User login
Stuck in a rut with the wrong diagnosis
CASE Aggressive behaviors, psychosis
Ms. N, age 58, has a long history of bipolar disorder with psychotic features. She presents to our emergency department (ED) after an acute fall and frequent violent behaviors at her nursing home, where she had resided since being diagnosed with an unspecified neurocognitive disorder. For several weeks before her fall, she was physically aggressive, throwing objects at nursing home staff, and was unable to have her behavior redirected.
While in the ED, Ms. N rambles and appears to be responding to internal stimuli. Suddenly, she stops responding and begins to stare.
HISTORY Severe, chronic psychosis and hospitalization
Ms. N is well-known at our inpatient psychiatry and electroconvulsive therapy (ECT) services. During the last 10 years, she has had worsening manic, psychotic, and catatonic (both excited and stuporous subtype) episodes. Three years ago, she had experienced a period of severe, chronic psychosis and excited catatonia that required extended inpatient treatment. While hospitalized, Ms. N had marginal responses to clozapine and benzodiazepines, but improved dramatically with ECT. After Ms. N left the hospital, she went to live with her boyfriend. She remained stable on monthly maintenance ECT treatments (bifrontal) before she was lost to follow-up 14 months prior to the current presentation. Ms. N’s family reports that she needed a cardiac clearance before continuing ECT treatment; however, she was hospitalized at another hospital with pneumonia and subsequent complications that interrupted the maintenance ECT treatments.
Approximately 3 months after medical issues requiring hospitalization began, Ms. N received a diagnosis of neurocognitive disorder due to difficulty with activities of daily living and cognitive decline. She was transferred to a nursing home by the outside hospital. When Ms. N’s symptoms of psychosis returned and she required inpatient psychiatric care, she was transferred to a nearby facility that did not have ECT available or knowledge of her history of catatonia resistant to pharmacologic management. Ms. N had a documented history of catatonia that spanned 10 years. During the last 4 years, Ms. N often required ECT treatment. Her current medication regimen prescribed by an outpatient psychiatrist includes clozapine, 300 mg twice daily, and clonazepam, 0.5 mg twice daily, both for bipolar disorder.
EVALUATION An unusual mix of symptoms
In the ED, Ms. N undergoes a CT of the head, which is found to be nonacute. Laboratory results show that her white blood cell count is 14.3 K/µL, which is mildly elevated. Results from a urinalysis and electrocardiogram (ECG) are unremarkable.
After Ms. N punches a radiology technician, she is administered IV lorazepam, 2 mg once, for her agitation. Twenty minutes after receiving IV lorazepam, she is calm and cooperative. However, approximately 4 hours later, Ms. N is yelling, tearful, and expressing delusions of grandeur—she believes she is God.
After she is admitted to the medical floor, Ms. N is seen by our consultation and liaison psychiatry service. She exhibits several signs of catatonia, including grasp reflex, gegenhalten (oppositional paratonia), waxy flexibility, and echolalia. Ms. N also has an episode of urinary incontinence. At some parts of the day, she is alert and oriented to self and location; at other times, she is somnolent and disoriented. The treatment team continues Ms. N’s previous medication regimen of clozapine, 300 mg twice daily, and clonazepam, 0.5 mg twice daily. Unfortunately, at times Ms. N spits out and hides her administered oral medications, which leads to the decision to discontinue clozapine. Once medically cleared, Ms. N is transferred to the psychiatric floor.
[polldaddy:10869949]
Continue to: TREATMENT
TREATMENT Bifrontal ECT initiated
On hospital Day 3 Ms. N is administered a trial of IM lorazepam, titrated up to 6 mg/d (maximum tolerated dose) while the treatment team initiates the legal process to conduct ECT because she is unable to give consent. Once Ms. N begins tolerating oral medications, amantadine, 100 mg twice daily, is added to treat her catatonia. As in prior hospitalizations, Ms. N is unresponsive to pharmacotherapy alone for her catatonic symptoms. On hospital Day 8, forced ECT is granted, which is 5 days after the process of filing paperwork was started. Bifrontal ECT is utilized with the following settings: frequency 70 Hz, pulse width 1.5 ms, 100% energy dose, 504 mC. Ms. N does not experience a significant improvement until she receives 10 ECT treatments as part of a 3-times-per-week acute series protocol. The Bush-Francis Catatonia Rating Scale (BFCRS) and the KANNER scale are used to monitor her progress. Her initial BFCRS score is 17 and initial KANNER scale, part 2 score is 26.
Ms. N spends a total of 61 days in the hospital, which is significantly longer than her previous hospital admissions on our psychiatric unit; these previous admissions were for treatment of both stuporous and excited subtypes of catatonia. This increased length of stay coincides with a significantly longer duration of untreated catatonia. Knowledge of her history of both the stuporous and excited subtypes of catatonia would have allowed for faster diagnosis and treatment.1
The authors’ observations
Originally conceptualized as a separate syndrome by Karl Kahlbaum, catatonia was considered only as a specifier for neuropsychiatric conditions (primarily schizophrenia) as recently as DSM-IV-TR.2 DSM-5 describes catatonia as a marked psychomotor disturbance and acknowledges its connection to schizophrenia by keeping it in the same chapter.3 DSM-5 includes separate diagnoses for catatonia, catatonia due to a general medical condition, and unspecified catatonia (for catatonia without a known underlying disorder).3 A recent meta-analysis found the prevalence of catatonia is higher in patients with medical/neurologic illness, bipolar disorder, and autism than in those with schizophrenia.4
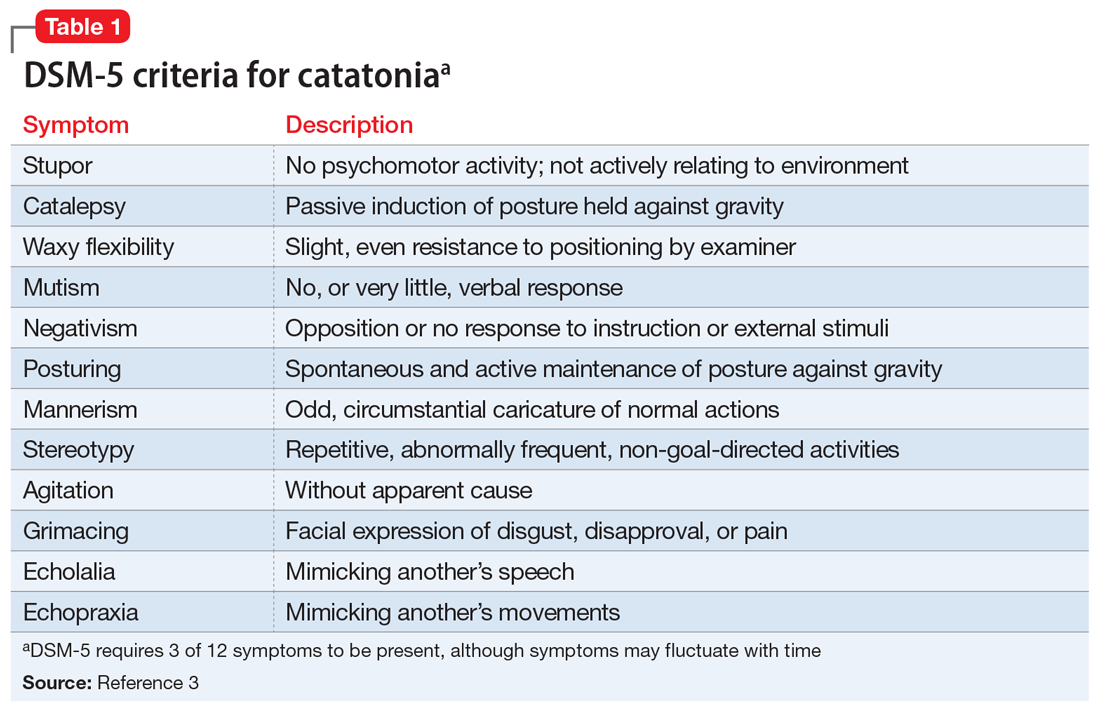
Table 13 highlights the DSM-5 criteria for catatonia. DSM-5 requires 3 of 12 symptoms to be present, although symptoms may fluctuate with time.3 If a clinician is not specifically looking for catatonia, it can be a difficult syndrome to diagnose. Does rigidity indicate catatonia, or excessive dopamine blockade from an antipsychotic? How can seemingly contradictory symptoms be part of the same syndrome? Many clinicians associate catatonia with the stuporous subtype (immobility, posturing, catalepsy), which is more prevalent, but the excited subtype, which may involve severe agitation, autonomic dysfunction, and impaired consciousness, can be lethal.2 The diversity in presentation of catatonia is not unlike the challenging variety of symptoms of heart attacks.
A retrospective study of all adults admitted to a hospital found that only 41% of patients who met criteria for catatonia received this diagnosis.5 Further complicating the diagnosis, delirium and catatonia can co-exist; one study found this was the case in 1 of 3 critically ill patients.6 DSM-5 criteria for catatonia due to another medical condition exclude the diagnosis if delirium is present, but this study and others suggest this needs to be reconsidered.3
Continue to: A standardized evaluation is key
A standardized evaluation is key
Just as a patient who presents with chest pain requires a standardized evaluation, including a pertinent history, laboratory workup, and ECG, psychiatrists may also use standardized diagnostic instruments to aid in the diagnosis of catatonia. One study of hospitalized patients with schizophrenia found that using a standardized diagnostic procedure for catatonia resulted in a 7-fold increase in the diagnosis.7 The BFCRS is the most common standardized instrument for catatonia, likely due to its high inter-rater reliability.8 Other scales include the KANNER scale and Northoff Catatonia Scale, which emphasize different aspects of the disease or for certain clinical populations (eg, the KANNER scale adjusts for patients who are nonverbal at baseline). One study suggested that BFCRS has lower reliability for less-severe illness.9 These differences emphasize that psychiatry does not have a thorough understanding of the intricacies of catatonia. However, using validated screening tools can lead to more consistent diagnoses and continue important research on this often-misunderstood illness.
Dangers of untreated catatonia
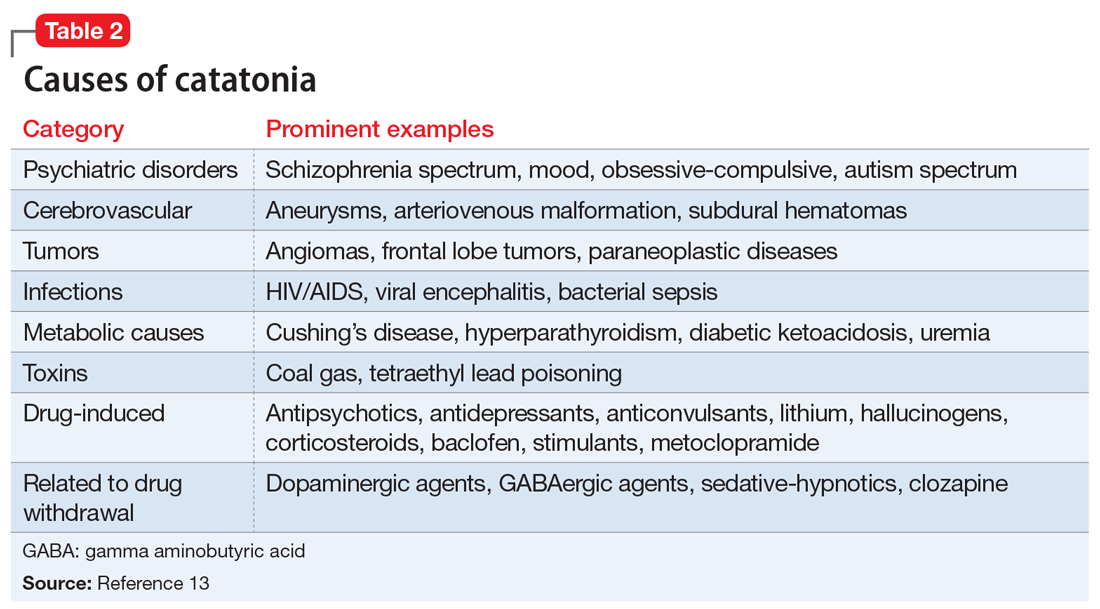
Rapid treatment of catatonia is necessary to prevent mortality. A study of patients in Kentucky’s state psychiatric hospitals found that untreated catatonia with resultant death from pulmonary embolism was the leading cause of preventable death.10 A 17-year retrospective study of patients with schizophrenia admitted to 1 hospital found that those with catatonia were >4 times as likely to die during hospitalization than those without catatonia.11 The significant morbidity and mortality from untreated catatonia are typically attributed to the consequences of poorly controlled movements, immobility, autonomic instability, and poor/no oral intake. Reduced oral intake can result in malnutrition, dehydration, arrhythmias, and increased risk of infections. Furthermore, chronic catatonic episodes are more difficult to treat.12 In addition to the aggressive management of neuropsychiatric symptoms, it is vital to evaluate relevant medical etiologies that may be contributing to the syndrome (Table 213). Tracking vital signs and laboratory values, such as creatine kinase, electrolytes, and complete blood count, is required to ensure the medical condition does not become life-threatening.
Treatment options
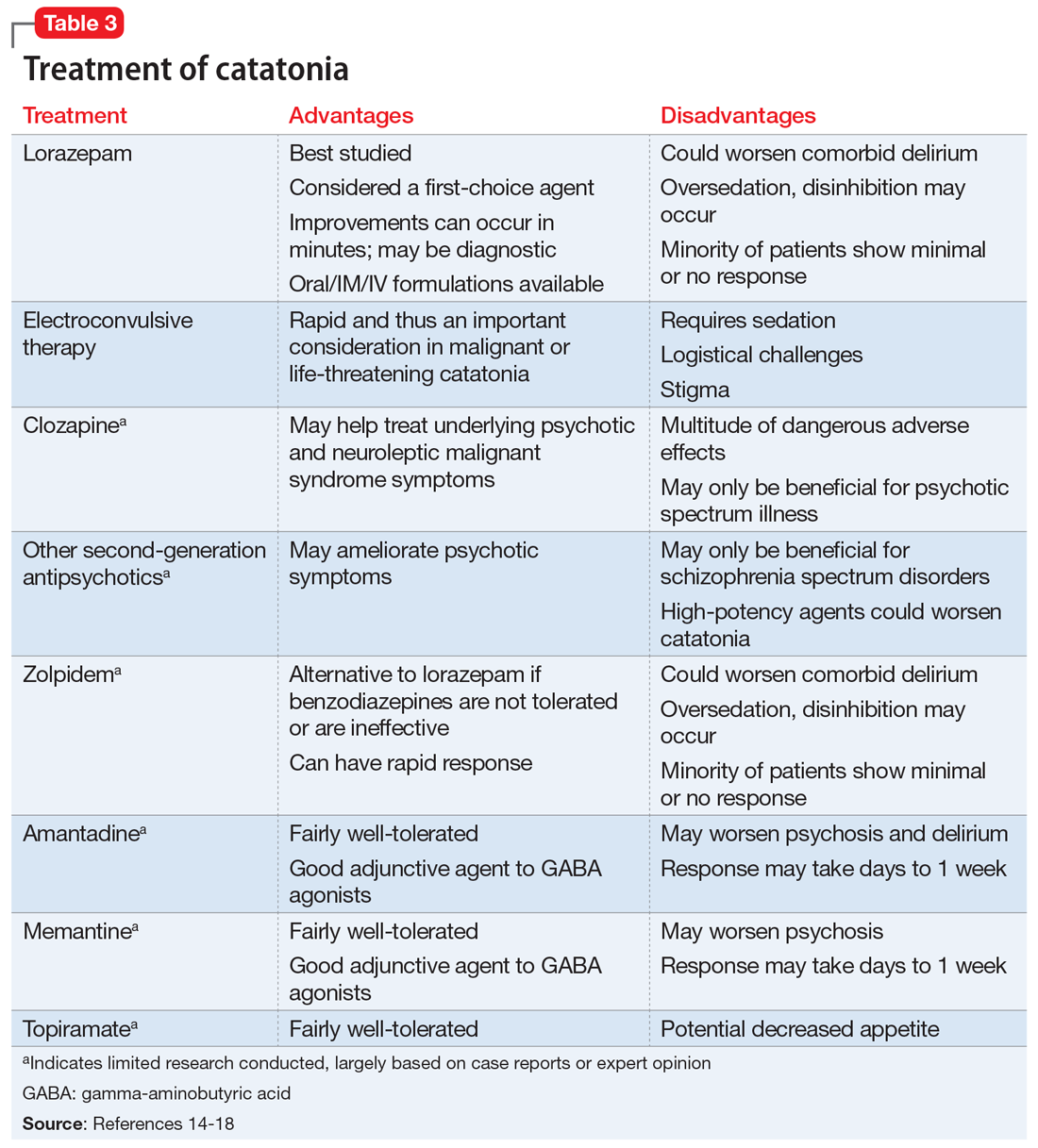
Studies and expert opinion suggest that benzodiazepines (specifically lorazepam, because it is the most studied agent) are the first-line treatment for catatonia. A lorazepam challenge test—providing 1 or 2 mg of IV lorazepam—is considered diagnostic and therapeutic given the high rate of response within 10 minutes.14 Patients with limited response to lorazepam or who are medically compromised should undergo ECT. Electroconvulsive therapy is considered the gold-standard treatment for catatonia; estimated response rates range from 59% to 100%, even in patients who fail to respond to pharmacotherapy.15 Although highly effective, ECT is often hindered by the time required to initiate treatment, stigma, lack of access, and other logistical challenges.
Table 314-18 highlights the advantages and disadvantages of treatment options for catatonia. Some researchers have suggested a zolpidem challenge test could augment lorazepam because some patients respond only to zolpidem.14 The efficacy of these medications along with some evidence of anti-N-methyl-
Ms. N was ultimately diagnosed with bipolar disorder, current episode mixed, with psychotic and catatonic features. Ms. N had symptoms of mania including grandiosity, periods of lack of sleep, delusions as well as depressive symptoms of tearfulness and low mood. The treatment team had considered that Ms. N had delirious mania because she had fluctuating sensorium, which included varying degrees of orientation and ability to answer questioning. However, the literature supporting the differentiation between delirious mania and excited catatonia is unclear, and both conditions may respond to ECT.18 A diagnosis of catatonia allowed the team to use rating scales to track Ms. N’s progress by monitoring for specific signs, such as grasp reflex and waxy flexibility.
Continue to: OUTCOME
OUTCOME Return to baseline
Before discharge, Ms. N’s BFCRS score decreases from the initial score of 17 to 0, and her KANNER scale score decreases from the initial score of 26 to 4, which correlates with vast improvement in clinical presentation. Once Ms. N completes the acute ECT treatment, she returns to her baseline level of functioning, and is discharged to live with her boyfriend. She is advised to continue weekly ECT for the first several months to ensure clinical stability. This regimen is later transitioned to biweekly and then monthly. Electroconvulsive therapy protocols from previous research were utilized in Ms. N’s case, but ultimately the lowest number of ECT treatments needed to maintain stability is determined clinically over many years.19 Ms. N is discharged on aripiprazole, 15 mg/d; bupropion ER, 300 mg/d (added after depressive symptoms emerge while catatonia symptoms improve midway through her lengthy hospitalization); and memantine, 10 mg/d. Ideally, clozapine would have been continued; however, due to her history of nonadherence and frequent restarting of the medication at a low dose, clozapine was discontinued and aripiprazole initiated.
More than 1 year later, Ms. N remains stable and continues to receive monthly ECT maintenance treatments.
Bottom Line
Catatonia should always be considered in a patient who presents with acute neuropsychiatric symptoms. Rapid diagnosis with standardized screening instruments and aggressive treatment are vital to prevent morbidity and mortality.
Related Resource
- Freudenreich O, Francis A, Fricchione GL. Chapter 9. Psychosis, mania, and catatonia. In: Levenson, James L, ed. The American Psychiatric Association Publishing textbook of psychosomatic medicine and consultation-liaison psychiatry. 3rd ed. American Psychiatric Association Publishing; 2019.
Drug Brand Names
Amantadine • Symmetrel
Aripiprazole • Abilify
Baclofen • Ozobax
Bupropion ER • Wellbutrin XL
Clonazepam • Klonopin
Clozapine • Clozaril
Lithium • Eskalith, Lithobid
Lorazepam • Ativan
Metoclopramide • Reglan
Memantine • Namenda
Topiramate • Topamax
Zolpidem • Ambien
1. Carroll BT. The universal field hypothesis of catatonia and neuroleptic malignant syndrome. CNS Spectrums. 2000;5(7):26-33.
2. Rasmussen SA, Mazurek MF, Rosebush PI. Catatonia: our current understanding of its diagnosis, treatment and pathophysiology. World J Psychiatry. 2016;6(4):391‐398.
3. Diagnostic and statistical manual of mental disorders, 5th ed. American Psychiatric Association; 2013. 119-121.
4. Solmi M, Pigato GG, Roiter B, et al. Prevalence of catatonia and its moderators in clinical samples: results from a meta-analysis and meta-regression analysis. Schizophrenia Bulletin. 2017;44(5):1133-1150.
5. Llesuy JR, Medina M, Jacobson KC, et al. Catatonia under-diagnosis in the general hospital. J Neuropsychiatry Clin Neurosci. 2018;30(2):145-151.
6. Wilson JE, Carlson R, Duggan MC, et al. Delirium and catatonia in critically ill patients. Crit Care Med. 2017;45(11):1837-1844.
7. Heijden FVD, Tuinier S, Arts N, et al. Catatonia: disappeared or under-diagnosed? Psychopathology. 2005;38(1):3-8.
8. Sarkar S, Sakey S, Mathan K, et al. Assessing catatonia using four different instruments: inter-rater reliability and prevalence in inpatient clinical population. Asian J Psychiatr. 2016;23:27-31.
9. Wilson JE, Niu K, Nicolson SE, et al. The diagnostic criteria and structure of catatonia. Schizophr Res. 2015;164(1-3):256-262.
10. Puentes R, Brenzel A, Leon JD. Pulmonary embolism during stuporous episodes of catatonia was found to be the most frequent cause of preventable death according to a state mortality review: 6 deaths in 15 years. Clin Schizophr Relat Psychoses. 2017; doi:10.3371/csrp.rpab.071317
11. Funayama M, Takata T, Koreki A, et al. Catatonic stupor in schizophrenic disorders and subsequent medical complications and mortality. Psychosomatic Medicine. 2018:80(4):370-376.
12. Perugi G, Medda P, Toni C, et al. The role of electroconvulsive therapy (ECT) in bipolar disorder: effectiveness in 522 patients with bipolar depression, mixed-state, mania and catatonic features. Curr Neuropharmacol. 2017;15(3):359-371.
13. Freudenreich O, Francis A, Fricchione GL. Chapter 9. Psychosis, mania, and catatonia. In: Levenson, James L, ed. The American Psychiatric Association Publishing Textbook of Psychosomatic medicine and Consultation-Liaison Psychiatry. 3rd ed. American Psychiatric Association Publishing; 2019.
14. Sienaert P, Dhossche DM, Vancampfort D, et al. A clinical review of the treatment of catatonia. Front Psychiatry. 2014;5:181.
15. Pelzer A, Heijden FVD, Boer ED. Systematic review of catatonia treatment. Neuropsychiatr Dis Treat. 2018;14:317-326.
16. Carroll BT, Goforth HW, Thomas C, et al. Review of adjunctive glutamate antagonist therapy in the treatment of catatonic syndromes. J Neuropsychiatry and Clin Neurosci. 2007;19(4):406-412.
17. Fink M. Rediscovering catatonia: the biography of a treatable syndrome. Acta Psychiatr Scand Suppl. 2013;(441):1-47.
18. Fink M, Taylor MA. Catatonia: a clinician’s guide to diagnosis and treatment. Cambridge University Press; 2006.
19. Petrides G, Tobias KG, Kellner CH, et al. Continuation and maintenance electroconvulsive therapy for mood disorders: review of the literature. Neuropsychobiology. 2011;64(3):129-140.
CASE Aggressive behaviors, psychosis
Ms. N, age 58, has a long history of bipolar disorder with psychotic features. She presents to our emergency department (ED) after an acute fall and frequent violent behaviors at her nursing home, where she had resided since being diagnosed with an unspecified neurocognitive disorder. For several weeks before her fall, she was physically aggressive, throwing objects at nursing home staff, and was unable to have her behavior redirected.
While in the ED, Ms. N rambles and appears to be responding to internal stimuli. Suddenly, she stops responding and begins to stare.
HISTORY Severe, chronic psychosis and hospitalization
Ms. N is well-known at our inpatient psychiatry and electroconvulsive therapy (ECT) services. During the last 10 years, she has had worsening manic, psychotic, and catatonic (both excited and stuporous subtype) episodes. Three years ago, she had experienced a period of severe, chronic psychosis and excited catatonia that required extended inpatient treatment. While hospitalized, Ms. N had marginal responses to clozapine and benzodiazepines, but improved dramatically with ECT. After Ms. N left the hospital, she went to live with her boyfriend. She remained stable on monthly maintenance ECT treatments (bifrontal) before she was lost to follow-up 14 months prior to the current presentation. Ms. N’s family reports that she needed a cardiac clearance before continuing ECT treatment; however, she was hospitalized at another hospital with pneumonia and subsequent complications that interrupted the maintenance ECT treatments.
Approximately 3 months after medical issues requiring hospitalization began, Ms. N received a diagnosis of neurocognitive disorder due to difficulty with activities of daily living and cognitive decline. She was transferred to a nursing home by the outside hospital. When Ms. N’s symptoms of psychosis returned and she required inpatient psychiatric care, she was transferred to a nearby facility that did not have ECT available or knowledge of her history of catatonia resistant to pharmacologic management. Ms. N had a documented history of catatonia that spanned 10 years. During the last 4 years, Ms. N often required ECT treatment. Her current medication regimen prescribed by an outpatient psychiatrist includes clozapine, 300 mg twice daily, and clonazepam, 0.5 mg twice daily, both for bipolar disorder.
EVALUATION An unusual mix of symptoms
In the ED, Ms. N undergoes a CT of the head, which is found to be nonacute. Laboratory results show that her white blood cell count is 14.3 K/µL, which is mildly elevated. Results from a urinalysis and electrocardiogram (ECG) are unremarkable.
After Ms. N punches a radiology technician, she is administered IV lorazepam, 2 mg once, for her agitation. Twenty minutes after receiving IV lorazepam, she is calm and cooperative. However, approximately 4 hours later, Ms. N is yelling, tearful, and expressing delusions of grandeur—she believes she is God.
After she is admitted to the medical floor, Ms. N is seen by our consultation and liaison psychiatry service. She exhibits several signs of catatonia, including grasp reflex, gegenhalten (oppositional paratonia), waxy flexibility, and echolalia. Ms. N also has an episode of urinary incontinence. At some parts of the day, she is alert and oriented to self and location; at other times, she is somnolent and disoriented. The treatment team continues Ms. N’s previous medication regimen of clozapine, 300 mg twice daily, and clonazepam, 0.5 mg twice daily. Unfortunately, at times Ms. N spits out and hides her administered oral medications, which leads to the decision to discontinue clozapine. Once medically cleared, Ms. N is transferred to the psychiatric floor.
