User login
An unexplained exacerbation of depression, anxiety, and panic
CASE Depression, anxiety, and panic attacks
At the urging of his parents Mr. P, age 33, presents to the partial hospitalization program (PHP) for worsening depression and anxiety, daily panic attacks with accompanying diaphoresis and headache, and the possibility that he may have taken an overdose of zolpidem. Mr. P denies taking an intentional overdose of zolpidem, claiming instead that he was having a sleep-walking episode and did not realize how many pills he took.
In addition to daily panic attacks, Mr. P reports having trouble falling asleep, overwhelming sadness, and daily passive suicidal ideation without a plan or active intent.
Mr. P cannot identify a specific trigger to this most recent exacerbation of depressed/anxious mood, but instead describes it as slowly building over the past 6 to 8 months. Mr. P says the panic attacks occur without warning and states, “I feel like my heart is going to jump out of my chest; I get a terrible headache, and I sweat like crazy. Sometimes I just feel like I’m about to pass out or die.” Although these episodes had been present for approximately 2 years, they now occur almost daily.
HISTORY Inconsistent adherence
For the last year, Mr. P had been taking alprazolam, 0.5 mg twice daily, and paroxetine, 20 mg/d, and these medications provided moderate relief of his depressive/anxious symptoms. However, he stopped taking both medications approximately 3 or 4 weeks ago when he ran out. He also takes propranolol, 20 mg/d, sporadically, for hypertension. In the past, he had been prescribed carvedilol, clonidine, and lisinopril—all with varying degrees of relief of his hypertension. He denies a family history of hypertension or any other chronic or acute health problems. He reports that he has been sober from alcohol for 19 months but smokes 1 to 2 marijuana cigarettes a day.
EVALUATION Elevated blood pressure and pulse
Mr. P’s physical examination and medical review of systems are unremarkable, except for an elevated blood pressure (190/110 mm Hg) and pulse (92 beats per minute); he also has a headache. A repeat blood pressure test later in the day is 172/94 mm Hg, with a pulse of 100 beats per minute. His urine drug screen is positive only for delta-9-tetrahydrocannabinol (THC).
[polldaddy:10558304]
The author’s observations
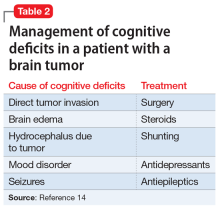
A CBC with differential is helpful for ruling out infection and anemia as causes of anxiety and depression.1 In Mr. P’s case, there were no concerning symptoms that pointed to anemia or infection as likely causes of his anxiety, depression, or panic attacks. A TSH level also would be reasonable, because hyperthyroidism can present as anxiety, while hyperthyroidism or hypothyroidism each can present as depression.1 However, both Mr. P’s medical history and physical examination were not concerning for thyroid disease, making it unlikely that he had either of those conditions. A review of Mr. P’s medical records indicated that within the past 6 months, his primary care physician (PCP) had ordered a CBC and TSH test; the results of both were within normal limits.
Serum porphyrin tests can exclude porphyria as a contributor to Mr. P’s anxiety and depression. Porphyrias are a group of 8 inherited disorders that involve accumulation of heme precursors (porphyrins) in the CNS and subcutaneous tissue.2 Collectively, porphyrias affect approximately 1 in 200,000 people.2 Anxiety and depression are strongly associated with porphyria, but do not occur secondary to the illness; depression and anxiety appear to be intrinsic personality features in people with porphyria.3 Skin lesions and abdominal pain are the most common symptoms,3 and there is a higher incidence of hypertension in people with porphyria than in the general population.4 Mr. P does not report any heritable disorders, nor does he appear to have any CNS disturbance or unusual cutaneous lesions, which makes it unlikely that this disorder is related to his psychiatric symptoms.
Continue to: A serum metanephrines test measures...
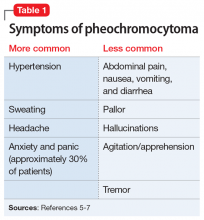
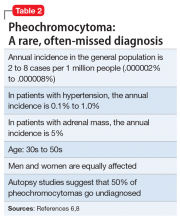
A serum metanephrines test measures the metabolites of epinephrine and norepinephrine. These catecholamines are produced in excess by an adrenal or extra-adrenal tumor seen in pheochromocytoma. The classic triad of symptoms of pheochromocytoma are hypertension, sweating, and headache; approximately 30% of patients report significant anxiety and panic (Table 15-7). This type of tumor is rare, with an annual incidence of only 2 to 8 cases per 1 million individuals. Among people with hypertension, the annual incidence is 0.1% to 1.0%, and for those with an adrenal mass, the annual incidence is 5% (Table 26,8). Autopsy studies suggest that up to 50% of pheochromocytomas are undiagnosed.8 Left untreated, pheochromocytoma can result in hypertensive crisis, arrhythmia, myocardial infarction, multisystem organ failure, and premature death.7 Table 36,7 highlights some causes of false-positive serum on metanephrines testing.
EVALUATION Metanephrines testing
Mr. P has what appears to be treatment-resistant hypertension, accompanied by the classic symptoms observed in most patients with pheochromocytoma. Because Mr. P is participating in the PHP 6 days per week for 6 hours each day, visiting his PCP would be inconvenient, so the treatment team orders the serum metanephrines test. If a positive result is found, Mr. P will be referred to his PCP for further assessment and follow-up care with endocrinology.
TREATMENT Pharmacotherapy to target anxiety and panic
Next, the treatment team establishes a safety plan for Mr. P, and restarts paroxetine, 20 mg/d, to target his depressed and anxious mood. Alprazolam, 0.5 mg twice daily, is started to target anxious mood and panic symptoms, and to allow time for the anxiolytic properties of the paroxetine to become fully effective. The alprazolam will be tapered and stopped after 2 weeks. Mr. P is started on hydroxyzine, 1 to 2 25-mg tablets 2 to 3 times daily as needed for anxious mood and panic symptoms.
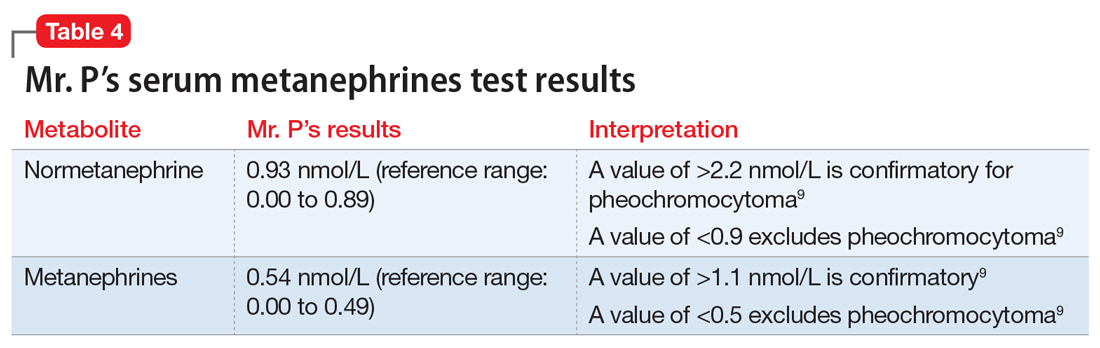
The serum metanephrines test results are equivocal, with a slight elevation of both epinephrine and norepinephrine that is too low to confirm a diagnosis of pheochromocytoma but too elevated to exclude it (Table 49). With Mr. P’s consent, the treatment team contacts his PCP and convey the results of this test. Mr. P schedules an appointment with his PCP for the following week for further assessment and confirmatory pheochromocytoma testing.
After 1 week, Mr. P remains anxious, with a slight reduction in panic attacks from multiple attacks each day to 3 or 4 attacks per week. The team considers adding an additional anxiolytic agent.
[polldaddy:10558305]
Continue to: The author's observations
The author’s observations
The triad of symptoms in pheochromocytoma results directly from the intermittent release of catecholamines into systemic circulation. Surges of epinephrine and norepinephrine lead to headaches, palpitations, diaphoresis, and (less commonly) gastrointestinal symptoms such as nausea, vomiting, and constipation. Persistent or episodic hypertension may be present, with 13% of patients maintaining a normal blood pressure.5-7 Patients with pheochromocytoma-related anxiety typically have substantial or complete resolution of anxiety and panic attacks after tumor resection.6,8,10
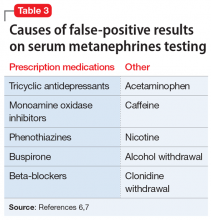
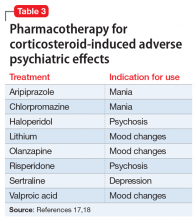
Because of their ability to raise catecholamine levels, several medications, including some psychotropics, can lead to false-positive results on serum and urine metanephrines testing. Tricyclic antidepressants and beta-blockers can cause false-positive results on plasma assays, while buspirone can cause false-positives on urinalysis assays.5 Trazodone, on the other hand, exhibits no catecholaminergic activity and its alpha-1 adrenergic antagonism may actually have some benefit in pheochromocytoma.11 Alpha-1 adrenergic antagonism with doxazosin, prazosin, or terazosin is the first-line of treatment in reducing pheochromocytoma-related hypertension.7 Treatment with a beta-blocker is safe only after alpha-adrenergic blockade occurs. While beta-blockers are useful for reducing the palpitations and anxiety observed in patients with pheochromocytoma, they must not be used alone due to the risk of hypertensive crisis resulting from unopposed alpha-adrenergic agonist activated vasoconstriction.5,7
TREATMENT CBT provides benefit
Mr. P decides against receiving an additional agent for anxiety and instead decides to wait for the outcome of the confirmatory pheochromocytoma testing. He continues to take alprazolam, and both his depressed mood and anxiety improve. His panic attacks continue to lessen, and he appears to benefit from cognitive-behavioral therapy provided during group therapy. Mr. P is advised by his PCP to taper and stop the alprazolam 3 to 5 days before his 24-hour urine metanephrines test because benzodiazepines can lead to false-positive results on a urinalysis assay.7
OUTCOME Remission of anxiety and depression
Mr. P has a repeat serum metanephrines test and a 24-hour urinalysis assay. Both are negative for pheochromocytoma. His PCP refers him to cardiology for management of treatment-resistant hypertension. He is discharged from the PHP and continues psychotherapy for depression and anxiety in an intensive outpatient program (IOP). Throughout his PHP and IOP treatments, he continues to take paroxetine and hydroxyzine. He achieves a successful remission of his anxiety and depression, with partial but significant remission of his panic attacks.
The author’s observations
Although Mr. P did not have pheochromocytoma, it is important to rule out this rare condition in patients who present with treatment-resistant hypertension and/or treatment-resistant anxiety.
Continue to: Bottom Line
Bottom Line
Pheochromocytoma is a tumor of the adrenal gland. The classic triad of symptoms of this rare condition is hypertension, sweating, and headache; approximately 30% of patients report significant anxiety and panic. Several medications, including tricyclic antidepressants, beta-blockers, and buspirone, can lead to false-positive results on the serum and urine metanephrines testing used to diagnose pheochromocytoma.
Related Resources
- National Organization for Rare Disorders. Rare Disease Database: pheochromocytoma. www.rarediseases.org/rare-diseases/pheochromocytoma/.
- Young WF Jr. Clinical presentation and diagnosis of pheochromocytoma. UpToDate. www.uptodate.com/contents/clinical-presentation-and-diagnosis-of-pheochromocytoma. Published January 2020.
Drug Brand Names
Alprazolam • Xanax
Amitriptyline • Elavil
Buspirone • Buspar
Carvedilol • Coreg
Clonidine • Catapres
Doxazosin • Cardura
Hydroxyzine • Vistaril
Lisinopril • Prinivil, Zestril
Paroxetine • Paxil
Prazosin • Minipress
Propranolol • Inderal
Terazosin • Hytrin
Trazodone • Desyrel
Zolpidem • Ambien
1. Morrison J. When psychological problems mask medical disorders: a guide for psychotherapists. 2nd ed. New York, NY: The Guilford Press; 2015.
2. American Porphyria Foundation. About porphyria. https://porphyriafoundation.org/patients/about-porphyria. Accessed May 13, 2020.
3. Millward L, Kelly P, King A, et al. Anxiety and depression in the acute porphyrias. J Inherit Metab Dis. 2005;28(6):1099-1107.
4. Bonkovsky H, Maddukuri VC, Yazici C, et al. Acute porphyrias in the USA: features of 108 subjects from porphyria consortium. Am J Med. 2014;127(12):1233-1241.
5. Tsirlin A, Oo Y, Sharma R, et al. Pheochromocytoma: a review. Maturitas. 2014;77(3):229-238.
6. Leung A, Zun L, Nordstrom K, et al. Psychiatric emergencies for physicians: clinical management and approach to distinguishing pheochromocytoma from psychiatric and thyrotoxic diseases in the emergency room. J Emerg Med. 2017;53(5):712-716.
7. Garg M, Kharb S, Brar KS, et al. Medical management of pheochromocytoma: role of the endocrinologist. Indian J Endocrinol and Metab. 2011;15(suppl 4):S329-S336. doi: 10.4103/2230-8210.86976.
8. Zardawi I. Phaeochromocytoma masquerading as anxiety and depression. Am J Case Rep. 2013;14:161-163.
9. ARUP Laboratories. Test directory. https://www.aruplab.com. Accessed February 11, 2020.
10. Sriram P, Raghavan V. Pheochromocytoma presenting as anxiety disorder: a case report. Asian J Psychiatr. 2017;29:83-84.
11. Stahl SM. Stahl’s essential psychopharmacology: neuroscientific basis and practical applications. Cambridge, UK: Cambridge University Press; 2013.
CASE Depression, anxiety, and panic attacks
At the urging of his parents Mr. P, age 33, presents to the partial hospitalization program (PHP) for worsening depression and anxiety, daily panic attacks with accompanying diaphoresis and headache, and the possibility that he may have taken an overdose of zolpidem. Mr. P denies taking an intentional overdose of zolpidem, claiming instead that he was having a sleep-walking episode and did not realize how many pills he took.
In addition to daily panic attacks, Mr. P reports having trouble falling asleep, overwhelming sadness, and daily passive suicidal ideation without a plan or active intent.
Mr. P cannot identify a specific trigger to this most recent exacerbation of depressed/anxious mood, but instead describes it as slowly building over the past 6 to 8 months. Mr. P says the panic attacks occur without warning and states, “I feel like my heart is going to jump out of my chest; I get a terrible headache, and I sweat like crazy. Sometimes I just feel like I’m about to pass out or die.” Although these episodes had been present for approximately 2 years, they now occur almost daily.
HISTORY Inconsistent adherence
For the last year, Mr. P had been taking alprazolam, 0.5 mg twice daily, and paroxetine, 20 mg/d, and these medications provided moderate relief of his depressive/anxious symptoms. However, he stopped taking both medications approximately 3 or 4 weeks ago when he ran out. He also takes propranolol, 20 mg/d, sporadically, for hypertension. In the past, he had been prescribed carvedilol, clonidine, and lisinopril—all with varying degrees of relief of his hypertension. He denies a family history of hypertension or any other chronic or acute health problems. He reports that he has been sober from alcohol for 19 months but smokes 1 to 2 marijuana cigarettes a day.
EVALUATION Elevated blood pressure and pulse
Mr. P’s physical examination and medical review of systems are unremarkable, except for an elevated blood pressure (190/110 mm Hg) and pulse (92 beats per minute); he also has a headache. A repeat blood pressure test later in the day is 172/94 mm Hg, with a pulse of 100 beats per minute. His urine drug screen is positive only for delta-9-tetrahydrocannabinol (THC).
[polldaddy:10558304]
The author’s observations
A CBC with differential is helpful for ruling out infection and anemia as causes of anxiety and depression.1 In Mr. P’s case, there were no concerning symptoms that pointed to anemia or infection as likely causes of his anxiety, depression, or panic attacks. A TSH level also would be reasonable, because hyperthyroidism can present as anxiety, while hyperthyroidism or hypothyroidism each can present as depression.1 However, both Mr. P’s medical history and physical examination were not concerning for thyroid disease, making it unlikely that he had either of those conditions. A review of Mr. P’s medical records indicated that within the past 6 months, his primary care physician (PCP) had ordered a CBC and TSH test; the results of both were within normal limits.
Serum porphyrin tests can exclude porphyria as a contributor to Mr. P’s anxiety and depression. Porphyrias are a group of 8 inherited disorders that involve accumulation of heme precursors (porphyrins) in the CNS and subcutaneous tissue.2 Collectively, porphyrias affect approximately 1 in 200,000 people.2 Anxiety and depression are strongly associated with porphyria, but do not occur secondary to the illness; depression and anxiety appear to be intrinsic personality features in people with porphyria.3 Skin lesions and abdominal pain are the most common symptoms,3 and there is a higher incidence of hypertension in people with porphyria than in the general population.4 Mr. P does not report any heritable disorders, nor does he appear to have any CNS disturbance or unusual cutaneous lesions, which makes it unlikely that this disorder is related to his psychiatric symptoms.
Continue to: A serum metanephrines test measures...
A serum metanephrines test measures the metabolites of epinephrine and norepinephrine. These catecholamines are produced in excess by an adrenal or extra-adrenal tumor seen in pheochromocytoma. The classic triad of symptoms of pheochromocytoma are hypertension, sweating, and headache; approximately 30% of patients report significant anxiety and panic (Table 15-7). This type of tumor is rare, with an annual incidence of only 2 to 8 cases per 1 million individuals. Among people with hypertension, the annual incidence is 0.1% to 1.0%, and for those with an adrenal mass, the annual incidence is 5% (Table 26,8). Autopsy studies suggest that up to 50% of pheochromocytomas are undiagnosed.8 Left untreated, pheochromocytoma can result in hypertensive crisis, arrhythmia, myocardial infarction, multisystem organ failure, and premature death.7 Table 36,7 highlights some causes of false-positive serum on metanephrines testing.
EVALUATION Metanephrines testing
Mr. P has what appears to be treatment-resistant hypertension, accompanied by the classic symptoms observed in most patients with pheochromocytoma. Because Mr. P is participating in the PHP 6 days per week for 6 hours each day, visiting his PCP would be inconvenient, so the treatment team orders the serum metanephrines test. If a positive result is found, Mr. P will be referred to his PCP for further assessment and follow-up care with endocrinology.
TREATMENT Pharmacotherapy to target anxiety and panic
Next, the treatment team establishes a safety plan for Mr. P, and restarts paroxetine, 20 mg/d, to target his depressed and anxious mood. Alprazolam, 0.5 mg twice daily, is started to target anxious mood and panic symptoms, and to allow time for the anxiolytic properties of the paroxetine to become fully effective. The alprazolam will be tapered and stopped after 2 weeks. Mr. P is started on hydroxyzine, 1 to 2 25-mg tablets 2 to 3 times daily as needed for anxious mood and panic symptoms.
The serum metanephrines test results are equivocal, with a slight elevation of both epinephrine and norepinephrine that is too low to confirm a diagnosis of pheochromocytoma but too elevated to exclude it (Table 49). With Mr. P’s consent, the treatment team contacts his PCP and convey the results of this test. Mr. P schedules an appointment with his PCP for the following week for further assessment and confirmatory pheochromocytoma testing.

After 1 week, Mr. P remains anxious, with a slight reduction in panic attacks from multiple attacks each day to 3 or 4 attacks per week. The team considers adding an additional anxiolytic agent.
[polldaddy:10558305]
Continue to: The author's observations
The author’s observations
The triad of symptoms in pheochromocytoma results directly from the intermittent release of catecholamines into systemic circulation. Surges of epinephrine and norepinephrine lead to headaches, palpitations, diaphoresis, and (less commonly) gastrointestinal symptoms such as nausea, vomiting, and constipation. Persistent or episodic hypertension may be present, with 13% of patients maintaining a normal blood pressure.5-7 Patients with pheochromocytoma-related anxiety typically have substantial or complete resolution of anxiety and panic attacks after tumor resection.6,8,10
Because of their ability to raise catecholamine levels, several medications, including some psychotropics, can lead to false-positive results on serum and urine metanephrines testing. Tricyclic antidepressants and beta-blockers can cause false-positive results on plasma assays, while buspirone can cause false-positives on urinalysis assays.5 Trazodone, on the other hand, exhibits no catecholaminergic activity and its alpha-1 adrenergic antagonism may actually have some benefit in pheochromocytoma.11 Alpha-1 adrenergic antagonism with doxazosin, prazosin, or terazosin is the first-line of treatment in reducing pheochromocytoma-related hypertension.7 Treatment with a beta-blocker is safe only after alpha-adrenergic blockade occurs. While beta-blockers are useful for reducing the palpitations and anxiety observed in patients with pheochromocytoma, they must not be used alone due to the risk of hypertensive crisis resulting from unopposed alpha-adrenergic agonist activated vasoconstriction.5,7
TREATMENT CBT provides benefit
Mr. P decides against receiving an additional agent for anxiety and instead decides to wait for the outcome of the confirmatory pheochromocytoma testing. He continues to take alprazolam, and both his depressed mood and anxiety improve. His panic attacks continue to lessen, and he appears to benefit from cognitive-behavioral therapy provided during group therapy. Mr. P is advised by his PCP to taper and stop the alprazolam 3 to 5 days before his 24-hour urine metanephrines test because benzodiazepines can lead to false-positive results on a urinalysis assay.7
OUTCOME Remission of anxiety and depression
Mr. P has a repeat serum metanephrines test and a 24-hour urinalysis assay. Both are negative for pheochromocytoma. His PCP refers him to cardiology for management of treatment-resistant hypertension. He is discharged from the PHP and continues psychotherapy for depression and anxiety in an intensive outpatient program (IOP). Throughout his PHP and IOP treatments, he continues to take paroxetine and hydroxyzine. He achieves a successful remission of his anxiety and depression, with partial but significant remission of his panic attacks.
The author’s observations
Although Mr. P did not have pheochromocytoma, it is important to rule out this rare condition in patients who present with treatment-resistant hypertension and/or treatment-resistant anxiety.
Continue to: Bottom Line
Bottom Line
Pheochromocytoma is a tumor of the adrenal gland. The classic triad of symptoms of this rare condition is hypertension, sweating, and headache; approximately 30% of patients report significant anxiety and panic. Several medications, including tricyclic antidepressants, beta-blockers, and buspirone, can lead to false-positive results on the serum and urine metanephrines testing used to diagnose pheochromocytoma.
Related Resources
- National Organization for Rare Disorders. Rare Disease Database: pheochromocytoma. www.rarediseases.org/rare-diseases/pheochromocytoma/.
- Young WF Jr. Clinical presentation and diagnosis of pheochromocytoma. UpToDate. www.uptodate.com/contents/clinical-presentation-and-diagnosis-of-pheochromocytoma. Published January 2020.
Drug Brand Names
Alprazolam • Xanax
Amitriptyline • Elavil
Buspirone • Buspar
Carvedilol • Coreg
Clonidine • Catapres
Doxazosin • Cardura
Hydroxyzine • Vistaril
Lisinopril • Prinivil, Zestril
Paroxetine • Paxil
Prazosin • Minipress
Propranolol • Inderal
Terazosin • Hytrin
Trazodone • Desyrel
Zolpidem • Ambien
CASE Depression, anxiety, and panic attacks
At the urging of his parents Mr. P, age 33, presents to the partial hospitalization program (PHP) for worsening depression and anxiety, daily panic attacks with accompanying diaphoresis and headache, and the possibility that he may have taken an overdose of zolpidem. Mr. P denies taking an intentional overdose of zolpidem, claiming instead that he was having a sleep-walking episode and did not realize how many pills he took.
In addition to daily panic attacks, Mr. P reports having trouble falling asleep, overwhelming sadness, and daily passive suicidal ideation without a plan or active intent.
Mr. P cannot identify a specific trigger to this most recent exacerbation of depressed/anxious mood, but instead describes it as slowly building over the past 6 to 8 months. Mr. P says the panic attacks occur without warning and states, “I feel like my heart is going to jump out of my chest; I get a terrible headache, and I sweat like crazy. Sometimes I just feel like I’m about to pass out or die.” Although these episodes had been present for approximately 2 years, they now occur almost daily.
HISTORY Inconsistent adherence
For the last year, Mr. P had been taking alprazolam, 0.5 mg twice daily, and paroxetine, 20 mg/d, and these medications provided moderate relief of his depressive/anxious symptoms. However, he stopped taking both medications approximately 3 or 4 weeks ago when he ran out. He also takes propranolol, 20 mg/d, sporadically, for hypertension. In the past, he had been prescribed carvedilol, clonidine, and lisinopril—all with varying degrees of relief of his hypertension. He denies a family history of hypertension or any other chronic or acute health problems. He reports that he has been sober from alcohol for 19 months but smokes 1 to 2 marijuana cigarettes a day.
EVALUATION Elevated blood pressure and pulse
Mr. P’s physical examination and medical review of systems are unremarkable, except for an elevated blood pressure (190/110 mm Hg) and pulse (92 beats per minute); he also has a headache. A repeat blood pressure test later in the day is 172/94 mm Hg, with a pulse of 100 beats per minute. His urine drug screen is positive only for delta-9-tetrahydrocannabinol (THC).
[polldaddy:10558304]
The author’s observations
A CBC with differential is helpful for ruling out infection and anemia as causes of anxiety and depression.1 In Mr. P’s case, there were no concerning symptoms that pointed to anemia or infection as likely causes of his anxiety, depression, or panic attacks. A TSH level also would be reasonable, because hyperthyroidism can present as anxiety, while hyperthyroidism or hypothyroidism each can present as depression.1 However, both Mr. P’s medical history and physical examination were not concerning for thyroid disease, making it unlikely that he had either of those conditions. A review of Mr. P’s medical records indicated that within the past 6 months, his primary care physician (PCP) had ordered a CBC and TSH test; the results of both were within normal limits.
