakemore A, et al. Overview of the prevalence, impact, and management of depression and anxiety in chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2014;9:1289-1306.
3. Cafarella P, Effing T, Usmani ZA, et al. Treatments for anxiety and depression in patients with chronic obstructive pulmonary disease: a literature review. Respirology. 2012;17(4):627-638.
4. Heslop-Marshall K, Baker C, Carrick-Sen D, et al. Randomised controlled trial of cognitive behavioural therapy in COPD. ERJ Open Res. 2018;4:00094-2018. doi: 10.1183/23120541.00094-2018.
5. de Godoy DV, de Godoy RF. A randomized controlled trial of the effect of psychotherapy on anxiety and depression in chronic obstructive pulmonary disease. Arch Phys Med Rehabil. 2003;84(8):1154-1157.
6. Abernethy A, Currow D, Frith P, et al. Randomised, double blind, placebo controlled crossover trial of sustained release morphine for the management of refractory dyspnoea. BMJ. 2003;327(7414):523-528.
7. Janowiak P, Krajnik M, Podolec Z, et al. Dosimetrically administered nebulized morphine for breathlessness in very severe chronic obstructive pulmonary disease: a randomized, controlled trial. BMC Pulm Med. 2017;17:186.
8. Rocker G, Horton R, Currow D, et al. Palliation of dyspnoea in advanced COPD: revisiting a role for opioids. Thorax. 2009;64(10):910-915.
9. Pooler A, Beech R. Examining the relationship between anxiety and depression and exacerbations of COPD which result in hospital admission: a systematic review. Int J Chron Obstruct Pulmon Dis. 2014;9:315-330.
10. Carmen Valenza M, Valenza-Peña G, Torres-Sánchez I, et al. Effectiveness of controlled breathing techniques on anxiety and depression in hospitalized patients with COPD: a randomized clinical trial. Respir Care. 2014;59(2):209-215.
11. Pollok J, van Agteren J, Esterman A, et al. Psychological therapies for the treatment of depression in chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2019;3:CD012347. doi: 10.1002/14651858.CD012347.pub2.
12. Roberts N, Kidd L, Kirkwood K, et al. A systematic review of the content and delivery of education in pulmonary rehabilitation programmes. Respiratory Medicine. 2018;145:161-181.
13. Pumar M, Gray C, Walsh J, et al. Anxiety and depression-important psychological comorbidities of COPD. J Thorac Dis. 2014;6(11):1615-1631.
14. Alexopoulos G, Kiosses D, Sirey J, et al. Untangling therapeutic ingredients of a personalized intervention for patients with depression and severe COPD. Am J Geriatr Psychiatry. 2014;22(11):1316-1324.
15. Jackson D, Banerjee S, Sirey J, et al. Two interventions for patients with major depression and severe chronic obstructive pulmonary disease: impact on quality of life. Am J Geriatr Psychiatry. 2018;27(5):502-511.
1. Harnett D. The difficult-to-treat psychiatric patient with comorbid medical illness. In: Dewan M, Pies R, eds. The difficult-to-treat psychiatric patient. Washington, DC: American Psychiatric Association Publishing; 2001:325-357.
2. Panagioti M, Scott C, Blakemore A, et al. Overview of the prevalence, impact, and management of depression and anxiety in chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2014;9:1289-1306.
3. Cafarella P, Effing T, Usmani ZA, et al. Treatments for anxiety and depression in patients with chronic obstructive pulmonary disease: a literature review. Respirology. 2012;17(4):627-638.
4. Heslop-Marshall K, Baker C, Carrick-Sen D, et al. Randomised controlled trial of cognitive behavioural therapy in COPD. ERJ Open Res. 2018;4:00094-2018. doi: 10.1183/23120541.00094-2018.
5. de Godoy DV, de Godoy RF. A randomized controlled trial of the effect of psychotherapy on anxiety and depression in chronic obstructive pulmonary disease. Arch Phys Med Rehabil. 2003;84(8):1154-1157.
6. Abernethy A, Currow D, Frith P, et al. Randomised, double blind, placebo controlled crossover trial of sustained release morphine for the management of refractory dyspnoea. BMJ. 2003;327(7414):523-528.
7. Janowiak P, Krajnik M, Podolec Z, et al. Dosimetrically administered nebulized morphine for breathlessness in very severe chronic obstructive pulmonary disease: a randomized, controlled trial. BMC Pulm Med. 2017;17:186.
8. Rocker G, Horton R, Currow D, et al. Palliation of dyspnoea in advanced COPD: revisiting a role for opioids. Thorax. 2009;64(10):910-915.
9. Pooler A, Beech R. Examining the relationship between anxiety and depression and exacerbations of COPD which result in hospital admission: a systematic review. Int J Chron Obstruct Pulmon Dis. 2014;9:315-330.
10. Carmen Valenza M, Valenza-Peña G, Torres-Sánchez I, et al. Effectiveness of controlled breathing techniques on anxiety and depression in hospitalized patients with COPD: a randomized clinical trial. Respir Care. 2014;59(2):209-215.
11. Pollok J, van Agteren J, Esterman A, et al. Psychological therapies for the treatment of depression in chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2019;3:CD012347. doi: 10.1002/14651858.CD012347.pub2.
12. Roberts N, Kidd L, Kirkwood K, et al. A systematic review of the content and delivery of education in pulmonary rehabilitation programmes. Respiratory Medicine. 2018;145:161-181.
13. Pumar M, Gray C, Walsh J, et al. Anxiety and depression-important psychological comorbidities of COPD. J Thorac Dis. 2014;6(11):1615-1631.
14. Alexopoulos G, Kiosses D, Sirey J, et al. Untangling therapeutic ingredients of a personalized intervention for patients with depression and severe COPD. Am J Geriatr Psychiatry. 2014;22(11):1316-1324.
15. Jackson D, Banerjee S, Sirey J, et al. Two interventions for patients with major depression and severe chronic obstructive pulmonary disease: impact on quality of life. Am J Geriatr Psychiatry. 2018;27(5):502-511.
Painful erections while being treated for OCD
CASE Prolonged, painful erections
Mr. G, age 27, who has a history of obsessive-compulsive disorder (OCD), presents to his internist’s office with complaints of “masturbating several times a day” and having ejaculatory delay of up to 50 minutes with intercourse. The frequent masturbation was an attempt to “cure” the ejaculatory delay. In addition, Mr. G reports that for the past 5 nights, he has awoke every 3 hours with a painful erection that lasted 1.5 to 2.5 hours, after which he would fall asleep, only to wake once again to the same phenomenon.
Mr. G’s symptoms began 3 weeks ago after his psychiatrist adjusted the dose of his medication for OCD. Mr. G had been receiving fluoxetine, 10 mg/d, for the past 3 years to manage his OCD, without improvement. During a recent consultation, his psychiatrist increased the dose to 20 mg/d, with the expectation that further dose increases might be necessary to treat his OCD.
HISTORY Concurrent GAD
Mr. G is single and in a monogamous heterosexual relationship. Three weeks earlier, when he was examined by his psychiatrist, Mr. G’s Yale-Brown Obsessive Compulsive Scale score was 28 and his Beck Anxiety Inventory score was 24. Based on these scores, the psychiatrist concluded Mr. G had concurrent generalized anxiety disorder (GAD).
EVALUATION Workup is normal
On presentation to his internist’s office, Mr. G’s laboratory values are all within normal range, including a chemistry panel, complete blood count with differential, and electrocardiogram. A human immunodeficiency virus test is negative. His internist instructs Mr. G to return to his psychiatrist.
[polldaddy:10640161]
TREATMENT Dose adjustment
Based on Mr. G’s description of painful and persistent erections in the absence of sexual stimulation or arousal, and because these episodes have occurred 5 consecutive nights, the psychiatrist makes a provisional diagnosis of stuttering priapism and reduces the fluoxetine dose from 20 to 10 mg/d.
The author’s observations
Priapism is classically defined as a persistent, unwanted penile or clitoral engorgement in the absence of sexual desire/arousal or stimulation. It can last for up to 4 to 6 hours1 orit can take a so-called “stuttering form” characterized by brief, recurrent, self-limited episodes. Priapism is a urologic emergency resulting in erectile dysfunction in 30% to 90% of patients. It is multifactorial and can be characterized as low-flow (occlusive) or high-flow (nonischemic). Most priapism is primary or idiopathic in nature; the incidence is 1.5 per 100,000 individuals (primarily men), with bimodal peaks, and it can occur in all age groups.2 Secondary priapism can occur from many causes (Table).
Mechanism is unclear
The molecular mechanism of priapism is not completely understood. Normally, nitrous oxide mediates penile erection. However, cyclic guanosine monophosphate (cGMP) acts at several levels to create smooth muscle reaction, leading to either penile tumescence or, in some cases, priapism. Stuttering or intermittent ischemic priapism is thought to be a downregulation of phosphodiesterase type 5, causing excess cGMP with subsequent smooth muscle relaxation in the penis.3
Continue to: Drug-induced priapism
Drug-induced priapism
Drug-induced priapism is commonly believed to be associated with alpha-1 adrenergic receptor blockade.4 This also results in dizziness and orthostatic hypotension.5 Trazodone is commonly associated with the development of secondary priapism; however, in the last 30 years, multiple case reports have demonstrated that a variety of psychoactive agents have been associated with low-flowpriapism.6 Most case reports have focused on new-onset priapism associated with the introduction of a new medication. Based on a recent informal search of Medline, since 1989, there have been >36 case reports of priapism associated with psychotropic use. Stuttering priapism is less frequently discussed in the literature.7
Ischemic priapism accounts for 95% of all reports. It can be associated with medication use or hematologic disorders, or it can be triggered by sexual activity. Often, patients who experience an episode will abstain from sexual contact.
The etiology of stuttering priapism is less clear. Episodes of stuttering priapism often occur during sleep and can resolve spontaneously.8 They are a form of ischemic priapism and are seen in patients with sickle cell anemia. It is not known how many patients with stuttering priapism will convert to the nonremitting form, which may require chemical or surgical intervention.9 Stuttering priapism may go unreported and perhaps may be overlooked by patients based on its frequency and intensity.
The activating selective serotonin reuptake inhibitor fluoxetine has a long half-life and is a potent inhibitor of the cytochrome P450 2D6 isoenzyme system. It inhibits serotonin transporter proteins. It is also a weak norepinephrine reuptake inhibitor, an effect that increases with increasing doses of the medication. Its 5HT2C antagonism is proposed as the mechanism of its activating properties.10 In Mr. G’s case, it is possible that fluoxetine’s weak norepinephrine reuptake inhibition resulted in an intermittent priapism effect mediated through the pathways described above.
OUTCOME Symptoms resolve
Approximately 1 week after Mr. G’s fluoxetine dose is reduced, his symptoms of priapism abated. The fluoxetine is discontinued and his ejaculatory delay resolves. Mr. G is started on fluvoxamine, 150 mg/d, which results in a significant decrease of both GAD and OCD symptoms with no notable ejaculatory delay, and no recurrence of priapism.
Continue to: The author's observations
The author’s observations
Mr. G’s case and other case reports suggest that psychiatrists should caution patients who are prescribed antidepressants or antipsychotics that stuttering priapism is a possible adverse effect.11 As seen in Mr. G’s case, fluoxetine (when used chronically) can moderate vascular responses at the pre- and post-synaptic adrenergic receptor.11 Priapism induced by a psychotropic medication will not necessarily lead to a longer-term, unremitting priapism, but it can be dramatic, frightening, and lead to noncompliance. Along with obtaining a standard history that includes asking patients about prior adverse medication events, psychiatrists also should ask their patients if they have experienced any instances of transient priapism that may require further evaluation.
Bottom Line
Any psychotropic medication that has the capacity to act on alpha adrenergic receptors can cause priapism. Ask patients if they have had any unusual erections/ clitoral engorgement while taking any psychotropic medications, because many patients will be hesitant to volunteer such information.
Related Resource
- Thippaiah SM, Nagaraja S, Birur B, et al. Successful management of psychotropics induced stuttering priapism with pseudoephedrine in a patient with schizophrenia. Psychopharmacol Bull. 2018;48(2):29-33.
Drug Brand Names
Fluoxetine • Prozac
Fluvoxamine • Luvox
Trazodone • Desyrel, Oleptro
1. Kadioglu A, Sanli O, Celtik M, et al. Practical management of patients with priapism. EAU-EBU Update Series. 2006;4(4):150-160.
2. Eland IA, van der Lei J, Stricker BHC. Incidence of priapism in the general population. Urology. 2001;57(5):970-972.
3. Halls JE, Patel DV, Walkden M, et al. Priapism: pathophysiology and the role of the radiologist. Br J Radiol. 2012;85(Spec Iss 1):S79-S85.
4. Wang CS, Kao WT, Chen CD, et al. Priapism associated with typical and atypical antipsychotic medications. Int Clinical Psychopharmacology. 2006;21(4):245-248.
5. Khan Q, Tucker P, Lokhande A. Priapism: what cause: mental illness, psychotropic medications or polysubstance abuse? J Okla State Med Assoc. 2016;109(11):515-517.
6. Dent LA, Brown WC, Murney JD. Citalopram-induced priapism. Pharmacotherapy. 2002;22(4):538-541.
7. Wilkening GL, Kucherer SA, Douaihy AB. Priapism and renal colic in a patient treated with duloxetine. Mental Health Clinician. 2016;6(4):197-200.
8. Morrison BF, Burnett AL. Stuttering priapism: insight into its pathogenesis and management. Curr Urol Rep. 2012;13(4):268-276.
9. Burnett AL, Bivalacqua TJ. Priapism: current principles and practice. Urol Clin North Am. 2007;34(4):631-642.
10. Stahl SM. Stahl’s essential psychopharmacology: neuroscientific basis and practical applications. 4th ed. Cambridge, United Kingdom: Cambridge University Press; 2013.
11. Pereira CA, Rodrigues FL, Ruginsk SG, et al. Chronic treatment with fluoxetine modulates vascular adrenergic responses by inhibition of pre- and post-synaptic mechanisms. Eu J Pharmacol. 2017;800:70-80.
CASE Prolonged, painful erections
Mr. G, age 27, who has a history of obsessive-compulsive disorder (OCD), presents to his internist’s office with complaints of “masturbating several times a day” and having ejaculatory delay of up to 50 minutes with intercourse. The frequent masturbation was an attempt to “cure” the ejaculatory delay. In addition, Mr. G reports that for the past 5 nights, he has awoke every 3 hours with a painful erection that lasted 1.5 to 2.5 hours, after which he would fall asleep, only to wake once again to the same phenomenon.
Mr. G’s symptoms began 3 weeks ago after his psychiatrist adjusted the dose of his medication for OCD. Mr. G had been receiving fluoxetine, 10 mg/d, for the past 3 years to manage his OCD, without improvement. During a recent consultation, his psychiatrist increased the dose to 20 mg/d, with the expectation that further dose increases might be necessary to treat his OCD.
HISTORY Concurrent GAD
Mr. G is single and in a monogamous heterosexual relationship. Three weeks earlier, when he was examined by his psychiatrist, Mr. G’s Yale-Brown Obsessive Compulsive Scale score was 28 and his Beck Anxiety Inventory score was 24. Based on these scores, the psychiatrist concluded Mr. G had concurrent generalized anxiety disorder (GAD).
EVALUATION Workup is normal
On presentation to his internist’s office, Mr. G’s laboratory values are all within normal range, including a chemistry panel, complete blood count with differential, and electrocardiogram. A human immunodeficiency virus test is negative. His internist instructs Mr. G to return to his psychiatrist.
[polldaddy:10640161]
TREATMENT Dose adjustment
Based on Mr. G’s description of painful and persistent erections in the absence of sexual stimulation or arousal, and because these episodes have occurred 5 consecutive nights, the psychiatrist makes a provisional diagnosis of stuttering priapism and reduces the fluoxetine dose from 20 to 10 mg/d.
The author’s observations
Priapism is classically defined as a persistent, unwanted penile or clitoral engorgement in the absence of sexual desire/arousal or stimulation. It can last for up to 4 to 6 hours1 orit can take a so-called “stuttering form” characterized by brief, recurrent, self-limited episodes. Priapism is a urologic emergency resulting in erectile dysfunction in 30% to 90% of patients. It is multifactorial and can be characterized as low-flow (occlusive) or high-flow (nonischemic). Most priapism is primary or idiopathic in nature; the incidence is 1.5 per 100,000 individuals (primarily men), with bimodal peaks, and it can occur in all age groups.2 Secondary priapism can occur from many causes (Table).
Mechanism is unclear
The molecular mechanism of priapism is not completely understood. Normally, nitrous oxide mediates penile erection. However, cyclic guanosine monophosphate (cGMP) acts at several levels to create smooth muscle reaction, leading to either penile tumescence or, in some cases, priapism. Stuttering or intermittent ischemic priapism is thought to be a downregulation of phosphodiesterase type 5, causing excess cGMP with subsequent smooth muscle relaxation in the penis.3
Continue to: Drug-induced priapism
Drug-induced priapism
Drug-induced priapism is commonly believed to be associated with alpha-1 adrenergic receptor blockade.4 This also results in dizziness and orthostatic hypotension.5 Trazodone is commonly associated with the development of secondary priapism; however, in the last 30 years, multiple case reports have demonstrated that a variety of psychoactive agents have been associated with low-flowpriapism.6 Most case reports have focused on new-onset priapism associated with the introduction of a new medication. Based on a recent informal search of Medline, since 1989, there have been >36 case reports of priapism associated with psychotropic use. Stuttering priapism is less frequently discussed in the literature.7
Ischemic priapism accounts for 95% of all reports. It can be associated with medication use or hematologic disorders, or it can be triggered by sexual activity. Often, patients who experience an episode will abstain from sexual contact.
