User login
CYP450 interactions between illicit substances and prescription medications
Ms. L, age 37, presents to psychiatric emergency services with command auditory hallucinations, ideas of reference, and suicidal ideation.
Ms. L has a 22-year history of schizophrenia. Additionally, she has a history of cocaine use disorder (in remission for 12 years), cannabis use disorder (in remission for 6 months), type 2 diabetes mellitus, and hypertension. Her psychotic symptoms are well controlled on a regimen of
On interview, Ms. L reports smoking cannabis each day for the past month and using $400 worth of cocaine over 2 days. She is experiencing intense guilt over these relapses and is admitted to the inpatient adult psychiatry unit. On admission, Ms. L’s clozapine and norclozapine trough levels (drawn approximately 12 hours after last administration documented by the ACT member) are 300 and 275 ng/mL, respectively. Generally, clozapine levels >350 to 420 ng/mL are considered therapeutic, and a clozapine-to-norclozapine ratio of 2:1 is desirable for maximum efficacy and tolerability. Because Ms. L’s clozapine level is <350 and her ratio is approximately 1:1, her clozapine treatment is subtherapeutic.
Because Ms. L has a history of documented adherence to and benefit from her current medication regimen, no changes are made during her 3-week hospital stay. She notices a gradual reduction in auditory hallucinations, no longer wants to harm herself, and is motivated to regain sobriety.
At the time of discharge, Ms. L’s clozapine and norclozapine trough levels are 550 and 250 ng/mL, respectively, which indicates a more favorable clozapine-to-norclozapine ratio of approximately 2:1 and a clozapine level greater than the recommended minimum threshold of 350 ng/mL. While cocaine ingestion presumably played a role in her acute decompensation, the treatment team determined that Ms. L’s relapse to cannabis use likely contributed to low clozapine levels by induction of cytochrome P450 (CYP) 1A2, and subsequently led to the delayed recovery of symptom control.1
The use of illicit substances is a widespread, growing problem. According to the 2017 National Survey on Drug Use and Health, 11.5% of Americans age ≥12 had used an illicit substance (ie, use of marijuana, cocaine, heroin, hallucinogens, inhalants, or methamphetamine, or misuse of prescription psychotherapeutics) in the past month.2 While illicit substance use is of particular public health interest due to a known increase in mortality and health care spending, there has been little discussion of the impact of illicit drug use on concurrent pharmacologic therapy. Just as prescription medications have pharmacokinetic drug–drug interactions with each other, so do illicit substances, though far less is known about their impact on the treatment of medical conditions.

Pharmacokinetic interactions
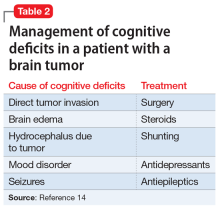
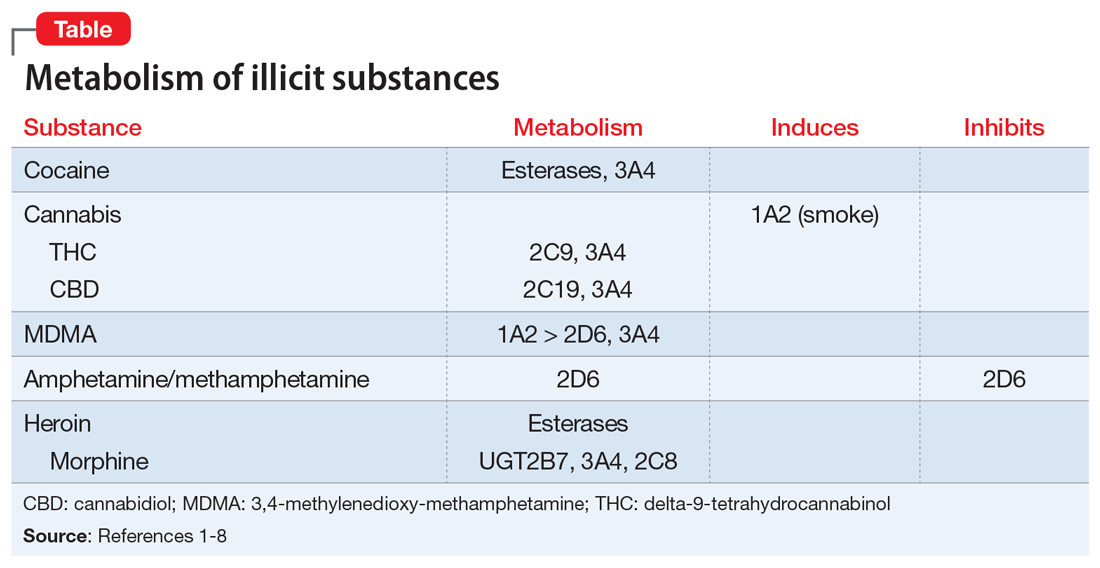
Key pharmacokinetic interactions have been reported with cocaine, marijuana, amphetamines, and opioids. The Table1-8 summarizes the metabolism of illicit substances.
Continue to: Cocaine
Cocaine is largely metabolized by serum esterases such as pseudocholinesterase, human carboxylesterase-1 (hCE-1), and human carboxylesterase-2 (hCE-2), to inactive metabolites benzoylecgonine (35% to 45%) and ecgonine (32% to 49%).2 However, a smaller portion (2.6% to 6.2%) undergoes hepatic N-demethylation by CYP3A4 to norcocaine.3 Norcocaine is an active metabolite responsible for some of the toxic effects of cocaine (eg, hepatotoxicity).4,5 Several commonly prescribed medications are known inducers of CYP3A4 (eg, phenytoin, carbamazepine) and may lead to increased levels of the toxic metabolite when used concurrently with cocaine. Additionally, the use of cocaine with acetylcholinesterase inhibitors, such as donepezil, may lead to reduction of serum esterases and shunt cocaine metabolism toward the hepatic pathway, thus increasing norcocaine formation.3
Cannabis. The metabolism and drug–drug interactions of cannabis can be separated by its 2 main components: delta-9-tetrahydrocannabinol (THC) and cannabidiol (CBD). A review conducted in 2014 concluded that THC is primarily metabolized by CYP2C9 and 3A4, while CBD is metabolized by CYP2C19 and 3A4.6 Oral administration of ketoconazole, a CYP3A4 inhibitor, along with cannabis extract has been shown to increase the maximum concentration (Cmax) and area under the curve (AUC) of THC by 1.2- and 1.8-fold, respectively, while increasing both Cmaxand AUC of CBD by 2-fold.6 In addition, CYP2C9 poor metabolizers have been shown to experience significant increases in THC exposure and reductions in metabolite formation, further supporting the role of CYP enzymes in cannabis metabolism.6
There is also evidence of enzyme induction by cannabis. Individuals who reported smoking marijuana experienced greater clearance of theophylline, a substrate of CYP1A2, than did those who reported not smoking marijuana.1,6 As with cigarette smoking, this effect appears to be a direct result of the hydrocarbons found in marijuana smoke rather than the cannabis itself, as there is a lack of evidence for enzyme induction when the drug is orally ingested.6
Amphetamine and methamphetamine appear to be both substrates and competitive inhibitors of CYP2D6.7 Rats administered quinidine (a strong 2D6 inhibitor) had 2-fold elevations in AUC and decreased clearance of amphetamine and its metabolites.8 Amphetamine-related recreational drugs, such as 3,4-methylenedioxy-methamphetamine (MDMA) and 3,4-methylenedioxyamphetamine (MDA), are substrates of CYP2D6 and CYP3A4, while MDMA also undergoes substantial metabolism by CYP1A2.3,7,9
Opioids. Heroin is metabolized to 6‑monoacetylmorphine (6-MAM) and morphine by hCE-1, hCE-2, and pseudocholinesterase, and has minimal impact on CYP enzymes. However, while morphine is primarily metabolized to inactive metabolites by UGT2B7, it does undergo minor metabolism through CYP3A4 and 2C8 pathways, creating potential for drug interactions with medications that inhibit or induce CYP3A4.10
Continue to: An underappreciated risk of illicit substance use
An underappreciated risk of illicit substance use
There is a paucity of evidence regarding the metabolism and pharmacokinetic interactions with illicit substances, and further research is needed. Despite the absence of comprehensive data on the subject, the available information indicates the use of illicit substances may have a significant impact on medications used to treat comorbid conditions. Alternatively, those medications may affect the kinetics of recreationally used substances. The risk of adverse consequences of drug–drug interactions is yet another reason patients should be encouraged to avoid use of substances and seek treatment for substance use disorders. When determining the most appropriate therapy for comorbid conditions for patients who are using illicit substances and are likely to continue to do so, clinicians should take into consideration potential interactions among prescription medications and the specific illicit substances the patient uses.
Related Resources
- Lindsey W, Stewart D, Childress D. Drug interactions between common illicit drugs and prescription therapies. Am J Drug Alcohol Abuse. 2012;38(4):334-343.
- Maurer H, Sauer C, Theobald D. Toxicokinetics of drugs of abuse: current knowledge of the isoenzymes involved in the human metabolism of tetrahydrocannabinol, cocaine, heroin, morphine, and codeine. Ther Drug Monit. 2006;28(3):447-453.
- Dean A. Illicit drugs and drug interactions. Pharmacist. 2006;25(9):684-689.
Drug Brand Names
Carbamazepine • Carbatrol, Tegretol
Clozapine • Clozaril
Donepezil • Aricept
Ketoconazole • Nizoral
Paliperidone palmitate • Invega sustenna
Phenytoin • Dilantin, Phenytek
Quinidine • Cardioquin, Duraquin
Theophylline • Elixophylline, Theochron
1. Jusko WJ, Schentag JJ, Clark JH, et al. Enhanced biotransformation of theophylline in marihuana and tobacco smokers. Clin Pharmacol Ther. 1978;24(4):405-410.
2. Substance Abuse and Mental Health Services Administration. Results from the 2017 National Survey on Drug Use and Health: Detailed Tables. https://www.samhsa.gov/data/sites/default/files/cbhsq-reports/NSDUHDetailedTabs2017/NSDUHDetailedTabs2017.htm#tab1-1A. Published 2019. Accessed February 7, 2020.
3. Quinn D, Wodak A, Day R. Pharmacokinetic and pharmacodynamic principles of illicit drug use and treatment of illicit drug users. Clin Pharmacokinet. 1997;33(5):344-400.
4. Ndikum-Moffor FM, Schoeb TR, Roberts SM. Liver toxicity from norcocaine nitroxide, an N-oxidative metabolite of cocaine. J Pharmacol Exp Ther. 1998;284(1):413-419.
5. Pellinen P, Honkakoski P, Stenbäck F, et al. Cocaine N-demethylation and the metabolism-related hepatotoxicity can be prevented by cytochrome P450 3A inhibitors. Eur J Pharmacol. 1994;270(1):35-43.
6. Stout S, Cimino N. Exogenous cannabinoids as substrates, inhibitors, and inducers of human drug metabolizing enzymes: a systematic review. Drug Metab Rev. 2013;46(1):86-95.
7. Kraemer T, Maurer H. Toxicokinetics of amphetamines: metabolism and toxicokinetic data of designer drugs, amphetamine, methamphetamine, and their N-alkyl derivatives. Ther Drug Monit. 2002;24(2):277-289.
8. Markowitz J, Patrick K. Pharmacokinetic and pharmacodynamic drug interactions in the treatment of attention-deficit hyperactivity disorder. Clin Pharmacokinet. 2001;40(10):753-772.
9. Kreth K, Kovar K, Schwab M, et al. Identification of the human cytochromes P450 involved in the oxidative metabolism of “ecstasy”-related designer drugs. Biochem Pharmacol. 2000;59(12):1563-1571.
10. Meyer M, Maurer H. Absorption, distribution, metabolism and excretion pharmacogenomics of drugs of abuse. Pharmacogenomics. 2011;12(2):215-233.
Ms. L, age 37, presents to psychiatric emergency services with command auditory hallucinations, ideas of reference, and suicidal ideation.
Ms. L has a 22-year history of schizophrenia. Additionally, she has a history of cocaine use disorder (in remission for 12 years), cannabis use disorder (in remission for 6 months), type 2 diabetes mellitus, and hypertension. Her psychotic symptoms are well controlled on a regimen of
On interview, Ms. L reports smoking cannabis each day for the past month and using $400 worth of cocaine over 2 days. She is experiencing intense guilt over these relapses and is admitted to the inpatient adult psychiatry unit. On admission, Ms. L’s clozapine and norclozapine trough levels (drawn approximately 12 hours after last administration documented by the ACT member) are 300 and 275 ng/mL, respectively. Generally, clozapine levels >350 to 420 ng/mL are considered therapeutic, and a clozapine-to-norclozapine ratio of 2:1 is desirable for maximum efficacy and tolerability. Because Ms. L’s clozapine level is <350 and her ratio is approximately 1:1, her clozapine treatment is subtherapeutic.
Because Ms. L has a history of documented adherence to and benefit from her current medication regimen, no changes are made during her 3-week hospital stay. She notices a gradual reduction in auditory hallucinations, no longer wants to harm herself, and is motivated to regain sobriety.
At the time of discharge, Ms. L’s clozapine and norclozapine trough levels are 550 and 250 ng/mL, respectively, which indicates a more favorable clozapine-to-norclozapine ratio of approximately 2:1 and a clozapine level greater than the recommended minimum threshold of 350 ng/mL. While cocaine ingestion presumably played a role in her acute decompensation, the treatment team determined that Ms. L’s relapse to cannabis use likely contributed to low clozapine levels by induction of cytochrome P450 (CYP) 1A2, and subsequently led to the delayed recovery of symptom control.1
The use of illicit substances is a widespread, growing problem. According to the 2017 National Survey on Drug Use and Health, 11.5% of Americans age ≥12 had used an illicit substance (ie, use of marijuana, cocaine, heroin, hallucinogens, inhalants, or methamphetamine, or misuse of prescription psychotherapeutics) in the past month.2 While illicit substance use is of particular public health interest due to a known increase in mortality and health care spending, there has been little discussion of the impact of illicit drug use on concurrent pharmacologic therapy. Just as prescription medications have pharmacokinetic drug–drug interactions with each other, so do illicit substances, though far less is known about their impact on the treatment of medical conditions.

Pharmacokinetic interactions
Key pharmacokinetic interactions have been reported with cocaine, marijuana, amphetamines, and opioids. The Table1-8 summarizes the metabolism of illicit substances.

Continue to: Cocaine
Cocaine is largely metabolized by serum esterases such as pseudocholinesterase, human carboxylesterase-1 (hCE-1), and human carboxylesterase-2 (hCE-2), to inactive metabolites benzoylecgonine (35% to 45%) and ecgonine (32% to 49%).2 However, a smaller portion (2.6% to 6.2%) undergoes hepatic N-demethylation by CYP3A4 to norcocaine.3 Norcocaine is an active metabolite responsible for some of the toxic effects of cocaine (eg, hepatotoxicity).4,5 Several commonly prescribed medications are known inducers of CYP3A4 (eg, phenytoin, carbamazepine) and may lead to increased levels of the toxic metabolite when used concurrently with cocaine. Additionally, the use of cocaine with acetylcholinesterase inhibitors, such as donepezil, may lead to reduction of serum esterases and shunt cocaine metabolism toward the hepatic pathway, thus increasing norcocaine formation.3
Cannabis. The metabolism and drug–drug interactions of cannabis can be separated by its 2 main components: delta-9-tetrahydrocannabinol (THC) and cannabidiol (CBD). A review conducted in 2014 concluded that THC is primarily metabolized by CYP2C9 and 3A4, while CBD is metabolized by CYP2C19 and 3A4.6 Oral administration of ketoconazole, a CYP3A4 inhibitor, along with cannabis extract has been shown to increase the maximum concentration (Cmax) and area under the curve (AUC) of THC by 1.2- and 1.8-fold, respectively, while increasing both Cmaxand AUC of CBD by 2-fold.6 In addition, CYP2C9 poor metabolizers have been shown to experience significant increases in THC exposure and reductions in metabolite formation, further supporting the role of CYP enzymes in cannabis metabolism.6
There is also evidence of enzyme induction by cannabis. Individuals who reported smoking marijuana experienced greater clearance of theophylline, a substrate of CYP1A2, than did those who reported not smoking marijuana.1,6 As with cigarette smoking, this effect appears to be a direct result of the hydrocarbons found in marijuana smoke rather than the cannabis itself, as there is a lack of evidence for enzyme induction when the drug is orally ingested.6
Amphetamine and methamphetamine appear to be both substrates and competitive inhibitors of CYP2D6.7 Rats administered quinidine (a strong 2D6 inhibitor) had 2-fold elevations in AUC and decreased clearance of amphetamine and its metabolites.8 Amphetamine-related recreational drugs, such as 3,4-methylenedioxy-methamphetamine (MDMA) and 3,4-methylenedioxyamphetamine (MDA), are substrates of CYP2D6 and CYP3A4, while MDMA also undergoes substantial metabolism by CYP1A2.3,7,9
Opioids. Heroin is metabolized to 6‑monoacetylmorphine (6-MAM) and morphine by hCE-1, hCE-2, and pseudocholinesterase, and has minimal impact on CYP enzymes. However, while morphine is primarily metabolized to inactive metabolites by UGT2B7, it does undergo minor metabolism through CYP3A4 and 2C8 pathways, creating potential for drug interactions with medications that inhibit or induce CYP3A4.10
Continue to: An underappreciated risk of illicit substance use
An underappreciated risk of illicit substance use
There is a paucity of evidence regarding the metabolism and pharmacokinetic interactions with illicit substances, and further research is needed. Despite the absence of comprehensive data on the subject, the available information indicates the use of illicit substances may have a significant impact on medications used to treat comorbid conditions. Alternatively, those medications may affect the kinetics of recreationally used substances. The risk of adverse consequences of drug–drug interactions is yet another reason patients should be encouraged to avoid use of substances and seek treatment for substance use disorders. When determining the most appropriate therapy for comorbid conditions for patients who are using illicit substances and are likely to continue to do so, clinicians should take into consideration potential interactions among prescription medications and the specific illicit substances the patient uses.
Related Resources
- Lindsey W, Stewart D, Childress D. Drug interactions between common illicit drugs and prescription therapies. Am J Drug Alcohol Abuse. 2012;38(4):334-343.
- Maurer H, Sauer C, Theobald D. Toxicokinetics of drugs of abuse: current knowledge of the isoenzymes involved in the human metabolism of tetrahydrocannabinol, cocaine, heroin, morphine, and codeine. Ther Drug Monit. 2006;28(3):447-453.
- Dean A. Illicit drugs and drug interactions. Pharmacist. 2006;25(9):684-689.
Drug Brand Names
Carbamazepine • Carbatrol, Tegretol
Clozapine • Clozaril
Donepezil • Aricept
Ketoconazole • Nizoral
Paliperidone palmitate • Invega sustenna
Phenytoin • Dilantin, Phenytek
Quinidine • Cardioquin, Duraquin
Theophylline • Elixophylline, Theochron
Ms. L, age 37, presents to psychiatric emergency services with command auditory hallucinations, ideas of reference, and suicidal ideation.
Ms. L has a 22-year history of schizophrenia. Additionally, she has a history of cocaine use disorder (in remission for 12 years), cannabis use disorder (in remission for 6 months), type 2 diabetes mellitus, and hypertension. Her psychotic symptoms are well controlled on a regimen of
On interview, Ms. L reports smoking cannabis each day for the past month and using $400 worth of cocaine over 2 days. She is experiencing intense guilt over these relapses and is admitted to the inpatient adult psychiatry unit. On admission, Ms. L’s clozapine and norclozapine trough levels (drawn approximately 12 hours after last administration documented by the ACT member) are 300 and 275 ng/mL, respectively. Generally, clozapine levels >350 to 420 ng/mL are considered therapeutic, and a clozapine-to-norclozapine ratio of 2:1 is desirable for maximum efficacy and tolerability. Because Ms. L’s clozapine level is <350 and her ratio is approximately 1:1, her clozapine treatment is subtherapeutic.
Because Ms. L has a history of documented adherence to and benefit from her current medication regimen, no changes are made during her 3-week hospital stay. She notices a gradual reduction in auditory hallucinations, no longer wants to harm herself, and is motivated to regain sobriety.
At the time of discharge, Ms. L’s clozapine and norclozapine trough levels are 550 and 250 ng/mL, respectively, which indicates a more favorable clozapine-to-norclozapine ratio of approximately 2:1 and a clozapine level greater than the recommended minimum threshold of 350 ng/mL. While cocaine ingestion presumably played a role in her acute decompensation, the treatment team determined that Ms. L’s relapse to cannabis use likely contributed to low clozapine levels by induction of cytochrome P450 (CYP) 1A2, and subsequently led to the delayed recovery of symptom control.1
The use of illicit substances is a widespread, growing problem. According to the 2017 National Survey on Drug Use and Health, 11.5% of Americans age ≥12 had used an illicit substance (ie, use of marijuana, cocaine, heroin, hallucinogens, inhalants, or methamphetamine, or misuse of prescription psychotherapeutics) in the past month.2 While illicit substance use is of particular public health interest due to a known increase in mortality and health care spending, there has been little discussion of the impact of illicit drug use on concurrent pharmacologic therapy. Just as prescription medications have pharmacokinetic drug–drug interactions with each other, so do illicit substances, though far less is known about their impact on the treatment of medical conditions.

Pharmacokinetic interactions
Key pharmacokinetic interactions have been reported with cocaine, marijuana, amphetamines, and opioids. The Table1-8 summarizes the metabolism of illicit substances.

Continue to: Cocaine
Cocaine is largely metabolized by serum esterases such as pseudocholinesterase, human carboxylesterase-1 (hCE-1), and human carboxylesterase-2 (hCE-2), to inactive metabolites benzoylecgonine (35% to 45%) and ecgonine (32% to 49%).2 However, a smaller portion (2.6% to 6.2%) undergoes hepatic N-demethylation by CYP3A4 to norcocaine.3 Norcocaine is an active metabolite responsible for some of the toxic effects of cocaine (eg, hepatotoxicity).4,5 Several commonly prescribed medications are known inducers of CYP3A4 (eg, phenytoin, carbamazepine) and may lead to increased levels of the toxic metabolite when used concurrently with cocaine. Additionally, the use of cocaine with acetylcholinesterase inhibitors, such as donepezil, may lead to reduction of serum esterases and shunt cocaine metabolism toward the hepatic pathway, thus increasing norcocaine formation.3
Cannabis. The metabolism and drug–drug interactions of cannabis can be separated by its 2 main components: delta-9-tetrahydrocannabinol (THC) and cannabidiol (CBD). A review conducted in 2014 concluded that THC is primarily metabolized by CYP2C9 and 3A4, while CBD is metabolized by CYP2C19 and 3A4.6 Oral administration of ketoconazole, a CYP3A4 inhibitor, along with cannabis extract has been shown to increase the maximum concentration (Cmax) and area under the curve (AUC) of THC by 1.2- and 1.8-fold, respectively, while increasing both Cmaxand AUC of CBD by 2-fold.6 In addition, CYP2C9 poor metabolizers have been shown to experience significant increases in THC exposure and reductions in metabolite formation, further supporting the role of CYP enzymes in cannabis metabolism.6
There is also evidence of enzyme induction by cannabis. Individuals who reported smoking marijuana experienced greater clearance of theophylline, a substrate of CYP1A2, than did those who reported not smoking marijuana.1,6 As with cigarette smoking, this effect appears to be a direct result of the hydrocarbons found in marijuana smoke rather than the cannabis itself, as there is a lack of evidence for enzyme induction when the drug is orally ingested.6
Amphetamine and methamphetamine appear to be both substrates and competitive inhibitors of CYP2D6.7 Rats administered quinidine (a strong 2D6 inhibitor) had 2-fold elevations in AUC and decreased clearance of amphetamine and its metabolites.8 Amphetamine-related recreational drugs, such as 3,4-methylenedioxy-methamphetamine (MDMA) and 3,4-methylenedioxyamphetamine (MDA), are substrates of CYP2D6 and CYP3A4, while MDMA also undergoes substantial metabolism by CYP1A2.3,7,9
Opioids. Heroin is metabolized to 6‑monoacetylmorphine (6-MAM) and morphine by hCE-1, hCE-2, and pseudocholinesterase, and has minimal impact on CYP enzymes. However, while morphine is primarily metabolized to inactive metabolites by UGT2B7, it does undergo minor metabolism through CYP3A4 and 2C8 pathways, creating potential for drug interactions with medications that inhibit or induce CYP3A4.10
Continue to: An underappreciated risk of illicit substance use
An underappreciated risk of illicit substance use
There is a paucity of evidence regarding the metabolism and pharmacokinetic interactions with illicit substances, and further research is needed. Despite the absence of comprehensive data on the subject, the available information indicates the use of illicit substances may have a significant impact on medications used to treat comorbid conditions. Alternatively, those medications may affect the kinetics of recreationally used substances. The risk of adverse consequences of drug–drug interactions is yet another reason patients should be encouraged to avoid use of substances and seek treatment for substance use disorders. When determining the most appropriate therapy for comorbid conditions for patients who are using illicit substances and are likely to continue to do so, clinicians should take into consideration potential interactions among prescription medications and the specific illicit substances the patient uses.
Related Resources
- Lindsey W, Stewart D, Childress D. Drug interactions between common illicit drugs and prescription therapies. Am J Drug Alcohol Abuse. 2012;38(4):334-343.
- Maurer H, Sauer C, Theobald D. Toxicokinetics of drugs of abuse: current knowledge of the isoenzymes involved in the human metabolism of tetrahydrocannabinol, cocaine, heroin, morphine, and codeine. Ther Drug Monit. 2006;28(3):447-453.
- Dean A. Illicit drugs and drug interactions. Pharmacist. 2006;25(9):684-689.
Drug Brand Names
Carbamazepine • Carbatrol, Tegretol
Clozapine • Clozaril
Donepezil • Aricept
Ketoconazole • Nizoral
Paliperidone palmitate • Invega sustenna
Phenytoin • Dilantin, Phenytek
Quinidine • Cardioquin, Duraquin
Theophylline • Elixophylline, Theochron
1. Jusko WJ, Schentag JJ, Clark JH, et al. Enhanced biotransformation of theophylline in marihuana and tobacco smokers. Clin Pharmacol Ther. 1978;24(4):405-410.
2. Substance Abuse and Mental Health Services Administration. Results from the 2017 National Survey on Drug Use and Health: Detailed Tables. https://www.samhsa.gov/data/sites/default/files/cbhsq-reports/NSDUHDetailedTabs2017/NSDUHDetailedTabs2017.htm#tab1-1A. Published 2019. Accessed February 7, 2020.
3. Quinn D, Wodak A, Day R. Pharmacokinetic and pharmacodynamic principles of illicit drug use and treatment of illicit drug users. Clin Pharmacokinet. 1997;33(5):344-400.
4. Ndikum-Moffor FM, Schoeb TR, Roberts SM. Liver toxicity from norcocaine nitroxide, an N-oxidative metabolite of cocaine. J Pharmacol Exp Ther. 1998;284(1):413-419.
5. Pellinen P, Honkakoski P, Stenbäck F, et al. Cocaine N-demethylation and the metabolism-related hepatotoxicity can be prevented by cytochrome P450 3A inhibitors. Eur J Pharmacol. 1994;270(1):35-43.
6. Stout S, Cimino N. Exogenous cannabinoids as substrates, inhibitors, and inducers of human drug metabolizing enzymes: a systematic review. Drug Metab Rev. 2013;46(1):86-95.
7. Kraemer T, Maurer H. Toxicokinetics of amphetamines: metabolism and toxicokinetic data of designer drugs, amphetamine, methamphetamine, and their N-alkyl derivatives. Ther Drug Monit. 2002;24(2):277-289.
8. Markowitz J, Patrick K. Pharmacokinetic and pharmacodynamic drug interactions in the treatment of attention-deficit hyperactivity disorder. Clin Pharmacokinet. 2001;40(10):753-772.
9. Kreth K, Kovar K, Schwab M, et al. Identification of the human cytochromes P450 involved in the oxidative metabolism of “ecstasy”-related designer drugs. Biochem Pharmacol. 2000;59(12):1563-1571.
10. Meyer M, Maurer H. Absorption, distribution, metabolism and excretion pharmacogenomics of drugs of abuse. Pharmacogenomics. 2011;12(2):215-233.
1. Jusko WJ, Schentag JJ, Clark JH, et al. Enhanced biotransformation of theophylline in marihuana and tobacco smokers. Clin Pharmacol Ther. 1978;24(4):405-410.
2. Substance Abuse and Mental Health Services Administration. Results from the 2017 National Survey on Drug Use and Health: Detailed Tables. https://www.samhsa.gov/data/sites/default/files/cbhsq-reports/NSDUHDetailedTabs2017/NSDUHDetailedTabs2017.htm#tab1-1A. Published 2019. Accessed February 7, 2020.
3. Quinn D, Wodak A, Day R. Pharmacokinetic and pharmacodynamic principles of illicit drug use and treatment of illicit drug users. Clin Pharmacokinet. 1997;33(5):344-400.
4. Ndikum-Moffor FM, Schoeb TR, Roberts SM. Liver toxicity from norcocaine nitroxide, an N-oxidative metabolite of cocaine. J Pharmacol Exp Ther. 1998;284(1):413-419.
5. Pellinen P, Honkakoski P, Stenbäck F, et al. Cocaine N-demethylation and the metabolism-related hepatotoxicity can be prevented by cytochrome P450 3A inhibitors. Eur J Pharmacol. 1994;270(1):35-43.
6. Stout S, Cimino N. Exogenous cannabinoids as substrates, inhibitors, and inducers of human drug metabolizing enzymes: a systematic review. Drug Metab Rev. 2013;46(1):86-95.
7. Kraemer T, Maurer H. Toxicokinetics of amphetamines: metabolism and toxicokinetic data of designer drugs, amphetamine, methamphetamine, and their N-alkyl derivatives. Ther Drug Monit. 2002;24(2):277-289.
8. Markowitz J, Patrick K. Pharmacokinetic and pharmacodynamic drug interactions in the treatment of attention-deficit hyperactivity disorder. Clin Pharmacokinet. 2001;40(10):753-772.
9. Kreth K, Kovar K, Schwab M, et al. Identification of the human cytochromes P450 involved in the oxidative metabolism of “ecstasy”-related designer drugs. Biochem Pharmacol. 2000;59(12):1563-1571.
10. Meyer M, Maurer H. Absorption, distribution, metabolism and excretion pharmacogenomics of drugs of abuse. Pharmacogenomics. 2011;12(2):215-233.
When the worry is worse than the actual illness
CASE Distraught over a medical illness
Ms. S, age 16, presents to the emergency department (ED) accompanied by her mother with superficial lacerations on her arm. Ms. S states, “I cut my arm because I was afraid I was going to do something serious if I didn’t get to go to the ED.” She says that 6 months earlier, she was diagnosed with superior mesenteric artery syndrome (SMAS), a rare, potentially life-threatening condition that occurs when the duodenum is compressed between the aorta and the superior mesenteric artery, causing a partial or complete blockage of the duodenum. Since receiving this diagnosis, Ms. S reports feeling anxious, depressed, and overwhelmed by both the pain she is experiencing from her illness and uncertainty about her prognosis.
HISTORY In pain and isolated
Since being diagnosed with SMAS, Ms. S has had approximately 30 medical and 7 ED visits for SMAS-related pain. Ms. S was referred to the outpatient clinic for ongoing support and treatment for SMAS.
Because of her pain and anxiety, Ms. S, a junior in high school, no longer attends school but has been working with a tutor. Ms. S says that some of her loneliness and hopelessness are due to the social isolation of being tutored at home. She states that she has been “out of sight and out of mind” from her friends. She also reports feeling different from them due to the pain brought on by SMAS.
Ms. S and her mother live in public housing. Ms. S says that overall, she has a good relationship with her mother, but that in certain situations, her mother’s anxiety causes her significant frustration and anxiety.
EVALUATION Transient suicidal thoughts
A physical examination reveals superficial lacerations to Ms. S’s left arm. Although she appears thin, her current body mass index (BMI) is 20.4 kg/m2, which is within normal range. She says she sees herself as “underweight” and “not fat at all.” Ms. S reports that she likes food and enjoyed eating until it became too painful following her SMAS diagnosis. Ms. S denies a history of binging or purging. Results from her laboratory workup and all values are within normal limits.
During the initial interview, Ms. S’s mother says they came to the ED because Ms. S urgently needs a psychiatric evaluation so she can be cleared for gastrointestinal (GI) surgery and placement of a nasogastric tube. Her mother says a surgeon from a different hospital told them that her insurance company required a psychiatric evaluation to rule out anorexia nervosa before they would authorize the GI surgery. When asked why psychiatry at this hospital was not consulted, Ms. S’s mother does not answer.
When asked about the symptoms she has been experiencing, Ms. S says that her sleep has been poor because of increased pain and excessive worrying about her health. She has limited her food intake. Ms. S reports that after eating, she lays on her left side to alleviate pain and help the food move through her body.
Continue to: Ms. S says...
Ms. S says she feels anxious and depressed due to her SMAS diagnosis, her mother’s online research and oversharing of poor prognoses, and being isolated from her friends. Most of her time outside the home is spent attending medical appointments with specialists. Several months ago, Ms. S had seen a psychotherapist, but her mother was unhappy with the treatment recommendations, which included seeking care from a nutritionist and joining group therapy. Ms. S’s mother says she ended her daughter’s psychotherapy because she was unable to obtain a signature ruling out anorexia nervosa within the first few appointments.
Ms. S also says she has had passive suicidal thoughts during the past month, usually twice a week. She reports that these thoughts lasted as long as several hours and were difficult to control, but she has no specific plan or intent. Ms. S denies current suicidal thoughts or ideation, and works with the treatment team to complete a safety plan, which she signs. Other than her recent visit to the ED, Ms. S denies any other thoughts or behaviors of self-injury or suicide.
[polldaddy:10586905]
The authors’ observations
The treatment team considered the following conditions as part of Ms. S’s differential diagnosis:
Major depressive disorder. The team was able to rule out MDD because Ms. S’s depression was attributed to SMAS. Ms. S reported that all depressive symptoms were manageable or nonexistent before the onset of pain from SMAS. There was no direct pathophysiological consequence of another medical condition. Ms. S was clear that her symptoms of anxiety and depression began after she was isolated from her friends and began having difficulty understanding her diagnosis and prognosis.
Anorexia nervosa also was ruled out. According to the DSM-5, a diagnosis of anorexia nervosa requires the following 3 criteria1:
- restriction of food intake resulting in significantly low body weight (defined as weight that is less than “minimally normal”) relative to age, gender, or development
- intense fear of gaining weight, or persistent behaviors that interfere with weight gain
- disturbance in the way in which one’s body weight or shape is experienced, undue influence of body weight or shape on self-evaluation, or lack of insight with regard to seriousness of current low body weight.
Continue to: Although Ms. S appeared...
Although Ms. S appeared thin, her BMI was within normal range. She added that she likes food and enjoyed eating, but that her medical condition made it too painful. Lastly, Ms. S denied a history of binging or purging.
Somatic symptom disorder.

Factitious disorder imposed on self. An individual with FDIS chronically stimulates, induces, or aggravates illnesses to gain the status of being a patient.
Factitious disorder imposed on another is the deliberate feigning or production of symptoms in another individual who is under the perpetrator’s supervision.1 Table 23 lists clinical indicators that raise suspicion for FDIA.
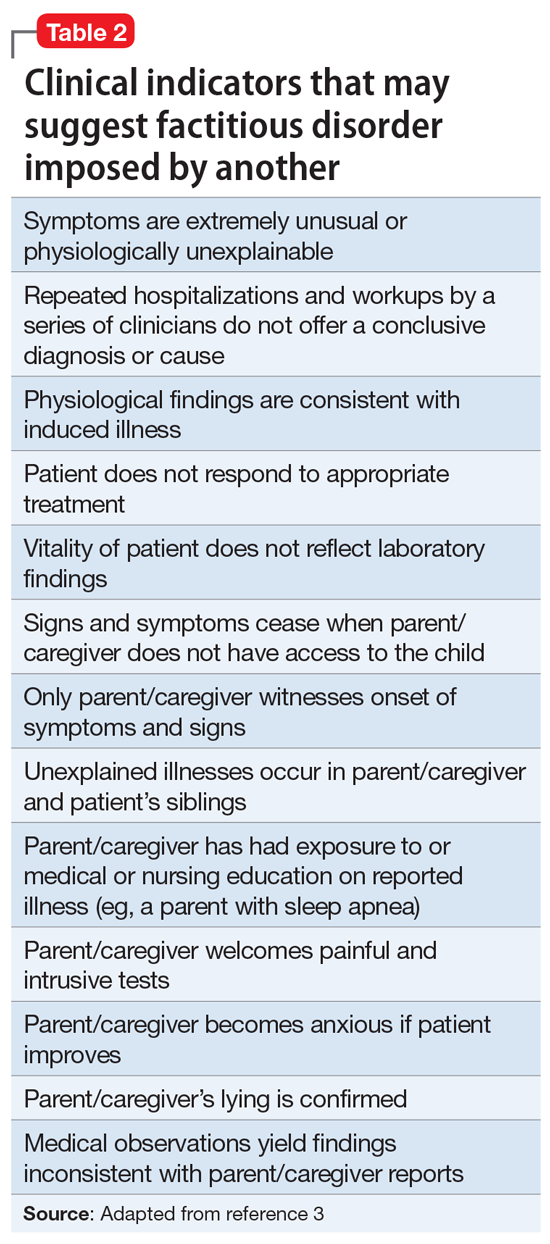
Before a diagnosis of somatic symptom disorder, FDIS, or FDIA could be established or ruled out, it was imperative to gather collateral information from other clinicians involved in Ms. S’s care. Ms. S and her mother had sought out help from a pediatric surgeon, a pediatric gastroenterologist, a pediatrician, and a psychotherapist.
Continue to: EVALUATION Collateral information
EVALUATION Collateral information
After Ms. S’s mother signs consent forms for exchange of information, the treatment team reaches out to the other clinicians. The therapist confirms that Ms. S’s mother had ended her daughter’s treatment after she was unable to quickly obtain documentation to rule out anorexia nervosa.
Both the pediatric surgeon and gastroenterologist report concerns of FDIA, which is why both clinicians had referred Ms. S and her mother to psychiatry. The pediatric surgeon states that on one occasion when he interviewed Ms. S separately from her mother, she seemed to be going down a checklist of symptoms. The surgeon reports that there was a partial occlusion of the superior mesenteric artery, confirming the diagnosis of SMAS, but he believed it was not severe enough to explain the symptoms Ms. S reported. The surgeon had scheduled another imaging appointment for 1 month later.
The pediatric gastroenterologist reports that Ms. S’s mother had demanded surgery and nasogastric tube placement for her daughter, which raised suspicion of FDIA. The gastroenterologist had convinced Ms. S and her mother to start low-dose doxepin, 20 mg twice a day, for anxiety, sleep, and abdominal pain.
Lastly, the pediatrician reports that she had not seen Ms. S for several months but stated that Ms. S always has been in the low normal BMI range. The pediatrician also reports that 6 months ago, the patient and her mother were frantically visiting EDs and scheduling doctor’s appointments.
[polldaddy:10586906]
The authors’ observations
The treatment team decided that Ms. S was not in imminent danger, and felt it was important to keep her in treatment without raising her mother’s suspicion. The team agreed to raise these concerns to the police, child protective services, and risk management if Ms. S’s health suddenly deteriorated or if her mother decided to remove Ms. S from our care.
Continue to: The treatment team...
The treatment team at the outpatient psychiatry clinic agreed that Ms. S did not currently meet criteria for anorexia nervosa, MDD, FDIS, or FDIA. However, Ms. S reported worries particular to persistent abdominal pain that was exacerbated by either eating or going to bed at night, which indicated that somatic symptom disorder was her likely diagnosis. Further, she endorsed a high level of anxiety and depression with regard to this somatic complaint that interfered with her daily activities and consumed an excessive amount of time, which also pointed to somatic symptom disorder. As a result of this diagnosis, the treatment team helped Ms. S manage her somatic symptoms and monitored for any other changes in her symptoms
Generally, cognitive-behavioral therapy (CBT) and mindfulness-based therapy may help relieve symptoms associated with somatic symptom disorder.4
TREATMENT Therapy sessions and medication management
At the psychiatric clinic, Ms. S is scheduled for biweekly therapy sessions with a social worker and biweekly appointments with a senior psychiatry resident for medication management. At each visit, Ms. S’s vital signs, height, and weight are measured. In the therapy sessions, she is taught mindfulness skills as well as CBT. The senior psychiatry resident maintains regular communication with the other clinicians involved in Ms. S’s care.
After the first month of treatment, Ms. S undergoes repeat imaging at the gastroenterologist’s office that indicates her SMAS is no longer occluded. Ms. S continues to report somatic symptoms, but with mild improvement.
Over the course of approximately 4 months, Ms. S begins to show signs of improvement in her pain, anxiety, and depression. Ms. S begins to feel well enough to get a summer job at a nursing home and expresses enthusiasm when her weight begins to increase. Her mother also became enthused and verbalized her appreciation that her daughter appeared to be improving.
Continue to: In the fall...
In the fall, Ms. S returns to high school for her senior year but has difficulty getting back into the routine and relating to her old friends. Ms. S continues to perseverate on thoughts of getting sick and her physical symptoms become overwhelming once again. She continues to be focused on any new symptoms she experiences, and to limit the types of foods she eats due to fear of the abdominal pain returning.
After several more months of psychiatric treatment, Ms. S reports significant relief from her abdominal pain, and no longer seeks corrective surgery for her SMAS. Although she occasionally struggles with perseverating thoughts and anxiety about her somatic symptoms such as abdominal pain and worrying about the types of foods she eats and becoming ill, she continues to work through symptoms of her somatic symptom disorder.
The authors’ observations
The main challenge of somatic symptom disorder is the patient’s “abnormal illness behavior.”2,5,6 For pediatric patients, there may an association between a parent’s psychological status and the patient’s somatic symptoms. Abdominal symptoms in a pediatric patient have a strong association with a parent who presents with depression, anxiety, or somatization. The effects of the parent’s psychological status could also manifest in the form of modeling catastrophic thinking or through reinforcement. Parents with certain traits, such as disproportionate worry about pain, may pay more attention to their child’s symptoms, and hence, reward the child when he/she reports somatic symptoms.7,8 In the case of Ms. S, her mother did not participate in therapy and the mother’s psychiatric history was never obtained.
OUTCOMES Making personal strides
Ms. S continues to use mindfulness skills as well as CBT to manage her symptoms of somatic symptom disorder. She continues to celebrate her weight gains, denies any thoughts of suicide or self-harm behaviors, and prepares for college by scheduling campus visits and completing admissions applications.
Bottom Line
Patients with somatic symptom disorder tend to have very high levels of worry about illness. Somatic symptoms in such patients may or may not have a medical explanation. Accurate diagnosis and careful management are necessary to reduce patient distress. Cognitive-behavioral therapy and mindfulness-based therapy may help relieve symptoms associated with this disorder.
Related Resources
- Henningsen P. Management of somatic symptom disorder. Dialogues Clin Neurosci. 2018;20(1):23-91.
- Rosic T, Kalra S, Samaan Z. Somatic symptom disorder, a new DSM-5 diagnosis of an old clinical challenge. BMJ Case Rep. 2016: bcr2015212553. doi: 10.1136/bcr-2015-212553.
Drug Brand Name
Doxepin • Silenor
1. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
2. Stern T, Freudenreich O, Smith F, et al. Massachusetts General Hospital Handbook of General Hospital Psychiatry, 7th ed. New York, NY: Elsevier; 2017.
3. Feldman MD, Eisendrath SJ. The spectrum of factitious disorders. Washington, DC: American Psychiatric Association; 1997.
4. Sadock BJ, Sadock VA, Ruiz P. Kaplan & Sadock’s synopsis of psychiatry: behavioral sciences/clinical psychiatry, 11th ed. Philadelphia, PA: Wolters Kluwer; 2014:470.
5. Pilowsky I. The concept of abnormal illness behavior. Psychosomatics. 1990;31(2):207-213.
6. Kirmayer LJ, Looper KJ. Abnormal illness behavior: physiological, psychological and social dimensions of coping with stress. Curr Opin Psychiatry. 2006;19(1):54-60.
7. Walker LS, Garber J, Greene JW. Somatic complaints in pediatric patients: a prospective study of the role of negative life events, child social and academic competence, and parental somatic symptoms. J Consult Clin Psychology. 1994;62(6):1213-1221.
8. Van Oudenhove L, Levy RL, Crowell MD, et al. Biopsychosocial aspects of functional gastrointestinal disorders: how central and environmental processes contribute to the development and expression of functional gastrointestinal disorders. Gastroenterology. 2016;150(6):1355-1367.
CASE Distraught over a medical illness
Ms. S, age 16, presents to the emergency department (ED) accompanied by her mother with superficial lacerations on her arm. Ms. S states, “I cut my arm because I was afraid I was going to do something serious if I didn’t get to go to the ED.” She says that 6 months earlier, she was diagnosed with superior mesenteric artery syndrome (SMAS), a rare, potentially life-threatening condition that occurs when the duodenum is compressed between the aorta and the superior mesenteric artery, causing a partial or complete blockage of the duodenum. Since receiving this diagnosis, Ms. S reports feeling anxious, depressed, and overwhelmed by both the pain she is experiencing from her illness and uncertainty about her prognosis.
HISTORY In pain and isolated
Since being diagnosed with SMAS, Ms. S has had approximately 30 medical and 7 ED visits for SMAS-related pain. Ms. S was referred to the outpatient clinic for ongoing support and treatment for SMAS.
Because of her pain and anxiety, Ms. S, a junior in high school, no longer attends school but has been working with a tutor. Ms. S says that some of her loneliness and hopelessness are due to the social isolation of being tutored at home. She states that she has been “out of sight and out of mind” from her friends. She also reports feeling different from them due to the pain brought on by SMAS.
Ms. S and her mother live in public housing. Ms. S says that overall, she has a good relationship with her mother, but that in certain situations, her mother’s anxiety causes her significant frustration and anxiety.
EVALUATION Transient suicidal thoughts
A physical examination reveals superficial lacerations to Ms. S’s left arm. Although she appears thin, her current body mass index (BMI) is 20.4 kg/m2, which is within normal range. She says she sees herself as “underweight” and “not fat at all.” Ms. S reports that she likes food and enjoyed eating until it became too painful following her SMAS diagnosis. Ms. S denies a history of binging or purging. Results from her laboratory workup and all values are within normal limits.
During the initial interview, Ms. S’s mother says they came to the ED because Ms. S urgently needs a psychiatric evaluation so she can be cleared for gastrointestinal (GI) surgery and placement of a nasogastric tube. Her mother says a surgeon from a different hospital told them that her insurance company required a psychiatric evaluation to rule out anorexia nervosa before they would authorize the GI surgery. When asked why psychiatry at this hospital was not consulted, Ms. S’s mother does not answer.
When asked about the symptoms she has been experiencing, Ms. S says that her sleep has been poor because of increased pain and excessive worrying about her health. She has limited her food intake. Ms. S reports that after eating, she lays on her left side to alleviate pain and help the food move through her body.
Continue to: Ms. S says...
Ms. S says she feels anxious and depressed due to her SMAS diagnosis, her mother’s online research and oversharing of poor prognoses, and being isolated from her friends. Most of her time outside the home is spent attending medical appointments with specialists. Several months ago, Ms. S had seen a psychotherapist, but her mother was unhappy with the treatment recommendations, which included seeking care from a nutritionist and joining group therapy. Ms. S’s mother says she ended her daughter’s psychotherapy because she was unable to obtain a signature ruling out anorexia nervosa within the first few appointments.
Ms. S also says she has had passive suicidal thoughts during the past month, usually twice a week. She reports that these thoughts lasted as long as several hours and were difficult to control, but she has no specific plan or intent. Ms. S denies current suicidal thoughts or ideation, and works with the treatment team to complete a safety plan, which she signs. Other than her recent visit to the ED, Ms. S denies any other thoughts or behaviors of self-injury or suicide.
[polldaddy:10586905]
The authors’ observations
The treatment team considered the following conditions as part of Ms. S’s differential diagnosis:
Major depressive disorder. The team was able to rule out MDD because Ms. S’s depression was attributed to SMAS. Ms. S reported that all depressive symptoms were manageable or nonexistent before the onset of pain from SMAS. There was no direct pathophysiological consequence of another medical condition. Ms. S was clear that her symptoms of anxiety and depression began after she was isolated from her friends and began having difficulty understanding her diagnosis and prognosis.
Anorexia nervosa also was ruled out. According to the DSM-5, a diagnosis of anorexia nervosa requires the following 3 criteria1:
- restriction of food intake resulting in significantly low body weight (defined as weight that is less than “minimally normal”) relative to age, gender, or development
- intense fear of gaining weight, or persistent behaviors that interfere with weight gain
- disturbance in the way in which one’s body weight or shape is experienced, undue influence of body weight or shape on self-evaluation, or lack of insight with regard to seriousness of current low body weight.
Continue to: Although Ms. S appeared...
Although Ms. S appeared thin, her BMI was within normal range. She added that she likes food and enjoyed eating, but that her medical condition made it too painful. Lastly, Ms. S denied a history of binging or purging.
Somatic symptom disorder.

Factitious disorder imposed on self. An individual with FDIS chronically stimulates, induces, or aggravates illnesses to gain the status of being a patient.
Factitious disorder imposed on another is the deliberate feigning or production of symptoms in another individual who is under the perpetrator’s supervision.1 Table 23 lists clinical indicators that raise suspicion for FDIA.

Before a diagnosis of somatic symptom disorder, FDIS, or FDIA could be established or ruled out, it was imperative to gather collateral information from other clinicians involved in Ms. S’s care. Ms. S and her mother had sought out help from a pediatric surgeon, a pediatric gastroenterologist, a pediatrician, and a psychotherapist.
Continue to: EVALUATION Collateral information
EVALUATION Collateral information
After Ms. S’s mother signs consent forms for exchange of information, the treatment team reaches out to the other clinicians. The therapist confirms that Ms. S’s mother had ended her daughter’s treatment after she was unable to quickly obtain documentation to rule out anorexia nervosa.
Both the pediatric surgeon and gastroenterologist report concerns of FDIA, which is why both clinicians had referred Ms. S and her mother to psychiatry. The pediatric surgeon states that on one occasion when he interviewed Ms. S separately from her mother, she seemed to be going down a checklist of symptoms. The surgeon reports that there was a partial occlusion of the superior mesenteric artery, confirming the diagnosis of SMAS, but he believed it was not severe enough to explain the symptoms Ms. S reported. The surgeon had scheduled another imaging appointment for 1 month later.
The pediatric gastroenterologist reports that Ms. S’s mother had demanded surgery and nasogastric tube placement for her daughter, which raised suspicion of FDIA. The gastroenterologist had convinced Ms. S and her mother to start low-dose doxepin, 20 mg twice a day, for anxiety, sleep, and abdominal pain.
Lastly, the pediatrician reports that she had not seen Ms. S for several months but stated that Ms. S always has been in the low normal BMI range. The pediatrician also reports that 6 months ago, the patient and her mother were frantically visiting EDs and scheduling doctor’s appointments.
[polldaddy:10586906]
The authors’ observations
The treatment team decided that Ms. S was not in imminent danger, and felt it was important to keep her in treatment without raising her mother’s suspicion. The team agreed to raise these concerns to the police, child protective services, and risk management if Ms. S’s health suddenly deteriorated or if her mother decided to remove Ms. S from our care.
Continue to: The treatment team...
The treatment team at the outpatient psychiatry clinic agreed that Ms. S did not currently meet criteria for anorexia nervosa, MDD, FDIS, or FDIA. However, Ms. S reported worries particular to persistent abdominal pain that was exacerbated by either eating or going to bed at night, which indicated that somatic symptom disorder was her likely diagnosis. Further, she endorsed a high level of anxiety and depression with regard to this somatic complaint that interfered with her daily activities and consumed an excessive amount of time, which also pointed to somatic symptom disorder. As a result of this diagnosis, the treatment team helped Ms. S manage her somatic symptoms and monitored for any other changes in her symptoms
Generally, cognitive-behavioral therapy (CBT) and mindfulness-based therapy may help relieve symptoms associated with somatic symptom disorder.4
TREATMENT Therapy sessions and medication management
At the psychiatric clinic, Ms. S is scheduled for biweekly therapy sessions with a social worker and biweekly appointments with a senior psychiatry resident for medication management. At each visit, Ms. S’s vital signs, height, and weight are measured. In the therapy sessions, she is taught mindfulness skills as well as CBT. The senior psychiatry resident maintains regular communication with the other clinicians involved in Ms. S’s care.
After the first month of treatment, Ms. S undergoes repeat imaging at the gastroenterologist’s office that indicates her SMAS is no longer occluded. Ms. S continues to report somatic symptoms, but with mild improvement.
Over the course of approximately 4 months, Ms. S begins to show signs of improvement in her pain, anxiety, and depression. Ms. S begins to feel well enough to get a summer job at a nursing home and expresses enthusiasm when her weight begins to increase. Her mother also became enthused and verbalized her appreciation that her daughter appeared to be improving.
Continue to: In the fall...
In the fall, Ms. S returns to high school for her senior year but has difficulty getting back into the routine and relating to her old friends. Ms. S continues to perseverate on thoughts of getting sick and her physical symptoms become overwhelming once again. She continues to be focused on any new symptoms she experiences, and to limit the types of foods she eats due to fear of the abdominal pain returning.
After several more months of psychiatric treatment, Ms. S reports significant relief from her abdominal pain, and no longer seeks corrective surgery for her SMAS. Although she occasionally struggles with perseverating thoughts and anxiety about her somatic symptoms such as abdominal pain and worrying about the types of foods she eats and becoming ill, she continues to work through symptoms of her somatic symptom disorder.
The authors’ observations
The main challenge of somatic symptom disorder is the patient’s “abnormal illness behavior.”2,5,6 For pediatric patients, there may an association between a parent’s psychological status and the patient’s somatic symptoms. Abdominal symptoms in a pediatric patient have a strong association with a parent who presents with depression, anxiety, or somatization. The effects of the parent’s psychological status could also manifest in the form of modeling catastrophic thinking or through reinforcement. Parents with certain traits, such as disproportionate worry about pain, may pay more attention to their child’s symptoms, and hence, reward the child when he/she reports somatic symptoms.7,8 In the case of Ms. S, her mother did not participate in therapy and the mother’s psychiatric history was never obtained.
OUTCOMES Making personal strides
Ms. S continues to use mindfulness skills as well as CBT to manage her symptoms of somatic symptom disorder. She continues to celebrate her weight gains, denies any thoughts of suicide or self-harm behaviors, and prepares for college by scheduling campus visits and completing admissions applications.
Bottom Line
Patients with somatic symptom disorder tend to have very high levels of worry about illness. Somatic symptoms in such patients may or may not have a medical explanation. Accurate diagnosis and careful management are necessary to reduce patient distress. Cognitive-behavioral therapy and mindfulness-based therapy may help relieve symptoms associated with this disorder.
Related Resources
- Henningsen P. Management of somatic symptom disorder. Dialogues Clin Neurosci. 2018;20(1):23-91.
- Rosic T, Kalra S, Samaan Z. Somatic symptom disorder, a new DSM-5 diagnosis of an old clinical challenge. BMJ Case Rep. 2016: bcr2015212553. doi: 10.1136/bcr-2015-212553.
Drug Brand Name
Doxepin • Silenor
CASE Distraught over a medical illness
Ms. S, age 16, presents to the emergency department (ED) accompanied by her mother with superficial lacerations on her arm. Ms. S states, “I cut my arm because I was afraid I was going to do something serious if I didn’t get to go to the ED.” She says that 6 months earlier, she was diagnosed with superior mesenteric artery syndrome (SMAS), a rare, potentially life-threatening condition that occurs when the duodenum is compressed between the aorta and the superior mesenteric artery, causing a partial or complete blockage of the duodenum. Since receiving this diagnosis, Ms. S reports feeling anxious, depressed, and overwhelmed by both the pain she is experiencing from her illness and uncertainty about her prognosis.
HISTORY In pain and isolated
Since being diagnosed with SMAS, Ms. S has had approximately 30 medical and 7 ED visits for SMAS-related pain. Ms. S was referred to the outpatient clinic for ongoing support and treatment for SMAS.
Because of her pain and anxiety, Ms. S, a junior in high school, no longer attends school but has been working with a tutor. Ms. S says that some of her loneliness and hopelessness are due to the social isolation of being tutored at home. She states that she has been “out of sight and out of mind” from her friends. She also reports feeling different from them due to the pain brought on by SMAS.
Ms. S and her mother live in public housing. Ms. S says that overall, she has a good relationship with her mother, but that in certain situations, her mother’s anxiety causes her significant frustration and anxiety.
EVALUATION Transient suicidal thoughts
A physical examination reveals superficial lacerations to Ms. S’s left arm. Although she appears thin, her current body mass index (BMI) is 20.4 kg/m2, which is within normal range. She says she sees herself as “underweight” and “not fat at all.” Ms. S reports that she likes food and enjoyed eating until it became too painful following her SMAS diagnosis. Ms. S denies a history of binging or purging. Results from her laboratory workup and all values are within normal limits.
During the initial interview, Ms. S’s mother says they came to the ED because Ms. S urgently needs a psychiatric evaluation so she can be cleared for gastrointestinal (GI) surgery and placement of a nasogastric tube. Her mother says a surgeon from a different hospital told them that her insurance company required a psychiatric evaluation to rule out anorexia nervosa before they would authorize the GI surgery. When asked why psychiatry at this hospital was not consulted, Ms. S’s mother does not answer.
When asked about the symptoms she has been experiencing, Ms. S says that her sleep has been poor because of increased pain and excessive worrying about her health. She has limited her food intake. Ms. S reports that after eating, she lays on her left side to alleviate pain and help the food move through her body.
Continue to: Ms. S says...
Ms. S says she feels anxious and depressed due to her SMAS diagnosis, her mother’s online research and oversharing of poor prognoses, and being isolated from her friends. Most of her time outside the home is spent attending medical appointments with specialists. Several months ago, Ms. S had seen a psychotherapist, but her mother was unhappy with the treatment recommendations, which included seeking care from a nutritionist and joining group therapy. Ms. S’s mother says she ended her daughter’s psychotherapy because she was unable to obtain a signature ruling out anorexia nervosa within the first few appointments.
Ms. S also says she has had passive suicidal thoughts during the past month, usually twice a week. She reports that these thoughts lasted as long as several hours and were difficult to control, but she has no specific plan or intent. Ms. S denies current suicidal thoughts or ideation, and works with the treatment team to complete a safety plan, which she signs. Other than her recent visit to the ED, Ms. S denies any other thoughts or behaviors of self-injury or suicide.
[polldaddy:10586905]
The authors’ observations
The treatment team considered the following conditions as part of Ms. S’s differential diagnosis:
Major depressive disorder. The team was able to rule out MDD because Ms. S’s depression was attributed to SMAS. Ms. S reported that all depressive symptoms were manageable or nonexistent before the onset of pain from SMAS. There was no direct pathophysiological consequence of another medical condition. Ms. S was clear that her symptoms of anxiety and depression began after she was isolated from her friends and began having difficulty understanding her diagnosis and prognosis.
Anorexia nervosa also was ruled out. According to the DSM-5, a diagnosis of anorexia nervosa requires the following 3 criteria1:
- restriction of food intake resulting in significantly low body weight (defined as weight that is less than “minimally normal”) relative to age, gender, or development
- intense fear of gaining weight, or persistent behaviors that interfere with weight gain
- disturbance in the way in which one’s body weight or shape is experienced, undue influence of body weight or shape on self-evaluation, or lack of insight with regard to seriousness of current low body weight.
Continue to: Although Ms. S appeared...
Although Ms. S appeared thin, her BMI was within normal range. She added that she likes food and enjoyed eating, but that her medical condition made it too painful. Lastly, Ms. S denied a history of binging or purging.
Somatic symptom disorder.

Factitious disorder imposed on self. An individual with FDIS chronically stimulates, induces, or aggravates illnesses to gain the status of being a patient.
Factitious disorder imposed on another is the deliberate feigning or production of symptoms in another individual who is under the perpetrator’s supervision.1 Table 23 lists clinical indicators that raise suspicion for FDIA.