Serum porphyrin tests can exclude porphyria as a contributor to Mr. P’s anxiety and depression. Porphyrias are a group of 8 inherited disorders that involve accumulation of heme precursors (porphyrins) in the CNS and subcutaneous tissue.2 Collectively, porphyrias affect approximately 1 in 200,000 people.2 Anxiety and depression are strongly associated with porphyria, but do not occur secondary to the illness; depression and anxiety appear to be intrinsic personality features in people with porphyria.3 Skin lesions and abdominal pain are the most common symptoms,3 and there is a higher incidence of hypertension in people with porphyria than in the general population.4 Mr. P does not report any heritable disorders, nor does he appear to have any CNS disturbance or unusual cutaneous lesions, which makes it unlikely that this disorder is related to his psychiatric symptoms.
Continue to: A serum metanephrines test measures...
A serum metanephrines test measures the metabolites of epinephrine and norepinephrine. These catecholamines are produced in excess by an adrenal or extra-adrenal tumor seen in pheochromocytoma. The classic triad of symptoms of pheochromocytoma are hypertension, sweating, and headache; approximately 30% of patients report significant anxiety and panic (Table 15-7). This type of tumor is rare, with an annual incidence of only 2 to 8 cases per 1 million individuals. Among people with hypertension, the annual incidence is 0.1% to 1.0%, and for those with an adrenal mass, the annual incidence is 5% (Table 26,8). Autopsy studies suggest that up to 50% of pheochromocytomas are undiagnosed.8 Left untreated, pheochromocytoma can result in hypertensive crisis, arrhythmia, myocardial infarction, multisystem organ failure, and premature death.7 Table 36,7 highlights some causes of false-positive serum on metanephrines testing.
EVALUATION Metanephrines testing
Mr. P has what appears to be treatment-resistant hypertension, accompanied by the classic symptoms observed in most patients with pheochromocytoma. Because Mr. P is participating in the PHP 6 days per week for 6 hours each day, visiting his PCP would be inconvenient, so the treatment team orders the serum metanephrines test. If a positive result is found, Mr. P will be referred to his PCP for further assessment and follow-up care with endocrinology.
TREATMENT Pharmacotherapy to target anxiety and panic
Next, the treatment team establishes a safety plan for Mr. P, and restarts paroxetine, 20 mg/d, to target his depressed and anxious mood. Alprazolam, 0.5 mg twice daily, is started to target anxious mood and panic symptoms, and to allow time for the anxiolytic properties of the paroxetine to become fully effective. The alprazolam will be tapered and stopped after 2 weeks. Mr. P is started on hydroxyzine, 1 to 2 25-mg tablets 2 to 3 times daily as needed for anxious mood and panic symptoms.
The serum metanephrines test results are equivocal, with a slight elevation of both epinephrine and norepinephrine that is too low to confirm a diagnosis of pheochromocytoma but too elevated to exclude it (Table 49). With Mr. P’s consent, the treatment team contacts his PCP and convey the results of this test. Mr. P schedules an appointment with his PCP for the following week for further assessment and confirmatory pheochromocytoma testing.

After 1 week, Mr. P remains anxious, with a slight reduction in panic attacks from multiple attacks each day to 3 or 4 attacks per week. The team considers adding an additional anxiolytic agent.
[polldaddy:10558305]
Continue to: The author's observations
The author’s observations
The triad of symptoms in pheochromocytoma results directly from the intermittent release of catecholamines into systemic circulation. Surges of epinephrine and norepinephrine lead to headaches, palpitations, diaphoresis, and (less commonly) gastrointestinal symptoms such as nausea, vomiting, and constipation. Persistent or episodic hypertension may be present, with 13% of patients maintaining a normal blood pressure.5-7 Patients with pheochromocytoma-related anxiety typically have substantial or complete resolution of anxiety and panic attacks after tumor resection.6,8,10
Because of their ability to raise catecholamine levels, several medications, including some psychotropics, can lead to false-positive results on serum and urine metanephrines testing. Tricyclic antidepressants and beta-blockers can cause false-positive results on plasma assays, while buspirone can cause false-positives on urinalysis assays.5 Trazodone, on the other hand, exhibits no catecholaminergic activity and its alpha-1 adrenergic antagonism may actually have some benefit in pheochromocytoma.11 Alpha-1 adrenergic antagonism with doxazosin, prazosin, or terazosin is the first-line of treatment in reducing pheochromocytoma-related hypertension.7 Treatment with a beta-blocker is safe only after alpha-adrenergic blockade occurs. While beta-blockers are useful for reducing the palpitations and anxiety observed in patients with pheochromocytoma, they must not be used alone due to the risk of hypertensive crisis resulting from unopposed alpha-adrenergic agonist activated vasoconstriction.5,7
TREATMENT CBT provides benefit
Mr. P decides against receiving an additional agent for anxiety and instead decides to wait for the outcome of the confirmatory pheochromocytoma testing. He continues to take alprazolam, and both his depressed mood and anxiety improve. His panic attacks continue to lessen, and he appears to benefit from cognitive-behavioral therapy provided during group therapy. Mr. P is advised by his PCP to taper and stop the alprazolam 3 to 5 days before his 24-hour urine metanephrines test because benzodiazepines can lead to false-positive results on a urinalysis assay.7
OUTCOME Remission of anxiety and depression
Mr. P has a repeat serum metanephrines test and a 24-hour urinalysis assay. Both are negative for pheochromocytoma. His PCP refers him to cardiology for management of treatment-resistant hypertension. He is discharged from the PHP and continues psychotherapy for depression and anxiety in an intensive outpatient program (IOP). Throughout his PHP and IOP treatments, he continues to take paroxetine and hydroxyzine. He achieves a successful remission of his anxiety and depression, with partial but significant remission of his panic attacks.
The author’s observations
Although Mr. P did not have pheochromocytoma, it is important to rule out this rare condition in patients who present with treatment-resistant hypertension and/or treatment-resistant anxiety.
Continue to: Bottom Line
Bottom Line
Pheochromocytoma is a tumor of the adrenal gland. The classic triad of symptoms of this rare condition is hypertension, sweating, and headache; approximately 30% of patients report significant anxiety and panic. Several medications, including tricyclic antidepressants, beta-blockers, and buspirone, can lead to false-positive results on the serum and urine metanephrines testing used to diagnose pheochromocytoma.
Related Resources
- National Organization for Rare Disorders. Rare Disease Database: pheochromocytoma. www.rarediseases.org/rare-diseases/pheochromocytoma/.
- Young WF Jr. Clinical presentation and diagnosis of pheochromocytoma. UpToDate. www.uptodate.com/contents/clinical-presentation-and-diagnosis-of-pheochromocytoma. Published January 2020.
Drug Brand Names
Alprazolam • Xanax
Amitriptyline • Elavil
Buspirone • Buspar
Carvedilol • Coreg
Clonidine • Catapres
Doxazosin • Cardura
Hydroxyzine • Vistaril
Lisinopril • Prinivil, Zestril
Paroxetine • Paxil
Prazosin • Minipress
Propranolol • Inderal
Terazosin • Hytrin
Trazodone • Desyrel
Zolpidem • Ambien
1. Morrison J. When psychological problems mask medical disorders: a guide for psychotherapists. 2nd ed. New York, NY: The Guilford Press; 2015.
2. American Porphyria Foundation. About porphyria. https://porphyriafoundation.org/patients/about-porphyria. Accessed May 13, 2020.
3. Millward L, Kelly P, King A, et al. Anxiety and depression in the acute porphyrias. J Inherit Metab Dis. 2005;28(6):1099-1107.
4. Bonkovsky H, Maddukuri VC, Yazici C, et al. Acute porphyrias in the USA: features of 108 subjects from porphyria consortium. Am J Med. 2014;127(12):1233-1241.
5. Tsirlin A, Oo Y, Sharma R, et al. Pheochromocytoma: a review. Maturitas. 2014;77(3):229-238.
6. Leung A, Zun L, Nordstrom K, et al. Psychiatric emergencies for physicians: clinical management and approach to distinguishing pheochromocytoma from psychiatric and thyrotoxic diseases in the emergency room. J Emerg Med. 2017;53(5):712-716.
7. Garg M, Kharb S, Brar KS, et al. Medical management of pheochromocytoma: role of the endocrinologist. Indian J Endocrinol and Metab. 2011;15(suppl 4):S329-S336. doi: 10.4103/2230-8210.86976.
8. Zardawi I. Phaeochromocytoma masquerading as anxiety and depression. Am J Case Rep. 2013;14:161-163.
9. ARUP Laboratories. Test directory. https://www.aruplab.com. Accessed February 11, 2020.
10. Sriram P, Raghavan V. Pheochromocytoma presenting as anxiety disorder: a case report. Asian J Psychiatr. 2017;29:83-84.
11. Stahl SM. Stahl’s essential psychopharmacology: neuroscientific basis and practical applications. Cambridge, UK: Cambridge University Press; 2013.
1. Morrison J. When psychological problems mask medical disorders: a guide for psychotherapists. 2nd ed. New York, NY: The Guilford Press; 2015.
2. American Porphyria Foundation. About porphyria. https://porphyriafoundation.org/patients/about-porphyria. Accessed May 13, 2020.
3. Millward L, Kelly P, King A, et al. Anxiety and depression in the acute porphyrias. J Inherit Metab Dis. 2005;28(6):1099-1107.
4. Bonkovsky H, Maddukuri VC, Yazici C, et al. Acute porphyrias in the USA: features of 108 subjects from porphyria consortium. Am J Med. 2014;127(12):1233-1241.
5. Tsirlin A, Oo Y, Sharma R, et al. Pheochromocytoma: a review. Maturitas. 2014;77(3):229-238.
6. Leung A, Zun L, Nordstrom K, et al. Psychiatric emergencies for physicians: clinical management and approach to distinguishing pheochromocytoma from psychiatric and thyrotoxic diseases in the emergency room. J Emerg Med. 2017;53(5):712-716.
7. Garg M, Kharb S, Brar KS, et al. Medical management of pheochromocytoma: role of the endocrinologist. Indian J Endocrinol and Metab. 2011;15(suppl 4):S329-S336. doi: 10.4103/2230-8210.86976.
8. Zardawi I. Phaeochromocytoma masquerading as anxiety and depression. Am J Case Rep. 2013;14:161-163.
9. ARUP Laboratories. Test directory. https://www.aruplab.com. Accessed February 11, 2020.
10. Sriram P, Raghavan V. Pheochromocytoma presenting as anxiety disorder: a case report. Asian J Psychiatr. 2017;29:83-84.
11. Stahl SM. Stahl’s essential psychopharmacology: neuroscientific basis and practical applications. Cambridge, UK: Cambridge University Press; 2013.
When mania isn’t what it seems
CASE Aggressive, impulsive, and not sleeping
Mr. S, age 22, is brought by his family to his outpatient psychiatrist because he has begun to
Mr. S has significant language impairment and is unreliable as a narrator. His family reports that Mr. S’s behavior has resulted in declining academic performance, and they have curtailed his social activities due to behavioral issues. Both his family and teachers report that it is increasingly difficult to redirect Mr. S’s behavior. Although not physically aggressive, Mr. S becomes verbally agitated when rituals are incomplete. He has gone from sleeping 8 hours each night to only 3 to 4 hours, but he does not appear tired during the day.
HISTORY Multiple hospitalizations
As a child, Mr. S had been diagnosed with autism and intellectual disability. When he was 13, he began exhibiting marked stereotypy, restlessness, impulsivity, frenzy, agitation, combativeness, and purposeless motor activity. At that time, he was not receiving any medications. Mr. S had not slept for 2 days and had been walking in circles nonstop. He became aggressive whenever anyone attempted to redirect his behavior. The family took Mr. S to the emergency department (ED), where clinicians ruled out organic causes for his behavioral disturbances, including infections, drug intoxication, and use of illicit substances. Mr. S was transferred from the ED to a child and adolescent psychiatry ward at a nearby university hospital for inpatient treatment.
On the inpatient unit, the treatment team diagnosed Mr. S with bipolar disorder and believed that he was experiencing a manic episode. He was prescribed quetiapine, 25 mg by mouth during the day and 75 mg by mouth at night, to stabilize his agitation, and was discharged with a plan to follow up with his outpatient psychiatrist. However, within 1 week, his symptoms returned, with markedly increased aggression and agitation, so he was readmitted, tapered off quetiapine, and prescribed valproic acid, 125 mg by mouth during the day and 375 mg by mouth at bedtime. With this regimen, Mr. S became calmer, but when he was discharged home, he was subdued and withdrawn, overly adherent to rules and routines, constantly irritable, and often unable to focus.
Two years later, Mr. S developed hyperammonemia. Valproic acid was discontinued, and many of his behavioral issues resolved. He flourished both academically and socially. He experienced no exacerbation of symptoms until his current presentation.
[polldaddy:10544547]
EVALUATION Pinpointing the cause
Mr. S’s physical examination reveals that his vital signs are within normal limits. Mr. S is mildly tachycardic (heart rate, 105 bpm), with regular rate and rhythm. No murmurs, gallops, or rubs are auscultated. The remainder of the physical exam, including a detailed neurologic exam, is normal.
On mental status examination, Mr. S makes limited eye contact. He has difficulty sitting in the chair, with increased rocking, finger flicking, and hand flapping from baseline. Some compulsive behaviors are noted, such as tapping his neck. He has increased tics (eye blinking and mouth opening) and increased verbigeration and repetitive verbal statements. He loudly and repeatedly demands to go home, and uses short sentences with incorrect pronouns. His affect is difficult to assess, but he is agitated. His thought process is concrete. There is no evidence of suicidal ideation, homicidal ideation, or psychosis. Mr. S denies auditory hallucinations. His insight and judgment are limited.
Continue to: The psychiatrist rules out...
The psychiatrist rules out a behavioral exacerbation of autism based on an interview with Mr. S’s family and established rapport from treating him for several years. Mr. S’s family reports that many of his behaviors are not new but that the increased drive and intensity is worrisome. Further, his family cannot identify any stressors or precipitants for the behaviors and reports that offering preferred reinforcers did not help. An anxiety disorder is ruled out because according to the family, Mr. S’s drive to constantly move and complete rituals is fueling his anxiety. Schizoaffective disorder is ruled out because Mr. S denies auditory hallucinations and has not been observed responding to internal stimuli.
His Bush-Francis Catatonia Rating Scale (BFCRS) score is 26, which suggests a high likelihood of catatonia. Based on the BFCRS score, Mr. S’s psychiatrist makes the diagnosis of hyperkinetic catatonia.
The authors’ observations
The psychiatrist determined that Mr. S had been misdiagnosed with bipolar disorder at age 13. At that time, he had experienced his first episode of hyperkinetic catatonia and his symptoms decreased after he received lorazepam in the ED. However, the treatment team did not correctly identify this, most likely due to limited knowledge of catatonia among emergency medicine clinicians.
This case exemplifies a cognitive error of premature closure. Rather than considering catatonia as a complication of autism when Mr. S was 13, the clinicians added a second psychiatric diagnosis of bipolar disorder.Although premature closure errors generally occur when the physician assumes the patient is having a common complication of a known illness,1 in Mr. S’s case, the opposite occurred.
Conceptualizing catatonia
One helpful model for conceptualizing catatonia is to think of it as a basal ganglia disorder, with lesions in the basal ganglia thalamocortical tracts and the anterior cingulate/medial orbitofrontal circuit. Disrupting these pathways can result in symptoms such as mutism or repetitive and imitative behaviors. This is likely due to decreased disinhibition by gamma-aminobutyric acid (GABA), resulting in a hypodopaminergic state. This explains why benzodiazepines, which act to increase GABA, are effective for treating catatonia, and antipsychotics that act to decrease dopamine can exacerbate symptoms. Fricchione et al2 developed a model to visually represent the neurobiologic pathophysiology of catatonia (Figure2).
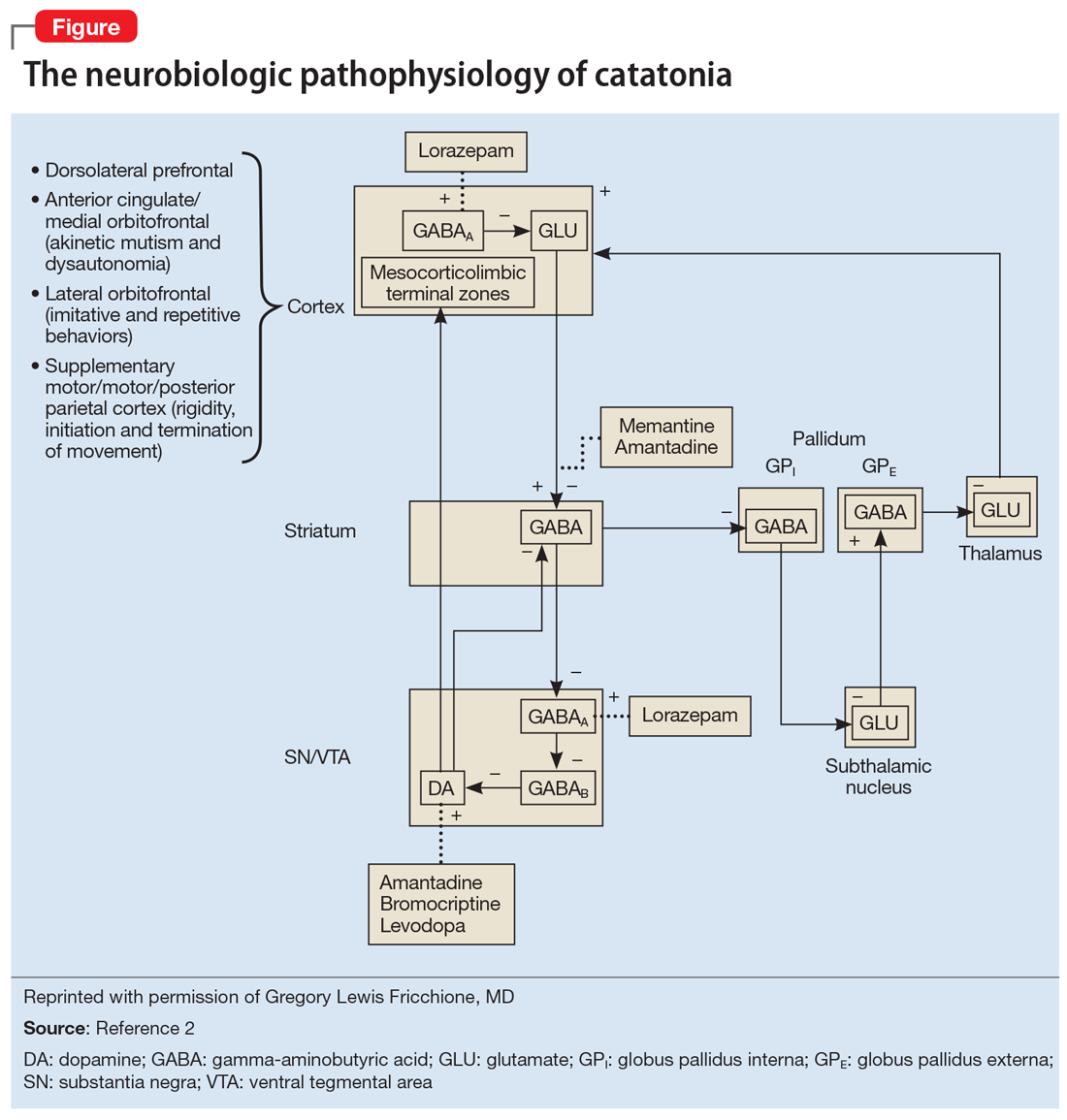
Continue to: Underlying causes of catatonia
Underlying causes of catatonia
Catatonia is most often seen in individuals with an underlying psychiatric condition such as schizophrenia, mood disorders, or autism. However, catatonia also occurs in the context of general neurologic and medical disorders, including (but not limited to) infections, metabolic disorders, endocrinopathies, epilepsy, neurodegenerative diseases, delirium, hypertensive encephalopathy, autoimmune encephalitis, and liver and kidney transplantation.3
Subtypes of catatonia include4:
- hypokinetic catatonia, which presents as stupor, mutism, and negativism
- hyperkinetic catatonia, which presents as hyperactivity, agitation, and stereotypy (as observed in Mr. S)
- malignant catatonia, which is a potentially lethal form of catatonia that occurs when hypo- or hyperkinetic catatonia is accompanied by autonomic instability such as tachycardia, tachypnea, hypertension, fever, and muscle rigidity
- periodic catatonia, which is characterized by brief episodes of stupor or excitatory catatonia lasting 4 to 10 days. These episodes recur over weeks to years, with patients remaining asymptomatic between episodes, or showing mild symptoms, such as facial grimacing or negativisms. Periodic catatonia often is autosomal dominant, involves linkage for the long arm of chromosome 15, and has a better prognosis than the other forms.
Autism and catatonia
Most individuals with autism who experience a catatonic episode first do so between age 10 and 19, and many episodes are precipitated by sudden changes in routine resulting in stress.5 An estimated 12% to 18% of patients with autism are diagnosed with catatonia in their lifetime, but the actual prevalence is likely higher.4
One of the reasons for this might be that although catatonia is well known in the psychiatric community, it is relatively unknown in the general medical community. Children and adolescents with psychiatric illness are likely to have symptoms of catatonia overlooked because catatonia often is not included in the differential diagnosis.6
In Mr. S’s case, it became clear that he did not have a mood disorder, but was prone to episodes of hyperkinetic catatonia due to his autism.
Continue to: Better recognition of catatonia
Better recognition of catatonia
As catatonia becomes better elucidated and more clearly described in the literature, there is increasing awareness that symptoms do not always involve stupor, mutism, and slowed motor activity, but can include increased motor activity, agitation, and stereotypies. The BFCRS is extremely useful for quantifying symptoms of catatonia. The best way to confirm the diagnosis is to use a lorazepam challenge in an inpatient setting, or a trial of lorazepam in an outpatient setting.5
[polldaddy:10544548]
The authors’ observations
Lorazepam is often considered the first-line treatment for catatonia because it is one of the most widely studied medications. Other benzodiazepines, such as oxazepam and clonazepam, and the sedative/hypnotic zolpidem have also been shown to be effective. Antipsychotics with dopamine-blocking mechanisms can exacerbate symptoms of catatonia and should be avoided in these patients. Furthermore, in cases of refractory catatonia, bilateral electroconvulsive therapy is an important and necessary treatment.7
TREATMENT Pharmacologic agents decrease BFCRS score
Mr. S is prescribed a regimen of lorazepam, 2 mg by mouth daily, and the supplement N-acetylcysteine, 600 mg by mouth daily. Within 2 weeks of starting this regimen, Mr. S’s BFCRS score decreases from 26 to 14. After 6 months of treatment with lorazepam, Mr. S shows considerable improvement. The stereotypic behaviors and impulsivity decrease significantly, leading to improved sleep and performance in school. After 6 months Mr. S is successfully tapered off the lorazepam, with a complete return to baseline.
Bottom Line
Hyperkinetic catatonia is easily overlooked, especially in the emergency setting. Catatonia should always be ruled out, particularly in patients with underlying conditions associated with it. Hyperkinetic catatonia is an underrecognized comorbidity in patients with autism.
Related Resources
- Dhossche DM, Wing L, Ohta M, et al. International Review of Neurobiology: Catatonia in autism spectrum disorders, vol 72. New York, NY: Academic Press/Elsevier; 2006.
- Taylor MA, Fink M. Catatonia in psychiatric classification: a home of its own. Am J Psychiatry. 2003;160(7):1233-1241.
Drug Brand Names
Amantadine • Symmetrel
Bromocriptine • Parlodel
Clonazepam • Klonopin
Lorazepam • Ativan
Memantine • Namenda
Oxazepam • Serax
Quetiapine • Seroquel
Valproic acid • Depakene, Depakote
Zolpidem • Ambien
1. McGee DL. Cognitive errors in clinical decision making. Merck Manual. https://www.merckmanuals.com/professional/special-subjects/clinical-decision-making/cognitive-errors-in-clinical-decision-making. Published November 2018. Accessed February 10, 2020.
2. Fricchione GL, Gross AF, Stern TA. Catatonia, neuroleptic malignant syndrome, and serotonin syndrome. Fricchione GL, Huffman JC, Stern TA, Bush G, eds. Massachusetts General Hospital Handbook of General Hospital Psychiatry. 6th ed. Philadelphia, PA: Saunders Elsevier; 2004:513-530.
3. Oldham MA, Lee HB. Catatonia vis-à-vis delirium: the significance of recognizing catatonia in altered mental status. Gen Hosp Psychiatry. 2015;37(6):554-559.
4. Wijemanne S, Jankovic J. Movement disorders in catatonia. J Neurol Neurosurg Psychiatry. 2014;86(8):825-832.
5. Dhossche DM, Shah A, Wing L. Blueprints for the assessment, treatment, and future study of catatonia in autism spectrum disorders. Int Rev Neurobiol. 2006:72;267-284.
6. Wing L, Shah A. Catatonia in autistic spectrum disorders. Br J Psychiatry. 2000:176(4):357-362.
7. Seinaert P, Dhossche DM, Vancampfort D, et al. A clinical review of the treatment of catatonia. Front Psychiatry. 2014;5:181.
CASE Aggressive, impulsive, and not sleeping
Mr. S, age 22, is brought by his family to his outpatient psychiatrist because he has begun to
Mr. S has significant language impairment and is unreliable as a narrator. His family reports that Mr. S’s behavior has resulted in declining academic performance, and they have curtailed his social activities due to behavioral issues. Both his family and teachers report that it is increasingly difficult to redirect Mr. S’s behavior. Although not physically aggressive, Mr. S becomes verbally agitated when rituals are incomplete. He has gone from sleeping 8 hours each night to only 3 to 4 hours, but he does not appear tired during the day.