The etiology of stuttering priapism is less clear. Episodes of stuttering priapism often occur during sleep and can resolve spontaneously.8 They are a form of ischemic priapism and are seen in patients with sickle cell anemia. It is not known how many patients with stuttering priapism will convert to the nonremitting form, which may require chemical or surgical intervention.9 Stuttering priapism may go unreported and perhaps may be overlooked by patients based on its frequency and intensity.
The activating selective serotonin reuptake inhibitor fluoxetine has a long half-life and is a potent inhibitor of the cytochrome P450 2D6 isoenzyme system. It inhibits serotonin transporter proteins. It is also a weak norepinephrine reuptake inhibitor, an effect that increases with increasing doses of the medication. Its 5HT2C antagonism is proposed as the mechanism of its activating properties.10 In Mr. G’s case, it is possible that fluoxetine’s weak norepinephrine reuptake inhibition resulted in an intermittent priapism effect mediated through the pathways described above.
OUTCOME Symptoms resolve
Approximately 1 week after Mr. G’s fluoxetine dose is reduced, his symptoms of priapism abated. The fluoxetine is discontinued and his ejaculatory delay resolves. Mr. G is started on fluvoxamine, 150 mg/d, which results in a significant decrease of both GAD and OCD symptoms with no notable ejaculatory delay, and no recurrence of priapism.
Continue to: The author's observations
The author’s observations
Mr. G’s case and other case reports suggest that psychiatrists should caution patients who are prescribed antidepressants or antipsychotics that stuttering priapism is a possible adverse effect.11 As seen in Mr. G’s case, fluoxetine (when used chronically) can moderate vascular responses at the pre- and post-synaptic adrenergic receptor.11 Priapism induced by a psychotropic medication will not necessarily lead to a longer-term, unremitting priapism, but it can be dramatic, frightening, and lead to noncompliance. Along with obtaining a standard history that includes asking patients about prior adverse medication events, psychiatrists also should ask their patients if they have experienced any instances of transient priapism that may require further evaluation.
Bottom Line
Any psychotropic medication that has the capacity to act on alpha adrenergic receptors can cause priapism. Ask patients if they have had any unusual erections/ clitoral engorgement while taking any psychotropic medications, because many patients will be hesitant to volunteer such information.
Related Resource
- Thippaiah SM, Nagaraja S, Birur B, et al. Successful management of psychotropics induced stuttering priapism with pseudoephedrine in a patient with schizophrenia. Psychopharmacol Bull. 2018;48(2):29-33.
Drug Brand Names
Fluoxetine • Prozac
Fluvoxamine • Luvox
Trazodone • Desyrel, Oleptro
CASE Prolonged, painful erections
Mr. G, age 27, who has a history of obsessive-compulsive disorder (OCD), presents to his internist’s office with complaints of “masturbating several times a day” and having ejaculatory delay of up to 50 minutes with intercourse. The frequent masturbation was an attempt to “cure” the ejaculatory delay. In addition, Mr. G reports that for the past 5 nights, he has awoke every 3 hours with a painful erection that lasted 1.5 to 2.5 hours, after which he would fall asleep, only to wake once again to the same phenomenon.
Mr. G’s symptoms began 3 weeks ago after his psychiatrist adjusted the dose of his medication for OCD. Mr. G had been receiving fluoxetine, 10 mg/d, for the past 3 years to manage his OCD, without improvement. During a recent consultation, his psychiatrist increased the dose to 20 mg/d, with the expectation that further dose increases might be necessary to treat his OCD.
HISTORY Concurrent GAD
Mr. G is single and in a monogamous heterosexual relationship. Three weeks earlier, when he was examined by his psychiatrist, Mr. G’s Yale-Brown Obsessive Compulsive Scale score was 28 and his Beck Anxiety Inventory score was 24. Based on these scores, the psychiatrist concluded Mr. G had concurrent generalized anxiety disorder (GAD).
EVALUATION Workup is normal
On presentation to his internist’s office, Mr. G’s laboratory values are all within normal range, including a chemistry panel, complete blood count with differential, and electrocardiogram. A human immunodeficiency virus test is negative. His internist instructs Mr. G to return to his psychiatrist.
[polldaddy:10640161]
TREATMENT Dose adjustment
Based on Mr. G’s description of painful and persistent erections in the absence of sexual stimulation or arousal, and because these episodes have occurred 5 consecutive nights, the psychiatrist makes a provisional diagnosis of stuttering priapism and reduces the fluoxetine dose from 20 to 10 mg/d.
The author’s observations
Priapism is classically defined as a persistent, unwanted penile or clitoral engorgement in the absence of sexual desire/arousal or stimulation. It can last for up to 4 to 6 hours1 orit can take a so-called “stuttering form” characterized by brief, recurrent, self-limited episodes. Priapism is a urologic emergency resulting in erectile dysfunction in 30% to 90% of patients. It is multifactorial and can be characterized as low-flow (occlusive) or high-flow (nonischemic). Most priapism is primary or idiopathic in nature; the incidence is 1.5 per 100,000 individuals (primarily men), with bimodal peaks, and it can occur in all age groups.2 Secondary priapism can occur from many causes (Table).
Mechanism is unclear
The molecular mechanism of priapism is not completely understood. Normally, nitrous oxide mediates penile erection. However, cyclic guanosine monophosphate (cGMP) acts at several levels to create smooth muscle reaction, leading to either penile tumescence or, in some cases, priapism. Stuttering or intermittent ischemic priapism is thought to be a downregulation of phosphodiesterase type 5, causing excess cGMP with subsequent smooth muscle relaxation in the penis.3
Continue to: Drug-induced priapism
Drug-induced priapism
Drug-induced priapism is commonly believed to be associated with alpha-1 adrenergic receptor blockade.4 This also results in dizziness and orthostatic hypotension.5 Trazodone is commonly associated with the development of secondary priapism; however, in the last 30 years, multiple case reports have demonstrated that a variety of psychoactive agents have been associated with low-flowpriapism.6 Most case reports have focused on new-onset priapism associated with the introduction of a new medication. Based on a recent informal search of Medline, since 1989, there have been >36 case reports of priapism associated with psychotropic use. Stuttering priapism is less frequently discussed in the literature.7
Ischemic priapism accounts for 95% of all reports. It can be associated with medication use or hematologic disorders, or it can be triggered by sexual activity. Often, patients who experience an episode will abstain from sexual contact.
The etiology of stuttering priapism is less clear. Episodes of stuttering priapism often occur during sleep and can resolve spontaneously.8 They are a form of ischemic priapism and are seen in patients with sickle cell anemia. It is not known how many patients with stuttering priapism will convert to the nonremitting form, which may require chemical or surgical intervention.9 Stuttering priapism may go unreported and perhaps may be overlooked by patients based on its frequency and intensity.
The activating selective serotonin reuptake inhibitor fluoxetine has a long half-life and is a potent inhibitor of the cytochrome P450 2D6 isoenzyme system. It inhibits serotonin transporter proteins. It is also a weak norepinephrine reuptake inhibitor, an effect that increases with increasing doses of the medication. Its 5HT2C antagonism is proposed as the mechanism of its activating properties.10 In Mr. G’s case, it is possible that fluoxetine’s weak norepinephrine reuptake inhibition resulted in an intermittent priapism effect mediated through the pathways described above.
OUTCOME Symptoms resolve
Approximately 1 week after Mr. G’s fluoxetine dose is reduced, his symptoms of priapism abated. The fluoxetine is discontinued and his ejaculatory delay resolves. Mr. G is started on fluvoxamine, 150 mg/d, which results in a significant decrease of both GAD and OCD symptoms with no notable ejaculatory delay, and no recurrence of priapism.
Continue to: The author's observations
The author’s observations
Mr. G’s case and other case reports suggest that psychiatrists should caution patients who are prescribed antidepressants or antipsychotics that stuttering priapism is a possible adverse effect.11 As seen in Mr. G’s case, fluoxetine (when used chronically) can moderate vascular responses at the pre- and post-synaptic adrenergic receptor.11 Priapism induced by a psychotropic medication will not necessarily lead to a longer-term, unremitting priapism, but it can be dramatic, frightening, and lead to noncompliance. Along with obtaining a standard history that includes asking patients about prior adverse medication events, psychiatrists also should ask their patients if they have experienced any instances of transient priapism that may require further evaluation.
Bottom Line
Any psychotropic medication that has the capacity to act on alpha adrenergic receptors can cause priapism. Ask patients if they have had any unusual erections/ clitoral engorgement while taking any psychotropic medications, because many patients will be hesitant to volunteer such information.
Related Resource
- Thippaiah SM, Nagaraja S, Birur B, et al. Successful management of psychotropics induced stuttering priapism with pseudoephedrine in a patient with schizophrenia. Psychopharmacol Bull. 2018;48(2):29-33.
Drug Brand Names
Fluoxetine • Prozac
Fluvoxamine • Luvox
Trazodone • Desyrel, Oleptro
1. Kadioglu A, Sanli O, Celtik M, et al. Practical management of patients with priapism. EAU-EBU Update Series. 2006;4(4):150-160.
2. Eland IA, van der Lei J, Stricker BHC. Incidence of priapism in the general population. Urology. 2001;57(5):970-972.
3. Halls JE, Patel DV, Walkden M, et al. Priapism: pathophysiology and the role of the radiologist. Br J Radiol. 2012;85(Spec Iss 1):S79-S85.
4. Wang CS, Kao WT, Chen CD, et al. Priapism associated with typical and atypical antipsychotic medications. Int Clinical Psychopharmacology. 2006;21(4):245-248.
5. Khan Q, Tucker P, Lokhande A. Priapism: what cause: mental illness, psychotropic medications or polysubstance abuse? J Okla State Med Assoc. 2016;109(11):515-517.
6. Dent LA, Brown WC, Murney JD. Citalopram-induced priapism. Pharmacotherapy. 2002;22(4):538-541.
7. Wilkening GL, Kucherer SA, Douaihy AB. Priapism and renal colic in a patient treated with duloxetine. Mental Health Clinician. 2016;6(4):197-200.
8. Morrison BF, Burnett AL. Stuttering priapism: insight into its pathogenesis and management. Curr Urol Rep. 2012;13(4):268-276.
9. Burnett AL, Bivalacqua TJ. Priapism: current principles and practice. Urol Clin North Am. 2007;34(4):631-642.
10. Stahl SM. Stahl’s essential psychopharmacology: neuroscientific basis and practical applications. 4th ed. Cambridge, United Kingdom: Cambridge University Press; 2013.
11. Pereira CA, Rodrigues FL, Ruginsk SG, et al. Chronic treatment with fluoxetine modulates vascular adrenergic responses by inhibition of pre- and post-synaptic mechanisms. Eu J Pharmacol. 2017;800:70-80.
1. Kadioglu A, Sanli O, Celtik M, et al. Practical management of patients with priapism. EAU-EBU Update Series. 2006;4(4):150-160.
2. Eland IA, van der Lei J, Stricker BHC. Incidence of priapism in the general population. Urology. 2001;57(5):970-972.
3. Halls JE, Patel DV, Walkden M, et al. Priapism: pathophysiology and the role of the radiologist. Br J Radiol. 2012;85(Spec Iss 1):S79-S85.
4. Wang CS, Kao WT, Chen CD, et al. Priapism associated with typical and atypical antipsychotic medications. Int Clinical Psychopharmacology. 2006;21(4):245-248.
5. Khan Q, Tucker P, Lokhande A. Priapism: what cause: mental illness, psychotropic medications or polysubstance abuse? J Okla State Med Assoc. 2016;109(11):515-517.
6. Dent LA, Brown WC, Murney JD. Citalopram-induced priapism. Pharmacotherapy. 2002;22(4):538-541.
7. Wilkening GL, Kucherer SA, Douaihy AB. Priapism and renal colic in a patient treated with duloxetine. Mental Health Clinician. 2016;6(4):197-200.
8. Morrison BF, Burnett AL. Stuttering priapism: insight into its pathogenesis and management. Curr Urol Rep. 2012;13(4):268-276.
9. Burnett AL, Bivalacqua TJ. Priapism: current principles and practice. Urol Clin North Am. 2007;34(4):631-642.
10. Stahl SM. Stahl’s essential psychopharmacology: neuroscientific basis and practical applications. 4th ed. Cambridge, United Kingdom: Cambridge University Press; 2013.
11. Pereira CA, Rodrigues FL, Ruginsk SG, et al. Chronic treatment with fluoxetine modulates vascular adrenergic responses by inhibition of pre- and post-synaptic mechanisms. Eu J Pharmacol. 2017;800:70-80.
Impact of the MTHFR C677T genetic variant on depression
Ms. T, age 55, presents to her psychiatrist’s clinic with a chief complaint of ongoing symptoms of anhedonia and lethargy related to her diagnosis of major depressive disorder (MDD). She also has a history of peripheral arterial disease, hypothyroidism, and generalized anxiety disorder. Her current antidepressant regimen is duloxetine, 60 mg/d, and mirtazapine, 15 mg at night. She recently elected to undergo pharmacogenetic testing, which showed that she is heterozygous for the methylenetetrahydrofolate reductase (MTHFR) C677T mutation (MTHFR C677T CT carrier). Her test report states that she may have impaired folate metabolism. Her psychiatrist adds L-methylfolate, 15 mg/d, to her current antidepressant regimen.
What is the relationship between folic acid and MTHFR?
Methylenetetrahydrofolate reductase is an intracellular enzyme responsible for one of several steps involved in converting dietary folic acid to its physiologically active form, L-methylfolate.1 Once active, L-methylfolate can be transported into the CNS, where it participates in one-carbon transfer reactions.2,3 Mutations in the MTHFR gene have been associated with decreased activity of the enzyme, which has been shown to result in accumulation of homocysteine and may lead to decreased synthesis of neurotransmitters.2,4Commercial pharmacogenetic testing panels may offer MTHFR genetic testing to assist with prescribing decisions for patients with mental illness. The most well-characterized mutation currently is C677T (rsID1801133), which is a single amino acid base pair change (cytosine [C] to thymine [T]) that leads to increased thermolability and instability of the enzyme.5 Carrying 1 or 2 T alleles can lead to a 35% or 70% reduction in enzyme activity, respectively. The T variant allele is most frequent in Hispanics (20% to 25%), Asians (up to 63%), and Caucasians (8% to 20%); however, it is relatively uncommon in African Americans (<2%).5,6 Another variant, A1289C (rs1801131), has also been associated with decreased enzyme function, particularly when analyzed in combination with C677T. However, carrying the 1289C variant allele does not appear to result in as large of a reduction of enzyme function as the 677T variant.7
What is the relationship between MTHFR C677T and depression?
Some researchers have proposed that the C677T mutation in MTHFR may be associated with depression as a result of decreased neurotransmitter synthesis, but studies have not consistently supported this hypothesis. Several studies suggest an association between MTHFR mutations and MDD8-10:
Jiang et al8 performed a meta-analysis of 13 studies including 1,295 Chinese patients and found that having at least 1 C677T variant allele was significantly associated with an increased risk of depression (for T vs C odds ratio 1.52, 95% confidence interval 1.24 to 1.85). The authors noted a stronger association identified in the Northern Chinese population compared with the Southern Chinese population.8
Bousman et al9 found that American patients with MDD and the 677CC genotype had greater Patient Health Questionnaire-9 (PHQ-9) scores at assessments at 24, 36, and 48 months post-baseline compared with those with the 677TT genotype (P = .024), which was unexpected based on previously reported associations.9
Schiepers et al10 also assessed the association between the MTHFR genotype in a Dutch ambulatory care population over 12 years. There was no association identified between scores on the depression subscale of the Symptom Checklist 90 and C677T diplotype.10
Table 16,8-12 provides summaries of these and other selected studies on MTHFR and MDD. Overall, although a pathophysiological basis for depression and decreased MTHFR function has been proposed, the current body of literature does not indicate a consistent link between MTHFR C677T genetic variants alone and depression.
