User login
Painful erections while being treated for OCD
CASE Prolonged, painful erections
Mr. G, age 27, who has a history of obsessive-compulsive disorder (OCD), presents to his internist’s office with complaints of “masturbating several times a day” and having ejaculatory delay of up to 50 minutes with intercourse. The frequent masturbation was an attempt to “cure” the ejaculatory delay. In addition, Mr. G reports that for the past 5 nights, he has awoke every 3 hours with a painful erection that lasted 1.5 to 2.5 hours, after which he would fall asleep, only to wake once again to the same phenomenon.
Mr. G’s symptoms began 3 weeks ago after his psychiatrist adjusted the dose of his medication for OCD. Mr. G had been receiving fluoxetine, 10 mg/d, for the past 3 years to manage his OCD, without improvement. During a recent consultation, his psychiatrist increased the dose to 20 mg/d, with the expectation that further dose increases might be necessary to treat his OCD.
HISTORY Concurrent GAD
Mr. G is single and in a monogamous heterosexual relationship. Three weeks earlier, when he was examined by his psychiatrist, Mr. G’s Yale-Brown Obsessive Compulsive Scale score was 28 and his Beck Anxiety Inventory score was 24. Based on these scores, the psychiatrist concluded Mr. G had concurrent generalized anxiety disorder (GAD).
EVALUATION Workup is normal
On presentation to his internist’s office, Mr. G’s laboratory values are all within normal range, including a chemistry panel, complete blood count with differential, and electrocardiogram. A human immunodeficiency virus test is negative. His internist instructs Mr. G to return to his psychiatrist.
[polldaddy:10640161]
TREATMENT Dose adjustment
Based on Mr. G’s description of painful and persistent erections in the absence of sexual stimulation or arousal, and because these episodes have occurred 5 consecutive nights, the psychiatrist makes a provisional diagnosis of stuttering priapism and reduces the fluoxetine dose from 20 to 10 mg/d.
The author’s observations
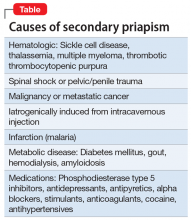
Priapism is classically defined as a persistent, unwanted penile or clitoral engorgement in the absence of sexual desire/arousal or stimulation. It can last for up to 4 to 6 hours1 orit can take a so-called “stuttering form” characterized by brief, recurrent, self-limited episodes. Priapism is a urologic emergency resulting in erectile dysfunction in 30% to 90% of patients. It is multifactorial and can be characterized as low-flow (occlusive) or high-flow (nonischemic). Most priapism is primary or idiopathic in nature; the incidence is 1.5 per 100,000 individuals (primarily men), with bimodal peaks, and it can occur in all age groups.2 Secondary priapism can occur from many causes (Table).
Mechanism is unclear
The molecular mechanism of priapism is not completely understood. Normally, nitrous oxide mediates penile erection. However, cyclic guanosine monophosphate (cGMP) acts at several levels to create smooth muscle reaction, leading to either penile tumescence or, in some cases, priapism. Stuttering or intermittent ischemic priapism is thought to be a downregulation of phosphodiesterase type 5, causing excess cGMP with subsequent smooth muscle relaxation in the penis.3
Continue to: Drug-induced priapism
Drug-induced priapism
Drug-induced priapism is commonly believed to be associated with alpha-1 adrenergic receptor blockade.4 This also results in dizziness and orthostatic hypotension.5 Trazodone is commonly associated with the development of secondary priapism; however, in the last 30 years, multiple case reports have demonstrated that a variety of psychoactive agents have been associated with low-flowpriapism.6 Most case reports have focused on new-onset priapism associated with the introduction of a new medication. Based on a recent informal search of Medline, since 1989, there have been >36 case reports of priapism associated with psychotropic use. Stuttering priapism is less frequently discussed in the literature.7
Ischemic priapism accounts for 95% of all reports. It can be associated with medication use or hematologic disorders, or it can be triggered by sexual activity. Often, patients who experience an episode will abstain from sexual contact.
The etiology of stuttering priapism is less clear. Episodes of stuttering priapism often occur during sleep and can resolve spontaneously.8 They are a form of ischemic priapism and are seen in patients with sickle cell anemia. It is not known how many patients with stuttering priapism will convert to the nonremitting form, which may require chemical or surgical intervention.9 Stuttering priapism may go unreported and perhaps may be overlooked by patients based on its frequency and intensity.
The activating selective serotonin reuptake inhibitor fluoxetine has a long half-life and is a potent inhibitor of the cytochrome P450 2D6 isoenzyme system. It inhibits serotonin transporter proteins. It is also a weak norepinephrine reuptake inhibitor, an effect that increases with increasing doses of the medication. Its 5HT2C antagonism is proposed as the mechanism of its activating properties.10 In Mr. G’s case, it is possible that fluoxetine’s weak norepinephrine reuptake inhibition resulted in an intermittent priapism effect mediated through the pathways described above.
OUTCOME Symptoms resolve
Approximately 1 week after Mr. G’s fluoxetine dose is reduced, his symptoms of priapism abated. The fluoxetine is discontinued and his ejaculatory delay resolves. Mr. G is started on fluvoxamine, 150 mg/d, which results in a significant decrease of both GAD and OCD symptoms with no notable ejaculatory delay, and no recurrence of priapism.
Continue to: The author's observations
The author’s observations
Mr. G’s case and other case reports suggest that psychiatrists should caution patients who are prescribed antidepressants or antipsychotics that stuttering priapism is a possible adverse effect.11 As seen in Mr. G’s case, fluoxetine (when used chronically) can moderate vascular responses at the pre- and post-synaptic adrenergic receptor.11 Priapism induced by a psychotropic medication will not necessarily lead to a longer-term, unremitting priapism, but it can be dramatic, frightening, and lead to noncompliance. Along with obtaining a standard history that includes asking patients about prior adverse medication events, psychiatrists also should ask their patients if they have experienced any instances of transient priapism that may require further evaluation.
Bottom Line
Any psychotropic medication that has the capacity to act on alpha adrenergic receptors can cause priapism. Ask patients if they have had any unusual erections/ clitoral engorgement while taking any psychotropic medications, because many patients will be hesitant to volunteer such information.
Related Resource
- Thippaiah SM, Nagaraja S, Birur B, et al. Successful management of psychotropics induced stuttering priapism with pseudoephedrine in a patient with schizophrenia. Psychopharmacol Bull. 2018;48(2):29-33.
Drug Brand Names
Fluoxetine • Prozac
Fluvoxamine • Luvox
Trazodone • Desyrel, Oleptro
1. Kadioglu A, Sanli O, Celtik M, et al. Practical management of patients with priapism. EAU-EBU Update Series. 2006;4(4):150-160.
2. Eland IA, van der Lei J, Stricker BHC. Incidence of priapism in the general population. Urology. 2001;57(5):970-972.
3. Halls JE, Patel DV, Walkden M, et al. Priapism: pathophysiology and the role of the radiologist. Br J Radiol. 2012;85(Spec Iss 1):S79-S85.
4. Wang CS, Kao WT, Chen CD, et al. Priapism associated with typical and atypical antipsychotic medications. Int Clinical Psychopharmacology. 2006;21(4):245-248.
5. Khan Q, Tucker P, Lokhande A. Priapism: what cause: mental illness, psychotropic medications or polysubstance abuse? J Okla State Med Assoc. 2016;109(11):515-517.
6. Dent LA, Brown WC, Murney JD. Citalopram-induced priapism. Pharmacotherapy. 2002;22(4):538-541.
7. Wilkening GL, Kucherer SA, Douaihy AB. Priapism and renal colic in a patient treated with duloxetine. Mental Health Clinician. 2016;6(4):197-200.
8. Morrison BF, Burnett AL. Stuttering priapism: insight into its pathogenesis and management. Curr Urol Rep. 2012;13(4):268-276.
9. Burnett AL, Bivalacqua TJ. Priapism: current principles and practice. Urol Clin North Am. 2007;34(4):631-642.
10. Stahl SM. Stahl’s essential psychopharmacology: neuroscientific basis and practical applications. 4th ed. Cambridge, United Kingdom: Cambridge University Press; 2013.
11. Pereira CA, Rodrigues FL, Ruginsk SG, et al. Chronic treatment with fluoxetine modulates vascular adrenergic responses by inhibition of pre- and post-synaptic mechanisms. Eu J Pharmacol. 2017;800:70-80.
CASE Prolonged, painful erections
Mr. G, age 27, who has a history of obsessive-compulsive disorder (OCD), presents to his internist’s office with complaints of “masturbating several times a day” and having ejaculatory delay of up to 50 minutes with intercourse. The frequent masturbation was an attempt to “cure” the ejaculatory delay. In addition, Mr. G reports that for the past 5 nights, he has awoke every 3 hours with a painful erection that lasted 1.5 to 2.5 hours, after which he would fall asleep, only to wake once again to the same phenomenon.
Mr. G’s symptoms began 3 weeks ago after his psychiatrist adjusted the dose of his medication for OCD. Mr. G had been receiving fluoxetine, 10 mg/d, for the past 3 years to manage his OCD, without improvement. During a recent consultation, his psychiatrist increased the dose to 20 mg/d, with the expectation that further dose increases might be necessary to treat his OCD.
HISTORY Concurrent GAD
Mr. G is single and in a monogamous heterosexual relationship. Three weeks earlier, when he was examined by his psychiatrist, Mr. G’s Yale-Brown Obsessive Compulsive Scale score was 28 and his Beck Anxiety Inventory score was 24. Based on these scores, the psychiatrist concluded Mr. G had concurrent generalized anxiety disorder (GAD).
EVALUATION Workup is normal
On presentation to his internist’s office, Mr. G’s laboratory values are all within normal range, including a chemistry panel, complete blood count with differential, and electrocardiogram. A human immunodeficiency virus test is negative. His internist instructs Mr. G to return to his psychiatrist.
[polldaddy:10640161]
TREATMENT Dose adjustment
Based on Mr. G’s description of painful and persistent erections in the absence of sexual stimulation or arousal, and because these episodes have occurred 5 consecutive nights, the psychiatrist makes a provisional diagnosis of stuttering priapism and reduces the fluoxetine dose from 20 to 10 mg/d.
The author’s observations
Priapism is classically defined as a persistent, unwanted penile or clitoral engorgement in the absence of sexual desire/arousal or stimulation. It can last for up to 4 to 6 hours1 orit can take a so-called “stuttering form” characterized by brief, recurrent, self-limited episodes. Priapism is a urologic emergency resulting in erectile dysfunction in 30% to 90% of patients. It is multifactorial and can be characterized as low-flow (occlusive) or high-flow (nonischemic). Most priapism is primary or idiopathic in nature; the incidence is 1.5 per 100,000 individuals (primarily men), with bimodal peaks, and it can occur in all age groups.2 Secondary priapism can occur from many causes (Table).
Mechanism is unclear
The molecular mechanism of priapism is not completely understood. Normally, nitrous oxide mediates penile erection. However, cyclic guanosine monophosphate (cGMP) acts at several levels to create smooth muscle reaction, leading to either penile tumescence or, in some cases, priapism. Stuttering or intermittent ischemic priapism is thought to be a downregulation of phosphodiesterase type 5, causing excess cGMP with subsequent smooth muscle relaxation in the penis.3
Continue to: Drug-induced priapism
Drug-induced priapism
Drug-induced priapism is commonly believed to be associated with alpha-1 adrenergic receptor blockade.4 This also results in dizziness and orthostatic hypotension.5 Trazodone is commonly associated with the development of secondary priapism; however, in the last 30 years, multiple case reports have demonstrated that a variety of psychoactive agents have been associated with low-flowpriapism.6 Most case reports have focused on new-onset priapism associated with the introduction of a new medication. Based on a recent informal search of Medline, since 1989, there have been >36 case reports of priapism associated with psychotropic use. Stuttering priapism is less frequently discussed in the literature.7
Ischemic priapism accounts for 95% of all reports. It can be associated with medication use or hematologic disorders, or it can be triggered by sexual activity. Often, patients who experience an episode will abstain from sexual contact.
The etiology of stuttering priapism is less clear. Episodes of stuttering priapism often occur during sleep and can resolve spontaneously.8 They are a form of ischemic priapism and are seen in patients with sickle cell anemia. It is not known how many patients with stuttering priapism will convert to the nonremitting form, which may require chemical or surgical intervention.9 Stuttering priapism may go unreported and perhaps may be overlooked by patients based on its frequency and intensity.
The activating selective serotonin reuptake inhibitor fluoxetine has a long half-life and is a potent inhibitor of the cytochrome P450 2D6 isoenzyme system. It inhibits serotonin transporter proteins. It is also a weak norepinephrine reuptake inhibitor, an effect that increases with increasing doses of the medication. Its 5HT2C antagonism is proposed as the mechanism of its activating properties.10 In Mr. G’s case, it is possible that fluoxetine’s weak norepinephrine reuptake inhibition resulted in an intermittent priapism effect mediated through the pathways described above.
OUTCOME Symptoms resolve
Approximately 1 week after Mr. G’s fluoxetine dose is reduced, his symptoms of priapism abated. The fluoxetine is discontinued and his ejaculatory delay resolves. Mr. G is started on fluvoxamine, 150 mg/d, which results in a significant decrease of both GAD and OCD symptoms with no notable ejaculatory delay, and no recurrence of priapism.
Continue to: The author's observations
The author’s observations
Mr. G’s case and other case reports suggest that psychiatrists should caution patients who are prescribed antidepressants or antipsychotics that stuttering priapism is a possible adverse effect.11 As seen in Mr. G’s case, fluoxetine (when used chronically) can moderate vascular responses at the pre- and post-synaptic adrenergic receptor.11 Priapism induced by a psychotropic medication will not necessarily lead to a longer-term, unremitting priapism, but it can be dramatic, frightening, and lead to noncompliance. Along with obtaining a standard history that includes asking patients about prior adverse medication events, psychiatrists also should ask their patients if they have experienced any instances of transient priapism that may require further evaluation.
Bottom Line
Any psychotropic medication that has the capacity to act on alpha adrenergic receptors can cause priapism. Ask patients if they have had any unusual erections/ clitoral engorgement while taking any psychotropic medications, because many patients will be hesitant to volunteer such information.
Related Resource
- Thippaiah SM, Nagaraja S, Birur B, et al. Successful management of psychotropics induced stuttering priapism with pseudoephedrine in a patient with schizophrenia. Psychopharmacol Bull. 2018;48(2):29-33.
Drug Brand Names
Fluoxetine • Prozac
Fluvoxamine • Luvox
Trazodone • Desyrel, Oleptro
CASE Prolonged, painful erections
Mr. G, age 27, who has a history of obsessive-compulsive disorder (OCD), presents to his internist’s office with complaints of “masturbating several times a day” and having ejaculatory delay of up to 50 minutes with intercourse. The frequent masturbation was an attempt to “cure” the ejaculatory delay. In addition, Mr. G reports that for the past 5 nights, he has awoke every 3 hours with a painful erection that lasted 1.5 to 2.5 hours, after which he would fall asleep, only to wake once again to the same phenomenon.
Mr. G’s symptoms began 3 weeks ago after his psychiatrist adjusted the dose of his medication for OCD. Mr. G had been receiving fluoxetine, 10 mg/d, for the past 3 years to manage his OCD, without improvement. During a recent consultation, his psychiatrist increased the dose to 20 mg/d, with the expectation that further dose increases might be necessary to treat his OCD.
HISTORY Concurrent GAD
Mr. G is single and in a monogamous heterosexual relationship. Three weeks earlier, when he was examined by his psychiatrist, Mr. G’s Yale-Brown Obsessive Compulsive Scale score was 28 and his Beck Anxiety Inventory score was 24. Based on these scores, the psychiatrist concluded Mr. G had concurrent generalized anxiety disorder (GAD).
EVALUATION Workup is normal
On presentation to his internist’s office, Mr. G’s laboratory values are all within normal range, including a chemistry panel, complete blood count with differential, and electrocardiogram. A human immunodeficiency virus test is negative. His internist instructs Mr. G to return to his psychiatrist.
[polldaddy:10640161]
TREATMENT Dose adjustment
Based on Mr. G’s description of painful and persistent erections in the absence of sexual stimulation or arousal, and because these episodes have occurred 5 consecutive nights, the psychiatrist makes a provisional diagnosis of stuttering priapism and reduces the fluoxetine dose from 20 to 10 mg/d.
The author’s observations
Priapism is classically defined as a persistent, unwanted penile or clitoral engorgement in the absence of sexual desire/arousal or stimulation. It can last for up to 4 to 6 hours1 orit can take a so-called “stuttering form” characterized by brief, recurrent, self-limited episodes. Priapism is a urologic emergency resulting in erectile dysfunction in 30% to 90% of patients. It is multifactorial and can be characterized as low-flow (occlusive) or high-flow (nonischemic). Most priapism is primary or idiopathic in nature; the incidence is 1.5 per 100,000 individuals (primarily men), with bimodal peaks, and it can occur in all age groups.2 Secondary priapism can occur from many causes (Table).
Mechanism is unclear
The molecular mechanism of priapism is not completely understood. Normally, nitrous oxide mediates penile erection. However, cyclic guanosine monophosphate (cGMP) acts at several levels to create smooth muscle reaction, leading to either penile tumescence or, in some cases, priapism. Stuttering or intermittent ischemic priapism is thought to be a downregulation of phosphodiesterase type 5, causing excess cGMP with subsequent smooth muscle relaxation in the penis.3
Continue to: Drug-induced priapism
Drug-induced priapism
Drug-induced priapism is commonly believed to be associated with alpha-1 adrenergic receptor blockade.4 This also results in dizziness and orthostatic hypotension.5 Trazodone is commonly associated with the development of secondary priapism; however, in the last 30 years, multiple case reports have demonstrated that a variety of psychoactive agents have been associated with low-flowpriapism.6 Most case reports have focused on new-onset priapism associated with the introduction of a new medication. Based on a recent informal search of Medline, since 1989, there have been >36 case reports of priapism associated with psychotropic use. Stuttering priapism is less frequently discussed in the literature.7
Ischemic priapism accounts for 95% of all reports. It can be associated with medication use or hematologic disorders, or it can be triggered by sexual activity. Often, patients who experience an episode will abstain from sexual contact.
The etiology of stuttering priapism is less clear. Episodes of stuttering priapism often occur during sleep and can resolve spontaneously.8 They are a form of ischemic priapism and are seen in patients with sickle cell anemia. It is not known how many patients with stuttering priapism will convert to the nonremitting form, which may require chemical or surgical intervention.9 Stuttering priapism may go unreported and perhaps may be overlooked by patients based on its frequency and intensity.
The activating selective serotonin reuptake inhibitor fluoxetine has a long half-life and is a potent inhibitor of the cytochrome P450 2D6 isoenzyme system. It inhibits serotonin transporter proteins. It is also a weak norepinephrine reuptake inhibitor, an effect that increases with increasing doses of the medication. Its 5HT2C antagonism is proposed as the mechanism of its activating properties.10 In Mr. G’s case, it is possible that fluoxetine’s weak norepinephrine reuptake inhibition resulted in an intermittent priapism effect mediated through the pathways described above.
OUTCOME Symptoms resolve
Approximately 1 week after Mr. G’s fluoxetine dose is reduced, his symptoms of priapism abated. The fluoxetine is discontinued and his ejaculatory delay resolves. Mr. G is started on fluvoxamine, 150 mg/d, which results in a significant decrease of both GAD and OCD symptoms with no notable ejaculatory delay, and no recurrence of priapism.
Continue to: The author's observations
The author’s observations
Mr. G’s case and other case reports suggest that psychiatrists should caution patients who are prescribed antidepressants or antipsychotics that stuttering priapism is a possible adverse effect.11 As seen in Mr. G’s case, fluoxetine (when used chronically) can moderate vascular responses at the pre- and post-synaptic adrenergic receptor.11 Priapism induced by a psychotropic medication will not necessarily lead to a longer-term, unremitting priapism, but it can be dramatic, frightening, and lead to noncompliance. Along with obtaining a standard history that includes asking patients about prior adverse medication events, psychiatrists also should ask their patients if they have experienced any instances of transient priapism that may require further evaluation.
Bottom Line
Any psychotropic medication that has the capacity to act on alpha adrenergic receptors can cause priapism. Ask patients if they have had any unusual erections/ clitoral engorgement while taking any psychotropic medications, because many patients will be hesitant to volunteer such information.
Related Resource
- Thippaiah SM, Nagaraja S, Birur B, et al. Successful management of psychotropics induced stuttering priapism with pseudoephedrine in a patient with schizophrenia. Psychopharmacol Bull. 2018;48(2):29-33.
Drug Brand Names
Fluoxetine • Prozac
Fluvoxamine • Luvox
Trazodone • Desyrel, Oleptro
1. Kadioglu A, Sanli O, Celtik M, et al. Practical management of patients with priapism. EAU-EBU Update Series. 2006;4(4):150-160.
2. Eland IA, van der Lei J, Stricker BHC. Incidence of priapism in the general population. Urology. 2001;57(5):970-972.
3. Halls JE, Patel DV, Walkden M, et al. Priapism: pathophysiology and the role of the radiologist. Br J Radiol. 2012;85(Spec Iss 1):S79-S85.
4. Wang CS, Kao WT, Chen CD, et al. Priapism associated with typical and atypical antipsychotic medications. Int Clinical Psychopharmacology. 2006;21(4):245-248.
5. Khan Q, Tucker P, Lokhande A. Priapism: what cause: mental illness, psychotropic medications or polysubstance abuse? J Okla State Med Assoc. 2016;109(11):515-517.
6. Dent LA, Brown WC, Murney JD. Citalopram-induced priapism. Pharmacotherapy. 2002;22(4):538-541.
7. Wilkening GL, Kucherer SA, Douaihy AB. Priapism and renal colic in a patient treated with duloxetine. Mental Health Clinician. 2016;6(4):197-200.
8. Morrison BF, Burnett AL. Stuttering priapism: insight into its pathogenesis and management. Curr Urol Rep. 2012;13(4):268-276.
9. Burnett AL, Bivalacqua TJ. Priapism: current principles and practice. Urol Clin North Am. 2007;34(4):631-642.
10. Stahl SM. Stahl’s essential psychopharmacology: neuroscientific basis and practical applications. 4th ed. Cambridge, United Kingdom: Cambridge University Press; 2013.
11. Pereira CA, Rodrigues FL, Ruginsk SG, et al. Chronic treatment with fluoxetine modulates vascular adrenergic responses by inhibition of pre- and post-synaptic mechanisms. Eu J Pharmacol. 2017;800:70-80.
1. Kadioglu A, Sanli O, Celtik M, et al. Practical management of patients with priapism. EAU-EBU Update Series. 2006;4(4):150-160.
2. Eland IA, van der Lei J, Stricker BHC. Incidence of priapism in the general population. Urology. 2001;57(5):970-972.
3. Halls JE, Patel DV, Walkden M, et al. Priapism: pathophysiology and the role of the radiologist. Br J Radiol. 2012;85(Spec Iss 1):S79-S85.
4. Wang CS, Kao WT, Chen CD, et al. Priapism associated with typical and atypical antipsychotic medications. Int Clinical Psychopharmacology. 2006;21(4):245-248.
5. Khan Q, Tucker P, Lokhande A. Priapism: what cause: mental illness, psychotropic medications or polysubstance abuse? J Okla State Med Assoc. 2016;109(11):515-517.
6. Dent LA, Brown WC, Murney JD. Citalopram-induced priapism. Pharmacotherapy. 2002;22(4):538-541.
7. Wilkening GL, Kucherer SA, Douaihy AB. Priapism and renal colic in a patient treated with duloxetine. Mental Health Clinician. 2016;6(4):197-200.
8. Morrison BF, Burnett AL. Stuttering priapism: insight into its pathogenesis and management. Curr Urol Rep. 2012;13(4):268-276.
9. Burnett AL, Bivalacqua TJ. Priapism: current principles and practice. Urol Clin North Am. 2007;34(4):631-642.
10. Stahl SM. Stahl’s essential psychopharmacology: neuroscientific basis and practical applications. 4th ed. Cambridge, United Kingdom: Cambridge University Press; 2013.
11. Pereira CA, Rodrigues FL, Ruginsk SG, et al. Chronic treatment with fluoxetine modulates vascular adrenergic responses by inhibition of pre- and post-synaptic mechanisms. Eu J Pharmacol. 2017;800:70-80.
The boy whose arm wouldn’t work
CASE Drooling, unsteady, and not himself
B, age 10, who is left handed and has autism spectrum disorder, is brought to the emergency department (ED) with a 1-day history of drooling, unsteady gait, and left wrist in sustained flexion. His parents report that for the past week, B has had cold symptoms, including rhinorrhea, a low-grade fever (100.0°F), and cough. Earlier in the day, he was seen at his pediatrician’s office, where he was diagnosed with an acute respiratory infection and started on amoxicillin, 500 mg twice daily for 7 days.
At baseline, B is nonverbal. He requires some assistance with his activities of daily living. He usually is able to walk without assistance and dress himself, but he is not toilet trained. His parents report that in the past day, he has had significant difficulties with tasks involving his left hand. Normally, B is able to feed himself “finger foods” but has been unable to do so today. His parents say that he has been unsteady on his feet, and has been “falling forward” when he tries to walk.
Two years ago, B was started on risperidone, 0.5 mg nightly, for behavioral aggression and self-mutilation. Over the next 12 months, the dosage was steadily increased to 1 mg twice daily, with good response. He has been taking his current dosage, 1 mg twice daily, for the past 12 months without adjustment. His parents report there have been no other medication changes, other than starting amoxicillin earlier that day.
As part of his initial ED evaluation, B is found to be mildly dehydrated, with an elevated sedimentation rate on urinalysis. His complete blood count (CBC) with differential is within normal limits. A comprehensive metabolic panel shows a slight increase in his creatinine level, indicating dehydration. B is administered IV fluid replacement because he is having difficulty drinking due to excessive drooling.
The ED physician is concerned that B may be experiencing an acute dystonic reaction from risperidone, so the team holds this medication, and gives B a one-time dose of IV diphenhydramine, 25 mg, for presumptive acute dystonic reaction. After several minutes, there is no improvement in the sustained flexion of his left wrist.
[polldaddy:10615848]
The authors’ observations
B presented with new-onset neurologic findings after a recently diagnosed upper respiratory viral illness. His symptoms appeared to be confined to his left upper extremity, specifically demonstrating left arm extension at the elbow with flexion of the left wrist. He also had new-onset unsteady gait with a stooped forward posture and required assistance with walking. Interestingly, despite B’s history of antipsychotic use, administering an anticholinergic agent did not lessen the dystonic posturing at his wrist and elbow.
EVALUATION Laboratory results reveal new clues
While in the ED, B undergoes MRI of the brain and spinal cord to rule out any mass lesions that could be impinging upon the motor pathways. Both brain and spinal cord imaging appear to be essentially normal, without evidence of impingement of the spinal nerves or lesions involving the brainstem or cerebellum.
Continue to: Due to concerns...
Due to concerns of possible airway obstruction, a CT scan of the neck is obtained to rule out any acute pathology, such as epiglottitis compromising his airway. The scan shows some inflammation and edema in the soft tissues that is thought to be secondary to his acute viral illness. B is able to maintain his airway and oxygenation, so intubation is not necessary.
A CPK test is ordered because there are concerns of sustained muscle contraction of B’s left wrist and elbow. The CPK level is 884 U/L (reference range 26 to 192 U/L). The elevation in CPK is consistent with prior laboratory findings of dehydration and indicating skeletal muscle breakdown from sustained muscle contraction. All other laboratory results, including a comprehensive metabolic panel, urine drug screen, and thyroid screening panel, are within normal limits.
[polldaddy:10615850]
EVALUATION No variation in facial expression
B is admitted to the general pediatrics service. Maintenance IV fluids are started due to concerns of dehydration and possible rhabdomyolysis due to his elevated CPK level. Risperidone is held throughout the hospital course due to concerns for an acute dystonic reaction. B is monitored for several days without clinical improvement and eventually discharged home with a diagnosis of inflammatory mononeuropathy due to viral infection. The patient is told to discontinue risperidone as part of discharge instructions.
Five days later, B returns to the hospital because there was no improvement in his left extremity or walking. His left elbow remains extended with left wrist in flexion. Psychiatry is consulted for further diagnostic clarity and evaluation.
On physical examination, B’s left arm remains unchanged. Despite discontinuing risperidone, there is evidence of cogwheel rigidity of the left wrist joint. Reflexes in the upper and lower extremities are 2+ and symmetrical bilaterally, suggesting intact upper and lower motor pathways. Babinski sign is absent bilaterally, which is a normal finding in B’s age group. B continues to have difficulty with ambulating and appears to “fall forward” while trying to walk with assistance. His parents also say that B is not laughing, smiling, or showing any variation in facial expression.
Continue to: Additional family history...
Additional family history is gathered from B’s parents for possible hereditary movement disorders such as Wilson’s disease. They report that no family members have developed involuntary movements or other neurologic syndromes. Additional considerations on the differential diagnosis for B include juvenile ALS or mononeuropathy involving the C5 and C6 nerve roots. B’s parents deny any recent shoulder trauma, and radiographic studies did not demonstrate any involvement of the nerve roots.
TREATMENT A trial of bromocriptine
At this point, B’s neurologic workup is essentially normal, and he is given a provisional diagnosis of antipsychotic-induced tardive dystonia vs tardive parkinsonism. Risperidone continues to be held, and B is monitored for clinical improvement. B is administered a one-time dose of diphenhydramine, 25 mg, for dystonia with no improvement in symptoms. He is then started on bromocriptine, 1.25 mg twice daily with meals, for parkinsonian symptoms secondary to antipsychotic medication use. After 1 day of treatment, B shows less sustained flexion of his left wrist. He is able to relax his left arm, shows improvements in ambulation, and requires less assistance. B continues to be observed closely and continues to improve toward his baseline.
At Day 4, he is discharged. B is able to walk mostly without assistance and demonstrates improvement in left wrist flexion. He is scheduled to see a movement disorders specialist a week after discharge. The initial diagnosis given by the movement disorder specialist is tardive dystonia.
The authors’ observations
Tardive dyskinesia is a well-known iatrogenic effect of antipsychotic medications that are commonly used to manage conditions such as schizophrenia or behavioral agitation associated with autism spectrum disorder. Symptoms of tardive dyskinesia typically emerge after 1 to 2 years of continuous exposure to dopamine receptor blocking agents (DRBAs). Tardive dyskinesia symptoms include involuntary, repetitive, purposeless movements of the tongue, jaw, lips, face, trunk, and upper and lower extremities, with significant functional impairment.1
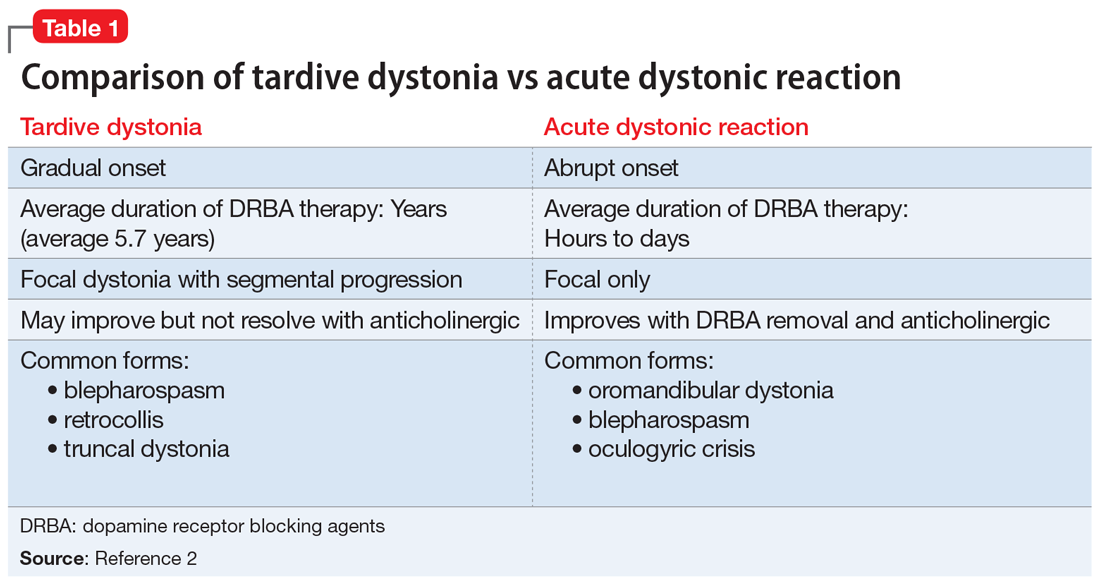
Tardive syndromes refer to a diverse array of hyperkinetic, hypokinetic, and sensory movement disorders resulting from at least 3 months of continuous DRBA therapy.2 Tardive dyskinesia is perhaps the most well-known of the tardive syndromes, but is not the only one to consider when assessing for antipsychotic-induced movement disorders. A key feature differentiating a tardive syndrome is the persistence of the movement disorder after the DRBA is discontinued. In this case, B had been receiving a stable dose of risperidone for >1 year. He developed dystonic posturing of his left wrist and elbow that was both unresponsive to anticholinergic medication and persisted after risperidone was discontinued. The term “tardive” emphasizes the delay in development of abnormal involuntary movement symptoms after initiating antipsychotic medications.3 Table 12 shows a comparison of tardive dystonia vs an acute dystonic reaction.
Continue to: Other tardive syndromes include...
Other tardive syndromes include:
- tardive tics
- tardive parkinsonism
- tardive pain
- tardive myoclonus
- tardive akathisia
- tardive tremors.
The incidence of tardive syndromes increases 5% annually for the first 5 years of treatment. At 10 years of treatment, the annual incidence is thought to be 49%, and at 25 years of treatment, 68%.4 The predominant theory of the pathophysiology of tardive syndromes is that the chronic use of DRBAs causes a gradual hypersensitization of dopamine receptors.4 The diagnosis of a tardive syndrome is based on history of exposure to a DRBA as well as clinical observation of symptoms.
Compared with classic tardive dyskinesia, tardive dystonia is more common among younger patients. The mean age of onset of tardive dystonia is 40, and it typically affects young males.5 Typical posturing observed in cases of tardive dystonia include extension of the arms and flexion at the wrists.6 In contrast to cases of primary dystonia, tardive dystonia is typically associated with stereotypies, akathisia, or other movement disorders. Anticholinergic agents, such as
The American Psychiatric Association has issued guidelines on screening for involuntary movement syndromes by using the Abnormal Involuntary Movement Scale (AIMS).7 The current recommendations include assessment every 6 months for patients receiving first-generation antipsychotics, and every 12 months for those receiving second-generation antipsychotics.7 Prescribers should also carefully assess for any pre-existing involuntary movements before prescribing a DRBA.7
[polldaddy:10615855]
The authors’ observations
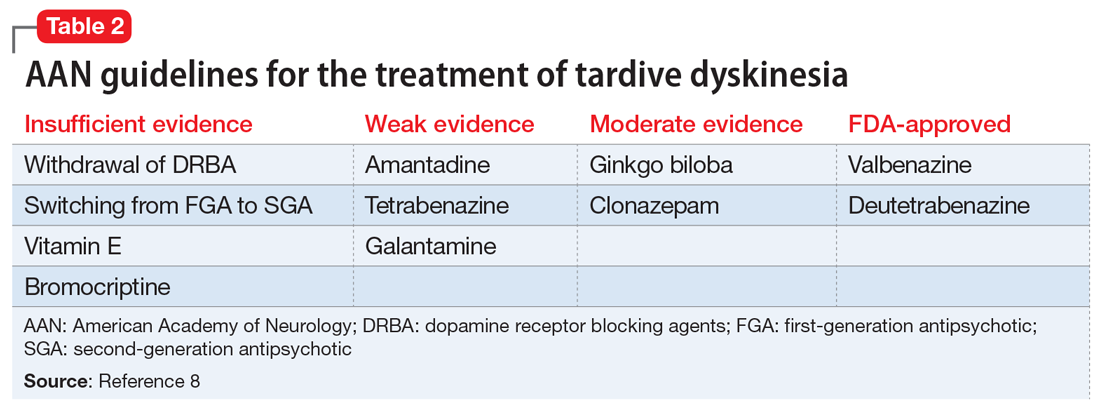
In 2013, the American Academy of Neurology (AAN) published guidelines on the treatment of tardive dyskinesia. According to these guidelines, at that time, the treatments with the most evidence supporting their use were clonazepam, ginkgo biloba,
Continue to: In 2017, valbenazine and deutetrabenazine...
In 2017, valbenazine and deutetrabenazine became the first FDA-approved treatments for tardive dyskinesia in adults. Both medications block the vesicular monoamine transporter 2 (VMAT2) system, which results in decreased synaptic dopamine and dopamine receptor stimulation. Both VMAT2 inhibitor medications have a category level A supporting their use for treating tardive dyskinesia.8-10
Currently, there are no published treatment guidelines on pharmacologic management of tardive dystonia. In B’s case, bromocriptine, a dopamine agonist, was used to counter the dopamine-blocking effects of risperidone on the nigrostriatal pathway and improve parkinsonian features of B’s presentation, including bradykinesia, stooped forward posture, and masked facies. Bromocriptine was found to be effective in alleviating parkinsonian features; however, to date there is no evidence demonstrating its effectiveness in countering delayed dystonic effects of DRBAs.
OUTCOME Improvement of dystonia symptoms
One week after discharge, B is seen for a follow-up visit. He continues taking bromocriptine, 1.25 mg twice daily, with meals after discharge. On examination, he has some evidence of tardive dystonia, including flexion of left wrist and posturing while ambulating. B’s parkinsonian features, including stooped forward posture, masked facies, and cogwheel rigidity of the left wrist muscle, have resolved. B is now able to walk on his own without unsteadiness. Bromocriptine is discontinued after 1 month, and his symptoms of dystonia continue to improve.
Two months after hospitalization, B is started on quetiapine, 25 mg twice daily, for behavioral aggression. Quetiapine is chosen because it has a lower dopamine receptor affinity compared with risperidone, and theoretically, quetiapine is associated with a lower risk of developing tardive symptoms. During the next 6 months, B is monitored closely for recurrence of tardive symptoms. Quetiapine is slowly titrated to 25 mg in the morning, and 50 mg at bedtime. His behavioral agitation improves significantly and he does not have a recurrence of tardive symptoms.
Bottom Line
Tardive dystonia is a possible iatrogenic adverse effect for patients receiving long-term dopamine receptor blocking agent (DRBA) therapy. Tardive syndromes encompass delayed-onset movement disorders caused by long-term blockade of the dopamine receptor by antipsychotic agents. Tardive dystonia can be contrasted from acute dystonic reaction based on the time course of development as well as by the persistence of symptoms after DRBAs are withheld.
Continue to: Related Resources
Related Resources
- American Academy of Neurology. Summary of evidence-based guideline for clinicians: treatment of tardive syndromes. https://www.aan.com/Guidelines/Home/GetGuidelineContent/613. Published 2013.
- Dystonia Medical Research Foundation. https://dystonia-foundation.org/.
Drug Brand Names
Amantadine • Gocovri, Symmetrel
Amoxicillin • Amoxil
Baclofen • Kemstro, Liroesal
Benztropine • Cogentin
Bromocriptine • Parlodel
Clonazepam • Klonopin
Deutetrabenazine • Austedo
Galantamine • Razadyne
Quetiapine • Seroquel
Risperidone • Risperdal
Tetrabenazine • Xenazine
Trihexyphenidyl • Artane, Tremin
Valbenazine • Ingrezza
1. Margolese HC, Chouinard G, Kolivakis TT, et al. Tardive dyskinesia in the era of typical and atypical antipsychotics. Part 1: pathophysiology and mechanisms of induction. Can J Psychiatr. 2005;50(9):541-547.
2. Truong D, Frei K. Setting the record straight: the nosology of tardive syndromes. Parkinsonism Relat Disord. 2019;59:146-150.
3. Cornett EM, Novitch M, Kaye AD, et al. Medication-induced tardive dyskinesia: a review and update. Ochsner J. 2017;17(2):162-174.
4. Schooler NR, Kane JM. Research diagnoses for tardive dyskinesia. Arch Gen Psychiatry. 1982;39(4):486-487.
5. Fahn S, Jankovic J, Hallett M. Principles and Practice of Movement Disorders. 2nd ed. Philadelphia, PA: Saunders; 2011:415-446.
6. Kang UJ, Burke RE, Fahn S. Natural history and treatment of tardive dystonia. Mov Disord. 1986;1(3):193-208.
7. Lehman AF, Lieberman JA, Dixon LB, et al. Practice guideline for the treatment of patients with schizophrenia, second edition. Am J Psychiatry. 2004;161(suppl 2):1-56.
8. Bhidayasiri R, Fahn S, Weiner WJ, et al, Evidence-based guideline: treatment of tardive syndromes: report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology. 2013;81(5):463-469.
9. Ingrezza [package insert]. San Diego, CA: Neurocrine Biosciences, Inc.; 2020.
10. Austedo [package insert]. North Wales, PA: Teva Pharmaceuticals; 2017.
CASE Drooling, unsteady, and not himself
B, age 10, who is left handed and has autism spectrum disorder, is brought to the emergency department (ED) with a 1-day history of drooling, unsteady gait, and left wrist in sustained flexion. His parents report that for the past week, B has had cold symptoms, including rhinorrhea, a low-grade fever (100.0°F), and cough. Earlier in the day, he was seen at his pediatrician’s office, where he was diagnosed with an acute respiratory infection and started on amoxicillin, 500 mg twice daily for 7 days.
At baseline, B is nonverbal. He requires some assistance with his activities of daily living. He usually is able to walk without assistance and dress himself, but he is not toilet trained. His parents report that in the past day, he has had significant difficulties with tasks involving his left hand. Normally, B is able to feed himself “finger foods” but has been unable to do so today. His parents say that he has been unsteady on his feet, and has been “falling forward” when he tries to walk.
Two years ago, B was started on risperidone, 0.5 mg nightly, for behavioral aggression and self-mutilation. Over the next 12 months, the dosage was steadily increased to 1 mg twice daily, with good response. He has been taking his current dosage, 1 mg twice daily, for the past 12 months without adjustment. His parents report there have been no other medication changes, other than starting amoxicillin earlier that day.
As part of his initial ED evaluation, B is found to be mildly dehydrated, with an elevated sedimentation rate on urinalysis. His complete blood count (CBC) with differential is within normal limits. A comprehensive metabolic panel shows a slight increase in his creatinine level, indicating dehydration. B is administered IV fluid replacement because he is having difficulty drinking due to excessive drooling.
The ED physician is concerned that B may be experiencing an acute dystonic reaction from risperidone, so the team holds this medication, and gives B a one-time dose of IV diphenhydramine, 25 mg, for presumptive acute dystonic reaction. After several minutes, there is no improvement in the sustained flexion of his left wrist.
[polldaddy:10615848]
The authors’ observations
B presented with new-onset neurologic findings after a recently diagnosed upper respiratory viral illness. His symptoms appeared to be confined to his left upper extremity, specifically demonstrating left arm extension at the elbow with flexion of the left wrist. He also had new-onset unsteady gait with a stooped forward posture and required assistance with walking. Interestingly, despite B’s history of antipsychotic use, administering an anticholinergic agent did not lessen the dystonic posturing at his wrist and elbow.
EVALUATION Laboratory results reveal new clues
While in the ED, B undergoes MRI of the brain and spinal cord to rule out any mass lesions that could be impinging upon the motor pathways. Both brain and spinal cord imaging appear to be essentially normal, without evidence of impingement of the spinal nerves or lesions involving the brainstem or cerebellum.
Continue to: Due to concerns...
Due to concerns of possible airway obstruction, a CT scan of the neck is obtained to rule out any acute pathology, such as epiglottitis compromising his airway. The scan shows some inflammation and edema in the soft tissues that is thought to be secondary to his acute viral illness. B is able to maintain his airway and oxygenation, so intubation is not necessary.
A CPK test is ordered because there are concerns of sustained muscle contraction of B’s left wrist and elbow. The CPK level is 884 U/L (reference range 26 to 192 U/L). The elevation in CPK is consistent with prior laboratory findings of dehydration and indicating skeletal muscle breakdown from sustained muscle contraction. All other laboratory results, including a comprehensive metabolic panel, urine drug screen, and thyroid screening panel, are within normal limits.
[polldaddy:10615850]
EVALUATION No variation in facial expression
B is admitted to the general pediatrics service. Maintenance IV fluids are started due to concerns of dehydration and possible rhabdomyolysis due to his elevated CPK level. Risperidone is held throughout the hospital course due to concerns for an acute dystonic reaction. B is monitored for several days without clinical improvement and eventually discharged home with a diagnosis of inflammatory mononeuropathy due to viral infection. The patient is told to discontinue risperidone as part of discharge instructions.
Five days later, B returns to the hospital because there was no improvement in his left extremity or walking. His left elbow remains extended with left wrist in flexion. Psychiatry is consulted for further diagnostic clarity and evaluation.
On physical examination, B’s left arm remains unchanged. Despite discontinuing risperidone, there is evidence of cogwheel rigidity of the left wrist joint. Reflexes in the upper and lower extremities are 2+ and symmetrical bilaterally, suggesting intact upper and lower motor pathways. Babinski sign is absent bilaterally, which is a normal finding in B’s age group. B continues to have difficulty with ambulating and appears to “fall forward” while trying to walk with assistance. His parents also say that B is not laughing, smiling, or showing any variation in facial expression.
Continue to: Additional family history...
Additional family history is gathered from B’s parents for possible hereditary movement disorders such as Wilson’s disease. They report that no family members have developed involuntary movements or other neurologic syndromes. Additional considerations on the differential diagnosis for B include juvenile ALS or mononeuropathy involving the C5 and C6 nerve roots. B’s parents deny any recent shoulder trauma, and radiographic studies did not demonstrate any involvement of the nerve roots.
TREATMENT A trial of bromocriptine
At this point, B’s neurologic workup is essentially normal, and he is given a provisional diagnosis of antipsychotic-induced tardive dystonia vs tardive parkinsonism. Risperidone continues to be held, and B is monitored for clinical improvement. B is administered a one-time dose of diphenhydramine, 25 mg, for dystonia with no improvement in symptoms. He is then started on bromocriptine, 1.25 mg twice daily with meals, for parkinsonian symptoms secondary to antipsychotic medication use. After 1 day of treatment, B shows less sustained flexion of his left wrist. He is able to relax his left arm, shows improvements in ambulation, and requires less assistance. B continues to be observed closely and continues to improve toward his baseline.
At Day 4, he is discharged. B is able to walk mostly without assistance and demonstrates improvement in left wrist flexion. He is scheduled to see a movement disorders specialist a week after discharge. The initial diagnosis given by the movement disorder specialist is tardive dystonia.
The authors’ observations
Tardive dyskinesia is a well-known iatrogenic effect of antipsychotic medications that are commonly used to manage conditions such as schizophrenia or behavioral agitation associated with autism spectrum disorder. Symptoms of tardive dyskinesia typically emerge after 1 to 2 years of continuous exposure to dopamine receptor blocking agents (DRBAs). Tardive dyskinesia symptoms include involuntary, repetitive, purposeless movements of the tongue, jaw, lips, face, trunk, and upper and lower extremities, with significant functional impairment.1
Tardive syndromes refer to a diverse array of hyperkinetic, hypokinetic, and sensory movement disorders resulting from at least 3 months of continuous DRBA therapy.2 Tardive dyskinesia is perhaps the most well-known of the tardive syndromes, but is not the only one to consider when assessing for antipsychotic-induced movement disorders. A key feature differentiating a tardive syndrome is the persistence of the movement disorder after the DRBA is discontinued. In this case, B had been receiving a stable dose of risperidone for >1 year. He developed dystonic posturing of his left wrist and elbow that was both unresponsive to anticholinergic medication and persisted after risperidone was discontinued. The term “tardive” emphasizes the delay in development of abnormal involuntary movement symptoms after initiating antipsychotic medications.3 Table 12 shows a comparison of tardive dystonia vs an acute dystonic reaction.

