User login
Food for thought: Dangerous weight loss in an older adult
CASE Fixated on health and nutrition
At the insistence of her daughter, Ms. L, age 75, presents to the emergency department (ED) for self-neglect and severe weight loss, with a body mass index (BMI) of 13.5 kg/m2 (normal: 18.5 to 24.9 kg/m2). When asked why she is in the ED, Ms. L says she doesn’t know. She attributes her significant weight loss (approximately 20 pounds in the last few months) to gastroesophageal reflux disease (GERD). She constantly worries about her esophagus. She had been diagnosed with esophageal dysphagia 7 years ago after undergoing radiofrequency ablation for esophageal cancer. Ms. L fixates on the negative effects certain foods and ingredients might have on her stomach and esophagus.
Following transfer from the ED, Ms. L is involuntarily admitted to our inpatient unit. Although she acknowledges weight loss, she minimizes the severity of her illness and indicates she would like to gain weight, but only by eating healthy foods she is comfortable with, including kale, quinoa, and vegetables. Ms. L says that she has always been interested in “healthful foods” and that she “loves sugar,” but “it’s bad for you,” mentioning that “sugar fuels cancer.” She has daily thoughts about sugar causing cancer. Ms. L also mentions that she stopped eating flour, sugar, fried food, and oils because those foods affect her “stomach acid” and cause “pimples on my face and weight loss.” While in the inpatient unit, Ms. L requests a special diet and demands to know the origin and ingredients of the foods she is offered. She emphasizes that her esophageal cancer diagnosis and dysphagia exacerbate worries that certain foods cause cancer, and wants to continue her diet restrictions. Nonetheless, she says she wants to get healthy, and denies an intense fear of gaining weight or feeling fat.
HISTORY Multiple psychiatric diagnoses
Ms. L lives alone and enjoys spending time with her grandchildren, visiting museums, and listening to classical music. However, her family, social workers, and records from a previous psychiatric hospitalization reveal that Ms. L has a history of psychiatric illness and fears regarding certain types of foods for much of her adult life. Ms. L’s family also described a range of compulsive behaviors, including shoplifting, hoarding art, multiple plastic surgeries, and phases where Ms. L ate only frozen yogurt without sugar.
Ms. L’s daughter reported that Ms. L had seen a psychologist in the late 1990s for depression and had been diagnosed with obsessive-compulsive disorder (OCD) and attention deficit/hyperactivity disorder in the early 2000s. In 2006, during a depressive episode after her divorce, Ms. L had a suicide attempt with pills and alcohol, and was hospitalized. Records from that stay described a history of mood dysregulation with fears regarding food and nutrition. Ms. L was treated with aripiprazole 5 mg/d. A trial of trazodone 25 mg/d did not have any effect. When discharged, she was receiving lamotrigine 100 mg/d. However, her daughter believes she stopped taking all psychiatric medications shortly after discharge.
Her daughter says that in the past 2 years, Ms. L has seen multiple doctors for treatment of somatic gastrointestinal (GI) complaints. A 2018 note from a social worker indicated that Ms. L endorsed taking >80 supplements per day and constantly researched nutrition online. In the months leading to her current hospitalization, Ms. L suffered from severe self-neglect and fear regarding foods she felt were not healthy for her. She had stopped leaving her apartment.
Continue to: EVALUATION Poor insight, normal lab results...
EVALUATION Poor insight, normal lab results
During her evaluation, Ms. L appears cachectic and frail. She has a heavily constricted affect and is guarded, dismissive, and vague. Although her thought processes are linear and goal-directed, her insight into her condition is extremely poor and she appears surprised when clinicians inform her that her self-neglect would lead to death. Instead, Ms. L insists she is eating healthily and demonstrates severe anxiety in relation to her GI symptoms.
Ms. L is oriented to person, place, and time. She scores 27/30 on the Montreal Cognitive Assessment, indicating normal cognition. She denies any depressive symptoms or suicidal intent. She does not appear to be internally preoccupied and denies having auditory or visual hallucinations or manic symptoms.
A neurologic examination reveals that her cranial nerves are normal, and cerebellar function, strength, and sensory testing are intact. Her gait is steady and she walks without a walker. Despite her severely low BMI and recent history of self-neglect, Ms. L’s laboratory results are remarkably normal and show no liver, metabolic, or electrolyte abnormalities, no signs of infection, and normal vitamin B12 levels. She has slightly elevated creatinine and blood urea nitrogen levels, but a normal glomerular filtration rate.
Her medical history is significant for squamous cell esophageal cancer, treated with radiofrequency ablation. Although Ms. L is constantly worried about the recurrence of cancer, pathology reports demonstrate no esophageal dysplasia. However, she does show evidence of an approximately 1 cm × 1 cm mild, noncircumferential esophageal stenosis, likely resulting from radiofrequency ablation.
[polldaddy:11079394]
The authors’ observations
Several health- and physical symptom-related psychiatric disorders have overlapping features, which can complicate the differential diagnosis (Table 11). Ms. L presented to the ED with a severely low BMI of 13.5 kg/m2, obsessions regarding specific types of food, and preoccupations regarding her esophagus. Despite her extensive psychiatric history (including intense fears regarding food), we ruled out a primary psychotic disorder because she did not describe auditory or visual hallucinations and never appeared internally preoccupied. While her BMI and persistent minimization of the extent of her disease meet criteria for anorexia nervosa, she denied body dysmorphia and did not have any fear of gaining weight.
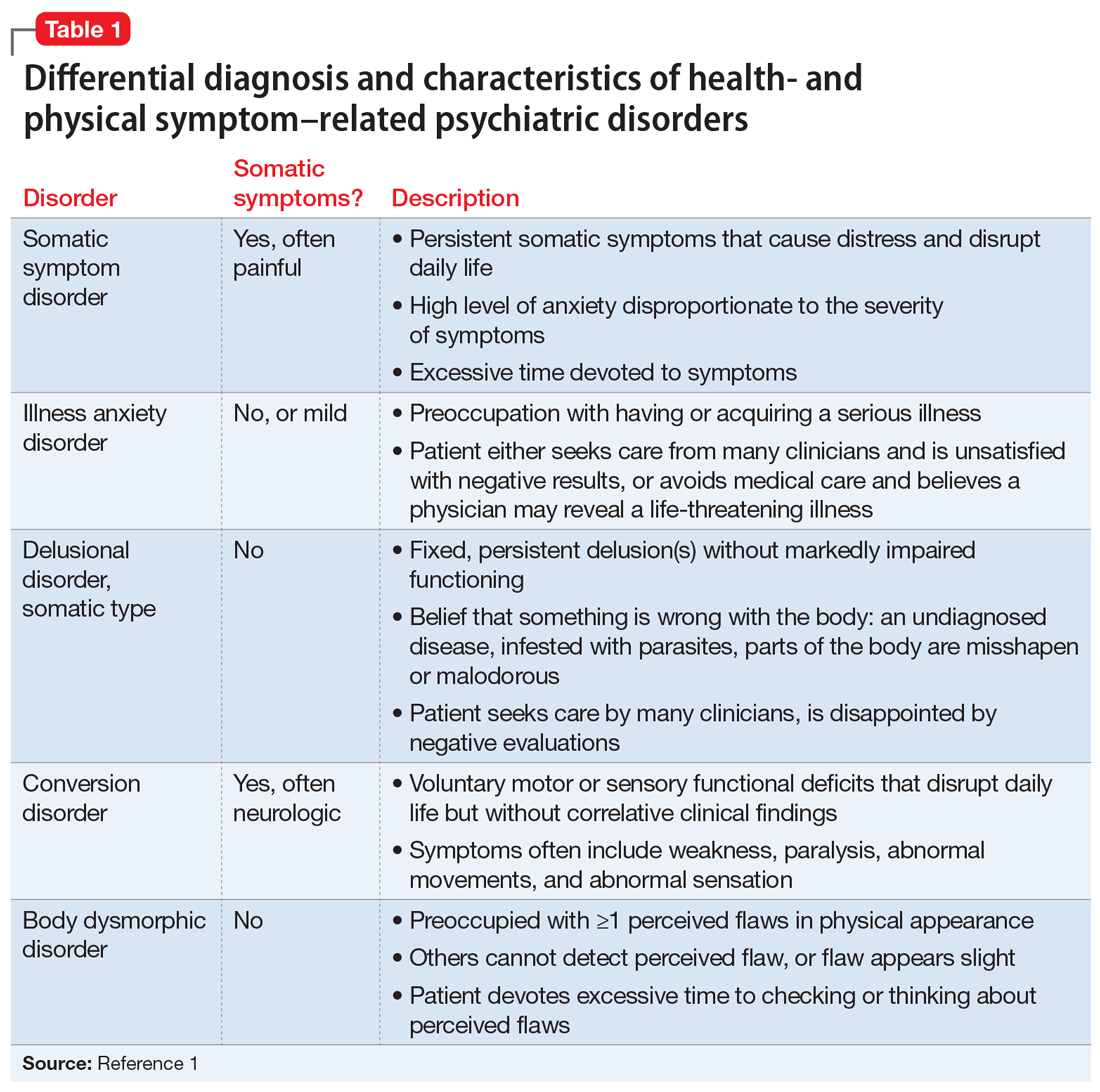
A central element of Ms. L’s presentation was her anxiety regarding how certain types of foods impact her health as well as her anxieties regarding her esophagus. While Ms. L was in remission from esophageal cancer and had a diagnosis of esophageal dysphagia, these preoccupations and obsessions regarding how certain types of foods affect her esophagus drove her to self-neglect and thus represent pathologic thought processes out of proportion to her symptoms. Illness anxiety disorder was considered because Ms. L met many of its criteria: preoccupation with having a serious illness, disproportionate preoccupation with somatic symptoms if they are present, extreme anxiety over health, and performance of health-related behaviors.1 However, illness anxiety disorder is a diagnosis of exclusion, and 1 criterion is that these symptoms cannot be explained by another mental disorder. We felt other diagnoses better fit Ms. L’s condition and ruled out illness anxiety disorder.
Ms. L’s long history of food and non-food–related obsessions and compulsions that interrupted her ability to perform daily activities were strongly suggestive for OCD. Additionally, her intense preoccupation, high level of anxiety, amount of time and energy spent seeking care for her esophagus and GERD symptoms, and the resulting significant disruption of daily life, met criteria for somatic symptom disorder (SSD). However, we did not believe that a diagnosis of OCD and SSD alone explained the entirety of Ms. L’s clinical picture. Despite ruling out anorexia nervosa, Ms. L nonetheless demonstrated disordered eating.
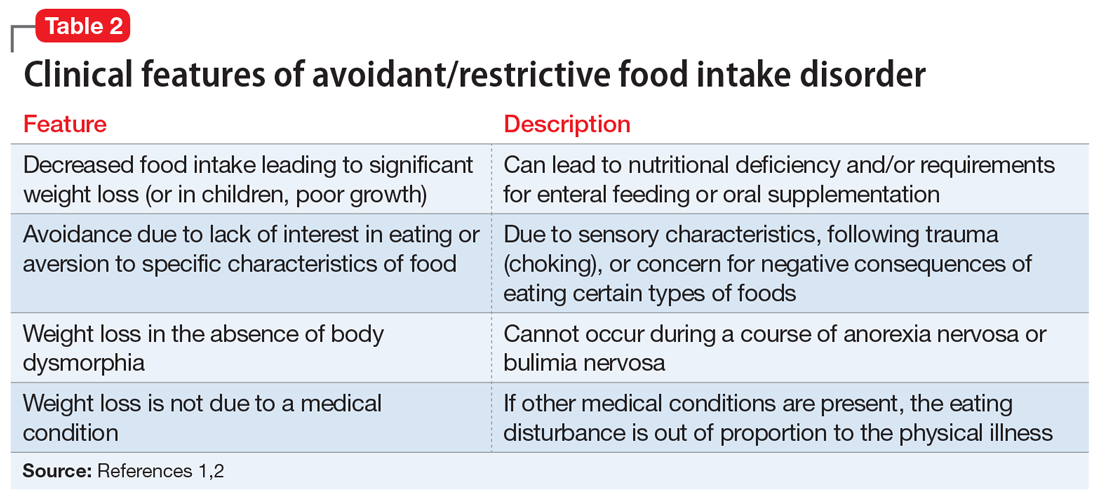
Avoidant/restrictive food intake disorder (ARFID) is an eating disorder in which patients restrict their diet and do not meet nutritional needs for any number of reasons, do not experience body dysmorphia, and do not fear weight gain.1 A common feature of ARFID is a fear of negative consequences from eating specific types of food.2 Table 21,2 summarizes additional clinical features of ARFID. Although ARFID is typically diagnosed in children and adolescents, particularly in individuals with autism with heightened sensory sensitivities, ARFID is also common among adult patients with GI disorders.3 In a retrospective chart review of 410 adults ages 18 to 90 (73% women) referred to a neurogastroenterology care center, 6.3% met the full criteria for ARFID and 17.3% had clinically significant avoidant or restrictive eating behaviors. Among patients with ARFID symptoms, 93% stated that a fear of GI symptoms was the driver of their avoidant or restrictive eating behaviors.2 Patients with GI diseases often develop dietary control and avoidance coping mechanisms to alleviate their symptoms.4 These strategies can exacerbate health anxieties and have a detrimental effect on mental health.5 Patients with GI disorders have a high degree of comorbidity with affective disorders, including anxiety disorders.6 These trends may arise from hypervigilance and the need to gain control over physical symptoms.7 Feeling a need for control, actions driven by anxiety and fear, and the need for compensatory behaviors are cardinal features of OCD and eating disorders.8 Multiple studies have demonstrated comorbidities between irritable bowel syndrome and eating disorders,9 SSD,10 and OCD.11 Taken together with observations that ARFID is also found in patients with GI disorders,2 these findings demonstrate that patients with a history of GI disease are at high risk of developing extreme health anxieties and behavioral coping strategies that can lead to disordered eating.
The rise in “healthy” eating materials online—particularly on social media—has created an atmosphere in which misinformation regarding diet and health is common and widespread. For patients with OCD and a predisposition to health anxiety, such as Ms. L, searching online for nutrition information and healthy living habits can exacerbate food-related anxieties and can lead to a pathological drive for purity and health.12Although not included in DSM-5, orthorexia nervosa was identified in 1997 as a proposed eating disorder best characterized as an obsession with healthy eating with associated restrictive behaviors.13 Patients with this disorder are rarely focused on losing weight, and orthorexic eating behaviors have been associated with both SSD and OCD.12,14 As in Ms. L’s case, patients with orthorexia nervosa demonstrate intrusive obsessions with nutrition, spend excessive amount of time researching nutrition, and fixate on food quality.12 Throughout Ms. L’s hospitalization, even as her food-related magical thinking symptoms improved, she constantly informed her care team that she had been “eating healthily” even though she was severely cachectic. Patients with SSD and OCD prone to health anxieties are at risk of developing pathologic food beliefs and dangerous eating behaviors. These patients may benefit from psychoeducation regarding nutrition and media literacy, which are components of effective eating disorder programs.15
[polldaddy:11079399]
Continue to: The authors' observations...
The authors’ observations
How do we approach the pharmacologic treatment of patients with co-occurring eating, somatic symptom, and anxiety disorders? Olanzapine facilitates recovery in children and adolescents with ARFID by promoting eating and weight gain, and decreasing symptoms of depression and anxiety.16 Patients with orthorexia nervosa also may benefit from treatment with olanzapine, which has decreased food-related fixations, magical thinking, and delusions regarding food.17 Further, orthorexic patients with ARFID have also been shown to respond to SSRIs due to those agents’ efficacy for treating intrusive thoughts, obsessions, and preoccupations from OCD and SSD.18,19 Thus, treating Ms. L’s symptoms with olanzapine and fluoxetine targeted the intersection of several diagnoses on our differential. Olanzapine’s propensity to cause weight gain is favorable in this population, particularly patients such as Ms. L, who do not exhibit body dysmorphia or fear of gaining weight.
OUTCOME Weight gain and fewer fears
Ms. L is prescribed olanzapine 5 mg/d and fluoxetine 20 mg/d. She gains 20.6 pounds in 4 weeks. Importantly, she endorses fewer fears related to foods and expands her palate to include foods she previously considered to be unhealthy, including white bread and farm-raised salmon. Further, she spends less time thinking about food and says she has less anxiety regarding the recurrence of GI symptoms.
1. Diagnostic and Statistical Manual of Mental Disorders: DSM-5. 5th ed. American Psychiatric Association; 2013.
2. Murray HB, Bailey AP, Keshishian AC. Prevalence and characteristics of avoidant/restrictive food intake disorder in adult neurogastroenterology patients. Clin Gastroenterol Hepatol. 2020;18(9):1995-2002.e1.
3. Görmez A, Kılıç A, Kırpınar İ. Avoidant/restrictive food intake disorder: an adult case responding to cognitive behavioral therapy. Clinical Case Studies. 2018;17(6):443-452.
4. Reed-Knight B, Squires M, Chitkara DK, et al. Adolescents with irritable bowel syndrome report increased eating-associated symptoms, changes in dietary composition, and altered eating behaviors: a pilot comparison study to healthy adolescents. Neurogastroenterol Motil. 2016;28(12):1915-1920.
5. Melchior C, Desprez C, Riachi G, et al. Anxiety and depression profile is associated with eating disorders in patients with irritable bowel syndrome. Front Psychiatry. 2020;10:928.
6. Mayer EA, Craske M, Naliboff BD. Depression, anxiety, and the gastrointestinal system. J Clin Psychiatry. 2001;62 Suppl 8:28-37.
7. Abraham S, Kellow J. Exploring eating disorder quality of life and functional gastrointestinal disorders among eating disorder patients. J Psychosom Res. 2011;70(4):372-377.
8. Swinbourne JM, Touyz SW. The co-morbidity of eating disorders and anxiety disorders: a review. Eur Eat Disord Rev. 2007;15(4):253-274.
9. Perkins SJ, Keville S, Schmidt U, et al. Eating disorders and irritable bowel syndrome: is there a link? J Psychosom Res. 2005;59(2):57-64.
10. Hausteiner-Wiehle C, Henningsen P. Irritable bowel syndrome: relations with functional, mental, and somatoform disorders. World J Gastroenterol. 2014;20(2):6024-6030.
11. Masand PS, Keuthen NJ, Gupta S, et al. Prevalence of irritable bowel syndrome in obsessive-compulsive disorder. CNS Spectr. 2006;11(1):21-25.
12. Koven NS, Abry AW. The clinical basis of orthorexia nervosa: emerging perspectives. Neuropsychiatr Dis Treat. 2015;11:385-394.
13. Bratman S. Health food junkie. Yoga Journal. 1997;136:42-50.
14. Barthels F, Müller R, Schüth T, et al. Orthorexic eating behavior in patients with somatoform disorders. Eat Weight Disord. 2021;26(1):135-143.
15. Ciao AC, Loth K, Neumark-Sztainer D. Preventing eating disorder pathology: common and unique features of successful eating disorders prevention programs. Curr Psychiatry Rep. 2014;16(7):453.
16. Brewerton TD, D’Agostino M. Adjunctive use of olanzapine in the treatment of avoidant restrictive food intake disorder in children and adolescents in an eating disorders program. J Child Adolesc Psychopharmacol. 2017;27(10):920-922.
17. Moroze RM, Dunn TM, Craig Holland J, et al. Microthinking about micronutrients: a case of transition from obsessions about healthy eating to near-fatal “orthorexia nervosa” and proposed diagnostic criteria. Psychosomatics. 2015;56(4):397-403.
18. Spettigue W, Norris ML, Santos A, et al. Treatment of children and adolescents with avoidant/restrictive food intake disorder: a case series examining the feasibility of family therapy and adjunctive treatments. J Eat Disord. 2018;6:20.
19. Niedzielski A, Kaźmierczak-Wojtaś N. Prevalence of Orthorexia Nervosa and Its Diagnostic Tools-A Literature Review. Int J Environ Res Public Health. 2021;18(10):5488. Published 2021 May 20. doi:10.3390/ijerph18105488 Prevalence of orthorexia nervosa and its diagnostic tools-a literature review. Int J Environ Res Public Health. 2021;18(10):5488.
CASE Fixated on health and nutrition
At the insistence of her daughter, Ms. L, age 75, presents to the emergency department (ED) for self-neglect and severe weight loss, with a body mass index (BMI) of 13.5 kg/m2 (normal: 18.5 to 24.9 kg/m2). When asked why she is in the ED, Ms. L says she doesn’t know. She attributes her significant weight loss (approximately 20 pounds in the last few months) to gastroesophageal reflux disease (GERD). She constantly worries about her esophagus. She had been diagnosed with esophageal dysphagia 7 years ago after undergoing radiofrequency ablation for esophageal cancer. Ms. L fixates on the negative effects certain foods and ingredients might have on her stomach and esophagus.
Following transfer from the ED, Ms. L is involuntarily admitted to our inpatient unit. Although she acknowledges weight loss, she minimizes the severity of her illness and indicates she would like to gain weight, but only by eating healthy foods she is comfortable with, including kale, quinoa, and vegetables. Ms. L says that she has always been interested in “healthful foods” and that she “loves sugar,” but “it’s bad for you,” mentioning that “sugar fuels cancer.” She has daily thoughts about sugar causing cancer. Ms. L also mentions that she stopped eating flour, sugar, fried food, and oils because those foods affect her “stomach acid” and cause “pimples on my face and weight loss.” While in the inpatient unit, Ms. L requests a special diet and demands to know the origin and ingredients of the foods she is offered. She emphasizes that her esophageal cancer diagnosis and dysphagia exacerbate worries that certain foods cause cancer, and wants to continue her diet restrictions. Nonetheless, she says she wants to get healthy, and denies an intense fear of gaining weight or feeling fat.
HISTORY Multiple psychiatric diagnoses
Ms. L lives alone and enjoys spending time with her grandchildren, visiting museums, and listening to classical music. However, her family, social workers, and records from a previous psychiatric hospitalization reveal that Ms. L has a history of psychiatric illness and fears regarding certain types of foods for much of her adult life. Ms. L’s family also described a range of compulsive behaviors, including shoplifting, hoarding art, multiple plastic surgeries, and phases where Ms. L ate only frozen yogurt without sugar.
Ms. L’s daughter reported that Ms. L had seen a psychologist in the late 1990s for depression and had been diagnosed with obsessive-compulsive disorder (OCD) and attention deficit/hyperactivity disorder in the early 2000s. In 2006, during a depressive episode after her divorce, Ms. L had a suicide attempt with pills and alcohol, and was hospitalized. Records from that stay described a history of mood dysregulation with fears regarding food and nutrition. Ms. L was treated with aripiprazole 5 mg/d. A trial of trazodone 25 mg/d did not have any effect. When discharged, she was receiving lamotrigine 100 mg/d. However, her daughter believes she stopped taking all psychiatric medications shortly after discharge.
Her daughter says that in the past 2 years, Ms. L has seen multiple doctors for treatment of somatic gastrointestinal (GI) complaints. A 2018 note from a social worker indicated that Ms. L endorsed taking >80 supplements per day and constantly researched nutrition online. In the months leading to her current hospitalization, Ms. L suffered from severe self-neglect and fear regarding foods she felt were not healthy for her. She had stopped leaving her apartment.
Continue to: EVALUATION Poor insight, normal lab results...
EVALUATION Poor insight, normal lab results
During her evaluation, Ms. L appears cachectic and frail. She has a heavily constricted affect and is guarded, dismissive, and vague. Although her thought processes are linear and goal-directed, her insight into her condition is extremely poor and she appears surprised when clinicians inform her that her self-neglect would lead to death. Instead, Ms. L insists she is eating healthily and demonstrates severe anxiety in relation to her GI symptoms.
Ms. L is oriented to person, place, and time. She scores 27/30 on the Montreal Cognitive Assessment, indicating normal cognition. She denies any depressive symptoms or suicidal intent. She does not appear to be internally preoccupied and denies having auditory or visual hallucinations or manic symptoms.
A neurologic examination reveals that her cranial nerves are normal, and cerebellar function, strength, and sensory testing are intact. Her gait is steady and she walks without a walker. Despite her severely low BMI and recent history of self-neglect, Ms. L’s laboratory results are remarkably normal and show no liver, metabolic, or electrolyte abnormalities, no signs of infection, and normal vitamin B12 levels. She has slightly elevated creatinine and blood urea nitrogen levels, but a normal glomerular filtration rate.
Her medical history is significant for squamous cell esophageal cancer, treated with radiofrequency ablation. Although Ms. L is constantly worried about the recurrence of cancer, pathology reports demonstrate no esophageal dysplasia. However, she does show evidence of an approximately 1 cm × 1 cm mild, noncircumferential esophageal stenosis, likely resulting from radiofrequency ablation.
[polldaddy:11079394]
The authors’ observations
Several health- and physical symptom-related psychiatric disorders have overlapping features, which can complicate the differential diagnosis (Table 11). Ms. L presented to the ED with a severely low BMI of 13.5 kg/m2, obsessions regarding specific types of food, and preoccupations regarding her esophagus. Despite her extensive psychiatric history (including intense fears regarding food), we ruled out a primary psychotic disorder because she did not describe auditory or visual hallucinations and never appeared internally preoccupied. While her BMI and persistent minimization of the extent of her disease meet criteria for anorexia nervosa, she denied body dysmorphia and did not have any fear of gaining weight.

A central element of Ms. L’s presentation was her anxiety regarding how certain types of foods impact her health as well as her anxieties regarding her esophagus. While Ms. L was in remission from esophageal cancer and had a diagnosis of esophageal dysphagia, these preoccupations and obsessions regarding how certain types of foods affect her esophagus drove her to self-neglect and thus represent pathologic thought processes out of proportion to her symptoms. Illness anxiety disorder was considered because Ms. L met many of its criteria: preoccupation with having a serious illness, disproportionate preoccupation with somatic symptoms if they are present, extreme anxiety over health, and performance of health-related behaviors.1 However, illness anxiety disorder is a diagnosis of exclusion, and 1 criterion is that these symptoms cannot be explained by another mental disorder. We felt other diagnoses better fit Ms. L’s condition and ruled out illness anxiety disorder.
Ms. L’s long history of food and non-food–related obsessions and compulsions that interrupted her ability to perform daily activities were strongly suggestive for OCD. Additionally, her intense preoccupation, high level of anxiety, amount of time and energy spent seeking care for her esophagus and GERD symptoms, and the resulting significant disruption of daily life, met criteria for somatic symptom disorder (SSD). However, we did not believe that a diagnosis of OCD and SSD alone explained the entirety of Ms. L’s clinical picture. Despite ruling out anorexia nervosa, Ms. L nonetheless demonstrated disordered eating.
Avoidant/restrictive food intake disorder (ARFID) is an eating disorder in which patients restrict their diet and do not meet nutritional needs for any number of reasons, do not experience body dysmorphia, and do not fear weight gain.1 A common feature of ARFID is a fear of negative consequences from eating specific types of food.2 Table 21,2 summarizes additional clinical features of ARFID. Although ARFID is typically diagnosed in children and adolescents, particularly in individuals with autism with heightened sensory sensitivities, ARFID is also common among adult patients with GI disorders.3 In a retrospective chart review of 410 adults ages 18 to 90 (73% women) referred to a neurogastroenterology care center, 6.3% met the full criteria for ARFID and 17.3% had clinically significant avoidant or restrictive eating behaviors. Among patients with ARFID symptoms, 93% stated that a fear of GI symptoms was the driver of their avoidant or restrictive eating behaviors.2 Patients with GI diseases often develop dietary control and avoidance coping mechanisms to alleviate their symptoms.4 These strategies can exacerbate health anxieties and have a detrimental effect on mental health.5 Patients with GI disorders have a high degree of comorbidity with affective disorders, including anxiety disorders.6 These trends may arise from hypervigilance and the need to gain control over physical symptoms.7 Feeling a need for control, actions driven by anxiety and fear, and the need for compensatory behaviors are cardinal features of OCD and eating disorders.8 Multiple studies have demonstrated comorbidities between irritable bowel syndrome and eating disorders,9 SSD,10 and OCD.11 Taken together with observations that ARFID is also found in patients with GI disorders,2 these findings demonstrate that patients with a history of GI disease are at high risk of developing extreme health anxieties and behavioral coping strategies that can lead to disordered eating.

The rise in “healthy” eating materials online—particularly on social media—has created an atmosphere in which misinformation regarding diet and health is common and widespread. For patients with OCD and a predisposition to health anxiety, such as Ms. L, searching online for nutrition information and healthy living habits can exacerbate food-related anxieties and can lead to a pathological drive for purity and health.12Although not included in DSM-5, orthorexia nervosa was identified in 1997 as a proposed eating disorder best characterized as an obsession with healthy eating with associated restrictive behaviors.13 Patients with this disorder are rarely focused on losing weight, and orthorexic eating behaviors have been associated with both SSD and OCD.12,14 As in Ms. L’s case, patients with orthorexia nervosa demonstrate intrusive obsessions with nutrition, spend excessive amount of time researching nutrition, and fixate on food quality.12 Throughout Ms. L’s hospitalization, even as her food-related magical thinking symptoms improved, she constantly informed her care team that she had been “eating healthily” even though she was severely cachectic. Patients with SSD and OCD prone to health anxieties are at risk of developing pathologic food beliefs and dangerous eating behaviors. These patients may benefit from psychoeducation regarding nutrition and media literacy, which are components of effective eating disorder programs.15
[polldaddy:11079399]
Continue to: The authors' observations...
The authors’ observations
How do we approach the pharmacologic treatment of patients with co-occurring eating, somatic symptom, and anxiety disorders? Olanzapine facilitates recovery in children and adolescents with ARFID by promoting eating and weight gain, and decreasing symptoms of depression and anxiety.16 Patients with orthorexia nervosa also may benefit from treatment with olanzapine, which has decreased food-related fixations, magical thinking, and delusions regarding food.17 Further, orthorexic patients with ARFID have also been shown to respond to SSRIs due to those agents’ efficacy for treating intrusive thoughts, obsessions, and preoccupations from OCD and SSD.18,19 Thus, treating Ms. L’s symptoms with olanzapine and fluoxetine targeted the intersection of several diagnoses on our differential. Olanzapine’s propensity to cause weight gain is favorable in this population, particularly patients such as Ms. L, who do not exhibit body dysmorphia or fear of gaining weight.
OUTCOME Weight gain and fewer fears
Ms. L is prescribed olanzapine 5 mg/d and fluoxetine 20 mg/d. She gains 20.6 pounds in 4 weeks. Importantly, she endorses fewer fears related to foods and expands her palate to include foods she previously considered to be unhealthy, including white bread and farm-raised salmon. Further, she spends less time thinking about food and says she has less anxiety regarding the recurrence of GI symptoms.
CASE Fixated on health and nutrition
At the insistence of her daughter, Ms. L, age 75, presents to the emergency department (ED) for self-neglect and severe weight loss, with a body mass index (BMI) of 13.5 kg/m2 (normal: 18.5 to 24.9 kg/m2). When asked why she is in the ED, Ms. L says she doesn’t know. She attributes her significant weight loss (approximately 20 pounds in the last few months) to gastroesophageal reflux disease (GERD). She constantly worries about her esophagus. She had been diagnosed with esophageal dysphagia 7 years ago after undergoing radiofrequency ablation for esophageal cancer. Ms. L fixates on the negative effects certain foods and ingredients might have on her stomach and esophagus.
Following transfer from the ED, Ms. L is involuntarily admitted to our inpatient unit. Although she acknowledges weight loss, she minimizes the severity of her illness and indicates she would like to gain weight, but only by eating healthy foods she is comfortable with, including kale, quinoa, and vegetables. Ms. L says that she has always been interested in “healthful foods” and that she “loves sugar,” but “it’s bad for you,” mentioning that “sugar fuels cancer.” She has daily thoughts about sugar causing cancer. Ms. L also mentions that she stopped eating flour, sugar, fried food, and oils because those foods affect her “stomach acid” and cause “pimples on my face and weight loss.” While in the inpatient unit, Ms. L requests a special diet and demands to know the origin and ingredients of the foods she is offered. She emphasizes that her esophageal cancer diagnosis and dysphagia exacerbate worries that certain foods cause cancer, and wants to continue her diet restrictions. Nonetheless, she says she wants to get healthy, and denies an intense fear of gaining weight or feeling fat.
HISTORY Multiple psychiatric diagnoses
Ms. L lives alone and enjoys spending time with her grandchildren, visiting museums, and listening to classical music. However, her family, social workers, and records from a previous psychiatric hospitalization reveal that Ms. L has a history of psychiatric illness and fears regarding certain types of foods for much of her adult life. Ms. L’s family also described a range of compulsive behaviors, including shoplifting, hoarding art, multiple plastic surgeries, and phases where Ms. L ate only frozen yogurt without sugar.
Ms. L’s daughter reported that Ms. L had seen a psychologist in the late 1990s for depression and had been diagnosed with obsessive-compulsive disorder (OCD) and attention deficit/hyperactivity disorder in the early 2000s. In 2006, during a depressive episode after her divorce, Ms. L had a suicide attempt with pills and alcohol, and was hospitalized. Records from that stay described a history of mood dysregulation with fears regarding food and nutrition. Ms. L was treated with aripiprazole 5 mg/d. A trial of trazodone 25 mg/d did not have any effect. When discharged, she was receiving lamotrigine 100 mg/d. However, her daughter believes she stopped taking all psychiatric medications shortly after discharge.
Her daughter says that in the past 2 years, Ms. L has seen multiple doctors for treatment of somatic gastrointestinal (GI) complaints. A 2018 note from a social worker indicated that Ms. L endorsed taking >80 supplements per day and constantly researched nutrition online. In the months leading to her current hospitalization, Ms. L suffered from severe self-neglect and fear regarding foods she felt were not healthy for her. She had stopped leaving her apartment.
Continue to: EVALUATION Poor insight, normal lab results...
EVALUATION Poor insight, normal lab results
During her evaluation, Ms. L appears cachectic and frail. She has a heavily constricted affect and is guarded, dismissive, and vague. Although her thought processes are linear and goal-directed, her insight into her condition is extremely poor and she appears surprised when clinicians inform her that her self-neglect would lead to death. Instead, Ms. L insists she is eating healthily and demonstrates severe anxiety in relation to her GI symptoms.
Ms. L is oriented to person, place, and time. She scores 27/30 on the Montreal Cognitive Assessment, indicating normal cognition. She denies any depressive symptoms or suicidal intent. She does not appear to be internally preoccupied and denies having auditory or visual hallucinations or manic symptoms.
A neurologic examination reveals that her cranial nerves are normal, and cerebellar function, strength, and sensory testing are intact. Her gait is steady and she walks without a walker. Despite her severely low BMI and recent history of self-neglect, Ms. L’s laboratory results are remarkably normal and show no liver, metabolic, or electrolyte abnormalities, no signs of infection, and normal vitamin B12 levels. She has slightly elevated creatinine and blood urea nitrogen levels, but a normal glomerular filtration rate.
Her medical history is significant for squamous cell esophageal cancer, treated with radiofrequency ablation. Although Ms. L is constantly worried about the recurrence of cancer, pathology reports demonstrate no esophageal dysplasia. However, she does show evidence of an approximately 1 cm × 1 cm mild, noncircumferential esophageal stenosis, likely resulting from radiofrequency ablation.
[polldaddy:11079394]
The authors’ observations
Several health- and physical symptom-related psychiatric disorders have overlapping features, which can complicate the differential diagnosis (Table 11). Ms. L presented to the ED with a severely low BMI of 13.5 kg/m2, obsessions regarding specific types of food, and preoccupations regarding her esophagus. Despite her extensive psychiatric history (including intense fears regarding food), we ruled out a primary psychotic disorder because she did not describe auditory or visual hallucinations and never appeared internally preoccupied. While her BMI and persistent minimization of the extent of her disease meet criteria for anorexia nervosa, she denied body dysmorphia and did not have any fear of gaining weight.

A central element of Ms. L’s presentation was her anxiety regarding how certain types of foods impact her health as well as her anxieties regarding her esophagus. While Ms. L was in remission from esophageal cancer and had a diagnosis of esophageal dysphagia, these preoccupations and obsessions regarding how certain types of foods affect her esophagus drove her to self-neglect and thus represent pathologic thought processes out of proportion to her symptoms. Illness anxiety disorder was considered because Ms. L met many of its criteria: preoccupation with having a serious illness, disproportionate preoccupation with somatic symptoms if they are present, extreme anxiety over health, and performance of health-related behaviors.1 However, illness anxiety disorder is a diagnosis of exclusion, and 1 criterion is that these symptoms cannot be explained by another mental disorder. We felt other diagnoses better fit Ms. L’s condition and ruled out illness anxiety disorder.
Ms. L’s long history of food and non-food–related obsessions and compulsions that interrupted her ability to perform daily activities were strongly suggestive for OCD. Additionally, her intense preoccupation, high level of anxiety, amount of time and energy spent seeking care for her esophagus and GERD symptoms, and the resulting significant disruption of daily life, met criteria for somatic symptom disorder (SSD). However, we did not believe that a diagnosis of OCD and SSD alone explained the entirety of Ms. L’s clinical picture. Despite ruling out anorexia nervosa, Ms. L nonetheless demonstrated disordered eating.
Avoidant/restrictive food intake disorder (ARFID) is an eating disorder in which patients restrict their diet and do not meet nutritional needs for any number of reasons, do not experience body dysmorphia, and do not fear weight gain.1 A common feature of ARFID is a fear of negative consequences from eating specific types of food.2 Table 21,2 summarizes additional clinical features of ARFID. Although ARFID is typically diagnosed in children and adolescents, particularly in individuals with autism with heightened sensory sensitivities, ARFID is also common among adult patients with GI disorders.3 In a retrospective chart review of 410 adults ages 18 to 90 (73% women) referred to a neurogastroenterology care center, 6.3% met the full criteria for ARFID and 17.3% had clinically significant avoidant or restrictive eating behaviors. Among patients with ARFID symptoms, 93% stated that a fear of GI symptoms was the driver of their avoidant or restrictive eating behaviors.2 Patients with GI diseases often develop dietary control and avoidance coping mechanisms to alleviate their symptoms.4 These strategies can exacerbate health anxieties and have a detrimental effect on mental health.5 Patients with GI disorders have a high degree of comorbidity with affective disorders, including anxiety disorders.6 These trends may arise from hypervigilance and the need to gain control over physical symptoms.7 Feeling a need for control, actions driven by anxiety and fear, and the need for compensatory behaviors are cardinal features of OCD and eating disorders.8 Multiple studies have demonstrated comorbidities between irritable bowel syndrome and eating disorders,9 SSD,10 and OCD.11 Taken together with observations that ARFID is also found in patients with GI disorders,2 these findings demonstrate that patients with a history of GI disease are at high risk of developing extreme health anxieties and behavioral coping strategies that can lead to disordered eating.