HISTORY Multiple hospitalizations
As a child, Mr. S had been diagnosed with autism and intellectual disability. When he was 13, he began exhibiting marked stereotypy, restlessness, impulsivity, frenzy, agitation, combativeness, and purposeless motor activity. At that time, he was not receiving any medications. Mr. S had not slept for 2 days and had been walking in circles nonstop. He became aggressive whenever anyone attempted to redirect his behavior. The family took Mr. S to the emergency department (ED), where clinicians ruled out organic causes for his behavioral disturbances, including infections, drug intoxication, and use of illicit substances. Mr. S was transferred from the ED to a child and adolescent psychiatry ward at a nearby university hospital for inpatient treatment.
On the inpatient unit, the treatment team diagnosed Mr. S with bipolar disorder and believed that he was experiencing a manic episode. He was prescribed quetiapine, 25 mg by mouth during the day and 75 mg by mouth at night, to stabilize his agitation, and was discharged with a plan to follow up with his outpatient psychiatrist. However, within 1 week, his symptoms returned, with markedly increased aggression and agitation, so he was readmitted, tapered off quetiapine, and prescribed valproic acid, 125 mg by mouth during the day and 375 mg by mouth at bedtime. With this regimen, Mr. S became calmer, but when he was discharged home, he was subdued and withdrawn, overly adherent to rules and routines, constantly irritable, and often unable to focus.
Two years later, Mr. S developed hyperammonemia. Valproic acid was discontinued, and many of his behavioral issues resolved. He flourished both academically and socially. He experienced no exacerbation of symptoms until his current presentation.
[polldaddy:10544547]
EVALUATION Pinpointing the cause
Mr. S’s physical examination reveals that his vital signs are within normal limits. Mr. S is mildly tachycardic (heart rate, 105 bpm), with regular rate and rhythm. No murmurs, gallops, or rubs are auscultated. The remainder of the physical exam, including a detailed neurologic exam, is normal.
On mental status examination, Mr. S makes limited eye contact. He has difficulty sitting in the chair, with increased rocking, finger flicking, and hand flapping from baseline. Some compulsive behaviors are noted, such as tapping his neck. He has increased tics (eye blinking and mouth opening) and increased verbigeration and repetitive verbal statements. He loudly and repeatedly demands to go home, and uses short sentences with incorrect pronouns. His affect is difficult to assess, but he is agitated. His thought process is concrete. There is no evidence of suicidal ideation, homicidal ideation, or psychosis. Mr. S denies auditory hallucinations. His insight and judgment are limited.
Continue to: The psychiatrist rules out...
The psychiatrist rules out a behavioral exacerbation of autism based on an interview with Mr. S’s family and established rapport from treating him for several years. Mr. S’s family reports that many of his behaviors are not new but that the increased drive and intensity is worrisome. Further, his family cannot identify any stressors or precipitants for the behaviors and reports that offering preferred reinforcers did not help. An anxiety disorder is ruled out because according to the family, Mr. S’s drive to constantly move and complete rituals is fueling his anxiety. Schizoaffective disorder is ruled out because Mr. S denies auditory hallucinations and has not been observed responding to internal stimuli.
His Bush-Francis Catatonia Rating Scale (BFCRS) score is 26, which suggests a high likelihood of catatonia. Based on the BFCRS score, Mr. S’s psychiatrist makes the diagnosis of hyperkinetic catatonia.
The authors’ observations
The psychiatrist determined that Mr. S had been misdiagnosed with bipolar disorder at age 13. At that time, he had experienced his first episode of hyperkinetic catatonia and his symptoms decreased after he received lorazepam in the ED. However, the treatment team did not correctly identify this, most likely due to limited knowledge of catatonia among emergency medicine clinicians.
This case exemplifies a cognitive error of premature closure. Rather than considering catatonia as a complication of autism when Mr. S was 13, the clinicians added a second psychiatric diagnosis of bipolar disorder.Although premature closure errors generally occur when the physician assumes the patient is having a common complication of a known illness,1 in Mr. S’s case, the opposite occurred.
Conceptualizing catatonia
One helpful model for conceptualizing catatonia is to think of it as a basal ganglia disorder, with lesions in the basal ganglia thalamocortical tracts and the anterior cingulate/medial orbitofrontal circuit. Disrupting these pathways can result in symptoms such as mutism or repetitive and imitative behaviors. This is likely due to decreased disinhibition by gamma-aminobutyric acid (GABA), resulting in a hypodopaminergic state. This explains why benzodiazepines, which act to increase GABA, are effective for treating catatonia, and antipsychotics that act to decrease dopamine can exacerbate symptoms. Fricchione et al2 developed a model to visually represent the neurobiologic pathophysiology of catatonia (Figure2).

Continue to: Underlying causes of catatonia
Underlying causes of catatonia
Catatonia is most often seen in individuals with an underlying psychiatric condition such as schizophrenia, mood disorders, or autism. However, catatonia also occurs in the context of general neurologic and medical disorders, including (but not limited to) infections, metabolic disorders, endocrinopathies, epilepsy, neurodegenerative diseases, delirium, hypertensive encephalopathy, autoimmune encephalitis, and liver and kidney transplantation.3
Subtypes of catatonia include4:
- hypokinetic catatonia, which presents as stupor, mutism, and negativism
- hyperkinetic catatonia, which presents as hyperactivity, agitation, and stereotypy (as observed in Mr. S)
- malignant catatonia, which is a potentially lethal form of catatonia that occurs when hypo- or hyperkinetic catatonia is accompanied by autonomic instability such as tachycardia, tachypnea, hypertension, fever, and muscle rigidity
- periodic catatonia, which is characterized by brief episodes of stupor or excitatory catatonia lasting 4 to 10 days. These episodes recur over weeks to years, with patients remaining asymptomatic between episodes, or showing mild symptoms, such as facial grimacing or negativisms. Periodic catatonia often is autosomal dominant, involves linkage for the long arm of chromosome 15, and has a better prognosis than the other forms.
Autism and catatonia
Most individuals with autism who experience a catatonic episode first do so between age 10 and 19, and many episodes are precipitated by sudden changes in routine resulting in stress.5 An estimated 12% to 18% of patients with autism are diagnosed with catatonia in their lifetime, but the actual prevalence is likely higher.4
One of the reasons for this might be that although catatonia is well known in the psychiatric community, it is relatively unknown in the general medical community. Children and adolescents with psychiatric illness are likely to have symptoms of catatonia overlooked because catatonia often is not included in the differential diagnosis.6
In Mr. S’s case, it became clear that he did not have a mood disorder, but was prone to episodes of hyperkinetic catatonia due to his autism.
Continue to: Better recognition of catatonia
Better recognition of catatonia
As catatonia becomes better elucidated and more clearly described in the literature, there is increasing awareness that symptoms do not always involve stupor, mutism, and slowed motor activity, but can include increased motor activity, agitation, and stereotypies. The BFCRS is extremely useful for quantifying symptoms of catatonia. The best way to confirm the diagnosis is to use a lorazepam challenge in an inpatient setting, or a trial of lorazepam in an outpatient setting.5
[polldaddy:10544548]
The authors’ observations
Lorazepam is often considered the first-line treatment for catatonia because it is one of the most widely studied medications. Other benzodiazepines, such as oxazepam and clonazepam, and the sedative/hypnotic zolpidem have also been shown to be effective. Antipsychotics with dopamine-blocking mechanisms can exacerbate symptoms of catatonia and should be avoided in these patients. Furthermore, in cases of refractory catatonia, bilateral electroconvulsive therapy is an important and necessary treatment.7
TREATMENT Pharmacologic agents decrease BFCRS score
Mr. S is prescribed a regimen of lorazepam, 2 mg by mouth daily, and the supplement N-acetylcysteine, 600 mg by mouth daily. Within 2 weeks of starting this regimen, Mr. S’s BFCRS score decreases from 26 to 14. After 6 months of treatment with lorazepam, Mr. S shows considerable improvement. The stereotypic behaviors and impulsivity decrease significantly, leading to improved sleep and performance in school. After 6 months Mr. S is successfully tapered off the lorazepam, with a complete return to baseline.
Bottom Line
Hyperkinetic catatonia is easily overlooked, especially in the emergency setting. Catatonia should always be ruled out, particularly in patients with underlying conditions associated with it. Hyperkinetic catatonia is an underrecognized comorbidity in patients with autism.
Related Resources
- Dhossche DM, Wing L, Ohta M, et al. International Review of Neurobiology: Catatonia in autism spectrum disorders, vol 72. New York, NY: Academic Press/Elsevier; 2006.
- Taylor MA, Fink M. Catatonia in psychiatric classification: a home of its own. Am J Psychiatry. 2003;160(7):1233-1241.
Drug Brand Names
Amantadine • Symmetrel
Bromocriptine • Parlodel
Clonazepam • Klonopin
Lorazepam • Ativan
Memantine • Namenda
Oxazepam • Serax
Quetiapine • Seroquel
Valproic acid • Depakene, Depakote
Zolpidem • Ambien
CASE Aggressive, impulsive, and not sleeping
Mr. S, age 22, is brought by his family to his outpatient psychiatrist because he has begun to
Mr. S has significant language impairment and is unreliable as a narrator. His family reports that Mr. S’s behavior has resulted in declining academic performance, and they have curtailed his social activities due to behavioral issues. Both his family and teachers report that it is increasingly difficult to redirect Mr. S’s behavior. Although not physically aggressive, Mr. S becomes verbally agitated when rituals are incomplete. He has gone from sleeping 8 hours each night to only 3 to 4 hours, but he does not appear tired during the day.
HISTORY Multiple hospitalizations
As a child, Mr. S had been diagnosed with autism and intellectual disability. When he was 13, he began exhibiting marked stereotypy, restlessness, impulsivity, frenzy, agitation, combativeness, and purposeless motor activity. At that time, he was not receiving any medications. Mr. S had not slept for 2 days and had been walking in circles nonstop. He became aggressive whenever anyone attempted to redirect his behavior. The family took Mr. S to the emergency department (ED), where clinicians ruled out organic causes for his behavioral disturbances, including infections, drug intoxication, and use of illicit substances. Mr. S was transferred from the ED to a child and adolescent psychiatry ward at a nearby university hospital for inpatient treatment.
On the inpatient unit, the treatment team diagnosed Mr. S with bipolar disorder and believed that he was experiencing a manic episode. He was prescribed quetiapine, 25 mg by mouth during the day and 75 mg by mouth at night, to stabilize his agitation, and was discharged with a plan to follow up with his outpatient psychiatrist. However, within 1 week, his symptoms returned, with markedly increased aggression and agitation, so he was readmitted, tapered off quetiapine, and prescribed valproic acid, 125 mg by mouth during the day and 375 mg by mouth at bedtime. With this regimen, Mr. S became calmer, but when he was discharged home, he was subdued and withdrawn, overly adherent to rules and routines, constantly irritable, and often unable to focus.
Two years later, Mr. S developed hyperammonemia. Valproic acid was discontinued, and many of his behavioral issues resolved. He flourished both academically and socially. He experienced no exacerbation of symptoms until his current presentation.
[polldaddy:10544547]
EVALUATION Pinpointing the cause
Mr. S’s physical examination reveals that his vital signs are within normal limits. Mr. S is mildly tachycardic (heart rate, 105 bpm), with regular rate and rhythm. No murmurs, gallops, or rubs are auscultated. The remainder of the physical exam, including a detailed neurologic exam, is normal.
On mental status examination, Mr. S makes limited eye contact. He has difficulty sitting in the chair, with increased rocking, finger flicking, and hand flapping from baseline. Some compulsive behaviors are noted, such as tapping his neck. He has increased tics (eye blinking and mouth opening) and increased verbigeration and repetitive verbal statements. He loudly and repeatedly demands to go home, and uses short sentences with incorrect pronouns. His affect is difficult to assess, but he is agitated. His thought process is concrete. There is no evidence of suicidal ideation, homicidal ideation, or psychosis. Mr. S denies auditory hallucinations. His insight and judgment are limited.
Continue to: The psychiatrist rules out...
The psychiatrist rules out a behavioral exacerbation of autism based on an interview with Mr. S’s family and established rapport from treating him for several years. Mr. S’s family reports that many of his behaviors are not new but that the increased drive and intensity is worrisome. Further, his family cannot identify any stressors or precipitants for the behaviors and reports that offering preferred reinforcers did not help. An anxiety disorder is ruled out because according to the family, Mr. S’s drive to constantly move and complete rituals is fueling his anxiety. Schizoaffective disorder is ruled out because Mr. S denies auditory hallucinations and has not been observed responding to internal stimuli.
His Bush-Francis Catatonia Rating Scale (BFCRS) score is 26, which suggests a high likelihood of catatonia. Based on the BFCRS score, Mr. S’s psychiatrist makes the diagnosis of hyperkinetic catatonia.
The authors’ observations
The psychiatrist determined that Mr. S had been misdiagnosed with bipolar disorder at age 13. At that time, he had experienced his first episode of hyperkinetic catatonia and his symptoms decreased after he received lorazepam in the ED. However, the treatment team did not correctly identify this, most likely due to limited knowledge of catatonia among emergency medicine clinicians.
This case exemplifies a cognitive error of premature closure. Rather than considering catatonia as a complication of autism when Mr. S was 13, the clinicians added a second psychiatric diagnosis of bipolar disorder.Although premature closure errors generally occur when the physician assumes the patient is having a common complication of a known illness,1 in Mr. S’s case, the opposite occurred.
Conceptualizing catatonia
One helpful model for conceptualizing catatonia is to think of it as a basal ganglia disorder, with lesions in the basal ganglia thalamocortical tracts and the anterior cingulate/medial orbitofrontal circuit. Disrupting these pathways can result in symptoms such as mutism or repetitive and imitative behaviors. This is likely due to decreased disinhibition by gamma-aminobutyric acid (GABA), resulting in a hypodopaminergic state. This explains why benzodiazepines, which act to increase GABA, are effective for treating catatonia, and antipsychotics that act to decrease dopamine can exacerbate symptoms. Fricchione et al2 developed a model to visually represent the neurobiologic pathophysiology of catatonia (Figure2).

Continue to: Underlying causes of catatonia
Underlying causes of catatonia
Catatonia is most often seen in individuals with an underlying psychiatric condition such as schizophrenia, mood disorders, or autism. However, catatonia also occurs in the context of general neurologic and medical disorders, including (but not limited to) infections, metabolic disorders, endocrinopathies, epilepsy, neurodegenerative diseases, delirium, hypertensive encephalopathy, autoimmune encephalitis, and liver and kidney transplantation.3
Subtypes of catatonia include4:
- hypokinetic catatonia, which presents as stupor, mutism, and negativism
- hyperkinetic catatonia, which presents as hyperactivity, agitation, and stereotypy (as observed in Mr. S)
- malignant catatonia, which is a potentially lethal form of catatonia that occurs when hypo- or hyperkinetic catatonia is accompanied by autonomic instability such as tachycardia, tachypnea, hypertension, fever, and muscle rigidity
- periodic catatonia, which is characterized by brief episodes of stupor or excitatory catatonia lasting 4 to 10 days. These episodes recur over weeks to years, with patients remaining asymptomatic between episodes, or showing mild symptoms, such as facial grimacing or negativisms. Periodic catatonia often is autosomal dominant, involves linkage for the long arm of chromosome 15, and has a better prognosis than the other forms.
Autism and catatonia
Most individuals with autism who experience a catatonic episode first do so between age 10 and 19, and many episodes are precipitated by sudden changes in routine resulting in stress.5 An estimated 12% to 18% of patients with autism are diagnosed with catatonia in their lifetime, but the actual prevalence is likely higher.4
One of the reasons for this might be that although catatonia is well known in the psychiatric community, it is relatively unknown in the general medical community. Children and adolescents with psychiatric illness are likely to have symptoms of catatonia overlooked because catatonia often is not included in the differential diagnosis.6
In Mr. S’s case, it became clear that he did not have a mood disorder, but was prone to episodes of hyperkinetic catatonia due to his autism.
Continue to: Better recognition of catatonia
Better recognition of catatonia
As catatonia becomes better elucidated and more clearly described in the literature, there is increasing awareness that symptoms do not always involve stupor, mutism, and slowed motor activity, but can include increased motor activity, agitation, and stereotypies. The BFCRS is extremely useful for quantifying symptoms of catatonia. The best way to confirm the diagnosis is to use a lorazepam challenge in an inpatient setting, or a trial of lorazepam in an outpatient setting.5
[polldaddy:10544548]
The authors’ observations
Lorazepam is often considered the first-line treatment for catatonia because it is one of the most widely studied medications. Other benzodiazepines, such as oxazepam and clonazepam, and the sedative/hypnotic zolpidem have also been shown to be effective. Antipsychotics with dopamine-blocking mechanisms can exacerbate symptoms of catatonia and should be avoided in these patients. Furthermore, in cases of refractory catatonia, bilateral electroconvulsive therapy is an important and necessary treatment.7
TREATMENT Pharmacologic agents decrease BFCRS score
Mr. S is prescribed a regimen of lorazepam, 2 mg by mouth daily, and the supplement N-acetylcysteine, 600 mg by mouth daily. Within 2 weeks of starting this regimen, Mr. S’s BFCRS score decreases from 26 to 14. After 6 months of treatment with lorazepam, Mr. S shows considerable improvement. The stereotypic behaviors and impulsivity decrease significantly, leading to improved sleep and performance in school. After 6 months Mr. S is successfully tapered off the lorazepam, with a complete return to baseline.
Bottom Line
Hyperkinetic catatonia is easily overlooked, especially in the emergency setting. Catatonia should always be ruled out, particularly in patients with underlying conditions associated with it. Hyperkinetic catatonia is an underrecognized comorbidity in patients with autism.
Related Resources
- Dhossche DM, Wing L, Ohta M, et al. International Review of Neurobiology: Catatonia in autism spectrum disorders, vol 72. New York, NY: Academic Press/Elsevier; 2006.
- Taylor MA, Fink M. Catatonia in psychiatric classification: a home of its own. Am J Psychiatry. 2003;160(7):1233-1241.
Drug Brand Names
Amantadine • Symmetrel
Bromocriptine • Parlodel
Clonazepam • Klonopin
Lorazepam • Ativan
Memantine • Namenda
Oxazepam • Serax
Quetiapine • Seroquel
Valproic acid • Depakene, Depakote
Zolpidem • Ambien
1. McGee DL. Cognitive errors in clinical decision making. Merck Manual. https://www.merckmanuals.com/professional/special-subjects/clinical-decision-making/cognitive-errors-in-clinical-decision-making. Published November 2018. Accessed February 10, 2020.
2. Fricchione GL, Gross AF, Stern TA. Catatonia, neuroleptic malignant syndrome, and serotonin syndrome. Fricchione GL, Huffman JC, Stern TA, Bush G, eds. Massachusetts General Hospital Handbook of General Hospital Psychiatry. 6th ed. Philadelphia, PA: Saunders Elsevier; 2004:513-530.
3. Oldham MA, Lee HB. Catatonia vis-à-vis delirium: the significance of recognizing catatonia in altered mental status. Gen Hosp Psychiatry. 2015;37(6):554-559.
4. Wijemanne S, Jankovic J. Movement disorders in catatonia. J Neurol Neurosurg Psychiatry. 2014;86(8):825-832.
5. Dhossche DM, Shah A, Wing L. Blueprints for the assessment, treatment, and future study of catatonia in autism spectrum disorders. Int Rev Neurobiol. 2006:72;267-284.
6. Wing L, Shah A. Catatonia in autistic spectrum disorders. Br J Psychiatry. 2000:176(4):357-362.
7. Seinaert P, Dhossche DM, Vancampfort D, et al. A clinical review of the treatment of catatonia. Front Psychiatry. 2014;5:181.
1. McGee DL. Cognitive errors in clinical decision making. Merck Manual. https://www.merckmanuals.com/professional/special-subjects/clinical-decision-making/cognitive-errors-in-clinical-decision-making. Published November 2018. Accessed February 10, 2020.
2. Fricchione GL, Gross AF, Stern TA. Catatonia, neuroleptic malignant syndrome, and serotonin syndrome. Fricchione GL, Huffman JC, Stern TA, Bush G, eds. Massachusetts General Hospital Handbook of General Hospital Psychiatry. 6th ed. Philadelphia, PA: Saunders Elsevier; 2004:513-530.
3. Oldham MA, Lee HB. Catatonia vis-à-vis delirium: the significance of recognizing catatonia in altered mental status. Gen Hosp Psychiatry. 2015;37(6):554-559.
4. Wijemanne S, Jankovic J. Movement disorders in catatonia. J Neurol Neurosurg Psychiatry. 2014;86(8):825-832.
5. Dhossche DM, Shah A, Wing L. Blueprints for the assessment, treatment, and future study of catatonia in autism spectrum disorders. Int Rev Neurobiol. 2006:72;267-284.
6. Wing L, Shah A. Catatonia in autistic spectrum disorders. Br J Psychiatry. 2000:176(4):357-362.
7. Seinaert P, Dhossche DM, Vancampfort D, et al. A clinical review of the treatment of catatonia. Front Psychiatry. 2014;5:181.
Command hallucinations, but is it really psychosis?
CASE Frequent hospitalizations
Ms. D, age 26, presents to the emergency department (ED) after drinking a bottle of hand sanitizer in a suicide attempt. She is admitted to an inpatient psychiatric unit, where she spends 50 days, followed by a transfer to a step-down unit, where she spends 26 days. Upon discharge, her diagnosis is schizoaffective disorder–bipolar type.
Shortly before this, Ms. D had intentionally ingested 20 vitamin pills to “make her heart stop” after a conflict at home. After ingesting the pills, Ms. D presented to the ED, where she stated that if she were discharged, she would kill herself by taking “better pills.” She was then admitted to an inpatient psychiatric unit, where she spent 60 days before being moved to an extended-care step-down facility, where she resided for 42 days.
HISTORY A challenging past
Ms. D has a history of >25 psychiatric hospitalizations with varying discharge diagnoses, including schizophrenia, schizoaffective disorder, borderline personality disorder (BPD), and borderline intellectual functioning.
Ms. D was raised in a 2-parent home with 3 older half-brothers and 3 sisters. She was sexually assaulted by a cousin when she was 12. Ms. D recalls one event of self-injury/cutting behavior at age 15 after she was bullied by peers. Her family history is significant for schizophrenia (mother), alcohol use disorder (both parents), and bipolar disorder (sister). Her mother, who is now deceased, was admitted to state psychiatric hospitals for extended periods.
Her medication regimen has changed with nearly every hospitalization but generally has included ≥1 antipsychotic, a mood stabilizer, an antidepressant, and a benzodiazepine (often prescribed on an as-needed basis). Ms. D is obese and has difficulty sleeping, hypothyroidism, gastroesophageal reflux disease (GERD), hypertension, and iron deficiency anemia. She receives medications to manage each of these conditions.
Ms. D’s previous psychotic symptoms included auditory command hallucinations. These occurred under stressful circumstances, such as during severe family conflicts that often led to her feeling abandoned. She reported that the “voice” she heard was usually her own instructing her to “take pills.” There was no prior evidence of bizarre delusions, negative symptoms, or disorganized thoughts or speech.
During episodes of decompensation, Ms. D did not report symptoms of mania, sustained depressed mood, or anxiety, nor were these symptoms observed. Although Ms. D endorsed suicidal ideation with a plan, intent, and means, during several of her previous ED presentations, she told clinicians that her intent was not to end her life but rather to evoke concern in her family members.
Continue to: After her mother died...
After her mother died when Ms. D was 19, she began to have nightmares of wanting to hurt herself and others and began experiencing multiple hospitalizations. In 2010, Ms. D was referred to an assertive community treatment (ACT) program for individuals age 16 to 27 because of her inability to participate in traditional community-based services and her historical need for advanced services, in order to provide psychiatric care in the least restrictive means possible.
Despite receiving intensive ACT services, and in addition to the numerous inpatient psychiatric hospitalizations, over 7 years, Ms. D accumulated 8 additional general-medical hospitalizations and >50 visits to hospital EDs and urgent care facilities. These hospitalizations typically followed arguments at home, strained family dynamics, and not feeling wanted. Ms. D would ingest large quantities of prescription or over-the-counter medications as a way of coping, which often occurred while she was residing in a step-down facility after hospital discharge.
[polldaddy:10528342]
The authors’ observations
The treatment team decided to transition Ms. D to an LTSR with full continuum of treatment. While some clinicians might be concerned with potential iatrogenic harm of LTSR placement and might instead recommend less restrictive residential support and an IOP. However, in Ms. D’s case, her numerous admissions to EDs, urgent care facilities, and medical and psychiatric hospitals, her failed step-down facility placements, and her family conflicts and poor dynamics limited the efficacy of her natural support system and drove the recommendation for an LTSR.
Previously, Ms. D’s experience with ACT services had centered on managing acute crises, with brief periods of stabilization that insufficiently engaged her in a consistent and meaningful treatment plan. Ms. D’s insurance company agreed to pay for the LTSR after lengthy discussions with the clinical leadership at the ACT program and the LTSR demonstrated that she was a high utilizer of health care services. They concluded that Ms. D’s stay at the LTSR would be less expensive than the frequent use of expensive hospital services and care.
EVALUATION A consensus on the diagnosis
During the first few weeks of Ms. D’s admission to the LTSR, the treatment team takes a thorough history and reviews her medical records, which they obtained from several past inpatient admissions and therapists who previously treated Ms. D. The team also collects collateral information from Ms. D’s family members. Based on this information, interviews, and composite behavioral observations from the first few weeks of Ms. D’s time at the LTSR, the psychiatrists and treatment team at the LTSR and ACT program determine that Ms. D meets the criteria for a primary diagnosis of BPD. Previous discharge diagnoses of schizoaffective disorder–bipolar type (Table 11), schizophrenia, or bipolar disorder could not be affirmed.

