User login
The paranoid business executive
CASE Bipolar-like symptoms
Mr. R, age 48, presents to the psychiatric emergency department (ED) for the third time in 4 days after a change in his behavior over the last 2.5 weeks. He exhibits heightened extroversion, pressured speech, and uncharacteristic irritability. Mr. R’s wife reports that her husband normally is reserved.
Mr. R’s wife first became concerned when she noticed he was not sleeping and spending his nights changing the locks on their home. Mr. R, who is a business executive, occupied his time by taking notes on ways to protect his identity from the senior partners at his company.
Three weeks before his first ED visit, Mr. R had been treated for a neck abscess with incision and drainage. He was sent home with a 10-day course of amoxicillin/clavulanate, 875/125 mg by mouth twice daily. There were no reports of steroid use during or after the procedure. Four days after starting the antibiotic, he stopped taking it because he and his wife felt it was contributing to his mood changes and bizarre behavior.
During his first visit to the ED, Mr. R received a 1-time dose of olanzapine, 5 mg by mouth, which helped temporarily reduce his anxiety; however, he returned the following day with the same anxiety symptoms and was discharged with a 30-day prescription for olanzapine, 5 mg/d, to manage symptoms until he could establish care with an outpatient psychiatrist. Two days later, he returned to the ED yet again convinced people were spying on him and that his coworkers were plotting to have him fired. He was not taking his phone to work due to fears that it would be hacked.
Mr. R’s only home medication is clomiphene citrate, 100 mg/d by mouth, which he’s received for the past 7 months to treat low testosterone. He has no personal or family history of psychiatric illness and no prior signs of mania or hypomania.
At the current ED visit, Mr. R’s testosterone level is checked and is within normal limits. His urine drug screen, head CT, and standard laboratory test results are unremarkable, except for mild transaminitis that does not warrant acute management.
The clinicians in the ED establish a diagnosis of mania, unspecified, and psychotic disorder, unspecified. They recommend that Mr. R be admitted for mood stabilization.
[polldaddy:10485725]
Continue to: The authors' observations
The authors’ observations
Our initial impression was that Mr. R was experiencing a manic episode from undiagnosed bipolar I disorder. The diagnosis was equivocal considering his age, lack of family history, and absence of prior psychiatric symptoms. In most cases, the mean age of onset for mania is late adolescence to early adulthood. It would be less common for a patient to experience a first manic episode at age 48, although mania may emerge at any age. Results from a large British study showed that the incidence of a first manic episode drops from 13.81% in men age 16 to 25 to 2.62% in men age 46 to 55.1 However, some estimates suggest that the prevalence of late-onset mania is much higher than previously expected; medical comorbidities, such as dementia and delirium, may play a significant role in posing as manic-type symptoms in these patients.2
In Mr. R’s case, he remained fully alert and oriented without waxing and waning attentional deficits, which made delirium less likely. His affective symptoms included a reduced need for sleep, anxiety, irritability, rapid speech, and grandiosity lasting at least 2 weeks. He also exhibited psychotic symptoms in the form of paranoia. Altogether, he fit diagnostic criteria for bipolar I disorder well.
At the time of his manic episode, Mr. R was taking clomiphene. Clomiphene-induced mania and psychosis has been reported scarcely in the literature.3 In these cases, behavioral changes occurred within the first month of clomiphene initiation, which is dissimilar from Mr. R’s timeline.4 However, there appeared to be a temporal relationship between Mr. R’s use of amoxicillin/clavulanate and his manic episode.
This led us to consider whether medication-induced bipolar disorder would be a more appropriate diagnosis. There are documented associations between mania and antibiotics5; however, to our knowledge, mania secondary specifically to amoxicillin/clavulanate has not been reported extensively in the American literature. We found 1 case of suspected amoxicillin-induced psychosis,6 as well as a case report from the Netherlands of possible amoxicillin/clavulanate-induced mania.7
EVALUATION Ongoing paranoia
During his psychiatric hospitalization, Mr. R remains cooperative and polite, but exhibits ongoing paranoia, pressured speech, and poor reality testing. He remains convinced that “people are out to get me,” and routinely scans the room for safety during daily evaluations. He reports that he feels safe in the hospital, but does not feel safe to leave. Mr. R does not recall if in the past he had taken any products containing amoxicillin, but he is able to appreciate changes in his mood after being prescribed the antibiotic. He reports that starting the antibiotic made him feel confident in social interactions.
Continue to: During Mr. R's psychiatric hospitalization...
During Mr. R’s psychiatric hospitalization, olanzapine is titrated to 10 mg at bedtime. Clomiphene citrate is discontinued to limit any potential precipitants of mania, and amoxicillin/clavulanate is not restarted.
Mr. R gradually shows improvement in sleep quality and duration and becomes less irritable. His speech returns to a regular rate and rhythm. He eventually begins to question whether his fears were reality-based. After 4 days, Mr. R is ready to be discharged home and return to work.
[polldaddy:10485726]
The authors’ observations
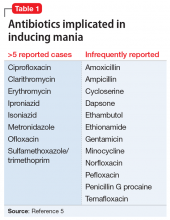
The term “antibiomania” is used to describe manic episodes that coincide with antibiotic usage.8 Clarithromycin and ciprofloxacin are the agents most frequently implicated in antibiomania.9 While numerous reports exist in the literature, antibiomania is still considered a rare or unusual adverse event.
The link between infections and neuropsychiatric symptoms is well documented, which makes it challenging to tease apart the role of the acute infection from the use of antibiotics in precipitating psychiatric symptoms. However, in most reported cases of antibiomania, the onset of manic symptoms typically occurs within the first week of antibiotic initiation and resolves 1 to 3 days after medication discontinuation. The temporal relationship between antibiotic initiation and onset of neuropsychiatric symptoms has been best highlighted in cases where clarithromycin is used to treat a chronic Helicobacter pylori infection.10
While reports of antibiomania date back more than 6 decades, the exact mechanism by which antibiotics cause psychiatric symptoms is mostly unknown, although there are several hypotheses.5 Many hypotheses suggest some antibiotics play a role in reducing gamma-aminobutyric acid (GABA) neurotransmission. Quinolones, for example, have been found to cross the blood–brain barrier and can inhibit GABA from binding to the receptor sites. This can result in hyper-excitability in the CNS. Several quinolones have been implicated in antibiomania (Table 15). Penicillins are also thought to interfere with GABA neurotransmission in a similar fashion; however, amoxicillin-clavulanate has poor CNS penetration in the absence of blood–brain barrier disruption,11 which makes this theory a less plausible explanation for Mr. R’s case.
Continue to: Another possible mechanism...
Another possible mechanism of antibiotic-induced CNS excitability is through the glutamatergic system. Cycloserine, an antitubercular agent, is an N-methyl-D-aspartate receptor (NMDA) partial agonist and has reported neuropsychiatric adverse effects.12 It has been proposed that quinolones may also have NMDA agonist activity.
The prostaglandin hypothesis suggests that a decrease in GABA may increase concentrations of steroid hormones in the rat CNS.13 Steroids have been implicated in the breakdown of prostaglandin E1 (PGE1).13 A disruption in steroid regulation may prevent PGE1 breakdown. Lithium’s antimanic properties are thought to be caused at least in part by limiting prostaglandin production.14 Thus, a shift in PGE1 may lead to mood dysregulation.
Bipolar disorder has been linked with mitochondrial function abnormalities.15 Antibiotics that target ribosomal RNA may disrupt normal mitochondrial function and increase risk for mania precipitation.15 However, amoxicillin exerts its antibiotic effects through binding to penicillin-binding proteins, which leads to inhibition of the cell wall biosynthesis.
Lastly, research into the microbiome has elucidated the gut-brain axis. In animal studies, the microbiome has been found to play a role in immunity, cognitive function, and behavior. Dysbiosis in the microbiome is currently being investigated for its role in schizophrenia and bipolar disorder.16 Both the microbiome and changes in mitochondrial function are thought to develop over time, so while these are plausible explanations, an onset within 4 days of antibiotic initiation is likely too short of an exposure time to produce these changes.
The most likely causes of Mr. R’s manic episode were clomiphene or amoxicillin-clavulanate, and the time course seems to indicate the antibiotic was the most likely culprit. Table 2 lists things to consider if you suspect your patient may be experiencing antibiomania.

Continue to: TREATMENT Stable on olanzapine
TREATMENT Stable on olanzapine
During his first visit to the outpatient clinic 4 weeks after being discharged, Mr. R reports that he has successfully returned to work, and his paranoia has completely resolved. He continues to take olanzapine, 10 mg nightly, and has restarted clomiphene, 100 mg/d.
During this outpatient follow-up visit, Mr. R attributes his manic episode to an adverse reaction to amoxicillin/clavulanate, and requests to be tapered off olanzapine. After he and his psychiatrist discuss the risk of relapse in untreated bipolar disorder, olanzapine is reduced to 7.5 mg at bedtime with a plan to taper to discontinuation.
At his second follow-up visit 1 month later, Mr. R has also stopped clomiphene and is taking a herbal supplement instead, which he reports is helpful for his fatigue.
[polldaddy:10485727]
OUTCOME Lasting euthymic mood
Mr. R agrees to our recommendation of continuing to monitor him every 3 months for at least 1 year. We provide him and his wife with education about early warning signs of mood instability. Eight months after his manic episode, Mr. R no longer receives any psychotropic medications and shows no signs of mood instability. His mood remains euthymic and he is able to function well at work and in his personal life.
Bottom Line
‘Antibiomania’ describes manic episodes that coincide with antibiotic usage. This adverse effect is rare but should be considered in patients who present with unexplained first-episode mania, particularly those with an initial onset of mania after early adulthood.
Continue to: Related Resources
Related Resources
- Rakofsky JJ, Dunlop BW. Nothing to sneeze at: Upper respiratory infections and mood disorders. Current Psychiatry. 2019;18(7):29-34.
- Adiba A, Jackson JC, Torrence CL. Older-age bipolar disorder: A case series. Current Psychiatry. 2019;18(2):24-29
Drug Brand Names
Amoxicillin • Amoxil
Amoxicillin/clavulanate • Augmentin
Ampicillin • Omnipen-N, Polycillin-N
Ciprofloxacin • Cipro
Clarithromycin • Biaxin
Clomiphene • Clomid
Cycloserine • Seromycin
Dapsone • Dapsone
Erythromycin • Erythrocin, Pediamycin
Ethambutol • Myambutol
Ethionamide • Trecator-SC
Gentamicin • Garamycin
Isoniazid • Hyzyd, Nydrazid
Lithium • Eskalith, Lithobid
Metronidazole • Flagyl
Minocycline • Dynacin, Solodyn
Norfloxacin • Noroxin
Ofloxacin • Floxin
Olanzapine • Zyprexa
Penicillin G procaine • Duracillin A-S, Pfizerpen
Sulfamethoxazole/trimethoprim • Bactrim, Septra
1. Kennedy M, Everitt B, Boydell J, et al. Incidence and distribution of first-episode mania by age: results for a 35-year study. Psychol Med. 2005;35(6):855-863.
2. Dols A, Kupka RW, van Lammeren A, et al. The prevalence of late-life mania: a review. Bipolar Disord. 2014;16:113-118.
3. Siedontopf F, Horstkamp B, Stief G, et al. Clomiphene citrate as a possible cause of a psychotic reaction during infertility treatment. Hum Reprod. 1997;12(4):706-707.
4. Oyffe T, Lerner A, Isaacs G, et al. Clomiphene-induced psychosis. Am J Psychiatry. 1997;154(8):1169-1170.
5. Lambrichts S, Van Oudenhove L, Sienaert P. Antibiotics and mania: a systematic review. J Affect Disord. 2017;219:149-156.
6. Beal DM, Hudson B, Zaiac M. Amoxicillin-induced psychosis? Am J Psychiatry. 1986;143(2):255-256.
7. Klain V, Timmerman L. Antibiomania, acute manic psychosis following the use of antibiotics. European Psychiatry. 2013;28(suppl 1):1.
8. Abouesh A, Stone C, Hobbs WR. Antimicrobial-induced mania (antibiomania): a review of spontaneous reports. J Clin Psychopharmacol. 2002;22(1):71-81.
9. Lally L, Mannion L. The potential for antimicrobials to adversely affect mental state. BMJ Case Rep. 2013. pii: bcr2013009659. doi: 10.1136/bcr-2013-009659.
10. Neufeld NH, Mohamed NS, Grujich N, et al. Acute neuropsychiatric symptoms associated with antibiotic treatment of Helicobactor Pylori infections: a review. J Psychiatr Pract. 2017;23(1):25-35.
11. Sutter R, Rüegg S, Tschudin-Sutter S. Seizures as adverse events of antibiotic drugs: a systematic review. Neurology. 2015;85(15):1332-1341.
12. Bakhla A, Gore P, Srivastava S. Cycloserine induced mania. Ind Psychiatry J. 2013;22(1):69-70.
13. Barbaccia ML, Roscetti G, Trabucchi M, et al. Isoniazid-induced inhibition of GABAergic transmission enhances neurosteroid content in the rat brain. Neuropharmacology. 1996;35(9-10):1299-1305.
14. Murphy D, Donnelly C, Moskowitz J. Inhibition by lithium of prostaglandin E1 and norepinephrine effects on cyclic adenosine monophosphate production in human platelets. Clin Pharmacol Ther. 1973;14(5):810-814.
15. Clay H, Sillivan S, Konradi C. Mitochondrial dysfunction and pathology in bipolar disorder and schizophrenia. Int J Dev Neurosci. 2011;29(3):311-324.
16. Dickerson F, Severance E, Yolken R. The microbiome, immunity, and schizophrenia and bipolar disorder. Brain Behav Immun. 2017;62:46-52.
CASE Bipolar-like symptoms
Mr. R, age 48, presents to the psychiatric emergency department (ED) for the third time in 4 days after a change in his behavior over the last 2.5 weeks. He exhibits heightened extroversion, pressured speech, and uncharacteristic irritability. Mr. R’s wife reports that her husband normally is reserved.
Mr. R’s wife first became concerned when she noticed he was not sleeping and spending his nights changing the locks on their home. Mr. R, who is a business executive, occupied his time by taking notes on ways to protect his identity from the senior partners at his company.
Three weeks before his first ED visit, Mr. R had been treated for a neck abscess with incision and drainage. He was sent home with a 10-day course of amoxicillin/clavulanate, 875/125 mg by mouth twice daily. There were no reports of steroid use during or after the procedure. Four days after starting the antibiotic, he stopped taking it because he and his wife felt it was contributing to his mood changes and bizarre behavior.
During his first visit to the ED, Mr. R received a 1-time dose of olanzapine, 5 mg by mouth, which helped temporarily reduce his anxiety; however, he returned the following day with the same anxiety symptoms and was discharged with a 30-day prescription for olanzapine, 5 mg/d, to manage symptoms until he could establish care with an outpatient psychiatrist. Two days later, he returned to the ED yet again convinced people were spying on him and that his coworkers were plotting to have him fired. He was not taking his phone to work due to fears that it would be hacked.
Mr. R’s only home medication is clomiphene citrate, 100 mg/d by mouth, which he’s received for the past 7 months to treat low testosterone. He has no personal or family history of psychiatric illness and no prior signs of mania or hypomania.
At the current ED visit, Mr. R’s testosterone level is checked and is within normal limits. His urine drug screen, head CT, and standard laboratory test results are unremarkable, except for mild transaminitis that does not warrant acute management.
The clinicians in the ED establish a diagnosis of mania, unspecified, and psychotic disorder, unspecified. They recommend that Mr. R be admitted for mood stabilization.
[polldaddy:10485725]
Continue to: The authors' observations
The authors’ observations
Our initial impression was that Mr. R was experiencing a manic episode from undiagnosed bipolar I disorder. The diagnosis was equivocal considering his age, lack of family history, and absence of prior psychiatric symptoms. In most cases, the mean age of onset for mania is late adolescence to early adulthood. It would be less common for a patient to experience a first manic episode at age 48, although mania may emerge at any age. Results from a large British study showed that the incidence of a first manic episode drops from 13.81% in men age 16 to 25 to 2.62% in men age 46 to 55.1 However, some estimates suggest that the prevalence of late-onset mania is much higher than previously expected; medical comorbidities, such as dementia and delirium, may play a significant role in posing as manic-type symptoms in these patients.2
In Mr. R’s case, he remained fully alert and oriented without waxing and waning attentional deficits, which made delirium less likely. His affective symptoms included a reduced need for sleep, anxiety, irritability, rapid speech, and grandiosity lasting at least 2 weeks. He also exhibited psychotic symptoms in the form of paranoia. Altogether, he fit diagnostic criteria for bipolar I disorder well.
At the time of his manic episode, Mr. R was taking clomiphene. Clomiphene-induced mania and psychosis has been reported scarcely in the literature.3 In these cases, behavioral changes occurred within the first month of clomiphene initiation, which is dissimilar from Mr. R’s timeline.4 However, there appeared to be a temporal relationship between Mr. R’s use of amoxicillin/clavulanate and his manic episode.
This led us to consider whether medication-induced bipolar disorder would be a more appropriate diagnosis. There are documented associations between mania and antibiotics5; however, to our knowledge, mania secondary specifically to amoxicillin/clavulanate has not been reported extensively in the American literature. We found 1 case of suspected amoxicillin-induced psychosis,6 as well as a case report from the Netherlands of possible amoxicillin/clavulanate-induced mania.7
EVALUATION Ongoing paranoia
During his psychiatric hospitalization, Mr. R remains cooperative and polite, but exhibits ongoing paranoia, pressured speech, and poor reality testing. He remains convinced that “people are out to get me,” and routinely scans the room for safety during daily evaluations. He reports that he feels safe in the hospital, but does not feel safe to leave. Mr. R does not recall if in the past he had taken any products containing amoxicillin, but he is able to appreciate changes in his mood after being prescribed the antibiotic. He reports that starting the antibiotic made him feel confident in social interactions.
Continue to: During Mr. R's psychiatric hospitalization...
During Mr. R’s psychiatric hospitalization, olanzapine is titrated to 10 mg at bedtime. Clomiphene citrate is discontinued to limit any potential precipitants of mania, and amoxicillin/clavulanate is not restarted.
Mr. R gradually shows improvement in sleep quality and duration and becomes less irritable. His speech returns to a regular rate and rhythm. He eventually begins to question whether his fears were reality-based. After 4 days, Mr. R is ready to be discharged home and return to work.
[polldaddy:10485726]
The authors’ observations
The term “antibiomania” is used to describe manic episodes that coincide with antibiotic usage.8 Clarithromycin and ciprofloxacin are the agents most frequently implicated in antibiomania.9 While numerous reports exist in the literature, antibiomania is still considered a rare or unusual adverse event.
The link between infections and neuropsychiatric symptoms is well documented, which makes it challenging to tease apart the role of the acute infection from the use of antibiotics in precipitating psychiatric symptoms. However, in most reported cases of antibiomania, the onset of manic symptoms typically occurs within the first week of antibiotic initiation and resolves 1 to 3 days after medication discontinuation. The temporal relationship between antibiotic initiation and onset of neuropsychiatric symptoms has been best highlighted in cases where clarithromycin is used to treat a chronic Helicobacter pylori infection.10
While reports of antibiomania date back more than 6 decades, the exact mechanism by which antibiotics cause psychiatric symptoms is mostly unknown, although there are several hypotheses.5 Many hypotheses suggest some antibiotics play a role in reducing gamma-aminobutyric acid (GABA) neurotransmission. Quinolones, for example, have been found to cross the blood–brain barrier and can inhibit GABA from binding to the receptor sites. This can result in hyper-excitability in the CNS. Several quinolones have been implicated in antibiomania (Table 15). Penicillins are also thought to interfere with GABA neurotransmission in a similar fashion; however, amoxicillin-clavulanate has poor CNS penetration in the absence of blood–brain barrier disruption,11 which makes this theory a less plausible explanation for Mr. R’s case.
Continue to: Another possible mechanism...
Another possible mechanism of antibiotic-induced CNS excitability is through the glutamatergic system. Cycloserine, an antitubercular agent, is an N-methyl-D-aspartate receptor (NMDA) partial agonist and has reported neuropsychiatric adverse effects.12 It has been proposed that quinolones may also have NMDA agonist activity.
The prostaglandin hypothesis suggests that a decrease in GABA may increase concentrations of steroid hormones in the rat CNS.13 Steroids have been implicated in the breakdown of prostaglandin E1 (PGE1).13 A disruption in steroid regulation may prevent PGE1 breakdown. Lithium’s antimanic properties are thought to be caused at least in part by limiting prostaglandin production.14 Thus, a shift in PGE1 may lead to mood dysregulation.
Bipolar disorder has been linked with mitochondrial function abnormalities.15 Antibiotics that target ribosomal RNA may disrupt normal mitochondrial function and increase risk for mania precipitation.15 However, amoxicillin exerts its antibiotic effects through binding to penicillin-binding proteins, which leads to inhibition of the cell wall biosynthesis.
Lastly, research into the microbiome has elucidated the gut-brain axis. In animal studies, the microbiome has been found to play a role in immunity, cognitive function, and behavior. Dysbiosis in the microbiome is currently being investigated for its role in schizophrenia and bipolar disorder.16 Both the microbiome and changes in mitochondrial function are thought to develop over time, so while these are plausible explanations, an onset within 4 days of antibiotic initiation is likely too short of an exposure time to produce these changes.
The most likely causes of Mr. R’s manic episode were clomiphene or amoxicillin-clavulanate, and the time course seems to indicate the antibiotic was the most likely culprit. Table 2 lists things to consider if you suspect your patient may be experiencing antibiomania.

Continue to: TREATMENT Stable on olanzapine
TREATMENT Stable on olanzapine
During his first visit to the outpatient clinic 4 weeks after being discharged, Mr. R reports that he has successfully returned to work, and his paranoia has completely resolved. He continues to take olanzapine, 10 mg nightly, and has restarted clomiphene, 100 mg/d.
During this outpatient follow-up visit, Mr. R attributes his manic episode to an adverse reaction to amoxicillin/clavulanate, and requests to be tapered off olanzapine. After he and his psychiatrist discuss the risk of relapse in untreated bipolar disorder, olanzapine is reduced to 7.5 mg at bedtime with a plan to taper to discontinuation.