The rise in “healthy” eating materials online—particularly on social media—has created an atmosphere in which misinformation regarding diet and health is common and widespread. For patients with OCD and a predisposition to health anxiety, such as Ms. L, searching online for nutrition information and healthy living habits can exacerbate food-related anxieties and can lead to a pathological drive for purity and health.12Although not included in DSM-5, orthorexia nervosa was identified in 1997 as a proposed eating disorder best characterized as an obsession with healthy eating with associated restrictive behaviors.13 Patients with this disorder are rarely focused on losing weight, and orthorexic eating behaviors have been associated with both SSD and OCD.12,14 As in Ms. L’s case, patients with orthorexia nervosa demonstrate intrusive obsessions with nutrition, spend excessive amount of time researching nutrition, and fixate on food quality.12 Throughout Ms. L’s hospitalization, even as her food-related magical thinking symptoms improved, she constantly informed her care team that she had been “eating healthily” even though she was severely cachectic. Patients with SSD and OCD prone to health anxieties are at risk of developing pathologic food beliefs and dangerous eating behaviors. These patients may benefit from psychoeducation regarding nutrition and media literacy, which are components of effective eating disorder programs.15
[polldaddy:11079399]
Continue to: The authors' observations...
The authors’ observations
How do we approach the pharmacologic treatment of patients with co-occurring eating, somatic symptom, and anxiety disorders? Olanzapine facilitates recovery in children and adolescents with ARFID by promoting eating and weight gain, and decreasing symptoms of depression and anxiety.16 Patients with orthorexia nervosa also may benefit from treatment with olanzapine, which has decreased food-related fixations, magical thinking, and delusions regarding food.17 Further, orthorexic patients with ARFID have also been shown to respond to SSRIs due to those agents’ efficacy for treating intrusive thoughts, obsessions, and preoccupations from OCD and SSD.18,19 Thus, treating Ms. L’s symptoms with olanzapine and fluoxetine targeted the intersection of several diagnoses on our differential. Olanzapine’s propensity to cause weight gain is favorable in this population, particularly patients such as Ms. L, who do not exhibit body dysmorphia or fear of gaining weight.
OUTCOME Weight gain and fewer fears
Ms. L is prescribed olanzapine 5 mg/d and fluoxetine 20 mg/d. She gains 20.6 pounds in 4 weeks. Importantly, she endorses fewer fears related to foods and expands her palate to include foods she previously considered to be unhealthy, including white bread and farm-raised salmon. Further, she spends less time thinking about food and says she has less anxiety regarding the recurrence of GI symptoms.
1. Diagnostic and Statistical Manual of Mental Disorders: DSM-5. 5th ed. American Psychiatric Association; 2013.
2. Murray HB, Bailey AP, Keshishian AC. Prevalence and characteristics of avoidant/restrictive food intake disorder in adult neurogastroenterology patients. Clin Gastroenterol Hepatol. 2020;18(9):1995-2002.e1.
3. Görmez A, Kılıç A, Kırpınar İ. Avoidant/restrictive food intake disorder: an adult case responding to cognitive behavioral therapy. Clinical Case Studies. 2018;17(6):443-452.
4. Reed-Knight B, Squires M, Chitkara DK, et al. Adolescents with irritable bowel syndrome report increased eating-associated symptoms, changes in dietary composition, and altered eating behaviors: a pilot comparison study to healthy adolescents. Neurogastroenterol Motil. 2016;28(12):1915-1920.
5. Melchior C, Desprez C, Riachi G, et al. Anxiety and depression profile is associated with eating disorders in patients with irritable bowel syndrome. Front Psychiatry. 2020;10:928.
6. Mayer EA, Craske M, Naliboff BD. Depression, anxiety, and the gastrointestinal system. J Clin Psychiatry. 2001;62 Suppl 8:28-37.
7. Abraham S, Kellow J. Exploring eating disorder quality of life and functional gastrointestinal disorders among eating disorder patients. J Psychosom Res. 2011;70(4):372-377.
8. Swinbourne JM, Touyz SW. The co-morbidity of eating disorders and anxiety disorders: a review. Eur Eat Disord Rev. 2007;15(4):253-274.
9. Perkins SJ, Keville S, Schmidt U, et al. Eating disorders and irritable bowel syndrome: is there a link? J Psychosom Res. 2005;59(2):57-64.
10. Hausteiner-Wiehle C, Henningsen P. Irritable bowel syndrome: relations with functional, mental, and somatoform disorders. World J Gastroenterol. 2014;20(2):6024-6030.
11. Masand PS, Keuthen NJ, Gupta S, et al. Prevalence of irritable bowel syndrome in obsessive-compulsive disorder. CNS Spectr. 2006;11(1):21-25.
12. Koven NS, Abry AW. The clinical basis of orthorexia nervosa: emerging perspectives. Neuropsychiatr Dis Treat. 2015;11:385-394.
13. Bratman S. Health food junkie. Yoga Journal. 1997;136:42-50.
14. Barthels F, Müller R, Schüth T, et al. Orthorexic eating behavior in patients with somatoform disorders. Eat Weight Disord. 2021;26(1):135-143.
15. Ciao AC, Loth K, Neumark-Sztainer D. Preventing eating disorder pathology: common and unique features of successful eating disorders prevention programs. Curr Psychiatry Rep. 2014;16(7):453.
16. Brewerton TD, D’Agostino M. Adjunctive use of olanzapine in the treatment of avoidant restrictive food intake disorder in children and adolescents in an eating disorders program. J Child Adolesc Psychopharmacol. 2017;27(10):920-922.
17. Moroze RM, Dunn TM, Craig Holland J, et al. Microthinking about micronutrients: a case of transition from obsessions about healthy eating to near-fatal “orthorexia nervosa” and proposed diagnostic criteria. Psychosomatics. 2015;56(4):397-403.
18. Spettigue W, Norris ML, Santos A, et al. Treatment of children and adolescents with avoidant/restrictive food intake disorder: a case series examining the feasibility of family therapy and adjunctive treatments. J Eat Disord. 2018;6:20.
19. Niedzielski A, Kaźmierczak-Wojtaś N. Prevalence of Orthorexia Nervosa and Its Diagnostic Tools-A Literature Review. Int J Environ Res Public Health. 2021;18(10):5488. Published 2021 May 20. doi:10.3390/ijerph18105488 Prevalence of orthorexia nervosa and its diagnostic tools-a literature review. Int J Environ Res Public Health. 2021;18(10):5488.
1. Diagnostic and Statistical Manual of Mental Disorders: DSM-5. 5th ed. American Psychiatric Association; 2013.
2. Murray HB, Bailey AP, Keshishian AC. Prevalence and characteristics of avoidant/restrictive food intake disorder in adult neurogastroenterology patients. Clin Gastroenterol Hepatol. 2020;18(9):1995-2002.e1.
3. Görmez A, Kılıç A, Kırpınar İ. Avoidant/restrictive food intake disorder: an adult case responding to cognitive behavioral therapy. Clinical Case Studies. 2018;17(6):443-452.
4. Reed-Knight B, Squires M, Chitkara DK, et al. Adolescents with irritable bowel syndrome report increased eating-associated symptoms, changes in dietary composition, and altered eating behaviors: a pilot comparison study to healthy adolescents. Neurogastroenterol Motil. 2016;28(12):1915-1920.
5. Melchior C, Desprez C, Riachi G, et al. Anxiety and depression profile is associated with eating disorders in patients with irritable bowel syndrome. Front Psychiatry. 2020;10:928.
6. Mayer EA, Craske M, Naliboff BD. Depression, anxiety, and the gastrointestinal system. J Clin Psychiatry. 2001;62 Suppl 8:28-37.
7. Abraham S, Kellow J. Exploring eating disorder quality of life and functional gastrointestinal disorders among eating disorder patients. J Psychosom Res. 2011;70(4):372-377.
8. Swinbourne JM, Touyz SW. The co-morbidity of eating disorders and anxiety disorders: a review. Eur Eat Disord Rev. 2007;15(4):253-274.
9. Perkins SJ, Keville S, Schmidt U, et al. Eating disorders and irritable bowel syndrome: is there a link? J Psychosom Res. 2005;59(2):57-64.
10. Hausteiner-Wiehle C, Henningsen P. Irritable bowel syndrome: relations with functional, mental, and somatoform disorders. World J Gastroenterol. 2014;20(2):6024-6030.
11. Masand PS, Keuthen NJ, Gupta S, et al. Prevalence of irritable bowel syndrome in obsessive-compulsive disorder. CNS Spectr. 2006;11(1):21-25.
12. Koven NS, Abry AW. The clinical basis of orthorexia nervosa: emerging perspectives. Neuropsychiatr Dis Treat. 2015;11:385-394.
13. Bratman S. Health food junkie. Yoga Journal. 1997;136:42-50.
14. Barthels F, Müller R, Schüth T, et al. Orthorexic eating behavior in patients with somatoform disorders. Eat Weight Disord. 2021;26(1):135-143.
15. Ciao AC, Loth K, Neumark-Sztainer D. Preventing eating disorder pathology: common and unique features of successful eating disorders prevention programs. Curr Psychiatry Rep. 2014;16(7):453.
16. Brewerton TD, D’Agostino M. Adjunctive use of olanzapine in the treatment of avoidant restrictive food intake disorder in children and adolescents in an eating disorders program. J Child Adolesc Psychopharmacol. 2017;27(10):920-922.
17. Moroze RM, Dunn TM, Craig Holland J, et al. Microthinking about micronutrients: a case of transition from obsessions about healthy eating to near-fatal “orthorexia nervosa” and proposed diagnostic criteria. Psychosomatics. 2015;56(4):397-403.
18. Spettigue W, Norris ML, Santos A, et al. Treatment of children and adolescents with avoidant/restrictive food intake disorder: a case series examining the feasibility of family therapy and adjunctive treatments. J Eat Disord. 2018;6:20.
19. Niedzielski A, Kaźmierczak-Wojtaś N. Prevalence of Orthorexia Nervosa and Its Diagnostic Tools-A Literature Review. Int J Environ Res Public Health. 2021;18(10):5488. Published 2021 May 20. doi:10.3390/ijerph18105488 Prevalence of orthorexia nervosa and its diagnostic tools-a literature review. Int J Environ Res Public Health. 2021;18(10):5488.
Depressed and awkward: Is it more than that?
CASE Treatment-resistant MDD
Ms. P, age 21, presents to the outpatient clinic. She has diagnoses of treatment-resistant major depressive disorder (MDD) and schizoid personality disorder (SPD). Ms. P was diagnosed with MDD 3 years ago after reporting symptoms of prevailing sadness for approximately 8 years, described as feelings of worthlessness, anhedonia, social withdrawal, and decreased hygiene and self-care behaviors, as well as suicidal ideation and self-harm. SPD was diagnosed 1 year earlier based on her “odd” behaviors and disheveled appearance following observation and in collateral with her family. Her odd behaviors are described as spending most of her time alone, preferring solitary activities, and having little contact with people other than her parents.
Ms. P reports that she was previously treated with citalopram, 20 mg/d, bupropion, 150 mg/d, aripiprazole, 3.75 mg/d, topiramate, 100 mg twice daily, and melatonin, 9 mg/d at bedtime, but discontinued follow-up appointments and medications after no significant improvement in symptoms.
[polldaddy:11027942]
The authors’ observations
The term “schizoid” first made its debut in the medical community to describe the prodromal social withdrawal and isolation observed in schizophrenia.1 The use of schizoid to describe a personality type first occurred in DSM-III in 1980.2 SPD is a Cluster A personality disorder that groups personalities characterized by common traits that are “odd” or “eccentric” and may resemble the positive and/or negative symptoms of schizophrenia.3,4 Relatively uncommon in clinical settings, SPD includes individuals who do not desire or enjoy close relationships. Those afflicted with SPD will be described as isolated, aloof, and detached from social relationships with others, even immediate family members. Individuals with SPD may appear indifferent to criticism and praise, and may take pleasure in only a few activities. They may exhibit a general absence of affective range, which contributes to their characterization as flat, blunted, or emotionally vacant. SPD is more commonly diagnosed in males and may be present in childhood and adolescence. These children are typified by solitariness, poor peer relationships, and underachievement in school. SPD impacts 3.1% to 4.9% of the United States population and approximately 1% of community populations.5,6
EVALUATION Persistent depressive symptoms
Ms. P is accompanied by her parents for the examination. She reports a chronic, persistent sad mood, hopelessness, anergia, insomnia, anhedonia, and decreased concentration and appetite. She says she experiences episodes of intense worry, along with tension, restlessness, feelings of being on the edge, irritability, and difficulty relaxing. Socially, she is withdrawn, preferring to stay alone in her room most of the day watching YouTube or trying to write stories. She has 2 friends with whom she does not interact with in person, but rather through digital means. Ms. P has never enjoyed attending school and feels “nervous” when she is around people. She has difficulty expressing her thoughts and often looks to her parents for help. Her parents add that getting Ms. P to attend school was a struggle, which resulted in periods of home schooling throughout high school.
The treating team prescribes citalopram, 10 mg/d, and aripiprazole, 2 mg/d. On subsequent follow-up visits, Ms. P’s depression improves with an increase in citalopram to 40 mg/d. Psychotherapy is added to her treatment plan to help address the persistent social deficits, odd behavior, and anxieties.
Continue to: Evaluation Psychological assessment...
EVALUATION Psychological assessment
At her psychotherapy intake appointment with the clinical neuropsychologist, Ms. P is dressed in purple from head to toe and sits clutching her purse and looking at the ground. She is overweight with clean, fitting clothing. Ms. P takes a secondary role during most of the interview, allowing her parents to answer most questions. When asked why she is starting therapy, Ms. P replies, “Well, I’ve been using the bathroom a lot.” She describes a feeling of comfort and calmness while in the restroom. Suddenly, she asks her parents to exit the exam room for a moment. Once they leave, she leans in and whispers, “Have you ever heard of self-sabotage? I think that’s what I’m doing.”
Her mood is euthymic, with a blunted affect. She scores 2 on the Patient Health Questionnaire-9 (PHQ-9) and 10 on the Generalized Anxiety Disorder 7-item scale (GAD-7), which indicates the positive impact of medication on her depressive symptoms but continuing moderate anxious distress. She endorses fear of the night, insomnia, and suicidal ideation. She reports an unusual “constant itching sensation,” resulting in hours of repetitive excoriation. Physical examination reveals several significant scars and scabs covering her bilateral upper and lower extremities. Her vocational history is brief; she had held 2 entry-level customer service positions that lasted <1 year. She was fired due to excessive bathroom use.
As the interview progresses, the intake clinician’s background in neuropsychological assessment facilitates screening for possible developmental disorders. Given the nature of the referral and psychotherapy intake, a full neuropsychological assessment is not conducted. The clinician emphasizes verbal abstraction and theory of mind. Ms. P’s IQ was estimated to be average by Wide Range Achievement Test 4 word reading and interview questions about her academic history. Questions are abstracted from the Autism Diagnostic Observation Schedule, Module 4, to assess for conversation ability, emotional insight, awareness and expression, relationships, and areas of functioning in daily living. Developmental history questions, such as those found on the Adaptive Behavior Assessment System, 3rd edition, help guide developmental information provided by parents in the areas of communication, emotion and eye-gaze, gestures, sensory function, language, social functioning, hygiene behavior, and specific interests.
Ms. P’s mother describes a normal pregnancy and delivery; however, she states that Ms. P was “born with problems,” including difficulty with rooting and sucking, and required gastrointestinal intubation until age 3. Cyclical vomiting followed normal food consumption. Ambulation, language acquisition, toilet training, and hygiene behavior were delayed. Ms. P experienced improvements with early intervention in intensive physical and occupational therapy.
Ms. P’s hygiene is well below average, and she requires cueing from her parents. She attended general education until she reached high school, when she began special education. She was sensitive to sensory stimulation from infancy, with sensory sensitivity to textures. Ms. P continues to report sensory sensitivity and lapses in hygiene.
She has difficulty establishing and maintaining relationships with her peers, and prefers solitary activities. Ms. P has no history of romantic relationships, although she does desire one. When asked about her understanding of various relationships, Ms. P’s responses are stereotyped, such as “I know someone is my friend because they are nice to me” and “People get married because they love each other.” She struggles to offer greater insight into the nuances that form lasting relationships and bonds. Ms. P struggles to imitate and describe the physical and internal cues of several basic emotions (eg, fear, joy, anger).
Her conversational and social skills are assessed by asking her to engage in a conversation with the examiner as if meeting for the first time. Her speech is reciprocal, aprosodic, and delayed. The conversation is one-sided, and the examiner fills in several awkward pauses. Ms. P’s gaze at times is intense and prolonged, especially when responding to questions. She tends to use descriptive statements (eg, “I like your purple pen, I like your shirt”) to engage in conversation, rather than gathering more information through reflective statements, questions, or expressing a shared interest.
Ms. P’s verbal abstraction is screened using questions from the Wechsler Adult Intelligence Scale, 4th edition Similarities subtest, to which she provides several responses within normal limits. Her understanding of colloquial speech is assessed by asking her the meaning of common phrases (eg, “Get knocked down 9 times, get up 10,” “Jack and Jill are 2 peas in a pod”). On many occasions, she is able to limit her response to 1 word, (eg, “resiliency”), demonstrating intact ability to decipher idioms.
[polldaddy:11027971]
The authors’ observations
Upon reflection of Ms. P’s clinical presentation and history of developmental delays, social deficits, sensory sensitivity since infancy, and repetitive behaviors (all which continue to impact her), the clinical team concluded that the diagnosis of autism spectrum disorder (ASD) helps explain the patient’s “odd” behaviors, more so than SPD.
ASD is a heterogenous, complex neuropsychiatric disorder characterized by a persistent deficit in social reciprocity, verbal, and nonverbal communication, and includes a pattern of restricted, repetitive and/or stereotyped behaviors and/or interests.5 The term “autismus” is Greek meaning “self,” and was first used to classify the qualities of “morbid self-admiration” observed in prodromal schizophrenia.7

To properly distinguish these disorders, keep in mind that patients with ASD have repetitive and restricted patterns of behaviors or interests that are not found in SPD, and experience deficits in forming, maintaining, and understanding relationships since they lack those skills, while patients with SPD are more prone to desire solitary activities and limited relationships.5,9
There has been an increased interest in determining why for some patients the diagnosis of ASD is delayed until they reach adulthood. Limited or no access to the patient’s childhood caregiver to obtain a developmental history, as well as generational differences on what constitutes typical childhood behavior, could contribute to a delayed diagnosis of ASD until adulthood. Some patients develop camouflaging strategies that allow them to navigate social expectations to a limited degree, such as learning stock phrases, imitating gestures, and telling anecdotes. Another factor to consider is that co-occurring psychiatric disorders may take center stage when patients present for mental health services.10 Fusar-Poli et al11 investigated the characteristics of patients who received a diagnosis of ASD in adulthood. They found that the median time from the initial clinical evaluation to diagnosis of ASD in adulthood was 11 years. In adults identified with ASD, their cognitive abilities ranged from average to above average, and they required less support. Additionally, they also had higher rates of being previously diagnosed with psychotic disorders and personality disorders.11
It is important to keep in mind that the wide spectrum of autism as currently defined by DSM-5 and its overlap of symptoms with other psychiatric disorders can make the diagnosis challenging for both child and adolescent psychiatrists and adult psychiatrists and might help explain why severe cases of ASD are more readily identified earlier than milder cases of ASD.10
Ms. P’s case is also an example of how women are more likely than men to be overlooked when evaluated for ASD. According to DSM-5, the estimated gender ratio for ASD is believed to be 4:1 (male:female).5 However, upon systematic review and meta-analysis, Loomes et al12 found that the gender ratio may be closer to 3:1 (male:female). These authors suggested that diagnostic bias and a failure of passive case ascertainment to estimate gender ratios as stated by DSM-5 in identifying ASD might explain the lower gender ratio.12 A growing body of evidence suggests that ASD is different in males and females. A 2019 qualitative study by Milner et al13 found that female participants reported using masking and camouflaging strategies to appear neurotypical. Compensatory behaviors were found to be linked to a delay in diagnosis and support for ASD.13
Cognitive ability as measured by IQ has also been found to be a factor in receiving a diagnosis of ASD. In a 2010 secondary analysis of a population-based study of the prevalence of ASD, Giarelli et al14found that girls with cognitive impairments as measured by IQ were less likely to be diagnosed with ASD than boys with cognitive impairment, despite meeting the criteria for ASD. Females tend to exhibit fewer repetitive behaviors than males, and tend to be more likely to show accompanying intellectual disability, which suggests that females with ASD may go unrecognized when they exhibit average intelligence with less impairment of behavior and subtler manifestation of social and communication deficits.15 Consequently, females tend to receive this diagnosis later than males.
Continue to: Treatment...
TREATMENT Adding CBT
At an interdisciplinary session several weeks later that includes Ms. P and her parents, the treatment team discusses the revised diagnoses of ASD and MDD, a treatment recommendation for cognitive-behavioral therapy (CBT), and continued use of medication. At this session, Ms. P discloses that she has not been consistent with her medication regimen since her last appointment, which helps explain the increase in her PHQ-9 score from 2 to 14 and GAD-7 score
[polldaddy:11027990]
The authors’ observations
CBT can be helpful in improving medication adherence, developing coping skills, and modifying maladaptive behaviors.

OUTCOME Improvement with psychotherapy
Ms. P and family agree with the team’s recommendations. The aims of Ms. P’s psychotherapy are to maintain medication compliance; implement behavioral modification, vocational rehabilitation, and community engagement; develop social skills; increase functional independence; and develop coping skills for depression and anxiety.
Bottom Line
The prevalence of schizoid personality disorder (SPD) is low, and its symptoms overlap with those of autism spectrum disorder. Therefore, before diagnosing SPD in an adult patient, it is important to obtain a detailed developmental history and include an interdisciplinary team to assess for autism spectrum disorder.
1. Fariba K, Gupta V. Schizoid personality disorder. StatPearls Publishing. Updated June 9, 2021. Accessed January 6, 2021. https://www.ncbi.nlm.nih.gov/books/NBK559234/
2. Diagnostic and Statistical Manual of Mental Disorders: DSM-III. 3rd ed rev. American Psychiatric Association; 1987.
3. Esterberg ML, Goulding SM, Walker EF. Cluster A personality disorders: schizotypal, schizoid and paranoid personality disorders in childhood and adolescence. J Psychopathol Behav Assess. 2010;32(4):515-528. doi:10.1007/s10862-010-9183-8
4. Kalus O, Bernstein DP, Siever LJ. Schizoid personality disorder: a review of current status and implications for DSM-IV. Journal of Personality Disorders. 1993;7(1), 43-52.
5. Diagnostic and Statistical Manual of Mental Disorders: DSM-5. 5th ed. American Psychiatric Association; 2013.
6. Eaton NR, Greene AL. Personality disorders: community prevalence and socio-demographic correlates. Curr Opin Psychol. 2018;21:28-32. doi:10.1016/j.copsyc.2017.09.001
7. Vatano
8. Ritsner MS. Handbook of Schizophrenia Spectrum Disorders, Volume I: Conceptual Issues and Neurobiological Advances. Springer; 2011.
9. Cook ML, Zhang Y, Constantino JN. On the continuity between autistic and schizoid personality disorder trait burden: a prospective study in adolescence. J Nerv Ment Dis. 2020;208(2):94-100. doi:10.1097/NMD.0000000000001105
10. Lai MC, Baron-Cohen S. Identifying the lost generation of adults with autism spectrum conditions. Lancet Psychiatry. 2015;2(11):1013-1027. doi:10.1016/S2215-0366(15)00277-1
11. Fusar-Poli L, Brondino N, Politi P, et al. Missed diagnoses and misdiagnoses of adults with autism spectrum disorder. Eur Arch Psychiatry Clin Neurosci. 2020;10.1007/s00406-020-01189-2. doi:10.1007/s00406-020-01189-w
12. Loomes R, Hull L, Mandy WPL. What is the male-to-female ratio in autism spectrum disorder? A systematic review and meta-analysis. J Am Acad Child Adolesc Psychiatry. 2017;56(6):466-474. doi:10.1016/j.jaac.2017.03.013
13. Milner V, McIntosh H, Colvert E, et al. A qualitative exploration of the female experience of autism spectrum disorder (ASD). J Autism Dev Disord. 2019;49(6):2389-2402. doi:10.1007/s10803-019-03906-4
14. Giarelli E, Wiggins LD, Rice CE, et al. Sex differences in the evaluation and diagnosis of autism spectrum disorders among children. Disabil Health J. 2010;3(2):107-116. doi:10.1016/j.dhjo.2009.07.001
15. Frazier TW, Georgiades S, Bishop SL, et al. Behavioral and cognitive characteristics of females and males with autism in the Simons Simplex Collection. J Am Acad Child Adolesc Psychiatry. 2014;53(3):329-40.e403. doi:10.1016/j.jaac.2013.12.004
16. Julius RJ, Novitsky MA Jr, et al. Medication adherence: a review of the literature and implications for clinical practice. J Psychiatr Pract. 2009;15(1):34-44. doi:10.1097/01.pra.0000344917.43780.77
17. Spain D, Sin J, Chalder T, et al. Cognitive behaviour therapy for adults with autism spectrum disorders and psychiatric co-morbidity: a review. Research in Autism Spectrum Disorders. 2015;9, 151-162. https://doi.org/10.1016/j.rasd.2014.10.019
18. Bishop-Fitzpatrick L, Minshew NJ, Eack SM. A systematic review of psychosocial interventions for adults with autism spectrum disorders. J Autism Dev Disord. 2013;43(3):687-694. doi:10.1007/s10803-012-1615-8
CASE Treatment-resistant MDD
Ms. P, age 21, presents to the outpatient clinic. She has diagnoses of treatment-resistant major depressive disorder (MDD) and schizoid personality disorder (SPD). Ms. P was diagnosed with MDD 3 years ago after reporting symptoms of prevailing sadness for approximately 8 years, described as feelings of worthlessness, anhedonia, social withdrawal, and decreased hygiene and self-care behaviors, as well as suicidal ideation and self-harm. SPD was diagnosed 1 year earlier based on her “odd” behaviors and disheveled appearance following observation and in collateral with her family. Her odd behaviors are described as spending most of her time alone, preferring solitary activities, and having little contact with people other than her parents.
Ms. P reports that she was previously treated with citalopram, 20 mg/d, bupropion, 150 mg/d, aripiprazole, 3.75 mg/d, topiramate, 100 mg twice daily, and melatonin, 9 mg/d at bedtime, but discontinued follow-up appointments and medications after no significant improvement in symptoms.
[polldaddy:11027942]
The authors’ observations
The term “schizoid” first made its debut in the medical community to describe the prodromal social withdrawal and isolation observed in schizophrenia.1 The use of schizoid to describe a personality type first occurred in DSM-III in 1980.2 SPD is a Cluster A personality disorder that groups personalities characterized by common traits that are “odd” or “eccentric” and may resemble the positive and/or negative symptoms of schizophrenia.3,4 Relatively uncommon in clinical settings, SPD includes individuals who do not desire or enjoy close relationships. Those afflicted with SPD will be described as isolated, aloof, and detached from social relationships with others, even immediate family members. Individuals with SPD may appear indifferent to criticism and praise, and may take pleasure in only a few activities. They may exhibit a general absence of affective range, which contributes to their characterization as flat, blunted, or emotionally vacant. SPD is more commonly diagnosed in males and may be present in childhood and adolescence. These children are typified by solitariness, poor peer relationships, and underachievement in school. SPD impacts 3.1% to 4.9% of the United States population and approximately 1% of community populations.5,6
EVALUATION Persistent depressive symptoms
Ms. P is accompanied by her parents for the examination. She reports a chronic, persistent sad mood, hopelessness, anergia, insomnia, anhedonia, and decreased concentration and appetite. She says she experiences episodes of intense worry, along with tension, restlessness, feelings of being on the edge, irritability, and difficulty relaxing. Socially, she is withdrawn, preferring to stay alone in her room most of the day watching YouTube or trying to write stories. She has 2 friends with whom she does not interact with in person, but rather through digital means. Ms. P has never enjoyed attending school and feels “nervous” when she is around people. She has difficulty expressing her thoughts and often looks to her parents for help. Her parents add that getting Ms. P to attend school was a struggle, which resulted in periods of home schooling throughout high school.
The treating team prescribes citalopram, 10 mg/d, and aripiprazole, 2 mg/d. On subsequent follow-up visits, Ms. P’s depression improves with an increase in citalopram to 40 mg/d. Psychotherapy is added to her treatment plan to help address the persistent social deficits, odd behavior, and anxieties.
Continue to: Evaluation Psychological assessment...
EVALUATION Psychological assessment
At her psychotherapy intake appointment with the clinical neuropsychologist, Ms. P is dressed in purple from head to toe and sits clutching her purse and looking at the ground. She is overweight with clean, fitting clothing. Ms. P takes a secondary role during most of the interview, allowing her parents to answer most questions. When asked why she is starting therapy, Ms. P replies, “Well, I’ve been using the bathroom a lot.” She describes a feeling of comfort and calmness while in the restroom. Suddenly, she asks her parents to exit the exam room for a moment. Once they leave, she leans in and whispers, “Have you ever heard of self-sabotage? I think that’s what I’m doing.”
Her mood is euthymic, with a blunted affect. She scores 2 on the Patient Health Questionnaire-9 (PHQ-9) and 10 on the Generalized Anxiety Disorder 7-item scale (GAD-7), which indicates the positive impact of medication on her depressive symptoms but continuing moderate anxious distress. She endorses fear of the night, insomnia, and suicidal ideation. She reports an unusual “constant itching sensation,” resulting in hours of repetitive excoriation. Physical examination reveals several significant scars and scabs covering her bilateral upper and lower extremities. Her vocational history is brief; she had held 2 entry-level customer service positions that lasted <1 year. She was fired due to excessive bathroom use.
As the interview progresses, the intake clinician’s background in neuropsychological assessment facilitates screening for possible developmental disorders. Given the nature of the referral and psychotherapy intake, a full neuropsychological assessment is not conducted. The clinician emphasizes verbal abstraction and theory of mind. Ms. P’s IQ was estimated to be average by Wide Range Achievement Test 4 word reading and interview questions about her academic history. Questions are abstracted from the Autism Diagnostic Observation Schedule, Module 4, to assess for conversation ability, emotional insight, awareness and expression, relationships, and areas of functioning in daily living. Developmental history questions, such as those found on the Adaptive Behavior Assessment System, 3rd edition, help guide developmental information provided by parents in the areas of communication, emotion and eye-gaze, gestures, sensory function, language, social functioning, hygiene behavior, and specific interests.
Ms. P’s mother describes a normal pregnancy and delivery; however, she states that Ms. P was “born with problems,” including difficulty with rooting and sucking, and required gastrointestinal intubation until age 3. Cyclical vomiting followed normal food consumption. Ambulation, language acquisition, toilet training, and hygiene behavior were delayed. Ms. P experienced improvements with early intervention in intensive physical and occupational therapy.
Ms. P’s hygiene is well below average, and she requires cueing from her parents. She attended general education until she reached high school, when she began special education. She was sensitive to sensory stimulation from infancy, with sensory sensitivity to textures. Ms. P continues to report sensory sensitivity and lapses in hygiene.
She has difficulty establishing and maintaining relationships with her peers, and prefers solitary activities. Ms. P has no history of romantic relationships, although she does desire one. When asked about her understanding of various relationships, Ms. P’s responses are stereotyped, such as “I know someone is my friend because they are nice to me” and “People get married because they love each other.” She struggles to offer greater insight into the nuances that form lasting relationships and bonds. Ms. P struggles to imitate and describe the physical and internal cues of several basic emotions (eg, fear, joy, anger).
Her conversational and social skills are assessed by asking her to engage in a conversation with the examiner as if meeting for the first time. Her speech is reciprocal, aprosodic, and delayed. The conversation is one-sided, and the examiner fills in several awkward pauses. Ms. P’s gaze at times is intense and prolonged, especially when responding to questions. She tends to use descriptive statements (eg, “I like your purple pen, I like your shirt”) to engage in conversation, rather than gathering more information through reflective statements, questions, or expressing a shared interest.
Ms. P’s verbal abstraction is screened using questions from the Wechsler Adult Intelligence Scale, 4th edition Similarities subtest, to which she provides several responses within normal limits. Her understanding of colloquial speech is assessed by asking her the meaning of common phrases (eg, “Get knocked down 9 times, get up 10,” “Jack and Jill are 2 peas in a pod”). On many occasions, she is able to limit her response to 1 word, (eg, “resiliency”), demonstrating intact ability to decipher idioms.
[polldaddy:11027971]
The authors’ observations
Upon reflection of Ms. P’s clinical presentation and history of developmental delays, social deficits, sensory sensitivity since infancy, and repetitive behaviors (all which continue to impact her), the clinical team concluded that the diagnosis of autism spectrum disorder (ASD) helps explain the patient’s “odd” behaviors, more so than SPD.
ASD is a heterogenous, complex neuropsychiatric disorder characterized by a persistent deficit in social reciprocity, verbal, and nonverbal communication, and includes a pattern of restricted, repetitive and/or stereotyped behaviors and/or interests.5 The term “autismus” is Greek meaning “self,” and was first used to classify the qualities of “morbid self-admiration” observed in prodromal schizophrenia.7

To properly distinguish these disorders, keep in mind that patients with ASD have repetitive and restricted patterns of behaviors or interests that are not found in SPD, and experience deficits in forming, maintaining, and understanding relationships since they lack those skills, while patients with SPD are more prone to desire solitary activities and limited relationships.5,9
There has been an increased interest in determining why for some patients the diagnosis of ASD is delayed until they reach adulthood. Limited or no access to the patient’s childhood caregiver to obtain a developmental history, as well as generational differences on what constitutes typical childhood behavior, could contribute to a delayed diagnosis of ASD until adulthood. Some patients develop camouflaging strategies that allow them to navigate social expectations to a limited degree, such as learning stock phrases, imitating gestures, and telling anecdotes. Another factor to consider is that co-occurring psychiatric disorders may take center stage when patients present for mental health services.10 Fusar-Poli et al11 investigated the characteristics of patients who received a diagnosis of ASD in adulthood. They found that the median time from the initial clinical evaluation to diagnosis of ASD in adulthood was 11 years. In adults identified with ASD, their cognitive abilities ranged from average to above average, and they required less support. Additionally, they also had higher rates of being previously diagnosed with psychotic disorders and personality disorders.11
It is important to keep in mind that the wide spectrum of autism as currently defined by DSM-5 and its overlap of symptoms with other psychiatric disorders can make the diagnosis challenging for both child and adolescent psychiatrists and adult psychiatrists and might help explain why severe cases of ASD are more readily identified earlier than milder cases of ASD.10
Ms. P’s case is also an example of how women are more likely than men to be overlooked when evaluated for ASD. According to DSM-5, the estimated gender ratio for ASD is believed to be 4:1 (male:female).5 However, upon systematic review and meta-analysis, Loomes et al12 found that the gender ratio may be closer to 3:1 (male:female). These authors suggested that diagnostic bias and a failure of passive case ascertainment to estimate gender ratios as stated by DSM-5 in identifying ASD might explain the lower gender ratio.12 A growing body of evidence suggests that ASD is different in males and females. A 2019 qualitative study by Milner et al13 found that female participants reported using masking and camouflaging strategies to appear neurotypical. Compensatory behaviors were found to be linked to a delay in diagnosis and support for ASD.13
Cognitive ability as measured by IQ has also been found to be a factor in receiving a diagnosis of ASD. In a 2010 secondary analysis of a population-based study of the prevalence of ASD, Giarelli et al14found that girls with cognitive impairments as measured by IQ were less likely to be diagnosed with ASD than boys with cognitive impairment, despite meeting the criteria for ASD. Females tend to exhibit fewer repetitive behaviors than males, and tend to be more likely to show accompanying intellectual disability, which suggests that females with ASD may go unrecognized when they exhibit average intelligence with less impairment of behavior and subtler manifestation of social and communication deficits.15 Consequently, females tend to receive this diagnosis later than males.
Continue to: Treatment...
TREATMENT Adding CBT
At an interdisciplinary session several weeks later that includes Ms. P and her parents, the treatment team discusses the revised diagnoses of ASD and MDD, a treatment recommendation for cognitive-behavioral therapy (CBT), and continued use of medication. At this session, Ms. P discloses that she has not been consistent with her medication regimen since her last appointment, which helps explain the increase in her PHQ-9 score from 2 to 14 and GAD-7 score
[polldaddy:11027990]
The authors’ observations
CBT can be helpful in improving medication adherence, developing coping skills, and modifying maladaptive behaviors.

OUTCOME Improvement with psychotherapy
Ms. P and family agree with the team’s recommendations. The aims of Ms. P’s psychotherapy are to maintain medication compliance; implement behavioral modification, vocational rehabilitation, and community engagement; develop social skills; increase functional independence; and develop coping skills for depression and anxiety.
Bottom Line
The prevalence of schizoid personality disorder (SPD) is low, and its symptoms overlap with those of autism spectrum disorder. Therefore, before diagnosing SPD in an adult patient, it is important to obtain a detailed developmental history and include an interdisciplinary team to assess for autism spectrum disorder.
CASE Treatment-resistant MDD
Ms. P, age 21, presents to the outpatient clinic. She has diagnoses of treatment-resistant major depressive disorder (MDD) and schizoid personality disorder (SPD). Ms. P was diagnosed with MDD 3 years ago after reporting symptoms of prevailing sadness for approximately 8 years, described as feelings of worthlessness, anhedonia, social withdrawal, and decreased hygiene and self-care behaviors, as well as suicidal ideation and self-harm. SPD was diagnosed 1 year earlier based on her “odd” behaviors and disheveled appearance following observation and in collateral with her family. Her odd behaviors are described as spending most of her time alone, preferring solitary activities, and having little contact with people other than her parents.
Ms. P reports that she was previously treated with citalopram, 20 mg/d, bupropion, 150 mg/d, aripiprazole, 3.75 mg/d, topiramate, 100 mg twice daily, and melatonin, 9 mg/d at bedtime, but discontinued follow-up appointments and medications after no significant improvement in symptoms.
[polldaddy:11027942]
The authors’ observations
The term “schizoid” first made its debut in the medical community to describe the prodromal social withdrawal and isolation observed in schizophrenia.1 The use of schizoid to describe a personality type first occurred in DSM-III in 1980.2 SPD is a Cluster A personality disorder that groups personalities characterized by common traits that are “odd” or “eccentric” and may resemble the positive and/or negative symptoms of schizophrenia.3,4 Relatively uncommon in clinical settings, SPD includes individuals who do not desire or enjoy close relationships. Those afflicted with SPD will be described as isolated, aloof, and detached from social relationships with others, even immediate family members. Individuals with SPD may appear indifferent to criticism and praise, and may take pleasure in only a few activities. They may exhibit a general absence of affective range, which contributes to their characterization as flat, blunted, or emotionally vacant. SPD is more commonly diagnosed in males and may be present in childhood and adolescence. These children are typified by solitariness, poor peer relationships, and underachievement in school. SPD impacts 3.1% to 4.9% of the United States population and approximately 1% of community populations.5,6
EVALUATION Persistent depressive symptoms
Ms. P is accompanied by her parents for the examination. She reports a chronic, persistent sad mood, hopelessness, anergia, insomnia, anhedonia, and decreased concentration and appetite. She says she experiences episodes of intense worry, along with tension, restlessness, feelings of being on the edge, irritability, and difficulty relaxing. Socially, she is withdrawn, preferring to stay alone in her room most of the day watching YouTube or trying to write stories. She has 2 friends with whom she does not interact with in person, but rather through digital means. Ms. P has never enjoyed attending school and feels “nervous” when she is around people. She has difficulty expressing her thoughts and often looks to her parents for help. Her parents add that getting Ms. P to attend school was a struggle, which resulted in periods of home schooling throughout high school.
The treating team prescribes citalopram, 10 mg/d, and aripiprazole, 2 mg/d. On subsequent follow-up visits, Ms. P’s depression improves with an increase in citalopram to 40 mg/d. Psychotherapy is added to her treatment plan to help address the persistent social deficits, odd behavior, and anxieties.
Continue to: Evaluation Psychological assessment...
EVALUATION Psychological assessment
At her psychotherapy intake appointment with the clinical neuropsychologist, Ms. P is dressed in purple from head to toe and sits clutching her purse and looking at the ground. She is overweight with clean, fitting clothing. Ms. P takes a secondary role during most of the interview, allowing her parents to answer most questions. When asked why she is starting therapy, Ms. P replies, “Well, I’ve been using the bathroom a lot.” She describes a feeling of comfort and calmness while in the restroom. Suddenly, she asks her parents to exit the exam room for a moment. Once they leave, she leans in and whispers, “Have you ever heard of self-sabotage? I think that’s what I’m doing.”
Her mood is euthymic, with a blunted affect. She scores 2 on the Patient Health Questionnaire-9 (PHQ-9) and 10 on the Generalized Anxiety Disorder 7-item scale (GAD-7), which indicates the positive impact of medication on her depressive symptoms but continuing moderate anxious distress. She endorses fear of the night, insomnia, and suicidal ideation. She reports an unusual “constant itching sensation,” resulting in hours of repetitive excoriation. Physical examination reveals several significant scars and scabs covering her bilateral upper and lower extremities. Her vocational history is brief; she had held 2 entry-level customer service positions that lasted <1 year. She was fired due to excessive bathroom use.
As the interview progresses, the intake clinician’s background in neuropsychological assessment facilitates screening for possible developmental disorders. Given the nature of the referral and psychotherapy intake, a full neuropsychological assessment is not conducted. The clinician emphasizes verbal abstraction and theory of mind. Ms. P’s IQ was estimated to be average by Wide Range Achievement Test 4 word reading and interview questions about her academic history. Questions are abstracted from the Autism Diagnostic Observation Schedule, Module 4, to assess for conversation ability, emotional insight, awareness and expression, relationships, and areas of functioning in daily living. Developmental history questions, such as those found on the Adaptive Behavior Assessment System, 3rd edition, help guide developmental information provided by parents in the areas of communication, emotion and eye-gaze, gestures, sensory function, language, social functioning, hygiene behavior, and specific interests.
Ms. P’s mother describes a normal pregnancy and delivery; however, she states that Ms. P was “born with problems,” including difficulty with rooting and sucking, and required gastrointestinal intubation until age 3. Cyclical vomiting followed normal food consumption. Ambulation, language acquisition, toilet training, and hygiene behavior were delayed. Ms. P experienced improvements with early intervention in intensive physical and occupational therapy.
Ms. P’s hygiene is well below average, and she requires cueing from her parents. She attended general education until she reached high school, when she began special education. She was sensitive to sensory stimulation from infancy, with sensory sensitivity to textures. Ms. P continues to report sensory sensitivity and lapses in hygiene.
She has difficulty establishing and maintaining relationships with her peers, and prefers solitary activities. Ms. P has no history of romantic relationships, although she does desire one. When asked about her understanding of various relationships, Ms. P’s responses are stereotyped, such as “I know someone is my friend because they are nice to me” and “People get married because they love each other.” She struggles to offer greater insight into the nuances that form lasting relationships and bonds. Ms. P struggles to imitate and describe the physical and internal cues of several basic emotions (eg, fear, joy, anger).
Her conversational and social skills are assessed by asking her to engage in a conversation with the examiner as if meeting for the first time. Her speech is reciprocal, aprosodic, and delayed. The conversation is one-sided, and the examiner fills in several awkward pauses. Ms. P’s gaze at times is intense and prolonged, especially when responding to questions. She tends to use descriptive statements (eg, “I like your purple pen, I like your shirt”) to engage in conversation, rather than gathering more information through reflective statements, questions, or expressing a shared interest.
Ms. P’s verbal abstraction is screened using questions from the Wechsler Adult Intelligence Scale, 4th edition Similarities subtest, to which she provides several responses within normal limits. Her understanding of colloquial speech is assessed by asking her the meaning of common phrases (eg, “Get knocked down 9 times, get up 10,” “Jack and Jill are 2 peas in a pod”). On many occasions, she is able to limit her response to 1 word, (eg, “resiliency”), demonstrating intact ability to decipher idioms.
[polldaddy:11027971]
The authors’ observations
Upon reflection of Ms. P’s clinical presentation and history of developmental delays, social deficits, sensory sensitivity since infancy, and repetitive behaviors (all which continue to impact her), the clinical team concluded that the diagnosis of autism spectrum disorder (ASD) helps explain the patient’s “odd” behaviors, more so than SPD.
ASD is a heterogenous, complex neuropsychiatric disorder characterized by a persistent deficit in social reciprocity, verbal, and nonverbal communication, and includes a pattern of restricted, repetitive and/or stereotyped behaviors and/or interests.5 The term “autismus” is Greek meaning “self,” and was first used to classify the qualities of “morbid self-admiration” observed in prodromal schizophrenia.7

To properly distinguish these disorders, keep in mind that patients with ASD have repetitive and restricted patterns of behaviors or interests that are not found in SPD, and experience deficits in forming, maintaining, and understanding relationships since they lack those skills, while patients with SPD are more prone to desire solitary activities and limited relationships.5,9
There has been an increased interest in determining why for some patients the diagnosis of ASD is delayed until they reach adulthood. Limited or no access to the patient’s childhood caregiver to obtain a developmental history, as well as generational differences on what constitutes typical childhood behavior, could contribute to a delayed diagnosis of ASD until adulthood. Some patients develop camouflaging strategies that allow them to navigate social expectations to a limited degree, such as learning stock phrases, imitating gestures, and telling anecdotes. Another factor to consider is that co-occurring psychiatric disorders may take center stage when patients present for mental health services.10 Fusar-Poli et al11 investigated the characteristics of patients who received a diagnosis of ASD in adulthood. They found that the median time from the initial clinical evaluation to diagnosis of ASD in adulthood was 11 years. In adults identified with ASD, their cognitive abilities ranged from average to above average, and they required less support. Additionally, they also had higher rates of being previously diagnosed with psychotic disorders and personality disorders.11
It is important to keep in mind that the wide spectrum of autism as currently defined by DSM-5 and its overlap of symptoms with other psychiatric disorders can make the diagnosis challenging for both child and adolescent psychiatrists and adult psychiatrists and might help explain why severe cases of ASD are more readily identified earlier than milder cases of ASD.10
Ms. P’s case is also an example of how women are more likely than men to be overlooked when evaluated for ASD. According to DSM-5, the estimated gender ratio for ASD is believed to be 4:1 (male:female).5 However, upon systematic review and meta-analysis, Loomes et al12 found that the gender ratio may be closer to 3:1 (male:female). These authors suggested that diagnostic bias and a failure of passive case ascertainment to estimate gender ratios as stated by DSM-5 in identifying ASD might explain the lower gender ratio.12 A growing body of evidence suggests that ASD is different in males and females. A 2019 qualitative study by Milner et al13 found that female participants reported using masking and camouflaging strategies to appear neurotypical. Compensatory behaviors were found to be linked to a delay in diagnosis and support for ASD.13
Cognitive ability as measured by IQ has also been found to be a factor in receiving a diagnosis of ASD. In a 2010 secondary analysis of a population-based study of the prevalence of ASD, Giarelli et al14found that girls with cognitive impairments as measured by IQ were less likely to be diagnosed with ASD than boys with cognitive impairment, despite meeting the criteria for ASD. Females tend to exhibit fewer repetitive behaviors than males, and tend to be more likely to show accompanying intellectual disability, which suggests that females with ASD may go unrecognized when they exhibit average intelligence with less impairment of behavior and subtler manifestation of social and communication deficits.15 Consequently, females tend to receive this diagnosis later than males.
Continue to: Treatment...
TREATMENT Adding CBT
At an interdisciplinary session several weeks later that includes Ms. P and her parents, the treatment team discusses the revised diagnoses of ASD and MDD, a treatment recommendation for cognitive-behavioral therapy (CBT), and continued use of medication. At this session, Ms. P discloses that she has not been consistent with her medication regimen since her last appointment, which helps explain the increase in her PHQ-9 score from 2 to 14 and GAD-7 score
[polldaddy:11027990]
The authors’ observations
CBT can be helpful in improving medication adherence, developing coping skills, and modifying maladaptive behaviors.