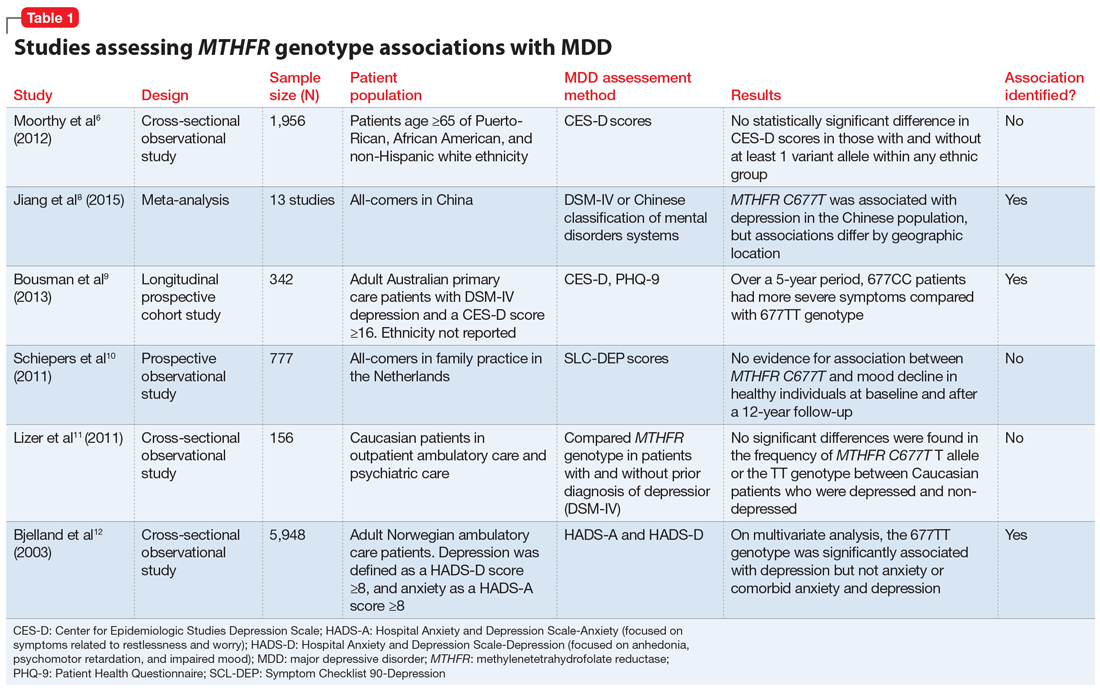
Continue to: Medication changes based on MTHFR: What is the evidence?
Medication changes based on MTHFR: What is the evidence?
Some evidence supports the use of active folate supplementation to improve symptoms of MDD.
Shelton et al3 conducted an observational study that assessed the effects of adding L-methylfolate (brand name: Deplin), 7.5 or 15 mg, to existing antidepressant therapy in 502 patients with MDD who had baseline PHQ-9 scores of at least 5. After an average 95 days of therapy, PHQ-9 scores were reduced by a mean of 8.5 points, with 67.9% of patients achieving at least a 50% reduction in PHQ-9 scores. The study did not take into account patients’ MTHFR genotype or differentiate results between the 2 doses of L-methylfolate.3
Papakostas et al13 performed 2 randomized, double-blind, parallel-sequential, placebo-controlled trials of L-methylfolate for patients with MDD. The first compared L-methylfolate, 7.5 and 15 mg, to placebo, without regard to MTHFR genotype.13 There was no significant difference between the 7.5-mg dose and placebo, or the 15-mg dose and placebo. However, among the group receiving the 15-mg dose, the response rate was 24%, vs 9% in the placebo group, which approached significance (P = .1). Papakostas et al13 followed up with a smaller trial comparing the 15-mg dose alone to placebo, and found the response rate was 32.3% in patients treated with L-methylfolate compared with 14.6% in the placebo group (P = .04).13
Although the Shelton et al3 and Papakostas et al13 studies showed some improvement in depressive symptom scores among patients who received L-methylfolate supplementation, an important consideration is if MTHFR genotype may predict patient response to this therapy.
Papakostas et al14 performed a post hoc analysis of their earlier study to assess potential associations amongst multiple other biomarkers of inflammation and metabolic disturbances hypothesized by the authors to be associated with MDD, as well as body mass index (BMI), with treatment outcome.14 When change in the Hamilton Depression Rating Scale-28 (HDRS-28) was analyzed by C677T and A1298C variant groups (677 CT vs TT and 1298 AC vs CC), no statistically significant improvements were identified (C677T mean change from baseline −3.8 points, P = .087; A1298C mean change from baseline −0.5 points, P = .807).14 However, statistically significant improvements in HDRS-28 scores were observed compared with baseline when the C677T genotype was pooled with other biomarkers, including methionine synthase (MTR 2756 AG/GG, −23.3 points vs baseline, P < .001) and a voltage-dependent calcium channel (CACNAIC AG/AA, −9 points vs baseline, P < .001), as well as with BMI ≥ 30 kg/m2 (−9.9 points vs baseline, P = .001).14
Continue to: Mech and Farah...
Mech and Farah15 performed a randomized, double-blind, placebo-controlled study of the use of EnLyte, a supplement containing 7-mg L-methylfolate, in patients with at least 1 variant of MTHFR (either C677T or A1298C) over an 8-week period. In addition to L-methylfolate, this supplement contains other active ingredients, including leucovorin (or folinic acid), magnesium ascorbate, and ferrous glycine cysteinate. Montgomery-Åsberg Depression Scale (MADRS) scores improved by 12 points in patients who received the supplement and by 1.3 points in patients who received placebo. However, because the supplement contained many ingredients, the response observed in this study cannot be attributed to L-methylfolate alone.15
Table 23,13,15,16 contains summaries of these and other selected studies assessing active folate supplementation in MDD.
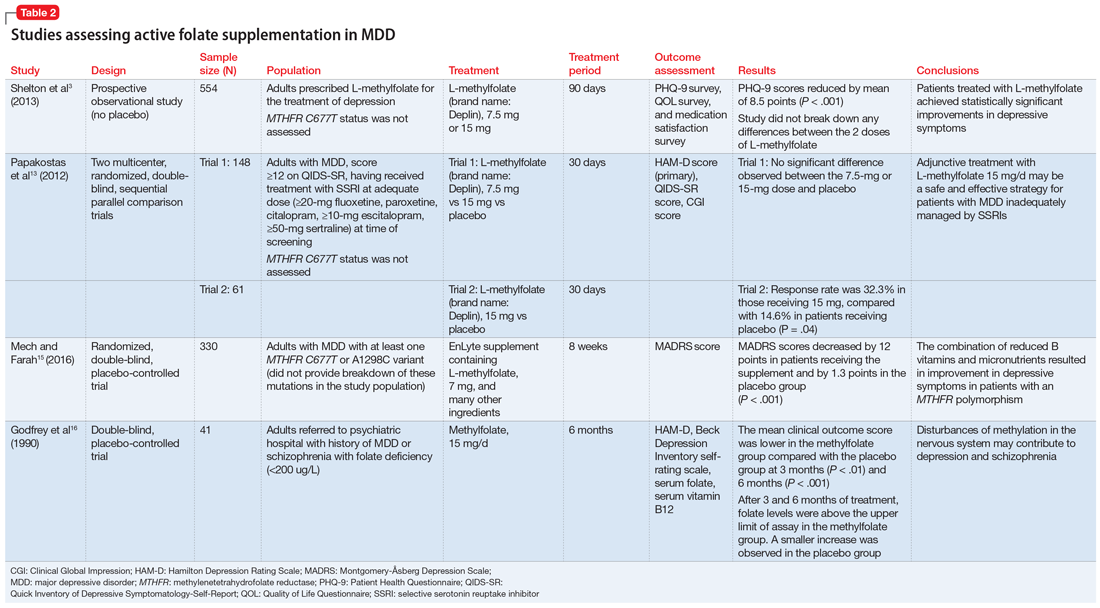
CASE CONTINUED
Over the next several weeks, Ms. T experiences some modest improvement in mood while taking L-methylfolate and her antidepressant regimen, and she experiences no notable adverse effects. Unfortunately, after 3 months, Ms. T discontinues the supplement due to the cost.
The value of MTHFR testing
Ms. T’s case is an example of how clinicians may respond to MTHFR pharmacogenetic testing. Although L-methylfolate has shown some benefit in several randomized clinical trials, available data do not confirm the relevance of MTHFR functional status to symptom response. Additionally, there is likely interplay among multiple factors affecting patients’ response to L-methylfolate. Larger randomized trials prospectively assessing other pharmacogenetic and lifestyle factors may shed more light on which patients would benefit.
Based on available data, the decision to prescribe L-methylfolate should not necessarily hinge on MTHFR genetics alone. Both patients and clinicians must be aware of the potentially prohibitive cost if L-methylfolate is recommended, as prescription insurance may not provide coverage (eg, a recent search on GoodRx.com showed that generic L-methylfolate was approximately $40 for 30 tablets; prices may vary). Additionally, clinicians should be aware that L-methylfolate is regulated as a medical food product and is not subject to strict quality standards required for prescription medications. Future prospective studies assessing the use of L-methylfolate specifically in patients with a MTHFR variants while investigating other relevant covariates may help identify which specific patient populations would benefit from supplementation.
Continue to: Related Resources
Related Resources
- Gilbody S, Lewis S, Lightfoot T. Methylenetetrahydrofolate reductase (MTHFR) genetic polymorphisms and psychiatric disorders: a HuGE review. Am J Epidemiol. 2007;165(1):1-13.
- Trimmer E. Methylenetetrahydrofolate reductase: biochemical characterization and medical significance. Current Pharmaceutical Design. 2013;19(4):2574-3595.
Drug Brand Names
Citalopram • Celexa
Duloxetine • Cymbalta
Escitalopram • Lexapro
Fluoxetine • Prozac
L-methylfolate • Deplin
Mirtazapine • Remeron
Paroxetine • Paxil
Sertraline • Zoloft
1. Scaglione F, Panzavolta G. Folate, folic acid and 5-methyltetrahydrofolate are not the same thing. Xenobiotica. 2014;44(5):480-488.
2. Jadavji N, Wieske F, Dirnagl U, et al. Methylenetetrahydrofolate reductase deficiency alters levels of glutamate and gamma-aminobutyric acid in brain tissue. Molecular Genetics and Metabolism Reports. 2015;3(Issue C):1-4.
3. Shelton R, Manning J, Barrentine L, et al. Assessing effects of L-methylfolate in depression management: results of a real-world patient experience trial. Prim Care Companion CNS Disord. 2013;15(4):pii:PCC.13m01520. doi: 10.4088/PCC.13m01520.
4. Brustolin S, Giugliani R, Felix T. Genetics of homocysteine metabolism and associated disorders. Braz J Med Biol Res. 2010;43(1):1-7.
5. Blom H, Smulders Y. Overview of homocysteine and folate metabolism. With special references to cardiovascular disease and neural tube defects. J Inherit Metab Dis. 2011;34:75-81.
6. Moorthy D, Peter I, Scott T, et al. Status of vitamins B-12 and B-6 but not of folate, homocysteine, and the methylenetetrahydrofolate reductase C677T polymorphism are associated with impaired cognition and depression in adults. J Nutr. 2012;142:1554-1560.
7. Lievers K, Boers G, Verhoef P, et al. A second common variant in the methylenetetrahydrofolate reductase (MTHFR) gene and its relationship to MTHFR enzyme activity, homocysteine, and cardiovascular disease risk. J Mol Med (Berl). 2001;79(9):522-528.
8. Jiang W, Xu J, Lu X, et al. Association between MTHFR C677T polymorphism and depression: a meta-analysis in the Chinese population. Psychol Health Med. 2015;21(6):675-685.
9. Bousman C, Potiriadis M, Everall I, et al. Methylenetetrahydrofolate reductase (MTHFR) genetic variation and major depressive disorder prognosis: a five-year prospective cohort study of primary care attendees. Am J Med Genet B Neuropsychiatr Genet. 2014;165B(1):68-76.
10. Schiepers O, Van Boxtel M, de Groot R, et al. Genetic variation in folate metabolism is not associated with cognitive functioning or mood in healthy adults. Prog Neuro-Psychopharmacol Biol Psychiatry. 2011;35(7):1682-1688.
11. Lizer M, Bogdan R, Kidd R. Comparison of the frequency of the methylenetetrahydrofolate reductase (MTHFR) C677T polymorphism in depressed versus nondepressed patients. J Psychiatr Pract. 2011;17(6):404-409.
12. Bjelland I, Tell G, Vollset S, et al. Folate, vitamin B12, homocysteine, and the MTHFR 677C->T polymorphism in anxiety and depression: the Hordaland Homocysteine Study. Arch Gen Psychiatry. 2003;60(6):618-626.
13. Papakostas G, Shelton R, Zajecka J, et al. L-methylfolate as adjunctive therapy for SSRI-resistant major depression: results of two randomized, double-blind, parallel sequential trials. Am J Psychiatry. 2012;169(12):1267-1274.
14. Papakostas G, Shelton R, Zajecka J, et al. Effect of adjunctive L-methylfolate 15 mg among inadequate responders to SSRIs in depressed patients who were stratified by biomarker levels and genotype: results from a randomized clinical trial. J Clin Psychiatry. 2014;75(8):855-863.
15. Mech A, Farah A. Correlation of clinical response with homocysteine reduction during therapy with reduced B vitamins in patients with MDD who are positive for MTHFR C677T or A1298C polymorphism: a randomized, double-blind, placebo-controlled study. J Clin Psychiatry. 2016;77(5):668-671.
16. Godfrey P, Toone B, Carney M, et al. Enhancement of recovery from psychiatric illness by methylfolate. Lancet. 1990;336(8712):392-395.
Ms. T, age 55, presents to her psychiatrist’s clinic with a chief complaint of ongoing symptoms of anhedonia and lethargy related to her diagnosis of major depressive disorder (MDD). She also has a history of peripheral arterial disease, hypothyroidism, and generalized anxiety disorder. Her current antidepressant regimen is duloxetine, 60 mg/d, and mirtazapine, 15 mg at night. She recently elected to undergo pharmacogenetic testing, which showed that she is heterozygous for the methylenetetrahydrofolate reductase (MTHFR) C677T mutation (MTHFR C677T CT carrier). Her test report states that she may have impaired folate metabolism. Her psychiatrist adds L-methylfolate, 15 mg/d, to her current antidepressant regimen.
What is the relationship between folic acid and MTHFR?
Methylenetetrahydrofolate reductase is an intracellular enzyme responsible for one of several steps involved in converting dietary folic acid to its physiologically active form, L-methylfolate.1 Once active, L-methylfolate can be transported into the CNS, where it participates in one-carbon transfer reactions.2,3 Mutations in the MTHFR gene have been associated with decreased activity of the enzyme, which has been shown to result in accumulation of homocysteine and may lead to decreased synthesis of neurotransmitters.2,4Commercial pharmacogenetic testing panels may offer MTHFR genetic testing to assist with prescribing decisions for patients with mental illness. The most well-characterized mutation currently is C677T (rsID1801133), which is a single amino acid base pair change (cytosine [C] to thymine [T]) that leads to increased thermolability and instability of the enzyme.5 Carrying 1 or 2 T alleles can lead to a 35% or 70% reduction in enzyme activity, respectively. The T variant allele is most frequent in Hispanics (20% to 25%), Asians (up to 63%), and Caucasians (8% to 20%); however, it is relatively uncommon in African Americans (<2%).5,6 Another variant, A1289C (rs1801131), has also been associated with decreased enzyme function, particularly when analyzed in combination with C677T. However, carrying the 1289C variant allele does not appear to result in as large of a reduction of enzyme function as the 677T variant.7
What is the relationship between MTHFR C677T and depression?
Some researchers have proposed that the C677T mutation in MTHFR may be associated with depression as a result of decreased neurotransmitter synthesis, but studies have not consistently supported this hypothesis. Several studies suggest an association between MTHFR mutations and MDD8-10:
Jiang et al8 performed a meta-analysis of 13 studies including 1,295 Chinese patients and found that having at least 1 C677T variant allele was significantly associated with an increased risk of depression (for T vs C odds ratio 1.52, 95% confidence interval 1.24 to 1.85). The authors noted a stronger association identified in the Northern Chinese population compared with the Southern Chinese population.8
Bousman et al9 found that American patients with MDD and the 677CC genotype had greater Patient Health Questionnaire-9 (PHQ-9) scores at assessments at 24, 36, and 48 months post-baseline compared with those with the 677TT genotype (P = .024), which was unexpected based on previously reported associations.9
Schiepers et al10 also assessed the association between the MTHFR genotype in a Dutch ambulatory care population over 12 years. There was no association identified between scores on the depression subscale of the Symptom Checklist 90 and C677T diplotype.10
Table 16,8-12 provides summaries of these and other selected studies on MTHFR and MDD. Overall, although a pathophysiological basis for depression and decreased MTHFR function has been proposed, the current body of literature does not indicate a consistent link between MTHFR C677T genetic variants alone and depression.

Continue to: Medication changes based on MTHFR: What is the evidence?
Medication changes based on MTHFR: What is the evidence?
Some evidence supports the use of active folate supplementation to improve symptoms of MDD.
Shelton et al3 conducted an observational study that assessed the effects of adding L-methylfolate (brand name: Deplin), 7.5 or 15 mg, to existing antidepressant therapy in 502 patients with MDD who had baseline PHQ-9 scores of at least 5. After an average 95 days of therapy, PHQ-9 scores were reduced by a mean of 8.5 points, with 67.9% of patients achieving at least a 50% reduction in PHQ-9 scores. The study did not take into account patients’ MTHFR genotype or differentiate results between the 2 doses of L-methylfolate.3
Papakostas et al13 performed 2 randomized, double-blind, parallel-sequential, placebo-controlled trials of L-methylfolate for patients with MDD. The first compared L-methylfolate, 7.5 and 15 mg, to placebo, without regard to MTHFR genotype.13 There was no significant difference between the 7.5-mg dose and placebo, or the 15-mg dose and placebo. However, among the group receiving the 15-mg dose, the response rate was 24%, vs 9% in the placebo group, which approached significance (P = .1). Papakostas et al13 followed up with a smaller trial comparing the 15-mg dose alone to placebo, and found the response rate was 32.3% in patients treated with L-methylfolate compared with 14.6% in the placebo group (P = .04).13
Although the Shelton et al3 and Papakostas et al13 studies showed some improvement in depressive symptom scores among patients who received L-methylfolate supplementation, an important consideration is if MTHFR genotype may predict patient response to this therapy.