At his second follow-up visit 1 month later, Mr. R has also stopped clomiphene and is taking a herbal supplement instead, which he reports is helpful for his fatigue.
[polldaddy:10485727]
OUTCOME Lasting euthymic mood
Mr. R agrees to our recommendation of continuing to monitor him every 3 months for at least 1 year. We provide him and his wife with education about early warning signs of mood instability. Eight months after his manic episode, Mr. R no longer receives any psychotropic medications and shows no signs of mood instability. His mood remains euthymic and he is able to function well at work and in his personal life.
Bottom Line
‘Antibiomania’ describes manic episodes that coincide with antibiotic usage. This adverse effect is rare but should be considered in patients who present with unexplained first-episode mania, particularly those with an initial onset of mania after early adulthood.
Continue to: Related Resources
Related Resources
- Rakofsky JJ, Dunlop BW. Nothing to sneeze at: Upper respiratory infections and mood disorders. Current Psychiatry. 2019;18(7):29-34.
- Adiba A, Jackson JC, Torrence CL. Older-age bipolar disorder: A case series. Current Psychiatry. 2019;18(2):24-29
Drug Brand Names
Amoxicillin • Amoxil
Amoxicillin/clavulanate • Augmentin
Ampicillin • Omnipen-N, Polycillin-N
Ciprofloxacin • Cipro
Clarithromycin • Biaxin
Clomiphene • Clomid
Cycloserine • Seromycin
Dapsone • Dapsone
Erythromycin • Erythrocin, Pediamycin
Ethambutol • Myambutol
Ethionamide • Trecator-SC
Gentamicin • Garamycin
Isoniazid • Hyzyd, Nydrazid
Lithium • Eskalith, Lithobid
Metronidazole • Flagyl
Minocycline • Dynacin, Solodyn
Norfloxacin • Noroxin
Ofloxacin • Floxin
Olanzapine • Zyprexa
Penicillin G procaine • Duracillin A-S, Pfizerpen
Sulfamethoxazole/trimethoprim • Bactrim, Septra
CASE Bipolar-like symptoms
Mr. R, age 48, presents to the psychiatric emergency department (ED) for the third time in 4 days after a change in his behavior over the last 2.5 weeks. He exhibits heightened extroversion, pressured speech, and uncharacteristic irritability. Mr. R’s wife reports that her husband normally is reserved.
Mr. R’s wife first became concerned when she noticed he was not sleeping and spending his nights changing the locks on their home. Mr. R, who is a business executive, occupied his time by taking notes on ways to protect his identity from the senior partners at his company.
Three weeks before his first ED visit, Mr. R had been treated for a neck abscess with incision and drainage. He was sent home with a 10-day course of amoxicillin/clavulanate, 875/125 mg by mouth twice daily. There were no reports of steroid use during or after the procedure. Four days after starting the antibiotic, he stopped taking it because he and his wife felt it was contributing to his mood changes and bizarre behavior.
During his first visit to the ED, Mr. R received a 1-time dose of olanzapine, 5 mg by mouth, which helped temporarily reduce his anxiety; however, he returned the following day with the same anxiety symptoms and was discharged with a 30-day prescription for olanzapine, 5 mg/d, to manage symptoms until he could establish care with an outpatient psychiatrist. Two days later, he returned to the ED yet again convinced people were spying on him and that his coworkers were plotting to have him fired. He was not taking his phone to work due to fears that it would be hacked.
Mr. R’s only home medication is clomiphene citrate, 100 mg/d by mouth, which he’s received for the past 7 months to treat low testosterone. He has no personal or family history of psychiatric illness and no prior signs of mania or hypomania.
At the current ED visit, Mr. R’s testosterone level is checked and is within normal limits. His urine drug screen, head CT, and standard laboratory test results are unremarkable, except for mild transaminitis that does not warrant acute management.
The clinicians in the ED establish a diagnosis of mania, unspecified, and psychotic disorder, unspecified. They recommend that Mr. R be admitted for mood stabilization.
[polldaddy:10485725]
Continue to: The authors' observations
The authors’ observations
Our initial impression was that Mr. R was experiencing a manic episode from undiagnosed bipolar I disorder. The diagnosis was equivocal considering his age, lack of family history, and absence of prior psychiatric symptoms. In most cases, the mean age of onset for mania is late adolescence to early adulthood. It would be less common for a patient to experience a first manic episode at age 48, although mania may emerge at any age. Results from a large British study showed that the incidence of a first manic episode drops from 13.81% in men age 16 to 25 to 2.62% in men age 46 to 55.1 However, some estimates suggest that the prevalence of late-onset mania is much higher than previously expected; medical comorbidities, such as dementia and delirium, may play a significant role in posing as manic-type symptoms in these patients.2
In Mr. R’s case, he remained fully alert and oriented without waxing and waning attentional deficits, which made delirium less likely. His affective symptoms included a reduced need for sleep, anxiety, irritability, rapid speech, and grandiosity lasting at least 2 weeks. He also exhibited psychotic symptoms in the form of paranoia. Altogether, he fit diagnostic criteria for bipolar I disorder well.
At the time of his manic episode, Mr. R was taking clomiphene. Clomiphene-induced mania and psychosis has been reported scarcely in the literature.3 In these cases, behavioral changes occurred within the first month of clomiphene initiation, which is dissimilar from Mr. R’s timeline.4 However, there appeared to be a temporal relationship between Mr. R’s use of amoxicillin/clavulanate and his manic episode.
This led us to consider whether medication-induced bipolar disorder would be a more appropriate diagnosis. There are documented associations between mania and antibiotics5; however, to our knowledge, mania secondary specifically to amoxicillin/clavulanate has not been reported extensively in the American literature. We found 1 case of suspected amoxicillin-induced psychosis,6 as well as a case report from the Netherlands of possible amoxicillin/clavulanate-induced mania.7
EVALUATION Ongoing paranoia
During his psychiatric hospitalization, Mr. R remains cooperative and polite, but exhibits ongoing paranoia, pressured speech, and poor reality testing. He remains convinced that “people are out to get me,” and routinely scans the room for safety during daily evaluations. He reports that he feels safe in the hospital, but does not feel safe to leave. Mr. R does not recall if in the past he had taken any products containing amoxicillin, but he is able to appreciate changes in his mood after being prescribed the antibiotic. He reports that starting the antibiotic made him feel confident in social interactions.
Continue to: During Mr. R's psychiatric hospitalization...
During Mr. R’s psychiatric hospitalization, olanzapine is titrated to 10 mg at bedtime. Clomiphene citrate is discontinued to limit any potential precipitants of mania, and amoxicillin/clavulanate is not restarted.
Mr. R gradually shows improvement in sleep quality and duration and becomes less irritable. His speech returns to a regular rate and rhythm. He eventually begins to question whether his fears were reality-based. After 4 days, Mr. R is ready to be discharged home and return to work.
[polldaddy:10485726]
The authors’ observations
The term “antibiomania” is used to describe manic episodes that coincide with antibiotic usage.8 Clarithromycin and ciprofloxacin are the agents most frequently implicated in antibiomania.9 While numerous reports exist in the literature, antibiomania is still considered a rare or unusual adverse event.
The link between infections and neuropsychiatric symptoms is well documented, which makes it challenging to tease apart the role of the acute infection from the use of antibiotics in precipitating psychiatric symptoms. However, in most reported cases of antibiomania, the onset of manic symptoms typically occurs within the first week of antibiotic initiation and resolves 1 to 3 days after medication discontinuation. The temporal relationship between antibiotic initiation and onset of neuropsychiatric symptoms has been best highlighted in cases where clarithromycin is used to treat a chronic Helicobacter pylori infection.10
While reports of antibiomania date back more than 6 decades, the exact mechanism by which antibiotics cause psychiatric symptoms is mostly unknown, although there are several hypotheses.5 Many hypotheses suggest some antibiotics play a role in reducing gamma-aminobutyric acid (GABA) neurotransmission. Quinolones, for example, have been found to cross the blood–brain barrier and can inhibit GABA from binding to the receptor sites. This can result in hyper-excitability in the CNS. Several quinolones have been implicated in antibiomania (Table 15). Penicillins are also thought to interfere with GABA neurotransmission in a similar fashion; however, amoxicillin-clavulanate has poor CNS penetration in the absence of blood–brain barrier disruption,11 which makes this theory a less plausible explanation for Mr. R’s case.
Continue to: Another possible mechanism...
Another possible mechanism of antibiotic-induced CNS excitability is through the glutamatergic system. Cycloserine, an antitubercular agent, is an N-methyl-D-aspartate receptor (NMDA) partial agonist and has reported neuropsychiatric adverse effects.12 It has been proposed that quinolones may also have NMDA agonist activity.
The prostaglandin hypothesis suggests that a decrease in GABA may increase concentrations of steroid hormones in the rat CNS.13 Steroids have been implicated in the breakdown of prostaglandin E1 (PGE1).13 A disruption in steroid regulation may prevent PGE1 breakdown. Lithium’s antimanic properties are thought to be caused at least in part by limiting prostaglandin production.14 Thus, a shift in PGE1 may lead to mood dysregulation.
Bipolar disorder has been linked with mitochondrial function abnormalities.15 Antibiotics that target ribosomal RNA may disrupt normal mitochondrial function and increase risk for mania precipitation.15 However, amoxicillin exerts its antibiotic effects through binding to penicillin-binding proteins, which leads to inhibition of the cell wall biosynthesis.
Lastly, research into the microbiome has elucidated the gut-brain axis. In animal studies, the microbiome has been found to play a role in immunity, cognitive function, and behavior. Dysbiosis in the microbiome is currently being investigated for its role in schizophrenia and bipolar disorder.16 Both the microbiome and changes in mitochondrial function are thought to develop over time, so while these are plausible explanations, an onset within 4 days of antibiotic initiation is likely too short of an exposure time to produce these changes.
The most likely causes of Mr. R’s manic episode were clomiphene or amoxicillin-clavulanate, and the time course seems to indicate the antibiotic was the most likely culprit. Table 2 lists things to consider if you suspect your patient may be experiencing antibiomania.

Continue to: TREATMENT Stable on olanzapine
TREATMENT Stable on olanzapine
During his first visit to the outpatient clinic 4 weeks after being discharged, Mr. R reports that he has successfully returned to work, and his paranoia has completely resolved. He continues to take olanzapine, 10 mg nightly, and has restarted clomiphene, 100 mg/d.
During this outpatient follow-up visit, Mr. R attributes his manic episode to an adverse reaction to amoxicillin/clavulanate, and requests to be tapered off olanzapine. After he and his psychiatrist discuss the risk of relapse in untreated bipolar disorder, olanzapine is reduced to 7.5 mg at bedtime with a plan to taper to discontinuation.
At his second follow-up visit 1 month later, Mr. R has also stopped clomiphene and is taking a herbal supplement instead, which he reports is helpful for his fatigue.
[polldaddy:10485727]
OUTCOME Lasting euthymic mood
Mr. R agrees to our recommendation of continuing to monitor him every 3 months for at least 1 year. We provide him and his wife with education about early warning signs of mood instability. Eight months after his manic episode, Mr. R no longer receives any psychotropic medications and shows no signs of mood instability. His mood remains euthymic and he is able to function well at work and in his personal life.
Bottom Line
‘Antibiomania’ describes manic episodes that coincide with antibiotic usage. This adverse effect is rare but should be considered in patients who present with unexplained first-episode mania, particularly those with an initial onset of mania after early adulthood.
Continue to: Related Resources
Related Resources
- Rakofsky JJ, Dunlop BW. Nothing to sneeze at: Upper respiratory infections and mood disorders. Current Psychiatry. 2019;18(7):29-34.
- Adiba A, Jackson JC, Torrence CL. Older-age bipolar disorder: A case series. Current Psychiatry. 2019;18(2):24-29
Drug Brand Names
Amoxicillin • Amoxil
Amoxicillin/clavulanate • Augmentin
Ampicillin • Omnipen-N, Polycillin-N
Ciprofloxacin • Cipro
Clarithromycin • Biaxin
Clomiphene • Clomid
Cycloserine • Seromycin
Dapsone • Dapsone
Erythromycin • Erythrocin, Pediamycin
Ethambutol • Myambutol
Ethionamide • Trecator-SC
Gentamicin • Garamycin
Isoniazid • Hyzyd, Nydrazid
Lithium • Eskalith, Lithobid
Metronidazole • Flagyl
Minocycline • Dynacin, Solodyn
Norfloxacin • Noroxin
Ofloxacin • Floxin
Olanzapine • Zyprexa
Penicillin G procaine • Duracillin A-S, Pfizerpen
Sulfamethoxazole/trimethoprim • Bactrim, Septra
1. Kennedy M, Everitt B, Boydell J, et al. Incidence and distribution of first-episode mania by age: results for a 35-year study. Psychol Med. 2005;35(6):855-863.
2. Dols A, Kupka RW, van Lammeren A, et al. The prevalence of late-life mania: a review. Bipolar Disord. 2014;16:113-118.
3. Siedontopf F, Horstkamp B, Stief G, et al. Clomiphene citrate as a possible cause of a psychotic reaction during infertility treatment. Hum Reprod. 1997;12(4):706-707.
4. Oyffe T, Lerner A, Isaacs G, et al. Clomiphene-induced psychosis. Am J Psychiatry. 1997;154(8):1169-1170.
5. Lambrichts S, Van Oudenhove L, Sienaert P. Antibiotics and mania: a systematic review. J Affect Disord. 2017;219:149-156.
6. Beal DM, Hudson B, Zaiac M. Amoxicillin-induced psychosis? Am J Psychiatry. 1986;143(2):255-256.
7. Klain V, Timmerman L. Antibiomania, acute manic psychosis following the use of antibiotics. European Psychiatry. 2013;28(suppl 1):1.
8. Abouesh A, Stone C, Hobbs WR. Antimicrobial-induced mania (antibiomania): a review of spontaneous reports. J Clin Psychopharmacol. 2002;22(1):71-81.
9. Lally L, Mannion L. The potential for antimicrobials to adversely affect mental state. BMJ Case Rep. 2013. pii: bcr2013009659. doi: 10.1136/bcr-2013-009659.
10. Neufeld NH, Mohamed NS, Grujich N, et al. Acute neuropsychiatric symptoms associated with antibiotic treatment of Helicobactor Pylori infections: a review. J Psychiatr Pract. 2017;23(1):25-35.
11. Sutter R, Rüegg S, Tschudin-Sutter S. Seizures as adverse events of antibiotic drugs: a systematic review. Neurology. 2015;85(15):1332-1341.
12. Bakhla A, Gore P, Srivastava S. Cycloserine induced mania. Ind Psychiatry J. 2013;22(1):69-70.
13. Barbaccia ML, Roscetti G, Trabucchi M, et al. Isoniazid-induced inhibition of GABAergic transmission enhances neurosteroid content in the rat brain. Neuropharmacology. 1996;35(9-10):1299-1305.
14. Murphy D, Donnelly C, Moskowitz J. Inhibition by lithium of prostaglandin E1 and norepinephrine effects on cyclic adenosine monophosphate production in human platelets. Clin Pharmacol Ther. 1973;14(5):810-814.
15. Clay H, Sillivan S, Konradi C. Mitochondrial dysfunction and pathology in bipolar disorder and schizophrenia. Int J Dev Neurosci. 2011;29(3):311-324.
16. Dickerson F, Severance E, Yolken R. The microbiome, immunity, and schizophrenia and bipolar disorder. Brain Behav Immun. 2017;62:46-52.
1. Kennedy M, Everitt B, Boydell J, et al. Incidence and distribution of first-episode mania by age: results for a 35-year study. Psychol Med. 2005;35(6):855-863.
2. Dols A, Kupka RW, van Lammeren A, et al. The prevalence of late-life mania: a review. Bipolar Disord. 2014;16:113-118.
3. Siedontopf F, Horstkamp B, Stief G, et al. Clomiphene citrate as a possible cause of a psychotic reaction during infertility treatment. Hum Reprod. 1997;12(4):706-707.
4. Oyffe T, Lerner A, Isaacs G, et al. Clomiphene-induced psychosis. Am J Psychiatry. 1997;154(8):1169-1170.
5. Lambrichts S, Van Oudenhove L, Sienaert P. Antibiotics and mania: a systematic review. J Affect Disord. 2017;219:149-156.
6. Beal DM, Hudson B, Zaiac M. Amoxicillin-induced psychosis? Am J Psychiatry. 1986;143(2):255-256.
7. Klain V, Timmerman L. Antibiomania, acute manic psychosis following the use of antibiotics. European Psychiatry. 2013;28(suppl 1):1.
8. Abouesh A, Stone C, Hobbs WR. Antimicrobial-induced mania (antibiomania): a review of spontaneous reports. J Clin Psychopharmacol. 2002;22(1):71-81.
9. Lally L, Mannion L. The potential for antimicrobials to adversely affect mental state. BMJ Case Rep. 2013. pii: bcr2013009659. doi: 10.1136/bcr-2013-009659.
10. Neufeld NH, Mohamed NS, Grujich N, et al. Acute neuropsychiatric symptoms associated with antibiotic treatment of Helicobactor Pylori infections: a review. J Psychiatr Pract. 2017;23(1):25-35.
11. Sutter R, Rüegg S, Tschudin-Sutter S. Seizures as adverse events of antibiotic drugs: a systematic review. Neurology. 2015;85(15):1332-1341.
12. Bakhla A, Gore P, Srivastava S. Cycloserine induced mania. Ind Psychiatry J. 2013;22(1):69-70.
13. Barbaccia ML, Roscetti G, Trabucchi M, et al. Isoniazid-induced inhibition of GABAergic transmission enhances neurosteroid content in the rat brain. Neuropharmacology. 1996;35(9-10):1299-1305.
14. Murphy D, Donnelly C, Moskowitz J. Inhibition by lithium of prostaglandin E1 and norepinephrine effects on cyclic adenosine monophosphate production in human platelets. Clin Pharmacol Ther. 1973;14(5):810-814.
15. Clay H, Sillivan S, Konradi C. Mitochondrial dysfunction and pathology in bipolar disorder and schizophrenia. Int J Dev Neurosci. 2011;29(3):311-324.
16. Dickerson F, Severance E, Yolken R. The microbiome, immunity, and schizophrenia and bipolar disorder. Brain Behav Immun. 2017;62:46-52.
Seeing snakes that aren’t there
CASE Disruptive and inattentive
R, age 9, is brought by his mother to our child/adolescent psychiatry clinic, where he has been receiving treatment for attention-deficit/hyperactivity disorder (ADHD), because he is experiencing visual hallucinations and exhibiting aggressive behavior. R had initially been prescribed (and had been taking) short-acting methylphenidate, 5 mg every morning for weeks. During this time, he responded well to the medication; he had reduced hyperactivity, talked less in class, and was able to give increased attention to his academic work. After 2 weeks, because R did not want to take short-acting methylphenidate in school, we switched him to osmotic-controlled release oral delivery system (OROS) methylphenidate, 18 mg every morning.
Two days after starting the OROS methylphenidate formulation, R develops visual hallucinations and aggressive behavior. His visual hallucinations—which occur both at home and at school—involve seeing snakes circling him. When hallucinating, he hits and pushes family members and throws objects at them. He refuses to go to school because he fears the snakes. The hallucinations continue throughout the day and persist for the next 3 to 4 days.
R does not have any comorbid medical or psychiatric illnesses; however, his father has a history of schizophrenia, polysubstance abuse, and multiple prior psychiatric hospitalizations due to medication noncompliance.
R undergoes laboratory workup, which includes a complete blood count, comprehensive metabolic panel, thyroid-stimulating hormone level, and urine drug screening. All results are within normal limits.
[polldaddy:10468215]
The authors’ observations
We ruled out delirium by ordering a basic laboratory workup. We considered the possibility of a new mood or psychotic disorder, but began to suspect the OROS methylphenidate might be causing R’s symptoms.
Attention-deficit/hyperactivity disorder is an increasingly prevalent diagnosis in the United States, affecting up to 6.4 million children age 4 to 17. While symptoms of ADHD often first appear in preschool-age children, the average age at which a child receives a diagnosis of ADHD is 7.
Stimulants are a clinically effective treatment for ADHD. In general, their use is safe and well tolerated, especially in pediatric patients. Some common adverse effects of stimulant medications include reduced appetite, headache, and insomnia.1 Psychotic symptoms such as paranoid delusions, visual hallucinations, auditory hallucinations, and tactile hallucinations are rare. In some cases, these psychotic symptoms can be accompanied by increased aggression.2-4
Continue to: Methylphenidate is one of the most...
Methylphenidate is one of the most commonly prescribed stimulants for treating ADHD. Methylphenidate has 2 known mechanisms of action: 1) inhibition of catecholamine reuptake at the presynaptic dopamine reuptake inhibitor, and 2) binding to and blocking intracellular dopamine transporters, inhibiting both dopamine and norepinephrine reuptake.5,6 Because increased levels of synaptic dopamine are implicated in the generation of psychotic symptoms, the pharmacologic mechanism of methylphenidate also implies a potential to induce psychotic symptoms.7
How common is this problem?
On the population level, there is no detectable difference in the event rate (incidence) of psychosis in children treated with stimulants or children not taking stimulants.8 However, there are reports that individual patients can experience psychosis due to treatment with stimulants as an unusual adverse medication reaction. In 1971, Lucas and Weiss9 were among the first to describe 3 cases of methylphenidate-induced psychosis. Since then, many articles in the scientific literature have reported cases of psychosis related to stimulant medications.