OUTCOME Improvement with psychotherapy
Ms. P and family agree with the team’s recommendations. The aims of Ms. P’s psychotherapy are to maintain medication compliance; implement behavioral modification, vocational rehabilitation, and community engagement; develop social skills; increase functional independence; and develop coping skills for depression and anxiety.
Bottom Line
The prevalence of schizoid personality disorder (SPD) is low, and its symptoms overlap with those of autism spectrum disorder. Therefore, before diagnosing SPD in an adult patient, it is important to obtain a detailed developmental history and include an interdisciplinary team to assess for autism spectrum disorder.
1. Fariba K, Gupta V. Schizoid personality disorder. StatPearls Publishing. Updated June 9, 2021. Accessed January 6, 2021. https://www.ncbi.nlm.nih.gov/books/NBK559234/
2. Diagnostic and Statistical Manual of Mental Disorders: DSM-III. 3rd ed rev. American Psychiatric Association; 1987.
3. Esterberg ML, Goulding SM, Walker EF. Cluster A personality disorders: schizotypal, schizoid and paranoid personality disorders in childhood and adolescence. J Psychopathol Behav Assess. 2010;32(4):515-528. doi:10.1007/s10862-010-9183-8
4. Kalus O, Bernstein DP, Siever LJ. Schizoid personality disorder: a review of current status and implications for DSM-IV. Journal of Personality Disorders. 1993;7(1), 43-52.
5. Diagnostic and Statistical Manual of Mental Disorders: DSM-5. 5th ed. American Psychiatric Association; 2013.
6. Eaton NR, Greene AL. Personality disorders: community prevalence and socio-demographic correlates. Curr Opin Psychol. 2018;21:28-32. doi:10.1016/j.copsyc.2017.09.001
7. Vatano
8. Ritsner MS. Handbook of Schizophrenia Spectrum Disorders, Volume I: Conceptual Issues and Neurobiological Advances. Springer; 2011.
9. Cook ML, Zhang Y, Constantino JN. On the continuity between autistic and schizoid personality disorder trait burden: a prospective study in adolescence. J Nerv Ment Dis. 2020;208(2):94-100. doi:10.1097/NMD.0000000000001105
10. Lai MC, Baron-Cohen S. Identifying the lost generation of adults with autism spectrum conditions. Lancet Psychiatry. 2015;2(11):1013-1027. doi:10.1016/S2215-0366(15)00277-1
11. Fusar-Poli L, Brondino N, Politi P, et al. Missed diagnoses and misdiagnoses of adults with autism spectrum disorder. Eur Arch Psychiatry Clin Neurosci. 2020;10.1007/s00406-020-01189-2. doi:10.1007/s00406-020-01189-w
12. Loomes R, Hull L, Mandy WPL. What is the male-to-female ratio in autism spectrum disorder? A systematic review and meta-analysis. J Am Acad Child Adolesc Psychiatry. 2017;56(6):466-474. doi:10.1016/j.jaac.2017.03.013
13. Milner V, McIntosh H, Colvert E, et al. A qualitative exploration of the female experience of autism spectrum disorder (ASD). J Autism Dev Disord. 2019;49(6):2389-2402. doi:10.1007/s10803-019-03906-4
14. Giarelli E, Wiggins LD, Rice CE, et al. Sex differences in the evaluation and diagnosis of autism spectrum disorders among children. Disabil Health J. 2010;3(2):107-116. doi:10.1016/j.dhjo.2009.07.001
15. Frazier TW, Georgiades S, Bishop SL, et al. Behavioral and cognitive characteristics of females and males with autism in the Simons Simplex Collection. J Am Acad Child Adolesc Psychiatry. 2014;53(3):329-40.e403. doi:10.1016/j.jaac.2013.12.004
16. Julius RJ, Novitsky MA Jr, et al. Medication adherence: a review of the literature and implications for clinical practice. J Psychiatr Pract. 2009;15(1):34-44. doi:10.1097/01.pra.0000344917.43780.77
17. Spain D, Sin J, Chalder T, et al. Cognitive behaviour therapy for adults with autism spectrum disorders and psychiatric co-morbidity: a review. Research in Autism Spectrum Disorders. 2015;9, 151-162. https://doi.org/10.1016/j.rasd.2014.10.019
18. Bishop-Fitzpatrick L, Minshew NJ, Eack SM. A systematic review of psychosocial interventions for adults with autism spectrum disorders. J Autism Dev Disord. 2013;43(3):687-694. doi:10.1007/s10803-012-1615-8
1. Fariba K, Gupta V. Schizoid personality disorder. StatPearls Publishing. Updated June 9, 2021. Accessed January 6, 2021. https://www.ncbi.nlm.nih.gov/books/NBK559234/
2. Diagnostic and Statistical Manual of Mental Disorders: DSM-III. 3rd ed rev. American Psychiatric Association; 1987.
3. Esterberg ML, Goulding SM, Walker EF. Cluster A personality disorders: schizotypal, schizoid and paranoid personality disorders in childhood and adolescence. J Psychopathol Behav Assess. 2010;32(4):515-528. doi:10.1007/s10862-010-9183-8
4. Kalus O, Bernstein DP, Siever LJ. Schizoid personality disorder: a review of current status and implications for DSM-IV. Journal of Personality Disorders. 1993;7(1), 43-52.
5. Diagnostic and Statistical Manual of Mental Disorders: DSM-5. 5th ed. American Psychiatric Association; 2013.
6. Eaton NR, Greene AL. Personality disorders: community prevalence and socio-demographic correlates. Curr Opin Psychol. 2018;21:28-32. doi:10.1016/j.copsyc.2017.09.001
7. Vatano
8. Ritsner MS. Handbook of Schizophrenia Spectrum Disorders, Volume I: Conceptual Issues and Neurobiological Advances. Springer; 2011.
9. Cook ML, Zhang Y, Constantino JN. On the continuity between autistic and schizoid personality disorder trait burden: a prospective study in adolescence. J Nerv Ment Dis. 2020;208(2):94-100. doi:10.1097/NMD.0000000000001105
10. Lai MC, Baron-Cohen S. Identifying the lost generation of adults with autism spectrum conditions. Lancet Psychiatry. 2015;2(11):1013-1027. doi:10.1016/S2215-0366(15)00277-1
11. Fusar-Poli L, Brondino N, Politi P, et al. Missed diagnoses and misdiagnoses of adults with autism spectrum disorder. Eur Arch Psychiatry Clin Neurosci. 2020;10.1007/s00406-020-01189-2. doi:10.1007/s00406-020-01189-w
12. Loomes R, Hull L, Mandy WPL. What is the male-to-female ratio in autism spectrum disorder? A systematic review and meta-analysis. J Am Acad Child Adolesc Psychiatry. 2017;56(6):466-474. doi:10.1016/j.jaac.2017.03.013
13. Milner V, McIntosh H, Colvert E, et al. A qualitative exploration of the female experience of autism spectrum disorder (ASD). J Autism Dev Disord. 2019;49(6):2389-2402. doi:10.1007/s10803-019-03906-4
14. Giarelli E, Wiggins LD, Rice CE, et al. Sex differences in the evaluation and diagnosis of autism spectrum disorders among children. Disabil Health J. 2010;3(2):107-116. doi:10.1016/j.dhjo.2009.07.001
15. Frazier TW, Georgiades S, Bishop SL, et al. Behavioral and cognitive characteristics of females and males with autism in the Simons Simplex Collection. J Am Acad Child Adolesc Psychiatry. 2014;53(3):329-40.e403. doi:10.1016/j.jaac.2013.12.004
16. Julius RJ, Novitsky MA Jr, et al. Medication adherence: a review of the literature and implications for clinical practice. J Psychiatr Pract. 2009;15(1):34-44. doi:10.1097/01.pra.0000344917.43780.77
17. Spain D, Sin J, Chalder T, et al. Cognitive behaviour therapy for adults with autism spectrum disorders and psychiatric co-morbidity: a review. Research in Autism Spectrum Disorders. 2015;9, 151-162. https://doi.org/10.1016/j.rasd.2014.10.019
18. Bishop-Fitzpatrick L, Minshew NJ, Eack SM. A systematic review of psychosocial interventions for adults with autism spectrum disorders. J Autism Dev Disord. 2013;43(3):687-694. doi:10.1007/s10803-012-1615-8
Severe GI distress: Is clozapine to blame?
CASE GI distress while taking clozapine
Mr. F, age 29, has a history of psychiatric hospitalizations for psychotic episodes. It took a herculean effort to get him to agree to try clozapine, to which he has experienced a modest to good response. Unfortunately, recently he has been experiencing significant upper gastrointestinal (GI) distress. He attributes this to clozapine, and asks if he can discontinue this medication.
HISTORY Nausea becomes severe
Mr. F, age 29, resides in a long-term residential setting for patients with serious mental illness who need additional support following acute hospitalization. He has treatment-refractory schizophrenia. He first developed symptoms at age 18, and experienced multiple psychotic episodes requiring psychiatric hospitalizations that lasted for months. He has had numerous antipsychotic trials and a course of electroconvulsive therapy, with limited benefit.
More recently, Mr. F’s symptoms began to stabilize on a medication regimen that includes clozapine, 350 mg/d at bedtime, and haloperidol, 2 mg/d. He has not required psychiatric hospitalization for the past year.
Within months of initiating clozapine, Mr. F starts to complain daily about symptoms of worsening abdominal pain, abdominal bloating, nausea, intermittent episodes of emesis, and heartburn. The symptoms begin when he wakes up, are worse in the morning, and persist throughout the morning. He has experienced occasional mild constipation, but no diarrhea or weight loss. There have been no major changes in his diet, addition of new medications, or significant use of nonsteroidal anti-inflammatory drugs.
Mr. F’s nausea worsens over the next several weeks, to the point he begins to significantly limit how much he eats to cope with it. His GI symptoms are also impacting his mood and daily functioning.
This is not Mr. F’s first experience with significant GI distress. A few months before his first psychotic episode, Mr. F began developing vision problems, joint and abdominal pain, and a general decline in social and academic functioning. At that time, he underwent a significant workup by both GI and integrative medicine, including stool testing, upper endoscopy, and a Cyrex panel (a complementary medicine approach to exploring for specific autoimmune conditions). Results were largely within expected parameters, though a hydrogen breath test was suggestive of possible small intestine bowel overgrowth. More recently, he has been adhering to a gluten-free diet, which his family felt may help prevent some of his physical symptoms as well as mitigate some of his psychotic symptoms. He now asks if he can stop taking clozapine.
[polldaddy:11008393]
EVALUATION Establishing the correct diagnosis
Initially, Mr. F is diagnosed with gastroesophageal reflux disease (GERD) and attempts to manage his symptoms with pharmacologic and diet-based interventions. He significantly cuts down on soda consumption, and undergoes trials of calcium carbonate, antiemetics, and a PPI. Unfortunately, no material improvements are noted, and he continued to experience significant upper GI distress, especially after meals.
The psychiatric treatment team, Mr. F, and his family seek consultation with a GI specialist, who recommends that Mr. F. undergo a nuclear medicine solid gastric emptying scintigraphy study to evaluate for gastroparesis (delayed gastric emptying).1 Results demonstrate grade 3 gastroparesis, with 56% radiotracer retainment at 4 hours. Mr. F is relieved to finally have an explanation for his persistent GI symptoms, and discusses his treatment options with the GI consultant and psychiatry team.
Continue to: The authors’ observations...
The authors’ observations
Mr. F and his family are opposed to starting a dopamine antagonist such as metoclopramide or domperidone (the latter is not FDA-approved but is available by special application to the FDA). These are first-line treatments for gastroparesis, but Mr. F and his family do not want them because of the risk of tardive dyskinesia. This is consistent with their previously expressed concerns regarding first-generation antipsychotics, and is why Mr. F has only been treated with a very low dose of haloperidol while the clozapine was titrated. Instead, Mr. F, his family, the psychiatry treatment team, and the GI specialist agree to pursue a combination of a GI hypomotility diet—which includes frequent small meals (4 to 6 per day), ideally with low fiber, low fat, and increased fluid intake—and a trial of the second line agent for gastroparesis, erythromycin, a medication with known hepatic cytochrome P450 (CYP) drug-drug interactions that impacts the clearance of clozapine.
Shared decision making is an evidence-based approach to engaging patients in medical decision making. It allows clinicians to provide education on potential treatment options and includes a discussion of risks and benefits. It also includes an assessment of the patient’s understanding of their condition, explores attitudes towards treatment, and elicits patient values specific to the desired outcome. Even in very ill patients with schizophrenia, shared decision making has been demonstrated to increase patient perception of involvement in their own care and knowledge about their condition.2 Using this framework, Mr. F and his family, as well as the GI and psychiatric teams, felt confident that the agreed-upon approach was the best one for Mr. F.
TREATMENT Erythromycin and continued clozapine
Mr. F. is started on erythromycin, 100 mg 3 times a day. Erythromycin is a prokinetic agent that acts as a motilin agonist and increases the rate of gastric emptying. The liquid formulation of the medication is a suspension typically taken in 3- to 4-week courses, with 1 week “off” to prevent tachyphylaxis.3 Compared to the tablet, the liquid suspension has higher bioavailability, allows for easier dose adjustment, and takes less time to reach peak serum concentrations, which make it the preferred formulation for gastroparesis treatment.
Per the GI consultant’s recommendation, Mr. F receives a total of 3 courses of erythromycin, with some improvement in the frequency of his nausea noted only during the third erythromycin course. His clozapine levels are closely monitored during this time, as well as symptoms of clozapine toxicity (ie, sedation, confusion, hypersalivation, seizures, myoclonic jerks), because erythromycin can directly affect clozapine levels.4,5 Case reports suggest that when these 2 medications are taken concomitantly, erythromycin inhibits the metabolism of hepatic enzyme CYP3A4, causing increased plasma concentrations of clozapine. Before starting erythromycin, Mr. F’s clozapine levels were 809 ng/mL at 350 mg/d. During the erythromycin courses, his levels are 1,043 to 1,074 ng/mL, despite reducing clozapine to 300 mg/d. However, he does not experience any adverse effects of clozapine (including seizures), which were being monitored closely.
The authors’ observations
Clozapine is the most effective medication for treatment-refractory schizophrenia.6 Compared to the other second-generation antipsychotics, it is associated with a lower risk of rehospitalization and treatment discontinuation, a significant decrease of positive symptom burden, and a reduction in suicidality.7,8 Unfortunately, clozapine use is not without significant risk. FDA black box warnings highlight severe neutropenia, myocarditis, seizures, and hypotension as potentially life-threatening adverse effects that require close monitoring.9
Recently, clinicians have increasingly focused on the underrecognized but well-established finding that clozapine can cause significant GI adverse effects. While constipation is a known adverse effect of other antipsychotics, a 2016 meta-analysis of 32 studies estimated that the pooled prevalence of clozapine-associated constipation was 31.2%, and showed that patients receiving clozapine were 3 times more likely to be constipated than patients receiving other antipsychotics (odds ratio 3.02, CI 1.91-4.77, P < .001, n = 11 studies).10 A 2012 review of 16 studies involving potentially lethal adverse effects of clozapine demonstrated that rates of agranulocytosis and GI hypomotility were nearly identical, but that mortality from constipation was 3.6 to 12.5 times higher than mortality from agranulocytosis.11
In 2020, the FDA issued an increased warning regarding severe bowel-related complications in patients receiving clozapine, ranging in severity from mild discomfort to ileus, bowel obstruction, toxic megacolon, and death.9
As exemplified by Mr. F’s case, upper GI symptoms associated with clozapine also are distressing and can have a significant impact on quality of life. Dyspepsia is a common complaint in patients with chronic psychiatric illness. A study of 79 psychiatric inpatients hospitalized long-term found that 80% reported at least 1 symptom of dyspepsia.12 There are few older studies describing the effect of clozapine on the upper GI system. We and others previously reported on significantly increased use of—not only antacids—but also H2 blockers and prokinetic agents after initiating clozapine, but sample sizes are small.13-15 These older data and newer studies suggest that GERD is a common upper GI disorder diagnosis following clozapine initiation, perhaps reflecting a knowledge gap and infrequent use of the more complex testing required to confirm a diagnosis of GI motility disorders such as gastroparesis.
In a study of 17 patients receiving clozapine, wireless motility capsules were used to measure whole gut motility, including gastric emptying time, small bowel transit time, and colonic transit time. In 82% of patients, there was demonstrated GI hypomotility in at least 1 region, and 41% of participants exhibited delayed gastric emptying, with a cut-off time of >5 hours required for a gastroparesis diagnosis.16 This is significantly higher than the prevalence of gastroparesis observed in studies of the general community.17 The Table18,19 summarizes the differences between GERD and gastroparesis.

OUTCOME Some improvement
Mr. F experiences limited improvement of some of his nausea symptoms during the third erythromycin cycle and returns to the gastroenterologist for a follow-up appointment. The GI specialist decides to discontinue erythromycin in view of potential drug-drug interactions and Mr. F’s elevated clozapine levels and the associated risks that might entail. Mr. F is again offered the D2 dopamine antagonist metoclopramide, but again refuses due to the risk for tardive dyskinesia. He is asked to continue the GI dysmotility diet. Mr. F finds some relief of nausea symptoms from an over-the-counter product for nausea (a nasal inhalant containing essential oils) and is advised to follow up with the GI specialist in 3 months. Shortly thereafter, he is discharged to live in a less restrictive supportive housing environment, and his follow-up psychiatric care is provided by an assertive community treatment team. Over the next several months, the dosage of clozapine is decreased to 250 mg/d. Mr. F initially experiences worsening psychiatric symptoms, but stabilizes thereafter. He then moves out of state to be closer to his family.
Bottom Line
In patients receiving clozapine, frequent nausea along with clustering of heartburn, abdominal pain, bloating, early satiety, and vomiting (especially after meals) may signal gastroparesis rather than gastroesophageal reflux disease. Such patients may require consultation with a gastroenterologist, a scintigraphy-based gastric emptying test, and treatment if gastroparesis is confirmed.
1. Camilleri M, Chedid V, Ford AC, et al. Gastroparesis. Nat Rev Dis Primers. 2018;4(1):41. doi:10.1038/s41572-018-0038-z
2. Hamann J, Langer B, Winkler V, et al. Shared decision making for in-patients with schizophrenia. Acta Psychiatr Scand. 2006;114(4):265-273. doi: 10.1111/j.1600-0447.2006.00798.x
3. Maganti K, Onyemere K, Jones MP. Oral erythromycin and symptomatic relief of gastroparesis: a systematic review. Am J Gastroenterol. 2003;98(2):259-263. doi:10.1111/j.1572-0241.2003.07167.x
4. Taylor D. Pharmacokinetic interactions involving clozapine. Br J Psychiatry. 1997;171:109-112. doi:10.1192/bjp.171.2.109
5. Edge SC, Markowitz JS, Devane CL. Clozapine drug-drug interactions: a review of the literature. Human Psychopharmacology: Clinical and Experimental. 1997;12(1):5-20.
6. Vanasse A, Blais L, Courteau J, et al. Comparative effectiveness and safety of antipsychotic drugs in schizophrenia treatment: a real-world observational study. Acta Psychiatr Scand. 2016;134(5):374-384. doi:10.1111/acps.12621
7. Siskind D, McCartney L, Goldschlager R, et al. Clozapine v. first- and second-generation antipsychotics in treatment-refractory schizophrenia: systematic review and meta-analysis. Br J Psychiatry. 2016;209(5):385-392. doi:10.1192/bjp.bp.115.177261
8. Azorin JM, Spiegel R, Remington G, et al. A double-blind comparative study of clozapine and risperidone in the management of severe chronic schizophrenia. Am J Psychiatry. 2001;158(8):1305-1313. doi:10.1176/appi.ajp.158.8.1305
9. National Alliance on Mental Illness. Clozapine. Accessed June 13, 2021. https://www.nami.org/About-Mental-Illness/Treatments/Mental-Health-Medications/Types-of-Medication/Clozapine-(Clozaril-and-FazaClo)
10. Shirazi A, Stubbs B, Gomez L, et al. Prevalence and predictors of clozapine-associated constipation: a systematic review and meta-analysis. Int J Mol Sci. 2016;17(6):863. doi:10.3390/ijms17060863
11. Cohen D, Bogers JP, van Dijk D, et al. Beyond white blood cell monitoring: screening in the initial phase of clozapine therapy. J Clin Psychiatry. 2012;73(10):1307-1312. doi:10.4088/JCP.11r06977
12. Mookhoek EJ, Meijs VM, Loonen AJ, et al. Dyspepsia in chronic psychiatric patients. Pharmacopsychiatry. 2005;38(3):125-127. doi:10.1055/s-2005-864123
13. John JP, Chengappa KN, Baker RW, et al. Assessment of changes in both weight and frequency of use of medications for the treatment of gastrointestinal symptoms among clozapine-treated patients. Ann Clin Psychiatry. 1995;7(3):119-125. doi: 10.3109/10401239509149038
14. Schwartz BJ, Frisolone JA. A case report of clozapine-induced gastric outlet obstruction. Am J Psychiatry. 1993;150(10):1563. doi:10.1176/ajp.150.10.1563a
15. Taylor D, Olofinjana O, Rahimi T. Use of antacid medication in patients receiving clozapine: a comparison with other second-generation antipsychotics. J Clin Psychopharmacol. 2010;30(4):460-461. doi:10.1097/JCP.0b013e3181e5c0f7
16. Every-Palmer S, Inns SJ, Grant E, et al. Effects of clozapine on the gut: cross-sectional study of delayed gastric emptying and small and large intestinal dysmotility. CNS Drugs. 2019;33(1):81-91. doi:10.1007/s40263-018-0587-4
17. Jung HK, Choung RS, Locke GR 3rd, et al. The incidence, prevalence, and outcomes of patients with gastroparesis in Olmsted County, Minnesota, from 1996 to 2006. Gastroenterology. 2009;136(4):1225-1233. doi: 10.1053/j.gastro.2008.12.047
18. Antunes C, Aleem A, Curtis SA. Gastroesophageal reflux disease. StatPearls Publishing. Updated July 7, 2021. Accessed December 8, 2021. https://www.ncbi.nlm.nih.gov/books/NBK441938/
19. Reddivari AKR, Mehta P. Gastroparesis. StatPearls Publishing. Updated June 30, 2021. Accessed December 8, 2021. https://www.ncbi.nlm.nih.gov/books/NBK551528/
CASE GI distress while taking clozapine
Mr. F, age 29, has a history of psychiatric hospitalizations for psychotic episodes. It took a herculean effort to get him to agree to try clozapine, to which he has experienced a modest to good response. Unfortunately, recently he has been experiencing significant upper gastrointestinal (GI) distress. He attributes this to clozapine, and asks if he can discontinue this medication.
HISTORY Nausea becomes severe
Mr. F, age 29, resides in a long-term residential setting for patients with serious mental illness who need additional support following acute hospitalization. He has treatment-refractory schizophrenia. He first developed symptoms at age 18, and experienced multiple psychotic episodes requiring psychiatric hospitalizations that lasted for months. He has had numerous antipsychotic trials and a course of electroconvulsive therapy, with limited benefit.
More recently, Mr. F’s symptoms began to stabilize on a medication regimen that includes clozapine, 350 mg/d at bedtime, and haloperidol, 2 mg/d. He has not required psychiatric hospitalization for the past year.
Within months of initiating clozapine, Mr. F starts to complain daily about symptoms of worsening abdominal pain, abdominal bloating, nausea, intermittent episodes of emesis, and heartburn. The symptoms begin when he wakes up, are worse in the morning, and persist throughout the morning. He has experienced occasional mild constipation, but no diarrhea or weight loss. There have been no major changes in his diet, addition of new medications, or significant use of nonsteroidal anti-inflammatory drugs.
Mr. F’s nausea worsens over the next several weeks, to the point he begins to significantly limit how much he eats to cope with it. His GI symptoms are also impacting his mood and daily functioning.
This is not Mr. F’s first experience with significant GI distress. A few months before his first psychotic episode, Mr. F began developing vision problems, joint and abdominal pain, and a general decline in social and academic functioning. At that time, he underwent a significant workup by both GI and integrative medicine, including stool testing, upper endoscopy, and a Cyrex panel (a complementary medicine approach to exploring for specific autoimmune conditions). Results were largely within expected parameters, though a hydrogen breath test was suggestive of possible small intestine bowel overgrowth. More recently, he has been adhering to a gluten-free diet, which his family felt may help prevent some of his physical symptoms as well as mitigate some of his psychotic symptoms. He now asks if he can stop taking clozapine.
[polldaddy:11008393]
EVALUATION Establishing the correct diagnosis
Initially, Mr. F is diagnosed with gastroesophageal reflux disease (GERD) and attempts to manage his symptoms with pharmacologic and diet-based interventions. He significantly cuts down on soda consumption, and undergoes trials of calcium carbonate, antiemetics, and a PPI. Unfortunately, no material improvements are noted, and he continued to experience significant upper GI distress, especially after meals.
The psychiatric treatment team, Mr. F, and his family seek consultation with a GI specialist, who recommends that Mr. F. undergo a nuclear medicine solid gastric emptying scintigraphy study to evaluate for gastroparesis (delayed gastric emptying).1 Results demonstrate grade 3 gastroparesis, with 56% radiotracer retainment at 4 hours. Mr. F is relieved to finally have an explanation for his persistent GI symptoms, and discusses his treatment options with the GI consultant and psychiatry team.
Continue to: The authors’ observations...
The authors’ observations
Mr. F and his family are opposed to starting a dopamine antagonist such as metoclopramide or domperidone (the latter is not FDA-approved but is available by special application to the FDA). These are first-line treatments for gastroparesis, but Mr. F and his family do not want them because of the risk of tardive dyskinesia. This is consistent with their previously expressed concerns regarding first-generation antipsychotics, and is why Mr. F has only been treated with a very low dose of haloperidol while the clozapine was titrated. Instead, Mr. F, his family, the psychiatry treatment team, and the GI specialist agree to pursue a combination of a GI hypomotility diet—which includes frequent small meals (4 to 6 per day), ideally with low fiber, low fat, and increased fluid intake—and a trial of the second line agent for gastroparesis, erythromycin, a medication with known hepatic cytochrome P450 (CYP) drug-drug interactions that impacts the clearance of clozapine.
Shared decision making is an evidence-based approach to engaging patients in medical decision making. It allows clinicians to provide education on potential treatment options and includes a discussion of risks and benefits. It also includes an assessment of the patient’s understanding of their condition, explores attitudes towards treatment, and elicits patient values specific to the desired outcome. Even in very ill patients with schizophrenia, shared decision making has been demonstrated to increase patient perception of involvement in their own care and knowledge about their condition.2 Using this framework, Mr. F and his family, as well as the GI and psychiatric teams, felt confident that the agreed-upon approach was the best one for Mr. F.
TREATMENT Erythromycin and continued clozapine
Mr. F. is started on erythromycin, 100 mg 3 times a day. Erythromycin is a prokinetic agent that acts as a motilin agonist and increases the rate of gastric emptying. The liquid formulation of the medication is a suspension typically taken in 3- to 4-week courses, with 1 week “off” to prevent tachyphylaxis.3 Compared to the tablet, the liquid suspension has higher bioavailability, allows for easier dose adjustment, and takes less time to reach peak serum concentrations, which make it the preferred formulation for gastroparesis treatment.
Per the GI consultant’s recommendation, Mr. F receives a total of 3 courses of erythromycin, with some improvement in the frequency of his nausea noted only during the third erythromycin course. His clozapine levels are closely monitored during this time, as well as symptoms of clozapine toxicity (ie, sedation, confusion, hypersalivation, seizures, myoclonic jerks), because erythromycin can directly affect clozapine levels.4,5 Case reports suggest that when these 2 medications are taken concomitantly, erythromycin inhibits the metabolism of hepatic enzyme CYP3A4, causing increased plasma concentrations of clozapine. Before starting erythromycin, Mr. F’s clozapine levels were 809 ng/mL at 350 mg/d. During the erythromycin courses, his levels are 1,043 to 1,074 ng/mL, despite reducing clozapine to 300 mg/d. However, he does not experience any adverse effects of clozapine (including seizures), which were being monitored closely.
The authors’ observations
Clozapine is the most effective medication for treatment-refractory schizophrenia.6 Compared to the other second-generation antipsychotics, it is associated with a lower risk of rehospitalization and treatment discontinuation, a significant decrease of positive symptom burden, and a reduction in suicidality.7,8 Unfortunately, clozapine use is not without significant risk. FDA black box warnings highlight severe neutropenia, myocarditis, seizures, and hypotension as potentially life-threatening adverse effects that require close monitoring.9
Recently, clinicians have increasingly focused on the underrecognized but well-established finding that clozapine can cause significant GI adverse effects. While constipation is a known adverse effect of other antipsychotics, a 2016 meta-analysis of 32 studies estimated that the pooled prevalence of clozapine-associated constipation was 31.2%, and showed that patients receiving clozapine were 3 times more likely to be constipated than patients receiving other antipsychotics (odds ratio 3.02, CI 1.91-4.77, P < .001, n = 11 studies).10 A 2012 review of 16 studies involving potentially lethal adverse effects of clozapine demonstrated that rates of agranulocytosis and GI hypomotility were nearly identical, but that mortality from constipation was 3.6 to 12.5 times higher than mortality from agranulocytosis.11
In 2020, the FDA issued an increased warning regarding severe bowel-related complications in patients receiving clozapine, ranging in severity from mild discomfort to ileus, bowel obstruction, toxic megacolon, and death.9
As exemplified by Mr. F’s case, upper GI symptoms associated with clozapine also are distressing and can have a significant impact on quality of life. Dyspepsia is a common complaint in patients with chronic psychiatric illness. A study of 79 psychiatric inpatients hospitalized long-term found that 80% reported at least 1 symptom of dyspepsia.12 There are few older studies describing the effect of clozapine on the upper GI system. We and others previously reported on significantly increased use of—not only antacids—but also H2 blockers and prokinetic agents after initiating clozapine, but sample sizes are small.13-15 These older data and newer studies suggest that GERD is a common upper GI disorder diagnosis following clozapine initiation, perhaps reflecting a knowledge gap and infrequent use of the more complex testing required to confirm a diagnosis of GI motility disorders such as gastroparesis.
In a study of 17 patients receiving clozapine, wireless motility capsules were used to measure whole gut motility, including gastric emptying time, small bowel transit time, and colonic transit time. In 82% of patients, there was demonstrated GI hypomotility in at least 1 region, and 41% of participants exhibited delayed gastric emptying, with a cut-off time of >5 hours required for a gastroparesis diagnosis.16 This is significantly higher than the prevalence of gastroparesis observed in studies of the general community.17 The Table18,19 summarizes the differences between GERD and gastroparesis.

OUTCOME Some improvement
Mr. F experiences limited improvement of some of his nausea symptoms during the third erythromycin cycle and returns to the gastroenterologist for a follow-up appointment. The GI specialist decides to discontinue erythromycin in view of potential drug-drug interactions and Mr. F’s elevated clozapine levels and the associated risks that might entail. Mr. F is again offered the D2 dopamine antagonist metoclopramide, but again refuses due to the risk for tardive dyskinesia. He is asked to continue the GI dysmotility diet. Mr. F finds some relief of nausea symptoms from an over-the-counter product for nausea (a nasal inhalant containing essential oils) and is advised to follow up with the GI specialist in 3 months. Shortly thereafter, he is discharged to live in a less restrictive supportive housing environment, and his follow-up psychiatric care is provided by an assertive community treatment team. Over the next several months, the dosage of clozapine is decreased to 250 mg/d. Mr. F initially experiences worsening psychiatric symptoms, but stabilizes thereafter. He then moves out of state to be closer to his family.
Bottom Line
In patients receiving clozapine, frequent nausea along with clustering of heartburn, abdominal pain, bloating, early satiety, and vomiting (especially after meals) may signal gastroparesis rather than gastroesophageal reflux disease. Such patients may require consultation with a gastroenterologist, a scintigraphy-based gastric emptying test, and treatment if gastroparesis is confirmed.
CASE GI distress while taking clozapine
Mr. F, age 29, has a history of psychiatric hospitalizations for psychotic episodes. It took a herculean effort to get him to agree to try clozapine, to which he has experienced a modest to good response. Unfortunately, recently he has been experiencing significant upper gastrointestinal (GI) distress. He attributes this to clozapine, and asks if he can discontinue this medication.
HISTORY Nausea becomes severe
Mr. F, age 29, resides in a long-term residential setting for patients with serious mental illness who need additional support following acute hospitalization. He has treatment-refractory schizophrenia. He first developed symptoms at age 18, and experienced multiple psychotic episodes requiring psychiatric hospitalizations that lasted for months. He has had numerous antipsychotic trials and a course of electroconvulsive therapy, with limited benefit.
More recently, Mr. F’s symptoms began to stabilize on a medication regimen that includes clozapine, 350 mg/d at bedtime, and haloperidol, 2 mg/d. He has not required psychiatric hospitalization for the past year.
Within months of initiating clozapine, Mr. F starts to complain daily about symptoms of worsening abdominal pain, abdominal bloating, nausea, intermittent episodes of emesis, and heartburn. The symptoms begin when he wakes up, are worse in the morning, and persist throughout the morning. He has experienced occasional mild constipation, but no diarrhea or weight loss. There have been no major changes in his diet, addition of new medications, or significant use of nonsteroidal anti-inflammatory drugs.
Mr. F’s nausea worsens over the next several weeks, to the point he begins to significantly limit how much he eats to cope with it. His GI symptoms are also impacting his mood and daily functioning.
This is not Mr. F’s first experience with significant GI distress. A few months before his first psychotic episode, Mr. F began developing vision problems, joint and abdominal pain, and a general decline in social and academic functioning. At that time, he underwent a significant workup by both GI and integrative medicine, including stool testing, upper endoscopy, and a Cyrex panel (a complementary medicine approach to exploring for specific autoimmune conditions). Results were largely within expected parameters, though a hydrogen breath test was suggestive of possible small intestine bowel overgrowth. More recently, he has been adhering to a gluten-free diet, which his family felt may help prevent some of his physical symptoms as well as mitigate some of his psychotic symptoms. He now asks if he can stop taking clozapine.
[polldaddy:11008393]
EVALUATION Establishing the correct diagnosis
Initially, Mr. F is diagnosed with gastroesophageal reflux disease (GERD) and attempts to manage his symptoms with pharmacologic and diet-based interventions. He significantly cuts down on soda consumption, and undergoes trials of calcium carbonate, antiemetics, and a PPI. Unfortunately, no material improvements are noted, and he continued to experience significant upper GI distress, especially after meals.
The psychiatric treatment team, Mr. F, and his family seek consultation with a GI specialist, who recommends that Mr. F. undergo a nuclear medicine solid gastric emptying scintigraphy study to evaluate for gastroparesis (delayed gastric emptying).1 Results demonstrate grade 3 gastroparesis, with 56% radiotracer retainment at 4 hours. Mr. F is relieved to finally have an explanation for his persistent GI symptoms, and discusses his treatment options with the GI consultant and psychiatry team.
Continue to: The authors’ observations...
The authors’ observations
Mr. F and his family are opposed to starting a dopamine antagonist such as metoclopramide or domperidone (the latter is not FDA-approved but is available by special application to the FDA). These are first-line treatments for gastroparesis, but Mr. F and his family do not want them because of the risk of tardive dyskinesia. This is consistent with their previously expressed concerns regarding first-generation antipsychotics, and is why Mr. F has only been treated with a very low dose of haloperidol while the clozapine was titrated. Instead, Mr. F, his family, the psychiatry treatment team, and the GI specialist agree to pursue a combination of a GI hypomotility diet—which includes frequent small meals (4 to 6 per day), ideally with low fiber, low fat, and increased fluid intake—and a trial of the second line agent for gastroparesis, erythromycin, a medication with known hepatic cytochrome P450 (CYP) drug-drug interactions that impacts the clearance of clozapine.
Shared decision making is an evidence-based approach to engaging patients in medical decision making. It allows clinicians to provide education on potential treatment options and includes a discussion of risks and benefits. It also includes an assessment of the patient’s understanding of their condition, explores attitudes towards treatment, and elicits patient values specific to the desired outcome. Even in very ill patients with schizophrenia, shared decision making has been demonstrated to increase patient perception of involvement in their own care and knowledge about their condition.2 Using this framework, Mr. F and his family, as well as the GI and psychiatric teams, felt confident that the agreed-upon approach was the best one for Mr. F.
TREATMENT Erythromycin and continued clozapine
Mr. F. is started on erythromycin, 100 mg 3 times a day. Erythromycin is a prokinetic agent that acts as a motilin agonist and increases the rate of gastric emptying. The liquid formulation of the medication is a suspension typically taken in 3- to 4-week courses, with 1 week “off” to prevent tachyphylaxis.3 Compared to the tablet, the liquid suspension has higher bioavailability, allows for easier dose adjustment, and takes less time to reach peak serum concentrations, which make it the preferred formulation for gastroparesis treatment.
Per the GI consultant’s recommendation, Mr. F receives a total of 3 courses of erythromycin, with some improvement in the frequency of his nausea noted only during the third erythromycin course. His clozapine levels are closely monitored during this time, as well as symptoms of clozapine toxicity (ie, sedation, confusion, hypersalivation, seizures, myoclonic jerks), because erythromycin can directly affect clozapine levels.4,5 Case reports suggest that when these 2 medications are taken concomitantly, erythromycin inhibits the metabolism of hepatic enzyme CYP3A4, causing increased plasma concentrations of clozapine. Before starting erythromycin, Mr. F’s clozapine levels were 809 ng/mL at 350 mg/d. During the erythromycin courses, his levels are 1,043 to 1,074 ng/mL, despite reducing clozapine to 300 mg/d. However, he does not experience any adverse effects of clozapine (including seizures), which were being monitored closely.
The authors’ observations
Clozapine is the most effective medication for treatment-refractory schizophrenia.6 Compared to the other second-generation antipsychotics, it is associated with a lower risk of rehospitalization and treatment discontinuation, a significant decrease of positive symptom burden, and a reduction in suicidality.7,8 Unfortunately, clozapine use is not without significant risk. FDA black box warnings highlight severe neutropenia, myocarditis, seizures, and hypotension as potentially life-threatening adverse effects that require close monitoring.9
Recently, clinicians have increasingly focused on the underrecognized but well-established finding that clozapine can cause significant GI adverse effects. While constipation is a known adverse effect of other antipsychotics, a 2016 meta-analysis of 32 studies estimated that the pooled prevalence of clozapine-associated constipation was 31.2%, and showed that patients receiving clozapine were 3 times more likely to be constipated than patients receiving other antipsychotics (odds ratio 3.02, CI 1.91-4.77, P < .001, n = 11 studies).10 A 2012 review of 16 studies involving potentially lethal adverse effects of clozapine demonstrated that rates of agranulocytosis and GI hypomotility were nearly identical, but that mortality from constipation was 3.6 to 12.5 times higher than mortality from agranulocytosis.11
In 2020, the FDA issued an increased warning regarding severe bowel-related complications in patients receiving clozapine, ranging in severity from mild discomfort to ileus, bowel obstruction, toxic megacolon, and death.9
As exemplified by Mr. F’s case, upper GI symptoms associated with clozapine also are distressing and can have a significant impact on quality of life. Dyspepsia is a common complaint in patients with chronic psychiatric illness. A study of 79 psychiatric inpatients hospitalized long-term found that 80% reported at least 1 symptom of dyspepsia.12 There are few older studies describing the effect of clozapine on the upper GI system. We and others previously reported on significantly increased use of—not only antacids—but also H2 blockers and prokinetic agents after initiating clozapine, but sample sizes are small.13-15 These older data and newer studies suggest that GERD is a common upper GI disorder diagnosis following clozapine initiation, perhaps reflecting a knowledge gap and infrequent use of the more complex testing required to confirm a diagnosis of GI motility disorders such as gastroparesis.
In a study of 17 patients receiving clozapine, wireless motility capsules were used to measure whole gut motility, including gastric emptying time, small bowel transit time, and colonic transit time. In 82% of patients, there was demonstrated GI hypomotility in at least 1 region, and 41% of participants exhibited delayed gastric emptying, with a cut-off time of >5 hours required for a gastroparesis diagnosis.16 This is significantly higher than the prevalence of gastroparesis observed in studies of the general community.17 The Table18,19 summarizes the differences between GERD and gastroparesis.