[polldaddy:10869949]
Continue to: TREATMENT
TREATMENT Bifrontal ECT initiated
On hospital Day 3 Ms. N is administered a trial of IM lorazepam, titrated up to 6 mg/d (maximum tolerated dose) while the treatment team initiates the legal process to conduct ECT because she is unable to give consent. Once Ms. N begins tolerating oral medications, amantadine, 100 mg twice daily, is added to treat her catatonia. As in prior hospitalizations, Ms. N is unresponsive to pharmacotherapy alone for her catatonic symptoms. On hospital Day 8, forced ECT is granted, which is 5 days after the process of filing paperwork was started. Bifrontal ECT is utilized with the following settings: frequency 70 Hz, pulse width 1.5 ms, 100% energy dose, 504 mC. Ms. N does not experience a significant improvement until she receives 10 ECT treatments as part of a 3-times-per-week acute series protocol. The Bush-Francis Catatonia Rating Scale (BFCRS) and the KANNER scale are used to monitor her progress. Her initial BFCRS score is 17 and initial KANNER scale, part 2 score is 26.
Ms. N spends a total of 61 days in the hospital, which is significantly longer than her previous hospital admissions on our psychiatric unit; these previous admissions were for treatment of both stuporous and excited subtypes of catatonia. This increased length of stay coincides with a significantly longer duration of untreated catatonia. Knowledge of her history of both the stuporous and excited subtypes of catatonia would have allowed for faster diagnosis and treatment.1
The authors’ observations
Originally conceptualized as a separate syndrome by Karl Kahlbaum, catatonia was considered only as a specifier for neuropsychiatric conditions (primarily schizophrenia) as recently as DSM-IV-TR.2 DSM-5 describes catatonia as a marked psychomotor disturbance and acknowledges its connection to schizophrenia by keeping it in the same chapter.3 DSM-5 includes separate diagnoses for catatonia, catatonia due to a general medical condition, and unspecified catatonia (for catatonia without a known underlying disorder).3 A recent meta-analysis found the prevalence of catatonia is higher in patients with medical/neurologic illness, bipolar disorder, and autism than in those with schizophrenia.4
Table 13 highlights the DSM-5 criteria for catatonia. DSM-5 requires 3 of 12 symptoms to be present, although symptoms may fluctuate with time.3 If a clinician is not specifically looking for catatonia, it can be a difficult syndrome to diagnose. Does rigidity indicate catatonia, or excessive dopamine blockade from an antipsychotic? How can seemingly contradictory symptoms be part of the same syndrome? Many clinicians associate catatonia with the stuporous subtype (immobility, posturing, catalepsy), which is more prevalent, but the excited subtype, which may involve severe agitation, autonomic dysfunction, and impaired consciousness, can be lethal.2 The diversity in presentation of catatonia is not unlike the challenging variety of symptoms of heart attacks.

A retrospective study of all adults admitted to a hospital found that only 41% of patients who met criteria for catatonia received this diagnosis.5 Further complicating the diagnosis, delirium and catatonia can co-exist; one study found this was the case in 1 of 3 critically ill patients.6 DSM-5 criteria for catatonia due to another medical condition exclude the diagnosis if delirium is present, but this study and others suggest this needs to be reconsidered.3
Continue to: A standardized evaluation is key
A standardized evaluation is key
Just as a patient who presents with chest pain requires a standardized evaluation, including a pertinent history, laboratory workup, and ECG, psychiatrists may also use standardized diagnostic instruments to aid in the diagnosis of catatonia. One study of hospitalized patients with schizophrenia found that using a standardized diagnostic procedure for catatonia resulted in a 7-fold increase in the diagnosis.7 The BFCRS is the most common standardized instrument for catatonia, likely due to its high inter-rater reliability.8 Other scales include the KANNER scale and Northoff Catatonia Scale, which emphasize different aspects of the disease or for certain clinical populations (eg, the KANNER scale adjusts for patients who are nonverbal at baseline). One study suggested that BFCRS has lower reliability for less-severe illness.9 These differences emphasize that psychiatry does not have a thorough understanding of the intricacies of catatonia. However, using validated screening tools can lead to more consistent diagnoses and continue important research on this often-misunderstood illness.
Dangers of untreated catatonia
Rapid treatment of catatonia is necessary to prevent mortality. A study of patients in Kentucky’s state psychiatric hospitals found that untreated catatonia with resultant death from pulmonary embolism was the leading cause of preventable death.10 A 17-year retrospective study of patients with schizophrenia admitted to 1 hospital found that those with catatonia were >4 times as likely to die during hospitalization than those without catatonia.11 The significant morbidity and mortality from untreated catatonia are typically attributed to the consequences of poorly controlled movements, immobility, autonomic instability, and poor/no oral intake. Reduced oral intake can result in malnutrition, dehydration, arrhythmias, and increased risk of infections. Furthermore, chronic catatonic episodes are more difficult to treat.12 In addition to the aggressive management of neuropsychiatric symptoms, it is vital to evaluate relevant medical etiologies that may be contributing to the syndrome (Table 213). Tracking vital signs and laboratory values, such as creatine kinase, electrolytes, and complete blood count, is required to ensure the medical condition does not become life-threatening.

Treatment options
Studies and expert opinion suggest that benzodiazepines (specifically lorazepam, because it is the most studied agent) are the first-line treatment for catatonia. A lorazepam challenge test—providing 1 or 2 mg of IV lorazepam—is considered diagnostic and therapeutic given the high rate of response within 10 minutes.14 Patients with limited response to lorazepam or who are medically compromised should undergo ECT. Electroconvulsive therapy is considered the gold-standard treatment for catatonia; estimated response rates range from 59% to 100%, even in patients who fail to respond to pharmacotherapy.15 Although highly effective, ECT is often hindered by the time required to initiate treatment, stigma, lack of access, and other logistical challenges.
Table 314-18 highlights the advantages and disadvantages of treatment options for catatonia. Some researchers have suggested a zolpidem challenge test could augment lorazepam because some patients respond only to zolpidem.14 The efficacy of these medications along with some evidence of anti-N-methyl-

Ms. N was ultimately diagnosed with bipolar disorder, current episode mixed, with psychotic and catatonic features. Ms. N had symptoms of mania including grandiosity, periods of lack of sleep, delusions as well as depressive symptoms of tearfulness and low mood. The treatment team had considered that Ms. N had delirious mania because she had fluctuating sensorium, which included varying degrees of orientation and ability to answer questioning. However, the literature supporting the differentiation between delirious mania and excited catatonia is unclear, and both conditions may respond to ECT.18 A diagnosis of catatonia allowed the team to use rating scales to track Ms. N’s progress by monitoring for specific signs, such as grasp reflex and waxy flexibility.
Continue to: OUTCOME
OUTCOME Return to baseline
Before discharge, Ms. N’s BFCRS score decreases from the initial score of 17 to 0, and her KANNER scale score decreases from the initial score of 26 to 4, which correlates with vast improvement in clinical presentation. Once Ms. N completes the acute ECT treatment, she returns to her baseline level of functioning, and is discharged to live with her boyfriend. She is advised to continue weekly ECT for the first several months to ensure clinical stability. This regimen is later transitioned to biweekly and then monthly. Electroconvulsive therapy protocols from previous research were utilized in Ms. N’s case, but ultimately the lowest number of ECT treatments needed to maintain stability is determined clinically over many years.19 Ms. N is discharged on aripiprazole, 15 mg/d; bupropion ER, 300 mg/d (added after depressive symptoms emerge while catatonia symptoms improve midway through her lengthy hospitalization); and memantine, 10 mg/d. Ideally, clozapine would have been continued; however, due to her history of nonadherence and frequent restarting of the medication at a low dose, clozapine was discontinued and aripiprazole initiated.
More than 1 year later, Ms. N remains stable and continues to receive monthly ECT maintenance treatments.
Bottom Line
Catatonia should always be considered in a patient who presents with acute neuropsychiatric symptoms. Rapid diagnosis with standardized screening instruments and aggressive treatment are vital to prevent morbidity and mortality.
Related Resource
- Freudenreich O, Francis A, Fricchione GL. Chapter 9. Psychosis, mania, and catatonia. In: Levenson, James L, ed. The American Psychiatric Association Publishing textbook of psychosomatic medicine and consultation-liaison psychiatry. 3rd ed. American Psychiatric Association Publishing; 2019.
Drug Brand Names
Amantadine • Symmetrel
Aripiprazole • Abilify
Baclofen • Ozobax
Bupropion ER • Wellbutrin XL
Clonazepam • Klonopin
Clozapine • Clozaril
Lithium • Eskalith, Lithobid
Lorazepam • Ativan
Metoclopramide • Reglan
Memantine • Namenda
Topiramate • Topamax
Zolpidem • Ambien
CASE Aggressive behaviors, psychosis
Ms. N, age 58, has a long history of bipolar disorder with psychotic features. She presents to our emergency department (ED) after an acute fall and frequent violent behaviors at her nursing home, where she had resided since being diagnosed with an unspecified neurocognitive disorder. For several weeks before her fall, she was physically aggressive, throwing objects at nursing home staff, and was unable to have her behavior redirected.
While in the ED, Ms. N rambles and appears to be responding to internal stimuli. Suddenly, she stops responding and begins to stare.
HISTORY Severe, chronic psychosis and hospitalization
Ms. N is well-known at our inpatient psychiatry and electroconvulsive therapy (ECT) services. During the last 10 years, she has had worsening manic, psychotic, and catatonic (both excited and stuporous subtype) episodes. Three years ago, she had experienced a period of severe, chronic psychosis and excited catatonia that required extended inpatient treatment. While hospitalized, Ms. N had marginal responses to clozapine and benzodiazepines, but improved dramatically with ECT. After Ms. N left the hospital, she went to live with her boyfriend. She remained stable on monthly maintenance ECT treatments (bifrontal) before she was lost to follow-up 14 months prior to the current presentation. Ms. N’s family reports that she needed a cardiac clearance before continuing ECT treatment; however, she was hospitalized at another hospital with pneumonia and subsequent complications that interrupted the maintenance ECT treatments.
Approximately 3 months after medical issues requiring hospitalization began, Ms. N received a diagnosis of neurocognitive disorder due to difficulty with activities of daily living and cognitive decline. She was transferred to a nursing home by the outside hospital. When Ms. N’s symptoms of psychosis returned and she required inpatient psychiatric care, she was transferred to a nearby facility that did not have ECT available or knowledge of her history of catatonia resistant to pharmacologic management. Ms. N had a documented history of catatonia that spanned 10 years. During the last 4 years, Ms. N often required ECT treatment. Her current medication regimen prescribed by an outpatient psychiatrist includes clozapine, 300 mg twice daily, and clonazepam, 0.5 mg twice daily, both for bipolar disorder.
EVALUATION An unusual mix of symptoms
In the ED, Ms. N undergoes a CT of the head, which is found to be nonacute. Laboratory results show that her white blood cell count is 14.3 K/µL, which is mildly elevated. Results from a urinalysis and electrocardiogram (ECG) are unremarkable.
After Ms. N punches a radiology technician, she is administered IV lorazepam, 2 mg once, for her agitation. Twenty minutes after receiving IV lorazepam, she is calm and cooperative. However, approximately 4 hours later, Ms. N is yelling, tearful, and expressing delusions of grandeur—she believes she is God.
After she is admitted to the medical floor, Ms. N is seen by our consultation and liaison psychiatry service. She exhibits several signs of catatonia, including grasp reflex, gegenhalten (oppositional paratonia), waxy flexibility, and echolalia. Ms. N also has an episode of urinary incontinence. At some parts of the day, she is alert and oriented to self and location; at other times, she is somnolent and disoriented. The treatment team continues Ms. N’s previous medication regimen of clozapine, 300 mg twice daily, and clonazepam, 0.5 mg twice daily. Unfortunately, at times Ms. N spits out and hides her administered oral medications, which leads to the decision to discontinue clozapine. Once medically cleared, Ms. N is transferred to the psychiatric floor.
[polldaddy:10869949]
Continue to: TREATMENT
TREATMENT Bifrontal ECT initiated
On hospital Day 3 Ms. N is administered a trial of IM lorazepam, titrated up to 6 mg/d (maximum tolerated dose) while the treatment team initiates the legal process to conduct ECT because she is unable to give consent. Once Ms. N begins tolerating oral medications, amantadine, 100 mg twice daily, is added to treat her catatonia. As in prior hospitalizations, Ms. N is unresponsive to pharmacotherapy alone for her catatonic symptoms. On hospital Day 8, forced ECT is granted, which is 5 days after the process of filing paperwork was started. Bifrontal ECT is utilized with the following settings: frequency 70 Hz, pulse width 1.5 ms, 100% energy dose, 504 mC. Ms. N does not experience a significant improvement until she receives 10 ECT treatments as part of a 3-times-per-week acute series protocol. The Bush-Francis Catatonia Rating Scale (BFCRS) and the KANNER scale are used to monitor her progress. Her initial BFCRS score is 17 and initial KANNER scale, part 2 score is 26.
Ms. N spends a total of 61 days in the hospital, which is significantly longer than her previous hospital admissions on our psychiatric unit; these previous admissions were for treatment of both stuporous and excited subtypes of catatonia. This increased length of stay coincides with a significantly longer duration of untreated catatonia. Knowledge of her history of both the stuporous and excited subtypes of catatonia would have allowed for faster diagnosis and treatment.1
The authors’ observations
Originally conceptualized as a separate syndrome by Karl Kahlbaum, catatonia was considered only as a specifier for neuropsychiatric conditions (primarily schizophrenia) as recently as DSM-IV-TR.2 DSM-5 describes catatonia as a marked psychomotor disturbance and acknowledges its connection to schizophrenia by keeping it in the same chapter.3 DSM-5 includes separate diagnoses for catatonia, catatonia due to a general medical condition, and unspecified catatonia (for catatonia without a known underlying disorder).3 A recent meta-analysis found the prevalence of catatonia is higher in patients with medical/neurologic illness, bipolar disorder, and autism than in those with schizophrenia.4
Table 13 highlights the DSM-5 criteria for catatonia. DSM-5 requires 3 of 12 symptoms to be present, although symptoms may fluctuate with time.3 If a clinician is not specifically looking for catatonia, it can be a difficult syndrome to diagnose. Does rigidity indicate catatonia, or excessive dopamine blockade from an antipsychotic? How can seemingly contradictory symptoms be part of the same syndrome? Many clinicians associate catatonia with the stuporous subtype (immobility, posturing, catalepsy), which is more prevalent, but the excited subtype, which may involve severe agitation, autonomic dysfunction, and impaired consciousness, can be lethal.2 The diversity in presentation of catatonia is not unlike the challenging variety of symptoms of heart attacks.

A retrospective study of all adults admitted to a hospital found that only 41% of patients who met criteria for catatonia received this diagnosis.5 Further complicating the diagnosis, delirium and catatonia can co-exist; one study found this was the case in 1 of 3 critically ill patients.6 DSM-5 criteria for catatonia due to another medical condition exclude the diagnosis if delirium is present, but this study and others suggest this needs to be reconsidered.3
Continue to: A standardized evaluation is key
A standardized evaluation is key
Just as a patient who presents with chest pain requires a standardized evaluation, including a pertinent history, laboratory workup, and ECG, psychiatrists may also use standardized diagnostic instruments to aid in the diagnosis of catatonia. One study of hospitalized patients with schizophrenia found that using a standardized diagnostic procedure for catatonia resulted in a 7-fold increase in the diagnosis.7 The BFCRS is the most common standardized instrument for catatonia, likely due to its high inter-rater reliability.8 Other scales include the KANNER scale and Northoff Catatonia Scale, which emphasize different aspects of the disease or for certain clinical populations (eg, the KANNER scale adjusts for patients who are nonverbal at baseline). One study suggested that BFCRS has lower reliability for less-severe illness.9 These differences emphasize that psychiatry does not have a thorough understanding of the intricacies of catatonia. However, using validated screening tools can lead to more consistent diagnoses and continue important research on this often-misunderstood illness.
Dangers of untreated catatonia
Rapid treatment of catatonia is necessary to prevent mortality. A study of patients in Kentucky’s state psychiatric hospitals found that untreated catatonia with resultant death from pulmonary embolism was the leading cause of preventable death.10 A 17-year retrospective study of patients with schizophrenia admitted to 1 hospital found that those with catatonia were >4 times as likely to die during hospitalization than those without catatonia.11 The significant morbidity and mortality from untreated catatonia are typically attributed to the consequences of poorly controlled movements, immobility, autonomic instability, and poor/no oral intake. Reduced oral intake can result in malnutrition, dehydration, arrhythmias, and increased risk of infections. Furthermore, chronic catatonic episodes are more difficult to treat.12 In addition to the aggressive management of neuropsychiatric symptoms, it is vital to evaluate relevant medical etiologies that may be contributing to the syndrome (Table 213). Tracking vital signs and laboratory values, such as creatine kinase, electrolytes, and complete blood count, is required to ensure the medical condition does not become life-threatening.

Treatment options
Studies and expert opinion suggest that benzodiazepines (specifically lorazepam, because it is the most studied agent) are the first-line treatment for catatonia. A lorazepam challenge test—providing 1 or 2 mg of IV lorazepam—is considered diagnostic and therapeutic given the high rate of response within 10 minutes.14 Patients with limited response to lorazepam or who are medically compromised should undergo ECT. Electroconvulsive therapy is considered the gold-standard treatment for catatonia; estimated response rates range from 59% to 100%, even in patients who fail to respond to pharmacotherapy.15 Although highly effective, ECT is often hindered by the time required to initiate treatment, stigma, lack of access, and other logistical challenges.
Table 314-18 highlights the advantages and disadvantages of treatment options for catatonia. Some researchers have suggested a zolpidem challenge test could augment lorazepam because some patients respond only to zolpidem.14 The efficacy of these medications along with some evidence of anti-N-methyl-

Ms. N was ultimately diagnosed with bipolar disorder, current episode mixed, with psychotic and catatonic features. Ms. N had symptoms of mania including grandiosity, periods of lack of sleep, delusions as well as depressive symptoms of tearfulness and low mood. The treatment team had considered that Ms. N had delirious mania because she had fluctuating sensorium, which included varying degrees of orientation and ability to answer questioning. However, the literature supporting the differentiation between delirious mania and excited catatonia is unclear, and both conditions may respond to ECT.18 A diagnosis of catatonia allowed the team to use rating scales to track Ms. N’s progress by monitoring for specific signs, such as grasp reflex and waxy flexibility.
Continue to: OUTCOME
OUTCOME Return to baseline
Before discharge, Ms. N’s BFCRS score decreases from the initial score of 17 to 0, and her KANNER scale score decreases from the initial score of 26 to 4, which correlates with vast improvement in clinical presentation. Once Ms. N completes the acute ECT treatment, she returns to her baseline level of functioning, and is discharged to live with her boyfriend. She is advised to continue weekly ECT for the first several months to ensure clinical stability. This regimen is later transitioned to biweekly and then monthly. Electroconvulsive therapy protocols from previous research were utilized in Ms. N’s case, but ultimately the lowest number of ECT treatments needed to maintain stability is determined clinically over many years.19 Ms. N is discharged on aripiprazole, 15 mg/d; bupropion ER, 300 mg/d (added after depressive symptoms emerge while catatonia symptoms improve midway through her lengthy hospitalization); and memantine, 10 mg/d. Ideally, clozapine would have been continued; however, due to her history of nonadherence and frequent restarting of the medication at a low dose, clozapine was discontinued and aripiprazole initiated.
More than 1 year later, Ms. N remains stable and continues to receive monthly ECT maintenance treatments.
Bottom Line
Catatonia should always be considered in a patient who presents with acute neuropsychiatric symptoms. Rapid diagnosis with standardized screening instruments and aggressive treatment are vital to prevent morbidity and mortality.
Related Resource
- Freudenreich O, Francis A, Fricchione GL. Chapter 9. Psychosis, mania, and catatonia. In: Levenson, James L, ed. The American Psychiatric Association Publishing textbook of psychosomatic medicine and consultation-liaison psychiatry. 3rd ed. American Psychiatric Association Publishing; 2019.
Drug Brand Names
Amantadine • Symmetrel
Aripiprazole • Abilify
Baclofen • Ozobax
Bupropion ER • Wellbutrin XL
Clonazepam • Klonopin
Clozapine • Clozaril
Lithium • Eskalith, Lithobid
Lorazepam • Ativan
Metoclopramide • Reglan
Memantine • Namenda
Topiramate • Topamax
Zolpidem • Ambien
1. Carroll BT. The universal field hypothesis of catatonia and neuroleptic malignant syndrome. CNS Spectrums. 2000;5(7):26-33.
2. Rasmussen SA, Mazurek MF, Rosebush PI. Catatonia: our current understanding of its diagnosis, treatment and pathophysiology. World J Psychiatry. 2016;6(4):391‐398.
3. Diagnostic and statistical manual of mental disorders, 5th ed. American Psychiatric Association; 2013. 119-121.
4. Solmi M, Pigato GG, Roiter B, et al. Prevalence of catatonia and its moderators in clinical samples: results from a meta-analysis and meta-regression analysis. Schizophrenia Bulletin. 2017;44(5):1133-1150.
5. Llesuy JR, Medina M, Jacobson KC, et al. Catatonia under-diagnosis in the general hospital. J Neuropsychiatry Clin Neurosci. 2018;30(2):145-151.
6. Wilson JE, Carlson R, Duggan MC, et al. Delirium and catatonia in critically ill patients. Crit Care Med. 2017;45(11):1837-1844.
7. Heijden FVD, Tuinier S, Arts N, et al. Catatonia: disappeared or under-diagnosed? Psychopathology. 2005;38(1):3-8.
8. Sarkar S, Sakey S, Mathan K, et al. Assessing catatonia using four different instruments: inter-rater reliability and prevalence in inpatient clinical population. Asian J Psychiatr. 2016;23:27-31.
9. Wilson JE, Niu K, Nicolson SE, et al. The diagnostic criteria and structure of catatonia. Schizophr Res. 2015;164(1-3):256-262.
10. Puentes R, Brenzel A, Leon JD. Pulmonary embolism during stuporous episodes of catatonia was found to be the most frequent cause of preventable death according to a state mortality review: 6 deaths in 15 years. Clin Schizophr Relat Psychoses. 2017; doi:10.3371/csrp.rpab.071317
11. Funayama M, Takata T, Koreki A, et al. Catatonic stupor in schizophrenic disorders and subsequent medical complications and mortality. Psychosomatic Medicine. 2018:80(4):370-376.
12. Perugi G, Medda P, Toni C, et al. The role of electroconvulsive therapy (ECT) in bipolar disorder: effectiveness in 522 patients with bipolar depression, mixed-state, mania and catatonic features. Curr Neuropharmacol. 2017;15(3):359-371.
13. Freudenreich O, Francis A, Fricchione GL. Chapter 9. Psychosis, mania, and catatonia. In: Levenson, James L, ed. The American Psychiatric Association Publishing Textbook of Psychosomatic medicine and Consultation-Liaison Psychiatry. 3rd ed. American Psychiatric Association Publishing; 2019.
14. Sienaert P, Dhossche DM, Vancampfort D, et al. A clinical review of the treatment of catatonia. Front Psychiatry. 2014;5:181.
15. Pelzer A, Heijden FVD, Boer ED. Systematic review of catatonia treatment. Neuropsychiatr Dis Treat. 2018;14:317-326.
16. Carroll BT, Goforth HW, Thomas C, et al. Review of adjunctive glutamate antagonist therapy in the treatment of catatonic syndromes. J Neuropsychiatry and Clin Neurosci. 2007;19(4):406-412.
17. Fink M. Rediscovering catatonia: the biography of a treatable syndrome. Acta Psychiatr Scand Suppl. 2013;(441):1-47.
18. Fink M, Taylor MA. Catatonia: a clinician’s guide to diagnosis and treatment. Cambridge University Press; 2006.
19. Petrides G, Tobias KG, Kellner CH, et al. Continuation and maintenance electroconvulsive therapy for mood disorders: review of the literature. Neuropsychobiology. 2011;64(3):129-140.
1. Carroll BT. The universal field hypothesis of catatonia and neuroleptic malignant syndrome. CNS Spectrums. 2000;5(7):26-33.
2. Rasmussen SA, Mazurek MF, Rosebush PI. Catatonia: our current understanding of its diagnosis, treatment and pathophysiology. World J Psychiatry. 2016;6(4):391‐398.
3. Diagnostic and statistical manual of mental disorders, 5th ed. American Psychiatric Association; 2013. 119-121.
4. Solmi M, Pigato GG, Roiter B, et al. Prevalence of catatonia and its moderators in clinical samples: results from a meta-analysis and meta-regression analysis. Schizophrenia Bulletin. 2017;44(5):1133-1150.
5. Llesuy JR, Medina M, Jacobson KC, et al. Catatonia under-diagnosis in the general hospital. J Neuropsychiatry Clin Neurosci. 2018;30(2):145-151.
6. Wilson JE, Carlson R, Duggan MC, et al. Delirium and catatonia in critically ill patients. Crit Care Med. 2017;45(11):1837-1844.
7. Heijden FVD, Tuinier S, Arts N, et al. Catatonia: disappeared or under-diagnosed? Psychopathology. 2005;38(1):3-8.
8. Sarkar S, Sakey S, Mathan K, et al. Assessing catatonia using four different instruments: inter-rater reliability and prevalence in inpatient clinical population. Asian J Psychiatr. 2016;23:27-31.
9. Wilson JE, Niu K, Nicolson SE, et al. The diagnostic criteria and structure of catatonia. Schizophr Res. 2015;164(1-3):256-262.
10. Puentes R, Brenzel A, Leon JD. Pulmonary embolism during stuporous episodes of catatonia was found to be the most frequent cause of preventable death according to a state mortality review: 6 deaths in 15 years. Clin Schizophr Relat Psychoses. 2017; doi:10.3371/csrp.rpab.071317
11. Funayama M, Takata T, Koreki A, et al. Catatonic stupor in schizophrenic disorders and subsequent medical complications and mortality. Psychosomatic Medicine. 2018:80(4):370-376.
12. Perugi G, Medda P, Toni C, et al. The role of electroconvulsive therapy (ECT) in bipolar disorder: effectiveness in 522 patients with bipolar depression, mixed-state, mania and catatonic features. Curr Neuropharmacol. 2017;15(3):359-371.
13. Freudenreich O, Francis A, Fricchione GL. Chapter 9. Psychosis, mania, and catatonia. In: Levenson, James L, ed. The American Psychiatric Association Publishing Textbook of Psychosomatic medicine and Consultation-Liaison Psychiatry. 3rd ed. American Psychiatric Association Publishing; 2019.
14. Sienaert P, Dhossche DM, Vancampfort D, et al. A clinical review of the treatment of catatonia. Front Psychiatry. 2014;5:181.
15. Pelzer A, Heijden FVD, Boer ED. Systematic review of catatonia treatment. Neuropsychiatr Dis Treat. 2018;14:317-326.
16. Carroll BT, Goforth HW, Thomas C, et al. Review of adjunctive glutamate antagonist therapy in the treatment of catatonic syndromes. J Neuropsychiatry and Clin Neurosci. 2007;19(4):406-412.