Continue to: Other tardive syndromes include...
Other tardive syndromes include:
- tardive tics
- tardive parkinsonism
- tardive pain
- tardive myoclonus
- tardive akathisia
- tardive tremors.
The incidence of tardive syndromes increases 5% annually for the first 5 years of treatment. At 10 years of treatment, the annual incidence is thought to be 49%, and at 25 years of treatment, 68%.4 The predominant theory of the pathophysiology of tardive syndromes is that the chronic use of DRBAs causes a gradual hypersensitization of dopamine receptors.4 The diagnosis of a tardive syndrome is based on history of exposure to a DRBA as well as clinical observation of symptoms.
Compared with classic tardive dyskinesia, tardive dystonia is more common among younger patients. The mean age of onset of tardive dystonia is 40, and it typically affects young males.5 Typical posturing observed in cases of tardive dystonia include extension of the arms and flexion at the wrists.6 In contrast to cases of primary dystonia, tardive dystonia is typically associated with stereotypies, akathisia, or other movement disorders. Anticholinergic agents, such as
The American Psychiatric Association has issued guidelines on screening for involuntary movement syndromes by using the Abnormal Involuntary Movement Scale (AIMS).7 The current recommendations include assessment every 6 months for patients receiving first-generation antipsychotics, and every 12 months for those receiving second-generation antipsychotics.7 Prescribers should also carefully assess for any pre-existing involuntary movements before prescribing a DRBA.7
[polldaddy:10615855]
The authors’ observations
In 2013, the American Academy of Neurology (AAN) published guidelines on the treatment of tardive dyskinesia. According to these guidelines, at that time, the treatments with the most evidence supporting their use were clonazepam, ginkgo biloba,