OUTCOME Some improvement
Mr. F experiences limited improvement of some of his nausea symptoms during the third erythromycin cycle and returns to the gastroenterologist for a follow-up appointment. The GI specialist decides to discontinue erythromycin in view of potential drug-drug interactions and Mr. F’s elevated clozapine levels and the associated risks that might entail. Mr. F is again offered the D2 dopamine antagonist metoclopramide, but again refuses due to the risk for tardive dyskinesia. He is asked to continue the GI dysmotility diet. Mr. F finds some relief of nausea symptoms from an over-the-counter product for nausea (a nasal inhalant containing essential oils) and is advised to follow up with the GI specialist in 3 months. Shortly thereafter, he is discharged to live in a less restrictive supportive housing environment, and his follow-up psychiatric care is provided by an assertive community treatment team. Over the next several months, the dosage of clozapine is decreased to 250 mg/d. Mr. F initially experiences worsening psychiatric symptoms, but stabilizes thereafter. He then moves out of state to be closer to his family.
Bottom Line
In patients receiving clozapine, frequent nausea along with clustering of heartburn, abdominal pain, bloating, early satiety, and vomiting (especially after meals) may signal gastroparesis rather than gastroesophageal reflux disease. Such patients may require consultation with a gastroenterologist, a scintigraphy-based gastric emptying test, and treatment if gastroparesis is confirmed.
1. Camilleri M, Chedid V, Ford AC, et al. Gastroparesis. Nat Rev Dis Primers. 2018;4(1):41. doi:10.1038/s41572-018-0038-z
2. Hamann J, Langer B, Winkler V, et al. Shared decision making for in-patients with schizophrenia. Acta Psychiatr Scand. 2006;114(4):265-273. doi: 10.1111/j.1600-0447.2006.00798.x
3. Maganti K, Onyemere K, Jones MP. Oral erythromycin and symptomatic relief of gastroparesis: a systematic review. Am J Gastroenterol. 2003;98(2):259-263. doi:10.1111/j.1572-0241.2003.07167.x
4. Taylor D. Pharmacokinetic interactions involving clozapine. Br J Psychiatry. 1997;171:109-112. doi:10.1192/bjp.171.2.109
5. Edge SC, Markowitz JS, Devane CL. Clozapine drug-drug interactions: a review of the literature. Human Psychopharmacology: Clinical and Experimental. 1997;12(1):5-20.
6. Vanasse A, Blais L, Courteau J, et al. Comparative effectiveness and safety of antipsychotic drugs in schizophrenia treatment: a real-world observational study. Acta Psychiatr Scand. 2016;134(5):374-384. doi:10.1111/acps.12621
7. Siskind D, McCartney L, Goldschlager R, et al. Clozapine v. first- and second-generation antipsychotics in treatment-refractory schizophrenia: systematic review and meta-analysis. Br J Psychiatry. 2016;209(5):385-392. doi:10.1192/bjp.bp.115.177261
8. Azorin JM, Spiegel R, Remington G, et al. A double-blind comparative study of clozapine and risperidone in the management of severe chronic schizophrenia. Am J Psychiatry. 2001;158(8):1305-1313. doi:10.1176/appi.ajp.158.8.1305
9. National Alliance on Mental Illness. Clozapine. Accessed June 13, 2021. https://www.nami.org/About-Mental-Illness/Treatments/Mental-Health-Medications/Types-of-Medication/Clozapine-(Clozaril-and-FazaClo)
10. Shirazi A, Stubbs B, Gomez L, et al. Prevalence and predictors of clozapine-associated constipation: a systematic review and meta-analysis. Int J Mol Sci. 2016;17(6):863. doi:10.3390/ijms17060863
11. Cohen D, Bogers JP, van Dijk D, et al. Beyond white blood cell monitoring: screening in the initial phase of clozapine therapy. J Clin Psychiatry. 2012;73(10):1307-1312. doi:10.4088/JCP.11r06977
12. Mookhoek EJ, Meijs VM, Loonen AJ, et al. Dyspepsia in chronic psychiatric patients. Pharmacopsychiatry. 2005;38(3):125-127. doi:10.1055/s-2005-864123
13. John JP, Chengappa KN, Baker RW, et al. Assessment of changes in both weight and frequency of use of medications for the treatment of gastrointestinal symptoms among clozapine-treated patients. Ann Clin Psychiatry. 1995;7(3):119-125. doi: 10.3109/10401239509149038
14. Schwartz BJ, Frisolone JA. A case report of clozapine-induced gastric outlet obstruction. Am J Psychiatry. 1993;150(10):1563. doi:10.1176/ajp.150.10.1563a
15. Taylor D, Olofinjana O, Rahimi T. Use of antacid medication in patients receiving clozapine: a comparison with other second-generation antipsychotics. J Clin Psychopharmacol. 2010;30(4):460-461. doi:10.1097/JCP.0b013e3181e5c0f7
16. Every-Palmer S, Inns SJ, Grant E, et al. Effects of clozapine on the gut: cross-sectional study of delayed gastric emptying and small and large intestinal dysmotility. CNS Drugs. 2019;33(1):81-91. doi:10.1007/s40263-018-0587-4
17. Jung HK, Choung RS, Locke GR 3rd, et al. The incidence, prevalence, and outcomes of patients with gastroparesis in Olmsted County, Minnesota, from 1996 to 2006. Gastroenterology. 2009;136(4):1225-1233. doi: 10.1053/j.gastro.2008.12.047
18. Antunes C, Aleem A, Curtis SA. Gastroesophageal reflux disease. StatPearls Publishing. Updated July 7, 2021. Accessed December 8, 2021. https://www.ncbi.nlm.nih.gov/books/NBK441938/
19. Reddivari AKR, Mehta P. Gastroparesis. StatPearls Publishing. Updated June 30, 2021. Accessed December 8, 2021. https://www.ncbi.nlm.nih.gov/books/NBK551528/
1. Camilleri M, Chedid V, Ford AC, et al. Gastroparesis. Nat Rev Dis Primers. 2018;4(1):41. doi:10.1038/s41572-018-0038-z
2. Hamann J, Langer B, Winkler V, et al. Shared decision making for in-patients with schizophrenia. Acta Psychiatr Scand. 2006;114(4):265-273. doi: 10.1111/j.1600-0447.2006.00798.x
3. Maganti K, Onyemere K, Jones MP. Oral erythromycin and symptomatic relief of gastroparesis: a systematic review. Am J Gastroenterol. 2003;98(2):259-263. doi:10.1111/j.1572-0241.2003.07167.x
4. Taylor D. Pharmacokinetic interactions involving clozapine. Br J Psychiatry. 1997;171:109-112. doi:10.1192/bjp.171.2.109
5. Edge SC, Markowitz JS, Devane CL. Clozapine drug-drug interactions: a review of the literature. Human Psychopharmacology: Clinical and Experimental. 1997;12(1):5-20.
6. Vanasse A, Blais L, Courteau J, et al. Comparative effectiveness and safety of antipsychotic drugs in schizophrenia treatment: a real-world observational study. Acta Psychiatr Scand. 2016;134(5):374-384. doi:10.1111/acps.12621
7. Siskind D, McCartney L, Goldschlager R, et al. Clozapine v. first- and second-generation antipsychotics in treatment-refractory schizophrenia: systematic review and meta-analysis. Br J Psychiatry. 2016;209(5):385-392. doi:10.1192/bjp.bp.115.177261
8. Azorin JM, Spiegel R, Remington G, et al. A double-blind comparative study of clozapine and risperidone in the management of severe chronic schizophrenia. Am J Psychiatry. 2001;158(8):1305-1313. doi:10.1176/appi.ajp.158.8.1305
9. National Alliance on Mental Illness. Clozapine. Accessed June 13, 2021. https://www.nami.org/About-Mental-Illness/Treatments/Mental-Health-Medications/Types-of-Medication/Clozapine-(Clozaril-and-FazaClo)
10. Shirazi A, Stubbs B, Gomez L, et al. Prevalence and predictors of clozapine-associated constipation: a systematic review and meta-analysis. Int J Mol Sci. 2016;17(6):863. doi:10.3390/ijms17060863
11. Cohen D, Bogers JP, van Dijk D, et al. Beyond white blood cell monitoring: screening in the initial phase of clozapine therapy. J Clin Psychiatry. 2012;73(10):1307-1312. doi:10.4088/JCP.11r06977
12. Mookhoek EJ, Meijs VM, Loonen AJ, et al. Dyspepsia in chronic psychiatric patients. Pharmacopsychiatry. 2005;38(3):125-127. doi:10.1055/s-2005-864123
13. John JP, Chengappa KN, Baker RW, et al. Assessment of changes in both weight and frequency of use of medications for the treatment of gastrointestinal symptoms among clozapine-treated patients. Ann Clin Psychiatry. 1995;7(3):119-125. doi: 10.3109/10401239509149038
14. Schwartz BJ, Frisolone JA. A case report of clozapine-induced gastric outlet obstruction. Am J Psychiatry. 1993;150(10):1563. doi:10.1176/ajp.150.10.1563a
15. Taylor D, Olofinjana O, Rahimi T. Use of antacid medication in patients receiving clozapine: a comparison with other second-generation antipsychotics. J Clin Psychopharmacol. 2010;30(4):460-461. doi:10.1097/JCP.0b013e3181e5c0f7
16. Every-Palmer S, Inns SJ, Grant E, et al. Effects of clozapine on the gut: cross-sectional study of delayed gastric emptying and small and large intestinal dysmotility. CNS Drugs. 2019;33(1):81-91. doi:10.1007/s40263-018-0587-4
17. Jung HK, Choung RS, Locke GR 3rd, et al. The incidence, prevalence, and outcomes of patients with gastroparesis in Olmsted County, Minnesota, from 1996 to 2006. Gastroenterology. 2009;136(4):1225-1233. doi: 10.1053/j.gastro.2008.12.047
18. Antunes C, Aleem A, Curtis SA. Gastroesophageal reflux disease. StatPearls Publishing. Updated July 7, 2021. Accessed December 8, 2021. https://www.ncbi.nlm.nih.gov/books/NBK441938/
19. Reddivari AKR, Mehta P. Gastroparesis. StatPearls Publishing. Updated June 30, 2021. Accessed December 8, 2021. https://www.ncbi.nlm.nih.gov/books/NBK551528/
Is it bipolar disorder, or a complex form of PTSD?
CASE A long history of suicidality
Mr. X, age 26, who has a history of bipolar II disorder and multiple inpatient admissions, presents to a state hospital after a suicide attempt by gunshot. He reports that throughout his lifetime, he has had >20 suicide attempts, often by overdose.
Mr. X is admitted to the hospital under a temporary detention order. He is initially adherent and cooperative with his psychiatric evaluations.
HISTORY Chronic physical and emotional pain
Mr. X is single, unemployed, and lives with his mother and nephew. He was diagnosed with bipolar II disorder during adolescence and receives sertraline, 50 mg twice a day, and lamotrigine, 100 mg twice a day, to which he reports adherence. He also was taking clonazepam and zolpidem, dosages unknown.
His medical history is significant for severe childhood liver disease and inflammatory bowel disease. He dropped out of school during high school due to his multiple medical conditions, which resulted in a significantly diminished overall childhood experience, interrupted developmental trajectory, and chronic physical and emotional pain. He has never been employed and receives financial support through disability benefits. He spends his days on the internet or watching television. He reports daily cigarette and marijuana use and occasional alcohol use, but no other substance use. His mother helps manage his medical conditions and is his main support. His biological father was abusive towards his mother and absent for most of Mr. X’s life. Beyond his mother and therapist, Mr. X has minimal other interpersonal interactions, and reports feeling isolated, lonely, and frustrated.
EVALUATION Agitated and aggressive while hospitalized
Upon learning that he is being involuntarily committed, Mr. X becomes physically aggressive, makes verbal threats, and throws objects across his room. He is given diphenhydramine, 50 mg, haloperidol, 5 mg, and lorazepam, 2 mg, all of which are ordered on an as-needed basis. Mr. X is placed in an emergency restraint chair and put in seclusion. The episode resolves within an hour with reassurance and attention from the treatment team; the rapid escalation from and return to a calmer state is indicative of situational, stress-induced mood lability and impulsivity. Mr. X is counseled on maintaining safety and appropriate behavior, and is advised to ask for medication if he feels agitated or unable to control his behaviors. To maintain safe and appropriate behavior, he requires daily counseling and expectation management regarding his treatment timeline. No further aggressive incidents are noted throughout his hospitalization, and he requires only minimal use of the as-needed medications.
[polldaddy:10983392]
The authors’ observations
The least appropriate therapy for Mr. X would be exposure and response prevention, which allows patients to face their fears without the need to soothe or relieve related feelings with a compulsive act. It is designed to improve specific behavioral deficits most often associated with obsessive-compulsive disorder, a diagnosis inconsistent with Mr. X’s history and presentation. Trauma-focused CBT could facilitate healing from Mr. X’s childhood trauma/adverse childhood experiences, and DBT might help with his anger, maladaptive coping strategies, and chronic suicidality. Motivational interviewing might help with his substance use and his apparent lack of motivation for other forms of social engagement, including seeking employment.
Based on Mr. X’s history of trauma and chronic physical and emotional pain, the treatment team reevaluated him and reconsidered his original diagnosis.
Continue to: EVALUATION A closer look at the diagnosis...
EVALUATION A closer look at the diagnosis
After meeting with Mr. X, the treatment team begins to piece together a more robust picture of him. They review his childhood trauma involving his biological father, his chronic and limiting medical illnesses, and his restricted and somewhat regressive level of functioning. Further, they consider his >20 suicide attempts, numerous psychiatric hospitalizations, and mood and behavioral lability and reactivity. Based on its review, the treatment team concludes that a diagnosis of bipolar disorder II or major depressive disorder is not fully adequate to describe Mr. X’s clinical picture.
At no point during his hospitalization does Mr. X meet full criteria for a major depressive episode or display mania or hypomania. The treatment team considers posttraumatic stress disorder (PTSD) in the setting of chronic, repetitive trauma given Mr. X’s nightmares, dissociative behavior, anger, negative cognitions, and intrusive symptoms. However, not all his symptoms fall within the diagnostic criteria of PTSD. There are also elements of borderline personality disorder in Mr. X’s history, most notably his multiple suicide attempts, emotional lability, and disrupted interpersonal attachments. In this context, a diagnosis of complex PTSD (CPTSD) seems most appropriate in capturing the array of trauma-related symptoms with which he presents.
Complex PTSD
Since at least the early to mid-1990s, there has been recognition of a qualitatively distinct clinical picture that can emerge when an individual’s exposure to trauma or adversity is chronic or repetitive, causing not only familiar PTSD symptomatology but also alterations in self-perception, interpersonal functioning, and affective instability. Complex PTSD was first described by Judith Herman, MD, in 1992 as a distinct entity from PTSD.1 She theorized that PTSD derives primarily from singular traumatic events, while a distinct clinical syndrome might arise after prolonged, repeated trauma.1 A diagnosis of CPTSD might arise in situations with more chronicity than a classic single circumscribed traumatic event, such as being held in captivity, under the control of perpetrators for extended periods of time, imprisoned, or subject to prolonged sexual abuse. Herman’s description of CPTSD identifies 3 areas of psychopathology that extend beyond PTSD1:
- symptomatic refers to the complex, diffuse, and tenacious symptom presentation
- characterological focuses on the personality changes in terms of dissociation, ego-fragmentation, and identity complications
- vulnerability describes characteristic repeated harm with respect to self-mutilation or other self-injurious behaviors, and suicidality.
Taxometrics, official recognition, and controversy
Complex PTSD was proposed for inclusion in DSM-IV as “Disorders of Extreme Stress Not Otherwise Specified,” or DESNOS. Reportedly, it was interpreted as a severe presentation of PTSD, and therefore not included in the manual as a separate diagnosis.2 In contrast, ICD-10 included a CPTSD-like entity of “Enduring Personality Change After Catastrophic Event” (EPCACE). Although the existence of CPTSD as a categorically distinct diagnosis in the psychiatric mainstream has been debated and discussed for years, with many arguably unaware of its existence, clinicians and researchers specializing in trauma are well-versed in its clinical utility. As such, CPTSD was again discussed during the development of DSM-5. In an apparent attempt to balance this clinical utility with ongoing concerns about its validity as a diagnostically distinct syndrome, DSM-5 did not officially recognize CPTSD, but added several criteria to PTSD referencing changes in self-perception, affective instability, and dysphoria, as well as a dissociative subtype, effectively expanding the scope of a PTSD diagnosis to also include CPTSD symptoms when applicable. ICD-11 has taken a different direction, and officially recognizes CPTSD as a distinct diagnosis.
ICD-11 presents CPTSD as a “sibling” disorder, which it distinguishes from PTSD with high levels of dissociation, depression, and borderline personality disorder traits.3 Within this framework, the diagnosis of CPTSD requires that the PTSD criteria be met in addition to symptoms that fall into a “disturbances of self-organization” category. When parsing the symptoms of the “disturbances of self-organization” category, the overlap with borderline personality disorder symptoms is apparent.4 This overlap has given rise to yet another controversy regarding CPTSD’s categorical validity; in addition to its distinctness from PTSD, its distinctness from borderline personality disorder has also been debated. In a study examining the similarity between CPTSD and borderline personality disorder, Jowett et al5 concluded that CPTSD was associated with greater exposure to multiple traumas earlier in life and resulted in higher functional impairment than borderline personality disorder, ultimately supporting CPTSD as a separate entity with features that overlap borderline personality disorder.5 According to Ford and Courtois6 “the evidence ... suggests that a sub-group of BPD patients—who often but not always have comorbid PTSD—may be best understood and treated if CPTSD is explicitly addressed as well—and in some cases, in lieu of—BPD.”
PTSD and CPTSD may therefore both be understood to fall within a spectrum of trauma diagnoses; this paradigm postulates that there exists a wide variety of posttraumatic patient presentations, perhaps on a continuum. On the less severe side of the trauma spectrum, the symptoms traditionally seen and characterized as PTSD (such as hypervigilance, nightmares, and flashbacks) may be found, while, with increasingly severe or prolonged trauma, there may be a tendency to see more complex elements (such as dissociation, personality changes mimicking borderline personality disorder, depression, anxiety, self-injurious behavior, and suicidality).7 Nevertheless, controversy about discriminant validity still exists. A review article by Resnick et al8 argued that the existing evidence is not strong enough to support CPTSD as a standalone entity. However, Resnick et al8 agreed that a singular PTSD diagnosis has limitations, and that there is a need for more research in the field of trauma psychiatry.
Continue to: Utility of the diagnostic conceptualization...
Utility of the diagnostic conceptualization
Although the controversy surrounding the distinction of CPTSD demands categorical clarity with respect to PTSD and borderline personality disorder as a means of resolution, the diagnosis has practical applications that should not limit its use in clinical formulation or treatment planning. Comorbid diagnoses do not prevent clinicians from diagnosing and treating patients who present with complicated manifestations of trauma.9 In fact, having overlapping diagnoses would highlight the array of patient presentations that can be seen in the posttraumatic condition. Furthermore, in the pursuit of individualized care approaches, the addition of CPTSD as a diagnostic conception would allow for more integrated treatment options using a multi-modular approach.10
The addition of CPTSD as a diagnosis is helpful in determining the etiology of a patient’s presentation and therefore formulating the most appropriate treatment plan. While the 2-pronged approach of psychopharmacology and therapy is the central dogma of psychiatric care, there are many specific options to consider for each. By viewing such patients through the lens of trauma as opposed to depression and anxiety, there is a clear shift in treatment that has the potential to make more lasting impacts and progress.11
CPTSD may coexist with PTSD, but it extends beyond it to include a pleomorphic symptom picture encompassing personality changes and a high risk for repeated harm. Failure to correctly classify a patient’s presentation as a response to repetitive, prolonged trauma may result in discrimination and inappropriate or ineffective treatment recommendations.
For a comparison of the diagnostic criteria of PTSD, CPTSD, and borderline personality disorder, see Table 112, Table 2,13,14, and Table 312.

Patients with CPTSD
One of the authors (NR) has cared for several similar individuals presenting for treatment with vague diagnoses of “chronic depression and anxiety” for years, sometimes with a speculative bipolar disorder diagnosis due to situational mood swings or reactivity, and a generally poor response to both medications and psychotherapy. These patients were frustrated because none of the diagnoses seemed to fully “fit” with their pattern of symptoms or subjective experience, and treatment seemed minimally helpful. Very often, their social history revealed a variety of adversities or traumatic events, such as childhood sexual or physical abuse, a home environment plagued by domestic violence, or being raised by one or both parents with their own history of trauma, or perhaps a personality or substance use disorder. Although many of these patients’ symptom profiles aligned only partially with “typical” PTSD, they were often better captured by CPTSD, with a focus on negative self-perception and impact on close relationships. Helping the patient “connect the dots” to create a more continuous narrative, and consequently reconceptualizing the diagnosis as a complex trauma disorder, has proven effective in a number of these cases, allowing the patient to make sense of their symptoms in the context of their personal history, reducing stigma, and allowing for different avenues with medication, therapy, and self-understanding. It can also help to validate the impact of a patient’s adverse experiences and encourage a patient to view their symptoms as an understandable or even once-adaptive response to traumatic stress, rather than a sign of personal weakness or defectiveness.
TREATMENT A trauma-focused approach
Once the treatment team considersMr. X’s significant childhood trauma and reconceptualizes his behaviors through this lens, treatment is adjusted accordingly. His significant reactivity, dissociative symptoms, social impairment, and repeated suicide attempts are better understood and have more significance through a trauma lens, which provides a better explanation than a primary mood disorder.
Therapeutic interventions in the hospital are tailored according to the treatment team’s new insight. Specific DBT skills are practiced, insight-oriented therapy and motivational interviewing are used, and Mr. X and his therapist begin to explore his trauma, both from his biological father and from his intense stressors experienced because of his medical issues.
Mr. X’s mother, who is very involved in his care, is provided with education on this conceptualization and given instruction on trauma-focused therapies in the outpatient setting. While Mr. X’s medication regimen is not changed significantly, for some patients, the reformulation from a primary mood or anxiety disorder to a trauma disorder might require a change in the pharmacotherapy regimen to address behavioral symptoms such as mood reactivity or issues with sleep.
OUTCOME Decreased intensity of suicidal thoughts
By the time of discharge, Mr. X has maintained safety, with no further outbursts, and subjectively reports feeling more understood and validated. Although chronic suicidal ideation can take months or years of treatment to resolve, at the time of discharge Mr. X reports a decreased intensity of these thoughts, and no acute suicidal ideation, plan, or intent. His discharge planning emphasizes ongoing work specifically related to coping with symptoms of traumatic stress, and the involvement of his main social support in facilitating this work.
The authors’ observations
As a caveat, it may be in some cases that chronic negative affect, dysphoria, and self-perception are better understood as a comorbid depressive disorder rather than subsumed into a PTSD/ CPTSD diagnosis. Also, because situational mood instability and impulsivity are often interpreted as bipolar disorder, a history of hypomania and mania should be ruled out. In Mr. X’s case, the diagnostic reformulation did not significantly impact pharmacotherapy because the target symptoms of mood instability, irritability, anxiety, and depression remained, despite the change in diagnosis.
Although the DSM-5 PTSD criteria effectively incorporate many CPTSD elements, we argue that this inclusivity comes at the expense of appreciating CPTSD as a qualitatively distinct condition, and we prefer ICD-11’s recognition of CPTSD as a separate diagnosis that incorporates PTSD criteria but extends the definition to include negative self-concept, affect dysregulation, and interpersonal difficulties.
Related Resources
- US Department of Veterans Affairs. PTSD: National Center for PTSD. Published January 1, 2007. https://www.ptsd.va.gov/ professional/treat/essentials/complex_ptsd.asp
- Jowett S, Karatzias T, Shevlin M, et al. Differentiating symptom profiles of ICD-11 PTSD, complex PTSD, and borderline personality disorder: a latent class analysis in a multiply traumatized sample. Personality disorders: theory, research, and treatment. 2020;11(1):36.
Drug Brand Names
Clonazepam • Klonopin
Haloperidol • Haldol
Lamotrigine • Lamictal
Lorazepam • Ativan
Sertraline • Zoloft
Zolpidem • Ambien
Bottom Line
Consider a diagnosis of complex posttraumatic stress disorder (CPTSD) when providing care for patients with chronic depression and suicidality with a history of trauma or childhood adversity. This reformulation can allow clinicians to understand the contributing factors more holistically; align with the patient more effectively; appreciate past and present interpersonal, psychological, and psychosocial factors that may precipitate and perpetuate symptoms; and allow for treatment recommendations beyond those of mood and anxiety disorders.
1. Herman JL. Complex PTSD: a syndrome in survivors of prolonged and repeated trauma. J Trauma Stress. 1992;5(3):377-391.
2. Friedman MJ. Finalizing PTSD in DSM-5: getting here from there and where to go next. J Trauma Stress. 2013;26(5):548-556. doi: 10.1002/jts.21840 3. Hyland P, Shevlin M, Fyvie C, et al. Posttraumatic stress disorder and complex posttraumatic stress disorder in DSM-5 and ICD-11: clinical and behavioral correlates. J Trauma Stress. 2018; 31(12):174-180.
4. Brand B, Loewenstein R. Dissociative disorders: an overview of assessment, phenomenology and treatment. Psychiatric Times. Published 2010. Accessed October 4, 2021. https://www.researchgate.net/profile/Bethany-Brand/publication/231337464_Dissociative_Disorders_An_Overview_of_Assessment_Phenomonology_and_Treatment/links/09e415068c721ef9b5000000/Dissociative-Disorders-An-Overview-of-Assessment-Phenomonology-and-Treatment.pdf
5. Jowett S, Karatzias T, Shevlin M, et al. Differentiating symptom profiles of ICD-11 PTSD, complex PTSD, and borderline personality disorder: a latent class analysis in a multiply traumatized sample. Personality Disorders: theory, research, and treatment. 2020;11(1):36.
6. Ford JD, Courtois CA. Complex PTSD, affect dysregulation, and borderline personality disorder. Bord Personal Disord Emot Dysregul. 2014;1:9. doi.org/10.1186/2051-6673-1-9
7. van der Kolk BA. The trauma spectrum: the interaction of biological and social events in the genesis of the trauma response. J Trauma Stress. 1998;1(3):273-290.
8. Resnick PA, Bovin MJ, Calloway AL, et al. A critical evaluation of the complex PTSD literature: implications for DSM-5. J Trauma Stress. 2012;25(3);241-251.
9. Herman J. CPTSD is a distinct entity: comment on Resick et al. J Trauma Stress. 2012;25(3): 256-257.
10. Karatzias T, Cloitre M. Treating adults with complex posttraumatic stress disorder using a modular approach to treatment: rationale, evidence, and directions for future research. J Trauma Stress. 2019;32(6):870-876.
11. Perry S, Cooper AM, Michels R. The psychodynamic formulation: its purpose, structure, and clinical application. Am J Psych. 1987;144(5):543-550.
12. Diagnostic and statistical manual of mental disorders, 5th ed. American Psychiatric Association; 2013.
13. International Classification of Diseases, 11th revision. 2019; World Health Organization.
14. US Department of Veterans Affairs. PTSD: National Center for PTSD. Complex PTSD. Published January 1, 2007. Accessed October 4, 2021. https://www.ptsd.va.gov/professional/treat/essentials/complex_ptsd.asp
CASE A long history of suicidality
Mr. X, age 26, who has a history of bipolar II disorder and multiple inpatient admissions, presents to a state hospital after a suicide attempt by gunshot. He reports that throughout his lifetime, he has had >20 suicide attempts, often by overdose.
Mr. X is admitted to the hospital under a temporary detention order. He is initially adherent and cooperative with his psychiatric evaluations.
HISTORY Chronic physical and emotional pain
Mr. X is single, unemployed, and lives with his mother and nephew. He was diagnosed with bipolar II disorder during adolescence and receives sertraline, 50 mg twice a day, and lamotrigine, 100 mg twice a day, to which he reports adherence. He also was taking clonazepam and zolpidem, dosages unknown.
His medical history is significant for severe childhood liver disease and inflammatory bowel disease. He dropped out of school during high school due to his multiple medical conditions, which resulted in a significantly diminished overall childhood experience, interrupted developmental trajectory, and chronic physical and emotional pain. He has never been employed and receives financial support through disability benefits. He spends his days on the internet or watching television. He reports daily cigarette and marijuana use and occasional alcohol use, but no other substance use. His mother helps manage his medical conditions and is his main support. His biological father was abusive towards his mother and absent for most of Mr. X’s life. Beyond his mother and therapist, Mr. X has minimal other interpersonal interactions, and reports feeling isolated, lonely, and frustrated.
EVALUATION Agitated and aggressive while hospitalized
Upon learning that he is being involuntarily committed, Mr. X becomes physically aggressive, makes verbal threats, and throws objects across his room. He is given diphenhydramine, 50 mg, haloperidol, 5 mg, and lorazepam, 2 mg, all of which are ordered on an as-needed basis. Mr. X is placed in an emergency restraint chair and put in seclusion. The episode resolves within an hour with reassurance and attention from the treatment team; the rapid escalation from and return to a calmer state is indicative of situational, stress-induced mood lability and impulsivity. Mr. X is counseled on maintaining safety and appropriate behavior, and is advised to ask for medication if he feels agitated or unable to control his behaviors. To maintain safe and appropriate behavior, he requires daily counseling and expectation management regarding his treatment timeline. No further aggressive incidents are noted throughout his hospitalization, and he requires only minimal use of the as-needed medications.
[polldaddy:10983392]
The authors’ observations
The least appropriate therapy for Mr. X would be exposure and response prevention, which allows patients to face their fears without the need to soothe or relieve related feelings with a compulsive act. It is designed to improve specific behavioral deficits most often associated with obsessive-compulsive disorder, a diagnosis inconsistent with Mr. X’s history and presentation. Trauma-focused CBT could facilitate healing from Mr. X’s childhood trauma/adverse childhood experiences, and DBT might help with his anger, maladaptive coping strategies, and chronic suicidality. Motivational interviewing might help with his substance use and his apparent lack of motivation for other forms of social engagement, including seeking employment.
Based on Mr. X’s history of trauma and chronic physical and emotional pain, the treatment team reevaluated him and reconsidered his original diagnosis.
Continue to: EVALUATION A closer look at the diagnosis...
EVALUATION A closer look at the diagnosis
After meeting with Mr. X, the treatment team begins to piece together a more robust picture of him. They review his childhood trauma involving his biological father, his chronic and limiting medical illnesses, and his restricted and somewhat regressive level of functioning. Further, they consider his >20 suicide attempts, numerous psychiatric hospitalizations, and mood and behavioral lability and reactivity. Based on its review, the treatment team concludes that a diagnosis of bipolar disorder II or major depressive disorder is not fully adequate to describe Mr. X’s clinical picture.
At no point during his hospitalization does Mr. X meet full criteria for a major depressive episode or display mania or hypomania. The treatment team considers posttraumatic stress disorder (PTSD) in the setting of chronic, repetitive trauma given Mr. X’s nightmares, dissociative behavior, anger, negative cognitions, and intrusive symptoms. However, not all his symptoms fall within the diagnostic criteria of PTSD. There are also elements of borderline personality disorder in Mr. X’s history, most notably his multiple suicide attempts, emotional lability, and disrupted interpersonal attachments. In this context, a diagnosis of complex PTSD (CPTSD) seems most appropriate in capturing the array of trauma-related symptoms with which he presents.
Complex PTSD
Since at least the early to mid-1990s, there has been recognition of a qualitatively distinct clinical picture that can emerge when an individual’s exposure to trauma or adversity is chronic or repetitive, causing not only familiar PTSD symptomatology but also alterations in self-perception, interpersonal functioning, and affective instability. Complex PTSD was first described by Judith Herman, MD, in 1992 as a distinct entity from PTSD.1 She theorized that PTSD derives primarily from singular traumatic events, while a distinct clinical syndrome might arise after prolonged, repeated trauma.1 A diagnosis of CPTSD might arise in situations with more chronicity than a classic single circumscribed traumatic event, such as being held in captivity, under the control of perpetrators for extended periods of time, imprisoned, or subject to prolonged sexual abuse. Herman’s description of CPTSD identifies 3 areas of psychopathology that extend beyond PTSD1:
- symptomatic refers to the complex, diffuse, and tenacious symptom presentation
- characterological focuses on the personality changes in terms of dissociation, ego-fragmentation, and identity complications
- vulnerability describes characteristic repeated harm with respect to self-mutilation or other self-injurious behaviors, and suicidality.
Taxometrics, official recognition, and controversy
Complex PTSD was proposed for inclusion in DSM-IV as “Disorders of Extreme Stress Not Otherwise Specified,” or DESNOS. Reportedly, it was interpreted as a severe presentation of PTSD, and therefore not included in the manual as a separate diagnosis.2 In contrast, ICD-10 included a CPTSD-like entity of “Enduring Personality Change After Catastrophic Event” (EPCACE). Although the existence of CPTSD as a categorically distinct diagnosis in the psychiatric mainstream has been debated and discussed for years, with many arguably unaware of its existence, clinicians and researchers specializing in trauma are well-versed in its clinical utility. As such, CPTSD was again discussed during the development of DSM-5. In an apparent attempt to balance this clinical utility with ongoing concerns about its validity as a diagnostically distinct syndrome, DSM-5 did not officially recognize CPTSD, but added several criteria to PTSD referencing changes in self-perception, affective instability, and dysphoria, as well as a dissociative subtype, effectively expanding the scope of a PTSD diagnosis to also include CPTSD symptoms when applicable. ICD-11 has taken a different direction, and officially recognizes CPTSD as a distinct diagnosis.
ICD-11 presents CPTSD as a “sibling” disorder, which it distinguishes from PTSD with high levels of dissociation, depression, and borderline personality disorder traits.3 Within this framework, the diagnosis of CPTSD requires that the PTSD criteria be met in addition to symptoms that fall into a “disturbances of self-organization” category. When parsing the symptoms of the “disturbances of self-organization” category, the overlap with borderline personality disorder symptoms is apparent.4 This overlap has given rise to yet another controversy regarding CPTSD’s categorical validity; in addition to its distinctness from PTSD, its distinctness from borderline personality disorder has also been debated. In a study examining the similarity between CPTSD and borderline personality disorder, Jowett et al5 concluded that CPTSD was associated with greater exposure to multiple traumas earlier in life and resulted in higher functional impairment than borderline personality disorder, ultimately supporting CPTSD as a separate entity with features that overlap borderline personality disorder.5 According to Ford and Courtois6 “the evidence ... suggests that a sub-group of BPD patients—who often but not always have comorbid PTSD—may be best understood and treated if CPTSD is explicitly addressed as well—and in some cases, in lieu of—BPD.”
PTSD and CPTSD may therefore both be understood to fall within a spectrum of trauma diagnoses; this paradigm postulates that there exists a wide variety of posttraumatic patient presentations, perhaps on a continuum. On the less severe side of the trauma spectrum, the symptoms traditionally seen and characterized as PTSD (such as hypervigilance, nightmares, and flashbacks) may be found, while, with increasingly severe or prolonged trauma, there may be a tendency to see more complex elements (such as dissociation, personality changes mimicking borderline personality disorder, depression, anxiety, self-injurious behavior, and suicidality).7 Nevertheless, controversy about discriminant validity still exists. A review article by Resnick et al8 argued that the existing evidence is not strong enough to support CPTSD as a standalone entity. However, Resnick et al8 agreed that a singular PTSD diagnosis has limitations, and that there is a need for more research in the field of trauma psychiatry.
Continue to: Utility of the diagnostic conceptualization...
Utility of the diagnostic conceptualization
Although the controversy surrounding the distinction of CPTSD demands categorical clarity with respect to PTSD and borderline personality disorder as a means of resolution, the diagnosis has practical applications that should not limit its use in clinical formulation or treatment planning. Comorbid diagnoses do not prevent clinicians from diagnosing and treating patients who present with complicated manifestations of trauma.9 In fact, having overlapping diagnoses would highlight the array of patient presentations that can be seen in the posttraumatic condition. Furthermore, in the pursuit of individualized care approaches, the addition of CPTSD as a diagnostic conception would allow for more integrated treatment options using a multi-modular approach.10
The addition of CPTSD as a diagnosis is helpful in determining the etiology of a patient’s presentation and therefore formulating the most appropriate treatment plan. While the 2-pronged approach of psychopharmacology and therapy is the central dogma of psychiatric care, there are many specific options to consider for each. By viewing such patients through the lens of trauma as opposed to depression and anxiety, there is a clear shift in treatment that has the potential to make more lasting impacts and progress.11
CPTSD may coexist with PTSD, but it extends beyond it to include a pleomorphic symptom picture encompassing personality changes and a high risk for repeated harm. Failure to correctly classify a patient’s presentation as a response to repetitive, prolonged trauma may result in discrimination and inappropriate or ineffective treatment recommendations.
For a comparison of the diagnostic criteria of PTSD, CPTSD, and borderline personality disorder, see Table 112, Table 2,13,14, and Table 312.