Papakostas et al14 performed a post hoc analysis of their earlier study to assess potential associations amongst multiple other biomarkers of inflammation and metabolic disturbances hypothesized by the authors to be associated with MDD, as well as body mass index (BMI), with treatment outcome.14 When change in the Hamilton Depression Rating Scale-28 (HDRS-28) was analyzed by C677T and A1298C variant groups (677 CT vs TT and 1298 AC vs CC), no statistically significant improvements were identified (C677T mean change from baseline −3.8 points, P = .087; A1298C mean change from baseline −0.5 points, P = .807).14 However, statistically significant improvements in HDRS-28 scores were observed compared with baseline when the C677T genotype was pooled with other biomarkers, including methionine synthase (MTR 2756 AG/GG, −23.3 points vs baseline, P < .001) and a voltage-dependent calcium channel (CACNAIC AG/AA, −9 points vs baseline, P < .001), as well as with BMI ≥ 30 kg/m2 (−9.9 points vs baseline, P = .001).14
Continue to: Mech and Farah...
Mech and Farah15 performed a randomized, double-blind, placebo-controlled study of the use of EnLyte, a supplement containing 7-mg L-methylfolate, in patients with at least 1 variant of MTHFR (either C677T or A1298C) over an 8-week period. In addition to L-methylfolate, this supplement contains other active ingredients, including leucovorin (or folinic acid), magnesium ascorbate, and ferrous glycine cysteinate. Montgomery-Åsberg Depression Scale (MADRS) scores improved by 12 points in patients who received the supplement and by 1.3 points in patients who received placebo. However, because the supplement contained many ingredients, the response observed in this study cannot be attributed to L-methylfolate alone.15
Table 23,13,15,16 contains summaries of these and other selected studies assessing active folate supplementation in MDD.

CASE CONTINUED
Over the next several weeks, Ms. T experiences some modest improvement in mood while taking L-methylfolate and her antidepressant regimen, and she experiences no notable adverse effects. Unfortunately, after 3 months, Ms. T discontinues the supplement due to the cost.
The value of MTHFR testing
Ms. T’s case is an example of how clinicians may respond to MTHFR pharmacogenetic testing. Although L-methylfolate has shown some benefit in several randomized clinical trials, available data do not confirm the relevance of MTHFR functional status to symptom response. Additionally, there is likely interplay among multiple factors affecting patients’ response to L-methylfolate. Larger randomized trials prospectively assessing other pharmacogenetic and lifestyle factors may shed more light on which patients would benefit.
Based on available data, the decision to prescribe L-methylfolate should not necessarily hinge on MTHFR genetics alone. Both patients and clinicians must be aware of the potentially prohibitive cost if L-methylfolate is recommended, as prescription insurance may not provide coverage (eg, a recent search on GoodRx.com showed that generic L-methylfolate was approximately $40 for 30 tablets; prices may vary). Additionally, clinicians should be aware that L-methylfolate is regulated as a medical food product and is not subject to strict quality standards required for prescription medications. Future prospective studies assessing the use of L-methylfolate specifically in patients with a MTHFR variants while investigating other relevant covariates may help identify which specific patient populations would benefit from supplementation.
Continue to: Related Resources
Related Resources
- Gilbody S, Lewis S, Lightfoot T. Methylenetetrahydrofolate reductase (MTHFR) genetic polymorphisms and psychiatric disorders: a HuGE review. Am J Epidemiol. 2007;165(1):1-13.
- Trimmer E. Methylenetetrahydrofolate reductase: biochemical characterization and medical significance. Current Pharmaceutical Design. 2013;19(4):2574-3595.
Drug Brand Names
Citalopram • Celexa
Duloxetine • Cymbalta
Escitalopram • Lexapro
Fluoxetine • Prozac
L-methylfolate • Deplin
Mirtazapine • Remeron
Paroxetine • Paxil
Sertraline • Zoloft
Ms. T, age 55, presents to her psychiatrist’s clinic with a chief complaint of ongoing symptoms of anhedonia and lethargy related to her diagnosis of major depressive disorder (MDD). She also has a history of peripheral arterial disease, hypothyroidism, and generalized anxiety disorder. Her current antidepressant regimen is duloxetine, 60 mg/d, and mirtazapine, 15 mg at night. She recently elected to undergo pharmacogenetic testing, which showed that she is heterozygous for the methylenetetrahydrofolate reductase (MTHFR) C677T mutation (MTHFR C677T CT carrier). Her test report states that she may have impaired folate metabolism. Her psychiatrist adds L-methylfolate, 15 mg/d, to her current antidepressant regimen.
What is the relationship between folic acid and MTHFR?
Methylenetetrahydrofolate reductase is an intracellular enzyme responsible for one of several steps involved in converting dietary folic acid to its physiologically active form, L-methylfolate.1 Once active, L-methylfolate can be transported into the CNS, where it participates in one-carbon transfer reactions.2,3 Mutations in the MTHFR gene have been associated with decreased activity of the enzyme, which has been shown to result in accumulation of homocysteine and may lead to decreased synthesis of neurotransmitters.2,4Commercial pharmacogenetic testing panels may offer MTHFR genetic testing to assist with prescribing decisions for patients with mental illness. The most well-characterized mutation currently is C677T (rsID1801133), which is a single amino acid base pair change (cytosine [C] to thymine [T]) that leads to increased thermolability and instability of the enzyme.5 Carrying 1 or 2 T alleles can lead to a 35% or 70% reduction in enzyme activity, respectively. The T variant allele is most frequent in Hispanics (20% to 25%), Asians (up to 63%), and Caucasians (8% to 20%); however, it is relatively uncommon in African Americans (<2%).5,6 Another variant, A1289C (rs1801131), has also been associated with decreased enzyme function, particularly when analyzed in combination with C677T. However, carrying the 1289C variant allele does not appear to result in as large of a reduction of enzyme function as the 677T variant.7
What is the relationship between MTHFR C677T and depression?
Some researchers have proposed that the C677T mutation in MTHFR may be associated with depression as a result of decreased neurotransmitter synthesis, but studies have not consistently supported this hypothesis. Several studies suggest an association between MTHFR mutations and MDD8-10:
Jiang et al8 performed a meta-analysis of 13 studies including 1,295 Chinese patients and found that having at least 1 C677T variant allele was significantly associated with an increased risk of depression (for T vs C odds ratio 1.52, 95% confidence interval 1.24 to 1.85). The authors noted a stronger association identified in the Northern Chinese population compared with the Southern Chinese population.8
Bousman et al9 found that American patients with MDD and the 677CC genotype had greater Patient Health Questionnaire-9 (PHQ-9) scores at assessments at 24, 36, and 48 months post-baseline compared with those with the 677TT genotype (P = .024), which was unexpected based on previously reported associations.9
Schiepers et al10 also assessed the association between the MTHFR genotype in a Dutch ambulatory care population over 12 years. There was no association identified between scores on the depression subscale of the Symptom Checklist 90 and C677T diplotype.10
Table 16,8-12 provides summaries of these and other selected studies on MTHFR and MDD. Overall, although a pathophysiological basis for depression and decreased MTHFR function has been proposed, the current body of literature does not indicate a consistent link between MTHFR C677T genetic variants alone and depression.

Continue to: Medication changes based on MTHFR: What is the evidence?
Medication changes based on MTHFR: What is the evidence?
Some evidence supports the use of active folate supplementation to improve symptoms of MDD.
Shelton et al3 conducted an observational study that assessed the effects of adding L-methylfolate (brand name: Deplin), 7.5 or 15 mg, to existing antidepressant therapy in 502 patients with MDD who had baseline PHQ-9 scores of at least 5. After an average 95 days of therapy, PHQ-9 scores were reduced by a mean of 8.5 points, with 67.9% of patients achieving at least a 50% reduction in PHQ-9 scores. The study did not take into account patients’ MTHFR genotype or differentiate results between the 2 doses of L-methylfolate.3
Papakostas et al13 performed 2 randomized, double-blind, parallel-sequential, placebo-controlled trials of L-methylfolate for patients with MDD. The first compared L-methylfolate, 7.5 and 15 mg, to placebo, without regard to MTHFR genotype.13 There was no significant difference between the 7.5-mg dose and placebo, or the 15-mg dose and placebo. However, among the group receiving the 15-mg dose, the response rate was 24%, vs 9% in the placebo group, which approached significance (P = .1). Papakostas et al13 followed up with a smaller trial comparing the 15-mg dose alone to placebo, and found the response rate was 32.3% in patients treated with L-methylfolate compared with 14.6% in the placebo group (P = .04).13
Although the Shelton et al3 and Papakostas et al13 studies showed some improvement in depressive symptom scores among patients who received L-methylfolate supplementation, an important consideration is if MTHFR genotype may predict patient response to this therapy.
Papakostas et al14 performed a post hoc analysis of their earlier study to assess potential associations amongst multiple other biomarkers of inflammation and metabolic disturbances hypothesized by the authors to be associated with MDD, as well as body mass index (BMI), with treatment outcome.14 When change in the Hamilton Depression Rating Scale-28 (HDRS-28) was analyzed by C677T and A1298C variant groups (677 CT vs TT and 1298 AC vs CC), no statistically significant improvements were identified (C677T mean change from baseline −3.8 points, P = .087; A1298C mean change from baseline −0.5 points, P = .807).14 However, statistically significant improvements in HDRS-28 scores were observed compared with baseline when the C677T genotype was pooled with other biomarkers, including methionine synthase (MTR 2756 AG/GG, −23.3 points vs baseline, P < .001) and a voltage-dependent calcium channel (CACNAIC AG/AA, −9 points vs baseline, P < .001), as well as with BMI ≥ 30 kg/m2 (−9.9 points vs baseline, P = .001).14
Continue to: Mech and Farah...
Mech and Farah15 performed a randomized, double-blind, placebo-controlled study of the use of EnLyte, a supplement containing 7-mg L-methylfolate, in patients with at least 1 variant of MTHFR (either C677T or A1298C) over an 8-week period. In addition to L-methylfolate, this supplement contains other active ingredients, including leucovorin (or folinic acid), magnesium ascorbate, and ferrous glycine cysteinate. Montgomery-Åsberg Depression Scale (MADRS) scores improved by 12 points in patients who received the supplement and by 1.3 points in patients who received placebo. However, because the supplement contained many ingredients, the response observed in this study cannot be attributed to L-methylfolate alone.15
Table 23,13,15,16 contains summaries of these and other selected studies assessing active folate supplementation in MDD.

CASE CONTINUED
Over the next several weeks, Ms. T experiences some modest improvement in mood while taking L-methylfolate and her antidepressant regimen, and she experiences no notable adverse effects. Unfortunately, after 3 months, Ms. T discontinues the supplement due to the cost.
The value of MTHFR testing
Ms. T’s case is an example of how clinicians may respond to MTHFR pharmacogenetic testing. Although L-methylfolate has shown some benefit in several randomized clinical trials, available data do not confirm the relevance of MTHFR functional status to symptom response. Additionally, there is likely interplay among multiple factors affecting patients’ response to L-methylfolate. Larger randomized trials prospectively assessing other pharmacogenetic and lifestyle factors may shed more light on which patients would benefit.
Based on available data, the decision to prescribe L-methylfolate should not necessarily hinge on MTHFR genetics alone. Both patients and clinicians must be aware of the potentially prohibitive cost if L-methylfolate is recommended, as prescription insurance may not provide coverage (eg, a recent search on GoodRx.com showed that generic L-methylfolate was approximately $40 for 30 tablets; prices may vary). Additionally, clinicians should be aware that L-methylfolate is regulated as a medical food product and is not subject to strict quality standards required for prescription medications. Future prospective studies assessing the use of L-methylfolate specifically in patients with a MTHFR variants while investigating other relevant covariates may help identify which specific patient populations would benefit from supplementation.
Continue to: Related Resources
Related Resources
- Gilbody S, Lewis S, Lightfoot T. Methylenetetrahydrofolate reductase (MTHFR) genetic polymorphisms and psychiatric disorders: a HuGE review. Am J Epidemiol. 2007;165(1):1-13.
- Trimmer E. Methylenetetrahydrofolate reductase: biochemical characterization and medical significance. Current Pharmaceutical Design. 2013;19(4):2574-3595.
Drug Brand Names
Citalopram • Celexa
Duloxetine • Cymbalta
Escitalopram • Lexapro
Fluoxetine • Prozac
L-methylfolate • Deplin
Mirtazapine • Remeron
Paroxetine • Paxil
Sertraline • Zoloft
1. Scaglione F, Panzavolta G. Folate, folic acid and 5-methyltetrahydrofolate are not the same thing. Xenobiotica. 2014;44(5):480-488.
2. Jadavji N, Wieske F, Dirnagl U, et al. Methylenetetrahydrofolate reductase deficiency alters levels of glutamate and gamma-aminobutyric acid in brain tissue. Molecular Genetics and Metabolism Reports. 2015;3(Issue C):1-4.
3. Shelton R, Manning J, Barrentine L, et al. Assessing effects of L-methylfolate in depression management: results of a real-world patient experience trial. Prim Care Companion CNS Disord. 2013;15(4):pii:PCC.13m01520. doi: 10.4088/PCC.13m01520.
4. Brustolin S, Giugliani R, Felix T. Genetics of homocysteine metabolism and associated disorders. Braz J Med Biol Res. 2010;43(1):1-7.
5. Blom H, Smulders Y. Overview of homocysteine and folate metabolism. With special references to cardiovascular disease and neural tube defects. J Inherit Metab Dis. 2011;34:75-81.
6. Moorthy D, Peter I, Scott T, et al. Status of vitamins B-12 and B-6 but not of folate, homocysteine, and the methylenetetrahydrofolate reductase C677T polymorphism are associated with impaired cognition and depression in adults. J Nutr. 2012;142:1554-1560.
7. Lievers K, Boers G, Verhoef P, et al. A second common variant in the methylenetetrahydrofolate reductase (MTHFR) gene and its relationship to MTHFR enzyme activity, homocysteine, and cardiovascular disease risk. J Mol Med (Berl). 2001;79(9):522-528.
8. Jiang W, Xu J, Lu X, et al. Association between MTHFR C677T polymorphism and depression: a meta-analysis in the Chinese population. Psychol Health Med. 2015;21(6):675-685.
9. Bousman C, Potiriadis M, Everall I, et al. Methylenetetrahydrofolate reductase (MTHFR) genetic variation and major depressive disorder prognosis: a five-year prospective cohort study of primary care attendees. Am J Med Genet B Neuropsychiatr Genet. 2014;165B(1):68-76.
10. Schiepers O, Van Boxtel M, de Groot R, et al. Genetic variation in folate metabolism is not associated with cognitive functioning or mood in healthy adults. Prog Neuro-Psychopharmacol Biol Psychiatry. 2011;35(7):1682-1688.
11. Lizer M, Bogdan R, Kidd R. Comparison of the frequency of the methylenetetrahydrofolate reductase (MTHFR) C677T polymorphism in depressed versus nondepressed patients. J Psychiatr Pract. 2011;17(6):404-409.
12. Bjelland I, Tell G, Vollset S, et al. Folate, vitamin B12, homocysteine, and the MTHFR 677C->T polymorphism in anxiety and depression: the Hordaland Homocysteine Study. Arch Gen Psychiatry. 2003;60(6):618-626.
13. Papakostas G, Shelton R, Zajecka J, et al. L-methylfolate as adjunctive therapy for SSRI-resistant major depression: results of two randomized, double-blind, parallel sequential trials. Am J Psychiatry. 2012;169(12):1267-1274.
14. Papakostas G, Shelton R, Zajecka J, et al. Effect of adjunctive L-methylfolate 15 mg among inadequate responders to SSRIs in depressed patients who were stratified by biomarker levels and genotype: results from a randomized clinical trial. J Clin Psychiatry. 2014;75(8):855-863.
15. Mech A, Farah A. Correlation of clinical response with homocysteine reduction during therapy with reduced B vitamins in patients with MDD who are positive for MTHFR C677T or A1298C polymorphism: a randomized, double-blind, placebo-controlled study. J Clin Psychiatry. 2016;77(5):668-671.
16. Godfrey P, Toone B, Carney M, et al. Enhancement of recovery from psychiatric illness by methylfolate. Lancet. 1990;336(8712):392-395.
1. Scaglione F, Panzavolta G. Folate, folic acid and 5-methyltetrahydrofolate are not the same thing. Xenobiotica. 2014;44(5):480-488.
2. Jadavji N, Wieske F, Dirnagl U, et al. Methylenetetrahydrofolate reductase deficiency alters levels of glutamate and gamma-aminobutyric acid in brain tissue. Molecular Genetics and Metabolism Reports. 2015;3(Issue C):1-4.
3. Shelton R, Manning J, Barrentine L, et al. Assessing effects of L-methylfolate in depression management: results of a real-world patient experience trial. Prim Care Companion CNS Disord. 2013;15(4):pii:PCC.13m01520. doi: 10.4088/PCC.13m01520.
4. Brustolin S, Giugliani R, Felix T. Genetics of homocysteine metabolism and associated disorders. Braz J Med Biol Res. 2010;43(1):1-7.