Continue to: The authors' observations
The authors’ observations
During Ms. D’s LTSR placement, it became clear that her self-harm behaviors and numerous visits to the ED and urgent care facilities involved severe and intense emotional dysregulation and maladaptive behaviors. These behaviors had developed over time in response to acute stressors and past trauma, and not as a result of a sustained mood or psychotic disorder. Before her LTSR placement, Ms. D was unable to use more adaptive coping skills, such as skills building, learning, and coaching. Ms. D typically “thrived” with medical attention in the ED or hospital, and once the stressor dissipated, she was discharged back to the same stressful living environment associated with her maladaptive coping.
Table 2 outlines the rationale for long-term residential treatment for Ms. D.

TREATMENT Developing more effective skills
Bolstered by a clearer diagnostic formulation of BPD, Ms. D’s initial treatment goals at the LTSR include developing effective skills (eg, mindfulness, interpersonal effectiveness, emotion regulation, and distress tolerance) to cope with family conflicts and other stressors while she is outside the facility on a therapeutic pass. Ms. D’s treatment focuses on skills learning and coaching, and behavior chain analyses, which are conducted by her therapist from the ACT program.
Ms. D remains clinically stable throughout her LTSR placement, and benefits from ongoing skills building and learning, coaching, and community integration efforts.
[polldaddy:10528348]
The authors’ observations
Several systematic reviews2-5 have found that there is a lack of high-quality evidence for the use of various psychotropic medications for patients with BPD, yet polypharmacy is common. Many patients with BPD receive ≥2 medications and >25% of patients receive ≥4 medications, typically for prolonged periods. Stoffers et al4 suggested that FGAs and antidepressants have marginal effects of for patients with BPD; however, their use cannot be ruled out because they may be helpful for comorbid symptoms that are often observed in patients with BPD. There is better evidence for SGAs, mood stabilizers, and omega-3 fatty acids; however, most effect estimates were based on single studies, and there is minimal data on long-term use of these agents.4
Continue to: A recent review highlighted...
A recent review highlighted 2 trends in medication prescribing for individuals with BPD3:
- a decrease in the use of benzodiazepines and antidepressants
- an increase in or preference for mood stabilizers and SGAs, especially valproate and quetiapine.
In terms of which medications can be used to target specific symptoms, the same researchers also noted from previous studies3:
- The prior use of SSRIs to target affective dysregulation, anxiety, and impulsive- behavior dyscontrol
- mood stabilizers (notably anticonvulsants) and SGAs to target “core symptoms” of BPD, including affective dysregulation, impulsive-behavioral dyscontrol, and cognitive-perceptual distortions
- omega-3 fatty acids for mood stabilization, impulsive-behavior dyscontrol, and possibly to reduce self-harm behaviors.
TREATMENT Medication adjustments
The treatment team reviews the lack of evidence for the long-term use of psychotropic medications in the treatment of BPD with Ms. D and her relatives,2-5 and develops a medication regimen that is clinically appropriate for managing the symptoms of BPD, while also being mindful of adverse effects.
When Ms. D was admitted to the LTSR from the hospital, her psychotropic medication regimen included haloperidol, 150 mg IM every month; olanzapine, 20 mg at bedtime; benztropine, 1 mg twice daily; and melatonin, 9 mg at bedtime.
Following discussions with Ms. D and her older sister, the team initiates a taper of olanzapine because of metabolic concerns. Ms. D has gained >40 lb while receiving this medication and had hypertension. Olanzapine was tapered and discontinued over the course of 3 months with no reemergence of sustained mood or psychotic symptoms (Table 3). During this period, Ms. D also participates in dietary counselling, follows a portion-controlled regimen, and loses >30 lb. Her wellness plan focuses on nutrition and exercise to improve her overall physical health.
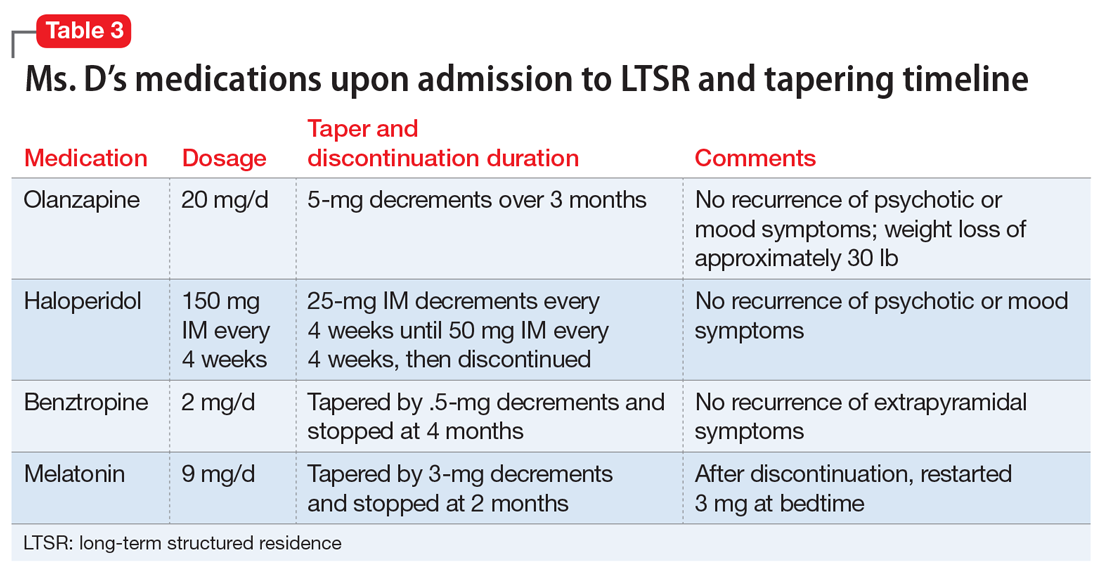
Continue to: Six months into her stay...
Six months into her stay at the LTSR, Ms. D remains clinically stable and is able to leave the LTSR placement to go on home passes. At this time, the team begins to taper the haloperidol long-acting injection. One month prior to discharge from the LTSR, haloperidol is discontinued entirely. The treatment team simultaneously tapers and discontinues benztropine. No recurrence of extrapyramidal symptoms is observed by staff or noted by the patient.
A treatment plan is developed to address Ms. D’s medical conditions, including hypothyroidism, GERD, and obesity. Ms. D does not appear to have difficulty sleeping at the LTSR, so melatonin is tapered by 3-mg decrements and stopped after 2 months. However, shortly thereafter, she develops insomnia, so a 3-mg dose is re-initiated, and her complaints abate. Her primary care physician discontinues hydrochlorothiazide, an antihypertensive medication.
Ms. D’s medication regimen consists of melatonin, 3 mg at bedtime; pantoprazole, 40 mg before breakfast, for GERD; senna, 8.6 mg at bedtime, and polyethylene glycol, 17 gm/d, for constipation; levothyroxine, 125 mcg/d, for hypothyroidism; metoprolol extended-release, 50 mg/d, for hypertension; and ferrous sulfate, 325 mg/d, for iron deficiency anemia.
OUTCOME Improved functioning
After 11 months at the LTSR, Ms. D is discharged home. She continues to receive outpatient services in the community through the ACT program, meeting with her therapist for cognitive-behavioral therapy, skills building and learning, and integration.
Approximately 9 months later, Ms. D is re-started on an SSRI (sertraline, 50 mg/d, which is increased to 100 mg/d 9 months later) to target symptoms of anxiety, which primarily manifest as excessive worrying. Hydroxyzine, 50 mg 3 times daily as needed, is added to this regimen, for breakthrough anxiety symptoms. Hydroxyzine is prescribed instead of a benzodiazepine to avoid potential addiction and abuse.
Continue to: Oral ziprasidone...
Oral ziprasidone, 20 mg/d twice daily, is initiated during 2 brief inpatient psychiatric admissions; however, it is successfully tapered within 1 week of discharge, in partnership with the ACT program.
In the 23 months after her discharge, Ms. D has had 1 ED visit and 2 brief inpatient psychiatric hospitalizations, which is markedly fewer encounters than she had in the 2 years before her LTSR placement. She has also lost an additional 30 lb since her LTSR discharge through a healthy diet and exercise.
Ms. D is now considering transitioning to living independently in the community through a residential supported housing program.
Bottom Line
Psychotic symptoms in patients with borderline personality disorder (BPD) are typically fleeting and mostly occur in the context of intense interpersonal conflicts and real or imagined abandonment. Long-term structured residence placement for patients with BPD can allow for careful formulation of a treatment plan, and help patients gain effective skills to cope with difficult family dynamics and other stressors, with the ultimate goal of gradual community integration.
Related Resource
- National Education Alliance for Borderline Personality Disorder. https://www.borderlinepersonalitydisorder.org.
Drug Brand Names
Benztropine • Cogentin
Haloperidol • Haldol
Hydrochlorothiazide • Microzide, HydroDiuril
Hydroxyzine • Vistaril
Levothyroxine • Synthroid,
Metoprolol ER • Toprol XL
Olanzapine • Zyprexa
Pantoprazole • Protonix
Polyethylene glycol • MiraLax, Glycolax
Quetiapine • Seroquel
Senna • Senokot
Sertraline • Zoloft
Valproate • Depakene, Depakote
Ziprasidone • Geodon
1. Diagnostic and Statistical Manual of Mental Disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
2. Hancock-Johnson E, Griffiths C, Picchioni M. A focused systematic review of pharmacological treatment for borderline personality disorder. CNS Drugs. 2017;31:345-356.
3. Starcevic V, Janca A. Pharmacotherapy of borderline personality disorder: replacing confusion with prudent pragmatism. Curr Opin Psychiatry. 2018;31(1):69-73.
4. Stoffers J, Völlm BA, Rücker G, et al. Pharmacological interventions for borderline personality disorder. Cochrane Database Syst Rev. 2010;6:CD005653. doi: 10.1002/14651858.CD005653.pub2.
5. Stoffers-Winterling JM, Storebo OJ, Völlm BA, et al. Pharmacological interventions for borderline personality disorder. Cochrane Database Syst Rev. 2018;3:CD012956. doi: 10.1002/14651858.CD012956.
CASE Frequent hospitalizations
Ms. D, age 26, presents to the emergency department (ED) after drinking a bottle of hand sanitizer in a suicide attempt. She is admitted to an inpatient psychiatric unit, where she spends 50 days, followed by a transfer to a step-down unit, where she spends 26 days. Upon discharge, her diagnosis is schizoaffective disorder–bipolar type.
Shortly before this, Ms. D had intentionally ingested 20 vitamin pills to “make her heart stop” after a conflict at home. After ingesting the pills, Ms. D presented to the ED, where she stated that if she were discharged, she would kill herself by taking “better pills.” She was then admitted to an inpatient psychiatric unit, where she spent 60 days before being moved to an extended-care step-down facility, where she resided for 42 days.
HISTORY A challenging past
Ms. D has a history of >25 psychiatric hospitalizations with varying discharge diagnoses, including schizophrenia, schizoaffective disorder, borderline personality disorder (BPD), and borderline intellectual functioning.
Ms. D was raised in a 2-parent home with 3 older half-brothers and 3 sisters. She was sexually assaulted by a cousin when she was 12. Ms. D recalls one event of self-injury/cutting behavior at age 15 after she was bullied by peers. Her family history is significant for schizophrenia (mother), alcohol use disorder (both parents), and bipolar disorder (sister). Her mother, who is now deceased, was admitted to state psychiatric hospitals for extended periods.
Her medication regimen has changed with nearly every hospitalization but generally has included ≥1 antipsychotic, a mood stabilizer, an antidepressant, and a benzodiazepine (often prescribed on an as-needed basis). Ms. D is obese and has difficulty sleeping, hypothyroidism, gastroesophageal reflux disease (GERD), hypertension, and iron deficiency anemia. She receives medications to manage each of these conditions.
Ms. D’s previous psychotic symptoms included auditory command hallucinations. These occurred under stressful circumstances, such as during severe family conflicts that often led to her feeling abandoned. She reported that the “voice” she heard was usually her own instructing her to “take pills.” There was no prior evidence of bizarre delusions, negative symptoms, or disorganized thoughts or speech.
During episodes of decompensation, Ms. D did not report symptoms of mania, sustained depressed mood, or anxiety, nor were these symptoms observed. Although Ms. D endorsed suicidal ideation with a plan, intent, and means, during several of her previous ED presentations, she told clinicians that her intent was not to end her life but rather to evoke concern in her family members.
Continue to: After her mother died...
After her mother died when Ms. D was 19, she began to have nightmares of wanting to hurt herself and others and began experiencing multiple hospitalizations. In 2010, Ms. D was referred to an assertive community treatment (ACT) program for individuals age 16 to 27 because of her inability to participate in traditional community-based services and her historical need for advanced services, in order to provide psychiatric care in the least restrictive means possible.
Despite receiving intensive ACT services, and in addition to the numerous inpatient psychiatric hospitalizations, over 7 years, Ms. D accumulated 8 additional general-medical hospitalizations and >50 visits to hospital EDs and urgent care facilities. These hospitalizations typically followed arguments at home, strained family dynamics, and not feeling wanted. Ms. D would ingest large quantities of prescription or over-the-counter medications as a way of coping, which often occurred while she was residing in a step-down facility after hospital discharge.
[polldaddy:10528342]
The authors’ observations
The treatment team decided to transition Ms. D to an LTSR with full continuum of treatment. While some clinicians might be concerned with potential iatrogenic harm of LTSR placement and might instead recommend less restrictive residential support and an IOP. However, in Ms. D’s case, her numerous admissions to EDs, urgent care facilities, and medical and psychiatric hospitals, her failed step-down facility placements, and her family conflicts and poor dynamics limited the efficacy of her natural support system and drove the recommendation for an LTSR.
Previously, Ms. D’s experience with ACT services had centered on managing acute crises, with brief periods of stabilization that insufficiently engaged her in a consistent and meaningful treatment plan. Ms. D’s insurance company agreed to pay for the LTSR after lengthy discussions with the clinical leadership at the ACT program and the LTSR demonstrated that she was a high utilizer of health care services. They concluded that Ms. D’s stay at the LTSR would be less expensive than the frequent use of expensive hospital services and care.
EVALUATION A consensus on the diagnosis
During the first few weeks of Ms. D’s admission to the LTSR, the treatment team takes a thorough history and reviews her medical records, which they obtained from several past inpatient admissions and therapists who previously treated Ms. D. The team also collects collateral information from Ms. D’s family members. Based on this information, interviews, and composite behavioral observations from the first few weeks of Ms. D’s time at the LTSR, the psychiatrists and treatment team at the LTSR and ACT program determine that Ms. D meets the criteria for a primary diagnosis of BPD. Previous discharge diagnoses of schizoaffective disorder–bipolar type (Table 11), schizophrenia, or bipolar disorder could not be affirmed.

Continue to: The authors' observations
The authors’ observations
During Ms. D’s LTSR placement, it became clear that her self-harm behaviors and numerous visits to the ED and urgent care facilities involved severe and intense emotional dysregulation and maladaptive behaviors. These behaviors had developed over time in response to acute stressors and past trauma, and not as a result of a sustained mood or psychotic disorder. Before her LTSR placement, Ms. D was unable to use more adaptive coping skills, such as skills building, learning, and coaching. Ms. D typically “thrived” with medical attention in the ED or hospital, and once the stressor dissipated, she was discharged back to the same stressful living environment associated with her maladaptive coping.
Table 2 outlines the rationale for long-term residential treatment for Ms. D.

TREATMENT Developing more effective skills
Bolstered by a clearer diagnostic formulation of BPD, Ms. D’s initial treatment goals at the LTSR include developing effective skills (eg, mindfulness, interpersonal effectiveness, emotion regulation, and distress tolerance) to cope with family conflicts and other stressors while she is outside the facility on a therapeutic pass. Ms. D’s treatment focuses on skills learning and coaching, and behavior chain analyses, which are conducted by her therapist from the ACT program.
Ms. D remains clinically stable throughout her LTSR placement, and benefits from ongoing skills building and learning, coaching, and community integration efforts.
[polldaddy:10528348]
The authors’ observations
Several systematic reviews2-5 have found that there is a lack of high-quality evidence for the use of various psychotropic medications for patients with BPD, yet polypharmacy is common. Many patients with BPD receive ≥2 medications and >25% of patients receive ≥4 medications, typically for prolonged periods. Stoffers et al4 suggested that FGAs and antidepressants have marginal effects of for patients with BPD; however, their use cannot be ruled out because they may be helpful for comorbid symptoms that are often observed in patients with BPD. There is better evidence for SGAs, mood stabilizers, and omega-3 fatty acids; however, most effect estimates were based on single studies, and there is minimal data on long-term use of these agents.4
Continue to: A recent review highlighted...
A recent review highlighted 2 trends in medication prescribing for individuals with BPD3:
- a decrease in the use of benzodiazepines and antidepressants
- an increase in or preference for mood stabilizers and SGAs, especially valproate and quetiapine.
In terms of which medications can be used to target specific symptoms, the same researchers also noted from previous studies3:
- The prior use of SSRIs to target affective dysregulation, anxiety, and impulsive- behavior dyscontrol
- mood stabilizers (notably anticonvulsants) and SGAs to target “core symptoms” of BPD, including affective dysregulation, impulsive-behavioral dyscontrol, and cognitive-perceptual distortions
- omega-3 fatty acids for mood stabilization, impulsive-behavior dyscontrol, and possibly to reduce self-harm behaviors.
TREATMENT Medication adjustments
The treatment team reviews the lack of evidence for the long-term use of psychotropic medications in the treatment of BPD with Ms. D and her relatives,2-5 and develops a medication regimen that is clinically appropriate for managing the symptoms of BPD, while also being mindful of adverse effects.
When Ms. D was admitted to the LTSR from the hospital, her psychotropic medication regimen included haloperidol, 150 mg IM every month; olanzapine, 20 mg at bedtime; benztropine, 1 mg twice daily; and melatonin, 9 mg at bedtime.
Following discussions with Ms. D and her older sister, the team initiates a taper of olanzapine because of metabolic concerns. Ms. D has gained >40 lb while receiving this medication and had hypertension. Olanzapine was tapered and discontinued over the course of 3 months with no reemergence of sustained mood or psychotic symptoms (Table 3). During this period, Ms. D also participates in dietary counselling, follows a portion-controlled regimen, and loses >30 lb. Her wellness plan focuses on nutrition and exercise to improve her overall physical health.

Continue to: Six months into her stay...
Six months into her stay at the LTSR, Ms. D remains clinically stable and is able to leave the LTSR placement to go on home passes. At this time, the team begins to taper the haloperidol long-acting injection. One month prior to discharge from the LTSR, haloperidol is discontinued entirely. The treatment team simultaneously tapers and discontinues benztropine. No recurrence of extrapyramidal symptoms is observed by staff or noted by the patient.
A treatment plan is developed to address Ms. D’s medical conditions, including hypothyroidism, GERD, and obesity. Ms. D does not appear to have difficulty sleeping at the LTSR, so melatonin is tapered by 3-mg decrements and stopped after 2 months. However, shortly thereafter, she develops insomnia, so a 3-mg dose is re-initiated, and her complaints abate. Her primary care physician discontinues hydrochlorothiazide, an antihypertensive medication.
Ms. D’s medication regimen consists of melatonin, 3 mg at bedtime; pantoprazole, 40 mg before breakfast, for GERD; senna, 8.6 mg at bedtime, and polyethylene glycol, 17 gm/d, for constipation; levothyroxine, 125 mcg/d, for hypothyroidism; metoprolol extended-release, 50 mg/d, for hypertension; and ferrous sulfate, 325 mg/d, for iron deficiency anemia.
OUTCOME Improved functioning
After 11 months at the LTSR, Ms. D is discharged home. She continues to receive outpatient services in the community through the ACT program, meeting with her therapist for cognitive-behavioral therapy, skills building and learning, and integration.
Approximately 9 months later, Ms. D is re-started on an SSRI (sertraline, 50 mg/d, which is increased to 100 mg/d 9 months later) to target symptoms of anxiety, which primarily manifest as excessive worrying. Hydroxyzine, 50 mg 3 times daily as needed, is added to this regimen, for breakthrough anxiety symptoms. Hydroxyzine is prescribed instead of a benzodiazepine to avoid potential addiction and abuse.
Continue to: Oral ziprasidone...
Oral ziprasidone, 20 mg/d twice daily, is initiated during 2 brief inpatient psychiatric admissions; however, it is successfully tapered within 1 week of discharge, in partnership with the ACT program.
In the 23 months after her discharge, Ms. D has had 1 ED visit and 2 brief inpatient psychiatric hospitalizations, which is markedly fewer encounters than she had in the 2 years before her LTSR placement. She has also lost an additional 30 lb since her LTSR discharge through a healthy diet and exercise.
Ms. D is now considering transitioning to living independently in the community through a residential supported housing program.
Bottom Line
Psychotic symptoms in patients with borderline personality disorder (BPD) are typically fleeting and mostly occur in the context of intense interpersonal conflicts and real or imagined abandonment. Long-term structured residence placement for patients with BPD can allow for careful formulation of a treatment plan, and help patients gain effective skills to cope with difficult family dynamics and other stressors, with the ultimate goal of gradual community integration.
Related Resource
- National Education Alliance for Borderline Personality Disorder. https://www.borderlinepersonalitydisorder.org.
Drug Brand Names
Benztropine • Cogentin
Haloperidol • Haldol
Hydrochlorothiazide • Microzide, HydroDiuril
Hydroxyzine • Vistaril
Levothyroxine • Synthroid,
Metoprolol ER • Toprol XL
Olanzapine • Zyprexa
Pantoprazole • Protonix
Polyethylene glycol • MiraLax, Glycolax
Quetiapine • Seroquel
Senna • Senokot
Sertraline • Zoloft
Valproate • Depakene, Depakote
Ziprasidone • Geodon
CASE Frequent hospitalizations
Ms. D, age 26, presents to the emergency department (ED) after drinking a bottle of hand sanitizer in a suicide attempt. She is admitted to an inpatient psychiatric unit, where she spends 50 days, followed by a transfer to a step-down unit, where she spends 26 days. Upon discharge, her diagnosis is schizoaffective disorder–bipolar type.
Shortly before this, Ms. D had intentionally ingested 20 vitamin pills to “make her heart stop” after a conflict at home. After ingesting the pills, Ms. D presented to the ED, where she stated that if she were discharged, she would kill herself by taking “better pills.” She was then admitted to an inpatient psychiatric unit, where she spent 60 days before being moved to an extended-care step-down facility, where she resided for 42 days.
HISTORY A challenging past
Ms. D has a history of >25 psychiatric hospitalizations with varying discharge diagnoses, including schizophrenia, schizoaffective disorder, borderline personality disorder (BPD), and borderline intellectual functioning.
Ms. D was raised in a 2-parent home with 3 older half-brothers and 3 sisters. She was sexually assaulted by a cousin when she was 12. Ms. D recalls one event of self-injury/cutting behavior at age 15 after she was bullied by peers. Her family history is significant for schizophrenia (mother), alcohol use disorder (both parents), and bipolar disorder (sister). Her mother, who is now deceased, was admitted to state psychiatric hospitals for extended periods.
Her medication regimen has changed with nearly every hospitalization but generally has included ≥1 antipsychotic, a mood stabilizer, an antidepressant, and a benzodiazepine (often prescribed on an as-needed basis). Ms. D is obese and has difficulty sleeping, hypothyroidism, gastroesophageal reflux disease (GERD), hypertension, and iron deficiency anemia. She receives medications to manage each of these conditions.
Ms. D’s previous psychotic symptoms included auditory command hallucinations. These occurred under stressful circumstances, such as during severe family conflicts that often led to her feeling abandoned. She reported that the “voice” she heard was usually her own instructing her to “take pills.” There was no prior evidence of bizarre delusions, negative symptoms, or disorganized thoughts or speech.
During episodes of decompensation, Ms. D did not report symptoms of mania, sustained depressed mood, or anxiety, nor were these symptoms observed. Although Ms. D endorsed suicidal ideation with a plan, intent, and means, during several of her previous ED presentations, she told clinicians that her intent was not to end her life but rather to evoke concern in her family members.
Continue to: After her mother died...
After her mother died when Ms. D was 19, she began to have nightmares of wanting to hurt herself and others and began experiencing multiple hospitalizations. In 2010, Ms. D was referred to an assertive community treatment (ACT) program for individuals age 16 to 27 because of her inability to participate in traditional community-based services and her historical need for advanced services, in order to provide psychiatric care in the least restrictive means possible.
Despite receiving intensive ACT services, and in addition to the numerous inpatient psychiatric hospitalizations, over 7 years, Ms. D accumulated 8 additional general-medical hospitalizations and >50 visits to hospital EDs and urgent care facilities. These hospitalizations typically followed arguments at home, strained family dynamics, and not feeling wanted. Ms. D would ingest large quantities of prescription or over-the-counter medications as a way of coping, which often occurred while she was residing in a step-down facility after hospital discharge.
[polldaddy:10528342]
The authors’ observations
The treatment team decided to transition Ms. D to an LTSR with full continuum of treatment. While some clinicians might be concerned with potential iatrogenic harm of LTSR placement and might instead recommend less restrictive residential support and an IOP. However, in Ms. D’s case, her numerous admissions to EDs, urgent care facilities, and medical and psychiatric hospitals, her failed step-down facility placements, and her family conflicts and poor dynamics limited the efficacy of her natural support system and drove the recommendation for an LTSR.