A brief review of the literature between 2002 and 2010 revealed 14 cases of stimulant-related psychosis, in patients ranging from age 7 to 45. Six of the patients were children, age 7 to 12; 1 patient was an adolescent, age 15; 4 were young adults, age 18 to 25; and 3 were older adults. Of all 14 individuals, 7 reported visual hallucinations, 4 had tactile hallucinations, 4 had auditory hallucinations, and 3 displayed paranoid delusions.10 With the aim of exploring possible etiologic factors associated with psychotic symptoms, such as type of drug and dosage, it was found that 9 patients received methylphenidate, with total daily doses ranging from 7.5 to 74 mg (3 patients received short-acting methylphenidate; 1 patient received methylphenidate extended release (ER); 1 patient received both; 4 patients received dextroamphetamine, with doses of 30 to 50 mg/d; and 1 patient received amphetamine, 10 mg/d). In terms of family history, 1 patient had a positive family history of schizophrenia; 1 patient had a family history of bipolar disorder; and 6 patients were negative for family history of any psychotic disorder.10
In 2006, due to growing concerns about adverse psychiatric effects of ADHD medications, the FDA Center for Drug Evaluation and Research Office of Surveillance and Epidemiology requested the electronic clinical trial databases of manufacturers of drugs approved for the treatment of ADHD, or those with active clinical development programs for the same indication.11 In that study, Mosholder et al11 analyzed data from 49 randomized, controlled clinical trials that were in pediatric development programs and found that there were psychotic or manic adverse events in 11 individuals in the pooled active drug group. These were observed with methylphenidate, dexmethylphenidate, and atomoxetine. There were no events in the placebo group, which reinforced the causality between the ADHD medication and these symptoms, as participants with untreated ADHD did not develop them.11
It is important to note that ADHD medications taken in excessive doses are much more likely to provoke psychotic adverse effects than when taken at therapeutic doses. However, as seen in our clinical case, patients such as R could develop acute psychosis even with a lower dosage of stimulant medications. An article by Ross2 suggested rates of .25% for this psychiatric adverse effect (1 in 400 children treated with therapeutic doses of stimulants will develop psychosis), which is consistent with the data from the Mosholder et al11 study.
Continue to: TREATMENT Discontinuation and re-challenge
TREATMENT Discontinuation and re-challenge
After 3 days, we discontinue OROS methylphenidate. Five days after discontinuation, R’s visual hallucinations and aggressive behaviors completely resolve. After not receiving stimulants for 2 weeks, R is restarted on short-acting methylphenidate, 5 mg/d, because he had a relatively good clinical response to short-acting methylphenidate previously. After 14 days, the short-acting methylphenidate dosage is increased to 5 mg twice daily without the re-emergence of psychosis or aggressive behaviors.
The authors’ observations
Although stimulant-induced psychosis can be a disturbing adverse effect, severe ADHD greatly affects a person’s functioning at school and at home and can lead to several comorbidities, including depression, anxiety, and substance abuse. For these reasons, most patients with ADHD who experience psychotic symptoms are re-challenged with stimulants.10 Out of the 14 cases discussed above, 4 patients were restarted on the same stimulant or a different ADHD medication; 2 of them had the same psychotic symptoms days after the reintroduction of the drug and the other 2 had no recurrence.10,12,13
Stimulant-induced hallucinations
The emergence of hallucinations with methylphenidate or amphetamines has been attributed to a chronic increase of dopamine levels in the synaptic cleft, while the pathophysiological mechanisms are not clearly known. In some cases, hallucinations emerged after taking the first low dose, which has been thought to be an effect of idiosyncratic mechanism. Stimulants cause an increase of the releasing of catecholamines. Porfirio et al14 argue that high-dose stimulants can deteriorate the response to visual stimuli, causing a different perception of visual stimuli in susceptible children, based on the information that norepinephrine is released in the lateral geniculate nucleus, and it increases the transmission of visual information.
An idiosyncratic drug reaction
Despite the existence of many theories on the pathophysiology of stimulant-induced psychosis (Box15-18), its actual mechanism remains unknown. In R’s case, given the speed with which his symptoms developed, the proposed mechanisms of action may not explain his psychotic symptoms. We must consider an idiosyncratic drug reaction as an explanation. This suggestion is supported by the fact that re-challenging with a stimulant did not re-induce psychosis in 2 out of the 4 cases described in the literature,10,12,13 as well as in R’s case.
Box
Although the subjective effects of methylphenidate and amphetamines are similar, neurochemical effects of the 2 stimulants are distinct, with different mechanisms of action. Methylphenidate targets the dopamine transporter (DAT) and the noradrenaline transporter (NET), inhibiting DA and NA reuptake, and therefore increasing DA and NA levels in the synaptic cleft. Amphetamine targets DAT and NET, inhibiting DA and NA reuptake, and therefore increasing DA and NA levels in the synaptic cleft. It also enters the presynaptic neuron, preventing DA/NA from storing in the vesicles. In addition, it promotes the release of catecholamines from vesicles into the cytosol and ultimately from the cytosol into the synaptic cleft.18
Generally, amphetamines are twice as potent as methylphenidate. As such, lower doses of amphetamine preparations can cause psychotic symptoms when compared with methamphetamine products.17 Griffith15 showed that paranoia manifested itself in all participants who were previously healthy as they underwent repeated administration of 5 to 15 mg of oral dextroamphetamine many times per day for up to 5 days in a row, leading to cumulative doses ranging from 200 to 800 mg.15 At such doses, the effects are similar to those obtained with illicit use of methamphetamine, a drug of abuse for which psychosis-inducing effects are well documented.
Psychosis in reaction to therapeutic doses of methylphenidate may have a mechanism of action that is shared by psychosis in response to chronic use of methamphetamine. Several hypotheses have been suggested to explain the mechanism behind stimulantinduced psychosis in cases of chronic methamphetamine use:
- Young,16 who had one of the first proposed theories in 1981, hypothesized attributing symptoms to dose-related effects at pre- and post-synaptic noradrenergic and dopaminergic receptors.
- Hsieh et al18 hypothesized that methamphetamine use causes an increased flow of dopamine in the striatum, which leads to excessive glutamate release into the cortex. Excess glutamate in the cortex might, over time, cause damage to cortical interneurons. This damage may dysregulate thalamocortical signals, resulting in psychotic symptoms.18
Although the mechanisms by which psychotic symptoms associated with stimulants occur remain unknown, possibilities include10,19:
- genetic predisposition
- changes induced by stimulants at the level of neurotransmitters, synapses, and brain circuits
- an idiosyncratic drug reaction.
Continue to: What to consider before prescribing stimulants
What to consider before prescribing stimulants
While stimulants are clearly beneficial for the vast majority of children with ADHD, there may be a small subgroup of patients for whom stimulants carry increased risk. For example, it is possible that patients with a family history of mood and psychotic disorders may be more vulnerable to stimulant-induced psychotic symptoms that are reversible on discontinuation.20 In our case, R had a first-degree relative (his father) with treatment-refractory schizophrenia.
Attentional dysfunction is a common premorbid presentation for children who later develop schizophrenia or bipolar disorder. Retrospective data from patients with schizophrenia or bipolar disorder document high rates of childhood stimulant use—generally higher even than other groups with attentional dysfunction21 and histories of stimulant-associated adverse behavioral effects.22 In these patients, a history of stimulant use is also associated with an earlier age at onset23 and a more severe course of illness during hospitalization.24 Stimulant exposure in vulnerable individuals may hasten the onset or worsen the course of bipolar or psychotic illnesses.21,25,26
OUTCOME Well-controlled symptoms
R continues to receive short-acting methylphenidate, 5 mg twice a day. His ADHD symptoms remain well-controlled, and he is able to do well academically.
The authors’ observations
Although stimulant-induced psychosis is a rare and unpredictable occurrence, carefully monitoring all patients for any adverse effects of ADHD medication is recommended. When present, psychotic symptoms may quickly remit upon discontinuation of the medication. The question of subsequently reintroducing stimulant medication for a patient with severe ADHD is complicated. One needs to measure the possible risk of a reoccurrence of the psychotic symptoms against the consequences of untreated ADHD. These consequences include increased risk for academic and occupational failure, depression, anxiety, and substance abuse. Psychosocial interventions for ADHD should be implemented, but for optimal results, they often need to be combined with medication. However, if a stimulant medication is to be reintroduced, this should be done with extreme care. Starting dosages need to be low, and increases should be gradual, with frequent monitoring.
Bottom Line
Although stimulant-induced psychosis is a rare occurrence, determine if your pediatric patient with attention-deficit/hyperactivity disorder (ADHD) has a family history of mood or psychotic disorders before initiating stimulants. Carefully monitor all patients for any adverse effects of stimulant medications prescribed for ADHD. If psychotic symptoms occur at therapeutic doses, reduce the dose or discontinue the medication. Once the psychotic or manic symptoms resolve, it may be appropriate to re-challenge with a stimulant.
Related Resource
- Man KK, Coghill D, Chan EW, et al. Methylphenidate and the risk of psychotic disorders and hallucinations in children and adolescents in a large health system. Transl Psychiatry. 2016;6(11):e956. doi: 10.1038/tp.2016.216.
Drug Brand Names
Atomoxetine • Strattera
Dexmethylphenidate • Focalin
Dextroamphetamine/amphetamine • Adderall
Methylphenidate • Metadate, Ritalin
Methylphenidate ER • Concerta
1. Cherland E, Fitzpatrick R. Psychotic side effects of psychostimulants: a 5-year review. Can J Psychiatry. 1999; 44(8):811-813.
2. Ross RG. Psychotic and manic-like symptoms during stimulant treatment of attention deficit hyperactivity disorder. Am. J. Psychiatry. 2006;163(7):1149-1152.
3. Rashid J, Mitelman S. Methylphenidate and somatic hallucinations. J Am Acad Child Adolesc Psychiatry. 2007;46(8):945-946.
4. Rubio JM, Sanjuán J, Flórez-Salamanca L, et al. Examining the course of hallucinatory experiences in children and adolescents: a systematic review. Schizophr Res. 2012;138(2-3):248-254.
5. Iversen L. Neurotransmitter transporters and their impact on the development of psychopharmacology. Br J Pharmacol. 2006;147(Suppl 1):S82-S88.
6. Howes OD, Kambeitz J, Kim E, et al. The nature of dopamine dysfunction in schizophrenia and what this means for treatment. Arch Gen Psychiatry. 2012;69(8):776-786.
7. Bloom AS, Russell LJ, Weisskopf B, et al. Methylphenidate-induced delusional disorder in a child with attention deficit disorder with hyperactivity. J Am Acad Child Adolesc Psychiatry. 1988;27(1):88-89.
8. Shibib S, Chaloub N. Stimulant induced psychosis. Child Adolesc Ment Health. 2009;14(1):1420-1423.
9. Lucas AR, Weiss M. Methylphenidate hallucinosis. JAMA. 1971;217(8):1079-1081.
10. Kraemer M, Uekermann J, Wiltfang J, et al. Methylphenidate-induced psychosis in adult attention-deficit/hyperactivity disorder: report of 3 new cases and review of the literature. Clin Neuropharmacol. 2010;33(4):204-206.
11. Mosholder AD, Gelperin K, Hammad TA, et al. Hallucinations and other psychotic symptoms associated with the use of attention-deficit/hyperactivity disorder drugs in children. Pediatrics. 2009; 123:611-616.
12. Gross-Tsur V, Joseph A, Shalev RS. Hallucinations during methylphenidate therapy. Neurology. 2004;63(4):753-754.
13. Halevy A, Shuper A. Methylphenidate induction of complex visual hallucinations. J Child Neurol. 2009;24(8):1005-1007.
14. Porfirio MC, Giana G, Giovinazzo S, et al. Methylphenidate-induced visual hallucinations. Neuropediatrics. 2011;42(1):30-31.
15. Griffith J. A study of illicit amphetamine drug traffic in Oklahoma City. Am J Psychiatry. 1966;123(5):560-569.
16. Young JG. Methylphenidate-induced hallucinosis: case histories and possible mechanisms of action. J Dev Behav Pediatr. 1981;2(2):35-38.
17. Stein MA, Sarampote CS, Waldman ID, et al. A dose-response study of OROS methylphenidate in children with attention-deficit/hyperactivity disorder. Pediatrics. 2003; 112(5):e404. PMID: 14595084.
18. Hsieh JH, Stein DJ, Howells FM. The neurobiology of methamphetamine induced psychosis. Front Hum Neurosci. 2014;8:537. doi:10.3389/fnhum.2014.00537.
19. Shyu YC, Yuan SS, Lee SY, et al. Attention-deficit/hyperactivity disorder, methylphenidate use and the risk of developing schizophrenia spectrum disorders: a nationwide population-based study in Taiwan. Schizophrenia Res. 2015;168(1-2):161-167.
20. MacKenzie LE, Abidi S, Fisher HL, et al. Stimulant medication and psychotic symptoms in offspring of parents with mental illness. Pediatrics. 2016;137(1). doi: 10.1542/peds.2015-2486.
21. Schaeffer J, Ross RG. Childhood-onset schizophrenia: premorbid and prodromal diagnosis and treatment histories. J Am Acad Child Adolesc Psychiatry. 2002;41(5):538-545.
22. Faedda GL, Baldessarini RJ, Blovinsky IP, et al. Treatment-emergent mania in pediatric bipolar disorder: a retrospective case review. J Affect Disord. 2004;82(1):149-158.
23. DelBello MP, Soutullo CA, Hendricks W, et al. Prior stimulant treatment in adolescents with bipolar disorder: association with age at onset. Bipolar Disord. 2001;3(2):53-57.
24. Soutullo CA, DelBello MP, Ochsner BS, et al. Severity of bipolarity in hospitalized manic adolescents with history of stimulant or antidepressant treatment. J Affect Disord. 2002;70(3):323-327.
25. Reichart CG, Nolen WA. Earlier onset of bipolar disorder in children by antidepressants or stimulants? An hypothesis. J Affect Disord. 2004;78(1):81-84.
26. Ikeda M, Okahisa Y, Aleksic B, et al. Evidence for shared genetic risk between methamphetamine-induced psychosis and schizophrenia. Neuropsychopharmacology. 2013;38(10):1864-1870.
CASE Disruptive and inattentive
R, age 9, is brought by his mother to our child/adolescent psychiatry clinic, where he has been receiving treatment for attention-deficit/hyperactivity disorder (ADHD), because he is experiencing visual hallucinations and exhibiting aggressive behavior. R had initially been prescribed (and had been taking) short-acting methylphenidate, 5 mg every morning for weeks. During this time, he responded well to the medication; he had reduced hyperactivity, talked less in class, and was able to give increased attention to his academic work. After 2 weeks, because R did not want to take short-acting methylphenidate in school, we switched him to osmotic-controlled release oral delivery system (OROS) methylphenidate, 18 mg every morning.
Two days after starting the OROS methylphenidate formulation, R develops visual hallucinations and aggressive behavior. His visual hallucinations—which occur both at home and at school—involve seeing snakes circling him. When hallucinating, he hits and pushes family members and throws objects at them. He refuses to go to school because he fears the snakes. The hallucinations continue throughout the day and persist for the next 3 to 4 days.
R does not have any comorbid medical or psychiatric illnesses; however, his father has a history of schizophrenia, polysubstance abuse, and multiple prior psychiatric hospitalizations due to medication noncompliance.
R undergoes laboratory workup, which includes a complete blood count, comprehensive metabolic panel, thyroid-stimulating hormone level, and urine drug screening. All results are within normal limits.
[polldaddy:10468215]
The authors’ observations
We ruled out delirium by ordering a basic laboratory workup. We considered the possibility of a new mood or psychotic disorder, but began to suspect the OROS methylphenidate might be causing R’s symptoms.
Attention-deficit/hyperactivity disorder is an increasingly prevalent diagnosis in the United States, affecting up to 6.4 million children age 4 to 17. While symptoms of ADHD often first appear in preschool-age children, the average age at which a child receives a diagnosis of ADHD is 7.
Stimulants are a clinically effective treatment for ADHD. In general, their use is safe and well tolerated, especially in pediatric patients. Some common adverse effects of stimulant medications include reduced appetite, headache, and insomnia.1 Psychotic symptoms such as paranoid delusions, visual hallucinations, auditory hallucinations, and tactile hallucinations are rare. In some cases, these psychotic symptoms can be accompanied by increased aggression.2-4
Continue to: Methylphenidate is one of the most...
Methylphenidate is one of the most commonly prescribed stimulants for treating ADHD. Methylphenidate has 2 known mechanisms of action: 1) inhibition of catecholamine reuptake at the presynaptic dopamine reuptake inhibitor, and 2) binding to and blocking intracellular dopamine transporters, inhibiting both dopamine and norepinephrine reuptake.5,6 Because increased levels of synaptic dopamine are implicated in the generation of psychotic symptoms, the pharmacologic mechanism of methylphenidate also implies a potential to induce psychotic symptoms.7
How common is this problem?
On the population level, there is no detectable difference in the event rate (incidence) of psychosis in children treated with stimulants or children not taking stimulants.8 However, there are reports that individual patients can experience psychosis due to treatment with stimulants as an unusual adverse medication reaction. In 1971, Lucas and Weiss9 were among the first to describe 3 cases of methylphenidate-induced psychosis. Since then, many articles in the scientific literature have reported cases of psychosis related to stimulant medications.
A brief review of the literature between 2002 and 2010 revealed 14 cases of stimulant-related psychosis, in patients ranging from age 7 to 45. Six of the patients were children, age 7 to 12; 1 patient was an adolescent, age 15; 4 were young adults, age 18 to 25; and 3 were older adults. Of all 14 individuals, 7 reported visual hallucinations, 4 had tactile hallucinations, 4 had auditory hallucinations, and 3 displayed paranoid delusions.10 With the aim of exploring possible etiologic factors associated with psychotic symptoms, such as type of drug and dosage, it was found that 9 patients received methylphenidate, with total daily doses ranging from 7.5 to 74 mg (3 patients received short-acting methylphenidate; 1 patient received methylphenidate extended release (ER); 1 patient received both; 4 patients received dextroamphetamine, with doses of 30 to 50 mg/d; and 1 patient received amphetamine, 10 mg/d). In terms of family history, 1 patient had a positive family history of schizophrenia; 1 patient had a family history of bipolar disorder; and 6 patients were negative for family history of any psychotic disorder.10
In 2006, due to growing concerns about adverse psychiatric effects of ADHD medications, the FDA Center for Drug Evaluation and Research Office of Surveillance and Epidemiology requested the electronic clinical trial databases of manufacturers of drugs approved for the treatment of ADHD, or those with active clinical development programs for the same indication.11 In that study, Mosholder et al11 analyzed data from 49 randomized, controlled clinical trials that were in pediatric development programs and found that there were psychotic or manic adverse events in 11 individuals in the pooled active drug group. These were observed with methylphenidate, dexmethylphenidate, and atomoxetine. There were no events in the placebo group, which reinforced the causality between the ADHD medication and these symptoms, as participants with untreated ADHD did not develop them.11
It is important to note that ADHD medications taken in excessive doses are much more likely to provoke psychotic adverse effects than when taken at therapeutic doses. However, as seen in our clinical case, patients such as R could develop acute psychosis even with a lower dosage of stimulant medications. An article by Ross2 suggested rates of .25% for this psychiatric adverse effect (1 in 400 children treated with therapeutic doses of stimulants will develop psychosis), which is consistent with the data from the Mosholder et al11 study.
Continue to: TREATMENT Discontinuation and re-challenge
TREATMENT Discontinuation and re-challenge
After 3 days, we discontinue OROS methylphenidate. Five days after discontinuation, R’s visual hallucinations and aggressive behaviors completely resolve. After not receiving stimulants for 2 weeks, R is restarted on short-acting methylphenidate, 5 mg/d, because he had a relatively good clinical response to short-acting methylphenidate previously. After 14 days, the short-acting methylphenidate dosage is increased to 5 mg twice daily without the re-emergence of psychosis or aggressive behaviors.
The authors’ observations
Although stimulant-induced psychosis can be a disturbing adverse effect, severe ADHD greatly affects a person’s functioning at school and at home and can lead to several comorbidities, including depression, anxiety, and substance abuse. For these reasons, most patients with ADHD who experience psychotic symptoms are re-challenged with stimulants.10 Out of the 14 cases discussed above, 4 patients were restarted on the same stimulant or a different ADHD medication; 2 of them had the same psychotic symptoms days after the reintroduction of the drug and the other 2 had no recurrence.10,12,13
Stimulant-induced hallucinations
The emergence of hallucinations with methylphenidate or amphetamines has been attributed to a chronic increase of dopamine levels in the synaptic cleft, while the pathophysiological mechanisms are not clearly known. In some cases, hallucinations emerged after taking the first low dose, which has been thought to be an effect of idiosyncratic mechanism. Stimulants cause an increase of the releasing of catecholamines. Porfirio et al14 argue that high-dose stimulants can deteriorate the response to visual stimuli, causing a different perception of visual stimuli in susceptible children, based on the information that norepinephrine is released in the lateral geniculate nucleus, and it increases the transmission of visual information.
An idiosyncratic drug reaction
Despite the existence of many theories on the pathophysiology of stimulant-induced psychosis (Box15-18), its actual mechanism remains unknown. In R’s case, given the speed with which his symptoms developed, the proposed mechanisms of action may not explain his psychotic symptoms. We must consider an idiosyncratic drug reaction as an explanation. This suggestion is supported by the fact that re-challenging with a stimulant did not re-induce psychosis in 2 out of the 4 cases described in the literature,10,12,13 as well as in R’s case.
Box
Although the subjective effects of methylphenidate and amphetamines are similar, neurochemical effects of the 2 stimulants are distinct, with different mechanisms of action. Methylphenidate targets the dopamine transporter (DAT) and the noradrenaline transporter (NET), inhibiting DA and NA reuptake, and therefore increasing DA and NA levels in the synaptic cleft. Amphetamine targets DAT and NET, inhibiting DA and NA reuptake, and therefore increasing DA and NA levels in the synaptic cleft. It also enters the presynaptic neuron, preventing DA/NA from storing in the vesicles. In addition, it promotes the release of catecholamines from vesicles into the cytosol and ultimately from the cytosol into the synaptic cleft.18
Generally, amphetamines are twice as potent as methylphenidate. As such, lower doses of amphetamine preparations can cause psychotic symptoms when compared with methamphetamine products.17 Griffith15 showed that paranoia manifested itself in all participants who were previously healthy as they underwent repeated administration of 5 to 15 mg of oral dextroamphetamine many times per day for up to 5 days in a row, leading to cumulative doses ranging from 200 to 800 mg.15 At such doses, the effects are similar to those obtained with illicit use of methamphetamine, a drug of abuse for which psychosis-inducing effects are well documented.