17. Fink M. Rediscovering catatonia: the biography of a treatable syndrome. Acta Psychiatr Scand Suppl. 2013;(441):1-47.
18. Fink M, Taylor MA. Catatonia: a clinician’s guide to diagnosis and treatment. Cambridge University Press; 2006.
19. Petrides G, Tobias KG, Kellner CH, et al. Continuation and maintenance electroconvulsive therapy for mood disorders: review of the literature. Neuropsychobiology. 2011;64(3):129-140.
Nothing up his sleeve: Decompensation after bariatric surgery
CASE Sudden-onset low mood
Mr. G, age 64, is obese (body mass index [BMI] 37 kg/m2) and has a history of schizoaffective disorder. He is recovering from a sleeve gastrectomy, a surgical weight-loss procedure in which a large portion of the stomach is removed. Seven weeks after his surgery, he experiences a sudden onset of “low mood” and fears that he will become suicidal; he has a history of suicide attempts. Mr. G calls his long-term outpatient clinic and is advised to go to the emergency department (ED).
For years, Mr. G had been stable in a group home setting, and had always been adherent to treatment and forthcoming about his medications with both his bariatric surgeon and psychiatrist. Within the last month, he had been seen at the clinic, had no psychiatric symptoms, and was recovering well from the sleeve gastrectomy.
HISTORY A stable regimen
Mr. G’s psychiatric symptoms initially developed when he was in his 20s, during a time in which he reported using “a lot of drugs.” He had multiple suicide attempts, and multiple inpatient and outpatient treatments. He was diagnosed with schizoaffective disorder.
Mr. G has been stable on medications for the last 2 years. His outpatient psychotropic regimen is divalproex sodium extended-release (ER), 2,500 mg every night at bedtime; iloperidone, 8 mg twice a day; escitalopram, 10 mg/d; and mirtazapine, 30 mg every night at bedtime.
In the group home, Mr. G spends his days socializing, studying philosophy, and writing essays. He hopes to find a job in the craftsman industry.
Mr. G’s medical history includes obesity (BMI: 37 kg/m2). Since the surgery, he has been receiving omeprazole, 40 mg/d, a proton pump inhibitor (PPI), to decrease the amount of acid in his stomach. Three weeks after surgery, he had an unremarkable postoperative outpatient psychiatry visit. Divalproex sodium ER was maintained at the pre-surgical dose of 2,500 mg/d.
EVALUATION Depressed and frightened
In the ED, Mr. G’s vitals are normal, but his serum valproic acid (VPA) level is 33.68 µg/mL (therapeutic range: 50 to 125 µg/mL), despite being compliant with treatment. Mr. G is discharged from the ED and told to follow up with his outpatient psychiatrist the next day.
Continue to: At his outpatient psychiatry appointment...
At his outpatient psychiatry appointment, Mr. G’s vital signs are normal, but he reports increasing depression and worsened mood. On mental status examination, Mr. G’s appearance is well groomed, and no agitation nor fidgeting are observed. His behavior is cooperative but somewhat disorganized. He is perseverative on “feeling so low.” He has poor eye contact, which is unusual for him. Mr. G’s speech is loud compared with his baseline. Affect is congruent to mood, which he describes as “depressed and frightened.” He is also noted to be irritable. His thought process is abstract and tangential, which is within his baseline. Mr. G’s thought content is fearful and negativistic, despite his usual positivity and optimism. He denies hallucinations and is oriented to time, place, and person. His judgment, attention, and memory are all within normal limits.
[polldaddy:10790537]
The authors’ observations
The psychiatrist rules out malingering/nonadherence due to Mr. G’s long history of treatment compliance, as evidenced by his past symptom control and therapeutic serum VPA levels. Mr. G was compliant with his postoperative appointments and has been healing well. Therefore, the treatment team believed that Mr. G’s intense and acute decompensation had to be related to a recent change. The notable changes in Mr. G’s case included his sleeve gastrectomy, and the addition of omeprazole to his medication regimen.
The treatment team observed that Mr. G had a long history of compliance with his medications and his symptoms were consistent with a low serum VPA level, which led to the conclusion that the low serum VPA level measured while he was in the ED was likely accurate. This prompted the team to consider Mr. G’s recent surgery. It is well documented that some bariatric surgeries can cause poor absorption of certain vitamins, minerals, and medications. However, Mr. G had a sleeve gastrectomy, which preserves absorption. At this point, the team considered if the patient’s recent medication change was the source of his low VPA level.
The psychiatrist concluded that the issue must have been with the addition of omeprazole because Mr. G’s sleeve gastrectomy was noneventful, he was healing well and being closely monitored by his bariatric surgeon, and this type of surgery preserves absorption. Fortunately, Mr. G was a good historian and had informed his psychiatrist about the addition of omeprazole after his sleeve gastrectomy. The psychiatrist knew acidity was important for the absorption of some medications. Although she was unsure as to whether the problem was a lack of absorption or lack of delivery, the psychiatrist knew a medication change was necessary to raise Mr. G’s serum VPA levels.
TREATMENT A change in divalproex formulation
The psychiatrist switches Mr. G’s formulation of divalproex sodium ER, 2,500 mg/d, to valproic acid immediate-release (IR) liquid capsules. He receives a total daily dose of 2,500 mg, but the dosage is split into 3 times a day. The omeprazole is continued to maintain the postoperative healing process, and he receives his other medications as well (iloperidone, 8 mg twice a day; escitalopram, 10 mg/d; and mirtazapine, 30 mg every night at bedtime).
[polldaddy:10790540]
Continue to: The authors' observations
The authors’ observations
The key component to creating a treatment plan for Mr. G centered on understanding drug metabolism and delivery. Acidity plays a role in dissolution of many medications, which led the team to surmise that the PPI, omeprazole, was the culprit. Through research, they understood that the divalproex sodium ER formulation needed a more acidic environment to dissolve, and therefore, an IR formulation was needed.
Different formulations, different characteristics
Medications can be produced in different formulations such as IR, delayed-release (DR), and ER formulations. Different formulations may contain the same medication at identical strengths; however, they may not be bioequivalent and should be titrated based on both the properties of the medication and the release type.1
Immediate-release formulations are developed to dissolve without delaying or prolonging absorption of the medication. These formulations typically include “superdisintegrants” containing croscarmellose sodium2 so that they disintegrate, de-aggregate, and/or dissolve when they come into contact with water or the gastrointestinal tract.3-7
Delayed-release formulations rely on the gastrointestinal pH to release the medication after a certain amount of time has elapsed due to the enteric coating surrounding the tablet. This enteric coating prevents gastric mucosa/gastric juices from inactivating an acid-labile medication.8
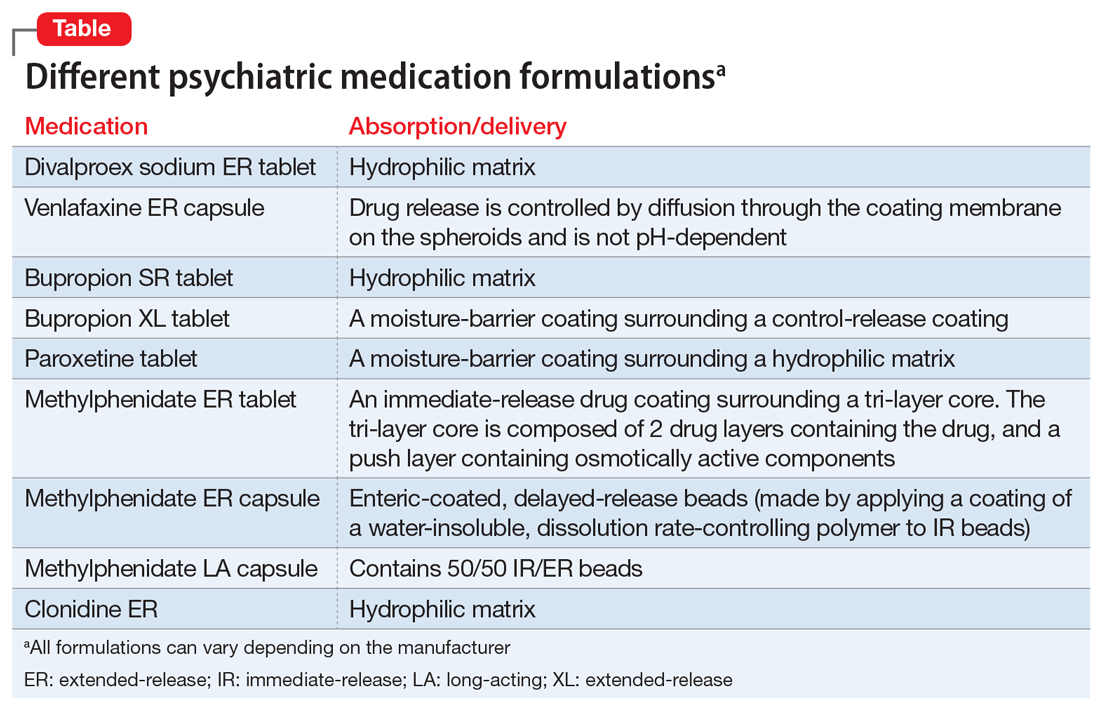
Extended-release formulations, such as the divalproex sodium ER that was originally prescribed to Mr. G, are designed to release the medication in a controlled manner over an extended period of time, and at a predetermined rate and location following administration.8-9 The advantage of this type of formulation is that it can be used to reduce dose frequency and improve adherence.10 Extended-release formulations are designed to minimize fluctuations in serum drug concentration between doses,11 thereby reducing the risk of adverse effects.12,13 A list of some common extended-release psychiatric medications is shown in the Table.
Continue to: The 5 oral formulations...
The 5 oral formulations of medications that contain valproic acid include:
- syrup
- capsule
- sprinkle
- enteric-coated delayed-release and extended-release
A parenteral form via IV is available for patients who are unable to swallow.
Absorption vs delivery
Any gastric bypass surgery can have postoperative complications, one of which can include absorption deficiencies of vitamins and minerals. Sleeve gastrectomy has the least amount of absorption-related nutritional deficiencies.14 Additionally, this procedure preserves the stomach’s ability to produce gastric acid. Therefore, regardless of formulation, there should be no initial postsurgical need to change psychotropic medication formulations. However, because VPA is related to B-vitamin deficiency, supplementation can be considered.
Omeprazole is a PPI that increases pH in the stomach and is often prescribed to promote healing of gastric surgery. However, in Mr. G’s case, omeprazole created a non-acidic environment in his stomach, which prevented the divalproex sodium ER formulation from being dissolved and the medication from being delivered. Mr. G’s absorption ability was preserved, which was confirmed by his rapid recovery and increased serum VPA levels once he was switched to the IR formulation. There is no literature supporting a recommended length of time a patient can receive omeprazole therapy for sleeve gastrectomy; this is at the surgeon’s discretion. Mr. G’s prescription for omeprazole was for 3 months.
Proper valproate dosing
In Mr. G’s case, it could be hypothesized that the VPA dosing was incorrect. For mood disorders, oral VPA dosing is 25 mg/kg/d. Mr. G lost 40 pounds, which would translate to a 450-mg reduction in dose. Despite maintaining his original dose, his serum VPA levels decreased by almost 50% and could not be attributed to trough measurement. In this case, Mr. G was prescribed a higher dose than needed given his weight loss.
Continue to: Divalproex sodium ER...
Divalproex sodium ER is a hydrophilic matrix tablet that requires a low pH to dissolve. Switching to an IR formulation bypassed the need for a low pH and the VPA was delivered and absorbed. Mr. G was always able to absorb the medication, but only when delivered. The Table lists other psychiatric medications that clinicians should be aware of that utilize similar hydrophilic matrix technology to slowly release medications through the gastrointestinal tract and also require low pH to release the medication from the tablet.
OUTCOME Stable once again
Two and a half weeks after his medication formulation is changed from divalproex sodium ER to IR, Mr. G shows improvement in his symptoms. His serum VPA level is 52 µg/mL, which is within therapeutic limits. He continues receiving his previous medications as well. He reports “feeling much better” and denies having any depressive symptoms nor anxiety. Mr. G is able to maintain eye contact, and has positive thought content, improved organization of thinking, and retained abstraction.
Bottom Line
All medication changes should be identified at each visit. Many extended-release psychiatric medications require lower pH to release the medication from the tablet. When evaluating nonresponse to psychotropic medications, anything that affects pH in the stomach should be considered.
Related Resources
- Monte SV, Russo KM, Mustafa E. Impact of sleeve gastrectomy on psychiatric medication use and symptoms. J Obes. 2018; 2018:8532602. doi: 10.1155/2018/8532602
- Qiu Y, Zhou D. Understanding design and development of modified release solid oral dosage forms. J Validation Technol. 2011;17(2):23-32.
- ObesityHelp, Inc. https://www.obesityhelp.com/medications-after-bariatric-surgery-wls/
Drug Brand Names
Bupropion • Wellbutrin, Zyban
Clonidine ER • Kapvay
Divalproex sodium extended- release tablets • Depakote ER
Escitalopram • Lexapro
Iloperidone • Fanapt
Methylphenidate ER tablet • Concerta
Methylphenidate ER capsule • Metadate, Jornay
Methylphenidate LA capsule • Ritalin LA
Mirtazapine • Remeron
Omeprazole • Prilosec, Zegerid
Paroxetine • Paxil
Valproic acid immediate- release capsules and solution • Depakene
Valproate sodium IV • Depacon
Venlafaxine • Effexor
1. Wheless JW, Phelps SJ. A clinician’s guide to oral extended-release drug delivery systems in epilepsy. J Pediatr Pharmacol Ther. 2018;23(4):277-292.
2. Jaimini M, Ranga S, Kumar A, et al. A review on immediate release drug delivery system by using design of experiment. J Drug Discov Therap. 2013;1(12):21-27.
3. Bhandari N, Kumar A, Choudhary A, et al. A review on immediate release drug delivery system. Int Res J Pharm App Sci. 2014;49(1):78-87.
4. Eatock J, Baker GA. Managing patient adherence and quality of life in epilepsy. Neuropsychiatr Dis Treat. 2007;3(1):117-131.
5. Manjunath R, Davis KL, Candrilli SD, et al. Association of antiepileptic drug nonadherence with risk of seizures in adults with epilepsy. Epilepsy Behav. 2009;14(2):372-378.
6. Samsonsen C, Reimers A, Bråthen G, et al. Nonadherence to treatment causing acute hospitalizations in people with epilepsy: an observational, prospective study. Epilepsia. 2014;55(11):e125-e128. doi: 10.1111/epi.12801
7. Mangal M, Thakral S, Goswami M, et al. Superdisintegrants: an updated review. Int Pharmacy Pharmaceut Sci Res. 2012;2(2):26-35.
8. Tablets. United States Pharmacopeia. Accessed January 21, 2021. http://www.pharmacopeia.cn/v29240/usp29nf24s0_c1151s87.html
9. Holquist C, Fava W. FDA safety page: delayed- vs. extended-release Rxs. Drug Topics. Published July 23, 2007. Accessed January 21, 2021. https://www.drugtopics.com/view/fda-safety-page-delayed-release-vs-extended-release-rxs
10. Qiu Y, Zhou D. Understanding design and development of modified release solid oral dosage forms. J Validation Technol. 2011;17(2):23-32.
11. Perucca E. Extended-release formulations of antiepileptic drugs: rationale and comparative value. Epilepsy Curr. 2009;9(6):153-157.
12. Bialer M. Extended-release formulations for the treatment of epilepsy. CNS Drugs. 2007;21(9):765-774.
13. Pellock JM, Smith MC, Cloyd JC, et al. Extended-release formulations: simplifying strategies in the management of antiepileptic drug therapy. Epilepsy Behav. 2004;5(3):301-307.
14. Sarkhosh K, Birch DW, Sharma A, et al. Complications associated with laparoscopic sleeve gastrectomy for morbid obesity: a surgeon’s guide. Can J Surg 2013;56(5):347-352.
CASE Sudden-onset low mood
Mr. G, age 64, is obese (body mass index [BMI] 37 kg/m2) and has a history of schizoaffective disorder. He is recovering from a sleeve gastrectomy, a surgical weight-loss procedure in which a large portion of the stomach is removed. Seven weeks after his surgery, he experiences a sudden onset of “low mood” and fears that he will become suicidal; he has a history of suicide attempts. Mr. G calls his long-term outpatient clinic and is advised to go to the emergency department (ED).
For years, Mr. G had been stable in a group home setting, and had always been adherent to treatment and forthcoming about his medications with both his bariatric surgeon and psychiatrist. Within the last month, he had been seen at the clinic, had no psychiatric symptoms, and was recovering well from the sleeve gastrectomy.
HISTORY A stable regimen
Mr. G’s psychiatric symptoms initially developed when he was in his 20s, during a time in which he reported using “a lot of drugs.” He had multiple suicide attempts, and multiple inpatient and outpatient treatments. He was diagnosed with schizoaffective disorder.
Mr. G has been stable on medications for the last 2 years. His outpatient psychotropic regimen is divalproex sodium extended-release (ER), 2,500 mg every night at bedtime; iloperidone, 8 mg twice a day; escitalopram, 10 mg/d; and mirtazapine, 30 mg every night at bedtime.
In the group home, Mr. G spends his days socializing, studying philosophy, and writing essays. He hopes to find a job in the craftsman industry.
Mr. G’s medical history includes obesity (BMI: 37 kg/m2). Since the surgery, he has been receiving omeprazole, 40 mg/d, a proton pump inhibitor (PPI), to decrease the amount of acid in his stomach. Three weeks after surgery, he had an unremarkable postoperative outpatient psychiatry visit. Divalproex sodium ER was maintained at the pre-surgical dose of 2,500 mg/d.
EVALUATION Depressed and frightened
In the ED, Mr. G’s vitals are normal, but his serum valproic acid (VPA) level is 33.68 µg/mL (therapeutic range: 50 to 125 µg/mL), despite being compliant with treatment. Mr. G is discharged from the ED and told to follow up with his outpatient psychiatrist the next day.
Continue to: At his outpatient psychiatry appointment...
At his outpatient psychiatry appointment, Mr. G’s vital signs are normal, but he reports increasing depression and worsened mood. On mental status examination, Mr. G’s appearance is well groomed, and no agitation nor fidgeting are observed. His behavior is cooperative but somewhat disorganized. He is perseverative on “feeling so low.” He has poor eye contact, which is unusual for him. Mr. G’s speech is loud compared with his baseline. Affect is congruent to mood, which he describes as “depressed and frightened.” He is also noted to be irritable. His thought process is abstract and tangential, which is within his baseline. Mr. G’s thought content is fearful and negativistic, despite his usual positivity and optimism. He denies hallucinations and is oriented to time, place, and person. His judgment, attention, and memory are all within normal limits.
[polldaddy:10790537]
The authors’ observations
The psychiatrist rules out malingering/nonadherence due to Mr. G’s long history of treatment compliance, as evidenced by his past symptom control and therapeutic serum VPA levels. Mr. G was compliant with his postoperative appointments and has been healing well. Therefore, the treatment team believed that Mr. G’s intense and acute decompensation had to be related to a recent change. The notable changes in Mr. G’s case included his sleeve gastrectomy, and the addition of omeprazole to his medication regimen.
The treatment team observed that Mr. G had a long history of compliance with his medications and his symptoms were consistent with a low serum VPA level, which led to the conclusion that the low serum VPA level measured while he was in the ED was likely accurate. This prompted the team to consider Mr. G’s recent surgery. It is well documented that some bariatric surgeries can cause poor absorption of certain vitamins, minerals, and medications. However, Mr. G had a sleeve gastrectomy, which preserves absorption. At this point, the team considered if the patient’s recent medication change was the source of his low VPA level.
The psychiatrist concluded that the issue must have been with the addition of omeprazole because Mr. G’s sleeve gastrectomy was noneventful, he was healing well and being closely monitored by his bariatric surgeon, and this type of surgery preserves absorption. Fortunately, Mr. G was a good historian and had informed his psychiatrist about the addition of omeprazole after his sleeve gastrectomy. The psychiatrist knew acidity was important for the absorption of some medications. Although she was unsure as to whether the problem was a lack of absorption or lack of delivery, the psychiatrist knew a medication change was necessary to raise Mr. G’s serum VPA levels.
TREATMENT A change in divalproex formulation
The psychiatrist switches Mr. G’s formulation of divalproex sodium ER, 2,500 mg/d, to valproic acid immediate-release (IR) liquid capsules. He receives a total daily dose of 2,500 mg, but the dosage is split into 3 times a day. The omeprazole is continued to maintain the postoperative healing process, and he receives his other medications as well (iloperidone, 8 mg twice a day; escitalopram, 10 mg/d; and mirtazapine, 30 mg every night at bedtime).
[polldaddy:10790540]
Continue to: The authors' observations
The authors’ observations
The key component to creating a treatment plan for Mr. G centered on understanding drug metabolism and delivery. Acidity plays a role in dissolution of many medications, which led the team to surmise that the PPI, omeprazole, was the culprit. Through research, they understood that the divalproex sodium ER formulation needed a more acidic environment to dissolve, and therefore, an IR formulation was needed.
Different formulations, different characteristics
Medications can be produced in different formulations such as IR, delayed-release (DR), and ER formulations. Different formulations may contain the same medication at identical strengths; however, they may not be bioequivalent and should be titrated based on both the properties of the medication and the release type.1
Immediate-release formulations are developed to dissolve without delaying or prolonging absorption of the medication. These formulations typically include “superdisintegrants” containing croscarmellose sodium2 so that they disintegrate, de-aggregate, and/or dissolve when they come into contact with water or the gastrointestinal tract.3-7
Delayed-release formulations rely on the gastrointestinal pH to release the medication after a certain amount of time has elapsed due to the enteric coating surrounding the tablet. This enteric coating prevents gastric mucosa/gastric juices from inactivating an acid-labile medication.8
Extended-release formulations, such as the divalproex sodium ER that was originally prescribed to Mr. G, are designed to release the medication in a controlled manner over an extended period of time, and at a predetermined rate and location following administration.8-9 The advantage of this type of formulation is that it can be used to reduce dose frequency and improve adherence.10 Extended-release formulations are designed to minimize fluctuations in serum drug concentration between doses,11 thereby reducing the risk of adverse effects.12,13 A list of some common extended-release psychiatric medications is shown in the Table.

Continue to: The 5 oral formulations...
The 5 oral formulations of medications that contain valproic acid include:
- syrup
- capsule
- sprinkle
- enteric-coated delayed-release and extended-release
A parenteral form via IV is available for patients who are unable to swallow.
Absorption vs delivery
Any gastric bypass surgery can have postoperative complications, one of which can include absorption deficiencies of vitamins and minerals. Sleeve gastrectomy has the least amount of absorption-related nutritional deficiencies.14 Additionally, this procedure preserves the stomach’s ability to produce gastric acid. Therefore, regardless of formulation, there should be no initial postsurgical need to change psychotropic medication formulations. However, because VPA is related to B-vitamin deficiency, supplementation can be considered.
Omeprazole is a PPI that increases pH in the stomach and is often prescribed to promote healing of gastric surgery. However, in Mr. G’s case, omeprazole created a non-acidic environment in his stomach, which prevented the divalproex sodium ER formulation from being dissolved and the medication from being delivered. Mr. G’s absorption ability was preserved, which was confirmed by his rapid recovery and increased serum VPA levels once he was switched to the IR formulation. There is no literature supporting a recommended length of time a patient can receive omeprazole therapy for sleeve gastrectomy; this is at the surgeon’s discretion. Mr. G’s prescription for omeprazole was for 3 months.
Proper valproate dosing
In Mr. G’s case, it could be hypothesized that the VPA dosing was incorrect. For mood disorders, oral VPA dosing is 25 mg/kg/d. Mr. G lost 40 pounds, which would translate to a 450-mg reduction in dose. Despite maintaining his original dose, his serum VPA levels decreased by almost 50% and could not be attributed to trough measurement. In this case, Mr. G was prescribed a higher dose than needed given his weight loss.
Continue to: Divalproex sodium ER...
Divalproex sodium ER is a hydrophilic matrix tablet that requires a low pH to dissolve. Switching to an IR formulation bypassed the need for a low pH and the VPA was delivered and absorbed. Mr. G was always able to absorb the medication, but only when delivered. The Table lists other psychiatric medications that clinicians should be aware of that utilize similar hydrophilic matrix technology to slowly release medications through the gastrointestinal tract and also require low pH to release the medication from the tablet.
OUTCOME Stable once again
Two and a half weeks after his medication formulation is changed from divalproex sodium ER to IR, Mr. G shows improvement in his symptoms. His serum VPA level is 52 µg/mL, which is within therapeutic limits. He continues receiving his previous medications as well. He reports “feeling much better” and denies having any depressive symptoms nor anxiety. Mr. G is able to maintain eye contact, and has positive thought content, improved organization of thinking, and retained abstraction.
Bottom Line
All medication changes should be identified at each visit. Many extended-release psychiatric medications require lower pH to release the medication from the tablet. When evaluating nonresponse to psychotropic medications, anything that affects pH in the stomach should be considered.
Related Resources
- Monte SV, Russo KM, Mustafa E. Impact of sleeve gastrectomy on psychiatric medication use and symptoms. J Obes. 2018; 2018:8532602. doi: 10.1155/2018/8532602
- Qiu Y, Zhou D. Understanding design and development of modified release solid oral dosage forms. J Validation Technol. 2011;17(2):23-32.
- ObesityHelp, Inc. https://www.obesityhelp.com/medications-after-bariatric-surgery-wls/
Drug Brand Names
Bupropion • Wellbutrin, Zyban
Clonidine ER • Kapvay
Divalproex sodium extended- release tablets • Depakote ER
Escitalopram • Lexapro
Iloperidone • Fanapt
Methylphenidate ER tablet • Concerta
Methylphenidate ER capsule • Metadate, Jornay
Methylphenidate LA capsule • Ritalin LA
Mirtazapine • Remeron
Omeprazole • Prilosec, Zegerid
Paroxetine • Paxil
Valproic acid immediate- release capsules and solution • Depakene
Valproate sodium IV • Depacon
Venlafaxine • Effexor
CASE Sudden-onset low mood
Mr. G, age 64, is obese (body mass index [BMI] 37 kg/m2) and has a history of schizoaffective disorder. He is recovering from a sleeve gastrectomy, a surgical weight-loss procedure in which a large portion of the stomach is removed. Seven weeks after his surgery, he experiences a sudden onset of “low mood” and fears that he will become suicidal; he has a history of suicide attempts. Mr. G calls his long-term outpatient clinic and is advised to go to the emergency department (ED).
For years, Mr. G had been stable in a group home setting, and had always been adherent to treatment and forthcoming about his medications with both his bariatric surgeon and psychiatrist. Within the last month, he had been seen at the clinic, had no psychiatric symptoms, and was recovering well from the sleeve gastrectomy.
HISTORY A stable regimen
Mr. G’s psychiatric symptoms initially developed when he was in his 20s, during a time in which he reported using “a lot of drugs.” He had multiple suicide attempts, and multiple inpatient and outpatient treatments. He was diagnosed with schizoaffective disorder.