Previously, Ms. D’s experience with ACT services had centered on managing acute crises, with brief periods of stabilization that insufficiently engaged her in a consistent and meaningful treatment plan. Ms. D’s insurance company agreed to pay for the LTSR after lengthy discussions with the clinical leadership at the ACT program and the LTSR demonstrated that she was a high utilizer of health care services. They concluded that Ms. D’s stay at the LTSR would be less expensive than the frequent use of expensive hospital services and care.
EVALUATION A consensus on the diagnosis
During the first few weeks of Ms. D’s admission to the LTSR, the treatment team takes a thorough history and reviews her medical records, which they obtained from several past inpatient admissions and therapists who previously treated Ms. D. The team also collects collateral information from Ms. D’s family members. Based on this information, interviews, and composite behavioral observations from the first few weeks of Ms. D’s time at the LTSR, the psychiatrists and treatment team at the LTSR and ACT program determine that Ms. D meets the criteria for a primary diagnosis of BPD. Previous discharge diagnoses of schizoaffective disorder–bipolar type (Table 11), schizophrenia, or bipolar disorder could not be affirmed.

Continue to: The authors' observations
The authors’ observations
During Ms. D’s LTSR placement, it became clear that her self-harm behaviors and numerous visits to the ED and urgent care facilities involved severe and intense emotional dysregulation and maladaptive behaviors. These behaviors had developed over time in response to acute stressors and past trauma, and not as a result of a sustained mood or psychotic disorder. Before her LTSR placement, Ms. D was unable to use more adaptive coping skills, such as skills building, learning, and coaching. Ms. D typically “thrived” with medical attention in the ED or hospital, and once the stressor dissipated, she was discharged back to the same stressful living environment associated with her maladaptive coping.
Table 2 outlines the rationale for long-term residential treatment for Ms. D.

TREATMENT Developing more effective skills
Bolstered by a clearer diagnostic formulation of BPD, Ms. D’s initial treatment goals at the LTSR include developing effective skills (eg, mindfulness, interpersonal effectiveness, emotion regulation, and distress tolerance) to cope with family conflicts and other stressors while she is outside the facility on a therapeutic pass. Ms. D’s treatment focuses on skills learning and coaching, and behavior chain analyses, which are conducted by her therapist from the ACT program.
Ms. D remains clinically stable throughout her LTSR placement, and benefits from ongoing skills building and learning, coaching, and community integration efforts.
[polldaddy:10528348]
The authors’ observations
Several systematic reviews2-5 have found that there is a lack of high-quality evidence for the use of various psychotropic medications for patients with BPD, yet polypharmacy is common. Many patients with BPD receive ≥2 medications and >25% of patients receive ≥4 medications, typically for prolonged periods. Stoffers et al4 suggested that FGAs and antidepressants have marginal effects of for patients with BPD; however, their use cannot be ruled out because they may be helpful for comorbid symptoms that are often observed in patients with BPD. There is better evidence for SGAs, mood stabilizers, and omega-3 fatty acids; however, most effect estimates were based on single studies, and there is minimal data on long-term use of these agents.4
Continue to: A recent review highlighted...
A recent review highlighted 2 trends in medication prescribing for individuals with BPD3:
- a decrease in the use of benzodiazepines and antidepressants
- an increase in or preference for mood stabilizers and SGAs, especially valproate and quetiapine.
In terms of which medications can be used to target specific symptoms, the same researchers also noted from previous studies3:
- The prior use of SSRIs to target affective dysregulation, anxiety, and impulsive- behavior dyscontrol
- mood stabilizers (notably anticonvulsants) and SGAs to target “core symptoms” of BPD, including affective dysregulation, impulsive-behavioral dyscontrol, and cognitive-perceptual distortions
- omega-3 fatty acids for mood stabilization, impulsive-behavior dyscontrol, and possibly to reduce self-harm behaviors.
TREATMENT Medication adjustments
The treatment team reviews the lack of evidence for the long-term use of psychotropic medications in the treatment of BPD with Ms. D and her relatives,2-5 and develops a medication regimen that is clinically appropriate for managing the symptoms of BPD, while also being mindful of adverse effects.
When Ms. D was admitted to the LTSR from the hospital, her psychotropic medication regimen included haloperidol, 150 mg IM every month; olanzapine, 20 mg at bedtime; benztropine, 1 mg twice daily; and melatonin, 9 mg at bedtime.
Following discussions with Ms. D and her older sister, the team initiates a taper of olanzapine because of metabolic concerns. Ms. D has gained >40 lb while receiving this medication and had hypertension. Olanzapine was tapered and discontinued over the course of 3 months with no reemergence of sustained mood or psychotic symptoms (Table 3). During this period, Ms. D also participates in dietary counselling, follows a portion-controlled regimen, and loses >30 lb. Her wellness plan focuses on nutrition and exercise to improve her overall physical health.

Continue to: Six months into her stay...
Six months into her stay at the LTSR, Ms. D remains clinically stable and is able to leave the LTSR placement to go on home passes. At this time, the team begins to taper the haloperidol long-acting injection. One month prior to discharge from the LTSR, haloperidol is discontinued entirely. The treatment team simultaneously tapers and discontinues benztropine. No recurrence of extrapyramidal symptoms is observed by staff or noted by the patient.
A treatment plan is developed to address Ms. D’s medical conditions, including hypothyroidism, GERD, and obesity. Ms. D does not appear to have difficulty sleeping at the LTSR, so melatonin is tapered by 3-mg decrements and stopped after 2 months. However, shortly thereafter, she develops insomnia, so a 3-mg dose is re-initiated, and her complaints abate. Her primary care physician discontinues hydrochlorothiazide, an antihypertensive medication.
Ms. D’s medication regimen consists of melatonin, 3 mg at bedtime; pantoprazole, 40 mg before breakfast, for GERD; senna, 8.6 mg at bedtime, and polyethylene glycol, 17 gm/d, for constipation; levothyroxine, 125 mcg/d, for hypothyroidism; metoprolol extended-release, 50 mg/d, for hypertension; and ferrous sulfate, 325 mg/d, for iron deficiency anemia.
OUTCOME Improved functioning
After 11 months at the LTSR, Ms. D is discharged home. She continues to receive outpatient services in the community through the ACT program, meeting with her therapist for cognitive-behavioral therapy, skills building and learning, and integration.
Approximately 9 months later, Ms. D is re-started on an SSRI (sertraline, 50 mg/d, which is increased to 100 mg/d 9 months later) to target symptoms of anxiety, which primarily manifest as excessive worrying. Hydroxyzine, 50 mg 3 times daily as needed, is added to this regimen, for breakthrough anxiety symptoms. Hydroxyzine is prescribed instead of a benzodiazepine to avoid potential addiction and abuse.
Continue to: Oral ziprasidone...
Oral ziprasidone, 20 mg/d twice daily, is initiated during 2 brief inpatient psychiatric admissions; however, it is successfully tapered within 1 week of discharge, in partnership with the ACT program.
In the 23 months after her discharge, Ms. D has had 1 ED visit and 2 brief inpatient psychiatric hospitalizations, which is markedly fewer encounters than she had in the 2 years before her LTSR placement. She has also lost an additional 30 lb since her LTSR discharge through a healthy diet and exercise.
Ms. D is now considering transitioning to living independently in the community through a residential supported housing program.
Bottom Line
Psychotic symptoms in patients with borderline personality disorder (BPD) are typically fleeting and mostly occur in the context of intense interpersonal conflicts and real or imagined abandonment. Long-term structured residence placement for patients with BPD can allow for careful formulation of a treatment plan, and help patients gain effective skills to cope with difficult family dynamics and other stressors, with the ultimate goal of gradual community integration.
Related Resource
- National Education Alliance for Borderline Personality Disorder. https://www.borderlinepersonalitydisorder.org.
Drug Brand Names
Benztropine • Cogentin
Haloperidol • Haldol
Hydrochlorothiazide • Microzide, HydroDiuril
Hydroxyzine • Vistaril
Levothyroxine • Synthroid,
Metoprolol ER • Toprol XL
Olanzapine • Zyprexa
Pantoprazole • Protonix
Polyethylene glycol • MiraLax, Glycolax
Quetiapine • Seroquel
Senna • Senokot
Sertraline • Zoloft
Valproate • Depakene, Depakote
Ziprasidone • Geodon
1. Diagnostic and Statistical Manual of Mental Disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
2. Hancock-Johnson E, Griffiths C, Picchioni M. A focused systematic review of pharmacological treatment for borderline personality disorder. CNS Drugs. 2017;31:345-356.
3. Starcevic V, Janca A. Pharmacotherapy of borderline personality disorder: replacing confusion with prudent pragmatism. Curr Opin Psychiatry. 2018;31(1):69-73.
4. Stoffers J, Völlm BA, Rücker G, et al. Pharmacological interventions for borderline personality disorder. Cochrane Database Syst Rev. 2010;6:CD005653. doi: 10.1002/14651858.CD005653.pub2.
5. Stoffers-Winterling JM, Storebo OJ, Völlm BA, et al. Pharmacological interventions for borderline personality disorder. Cochrane Database Syst Rev. 2018;3:CD012956. doi: 10.1002/14651858.CD012956.
1. Diagnostic and Statistical Manual of Mental Disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
2. Hancock-Johnson E, Griffiths C, Picchioni M. A focused systematic review of pharmacological treatment for borderline personality disorder. CNS Drugs. 2017;31:345-356.
3. Starcevic V, Janca A. Pharmacotherapy of borderline personality disorder: replacing confusion with prudent pragmatism. Curr Opin Psychiatry. 2018;31(1):69-73.
4. Stoffers J, Völlm BA, Rücker G, et al. Pharmacological interventions for borderline personality disorder. Cochrane Database Syst Rev. 2010;6:CD005653. doi: 10.1002/14651858.CD005653.pub2.
5. Stoffers-Winterling JM, Storebo OJ, Völlm BA, et al. Pharmacological interventions for borderline personality disorder. Cochrane Database Syst Rev. 2018;3:CD012956. doi: 10.1002/14651858.CD012956.
Depression, or something else?
CASE Suicidal behavior, severe headaches
Ms. A, age 60, presents to the emergency department (ED) with depression, suicidal behavior, and 3 days of severe headaches. Neurology is consulted and an MRI is ordered, which shows a 3.0-cm mass lesion in the left temporal lobe with associated vasogenic edema that is suspicious for metastatic disease (Figure).
Ms. A is admitted to the hospital for further workup of her brain lesion. She is started on IV dexamethasone, 10 mg every 6 hours, a glucocorticosteroid, for brain edema, and levetiracetam, 500 mg twice a day, for seizure prophylaxis.
Upon admission, in addition to oncology and neurosurgery, psychiatry is also consulted to evaluate Ms. A for depression and suicidality.
EVALUATION Mood changes and poor judgment
Ms. A has a psychiatric history of depression and alcohol use disorder but says she has not consumed any alcohol in years. Her medical history includes hypertension, diabetes, and stage 4 non-small–cell lung cancer, for which she received surgery and adjuvant chemoradiotherapy 1 year ago.
On initial intake, Ms. A reports that in addition to the headaches, she has also been experiencing worsening depression and suicidal behavior. For the past 2 months, she has had a severely depressed mood, with notable anhedonia, poor appetite, insomnia, low energy, and decreased concentration. The changes in her mental health were triggered by her mother’s death. Three days prior to admission, the patient planned to overdose on antihypertensive pills, but her suicide attempt was interrupted when her family called. She denies any current suicidal ideation, intent, or plan.
According to her family, Ms. A has been increasingly irritable and her personality has changed in the past month. She also has been repeatedly sorting through her neighbors’ garbage.
Ms. A’s current psychiatric medications are duloxetine, 30 mg/d; quetiapine, 50 mg every night at bedtime; and buspirone, 10 mg/d. However, it is unclear if she is consistently taking these medications.
Continue to: On mental status examination...
On mental status examination, Ms. A is calm and she has no abnormal movements. She says she is depressed. Her affect is reactive and labile. She is alert and oriented to person, place, and time. Her attention, registration, and recall are intact. Her executive function is not tested. However, Ms. A’s insight and judgment seem poor.
To address Ms. A’s worsening depression, the psychiatry team increases her duloxetine from 30 to 60 mg/d, and she continues quetiapine, 50 mg every night at bedtime, for mood lability. Buspirone is not continued because she was not taking a therapeutic dosage in the community.
Within 4 days, Ms. A shows improvement in sleep, appetite, and mood. She has no further suicidal ideation.
[polldaddy:10511743]
The authors’ observations
Ms. A had a recurrence of what was presumed to be major depressive disorder (MDD) in the context of her mother’s death. However, she also exhibited irritability, mood lability, and impulsivity, all of which could be part of her depression, or a separate problem related to her brain tumor. Because Ms. A had never displayed bizarre behavior before the past few weeks, it is likely that her CNS lesion was directly affecting her personality and possibly underlying her planned suicide attempt.
Fifty to 80% of patients with CNS tumors, either primary or metastatic, present with psychiatric symptoms.1 Table 11-3 lists common psychiatric symptoms of brain tumors. Unfortunately, there is little reliable evidence that directly correlates tumor location with specific psychiatric symptoms. A 2010 meta-analysis found a statistically significant link between anorexia nervosa and hypothalamic tumors.1 However, for other brain regions, there is only an increased likelihood that any given tumor location will produce psychiatric symptoms.1,4 For instance, compared to patients with tumors in other locations, those with temporal lobe tumors are more likely to present with mood disorders, personality changes, and memory problems.1 In contrast, patients with frontal lobe tumors have an increased likelihood of psychosis, mood disorders, and personality changes.1 Patients with tumors in the pituitary region often present with anxiety.1
Continue to: When considering treatment options...
When considering treatment options for Ms. A, alcohol withdrawal was unlikely given the remote history of alcohol use, low alcohol blood level, and lack of evidence of unstable vital signs or tremor. Although she might have benefited from inpatient psychiatric treatment, this needed to wait until there was a definitive treatment plan for her brain tumor. Finally, although a paraneoplastic syndrome, such as limbic encephalitis, could be causing her psychiatric symptoms, this scenario is less likely with non-small–cell lung cancer.
Although uncommon, CNS tumors can present with psychiatric symptoms as the only manifestation. This is more likely when a patient exhibits new-onset or atypical symptoms, or fails to respond to standard psychiatric treatment.4 Case reports have described patients with brain tumors being misdiagnosed as having a primary psychiatric condition, which delays treatment of their CNS cancer.2 Additionally, frontal and limbic tumors are more likely to present with psychiatric manifestations; up to 90% of patients exhibit altered mental status or personality changes, as did Ms. A.1,4 Clearly, it is easier to identify patients with psychiatric symptoms resulting from a brain tumor when they also present with focal neurologic deficits or systemic symptoms, such as headache or nausea and vomiting. Ms. A presented with severe headaches, which is what led to her early imaging and prompt diagnosis.
Numerous proposed mechanisms might account for the psychiatric symptoms that occur during the course of a brain tumor, including direct injury to neuronal cells, secretion of hormones or other tumor-derived substances, and peri-ictal phenomena.3
TREATMENT Tumor is removed, but memory is impaired
Ms. A is scheduled for craniotomy and surgical resection of the frontal mass. Prior to surgery, Ms. A shows interest in improving her health, cooperates with staff, and seeks her daughter’s input on treatment. One week after admission, Ms. A has her mass resected, which is confirmed on biopsy to be a lung metastasis. Post-surgery, Ms. A receives codeine, 30 mg every 6 hours as needed, for pain; she continues dexamethasone, 4 mg IV every 6 hours, for brain edema and levetiracetam, 500 mg twice a day, for seizure prophylaxis.
On Day 2 after surgery, Ms. A attempts to elope. When she is approached by a psychiatrist on the treatment team, she does not recognize him. Although her long-term memory seems intact, she is unable to remember the details of recent events, including her medical and surgical treatments.
[polldaddy:10511745]
Continue to: The authors' observations
The authors’ observations
Ms. A’s memory impairment may be secondary to a surgically acquired neurocognitive deficit. In the United States, brain metastases represent a significant public health issue, affecting >100,000 patients per year.5 Metastatic lesions are the most common brain tumors. Lung cancer, breast cancer, and melanoma are the leading solid tumors to spread to the CNS.5 In cases of single brain metastasis, similar to Ms. A’s solitary left temporal lobe lesion, surgical resection plays a critical role in treatment. It provides histological confirmation of metastatic disease and can relieve mass effect if present. Studies have shown that combined surgical resection with radiation improves survival relative to patients who undergo radiation therapy alone.6,7
However, the benefits of surgical resection need to be balanced with preservation of neurologic function. Emerging evidence suggests that a majority of patients have surgically-acquired cognitive deficits due to damage of normal surrounding tissues, and these deficits are associated with reduced quality of life.8,9 Further, a study examining glioma surgical resections found that patients with left temporal lobe tumors exhibit more frequent and severe neurocognitive decline than patients with right temporal lobe tumors, especially in domains such as verbal memory.8 Ms. A’s memory impairment was persistent during her postoperative course, which suggests that it was not just an immediate post-surgical phenomenon, but a longer-lasting cognitive change directly related to the resection.
It is also possible that Ms. A had a prior neurocognitive disorder that manifested to a greater degree as a result of the CNS tumor. Ms. A might have had early-onset Alzheimer’s disease, although her intact memory before surgery makes this less likely. Alternatively, she could have had vascular dementia, especially given her long-standing hypertension and diabetes. This might have been missed in the initial evaluation because executive function was not tested. However, the relatively abrupt onset of memory problems after surgery suggests that she had no underlying neurocognitive disorder.
Ms. A’s presumed episode of MDD might also explain her memory changes. Major depressive disorder is increasingly common among geriatric patients, affecting approximately 5% of community-dwelling older adults.10 Its incidence increases with medical comorbidities, as suggested by depression rates of 5% to 10% in the primary care setting vs 37% in patients after critical-care hospitalizations.10 Late-life depression (LLD) occurs in adults age ≥60. Unlike depression in younger patients, LLD is more likely to be associated with cognitive impairment, specifically impairment of executive function and memory.11 The incidence of cognitive impairment in LLD is higher in patients with a history of depression, such as Ms. A.11,12 However, in general, patients who are depressed have memory complaints out of proportion to the clinical findings, and they show poor effort on cognitive testing. Ms. A exhibited neither of these, which makes it less likely that LLD was the exclusive cause of her memory loss.13 Table 214 outlines the management of cognitive deficits in a patient with a brain tumor.
EVALUATION Increasingly agitated and paranoid
After the tumor resection, Ms. A becomes increasingly irritable, uncooperative, and agitated. She repeatedly demands to be discharged. She insists she is fine and refuses medications and further laboratory workup. She becomes paranoid about the nursing staff and believes they are trying to kill her.
Continue to: On psychiatric re-evaluation...
On psychiatric re-evaluation, Ms. A demonstrates pressured speech, perseveration about going home, paranoid delusions, and anger at her family and physicians.
[polldaddy:10511747]
The authors’ observations
Ms. A’s refusal of medications and agitation may be explained by postoperative delirium, a surgical complication that is increasingly common among geriatric patients and is associated with poor clinical outcomes. Delirium is characterized by an acute onset and fluctuating course of symptoms that include inattention, motoric hypo- or hyperactivity, inappropriate behavior, emotional lability, cognitive dysfunction, and psychotic symptoms.15 Risk factors that contribute to postoperative delirium include older age, alcohol use, and poor baseline functional and cognitive status.16 The pathophysiology of delirium is not fully understood, but accumulating evidence suggests that different sets of interacting biologic factors (ie, neurotransmitters and inflammation) contribute to a disruption of large-scale neuronal networks in the brain, resulting in cognitive dysfunction.15 Patients who develop postoperative delirium are more likely to develop long-term cognitive dysfunction and have an increased risk of dementia.16
Another potential source of Ms. A’s agitation is steroid use. Ms. A received IV dexamethasone, 8 to 16 mg/d, around the time of her surgery. Steroids are commonly used to treat brain tumors, particularly when there is vasogenic edema. Steroid psychosis is a term loosely used to describe a wide range of psychiatric symptoms induced by corticosteroids that includes, but is not limited to, depression, mania, psychosis, delirium, and cognitive impairment.17 Steroid-induced psychiatric adverse effects occur in 5% to 18% of patients receiving corticosteroids and often happen early in treatment, although they can occur at any point.18 Corticosteroids influence brain activity via glucocorticoid and mineralocorticoid receptors. These receptors are widely distributed throughout the brain and affect neurotransmitter systems, such as the serotonergic system, that are associated with changes in mood, behavior, and cognition.17 While the adverse psychiatric manifestations of steroid use vary, higher dosages are associated with an increased risk of psychiatric complications; mania is more prevalent early in the course of treatment, and depression is more common with long-term use.17,19 Table 317,18 outlines the evidence-based treatment of corticosteroid-induced adverse psychiatric effects.
Although there are no clinical guidelines or FDA-approved medications for treating steroid-induced psychiatric adverse events, these are best managed by tapering and discontinuing steroids when possible and simultaneously using psychotropic medications to treat psychiatric symptoms. Case reports and limited evidence-based literature have demonstrated that steroid-induced mania responds to mood stabilizers or antipsychotics, while depression can be managed with antidepressants or lithium.17
Additionally, patients with CNS tumors are at risk for seizures and often are prescribed antiepileptics. Because it is easy to administer and does not need to be titrated, levetiracetam is a commonly used agent. However, levetiracetam can cause psychiatric adverse effects, including behavior changes and frank psychosis.20
Continue to: Finally, Ms. A's altered mental status...
Finally, Ms. A’s altered mental status could have been related to opioid intoxication. Opioids are used to manage postsurgical pain, and studies have shown these medications can be a precipitating factor for delirium in geriatric patients.21
TREATMENT Medication adjustments
At the request of the psychiatry team, levetiracetam is discontinued due to its potential for psychiatric adverse effects. The neurosurgery team replaces it with valproic acid, 500 mg every 12 hours. Ms. A is also tapered off steroids fairly rapidly because of the potential for steroid-induced psychiatric adverse effects. Her quetiapine is titrated from 50 to 150 mg every night at bedtime, and duloxetine is discontinued.
OUTCOME Agitation improves dramatically
Ms. A’s new medication regimen dramatically improves her agitation, which allows Ms. A, her family, and the medical team to work together to establish treatment goals. Ms. A ultimately returns home with the assistance of her family. She continues to have memory issues, but with improved emotion regulation. Several months later, Ms. A is readmitted to the hospital because her cancer has progressed despite treatment.
Bottom Line
Brain tumors may present with various psychiatric manifestations that can change during the course of the patient’s treatment. A comprehensive psychiatric evaluation should parse out the interplay between direct effects of the tumor and any adverse effects that are the result of medical and/or surgical interventions to determine the cause of psychiatric symptoms and their appropriate management.
Related Resource
Madhusoodanan S, Ting MB, Farah T, et al. Psychiatric aspects of brain tumors: a review. World J Psychiatry. 2015;5(3):273-285.
Drug Brand Names
Aripiprazole • Abilify
Buspirone • Buspar
Chlorpromazine • Thorazine
Codeine • Codeine systemic
Dexamethasone • Decadron
Duloxetine • Cymbalta
Haloperidol • Haldol
Levetiracetam • Keppra
Lorazepam • Ativan
Lithium • Eskalith, Lithobid
Olanzapine • Zyprexa
Quetiapine • Seroquel
Risperidone • Risperdal
Sertraline • Zoloft
Valproic acid • Depakene
1. Madhusoodanan S, Opler MG, Moise D, et al. Brain tumor location and psychiatric symptoms: is there any association? A meta-analysis of published case studies. Expert Rev Neurother. 2010;10(10):1529-1536.
2. Bunevicius A, Deltuva VP, Deltuviene D, et al. Brain lesions manifesting as psychiatric disorders: eight cases. CNS Spectr. 2008;13(11):950-958.
3. Pearl ML, Talgat G, Valea FA, et al. Psychiatric symptoms due to brain metastases. Med Update Psychiatr. 1998;3(4):91-94.
4. Madhusoodanan S, Danan D, Moise D. Psychiatric manifestations of brain tumors: diagnostic implications. Expert Rev Neurother. 2007;7(4):343-349.
5. Ferguson SD, Wagner KM, Prabhu SS, et al. Neurosurgical management of brain metastases. Clin Exp Metastasis. 2017;34(6-7):377-389.
6. Husain ZA, Regine WF, Kwok Y, et al. Brain metastases: contemporary management and future directions. Eur J Clin Med Oncol. 2011;3(3):38-45.
7. Vecht CJ, Haaxmareiche H, Noordijk EM, et al. Treatment of single brain metastasis - radiotherapy alone or combined with neurosurgery. Ann Neurol. 1993;33(6):583-590.
8. Barry RL, Byun NE, Tantawy MN, et al. In vivo neuroimaging and behavioral correlates in a rat model of chemotherapy-induced cognitive dysfunction. Brain Imaging Behav. 2018;12(1):87-95.
9. Wu AS, Witgert ME, Lang FF, et al. Neurocognitive function before and after surgery for insular gliomas. J Neurosurg. 2011;115(6):1115-1125.
10. Taylor WD. Depression in the elderly. N Engl J Med. 2014;371(13):1228-1236.
11. Liguori C, Pierantozzi M, Chiaravalloti A, et al. When cognitive decline and depression coexist in the elderly: CSF biomarkers analysis can differentiate Alzheimer’s disease from late-life depression. Front Aging Neurosci. 2018;10:38.
12. Luijendijk HJ, van den Berg JF, Dekker MJHJ, et al. Incidence and recurrence of late-life depression. Arch Gen Psychiatry. 2008;65(12):1394-1401.
13. Potter GG, Steffens DC. Contribution of depression to cognitive impairment and dementia in older adults. Neurologist. 2007;13(3):105-117.