Continue to: In 2017, valbenazine and deutetrabenazine...
In 2017, valbenazine and deutetrabenazine became the first FDA-approved treatments for tardive dyskinesia in adults. Both medications block the vesicular monoamine transporter 2 (VMAT2) system, which results in decreased synaptic dopamine and dopamine receptor stimulation. Both VMAT2 inhibitor medications have a category level A supporting their use for treating tardive dyskinesia.8-10
Currently, there are no published treatment guidelines on pharmacologic management of tardive dystonia. In B’s case, bromocriptine, a dopamine agonist, was used to counter the dopamine-blocking effects of risperidone on the nigrostriatal pathway and improve parkinsonian features of B’s presentation, including bradykinesia, stooped forward posture, and masked facies. Bromocriptine was found to be effective in alleviating parkinsonian features; however, to date there is no evidence demonstrating its effectiveness in countering delayed dystonic effects of DRBAs.
OUTCOME Improvement of dystonia symptoms
One week after discharge, B is seen for a follow-up visit. He continues taking bromocriptine, 1.25 mg twice daily, with meals after discharge. On examination, he has some evidence of tardive dystonia, including flexion of left wrist and posturing while ambulating. B’s parkinsonian features, including stooped forward posture, masked facies, and cogwheel rigidity of the left wrist muscle, have resolved. B is now able to walk on his own without unsteadiness. Bromocriptine is discontinued after 1 month, and his symptoms of dystonia continue to improve.
Two months after hospitalization, B is started on quetiapine, 25 mg twice daily, for behavioral aggression. Quetiapine is chosen because it has a lower dopamine receptor affinity compared with risperidone, and theoretically, quetiapine is associated with a lower risk of developing tardive symptoms. During the next 6 months, B is monitored closely for recurrence of tardive symptoms. Quetiapine is slowly titrated to 25 mg in the morning, and 50 mg at bedtime. His behavioral agitation improves significantly and he does not have a recurrence of tardive symptoms.
Bottom Line
Tardive dystonia is a possible iatrogenic adverse effect for patients receiving long-term dopamine receptor blocking agent (DRBA) therapy. Tardive syndromes encompass delayed-onset movement disorders caused by long-term blockade of the dopamine receptor by antipsychotic agents. Tardive dystonia can be contrasted from acute dystonic reaction based on the time course of development as well as by the persistence of symptoms after DRBAs are withheld.
Continue to: Related Resources
Related Resources
- American Academy of Neurology. Summary of evidence-based guideline for clinicians: treatment of tardive syndromes. https://www.aan.com/Guidelines/Home/GetGuidelineContent/613. Published 2013.
- Dystonia Medical Research Foundation. https://dystonia-foundation.org/.
Drug Brand Names
Amantadine • Gocovri, Symmetrel
Amoxicillin • Amoxil
Baclofen • Kemstro, Liroesal
Benztropine • Cogentin
Bromocriptine • Parlodel
Clonazepam • Klonopin
Deutetrabenazine • Austedo
Galantamine • Razadyne
Quetiapine • Seroquel
Risperidone • Risperdal
Tetrabenazine • Xenazine
Trihexyphenidyl • Artane, Tremin
Valbenazine • Ingrezza
CASE Drooling, unsteady, and not himself
B, age 10, who is left handed and has autism spectrum disorder, is brought to the emergency department (ED) with a 1-day history of drooling, unsteady gait, and left wrist in sustained flexion. His parents report that for the past week, B has had cold symptoms, including rhinorrhea, a low-grade fever (100.0°F), and cough. Earlier in the day, he was seen at his pediatrician’s office, where he was diagnosed with an acute respiratory infection and started on amoxicillin, 500 mg twice daily for 7 days.
At baseline, B is nonverbal. He requires some assistance with his activities of daily living. He usually is able to walk without assistance and dress himself, but he is not toilet trained. His parents report that in the past day, he has had significant difficulties with tasks involving his left hand. Normally, B is able to feed himself “finger foods” but has been unable to do so today. His parents say that he has been unsteady on his feet, and has been “falling forward” when he tries to walk.
Two years ago, B was started on risperidone, 0.5 mg nightly, for behavioral aggression and self-mutilation. Over the next 12 months, the dosage was steadily increased to 1 mg twice daily, with good response. He has been taking his current dosage, 1 mg twice daily, for the past 12 months without adjustment. His parents report there have been no other medication changes, other than starting amoxicillin earlier that day.
As part of his initial ED evaluation, B is found to be mildly dehydrated, with an elevated sedimentation rate on urinalysis. His complete blood count (CBC) with differential is within normal limits. A comprehensive metabolic panel shows a slight increase in his creatinine level, indicating dehydration. B is administered IV fluid replacement because he is having difficulty drinking due to excessive drooling.
The ED physician is concerned that B may be experiencing an acute dystonic reaction from risperidone, so the team holds this medication, and gives B a one-time dose of IV diphenhydramine, 25 mg, for presumptive acute dystonic reaction. After several minutes, there is no improvement in the sustained flexion of his left wrist.
[polldaddy:10615848]
The authors’ observations
B presented with new-onset neurologic findings after a recently diagnosed upper respiratory viral illness. His symptoms appeared to be confined to his left upper extremity, specifically demonstrating left arm extension at the elbow with flexion of the left wrist. He also had new-onset unsteady gait with a stooped forward posture and required assistance with walking. Interestingly, despite B’s history of antipsychotic use, administering an anticholinergic agent did not lessen the dystonic posturing at his wrist and elbow.
EVALUATION Laboratory results reveal new clues
While in the ED, B undergoes MRI of the brain and spinal cord to rule out any mass lesions that could be impinging upon the motor pathways. Both brain and spinal cord imaging appear to be essentially normal, without evidence of impingement of the spinal nerves or lesions involving the brainstem or cerebellum.
Continue to: Due to concerns...
Due to concerns of possible airway obstruction, a CT scan of the neck is obtained to rule out any acute pathology, such as epiglottitis compromising his airway. The scan shows some inflammation and edema in the soft tissues that is thought to be secondary to his acute viral illness. B is able to maintain his airway and oxygenation, so intubation is not necessary.
A CPK test is ordered because there are concerns of sustained muscle contraction of B’s left wrist and elbow. The CPK level is 884 U/L (reference range 26 to 192 U/L). The elevation in CPK is consistent with prior laboratory findings of dehydration and indicating skeletal muscle breakdown from sustained muscle contraction. All other laboratory results, including a comprehensive metabolic panel, urine drug screen, and thyroid screening panel, are within normal limits.
[polldaddy:10615850]
EVALUATION No variation in facial expression
B is admitted to the general pediatrics service. Maintenance IV fluids are started due to concerns of dehydration and possible rhabdomyolysis due to his elevated CPK level. Risperidone is held throughout the hospital course due to concerns for an acute dystonic reaction. B is monitored for several days without clinical improvement and eventually discharged home with a diagnosis of inflammatory mononeuropathy due to viral infection. The patient is told to discontinue risperidone as part of discharge instructions.
Five days later, B returns to the hospital because there was no improvement in his left extremity or walking. His left elbow remains extended with left wrist in flexion. Psychiatry is consulted for further diagnostic clarity and evaluation.
On physical examination, B’s left arm remains unchanged. Despite discontinuing risperidone, there is evidence of cogwheel rigidity of the left wrist joint. Reflexes in the upper and lower extremities are 2+ and symmetrical bilaterally, suggesting intact upper and lower motor pathways. Babinski sign is absent bilaterally, which is a normal finding in B’s age group. B continues to have difficulty with ambulating and appears to “fall forward” while trying to walk with assistance. His parents also say that B is not laughing, smiling, or showing any variation in facial expression.
Continue to: Additional family history...
Additional family history is gathered from B’s parents for possible hereditary movement disorders such as Wilson’s disease. They report that no family members have developed involuntary movements or other neurologic syndromes. Additional considerations on the differential diagnosis for B include juvenile ALS or mononeuropathy involving the C5 and C6 nerve roots. B’s parents deny any recent shoulder trauma, and radiographic studies did not demonstrate any involvement of the nerve roots.
TREATMENT A trial of bromocriptine
At this point, B’s neurologic workup is essentially normal, and he is given a provisional diagnosis of antipsychotic-induced tardive dystonia vs tardive parkinsonism. Risperidone continues to be held, and B is monitored for clinical improvement. B is administered a one-time dose of diphenhydramine, 25 mg, for dystonia with no improvement in symptoms. He is then started on bromocriptine, 1.25 mg twice daily with meals, for parkinsonian symptoms secondary to antipsychotic medication use. After 1 day of treatment, B shows less sustained flexion of his left wrist. He is able to relax his left arm, shows improvements in ambulation, and requires less assistance. B continues to be observed closely and continues to improve toward his baseline.
At Day 4, he is discharged. B is able to walk mostly without assistance and demonstrates improvement in left wrist flexion. He is scheduled to see a movement disorders specialist a week after discharge. The initial diagnosis given by the movement disorder specialist is tardive dystonia.
The authors’ observations
Tardive dyskinesia is a well-known iatrogenic effect of antipsychotic medications that are commonly used to manage conditions such as schizophrenia or behavioral agitation associated with autism spectrum disorder. Symptoms of tardive dyskinesia typically emerge after 1 to 2 years of continuous exposure to dopamine receptor blocking agents (DRBAs). Tardive dyskinesia symptoms include involuntary, repetitive, purposeless movements of the tongue, jaw, lips, face, trunk, and upper and lower extremities, with significant functional impairment.1
Tardive syndromes refer to a diverse array of hyperkinetic, hypokinetic, and sensory movement disorders resulting from at least 3 months of continuous DRBA therapy.2 Tardive dyskinesia is perhaps the most well-known of the tardive syndromes, but is not the only one to consider when assessing for antipsychotic-induced movement disorders. A key feature differentiating a tardive syndrome is the persistence of the movement disorder after the DRBA is discontinued. In this case, B had been receiving a stable dose of risperidone for >1 year. He developed dystonic posturing of his left wrist and elbow that was both unresponsive to anticholinergic medication and persisted after risperidone was discontinued. The term “tardive” emphasizes the delay in development of abnormal involuntary movement symptoms after initiating antipsychotic medications.3 Table 12 shows a comparison of tardive dystonia vs an acute dystonic reaction.

Continue to: Other tardive syndromes include...
Other tardive syndromes include:
- tardive tics
- tardive parkinsonism
- tardive pain
- tardive myoclonus
- tardive akathisia
- tardive tremors.
The incidence of tardive syndromes increases 5% annually for the first 5 years of treatment. At 10 years of treatment, the annual incidence is thought to be 49%, and at 25 years of treatment, 68%.4 The predominant theory of the pathophysiology of tardive syndromes is that the chronic use of DRBAs causes a gradual hypersensitization of dopamine receptors.4 The diagnosis of a tardive syndrome is based on history of exposure to a DRBA as well as clinical observation of symptoms.
Compared with classic tardive dyskinesia, tardive dystonia is more common among younger patients. The mean age of onset of tardive dystonia is 40, and it typically affects young males.5 Typical posturing observed in cases of tardive dystonia include extension of the arms and flexion at the wrists.6 In contrast to cases of primary dystonia, tardive dystonia is typically associated with stereotypies, akathisia, or other movement disorders. Anticholinergic agents, such as
The American Psychiatric Association has issued guidelines on screening for involuntary movement syndromes by using the Abnormal Involuntary Movement Scale (AIMS).7 The current recommendations include assessment every 6 months for patients receiving first-generation antipsychotics, and every 12 months for those receiving second-generation antipsychotics.7 Prescribers should also carefully assess for any pre-existing involuntary movements before prescribing a DRBA.7
[polldaddy:10615855]
The authors’ observations
In 2013, the American Academy of Neurology (AAN) published guidelines on the treatment of tardive dyskinesia. According to these guidelines, at that time, the treatments with the most evidence supporting their use were clonazepam, ginkgo biloba,