Patients with CPTSD
One of the authors (NR) has cared for several similar individuals presenting for treatment with vague diagnoses of “chronic depression and anxiety” for years, sometimes with a speculative bipolar disorder diagnosis due to situational mood swings or reactivity, and a generally poor response to both medications and psychotherapy. These patients were frustrated because none of the diagnoses seemed to fully “fit” with their pattern of symptoms or subjective experience, and treatment seemed minimally helpful. Very often, their social history revealed a variety of adversities or traumatic events, such as childhood sexual or physical abuse, a home environment plagued by domestic violence, or being raised by one or both parents with their own history of trauma, or perhaps a personality or substance use disorder. Although many of these patients’ symptom profiles aligned only partially with “typical” PTSD, they were often better captured by CPTSD, with a focus on negative self-perception and impact on close relationships. Helping the patient “connect the dots” to create a more continuous narrative, and consequently reconceptualizing the diagnosis as a complex trauma disorder, has proven effective in a number of these cases, allowing the patient to make sense of their symptoms in the context of their personal history, reducing stigma, and allowing for different avenues with medication, therapy, and self-understanding. It can also help to validate the impact of a patient’s adverse experiences and encourage a patient to view their symptoms as an understandable or even once-adaptive response to traumatic stress, rather than a sign of personal weakness or defectiveness.
TREATMENT A trauma-focused approach
Once the treatment team considersMr. X’s significant childhood trauma and reconceptualizes his behaviors through this lens, treatment is adjusted accordingly. His significant reactivity, dissociative symptoms, social impairment, and repeated suicide attempts are better understood and have more significance through a trauma lens, which provides a better explanation than a primary mood disorder.
Therapeutic interventions in the hospital are tailored according to the treatment team’s new insight. Specific DBT skills are practiced, insight-oriented therapy and motivational interviewing are used, and Mr. X and his therapist begin to explore his trauma, both from his biological father and from his intense stressors experienced because of his medical issues.
Mr. X’s mother, who is very involved in his care, is provided with education on this conceptualization and given instruction on trauma-focused therapies in the outpatient setting. While Mr. X’s medication regimen is not changed significantly, for some patients, the reformulation from a primary mood or anxiety disorder to a trauma disorder might require a change in the pharmacotherapy regimen to address behavioral symptoms such as mood reactivity or issues with sleep.
OUTCOME Decreased intensity of suicidal thoughts
By the time of discharge, Mr. X has maintained safety, with no further outbursts, and subjectively reports feeling more understood and validated. Although chronic suicidal ideation can take months or years of treatment to resolve, at the time of discharge Mr. X reports a decreased intensity of these thoughts, and no acute suicidal ideation, plan, or intent. His discharge planning emphasizes ongoing work specifically related to coping with symptoms of traumatic stress, and the involvement of his main social support in facilitating this work.
The authors’ observations
As a caveat, it may be in some cases that chronic negative affect, dysphoria, and self-perception are better understood as a comorbid depressive disorder rather than subsumed into a PTSD/ CPTSD diagnosis. Also, because situational mood instability and impulsivity are often interpreted as bipolar disorder, a history of hypomania and mania should be ruled out. In Mr. X’s case, the diagnostic reformulation did not significantly impact pharmacotherapy because the target symptoms of mood instability, irritability, anxiety, and depression remained, despite the change in diagnosis.
Although the DSM-5 PTSD criteria effectively incorporate many CPTSD elements, we argue that this inclusivity comes at the expense of appreciating CPTSD as a qualitatively distinct condition, and we prefer ICD-11’s recognition of CPTSD as a separate diagnosis that incorporates PTSD criteria but extends the definition to include negative self-concept, affect dysregulation, and interpersonal difficulties.
Related Resources
- US Department of Veterans Affairs. PTSD: National Center for PTSD. Published January 1, 2007. https://www.ptsd.va.gov/ professional/treat/essentials/complex_ptsd.asp
- Jowett S, Karatzias T, Shevlin M, et al. Differentiating symptom profiles of ICD-11 PTSD, complex PTSD, and borderline personality disorder: a latent class analysis in a multiply traumatized sample. Personality disorders: theory, research, and treatment. 2020;11(1):36.
Drug Brand Names
Clonazepam • Klonopin
Haloperidol • Haldol
Lamotrigine • Lamictal
Lorazepam • Ativan
Sertraline • Zoloft
Zolpidem • Ambien
Bottom Line
Consider a diagnosis of complex posttraumatic stress disorder (CPTSD) when providing care for patients with chronic depression and suicidality with a history of trauma or childhood adversity. This reformulation can allow clinicians to understand the contributing factors more holistically; align with the patient more effectively; appreciate past and present interpersonal, psychological, and psychosocial factors that may precipitate and perpetuate symptoms; and allow for treatment recommendations beyond those of mood and anxiety disorders.
CASE A long history of suicidality
Mr. X, age 26, who has a history of bipolar II disorder and multiple inpatient admissions, presents to a state hospital after a suicide attempt by gunshot. He reports that throughout his lifetime, he has had >20 suicide attempts, often by overdose.
Mr. X is admitted to the hospital under a temporary detention order. He is initially adherent and cooperative with his psychiatric evaluations.
HISTORY Chronic physical and emotional pain
Mr. X is single, unemployed, and lives with his mother and nephew. He was diagnosed with bipolar II disorder during adolescence and receives sertraline, 50 mg twice a day, and lamotrigine, 100 mg twice a day, to which he reports adherence. He also was taking clonazepam and zolpidem, dosages unknown.
His medical history is significant for severe childhood liver disease and inflammatory bowel disease. He dropped out of school during high school due to his multiple medical conditions, which resulted in a significantly diminished overall childhood experience, interrupted developmental trajectory, and chronic physical and emotional pain. He has never been employed and receives financial support through disability benefits. He spends his days on the internet or watching television. He reports daily cigarette and marijuana use and occasional alcohol use, but no other substance use. His mother helps manage his medical conditions and is his main support. His biological father was abusive towards his mother and absent for most of Mr. X’s life. Beyond his mother and therapist, Mr. X has minimal other interpersonal interactions, and reports feeling isolated, lonely, and frustrated.
EVALUATION Agitated and aggressive while hospitalized
Upon learning that he is being involuntarily committed, Mr. X becomes physically aggressive, makes verbal threats, and throws objects across his room. He is given diphenhydramine, 50 mg, haloperidol, 5 mg, and lorazepam, 2 mg, all of which are ordered on an as-needed basis. Mr. X is placed in an emergency restraint chair and put in seclusion. The episode resolves within an hour with reassurance and attention from the treatment team; the rapid escalation from and return to a calmer state is indicative of situational, stress-induced mood lability and impulsivity. Mr. X is counseled on maintaining safety and appropriate behavior, and is advised to ask for medication if he feels agitated or unable to control his behaviors. To maintain safe and appropriate behavior, he requires daily counseling and expectation management regarding his treatment timeline. No further aggressive incidents are noted throughout his hospitalization, and he requires only minimal use of the as-needed medications.
[polldaddy:10983392]
The authors’ observations
The least appropriate therapy for Mr. X would be exposure and response prevention, which allows patients to face their fears without the need to soothe or relieve related feelings with a compulsive act. It is designed to improve specific behavioral deficits most often associated with obsessive-compulsive disorder, a diagnosis inconsistent with Mr. X’s history and presentation. Trauma-focused CBT could facilitate healing from Mr. X’s childhood trauma/adverse childhood experiences, and DBT might help with his anger, maladaptive coping strategies, and chronic suicidality. Motivational interviewing might help with his substance use and his apparent lack of motivation for other forms of social engagement, including seeking employment.
Based on Mr. X’s history of trauma and chronic physical and emotional pain, the treatment team reevaluated him and reconsidered his original diagnosis.
Continue to: EVALUATION A closer look at the diagnosis...
EVALUATION A closer look at the diagnosis
After meeting with Mr. X, the treatment team begins to piece together a more robust picture of him. They review his childhood trauma involving his biological father, his chronic and limiting medical illnesses, and his restricted and somewhat regressive level of functioning. Further, they consider his >20 suicide attempts, numerous psychiatric hospitalizations, and mood and behavioral lability and reactivity. Based on its review, the treatment team concludes that a diagnosis of bipolar disorder II or major depressive disorder is not fully adequate to describe Mr. X’s clinical picture.
At no point during his hospitalization does Mr. X meet full criteria for a major depressive episode or display mania or hypomania. The treatment team considers posttraumatic stress disorder (PTSD) in the setting of chronic, repetitive trauma given Mr. X’s nightmares, dissociative behavior, anger, negative cognitions, and intrusive symptoms. However, not all his symptoms fall within the diagnostic criteria of PTSD. There are also elements of borderline personality disorder in Mr. X’s history, most notably his multiple suicide attempts, emotional lability, and disrupted interpersonal attachments. In this context, a diagnosis of complex PTSD (CPTSD) seems most appropriate in capturing the array of trauma-related symptoms with which he presents.
Complex PTSD
Since at least the early to mid-1990s, there has been recognition of a qualitatively distinct clinical picture that can emerge when an individual’s exposure to trauma or adversity is chronic or repetitive, causing not only familiar PTSD symptomatology but also alterations in self-perception, interpersonal functioning, and affective instability. Complex PTSD was first described by Judith Herman, MD, in 1992 as a distinct entity from PTSD.1 She theorized that PTSD derives primarily from singular traumatic events, while a distinct clinical syndrome might arise after prolonged, repeated trauma.1 A diagnosis of CPTSD might arise in situations with more chronicity than a classic single circumscribed traumatic event, such as being held in captivity, under the control of perpetrators for extended periods of time, imprisoned, or subject to prolonged sexual abuse. Herman’s description of CPTSD identifies 3 areas of psychopathology that extend beyond PTSD1:
- symptomatic refers to the complex, diffuse, and tenacious symptom presentation
- characterological focuses on the personality changes in terms of dissociation, ego-fragmentation, and identity complications
- vulnerability describes characteristic repeated harm with respect to self-mutilation or other self-injurious behaviors, and suicidality.
Taxometrics, official recognition, and controversy
Complex PTSD was proposed for inclusion in DSM-IV as “Disorders of Extreme Stress Not Otherwise Specified,” or DESNOS. Reportedly, it was interpreted as a severe presentation of PTSD, and therefore not included in the manual as a separate diagnosis.2 In contrast, ICD-10 included a CPTSD-like entity of “Enduring Personality Change After Catastrophic Event” (EPCACE). Although the existence of CPTSD as a categorically distinct diagnosis in the psychiatric mainstream has been debated and discussed for years, with many arguably unaware of its existence, clinicians and researchers specializing in trauma are well-versed in its clinical utility. As such, CPTSD was again discussed during the development of DSM-5. In an apparent attempt to balance this clinical utility with ongoing concerns about its validity as a diagnostically distinct syndrome, DSM-5 did not officially recognize CPTSD, but added several criteria to PTSD referencing changes in self-perception, affective instability, and dysphoria, as well as a dissociative subtype, effectively expanding the scope of a PTSD diagnosis to also include CPTSD symptoms when applicable. ICD-11 has taken a different direction, and officially recognizes CPTSD as a distinct diagnosis.
ICD-11 presents CPTSD as a “sibling” disorder, which it distinguishes from PTSD with high levels of dissociation, depression, and borderline personality disorder traits.3 Within this framework, the diagnosis of CPTSD requires that the PTSD criteria be met in addition to symptoms that fall into a “disturbances of self-organization” category. When parsing the symptoms of the “disturbances of self-organization” category, the overlap with borderline personality disorder symptoms is apparent.4 This overlap has given rise to yet another controversy regarding CPTSD’s categorical validity; in addition to its distinctness from PTSD, its distinctness from borderline personality disorder has also been debated. In a study examining the similarity between CPTSD and borderline personality disorder, Jowett et al5 concluded that CPTSD was associated with greater exposure to multiple traumas earlier in life and resulted in higher functional impairment than borderline personality disorder, ultimately supporting CPTSD as a separate entity with features that overlap borderline personality disorder.5 According to Ford and Courtois6 “the evidence ... suggests that a sub-group of BPD patients—who often but not always have comorbid PTSD—may be best understood and treated if CPTSD is explicitly addressed as well—and in some cases, in lieu of—BPD.”
PTSD and CPTSD may therefore both be understood to fall within a spectrum of trauma diagnoses; this paradigm postulates that there exists a wide variety of posttraumatic patient presentations, perhaps on a continuum. On the less severe side of the trauma spectrum, the symptoms traditionally seen and characterized as PTSD (such as hypervigilance, nightmares, and flashbacks) may be found, while, with increasingly severe or prolonged trauma, there may be a tendency to see more complex elements (such as dissociation, personality changes mimicking borderline personality disorder, depression, anxiety, self-injurious behavior, and suicidality).7 Nevertheless, controversy about discriminant validity still exists. A review article by Resnick et al8 argued that the existing evidence is not strong enough to support CPTSD as a standalone entity. However, Resnick et al8 agreed that a singular PTSD diagnosis has limitations, and that there is a need for more research in the field of trauma psychiatry.
Continue to: Utility of the diagnostic conceptualization...
Utility of the diagnostic conceptualization
Although the controversy surrounding the distinction of CPTSD demands categorical clarity with respect to PTSD and borderline personality disorder as a means of resolution, the diagnosis has practical applications that should not limit its use in clinical formulation or treatment planning. Comorbid diagnoses do not prevent clinicians from diagnosing and treating patients who present with complicated manifestations of trauma.9 In fact, having overlapping diagnoses would highlight the array of patient presentations that can be seen in the posttraumatic condition. Furthermore, in the pursuit of individualized care approaches, the addition of CPTSD as a diagnostic conception would allow for more integrated treatment options using a multi-modular approach.10
The addition of CPTSD as a diagnosis is helpful in determining the etiology of a patient’s presentation and therefore formulating the most appropriate treatment plan. While the 2-pronged approach of psychopharmacology and therapy is the central dogma of psychiatric care, there are many specific options to consider for each. By viewing such patients through the lens of trauma as opposed to depression and anxiety, there is a clear shift in treatment that has the potential to make more lasting impacts and progress.11
CPTSD may coexist with PTSD, but it extends beyond it to include a pleomorphic symptom picture encompassing personality changes and a high risk for repeated harm. Failure to correctly classify a patient’s presentation as a response to repetitive, prolonged trauma may result in discrimination and inappropriate or ineffective treatment recommendations.
For a comparison of the diagnostic criteria of PTSD, CPTSD, and borderline personality disorder, see Table 112, Table 2,13,14, and Table 312.



Patients with CPTSD
One of the authors (NR) has cared for several similar individuals presenting for treatment with vague diagnoses of “chronic depression and anxiety” for years, sometimes with a speculative bipolar disorder diagnosis due to situational mood swings or reactivity, and a generally poor response to both medications and psychotherapy. These patients were frustrated because none of the diagnoses seemed to fully “fit” with their pattern of symptoms or subjective experience, and treatment seemed minimally helpful. Very often, their social history revealed a variety of adversities or traumatic events, such as childhood sexual or physical abuse, a home environment plagued by domestic violence, or being raised by one or both parents with their own history of trauma, or perhaps a personality or substance use disorder. Although many of these patients’ symptom profiles aligned only partially with “typical” PTSD, they were often better captured by CPTSD, with a focus on negative self-perception and impact on close relationships. Helping the patient “connect the dots” to create a more continuous narrative, and consequently reconceptualizing the diagnosis as a complex trauma disorder, has proven effective in a number of these cases, allowing the patient to make sense of their symptoms in the context of their personal history, reducing stigma, and allowing for different avenues with medication, therapy, and self-understanding. It can also help to validate the impact of a patient’s adverse experiences and encourage a patient to view their symptoms as an understandable or even once-adaptive response to traumatic stress, rather than a sign of personal weakness or defectiveness.
TREATMENT A trauma-focused approach
Once the treatment team considersMr. X’s significant childhood trauma and reconceptualizes his behaviors through this lens, treatment is adjusted accordingly. His significant reactivity, dissociative symptoms, social impairment, and repeated suicide attempts are better understood and have more significance through a trauma lens, which provides a better explanation than a primary mood disorder.
Therapeutic interventions in the hospital are tailored according to the treatment team’s new insight. Specific DBT skills are practiced, insight-oriented therapy and motivational interviewing are used, and Mr. X and his therapist begin to explore his trauma, both from his biological father and from his intense stressors experienced because of his medical issues.
Mr. X’s mother, who is very involved in his care, is provided with education on this conceptualization and given instruction on trauma-focused therapies in the outpatient setting. While Mr. X’s medication regimen is not changed significantly, for some patients, the reformulation from a primary mood or anxiety disorder to a trauma disorder might require a change in the pharmacotherapy regimen to address behavioral symptoms such as mood reactivity or issues with sleep.
OUTCOME Decreased intensity of suicidal thoughts
By the time of discharge, Mr. X has maintained safety, with no further outbursts, and subjectively reports feeling more understood and validated. Although chronic suicidal ideation can take months or years of treatment to resolve, at the time of discharge Mr. X reports a decreased intensity of these thoughts, and no acute suicidal ideation, plan, or intent. His discharge planning emphasizes ongoing work specifically related to coping with symptoms of traumatic stress, and the involvement of his main social support in facilitating this work.
The authors’ observations
As a caveat, it may be in some cases that chronic negative affect, dysphoria, and self-perception are better understood as a comorbid depressive disorder rather than subsumed into a PTSD/ CPTSD diagnosis. Also, because situational mood instability and impulsivity are often interpreted as bipolar disorder, a history of hypomania and mania should be ruled out. In Mr. X’s case, the diagnostic reformulation did not significantly impact pharmacotherapy because the target symptoms of mood instability, irritability, anxiety, and depression remained, despite the change in diagnosis.
Although the DSM-5 PTSD criteria effectively incorporate many CPTSD elements, we argue that this inclusivity comes at the expense of appreciating CPTSD as a qualitatively distinct condition, and we prefer ICD-11’s recognition of CPTSD as a separate diagnosis that incorporates PTSD criteria but extends the definition to include negative self-concept, affect dysregulation, and interpersonal difficulties.
Related Resources
- US Department of Veterans Affairs. PTSD: National Center for PTSD. Published January 1, 2007. https://www.ptsd.va.gov/ professional/treat/essentials/complex_ptsd.asp
- Jowett S, Karatzias T, Shevlin M, et al. Differentiating symptom profiles of ICD-11 PTSD, complex PTSD, and borderline personality disorder: a latent class analysis in a multiply traumatized sample. Personality disorders: theory, research, and treatment. 2020;11(1):36.
Drug Brand Names
Clonazepam • Klonopin
Haloperidol • Haldol
Lamotrigine • Lamictal
Lorazepam • Ativan
Sertraline • Zoloft
Zolpidem • Ambien
Bottom Line
Consider a diagnosis of complex posttraumatic stress disorder (CPTSD) when providing care for patients with chronic depression and suicidality with a history of trauma or childhood adversity. This reformulation can allow clinicians to understand the contributing factors more holistically; align with the patient more effectively; appreciate past and present interpersonal, psychological, and psychosocial factors that may precipitate and perpetuate symptoms; and allow for treatment recommendations beyond those of mood and anxiety disorders.
1. Herman JL. Complex PTSD: a syndrome in survivors of prolonged and repeated trauma. J Trauma Stress. 1992;5(3):377-391.
2. Friedman MJ. Finalizing PTSD in DSM-5: getting here from there and where to go next. J Trauma Stress. 2013;26(5):548-556. doi: 10.1002/jts.21840 3. Hyland P, Shevlin M, Fyvie C, et al. Posttraumatic stress disorder and complex posttraumatic stress disorder in DSM-5 and ICD-11: clinical and behavioral correlates. J Trauma Stress. 2018; 31(12):174-180.
4. Brand B, Loewenstein R. Dissociative disorders: an overview of assessment, phenomenology and treatment. Psychiatric Times. Published 2010. Accessed October 4, 2021. https://www.researchgate.net/profile/Bethany-Brand/publication/231337464_Dissociative_Disorders_An_Overview_of_Assessment_Phenomonology_and_Treatment/links/09e415068c721ef9b5000000/Dissociative-Disorders-An-Overview-of-Assessment-Phenomonology-and-Treatment.pdf
5. Jowett S, Karatzias T, Shevlin M, et al. Differentiating symptom profiles of ICD-11 PTSD, complex PTSD, and borderline personality disorder: a latent class analysis in a multiply traumatized sample. Personality Disorders: theory, research, and treatment. 2020;11(1):36.
6. Ford JD, Courtois CA. Complex PTSD, affect dysregulation, and borderline personality disorder. Bord Personal Disord Emot Dysregul. 2014;1:9. doi.org/10.1186/2051-6673-1-9
7. van der Kolk BA. The trauma spectrum: the interaction of biological and social events in the genesis of the trauma response. J Trauma Stress. 1998;1(3):273-290.
8. Resnick PA, Bovin MJ, Calloway AL, et al. A critical evaluation of the complex PTSD literature: implications for DSM-5. J Trauma Stress. 2012;25(3);241-251.
9. Herman J. CPTSD is a distinct entity: comment on Resick et al. J Trauma Stress. 2012;25(3): 256-257.
10. Karatzias T, Cloitre M. Treating adults with complex posttraumatic stress disorder using a modular approach to treatment: rationale, evidence, and directions for future research. J Trauma Stress. 2019;32(6):870-876.
11. Perry S, Cooper AM, Michels R. The psychodynamic formulation: its purpose, structure, and clinical application. Am J Psych. 1987;144(5):543-550.
12. Diagnostic and statistical manual of mental disorders, 5th ed. American Psychiatric Association; 2013.
13. International Classification of Diseases, 11th revision. 2019; World Health Organization.
14. US Department of Veterans Affairs. PTSD: National Center for PTSD. Complex PTSD. Published January 1, 2007. Accessed October 4, 2021. https://www.ptsd.va.gov/professional/treat/essentials/complex_ptsd.asp
1. Herman JL. Complex PTSD: a syndrome in survivors of prolonged and repeated trauma. J Trauma Stress. 1992;5(3):377-391.
2. Friedman MJ. Finalizing PTSD in DSM-5: getting here from there and where to go next. J Trauma Stress. 2013;26(5):548-556. doi: 10.1002/jts.21840 3. Hyland P, Shevlin M, Fyvie C, et al. Posttraumatic stress disorder and complex posttraumatic stress disorder in DSM-5 and ICD-11: clinical and behavioral correlates. J Trauma Stress. 2018; 31(12):174-180.
4. Brand B, Loewenstein R. Dissociative disorders: an overview of assessment, phenomenology and treatment. Psychiatric Times. Published 2010. Accessed October 4, 2021. https://www.researchgate.net/profile/Bethany-Brand/publication/231337464_Dissociative_Disorders_An_Overview_of_Assessment_Phenomonology_and_Treatment/links/09e415068c721ef9b5000000/Dissociative-Disorders-An-Overview-of-Assessment-Phenomonology-and-Treatment.pdf
5. Jowett S, Karatzias T, Shevlin M, et al. Differentiating symptom profiles of ICD-11 PTSD, complex PTSD, and borderline personality disorder: a latent class analysis in a multiply traumatized sample. Personality Disorders: theory, research, and treatment. 2020;11(1):36.
6. Ford JD, Courtois CA. Complex PTSD, affect dysregulation, and borderline personality disorder. Bord Personal Disord Emot Dysregul. 2014;1:9. doi.org/10.1186/2051-6673-1-9
7. van der Kolk BA. The trauma spectrum: the interaction of biological and social events in the genesis of the trauma response. J Trauma Stress. 1998;1(3):273-290.
8. Resnick PA, Bovin MJ, Calloway AL, et al. A critical evaluation of the complex PTSD literature: implications for DSM-5. J Trauma Stress. 2012;25(3);241-251.
9. Herman J. CPTSD is a distinct entity: comment on Resick et al. J Trauma Stress. 2012;25(3): 256-257.
10. Karatzias T, Cloitre M. Treating adults with complex posttraumatic stress disorder using a modular approach to treatment: rationale, evidence, and directions for future research. J Trauma Stress. 2019;32(6):870-876.
11. Perry S, Cooper AM, Michels R. The psychodynamic formulation: its purpose, structure, and clinical application. Am J Psych. 1987;144(5):543-550.
12. Diagnostic and statistical manual of mental disorders, 5th ed. American Psychiatric Association; 2013.
13. International Classification of Diseases, 11th revision. 2019; World Health Organization.
14. US Department of Veterans Affairs. PTSD: National Center for PTSD. Complex PTSD. Published January 1, 2007. Accessed October 4, 2021. https://www.ptsd.va.gov/professional/treat/essentials/complex_ptsd.asp
Persistent altered mental status
CASE Sluggish, weak, and incoherent
Mr. O, age 24, who has a history of schizophrenia and obesity, presents to the emergency department (ED) for altered mental status (AMS). His mother reports that he has been sluggish, weak, incoherent, had no appetite, and that on the day before admission, he was drinking excessive amounts of water and urinating every 10 minutes.
HISTORY Multiple ineffective antipsychotics
Mr. O was diagnosed with schizophrenia at age 21 and struggled with medication adherence, which resulted in multiple hospitalizations for stabilization. Trials of haloperidol, risperidone, paliperidone palmitate, and valproic acid had been ineffective. At the time of admission, his psychotropic medication regimen is fluphenazine decanoate, 25 mg injection every 2 weeks; clozapine, 50 mg/d; lithium carbonate, 300 mg twice a day; benztropine, 2 mg every night; and trazodone, 50 mg every night.
EVALUATION Fever, tachycardia, and diabetic ketoacidosis
Upon arrival to the ED, Mr. O is obtunded, unable to follow commands, and does not respond to painful stimuli. On physical exam, he has a fever of 38.4°C (reference range 35.1°C to 37.9°C); tachycardia with a heart rate of 142 beats per minute (bpm) (reference range 60 to 100); tachypnea with a respiratory rate of 35 breaths per minute (reference range 12 to 20); a blood pressure of 116/76 mmHg (reference range 90/60 to 130/80); and hypoxemia with an oxygen saturation of 90% on room air (reference range 94% to 100%).
Mr. O is admitted to the hospital and his laboratory workup indicates diabetic ketoacidosis (DKA), with a glucose of 1,700 mg/dL; anion gap of 30 (reference range 4 to 12 mmol/L); pH 7.04 (reference range 7.32 to 7.42); serum bicarbonate 6 (reference range 20 to 24 mEq/L); beta-hydroxybutyrate 11.04 (reference range 0 to 0.27 mmol/L); urine ketones, serum osmolality 407 (reference range 280 to 300 mOsm/kg); and an elevated white blood cell count of 18.4 (reference range 4.5 to 11.0 × 109/L). A CT scan of the head is negative for acute pathology.
Initially, all psychotropic medications are held. On Day 3 of hospitalization, psychiatry is consulted and clozapine, 50 mg/d; lithium, 300 mg/d; and benztropine, 1 mg at night, are restarted; however, fluphenazine decanoate and trazodone are held. The team recommends IV haloperidol, 2 mg as needed for agitation; however, it is never administered.
Imaging rules out deep vein thrombosis, cardiac dysfunction, and stroke, but a CT chest scan is notable for bilateral lung infiltrates, which suggests aspiration pneumonia.
Mr. O is diagnosed with diabetes, complicated by DKA, and is treated in the intensive care unit (ICU). Despite resolution of the DKA, he remains altered with fever and tachycardia.
Continue to: On Day 6 of hospitalization...
On Day 6 of hospitalization, Mr. O continues to be tachycardic and obtunded with nuchal rigidity. The team decides to transfer Mr. O to another hospital for a higher level of care and continued workup of his persistent AMS.
Immediately upon arrival at the second hospital, infectious disease and neurology teams are consulted for further evaluation. Mr. O’s AMS continues despite no clear signs of infection or other neurologic insults.
[polldaddy:10930631]
The authors’ observations
Based on Mr. O’s psychiatric history and laboratory results, the first medical team concluded his initial AMS was likely secondary to DKA; however, the AMS continued after the DKA resolved. At the second hospital, Mr. O’s treatment team continued to dig for answers.
EVALUATION Exploring the differential diagnosis
At the second hospital, Mr. O is admitted to the ICU with fever (37.8°C), tachycardia (120 bpm), tachypnea, withdrawal from painful stimuli, decreased reflexes, and muscle rigidity, including clenched jaw. The differential diagnoses include meningitis, sepsis from aspiration pneumonia, severe metabolic encephalopathy with prolonged recovery, central pontine myelinolysis, anoxic brain injury, and subclinical seizures.
Empiric vancomycin, 1.75 g every 12 hours; ceftriaxone, 2 g/d; and acyclovir, 900 mg every 8 hours are started for meningoencephalitis, and all psychotropic medications are discontinued. Case reports have documented a relationship between hyperglycemic hyperosmolar syndrome (HHS) and malignant hyperthermia in rare cases1; however, HHS is ruled out based on Mr. O’s laboratory results.A lumbar puncture and imaging rules out CNS infection. Antibiotic treatment is narrowed to ampicillin-sulbactam due to Mr. O’s prior CT chest showing concern for aspiration pneumonia. An MRI of the brain rules out central pontine myelinolysis, acute stroke, and anoxic brain injury, and an EEG shows nonspecific encephalopathy. On Day 10 of hospitalization, a neurologic exam shows flaccid paralysis and bilateral clonus, and Mr. O is mute. On Day 14 of hospitalization, his fever resolves, and his blood cultures are negative. On Day 15 of hospitalization, Mr. O’s creatine kinase (CK) level is elevated at 1,308 U/L (reference range 26 to 192 U/L), suggesting rhabdomyolysis.
Continue to: Given the neurologic exam findings...
Given the neurologic exam findings, and the limited evidence of infection, the differential diagnosis for Mr. O’s AMS is broadened to include catatonia, neuroleptic malignant syndrome (NMS), serotonin syndrome, and autoimmune encephalitis. The psychiatry team evaluates Mr. O for catatonia. He scores 14 on the Bush-Francis Catatonia Rating Scale, with findings of immobility/stupor, mutism, staring, autonomic instability, and withdrawal indicating the presence of catatonia.2
The authors’ observations
When Mr. O was transferred to the second hospital, the primary concern was to rule out meningitis due to his unstable vitals, obtunded mental state, and nuchal rigidity. A comprehensive infectious workup, including lumbar puncture, was imperative because infection can not only lead to AMS, but also precipitate episodes of DKA. Mr. O’s persistently abnormal vital signs indicated an underlying process may have been missed by focusing on treating DKA.
TREATMENT Finally, the diagnosis is established
A lorazepam challenge is performed, and Mr. O receives 4 mg of lorazepam over 24 hours with little change in his catatonia symptoms. Given his persistent fever, tachycardia, and an elevated CK levels in the context of recent exposure to antipsychotic medications, Mr. O is diagnosed with NMS (Table 13,4 ) and is started on bromocriptine, 5 mg 3 times daily.
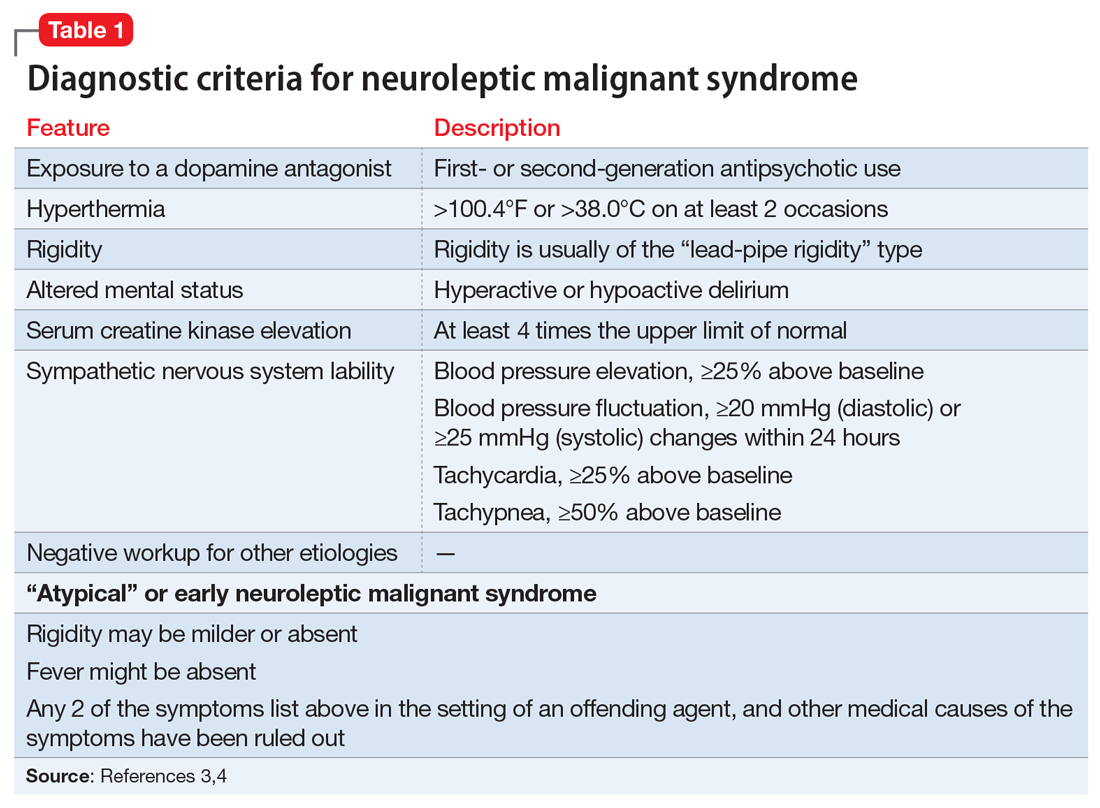
[polldaddy:10930632]
The authors’ observations
Mr. O’s complicated medical state—starting with DKA, halting the use of antipsychotic medications, and the suspicion of catatonia due to his history of schizophrenia—all distracted from the ultimate diagnosis of NMS as the cause of his enduring AMS and autonomic instability. Catatonia and NMS have overlapping symptomatology, including rigidity, autonomic instability, and stupor, which make the diagnosis of either condition complicated. A positive lorazepam test to diagnose catatonia is defined as a marked reduction in catatonia symptoms (typically a 50% reduction) as measured on a standardized rating scale.5 However, a negative lorazepam challenge does not definitely rule out catatonia because some cases are resistant to benzodiazepines.6
NMS risk factors relevant in this case include male sex, young age, acute medical illness, dehydration, and exposure to multiple psychotropic medications, including 2 antipsychotics, clozapine and fluphenazine.7 DKA is especially pertinent due to its acute onset and cause of significant dehydration. NMS can occur at any point of antipsychotic exposure, although the risk is highest during the initial weeks of treatment and during dosage changes. Unfortunately, Mr. O’s treatment team was unable to determine whether his medication had been recently changed, so it is not known what role this may have played in the development of NMS. Although first-generation antipsychotics are considered more likely to cause NMS, second-generation antipsychotics (SGAs) dominate the treatment of schizophrenia and bipolar disorder, and these medications also can cause NMS.8 As occurred in this case, long-acting injectable antipsychotics can be easily forgotten when not administered in the hospital, and their presence in the body persists for weeks. For example, the half-life of fluphenazine decanoate is approximately 10 days, and the half-life of haloperidol decanoate is 21 days.9
Continue to: OUTCOME Improvement with bromocriptine
OUTCOME Improvement with bromocriptine
After 4 days of bromocriptine, 5 mg 3 times daily, Mr. O is more alert, able to say “hello,” and can follow 1-step commands. By Day 26 of hospitalization, his CK levels decrease to 296 U/L, his CSF autoimmune panel is negative, and he is able to participate in physical therapy. After failing multiple swallow tests, Mr. O requires a percutaneous endoscopic gastrostomy (PEG) tube. He is discharged from the hospital to a long-term acute care facility with the plan to taper bromocriptine and restart a psychotropic regimen with his outpatient psychiatrist. At the time of discharge, he is able to sit at the edge of the bed independently, state his name, and respond to questions with multiple-word answers.
[polldaddy:10930633]
The authors’ observations
The most common pharmacologic treatments for NMS are dantrolene, bromocriptine, benzodiazepines (lorazepam or diazepam), and amantadine.3 Mild cases of NMS should be treated with discontinuation of all antipsychotics, supportive care, and benzodiazepines.3 Bromocriptine or amantadine are more appropriate for moderate cases and dantrolene for severe cases of NMS.3 All antipsychotics should be discontinued while a patient is experiencing an episode of NMS; however, once the NMS has resolved, clinicians must thoroughly evaluate the risks and benefits of restarting antipsychotic medication. After a patient has experienced an episode of NMS, clinicians generally should avoid prescribing the agent(s) that caused NMS and long-acting injections, and slowly titrate a low-potency SGA such as quetiapine.10Table 23,11,12 outlines the pharmacologic treatment of NMS.
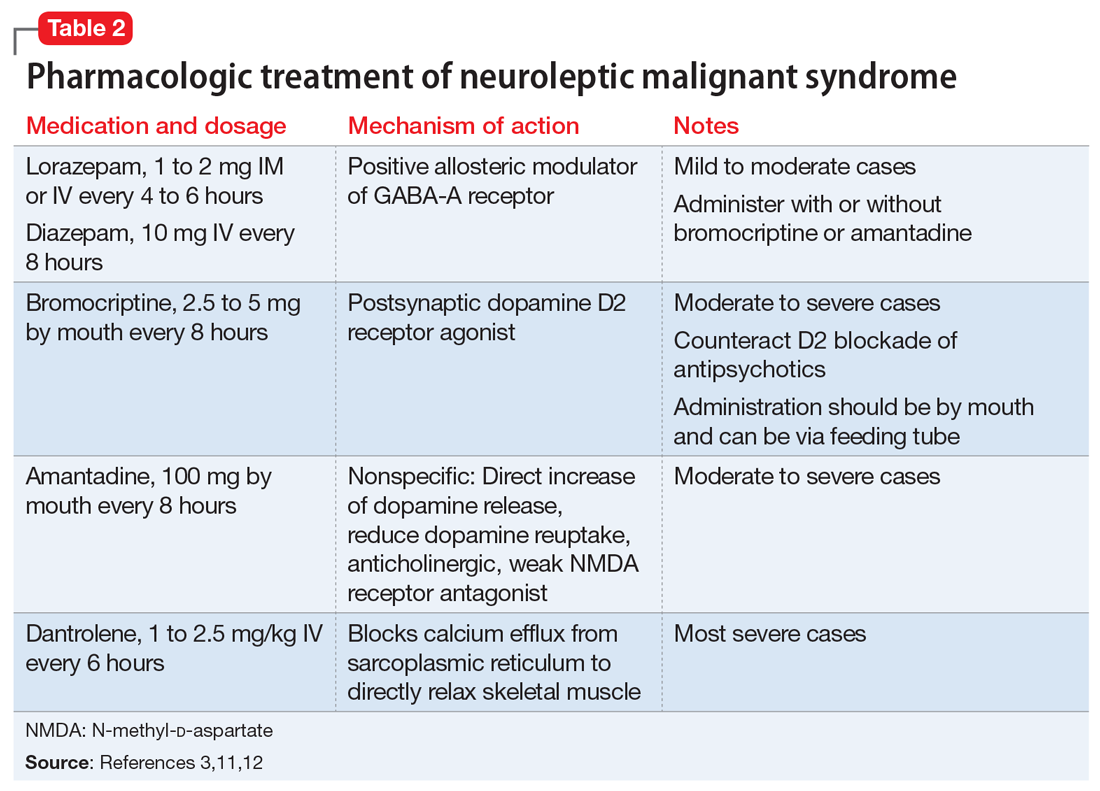
Bottom Line
Neuroleptic malignant syndrome (NMS) should always be part of the differential diagnosis in patients with mental illness and altered mental status. The risk of NMS is especially high in patients with acute medical illness and exposure to antipsychotic medications.
Related Resource
- Turner AH, Kim JJ, McCarron RM. Differentiating serotonin syndrome and neuroleptic malignant syndrome. Current Psychiatry. 2019;18(2):30-36.
Drug Brand Names
Acyclovir • Zovirax
Amantadine • Gocovri
Ampicillin-sulbactam • Unasyn
Aripiprazole • Abilify Maintena
Benztropine • Cogentin
Bromocriptine • Cycloset, Parlodel
Ceftriaxone • Rocephin
Clozapine • Clozaril
Dantrolene • Dantrium
Diazepam • Valium
Haloperidol • Haldol
Lithium • Eskalith, Lithobid
Lorazepam • Ativan
Paliperidone palmitate • Invega Sustenna
Quetiapine • Seroquel
Risperidone • Risperdal
Valproate sodium • Depakote
Trazodone • Oleptro
Vancomycin • Vancocin
1. Zeitler P, Haqq A, Rosenbloom A, et al. Hyperglycemic hyperosmolar syndrome in children: pathophysiological considerations and suggested guidelines for treatment. J Pediatr. 2011;158(1):9-14.e1-2. doi: 10.1016/j.jpeds.2010.09.048
2. Francis A. Catatonia: diagnosis, classification, and treatment. Curr Psychiatry Rep. 2010;12(3):180-185. doi: 10.1007/s11920-010-0113-y
3. Pileggi DJ, Cook AM. Neuroleptic malignant syndrome. Ann Pharmacother. 2016;50(11):973-981. doi:10.1177/1060028016657553
4. Gurrera RJ, Caroff SN, Cohen A, et al. An international consensus study of neuroleptic malignant syndrome diagnostic criteria using the Delphi method. J Clin Psychiatry. 2011;72(9):1222-1228. doi:10.4088/JCP.10m06438
5. Sienaert P, Dhossche DM, Vancampfort D, et al. A clinical review of the treatment of catatonia. Front Psychiatry. 2014;5:181. doi:10.3389/fpsyt.2014.00181
6. Daniels J. Catatonia: clinical aspects and neurobiological correlates. J Neuropsychiatry Clin Neurosci. 2009;21(4):371-380. doi:10.1176/jnp.2009.21.4.371
7. Bhanushali MJ, Tuite PJ. The evaluation and management of patients with neuroleptic malignant syndrome. Neurol Clin. 2004;22(2):389-411. doi:10.1016/j.ncl.2003.12.006
8. Tse L, Barr AM, Scarapicchia V, et al. Neuroleptic malignant syndrome: a review from a clinically oriented perspective. Curr Neuropharmacol. 2015;13(3):395-406. doi:10.2174/1570159x13999150424113345
9. Correll CU, Kim E, Sliwa JK, et al. Pharmacokinetic characteristics of long-acting injectable antipsychotics for schizophrenia: an overview. CNS Drugs. 2021;35(1):39-59. doi:10.1007/s40263-020-00779-5
10. Strawn JR, Keck PE Jr, Caroff SN. Neuroleptic malignant syndrome. Am J Psychiatry. 2007;164(6):870-876. doi:10.1176/ajp.2007.164.6.870
11. Griffin CE 3rd, Kaye AM, Bueno FR, et al. Benzodiazepine pharmacology and central nervous system-mediated effects. Ochsner J. 2013;13(2):214-223.
12. Reulbach U, Dütsch C, Biermann T, et al. Managing an effective treatment for neuroleptic malignant syndrome. Crit Care. 2007;11(1):R4. doi:10.1186/cc5148
CASE Sluggish, weak, and incoherent
Mr. O, age 24, who has a history of schizophrenia and obesity, presents to the emergency department (ED) for altered mental status (AMS). His mother reports that he has been sluggish, weak, incoherent, had no appetite, and that on the day before admission, he was drinking excessive amounts of water and urinating every 10 minutes.
HISTORY Multiple ineffective antipsychotics
Mr. O was diagnosed with schizophrenia at age 21 and struggled with medication adherence, which resulted in multiple hospitalizations for stabilization. Trials of haloperidol, risperidone, paliperidone palmitate, and valproic acid had been ineffective. At the time of admission, his psychotropic medication regimen is fluphenazine decanoate, 25 mg injection every 2 weeks; clozapine, 50 mg/d; lithium carbonate, 300 mg twice a day; benztropine, 2 mg every night; and trazodone, 50 mg every night.
EVALUATION Fever, tachycardia, and diabetic ketoacidosis
Upon arrival to the ED, Mr. O is obtunded, unable to follow commands, and does not respond to painful stimuli. On physical exam, he has a fever of 38.4°C (reference range 35.1°C to 37.9°C); tachycardia with a heart rate of 142 beats per minute (bpm) (reference range 60 to 100); tachypnea with a respiratory rate of 35 breaths per minute (reference range 12 to 20); a blood pressure of 116/76 mmHg (reference range 90/60 to 130/80); and hypoxemia with an oxygen saturation of 90% on room air (reference range 94% to 100%).
Mr. O is admitted to the hospital and his laboratory workup indicates diabetic ketoacidosis (DKA), with a glucose of 1,700 mg/dL; anion gap of 30 (reference range 4 to 12 mmol/L); pH 7.04 (reference range 7.32 to 7.42); serum bicarbonate 6 (reference range 20 to 24 mEq/L); beta-hydroxybutyrate 11.04 (reference range 0 to 0.27 mmol/L); urine ketones, serum osmolality 407 (reference range 280 to 300 mOsm/kg); and an elevated white blood cell count of 18.4 (reference range 4.5 to 11.0 × 109/L). A CT scan of the head is negative for acute pathology.
Initially, all psychotropic medications are held. On Day 3 of hospitalization, psychiatry is consulted and clozapine, 50 mg/d; lithium, 300 mg/d; and benztropine, 1 mg at night, are restarted; however, fluphenazine decanoate and trazodone are held. The team recommends IV haloperidol, 2 mg as needed for agitation; however, it is never administered.
Imaging rules out deep vein thrombosis, cardiac dysfunction, and stroke, but a CT chest scan is notable for bilateral lung infiltrates, which suggests aspiration pneumonia.
Mr. O is diagnosed with diabetes, complicated by DKA, and is treated in the intensive care unit (ICU). Despite resolution of the DKA, he remains altered with fever and tachycardia.
Continue to: On Day 6 of hospitalization...
On Day 6 of hospitalization, Mr. O continues to be tachycardic and obtunded with nuchal rigidity. The team decides to transfer Mr. O to another hospital for a higher level of care and continued workup of his persistent AMS.
Immediately upon arrival at the second hospital, infectious disease and neurology teams are consulted for further evaluation. Mr. O’s AMS continues despite no clear signs of infection or other neurologic insults.
[polldaddy:10930631]
The authors’ observations
Based on Mr. O’s psychiatric history and laboratory results, the first medical team concluded his initial AMS was likely secondary to DKA; however, the AMS continued after the DKA resolved. At the second hospital, Mr. O’s treatment team continued to dig for answers.
EVALUATION Exploring the differential diagnosis
At the second hospital, Mr. O is admitted to the ICU with fever (37.8°C), tachycardia (120 bpm), tachypnea, withdrawal from painful stimuli, decreased reflexes, and muscle rigidity, including clenched jaw. The differential diagnoses include meningitis, sepsis from aspiration pneumonia, severe metabolic encephalopathy with prolonged recovery, central pontine myelinolysis, anoxic brain injury, and subclinical seizures.
Empiric vancomycin, 1.75 g every 12 hours; ceftriaxone, 2 g/d; and acyclovir, 900 mg every 8 hours are started for meningoencephalitis, and all psychotropic medications are discontinued. Case reports have documented a relationship between hyperglycemic hyperosmolar syndrome (HHS) and malignant hyperthermia in rare cases1; however, HHS is ruled out based on Mr. O’s laboratory results.A lumbar puncture and imaging rules out CNS infection. Antibiotic treatment is narrowed to ampicillin-sulbactam due to Mr. O’s prior CT chest showing concern for aspiration pneumonia. An MRI of the brain rules out central pontine myelinolysis, acute stroke, and anoxic brain injury, and an EEG shows nonspecific encephalopathy. On Day 10 of hospitalization, a neurologic exam shows flaccid paralysis and bilateral clonus, and Mr. O is mute. On Day 14 of hospitalization, his fever resolves, and his blood cultures are negative. On Day 15 of hospitalization, Mr. O’s creatine kinase (CK) level is elevated at 1,308 U/L (reference range 26 to 192 U/L), suggesting rhabdomyolysis.
Continue to: Given the neurologic exam findings...
Given the neurologic exam findings, and the limited evidence of infection, the differential diagnosis for Mr. O’s AMS is broadened to include catatonia, neuroleptic malignant syndrome (NMS), serotonin syndrome, and autoimmune encephalitis. The psychiatry team evaluates Mr. O for catatonia. He scores 14 on the Bush-Francis Catatonia Rating Scale, with findings of immobility/stupor, mutism, staring, autonomic instability, and withdrawal indicating the presence of catatonia.2
The authors’ observations
When Mr. O was transferred to the second hospital, the primary concern was to rule out meningitis due to his unstable vitals, obtunded mental state, and nuchal rigidity. A comprehensive infectious workup, including lumbar puncture, was imperative because infection can not only lead to AMS, but also precipitate episodes of DKA. Mr. O’s persistently abnormal vital signs indicated an underlying process may have been missed by focusing on treating DKA.
TREATMENT Finally, the diagnosis is established
A lorazepam challenge is performed, and Mr. O receives 4 mg of lorazepam over 24 hours with little change in his catatonia symptoms. Given his persistent fever, tachycardia, and an elevated CK levels in the context of recent exposure to antipsychotic medications, Mr. O is diagnosed with NMS (Table 13,4 ) and is started on bromocriptine, 5 mg 3 times daily.