Mr. G has been stable on medications for the last 2 years. His outpatient psychotropic regimen is divalproex sodium extended-release (ER), 2,500 mg every night at bedtime; iloperidone, 8 mg twice a day; escitalopram, 10 mg/d; and mirtazapine, 30 mg every night at bedtime.
In the group home, Mr. G spends his days socializing, studying philosophy, and writing essays. He hopes to find a job in the craftsman industry.
Mr. G’s medical history includes obesity (BMI: 37 kg/m2). Since the surgery, he has been receiving omeprazole, 40 mg/d, a proton pump inhibitor (PPI), to decrease the amount of acid in his stomach. Three weeks after surgery, he had an unremarkable postoperative outpatient psychiatry visit. Divalproex sodium ER was maintained at the pre-surgical dose of 2,500 mg/d.
EVALUATION Depressed and frightened
In the ED, Mr. G’s vitals are normal, but his serum valproic acid (VPA) level is 33.68 µg/mL (therapeutic range: 50 to 125 µg/mL), despite being compliant with treatment. Mr. G is discharged from the ED and told to follow up with his outpatient psychiatrist the next day.
Continue to: At his outpatient psychiatry appointment...
At his outpatient psychiatry appointment, Mr. G’s vital signs are normal, but he reports increasing depression and worsened mood. On mental status examination, Mr. G’s appearance is well groomed, and no agitation nor fidgeting are observed. His behavior is cooperative but somewhat disorganized. He is perseverative on “feeling so low.” He has poor eye contact, which is unusual for him. Mr. G’s speech is loud compared with his baseline. Affect is congruent to mood, which he describes as “depressed and frightened.” He is also noted to be irritable. His thought process is abstract and tangential, which is within his baseline. Mr. G’s thought content is fearful and negativistic, despite his usual positivity and optimism. He denies hallucinations and is oriented to time, place, and person. His judgment, attention, and memory are all within normal limits.
[polldaddy:10790537]
The authors’ observations
The psychiatrist rules out malingering/nonadherence due to Mr. G’s long history of treatment compliance, as evidenced by his past symptom control and therapeutic serum VPA levels. Mr. G was compliant with his postoperative appointments and has been healing well. Therefore, the treatment team believed that Mr. G’s intense and acute decompensation had to be related to a recent change. The notable changes in Mr. G’s case included his sleeve gastrectomy, and the addition of omeprazole to his medication regimen.
The treatment team observed that Mr. G had a long history of compliance with his medications and his symptoms were consistent with a low serum VPA level, which led to the conclusion that the low serum VPA level measured while he was in the ED was likely accurate. This prompted the team to consider Mr. G’s recent surgery. It is well documented that some bariatric surgeries can cause poor absorption of certain vitamins, minerals, and medications. However, Mr. G had a sleeve gastrectomy, which preserves absorption. At this point, the team considered if the patient’s recent medication change was the source of his low VPA level.
The psychiatrist concluded that the issue must have been with the addition of omeprazole because Mr. G’s sleeve gastrectomy was noneventful, he was healing well and being closely monitored by his bariatric surgeon, and this type of surgery preserves absorption. Fortunately, Mr. G was a good historian and had informed his psychiatrist about the addition of omeprazole after his sleeve gastrectomy. The psychiatrist knew acidity was important for the absorption of some medications. Although she was unsure as to whether the problem was a lack of absorption or lack of delivery, the psychiatrist knew a medication change was necessary to raise Mr. G’s serum VPA levels.
TREATMENT A change in divalproex formulation
The psychiatrist switches Mr. G’s formulation of divalproex sodium ER, 2,500 mg/d, to valproic acid immediate-release (IR) liquid capsules. He receives a total daily dose of 2,500 mg, but the dosage is split into 3 times a day. The omeprazole is continued to maintain the postoperative healing process, and he receives his other medications as well (iloperidone, 8 mg twice a day; escitalopram, 10 mg/d; and mirtazapine, 30 mg every night at bedtime).
[polldaddy:10790540]
Continue to: The authors' observations
The authors’ observations
The key component to creating a treatment plan for Mr. G centered on understanding drug metabolism and delivery. Acidity plays a role in dissolution of many medications, which led the team to surmise that the PPI, omeprazole, was the culprit. Through research, they understood that the divalproex sodium ER formulation needed a more acidic environment to dissolve, and therefore, an IR formulation was needed.
Different formulations, different characteristics
Medications can be produced in different formulations such as IR, delayed-release (DR), and ER formulations. Different formulations may contain the same medication at identical strengths; however, they may not be bioequivalent and should be titrated based on both the properties of the medication and the release type.1
Immediate-release formulations are developed to dissolve without delaying or prolonging absorption of the medication. These formulations typically include “superdisintegrants” containing croscarmellose sodium2 so that they disintegrate, de-aggregate, and/or dissolve when they come into contact with water or the gastrointestinal tract.3-7
Delayed-release formulations rely on the gastrointestinal pH to release the medication after a certain amount of time has elapsed due to the enteric coating surrounding the tablet. This enteric coating prevents gastric mucosa/gastric juices from inactivating an acid-labile medication.8
Extended-release formulations, such as the divalproex sodium ER that was originally prescribed to Mr. G, are designed to release the medication in a controlled manner over an extended period of time, and at a predetermined rate and location following administration.8-9 The advantage of this type of formulation is that it can be used to reduce dose frequency and improve adherence.10 Extended-release formulations are designed to minimize fluctuations in serum drug concentration between doses,11 thereby reducing the risk of adverse effects.12,13 A list of some common extended-release psychiatric medications is shown in the Table.

Continue to: The 5 oral formulations...
The 5 oral formulations of medications that contain valproic acid include:
- syrup
- capsule
- sprinkle
- enteric-coated delayed-release and extended-release
A parenteral form via IV is available for patients who are unable to swallow.
Absorption vs delivery
Any gastric bypass surgery can have postoperative complications, one of which can include absorption deficiencies of vitamins and minerals. Sleeve gastrectomy has the least amount of absorption-related nutritional deficiencies.14 Additionally, this procedure preserves the stomach’s ability to produce gastric acid. Therefore, regardless of formulation, there should be no initial postsurgical need to change psychotropic medication formulations. However, because VPA is related to B-vitamin deficiency, supplementation can be considered.
Omeprazole is a PPI that increases pH in the stomach and is often prescribed to promote healing of gastric surgery. However, in Mr. G’s case, omeprazole created a non-acidic environment in his stomach, which prevented the divalproex sodium ER formulation from being dissolved and the medication from being delivered. Mr. G’s absorption ability was preserved, which was confirmed by his rapid recovery and increased serum VPA levels once he was switched to the IR formulation. There is no literature supporting a recommended length of time a patient can receive omeprazole therapy for sleeve gastrectomy; this is at the surgeon’s discretion. Mr. G’s prescription for omeprazole was for 3 months.
Proper valproate dosing
In Mr. G’s case, it could be hypothesized that the VPA dosing was incorrect. For mood disorders, oral VPA dosing is 25 mg/kg/d. Mr. G lost 40 pounds, which would translate to a 450-mg reduction in dose. Despite maintaining his original dose, his serum VPA levels decreased by almost 50% and could not be attributed to trough measurement. In this case, Mr. G was prescribed a higher dose than needed given his weight loss.
Continue to: Divalproex sodium ER...
Divalproex sodium ER is a hydrophilic matrix tablet that requires a low pH to dissolve. Switching to an IR formulation bypassed the need for a low pH and the VPA was delivered and absorbed. Mr. G was always able to absorb the medication, but only when delivered. The Table lists other psychiatric medications that clinicians should be aware of that utilize similar hydrophilic matrix technology to slowly release medications through the gastrointestinal tract and also require low pH to release the medication from the tablet.
OUTCOME Stable once again
Two and a half weeks after his medication formulation is changed from divalproex sodium ER to IR, Mr. G shows improvement in his symptoms. His serum VPA level is 52 µg/mL, which is within therapeutic limits. He continues receiving his previous medications as well. He reports “feeling much better” and denies having any depressive symptoms nor anxiety. Mr. G is able to maintain eye contact, and has positive thought content, improved organization of thinking, and retained abstraction.
Bottom Line
All medication changes should be identified at each visit. Many extended-release psychiatric medications require lower pH to release the medication from the tablet. When evaluating nonresponse to psychotropic medications, anything that affects pH in the stomach should be considered.
Related Resources
- Monte SV, Russo KM, Mustafa E. Impact of sleeve gastrectomy on psychiatric medication use and symptoms. J Obes. 2018; 2018:8532602. doi: 10.1155/2018/8532602
- Qiu Y, Zhou D. Understanding design and development of modified release solid oral dosage forms. J Validation Technol. 2011;17(2):23-32.
- ObesityHelp, Inc. https://www.obesityhelp.com/medications-after-bariatric-surgery-wls/
Drug Brand Names
Bupropion • Wellbutrin, Zyban
Clonidine ER • Kapvay
Divalproex sodium extended- release tablets • Depakote ER
Escitalopram • Lexapro
Iloperidone • Fanapt
Methylphenidate ER tablet • Concerta
Methylphenidate ER capsule • Metadate, Jornay
Methylphenidate LA capsule • Ritalin LA
Mirtazapine • Remeron
Omeprazole • Prilosec, Zegerid
Paroxetine • Paxil
Valproic acid immediate- release capsules and solution • Depakene
Valproate sodium IV • Depacon
Venlafaxine • Effexor
1. Wheless JW, Phelps SJ. A clinician’s guide to oral extended-release drug delivery systems in epilepsy. J Pediatr Pharmacol Ther. 2018;23(4):277-292.
2. Jaimini M, Ranga S, Kumar A, et al. A review on immediate release drug delivery system by using design of experiment. J Drug Discov Therap. 2013;1(12):21-27.
3. Bhandari N, Kumar A, Choudhary A, et al. A review on immediate release drug delivery system. Int Res J Pharm App Sci. 2014;49(1):78-87.
4. Eatock J, Baker GA. Managing patient adherence and quality of life in epilepsy. Neuropsychiatr Dis Treat. 2007;3(1):117-131.
5. Manjunath R, Davis KL, Candrilli SD, et al. Association of antiepileptic drug nonadherence with risk of seizures in adults with epilepsy. Epilepsy Behav. 2009;14(2):372-378.
6. Samsonsen C, Reimers A, Bråthen G, et al. Nonadherence to treatment causing acute hospitalizations in people with epilepsy: an observational, prospective study. Epilepsia. 2014;55(11):e125-e128. doi: 10.1111/epi.12801
7. Mangal M, Thakral S, Goswami M, et al. Superdisintegrants: an updated review. Int Pharmacy Pharmaceut Sci Res. 2012;2(2):26-35.
8. Tablets. United States Pharmacopeia. Accessed January 21, 2021. http://www.pharmacopeia.cn/v29240/usp29nf24s0_c1151s87.html
9. Holquist C, Fava W. FDA safety page: delayed- vs. extended-release Rxs. Drug Topics. Published July 23, 2007. Accessed January 21, 2021. https://www.drugtopics.com/view/fda-safety-page-delayed-release-vs-extended-release-rxs
10. Qiu Y, Zhou D. Understanding design and development of modified release solid oral dosage forms. J Validation Technol. 2011;17(2):23-32.
11. Perucca E. Extended-release formulations of antiepileptic drugs: rationale and comparative value. Epilepsy Curr. 2009;9(6):153-157.
12. Bialer M. Extended-release formulations for the treatment of epilepsy. CNS Drugs. 2007;21(9):765-774.
13. Pellock JM, Smith MC, Cloyd JC, et al. Extended-release formulations: simplifying strategies in the management of antiepileptic drug therapy. Epilepsy Behav. 2004;5(3):301-307.
14. Sarkhosh K, Birch DW, Sharma A, et al. Complications associated with laparoscopic sleeve gastrectomy for morbid obesity: a surgeon’s guide. Can J Surg 2013;56(5):347-352.
1. Wheless JW, Phelps SJ. A clinician’s guide to oral extended-release drug delivery systems in epilepsy. J Pediatr Pharmacol Ther. 2018;23(4):277-292.
2. Jaimini M, Ranga S, Kumar A, et al. A review on immediate release drug delivery system by using design of experiment. J Drug Discov Therap. 2013;1(12):21-27.
3. Bhandari N, Kumar A, Choudhary A, et al. A review on immediate release drug delivery system. Int Res J Pharm App Sci. 2014;49(1):78-87.
4. Eatock J, Baker GA. Managing patient adherence and quality of life in epilepsy. Neuropsychiatr Dis Treat. 2007;3(1):117-131.
5. Manjunath R, Davis KL, Candrilli SD, et al. Association of antiepileptic drug nonadherence with risk of seizures in adults with epilepsy. Epilepsy Behav. 2009;14(2):372-378.
6. Samsonsen C, Reimers A, Bråthen G, et al. Nonadherence to treatment causing acute hospitalizations in people with epilepsy: an observational, prospective study. Epilepsia. 2014;55(11):e125-e128. doi: 10.1111/epi.12801
7. Mangal M, Thakral S, Goswami M, et al. Superdisintegrants: an updated review. Int Pharmacy Pharmaceut Sci Res. 2012;2(2):26-35.
8. Tablets. United States Pharmacopeia. Accessed January 21, 2021. http://www.pharmacopeia.cn/v29240/usp29nf24s0_c1151s87.html
9. Holquist C, Fava W. FDA safety page: delayed- vs. extended-release Rxs. Drug Topics. Published July 23, 2007. Accessed January 21, 2021. https://www.drugtopics.com/view/fda-safety-page-delayed-release-vs-extended-release-rxs
10. Qiu Y, Zhou D. Understanding design and development of modified release solid oral dosage forms. J Validation Technol. 2011;17(2):23-32.
11. Perucca E. Extended-release formulations of antiepileptic drugs: rationale and comparative value. Epilepsy Curr. 2009;9(6):153-157.
12. Bialer M. Extended-release formulations for the treatment of epilepsy. CNS Drugs. 2007;21(9):765-774.
13. Pellock JM, Smith MC, Cloyd JC, et al. Extended-release formulations: simplifying strategies in the management of antiepileptic drug therapy. Epilepsy Behav. 2004;5(3):301-307.
14. Sarkhosh K, Birch DW, Sharma A, et al. Complications associated with laparoscopic sleeve gastrectomy for morbid obesity: a surgeon’s guide. Can J Surg 2013;56(5):347-352.
Elaborate hallucinations, but is it a psychotic disorder?
CASE Visual, auditory, and tactile hallucinations
Mr. B, age 93, is brought to the emergency department by his son after experiencing hallucinations where he reportedly saw and heard individuals in his home. In frustration, Mr. B wielded a knife because he “wanted them to go away.”
Mr. B and his son report that the hallucinations had begun 2 years ago, without prior trauma, medication changes, changes in social situation, or other apparent precipitating events. The hallucinations “come and go,” without preceding symptoms, but have recurring content involving a friendly man named “Harry,” people coming out of the television, 2 children playing, and water covering the floor. Mr. B acknowledges these are hallucinations and had not felt threatened by them until recently, when he wielded the knife. He often tries to talk to them, but they do not reply.
Mr. B also reports intermittent auditory hallucinations including voices at home (non-command) and papers rustling. He also describes tactile hallucinations, where he says he can feel Harry and others prodding him, knocking things out of his hands, or splashing him with water.
Mr. B is admitted to the hospital because he is a danger to himself and others. While on the inpatient unit, Mr. B is pleasant with staff, and eats and sleeps normally; however, he continues to have hallucinations of Harry. Mr. B reports seeing Harry in the hall, and says that Harry pulls out Mr. B’s earpiece and steals his fork. Mr. B also reports hearing a sound “like a bee buzzing.” Mr. B is started on risperidone, 1 mg nightly, for a presumed psychotic disorder.
HISTORY Independent and in good health
Mr. B lives alone and is independent in his activities of daily living. He spends his days at home, often visited by his children, who bring him groceries and other necessities.
Mr. B takes no medications, and has no history of psychiatric treatment; psychotic, manic, or depressive episodes; posttraumatic stress disorder; obsessive-compulsive disorder; or recent emotional stress. His medical history includes chronic progressive hearing loss, which is managed with hearing aids; macular degeneration; and prior bilateral cataract surgeries.
EVALUATION Mental status exam and objective findings
During his evaluation, Mr. B appears well-nourished, and wears glasses and hearing aids. During the interview, he is euthymic with appropriately reactive affect. He is talkative but redirectable, with a goal-directed thought process. Mr. B does not appear to be internally preoccupied. His hearing is impaired, and he often requires questions to be repeated loudly. He is oriented to person, place, and time. There are no signs of delusions, paranoia, thought blocking, thought broadcasting/insertion, or referential thinking. He denies depressed mood, anhedonia, fatigue, sleep changes, or manic symptoms. He denies the occurrence of auditory or visual hallucinations during the evaluation.
Continue to: A neurologic exam shows...
A neurologic exam shows impaired hearing bilaterally and impaired visual acuity. Even with glasses, both eyes have acuity only to finger counting. All other cranial nerves are normal, and Mr. B’s strength, sensation, and cerebellar function are all intact, without rigidity, numbness, or tingling. His gait is steady without a walker, with symmetric arm swing and slight dragging of his feet. His vitals are stable, with normal orthostatic pressures.
Other objective data include a score of 24/30 on the Mini-Mental State Examination, notable for deficits in visuospatial orientation, attention, and calculation, with language and copying limited by poor vision. Mr. B scores 16/22 on the Montreal Cognitive Assessment (MoCA)-Blind (adapted version of MoCA), which is equivalent to a 22/30 on the MoCA, indicating some mild cognitive impairment; however, this modified test is still limited by his poor hearing. His serum and urine laboratory workup show no liver, kidney, metabolic, or electrolyte abnormalities, no sign of infection, negative urine drug screen, and normal B12 and thyroid-stimulating hormone levels. He undergoes a brain MRI, which shows chronic microvascular ischemic change, without mass lesions, infarction, or other pathology.
[polldaddy:10729178]
The authors’ observations
Given Mr. B’s presentation, we ruled out a primary psychotic disorder. He had no psychiatric history, with organized thought, a reactive affect, and no delusions, paranoia, or other psychotic symptoms, all pointing against psychosis. His brain MRI showed no malignancy or other lesions. He had no substance use history to suggest intoxication/withdrawal. His intact attention and orientation did not suggest delirium, and his serum and urine studies were all negative. Although his blaming Harry for knocking things out of his hands could suggest confabulation, Mr. B had no other signs of Korsakoff syndrome, such as ataxia, general confusion, or malnourishment.
We also considered early dementia. There was suspicion for Lewy body dementia given Mr. B’s prominent fluctuating visual hallucinations; however, he displayed no other signs of the disorder, such as parkinsonism, dysautonomia, or sensitivity to the antipsychotic (risperidone 1 mg nightly) started on admission. The presence of 1 core feature of Lewy body dementia—visual hallucinations—indicated a possible, but not probable, diagnosis. Additionally, Mr. B did not have the characteristic features of other types of dementia, such as the stepwise progression of vascular dementia, the behavioral disinhibition of frontotemporal dementia, or the insidious forgetfulness, confusion, language problems, or paranoia that may appear in Alzheimer’s disease. Remarkably, he had a relatively normal brain MRI for his age, given chronic microvascular ischemic changes, and cognitive testing that indicated only mild impairment further pointed against a dementia process.
Charles Bonnet syndrome
Based on Mr. B’s severe vision loss and history of ocular surgeries, we diagnosed him with CBS, described as visual hallucinations in the presence of impaired vision. Charles Bonnet syndrome has been observed in several disorders that affect vision, most commonly macular degeneration, diabetic retinopathy, and glaucoma, with an estimated prevalence of 11% to 39% in older patients with ocular disease.1,2 Visual hallucinations in CBS occur due to ocular disease, likely resulting from changes in afferent sensory input to visual cortical regions of the brain. Table 13 outlines the features of visual hallucinations in patients with CBS. The subsequent disinhibition and spontaneous firing of the visual association cortices leads to the “release hallucinations” of the syndrome.4 The disorder is thought to be significantly underdiagnosed—in a survey of patients with CBS, only 15% had reported their visual hallucinations to a physician.5

Continue to: Mr. B's symptoms...
Mr. B’s symptoms are atypical for CBS, but they fit the diagnosis when considering the entire clinical picture. While hallucinations in CBS are more often simple shapes, complex hallucinations including people and scenes have been noted in several instances.6
Similar to Mr. B’s case, patients with CBS can have recurring figures in their hallucinations, and the images may even move across the visual field.1 Patients with CBS also frequently recognize that their hallucinations are not real, and may or may not be distressed by them.4 Patients with CBS often have hallucinations multiple times daily, lasting from a few seconds to many minutes,7 consistent with Mr. B’s temporary symptoms.
Although auditory and tactile hallucinations are typically not included in CBS, they can also be explained by Mr. B’s significant sensory impairment. Severe hearing impairment in geriatric adults has been associated with auditory hallucinations8; in 1 survey, half of these hallucinations consisted of voices.9 In contrast, tactile hallucinations are not described in sensory deprivation literature. However, in the context of Mr. B’s severe comorbid hearing and vision loss, we propose that these hallucinations reflect his interpretation of sensory events around him, and their integration into his extensive hallucination framework. In other words, Harry poking him and causing him to drop things may be Mr. B’s way of rationalizing events that he has trouble perceiving entirely, or his mild forgetfulness. Mr. B’s social isolation is another factor that may worsen his sensory deprivation and contribute to his extensive hallucinations.10 Additionally, his mild cognitive deficits on testing with chronic microvascular changes on the MRI may suggest a mild vascular-related dementia process, which could also exacerbate his hallucinations. While classic CBS occurs without cognitive impairment, dementia can often co-occur with CBS.11
TREATMENT No significant improvement with medications
During his inpatient stay, Mr. B is treated with risperidone, 1 mg nightly, and is also started on donepezil, 5 mg/d, to treat a possible comorbid dementia. However, he continues to hallucinate without significant improvement.
[polldaddy:10729181]
The authors’ observations
There is no definitive treatment for CBS, and while the hallucinations may spontaneously resolve, per case reports, this typically occurs only as visual loss progresses to total blindness.12 However, many patients can have the hallucinations remit after the underlying ocular etiology is corrected, such as through ocular surgery.13 Other optical interventions, such as special glasses or contact lenses, may help maximize remaining vision.8 In patients without this option, such as Mr. B, there are limited data on beneficial medications for CBS.
Continue to: Evidence for treatment of CBS...
Evidence for treatment of CBS with antipsychotic medications is mixed. Some case studies have found them to be ineffective, while others have found agents such as olanzapine or risperidone to be partially helpful in reducing symptoms.14 There are also data from case reports that may support the use of cholinesterase inhibitors such as donepezil, antiepileptics (carbamazepine, valproate, gabapentin, and clonazepam), and certain antidepressants (escitalopram, venlafaxine) (Table 28,11).3
Addressing loneliness and social isolation
With minimal definitive evidence for pharmacologic management, the most important intervention for treating CBS may be changing the patient’s sensory environment. Specifically, loneliness and social isolation are major exacerbating factors of CBS, and many clinicians advocate for the consistent presence of a sympathetic professional. Reassurance that hallucinations are from ocular disease rather than a primary mental disorder may be extremely relieving for patients.11 A psychoeducation or support group may also be beneficial, not only for giving patients more social contact, but also for teaching them coping skills or strategies to reduce hallucinations, such as distraction, turning on more lights, or even certain eye/blinking movements.11 Table 28,11 (page 49) outlines behavioral interventions for CBS.
Regardless of etiology, Mr. B’s hallucinations significantly affected his quality of life. During his inpatient stay, he was treated with
OUTCOME Home care and family involvement
After discussion with Mr. B and his family about the risks and benefits of medication, the risperidone and donepezil are discontinued. Ultimately, it is determined that Mr. B requires a higher level of home care, both for his safety and to improve his social contact. Mr. B returns home with a combination of a professional home health aide and increased family involvement.
Bottom Line
When evaluating visual hallucinations in older adults, Charles Bonnet syndrome (CBS) should be considered. Sensory deprivation and social isolation are significant risk factors for CBS. While evidence is inconclusive for medical treatment, reassurance and behavioral interventions can often improve symptoms.
Continue to: Related Resources
Related Resources
- Charles Bonnet Syndrome Foundation. http://www.charlesbonnetsyndrome.org
- Schultz G, Melzack R. The Charles Bonnet syndrome: ‘phantom visual images’. Perception. 1991;20:809-825.
- Menon GJ, Rahman I, Menon SJ, et al. Complex visual hallucinations in the visually impaired: the Charles Bonnet syndrome. Surv Ophthalmol. 2003;48(1):58-72.
Drug Brand Names
Carbamazepine • Tegretol
Clonazepam • Klonopin
Donepezil • Aricept
Escitalopram • Lexapro
Gabapentin • Neurontin
Olanzapine • Zyprexa
Risperidone • Risperdal
Valproate • Depakote
Venlafaxine • Effexor
1. Menon GJ, Rahman I, Menon SJ, et al. Complex visual hallucinations in the visually impaired: the Charles Bonnet syndrome. Surv Ophthalmol. 2003;48(1):58-72.
2. Cox TM, Ffytche DH. Negative outcome Charles Bonnet syndrome. Br J Ophthalmol. 2014;98(9):1236-1239.
3. Pelak VS. Visual release hallucinations (Charles Bonnet syndrome). UpToDate. Updated February 5, 2019. Accessed September 17, 2020. https://www.uptodate.com/contents/visual-release-hallucinations-charles-bonnet-syndrome
4. Burke W. The neural basis of Charles Bonnet hallucinations: a hypothesis. J Neurol Neurosurg Psychiatry. 2002;73(5):535-541.
5. Scott IU, Schein OD, Feuer WJ, et al. Visual hallucinations in patients with retinal disease. Am J Ophthalmol. 2001;131(5):590-598.
6. Lepore FE. Spontaneous visual phenomena with visual loss: 104 patients with lesions of retinal and neural afferent pathways. Neurology. 1990;40(3 Pt 1):444-447.
7. Nesher R, Nesher G, Epstein E, et al. Charles Bonnet syndrome in glaucoma patients with low vision. J Glaucoma. 2001;10(5):396-400.
8. Pang L. Hallucinations experienced by visually impaired: Charles Bonnet syndrome. Optom Vis Sci. 2016;93(12):1466-1478.
9. Linszen M, Van Zanten G, Teunisse R, et al. Auditory hallucinations in adults with hearing impairment: a large prevalence study. Psychological Medicine. 2019;49(1):132-139.
10. Teunisse RJ, Cruysberg JR, Hoefnagels WH, et al. Social and psychological characteristics of elderly visually handicapped patients with the Charles Bonnet syndrome. Compr Psychiatry. 1999;40(4):315-319.
11. Eperjesi F, Akbarali A. Rehabilitation in Charles Bonnet syndrome: a review of treatment options. Clin Exp Optom. 2004;87(3):149-152.
12. Fernandez A, Lichtshein G, Vieweg WVR. The Charles Bonnet syndrome: a review. J Nen Ment Dis. 1997;185(3):195-200.
13. Rosenbaum F, Harati Y, Rolak L, et al. Visual hallucinations in sane people: Charles Bonnet syndrome. J Am Geriatr Soc. 1987;35(1):66-68.