5. Blom H, Smulders Y. Overview of homocysteine and folate metabolism. With special references to cardiovascular disease and neural tube defects. J Inherit Metab Dis. 2011;34:75-81.
6. Moorthy D, Peter I, Scott T, et al. Status of vitamins B-12 and B-6 but not of folate, homocysteine, and the methylenetetrahydrofolate reductase C677T polymorphism are associated with impaired cognition and depression in adults. J Nutr. 2012;142:1554-1560.
7. Lievers K, Boers G, Verhoef P, et al. A second common variant in the methylenetetrahydrofolate reductase (MTHFR) gene and its relationship to MTHFR enzyme activity, homocysteine, and cardiovascular disease risk. J Mol Med (Berl). 2001;79(9):522-528.
8. Jiang W, Xu J, Lu X, et al. Association between MTHFR C677T polymorphism and depression: a meta-analysis in the Chinese population. Psychol Health Med. 2015;21(6):675-685.
9. Bousman C, Potiriadis M, Everall I, et al. Methylenetetrahydrofolate reductase (MTHFR) genetic variation and major depressive disorder prognosis: a five-year prospective cohort study of primary care attendees. Am J Med Genet B Neuropsychiatr Genet. 2014;165B(1):68-76.
10. Schiepers O, Van Boxtel M, de Groot R, et al. Genetic variation in folate metabolism is not associated with cognitive functioning or mood in healthy adults. Prog Neuro-Psychopharmacol Biol Psychiatry. 2011;35(7):1682-1688.
11. Lizer M, Bogdan R, Kidd R. Comparison of the frequency of the methylenetetrahydrofolate reductase (MTHFR) C677T polymorphism in depressed versus nondepressed patients. J Psychiatr Pract. 2011;17(6):404-409.
12. Bjelland I, Tell G, Vollset S, et al. Folate, vitamin B12, homocysteine, and the MTHFR 677C->T polymorphism in anxiety and depression: the Hordaland Homocysteine Study. Arch Gen Psychiatry. 2003;60(6):618-626.
13. Papakostas G, Shelton R, Zajecka J, et al. L-methylfolate as adjunctive therapy for SSRI-resistant major depression: results of two randomized, double-blind, parallel sequential trials. Am J Psychiatry. 2012;169(12):1267-1274.
14. Papakostas G, Shelton R, Zajecka J, et al. Effect of adjunctive L-methylfolate 15 mg among inadequate responders to SSRIs in depressed patients who were stratified by biomarker levels and genotype: results from a randomized clinical trial. J Clin Psychiatry. 2014;75(8):855-863.
15. Mech A, Farah A. Correlation of clinical response with homocysteine reduction during therapy with reduced B vitamins in patients with MDD who are positive for MTHFR C677T or A1298C polymorphism: a randomized, double-blind, placebo-controlled study. J Clin Psychiatry. 2016;77(5):668-671.
16. Godfrey P, Toone B, Carney M, et al. Enhancement of recovery from psychiatric illness by methylfolate. Lancet. 1990;336(8712):392-395.
The boy whose arm wouldn’t work
CASE Drooling, unsteady, and not himself
B, age 10, who is left handed and has autism spectrum disorder, is brought to the emergency department (ED) with a 1-day history of drooling, unsteady gait, and left wrist in sustained flexion. His parents report that for the past week, B has had cold symptoms, including rhinorrhea, a low-grade fever (100.0°F), and cough. Earlier in the day, he was seen at his pediatrician’s office, where he was diagnosed with an acute respiratory infection and started on amoxicillin, 500 mg twice daily for 7 days.
At baseline, B is nonverbal. He requires some assistance with his activities of daily living. He usually is able to walk without assistance and dress himself, but he is not toilet trained. His parents report that in the past day, he has had significant difficulties with tasks involving his left hand. Normally, B is able to feed himself “finger foods” but has been unable to do so today. His parents say that he has been unsteady on his feet, and has been “falling forward” when he tries to walk.
Two years ago, B was started on risperidone, 0.5 mg nightly, for behavioral aggression and self-mutilation. Over the next 12 months, the dosage was steadily increased to 1 mg twice daily, with good response. He has been taking his current dosage, 1 mg twice daily, for the past 12 months without adjustment. His parents report there have been no other medication changes, other than starting amoxicillin earlier that day.
As part of his initial ED evaluation, B is found to be mildly dehydrated, with an elevated sedimentation rate on urinalysis. His complete blood count (CBC) with differential is within normal limits. A comprehensive metabolic panel shows a slight increase in his creatinine level, indicating dehydration. B is administered IV fluid replacement because he is having difficulty drinking due to excessive drooling.
The ED physician is concerned that B may be experiencing an acute dystonic reaction from risperidone, so the team holds this medication, and gives B a one-time dose of IV diphenhydramine, 25 mg, for presumptive acute dystonic reaction. After several minutes, there is no improvement in the sustained flexion of his left wrist.
[polldaddy:10615848]
The authors’ observations
B presented with new-onset neurologic findings after a recently diagnosed upper respiratory viral illness. His symptoms appeared to be confined to his left upper extremity, specifically demonstrating left arm extension at the elbow with flexion of the left wrist. He also had new-onset unsteady gait with a stooped forward posture and required assistance with walking. Interestingly, despite B’s history of antipsychotic use, administering an anticholinergic agent did not lessen the dystonic posturing at his wrist and elbow.
EVALUATION Laboratory results reveal new clues
While in the ED, B undergoes MRI of the brain and spinal cord to rule out any mass lesions that could be impinging upon the motor pathways. Both brain and spinal cord imaging appear to be essentially normal, without evidence of impingement of the spinal nerves or lesions involving the brainstem or cerebellum.
Continue to: Due to concerns...
Due to concerns of possible airway obstruction, a CT scan of the neck is obtained to rule out any acute pathology, such as epiglottitis compromising his airway. The scan shows some inflammation and edema in the soft tissues that is thought to be secondary to his acute viral illness. B is able to maintain his airway and oxygenation, so intubation is not necessary.
A CPK test is ordered because there are concerns of sustained muscle contraction of B’s left wrist and elbow. The CPK level is 884 U/L (reference range 26 to 192 U/L). The elevation in CPK is consistent with prior laboratory findings of dehydration and indicating skeletal muscle breakdown from sustained muscle contraction. All other laboratory results, including a comprehensive metabolic panel, urine drug screen, and thyroid screening panel, are within normal limits.
[polldaddy:10615850]
EVALUATION No variation in facial expression
B is admitted to the general pediatrics service. Maintenance IV fluids are started due to concerns of dehydration and possible rhabdomyolysis due to his elevated CPK level. Risperidone is held throughout the hospital course due to concerns for an acute dystonic reaction. B is monitored for several days without clinical improvement and eventually discharged home with a diagnosis of inflammatory mononeuropathy due to viral infection. The patient is told to discontinue risperidone as part of discharge instructions.
Five days later, B returns to the hospital because there was no improvement in his left extremity or walking. His left elbow remains extended with left wrist in flexion. Psychiatry is consulted for further diagnostic clarity and evaluation.
On physical examination, B’s left arm remains unchanged. Despite discontinuing risperidone, there is evidence of cogwheel rigidity of the left wrist joint. Reflexes in the upper and lower extremities are 2+ and symmetrical bilaterally, suggesting intact upper and lower motor pathways. Babinski sign is absent bilaterally, which is a normal finding in B’s age group. B continues to have difficulty with ambulating and appears to “fall forward” while trying to walk with assistance. His parents also say that B is not laughing, smiling, or showing any variation in facial expression.
Continue to: Additional family history...
Additional family history is gathered from B’s parents for possible hereditary movement disorders such as Wilson’s disease. They report that no family members have developed involuntary movements or other neurologic syndromes. Additional considerations on the differential diagnosis for B include juvenile ALS or mononeuropathy involving the C5 and C6 nerve roots. B’s parents deny any recent shoulder trauma, and radiographic studies did not demonstrate any involvement of the nerve roots.
TREATMENT A trial of bromocriptine
At this point, B’s neurologic workup is essentially normal, and he is given a provisional diagnosis of antipsychotic-induced tardive dystonia vs tardive parkinsonism. Risperidone continues to be held, and B is monitored for clinical improvement. B is administered a one-time dose of diphenhydramine, 25 mg, for dystonia with no improvement in symptoms. He is then started on bromocriptine, 1.25 mg twice daily with meals, for parkinsonian symptoms secondary to antipsychotic medication use. After 1 day of treatment, B shows less sustained flexion of his left wrist. He is able to relax his left arm, shows improvements in ambulation, and requires less assistance. B continues to be observed closely and continues to improve toward his baseline.
At Day 4, he is discharged. B is able to walk mostly without assistance and demonstrates improvement in left wrist flexion. He is scheduled to see a movement disorders specialist a week after discharge. The initial diagnosis given by the movement disorder specialist is tardive dystonia.
The authors’ observations
Tardive dyskinesia is a well-known iatrogenic effect of antipsychotic medications that are commonly used to manage conditions such as schizophrenia or behavioral agitation associated with autism spectrum disorder. Symptoms of tardive dyskinesia typically emerge after 1 to 2 years of continuous exposure to dopamine receptor blocking agents (DRBAs). Tardive dyskinesia symptoms include involuntary, repetitive, purposeless movements of the tongue, jaw, lips, face, trunk, and upper and lower extremities, with significant functional impairment.1
Tardive syndromes refer to a diverse array of hyperkinetic, hypokinetic, and sensory movement disorders resulting from at least 3 months of continuous DRBA therapy.2 Tardive dyskinesia is perhaps the most well-known of the tardive syndromes, but is not the only one to consider when assessing for antipsychotic-induced movement disorders. A key feature differentiating a tardive syndrome is the persistence of the movement disorder after the DRBA is discontinued. In this case, B had been receiving a stable dose of risperidone for >1 year. He developed dystonic posturing of his left wrist and elbow that was both unresponsive to anticholinergic medication and persisted after risperidone was discontinued. The term “tardive” emphasizes the delay in development of abnormal involuntary movement symptoms after initiating antipsychotic medications.3 Table 12 shows a comparison of tardive dystonia vs an acute dystonic reaction.
Continue to: Other tardive syndromes include...
Other tardive syndromes include:
- tardive tics
- tardive parkinsonism
- tardive pain
- tardive myoclonus
- tardive akathisia
- tardive tremors.
The incidence of tardive syndromes increases 5% annually for the first 5 years of treatment. At 10 years of treatment, the annual incidence is thought to be 49%, and at 25 years of treatment, 68%.4 The predominant theory of the pathophysiology of tardive syndromes is that the chronic use of DRBAs causes a gradual hypersensitization of dopamine receptors.4 The diagnosis of a tardive syndrome is based on history of exposure to a DRBA as well as clinical observation of symptoms.
Compared with classic tardive dyskinesia, tardive dystonia is more common among younger patients. The mean age of onset of tardive dystonia is 40, and it typically affects young males.5 Typical posturing observed in cases of tardive dystonia include extension of the arms and flexion at the wrists.6 In contrast to cases of primary dystonia, tardive dystonia is typically associated with stereotypies, akathisia, or other movement disorders. Anticholinergic agents, such as
The American Psychiatric Association has issued guidelines on screening for involuntary movement syndromes by using the Abnormal Involuntary Movement Scale (AIMS).7 The current recommendations include assessment every 6 months for patients receiving first-generation antipsychotics, and every 12 months for those receiving second-generation antipsychotics.7 Prescribers should also carefully assess for any pre-existing involuntary movements before prescribing a DRBA.7
[polldaddy:10615855]
The authors’ observations
In 2013, the American Academy of Neurology (AAN) published guidelines on the treatment of tardive dyskinesia. According to these guidelines, at that time, the treatments with the most evidence supporting their use were clonazepam, ginkgo biloba,
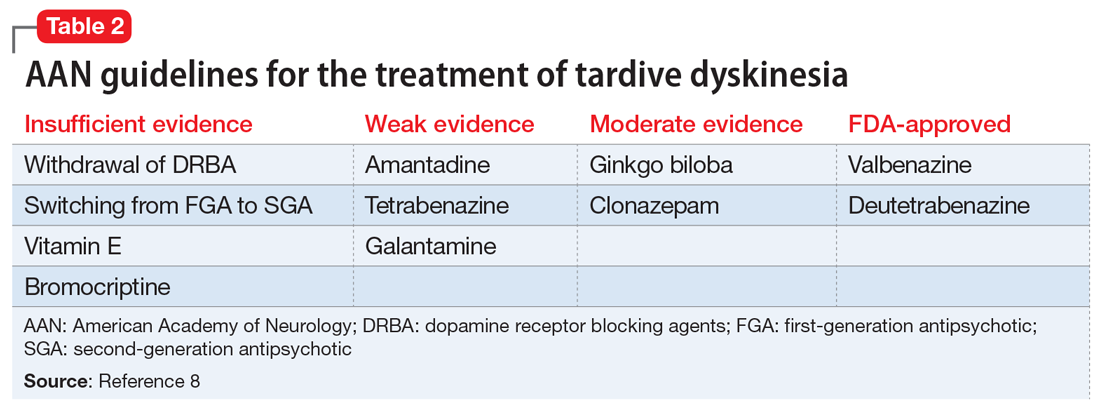
Continue to: In 2017, valbenazine and deutetrabenazine...
In 2017, valbenazine and deutetrabenazine became the first FDA-approved treatments for tardive dyskinesia in adults. Both medications block the vesicular monoamine transporter 2 (VMAT2) system, which results in decreased synaptic dopamine and dopamine receptor stimulation. Both VMAT2 inhibitor medications have a category level A supporting their use for treating tardive dyskinesia.8-10
Currently, there are no published treatment guidelines on pharmacologic management of tardive dystonia. In B’s case, bromocriptine, a dopamine agonist, was used to counter the dopamine-blocking effects of risperidone on the nigrostriatal pathway and improve parkinsonian features of B’s presentation, including bradykinesia, stooped forward posture, and masked facies. Bromocriptine was found to be effective in alleviating parkinsonian features; however, to date there is no evidence demonstrating its effectiveness in countering delayed dystonic effects of DRBAs.
OUTCOME Improvement of dystonia symptoms
One week after discharge, B is seen for a follow-up visit. He continues taking bromocriptine, 1.25 mg twice daily, with meals after discharge. On examination, he has some evidence of tardive dystonia, including flexion of left wrist and posturing while ambulating. B’s parkinsonian features, including stooped forward posture, masked facies, and cogwheel rigidity of the left wrist muscle, have resolved. B is now able to walk on his own without unsteadiness. Bromocriptine is discontinued after 1 month, and his symptoms of dystonia continue to improve.
Two months after hospitalization, B is started on quetiapine, 25 mg twice daily, for behavioral aggression. Quetiapine is chosen because it has a lower dopamine receptor affinity compared with risperidone, and theoretically, quetiapine is associated with a lower risk of developing tardive symptoms. During the next 6 months, B is monitored closely for recurrence of tardive symptoms. Quetiapine is slowly titrated to 25 mg in the morning, and 50 mg at bedtime. His behavioral agitation improves significantly and he does not have a recurrence of tardive symptoms.
Bottom Line
Tardive dystonia is a possible iatrogenic adverse effect for patients receiving long-term dopamine receptor blocking agent (DRBA) therapy. Tardive syndromes encompass delayed-onset movement disorders caused by long-term blockade of the dopamine receptor by antipsychotic agents. Tardive dystonia can be contrasted from acute dystonic reaction based on the time course of development as well as by the persistence of symptoms after DRBAs are withheld.
Continue to: Related Resources
Related Resources
- American Academy of Neurology. Summary of evidence-based guideline for clinicians: treatment of tardive syndromes. https://www.aan.com/Guidelines/Home/GetGuidelineContent/613. Published 2013.
- Dystonia Medical Research Foundation. https://dystonia-foundation.org/.
Drug Brand Names
Amantadine • Gocovri, Symmetrel
Amoxicillin • Amoxil
Baclofen • Kemstro, Liroesal
Benztropine • Cogentin
Bromocriptine • Parlodel
Clonazepam • Klonopin
Deutetrabenazine • Austedo
Galantamine • Razadyne
Quetiapine • Seroquel
Risperidone • Risperdal
Tetrabenazine • Xenazine
Trihexyphenidyl • Artane, Tremin
Valbenazine • Ingrezza
1. Margolese HC, Chouinard G, Kolivakis TT, et al. Tardive dyskinesia in the era of typical and atypical antipsychotics. Part 1: pathophysiology and mechanisms of induction. Can J Psychiatr. 2005;50(9):541-547.
2. Truong D, Frei K. Setting the record straight: the nosology of tardive syndromes. Parkinsonism Relat Disord. 2019;59:146-150.
3. Cornett EM, Novitch M, Kaye AD, et al. Medication-induced tardive dyskinesia: a review and update. Ochsner J. 2017;17(2):162-174.
4. Schooler NR, Kane JM. Research diagnoses for tardive dyskinesia. Arch Gen Psychiatry. 1982;39(4):486-487.
5. Fahn S, Jankovic J, Hallett M. Principles and Practice of Movement Disorders. 2nd ed. Philadelphia, PA: Saunders; 2011:415-446.
6. Kang UJ, Burke RE, Fahn S. Natural history and treatment of tardive dystonia. Mov Disord. 1986;1(3):193-208.
7. Lehman AF, Lieberman JA, Dixon LB, et al. Practice guideline for the treatment of patients with schizophrenia, second edition. Am J Psychiatry. 2004;161(suppl 2):1-56.