[polldaddy:10930632]
The authors’ observations
Mr. O’s complicated medical state—starting with DKA, halting the use of antipsychotic medications, and the suspicion of catatonia due to his history of schizophrenia—all distracted from the ultimate diagnosis of NMS as the cause of his enduring AMS and autonomic instability. Catatonia and NMS have overlapping symptomatology, including rigidity, autonomic instability, and stupor, which make the diagnosis of either condition complicated. A positive lorazepam test to diagnose catatonia is defined as a marked reduction in catatonia symptoms (typically a 50% reduction) as measured on a standardized rating scale.5 However, a negative lorazepam challenge does not definitely rule out catatonia because some cases are resistant to benzodiazepines.6
NMS risk factors relevant in this case include male sex, young age, acute medical illness, dehydration, and exposure to multiple psychotropic medications, including 2 antipsychotics, clozapine and fluphenazine.7 DKA is especially pertinent due to its acute onset and cause of significant dehydration. NMS can occur at any point of antipsychotic exposure, although the risk is highest during the initial weeks of treatment and during dosage changes. Unfortunately, Mr. O’s treatment team was unable to determine whether his medication had been recently changed, so it is not known what role this may have played in the development of NMS. Although first-generation antipsychotics are considered more likely to cause NMS, second-generation antipsychotics (SGAs) dominate the treatment of schizophrenia and bipolar disorder, and these medications also can cause NMS.8 As occurred in this case, long-acting injectable antipsychotics can be easily forgotten when not administered in the hospital, and their presence in the body persists for weeks. For example, the half-life of fluphenazine decanoate is approximately 10 days, and the half-life of haloperidol decanoate is 21 days.9
Continue to: OUTCOME Improvement with bromocriptine
OUTCOME Improvement with bromocriptine
After 4 days of bromocriptine, 5 mg 3 times daily, Mr. O is more alert, able to say “hello,” and can follow 1-step commands. By Day 26 of hospitalization, his CK levels decrease to 296 U/L, his CSF autoimmune panel is negative, and he is able to participate in physical therapy. After failing multiple swallow tests, Mr. O requires a percutaneous endoscopic gastrostomy (PEG) tube. He is discharged from the hospital to a long-term acute care facility with the plan to taper bromocriptine and restart a psychotropic regimen with his outpatient psychiatrist. At the time of discharge, he is able to sit at the edge of the bed independently, state his name, and respond to questions with multiple-word answers.
[polldaddy:10930633]
The authors’ observations
The most common pharmacologic treatments for NMS are dantrolene, bromocriptine, benzodiazepines (lorazepam or diazepam), and amantadine.3 Mild cases of NMS should be treated with discontinuation of all antipsychotics, supportive care, and benzodiazepines.3 Bromocriptine or amantadine are more appropriate for moderate cases and dantrolene for severe cases of NMS.3 All antipsychotics should be discontinued while a patient is experiencing an episode of NMS; however, once the NMS has resolved, clinicians must thoroughly evaluate the risks and benefits of restarting antipsychotic medication. After a patient has experienced an episode of NMS, clinicians generally should avoid prescribing the agent(s) that caused NMS and long-acting injections, and slowly titrate a low-potency SGA such as quetiapine.10Table 23,11,12 outlines the pharmacologic treatment of NMS.

Bottom Line
Neuroleptic malignant syndrome (NMS) should always be part of the differential diagnosis in patients with mental illness and altered mental status. The risk of NMS is especially high in patients with acute medical illness and exposure to antipsychotic medications.
Related Resource
- Turner AH, Kim JJ, McCarron RM. Differentiating serotonin syndrome and neuroleptic malignant syndrome. Current Psychiatry. 2019;18(2):30-36.
Drug Brand Names
Acyclovir • Zovirax
Amantadine • Gocovri
Ampicillin-sulbactam • Unasyn
Aripiprazole • Abilify Maintena
Benztropine • Cogentin
Bromocriptine • Cycloset, Parlodel
Ceftriaxone • Rocephin
Clozapine • Clozaril
Dantrolene • Dantrium
Diazepam • Valium
Haloperidol • Haldol
Lithium • Eskalith, Lithobid
Lorazepam • Ativan
Paliperidone palmitate • Invega Sustenna
Quetiapine • Seroquel
Risperidone • Risperdal
Valproate sodium • Depakote
Trazodone • Oleptro
Vancomycin • Vancocin
CASE Sluggish, weak, and incoherent
Mr. O, age 24, who has a history of schizophrenia and obesity, presents to the emergency department (ED) for altered mental status (AMS). His mother reports that he has been sluggish, weak, incoherent, had no appetite, and that on the day before admission, he was drinking excessive amounts of water and urinating every 10 minutes.
HISTORY Multiple ineffective antipsychotics
Mr. O was diagnosed with schizophrenia at age 21 and struggled with medication adherence, which resulted in multiple hospitalizations for stabilization. Trials of haloperidol, risperidone, paliperidone palmitate, and valproic acid had been ineffective. At the time of admission, his psychotropic medication regimen is fluphenazine decanoate, 25 mg injection every 2 weeks; clozapine, 50 mg/d; lithium carbonate, 300 mg twice a day; benztropine, 2 mg every night; and trazodone, 50 mg every night.
EVALUATION Fever, tachycardia, and diabetic ketoacidosis
Upon arrival to the ED, Mr. O is obtunded, unable to follow commands, and does not respond to painful stimuli. On physical exam, he has a fever of 38.4°C (reference range 35.1°C to 37.9°C); tachycardia with a heart rate of 142 beats per minute (bpm) (reference range 60 to 100); tachypnea with a respiratory rate of 35 breaths per minute (reference range 12 to 20); a blood pressure of 116/76 mmHg (reference range 90/60 to 130/80); and hypoxemia with an oxygen saturation of 90% on room air (reference range 94% to 100%).
Mr. O is admitted to the hospital and his laboratory workup indicates diabetic ketoacidosis (DKA), with a glucose of 1,700 mg/dL; anion gap of 30 (reference range 4 to 12 mmol/L); pH 7.04 (reference range 7.32 to 7.42); serum bicarbonate 6 (reference range 20 to 24 mEq/L); beta-hydroxybutyrate 11.04 (reference range 0 to 0.27 mmol/L); urine ketones, serum osmolality 407 (reference range 280 to 300 mOsm/kg); and an elevated white blood cell count of 18.4 (reference range 4.5 to 11.0 × 109/L). A CT scan of the head is negative for acute pathology.
Initially, all psychotropic medications are held. On Day 3 of hospitalization, psychiatry is consulted and clozapine, 50 mg/d; lithium, 300 mg/d; and benztropine, 1 mg at night, are restarted; however, fluphenazine decanoate and trazodone are held. The team recommends IV haloperidol, 2 mg as needed for agitation; however, it is never administered.
Imaging rules out deep vein thrombosis, cardiac dysfunction, and stroke, but a CT chest scan is notable for bilateral lung infiltrates, which suggests aspiration pneumonia.
Mr. O is diagnosed with diabetes, complicated by DKA, and is treated in the intensive care unit (ICU). Despite resolution of the DKA, he remains altered with fever and tachycardia.
Continue to: On Day 6 of hospitalization...
On Day 6 of hospitalization, Mr. O continues to be tachycardic and obtunded with nuchal rigidity. The team decides to transfer Mr. O to another hospital for a higher level of care and continued workup of his persistent AMS.
Immediately upon arrival at the second hospital, infectious disease and neurology teams are consulted for further evaluation. Mr. O’s AMS continues despite no clear signs of infection or other neurologic insults.
[polldaddy:10930631]
The authors’ observations
Based on Mr. O’s psychiatric history and laboratory results, the first medical team concluded his initial AMS was likely secondary to DKA; however, the AMS continued after the DKA resolved. At the second hospital, Mr. O’s treatment team continued to dig for answers.
EVALUATION Exploring the differential diagnosis
At the second hospital, Mr. O is admitted to the ICU with fever (37.8°C), tachycardia (120 bpm), tachypnea, withdrawal from painful stimuli, decreased reflexes, and muscle rigidity, including clenched jaw. The differential diagnoses include meningitis, sepsis from aspiration pneumonia, severe metabolic encephalopathy with prolonged recovery, central pontine myelinolysis, anoxic brain injury, and subclinical seizures.
Empiric vancomycin, 1.75 g every 12 hours; ceftriaxone, 2 g/d; and acyclovir, 900 mg every 8 hours are started for meningoencephalitis, and all psychotropic medications are discontinued. Case reports have documented a relationship between hyperglycemic hyperosmolar syndrome (HHS) and malignant hyperthermia in rare cases1; however, HHS is ruled out based on Mr. O’s laboratory results.A lumbar puncture and imaging rules out CNS infection. Antibiotic treatment is narrowed to ampicillin-sulbactam due to Mr. O’s prior CT chest showing concern for aspiration pneumonia. An MRI of the brain rules out central pontine myelinolysis, acute stroke, and anoxic brain injury, and an EEG shows nonspecific encephalopathy. On Day 10 of hospitalization, a neurologic exam shows flaccid paralysis and bilateral clonus, and Mr. O is mute. On Day 14 of hospitalization, his fever resolves, and his blood cultures are negative. On Day 15 of hospitalization, Mr. O’s creatine kinase (CK) level is elevated at 1,308 U/L (reference range 26 to 192 U/L), suggesting rhabdomyolysis.
Continue to: Given the neurologic exam findings...
Given the neurologic exam findings, and the limited evidence of infection, the differential diagnosis for Mr. O’s AMS is broadened to include catatonia, neuroleptic malignant syndrome (NMS), serotonin syndrome, and autoimmune encephalitis. The psychiatry team evaluates Mr. O for catatonia. He scores 14 on the Bush-Francis Catatonia Rating Scale, with findings of immobility/stupor, mutism, staring, autonomic instability, and withdrawal indicating the presence of catatonia.2
The authors’ observations
When Mr. O was transferred to the second hospital, the primary concern was to rule out meningitis due to his unstable vitals, obtunded mental state, and nuchal rigidity. A comprehensive infectious workup, including lumbar puncture, was imperative because infection can not only lead to AMS, but also precipitate episodes of DKA. Mr. O’s persistently abnormal vital signs indicated an underlying process may have been missed by focusing on treating DKA.
TREATMENT Finally, the diagnosis is established
A lorazepam challenge is performed, and Mr. O receives 4 mg of lorazepam over 24 hours with little change in his catatonia symptoms. Given his persistent fever, tachycardia, and an elevated CK levels in the context of recent exposure to antipsychotic medications, Mr. O is diagnosed with NMS (Table 13,4 ) and is started on bromocriptine, 5 mg 3 times daily.

[polldaddy:10930632]
The authors’ observations
Mr. O’s complicated medical state—starting with DKA, halting the use of antipsychotic medications, and the suspicion of catatonia due to his history of schizophrenia—all distracted from the ultimate diagnosis of NMS as the cause of his enduring AMS and autonomic instability. Catatonia and NMS have overlapping symptomatology, including rigidity, autonomic instability, and stupor, which make the diagnosis of either condition complicated. A positive lorazepam test to diagnose catatonia is defined as a marked reduction in catatonia symptoms (typically a 50% reduction) as measured on a standardized rating scale.5 However, a negative lorazepam challenge does not definitely rule out catatonia because some cases are resistant to benzodiazepines.6
NMS risk factors relevant in this case include male sex, young age, acute medical illness, dehydration, and exposure to multiple psychotropic medications, including 2 antipsychotics, clozapine and fluphenazine.7 DKA is especially pertinent due to its acute onset and cause of significant dehydration. NMS can occur at any point of antipsychotic exposure, although the risk is highest during the initial weeks of treatment and during dosage changes. Unfortunately, Mr. O’s treatment team was unable to determine whether his medication had been recently changed, so it is not known what role this may have played in the development of NMS. Although first-generation antipsychotics are considered more likely to cause NMS, second-generation antipsychotics (SGAs) dominate the treatment of schizophrenia and bipolar disorder, and these medications also can cause NMS.8 As occurred in this case, long-acting injectable antipsychotics can be easily forgotten when not administered in the hospital, and their presence in the body persists for weeks. For example, the half-life of fluphenazine decanoate is approximately 10 days, and the half-life of haloperidol decanoate is 21 days.9
Continue to: OUTCOME Improvement with bromocriptine
OUTCOME Improvement with bromocriptine
After 4 days of bromocriptine, 5 mg 3 times daily, Mr. O is more alert, able to say “hello,” and can follow 1-step commands. By Day 26 of hospitalization, his CK levels decrease to 296 U/L, his CSF autoimmune panel is negative, and he is able to participate in physical therapy. After failing multiple swallow tests, Mr. O requires a percutaneous endoscopic gastrostomy (PEG) tube. He is discharged from the hospital to a long-term acute care facility with the plan to taper bromocriptine and restart a psychotropic regimen with his outpatient psychiatrist. At the time of discharge, he is able to sit at the edge of the bed independently, state his name, and respond to questions with multiple-word answers.
[polldaddy:10930633]
The authors’ observations
The most common pharmacologic treatments for NMS are dantrolene, bromocriptine, benzodiazepines (lorazepam or diazepam), and amantadine.3 Mild cases of NMS should be treated with discontinuation of all antipsychotics, supportive care, and benzodiazepines.3 Bromocriptine or amantadine are more appropriate for moderate cases and dantrolene for severe cases of NMS.3 All antipsychotics should be discontinued while a patient is experiencing an episode of NMS; however, once the NMS has resolved, clinicians must thoroughly evaluate the risks and benefits of restarting antipsychotic medication. After a patient has experienced an episode of NMS, clinicians generally should avoid prescribing the agent(s) that caused NMS and long-acting injections, and slowly titrate a low-potency SGA such as quetiapine.10Table 23,11,12 outlines the pharmacologic treatment of NMS.

Bottom Line
Neuroleptic malignant syndrome (NMS) should always be part of the differential diagnosis in patients with mental illness and altered mental status. The risk of NMS is especially high in patients with acute medical illness and exposure to antipsychotic medications.
Related Resource
- Turner AH, Kim JJ, McCarron RM. Differentiating serotonin syndrome and neuroleptic malignant syndrome. Current Psychiatry. 2019;18(2):30-36.
Drug Brand Names
Acyclovir • Zovirax
Amantadine • Gocovri
Ampicillin-sulbactam • Unasyn
Aripiprazole • Abilify Maintena
Benztropine • Cogentin
Bromocriptine • Cycloset, Parlodel
Ceftriaxone • Rocephin
Clozapine • Clozaril
Dantrolene • Dantrium
Diazepam • Valium
Haloperidol • Haldol
Lithium • Eskalith, Lithobid
Lorazepam • Ativan
Paliperidone palmitate • Invega Sustenna
Quetiapine • Seroquel
Risperidone • Risperdal
Valproate sodium • Depakote
Trazodone • Oleptro
Vancomycin • Vancocin
1. Zeitler P, Haqq A, Rosenbloom A, et al. Hyperglycemic hyperosmolar syndrome in children: pathophysiological considerations and suggested guidelines for treatment. J Pediatr. 2011;158(1):9-14.e1-2. doi: 10.1016/j.jpeds.2010.09.048
2. Francis A. Catatonia: diagnosis, classification, and treatment. Curr Psychiatry Rep. 2010;12(3):180-185. doi: 10.1007/s11920-010-0113-y
3. Pileggi DJ, Cook AM. Neuroleptic malignant syndrome. Ann Pharmacother. 2016;50(11):973-981. doi:10.1177/1060028016657553
4. Gurrera RJ, Caroff SN, Cohen A, et al. An international consensus study of neuroleptic malignant syndrome diagnostic criteria using the Delphi method. J Clin Psychiatry. 2011;72(9):1222-1228. doi:10.4088/JCP.10m06438
5. Sienaert P, Dhossche DM, Vancampfort D, et al. A clinical review of the treatment of catatonia. Front Psychiatry. 2014;5:181. doi:10.3389/fpsyt.2014.00181
6. Daniels J. Catatonia: clinical aspects and neurobiological correlates. J Neuropsychiatry Clin Neurosci. 2009;21(4):371-380. doi:10.1176/jnp.2009.21.4.371
7. Bhanushali MJ, Tuite PJ. The evaluation and management of patients with neuroleptic malignant syndrome. Neurol Clin. 2004;22(2):389-411. doi:10.1016/j.ncl.2003.12.006
8. Tse L, Barr AM, Scarapicchia V, et al. Neuroleptic malignant syndrome: a review from a clinically oriented perspective. Curr Neuropharmacol. 2015;13(3):395-406. doi:10.2174/1570159x13999150424113345
9. Correll CU, Kim E, Sliwa JK, et al. Pharmacokinetic characteristics of long-acting injectable antipsychotics for schizophrenia: an overview. CNS Drugs. 2021;35(1):39-59. doi:10.1007/s40263-020-00779-5
10. Strawn JR, Keck PE Jr, Caroff SN. Neuroleptic malignant syndrome. Am J Psychiatry. 2007;164(6):870-876. doi:10.1176/ajp.2007.164.6.870
11. Griffin CE 3rd, Kaye AM, Bueno FR, et al. Benzodiazepine pharmacology and central nervous system-mediated effects. Ochsner J. 2013;13(2):214-223.
12. Reulbach U, Dütsch C, Biermann T, et al. Managing an effective treatment for neuroleptic malignant syndrome. Crit Care. 2007;11(1):R4. doi:10.1186/cc5148
1. Zeitler P, Haqq A, Rosenbloom A, et al. Hyperglycemic hyperosmolar syndrome in children: pathophysiological considerations and suggested guidelines for treatment. J Pediatr. 2011;158(1):9-14.e1-2. doi: 10.1016/j.jpeds.2010.09.048
2. Francis A. Catatonia: diagnosis, classification, and treatment. Curr Psychiatry Rep. 2010;12(3):180-185. doi: 10.1007/s11920-010-0113-y
3. Pileggi DJ, Cook AM. Neuroleptic malignant syndrome. Ann Pharmacother. 2016;50(11):973-981. doi:10.1177/1060028016657553
4. Gurrera RJ, Caroff SN, Cohen A, et al. An international consensus study of neuroleptic malignant syndrome diagnostic criteria using the Delphi method. J Clin Psychiatry. 2011;72(9):1222-1228. doi:10.4088/JCP.10m06438
5. Sienaert P, Dhossche DM, Vancampfort D, et al. A clinical review of the treatment of catatonia. Front Psychiatry. 2014;5:181. doi:10.3389/fpsyt.2014.00181
6. Daniels J. Catatonia: clinical aspects and neurobiological correlates. J Neuropsychiatry Clin Neurosci. 2009;21(4):371-380. doi:10.1176/jnp.2009.21.4.371
7. Bhanushali MJ, Tuite PJ. The evaluation and management of patients with neuroleptic malignant syndrome. Neurol Clin. 2004;22(2):389-411. doi:10.1016/j.ncl.2003.12.006
8. Tse L, Barr AM, Scarapicchia V, et al. Neuroleptic malignant syndrome: a review from a clinically oriented perspective. Curr Neuropharmacol. 2015;13(3):395-406. doi:10.2174/1570159x13999150424113345
9. Correll CU, Kim E, Sliwa JK, et al. Pharmacokinetic characteristics of long-acting injectable antipsychotics for schizophrenia: an overview. CNS Drugs. 2021;35(1):39-59. doi:10.1007/s40263-020-00779-5
10. Strawn JR, Keck PE Jr, Caroff SN. Neuroleptic malignant syndrome. Am J Psychiatry. 2007;164(6):870-876. doi:10.1176/ajp.2007.164.6.870
11. Griffin CE 3rd, Kaye AM, Bueno FR, et al. Benzodiazepine pharmacology and central nervous system-mediated effects. Ochsner J. 2013;13(2):214-223.
12. Reulbach U, Dütsch C, Biermann T, et al. Managing an effective treatment for neuroleptic malignant syndrome. Crit Care. 2007;11(1):R4. doi:10.1186/cc5148
An unquenchable thirst
CASE Unresponsive after a presumed seizure
Mr. F, age 44, has schizophrenia. He is brought to the hospital by ambulance after he is found on the ground outside of his mother’s house following a presumed seizure and fall. On arrival to the emergency department, he is unresponsive. His laboratory values are significant for a sodium level of 110 mEq/L (reference range: 135 to 145 mEq/L), indicating hyponatremia.
HISTORY Fixated on purity
Mr. F’s mother reports that Mr. F had an unremarkable childhood. He was raised in a household with both parents and a younger sister. Mr. F did well academically and studied engineering and physics in college. There was no reported history of trauma or substance use.
During his senior year of college, Mr. F began experiencing paranoia, auditory hallucinations, and religious delusions. He required hospitalization and was diagnosed with schizophrenia. Following multiple hospitalizations over 5 years, he moved in with his mother, who was granted guardianship.
His mother said Mr. F’s religious delusions were of purity and cleansing the soul. He spent hours memorizing the Bible and would go for days without eating but would drink large amounts of water. She said she thought this was due to his desire to flush out imperfections.
In the past 3 years, Mr. F has been hospitalized several times for severe hyponatremia. At home, his mother attempted to restrict his water intake. However, Mr. F would still drink out of sinks and hoses. Mr. F’s mother reports that over the past month he had become more isolated. He would spend entire days reading the Bible, and his water intake had further increased.
Prior medication trials for Mr. F included haloperidol, up to 10 mg twice per day; aripiprazole, up to 20 mg/d; and risperidone, up to 6 mg nightly. These had been effective, but Mr. F had difficulty with adherence. He did not receive a long-acting injectable (LAI) antipsychotic initially due to lack of access at the rural clinic where he was treated, and later due to his mother’s preference for her son to receive oral medications. Prior to his current presentation, Mr. F’s medication regimen was olanzapine, 10 mg twice a day; perphenazine, 8 mg twice a day; and long-acting propranolol, 60 mg/d. Mr. F had no other chronic medical problems.
EVALUATION Hyponatremia, but why?
Mr. F is intubated and admitted to the surgical service for stabilization due to injuries from his fall. He has fractures of his right sinus and bilateral nasal bones, which are managed nonoperatively. He is delirious, with waxing and waning attention, memory disturbances, and disorientation. His psychotropic medications are held.
Continue to: Imaging of his head...
Imaging of his head does not reveal acute abnormalities suggesting a malignant or paraneoplastic process, and there are no concerns for ongoing seizures. An infection workup is negative. His urine toxicology is negative and blood alcohol level is 0. His sodium normalizes after 3 days of IV fluids and fluid restriction. Therefore, further tests to differentiate the causes of hyponatremia, such as urine electrolytes and urine osmolality, are not pursued.
[polldaddy:10910406]
The authors’ observations
The differential diagnosis for hyponatremia is broad in the setting of psychiatric illness. Low sodium levels could be due to psychotropic medications, psychiatrically-driven behaviors, or an underlying medical problem. Our differential diagnosis for Mr. F included iatrogenic syndrome of inappropriate antidiuretic hormone (SIADH), diabetes insipidus, or psychogenic polydipsia, a form of primary polydipsia. Other causes of primary polydipsia are related to substances, such as heavy beer intakeor use of 3,4-methylenedioxymethamphetamine (MDMA, also known as “ecstasy”), or brain lesions,1 but these causes were less likely given Mr. F’s negative urine toxicology and head imaging.
While psychogenic polydipsia is due to increased water consumption, both SIADH and diabetes insipidus are due to alterations in fluid homeostasis.2,3 Table 12-4 outlines distinguishing characteristics of SIADH, diabetes insipidus, and psychogenic polydipsia. Urine studies were not pursued because Mr. F’s sodium resolved and acute concerns, such as malignancy or infection, were ruled out. Mr. F’s hyponatremia was presumed to be due to psychogenic polydipsia because of his increased fluid intake and normalization of sodium with hypertonic fluids and subsequent fluid restriction. During this time, he was managed on the surgical service; the plan was to pursue urine studies and possibly a fluid challenge if his hyponatremia persisted.
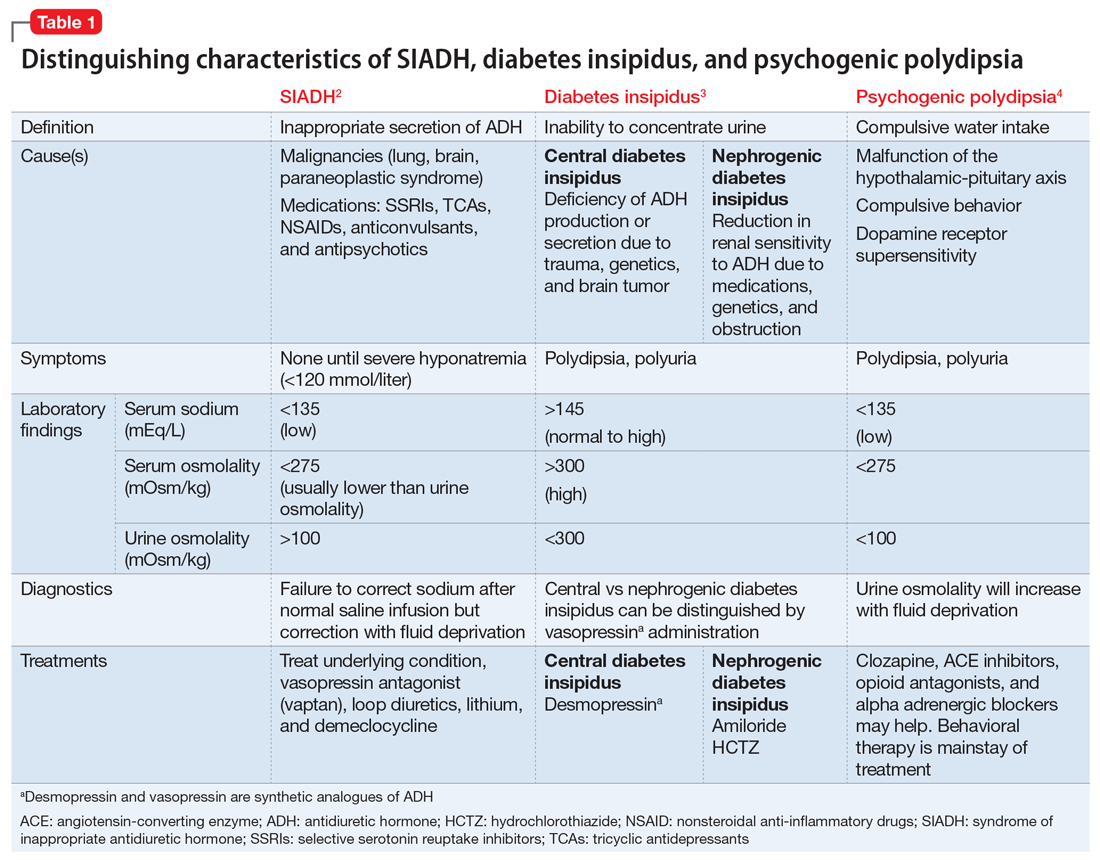
EVALUATION Delirium resolves, delusions persist
While Mr. F is on the surgical service, the treatment team focuses on stabilizing his sodium level and assessing for causes of altered mental status that led to his fall. Psychiatry is consulted for management of his agitation. Following the gradual correction of his sodium level and extubation, his sensorium improves. By hospital Day 5, Mr. F’s delirium resolves.
During this time, Mr. F’s disorganization and religious delusions become apparent. He spends much of his time reading his Bible. He has poor hygiene and limited engagement in activities of daily living. Due to his psychosis and inability to care for himself, Mr. F is transferred to the psychiatric unit with consent from his mother.
Continue to: TREATMENT Olanzapine and fluid restriction
TREATMENT Olanzapine and fluid restriction
In the psychiatric unit, Mr. F is restarted on olanzapine, but not on perphenazine due to anticholinergic effects and not on propranolol due to continued orthostatic hypotension. Five days later, he is at his baseline level of functioning with residual psychosis. His fluid intake is restricted to <1.5 L per day and he is easily compliant.
Mr. F’s mother is comfortable with his discharge home on a regimen of olanzapine, 25 mg/d, and the team discusses the fluid restrictions with her. The treatment team suggests initiating an LAI before Mr. F is discharged, but this is not pursued because his mother thinks he is doing well with the oral medication. She wants to monitor him with the medication changes in the clinic before pursuing an LAI; however, she is open to it in the future.
The authors’ observations
Approximately 20% of patients with schizophrenia may experience psychogenic polydipsia.4,5 The cause of psychogenic polydipsia in patients with serious mental illness is multifactorial. It may stem from malfunction of the hypothalamic-pituitary axis, which leads to alterations in antidiuretic hormone secretion and function.4-6
Mr. F’s case highlights several challenges associated with treating psychogenic polydipsia in patients with serious mental illness. Antipsychotics with high dopamine affinity, such as risperidone and haloperidol, may increase the risk of psychogenic polydipsia, while antipsychotics with lower dopamine affinity, such as clozapine, may decrease the occurrence.5 Antipsychotics block postsynaptic dopamine receptors, which can induce supersensitivity by increasing presynaptic dopamine release in the hypothalamic areas, where thirst regulation occurs. This increase in dopamine leads to increased thirst drive and fluid intake.3
Quetiapine or clozapine may have been a better antipsychotic choice because these agents have lower D2 receptor affinity, whereas olanzapine has intermediate binding to D2 receptors.6,7 However, quetiapine and clozapine are more strongly associated with orthostasis, which was a concern during Mr. F’s hospitalization. The weekly laboratory testing required with clozapine use would have been an unfeasible burden for Mr. F because he lived in a rural environment. Perphenazine was not continued due to higher D2 affinity and anticholinergic effects, which can increase thirst.6
Continue to: In addition to switching...
In addition to switching to an antipsychotic with looser D2 binding, other medications for treating polydipsia have been studied. It is hypothesized that the alpha-2 adrenergic system may play a role in thirst regulation. For example, mianserin, an alpha-2 antagonist, may decrease water intake. However, studies have been small and inconsistent.8,9 Propranolol,10 a beta adrenergic receptor blocker; irbesartan,11 an angiotensin-II receptor blocker; demeclocycline,12 a tetracycline that inhibits antidiuretic hormone action; and naltrexone,9 a mu opioid antagonist, have been studied with inconclusive results and a variety of adverse effects5,7,13 (Table 28-13).
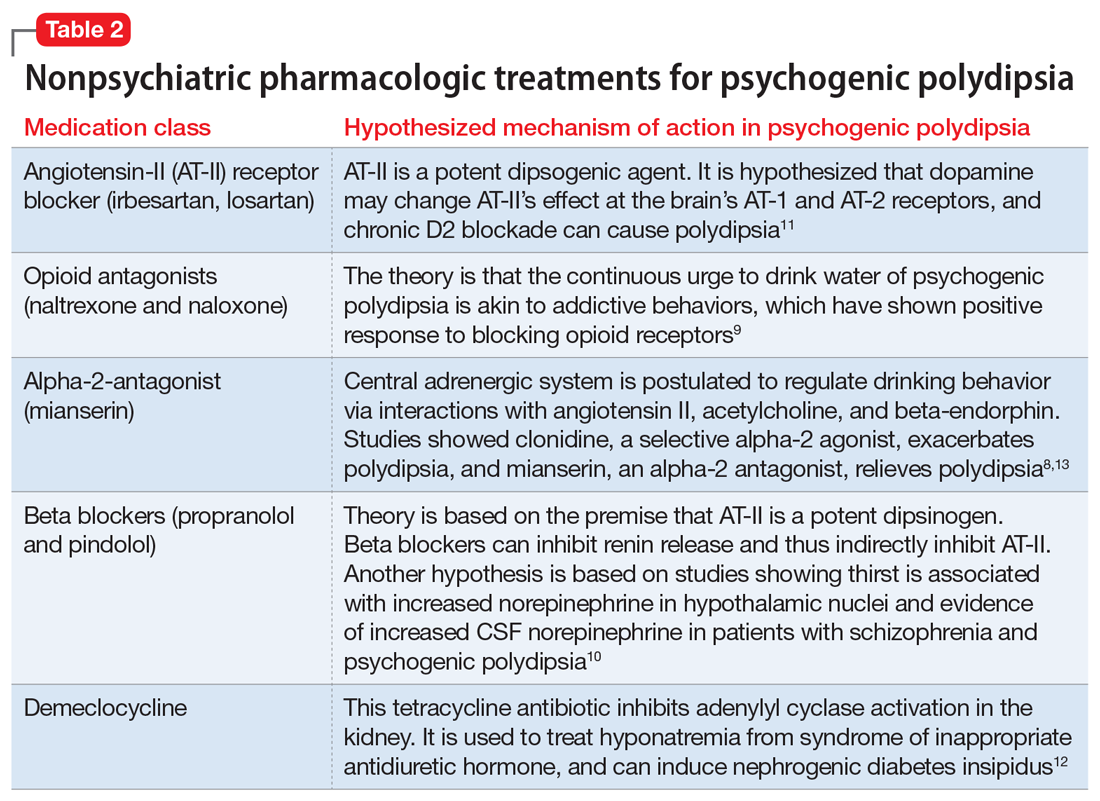
Behavioral interventions for patients with psychogenic polydipsia include fluid restriction, twice-daily weight checks, cognitive-behavioral therapy, and reinforcement schedules, which may be useful but less realistic due to need for increased supervision.11,12 Patient and family education on the signs of hyponatremia are important to prevent serious complications, such as those Mr. F experienced.
OUTCOME Repeated hospitalizations
Mr. F is discharged with follow-up in our psychiatry clinic and attends 1 appointment. At that time, his mother reports that Mr. F is compliant with his medication and has limited fluid intake. However, over the next 2 months, he is admitted to our psychiatric unit twice with similar presentations. Each time, the treatment team has extensive discussions with Mr. F’s mother about strategies to limit his water intake and the possibility of residential placement due to his need for a higher level of care. Although she acknowledges that nursing home placement may be needed in the future, she is not ready to take this step.
Three months later, Mr. F returns to our hospital with severe abdominal pain and is found to have a perforated bowel obstruction. His sodium is within normal limits on presentation, and the psychiatry team is not involved during this hospitalization. Mr. F is treated for sepsis and undergoes 3 exploratory laparotomies with continued decline in his health. He dies during this hospitalization. The cause of Mr. F’s perforated bowel obstruction is not determined, and his family does not pursue an autopsy.
The authors’ observations
At Mr. F’s final hospital presentation, his sodium was normal. It is possible Mr. F and his mother had found an acceptable fluid restriction routine, and he may have been doing better from a psychiatric perspective, but this will remain unknown.
Continue to: This case highlights...
This case highlights the clinical and ethical complexity of treating patients with psychogenic polydipsia. Because Mr. F no longer had autonomy, we had to determine if his mother was acting in his best interest as his guardian. Guardianship requirements and expectations vary by state. In our state of Missouri, a guardian is appointed by the court to act in the best interest of the ward, and may be a family member (preferred) or state-appointed. The guardian is responsible for providing the ward’s care and is in charge of financial and medical decisions. In Missouri, the guardian must assure the ward resides in the “least restrictive setting reasonably available,” which is the minimum necessary to provide the ward safe care and housing.14 Full guardianship, as in Mr. F’s case, is different from limited guardianship, which is an option in states such as Missouri. In limited guardianship, the court decides the extent of the guardian’s role in decisions for the ward.14,15
Mr. F’s mother believed she was acting in her son’s best interest by having him home with his family. She believed by living at home, he would derive more enjoyment from life than living in a nursing home. By the time Mr. F presented to our hospital, he had been living with decompensated schizophrenia for years, so some level of psychosis was likely to persist, even with treatment. Given his increasingly frequent hospitalizations for hyponatremia due to increased water intake, more intense supervision may have been needed to maintain his safety, in line with nonmaleficence. The treatment team considered Mr. F’s best interest when discussing placement and worked to understand his mother’s preferences.
His mother continued to acknowledge the need for changes and adjustments at home. She was receptive to the need for fluid restriction and increased structure at home. Therefore, we felt she continued to be acting in his best interest, and his home would be the least restrictive setting for his care. If Mr. F had continued to require repeated hospitalizations and had not passed away, we would have pursued an ethics consult to discuss the need for nursing home placement and how to best approach this with Mr. F’s mother.
Bottom Line
Patients with serious mental illness who present with hyponatremia should be evaluated for psychogenic polydipsia by assessing their dietary and fluid intakes, along with collateral from family. The use of antipsychotics with high dopamine affinity may increase the risk of psychogenic polydipsia. Behavioral interventions include fluid restriction, weight checks, cognitive-behavioral therapy, and reinforcement schedules.
Related Resources
- Sharp CS, Wilson MP. Hyponatremia. In: Nordstrom KD, Wilson MP, eds. Quick guide to psychiatric emergencies. Springer International Publishing; 2018:115-119. doi:10.1007/ 978-3-319-58260-3_21
- Sailer C, Winzeler B, Christ-Crain M. Primary polydipsia in the medical and psychiatric patient: characteristics, complications and therapy. Swiss Med Wkly. 2017;147:w14514. doi:10.4414/ smw.2017.14514
Drug Brand Names
Amiloride • Midamor
Aripiprazole • Abilify
Clonidine • Catapres
Clozapine • Clozaril
Demeclocycline • Declomycin
Desmopressin • DDAVP
Haloperidol • Haldol
Irbesartan • Avapro
Lithium • Eskalith, Lithobid
Losartan • Cozaar
Mianserin • Tolvon
Naloxone • Narcan
Naltrexone • Revia
Olanzapine • Zyprexa
Perphenazine • Trilafon
Propranolol • Inderal LA
Quetiapine • Seroquel
Risperidone • Risperda
1. Sharp CS, Wilson MP. Hyponatremia. In: Nordstrom KD, Wilson MP, eds. Quick guide to psychiatric emergencies. Springer International Publishing; 2018:115-119. doi:10.1007/978-3-319-58260-3_21
2. Gross P. Clinical management of SIADH. Ther Adv Endocrinol Metab. 2012;3(2):61-73. doi:10.1177/2042018812437561
3. Christ-Crain M, Bichet DG, Fenske WK, et al. Diabetes insipidus. Nat Rev Dis Primer. 2019;5(1):54. doi:10.1038/s41572-019-0103-2
4. Ahmadi L, Goldman MB. Primary polydipsia: update. Best Pract Res Clin Endocrinol Metab. 2020;34(5):101469. doi:10.1016/j.beem.2020.101469
5. Kirino S, Sakuma M, Misawa F, et al. Relationship between polydipsia and antipsychotics: a systematic review of clinical studies and case reports. Prog Neuropsychopharmacol Biol Psychiatry. 2020;96:109756. doi:10.1016/j.pnpbp.2019.109756
6. Siafis S, Tzachanis D, Samara M, et al. Antipsychotic drugs: from receptor-binding profiles to metabolic side effects. Curr Neuropharmacol. 2018;16(8):1210-1223. doi:10.2174/1570159X15666170630163616
7. Seeman P, Tallerico T. Antipsychotic drugs which elicit little or no parkinsonism bind more loosely than dopamine to brain D2 receptors, yet occupy high levels of these receptors. Mol Psychiatry. 1998;3(2):123-134. doi:10.1038/sj.mp.4000336
8. Hayashi T, Nishikawa T, Koga I, et al. Involvement of the α 2 -adrenergic system in polydipsia in schizophrenic patients: a pilot study. Psychopharmacology (Berl). 1997;130(4):382-386. doi:10.1007/s002130050254
9. Rizvi S, Gold J, Khan AM. Role of naltrexone in improving compulsive drinking in psychogenic polydipsia. Cureus. 2019;11(8):e5320. doi:10.7759/cureus.5320
10. Kishi Y, Kurosawa H, Endo S. Is propranolol effective in primary polydipsia? Int J Psychiatry Med. 1998;28(3):315-325. doi:10.2190/QPWL-14H7-HPGG-A29D
11. Kruse D, Pantelis C, Rudd R, et al. Treatment of psychogenic polydipsia: comparison of risperidone and olanzapine, and the effects of an adjunctive angiotensin-II receptor blocking drug (irbesartan). Aust N Z J Psychiatry. 2001;35(1):65-68. doi:10.1046/j.1440-1614.2001.00847.x
12. Alexander RC, Karp BI, Thompson S, et al. A double blind, placebo-controlled trial of demeclocycline treatment of polydipsia-hyponatremia in chronically psychotic patients. Biol Psychiatry. 1991;30(4):417-420. doi:10.1016/0006-3223(91)90300-B
13. Valente S, Fisher D. Recognizing and managing psychogenic polydipsia in mental health. J Nurse Pract. 2010;6(7):546-550. doi:10.1016/j.nurpra.2010.03.004
14. Barton R, Esq SL, Lockett LL. The use of conservatorships and adult guardianships and other options in the care of the mentally ill in the United States. World Guard Congr. Published May 29, 2014. Accessed June 18, 2021. http://www.guardianship.org/IRL/Resources/Handouts/Family%20Members%20as%20Guardians_Handout.pdf
15. ABA Commission on Law & Aging. Adult Guardianship Statutory Table of Authorities. ABA. Published January 2021. Accessed June 17, 2021. https://www.americanbar.org/content/dam/aba/administrative/law_aging/2019-adult-guardianship-statutory-table-of-authorities.pdf
CASE Unresponsive after a presumed seizure
Mr. F, age 44, has schizophrenia. He is brought to the hospital by ambulance after he is found on the ground outside of his mother’s house following a presumed seizure and fall. On arrival to the emergency department, he is unresponsive. His laboratory values are significant for a sodium level of 110 mEq/L (reference range: 135 to 145 mEq/L), indicating hyponatremia.
HISTORY Fixated on purity
Mr. F’s mother reports that Mr. F had an unremarkable childhood. He was raised in a household with both parents and a younger sister. Mr. F did well academically and studied engineering and physics in college. There was no reported history of trauma or substance use.
During his senior year of college, Mr. F began experiencing paranoia, auditory hallucinations, and religious delusions. He required hospitalization and was diagnosed with schizophrenia. Following multiple hospitalizations over 5 years, he moved in with his mother, who was granted guardianship.
His mother said Mr. F’s religious delusions were of purity and cleansing the soul. He spent hours memorizing the Bible and would go for days without eating but would drink large amounts of water. She said she thought this was due to his desire to flush out imperfections.
In the past 3 years, Mr. F has been hospitalized several times for severe hyponatremia. At home, his mother attempted to restrict his water intake. However, Mr. F would still drink out of sinks and hoses. Mr. F’s mother reports that over the past month he had become more isolated. He would spend entire days reading the Bible, and his water intake had further increased.
Prior medication trials for Mr. F included haloperidol, up to 10 mg twice per day; aripiprazole, up to 20 mg/d; and risperidone, up to 6 mg nightly. These had been effective, but Mr. F had difficulty with adherence. He did not receive a long-acting injectable (LAI) antipsychotic initially due to lack of access at the rural clinic where he was treated, and later due to his mother’s preference for her son to receive oral medications. Prior to his current presentation, Mr. F’s medication regimen was olanzapine, 10 mg twice a day; perphenazine, 8 mg twice a day; and long-acting propranolol, 60 mg/d. Mr. F had no other chronic medical problems.
EVALUATION Hyponatremia, but why?
Mr. F is intubated and admitted to the surgical service for stabilization due to injuries from his fall. He has fractures of his right sinus and bilateral nasal bones, which are managed nonoperatively. He is delirious, with waxing and waning attention, memory disturbances, and disorientation. His psychotropic medications are held.
Continue to: Imaging of his head...
Imaging of his head does not reveal acute abnormalities suggesting a malignant or paraneoplastic process, and there are no concerns for ongoing seizures. An infection workup is negative. His urine toxicology is negative and blood alcohol level is 0. His sodium normalizes after 3 days of IV fluids and fluid restriction. Therefore, further tests to differentiate the causes of hyponatremia, such as urine electrolytes and urine osmolality, are not pursued.
[polldaddy:10910406]
The authors’ observations
The differential diagnosis for hyponatremia is broad in the setting of psychiatric illness. Low sodium levels could be due to psychotropic medications, psychiatrically-driven behaviors, or an underlying medical problem. Our differential diagnosis for Mr. F included iatrogenic syndrome of inappropriate antidiuretic hormone (SIADH), diabetes insipidus, or psychogenic polydipsia, a form of primary polydipsia. Other causes of primary polydipsia are related to substances, such as heavy beer intakeor use of 3,4-methylenedioxymethamphetamine (MDMA, also known as “ecstasy”), or brain lesions,1 but these causes were less likely given Mr. F’s negative urine toxicology and head imaging.
While psychogenic polydipsia is due to increased water consumption, both SIADH and diabetes insipidus are due to alterations in fluid homeostasis.2,3 Table 12-4 outlines distinguishing characteristics of SIADH, diabetes insipidus, and psychogenic polydipsia. Urine studies were not pursued because Mr. F’s sodium resolved and acute concerns, such as malignancy or infection, were ruled out. Mr. F’s hyponatremia was presumed to be due to psychogenic polydipsia because of his increased fluid intake and normalization of sodium with hypertonic fluids and subsequent fluid restriction. During this time, he was managed on the surgical service; the plan was to pursue urine studies and possibly a fluid challenge if his hyponatremia persisted.