Before a diagnosis of somatic symptom disorder, FDIS, or FDIA could be established or ruled out, it was imperative to gather collateral information from other clinicians involved in Ms. S’s care. Ms. S and her mother had sought out help from a pediatric surgeon, a pediatric gastroenterologist, a pediatrician, and a psychotherapist.
Continue to: EVALUATION Collateral information
EVALUATION Collateral information
After Ms. S’s mother signs consent forms for exchange of information, the treatment team reaches out to the other clinicians. The therapist confirms that Ms. S’s mother had ended her daughter’s treatment after she was unable to quickly obtain documentation to rule out anorexia nervosa.
Both the pediatric surgeon and gastroenterologist report concerns of FDIA, which is why both clinicians had referred Ms. S and her mother to psychiatry. The pediatric surgeon states that on one occasion when he interviewed Ms. S separately from her mother, she seemed to be going down a checklist of symptoms. The surgeon reports that there was a partial occlusion of the superior mesenteric artery, confirming the diagnosis of SMAS, but he believed it was not severe enough to explain the symptoms Ms. S reported. The surgeon had scheduled another imaging appointment for 1 month later.
The pediatric gastroenterologist reports that Ms. S’s mother had demanded surgery and nasogastric tube placement for her daughter, which raised suspicion of FDIA. The gastroenterologist had convinced Ms. S and her mother to start low-dose doxepin, 20 mg twice a day, for anxiety, sleep, and abdominal pain.
Lastly, the pediatrician reports that she had not seen Ms. S for several months but stated that Ms. S always has been in the low normal BMI range. The pediatrician also reports that 6 months ago, the patient and her mother were frantically visiting EDs and scheduling doctor’s appointments.
[polldaddy:10586906]
The authors’ observations
The treatment team decided that Ms. S was not in imminent danger, and felt it was important to keep her in treatment without raising her mother’s suspicion. The team agreed to raise these concerns to the police, child protective services, and risk management if Ms. S’s health suddenly deteriorated or if her mother decided to remove Ms. S from our care.
Continue to: The treatment team...
The treatment team at the outpatient psychiatry clinic agreed that Ms. S did not currently meet criteria for anorexia nervosa, MDD, FDIS, or FDIA. However, Ms. S reported worries particular to persistent abdominal pain that was exacerbated by either eating or going to bed at night, which indicated that somatic symptom disorder was her likely diagnosis. Further, she endorsed a high level of anxiety and depression with regard to this somatic complaint that interfered with her daily activities and consumed an excessive amount of time, which also pointed to somatic symptom disorder. As a result of this diagnosis, the treatment team helped Ms. S manage her somatic symptoms and monitored for any other changes in her symptoms
Generally, cognitive-behavioral therapy (CBT) and mindfulness-based therapy may help relieve symptoms associated with somatic symptom disorder.4
TREATMENT Therapy sessions and medication management
At the psychiatric clinic, Ms. S is scheduled for biweekly therapy sessions with a social worker and biweekly appointments with a senior psychiatry resident for medication management. At each visit, Ms. S’s vital signs, height, and weight are measured. In the therapy sessions, she is taught mindfulness skills as well as CBT. The senior psychiatry resident maintains regular communication with the other clinicians involved in Ms. S’s care.
After the first month of treatment, Ms. S undergoes repeat imaging at the gastroenterologist’s office that indicates her SMAS is no longer occluded. Ms. S continues to report somatic symptoms, but with mild improvement.
Over the course of approximately 4 months, Ms. S begins to show signs of improvement in her pain, anxiety, and depression. Ms. S begins to feel well enough to get a summer job at a nursing home and expresses enthusiasm when her weight begins to increase. Her mother also became enthused and verbalized her appreciation that her daughter appeared to be improving.
Continue to: In the fall...
In the fall, Ms. S returns to high school for her senior year but has difficulty getting back into the routine and relating to her old friends. Ms. S continues to perseverate on thoughts of getting sick and her physical symptoms become overwhelming once again. She continues to be focused on any new symptoms she experiences, and to limit the types of foods she eats due to fear of the abdominal pain returning.
After several more months of psychiatric treatment, Ms. S reports significant relief from her abdominal pain, and no longer seeks corrective surgery for her SMAS. Although she occasionally struggles with perseverating thoughts and anxiety about her somatic symptoms such as abdominal pain and worrying about the types of foods she eats and becoming ill, she continues to work through symptoms of her somatic symptom disorder.
The authors’ observations
The main challenge of somatic symptom disorder is the patient’s “abnormal illness behavior.”2,5,6 For pediatric patients, there may an association between a parent’s psychological status and the patient’s somatic symptoms. Abdominal symptoms in a pediatric patient have a strong association with a parent who presents with depression, anxiety, or somatization. The effects of the parent’s psychological status could also manifest in the form of modeling catastrophic thinking or through reinforcement. Parents with certain traits, such as disproportionate worry about pain, may pay more attention to their child’s symptoms, and hence, reward the child when he/she reports somatic symptoms.7,8 In the case of Ms. S, her mother did not participate in therapy and the mother’s psychiatric history was never obtained.
OUTCOMES Making personal strides
Ms. S continues to use mindfulness skills as well as CBT to manage her symptoms of somatic symptom disorder. She continues to celebrate her weight gains, denies any thoughts of suicide or self-harm behaviors, and prepares for college by scheduling campus visits and completing admissions applications.
Bottom Line
Patients with somatic symptom disorder tend to have very high levels of worry about illness. Somatic symptoms in such patients may or may not have a medical explanation. Accurate diagnosis and careful management are necessary to reduce patient distress. Cognitive-behavioral therapy and mindfulness-based therapy may help relieve symptoms associated with this disorder.
Related Resources
- Henningsen P. Management of somatic symptom disorder. Dialogues Clin Neurosci. 2018;20(1):23-91.
- Rosic T, Kalra S, Samaan Z. Somatic symptom disorder, a new DSM-5 diagnosis of an old clinical challenge. BMJ Case Rep. 2016: bcr2015212553. doi: 10.1136/bcr-2015-212553.
Drug Brand Name
Doxepin • Silenor
1. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
2. Stern T, Freudenreich O, Smith F, et al. Massachusetts General Hospital Handbook of General Hospital Psychiatry, 7th ed. New York, NY: Elsevier; 2017.
3. Feldman MD, Eisendrath SJ. The spectrum of factitious disorders. Washington, DC: American Psychiatric Association; 1997.
4. Sadock BJ, Sadock VA, Ruiz P. Kaplan & Sadock’s synopsis of psychiatry: behavioral sciences/clinical psychiatry, 11th ed. Philadelphia, PA: Wolters Kluwer; 2014:470.
5. Pilowsky I. The concept of abnormal illness behavior. Psychosomatics. 1990;31(2):207-213.
6. Kirmayer LJ, Looper KJ. Abnormal illness behavior: physiological, psychological and social dimensions of coping with stress. Curr Opin Psychiatry. 2006;19(1):54-60.
7. Walker LS, Garber J, Greene JW. Somatic complaints in pediatric patients: a prospective study of the role of negative life events, child social and academic competence, and parental somatic symptoms. J Consult Clin Psychology. 1994;62(6):1213-1221.
8. Van Oudenhove L, Levy RL, Crowell MD, et al. Biopsychosocial aspects of functional gastrointestinal disorders: how central and environmental processes contribute to the development and expression of functional gastrointestinal disorders. Gastroenterology. 2016;150(6):1355-1367.
1. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
2. Stern T, Freudenreich O, Smith F, et al. Massachusetts General Hospital Handbook of General Hospital Psychiatry, 7th ed. New York, NY: Elsevier; 2017.
3. Feldman MD, Eisendrath SJ. The spectrum of factitious disorders. Washington, DC: American Psychiatric Association; 1997.
4. Sadock BJ, Sadock VA, Ruiz P. Kaplan & Sadock’s synopsis of psychiatry: behavioral sciences/clinical psychiatry, 11th ed. Philadelphia, PA: Wolters Kluwer; 2014:470.
5. Pilowsky I. The concept of abnormal illness behavior. Psychosomatics. 1990;31(2):207-213.
6. Kirmayer LJ, Looper KJ. Abnormal illness behavior: physiological, psychological and social dimensions of coping with stress. Curr Opin Psychiatry. 2006;19(1):54-60.
7. Walker LS, Garber J, Greene JW. Somatic complaints in pediatric patients: a prospective study of the role of negative life events, child social and academic competence, and parental somatic symptoms. J Consult Clin Psychology. 1994;62(6):1213-1221.
8. Van Oudenhove L, Levy RL, Crowell MD, et al. Biopsychosocial aspects of functional gastrointestinal disorders: how central and environmental processes contribute to the development and expression of functional gastrointestinal disorders. Gastroenterology. 2016;150(6):1355-1367.
New-onset psychosis while being treated for coronavirus
CASE Agitated, psychotic, and COVID-19–positive
Mr. G, age 56, is brought to the emergency department (ED) by emergency medical services (EMS) after his girlfriend reports that he was trying to climb into the “fiery furnace” to “burn the devil within him.” Mr. G had recently tested positive for coronavirus disease 2019 (COVID-19) via polymerase chain reaction and had been receiving treatment for it. In the ED, he is distressed and repeatedly exclaims, “The devil is alive!” He insists on covering himself with blankets, despite diaphoresis and soaking through his clothing within minutes. Because he does not respond to attempted redirection, the ED clinicians administer a single dose of IM haloperidol, 2 mg, for agitation.
HISTORY Multiple ED visits and hospitalizations
Mr. G, who has no known psychiatric history, lives with his girlfriend of 10 years. His medical history includes chronic obstructive pulmonary disease and prostate cancer. In 2015, he had a radical prostatectomy, without chemotherapy. His social history includes childhood neglect, which prompted him to leave home when he was a teenager. Mr. G had earned his general education development certificate and worked at a small retail store.
Mr. G had no previous history of mental health treatment per self-report, collateral information from his girlfriend, and chart review. He reported no known family psychiatric history. He did not endorse past psychiatric admissions or suicide attempts, nor previous periods of mania, depression, or psychosis. He said he used illicit substances as a teen, but denied using alcohol, tobacco products, or illicit substances in the past 20 years.
Mr. G recently had multiple ED visits and hospitalizations due to ongoing signs and symptoms associated with his COVID-19 diagnosis, primarily worsening shortness of breath and cough. Eleven days before EMS brought him to the ED at his girlfriend’s request, Mr. G had presented to the ED with chief complaints of shortness of breath and dry cough (Day 0). He reported that he had been “running a fever” for 2 days. In the ED, his initial vital signs were notable only for a temperature of 100.9°F (38.28°C). He was diagnosed with “acute viral syndrome” and received 1 dose of IV ceftriaxone, 2 g, and IV azithromycin, 500 mg. On Day 2, the ED clinicians prescribed a 4-day course of oral azithromycin, 250 mg/d, and discharged him home.
On Day 3, Mr. G returned to the ED with similar complaints—congestion and productive cough. He tested positive for COVID-19, and the ED discharged him home with quarantine instructions. Hours later, he returned to the ED via EMS with chief complaints of chest pain, diarrhea, and myalgias. He was prescribed a 5-day course ofoseltamivir, 75 mg twice daily, and azithromycin, 250 mg/d. The ED again discharged him home.
On Day 4, Mr. G returned to the ED for a fourth time. His chief complaint was worsening shortness of breath. His oxygen saturation was 94% on room air; it improved to 96% on 2 L of oxygen. His chest X-ray showed diffuse reticulonodular opacities throughout his bilateral lung fields and increased airspace opacification in the bilateral lower lobes. The ED admitted Mr. G to an internal medicine unit, where the primary treatment team enrolled him in a clinical trial. As part of the trial, Mr. G received hydroxychloroquine, 400 mg, on Day 4 and Day 5. The placebo-controlled component of the trial involved Mr. G receiving daily infusions of either remdesivir or placebo on Day 6 through Day 8. On Day 8, Mr. G was discharged home.
On Day 9, Mr. G returned to the ED with a chief complaint that his “thermometer wasn’t working” at home. The ED readmitted him to the internal medicine unit. On Day 9 through Day 11, Mr. G received daily doses of
Continue to: During the second hospitalization...
During the second hospitalization, nursing staff reported that Mr. G seemed religiously preoccupied and once reported seeing angels and demons. He was observed sitting in a chair praying to Allah that he would “come in on a horse to chop all the workers’ heads off.”
On Day 11, Mr. G was discharged home. Later that evening, the EMS brought him back in the ED due to his girlfriend’s concerns about his mental state.
EVALUATION Talks to God
On Day 12, psychiatry is consulted to evaluate Mr. G’s new-onset psychosis. Mr. G is alert and oriented to person, place, and time. His speech is loud, though the amount and rate are unremarkable. He displays no psychomotor agitation. His thought process is tangential and focuses on religious themes, specifically referring to Islam. He reports auditory hallucinations of God speaking directly to him. Mr. G states, “I am here because of a miraculous transformation from death back to life. Do you believe in God? Which God do you believe in? There are 2 Gods and only one of them is the true God. He is the God of all the 7 heavens and His true name is Allah, only one God, one faith. Allah is a ball of energy.”
Mr. G’s girlfriend provides collateral information that Mr. G had been raised Christian but was not religious as an adult. She says that he had never spoken about being Muslim. She adds that she had never known him to speak much about religion.
[polldaddy:10572249]
The authors’ observations
The etiology of new-onset psychosis can be related to several factors, including primary psychiatric illnesses, use of illicit substances, sequelae of general medical conditions, or adverse effects of prescribed medications. We considered each of these in the differential diagnosis for Mr. G.
Continue to: Psychiatric illness or illicit substance use
Psychiatric illness or illicit substance use. Because Mr. G was 56 years old and had no known psychiatric history or family psychiatric history, a primary psychiatric illness seemed less likely. Substance-induced psychosis related to illicit substance use also seemed unlikely because he denied using illicit substances, and an expanded urine drug screen was negative.
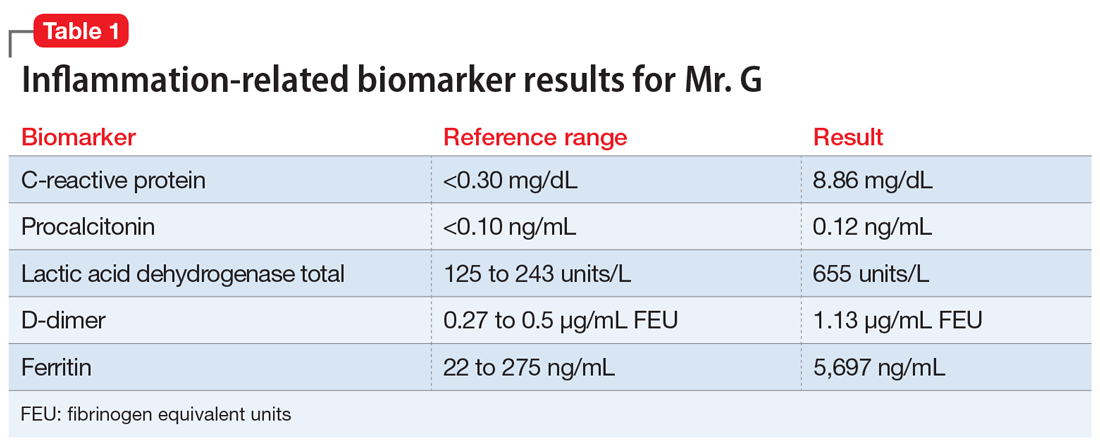
Psychosis due to a general medical condition. Results from Mr. G’s laboratory workup show marked elevation in multiple inflammation-related biomarkers (Table 1), consistent with the inflammatory profile seen with COVID-19 infection. However, results from several laboratory tests for potential etiologies of new-onset psychosis due to a general medical condition were negative (Table 2). Based on Mr. G’s history of prostate cancer, we considered the possibility of metastatic space-occupying lesions of the brain; however, Mr. G’s head CT showed no acute intracranial abnormalities. Another possible etiology we considered was COVID-19–induced encephalitis; however, Mr. G’s brain MRI with and without contrast showed no evidence of acute or chronic intracranial changes.
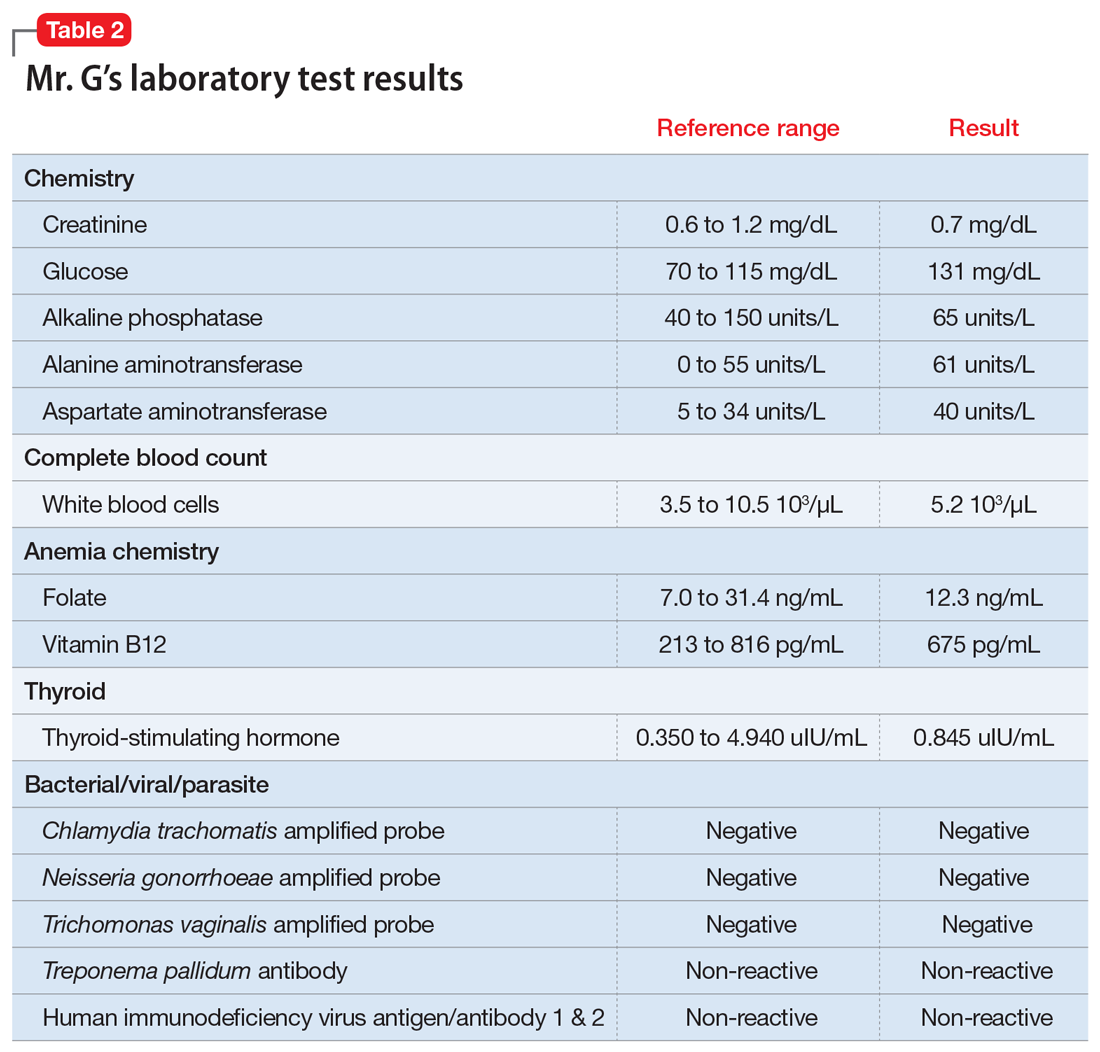
Medication-induced psychosis. After largely ruling out primary psychiatric illnesses, illicit substances, and sequelae of general medical conditions, we turned our attention to prescribed medications as a potential etiology of Mr. G’s new-onset psychosis. During his initial hospitalization, Mr. G had been prescribed 2 doses of hydroxychloroquine, 400 mg, to treat his diagnosis of COVID-19. Because none of the other medications he received were reported to have neuropsychiatric adverse effects, including psychosis, hydroxychloroquine-induced psychosis was therefore the primary team’s working diagnosis.
EVALUATION Request to leave AMA
On Day 13, Mr. G requests to leave the hospital against medical advice (AMA). Until this point, he had voluntarily remained in the hospital, which he repeatedly referred to as “Heaven.” When asked to describe his medical condition, Mr. G replies, “God told me my condition is far beyond man’s understanding.” He denies that he is positive for COVID-19. He states, “I am cured, and the real fight has just begun.”
At the recommendation of the psychiatry consultation-liaison (C-L) service, the primary treatment team determines that Mr. G does not have capacity to leave AMA. The team is concerned that because of his psychotic symptoms, Mr. G would be unable to understand and follow his quarantine instructions. He remains hospitalized on a medical hold.
Continue to: The authors' observations
The authors’ observations
One important consideration this case highlighted was potential third-party responsibility clinicians and hospital systems may face if they discharge a patient with a communicable illness who is unable to follow precautions based on a psychiatric condition.1 That concern was based on Mr. G’s reported desire to pursue missions “beyond man’s understanding,” which he felt compelled to complete, and which could unnecessarily place the public at risk. The psychiatry C-L service consulted the local health department and conferred with the hospital’s legal representatives, who agreed with the plan to keep Mr. G in the hospital for his safety as well as for the public’s safety.
TREATMENT Oral haloperidol
The psychiatry C-L service recommends initiating an antipsychotic. On Day 13, Mr. G starts oral haloperidol, 2.5 mg twice a day, to address his ongoing psychotic symptoms. On Day 14, the treatment team increases the dosage to 5 mg twice a day. Mr. G tolerates the haloperidol and gradually begins to improve. He demonstrates improved sleep, normal speech volume, less religious preoccupation, and a considerably improved understanding of his medical condition.
The authors’ observations
Mr. G’s initial psychiatric evaluation demonstrated an acute onset of psychotic symptoms, without evidence of delirium. Psychosis secondary to a general medical condition (such as COVID-19) and hydroxychloroquine-induced psychotic disorder topped our initial considerations in the differential diagnosis of this case. While the exact neuropsychiatric sequelae of COVID-19 are not yet clear, previous experiences with viral pandemics and case studies from the current pandemic demonstrate a wide variety of possible neuropsychiatric manifestations. Mood symptoms, psychosis, and encephalopathy represent some of the neuropsychiatric complications observed with past viral pandemics.2 Neuropsychiatric symptoms may be triggered by the virus itself, or from the host’s immune response to the infection.3 To further complicate matters, neuropsychiatric symptoms may manifest during the acute viral infection, or may surface later, as subacute or chronic neuropsychiatric illness.
Neuropsychiatric adverse events
Mr. G developed psychotic symptoms within the first few days of receiving hydroxychloroquine, which is consistent with the scant literature on this topic.8 Based on the available information, hydroxychloroquine remains the most likely etiology of his new-onset psychotic symptoms. Mr. G’s case is one example of the possible neuropsychiatric presentations clinicians may face while treating a novel viral illness.
Continue to: OUTCOME Homeward-bound
OUTCOME Homeward-bound
By Day 18, Mr. G’s psychotic symptoms have significantly improved. He is able to rationally process information about his COVID-19 diagnosis and the recommended quarantine instructions he needs to follow after discharge. He is cleared by infection control and discharged home to return to living with his girlfriend.
Mr. G attends his follow-up psychiatric appointment remotely 2 weeks after discharge. He reports that since discharge, he has continued taking his prescribed haloperidol, 5 mg twice a day. He demonstrates improved insight into his medical condition, acknowledging his COVID-19–positive status, and confirms that he has been following quarantine instructions. He does not report ongoing auditory or visual hallucinations, and is no longer religiously preoccupied. He says he is looking forward to being medically cleared to return to work.
The authors’ observations
This case highlights the need for prospective, longitudinal screening and monitoring of neuropsychiatric symptoms as part of the public health response to COVID-19. The case also highlights the importance of careful monitoring for adverse events, including neuropsychiatric symptoms, during clinical trials that involve experimental treatments. The long-term prognosis for individuals such as Mr. G who develop neuropsychiatric symptoms during acute COVID-19 infection remains unknown. Similarly, subacute and chronic neuropsychiatric manifestations that may develop after resolution of acute COVID-19 infection are unknown at this time. However, we can learn from past viral pandemics and anticipate that neuropsychiatric sequelae are likely to occur and should be part of the public health response to the pandemic.
Bottom Line
The coronavirus disease 2019 pandemic provides multiple clinical challenges pertinent to psychiatry. Neuropsychiatric symptoms may manifest from delirium, viral infection, host immune response, or adverse reactions to experimental treatments. These potential neuropsychiatric symptoms may complicate medical treatment. They can also raise important ethical and legal considerations, such as weighing patient autonomy vs third-party responsibility to the public at large.
Related Resources
- Ferrando SJ, Klepacz L, Lynch S, et al. COVID-19 psychosis: a potential new neuropsychiatric condition triggered by novel coronavirus infection and the inflammatory response? [published online May 19, 2020]. Psychosomatics. 2020. doi: 10.1016/j.psym.2020.05.012.
- Vlessides M. COVID-19 and psychosis: is there a link? Medscape Medical News. https://www.medscape.com/viewarticle/930224. Published May 8, 2020.
Drug Brand Names
Azithromycin • Zithromax
Ceftriaxone • Rocephin
Chloroquine • Aralen
Haloperidol • Haldol
Hydroxychloroquine • Plaquenil
Levofloxacin • Levaquin
Oseltamivir • Tamiflu
1. Ghossoub E, Newman WJ. COVID-19 and the duty to protect from communicable diseases. [published online ahead of print, May 8, 2020]. J Am Acad Psychiatry Law.
2. Menninger Ka. Psychoses associated with influenza: I. general data: statistical analysis. JAMA. 1919;72(4):235-241.
3. Troyer EA, Kohn JN, Hong S. Are we facing a crashing wave of neuropsychiatric sequelae of COVID-19? Neuropsychiatric symptoms and potential immunologic mechanisms. Brain, Behavior, and Immunity. 2020. doi:10.1016/j.bbi.2020.04.027.
4. Alkadi HO. Antimalarial drug toxicity: a review. Chemotherapy. 2007;53(6):385-391.
5. Bogaczewicz A, Sobów T. Psychiatric adverse effects of chloroquine. Psychiatria i Psychologia Kliniczna. 2017;17(2):111-114.
6. Sato K, Mano T, Iwata A, et al. Neuropsychiatric adverse events of chloroquine: a real-world pharmacovigilance study using the FDA Adverse Event Reporting System (FAERS) database. Biosci Trends. 2020;14(2):139-143.
7. Cortegiani A, Ingoglia G, Ippolito M, et al. A systematic review on the efficacy and safety of chloroquine for the treatment of COVID-19. J Crit Care. 2020;57:279-283.
8. Das P, Rai A, Chopra A, et al. Psychosis likely induced by hydroxychloroquine in a patient with chronic Q fever: a case report and clinically relevant review of pharmacology. Psychosomatics. 2014;55(4):409-413.
CASE Agitated, psychotic, and COVID-19–positive
Mr. G, age 56, is brought to the emergency department (ED) by emergency medical services (EMS) after his girlfriend reports that he was trying to climb into the “fiery furnace” to “burn the devil within him.” Mr. G had recently tested positive for coronavirus disease 2019 (COVID-19) via polymerase chain reaction and had been receiving treatment for it. In the ED, he is distressed and repeatedly exclaims, “The devil is alive!” He insists on covering himself with blankets, despite diaphoresis and soaking through his clothing within minutes. Because he does not respond to attempted redirection, the ED clinicians administer a single dose of IM haloperidol, 2 mg, for agitation.
HISTORY Multiple ED visits and hospitalizations
Mr. G, who has no known psychiatric history, lives with his girlfriend of 10 years. His medical history includes chronic obstructive pulmonary disease and prostate cancer. In 2015, he had a radical prostatectomy, without chemotherapy. His social history includes childhood neglect, which prompted him to leave home when he was a teenager. Mr. G had earned his general education development certificate and worked at a small retail store.
Mr. G had no previous history of mental health treatment per self-report, collateral information from his girlfriend, and chart review. He reported no known family psychiatric history. He did not endorse past psychiatric admissions or suicide attempts, nor previous periods of mania, depression, or psychosis. He said he used illicit substances as a teen, but denied using alcohol, tobacco products, or illicit substances in the past 20 years.
Mr. G recently had multiple ED visits and hospitalizations due to ongoing signs and symptoms associated with his COVID-19 diagnosis, primarily worsening shortness of breath and cough. Eleven days before EMS brought him to the ED at his girlfriend’s request, Mr. G had presented to the ED with chief complaints of shortness of breath and dry cough (Day 0). He reported that he had been “running a fever” for 2 days. In the ED, his initial vital signs were notable only for a temperature of 100.9°F (38.28°C). He was diagnosed with “acute viral syndrome” and received 1 dose of IV ceftriaxone, 2 g, and IV azithromycin, 500 mg. On Day 2, the ED clinicians prescribed a 4-day course of oral azithromycin, 250 mg/d, and discharged him home.
On Day 3, Mr. G returned to the ED with similar complaints—congestion and productive cough. He tested positive for COVID-19, and the ED discharged him home with quarantine instructions. Hours later, he returned to the ED via EMS with chief complaints of chest pain, diarrhea, and myalgias. He was prescribed a 5-day course ofoseltamivir, 75 mg twice daily, and azithromycin, 250 mg/d. The ED again discharged him home.
On Day 4, Mr. G returned to the ED for a fourth time. His chief complaint was worsening shortness of breath. His oxygen saturation was 94% on room air; it improved to 96% on 2 L of oxygen. His chest X-ray showed diffuse reticulonodular opacities throughout his bilateral lung fields and increased airspace opacification in the bilateral lower lobes. The ED admitted Mr. G to an internal medicine unit, where the primary treatment team enrolled him in a clinical trial. As part of the trial, Mr. G received hydroxychloroquine, 400 mg, on Day 4 and Day 5. The placebo-controlled component of the trial involved Mr. G receiving daily infusions of either remdesivir or placebo on Day 6 through Day 8. On Day 8, Mr. G was discharged home.
On Day 9, Mr. G returned to the ED with a chief complaint that his “thermometer wasn’t working” at home. The ED readmitted him to the internal medicine unit. On Day 9 through Day 11, Mr. G received daily doses of
Continue to: During the second hospitalization...
During the second hospitalization, nursing staff reported that Mr. G seemed religiously preoccupied and once reported seeing angels and demons. He was observed sitting in a chair praying to Allah that he would “come in on a horse to chop all the workers’ heads off.”
On Day 11, Mr. G was discharged home. Later that evening, the EMS brought him back in the ED due to his girlfriend’s concerns about his mental state.
EVALUATION Talks to God
On Day 12, psychiatry is consulted to evaluate Mr. G’s new-onset psychosis. Mr. G is alert and oriented to person, place, and time. His speech is loud, though the amount and rate are unremarkable. He displays no psychomotor agitation. His thought process is tangential and focuses on religious themes, specifically referring to Islam. He reports auditory hallucinations of God speaking directly to him. Mr. G states, “I am here because of a miraculous transformation from death back to life. Do you believe in God? Which God do you believe in? There are 2 Gods and only one of them is the true God. He is the God of all the 7 heavens and His true name is Allah, only one God, one faith. Allah is a ball of energy.”
Mr. G’s girlfriend provides collateral information that Mr. G had been raised Christian but was not religious as an adult. She says that he had never spoken about being Muslim. She adds that she had never known him to speak much about religion.
[polldaddy:10572249]
The authors’ observations
The etiology of new-onset psychosis can be related to several factors, including primary psychiatric illnesses, use of illicit substances, sequelae of general medical conditions, or adverse effects of prescribed medications. We considered each of these in the differential diagnosis for Mr. G.
Continue to: Psychiatric illness or illicit substance use
Psychiatric illness or illicit substance use. Because Mr. G was 56 years old and had no known psychiatric history or family psychiatric history, a primary psychiatric illness seemed less likely. Substance-induced psychosis related to illicit substance use also seemed unlikely because he denied using illicit substances, and an expanded urine drug screen was negative.

Psychosis due to a general medical condition. Results from Mr. G’s laboratory workup show marked elevation in multiple inflammation-related biomarkers (Table 1), consistent with the inflammatory profile seen with COVID-19 infection. However, results from several laboratory tests for potential etiologies of new-onset psychosis due to a general medical condition were negative (Table 2). Based on Mr. G’s history of prostate cancer, we considered the possibility of metastatic space-occupying lesions of the brain; however, Mr. G’s head CT showed no acute intracranial abnormalities. Another possible etiology we considered was COVID-19–induced encephalitis; however, Mr. G’s brain MRI with and without contrast showed no evidence of acute or chronic intracranial changes.

Medication-induced psychosis. After largely ruling out primary psychiatric illnesses, illicit substances, and sequelae of general medical conditions, we turned our attention to prescribed medications as a potential etiology of Mr. G’s new-onset psychosis. During his initial hospitalization, Mr. G had been prescribed 2 doses of hydroxychloroquine, 400 mg, to treat his diagnosis of COVID-19. Because none of the other medications he received were reported to have neuropsychiatric adverse effects, including psychosis, hydroxychloroquine-induced psychosis was therefore the primary team’s working diagnosis.
EVALUATION Request to leave AMA
On Day 13, Mr. G requests to leave the hospital against medical advice (AMA). Until this point, he had voluntarily remained in the hospital, which he repeatedly referred to as “Heaven.” When asked to describe his medical condition, Mr. G replies, “God told me my condition is far beyond man’s understanding.” He denies that he is positive for COVID-19. He states, “I am cured, and the real fight has just begun.”
At the recommendation of the psychiatry consultation-liaison (C-L) service, the primary treatment team determines that Mr. G does not have capacity to leave AMA. The team is concerned that because of his psychotic symptoms, Mr. G would be unable to understand and follow his quarantine instructions. He remains hospitalized on a medical hold.
Continue to: The authors' observations
The authors’ observations
One important consideration this case highlighted was potential third-party responsibility clinicians and hospital systems may face if they discharge a patient with a communicable illness who is unable to follow precautions based on a psychiatric condition.1 That concern was based on Mr. G’s reported desire to pursue missions “beyond man’s understanding,” which he felt compelled to complete, and which could unnecessarily place the public at risk. The psychiatry C-L service consulted the local health department and conferred with the hospital’s legal representatives, who agreed with the plan to keep Mr. G in the hospital for his safety as well as for the public’s safety.
TREATMENT Oral haloperidol
The psychiatry C-L service recommends initiating an antipsychotic. On Day 13, Mr. G starts oral haloperidol, 2.5 mg twice a day, to address his ongoing psychotic symptoms. On Day 14, the treatment team increases the dosage to 5 mg twice a day. Mr. G tolerates the haloperidol and gradually begins to improve. He demonstrates improved sleep, normal speech volume, less religious preoccupation, and a considerably improved understanding of his medical condition.
The authors’ observations
Mr. G’s initial psychiatric evaluation demonstrated an acute onset of psychotic symptoms, without evidence of delirium. Psychosis secondary to a general medical condition (such as COVID-19) and hydroxychloroquine-induced psychotic disorder topped our initial considerations in the differential diagnosis of this case. While the exact neuropsychiatric sequelae of COVID-19 are not yet clear, previous experiences with viral pandemics and case studies from the current pandemic demonstrate a wide variety of possible neuropsychiatric manifestations. Mood symptoms, psychosis, and encephalopathy represent some of the neuropsychiatric complications observed with past viral pandemics.2 Neuropsychiatric symptoms may be triggered by the virus itself, or from the host’s immune response to the infection.3 To further complicate matters, neuropsychiatric symptoms may manifest during the acute viral infection, or may surface later, as subacute or chronic neuropsychiatric illness.
Neuropsychiatric adverse events
Mr. G developed psychotic symptoms within the first few days of receiving hydroxychloroquine, which is consistent with the scant literature on this topic.8 Based on the available information, hydroxychloroquine remains the most likely etiology of his new-onset psychotic symptoms. Mr. G’s case is one example of the possible neuropsychiatric presentations clinicians may face while treating a novel viral illness.
Continue to: OUTCOME Homeward-bound
OUTCOME Homeward-bound
By Day 18, Mr. G’s psychotic symptoms have significantly improved. He is able to rationally process information about his COVID-19 diagnosis and the recommended quarantine instructions he needs to follow after discharge. He is cleared by infection control and discharged home to return to living with his girlfriend.
Mr. G attends his follow-up psychiatric appointment remotely 2 weeks after discharge. He reports that since discharge, he has continued taking his prescribed haloperidol, 5 mg twice a day. He demonstrates improved insight into his medical condition, acknowledging his COVID-19–positive status, and confirms that he has been following quarantine instructions. He does not report ongoing auditory or visual hallucinations, and is no longer religiously preoccupied. He says he is looking forward to being medically cleared to return to work.
The authors’ observations
This case highlights the need for prospective, longitudinal screening and monitoring of neuropsychiatric symptoms as part of the public health response to COVID-19. The case also highlights the importance of careful monitoring for adverse events, including neuropsychiatric symptoms, during clinical trials that involve experimental treatments. The long-term prognosis for individuals such as Mr. G who develop neuropsychiatric symptoms during acute COVID-19 infection remains unknown. Similarly, subacute and chronic neuropsychiatric manifestations that may develop after resolution of acute COVID-19 infection are unknown at this time. However, we can learn from past viral pandemics and anticipate that neuropsychiatric sequelae are likely to occur and should be part of the public health response to the pandemic.
Bottom Line
The coronavirus disease 2019 pandemic provides multiple clinical challenges pertinent to psychiatry. Neuropsychiatric symptoms may manifest from delirium, viral infection, host immune response, or adverse reactions to experimental treatments. These potential neuropsychiatric symptoms may complicate medical treatment. They can also raise important ethical and legal considerations, such as weighing patient autonomy vs third-party responsibility to the public at large.
Related Resources
- Ferrando SJ, Klepacz L, Lynch S, et al. COVID-19 psychosis: a potential new neuropsychiatric condition triggered by novel coronavirus infection and the inflammatory response? [published online May 19, 2020]. Psychosomatics. 2020. doi: 10.1016/j.psym.2020.05.012.
- Vlessides M. COVID-19 and psychosis: is there a link? Medscape Medical News. https://www.medscape.com/viewarticle/930224. Published May 8, 2020.
Drug Brand Names
Azithromycin • Zithromax
Ceftriaxone • Rocephin
Chloroquine • Aralen
Haloperidol • Haldol
Hydroxychloroquine • Plaquenil
Levofloxacin • Levaquin
Oseltamivir • Tamiflu
CASE Agitated, psychotic, and COVID-19–positive
Mr. G, age 56, is brought to the emergency department (ED) by emergency medical services (EMS) after his girlfriend reports that he was trying to climb into the “fiery furnace” to “burn the devil within him.” Mr. G had recently tested positive for coronavirus disease 2019 (COVID-19) via polymerase chain reaction and had been receiving treatment for it. In the ED, he is distressed and repeatedly exclaims, “The devil is alive!” He insists on covering himself with blankets, despite diaphoresis and soaking through his clothing within minutes. Because he does not respond to attempted redirection, the ED clinicians administer a single dose of IM haloperidol, 2 mg, for agitation.
HISTORY Multiple ED visits and hospitalizations
Mr. G, who has no known psychiatric history, lives with his girlfriend of 10 years. His medical history includes chronic obstructive pulmonary disease and prostate cancer. In 2015, he had a radical prostatectomy, without chemotherapy. His social history includes childhood neglect, which prompted him to leave home when he was a teenager. Mr. G had earned his general education development certificate and worked at a small retail store.
Mr. G had no previous history of mental health treatment per self-report, collateral information from his girlfriend, and chart review. He reported no known family psychiatric history. He did not endorse past psychiatric admissions or suicide attempts, nor previous periods of mania, depression, or psychosis. He said he used illicit substances as a teen, but denied using alcohol, tobacco products, or illicit substances in the past 20 years.
Mr. G recently had multiple ED visits and hospitalizations due to ongoing signs and symptoms associated with his COVID-19 diagnosis, primarily worsening shortness of breath and cough. Eleven days before EMS brought him to the ED at his girlfriend’s request, Mr. G had presented to the ED with chief complaints of shortness of breath and dry cough (Day 0). He reported that he had been “running a fever” for 2 days. In the ED, his initial vital signs were notable only for a temperature of 100.9°F (38.28°C). He was diagnosed with “acute viral syndrome” and received 1 dose of IV ceftriaxone, 2 g, and IV azithromycin, 500 mg. On Day 2, the ED clinicians prescribed a 4-day course of oral azithromycin, 250 mg/d, and discharged him home.
On Day 3, Mr. G returned to the ED with similar complaints—congestion and productive cough. He tested positive for COVID-19, and the ED discharged him home with quarantine instructions. Hours later, he returned to the ED via EMS with chief complaints of chest pain, diarrhea, and myalgias. He was prescribed a 5-day course ofoseltamivir, 75 mg twice daily, and azithromycin, 250 mg/d. The ED again discharged him home.
On Day 4, Mr. G returned to the ED for a fourth time. His chief complaint was worsening shortness of breath. His oxygen saturation was 94% on room air; it improved to 96% on 2 L of oxygen. His chest X-ray showed diffuse reticulonodular opacities throughout his bilateral lung fields and increased airspace opacification in the bilateral lower lobes. The ED admitted Mr. G to an internal medicine unit, where the primary treatment team enrolled him in a clinical trial. As part of the trial, Mr. G received hydroxychloroquine, 400 mg, on Day 4 and Day 5. The placebo-controlled component of the trial involved Mr. G receiving daily infusions of either remdesivir or placebo on Day 6 through Day 8. On Day 8, Mr. G was discharged home.
On Day 9, Mr. G returned to the ED with a chief complaint that his “thermometer wasn’t working” at home. The ED readmitted him to the internal medicine unit. On Day 9 through Day 11, Mr. G received daily doses of
Continue to: During the second hospitalization...
During the second hospitalization, nursing staff reported that Mr. G seemed religiously preoccupied and once reported seeing angels and demons. He was observed sitting in a chair praying to Allah that he would “come in on a horse to chop all the workers’ heads off.”
On Day 11, Mr. G was discharged home. Later that evening, the EMS brought him back in the ED due to his girlfriend’s concerns about his mental state.
EVALUATION Talks to God
On Day 12, psychiatry is consulted to evaluate Mr. G’s new-onset psychosis. Mr. G is alert and oriented to person, place, and time. His speech is loud, though the amount and rate are unremarkable. He displays no psychomotor agitation. His thought process is tangential and focuses on religious themes, specifically referring to Islam. He reports auditory hallucinations of God speaking directly to him. Mr. G states, “I am here because of a miraculous transformation from death back to life. Do you believe in God? Which God do you believe in? There are 2 Gods and only one of them is the true God. He is the God of all the 7 heavens and His true name is Allah, only one God, one faith. Allah is a ball of energy.”
Mr. G’s girlfriend provides collateral information that Mr. G had been raised Christian but was not religious as an adult. She says that he had never spoken about being Muslim. She adds that she had never known him to speak much about religion.
[polldaddy:10572249]
The authors’ observations
The etiology of new-onset psychosis can be related to several factors, including primary psychiatric illnesses, use of illicit substances, sequelae of general medical conditions, or adverse effects of prescribed medications. We considered each of these in the differential diagnosis for Mr. G.
Continue to: Psychiatric illness or illicit substance use
Psychiatric illness or illicit substance use. Because Mr. G was 56 years old and had no known psychiatric history or family psychiatric history, a primary psychiatric illness seemed less likely. Substance-induced psychosis related to illicit substance use also seemed unlikely because he denied using illicit substances, and an expanded urine drug screen was negative.

Psychosis due to a general medical condition. Results from Mr. G’s laboratory workup show marked elevation in multiple inflammation-related biomarkers (Table 1), consistent with the inflammatory profile seen with COVID-19 infection. However, results from several laboratory tests for potential etiologies of new-onset psychosis due to a general medical condition were negative (Table 2). Based on Mr. G’s history of prostate cancer, we considered the possibility of metastatic space-occupying lesions of the brain; however, Mr. G’s head CT showed no acute intracranial abnormalities. Another possible etiology we considered was COVID-19–induced encephalitis; however, Mr. G’s brain MRI with and without contrast showed no evidence of acute or chronic intracranial changes.

Medication-induced psychosis. After largely ruling out primary psychiatric illnesses, illicit substances, and sequelae of general medical conditions, we turned our attention to prescribed medications as a potential etiology of Mr. G’s new-onset psychosis. During his initial hospitalization, Mr. G had been prescribed 2 doses of hydroxychloroquine, 400 mg, to treat his diagnosis of COVID-19. Because none of the other medications he received were reported to have neuropsychiatric adverse effects, including psychosis, hydroxychloroquine-induced psychosis was therefore the primary team’s working diagnosis.
EVALUATION Request to leave AMA
On Day 13, Mr. G requests to leave the hospital against medical advice (AMA). Until this point, he had voluntarily remained in the hospital, which he repeatedly referred to as “Heaven.” When asked to describe his medical condition, Mr. G replies, “God told me my condition is far beyond man’s understanding.” He denies that he is positive for COVID-19. He states, “I am cured, and the real fight has just begun.”
At the recommendation of the psychiatry consultation-liaison (C-L) service, the primary treatment team determines that Mr. G does not have capacity to leave AMA. The team is concerned that because of his psychotic symptoms, Mr. G would be unable to understand and follow his quarantine instructions. He remains hospitalized on a medical hold.
Continue to: The authors' observations
The authors’ observations
One important consideration this case highlighted was potential third-party responsibility clinicians and hospital systems may face if they discharge a patient with a communicable illness who is unable to follow precautions based on a psychiatric condition.1 That concern was based on Mr. G’s reported desire to pursue missions “beyond man’s understanding,” which he felt compelled to complete, and which could unnecessarily place the public at risk. The psychiatry C-L service consulted the local health department and conferred with the hospital’s legal representatives, who agreed with the plan to keep Mr. G in the hospital for his safety as well as for the public’s safety.
TREATMENT Oral haloperidol
The psychiatry C-L service recommends initiating an antipsychotic. On Day 13, Mr. G starts oral haloperidol, 2.5 mg twice a day, to address his ongoing psychotic symptoms. On Day 14, the treatment team increases the dosage to 5 mg twice a day. Mr. G tolerates the haloperidol and gradually begins to improve. He demonstrates improved sleep, normal speech volume, less religious preoccupation, and a considerably improved understanding of his medical condition.
The authors’ observations
Mr. G’s initial psychiatric evaluation demonstrated an acute onset of psychotic symptoms, without evidence of delirium. Psychosis secondary to a general medical condition (such as COVID-19) and hydroxychloroquine-induced psychotic disorder topped our initial considerations in the differential diagnosis of this case. While the exact neuropsychiatric sequelae of COVID-19 are not yet clear, previous experiences with viral pandemics and case studies from the current pandemic demonstrate a wide variety of possible neuropsychiatric manifestations. Mood symptoms, psychosis, and encephalopathy represent some of the neuropsychiatric complications observed with past viral pandemics.2 Neuropsychiatric symptoms may be triggered by the virus itself, or from the host’s immune response to the infection.3 To further complicate matters, neuropsychiatric symptoms may manifest during the acute viral infection, or may surface later, as subacute or chronic neuropsychiatric illness.
Neuropsychiatric adverse events
Mr. G developed psychotic symptoms within the first few days of receiving hydroxychloroquine, which is consistent with the scant literature on this topic.8 Based on the available information, hydroxychloroquine remains the most likely etiology of his new-onset psychotic symptoms. Mr. G’s case is one example of the possible neuropsychiatric presentations clinicians may face while treating a novel viral illness.
Continue to: OUTCOME Homeward-bound
OUTCOME Homeward-bound
By Day 18, Mr. G’s psychotic symptoms have significantly improved. He is able to rationally process information about his COVID-19 diagnosis and the recommended quarantine instructions he needs to follow after discharge. He is cleared by infection control and discharged home to return to living with his girlfriend.
Mr. G attends his follow-up psychiatric appointment remotely 2 weeks after discharge. He reports that since discharge, he has continued taking his prescribed haloperidol, 5 mg twice a day. He demonstrates improved insight into his medical condition, acknowledging his COVID-19–positive status, and confirms that he has been following quarantine instructions. He does not report ongoing auditory or visual hallucinations, and is no longer religiously preoccupied. He says he is looking forward to being medically cleared to return to work.
The authors’ observations
This case highlights the need for prospective, longitudinal screening and monitoring of neuropsychiatric symptoms as part of the public health response to COVID-19. The case also highlights the importance of careful monitoring for adverse events, including neuropsychiatric symptoms, during clinical trials that involve experimental treatments. The long-term prognosis for individuals such as Mr. G who develop neuropsychiatric symptoms during acute COVID-19 infection remains unknown. Similarly, subacute and chronic neuropsychiatric manifestations that may develop after resolution of acute COVID-19 infection are unknown at this time. However, we can learn from past viral pandemics and anticipate that neuropsychiatric sequelae are likely to occur and should be part of the public health response to the pandemic.
Bottom Line
The coronavirus disease 2019 pandemic provides multiple clinical challenges pertinent to psychiatry. Neuropsychiatric symptoms may manifest from delirium, viral infection, host immune response, or adverse reactions to experimental treatments. These potential neuropsychiatric symptoms may complicate medical treatment. They can also raise important ethical and legal considerations, such as weighing patient autonomy vs third-party responsibility to the public at large.
Related Resources
- Ferrando SJ, Klepacz L, Lynch S, et al. COVID-19 psychosis: a potential new neuropsychiatric condition triggered by novel coronavirus infection and the inflammatory response? [published online May 19, 2020]. Psychosomatics. 2020. doi: 10.1016/j.psym.2020.05.012.
- Vlessides M. COVID-19 and psychosis: is there a link? Medscape Medical News. https://www.medscape.com/viewarticle/930224. Published May 8, 2020.
Drug Brand Names
Azithromycin • Zithromax
Ceftriaxone • Rocephin
Chloroquine • Aralen
Haloperidol • Haldol
Hydroxychloroquine • Plaquenil
Levofloxacin • Levaquin
Oseltamivir • Tamiflu
1. Ghossoub E, Newman WJ. COVID-19 and the duty to protect from communicable diseases. [published online ahead of print, May 8, 2020]. J Am Acad Psychiatry Law.
2. Menninger Ka. Psychoses associated with influenza: I. general data: statistical analysis. JAMA. 1919;72(4):235-241.
3. Troyer EA, Kohn JN, Hong S. Are we facing a crashing wave of neuropsychiatric sequelae of COVID-19? Neuropsychiatric symptoms and potential immunologic mechanisms. Brain, Behavior, and Immunity. 2020. doi:10.1016/j.bbi.2020.04.027.
4. Alkadi HO. Antimalarial drug toxicity: a review. Chemotherapy. 2007;53(6):385-391.
5. Bogaczewicz A, Sobów T. Psychiatric adverse effects of chloroquine. Psychiatria i Psychologia Kliniczna. 2017;17(2):111-114.
6. Sato K, Mano T, Iwata A, et al. Neuropsychiatric adverse events of chloroquine: a real-world pharmacovigilance study using the FDA Adverse Event Reporting System (FAERS) database. Biosci Trends. 2020;14(2):139-143.
7. Cortegiani A, Ingoglia G, Ippolito M, et al. A systematic review on the efficacy and safety of chloroquine for the treatment of COVID-19. J Crit Care. 2020;57:279-283.
8. Das P, Rai A, Chopra A, et al. Psychosis likely induced by hydroxychloroquine in a patient with chronic Q fever: a case report and clinically relevant review of pharmacology. Psychosomatics. 2014;55(4):409-413.
1. Ghossoub E, Newman WJ. COVID-19 and the duty to protect from communicable diseases. [published online ahead of print, May 8, 2020]. J Am Acad Psychiatry Law.
2. Menninger Ka. Psychoses associated with influenza: I. general data: statistical analysis. JAMA. 1919;72(4):235-241.
3. Troyer EA, Kohn JN, Hong S. Are we facing a crashing wave of neuropsychiatric sequelae of COVID-19? Neuropsychiatric symptoms and potential immunologic mechanisms. Brain, Behavior, and Immunity. 2020. doi:10.1016/j.bbi.2020.04.027.
4. Alkadi HO. Antimalarial drug toxicity: a review. Chemotherapy. 2007;53(6):385-391.
5. Bogaczewicz A, Sobów T. Psychiatric adverse effects of chloroquine. Psychiatria i Psychologia Kliniczna. 2017;17(2):111-114.
6. Sato K, Mano T, Iwata A, et al. Neuropsychiatric adverse events of chloroquine: a real-world pharmacovigilance study using the FDA Adverse Event Reporting System (FAERS) database. Biosci Trends. 2020;14(2):139-143.
7. Cortegiani A, Ingoglia G, Ippolito M, et al. A systematic review on the efficacy and safety of chloroquine for the treatment of COVID-19. J Crit Care. 2020;57:279-283.
8. Das P, Rai A, Chopra A, et al. Psychosis likely induced by hydroxychloroquine in a patient with chronic Q fever: a case report and clinically relevant review of pharmacology. Psychosomatics. 2014;55(4):409-413.
An unexplained exacerbation of depression, anxiety, and panic
CASE Depression, anxiety, and panic attacks
At the urging of his parents Mr. P, age 33, presents to the partial hospitalization program (PHP) for worsening depression and anxiety, daily panic attacks with accompanying diaphoresis and headache, and the possibility that he may have taken an overdose of zolpidem. Mr. P denies taking an intentional overdose of zolpidem, claiming instead that he was having a sleep-walking episode and did not realize how many pills he took.
In addition to daily panic attacks, Mr. P reports having trouble falling asleep, overwhelming sadness, and daily passive suicidal ideation without a plan or active intent.
Mr. P cannot identify a specific trigger to this most recent exacerbation of depressed/anxious mood, but instead describes it as slowly building over the past 6 to 8 months. Mr. P says the panic attacks occur without warning and states, “I feel like my heart is going to jump out of my chest; I get a terrible headache, and I sweat like crazy. Sometimes I just feel like I’m about to pass out or die.” Although these episodes had been present for approximately 2 years, they now occur almost daily.
HISTORY Inconsistent adherence
For the last year, Mr. P had been taking alprazolam, 0.5 mg twice daily, and paroxetine, 20 mg/d, and these medications provided moderate relief of his depressive/anxious symptoms. However, he stopped taking both medications approximately 3 or 4 weeks ago when he ran out. He also takes propranolol, 20 mg/d, sporadically, for hypertension. In the past, he had been prescribed carvedilol, clonidine, and lisinopril—all with varying degrees of relief of his hypertension. He denies a family history of hypertension or any other chronic or acute health problems. He reports that he has been sober from alcohol for 19 months but smokes 1 to 2 marijuana cigarettes a day.
EVALUATION Elevated blood pressure and pulse
Mr. P’s physical examination and medical review of systems are unremarkable, except for an elevated blood pressure (190/110 mm Hg) and pulse (92 beats per minute); he also has a headache. A repeat blood pressure test later in the day is 172/94 mm Hg, with a pulse of 100 beats per minute. His urine drug screen is positive only for delta-9-tetrahydrocannabinol (THC).
[polldaddy:10558304]
The author’s observations
A CBC with differential is helpful for ruling out infection and anemia as causes of anxiety and depression.1 In Mr. P’s case, there were no concerning symptoms that pointed to anemia or infection as likely causes of his anxiety, depression, or panic attacks. A TSH level also would be reasonable, because hyperthyroidism can present as anxiety, while hyperthyroidism or hypothyroidism each can present as depression.1 However, both Mr. P’s medical history and physical examination were not concerning for thyroid disease, making it unlikely that he had either of those conditions. A review of Mr. P’s medical records indicated that within the past 6 months, his primary care physician (PCP) had ordered a CBC and TSH test; the results of both were within normal limits.
Serum porphyrin tests can exclude porphyria as a contributor to Mr. P’s anxiety and depression. Porphyrias are a group of 8 inherited disorders that involve accumulation of heme precursors (porphyrins) in the CNS and subcutaneous tissue.2 Collectively, porphyrias affect approximately 1 in 200,000 people.2 Anxiety and depression are strongly associated with porphyria, but do not occur secondary to the illness; depression and anxiety appear to be intrinsic personality features in people with porphyria.3 Skin lesions and abdominal pain are the most common symptoms,3 and there is a higher incidence of hypertension in people with porphyria than in the general population.4 Mr. P does not report any heritable disorders, nor does he appear to have any CNS disturbance or unusual cutaneous lesions, which makes it unlikely that this disorder is related to his psychiatric symptoms.
Continue to: A serum metanephrines test measures...
A serum metanephrines test measures the metabolites of epinephrine and norepinephrine. These catecholamines are produced in excess by an adrenal or extra-adrenal tumor seen in pheochromocytoma. The classic triad of symptoms of pheochromocytoma are hypertension, sweating, and headache; approximately 30% of patients report significant anxiety and panic (Table 15-7). This type of tumor is rare, with an annual incidence of only 2 to 8 cases per 1 million individuals. Among people with hypertension, the annual incidence is 0.1% to 1.0%, and for those with an adrenal mass, the annual incidence is 5% (Table 26,8). Autopsy studies suggest that up to 50% of pheochromocytomas are undiagnosed.8 Left untreated, pheochromocytoma can result in hypertensive crisis, arrhythmia, myocardial infarction, multisystem organ failure, and premature death.7 Table 36,7 highlights some causes of false-positive serum on metanephrines testing.
EVALUATION Metanephrines testing
Mr. P has what appears to be treatment-resistant hypertension, accompanied by the classic symptoms observed in most patients with pheochromocytoma. Because Mr. P is participating in the PHP 6 days per week for 6 hours each day, visiting his PCP would be inconvenient, so the treatment team orders the serum metanephrines test. If a positive result is found, Mr. P will be referred to his PCP for further assessment and follow-up care with endocrinology.
TREATMENT Pharmacotherapy to target anxiety and panic
Next, the treatment team establishes a safety plan for Mr. P, and restarts paroxetine, 20 mg/d, to target his depressed and anxious mood. Alprazolam, 0.5 mg twice daily, is started to target anxious mood and panic symptoms, and to allow time for the anxiolytic properties of the paroxetine to become fully effective. The alprazolam will be tapered and stopped after 2 weeks. Mr. P is started on hydroxyzine, 1 to 2 25-mg tablets 2 to 3 times daily as needed for anxious mood and panic symptoms.
The serum metanephrines test results are equivocal, with a slight elevation of both epinephrine and norepinephrine that is too low to confirm a diagnosis of pheochromocytoma but too elevated to exclude it (Table 49). With Mr. P’s consent, the treatment team contacts his PCP and convey the results of this test. Mr. P schedules an appointment with his PCP for the following week for further assessment and confirmatory pheochromocytoma testing.
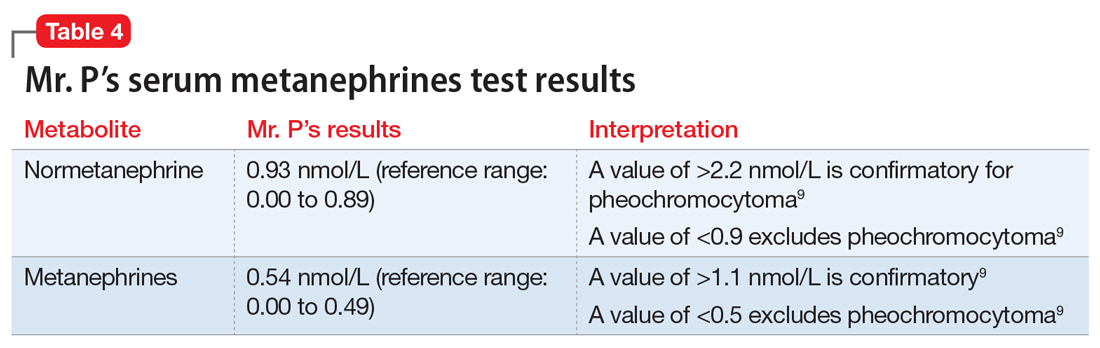
After 1 week, Mr. P remains anxious, with a slight reduction in panic attacks from multiple attacks each day to 3 or 4 attacks per week. The team considers adding an additional anxiolytic agent.
[polldaddy:10558305]
Continue to: The author's observations
The author’s observations
The triad of symptoms in pheochromocytoma results directly from the intermittent release of catecholamines into systemic circulation. Surges of epinephrine and norepinephrine lead to headaches, palpitations, diaphoresis, and (less commonly) gastrointestinal symptoms such as nausea, vomiting, and constipation. Persistent or episodic hypertension may be present, with 13% of patients maintaining a normal blood pressure.5-7 Patients with pheochromocytoma-related anxiety typically have substantial or complete resolution of anxiety and panic attacks after tumor resection.6,8,10
Because of their ability to raise catecholamine levels, several medications, including some psychotropics, can lead to false-positive results on serum and urine metanephrines testing. Tricyclic antidepressants and beta-blockers can cause false-positive results on plasma assays, while buspirone can cause false-positives on urinalysis assays.5 Trazodone, on the other hand, exhibits no catecholaminergic activity and its alpha-1 adrenergic antagonism may actually have some benefit in pheochromocytoma.11 Alpha-1 adrenergic antagonism with doxazosin, prazosin, or terazosin is the first-line of treatment in reducing pheochromocytoma-related hypertension.7 Treatment with a beta-blocker is safe only after alpha-adrenergic blockade occurs. While beta-blockers are useful for reducing the palpitations and anxiety observed in patients with pheochromocytoma, they must not be used alone due to the risk of hypertensive crisis resulting from unopposed alpha-adrenergic agonist activated vasoconstriction.5,7
TREATMENT CBT provides benefit
Mr. P decides against receiving an additional agent for anxiety and instead decides to wait for the outcome of the confirmatory pheochromocytoma testing. He continues to take alprazolam, and both his depressed mood and anxiety improve. His panic attacks continue to lessen, and he appears to benefit from cognitive-behavioral therapy provided during group therapy. Mr. P is advised by his PCP to taper and stop the alprazolam 3 to 5 days before his 24-hour urine metanephrines test because benzodiazepines can lead to false-positive results on a urinalysis assay.7
OUTCOME Remission of anxiety and depression
Mr. P has a repeat serum metanephrines test and a 24-hour urinalysis assay. Both are negative for pheochromocytoma. His PCP refers him to cardiology for management of treatment-resistant hypertension. He is discharged from the PHP and continues psychotherapy for depression and anxiety in an intensive outpatient program (IOP). Throughout his PHP and IOP treatments, he continues to take paroxetine and hydroxyzine. He achieves a successful remission of his anxiety and depression, with partial but significant remission of his panic attacks.
The author’s observations
Although Mr. P did not have pheochromocytoma, it is important to rule out this rare condition in patients who present with treatment-resistant hypertension and/or treatment-resistant anxiety.
Continue to: Bottom Line
Bottom Line
Pheochromocytoma is a tumor of the adrenal gland. The classic triad of symptoms of this rare condition is hypertension, sweating, and headache; approximately 30% of patients report significant anxiety and panic. Several medications, including tricyclic antidepressants, beta-blockers, and buspirone, can lead to false-positive results on the serum and urine metanephrines testing used to diagnose pheochromocytoma.
Related Resources
- National Organization for Rare Disorders. Rare Disease Database: pheochromocytoma. www.rarediseases.org/rare-diseases/pheochromocytoma/.
- Young WF Jr. Clinical presentation and diagnosis of pheochromocytoma. UpToDate. www.uptodate.com/contents/clinical-presentation-and-diagnosis-of-pheochromocytoma. Published January 2020.
Drug Brand Names
Alprazolam • Xanax
Amitriptyline • Elavil
Buspirone • Buspar
Carvedilol • Coreg
Clonidine • Catapres
Doxazosin • Cardura
Hydroxyzine • Vistaril
Lisinopril • Prinivil, Zestril
Paroxetine • Paxil
Prazosin • Minipress
Propranolol • Inderal
Terazosin • Hytrin
Trazodone • Desyrel
Zolpidem • Ambien
1. Morrison J. When psychological problems mask medical disorders: a guide for psychotherapists. 2nd ed. New York, NY: The Guilford Press; 2015.
2. American Porphyria Foundation. About porphyria. https://porphyriafoundation.org/patients/about-porphyria. Accessed May 13, 2020.
3. Millward L, Kelly P, King A, et al. Anxiety and depression in the acute porphyrias. J Inherit Metab Dis. 2005;28(6):1099-1107.
4. Bonkovsky H, Maddukuri VC, Yazici C, et al. Acute porphyrias in the USA: features of 108 subjects from porphyria consortium. Am J Med. 2014;127(12):1233-1241.
5. Tsirlin A, Oo Y, Sharma R, et al. Pheochromocytoma: a review. Maturitas. 2014;77(3):229-238.
6. Leung A, Zun L, Nordstrom K, et al. Psychiatric emergencies for physicians: clinical management and approach to distinguishing pheochromocytoma from psychiatric and thyrotoxic diseases in the emergency room. J Emerg Med. 2017;53(5):712-716.
7. Garg M, Kharb S, Brar KS, et al. Medical management of pheochromocytoma: role of the endocrinologist. Indian J Endocrinol and Metab. 2011;15(suppl 4):S329-S336. doi: 10.4103/2230-8210.86976.
8. Zardawi I. Phaeochromocytoma masquerading as anxiety and depression. Am J Case Rep. 2013;14:161-163.
9. ARUP Laboratories. Test directory. https://www.aruplab.com. Accessed February 11, 2020.
10. Sriram P, Raghavan V. Pheochromocytoma presenting as anxiety disorder: a case report. Asian J Psychiatr. 2017;29:83-84.
11. Stahl SM. Stahl’s essential psychopharmacology: neuroscientific basis and practical applications. Cambridge, UK: Cambridge University Press; 2013.
CASE Depression, anxiety, and panic attacks
At the urging of his parents Mr. P, age 33, presents to the partial hospitalization program (PHP) for worsening depression and anxiety, daily panic attacks with accompanying diaphoresis and headache, and the possibility that he may have taken an overdose of zolpidem. Mr. P denies taking an intentional overdose of zolpidem, claiming instead that he was having a sleep-walking episode and did not realize how many pills he took.
In addition to daily panic attacks, Mr. P reports having trouble falling asleep, overwhelming sadness, and daily passive suicidal ideation without a plan or active intent.
Mr. P cannot identify a specific trigger to this most recent exacerbation of depressed/anxious mood, but instead describes it as slowly building over the past 6 to 8 months. Mr. P says the panic attacks occur without warning and states, “I feel like my heart is going to jump out of my chest; I get a terrible headache, and I sweat like crazy. Sometimes I just feel like I’m about to pass out or die.” Although these episodes had been present for approximately 2 years, they now occur almost daily.
HISTORY Inconsistent adherence
For the last year, Mr. P had been taking alprazolam, 0.5 mg twice daily, and paroxetine, 20 mg/d, and these medications provided moderate relief of his depressive/anxious symptoms. However, he stopped taking both medications approximately 3 or 4 weeks ago when he ran out. He also takes propranolol, 20 mg/d, sporadically, for hypertension. In the past, he had been prescribed carvedilol, clonidine, and lisinopril—all with varying degrees of relief of his hypertension. He denies a family history of hypertension or any other chronic or acute health problems. He reports that he has been sober from alcohol for 19 months but smokes 1 to 2 marijuana cigarettes a day.
EVALUATION Elevated blood pressure and pulse
Mr. P’s physical examination and medical review of systems are unremarkable, except for an elevated blood pressure (190/110 mm Hg) and pulse (92 beats per minute); he also has a headache. A repeat blood pressure test later in the day is 172/94 mm Hg, with a pulse of 100 beats per minute. His urine drug screen is positive only for delta-9-tetrahydrocannabinol (THC).
[polldaddy:10558304]
The author’s observations
A CBC with differential is helpful for ruling out infection and anemia as causes of anxiety and depression.1 In Mr. P’s case, there were no concerning symptoms that pointed to anemia or infection as likely causes of his anxiety, depression, or panic attacks. A TSH level also would be reasonable, because hyperthyroidism can present as anxiety, while hyperthyroidism or hypothyroidism each can present as depression.1 However, both Mr. P’s medical history and physical examination were not concerning for thyroid disease, making it unlikely that he had either of those conditions. A review of Mr. P’s medical records indicated that within the past 6 months, his primary care physician (PCP) had ordered a CBC and TSH test; the results of both were within normal limits.
Serum porphyrin tests can exclude porphyria as a contributor to Mr. P’s anxiety and depression. Porphyrias are a group of 8 inherited disorders that involve accumulation of heme precursors (porphyrins) in the CNS and subcutaneous tissue.2 Collectively, porphyrias affect approximately 1 in 200,000 people.2 Anxiety and depression are strongly associated with porphyria, but do not occur secondary to the illness; depression and anxiety appear to be intrinsic personality features in people with porphyria.3 Skin lesions and abdominal pain are the most common symptoms,3 and there is a higher incidence of hypertension in people with porphyria than in the general population.4 Mr. P does not report any heritable disorders, nor does he appear to have any CNS disturbance or unusual cutaneous lesions, which makes it unlikely that this disorder is related to his psychiatric symptoms.
Continue to: A serum metanephrines test measures...
A serum metanephrines test measures the metabolites of epinephrine and norepinephrine. These catecholamines are produced in excess by an adrenal or extra-adrenal tumor seen in pheochromocytoma. The classic triad of symptoms of pheochromocytoma are hypertension, sweating, and headache; approximately 30% of patients report significant anxiety and panic (Table 15-7). This type of tumor is rare, with an annual incidence of only 2 to 8 cases per 1 million individuals. Among people with hypertension, the annual incidence is 0.1% to 1.0%, and for those with an adrenal mass, the annual incidence is 5% (Table 26,8). Autopsy studies suggest that up to 50% of pheochromocytomas are undiagnosed.8 Left untreated, pheochromocytoma can result in hypertensive crisis, arrhythmia, myocardial infarction, multisystem organ failure, and premature death.7 Table 36,7 highlights some causes of false-positive serum on metanephrines testing.
EVALUATION Metanephrines testing
Mr. P has what appears to be treatment-resistant hypertension, accompanied by the classic symptoms observed in most patients with pheochromocytoma. Because Mr. P is participating in the PHP 6 days per week for 6 hours each day, visiting his PCP would be inconvenient, so the treatment team orders the serum metanephrines test. If a positive result is found, Mr. P will be referred to his PCP for further assessment and follow-up care with endocrinology.
TREATMENT Pharmacotherapy to target anxiety and panic
Next, the treatment team establishes a safety plan for Mr. P, and restarts paroxetine, 20 mg/d, to target his depressed and anxious mood. Alprazolam, 0.5 mg twice daily, is started to target anxious mood and panic symptoms, and to allow time for the anxiolytic properties of the paroxetine to become fully effective. The alprazolam will be tapered and stopped after 2 weeks. Mr. P is started on hydroxyzine, 1 to 2 25-mg tablets 2 to 3 times daily as needed for anxious mood and panic symptoms.
The serum metanephrines test results are equivocal, with a slight elevation of both epinephrine and norepinephrine that is too low to confirm a diagnosis of pheochromocytoma but too elevated to exclude it (Table 49). With Mr. P’s consent, the treatment team contacts his PCP and convey the results of this test. Mr. P schedules an appointment with his PCP for the following week for further assessment and confirmatory pheochromocytoma testing.