Continue to: In 2017, valbenazine and deutetrabenazine...
In 2017, valbenazine and deutetrabenazine became the first FDA-approved treatments for tardive dyskinesia in adults. Both medications block the vesicular monoamine transporter 2 (VMAT2) system, which results in decreased synaptic dopamine and dopamine receptor stimulation. Both VMAT2 inhibitor medications have a category level A supporting their use for treating tardive dyskinesia.8-10
Currently, there are no published treatment guidelines on pharmacologic management of tardive dystonia. In B’s case, bromocriptine, a dopamine agonist, was used to counter the dopamine-blocking effects of risperidone on the nigrostriatal pathway and improve parkinsonian features of B’s presentation, including bradykinesia, stooped forward posture, and masked facies. Bromocriptine was found to be effective in alleviating parkinsonian features; however, to date there is no evidence demonstrating its effectiveness in countering delayed dystonic effects of DRBAs.
OUTCOME Improvement of dystonia symptoms
One week after discharge, B is seen for a follow-up visit. He continues taking bromocriptine, 1.25 mg twice daily, with meals after discharge. On examination, he has some evidence of tardive dystonia, including flexion of left wrist and posturing while ambulating. B’s parkinsonian features, including stooped forward posture, masked facies, and cogwheel rigidity of the left wrist muscle, have resolved. B is now able to walk on his own without unsteadiness. Bromocriptine is discontinued after 1 month, and his symptoms of dystonia continue to improve.
Two months after hospitalization, B is started on quetiapine, 25 mg twice daily, for behavioral aggression. Quetiapine is chosen because it has a lower dopamine receptor affinity compared with risperidone, and theoretically, quetiapine is associated with a lower risk of developing tardive symptoms. During the next 6 months, B is monitored closely for recurrence of tardive symptoms. Quetiapine is slowly titrated to 25 mg in the morning, and 50 mg at bedtime. His behavioral agitation improves significantly and he does not have a recurrence of tardive symptoms.
Bottom Line
Tardive dystonia is a possible iatrogenic adverse effect for patients receiving long-term dopamine receptor blocking agent (DRBA) therapy. Tardive syndromes encompass delayed-onset movement disorders caused by long-term blockade of the dopamine receptor by antipsychotic agents. Tardive dystonia can be contrasted from acute dystonic reaction based on the time course of development as well as by the persistence of symptoms after DRBAs are withheld.
Continue to: Related Resources
Related Resources
- American Academy of Neurology. Summary of evidence-based guideline for clinicians: treatment of tardive syndromes. https://www.aan.com/Guidelines/Home/GetGuidelineContent/613. Published 2013.
- Dystonia Medical Research Foundation. https://dystonia-foundation.org/.
Drug Brand Names
Amantadine • Gocovri, Symmetrel
Amoxicillin • Amoxil
Baclofen • Kemstro, Liroesal
Benztropine • Cogentin
Bromocriptine • Parlodel
Clonazepam • Klonopin
Deutetrabenazine • Austedo
Galantamine • Razadyne
Quetiapine • Seroquel
Risperidone • Risperdal
Tetrabenazine • Xenazine
Trihexyphenidyl • Artane, Tremin
Valbenazine • Ingrezza
1. Margolese HC, Chouinard G, Kolivakis TT, et al. Tardive dyskinesia in the era of typical and atypical antipsychotics. Part 1: pathophysiology and mechanisms of induction. Can J Psychiatr. 2005;50(9):541-547.
2. Truong D, Frei K. Setting the record straight: the nosology of tardive syndromes. Parkinsonism Relat Disord. 2019;59:146-150.
3. Cornett EM, Novitch M, Kaye AD, et al. Medication-induced tardive dyskinesia: a review and update. Ochsner J. 2017;17(2):162-174.
4. Schooler NR, Kane JM. Research diagnoses for tardive dyskinesia. Arch Gen Psychiatry. 1982;39(4):486-487.
5. Fahn S, Jankovic J, Hallett M. Principles and Practice of Movement Disorders. 2nd ed. Philadelphia, PA: Saunders; 2011:415-446.
6. Kang UJ, Burke RE, Fahn S. Natural history and treatment of tardive dystonia. Mov Disord. 1986;1(3):193-208.
7. Lehman AF, Lieberman JA, Dixon LB, et al. Practice guideline for the treatment of patients with schizophrenia, second edition. Am J Psychiatry. 2004;161(suppl 2):1-56.
8. Bhidayasiri R, Fahn S, Weiner WJ, et al, Evidence-based guideline: treatment of tardive syndromes: report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology. 2013;81(5):463-469.
9. Ingrezza [package insert]. San Diego, CA: Neurocrine Biosciences, Inc.; 2020.
10. Austedo [package insert]. North Wales, PA: Teva Pharmaceuticals; 2017.
1. Margolese HC, Chouinard G, Kolivakis TT, et al. Tardive dyskinesia in the era of typical and atypical antipsychotics. Part 1: pathophysiology and mechanisms of induction. Can J Psychiatr. 2005;50(9):541-547.
2. Truong D, Frei K. Setting the record straight: the nosology of tardive syndromes. Parkinsonism Relat Disord. 2019;59:146-150.
3. Cornett EM, Novitch M, Kaye AD, et al. Medication-induced tardive dyskinesia: a review and update. Ochsner J. 2017;17(2):162-174.
4. Schooler NR, Kane JM. Research diagnoses for tardive dyskinesia. Arch Gen Psychiatry. 1982;39(4):486-487.
5. Fahn S, Jankovic J, Hallett M. Principles and Practice of Movement Disorders. 2nd ed. Philadelphia, PA: Saunders; 2011:415-446.
6. Kang UJ, Burke RE, Fahn S. Natural history and treatment of tardive dystonia. Mov Disord. 1986;1(3):193-208.
7. Lehman AF, Lieberman JA, Dixon LB, et al. Practice guideline for the treatment of patients with schizophrenia, second edition. Am J Psychiatry. 2004;161(suppl 2):1-56.
8. Bhidayasiri R, Fahn S, Weiner WJ, et al, Evidence-based guideline: treatment of tardive syndromes: report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology. 2013;81(5):463-469.
9. Ingrezza [package insert]. San Diego, CA: Neurocrine Biosciences, Inc.; 2020.
10. Austedo [package insert]. North Wales, PA: Teva Pharmaceuticals; 2017.
Obsessions or psychosis?
CASE Perseverating on nonexistent sexual assaults
Mr. R, age 17, who has been diagnosed with obsessive-compulsive disorder (OCD), presents to the emergency department (ED) because he thinks that he is being sexually assaulted and is concerned that he is sexually assaulting other people. His family reports that Mr. R has perseverated over these thoughts for months, although there is no evidence to suggest these events have occurred. In order to ameliorate his distress, he performs rituals of looking upwards and repeatedly saying, “It didn’t happen.”
Mr. R is admitted to the inpatient psychiatry unit for further evaluation.
HISTORY Decompensation while attending a PHP
Mr. R had been diagnosed with bipolar disorder and attention-deficit/hyperactivity disorder when he was 13. During that time, he was treated with divalproex sodium and dextroamphetamine. At age 15, Mr. R’s diagnosis was changed to OCD. Seven months before coming to the ED, his symptoms had been getting worse. On one occasion, Mr. R was talking in a nonsensical fashion at school, and the police were called. Mr. R became physically aggressive with the police and was subsequently hospitalized, after which he attended a partial hospitalization program (PHP). At the PHP, Mr. R received exposure and response prevention therapy for OCD, but did not improve, and his symptoms deteriorated until he was unable to brush his teeth or shower regularly. While attending the PHP, Mr. R also developed disorganized speech. The PHP clinicians became concerned that Mr. R’s symptoms may have been prodromal symptoms of schizophrenia because he did not respond to the OCD treatment and his symptoms had worsened over the 3 months he attended the PHP.
EVALUATION Normal laboratory results
Upon admission to the inpatient psychiatric unit, Mr. R is restarted on his home medications, which include
His laboratory workup, including a complete blood count, comprehensive metabolic panel, urine drug screen, and blood ethanol, are all within normal limits. Previous laboratory results, including a thyroid function panel, vitamin D level, and various autoimmune panels, were also within normal limits.
His family reports that Mr. R’s symptoms seem to worsen when he is under increased stress from school and prepping for standardized college admission examinations. The family also says that while he is playing tennis, Mr. R will posture himself in a crouched down position and at times will remain in this position for 30 minutes.
Mr. R says he eventually wants to go to college and have a professional career.
[polldaddy:10600530]
Continue to: The authors' observations
The authors’ observations
When considering Mr. R’s diagnosis, our treatment team considered the possibility of OCD with absent insight/delusional beliefs, OCD with comorbid schizophrenia, bipolar disorder, and psychotic disorder due to another medical condition.
Overlap between OCD and schizophrenia
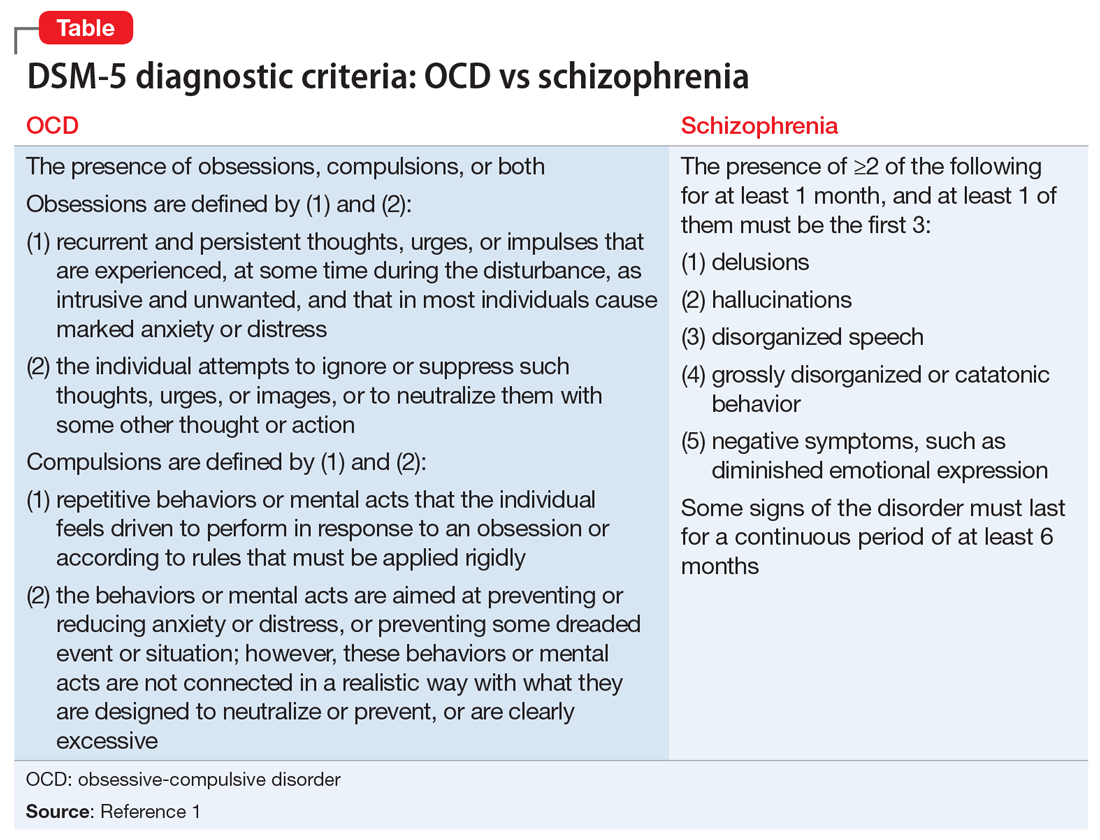
Much of the literature about OCD examines its functional impairment in adults, with findings extrapolated to pediatric patients. Children differ from adults in a variety of meaningful ways. Baytunca et al4 examined adolescents with early-onset schizophrenia, with and without comorbid OCD. Patients with comorbid OCD required higher doses of antipsychotic medication to treat acute psychotic symptoms and maintain a reduction in symptoms. The study controlled for the severity of schizophrenia, and its findings suggest that schizophrenia with comorbid OCD is more treatment-resistant than schizophrenia alone.4
Some researchers have theorized that in adolescents, OCD and psychosis are integrally related such that one disorder could represent a prodrome or a cause of the other disorder. Niendam et al5 studied OCS in the psychosis prodrome. They found that OCS can present as a part of the prodromal picture in youth at high risk for psychosis. However, because none of the patients experiencing OCS converted to full-blown psychosis, these results suggest that OCS may not represent a prodrome to psychosis per se. Instead, these individuals may represent a subset of false positives over the follow-up period.5 Another possible explanation for the increased emergence of pre-psychotic symptoms in adolescents with OCD could be a difference in their threshold of perception. OCS compels adolescents with OCD to self-analyze more critically and frequently. As a result, these patients may more often report depressive symptoms, distress, and exacerbations of pre-psychotic symptoms. These findings highlight that
[polldaddy:10600532]
Continue to: TREATMENT Improvement after switching to haloperidol
TREATMENT Improvement after switching to haloperidol
The treatment team decides to change Mr. R’s medications by cross-titrating risperidone to
The treatment team obtains a consultation on whether electroconvulsive therapy would be appropriate, but this treatment is not recommended. Instead, the team considers
Throughout admission, Mr. R focuses on his lack of improvement and how this episode is negatively impacting his grades and his dream of going to college and having a professional career.
OUTCOME Relief at last
Mr. R improves with the addition of sertraline and tolerates rapid titration well. He continues haloperidol without adverse effects, and is discharged home with close follow-up in a PHP and outpatient psychiatry.
However, after discharge, Mr. R’s symptoms get worse, and he is admitted to a different inpatient facility. At this facility, he continues sertraline, but haloperidol is cross-titrated to
Continue to: Currently...
Currently, Mr. R has greatly improved and is able to function in school. He takes sertraline, 100 mg twice a day, and olanzapine, 7.5 mg twice a day. Mr. R reports his rituals have reduced in frequency to less than 15 minutes each day. His thought processes are organized, and he is confident he will be able to achieve his goals.
The authors’ observations
Given Mr. R’s rapid improvement once an effective pharmacologic regimen was established, we concluded that he had a severe case of OCD with absent insight/delusional beliefs, and that he did not have schizophrenia. Mr. R’s case highlights how a psychiatric diagnosis can produce anxiety as a result of the psychosocial stressors and limitations associated with that diagnosis.
Bottom Line
There is both an epidemiologic and biologic overlap between obsessive-compulsive disorder and schizophrenia. In adolescents, either disorder could represent a prodrome or a cause of the other. It is essential to perform a thorough assessment of individuals with obsessive-compulsive disorder because these patients may exhibit subtle psychotic symptoms.
Related Resources
- Cunill R, Castells X, Simeon D. Relationships between obsessivecompulsive symptomatology and severity of psychosis in schizophrenia: a systematic review and meta-analysis. J Clin Psychiatry. 2009;70(1):70-82.
- Harris E, Delgado SV. Treatment-resistant OCD: there’s more we can do. Current Psychiatry. 2018;17(11):10-12,14-18,51.
Drug Brand Names
Clozapine • Clozaril
Dextroamphetamine • Dexedrine
Divalproex sodium • Depakote
Fluvoxamine • Luvox
Haloperidol • Haldol
Hydroxyzine • Atarax, Vistaril
Lurasidone • Latuda
Olanzapine • Zyprexa
Risperidone • Risperdal
Sertraline • Zoloft
1. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
2. Schirmbeck F, Swets M, de Haan L. Obsessive-compulsive symptoms in schizophrenia. In: De Haan L, Schirmbeck F, Zink M. Epidemiology: prevalence and clinical characteristics of obsessive-compulsive disorder and obsessive-compulsive symptoms in patients with psychotic disorders. New York, NY: Springer International Publishing; 2015:47-61.
3. de Haan L, Sterk B, Wouters L, et al. The 5-year course of obsessive-compulsive symptoms and obsessive-compulsive disorder in first-episode schizophrenia and related disorders. Schizophr Bull. 2011;39(1):151-160.
4. Baytunca B, Kalyoncu T, Ozel I, et al. Early onset schizophrenia associated with obsessive-compulsive disorder: clinical features and correlates. Clin Neuropharmacol. 2017;40(6):243-245.
5. Niendam TA, Berzak J, Cannon TD, et al. Obsessive compulsive symptoms in the psychosis prodrome: correlates of clinical and functional outcome. Schizophr Res. 2009;108(1-3):170-175.
CASE Perseverating on nonexistent sexual assaults
Mr. R, age 17, who has been diagnosed with obsessive-compulsive disorder (OCD), presents to the emergency department (ED) because he thinks that he is being sexually assaulted and is concerned that he is sexually assaulting other people. His family reports that Mr. R has perseverated over these thoughts for months, although there is no evidence to suggest these events have occurred. In order to ameliorate his distress, he performs rituals of looking upwards and repeatedly saying, “It didn’t happen.”
Mr. R is admitted to the inpatient psychiatry unit for further evaluation.
HISTORY Decompensation while attending a PHP
Mr. R had been diagnosed with bipolar disorder and attention-deficit/hyperactivity disorder when he was 13. During that time, he was treated with divalproex sodium and dextroamphetamine. At age 15, Mr. R’s diagnosis was changed to OCD. Seven months before coming to the ED, his symptoms had been getting worse. On one occasion, Mr. R was talking in a nonsensical fashion at school, and the police were called. Mr. R became physically aggressive with the police and was subsequently hospitalized, after which he attended a partial hospitalization program (PHP). At the PHP, Mr. R received exposure and response prevention therapy for OCD, but did not improve, and his symptoms deteriorated until he was unable to brush his teeth or shower regularly. While attending the PHP, Mr. R also developed disorganized speech. The PHP clinicians became concerned that Mr. R’s symptoms may have been prodromal symptoms of schizophrenia because he did not respond to the OCD treatment and his symptoms had worsened over the 3 months he attended the PHP.
EVALUATION Normal laboratory results
Upon admission to the inpatient psychiatric unit, Mr. R is restarted on his home medications, which include
His laboratory workup, including a complete blood count, comprehensive metabolic panel, urine drug screen, and blood ethanol, are all within normal limits. Previous laboratory results, including a thyroid function panel, vitamin D level, and various autoimmune panels, were also within normal limits.
His family reports that Mr. R’s symptoms seem to worsen when he is under increased stress from school and prepping for standardized college admission examinations. The family also says that while he is playing tennis, Mr. R will posture himself in a crouched down position and at times will remain in this position for 30 minutes.
Mr. R says he eventually wants to go to college and have a professional career.
[polldaddy:10600530]
Continue to: The authors' observations
The authors’ observations
When considering Mr. R’s diagnosis, our treatment team considered the possibility of OCD with absent insight/delusional beliefs, OCD with comorbid schizophrenia, bipolar disorder, and psychotic disorder due to another medical condition.
Overlap between OCD and schizophrenia

Much of the literature about OCD examines its functional impairment in adults, with findings extrapolated to pediatric patients. Children differ from adults in a variety of meaningful ways. Baytunca et al4 examined adolescents with early-onset schizophrenia, with and without comorbid OCD. Patients with comorbid OCD required higher doses of antipsychotic medication to treat acute psychotic symptoms and maintain a reduction in symptoms. The study controlled for the severity of schizophrenia, and its findings suggest that schizophrenia with comorbid OCD is more treatment-resistant than schizophrenia alone.4
Some researchers have theorized that in adolescents, OCD and psychosis are integrally related such that one disorder could represent a prodrome or a cause of the other disorder. Niendam et al5 studied OCS in the psychosis prodrome. They found that OCS can present as a part of the prodromal picture in youth at high risk for psychosis. However, because none of the patients experiencing OCS converted to full-blown psychosis, these results suggest that OCS may not represent a prodrome to psychosis per se. Instead, these individuals may represent a subset of false positives over the follow-up period.5 Another possible explanation for the increased emergence of pre-psychotic symptoms in adolescents with OCD could be a difference in their threshold of perception. OCS compels adolescents with OCD to self-analyze more critically and frequently. As a result, these patients may more often report depressive symptoms, distress, and exacerbations of pre-psychotic symptoms. These findings highlight that
[polldaddy:10600532]
Continue to: TREATMENT Improvement after switching to haloperidol
TREATMENT Improvement after switching to haloperidol
The treatment team decides to change Mr. R’s medications by cross-titrating risperidone to
The treatment team obtains a consultation on whether electroconvulsive therapy would be appropriate, but this treatment is not recommended. Instead, the team considers
Throughout admission, Mr. R focuses on his lack of improvement and how this episode is negatively impacting his grades and his dream of going to college and having a professional career.
OUTCOME Relief at last
Mr. R improves with the addition of sertraline and tolerates rapid titration well. He continues haloperidol without adverse effects, and is discharged home with close follow-up in a PHP and outpatient psychiatry.
However, after discharge, Mr. R’s symptoms get worse, and he is admitted to a different inpatient facility. At this facility, he continues sertraline, but haloperidol is cross-titrated to
Continue to: Currently...
Currently, Mr. R has greatly improved and is able to function in school. He takes sertraline, 100 mg twice a day, and olanzapine, 7.5 mg twice a day. Mr. R reports his rituals have reduced in frequency to less than 15 minutes each day. His thought processes are organized, and he is confident he will be able to achieve his goals.
The authors’ observations
Given Mr. R’s rapid improvement once an effective pharmacologic regimen was established, we concluded that he had a severe case of OCD with absent insight/delusional beliefs, and that he did not have schizophrenia. Mr. R’s case highlights how a psychiatric diagnosis can produce anxiety as a result of the psychosocial stressors and limitations associated with that diagnosis.
Bottom Line
There is both an epidemiologic and biologic overlap between obsessive-compulsive disorder and schizophrenia. In adolescents, either disorder could represent a prodrome or a cause of the other. It is essential to perform a thorough assessment of individuals with obsessive-compulsive disorder because these patients may exhibit subtle psychotic symptoms.
Related Resources
- Cunill R, Castells X, Simeon D. Relationships between obsessivecompulsive symptomatology and severity of psychosis in schizophrenia: a systematic review and meta-analysis. J Clin Psychiatry. 2009;70(1):70-82.
- Harris E, Delgado SV. Treatment-resistant OCD: there’s more we can do. Current Psychiatry. 2018;17(11):10-12,14-18,51.
Drug Brand Names
Clozapine • Clozaril
Dextroamphetamine • Dexedrine
Divalproex sodium • Depakote
Fluvoxamine • Luvox
Haloperidol • Haldol
Hydroxyzine • Atarax, Vistaril
Lurasidone • Latuda
Olanzapine • Zyprexa
Risperidone • Risperdal
Sertraline • Zoloft
CASE Perseverating on nonexistent sexual assaults
Mr. R, age 17, who has been diagnosed with obsessive-compulsive disorder (OCD), presents to the emergency department (ED) because he thinks that he is being sexually assaulted and is concerned that he is sexually assaulting other people. His family reports that Mr. R has perseverated over these thoughts for months, although there is no evidence to suggest these events have occurred. In order to ameliorate his distress, he performs rituals of looking upwards and repeatedly saying, “It didn’t happen.”
Mr. R is admitted to the inpatient psychiatry unit for further evaluation.
HISTORY Decompensation while attending a PHP
Mr. R had been diagnosed with bipolar disorder and attention-deficit/hyperactivity disorder when he was 13. During that time, he was treated with divalproex sodium and dextroamphetamine. At age 15, Mr. R’s diagnosis was changed to OCD. Seven months before coming to the ED, his symptoms had been getting worse. On one occasion, Mr. R was talking in a nonsensical fashion at school, and the police were called. Mr. R became physically aggressive with the police and was subsequently hospitalized, after which he attended a partial hospitalization program (PHP). At the PHP, Mr. R received exposure and response prevention therapy for OCD, but did not improve, and his symptoms deteriorated until he was unable to brush his teeth or shower regularly. While attending the PHP, Mr. R also developed disorganized speech. The PHP clinicians became concerned that Mr. R’s symptoms may have been prodromal symptoms of schizophrenia because he did not respond to the OCD treatment and his symptoms had worsened over the 3 months he attended the PHP.
EVALUATION Normal laboratory results
Upon admission to the inpatient psychiatric unit, Mr. R is restarted on his home medications, which include
His laboratory workup, including a complete blood count, comprehensive metabolic panel, urine drug screen, and blood ethanol, are all within normal limits. Previous laboratory results, including a thyroid function panel, vitamin D level, and various autoimmune panels, were also within normal limits.
His family reports that Mr. R’s symptoms seem to worsen when he is under increased stress from school and prepping for standardized college admission examinations. The family also says that while he is playing tennis, Mr. R will posture himself in a crouched down position and at times will remain in this position for 30 minutes.
Mr. R says he eventually wants to go to college and have a professional career.
[polldaddy:10600530]
Continue to: The authors' observations
The authors’ observations
When considering Mr. R’s diagnosis, our treatment team considered the possibility of OCD with absent insight/delusional beliefs, OCD with comorbid schizophrenia, bipolar disorder, and psychotic disorder due to another medical condition.
Overlap between OCD and schizophrenia