14. Coletti Moja M, Milano E, Gasverde S, et al. Olanzapine therapy in hallucinatory visions related to Bonnet syndrome. Neurol Sci. 2005;26(3):168-170.
CASE Visual, auditory, and tactile hallucinations
Mr. B, age 93, is brought to the emergency department by his son after experiencing hallucinations where he reportedly saw and heard individuals in his home. In frustration, Mr. B wielded a knife because he “wanted them to go away.”
Mr. B and his son report that the hallucinations had begun 2 years ago, without prior trauma, medication changes, changes in social situation, or other apparent precipitating events. The hallucinations “come and go,” without preceding symptoms, but have recurring content involving a friendly man named “Harry,” people coming out of the television, 2 children playing, and water covering the floor. Mr. B acknowledges these are hallucinations and had not felt threatened by them until recently, when he wielded the knife. He often tries to talk to them, but they do not reply.
Mr. B also reports intermittent auditory hallucinations including voices at home (non-command) and papers rustling. He also describes tactile hallucinations, where he says he can feel Harry and others prodding him, knocking things out of his hands, or splashing him with water.
Mr. B is admitted to the hospital because he is a danger to himself and others. While on the inpatient unit, Mr. B is pleasant with staff, and eats and sleeps normally; however, he continues to have hallucinations of Harry. Mr. B reports seeing Harry in the hall, and says that Harry pulls out Mr. B’s earpiece and steals his fork. Mr. B also reports hearing a sound “like a bee buzzing.” Mr. B is started on risperidone, 1 mg nightly, for a presumed psychotic disorder.
HISTORY Independent and in good health
Mr. B lives alone and is independent in his activities of daily living. He spends his days at home, often visited by his children, who bring him groceries and other necessities.
Mr. B takes no medications, and has no history of psychiatric treatment; psychotic, manic, or depressive episodes; posttraumatic stress disorder; obsessive-compulsive disorder; or recent emotional stress. His medical history includes chronic progressive hearing loss, which is managed with hearing aids; macular degeneration; and prior bilateral cataract surgeries.
EVALUATION Mental status exam and objective findings
During his evaluation, Mr. B appears well-nourished, and wears glasses and hearing aids. During the interview, he is euthymic with appropriately reactive affect. He is talkative but redirectable, with a goal-directed thought process. Mr. B does not appear to be internally preoccupied. His hearing is impaired, and he often requires questions to be repeated loudly. He is oriented to person, place, and time. There are no signs of delusions, paranoia, thought blocking, thought broadcasting/insertion, or referential thinking. He denies depressed mood, anhedonia, fatigue, sleep changes, or manic symptoms. He denies the occurrence of auditory or visual hallucinations during the evaluation.
Continue to: A neurologic exam shows...
A neurologic exam shows impaired hearing bilaterally and impaired visual acuity. Even with glasses, both eyes have acuity only to finger counting. All other cranial nerves are normal, and Mr. B’s strength, sensation, and cerebellar function are all intact, without rigidity, numbness, or tingling. His gait is steady without a walker, with symmetric arm swing and slight dragging of his feet. His vitals are stable, with normal orthostatic pressures.
Other objective data include a score of 24/30 on the Mini-Mental State Examination, notable for deficits in visuospatial orientation, attention, and calculation, with language and copying limited by poor vision. Mr. B scores 16/22 on the Montreal Cognitive Assessment (MoCA)-Blind (adapted version of MoCA), which is equivalent to a 22/30 on the MoCA, indicating some mild cognitive impairment; however, this modified test is still limited by his poor hearing. His serum and urine laboratory workup show no liver, kidney, metabolic, or electrolyte abnormalities, no sign of infection, negative urine drug screen, and normal B12 and thyroid-stimulating hormone levels. He undergoes a brain MRI, which shows chronic microvascular ischemic change, without mass lesions, infarction, or other pathology.
[polldaddy:10729178]
The authors’ observations
Given Mr. B’s presentation, we ruled out a primary psychotic disorder. He had no psychiatric history, with organized thought, a reactive affect, and no delusions, paranoia, or other psychotic symptoms, all pointing against psychosis. His brain MRI showed no malignancy or other lesions. He had no substance use history to suggest intoxication/withdrawal. His intact attention and orientation did not suggest delirium, and his serum and urine studies were all negative. Although his blaming Harry for knocking things out of his hands could suggest confabulation, Mr. B had no other signs of Korsakoff syndrome, such as ataxia, general confusion, or malnourishment.
We also considered early dementia. There was suspicion for Lewy body dementia given Mr. B’s prominent fluctuating visual hallucinations; however, he displayed no other signs of the disorder, such as parkinsonism, dysautonomia, or sensitivity to the antipsychotic (risperidone 1 mg nightly) started on admission. The presence of 1 core feature of Lewy body dementia—visual hallucinations—indicated a possible, but not probable, diagnosis. Additionally, Mr. B did not have the characteristic features of other types of dementia, such as the stepwise progression of vascular dementia, the behavioral disinhibition of frontotemporal dementia, or the insidious forgetfulness, confusion, language problems, or paranoia that may appear in Alzheimer’s disease. Remarkably, he had a relatively normal brain MRI for his age, given chronic microvascular ischemic changes, and cognitive testing that indicated only mild impairment further pointed against a dementia process.
Charles Bonnet syndrome
Based on Mr. B’s severe vision loss and history of ocular surgeries, we diagnosed him with CBS, described as visual hallucinations in the presence of impaired vision. Charles Bonnet syndrome has been observed in several disorders that affect vision, most commonly macular degeneration, diabetic retinopathy, and glaucoma, with an estimated prevalence of 11% to 39% in older patients with ocular disease.1,2 Visual hallucinations in CBS occur due to ocular disease, likely resulting from changes in afferent sensory input to visual cortical regions of the brain. Table 13 outlines the features of visual hallucinations in patients with CBS. The subsequent disinhibition and spontaneous firing of the visual association cortices leads to the “release hallucinations” of the syndrome.4 The disorder is thought to be significantly underdiagnosed—in a survey of patients with CBS, only 15% had reported their visual hallucinations to a physician.5

Continue to: Mr. B's symptoms...
Mr. B’s symptoms are atypical for CBS, but they fit the diagnosis when considering the entire clinical picture. While hallucinations in CBS are more often simple shapes, complex hallucinations including people and scenes have been noted in several instances.6
Similar to Mr. B’s case, patients with CBS can have recurring figures in their hallucinations, and the images may even move across the visual field.1 Patients with CBS also frequently recognize that their hallucinations are not real, and may or may not be distressed by them.4 Patients with CBS often have hallucinations multiple times daily, lasting from a few seconds to many minutes,7 consistent with Mr. B’s temporary symptoms.
Although auditory and tactile hallucinations are typically not included in CBS, they can also be explained by Mr. B’s significant sensory impairment. Severe hearing impairment in geriatric adults has been associated with auditory hallucinations8; in 1 survey, half of these hallucinations consisted of voices.9 In contrast, tactile hallucinations are not described in sensory deprivation literature. However, in the context of Mr. B’s severe comorbid hearing and vision loss, we propose that these hallucinations reflect his interpretation of sensory events around him, and their integration into his extensive hallucination framework. In other words, Harry poking him and causing him to drop things may be Mr. B’s way of rationalizing events that he has trouble perceiving entirely, or his mild forgetfulness. Mr. B’s social isolation is another factor that may worsen his sensory deprivation and contribute to his extensive hallucinations.10 Additionally, his mild cognitive deficits on testing with chronic microvascular changes on the MRI may suggest a mild vascular-related dementia process, which could also exacerbate his hallucinations. While classic CBS occurs without cognitive impairment, dementia can often co-occur with CBS.11
TREATMENT No significant improvement with medications
During his inpatient stay, Mr. B is treated with risperidone, 1 mg nightly, and is also started on donepezil, 5 mg/d, to treat a possible comorbid dementia. However, he continues to hallucinate without significant improvement.
[polldaddy:10729181]
The authors’ observations
There is no definitive treatment for CBS, and while the hallucinations may spontaneously resolve, per case reports, this typically occurs only as visual loss progresses to total blindness.12 However, many patients can have the hallucinations remit after the underlying ocular etiology is corrected, such as through ocular surgery.13 Other optical interventions, such as special glasses or contact lenses, may help maximize remaining vision.8 In patients without this option, such as Mr. B, there are limited data on beneficial medications for CBS.
Continue to: Evidence for treatment of CBS...
Evidence for treatment of CBS with antipsychotic medications is mixed. Some case studies have found them to be ineffective, while others have found agents such as olanzapine or risperidone to be partially helpful in reducing symptoms.14 There are also data from case reports that may support the use of cholinesterase inhibitors such as donepezil, antiepileptics (carbamazepine, valproate, gabapentin, and clonazepam), and certain antidepressants (escitalopram, venlafaxine) (Table 28,11).3
Addressing loneliness and social isolation
With minimal definitive evidence for pharmacologic management, the most important intervention for treating CBS may be changing the patient’s sensory environment. Specifically, loneliness and social isolation are major exacerbating factors of CBS, and many clinicians advocate for the consistent presence of a sympathetic professional. Reassurance that hallucinations are from ocular disease rather than a primary mental disorder may be extremely relieving for patients.11 A psychoeducation or support group may also be beneficial, not only for giving patients more social contact, but also for teaching them coping skills or strategies to reduce hallucinations, such as distraction, turning on more lights, or even certain eye/blinking movements.11 Table 28,11 (page 49) outlines behavioral interventions for CBS.
Regardless of etiology, Mr. B’s hallucinations significantly affected his quality of life. During his inpatient stay, he was treated with
OUTCOME Home care and family involvement
After discussion with Mr. B and his family about the risks and benefits of medication, the risperidone and donepezil are discontinued. Ultimately, it is determined that Mr. B requires a higher level of home care, both for his safety and to improve his social contact. Mr. B returns home with a combination of a professional home health aide and increased family involvement.
Bottom Line
When evaluating visual hallucinations in older adults, Charles Bonnet syndrome (CBS) should be considered. Sensory deprivation and social isolation are significant risk factors for CBS. While evidence is inconclusive for medical treatment, reassurance and behavioral interventions can often improve symptoms.
Continue to: Related Resources
Related Resources
- Charles Bonnet Syndrome Foundation. http://www.charlesbonnetsyndrome.org
- Schultz G, Melzack R. The Charles Bonnet syndrome: ‘phantom visual images’. Perception. 1991;20:809-825.
- Menon GJ, Rahman I, Menon SJ, et al. Complex visual hallucinations in the visually impaired: the Charles Bonnet syndrome. Surv Ophthalmol. 2003;48(1):58-72.
Drug Brand Names
Carbamazepine • Tegretol
Clonazepam • Klonopin
Donepezil • Aricept
Escitalopram • Lexapro
Gabapentin • Neurontin
Olanzapine • Zyprexa
Risperidone • Risperdal
Valproate • Depakote
Venlafaxine • Effexor
CASE Visual, auditory, and tactile hallucinations
Mr. B, age 93, is brought to the emergency department by his son after experiencing hallucinations where he reportedly saw and heard individuals in his home. In frustration, Mr. B wielded a knife because he “wanted them to go away.”
Mr. B and his son report that the hallucinations had begun 2 years ago, without prior trauma, medication changes, changes in social situation, or other apparent precipitating events. The hallucinations “come and go,” without preceding symptoms, but have recurring content involving a friendly man named “Harry,” people coming out of the television, 2 children playing, and water covering the floor. Mr. B acknowledges these are hallucinations and had not felt threatened by them until recently, when he wielded the knife. He often tries to talk to them, but they do not reply.
Mr. B also reports intermittent auditory hallucinations including voices at home (non-command) and papers rustling. He also describes tactile hallucinations, where he says he can feel Harry and others prodding him, knocking things out of his hands, or splashing him with water.
Mr. B is admitted to the hospital because he is a danger to himself and others. While on the inpatient unit, Mr. B is pleasant with staff, and eats and sleeps normally; however, he continues to have hallucinations of Harry. Mr. B reports seeing Harry in the hall, and says that Harry pulls out Mr. B’s earpiece and steals his fork. Mr. B also reports hearing a sound “like a bee buzzing.” Mr. B is started on risperidone, 1 mg nightly, for a presumed psychotic disorder.
HISTORY Independent and in good health
Mr. B lives alone and is independent in his activities of daily living. He spends his days at home, often visited by his children, who bring him groceries and other necessities.
Mr. B takes no medications, and has no history of psychiatric treatment; psychotic, manic, or depressive episodes; posttraumatic stress disorder; obsessive-compulsive disorder; or recent emotional stress. His medical history includes chronic progressive hearing loss, which is managed with hearing aids; macular degeneration; and prior bilateral cataract surgeries.
EVALUATION Mental status exam and objective findings
During his evaluation, Mr. B appears well-nourished, and wears glasses and hearing aids. During the interview, he is euthymic with appropriately reactive affect. He is talkative but redirectable, with a goal-directed thought process. Mr. B does not appear to be internally preoccupied. His hearing is impaired, and he often requires questions to be repeated loudly. He is oriented to person, place, and time. There are no signs of delusions, paranoia, thought blocking, thought broadcasting/insertion, or referential thinking. He denies depressed mood, anhedonia, fatigue, sleep changes, or manic symptoms. He denies the occurrence of auditory or visual hallucinations during the evaluation.
Continue to: A neurologic exam shows...
A neurologic exam shows impaired hearing bilaterally and impaired visual acuity. Even with glasses, both eyes have acuity only to finger counting. All other cranial nerves are normal, and Mr. B’s strength, sensation, and cerebellar function are all intact, without rigidity, numbness, or tingling. His gait is steady without a walker, with symmetric arm swing and slight dragging of his feet. His vitals are stable, with normal orthostatic pressures.
Other objective data include a score of 24/30 on the Mini-Mental State Examination, notable for deficits in visuospatial orientation, attention, and calculation, with language and copying limited by poor vision. Mr. B scores 16/22 on the Montreal Cognitive Assessment (MoCA)-Blind (adapted version of MoCA), which is equivalent to a 22/30 on the MoCA, indicating some mild cognitive impairment; however, this modified test is still limited by his poor hearing. His serum and urine laboratory workup show no liver, kidney, metabolic, or electrolyte abnormalities, no sign of infection, negative urine drug screen, and normal B12 and thyroid-stimulating hormone levels. He undergoes a brain MRI, which shows chronic microvascular ischemic change, without mass lesions, infarction, or other pathology.
[polldaddy:10729178]
The authors’ observations
Given Mr. B’s presentation, we ruled out a primary psychotic disorder. He had no psychiatric history, with organized thought, a reactive affect, and no delusions, paranoia, or other psychotic symptoms, all pointing against psychosis. His brain MRI showed no malignancy or other lesions. He had no substance use history to suggest intoxication/withdrawal. His intact attention and orientation did not suggest delirium, and his serum and urine studies were all negative. Although his blaming Harry for knocking things out of his hands could suggest confabulation, Mr. B had no other signs of Korsakoff syndrome, such as ataxia, general confusion, or malnourishment.
We also considered early dementia. There was suspicion for Lewy body dementia given Mr. B’s prominent fluctuating visual hallucinations; however, he displayed no other signs of the disorder, such as parkinsonism, dysautonomia, or sensitivity to the antipsychotic (risperidone 1 mg nightly) started on admission. The presence of 1 core feature of Lewy body dementia—visual hallucinations—indicated a possible, but not probable, diagnosis. Additionally, Mr. B did not have the characteristic features of other types of dementia, such as the stepwise progression of vascular dementia, the behavioral disinhibition of frontotemporal dementia, or the insidious forgetfulness, confusion, language problems, or paranoia that may appear in Alzheimer’s disease. Remarkably, he had a relatively normal brain MRI for his age, given chronic microvascular ischemic changes, and cognitive testing that indicated only mild impairment further pointed against a dementia process.
Charles Bonnet syndrome
Based on Mr. B’s severe vision loss and history of ocular surgeries, we diagnosed him with CBS, described as visual hallucinations in the presence of impaired vision. Charles Bonnet syndrome has been observed in several disorders that affect vision, most commonly macular degeneration, diabetic retinopathy, and glaucoma, with an estimated prevalence of 11% to 39% in older patients with ocular disease.1,2 Visual hallucinations in CBS occur due to ocular disease, likely resulting from changes in afferent sensory input to visual cortical regions of the brain. Table 13 outlines the features of visual hallucinations in patients with CBS. The subsequent disinhibition and spontaneous firing of the visual association cortices leads to the “release hallucinations” of the syndrome.4 The disorder is thought to be significantly underdiagnosed—in a survey of patients with CBS, only 15% had reported their visual hallucinations to a physician.5

Continue to: Mr. B's symptoms...
Mr. B’s symptoms are atypical for CBS, but they fit the diagnosis when considering the entire clinical picture. While hallucinations in CBS are more often simple shapes, complex hallucinations including people and scenes have been noted in several instances.6
Similar to Mr. B’s case, patients with CBS can have recurring figures in their hallucinations, and the images may even move across the visual field.1 Patients with CBS also frequently recognize that their hallucinations are not real, and may or may not be distressed by them.4 Patients with CBS often have hallucinations multiple times daily, lasting from a few seconds to many minutes,7 consistent with Mr. B’s temporary symptoms.
Although auditory and tactile hallucinations are typically not included in CBS, they can also be explained by Mr. B’s significant sensory impairment. Severe hearing impairment in geriatric adults has been associated with auditory hallucinations8; in 1 survey, half of these hallucinations consisted of voices.9 In contrast, tactile hallucinations are not described in sensory deprivation literature. However, in the context of Mr. B’s severe comorbid hearing and vision loss, we propose that these hallucinations reflect his interpretation of sensory events around him, and their integration into his extensive hallucination framework. In other words, Harry poking him and causing him to drop things may be Mr. B’s way of rationalizing events that he has trouble perceiving entirely, or his mild forgetfulness. Mr. B’s social isolation is another factor that may worsen his sensory deprivation and contribute to his extensive hallucinations.10 Additionally, his mild cognitive deficits on testing with chronic microvascular changes on the MRI may suggest a mild vascular-related dementia process, which could also exacerbate his hallucinations. While classic CBS occurs without cognitive impairment, dementia can often co-occur with CBS.11
TREATMENT No significant improvement with medications
During his inpatient stay, Mr. B is treated with risperidone, 1 mg nightly, and is also started on donepezil, 5 mg/d, to treat a possible comorbid dementia. However, he continues to hallucinate without significant improvement.
[polldaddy:10729181]
The authors’ observations
There is no definitive treatment for CBS, and while the hallucinations may spontaneously resolve, per case reports, this typically occurs only as visual loss progresses to total blindness.12 However, many patients can have the hallucinations remit after the underlying ocular etiology is corrected, such as through ocular surgery.13 Other optical interventions, such as special glasses or contact lenses, may help maximize remaining vision.8 In patients without this option, such as Mr. B, there are limited data on beneficial medications for CBS.
Continue to: Evidence for treatment of CBS...
Evidence for treatment of CBS with antipsychotic medications is mixed. Some case studies have found them to be ineffective, while others have found agents such as olanzapine or risperidone to be partially helpful in reducing symptoms.14 There are also data from case reports that may support the use of cholinesterase inhibitors such as donepezil, antiepileptics (carbamazepine, valproate, gabapentin, and clonazepam), and certain antidepressants (escitalopram, venlafaxine) (Table 28,11).3
Addressing loneliness and social isolation
With minimal definitive evidence for pharmacologic management, the most important intervention for treating CBS may be changing the patient’s sensory environment. Specifically, loneliness and social isolation are major exacerbating factors of CBS, and many clinicians advocate for the consistent presence of a sympathetic professional. Reassurance that hallucinations are from ocular disease rather than a primary mental disorder may be extremely relieving for patients.11 A psychoeducation or support group may also be beneficial, not only for giving patients more social contact, but also for teaching them coping skills or strategies to reduce hallucinations, such as distraction, turning on more lights, or even certain eye/blinking movements.11 Table 28,11 (page 49) outlines behavioral interventions for CBS.
Regardless of etiology, Mr. B’s hallucinations significantly affected his quality of life. During his inpatient stay, he was treated with
OUTCOME Home care and family involvement
After discussion with Mr. B and his family about the risks and benefits of medication, the risperidone and donepezil are discontinued. Ultimately, it is determined that Mr. B requires a higher level of home care, both for his safety and to improve his social contact. Mr. B returns home with a combination of a professional home health aide and increased family involvement.
Bottom Line
When evaluating visual hallucinations in older adults, Charles Bonnet syndrome (CBS) should be considered. Sensory deprivation and social isolation are significant risk factors for CBS. While evidence is inconclusive for medical treatment, reassurance and behavioral interventions can often improve symptoms.
Continue to: Related Resources
Related Resources
- Charles Bonnet Syndrome Foundation. http://www.charlesbonnetsyndrome.org
- Schultz G, Melzack R. The Charles Bonnet syndrome: ‘phantom visual images’. Perception. 1991;20:809-825.
- Menon GJ, Rahman I, Menon SJ, et al. Complex visual hallucinations in the visually impaired: the Charles Bonnet syndrome. Surv Ophthalmol. 2003;48(1):58-72.
Drug Brand Names
Carbamazepine • Tegretol
Clonazepam • Klonopin
Donepezil • Aricept
Escitalopram • Lexapro
Gabapentin • Neurontin
Olanzapine • Zyprexa
Risperidone • Risperdal
Valproate • Depakote
Venlafaxine • Effexor
1. Menon GJ, Rahman I, Menon SJ, et al. Complex visual hallucinations in the visually impaired: the Charles Bonnet syndrome. Surv Ophthalmol. 2003;48(1):58-72.
2. Cox TM, Ffytche DH. Negative outcome Charles Bonnet syndrome. Br J Ophthalmol. 2014;98(9):1236-1239.
3. Pelak VS. Visual release hallucinations (Charles Bonnet syndrome). UpToDate. Updated February 5, 2019. Accessed September 17, 2020. https://www.uptodate.com/contents/visual-release-hallucinations-charles-bonnet-syndrome
4. Burke W. The neural basis of Charles Bonnet hallucinations: a hypothesis. J Neurol Neurosurg Psychiatry. 2002;73(5):535-541.
5. Scott IU, Schein OD, Feuer WJ, et al. Visual hallucinations in patients with retinal disease. Am J Ophthalmol. 2001;131(5):590-598.
6. Lepore FE. Spontaneous visual phenomena with visual loss: 104 patients with lesions of retinal and neural afferent pathways. Neurology. 1990;40(3 Pt 1):444-447.
7. Nesher R, Nesher G, Epstein E, et al. Charles Bonnet syndrome in glaucoma patients with low vision. J Glaucoma. 2001;10(5):396-400.
8. Pang L. Hallucinations experienced by visually impaired: Charles Bonnet syndrome. Optom Vis Sci. 2016;93(12):1466-1478.
9. Linszen M, Van Zanten G, Teunisse R, et al. Auditory hallucinations in adults with hearing impairment: a large prevalence study. Psychological Medicine. 2019;49(1):132-139.
10. Teunisse RJ, Cruysberg JR, Hoefnagels WH, et al. Social and psychological characteristics of elderly visually handicapped patients with the Charles Bonnet syndrome. Compr Psychiatry. 1999;40(4):315-319.
11. Eperjesi F, Akbarali A. Rehabilitation in Charles Bonnet syndrome: a review of treatment options. Clin Exp Optom. 2004;87(3):149-152.
12. Fernandez A, Lichtshein G, Vieweg WVR. The Charles Bonnet syndrome: a review. J Nen Ment Dis. 1997;185(3):195-200.
13. Rosenbaum F, Harati Y, Rolak L, et al. Visual hallucinations in sane people: Charles Bonnet syndrome. J Am Geriatr Soc. 1987;35(1):66-68.
14. Coletti Moja M, Milano E, Gasverde S, et al. Olanzapine therapy in hallucinatory visions related to Bonnet syndrome. Neurol Sci. 2005;26(3):168-170.
1. Menon GJ, Rahman I, Menon SJ, et al. Complex visual hallucinations in the visually impaired: the Charles Bonnet syndrome. Surv Ophthalmol. 2003;48(1):58-72.
2. Cox TM, Ffytche DH. Negative outcome Charles Bonnet syndrome. Br J Ophthalmol. 2014;98(9):1236-1239.
3. Pelak VS. Visual release hallucinations (Charles Bonnet syndrome). UpToDate. Updated February 5, 2019. Accessed September 17, 2020. https://www.uptodate.com/contents/visual-release-hallucinations-charles-bonnet-syndrome
4. Burke W. The neural basis of Charles Bonnet hallucinations: a hypothesis. J Neurol Neurosurg Psychiatry. 2002;73(5):535-541.
5. Scott IU, Schein OD, Feuer WJ, et al. Visual hallucinations in patients with retinal disease. Am J Ophthalmol. 2001;131(5):590-598.
6. Lepore FE. Spontaneous visual phenomena with visual loss: 104 patients with lesions of retinal and neural afferent pathways. Neurology. 1990;40(3 Pt 1):444-447.
7. Nesher R, Nesher G, Epstein E, et al. Charles Bonnet syndrome in glaucoma patients with low vision. J Glaucoma. 2001;10(5):396-400.
8. Pang L. Hallucinations experienced by visually impaired: Charles Bonnet syndrome. Optom Vis Sci. 2016;93(12):1466-1478.
9. Linszen M, Van Zanten G, Teunisse R, et al. Auditory hallucinations in adults with hearing impairment: a large prevalence study. Psychological Medicine. 2019;49(1):132-139.
10. Teunisse RJ, Cruysberg JR, Hoefnagels WH, et al. Social and psychological characteristics of elderly visually handicapped patients with the Charles Bonnet syndrome. Compr Psychiatry. 1999;40(4):315-319.
11. Eperjesi F, Akbarali A. Rehabilitation in Charles Bonnet syndrome: a review of treatment options. Clin Exp Optom. 2004;87(3):149-152.
12. Fernandez A, Lichtshein G, Vieweg WVR. The Charles Bonnet syndrome: a review. J Nen Ment Dis. 1997;185(3):195-200.
13. Rosenbaum F, Harati Y, Rolak L, et al. Visual hallucinations in sane people: Charles Bonnet syndrome. J Am Geriatr Soc. 1987;35(1):66-68.
14. Coletti Moja M, Milano E, Gasverde S, et al. Olanzapine therapy in hallucinatory visions related to Bonnet syndrome. Neurol Sci. 2005;26(3):168-170.
Threatening to burn the house down
CASE Agitated and aggressive
Mr. X, age 61, who has Alzheimer’s disease, is brought to the emergency department (ED) by his family after he is found to be confused, becomes physically aggressive with family members, and threatens to burn the house down. His family reports that earlier that day, he was paranoid that somebody was trying to kill him and he tried to leave the house. Mr. X has been experiencing visual hallucinations and delusional thoughts that made him aggressive towards his son. After an initial laboratory workup in the ED, Mr. X’s bloodwork comes back positive for mild leukocytosis, indicating the possibility of an infectious etiology. Mr. X is admitted to the hospital for further evaluation of his altered mental status.