After 1 week, Mr. P remains anxious, with a slight reduction in panic attacks from multiple attacks each day to 3 or 4 attacks per week. The team considers adding an additional anxiolytic agent.
[polldaddy:10558305]
Continue to: The author's observations
The author’s observations
The triad of symptoms in pheochromocytoma results directly from the intermittent release of catecholamines into systemic circulation. Surges of epinephrine and norepinephrine lead to headaches, palpitations, diaphoresis, and (less commonly) gastrointestinal symptoms such as nausea, vomiting, and constipation. Persistent or episodic hypertension may be present, with 13% of patients maintaining a normal blood pressure.5-7 Patients with pheochromocytoma-related anxiety typically have substantial or complete resolution of anxiety and panic attacks after tumor resection.6,8,10
Because of their ability to raise catecholamine levels, several medications, including some psychotropics, can lead to false-positive results on serum and urine metanephrines testing. Tricyclic antidepressants and beta-blockers can cause false-positive results on plasma assays, while buspirone can cause false-positives on urinalysis assays.5 Trazodone, on the other hand, exhibits no catecholaminergic activity and its alpha-1 adrenergic antagonism may actually have some benefit in pheochromocytoma.11 Alpha-1 adrenergic antagonism with doxazosin, prazosin, or terazosin is the first-line of treatment in reducing pheochromocytoma-related hypertension.7 Treatment with a beta-blocker is safe only after alpha-adrenergic blockade occurs. While beta-blockers are useful for reducing the palpitations and anxiety observed in patients with pheochromocytoma, they must not be used alone due to the risk of hypertensive crisis resulting from unopposed alpha-adrenergic agonist activated vasoconstriction.5,7
TREATMENT CBT provides benefit
Mr. P decides against receiving an additional agent for anxiety and instead decides to wait for the outcome of the confirmatory pheochromocytoma testing. He continues to take alprazolam, and both his depressed mood and anxiety improve. His panic attacks continue to lessen, and he appears to benefit from cognitive-behavioral therapy provided during group therapy. Mr. P is advised by his PCP to taper and stop the alprazolam 3 to 5 days before his 24-hour urine metanephrines test because benzodiazepines can lead to false-positive results on a urinalysis assay.7
OUTCOME Remission of anxiety and depression
Mr. P has a repeat serum metanephrines test and a 24-hour urinalysis assay. Both are negative for pheochromocytoma. His PCP refers him to cardiology for management of treatment-resistant hypertension. He is discharged from the PHP and continues psychotherapy for depression and anxiety in an intensive outpatient program (IOP). Throughout his PHP and IOP treatments, he continues to take paroxetine and hydroxyzine. He achieves a successful remission of his anxiety and depression, with partial but significant remission of his panic attacks.
The author’s observations
Although Mr. P did not have pheochromocytoma, it is important to rule out this rare condition in patients who present with treatment-resistant hypertension and/or treatment-resistant anxiety.
Continue to: Bottom Line
Bottom Line
Pheochromocytoma is a tumor of the adrenal gland. The classic triad of symptoms of this rare condition is hypertension, sweating, and headache; approximately 30% of patients report significant anxiety and panic. Several medications, including tricyclic antidepressants, beta-blockers, and buspirone, can lead to false-positive results on the serum and urine metanephrines testing used to diagnose pheochromocytoma.
Related Resources
- National Organization for Rare Disorders. Rare Disease Database: pheochromocytoma. www.rarediseases.org/rare-diseases/pheochromocytoma/.
- Young WF Jr. Clinical presentation and diagnosis of pheochromocytoma. UpToDate. www.uptodate.com/contents/clinical-presentation-and-diagnosis-of-pheochromocytoma. Published January 2020.
Drug Brand Names
Alprazolam • Xanax
Amitriptyline • Elavil
Buspirone • Buspar
Carvedilol • Coreg
Clonidine • Catapres
Doxazosin • Cardura
Hydroxyzine • Vistaril
Lisinopril • Prinivil, Zestril
Paroxetine • Paxil
Prazosin • Minipress
Propranolol • Inderal
Terazosin • Hytrin
Trazodone • Desyrel
Zolpidem • Ambien
CASE Depression, anxiety, and panic attacks
At the urging of his parents Mr. P, age 33, presents to the partial hospitalization program (PHP) for worsening depression and anxiety, daily panic attacks with accompanying diaphoresis and headache, and the possibility that he may have taken an overdose of zolpidem. Mr. P denies taking an intentional overdose of zolpidem, claiming instead that he was having a sleep-walking episode and did not realize how many pills he took.
In addition to daily panic attacks, Mr. P reports having trouble falling asleep, overwhelming sadness, and daily passive suicidal ideation without a plan or active intent.
Mr. P cannot identify a specific trigger to this most recent exacerbation of depressed/anxious mood, but instead describes it as slowly building over the past 6 to 8 months. Mr. P says the panic attacks occur without warning and states, “I feel like my heart is going to jump out of my chest; I get a terrible headache, and I sweat like crazy. Sometimes I just feel like I’m about to pass out or die.” Although these episodes had been present for approximately 2 years, they now occur almost daily.
HISTORY Inconsistent adherence
For the last year, Mr. P had been taking alprazolam, 0.5 mg twice daily, and paroxetine, 20 mg/d, and these medications provided moderate relief of his depressive/anxious symptoms. However, he stopped taking both medications approximately 3 or 4 weeks ago when he ran out. He also takes propranolol, 20 mg/d, sporadically, for hypertension. In the past, he had been prescribed carvedilol, clonidine, and lisinopril—all with varying degrees of relief of his hypertension. He denies a family history of hypertension or any other chronic or acute health problems. He reports that he has been sober from alcohol for 19 months but smokes 1 to 2 marijuana cigarettes a day.
EVALUATION Elevated blood pressure and pulse
Mr. P’s physical examination and medical review of systems are unremarkable, except for an elevated blood pressure (190/110 mm Hg) and pulse (92 beats per minute); he also has a headache. A repeat blood pressure test later in the day is 172/94 mm Hg, with a pulse of 100 beats per minute. His urine drug screen is positive only for delta-9-tetrahydrocannabinol (THC).
[polldaddy:10558304]
The author’s observations
A CBC with differential is helpful for ruling out infection and anemia as causes of anxiety and depression.1 In Mr. P’s case, there were no concerning symptoms that pointed to anemia or infection as likely causes of his anxiety, depression, or panic attacks. A TSH level also would be reasonable, because hyperthyroidism can present as anxiety, while hyperthyroidism or hypothyroidism each can present as depression.1 However, both Mr. P’s medical history and physical examination were not concerning for thyroid disease, making it unlikely that he had either of those conditions. A review of Mr. P’s medical records indicated that within the past 6 months, his primary care physician (PCP) had ordered a CBC and TSH test; the results of both were within normal limits.
Serum porphyrin tests can exclude porphyria as a contributor to Mr. P’s anxiety and depression. Porphyrias are a group of 8 inherited disorders that involve accumulation of heme precursors (porphyrins) in the CNS and subcutaneous tissue.2 Collectively, porphyrias affect approximately 1 in 200,000 people.2 Anxiety and depression are strongly associated with porphyria, but do not occur secondary to the illness; depression and anxiety appear to be intrinsic personality features in people with porphyria.3 Skin lesions and abdominal pain are the most common symptoms,3 and there is a higher incidence of hypertension in people with porphyria than in the general population.4 Mr. P does not report any heritable disorders, nor does he appear to have any CNS disturbance or unusual cutaneous lesions, which makes it unlikely that this disorder is related to his psychiatric symptoms.
Continue to: A serum metanephrines test measures...
A serum metanephrines test measures the metabolites of epinephrine and norepinephrine. These catecholamines are produced in excess by an adrenal or extra-adrenal tumor seen in pheochromocytoma. The classic triad of symptoms of pheochromocytoma are hypertension, sweating, and headache; approximately 30% of patients report significant anxiety and panic (Table 15-7). This type of tumor is rare, with an annual incidence of only 2 to 8 cases per 1 million individuals. Among people with hypertension, the annual incidence is 0.1% to 1.0%, and for those with an adrenal mass, the annual incidence is 5% (Table 26,8). Autopsy studies suggest that up to 50% of pheochromocytomas are undiagnosed.8 Left untreated, pheochromocytoma can result in hypertensive crisis, arrhythmia, myocardial infarction, multisystem organ failure, and premature death.7 Table 36,7 highlights some causes of false-positive serum on metanephrines testing.
EVALUATION Metanephrines testing
Mr. P has what appears to be treatment-resistant hypertension, accompanied by the classic symptoms observed in most patients with pheochromocytoma. Because Mr. P is participating in the PHP 6 days per week for 6 hours each day, visiting his PCP would be inconvenient, so the treatment team orders the serum metanephrines test. If a positive result is found, Mr. P will be referred to his PCP for further assessment and follow-up care with endocrinology.
TREATMENT Pharmacotherapy to target anxiety and panic
Next, the treatment team establishes a safety plan for Mr. P, and restarts paroxetine, 20 mg/d, to target his depressed and anxious mood. Alprazolam, 0.5 mg twice daily, is started to target anxious mood and panic symptoms, and to allow time for the anxiolytic properties of the paroxetine to become fully effective. The alprazolam will be tapered and stopped after 2 weeks. Mr. P is started on hydroxyzine, 1 to 2 25-mg tablets 2 to 3 times daily as needed for anxious mood and panic symptoms.
The serum metanephrines test results are equivocal, with a slight elevation of both epinephrine and norepinephrine that is too low to confirm a diagnosis of pheochromocytoma but too elevated to exclude it (Table 49). With Mr. P’s consent, the treatment team contacts his PCP and convey the results of this test. Mr. P schedules an appointment with his PCP for the following week for further assessment and confirmatory pheochromocytoma testing.

After 1 week, Mr. P remains anxious, with a slight reduction in panic attacks from multiple attacks each day to 3 or 4 attacks per week. The team considers adding an additional anxiolytic agent.
[polldaddy:10558305]
Continue to: The author's observations
The author’s observations
The triad of symptoms in pheochromocytoma results directly from the intermittent release of catecholamines into systemic circulation. Surges of epinephrine and norepinephrine lead to headaches, palpitations, diaphoresis, and (less commonly) gastrointestinal symptoms such as nausea, vomiting, and constipation. Persistent or episodic hypertension may be present, with 13% of patients maintaining a normal blood pressure.5-7 Patients with pheochromocytoma-related anxiety typically have substantial or complete resolution of anxiety and panic attacks after tumor resection.6,8,10
Because of their ability to raise catecholamine levels, several medications, including some psychotropics, can lead to false-positive results on serum and urine metanephrines testing. Tricyclic antidepressants and beta-blockers can cause false-positive results on plasma assays, while buspirone can cause false-positives on urinalysis assays.5 Trazodone, on the other hand, exhibits no catecholaminergic activity and its alpha-1 adrenergic antagonism may actually have some benefit in pheochromocytoma.11 Alpha-1 adrenergic antagonism with doxazosin, prazosin, or terazosin is the first-line of treatment in reducing pheochromocytoma-related hypertension.7 Treatment with a beta-blocker is safe only after alpha-adrenergic blockade occurs. While beta-blockers are useful for reducing the palpitations and anxiety observed in patients with pheochromocytoma, they must not be used alone due to the risk of hypertensive crisis resulting from unopposed alpha-adrenergic agonist activated vasoconstriction.5,7
TREATMENT CBT provides benefit
Mr. P decides against receiving an additional agent for anxiety and instead decides to wait for the outcome of the confirmatory pheochromocytoma testing. He continues to take alprazolam, and both his depressed mood and anxiety improve. His panic attacks continue to lessen, and he appears to benefit from cognitive-behavioral therapy provided during group therapy. Mr. P is advised by his PCP to taper and stop the alprazolam 3 to 5 days before his 24-hour urine metanephrines test because benzodiazepines can lead to false-positive results on a urinalysis assay.7
OUTCOME Remission of anxiety and depression
Mr. P has a repeat serum metanephrines test and a 24-hour urinalysis assay. Both are negative for pheochromocytoma. His PCP refers him to cardiology for management of treatment-resistant hypertension. He is discharged from the PHP and continues psychotherapy for depression and anxiety in an intensive outpatient program (IOP). Throughout his PHP and IOP treatments, he continues to take paroxetine and hydroxyzine. He achieves a successful remission of his anxiety and depression, with partial but significant remission of his panic attacks.
The author’s observations
Although Mr. P did not have pheochromocytoma, it is important to rule out this rare condition in patients who present with treatment-resistant hypertension and/or treatment-resistant anxiety.
Continue to: Bottom Line
Bottom Line
Pheochromocytoma is a tumor of the adrenal gland. The classic triad of symptoms of this rare condition is hypertension, sweating, and headache; approximately 30% of patients report significant anxiety and panic. Several medications, including tricyclic antidepressants, beta-blockers, and buspirone, can lead to false-positive results on the serum and urine metanephrines testing used to diagnose pheochromocytoma.
Related Resources
- National Organization for Rare Disorders. Rare Disease Database: pheochromocytoma. www.rarediseases.org/rare-diseases/pheochromocytoma/.
- Young WF Jr. Clinical presentation and diagnosis of pheochromocytoma. UpToDate. www.uptodate.com/contents/clinical-presentation-and-diagnosis-of-pheochromocytoma. Published January 2020.
Drug Brand Names
Alprazolam • Xanax
Amitriptyline • Elavil
Buspirone • Buspar
Carvedilol • Coreg
Clonidine • Catapres
Doxazosin • Cardura
Hydroxyzine • Vistaril
Lisinopril • Prinivil, Zestril
Paroxetine • Paxil
Prazosin • Minipress
Propranolol • Inderal
Terazosin • Hytrin
Trazodone • Desyrel
Zolpidem • Ambien
1. Morrison J. When psychological problems mask medical disorders: a guide for psychotherapists. 2nd ed. New York, NY: The Guilford Press; 2015.
2. American Porphyria Foundation. About porphyria. https://porphyriafoundation.org/patients/about-porphyria. Accessed May 13, 2020.
3. Millward L, Kelly P, King A, et al. Anxiety and depression in the acute porphyrias. J Inherit Metab Dis. 2005;28(6):1099-1107.
4. Bonkovsky H, Maddukuri VC, Yazici C, et al. Acute porphyrias in the USA: features of 108 subjects from porphyria consortium. Am J Med. 2014;127(12):1233-1241.
5. Tsirlin A, Oo Y, Sharma R, et al. Pheochromocytoma: a review. Maturitas. 2014;77(3):229-238.
6. Leung A, Zun L, Nordstrom K, et al. Psychiatric emergencies for physicians: clinical management and approach to distinguishing pheochromocytoma from psychiatric and thyrotoxic diseases in the emergency room. J Emerg Med. 2017;53(5):712-716.
7. Garg M, Kharb S, Brar KS, et al. Medical management of pheochromocytoma: role of the endocrinologist. Indian J Endocrinol and Metab. 2011;15(suppl 4):S329-S336. doi: 10.4103/2230-8210.86976.
8. Zardawi I. Phaeochromocytoma masquerading as anxiety and depression. Am J Case Rep. 2013;14:161-163.
9. ARUP Laboratories. Test directory. https://www.aruplab.com. Accessed February 11, 2020.
10. Sriram P, Raghavan V. Pheochromocytoma presenting as anxiety disorder: a case report. Asian J Psychiatr. 2017;29:83-84.
11. Stahl SM. Stahl’s essential psychopharmacology: neuroscientific basis and practical applications. Cambridge, UK: Cambridge University Press; 2013.
1. Morrison J. When psychological problems mask medical disorders: a guide for psychotherapists. 2nd ed. New York, NY: The Guilford Press; 2015.
2. American Porphyria Foundation. About porphyria. https://porphyriafoundation.org/patients/about-porphyria. Accessed May 13, 2020.
3. Millward L, Kelly P, King A, et al. Anxiety and depression in the acute porphyrias. J Inherit Metab Dis. 2005;28(6):1099-1107.
4. Bonkovsky H, Maddukuri VC, Yazici C, et al. Acute porphyrias in the USA: features of 108 subjects from porphyria consortium. Am J Med. 2014;127(12):1233-1241.
5. Tsirlin A, Oo Y, Sharma R, et al. Pheochromocytoma: a review. Maturitas. 2014;77(3):229-238.
6. Leung A, Zun L, Nordstrom K, et al. Psychiatric emergencies for physicians: clinical management and approach to distinguishing pheochromocytoma from psychiatric and thyrotoxic diseases in the emergency room. J Emerg Med. 2017;53(5):712-716.
7. Garg M, Kharb S, Brar KS, et al. Medical management of pheochromocytoma: role of the endocrinologist. Indian J Endocrinol and Metab. 2011;15(suppl 4):S329-S336. doi: 10.4103/2230-8210.86976.
8. Zardawi I. Phaeochromocytoma masquerading as anxiety and depression. Am J Case Rep. 2013;14:161-163.
9. ARUP Laboratories. Test directory. https://www.aruplab.com. Accessed February 11, 2020.
10. Sriram P, Raghavan V. Pheochromocytoma presenting as anxiety disorder: a case report. Asian J Psychiatr. 2017;29:83-84.
11. Stahl SM. Stahl’s essential psychopharmacology: neuroscientific basis and practical applications. Cambridge, UK: Cambridge University Press; 2013.
When mania isn’t what it seems
CASE Aggressive, impulsive, and not sleeping
Mr. S, age 22, is brought by his family to his outpatient psychiatrist because he has begun to
Mr. S has significant language impairment and is unreliable as a narrator. His family reports that Mr. S’s behavior has resulted in declining academic performance, and they have curtailed his social activities due to behavioral issues. Both his family and teachers report that it is increasingly difficult to redirect Mr. S’s behavior. Although not physically aggressive, Mr. S becomes verbally agitated when rituals are incomplete. He has gone from sleeping 8 hours each night to only 3 to 4 hours, but he does not appear tired during the day.
HISTORY Multiple hospitalizations
As a child, Mr. S had been diagnosed with autism and intellectual disability. When he was 13, he began exhibiting marked stereotypy, restlessness, impulsivity, frenzy, agitation, combativeness, and purposeless motor activity. At that time, he was not receiving any medications. Mr. S had not slept for 2 days and had been walking in circles nonstop. He became aggressive whenever anyone attempted to redirect his behavior. The family took Mr. S to the emergency department (ED), where clinicians ruled out organic causes for his behavioral disturbances, including infections, drug intoxication, and use of illicit substances. Mr. S was transferred from the ED to a child and adolescent psychiatry ward at a nearby university hospital for inpatient treatment.
On the inpatient unit, the treatment team diagnosed Mr. S with bipolar disorder and believed that he was experiencing a manic episode. He was prescribed quetiapine, 25 mg by mouth during the day and 75 mg by mouth at night, to stabilize his agitation, and was discharged with a plan to follow up with his outpatient psychiatrist. However, within 1 week, his symptoms returned, with markedly increased aggression and agitation, so he was readmitted, tapered off quetiapine, and prescribed valproic acid, 125 mg by mouth during the day and 375 mg by mouth at bedtime. With this regimen, Mr. S became calmer, but when he was discharged home, he was subdued and withdrawn, overly adherent to rules and routines, constantly irritable, and often unable to focus.
Two years later, Mr. S developed hyperammonemia. Valproic acid was discontinued, and many of his behavioral issues resolved. He flourished both academically and socially. He experienced no exacerbation of symptoms until his current presentation.
[polldaddy:10544547]
EVALUATION Pinpointing the cause
Mr. S’s physical examination reveals that his vital signs are within normal limits. Mr. S is mildly tachycardic (heart rate, 105 bpm), with regular rate and rhythm. No murmurs, gallops, or rubs are auscultated. The remainder of the physical exam, including a detailed neurologic exam, is normal.
On mental status examination, Mr. S makes limited eye contact. He has difficulty sitting in the chair, with increased rocking, finger flicking, and hand flapping from baseline. Some compulsive behaviors are noted, such as tapping his neck. He has increased tics (eye blinking and mouth opening) and increased verbigeration and repetitive verbal statements. He loudly and repeatedly demands to go home, and uses short sentences with incorrect pronouns. His affect is difficult to assess, but he is agitated. His thought process is concrete. There is no evidence of suicidal ideation, homicidal ideation, or psychosis. Mr. S denies auditory hallucinations. His insight and judgment are limited.
Continue to: The psychiatrist rules out...
The psychiatrist rules out a behavioral exacerbation of autism based on an interview with Mr. S’s family and established rapport from treating him for several years. Mr. S’s family reports that many of his behaviors are not new but that the increased drive and intensity is worrisome. Further, his family cannot identify any stressors or precipitants for the behaviors and reports that offering preferred reinforcers did not help. An anxiety disorder is ruled out because according to the family, Mr. S’s drive to constantly move and complete rituals is fueling his anxiety. Schizoaffective disorder is ruled out because Mr. S denies auditory hallucinations and has not been observed responding to internal stimuli.
His Bush-Francis Catatonia Rating Scale (BFCRS) score is 26, which suggests a high likelihood of catatonia. Based on the BFCRS score, Mr. S’s psychiatrist makes the diagnosis of hyperkinetic catatonia.
The authors’ observations
The psychiatrist determined that Mr. S had been misdiagnosed with bipolar disorder at age 13. At that time, he had experienced his first episode of hyperkinetic catatonia and his symptoms decreased after he received lorazepam in the ED. However, the treatment team did not correctly identify this, most likely due to limited knowledge of catatonia among emergency medicine clinicians.
This case exemplifies a cognitive error of premature closure. Rather than considering catatonia as a complication of autism when Mr. S was 13, the clinicians added a second psychiatric diagnosis of bipolar disorder.Although premature closure errors generally occur when the physician assumes the patient is having a common complication of a known illness,1 in Mr. S’s case, the opposite occurred.
Conceptualizing catatonia
One helpful model for conceptualizing catatonia is to think of it as a basal ganglia disorder, with lesions in the basal ganglia thalamocortical tracts and the anterior cingulate/medial orbitofrontal circuit. Disrupting these pathways can result in symptoms such as mutism or repetitive and imitative behaviors. This is likely due to decreased disinhibition by gamma-aminobutyric acid (GABA), resulting in a hypodopaminergic state. This explains why benzodiazepines, which act to increase GABA, are effective for treating catatonia, and antipsychotics that act to decrease dopamine can exacerbate symptoms. Fricchione et al2 developed a model to visually represent the neurobiologic pathophysiology of catatonia (Figure2).
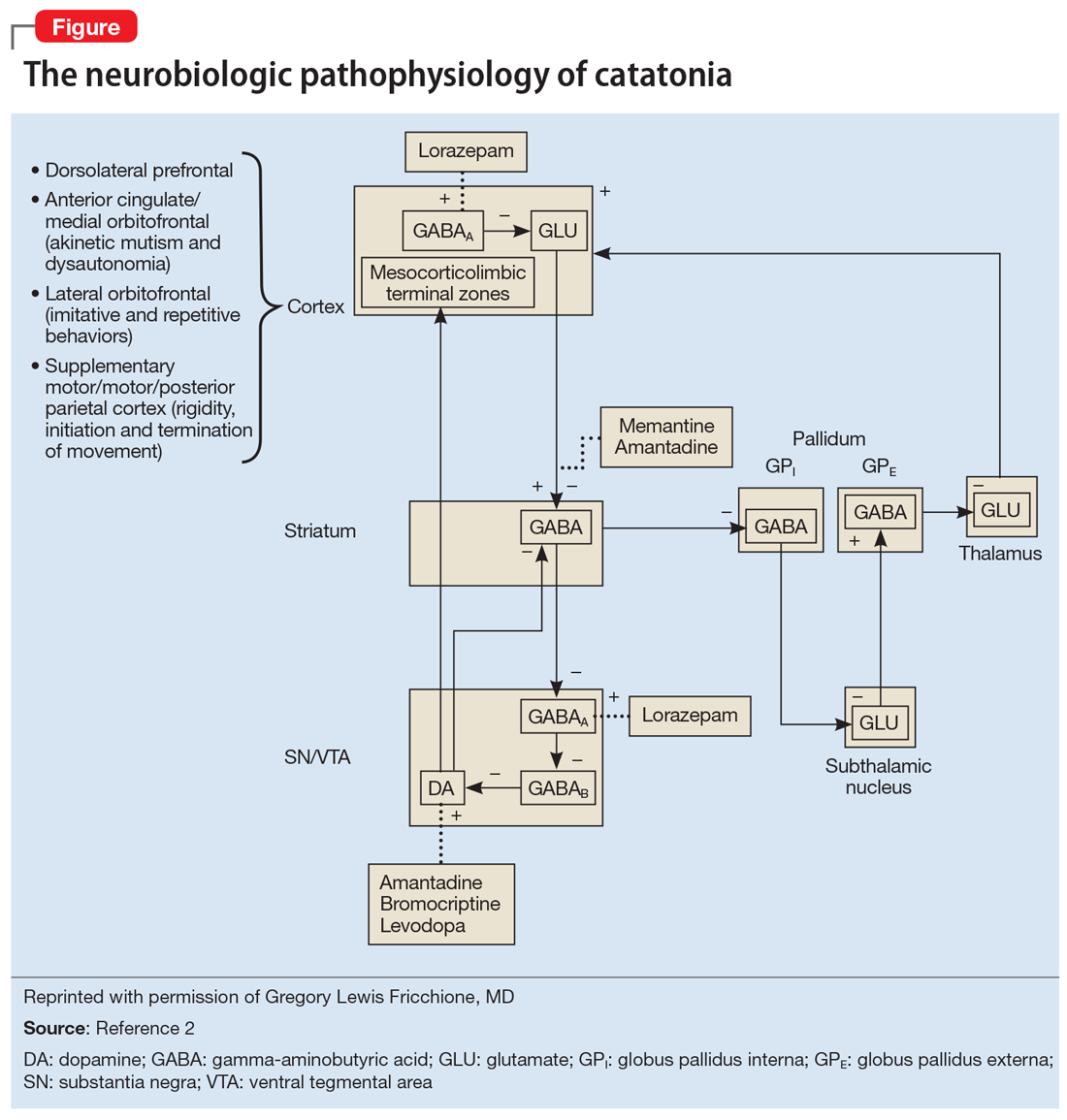
Continue to: Underlying causes of catatonia
Underlying causes of catatonia
Catatonia is most often seen in individuals with an underlying psychiatric condition such as schizophrenia, mood disorders, or autism. However, catatonia also occurs in the context of general neurologic and medical disorders, including (but not limited to) infections, metabolic disorders, endocrinopathies, epilepsy, neurodegenerative diseases, delirium, hypertensive encephalopathy, autoimmune encephalitis, and liver and kidney transplantation.3
Subtypes of catatonia include4:
- hypokinetic catatonia, which presents as stupor, mutism, and negativism
- hyperkinetic catatonia, which presents as hyperactivity, agitation, and stereotypy (as observed in Mr. S)
- malignant catatonia, which is a potentially lethal form of catatonia that occurs when hypo- or hyperkinetic catatonia is accompanied by autonomic instability such as tachycardia, tachypnea, hypertension, fever, and muscle rigidity
- periodic catatonia, which is characterized by brief episodes of stupor or excitatory catatonia lasting 4 to 10 days. These episodes recur over weeks to years, with patients remaining asymptomatic between episodes, or showing mild symptoms, such as facial grimacing or negativisms. Periodic catatonia often is autosomal dominant, involves linkage for the long arm of chromosome 15, and has a better prognosis than the other forms.
Autism and catatonia
Most individuals with autism who experience a catatonic episode first do so between age 10 and 19, and many episodes are precipitated by sudden changes in routine resulting in stress.5 An estimated 12% to 18% of patients with autism are diagnosed with catatonia in their lifetime, but the actual prevalence is likely higher.4
One of the reasons for this might be that although catatonia is well known in the psychiatric community, it is relatively unknown in the general medical community. Children and adolescents with psychiatric illness are likely to have symptoms of catatonia overlooked because catatonia often is not included in the differential diagnosis.6
In Mr. S’s case, it became clear that he did not have a mood disorder, but was prone to episodes of hyperkinetic catatonia due to his autism.
Continue to: Better recognition of catatonia
Better recognition of catatonia
As catatonia becomes better elucidated and more clearly described in the literature, there is increasing awareness that symptoms do not always involve stupor, mutism, and slowed motor activity, but can include increased motor activity, agitation, and stereotypies. The BFCRS is extremely useful for quantifying symptoms of catatonia. The best way to confirm the diagnosis is to use a lorazepam challenge in an inpatient setting, or a trial of lorazepam in an outpatient setting.5
[polldaddy:10544548]
The authors’ observations
Lorazepam is often considered the first-line treatment for catatonia because it is one of the most widely studied medications. Other benzodiazepines, such as oxazepam and clonazepam, and the sedative/hypnotic zolpidem have also been shown to be effective. Antipsychotics with dopamine-blocking mechanisms can exacerbate symptoms of catatonia and should be avoided in these patients. Furthermore, in cases of refractory catatonia, bilateral electroconvulsive therapy is an important and necessary treatment.7
TREATMENT Pharmacologic agents decrease BFCRS score
Mr. S is prescribed a regimen of lorazepam, 2 mg by mouth daily, and the supplement N-acetylcysteine, 600 mg by mouth daily. Within 2 weeks of starting this regimen, Mr. S’s BFCRS score decreases from 26 to 14. After 6 months of treatment with lorazepam, Mr. S shows considerable improvement. The stereotypic behaviors and impulsivity decrease significantly, leading to improved sleep and performance in school. After 6 months Mr. S is successfully tapered off the lorazepam, with a complete return to baseline.
Bottom Line
Hyperkinetic catatonia is easily overlooked, especially in the emergency setting. Catatonia should always be ruled out, particularly in patients with underlying conditions associated with it. Hyperkinetic catatonia is an underrecognized comorbidity in patients with autism.
Related Resources
- Dhossche DM, Wing L, Ohta M, et al. International Review of Neurobiology: Catatonia in autism spectrum disorders, vol 72. New York, NY: Academic Press/Elsevier; 2006.
- Taylor MA, Fink M. Catatonia in psychiatric classification: a home of its own. Am J Psychiatry. 2003;160(7):1233-1241.
Drug Brand Names
Amantadine • Symmetrel
Bromocriptine • Parlodel
Clonazepam • Klonopin
Lorazepam • Ativan
Memantine • Namenda
Oxazepam • Serax
Quetiapine • Seroquel
Valproic acid • Depakene, Depakote
Zolpidem • Ambien
1. McGee DL. Cognitive errors in clinical decision making. Merck Manual. https://www.merckmanuals.com/professional/special-subjects/clinical-decision-making/cognitive-errors-in-clinical-decision-making. Published November 2018. Accessed February 10, 2020.
2. Fricchione GL, Gross AF, Stern TA. Catatonia, neuroleptic malignant syndrome, and serotonin syndrome. Fricchione GL, Huffman JC, Stern TA, Bush G, eds. Massachusetts General Hospital Handbook of General Hospital Psychiatry. 6th ed. Philadelphia, PA: Saunders Elsevier; 2004:513-530.
3. Oldham MA, Lee HB. Catatonia vis-à-vis delirium: the significance of recognizing catatonia in altered mental status. Gen Hosp Psychiatry. 2015;37(6):554-559.
4. Wijemanne S, Jankovic J. Movement disorders in catatonia. J Neurol Neurosurg Psychiatry. 2014;86(8):825-832.
5. Dhossche DM, Shah A, Wing L. Blueprints for the assessment, treatment, and future study of catatonia in autism spectrum disorders. Int Rev Neurobiol. 2006:72;267-284.
6. Wing L, Shah A. Catatonia in autistic spectrum disorders. Br J Psychiatry. 2000:176(4):357-362.
7. Seinaert P, Dhossche DM, Vancampfort D, et al. A clinical review of the treatment of catatonia. Front Psychiatry. 2014;5:181.
CASE Aggressive, impulsive, and not sleeping
Mr. S, age 22, is brought by his family to his outpatient psychiatrist because he has begun to
Mr. S has significant language impairment and is unreliable as a narrator. His family reports that Mr. S’s behavior has resulted in declining academic performance, and they have curtailed his social activities due to behavioral issues. Both his family and teachers report that it is increasingly difficult to redirect Mr. S’s behavior. Although not physically aggressive, Mr. S becomes verbally agitated when rituals are incomplete. He has gone from sleeping 8 hours each night to only 3 to 4 hours, but he does not appear tired during the day.
HISTORY Multiple hospitalizations
As a child, Mr. S had been diagnosed with autism and intellectual disability. When he was 13, he began exhibiting marked stereotypy, restlessness, impulsivity, frenzy, agitation, combativeness, and purposeless motor activity. At that time, he was not receiving any medications. Mr. S had not slept for 2 days and had been walking in circles nonstop. He became aggressive whenever anyone attempted to redirect his behavior. The family took Mr. S to the emergency department (ED), where clinicians ruled out organic causes for his behavioral disturbances, including infections, drug intoxication, and use of illicit substances. Mr. S was transferred from the ED to a child and adolescent psychiatry ward at a nearby university hospital for inpatient treatment.
On the inpatient unit, the treatment team diagnosed Mr. S with bipolar disorder and believed that he was experiencing a manic episode. He was prescribed quetiapine, 25 mg by mouth during the day and 75 mg by mouth at night, to stabilize his agitation, and was discharged with a plan to follow up with his outpatient psychiatrist. However, within 1 week, his symptoms returned, with markedly increased aggression and agitation, so he was readmitted, tapered off quetiapine, and prescribed valproic acid, 125 mg by mouth during the day and 375 mg by mouth at bedtime. With this regimen, Mr. S became calmer, but when he was discharged home, he was subdued and withdrawn, overly adherent to rules and routines, constantly irritable, and often unable to focus.
Two years later, Mr. S developed hyperammonemia. Valproic acid was discontinued, and many of his behavioral issues resolved. He flourished both academically and socially. He experienced no exacerbation of symptoms until his current presentation.
[polldaddy:10544547]
EVALUATION Pinpointing the cause
Mr. S’s physical examination reveals that his vital signs are within normal limits. Mr. S is mildly tachycardic (heart rate, 105 bpm), with regular rate and rhythm. No murmurs, gallops, or rubs are auscultated. The remainder of the physical exam, including a detailed neurologic exam, is normal.
On mental status examination, Mr. S makes limited eye contact. He has difficulty sitting in the chair, with increased rocking, finger flicking, and hand flapping from baseline. Some compulsive behaviors are noted, such as tapping his neck. He has increased tics (eye blinking and mouth opening) and increased verbigeration and repetitive verbal statements. He loudly and repeatedly demands to go home, and uses short sentences with incorrect pronouns. His affect is difficult to assess, but he is agitated. His thought process is concrete. There is no evidence of suicidal ideation, homicidal ideation, or psychosis. Mr. S denies auditory hallucinations. His insight and judgment are limited.
Continue to: The psychiatrist rules out...
The psychiatrist rules out a behavioral exacerbation of autism based on an interview with Mr. S’s family and established rapport from treating him for several years. Mr. S’s family reports that many of his behaviors are not new but that the increased drive and intensity is worrisome. Further, his family cannot identify any stressors or precipitants for the behaviors and reports that offering preferred reinforcers did not help. An anxiety disorder is ruled out because according to the family, Mr. S’s drive to constantly move and complete rituals is fueling his anxiety. Schizoaffective disorder is ruled out because Mr. S denies auditory hallucinations and has not been observed responding to internal stimuli.
His Bush-Francis Catatonia Rating Scale (BFCRS) score is 26, which suggests a high likelihood of catatonia. Based on the BFCRS score, Mr. S’s psychiatrist makes the diagnosis of hyperkinetic catatonia.
The authors’ observations
The psychiatrist determined that Mr. S had been misdiagnosed with bipolar disorder at age 13. At that time, he had experienced his first episode of hyperkinetic catatonia and his symptoms decreased after he received lorazepam in the ED. However, the treatment team did not correctly identify this, most likely due to limited knowledge of catatonia among emergency medicine clinicians.
This case exemplifies a cognitive error of premature closure. Rather than considering catatonia as a complication of autism when Mr. S was 13, the clinicians added a second psychiatric diagnosis of bipolar disorder.Although premature closure errors generally occur when the physician assumes the patient is having a common complication of a known illness,1 in Mr. S’s case, the opposite occurred.
Conceptualizing catatonia
One helpful model for conceptualizing catatonia is to think of it as a basal ganglia disorder, with lesions in the basal ganglia thalamocortical tracts and the anterior cingulate/medial orbitofrontal circuit. Disrupting these pathways can result in symptoms such as mutism or repetitive and imitative behaviors. This is likely due to decreased disinhibition by gamma-aminobutyric acid (GABA), resulting in a hypodopaminergic state. This explains why benzodiazepines, which act to increase GABA, are effective for treating catatonia, and antipsychotics that act to decrease dopamine can exacerbate symptoms. Fricchione et al2 developed a model to visually represent the neurobiologic pathophysiology of catatonia (Figure2).

Continue to: Underlying causes of catatonia
Underlying causes of catatonia
Catatonia is most often seen in individuals with an underlying psychiatric condition such as schizophrenia, mood disorders, or autism. However, catatonia also occurs in the context of general neurologic and medical disorders, including (but not limited to) infections, metabolic disorders, endocrinopathies, epilepsy, neurodegenerative diseases, delirium, hypertensive encephalopathy, autoimmune encephalitis, and liver and kidney transplantation.3
Subtypes of catatonia include4:
- hypokinetic catatonia, which presents as stupor, mutism, and negativism
- hyperkinetic catatonia, which presents as hyperactivity, agitation, and stereotypy (as observed in Mr. S)
- malignant catatonia, which is a potentially lethal form of catatonia that occurs when hypo- or hyperkinetic catatonia is accompanied by autonomic instability such as tachycardia, tachypnea, hypertension, fever, and muscle rigidity
- periodic catatonia, which is characterized by brief episodes of stupor or excitatory catatonia lasting 4 to 10 days. These episodes recur over weeks to years, with patients remaining asymptomatic between episodes, or showing mild symptoms, such as facial grimacing or negativisms. Periodic catatonia often is autosomal dominant, involves linkage for the long arm of chromosome 15, and has a better prognosis than the other forms.
Autism and catatonia
Most individuals with autism who experience a catatonic episode first do so between age 10 and 19, and many episodes are precipitated by sudden changes in routine resulting in stress.5 An estimated 12% to 18% of patients with autism are diagnosed with catatonia in their lifetime, but the actual prevalence is likely higher.4
One of the reasons for this might be that although catatonia is well known in the psychiatric community, it is relatively unknown in the general medical community. Children and adolescents with psychiatric illness are likely to have symptoms of catatonia overlooked because catatonia often is not included in the differential diagnosis.6
In Mr. S’s case, it became clear that he did not have a mood disorder, but was prone to episodes of hyperkinetic catatonia due to his autism.
Continue to: Better recognition of catatonia
Better recognition of catatonia
As catatonia becomes better elucidated and more clearly described in the literature, there is increasing awareness that symptoms do not always involve stupor, mutism, and slowed motor activity, but can include increased motor activity, agitation, and stereotypies. The BFCRS is extremely useful for quantifying symptoms of catatonia. The best way to confirm the diagnosis is to use a lorazepam challenge in an inpatient setting, or a trial of lorazepam in an outpatient setting.5
[polldaddy:10544548]
The authors’ observations
Lorazepam is often considered the first-line treatment for catatonia because it is one of the most widely studied medications. Other benzodiazepines, such as oxazepam and clonazepam, and the sedative/hypnotic zolpidem have also been shown to be effective. Antipsychotics with dopamine-blocking mechanisms can exacerbate symptoms of catatonia and should be avoided in these patients. Furthermore, in cases of refractory catatonia, bilateral electroconvulsive therapy is an important and necessary treatment.7
TREATMENT Pharmacologic agents decrease BFCRS score
Mr. S is prescribed a regimen of lorazepam, 2 mg by mouth daily, and the supplement N-acetylcysteine, 600 mg by mouth daily. Within 2 weeks of starting this regimen, Mr. S’s BFCRS score decreases from 26 to 14. After 6 months of treatment with lorazepam, Mr. S shows considerable improvement. The stereotypic behaviors and impulsivity decrease significantly, leading to improved sleep and performance in school. After 6 months Mr. S is successfully tapered off the lorazepam, with a complete return to baseline.
Bottom Line
Hyperkinetic catatonia is easily overlooked, especially in the emergency setting. Catatonia should always be ruled out, particularly in patients with underlying conditions associated with it. Hyperkinetic catatonia is an underrecognized comorbidity in patients with autism.
Related Resources
- Dhossche DM, Wing L, Ohta M, et al. International Review of Neurobiology: Catatonia in autism spectrum disorders, vol 72. New York, NY: Academic Press/Elsevier; 2006.
- Taylor MA, Fink M. Catatonia in psychiatric classification: a home of its own. Am J Psychiatry. 2003;160(7):1233-1241.
Drug Brand Names
Amantadine • Symmetrel
Bromocriptine • Parlodel
Clonazepam • Klonopin
Lorazepam • Ativan
Memantine • Namenda
Oxazepam • Serax
Quetiapine • Seroquel
Valproic acid • Depakene, Depakote
Zolpidem • Ambien
CASE Aggressive, impulsive, and not sleeping
Mr. S, age 22, is brought by his family to his outpatient psychiatrist because he has begun to
Mr. S has significant language impairment and is unreliable as a narrator. His family reports that Mr. S’s behavior has resulted in declining academic performance, and they have curtailed his social activities due to behavioral issues. Both his family and teachers report that it is increasingly difficult to redirect Mr. S’s behavior. Although not physically aggressive, Mr. S becomes verbally agitated when rituals are incomplete. He has gone from sleeping 8 hours each night to only 3 to 4 hours, but he does not appear tired during the day.
HISTORY Multiple hospitalizations
As a child, Mr. S had been diagnosed with autism and intellectual disability. When he was 13, he began exhibiting marked stereotypy, restlessness, impulsivity, frenzy, agitation, combativeness, and purposeless motor activity. At that time, he was not receiving any medications. Mr. S had not slept for 2 days and had been walking in circles nonstop. He became aggressive whenever anyone attempted to redirect his behavior. The family took Mr. S to the emergency department (ED), where clinicians ruled out organic causes for his behavioral disturbances, including infections, drug intoxication, and use of illicit substances. Mr. S was transferred from the ED to a child and adolescent psychiatry ward at a nearby university hospital for inpatient treatment.
On the inpatient unit, the treatment team diagnosed Mr. S with bipolar disorder and believed that he was experiencing a manic episode. He was prescribed quetiapine, 25 mg by mouth during the day and 75 mg by mouth at night, to stabilize his agitation, and was discharged with a plan to follow up with his outpatient psychiatrist. However, within 1 week, his symptoms returned, with markedly increased aggression and agitation, so he was readmitted, tapered off quetiapine, and prescribed valproic acid, 125 mg by mouth during the day and 375 mg by mouth at bedtime. With this regimen, Mr. S became calmer, but when he was discharged home, he was subdued and withdrawn, overly adherent to rules and routines, constantly irritable, and often unable to focus.
Two years later, Mr. S developed hyperammonemia. Valproic acid was discontinued, and many of his behavioral issues resolved. He flourished both academically and socially. He experienced no exacerbation of symptoms until his current presentation.
[polldaddy:10544547]
EVALUATION Pinpointing the cause
Mr. S’s physical examination reveals that his vital signs are within normal limits. Mr. S is mildly tachycardic (heart rate, 105 bpm), with regular rate and rhythm. No murmurs, gallops, or rubs are auscultated. The remainder of the physical exam, including a detailed neurologic exam, is normal.
On mental status examination, Mr. S makes limited eye contact. He has difficulty sitting in the chair, with increased rocking, finger flicking, and hand flapping from baseline. Some compulsive behaviors are noted, such as tapping his neck. He has increased tics (eye blinking and mouth opening) and increased verbigeration and repetitive verbal statements. He loudly and repeatedly demands to go home, and uses short sentences with incorrect pronouns. His affect is difficult to assess, but he is agitated. His thought process is concrete. There is no evidence of suicidal ideation, homicidal ideation, or psychosis. Mr. S denies auditory hallucinations. His insight and judgment are limited.
Continue to: The psychiatrist rules out...
The psychiatrist rules out a behavioral exacerbation of autism based on an interview with Mr. S’s family and established rapport from treating him for several years. Mr. S’s family reports that many of his behaviors are not new but that the increased drive and intensity is worrisome. Further, his family cannot identify any stressors or precipitants for the behaviors and reports that offering preferred reinforcers did not help. An anxiety disorder is ruled out because according to the family, Mr. S’s drive to constantly move and complete rituals is fueling his anxiety. Schizoaffective disorder is ruled out because Mr. S denies auditory hallucinations and has not been observed responding to internal stimuli.
His Bush-Francis Catatonia Rating Scale (BFCRS) score is 26, which suggests a high likelihood of catatonia. Based on the BFCRS score, Mr. S’s psychiatrist makes the diagnosis of hyperkinetic catatonia.
The authors’ observations
The psychiatrist determined that Mr. S had been misdiagnosed with bipolar disorder at age 13. At that time, he had experienced his first episode of hyperkinetic catatonia and his symptoms decreased after he received lorazepam in the ED. However, the treatment team did not correctly identify this, most likely due to limited knowledge of catatonia among emergency medicine clinicians.
This case exemplifies a cognitive error of premature closure. Rather than considering catatonia as a complication of autism when Mr. S was 13, the clinicians added a second psychiatric diagnosis of bipolar disorder.Although premature closure errors generally occur when the physician assumes the patient is having a common complication of a known illness,1 in Mr. S’s case, the opposite occurred.
Conceptualizing catatonia
One helpful model for conceptualizing catatonia is to think of it as a basal ganglia disorder, with lesions in the basal ganglia thalamocortical tracts and the anterior cingulate/medial orbitofrontal circuit. Disrupting these pathways can result in symptoms such as mutism or repetitive and imitative behaviors. This is likely due to decreased disinhibition by gamma-aminobutyric acid (GABA), resulting in a hypodopaminergic state. This explains why benzodiazepines, which act to increase GABA, are effective for treating catatonia, and antipsychotics that act to decrease dopamine can exacerbate symptoms. Fricchione et al2 developed a model to visually represent the neurobiologic pathophysiology of catatonia (Figure2).

Continue to: Underlying causes of catatonia
Underlying causes of catatonia
Catatonia is most often seen in individuals with an underlying psychiatric condition such as schizophrenia, mood disorders, or autism. However, catatonia also occurs in the context of general neurologic and medical disorders, including (but not limited to) infections, metabolic disorders, endocrinopathies, epilepsy, neurodegenerative diseases, delirium, hypertensive encephalopathy, autoimmune encephalitis, and liver and kidney transplantation.3
Subtypes of catatonia include4:
- hypokinetic catatonia, which presents as stupor, mutism, and negativism
- hyperkinetic catatonia, which presents as hyperactivity, agitation, and stereotypy (as observed in Mr. S)
- malignant catatonia, which is a potentially lethal form of catatonia that occurs when hypo- or hyperkinetic catatonia is accompanied by autonomic instability such as tachycardia, tachypnea, hypertension, fever, and muscle rigidity
- periodic catatonia, which is characterized by brief episodes of stupor or excitatory catatonia lasting 4 to 10 days. These episodes recur over weeks to years, with patients remaining asymptomatic between episodes, or showing mild symptoms, such as facial grimacing or negativisms. Periodic catatonia often is autosomal dominant, involves linkage for the long arm of chromosome 15, and has a better prognosis than the other forms.
Autism and catatonia
Most individuals with autism who experience a catatonic episode first do so between age 10 and 19, and many episodes are precipitated by sudden changes in routine resulting in stress.5 An estimated 12% to 18% of patients with autism are diagnosed with catatonia in their lifetime, but the actual prevalence is likely higher.4
One of the reasons for this might be that although catatonia is well known in the psychiatric community, it is relatively unknown in the general medical community. Children and adolescents with psychiatric illness are likely to have symptoms of catatonia overlooked because catatonia often is not included in the differential diagnosis.6
In Mr. S’s case, it became clear that he did not have a mood disorder, but was prone to episodes of hyperkinetic catatonia due to his autism.
Continue to: Better recognition of catatonia
Better recognition of catatonia
As catatonia becomes better elucidated and more clearly described in the literature, there is increasing awareness that symptoms do not always involve stupor, mutism, and slowed motor activity, but can include increased motor activity, agitation, and stereotypies. The BFCRS is extremely useful for quantifying symptoms of catatonia. The best way to confirm the diagnosis is to use a lorazepam challenge in an inpatient setting, or a trial of lorazepam in an outpatient setting.5
[polldaddy:10544548]
The authors’ observations
Lorazepam is often considered the first-line treatment for catatonia because it is one of the most widely studied medications. Other benzodiazepines, such as oxazepam and clonazepam, and the sedative/hypnotic zolpidem have also been shown to be effective. Antipsychotics with dopamine-blocking mechanisms can exacerbate symptoms of catatonia and should be avoided in these patients. Furthermore, in cases of refractory catatonia, bilateral electroconvulsive therapy is an important and necessary treatment.7
TREATMENT Pharmacologic agents decrease BFCRS score
Mr. S is prescribed a regimen of lorazepam, 2 mg by mouth daily, and the supplement N-acetylcysteine, 600 mg by mouth daily. Within 2 weeks of starting this regimen, Mr. S’s BFCRS score decreases from 26 to 14. After 6 months of treatment with lorazepam, Mr. S shows considerable improvement. The stereotypic behaviors and impulsivity decrease significantly, leading to improved sleep and performance in school. After 6 months Mr. S is successfully tapered off the lorazepam, with a complete return to baseline.
Bottom Line
Hyperkinetic catatonia is easily overlooked, especially in the emergency setting. Catatonia should always be ruled out, particularly in patients with underlying conditions associated with it. Hyperkinetic catatonia is an underrecognized comorbidity in patients with autism.
Related Resources
- Dhossche DM, Wing L, Ohta M, et al. International Review of Neurobiology: Catatonia in autism spectrum disorders, vol 72. New York, NY: Academic Press/Elsevier; 2006.
- Taylor MA, Fink M. Catatonia in psychiatric classification: a home of its own. Am J Psychiatry. 2003;160(7):1233-1241.
Drug Brand Names
Amantadine • Symmetrel
Bromocriptine • Parlodel
Clonazepam • Klonopin
Lorazepam • Ativan
Memantine • Namenda
Oxazepam • Serax
Quetiapine • Seroquel
Valproic acid • Depakene, Depakote
Zolpidem • Ambien
1. McGee DL. Cognitive errors in clinical decision making. Merck Manual. https://www.merckmanuals.com/professional/special-subjects/clinical-decision-making/cognitive-errors-in-clinical-decision-making. Published November 2018. Accessed February 10, 2020.
2. Fricchione GL, Gross AF, Stern TA. Catatonia, neuroleptic malignant syndrome, and serotonin syndrome. Fricchione GL, Huffman JC, Stern TA, Bush G, eds. Massachusetts General Hospital Handbook of General Hospital Psychiatry. 6th ed. Philadelphia, PA: Saunders Elsevier; 2004:513-530.
3. Oldham MA, Lee HB. Catatonia vis-à-vis delirium: the significance of recognizing catatonia in altered mental status. Gen Hosp Psychiatry. 2015;37(6):554-559.
4. Wijemanne S, Jankovic J. Movement disorders in catatonia. J Neurol Neurosurg Psychiatry. 2014;86(8):825-832.
5. Dhossche DM, Shah A, Wing L. Blueprints for the assessment, treatment, and future study of catatonia in autism spectrum disorders. Int Rev Neurobiol. 2006:72;267-284.
6. Wing L, Shah A. Catatonia in autistic spectrum disorders. Br J Psychiatry. 2000:176(4):357-362.
7. Seinaert P, Dhossche DM, Vancampfort D, et al. A clinical review of the treatment of catatonia. Front Psychiatry. 2014;5:181.
1. McGee DL. Cognitive errors in clinical decision making. Merck Manual. https://www.merckmanuals.com/professional/special-subjects/clinical-decision-making/cognitive-errors-in-clinical-decision-making. Published November 2018. Accessed February 10, 2020.
2. Fricchione GL, Gross AF, Stern TA. Catatonia, neuroleptic malignant syndrome, and serotonin syndrome. Fricchione GL, Huffman JC, Stern TA, Bush G, eds. Massachusetts General Hospital Handbook of General Hospital Psychiatry. 6th ed. Philadelphia, PA: Saunders Elsevier; 2004:513-530.
3. Oldham MA, Lee HB. Catatonia vis-à-vis delirium: the significance of recognizing catatonia in altered mental status. Gen Hosp Psychiatry. 2015;37(6):554-559.
4. Wijemanne S, Jankovic J. Movement disorders in catatonia. J Neurol Neurosurg Psychiatry. 2014;86(8):825-832.
5. Dhossche DM, Shah A, Wing L. Blueprints for the assessment, treatment, and future study of catatonia in autism spectrum disorders. Int Rev Neurobiol. 2006:72;267-284.
6. Wing L, Shah A. Catatonia in autistic spectrum disorders. Br J Psychiatry. 2000:176(4):357-362.
7. Seinaert P, Dhossche DM, Vancampfort D, et al. A clinical review of the treatment of catatonia. Front Psychiatry. 2014;5:181.
Command hallucinations, but is it really psychosis?
CASE Frequent hospitalizations
Ms. D, age 26, presents to the emergency department (ED) after drinking a bottle of hand sanitizer in a suicide attempt. She is admitted to an inpatient psychiatric unit, where she spends 50 days, followed by a transfer to a step-down unit, where she spends 26 days. Upon discharge, her diagnosis is schizoaffective disorder–bipolar type.
Shortly before this, Ms. D had intentionally ingested 20 vitamin pills to “make her heart stop” after a conflict at home. After ingesting the pills, Ms. D presented to the ED, where she stated that if she were discharged, she would kill herself by taking “better pills.” She was then admitted to an inpatient psychiatric unit, where she spent 60 days before being moved to an extended-care step-down facility, where she resided for 42 days.
HISTORY A challenging past
Ms. D has a history of >25 psychiatric hospitalizations with varying discharge diagnoses, including schizophrenia, schizoaffective disorder, borderline personality disorder (BPD), and borderline intellectual functioning.
Ms. D was raised in a 2-parent home with 3 older half-brothers and 3 sisters. She was sexually assaulted by a cousin when she was 12. Ms. D recalls one event of self-injury/cutting behavior at age 15 after she was bullied by peers. Her family history is significant for schizophrenia (mother), alcohol use disorder (both parents), and bipolar disorder (sister). Her mother, who is now deceased, was admitted to state psychiatric hospitals for extended periods.
Her medication regimen has changed with nearly every hospitalization but generally has included ≥1 antipsychotic, a mood stabilizer, an antidepressant, and a benzodiazepine (often prescribed on an as-needed basis). Ms. D is obese and has difficulty sleeping, hypothyroidism, gastroesophageal reflux disease (GERD), hypertension, and iron deficiency anemia. She receives medications to manage each of these conditions.
Ms. D’s previous psychotic symptoms included auditory command hallucinations. These occurred under stressful circumstances, such as during severe family conflicts that often led to her feeling abandoned. She reported that the “voice” she heard was usually her own instructing her to “take pills.” There was no prior evidence of bizarre delusions, negative symptoms, or disorganized thoughts or speech.
During episodes of decompensation, Ms. D did not report symptoms of mania, sustained depressed mood, or anxiety, nor were these symptoms observed. Although Ms. D endorsed suicidal ideation with a plan, intent, and means, during several of her previous ED presentations, she told clinicians that her intent was not to end her life but rather to evoke concern in her family members.
Continue to: After her mother died...
After her mother died when Ms. D was 19, she began to have nightmares of wanting to hurt herself and others and began experiencing multiple hospitalizations. In 2010, Ms. D was referred to an assertive community treatment (ACT) program for individuals age 16 to 27 because of her inability to participate in traditional community-based services and her historical need for advanced services, in order to provide psychiatric care in the least restrictive means possible.
Despite receiving intensive ACT services, and in addition to the numerous inpatient psychiatric hospitalizations, over 7 years, Ms. D accumulated 8 additional general-medical hospitalizations and >50 visits to hospital EDs and urgent care facilities. These hospitalizations typically followed arguments at home, strained family dynamics, and not feeling wanted. Ms. D would ingest large quantities of prescription or over-the-counter medications as a way of coping, which often occurred while she was residing in a step-down facility after hospital discharge.
[polldaddy:10528342]
The authors’ observations
The treatment team decided to transition Ms. D to an LTSR with full continuum of treatment. While some clinicians might be concerned with potential iatrogenic harm of LTSR placement and might instead recommend less restrictive residential support and an IOP. However, in Ms. D’s case, her numerous admissions to EDs, urgent care facilities, and medical and psychiatric hospitals, her failed step-down facility placements, and her family conflicts and poor dynamics limited the efficacy of her natural support system and drove the recommendation for an LTSR.
Previously, Ms. D’s experience with ACT services had centered on managing acute crises, with brief periods of stabilization that insufficiently engaged her in a consistent and meaningful treatment plan. Ms. D’s insurance company agreed to pay for the LTSR after lengthy discussions with the clinical leadership at the ACT program and the LTSR demonstrated that she was a high utilizer of health care services. They concluded that Ms. D’s stay at the LTSR would be less expensive than the frequent use of expensive hospital services and care.
EVALUATION A consensus on the diagnosis
During the first few weeks of Ms. D’s admission to the LTSR, the treatment team takes a thorough history and reviews her medical records, which they obtained from several past inpatient admissions and therapists who previously treated Ms. D. The team also collects collateral information from Ms. D’s family members. Based on this information, interviews, and composite behavioral observations from the first few weeks of Ms. D’s time at the LTSR, the psychiatrists and treatment team at the LTSR and ACT program determine that Ms. D meets the criteria for a primary diagnosis of BPD. Previous discharge diagnoses of schizoaffective disorder–bipolar type (Table 11), schizophrenia, or bipolar disorder could not be affirmed.

Continue to: The authors' observations
The authors’ observations
During Ms. D’s LTSR placement, it became clear that her self-harm behaviors and numerous visits to the ED and urgent care facilities involved severe and intense emotional dysregulation and maladaptive behaviors. These behaviors had developed over time in response to acute stressors and past trauma, and not as a result of a sustained mood or psychotic disorder. Before her LTSR placement, Ms. D was unable to use more adaptive coping skills, such as skills building, learning, and coaching. Ms. D typically “thrived” with medical attention in the ED or hospital, and once the stressor dissipated, she was discharged back to the same stressful living environment associated with her maladaptive coping.
Table 2 outlines the rationale for long-term residential treatment for Ms. D.