14. Taphoorn MJB, Klein M. Cognitive deficits in adult patients with brain tumours. Lancet Neurol. 2004;3(3):159-168.
15. Inouye SK, Westendorp RGJ, Saczynski JS. Delirium in elderly people. Lancet. 2014;383(9920):911-922.
16. Sprung J, Roberts RO, Weingarten TN, et al. Postoperative delirium in elderly patients is associated with subsequent cognitive impairment. Br J Anaesth. 2017;119(2):316-323.
17. Kusljic S, Manias E, Gogos A. Corticosteroid-induced psychiatric disturbances: it is time for pharmacists to take notice. Res Soc Adm Pharm. 2016;12(2):355-360.
18. Cerullo MA. Corticosteroid-induced mania: prepare for the unpredictable. Current Psychiatry. 2006;5(6):43-50.
19. Dubovsky AN, Arvikar S, Stern TA, et al. Steroid psychosis revisited. Psychosomatics. 2012;53(2):103-115.
20. Habets JGV, Leentjens AFG, Schijns OEMG. Serious and reversible levetiracetam-induced psychiatric symptoms after resection of frontal low-grade glioma: two case histories. Br J Neurosurg. 2017;31(4):471-473.
21
CASE Suicidal behavior, severe headaches
Ms. A, age 60, presents to the emergency department (ED) with depression, suicidal behavior, and 3 days of severe headaches. Neurology is consulted and an MRI is ordered, which shows a 3.0-cm mass lesion in the left temporal lobe with associated vasogenic edema that is suspicious for metastatic disease (Figure).
Ms. A is admitted to the hospital for further workup of her brain lesion. She is started on IV dexamethasone, 10 mg every 6 hours, a glucocorticosteroid, for brain edema, and levetiracetam, 500 mg twice a day, for seizure prophylaxis.
Upon admission, in addition to oncology and neurosurgery, psychiatry is also consulted to evaluate Ms. A for depression and suicidality.
EVALUATION Mood changes and poor judgment
Ms. A has a psychiatric history of depression and alcohol use disorder but says she has not consumed any alcohol in years. Her medical history includes hypertension, diabetes, and stage 4 non-small–cell lung cancer, for which she received surgery and adjuvant chemoradiotherapy 1 year ago.
On initial intake, Ms. A reports that in addition to the headaches, she has also been experiencing worsening depression and suicidal behavior. For the past 2 months, she has had a severely depressed mood, with notable anhedonia, poor appetite, insomnia, low energy, and decreased concentration. The changes in her mental health were triggered by her mother’s death. Three days prior to admission, the patient planned to overdose on antihypertensive pills, but her suicide attempt was interrupted when her family called. She denies any current suicidal ideation, intent, or plan.
According to her family, Ms. A has been increasingly irritable and her personality has changed in the past month. She also has been repeatedly sorting through her neighbors’ garbage.
Ms. A’s current psychiatric medications are duloxetine, 30 mg/d; quetiapine, 50 mg every night at bedtime; and buspirone, 10 mg/d. However, it is unclear if she is consistently taking these medications.
Continue to: On mental status examination...
On mental status examination, Ms. A is calm and she has no abnormal movements. She says she is depressed. Her affect is reactive and labile. She is alert and oriented to person, place, and time. Her attention, registration, and recall are intact. Her executive function is not tested. However, Ms. A’s insight and judgment seem poor.
To address Ms. A’s worsening depression, the psychiatry team increases her duloxetine from 30 to 60 mg/d, and she continues quetiapine, 50 mg every night at bedtime, for mood lability. Buspirone is not continued because she was not taking a therapeutic dosage in the community.
Within 4 days, Ms. A shows improvement in sleep, appetite, and mood. She has no further suicidal ideation.
[polldaddy:10511743]
The authors’ observations
Ms. A had a recurrence of what was presumed to be major depressive disorder (MDD) in the context of her mother’s death. However, she also exhibited irritability, mood lability, and impulsivity, all of which could be part of her depression, or a separate problem related to her brain tumor. Because Ms. A had never displayed bizarre behavior before the past few weeks, it is likely that her CNS lesion was directly affecting her personality and possibly underlying her planned suicide attempt.
Fifty to 80% of patients with CNS tumors, either primary or metastatic, present with psychiatric symptoms.1 Table 11-3 lists common psychiatric symptoms of brain tumors. Unfortunately, there is little reliable evidence that directly correlates tumor location with specific psychiatric symptoms. A 2010 meta-analysis found a statistically significant link between anorexia nervosa and hypothalamic tumors.1 However, for other brain regions, there is only an increased likelihood that any given tumor location will produce psychiatric symptoms.1,4 For instance, compared to patients with tumors in other locations, those with temporal lobe tumors are more likely to present with mood disorders, personality changes, and memory problems.1 In contrast, patients with frontal lobe tumors have an increased likelihood of psychosis, mood disorders, and personality changes.1 Patients with tumors in the pituitary region often present with anxiety.1
Continue to: When considering treatment options...
When considering treatment options for Ms. A, alcohol withdrawal was unlikely given the remote history of alcohol use, low alcohol blood level, and lack of evidence of unstable vital signs or tremor. Although she might have benefited from inpatient psychiatric treatment, this needed to wait until there was a definitive treatment plan for her brain tumor. Finally, although a paraneoplastic syndrome, such as limbic encephalitis, could be causing her psychiatric symptoms, this scenario is less likely with non-small–cell lung cancer.
Although uncommon, CNS tumors can present with psychiatric symptoms as the only manifestation. This is more likely when a patient exhibits new-onset or atypical symptoms, or fails to respond to standard psychiatric treatment.4 Case reports have described patients with brain tumors being misdiagnosed as having a primary psychiatric condition, which delays treatment of their CNS cancer.2 Additionally, frontal and limbic tumors are more likely to present with psychiatric manifestations; up to 90% of patients exhibit altered mental status or personality changes, as did Ms. A.1,4 Clearly, it is easier to identify patients with psychiatric symptoms resulting from a brain tumor when they also present with focal neurologic deficits or systemic symptoms, such as headache or nausea and vomiting. Ms. A presented with severe headaches, which is what led to her early imaging and prompt diagnosis.
Numerous proposed mechanisms might account for the psychiatric symptoms that occur during the course of a brain tumor, including direct injury to neuronal cells, secretion of hormones or other tumor-derived substances, and peri-ictal phenomena.3
TREATMENT Tumor is removed, but memory is impaired
Ms. A is scheduled for craniotomy and surgical resection of the frontal mass. Prior to surgery, Ms. A shows interest in improving her health, cooperates with staff, and seeks her daughter’s input on treatment. One week after admission, Ms. A has her mass resected, which is confirmed on biopsy to be a lung metastasis. Post-surgery, Ms. A receives codeine, 30 mg every 6 hours as needed, for pain; she continues dexamethasone, 4 mg IV every 6 hours, for brain edema and levetiracetam, 500 mg twice a day, for seizure prophylaxis.
On Day 2 after surgery, Ms. A attempts to elope. When she is approached by a psychiatrist on the treatment team, she does not recognize him. Although her long-term memory seems intact, she is unable to remember the details of recent events, including her medical and surgical treatments.
[polldaddy:10511745]
Continue to: The authors' observations
The authors’ observations
Ms. A’s memory impairment may be secondary to a surgically acquired neurocognitive deficit. In the United States, brain metastases represent a significant public health issue, affecting >100,000 patients per year.5 Metastatic lesions are the most common brain tumors. Lung cancer, breast cancer, and melanoma are the leading solid tumors to spread to the CNS.5 In cases of single brain metastasis, similar to Ms. A’s solitary left temporal lobe lesion, surgical resection plays a critical role in treatment. It provides histological confirmation of metastatic disease and can relieve mass effect if present. Studies have shown that combined surgical resection with radiation improves survival relative to patients who undergo radiation therapy alone.6,7
However, the benefits of surgical resection need to be balanced with preservation of neurologic function. Emerging evidence suggests that a majority of patients have surgically-acquired cognitive deficits due to damage of normal surrounding tissues, and these deficits are associated with reduced quality of life.8,9 Further, a study examining glioma surgical resections found that patients with left temporal lobe tumors exhibit more frequent and severe neurocognitive decline than patients with right temporal lobe tumors, especially in domains such as verbal memory.8 Ms. A’s memory impairment was persistent during her postoperative course, which suggests that it was not just an immediate post-surgical phenomenon, but a longer-lasting cognitive change directly related to the resection.
It is also possible that Ms. A had a prior neurocognitive disorder that manifested to a greater degree as a result of the CNS tumor. Ms. A might have had early-onset Alzheimer’s disease, although her intact memory before surgery makes this less likely. Alternatively, she could have had vascular dementia, especially given her long-standing hypertension and diabetes. This might have been missed in the initial evaluation because executive function was not tested. However, the relatively abrupt onset of memory problems after surgery suggests that she had no underlying neurocognitive disorder.
Ms. A’s presumed episode of MDD might also explain her memory changes. Major depressive disorder is increasingly common among geriatric patients, affecting approximately 5% of community-dwelling older adults.10 Its incidence increases with medical comorbidities, as suggested by depression rates of 5% to 10% in the primary care setting vs 37% in patients after critical-care hospitalizations.10 Late-life depression (LLD) occurs in adults age ≥60. Unlike depression in younger patients, LLD is more likely to be associated with cognitive impairment, specifically impairment of executive function and memory.11 The incidence of cognitive impairment in LLD is higher in patients with a history of depression, such as Ms. A.11,12 However, in general, patients who are depressed have memory complaints out of proportion to the clinical findings, and they show poor effort on cognitive testing. Ms. A exhibited neither of these, which makes it less likely that LLD was the exclusive cause of her memory loss.13 Table 214 outlines the management of cognitive deficits in a patient with a brain tumor.
EVALUATION Increasingly agitated and paranoid
After the tumor resection, Ms. A becomes increasingly irritable, uncooperative, and agitated. She repeatedly demands to be discharged. She insists she is fine and refuses medications and further laboratory workup. She becomes paranoid about the nursing staff and believes they are trying to kill her.
Continue to: On psychiatric re-evaluation...
On psychiatric re-evaluation, Ms. A demonstrates pressured speech, perseveration about going home, paranoid delusions, and anger at her family and physicians.
[polldaddy:10511747]
The authors’ observations
Ms. A’s refusal of medications and agitation may be explained by postoperative delirium, a surgical complication that is increasingly common among geriatric patients and is associated with poor clinical outcomes. Delirium is characterized by an acute onset and fluctuating course of symptoms that include inattention, motoric hypo- or hyperactivity, inappropriate behavior, emotional lability, cognitive dysfunction, and psychotic symptoms.15 Risk factors that contribute to postoperative delirium include older age, alcohol use, and poor baseline functional and cognitive status.16 The pathophysiology of delirium is not fully understood, but accumulating evidence suggests that different sets of interacting biologic factors (ie, neurotransmitters and inflammation) contribute to a disruption of large-scale neuronal networks in the brain, resulting in cognitive dysfunction.15 Patients who develop postoperative delirium are more likely to develop long-term cognitive dysfunction and have an increased risk of dementia.16
Another potential source of Ms. A’s agitation is steroid use. Ms. A received IV dexamethasone, 8 to 16 mg/d, around the time of her surgery. Steroids are commonly used to treat brain tumors, particularly when there is vasogenic edema. Steroid psychosis is a term loosely used to describe a wide range of psychiatric symptoms induced by corticosteroids that includes, but is not limited to, depression, mania, psychosis, delirium, and cognitive impairment.17 Steroid-induced psychiatric adverse effects occur in 5% to 18% of patients receiving corticosteroids and often happen early in treatment, although they can occur at any point.18 Corticosteroids influence brain activity via glucocorticoid and mineralocorticoid receptors. These receptors are widely distributed throughout the brain and affect neurotransmitter systems, such as the serotonergic system, that are associated with changes in mood, behavior, and cognition.17 While the adverse psychiatric manifestations of steroid use vary, higher dosages are associated with an increased risk of psychiatric complications; mania is more prevalent early in the course of treatment, and depression is more common with long-term use.17,19 Table 317,18 outlines the evidence-based treatment of corticosteroid-induced adverse psychiatric effects.
Although there are no clinical guidelines or FDA-approved medications for treating steroid-induced psychiatric adverse events, these are best managed by tapering and discontinuing steroids when possible and simultaneously using psychotropic medications to treat psychiatric symptoms. Case reports and limited evidence-based literature have demonstrated that steroid-induced mania responds to mood stabilizers or antipsychotics, while depression can be managed with antidepressants or lithium.17
Additionally, patients with CNS tumors are at risk for seizures and often are prescribed antiepileptics. Because it is easy to administer and does not need to be titrated, levetiracetam is a commonly used agent. However, levetiracetam can cause psychiatric adverse effects, including behavior changes and frank psychosis.20
Continue to: Finally, Ms. A's altered mental status...
Finally, Ms. A’s altered mental status could have been related to opioid intoxication. Opioids are used to manage postsurgical pain, and studies have shown these medications can be a precipitating factor for delirium in geriatric patients.21
TREATMENT Medication adjustments
At the request of the psychiatry team, levetiracetam is discontinued due to its potential for psychiatric adverse effects. The neurosurgery team replaces it with valproic acid, 500 mg every 12 hours. Ms. A is also tapered off steroids fairly rapidly because of the potential for steroid-induced psychiatric adverse effects. Her quetiapine is titrated from 50 to 150 mg every night at bedtime, and duloxetine is discontinued.
OUTCOME Agitation improves dramatically
Ms. A’s new medication regimen dramatically improves her agitation, which allows Ms. A, her family, and the medical team to work together to establish treatment goals. Ms. A ultimately returns home with the assistance of her family. She continues to have memory issues, but with improved emotion regulation. Several months later, Ms. A is readmitted to the hospital because her cancer has progressed despite treatment.
Bottom Line
Brain tumors may present with various psychiatric manifestations that can change during the course of the patient’s treatment. A comprehensive psychiatric evaluation should parse out the interplay between direct effects of the tumor and any adverse effects that are the result of medical and/or surgical interventions to determine the cause of psychiatric symptoms and their appropriate management.
Related Resource
Madhusoodanan S, Ting MB, Farah T, et al. Psychiatric aspects of brain tumors: a review. World J Psychiatry. 2015;5(3):273-285.
Drug Brand Names
Aripiprazole • Abilify
Buspirone • Buspar
Chlorpromazine • Thorazine
Codeine • Codeine systemic
Dexamethasone • Decadron
Duloxetine • Cymbalta
Haloperidol • Haldol
Levetiracetam • Keppra
Lorazepam • Ativan
Lithium • Eskalith, Lithobid
Olanzapine • Zyprexa
Quetiapine • Seroquel
Risperidone • Risperdal
Sertraline • Zoloft
Valproic acid • Depakene
CASE Suicidal behavior, severe headaches
Ms. A, age 60, presents to the emergency department (ED) with depression, suicidal behavior, and 3 days of severe headaches. Neurology is consulted and an MRI is ordered, which shows a 3.0-cm mass lesion in the left temporal lobe with associated vasogenic edema that is suspicious for metastatic disease (Figure).
Ms. A is admitted to the hospital for further workup of her brain lesion. She is started on IV dexamethasone, 10 mg every 6 hours, a glucocorticosteroid, for brain edema, and levetiracetam, 500 mg twice a day, for seizure prophylaxis.
Upon admission, in addition to oncology and neurosurgery, psychiatry is also consulted to evaluate Ms. A for depression and suicidality.
EVALUATION Mood changes and poor judgment
Ms. A has a psychiatric history of depression and alcohol use disorder but says she has not consumed any alcohol in years. Her medical history includes hypertension, diabetes, and stage 4 non-small–cell lung cancer, for which she received surgery and adjuvant chemoradiotherapy 1 year ago.
On initial intake, Ms. A reports that in addition to the headaches, she has also been experiencing worsening depression and suicidal behavior. For the past 2 months, she has had a severely depressed mood, with notable anhedonia, poor appetite, insomnia, low energy, and decreased concentration. The changes in her mental health were triggered by her mother’s death. Three days prior to admission, the patient planned to overdose on antihypertensive pills, but her suicide attempt was interrupted when her family called. She denies any current suicidal ideation, intent, or plan.
According to her family, Ms. A has been increasingly irritable and her personality has changed in the past month. She also has been repeatedly sorting through her neighbors’ garbage.
Ms. A’s current psychiatric medications are duloxetine, 30 mg/d; quetiapine, 50 mg every night at bedtime; and buspirone, 10 mg/d. However, it is unclear if she is consistently taking these medications.
Continue to: On mental status examination...
On mental status examination, Ms. A is calm and she has no abnormal movements. She says she is depressed. Her affect is reactive and labile. She is alert and oriented to person, place, and time. Her attention, registration, and recall are intact. Her executive function is not tested. However, Ms. A’s insight and judgment seem poor.
To address Ms. A’s worsening depression, the psychiatry team increases her duloxetine from 30 to 60 mg/d, and she continues quetiapine, 50 mg every night at bedtime, for mood lability. Buspirone is not continued because she was not taking a therapeutic dosage in the community.
Within 4 days, Ms. A shows improvement in sleep, appetite, and mood. She has no further suicidal ideation.
[polldaddy:10511743]
The authors’ observations
Ms. A had a recurrence of what was presumed to be major depressive disorder (MDD) in the context of her mother’s death. However, she also exhibited irritability, mood lability, and impulsivity, all of which could be part of her depression, or a separate problem related to her brain tumor. Because Ms. A had never displayed bizarre behavior before the past few weeks, it is likely that her CNS lesion was directly affecting her personality and possibly underlying her planned suicide attempt.
Fifty to 80% of patients with CNS tumors, either primary or metastatic, present with psychiatric symptoms.1 Table 11-3 lists common psychiatric symptoms of brain tumors. Unfortunately, there is little reliable evidence that directly correlates tumor location with specific psychiatric symptoms. A 2010 meta-analysis found a statistically significant link between anorexia nervosa and hypothalamic tumors.1 However, for other brain regions, there is only an increased likelihood that any given tumor location will produce psychiatric symptoms.1,4 For instance, compared to patients with tumors in other locations, those with temporal lobe tumors are more likely to present with mood disorders, personality changes, and memory problems.1 In contrast, patients with frontal lobe tumors have an increased likelihood of psychosis, mood disorders, and personality changes.1 Patients with tumors in the pituitary region often present with anxiety.1
Continue to: When considering treatment options...
When considering treatment options for Ms. A, alcohol withdrawal was unlikely given the remote history of alcohol use, low alcohol blood level, and lack of evidence of unstable vital signs or tremor. Although she might have benefited from inpatient psychiatric treatment, this needed to wait until there was a definitive treatment plan for her brain tumor. Finally, although a paraneoplastic syndrome, such as limbic encephalitis, could be causing her psychiatric symptoms, this scenario is less likely with non-small–cell lung cancer.
Although uncommon, CNS tumors can present with psychiatric symptoms as the only manifestation. This is more likely when a patient exhibits new-onset or atypical symptoms, or fails to respond to standard psychiatric treatment.4 Case reports have described patients with brain tumors being misdiagnosed as having a primary psychiatric condition, which delays treatment of their CNS cancer.2 Additionally, frontal and limbic tumors are more likely to present with psychiatric manifestations; up to 90% of patients exhibit altered mental status or personality changes, as did Ms. A.1,4 Clearly, it is easier to identify patients with psychiatric symptoms resulting from a brain tumor when they also present with focal neurologic deficits or systemic symptoms, such as headache or nausea and vomiting. Ms. A presented with severe headaches, which is what led to her early imaging and prompt diagnosis.
Numerous proposed mechanisms might account for the psychiatric symptoms that occur during the course of a brain tumor, including direct injury to neuronal cells, secretion of hormones or other tumor-derived substances, and peri-ictal phenomena.3
TREATMENT Tumor is removed, but memory is impaired
Ms. A is scheduled for craniotomy and surgical resection of the frontal mass. Prior to surgery, Ms. A shows interest in improving her health, cooperates with staff, and seeks her daughter’s input on treatment. One week after admission, Ms. A has her mass resected, which is confirmed on biopsy to be a lung metastasis. Post-surgery, Ms. A receives codeine, 30 mg every 6 hours as needed, for pain; she continues dexamethasone, 4 mg IV every 6 hours, for brain edema and levetiracetam, 500 mg twice a day, for seizure prophylaxis.
On Day 2 after surgery, Ms. A attempts to elope. When she is approached by a psychiatrist on the treatment team, she does not recognize him. Although her long-term memory seems intact, she is unable to remember the details of recent events, including her medical and surgical treatments.
[polldaddy:10511745]
Continue to: The authors' observations
The authors’ observations
Ms. A’s memory impairment may be secondary to a surgically acquired neurocognitive deficit. In the United States, brain metastases represent a significant public health issue, affecting >100,000 patients per year.5 Metastatic lesions are the most common brain tumors. Lung cancer, breast cancer, and melanoma are the leading solid tumors to spread to the CNS.5 In cases of single brain metastasis, similar to Ms. A’s solitary left temporal lobe lesion, surgical resection plays a critical role in treatment. It provides histological confirmation of metastatic disease and can relieve mass effect if present. Studies have shown that combined surgical resection with radiation improves survival relative to patients who undergo radiation therapy alone.6,7
However, the benefits of surgical resection need to be balanced with preservation of neurologic function. Emerging evidence suggests that a majority of patients have surgically-acquired cognitive deficits due to damage of normal surrounding tissues, and these deficits are associated with reduced quality of life.8,9 Further, a study examining glioma surgical resections found that patients with left temporal lobe tumors exhibit more frequent and severe neurocognitive decline than patients with right temporal lobe tumors, especially in domains such as verbal memory.8 Ms. A’s memory impairment was persistent during her postoperative course, which suggests that it was not just an immediate post-surgical phenomenon, but a longer-lasting cognitive change directly related to the resection.
It is also possible that Ms. A had a prior neurocognitive disorder that manifested to a greater degree as a result of the CNS tumor. Ms. A might have had early-onset Alzheimer’s disease, although her intact memory before surgery makes this less likely. Alternatively, she could have had vascular dementia, especially given her long-standing hypertension and diabetes. This might have been missed in the initial evaluation because executive function was not tested. However, the relatively abrupt onset of memory problems after surgery suggests that she had no underlying neurocognitive disorder.
Ms. A’s presumed episode of MDD might also explain her memory changes. Major depressive disorder is increasingly common among geriatric patients, affecting approximately 5% of community-dwelling older adults.10 Its incidence increases with medical comorbidities, as suggested by depression rates of 5% to 10% in the primary care setting vs 37% in patients after critical-care hospitalizations.10 Late-life depression (LLD) occurs in adults age ≥60. Unlike depression in younger patients, LLD is more likely to be associated with cognitive impairment, specifically impairment of executive function and memory.11 The incidence of cognitive impairment in LLD is higher in patients with a history of depression, such as Ms. A.11,12 However, in general, patients who are depressed have memory complaints out of proportion to the clinical findings, and they show poor effort on cognitive testing. Ms. A exhibited neither of these, which makes it less likely that LLD was the exclusive cause of her memory loss.13 Table 214 outlines the management of cognitive deficits in a patient with a brain tumor.
EVALUATION Increasingly agitated and paranoid
After the tumor resection, Ms. A becomes increasingly irritable, uncooperative, and agitated. She repeatedly demands to be discharged. She insists she is fine and refuses medications and further laboratory workup. She becomes paranoid about the nursing staff and believes they are trying to kill her.
Continue to: On psychiatric re-evaluation...
On psychiatric re-evaluation, Ms. A demonstrates pressured speech, perseveration about going home, paranoid delusions, and anger at her family and physicians.
[polldaddy:10511747]
The authors’ observations
Ms. A’s refusal of medications and agitation may be explained by postoperative delirium, a surgical complication that is increasingly common among geriatric patients and is associated with poor clinical outcomes. Delirium is characterized by an acute onset and fluctuating course of symptoms that include inattention, motoric hypo- or hyperactivity, inappropriate behavior, emotional lability, cognitive dysfunction, and psychotic symptoms.15 Risk factors that contribute to postoperative delirium include older age, alcohol use, and poor baseline functional and cognitive status.16 The pathophysiology of delirium is not fully understood, but accumulating evidence suggests that different sets of interacting biologic factors (ie, neurotransmitters and inflammation) contribute to a disruption of large-scale neuronal networks in the brain, resulting in cognitive dysfunction.15 Patients who develop postoperative delirium are more likely to develop long-term cognitive dysfunction and have an increased risk of dementia.16
Another potential source of Ms. A’s agitation is steroid use. Ms. A received IV dexamethasone, 8 to 16 mg/d, around the time of her surgery. Steroids are commonly used to treat brain tumors, particularly when there is vasogenic edema. Steroid psychosis is a term loosely used to describe a wide range of psychiatric symptoms induced by corticosteroids that includes, but is not limited to, depression, mania, psychosis, delirium, and cognitive impairment.17 Steroid-induced psychiatric adverse effects occur in 5% to 18% of patients receiving corticosteroids and often happen early in treatment, although they can occur at any point.18 Corticosteroids influence brain activity via glucocorticoid and mineralocorticoid receptors. These receptors are widely distributed throughout the brain and affect neurotransmitter systems, such as the serotonergic system, that are associated with changes in mood, behavior, and cognition.17 While the adverse psychiatric manifestations of steroid use vary, higher dosages are associated with an increased risk of psychiatric complications; mania is more prevalent early in the course of treatment, and depression is more common with long-term use.17,19 Table 317,18 outlines the evidence-based treatment of corticosteroid-induced adverse psychiatric effects.
Although there are no clinical guidelines or FDA-approved medications for treating steroid-induced psychiatric adverse events, these are best managed by tapering and discontinuing steroids when possible and simultaneously using psychotropic medications to treat psychiatric symptoms. Case reports and limited evidence-based literature have demonstrated that steroid-induced mania responds to mood stabilizers or antipsychotics, while depression can be managed with antidepressants or lithium.17
Additionally, patients with CNS tumors are at risk for seizures and often are prescribed antiepileptics. Because it is easy to administer and does not need to be titrated, levetiracetam is a commonly used agent. However, levetiracetam can cause psychiatric adverse effects, including behavior changes and frank psychosis.20
Continue to: Finally, Ms. A's altered mental status...