EVALUATION Delirium resolves, delusions persist
While Mr. F is on the surgical service, the treatment team focuses on stabilizing his sodium level and assessing for causes of altered mental status that led to his fall. Psychiatry is consulted for management of his agitation. Following the gradual correction of his sodium level and extubation, his sensorium improves. By hospital Day 5, Mr. F’s delirium resolves.
During this time, Mr. F’s disorganization and religious delusions become apparent. He spends much of his time reading his Bible. He has poor hygiene and limited engagement in activities of daily living. Due to his psychosis and inability to care for himself, Mr. F is transferred to the psychiatric unit with consent from his mother.
Continue to: TREATMENT Olanzapine and fluid restriction
TREATMENT Olanzapine and fluid restriction
In the psychiatric unit, Mr. F is restarted on olanzapine, but not on perphenazine due to anticholinergic effects and not on propranolol due to continued orthostatic hypotension. Five days later, he is at his baseline level of functioning with residual psychosis. His fluid intake is restricted to <1.5 L per day and he is easily compliant.
Mr. F’s mother is comfortable with his discharge home on a regimen of olanzapine, 25 mg/d, and the team discusses the fluid restrictions with her. The treatment team suggests initiating an LAI before Mr. F is discharged, but this is not pursued because his mother thinks he is doing well with the oral medication. She wants to monitor him with the medication changes in the clinic before pursuing an LAI; however, she is open to it in the future.
The authors’ observations
Approximately 20% of patients with schizophrenia may experience psychogenic polydipsia.4,5 The cause of psychogenic polydipsia in patients with serious mental illness is multifactorial. It may stem from malfunction of the hypothalamic-pituitary axis, which leads to alterations in antidiuretic hormone secretion and function.4-6
Mr. F’s case highlights several challenges associated with treating psychogenic polydipsia in patients with serious mental illness. Antipsychotics with high dopamine affinity, such as risperidone and haloperidol, may increase the risk of psychogenic polydipsia, while antipsychotics with lower dopamine affinity, such as clozapine, may decrease the occurrence.5 Antipsychotics block postsynaptic dopamine receptors, which can induce supersensitivity by increasing presynaptic dopamine release in the hypothalamic areas, where thirst regulation occurs. This increase in dopamine leads to increased thirst drive and fluid intake.3
Quetiapine or clozapine may have been a better antipsychotic choice because these agents have lower D2 receptor affinity, whereas olanzapine has intermediate binding to D2 receptors.6,7 However, quetiapine and clozapine are more strongly associated with orthostasis, which was a concern during Mr. F’s hospitalization. The weekly laboratory testing required with clozapine use would have been an unfeasible burden for Mr. F because he lived in a rural environment. Perphenazine was not continued due to higher D2 affinity and anticholinergic effects, which can increase thirst.6
Continue to: In addition to switching...
In addition to switching to an antipsychotic with looser D2 binding, other medications for treating polydipsia have been studied. It is hypothesized that the alpha-2 adrenergic system may play a role in thirst regulation. For example, mianserin, an alpha-2 antagonist, may decrease water intake. However, studies have been small and inconsistent.8,9 Propranolol,10 a beta adrenergic receptor blocker; irbesartan,11 an angiotensin-II receptor blocker; demeclocycline,12 a tetracycline that inhibits antidiuretic hormone action; and naltrexone,9 a mu opioid antagonist, have been studied with inconclusive results and a variety of adverse effects5,7,13 (Table 28-13).

Behavioral interventions for patients with psychogenic polydipsia include fluid restriction, twice-daily weight checks, cognitive-behavioral therapy, and reinforcement schedules, which may be useful but less realistic due to need for increased supervision.11,12 Patient and family education on the signs of hyponatremia are important to prevent serious complications, such as those Mr. F experienced.
OUTCOME Repeated hospitalizations
Mr. F is discharged with follow-up in our psychiatry clinic and attends 1 appointment. At that time, his mother reports that Mr. F is compliant with his medication and has limited fluid intake. However, over the next 2 months, he is admitted to our psychiatric unit twice with similar presentations. Each time, the treatment team has extensive discussions with Mr. F’s mother about strategies to limit his water intake and the possibility of residential placement due to his need for a higher level of care. Although she acknowledges that nursing home placement may be needed in the future, she is not ready to take this step.
Three months later, Mr. F returns to our hospital with severe abdominal pain and is found to have a perforated bowel obstruction. His sodium is within normal limits on presentation, and the psychiatry team is not involved during this hospitalization. Mr. F is treated for sepsis and undergoes 3 exploratory laparotomies with continued decline in his health. He dies during this hospitalization. The cause of Mr. F’s perforated bowel obstruction is not determined, and his family does not pursue an autopsy.
The authors’ observations
At Mr. F’s final hospital presentation, his sodium was normal. It is possible Mr. F and his mother had found an acceptable fluid restriction routine, and he may have been doing better from a psychiatric perspective, but this will remain unknown.
Continue to: This case highlights...
This case highlights the clinical and ethical complexity of treating patients with psychogenic polydipsia. Because Mr. F no longer had autonomy, we had to determine if his mother was acting in his best interest as his guardian. Guardianship requirements and expectations vary by state. In our state of Missouri, a guardian is appointed by the court to act in the best interest of the ward, and may be a family member (preferred) or state-appointed. The guardian is responsible for providing the ward’s care and is in charge of financial and medical decisions. In Missouri, the guardian must assure the ward resides in the “least restrictive setting reasonably available,” which is the minimum necessary to provide the ward safe care and housing.14 Full guardianship, as in Mr. F’s case, is different from limited guardianship, which is an option in states such as Missouri. In limited guardianship, the court decides the extent of the guardian’s role in decisions for the ward.14,15
Mr. F’s mother believed she was acting in her son’s best interest by having him home with his family. She believed by living at home, he would derive more enjoyment from life than living in a nursing home. By the time Mr. F presented to our hospital, he had been living with decompensated schizophrenia for years, so some level of psychosis was likely to persist, even with treatment. Given his increasingly frequent hospitalizations for hyponatremia due to increased water intake, more intense supervision may have been needed to maintain his safety, in line with nonmaleficence. The treatment team considered Mr. F’s best interest when discussing placement and worked to understand his mother’s preferences.
His mother continued to acknowledge the need for changes and adjustments at home. She was receptive to the need for fluid restriction and increased structure at home. Therefore, we felt she continued to be acting in his best interest, and his home would be the least restrictive setting for his care. If Mr. F had continued to require repeated hospitalizations and had not passed away, we would have pursued an ethics consult to discuss the need for nursing home placement and how to best approach this with Mr. F’s mother.
Bottom Line
Patients with serious mental illness who present with hyponatremia should be evaluated for psychogenic polydipsia by assessing their dietary and fluid intakes, along with collateral from family. The use of antipsychotics with high dopamine affinity may increase the risk of psychogenic polydipsia. Behavioral interventions include fluid restriction, weight checks, cognitive-behavioral therapy, and reinforcement schedules.
Related Resources
- Sharp CS, Wilson MP. Hyponatremia. In: Nordstrom KD, Wilson MP, eds. Quick guide to psychiatric emergencies. Springer International Publishing; 2018:115-119. doi:10.1007/ 978-3-319-58260-3_21
- Sailer C, Winzeler B, Christ-Crain M. Primary polydipsia in the medical and psychiatric patient: characteristics, complications and therapy. Swiss Med Wkly. 2017;147:w14514. doi:10.4414/ smw.2017.14514
Drug Brand Names
Amiloride • Midamor
Aripiprazole • Abilify
Clonidine • Catapres
Clozapine • Clozaril
Demeclocycline • Declomycin
Desmopressin • DDAVP
Haloperidol • Haldol
Irbesartan • Avapro
Lithium • Eskalith, Lithobid
Losartan • Cozaar
Mianserin • Tolvon
Naloxone • Narcan
Naltrexone • Revia
Olanzapine • Zyprexa
Perphenazine • Trilafon
Propranolol • Inderal LA
Quetiapine • Seroquel
Risperidone • Risperda
CASE Unresponsive after a presumed seizure
Mr. F, age 44, has schizophrenia. He is brought to the hospital by ambulance after he is found on the ground outside of his mother’s house following a presumed seizure and fall. On arrival to the emergency department, he is unresponsive. His laboratory values are significant for a sodium level of 110 mEq/L (reference range: 135 to 145 mEq/L), indicating hyponatremia.
HISTORY Fixated on purity
Mr. F’s mother reports that Mr. F had an unremarkable childhood. He was raised in a household with both parents and a younger sister. Mr. F did well academically and studied engineering and physics in college. There was no reported history of trauma or substance use.
During his senior year of college, Mr. F began experiencing paranoia, auditory hallucinations, and religious delusions. He required hospitalization and was diagnosed with schizophrenia. Following multiple hospitalizations over 5 years, he moved in with his mother, who was granted guardianship.
His mother said Mr. F’s religious delusions were of purity and cleansing the soul. He spent hours memorizing the Bible and would go for days without eating but would drink large amounts of water. She said she thought this was due to his desire to flush out imperfections.
In the past 3 years, Mr. F has been hospitalized several times for severe hyponatremia. At home, his mother attempted to restrict his water intake. However, Mr. F would still drink out of sinks and hoses. Mr. F’s mother reports that over the past month he had become more isolated. He would spend entire days reading the Bible, and his water intake had further increased.
Prior medication trials for Mr. F included haloperidol, up to 10 mg twice per day; aripiprazole, up to 20 mg/d; and risperidone, up to 6 mg nightly. These had been effective, but Mr. F had difficulty with adherence. He did not receive a long-acting injectable (LAI) antipsychotic initially due to lack of access at the rural clinic where he was treated, and later due to his mother’s preference for her son to receive oral medications. Prior to his current presentation, Mr. F’s medication regimen was olanzapine, 10 mg twice a day; perphenazine, 8 mg twice a day; and long-acting propranolol, 60 mg/d. Mr. F had no other chronic medical problems.
EVALUATION Hyponatremia, but why?
Mr. F is intubated and admitted to the surgical service for stabilization due to injuries from his fall. He has fractures of his right sinus and bilateral nasal bones, which are managed nonoperatively. He is delirious, with waxing and waning attention, memory disturbances, and disorientation. His psychotropic medications are held.
Continue to: Imaging of his head...
Imaging of his head does not reveal acute abnormalities suggesting a malignant or paraneoplastic process, and there are no concerns for ongoing seizures. An infection workup is negative. His urine toxicology is negative and blood alcohol level is 0. His sodium normalizes after 3 days of IV fluids and fluid restriction. Therefore, further tests to differentiate the causes of hyponatremia, such as urine electrolytes and urine osmolality, are not pursued.
[polldaddy:10910406]
The authors’ observations
The differential diagnosis for hyponatremia is broad in the setting of psychiatric illness. Low sodium levels could be due to psychotropic medications, psychiatrically-driven behaviors, or an underlying medical problem. Our differential diagnosis for Mr. F included iatrogenic syndrome of inappropriate antidiuretic hormone (SIADH), diabetes insipidus, or psychogenic polydipsia, a form of primary polydipsia. Other causes of primary polydipsia are related to substances, such as heavy beer intakeor use of 3,4-methylenedioxymethamphetamine (MDMA, also known as “ecstasy”), or brain lesions,1 but these causes were less likely given Mr. F’s negative urine toxicology and head imaging.
While psychogenic polydipsia is due to increased water consumption, both SIADH and diabetes insipidus are due to alterations in fluid homeostasis.2,3 Table 12-4 outlines distinguishing characteristics of SIADH, diabetes insipidus, and psychogenic polydipsia. Urine studies were not pursued because Mr. F’s sodium resolved and acute concerns, such as malignancy or infection, were ruled out. Mr. F’s hyponatremia was presumed to be due to psychogenic polydipsia because of his increased fluid intake and normalization of sodium with hypertonic fluids and subsequent fluid restriction. During this time, he was managed on the surgical service; the plan was to pursue urine studies and possibly a fluid challenge if his hyponatremia persisted.

EVALUATION Delirium resolves, delusions persist
While Mr. F is on the surgical service, the treatment team focuses on stabilizing his sodium level and assessing for causes of altered mental status that led to his fall. Psychiatry is consulted for management of his agitation. Following the gradual correction of his sodium level and extubation, his sensorium improves. By hospital Day 5, Mr. F’s delirium resolves.
During this time, Mr. F’s disorganization and religious delusions become apparent. He spends much of his time reading his Bible. He has poor hygiene and limited engagement in activities of daily living. Due to his psychosis and inability to care for himself, Mr. F is transferred to the psychiatric unit with consent from his mother.
Continue to: TREATMENT Olanzapine and fluid restriction
TREATMENT Olanzapine and fluid restriction
In the psychiatric unit, Mr. F is restarted on olanzapine, but not on perphenazine due to anticholinergic effects and not on propranolol due to continued orthostatic hypotension. Five days later, he is at his baseline level of functioning with residual psychosis. His fluid intake is restricted to <1.5 L per day and he is easily compliant.
Mr. F’s mother is comfortable with his discharge home on a regimen of olanzapine, 25 mg/d, and the team discusses the fluid restrictions with her. The treatment team suggests initiating an LAI before Mr. F is discharged, but this is not pursued because his mother thinks he is doing well with the oral medication. She wants to monitor him with the medication changes in the clinic before pursuing an LAI; however, she is open to it in the future.
The authors’ observations
Approximately 20% of patients with schizophrenia may experience psychogenic polydipsia.4,5 The cause of psychogenic polydipsia in patients with serious mental illness is multifactorial. It may stem from malfunction of the hypothalamic-pituitary axis, which leads to alterations in antidiuretic hormone secretion and function.4-6
Mr. F’s case highlights several challenges associated with treating psychogenic polydipsia in patients with serious mental illness. Antipsychotics with high dopamine affinity, such as risperidone and haloperidol, may increase the risk of psychogenic polydipsia, while antipsychotics with lower dopamine affinity, such as clozapine, may decrease the occurrence.5 Antipsychotics block postsynaptic dopamine receptors, which can induce supersensitivity by increasing presynaptic dopamine release in the hypothalamic areas, where thirst regulation occurs. This increase in dopamine leads to increased thirst drive and fluid intake.3
Quetiapine or clozapine may have been a better antipsychotic choice because these agents have lower D2 receptor affinity, whereas olanzapine has intermediate binding to D2 receptors.6,7 However, quetiapine and clozapine are more strongly associated with orthostasis, which was a concern during Mr. F’s hospitalization. The weekly laboratory testing required with clozapine use would have been an unfeasible burden for Mr. F because he lived in a rural environment. Perphenazine was not continued due to higher D2 affinity and anticholinergic effects, which can increase thirst.6
Continue to: In addition to switching...
In addition to switching to an antipsychotic with looser D2 binding, other medications for treating polydipsia have been studied. It is hypothesized that the alpha-2 adrenergic system may play a role in thirst regulation. For example, mianserin, an alpha-2 antagonist, may decrease water intake. However, studies have been small and inconsistent.8,9 Propranolol,10 a beta adrenergic receptor blocker; irbesartan,11 an angiotensin-II receptor blocker; demeclocycline,12 a tetracycline that inhibits antidiuretic hormone action; and naltrexone,9 a mu opioid antagonist, have been studied with inconclusive results and a variety of adverse effects5,7,13 (Table 28-13).

Behavioral interventions for patients with psychogenic polydipsia include fluid restriction, twice-daily weight checks, cognitive-behavioral therapy, and reinforcement schedules, which may be useful but less realistic due to need for increased supervision.11,12 Patient and family education on the signs of hyponatremia are important to prevent serious complications, such as those Mr. F experienced.
OUTCOME Repeated hospitalizations
Mr. F is discharged with follow-up in our psychiatry clinic and attends 1 appointment. At that time, his mother reports that Mr. F is compliant with his medication and has limited fluid intake. However, over the next 2 months, he is admitted to our psychiatric unit twice with similar presentations. Each time, the treatment team has extensive discussions with Mr. F’s mother about strategies to limit his water intake and the possibility of residential placement due to his need for a higher level of care. Although she acknowledges that nursing home placement may be needed in the future, she is not ready to take this step.
Three months later, Mr. F returns to our hospital with severe abdominal pain and is found to have a perforated bowel obstruction. His sodium is within normal limits on presentation, and the psychiatry team is not involved during this hospitalization. Mr. F is treated for sepsis and undergoes 3 exploratory laparotomies with continued decline in his health. He dies during this hospitalization. The cause of Mr. F’s perforated bowel obstruction is not determined, and his family does not pursue an autopsy.
The authors’ observations
At Mr. F’s final hospital presentation, his sodium was normal. It is possible Mr. F and his mother had found an acceptable fluid restriction routine, and he may have been doing better from a psychiatric perspective, but this will remain unknown.
Continue to: This case highlights...
This case highlights the clinical and ethical complexity of treating patients with psychogenic polydipsia. Because Mr. F no longer had autonomy, we had to determine if his mother was acting in his best interest as his guardian. Guardianship requirements and expectations vary by state. In our state of Missouri, a guardian is appointed by the court to act in the best interest of the ward, and may be a family member (preferred) or state-appointed. The guardian is responsible for providing the ward’s care and is in charge of financial and medical decisions. In Missouri, the guardian must assure the ward resides in the “least restrictive setting reasonably available,” which is the minimum necessary to provide the ward safe care and housing.14 Full guardianship, as in Mr. F’s case, is different from limited guardianship, which is an option in states such as Missouri. In limited guardianship, the court decides the extent of the guardian’s role in decisions for the ward.14,15
Mr. F’s mother believed she was acting in her son’s best interest by having him home with his family. She believed by living at home, he would derive more enjoyment from life than living in a nursing home. By the time Mr. F presented to our hospital, he had been living with decompensated schizophrenia for years, so some level of psychosis was likely to persist, even with treatment. Given his increasingly frequent hospitalizations for hyponatremia due to increased water intake, more intense supervision may have been needed to maintain his safety, in line with nonmaleficence. The treatment team considered Mr. F’s best interest when discussing placement and worked to understand his mother’s preferences.
His mother continued to acknowledge the need for changes and adjustments at home. She was receptive to the need for fluid restriction and increased structure at home. Therefore, we felt she continued to be acting in his best interest, and his home would be the least restrictive setting for his care. If Mr. F had continued to require repeated hospitalizations and had not passed away, we would have pursued an ethics consult to discuss the need for nursing home placement and how to best approach this with Mr. F’s mother.
Bottom Line
Patients with serious mental illness who present with hyponatremia should be evaluated for psychogenic polydipsia by assessing their dietary and fluid intakes, along with collateral from family. The use of antipsychotics with high dopamine affinity may increase the risk of psychogenic polydipsia. Behavioral interventions include fluid restriction, weight checks, cognitive-behavioral therapy, and reinforcement schedules.
Related Resources
- Sharp CS, Wilson MP. Hyponatremia. In: Nordstrom KD, Wilson MP, eds. Quick guide to psychiatric emergencies. Springer International Publishing; 2018:115-119. doi:10.1007/ 978-3-319-58260-3_21
- Sailer C, Winzeler B, Christ-Crain M. Primary polydipsia in the medical and psychiatric patient: characteristics, complications and therapy. Swiss Med Wkly. 2017;147:w14514. doi:10.4414/ smw.2017.14514
Drug Brand Names
Amiloride • Midamor
Aripiprazole • Abilify
Clonidine • Catapres
Clozapine • Clozaril
Demeclocycline • Declomycin
Desmopressin • DDAVP
Haloperidol • Haldol
Irbesartan • Avapro
Lithium • Eskalith, Lithobid
Losartan • Cozaar
Mianserin • Tolvon
Naloxone • Narcan
Naltrexone • Revia
Olanzapine • Zyprexa
Perphenazine • Trilafon
Propranolol • Inderal LA
Quetiapine • Seroquel
Risperidone • Risperda
1. Sharp CS, Wilson MP. Hyponatremia. In: Nordstrom KD, Wilson MP, eds. Quick guide to psychiatric emergencies. Springer International Publishing; 2018:115-119. doi:10.1007/978-3-319-58260-3_21
2. Gross P. Clinical management of SIADH. Ther Adv Endocrinol Metab. 2012;3(2):61-73. doi:10.1177/2042018812437561
3. Christ-Crain M, Bichet DG, Fenske WK, et al. Diabetes insipidus. Nat Rev Dis Primer. 2019;5(1):54. doi:10.1038/s41572-019-0103-2
4. Ahmadi L, Goldman MB. Primary polydipsia: update. Best Pract Res Clin Endocrinol Metab. 2020;34(5):101469. doi:10.1016/j.beem.2020.101469
5. Kirino S, Sakuma M, Misawa F, et al. Relationship between polydipsia and antipsychotics: a systematic review of clinical studies and case reports. Prog Neuropsychopharmacol Biol Psychiatry. 2020;96:109756. doi:10.1016/j.pnpbp.2019.109756
6. Siafis S, Tzachanis D, Samara M, et al. Antipsychotic drugs: from receptor-binding profiles to metabolic side effects. Curr Neuropharmacol. 2018;16(8):1210-1223. doi:10.2174/1570159X15666170630163616
7. Seeman P, Tallerico T. Antipsychotic drugs which elicit little or no parkinsonism bind more loosely than dopamine to brain D2 receptors, yet occupy high levels of these receptors. Mol Psychiatry. 1998;3(2):123-134. doi:10.1038/sj.mp.4000336
8. Hayashi T, Nishikawa T, Koga I, et al. Involvement of the α 2 -adrenergic system in polydipsia in schizophrenic patients: a pilot study. Psychopharmacology (Berl). 1997;130(4):382-386. doi:10.1007/s002130050254
9. Rizvi S, Gold J, Khan AM. Role of naltrexone in improving compulsive drinking in psychogenic polydipsia. Cureus. 2019;11(8):e5320. doi:10.7759/cureus.5320
10. Kishi Y, Kurosawa H, Endo S. Is propranolol effective in primary polydipsia? Int J Psychiatry Med. 1998;28(3):315-325. doi:10.2190/QPWL-14H7-HPGG-A29D
11. Kruse D, Pantelis C, Rudd R, et al. Treatment of psychogenic polydipsia: comparison of risperidone and olanzapine, and the effects of an adjunctive angiotensin-II receptor blocking drug (irbesartan). Aust N Z J Psychiatry. 2001;35(1):65-68. doi:10.1046/j.1440-1614.2001.00847.x
12. Alexander RC, Karp BI, Thompson S, et al. A double blind, placebo-controlled trial of demeclocycline treatment of polydipsia-hyponatremia in chronically psychotic patients. Biol Psychiatry. 1991;30(4):417-420. doi:10.1016/0006-3223(91)90300-B
13. Valente S, Fisher D. Recognizing and managing psychogenic polydipsia in mental health. J Nurse Pract. 2010;6(7):546-550. doi:10.1016/j.nurpra.2010.03.004
14. Barton R, Esq SL, Lockett LL. The use of conservatorships and adult guardianships and other options in the care of the mentally ill in the United States. World Guard Congr. Published May 29, 2014. Accessed June 18, 2021. http://www.guardianship.org/IRL/Resources/Handouts/Family%20Members%20as%20Guardians_Handout.pdf
15. ABA Commission on Law & Aging. Adult Guardianship Statutory Table of Authorities. ABA. Published January 2021. Accessed June 17, 2021. https://www.americanbar.org/content/dam/aba/administrative/law_aging/2019-adult-guardianship-statutory-table-of-authorities.pdf
1. Sharp CS, Wilson MP. Hyponatremia. In: Nordstrom KD, Wilson MP, eds. Quick guide to psychiatric emergencies. Springer International Publishing; 2018:115-119. doi:10.1007/978-3-319-58260-3_21
2. Gross P. Clinical management of SIADH. Ther Adv Endocrinol Metab. 2012;3(2):61-73. doi:10.1177/2042018812437561
3. Christ-Crain M, Bichet DG, Fenske WK, et al. Diabetes insipidus. Nat Rev Dis Primer. 2019;5(1):54. doi:10.1038/s41572-019-0103-2
4. Ahmadi L, Goldman MB. Primary polydipsia: update. Best Pract Res Clin Endocrinol Metab. 2020;34(5):101469. doi:10.1016/j.beem.2020.101469
5. Kirino S, Sakuma M, Misawa F, et al. Relationship between polydipsia and antipsychotics: a systematic review of clinical studies and case reports. Prog Neuropsychopharmacol Biol Psychiatry. 2020;96:109756. doi:10.1016/j.pnpbp.2019.109756
6. Siafis S, Tzachanis D, Samara M, et al. Antipsychotic drugs: from receptor-binding profiles to metabolic side effects. Curr Neuropharmacol. 2018;16(8):1210-1223. doi:10.2174/1570159X15666170630163616
7. Seeman P, Tallerico T. Antipsychotic drugs which elicit little or no parkinsonism bind more loosely than dopamine to brain D2 receptors, yet occupy high levels of these receptors. Mol Psychiatry. 1998;3(2):123-134. doi:10.1038/sj.mp.4000336
8. Hayashi T, Nishikawa T, Koga I, et al. Involvement of the α 2 -adrenergic system in polydipsia in schizophrenic patients: a pilot study. Psychopharmacology (Berl). 1997;130(4):382-386. doi:10.1007/s002130050254
9. Rizvi S, Gold J, Khan AM. Role of naltrexone in improving compulsive drinking in psychogenic polydipsia. Cureus. 2019;11(8):e5320. doi:10.7759/cureus.5320
10. Kishi Y, Kurosawa H, Endo S. Is propranolol effective in primary polydipsia? Int J Psychiatry Med. 1998;28(3):315-325. doi:10.2190/QPWL-14H7-HPGG-A29D
11. Kruse D, Pantelis C, Rudd R, et al. Treatment of psychogenic polydipsia: comparison of risperidone and olanzapine, and the effects of an adjunctive angiotensin-II receptor blocking drug (irbesartan). Aust N Z J Psychiatry. 2001;35(1):65-68. doi:10.1046/j.1440-1614.2001.00847.x
12. Alexander RC, Karp BI, Thompson S, et al. A double blind, placebo-controlled trial of demeclocycline treatment of polydipsia-hyponatremia in chronically psychotic patients. Biol Psychiatry. 1991;30(4):417-420. doi:10.1016/0006-3223(91)90300-B
13. Valente S, Fisher D. Recognizing and managing psychogenic polydipsia in mental health. J Nurse Pract. 2010;6(7):546-550. doi:10.1016/j.nurpra.2010.03.004
14. Barton R, Esq SL, Lockett LL. The use of conservatorships and adult guardianships and other options in the care of the mentally ill in the United States. World Guard Congr. Published May 29, 2014. Accessed June 18, 2021. http://www.guardianship.org/IRL/Resources/Handouts/Family%20Members%20as%20Guardians_Handout.pdf
15. ABA Commission on Law & Aging. Adult Guardianship Statutory Table of Authorities. ABA. Published January 2021. Accessed June 17, 2021. https://www.americanbar.org/content/dam/aba/administrative/law_aging/2019-adult-guardianship-statutory-table-of-authorities.pdf
Late-onset, treatment-resistant anxiety and depression
CASE Anxious and can’t sleep
Mr. A, age 41, presents to his primary care physician (PCP) with anxiety and insomnia. He describes having generalized anxiety with initial and middle insomnia, and says he is sleeping an average of 2 hours per night. He denies any other psychiatric symptoms. Mr. A has no significant psychiatric or medical history.
Mr. A is initiated on zolpidem tartrate, 12.5 mg every night at bedtime, and paroxetine, 20 mg every night at bedtime, for anxiety and insomnia, but these medications result in little to no improvement.
During a 4-month period, he is treated with trials of alprazolam, 0.5 mg every 8 hours as needed; diazepam 5 mg twice a day as needed; diphenhydramine, 50 mg at bedtime; and eszopiclone, 3 mg at bedtime. Despite these treatments, he experiences increased anxiety and insomnia, and develops depressive symptoms, including depressed mood, poor concentration, general malaise, extreme fatigue, a 15-pound unintentional weight loss, erectile dysfunction, and decreased libido. Mr. A denies having suicidal or homicidal ideations. Additionally, he typically goes to the gym approximately 3 times per week, and has noticed that the amount of weight he is able to lift has decreased, which is distressing. Previously, he had been able to lift 300 pounds, but now he can only lift 200 pounds.
[polldaddy:10891920]
The authors’ observations
Insomnia, anxiety, and depression are common chief complaints in medical settings. However, some psychiatric presentations may have an underlying medical etiology.
DSM-5 requires that medical conditions be ruled out in order for a patient to meet criteria for a psychiatric diagnosis.1 Medical differential diagnoses for patients with psychiatric symptoms can include autoimmune, drug/toxin, metabolic, infectious, neoplastic, neurologic, and nutritional etiologies (Table 12). To rule out the possibility of an underlying medical etiology, general screening guidelines include complete blood count, complete metabolic panel, urinalysis, and urine drug screen with alcohol. Human immunodeficiency virus testing and thyroid hormone testing are also commonly ordered.3 Further laboratory testing and imaging is typically not warranted in the absence of historical or physical findings because they are not advocated as cost-effective, so health care professionals must use their clinical judgment to determine appropriate further evaluation. The onset of anxiety most commonly occurs in late adolescence early and adulthood, but Mr. A experienced his first symptoms of anxiety at age 41.2 Mr. A’s age, lack of psychiatric or family history of mental illness, acute onset of symptoms, and failure of symptoms to abate with standard psychiatric treatments warrant a more extensive workup.
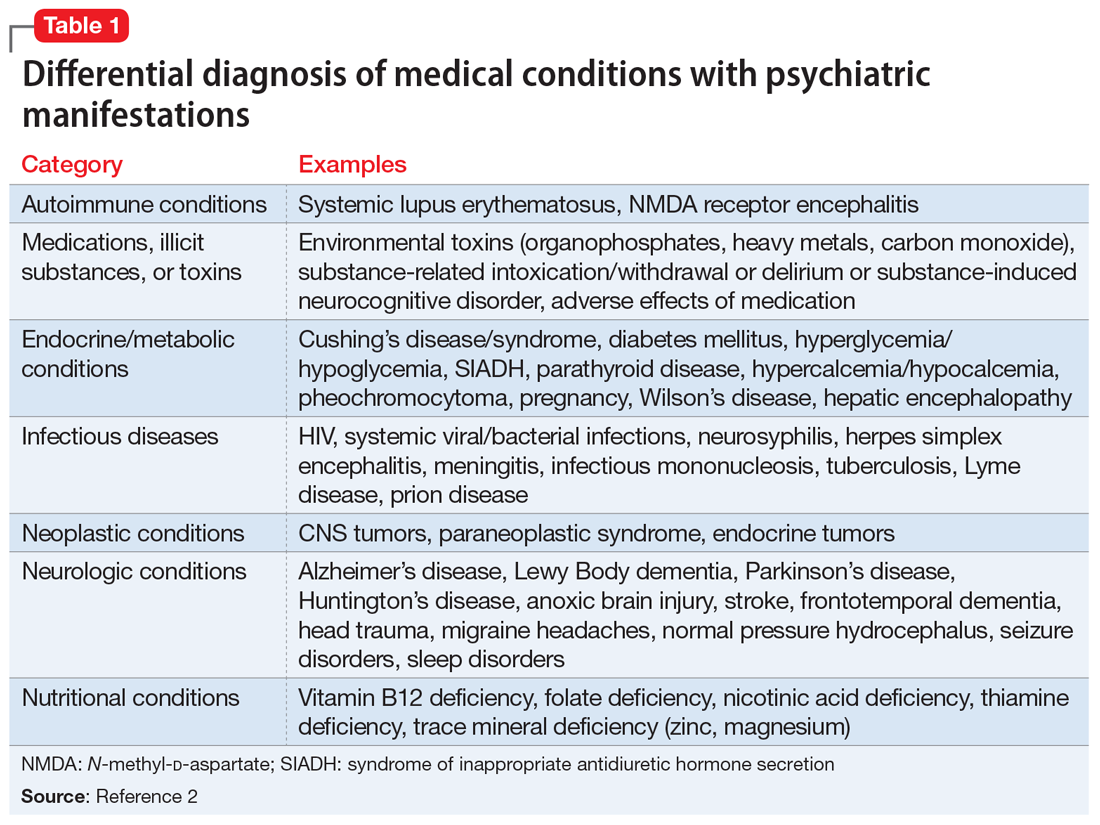
EVALUATION Imaging reveals an important finding
Because Mr. A’s symptoms do not improve with standard psychiatric treatments, his PCP orders standard laboratory bloodwork to investigate a possible medical etiology; however, his results are all within normal range.
After the PCP’s niece is coincidentally diagnosed with a pituitary macroadenoma, the PCP orders brain imaging for Mr. A. Results of an MRI show that Mr. A has a 1.6-cm macroadenoma of the pituitary. He is referred to an endocrinologist, who orders additional laboratory tests that show an elevated 24-hour free urine cortisol level of 73 μg/24 h (normal range: 3.5 to 45 μg/24 h), suggesting that Mr. A’s anxiety may be due to Cushing’s disease or that his anxiety caused falsely elevated urinary cortisol levels. Four weeks later, bloodwork is repeated and shows an abnormal dexamethasone suppression test, and 2 more elevated 24-hour free urine cortisol levels of 76 μg/24 h and 150 μg/24 h. A repeat MRI shows a 1.8-cm, mostly cystic sellar mass, indicating the need for surgical intervention. Although the tumor is large and shows optic nerve compression, Mr. A does not complain of headaches or changes in vision.
Continue to: Two months later...
Two months later, Mr. A undergoes a transsphenoidal tumor resection of the pituitary adenoma, and biopsy results confirm an adrenocorticotropic hormone (ACTH)-secreting pituitary macroadenoma, which is consistent with Cushing’s disease. Following surgery, steroid treatment with dexamethasone is discontinued due to a persistently elevated
[polldaddy:10891923]
The authors’ observations
Chronic excess glucocorticoid production is the underlying pathophysiology of Cushing’s disease, which is most commonly caused by an ACTH-producing adenoma.4,5 When these hormones become dysregulated, the result can be over- or underproduction of cortisol, which can lead to physical and psychiatric manifestations.6
Cushing’s disease most commonly manifests with the physical symptoms of centripetal fat deposition, abdominal striae, facial plethora, muscle atrophy, bone density loss, immunosuppression, and cardiovascular complications.5
Hypercortisolism can precipitate anxiety (12% to 79%), mood disorders (50% to 70%), and (less commonly) psychotic disorders; however, in a clinical setting, if a patient presented with one of these as a chief complaint, they would likely first be treated psychiatrically rather than worked up medically for a rare medical condition.5,7-13
Mr. A’s initial bloodwork was unremarkable, but cortisol levels were not obtained at that time because testing for cortisol levels to rule out an underlying medical condition is not routine in patients with depression and anxiety. In Mr. A’s case, a neuroendocrine workup was only ordered once his PCP’s niece coincidentally was diagnosed with a pituitary adenoma.
Continue to: For Mr. A...
For Mr. A, Cushing’s disease presented as a psychiatric disorder with anxiety and insomnia that were resistant to numerous psychiatric medications during an 8-month period. If Mr. A’s PCP had not ordered a brain MRI, he may have continued to receive ineffective psychiatric treatment for some time. Many of Mr. A’s physical symptoms were consistent with Cushing’s disease and mental illness, including erectile dysfunction, fatigue, and muscle weakness; however, his 15-pound weight loss pointed more toward psychiatric illness and further disguised his underlying medical diagnosis, because sudden weight gain is commonly seen in Cushing’s disease (Table 24,5,7,9).
TREATMENT Persistent psychiatric symptoms, then finally relief
Four weeks after surgery, Mr. A’s psychiatric symptoms gradually intensify, which prompts him to see a psychiatrist. A mental status examination (MSE) shows that he is well-nourished, with normal activity, appropriate behavior, and coherent thought process, but depressed mood and flat affect. He denies suicidal or homicidal ideation. He reports that despite being advised to have realistic expectations, he had high hopes that the surgery would lead to remission of all his symptoms, and expresses disappointment that he does not feel “back to normal.”
Six days later, Mr. A’s wife takes him to the hospital. His MSE shows that he has a tense appearance, fidgety activity, depressed and anxious mood, restricted affect, circumstantial thought process, and paranoid delusions that his wife was plotting against him. He says he still is experiencing insomnia. He also discloses having suicidal ideations with a plan and intent to overdose on medication, as well as homicidal ideations about killing his wife and children. Mr. A provides reasons for why he would want to hurt his family, and does not appear to be bothered by these thoughts.
Mr. A is admitted to the inpatient psychiatric unit and is prescribed quetiapine, 100 mg every night at bedtime. During the next 2 days, quetiapine is titrated to 300 mg every night at bedtime. On hospital Day 3, Mr. A says he is feeling worse than the previous days. He is still having vague suicidal thoughts and feels agitated, guilty, and depressed. To treat these persistent symptoms, quetiapine is further increased to 400 mg every night at bedtime, and he is initiated on bupropion XL, 150 mg, to treat persistent symptoms.
After 1 week of hospitalization, the treatment team meets with Mr. A and his wife, who has been supportive throughout her husband’s hospitalization. During the meeting, they both agree that Mr. A has experienced some improvement because he is no longer having suicidal or homicidal thoughts, but he is still feeling depressed and frustrated by his continued insomnia. Following the meeting, Mr. A’s quetiapine is further increased to 450 mg every night at bedtime to address continued insomnia, and bupropion XL is increased to 300 mg/d to address continued depressive symptoms. During the next few days, his affective symptoms improve; however, his initial insomnia continues, and quetiapine is further increased to 500 mg every night at bedtime.
Continue to: On hospital Day 20...
On hospital Day 20, Mr. A is discharged back to his outpatient psychiatrist and receives quetiapine, 500 mg every night at bedtime, and bupropion XL, 300 mg/d. Although Mr. A’s depression and anxiety continue to be well controlled, his insomnia persists. Sleep hygiene is addressed, and alprazolam, 0.5 mg every night at bedtime, is added to his regimen, which proves to be effective.
OUTCOME A slow remission
After a year of treatment, Mr. A is slowly tapered off of all medications. Two years later, he is in complete remission of all psychiatric symptoms and no longer requires any psychotropic medications.
The authors’ observations
Treatment for hypercortisolism in patients with psychiatric symptoms triggered by glucocorticoid imbalance has typically resulted in a decrease in the severity of their psychiatric symptoms.9,11 A prospective longitudinal study examining 33 patients found that correction of hypercortisolism in patients with Cushing’s syndrome often led to resolution of their psychiatric symptoms, with 87.9% of patients back to baseline within 1 year.14 However, to our knowledge, few reports have described the management of patients whose symptoms are resistant to treatment of hypercortisolism.
In our case, after transsphenoidal resection of an adenoma, Mr. A became suicidal and paranoid, and his anxiety and insomnia also persisted. A possible explanation for the worsening of Mr. A’s symptoms after surgery could be the slow recovery of the hypothalamic-pituitary-adrenal (HPA) axis and therefore a temporary deficiency in glucocorticoid, which caused an increase in catecholamines, leading to an increase in stress.14 This concept of a “slow recovery” is supported by the fact that Mr. A was successfully weaned off all medication after 1 year of treatment, and achieved complete remission of psychiatric symptoms for >2 years. Furthermore, the severity of Mr. A’s symptoms appeared to correlate with his 24-hour urine cortisol and
Future research should evaluate the utility of screening all patients with treatment-resistant anxiety and/or insomnia for hypercortisolism. Even without other clues to endocrinopathies, serum cortisol levels can be used as a screening tool for diagnosing underlying medical causes in patients with anxiety and depression.2 A greater understanding of the relationship between medical and psychiatric manifestations will allow clinicians to better care for patients. Further research is needed to elucidate the quantitative relationship between cortisol levels and anxiety to evaluate severity, guide treatment planning, and follow treatment response for patients with anxiety. It may be useful to determine the threshold between elevated cortisol levels due to anxiety vs elevated cortisol due to an underlying medical pathology such as Cushing’s disease. Additionally, little research has been conducted to compare how psychiatric symptoms respond to pituitary macroadenoma resection alone, pharmaceutical intervention alone, or a combination of these approaches. It would be beneficial to evaluate these treatment strategies to elucidate the most effective method to reduce psychiatric symptoms in patients with hypercortisolism, and perhaps to reduce the incidence of post-resection worsening of psychiatric symptoms.
Continue to: This case was challenging...
This case was challenging because Mr. A did not initially respond to psychiatric intervention, his psychiatric symptoms worsened after transsphenoidal resection of the pituitary adenoma, and his symptoms were alleviated only after psychiatric medications were re-initiated following surgery. This case highlights the importance of considering an underlying medically diagnosable and treatable cause of psychiatric illness, and illustrates the complex ongoing management that may be necessary to help a patient with this condition achieve their baseline. Further, Mr. A’s case shows that the absence of response to standard psychiatric therapies should warrant earlier laboratory and/or imaging evaluation prior to or in conjunction with psychiatric referral. Additionally, testing for cortisol levels is not typically done for a patient with treatment-resistant anxiety, and this case highlights the importance of considering hypercortisolism in such circumstances.
Bottom Line
Consider testing cortisol levels in patients with treatment-resistant anxiety and insomnia, because cortisol plays a role in Cushing’s disease and anxiety. The severity of psychiatric manifestations of Cushing’s disease may correlate with cortisol levels. Treatment should focus on symptomatic management and underlying etiology.
Related Resources
- Roberts LW, Hales RE, Yudofsky SC, ed. The American Psychiatric Association Publishing Textbook of Psychiatry. 7th ed. American Psychiatric Association Publishing; 2019.
- Rotham J. Cushing’s syndrome: a tale of frequent misdiagnosis. National Center for Health Research. 2020. www.center4research.org/cushings-syndrome-frequent-misdiagnosis/
- Middleman D. Psychiatric issues of Cushing’s patients: coping with Cushing’s. Cushing’s Support and Research Foundation. www.csrf.net/coping-with-cushings/psychiatric-issues-of-cushings-patients/
Drug Brand Names
Alprazolam • Xanax
Bupropion • Wellbutrin
Dexamethasone • Decadron
Diazepam • Valium
Eszopiclone • Lunesta
Paroxetine • Paxil
Quetiapine • Seroquel
Zolpidem tartrate • Ambien CR
1. Diagnostic and statistical manual of mental disorders, 5th ed. American Psychiatric Association; 2013.
2. Sadock BJ, Sadock VA, Ruiz P, et al. Neural sciences. In: Sadock BJ, Sadock VA, Ruiz P, et al. Kaplan and Sadock’s synopsis of psychiatry: behavioral sciences/clinical psychiatry. 11th ed. Wolters Kluwer; 2015.
3. Anfinson TJ, Kathol RG. Screening laboratory evaluation in psychiatric patients: a review. Gen Hosp Psychiatry. 1992;14(4):248-257.
4. Fehm HL, Voigt KH. Pathophysiology of Cushing’s disease. Pathobiol Annu. 1979;9:225-255.
5. Fujii Y, Mizoguchi Y, Masuoka J, et al. Cushing’s syndrome and psychosis: a case report and literature review. Prim Care Companion CNS Disord. 2018;20(5):18.
6. Raff H, Sharma ST, Nieman LK. Physiological basis for the etiology, diagnosis, and treatment of adrenal disorders: Cushing’s syndrome, adrenal insufficiency, and congenital adrenal hyperplasia. Compr Physiol. 2011;4(2):739-769.
7. Santos A, Resimini E, Pascual JC, et al. Psychiatric symptoms in patients with Cushing’s syndrome: prevalence diagnosis, and management. Drugs. 2017;77(8):829-842.
8. Arnaldi G, Angeli A, Atkinson B, et al. Diagnosis and complications of Cushing’s syndrome: a consensus statement. J Clin Endocrinol Metab. 2003;88(12):5593-5602.
9. Sonino N, Fava GA. Psychosomatic aspects of Cushing’s disease. Psychother Psychosom. 1998;67(3):140-146.
10. Loosen PT, Chambliss B, DeBold CR, et al. Psychiatric phenomenology in Cushing’s disease. Pharmacopsychiatry. 1992;25(4):192-198.
11. Kelly WF, Kelly MJ, Faragher B. A prospective study of psychiatric and psychological aspects of Cushing’s syndrome. Clin Endocrinol. 1996;45(6):715-720.
12. Katho RG, Delahunt JW, Hannah L. Transition from bipolar affective disorder to intermittent Cushing’s syndrome: case report. J Clin Psychiatry. 1985;46(5):194-196.
13. Hirsh D, Orr G, Kantarovich V, et al. Cushing’s syndrome presenting as a schizophrenia-like psychotic state. Isr J Psychiatry Relat Sci. 2000;37(1):46-50.
14. Dorn LD, Burgess ES, Friedman TC, et al. The longitudinal course of psychopathology in Cushing’s syndrome after correction of hypercortisolism. J Clin Endocrinol Metab. 1997;82(3):912-919.
15. Starkman MN, Schteingart DE, Schork MA. Cushing’s syndrome after treatment: changes in cortisol and ACTH levels, and amelioration of the depressive syndrome. Psychiatry Res. 1986;19(3):177-178.
CASE Anxious and can’t sleep
Mr. A, age 41, presents to his primary care physician (PCP) with anxiety and insomnia. He describes having generalized anxiety with initial and middle insomnia, and says he is sleeping an average of 2 hours per night. He denies any other psychiatric symptoms. Mr. A has no significant psychiatric or medical history.
Mr. A is initiated on zolpidem tartrate, 12.5 mg every night at bedtime, and paroxetine, 20 mg every night at bedtime, for anxiety and insomnia, but these medications result in little to no improvement.
During a 4-month period, he is treated with trials of alprazolam, 0.5 mg every 8 hours as needed; diazepam 5 mg twice a day as needed; diphenhydramine, 50 mg at bedtime; and eszopiclone, 3 mg at bedtime. Despite these treatments, he experiences increased anxiety and insomnia, and develops depressive symptoms, including depressed mood, poor concentration, general malaise, extreme fatigue, a 15-pound unintentional weight loss, erectile dysfunction, and decreased libido. Mr. A denies having suicidal or homicidal ideations. Additionally, he typically goes to the gym approximately 3 times per week, and has noticed that the amount of weight he is able to lift has decreased, which is distressing. Previously, he had been able to lift 300 pounds, but now he can only lift 200 pounds.
[polldaddy:10891920]
The authors’ observations
Insomnia, anxiety, and depression are common chief complaints in medical settings. However, some psychiatric presentations may have an underlying medical etiology.
DSM-5 requires that medical conditions be ruled out in order for a patient to meet criteria for a psychiatric diagnosis.1 Medical differential diagnoses for patients with psychiatric symptoms can include autoimmune, drug/toxin, metabolic, infectious, neoplastic, neurologic, and nutritional etiologies (Table 12). To rule out the possibility of an underlying medical etiology, general screening guidelines include complete blood count, complete metabolic panel, urinalysis, and urine drug screen with alcohol. Human immunodeficiency virus testing and thyroid hormone testing are also commonly ordered.3 Further laboratory testing and imaging is typically not warranted in the absence of historical or physical findings because they are not advocated as cost-effective, so health care professionals must use their clinical judgment to determine appropriate further evaluation. The onset of anxiety most commonly occurs in late adolescence early and adulthood, but Mr. A experienced his first symptoms of anxiety at age 41.2 Mr. A’s age, lack of psychiatric or family history of mental illness, acute onset of symptoms, and failure of symptoms to abate with standard psychiatric treatments warrant a more extensive workup.