TREATMENT Developing more effective skills
Bolstered by a clearer diagnostic formulation of BPD, Ms. D’s initial treatment goals at the LTSR include developing effective skills (eg, mindfulness, interpersonal effectiveness, emotion regulation, and distress tolerance) to cope with family conflicts and other stressors while she is outside the facility on a therapeutic pass. Ms. D’s treatment focuses on skills learning and coaching, and behavior chain analyses, which are conducted by her therapist from the ACT program.
Ms. D remains clinically stable throughout her LTSR placement, and benefits from ongoing skills building and learning, coaching, and community integration efforts.
[polldaddy:10528348]
The authors’ observations
Several systematic reviews2-5 have found that there is a lack of high-quality evidence for the use of various psychotropic medications for patients with BPD, yet polypharmacy is common. Many patients with BPD receive ≥2 medications and >25% of patients receive ≥4 medications, typically for prolonged periods. Stoffers et al4 suggested that FGAs and antidepressants have marginal effects of for patients with BPD; however, their use cannot be ruled out because they may be helpful for comorbid symptoms that are often observed in patients with BPD. There is better evidence for SGAs, mood stabilizers, and omega-3 fatty acids; however, most effect estimates were based on single studies, and there is minimal data on long-term use of these agents.4
Continue to: A recent review highlighted...
A recent review highlighted 2 trends in medication prescribing for individuals with BPD3:
- a decrease in the use of benzodiazepines and antidepressants
- an increase in or preference for mood stabilizers and SGAs, especially valproate and quetiapine.
In terms of which medications can be used to target specific symptoms, the same researchers also noted from previous studies3:
- The prior use of SSRIs to target affective dysregulation, anxiety, and impulsive- behavior dyscontrol
- mood stabilizers (notably anticonvulsants) and SGAs to target “core symptoms” of BPD, including affective dysregulation, impulsive-behavioral dyscontrol, and cognitive-perceptual distortions
- omega-3 fatty acids for mood stabilization, impulsive-behavior dyscontrol, and possibly to reduce self-harm behaviors.
TREATMENT Medication adjustments
The treatment team reviews the lack of evidence for the long-term use of psychotropic medications in the treatment of BPD with Ms. D and her relatives,2-5 and develops a medication regimen that is clinically appropriate for managing the symptoms of BPD, while also being mindful of adverse effects.
When Ms. D was admitted to the LTSR from the hospital, her psychotropic medication regimen included haloperidol, 150 mg IM every month; olanzapine, 20 mg at bedtime; benztropine, 1 mg twice daily; and melatonin, 9 mg at bedtime.
Following discussions with Ms. D and her older sister, the team initiates a taper of olanzapine because of metabolic concerns. Ms. D has gained >40 lb while receiving this medication and had hypertension. Olanzapine was tapered and discontinued over the course of 3 months with no reemergence of sustained mood or psychotic symptoms (Table 3). During this period, Ms. D also participates in dietary counselling, follows a portion-controlled regimen, and loses >30 lb. Her wellness plan focuses on nutrition and exercise to improve her overall physical health.
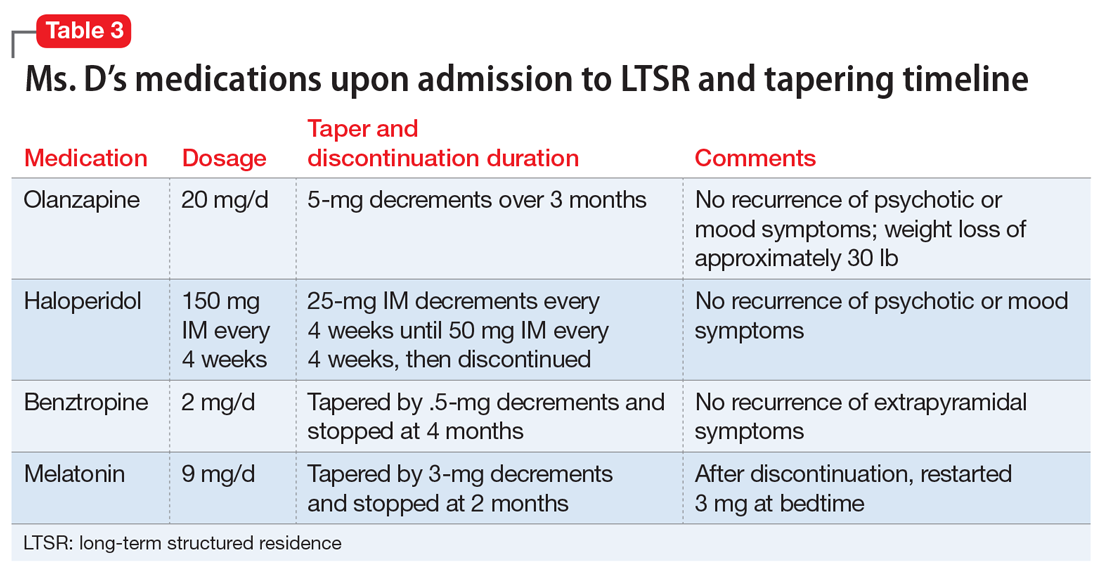
Continue to: Six months into her stay...
Six months into her stay at the LTSR, Ms. D remains clinically stable and is able to leave the LTSR placement to go on home passes. At this time, the team begins to taper the haloperidol long-acting injection. One month prior to discharge from the LTSR, haloperidol is discontinued entirely. The treatment team simultaneously tapers and discontinues benztropine. No recurrence of extrapyramidal symptoms is observed by staff or noted by the patient.
A treatment plan is developed to address Ms. D’s medical conditions, including hypothyroidism, GERD, and obesity. Ms. D does not appear to have difficulty sleeping at the LTSR, so melatonin is tapered by 3-mg decrements and stopped after 2 months. However, shortly thereafter, she develops insomnia, so a 3-mg dose is re-initiated, and her complaints abate. Her primary care physician discontinues hydrochlorothiazide, an antihypertensive medication.
Ms. D’s medication regimen consists of melatonin, 3 mg at bedtime; pantoprazole, 40 mg before breakfast, for GERD; senna, 8.6 mg at bedtime, and polyethylene glycol, 17 gm/d, for constipation; levothyroxine, 125 mcg/d, for hypothyroidism; metoprolol extended-release, 50 mg/d, for hypertension; and ferrous sulfate, 325 mg/d, for iron deficiency anemia.
OUTCOME Improved functioning
After 11 months at the LTSR, Ms. D is discharged home. She continues to receive outpatient services in the community through the ACT program, meeting with her therapist for cognitive-behavioral therapy, skills building and learning, and integration.
Approximately 9 months later, Ms. D is re-started on an SSRI (sertraline, 50 mg/d, which is increased to 100 mg/d 9 months later) to target symptoms of anxiety, which primarily manifest as excessive worrying. Hydroxyzine, 50 mg 3 times daily as needed, is added to this regimen, for breakthrough anxiety symptoms. Hydroxyzine is prescribed instead of a benzodiazepine to avoid potential addiction and abuse.
Continue to: Oral ziprasidone...
Oral ziprasidone, 20 mg/d twice daily, is initiated during 2 brief inpatient psychiatric admissions; however, it is successfully tapered within 1 week of discharge, in partnership with the ACT program.
In the 23 months after her discharge, Ms. D has had 1 ED visit and 2 brief inpatient psychiatric hospitalizations, which is markedly fewer encounters than she had in the 2 years before her LTSR placement. She has also lost an additional 30 lb since her LTSR discharge through a healthy diet and exercise.
Ms. D is now considering transitioning to living independently in the community through a residential supported housing program.
Bottom Line
Psychotic symptoms in patients with borderline personality disorder (BPD) are typically fleeting and mostly occur in the context of intense interpersonal conflicts and real or imagined abandonment. Long-term structured residence placement for patients with BPD can allow for careful formulation of a treatment plan, and help patients gain effective skills to cope with difficult family dynamics and other stressors, with the ultimate goal of gradual community integration.
Related Resource
- National Education Alliance for Borderline Personality Disorder. https://www.borderlinepersonalitydisorder.org.
Drug Brand Names
Benztropine • Cogentin
Haloperidol • Haldol
Hydrochlorothiazide • Microzide, HydroDiuril
Hydroxyzine • Vistaril
Levothyroxine • Synthroid,
Metoprolol ER • Toprol XL
Olanzapine • Zyprexa
Pantoprazole • Protonix
Polyethylene glycol • MiraLax, Glycolax
Quetiapine • Seroquel
Senna • Senokot
Sertraline • Zoloft
Valproate • Depakene, Depakote
Ziprasidone • Geodon
1. Diagnostic and Statistical Manual of Mental Disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
2. Hancock-Johnson E, Griffiths C, Picchioni M. A focused systematic review of pharmacological treatment for borderline personality disorder. CNS Drugs. 2017;31:345-356.
3. Starcevic V, Janca A. Pharmacotherapy of borderline personality disorder: replacing confusion with prudent pragmatism. Curr Opin Psychiatry. 2018;31(1):69-73.
4. Stoffers J, Völlm BA, Rücker G, et al. Pharmacological interventions for borderline personality disorder. Cochrane Database Syst Rev. 2010;6:CD005653. doi: 10.1002/14651858.CD005653.pub2.
5. Stoffers-Winterling JM, Storebo OJ, Völlm BA, et al. Pharmacological interventions for borderline personality disorder. Cochrane Database Syst Rev. 2018;3:CD012956. doi: 10.1002/14651858.CD012956.
CASE Frequent hospitalizations
Ms. D, age 26, presents to the emergency department (ED) after drinking a bottle of hand sanitizer in a suicide attempt. She is admitted to an inpatient psychiatric unit, where she spends 50 days, followed by a transfer to a step-down unit, where she spends 26 days. Upon discharge, her diagnosis is schizoaffective disorder–bipolar type.
Shortly before this, Ms. D had intentionally ingested 20 vitamin pills to “make her heart stop” after a conflict at home. After ingesting the pills, Ms. D presented to the ED, where she stated that if she were discharged, she would kill herself by taking “better pills.” She was then admitted to an inpatient psychiatric unit, where she spent 60 days before being moved to an extended-care step-down facility, where she resided for 42 days.
HISTORY A challenging past
Ms. D has a history of >25 psychiatric hospitalizations with varying discharge diagnoses, including schizophrenia, schizoaffective disorder, borderline personality disorder (BPD), and borderline intellectual functioning.
Ms. D was raised in a 2-parent home with 3 older half-brothers and 3 sisters. She was sexually assaulted by a cousin when she was 12. Ms. D recalls one event of self-injury/cutting behavior at age 15 after she was bullied by peers. Her family history is significant for schizophrenia (mother), alcohol use disorder (both parents), and bipolar disorder (sister). Her mother, who is now deceased, was admitted to state psychiatric hospitals for extended periods.
Her medication regimen has changed with nearly every hospitalization but generally has included ≥1 antipsychotic, a mood stabilizer, an antidepressant, and a benzodiazepine (often prescribed on an as-needed basis). Ms. D is obese and has difficulty sleeping, hypothyroidism, gastroesophageal reflux disease (GERD), hypertension, and iron deficiency anemia. She receives medications to manage each of these conditions.
Ms. D’s previous psychotic symptoms included auditory command hallucinations. These occurred under stressful circumstances, such as during severe family conflicts that often led to her feeling abandoned. She reported that the “voice” she heard was usually her own instructing her to “take pills.” There was no prior evidence of bizarre delusions, negative symptoms, or disorganized thoughts or speech.
During episodes of decompensation, Ms. D did not report symptoms of mania, sustained depressed mood, or anxiety, nor were these symptoms observed. Although Ms. D endorsed suicidal ideation with a plan, intent, and means, during several of her previous ED presentations, she told clinicians that her intent was not to end her life but rather to evoke concern in her family members.
Continue to: After her mother died...
After her mother died when Ms. D was 19, she began to have nightmares of wanting to hurt herself and others and began experiencing multiple hospitalizations. In 2010, Ms. D was referred to an assertive community treatment (ACT) program for individuals age 16 to 27 because of her inability to participate in traditional community-based services and her historical need for advanced services, in order to provide psychiatric care in the least restrictive means possible.
Despite receiving intensive ACT services, and in addition to the numerous inpatient psychiatric hospitalizations, over 7 years, Ms. D accumulated 8 additional general-medical hospitalizations and >50 visits to hospital EDs and urgent care facilities. These hospitalizations typically followed arguments at home, strained family dynamics, and not feeling wanted. Ms. D would ingest large quantities of prescription or over-the-counter medications as a way of coping, which often occurred while she was residing in a step-down facility after hospital discharge.
[polldaddy:10528342]
The authors’ observations
The treatment team decided to transition Ms. D to an LTSR with full continuum of treatment. While some clinicians might be concerned with potential iatrogenic harm of LTSR placement and might instead recommend less restrictive residential support and an IOP. However, in Ms. D’s case, her numerous admissions to EDs, urgent care facilities, and medical and psychiatric hospitals, her failed step-down facility placements, and her family conflicts and poor dynamics limited the efficacy of her natural support system and drove the recommendation for an LTSR.
Previously, Ms. D’s experience with ACT services had centered on managing acute crises, with brief periods of stabilization that insufficiently engaged her in a consistent and meaningful treatment plan. Ms. D’s insurance company agreed to pay for the LTSR after lengthy discussions with the clinical leadership at the ACT program and the LTSR demonstrated that she was a high utilizer of health care services. They concluded that Ms. D’s stay at the LTSR would be less expensive than the frequent use of expensive hospital services and care.
EVALUATION A consensus on the diagnosis
During the first few weeks of Ms. D’s admission to the LTSR, the treatment team takes a thorough history and reviews her medical records, which they obtained from several past inpatient admissions and therapists who previously treated Ms. D. The team also collects collateral information from Ms. D’s family members. Based on this information, interviews, and composite behavioral observations from the first few weeks of Ms. D’s time at the LTSR, the psychiatrists and treatment team at the LTSR and ACT program determine that Ms. D meets the criteria for a primary diagnosis of BPD. Previous discharge diagnoses of schizoaffective disorder–bipolar type (Table 11), schizophrenia, or bipolar disorder could not be affirmed.

Continue to: The authors' observations
The authors’ observations
During Ms. D’s LTSR placement, it became clear that her self-harm behaviors and numerous visits to the ED and urgent care facilities involved severe and intense emotional dysregulation and maladaptive behaviors. These behaviors had developed over time in response to acute stressors and past trauma, and not as a result of a sustained mood or psychotic disorder. Before her LTSR placement, Ms. D was unable to use more adaptive coping skills, such as skills building, learning, and coaching. Ms. D typically “thrived” with medical attention in the ED or hospital, and once the stressor dissipated, she was discharged back to the same stressful living environment associated with her maladaptive coping.
Table 2 outlines the rationale for long-term residential treatment for Ms. D.

TREATMENT Developing more effective skills
Bolstered by a clearer diagnostic formulation of BPD, Ms. D’s initial treatment goals at the LTSR include developing effective skills (eg, mindfulness, interpersonal effectiveness, emotion regulation, and distress tolerance) to cope with family conflicts and other stressors while she is outside the facility on a therapeutic pass. Ms. D’s treatment focuses on skills learning and coaching, and behavior chain analyses, which are conducted by her therapist from the ACT program.
Ms. D remains clinically stable throughout her LTSR placement, and benefits from ongoing skills building and learning, coaching, and community integration efforts.
[polldaddy:10528348]
The authors’ observations
Several systematic reviews2-5 have found that there is a lack of high-quality evidence for the use of various psychotropic medications for patients with BPD, yet polypharmacy is common. Many patients with BPD receive ≥2 medications and >25% of patients receive ≥4 medications, typically for prolonged periods. Stoffers et al4 suggested that FGAs and antidepressants have marginal effects of for patients with BPD; however, their use cannot be ruled out because they may be helpful for comorbid symptoms that are often observed in patients with BPD. There is better evidence for SGAs, mood stabilizers, and omega-3 fatty acids; however, most effect estimates were based on single studies, and there is minimal data on long-term use of these agents.4
Continue to: A recent review highlighted...
A recent review highlighted 2 trends in medication prescribing for individuals with BPD3:
- a decrease in the use of benzodiazepines and antidepressants
- an increase in or preference for mood stabilizers and SGAs, especially valproate and quetiapine.
In terms of which medications can be used to target specific symptoms, the same researchers also noted from previous studies3:
- The prior use of SSRIs to target affective dysregulation, anxiety, and impulsive- behavior dyscontrol
- mood stabilizers (notably anticonvulsants) and SGAs to target “core symptoms” of BPD, including affective dysregulation, impulsive-behavioral dyscontrol, and cognitive-perceptual distortions
- omega-3 fatty acids for mood stabilization, impulsive-behavior dyscontrol, and possibly to reduce self-harm behaviors.
TREATMENT Medication adjustments
The treatment team reviews the lack of evidence for the long-term use of psychotropic medications in the treatment of BPD with Ms. D and her relatives,2-5 and develops a medication regimen that is clinically appropriate for managing the symptoms of BPD, while also being mindful of adverse effects.
When Ms. D was admitted to the LTSR from the hospital, her psychotropic medication regimen included haloperidol, 150 mg IM every month; olanzapine, 20 mg at bedtime; benztropine, 1 mg twice daily; and melatonin, 9 mg at bedtime.
Following discussions with Ms. D and her older sister, the team initiates a taper of olanzapine because of metabolic concerns. Ms. D has gained >40 lb while receiving this medication and had hypertension. Olanzapine was tapered and discontinued over the course of 3 months with no reemergence of sustained mood or psychotic symptoms (Table 3). During this period, Ms. D also participates in dietary counselling, follows a portion-controlled regimen, and loses >30 lb. Her wellness plan focuses on nutrition and exercise to improve her overall physical health.

Continue to: Six months into her stay...
Six months into her stay at the LTSR, Ms. D remains clinically stable and is able to leave the LTSR placement to go on home passes. At this time, the team begins to taper the haloperidol long-acting injection. One month prior to discharge from the LTSR, haloperidol is discontinued entirely. The treatment team simultaneously tapers and discontinues benztropine. No recurrence of extrapyramidal symptoms is observed by staff or noted by the patient.
A treatment plan is developed to address Ms. D’s medical conditions, including hypothyroidism, GERD, and obesity. Ms. D does not appear to have difficulty sleeping at the LTSR, so melatonin is tapered by 3-mg decrements and stopped after 2 months. However, shortly thereafter, she develops insomnia, so a 3-mg dose is re-initiated, and her complaints abate. Her primary care physician discontinues hydrochlorothiazide, an antihypertensive medication.
Ms. D’s medication regimen consists of melatonin, 3 mg at bedtime; pantoprazole, 40 mg before breakfast, for GERD; senna, 8.6 mg at bedtime, and polyethylene glycol, 17 gm/d, for constipation; levothyroxine, 125 mcg/d, for hypothyroidism; metoprolol extended-release, 50 mg/d, for hypertension; and ferrous sulfate, 325 mg/d, for iron deficiency anemia.
OUTCOME Improved functioning
After 11 months at the LTSR, Ms. D is discharged home. She continues to receive outpatient services in the community through the ACT program, meeting with her therapist for cognitive-behavioral therapy, skills building and learning, and integration.
Approximately 9 months later, Ms. D is re-started on an SSRI (sertraline, 50 mg/d, which is increased to 100 mg/d 9 months later) to target symptoms of anxiety, which primarily manifest as excessive worrying. Hydroxyzine, 50 mg 3 times daily as needed, is added to this regimen, for breakthrough anxiety symptoms. Hydroxyzine is prescribed instead of a benzodiazepine to avoid potential addiction and abuse.
Continue to: Oral ziprasidone...
Oral ziprasidone, 20 mg/d twice daily, is initiated during 2 brief inpatient psychiatric admissions; however, it is successfully tapered within 1 week of discharge, in partnership with the ACT program.
In the 23 months after her discharge, Ms. D has had 1 ED visit and 2 brief inpatient psychiatric hospitalizations, which is markedly fewer encounters than she had in the 2 years before her LTSR placement. She has also lost an additional 30 lb since her LTSR discharge through a healthy diet and exercise.
Ms. D is now considering transitioning to living independently in the community through a residential supported housing program.
Bottom Line
Psychotic symptoms in patients with borderline personality disorder (BPD) are typically fleeting and mostly occur in the context of intense interpersonal conflicts and real or imagined abandonment. Long-term structured residence placement for patients with BPD can allow for careful formulation of a treatment plan, and help patients gain effective skills to cope with difficult family dynamics and other stressors, with the ultimate goal of gradual community integration.
Related Resource
- National Education Alliance for Borderline Personality Disorder. https://www.borderlinepersonalitydisorder.org.
Drug Brand Names
Benztropine • Cogentin
Haloperidol • Haldol
Hydrochlorothiazide • Microzide, HydroDiuril
Hydroxyzine • Vistaril
Levothyroxine • Synthroid,
Metoprolol ER • Toprol XL
Olanzapine • Zyprexa
Pantoprazole • Protonix
Polyethylene glycol • MiraLax, Glycolax
Quetiapine • Seroquel
Senna • Senokot
Sertraline • Zoloft
Valproate • Depakene, Depakote
Ziprasidone • Geodon
CASE Frequent hospitalizations
Ms. D, age 26, presents to the emergency department (ED) after drinking a bottle of hand sanitizer in a suicide attempt. She is admitted to an inpatient psychiatric unit, where she spends 50 days, followed by a transfer to a step-down unit, where she spends 26 days. Upon discharge, her diagnosis is schizoaffective disorder–bipolar type.
Shortly before this, Ms. D had intentionally ingested 20 vitamin pills to “make her heart stop” after a conflict at home. After ingesting the pills, Ms. D presented to the ED, where she stated that if she were discharged, she would kill herself by taking “better pills.” She was then admitted to an inpatient psychiatric unit, where she spent 60 days before being moved to an extended-care step-down facility, where she resided for 42 days.
HISTORY A challenging past
Ms. D has a history of >25 psychiatric hospitalizations with varying discharge diagnoses, including schizophrenia, schizoaffective disorder, borderline personality disorder (BPD), and borderline intellectual functioning.
Ms. D was raised in a 2-parent home with 3 older half-brothers and 3 sisters. She was sexually assaulted by a cousin when she was 12. Ms. D recalls one event of self-injury/cutting behavior at age 15 after she was bullied by peers. Her family history is significant for schizophrenia (mother), alcohol use disorder (both parents), and bipolar disorder (sister). Her mother, who is now deceased, was admitted to state psychiatric hospitals for extended periods.
Her medication regimen has changed with nearly every hospitalization but generally has included ≥1 antipsychotic, a mood stabilizer, an antidepressant, and a benzodiazepine (often prescribed on an as-needed basis). Ms. D is obese and has difficulty sleeping, hypothyroidism, gastroesophageal reflux disease (GERD), hypertension, and iron deficiency anemia. She receives medications to manage each of these conditions.
Ms. D’s previous psychotic symptoms included auditory command hallucinations. These occurred under stressful circumstances, such as during severe family conflicts that often led to her feeling abandoned. She reported that the “voice” she heard was usually her own instructing her to “take pills.” There was no prior evidence of bizarre delusions, negative symptoms, or disorganized thoughts or speech.
During episodes of decompensation, Ms. D did not report symptoms of mania, sustained depressed mood, or anxiety, nor were these symptoms observed. Although Ms. D endorsed suicidal ideation with a plan, intent, and means, during several of her previous ED presentations, she told clinicians that her intent was not to end her life but rather to evoke concern in her family members.
Continue to: After her mother died...
After her mother died when Ms. D was 19, she began to have nightmares of wanting to hurt herself and others and began experiencing multiple hospitalizations. In 2010, Ms. D was referred to an assertive community treatment (ACT) program for individuals age 16 to 27 because of her inability to participate in traditional community-based services and her historical need for advanced services, in order to provide psychiatric care in the least restrictive means possible.
Despite receiving intensive ACT services, and in addition to the numerous inpatient psychiatric hospitalizations, over 7 years, Ms. D accumulated 8 additional general-medical hospitalizations and >50 visits to hospital EDs and urgent care facilities. These hospitalizations typically followed arguments at home, strained family dynamics, and not feeling wanted. Ms. D would ingest large quantities of prescription or over-the-counter medications as a way of coping, which often occurred while she was residing in a step-down facility after hospital discharge.
[polldaddy:10528342]
The authors’ observations
The treatment team decided to transition Ms. D to an LTSR with full continuum of treatment. While some clinicians might be concerned with potential iatrogenic harm of LTSR placement and might instead recommend less restrictive residential support and an IOP. However, in Ms. D’s case, her numerous admissions to EDs, urgent care facilities, and medical and psychiatric hospitals, her failed step-down facility placements, and her family conflicts and poor dynamics limited the efficacy of her natural support system and drove the recommendation for an LTSR.
Previously, Ms. D’s experience with ACT services had centered on managing acute crises, with brief periods of stabilization that insufficiently engaged her in a consistent and meaningful treatment plan. Ms. D’s insurance company agreed to pay for the LTSR after lengthy discussions with the clinical leadership at the ACT program and the LTSR demonstrated that she was a high utilizer of health care services. They concluded that Ms. D’s stay at the LTSR would be less expensive than the frequent use of expensive hospital services and care.
EVALUATION A consensus on the diagnosis
During the first few weeks of Ms. D’s admission to the LTSR, the treatment team takes a thorough history and reviews her medical records, which they obtained from several past inpatient admissions and therapists who previously treated Ms. D. The team also collects collateral information from Ms. D’s family members. Based on this information, interviews, and composite behavioral observations from the first few weeks of Ms. D’s time at the LTSR, the psychiatrists and treatment team at the LTSR and ACT program determine that Ms. D meets the criteria for a primary diagnosis of BPD. Previous discharge diagnoses of schizoaffective disorder–bipolar type (Table 11), schizophrenia, or bipolar disorder could not be affirmed.

Continue to: The authors' observations
The authors’ observations
During Ms. D’s LTSR placement, it became clear that her self-harm behaviors and numerous visits to the ED and urgent care facilities involved severe and intense emotional dysregulation and maladaptive behaviors. These behaviors had developed over time in response to acute stressors and past trauma, and not as a result of a sustained mood or psychotic disorder. Before her LTSR placement, Ms. D was unable to use more adaptive coping skills, such as skills building, learning, and coaching. Ms. D typically “thrived” with medical attention in the ED or hospital, and once the stressor dissipated, she was discharged back to the same stressful living environment associated with her maladaptive coping.
Table 2 outlines the rationale for long-term residential treatment for Ms. D.

TREATMENT Developing more effective skills
Bolstered by a clearer diagnostic formulation of BPD, Ms. D’s initial treatment goals at the LTSR include developing effective skills (eg, mindfulness, interpersonal effectiveness, emotion regulation, and distress tolerance) to cope with family conflicts and other stressors while she is outside the facility on a therapeutic pass. Ms. D’s treatment focuses on skills learning and coaching, and behavior chain analyses, which are conducted by her therapist from the ACT program.
Ms. D remains clinically stable throughout her LTSR placement, and benefits from ongoing skills building and learning, coaching, and community integration efforts.
[polldaddy:10528348]
The authors’ observations
Several systematic reviews2-5 have found that there is a lack of high-quality evidence for the use of various psychotropic medications for patients with BPD, yet polypharmacy is common. Many patients with BPD receive ≥2 medications and >25% of patients receive ≥4 medications, typically for prolonged periods. Stoffers et al4 suggested that FGAs and antidepressants have marginal effects of for patients with BPD; however, their use cannot be ruled out because they may be helpful for comorbid symptoms that are often observed in patients with BPD. There is better evidence for SGAs, mood stabilizers, and omega-3 fatty acids; however, most effect estimates were based on single studies, and there is minimal data on long-term use of these agents.4
Continue to: A recent review highlighted...
A recent review highlighted 2 trends in medication prescribing for individuals with BPD3:
- a decrease in the use of benzodiazepines and antidepressants
- an increase in or preference for mood stabilizers and SGAs, especially valproate and quetiapine.
In terms of which medications can be used to target specific symptoms, the same researchers also noted from previous studies3:
- The prior use of SSRIs to target affective dysregulation, anxiety, and impulsive- behavior dyscontrol
- mood stabilizers (notably anticonvulsants) and SGAs to target “core symptoms” of BPD, including affective dysregulation, impulsive-behavioral dyscontrol, and cognitive-perceptual distortions
- omega-3 fatty acids for mood stabilization, impulsive-behavior dyscontrol, and possibly to reduce self-harm behaviors.
TREATMENT Medication adjustments
The treatment team reviews the lack of evidence for the long-term use of psychotropic medications in the treatment of BPD with Ms. D and her relatives,2-5 and develops a medication regimen that is clinically appropriate for managing the symptoms of BPD, while also being mindful of adverse effects.
When Ms. D was admitted to the LTSR from the hospital, her psychotropic medication regimen included haloperidol, 150 mg IM every month; olanzapine, 20 mg at bedtime; benztropine, 1 mg twice daily; and melatonin, 9 mg at bedtime.
Following discussions with Ms. D and her older sister, the team initiates a taper of olanzapine because of metabolic concerns. Ms. D has gained >40 lb while receiving this medication and had hypertension. Olanzapine was tapered and discontinued over the course of 3 months with no reemergence of sustained mood or psychotic symptoms (Table 3). During this period, Ms. D also participates in dietary counselling, follows a portion-controlled regimen, and loses >30 lb. Her wellness plan focuses on nutrition and exercise to improve her overall physical health.

Continue to: Six months into her stay...
Six months into her stay at the LTSR, Ms. D remains clinically stable and is able to leave the LTSR placement to go on home passes. At this time, the team begins to taper the haloperidol long-acting injection. One month prior to discharge from the LTSR, haloperidol is discontinued entirely. The treatment team simultaneously tapers and discontinues benztropine. No recurrence of extrapyramidal symptoms is observed by staff or noted by the patient.
A treatment plan is developed to address Ms. D’s medical conditions, including hypothyroidism, GERD, and obesity. Ms. D does not appear to have difficulty sleeping at the LTSR, so melatonin is tapered by 3-mg decrements and stopped after 2 months. However, shortly thereafter, she develops insomnia, so a 3-mg dose is re-initiated, and her complaints abate. Her primary care physician discontinues hydrochlorothiazide, an antihypertensive medication.
Ms. D’s medication regimen consists of melatonin, 3 mg at bedtime; pantoprazole, 40 mg before breakfast, for GERD; senna, 8.6 mg at bedtime, and polyethylene glycol, 17 gm/d, for constipation; levothyroxine, 125 mcg/d, for hypothyroidism; metoprolol extended-release, 50 mg/d, for hypertension; and ferrous sulfate, 325 mg/d, for iron deficiency anemia.
OUTCOME Improved functioning
After 11 months at the LTSR, Ms. D is discharged home. She continues to receive outpatient services in the community through the ACT program, meeting with her therapist for cognitive-behavioral therapy, skills building and learning, and integration.
Approximately 9 months later, Ms. D is re-started on an SSRI (sertraline, 50 mg/d, which is increased to 100 mg/d 9 months later) to target symptoms of anxiety, which primarily manifest as excessive worrying. Hydroxyzine, 50 mg 3 times daily as needed, is added to this regimen, for breakthrough anxiety symptoms. Hydroxyzine is prescribed instead of a benzodiazepine to avoid potential addiction and abuse.
Continue to: Oral ziprasidone...
Oral ziprasidone, 20 mg/d twice daily, is initiated during 2 brief inpatient psychiatric admissions; however, it is successfully tapered within 1 week of discharge, in partnership with the ACT program.
In the 23 months after her discharge, Ms. D has had 1 ED visit and 2 brief inpatient psychiatric hospitalizations, which is markedly fewer encounters than she had in the 2 years before her LTSR placement. She has also lost an additional 30 lb since her LTSR discharge through a healthy diet and exercise.
Ms. D is now considering transitioning to living independently in the community through a residential supported housing program.
Bottom Line
Psychotic symptoms in patients with borderline personality disorder (BPD) are typically fleeting and mostly occur in the context of intense interpersonal conflicts and real or imagined abandonment. Long-term structured residence placement for patients with BPD can allow for careful formulation of a treatment plan, and help patients gain effective skills to cope with difficult family dynamics and other stressors, with the ultimate goal of gradual community integration.
Related Resource
- National Education Alliance for Borderline Personality Disorder. https://www.borderlinepersonalitydisorder.org.
Drug Brand Names
Benztropine • Cogentin
Haloperidol • Haldol
Hydrochlorothiazide • Microzide, HydroDiuril
Hydroxyzine • Vistaril
Levothyroxine • Synthroid,
Metoprolol ER • Toprol XL
Olanzapine • Zyprexa
Pantoprazole • Protonix
Polyethylene glycol • MiraLax, Glycolax
Quetiapine • Seroquel
Senna • Senokot
Sertraline • Zoloft
Valproate • Depakene, Depakote
Ziprasidone • Geodon
1. Diagnostic and Statistical Manual of Mental Disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
2. Hancock-Johnson E, Griffiths C, Picchioni M. A focused systematic review of pharmacological treatment for borderline personality disorder. CNS Drugs. 2017;31:345-356.
3. Starcevic V, Janca A. Pharmacotherapy of borderline personality disorder: replacing confusion with prudent pragmatism. Curr Opin Psychiatry. 2018;31(1):69-73.
4. Stoffers J, Völlm BA, Rücker G, et al. Pharmacological interventions for borderline personality disorder. Cochrane Database Syst Rev. 2010;6:CD005653. doi: 10.1002/14651858.CD005653.pub2.
5. Stoffers-Winterling JM, Storebo OJ, Völlm BA, et al. Pharmacological interventions for borderline personality disorder. Cochrane Database Syst Rev. 2018;3:CD012956. doi: 10.1002/14651858.CD012956.
1. Diagnostic and Statistical Manual of Mental Disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
2. Hancock-Johnson E, Griffiths C, Picchioni M. A focused systematic review of pharmacological treatment for borderline personality disorder. CNS Drugs. 2017;31:345-356.
3. Starcevic V, Janca A. Pharmacotherapy of borderline personality disorder: replacing confusion with prudent pragmatism. Curr Opin Psychiatry. 2018;31(1):69-73.
4. Stoffers J, Völlm BA, Rücker G, et al. Pharmacological interventions for borderline personality disorder. Cochrane Database Syst Rev. 2010;6:CD005653. doi: 10.1002/14651858.CD005653.pub2.
5. Stoffers-Winterling JM, Storebo OJ, Völlm BA, et al. Pharmacological interventions for borderline personality disorder. Cochrane Database Syst Rev. 2018;3:CD012956. doi: 10.1002/14651858.CD012956.
Polypharmacy in older adults
Mrs. B, age 66, presents to the emergency department with altered mental status, impaired gait, and tremors. Her son says she has had these symptoms for 3 days. He adds that she has been experiencing more knee pain than usual, and began taking naproxen, 220 mg twice daily, approximately 1 week ago.
Mrs. B’s medical history includes coronary artery disease (CAD), gastroesophageal reflux disease (GERD), hip fracture, osteoarthritis, and osteoporosis. She also has a history of insomnia and bipolar disorder.
Further, Mrs. B reports that 2 months ago, after watching a television program about mental health, she began taking ginkgo biloba, 60 mg/d by mouth for “memory,” and kava kava, 100 mg by mouth 3 times a day for “anxiety.” She did not tell her physician or pharmacist that she began using these supplements because she believes that “natural supplements wouldn’t affect her prescription medications.”
In addition to naproxen, gingko biloba, and kava kava, Mrs. B takes the following medications orally:
Mrs. B’s blood pressure is 132/74 mm Hg (at goal for her age) and her laboratory workup is unremarkable, except for the following results: serum creatinine level of 1.1 mg/dL, blood urea nitrogen/serum creatinine ratio of 40, and creatinine clearance rate of approximately 85 mL/min. An electrocardiogram shows normal sinus rhythm with a QTc of 489 ms. A lithium serum concentration level, drawn randomly, is 1.6 mEq/mL, suggesting lithium toxicity.
Although there is no consensus definition of polypharmacy, the most commonly referenced is concurrent use of ≥5 medications.1 During the last 2 decades, the percentage of adults who report receiving polypharmacy has markedly increased, from 8.2% to 15%.2 Geriatric patients, defined as those age >65, typically receive ≥5 prescription medications.2 Polypharmacy is associated with increased1:
- mortality
- adverse drug reactions
- falls
- length of hospital stay
- readmission rates.
Older adults are particularly vulnerable to the negative outcomes associated with polypharmacy because both increasing age and number of medications received are positively correlated with the risk of adverse events.3 However, the use of multiple medications may be clinically appropriate and necessary in patients with multiple chronic conditions. Recent research suggests that in addition to prescription medications, over-the-counter (OTC) medications and dietary supplements also pose polypharmacy concerns for geriatric patients.3 Here we discuss the risks of OTC medications and dietary supplements for older patients who may be receiving polypharmacy, and highlight specific agents and interactions to watch for in these individuals based on Mrs. B’s case.
Continue to: Factors that increase the risks of OTC medications
Factors that increase the risks of OTC medications
Although older adults account for only 15% of the present population, they purchase 40% of all OTC medications.4 These patients may inadvertently use OTC medications containing unnecessary or potentially harmful active ingredients because of unfamiliarity with the specific product, variability among products, or decreased health literacy. According to research presented at a 2010 Institute of Medicine Workshop on Safe Use Initiative and Health Literacy, many patients have a limited understanding of OTC medication indications and therapeutic duplication.5 For example, researchers found that almost 70% of patients thought they could take 2 products containing the same ingredient.5 Most patients were not able to determine the active ingredients or maximum daily dose of an OTC medication. Patients who were older, had lower literacy, or were African American were more likely to misunderstand medication labeling.5 Additional literature suggests that up to 20% of medical admissions can be attributed to adverse effects of OTC medications.6
Misconceptions regarding dietary supplements
The use of alternative and complementary medicine also is on the rise among geriatric patients.7-9 A recent study found that 70% of older adults in the United States consumed at least 1 dietary supplement in the past 30 days, with 29% consuming ≥4 natural products. Women consumed twice as many supplements as men.10
The perceived safety of natural medicines and dietary supplements is a common and potentially dangerous misconception.11 Because patients typically assume dietary supplements are safe, they often do not report their use to their clinicians, especially if clinicians do not explicitly ask them about supplement use.12 This is especially concerning because the FDA does not have the authority to review or regulate natural medicines or dietary supplements.13,14
With no requirements or regulations regarding quality control of these products, the obvious question is: “How do patients know what they’re ingesting?” The uncertainty regarding the true composition of dietary supplements is a cause for concern because federal regulations do not provide a standard way to verify the purity, quality, and safety. As a result, there is a dearth of information regarding drug–dietary supplement interactions and drug–dietary supplement–disease state interactions.8,15

What to watch for
Table 116-22 outlines OTC medication classes and potential medication and/or disease state interactions. Table 223-45 outlines potential interactions between select dietary supplements, medications, and disease states. Here we discuss several of these potential interactions based on the medications that Mrs. B was taking.
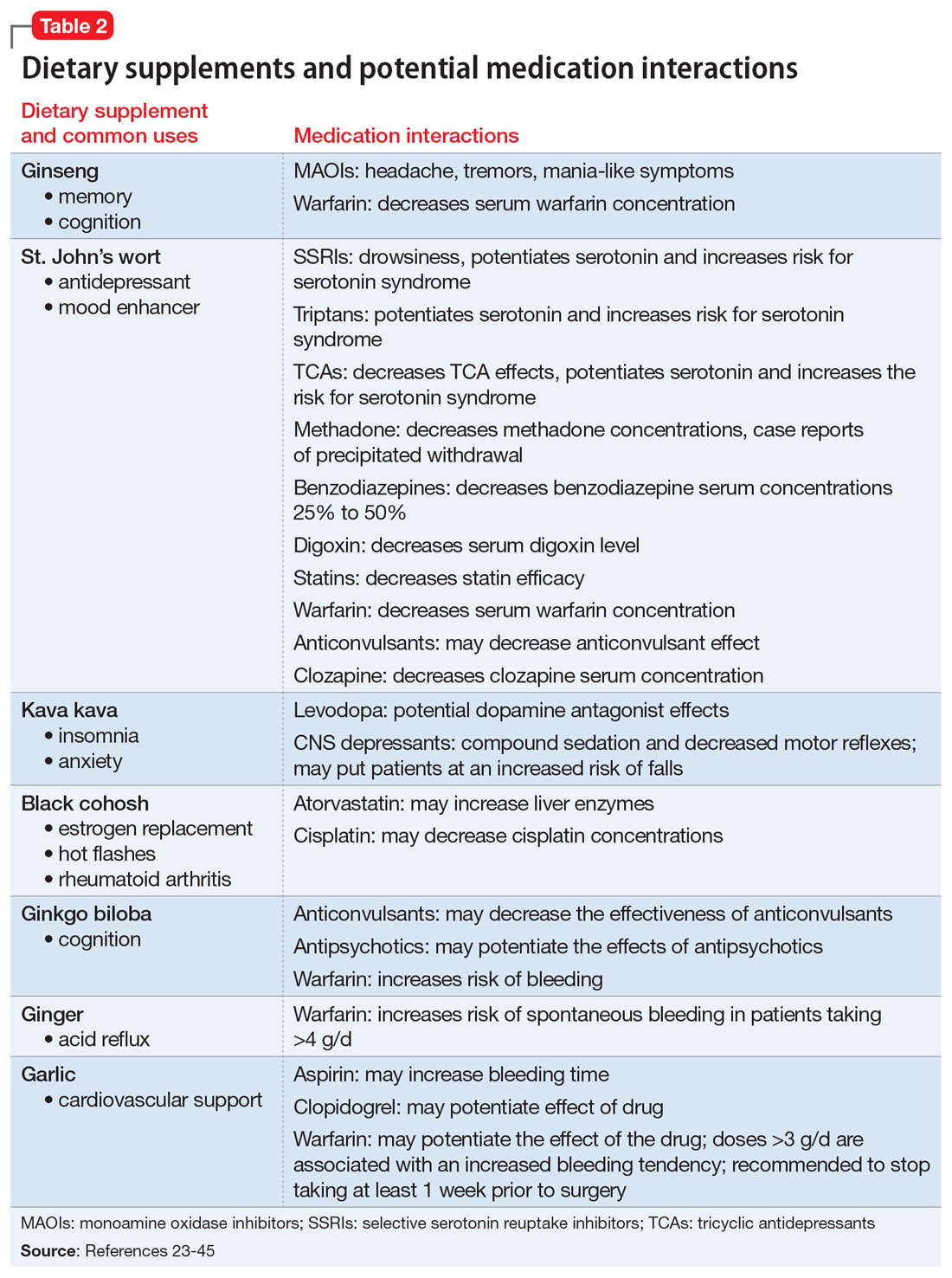
Continue to: Nonsteroidal anti-inflammatory drugs (NSAIDs)
Nonsteroidal anti-inflammatory drugs (NSAIDs). All OTC NSAIDs, except aspirin and salicylates, increase the risk for lithium toxicity by decreasing glomerular filtration rate and promoting lithium reabsorption in the kidneys.16 Additionally, NSAIDs increase the risk of developing gastric ulcers and may initiate or exacerbate GERD by suppressing gastric prostaglandin synthesis. Gastric prostaglandins facilitate the formation of a protective lipid-layer in the gastrointestinal (GI) tract.18,46-48 For Mrs. B, the naproxen she was taking resulted in lithium toxicity.
Ginkgo biloba is a plant used most commonly for its reported effect on memory. However, many drug–dietary supplement interactions have been associated with ginkgo biloba that may pose a problem for geriatric patients who receive polypharmacy.49 Mrs. B may have experienced decreased effectiveness of omeprazole and increased sedation or orthostatic hypotension with trazodone.
Kava kava is a natural sedative that can worsen cognition, increase the risk of falls, and potentially cause hepatotoxicity.50 The sedative effects of kava kava are thought to be a direct result of gamma-aminobutyric acid (GABA) modulation via the blockage of voltage-gated sodium ion channels.51 In Mrs. B’s case, when used in combination with diphenhydramine and trazodone, kava kava had the potential to further increase her risk of sedation and falls.
Gastroesophageal reflux disease medications. Older adults may be at an increased risk of GERD due to diseases that affect the esophagus and GI tract, such as diabetes, Parkinson’s disease, and Alzheimer’s disease. Medications may also contribute to gastric reflux by loosening the esophageal tone. Nitrates, benzodiazepines, anticholinergics, antidepressants, and lidocaine have been implicated in precipitating or exacerbating GERD.52
Numerous OTC products can be used to treat heartburn. Calcium carbonate supplements are typically recommended as first-line agents to treat occasional heartburn; histamine-2 receptor antagonists (H2RAs) and proton pump inhibitors (PPIs) generally are reserved for patients who experience heartburn more frequently.47 Per the American Geriatrics Society Beers Criteria for Potentially Inappropriate Medication Use in Older Adults, H2RAs were removed from the “avoid” list for patients with dementia or cognitive impairment due to a lack of strong evidence; however, H2RAs remain on the “avoid” list for patients with delirium.17 Low-dose H2RAs can be used safely in geriatric patients who have renal impairment. Although PPIs are not listedon the Beers Criteria, they have been associated with an increased risk of dementia, osteoporosis, and infections.53,54 There is robust evidence to support bone loss and fractures associated with chronic use of PPIs. However, the data linking PPI use and dementia is controversial due to multiple confounders identified in the studies, such as concomitant use of benzodiazepines.48 PPIs should be prescribed sparingly and judiciously in geriatric patients, and the need for continued PPI therapy should frequently be reassessed.48 Mrs. B’s use of omeprazole, a PPI, may put her at an increased risk for hip fracture compounded by an elevated fall risk associated with other medications she was taking.
Continue to: Trazodone
Trazodone causes sedative effects via anti-alpha 1 activity, which is thought to be responsible for orthostasis and may further increase the risk of falls.51 Mrs. B’s use of trazodone may have increased her risk of sedation and falls.
Antihistaminergic medications are associated with sedation, confusion, cognitive dysfunction, falls, and delirium in geriatric patients. Medications that act on histamine receptors can be particularly detrimental in the geriatric population because of their decreased clearance, smaller volume of distribution, and decreased tolerance.17,18
Anticholinergic medications. Although atropine and benztropine are widely recognized as anticholinergic agents, other medications, such as digoxin, paroxetine, and colchicine, also demonstrate anticholinergic activity that can cause problematic central and peripheral effects in geriatric patients.55 Central anticholinergic inhibition can lead to reduced cognitive function and impairments in attention and short-term memory. The peripheral effects of anticholinergic medications are similar to those of antihistamines and may include, but are not limited to, dry eyes and mouth via increased inhibition of acetylcholine-mediated muscle contraction of salivary glands.55 These effects can be compounded by the use of OTC medications that exhibit anticholinergic activity.
Diphenhydramine causes sedation through its activity on cholinergic and histaminergic receptors. Patients may not be aware that many OTC cough-and-cold combination products (such as NyQuil, Theraflu, etc.) and OTC nighttime analgesic products (such as Tylenol PM, Aleve PM, Motrin PM, etc.) contain diphenhydramine. For a geriatric patient, such as Mrs. B, diphenhydramine may increase the risk of falls and worsen cognition.
Teach patients to disclose everything they take
Polypharmacy can be detrimental to older patients’ health due to the increased risk of toxicity caused by therapeutic duplication, drug–drug interactions, and drug-disease interactions. Most patients are unable to navigate the nuances of medication indications, maximum dosages, and therapeutic duplications. Older adults frequently take OTC medications and have the greatest risk of developing adverse effects from these medications due to decreased renal and hepatic clearance, increased drug sensitivity, and decreased volume of distribution. Dietary supplements pose a unique risk because they are not FDA-regulated and their purity, quality, and content cannot be verified. Educating patients and family members about the importance of reporting all their prescription medications, OTC medications, and dietary supplements to their pharmacists and clinicians is critical in order to identify and mitigate the risks associated with polypharmacy in geriatric patients.
Continue to: CASE
CASE CONTINUED
Mrs. B is diagnosed with lithium toxicity due to a drug–drug interaction with naproxen. Her lithium is held, and IV fluids are administered. Her symptoms resolve over the next few days. Mrs. B and her son are taught about the interaction between lithium and NSAIDs, and she is counseled to avoid all OTC NSAIDs other than aspirin. Her clinician recommends taking acetaminophen because it will not interact with her medications and is the recommended OTC treatment for mild or moderate pain in geriatric patients.17,56
Next, the clinician addresses Mrs. B’s GERD. Although Mrs. B had been taking PPIs twice daily, her physician recommends decreasing the omeprazole frequency to once daily to minimize adverse effects and pill burden. She also decreases Mrs. B’s aspirin from 325 to 81 mg/d because evidence suggests that when used to prevent CAD, lower-dose aspirin is effective as high-dose aspirin and has fewer adverse effects.57 Finally, she advises Mrs. B to stop taking ginkgo biloba and kava kava and to always check with her primary care physician or pharmacist before beginning any new medication, dietary supplement, or vitamin.
Mrs. B agrees to first check with her clinicians before following advice from mass media. A follow-up appointment is scheduled for 2 weeks to assess renal function, a lithium serum concentration, and adherence to her simplified medication regimen.
Related Resources
- US Department of Health and Human Services. National Institutes of Health. MedlinePlus. Herbs and supplements. https://medlineplus.gov/druginfo/herb_All.html.
- US Department of Health and Human Services. National Center for Complementary and Integrative Health. https://nccih.nih.gov/.
Drug Brand Names
Atorvastatin • Lipitor
Atropine • Atropen
Benztropine • Cogentin
Clozapine • Clozaril
Clopidogrel • Plavix
Colchicine • Colcrys, Gloperba
Digoxin • Cardoxin, Digitek
Lidocaine • Lidoderm, Xylocaine Viscous
Lithium • Eskalith, Lithobid
Methadone • Methadose
Morphine • Kadian, Morphabond
Paroxetine • Paxil
Trazodone • Desyrel
Warfarin • Coumadin, Jantoven
1. Masnoon N, Shakib S, Kalisch-Ellett, et al. What is polypharmacy? A systematic review of definitions. BMC Geriatr. 2017;17:230.
2. Kantor ED, Rehm CD, Haas JS, et al. Trends in prescription drug use among adults in the United States from 1999-2012. JAMA. 2015;314(17):1818-1831.
3. Maher RL, Hanlon J, Hajjar ER. Clinical consequences of polypharmacy in elderly. Expert Opin Drug Saf. 2014;13(1):57-65.
4. Maiese DR. Healthy People 2010-leading health indicators for women. Womens Health Issues. 2002;12(4):155-164.
5. National Academy of Sciences. Institute of Medicine (US) Roundtable on Health Literacy. The Safe Use Initiative and Health Literacy: workshop summary. https://www.ncbi.nlm.nih.gov/books/NBK209756/. Published 2010. Accessed January 22, 2020.
6. Caranasos GJ, Stewart RB, Cluff LE. Drug-induced illness leading to hospitalisation. JAMA. 1974;228(6):713-717.
7. Agbabiaka T. Prevalence of drug–herb and drug-supplement interactions in older adults: a cross-sectional survey. Br J Gen Pract. 2018;68(675):e711-e717. doi: 10.3399/bjgp18X699101.
8. Agbabiaka T, Wider B, Watson L, et al. Concurrent use of prescription drugs and herbal medicinal products in older adults: a systematic review. Drugs Aging. 2017;34(12):891-905.
9. de Souza Silva JE, Santos Souza CA, da Silva TB, et al. Use of herbal medicines by elderly patients: a systematic review. Arch Gerontol Geriatr. 2014;59(2):227-233.
10. Gahche J, Bailey RL, Potischman N, et al. Dietary supplement use was very high among older adults in the United States in 2011-2014. J Nutr. 2017;147(10):1968-1976.
11. Nisly NL, Gryzlak BM, Zimmerman MB et al. Dietary supplement polypharmacy: an unrecognized public health problem? Evid Based Complement Alternat Med. 2010;7(1):107-113.
12. Kennedy J, Wang CC, Wu CH. Patient disclosure about herb and supplement use among adults in the US. Evid Based Complement Alternat Med. 2008;5(4):451-456.
13. Dickinson A. History and overview of DSHEA. Fitoterapia. 2011;82(1):5-10.
14. Dietary Supplement Health and Education Act of 1994. Public Law 103-417,103rd Congress. https://www.congress.gov/bill/103rd-congress/senate-bill/784. Accessed February 20, 2020.
15. US Department of Health & Human Services. National Institute on Aging. Dietary supplements. https://www.nia.nih.gov/health/dietary-supplements. Reviewed November 30, 2017. Accessed January 22, 2020.
16. Ragheb M. The clinical significance of lithium-nonsteroidal. J Clin Psychopharmacol. 1990;10(5):350-354.
17. 2019 American Geriatrics Society Beers Criteria® Update Expert Panel. American Geriatrics Society 2019 Updated AGS Beers Criteria® for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2019;67(4):674-694.
18. Cho H, Myung J, Suh HS, et al. Antihistamine use and the risk of injurious falls or fracture in elderly patients: a systematic review and meta-analysis. Osteoporos Int. 2018;29(10):2163-2170.
19. Manlucu J, Tonelli M, Ray JG, et al. Dose-reducing H2 receptor antagonists in the presence of low glomerular filtration rate: a systematic review of the evidence. Nephrol Dial Transplant. 2005;20(11):2376-2384.
20. Sudafed [package insert]. Fort Washington, PA: McNeil Consumer Healthcare Division; 2018.
21. US National Library of Medicine. National Center for Biotechnology Information. PubChem Compound Summary: Dextromethorphan; CID=5360696. https://pubchem.ncbi.nlm.nih.gov/compound/5360696. Accessed January 22, 2020.
22. Hedya SA, Swoboda HD. Lithium toxicity. https://www.ncbi.nlm.nih.gov/books/NBK499992/. Updated August 14, 2019. Accessed January 22, 2020.
23. US Department of Health & Human Services. National Center for Complementary and Integrative Health. Herb-drug interactions: what the science says. https://www.nccih.nih.gov/health/providers/digest/herb-drug-interactions-science. Published September 2015. Accessed January 22, 2020.
24. Shader RI, Greenblatt DJ. Bees, ginseng and MAOIs revisited. J Clin Psychopharmacol. 1988;8(4):235.
25. Chua YT. Interaction between warfarin and Chinese herbal medicines. Singapore Med J. 2015;56(1):11-18.
26. Bonetto N, Santelli L, Battistin L, et al. Serotonin syndrome and rhabdomyolysis induced by concomitant use of triptans, fluoxetine and hypericum. Cephalalgia. 2007;27(12):1421-1423.
27. Henderson L, Yue QY, Bergquist C, et al. St John’s wort (Hypericum perforatum): drug interactions and clinical outcomes. Br J Clin Pharmacol. 2002;54(4):349-356.
28. Johne A, Schmider J, Brockmöller J, et al. Decreased plasma levels of amitriptyline and its metabolites on comedication with an extract from St John’s wort (Hypericum perforatum). J Clin Psychopharmacol. 2002;22(1):46-54.
29. Eich-Höchli D, Oppliger R, Golay KP, et al. Methadone maintenance treatment and St John’s wort: a case report. Pharmacopsychiatry. 2003;36(1):35-37.
30. Johne A, Brockmöller J, Bauer S, et al. Pharmacokinetic interaction of digoxin with an herbal extract from St John’s wort (Hypericum perforatum). Clin Pharmacol Ther. 1999;66(4):338-345.
31. Andrén L, Andreasson A, Eggertsen R. Interaction between a commercially available St John’s wort product (Movina) and atorvastatin in patients with hypercholesterolemia. Eur J Clin Pharmacol. 2007;63(10):913-916.
32. Van Strater AC. Interaction of St John’s wort (Hypericum perforatum) with clozapine. Int Clin Psychopharmacol. 2012;27(2):121-124.
33. Nöldner M, Chatterjee SS. Inhibition of haloperidol-induced catalepsy in rats by root extracts from Piper methysticum F. Phytomedicine. 1999;6(4):285-286.
34. Boerner RJ, Klement S. Attenuation of neuroleptic-induced extrapyramidal side effects by kava special extract WS 1490. Wien Med Wochenschr. 2004;154(21-22):508-510.
35. Schelosky L, Raffauf C, Jendroska K, et al. Kava and dopamine antagonism. J Neurol Neurosurg Psychiatry. 1995;58(5):639-640.
36. Singh YN. Potential for interaction of kava and St. John’s wort with drugs. J Ethnopharmacol. 2005;100(1-2):108-113.
37. Patel NM, Derkits R. Possible increase in liver enzymes secondary to atorvastatin and black cohosh administration. J Pharm Prac. 2007;20(4):341-346.
38. Rockwell S, Liu Y, Higgins SA. Alteration of the effects of cancer therapy agents on breast cancer cells by the herbal medicine black cohosh. Breast Cancer Res Treat. 2005;90(3):233-239.
39. Granger AS. Ginkgo biloba precipitating epileptic seizures. Age Ageing. 2001;30(6):523-525.
40. Mohutsky MA, Anderson GD, Miller JW, et al. Ginkgo biloba: evaluation of CYP2C9 drug interactions in vitro and in vivo. Am J Ther. 2006;13(1):24-31.
41. Zhang XY, Zhou DF, Zhang PY, et al. A double-blind, placebo controlled trial of extract of Ginkgo biloba added to haloperidol in treatment-resistant patients with schizophrenia. J Clin Psychiatry. 2001;62(11):878-883.
42. Atmaca M, Tezcan E, Kuloglu M, et al. The effect of extract of ginkgo biloba addition to olanzapine on therapeutic effect and antioxidant enzyme levels in patients with schizophrenia. Psychiatry Clin Neurosci. 2005;59(6):652-656.
43. Doruk A, Uzun O, Ozsahin A. A placebo-controlled study of extract of ginkgo biloba added to clozapine in patients with treatment-resistant schizophrenia. Int Clin Psychopharmacol. 2008;23(4):223-237.
44. Vaes LP. Interactions of warfarin with garlic, ginger, ginkgo, or ginseng: nature of the evidence. Ann Pharmacother. 2000;34(12):1478-1482.
45. Kanji S, Seely D, Yazdi F, et al. Interactions of commonly used dietary supplements with cardiovascular drugs: a systematic review. Syst Rev. 2012;1:26.
46. Wallace JL. Pathogenesis of NSAID-induced gastroduodenal mucosal injury. Best Pract Res Clin Gastroenterol. 2001;15(5):691-703.
47. Triadafilopoulos G, Sharma R. Features of symptomatic gastroesophageal reflux disease in elderly patients. Am J Gastroenterol. 1997;92(11):2007-2011.
48. Haastrup PF, Thompson W, Søndergaard J, et al. Side effects of long-term proton pump inhibitor use: a review. Basic Clin Pharmacol Toxicol. 2018;123(2):114-121.
49. Diamond BJ, Bailey MR. Ginkgo biloba: indications, mechanisms and safety. Psychiatr Clin N Am. 2013;36:73-83.
50. White CM. The pharmacology, pharmacokinetics, efficacy, and adverse events associated with kava. J Clin Pharmacol. 2018;58(11):1396-1405.
51. Gleitz J, Beile A, Peters T. (+/-)-Kavain inhibits veratridine-activated voltage-dependent Na(+)-channels in synaptosomes prepared from rat cerebral cortex. Neuropharmacology. 1995;34(9):1133-1138.
52. Kahrilas PJ. Gastroesophageal reflux disease and its complications. In: Feldman M, ed. Sleisenger & Fordtran’s Gastrointestinal and Liver Disease. 6th ed. Philadelphia, PA: WB Saunders Company; 1998:498-516.
53. Haenisch B, von Holt K, Wiese B, et al. Risk of dementia in elderly patients with the use of proton pump inhibitors. Eur Arch Psychiatry Clin Neurosci. 2015;265(5):419-428.
54. Sheen E, Triadafilopoulos G. Adverse effects of long-term proton pump inhibitor therapy. Dig Dis Sci. 2011;56(4):931-950.
55. Pitkälä KH, Suominen MH, Bell JS, et al. Herbal medications and other dietary supplements. A clinical review for physicians caring for older people. Ann Medicine. 2016;48(8):586-602.
56. American College of Rheumatology Subcommittee on Osteoarthritis Guidelines. Recommendations for the medical management of osteoarthritis of the hip and knee: 2000 update. Arthritis Rheum. 2000;43(9):1905-1915.
57. Vandvik PO, Lincoff AM, Core JM, et al. Primary and secondary prevention of cardiovascular disease: Antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141(2 Suppl):e637S-e668S. doi: 10.1378/chest.11-2306.
Mrs. B, age 66, presents to the emergency department with altered mental status, impaired gait, and tremors. Her son says she has had these symptoms for 3 days. He adds that she has been experiencing more knee pain than usual, and began taking naproxen, 220 mg twice daily, approximately 1 week ago.
Mrs. B’s medical history includes coronary artery disease (CAD), gastroesophageal reflux disease (GERD), hip fracture, osteoarthritis, and osteoporosis. She also has a history of insomnia and bipolar disorder.
Further, Mrs. B reports that 2 months ago, after watching a television program about mental health, she began taking ginkgo biloba, 60 mg/d by mouth for “memory,” and kava kava, 100 mg by mouth 3 times a day for “anxiety.” She did not tell her physician or pharmacist that she began using these supplements because she believes that “natural supplements wouldn’t affect her prescription medications.”
In addition to naproxen, gingko biloba, and kava kava, Mrs. B takes the following medications orally:
Mrs. B’s blood pressure is 132/74 mm Hg (at goal for her age) and her laboratory workup is unremarkable, except for the following results: serum creatinine level of 1.1 mg/dL, blood urea nitrogen/serum creatinine ratio of 40, and creatinine clearance rate of approximately 85 mL/min. An electrocardiogram shows normal sinus rhythm with a QTc of 489 ms. A lithium serum concentration level, drawn randomly, is 1.6 mEq/mL, suggesting lithium toxicity.
Although there is no consensus definition of polypharmacy, the most commonly referenced is concurrent use of ≥5 medications.1 During the last 2 decades, the percentage of adults who report receiving polypharmacy has markedly increased, from 8.2% to 15%.2 Geriatric patients, defined as those age >65, typically receive ≥5 prescription medications.2 Polypharmacy is associated with increased1:
- mortality
- adverse drug reactions
- falls
- length of hospital stay
- readmission rates.
Older adults are particularly vulnerable to the negative outcomes associated with polypharmacy because both increasing age and number of medications received are positively correlated with the risk of adverse events.3 However, the use of multiple medications may be clinically appropriate and necessary in patients with multiple chronic conditions. Recent research suggests that in addition to prescription medications, over-the-counter (OTC) medications and dietary supplements also pose polypharmacy concerns for geriatric patients.3 Here we discuss the risks of OTC medications and dietary supplements for older patients who may be receiving polypharmacy, and highlight specific agents and interactions to watch for in these individuals based on Mrs. B’s case.
Continue to: Factors that increase the risks of OTC medications
Factors that increase the risks of OTC medications
Although older adults account for only 15% of the present population, they purchase 40% of all OTC medications.4 These patients may inadvertently use OTC medications containing unnecessary or potentially harmful active ingredients because of unfamiliarity with the specific product, variability among products, or decreased health literacy. According to research presented at a 2010 Institute of Medicine Workshop on Safe Use Initiative and Health Literacy, many patients have a limited understanding of OTC medication indications and therapeutic duplication.5 For example, researchers found that almost 70% of patients thought they could take 2 products containing the same ingredient.5 Most patients were not able to determine the active ingredients or maximum daily dose of an OTC medication. Patients who were older, had lower literacy, or were African American were more likely to misunderstand medication labeling.5 Additional literature suggests that up to 20% of medical admissions can be attributed to adverse effects of OTC medications.6
Misconceptions regarding dietary supplements
The use of alternative and complementary medicine also is on the rise among geriatric patients.7-9 A recent study found that 70% of older adults in the United States consumed at least 1 dietary supplement in the past 30 days, with 29% consuming ≥4 natural products. Women consumed twice as many supplements as men.10
The perceived safety of natural medicines and dietary supplements is a common and potentially dangerous misconception.11 Because patients typically assume dietary supplements are safe, they often do not report their use to their clinicians, especially if clinicians do not explicitly ask them about supplement use.12 This is especially concerning because the FDA does not have the authority to review or regulate natural medicines or dietary supplements.13,14
With no requirements or regulations regarding quality control of these products, the obvious question is: “How do patients know what they’re ingesting?” The uncertainty regarding the true composition of dietary supplements is a cause for concern because federal regulations do not provide a standard way to verify the purity, quality, and safety. As a result, there is a dearth of information regarding drug–dietary supplement interactions and drug–dietary supplement–disease state interactions.8,15

What to watch for
Table 116-22 outlines OTC medication classes and potential medication and/or disease state interactions. Table 223-45 outlines potential interactions between select dietary supplements, medications, and disease states. Here we discuss several of these potential interactions based on the medications that Mrs. B was taking.