Finally, Ms. A’s altered mental status could have been related to opioid intoxication. Opioids are used to manage postsurgical pain, and studies have shown these medications can be a precipitating factor for delirium in geriatric patients.21
TREATMENT Medication adjustments
At the request of the psychiatry team, levetiracetam is discontinued due to its potential for psychiatric adverse effects. The neurosurgery team replaces it with valproic acid, 500 mg every 12 hours. Ms. A is also tapered off steroids fairly rapidly because of the potential for steroid-induced psychiatric adverse effects. Her quetiapine is titrated from 50 to 150 mg every night at bedtime, and duloxetine is discontinued.
OUTCOME Agitation improves dramatically
Ms. A’s new medication regimen dramatically improves her agitation, which allows Ms. A, her family, and the medical team to work together to establish treatment goals. Ms. A ultimately returns home with the assistance of her family. She continues to have memory issues, but with improved emotion regulation. Several months later, Ms. A is readmitted to the hospital because her cancer has progressed despite treatment.
Bottom Line
Brain tumors may present with various psychiatric manifestations that can change during the course of the patient’s treatment. A comprehensive psychiatric evaluation should parse out the interplay between direct effects of the tumor and any adverse effects that are the result of medical and/or surgical interventions to determine the cause of psychiatric symptoms and their appropriate management.
Related Resource
Madhusoodanan S, Ting MB, Farah T, et al. Psychiatric aspects of brain tumors: a review. World J Psychiatry. 2015;5(3):273-285.
Drug Brand Names
Aripiprazole • Abilify
Buspirone • Buspar
Chlorpromazine • Thorazine
Codeine • Codeine systemic
Dexamethasone • Decadron
Duloxetine • Cymbalta
Haloperidol • Haldol
Levetiracetam • Keppra
Lorazepam • Ativan
Lithium • Eskalith, Lithobid
Olanzapine • Zyprexa
Quetiapine • Seroquel
Risperidone • Risperdal
Sertraline • Zoloft
Valproic acid • Depakene
1. Madhusoodanan S, Opler MG, Moise D, et al. Brain tumor location and psychiatric symptoms: is there any association? A meta-analysis of published case studies. Expert Rev Neurother. 2010;10(10):1529-1536.
2. Bunevicius A, Deltuva VP, Deltuviene D, et al. Brain lesions manifesting as psychiatric disorders: eight cases. CNS Spectr. 2008;13(11):950-958.
3. Pearl ML, Talgat G, Valea FA, et al. Psychiatric symptoms due to brain metastases. Med Update Psychiatr. 1998;3(4):91-94.
4. Madhusoodanan S, Danan D, Moise D. Psychiatric manifestations of brain tumors: diagnostic implications. Expert Rev Neurother. 2007;7(4):343-349.
5. Ferguson SD, Wagner KM, Prabhu SS, et al. Neurosurgical management of brain metastases. Clin Exp Metastasis. 2017;34(6-7):377-389.
6. Husain ZA, Regine WF, Kwok Y, et al. Brain metastases: contemporary management and future directions. Eur J Clin Med Oncol. 2011;3(3):38-45.
7. Vecht CJ, Haaxmareiche H, Noordijk EM, et al. Treatment of single brain metastasis - radiotherapy alone or combined with neurosurgery. Ann Neurol. 1993;33(6):583-590.
8. Barry RL, Byun NE, Tantawy MN, et al. In vivo neuroimaging and behavioral correlates in a rat model of chemotherapy-induced cognitive dysfunction. Brain Imaging Behav. 2018;12(1):87-95.
9. Wu AS, Witgert ME, Lang FF, et al. Neurocognitive function before and after surgery for insular gliomas. J Neurosurg. 2011;115(6):1115-1125.
10. Taylor WD. Depression in the elderly. N Engl J Med. 2014;371(13):1228-1236.
11. Liguori C, Pierantozzi M, Chiaravalloti A, et al. When cognitive decline and depression coexist in the elderly: CSF biomarkers analysis can differentiate Alzheimer’s disease from late-life depression. Front Aging Neurosci. 2018;10:38.
12. Luijendijk HJ, van den Berg JF, Dekker MJHJ, et al. Incidence and recurrence of late-life depression. Arch Gen Psychiatry. 2008;65(12):1394-1401.
13. Potter GG, Steffens DC. Contribution of depression to cognitive impairment and dementia in older adults. Neurologist. 2007;13(3):105-117.
14. Taphoorn MJB, Klein M. Cognitive deficits in adult patients with brain tumours. Lancet Neurol. 2004;3(3):159-168.
15. Inouye SK, Westendorp RGJ, Saczynski JS. Delirium in elderly people. Lancet. 2014;383(9920):911-922.
16. Sprung J, Roberts RO, Weingarten TN, et al. Postoperative delirium in elderly patients is associated with subsequent cognitive impairment. Br J Anaesth. 2017;119(2):316-323.
17. Kusljic S, Manias E, Gogos A. Corticosteroid-induced psychiatric disturbances: it is time for pharmacists to take notice. Res Soc Adm Pharm. 2016;12(2):355-360.
18. Cerullo MA. Corticosteroid-induced mania: prepare for the unpredictable. Current Psychiatry. 2006;5(6):43-50.
19. Dubovsky AN, Arvikar S, Stern TA, et al. Steroid psychosis revisited. Psychosomatics. 2012;53(2):103-115.
20. Habets JGV, Leentjens AFG, Schijns OEMG. Serious and reversible levetiracetam-induced psychiatric symptoms after resection of frontal low-grade glioma: two case histories. Br J Neurosurg. 2017;31(4):471-473.
21
1. Madhusoodanan S, Opler MG, Moise D, et al. Brain tumor location and psychiatric symptoms: is there any association? A meta-analysis of published case studies. Expert Rev Neurother. 2010;10(10):1529-1536.
2. Bunevicius A, Deltuva VP, Deltuviene D, et al. Brain lesions manifesting as psychiatric disorders: eight cases. CNS Spectr. 2008;13(11):950-958.
3. Pearl ML, Talgat G, Valea FA, et al. Psychiatric symptoms due to brain metastases. Med Update Psychiatr. 1998;3(4):91-94.
4. Madhusoodanan S, Danan D, Moise D. Psychiatric manifestations of brain tumors: diagnostic implications. Expert Rev Neurother. 2007;7(4):343-349.
5. Ferguson SD, Wagner KM, Prabhu SS, et al. Neurosurgical management of brain metastases. Clin Exp Metastasis. 2017;34(6-7):377-389.
6. Husain ZA, Regine WF, Kwok Y, et al. Brain metastases: contemporary management and future directions. Eur J Clin Med Oncol. 2011;3(3):38-45.
7. Vecht CJ, Haaxmareiche H, Noordijk EM, et al. Treatment of single brain metastasis - radiotherapy alone or combined with neurosurgery. Ann Neurol. 1993;33(6):583-590.
8. Barry RL, Byun NE, Tantawy MN, et al. In vivo neuroimaging and behavioral correlates in a rat model of chemotherapy-induced cognitive dysfunction. Brain Imaging Behav. 2018;12(1):87-95.
9. Wu AS, Witgert ME, Lang FF, et al. Neurocognitive function before and after surgery for insular gliomas. J Neurosurg. 2011;115(6):1115-1125.
10. Taylor WD. Depression in the elderly. N Engl J Med. 2014;371(13):1228-1236.
11. Liguori C, Pierantozzi M, Chiaravalloti A, et al. When cognitive decline and depression coexist in the elderly: CSF biomarkers analysis can differentiate Alzheimer’s disease from late-life depression. Front Aging Neurosci. 2018;10:38.
12. Luijendijk HJ, van den Berg JF, Dekker MJHJ, et al. Incidence and recurrence of late-life depression. Arch Gen Psychiatry. 2008;65(12):1394-1401.
13. Potter GG, Steffens DC. Contribution of depression to cognitive impairment and dementia in older adults. Neurologist. 2007;13(3):105-117.
14. Taphoorn MJB, Klein M. Cognitive deficits in adult patients with brain tumours. Lancet Neurol. 2004;3(3):159-168.
15. Inouye SK, Westendorp RGJ, Saczynski JS. Delirium in elderly people. Lancet. 2014;383(9920):911-922.
16. Sprung J, Roberts RO, Weingarten TN, et al. Postoperative delirium in elderly patients is associated with subsequent cognitive impairment. Br J Anaesth. 2017;119(2):316-323.
17. Kusljic S, Manias E, Gogos A. Corticosteroid-induced psychiatric disturbances: it is time for pharmacists to take notice. Res Soc Adm Pharm. 2016;12(2):355-360.
18. Cerullo MA. Corticosteroid-induced mania: prepare for the unpredictable. Current Psychiatry. 2006;5(6):43-50.
19. Dubovsky AN, Arvikar S, Stern TA, et al. Steroid psychosis revisited. Psychosomatics. 2012;53(2):103-115.
20. Habets JGV, Leentjens AFG, Schijns OEMG. Serious and reversible levetiracetam-induced psychiatric symptoms after resection of frontal low-grade glioma: two case histories. Br J Neurosurg. 2017;31(4):471-473.
21
Suicidal while receiving treatment for breast cancer
CASE Worsening mood symptoms and suicidal ideation
On a recent visit to the oncology clinic, where she has been receiving treatment for breast cancer for 11 months, Mrs. L, age 46, reports the abrupt onset of sadness, irritability, difficulty sleeping, and negative self-thoughts.
Eleven months ago, Mrs. L was diagnosed with invasive lobular carcinoma of the right breast that was classified as T2N0MX, representing relatively early-stage disease. Shortly after her diagnosis, Mrs. L completed 4 cycles of neoadjuvant chemotherapy with doxorubicin and cyclophosphamide, followed by treatment with trastuzumab. Subsequently, she underwent a right segmental mastectomy with bilateral mastopexy and radiation therapy. Recently, Mrs. L’s oncology team prescribed tamoxifen, 20 mg/d, and trastuzumab, 420 mg IV every 3 weeks; however, within 3 weeks after starting tamoxifen, Mrs. L’s mood symptoms worsened to the point where she says she is considering suicide—with a plan to use her husband’s gun to kill herself.
Mrs. L has no other pertinent medical history and no reported history of psychiatric disease.
The primary oncology team discontinues tamoxifen (after 5 weeks of treatment) and refers Mrs. L to psychiatry for further mood evaluation.
[polldaddy:10497042]
The authors’ observations
The prevalence of depression is higher in patients with cancer than in the general population.1 The etiology of depression is often multifactorial.2 In Mrs. L’s case, we hypothesized that the possible cause of her depressive symptoms included concerns about her self-image after mastectomy and the adverse effects of chemotherapy and tamoxifen.
Among these possible causes, estrogen level is particularly important. Estrogen affects the brain in numerous ways, including by modulating different neurotransmitters,3,4 regulating neuroplasticity, providing neuroprotection by preventing formation of oxidative free radicals and of beta amyloid, and possibly avoiding inflammation. From a behavioral standpoint, estrogen acts as an antidepressant while enhancing memory and modulating maternal behavior.4 Therefore, decreased estrogen levels could result in depression and other neuropsychiatric problems. This is illustrated in Mrs. L’s case, where tamoxifen administered after breast cancer treatment coincided with the abrupt onset of depression with suicidal ideation.
Depression in patients receiving tamoxifen might be explained by the fact that tamoxifen is a selective estrogen receptor blocker with dual properties. Specifically, while it has antagonistic action in breast tissue, diminishing the growth-promoting action of estrogen on breast cancer cells, it additionally crosses the blood-brain barrier, so it may block the neuroprotective action of estrogen in the brain.
EXAMINATION Improvement in depression but slightly anxious
During her psychiatric examination, Mrs. L is fairly well-groomed and cooperative. Her speech is normal, thought process is organized, and she has fair insight into her medical situation, with fair judgment. She is alert, attentive, and oriented to time, place, as well as person. She confirms that she has no prior psychiatric history, including no prior suicide attempts. She lives with her husband, who has been supportive. Mrs. L has no children, and she continues to work.
Continue to: Mrs. L reports that per her oncology...
Mrs. L reports that per her oncology team’s instruction, she has not taken tamoxifen for almost 1 week, and notes improvement in her mood. She describes her mood as “fine now,” but appears slightly anxious. She adamantly denies suicidal ideation since stopping tamoxifen; however, she confirms that prior to stopping tamoxifen, she experienced low mood, suicidal thoughts, and a decreased interest in activities. Mrs. L’s Patient Health Questionnaire–9 score is 13, indicating moderate depression. She says she is constantly preoccupied with thoughts about the adverse effects of hormone therapy, and specifically about the oncology team’s suggestion of a retrial of tamoxifen. Due to her constant worry, she has difficulty relaxing; her Generalized Anxiety Disorder–7 item scale score is 12, indicating moderate anxiety. She has a history of cigarette smoking but stopped after her breast cancer diagnosis. She also reports gaining weight since beginning cancer treatment (body mass index: 28.0 kg/m2) and experiencing breast pain.
Mrs. L’s vital signs are normal. Results of her laboratory workup reveal a thyroid-stimulating hormone level of 1.40 µU/mL (reference range: 0.27 to 4.20 µU/mL); a follicle-stimulating hormone (FSH) level of 78.4 mIU/mL (postmenopausal reference range: 25.8 to 134.8 mIU/mL); and an estradiol level of <12.0 pg/mL (postmenopausal range: <55 pg/mL).
The authors’ observations
Studies investigating the effects of tamoxifen on mood have produced varying results (Table5-16). Some researchers have found a significant relationship between depression and tamoxifen in patients with breast cancer. In a case-control study, 42 postmenopausal women with breast cancer who received tamoxifen reported statistically significant elevated depression scores.5 Similarly, in a prospective trial that assessed mood symptoms in 21 pre- and postmenopausal women who developed estrogen deficiency during breast cancer treatment (including treatment with tamoxifen and chemotherapy), 38% of patients met the criteria for major depressive disorder (MDD) in the first 6 months of treatment. Sixty-six percent of these patients were postmenopausal, and 38% were premenopausal. Twenty-five percent of the premenopausal women who experienced MDD symptoms had been treated with tamoxifen and chemotherapy.6
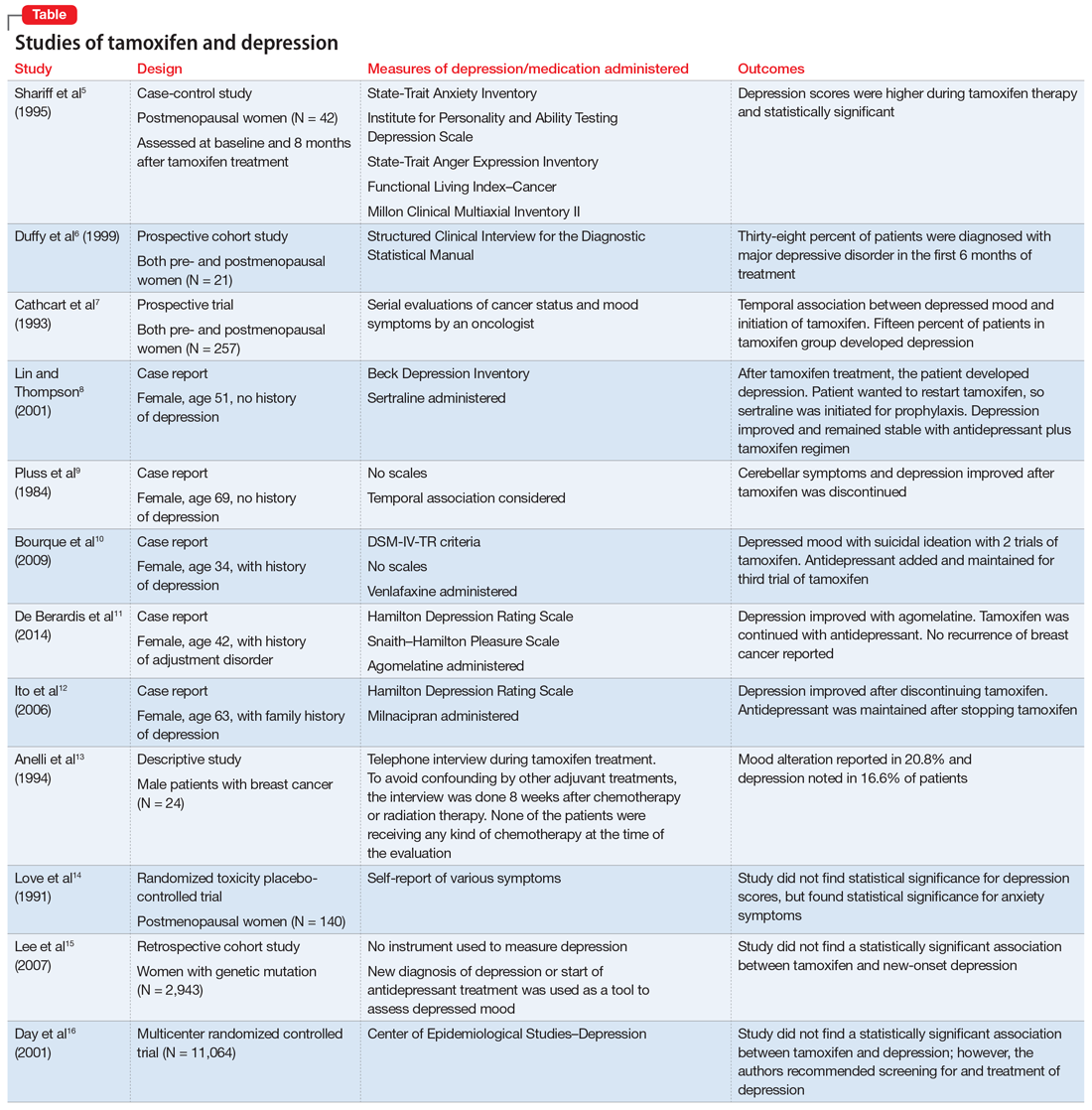
In a larger prospective trial (N = 257), an oncologist assessed mood symptoms in 2 groups of patients with breast cancer: individuals who received tamoxifen, and those who did not receive tamoxifen.7 They found that 15% of patients who received tamoxifen experienced depression, compared with 3% of patients who did not receive tamoxifen; this difference was statistically significant.7 Overall, 31% of the patients had “significant depression” and 27% discontinued tamoxifen because of adverse effects.7 There have been 2 case reports of tamoxifen use and severe depression in patients with no prior psychiatry history8,9 and 3 case reports of tamoxifen use and severe depression in patients who had a psychiatric history.10-12
One study that examined 24 men with breast cancer found that 62.5% of these patients experienced adverse effects related to tamoxifen, and 25% discontinued tamoxifen because of its adverse effects.13 Among the various adverse effects related to tamoxifen, mood alteration was reported in 20.8% of cases, and depressed feelings were reported in 16.6%.13
Continue to: Despite the evidence...
Despite this evidence, other studies have not found an association between tamoxifen and depressed mood in patients with breast cancer. One group of researchers who assessed various symptoms self-reported by postmenopausal women who were breast cancer survivors found that the depression scores were not significant.14 A retrospective cohort study assessed the onset of depression in patients with breast cancer with positive hormone receptor status (who received tamoxifen) vs negative hormone receptor status (who did not receive tamoxifen). These researchers did not find a statistically significant hazard ratio for “new-onset depression.”15 Unfortunately, the criteria for “new-onset depression” used in this study was the diagnosis of depression or use of an antidepressant given or ordered by a clinician, which is not a sensitive assessment of depressed mood.15
A multicenter randomized, placebo-controlled trial (the National Surgical Adjuvant Breast and Bowel Project) assessed the incidence of negative health outcomes, including depression, in a secondary outcome analysis.16 These researchers did not find a statistically significantassociation between tamoxifen and depression. However, in this study, assessment of depression was based on self-report using the Center of Epidemiologic Studies Depression (CES-D) scale, which does not clinically categorize depression. Furthermore, these researchers strongly recommended screening for mood disorders in routine clinical practice. In this study, 3 women completed suicide, 2 of whom were in the tamoxifen arm.16
[polldaddy:10497045]
The authors’ observations
Tamoxifen is a prodrug that converts to the active metabolite, endoxifen, via cytochrome P450 2D6 (CYP2D6) activity. Antidepressants with strong 2D6-inhibiting properties, such as fluoxetine, duloxetine, bupropion, and paroxetine, should be avoided in patients receiving tamoxifen because they interfere with the formation of the active metabolite and could reduce the effectiveness of tamoxifen and its ability to reduce the risk of cancer recurrence.17 Antidepressants can help treat psychological distress, especially depression, which is common in patients with cancer, and vasomotor symptoms, which may impair quality of life and adherence to long-term endocrine therapy. Because tamoxifen can decrease cancer recurrence and associated mortality,18 adherence with treatment is crucial.
TREATMENT Starting an antidepressant
The psychiatry team initiates venlafaxine, 37.5 mg/d, to treat Mrs. L’s anxiety and help prevent the recurrence of severe depression. They prescribe venlafaxine because they anticipate that, based on Mrs. L’s age, the oncology team might reconsider treatment with tamoxifen. Venlafaxine is preferred because it has a favorable pharmacodynamic profile and does not interfere with the metabolism of tamoxifen, as is the case with many selective serotonin reuptake inhibitors.17
Although Mrs. L’s depression had abated once she stopped receiving tamoxifen, she continues to experience anxiety and tearfulness, primarily due to fear of adverse effects of hormone therapy, and due to family as well as work stressors. Therefore, venlafaxine is gradually titrated up to 150 mg/d.
Continue to: The oncology team proposes...
The oncology team proposes a trial of leuprolide, a gonadotropin-releasing hormone agonist that downregulates pituitary receptors, subsequently suppressing female reproductive hormones, which in turn stops the ovaries from producing estrogen so there is a minimal amount of estrogen to promote the growth of estrogen–receptor-positive breast cancer. Mrs. L declines this agent because she is concerned that she will gain weight. Instead, Mrs. L expresses interest in undergoing an oophorectomy to reduce her estrogen level. In the meantime, based on her reproductive hormone levels (FSH and estradiol levels) which are indicative of postmenopausal status, the oncology team prescribes the aromatase inhibitor (AI) exemestane 25 mg/d. The AI helps to decrease the amount of estrogen the body makes peripherally, which is the main source of estrogen in postmenopausal women.
The authors’ observations
Estrogen originates in the ovaries in premenopausal women; it is also produced by peripheral conversion of androgens to estrogen in adipose tissues and muscle in postmenopausal women.19 Aromatase inhibitors block the enzyme aromatase that converts androgen to estrogen, which leads to estrogen deficiency in postmenopausal women and possibly to neuropsychiatric effects.19
The results of studies assessing the adverse psychiatric effects of AIs are mixed. When the results of studies evaluating tamoxifen are compared with those evaluating AIs, overall patients who received AIs had less severe or less frequent mood symptoms. One possible explanation could be that AIs are relatively new compared with tamoxifen. Second, AIs are more commonly used in postmenopausal women with breast cancer, and these patients’ overall estrogen level is significantly lower than that of premenopausal women with breast cancer. Therefore, the degree of hormone fluctuation is less intense in postmenopausal breast cancer survivors.
OUTCOME
After starting exemestane, and while still receiving venlafaxine, Mrs. L no longer experiences severe depressive symptoms. After 8 months, venlafaxine is discontinued. She continues to deny depressive symptoms but has intermittent anxiety, which she is able to manage without psychiatric medication. She continues to remain adherent with ongoing exemestane treatment, with no evidence of disease progression or recurrence.
The authors’ observations
For patients with estrogen-positive breast cancer, the decision to discontinue tamoxifen because of unacceptable adverse effects is an important one because it may increase the risk of cancer recurrence. Psychiatrists have an important role in supporting the patient through this process, helping patients understand alternatives, and working with the oncology team to formulate a plan that is acceptable to everyone.
Continue to: Bottom Line
Bottom Line
For patients with estrogen–positive breast cancer, anti-estrogen treatment can reduce the risk of cancer recurrence. However, it can cause adverse effects, including depression, that might impair quality of life and treatment adherence. For patients with severe depression, stopping estrogen blockers may be warranted. Initiating an antidepressant that does not interfere with the metabolism of tamoxifen may help treat depression and vasomotor symptoms.
Related Resource
- Agarwala P. Tailoring depression treatment for women with breast cancer. Current Psychiatry. 2010;9(11):39-40,45-46,48-49.
Drug Brand Names
Agomelatine • Valdoxan
Bupropion • Wellbutrin, Zyban
Cyclophosphamide • Cytoxan
Doxorubicin • Adriamycin
Duloxetine • Cymbalta
Exemestane • Aromasin
Fluoxetine • Prozac
Leuprolide • Eligard, Lupron
Milnacipran • Savella
Paroxetine • Paxil
Sertraline • Zoloft
Tamoxifen • Soltamox
Trastuzumab • Herceptin
Venlafaxine • Effexor
1. Zabora J, BrintzenhofeSzoc K, Curbow B, et al. The prevalence of psychological distress by cancer site. Psychooncology. 2001;10(1):19-28.
2. Thompson DS, Spanier CA, Vogel VG. The relationship between tamoxifen, estrogen, and depressive symptoms. Breast J. 1999;5(6):375-382.
3. Halbreich U. Role of estrogen in postmenopausal depression. Neurology. 1997;48(5 suppl 7):S16-S19.
4. Schiller CE, Johnson SL, Abate AC, et al. Reproductive steroid regulation of mood and behavior. Compr Physiol. 2016;6(3):1135-1160.