Psychosis in reaction to therapeutic doses of methylphenidate may have a mechanism of action that is shared by psychosis in response to chronic use of methamphetamine. Several hypotheses have been suggested to explain the mechanism behind stimulantinduced psychosis in cases of chronic methamphetamine use:
- Young,16 who had one of the first proposed theories in 1981, hypothesized attributing symptoms to dose-related effects at pre- and post-synaptic noradrenergic and dopaminergic receptors.
- Hsieh et al18 hypothesized that methamphetamine use causes an increased flow of dopamine in the striatum, which leads to excessive glutamate release into the cortex. Excess glutamate in the cortex might, over time, cause damage to cortical interneurons. This damage may dysregulate thalamocortical signals, resulting in psychotic symptoms.18
Although the mechanisms by which psychotic symptoms associated with stimulants occur remain unknown, possibilities include10,19:
- genetic predisposition
- changes induced by stimulants at the level of neurotransmitters, synapses, and brain circuits
- an idiosyncratic drug reaction.
Continue to: What to consider before prescribing stimulants
What to consider before prescribing stimulants
While stimulants are clearly beneficial for the vast majority of children with ADHD, there may be a small subgroup of patients for whom stimulants carry increased risk. For example, it is possible that patients with a family history of mood and psychotic disorders may be more vulnerable to stimulant-induced psychotic symptoms that are reversible on discontinuation.20 In our case, R had a first-degree relative (his father) with treatment-refractory schizophrenia.
Attentional dysfunction is a common premorbid presentation for children who later develop schizophrenia or bipolar disorder. Retrospective data from patients with schizophrenia or bipolar disorder document high rates of childhood stimulant use—generally higher even than other groups with attentional dysfunction21 and histories of stimulant-associated adverse behavioral effects.22 In these patients, a history of stimulant use is also associated with an earlier age at onset23 and a more severe course of illness during hospitalization.24 Stimulant exposure in vulnerable individuals may hasten the onset or worsen the course of bipolar or psychotic illnesses.21,25,26
OUTCOME Well-controlled symptoms
R continues to receive short-acting methylphenidate, 5 mg twice a day. His ADHD symptoms remain well-controlled, and he is able to do well academically.
The authors’ observations
Although stimulant-induced psychosis is a rare and unpredictable occurrence, carefully monitoring all patients for any adverse effects of ADHD medication is recommended. When present, psychotic symptoms may quickly remit upon discontinuation of the medication. The question of subsequently reintroducing stimulant medication for a patient with severe ADHD is complicated. One needs to measure the possible risk of a reoccurrence of the psychotic symptoms against the consequences of untreated ADHD. These consequences include increased risk for academic and occupational failure, depression, anxiety, and substance abuse. Psychosocial interventions for ADHD should be implemented, but for optimal results, they often need to be combined with medication. However, if a stimulant medication is to be reintroduced, this should be done with extreme care. Starting dosages need to be low, and increases should be gradual, with frequent monitoring.
Bottom Line
Although stimulant-induced psychosis is a rare occurrence, determine if your pediatric patient with attention-deficit/hyperactivity disorder (ADHD) has a family history of mood or psychotic disorders before initiating stimulants. Carefully monitor all patients for any adverse effects of stimulant medications prescribed for ADHD. If psychotic symptoms occur at therapeutic doses, reduce the dose or discontinue the medication. Once the psychotic or manic symptoms resolve, it may be appropriate to re-challenge with a stimulant.
Related Resource
- Man KK, Coghill D, Chan EW, et al. Methylphenidate and the risk of psychotic disorders and hallucinations in children and adolescents in a large health system. Transl Psychiatry. 2016;6(11):e956. doi: 10.1038/tp.2016.216.
Drug Brand Names
Atomoxetine • Strattera
Dexmethylphenidate • Focalin
Dextroamphetamine/amphetamine • Adderall
Methylphenidate • Metadate, Ritalin
Methylphenidate ER • Concerta
CASE Disruptive and inattentive
R, age 9, is brought by his mother to our child/adolescent psychiatry clinic, where he has been receiving treatment for attention-deficit/hyperactivity disorder (ADHD), because he is experiencing visual hallucinations and exhibiting aggressive behavior. R had initially been prescribed (and had been taking) short-acting methylphenidate, 5 mg every morning for weeks. During this time, he responded well to the medication; he had reduced hyperactivity, talked less in class, and was able to give increased attention to his academic work. After 2 weeks, because R did not want to take short-acting methylphenidate in school, we switched him to osmotic-controlled release oral delivery system (OROS) methylphenidate, 18 mg every morning.
Two days after starting the OROS methylphenidate formulation, R develops visual hallucinations and aggressive behavior. His visual hallucinations—which occur both at home and at school—involve seeing snakes circling him. When hallucinating, he hits and pushes family members and throws objects at them. He refuses to go to school because he fears the snakes. The hallucinations continue throughout the day and persist for the next 3 to 4 days.
R does not have any comorbid medical or psychiatric illnesses; however, his father has a history of schizophrenia, polysubstance abuse, and multiple prior psychiatric hospitalizations due to medication noncompliance.
R undergoes laboratory workup, which includes a complete blood count, comprehensive metabolic panel, thyroid-stimulating hormone level, and urine drug screening. All results are within normal limits.
[polldaddy:10468215]
The authors’ observations
We ruled out delirium by ordering a basic laboratory workup. We considered the possibility of a new mood or psychotic disorder, but began to suspect the OROS methylphenidate might be causing R’s symptoms.
Attention-deficit/hyperactivity disorder is an increasingly prevalent diagnosis in the United States, affecting up to 6.4 million children age 4 to 17. While symptoms of ADHD often first appear in preschool-age children, the average age at which a child receives a diagnosis of ADHD is 7.
Stimulants are a clinically effective treatment for ADHD. In general, their use is safe and well tolerated, especially in pediatric patients. Some common adverse effects of stimulant medications include reduced appetite, headache, and insomnia.1 Psychotic symptoms such as paranoid delusions, visual hallucinations, auditory hallucinations, and tactile hallucinations are rare. In some cases, these psychotic symptoms can be accompanied by increased aggression.2-4
Continue to: Methylphenidate is one of the most...
Methylphenidate is one of the most commonly prescribed stimulants for treating ADHD. Methylphenidate has 2 known mechanisms of action: 1) inhibition of catecholamine reuptake at the presynaptic dopamine reuptake inhibitor, and 2) binding to and blocking intracellular dopamine transporters, inhibiting both dopamine and norepinephrine reuptake.5,6 Because increased levels of synaptic dopamine are implicated in the generation of psychotic symptoms, the pharmacologic mechanism of methylphenidate also implies a potential to induce psychotic symptoms.7
How common is this problem?
On the population level, there is no detectable difference in the event rate (incidence) of psychosis in children treated with stimulants or children not taking stimulants.8 However, there are reports that individual patients can experience psychosis due to treatment with stimulants as an unusual adverse medication reaction. In 1971, Lucas and Weiss9 were among the first to describe 3 cases of methylphenidate-induced psychosis. Since then, many articles in the scientific literature have reported cases of psychosis related to stimulant medications.
A brief review of the literature between 2002 and 2010 revealed 14 cases of stimulant-related psychosis, in patients ranging from age 7 to 45. Six of the patients were children, age 7 to 12; 1 patient was an adolescent, age 15; 4 were young adults, age 18 to 25; and 3 were older adults. Of all 14 individuals, 7 reported visual hallucinations, 4 had tactile hallucinations, 4 had auditory hallucinations, and 3 displayed paranoid delusions.10 With the aim of exploring possible etiologic factors associated with psychotic symptoms, such as type of drug and dosage, it was found that 9 patients received methylphenidate, with total daily doses ranging from 7.5 to 74 mg (3 patients received short-acting methylphenidate; 1 patient received methylphenidate extended release (ER); 1 patient received both; 4 patients received dextroamphetamine, with doses of 30 to 50 mg/d; and 1 patient received amphetamine, 10 mg/d). In terms of family history, 1 patient had a positive family history of schizophrenia; 1 patient had a family history of bipolar disorder; and 6 patients were negative for family history of any psychotic disorder.10
In 2006, due to growing concerns about adverse psychiatric effects of ADHD medications, the FDA Center for Drug Evaluation and Research Office of Surveillance and Epidemiology requested the electronic clinical trial databases of manufacturers of drugs approved for the treatment of ADHD, or those with active clinical development programs for the same indication.11 In that study, Mosholder et al11 analyzed data from 49 randomized, controlled clinical trials that were in pediatric development programs and found that there were psychotic or manic adverse events in 11 individuals in the pooled active drug group. These were observed with methylphenidate, dexmethylphenidate, and atomoxetine. There were no events in the placebo group, which reinforced the causality between the ADHD medication and these symptoms, as participants with untreated ADHD did not develop them.11
It is important to note that ADHD medications taken in excessive doses are much more likely to provoke psychotic adverse effects than when taken at therapeutic doses. However, as seen in our clinical case, patients such as R could develop acute psychosis even with a lower dosage of stimulant medications. An article by Ross2 suggested rates of .25% for this psychiatric adverse effect (1 in 400 children treated with therapeutic doses of stimulants will develop psychosis), which is consistent with the data from the Mosholder et al11 study.
Continue to: TREATMENT Discontinuation and re-challenge
TREATMENT Discontinuation and re-challenge
After 3 days, we discontinue OROS methylphenidate. Five days after discontinuation, R’s visual hallucinations and aggressive behaviors completely resolve. After not receiving stimulants for 2 weeks, R is restarted on short-acting methylphenidate, 5 mg/d, because he had a relatively good clinical response to short-acting methylphenidate previously. After 14 days, the short-acting methylphenidate dosage is increased to 5 mg twice daily without the re-emergence of psychosis or aggressive behaviors.
The authors’ observations
Although stimulant-induced psychosis can be a disturbing adverse effect, severe ADHD greatly affects a person’s functioning at school and at home and can lead to several comorbidities, including depression, anxiety, and substance abuse. For these reasons, most patients with ADHD who experience psychotic symptoms are re-challenged with stimulants.10 Out of the 14 cases discussed above, 4 patients were restarted on the same stimulant or a different ADHD medication; 2 of them had the same psychotic symptoms days after the reintroduction of the drug and the other 2 had no recurrence.10,12,13
Stimulant-induced hallucinations
The emergence of hallucinations with methylphenidate or amphetamines has been attributed to a chronic increase of dopamine levels in the synaptic cleft, while the pathophysiological mechanisms are not clearly known. In some cases, hallucinations emerged after taking the first low dose, which has been thought to be an effect of idiosyncratic mechanism. Stimulants cause an increase of the releasing of catecholamines. Porfirio et al14 argue that high-dose stimulants can deteriorate the response to visual stimuli, causing a different perception of visual stimuli in susceptible children, based on the information that norepinephrine is released in the lateral geniculate nucleus, and it increases the transmission of visual information.
An idiosyncratic drug reaction
Despite the existence of many theories on the pathophysiology of stimulant-induced psychosis (Box15-18), its actual mechanism remains unknown. In R’s case, given the speed with which his symptoms developed, the proposed mechanisms of action may not explain his psychotic symptoms. We must consider an idiosyncratic drug reaction as an explanation. This suggestion is supported by the fact that re-challenging with a stimulant did not re-induce psychosis in 2 out of the 4 cases described in the literature,10,12,13 as well as in R’s case.
Box
Although the subjective effects of methylphenidate and amphetamines are similar, neurochemical effects of the 2 stimulants are distinct, with different mechanisms of action. Methylphenidate targets the dopamine transporter (DAT) and the noradrenaline transporter (NET), inhibiting DA and NA reuptake, and therefore increasing DA and NA levels in the synaptic cleft. Amphetamine targets DAT and NET, inhibiting DA and NA reuptake, and therefore increasing DA and NA levels in the synaptic cleft. It also enters the presynaptic neuron, preventing DA/NA from storing in the vesicles. In addition, it promotes the release of catecholamines from vesicles into the cytosol and ultimately from the cytosol into the synaptic cleft.18
Generally, amphetamines are twice as potent as methylphenidate. As such, lower doses of amphetamine preparations can cause psychotic symptoms when compared with methamphetamine products.17 Griffith15 showed that paranoia manifested itself in all participants who were previously healthy as they underwent repeated administration of 5 to 15 mg of oral dextroamphetamine many times per day for up to 5 days in a row, leading to cumulative doses ranging from 200 to 800 mg.15 At such doses, the effects are similar to those obtained with illicit use of methamphetamine, a drug of abuse for which psychosis-inducing effects are well documented.
Psychosis in reaction to therapeutic doses of methylphenidate may have a mechanism of action that is shared by psychosis in response to chronic use of methamphetamine. Several hypotheses have been suggested to explain the mechanism behind stimulantinduced psychosis in cases of chronic methamphetamine use:
- Young,16 who had one of the first proposed theories in 1981, hypothesized attributing symptoms to dose-related effects at pre- and post-synaptic noradrenergic and dopaminergic receptors.
- Hsieh et al18 hypothesized that methamphetamine use causes an increased flow of dopamine in the striatum, which leads to excessive glutamate release into the cortex. Excess glutamate in the cortex might, over time, cause damage to cortical interneurons. This damage may dysregulate thalamocortical signals, resulting in psychotic symptoms.18
Although the mechanisms by which psychotic symptoms associated with stimulants occur remain unknown, possibilities include10,19:
- genetic predisposition
- changes induced by stimulants at the level of neurotransmitters, synapses, and brain circuits
- an idiosyncratic drug reaction.
Continue to: What to consider before prescribing stimulants
What to consider before prescribing stimulants
While stimulants are clearly beneficial for the vast majority of children with ADHD, there may be a small subgroup of patients for whom stimulants carry increased risk. For example, it is possible that patients with a family history of mood and psychotic disorders may be more vulnerable to stimulant-induced psychotic symptoms that are reversible on discontinuation.20 In our case, R had a first-degree relative (his father) with treatment-refractory schizophrenia.
Attentional dysfunction is a common premorbid presentation for children who later develop schizophrenia or bipolar disorder. Retrospective data from patients with schizophrenia or bipolar disorder document high rates of childhood stimulant use—generally higher even than other groups with attentional dysfunction21 and histories of stimulant-associated adverse behavioral effects.22 In these patients, a history of stimulant use is also associated with an earlier age at onset23 and a more severe course of illness during hospitalization.24 Stimulant exposure in vulnerable individuals may hasten the onset or worsen the course of bipolar or psychotic illnesses.21,25,26
OUTCOME Well-controlled symptoms
R continues to receive short-acting methylphenidate, 5 mg twice a day. His ADHD symptoms remain well-controlled, and he is able to do well academically.
The authors’ observations
Although stimulant-induced psychosis is a rare and unpredictable occurrence, carefully monitoring all patients for any adverse effects of ADHD medication is recommended. When present, psychotic symptoms may quickly remit upon discontinuation of the medication. The question of subsequently reintroducing stimulant medication for a patient with severe ADHD is complicated. One needs to measure the possible risk of a reoccurrence of the psychotic symptoms against the consequences of untreated ADHD. These consequences include increased risk for academic and occupational failure, depression, anxiety, and substance abuse. Psychosocial interventions for ADHD should be implemented, but for optimal results, they often need to be combined with medication. However, if a stimulant medication is to be reintroduced, this should be done with extreme care. Starting dosages need to be low, and increases should be gradual, with frequent monitoring.
Bottom Line
Although stimulant-induced psychosis is a rare occurrence, determine if your pediatric patient with attention-deficit/hyperactivity disorder (ADHD) has a family history of mood or psychotic disorders before initiating stimulants. Carefully monitor all patients for any adverse effects of stimulant medications prescribed for ADHD. If psychotic symptoms occur at therapeutic doses, reduce the dose or discontinue the medication. Once the psychotic or manic symptoms resolve, it may be appropriate to re-challenge with a stimulant.
Related Resource
- Man KK, Coghill D, Chan EW, et al. Methylphenidate and the risk of psychotic disorders and hallucinations in children and adolescents in a large health system. Transl Psychiatry. 2016;6(11):e956. doi: 10.1038/tp.2016.216.
Drug Brand Names
Atomoxetine • Strattera
Dexmethylphenidate • Focalin
Dextroamphetamine/amphetamine • Adderall
Methylphenidate • Metadate, Ritalin
Methylphenidate ER • Concerta
1. Cherland E, Fitzpatrick R. Psychotic side effects of psychostimulants: a 5-year review. Can J Psychiatry. 1999; 44(8):811-813.
2. Ross RG. Psychotic and manic-like symptoms during stimulant treatment of attention deficit hyperactivity disorder. Am. J. Psychiatry. 2006;163(7):1149-1152.
3. Rashid J, Mitelman S. Methylphenidate and somatic hallucinations. J Am Acad Child Adolesc Psychiatry. 2007;46(8):945-946.
4. Rubio JM, Sanjuán J, Flórez-Salamanca L, et al. Examining the course of hallucinatory experiences in children and adolescents: a systematic review. Schizophr Res. 2012;138(2-3):248-254.
5. Iversen L. Neurotransmitter transporters and their impact on the development of psychopharmacology. Br J Pharmacol. 2006;147(Suppl 1):S82-S88.
6. Howes OD, Kambeitz J, Kim E, et al. The nature of dopamine dysfunction in schizophrenia and what this means for treatment. Arch Gen Psychiatry. 2012;69(8):776-786.
7. Bloom AS, Russell LJ, Weisskopf B, et al. Methylphenidate-induced delusional disorder in a child with attention deficit disorder with hyperactivity. J Am Acad Child Adolesc Psychiatry. 1988;27(1):88-89.
8. Shibib S, Chaloub N. Stimulant induced psychosis. Child Adolesc Ment Health. 2009;14(1):1420-1423.
9. Lucas AR, Weiss M. Methylphenidate hallucinosis. JAMA. 1971;217(8):1079-1081.
10. Kraemer M, Uekermann J, Wiltfang J, et al. Methylphenidate-induced psychosis in adult attention-deficit/hyperactivity disorder: report of 3 new cases and review of the literature. Clin Neuropharmacol. 2010;33(4):204-206.
11. Mosholder AD, Gelperin K, Hammad TA, et al. Hallucinations and other psychotic symptoms associated with the use of attention-deficit/hyperactivity disorder drugs in children. Pediatrics. 2009; 123:611-616.
12. Gross-Tsur V, Joseph A, Shalev RS. Hallucinations during methylphenidate therapy. Neurology. 2004;63(4):753-754.
13. Halevy A, Shuper A. Methylphenidate induction of complex visual hallucinations. J Child Neurol. 2009;24(8):1005-1007.
14. Porfirio MC, Giana G, Giovinazzo S, et al. Methylphenidate-induced visual hallucinations. Neuropediatrics. 2011;42(1):30-31.
15. Griffith J. A study of illicit amphetamine drug traffic in Oklahoma City. Am J Psychiatry. 1966;123(5):560-569.
16. Young JG. Methylphenidate-induced hallucinosis: case histories and possible mechanisms of action. J Dev Behav Pediatr. 1981;2(2):35-38.
17. Stein MA, Sarampote CS, Waldman ID, et al. A dose-response study of OROS methylphenidate in children with attention-deficit/hyperactivity disorder. Pediatrics. 2003; 112(5):e404. PMID: 14595084.
18. Hsieh JH, Stein DJ, Howells FM. The neurobiology of methamphetamine induced psychosis. Front Hum Neurosci. 2014;8:537. doi:10.3389/fnhum.2014.00537.
19. Shyu YC, Yuan SS, Lee SY, et al. Attention-deficit/hyperactivity disorder, methylphenidate use and the risk of developing schizophrenia spectrum disorders: a nationwide population-based study in Taiwan. Schizophrenia Res. 2015;168(1-2):161-167.
20. MacKenzie LE, Abidi S, Fisher HL, et al. Stimulant medication and psychotic symptoms in offspring of parents with mental illness. Pediatrics. 2016;137(1). doi: 10.1542/peds.2015-2486.
21. Schaeffer J, Ross RG. Childhood-onset schizophrenia: premorbid and prodromal diagnosis and treatment histories. J Am Acad Child Adolesc Psychiatry. 2002;41(5):538-545.
22. Faedda GL, Baldessarini RJ, Blovinsky IP, et al. Treatment-emergent mania in pediatric bipolar disorder: a retrospective case review. J Affect Disord. 2004;82(1):149-158.
23. DelBello MP, Soutullo CA, Hendricks W, et al. Prior stimulant treatment in adolescents with bipolar disorder: association with age at onset. Bipolar Disord. 2001;3(2):53-57.
24. Soutullo CA, DelBello MP, Ochsner BS, et al. Severity of bipolarity in hospitalized manic adolescents with history of stimulant or antidepressant treatment. J Affect Disord. 2002;70(3):323-327.
25. Reichart CG, Nolen WA. Earlier onset of bipolar disorder in children by antidepressants or stimulants? An hypothesis. J Affect Disord. 2004;78(1):81-84.
26. Ikeda M, Okahisa Y, Aleksic B, et al. Evidence for shared genetic risk between methamphetamine-induced psychosis and schizophrenia. Neuropsychopharmacology. 2013;38(10):1864-1870.
1. Cherland E, Fitzpatrick R. Psychotic side effects of psychostimulants: a 5-year review. Can J Psychiatry. 1999; 44(8):811-813.
2. Ross RG. Psychotic and manic-like symptoms during stimulant treatment of attention deficit hyperactivity disorder. Am. J. Psychiatry. 2006;163(7):1149-1152.
3. Rashid J, Mitelman S. Methylphenidate and somatic hallucinations. J Am Acad Child Adolesc Psychiatry. 2007;46(8):945-946.
4. Rubio JM, Sanjuán J, Flórez-Salamanca L, et al. Examining the course of hallucinatory experiences in children and adolescents: a systematic review. Schizophr Res. 2012;138(2-3):248-254.
5. Iversen L. Neurotransmitter transporters and their impact on the development of psychopharmacology. Br J Pharmacol. 2006;147(Suppl 1):S82-S88.