Continue to: Nonsteroidal anti-inflammatory drugs (NSAIDs)
Nonsteroidal anti-inflammatory drugs (NSAIDs). All OTC NSAIDs, except aspirin and salicylates, increase the risk for lithium toxicity by decreasing glomerular filtration rate and promoting lithium reabsorption in the kidneys.16 Additionally, NSAIDs increase the risk of developing gastric ulcers and may initiate or exacerbate GERD by suppressing gastric prostaglandin synthesis. Gastric prostaglandins facilitate the formation of a protective lipid-layer in the gastrointestinal (GI) tract.18,46-48 For Mrs. B, the naproxen she was taking resulted in lithium toxicity.
Ginkgo biloba is a plant used most commonly for its reported effect on memory. However, many drug–dietary supplement interactions have been associated with ginkgo biloba that may pose a problem for geriatric patients who receive polypharmacy.49 Mrs. B may have experienced decreased effectiveness of omeprazole and increased sedation or orthostatic hypotension with trazodone.
Kava kava is a natural sedative that can worsen cognition, increase the risk of falls, and potentially cause hepatotoxicity.50 The sedative effects of kava kava are thought to be a direct result of gamma-aminobutyric acid (GABA) modulation via the blockage of voltage-gated sodium ion channels.51 In Mrs. B’s case, when used in combination with diphenhydramine and trazodone, kava kava had the potential to further increase her risk of sedation and falls.
Gastroesophageal reflux disease medications. Older adults may be at an increased risk of GERD due to diseases that affect the esophagus and GI tract, such as diabetes, Parkinson’s disease, and Alzheimer’s disease. Medications may also contribute to gastric reflux by loosening the esophageal tone. Nitrates, benzodiazepines, anticholinergics, antidepressants, and lidocaine have been implicated in precipitating or exacerbating GERD.52
Numerous OTC products can be used to treat heartburn. Calcium carbonate supplements are typically recommended as first-line agents to treat occasional heartburn; histamine-2 receptor antagonists (H2RAs) and proton pump inhibitors (PPIs) generally are reserved for patients who experience heartburn more frequently.47 Per the American Geriatrics Society Beers Criteria for Potentially Inappropriate Medication Use in Older Adults, H2RAs were removed from the “avoid” list for patients with dementia or cognitive impairment due to a lack of strong evidence; however, H2RAs remain on the “avoid” list for patients with delirium.17 Low-dose H2RAs can be used safely in geriatric patients who have renal impairment. Although PPIs are not listedon the Beers Criteria, they have been associated with an increased risk of dementia, osteoporosis, and infections.53,54 There is robust evidence to support bone loss and fractures associated with chronic use of PPIs. However, the data linking PPI use and dementia is controversial due to multiple confounders identified in the studies, such as concomitant use of benzodiazepines.48 PPIs should be prescribed sparingly and judiciously in geriatric patients, and the need for continued PPI therapy should frequently be reassessed.48 Mrs. B’s use of omeprazole, a PPI, may put her at an increased risk for hip fracture compounded by an elevated fall risk associated with other medications she was taking.
Continue to: Trazodone
Trazodone causes sedative effects via anti-alpha 1 activity, which is thought to be responsible for orthostasis and may further increase the risk of falls.51 Mrs. B’s use of trazodone may have increased her risk of sedation and falls.
Antihistaminergic medications are associated with sedation, confusion, cognitive dysfunction, falls, and delirium in geriatric patients. Medications that act on histamine receptors can be particularly detrimental in the geriatric population because of their decreased clearance, smaller volume of distribution, and decreased tolerance.17,18
Anticholinergic medications. Although atropine and benztropine are widely recognized as anticholinergic agents, other medications, such as digoxin, paroxetine, and colchicine, also demonstrate anticholinergic activity that can cause problematic central and peripheral effects in geriatric patients.55 Central anticholinergic inhibition can lead to reduced cognitive function and impairments in attention and short-term memory. The peripheral effects of anticholinergic medications are similar to those of antihistamines and may include, but are not limited to, dry eyes and mouth via increased inhibition of acetylcholine-mediated muscle contraction of salivary glands.55 These effects can be compounded by the use of OTC medications that exhibit anticholinergic activity.
Diphenhydramine causes sedation through its activity on cholinergic and histaminergic receptors. Patients may not be aware that many OTC cough-and-cold combination products (such as NyQuil, Theraflu, etc.) and OTC nighttime analgesic products (such as Tylenol PM, Aleve PM, Motrin PM, etc.) contain diphenhydramine. For a geriatric patient, such as Mrs. B, diphenhydramine may increase the risk of falls and worsen cognition.
Teach patients to disclose everything they take
Polypharmacy can be detrimental to older patients’ health due to the increased risk of toxicity caused by therapeutic duplication, drug–drug interactions, and drug-disease interactions. Most patients are unable to navigate the nuances of medication indications, maximum dosages, and therapeutic duplications. Older adults frequently take OTC medications and have the greatest risk of developing adverse effects from these medications due to decreased renal and hepatic clearance, increased drug sensitivity, and decreased volume of distribution. Dietary supplements pose a unique risk because they are not FDA-regulated and their purity, quality, and content cannot be verified. Educating patients and family members about the importance of reporting all their prescription medications, OTC medications, and dietary supplements to their pharmacists and clinicians is critical in order to identify and mitigate the risks associated with polypharmacy in geriatric patients.
Continue to: CASE
CASE CONTINUED
Mrs. B is diagnosed with lithium toxicity due to a drug–drug interaction with naproxen. Her lithium is held, and IV fluids are administered. Her symptoms resolve over the next few days. Mrs. B and her son are taught about the interaction between lithium and NSAIDs, and she is counseled to avoid all OTC NSAIDs other than aspirin. Her clinician recommends taking acetaminophen because it will not interact with her medications and is the recommended OTC treatment for mild or moderate pain in geriatric patients.17,56
Next, the clinician addresses Mrs. B’s GERD. Although Mrs. B had been taking PPIs twice daily, her physician recommends decreasing the omeprazole frequency to once daily to minimize adverse effects and pill burden. She also decreases Mrs. B’s aspirin from 325 to 81 mg/d because evidence suggests that when used to prevent CAD, lower-dose aspirin is effective as high-dose aspirin and has fewer adverse effects.57 Finally, she advises Mrs. B to stop taking ginkgo biloba and kava kava and to always check with her primary care physician or pharmacist before beginning any new medication, dietary supplement, or vitamin.
Mrs. B agrees to first check with her clinicians before following advice from mass media. A follow-up appointment is scheduled for 2 weeks to assess renal function, a lithium serum concentration, and adherence to her simplified medication regimen.
Related Resources
- US Department of Health and Human Services. National Institutes of Health. MedlinePlus. Herbs and supplements. https://medlineplus.gov/druginfo/herb_All.html.
- US Department of Health and Human Services. National Center for Complementary and Integrative Health. https://nccih.nih.gov/.
Drug Brand Names
Atorvastatin • Lipitor
Atropine • Atropen
Benztropine • Cogentin
Clozapine • Clozaril
Clopidogrel • Plavix
Colchicine • Colcrys, Gloperba
Digoxin • Cardoxin, Digitek
Lidocaine • Lidoderm, Xylocaine Viscous
Lithium • Eskalith, Lithobid
Methadone • Methadose
Morphine • Kadian, Morphabond
Paroxetine • Paxil
Trazodone • Desyrel
Warfarin • Coumadin, Jantoven
Mrs. B, age 66, presents to the emergency department with altered mental status, impaired gait, and tremors. Her son says she has had these symptoms for 3 days. He adds that she has been experiencing more knee pain than usual, and began taking naproxen, 220 mg twice daily, approximately 1 week ago.
Mrs. B’s medical history includes coronary artery disease (CAD), gastroesophageal reflux disease (GERD), hip fracture, osteoarthritis, and osteoporosis. She also has a history of insomnia and bipolar disorder.
Further, Mrs. B reports that 2 months ago, after watching a television program about mental health, she began taking ginkgo biloba, 60 mg/d by mouth for “memory,” and kava kava, 100 mg by mouth 3 times a day for “anxiety.” She did not tell her physician or pharmacist that she began using these supplements because she believes that “natural supplements wouldn’t affect her prescription medications.”
In addition to naproxen, gingko biloba, and kava kava, Mrs. B takes the following medications orally:
Mrs. B’s blood pressure is 132/74 mm Hg (at goal for her age) and her laboratory workup is unremarkable, except for the following results: serum creatinine level of 1.1 mg/dL, blood urea nitrogen/serum creatinine ratio of 40, and creatinine clearance rate of approximately 85 mL/min. An electrocardiogram shows normal sinus rhythm with a QTc of 489 ms. A lithium serum concentration level, drawn randomly, is 1.6 mEq/mL, suggesting lithium toxicity.
Although there is no consensus definition of polypharmacy, the most commonly referenced is concurrent use of ≥5 medications.1 During the last 2 decades, the percentage of adults who report receiving polypharmacy has markedly increased, from 8.2% to 15%.2 Geriatric patients, defined as those age >65, typically receive ≥5 prescription medications.2 Polypharmacy is associated with increased1:
- mortality
- adverse drug reactions
- falls
- length of hospital stay
- readmission rates.
Older adults are particularly vulnerable to the negative outcomes associated with polypharmacy because both increasing age and number of medications received are positively correlated with the risk of adverse events.3 However, the use of multiple medications may be clinically appropriate and necessary in patients with multiple chronic conditions. Recent research suggests that in addition to prescription medications, over-the-counter (OTC) medications and dietary supplements also pose polypharmacy concerns for geriatric patients.3 Here we discuss the risks of OTC medications and dietary supplements for older patients who may be receiving polypharmacy, and highlight specific agents and interactions to watch for in these individuals based on Mrs. B’s case.
Continue to: Factors that increase the risks of OTC medications
Factors that increase the risks of OTC medications
Although older adults account for only 15% of the present population, they purchase 40% of all OTC medications.4 These patients may inadvertently use OTC medications containing unnecessary or potentially harmful active ingredients because of unfamiliarity with the specific product, variability among products, or decreased health literacy. According to research presented at a 2010 Institute of Medicine Workshop on Safe Use Initiative and Health Literacy, many patients have a limited understanding of OTC medication indications and therapeutic duplication.5 For example, researchers found that almost 70% of patients thought they could take 2 products containing the same ingredient.5 Most patients were not able to determine the active ingredients or maximum daily dose of an OTC medication. Patients who were older, had lower literacy, or were African American were more likely to misunderstand medication labeling.5 Additional literature suggests that up to 20% of medical admissions can be attributed to adverse effects of OTC medications.6
Misconceptions regarding dietary supplements
The use of alternative and complementary medicine also is on the rise among geriatric patients.7-9 A recent study found that 70% of older adults in the United States consumed at least 1 dietary supplement in the past 30 days, with 29% consuming ≥4 natural products. Women consumed twice as many supplements as men.10
The perceived safety of natural medicines and dietary supplements is a common and potentially dangerous misconception.11 Because patients typically assume dietary supplements are safe, they often do not report their use to their clinicians, especially if clinicians do not explicitly ask them about supplement use.12 This is especially concerning because the FDA does not have the authority to review or regulate natural medicines or dietary supplements.13,14
With no requirements or regulations regarding quality control of these products, the obvious question is: “How do patients know what they’re ingesting?” The uncertainty regarding the true composition of dietary supplements is a cause for concern because federal regulations do not provide a standard way to verify the purity, quality, and safety. As a result, there is a dearth of information regarding drug–dietary supplement interactions and drug–dietary supplement–disease state interactions.8,15

What to watch for
Table 116-22 outlines OTC medication classes and potential medication and/or disease state interactions. Table 223-45 outlines potential interactions between select dietary supplements, medications, and disease states. Here we discuss several of these potential interactions based on the medications that Mrs. B was taking.

Continue to: Nonsteroidal anti-inflammatory drugs (NSAIDs)
Nonsteroidal anti-inflammatory drugs (NSAIDs). All OTC NSAIDs, except aspirin and salicylates, increase the risk for lithium toxicity by decreasing glomerular filtration rate and promoting lithium reabsorption in the kidneys.16 Additionally, NSAIDs increase the risk of developing gastric ulcers and may initiate or exacerbate GERD by suppressing gastric prostaglandin synthesis. Gastric prostaglandins facilitate the formation of a protective lipid-layer in the gastrointestinal (GI) tract.18,46-48 For Mrs. B, the naproxen she was taking resulted in lithium toxicity.
Ginkgo biloba is a plant used most commonly for its reported effect on memory. However, many drug–dietary supplement interactions have been associated with ginkgo biloba that may pose a problem for geriatric patients who receive polypharmacy.49 Mrs. B may have experienced decreased effectiveness of omeprazole and increased sedation or orthostatic hypotension with trazodone.
Kava kava is a natural sedative that can worsen cognition, increase the risk of falls, and potentially cause hepatotoxicity.50 The sedative effects of kava kava are thought to be a direct result of gamma-aminobutyric acid (GABA) modulation via the blockage of voltage-gated sodium ion channels.51 In Mrs. B’s case, when used in combination with diphenhydramine and trazodone, kava kava had the potential to further increase her risk of sedation and falls.
Gastroesophageal reflux disease medications. Older adults may be at an increased risk of GERD due to diseases that affect the esophagus and GI tract, such as diabetes, Parkinson’s disease, and Alzheimer’s disease. Medications may also contribute to gastric reflux by loosening the esophageal tone. Nitrates, benzodiazepines, anticholinergics, antidepressants, and lidocaine have been implicated in precipitating or exacerbating GERD.52
Numerous OTC products can be used to treat heartburn. Calcium carbonate supplements are typically recommended as first-line agents to treat occasional heartburn; histamine-2 receptor antagonists (H2RAs) and proton pump inhibitors (PPIs) generally are reserved for patients who experience heartburn more frequently.47 Per the American Geriatrics Society Beers Criteria for Potentially Inappropriate Medication Use in Older Adults, H2RAs were removed from the “avoid” list for patients with dementia or cognitive impairment due to a lack of strong evidence; however, H2RAs remain on the “avoid” list for patients with delirium.17 Low-dose H2RAs can be used safely in geriatric patients who have renal impairment. Although PPIs are not listedon the Beers Criteria, they have been associated with an increased risk of dementia, osteoporosis, and infections.53,54 There is robust evidence to support bone loss and fractures associated with chronic use of PPIs. However, the data linking PPI use and dementia is controversial due to multiple confounders identified in the studies, such as concomitant use of benzodiazepines.48 PPIs should be prescribed sparingly and judiciously in geriatric patients, and the need for continued PPI therapy should frequently be reassessed.48 Mrs. B’s use of omeprazole, a PPI, may put her at an increased risk for hip fracture compounded by an elevated fall risk associated with other medications she was taking.
Continue to: Trazodone
Trazodone causes sedative effects via anti-alpha 1 activity, which is thought to be responsible for orthostasis and may further increase the risk of falls.51 Mrs. B’s use of trazodone may have increased her risk of sedation and falls.
Antihistaminergic medications are associated with sedation, confusion, cognitive dysfunction, falls, and delirium in geriatric patients. Medications that act on histamine receptors can be particularly detrimental in the geriatric population because of their decreased clearance, smaller volume of distribution, and decreased tolerance.17,18
Anticholinergic medications. Although atropine and benztropine are widely recognized as anticholinergic agents, other medications, such as digoxin, paroxetine, and colchicine, also demonstrate anticholinergic activity that can cause problematic central and peripheral effects in geriatric patients.55 Central anticholinergic inhibition can lead to reduced cognitive function and impairments in attention and short-term memory. The peripheral effects of anticholinergic medications are similar to those of antihistamines and may include, but are not limited to, dry eyes and mouth via increased inhibition of acetylcholine-mediated muscle contraction of salivary glands.55 These effects can be compounded by the use of OTC medications that exhibit anticholinergic activity.
Diphenhydramine causes sedation through its activity on cholinergic and histaminergic receptors. Patients may not be aware that many OTC cough-and-cold combination products (such as NyQuil, Theraflu, etc.) and OTC nighttime analgesic products (such as Tylenol PM, Aleve PM, Motrin PM, etc.) contain diphenhydramine. For a geriatric patient, such as Mrs. B, diphenhydramine may increase the risk of falls and worsen cognition.
Teach patients to disclose everything they take
Polypharmacy can be detrimental to older patients’ health due to the increased risk of toxicity caused by therapeutic duplication, drug–drug interactions, and drug-disease interactions. Most patients are unable to navigate the nuances of medication indications, maximum dosages, and therapeutic duplications. Older adults frequently take OTC medications and have the greatest risk of developing adverse effects from these medications due to decreased renal and hepatic clearance, increased drug sensitivity, and decreased volume of distribution. Dietary supplements pose a unique risk because they are not FDA-regulated and their purity, quality, and content cannot be verified. Educating patients and family members about the importance of reporting all their prescription medications, OTC medications, and dietary supplements to their pharmacists and clinicians is critical in order to identify and mitigate the risks associated with polypharmacy in geriatric patients.
Continue to: CASE
CASE CONTINUED
Mrs. B is diagnosed with lithium toxicity due to a drug–drug interaction with naproxen. Her lithium is held, and IV fluids are administered. Her symptoms resolve over the next few days. Mrs. B and her son are taught about the interaction between lithium and NSAIDs, and she is counseled to avoid all OTC NSAIDs other than aspirin. Her clinician recommends taking acetaminophen because it will not interact with her medications and is the recommended OTC treatment for mild or moderate pain in geriatric patients.17,56
Next, the clinician addresses Mrs. B’s GERD. Although Mrs. B had been taking PPIs twice daily, her physician recommends decreasing the omeprazole frequency to once daily to minimize adverse effects and pill burden. She also decreases Mrs. B’s aspirin from 325 to 81 mg/d because evidence suggests that when used to prevent CAD, lower-dose aspirin is effective as high-dose aspirin and has fewer adverse effects.57 Finally, she advises Mrs. B to stop taking ginkgo biloba and kava kava and to always check with her primary care physician or pharmacist before beginning any new medication, dietary supplement, or vitamin.
Mrs. B agrees to first check with her clinicians before following advice from mass media. A follow-up appointment is scheduled for 2 weeks to assess renal function, a lithium serum concentration, and adherence to her simplified medication regimen.
Related Resources
- US Department of Health and Human Services. National Institutes of Health. MedlinePlus. Herbs and supplements. https://medlineplus.gov/druginfo/herb_All.html.
- US Department of Health and Human Services. National Center for Complementary and Integrative Health. https://nccih.nih.gov/.
Drug Brand Names
Atorvastatin • Lipitor
Atropine • Atropen
Benztropine • Cogentin
Clozapine • Clozaril
Clopidogrel • Plavix
Colchicine • Colcrys, Gloperba
Digoxin • Cardoxin, Digitek
Lidocaine • Lidoderm, Xylocaine Viscous
Lithium • Eskalith, Lithobid
Methadone • Methadose
Morphine • Kadian, Morphabond
Paroxetine • Paxil
Trazodone • Desyrel
Warfarin • Coumadin, Jantoven
1. Masnoon N, Shakib S, Kalisch-Ellett, et al. What is polypharmacy? A systematic review of definitions. BMC Geriatr. 2017;17:230.
2. Kantor ED, Rehm CD, Haas JS, et al. Trends in prescription drug use among adults in the United States from 1999-2012. JAMA. 2015;314(17):1818-1831.
3. Maher RL, Hanlon J, Hajjar ER. Clinical consequences of polypharmacy in elderly. Expert Opin Drug Saf. 2014;13(1):57-65.
4. Maiese DR. Healthy People 2010-leading health indicators for women. Womens Health Issues. 2002;12(4):155-164.
5. National Academy of Sciences. Institute of Medicine (US) Roundtable on Health Literacy. The Safe Use Initiative and Health Literacy: workshop summary. https://www.ncbi.nlm.nih.gov/books/NBK209756/. Published 2010. Accessed January 22, 2020.
6. Caranasos GJ, Stewart RB, Cluff LE. Drug-induced illness leading to hospitalisation. JAMA. 1974;228(6):713-717.
7. Agbabiaka T. Prevalence of drug–herb and drug-supplement interactions in older adults: a cross-sectional survey. Br J Gen Pract. 2018;68(675):e711-e717. doi: 10.3399/bjgp18X699101.
8. Agbabiaka T, Wider B, Watson L, et al. Concurrent use of prescription drugs and herbal medicinal products in older adults: a systematic review. Drugs Aging. 2017;34(12):891-905.
9. de Souza Silva JE, Santos Souza CA, da Silva TB, et al. Use of herbal medicines by elderly patients: a systematic review. Arch Gerontol Geriatr. 2014;59(2):227-233.
10. Gahche J, Bailey RL, Potischman N, et al. Dietary supplement use was very high among older adults in the United States in 2011-2014. J Nutr. 2017;147(10):1968-1976.
11. Nisly NL, Gryzlak BM, Zimmerman MB et al. Dietary supplement polypharmacy: an unrecognized public health problem? Evid Based Complement Alternat Med. 2010;7(1):107-113.
12. Kennedy J, Wang CC, Wu CH. Patient disclosure about herb and supplement use among adults in the US. Evid Based Complement Alternat Med. 2008;5(4):451-456.
13. Dickinson A. History and overview of DSHEA. Fitoterapia. 2011;82(1):5-10.
14. Dietary Supplement Health and Education Act of 1994. Public Law 103-417,103rd Congress. https://www.congress.gov/bill/103rd-congress/senate-bill/784. Accessed February 20, 2020.
15. US Department of Health & Human Services. National Institute on Aging. Dietary supplements. https://www.nia.nih.gov/health/dietary-supplements. Reviewed November 30, 2017. Accessed January 22, 2020.
16. Ragheb M. The clinical significance of lithium-nonsteroidal. J Clin Psychopharmacol. 1990;10(5):350-354.
17. 2019 American Geriatrics Society Beers Criteria® Update Expert Panel. American Geriatrics Society 2019 Updated AGS Beers Criteria® for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2019;67(4):674-694.
18. Cho H, Myung J, Suh HS, et al. Antihistamine use and the risk of injurious falls or fracture in elderly patients: a systematic review and meta-analysis. Osteoporos Int. 2018;29(10):2163-2170.
19. Manlucu J, Tonelli M, Ray JG, et al. Dose-reducing H2 receptor antagonists in the presence of low glomerular filtration rate: a systematic review of the evidence. Nephrol Dial Transplant. 2005;20(11):2376-2384.
20. Sudafed [package insert]. Fort Washington, PA: McNeil Consumer Healthcare Division; 2018.
21. US National Library of Medicine. National Center for Biotechnology Information. PubChem Compound Summary: Dextromethorphan; CID=5360696. https://pubchem.ncbi.nlm.nih.gov/compound/5360696. Accessed January 22, 2020.
22. Hedya SA, Swoboda HD. Lithium toxicity. https://www.ncbi.nlm.nih.gov/books/NBK499992/. Updated August 14, 2019. Accessed January 22, 2020.
23. US Department of Health & Human Services. National Center for Complementary and Integrative Health. Herb-drug interactions: what the science says. https://www.nccih.nih.gov/health/providers/digest/herb-drug-interactions-science. Published September 2015. Accessed January 22, 2020.
24. Shader RI, Greenblatt DJ. Bees, ginseng and MAOIs revisited. J Clin Psychopharmacol. 1988;8(4):235.
25. Chua YT. Interaction between warfarin and Chinese herbal medicines. Singapore Med J. 2015;56(1):11-18.
26. Bonetto N, Santelli L, Battistin L, et al. Serotonin syndrome and rhabdomyolysis induced by concomitant use of triptans, fluoxetine and hypericum. Cephalalgia. 2007;27(12):1421-1423.
27. Henderson L, Yue QY, Bergquist C, et al. St John’s wort (Hypericum perforatum): drug interactions and clinical outcomes. Br J Clin Pharmacol. 2002;54(4):349-356.
28. Johne A, Schmider J, Brockmöller J, et al. Decreased plasma levels of amitriptyline and its metabolites on comedication with an extract from St John’s wort (Hypericum perforatum). J Clin Psychopharmacol. 2002;22(1):46-54.
29. Eich-Höchli D, Oppliger R, Golay KP, et al. Methadone maintenance treatment and St John’s wort: a case report. Pharmacopsychiatry. 2003;36(1):35-37.
30. Johne A, Brockmöller J, Bauer S, et al. Pharmacokinetic interaction of digoxin with an herbal extract from St John’s wort (Hypericum perforatum). Clin Pharmacol Ther. 1999;66(4):338-345.
31. Andrén L, Andreasson A, Eggertsen R. Interaction between a commercially available St John’s wort product (Movina) and atorvastatin in patients with hypercholesterolemia. Eur J Clin Pharmacol. 2007;63(10):913-916.
32. Van Strater AC. Interaction of St John’s wort (Hypericum perforatum) with clozapine. Int Clin Psychopharmacol. 2012;27(2):121-124.
33. Nöldner M, Chatterjee SS. Inhibition of haloperidol-induced catalepsy in rats by root extracts from Piper methysticum F. Phytomedicine. 1999;6(4):285-286.
34. Boerner RJ, Klement S. Attenuation of neuroleptic-induced extrapyramidal side effects by kava special extract WS 1490. Wien Med Wochenschr. 2004;154(21-22):508-510.
35. Schelosky L, Raffauf C, Jendroska K, et al. Kava and dopamine antagonism. J Neurol Neurosurg Psychiatry. 1995;58(5):639-640.
36. Singh YN. Potential for interaction of kava and St. John’s wort with drugs. J Ethnopharmacol. 2005;100(1-2):108-113.
37. Patel NM, Derkits R. Possible increase in liver enzymes secondary to atorvastatin and black cohosh administration. J Pharm Prac. 2007;20(4):341-346.
38. Rockwell S, Liu Y, Higgins SA. Alteration of the effects of cancer therapy agents on breast cancer cells by the herbal medicine black cohosh. Breast Cancer Res Treat. 2005;90(3):233-239.
39. Granger AS. Ginkgo biloba precipitating epileptic seizures. Age Ageing. 2001;30(6):523-525.
40. Mohutsky MA, Anderson GD, Miller JW, et al. Ginkgo biloba: evaluation of CYP2C9 drug interactions in vitro and in vivo. Am J Ther. 2006;13(1):24-31.
41. Zhang XY, Zhou DF, Zhang PY, et al. A double-blind, placebo controlled trial of extract of Ginkgo biloba added to haloperidol in treatment-resistant patients with schizophrenia. J Clin Psychiatry. 2001;62(11):878-883.
42. Atmaca M, Tezcan E, Kuloglu M, et al. The effect of extract of ginkgo biloba addition to olanzapine on therapeutic effect and antioxidant enzyme levels in patients with schizophrenia. Psychiatry Clin Neurosci. 2005;59(6):652-656.
43. Doruk A, Uzun O, Ozsahin A. A placebo-controlled study of extract of ginkgo biloba added to clozapine in patients with treatment-resistant schizophrenia. Int Clin Psychopharmacol. 2008;23(4):223-237.
44. Vaes LP. Interactions of warfarin with garlic, ginger, ginkgo, or ginseng: nature of the evidence. Ann Pharmacother. 2000;34(12):1478-1482.
45. Kanji S, Seely D, Yazdi F, et al. Interactions of commonly used dietary supplements with cardiovascular drugs: a systematic review. Syst Rev. 2012;1:26.
46. Wallace JL. Pathogenesis of NSAID-induced gastroduodenal mucosal injury. Best Pract Res Clin Gastroenterol. 2001;15(5):691-703.
47. Triadafilopoulos G, Sharma R. Features of symptomatic gastroesophageal reflux disease in elderly patients. Am J Gastroenterol. 1997;92(11):2007-2011.
48. Haastrup PF, Thompson W, Søndergaard J, et al. Side effects of long-term proton pump inhibitor use: a review. Basic Clin Pharmacol Toxicol. 2018;123(2):114-121.
49. Diamond BJ, Bailey MR. Ginkgo biloba: indications, mechanisms and safety. Psychiatr Clin N Am. 2013;36:73-83.
50. White CM. The pharmacology, pharmacokinetics, efficacy, and adverse events associated with kava. J Clin Pharmacol. 2018;58(11):1396-1405.
51. Gleitz J, Beile A, Peters T. (+/-)-Kavain inhibits veratridine-activated voltage-dependent Na(+)-channels in synaptosomes prepared from rat cerebral cortex. Neuropharmacology. 1995;34(9):1133-1138.
52. Kahrilas PJ. Gastroesophageal reflux disease and its complications. In: Feldman M, ed. Sleisenger & Fordtran’s Gastrointestinal and Liver Disease. 6th ed. Philadelphia, PA: WB Saunders Company; 1998:498-516.
53. Haenisch B, von Holt K, Wiese B, et al. Risk of dementia in elderly patients with the use of proton pump inhibitors. Eur Arch Psychiatry Clin Neurosci. 2015;265(5):419-428.
54. Sheen E, Triadafilopoulos G. Adverse effects of long-term proton pump inhibitor therapy. Dig Dis Sci. 2011;56(4):931-950.
55. Pitkälä KH, Suominen MH, Bell JS, et al. Herbal medications and other dietary supplements. A clinical review for physicians caring for older people. Ann Medicine. 2016;48(8):586-602.
56. American College of Rheumatology Subcommittee on Osteoarthritis Guidelines. Recommendations for the medical management of osteoarthritis of the hip and knee: 2000 update. Arthritis Rheum. 2000;43(9):1905-1915.
57. Vandvik PO, Lincoff AM, Core JM, et al. Primary and secondary prevention of cardiovascular disease: Antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141(2 Suppl):e637S-e668S. doi: 10.1378/chest.11-2306.
1. Masnoon N, Shakib S, Kalisch-Ellett, et al. What is polypharmacy? A systematic review of definitions. BMC Geriatr. 2017;17:230.
2. Kantor ED, Rehm CD, Haas JS, et al. Trends in prescription drug use among adults in the United States from 1999-2012. JAMA. 2015;314(17):1818-1831.
3. Maher RL, Hanlon J, Hajjar ER. Clinical consequences of polypharmacy in elderly. Expert Opin Drug Saf. 2014;13(1):57-65.
4. Maiese DR. Healthy People 2010-leading health indicators for women. Womens Health Issues. 2002;12(4):155-164.
5. National Academy of Sciences. Institute of Medicine (US) Roundtable on Health Literacy. The Safe Use Initiative and Health Literacy: workshop summary. https://www.ncbi.nlm.nih.gov/books/NBK209756/. Published 2010. Accessed January 22, 2020.
6. Caranasos GJ, Stewart RB, Cluff LE. Drug-induced illness leading to hospitalisation. JAMA. 1974;228(6):713-717.
7. Agbabiaka T. Prevalence of drug–herb and drug-supplement interactions in older adults: a cross-sectional survey. Br J Gen Pract. 2018;68(675):e711-e717. doi: 10.3399/bjgp18X699101.
8. Agbabiaka T, Wider B, Watson L, et al. Concurrent use of prescription drugs and herbal medicinal products in older adults: a systematic review. Drugs Aging. 2017;34(12):891-905.
9. de Souza Silva JE, Santos Souza CA, da Silva TB, et al. Use of herbal medicines by elderly patients: a systematic review. Arch Gerontol Geriatr. 2014;59(2):227-233.
10. Gahche J, Bailey RL, Potischman N, et al. Dietary supplement use was very high among older adults in the United States in 2011-2014. J Nutr. 2017;147(10):1968-1976.
11. Nisly NL, Gryzlak BM, Zimmerman MB et al. Dietary supplement polypharmacy: an unrecognized public health problem? Evid Based Complement Alternat Med. 2010;7(1):107-113.
12. Kennedy J, Wang CC, Wu CH. Patient disclosure about herb and supplement use among adults in the US. Evid Based Complement Alternat Med. 2008;5(4):451-456.
13. Dickinson A. History and overview of DSHEA. Fitoterapia. 2011;82(1):5-10.
14. Dietary Supplement Health and Education Act of 1994. Public Law 103-417,103rd Congress. https://www.congress.gov/bill/103rd-congress/senate-bill/784. Accessed February 20, 2020.
15. US Department of Health & Human Services. National Institute on Aging. Dietary supplements. https://www.nia.nih.gov/health/dietary-supplements. Reviewed November 30, 2017. Accessed January 22, 2020.
16. Ragheb M. The clinical significance of lithium-nonsteroidal. J Clin Psychopharmacol. 1990;10(5):350-354.
17. 2019 American Geriatrics Society Beers Criteria® Update Expert Panel. American Geriatrics Society 2019 Updated AGS Beers Criteria® for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2019;67(4):674-694.
18. Cho H, Myung J, Suh HS, et al. Antihistamine use and the risk of injurious falls or fracture in elderly patients: a systematic review and meta-analysis. Osteoporos Int. 2018;29(10):2163-2170.
19. Manlucu J, Tonelli M, Ray JG, et al. Dose-reducing H2 receptor antagonists in the presence of low glomerular filtration rate: a systematic review of the evidence. Nephrol Dial Transplant. 2005;20(11):2376-2384.
20. Sudafed [package insert]. Fort Washington, PA: McNeil Consumer Healthcare Division; 2018.
21. US National Library of Medicine. National Center for Biotechnology Information. PubChem Compound Summary: Dextromethorphan; CID=5360696. https://pubchem.ncbi.nlm.nih.gov/compound/5360696. Accessed January 22, 2020.
22. Hedya SA, Swoboda HD. Lithium toxicity. https://www.ncbi.nlm.nih.gov/books/NBK499992/. Updated August 14, 2019. Accessed January 22, 2020.
23. US Department of Health & Human Services. National Center for Complementary and Integrative Health. Herb-drug interactions: what the science says. https://www.nccih.nih.gov/health/providers/digest/herb-drug-interactions-science. Published September 2015. Accessed January 22, 2020.
24. Shader RI, Greenblatt DJ. Bees, ginseng and MAOIs revisited. J Clin Psychopharmacol. 1988;8(4):235.
25. Chua YT. Interaction between warfarin and Chinese herbal medicines. Singapore Med J. 2015;56(1):11-18.
26. Bonetto N, Santelli L, Battistin L, et al. Serotonin syndrome and rhabdomyolysis induced by concomitant use of triptans, fluoxetine and hypericum. Cephalalgia. 2007;27(12):1421-1423.
27. Henderson L, Yue QY, Bergquist C, et al. St John’s wort (Hypericum perforatum): drug interactions and clinical outcomes. Br J Clin Pharmacol. 2002;54(4):349-356.
28. Johne A, Schmider J, Brockmöller J, et al. Decreased plasma levels of amitriptyline and its metabolites on comedication with an extract from St John’s wort (Hypericum perforatum). J Clin Psychopharmacol. 2002;22(1):46-54.
29. Eich-Höchli D, Oppliger R, Golay KP, et al. Methadone maintenance treatment and St John’s wort: a case report. Pharmacopsychiatry. 2003;36(1):35-37.
30. Johne A, Brockmöller J, Bauer S, et al. Pharmacokinetic interaction of digoxin with an herbal extract from St John’s wort (Hypericum perforatum). Clin Pharmacol Ther. 1999;66(4):338-345.
31. Andrén L, Andreasson A, Eggertsen R. Interaction between a commercially available St John’s wort product (Movina) and atorvastatin in patients with hypercholesterolemia. Eur J Clin Pharmacol. 2007;63(10):913-916.
32. Van Strater AC. Interaction of St John’s wort (Hypericum perforatum) with clozapine. Int Clin Psychopharmacol. 2012;27(2):121-124.
33. Nöldner M, Chatterjee SS. Inhibition of haloperidol-induced catalepsy in rats by root extracts from Piper methysticum F. Phytomedicine. 1999;6(4):285-286.
34. Boerner RJ, Klement S. Attenuation of neuroleptic-induced extrapyramidal side effects by kava special extract WS 1490. Wien Med Wochenschr. 2004;154(21-22):508-510.
35. Schelosky L, Raffauf C, Jendroska K, et al. Kava and dopamine antagonism. J Neurol Neurosurg Psychiatry. 1995;58(5):639-640.
36. Singh YN. Potential for interaction of kava and St. John’s wort with drugs. J Ethnopharmacol. 2005;100(1-2):108-113.
37. Patel NM, Derkits R. Possible increase in liver enzymes secondary to atorvastatin and black cohosh administration. J Pharm Prac. 2007;20(4):341-346.
38. Rockwell S, Liu Y, Higgins SA. Alteration of the effects of cancer therapy agents on breast cancer cells by the herbal medicine black cohosh. Breast Cancer Res Treat. 2005;90(3):233-239.
39. Granger AS. Ginkgo biloba precipitating epileptic seizures. Age Ageing. 2001;30(6):523-525.
40. Mohutsky MA, Anderson GD, Miller JW, et al. Ginkgo biloba: evaluation of CYP2C9 drug interactions in vitro and in vivo. Am J Ther. 2006;13(1):24-31.
41. Zhang XY, Zhou DF, Zhang PY, et al. A double-blind, placebo controlled trial of extract of Ginkgo biloba added to haloperidol in treatment-resistant patients with schizophrenia. J Clin Psychiatry. 2001;62(11):878-883.
42. Atmaca M, Tezcan E, Kuloglu M, et al. The effect of extract of ginkgo biloba addition to olanzapine on therapeutic effect and antioxidant enzyme levels in patients with schizophrenia. Psychiatry Clin Neurosci. 2005;59(6):652-656.
43. Doruk A, Uzun O, Ozsahin A. A placebo-controlled study of extract of ginkgo biloba added to clozapine in patients with treatment-resistant schizophrenia. Int Clin Psychopharmacol. 2008;23(4):223-237.
44. Vaes LP. Interactions of warfarin with garlic, ginger, ginkgo, or ginseng: nature of the evidence. Ann Pharmacother. 2000;34(12):1478-1482.
45. Kanji S, Seely D, Yazdi F, et al. Interactions of commonly used dietary supplements with cardiovascular drugs: a systematic review. Syst Rev. 2012;1:26.
46. Wallace JL. Pathogenesis of NSAID-induced gastroduodenal mucosal injury. Best Pract Res Clin Gastroenterol. 2001;15(5):691-703.
47. Triadafilopoulos G, Sharma R. Features of symptomatic gastroesophageal reflux disease in elderly patients. Am J Gastroenterol. 1997;92(11):2007-2011.
48. Haastrup PF, Thompson W, Søndergaard J, et al. Side effects of long-term proton pump inhibitor use: a review. Basic Clin Pharmacol Toxicol. 2018;123(2):114-121.
49. Diamond BJ, Bailey MR. Ginkgo biloba: indications, mechanisms and safety. Psychiatr Clin N Am. 2013;36:73-83.
50. White CM. The pharmacology, pharmacokinetics, efficacy, and adverse events associated with kava. J Clin Pharmacol. 2018;58(11):1396-1405.
51. Gleitz J, Beile A, Peters T. (+/-)-Kavain inhibits veratridine-activated voltage-dependent Na(+)-channels in synaptosomes prepared from rat cerebral cortex. Neuropharmacology. 1995;34(9):1133-1138.
52. Kahrilas PJ. Gastroesophageal reflux disease and its complications. In: Feldman M, ed. Sleisenger & Fordtran’s Gastrointestinal and Liver Disease. 6th ed. Philadelphia, PA: WB Saunders Company; 1998:498-516.
53. Haenisch B, von Holt K, Wiese B, et al. Risk of dementia in elderly patients with the use of proton pump inhibitors. Eur Arch Psychiatry Clin Neurosci. 2015;265(5):419-428.
54. Sheen E, Triadafilopoulos G. Adverse effects of long-term proton pump inhibitor therapy. Dig Dis Sci. 2011;56(4):931-950.
55. Pitkälä KH, Suominen MH, Bell JS, et al. Herbal medications and other dietary supplements. A clinical review for physicians caring for older people. Ann Medicine. 2016;48(8):586-602.
56. American College of Rheumatology Subcommittee on Osteoarthritis Guidelines. Recommendations for the medical management of osteoarthritis of the hip and knee: 2000 update. Arthritis Rheum. 2000;43(9):1905-1915.
57. Vandvik PO, Lincoff AM, Core JM, et al. Primary and secondary prevention of cardiovascular disease: Antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141(2 Suppl):e637S-e668S. doi: 10.1378/chest.11-2306.
Systemic Treatment for Advanced Hepatocellular Carcinoma
From the University of Alabama at Birmingham, Division of Hematology Oncology, Birmingham, AL, and the University of South Alabama, Division of Hematology Oncology, Mobile, AL. Dr. Paluri and Dr. Hatic contributed equally to this article.
Abstract
- Objective: To review systemic treatment options for patients with locally advanced unresectable hepatocellular carcinoma (HCC).
- Methods: Review of the literature.
- Results: The paradigm of what constitutes first-line treatment for advanced HCC is shifting. Until recently, many patients with advanced HCC were treated with repeated locoregional therapies, such as transartertial embolization (TACE). However, retrospective studies suggest that continuing TACE after refractoriness or failure may not be beneficial and may delay subsequent treatments because of deterioration of liver function or declines in performance status. With recent approvals of several systemic therapy options, including immunotherapy, it is vital to conduct a risk-benefit assessment prior to repeating TACE after failure, so that patients are not denied the use of available systemic therapeutic options due to declined performance status or organ function from these procedures. The optimal timing and the sequence of systemic and locoregional therapy must be carefully evaluated by a multidisciplinary team.
- Conclusion: Randomized clinical trials to improve patient selection and determine the proper sequence of treatments are needed. Given the heterogeneity of HCC, molecular profiling of the tumor to differentiate responders from nonresponders may elucidate potential biomarkers to effectively guide treatment recommendations.
Keywords: liver cancer; molecular therapy; immunotherapy.
Hepatocellular carcinoma (HCC) represents 90% of primary liver malignancies. It is the fifth most common malignancy in males and the ninth most common in females worldwide.1 In contrast to other major cancers (colon, breast, prostate), the incidence of and mortality from HCC has increased over the past decade, following a brief decline between 1999 and 2004.2 The epidemiology and incidence of HCC is closely linked to chronic liver disease and conditions predisposing to liver cirrhosis. Worldwide, hepatitis B virus infection is the leading cause of liver cirrhosis and, hence, HCC. In the United States, 50% of HCC cases are linked to hepatitis C virus (HCV) infection. Diabetes mellitus and alcoholic and nonalcoholic steatohepatitis are the other major etiologies of HCC. Indeed, the metabolic syndrome, independent of other factors, is associated with a 2-fold increase in the risk of HCC.3
Although most cases of HCC are predated by liver cirrhosis, in about 20% of patients HCC occurs without liver cirrhosis.4 Similar to other malignancies, surgery in the form of resection (for isolated lesions in the context of good liver function) or liver transplant (for low-volume disease with mildly impaired liver function) provides the best chance of a cure. Locoregional therapies involving hepatic artery–directed therapy are offered for patients with more advanced disease that is limited to the liver, while systemic therapy is offered for advanced unresectable disease that involves portal vein invasion, lymph nodes, and distant metastasis. The
Molecular Pathogenesis
Similar to other malignancies, a multistep process of carcinogenesis, with accumulation of genomic alterations at the molecular and cellular levels, is recognized in HCC. In about 80% of cases, repeated and chronic injury, inflammation, and repair lead to a distortion of normal liver architecture and development of cirrhotic nodules. Exome sequencing of HCC tissues has identified risk factor–specific mutational signatures, including those related to the tumor microenvironment, and defined the extensive landscape of altered genes and pathways in HCC (eg, angiogenic and MET pathways).7 In the Schulze et al study, the frequency of alterations that could be targeted by available Food and Drug Administration (FDA)–approved drugs comprised either amplifications or mutations of FLTs (6%), FGF3 or 4 or 19 (4%), PDGFRs (3%), VEGFA (1%), HGF (3%), MTOR (2%), EGFR (1%), FGFRs (1%), and MET (1%).7 Epigenetic modification of growth-factor expression, particularly insulin-like growth factor 2 and transforming growth factor alpha, and structural alterations that lead to loss of heterozygosity are early events that cause hepatocyte proliferation and progression of dysplastic nodules.8,9 Advances in whole-exome sequencing have identified TERT promoter mutations, leading to activation of telomerase, as an early event in HCC pathogenesis. Other commonly altered genes include CTNNB1 (B-Catenin) and TP53. As a group, alterations in the MAP kinase pathway genes occur in about 40% of HCC cases.
Actionable oncogenic driver alterations are not as common as tumor suppressor pathway alterations in HCC, making targeted drug development challenging.10,11 The FGFR (fibroblast growth factor receptor) pathway, which plays a critical role in carcinogenesis-related cell growth, survival, neo-angiogenesis, and acquired resistance to other cancer treatments, is being explored as a treatment target.12 The molecular characterization of HCC may help with identifying new biomarkers and present opportunities for developing therapeutic targets.
CASE PRESENTATION
A 61-year-old man with a history of chronic hepatitis C and hypertension presents to his primary care physician due to right upper quadrant pain. Laboratory evaluation shows transaminases elevated 2 times the upper limit of normal. This leads to an ultrasound and follow-up magnetic resonance imaging. Imaging shows diffuse cirrhotic changes, with a 6-cm, well-circumscribed lesion within the left lobe of the liver that shows rapid arterial enhancement with venous washout. These vascular characteristics are consistent with HCC. In addition, 2 satellite lesions in the left lobe and sonographic evidence of invasion into the portal vein are present. Periportal lymph nodes are pathologically enlarged.
The physical examination is unremarkable, except for mild tenderness over the right upper quadrant of the abdomen. Serum bilirubin, albumin, platelets, and international normalized ratio are normal, and alpha fetoprotein (AFP) is elevated at 1769 ng/mL. The patient’s family history is unremarkable for any major illness or cancer. Computed tomography scan of the chest and pelvis shows no evidence of other lesions. His liver disease is classified as Child–Pugh A. Due to locally advanced presentation, the tumor is deemed to be nontransplantable and unresectable, and is staged as BCLC-C. The patient continues to work and his performance status is ECOG (
What systemic treatment would you recommend for this patient with locally advanced unresectable HCC with nodal metastasis?
First-Line Therapeutic Options
Systemic treatment of HCC is challenging because of the underlying liver cirrhosis and hepatic dysfunction present in most patients. Overall prognosis is therefore dependent on the disease biology and burden and on the degree of hepatic dysfunction. These factors must be considered in patients with advanced disease who present for systemic therapy. As such, patients with BCLC class D HCC with poor performance status and impaired liver function are better off with best supportive care and hospice services (Figure). Table 1 and Table 2 outline the landmark trials that led to the approval of agents for advanced HCC treatment.
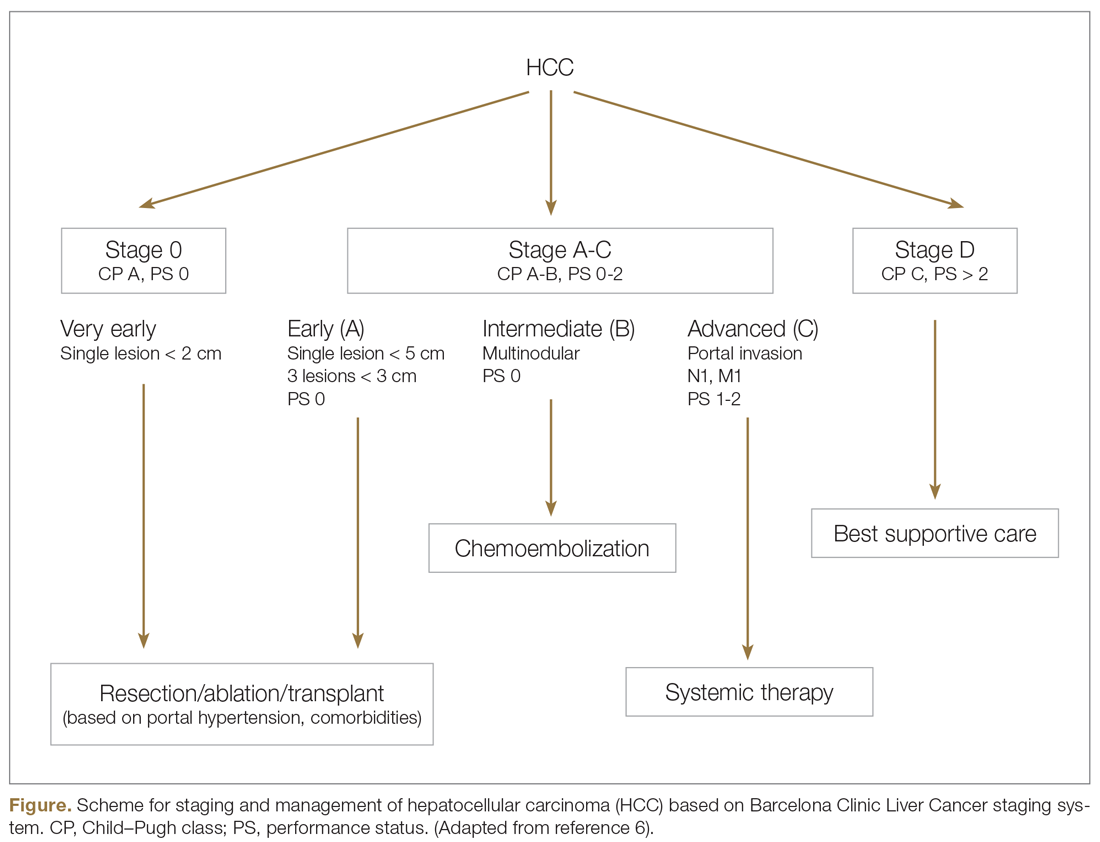
Sorafenib
In the patient with BCLC class C HCC who has preserved liver function (traditionally based on a Child–Pugh score of ≤ 6 and a decent functional status [ECOG performance status 1-2]), sorafenib is the first FDA-approved first-line treatment. Sorafenib is a small-molecule tyrosine kinase inhibitor that targets vascular endothelial growth factor receptor (VEGFR) kinase signaling, in addition to many other tyrosine kinase pathways (including the platelet-derived growth factor and Raf-Ras pathways). Evidence for the clinical benefit of sorafenib comes from the SHARP trial.13 This was a multinational, but primarily European, randomized phase 3 study that compared sorafenib to best supportive care for advanced HCC in patients with a Child–Pugh score ≤ 6A and a robust performance status (ECOG 0 and 1). Overall survival (OS) with placebo and sorafenib was 7.9 months and 10.7 months, respectively. There was no difference in time to radiologic progression, and the progression-free survival (PFS) at 4 months was 62% with sorafenib and 42% with placebo. Patients with HCV-associated HCC appeared to derive a more substantial benefit from sorafenib.14 In a smaller randomized study of sorafenib in Asian patients with predominantly hepatitis B–associated HCC, OS in the sorafenib and best supportive care arms was lower than that reported in the SHARP study (6.5 months vs 4.2 months), although OS still was longer in the sorafenib group.15
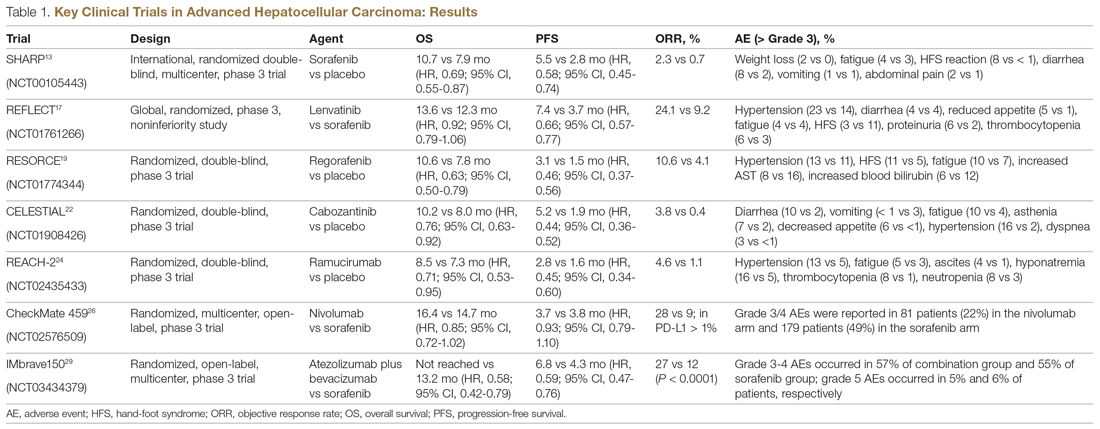
Significant adverse events reported with sorafenib include fatigue (30%), hand and foot syndrome (30%), diarrhea (15%), and mucositis (10%). Major proportions of patients in the clinical setting have not tolerated the standard dose of 400 mg twice daily. Dose-adjusted administration of sorafenib has been advocated in patients with more impaired liver function (Child–Pugh class 7B) and bilirubin of 1.5 to 3 times the upper limit of normal, although it is unclear whether these patients are deriving any benefit from sorafenib.16 At this time, in a patient with preserved liver function, starting with 400 mg twice daily, followed by dose reduction based on toxicity, remains standard.
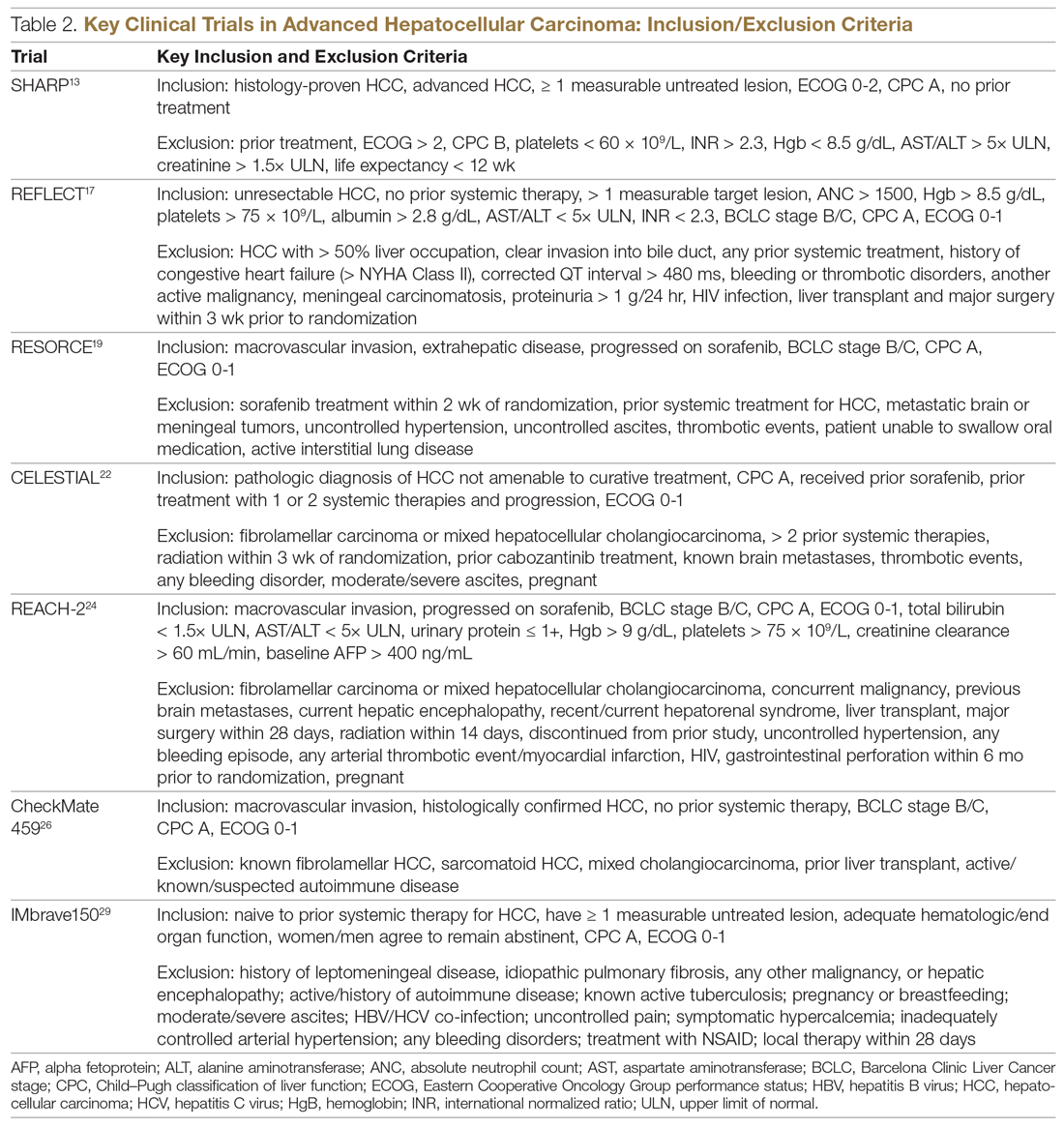
Lenvatinib
After multiple attempts to develop newer first-line treatments for HCC,
Second-Line Therapeutic Options
Following the sorafenib approval, clinical studies of several other agents did not meet their primary endpoint and failed to show improvement in clinical outcomes compared to sorafenib. However, over the past years the treatment landscape for advanced HCC has been changed with the approval of several agents in the second line. The overall response rate (ORR) has become the new theme for management of advanced disease. With multiple therapeutic options available, optimal sequencing now plays a critical role, especially for transitioning from locoregional to systemic therapy. Five drugs are now indicated for second-line treatment of patients who progressed on or were intolerant to sorafenib: regorafenib, cabozantinib, ramucirumab, nivolumab, and pembrolizumab.
Regorafenib
Regorafenib was evaluated in the advanced HCC setting in a single-arm, phase 2 trial involving 36 patients with Child–Pugh class A liver disease who had progressed on prior sorafenib.18 Patients received regorafenib 160 mg orally once daily for 3 weeks on/1 week off cycles. Disease control was achieved in 72% of patients, with stable disease in 25 patients (69%). Based on these results, regorafenib was further evaluated in the multicenter, phase 3, 2:1 randomized, double-blind, placebo-controlled RESORCE study, which enrolled 573 patients.19 Due to the overlapping safety profiles of sorafenib and regorafenib, the inclusion criteria required patients to have tolerated a sorafenib dose of at least 400 mg daily for 20 of the past 28 days of treatment prior to enrollment. The primary endpoint of the study, OS, was met (median OS of 10.6 months in regorafenib arm versus 7.8 months in placebo arm; hazard ratio [HR], 0.63; P < 0.0001).
Cabozantinib
CELESTIAL was a phase 3, double-blind study that assessed the efficacy of cabozantinib versus placebo in patients with advanced HCC who had received prior sorafenib.22 In this study, 707 patients with Child–Pugh class A liver disease who progressed on at least 1 prior systemic therapy were randomized in a 2:1 ratio to treatment with cabozantinib at 60 mg daily or placebo. Patients treated with cabozantinib had a longer OS (10.2 months vs 8.0 months), resulting in a 24% reduction in the risk of death (HR, 0.76), and a longer median PFS (5.2 months versus 1.9 months). The disease control rate was higher with cabozantinib (64% vs 33%) as well. The incidence of high‐grade adverse events in the cabozantinib group was twice that of the placebo group. Common adverse events reported with cabozantinib included HFSR (17%), hypertension (16%), increased aspartate aminotransferase (12%), fatigue (10%), and diarrhea (10%).
Ramucirumab
REACH was a phase 3 study exploring the efficacy of ramucirumab that did not meet its primary endpoint; however, the subgroup analysis in AFP-high patients showed an OS improvement with ramucirumab.23 This led to the phase 3 REACH-2 trial, a multicenter, randomized, double-blind biomarker study in patients with advanced HCC who either progressed on or were intolerant to sorafenib and had an AFP level ≥ 400 ng/mL.24 Patients were randomized to ramucirumab 8 mg/kg every 2 weeks or placebo. The study met its primary endpoint, showing improved OS (8.5 months vs 7.3 months; P = 0.0059). The most common treatment-related adverse events in the ramucirumab group were ascites (5%), hypertension (12%), asthenia (5%), malignant neoplasm progression (6%), increased aspartate aminotransferase concentration (5%), and thrombocytopenia.
Immunotherapy
HCC is considered an inflammation-induced cancer, which renders immunotherapeutic strategies more appealing. The PD-L1/PD-1 pathway is the critical immune checkpoint mechanism and is an important target for treatment. HCC uses a complex, overlapping set of mechanisms to evade cancer-specific immunity and to suppress the immune system. Initial efforts to develop immunotherapies for HCC focused on anti-PD-1 and anti-PD-L1 antibodies. CheckMate 040 evaluated nivolumab in 262 sorafenib-naïve and -treated patients with advanced HCC (98% with Child–Pugh scores of 5 or 6), with a median follow-up of 12.9 months.25 In sorafenib-naïve patients (n = 80), the ORR was 23%, and the disease control rate was 63%. In sorafenib-treated patients (n = 182), the ORR was 18%. Response was not associated with PD-L1 expression. Durable objective responses, a manageable safety profile, and promising efficacy led the FDA to grant accelerated approval of nivolumab for the treatment of patients with HCC who have been previously treated with sorafenib. Based on this, the phase 3 randomized CheckMate-459 trial evaluated the efficacy of nivolumab versus sorafenib in the first-line. Median OS and ORR were better with nivolumab (16.4 months vs 14.7 months; HR 0.85; P = 0.752; and 15% [with 5 complete responses] vs 7%), as was the safety profile (22% vs 49% reporting grade 3 and 4 adverse events). 26
The KEYNOTE-224 study27 evaluated pembrolizumab in 104 patients with previously treated advanced HCC. This study showed an ORR of 17%, with 1 complete response and 17 partial responses. One-third of the patients had progressive disease, while 46 had stable disease. Among those who responded, 56% maintained a durable response for more than 1 year. Subsequently, in KEYNOTE 240, pembrolizumab showed an improvement in OS (13.9 months vs 10.6 months; HR, 0.78; P = 0.0238) and PFS (3.0 months versus 2.8 months; HR, 0.78; P = 0.0186) compared with placebo.28 The ORR for pembrolizumab was 16.9% (95% confidence interval [CI], 12.7%-21.8%) versus 2.2% (95% CI, 0.5%-6.4%; P = 0.00001) for placebo. Mean duration of response was 13.8 months.
In the IMbrave150 trial, atezolizumab/bevacizumab combination, compared to sorafenib, had better OS (not estimable vs 13.2 months; P = 0.0006), PFS (6.8 months vs 4.5 months, P < 0.0001), and ORR (33% vs 13%, P < 0.0001), but grade 3-4 events were similar.29 This combination has potential for first-line approval. The COSMIC–312 study is comparing the combination of cabozantinib and atezolizumab to sorafenib monotherapy and cabozantinib monotherapy in advanced HCC.
Resistance to immunotherapy can be extrinsic, associated with activation mechanisms of T-cells, or intrinsic, related to immune recognition, gene expression, and cell-signaling pathways.30 Tumor-immune heterogeneity and antigen presentation contribute to complex resistance mechanisms.31,32 Although clinical outcomes have improved with immune checkpoint inhibitors, the response rate is low and responses are inconsistent, likely due to an immunosuppressive tumor microenvironment.33 Therefore, several novel combinations of checkpoint inhibitors and targeted drugs are being evaluated to bypass some of the resistance mechanisms (Table 3).
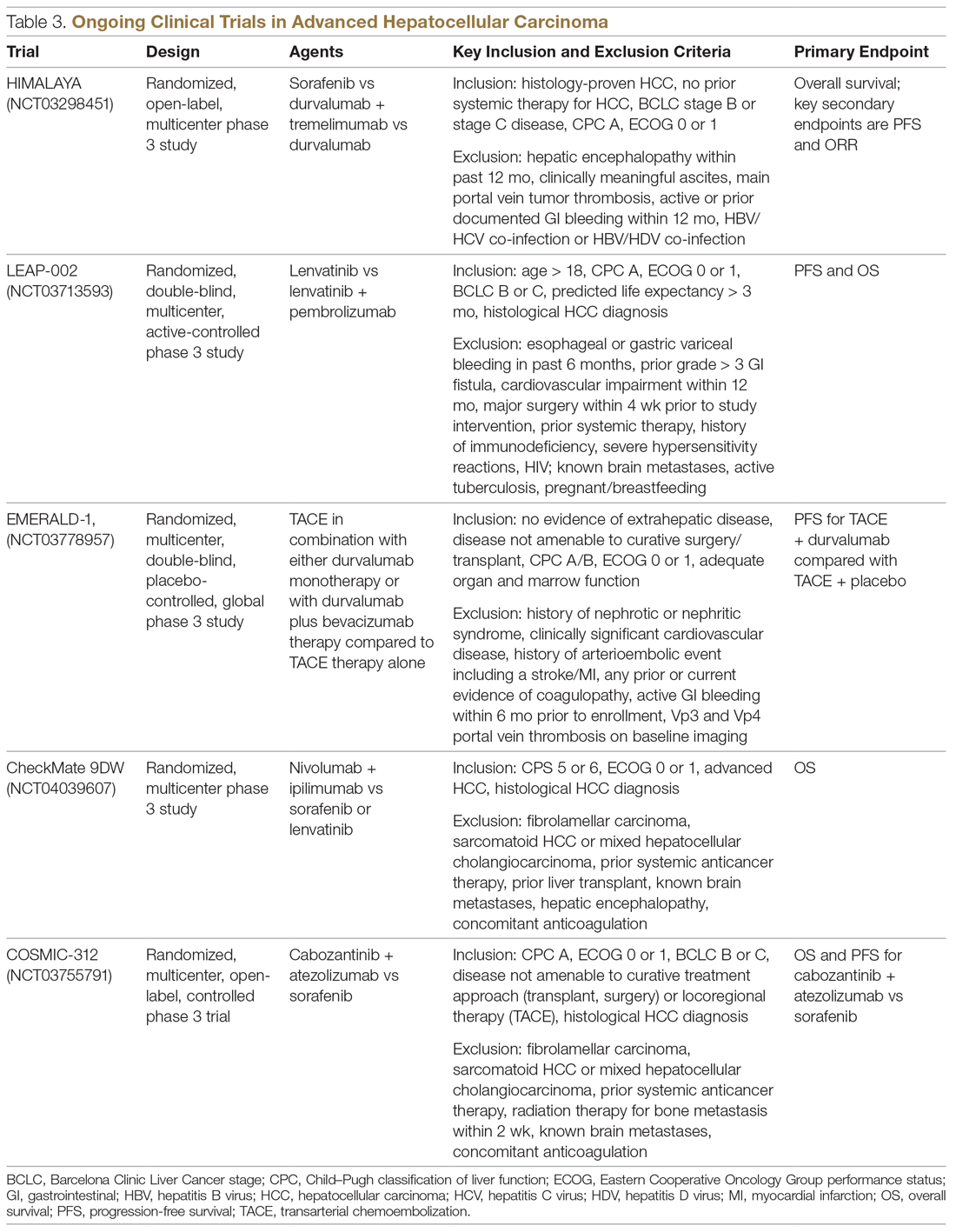
Chemotherapy
Multiple combinations of cytotoxic regimens have been evaluated, but efficacy has been modest, suggesting the limited role for traditional chemotherapy in the systemic management of advanced HCC. Response rates to chemotherapy are low and responses are not durable. Gemcitabine- and doxorubicin-based treatment and FOLFOX (5-fluorouracil, leucovorin, oxaliplatin) are some regimens that have been studied, with a median OS of less than 1 year for these regimens.34-36 FOLFOX had a higher response rate (8.15% vs 2.67%; P = 0.02) and longer median OS (6.40 months versus 4.97 months; HR, 0.80; 95% CI, 0.63-1.02; P = 0.07) than doxorubicin.34 With the gemcitabine/oxaliplatin combination, ORR was 18%, with stable disease in 58% of patients, and median PFS and OS were 6.3 months and 11.5 months, respectively.35 In a study that compared doxorubicin and PIAF (cisplatin/interferon a-2b/doxorubicin/5-fluorouracil), median OS was 6.83 months and 8.67 months, respectively (P = 0.83). The hazard ratio for death from any cause in the PIAF group compared with the doxorubicin group was 0.97 (95% CI, 0.71-1.32). PIAF had a higher ORR (20.9%; 95% CI, 12.5%-29.2%) than doxorubicin (10.5%; 95% CI, 3.9%-16.9%).
The phase 3 ALLIANCE study evaluated the combination of sorafenib and doxorubicin in treatment-naïve HCC patients with Child–Pugh class A liver disease, and did not demonstrate superiority with the addition of cytotoxic chemotherapy.37 Indeed, the combination of chemotherapy with sorafenib appears harmful in terms of lower OS (9.3 months vs 10.6 months; HR, 1.06; 95% CI, 0.8-1.4) and worse toxicity. Patients treated with the combination experienced more hematologic (37.8% vs 8.1%) and nonhematologic adverse events (63.6% vs 61.5%).
Locoregional Therapy
The role of locoregional therapy in advanced HCC remains the subject of intense debate. Patients with BCLC stage C HCC with metastatic disease and those with lymph node involvement are candidates for systemic therapy. The optimal candidate for locoregional therapy is the patient with localized intermediate-stage disease, particularly hepatic artery–delivered therapeutic interventions. However, the presence of a solitary large tumor or portal vein involvement constitutes gray areas regarding which therapy to deliver directly to the tumor via the hepatic artery, and increasingly stereotactic body radiation therapy is being offered.
Transarterial Chemoembolization
Transarterial chemoembolization (TACE), with or without chemotherapy, is the most widely adopted locoregional therapy in the management of HCC. TACE exploits the differential vascular supply to the HCC and normal liver parenchyma. Normal liver receives only one-fourth of its blood supply from the hepatic artery (three-fourths from the portal vein), whereas HCC is mainly supplied by the hepatic artery. A survival benefit for TACE compared to best supportive care is widely acknowledged for intermediate-stage HCC, and transarterial embolization (TAE) with gelatin sponge or microspheres is noninferior to TACE.38,39 Overall safety profile and efficacy inform therapy selection in advanced HCC, although the evidence for TACE in advanced HCC is less robust. Although single-institution experiences suggest survival numbers similar to and even superior to sorafenib,40,41 there is a scarcity of large randomized clinical trial data to back this up. Based on this, patients with advanced HCC should only be offered liver-directed therapy within a clinical trial or on a case-by-case basis under multidisciplinary tumor board consensus.
A serious adverse effect of TACE is post-embolization syndrome, which occurs in about 30% of patients and may be associated with poor prognosis.42 The syndrome consists of right upper quadrant abdominal pain, malaise, and worsening nausea/vomiting following the embolization procedure. Laboratory abnormalities and other complications may persist for up to 30 days after the procedure. This is a concern, because post-embolization syndrome may affect the ability to deliver systemic therapy.
Transarterial Radioembolization
In the past few years, there has been an uptick in the utilization of transarterial radioembolization (TARE), which instead of delivering glass beads, as done in TAE, or chemotherapy-infused beads, as done in TACE, delivers the radioisotope Y-90 to the tumor via the hepatic artery. TARE is able to administer larger doses of radiation to the tumor while avoiding normal liver tissue, as compared to external-beam radiation. There has been no head-to-head comparison of these different intra-arterial therapy approaches, but TARE with Y-90 has been shown to be safe in patients with portal vein thrombosis. A recent multicenter retrospective study of TARE demonstrated a median OS of 8.8 to 10.8 months in patients with BCLC C HCC,43 and in a large randomized study of Y-90 compared to sorafenib in advanced and previously treated intermediate HCC, there was no difference in median OS between the treatment modalities (8 months for selective internal radiotherapy, 9 months for sorafenib; P = 0.18). Treatment with Y-90 was better tolerated.44 A major impediment to the adoption of TARE is the time it takes to order, plan, and deliver Y-90 to patients. Radio-embolization-induced liver disease, similar to post-embolization syndrome, is characterized by jaundice and ascites, which may occur 4 to 8 weeks postprocedure and is more common in patients with HCC who do not have cirrhosis. Compared to TACE, TARE may offer a better adverse effect profile, with improvement in quality of life.
Combination of Systemic and Locoregional Therapy
Even in carefully selected patients with intermediate- and advanced-stage HCC, locoregional therapy is not curative. Tumor embolization may promote more angiogenesis, and hence tumor progression, by causing hypoxia and upregulation of hypoxia-inducible factor.45 This upregulation of angiogenesis as a resistance mechanism to tumor embolization provides a rationale for combining systemic therapy (typically based on abrogating angiogenesis) with TACE/TAE. Most of the experience has been with sorafenib in intermediate-stage disease, and the results have been disappointing. The administration of sorafenib after at least a partial response with TACE did not provide additional benefit in terms of time to progression.46 Similarly, in the SPACE trial, concurrent therapy with TACE-doxorubicin-eluting beads and sorafenib compared to TACE-doxorubicin-eluting beads and placebo yielded similar time to progression numbers for both treatment modalities.47 While the data have been disappointing in intermediate-stage disease, as described earlier, registry data suggest that patients with advanced-stage disease may benefit from this approach.48
In the phase 2 TACTICS trial, 156 patients with unresectable HCC were randomized to receive TACE alone or sorafenib plus TACE, with a novel endpoint, time to untreatable progression (TTUP) and/or progression to TACE refractoriness.49 Treatment with sorafenib following TACE was continued until TTUP, decline in liver function to Child–Pugh class C, or the development of vascular invasion or extrahepatic spread. Development of new lesions while on sorafenib was not considered as progressive disease as long as the lesions were amenable to TACE. In this study, PFS was longer with sorafenib-TACE compared to TACE alone (26.7 months vs 20.6 months; P = 0.02). However, the TTUP endpoint needs further validation, and we are still awaiting the survival outcomes of this study. At this time, there are insufficient data to recommend the combination of liver-directed locoregional therapy and sorafenib or other systemic therapy options outside of a clinical trial setting.
Current Treatment Approach for Advanced HCC (BCLC-C)
Although progress is being made in the development of effective therapies, advanced HCC is generally incurable. These patients experience significant symptom burden throughout the course of the disease. Therefore, the optimal treatment plan must focus on improving or maintaining quality of life, in addition to overall efficacy. It is important to actively involve patients in treatment decisions for an individualized treatment plan, and to discuss the best strategy for incorporating current advances in targeted and immunotherapies. The paradigm of what constitutes first-line treatment for advanced HCC is shifting due to the recent systemic therapy approvals. Prior to the availability of these therapies, many patients with advanced HCC were treated with repeated locoregional therapies. For instance, TACE was often used to treat unresectable HCC multiple times beyond progression. There was no consensus on the definition of TACE failure, and hence it was used in broader, unselected populations. Retrospective studies suggest that continuing TACE after refractoriness or failure may not be beneficial, and may delay subsequent treatments because of deterioration of liver function or declines in performance status. With recent approvals of several systemic therapy options, including immunotherapy, it is vital to conduct a risk-benefit assessment prior to repeating TACE after failure, so that patients are not denied the use of available systemic therapeutic options due to declined performance status or organ function from these procedures. The optimal timing and the sequence of systemic and locoregional therapy must be carefully evaluated by a multidisciplinary team.
CASE CONCLUSION
An important part of evaluating a new patient with HCC is to determine whether they are a candidate for curative therapies, such as transplant or surgical resection. These are no longer an option for patients with intermediate disease. For patients with advanced disease characteristics, such as vascular invasion or systemic metastasis, the evidence supports using systemic therapy with sorafenib or lenvatinib. Lenvatinib, with a better tolerance profile and response rate, is the treatment of choice for the patient described in the case scenario. Lenvatinib is also indicated for first-line treatment of advanced HCC, and is useful in very aggressive tumors, such as those with an AFP level exceeding 200 ng/mL.
Future Directions
The emerging role of novel systemic therapeutics, including immunotherapy, has drastically changed the treatment landscape for hepatocellular cancers, with 6 new drugs for treating advanced hepatocellular cancers approved recently. While these systemic drugs have improved survival in advanced HCC in the past decade, patient selection and treatment sequencing remain a challenge, due to a lack of biomarkers capable of predicting antitumor responses. In addition, there is an unmet need for treatment options for patients with Child–Pugh class B7 and C liver disease and poor performance status.
The goal of future management should be to achieve personalized care aimed at improved safety and efficacy by targeting multiple cancer pathways in the HCC cascade with combination treatments. Randomized clinical trials to improve patient selection and determine the proper sequence of treatments are needed. Given the heterogeneity of HCC, molecular profiling of the tumor to differentiate responders from nonresponders may elucidate potential biomarkers to effectively guide treatment recommendations.
1. Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424.
2. Altekruse SF, McGlynn KA, Reichman ME. Hepatocellular carcinoma incidence, mortality, and survival trends in the United States from 1975 to 2005. J Clin Oncol. 2009;27:1485-1491.
3. Welzel TM, Graubard BI, Zeuzem S, et al. Metabolic syndrome increases the risk of primary liver cancer in the United States: a study in the SEER-Medicare database. Hepatology. 2011;54:463-471.
4. Schutte K, Schulz C, Poranzke J, et al. Characterization and prognosis of patients with hepatocellular carcinoma (HCC) in the non-cirrhotic liver. BMC Gastroenterol. 2014;14:117.
5. Llovet JM, Bru C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis. 1999;19:329-338.
6. Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet. 2018;391:1301-1314.
7. Schulze K, Imbeaud S, Letouzé E, et al. Exome sequencing of hepatocellular carcinomas identifies new mutational signatures and potential therapeutic targets. Nat Genet. 2015;47:505-511.
8. Thorgeirsson SS, Grisham JW. Molecular pathogenesis of human hepatocellular carcinoma. Nat Genet. 2002;31:339-346.
9. Dhanasekaran R, Bandoh S, Roberts LR. Molecular pathogenesis of hepatocellular carcinoma and impact of therapeutic advances. F1000Res. 2016;5.
10. Schulze K, Imbeaud S, Letouze E, et al. Exome sequencing of hepatocellular carcinomas identifies new mutational signatures and potential therapeutic targets. Nat Genet. 2015;47:505-511.
11. Cancer Genome Atlas Research Network. Electronic address: [email protected]; Cancer Genome Atlas Research Network. Comprehensive and integrative genomic characterization of hepatocellular carcinoma. Cell. 2017;169:1327-1134.
12. Chae YK, Ranganath K, Hammerman PS, et al: Inhibition of the fibroblast growth factor receptor (FGFR) pathway: the current landscape and barriers to clinical application. Oncotarget. 2016;8:16052-16074.
13. Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378-390.
14. Jackson R, Psarelli EE, Berhane S, et al. Impact of viral status on survival in patients receiving sorafenib for advanced hepatocellular cancer: a meta-analysis of randomized phase III trials. J Clin Oncol. 2017;35:622-628.
15. Cheng AL, Kang YK, Chen Z, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25-34.
16. Da Fonseca LG, Barroso-Sousa R, Bento AD, et al. Safety and efficacy of sorafenib in patients with Child-Pugh B advanced hepatocellular carcinoma. Mol Clin Oncol. 2015;3:793-796.
17. Kudo M, Finn RS, Qin S, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018;391:1163-1173.
18. Bruix J, Tak W-Y, Gasbarrini A, et al. Regorafenib as second-line therapy for intermediate or advanced hepatocellular carcinoma: Multicentre, open-label, phase II safety study. Eur J Cancer. 2013;49:3412-3419.
19. Bruix J, Qin S, Merle P, et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;389:56-66.
20. Bruix J, Merle P, Granito A, et al. Hand-foot skin reaction (HFSR) and overall survival (OS) in the phase 3 RESORCE trial of regorafenib for treatment of hepatocellular carcinoma (HCC) progressing on sorafenib. J Clin Oncol. 2018;36:412-412.
21. Finn RS, Merle P, Granito A, et al. Outcomes of sequential treatment with sorafenib followed by regorafenib for HCC: Additional analyses from the phase III RESORCE trial. J Hepatol. 2018;69:353-358.
22. Abou-Alfa GK, Meyer T, Cheng A-L, et al. Cabozantinib (C) versus placebo (P) in patients (pts) with advanced hepatocellular carcinoma (HCC) who have received prior sorafenib: Results from the randomized phase III CELESTIAL trial. J Clin Oncol. 2018;36:207-207.
23. Zhu AX, Park JO, Ryoo B-Y, et al. Ramucirumab versus placebo as second-line treatment in patients with advanced hepatocellular carcinoma following first-line therapy with sorafenib (REACH): a randomised, double-blind, multicentre, phase 3 trial. Lancet Oncology. 2015;16:859-870.
24. Zhu AX, Kang Y-K, Yen C-J, et al. Ramucirumab after sorafenib in patients with advanced hepatocellular carcinoma and increased αfetoprotein concentrations (REACH-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncology. 2019;20:282-296.
25. El-Khoueiry AB, Sangro B, Yau T, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet. 2017;389:2492-2502.
26. Yau T, Park JW, Finn RS, et al. CheckMate 459: A randomized, multi-center phase III study of nivolumab (NIVO) vs sorafenib (SOR) as first-line (1L) treatment in patients (pts) with advanced hepatocellular carcinoma (aHCC). Ann Oncol. 2020;30:v874-v875.
27. Zhu AX, Finn RS, Cattan S, et al. KEYNOTE-224: Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib. J Clin Oncol. 2018;36:942-952.
28. Finn RS, Ryoo BY, Merle P, et al. Pembrolizumab as second-line therapy in patients with advanced hepatocellular carcinoma in KEYNOTE-240: a randomized, double-blind, phase III trial. J Clin Oncol. 2020;38:193-202.
29. Cheng A-L, Qin S, Ikeda M, et al. IMbrave150: efficacy and safety results from a ph III study evaluating atezolizumab (atezo) + bevacizumab (bev) vs sorafenib (sor) as first treatment (tx) for patients (pts) with unresectable hepatocellular carcinoma (HCC). Ann Oncol. 2019;30 (suppl_9):ix183-ix202.
30. Jiang Y, Han Q-J, Zhang J. Hepatocellular carcinoma: Mechanisms of progression and immunotherapy. World J Gastroenterol. 2019;25:3151-3167.
31. Xu F, Jin T, Zhu Y, et al. Immune checkpoint therapy in liver cancer. J Exp Clin Cancer Res. 2018;37:110.
32. Koyama S, Akbay EA, Li YY, et al. Adaptive resistance to therapeutic PD-1 blockade is associated with upregulation of alternative immune checkpoints. Nat Commun. 2016;7:10501.
33. Prieto J, Melero I, Sangro B. Immunological landscape and immunotherapy of hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol. 2015;12:681-700.
34. Qin S, Bai Y, Lim HY, et al. Randomized, multicenter, open-label study of oxaliplatin plus fluorouracil/leucovorin versus doxorubicin as palliative chemotherapy in patients with advanced hepatocellular carcinoma from Asia. J Clin Oncol. 2013;31:3501-3508.
35. Louafi S, Boige V, Ducreux M, et al. Gemcitabine plus oxaliplatin (GEMOX) in patients with advanced hepatocellular carcinoma (HCC). Cancer. 2007;109:1384-1390.
36. Tang A, Chan AT, Zee B, et al. A randomized phase iii study of doxorubicin versus cisplatin/interferon α-2b/doxorubicin/fluorouracil (PIAF) combination chemotherapy for unresectable hepatocellular carcinoma. J Natl Cancer Inst. 2005;97:1532-1538.
37. Abou-Alfa GK, Niedzwieski D, Knox JJ, et al. Phase III randomized study of sorafenib plus doxorubicin versus sorafenib in patients with advanced hepatocellular carcinoma (HCC): CALGB 80802 (Alliance). J Clin Oncol. 2016;34:192.
38. Llovet JM, Real MI, Montana X, et al. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet. 2002;359:1734-1739.
39. Brown KT, Do RK, Gonen M, et al. randomized trial of hepatic artery embolization for hepatocellular carcinoma using doxorubicin-eluting microspheres compared with embolization with microspheres alone. J Clin Oncol. 2016;34:2046-2053.
40. Kirstein MM, Voigtlander T, Schweitzer N, et al. Transarterial chemoembolization versus sorafenib in patients with hepatocellular carcinoma and extrahepatic disease. United European Gastroenterol J. 2018;6:238-246.
41. Pinter M, Hucke F, Graziadei I, et al. Advanced-stage hepatocellular carcinoma: transarterial chemoembolization versus sorafenib. Radiology. 2012;263:590-599.
42. Mason MC, Massarweh NN, Salami A, et al. Post-embolization syndrome as an early predictor of overall survival after transarterial chemoembolization for hepatocellular carcinoma. HPB (Oxford). 2015;17:1137-1144.
43. Sangro B, Maini CL, Ettorre GM, et al. Radioembolisation in patients with hepatocellular carcinoma that have previously received liver-directed therapies. Eur J Nucl Med Mol Imaging. 2018;45:1721-1730.
44. Vilgrain V, Pereira H, Assenat E, et al. Efficacy and safety of selective internal radiotherapy with yttrium-90 resin microspheres compared with sorafenib in locally advanced and inoperable hepatocellular carcinoma (SARAH): an open-label randomised controlled phase 3 trial. Lancet Oncol. 2017;18:1624-1636.
45. Sergio A, Cristofori C, Cardin R, et al. Transcatheter arterial chemoembolization (TACE) in hepatocellular carcinoma (HCC): the role of angiogenesis and invasiveness. Am J Gastroenterol. 2008;103:914-921.
46. Kudo M, Imanaka K, Chida N, et al. Phase III study of sorafenib after transarterial chemoembolisation in Japanese and Korean patients with unresectable hepatocellular carcinoma. Eur J Cancer. 2011;47:2117-2127.
47. Lencioni R, Llovet JM, Han G, et al. Sorafenib or placebo plus TACE with doxorubicin-eluting beads for intermediate stage HCC: The SPACE trial. J Hepatol. 2016;64:1090-1098.
48. Geschwind JF, Chapiro J. Sorafenib in combination with transarterial chemoembolization for the treatment of hepatocellular carcinoma. Clin Adv Hematol Oncol. 2016;14:585-587.
49. Kudo M, Ueshima K, Ikeda M, et al. Randomized, open label, multicenter, phase II trial comparing transarterial chemoembolization (TACE) plus sorafenib with TACE alone in patients with hepatocellular carcinoma (HCC): TACTICS trial. J Clin Oncol. 2018;36:206.
From the University of Alabama at Birmingham, Division of Hematology Oncology, Birmingham, AL, and the University of South Alabama, Division of Hematology Oncology, Mobile, AL. Dr. Paluri and Dr. Hatic contributed equally to this article.
Abstract
- Objective: To review systemic treatment options for patients with locally advanced unresectable hepatocellular carcinoma (HCC).
- Methods: Review of the literature.
- Results: The paradigm of what constitutes first-line treatment for advanced HCC is shifting. Until recently, many patients with advanced HCC were treated with repeated locoregional therapies, such as transartertial embolization (TACE). However, retrospective studies suggest that continuing TACE after refractoriness or failure may not be beneficial and may delay subsequent treatments because of deterioration of liver function or declines in performance status. With recent approvals of several systemic therapy options, including immunotherapy, it is vital to conduct a risk-benefit assessment prior to repeating TACE after failure, so that patients are not denied the use of available systemic therapeutic options due to declined performance status or organ function from these procedures. The optimal timing and the sequence of systemic and locoregional therapy must be carefully evaluated by a multidisciplinary team.
- Conclusion: Randomized clinical trials to improve patient selection and determine the proper sequence of treatments are needed. Given the heterogeneity of HCC, molecular profiling of the tumor to differentiate responders from nonresponders may elucidate potential biomarkers to effectively guide treatment recommendations.
Keywords: liver cancer; molecular therapy; immunotherapy.
Hepatocellular carcinoma (HCC) represents 90% of primary liver malignancies. It is the fifth most common malignancy in males and the ninth most common in females worldwide.1 In contrast to other major cancers (colon, breast, prostate), the incidence of and mortality from HCC has increased over the past decade, following a brief decline between 1999 and 2004.2 The epidemiology and incidence of HCC is closely linked to chronic liver disease and conditions predisposing to liver cirrhosis. Worldwide, hepatitis B virus infection is the leading cause of liver cirrhosis and, hence, HCC. In the United States, 50% of HCC cases are linked to hepatitis C virus (HCV) infection. Diabetes mellitus and alcoholic and nonalcoholic steatohepatitis are the other major etiologies of HCC. Indeed, the metabolic syndrome, independent of other factors, is associated with a 2-fold increase in the risk of HCC.3
Although most cases of HCC are predated by liver cirrhosis, in about 20% of patients HCC occurs without liver cirrhosis.4 Similar to other malignancies, surgery in the form of resection (for isolated lesions in the context of good liver function) or liver transplant (for low-volume disease with mildly impaired liver function) provides the best chance of a cure. Locoregional therapies involving hepatic artery–directed therapy are offered for patients with more advanced disease that is limited to the liver, while systemic therapy is offered for advanced unresectable disease that involves portal vein invasion, lymph nodes, and distant metastasis. The
Molecular Pathogenesis
Similar to other malignancies, a multistep process of carcinogenesis, with accumulation of genomic alterations at the molecular and cellular levels, is recognized in HCC. In about 80% of cases, repeated and chronic injury, inflammation, and repair lead to a distortion of normal liver architecture and development of cirrhotic nodules. Exome sequencing of HCC tissues has identified risk factor–specific mutational signatures, including those related to the tumor microenvironment, and defined the extensive landscape of altered genes and pathways in HCC (eg, angiogenic and MET pathways).7 In the Schulze et al study, the frequency of alterations that could be targeted by available Food and Drug Administration (FDA)–approved drugs comprised either amplifications or mutations of FLTs (6%), FGF3 or 4 or 19 (4%), PDGFRs (3%), VEGFA (1%), HGF (3%), MTOR (2%), EGFR (1%), FGFRs (1%), and MET (1%).7 Epigenetic modification of growth-factor expression, particularly insulin-like growth factor 2 and transforming growth factor alpha, and structural alterations that lead to loss of heterozygosity are early events that cause hepatocyte proliferation and progression of dysplastic nodules.8,9 Advances in whole-exome sequencing have identified TERT promoter mutations, leading to activation of telomerase, as an early event in HCC pathogenesis. Other commonly altered genes include CTNNB1 (B-Catenin) and TP53. As a group, alterations in the MAP kinase pathway genes occur in about 40% of HCC cases.
Actionable oncogenic driver alterations are not as common as tumor suppressor pathway alterations in HCC, making targeted drug development challenging.10,11 The FGFR (fibroblast growth factor receptor) pathway, which plays a critical role in carcinogenesis-related cell growth, survival, neo-angiogenesis, and acquired resistance to other cancer treatments, is being explored as a treatment target.12 The molecular characterization of HCC may help with identifying new biomarkers and present opportunities for developing therapeutic targets.
CASE PRESENTATION
A 61-year-old man with a history of chronic hepatitis C and hypertension presents to his primary care physician due to right upper quadrant pain. Laboratory evaluation shows transaminases elevated 2 times the upper limit of normal. This leads to an ultrasound and follow-up magnetic resonance imaging. Imaging shows diffuse cirrhotic changes, with a 6-cm, well-circumscribed lesion within the left lobe of the liver that shows rapid arterial enhancement with venous washout. These vascular characteristics are consistent with HCC. In addition, 2 satellite lesions in the left lobe and sonographic evidence of invasion into the portal vein are present. Periportal lymph nodes are pathologically enlarged.
The physical examination is unremarkable, except for mild tenderness over the right upper quadrant of the abdomen. Serum bilirubin, albumin, platelets, and international normalized ratio are normal, and alpha fetoprotein (AFP) is elevated at 1769 ng/mL. The patient’s family history is unremarkable for any major illness or cancer. Computed tomography scan of the chest and pelvis shows no evidence of other lesions. His liver disease is classified as Child–Pugh A. Due to locally advanced presentation, the tumor is deemed to be nontransplantable and unresectable, and is staged as BCLC-C. The patient continues to work and his performance status is ECOG (
What systemic treatment would you recommend for this patient with locally advanced unresectable HCC with nodal metastasis?
First-Line Therapeutic Options
Systemic treatment of HCC is challenging because of the underlying liver cirrhosis and hepatic dysfunction present in most patients. Overall prognosis is therefore dependent on the disease biology and burden and on the degree of hepatic dysfunction. These factors must be considered in patients with advanced disease who present for systemic therapy. As such, patients with BCLC class D HCC with poor performance status and impaired liver function are better off with best supportive care and hospice services (Figure). Table 1 and Table 2 outline the landmark trials that led to the approval of agents for advanced HCC treatment.