Much of the literature about OCD examines its functional impairment in adults, with findings extrapolated to pediatric patients. Children differ from adults in a variety of meaningful ways. Baytunca et al4 examined adolescents with early-onset schizophrenia, with and without comorbid OCD. Patients with comorbid OCD required higher doses of antipsychotic medication to treat acute psychotic symptoms and maintain a reduction in symptoms. The study controlled for the severity of schizophrenia, and its findings suggest that schizophrenia with comorbid OCD is more treatment-resistant than schizophrenia alone.4
Some researchers have theorized that in adolescents, OCD and psychosis are integrally related such that one disorder could represent a prodrome or a cause of the other disorder. Niendam et al5 studied OCS in the psychosis prodrome. They found that OCS can present as a part of the prodromal picture in youth at high risk for psychosis. However, because none of the patients experiencing OCS converted to full-blown psychosis, these results suggest that OCS may not represent a prodrome to psychosis per se. Instead, these individuals may represent a subset of false positives over the follow-up period.5 Another possible explanation for the increased emergence of pre-psychotic symptoms in adolescents with OCD could be a difference in their threshold of perception. OCS compels adolescents with OCD to self-analyze more critically and frequently. As a result, these patients may more often report depressive symptoms, distress, and exacerbations of pre-psychotic symptoms. These findings highlight that
[polldaddy:10600532]
Continue to: TREATMENT Improvement after switching to haloperidol
TREATMENT Improvement after switching to haloperidol
The treatment team decides to change Mr. R’s medications by cross-titrating risperidone to
The treatment team obtains a consultation on whether electroconvulsive therapy would be appropriate, but this treatment is not recommended. Instead, the team considers
Throughout admission, Mr. R focuses on his lack of improvement and how this episode is negatively impacting his grades and his dream of going to college and having a professional career.
OUTCOME Relief at last
Mr. R improves with the addition of sertraline and tolerates rapid titration well. He continues haloperidol without adverse effects, and is discharged home with close follow-up in a PHP and outpatient psychiatry.
However, after discharge, Mr. R’s symptoms get worse, and he is admitted to a different inpatient facility. At this facility, he continues sertraline, but haloperidol is cross-titrated to
Continue to: Currently...
Currently, Mr. R has greatly improved and is able to function in school. He takes sertraline, 100 mg twice a day, and olanzapine, 7.5 mg twice a day. Mr. R reports his rituals have reduced in frequency to less than 15 minutes each day. His thought processes are organized, and he is confident he will be able to achieve his goals.
The authors’ observations
Given Mr. R’s rapid improvement once an effective pharmacologic regimen was established, we concluded that he had a severe case of OCD with absent insight/delusional beliefs, and that he did not have schizophrenia. Mr. R’s case highlights how a psychiatric diagnosis can produce anxiety as a result of the psychosocial stressors and limitations associated with that diagnosis.
Bottom Line
There is both an epidemiologic and biologic overlap between obsessive-compulsive disorder and schizophrenia. In adolescents, either disorder could represent a prodrome or a cause of the other. It is essential to perform a thorough assessment of individuals with obsessive-compulsive disorder because these patients may exhibit subtle psychotic symptoms.
Related Resources
- Cunill R, Castells X, Simeon D. Relationships between obsessivecompulsive symptomatology and severity of psychosis in schizophrenia: a systematic review and meta-analysis. J Clin Psychiatry. 2009;70(1):70-82.
- Harris E, Delgado SV. Treatment-resistant OCD: there’s more we can do. Current Psychiatry. 2018;17(11):10-12,14-18,51.
Drug Brand Names
Clozapine • Clozaril
Dextroamphetamine • Dexedrine
Divalproex sodium • Depakote
Fluvoxamine • Luvox
Haloperidol • Haldol
Hydroxyzine • Atarax, Vistaril
Lurasidone • Latuda
Olanzapine • Zyprexa
Risperidone • Risperdal
Sertraline • Zoloft
1. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
2. Schirmbeck F, Swets M, de Haan L. Obsessive-compulsive symptoms in schizophrenia. In: De Haan L, Schirmbeck F, Zink M. Epidemiology: prevalence and clinical characteristics of obsessive-compulsive disorder and obsessive-compulsive symptoms in patients with psychotic disorders. New York, NY: Springer International Publishing; 2015:47-61.
3. de Haan L, Sterk B, Wouters L, et al. The 5-year course of obsessive-compulsive symptoms and obsessive-compulsive disorder in first-episode schizophrenia and related disorders. Schizophr Bull. 2011;39(1):151-160.
4. Baytunca B, Kalyoncu T, Ozel I, et al. Early onset schizophrenia associated with obsessive-compulsive disorder: clinical features and correlates. Clin Neuropharmacol. 2017;40(6):243-245.
5. Niendam TA, Berzak J, Cannon TD, et al. Obsessive compulsive symptoms in the psychosis prodrome: correlates of clinical and functional outcome. Schizophr Res. 2009;108(1-3):170-175.
1. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
2. Schirmbeck F, Swets M, de Haan L. Obsessive-compulsive symptoms in schizophrenia. In: De Haan L, Schirmbeck F, Zink M. Epidemiology: prevalence and clinical characteristics of obsessive-compulsive disorder and obsessive-compulsive symptoms in patients with psychotic disorders. New York, NY: Springer International Publishing; 2015:47-61.
3. de Haan L, Sterk B, Wouters L, et al. The 5-year course of obsessive-compulsive symptoms and obsessive-compulsive disorder in first-episode schizophrenia and related disorders. Schizophr Bull. 2011;39(1):151-160.
4. Baytunca B, Kalyoncu T, Ozel I, et al. Early onset schizophrenia associated with obsessive-compulsive disorder: clinical features and correlates. Clin Neuropharmacol. 2017;40(6):243-245.
5. Niendam TA, Berzak J, Cannon TD, et al. Obsessive compulsive symptoms in the psychosis prodrome: correlates of clinical and functional outcome. Schizophr Res. 2009;108(1-3):170-175.
When the worry is worse than the actual illness
CASE Distraught over a medical illness
Ms. S, age 16, presents to the emergency department (ED) accompanied by her mother with superficial lacerations on her arm. Ms. S states, “I cut my arm because I was afraid I was going to do something serious if I didn’t get to go to the ED.” She says that 6 months earlier, she was diagnosed with superior mesenteric artery syndrome (SMAS), a rare, potentially life-threatening condition that occurs when the duodenum is compressed between the aorta and the superior mesenteric artery, causing a partial or complete blockage of the duodenum. Since receiving this diagnosis, Ms. S reports feeling anxious, depressed, and overwhelmed by both the pain she is experiencing from her illness and uncertainty about her prognosis.
HISTORY In pain and isolated
Since being diagnosed with SMAS, Ms. S has had approximately 30 medical and 7 ED visits for SMAS-related pain. Ms. S was referred to the outpatient clinic for ongoing support and treatment for SMAS.
Because of her pain and anxiety, Ms. S, a junior in high school, no longer attends school but has been working with a tutor. Ms. S says that some of her loneliness and hopelessness are due to the social isolation of being tutored at home. She states that she has been “out of sight and out of mind” from her friends. She also reports feeling different from them due to the pain brought on by SMAS.
Ms. S and her mother live in public housing. Ms. S says that overall, she has a good relationship with her mother, but that in certain situations, her mother’s anxiety causes her significant frustration and anxiety.
EVALUATION Transient suicidal thoughts
A physical examination reveals superficial lacerations to Ms. S’s left arm. Although she appears thin, her current body mass index (BMI) is 20.4 kg/m2, which is within normal range. She says she sees herself as “underweight” and “not fat at all.” Ms. S reports that she likes food and enjoyed eating until it became too painful following her SMAS diagnosis. Ms. S denies a history of binging or purging. Results from her laboratory workup and all values are within normal limits.
During the initial interview, Ms. S’s mother says they came to the ED because Ms. S urgently needs a psychiatric evaluation so she can be cleared for gastrointestinal (GI) surgery and placement of a nasogastric tube. Her mother says a surgeon from a different hospital told them that her insurance company required a psychiatric evaluation to rule out anorexia nervosa before they would authorize the GI surgery. When asked why psychiatry at this hospital was not consulted, Ms. S’s mother does not answer.
When asked about the symptoms she has been experiencing, Ms. S says that her sleep has been poor because of increased pain and excessive worrying about her health. She has limited her food intake. Ms. S reports that after eating, she lays on her left side to alleviate pain and help the food move through her body.
Continue to: Ms. S says...
Ms. S says she feels anxious and depressed due to her SMAS diagnosis, her mother’s online research and oversharing of poor prognoses, and being isolated from her friends. Most of her time outside the home is spent attending medical appointments with specialists. Several months ago, Ms. S had seen a psychotherapist, but her mother was unhappy with the treatment recommendations, which included seeking care from a nutritionist and joining group therapy. Ms. S’s mother says she ended her daughter’s psychotherapy because she was unable to obtain a signature ruling out anorexia nervosa within the first few appointments.
Ms. S also says she has had passive suicidal thoughts during the past month, usually twice a week. She reports that these thoughts lasted as long as several hours and were difficult to control, but she has no specific plan or intent. Ms. S denies current suicidal thoughts or ideation, and works with the treatment team to complete a safety plan, which she signs. Other than her recent visit to the ED, Ms. S denies any other thoughts or behaviors of self-injury or suicide.
[polldaddy:10586905]
The authors’ observations
The treatment team considered the following conditions as part of Ms. S’s differential diagnosis:
Major depressive disorder. The team was able to rule out MDD because Ms. S’s depression was attributed to SMAS. Ms. S reported that all depressive symptoms were manageable or nonexistent before the onset of pain from SMAS. There was no direct pathophysiological consequence of another medical condition. Ms. S was clear that her symptoms of anxiety and depression began after she was isolated from her friends and began having difficulty understanding her diagnosis and prognosis.
Anorexia nervosa also was ruled out. According to the DSM-5, a diagnosis of anorexia nervosa requires the following 3 criteria1:
- restriction of food intake resulting in significantly low body weight (defined as weight that is less than “minimally normal”) relative to age, gender, or development
- intense fear of gaining weight, or persistent behaviors that interfere with weight gain
- disturbance in the way in which one’s body weight or shape is experienced, undue influence of body weight or shape on self-evaluation, or lack of insight with regard to seriousness of current low body weight.
Continue to: Although Ms. S appeared...
Although Ms. S appeared thin, her BMI was within normal range. She added that she likes food and enjoyed eating, but that her medical condition made it too painful. Lastly, Ms. S denied a history of binging or purging.
Somatic symptom disorder.

Factitious disorder imposed on self. An individual with FDIS chronically stimulates, induces, or aggravates illnesses to gain the status of being a patient.
Factitious disorder imposed on another is the deliberate feigning or production of symptoms in another individual who is under the perpetrator’s supervision.1 Table 23 lists clinical indicators that raise suspicion for FDIA.
Before a diagnosis of somatic symptom disorder, FDIS, or FDIA could be established or ruled out, it was imperative to gather collateral information from other clinicians involved in Ms. S’s care. Ms. S and her mother had sought out help from a pediatric surgeon, a pediatric gastroenterologist, a pediatrician, and a psychotherapist.
Continue to: EVALUATION Collateral information
EVALUATION Collateral information
After Ms. S’s mother signs consent forms for exchange of information, the treatment team reaches out to the other clinicians. The therapist confirms that Ms. S’s mother had ended her daughter’s treatment after she was unable to quickly obtain documentation to rule out anorexia nervosa.
Both the pediatric surgeon and gastroenterologist report concerns of FDIA, which is why both clinicians had referred Ms. S and her mother to psychiatry. The pediatric surgeon states that on one occasion when he interviewed Ms. S separately from her mother, she seemed to be going down a checklist of symptoms. The surgeon reports that there was a partial occlusion of the superior mesenteric artery, confirming the diagnosis of SMAS, but he believed it was not severe enough to explain the symptoms Ms. S reported. The surgeon had scheduled another imaging appointment for 1 month later.
The pediatric gastroenterologist reports that Ms. S’s mother had demanded surgery and nasogastric tube placement for her daughter, which raised suspicion of FDIA. The gastroenterologist had convinced Ms. S and her mother to start low-dose doxepin, 20 mg twice a day, for anxiety, sleep, and abdominal pain.
Lastly, the pediatrician reports that she had not seen Ms. S for several months but stated that Ms. S always has been in the low normal BMI range. The pediatrician also reports that 6 months ago, the patient and her mother were frantically visiting EDs and scheduling doctor’s appointments.
[polldaddy:10586906]
The authors’ observations
The treatment team decided that Ms. S was not in imminent danger, and felt it was important to keep her in treatment without raising her mother’s suspicion. The team agreed to raise these concerns to the police, child protective services, and risk management if Ms. S’s health suddenly deteriorated or if her mother decided to remove Ms. S from our care.
Continue to: The treatment team...
The treatment team at the outpatient psychiatry clinic agreed that Ms. S did not currently meet criteria for anorexia nervosa, MDD, FDIS, or FDIA. However, Ms. S reported worries particular to persistent abdominal pain that was exacerbated by either eating or going to bed at night, which indicated that somatic symptom disorder was her likely diagnosis. Further, she endorsed a high level of anxiety and depression with regard to this somatic complaint that interfered with her daily activities and consumed an excessive amount of time, which also pointed to somatic symptom disorder. As a result of this diagnosis, the treatment team helped Ms. S manage her somatic symptoms and monitored for any other changes in her symptoms
Generally, cognitive-behavioral therapy (CBT) and mindfulness-based therapy may help relieve symptoms associated with somatic symptom disorder.4
TREATMENT Therapy sessions and medication management
At the psychiatric clinic, Ms. S is scheduled for biweekly therapy sessions with a social worker and biweekly appointments with a senior psychiatry resident for medication management. At each visit, Ms. S’s vital signs, height, and weight are measured. In the therapy sessions, she is taught mindfulness skills as well as CBT. The senior psychiatry resident maintains regular communication with the other clinicians involved in Ms. S’s care.
After the first month of treatment, Ms. S undergoes repeat imaging at the gastroenterologist’s office that indicates her SMAS is no longer occluded. Ms. S continues to report somatic symptoms, but with mild improvement.
Over the course of approximately 4 months, Ms. S begins to show signs of improvement in her pain, anxiety, and depression. Ms. S begins to feel well enough to get a summer job at a nursing home and expresses enthusiasm when her weight begins to increase. Her mother also became enthused and verbalized her appreciation that her daughter appeared to be improving.
Continue to: In the fall...
In the fall, Ms. S returns to high school for her senior year but has difficulty getting back into the routine and relating to her old friends. Ms. S continues to perseverate on thoughts of getting sick and her physical symptoms become overwhelming once again. She continues to be focused on any new symptoms she experiences, and to limit the types of foods she eats due to fear of the abdominal pain returning.
After several more months of psychiatric treatment, Ms. S reports significant relief from her abdominal pain, and no longer seeks corrective surgery for her SMAS. Although she occasionally struggles with perseverating thoughts and anxiety about her somatic symptoms such as abdominal pain and worrying about the types of foods she eats and becoming ill, she continues to work through symptoms of her somatic symptom disorder.
The authors’ observations
The main challenge of somatic symptom disorder is the patient’s “abnormal illness behavior.”2,5,6 For pediatric patients, there may an association between a parent’s psychological status and the patient’s somatic symptoms. Abdominal symptoms in a pediatric patient have a strong association with a parent who presents with depression, anxiety, or somatization. The effects of the parent’s psychological status could also manifest in the form of modeling catastrophic thinking or through reinforcement. Parents with certain traits, such as disproportionate worry about pain, may pay more attention to their child’s symptoms, and hence, reward the child when he/she reports somatic symptoms.7,8 In the case of Ms. S, her mother did not participate in therapy and the mother’s psychiatric history was never obtained.
OUTCOMES Making personal strides
Ms. S continues to use mindfulness skills as well as CBT to manage her symptoms of somatic symptom disorder. She continues to celebrate her weight gains, denies any thoughts of suicide or self-harm behaviors, and prepares for college by scheduling campus visits and completing admissions applications.
Bottom Line
Patients with somatic symptom disorder tend to have very high levels of worry about illness. Somatic symptoms in such patients may or may not have a medical explanation. Accurate diagnosis and careful management are necessary to reduce patient distress. Cognitive-behavioral therapy and mindfulness-based therapy may help relieve symptoms associated with this disorder.
Related Resources
- Henningsen P. Management of somatic symptom disorder. Dialogues Clin Neurosci. 2018;20(1):23-91.
- Rosic T, Kalra S, Samaan Z. Somatic symptom disorder, a new DSM-5 diagnosis of an old clinical challenge. BMJ Case Rep. 2016: bcr2015212553. doi: 10.1136/bcr-2015-212553.
Drug Brand Name
Doxepin • Silenor
1. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
2. Stern T, Freudenreich O, Smith F, et al. Massachusetts General Hospital Handbook of General Hospital Psychiatry, 7th ed. New York, NY: Elsevier; 2017.
3. Feldman MD, Eisendrath SJ. The spectrum of factitious disorders. Washington, DC: American Psychiatric Association; 1997.
4. Sadock BJ, Sadock VA, Ruiz P. Kaplan & Sadock’s synopsis of psychiatry: behavioral sciences/clinical psychiatry, 11th ed. Philadelphia, PA: Wolters Kluwer; 2014:470.
5. Pilowsky I. The concept of abnormal illness behavior. Psychosomatics. 1990;31(2):207-213.
6. Kirmayer LJ, Looper KJ. Abnormal illness behavior: physiological, psychological and social dimensions of coping with stress. Curr Opin Psychiatry. 2006;19(1):54-60.
7. Walker LS, Garber J, Greene JW. Somatic complaints in pediatric patients: a prospective study of the role of negative life events, child social and academic competence, and parental somatic symptoms. J Consult Clin Psychology. 1994;62(6):1213-1221.
8. Van Oudenhove L, Levy RL, Crowell MD, et al. Biopsychosocial aspects of functional gastrointestinal disorders: how central and environmental processes contribute to the development and expression of functional gastrointestinal disorders. Gastroenterology. 2016;150(6):1355-1367.
CASE Distraught over a medical illness
Ms. S, age 16, presents to the emergency department (ED) accompanied by her mother with superficial lacerations on her arm. Ms. S states, “I cut my arm because I was afraid I was going to do something serious if I didn’t get to go to the ED.” She says that 6 months earlier, she was diagnosed with superior mesenteric artery syndrome (SMAS), a rare, potentially life-threatening condition that occurs when the duodenum is compressed between the aorta and the superior mesenteric artery, causing a partial or complete blockage of the duodenum. Since receiving this diagnosis, Ms. S reports feeling anxious, depressed, and overwhelmed by both the pain she is experiencing from her illness and uncertainty about her prognosis.
HISTORY In pain and isolated
Since being diagnosed with SMAS, Ms. S has had approximately 30 medical and 7 ED visits for SMAS-related pain. Ms. S was referred to the outpatient clinic for ongoing support and treatment for SMAS.
Because of her pain and anxiety, Ms. S, a junior in high school, no longer attends school but has been working with a tutor. Ms. S says that some of her loneliness and hopelessness are due to the social isolation of being tutored at home. She states that she has been “out of sight and out of mind” from her friends. She also reports feeling different from them due to the pain brought on by SMAS.
Ms. S and her mother live in public housing. Ms. S says that overall, she has a good relationship with her mother, but that in certain situations, her mother’s anxiety causes her significant frustration and anxiety.
EVALUATION Transient suicidal thoughts
A physical examination reveals superficial lacerations to Ms. S’s left arm. Although she appears thin, her current body mass index (BMI) is 20.4 kg/m2, which is within normal range. She says she sees herself as “underweight” and “not fat at all.” Ms. S reports that she likes food and enjoyed eating until it became too painful following her SMAS diagnosis. Ms. S denies a history of binging or purging. Results from her laboratory workup and all values are within normal limits.
During the initial interview, Ms. S’s mother says they came to the ED because Ms. S urgently needs a psychiatric evaluation so she can be cleared for gastrointestinal (GI) surgery and placement of a nasogastric tube. Her mother says a surgeon from a different hospital told them that her insurance company required a psychiatric evaluation to rule out anorexia nervosa before they would authorize the GI surgery. When asked why psychiatry at this hospital was not consulted, Ms. S’s mother does not answer.
When asked about the symptoms she has been experiencing, Ms. S says that her sleep has been poor because of increased pain and excessive worrying about her health. She has limited her food intake. Ms. S reports that after eating, she lays on her left side to alleviate pain and help the food move through her body.
Continue to: Ms. S says...
Ms. S says she feels anxious and depressed due to her SMAS diagnosis, her mother’s online research and oversharing of poor prognoses, and being isolated from her friends. Most of her time outside the home is spent attending medical appointments with specialists. Several months ago, Ms. S had seen a psychotherapist, but her mother was unhappy with the treatment recommendations, which included seeking care from a nutritionist and joining group therapy. Ms. S’s mother says she ended her daughter’s psychotherapy because she was unable to obtain a signature ruling out anorexia nervosa within the first few appointments.
Ms. S also says she has had passive suicidal thoughts during the past month, usually twice a week. She reports that these thoughts lasted as long as several hours and were difficult to control, but she has no specific plan or intent. Ms. S denies current suicidal thoughts or ideation, and works with the treatment team to complete a safety plan, which she signs. Other than her recent visit to the ED, Ms. S denies any other thoughts or behaviors of self-injury or suicide.
[polldaddy:10586905]
The authors’ observations
The treatment team considered the following conditions as part of Ms. S’s differential diagnosis:
Major depressive disorder. The team was able to rule out MDD because Ms. S’s depression was attributed to SMAS. Ms. S reported that all depressive symptoms were manageable or nonexistent before the onset of pain from SMAS. There was no direct pathophysiological consequence of another medical condition. Ms. S was clear that her symptoms of anxiety and depression began after she was isolated from her friends and began having difficulty understanding her diagnosis and prognosis.
Anorexia nervosa also was ruled out. According to the DSM-5, a diagnosis of anorexia nervosa requires the following 3 criteria1:
- restriction of food intake resulting in significantly low body weight (defined as weight that is less than “minimally normal”) relative to age, gender, or development
- intense fear of gaining weight, or persistent behaviors that interfere with weight gain
- disturbance in the way in which one’s body weight or shape is experienced, undue influence of body weight or shape on self-evaluation, or lack of insight with regard to seriousness of current low body weight.
Continue to: Although Ms. S appeared...
Although Ms. S appeared thin, her BMI was within normal range. She added that she likes food and enjoyed eating, but that her medical condition made it too painful. Lastly, Ms. S denied a history of binging or purging.
Somatic symptom disorder.