6. Howes OD, Kambeitz J, Kim E, et al. The nature of dopamine dysfunction in schizophrenia and what this means for treatment. Arch Gen Psychiatry. 2012;69(8):776-786.
7. Bloom AS, Russell LJ, Weisskopf B, et al. Methylphenidate-induced delusional disorder in a child with attention deficit disorder with hyperactivity. J Am Acad Child Adolesc Psychiatry. 1988;27(1):88-89.
8. Shibib S, Chaloub N. Stimulant induced psychosis. Child Adolesc Ment Health. 2009;14(1):1420-1423.
9. Lucas AR, Weiss M. Methylphenidate hallucinosis. JAMA. 1971;217(8):1079-1081.
10. Kraemer M, Uekermann J, Wiltfang J, et al. Methylphenidate-induced psychosis in adult attention-deficit/hyperactivity disorder: report of 3 new cases and review of the literature. Clin Neuropharmacol. 2010;33(4):204-206.
11. Mosholder AD, Gelperin K, Hammad TA, et al. Hallucinations and other psychotic symptoms associated with the use of attention-deficit/hyperactivity disorder drugs in children. Pediatrics. 2009; 123:611-616.
12. Gross-Tsur V, Joseph A, Shalev RS. Hallucinations during methylphenidate therapy. Neurology. 2004;63(4):753-754.
13. Halevy A, Shuper A. Methylphenidate induction of complex visual hallucinations. J Child Neurol. 2009;24(8):1005-1007.
14. Porfirio MC, Giana G, Giovinazzo S, et al. Methylphenidate-induced visual hallucinations. Neuropediatrics. 2011;42(1):30-31.
15. Griffith J. A study of illicit amphetamine drug traffic in Oklahoma City. Am J Psychiatry. 1966;123(5):560-569.
16. Young JG. Methylphenidate-induced hallucinosis: case histories and possible mechanisms of action. J Dev Behav Pediatr. 1981;2(2):35-38.
17. Stein MA, Sarampote CS, Waldman ID, et al. A dose-response study of OROS methylphenidate in children with attention-deficit/hyperactivity disorder. Pediatrics. 2003; 112(5):e404. PMID: 14595084.
18. Hsieh JH, Stein DJ, Howells FM. The neurobiology of methamphetamine induced psychosis. Front Hum Neurosci. 2014;8:537. doi:10.3389/fnhum.2014.00537.
19. Shyu YC, Yuan SS, Lee SY, et al. Attention-deficit/hyperactivity disorder, methylphenidate use and the risk of developing schizophrenia spectrum disorders: a nationwide population-based study in Taiwan. Schizophrenia Res. 2015;168(1-2):161-167.
20. MacKenzie LE, Abidi S, Fisher HL, et al. Stimulant medication and psychotic symptoms in offspring of parents with mental illness. Pediatrics. 2016;137(1). doi: 10.1542/peds.2015-2486.
21. Schaeffer J, Ross RG. Childhood-onset schizophrenia: premorbid and prodromal diagnosis and treatment histories. J Am Acad Child Adolesc Psychiatry. 2002;41(5):538-545.
22. Faedda GL, Baldessarini RJ, Blovinsky IP, et al. Treatment-emergent mania in pediatric bipolar disorder: a retrospective case review. J Affect Disord. 2004;82(1):149-158.
23. DelBello MP, Soutullo CA, Hendricks W, et al. Prior stimulant treatment in adolescents with bipolar disorder: association with age at onset. Bipolar Disord. 2001;3(2):53-57.
24. Soutullo CA, DelBello MP, Ochsner BS, et al. Severity of bipolarity in hospitalized manic adolescents with history of stimulant or antidepressant treatment. J Affect Disord. 2002;70(3):323-327.
25. Reichart CG, Nolen WA. Earlier onset of bipolar disorder in children by antidepressants or stimulants? An hypothesis. J Affect Disord. 2004;78(1):81-84.
26. Ikeda M, Okahisa Y, Aleksic B, et al. Evidence for shared genetic risk between methamphetamine-induced psychosis and schizophrenia. Neuropsychopharmacology. 2013;38(10):1864-1870.
The woman who couldn’t stop eating
CASE Uncontrollable eating and weight gain
Ms. C, age 33, presents to an outpatient clinic with complaints of weight gain and “uncontrollable eating.” Ms. C says she’s gained >50 lb over the last year. She describes progressively frequent episodes of overeating during which she feels that she has no control over the amount of food she consumes. She reports eating as often as 10 times a day, and overeating to the point of physical discomfort during most meals. She gives an example of having recently consumed a large pizza, several portions of Chinese food, approximately 20 chicken wings, and half a chocolate cake for dinner. Ms. C admits that on several occasions she has vomited after meals due to feeling extremely full; however, she denies having done so intentionally. She also denies restricting her food intake, misusing laxatives or diuretics, or exercising excessively.
Ms. C expresses frustration and embarrassment with her eating and resulting weight gain. She says she has poor self-esteem, low energy and motivation, and poor concentration. She feels that her condition has significantly impacted her social life, romantic relationships, and family life. She admits she’s been avoiding dating and seeing friends due to her weight gain, and has been irritable with her teenage daughter.
During her initial evaluation, Ms. C is alert and oriented, with a linear and goal-directed thought process. She is somewhat irritable and guarded, wearing large sunglasses that cover most of her face, but is not overtly paranoid. Although she appears frustrated when discussing her condition, she denies feeling hopeless or helpless.
HISTORY Thyroid cancer and mood swings
Ms. C, who is single and unemployed, lives in an apartment with her teenage daughter, with whom she describes having a good relationship. She has been receiving disability benefits for the past 2 years after a motor vehicle accident resulted in multiple fractures of her arm and elbow, and subsequent chronic pain. Ms. C reports a distant history of “problems with alcohol,” but denies drinking any alcohol since being charged with driving under the influence several years ago. She has a 10 pack-year history of smoking and denies any history of illicit drug use.
Two years ago, Ms. C was diagnosed with thyroid carcinoma, and treated with surgical resection and a course of radiation. She has regular visits with her endocrinologist and has been prescribed oral levothyroxine, 150 mcg/d.
Ms. C reports a history of “mood swings” characterized by “snapping at people” and becoming irritable in response to stressful situations, but denies any past symptoms consistent with a manic or hypomanic episode. Ms. C has not been admitted to a psychiatric hospital, nor has she received any prior psychiatric treatment. She reluctantly discloses that approximately 3 years ago she had a less severe episode of uncontrollable eating and weight gain (20 to 30 lb). At that time, she was able to regain her desired physical appearance by going on the “Subway diet” and undergoing liposuction and plastic surgery.
At her current outpatient clinic visit, Ms. C expresses an interest in exploring bariatric surgery as a potential solution to her weight gain.
[polldaddy:10446186]
Continue to: EVALUATION Obese; stable thyroid function
EVALUATION Obese; stable thyroid function
We refer Ms. C for a physical examination and routine blood analysis to rule out any medical contributors to her condition. Her physical examination is reported as normal, with no signs of skin changes, goiter, or exophthalmos. Ms. C is noted to be obese, with a body mass index of 37.2 kg/m2, and an abdominal circumference of 38.5 in.
A blood analysis shows that Ms. C has elevated triglyceride levels (202 mg/dL) and elevated cholesterol levels (210 mg/dL). Her thyroid function tests are within normal limits based on the dose of levothyroxine she’s been receiving. A pregnancy test is negative.
Ms. C gives the team at the clinic permission to contact her endocrinologist, who reports that he does not suspect that Ms. C’s drastic weight gain and abnormal eating patterns are attributable to her history of thyroid carcinoma because her thyroid function tests have been stable on her current regimen.
The authors’ observations
Based on Ms. C’s initial presentation, we strongly suspected a diagnosis of binge eating disorder (BED). Several differential diagnoses were considered and carefully ruled out; Ms. C’s medical workup did not suggest that her weight gain was due to an active medical condition, and she did not meet DSM-5 criteria for a mood or psychotic disorder or anorexia nervosa or bulimia nervosa.
With an estimated lifetime prevalence in the United States of 2.6%, BED is the most prevalent eating disorder (compared with 0.6% for anorexia nervosa and 1% for bulimia nervosa).1 BED is more prevalent in women than in men, and the mean age of onset is mid-20s.
Continue to: BED may be difficult...
BED may be difficult to detect because patients may feel ashamed or guilty and are often hesitant to disclose and discuss their symptoms. Furthermore, they are frequently frustrated by the subjective loss of control over their behaviors. Patients with BED often present to medical facilities seeking weight loss solutions rather than to psychiatric clinics.
Screening for eating disorders
Several screening instruments have been developed to help clinicians identify patients who may need further evaluation for possible diagnosis of an eating disorder, including anorexia nervosa, bulimia nervosa, and BED.2 The SCOFF questionnaire is composed of 5 brief clinician-administered questions to screen for eating disorders.2 The 7-item Binge Eating Disorder Screener (BED-7) is a screening instrument specific for BED that examines a patient’s eating patterns and behaviors during the past 3 months.3
In general, suspect BED in patients who have significant weight dissatisfaction, fluctuation in weight, and depressive symptoms. The DSM-5 criteria for binge eating disorder are shown in Table 14.
BED and comorbid psychiatric disorders
Patients with BED are more likely than the general population to have comorbid psychiatric disorders, including mood and anxiety disorders, attention-deficit/hyperactivity disorder, posttraumatic stress disorder, and substance use disorders. Swanson et al5 found that 83.5% of adolescents who met criteria for BED also met criteria for at least 1 other psychiatric disorder, and 37% endorsed >3 concurrent psychiatric conditions. Once BED is confirmed, it is important to screen for other psychiatric and medical comorbidities that are often present in individuals with BED (Table 21,6).
The rates of diagnosis and treatment of BED remain low. This is likely due to patient factors such as shame and fear of stigma and clinician factors such as lack of awareness, ineffective communication, hesitation to discuss the sensitive topic, or insufficient knowledge about treatment options once BED is diagnosed.
[polldaddy:10446187]
Continue to: TREATMENT Combination therapy
TREATMENT Combination therapy
Ms. C is ambivalent about her BED diagnosis, and becomes angry about it when the proposed treatments do not involve bariatric surgery or cosmetic procedures. Ms. C is enrolled in weekly individual psychotherapy, where she receives a combination of CBT and psychodynamic therapy; however, her attendance is inconsistent. Ms. C is offered a trial of fluoxetine, but adamantly refuses, citing a relative who experienced adverse effects while receiving this type of antidepressant. Ms. C also refuses a trial of topiramate due to concerns of feeling sedated. Finally, she is offered a trial of lisdexamfetamine, 30 mg/d, which was FDA-approved in 2015 to treat moderate to severe BED. We discuss the risks, benefits, and adverse effects of lisdexamfetamine with Ms. C; however, she is hesitant to start this medication and expresses increasing interest in obtaining a consultation for bariatric surgery. Ms. C is provided with extensive education about the risks and dangers of surgery before addressing her eating patterns, and the clinician provides validation, verbal support, and counseling. Ms. C eventually agrees to a trial of lisdexamfetamine, but her insurance denies coverage of this medication.
The authors’ observations
When developing an individualized treatment plan for a patient with BED, the patient’s psychiatric and medical comorbidities should be considered. Treatment goals for patients with BED include:
- abstinence from binge eating
- sustainable weight loss and metabolic health
- reduction in symptoms associated with comorbid conditions
- improvement in self-esteem and overall quality of life.
A 2015 comparative effectiveness review of management and outcomes for patients with BED evaluated pharmacologic, psychologic, behavioral, and combined approaches for treating patients with BED.7 The results suggested that second-generation antidepressants, topiramate, and lisdexamfetamine were superior to placebo in reducing binge-eating episodes and achieving abstinence from binge-eating. Weight reduction was also achieved with topiramate and lisdexamfetamine, and antidepressants helped relieve symptoms of comorbid depression.
Various formats of CBT, including therapist-led and guided self-help, were also superior to placebo in reducing the frequency of binge-eating and promoting abstinence; however, they were generally not effective in treating depression or reducing patients’ weight.7
OUTCOME Fixated on surgery
We appeal the decision of Ms. C’s insurance company; however, during the appeals process, Ms. C becomes increasingly irritable and informs us that she has changed her mind and, with the reported support of her medical doctors, wishes to undergo bariatric surgery. Although we made multiple attempts to engage Ms. C in further treatment, she is lost to follow-up.
Continue to: Bottom Line
Bottom Line
Diagnosing and managing patients with binge eating disorder (BED) can be challenging because patients may hesitate to seek help, and/or have psychiatric and medical comorbidities. They often present to medical facilities seeking weight loss solutions rather than to psychiatric clinics. Once BED is confirmed, screen for other psychiatric and medical comorbidities. A combination of pharmacologic and psychotherapeutic interventions can benefit some patients with BED, but treatment should be individualized.
Related Resources
- National Eating Disorders Association. NEDA. www.nationaleatingdisorders.org/.
- Safer D, Telch C, Chen EY. Dialectical behavior therapy for binge eating and bulimia. New York, NY: Guilford Press; 2017.
Drug Brand Names
Fluoxetine • Prozac
Levothyroxine • Synthroid
Lisdexamfetamine • Vyvanse
Topiramate • Topamax
1. Hudson JI, Hiripi E, Pope HG Jr, et al. The prevalence and correlates of eating disorders in the National Comorbidity Survey Replication. Biol Psychiatry. 2007;61(3):348-358.
2. Morgan JF, Reid F, Lacey JH. The SCOFF questionnaire: assessment of a new screening tool for eating disorders. BMJ. 1999;319(7223):1467-1468.
3. Herman BK, Deal LS, DiBenedetti DB, et al. Development of the 7-Item Binge-Eating Disorder screener (BEDS-7). Prim Care Companion CNS Disord. 2016;18(2):10.4088/PCC.15m01896. doi:10.4088/PCC.15m01896.
4. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
5. Swanson SA, Crow SJ, Le Grange D, et al. Prevalence and correlates of eating disorders in adolescents. Results from the national comorbidity survey replication adolescent supplement. Arch Gen Psychiatry. 2011;68(7):714.
6. Guerdjikova AI, Mori N, Casuto LS, et al. Binge eating disorder. Psychiatric Clinics of North America. 2017;40(2):255-266.
7. Berkman ND, Brownley KA, Peat CM, et al. Management and outcomes of binge-eating disorder. Comparative Effectiveness Reviews, No. 160. Agency for Healthcare Research and Quality (US). https://www.ncbi.nlm.nih.gov/books/NBK338312/. Published December 2015. Accessed July 29, 2019.
CASE Uncontrollable eating and weight gain
Ms. C, age 33, presents to an outpatient clinic with complaints of weight gain and “uncontrollable eating.” Ms. C says she’s gained >50 lb over the last year. She describes progressively frequent episodes of overeating during which she feels that she has no control over the amount of food she consumes. She reports eating as often as 10 times a day, and overeating to the point of physical discomfort during most meals. She gives an example of having recently consumed a large pizza, several portions of Chinese food, approximately 20 chicken wings, and half a chocolate cake for dinner. Ms. C admits that on several occasions she has vomited after meals due to feeling extremely full; however, she denies having done so intentionally. She also denies restricting her food intake, misusing laxatives or diuretics, or exercising excessively.
Ms. C expresses frustration and embarrassment with her eating and resulting weight gain. She says she has poor self-esteem, low energy and motivation, and poor concentration. She feels that her condition has significantly impacted her social life, romantic relationships, and family life. She admits she’s been avoiding dating and seeing friends due to her weight gain, and has been irritable with her teenage daughter.
During her initial evaluation, Ms. C is alert and oriented, with a linear and goal-directed thought process. She is somewhat irritable and guarded, wearing large sunglasses that cover most of her face, but is not overtly paranoid. Although she appears frustrated when discussing her condition, she denies feeling hopeless or helpless.
HISTORY Thyroid cancer and mood swings
Ms. C, who is single and unemployed, lives in an apartment with her teenage daughter, with whom she describes having a good relationship. She has been receiving disability benefits for the past 2 years after a motor vehicle accident resulted in multiple fractures of her arm and elbow, and subsequent chronic pain. Ms. C reports a distant history of “problems with alcohol,” but denies drinking any alcohol since being charged with driving under the influence several years ago. She has a 10 pack-year history of smoking and denies any history of illicit drug use.
Two years ago, Ms. C was diagnosed with thyroid carcinoma, and treated with surgical resection and a course of radiation. She has regular visits with her endocrinologist and has been prescribed oral levothyroxine, 150 mcg/d.
Ms. C reports a history of “mood swings” characterized by “snapping at people” and becoming irritable in response to stressful situations, but denies any past symptoms consistent with a manic or hypomanic episode. Ms. C has not been admitted to a psychiatric hospital, nor has she received any prior psychiatric treatment. She reluctantly discloses that approximately 3 years ago she had a less severe episode of uncontrollable eating and weight gain (20 to 30 lb). At that time, she was able to regain her desired physical appearance by going on the “Subway diet” and undergoing liposuction and plastic surgery.
At her current outpatient clinic visit, Ms. C expresses an interest in exploring bariatric surgery as a potential solution to her weight gain.
[polldaddy:10446186]
Continue to: EVALUATION Obese; stable thyroid function
EVALUATION Obese; stable thyroid function
We refer Ms. C for a physical examination and routine blood analysis to rule out any medical contributors to her condition. Her physical examination is reported as normal, with no signs of skin changes, goiter, or exophthalmos. Ms. C is noted to be obese, with a body mass index of 37.2 kg/m2, and an abdominal circumference of 38.5 in.
A blood analysis shows that Ms. C has elevated triglyceride levels (202 mg/dL) and elevated cholesterol levels (210 mg/dL). Her thyroid function tests are within normal limits based on the dose of levothyroxine she’s been receiving. A pregnancy test is negative.
Ms. C gives the team at the clinic permission to contact her endocrinologist, who reports that he does not suspect that Ms. C’s drastic weight gain and abnormal eating patterns are attributable to her history of thyroid carcinoma because her thyroid function tests have been stable on her current regimen.
The authors’ observations
Based on Ms. C’s initial presentation, we strongly suspected a diagnosis of binge eating disorder (BED). Several differential diagnoses were considered and carefully ruled out; Ms. C’s medical workup did not suggest that her weight gain was due to an active medical condition, and she did not meet DSM-5 criteria for a mood or psychotic disorder or anorexia nervosa or bulimia nervosa.
With an estimated lifetime prevalence in the United States of 2.6%, BED is the most prevalent eating disorder (compared with 0.6% for anorexia nervosa and 1% for bulimia nervosa).1 BED is more prevalent in women than in men, and the mean age of onset is mid-20s.
Continue to: BED may be difficult...
BED may be difficult to detect because patients may feel ashamed or guilty and are often hesitant to disclose and discuss their symptoms. Furthermore, they are frequently frustrated by the subjective loss of control over their behaviors. Patients with BED often present to medical facilities seeking weight loss solutions rather than to psychiatric clinics.
Screening for eating disorders
Several screening instruments have been developed to help clinicians identify patients who may need further evaluation for possible diagnosis of an eating disorder, including anorexia nervosa, bulimia nervosa, and BED.2 The SCOFF questionnaire is composed of 5 brief clinician-administered questions to screen for eating disorders.2 The 7-item Binge Eating Disorder Screener (BED-7) is a screening instrument specific for BED that examines a patient’s eating patterns and behaviors during the past 3 months.3
In general, suspect BED in patients who have significant weight dissatisfaction, fluctuation in weight, and depressive symptoms. The DSM-5 criteria for binge eating disorder are shown in Table 14.
BED and comorbid psychiatric disorders
Patients with BED are more likely than the general population to have comorbid psychiatric disorders, including mood and anxiety disorders, attention-deficit/hyperactivity disorder, posttraumatic stress disorder, and substance use disorders. Swanson et al5 found that 83.5% of adolescents who met criteria for BED also met criteria for at least 1 other psychiatric disorder, and 37% endorsed >3 concurrent psychiatric conditions. Once BED is confirmed, it is important to screen for other psychiatric and medical comorbidities that are often present in individuals with BED (Table 21,6).
The rates of diagnosis and treatment of BED remain low. This is likely due to patient factors such as shame and fear of stigma and clinician factors such as lack of awareness, ineffective communication, hesitation to discuss the sensitive topic, or insufficient knowledge about treatment options once BED is diagnosed.
[polldaddy:10446187]
Continue to: TREATMENT Combination therapy
TREATMENT Combination therapy
Ms. C is ambivalent about her BED diagnosis, and becomes angry about it when the proposed treatments do not involve bariatric surgery or cosmetic procedures. Ms. C is enrolled in weekly individual psychotherapy, where she receives a combination of CBT and psychodynamic therapy; however, her attendance is inconsistent. Ms. C is offered a trial of fluoxetine, but adamantly refuses, citing a relative who experienced adverse effects while receiving this type of antidepressant. Ms. C also refuses a trial of topiramate due to concerns of feeling sedated. Finally, she is offered a trial of lisdexamfetamine, 30 mg/d, which was FDA-approved in 2015 to treat moderate to severe BED. We discuss the risks, benefits, and adverse effects of lisdexamfetamine with Ms. C; however, she is hesitant to start this medication and expresses increasing interest in obtaining a consultation for bariatric surgery. Ms. C is provided with extensive education about the risks and dangers of surgery before addressing her eating patterns, and the clinician provides validation, verbal support, and counseling. Ms. C eventually agrees to a trial of lisdexamfetamine, but her insurance denies coverage of this medication.
The authors’ observations
When developing an individualized treatment plan for a patient with BED, the patient’s psychiatric and medical comorbidities should be considered. Treatment goals for patients with BED include:
- abstinence from binge eating
- sustainable weight loss and metabolic health
- reduction in symptoms associated with comorbid conditions
- improvement in self-esteem and overall quality of life.
A 2015 comparative effectiveness review of management and outcomes for patients with BED evaluated pharmacologic, psychologic, behavioral, and combined approaches for treating patients with BED.7 The results suggested that second-generation antidepressants, topiramate, and lisdexamfetamine were superior to placebo in reducing binge-eating episodes and achieving abstinence from binge-eating. Weight reduction was also achieved with topiramate and lisdexamfetamine, and antidepressants helped relieve symptoms of comorbid depression.