Sorafenib
In the patient with BCLC class C HCC who has preserved liver function (traditionally based on a Child–Pugh score of ≤ 6 and a decent functional status [ECOG performance status 1-2]), sorafenib is the first FDA-approved first-line treatment. Sorafenib is a small-molecule tyrosine kinase inhibitor that targets vascular endothelial growth factor receptor (VEGFR) kinase signaling, in addition to many other tyrosine kinase pathways (including the platelet-derived growth factor and Raf-Ras pathways). Evidence for the clinical benefit of sorafenib comes from the SHARP trial.13 This was a multinational, but primarily European, randomized phase 3 study that compared sorafenib to best supportive care for advanced HCC in patients with a Child–Pugh score ≤ 6A and a robust performance status (ECOG 0 and 1). Overall survival (OS) with placebo and sorafenib was 7.9 months and 10.7 months, respectively. There was no difference in time to radiologic progression, and the progression-free survival (PFS) at 4 months was 62% with sorafenib and 42% with placebo. Patients with HCV-associated HCC appeared to derive a more substantial benefit from sorafenib.14 In a smaller randomized study of sorafenib in Asian patients with predominantly hepatitis B–associated HCC, OS in the sorafenib and best supportive care arms was lower than that reported in the SHARP study (6.5 months vs 4.2 months), although OS still was longer in the sorafenib group.15

Significant adverse events reported with sorafenib include fatigue (30%), hand and foot syndrome (30%), diarrhea (15%), and mucositis (10%). Major proportions of patients in the clinical setting have not tolerated the standard dose of 400 mg twice daily. Dose-adjusted administration of sorafenib has been advocated in patients with more impaired liver function (Child–Pugh class 7B) and bilirubin of 1.5 to 3 times the upper limit of normal, although it is unclear whether these patients are deriving any benefit from sorafenib.16 At this time, in a patient with preserved liver function, starting with 400 mg twice daily, followed by dose reduction based on toxicity, remains standard.

Lenvatinib
After multiple attempts to develop newer first-line treatments for HCC,
Second-Line Therapeutic Options
Following the sorafenib approval, clinical studies of several other agents did not meet their primary endpoint and failed to show improvement in clinical outcomes compared to sorafenib. However, over the past years the treatment landscape for advanced HCC has been changed with the approval of several agents in the second line. The overall response rate (ORR) has become the new theme for management of advanced disease. With multiple therapeutic options available, optimal sequencing now plays a critical role, especially for transitioning from locoregional to systemic therapy. Five drugs are now indicated for second-line treatment of patients who progressed on or were intolerant to sorafenib: regorafenib, cabozantinib, ramucirumab, nivolumab, and pembrolizumab.
Regorafenib
Regorafenib was evaluated in the advanced HCC setting in a single-arm, phase 2 trial involving 36 patients with Child–Pugh class A liver disease who had progressed on prior sorafenib.18 Patients received regorafenib 160 mg orally once daily for 3 weeks on/1 week off cycles. Disease control was achieved in 72% of patients, with stable disease in 25 patients (69%). Based on these results, regorafenib was further evaluated in the multicenter, phase 3, 2:1 randomized, double-blind, placebo-controlled RESORCE study, which enrolled 573 patients.19 Due to the overlapping safety profiles of sorafenib and regorafenib, the inclusion criteria required patients to have tolerated a sorafenib dose of at least 400 mg daily for 20 of the past 28 days of treatment prior to enrollment. The primary endpoint of the study, OS, was met (median OS of 10.6 months in regorafenib arm versus 7.8 months in placebo arm; hazard ratio [HR], 0.63; P < 0.0001).
Cabozantinib
CELESTIAL was a phase 3, double-blind study that assessed the efficacy of cabozantinib versus placebo in patients with advanced HCC who had received prior sorafenib.22 In this study, 707 patients with Child–Pugh class A liver disease who progressed on at least 1 prior systemic therapy were randomized in a 2:1 ratio to treatment with cabozantinib at 60 mg daily or placebo. Patients treated with cabozantinib had a longer OS (10.2 months vs 8.0 months), resulting in a 24% reduction in the risk of death (HR, 0.76), and a longer median PFS (5.2 months versus 1.9 months). The disease control rate was higher with cabozantinib (64% vs 33%) as well. The incidence of high‐grade adverse events in the cabozantinib group was twice that of the placebo group. Common adverse events reported with cabozantinib included HFSR (17%), hypertension (16%), increased aspartate aminotransferase (12%), fatigue (10%), and diarrhea (10%).
Ramucirumab
REACH was a phase 3 study exploring the efficacy of ramucirumab that did not meet its primary endpoint; however, the subgroup analysis in AFP-high patients showed an OS improvement with ramucirumab.23 This led to the phase 3 REACH-2 trial, a multicenter, randomized, double-blind biomarker study in patients with advanced HCC who either progressed on or were intolerant to sorafenib and had an AFP level ≥ 400 ng/mL.24 Patients were randomized to ramucirumab 8 mg/kg every 2 weeks or placebo. The study met its primary endpoint, showing improved OS (8.5 months vs 7.3 months; P = 0.0059). The most common treatment-related adverse events in the ramucirumab group were ascites (5%), hypertension (12%), asthenia (5%), malignant neoplasm progression (6%), increased aspartate aminotransferase concentration (5%), and thrombocytopenia.
Immunotherapy
HCC is considered an inflammation-induced cancer, which renders immunotherapeutic strategies more appealing. The PD-L1/PD-1 pathway is the critical immune checkpoint mechanism and is an important target for treatment. HCC uses a complex, overlapping set of mechanisms to evade cancer-specific immunity and to suppress the immune system. Initial efforts to develop immunotherapies for HCC focused on anti-PD-1 and anti-PD-L1 antibodies. CheckMate 040 evaluated nivolumab in 262 sorafenib-naïve and -treated patients with advanced HCC (98% with Child–Pugh scores of 5 or 6), with a median follow-up of 12.9 months.25 In sorafenib-naïve patients (n = 80), the ORR was 23%, and the disease control rate was 63%. In sorafenib-treated patients (n = 182), the ORR was 18%. Response was not associated with PD-L1 expression. Durable objective responses, a manageable safety profile, and promising efficacy led the FDA to grant accelerated approval of nivolumab for the treatment of patients with HCC who have been previously treated with sorafenib. Based on this, the phase 3 randomized CheckMate-459 trial evaluated the efficacy of nivolumab versus sorafenib in the first-line. Median OS and ORR were better with nivolumab (16.4 months vs 14.7 months; HR 0.85; P = 0.752; and 15% [with 5 complete responses] vs 7%), as was the safety profile (22% vs 49% reporting grade 3 and 4 adverse events). 26
The KEYNOTE-224 study27 evaluated pembrolizumab in 104 patients with previously treated advanced HCC. This study showed an ORR of 17%, with 1 complete response and 17 partial responses. One-third of the patients had progressive disease, while 46 had stable disease. Among those who responded, 56% maintained a durable response for more than 1 year. Subsequently, in KEYNOTE 240, pembrolizumab showed an improvement in OS (13.9 months vs 10.6 months; HR, 0.78; P = 0.0238) and PFS (3.0 months versus 2.8 months; HR, 0.78; P = 0.0186) compared with placebo.28 The ORR for pembrolizumab was 16.9% (95% confidence interval [CI], 12.7%-21.8%) versus 2.2% (95% CI, 0.5%-6.4%; P = 0.00001) for placebo. Mean duration of response was 13.8 months.
In the IMbrave150 trial, atezolizumab/bevacizumab combination, compared to sorafenib, had better OS (not estimable vs 13.2 months; P = 0.0006), PFS (6.8 months vs 4.5 months, P < 0.0001), and ORR (33% vs 13%, P < 0.0001), but grade 3-4 events were similar.29 This combination has potential for first-line approval. The COSMIC–312 study is comparing the combination of cabozantinib and atezolizumab to sorafenib monotherapy and cabozantinib monotherapy in advanced HCC.
Resistance to immunotherapy can be extrinsic, associated with activation mechanisms of T-cells, or intrinsic, related to immune recognition, gene expression, and cell-signaling pathways.30 Tumor-immune heterogeneity and antigen presentation contribute to complex resistance mechanisms.31,32 Although clinical outcomes have improved with immune checkpoint inhibitors, the response rate is low and responses are inconsistent, likely due to an immunosuppressive tumor microenvironment.33 Therefore, several novel combinations of checkpoint inhibitors and targeted drugs are being evaluated to bypass some of the resistance mechanisms (Table 3).

Chemotherapy
Multiple combinations of cytotoxic regimens have been evaluated, but efficacy has been modest, suggesting the limited role for traditional chemotherapy in the systemic management of advanced HCC. Response rates to chemotherapy are low and responses are not durable. Gemcitabine- and doxorubicin-based treatment and FOLFOX (5-fluorouracil, leucovorin, oxaliplatin) are some regimens that have been studied, with a median OS of less than 1 year for these regimens.34-36 FOLFOX had a higher response rate (8.15% vs 2.67%; P = 0.02) and longer median OS (6.40 months versus 4.97 months; HR, 0.80; 95% CI, 0.63-1.02; P = 0.07) than doxorubicin.34 With the gemcitabine/oxaliplatin combination, ORR was 18%, with stable disease in 58% of patients, and median PFS and OS were 6.3 months and 11.5 months, respectively.35 In a study that compared doxorubicin and PIAF (cisplatin/interferon a-2b/doxorubicin/5-fluorouracil), median OS was 6.83 months and 8.67 months, respectively (P = 0.83). The hazard ratio for death from any cause in the PIAF group compared with the doxorubicin group was 0.97 (95% CI, 0.71-1.32). PIAF had a higher ORR (20.9%; 95% CI, 12.5%-29.2%) than doxorubicin (10.5%; 95% CI, 3.9%-16.9%).
The phase 3 ALLIANCE study evaluated the combination of sorafenib and doxorubicin in treatment-naïve HCC patients with Child–Pugh class A liver disease, and did not demonstrate superiority with the addition of cytotoxic chemotherapy.37 Indeed, the combination of chemotherapy with sorafenib appears harmful in terms of lower OS (9.3 months vs 10.6 months; HR, 1.06; 95% CI, 0.8-1.4) and worse toxicity. Patients treated with the combination experienced more hematologic (37.8% vs 8.1%) and nonhematologic adverse events (63.6% vs 61.5%).
Locoregional Therapy
The role of locoregional therapy in advanced HCC remains the subject of intense debate. Patients with BCLC stage C HCC with metastatic disease and those with lymph node involvement are candidates for systemic therapy. The optimal candidate for locoregional therapy is the patient with localized intermediate-stage disease, particularly hepatic artery–delivered therapeutic interventions. However, the presence of a solitary large tumor or portal vein involvement constitutes gray areas regarding which therapy to deliver directly to the tumor via the hepatic artery, and increasingly stereotactic body radiation therapy is being offered.
Transarterial Chemoembolization
Transarterial chemoembolization (TACE), with or without chemotherapy, is the most widely adopted locoregional therapy in the management of HCC. TACE exploits the differential vascular supply to the HCC and normal liver parenchyma. Normal liver receives only one-fourth of its blood supply from the hepatic artery (three-fourths from the portal vein), whereas HCC is mainly supplied by the hepatic artery. A survival benefit for TACE compared to best supportive care is widely acknowledged for intermediate-stage HCC, and transarterial embolization (TAE) with gelatin sponge or microspheres is noninferior to TACE.38,39 Overall safety profile and efficacy inform therapy selection in advanced HCC, although the evidence for TACE in advanced HCC is less robust. Although single-institution experiences suggest survival numbers similar to and even superior to sorafenib,40,41 there is a scarcity of large randomized clinical trial data to back this up. Based on this, patients with advanced HCC should only be offered liver-directed therapy within a clinical trial or on a case-by-case basis under multidisciplinary tumor board consensus.
A serious adverse effect of TACE is post-embolization syndrome, which occurs in about 30% of patients and may be associated with poor prognosis.42 The syndrome consists of right upper quadrant abdominal pain, malaise, and worsening nausea/vomiting following the embolization procedure. Laboratory abnormalities and other complications may persist for up to 30 days after the procedure. This is a concern, because post-embolization syndrome may affect the ability to deliver systemic therapy.
Transarterial Radioembolization
In the past few years, there has been an uptick in the utilization of transarterial radioembolization (TARE), which instead of delivering glass beads, as done in TAE, or chemotherapy-infused beads, as done in TACE, delivers the radioisotope Y-90 to the tumor via the hepatic artery. TARE is able to administer larger doses of radiation to the tumor while avoiding normal liver tissue, as compared to external-beam radiation. There has been no head-to-head comparison of these different intra-arterial therapy approaches, but TARE with Y-90 has been shown to be safe in patients with portal vein thrombosis. A recent multicenter retrospective study of TARE demonstrated a median OS of 8.8 to 10.8 months in patients with BCLC C HCC,43 and in a large randomized study of Y-90 compared to sorafenib in advanced and previously treated intermediate HCC, there was no difference in median OS between the treatment modalities (8 months for selective internal radiotherapy, 9 months for sorafenib; P = 0.18). Treatment with Y-90 was better tolerated.44 A major impediment to the adoption of TARE is the time it takes to order, plan, and deliver Y-90 to patients. Radio-embolization-induced liver disease, similar to post-embolization syndrome, is characterized by jaundice and ascites, which may occur 4 to 8 weeks postprocedure and is more common in patients with HCC who do not have cirrhosis. Compared to TACE, TARE may offer a better adverse effect profile, with improvement in quality of life.
Combination of Systemic and Locoregional Therapy
Even in carefully selected patients with intermediate- and advanced-stage HCC, locoregional therapy is not curative. Tumor embolization may promote more angiogenesis, and hence tumor progression, by causing hypoxia and upregulation of hypoxia-inducible factor.45 This upregulation of angiogenesis as a resistance mechanism to tumor embolization provides a rationale for combining systemic therapy (typically based on abrogating angiogenesis) with TACE/TAE. Most of the experience has been with sorafenib in intermediate-stage disease, and the results have been disappointing. The administration of sorafenib after at least a partial response with TACE did not provide additional benefit in terms of time to progression.46 Similarly, in the SPACE trial, concurrent therapy with TACE-doxorubicin-eluting beads and sorafenib compared to TACE-doxorubicin-eluting beads and placebo yielded similar time to progression numbers for both treatment modalities.47 While the data have been disappointing in intermediate-stage disease, as described earlier, registry data suggest that patients with advanced-stage disease may benefit from this approach.48
In the phase 2 TACTICS trial, 156 patients with unresectable HCC were randomized to receive TACE alone or sorafenib plus TACE, with a novel endpoint, time to untreatable progression (TTUP) and/or progression to TACE refractoriness.49 Treatment with sorafenib following TACE was continued until TTUP, decline in liver function to Child–Pugh class C, or the development of vascular invasion or extrahepatic spread. Development of new lesions while on sorafenib was not considered as progressive disease as long as the lesions were amenable to TACE. In this study, PFS was longer with sorafenib-TACE compared to TACE alone (26.7 months vs 20.6 months; P = 0.02). However, the TTUP endpoint needs further validation, and we are still awaiting the survival outcomes of this study. At this time, there are insufficient data to recommend the combination of liver-directed locoregional therapy and sorafenib or other systemic therapy options outside of a clinical trial setting.
Current Treatment Approach for Advanced HCC (BCLC-C)
Although progress is being made in the development of effective therapies, advanced HCC is generally incurable. These patients experience significant symptom burden throughout the course of the disease. Therefore, the optimal treatment plan must focus on improving or maintaining quality of life, in addition to overall efficacy. It is important to actively involve patients in treatment decisions for an individualized treatment plan, and to discuss the best strategy for incorporating current advances in targeted and immunotherapies. The paradigm of what constitutes first-line treatment for advanced HCC is shifting due to the recent systemic therapy approvals. Prior to the availability of these therapies, many patients with advanced HCC were treated with repeated locoregional therapies. For instance, TACE was often used to treat unresectable HCC multiple times beyond progression. There was no consensus on the definition of TACE failure, and hence it was used in broader, unselected populations. Retrospective studies suggest that continuing TACE after refractoriness or failure may not be beneficial, and may delay subsequent treatments because of deterioration of liver function or declines in performance status. With recent approvals of several systemic therapy options, including immunotherapy, it is vital to conduct a risk-benefit assessment prior to repeating TACE after failure, so that patients are not denied the use of available systemic therapeutic options due to declined performance status or organ function from these procedures. The optimal timing and the sequence of systemic and locoregional therapy must be carefully evaluated by a multidisciplinary team.
CASE CONCLUSION
An important part of evaluating a new patient with HCC is to determine whether they are a candidate for curative therapies, such as transplant or surgical resection. These are no longer an option for patients with intermediate disease. For patients with advanced disease characteristics, such as vascular invasion or systemic metastasis, the evidence supports using systemic therapy with sorafenib or lenvatinib. Lenvatinib, with a better tolerance profile and response rate, is the treatment of choice for the patient described in the case scenario. Lenvatinib is also indicated for first-line treatment of advanced HCC, and is useful in very aggressive tumors, such as those with an AFP level exceeding 200 ng/mL.
Future Directions
The emerging role of novel systemic therapeutics, including immunotherapy, has drastically changed the treatment landscape for hepatocellular cancers, with 6 new drugs for treating advanced hepatocellular cancers approved recently. While these systemic drugs have improved survival in advanced HCC in the past decade, patient selection and treatment sequencing remain a challenge, due to a lack of biomarkers capable of predicting antitumor responses. In addition, there is an unmet need for treatment options for patients with Child–Pugh class B7 and C liver disease and poor performance status.
The goal of future management should be to achieve personalized care aimed at improved safety and efficacy by targeting multiple cancer pathways in the HCC cascade with combination treatments. Randomized clinical trials to improve patient selection and determine the proper sequence of treatments are needed. Given the heterogeneity of HCC, molecular profiling of the tumor to differentiate responders from nonresponders may elucidate potential biomarkers to effectively guide treatment recommendations.
From the University of Alabama at Birmingham, Division of Hematology Oncology, Birmingham, AL, and the University of South Alabama, Division of Hematology Oncology, Mobile, AL. Dr. Paluri and Dr. Hatic contributed equally to this article.
Abstract
- Objective: To review systemic treatment options for patients with locally advanced unresectable hepatocellular carcinoma (HCC).
- Methods: Review of the literature.
- Results: The paradigm of what constitutes first-line treatment for advanced HCC is shifting. Until recently, many patients with advanced HCC were treated with repeated locoregional therapies, such as transartertial embolization (TACE). However, retrospective studies suggest that continuing TACE after refractoriness or failure may not be beneficial and may delay subsequent treatments because of deterioration of liver function or declines in performance status. With recent approvals of several systemic therapy options, including immunotherapy, it is vital to conduct a risk-benefit assessment prior to repeating TACE after failure, so that patients are not denied the use of available systemic therapeutic options due to declined performance status or organ function from these procedures. The optimal timing and the sequence of systemic and locoregional therapy must be carefully evaluated by a multidisciplinary team.
- Conclusion: Randomized clinical trials to improve patient selection and determine the proper sequence of treatments are needed. Given the heterogeneity of HCC, molecular profiling of the tumor to differentiate responders from nonresponders may elucidate potential biomarkers to effectively guide treatment recommendations.
Keywords: liver cancer; molecular therapy; immunotherapy.
Hepatocellular carcinoma (HCC) represents 90% of primary liver malignancies. It is the fifth most common malignancy in males and the ninth most common in females worldwide.1 In contrast to other major cancers (colon, breast, prostate), the incidence of and mortality from HCC has increased over the past decade, following a brief decline between 1999 and 2004.2 The epidemiology and incidence of HCC is closely linked to chronic liver disease and conditions predisposing to liver cirrhosis. Worldwide, hepatitis B virus infection is the leading cause of liver cirrhosis and, hence, HCC. In the United States, 50% of HCC cases are linked to hepatitis C virus (HCV) infection. Diabetes mellitus and alcoholic and nonalcoholic steatohepatitis are the other major etiologies of HCC. Indeed, the metabolic syndrome, independent of other factors, is associated with a 2-fold increase in the risk of HCC.3
Although most cases of HCC are predated by liver cirrhosis, in about 20% of patients HCC occurs without liver cirrhosis.4 Similar to other malignancies, surgery in the form of resection (for isolated lesions in the context of good liver function) or liver transplant (for low-volume disease with mildly impaired liver function) provides the best chance of a cure. Locoregional therapies involving hepatic artery–directed therapy are offered for patients with more advanced disease that is limited to the liver, while systemic therapy is offered for advanced unresectable disease that involves portal vein invasion, lymph nodes, and distant metastasis. The
Molecular Pathogenesis
Similar to other malignancies, a multistep process of carcinogenesis, with accumulation of genomic alterations at the molecular and cellular levels, is recognized in HCC. In about 80% of cases, repeated and chronic injury, inflammation, and repair lead to a distortion of normal liver architecture and development of cirrhotic nodules. Exome sequencing of HCC tissues has identified risk factor–specific mutational signatures, including those related to the tumor microenvironment, and defined the extensive landscape of altered genes and pathways in HCC (eg, angiogenic and MET pathways).7 In the Schulze et al study, the frequency of alterations that could be targeted by available Food and Drug Administration (FDA)–approved drugs comprised either amplifications or mutations of FLTs (6%), FGF3 or 4 or 19 (4%), PDGFRs (3%), VEGFA (1%), HGF (3%), MTOR (2%), EGFR (1%), FGFRs (1%), and MET (1%).7 Epigenetic modification of growth-factor expression, particularly insulin-like growth factor 2 and transforming growth factor alpha, and structural alterations that lead to loss of heterozygosity are early events that cause hepatocyte proliferation and progression of dysplastic nodules.8,9 Advances in whole-exome sequencing have identified TERT promoter mutations, leading to activation of telomerase, as an early event in HCC pathogenesis. Other commonly altered genes include CTNNB1 (B-Catenin) and TP53. As a group, alterations in the MAP kinase pathway genes occur in about 40% of HCC cases.
Actionable oncogenic driver alterations are not as common as tumor suppressor pathway alterations in HCC, making targeted drug development challenging.10,11 The FGFR (fibroblast growth factor receptor) pathway, which plays a critical role in carcinogenesis-related cell growth, survival, neo-angiogenesis, and acquired resistance to other cancer treatments, is being explored as a treatment target.12 The molecular characterization of HCC may help with identifying new biomarkers and present opportunities for developing therapeutic targets.
CASE PRESENTATION
A 61-year-old man with a history of chronic hepatitis C and hypertension presents to his primary care physician due to right upper quadrant pain. Laboratory evaluation shows transaminases elevated 2 times the upper limit of normal. This leads to an ultrasound and follow-up magnetic resonance imaging. Imaging shows diffuse cirrhotic changes, with a 6-cm, well-circumscribed lesion within the left lobe of the liver that shows rapid arterial enhancement with venous washout. These vascular characteristics are consistent with HCC. In addition, 2 satellite lesions in the left lobe and sonographic evidence of invasion into the portal vein are present. Periportal lymph nodes are pathologically enlarged.
The physical examination is unremarkable, except for mild tenderness over the right upper quadrant of the abdomen. Serum bilirubin, albumin, platelets, and international normalized ratio are normal, and alpha fetoprotein (AFP) is elevated at 1769 ng/mL. The patient’s family history is unremarkable for any major illness or cancer. Computed tomography scan of the chest and pelvis shows no evidence of other lesions. His liver disease is classified as Child–Pugh A. Due to locally advanced presentation, the tumor is deemed to be nontransplantable and unresectable, and is staged as BCLC-C. The patient continues to work and his performance status is ECOG (
What systemic treatment would you recommend for this patient with locally advanced unresectable HCC with nodal metastasis?
First-Line Therapeutic Options
Systemic treatment of HCC is challenging because of the underlying liver cirrhosis and hepatic dysfunction present in most patients. Overall prognosis is therefore dependent on the disease biology and burden and on the degree of hepatic dysfunction. These factors must be considered in patients with advanced disease who present for systemic therapy. As such, patients with BCLC class D HCC with poor performance status and impaired liver function are better off with best supportive care and hospice services (Figure). Table 1 and Table 2 outline the landmark trials that led to the approval of agents for advanced HCC treatment.

Sorafenib
In the patient with BCLC class C HCC who has preserved liver function (traditionally based on a Child–Pugh score of ≤ 6 and a decent functional status [ECOG performance status 1-2]), sorafenib is the first FDA-approved first-line treatment. Sorafenib is a small-molecule tyrosine kinase inhibitor that targets vascular endothelial growth factor receptor (VEGFR) kinase signaling, in addition to many other tyrosine kinase pathways (including the platelet-derived growth factor and Raf-Ras pathways). Evidence for the clinical benefit of sorafenib comes from the SHARP trial.13 This was a multinational, but primarily European, randomized phase 3 study that compared sorafenib to best supportive care for advanced HCC in patients with a Child–Pugh score ≤ 6A and a robust performance status (ECOG 0 and 1). Overall survival (OS) with placebo and sorafenib was 7.9 months and 10.7 months, respectively. There was no difference in time to radiologic progression, and the progression-free survival (PFS) at 4 months was 62% with sorafenib and 42% with placebo. Patients with HCV-associated HCC appeared to derive a more substantial benefit from sorafenib.14 In a smaller randomized study of sorafenib in Asian patients with predominantly hepatitis B–associated HCC, OS in the sorafenib and best supportive care arms was lower than that reported in the SHARP study (6.5 months vs 4.2 months), although OS still was longer in the sorafenib group.15

Significant adverse events reported with sorafenib include fatigue (30%), hand and foot syndrome (30%), diarrhea (15%), and mucositis (10%). Major proportions of patients in the clinical setting have not tolerated the standard dose of 400 mg twice daily. Dose-adjusted administration of sorafenib has been advocated in patients with more impaired liver function (Child–Pugh class 7B) and bilirubin of 1.5 to 3 times the upper limit of normal, although it is unclear whether these patients are deriving any benefit from sorafenib.16 At this time, in a patient with preserved liver function, starting with 400 mg twice daily, followed by dose reduction based on toxicity, remains standard.

Lenvatinib
After multiple attempts to develop newer first-line treatments for HCC,
Second-Line Therapeutic Options
Following the sorafenib approval, clinical studies of several other agents did not meet their primary endpoint and failed to show improvement in clinical outcomes compared to sorafenib. However, over the past years the treatment landscape for advanced HCC has been changed with the approval of several agents in the second line. The overall response rate (ORR) has become the new theme for management of advanced disease. With multiple therapeutic options available, optimal sequencing now plays a critical role, especially for transitioning from locoregional to systemic therapy. Five drugs are now indicated for second-line treatment of patients who progressed on or were intolerant to sorafenib: regorafenib, cabozantinib, ramucirumab, nivolumab, and pembrolizumab.
Regorafenib
Regorafenib was evaluated in the advanced HCC setting in a single-arm, phase 2 trial involving 36 patients with Child–Pugh class A liver disease who had progressed on prior sorafenib.18 Patients received regorafenib 160 mg orally once daily for 3 weeks on/1 week off cycles. Disease control was achieved in 72% of patients, with stable disease in 25 patients (69%). Based on these results, regorafenib was further evaluated in the multicenter, phase 3, 2:1 randomized, double-blind, placebo-controlled RESORCE study, which enrolled 573 patients.19 Due to the overlapping safety profiles of sorafenib and regorafenib, the inclusion criteria required patients to have tolerated a sorafenib dose of at least 400 mg daily for 20 of the past 28 days of treatment prior to enrollment. The primary endpoint of the study, OS, was met (median OS of 10.6 months in regorafenib arm versus 7.8 months in placebo arm; hazard ratio [HR], 0.63; P < 0.0001).
Cabozantinib
CELESTIAL was a phase 3, double-blind study that assessed the efficacy of cabozantinib versus placebo in patients with advanced HCC who had received prior sorafenib.22 In this study, 707 patients with Child–Pugh class A liver disease who progressed on at least 1 prior systemic therapy were randomized in a 2:1 ratio to treatment with cabozantinib at 60 mg daily or placebo. Patients treated with cabozantinib had a longer OS (10.2 months vs 8.0 months), resulting in a 24% reduction in the risk of death (HR, 0.76), and a longer median PFS (5.2 months versus 1.9 months). The disease control rate was higher with cabozantinib (64% vs 33%) as well. The incidence of high‐grade adverse events in the cabozantinib group was twice that of the placebo group. Common adverse events reported with cabozantinib included HFSR (17%), hypertension (16%), increased aspartate aminotransferase (12%), fatigue (10%), and diarrhea (10%).
Ramucirumab
REACH was a phase 3 study exploring the efficacy of ramucirumab that did not meet its primary endpoint; however, the subgroup analysis in AFP-high patients showed an OS improvement with ramucirumab.23 This led to the phase 3 REACH-2 trial, a multicenter, randomized, double-blind biomarker study in patients with advanced HCC who either progressed on or were intolerant to sorafenib and had an AFP level ≥ 400 ng/mL.24 Patients were randomized to ramucirumab 8 mg/kg every 2 weeks or placebo. The study met its primary endpoint, showing improved OS (8.5 months vs 7.3 months; P = 0.0059). The most common treatment-related adverse events in the ramucirumab group were ascites (5%), hypertension (12%), asthenia (5%), malignant neoplasm progression (6%), increased aspartate aminotransferase concentration (5%), and thrombocytopenia.
Immunotherapy
HCC is considered an inflammation-induced cancer, which renders immunotherapeutic strategies more appealing. The PD-L1/PD-1 pathway is the critical immune checkpoint mechanism and is an important target for treatment. HCC uses a complex, overlapping set of mechanisms to evade cancer-specific immunity and to suppress the immune system. Initial efforts to develop immunotherapies for HCC focused on anti-PD-1 and anti-PD-L1 antibodies. CheckMate 040 evaluated nivolumab in 262 sorafenib-naïve and -treated patients with advanced HCC (98% with Child–Pugh scores of 5 or 6), with a median follow-up of 12.9 months.25 In sorafenib-naïve patients (n = 80), the ORR was 23%, and the disease control rate was 63%. In sorafenib-treated patients (n = 182), the ORR was 18%. Response was not associated with PD-L1 expression. Durable objective responses, a manageable safety profile, and promising efficacy led the FDA to grant accelerated approval of nivolumab for the treatment of patients with HCC who have been previously treated with sorafenib. Based on this, the phase 3 randomized CheckMate-459 trial evaluated the efficacy of nivolumab versus sorafenib in the first-line. Median OS and ORR were better with nivolumab (16.4 months vs 14.7 months; HR 0.85; P = 0.752; and 15% [with 5 complete responses] vs 7%), as was the safety profile (22% vs 49% reporting grade 3 and 4 adverse events). 26
The KEYNOTE-224 study27 evaluated pembrolizumab in 104 patients with previously treated advanced HCC. This study showed an ORR of 17%, with 1 complete response and 17 partial responses. One-third of the patients had progressive disease, while 46 had stable disease. Among those who responded, 56% maintained a durable response for more than 1 year. Subsequently, in KEYNOTE 240, pembrolizumab showed an improvement in OS (13.9 months vs 10.6 months; HR, 0.78; P = 0.0238) and PFS (3.0 months versus 2.8 months; HR, 0.78; P = 0.0186) compared with placebo.28 The ORR for pembrolizumab was 16.9% (95% confidence interval [CI], 12.7%-21.8%) versus 2.2% (95% CI, 0.5%-6.4%; P = 0.00001) for placebo. Mean duration of response was 13.8 months.
In the IMbrave150 trial, atezolizumab/bevacizumab combination, compared to sorafenib, had better OS (not estimable vs 13.2 months; P = 0.0006), PFS (6.8 months vs 4.5 months, P < 0.0001), and ORR (33% vs 13%, P < 0.0001), but grade 3-4 events were similar.29 This combination has potential for first-line approval. The COSMIC–312 study is comparing the combination of cabozantinib and atezolizumab to sorafenib monotherapy and cabozantinib monotherapy in advanced HCC.
Resistance to immunotherapy can be extrinsic, associated with activation mechanisms of T-cells, or intrinsic, related to immune recognition, gene expression, and cell-signaling pathways.30 Tumor-immune heterogeneity and antigen presentation contribute to complex resistance mechanisms.31,32 Although clinical outcomes have improved with immune checkpoint inhibitors, the response rate is low and responses are inconsistent, likely due to an immunosuppressive tumor microenvironment.33 Therefore, several novel combinations of checkpoint inhibitors and targeted drugs are being evaluated to bypass some of the resistance mechanisms (Table 3).