8. Bhidayasiri R, Fahn S, Weiner WJ, et al, Evidence-based guideline: treatment of tardive syndromes: report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology. 2013;81(5):463-469.
9. Ingrezza [package insert]. San Diego, CA: Neurocrine Biosciences, Inc.; 2020.
10. Austedo [package insert]. North Wales, PA: Teva Pharmaceuticals; 2017.
CASE Drooling, unsteady, and not himself
B, age 10, who is left handed and has autism spectrum disorder, is brought to the emergency department (ED) with a 1-day history of drooling, unsteady gait, and left wrist in sustained flexion. His parents report that for the past week, B has had cold symptoms, including rhinorrhea, a low-grade fever (100.0°F), and cough. Earlier in the day, he was seen at his pediatrician’s office, where he was diagnosed with an acute respiratory infection and started on amoxicillin, 500 mg twice daily for 7 days.
At baseline, B is nonverbal. He requires some assistance with his activities of daily living. He usually is able to walk without assistance and dress himself, but he is not toilet trained. His parents report that in the past day, he has had significant difficulties with tasks involving his left hand. Normally, B is able to feed himself “finger foods” but has been unable to do so today. His parents say that he has been unsteady on his feet, and has been “falling forward” when he tries to walk.
Two years ago, B was started on risperidone, 0.5 mg nightly, for behavioral aggression and self-mutilation. Over the next 12 months, the dosage was steadily increased to 1 mg twice daily, with good response. He has been taking his current dosage, 1 mg twice daily, for the past 12 months without adjustment. His parents report there have been no other medication changes, other than starting amoxicillin earlier that day.
As part of his initial ED evaluation, B is found to be mildly dehydrated, with an elevated sedimentation rate on urinalysis. His complete blood count (CBC) with differential is within normal limits. A comprehensive metabolic panel shows a slight increase in his creatinine level, indicating dehydration. B is administered IV fluid replacement because he is having difficulty drinking due to excessive drooling.
The ED physician is concerned that B may be experiencing an acute dystonic reaction from risperidone, so the team holds this medication, and gives B a one-time dose of IV diphenhydramine, 25 mg, for presumptive acute dystonic reaction. After several minutes, there is no improvement in the sustained flexion of his left wrist.
[polldaddy:10615848]
The authors’ observations
B presented with new-onset neurologic findings after a recently diagnosed upper respiratory viral illness. His symptoms appeared to be confined to his left upper extremity, specifically demonstrating left arm extension at the elbow with flexion of the left wrist. He also had new-onset unsteady gait with a stooped forward posture and required assistance with walking. Interestingly, despite B’s history of antipsychotic use, administering an anticholinergic agent did not lessen the dystonic posturing at his wrist and elbow.
EVALUATION Laboratory results reveal new clues
While in the ED, B undergoes MRI of the brain and spinal cord to rule out any mass lesions that could be impinging upon the motor pathways. Both brain and spinal cord imaging appear to be essentially normal, without evidence of impingement of the spinal nerves or lesions involving the brainstem or cerebellum.
Continue to: Due to concerns...
Due to concerns of possible airway obstruction, a CT scan of the neck is obtained to rule out any acute pathology, such as epiglottitis compromising his airway. The scan shows some inflammation and edema in the soft tissues that is thought to be secondary to his acute viral illness. B is able to maintain his airway and oxygenation, so intubation is not necessary.
A CPK test is ordered because there are concerns of sustained muscle contraction of B’s left wrist and elbow. The CPK level is 884 U/L (reference range 26 to 192 U/L). The elevation in CPK is consistent with prior laboratory findings of dehydration and indicating skeletal muscle breakdown from sustained muscle contraction. All other laboratory results, including a comprehensive metabolic panel, urine drug screen, and thyroid screening panel, are within normal limits.
[polldaddy:10615850]
EVALUATION No variation in facial expression
B is admitted to the general pediatrics service. Maintenance IV fluids are started due to concerns of dehydration and possible rhabdomyolysis due to his elevated CPK level. Risperidone is held throughout the hospital course due to concerns for an acute dystonic reaction. B is monitored for several days without clinical improvement and eventually discharged home with a diagnosis of inflammatory mononeuropathy due to viral infection. The patient is told to discontinue risperidone as part of discharge instructions.
Five days later, B returns to the hospital because there was no improvement in his left extremity or walking. His left elbow remains extended with left wrist in flexion. Psychiatry is consulted for further diagnostic clarity and evaluation.
On physical examination, B’s left arm remains unchanged. Despite discontinuing risperidone, there is evidence of cogwheel rigidity of the left wrist joint. Reflexes in the upper and lower extremities are 2+ and symmetrical bilaterally, suggesting intact upper and lower motor pathways. Babinski sign is absent bilaterally, which is a normal finding in B’s age group. B continues to have difficulty with ambulating and appears to “fall forward” while trying to walk with assistance. His parents also say that B is not laughing, smiling, or showing any variation in facial expression.
Continue to: Additional family history...
Additional family history is gathered from B’s parents for possible hereditary movement disorders such as Wilson’s disease. They report that no family members have developed involuntary movements or other neurologic syndromes. Additional considerations on the differential diagnosis for B include juvenile ALS or mononeuropathy involving the C5 and C6 nerve roots. B’s parents deny any recent shoulder trauma, and radiographic studies did not demonstrate any involvement of the nerve roots.
TREATMENT A trial of bromocriptine
At this point, B’s neurologic workup is essentially normal, and he is given a provisional diagnosis of antipsychotic-induced tardive dystonia vs tardive parkinsonism. Risperidone continues to be held, and B is monitored for clinical improvement. B is administered a one-time dose of diphenhydramine, 25 mg, for dystonia with no improvement in symptoms. He is then started on bromocriptine, 1.25 mg twice daily with meals, for parkinsonian symptoms secondary to antipsychotic medication use. After 1 day of treatment, B shows less sustained flexion of his left wrist. He is able to relax his left arm, shows improvements in ambulation, and requires less assistance. B continues to be observed closely and continues to improve toward his baseline.
At Day 4, he is discharged. B is able to walk mostly without assistance and demonstrates improvement in left wrist flexion. He is scheduled to see a movement disorders specialist a week after discharge. The initial diagnosis given by the movement disorder specialist is tardive dystonia.
The authors’ observations
Tardive dyskinesia is a well-known iatrogenic effect of antipsychotic medications that are commonly used to manage conditions such as schizophrenia or behavioral agitation associated with autism spectrum disorder. Symptoms of tardive dyskinesia typically emerge after 1 to 2 years of continuous exposure to dopamine receptor blocking agents (DRBAs). Tardive dyskinesia symptoms include involuntary, repetitive, purposeless movements of the tongue, jaw, lips, face, trunk, and upper and lower extremities, with significant functional impairment.1
Tardive syndromes refer to a diverse array of hyperkinetic, hypokinetic, and sensory movement disorders resulting from at least 3 months of continuous DRBA therapy.2 Tardive dyskinesia is perhaps the most well-known of the tardive syndromes, but is not the only one to consider when assessing for antipsychotic-induced movement disorders. A key feature differentiating a tardive syndrome is the persistence of the movement disorder after the DRBA is discontinued. In this case, B had been receiving a stable dose of risperidone for >1 year. He developed dystonic posturing of his left wrist and elbow that was both unresponsive to anticholinergic medication and persisted after risperidone was discontinued. The term “tardive” emphasizes the delay in development of abnormal involuntary movement symptoms after initiating antipsychotic medications.3 Table 12 shows a comparison of tardive dystonia vs an acute dystonic reaction.

Continue to: Other tardive syndromes include...
Other tardive syndromes include:
- tardive tics
- tardive parkinsonism
- tardive pain
- tardive myoclonus
- tardive akathisia
- tardive tremors.
The incidence of tardive syndromes increases 5% annually for the first 5 years of treatment. At 10 years of treatment, the annual incidence is thought to be 49%, and at 25 years of treatment, 68%.4 The predominant theory of the pathophysiology of tardive syndromes is that the chronic use of DRBAs causes a gradual hypersensitization of dopamine receptors.4 The diagnosis of a tardive syndrome is based on history of exposure to a DRBA as well as clinical observation of symptoms.
Compared with classic tardive dyskinesia, tardive dystonia is more common among younger patients. The mean age of onset of tardive dystonia is 40, and it typically affects young males.5 Typical posturing observed in cases of tardive dystonia include extension of the arms and flexion at the wrists.6 In contrast to cases of primary dystonia, tardive dystonia is typically associated with stereotypies, akathisia, or other movement disorders. Anticholinergic agents, such as
The American Psychiatric Association has issued guidelines on screening for involuntary movement syndromes by using the Abnormal Involuntary Movement Scale (AIMS).7 The current recommendations include assessment every 6 months for patients receiving first-generation antipsychotics, and every 12 months for those receiving second-generation antipsychotics.7 Prescribers should also carefully assess for any pre-existing involuntary movements before prescribing a DRBA.7
[polldaddy:10615855]
The authors’ observations
In 2013, the American Academy of Neurology (AAN) published guidelines on the treatment of tardive dyskinesia. According to these guidelines, at that time, the treatments with the most evidence supporting their use were clonazepam, ginkgo biloba,

Continue to: In 2017, valbenazine and deutetrabenazine...
In 2017, valbenazine and deutetrabenazine became the first FDA-approved treatments for tardive dyskinesia in adults. Both medications block the vesicular monoamine transporter 2 (VMAT2) system, which results in decreased synaptic dopamine and dopamine receptor stimulation. Both VMAT2 inhibitor medications have a category level A supporting their use for treating tardive dyskinesia.8-10
Currently, there are no published treatment guidelines on pharmacologic management of tardive dystonia. In B’s case, bromocriptine, a dopamine agonist, was used to counter the dopamine-blocking effects of risperidone on the nigrostriatal pathway and improve parkinsonian features of B’s presentation, including bradykinesia, stooped forward posture, and masked facies. Bromocriptine was found to be effective in alleviating parkinsonian features; however, to date there is no evidence demonstrating its effectiveness in countering delayed dystonic effects of DRBAs.
OUTCOME Improvement of dystonia symptoms
One week after discharge, B is seen for a follow-up visit. He continues taking bromocriptine, 1.25 mg twice daily, with meals after discharge. On examination, he has some evidence of tardive dystonia, including flexion of left wrist and posturing while ambulating. B’s parkinsonian features, including stooped forward posture, masked facies, and cogwheel rigidity of the left wrist muscle, have resolved. B is now able to walk on his own without unsteadiness. Bromocriptine is discontinued after 1 month, and his symptoms of dystonia continue to improve.
Two months after hospitalization, B is started on quetiapine, 25 mg twice daily, for behavioral aggression. Quetiapine is chosen because it has a lower dopamine receptor affinity compared with risperidone, and theoretically, quetiapine is associated with a lower risk of developing tardive symptoms. During the next 6 months, B is monitored closely for recurrence of tardive symptoms. Quetiapine is slowly titrated to 25 mg in the morning, and 50 mg at bedtime. His behavioral agitation improves significantly and he does not have a recurrence of tardive symptoms.
Bottom Line
Tardive dystonia is a possible iatrogenic adverse effect for patients receiving long-term dopamine receptor blocking agent (DRBA) therapy. Tardive syndromes encompass delayed-onset movement disorders caused by long-term blockade of the dopamine receptor by antipsychotic agents. Tardive dystonia can be contrasted from acute dystonic reaction based on the time course of development as well as by the persistence of symptoms after DRBAs are withheld.
Continue to: Related Resources
Related Resources
- American Academy of Neurology. Summary of evidence-based guideline for clinicians: treatment of tardive syndromes. https://www.aan.com/Guidelines/Home/GetGuidelineContent/613. Published 2013.
- Dystonia Medical Research Foundation. https://dystonia-foundation.org/.
Drug Brand Names
Amantadine • Gocovri, Symmetrel
Amoxicillin • Amoxil
Baclofen • Kemstro, Liroesal
Benztropine • Cogentin
Bromocriptine • Parlodel
Clonazepam • Klonopin
Deutetrabenazine • Austedo
Galantamine • Razadyne
Quetiapine • Seroquel
Risperidone • Risperdal
Tetrabenazine • Xenazine
Trihexyphenidyl • Artane, Tremin
Valbenazine • Ingrezza
CASE Drooling, unsteady, and not himself
B, age 10, who is left handed and has autism spectrum disorder, is brought to the emergency department (ED) with a 1-day history of drooling, unsteady gait, and left wrist in sustained flexion. His parents report that for the past week, B has had cold symptoms, including rhinorrhea, a low-grade fever (100.0°F), and cough. Earlier in the day, he was seen at his pediatrician’s office, where he was diagnosed with an acute respiratory infection and started on amoxicillin, 500 mg twice daily for 7 days.
At baseline, B is nonverbal. He requires some assistance with his activities of daily living. He usually is able to walk without assistance and dress himself, but he is not toilet trained. His parents report that in the past day, he has had significant difficulties with tasks involving his left hand. Normally, B is able to feed himself “finger foods” but has been unable to do so today. His parents say that he has been unsteady on his feet, and has been “falling forward” when he tries to walk.
Two years ago, B was started on risperidone, 0.5 mg nightly, for behavioral aggression and self-mutilation. Over the next 12 months, the dosage was steadily increased to 1 mg twice daily, with good response. He has been taking his current dosage, 1 mg twice daily, for the past 12 months without adjustment. His parents report there have been no other medication changes, other than starting amoxicillin earlier that day.
As part of his initial ED evaluation, B is found to be mildly dehydrated, with an elevated sedimentation rate on urinalysis. His complete blood count (CBC) with differential is within normal limits. A comprehensive metabolic panel shows a slight increase in his creatinine level, indicating dehydration. B is administered IV fluid replacement because he is having difficulty drinking due to excessive drooling.
The ED physician is concerned that B may be experiencing an acute dystonic reaction from risperidone, so the team holds this medication, and gives B a one-time dose of IV diphenhydramine, 25 mg, for presumptive acute dystonic reaction. After several minutes, there is no improvement in the sustained flexion of his left wrist.
[polldaddy:10615848]
The authors’ observations
B presented with new-onset neurologic findings after a recently diagnosed upper respiratory viral illness. His symptoms appeared to be confined to his left upper extremity, specifically demonstrating left arm extension at the elbow with flexion of the left wrist. He also had new-onset unsteady gait with a stooped forward posture and required assistance with walking. Interestingly, despite B’s history of antipsychotic use, administering an anticholinergic agent did not lessen the dystonic posturing at his wrist and elbow.
EVALUATION Laboratory results reveal new clues
While in the ED, B undergoes MRI of the brain and spinal cord to rule out any mass lesions that could be impinging upon the motor pathways. Both brain and spinal cord imaging appear to be essentially normal, without evidence of impingement of the spinal nerves or lesions involving the brainstem or cerebellum.
Continue to: Due to concerns...
Due to concerns of possible airway obstruction, a CT scan of the neck is obtained to rule out any acute pathology, such as epiglottitis compromising his airway. The scan shows some inflammation and edema in the soft tissues that is thought to be secondary to his acute viral illness. B is able to maintain his airway and oxygenation, so intubation is not necessary.
A CPK test is ordered because there are concerns of sustained muscle contraction of B’s left wrist and elbow. The CPK level is 884 U/L (reference range 26 to 192 U/L). The elevation in CPK is consistent with prior laboratory findings of dehydration and indicating skeletal muscle breakdown from sustained muscle contraction. All other laboratory results, including a comprehensive metabolic panel, urine drug screen, and thyroid screening panel, are within normal limits.
[polldaddy:10615850]
EVALUATION No variation in facial expression
B is admitted to the general pediatrics service. Maintenance IV fluids are started due to concerns of dehydration and possible rhabdomyolysis due to his elevated CPK level. Risperidone is held throughout the hospital course due to concerns for an acute dystonic reaction. B is monitored for several days without clinical improvement and eventually discharged home with a diagnosis of inflammatory mononeuropathy due to viral infection. The patient is told to discontinue risperidone as part of discharge instructions.
Five days later, B returns to the hospital because there was no improvement in his left extremity or walking. His left elbow remains extended with left wrist in flexion. Psychiatry is consulted for further diagnostic clarity and evaluation.
On physical examination, B’s left arm remains unchanged. Despite discontinuing risperidone, there is evidence of cogwheel rigidity of the left wrist joint. Reflexes in the upper and lower extremities are 2+ and symmetrical bilaterally, suggesting intact upper and lower motor pathways. Babinski sign is absent bilaterally, which is a normal finding in B’s age group. B continues to have difficulty with ambulating and appears to “fall forward” while trying to walk with assistance. His parents also say that B is not laughing, smiling, or showing any variation in facial expression.
Continue to: Additional family history...
Additional family history is gathered from B’s parents for possible hereditary movement disorders such as Wilson’s disease. They report that no family members have developed involuntary movements or other neurologic syndromes. Additional considerations on the differential diagnosis for B include juvenile ALS or mononeuropathy involving the C5 and C6 nerve roots. B’s parents deny any recent shoulder trauma, and radiographic studies did not demonstrate any involvement of the nerve roots.