Various formats of CBT, including therapist-led and guided self-help, were also superior to placebo in reducing the frequency of binge-eating and promoting abstinence; however, they were generally not effective in treating depression or reducing patients’ weight.7
OUTCOME Fixated on surgery
We appeal the decision of Ms. C’s insurance company; however, during the appeals process, Ms. C becomes increasingly irritable and informs us that she has changed her mind and, with the reported support of her medical doctors, wishes to undergo bariatric surgery. Although we made multiple attempts to engage Ms. C in further treatment, she is lost to follow-up.
Continue to: Bottom Line
Bottom Line
Diagnosing and managing patients with binge eating disorder (BED) can be challenging because patients may hesitate to seek help, and/or have psychiatric and medical comorbidities. They often present to medical facilities seeking weight loss solutions rather than to psychiatric clinics. Once BED is confirmed, screen for other psychiatric and medical comorbidities. A combination of pharmacologic and psychotherapeutic interventions can benefit some patients with BED, but treatment should be individualized.
Related Resources
- National Eating Disorders Association. NEDA. www.nationaleatingdisorders.org/.
- Safer D, Telch C, Chen EY. Dialectical behavior therapy for binge eating and bulimia. New York, NY: Guilford Press; 2017.
Drug Brand Names
Fluoxetine • Prozac
Levothyroxine • Synthroid
Lisdexamfetamine • Vyvanse
Topiramate • Topamax
CASE Uncontrollable eating and weight gain
Ms. C, age 33, presents to an outpatient clinic with complaints of weight gain and “uncontrollable eating.” Ms. C says she’s gained >50 lb over the last year. She describes progressively frequent episodes of overeating during which she feels that she has no control over the amount of food she consumes. She reports eating as often as 10 times a day, and overeating to the point of physical discomfort during most meals. She gives an example of having recently consumed a large pizza, several portions of Chinese food, approximately 20 chicken wings, and half a chocolate cake for dinner. Ms. C admits that on several occasions she has vomited after meals due to feeling extremely full; however, she denies having done so intentionally. She also denies restricting her food intake, misusing laxatives or diuretics, or exercising excessively.
Ms. C expresses frustration and embarrassment with her eating and resulting weight gain. She says she has poor self-esteem, low energy and motivation, and poor concentration. She feels that her condition has significantly impacted her social life, romantic relationships, and family life. She admits she’s been avoiding dating and seeing friends due to her weight gain, and has been irritable with her teenage daughter.
During her initial evaluation, Ms. C is alert and oriented, with a linear and goal-directed thought process. She is somewhat irritable and guarded, wearing large sunglasses that cover most of her face, but is not overtly paranoid. Although she appears frustrated when discussing her condition, she denies feeling hopeless or helpless.
HISTORY Thyroid cancer and mood swings
Ms. C, who is single and unemployed, lives in an apartment with her teenage daughter, with whom she describes having a good relationship. She has been receiving disability benefits for the past 2 years after a motor vehicle accident resulted in multiple fractures of her arm and elbow, and subsequent chronic pain. Ms. C reports a distant history of “problems with alcohol,” but denies drinking any alcohol since being charged with driving under the influence several years ago. She has a 10 pack-year history of smoking and denies any history of illicit drug use.
Two years ago, Ms. C was diagnosed with thyroid carcinoma, and treated with surgical resection and a course of radiation. She has regular visits with her endocrinologist and has been prescribed oral levothyroxine, 150 mcg/d.
Ms. C reports a history of “mood swings” characterized by “snapping at people” and becoming irritable in response to stressful situations, but denies any past symptoms consistent with a manic or hypomanic episode. Ms. C has not been admitted to a psychiatric hospital, nor has she received any prior psychiatric treatment. She reluctantly discloses that approximately 3 years ago she had a less severe episode of uncontrollable eating and weight gain (20 to 30 lb). At that time, she was able to regain her desired physical appearance by going on the “Subway diet” and undergoing liposuction and plastic surgery.
At her current outpatient clinic visit, Ms. C expresses an interest in exploring bariatric surgery as a potential solution to her weight gain.
[polldaddy:10446186]
Continue to: EVALUATION Obese; stable thyroid function
EVALUATION Obese; stable thyroid function
We refer Ms. C for a physical examination and routine blood analysis to rule out any medical contributors to her condition. Her physical examination is reported as normal, with no signs of skin changes, goiter, or exophthalmos. Ms. C is noted to be obese, with a body mass index of 37.2 kg/m2, and an abdominal circumference of 38.5 in.
A blood analysis shows that Ms. C has elevated triglyceride levels (202 mg/dL) and elevated cholesterol levels (210 mg/dL). Her thyroid function tests are within normal limits based on the dose of levothyroxine she’s been receiving. A pregnancy test is negative.
Ms. C gives the team at the clinic permission to contact her endocrinologist, who reports that he does not suspect that Ms. C’s drastic weight gain and abnormal eating patterns are attributable to her history of thyroid carcinoma because her thyroid function tests have been stable on her current regimen.
The authors’ observations
Based on Ms. C’s initial presentation, we strongly suspected a diagnosis of binge eating disorder (BED). Several differential diagnoses were considered and carefully ruled out; Ms. C’s medical workup did not suggest that her weight gain was due to an active medical condition, and she did not meet DSM-5 criteria for a mood or psychotic disorder or anorexia nervosa or bulimia nervosa.
With an estimated lifetime prevalence in the United States of 2.6%, BED is the most prevalent eating disorder (compared with 0.6% for anorexia nervosa and 1% for bulimia nervosa).1 BED is more prevalent in women than in men, and the mean age of onset is mid-20s.
Continue to: BED may be difficult...
BED may be difficult to detect because patients may feel ashamed or guilty and are often hesitant to disclose and discuss their symptoms. Furthermore, they are frequently frustrated by the subjective loss of control over their behaviors. Patients with BED often present to medical facilities seeking weight loss solutions rather than to psychiatric clinics.
Screening for eating disorders
Several screening instruments have been developed to help clinicians identify patients who may need further evaluation for possible diagnosis of an eating disorder, including anorexia nervosa, bulimia nervosa, and BED.2 The SCOFF questionnaire is composed of 5 brief clinician-administered questions to screen for eating disorders.2 The 7-item Binge Eating Disorder Screener (BED-7) is a screening instrument specific for BED that examines a patient’s eating patterns and behaviors during the past 3 months.3
In general, suspect BED in patients who have significant weight dissatisfaction, fluctuation in weight, and depressive symptoms. The DSM-5 criteria for binge eating disorder are shown in Table 14.
BED and comorbid psychiatric disorders
Patients with BED are more likely than the general population to have comorbid psychiatric disorders, including mood and anxiety disorders, attention-deficit/hyperactivity disorder, posttraumatic stress disorder, and substance use disorders. Swanson et al5 found that 83.5% of adolescents who met criteria for BED also met criteria for at least 1 other psychiatric disorder, and 37% endorsed >3 concurrent psychiatric conditions. Once BED is confirmed, it is important to screen for other psychiatric and medical comorbidities that are often present in individuals with BED (Table 21,6).
The rates of diagnosis and treatment of BED remain low. This is likely due to patient factors such as shame and fear of stigma and clinician factors such as lack of awareness, ineffective communication, hesitation to discuss the sensitive topic, or insufficient knowledge about treatment options once BED is diagnosed.
[polldaddy:10446187]
Continue to: TREATMENT Combination therapy
TREATMENT Combination therapy
Ms. C is ambivalent about her BED diagnosis, and becomes angry about it when the proposed treatments do not involve bariatric surgery or cosmetic procedures. Ms. C is enrolled in weekly individual psychotherapy, where she receives a combination of CBT and psychodynamic therapy; however, her attendance is inconsistent. Ms. C is offered a trial of fluoxetine, but adamantly refuses, citing a relative who experienced adverse effects while receiving this type of antidepressant. Ms. C also refuses a trial of topiramate due to concerns of feeling sedated. Finally, she is offered a trial of lisdexamfetamine, 30 mg/d, which was FDA-approved in 2015 to treat moderate to severe BED. We discuss the risks, benefits, and adverse effects of lisdexamfetamine with Ms. C; however, she is hesitant to start this medication and expresses increasing interest in obtaining a consultation for bariatric surgery. Ms. C is provided with extensive education about the risks and dangers of surgery before addressing her eating patterns, and the clinician provides validation, verbal support, and counseling. Ms. C eventually agrees to a trial of lisdexamfetamine, but her insurance denies coverage of this medication.
The authors’ observations
When developing an individualized treatment plan for a patient with BED, the patient’s psychiatric and medical comorbidities should be considered. Treatment goals for patients with BED include:
- abstinence from binge eating
- sustainable weight loss and metabolic health
- reduction in symptoms associated with comorbid conditions
- improvement in self-esteem and overall quality of life.
A 2015 comparative effectiveness review of management and outcomes for patients with BED evaluated pharmacologic, psychologic, behavioral, and combined approaches for treating patients with BED.7 The results suggested that second-generation antidepressants, topiramate, and lisdexamfetamine were superior to placebo in reducing binge-eating episodes and achieving abstinence from binge-eating. Weight reduction was also achieved with topiramate and lisdexamfetamine, and antidepressants helped relieve symptoms of comorbid depression.
Various formats of CBT, including therapist-led and guided self-help, were also superior to placebo in reducing the frequency of binge-eating and promoting abstinence; however, they were generally not effective in treating depression or reducing patients’ weight.7
OUTCOME Fixated on surgery
We appeal the decision of Ms. C’s insurance company; however, during the appeals process, Ms. C becomes increasingly irritable and informs us that she has changed her mind and, with the reported support of her medical doctors, wishes to undergo bariatric surgery. Although we made multiple attempts to engage Ms. C in further treatment, she is lost to follow-up.
Continue to: Bottom Line
Bottom Line
Diagnosing and managing patients with binge eating disorder (BED) can be challenging because patients may hesitate to seek help, and/or have psychiatric and medical comorbidities. They often present to medical facilities seeking weight loss solutions rather than to psychiatric clinics. Once BED is confirmed, screen for other psychiatric and medical comorbidities. A combination of pharmacologic and psychotherapeutic interventions can benefit some patients with BED, but treatment should be individualized.
Related Resources
- National Eating Disorders Association. NEDA. www.nationaleatingdisorders.org/.
- Safer D, Telch C, Chen EY. Dialectical behavior therapy for binge eating and bulimia. New York, NY: Guilford Press; 2017.
Drug Brand Names
Fluoxetine • Prozac
Levothyroxine • Synthroid
Lisdexamfetamine • Vyvanse
Topiramate • Topamax
1. Hudson JI, Hiripi E, Pope HG Jr, et al. The prevalence and correlates of eating disorders in the National Comorbidity Survey Replication. Biol Psychiatry. 2007;61(3):348-358.
2. Morgan JF, Reid F, Lacey JH. The SCOFF questionnaire: assessment of a new screening tool for eating disorders. BMJ. 1999;319(7223):1467-1468.
3. Herman BK, Deal LS, DiBenedetti DB, et al. Development of the 7-Item Binge-Eating Disorder screener (BEDS-7). Prim Care Companion CNS Disord. 2016;18(2):10.4088/PCC.15m01896. doi:10.4088/PCC.15m01896.
4. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
5. Swanson SA, Crow SJ, Le Grange D, et al. Prevalence and correlates of eating disorders in adolescents. Results from the national comorbidity survey replication adolescent supplement. Arch Gen Psychiatry. 2011;68(7):714.
6. Guerdjikova AI, Mori N, Casuto LS, et al. Binge eating disorder. Psychiatric Clinics of North America. 2017;40(2):255-266.
7. Berkman ND, Brownley KA, Peat CM, et al. Management and outcomes of binge-eating disorder. Comparative Effectiveness Reviews, No. 160. Agency for Healthcare Research and Quality (US). https://www.ncbi.nlm.nih.gov/books/NBK338312/. Published December 2015. Accessed July 29, 2019.
1. Hudson JI, Hiripi E, Pope HG Jr, et al. The prevalence and correlates of eating disorders in the National Comorbidity Survey Replication. Biol Psychiatry. 2007;61(3):348-358.
2. Morgan JF, Reid F, Lacey JH. The SCOFF questionnaire: assessment of a new screening tool for eating disorders. BMJ. 1999;319(7223):1467-1468.
3. Herman BK, Deal LS, DiBenedetti DB, et al. Development of the 7-Item Binge-Eating Disorder screener (BEDS-7). Prim Care Companion CNS Disord. 2016;18(2):10.4088/PCC.15m01896. doi:10.4088/PCC.15m01896.
4. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
5. Swanson SA, Crow SJ, Le Grange D, et al. Prevalence and correlates of eating disorders in adolescents. Results from the national comorbidity survey replication adolescent supplement. Arch Gen Psychiatry. 2011;68(7):714.
6. Guerdjikova AI, Mori N, Casuto LS, et al. Binge eating disorder. Psychiatric Clinics of North America. 2017;40(2):255-266.
7. Berkman ND, Brownley KA, Peat CM, et al. Management and outcomes of binge-eating disorder. Comparative Effectiveness Reviews, No. 160. Agency for Healthcare Research and Quality (US). https://www.ncbi.nlm.nih.gov/books/NBK338312/. Published December 2015. Accessed July 29, 2019.
The 84-year-old state boxing champ: Bipolar disorder, or something else?
CASE Agitated, uncooperative, and irritable
Mr. X, age 84, presents to the emergency department with agitation, mania-like symptoms, and mood-congruent psychotic symptoms that started 2 weeks ago. Mr. X, who is accompanied by his wife, has no psychiatric history.
On examination, Mr. X is easily agitated and uncooperative. His speech is fast, but not pressured, with increased volume and tone. He states, “My mood is fantastic” with mood-congruent affect. His thought process reveals circumstantiality and loose association. Mr. X’s thought content includes flight of ideas and delusions of grandeur; he claims to be a state boxing champion and a psychologist. He also claims that he will run for Congress in the near future. He reports that he’s started knocking on his neighbors’ doors, pitched the idea to buy their house, and convinced them to vote for him as their congressman. He denies any suicidal or homicidal ideations. There is no evidence of perceptual disturbance. Mr. X undergoes a Mini-Mental State Examination (MMSE) and scores 26/30, which suggests no cognitive impairment. However, his insight and judgment are poor.
Mr. X’s physical examination is unremarkable. His laboratory workup includes a complete blood count, comprehensive metabolic panel, urinalysis, thyroid function test, vitamin B12 and folate levels, urine drug screen, and blood alcohol level. All results are within normal limits. He has no history of alcohol or recreational drug use as evident by the laboratory results and collateral information from his wife. Further, a non-contrast CT scan of his head shows no abnormality.
Approximately 1 month ago, Mr. X was diagnosed with restless leg syndrome (RLS). Mr. X’s medication regimen consists of gabapentin, 300 mg 3 times daily, prescribed years ago by his neurologist for neuropathic pain; and ropinirole, 3 mg/d, for RLS. His neurologist had prescribed him ropinirole, which was started at 1 mg/d and titrated to 3 mg/d within a 1-week span. Two weeks after Mr. X started this medication regimen, his wife reports that she noticed changes in his behavior, including severe agitation, irritability, delusions of grandeur, decreased need for sleep, and racing of thoughts.
[polldaddy:10417490]
The authors’ observations
Mr. X was diagnosed with medication (ropinirole)-induced bipolar and related disorder with mood-congruent psychotic features.
To determine this diagnosis, we initially considered Mr. X’s age and medical conditions, including stroke and space-occupying lesions of the brain. However, the laboratory and neuroimaging studies, which included a CT scan of the head and MRI of the brain, were negative. Next, because Mr. X had sudden onset manic symptoms after ropinirole was initiated, we considered the possibility of a substance/medication-induced bipolar and related disorder. Further, ropinirole is capable of producing the symptoms in criterion A of DSM-5 criteria for substance/medication-induced bipolar and related disorder. Mr. X met all DSM-5 criteria for substance/medication-induced bipolar and related disorder (Table1).

[polldaddy:10417494]
TREATMENT Medication adjustments and improvement
The admitting clinician discontinues ropinirole and initiates divalproex sodium, 500 mg twice a day. By Day 4, Mr. X shows significant improvement, including no irritable mood and regression of delusions of grandeur, and his sleep cycle returns to normal. At this time, the divalproex sodium is also discontinued.
Continue to: The authors' observations
The authors’ observations
Dopamine agonist agents are a standard treatment in the management of Parkinson’s disease and RLS.2-5 Ropinirole, a dopamine receptor agonist, has a high affinity for dopamine D2 and D3 receptor subtypes.4 Published reports have linked dopamine agonists to mania with psychotic features.6,7 In a study by Stoner et al,8 of 95 patients treated with ropinirole, 13 patients developed psychotic features that necessitated the use of antipsychotic medications or a lower dose of ropinirole.
The recommended starting dose for ropinirole is 0.25 mg/d. The dose can be increased to 0.5 mg in the next 2 days, and to 1 mg/d at the end of the first week.9 The mean effective daily dose is 2 mg/d, and maximum recommended dose is 4 mg/d.9 For Mr. X, ropinirole was quickly titrated to 3 mg/d over 1 week, which resulted in mania and psychosis. We suggest that when treating geriatric patients, clinicians should consider prescribing the lowest effective dose of psychotropic medications, such as ropinirole, to prevent adverse effects. Higher doses of dopamine agonists, especially in geriatric patients, increase the risk of common adverse effects, such as nausea (25% to 50%), headache (7% to 22%), fatigue (1% to 19%), dizziness (6% to 18%), and vomiting (5% to 11%).10 When prescribing dopamine agonists, clinicians should educate patients and their caregivers about the rare but potential risk of medication-induced mania and psychosis.
Mr. X’s case emphasizes the importance of a comprehensive psychiatric evaluation and medical workup to rule out a wide differential diagnosis when approaching new-onset mania and psychosis in geriatric patients.11 Our case contributes to the evidence that dopamine agonist medications are associated with mania and psychotic symptoms.
OUTCOME A return to baseline
On Day 12, Mr. X is discharged home in a stable condition. Two weeks later, at an outpatient follow-up visit, Mr. X is asymptomatic and has returned to his baseline functioning.
Bottom Line
When approaching new-onset mania and psychosis in geriatric patients, a comprehensive psychiatric evaluation and medical workup are necessary to rule out a wide differential diagnosis. Ropinirole use can lead to mania and psychotic symptoms, especially in geriatric patients. As should be done with all other dopaminergic agents, increase the dose of ropinirole with caution, and be vigilant for the emergence of signs of mania and/or psychosis.
Continue to: Related Resources
Related Resources
- Adabie A, Jackson JC, Torrence CL. Older-age bipolar disorder: A case series. Current Psychiatry. 2019;18(2):24-29.
- Chen P, Dols A, Rej S, et al. Update on the epidemiology, diagnosis, and treatment of mania in older-age bipolar disorder. Curr Psychiatry Rep. 2017;19(8):46.
Drug Brand Names
Divalproex sodium • Depakote
Gabapentin • Neurontin
Ropinirole • Requip
1. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
2. Singh A, Althoff R, Martineau RJ, et al. Pramipexole, ropinirole, and mania in Parkinson’s disease. Am J Psychiatry. 2005;162(4):814-815.
3. Weiss HD, Pontone GM. Dopamine receptor agonist drugs and impulse control disorders. JAMA Intern Med. 2014;174(12):1935-1937.
4 Shill HA, Stacy M. Update on ropinirole in the treatment of Parkinson’s disease. Neuropsychiatr Dis Treat. 2009;5:33-36.
5. Borovac JA. Side effects of a dopamine agonist therapy for Parkinson’s disease: a mini-review of clinical pharmacology. Yale J Biol Med. 2016;89(1):37-47.
6. Yüksel RN, Elyas Kaya Z, Dilbaz N, et al. Cabergoline-induced manic episode: case report. Ther Adv Psychopharmacol. 2016;6(3):229-231.
7. Perea E, Robbins BV, Hutto B. Psychosis related to ropinirole. Am J Psychiatry. 2006;163(3):547-548.
8. Stoner SC, Dahmen MM, Makos M, et al. An exploratory retrospective evaluation of ropinirole-associated psychotic symptoms in an outpatient population treated for restless legs syndrome or Parkinson’s disease. Ann Pharmacother. 2009;43(9):1426-1432.
9. Trenkwalder C, Hening WA, Montagna P, et al. Treatment of restless legs syndrome: an evidence-based review and implications for clinical practice. Mov Disord. 2008;23(16):2267-2302.
10. Garcia-Borreguero D, Kohnen R, Silber MH, et al. The long-term treatment of restless legs syndrome/Willis-Ekbom disease: evidence-based guidelines and clinical consensus best practice guidance: a report from the International Restless Legs Syndrome Study Group. Sleep Med. 2013;14(7):675-684.
11. Dols A, Beekman A. Older age bipolar disorder. Psychiatr Clin North Am. 2018;41(1):95-110.
CASE Agitated, uncooperative, and irritable
Mr. X, age 84, presents to the emergency department with agitation, mania-like symptoms, and mood-congruent psychotic symptoms that started 2 weeks ago. Mr. X, who is accompanied by his wife, has no psychiatric history.
On examination, Mr. X is easily agitated and uncooperative. His speech is fast, but not pressured, with increased volume and tone. He states, “My mood is fantastic” with mood-congruent affect. His thought process reveals circumstantiality and loose association. Mr. X’s thought content includes flight of ideas and delusions of grandeur; he claims to be a state boxing champion and a psychologist. He also claims that he will run for Congress in the near future. He reports that he’s started knocking on his neighbors’ doors, pitched the idea to buy their house, and convinced them to vote for him as their congressman. He denies any suicidal or homicidal ideations. There is no evidence of perceptual disturbance. Mr. X undergoes a Mini-Mental State Examination (MMSE) and scores 26/30, which suggests no cognitive impairment. However, his insight and judgment are poor.