Factitious disorder imposed on self. An individual with FDIS chronically stimulates, induces, or aggravates illnesses to gain the status of being a patient.
Factitious disorder imposed on another is the deliberate feigning or production of symptoms in another individual who is under the perpetrator’s supervision.1 Table 23 lists clinical indicators that raise suspicion for FDIA.

Before a diagnosis of somatic symptom disorder, FDIS, or FDIA could be established or ruled out, it was imperative to gather collateral information from other clinicians involved in Ms. S’s care. Ms. S and her mother had sought out help from a pediatric surgeon, a pediatric gastroenterologist, a pediatrician, and a psychotherapist.
Continue to: EVALUATION Collateral information
EVALUATION Collateral information
After Ms. S’s mother signs consent forms for exchange of information, the treatment team reaches out to the other clinicians. The therapist confirms that Ms. S’s mother had ended her daughter’s treatment after she was unable to quickly obtain documentation to rule out anorexia nervosa.
Both the pediatric surgeon and gastroenterologist report concerns of FDIA, which is why both clinicians had referred Ms. S and her mother to psychiatry. The pediatric surgeon states that on one occasion when he interviewed Ms. S separately from her mother, she seemed to be going down a checklist of symptoms. The surgeon reports that there was a partial occlusion of the superior mesenteric artery, confirming the diagnosis of SMAS, but he believed it was not severe enough to explain the symptoms Ms. S reported. The surgeon had scheduled another imaging appointment for 1 month later.
The pediatric gastroenterologist reports that Ms. S’s mother had demanded surgery and nasogastric tube placement for her daughter, which raised suspicion of FDIA. The gastroenterologist had convinced Ms. S and her mother to start low-dose doxepin, 20 mg twice a day, for anxiety, sleep, and abdominal pain.
Lastly, the pediatrician reports that she had not seen Ms. S for several months but stated that Ms. S always has been in the low normal BMI range. The pediatrician also reports that 6 months ago, the patient and her mother were frantically visiting EDs and scheduling doctor’s appointments.
[polldaddy:10586906]
The authors’ observations
The treatment team decided that Ms. S was not in imminent danger, and felt it was important to keep her in treatment without raising her mother’s suspicion. The team agreed to raise these concerns to the police, child protective services, and risk management if Ms. S’s health suddenly deteriorated or if her mother decided to remove Ms. S from our care.
Continue to: The treatment team...
The treatment team at the outpatient psychiatry clinic agreed that Ms. S did not currently meet criteria for anorexia nervosa, MDD, FDIS, or FDIA. However, Ms. S reported worries particular to persistent abdominal pain that was exacerbated by either eating or going to bed at night, which indicated that somatic symptom disorder was her likely diagnosis. Further, she endorsed a high level of anxiety and depression with regard to this somatic complaint that interfered with her daily activities and consumed an excessive amount of time, which also pointed to somatic symptom disorder. As a result of this diagnosis, the treatment team helped Ms. S manage her somatic symptoms and monitored for any other changes in her symptoms
Generally, cognitive-behavioral therapy (CBT) and mindfulness-based therapy may help relieve symptoms associated with somatic symptom disorder.4
TREATMENT Therapy sessions and medication management
At the psychiatric clinic, Ms. S is scheduled for biweekly therapy sessions with a social worker and biweekly appointments with a senior psychiatry resident for medication management. At each visit, Ms. S’s vital signs, height, and weight are measured. In the therapy sessions, she is taught mindfulness skills as well as CBT. The senior psychiatry resident maintains regular communication with the other clinicians involved in Ms. S’s care.
After the first month of treatment, Ms. S undergoes repeat imaging at the gastroenterologist’s office that indicates her SMAS is no longer occluded. Ms. S continues to report somatic symptoms, but with mild improvement.
Over the course of approximately 4 months, Ms. S begins to show signs of improvement in her pain, anxiety, and depression. Ms. S begins to feel well enough to get a summer job at a nursing home and expresses enthusiasm when her weight begins to increase. Her mother also became enthused and verbalized her appreciation that her daughter appeared to be improving.
Continue to: In the fall...
In the fall, Ms. S returns to high school for her senior year but has difficulty getting back into the routine and relating to her old friends. Ms. S continues to perseverate on thoughts of getting sick and her physical symptoms become overwhelming once again. She continues to be focused on any new symptoms she experiences, and to limit the types of foods she eats due to fear of the abdominal pain returning.
After several more months of psychiatric treatment, Ms. S reports significant relief from her abdominal pain, and no longer seeks corrective surgery for her SMAS. Although she occasionally struggles with perseverating thoughts and anxiety about her somatic symptoms such as abdominal pain and worrying about the types of foods she eats and becoming ill, she continues to work through symptoms of her somatic symptom disorder.
The authors’ observations
The main challenge of somatic symptom disorder is the patient’s “abnormal illness behavior.”2,5,6 For pediatric patients, there may an association between a parent’s psychological status and the patient’s somatic symptoms. Abdominal symptoms in a pediatric patient have a strong association with a parent who presents with depression, anxiety, or somatization. The effects of the parent’s psychological status could also manifest in the form of modeling catastrophic thinking or through reinforcement. Parents with certain traits, such as disproportionate worry about pain, may pay more attention to their child’s symptoms, and hence, reward the child when he/she reports somatic symptoms.7,8 In the case of Ms. S, her mother did not participate in therapy and the mother’s psychiatric history was never obtained.
OUTCOMES Making personal strides
Ms. S continues to use mindfulness skills as well as CBT to manage her symptoms of somatic symptom disorder. She continues to celebrate her weight gains, denies any thoughts of suicide or self-harm behaviors, and prepares for college by scheduling campus visits and completing admissions applications.
Bottom Line
Patients with somatic symptom disorder tend to have very high levels of worry about illness. Somatic symptoms in such patients may or may not have a medical explanation. Accurate diagnosis and careful management are necessary to reduce patient distress. Cognitive-behavioral therapy and mindfulness-based therapy may help relieve symptoms associated with this disorder.
Related Resources
- Henningsen P. Management of somatic symptom disorder. Dialogues Clin Neurosci. 2018;20(1):23-91.
- Rosic T, Kalra S, Samaan Z. Somatic symptom disorder, a new DSM-5 diagnosis of an old clinical challenge. BMJ Case Rep. 2016: bcr2015212553. doi: 10.1136/bcr-2015-212553.
Drug Brand Name
Doxepin • Silenor
CASE Distraught over a medical illness
Ms. S, age 16, presents to the emergency department (ED) accompanied by her mother with superficial lacerations on her arm. Ms. S states, “I cut my arm because I was afraid I was going to do something serious if I didn’t get to go to the ED.” She says that 6 months earlier, she was diagnosed with superior mesenteric artery syndrome (SMAS), a rare, potentially life-threatening condition that occurs when the duodenum is compressed between the aorta and the superior mesenteric artery, causing a partial or complete blockage of the duodenum. Since receiving this diagnosis, Ms. S reports feeling anxious, depressed, and overwhelmed by both the pain she is experiencing from her illness and uncertainty about her prognosis.
HISTORY In pain and isolated
Since being diagnosed with SMAS, Ms. S has had approximately 30 medical and 7 ED visits for SMAS-related pain. Ms. S was referred to the outpatient clinic for ongoing support and treatment for SMAS.
Because of her pain and anxiety, Ms. S, a junior in high school, no longer attends school but has been working with a tutor. Ms. S says that some of her loneliness and hopelessness are due to the social isolation of being tutored at home. She states that she has been “out of sight and out of mind” from her friends. She also reports feeling different from them due to the pain brought on by SMAS.
Ms. S and her mother live in public housing. Ms. S says that overall, she has a good relationship with her mother, but that in certain situations, her mother’s anxiety causes her significant frustration and anxiety.
EVALUATION Transient suicidal thoughts
A physical examination reveals superficial lacerations to Ms. S’s left arm. Although she appears thin, her current body mass index (BMI) is 20.4 kg/m2, which is within normal range. She says she sees herself as “underweight” and “not fat at all.” Ms. S reports that she likes food and enjoyed eating until it became too painful following her SMAS diagnosis. Ms. S denies a history of binging or purging. Results from her laboratory workup and all values are within normal limits.
During the initial interview, Ms. S’s mother says they came to the ED because Ms. S urgently needs a psychiatric evaluation so she can be cleared for gastrointestinal (GI) surgery and placement of a nasogastric tube. Her mother says a surgeon from a different hospital told them that her insurance company required a psychiatric evaluation to rule out anorexia nervosa before they would authorize the GI surgery. When asked why psychiatry at this hospital was not consulted, Ms. S’s mother does not answer.
When asked about the symptoms she has been experiencing, Ms. S says that her sleep has been poor because of increased pain and excessive worrying about her health. She has limited her food intake. Ms. S reports that after eating, she lays on her left side to alleviate pain and help the food move through her body.
Continue to: Ms. S says...
Ms. S says she feels anxious and depressed due to her SMAS diagnosis, her mother’s online research and oversharing of poor prognoses, and being isolated from her friends. Most of her time outside the home is spent attending medical appointments with specialists. Several months ago, Ms. S had seen a psychotherapist, but her mother was unhappy with the treatment recommendations, which included seeking care from a nutritionist and joining group therapy. Ms. S’s mother says she ended her daughter’s psychotherapy because she was unable to obtain a signature ruling out anorexia nervosa within the first few appointments.
Ms. S also says she has had passive suicidal thoughts during the past month, usually twice a week. She reports that these thoughts lasted as long as several hours and were difficult to control, but she has no specific plan or intent. Ms. S denies current suicidal thoughts or ideation, and works with the treatment team to complete a safety plan, which she signs. Other than her recent visit to the ED, Ms. S denies any other thoughts or behaviors of self-injury or suicide.
[polldaddy:10586905]
The authors’ observations
The treatment team considered the following conditions as part of Ms. S’s differential diagnosis:
Major depressive disorder. The team was able to rule out MDD because Ms. S’s depression was attributed to SMAS. Ms. S reported that all depressive symptoms were manageable or nonexistent before the onset of pain from SMAS. There was no direct pathophysiological consequence of another medical condition. Ms. S was clear that her symptoms of anxiety and depression began after she was isolated from her friends and began having difficulty understanding her diagnosis and prognosis.
Anorexia nervosa also was ruled out. According to the DSM-5, a diagnosis of anorexia nervosa requires the following 3 criteria1:
- restriction of food intake resulting in significantly low body weight (defined as weight that is less than “minimally normal”) relative to age, gender, or development
- intense fear of gaining weight, or persistent behaviors that interfere with weight gain
- disturbance in the way in which one’s body weight or shape is experienced, undue influence of body weight or shape on self-evaluation, or lack of insight with regard to seriousness of current low body weight.
Continue to: Although Ms. S appeared...
Although Ms. S appeared thin, her BMI was within normal range. She added that she likes food and enjoyed eating, but that her medical condition made it too painful. Lastly, Ms. S denied a history of binging or purging.
Somatic symptom disorder.

Factitious disorder imposed on self. An individual with FDIS chronically stimulates, induces, or aggravates illnesses to gain the status of being a patient.
Factitious disorder imposed on another is the deliberate feigning or production of symptoms in another individual who is under the perpetrator’s supervision.1 Table 23 lists clinical indicators that raise suspicion for FDIA.