Chemotherapy
Multiple combinations of cytotoxic regimens have been evaluated, but efficacy has been modest, suggesting the limited role for traditional chemotherapy in the systemic management of advanced HCC. Response rates to chemotherapy are low and responses are not durable. Gemcitabine- and doxorubicin-based treatment and FOLFOX (5-fluorouracil, leucovorin, oxaliplatin) are some regimens that have been studied, with a median OS of less than 1 year for these regimens.34-36 FOLFOX had a higher response rate (8.15% vs 2.67%; P = 0.02) and longer median OS (6.40 months versus 4.97 months; HR, 0.80; 95% CI, 0.63-1.02; P = 0.07) than doxorubicin.34 With the gemcitabine/oxaliplatin combination, ORR was 18%, with stable disease in 58% of patients, and median PFS and OS were 6.3 months and 11.5 months, respectively.35 In a study that compared doxorubicin and PIAF (cisplatin/interferon a-2b/doxorubicin/5-fluorouracil), median OS was 6.83 months and 8.67 months, respectively (P = 0.83). The hazard ratio for death from any cause in the PIAF group compared with the doxorubicin group was 0.97 (95% CI, 0.71-1.32). PIAF had a higher ORR (20.9%; 95% CI, 12.5%-29.2%) than doxorubicin (10.5%; 95% CI, 3.9%-16.9%).
The phase 3 ALLIANCE study evaluated the combination of sorafenib and doxorubicin in treatment-naïve HCC patients with Child–Pugh class A liver disease, and did not demonstrate superiority with the addition of cytotoxic chemotherapy.37 Indeed, the combination of chemotherapy with sorafenib appears harmful in terms of lower OS (9.3 months vs 10.6 months; HR, 1.06; 95% CI, 0.8-1.4) and worse toxicity. Patients treated with the combination experienced more hematologic (37.8% vs 8.1%) and nonhematologic adverse events (63.6% vs 61.5%).
Locoregional Therapy
The role of locoregional therapy in advanced HCC remains the subject of intense debate. Patients with BCLC stage C HCC with metastatic disease and those with lymph node involvement are candidates for systemic therapy. The optimal candidate for locoregional therapy is the patient with localized intermediate-stage disease, particularly hepatic artery–delivered therapeutic interventions. However, the presence of a solitary large tumor or portal vein involvement constitutes gray areas regarding which therapy to deliver directly to the tumor via the hepatic artery, and increasingly stereotactic body radiation therapy is being offered.
Transarterial Chemoembolization
Transarterial chemoembolization (TACE), with or without chemotherapy, is the most widely adopted locoregional therapy in the management of HCC. TACE exploits the differential vascular supply to the HCC and normal liver parenchyma. Normal liver receives only one-fourth of its blood supply from the hepatic artery (three-fourths from the portal vein), whereas HCC is mainly supplied by the hepatic artery. A survival benefit for TACE compared to best supportive care is widely acknowledged for intermediate-stage HCC, and transarterial embolization (TAE) with gelatin sponge or microspheres is noninferior to TACE.38,39 Overall safety profile and efficacy inform therapy selection in advanced HCC, although the evidence for TACE in advanced HCC is less robust. Although single-institution experiences suggest survival numbers similar to and even superior to sorafenib,40,41 there is a scarcity of large randomized clinical trial data to back this up. Based on this, patients with advanced HCC should only be offered liver-directed therapy within a clinical trial or on a case-by-case basis under multidisciplinary tumor board consensus.
A serious adverse effect of TACE is post-embolization syndrome, which occurs in about 30% of patients and may be associated with poor prognosis.42 The syndrome consists of right upper quadrant abdominal pain, malaise, and worsening nausea/vomiting following the embolization procedure. Laboratory abnormalities and other complications may persist for up to 30 days after the procedure. This is a concern, because post-embolization syndrome may affect the ability to deliver systemic therapy.
Transarterial Radioembolization
In the past few years, there has been an uptick in the utilization of transarterial radioembolization (TARE), which instead of delivering glass beads, as done in TAE, or chemotherapy-infused beads, as done in TACE, delivers the radioisotope Y-90 to the tumor via the hepatic artery. TARE is able to administer larger doses of radiation to the tumor while avoiding normal liver tissue, as compared to external-beam radiation. There has been no head-to-head comparison of these different intra-arterial therapy approaches, but TARE with Y-90 has been shown to be safe in patients with portal vein thrombosis. A recent multicenter retrospective study of TARE demonstrated a median OS of 8.8 to 10.8 months in patients with BCLC C HCC,43 and in a large randomized study of Y-90 compared to sorafenib in advanced and previously treated intermediate HCC, there was no difference in median OS between the treatment modalities (8 months for selective internal radiotherapy, 9 months for sorafenib; P = 0.18). Treatment with Y-90 was better tolerated.44 A major impediment to the adoption of TARE is the time it takes to order, plan, and deliver Y-90 to patients. Radio-embolization-induced liver disease, similar to post-embolization syndrome, is characterized by jaundice and ascites, which may occur 4 to 8 weeks postprocedure and is more common in patients with HCC who do not have cirrhosis. Compared to TACE, TARE may offer a better adverse effect profile, with improvement in quality of life.
Combination of Systemic and Locoregional Therapy
Even in carefully selected patients with intermediate- and advanced-stage HCC, locoregional therapy is not curative. Tumor embolization may promote more angiogenesis, and hence tumor progression, by causing hypoxia and upregulation of hypoxia-inducible factor.45 This upregulation of angiogenesis as a resistance mechanism to tumor embolization provides a rationale for combining systemic therapy (typically based on abrogating angiogenesis) with TACE/TAE. Most of the experience has been with sorafenib in intermediate-stage disease, and the results have been disappointing. The administration of sorafenib after at least a partial response with TACE did not provide additional benefit in terms of time to progression.46 Similarly, in the SPACE trial, concurrent therapy with TACE-doxorubicin-eluting beads and sorafenib compared to TACE-doxorubicin-eluting beads and placebo yielded similar time to progression numbers for both treatment modalities.47 While the data have been disappointing in intermediate-stage disease, as described earlier, registry data suggest that patients with advanced-stage disease may benefit from this approach.48
In the phase 2 TACTICS trial, 156 patients with unresectable HCC were randomized to receive TACE alone or sorafenib plus TACE, with a novel endpoint, time to untreatable progression (TTUP) and/or progression to TACE refractoriness.49 Treatment with sorafenib following TACE was continued until TTUP, decline in liver function to Child–Pugh class C, or the development of vascular invasion or extrahepatic spread. Development of new lesions while on sorafenib was not considered as progressive disease as long as the lesions were amenable to TACE. In this study, PFS was longer with sorafenib-TACE compared to TACE alone (26.7 months vs 20.6 months; P = 0.02). However, the TTUP endpoint needs further validation, and we are still awaiting the survival outcomes of this study. At this time, there are insufficient data to recommend the combination of liver-directed locoregional therapy and sorafenib or other systemic therapy options outside of a clinical trial setting.
Current Treatment Approach for Advanced HCC (BCLC-C)
Although progress is being made in the development of effective therapies, advanced HCC is generally incurable. These patients experience significant symptom burden throughout the course of the disease. Therefore, the optimal treatment plan must focus on improving or maintaining quality of life, in addition to overall efficacy. It is important to actively involve patients in treatment decisions for an individualized treatment plan, and to discuss the best strategy for incorporating current advances in targeted and immunotherapies. The paradigm of what constitutes first-line treatment for advanced HCC is shifting due to the recent systemic therapy approvals. Prior to the availability of these therapies, many patients with advanced HCC were treated with repeated locoregional therapies. For instance, TACE was often used to treat unresectable HCC multiple times beyond progression. There was no consensus on the definition of TACE failure, and hence it was used in broader, unselected populations. Retrospective studies suggest that continuing TACE after refractoriness or failure may not be beneficial, and may delay subsequent treatments because of deterioration of liver function or declines in performance status. With recent approvals of several systemic therapy options, including immunotherapy, it is vital to conduct a risk-benefit assessment prior to repeating TACE after failure, so that patients are not denied the use of available systemic therapeutic options due to declined performance status or organ function from these procedures. The optimal timing and the sequence of systemic and locoregional therapy must be carefully evaluated by a multidisciplinary team.
CASE CONCLUSION
An important part of evaluating a new patient with HCC is to determine whether they are a candidate for curative therapies, such as transplant or surgical resection. These are no longer an option for patients with intermediate disease. For patients with advanced disease characteristics, such as vascular invasion or systemic metastasis, the evidence supports using systemic therapy with sorafenib or lenvatinib. Lenvatinib, with a better tolerance profile and response rate, is the treatment of choice for the patient described in the case scenario. Lenvatinib is also indicated for first-line treatment of advanced HCC, and is useful in very aggressive tumors, such as those with an AFP level exceeding 200 ng/mL.
Future Directions
The emerging role of novel systemic therapeutics, including immunotherapy, has drastically changed the treatment landscape for hepatocellular cancers, with 6 new drugs for treating advanced hepatocellular cancers approved recently. While these systemic drugs have improved survival in advanced HCC in the past decade, patient selection and treatment sequencing remain a challenge, due to a lack of biomarkers capable of predicting antitumor responses. In addition, there is an unmet need for treatment options for patients with Child–Pugh class B7 and C liver disease and poor performance status.
The goal of future management should be to achieve personalized care aimed at improved safety and efficacy by targeting multiple cancer pathways in the HCC cascade with combination treatments. Randomized clinical trials to improve patient selection and determine the proper sequence of treatments are needed. Given the heterogeneity of HCC, molecular profiling of the tumor to differentiate responders from nonresponders may elucidate potential biomarkers to effectively guide treatment recommendations.
1. Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424.
2. Altekruse SF, McGlynn KA, Reichman ME. Hepatocellular carcinoma incidence, mortality, and survival trends in the United States from 1975 to 2005. J Clin Oncol. 2009;27:1485-1491.
3. Welzel TM, Graubard BI, Zeuzem S, et al. Metabolic syndrome increases the risk of primary liver cancer in the United States: a study in the SEER-Medicare database. Hepatology. 2011;54:463-471.
4. Schutte K, Schulz C, Poranzke J, et al. Characterization and prognosis of patients with hepatocellular carcinoma (HCC) in the non-cirrhotic liver. BMC Gastroenterol. 2014;14:117.
5. Llovet JM, Bru C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis. 1999;19:329-338.
6. Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet. 2018;391:1301-1314.
7. Schulze K, Imbeaud S, Letouzé E, et al. Exome sequencing of hepatocellular carcinomas identifies new mutational signatures and potential therapeutic targets. Nat Genet. 2015;47:505-511.
8. Thorgeirsson SS, Grisham JW. Molecular pathogenesis of human hepatocellular carcinoma. Nat Genet. 2002;31:339-346.
9. Dhanasekaran R, Bandoh S, Roberts LR. Molecular pathogenesis of hepatocellular carcinoma and impact of therapeutic advances. F1000Res. 2016;5.
10. Schulze K, Imbeaud S, Letouze E, et al. Exome sequencing of hepatocellular carcinomas identifies new mutational signatures and potential therapeutic targets. Nat Genet. 2015;47:505-511.
11. Cancer Genome Atlas Research Network. Electronic address: [email protected]; Cancer Genome Atlas Research Network. Comprehensive and integrative genomic characterization of hepatocellular carcinoma. Cell. 2017;169:1327-1134.
12. Chae YK, Ranganath K, Hammerman PS, et al: Inhibition of the fibroblast growth factor receptor (FGFR) pathway: the current landscape and barriers to clinical application. Oncotarget. 2016;8:16052-16074.
13. Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378-390.
14. Jackson R, Psarelli EE, Berhane S, et al. Impact of viral status on survival in patients receiving sorafenib for advanced hepatocellular cancer: a meta-analysis of randomized phase III trials. J Clin Oncol. 2017;35:622-628.
15. Cheng AL, Kang YK, Chen Z, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25-34.
16. Da Fonseca LG, Barroso-Sousa R, Bento AD, et al. Safety and efficacy of sorafenib in patients with Child-Pugh B advanced hepatocellular carcinoma. Mol Clin Oncol. 2015;3:793-796.
17. Kudo M, Finn RS, Qin S, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018;391:1163-1173.
18. Bruix J, Tak W-Y, Gasbarrini A, et al. Regorafenib as second-line therapy for intermediate or advanced hepatocellular carcinoma: Multicentre, open-label, phase II safety study. Eur J Cancer. 2013;49:3412-3419.
19. Bruix J, Qin S, Merle P, et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;389:56-66.
20. Bruix J, Merle P, Granito A, et al. Hand-foot skin reaction (HFSR) and overall survival (OS) in the phase 3 RESORCE trial of regorafenib for treatment of hepatocellular carcinoma (HCC) progressing on sorafenib. J Clin Oncol. 2018;36:412-412.
21. Finn RS, Merle P, Granito A, et al. Outcomes of sequential treatment with sorafenib followed by regorafenib for HCC: Additional analyses from the phase III RESORCE trial. J Hepatol. 2018;69:353-358.
22. Abou-Alfa GK, Meyer T, Cheng A-L, et al. Cabozantinib (C) versus placebo (P) in patients (pts) with advanced hepatocellular carcinoma (HCC) who have received prior sorafenib: Results from the randomized phase III CELESTIAL trial. J Clin Oncol. 2018;36:207-207.
23. Zhu AX, Park JO, Ryoo B-Y, et al. Ramucirumab versus placebo as second-line treatment in patients with advanced hepatocellular carcinoma following first-line therapy with sorafenib (REACH): a randomised, double-blind, multicentre, phase 3 trial. Lancet Oncology. 2015;16:859-870.
24. Zhu AX, Kang Y-K, Yen C-J, et al. Ramucirumab after sorafenib in patients with advanced hepatocellular carcinoma and increased αfetoprotein concentrations (REACH-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncology. 2019;20:282-296.
25. El-Khoueiry AB, Sangro B, Yau T, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet. 2017;389:2492-2502.
26. Yau T, Park JW, Finn RS, et al. CheckMate 459: A randomized, multi-center phase III study of nivolumab (NIVO) vs sorafenib (SOR) as first-line (1L) treatment in patients (pts) with advanced hepatocellular carcinoma (aHCC). Ann Oncol. 2020;30:v874-v875.
27. Zhu AX, Finn RS, Cattan S, et al. KEYNOTE-224: Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib. J Clin Oncol. 2018;36:942-952.
28. Finn RS, Ryoo BY, Merle P, et al. Pembrolizumab as second-line therapy in patients with advanced hepatocellular carcinoma in KEYNOTE-240: a randomized, double-blind, phase III trial. J Clin Oncol. 2020;38:193-202.
29. Cheng A-L, Qin S, Ikeda M, et al. IMbrave150: efficacy and safety results from a ph III study evaluating atezolizumab (atezo) + bevacizumab (bev) vs sorafenib (sor) as first treatment (tx) for patients (pts) with unresectable hepatocellular carcinoma (HCC). Ann Oncol. 2019;30 (suppl_9):ix183-ix202.
30. Jiang Y, Han Q-J, Zhang J. Hepatocellular carcinoma: Mechanisms of progression and immunotherapy. World J Gastroenterol. 2019;25:3151-3167.
31. Xu F, Jin T, Zhu Y, et al. Immune checkpoint therapy in liver cancer. J Exp Clin Cancer Res. 2018;37:110.
32. Koyama S, Akbay EA, Li YY, et al. Adaptive resistance to therapeutic PD-1 blockade is associated with upregulation of alternative immune checkpoints. Nat Commun. 2016;7:10501.
33. Prieto J, Melero I, Sangro B. Immunological landscape and immunotherapy of hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol. 2015;12:681-700.
34. Qin S, Bai Y, Lim HY, et al. Randomized, multicenter, open-label study of oxaliplatin plus fluorouracil/leucovorin versus doxorubicin as palliative chemotherapy in patients with advanced hepatocellular carcinoma from Asia. J Clin Oncol. 2013;31:3501-3508.
35. Louafi S, Boige V, Ducreux M, et al. Gemcitabine plus oxaliplatin (GEMOX) in patients with advanced hepatocellular carcinoma (HCC). Cancer. 2007;109:1384-1390.
36. Tang A, Chan AT, Zee B, et al. A randomized phase iii study of doxorubicin versus cisplatin/interferon α-2b/doxorubicin/fluorouracil (PIAF) combination chemotherapy for unresectable hepatocellular carcinoma. J Natl Cancer Inst. 2005;97:1532-1538.
37. Abou-Alfa GK, Niedzwieski D, Knox JJ, et al. Phase III randomized study of sorafenib plus doxorubicin versus sorafenib in patients with advanced hepatocellular carcinoma (HCC): CALGB 80802 (Alliance). J Clin Oncol. 2016;34:192.
38. Llovet JM, Real MI, Montana X, et al. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet. 2002;359:1734-1739.
39. Brown KT, Do RK, Gonen M, et al. randomized trial of hepatic artery embolization for hepatocellular carcinoma using doxorubicin-eluting microspheres compared with embolization with microspheres alone. J Clin Oncol. 2016;34:2046-2053.
40. Kirstein MM, Voigtlander T, Schweitzer N, et al. Transarterial chemoembolization versus sorafenib in patients with hepatocellular carcinoma and extrahepatic disease. United European Gastroenterol J. 2018;6:238-246.
41. Pinter M, Hucke F, Graziadei I, et al. Advanced-stage hepatocellular carcinoma: transarterial chemoembolization versus sorafenib. Radiology. 2012;263:590-599.
42. Mason MC, Massarweh NN, Salami A, et al. Post-embolization syndrome as an early predictor of overall survival after transarterial chemoembolization for hepatocellular carcinoma. HPB (Oxford). 2015;17:1137-1144.
43. Sangro B, Maini CL, Ettorre GM, et al. Radioembolisation in patients with hepatocellular carcinoma that have previously received liver-directed therapies. Eur J Nucl Med Mol Imaging. 2018;45:1721-1730.
44. Vilgrain V, Pereira H, Assenat E, et al. Efficacy and safety of selective internal radiotherapy with yttrium-90 resin microspheres compared with sorafenib in locally advanced and inoperable hepatocellular carcinoma (SARAH): an open-label randomised controlled phase 3 trial. Lancet Oncol. 2017;18:1624-1636.
45. Sergio A, Cristofori C, Cardin R, et al. Transcatheter arterial chemoembolization (TACE) in hepatocellular carcinoma (HCC): the role of angiogenesis and invasiveness. Am J Gastroenterol. 2008;103:914-921.
46. Kudo M, Imanaka K, Chida N, et al. Phase III study of sorafenib after transarterial chemoembolisation in Japanese and Korean patients with unresectable hepatocellular carcinoma. Eur J Cancer. 2011;47:2117-2127.
47. Lencioni R, Llovet JM, Han G, et al. Sorafenib or placebo plus TACE with doxorubicin-eluting beads for intermediate stage HCC: The SPACE trial. J Hepatol. 2016;64:1090-1098.
48. Geschwind JF, Chapiro J. Sorafenib in combination with transarterial chemoembolization for the treatment of hepatocellular carcinoma. Clin Adv Hematol Oncol. 2016;14:585-587.
49. Kudo M, Ueshima K, Ikeda M, et al. Randomized, open label, multicenter, phase II trial comparing transarterial chemoembolization (TACE) plus sorafenib with TACE alone in patients with hepatocellular carcinoma (HCC): TACTICS trial. J Clin Oncol. 2018;36:206.
1. Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424.
2. Altekruse SF, McGlynn KA, Reichman ME. Hepatocellular carcinoma incidence, mortality, and survival trends in the United States from 1975 to 2005. J Clin Oncol. 2009;27:1485-1491.
3. Welzel TM, Graubard BI, Zeuzem S, et al. Metabolic syndrome increases the risk of primary liver cancer in the United States: a study in the SEER-Medicare database. Hepatology. 2011;54:463-471.
4. Schutte K, Schulz C, Poranzke J, et al. Characterization and prognosis of patients with hepatocellular carcinoma (HCC) in the non-cirrhotic liver. BMC Gastroenterol. 2014;14:117.
5. Llovet JM, Bru C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis. 1999;19:329-338.
6. Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet. 2018;391:1301-1314.
7. Schulze K, Imbeaud S, Letouzé E, et al. Exome sequencing of hepatocellular carcinomas identifies new mutational signatures and potential therapeutic targets. Nat Genet. 2015;47:505-511.
8. Thorgeirsson SS, Grisham JW. Molecular pathogenesis of human hepatocellular carcinoma. Nat Genet. 2002;31:339-346.
9. Dhanasekaran R, Bandoh S, Roberts LR. Molecular pathogenesis of hepatocellular carcinoma and impact of therapeutic advances. F1000Res. 2016;5.
10. Schulze K, Imbeaud S, Letouze E, et al. Exome sequencing of hepatocellular carcinomas identifies new mutational signatures and potential therapeutic targets. Nat Genet. 2015;47:505-511.
11. Cancer Genome Atlas Research Network. Electronic address: [email protected]; Cancer Genome Atlas Research Network. Comprehensive and integrative genomic characterization of hepatocellular carcinoma. Cell. 2017;169:1327-1134.
12. Chae YK, Ranganath K, Hammerman PS, et al: Inhibition of the fibroblast growth factor receptor (FGFR) pathway: the current landscape and barriers to clinical application. Oncotarget. 2016;8:16052-16074.
13. Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378-390.
14. Jackson R, Psarelli EE, Berhane S, et al. Impact of viral status on survival in patients receiving sorafenib for advanced hepatocellular cancer: a meta-analysis of randomized phase III trials. J Clin Oncol. 2017;35:622-628.
15. Cheng AL, Kang YK, Chen Z, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25-34.
16. Da Fonseca LG, Barroso-Sousa R, Bento AD, et al. Safety and efficacy of sorafenib in patients with Child-Pugh B advanced hepatocellular carcinoma. Mol Clin Oncol. 2015;3:793-796.
17. Kudo M, Finn RS, Qin S, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018;391:1163-1173.
18. Bruix J, Tak W-Y, Gasbarrini A, et al. Regorafenib as second-line therapy for intermediate or advanced hepatocellular carcinoma: Multicentre, open-label, phase II safety study. Eur J Cancer. 2013;49:3412-3419.
19. Bruix J, Qin S, Merle P, et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;389:56-66.
20. Bruix J, Merle P, Granito A, et al. Hand-foot skin reaction (HFSR) and overall survival (OS) in the phase 3 RESORCE trial of regorafenib for treatment of hepatocellular carcinoma (HCC) progressing on sorafenib. J Clin Oncol. 2018;36:412-412.
21. Finn RS, Merle P, Granito A, et al. Outcomes of sequential treatment with sorafenib followed by regorafenib for HCC: Additional analyses from the phase III RESORCE trial. J Hepatol. 2018;69:353-358.
22. Abou-Alfa GK, Meyer T, Cheng A-L, et al. Cabozantinib (C) versus placebo (P) in patients (pts) with advanced hepatocellular carcinoma (HCC) who have received prior sorafenib: Results from the randomized phase III CELESTIAL trial. J Clin Oncol. 2018;36:207-207.
23. Zhu AX, Park JO, Ryoo B-Y, et al. Ramucirumab versus placebo as second-line treatment in patients with advanced hepatocellular carcinoma following first-line therapy with sorafenib (REACH): a randomised, double-blind, multicentre, phase 3 trial. Lancet Oncology. 2015;16:859-870.
24. Zhu AX, Kang Y-K, Yen C-J, et al. Ramucirumab after sorafenib in patients with advanced hepatocellular carcinoma and increased αfetoprotein concentrations (REACH-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncology. 2019;20:282-296.
25. El-Khoueiry AB, Sangro B, Yau T, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet. 2017;389:2492-2502.
26. Yau T, Park JW, Finn RS, et al. CheckMate 459: A randomized, multi-center phase III study of nivolumab (NIVO) vs sorafenib (SOR) as first-line (1L) treatment in patients (pts) with advanced hepatocellular carcinoma (aHCC). Ann Oncol. 2020;30:v874-v875.
27. Zhu AX, Finn RS, Cattan S, et al. KEYNOTE-224: Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib. J Clin Oncol. 2018;36:942-952.
28. Finn RS, Ryoo BY, Merle P, et al. Pembrolizumab as second-line therapy in patients with advanced hepatocellular carcinoma in KEYNOTE-240: a randomized, double-blind, phase III trial. J Clin Oncol. 2020;38:193-202.
29. Cheng A-L, Qin S, Ikeda M, et al. IMbrave150: efficacy and safety results from a ph III study evaluating atezolizumab (atezo) + bevacizumab (bev) vs sorafenib (sor) as first treatment (tx) for patients (pts) with unresectable hepatocellular carcinoma (HCC). Ann Oncol. 2019;30 (suppl_9):ix183-ix202.
30. Jiang Y, Han Q-J, Zhang J. Hepatocellular carcinoma: Mechanisms of progression and immunotherapy. World J Gastroenterol. 2019;25:3151-3167.
31. Xu F, Jin T, Zhu Y, et al. Immune checkpoint therapy in liver cancer. J Exp Clin Cancer Res. 2018;37:110.
32. Koyama S, Akbay EA, Li YY, et al. Adaptive resistance to therapeutic PD-1 blockade is associated with upregulation of alternative immune checkpoints. Nat Commun. 2016;7:10501.
33. Prieto J, Melero I, Sangro B. Immunological landscape and immunotherapy of hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol. 2015;12:681-700.
34. Qin S, Bai Y, Lim HY, et al. Randomized, multicenter, open-label study of oxaliplatin plus fluorouracil/leucovorin versus doxorubicin as palliative chemotherapy in patients with advanced hepatocellular carcinoma from Asia. J Clin Oncol. 2013;31:3501-3508.
35. Louafi S, Boige V, Ducreux M, et al. Gemcitabine plus oxaliplatin (GEMOX) in patients with advanced hepatocellular carcinoma (HCC). Cancer. 2007;109:1384-1390.
36. Tang A, Chan AT, Zee B, et al. A randomized phase iii study of doxorubicin versus cisplatin/interferon α-2b/doxorubicin/fluorouracil (PIAF) combination chemotherapy for unresectable hepatocellular carcinoma. J Natl Cancer Inst. 2005;97:1532-1538.
37. Abou-Alfa GK, Niedzwieski D, Knox JJ, et al. Phase III randomized study of sorafenib plus doxorubicin versus sorafenib in patients with advanced hepatocellular carcinoma (HCC): CALGB 80802 (Alliance). J Clin Oncol. 2016;34:192.
38. Llovet JM, Real MI, Montana X, et al. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet. 2002;359:1734-1739.
39. Brown KT, Do RK, Gonen M, et al. randomized trial of hepatic artery embolization for hepatocellular carcinoma using doxorubicin-eluting microspheres compared with embolization with microspheres alone. J Clin Oncol. 2016;34:2046-2053.
40. Kirstein MM, Voigtlander T, Schweitzer N, et al. Transarterial chemoembolization versus sorafenib in patients with hepatocellular carcinoma and extrahepatic disease. United European Gastroenterol J. 2018;6:238-246.
41. Pinter M, Hucke F, Graziadei I, et al. Advanced-stage hepatocellular carcinoma: transarterial chemoembolization versus sorafenib. Radiology. 2012;263:590-599.
42. Mason MC, Massarweh NN, Salami A, et al. Post-embolization syndrome as an early predictor of overall survival after transarterial chemoembolization for hepatocellular carcinoma. HPB (Oxford). 2015;17:1137-1144.
43. Sangro B, Maini CL, Ettorre GM, et al. Radioembolisation in patients with hepatocellular carcinoma that have previously received liver-directed therapies. Eur J Nucl Med Mol Imaging. 2018;45:1721-1730.
44. Vilgrain V, Pereira H, Assenat E, et al. Efficacy and safety of selective internal radiotherapy with yttrium-90 resin microspheres compared with sorafenib in locally advanced and inoperable hepatocellular carcinoma (SARAH): an open-label randomised controlled phase 3 trial. Lancet Oncol. 2017;18:1624-1636.
45. Sergio A, Cristofori C, Cardin R, et al. Transcatheter arterial chemoembolization (TACE) in hepatocellular carcinoma (HCC): the role of angiogenesis and invasiveness. Am J Gastroenterol. 2008;103:914-921.
46. Kudo M, Imanaka K, Chida N, et al. Phase III study of sorafenib after transarterial chemoembolisation in Japanese and Korean patients with unresectable hepatocellular carcinoma. Eur J Cancer. 2011;47:2117-2127.
47. Lencioni R, Llovet JM, Han G, et al. Sorafenib or placebo plus TACE with doxorubicin-eluting beads for intermediate stage HCC: The SPACE trial. J Hepatol. 2016;64:1090-1098.
48. Geschwind JF, Chapiro J. Sorafenib in combination with transarterial chemoembolization for the treatment of hepatocellular carcinoma. Clin Adv Hematol Oncol. 2016;14:585-587.
49. Kudo M, Ueshima K, Ikeda M, et al. Randomized, open label, multicenter, phase II trial comparing transarterial chemoembolization (TACE) plus sorafenib with TACE alone in patients with hepatocellular carcinoma (HCC): TACTICS trial. J Clin Oncol. 2018;36:206.
Refining your approach to hypothyroidism treatment
CASE
A 38-year-old woman presents for a routine physical. Other than urgent care visits for 1 episode of influenza and 2 upper respiratory illnesses, she has not seen a physician for a physical in 5 years. She denies any significant medical history. She takes naproxen occasionally for chronic right knee pain. She does not use tobacco or alcohol. Recently, she has started using a meal replacement shake at lunchtime for weight management. She performs aerobic exercise 30 to 40 minutes per day, 5 days per week. Her family history is significant for type 2 diabetes mellitus, arthritis, heart disease, and hyperlipidemia on her mother’s side. She is single, is not currently sexually active, works as a pharmacy technician, and has no children. A high-risk human papillomavirus test was normal 4 years ago.
A review of systems is notable for a 20-pound weight gain over the past year, worsening heartburn over the past 2 weeks, and chronic knee pain, which is greater in the right knee than the left. She denies weakness, fatigue, nausea, diarrhea, constipation, or abdominal pain. Vital signs reveal a blood pressure of 146/88 mm Hg, a heart rate of 63 bpm, a temperature of 98°F (36.7°C), a respiratory rate of 16, a height of 5’7’’ (1.7 m), a weight of 217 lbs (98.4 kg), and a peripheral capillary oxygen saturation (SpO2) of 99% on room air. The physical exam reveals a body mass index (BMI) of 34, warm dry skin, and coarse brittle hair.
Lab results reveal a thyroid-stimulating hormone (TSH) level of 11.17 mIU/L (reference range, 0.45-4.5 mIU/L) and a free thyroxine (T4) of 0.58 ng/dL (reference range, 0.8-2.8 ng/dL). A basic metabolic panel and hemoglobin A1C level are normal.
What would you recommend?
In the United States, the prevalence of overt hypothyroidism (defined as a TSH level > 4.5 mIU/L and a low free T4) among people ≥ 12 years of age was estimated at 0.3% based on National Health and Nutrition Examination Survey (NHANES) data from 1999-2002.1 Subclinical hypothyroidism (TSH level > 4.5 mIU/L but < 10 mIU/L and a normal T4 level) is even more common, with an estimated prevalence of 3.4%.1 Hypothyroidism is more common in females and occurs more frequently in Caucasian Americans and Mexican Americans than in African Americans.1
The most common etiologies of hypothyroidism include autoimmune thyroiditis (eg, Hashimoto thyroiditis, atrophic autoimmune thyroiditis) and iatrogenic causes (eg, after radioactive iodine ablation or thyroidectomy) (TABLE 1).2-4
Initiating thyroid hormone replacement
Factors to consider when starting a patient on thyroid hormone replacement include age, weight, symptom severity, TSH level, goal TSH value, adverse effects from thyroid supplements, history of cardiac disease, and, for women of child-bearing age, the desire for pregnancy vs the use of contraceptives. Most adult patients < 50 years with overt hypothyroidism can begin a weight-based dose of levothyroxine: ~1.6 mcg/kg/d (based on ideal body weight).3
Continue to: For adults with cardiac disease...
For adults with cardiac disease, the risk of over-replacement limits initial dosing to 25 to 50 mcg/d for patients < 50 years (12.5-25 mcg/d; ≥ 50 years).3 For adults with subclinical hypothyroidism, it is reasonable to begin therapy at a lower daily dose (eg, 25-75 mcg/d) depending on baseline TSH level, symptoms (the patient may be asymptomatic), and the presence of cardiac disease (TABLE 23,4). Consider treatment in patients with subclinical hypothyroidism particularly when patients have a goiter or dyslipidemia and in women contemplating pregnancy in the near future. Elderly patients may require a dose 20% to 25% lower than younger adults because of decreased body mass.3
Levothyroxine is considered first-line therapy for hypothyroidism because of its low cost, dose consistency, low risk of allergic reactions, and potential to cause fewer cardiac adverse effects than triiodothyronine (T3) products such as desiccated thyroid extract.5 Although data have not shown an absolute increase in cardiovascular adverse effects, T3 products have a higher T3 vs T4 ratio, giving them a theoretically increased risk.5,6 Desiccated thyroid extract also has been associated with allergic reactions.5
Use of liothyronine alone or in combination with levothyroxine lacks evidence and guideline support.4 Furthermore, it is dosed twice daily, which makes it less convenient, and concerns still exist that there may be an increase in cardiovascular adverse effects.4,6 See TABLE 37 for a summary of available products and their equivalent doses.
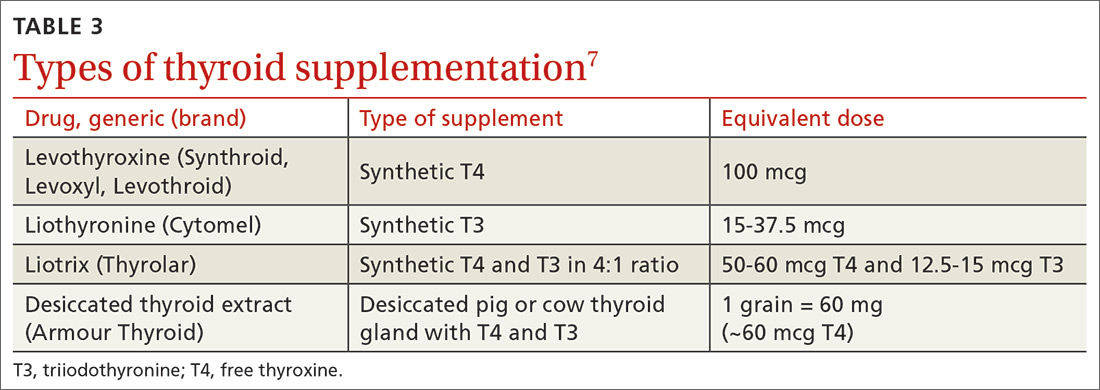
Maintaining patients on therapy
The maintenance phase begins once hypothyroidism is diagnosed and treatment is initiated. This phase includes regular monitoring with laboratory studies, office visits, and as-needed adjustments in hormone replacement dosing. The frequency at which all of these occur is variable and based on a number of factors including the patient’s other medical conditions, use of other medications including over-the-counter agents, the patient’s age, weight changes, and pregnancy status.3,4,8 In general, dosage adjustments of 12.5 to 25 mcg can be made at 6- to 8-week intervals based on repeat TSH measurements, patient symptoms, and comorbidities.3
Once a patient is symptomatically stable and laboratory values have normalized, the recommended frequency of laboratory evaluation and office visits is every 12 months, barring significant changes in any of the factors mentioned above. At each visit, physicians should perform medication (including supplements) reconciliation and discuss any health condition updates. Changes to the therapy plan, including frequency or timing of laboratory tests, may be necessary if patients begin taking medications that alter the absorption or function of levothyroxine (eg, steroids).
Continue to: To maximize absorption...
To maximize absorption, providers should review with patients the optimal way to take thyroid hormones. Levothyroxine is approximately 70% to 80% absorbed under ideal conditions, which means taking it in the morning at least 30 to 60 minutes before eating or 3 to 4 hours after the last meal of the day.3,9-13 Of note, TSH levels may increase slightly in patients taking proton pump inhibitors, but this does not usually require a dose increase of thyroid hormone.11 Given that some supplements, particularly iron and calcium, can interfere with absorption, it is recommended to maintain a 3- to 4-hour gap between taking those supplements and taking levothyroxine.12-14 For those patients unable or unwilling to adhere to these recommendations, an increase in levothyroxine dose may be required in order to compensate for the decreased absorption.
Don’t adjust hormone therapy based on clinical presentation alone. While clinical symptoms are important, it is not recommended to adjust hormone therapy based solely on clinical presentation. Common hypothyroid symptoms of dry skin, edema, weight gain, and fatigue may be caused by other medical conditions. While indices including Achilles reflex time and basal metabolic rate have shown some correlation to thyroid dysfunction, there has been limited evidence to show that longitudinal index changes reflect subtle changes in thyroid hormone levels.3
The most recent guidelines from the American Thyroid Association recommend that, “Symptoms should be followed, but considered in the context of serum thyrotropin values, relevant comorbidities, and other potential causes.”3
Special populations/circumstances to keep in mind
Malabsorption conditions. When a higher than expected weight-based dose of levothyroxine is required, physicians should review administration timing, adherence, and comorbid medical conditions that can affect absorption.
Several studies, for example, have demonstrated the impact of Helicobacter pylori gastritis on levothyroxine absorption and subsequent TSH levels.15-17 In one nonrandomized prospective study, patients with H pylori and hypothyroidism who were previously thought to be unresponsive to levothyroxine therapy had a decrease in average TSH level from 30.5 mIU/L to 4.2 mIU/L after H pylori was eradicated.15 Autoimmune atrophic gastritis and celiac disease, both of which are more common in those with other autoimmune diseases, are also associated with the need for higher than expected levothyroxine doses.17,18
Continue to: A history of gastric bypass surgery...
A history of gastric bypass surgery alone is not considered a risk factor for poor absorption of thyroid hormone, given that the majority of levothyroxine absorption occurs in the ileum.19,20 However, advancing age (> 70 years) and extreme obesity (BMI > 40) are independent risk factors for decreased levothyroxine absorption.20,21
Women of reproductive age and pregnant women. Overt untreated or undertreated hypothyroidism can be associated with increased risk of maternal and fetal complications including decreased fertility, miscarriage, preterm delivery, lower birth rates, and infant cognitive deficits.3,22 Therefore, the main focus should be optimization of thyroid hormone levels prior to and during pregnancy.3,4,8,22 Thyroid hormone replacement needs to be increased during pregnancy in approximately 50% to 85% of women using thyroid replacement prior to pregnancy, but the dose requirements vary based on the underlying etiology of thyroid dysfunction.
One initial option for patients on a stable dose before pregnancy is to increase their daily dose by a half tablet (1.5 × daily dose) immediately after home confirmation of pregnancy, until finer dose adjustments (usually increases of 25%-60% ) can be made by a physician. Experts recommend that a TSH level be obtained every 4 weeks until mid-gestation and then at least once around 30 weeks’ gestation to ensure specific targets are being met with dose adjustments.22 Optimal thyrotropin reference ranges during conception and pregnancy can be found in the literature.23
Patients who have positive antibodies and normal thyroid function tests. Patients who are screened for thyroid disorders may demonstrate normal thyroid function (ie, euthyroid) with TSH, free T4, and, if checked, free T3, all within normal ranges. Despite these normal lab results, patients may have additional test results that demonstrate positive thyroid autoantibodies including thyroglobulin antibodies and/or thyroid peroxidase antibodies. Thyroid autoimmunity itself has been associated with a range of other autoimmune conditions as well as an increased risk of thyroid cancer in those with Hashimoto thyroiditis.24 Two studies showed that prophylactic treatment of euthyroid patients with levothyroxine led to a reduction in antibody levels and a lower TSH level.25,26 However, no studies have focused on patient-oriented outcomes such as hospitalizations, quality of life, or symptoms. If the patient remains asymptomatic, we recommend no treatment, but that the patient’s TSH levels be monitored every 12 months.27
Elderly patients. Population data have shown that TSH increases normally with age, with a TSH level of 7.5 mIU/L being the upper limit of normal for a population of healthy adults > 80 years of age.28,29 Overall, studies have failed to show any benefit in treating elderly patients with subclinical hypothyroidism unless their TSH level exceeds 10 mIU/L.6,21 The one exception is elderly patients with heart failure in whom untreated subclinical hypothyroidism has been shown to be associated with higher mortality.30
Continue to: Elderly patients are at higher risk...
Elderly patients are at higher risk for adverse effects of thyroid over-replacement, including atrial fibrillation and osteoporosis. While there have been no randomized trials examining target TSH levels in this population, a reasonable recommendation is a goal TSH level of 4 to 6 mIU/L for elderly patients ≥ 70 years.4
CASE
As a result of the patient’s elevated TSH level and symptoms of hypothyroidism, you start levothyroxine 150 mcg/d by mouth, counsel her on potential adverse effects, and schedule a follow-up visit with another TSH check in 6 weeks.
Follow-up laboratory studies 6 weeks later reveal a TSH level of 5.86 mIU/L (reference range, 0.45-4.5 mIU/L) and a free T4 level of 0.74 ng/dL (reference range, 0.8-2.8 ng/dL). Based on those results, you increase the dose of levothyroxine to 175 mcg/d.
At her follow-up visit 12 weeks after initial presentation, her TSH level is 3.85 mIU/L. She reports feeling better overall with less fatigue, and she has lost 5 pounds since her last visit. You recommend she continue levothyroxine 175 mcg/d after reviewing medication compliance with the patient and ensuring she is indeed taking it in the morning, at least 30 minutes prior to eating. With improved but not resolved symptoms, she agrees to follow-up with repeat TSH laboratory studies in 6 weeks to determine whether further dose adjustments are necessary. Given that she is of reproductive age and her TSH level is suboptimal for pregnancy, you caution her about heightened pregnancy/fetal risks with a suboptimal TSH and recommend that she use reliable contraception.
CORRESPONDENCE
Christopher Bunt, MD, FAAFP, 5 Charleston Center Drive, Suite 263, MSC 192,Charleston, SC 29425; [email protected]
1. Aoki Y, Belin RM, Clickner R, et al. Serum TSH and total T4 in the United States population and their association with participant characteristics: National Health and Nutrition Examination Survey (NHANES 1999-2002). Thyroid. 2007;17:1211-1223.
2. Vaidya B, Pearce SH. Management of hypothyroidism in adults. BMJ. 2008;337:a801.
3. Garber JR, Cobin RH, Gharib H, et al. Clinical practice guidelines for hypothyroidism in adults: cosponsored by the American Association of Clinical Endocrinologists and the American Thyroid Association. Endocr Pract. 2012;18:988-1028.
4. Jonklaas J, Bianco AC, Bauer AJ, et al. Guidelines for the treatment of hypothyroidism: prepared by the American Thyroid Association task force on thyroid hormone replacement. Thyroid. 2014;24:1670-1751.
5. Toft AD. Thyroxine therapy. N Engl J Med. 1994;331:174-180.
6. Floriani C, Gencer B, Collet TH, et al. Subclinical thyroid dysfunction and cardiovascular diseases: 2016 update. Eur Heart J. 2018;39:503-507.
7. Lexi-Comp, Inc. (Lexi-Drugs®). https://online.lexi.com/lco/action/login. Accessed July 7, 2017.
8. Okosieme O, Gilbert J, Abraham P, et al. Management of primary hypothyroidism: statement by the British Thyroid Association Executive Committee. Clin Endocrinol (Oxf). 2016;84:799-808.
9. Fish LH, Schwartz HL, Cavanaugh J, et al. Replacement dose, metabolism, and bioavailability of levothyroxine in the treatment of hypothyroidism. Role of triiodothyronine in pituitary feedback in humans. N Engl J Med. 1987;316:764-770.
10. John-Kalarickal J, Pearlman G, Carlson HE. New medications which decrease levothyroxine absorption. Thyroid. 2007;17:763-765.
11. Sachmechi I, Reich DM, Aninyei M, et al. Effect of proton pump inhibitors on serum thyroid-stimulating hormone level in euthyroid patients treated with levothyroxine for hypothyroidism. Endocr Pract. 2007;13:345-349.
12. Sperber AD, Liel Y. Evidence for interference with the intestinal absorption of levothyroxine sodium by aluminum hydroxide. Arch Intern Med. 1992;152:183-184.
13. Zamfirescu I, Carlson HE. Absorption of levothyroxine when coadministered with various calcium formulations. Thyroid. 2011;21:483-486.
14. Campbell NR, Hasinoff BB, Stalts H, et al. Ferrous sulfate reduces thyroxine efficacy in patients with hypothyroidism. Ann Intern Med. 1992;117:1010-1013.
15. Bugdaci MS, Zuhur SS, Sokmen M, et al. The role of Helicobacter pylori in patients with hypothyroidism in whom could not be achieved normal thyrotropin levels despite treatment with high doses of thyroxine. Helicobacter. 2011;16:124-130.
16. Centanni M, Gargano L, Canettieri G, et al. Thyroxine in goiter, Helicobacter pylori infection, and chronic gastritis. N Engl J Med. 2006;354:1787-1795.
17. Centanni M, Marignani M, Gargano L, et al. Atrophic body gastritis in patients with autoimmune thyroid disease: an underdiagnosed association. Arch Intern Med. 1999;159:1726-1730.
18. Collins D, Wilcox R, Nathan M, et al. Celiac disease and hypothyroidism. Am J Med. 2012;125:278-282.
19. Azizi F, Belur R, Albano J. Malabsorption of thyroid hormones after jejunoileal bypass for obesity. Ann Intern Med. 1979;90:941-942.
20. Gkotsina M, Michalaki M, Mamali I, et al. Improved levothyroxine pharmacokinetics after bariatric surgery. Thyroid. 2013;23:414-419.
21. Hennessey JV, Espaillat R. Diagnosis and management of subclinical hypothyroidism in elderly adults: a review of the literature. J Am Geriatr Soc. 2015;63:1663-1673.
22. Alexander EK, Pearce EN, Brent GA, et al. 2017 Guidelines of the American Thyroid Association for the diagnosis and management of thyroid disease during pregnancy and the postpartum. Thyroid. 2017;27:315-389.
23. Carney LA, Quinlan JD, West JM. Thyroid disease in pregnancy. Am Fam Physician. 2014;89:273-278.
24. Fröhlich E, Wahl R. Thyroid autoimmunity: role of anti-thyroid antibodies in thyroid and extra-thyroidal diseases. Front Immunol. 2017;8:521.
25. Aksoy DY, Kerimoglu U, Okur H, et al. Effects of prophylactic thyroid hormone replacement in euthyroid Hashimoto’s thyroiditis. Endocr J. 2005;52:337-343.
26. Padberg S, Heller K, Usadel KH, et al. One-year prophylactic treatment of euthyroid Hashimoto’s thyroiditis patients with levothyroxine: is there a benefit? Thyroid. 2001;11:249-255.
27. Rugge B, Balshem H, Sehgal R, et al. Screening and Treatment of Subclinical Hypothyroidism or Hyperthyroidism [Internet]. Comparative Effectiveness Reviews, No. 24. Rockville, MD: Agency for Healthcare Research and Quality; October 2011. www.ncbi.nlm.nih.gov/books/NBK83492/. Accessed February 21, 2020.
28. Hollowell JG, Staehling NW, Flanders WD, et al. Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). J Clin Endocrinol Metab. 2002;87:489-499.
29. Surks MI, Hollowell JG. Age-specific distribution of serum thyrotropin and antithyroid antibodies in the US population: implications for the prevalence of subclinical hypothyroidism. J Clin Endocrinol Metab. 2007;92:4575-4582.
30. Pasqualetti G, Tognini S, Polini A, et al. Is subclinical hypothyroidism a cardiovascular risk factor in the elderly? J Clin Endocrinol Metab. 2013;98:2256-2266.
CASE
A 38-year-old woman presents for a routine physical. Other than urgent care visits for 1 episode of influenza and 2 upper respiratory illnesses, she has not seen a physician for a physical in 5 years. She denies any significant medical history. She takes naproxen occasionally for chronic right knee pain. She does not use tobacco or alcohol. Recently, she has started using a meal replacement shake at lunchtime for weight management. She performs aerobic exercise 30 to 40 minutes per day, 5 days per week. Her family history is significant for type 2 diabetes mellitus, arthritis, heart disease, and hyperlipidemia on her mother’s side. She is single, is not currently sexually active, works as a pharmacy technician, and has no children. A high-risk human papillomavirus test was normal 4 years ago.
A review of systems is notable for a 20-pound weight gain over the past year, worsening heartburn over the past 2 weeks, and chronic knee pain, which is greater in the right knee than the left. She denies weakness, fatigue, nausea, diarrhea, constipation, or abdominal pain. Vital signs reveal a blood pressure of 146/88 mm Hg, a heart rate of 63 bpm, a temperature of 98°F (36.7°C), a respiratory rate of 16, a height of 5’7’’ (1.7 m), a weight of 217 lbs (98.4 kg), and a peripheral capillary oxygen saturation (SpO2) of 99% on room air. The physical exam reveals a body mass index (BMI) of 34, warm dry skin, and coarse brittle hair.
Lab results reveal a thyroid-stimulating hormone (TSH) level of 11.17 mIU/L (reference range, 0.45-4.5 mIU/L) and a free thyroxine (T4) of 0.58 ng/dL (reference range, 0.8-2.8 ng/dL). A basic metabolic panel and hemoglobin A1C level are normal.
What would you recommend?
In the United States, the prevalence of overt hypothyroidism (defined as a TSH level > 4.5 mIU/L and a low free T4) among people ≥ 12 years of age was estimated at 0.3% based on National Health and Nutrition Examination Survey (NHANES) data from 1999-2002.1 Subclinical hypothyroidism (TSH level > 4.5 mIU/L but < 10 mIU/L and a normal T4 level) is even more common, with an estimated prevalence of 3.4%.1 Hypothyroidism is more common in females and occurs more frequently in Caucasian Americans and Mexican Americans than in African Americans.1
The most common etiologies of hypothyroidism include autoimmune thyroiditis (eg, Hashimoto thyroiditis, atrophic autoimmune thyroiditis) and iatrogenic causes (eg, after radioactive iodine ablation or thyroidectomy) (TABLE 1).2-4

Initiating thyroid hormone replacement
Factors to consider when starting a patient on thyroid hormone replacement include age, weight, symptom severity, TSH level, goal TSH value, adverse effects from thyroid supplements, history of cardiac disease, and, for women of child-bearing age, the desire for pregnancy vs the use of contraceptives. Most adult patients < 50 years with overt hypothyroidism can begin a weight-based dose of levothyroxine: ~1.6 mcg/kg/d (based on ideal body weight).3
Continue to: For adults with cardiac disease...
For adults with cardiac disease, the risk of over-replacement limits initial dosing to 25 to 50 mcg/d for patients < 50 years (12.5-25 mcg/d; ≥ 50 years).3 For adults with subclinical hypothyroidism, it is reasonable to begin therapy at a lower daily dose (eg, 25-75 mcg/d) depending on baseline TSH level, symptoms (the patient may be asymptomatic), and the presence of cardiac disease (TABLE 23,4). Consider treatment in patients with subclinical hypothyroidism particularly when patients have a goiter or dyslipidemia and in women contemplating pregnancy in the near future. Elderly patients may require a dose 20% to 25% lower than younger adults because of decreased body mass.3

Levothyroxine is considered first-line therapy for hypothyroidism because of its low cost, dose consistency, low risk of allergic reactions, and potential to cause fewer cardiac adverse effects than triiodothyronine (T3) products such as desiccated thyroid extract.5 Although data have not shown an absolute increase in cardiovascular adverse effects, T3 products have a higher T3 vs T4 ratio, giving them a theoretically increased risk.5,6 Desiccated thyroid extract also has been associated with allergic reactions.5
Use of liothyronine alone or in combination with levothyroxine lacks evidence and guideline support.4 Furthermore, it is dosed twice daily, which makes it less convenient, and concerns still exist that there may be an increase in cardiovascular adverse effects.4,6 See TABLE 37 for a summary of available products and their equivalent doses.

Maintaining patients on therapy
The maintenance phase begins once hypothyroidism is diagnosed and treatment is initiated. This phase includes regular monitoring with laboratory studies, office visits, and as-needed adjustments in hormone replacement dosing. The frequency at which all of these occur is variable and based on a number of factors including the patient’s other medical conditions, use of other medications including over-the-counter agents, the patient’s age, weight changes, and pregnancy status.3,4,8 In general, dosage adjustments of 12.5 to 25 mcg can be made at 6- to 8-week intervals based on repeat TSH measurements, patient symptoms, and comorbidities.3
Once a patient is symptomatically stable and laboratory values have normalized, the recommended frequency of laboratory evaluation and office visits is every 12 months, barring significant changes in any of the factors mentioned above. At each visit, physicians should perform medication (including supplements) reconciliation and discuss any health condition updates. Changes to the therapy plan, including frequency or timing of laboratory tests, may be necessary if patients begin taking medications that alter the absorption or function of levothyroxine (eg, steroids).
Continue to: To maximize absorption...
To maximize absorption, providers should review with patients the optimal way to take thyroid hormones. Levothyroxine is approximately 70% to 80% absorbed under ideal conditions, which means taking it in the morning at least 30 to 60 minutes before eating or 3 to 4 hours after the last meal of the day.3,9-13 Of note, TSH levels may increase slightly in patients taking proton pump inhibitors, but this does not usually require a dose increase of thyroid hormone.11 Given that some supplements, particularly iron and calcium, can interfere with absorption, it is recommended to maintain a 3- to 4-hour gap between taking those supplements and taking levothyroxine.12-14 For those patients unable or unwilling to adhere to these recommendations, an increase in levothyroxine dose may be required in order to compensate for the decreased absorption.
Don’t adjust hormone therapy based on clinical presentation alone. While clinical symptoms are important, it is not recommended to adjust hormone therapy based solely on clinical presentation. Common hypothyroid symptoms of dry skin, edema, weight gain, and fatigue may be caused by other medical conditions. While indices including Achilles reflex time and basal metabolic rate have shown some correlation to thyroid dysfunction, there has been limited evidence to show that longitudinal index changes reflect subtle changes in thyroid hormone levels.3
The most recent guidelines from the American Thyroid Association recommend that, “Symptoms should be followed, but considered in the context of serum thyrotropin values, relevant comorbidities, and other potential causes.”3
Special populations/circumstances to keep in mind
Malabsorption conditions. When a higher than expected weight-based dose of levothyroxine is required, physicians should review administration timing, adherence, and comorbid medical conditions that can affect absorption.
Several studies, for example, have demonstrated the impact of Helicobacter pylori gastritis on levothyroxine absorption and subsequent TSH levels.15-17 In one nonrandomized prospective study, patients with H pylori and hypothyroidism who were previously thought to be unresponsive to levothyroxine therapy had a decrease in average TSH level from 30.5 mIU/L to 4.2 mIU/L after H pylori was eradicated.15 Autoimmune atrophic gastritis and celiac disease, both of which are more common in those with other autoimmune diseases, are also associated with the need for higher than expected levothyroxine doses.17,18
Continue to: A history of gastric bypass surgery...
A history of gastric bypass surgery alone is not considered a risk factor for poor absorption of thyroid hormone, given that the majority of levothyroxine absorption occurs in the ileum.19,20 However, advancing age (> 70 years) and extreme obesity (BMI > 40) are independent risk factors for decreased levothyroxine absorption.20,21
Women of reproductive age and pregnant women. Overt untreated or undertreated hypothyroidism can be associated with increased risk of maternal and fetal complications including decreased fertility, miscarriage, preterm delivery, lower birth rates, and infant cognitive deficits.3,22 Therefore, the main focus should be optimization of thyroid hormone levels prior to and during pregnancy.3,4,8,22 Thyroid hormone replacement needs to be increased during pregnancy in approximately 50% to 85% of women using thyroid replacement prior to pregnancy, but the dose requirements vary based on the underlying etiology of thyroid dysfunction.
One initial option for patients on a stable dose before pregnancy is to increase their daily dose by a half tablet (1.5 × daily dose) immediately after home confirmation of pregnancy, until finer dose adjustments (usually increases of 25%-60% ) can be made by a physician. Experts recommend that a TSH level be obtained every 4 weeks until mid-gestation and then at least once around 30 weeks’ gestation to ensure specific targets are being met with dose adjustments.22 Optimal thyrotropin reference ranges during conception and pregnancy can be found in the literature.23
Patients who have positive antibodies and normal thyroid function tests. Patients who are screened for thyroid disorders may demonstrate normal thyroid function (ie, euthyroid) with TSH, free T4, and, if checked, free T3, all within normal ranges. Despite these normal lab results, patients may have additional test results that demonstrate positive thyroid autoantibodies including thyroglobulin antibodies and/or thyroid peroxidase antibodies. Thyroid autoimmunity itself has been associated with a range of other autoimmune conditions as well as an increased risk of thyroid cancer in those with Hashimoto thyroiditis.24 Two studies showed that prophylactic treatment of euthyroid patients with levothyroxine led to a reduction in antibody levels and a lower TSH level.25,26 However, no studies have focused on patient-oriented outcomes such as hospitalizations, quality of life, or symptoms. If the patient remains asymptomatic, we recommend no treatment, but that the patient’s TSH levels be monitored every 12 months.27
Elderly patients. Population data have shown that TSH increases normally with age, with a TSH level of 7.5 mIU/L being the upper limit of normal for a population of healthy adults > 80 years of age.28,29 Overall, studies have failed to show any benefit in treating elderly patients with subclinical hypothyroidism unless their TSH level exceeds 10 mIU/L.6,21 The one exception is elderly patients with heart failure in whom untreated subclinical hypothyroidism has been shown to be associated with higher mortality.30
Continue to: Elderly patients are at higher risk...
Elderly patients are at higher risk for adverse effects of thyroid over-replacement, including atrial fibrillation and osteoporosis. While there have been no randomized trials examining target TSH levels in this population, a reasonable recommendation is a goal TSH level of 4 to 6 mIU/L for elderly patients ≥ 70 years.4
CASE
As a result of the patient’s elevated TSH level and symptoms of hypothyroidism, you start levothyroxine 150 mcg/d by mouth, counsel her on potential adverse effects, and schedule a follow-up visit with another TSH check in 6 weeks.
Follow-up laboratory studies 6 weeks later reveal a TSH level of 5.86 mIU/L (reference range, 0.45-4.5 mIU/L) and a free T4 level of 0.74 ng/dL (reference range, 0.8-2.8 ng/dL). Based on those results, you increase the dose of levothyroxine to 175 mcg/d.
At her follow-up visit 12 weeks after initial presentation, her TSH level is 3.85 mIU/L. She reports feeling better overall with less fatigue, and she has lost 5 pounds since her last visit. You recommend she continue levothyroxine 175 mcg/d after reviewing medication compliance with the patient and ensuring she is indeed taking it in the morning, at least 30 minutes prior to eating. With improved but not resolved symptoms, she agrees to follow-up with repeat TSH laboratory studies in 6 weeks to determine whether further dose adjustments are necessary. Given that she is of reproductive age and her TSH level is suboptimal for pregnancy, you caution her about heightened pregnancy/fetal risks with a suboptimal TSH and recommend that she use reliable contraception.
CORRESPONDENCE
Christopher Bunt, MD, FAAFP, 5 Charleston Center Drive, Suite 263, MSC 192,Charleston, SC 29425; [email protected]
CASE
A 38-year-old woman presents for a routine physical. Other than urgent care visits for 1 episode of influenza and 2 upper respiratory illnesses, she has not seen a physician for a physical in 5 years. She denies any significant medical history. She takes naproxen occasionally for chronic right knee pain. She does not use tobacco or alcohol. Recently, she has started using a meal replacement shake at lunchtime for weight management. She performs aerobic exercise 30 to 40 minutes per day, 5 days per week. Her family history is significant for type 2 diabetes mellitus, arthritis, heart disease, and hyperlipidemia on her mother’s side. She is single, is not currently sexually active, works as a pharmacy technician, and has no children. A high-risk human papillomavirus test was normal 4 years ago.
A review of systems is notable for a 20-pound weight gain over the past year, worsening heartburn over the past 2 weeks, and chronic knee pain, which is greater in the right knee than the left. She denies weakness, fatigue, nausea, diarrhea, constipation, or abdominal pain. Vital signs reveal a blood pressure of 146/88 mm Hg, a heart rate of 63 bpm, a temperature of 98°F (36.7°C), a respiratory rate of 16, a height of 5’7’’ (1.7 m), a weight of 217 lbs (98.4 kg), and a peripheral capillary oxygen saturation (SpO2) of 99% on room air. The physical exam reveals a body mass index (BMI) of 34, warm dry skin, and coarse brittle hair.
Lab results reveal a thyroid-stimulating hormone (TSH) level of 11.17 mIU/L (reference range, 0.45-4.5 mIU/L) and a free thyroxine (T4) of 0.58 ng/dL (reference range, 0.8-2.8 ng/dL). A basic metabolic panel and hemoglobin A1C level are normal.
What would you recommend?
In the United States, the prevalence of overt hypothyroidism (defined as a TSH level > 4.5 mIU/L and a low free T4) among people ≥ 12 years of age was estimated at 0.3% based on National Health and Nutrition Examination Survey (NHANES) data from 1999-2002.1 Subclinical hypothyroidism (TSH level > 4.5 mIU/L but < 10 mIU/L and a normal T4 level) is even more common, with an estimated prevalence of 3.4%.1 Hypothyroidism is more common in females and occurs more frequently in Caucasian Americans and Mexican Americans than in African Americans.1
The most common etiologies of hypothyroidism include autoimmune thyroiditis (eg, Hashimoto thyroiditis, atrophic autoimmune thyroiditis) and iatrogenic causes (eg, after radioactive iodine ablation or thyroidectomy) (TABLE 1).2-4

Initiating thyroid hormone replacement
Factors to consider when starting a patient on thyroid hormone replacement include age, weight, symptom severity, TSH level, goal TSH value, adverse effects from thyroid supplements, history of cardiac disease, and, for women of child-bearing age, the desire for pregnancy vs the use of contraceptives. Most adult patients < 50 years with overt hypothyroidism can begin a weight-based dose of levothyroxine: ~1.6 mcg/kg/d (based on ideal body weight).3
Continue to: For adults with cardiac disease...
For adults with cardiac disease, the risk of over-replacement limits initial dosing to 25 to 50 mcg/d for patients < 50 years (12.5-25 mcg/d; ≥ 50 years).3 For adults with subclinical hypothyroidism, it is reasonable to begin therapy at a lower daily dose (eg, 25-75 mcg/d) depending on baseline TSH level, symptoms (the patient may be asymptomatic), and the presence of cardiac disease (TABLE 23,4). Consider treatment in patients with subclinical hypothyroidism particularly when patients have a goiter or dyslipidemia and in women contemplating pregnancy in the near future. Elderly patients may require a dose 20% to 25% lower than younger adults because of decreased body mass.3

Levothyroxine is considered first-line therapy for hypothyroidism because of its low cost, dose consistency, low risk of allergic reactions, and potential to cause fewer cardiac adverse effects than triiodothyronine (T3) products such as desiccated thyroid extract.5 Although data have not shown an absolute increase in cardiovascular adverse effects, T3 products have a higher T3 vs T4 ratio, giving them a theoretically increased risk.5,6 Desiccated thyroid extract also has been associated with allergic reactions.5
Use of liothyronine alone or in combination with levothyroxine lacks evidence and guideline support.4 Furthermore, it is dosed twice daily, which makes it less convenient, and concerns still exist that there may be an increase in cardiovascular adverse effects.4,6 See TABLE 37 for a summary of available products and their equivalent doses.