EVALUATION Imaging reveals an important finding
Because Mr. A’s symptoms do not improve with standard psychiatric treatments, his PCP orders standard laboratory bloodwork to investigate a possible medical etiology; however, his results are all within normal range.
After the PCP’s niece is coincidentally diagnosed with a pituitary macroadenoma, the PCP orders brain imaging for Mr. A. Results of an MRI show that Mr. A has a 1.6-cm macroadenoma of the pituitary. He is referred to an endocrinologist, who orders additional laboratory tests that show an elevated 24-hour free urine cortisol level of 73 μg/24 h (normal range: 3.5 to 45 μg/24 h), suggesting that Mr. A’s anxiety may be due to Cushing’s disease or that his anxiety caused falsely elevated urinary cortisol levels. Four weeks later, bloodwork is repeated and shows an abnormal dexamethasone suppression test, and 2 more elevated 24-hour free urine cortisol levels of 76 μg/24 h and 150 μg/24 h. A repeat MRI shows a 1.8-cm, mostly cystic sellar mass, indicating the need for surgical intervention. Although the tumor is large and shows optic nerve compression, Mr. A does not complain of headaches or changes in vision.
Continue to: Two months later...
Two months later, Mr. A undergoes a transsphenoidal tumor resection of the pituitary adenoma, and biopsy results confirm an adrenocorticotropic hormone (ACTH)-secreting pituitary macroadenoma, which is consistent with Cushing’s disease. Following surgery, steroid treatment with dexamethasone is discontinued due to a persistently elevated
[polldaddy:10891923]
The authors’ observations
Chronic excess glucocorticoid production is the underlying pathophysiology of Cushing’s disease, which is most commonly caused by an ACTH-producing adenoma.4,5 When these hormones become dysregulated, the result can be over- or underproduction of cortisol, which can lead to physical and psychiatric manifestations.6
Cushing’s disease most commonly manifests with the physical symptoms of centripetal fat deposition, abdominal striae, facial plethora, muscle atrophy, bone density loss, immunosuppression, and cardiovascular complications.5
Hypercortisolism can precipitate anxiety (12% to 79%), mood disorders (50% to 70%), and (less commonly) psychotic disorders; however, in a clinical setting, if a patient presented with one of these as a chief complaint, they would likely first be treated psychiatrically rather than worked up medically for a rare medical condition.5,7-13
Mr. A’s initial bloodwork was unremarkable, but cortisol levels were not obtained at that time because testing for cortisol levels to rule out an underlying medical condition is not routine in patients with depression and anxiety. In Mr. A’s case, a neuroendocrine workup was only ordered once his PCP’s niece coincidentally was diagnosed with a pituitary adenoma.
Continue to: For Mr. A...
For Mr. A, Cushing’s disease presented as a psychiatric disorder with anxiety and insomnia that were resistant to numerous psychiatric medications during an 8-month period. If Mr. A’s PCP had not ordered a brain MRI, he may have continued to receive ineffective psychiatric treatment for some time. Many of Mr. A’s physical symptoms were consistent with Cushing’s disease and mental illness, including erectile dysfunction, fatigue, and muscle weakness; however, his 15-pound weight loss pointed more toward psychiatric illness and further disguised his underlying medical diagnosis, because sudden weight gain is commonly seen in Cushing’s disease (Table 24,5,7,9).
TREATMENT Persistent psychiatric symptoms, then finally relief
Four weeks after surgery, Mr. A’s psychiatric symptoms gradually intensify, which prompts him to see a psychiatrist. A mental status examination (MSE) shows that he is well-nourished, with normal activity, appropriate behavior, and coherent thought process, but depressed mood and flat affect. He denies suicidal or homicidal ideation. He reports that despite being advised to have realistic expectations, he had high hopes that the surgery would lead to remission of all his symptoms, and expresses disappointment that he does not feel “back to normal.”
Six days later, Mr. A’s wife takes him to the hospital. His MSE shows that he has a tense appearance, fidgety activity, depressed and anxious mood, restricted affect, circumstantial thought process, and paranoid delusions that his wife was plotting against him. He says he still is experiencing insomnia. He also discloses having suicidal ideations with a plan and intent to overdose on medication, as well as homicidal ideations about killing his wife and children. Mr. A provides reasons for why he would want to hurt his family, and does not appear to be bothered by these thoughts.
Mr. A is admitted to the inpatient psychiatric unit and is prescribed quetiapine, 100 mg every night at bedtime. During the next 2 days, quetiapine is titrated to 300 mg every night at bedtime. On hospital Day 3, Mr. A says he is feeling worse than the previous days. He is still having vague suicidal thoughts and feels agitated, guilty, and depressed. To treat these persistent symptoms, quetiapine is further increased to 400 mg every night at bedtime, and he is initiated on bupropion XL, 150 mg, to treat persistent symptoms.
After 1 week of hospitalization, the treatment team meets with Mr. A and his wife, who has been supportive throughout her husband’s hospitalization. During the meeting, they both agree that Mr. A has experienced some improvement because he is no longer having suicidal or homicidal thoughts, but he is still feeling depressed and frustrated by his continued insomnia. Following the meeting, Mr. A’s quetiapine is further increased to 450 mg every night at bedtime to address continued insomnia, and bupropion XL is increased to 300 mg/d to address continued depressive symptoms. During the next few days, his affective symptoms improve; however, his initial insomnia continues, and quetiapine is further increased to 500 mg every night at bedtime.
Continue to: On hospital Day 20...
On hospital Day 20, Mr. A is discharged back to his outpatient psychiatrist and receives quetiapine, 500 mg every night at bedtime, and bupropion XL, 300 mg/d. Although Mr. A’s depression and anxiety continue to be well controlled, his insomnia persists. Sleep hygiene is addressed, and alprazolam, 0.5 mg every night at bedtime, is added to his regimen, which proves to be effective.
OUTCOME A slow remission
After a year of treatment, Mr. A is slowly tapered off of all medications. Two years later, he is in complete remission of all psychiatric symptoms and no longer requires any psychotropic medications.
The authors’ observations
Treatment for hypercortisolism in patients with psychiatric symptoms triggered by glucocorticoid imbalance has typically resulted in a decrease in the severity of their psychiatric symptoms.9,11 A prospective longitudinal study examining 33 patients found that correction of hypercortisolism in patients with Cushing’s syndrome often led to resolution of their psychiatric symptoms, with 87.9% of patients back to baseline within 1 year.14 However, to our knowledge, few reports have described the management of patients whose symptoms are resistant to treatment of hypercortisolism.
In our case, after transsphenoidal resection of an adenoma, Mr. A became suicidal and paranoid, and his anxiety and insomnia also persisted. A possible explanation for the worsening of Mr. A’s symptoms after surgery could be the slow recovery of the hypothalamic-pituitary-adrenal (HPA) axis and therefore a temporary deficiency in glucocorticoid, which caused an increase in catecholamines, leading to an increase in stress.14 This concept of a “slow recovery” is supported by the fact that Mr. A was successfully weaned off all medication after 1 year of treatment, and achieved complete remission of psychiatric symptoms for >2 years. Furthermore, the severity of Mr. A’s symptoms appeared to correlate with his 24-hour urine cortisol and
Future research should evaluate the utility of screening all patients with treatment-resistant anxiety and/or insomnia for hypercortisolism. Even without other clues to endocrinopathies, serum cortisol levels can be used as a screening tool for diagnosing underlying medical causes in patients with anxiety and depression.2 A greater understanding of the relationship between medical and psychiatric manifestations will allow clinicians to better care for patients. Further research is needed to elucidate the quantitative relationship between cortisol levels and anxiety to evaluate severity, guide treatment planning, and follow treatment response for patients with anxiety. It may be useful to determine the threshold between elevated cortisol levels due to anxiety vs elevated cortisol due to an underlying medical pathology such as Cushing’s disease. Additionally, little research has been conducted to compare how psychiatric symptoms respond to pituitary macroadenoma resection alone, pharmaceutical intervention alone, or a combination of these approaches. It would be beneficial to evaluate these treatment strategies to elucidate the most effective method to reduce psychiatric symptoms in patients with hypercortisolism, and perhaps to reduce the incidence of post-resection worsening of psychiatric symptoms.
Continue to: This case was challenging...
This case was challenging because Mr. A did not initially respond to psychiatric intervention, his psychiatric symptoms worsened after transsphenoidal resection of the pituitary adenoma, and his symptoms were alleviated only after psychiatric medications were re-initiated following surgery. This case highlights the importance of considering an underlying medically diagnosable and treatable cause of psychiatric illness, and illustrates the complex ongoing management that may be necessary to help a patient with this condition achieve their baseline. Further, Mr. A’s case shows that the absence of response to standard psychiatric therapies should warrant earlier laboratory and/or imaging evaluation prior to or in conjunction with psychiatric referral. Additionally, testing for cortisol levels is not typically done for a patient with treatment-resistant anxiety, and this case highlights the importance of considering hypercortisolism in such circumstances.
Bottom Line
Consider testing cortisol levels in patients with treatment-resistant anxiety and insomnia, because cortisol plays a role in Cushing’s disease and anxiety. The severity of psychiatric manifestations of Cushing’s disease may correlate with cortisol levels. Treatment should focus on symptomatic management and underlying etiology.
Related Resources
- Roberts LW, Hales RE, Yudofsky SC, ed. The American Psychiatric Association Publishing Textbook of Psychiatry. 7th ed. American Psychiatric Association Publishing; 2019.
- Rotham J. Cushing’s syndrome: a tale of frequent misdiagnosis. National Center for Health Research. 2020. www.center4research.org/cushings-syndrome-frequent-misdiagnosis/
- Middleman D. Psychiatric issues of Cushing’s patients: coping with Cushing’s. Cushing’s Support and Research Foundation. www.csrf.net/coping-with-cushings/psychiatric-issues-of-cushings-patients/
Drug Brand Names
Alprazolam • Xanax
Bupropion • Wellbutrin
Dexamethasone • Decadron
Diazepam • Valium
Eszopiclone • Lunesta
Paroxetine • Paxil
Quetiapine • Seroquel
Zolpidem tartrate • Ambien CR
CASE Anxious and can’t sleep
Mr. A, age 41, presents to his primary care physician (PCP) with anxiety and insomnia. He describes having generalized anxiety with initial and middle insomnia, and says he is sleeping an average of 2 hours per night. He denies any other psychiatric symptoms. Mr. A has no significant psychiatric or medical history.
Mr. A is initiated on zolpidem tartrate, 12.5 mg every night at bedtime, and paroxetine, 20 mg every night at bedtime, for anxiety and insomnia, but these medications result in little to no improvement.
During a 4-month period, he is treated with trials of alprazolam, 0.5 mg every 8 hours as needed; diazepam 5 mg twice a day as needed; diphenhydramine, 50 mg at bedtime; and eszopiclone, 3 mg at bedtime. Despite these treatments, he experiences increased anxiety and insomnia, and develops depressive symptoms, including depressed mood, poor concentration, general malaise, extreme fatigue, a 15-pound unintentional weight loss, erectile dysfunction, and decreased libido. Mr. A denies having suicidal or homicidal ideations. Additionally, he typically goes to the gym approximately 3 times per week, and has noticed that the amount of weight he is able to lift has decreased, which is distressing. Previously, he had been able to lift 300 pounds, but now he can only lift 200 pounds.
[polldaddy:10891920]
The authors’ observations
Insomnia, anxiety, and depression are common chief complaints in medical settings. However, some psychiatric presentations may have an underlying medical etiology.
DSM-5 requires that medical conditions be ruled out in order for a patient to meet criteria for a psychiatric diagnosis.1 Medical differential diagnoses for patients with psychiatric symptoms can include autoimmune, drug/toxin, metabolic, infectious, neoplastic, neurologic, and nutritional etiologies (Table 12). To rule out the possibility of an underlying medical etiology, general screening guidelines include complete blood count, complete metabolic panel, urinalysis, and urine drug screen with alcohol. Human immunodeficiency virus testing and thyroid hormone testing are also commonly ordered.3 Further laboratory testing and imaging is typically not warranted in the absence of historical or physical findings because they are not advocated as cost-effective, so health care professionals must use their clinical judgment to determine appropriate further evaluation. The onset of anxiety most commonly occurs in late adolescence early and adulthood, but Mr. A experienced his first symptoms of anxiety at age 41.2 Mr. A’s age, lack of psychiatric or family history of mental illness, acute onset of symptoms, and failure of symptoms to abate with standard psychiatric treatments warrant a more extensive workup.

EVALUATION Imaging reveals an important finding
Because Mr. A’s symptoms do not improve with standard psychiatric treatments, his PCP orders standard laboratory bloodwork to investigate a possible medical etiology; however, his results are all within normal range.
After the PCP’s niece is coincidentally diagnosed with a pituitary macroadenoma, the PCP orders brain imaging for Mr. A. Results of an MRI show that Mr. A has a 1.6-cm macroadenoma of the pituitary. He is referred to an endocrinologist, who orders additional laboratory tests that show an elevated 24-hour free urine cortisol level of 73 μg/24 h (normal range: 3.5 to 45 μg/24 h), suggesting that Mr. A’s anxiety may be due to Cushing’s disease or that his anxiety caused falsely elevated urinary cortisol levels. Four weeks later, bloodwork is repeated and shows an abnormal dexamethasone suppression test, and 2 more elevated 24-hour free urine cortisol levels of 76 μg/24 h and 150 μg/24 h. A repeat MRI shows a 1.8-cm, mostly cystic sellar mass, indicating the need for surgical intervention. Although the tumor is large and shows optic nerve compression, Mr. A does not complain of headaches or changes in vision.
Continue to: Two months later...
Two months later, Mr. A undergoes a transsphenoidal tumor resection of the pituitary adenoma, and biopsy results confirm an adrenocorticotropic hormone (ACTH)-secreting pituitary macroadenoma, which is consistent with Cushing’s disease. Following surgery, steroid treatment with dexamethasone is discontinued due to a persistently elevated
[polldaddy:10891923]
The authors’ observations
Chronic excess glucocorticoid production is the underlying pathophysiology of Cushing’s disease, which is most commonly caused by an ACTH-producing adenoma.4,5 When these hormones become dysregulated, the result can be over- or underproduction of cortisol, which can lead to physical and psychiatric manifestations.6
Cushing’s disease most commonly manifests with the physical symptoms of centripetal fat deposition, abdominal striae, facial plethora, muscle atrophy, bone density loss, immunosuppression, and cardiovascular complications.5
Hypercortisolism can precipitate anxiety (12% to 79%), mood disorders (50% to 70%), and (less commonly) psychotic disorders; however, in a clinical setting, if a patient presented with one of these as a chief complaint, they would likely first be treated psychiatrically rather than worked up medically for a rare medical condition.5,7-13
Mr. A’s initial bloodwork was unremarkable, but cortisol levels were not obtained at that time because testing for cortisol levels to rule out an underlying medical condition is not routine in patients with depression and anxiety. In Mr. A’s case, a neuroendocrine workup was only ordered once his PCP’s niece coincidentally was diagnosed with a pituitary adenoma.
Continue to: For Mr. A...
For Mr. A, Cushing’s disease presented as a psychiatric disorder with anxiety and insomnia that were resistant to numerous psychiatric medications during an 8-month period. If Mr. A’s PCP had not ordered a brain MRI, he may have continued to receive ineffective psychiatric treatment for some time. Many of Mr. A’s physical symptoms were consistent with Cushing’s disease and mental illness, including erectile dysfunction, fatigue, and muscle weakness; however, his 15-pound weight loss pointed more toward psychiatric illness and further disguised his underlying medical diagnosis, because sudden weight gain is commonly seen in Cushing’s disease (Table 24,5,7,9).
TREATMENT Persistent psychiatric symptoms, then finally relief
Four weeks after surgery, Mr. A’s psychiatric symptoms gradually intensify, which prompts him to see a psychiatrist. A mental status examination (MSE) shows that he is well-nourished, with normal activity, appropriate behavior, and coherent thought process, but depressed mood and flat affect. He denies suicidal or homicidal ideation. He reports that despite being advised to have realistic expectations, he had high hopes that the surgery would lead to remission of all his symptoms, and expresses disappointment that he does not feel “back to normal.”
Six days later, Mr. A’s wife takes him to the hospital. His MSE shows that he has a tense appearance, fidgety activity, depressed and anxious mood, restricted affect, circumstantial thought process, and paranoid delusions that his wife was plotting against him. He says he still is experiencing insomnia. He also discloses having suicidal ideations with a plan and intent to overdose on medication, as well as homicidal ideations about killing his wife and children. Mr. A provides reasons for why he would want to hurt his family, and does not appear to be bothered by these thoughts.
Mr. A is admitted to the inpatient psychiatric unit and is prescribed quetiapine, 100 mg every night at bedtime. During the next 2 days, quetiapine is titrated to 300 mg every night at bedtime. On hospital Day 3, Mr. A says he is feeling worse than the previous days. He is still having vague suicidal thoughts and feels agitated, guilty, and depressed. To treat these persistent symptoms, quetiapine is further increased to 400 mg every night at bedtime, and he is initiated on bupropion XL, 150 mg, to treat persistent symptoms.
After 1 week of hospitalization, the treatment team meets with Mr. A and his wife, who has been supportive throughout her husband’s hospitalization. During the meeting, they both agree that Mr. A has experienced some improvement because he is no longer having suicidal or homicidal thoughts, but he is still feeling depressed and frustrated by his continued insomnia. Following the meeting, Mr. A’s quetiapine is further increased to 450 mg every night at bedtime to address continued insomnia, and bupropion XL is increased to 300 mg/d to address continued depressive symptoms. During the next few days, his affective symptoms improve; however, his initial insomnia continues, and quetiapine is further increased to 500 mg every night at bedtime.
Continue to: On hospital Day 20...
On hospital Day 20, Mr. A is discharged back to his outpatient psychiatrist and receives quetiapine, 500 mg every night at bedtime, and bupropion XL, 300 mg/d. Although Mr. A’s depression and anxiety continue to be well controlled, his insomnia persists. Sleep hygiene is addressed, and alprazolam, 0.5 mg every night at bedtime, is added to his regimen, which proves to be effective.
OUTCOME A slow remission
After a year of treatment, Mr. A is slowly tapered off of all medications. Two years later, he is in complete remission of all psychiatric symptoms and no longer requires any psychotropic medications.
The authors’ observations
Treatment for hypercortisolism in patients with psychiatric symptoms triggered by glucocorticoid imbalance has typically resulted in a decrease in the severity of their psychiatric symptoms.9,11 A prospective longitudinal study examining 33 patients found that correction of hypercortisolism in patients with Cushing’s syndrome often led to resolution of their psychiatric symptoms, with 87.9% of patients back to baseline within 1 year.14 However, to our knowledge, few reports have described the management of patients whose symptoms are resistant to treatment of hypercortisolism.
In our case, after transsphenoidal resection of an adenoma, Mr. A became suicidal and paranoid, and his anxiety and insomnia also persisted. A possible explanation for the worsening of Mr. A’s symptoms after surgery could be the slow recovery of the hypothalamic-pituitary-adrenal (HPA) axis and therefore a temporary deficiency in glucocorticoid, which caused an increase in catecholamines, leading to an increase in stress.14 This concept of a “slow recovery” is supported by the fact that Mr. A was successfully weaned off all medication after 1 year of treatment, and achieved complete remission of psychiatric symptoms for >2 years. Furthermore, the severity of Mr. A’s symptoms appeared to correlate with his 24-hour urine cortisol and
Future research should evaluate the utility of screening all patients with treatment-resistant anxiety and/or insomnia for hypercortisolism. Even without other clues to endocrinopathies, serum cortisol levels can be used as a screening tool for diagnosing underlying medical causes in patients with anxiety and depression.2 A greater understanding of the relationship between medical and psychiatric manifestations will allow clinicians to better care for patients. Further research is needed to elucidate the quantitative relationship between cortisol levels and anxiety to evaluate severity, guide treatment planning, and follow treatment response for patients with anxiety. It may be useful to determine the threshold between elevated cortisol levels due to anxiety vs elevated cortisol due to an underlying medical pathology such as Cushing’s disease. Additionally, little research has been conducted to compare how psychiatric symptoms respond to pituitary macroadenoma resection alone, pharmaceutical intervention alone, or a combination of these approaches. It would be beneficial to evaluate these treatment strategies to elucidate the most effective method to reduce psychiatric symptoms in patients with hypercortisolism, and perhaps to reduce the incidence of post-resection worsening of psychiatric symptoms.
Continue to: This case was challenging...
This case was challenging because Mr. A did not initially respond to psychiatric intervention, his psychiatric symptoms worsened after transsphenoidal resection of the pituitary adenoma, and his symptoms were alleviated only after psychiatric medications were re-initiated following surgery. This case highlights the importance of considering an underlying medically diagnosable and treatable cause of psychiatric illness, and illustrates the complex ongoing management that may be necessary to help a patient with this condition achieve their baseline. Further, Mr. A’s case shows that the absence of response to standard psychiatric therapies should warrant earlier laboratory and/or imaging evaluation prior to or in conjunction with psychiatric referral. Additionally, testing for cortisol levels is not typically done for a patient with treatment-resistant anxiety, and this case highlights the importance of considering hypercortisolism in such circumstances.
Bottom Line
Consider testing cortisol levels in patients with treatment-resistant anxiety and insomnia, because cortisol plays a role in Cushing’s disease and anxiety. The severity of psychiatric manifestations of Cushing’s disease may correlate with cortisol levels. Treatment should focus on symptomatic management and underlying etiology.
Related Resources
- Roberts LW, Hales RE, Yudofsky SC, ed. The American Psychiatric Association Publishing Textbook of Psychiatry. 7th ed. American Psychiatric Association Publishing; 2019.
- Rotham J. Cushing’s syndrome: a tale of frequent misdiagnosis. National Center for Health Research. 2020. www.center4research.org/cushings-syndrome-frequent-misdiagnosis/
- Middleman D. Psychiatric issues of Cushing’s patients: coping with Cushing’s. Cushing’s Support and Research Foundation. www.csrf.net/coping-with-cushings/psychiatric-issues-of-cushings-patients/
Drug Brand Names
Alprazolam • Xanax
Bupropion • Wellbutrin
Dexamethasone • Decadron
Diazepam • Valium
Eszopiclone • Lunesta
Paroxetine • Paxil
Quetiapine • Seroquel
Zolpidem tartrate • Ambien CR
1. Diagnostic and statistical manual of mental disorders, 5th ed. American Psychiatric Association; 2013.
2. Sadock BJ, Sadock VA, Ruiz P, et al. Neural sciences. In: Sadock BJ, Sadock VA, Ruiz P, et al. Kaplan and Sadock’s synopsis of psychiatry: behavioral sciences/clinical psychiatry. 11th ed. Wolters Kluwer; 2015.
3. Anfinson TJ, Kathol RG. Screening laboratory evaluation in psychiatric patients: a review. Gen Hosp Psychiatry. 1992;14(4):248-257.
4. Fehm HL, Voigt KH. Pathophysiology of Cushing’s disease. Pathobiol Annu. 1979;9:225-255.
5. Fujii Y, Mizoguchi Y, Masuoka J, et al. Cushing’s syndrome and psychosis: a case report and literature review. Prim Care Companion CNS Disord. 2018;20(5):18.
6. Raff H, Sharma ST, Nieman LK. Physiological basis for the etiology, diagnosis, and treatment of adrenal disorders: Cushing’s syndrome, adrenal insufficiency, and congenital adrenal hyperplasia. Compr Physiol. 2011;4(2):739-769.
7. Santos A, Resimini E, Pascual JC, et al. Psychiatric symptoms in patients with Cushing’s syndrome: prevalence diagnosis, and management. Drugs. 2017;77(8):829-842.
8. Arnaldi G, Angeli A, Atkinson B, et al. Diagnosis and complications of Cushing’s syndrome: a consensus statement. J Clin Endocrinol Metab. 2003;88(12):5593-5602.
9. Sonino N, Fava GA. Psychosomatic aspects of Cushing’s disease. Psychother Psychosom. 1998;67(3):140-146.
10. Loosen PT, Chambliss B, DeBold CR, et al. Psychiatric phenomenology in Cushing’s disease. Pharmacopsychiatry. 1992;25(4):192-198.
11. Kelly WF, Kelly MJ, Faragher B. A prospective study of psychiatric and psychological aspects of Cushing’s syndrome. Clin Endocrinol. 1996;45(6):715-720.
12. Katho RG, Delahunt JW, Hannah L. Transition from bipolar affective disorder to intermittent Cushing’s syndrome: case report. J Clin Psychiatry. 1985;46(5):194-196.
13. Hirsh D, Orr G, Kantarovich V, et al. Cushing’s syndrome presenting as a schizophrenia-like psychotic state. Isr J Psychiatry Relat Sci. 2000;37(1):46-50.
14. Dorn LD, Burgess ES, Friedman TC, et al. The longitudinal course of psychopathology in Cushing’s syndrome after correction of hypercortisolism. J Clin Endocrinol Metab. 1997;82(3):912-919.
15. Starkman MN, Schteingart DE, Schork MA. Cushing’s syndrome after treatment: changes in cortisol and ACTH levels, and amelioration of the depressive syndrome. Psychiatry Res. 1986;19(3):177-178.
1. Diagnostic and statistical manual of mental disorders, 5th ed. American Psychiatric Association; 2013.
2. Sadock BJ, Sadock VA, Ruiz P, et al. Neural sciences. In: Sadock BJ, Sadock VA, Ruiz P, et al. Kaplan and Sadock’s synopsis of psychiatry: behavioral sciences/clinical psychiatry. 11th ed. Wolters Kluwer; 2015.
3. Anfinson TJ, Kathol RG. Screening laboratory evaluation in psychiatric patients: a review. Gen Hosp Psychiatry. 1992;14(4):248-257.
4. Fehm HL, Voigt KH. Pathophysiology of Cushing’s disease. Pathobiol Annu. 1979;9:225-255.
5. Fujii Y, Mizoguchi Y, Masuoka J, et al. Cushing’s syndrome and psychosis: a case report and literature review. Prim Care Companion CNS Disord. 2018;20(5):18.
6. Raff H, Sharma ST, Nieman LK. Physiological basis for the etiology, diagnosis, and treatment of adrenal disorders: Cushing’s syndrome, adrenal insufficiency, and congenital adrenal hyperplasia. Compr Physiol. 2011;4(2):739-769.
7. Santos A, Resimini E, Pascual JC, et al. Psychiatric symptoms in patients with Cushing’s syndrome: prevalence diagnosis, and management. Drugs. 2017;77(8):829-842.
8. Arnaldi G, Angeli A, Atkinson B, et al. Diagnosis and complications of Cushing’s syndrome: a consensus statement. J Clin Endocrinol Metab. 2003;88(12):5593-5602.
9. Sonino N, Fava GA. Psychosomatic aspects of Cushing’s disease. Psychother Psychosom. 1998;67(3):140-146.
10. Loosen PT, Chambliss B, DeBold CR, et al. Psychiatric phenomenology in Cushing’s disease. Pharmacopsychiatry. 1992;25(4):192-198.
11. Kelly WF, Kelly MJ, Faragher B. A prospective study of psychiatric and psychological aspects of Cushing’s syndrome. Clin Endocrinol. 1996;45(6):715-720.
12. Katho RG, Delahunt JW, Hannah L. Transition from bipolar affective disorder to intermittent Cushing’s syndrome: case report. J Clin Psychiatry. 1985;46(5):194-196.
13. Hirsh D, Orr G, Kantarovich V, et al. Cushing’s syndrome presenting as a schizophrenia-like psychotic state. Isr J Psychiatry Relat Sci. 2000;37(1):46-50.
14. Dorn LD, Burgess ES, Friedman TC, et al. The longitudinal course of psychopathology in Cushing’s syndrome after correction of hypercortisolism. J Clin Endocrinol Metab. 1997;82(3):912-919.
15. Starkman MN, Schteingart DE, Schork MA. Cushing’s syndrome after treatment: changes in cortisol and ACTH levels, and amelioration of the depressive syndrome. Psychiatry Res. 1986;19(3):177-178.