Before a diagnosis of somatic symptom disorder, FDIS, or FDIA could be established or ruled out, it was imperative to gather collateral information from other clinicians involved in Ms. S’s care. Ms. S and her mother had sought out help from a pediatric surgeon, a pediatric gastroenterologist, a pediatrician, and a psychotherapist.
Continue to: EVALUATION Collateral information
EVALUATION Collateral information
After Ms. S’s mother signs consent forms for exchange of information, the treatment team reaches out to the other clinicians. The therapist confirms that Ms. S’s mother had ended her daughter’s treatment after she was unable to quickly obtain documentation to rule out anorexia nervosa.
Both the pediatric surgeon and gastroenterologist report concerns of FDIA, which is why both clinicians had referred Ms. S and her mother to psychiatry. The pediatric surgeon states that on one occasion when he interviewed Ms. S separately from her mother, she seemed to be going down a checklist of symptoms. The surgeon reports that there was a partial occlusion of the superior mesenteric artery, confirming the diagnosis of SMAS, but he believed it was not severe enough to explain the symptoms Ms. S reported. The surgeon had scheduled another imaging appointment for 1 month later.
The pediatric gastroenterologist reports that Ms. S’s mother had demanded surgery and nasogastric tube placement for her daughter, which raised suspicion of FDIA. The gastroenterologist had convinced Ms. S and her mother to start low-dose doxepin, 20 mg twice a day, for anxiety, sleep, and abdominal pain.
Lastly, the pediatrician reports that she had not seen Ms. S for several months but stated that Ms. S always has been in the low normal BMI range. The pediatrician also reports that 6 months ago, the patient and her mother were frantically visiting EDs and scheduling doctor’s appointments.
[polldaddy:10586906]
The authors’ observations
The treatment team decided that Ms. S was not in imminent danger, and felt it was important to keep her in treatment without raising her mother’s suspicion. The team agreed to raise these concerns to the police, child protective services, and risk management if Ms. S’s health suddenly deteriorated or if her mother decided to remove Ms. S from our care.
Continue to: The treatment team...
The treatment team at the outpatient psychiatry clinic agreed that Ms. S did not currently meet criteria for anorexia nervosa, MDD, FDIS, or FDIA. However, Ms. S reported worries particular to persistent abdominal pain that was exacerbated by either eating or going to bed at night, which indicated that somatic symptom disorder was her likely diagnosis. Further, she endorsed a high level of anxiety and depression with regard to this somatic complaint that interfered with her daily activities and consumed an excessive amount of time, which also pointed to somatic symptom disorder. As a result of this diagnosis, the treatment team helped Ms. S manage her somatic symptoms and monitored for any other changes in her symptoms
Generally, cognitive-behavioral therapy (CBT) and mindfulness-based therapy may help relieve symptoms associated with somatic symptom disorder.4
TREATMENT Therapy sessions and medication management
At the psychiatric clinic, Ms. S is scheduled for biweekly therapy sessions with a social worker and biweekly appointments with a senior psychiatry resident for medication management. At each visit, Ms. S’s vital signs, height, and weight are measured. In the therapy sessions, she is taught mindfulness skills as well as CBT. The senior psychiatry resident maintains regular communication with the other clinicians involved in Ms. S’s care.
After the first month of treatment, Ms. S undergoes repeat imaging at the gastroenterologist’s office that indicates her SMAS is no longer occluded. Ms. S continues to report somatic symptoms, but with mild improvement.
Over the course of approximately 4 months, Ms. S begins to show signs of improvement in her pain, anxiety, and depression. Ms. S begins to feel well enough to get a summer job at a nursing home and expresses enthusiasm when her weight begins to increase. Her mother also became enthused and verbalized her appreciation that her daughter appeared to be improving.
Continue to: In the fall...
In the fall, Ms. S returns to high school for her senior year but has difficulty getting back into the routine and relating to her old friends. Ms. S continues to perseverate on thoughts of getting sick and her physical symptoms become overwhelming once again. She continues to be focused on any new symptoms she experiences, and to limit the types of foods she eats due to fear of the abdominal pain returning.
After several more months of psychiatric treatment, Ms. S reports significant relief from her abdominal pain, and no longer seeks corrective surgery for her SMAS. Although she occasionally struggles with perseverating thoughts and anxiety about her somatic symptoms such as abdominal pain and worrying about the types of foods she eats and becoming ill, she continues to work through symptoms of her somatic symptom disorder.
The authors’ observations
The main challenge of somatic symptom disorder is the patient’s “abnormal illness behavior.”2,5,6 For pediatric patients, there may an association between a parent’s psychological status and the patient’s somatic symptoms. Abdominal symptoms in a pediatric patient have a strong association with a parent who presents with depression, anxiety, or somatization. The effects of the parent’s psychological status could also manifest in the form of modeling catastrophic thinking or through reinforcement. Parents with certain traits, such as disproportionate worry about pain, may pay more attention to their child’s symptoms, and hence, reward the child when he/she reports somatic symptoms.7,8 In the case of Ms. S, her mother did not participate in therapy and the mother’s psychiatric history was never obtained.
OUTCOMES Making personal strides
Ms. S continues to use mindfulness skills as well as CBT to manage her symptoms of somatic symptom disorder. She continues to celebrate her weight gains, denies any thoughts of suicide or self-harm behaviors, and prepares for college by scheduling campus visits and completing admissions applications.
Bottom Line
Patients with somatic symptom disorder tend to have very high levels of worry about illness. Somatic symptoms in such patients may or may not have a medical explanation. Accurate diagnosis and careful management are necessary to reduce patient distress. Cognitive-behavioral therapy and mindfulness-based therapy may help relieve symptoms associated with this disorder.
Related Resources
- Henningsen P. Management of somatic symptom disorder. Dialogues Clin Neurosci. 2018;20(1):23-91.
- Rosic T, Kalra S, Samaan Z. Somatic symptom disorder, a new DSM-5 diagnosis of an old clinical challenge. BMJ Case Rep. 2016: bcr2015212553. doi: 10.1136/bcr-2015-212553.
Drug Brand Name
Doxepin • Silenor
1. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
2. Stern T, Freudenreich O, Smith F, et al. Massachusetts General Hospital Handbook of General Hospital Psychiatry, 7th ed. New York, NY: Elsevier; 2017.
3. Feldman MD, Eisendrath SJ. The spectrum of factitious disorders. Washington, DC: American Psychiatric Association; 1997.
4. Sadock BJ, Sadock VA, Ruiz P. Kaplan & Sadock’s synopsis of psychiatry: behavioral sciences/clinical psychiatry, 11th ed. Philadelphia, PA: Wolters Kluwer; 2014:470.
5. Pilowsky I. The concept of abnormal illness behavior. Psychosomatics. 1990;31(2):207-213.
6. Kirmayer LJ, Looper KJ. Abnormal illness behavior: physiological, psychological and social dimensions of coping with stress. Curr Opin Psychiatry. 2006;19(1):54-60.
7. Walker LS, Garber J, Greene JW. Somatic complaints in pediatric patients: a prospective study of the role of negative life events, child social and academic competence, and parental somatic symptoms. J Consult Clin Psychology. 1994;62(6):1213-1221.
8. Van Oudenhove L, Levy RL, Crowell MD, et al. Biopsychosocial aspects of functional gastrointestinal disorders: how central and environmental processes contribute to the development and expression of functional gastrointestinal disorders. Gastroenterology. 2016;150(6):1355-1367.
1. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
2. Stern T, Freudenreich O, Smith F, et al. Massachusetts General Hospital Handbook of General Hospital Psychiatry, 7th ed. New York, NY: Elsevier; 2017.
3. Feldman MD, Eisendrath SJ. The spectrum of factitious disorders. Washington, DC: American Psychiatric Association; 1997.
4. Sadock BJ, Sadock VA, Ruiz P. Kaplan & Sadock’s synopsis of psychiatry: behavioral sciences/clinical psychiatry, 11th ed. Philadelphia, PA: Wolters Kluwer; 2014:470.
5. Pilowsky I. The concept of abnormal illness behavior. Psychosomatics. 1990;31(2):207-213.
6. Kirmayer LJ, Looper KJ. Abnormal illness behavior: physiological, psychological and social dimensions of coping with stress. Curr Opin Psychiatry. 2006;19(1):54-60.
7. Walker LS, Garber J, Greene JW. Somatic complaints in pediatric patients: a prospective study of the role of negative life events, child social and academic competence, and parental somatic symptoms. J Consult Clin Psychology. 1994;62(6):1213-1221.
8. Van Oudenhove L, Levy RL, Crowell MD, et al. Biopsychosocial aspects of functional gastrointestinal disorders: how central and environmental processes contribute to the development and expression of functional gastrointestinal disorders. Gastroenterology. 2016;150(6):1355-1367.
New-onset psychosis while being treated for coronavirus
CASE Agitated, psychotic, and COVID-19–positive
Mr. G, age 56, is brought to the emergency department (ED) by emergency medical services (EMS) after his girlfriend reports that he was trying to climb into the “fiery furnace” to “burn the devil within him.” Mr. G had recently tested positive for coronavirus disease 2019 (COVID-19) via polymerase chain reaction and had been receiving treatment for it. In the ED, he is distressed and repeatedly exclaims, “The devil is alive!” He insists on covering himself with blankets, despite diaphoresis and soaking through his clothing within minutes. Because he does not respond to attempted redirection, the ED clinicians administer a single dose of IM haloperidol, 2 mg, for agitation.
HISTORY Multiple ED visits and hospitalizations
Mr. G, who has no known psychiatric history, lives with his girlfriend of 10 years. His medical history includes chronic obstructive pulmonary disease and prostate cancer. In 2015, he had a radical prostatectomy, without chemotherapy. His social history includes childhood neglect, which prompted him to leave home when he was a teenager. Mr. G had earned his general education development certificate and worked at a small retail store.
Mr. G had no previous history of mental health treatment per self-report, collateral information from his girlfriend, and chart review. He reported no known family psychiatric history. He did not endorse past psychiatric admissions or suicide attempts, nor previous periods of mania, depression, or psychosis. He said he used illicit substances as a teen, but denied using alcohol, tobacco products, or illicit substances in the past 20 years.
Mr. G recently had multiple ED visits and hospitalizations due to ongoing signs and symptoms associated with his COVID-19 diagnosis, primarily worsening shortness of breath and cough. Eleven days before EMS brought him to the ED at his girlfriend’s request, Mr. G had presented to the ED with chief complaints of shortness of breath and dry cough (Day 0). He reported that he had been “running a fever” for 2 days. In the ED, his initial vital signs were notable only for a temperature of 100.9°F (38.28°C). He was diagnosed with “acute viral syndrome” and received 1 dose of IV ceftriaxone, 2 g, and IV azithromycin, 500 mg. On Day 2, the ED clinicians prescribed a 4-day course of oral azithromycin, 250 mg/d, and discharged him home.
On Day 3, Mr. G returned to the ED with similar complaints—congestion and productive cough. He tested positive for COVID-19, and the ED discharged him home with quarantine instructions. Hours later, he returned to the ED via EMS with chief complaints of chest pain, diarrhea, and myalgias. He was prescribed a 5-day course ofoseltamivir, 75 mg twice daily, and azithromycin, 250 mg/d. The ED again discharged him home.
On Day 4, Mr. G returned to the ED for a fourth time. His chief complaint was worsening shortness of breath. His oxygen saturation was 94% on room air; it improved to 96% on 2 L of oxygen. His chest X-ray showed diffuse reticulonodular opacities throughout his bilateral lung fields and increased airspace opacification in the bilateral lower lobes. The ED admitted Mr. G to an internal medicine unit, where the primary treatment team enrolled him in a clinical trial. As part of the trial, Mr. G received hydroxychloroquine, 400 mg, on Day 4 and Day 5. The placebo-controlled component of the trial involved Mr. G receiving daily infusions of either remdesivir or placebo on Day 6 through Day 8. On Day 8, Mr. G was discharged home.
On Day 9, Mr. G returned to the ED with a chief complaint that his “thermometer wasn’t working” at home. The ED readmitted him to the internal medicine unit. On Day 9 through Day 11, Mr. G received daily doses of
Continue to: During the second hospitalization...
During the second hospitalization, nursing staff reported that Mr. G seemed religiously preoccupied and once reported seeing angels and demons. He was observed sitting in a chair praying to Allah that he would “come in on a horse to chop all the workers’ heads off.”
On Day 11, Mr. G was discharged home. Later that evening, the EMS brought him back in the ED due to his girlfriend’s concerns about his mental state.
EVALUATION Talks to God
On Day 12, psychiatry is consulted to evaluate Mr. G’s new-onset psychosis. Mr. G is alert and oriented to person, place, and time. His speech is loud, though the amount and rate are unremarkable. He displays no psychomotor agitation. His thought process is tangential and focuses on religious themes, specifically referring to Islam. He reports auditory hallucinations of God speaking directly to him. Mr. G states, “I am here because of a miraculous transformation from death back to life. Do you believe in God? Which God do you believe in? There are 2 Gods and only one of them is the true God. He is the God of all the 7 heavens and His true name is Allah, only one God, one faith. Allah is a ball of energy.”
Mr. G’s girlfriend provides collateral information that Mr. G had been raised Christian but was not religious as an adult. She says that he had never spoken about being Muslim. She adds that she had never known him to speak much about religion.
[polldaddy:10572249]
The authors’ observations
The etiology of new-onset psychosis can be related to several factors, including primary psychiatric illnesses, use of illicit substances, sequelae of general medical conditions, or adverse effects of prescribed medications. We considered each of these in the differential diagnosis for Mr. G.
Continue to: Psychiatric illness or illicit substance use
Psychiatric illness or illicit substance use. Because Mr. G was 56 years old and had no known psychiatric history or family psychiatric history, a primary psychiatric illness seemed less likely. Substance-induced psychosis related to illicit substance use also seemed unlikely because he denied using illicit substances, and an expanded urine drug screen was negative.
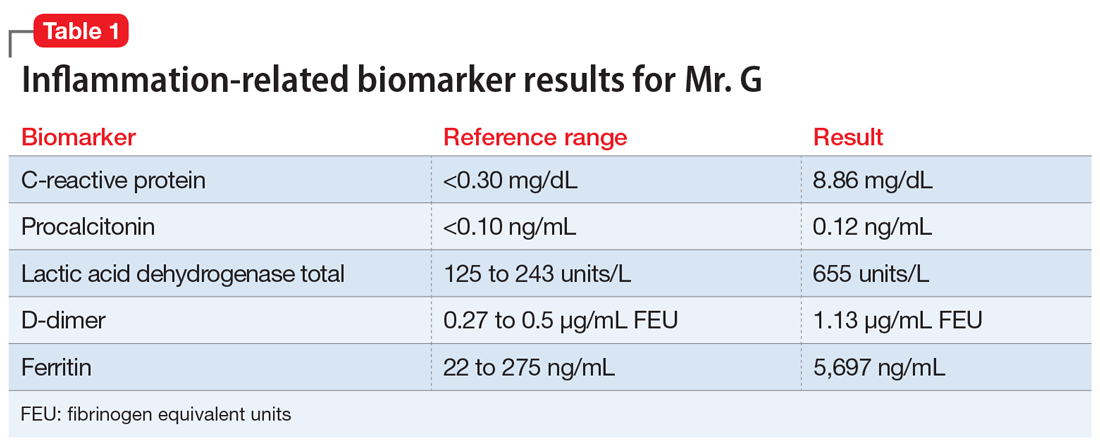
Psychosis due to a general medical condition. Results from Mr. G’s laboratory workup show marked elevation in multiple inflammation-related biomarkers (Table 1), consistent with the inflammatory profile seen with COVID-19 infection. However, results from several laboratory tests for potential etiologies of new-onset psychosis due to a general medical condition were negative (Table 2). Based on Mr. G’s history of prostate cancer, we considered the possibility of metastatic space-occupying lesions of the brain; however, Mr. G’s head CT showed no acute intracranial abnormalities. Another possible etiology we considered was COVID-19–induced encephalitis; however, Mr. G’s brain MRI with and without contrast showed no evidence of acute or chronic intracranial changes.
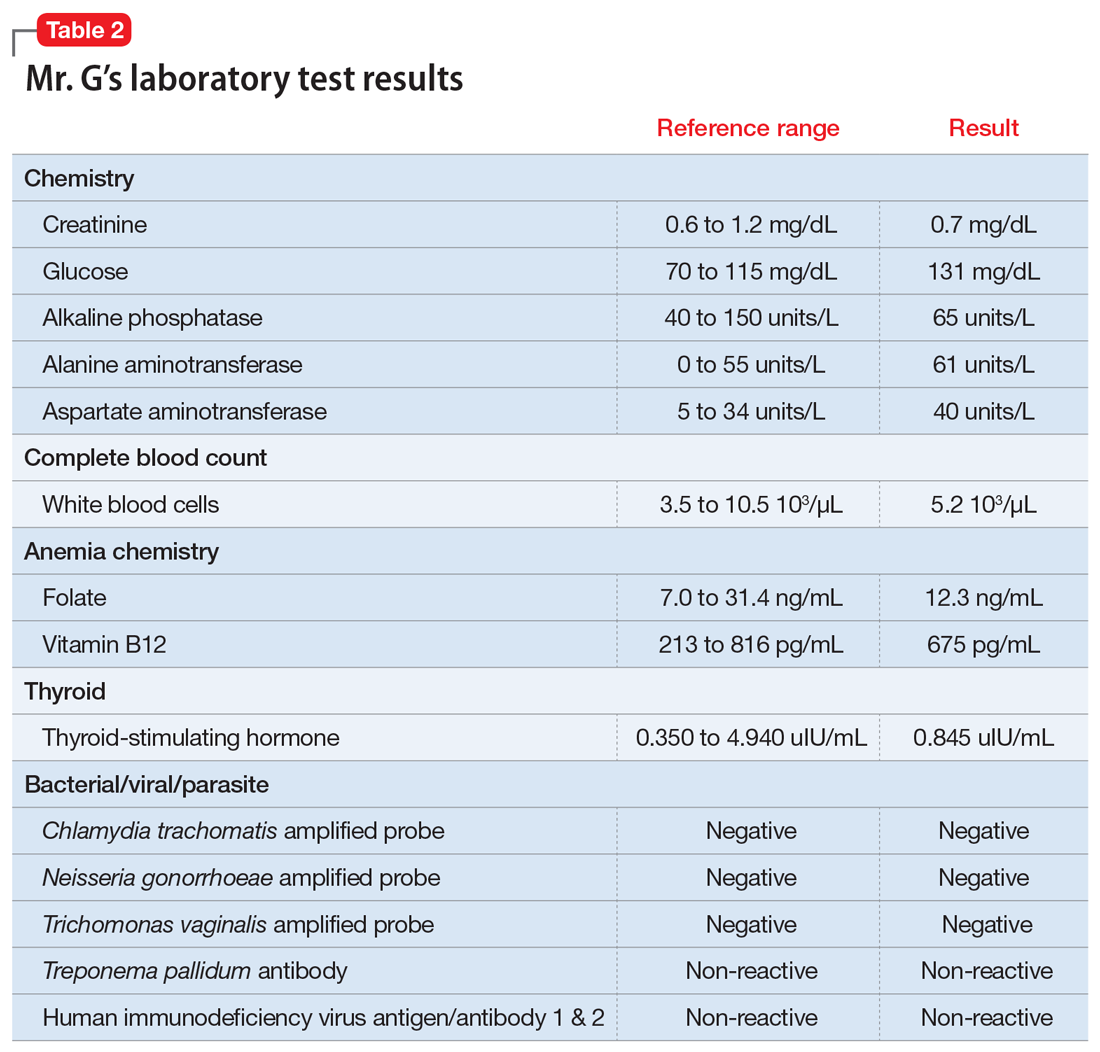
Medication-induced psychosis. After largely ruling out primary psychiatric illnesses, illicit substances, and sequelae of general medical conditions, we turned our attention to prescribed medications as a potential etiology of Mr. G’s new-onset psychosis. During his initial hospitalization, Mr. G had been prescribed 2 doses of hydroxychloroquine, 400 mg, to treat his diagnosis of COVID-19. Because none of the other medications he received were reported to have neuropsychiatric adverse effects, including psychosis, hydroxychloroquine-induced psychosis was therefore the primary team’s working diagnosis.
EVALUATION Request to leave AMA
On Day 13, Mr. G requests to leave the hospital against medical advice (AMA). Until this point, he had voluntarily remained in the hospital, which he repeatedly referred to as “Heaven.” When asked to describe his medical condition, Mr. G replies, “God told me my condition is far beyond man’s understanding.” He denies that he is positive for COVID-19. He states, “I am cured, and the real fight has just begun.”
At the recommendation of the psychiatry consultation-liaison (C-L) service, the primary treatment team determines that Mr. G does not have capacity to leave AMA. The team is concerned that because of his psychotic symptoms, Mr. G would be unable to understand and follow his quarantine instructions. He remains hospitalized on a medical hold.
Continue to: The authors' observations
The authors’ observations
One important consideration this case highlighted was potential third-party responsibility clinicians and hospital systems may face if they discharge a patient with a communicable illness who is unable to follow precautions based on a psychiatric condition.1 That concern was based on Mr. G’s reported desire to pursue missions “beyond man’s understanding,” which he felt compelled to complete, and which could unnecessarily place the public at risk. The psychiatry C-L service consulted the local health department and conferred with the hospital’s legal representatives, who agreed with the plan to keep Mr. G in the hospital for his safety as well as for the public’s safety.
TREATMENT Oral haloperidol
The psychiatry C-L service recommends initiating an antipsychotic. On Day 13, Mr. G starts oral haloperidol, 2.5 mg twice a day, to address his ongoing psychotic symptoms. On Day 14, the treatment team increases the dosage to 5 mg twice a day. Mr. G tolerates the haloperidol and gradually begins to improve. He demonstrates improved sleep, normal speech volume, less religious preoccupation, and a considerably improved understanding of his medical condition.
The authors’ observations
Mr. G’s initial psychiatric evaluation demonstrated an acute onset of psychotic symptoms, without evidence of delirium. Psychosis secondary to a general medical condition (such as COVID-19) and hydroxychloroquine-induced psychotic disorder topped our initial considerations in the differential diagnosis of this case. While the exact neuropsychiatric sequelae of COVID-19 are not yet clear, previous experiences with viral pandemics and case studies from the current pandemic demonstrate a wide variety of possible neuropsychiatric manifestations. Mood symptoms, psychosis, and encephalopathy represent some of the neuropsychiatric complications observed with past viral pandemics.2 Neuropsychiatric symptoms may be triggered by the virus itself, or from the host’s immune response to the infection.3 To further complicate matters, neuropsychiatric symptoms may manifest during the acute viral infection, or may surface later, as subacute or chronic neuropsychiatric illness.
Neuropsychiatric adverse events
Mr. G developed psychotic symptoms within the first few days of receiving hydroxychloroquine, which is consistent with the scant literature on this topic.8 Based on the available information, hydroxychloroquine remains the most likely etiology of his new-onset psychotic symptoms. Mr. G’s case is one example of the possible neuropsychiatric presentations clinicians may face while treating a novel viral illness.
Continue to: OUTCOME Homeward-bound
OUTCOME Homeward-bound
By Day 18, Mr. G’s psychotic symptoms have significantly improved. He is able to rationally process information about his COVID-19 diagnosis and the recommended quarantine instructions he needs to follow after discharge. He is cleared by infection control and discharged home to return to living with his girlfriend.
Mr. G attends his follow-up psychiatric appointment remotely 2 weeks after discharge. He reports that since discharge, he has continued taking his prescribed haloperidol, 5 mg twice a day. He demonstrates improved insight into his medical condition, acknowledging his COVID-19–positive status, and confirms that he has been following quarantine instructions. He does not report ongoing auditory or visual hallucinations, and is no longer religiously preoccupied. He says he is looking forward to being medically cleared to return to work.
The authors’ observations
This case highlights the need for prospective, longitudinal screening and monitoring of neuropsychiatric symptoms as part of the public health response to COVID-19. The case also highlights the importance of careful monitoring for adverse events, including neuropsychiatric symptoms, during clinical trials that involve experimental treatments. The long-term prognosis for individuals such as Mr. G who develop neuropsychiatric symptoms during acute COVID-19 infection remains unknown. Similarly, subacute and chronic neuropsychiatric manifestations that may develop after resolution of acute COVID-19 infection are unknown at this time. However, we can learn from past viral pandemics and anticipate that neuropsychiatric sequelae are likely to occur and should be part of the public health response to the pandemic.
Bottom Line
The coronavirus disease 2019 pandemic provides multiple clinical challenges pertinent to psychiatry. Neuropsychiatric symptoms may manifest from delirium, viral infection, host immune response, or adverse reactions to experimental treatments. These potential neuropsychiatric symptoms may complicate medical treatment. They can also raise important ethical and legal considerations, such as weighing patient autonomy vs third-party responsibility to the public at large.
Related Resources
- Ferrando SJ, Klepacz L, Lynch S, et al. COVID-19 psychosis: a potential new neuropsychiatric condition triggered by novel coronavirus infection and the inflammatory response? [published online May 19, 2020]. Psychosomatics. 2020. doi: 10.1016/j.psym.2020.05.012.
- Vlessides M. COVID-19 and psychosis: is there a link? Medscape Medical News. https://www.medscape.com/viewarticle/930224. Published May 8, 2020.
Drug Brand Names
Azithromycin • Zithromax
Ceftriaxone • Rocephin
Chloroquine • Aralen
Haloperidol • Haldol
Hydroxychloroquine • Plaquenil
Levofloxacin • Levaquin
Oseltamivir • Tamiflu
1. Ghossoub E, Newman WJ. COVID-19 and the duty to protect from communicable diseases. [published online ahead of print, May 8, 2020]. J Am Acad Psychiatry Law.
2. Menninger Ka. Psychoses associated with influenza: I. general data: statistical analysis. JAMA. 1919;72(4):235-241.
3. Troyer EA, Kohn JN, Hong S. Are we facing a crashing wave of neuropsychiatric sequelae of COVID-19? Neuropsychiatric symptoms and potential immunologic mechanisms. Brain, Behavior, and Immunity. 2020. doi:10.1016/j.bbi.2020.04.027.
4. Alkadi HO. Antimalarial drug toxicity: a review. Chemotherapy. 2007;53(6):385-391.
5. Bogaczewicz A, Sobów T. Psychiatric adverse effects of chloroquine. Psychiatria i Psychologia Kliniczna. 2017;17(2):111-114.
6. Sato K, Mano T, Iwata A, et al. Neuropsychiatric adverse events of chloroquine: a real-world pharmacovigilance study using the FDA Adverse Event Reporting System (FAERS) database. Biosci Trends. 2020;14(2):139-143.
7. Cortegiani A, Ingoglia G, Ippolito M, et al. A systematic review on the efficacy and safety of chloroquine for the treatment of COVID-19. J Crit Care. 2020;57:279-283.
8. Das P, Rai A, Chopra A, et al. Psychosis likely induced by hydroxychloroquine in a patient with chronic Q fever: a case report and clinically relevant review of pharmacology. Psychosomatics. 2014;55(4):409-413.
CASE Agitated, psychotic, and COVID-19–positive
Mr. G, age 56, is brought to the emergency department (ED) by emergency medical services (EMS) after his girlfriend reports that he was trying to climb into the “fiery furnace” to “burn the devil within him.” Mr. G had recently tested positive for coronavirus disease 2019 (COVID-19) via polymerase chain reaction and had been receiving treatment for it. In the ED, he is distressed and repeatedly exclaims, “The devil is alive!” He insists on covering himself with blankets, despite diaphoresis and soaking through his clothing within minutes. Because he does not respond to attempted redirection, the ED clinicians administer a single dose of IM haloperidol, 2 mg, for agitation.
HISTORY Multiple ED visits and hospitalizations
Mr. G, who has no known psychiatric history, lives with his girlfriend of 10 years. His medical history includes chronic obstructive pulmonary disease and prostate cancer. In 2015, he had a radical prostatectomy, without chemotherapy. His social history includes childhood neglect, which prompted him to leave home when he was a teenager. Mr. G had earned his general education development certificate and worked at a small retail store.
Mr. G had no previous history of mental health treatment per self-report, collateral information from his girlfriend, and chart review. He reported no known family psychiatric history. He did not endorse past psychiatric admissions or suicide attempts, nor previous periods of mania, depression, or psychosis. He said he used illicit substances as a teen, but denied using alcohol, tobacco products, or illicit substances in the past 20 years.
Mr. G recently had multiple ED visits and hospitalizations due to ongoing signs and symptoms associated with his COVID-19 diagnosis, primarily worsening shortness of breath and cough. Eleven days before EMS brought him to the ED at his girlfriend’s request, Mr. G had presented to the ED with chief complaints of shortness of breath and dry cough (Day 0). He reported that he had been “running a fever” for 2 days. In the ED, his initial vital signs were notable only for a temperature of 100.9°F (38.28°C). He was diagnosed with “acute viral syndrome” and received 1 dose of IV ceftriaxone, 2 g, and IV azithromycin, 500 mg. On Day 2, the ED clinicians prescribed a 4-day course of oral azithromycin, 250 mg/d, and discharged him home.
On Day 3, Mr. G returned to the ED with similar complaints—congestion and productive cough. He tested positive for COVID-19, and the ED discharged him home with quarantine instructions. Hours later, he returned to the ED via EMS with chief complaints of chest pain, diarrhea, and myalgias. He was prescribed a 5-day course ofoseltamivir, 75 mg twice daily, and azithromycin, 250 mg/d. The ED again discharged him home.
On Day 4, Mr. G returned to the ED for a fourth time. His chief complaint was worsening shortness of breath. His oxygen saturation was 94% on room air; it improved to 96% on 2 L of oxygen. His chest X-ray showed diffuse reticulonodular opacities throughout his bilateral lung fields and increased airspace opacification in the bilateral lower lobes. The ED admitted Mr. G to an internal medicine unit, where the primary treatment team enrolled him in a clinical trial. As part of the trial, Mr. G received hydroxychloroquine, 400 mg, on Day 4 and Day 5. The placebo-controlled component of the trial involved Mr. G receiving daily infusions of either remdesivir or placebo on Day 6 through Day 8. On Day 8, Mr. G was discharged home.
On Day 9, Mr. G returned to the ED with a chief complaint that his “thermometer wasn’t working” at home. The ED readmitted him to the internal medicine unit. On Day 9 through Day 11, Mr. G received daily doses of
Continue to: During the second hospitalization...
During the second hospitalization, nursing staff reported that Mr. G seemed religiously preoccupied and once reported seeing angels and demons. He was observed sitting in a chair praying to Allah that he would “come in on a horse to chop all the workers’ heads off.”
On Day 11, Mr. G was discharged home. Later that evening, the EMS brought him back in the ED due to his girlfriend’s concerns about his mental state.
EVALUATION Talks to God
On Day 12, psychiatry is consulted to evaluate Mr. G’s new-onset psychosis. Mr. G is alert and oriented to person, place, and time. His speech is loud, though the amount and rate are unremarkable. He displays no psychomotor agitation. His thought process is tangential and focuses on religious themes, specifically referring to Islam. He reports auditory hallucinations of God speaking directly to him. Mr. G states, “I am here because of a miraculous transformation from death back to life. Do you believe in God? Which God do you believe in? There are 2 Gods and only one of them is the true God. He is the God of all the 7 heavens and His true name is Allah, only one God, one faith. Allah is a ball of energy.”
Mr. G’s girlfriend provides collateral information that Mr. G had been raised Christian but was not religious as an adult. She says that he had never spoken about being Muslim. She adds that she had never known him to speak much about religion.
[polldaddy:10572249]
The authors’ observations
The etiology of new-onset psychosis can be related to several factors, including primary psychiatric illnesses, use of illicit substances, sequelae of general medical conditions, or adverse effects of prescribed medications. We considered each of these in the differential diagnosis for Mr. G.
Continue to: Psychiatric illness or illicit substance use
Psychiatric illness or illicit substance use. Because Mr. G was 56 years old and had no known psychiatric history or family psychiatric history, a primary psychiatric illness seemed less likely. Substance-induced psychosis related to illicit substance use also seemed unlikely because he denied using illicit substances, and an expanded urine drug screen was negative.