HISTORY Decline over 2 years
This is Mr. X’s third inpatient admission for agitation and psychosis. His current medications—twice daily divalproex sodium extended release (ER), 250 mg every morning and 500 mg at every bedtime, and prazosin, 2 mg/d at bedtime—have been only partially effective. His medical history includes osteoarthritis, back pain, and heterozygous factor V Leiden (not on anticoagulation). He quit smoking tobacco several years ago and has no history of substance use. He has no family history of dementia. Previous trials of cholinesterase inhibitors, antipsychotics, and antidepressants resulted in only minimal improvement in his agitation and psychosis.
A chart review shows that 2 years before his current hospital admission, Mr. X had presented to his primary care physician with slurred speech, forgetfulness, missing words, and transient reading difficulties. His initial laboratory workup and MRI came back normal. He was placed on short-term disability due to work-related errors. He was referred to the hospital’s Memory Clinic 2 years ago, where his Mini-Mental State Exam score was 20/30, indicating mild cognitive impairment. Stroke workup was negative. Due to significant language deficits, a differential diagnosis for Alzheimer’s disease vs primary progressive aphasia vs frontotemporal dementia was made. He screened positive for amyloid PET scan, which confirmed the diagnosis of Alzheimer’s disease.
Neuropsychological testing showed similarities with logopenic variant of primary progressive aphasia, which in many cases is present in Alzheimer’s disease. Mr. X was prescribed anticholinesterase inhibitors, including donepezil, 10 mg/d, and rivastigmine patch, 9.5 mg/d; and memantine, 10 mg/d, which he could not tolerate because of adverse effects. During the next year, Mr. X deteriorated and presented to the ED a few times with significant psychotic symptoms and aggression. He had a poor response to various pharmacologic and nonpharmacologic interventions during this time.
EVALUATION Continued problematic behaviors
During his hospitalization, Mr. X continues to be agitated and paranoid and is placed in restraints. He is unable to respond to his name and cannot follow simple verbal commands. Results of his laboratory workup are within normal limits. His mild leukocytosis resolves with no active signs of infection. Psychiatry is consulted for management of his behavioral and psychological symptoms of dementia (BPSD).
Continue to: Mr. X is started on olanzapine...
Mr. X is started on olanzapine and lorazepam as needed for agitation, and his twice daily divalproex sodium ER is increased to 250 every morning and 750 mg at every bedtime. However, Mr. X remains agitated and requires restraints. Olanzapine is switched from an as-needed dose to scheduled doses of 10 mg every morning and 15 mg at every bedtime, to address his psychosis and agitation.
On Day 24 of hospitalization, Mr. X’s ammonia levels are checked and are found to be 69 µ/dL, which is high (normal range: 15 to 45 µ/dL). Divalproex sodium ER is eventually tapered and discontinued. Mr. X is started on carbamazepine, which is titrated to 400 mg twice daily and results in some improvement in his behavior. He continues to receive carbamazepine and is started on dextromethorphan-quinidine, 10 mg/d, and increased to 10 mg twice daily; however, Mr. X continues to be verbally aggressive with staff, throws food, wanders around, and tries to leave the hospital unit, so he is placed in restraints and continues to require a sitter.
[polldaddy:10698428]
The authors' observations
Dementia typically affects older adults, but its onset can occur before age 60. It is a syndrome rather than a specific illness; the most common types are Alzheimer’s disease, vascular dementia, dementia with Lewy bodies, and frontotemporal dementia. Diagnostic clarity and an evidence-based treatment plan are crucial for improving the quality of life for both the patient and their caregivers. The Table outlines the differential diagnosis of cognitive deficits. New-onset cognitive deficits warrant neuroimaging, and other testing may also be needed.
Behavioral and psychological symptoms of dementia
Noncognitive symptoms occur in 98% of individuals with dementia at some point in their disease and are often the most distressing to both caregivers and patients.1 Behavioral and psychological symptoms of dementia, including apathy, depression, sleep disorders, hallucinations, delusions, psychosis, agitation, and aggression, are exceedingly prevalent.2 Although these symptoms pose a significant burden, there are no clear published treatment guidelines; however, the American Psychiatric Association and the American Geriatric Society recommend using nonpharmacologic approaches as the first-line of treatment for patients with BPSD.3,4
Nonpharmacologic treatments
Due to the unfavorable adverse effects profiles of medications commonly used to treat dementia, nonpharmacologic treatment approaches have always played a crucial role for managing BPSD. Interventions such as music therapy, aromatherapy, art therapy, behavioral therapy, reality orientation, tailored activities, and physical exercises, have shown promising results for alleviating BPSD.5-7
Continue to: Pharmacologic therapies should be used...
Pharmacologic treatments
Pharmacologic therapies should be used when nonpharmacologic approaches are unsuccessful, or when a patient is at imminent risk to harm themselves or others.
Antipsychotics. Although there is conflicting data regarding the use of antipsychotics in older adults, these agents are the most common pharmacologic treatment for patients with BPSD. Several studies examining the efficacy of antipsychotics for treating BPSD have demonstrated an increased risk of cerebrovascular events, including stroke and death due to any cause.8 While the use of antipsychotics increases the risk of mortality in older adults, the absolute risk is still low.9
Antipsychotics used to treat BPSD include:
- Risperidone is well studied in older adults and has shown benefit for treating aggression, agitation, and psychosis.10
- Quetiapine has a favorable adverse effects profile and may help improve sleep and reduce anxiety.10
- Olanzapine. Low-dose olanzapine has been modestly effective in decreasing agitation and aggression in patients with Alzheimer’s and vascular dementias.11
- Aripiprazole has shown modest benefit in treating psychosis and agitation in patients with dementia but may be associated with insomnia or activation symptoms at lower doses.10
- Ziprasidone. Case reports have found benefit with oral and injectable forms.12
Antidepressants. In the CitAD study, which was a placebo-controlled randomized trial, citalopram titrated to a target of 30 mg/d was found to be effective in reducing BPSD.13 However, QTc prolongation limits the use of citalopram. Sertraline was studied in 1 small, randomized trial against haloperidol but showed no additional benefit.14
Mood stabilizers. In a small, randomized trial, carbamazepine was helpful for patients with BPSD who were resistant to treatment with antipsychotics, with efficacy demonstrated over 6 weeks.15 No other mood stabilizers have had significant positive results in treating BPSD.16
Anxiolytic medications. Some research suggests that the occasional use of lorazepam, as necessary, is acceptable for patients with extreme agitation or aggression when behavioral interventions or sleep aids are ineffective.17 Various case reports and case series have suggested gabapentin may be effective for BPSD.18
Prazosin. In a small randomized placebo-controlled trial, the commonly used antihypertensive agent prazosin reduced agitation and aggression in patients with Alzheimer’s dementia, at doses from 1 to 6 mg/d.19 Postural hypotension, the main adverse effect associated with prazosin, can limit its use.
Trazodone. Some research suggests trazodone can reduce irritability and aggression in patients with Alzheimer’s disease.20
Dextromethorphan/quinidine. In a 10-week phase 2 randomized clinical trial of patients with probable Alzheimer’s disease dementia, combination dextromethorphan/quinidine reduced agitation and was generally well tolerated.21
For patients such as Mr. X who do not respond to multiple pharmacologic treatments, electroconvulsive therapy (ECT) may be an option.
Continue to: Because Mr. X does not respond...
TREATMENT A trial of ECT
Because Mr. X does not respond to the standard treatment protocols, the treatment team and Mr. X’s family discuss the use of ECT to control his agitation. Consent is obtained from his legal guardian and Mr. X is medically cleared to receive ECT. Mr. X receives 3 ECT treatments per week. During the first week, Mr. X experiences post-treatment agitation and confusion. The frequency of ECT treatments is reduced to 2 treatments per week, and then 1 session per week. Mr. X starts to show improvement in his agitation and ECT is continued at 1 session per week for 7 weeks.
The authors’ observations
Electroconvulsive therapy has been an effective treatment for patients with treatment-resistant depression and has shown benefit in treating other psychiatric conditions such as acute mania, catatonia, psychotic disorders, and Parkinson’s disease.22 Its use as an off-label treatment for chronic neuropathic pain has also been well documented.23 Although ECT is not indicated for treating agitation and aggression in patients with dementia, its effectiveness for these symptoms has been discussed extensively in the literature.22,24-26
Electroconvulsive therapy treatment can be divided into 2 phases: an acute phase during which ECT is administered 2 to 3 times a week for 4 to 5 weeks, and a maintenance phase of weekly treatments for 4 weeks and then biweekly treatments for 8 weeks.26 Although extensive research supports the safe use of ECT in older adults, concerns for worsening cognitive impairment can deter patients and families from agreeing to this treatment.
Adverse effects of ECT such as headaches and postictal confusion are generally mild and transient. Severe adverse effects such as seizures, severe confusion, and delirium are uncommon.25 The number of ECT treatments required for a good effect ranges from 2 to 18, and the most common position for electrodes placement is bilateral. Outcomes can be measured by using rating scales such as the Cohen-Mansfield Agitation Inventory, Neuropsychiatric Inventory, Social Dysfunction and Aggression Scale, Clinical Global Impression scale, and Pittsford Agitation Scale.25 Obtaining consent from patients with dementia is generally not possible because these patients generally lack the capacity to make medical decisions. Clinicians should refer to their state laws regarding medical-decision making in such cases. The patient’s next of kin or medical power of attorney should be contacted, and the risks and benefits should be discussed before starting ECT.
OUTCOME Lasting improvement
Due to Mr. X’s improvement after ECT, on hospital Day 124, the restraints are removed and he no longer requires a sitter. He starts responding to his name and following simple verbal commands. Electroconvulsive therapy is tapered to every other week, and eventually stopped as his status improves. Mr. X continues to do well and is maintained on the same dosages of olanzapine, carbamazepine, and dextromethorphan-quinidine he had been receiving prior to discharge.
Related Resources
• Van den Berg JF, Kruithof HC, Kok RM, et al. Electroconvulsive therapy for agitation and aggression in dementia: a systematic review. Am J Geriatr Psychiatry. 2018;26(4):419-434.
• Kales HC, Mulsant BH, Sajatovic M. Prescribing antipsychotics in geriatric patients: Focus on dementia. Current Psychiatry. 2017;16(12):24-30.
Drug Brand Names
Aripiprazole • Abilify
Carbamazepine • Tegretol
Citalopram • Celexa
Dextromethorphan- quinidine • Nuedexta
Divalproex sodium ER • Depakote
Donepezil • Aricept
Gabapentin • Neurontin
Haloperidol • Haldol
Lorazepam • Ativan
Memantine • Namenda
Olanzapine • Zyprexa
Prazosin • Minipress
Quetiapine • Seroquel
Risperidone • Risperdal
Rivastigmine • Exelon
Sertraline • Zoloft
Trazodone • Desyrel, Oleptro
Ziprasidone • Geodon
1. Kales HC, Gitlin LN, Lyketsos CG. Management of neuropsychiatric symptoms of dementia in clinical settings: recommendations from a multidisciplinary expert panel. J Am Geriatr Soc. 2014;62(4):762-769.
2. Scarmeas N, Brandt J, Albert M, et al. Delusions and hallucinations are associated with worse outcome in Alzheimer disease. Arch Neurol. 2005;62(10):1601-1608.
3. Reus VI, Fochtmann LJ, Eyler AE, et al. The American Psychiatric Association Practice Guideline on the use of antipsychotics to treat agitation or psychosis in patients with dementia. Am J Psychiatry. 2016;173(5):543-546.
4. AGS Executive Committee. A guide to the management of psychotic disorders and neuropsychiatric symptoms of dementia in older adults. The American Geriatrics Society. Published April 2011. Accessed September 24, 2020. https://qioprogram.org/sites/default/files/AGS_Guidelines_for_Telligen.pdf
5. Yang MH, Lin LC, Wu SC, et al. Comparison of the efficacy of aroma-acupressure and aromatherapy for the treatment of dementia-associated agitation. BMC Complement Altern Med. 2015;15:93.
6. Cerga-Pashoja A, Lowery D, Bhattacharya R, et al. Evaluation of exercise on individuals with dementia and their carers: a randomised controlled trial. Trials. 2010;11:53.
7. Chen RC, Liu CL, Lin MH, et al. Non-pharmacological treatment reducing not only behavioral symptoms, but also psychotic symptoms of older adults with dementia: a prospective cohort study in Taiwan. Geriatr Gerontol Int. 2014;14(2):440-446.
8. Schneider LS, Dagerman KS, Insel P. Risk of death with atypical antipsychotic drug treatment for dementia: meta-analysis of randomized placebo-controlled trials. JAMA. 2005;294(15):1934-1943.
9. Lenzer J. FDA warns about using antipsychotic drugs for dementia. BMJ. 2005;330(7497):922.
10. Burke AD, Tariot PN. Atypical antipsychotics in the elderly: a review of therapeutic trends and clinical outcomes. Expert Opin Pharmacother. 2009;10(15):2407-2414.
11. Moretti R, Torre R, Antonello T, et al. Olanzapine as a possible treatment of behavioral symptoms in vascular dementia: risks of cerebrovascular events. J Neurol. 2005;252(10):1186-1193.
12. Cole SA, Saleem R, Shea WP, et al. Ziprasidone for agitation or psychosis in dementia: four cases. Int J Psychiatry Med. 2005;35(1):91-98.
13. Porsteinsson AP, Drye LT, Pollock BG, et al. Effect of citalopram on agitation in Alzheimer disease: the CitAD randomized clinical trial. JAMA. 2014;311(7):682-691.
14. Gaber S, Ronzoli S, Bruno A, et al. Sertraline versus small doses of haloperidol in the treatment of agitated behavior in patients with dementia. Arch Gerontol Geriatr Suppl. 2001; 7:159-162.
15. Olin JT, Fox LS, Pawluczyk S, et al. A pilot randomized trial of carbamazepine for behavioral symptoms in treatment-resistant outpatients with Alzheimer disease. Am J Geriatr Psychiatry. 2001;9(4):400-405.
16. Konovalov S, Muralee S, Tampi RR. Anticonvulsants for the treatment of behavioral and psychological symptoms of dementia: a literature review. Int Psychogeriatr. 2008;20(2):293-308.
17. Davies SJC, Burhan AM, Kim D. Sequential drug treatment algorithm for agitation and aggression in Alzheimer’s and mixed dementia. J Psychopharmacol. 2018;32(5):509-523.
18. Kim Y, Wilkins KM, Tampi RR. Use of gabapentin in the treatment of behavioural and psychological symptoms of dementia: a review of the evidence. Drugs Aging. 2008;25(3):187-196.
19. Wang LY, Shofer JB, Rohde K, et al. Prazosin for the treatment of behavioral symptoms in patients with Alzheimer disease with agitation and aggression. Am J Geriatr Psychiatry. 2009; 17(9):744-751.
20. López-Pousa S, Garre-Olmo J, Vilalta-Franch J, et al. Trazodone for Alzheimer’s disease: a naturalistic follow-up study. Arch Gerontol Geriatr. 2008;47(2):207-215.
21. Cummings JL, Lyketsos CG, Peskind ER. Effect of dextromethorphan-quinidine on agitation in patients with Alzheimer disease dementia: a randomized clinical trial. JAMA. 2015;314(12):1242-1254.
22. Ujkaj M, Davidoff DA, Seiner SJ, et al. Safety and efficacy of electroconvulsive therapy for the treatment of agitation and aggression in patients with dementia. Am J Geriatr Psychiatry. 2012;20(1):61-72.
23. McDaniel WW. Electroconvulsive therapy in complex regional pain syndromes. J ECT. 2003;19(4):226-229.
24. Glass OM, Forester BP, Hermida AP. Electroconvulsive therapy (ECT) for treating agitation in dementia (major neurocognitive disorder)–a promising option. Int Psychogeriatr. 2017;29(5):717-726.
25. Van den Berg JF, Kruithof HC, Kok RM, et al. Electroconvulsive therapy for agitation and aggression in dementia: a systematic review. Am J Geriatr Psychiatry. 2018;26(4):419-434.
26. Isserles M, Daskalakis ZJ, Kumar S, et al. Clinical effectiveness and tolerability of electroconvulsive therapy in patients with neuropsychiatric symptoms of dementia. J Alzheimers Dis. 2017;57(1):45-51.
CASE Agitated and aggressive
Mr. X, age 61, who has Alzheimer’s disease, is brought to the emergency department (ED) by his family after he is found to be confused, becomes physically aggressive with family members, and threatens to burn the house down. His family reports that earlier that day, he was paranoid that somebody was trying to kill him and he tried to leave the house. Mr. X has been experiencing visual hallucinations and delusional thoughts that made him aggressive towards his son. After an initial laboratory workup in the ED, Mr. X’s bloodwork comes back positive for mild leukocytosis, indicating the possibility of an infectious etiology. Mr. X is admitted to the hospital for further evaluation of his altered mental status.
HISTORY Decline over 2 years
This is Mr. X’s third inpatient admission for agitation and psychosis. His current medications—twice daily divalproex sodium extended release (ER), 250 mg every morning and 500 mg at every bedtime, and prazosin, 2 mg/d at bedtime—have been only partially effective. His medical history includes osteoarthritis, back pain, and heterozygous factor V Leiden (not on anticoagulation). He quit smoking tobacco several years ago and has no history of substance use. He has no family history of dementia. Previous trials of cholinesterase inhibitors, antipsychotics, and antidepressants resulted in only minimal improvement in his agitation and psychosis.
A chart review shows that 2 years before his current hospital admission, Mr. X had presented to his primary care physician with slurred speech, forgetfulness, missing words, and transient reading difficulties. His initial laboratory workup and MRI came back normal. He was placed on short-term disability due to work-related errors. He was referred to the hospital’s Memory Clinic 2 years ago, where his Mini-Mental State Exam score was 20/30, indicating mild cognitive impairment. Stroke workup was negative. Due to significant language deficits, a differential diagnosis for Alzheimer’s disease vs primary progressive aphasia vs frontotemporal dementia was made. He screened positive for amyloid PET scan, which confirmed the diagnosis of Alzheimer’s disease.
Neuropsychological testing showed similarities with logopenic variant of primary progressive aphasia, which in many cases is present in Alzheimer’s disease. Mr. X was prescribed anticholinesterase inhibitors, including donepezil, 10 mg/d, and rivastigmine patch, 9.5 mg/d; and memantine, 10 mg/d, which he could not tolerate because of adverse effects. During the next year, Mr. X deteriorated and presented to the ED a few times with significant psychotic symptoms and aggression. He had a poor response to various pharmacologic and nonpharmacologic interventions during this time.
EVALUATION Continued problematic behaviors
During his hospitalization, Mr. X continues to be agitated and paranoid and is placed in restraints. He is unable to respond to his name and cannot follow simple verbal commands. Results of his laboratory workup are within normal limits. His mild leukocytosis resolves with no active signs of infection. Psychiatry is consulted for management of his behavioral and psychological symptoms of dementia (BPSD).
Continue to: Mr. X is started on olanzapine...
Mr. X is started on olanzapine and lorazepam as needed for agitation, and his twice daily divalproex sodium ER is increased to 250 every morning and 750 mg at every bedtime. However, Mr. X remains agitated and requires restraints. Olanzapine is switched from an as-needed dose to scheduled doses of 10 mg every morning and 15 mg at every bedtime, to address his psychosis and agitation.
On Day 24 of hospitalization, Mr. X’s ammonia levels are checked and are found to be 69 µ/dL, which is high (normal range: 15 to 45 µ/dL). Divalproex sodium ER is eventually tapered and discontinued. Mr. X is started on carbamazepine, which is titrated to 400 mg twice daily and results in some improvement in his behavior. He continues to receive carbamazepine and is started on dextromethorphan-quinidine, 10 mg/d, and increased to 10 mg twice daily; however, Mr. X continues to be verbally aggressive with staff, throws food, wanders around, and tries to leave the hospital unit, so he is placed in restraints and continues to require a sitter.
[polldaddy:10698428]
The authors' observations
Dementia typically affects older adults, but its onset can occur before age 60. It is a syndrome rather than a specific illness; the most common types are Alzheimer’s disease, vascular dementia, dementia with Lewy bodies, and frontotemporal dementia. Diagnostic clarity and an evidence-based treatment plan are crucial for improving the quality of life for both the patient and their caregivers. The Table outlines the differential diagnosis of cognitive deficits. New-onset cognitive deficits warrant neuroimaging, and other testing may also be needed.

Behavioral and psychological symptoms of dementia
Noncognitive symptoms occur in 98% of individuals with dementia at some point in their disease and are often the most distressing to both caregivers and patients.1 Behavioral and psychological symptoms of dementia, including apathy, depression, sleep disorders, hallucinations, delusions, psychosis, agitation, and aggression, are exceedingly prevalent.2 Although these symptoms pose a significant burden, there are no clear published treatment guidelines; however, the American Psychiatric Association and the American Geriatric Society recommend using nonpharmacologic approaches as the first-line of treatment for patients with BPSD.3,4
Nonpharmacologic treatments
Due to the unfavorable adverse effects profiles of medications commonly used to treat dementia, nonpharmacologic treatment approaches have always played a crucial role for managing BPSD. Interventions such as music therapy, aromatherapy, art therapy, behavioral therapy, reality orientation, tailored activities, and physical exercises, have shown promising results for alleviating BPSD.5-7
Continue to: Pharmacologic therapies should be used...
Pharmacologic treatments
Pharmacologic therapies should be used when nonpharmacologic approaches are unsuccessful, or when a patient is at imminent risk to harm themselves or others.
Antipsychotics. Although there is conflicting data regarding the use of antipsychotics in older adults, these agents are the most common pharmacologic treatment for patients with BPSD. Several studies examining the efficacy of antipsychotics for treating BPSD have demonstrated an increased risk of cerebrovascular events, including stroke and death due to any cause.8 While the use of antipsychotics increases the risk of mortality in older adults, the absolute risk is still low.9
Antipsychotics used to treat BPSD include:
- Risperidone is well studied in older adults and has shown benefit for treating aggression, agitation, and psychosis.10
- Quetiapine has a favorable adverse effects profile and may help improve sleep and reduce anxiety.10
- Olanzapine. Low-dose olanzapine has been modestly effective in decreasing agitation and aggression in patients with Alzheimer’s and vascular dementias.11
- Aripiprazole has shown modest benefit in treating psychosis and agitation in patients with dementia but may be associated with insomnia or activation symptoms at lower doses.10
- Ziprasidone. Case reports have found benefit with oral and injectable forms.12
Antidepressants. In the CitAD study, which was a placebo-controlled randomized trial, citalopram titrated to a target of 30 mg/d was found to be effective in reducing BPSD.13 However, QTc prolongation limits the use of citalopram. Sertraline was studied in 1 small, randomized trial against haloperidol but showed no additional benefit.14
Mood stabilizers. In a small, randomized trial, carbamazepine was helpful for patients with BPSD who were resistant to treatment with antipsychotics, with efficacy demonstrated over 6 weeks.15 No other mood stabilizers have had significant positive results in treating BPSD.16
Anxiolytic medications. Some research suggests that the occasional use of lorazepam, as necessary, is acceptable for patients with extreme agitation or aggression when behavioral interventions or sleep aids are ineffective.17 Various case reports and case series have suggested gabapentin may be effective for BPSD.18
Prazosin. In a small randomized placebo-controlled trial, the commonly used antihypertensive agent prazosin reduced agitation and aggression in patients with Alzheimer’s dementia, at doses from 1 to 6 mg/d.19 Postural hypotension, the main adverse effect associated with prazosin, can limit its use.
Trazodone. Some research suggests trazodone can reduce irritability and aggression in patients with Alzheimer’s disease.20
Dextromethorphan/quinidine. In a 10-week phase 2 randomized clinical trial of patients with probable Alzheimer’s disease dementia, combination dextromethorphan/quinidine reduced agitation and was generally well tolerated.21
For patients such as Mr. X who do not respond to multiple pharmacologic treatments, electroconvulsive therapy (ECT) may be an option.
Continue to: Because Mr. X does not respond...
TREATMENT A trial of ECT
Because Mr. X does not respond to the standard treatment protocols, the treatment team and Mr. X’s family discuss the use of ECT to control his agitation. Consent is obtained from his legal guardian and Mr. X is medically cleared to receive ECT. Mr. X receives 3 ECT treatments per week. During the first week, Mr. X experiences post-treatment agitation and confusion. The frequency of ECT treatments is reduced to 2 treatments per week, and then 1 session per week. Mr. X starts to show improvement in his agitation and ECT is continued at 1 session per week for 7 weeks.
The authors’ observations
Electroconvulsive therapy has been an effective treatment for patients with treatment-resistant depression and has shown benefit in treating other psychiatric conditions such as acute mania, catatonia, psychotic disorders, and Parkinson’s disease.22 Its use as an off-label treatment for chronic neuropathic pain has also been well documented.23 Although ECT is not indicated for treating agitation and aggression in patients with dementia, its effectiveness for these symptoms has been discussed extensively in the literature.22,24-26
Electroconvulsive therapy treatment can be divided into 2 phases: an acute phase during which ECT is administered 2 to 3 times a week for 4 to 5 weeks, and a maintenance phase of weekly treatments for 4 weeks and then biweekly treatments for 8 weeks.26 Although extensive research supports the safe use of ECT in older adults, concerns for worsening cognitive impairment can deter patients and families from agreeing to this treatment.
Adverse effects of ECT such as headaches and postictal confusion are generally mild and transient. Severe adverse effects such as seizures, severe confusion, and delirium are uncommon.25 The number of ECT treatments required for a good effect ranges from 2 to 18, and the most common position for electrodes placement is bilateral. Outcomes can be measured by using rating scales such as the Cohen-Mansfield Agitation Inventory, Neuropsychiatric Inventory, Social Dysfunction and Aggression Scale, Clinical Global Impression scale, and Pittsford Agitation Scale.25 Obtaining consent from patients with dementia is generally not possible because these patients generally lack the capacity to make medical decisions. Clinicians should refer to their state laws regarding medical-decision making in such cases. The patient’s next of kin or medical power of attorney should be contacted, and the risks and benefits should be discussed before starting ECT.
OUTCOME Lasting improvement
Due to Mr. X’s improvement after ECT, on hospital Day 124, the restraints are removed and he no longer requires a sitter. He starts responding to his name and following simple verbal commands. Electroconvulsive therapy is tapered to every other week, and eventually stopped as his status improves. Mr. X continues to do well and is maintained on the same dosages of olanzapine, carbamazepine, and dextromethorphan-quinidine he had been receiving prior to discharge.
Related Resources
• Van den Berg JF, Kruithof HC, Kok RM, et al. Electroconvulsive therapy for agitation and aggression in dementia: a systematic review. Am J Geriatr Psychiatry. 2018;26(4):419-434.
• Kales HC, Mulsant BH, Sajatovic M. Prescribing antipsychotics in geriatric patients: Focus on dementia. Current Psychiatry. 2017;16(12):24-30.