Mr. X’s physical examination is unremarkable. His laboratory workup includes a complete blood count, comprehensive metabolic panel, urinalysis, thyroid function test, vitamin B12 and folate levels, urine drug screen, and blood alcohol level. All results are within normal limits. He has no history of alcohol or recreational drug use as evident by the laboratory results and collateral information from his wife. Further, a non-contrast CT scan of his head shows no abnormality.
Approximately 1 month ago, Mr. X was diagnosed with restless leg syndrome (RLS). Mr. X’s medication regimen consists of gabapentin, 300 mg 3 times daily, prescribed years ago by his neurologist for neuropathic pain; and ropinirole, 3 mg/d, for RLS. His neurologist had prescribed him ropinirole, which was started at 1 mg/d and titrated to 3 mg/d within a 1-week span. Two weeks after Mr. X started this medication regimen, his wife reports that she noticed changes in his behavior, including severe agitation, irritability, delusions of grandeur, decreased need for sleep, and racing of thoughts.
[polldaddy:10417490]
The authors’ observations
Mr. X was diagnosed with medication (ropinirole)-induced bipolar and related disorder with mood-congruent psychotic features.
To determine this diagnosis, we initially considered Mr. X’s age and medical conditions, including stroke and space-occupying lesions of the brain. However, the laboratory and neuroimaging studies, which included a CT scan of the head and MRI of the brain, were negative. Next, because Mr. X had sudden onset manic symptoms after ropinirole was initiated, we considered the possibility of a substance/medication-induced bipolar and related disorder. Further, ropinirole is capable of producing the symptoms in criterion A of DSM-5 criteria for substance/medication-induced bipolar and related disorder. Mr. X met all DSM-5 criteria for substance/medication-induced bipolar and related disorder (Table1).

[polldaddy:10417494]
TREATMENT Medication adjustments and improvement
The admitting clinician discontinues ropinirole and initiates divalproex sodium, 500 mg twice a day. By Day 4, Mr. X shows significant improvement, including no irritable mood and regression of delusions of grandeur, and his sleep cycle returns to normal. At this time, the divalproex sodium is also discontinued.
Continue to: The authors' observations
The authors’ observations
Dopamine agonist agents are a standard treatment in the management of Parkinson’s disease and RLS.2-5 Ropinirole, a dopamine receptor agonist, has a high affinity for dopamine D2 and D3 receptor subtypes.4 Published reports have linked dopamine agonists to mania with psychotic features.6,7 In a study by Stoner et al,8 of 95 patients treated with ropinirole, 13 patients developed psychotic features that necessitated the use of antipsychotic medications or a lower dose of ropinirole.
The recommended starting dose for ropinirole is 0.25 mg/d. The dose can be increased to 0.5 mg in the next 2 days, and to 1 mg/d at the end of the first week.9 The mean effective daily dose is 2 mg/d, and maximum recommended dose is 4 mg/d.9 For Mr. X, ropinirole was quickly titrated to 3 mg/d over 1 week, which resulted in mania and psychosis. We suggest that when treating geriatric patients, clinicians should consider prescribing the lowest effective dose of psychotropic medications, such as ropinirole, to prevent adverse effects. Higher doses of dopamine agonists, especially in geriatric patients, increase the risk of common adverse effects, such as nausea (25% to 50%), headache (7% to 22%), fatigue (1% to 19%), dizziness (6% to 18%), and vomiting (5% to 11%).10 When prescribing dopamine agonists, clinicians should educate patients and their caregivers about the rare but potential risk of medication-induced mania and psychosis.
Mr. X’s case emphasizes the importance of a comprehensive psychiatric evaluation and medical workup to rule out a wide differential diagnosis when approaching new-onset mania and psychosis in geriatric patients.11 Our case contributes to the evidence that dopamine agonist medications are associated with mania and psychotic symptoms.
OUTCOME A return to baseline
On Day 12, Mr. X is discharged home in a stable condition. Two weeks later, at an outpatient follow-up visit, Mr. X is asymptomatic and has returned to his baseline functioning.
Bottom Line
When approaching new-onset mania and psychosis in geriatric patients, a comprehensive psychiatric evaluation and medical workup are necessary to rule out a wide differential diagnosis. Ropinirole use can lead to mania and psychotic symptoms, especially in geriatric patients. As should be done with all other dopaminergic agents, increase the dose of ropinirole with caution, and be vigilant for the emergence of signs of mania and/or psychosis.
Continue to: Related Resources
Related Resources
- Adabie A, Jackson JC, Torrence CL. Older-age bipolar disorder: A case series. Current Psychiatry. 2019;18(2):24-29.
- Chen P, Dols A, Rej S, et al. Update on the epidemiology, diagnosis, and treatment of mania in older-age bipolar disorder. Curr Psychiatry Rep. 2017;19(8):46.
Drug Brand Names
Divalproex sodium • Depakote
Gabapentin • Neurontin
Ropinirole • Requip
CASE Agitated, uncooperative, and irritable
Mr. X, age 84, presents to the emergency department with agitation, mania-like symptoms, and mood-congruent psychotic symptoms that started 2 weeks ago. Mr. X, who is accompanied by his wife, has no psychiatric history.
On examination, Mr. X is easily agitated and uncooperative. His speech is fast, but not pressured, with increased volume and tone. He states, “My mood is fantastic” with mood-congruent affect. His thought process reveals circumstantiality and loose association. Mr. X’s thought content includes flight of ideas and delusions of grandeur; he claims to be a state boxing champion and a psychologist. He also claims that he will run for Congress in the near future. He reports that he’s started knocking on his neighbors’ doors, pitched the idea to buy their house, and convinced them to vote for him as their congressman. He denies any suicidal or homicidal ideations. There is no evidence of perceptual disturbance. Mr. X undergoes a Mini-Mental State Examination (MMSE) and scores 26/30, which suggests no cognitive impairment. However, his insight and judgment are poor.
Mr. X’s physical examination is unremarkable. His laboratory workup includes a complete blood count, comprehensive metabolic panel, urinalysis, thyroid function test, vitamin B12 and folate levels, urine drug screen, and blood alcohol level. All results are within normal limits. He has no history of alcohol or recreational drug use as evident by the laboratory results and collateral information from his wife. Further, a non-contrast CT scan of his head shows no abnormality.
Approximately 1 month ago, Mr. X was diagnosed with restless leg syndrome (RLS). Mr. X’s medication regimen consists of gabapentin, 300 mg 3 times daily, prescribed years ago by his neurologist for neuropathic pain; and ropinirole, 3 mg/d, for RLS. His neurologist had prescribed him ropinirole, which was started at 1 mg/d and titrated to 3 mg/d within a 1-week span. Two weeks after Mr. X started this medication regimen, his wife reports that she noticed changes in his behavior, including severe agitation, irritability, delusions of grandeur, decreased need for sleep, and racing of thoughts.
[polldaddy:10417490]
The authors’ observations
Mr. X was diagnosed with medication (ropinirole)-induced bipolar and related disorder with mood-congruent psychotic features.
To determine this diagnosis, we initially considered Mr. X’s age and medical conditions, including stroke and space-occupying lesions of the brain. However, the laboratory and neuroimaging studies, which included a CT scan of the head and MRI of the brain, were negative. Next, because Mr. X had sudden onset manic symptoms after ropinirole was initiated, we considered the possibility of a substance/medication-induced bipolar and related disorder. Further, ropinirole is capable of producing the symptoms in criterion A of DSM-5 criteria for substance/medication-induced bipolar and related disorder. Mr. X met all DSM-5 criteria for substance/medication-induced bipolar and related disorder (Table1).

[polldaddy:10417494]
TREATMENT Medication adjustments and improvement
The admitting clinician discontinues ropinirole and initiates divalproex sodium, 500 mg twice a day. By Day 4, Mr. X shows significant improvement, including no irritable mood and regression of delusions of grandeur, and his sleep cycle returns to normal. At this time, the divalproex sodium is also discontinued.
Continue to: The authors' observations
The authors’ observations
Dopamine agonist agents are a standard treatment in the management of Parkinson’s disease and RLS.2-5 Ropinirole, a dopamine receptor agonist, has a high affinity for dopamine D2 and D3 receptor subtypes.4 Published reports have linked dopamine agonists to mania with psychotic features.6,7 In a study by Stoner et al,8 of 95 patients treated with ropinirole, 13 patients developed psychotic features that necessitated the use of antipsychotic medications or a lower dose of ropinirole.
The recommended starting dose for ropinirole is 0.25 mg/d. The dose can be increased to 0.5 mg in the next 2 days, and to 1 mg/d at the end of the first week.9 The mean effective daily dose is 2 mg/d, and maximum recommended dose is 4 mg/d.9 For Mr. X, ropinirole was quickly titrated to 3 mg/d over 1 week, which resulted in mania and psychosis. We suggest that when treating geriatric patients, clinicians should consider prescribing the lowest effective dose of psychotropic medications, such as ropinirole, to prevent adverse effects. Higher doses of dopamine agonists, especially in geriatric patients, increase the risk of common adverse effects, such as nausea (25% to 50%), headache (7% to 22%), fatigue (1% to 19%), dizziness (6% to 18%), and vomiting (5% to 11%).10 When prescribing dopamine agonists, clinicians should educate patients and their caregivers about the rare but potential risk of medication-induced mania and psychosis.
Mr. X’s case emphasizes the importance of a comprehensive psychiatric evaluation and medical workup to rule out a wide differential diagnosis when approaching new-onset mania and psychosis in geriatric patients.11 Our case contributes to the evidence that dopamine agonist medications are associated with mania and psychotic symptoms.
OUTCOME A return to baseline
On Day 12, Mr. X is discharged home in a stable condition. Two weeks later, at an outpatient follow-up visit, Mr. X is asymptomatic and has returned to his baseline functioning.
Bottom Line
When approaching new-onset mania and psychosis in geriatric patients, a comprehensive psychiatric evaluation and medical workup are necessary to rule out a wide differential diagnosis. Ropinirole use can lead to mania and psychotic symptoms, especially in geriatric patients. As should be done with all other dopaminergic agents, increase the dose of ropinirole with caution, and be vigilant for the emergence of signs of mania and/or psychosis.
Continue to: Related Resources
Related Resources
- Adabie A, Jackson JC, Torrence CL. Older-age bipolar disorder: A case series. Current Psychiatry. 2019;18(2):24-29.
- Chen P, Dols A, Rej S, et al. Update on the epidemiology, diagnosis, and treatment of mania in older-age bipolar disorder. Curr Psychiatry Rep. 2017;19(8):46.
Drug Brand Names
Divalproex sodium • Depakote
Gabapentin • Neurontin
Ropinirole • Requip
1. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
2. Singh A, Althoff R, Martineau RJ, et al. Pramipexole, ropinirole, and mania in Parkinson’s disease. Am J Psychiatry. 2005;162(4):814-815.
3. Weiss HD, Pontone GM. Dopamine receptor agonist drugs and impulse control disorders. JAMA Intern Med. 2014;174(12):1935-1937.
4 Shill HA, Stacy M. Update on ropinirole in the treatment of Parkinson’s disease. Neuropsychiatr Dis Treat. 2009;5:33-36.
5. Borovac JA. Side effects of a dopamine agonist therapy for Parkinson’s disease: a mini-review of clinical pharmacology. Yale J Biol Med. 2016;89(1):37-47.
6. Yüksel RN, Elyas Kaya Z, Dilbaz N, et al. Cabergoline-induced manic episode: case report. Ther Adv Psychopharmacol. 2016;6(3):229-231.
7. Perea E, Robbins BV, Hutto B. Psychosis related to ropinirole. Am J Psychiatry. 2006;163(3):547-548.
8. Stoner SC, Dahmen MM, Makos M, et al. An exploratory retrospective evaluation of ropinirole-associated psychotic symptoms in an outpatient population treated for restless legs syndrome or Parkinson’s disease. Ann Pharmacother. 2009;43(9):1426-1432.
9. Trenkwalder C, Hening WA, Montagna P, et al. Treatment of restless legs syndrome: an evidence-based review and implications for clinical practice. Mov Disord. 2008;23(16):2267-2302.
10. Garcia-Borreguero D, Kohnen R, Silber MH, et al. The long-term treatment of restless legs syndrome/Willis-Ekbom disease: evidence-based guidelines and clinical consensus best practice guidance: a report from the International Restless Legs Syndrome Study Group. Sleep Med. 2013;14(7):675-684.
11. Dols A, Beekman A. Older age bipolar disorder. Psychiatr Clin North Am. 2018;41(1):95-110.
1. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
2. Singh A, Althoff R, Martineau RJ, et al. Pramipexole, ropinirole, and mania in Parkinson’s disease. Am J Psychiatry. 2005;162(4):814-815.
3. Weiss HD, Pontone GM. Dopamine receptor agonist drugs and impulse control disorders. JAMA Intern Med. 2014;174(12):1935-1937.
4 Shill HA, Stacy M. Update on ropinirole in the treatment of Parkinson’s disease. Neuropsychiatr Dis Treat. 2009;5:33-36.
5. Borovac JA. Side effects of a dopamine agonist therapy for Parkinson’s disease: a mini-review of clinical pharmacology. Yale J Biol Med. 2016;89(1):37-47.
6. Yüksel RN, Elyas Kaya Z, Dilbaz N, et al. Cabergoline-induced manic episode: case report. Ther Adv Psychopharmacol. 2016;6(3):229-231.
7. Perea E, Robbins BV, Hutto B. Psychosis related to ropinirole. Am J Psychiatry. 2006;163(3):547-548.
8. Stoner SC, Dahmen MM, Makos M, et al. An exploratory retrospective evaluation of ropinirole-associated psychotic symptoms in an outpatient population treated for restless legs syndrome or Parkinson’s disease. Ann Pharmacother. 2009;43(9):1426-1432.
9. Trenkwalder C, Hening WA, Montagna P, et al. Treatment of restless legs syndrome: an evidence-based review and implications for clinical practice. Mov Disord. 2008;23(16):2267-2302.
10. Garcia-Borreguero D, Kohnen R, Silber MH, et al. The long-term treatment of restless legs syndrome/Willis-Ekbom disease: evidence-based guidelines and clinical consensus best practice guidance: a report from the International Restless Legs Syndrome Study Group. Sleep Med. 2013;14(7):675-684.
11. Dols A, Beekman A. Older age bipolar disorder. Psychiatr Clin North Am. 2018;41(1):95-110.
Sick, or faking it?
CASE Vague symptoms; no clear etiology
Mr. W, age 53, presents to the emergency department (ED) describing acute mid-sternal chest pain (severity: 8 out of 10). His medical history is significant for pulmonary embolism and ascending aortic aneurysm in the context of Takayasu’s arteritis, an inflammatory condition of the large arterial blood vessels characterized by lesions that can lead to vascular stenosis, occlusion, or aneurysm. Takayasu’s arteritis is also known as pulseless disease due to the weak or absent pulses the condition produces.
A review of Mr. W’s medical records reveals that this is his 23rd visit to this hospital within a year; the year before that, he had 22 visits. At each of these previous visits, he had similar vague symptoms, including dizziness, chest pain, lightheadedness, fainting, bilateral knee weakness, and left-arm numbness/weakness, and no clear acute etiology for his reported symptoms. Each time, after the treating clinicians ruled out possible acute complications of a flare-up of Takayasu’s arteritis through a physical examination, laboratory tests, and imaging studies, Mr. W was discharged with recommendations that he follow-up with his primary care physician and specialists. At each discharge, he would leave the hospital with hesitation.
At this present visit, the ED physician recognizes Mr. W as someone who visits the ED often with no profound acute issues, and reviews the substantial medical records available to the hospital. He suspects Mr. W is feigning symptoms, and orders a psychiatric consultation.
EVALUATION Psychiatric interview and mental status exam
On examination, Mr. W is not in acute distress. Despite reporting an 8 out of 10 for chest pain severity, he displays no psychomotor agitation, and his pulse rate and blood pressure are within normal limits. He makes appropriate eye contact and describes his mood as “great.” He reports no problems with sleep, appetite, or disinterest in pleasurable activities, and denies being depressed or having any symptoms consistent with a mood disorder, anxiety disorder, or psychosis. He denies a history of panic attacks or excessive worrying that interferes with his sleep or activities of daily living. Additionally, Mr. W describes a stable, peaceful, and stress-free life within the limitations of his Takayasu’s arteritis, which he has been managing well since his diagnosis 6 years earlier.
Mr. W denies having any psychiatric symptoms, apprehensive feelings, or beliefs/fears that would be considered delusional, and he has no previous legal issues aside from an occasional driving citation. During the assessment, his affect remains broad and he denies having thoughts of suicide or homicide, or auditory or visual hallucinations.
Mr. W’s drug screen results are negative, and he denies using any illicit drugs. He uses only the medications that are prescribed by his clinicians. Overall, he seems to be a well-functioning individual. Mr. W reports that work is generally not stressful.
When the psychiatric team asks him about his frequent hospitalizations and ED visits, Mr. W is insistent that he is “just doing what my doctors said for me to do.” He repeats that he does not have any mental illness and did not see the point of seeing a psychiatrist.
In pursuit of collateral information, the psychiatry team accesses a regional medical record database that allows registered medical institutions and practices to track patients’ medical encounters within the region. According to this database, within approximately 5.5 years, Mr. W had 163 clinical encounters (ED visits and inpatient admissions) and 376 radiological studies in our region (Table 1 and Table 2).
[polldaddy:10394110]
Continue to: The authors' observations
The authors’ observations
The psychiatry team’s investigation of Mr. W’s medical records revealed the extent of his care-seeking behavior, and provided evidence for a diagnosis of factitious disorder.
Factitious disorder is an elusive psychiatric condition in which an individual chronically stimulates, induces, or aggravates illnesses to gain the status of being a patient. Although its exact cause has not been fully deciphered, it is seen mostly among individuals with knowledge of the workings of the medical field, such as a health care worker.1 Factitious disorder is taxing on the health care system, with an estimated cost in the thousands of dollars per patient visit.2 The condition has an estimated prevalence of 0.8% to 1.0% of patients seen by psychiatric consult services3 and is reported to be more prevalent among women than men.1 Its cardinal features include health care site hopping and hospital shopping, vagueness about the patient’s history and symptoms, and discrepancy among reported symptoms, the patient’s behaviors, and objective clinical findings.4,5 Although not all patients with factitious disorder have a legitimate medical reason for seeking care, some individuals with an established medical diagnosis use their condition as a tool to chronically seek care and play the sick role.
Factitious disorder should not be confused with malingering, which is differentiated by the patient’s search for a secondary gain, such as financial reward or avoiding jail; or conversion disorder, which is marked by true physical or neurologic symptoms and clinical findings triggered by psychological stressors. Patients with factitious disorder usually are cooperative during hospital stays and resume their normal daily routine shortly after discharge.4 In this case, Mr. W denied any psychiatric symptoms, apprehensive feelings, or beliefs or fears that would be considered delusional. He had no previous or pending legal issues, which ruled out malingering to avoid legal repercussions.
Mr. W’s presentation was complicated by his Takayasu’s arteritis diagnosis. Because Takayasu’s arteritis has a serious list of potential complications, ED physicians have a low threshold for ordering diagnostic studies for a patient with Takayasu’s arteritis who presents with a chief complaint of chest pain. In other words, when a patient with this condition presents to an acute setting (such as the ED) with chest pain, his/her chief complaint is taken with extreme seriousness. Conventional angiography is the standard diagnostic tool for Takayasu’s arteritis; CT angiography and magnetic resonance angiography are used for monitoring the disease’s progression.6
[polldaddy:10394113]
The authors’ observations
Currently, there are no FDA-approved treatments for factitious disorder, and patients with this condition generally are resistant to psychiatric and/or psychological care when discovered and offered treatment.7 Among those who consent to psychiatric care, psychoeducation, or psychotherapy, which have shown some efficacy for the condition, the dropout rate is high.8
Continue to: Although the instinctive approach...
Although the instinctive approach is to confront the patient once the deception has been uncovered, expert recommendations are contradictory. Some recommend confrontation as part of a treatment protocol,8 while others advise against such an approach.9
Because of how often patients with factitious disorder seek medical care, secondary iatrogenic consequences are possible. For example, for years, Mr. W has been unknowingly exposing himself to the iatrogenic consequences of the cumulative effect of diagnostic imaging for years. In 3 years alone, Mr. W had undergone an average of 125 diagnostic imaging studies per year—with and without contrast—and many unnecessary rounds of treatment with steroids and other interventions known to have secondary iatrogenic consequences.10 Excessive radiation exposure is known to be carcinogenic over time,10 and excessive use of steroids is associated with weight gain, physical habitus changes, and increased risk of infections.11 In addition, the renal effects of the contrast materials from repeated imaging studies over so many years on Mr. W’s future kidney function are unknown.
TREATMENT Psychoeducation and referral for psychotherapy
We counsel Mr. W about factitious disorder and the risks of excessive hospitalizations, and refer him for follow-up at our local psychiatric clinic, as well as for individual psychotherapy. Mr. W is discharged because his medical work-up does not reveal any significant acute medical issues.
The authors’ observations
Because of the poor insight associated with factitious disorder and the limited treatment options available, a patient with factitious disorder is unlikely to enter psychiatric treatment on his/her own. The prognosis for a patient with factitious disorder remains poor unless the patient is forced into treatment. More intervention-focused research is needed to help improve outcomes for patients with factitious disorder.
OUTCOME Failure to follow up
Mr. W fails to attend individual psychotherapy as recommended. According to our regional record database, Mr. W continues to present to other EDs regularly.
Continue to: Bottom Line
Bottom Line
A patient with factitious disorder stimulates, induces, or aggravates illnesses to gain the status of being a patient. Treatment options include psychiatric care, psychoeducation, or psychotherapy. However, due to poor insight, a patient with factitious disorder is unlikely to enter psychiatric treatment on his/her own.
Related Resources
- Yates GP, Feldman MD. Factitious disorder: a systematic review of 455 cases in the professional literature. Gen Hosp Psychiatry. 2016;41:20-28.
- Galli S, Tatu L, Bogousslavsky J, et al. Conversion, factitious disorder and malingering: a distinct pattern or a continuum? Front Neurol Neurosci. 2018;42:72-80.