Maintaining patients on therapy
The maintenance phase begins once hypothyroidism is diagnosed and treatment is initiated. This phase includes regular monitoring with laboratory studies, office visits, and as-needed adjustments in hormone replacement dosing. The frequency at which all of these occur is variable and based on a number of factors including the patient’s other medical conditions, use of other medications including over-the-counter agents, the patient’s age, weight changes, and pregnancy status.3,4,8 In general, dosage adjustments of 12.5 to 25 mcg can be made at 6- to 8-week intervals based on repeat TSH measurements, patient symptoms, and comorbidities.3
Once a patient is symptomatically stable and laboratory values have normalized, the recommended frequency of laboratory evaluation and office visits is every 12 months, barring significant changes in any of the factors mentioned above. At each visit, physicians should perform medication (including supplements) reconciliation and discuss any health condition updates. Changes to the therapy plan, including frequency or timing of laboratory tests, may be necessary if patients begin taking medications that alter the absorption or function of levothyroxine (eg, steroids).
Continue to: To maximize absorption...
To maximize absorption, providers should review with patients the optimal way to take thyroid hormones. Levothyroxine is approximately 70% to 80% absorbed under ideal conditions, which means taking it in the morning at least 30 to 60 minutes before eating or 3 to 4 hours after the last meal of the day.3,9-13 Of note, TSH levels may increase slightly in patients taking proton pump inhibitors, but this does not usually require a dose increase of thyroid hormone.11 Given that some supplements, particularly iron and calcium, can interfere with absorption, it is recommended to maintain a 3- to 4-hour gap between taking those supplements and taking levothyroxine.12-14 For those patients unable or unwilling to adhere to these recommendations, an increase in levothyroxine dose may be required in order to compensate for the decreased absorption.
Don’t adjust hormone therapy based on clinical presentation alone. While clinical symptoms are important, it is not recommended to adjust hormone therapy based solely on clinical presentation. Common hypothyroid symptoms of dry skin, edema, weight gain, and fatigue may be caused by other medical conditions. While indices including Achilles reflex time and basal metabolic rate have shown some correlation to thyroid dysfunction, there has been limited evidence to show that longitudinal index changes reflect subtle changes in thyroid hormone levels.3
The most recent guidelines from the American Thyroid Association recommend that, “Symptoms should be followed, but considered in the context of serum thyrotropin values, relevant comorbidities, and other potential causes.”3
Special populations/circumstances to keep in mind
Malabsorption conditions. When a higher than expected weight-based dose of levothyroxine is required, physicians should review administration timing, adherence, and comorbid medical conditions that can affect absorption.
Several studies, for example, have demonstrated the impact of Helicobacter pylori gastritis on levothyroxine absorption and subsequent TSH levels.15-17 In one nonrandomized prospective study, patients with H pylori and hypothyroidism who were previously thought to be unresponsive to levothyroxine therapy had a decrease in average TSH level from 30.5 mIU/L to 4.2 mIU/L after H pylori was eradicated.15 Autoimmune atrophic gastritis and celiac disease, both of which are more common in those with other autoimmune diseases, are also associated with the need for higher than expected levothyroxine doses.17,18
Continue to: A history of gastric bypass surgery...
A history of gastric bypass surgery alone is not considered a risk factor for poor absorption of thyroid hormone, given that the majority of levothyroxine absorption occurs in the ileum.19,20 However, advancing age (> 70 years) and extreme obesity (BMI > 40) are independent risk factors for decreased levothyroxine absorption.20,21
Women of reproductive age and pregnant women. Overt untreated or undertreated hypothyroidism can be associated with increased risk of maternal and fetal complications including decreased fertility, miscarriage, preterm delivery, lower birth rates, and infant cognitive deficits.3,22 Therefore, the main focus should be optimization of thyroid hormone levels prior to and during pregnancy.3,4,8,22 Thyroid hormone replacement needs to be increased during pregnancy in approximately 50% to 85% of women using thyroid replacement prior to pregnancy, but the dose requirements vary based on the underlying etiology of thyroid dysfunction.
One initial option for patients on a stable dose before pregnancy is to increase their daily dose by a half tablet (1.5 × daily dose) immediately after home confirmation of pregnancy, until finer dose adjustments (usually increases of 25%-60% ) can be made by a physician. Experts recommend that a TSH level be obtained every 4 weeks until mid-gestation and then at least once around 30 weeks’ gestation to ensure specific targets are being met with dose adjustments.22 Optimal thyrotropin reference ranges during conception and pregnancy can be found in the literature.23
Patients who have positive antibodies and normal thyroid function tests. Patients who are screened for thyroid disorders may demonstrate normal thyroid function (ie, euthyroid) with TSH, free T4, and, if checked, free T3, all within normal ranges. Despite these normal lab results, patients may have additional test results that demonstrate positive thyroid autoantibodies including thyroglobulin antibodies and/or thyroid peroxidase antibodies. Thyroid autoimmunity itself has been associated with a range of other autoimmune conditions as well as an increased risk of thyroid cancer in those with Hashimoto thyroiditis.24 Two studies showed that prophylactic treatment of euthyroid patients with levothyroxine led to a reduction in antibody levels and a lower TSH level.25,26 However, no studies have focused on patient-oriented outcomes such as hospitalizations, quality of life, or symptoms. If the patient remains asymptomatic, we recommend no treatment, but that the patient’s TSH levels be monitored every 12 months.27
Elderly patients. Population data have shown that TSH increases normally with age, with a TSH level of 7.5 mIU/L being the upper limit of normal for a population of healthy adults > 80 years of age.28,29 Overall, studies have failed to show any benefit in treating elderly patients with subclinical hypothyroidism unless their TSH level exceeds 10 mIU/L.6,21 The one exception is elderly patients with heart failure in whom untreated subclinical hypothyroidism has been shown to be associated with higher mortality.30
Continue to: Elderly patients are at higher risk...
Elderly patients are at higher risk for adverse effects of thyroid over-replacement, including atrial fibrillation and osteoporosis. While there have been no randomized trials examining target TSH levels in this population, a reasonable recommendation is a goal TSH level of 4 to 6 mIU/L for elderly patients ≥ 70 years.4
CASE
As a result of the patient’s elevated TSH level and symptoms of hypothyroidism, you start levothyroxine 150 mcg/d by mouth, counsel her on potential adverse effects, and schedule a follow-up visit with another TSH check in 6 weeks.
Follow-up laboratory studies 6 weeks later reveal a TSH level of 5.86 mIU/L (reference range, 0.45-4.5 mIU/L) and a free T4 level of 0.74 ng/dL (reference range, 0.8-2.8 ng/dL). Based on those results, you increase the dose of levothyroxine to 175 mcg/d.
At her follow-up visit 12 weeks after initial presentation, her TSH level is 3.85 mIU/L. She reports feeling better overall with less fatigue, and she has lost 5 pounds since her last visit. You recommend she continue levothyroxine 175 mcg/d after reviewing medication compliance with the patient and ensuring she is indeed taking it in the morning, at least 30 minutes prior to eating. With improved but not resolved symptoms, she agrees to follow-up with repeat TSH laboratory studies in 6 weeks to determine whether further dose adjustments are necessary. Given that she is of reproductive age and her TSH level is suboptimal for pregnancy, you caution her about heightened pregnancy/fetal risks with a suboptimal TSH and recommend that she use reliable contraception.
CORRESPONDENCE
Christopher Bunt, MD, FAAFP, 5 Charleston Center Drive, Suite 263, MSC 192,Charleston, SC 29425; [email protected]
1. Aoki Y, Belin RM, Clickner R, et al. Serum TSH and total T4 in the United States population and their association with participant characteristics: National Health and Nutrition Examination Survey (NHANES 1999-2002). Thyroid. 2007;17:1211-1223.
2. Vaidya B, Pearce SH. Management of hypothyroidism in adults. BMJ. 2008;337:a801.
3. Garber JR, Cobin RH, Gharib H, et al. Clinical practice guidelines for hypothyroidism in adults: cosponsored by the American Association of Clinical Endocrinologists and the American Thyroid Association. Endocr Pract. 2012;18:988-1028.
4. Jonklaas J, Bianco AC, Bauer AJ, et al. Guidelines for the treatment of hypothyroidism: prepared by the American Thyroid Association task force on thyroid hormone replacement. Thyroid. 2014;24:1670-1751.
5. Toft AD. Thyroxine therapy. N Engl J Med. 1994;331:174-180.
6. Floriani C, Gencer B, Collet TH, et al. Subclinical thyroid dysfunction and cardiovascular diseases: 2016 update. Eur Heart J. 2018;39:503-507.
7. Lexi-Comp, Inc. (Lexi-Drugs®). https://online.lexi.com/lco/action/login. Accessed July 7, 2017.
8. Okosieme O, Gilbert J, Abraham P, et al. Management of primary hypothyroidism: statement by the British Thyroid Association Executive Committee. Clin Endocrinol (Oxf). 2016;84:799-808.
9. Fish LH, Schwartz HL, Cavanaugh J, et al. Replacement dose, metabolism, and bioavailability of levothyroxine in the treatment of hypothyroidism. Role of triiodothyronine in pituitary feedback in humans. N Engl J Med. 1987;316:764-770.
10. John-Kalarickal J, Pearlman G, Carlson HE. New medications which decrease levothyroxine absorption. Thyroid. 2007;17:763-765.
11. Sachmechi I, Reich DM, Aninyei M, et al. Effect of proton pump inhibitors on serum thyroid-stimulating hormone level in euthyroid patients treated with levothyroxine for hypothyroidism. Endocr Pract. 2007;13:345-349.
12. Sperber AD, Liel Y. Evidence for interference with the intestinal absorption of levothyroxine sodium by aluminum hydroxide. Arch Intern Med. 1992;152:183-184.
13. Zamfirescu I, Carlson HE. Absorption of levothyroxine when coadministered with various calcium formulations. Thyroid. 2011;21:483-486.
14. Campbell NR, Hasinoff BB, Stalts H, et al. Ferrous sulfate reduces thyroxine efficacy in patients with hypothyroidism. Ann Intern Med. 1992;117:1010-1013.
15. Bugdaci MS, Zuhur SS, Sokmen M, et al. The role of Helicobacter pylori in patients with hypothyroidism in whom could not be achieved normal thyrotropin levels despite treatment with high doses of thyroxine. Helicobacter. 2011;16:124-130.
16. Centanni M, Gargano L, Canettieri G, et al. Thyroxine in goiter, Helicobacter pylori infection, and chronic gastritis. N Engl J Med. 2006;354:1787-1795.
17. Centanni M, Marignani M, Gargano L, et al. Atrophic body gastritis in patients with autoimmune thyroid disease: an underdiagnosed association. Arch Intern Med. 1999;159:1726-1730.
18. Collins D, Wilcox R, Nathan M, et al. Celiac disease and hypothyroidism. Am J Med. 2012;125:278-282.
19. Azizi F, Belur R, Albano J. Malabsorption of thyroid hormones after jejunoileal bypass for obesity. Ann Intern Med. 1979;90:941-942.
20. Gkotsina M, Michalaki M, Mamali I, et al. Improved levothyroxine pharmacokinetics after bariatric surgery. Thyroid. 2013;23:414-419.
21. Hennessey JV, Espaillat R. Diagnosis and management of subclinical hypothyroidism in elderly adults: a review of the literature. J Am Geriatr Soc. 2015;63:1663-1673.
22. Alexander EK, Pearce EN, Brent GA, et al. 2017 Guidelines of the American Thyroid Association for the diagnosis and management of thyroid disease during pregnancy and the postpartum. Thyroid. 2017;27:315-389.
23. Carney LA, Quinlan JD, West JM. Thyroid disease in pregnancy. Am Fam Physician. 2014;89:273-278.
24. Fröhlich E, Wahl R. Thyroid autoimmunity: role of anti-thyroid antibodies in thyroid and extra-thyroidal diseases. Front Immunol. 2017;8:521.
25. Aksoy DY, Kerimoglu U, Okur H, et al. Effects of prophylactic thyroid hormone replacement in euthyroid Hashimoto’s thyroiditis. Endocr J. 2005;52:337-343.
26. Padberg S, Heller K, Usadel KH, et al. One-year prophylactic treatment of euthyroid Hashimoto’s thyroiditis patients with levothyroxine: is there a benefit? Thyroid. 2001;11:249-255.
27. Rugge B, Balshem H, Sehgal R, et al. Screening and Treatment of Subclinical Hypothyroidism or Hyperthyroidism [Internet]. Comparative Effectiveness Reviews, No. 24. Rockville, MD: Agency for Healthcare Research and Quality; October 2011. www.ncbi.nlm.nih.gov/books/NBK83492/. Accessed February 21, 2020.
28. Hollowell JG, Staehling NW, Flanders WD, et al. Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). J Clin Endocrinol Metab. 2002;87:489-499.
29. Surks MI, Hollowell JG. Age-specific distribution of serum thyrotropin and antithyroid antibodies in the US population: implications for the prevalence of subclinical hypothyroidism. J Clin Endocrinol Metab. 2007;92:4575-4582.
30. Pasqualetti G, Tognini S, Polini A, et al. Is subclinical hypothyroidism a cardiovascular risk factor in the elderly? J Clin Endocrinol Metab. 2013;98:2256-2266.
1. Aoki Y, Belin RM, Clickner R, et al. Serum TSH and total T4 in the United States population and their association with participant characteristics: National Health and Nutrition Examination Survey (NHANES 1999-2002). Thyroid. 2007;17:1211-1223.
2. Vaidya B, Pearce SH. Management of hypothyroidism in adults. BMJ. 2008;337:a801.
3. Garber JR, Cobin RH, Gharib H, et al. Clinical practice guidelines for hypothyroidism in adults: cosponsored by the American Association of Clinical Endocrinologists and the American Thyroid Association. Endocr Pract. 2012;18:988-1028.
4. Jonklaas J, Bianco AC, Bauer AJ, et al. Guidelines for the treatment of hypothyroidism: prepared by the American Thyroid Association task force on thyroid hormone replacement. Thyroid. 2014;24:1670-1751.
5. Toft AD. Thyroxine therapy. N Engl J Med. 1994;331:174-180.
6. Floriani C, Gencer B, Collet TH, et al. Subclinical thyroid dysfunction and cardiovascular diseases: 2016 update. Eur Heart J. 2018;39:503-507.
7. Lexi-Comp, Inc. (Lexi-Drugs®). https://online.lexi.com/lco/action/login. Accessed July 7, 2017.
8. Okosieme O, Gilbert J, Abraham P, et al. Management of primary hypothyroidism: statement by the British Thyroid Association Executive Committee. Clin Endocrinol (Oxf). 2016;84:799-808.
9. Fish LH, Schwartz HL, Cavanaugh J, et al. Replacement dose, metabolism, and bioavailability of levothyroxine in the treatment of hypothyroidism. Role of triiodothyronine in pituitary feedback in humans. N Engl J Med. 1987;316:764-770.
10. John-Kalarickal J, Pearlman G, Carlson HE. New medications which decrease levothyroxine absorption. Thyroid. 2007;17:763-765.
11. Sachmechi I, Reich DM, Aninyei M, et al. Effect of proton pump inhibitors on serum thyroid-stimulating hormone level in euthyroid patients treated with levothyroxine for hypothyroidism. Endocr Pract. 2007;13:345-349.
12. Sperber AD, Liel Y. Evidence for interference with the intestinal absorption of levothyroxine sodium by aluminum hydroxide. Arch Intern Med. 1992;152:183-184.
13. Zamfirescu I, Carlson HE. Absorption of levothyroxine when coadministered with various calcium formulations. Thyroid. 2011;21:483-486.
14. Campbell NR, Hasinoff BB, Stalts H, et al. Ferrous sulfate reduces thyroxine efficacy in patients with hypothyroidism. Ann Intern Med. 1992;117:1010-1013.
15. Bugdaci MS, Zuhur SS, Sokmen M, et al. The role of Helicobacter pylori in patients with hypothyroidism in whom could not be achieved normal thyrotropin levels despite treatment with high doses of thyroxine. Helicobacter. 2011;16:124-130.
16. Centanni M, Gargano L, Canettieri G, et al. Thyroxine in goiter, Helicobacter pylori infection, and chronic gastritis. N Engl J Med. 2006;354:1787-1795.
17. Centanni M, Marignani M, Gargano L, et al. Atrophic body gastritis in patients with autoimmune thyroid disease: an underdiagnosed association. Arch Intern Med. 1999;159:1726-1730.
18. Collins D, Wilcox R, Nathan M, et al. Celiac disease and hypothyroidism. Am J Med. 2012;125:278-282.
19. Azizi F, Belur R, Albano J. Malabsorption of thyroid hormones after jejunoileal bypass for obesity. Ann Intern Med. 1979;90:941-942.
20. Gkotsina M, Michalaki M, Mamali I, et al. Improved levothyroxine pharmacokinetics after bariatric surgery. Thyroid. 2013;23:414-419.
21. Hennessey JV, Espaillat R. Diagnosis and management of subclinical hypothyroidism in elderly adults: a review of the literature. J Am Geriatr Soc. 2015;63:1663-1673.
22. Alexander EK, Pearce EN, Brent GA, et al. 2017 Guidelines of the American Thyroid Association for the diagnosis and management of thyroid disease during pregnancy and the postpartum. Thyroid. 2017;27:315-389.
23. Carney LA, Quinlan JD, West JM. Thyroid disease in pregnancy. Am Fam Physician. 2014;89:273-278.
24. Fröhlich E, Wahl R. Thyroid autoimmunity: role of anti-thyroid antibodies in thyroid and extra-thyroidal diseases. Front Immunol. 2017;8:521.
25. Aksoy DY, Kerimoglu U, Okur H, et al. Effects of prophylactic thyroid hormone replacement in euthyroid Hashimoto’s thyroiditis. Endocr J. 2005;52:337-343.
26. Padberg S, Heller K, Usadel KH, et al. One-year prophylactic treatment of euthyroid Hashimoto’s thyroiditis patients with levothyroxine: is there a benefit? Thyroid. 2001;11:249-255.
27. Rugge B, Balshem H, Sehgal R, et al. Screening and Treatment of Subclinical Hypothyroidism or Hyperthyroidism [Internet]. Comparative Effectiveness Reviews, No. 24. Rockville, MD: Agency for Healthcare Research and Quality; October 2011. www.ncbi.nlm.nih.gov/books/NBK83492/. Accessed February 21, 2020.
28. Hollowell JG, Staehling NW, Flanders WD, et al. Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). J Clin Endocrinol Metab. 2002;87:489-499.
29. Surks MI, Hollowell JG. Age-specific distribution of serum thyrotropin and antithyroid antibodies in the US population: implications for the prevalence of subclinical hypothyroidism. J Clin Endocrinol Metab. 2007;92:4575-4582.
30. Pasqualetti G, Tognini S, Polini A, et al. Is subclinical hypothyroidism a cardiovascular risk factor in the elderly? J Clin Endocrinol Metab. 2013;98:2256-2266.
PRACTICE RECOMMENDATIONS
› Prescribe levothyroxine 1.6 mcg/kg/d for healthy adult patients < 50 years of age with overt hypothyroidism. B
› Consider lower initial doses of levothyroxine in patients with cardiac disease (12.5-50 mcg/d) or subclinical hypothyroidism (25-75 mcg/d). B
› Titrate levothyroxine by 12.5 to 25 mcg/d at 6- to 8-week intervals based on thyroid-stimulating hormone measurements, comorbidities, and symptoms. C
› Closely monitor and provide thyroid supplementation to female patients who are pregnant or of reproductive age with concomitant hypothyroidism. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Depression, or something else?
CASE Suicidal behavior, severe headaches
Ms. A, age 60, presents to the emergency department (ED) with depression, suicidal behavior, and 3 days of severe headaches. Neurology is consulted and an MRI is ordered, which shows a 3.0-cm mass lesion in the left temporal lobe with associated vasogenic edema that is suspicious for metastatic disease (Figure).
Ms. A is admitted to the hospital for further workup of her brain lesion. She is started on IV dexamethasone, 10 mg every 6 hours, a glucocorticosteroid, for brain edema, and levetiracetam, 500 mg twice a day, for seizure prophylaxis.
Upon admission, in addition to oncology and neurosurgery, psychiatry is also consulted to evaluate Ms. A for depression and suicidality.
EVALUATION Mood changes and poor judgment
Ms. A has a psychiatric history of depression and alcohol use disorder but says she has not consumed any alcohol in years. Her medical history includes hypertension, diabetes, and stage 4 non-small–cell lung cancer, for which she received surgery and adjuvant chemoradiotherapy 1 year ago.
On initial intake, Ms. A reports that in addition to the headaches, she has also been experiencing worsening depression and suicidal behavior. For the past 2 months, she has had a severely depressed mood, with notable anhedonia, poor appetite, insomnia, low energy, and decreased concentration. The changes in her mental health were triggered by her mother’s death. Three days prior to admission, the patient planned to overdose on antihypertensive pills, but her suicide attempt was interrupted when her family called. She denies any current suicidal ideation, intent, or plan.
According to her family, Ms. A has been increasingly irritable and her personality has changed in the past month. She also has been repeatedly sorting through her neighbors’ garbage.
Ms. A’s current psychiatric medications are duloxetine, 30 mg/d; quetiapine, 50 mg every night at bedtime; and buspirone, 10 mg/d. However, it is unclear if she is consistently taking these medications.
Continue to: On mental status examination...
On mental status examination, Ms. A is calm and she has no abnormal movements. She says she is depressed. Her affect is reactive and labile. She is alert and oriented to person, place, and time. Her attention, registration, and recall are intact. Her executive function is not tested. However, Ms. A’s insight and judgment seem poor.
To address Ms. A’s worsening depression, the psychiatry team increases her duloxetine from 30 to 60 mg/d, and she continues quetiapine, 50 mg every night at bedtime, for mood lability. Buspirone is not continued because she was not taking a therapeutic dosage in the community.
Within 4 days, Ms. A shows improvement in sleep, appetite, and mood. She has no further suicidal ideation.
[polldaddy:10511743]
The authors’ observations
Ms. A had a recurrence of what was presumed to be major depressive disorder (MDD) in the context of her mother’s death. However, she also exhibited irritability, mood lability, and impulsivity, all of which could be part of her depression, or a separate problem related to her brain tumor. Because Ms. A had never displayed bizarre behavior before the past few weeks, it is likely that her CNS lesion was directly affecting her personality and possibly underlying her planned suicide attempt.
Fifty to 80% of patients with CNS tumors, either primary or metastatic, present with psychiatric symptoms.1 Table 11-3 lists common psychiatric symptoms of brain tumors. Unfortunately, there is little reliable evidence that directly correlates tumor location with specific psychiatric symptoms. A 2010 meta-analysis found a statistically significant link between anorexia nervosa and hypothalamic tumors.1 However, for other brain regions, there is only an increased likelihood that any given tumor location will produce psychiatric symptoms.1,4 For instance, compared to patients with tumors in other locations, those with temporal lobe tumors are more likely to present with mood disorders, personality changes, and memory problems.1 In contrast, patients with frontal lobe tumors have an increased likelihood of psychosis, mood disorders, and personality changes.1 Patients with tumors in the pituitary region often present with anxiety.1
Continue to: When considering treatment options...
When considering treatment options for Ms. A, alcohol withdrawal was unlikely given the remote history of alcohol use, low alcohol blood level, and lack of evidence of unstable vital signs or tremor. Although she might have benefited from inpatient psychiatric treatment, this needed to wait until there was a definitive treatment plan for her brain tumor. Finally, although a paraneoplastic syndrome, such as limbic encephalitis, could be causing her psychiatric symptoms, this scenario is less likely with non-small–cell lung cancer.
Although uncommon, CNS tumors can present with psychiatric symptoms as the only manifestation. This is more likely when a patient exhibits new-onset or atypical symptoms, or fails to respond to standard psychiatric treatment.4 Case reports have described patients with brain tumors being misdiagnosed as having a primary psychiatric condition, which delays treatment of their CNS cancer.2 Additionally, frontal and limbic tumors are more likely to present with psychiatric manifestations; up to 90% of patients exhibit altered mental status or personality changes, as did Ms. A.1,4 Clearly, it is easier to identify patients with psychiatric symptoms resulting from a brain tumor when they also present with focal neurologic deficits or systemic symptoms, such as headache or nausea and vomiting. Ms. A presented with severe headaches, which is what led to her early imaging and prompt diagnosis.
Numerous proposed mechanisms might account for the psychiatric symptoms that occur during the course of a brain tumor, including direct injury to neuronal cells, secretion of hormones or other tumor-derived substances, and peri-ictal phenomena.3
TREATMENT Tumor is removed, but memory is impaired
Ms. A is scheduled for craniotomy and surgical resection of the frontal mass. Prior to surgery, Ms. A shows interest in improving her health, cooperates with staff, and seeks her daughter’s input on treatment. One week after admission, Ms. A has her mass resected, which is confirmed on biopsy to be a lung metastasis. Post-surgery, Ms. A receives codeine, 30 mg every 6 hours as needed, for pain; she continues dexamethasone, 4 mg IV every 6 hours, for brain edema and levetiracetam, 500 mg twice a day, for seizure prophylaxis.
On Day 2 after surgery, Ms. A attempts to elope. When she is approached by a psychiatrist on the treatment team, she does not recognize him. Although her long-term memory seems intact, she is unable to remember the details of recent events, including her medical and surgical treatments.
[polldaddy:10511745]
Continue to: The authors' observations
The authors’ observations
Ms. A’s memory impairment may be secondary to a surgically acquired neurocognitive deficit. In the United States, brain metastases represent a significant public health issue, affecting >100,000 patients per year.5 Metastatic lesions are the most common brain tumors. Lung cancer, breast cancer, and melanoma are the leading solid tumors to spread to the CNS.5 In cases of single brain metastasis, similar to Ms. A’s solitary left temporal lobe lesion, surgical resection plays a critical role in treatment. It provides histological confirmation of metastatic disease and can relieve mass effect if present. Studies have shown that combined surgical resection with radiation improves survival relative to patients who undergo radiation therapy alone.6,7
However, the benefits of surgical resection need to be balanced with preservation of neurologic function. Emerging evidence suggests that a majority of patients have surgically-acquired cognitive deficits due to damage of normal surrounding tissues, and these deficits are associated with reduced quality of life.8,9 Further, a study examining glioma surgical resections found that patients with left temporal lobe tumors exhibit more frequent and severe neurocognitive decline than patients with right temporal lobe tumors, especially in domains such as verbal memory.8 Ms. A’s memory impairment was persistent during her postoperative course, which suggests that it was not just an immediate post-surgical phenomenon, but a longer-lasting cognitive change directly related to the resection.
It is also possible that Ms. A had a prior neurocognitive disorder that manifested to a greater degree as a result of the CNS tumor. Ms. A might have had early-onset Alzheimer’s disease, although her intact memory before surgery makes this less likely. Alternatively, she could have had vascular dementia, especially given her long-standing hypertension and diabetes. This might have been missed in the initial evaluation because executive function was not tested. However, the relatively abrupt onset of memory problems after surgery suggests that she had no underlying neurocognitive disorder.
Ms. A’s presumed episode of MDD might also explain her memory changes. Major depressive disorder is increasingly common among geriatric patients, affecting approximately 5% of community-dwelling older adults.10 Its incidence increases with medical comorbidities, as suggested by depression rates of 5% to 10% in the primary care setting vs 37% in patients after critical-care hospitalizations.10 Late-life depression (LLD) occurs in adults age ≥60. Unlike depression in younger patients, LLD is more likely to be associated with cognitive impairment, specifically impairment of executive function and memory.11 The incidence of cognitive impairment in LLD is higher in patients with a history of depression, such as Ms. A.11,12 However, in general, patients who are depressed have memory complaints out of proportion to the clinical findings, and they show poor effort on cognitive testing. Ms. A exhibited neither of these, which makes it less likely that LLD was the exclusive cause of her memory loss.13 Table 214 outlines the management of cognitive deficits in a patient with a brain tumor.
EVALUATION Increasingly agitated and paranoid
After the tumor resection, Ms. A becomes increasingly irritable, uncooperative, and agitated. She repeatedly demands to be discharged. She insists she is fine and refuses medications and further laboratory workup. She becomes paranoid about the nursing staff and believes they are trying to kill her.
Continue to: On psychiatric re-evaluation...
On psychiatric re-evaluation, Ms. A demonstrates pressured speech, perseveration about going home, paranoid delusions, and anger at her family and physicians.
[polldaddy:10511747]
The authors’ observations
Ms. A’s refusal of medications and agitation may be explained by postoperative delirium, a surgical complication that is increasingly common among geriatric patients and is associated with poor clinical outcomes. Delirium is characterized by an acute onset and fluctuating course of symptoms that include inattention, motoric hypo- or hyperactivity, inappropriate behavior, emotional lability, cognitive dysfunction, and psychotic symptoms.15 Risk factors that contribute to postoperative delirium include older age, alcohol use, and poor baseline functional and cognitive status.16 The pathophysiology of delirium is not fully understood, but accumulating evidence suggests that different sets of interacting biologic factors (ie, neurotransmitters and inflammation) contribute to a disruption of large-scale neuronal networks in the brain, resulting in cognitive dysfunction.15 Patients who develop postoperative delirium are more likely to develop long-term cognitive dysfunction and have an increased risk of dementia.16
Another potential source of Ms. A’s agitation is steroid use. Ms. A received IV dexamethasone, 8 to 16 mg/d, around the time of her surgery. Steroids are commonly used to treat brain tumors, particularly when there is vasogenic edema. Steroid psychosis is a term loosely used to describe a wide range of psychiatric symptoms induced by corticosteroids that includes, but is not limited to, depression, mania, psychosis, delirium, and cognitive impairment.17 Steroid-induced psychiatric adverse effects occur in 5% to 18% of patients receiving corticosteroids and often happen early in treatment, although they can occur at any point.18 Corticosteroids influence brain activity via glucocorticoid and mineralocorticoid receptors. These receptors are widely distributed throughout the brain and affect neurotransmitter systems, such as the serotonergic system, that are associated with changes in mood, behavior, and cognition.17 While the adverse psychiatric manifestations of steroid use vary, higher dosages are associated with an increased risk of psychiatric complications; mania is more prevalent early in the course of treatment, and depression is more common with long-term use.17,19 Table 317,18 outlines the evidence-based treatment of corticosteroid-induced adverse psychiatric effects.
Although there are no clinical guidelines or FDA-approved medications for treating steroid-induced psychiatric adverse events, these are best managed by tapering and discontinuing steroids when possible and simultaneously using psychotropic medications to treat psychiatric symptoms. Case reports and limited evidence-based literature have demonstrated that steroid-induced mania responds to mood stabilizers or antipsychotics, while depression can be managed with antidepressants or lithium.17
Additionally, patients with CNS tumors are at risk for seizures and often are prescribed antiepileptics. Because it is easy to administer and does not need to be titrated, levetiracetam is a commonly used agent. However, levetiracetam can cause psychiatric adverse effects, including behavior changes and frank psychosis.20
Continue to: Finally, Ms. A's altered mental status...
Finally, Ms. A’s altered mental status could have been related to opioid intoxication. Opioids are used to manage postsurgical pain, and studies have shown these medications can be a precipitating factor for delirium in geriatric patients.21
TREATMENT Medication adjustments
At the request of the psychiatry team, levetiracetam is discontinued due to its potential for psychiatric adverse effects. The neurosurgery team replaces it with valproic acid, 500 mg every 12 hours. Ms. A is also tapered off steroids fairly rapidly because of the potential for steroid-induced psychiatric adverse effects. Her quetiapine is titrated from 50 to 150 mg every night at bedtime, and duloxetine is discontinued.
OUTCOME Agitation improves dramatically
Ms. A’s new medication regimen dramatically improves her agitation, which allows Ms. A, her family, and the medical team to work together to establish treatment goals. Ms. A ultimately returns home with the assistance of her family. She continues to have memory issues, but with improved emotion regulation. Several months later, Ms. A is readmitted to the hospital because her cancer has progressed despite treatment.
Bottom Line
Brain tumors may present with various psychiatric manifestations that can change during the course of the patient’s treatment. A comprehensive psychiatric evaluation should parse out the interplay between direct effects of the tumor and any adverse effects that are the result of medical and/or surgical interventions to determine the cause of psychiatric symptoms and their appropriate management.
Related Resource
Madhusoodanan S, Ting MB, Farah T, et al. Psychiatric aspects of brain tumors: a review. World J Psychiatry. 2015;5(3):273-285.
Drug Brand Names
Aripiprazole • Abilify
Buspirone • Buspar
Chlorpromazine • Thorazine
Codeine • Codeine systemic
Dexamethasone • Decadron
Duloxetine • Cymbalta
Haloperidol • Haldol
Levetiracetam • Keppra
Lorazepam • Ativan
Lithium • Eskalith, Lithobid
Olanzapine • Zyprexa
Quetiapine • Seroquel
Risperidone • Risperdal
Sertraline • Zoloft
Valproic acid • Depakene
1. Madhusoodanan S, Opler MG, Moise D, et al. Brain tumor location and psychiatric symptoms: is there any association? A meta-analysis of published case studies. Expert Rev Neurother. 2010;10(10):1529-1536.
2. Bunevicius A, Deltuva VP, Deltuviene D, et al. Brain lesions manifesting as psychiatric disorders: eight cases. CNS Spectr. 2008;13(11):950-958.
3. Pearl ML, Talgat G, Valea FA, et al. Psychiatric symptoms due to brain metastases. Med Update Psychiatr. 1998;3(4):91-94.
4. Madhusoodanan S, Danan D, Moise D. Psychiatric manifestations of brain tumors: diagnostic implications. Expert Rev Neurother. 2007;7(4):343-349.
5. Ferguson SD, Wagner KM, Prabhu SS, et al. Neurosurgical management of brain metastases. Clin Exp Metastasis. 2017;34(6-7):377-389.
6. Husain ZA, Regine WF, Kwok Y, et al. Brain metastases: contemporary management and future directions. Eur J Clin Med Oncol. 2011;3(3):38-45.
7. Vecht CJ, Haaxmareiche H, Noordijk EM, et al. Treatment of single brain metastasis - radiotherapy alone or combined with neurosurgery. Ann Neurol. 1993;33(6):583-590.
8. Barry RL, Byun NE, Tantawy MN, et al. In vivo neuroimaging and behavioral correlates in a rat model of chemotherapy-induced cognitive dysfunction. Brain Imaging Behav. 2018;12(1):87-95.
9. Wu AS, Witgert ME, Lang FF, et al. Neurocognitive function before and after surgery for insular gliomas. J Neurosurg. 2011;115(6):1115-1125.
10. Taylor WD. Depression in the elderly. N Engl J Med. 2014;371(13):1228-1236.
11. Liguori C, Pierantozzi M, Chiaravalloti A, et al. When cognitive decline and depression coexist in the elderly: CSF biomarkers analysis can differentiate Alzheimer’s disease from late-life depression. Front Aging Neurosci. 2018;10:38.
12. Luijendijk HJ, van den Berg JF, Dekker MJHJ, et al. Incidence and recurrence of late-life depression. Arch Gen Psychiatry. 2008;65(12):1394-1401.
13. Potter GG, Steffens DC. Contribution of depression to cognitive impairment and dementia in older adults. Neurologist. 2007;13(3):105-117.
14. Taphoorn MJB, Klein M. Cognitive deficits in adult patients with brain tumours. Lancet Neurol. 2004;3(3):159-168.
15. Inouye SK, Westendorp RGJ, Saczynski JS. Delirium in elderly people. Lancet. 2014;383(9920):911-922.
16. Sprung J, Roberts RO, Weingarten TN, et al. Postoperative delirium in elderly patients is associated with subsequent cognitive impairment. Br J Anaesth. 2017;119(2):316-323.
17. Kusljic S, Manias E, Gogos A. Corticosteroid-induced psychiatric disturbances: it is time for pharmacists to take notice. Res Soc Adm Pharm. 2016;12(2):355-360.
18. Cerullo MA. Corticosteroid-induced mania: prepare for the unpredictable. Current Psychiatry. 2006;5(6):43-50.
19. Dubovsky AN, Arvikar S, Stern TA, et al. Steroid psychosis revisited. Psychosomatics. 2012;53(2):103-115.
20. Habets JGV, Leentjens AFG, Schijns OEMG. Serious and reversible levetiracetam-induced psychiatric symptoms after resection of frontal low-grade glioma: two case histories. Br J Neurosurg. 2017;31(4):471-473.
21
CASE Suicidal behavior, severe headaches
Ms. A, age 60, presents to the emergency department (ED) with depression, suicidal behavior, and 3 days of severe headaches. Neurology is consulted and an MRI is ordered, which shows a 3.0-cm mass lesion in the left temporal lobe with associated vasogenic edema that is suspicious for metastatic disease (Figure).
Ms. A is admitted to the hospital for further workup of her brain lesion. She is started on IV dexamethasone, 10 mg every 6 hours, a glucocorticosteroid, for brain edema, and levetiracetam, 500 mg twice a day, for seizure prophylaxis.
Upon admission, in addition to oncology and neurosurgery, psychiatry is also consulted to evaluate Ms. A for depression and suicidality.
EVALUATION Mood changes and poor judgment
Ms. A has a psychiatric history of depression and alcohol use disorder but says she has not consumed any alcohol in years. Her medical history includes hypertension, diabetes, and stage 4 non-small–cell lung cancer, for which she received surgery and adjuvant chemoradiotherapy 1 year ago.
On initial intake, Ms. A reports that in addition to the headaches, she has also been experiencing worsening depression and suicidal behavior. For the past 2 months, she has had a severely depressed mood, with notable anhedonia, poor appetite, insomnia, low energy, and decreased concentration. The changes in her mental health were triggered by her mother’s death. Three days prior to admission, the patient planned to overdose on antihypertensive pills, but her suicide attempt was interrupted when her family called. She denies any current suicidal ideation, intent, or plan.
According to her family, Ms. A has been increasingly irritable and her personality has changed in the past month. She also has been repeatedly sorting through her neighbors’ garbage.
Ms. A’s current psychiatric medications are duloxetine, 30 mg/d; quetiapine, 50 mg every night at bedtime; and buspirone, 10 mg/d. However, it is unclear if she is consistently taking these medications.
Continue to: On mental status examination...
On mental status examination, Ms. A is calm and she has no abnormal movements. She says she is depressed. Her affect is reactive and labile. She is alert and oriented to person, place, and time. Her attention, registration, and recall are intact. Her executive function is not tested. However, Ms. A’s insight and judgment seem poor.
To address Ms. A’s worsening depression, the psychiatry team increases her duloxetine from 30 to 60 mg/d, and she continues quetiapine, 50 mg every night at bedtime, for mood lability. Buspirone is not continued because she was not taking a therapeutic dosage in the community.
Within 4 days, Ms. A shows improvement in sleep, appetite, and mood. She has no further suicidal ideation.
[polldaddy:10511743]
The authors’ observations
Ms. A had a recurrence of what was presumed to be major depressive disorder (MDD) in the context of her mother’s death. However, she also exhibited irritability, mood lability, and impulsivity, all of which could be part of her depression, or a separate problem related to her brain tumor. Because Ms. A had never displayed bizarre behavior before the past few weeks, it is likely that her CNS lesion was directly affecting her personality and possibly underlying her planned suicide attempt.
Fifty to 80% of patients with CNS tumors, either primary or metastatic, present with psychiatric symptoms.1 Table 11-3 lists common psychiatric symptoms of brain tumors. Unfortunately, there is little reliable evidence that directly correlates tumor location with specific psychiatric symptoms. A 2010 meta-analysis found a statistically significant link between anorexia nervosa and hypothalamic tumors.1 However, for other brain regions, there is only an increased likelihood that any given tumor location will produce psychiatric symptoms.1,4 For instance, compared to patients with tumors in other locations, those with temporal lobe tumors are more likely to present with mood disorders, personality changes, and memory problems.1 In contrast, patients with frontal lobe tumors have an increased likelihood of psychosis, mood disorders, and personality changes.1 Patients with tumors in the pituitary region often present with anxiety.1
Continue to: When considering treatment options...
When considering treatment options for Ms. A, alcohol withdrawal was unlikely given the remote history of alcohol use, low alcohol blood level, and lack of evidence of unstable vital signs or tremor. Although she might have benefited from inpatient psychiatric treatment, this needed to wait until there was a definitive treatment plan for her brain tumor. Finally, although a paraneoplastic syndrome, such as limbic encephalitis, could be causing her psychiatric symptoms, this scenario is less likely with non-small–cell lung cancer.
Although uncommon, CNS tumors can present with psychiatric symptoms as the only manifestation. This is more likely when a patient exhibits new-onset or atypical symptoms, or fails to respond to standard psychiatric treatment.4 Case reports have described patients with brain tumors being misdiagnosed as having a primary psychiatric condition, which delays treatment of their CNS cancer.2 Additionally, frontal and limbic tumors are more likely to present with psychiatric manifestations; up to 90% of patients exhibit altered mental status or personality changes, as did Ms. A.1,4 Clearly, it is easier to identify patients with psychiatric symptoms resulting from a brain tumor when they also present with focal neurologic deficits or systemic symptoms, such as headache or nausea and vomiting. Ms. A presented with severe headaches, which is what led to her early imaging and prompt diagnosis.
Numerous proposed mechanisms might account for the psychiatric symptoms that occur during the course of a brain tumor, including direct injury to neuronal cells, secretion of hormones or other tumor-derived substances, and peri-ictal phenomena.3
TREATMENT Tumor is removed, but memory is impaired
Ms. A is scheduled for craniotomy and surgical resection of the frontal mass. Prior to surgery, Ms. A shows interest in improving her health, cooperates with staff, and seeks her daughter’s input on treatment. One week after admission, Ms. A has her mass resected, which is confirmed on biopsy to be a lung metastasis. Post-surgery, Ms. A receives codeine, 30 mg every 6 hours as needed, for pain; she continues dexamethasone, 4 mg IV every 6 hours, for brain edema and levetiracetam, 500 mg twice a day, for seizure prophylaxis.
On Day 2 after surgery, Ms. A attempts to elope. When she is approached by a psychiatrist on the treatment team, she does not recognize him. Although her long-term memory seems intact, she is unable to remember the details of recent events, including her medical and surgical treatments.
[polldaddy:10511745]
Continue to: The authors' observations
The authors’ observations
Ms. A’s memory impairment may be secondary to a surgically acquired neurocognitive deficit. In the United States, brain metastases represent a significant public health issue, affecting >100,000 patients per year.5 Metastatic lesions are the most common brain tumors. Lung cancer, breast cancer, and melanoma are the leading solid tumors to spread to the CNS.5 In cases of single brain metastasis, similar to Ms. A’s solitary left temporal lobe lesion, surgical resection plays a critical role in treatment. It provides histological confirmation of metastatic disease and can relieve mass effect if present. Studies have shown that combined surgical resection with radiation improves survival relative to patients who undergo radiation therapy alone.6,7
However, the benefits of surgical resection need to be balanced with preservation of neurologic function. Emerging evidence suggests that a majority of patients have surgically-acquired cognitive deficits due to damage of normal surrounding tissues, and these deficits are associated with reduced quality of life.8,9 Further, a study examining glioma surgical resections found that patients with left temporal lobe tumors exhibit more frequent and severe neurocognitive decline than patients with right temporal lobe tumors, especially in domains such as verbal memory.8 Ms. A’s memory impairment was persistent during her postoperative course, which suggests that it was not just an immediate post-surgical phenomenon, but a longer-lasting cognitive change directly related to the resection.
It is also possible that Ms. A had a prior neurocognitive disorder that manifested to a greater degree as a result of the CNS tumor. Ms. A might have had early-onset Alzheimer’s disease, although her intact memory before surgery makes this less likely. Alternatively, she could have had vascular dementia, especially given her long-standing hypertension and diabetes. This might have been missed in the initial evaluation because executive function was not tested. However, the relatively abrupt onset of memory problems after surgery suggests that she had no underlying neurocognitive disorder.
Ms. A’s presumed episode of MDD might also explain her memory changes. Major depressive disorder is increasingly common among geriatric patients, affecting approximately 5% of community-dwelling older adults.10 Its incidence increases with medical comorbidities, as suggested by depression rates of 5% to 10% in the primary care setting vs 37% in patients after critical-care hospitalizations.10 Late-life depression (LLD) occurs in adults age ≥60. Unlike depression in younger patients, LLD is more likely to be associated with cognitive impairment, specifically impairment of executive function and memory.11 The incidence of cognitive impairment in LLD is higher in patients with a history of depression, such as Ms. A.11,12 However, in general, patients who are depressed have memory complaints out of proportion to the clinical findings, and they show poor effort on cognitive testing. Ms. A exhibited neither of these, which makes it less likely that LLD was the exclusive cause of her memory loss.13 Table 214 outlines the management of cognitive deficits in a patient with a brain tumor.
EVALUATION Increasingly agitated and paranoid
After the tumor resection, Ms. A becomes increasingly irritable, uncooperative, and agitated. She repeatedly demands to be discharged. She insists she is fine and refuses medications and further laboratory workup. She becomes paranoid about the nursing staff and believes they are trying to kill her.
Continue to: On psychiatric re-evaluation...
On psychiatric re-evaluation, Ms. A demonstrates pressured speech, perseveration about going home, paranoid delusions, and anger at her family and physicians.
[polldaddy:10511747]
The authors’ observations
Ms. A’s refusal of medications and agitation may be explained by postoperative delirium, a surgical complication that is increasingly common among geriatric patients and is associated with poor clinical outcomes. Delirium is characterized by an acute onset and fluctuating course of symptoms that include inattention, motoric hypo- or hyperactivity, inappropriate behavior, emotional lability, cognitive dysfunction, and psychotic symptoms.15 Risk factors that contribute to postoperative delirium include older age, alcohol use, and poor baseline functional and cognitive status.16 The pathophysiology of delirium is not fully understood, but accumulating evidence suggests that different sets of interacting biologic factors (ie, neurotransmitters and inflammation) contribute to a disruption of large-scale neuronal networks in the brain, resulting in cognitive dysfunction.15 Patients who develop postoperative delirium are more likely to develop long-term cognitive dysfunction and have an increased risk of dementia.16
Another potential source of Ms. A’s agitation is steroid use. Ms. A received IV dexamethasone, 8 to 16 mg/d, around the time of her surgery. Steroids are commonly used to treat brain tumors, particularly when there is vasogenic edema. Steroid psychosis is a term loosely used to describe a wide range of psychiatric symptoms induced by corticosteroids that includes, but is not limited to, depression, mania, psychosis, delirium, and cognitive impairment.17 Steroid-induced psychiatric adverse effects occur in 5% to 18% of patients receiving corticosteroids and often happen early in treatment, although they can occur at any point.18 Corticosteroids influence brain activity via glucocorticoid and mineralocorticoid receptors. These receptors are widely distributed throughout the brain and affect neurotransmitter systems, such as the serotonergic system, that are associated with changes in mood, behavior, and cognition.17 While the adverse psychiatric manifestations of steroid use vary, higher dosages are associated with an increased risk of psychiatric complications; mania is more prevalent early in the course of treatment, and depression is more common with long-term use.17,19 Table 317,18 outlines the evidence-based treatment of corticosteroid-induced adverse psychiatric effects.
Although there are no clinical guidelines or FDA-approved medications for treating steroid-induced psychiatric adverse events, these are best managed by tapering and discontinuing steroids when possible and simultaneously using psychotropic medications to treat psychiatric symptoms. Case reports and limited evidence-based literature have demonstrated that steroid-induced mania responds to mood stabilizers or antipsychotics, while depression can be managed with antidepressants or lithium.17
Additionally, patients with CNS tumors are at risk for seizures and often are prescribed antiepileptics. Because it is easy to administer and does not need to be titrated, levetiracetam is a commonly used agent. However, levetiracetam can cause psychiatric adverse effects, including behavior changes and frank psychosis.20
Continue to: Finally, Ms. A's altered mental status...
Finally, Ms. A’s altered mental status could have been related to opioid intoxication. Opioids are used to manage postsurgical pain, and studies have shown these medications can be a precipitating factor for delirium in geriatric patients.21
TREATMENT Medication adjustments
At the request of the psychiatry team, levetiracetam is discontinued due to its potential for psychiatric adverse effects. The neurosurgery team replaces it with valproic acid, 500 mg every 12 hours. Ms. A is also tapered off steroids fairly rapidly because of the potential for steroid-induced psychiatric adverse effects. Her quetiapine is titrated from 50 to 150 mg every night at bedtime, and duloxetine is discontinued.
OUTCOME Agitation improves dramatically
Ms. A’s new medication regimen dramatically improves her agitation, which allows Ms. A, her family, and the medical team to work together to establish treatment goals. Ms. A ultimately returns home with the assistance of her family. She continues to have memory issues, but with improved emotion regulation. Several months later, Ms. A is readmitted to the hospital because her cancer has progressed despite treatment.
Bottom Line
Brain tumors may present with various psychiatric manifestations that can change during the course of the patient’s treatment. A comprehensive psychiatric evaluation should parse out the interplay between direct effects of the tumor and any adverse effects that are the result of medical and/or surgical interventions to determine the cause of psychiatric symptoms and their appropriate management.
Related Resource
Madhusoodanan S, Ting MB, Farah T, et al. Psychiatric aspects of brain tumors: a review. World J Psychiatry. 2015;5(3):273-285.
Drug Brand Names
Aripiprazole • Abilify
Buspirone • Buspar
Chlorpromazine • Thorazine
Codeine • Codeine systemic
Dexamethasone • Decadron
Duloxetine • Cymbalta
Haloperidol • Haldol
Levetiracetam • Keppra
Lorazepam • Ativan
Lithium • Eskalith, Lithobid
Olanzapine • Zyprexa
Quetiapine • Seroquel
Risperidone • Risperdal
Sertraline • Zoloft
Valproic acid • Depakene
CASE Suicidal behavior, severe headaches
Ms. A, age 60, presents to the emergency department (ED) with depression, suicidal behavior, and 3 days of severe headaches. Neurology is consulted and an MRI is ordered, which shows a 3.0-cm mass lesion in the left temporal lobe with associated vasogenic edema that is suspicious for metastatic disease (Figure).
Ms. A is admitted to the hospital for further workup of her brain lesion. She is started on IV dexamethasone, 10 mg every 6 hours, a glucocorticosteroid, for brain edema, and levetiracetam, 500 mg twice a day, for seizure prophylaxis.
Upon admission, in addition to oncology and neurosurgery, psychiatry is also consulted to evaluate Ms. A for depression and suicidality.
EVALUATION Mood changes and poor judgment
Ms. A has a psychiatric history of depression and alcohol use disorder but says she has not consumed any alcohol in years. Her medical history includes hypertension, diabetes, and stage 4 non-small–cell lung cancer, for which she received surgery and adjuvant chemoradiotherapy 1 year ago.
On initial intake, Ms. A reports that in addition to the headaches, she has also been experiencing worsening depression and suicidal behavior. For the past 2 months, she has had a severely depressed mood, with notable anhedonia, poor appetite, insomnia, low energy, and decreased concentration. The changes in her mental health were triggered by her mother’s death. Three days prior to admission, the patient planned to overdose on antihypertensive pills, but her suicide attempt was interrupted when her family called. She denies any current suicidal ideation, intent, or plan.
According to her family, Ms. A has been increasingly irritable and her personality has changed in the past month. She also has been repeatedly sorting through her neighbors’ garbage.
Ms. A’s current psychiatric medications are duloxetine, 30 mg/d; quetiapine, 50 mg every night at bedtime; and buspirone, 10 mg/d. However, it is unclear if she is consistently taking these medications.
Continue to: On mental status examination...
On mental status examination, Ms. A is calm and she has no abnormal movements. She says she is depressed. Her affect is reactive and labile. She is alert and oriented to person, place, and time. Her attention, registration, and recall are intact. Her executive function is not tested. However, Ms. A’s insight and judgment seem poor.
To address Ms. A’s worsening depression, the psychiatry team increases her duloxetine from 30 to 60 mg/d, and she continues quetiapine, 50 mg every night at bedtime, for mood lability. Buspirone is not continued because she was not taking a therapeutic dosage in the community.
Within 4 days, Ms. A shows improvement in sleep, appetite, and mood. She has no further suicidal ideation.
[polldaddy:10511743]
The authors’ observations
Ms. A had a recurrence of what was presumed to be major depressive disorder (MDD) in the context of her mother’s death. However, she also exhibited irritability, mood lability, and impulsivity, all of which could be part of her depression, or a separate problem related to her brain tumor. Because Ms. A had never displayed bizarre behavior before the past few weeks, it is likely that her CNS lesion was directly affecting her personality and possibly underlying her planned suicide attempt.
Fifty to 80% of patients with CNS tumors, either primary or metastatic, present with psychiatric symptoms.1 Table 11-3 lists common psychiatric symptoms of brain tumors. Unfortunately, there is little reliable evidence that directly correlates tumor location with specific psychiatric symptoms. A 2010 meta-analysis found a statistically significant link between anorexia nervosa and hypothalamic tumors.1 However, for other brain regions, there is only an increased likelihood that any given tumor location will produce psychiatric symptoms.1,4 For instance, compared to patients with tumors in other locations, those with temporal lobe tumors are more likely to present with mood disorders, personality changes, and memory problems.1 In contrast, patients with frontal lobe tumors have an increased likelihood of psychosis, mood disorders, and personality changes.1 Patients with tumors in the pituitary region often present with anxiety.1
Continue to: When considering treatment options...
When considering treatment options for Ms. A, alcohol withdrawal was unlikely given the remote history of alcohol use, low alcohol blood level, and lack of evidence of unstable vital signs or tremor. Although she might have benefited from inpatient psychiatric treatment, this needed to wait until there was a definitive treatment plan for her brain tumor. Finally, although a paraneoplastic syndrome, such as limbic encephalitis, could be causing her psychiatric symptoms, this scenario is less likely with non-small–cell lung cancer.
Although uncommon, CNS tumors can present with psychiatric symptoms as the only manifestation. This is more likely when a patient exhibits new-onset or atypical symptoms, or fails to respond to standard psychiatric treatment.4 Case reports have described patients with brain tumors being misdiagnosed as having a primary psychiatric condition, which delays treatment of their CNS cancer.2 Additionally, frontal and limbic tumors are more likely to present with psychiatric manifestations; up to 90% of patients exhibit altered mental status or personality changes, as did Ms. A.1,4 Clearly, it is easier to identify patients with psychiatric symptoms resulting from a brain tumor when they also present with focal neurologic deficits or systemic symptoms, such as headache or nausea and vomiting. Ms. A presented with severe headaches, which is what led to her early imaging and prompt diagnosis.
Numerous proposed mechanisms might account for the psychiatric symptoms that occur during the course of a brain tumor, including direct injury to neuronal cells, secretion of hormones or other tumor-derived substances, and peri-ictal phenomena.3
TREATMENT Tumor is removed, but memory is impaired
Ms. A is scheduled for craniotomy and surgical resection of the frontal mass. Prior to surgery, Ms. A shows interest in improving her health, cooperates with staff, and seeks her daughter’s input on treatment. One week after admission, Ms. A has her mass resected, which is confirmed on biopsy to be a lung metastasis. Post-surgery, Ms. A receives codeine, 30 mg every 6 hours as needed, for pain; she continues dexamethasone, 4 mg IV every 6 hours, for brain edema and levetiracetam, 500 mg twice a day, for seizure prophylaxis.
On Day 2 after surgery, Ms. A attempts to elope. When she is approached by a psychiatrist on the treatment team, she does not recognize him. Although her long-term memory seems intact, she is unable to remember the details of recent events, including her medical and surgical treatments.
[polldaddy:10511745]
Continue to: The authors' observations
The authors’ observations
Ms. A’s memory impairment may be secondary to a surgically acquired neurocognitive deficit. In the United States, brain metastases represent a significant public health issue, affecting >100,000 patients per year.5 Metastatic lesions are the most common brain tumors. Lung cancer, breast cancer, and melanoma are the leading solid tumors to spread to the CNS.5 In cases of single brain metastasis, similar to Ms. A’s solitary left temporal lobe lesion, surgical resection plays a critical role in treatment. It provides histological confirmation of metastatic disease and can relieve mass effect if present. Studies have shown that combined surgical resection with radiation improves survival relative to patients who undergo radiation therapy alone.6,7
However, the benefits of surgical resection need to be balanced with preservation of neurologic function. Emerging evidence suggests that a majority of patients have surgically-acquired cognitive deficits due to damage of normal surrounding tissues, and these deficits are associated with reduced quality of life.8,9 Further, a study examining glioma surgical resections found that patients with left temporal lobe tumors exhibit more frequent and severe neurocognitive decline than patients with right temporal lobe tumors, especially in domains such as verbal memory.8 Ms. A’s memory impairment was persistent during her postoperative course, which suggests that it was not just an immediate post-surgical phenomenon, but a longer-lasting cognitive change directly related to the resection.
It is also possible that Ms. A had a prior neurocognitive disorder that manifested to a greater degree as a result of the CNS tumor. Ms. A might have had early-onset Alzheimer’s disease, although her intact memory before surgery makes this less likely. Alternatively, she could have had vascular dementia, especially given her long-standing hypertension and diabetes. This might have been missed in the initial evaluation because executive function was not tested. However, the relatively abrupt onset of memory problems after surgery suggests that she had no underlying neurocognitive disorder.
Ms. A’s presumed episode of MDD might also explain her memory changes. Major depressive disorder is increasingly common among geriatric patients, affecting approximately 5% of community-dwelling older adults.10 Its incidence increases with medical comorbidities, as suggested by depression rates of 5% to 10% in the primary care setting vs 37% in patients after critical-care hospitalizations.10 Late-life depression (LLD) occurs in adults age ≥60. Unlike depression in younger patients, LLD is more likely to be associated with cognitive impairment, specifically impairment of executive function and memory.11 The incidence of cognitive impairment in LLD is higher in patients with a history of depression, such as Ms. A.11,12 However, in general, patients who are depressed have memory complaints out of proportion to the clinical findings, and they show poor effort on cognitive testing. Ms. A exhibited neither of these, which makes it less likely that LLD was the exclusive cause of her memory loss.13 Table 214 outlines the management of cognitive deficits in a patient with a brain tumor.
EVALUATION Increasingly agitated and paranoid
After the tumor resection, Ms. A becomes increasingly irritable, uncooperative, and agitated. She repeatedly demands to be discharged. She insists she is fine and refuses medications and further laboratory workup. She becomes paranoid about the nursing staff and believes they are trying to kill her.
Continue to: On psychiatric re-evaluation...
On psychiatric re-evaluation, Ms. A demonstrates pressured speech, perseveration about going home, paranoid delusions, and anger at her family and physicians.
[polldaddy:10511747]
The authors’ observations
Ms. A’s refusal of medications and agitation may be explained by postoperative delirium, a surgical complication that is increasingly common among geriatric patients and is associated with poor clinical outcomes. Delirium is characterized by an acute onset and fluctuating course of symptoms that include inattention, motoric hypo- or hyperactivity, inappropriate behavior, emotional lability, cognitive dysfunction, and psychotic symptoms.15 Risk factors that contribute to postoperative delirium include older age, alcohol use, and poor baseline functional and cognitive status.16 The pathophysiology of delirium is not fully understood, but accumulating evidence suggests that different sets of interacting biologic factors (ie, neurotransmitters and inflammation) contribute to a disruption of large-scale neuronal networks in the brain, resulting in cognitive dysfunction.15 Patients who develop postoperative delirium are more likely to develop long-term cognitive dysfunction and have an increased risk of dementia.16
Another potential source of Ms. A’s agitation is steroid use. Ms. A received IV dexamethasone, 8 to 16 mg/d, around the time of her surgery. Steroids are commonly used to treat brain tumors, particularly when there is vasogenic edema. Steroid psychosis is a term loosely used to describe a wide range of psychiatric symptoms induced by corticosteroids that includes, but is not limited to, depression, mania, psychosis, delirium, and cognitive impairment.17 Steroid-induced psychiatric adverse effects occur in 5% to 18% of patients receiving corticosteroids and often happen early in treatment, although they can occur at any point.18 Corticosteroids influence brain activity via glucocorticoid and mineralocorticoid receptors. These receptors are widely distributed throughout the brain and affect neurotransmitter systems, such as the serotonergic system, that are associated with changes in mood, behavior, and cognition.17 While the adverse psychiatric manifestations of steroid use vary, higher dosages are associated with an increased risk of psychiatric complications; mania is more prevalent early in the course of treatment, and depression is more common with long-term use.17,19 Table 317,18 outlines the evidence-based treatment of corticosteroid-induced adverse psychiatric effects.
Although there are no clinical guidelines or FDA-approved medications for treating steroid-induced psychiatric adverse events, these are best managed by tapering and discontinuing steroids when possible and simultaneously using psychotropic medications to treat psychiatric symptoms. Case reports and limited evidence-based literature have demonstrated that steroid-induced mania responds to mood stabilizers or antipsychotics, while depression can be managed with antidepressants or lithium.17
Additionally, patients with CNS tumors are at risk for seizures and often are prescribed antiepileptics. Because it is easy to administer and does not need to be titrated, levetiracetam is a commonly used agent. However, levetiracetam can cause psychiatric adverse effects, including behavior changes and frank psychosis.20
Continue to: Finally, Ms. A's altered mental status...
Finally, Ms. A’s altered mental status could have been related to opioid intoxication. Opioids are used to manage postsurgical pain, and studies have shown these medications can be a precipitating factor for delirium in geriatric patients.21
TREATMENT Medication adjustments
At the request of the psychiatry team, levetiracetam is discontinued due to its potential for psychiatric adverse effects. The neurosurgery team replaces it with valproic acid, 500 mg every 12 hours. Ms. A is also tapered off steroids fairly rapidly because of the potential for steroid-induced psychiatric adverse effects. Her quetiapine is titrated from 50 to 150 mg every night at bedtime, and duloxetine is discontinued.
OUTCOME Agitation improves dramatically
Ms. A’s new medication regimen dramatically improves her agitation, which allows Ms. A, her family, and the medical team to work together to establish treatment goals. Ms. A ultimately returns home with the assistance of her family. She continues to have memory issues, but with improved emotion regulation. Several months later, Ms. A is readmitted to the hospital because her cancer has progressed despite treatment.
Bottom Line
Brain tumors may present with various psychiatric manifestations that can change during the course of the patient’s treatment. A comprehensive psychiatric evaluation should parse out the interplay between direct effects of the tumor and any adverse effects that are the result of medical and/or surgical interventions to determine the cause of psychiatric symptoms and their appropriate management.
Related Resource
Madhusoodanan S, Ting MB, Farah T, et al. Psychiatric aspects of brain tumors: a review. World J Psychiatry. 2015;5(3):273-285.
Drug Brand Names
Aripiprazole • Abilify
Buspirone • Buspar
Chlorpromazine • Thorazine
Codeine • Codeine systemic
Dexamethasone • Decadron
Duloxetine • Cymbalta
Haloperidol • Haldol
Levetiracetam • Keppra
Lorazepam • Ativan
Lithium • Eskalith, Lithobid
Olanzapine • Zyprexa
Quetiapine • Seroquel
Risperidone • Risperdal
Sertraline • Zoloft
Valproic acid • Depakene
1. Madhusoodanan S, Opler MG, Moise D, et al. Brain tumor location and psychiatric symptoms: is there any association? A meta-analysis of published case studies. Expert Rev Neurother. 2010;10(10):1529-1536.
2. Bunevicius A, Deltuva VP, Deltuviene D, et al. Brain lesions manifesting as psychiatric disorders: eight cases. CNS Spectr. 2008;13(11):950-958.
3. Pearl ML, Talgat G, Valea FA, et al. Psychiatric symptoms due to brain metastases. Med Update Psychiatr. 1998;3(4):91-94.
4. Madhusoodanan S, Danan D, Moise D. Psychiatric manifestations of brain tumors: diagnostic implications. Expert Rev Neurother. 2007;7(4):343-349.
5. Ferguson SD, Wagner KM, Prabhu SS, et al. Neurosurgical management of brain metastases. Clin Exp Metastasis. 2017;34(6-7):377-389.
6. Husain ZA, Regine WF, Kwok Y, et al. Brain metastases: contemporary management and future directions. Eur J Clin Med Oncol. 2011;3(3):38-45.
7. Vecht CJ, Haaxmareiche H, Noordijk EM, et al. Treatment of single brain metastasis - radiotherapy alone or combined with neurosurgery. Ann Neurol. 1993;33(6):583-590.
8. Barry RL, Byun NE, Tantawy MN, et al. In vivo neuroimaging and behavioral correlates in a rat model of chemotherapy-induced cognitive dysfunction. Brain Imaging Behav. 2018;12(1):87-95.
9. Wu AS, Witgert ME, Lang FF, et al. Neurocognitive function before and after surgery for insular gliomas. J Neurosurg. 2011;115(6):1115-1125.
10. Taylor WD. Depression in the elderly. N Engl J Med. 2014;371(13):1228-1236.
11. Liguori C, Pierantozzi M, Chiaravalloti A, et al. When cognitive decline and depression coexist in the elderly: CSF biomarkers analysis can differentiate Alzheimer’s disease from late-life depression. Front Aging Neurosci. 2018;10:38.
12. Luijendijk HJ, van den Berg JF, Dekker MJHJ, et al. Incidence and recurrence of late-life depression. Arch Gen Psychiatry. 2008;65(12):1394-1401.
13. Potter GG, Steffens DC. Contribution of depression to cognitive impairment and dementia in older adults. Neurologist. 2007;13(3):105-117.
14. Taphoorn MJB, Klein M. Cognitive deficits in adult patients with brain tumours. Lancet Neurol. 2004;3(3):159-168.
15. Inouye SK, Westendorp RGJ, Saczynski JS. Delirium in elderly people. Lancet. 2014;383(9920):911-922.
16. Sprung J, Roberts RO, Weingarten TN, et al. Postoperative delirium in elderly patients is associated with subsequent cognitive impairment. Br J Anaesth. 2017;119(2):316-323.
17. Kusljic S, Manias E, Gogos A. Corticosteroid-induced psychiatric disturbances: it is time for pharmacists to take notice. Res Soc Adm Pharm. 2016;12(2):355-360.
18. Cerullo MA. Corticosteroid-induced mania: prepare for the unpredictable. Current Psychiatry. 2006;5(6):43-50.
19. Dubovsky AN, Arvikar S, Stern TA, et al. Steroid psychosis revisited. Psychosomatics. 2012;53(2):103-115.
20. Habets JGV, Leentjens AFG, Schijns OEMG. Serious and reversible levetiracetam-induced psychiatric symptoms after resection of frontal low-grade glioma: two case histories. Br J Neurosurg. 2017;31(4):471-473.
21
1. Madhusoodanan S, Opler MG, Moise D, et al. Brain tumor location and psychiatric symptoms: is there any association? A meta-analysis of published case studies. Expert Rev Neurother. 2010;10(10):1529-1536.
2. Bunevicius A, Deltuva VP, Deltuviene D, et al. Brain lesions manifesting as psychiatric disorders: eight cases. CNS Spectr. 2008;13(11):950-958.
3. Pearl ML, Talgat G, Valea FA, et al. Psychiatric symptoms due to brain metastases. Med Update Psychiatr. 1998;3(4):91-94.
4. Madhusoodanan S, Danan D, Moise D. Psychiatric manifestations of brain tumors: diagnostic implications. Expert Rev Neurother. 2007;7(4):343-349.
5. Ferguson SD, Wagner KM, Prabhu SS, et al. Neurosurgical management of brain metastases. Clin Exp Metastasis. 2017;34(6-7):377-389.
6. Husain ZA, Regine WF, Kwok Y, et al. Brain metastases: contemporary management and future directions. Eur J Clin Med Oncol. 2011;3(3):38-45.
7. Vecht CJ, Haaxmareiche H, Noordijk EM, et al. Treatment of single brain metastasis - radiotherapy alone or combined with neurosurgery. Ann Neurol. 1993;33(6):583-590.
8. Barry RL, Byun NE, Tantawy MN, et al. In vivo neuroimaging and behavioral correlates in a rat model of chemotherapy-induced cognitive dysfunction. Brain Imaging Behav. 2018;12(1):87-95.
9. Wu AS, Witgert ME, Lang FF, et al. Neurocognitive function before and after surgery for insular gliomas. J Neurosurg. 2011;115(6):1115-1125.
10. Taylor WD. Depression in the elderly. N Engl J Med. 2014;371(13):1228-1236.
11. Liguori C, Pierantozzi M, Chiaravalloti A, et al. When cognitive decline and depression coexist in the elderly: CSF biomarkers analysis can differentiate Alzheimer’s disease from late-life depression. Front Aging Neurosci. 2018;10:38.
12. Luijendijk HJ, van den Berg JF, Dekker MJHJ, et al. Incidence and recurrence of late-life depression. Arch Gen Psychiatry. 2008;65(12):1394-1401.
13. Potter GG, Steffens DC. Contribution of depression to cognitive impairment and dementia in older adults. Neurologist. 2007;13(3):105-117.
14. Taphoorn MJB, Klein M. Cognitive deficits in adult patients with brain tumours. Lancet Neurol. 2004;3(3):159-168.
15. Inouye SK, Westendorp RGJ, Saczynski JS. Delirium in elderly people. Lancet. 2014;383(9920):911-922.
16. Sprung J, Roberts RO, Weingarten TN, et al. Postoperative delirium in elderly patients is associated with subsequent cognitive impairment. Br J Anaesth. 2017;119(2):316-323.
17. Kusljic S, Manias E, Gogos A. Corticosteroid-induced psychiatric disturbances: it is time for pharmacists to take notice. Res Soc Adm Pharm. 2016;12(2):355-360.
18. Cerullo MA. Corticosteroid-induced mania: prepare for the unpredictable. Current Psychiatry. 2006;5(6):43-50.
19. Dubovsky AN, Arvikar S, Stern TA, et al. Steroid psychosis revisited. Psychosomatics. 2012;53(2):103-115.
20. Habets JGV, Leentjens AFG, Schijns OEMG. Serious and reversible levetiracetam-induced psychiatric symptoms after resection of frontal low-grade glioma: two case histories. Br J Neurosurg. 2017;31(4):471-473.
21