TREATMENT A trial of bromocriptine
At this point, B’s neurologic workup is essentially normal, and he is given a provisional diagnosis of antipsychotic-induced tardive dystonia vs tardive parkinsonism. Risperidone continues to be held, and B is monitored for clinical improvement. B is administered a one-time dose of diphenhydramine, 25 mg, for dystonia with no improvement in symptoms. He is then started on bromocriptine, 1.25 mg twice daily with meals, for parkinsonian symptoms secondary to antipsychotic medication use. After 1 day of treatment, B shows less sustained flexion of his left wrist. He is able to relax his left arm, shows improvements in ambulation, and requires less assistance. B continues to be observed closely and continues to improve toward his baseline.
At Day 4, he is discharged. B is able to walk mostly without assistance and demonstrates improvement in left wrist flexion. He is scheduled to see a movement disorders specialist a week after discharge. The initial diagnosis given by the movement disorder specialist is tardive dystonia.
The authors’ observations
Tardive dyskinesia is a well-known iatrogenic effect of antipsychotic medications that are commonly used to manage conditions such as schizophrenia or behavioral agitation associated with autism spectrum disorder. Symptoms of tardive dyskinesia typically emerge after 1 to 2 years of continuous exposure to dopamine receptor blocking agents (DRBAs). Tardive dyskinesia symptoms include involuntary, repetitive, purposeless movements of the tongue, jaw, lips, face, trunk, and upper and lower extremities, with significant functional impairment.1
Tardive syndromes refer to a diverse array of hyperkinetic, hypokinetic, and sensory movement disorders resulting from at least 3 months of continuous DRBA therapy.2 Tardive dyskinesia is perhaps the most well-known of the tardive syndromes, but is not the only one to consider when assessing for antipsychotic-induced movement disorders. A key feature differentiating a tardive syndrome is the persistence of the movement disorder after the DRBA is discontinued. In this case, B had been receiving a stable dose of risperidone for >1 year. He developed dystonic posturing of his left wrist and elbow that was both unresponsive to anticholinergic medication and persisted after risperidone was discontinued. The term “tardive” emphasizes the delay in development of abnormal involuntary movement symptoms after initiating antipsychotic medications.3 Table 12 shows a comparison of tardive dystonia vs an acute dystonic reaction.

Continue to: Other tardive syndromes include...
Other tardive syndromes include:
- tardive tics
- tardive parkinsonism
- tardive pain
- tardive myoclonus
- tardive akathisia
- tardive tremors.
The incidence of tardive syndromes increases 5% annually for the first 5 years of treatment. At 10 years of treatment, the annual incidence is thought to be 49%, and at 25 years of treatment, 68%.4 The predominant theory of the pathophysiology of tardive syndromes is that the chronic use of DRBAs causes a gradual hypersensitization of dopamine receptors.4 The diagnosis of a tardive syndrome is based on history of exposure to a DRBA as well as clinical observation of symptoms.
Compared with classic tardive dyskinesia, tardive dystonia is more common among younger patients. The mean age of onset of tardive dystonia is 40, and it typically affects young males.5 Typical posturing observed in cases of tardive dystonia include extension of the arms and flexion at the wrists.6 In contrast to cases of primary dystonia, tardive dystonia is typically associated with stereotypies, akathisia, or other movement disorders. Anticholinergic agents, such as
The American Psychiatric Association has issued guidelines on screening for involuntary movement syndromes by using the Abnormal Involuntary Movement Scale (AIMS).7 The current recommendations include assessment every 6 months for patients receiving first-generation antipsychotics, and every 12 months for those receiving second-generation antipsychotics.7 Prescribers should also carefully assess for any pre-existing involuntary movements before prescribing a DRBA.7
[polldaddy:10615855]
The authors’ observations
In 2013, the American Academy of Neurology (AAN) published guidelines on the treatment of tardive dyskinesia. According to these guidelines, at that time, the treatments with the most evidence supporting their use were clonazepam, ginkgo biloba,

Continue to: In 2017, valbenazine and deutetrabenazine...
In 2017, valbenazine and deutetrabenazine became the first FDA-approved treatments for tardive dyskinesia in adults. Both medications block the vesicular monoamine transporter 2 (VMAT2) system, which results in decreased synaptic dopamine and dopamine receptor stimulation. Both VMAT2 inhibitor medications have a category level A supporting their use for treating tardive dyskinesia.8-10
Currently, there are no published treatment guidelines on pharmacologic management of tardive dystonia. In B’s case, bromocriptine, a dopamine agonist, was used to counter the dopamine-blocking effects of risperidone on the nigrostriatal pathway and improve parkinsonian features of B’s presentation, including bradykinesia, stooped forward posture, and masked facies. Bromocriptine was found to be effective in alleviating parkinsonian features; however, to date there is no evidence demonstrating its effectiveness in countering delayed dystonic effects of DRBAs.
OUTCOME Improvement of dystonia symptoms
One week after discharge, B is seen for a follow-up visit. He continues taking bromocriptine, 1.25 mg twice daily, with meals after discharge. On examination, he has some evidence of tardive dystonia, including flexion of left wrist and posturing while ambulating. B’s parkinsonian features, including stooped forward posture, masked facies, and cogwheel rigidity of the left wrist muscle, have resolved. B is now able to walk on his own without unsteadiness. Bromocriptine is discontinued after 1 month, and his symptoms of dystonia continue to improve.
Two months after hospitalization, B is started on quetiapine, 25 mg twice daily, for behavioral aggression. Quetiapine is chosen because it has a lower dopamine receptor affinity compared with risperidone, and theoretically, quetiapine is associated with a lower risk of developing tardive symptoms. During the next 6 months, B is monitored closely for recurrence of tardive symptoms. Quetiapine is slowly titrated to 25 mg in the morning, and 50 mg at bedtime. His behavioral agitation improves significantly and he does not have a recurrence of tardive symptoms.
Bottom Line
Tardive dystonia is a possible iatrogenic adverse effect for patients receiving long-term dopamine receptor blocking agent (DRBA) therapy. Tardive syndromes encompass delayed-onset movement disorders caused by long-term blockade of the dopamine receptor by antipsychotic agents. Tardive dystonia can be contrasted from acute dystonic reaction based on the time course of development as well as by the persistence of symptoms after DRBAs are withheld.
Continue to: Related Resources
Related Resources
- American Academy of Neurology. Summary of evidence-based guideline for clinicians: treatment of tardive syndromes. https://www.aan.com/Guidelines/Home/GetGuidelineContent/613. Published 2013.
- Dystonia Medical Research Foundation. https://dystonia-foundation.org/.
Drug Brand Names
Amantadine • Gocovri, Symmetrel
Amoxicillin • Amoxil
Baclofen • Kemstro, Liroesal
Benztropine • Cogentin
Bromocriptine • Parlodel
Clonazepam • Klonopin
Deutetrabenazine • Austedo
Galantamine • Razadyne
Quetiapine • Seroquel
Risperidone • Risperdal
Tetrabenazine • Xenazine
Trihexyphenidyl • Artane, Tremin
Valbenazine • Ingrezza
1. Margolese HC, Chouinard G, Kolivakis TT, et al. Tardive dyskinesia in the era of typical and atypical antipsychotics. Part 1: pathophysiology and mechanisms of induction. Can J Psychiatr. 2005;50(9):541-547.
2. Truong D, Frei K. Setting the record straight: the nosology of tardive syndromes. Parkinsonism Relat Disord. 2019;59:146-150.
3. Cornett EM, Novitch M, Kaye AD, et al. Medication-induced tardive dyskinesia: a review and update. Ochsner J. 2017;17(2):162-174.
4. Schooler NR, Kane JM. Research diagnoses for tardive dyskinesia. Arch Gen Psychiatry. 1982;39(4):486-487.
5. Fahn S, Jankovic J, Hallett M. Principles and Practice of Movement Disorders. 2nd ed. Philadelphia, PA: Saunders; 2011:415-446.
6. Kang UJ, Burke RE, Fahn S. Natural history and treatment of tardive dystonia. Mov Disord. 1986;1(3):193-208.
7. Lehman AF, Lieberman JA, Dixon LB, et al. Practice guideline for the treatment of patients with schizophrenia, second edition. Am J Psychiatry. 2004;161(suppl 2):1-56.
8. Bhidayasiri R, Fahn S, Weiner WJ, et al, Evidence-based guideline: treatment of tardive syndromes: report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology. 2013;81(5):463-469.
9. Ingrezza [package insert]. San Diego, CA: Neurocrine Biosciences, Inc.; 2020.
10. Austedo [package insert]. North Wales, PA: Teva Pharmaceuticals; 2017.
1. Margolese HC, Chouinard G, Kolivakis TT, et al. Tardive dyskinesia in the era of typical and atypical antipsychotics. Part 1: pathophysiology and mechanisms of induction. Can J Psychiatr. 2005;50(9):541-547.
2. Truong D, Frei K. Setting the record straight: the nosology of tardive syndromes. Parkinsonism Relat Disord. 2019;59:146-150.
3. Cornett EM, Novitch M, Kaye AD, et al. Medication-induced tardive dyskinesia: a review and update. Ochsner J. 2017;17(2):162-174.
4. Schooler NR, Kane JM. Research diagnoses for tardive dyskinesia. Arch Gen Psychiatry. 1982;39(4):486-487.
5. Fahn S, Jankovic J, Hallett M. Principles and Practice of Movement Disorders. 2nd ed. Philadelphia, PA: Saunders; 2011:415-446.
6. Kang UJ, Burke RE, Fahn S. Natural history and treatment of tardive dystonia. Mov Disord. 1986;1(3):193-208.
7. Lehman AF, Lieberman JA, Dixon LB, et al. Practice guideline for the treatment of patients with schizophrenia, second edition. Am J Psychiatry. 2004;161(suppl 2):1-56.
8. Bhidayasiri R, Fahn S, Weiner WJ, et al, Evidence-based guideline: treatment of tardive syndromes: report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology. 2013;81(5):463-469.
9. Ingrezza [package insert]. San Diego, CA: Neurocrine Biosciences, Inc.; 2020.
10. Austedo [package insert]. North Wales, PA: Teva Pharmaceuticals; 2017.
Obsessions or psychosis?
CASE Perseverating on nonexistent sexual assaults
Mr. R, age 17, who has been diagnosed with obsessive-compulsive disorder (OCD), presents to the emergency department (ED) because he thinks that he is being sexually assaulted and is concerned that he is sexually assaulting other people. His family reports that Mr. R has perseverated over these thoughts for months, although there is no evidence to suggest these events have occurred. In order to ameliorate his distress, he performs rituals of looking upwards and repeatedly saying, “It didn’t happen.”
Mr. R is admitted to the inpatient psychiatry unit for further evaluation.
HISTORY Decompensation while attending a PHP
Mr. R had been diagnosed with bipolar disorder and attention-deficit/hyperactivity disorder when he was 13. During that time, he was treated with divalproex sodium and dextroamphetamine. At age 15, Mr. R’s diagnosis was changed to OCD. Seven months before coming to the ED, his symptoms had been getting worse. On one occasion, Mr. R was talking in a nonsensical fashion at school, and the police were called. Mr. R became physically aggressive with the police and was subsequently hospitalized, after which he attended a partial hospitalization program (PHP). At the PHP, Mr. R received exposure and response prevention therapy for OCD, but did not improve, and his symptoms deteriorated until he was unable to brush his teeth or shower regularly. While attending the PHP, Mr. R also developed disorganized speech. The PHP clinicians became concerned that Mr. R’s symptoms may have been prodromal symptoms of schizophrenia because he did not respond to the OCD treatment and his symptoms had worsened over the 3 months he attended the PHP.
EVALUATION Normal laboratory results
Upon admission to the inpatient psychiatric unit, Mr. R is restarted on his home medications, which include
His laboratory workup, including a complete blood count, comprehensive metabolic panel, urine drug screen, and blood ethanol, are all within normal limits. Previous laboratory results, including a thyroid function panel, vitamin D level, and various autoimmune panels, were also within normal limits.
His family reports that Mr. R’s symptoms seem to worsen when he is under increased stress from school and prepping for standardized college admission examinations. The family also says that while he is playing tennis, Mr. R will posture himself in a crouched down position and at times will remain in this position for 30 minutes.
Mr. R says he eventually wants to go to college and have a professional career.
[polldaddy:10600530]
Continue to: The authors' observations
The authors’ observations
When considering Mr. R’s diagnosis, our treatment team considered the possibility of OCD with absent insight/delusional beliefs, OCD with comorbid schizophrenia, bipolar disorder, and psychotic disorder due to another medical condition.
Overlap between OCD and schizophrenia
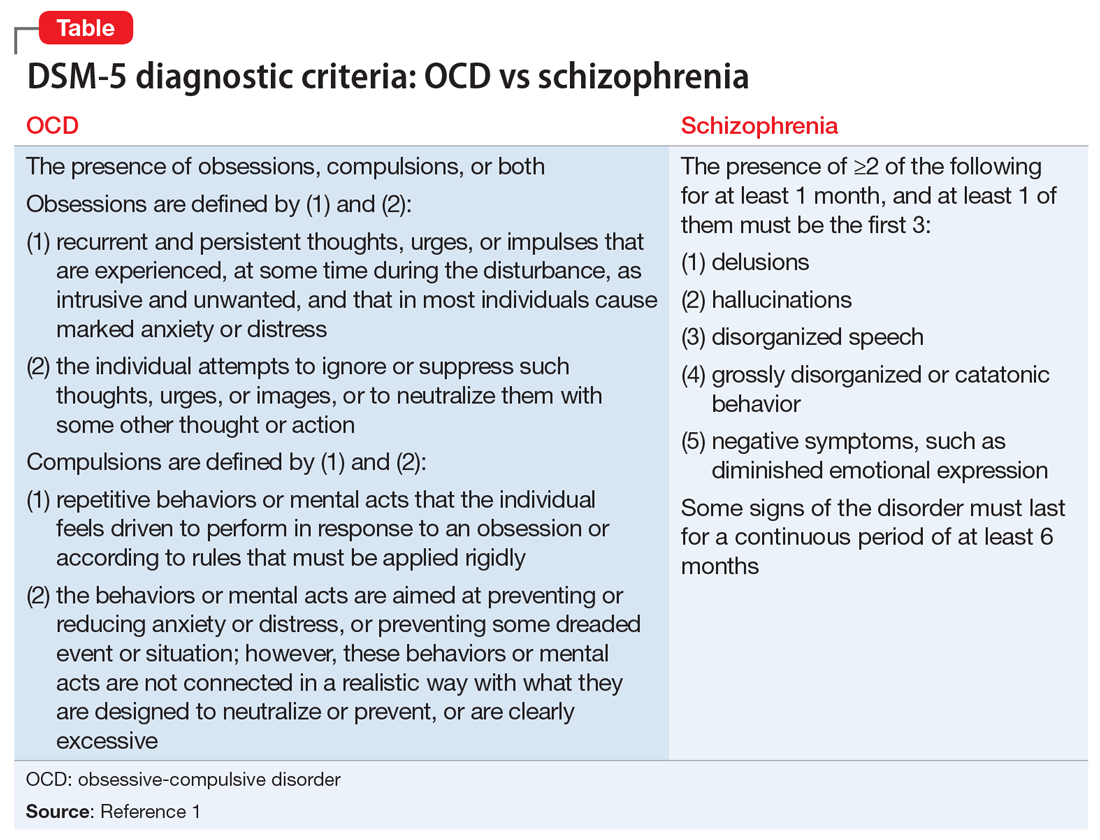
Much of the literature about OCD examines its functional impairment in adults, with findings extrapolated to pediatric patients. Children differ from adults in a variety of meaningful ways. Baytunca et al4 examined adolescents with early-onset schizophrenia, with and without comorbid OCD. Patients with comorbid OCD required higher doses of antipsychotic medication to treat acute psychotic symptoms and maintain a reduction in symptoms. The study controlled for the severity of schizophrenia, and its findings suggest that schizophrenia with comorbid OCD is more treatment-resistant than schizophrenia alone.4
Some researchers have theorized that in adolescents, OCD and psychosis are integrally related such that one disorder could represent a prodrome or a cause of the other disorder. Niendam et al5 studied OCS in the psychosis prodrome. They found that OCS can present as a part of the prodromal picture in youth at high risk for psychosis. However, because none of the patients experiencing OCS converted to full-blown psychosis, these results suggest that OCS may not represent a prodrome to psychosis per se. Instead, these individuals may represent a subset of false positives over the follow-up period.5 Another possible explanation for the increased emergence of pre-psychotic symptoms in adolescents with OCD could be a difference in their threshold of perception. OCS compels adolescents with OCD to self-analyze more critically and frequently. As a result, these patients may more often report depressive symptoms, distress, and exacerbations of pre-psychotic symptoms. These findings highlight that
[polldaddy:10600532]
Continue to: TREATMENT Improvement after switching to haloperidol
TREATMENT Improvement after switching to haloperidol
The treatment team decides to change Mr. R’s medications by cross-titrating risperidone to
The treatment team obtains a consultation on whether electroconvulsive therapy would be appropriate, but this treatment is not recommended. Instead, the team considers
Throughout admission, Mr. R focuses on his lack of improvement and how this episode is negatively impacting his grades and his dream of going to college and having a professional career.
OUTCOME Relief at last
Mr. R improves with the addition of sertraline and tolerates rapid titration well. He continues haloperidol without adverse effects, and is discharged home with close follow-up in a PHP and outpatient psychiatry.
However, after discharge, Mr. R’s symptoms get worse, and he is admitted to a different inpatient facility. At this facility, he continues sertraline, but haloperidol is cross-titrated to
Continue to: Currently...