1. Krahn LE, Li H, O’Connor MK. Patients who strive to be ill: factitious disorder with physical symptoms. Am J Psychiatry. 2003;160(6):1163-1168.
2. Hoertel N, Lavaud P, Le Strat Y, et al. Estimated cost of a factitious disorder with 6-year follow-up. Psychiatry Res. 2012;200(2):1077-1078.
3. Sadock BJ, Sadock VA, Ruiz P. Psychosomatic medicine; factitious disorder. In: Pataki CS, Sussman N, eds. Synopsis of psychiatry: Behavioral sciences/clinical psychiatry. 11th ed. Philadelphia, PA: Wolters Kluwer; 2015:34-45.
4 . Savino AC, Fordtran JS. Factitious disease: clinical lessons from case studies at Baylor University Medical Center. Proc (Bayl Univ Med Cent). 2006;19(3):195-208.
5. Burnel A. Recognition and management of factitious disorder. Prescriber. 2015;26(21):37-39.
6. Duftner C, Dejaco C, Sepriano A, et al. Imaging in diagnosis, outcome prediction and monitoring of large vessel vasculitis: a systematic literature review and meta-analysis informing the EULAR recommendations. RMD Open. 2018;4(1):e000612. doi: 10.1136/rmdopen-2017-000612.
7. Jafferany M, Khalid Z, McDonald KA, et al. Psychological aspects of factitious disorder. Prim Care Companion CNS Disord. 2018;20(1). doi: 10.4088/PCC.17nr02229.
8. Bolat N, Yalçin O. Factitious disorder presenting with stuttering in two adolescents: the importance of psychoeducation. Noro Psikiyatri Arsivi. 2017;54(1):87-89.
9. Eisendrath SJ. Factitious physical disorders. West J Med. 1994;160(2):177-179.
10. Sodickson A, Baeyens PF, Andriole KP, et al. Recurrent CT, cumulative radiation exposure, and associated radiation-induced cancer risks from CT of adults. Radiology. 2009;251(1):175-184.
11. Oray M, Abu Samra K, Ebrahimiadib N, et al. Long-term side effects of glucocorticoids. Expert Opin Drug Saf. 2016;15(4):457-465.
CASE Vague symptoms; no clear etiology
Mr. W, age 53, presents to the emergency department (ED) describing acute mid-sternal chest pain (severity: 8 out of 10). His medical history is significant for pulmonary embolism and ascending aortic aneurysm in the context of Takayasu’s arteritis, an inflammatory condition of the large arterial blood vessels characterized by lesions that can lead to vascular stenosis, occlusion, or aneurysm. Takayasu’s arteritis is also known as pulseless disease due to the weak or absent pulses the condition produces.
A review of Mr. W’s medical records reveals that this is his 23rd visit to this hospital within a year; the year before that, he had 22 visits. At each of these previous visits, he had similar vague symptoms, including dizziness, chest pain, lightheadedness, fainting, bilateral knee weakness, and left-arm numbness/weakness, and no clear acute etiology for his reported symptoms. Each time, after the treating clinicians ruled out possible acute complications of a flare-up of Takayasu’s arteritis through a physical examination, laboratory tests, and imaging studies, Mr. W was discharged with recommendations that he follow-up with his primary care physician and specialists. At each discharge, he would leave the hospital with hesitation.
At this present visit, the ED physician recognizes Mr. W as someone who visits the ED often with no profound acute issues, and reviews the substantial medical records available to the hospital. He suspects Mr. W is feigning symptoms, and orders a psychiatric consultation.
EVALUATION Psychiatric interview and mental status exam
On examination, Mr. W is not in acute distress. Despite reporting an 8 out of 10 for chest pain severity, he displays no psychomotor agitation, and his pulse rate and blood pressure are within normal limits. He makes appropriate eye contact and describes his mood as “great.” He reports no problems with sleep, appetite, or disinterest in pleasurable activities, and denies being depressed or having any symptoms consistent with a mood disorder, anxiety disorder, or psychosis. He denies a history of panic attacks or excessive worrying that interferes with his sleep or activities of daily living. Additionally, Mr. W describes a stable, peaceful, and stress-free life within the limitations of his Takayasu’s arteritis, which he has been managing well since his diagnosis 6 years earlier.
Mr. W denies having any psychiatric symptoms, apprehensive feelings, or beliefs/fears that would be considered delusional, and he has no previous legal issues aside from an occasional driving citation. During the assessment, his affect remains broad and he denies having thoughts of suicide or homicide, or auditory or visual hallucinations.
Mr. W’s drug screen results are negative, and he denies using any illicit drugs. He uses only the medications that are prescribed by his clinicians. Overall, he seems to be a well-functioning individual. Mr. W reports that work is generally not stressful.
When the psychiatric team asks him about his frequent hospitalizations and ED visits, Mr. W is insistent that he is “just doing what my doctors said for me to do.” He repeats that he does not have any mental illness and did not see the point of seeing a psychiatrist.
In pursuit of collateral information, the psychiatry team accesses a regional medical record database that allows registered medical institutions and practices to track patients’ medical encounters within the region. According to this database, within approximately 5.5 years, Mr. W had 163 clinical encounters (ED visits and inpatient admissions) and 376 radiological studies in our region (Table 1 and Table 2).

[polldaddy:10394110]
Continue to: The authors' observations
The authors’ observations
The psychiatry team’s investigation of Mr. W’s medical records revealed the extent of his care-seeking behavior, and provided evidence for a diagnosis of factitious disorder.
Factitious disorder is an elusive psychiatric condition in which an individual chronically stimulates, induces, or aggravates illnesses to gain the status of being a patient. Although its exact cause has not been fully deciphered, it is seen mostly among individuals with knowledge of the workings of the medical field, such as a health care worker.1 Factitious disorder is taxing on the health care system, with an estimated cost in the thousands of dollars per patient visit.2 The condition has an estimated prevalence of 0.8% to 1.0% of patients seen by psychiatric consult services3 and is reported to be more prevalent among women than men.1 Its cardinal features include health care site hopping and hospital shopping, vagueness about the patient’s history and symptoms, and discrepancy among reported symptoms, the patient’s behaviors, and objective clinical findings.4,5 Although not all patients with factitious disorder have a legitimate medical reason for seeking care, some individuals with an established medical diagnosis use their condition as a tool to chronically seek care and play the sick role.
Factitious disorder should not be confused with malingering, which is differentiated by the patient’s search for a secondary gain, such as financial reward or avoiding jail; or conversion disorder, which is marked by true physical or neurologic symptoms and clinical findings triggered by psychological stressors. Patients with factitious disorder usually are cooperative during hospital stays and resume their normal daily routine shortly after discharge.4 In this case, Mr. W denied any psychiatric symptoms, apprehensive feelings, or beliefs or fears that would be considered delusional. He had no previous or pending legal issues, which ruled out malingering to avoid legal repercussions.
Mr. W’s presentation was complicated by his Takayasu’s arteritis diagnosis. Because Takayasu’s arteritis has a serious list of potential complications, ED physicians have a low threshold for ordering diagnostic studies for a patient with Takayasu’s arteritis who presents with a chief complaint of chest pain. In other words, when a patient with this condition presents to an acute setting (such as the ED) with chest pain, his/her chief complaint is taken with extreme seriousness. Conventional angiography is the standard diagnostic tool for Takayasu’s arteritis; CT angiography and magnetic resonance angiography are used for monitoring the disease’s progression.6
[polldaddy:10394113]
The authors’ observations
Currently, there are no FDA-approved treatments for factitious disorder, and patients with this condition generally are resistant to psychiatric and/or psychological care when discovered and offered treatment.7 Among those who consent to psychiatric care, psychoeducation, or psychotherapy, which have shown some efficacy for the condition, the dropout rate is high.8
Continue to: Although the instinctive approach...
Although the instinctive approach is to confront the patient once the deception has been uncovered, expert recommendations are contradictory. Some recommend confrontation as part of a treatment protocol,8 while others advise against such an approach.9
Because of how often patients with factitious disorder seek medical care, secondary iatrogenic consequences are possible. For example, for years, Mr. W has been unknowingly exposing himself to the iatrogenic consequences of the cumulative effect of diagnostic imaging for years. In 3 years alone, Mr. W had undergone an average of 125 diagnostic imaging studies per year—with and without contrast—and many unnecessary rounds of treatment with steroids and other interventions known to have secondary iatrogenic consequences.10 Excessive radiation exposure is known to be carcinogenic over time,10 and excessive use of steroids is associated with weight gain, physical habitus changes, and increased risk of infections.11 In addition, the renal effects of the contrast materials from repeated imaging studies over so many years on Mr. W’s future kidney function are unknown.
TREATMENT Psychoeducation and referral for psychotherapy
We counsel Mr. W about factitious disorder and the risks of excessive hospitalizations, and refer him for follow-up at our local psychiatric clinic, as well as for individual psychotherapy. Mr. W is discharged because his medical work-up does not reveal any significant acute medical issues.
The authors’ observations
Because of the poor insight associated with factitious disorder and the limited treatment options available, a patient with factitious disorder is unlikely to enter psychiatric treatment on his/her own. The prognosis for a patient with factitious disorder remains poor unless the patient is forced into treatment. More intervention-focused research is needed to help improve outcomes for patients with factitious disorder.
OUTCOME Failure to follow up
Mr. W fails to attend individual psychotherapy as recommended. According to our regional record database, Mr. W continues to present to other EDs regularly.
Continue to: Bottom Line
Bottom Line
A patient with factitious disorder stimulates, induces, or aggravates illnesses to gain the status of being a patient. Treatment options include psychiatric care, psychoeducation, or psychotherapy. However, due to poor insight, a patient with factitious disorder is unlikely to enter psychiatric treatment on his/her own.
Related Resources
- Yates GP, Feldman MD. Factitious disorder: a systematic review of 455 cases in the professional literature. Gen Hosp Psychiatry. 2016;41:20-28.
- Galli S, Tatu L, Bogousslavsky J, et al. Conversion, factitious disorder and malingering: a distinct pattern or a continuum? Front Neurol Neurosci. 2018;42:72-80.
CASE Vague symptoms; no clear etiology
Mr. W, age 53, presents to the emergency department (ED) describing acute mid-sternal chest pain (severity: 8 out of 10). His medical history is significant for pulmonary embolism and ascending aortic aneurysm in the context of Takayasu’s arteritis, an inflammatory condition of the large arterial blood vessels characterized by lesions that can lead to vascular stenosis, occlusion, or aneurysm. Takayasu’s arteritis is also known as pulseless disease due to the weak or absent pulses the condition produces.
A review of Mr. W’s medical records reveals that this is his 23rd visit to this hospital within a year; the year before that, he had 22 visits. At each of these previous visits, he had similar vague symptoms, including dizziness, chest pain, lightheadedness, fainting, bilateral knee weakness, and left-arm numbness/weakness, and no clear acute etiology for his reported symptoms. Each time, after the treating clinicians ruled out possible acute complications of a flare-up of Takayasu’s arteritis through a physical examination, laboratory tests, and imaging studies, Mr. W was discharged with recommendations that he follow-up with his primary care physician and specialists. At each discharge, he would leave the hospital with hesitation.
At this present visit, the ED physician recognizes Mr. W as someone who visits the ED often with no profound acute issues, and reviews the substantial medical records available to the hospital. He suspects Mr. W is feigning symptoms, and orders a psychiatric consultation.
EVALUATION Psychiatric interview and mental status exam
On examination, Mr. W is not in acute distress. Despite reporting an 8 out of 10 for chest pain severity, he displays no psychomotor agitation, and his pulse rate and blood pressure are within normal limits. He makes appropriate eye contact and describes his mood as “great.” He reports no problems with sleep, appetite, or disinterest in pleasurable activities, and denies being depressed or having any symptoms consistent with a mood disorder, anxiety disorder, or psychosis. He denies a history of panic attacks or excessive worrying that interferes with his sleep or activities of daily living. Additionally, Mr. W describes a stable, peaceful, and stress-free life within the limitations of his Takayasu’s arteritis, which he has been managing well since his diagnosis 6 years earlier.
Mr. W denies having any psychiatric symptoms, apprehensive feelings, or beliefs/fears that would be considered delusional, and he has no previous legal issues aside from an occasional driving citation. During the assessment, his affect remains broad and he denies having thoughts of suicide or homicide, or auditory or visual hallucinations.
Mr. W’s drug screen results are negative, and he denies using any illicit drugs. He uses only the medications that are prescribed by his clinicians. Overall, he seems to be a well-functioning individual. Mr. W reports that work is generally not stressful.
When the psychiatric team asks him about his frequent hospitalizations and ED visits, Mr. W is insistent that he is “just doing what my doctors said for me to do.” He repeats that he does not have any mental illness and did not see the point of seeing a psychiatrist.
In pursuit of collateral information, the psychiatry team accesses a regional medical record database that allows registered medical institutions and practices to track patients’ medical encounters within the region. According to this database, within approximately 5.5 years, Mr. W had 163 clinical encounters (ED visits and inpatient admissions) and 376 radiological studies in our region (Table 1 and Table 2).

[polldaddy:10394110]
Continue to: The authors' observations
The authors’ observations
The psychiatry team’s investigation of Mr. W’s medical records revealed the extent of his care-seeking behavior, and provided evidence for a diagnosis of factitious disorder.
Factitious disorder is an elusive psychiatric condition in which an individual chronically stimulates, induces, or aggravates illnesses to gain the status of being a patient. Although its exact cause has not been fully deciphered, it is seen mostly among individuals with knowledge of the workings of the medical field, such as a health care worker.1 Factitious disorder is taxing on the health care system, with an estimated cost in the thousands of dollars per patient visit.2 The condition has an estimated prevalence of 0.8% to 1.0% of patients seen by psychiatric consult services3 and is reported to be more prevalent among women than men.1 Its cardinal features include health care site hopping and hospital shopping, vagueness about the patient’s history and symptoms, and discrepancy among reported symptoms, the patient’s behaviors, and objective clinical findings.4,5 Although not all patients with factitious disorder have a legitimate medical reason for seeking care, some individuals with an established medical diagnosis use their condition as a tool to chronically seek care and play the sick role.
Factitious disorder should not be confused with malingering, which is differentiated by the patient’s search for a secondary gain, such as financial reward or avoiding jail; or conversion disorder, which is marked by true physical or neurologic symptoms and clinical findings triggered by psychological stressors. Patients with factitious disorder usually are cooperative during hospital stays and resume their normal daily routine shortly after discharge.4 In this case, Mr. W denied any psychiatric symptoms, apprehensive feelings, or beliefs or fears that would be considered delusional. He had no previous or pending legal issues, which ruled out malingering to avoid legal repercussions.
Mr. W’s presentation was complicated by his Takayasu’s arteritis diagnosis. Because Takayasu’s arteritis has a serious list of potential complications, ED physicians have a low threshold for ordering diagnostic studies for a patient with Takayasu’s arteritis who presents with a chief complaint of chest pain. In other words, when a patient with this condition presents to an acute setting (such as the ED) with chest pain, his/her chief complaint is taken with extreme seriousness. Conventional angiography is the standard diagnostic tool for Takayasu’s arteritis; CT angiography and magnetic resonance angiography are used for monitoring the disease’s progression.6
[polldaddy:10394113]
The authors’ observations
Currently, there are no FDA-approved treatments for factitious disorder, and patients with this condition generally are resistant to psychiatric and/or psychological care when discovered and offered treatment.7 Among those who consent to psychiatric care, psychoeducation, or psychotherapy, which have shown some efficacy for the condition, the dropout rate is high.8
Continue to: Although the instinctive approach...
Although the instinctive approach is to confront the patient once the deception has been uncovered, expert recommendations are contradictory. Some recommend confrontation as part of a treatment protocol,8 while others advise against such an approach.9
Because of how often patients with factitious disorder seek medical care, secondary iatrogenic consequences are possible. For example, for years, Mr. W has been unknowingly exposing himself to the iatrogenic consequences of the cumulative effect of diagnostic imaging for years. In 3 years alone, Mr. W had undergone an average of 125 diagnostic imaging studies per year—with and without contrast—and many unnecessary rounds of treatment with steroids and other interventions known to have secondary iatrogenic consequences.10 Excessive radiation exposure is known to be carcinogenic over time,10 and excessive use of steroids is associated with weight gain, physical habitus changes, and increased risk of infections.11 In addition, the renal effects of the contrast materials from repeated imaging studies over so many years on Mr. W’s future kidney function are unknown.
TREATMENT Psychoeducation and referral for psychotherapy
We counsel Mr. W about factitious disorder and the risks of excessive hospitalizations, and refer him for follow-up at our local psychiatric clinic, as well as for individual psychotherapy. Mr. W is discharged because his medical work-up does not reveal any significant acute medical issues.
The authors’ observations
Because of the poor insight associated with factitious disorder and the limited treatment options available, a patient with factitious disorder is unlikely to enter psychiatric treatment on his/her own. The prognosis for a patient with factitious disorder remains poor unless the patient is forced into treatment. More intervention-focused research is needed to help improve outcomes for patients with factitious disorder.
OUTCOME Failure to follow up
Mr. W fails to attend individual psychotherapy as recommended. According to our regional record database, Mr. W continues to present to other EDs regularly.
Continue to: Bottom Line
Bottom Line
A patient with factitious disorder stimulates, induces, or aggravates illnesses to gain the status of being a patient. Treatment options include psychiatric care, psychoeducation, or psychotherapy. However, due to poor insight, a patient with factitious disorder is unlikely to enter psychiatric treatment on his/her own.
Related Resources
- Yates GP, Feldman MD. Factitious disorder: a systematic review of 455 cases in the professional literature. Gen Hosp Psychiatry. 2016;41:20-28.
- Galli S, Tatu L, Bogousslavsky J, et al. Conversion, factitious disorder and malingering: a distinct pattern or a continuum? Front Neurol Neurosci. 2018;42:72-80.
1. Krahn LE, Li H, O’Connor MK. Patients who strive to be ill: factitious disorder with physical symptoms. Am J Psychiatry. 2003;160(6):1163-1168.
2. Hoertel N, Lavaud P, Le Strat Y, et al. Estimated cost of a factitious disorder with 6-year follow-up. Psychiatry Res. 2012;200(2):1077-1078.
3. Sadock BJ, Sadock VA, Ruiz P. Psychosomatic medicine; factitious disorder. In: Pataki CS, Sussman N, eds. Synopsis of psychiatry: Behavioral sciences/clinical psychiatry. 11th ed. Philadelphia, PA: Wolters Kluwer; 2015:34-45.
4 . Savino AC, Fordtran JS. Factitious disease: clinical lessons from case studies at Baylor University Medical Center. Proc (Bayl Univ Med Cent). 2006;19(3):195-208.
5. Burnel A. Recognition and management of factitious disorder. Prescriber. 2015;26(21):37-39.
6. Duftner C, Dejaco C, Sepriano A, et al. Imaging in diagnosis, outcome prediction and monitoring of large vessel vasculitis: a systematic literature review and meta-analysis informing the EULAR recommendations. RMD Open. 2018;4(1):e000612. doi: 10.1136/rmdopen-2017-000612.
7. Jafferany M, Khalid Z, McDonald KA, et al. Psychological aspects of factitious disorder. Prim Care Companion CNS Disord. 2018;20(1). doi: 10.4088/PCC.17nr02229.
8. Bolat N, Yalçin O. Factitious disorder presenting with stuttering in two adolescents: the importance of psychoeducation. Noro Psikiyatri Arsivi. 2017;54(1):87-89.
9. Eisendrath SJ. Factitious physical disorders. West J Med. 1994;160(2):177-179.
10. Sodickson A, Baeyens PF, Andriole KP, et al. Recurrent CT, cumulative radiation exposure, and associated radiation-induced cancer risks from CT of adults. Radiology. 2009;251(1):175-184.
11. Oray M, Abu Samra K, Ebrahimiadib N, et al. Long-term side effects of glucocorticoids. Expert Opin Drug Saf. 2016;15(4):457-465.
1. Krahn LE, Li H, O’Connor MK. Patients who strive to be ill: factitious disorder with physical symptoms. Am J Psychiatry. 2003;160(6):1163-1168.
2. Hoertel N, Lavaud P, Le Strat Y, et al. Estimated cost of a factitious disorder with 6-year follow-up. Psychiatry Res. 2012;200(2):1077-1078.
3. Sadock BJ, Sadock VA, Ruiz P. Psychosomatic medicine; factitious disorder. In: Pataki CS, Sussman N, eds. Synopsis of psychiatry: Behavioral sciences/clinical psychiatry. 11th ed. Philadelphia, PA: Wolters Kluwer; 2015:34-45.
4 . Savino AC, Fordtran JS. Factitious disease: clinical lessons from case studies at Baylor University Medical Center. Proc (Bayl Univ Med Cent). 2006;19(3):195-208.
5. Burnel A. Recognition and management of factitious disorder. Prescriber. 2015;26(21):37-39.
6. Duftner C, Dejaco C, Sepriano A, et al. Imaging in diagnosis, outcome prediction and monitoring of large vessel vasculitis: a systematic literature review and meta-analysis informing the EULAR recommendations. RMD Open. 2018;4(1):e000612. doi: 10.1136/rmdopen-2017-000612.
7. Jafferany M, Khalid Z, McDonald KA, et al. Psychological aspects of factitious disorder. Prim Care Companion CNS Disord. 2018;20(1). doi: 10.4088/PCC.17nr02229.
8. Bolat N, Yalçin O. Factitious disorder presenting with stuttering in two adolescents: the importance of psychoeducation. Noro Psikiyatri Arsivi. 2017;54(1):87-89.
9. Eisendrath SJ. Factitious physical disorders. West J Med. 1994;160(2):177-179.
10. Sodickson A, Baeyens PF, Andriole KP, et al. Recurrent CT, cumulative radiation exposure, and associated radiation-induced cancer risks from CT of adults. Radiology. 2009;251(1):175-184.
11. Oray M, Abu Samra K, Ebrahimiadib N, et al. Long-term side effects of glucocorticoids. Expert Opin Drug Saf. 2016;15(4):457-465.