Drug Brand Names
Aripiprazole • Abilify
Carbamazepine • Tegretol
Citalopram • Celexa
Dextromethorphan- quinidine • Nuedexta
Divalproex sodium ER • Depakote
Donepezil • Aricept
Gabapentin • Neurontin
Haloperidol • Haldol
Lorazepam • Ativan
Memantine • Namenda
Olanzapine • Zyprexa
Prazosin • Minipress
Quetiapine • Seroquel
Risperidone • Risperdal
Rivastigmine • Exelon
Sertraline • Zoloft
Trazodone • Desyrel, Oleptro
Ziprasidone • Geodon
CASE Agitated and aggressive
Mr. X, age 61, who has Alzheimer’s disease, is brought to the emergency department (ED) by his family after he is found to be confused, becomes physically aggressive with family members, and threatens to burn the house down. His family reports that earlier that day, he was paranoid that somebody was trying to kill him and he tried to leave the house. Mr. X has been experiencing visual hallucinations and delusional thoughts that made him aggressive towards his son. After an initial laboratory workup in the ED, Mr. X’s bloodwork comes back positive for mild leukocytosis, indicating the possibility of an infectious etiology. Mr. X is admitted to the hospital for further evaluation of his altered mental status.
HISTORY Decline over 2 years
This is Mr. X’s third inpatient admission for agitation and psychosis. His current medications—twice daily divalproex sodium extended release (ER), 250 mg every morning and 500 mg at every bedtime, and prazosin, 2 mg/d at bedtime—have been only partially effective. His medical history includes osteoarthritis, back pain, and heterozygous factor V Leiden (not on anticoagulation). He quit smoking tobacco several years ago and has no history of substance use. He has no family history of dementia. Previous trials of cholinesterase inhibitors, antipsychotics, and antidepressants resulted in only minimal improvement in his agitation and psychosis.
A chart review shows that 2 years before his current hospital admission, Mr. X had presented to his primary care physician with slurred speech, forgetfulness, missing words, and transient reading difficulties. His initial laboratory workup and MRI came back normal. He was placed on short-term disability due to work-related errors. He was referred to the hospital’s Memory Clinic 2 years ago, where his Mini-Mental State Exam score was 20/30, indicating mild cognitive impairment. Stroke workup was negative. Due to significant language deficits, a differential diagnosis for Alzheimer’s disease vs primary progressive aphasia vs frontotemporal dementia was made. He screened positive for amyloid PET scan, which confirmed the diagnosis of Alzheimer’s disease.
Neuropsychological testing showed similarities with logopenic variant of primary progressive aphasia, which in many cases is present in Alzheimer’s disease. Mr. X was prescribed anticholinesterase inhibitors, including donepezil, 10 mg/d, and rivastigmine patch, 9.5 mg/d; and memantine, 10 mg/d, which he could not tolerate because of adverse effects. During the next year, Mr. X deteriorated and presented to the ED a few times with significant psychotic symptoms and aggression. He had a poor response to various pharmacologic and nonpharmacologic interventions during this time.
EVALUATION Continued problematic behaviors
During his hospitalization, Mr. X continues to be agitated and paranoid and is placed in restraints. He is unable to respond to his name and cannot follow simple verbal commands. Results of his laboratory workup are within normal limits. His mild leukocytosis resolves with no active signs of infection. Psychiatry is consulted for management of his behavioral and psychological symptoms of dementia (BPSD).
Continue to: Mr. X is started on olanzapine...
Mr. X is started on olanzapine and lorazepam as needed for agitation, and his twice daily divalproex sodium ER is increased to 250 every morning and 750 mg at every bedtime. However, Mr. X remains agitated and requires restraints. Olanzapine is switched from an as-needed dose to scheduled doses of 10 mg every morning and 15 mg at every bedtime, to address his psychosis and agitation.
On Day 24 of hospitalization, Mr. X’s ammonia levels are checked and are found to be 69 µ/dL, which is high (normal range: 15 to 45 µ/dL). Divalproex sodium ER is eventually tapered and discontinued. Mr. X is started on carbamazepine, which is titrated to 400 mg twice daily and results in some improvement in his behavior. He continues to receive carbamazepine and is started on dextromethorphan-quinidine, 10 mg/d, and increased to 10 mg twice daily; however, Mr. X continues to be verbally aggressive with staff, throws food, wanders around, and tries to leave the hospital unit, so he is placed in restraints and continues to require a sitter.
[polldaddy:10698428]
The authors' observations
Dementia typically affects older adults, but its onset can occur before age 60. It is a syndrome rather than a specific illness; the most common types are Alzheimer’s disease, vascular dementia, dementia with Lewy bodies, and frontotemporal dementia. Diagnostic clarity and an evidence-based treatment plan are crucial for improving the quality of life for both the patient and their caregivers. The Table outlines the differential diagnosis of cognitive deficits. New-onset cognitive deficits warrant neuroimaging, and other testing may also be needed.

Behavioral and psychological symptoms of dementia
Noncognitive symptoms occur in 98% of individuals with dementia at some point in their disease and are often the most distressing to both caregivers and patients.1 Behavioral and psychological symptoms of dementia, including apathy, depression, sleep disorders, hallucinations, delusions, psychosis, agitation, and aggression, are exceedingly prevalent.2 Although these symptoms pose a significant burden, there are no clear published treatment guidelines; however, the American Psychiatric Association and the American Geriatric Society recommend using nonpharmacologic approaches as the first-line of treatment for patients with BPSD.3,4
Nonpharmacologic treatments
Due to the unfavorable adverse effects profiles of medications commonly used to treat dementia, nonpharmacologic treatment approaches have always played a crucial role for managing BPSD. Interventions such as music therapy, aromatherapy, art therapy, behavioral therapy, reality orientation, tailored activities, and physical exercises, have shown promising results for alleviating BPSD.5-7
Continue to: Pharmacologic therapies should be used...
Pharmacologic treatments
Pharmacologic therapies should be used when nonpharmacologic approaches are unsuccessful, or when a patient is at imminent risk to harm themselves or others.
Antipsychotics. Although there is conflicting data regarding the use of antipsychotics in older adults, these agents are the most common pharmacologic treatment for patients with BPSD. Several studies examining the efficacy of antipsychotics for treating BPSD have demonstrated an increased risk of cerebrovascular events, including stroke and death due to any cause.8 While the use of antipsychotics increases the risk of mortality in older adults, the absolute risk is still low.9
Antipsychotics used to treat BPSD include:
- Risperidone is well studied in older adults and has shown benefit for treating aggression, agitation, and psychosis.10
- Quetiapine has a favorable adverse effects profile and may help improve sleep and reduce anxiety.10
- Olanzapine. Low-dose olanzapine has been modestly effective in decreasing agitation and aggression in patients with Alzheimer’s and vascular dementias.11
- Aripiprazole has shown modest benefit in treating psychosis and agitation in patients with dementia but may be associated with insomnia or activation symptoms at lower doses.10
- Ziprasidone. Case reports have found benefit with oral and injectable forms.12
Antidepressants. In the CitAD study, which was a placebo-controlled randomized trial, citalopram titrated to a target of 30 mg/d was found to be effective in reducing BPSD.13 However, QTc prolongation limits the use of citalopram. Sertraline was studied in 1 small, randomized trial against haloperidol but showed no additional benefit.14
Mood stabilizers. In a small, randomized trial, carbamazepine was helpful for patients with BPSD who were resistant to treatment with antipsychotics, with efficacy demonstrated over 6 weeks.15 No other mood stabilizers have had significant positive results in treating BPSD.16
Anxiolytic medications. Some research suggests that the occasional use of lorazepam, as necessary, is acceptable for patients with extreme agitation or aggression when behavioral interventions or sleep aids are ineffective.17 Various case reports and case series have suggested gabapentin may be effective for BPSD.18
Prazosin. In a small randomized placebo-controlled trial, the commonly used antihypertensive agent prazosin reduced agitation and aggression in patients with Alzheimer’s dementia, at doses from 1 to 6 mg/d.19 Postural hypotension, the main adverse effect associated with prazosin, can limit its use.
Trazodone. Some research suggests trazodone can reduce irritability and aggression in patients with Alzheimer’s disease.20
Dextromethorphan/quinidine. In a 10-week phase 2 randomized clinical trial of patients with probable Alzheimer’s disease dementia, combination dextromethorphan/quinidine reduced agitation and was generally well tolerated.21
For patients such as Mr. X who do not respond to multiple pharmacologic treatments, electroconvulsive therapy (ECT) may be an option.
Continue to: Because Mr. X does not respond...
TREATMENT A trial of ECT
Because Mr. X does not respond to the standard treatment protocols, the treatment team and Mr. X’s family discuss the use of ECT to control his agitation. Consent is obtained from his legal guardian and Mr. X is medically cleared to receive ECT. Mr. X receives 3 ECT treatments per week. During the first week, Mr. X experiences post-treatment agitation and confusion. The frequency of ECT treatments is reduced to 2 treatments per week, and then 1 session per week. Mr. X starts to show improvement in his agitation and ECT is continued at 1 session per week for 7 weeks.
The authors’ observations
Electroconvulsive therapy has been an effective treatment for patients with treatment-resistant depression and has shown benefit in treating other psychiatric conditions such as acute mania, catatonia, psychotic disorders, and Parkinson’s disease.22 Its use as an off-label treatment for chronic neuropathic pain has also been well documented.23 Although ECT is not indicated for treating agitation and aggression in patients with dementia, its effectiveness for these symptoms has been discussed extensively in the literature.22,24-26
Electroconvulsive therapy treatment can be divided into 2 phases: an acute phase during which ECT is administered 2 to 3 times a week for 4 to 5 weeks, and a maintenance phase of weekly treatments for 4 weeks and then biweekly treatments for 8 weeks.26 Although extensive research supports the safe use of ECT in older adults, concerns for worsening cognitive impairment can deter patients and families from agreeing to this treatment.
Adverse effects of ECT such as headaches and postictal confusion are generally mild and transient. Severe adverse effects such as seizures, severe confusion, and delirium are uncommon.25 The number of ECT treatments required for a good effect ranges from 2 to 18, and the most common position for electrodes placement is bilateral. Outcomes can be measured by using rating scales such as the Cohen-Mansfield Agitation Inventory, Neuropsychiatric Inventory, Social Dysfunction and Aggression Scale, Clinical Global Impression scale, and Pittsford Agitation Scale.25 Obtaining consent from patients with dementia is generally not possible because these patients generally lack the capacity to make medical decisions. Clinicians should refer to their state laws regarding medical-decision making in such cases. The patient’s next of kin or medical power of attorney should be contacted, and the risks and benefits should be discussed before starting ECT.
OUTCOME Lasting improvement
Due to Mr. X’s improvement after ECT, on hospital Day 124, the restraints are removed and he no longer requires a sitter. He starts responding to his name and following simple verbal commands. Electroconvulsive therapy is tapered to every other week, and eventually stopped as his status improves. Mr. X continues to do well and is maintained on the same dosages of olanzapine, carbamazepine, and dextromethorphan-quinidine he had been receiving prior to discharge.
Related Resources
• Van den Berg JF, Kruithof HC, Kok RM, et al. Electroconvulsive therapy for agitation and aggression in dementia: a systematic review. Am J Geriatr Psychiatry. 2018;26(4):419-434.
• Kales HC, Mulsant BH, Sajatovic M. Prescribing antipsychotics in geriatric patients: Focus on dementia. Current Psychiatry. 2017;16(12):24-30.
Drug Brand Names
Aripiprazole • Abilify
Carbamazepine • Tegretol
Citalopram • Celexa
Dextromethorphan- quinidine • Nuedexta
Divalproex sodium ER • Depakote
Donepezil • Aricept
Gabapentin • Neurontin
Haloperidol • Haldol
Lorazepam • Ativan
Memantine • Namenda
Olanzapine • Zyprexa
Prazosin • Minipress
Quetiapine • Seroquel
Risperidone • Risperdal
Rivastigmine • Exelon
Sertraline • Zoloft
Trazodone • Desyrel, Oleptro
Ziprasidone • Geodon
1. Kales HC, Gitlin LN, Lyketsos CG. Management of neuropsychiatric symptoms of dementia in clinical settings: recommendations from a multidisciplinary expert panel. J Am Geriatr Soc. 2014;62(4):762-769.
2. Scarmeas N, Brandt J, Albert M, et al. Delusions and hallucinations are associated with worse outcome in Alzheimer disease. Arch Neurol. 2005;62(10):1601-1608.
3. Reus VI, Fochtmann LJ, Eyler AE, et al. The American Psychiatric Association Practice Guideline on the use of antipsychotics to treat agitation or psychosis in patients with dementia. Am J Psychiatry. 2016;173(5):543-546.
4. AGS Executive Committee. A guide to the management of psychotic disorders and neuropsychiatric symptoms of dementia in older adults. The American Geriatrics Society. Published April 2011. Accessed September 24, 2020. https://qioprogram.org/sites/default/files/AGS_Guidelines_for_Telligen.pdf
5. Yang MH, Lin LC, Wu SC, et al. Comparison of the efficacy of aroma-acupressure and aromatherapy for the treatment of dementia-associated agitation. BMC Complement Altern Med. 2015;15:93.
6. Cerga-Pashoja A, Lowery D, Bhattacharya R, et al. Evaluation of exercise on individuals with dementia and their carers: a randomised controlled trial. Trials. 2010;11:53.
7. Chen RC, Liu CL, Lin MH, et al. Non-pharmacological treatment reducing not only behavioral symptoms, but also psychotic symptoms of older adults with dementia: a prospective cohort study in Taiwan. Geriatr Gerontol Int. 2014;14(2):440-446.
8. Schneider LS, Dagerman KS, Insel P. Risk of death with atypical antipsychotic drug treatment for dementia: meta-analysis of randomized placebo-controlled trials. JAMA. 2005;294(15):1934-1943.
9. Lenzer J. FDA warns about using antipsychotic drugs for dementia. BMJ. 2005;330(7497):922.
10. Burke AD, Tariot PN. Atypical antipsychotics in the elderly: a review of therapeutic trends and clinical outcomes. Expert Opin Pharmacother. 2009;10(15):2407-2414.
11. Moretti R, Torre R, Antonello T, et al. Olanzapine as a possible treatment of behavioral symptoms in vascular dementia: risks of cerebrovascular events. J Neurol. 2005;252(10):1186-1193.
12. Cole SA, Saleem R, Shea WP, et al. Ziprasidone for agitation or psychosis in dementia: four cases. Int J Psychiatry Med. 2005;35(1):91-98.
13. Porsteinsson AP, Drye LT, Pollock BG, et al. Effect of citalopram on agitation in Alzheimer disease: the CitAD randomized clinical trial. JAMA. 2014;311(7):682-691.
14. Gaber S, Ronzoli S, Bruno A, et al. Sertraline versus small doses of haloperidol in the treatment of agitated behavior in patients with dementia. Arch Gerontol Geriatr Suppl. 2001; 7:159-162.
15. Olin JT, Fox LS, Pawluczyk S, et al. A pilot randomized trial of carbamazepine for behavioral symptoms in treatment-resistant outpatients with Alzheimer disease. Am J Geriatr Psychiatry. 2001;9(4):400-405.
16. Konovalov S, Muralee S, Tampi RR. Anticonvulsants for the treatment of behavioral and psychological symptoms of dementia: a literature review. Int Psychogeriatr. 2008;20(2):293-308.
17. Davies SJC, Burhan AM, Kim D. Sequential drug treatment algorithm for agitation and aggression in Alzheimer’s and mixed dementia. J Psychopharmacol. 2018;32(5):509-523.
18. Kim Y, Wilkins KM, Tampi RR. Use of gabapentin in the treatment of behavioural and psychological symptoms of dementia: a review of the evidence. Drugs Aging. 2008;25(3):187-196.
19. Wang LY, Shofer JB, Rohde K, et al. Prazosin for the treatment of behavioral symptoms in patients with Alzheimer disease with agitation and aggression. Am J Geriatr Psychiatry. 2009; 17(9):744-751.
20. López-Pousa S, Garre-Olmo J, Vilalta-Franch J, et al. Trazodone for Alzheimer’s disease: a naturalistic follow-up study. Arch Gerontol Geriatr. 2008;47(2):207-215.
21. Cummings JL, Lyketsos CG, Peskind ER. Effect of dextromethorphan-quinidine on agitation in patients with Alzheimer disease dementia: a randomized clinical trial. JAMA. 2015;314(12):1242-1254.
22. Ujkaj M, Davidoff DA, Seiner SJ, et al. Safety and efficacy of electroconvulsive therapy for the treatment of agitation and aggression in patients with dementia. Am J Geriatr Psychiatry. 2012;20(1):61-72.
23. McDaniel WW. Electroconvulsive therapy in complex regional pain syndromes. J ECT. 2003;19(4):226-229.
24. Glass OM, Forester BP, Hermida AP. Electroconvulsive therapy (ECT) for treating agitation in dementia (major neurocognitive disorder)–a promising option. Int Psychogeriatr. 2017;29(5):717-726.
25. Van den Berg JF, Kruithof HC, Kok RM, et al. Electroconvulsive therapy for agitation and aggression in dementia: a systematic review. Am J Geriatr Psychiatry. 2018;26(4):419-434.
26. Isserles M, Daskalakis ZJ, Kumar S, et al. Clinical effectiveness and tolerability of electroconvulsive therapy in patients with neuropsychiatric symptoms of dementia. J Alzheimers Dis. 2017;57(1):45-51.
1. Kales HC, Gitlin LN, Lyketsos CG. Management of neuropsychiatric symptoms of dementia in clinical settings: recommendations from a multidisciplinary expert panel. J Am Geriatr Soc. 2014;62(4):762-769.
2. Scarmeas N, Brandt J, Albert M, et al. Delusions and hallucinations are associated with worse outcome in Alzheimer disease. Arch Neurol. 2005;62(10):1601-1608.
3. Reus VI, Fochtmann LJ, Eyler AE, et al. The American Psychiatric Association Practice Guideline on the use of antipsychotics to treat agitation or psychosis in patients with dementia. Am J Psychiatry. 2016;173(5):543-546.
4. AGS Executive Committee. A guide to the management of psychotic disorders and neuropsychiatric symptoms of dementia in older adults. The American Geriatrics Society. Published April 2011. Accessed September 24, 2020. https://qioprogram.org/sites/default/files/AGS_Guidelines_for_Telligen.pdf
5. Yang MH, Lin LC, Wu SC, et al. Comparison of the efficacy of aroma-acupressure and aromatherapy for the treatment of dementia-associated agitation. BMC Complement Altern Med. 2015;15:93.
6. Cerga-Pashoja A, Lowery D, Bhattacharya R, et al. Evaluation of exercise on individuals with dementia and their carers: a randomised controlled trial. Trials. 2010;11:53.
7. Chen RC, Liu CL, Lin MH, et al. Non-pharmacological treatment reducing not only behavioral symptoms, but also psychotic symptoms of older adults with dementia: a prospective cohort study in Taiwan. Geriatr Gerontol Int. 2014;14(2):440-446.
8. Schneider LS, Dagerman KS, Insel P. Risk of death with atypical antipsychotic drug treatment for dementia: meta-analysis of randomized placebo-controlled trials. JAMA. 2005;294(15):1934-1943.
9. Lenzer J. FDA warns about using antipsychotic drugs for dementia. BMJ. 2005;330(7497):922.
10. Burke AD, Tariot PN. Atypical antipsychotics in the elderly: a review of therapeutic trends and clinical outcomes. Expert Opin Pharmacother. 2009;10(15):2407-2414.
11. Moretti R, Torre R, Antonello T, et al. Olanzapine as a possible treatment of behavioral symptoms in vascular dementia: risks of cerebrovascular events. J Neurol. 2005;252(10):1186-1193.
12. Cole SA, Saleem R, Shea WP, et al. Ziprasidone for agitation or psychosis in dementia: four cases. Int J Psychiatry Med. 2005;35(1):91-98.
13. Porsteinsson AP, Drye LT, Pollock BG, et al. Effect of citalopram on agitation in Alzheimer disease: the CitAD randomized clinical trial. JAMA. 2014;311(7):682-691.
14. Gaber S, Ronzoli S, Bruno A, et al. Sertraline versus small doses of haloperidol in the treatment of agitated behavior in patients with dementia. Arch Gerontol Geriatr Suppl. 2001; 7:159-162.
15. Olin JT, Fox LS, Pawluczyk S, et al. A pilot randomized trial of carbamazepine for behavioral symptoms in treatment-resistant outpatients with Alzheimer disease. Am J Geriatr Psychiatry. 2001;9(4):400-405.
16. Konovalov S, Muralee S, Tampi RR. Anticonvulsants for the treatment of behavioral and psychological symptoms of dementia: a literature review. Int Psychogeriatr. 2008;20(2):293-308.
17. Davies SJC, Burhan AM, Kim D. Sequential drug treatment algorithm for agitation and aggression in Alzheimer’s and mixed dementia. J Psychopharmacol. 2018;32(5):509-523.
18. Kim Y, Wilkins KM, Tampi RR. Use of gabapentin in the treatment of behavioural and psychological symptoms of dementia: a review of the evidence. Drugs Aging. 2008;25(3):187-196.
19. Wang LY, Shofer JB, Rohde K, et al. Prazosin for the treatment of behavioral symptoms in patients with Alzheimer disease with agitation and aggression. Am J Geriatr Psychiatry. 2009; 17(9):744-751.
20. López-Pousa S, Garre-Olmo J, Vilalta-Franch J, et al. Trazodone for Alzheimer’s disease: a naturalistic follow-up study. Arch Gerontol Geriatr. 2008;47(2):207-215.
21. Cummings JL, Lyketsos CG, Peskind ER. Effect of dextromethorphan-quinidine on agitation in patients with Alzheimer disease dementia: a randomized clinical trial. JAMA. 2015;314(12):1242-1254.
22. Ujkaj M, Davidoff DA, Seiner SJ, et al. Safety and efficacy of electroconvulsive therapy for the treatment of agitation and aggression in patients with dementia. Am J Geriatr Psychiatry. 2012;20(1):61-72.
23. McDaniel WW. Electroconvulsive therapy in complex regional pain syndromes. J ECT. 2003;19(4):226-229.
24. Glass OM, Forester BP, Hermida AP. Electroconvulsive therapy (ECT) for treating agitation in dementia (major neurocognitive disorder)–a promising option. Int Psychogeriatr. 2017;29(5):717-726.
25. Van den Berg JF, Kruithof HC, Kok RM, et al. Electroconvulsive therapy for agitation and aggression in dementia: a systematic review. Am J Geriatr Psychiatry. 2018;26(4):419-434.
26. Isserles M, Daskalakis ZJ, Kumar S, et al. Clinical effectiveness and tolerability of electroconvulsive therapy in patients with neuropsychiatric symptoms of dementia. J Alzheimers Dis. 2017;57(1):45-51.
The COPD patient who couldn’t stop worrying
CASE A passive wish to die
Ms. M, age 76, has a history of major depressive disorder, unspecified anxiety disorder, and severe chronic obstructive pulmonary disease (COPD), for which she requires supplemental oxygen. She is admitted to a psychiatric hospital after several months of increased dysphoria, rumination, anhedonia, and a passive wish to die. She also has a decreased appetite and has lost 10 lb, experiences frequent daily episodes of shortness of breath and associated racing thoughts, and has a rapid heart rate.
HISTORY Past medication trials
In addition to COPD, Ms. M’s medical history includes hypertension. Past psychotropic medication trials used to treat her depression and anxiety have included aripiprazole, 5 mg/d; duloxetine, 60 mg/d; fluoxetine, 40 mg/d; mirtazapine, 30 mg nightly; buspirone, 10 mg twice daily; and clonazepam, 0.5 mg twice daily. She has no history of psychotherapy, and because of her uncontrolled anxiety and depression, she has never completed a pulmonary rehabilitation program.
Her current medications include salmeterol, 50 mcg inhaled twice daily, for COPD; amlodipine, 10 mg/d, for hypertension; buspirone, 10 mg twice daily, for anxiety; and duloxetine, 60 mg/d, for depression.
EXAMINATION No evidence of dementia
On examination, Ms. M is alert and oriented to person, place, date, and situation. Overall, she has mild difficulty with attention and short-term recall, which appears to be due to poor effort; intact long-term memory; and is able to abstract appropriately. There is no evidence of dementia.
A mental status exam reveals a frail, elderly woman with fair-to-poor hygiene, cooperative behavior, slowed motor activity, slowed speech with low volume, low mood, and depressed affect with constricted range. Her thought process is linear, her thought content includes passive death wishes, and she does not have hallucinations.
Bitemporal electroconvulsive therapy (ECT), 1.0 ms pulse width at 1.5 times Ms. M’s seizure threshold 3 times weekly, is initiated to treat her depression, with seizure duration averaging 45 seconds for each session. She receives a total of 8 treatments over the course of admission. Buspirone, 10 mg twice daily, is stopped shortly after admission, but she continues to receive duloxetine, 60 mg/d. Ms. M continues to have shortness of breath, palpitations, fearful ruminations about the future, and difficulty falling asleep.
[polldaddy:10673878]
The authors’ observations
The treatment team explores other options, such as benzodiazepines, psychotherapy modalities, and mindfulness exercises, to treat Ms. M’s anxiety and comorbid COPD. Lorazepam, 0.5 mg twice daily, was chosen to treat her acute anxiety. Due to Ms. M’s need for supplemental oxygen, the treatment team attempted to mitigate the risk of using a benzodiazepine by limiting its use to the minimum effective dose. The teams also looked for alternative therapies.
Continue to: Evalution of anxiety...
Evaluation of anxiety and depression in a patient with COPD is complicated by a high degree of symptom overlap. Patients with COPD may experience anxiety symptoms such as shortness of breath, rapid heart rate, numbness/tingling, and racing thoughts, and/or depressive symptoms such as decreased energy, impaired sleep, and impaired concentration. It can therefore be difficult to discern if a symptom is attributable to the physical diagnosis, the psychiatric diagnosis, or a combination of both. Catastrophic thinking about mild physical symptoms is common in patients with COPD. This can lead to hyperventilation and hypocapnia (manifested by lightheadedness, dizziness, paresthesia, and altered consciousness), with a reciprocally escalating cascade of anxiety and somatic symptoms.1
First-line therapy for anxiety disorders with comorbid COPD is CBT and other nonpharmacologic interventions.2,3
Although there is little evidence that traditional pharmacologic treatments (eg, antidepressants, benzodiazepines) have a statistically significant effect on anxiety and depression in COPD, studies have found that they have some clinical benefit.3 Risks, however, limit the utility of certain agents. Sedative-hypnotics potentially decrease respiratory drive and, particularly in older patients, antidepressants’ sedating effects can increase the risk of falls3 leading to increased morbidity, hospitalization, and mortality.
TREATMENT Mindfulness techniques and meditation
Ms. M’s symptoms show no improvement with the addition of lorazepam, 0.5 mg twice daily. A clinician teaches Ms. M mindfulness techniques, and she begins a trial of daily, individual, guided meditation using a meditation app. Respiratory therapists also instruct her on controlled breathing techniques such as pursed-lips breathing, diaphragmatic breathing, and deep breathing. They also encourage Ms. M to participate in the daily exercise group while on the unit.
[polldaddy:10673881]
The authors’ observations
Research indicates that low doses of opioids are safe and effective for refractory breathlessness in patients with severe COPD(those with an arterial partial pressure of oxygen ≤55 mm Hg or arterial oxygen saturation ≤88%).6,7
Continue to: The current opioid crisis...