Psychosis due to a general medical condition. Results from Mr. G’s laboratory workup show marked elevation in multiple inflammation-related biomarkers (Table 1), consistent with the inflammatory profile seen with COVID-19 infection. However, results from several laboratory tests for potential etiologies of new-onset psychosis due to a general medical condition were negative (Table 2). Based on Mr. G’s history of prostate cancer, we considered the possibility of metastatic space-occupying lesions of the brain; however, Mr. G’s head CT showed no acute intracranial abnormalities. Another possible etiology we considered was COVID-19–induced encephalitis; however, Mr. G’s brain MRI with and without contrast showed no evidence of acute or chronic intracranial changes.

Medication-induced psychosis. After largely ruling out primary psychiatric illnesses, illicit substances, and sequelae of general medical conditions, we turned our attention to prescribed medications as a potential etiology of Mr. G’s new-onset psychosis. During his initial hospitalization, Mr. G had been prescribed 2 doses of hydroxychloroquine, 400 mg, to treat his diagnosis of COVID-19. Because none of the other medications he received were reported to have neuropsychiatric adverse effects, including psychosis, hydroxychloroquine-induced psychosis was therefore the primary team’s working diagnosis.
EVALUATION Request to leave AMA
On Day 13, Mr. G requests to leave the hospital against medical advice (AMA). Until this point, he had voluntarily remained in the hospital, which he repeatedly referred to as “Heaven.” When asked to describe his medical condition, Mr. G replies, “God told me my condition is far beyond man’s understanding.” He denies that he is positive for COVID-19. He states, “I am cured, and the real fight has just begun.”
At the recommendation of the psychiatry consultation-liaison (C-L) service, the primary treatment team determines that Mr. G does not have capacity to leave AMA. The team is concerned that because of his psychotic symptoms, Mr. G would be unable to understand and follow his quarantine instructions. He remains hospitalized on a medical hold.
Continue to: The authors' observations
The authors’ observations
One important consideration this case highlighted was potential third-party responsibility clinicians and hospital systems may face if they discharge a patient with a communicable illness who is unable to follow precautions based on a psychiatric condition.1 That concern was based on Mr. G’s reported desire to pursue missions “beyond man’s understanding,” which he felt compelled to complete, and which could unnecessarily place the public at risk. The psychiatry C-L service consulted the local health department and conferred with the hospital’s legal representatives, who agreed with the plan to keep Mr. G in the hospital for his safety as well as for the public’s safety.
TREATMENT Oral haloperidol
The psychiatry C-L service recommends initiating an antipsychotic. On Day 13, Mr. G starts oral haloperidol, 2.5 mg twice a day, to address his ongoing psychotic symptoms. On Day 14, the treatment team increases the dosage to 5 mg twice a day. Mr. G tolerates the haloperidol and gradually begins to improve. He demonstrates improved sleep, normal speech volume, less religious preoccupation, and a considerably improved understanding of his medical condition.
The authors’ observations
Mr. G’s initial psychiatric evaluation demonstrated an acute onset of psychotic symptoms, without evidence of delirium. Psychosis secondary to a general medical condition (such as COVID-19) and hydroxychloroquine-induced psychotic disorder topped our initial considerations in the differential diagnosis of this case. While the exact neuropsychiatric sequelae of COVID-19 are not yet clear, previous experiences with viral pandemics and case studies from the current pandemic demonstrate a wide variety of possible neuropsychiatric manifestations. Mood symptoms, psychosis, and encephalopathy represent some of the neuropsychiatric complications observed with past viral pandemics.2 Neuropsychiatric symptoms may be triggered by the virus itself, or from the host’s immune response to the infection.3 To further complicate matters, neuropsychiatric symptoms may manifest during the acute viral infection, or may surface later, as subacute or chronic neuropsychiatric illness.
Neuropsychiatric adverse events
Mr. G developed psychotic symptoms within the first few days of receiving hydroxychloroquine, which is consistent with the scant literature on this topic.8 Based on the available information, hydroxychloroquine remains the most likely etiology of his new-onset psychotic symptoms. Mr. G’s case is one example of the possible neuropsychiatric presentations clinicians may face while treating a novel viral illness.
Continue to: OUTCOME Homeward-bound
OUTCOME Homeward-bound
By Day 18, Mr. G’s psychotic symptoms have significantly improved. He is able to rationally process information about his COVID-19 diagnosis and the recommended quarantine instructions he needs to follow after discharge. He is cleared by infection control and discharged home to return to living with his girlfriend.
Mr. G attends his follow-up psychiatric appointment remotely 2 weeks after discharge. He reports that since discharge, he has continued taking his prescribed haloperidol, 5 mg twice a day. He demonstrates improved insight into his medical condition, acknowledging his COVID-19–positive status, and confirms that he has been following quarantine instructions. He does not report ongoing auditory or visual hallucinations, and is no longer religiously preoccupied. He says he is looking forward to being medically cleared to return to work.
The authors’ observations
This case highlights the need for prospective, longitudinal screening and monitoring of neuropsychiatric symptoms as part of the public health response to COVID-19. The case also highlights the importance of careful monitoring for adverse events, including neuropsychiatric symptoms, during clinical trials that involve experimental treatments. The long-term prognosis for individuals such as Mr. G who develop neuropsychiatric symptoms during acute COVID-19 infection remains unknown. Similarly, subacute and chronic neuropsychiatric manifestations that may develop after resolution of acute COVID-19 infection are unknown at this time. However, we can learn from past viral pandemics and anticipate that neuropsychiatric sequelae are likely to occur and should be part of the public health response to the pandemic.
Bottom Line
The coronavirus disease 2019 pandemic provides multiple clinical challenges pertinent to psychiatry. Neuropsychiatric symptoms may manifest from delirium, viral infection, host immune response, or adverse reactions to experimental treatments. These potential neuropsychiatric symptoms may complicate medical treatment. They can also raise important ethical and legal considerations, such as weighing patient autonomy vs third-party responsibility to the public at large.
Related Resources
- Ferrando SJ, Klepacz L, Lynch S, et al. COVID-19 psychosis: a potential new neuropsychiatric condition triggered by novel coronavirus infection and the inflammatory response? [published online May 19, 2020]. Psychosomatics. 2020. doi: 10.1016/j.psym.2020.05.012.
- Vlessides M. COVID-19 and psychosis: is there a link? Medscape Medical News. https://www.medscape.com/viewarticle/930224. Published May 8, 2020.
Drug Brand Names
Azithromycin • Zithromax
Ceftriaxone • Rocephin
Chloroquine • Aralen
Haloperidol • Haldol
Hydroxychloroquine • Plaquenil
Levofloxacin • Levaquin
Oseltamivir • Tamiflu
CASE Agitated, psychotic, and COVID-19–positive
Mr. G, age 56, is brought to the emergency department (ED) by emergency medical services (EMS) after his girlfriend reports that he was trying to climb into the “fiery furnace” to “burn the devil within him.” Mr. G had recently tested positive for coronavirus disease 2019 (COVID-19) via polymerase chain reaction and had been receiving treatment for it. In the ED, he is distressed and repeatedly exclaims, “The devil is alive!” He insists on covering himself with blankets, despite diaphoresis and soaking through his clothing within minutes. Because he does not respond to attempted redirection, the ED clinicians administer a single dose of IM haloperidol, 2 mg, for agitation.
HISTORY Multiple ED visits and hospitalizations
Mr. G, who has no known psychiatric history, lives with his girlfriend of 10 years. His medical history includes chronic obstructive pulmonary disease and prostate cancer. In 2015, he had a radical prostatectomy, without chemotherapy. His social history includes childhood neglect, which prompted him to leave home when he was a teenager. Mr. G had earned his general education development certificate and worked at a small retail store.
Mr. G had no previous history of mental health treatment per self-report, collateral information from his girlfriend, and chart review. He reported no known family psychiatric history. He did not endorse past psychiatric admissions or suicide attempts, nor previous periods of mania, depression, or psychosis. He said he used illicit substances as a teen, but denied using alcohol, tobacco products, or illicit substances in the past 20 years.
Mr. G recently had multiple ED visits and hospitalizations due to ongoing signs and symptoms associated with his COVID-19 diagnosis, primarily worsening shortness of breath and cough. Eleven days before EMS brought him to the ED at his girlfriend’s request, Mr. G had presented to the ED with chief complaints of shortness of breath and dry cough (Day 0). He reported that he had been “running a fever” for 2 days. In the ED, his initial vital signs were notable only for a temperature of 100.9°F (38.28°C). He was diagnosed with “acute viral syndrome” and received 1 dose of IV ceftriaxone, 2 g, and IV azithromycin, 500 mg. On Day 2, the ED clinicians prescribed a 4-day course of oral azithromycin, 250 mg/d, and discharged him home.
On Day 3, Mr. G returned to the ED with similar complaints—congestion and productive cough. He tested positive for COVID-19, and the ED discharged him home with quarantine instructions. Hours later, he returned to the ED via EMS with chief complaints of chest pain, diarrhea, and myalgias. He was prescribed a 5-day course ofoseltamivir, 75 mg twice daily, and azithromycin, 250 mg/d. The ED again discharged him home.
On Day 4, Mr. G returned to the ED for a fourth time. His chief complaint was worsening shortness of breath. His oxygen saturation was 94% on room air; it improved to 96% on 2 L of oxygen. His chest X-ray showed diffuse reticulonodular opacities throughout his bilateral lung fields and increased airspace opacification in the bilateral lower lobes. The ED admitted Mr. G to an internal medicine unit, where the primary treatment team enrolled him in a clinical trial. As part of the trial, Mr. G received hydroxychloroquine, 400 mg, on Day 4 and Day 5. The placebo-controlled component of the trial involved Mr. G receiving daily infusions of either remdesivir or placebo on Day 6 through Day 8. On Day 8, Mr. G was discharged home.
On Day 9, Mr. G returned to the ED with a chief complaint that his “thermometer wasn’t working” at home. The ED readmitted him to the internal medicine unit. On Day 9 through Day 11, Mr. G received daily doses of
Continue to: During the second hospitalization...
During the second hospitalization, nursing staff reported that Mr. G seemed religiously preoccupied and once reported seeing angels and demons. He was observed sitting in a chair praying to Allah that he would “come in on a horse to chop all the workers’ heads off.”
On Day 11, Mr. G was discharged home. Later that evening, the EMS brought him back in the ED due to his girlfriend’s concerns about his mental state.
EVALUATION Talks to God
On Day 12, psychiatry is consulted to evaluate Mr. G’s new-onset psychosis. Mr. G is alert and oriented to person, place, and time. His speech is loud, though the amount and rate are unremarkable. He displays no psychomotor agitation. His thought process is tangential and focuses on religious themes, specifically referring to Islam. He reports auditory hallucinations of God speaking directly to him. Mr. G states, “I am here because of a miraculous transformation from death back to life. Do you believe in God? Which God do you believe in? There are 2 Gods and only one of them is the true God. He is the God of all the 7 heavens and His true name is Allah, only one God, one faith. Allah is a ball of energy.”
Mr. G’s girlfriend provides collateral information that Mr. G had been raised Christian but was not religious as an adult. She says that he had never spoken about being Muslim. She adds that she had never known him to speak much about religion.
[polldaddy:10572249]
The authors’ observations
The etiology of new-onset psychosis can be related to several factors, including primary psychiatric illnesses, use of illicit substances, sequelae of general medical conditions, or adverse effects of prescribed medications. We considered each of these in the differential diagnosis for Mr. G.
Continue to: Psychiatric illness or illicit substance use
Psychiatric illness or illicit substance use. Because Mr. G was 56 years old and had no known psychiatric history or family psychiatric history, a primary psychiatric illness seemed less likely. Substance-induced psychosis related to illicit substance use also seemed unlikely because he denied using illicit substances, and an expanded urine drug screen was negative.

Psychosis due to a general medical condition. Results from Mr. G’s laboratory workup show marked elevation in multiple inflammation-related biomarkers (Table 1), consistent with the inflammatory profile seen with COVID-19 infection. However, results from several laboratory tests for potential etiologies of new-onset psychosis due to a general medical condition were negative (Table 2). Based on Mr. G’s history of prostate cancer, we considered the possibility of metastatic space-occupying lesions of the brain; however, Mr. G’s head CT showed no acute intracranial abnormalities. Another possible etiology we considered was COVID-19–induced encephalitis; however, Mr. G’s brain MRI with and without contrast showed no evidence of acute or chronic intracranial changes.

Medication-induced psychosis. After largely ruling out primary psychiatric illnesses, illicit substances, and sequelae of general medical conditions, we turned our attention to prescribed medications as a potential etiology of Mr. G’s new-onset psychosis. During his initial hospitalization, Mr. G had been prescribed 2 doses of hydroxychloroquine, 400 mg, to treat his diagnosis of COVID-19. Because none of the other medications he received were reported to have neuropsychiatric adverse effects, including psychosis, hydroxychloroquine-induced psychosis was therefore the primary team’s working diagnosis.
EVALUATION Request to leave AMA
On Day 13, Mr. G requests to leave the hospital against medical advice (AMA). Until this point, he had voluntarily remained in the hospital, which he repeatedly referred to as “Heaven.” When asked to describe his medical condition, Mr. G replies, “God told me my condition is far beyond man’s understanding.” He denies that he is positive for COVID-19. He states, “I am cured, and the real fight has just begun.”
At the recommendation of the psychiatry consultation-liaison (C-L) service, the primary treatment team determines that Mr. G does not have capacity to leave AMA. The team is concerned that because of his psychotic symptoms, Mr. G would be unable to understand and follow his quarantine instructions. He remains hospitalized on a medical hold.
Continue to: The authors' observations
The authors’ observations
One important consideration this case highlighted was potential third-party responsibility clinicians and hospital systems may face if they discharge a patient with a communicable illness who is unable to follow precautions based on a psychiatric condition.1 That concern was based on Mr. G’s reported desire to pursue missions “beyond man’s understanding,” which he felt compelled to complete, and which could unnecessarily place the public at risk. The psychiatry C-L service consulted the local health department and conferred with the hospital’s legal representatives, who agreed with the plan to keep Mr. G in the hospital for his safety as well as for the public’s safety.
TREATMENT Oral haloperidol
The psychiatry C-L service recommends initiating an antipsychotic. On Day 13, Mr. G starts oral haloperidol, 2.5 mg twice a day, to address his ongoing psychotic symptoms. On Day 14, the treatment team increases the dosage to 5 mg twice a day. Mr. G tolerates the haloperidol and gradually begins to improve. He demonstrates improved sleep, normal speech volume, less religious preoccupation, and a considerably improved understanding of his medical condition.
The authors’ observations
Mr. G’s initial psychiatric evaluation demonstrated an acute onset of psychotic symptoms, without evidence of delirium. Psychosis secondary to a general medical condition (such as COVID-19) and hydroxychloroquine-induced psychotic disorder topped our initial considerations in the differential diagnosis of this case. While the exact neuropsychiatric sequelae of COVID-19 are not yet clear, previous experiences with viral pandemics and case studies from the current pandemic demonstrate a wide variety of possible neuropsychiatric manifestations. Mood symptoms, psychosis, and encephalopathy represent some of the neuropsychiatric complications observed with past viral pandemics.2 Neuropsychiatric symptoms may be triggered by the virus itself, or from the host’s immune response to the infection.3 To further complicate matters, neuropsychiatric symptoms may manifest during the acute viral infection, or may surface later, as subacute or chronic neuropsychiatric illness.
Neuropsychiatric adverse events
Mr. G developed psychotic symptoms within the first few days of receiving hydroxychloroquine, which is consistent with the scant literature on this topic.8 Based on the available information, hydroxychloroquine remains the most likely etiology of his new-onset psychotic symptoms. Mr. G’s case is one example of the possible neuropsychiatric presentations clinicians may face while treating a novel viral illness.
Continue to: OUTCOME Homeward-bound
OUTCOME Homeward-bound
By Day 18, Mr. G’s psychotic symptoms have significantly improved. He is able to rationally process information about his COVID-19 diagnosis and the recommended quarantine instructions he needs to follow after discharge. He is cleared by infection control and discharged home to return to living with his girlfriend.
Mr. G attends his follow-up psychiatric appointment remotely 2 weeks after discharge. He reports that since discharge, he has continued taking his prescribed haloperidol, 5 mg twice a day. He demonstrates improved insight into his medical condition, acknowledging his COVID-19–positive status, and confirms that he has been following quarantine instructions. He does not report ongoing auditory or visual hallucinations, and is no longer religiously preoccupied. He says he is looking forward to being medically cleared to return to work.
The authors’ observations
This case highlights the need for prospective, longitudinal screening and monitoring of neuropsychiatric symptoms as part of the public health response to COVID-19. The case also highlights the importance of careful monitoring for adverse events, including neuropsychiatric symptoms, during clinical trials that involve experimental treatments. The long-term prognosis for individuals such as Mr. G who develop neuropsychiatric symptoms during acute COVID-19 infection remains unknown. Similarly, subacute and chronic neuropsychiatric manifestations that may develop after resolution of acute COVID-19 infection are unknown at this time. However, we can learn from past viral pandemics and anticipate that neuropsychiatric sequelae are likely to occur and should be part of the public health response to the pandemic.
Bottom Line
The coronavirus disease 2019 pandemic provides multiple clinical challenges pertinent to psychiatry. Neuropsychiatric symptoms may manifest from delirium, viral infection, host immune response, or adverse reactions to experimental treatments. These potential neuropsychiatric symptoms may complicate medical treatment. They can also raise important ethical and legal considerations, such as weighing patient autonomy vs third-party responsibility to the public at large.
Related Resources
- Ferrando SJ, Klepacz L, Lynch S, et al. COVID-19 psychosis: a potential new neuropsychiatric condition triggered by novel coronavirus infection and the inflammatory response? [published online May 19, 2020]. Psychosomatics. 2020. doi: 10.1016/j.psym.2020.05.012.
- Vlessides M. COVID-19 and psychosis: is there a link? Medscape Medical News. https://www.medscape.com/viewarticle/930224. Published May 8, 2020.
Drug Brand Names
Azithromycin • Zithromax
Ceftriaxone • Rocephin
Chloroquine • Aralen
Haloperidol • Haldol
Hydroxychloroquine • Plaquenil
Levofloxacin • Levaquin
Oseltamivir • Tamiflu
1. Ghossoub E, Newman WJ. COVID-19 and the duty to protect from communicable diseases. [published online ahead of print, May 8, 2020]. J Am Acad Psychiatry Law.
2. Menninger Ka. Psychoses associated with influenza: I. general data: statistical analysis. JAMA. 1919;72(4):235-241.
3. Troyer EA, Kohn JN, Hong S. Are we facing a crashing wave of neuropsychiatric sequelae of COVID-19? Neuropsychiatric symptoms and potential immunologic mechanisms. Brain, Behavior, and Immunity. 2020. doi:10.1016/j.bbi.2020.04.027.
4. Alkadi HO. Antimalarial drug toxicity: a review. Chemotherapy. 2007;53(6):385-391.
5. Bogaczewicz A, Sobów T. Psychiatric adverse effects of chloroquine. Psychiatria i Psychologia Kliniczna. 2017;17(2):111-114.
6. Sato K, Mano T, Iwata A, et al. Neuropsychiatric adverse events of chloroquine: a real-world pharmacovigilance study using the FDA Adverse Event Reporting System (FAERS) database. Biosci Trends. 2020;14(2):139-143.
7. Cortegiani A, Ingoglia G, Ippolito M, et al. A systematic review on the efficacy and safety of chloroquine for the treatment of COVID-19. J Crit Care. 2020;57:279-283.
8. Das P, Rai A, Chopra A, et al. Psychosis likely induced by hydroxychloroquine in a patient with chronic Q fever: a case report and clinically relevant review of pharmacology. Psychosomatics. 2014;55(4):409-413.
1. Ghossoub E, Newman WJ. COVID-19 and the duty to protect from communicable diseases. [published online ahead of print, May 8, 2020]. J Am Acad Psychiatry Law.
2. Menninger Ka. Psychoses associated with influenza: I. general data: statistical analysis. JAMA. 1919;72(4):235-241.
3. Troyer EA, Kohn JN, Hong S. Are we facing a crashing wave of neuropsychiatric sequelae of COVID-19? Neuropsychiatric symptoms and potential immunologic mechanisms. Brain, Behavior, and Immunity. 2020. doi:10.1016/j.bbi.2020.04.027.
4. Alkadi HO. Antimalarial drug toxicity: a review. Chemotherapy. 2007;53(6):385-391.
5. Bogaczewicz A, Sobów T. Psychiatric adverse effects of chloroquine. Psychiatria i Psychologia Kliniczna. 2017;17(2):111-114.
6. Sato K, Mano T, Iwata A, et al. Neuropsychiatric adverse events of chloroquine: a real-world pharmacovigilance study using the FDA Adverse Event Reporting System (FAERS) database. Biosci Trends. 2020;14(2):139-143.
7. Cortegiani A, Ingoglia G, Ippolito M, et al. A systematic review on the efficacy and safety of chloroquine for the treatment of COVID-19. J Crit Care. 2020;57:279-283.
8. Das P, Rai A, Chopra A, et al. Psychosis likely induced by hydroxychloroquine in a patient with chronic Q fever: a case report and clinically relevant review of pharmacology. Psychosomatics. 2014;55(4):409-413.