Currently, Mr. R has greatly improved and is able to function in school. He takes sertraline, 100 mg twice a day, and olanzapine, 7.5 mg twice a day. Mr. R reports his rituals have reduced in frequency to less than 15 minutes each day. His thought processes are organized, and he is confident he will be able to achieve his goals.
The authors’ observations
Given Mr. R’s rapid improvement once an effective pharmacologic regimen was established, we concluded that he had a severe case of OCD with absent insight/delusional beliefs, and that he did not have schizophrenia. Mr. R’s case highlights how a psychiatric diagnosis can produce anxiety as a result of the psychosocial stressors and limitations associated with that diagnosis.
Bottom Line
There is both an epidemiologic and biologic overlap between obsessive-compulsive disorder and schizophrenia. In adolescents, either disorder could represent a prodrome or a cause of the other. It is essential to perform a thorough assessment of individuals with obsessive-compulsive disorder because these patients may exhibit subtle psychotic symptoms.
Related Resources
- Cunill R, Castells X, Simeon D. Relationships between obsessivecompulsive symptomatology and severity of psychosis in schizophrenia: a systematic review and meta-analysis. J Clin Psychiatry. 2009;70(1):70-82.
- Harris E, Delgado SV. Treatment-resistant OCD: there’s more we can do. Current Psychiatry. 2018;17(11):10-12,14-18,51.
Drug Brand Names
Clozapine • Clozaril
Dextroamphetamine • Dexedrine
Divalproex sodium • Depakote
Fluvoxamine • Luvox
Haloperidol • Haldol
Hydroxyzine • Atarax, Vistaril
Lurasidone • Latuda
Olanzapine • Zyprexa
Risperidone • Risperdal
Sertraline • Zoloft
1. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
2. Schirmbeck F, Swets M, de Haan L. Obsessive-compulsive symptoms in schizophrenia. In: De Haan L, Schirmbeck F, Zink M. Epidemiology: prevalence and clinical characteristics of obsessive-compulsive disorder and obsessive-compulsive symptoms in patients with psychotic disorders. New York, NY: Springer International Publishing; 2015:47-61.
3. de Haan L, Sterk B, Wouters L, et al. The 5-year course of obsessive-compulsive symptoms and obsessive-compulsive disorder in first-episode schizophrenia and related disorders. Schizophr Bull. 2011;39(1):151-160.
4. Baytunca B, Kalyoncu T, Ozel I, et al. Early onset schizophrenia associated with obsessive-compulsive disorder: clinical features and correlates. Clin Neuropharmacol. 2017;40(6):243-245.
5. Niendam TA, Berzak J, Cannon TD, et al. Obsessive compulsive symptoms in the psychosis prodrome: correlates of clinical and functional outcome. Schizophr Res. 2009;108(1-3):170-175.
CASE Perseverating on nonexistent sexual assaults
Mr. R, age 17, who has been diagnosed with obsessive-compulsive disorder (OCD), presents to the emergency department (ED) because he thinks that he is being sexually assaulted and is concerned that he is sexually assaulting other people. His family reports that Mr. R has perseverated over these thoughts for months, although there is no evidence to suggest these events have occurred. In order to ameliorate his distress, he performs rituals of looking upwards and repeatedly saying, “It didn’t happen.”
Mr. R is admitted to the inpatient psychiatry unit for further evaluation.
HISTORY Decompensation while attending a PHP
Mr. R had been diagnosed with bipolar disorder and attention-deficit/hyperactivity disorder when he was 13. During that time, he was treated with divalproex sodium and dextroamphetamine. At age 15, Mr. R’s diagnosis was changed to OCD. Seven months before coming to the ED, his symptoms had been getting worse. On one occasion, Mr. R was talking in a nonsensical fashion at school, and the police were called. Mr. R became physically aggressive with the police and was subsequently hospitalized, after which he attended a partial hospitalization program (PHP). At the PHP, Mr. R received exposure and response prevention therapy for OCD, but did not improve, and his symptoms deteriorated until he was unable to brush his teeth or shower regularly. While attending the PHP, Mr. R also developed disorganized speech. The PHP clinicians became concerned that Mr. R’s symptoms may have been prodromal symptoms of schizophrenia because he did not respond to the OCD treatment and his symptoms had worsened over the 3 months he attended the PHP.
EVALUATION Normal laboratory results
Upon admission to the inpatient psychiatric unit, Mr. R is restarted on his home medications, which include
His laboratory workup, including a complete blood count, comprehensive metabolic panel, urine drug screen, and blood ethanol, are all within normal limits. Previous laboratory results, including a thyroid function panel, vitamin D level, and various autoimmune panels, were also within normal limits.
His family reports that Mr. R’s symptoms seem to worsen when he is under increased stress from school and prepping for standardized college admission examinations. The family also says that while he is playing tennis, Mr. R will posture himself in a crouched down position and at times will remain in this position for 30 minutes.
Mr. R says he eventually wants to go to college and have a professional career.
[polldaddy:10600530]
Continue to: The authors' observations
The authors’ observations
When considering Mr. R’s diagnosis, our treatment team considered the possibility of OCD with absent insight/delusional beliefs, OCD with comorbid schizophrenia, bipolar disorder, and psychotic disorder due to another medical condition.
Overlap between OCD and schizophrenia

Much of the literature about OCD examines its functional impairment in adults, with findings extrapolated to pediatric patients. Children differ from adults in a variety of meaningful ways. Baytunca et al4 examined adolescents with early-onset schizophrenia, with and without comorbid OCD. Patients with comorbid OCD required higher doses of antipsychotic medication to treat acute psychotic symptoms and maintain a reduction in symptoms. The study controlled for the severity of schizophrenia, and its findings suggest that schizophrenia with comorbid OCD is more treatment-resistant than schizophrenia alone.4
Some researchers have theorized that in adolescents, OCD and psychosis are integrally related such that one disorder could represent a prodrome or a cause of the other disorder. Niendam et al5 studied OCS in the psychosis prodrome. They found that OCS can present as a part of the prodromal picture in youth at high risk for psychosis. However, because none of the patients experiencing OCS converted to full-blown psychosis, these results suggest that OCS may not represent a prodrome to psychosis per se. Instead, these individuals may represent a subset of false positives over the follow-up period.5 Another possible explanation for the increased emergence of pre-psychotic symptoms in adolescents with OCD could be a difference in their threshold of perception. OCS compels adolescents with OCD to self-analyze more critically and frequently. As a result, these patients may more often report depressive symptoms, distress, and exacerbations of pre-psychotic symptoms. These findings highlight that
[polldaddy:10600532]
Continue to: TREATMENT Improvement after switching to haloperidol
TREATMENT Improvement after switching to haloperidol
The treatment team decides to change Mr. R’s medications by cross-titrating risperidone to
The treatment team obtains a consultation on whether electroconvulsive therapy would be appropriate, but this treatment is not recommended. Instead, the team considers
Throughout admission, Mr. R focuses on his lack of improvement and how this episode is negatively impacting his grades and his dream of going to college and having a professional career.
OUTCOME Relief at last
Mr. R improves with the addition of sertraline and tolerates rapid titration well. He continues haloperidol without adverse effects, and is discharged home with close follow-up in a PHP and outpatient psychiatry.
However, after discharge, Mr. R’s symptoms get worse, and he is admitted to a different inpatient facility. At this facility, he continues sertraline, but haloperidol is cross-titrated to
Continue to: Currently...
Currently, Mr. R has greatly improved and is able to function in school. He takes sertraline, 100 mg twice a day, and olanzapine, 7.5 mg twice a day. Mr. R reports his rituals have reduced in frequency to less than 15 minutes each day. His thought processes are organized, and he is confident he will be able to achieve his goals.
The authors’ observations
Given Mr. R’s rapid improvement once an effective pharmacologic regimen was established, we concluded that he had a severe case of OCD with absent insight/delusional beliefs, and that he did not have schizophrenia. Mr. R’s case highlights how a psychiatric diagnosis can produce anxiety as a result of the psychosocial stressors and limitations associated with that diagnosis.
Bottom Line
There is both an epidemiologic and biologic overlap between obsessive-compulsive disorder and schizophrenia. In adolescents, either disorder could represent a prodrome or a cause of the other. It is essential to perform a thorough assessment of individuals with obsessive-compulsive disorder because these patients may exhibit subtle psychotic symptoms.
Related Resources
- Cunill R, Castells X, Simeon D. Relationships between obsessivecompulsive symptomatology and severity of psychosis in schizophrenia: a systematic review and meta-analysis. J Clin Psychiatry. 2009;70(1):70-82.
- Harris E, Delgado SV. Treatment-resistant OCD: there’s more we can do. Current Psychiatry. 2018;17(11):10-12,14-18,51.
Drug Brand Names
Clozapine • Clozaril
Dextroamphetamine • Dexedrine
Divalproex sodium • Depakote
Fluvoxamine • Luvox
Haloperidol • Haldol
Hydroxyzine • Atarax, Vistaril
Lurasidone • Latuda
Olanzapine • Zyprexa
Risperidone • Risperdal
Sertraline • Zoloft
CASE Perseverating on nonexistent sexual assaults
Mr. R, age 17, who has been diagnosed with obsessive-compulsive disorder (OCD), presents to the emergency department (ED) because he thinks that he is being sexually assaulted and is concerned that he is sexually assaulting other people. His family reports that Mr. R has perseverated over these thoughts for months, although there is no evidence to suggest these events have occurred. In order to ameliorate his distress, he performs rituals of looking upwards and repeatedly saying, “It didn’t happen.”
Mr. R is admitted to the inpatient psychiatry unit for further evaluation.
HISTORY Decompensation while attending a PHP
Mr. R had been diagnosed with bipolar disorder and attention-deficit/hyperactivity disorder when he was 13. During that time, he was treated with divalproex sodium and dextroamphetamine. At age 15, Mr. R’s diagnosis was changed to OCD. Seven months before coming to the ED, his symptoms had been getting worse. On one occasion, Mr. R was talking in a nonsensical fashion at school, and the police were called. Mr. R became physically aggressive with the police and was subsequently hospitalized, after which he attended a partial hospitalization program (PHP). At the PHP, Mr. R received exposure and response prevention therapy for OCD, but did not improve, and his symptoms deteriorated until he was unable to brush his teeth or shower regularly. While attending the PHP, Mr. R also developed disorganized speech. The PHP clinicians became concerned that Mr. R’s symptoms may have been prodromal symptoms of schizophrenia because he did not respond to the OCD treatment and his symptoms had worsened over the 3 months he attended the PHP.
EVALUATION Normal laboratory results
Upon admission to the inpatient psychiatric unit, Mr. R is restarted on his home medications, which include
His laboratory workup, including a complete blood count, comprehensive metabolic panel, urine drug screen, and blood ethanol, are all within normal limits. Previous laboratory results, including a thyroid function panel, vitamin D level, and various autoimmune panels, were also within normal limits.
His family reports that Mr. R’s symptoms seem to worsen when he is under increased stress from school and prepping for standardized college admission examinations. The family also says that while he is playing tennis, Mr. R will posture himself in a crouched down position and at times will remain in this position for 30 minutes.
Mr. R says he eventually wants to go to college and have a professional career.
[polldaddy:10600530]
Continue to: The authors' observations
The authors’ observations
When considering Mr. R’s diagnosis, our treatment team considered the possibility of OCD with absent insight/delusional beliefs, OCD with comorbid schizophrenia, bipolar disorder, and psychotic disorder due to another medical condition.
Overlap between OCD and schizophrenia

Much of the literature about OCD examines its functional impairment in adults, with findings extrapolated to pediatric patients. Children differ from adults in a variety of meaningful ways. Baytunca et al4 examined adolescents with early-onset schizophrenia, with and without comorbid OCD. Patients with comorbid OCD required higher doses of antipsychotic medication to treat acute psychotic symptoms and maintain a reduction in symptoms. The study controlled for the severity of schizophrenia, and its findings suggest that schizophrenia with comorbid OCD is more treatment-resistant than schizophrenia alone.4
Some researchers have theorized that in adolescents, OCD and psychosis are integrally related such that one disorder could represent a prodrome or a cause of the other disorder. Niendam et al5 studied OCS in the psychosis prodrome. They found that OCS can present as a part of the prodromal picture in youth at high risk for psychosis. However, because none of the patients experiencing OCS converted to full-blown psychosis, these results suggest that OCS may not represent a prodrome to psychosis per se. Instead, these individuals may represent a subset of false positives over the follow-up period.5 Another possible explanation for the increased emergence of pre-psychotic symptoms in adolescents with OCD could be a difference in their threshold of perception. OCS compels adolescents with OCD to self-analyze more critically and frequently. As a result, these patients may more often report depressive symptoms, distress, and exacerbations of pre-psychotic symptoms. These findings highlight that
[polldaddy:10600532]
Continue to: TREATMENT Improvement after switching to haloperidol
TREATMENT Improvement after switching to haloperidol
The treatment team decides to change Mr. R’s medications by cross-titrating risperidone to
The treatment team obtains a consultation on whether electroconvulsive therapy would be appropriate, but this treatment is not recommended. Instead, the team considers
Throughout admission, Mr. R focuses on his lack of improvement and how this episode is negatively impacting his grades and his dream of going to college and having a professional career.
OUTCOME Relief at last
Mr. R improves with the addition of sertraline and tolerates rapid titration well. He continues haloperidol without adverse effects, and is discharged home with close follow-up in a PHP and outpatient psychiatry.
However, after discharge, Mr. R’s symptoms get worse, and he is admitted to a different inpatient facility. At this facility, he continues sertraline, but haloperidol is cross-titrated to
Continue to: Currently...
Currently, Mr. R has greatly improved and is able to function in school. He takes sertraline, 100 mg twice a day, and olanzapine, 7.5 mg twice a day. Mr. R reports his rituals have reduced in frequency to less than 15 minutes each day. His thought processes are organized, and he is confident he will be able to achieve his goals.
The authors’ observations
Given Mr. R’s rapid improvement once an effective pharmacologic regimen was established, we concluded that he had a severe case of OCD with absent insight/delusional beliefs, and that he did not have schizophrenia. Mr. R’s case highlights how a psychiatric diagnosis can produce anxiety as a result of the psychosocial stressors and limitations associated with that diagnosis.
Bottom Line
There is both an epidemiologic and biologic overlap between obsessive-compulsive disorder and schizophrenia. In adolescents, either disorder could represent a prodrome or a cause of the other. It is essential to perform a thorough assessment of individuals with obsessive-compulsive disorder because these patients may exhibit subtle psychotic symptoms.
Related Resources
- Cunill R, Castells X, Simeon D. Relationships between obsessivecompulsive symptomatology and severity of psychosis in schizophrenia: a systematic review and meta-analysis. J Clin Psychiatry. 2009;70(1):70-82.
- Harris E, Delgado SV. Treatment-resistant OCD: there’s more we can do. Current Psychiatry. 2018;17(11):10-12,14-18,51.
Drug Brand Names
Clozapine • Clozaril
Dextroamphetamine • Dexedrine
Divalproex sodium • Depakote
Fluvoxamine • Luvox
Haloperidol • Haldol
Hydroxyzine • Atarax, Vistaril
Lurasidone • Latuda
Olanzapine • Zyprexa
Risperidone • Risperdal
Sertraline • Zoloft
1. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
2. Schirmbeck F, Swets M, de Haan L. Obsessive-compulsive symptoms in schizophrenia. In: De Haan L, Schirmbeck F, Zink M. Epidemiology: prevalence and clinical characteristics of obsessive-compulsive disorder and obsessive-compulsive symptoms in patients with psychotic disorders. New York, NY: Springer International Publishing; 2015:47-61.
3. de Haan L, Sterk B, Wouters L, et al. The 5-year course of obsessive-compulsive symptoms and obsessive-compulsive disorder in first-episode schizophrenia and related disorders. Schizophr Bull. 2011;39(1):151-160.
4. Baytunca B, Kalyoncu T, Ozel I, et al. Early onset schizophrenia associated with obsessive-compulsive disorder: clinical features and correlates. Clin Neuropharmacol. 2017;40(6):243-245.
5. Niendam TA, Berzak J, Cannon TD, et al. Obsessive compulsive symptoms in the psychosis prodrome: correlates of clinical and functional outcome. Schizophr Res. 2009;108(1-3):170-175.
1. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
2. Schirmbeck F, Swets M, de Haan L. Obsessive-compulsive symptoms in schizophrenia. In: De Haan L, Schirmbeck F, Zink M. Epidemiology: prevalence and clinical characteristics of obsessive-compulsive disorder and obsessive-compulsive symptoms in patients with psychotic disorders. New York, NY: Springer International Publishing; 2015:47-61.
3. de Haan L, Sterk B, Wouters L, et al. The 5-year course of obsessive-compulsive symptoms and obsessive-compulsive disorder in first-episode schizophrenia and related disorders. Schizophr Bull. 2011;39(1):151-160.
4. Baytunca B, Kalyoncu T, Ozel I, et al. Early onset schizophrenia associated with obsessive-compulsive disorder: clinical features and correlates. Clin Neuropharmacol. 2017;40(6):243-245.
5. Niendam TA, Berzak J, Cannon TD, et al. Obsessive compulsive symptoms in the psychosis prodrome: correlates of clinical and functional outcome. Schizophr Res. 2009;108(1-3):170-175.