The current opioid crisis prompts additional caution in prescribing, especially when considering using short-acting, immediate-release opioids such as morphine, which have a greater potential for abuse and dependence.
Many patients with COPD in the end-of-life phase and in severe pain or discomfort due to the advanced stages of their illness receive opioids as part of palliative care. Patients with COPD whose medical care is predominantly palliative may benefit greatly from being prescribed opioids. Most patients with COPD who find relief from low-dose opioids usually have 6 to 12 months to live, and low-dose opioids may help them obtain the best possible quality of life.
Choosing opioids as a treatment involves the risk of physiologic dependence and opioid use disorder. For Ms. M, the potential benefits were thought to outweigh such risks.
OUTCOME Breathlessness improves, anxiety decreases
Ms. M’s lorazepam is discontinued, and immediate-release morphine is prescribed at a low dose of 1 mg/d on an as-needed basis for anxiety with good effect. Ms. M’s breathlessness improves, leading to an overall decrease in anxiety. She does not experience sedation, confusion, or adverse respiratory effects.
Ms. M’s anxiety and depression improve over the course of the hospitalization with this regimen. On hospital Day 25, she is discharged with a plan to continue duloxetine, 60 mg/d, ECT twice weekly, and low-dose morphine, 1 mg/d, as needed for anxiety. She is referred for pulmonary rehabilitation and CBT to maintain remission.
[polldaddy:10673882]
Continue to: The authors' observations
The authors’ observations
Ms. M’s case highlights several challenges associated with treating psychiatric illness in a patient with a chronic medical illness. The relationship between COPD, anxiety, and depression is complex, and is associated with reduced quality of life, increasing severity of pulmonary disease, increased dyspnea, a sense of loss and inability to cope, and decreased self-efficacy and adherence to treatment.9-11
Bottom Line
When traditional antidepressant and anxiolytic therapies have not sufficiently helped, consider low-dose, once-daily opioids to address refractory breathlessness in a patient with COPD with comorbid anxiety and depression. This treatment can lead patients to participate in rehabilitation therapies and improve their quality of life.
Related Resources
- Alexopoulos G, Kiosses D, Sirey J, et al. Untangling therapeutic ingredients of a personalized intervention for patients with depression and severe COPD. Am J Geriatr Psychiatry. 2014;22(11):1316-1324.
- Jackson D, Banerjee S, Sirey J, et al. Two interventions for patients with major depression and severe chronic obstructive pulmonary disease: impact on quality of life. Am J Geriatr Psychiatry. 2018;27(5):502-511.
Drug Brand Names
Amlodipine • Norvasc
Aripiprazole • Abilify
Buspirone • Buspar
Clonazepam • Klonopin
Duloxetine • Cymbalta
Fluoxetine • Prozac
Hydromorphone • Dilaudid
Levodopa • Sinemet
Lorazepam • Ativan
Mirtazapine • Remeron
Morphine • MS Contin
Naloxone • Narcan
Oxycodone • Oxycontin
Salmeterol • Serevent Diskus
1. Harnett D. The difficult-to-treat psychiatric patient with comorbid medical illness. In: Dewan M, Pies R, eds. The difficult-to-treat psychiatric patient. Washington, DC: American Psychiatric Association Publishing; 2001:325-357.
2. Panagioti M, Scott C, Blakemore A, et al. Overview of the prevalence, impact, and management of depression and anxiety in chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2014;9:1289-1306.
3. Cafarella P, Effing T, Usmani ZA, et al. Treatments for anxiety and depression in patients with chronic obstructive pulmonary disease: a literature review. Respirology. 2012;17(4):627-638.
4. Heslop-Marshall K, Baker C, Carrick-Sen D, et al. Randomised controlled trial of cognitive behavioural therapy in COPD. ERJ Open Res. 2018;4:00094-2018. doi: 10.1183/23120541.00094-2018.
5. de Godoy DV, de Godoy RF. A randomized controlled trial of the effect of psychotherapy on anxiety and depression in chronic obstructive pulmonary disease. Arch Phys Med Rehabil. 2003;84(8):1154-1157.
6. Abernethy A, Currow D, Frith P, et al. Randomised, double blind, placebo controlled crossover trial of sustained release morphine for the management of refractory dyspnoea. BMJ. 2003;327(7414):523-528.
7. Janowiak P, Krajnik M, Podolec Z, et al. Dosimetrically administered nebulized morphine for breathlessness in very severe chronic obstructive pulmonary disease: a randomized, controlled trial. BMC Pulm Med. 2017;17:186.
8. Rocker G, Horton R, Currow D, et al. Palliation of dyspnoea in advanced COPD: revisiting a role for opioids. Thorax. 2009;64(10):910-915.
9. Pooler A, Beech R. Examining the relationship between anxiety and depression and exacerbations of COPD which result in hospital admission: a systematic review. Int J Chron Obstruct Pulmon Dis. 2014;9:315-330.
10. Carmen Valenza M, Valenza-Peña G, Torres-Sánchez I, et al. Effectiveness of controlled breathing techniques on anxiety and depression in hospitalized patients with COPD: a randomized clinical trial. Respir Care. 2014;59(2):209-215.
11. Pollok J, van Agteren J, Esterman A, et al. Psychological therapies for the treatment of depression in chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2019;3:CD012347. doi: 10.1002/14651858.CD012347.pub2.
12. Roberts N, Kidd L, Kirkwood K, et al. A systematic review of the content and delivery of education in pulmonary rehabilitation programmes. Respiratory Medicine. 2018;145:161-181.
13. Pumar M, Gray C, Walsh J, et al. Anxiety and depression-important psychological comorbidities of COPD. J Thorac Dis. 2014;6(11):1615-1631.
14. Alexopoulos G, Kiosses D, Sirey J, et al. Untangling therapeutic ingredients of a personalized intervention for patients with depression and severe COPD. Am J Geriatr Psychiatry. 2014;22(11):1316-1324.
15. Jackson D, Banerjee S, Sirey J, et al. Two interventions for patients with major depression and severe chronic obstructive pulmonary disease: impact on quality of life. Am J Geriatr Psychiatry. 2018;27(5):502-511.
CASE A passive wish to die
Ms. M, age 76, has a history of major depressive disorder, unspecified anxiety disorder, and severe chronic obstructive pulmonary disease (COPD), for which she requires supplemental oxygen. She is admitted to a psychiatric hospital after several months of increased dysphoria, rumination, anhedonia, and a passive wish to die. She also has a decreased appetite and has lost 10 lb, experiences frequent daily episodes of shortness of breath and associated racing thoughts, and has a rapid heart rate.
HISTORY Past medication trials
In addition to COPD, Ms. M’s medical history includes hypertension. Past psychotropic medication trials used to treat her depression and anxiety have included aripiprazole, 5 mg/d; duloxetine, 60 mg/d; fluoxetine, 40 mg/d; mirtazapine, 30 mg nightly; buspirone, 10 mg twice daily; and clonazepam, 0.5 mg twice daily. She has no history of psychotherapy, and because of her uncontrolled anxiety and depression, she has never completed a pulmonary rehabilitation program.
Her current medications include salmeterol, 50 mcg inhaled twice daily, for COPD; amlodipine, 10 mg/d, for hypertension; buspirone, 10 mg twice daily, for anxiety; and duloxetine, 60 mg/d, for depression.
EXAMINATION No evidence of dementia
On examination, Ms. M is alert and oriented to person, place, date, and situation. Overall, she has mild difficulty with attention and short-term recall, which appears to be due to poor effort; intact long-term memory; and is able to abstract appropriately. There is no evidence of dementia.
A mental status exam reveals a frail, elderly woman with fair-to-poor hygiene, cooperative behavior, slowed motor activity, slowed speech with low volume, low mood, and depressed affect with constricted range. Her thought process is linear, her thought content includes passive death wishes, and she does not have hallucinations.
Bitemporal electroconvulsive therapy (ECT), 1.0 ms pulse width at 1.5 times Ms. M’s seizure threshold 3 times weekly, is initiated to treat her depression, with seizure duration averaging 45 seconds for each session. She receives a total of 8 treatments over the course of admission. Buspirone, 10 mg twice daily, is stopped shortly after admission, but she continues to receive duloxetine, 60 mg/d. Ms. M continues to have shortness of breath, palpitations, fearful ruminations about the future, and difficulty falling asleep.
[polldaddy:10673878]
The authors’ observations
The treatment team explores other options, such as benzodiazepines, psychotherapy modalities, and mindfulness exercises, to treat Ms. M’s anxiety and comorbid COPD. Lorazepam, 0.5 mg twice daily, was chosen to treat her acute anxiety. Due to Ms. M’s need for supplemental oxygen, the treatment team attempted to mitigate the risk of using a benzodiazepine by limiting its use to the minimum effective dose. The teams also looked for alternative therapies.
Continue to: Evalution of anxiety...
Evaluation of anxiety and depression in a patient with COPD is complicated by a high degree of symptom overlap. Patients with COPD may experience anxiety symptoms such as shortness of breath, rapid heart rate, numbness/tingling, and racing thoughts, and/or depressive symptoms such as decreased energy, impaired sleep, and impaired concentration. It can therefore be difficult to discern if a symptom is attributable to the physical diagnosis, the psychiatric diagnosis, or a combination of both. Catastrophic thinking about mild physical symptoms is common in patients with COPD. This can lead to hyperventilation and hypocapnia (manifested by lightheadedness, dizziness, paresthesia, and altered consciousness), with a reciprocally escalating cascade of anxiety and somatic symptoms.1
First-line therapy for anxiety disorders with comorbid COPD is CBT and other nonpharmacologic interventions.2,3
Although there is little evidence that traditional pharmacologic treatments (eg, antidepressants, benzodiazepines) have a statistically significant effect on anxiety and depression in COPD, studies have found that they have some clinical benefit.3 Risks, however, limit the utility of certain agents. Sedative-hypnotics potentially decrease respiratory drive and, particularly in older patients, antidepressants’ sedating effects can increase the risk of falls3 leading to increased morbidity, hospitalization, and mortality.
TREATMENT Mindfulness techniques and meditation
Ms. M’s symptoms show no improvement with the addition of lorazepam, 0.5 mg twice daily. A clinician teaches Ms. M mindfulness techniques, and she begins a trial of daily, individual, guided meditation using a meditation app. Respiratory therapists also instruct her on controlled breathing techniques such as pursed-lips breathing, diaphragmatic breathing, and deep breathing. They also encourage Ms. M to participate in the daily exercise group while on the unit.
[polldaddy:10673881]
The authors’ observations
Research indicates that low doses of opioids are safe and effective for refractory breathlessness in patients with severe COPD(those with an arterial partial pressure of oxygen ≤55 mm Hg or arterial oxygen saturation ≤88%).6,7
Continue to: The current opioid crisis...
The current opioid crisis prompts additional caution in prescribing, especially when considering using short-acting, immediate-release opioids such as morphine, which have a greater potential for abuse and dependence.
Many patients with COPD in the end-of-life phase and in severe pain or discomfort due to the advanced stages of their illness receive opioids as part of palliative care. Patients with COPD whose medical care is predominantly palliative may benefit greatly from being prescribed opioids. Most patients with COPD who find relief from low-dose opioids usually have 6 to 12 months to live, and low-dose opioids may help them obtain the best possible quality of life.
Choosing opioids as a treatment involves the risk of physiologic dependence and opioid use disorder. For Ms. M, the potential benefits were thought to outweigh such risks.
OUTCOME Breathlessness improves, anxiety decreases
Ms. M’s lorazepam is discontinued, and immediate-release morphine is prescribed at a low dose of 1 mg/d on an as-needed basis for anxiety with good effect. Ms. M’s breathlessness improves, leading to an overall decrease in anxiety. She does not experience sedation, confusion, or adverse respiratory effects.
Ms. M’s anxiety and depression improve over the course of the hospitalization with this regimen. On hospital Day 25, she is discharged with a plan to continue duloxetine, 60 mg/d, ECT twice weekly, and low-dose morphine, 1 mg/d, as needed for anxiety. She is referred for pulmonary rehabilitation and CBT to maintain remission.
[polldaddy:10673882]
Continue to: The authors' observations
The authors’ observations
Ms. M’s case highlights several challenges associated with treating psychiatric illness in a patient with a chronic medical illness. The relationship between COPD, anxiety, and depression is complex, and is associated with reduced quality of life, increasing severity of pulmonary disease, increased dyspnea, a sense of loss and inability to cope, and decreased self-efficacy and adherence to treatment.9-11
Bottom Line
When traditional antidepressant and anxiolytic therapies have not sufficiently helped, consider low-dose, once-daily opioids to address refractory breathlessness in a patient with COPD with comorbid anxiety and depression. This treatment can lead patients to participate in rehabilitation therapies and improve their quality of life.
Related Resources
- Alexopoulos G, Kiosses D, Sirey J, et al. Untangling therapeutic ingredients of a personalized intervention for patients with depression and severe COPD. Am J Geriatr Psychiatry. 2014;22(11):1316-1324.
- Jackson D, Banerjee S, Sirey J, et al. Two interventions for patients with major depression and severe chronic obstructive pulmonary disease: impact on quality of life. Am J Geriatr Psychiatry. 2018;27(5):502-511.
Drug Brand Names
Amlodipine • Norvasc
Aripiprazole • Abilify
Buspirone • Buspar
Clonazepam • Klonopin
Duloxetine • Cymbalta
Fluoxetine • Prozac
Hydromorphone • Dilaudid
Levodopa • Sinemet
Lorazepam • Ativan
Mirtazapine • Remeron
Morphine • MS Contin
Naloxone • Narcan
Oxycodone • Oxycontin
Salmeterol • Serevent Diskus
CASE A passive wish to die
Ms. M, age 76, has a history of major depressive disorder, unspecified anxiety disorder, and severe chronic obstructive pulmonary disease (COPD), for which she requires supplemental oxygen. She is admitted to a psychiatric hospital after several months of increased dysphoria, rumination, anhedonia, and a passive wish to die. She also has a decreased appetite and has lost 10 lb, experiences frequent daily episodes of shortness of breath and associated racing thoughts, and has a rapid heart rate.
HISTORY Past medication trials
In addition to COPD, Ms. M’s medical history includes hypertension. Past psychotropic medication trials used to treat her depression and anxiety have included aripiprazole, 5 mg/d; duloxetine, 60 mg/d; fluoxetine, 40 mg/d; mirtazapine, 30 mg nightly; buspirone, 10 mg twice daily; and clonazepam, 0.5 mg twice daily. She has no history of psychotherapy, and because of her uncontrolled anxiety and depression, she has never completed a pulmonary rehabilitation program.
Her current medications include salmeterol, 50 mcg inhaled twice daily, for COPD; amlodipine, 10 mg/d, for hypertension; buspirone, 10 mg twice daily, for anxiety; and duloxetine, 60 mg/d, for depression.
EXAMINATION No evidence of dementia
On examination, Ms. M is alert and oriented to person, place, date, and situation. Overall, she has mild difficulty with attention and short-term recall, which appears to be due to poor effort; intact long-term memory; and is able to abstract appropriately. There is no evidence of dementia.
A mental status exam reveals a frail, elderly woman with fair-to-poor hygiene, cooperative behavior, slowed motor activity, slowed speech with low volume, low mood, and depressed affect with constricted range. Her thought process is linear, her thought content includes passive death wishes, and she does not have hallucinations.
Bitemporal electroconvulsive therapy (ECT), 1.0 ms pulse width at 1.5 times Ms. M’s seizure threshold 3 times weekly, is initiated to treat her depression, with seizure duration averaging 45 seconds for each session. She receives a total of 8 treatments over the course of admission. Buspirone, 10 mg twice daily, is stopped shortly after admission, but she continues to receive duloxetine, 60 mg/d. Ms. M continues to have shortness of breath, palpitations, fearful ruminations about the future, and difficulty falling asleep.
[polldaddy:10673878]
The authors’ observations
The treatment team explores other options, such as benzodiazepines, psychotherapy modalities, and mindfulness exercises, to treat Ms. M’s anxiety and comorbid COPD. Lorazepam, 0.5 mg twice daily, was chosen to treat her acute anxiety. Due to Ms. M’s need for supplemental oxygen, the treatment team attempted to mitigate the risk of using a benzodiazepine by limiting its use to the minimum effective dose. The teams also looked for alternative therapies.
Continue to: Evalution of anxiety...
Evaluation of anxiety and depression in a patient with COPD is complicated by a high degree of symptom overlap. Patients with COPD may experience anxiety symptoms such as shortness of breath, rapid heart rate, numbness/tingling, and racing thoughts, and/or depressive symptoms such as decreased energy, impaired sleep, and impaired concentration. It can therefore be difficult to discern if a symptom is attributable to the physical diagnosis, the psychiatric diagnosis, or a combination of both. Catastrophic thinking about mild physical symptoms is common in patients with COPD. This can lead to hyperventilation and hypocapnia (manifested by lightheadedness, dizziness, paresthesia, and altered consciousness), with a reciprocally escalating cascade of anxiety and somatic symptoms.1
First-line therapy for anxiety disorders with comorbid COPD is CBT and other nonpharmacologic interventions.2,3
Although there is little evidence that traditional pharmacologic treatments (eg, antidepressants, benzodiazepines) have a statistically significant effect on anxiety and depression in COPD, studies have found that they have some clinical benefit.3 Risks, however, limit the utility of certain agents. Sedative-hypnotics potentially decrease respiratory drive and, particularly in older patients, antidepressants’ sedating effects can increase the risk of falls3 leading to increased morbidity, hospitalization, and mortality.
TREATMENT Mindfulness techniques and meditation
Ms. M’s symptoms show no improvement with the addition of lorazepam, 0.5 mg twice daily. A clinician teaches Ms. M mindfulness techniques, and she begins a trial of daily, individual, guided meditation using a meditation app. Respiratory therapists also instruct her on controlled breathing techniques such as pursed-lips breathing, diaphragmatic breathing, and deep breathing. They also encourage Ms. M to participate in the daily exercise group while on the unit.
[polldaddy:10673881]
The authors’ observations
Research indicates that low doses of opioids are safe and effective for refractory breathlessness in patients with severe COPD(those with an arterial partial pressure of oxygen ≤55 mm Hg or arterial oxygen saturation ≤88%).6,7
Continue to: The current opioid crisis...
The current opioid crisis prompts additional caution in prescribing, especially when considering using short-acting, immediate-release opioids such as morphine, which have a greater potential for abuse and dependence.
Many patients with COPD in the end-of-life phase and in severe pain or discomfort due to the advanced stages of their illness receive opioids as part of palliative care. Patients with COPD whose medical care is predominantly palliative may benefit greatly from being prescribed opioids. Most patients with COPD who find relief from low-dose opioids usually have 6 to 12 months to live, and low-dose opioids may help them obtain the best possible quality of life.
Choosing opioids as a treatment involves the risk of physiologic dependence and opioid use disorder. For Ms. M, the potential benefits were thought to outweigh such risks.
OUTCOME Breathlessness improves, anxiety decreases
Ms. M’s lorazepam is discontinued, and immediate-release morphine is prescribed at a low dose of 1 mg/d on an as-needed basis for anxiety with good effect. Ms. M’s breathlessness improves, leading to an overall decrease in anxiety. She does not experience sedation, confusion, or adverse respiratory effects.
Ms. M’s anxiety and depression improve over the course of the hospitalization with this regimen. On hospital Day 25, she is discharged with a plan to continue duloxetine, 60 mg/d, ECT twice weekly, and low-dose morphine, 1 mg/d, as needed for anxiety. She is referred for pulmonary rehabilitation and CBT to maintain remission.
[polldaddy:10673882]
Continue to: The authors' observations
The authors’ observations
Ms. M’s case highlights several challenges associated with treating psychiatric illness in a patient with a chronic medical illness. The relationship between COPD, anxiety, and depression is complex, and is associated with reduced quality of life, increasing severity of pulmonary disease, increased dyspnea, a sense of loss and inability to cope, and decreased self-efficacy and adherence to treatment.9-11
Bottom Line
When traditional antidepressant and anxiolytic therapies have not sufficiently helped, consider low-dose, once-daily opioids to address refractory breathlessness in a patient with COPD with comorbid anxiety and depression. This treatment can lead patients to participate in rehabilitation therapies and improve their quality of life.
Related Resources
- Alexopoulos G, Kiosses D, Sirey J, et al. Untangling therapeutic ingredients of a personalized intervention for patients with depression and severe COPD. Am J Geriatr Psychiatry. 2014;22(11):1316-1324.
- Jackson D, Banerjee S, Sirey J, et al. Two interventions for patients with major depression and severe chronic obstructive pulmonary disease: impact on quality of life. Am J Geriatr Psychiatry. 2018;27(5):502-511.
Drug Brand Names
Amlodipine • Norvasc
Aripiprazole • Abilify
Buspirone • Buspar
Clonazepam • Klonopin
Duloxetine • Cymbalta
Fluoxetine • Prozac
Hydromorphone • Dilaudid
Levodopa • Sinemet
Lorazepam • Ativan
Mirtazapine • Remeron
Morphine • MS Contin
Naloxone • Narcan
Oxycodone • Oxycontin
Salmeterol • Serevent Diskus
1. Harnett D. The difficult-to-treat psychiatric patient with comorbid medical illness. In: Dewan M, Pies R, eds. The difficult-to-treat psychiatric patient. Washington, DC: American Psychiatric Association Publishing; 2001:325-357.
2. Panagioti M, Scott C, Blakemore A, et al. Overview of the prevalence, impact, and management of depression and anxiety in chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2014;9:1289-1306.
3. Cafarella P, Effing T, Usmani ZA, et al. Treatments for anxiety and depression in patients with chronic obstructive pulmonary disease: a literature review. Respirology. 2012;17(4):627-638.
4. Heslop-Marshall K, Baker C, Carrick-Sen D, et al. Randomised controlled trial of cognitive behavioural therapy in COPD. ERJ Open Res. 2018;4:00094-2018. doi: 10.1183/23120541.00094-2018.
5. de Godoy DV, de Godoy RF. A randomized controlled trial of the effect of psychotherapy on anxiety and depression in chronic obstructive pulmonary disease. Arch Phys Med Rehabil. 2003;84(8):1154-1157.
6. Abernethy A, Currow D, Frith P, et al. Randomised, double blind, placebo controlled crossover trial of sustained release morphine for the management of refractory dyspnoea. BMJ. 2003;327(7414):523-528.
7. Janowiak P, Krajnik M, Podolec Z, et al. Dosimetrically administered nebulized morphine for breathlessness in very severe chronic obstructive pulmonary disease: a randomized, controlled trial. BMC Pulm Med. 2017;17:186.
8. Rocker G, Horton R, Currow D, et al. Palliation of dyspnoea in advanced COPD: revisiting a role for opioids. Thorax. 2009;64(10):910-915.
9. Pooler A, Beech R. Examining the relationship between anxiety and depression and exacerbations of COPD which result in hospital admission: a systematic review. Int J Chron Obstruct Pulmon Dis. 2014;9:315-330.
10. Carmen Valenza M, Valenza-Peña G, Torres-Sánchez I, et al. Effectiveness of controlled breathing techniques on anxiety and depression in hospitalized patients with COPD: a randomized clinical trial. Respir Care. 2014;59(2):209-215.
11. Pollok J, van Agteren J, Esterman A, et al. Psychological therapies for the treatment of depression in chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2019;3:CD012347. doi: 10.1002/14651858.CD012347.pub2.
12. Roberts N, Kidd L, Kirkwood K, et al. A systematic review of the content and delivery of education in pulmonary rehabilitation programmes. Respiratory Medicine. 2018;145:161-181.
13. Pumar M, Gray C, Walsh J, et al. Anxiety and depression-important psychological comorbidities of COPD. J Thorac Dis. 2014;6(11):1615-1631.
14. Alexopoulos G, Kiosses D, Sirey J, et al. Untangling therapeutic ingredients of a personalized intervention for patients with depression and severe COPD. Am J Geriatr Psychiatry. 2014;22(11):1316-1324.
15. Jackson D, Banerjee S, Sirey J, et al. Two interventions for patients with major depression and severe chronic obstructive pulmonary disease: impact on quality of life. Am J Geriatr Psychiatry. 2018;27(5):502-511.
1. Harnett D. The difficult-to-treat psychiatric patient with comorbid medical illness. In: Dewan M, Pies R, eds. The difficult-to-treat psychiatric patient. Washington, DC: American Psychiatric Association Publishing; 2001:325-357.
2. Panagioti M, Scott C, Blakemore A, et al. Overview of the prevalence, impact, and management of depression and anxiety in chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2014;9:1289-1306.
3. Cafarella P, Effing T, Usmani ZA, et al. Treatments for anxiety and depression in patients with chronic obstructive pulmonary disease: a literature review. Respirology. 2012;17(4):627-638.
4. Heslop-Marshall K, Baker C, Carrick-Sen D, et al. Randomised controlled trial of cognitive behavioural therapy in COPD. ERJ Open Res. 2018;4:00094-2018. doi: 10.1183/23120541.00094-2018.
5. de Godoy DV, de Godoy RF. A randomized controlled trial of the effect of psychotherapy on anxiety and depression in chronic obstructive pulmonary disease. Arch Phys Med Rehabil. 2003;84(8):1154-1157.
6. Abernethy A, Currow D, Frith P, et al. Randomised, double blind, placebo controlled crossover trial of sustained release morphine for the management of refractory dyspnoea. BMJ. 2003;327(7414):523-528.
7. Janowiak P, Krajnik M, Podolec Z, et al. Dosimetrically administered nebulized morphine for breathlessness in very severe chronic obstructive pulmonary disease: a randomized, controlled trial. BMC Pulm Med. 2017;17:186.
8. Rocker G, Horton R, Currow D, et al. Palliation of dyspnoea in advanced COPD: revisiting a role for opioids. Thorax. 2009;64(10):910-915.
9. Pooler A, Beech R. Examining the relationship between anxiety and depression and exacerbations of COPD which result in hospital admission: a systematic review. Int J Chron Obstruct Pulmon Dis. 2014;9:315-330.
10. Carmen Valenza M, Valenza-Peña G, Torres-Sánchez I, et al. Effectiveness of controlled breathing techniques on anxiety and depression in hospitalized patients with COPD: a randomized clinical trial. Respir Care. 2014;59(2):209-215.
11. Pollok J, van Agteren J, Esterman A, et al. Psychological therapies for the treatment of depression in chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2019;3:CD012347. doi: 10.1002/14651858.CD012347.pub2.
12. Roberts N, Kidd L, Kirkwood K, et al. A systematic review of the content and delivery of education in pulmonary rehabilitation programmes. Respiratory Medicine. 2018;145:161-181.
13. Pumar M, Gray C, Walsh J, et al. Anxiety and depression-important psychological comorbidities of COPD. J Thorac Dis. 2014;6(11):1615-1631.
14. Alexopoulos G, Kiosses D, Sirey J, et al. Untangling therapeutic ingredients of a personalized intervention for patients with depression and severe COPD. Am J Geriatr Psychiatry. 2014;22(11):1316-1324.
15. Jackson D, Banerjee S, Sirey J, et al. Two interventions for patients with major depression and severe chronic obstructive pulmonary disease: impact on quality of life. Am J Geriatr Psychiatry. 2018;27(5):502-511.