5. Shariff S, Cumming CE, Lees A, et al. Mood disorder in women with early breast cancer taking tamoxifen, an estradiol receptor antagonist. An expected or unexpected effect? Ann N Y Acad Sci. 1995;761:365-368.
6. Duffy LS, Greenberg DB, Younger J, et al. Iatrogenic acute estrogen deficiency and psychiatric syndromes in breast cancer patients. Psychosomatics. 1999;40(4):304-308.
7. Cathcart CK, Jones SE, Pumroy CS, et al. Clinical recognition and management of depression in node negative breast cancer patients treated with tamoxifen. Breast Cancer Res Treat. 1993;27(3):277-281.
8. Lin J, Thompson DS. Case report: tamoxifen-induced depression. Primary Care Update for Ob/Gyns. 2001;8(5):207-208.
9. Pluss JL, DiBella NJ. Reversible central nervous system dysfunction due to tamoxifen in a patient with breast cancer. Ann Intern Med. 1984;101(5):652.
10. Bourque F, Karama S, Looper K, et al. Acute tamoxifen-induced depression and its prevention with venlafaxine. Psychosomatics. 2009;50(2):162-165.
11. De Berardis D, Brucchi M, Serroni N, et al. Successful use of agomelatine in the treatment of major depression in a woman taking tamoxifen: a case report. Clin Neuropharmacol. 2014;37(1):31-33.
12. Ito M, Baba H, Kawashima R, et al. A case of prolonged depression with tamoxifen. Japan Med Assoc J. 2006;49(4):167-172.
13. Anelli TF, Anelli A, Tran KN, et al. Tamoxifen administration is associated with a high rate of treatment-limiting symptoms in male breast cancer patients. Cancer. 1994;74(1):74-77.
14. Love RR, Cameron L, Connell BL, et al. Symptoms associated with tamoxifen treatment in postmenopausal women. Arch Intern Med. 1991;151(9):1842-1847.
15. Lee KC, Ray GT, Hunkeler EM, et al. Tamoxifen treatment and new-onset depression in breast cancer patients. Psychosomatics. 2007;48(3):205-210.
16. Day R, Ganz PA, Costantino JP. Tamoxifen and depression: more evidence from the National Surgical Adjuvant Breast and Bowel Project’s Breast Cancer Prevention (P-1) Randomized Study. J Natl Cancer Inst. 2001;93(21):1615-1623.
17. Juurlink D. Revisiting the drug interaction between tamoxifen and SSRI antidepressants. BMJ. 2016;354:i5309.
18. Davies C, Godwin J, Gray R, et al. Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of randomised trials. Lancet. 2011;378(9793):771-784.
19. Buijs C, de Vries EGE, Mourits MJE, et al. The influence of endocrine treatments for breast cancer on health-related quality of life. Cancer Treat Rev. 2008;34(7):640-655.
CASE Worsening mood symptoms and suicidal ideation
On a recent visit to the oncology clinic, where she has been receiving treatment for breast cancer for 11 months, Mrs. L, age 46, reports the abrupt onset of sadness, irritability, difficulty sleeping, and negative self-thoughts.
Eleven months ago, Mrs. L was diagnosed with invasive lobular carcinoma of the right breast that was classified as T2N0MX, representing relatively early-stage disease. Shortly after her diagnosis, Mrs. L completed 4 cycles of neoadjuvant chemotherapy with doxorubicin and cyclophosphamide, followed by treatment with trastuzumab. Subsequently, she underwent a right segmental mastectomy with bilateral mastopexy and radiation therapy. Recently, Mrs. L’s oncology team prescribed tamoxifen, 20 mg/d, and trastuzumab, 420 mg IV every 3 weeks; however, within 3 weeks after starting tamoxifen, Mrs. L’s mood symptoms worsened to the point where she says she is considering suicide—with a plan to use her husband’s gun to kill herself.
Mrs. L has no other pertinent medical history and no reported history of psychiatric disease.
The primary oncology team discontinues tamoxifen (after 5 weeks of treatment) and refers Mrs. L to psychiatry for further mood evaluation.
[polldaddy:10497042]
The authors’ observations
The prevalence of depression is higher in patients with cancer than in the general population.1 The etiology of depression is often multifactorial.2 In Mrs. L’s case, we hypothesized that the possible cause of her depressive symptoms included concerns about her self-image after mastectomy and the adverse effects of chemotherapy and tamoxifen.
Among these possible causes, estrogen level is particularly important. Estrogen affects the brain in numerous ways, including by modulating different neurotransmitters,3,4 regulating neuroplasticity, providing neuroprotection by preventing formation of oxidative free radicals and of beta amyloid, and possibly avoiding inflammation. From a behavioral standpoint, estrogen acts as an antidepressant while enhancing memory and modulating maternal behavior.4 Therefore, decreased estrogen levels could result in depression and other neuropsychiatric problems. This is illustrated in Mrs. L’s case, where tamoxifen administered after breast cancer treatment coincided with the abrupt onset of depression with suicidal ideation.
Depression in patients receiving tamoxifen might be explained by the fact that tamoxifen is a selective estrogen receptor blocker with dual properties. Specifically, while it has antagonistic action in breast tissue, diminishing the growth-promoting action of estrogen on breast cancer cells, it additionally crosses the blood-brain barrier, so it may block the neuroprotective action of estrogen in the brain.
EXAMINATION Improvement in depression but slightly anxious
During her psychiatric examination, Mrs. L is fairly well-groomed and cooperative. Her speech is normal, thought process is organized, and she has fair insight into her medical situation, with fair judgment. She is alert, attentive, and oriented to time, place, as well as person. She confirms that she has no prior psychiatric history, including no prior suicide attempts. She lives with her husband, who has been supportive. Mrs. L has no children, and she continues to work.
Continue to: Mrs. L reports that per her oncology...
Mrs. L reports that per her oncology team’s instruction, she has not taken tamoxifen for almost 1 week, and notes improvement in her mood. She describes her mood as “fine now,” but appears slightly anxious. She adamantly denies suicidal ideation since stopping tamoxifen; however, she confirms that prior to stopping tamoxifen, she experienced low mood, suicidal thoughts, and a decreased interest in activities. Mrs. L’s Patient Health Questionnaire–9 score is 13, indicating moderate depression. She says she is constantly preoccupied with thoughts about the adverse effects of hormone therapy, and specifically about the oncology team’s suggestion of a retrial of tamoxifen. Due to her constant worry, she has difficulty relaxing; her Generalized Anxiety Disorder–7 item scale score is 12, indicating moderate anxiety. She has a history of cigarette smoking but stopped after her breast cancer diagnosis. She also reports gaining weight since beginning cancer treatment (body mass index: 28.0 kg/m2) and experiencing breast pain.
Mrs. L’s vital signs are normal. Results of her laboratory workup reveal a thyroid-stimulating hormone level of 1.40 µU/mL (reference range: 0.27 to 4.20 µU/mL); a follicle-stimulating hormone (FSH) level of 78.4 mIU/mL (postmenopausal reference range: 25.8 to 134.8 mIU/mL); and an estradiol level of <12.0 pg/mL (postmenopausal range: <55 pg/mL).
The authors’ observations
Studies investigating the effects of tamoxifen on mood have produced varying results (Table5-16). Some researchers have found a significant relationship between depression and tamoxifen in patients with breast cancer. In a case-control study, 42 postmenopausal women with breast cancer who received tamoxifen reported statistically significant elevated depression scores.5 Similarly, in a prospective trial that assessed mood symptoms in 21 pre- and postmenopausal women who developed estrogen deficiency during breast cancer treatment (including treatment with tamoxifen and chemotherapy), 38% of patients met the criteria for major depressive disorder (MDD) in the first 6 months of treatment. Sixty-six percent of these patients were postmenopausal, and 38% were premenopausal. Twenty-five percent of the premenopausal women who experienced MDD symptoms had been treated with tamoxifen and chemotherapy.6

In a larger prospective trial (N = 257), an oncologist assessed mood symptoms in 2 groups of patients with breast cancer: individuals who received tamoxifen, and those who did not receive tamoxifen.7 They found that 15% of patients who received tamoxifen experienced depression, compared with 3% of patients who did not receive tamoxifen; this difference was statistically significant.7 Overall, 31% of the patients had “significant depression” and 27% discontinued tamoxifen because of adverse effects.7 There have been 2 case reports of tamoxifen use and severe depression in patients with no prior psychiatry history8,9 and 3 case reports of tamoxifen use and severe depression in patients who had a psychiatric history.10-12
One study that examined 24 men with breast cancer found that 62.5% of these patients experienced adverse effects related to tamoxifen, and 25% discontinued tamoxifen because of its adverse effects.13 Among the various adverse effects related to tamoxifen, mood alteration was reported in 20.8% of cases, and depressed feelings were reported in 16.6%.13
Continue to: Despite the evidence...
Despite this evidence, other studies have not found an association between tamoxifen and depressed mood in patients with breast cancer. One group of researchers who assessed various symptoms self-reported by postmenopausal women who were breast cancer survivors found that the depression scores were not significant.14 A retrospective cohort study assessed the onset of depression in patients with breast cancer with positive hormone receptor status (who received tamoxifen) vs negative hormone receptor status (who did not receive tamoxifen). These researchers did not find a statistically significant hazard ratio for “new-onset depression.”15 Unfortunately, the criteria for “new-onset depression” used in this study was the diagnosis of depression or use of an antidepressant given or ordered by a clinician, which is not a sensitive assessment of depressed mood.15
A multicenter randomized, placebo-controlled trial (the National Surgical Adjuvant Breast and Bowel Project) assessed the incidence of negative health outcomes, including depression, in a secondary outcome analysis.16 These researchers did not find a statistically significantassociation between tamoxifen and depression. However, in this study, assessment of depression was based on self-report using the Center of Epidemiologic Studies Depression (CES-D) scale, which does not clinically categorize depression. Furthermore, these researchers strongly recommended screening for mood disorders in routine clinical practice. In this study, 3 women completed suicide, 2 of whom were in the tamoxifen arm.16
[polldaddy:10497045]
The authors’ observations
Tamoxifen is a prodrug that converts to the active metabolite, endoxifen, via cytochrome P450 2D6 (CYP2D6) activity. Antidepressants with strong 2D6-inhibiting properties, such as fluoxetine, duloxetine, bupropion, and paroxetine, should be avoided in patients receiving tamoxifen because they interfere with the formation of the active metabolite and could reduce the effectiveness of tamoxifen and its ability to reduce the risk of cancer recurrence.17 Antidepressants can help treat psychological distress, especially depression, which is common in patients with cancer, and vasomotor symptoms, which may impair quality of life and adherence to long-term endocrine therapy. Because tamoxifen can decrease cancer recurrence and associated mortality,18 adherence with treatment is crucial.
TREATMENT Starting an antidepressant
The psychiatry team initiates venlafaxine, 37.5 mg/d, to treat Mrs. L’s anxiety and help prevent the recurrence of severe depression. They prescribe venlafaxine because they anticipate that, based on Mrs. L’s age, the oncology team might reconsider treatment with tamoxifen. Venlafaxine is preferred because it has a favorable pharmacodynamic profile and does not interfere with the metabolism of tamoxifen, as is the case with many selective serotonin reuptake inhibitors.17
Although Mrs. L’s depression had abated once she stopped receiving tamoxifen, she continues to experience anxiety and tearfulness, primarily due to fear of adverse effects of hormone therapy, and due to family as well as work stressors. Therefore, venlafaxine is gradually titrated up to 150 mg/d.
Continue to: The oncology team proposes...
The oncology team proposes a trial of leuprolide, a gonadotropin-releasing hormone agonist that downregulates pituitary receptors, subsequently suppressing female reproductive hormones, which in turn stops the ovaries from producing estrogen so there is a minimal amount of estrogen to promote the growth of estrogen–receptor-positive breast cancer. Mrs. L declines this agent because she is concerned that she will gain weight. Instead, Mrs. L expresses interest in undergoing an oophorectomy to reduce her estrogen level. In the meantime, based on her reproductive hormone levels (FSH and estradiol levels) which are indicative of postmenopausal status, the oncology team prescribes the aromatase inhibitor (AI) exemestane 25 mg/d. The AI helps to decrease the amount of estrogen the body makes peripherally, which is the main source of estrogen in postmenopausal women.
The authors’ observations
Estrogen originates in the ovaries in premenopausal women; it is also produced by peripheral conversion of androgens to estrogen in adipose tissues and muscle in postmenopausal women.19 Aromatase inhibitors block the enzyme aromatase that converts androgen to estrogen, which leads to estrogen deficiency in postmenopausal women and possibly to neuropsychiatric effects.19
The results of studies assessing the adverse psychiatric effects of AIs are mixed. When the results of studies evaluating tamoxifen are compared with those evaluating AIs, overall patients who received AIs had less severe or less frequent mood symptoms. One possible explanation could be that AIs are relatively new compared with tamoxifen. Second, AIs are more commonly used in postmenopausal women with breast cancer, and these patients’ overall estrogen level is significantly lower than that of premenopausal women with breast cancer. Therefore, the degree of hormone fluctuation is less intense in postmenopausal breast cancer survivors.
OUTCOME
After starting exemestane, and while still receiving venlafaxine, Mrs. L no longer experiences severe depressive symptoms. After 8 months, venlafaxine is discontinued. She continues to deny depressive symptoms but has intermittent anxiety, which she is able to manage without psychiatric medication. She continues to remain adherent with ongoing exemestane treatment, with no evidence of disease progression or recurrence.
The authors’ observations
For patients with estrogen-positive breast cancer, the decision to discontinue tamoxifen because of unacceptable adverse effects is an important one because it may increase the risk of cancer recurrence. Psychiatrists have an important role in supporting the patient through this process, helping patients understand alternatives, and working with the oncology team to formulate a plan that is acceptable to everyone.
Continue to: Bottom Line
Bottom Line
For patients with estrogen–positive breast cancer, anti-estrogen treatment can reduce the risk of cancer recurrence. However, it can cause adverse effects, including depression, that might impair quality of life and treatment adherence. For patients with severe depression, stopping estrogen blockers may be warranted. Initiating an antidepressant that does not interfere with the metabolism of tamoxifen may help treat depression and vasomotor symptoms.
Related Resource
- Agarwala P. Tailoring depression treatment for women with breast cancer. Current Psychiatry. 2010;9(11):39-40,45-46,48-49.
Drug Brand Names
Agomelatine • Valdoxan
Bupropion • Wellbutrin, Zyban
Cyclophosphamide • Cytoxan
Doxorubicin • Adriamycin
Duloxetine • Cymbalta
Exemestane • Aromasin
Fluoxetine • Prozac
Leuprolide • Eligard, Lupron
Milnacipran • Savella
Paroxetine • Paxil
Sertraline • Zoloft
Tamoxifen • Soltamox
Trastuzumab • Herceptin
Venlafaxine • Effexor
CASE Worsening mood symptoms and suicidal ideation
On a recent visit to the oncology clinic, where she has been receiving treatment for breast cancer for 11 months, Mrs. L, age 46, reports the abrupt onset of sadness, irritability, difficulty sleeping, and negative self-thoughts.
Eleven months ago, Mrs. L was diagnosed with invasive lobular carcinoma of the right breast that was classified as T2N0MX, representing relatively early-stage disease. Shortly after her diagnosis, Mrs. L completed 4 cycles of neoadjuvant chemotherapy with doxorubicin and cyclophosphamide, followed by treatment with trastuzumab. Subsequently, she underwent a right segmental mastectomy with bilateral mastopexy and radiation therapy. Recently, Mrs. L’s oncology team prescribed tamoxifen, 20 mg/d, and trastuzumab, 420 mg IV every 3 weeks; however, within 3 weeks after starting tamoxifen, Mrs. L’s mood symptoms worsened to the point where she says she is considering suicide—with a plan to use her husband’s gun to kill herself.
Mrs. L has no other pertinent medical history and no reported history of psychiatric disease.
The primary oncology team discontinues tamoxifen (after 5 weeks of treatment) and refers Mrs. L to psychiatry for further mood evaluation.
[polldaddy:10497042]
The authors’ observations
The prevalence of depression is higher in patients with cancer than in the general population.1 The etiology of depression is often multifactorial.2 In Mrs. L’s case, we hypothesized that the possible cause of her depressive symptoms included concerns about her self-image after mastectomy and the adverse effects of chemotherapy and tamoxifen.
Among these possible causes, estrogen level is particularly important. Estrogen affects the brain in numerous ways, including by modulating different neurotransmitters,3,4 regulating neuroplasticity, providing neuroprotection by preventing formation of oxidative free radicals and of beta amyloid, and possibly avoiding inflammation. From a behavioral standpoint, estrogen acts as an antidepressant while enhancing memory and modulating maternal behavior.4 Therefore, decreased estrogen levels could result in depression and other neuropsychiatric problems. This is illustrated in Mrs. L’s case, where tamoxifen administered after breast cancer treatment coincided with the abrupt onset of depression with suicidal ideation.
Depression in patients receiving tamoxifen might be explained by the fact that tamoxifen is a selective estrogen receptor blocker with dual properties. Specifically, while it has antagonistic action in breast tissue, diminishing the growth-promoting action of estrogen on breast cancer cells, it additionally crosses the blood-brain barrier, so it may block the neuroprotective action of estrogen in the brain.
EXAMINATION Improvement in depression but slightly anxious
During her psychiatric examination, Mrs. L is fairly well-groomed and cooperative. Her speech is normal, thought process is organized, and she has fair insight into her medical situation, with fair judgment. She is alert, attentive, and oriented to time, place, as well as person. She confirms that she has no prior psychiatric history, including no prior suicide attempts. She lives with her husband, who has been supportive. Mrs. L has no children, and she continues to work.
Continue to: Mrs. L reports that per her oncology...
Mrs. L reports that per her oncology team’s instruction, she has not taken tamoxifen for almost 1 week, and notes improvement in her mood. She describes her mood as “fine now,” but appears slightly anxious. She adamantly denies suicidal ideation since stopping tamoxifen; however, she confirms that prior to stopping tamoxifen, she experienced low mood, suicidal thoughts, and a decreased interest in activities. Mrs. L’s Patient Health Questionnaire–9 score is 13, indicating moderate depression. She says she is constantly preoccupied with thoughts about the adverse effects of hormone therapy, and specifically about the oncology team’s suggestion of a retrial of tamoxifen. Due to her constant worry, she has difficulty relaxing; her Generalized Anxiety Disorder–7 item scale score is 12, indicating moderate anxiety. She has a history of cigarette smoking but stopped after her breast cancer diagnosis. She also reports gaining weight since beginning cancer treatment (body mass index: 28.0 kg/m2) and experiencing breast pain.
Mrs. L’s vital signs are normal. Results of her laboratory workup reveal a thyroid-stimulating hormone level of 1.40 µU/mL (reference range: 0.27 to 4.20 µU/mL); a follicle-stimulating hormone (FSH) level of 78.4 mIU/mL (postmenopausal reference range: 25.8 to 134.8 mIU/mL); and an estradiol level of <12.0 pg/mL (postmenopausal range: <55 pg/mL).
The authors’ observations
Studies investigating the effects of tamoxifen on mood have produced varying results (Table5-16). Some researchers have found a significant relationship between depression and tamoxifen in patients with breast cancer. In a case-control study, 42 postmenopausal women with breast cancer who received tamoxifen reported statistically significant elevated depression scores.5 Similarly, in a prospective trial that assessed mood symptoms in 21 pre- and postmenopausal women who developed estrogen deficiency during breast cancer treatment (including treatment with tamoxifen and chemotherapy), 38% of patients met the criteria for major depressive disorder (MDD) in the first 6 months of treatment. Sixty-six percent of these patients were postmenopausal, and 38% were premenopausal. Twenty-five percent of the premenopausal women who experienced MDD symptoms had been treated with tamoxifen and chemotherapy.6

In a larger prospective trial (N = 257), an oncologist assessed mood symptoms in 2 groups of patients with breast cancer: individuals who received tamoxifen, and those who did not receive tamoxifen.7 They found that 15% of patients who received tamoxifen experienced depression, compared with 3% of patients who did not receive tamoxifen; this difference was statistically significant.7 Overall, 31% of the patients had “significant depression” and 27% discontinued tamoxifen because of adverse effects.7 There have been 2 case reports of tamoxifen use and severe depression in patients with no prior psychiatry history8,9 and 3 case reports of tamoxifen use and severe depression in patients who had a psychiatric history.10-12
One study that examined 24 men with breast cancer found that 62.5% of these patients experienced adverse effects related to tamoxifen, and 25% discontinued tamoxifen because of its adverse effects.13 Among the various adverse effects related to tamoxifen, mood alteration was reported in 20.8% of cases, and depressed feelings were reported in 16.6%.13
Continue to: Despite the evidence...
Despite this evidence, other studies have not found an association between tamoxifen and depressed mood in patients with breast cancer. One group of researchers who assessed various symptoms self-reported by postmenopausal women who were breast cancer survivors found that the depression scores were not significant.14 A retrospective cohort study assessed the onset of depression in patients with breast cancer with positive hormone receptor status (who received tamoxifen) vs negative hormone receptor status (who did not receive tamoxifen). These researchers did not find a statistically significant hazard ratio for “new-onset depression.”15 Unfortunately, the criteria for “new-onset depression” used in this study was the diagnosis of depression or use of an antidepressant given or ordered by a clinician, which is not a sensitive assessment of depressed mood.15
A multicenter randomized, placebo-controlled trial (the National Surgical Adjuvant Breast and Bowel Project) assessed the incidence of negative health outcomes, including depression, in a secondary outcome analysis.16 These researchers did not find a statistically significantassociation between tamoxifen and depression. However, in this study, assessment of depression was based on self-report using the Center of Epidemiologic Studies Depression (CES-D) scale, which does not clinically categorize depression. Furthermore, these researchers strongly recommended screening for mood disorders in routine clinical practice. In this study, 3 women completed suicide, 2 of whom were in the tamoxifen arm.16
[polldaddy:10497045]
The authors’ observations
Tamoxifen is a prodrug that converts to the active metabolite, endoxifen, via cytochrome P450 2D6 (CYP2D6) activity. Antidepressants with strong 2D6-inhibiting properties, such as fluoxetine, duloxetine, bupropion, and paroxetine, should be avoided in patients receiving tamoxifen because they interfere with the formation of the active metabolite and could reduce the effectiveness of tamoxifen and its ability to reduce the risk of cancer recurrence.17 Antidepressants can help treat psychological distress, especially depression, which is common in patients with cancer, and vasomotor symptoms, which may impair quality of life and adherence to long-term endocrine therapy. Because tamoxifen can decrease cancer recurrence and associated mortality,18 adherence with treatment is crucial.
TREATMENT Starting an antidepressant
The psychiatry team initiates venlafaxine, 37.5 mg/d, to treat Mrs. L’s anxiety and help prevent the recurrence of severe depression. They prescribe venlafaxine because they anticipate that, based on Mrs. L’s age, the oncology team might reconsider treatment with tamoxifen. Venlafaxine is preferred because it has a favorable pharmacodynamic profile and does not interfere with the metabolism of tamoxifen, as is the case with many selective serotonin reuptake inhibitors.17
Although Mrs. L’s depression had abated once she stopped receiving tamoxifen, she continues to experience anxiety and tearfulness, primarily due to fear of adverse effects of hormone therapy, and due to family as well as work stressors. Therefore, venlafaxine is gradually titrated up to 150 mg/d.
Continue to: The oncology team proposes...
The oncology team proposes a trial of leuprolide, a gonadotropin-releasing hormone agonist that downregulates pituitary receptors, subsequently suppressing female reproductive hormones, which in turn stops the ovaries from producing estrogen so there is a minimal amount of estrogen to promote the growth of estrogen–receptor-positive breast cancer. Mrs. L declines this agent because she is concerned that she will gain weight. Instead, Mrs. L expresses interest in undergoing an oophorectomy to reduce her estrogen level. In the meantime, based on her reproductive hormone levels (FSH and estradiol levels) which are indicative of postmenopausal status, the oncology team prescribes the aromatase inhibitor (AI) exemestane 25 mg/d. The AI helps to decrease the amount of estrogen the body makes peripherally, which is the main source of estrogen in postmenopausal women.
The authors’ observations
Estrogen originates in the ovaries in premenopausal women; it is also produced by peripheral conversion of androgens to estrogen in adipose tissues and muscle in postmenopausal women.19 Aromatase inhibitors block the enzyme aromatase that converts androgen to estrogen, which leads to estrogen deficiency in postmenopausal women and possibly to neuropsychiatric effects.19
The results of studies assessing the adverse psychiatric effects of AIs are mixed. When the results of studies evaluating tamoxifen are compared with those evaluating AIs, overall patients who received AIs had less severe or less frequent mood symptoms. One possible explanation could be that AIs are relatively new compared with tamoxifen. Second, AIs are more commonly used in postmenopausal women with breast cancer, and these patients’ overall estrogen level is significantly lower than that of premenopausal women with breast cancer. Therefore, the degree of hormone fluctuation is less intense in postmenopausal breast cancer survivors.
OUTCOME
After starting exemestane, and while still receiving venlafaxine, Mrs. L no longer experiences severe depressive symptoms. After 8 months, venlafaxine is discontinued. She continues to deny depressive symptoms but has intermittent anxiety, which she is able to manage without psychiatric medication. She continues to remain adherent with ongoing exemestane treatment, with no evidence of disease progression or recurrence.
The authors’ observations
For patients with estrogen-positive breast cancer, the decision to discontinue tamoxifen because of unacceptable adverse effects is an important one because it may increase the risk of cancer recurrence. Psychiatrists have an important role in supporting the patient through this process, helping patients understand alternatives, and working with the oncology team to formulate a plan that is acceptable to everyone.
Continue to: Bottom Line
Bottom Line
For patients with estrogen–positive breast cancer, anti-estrogen treatment can reduce the risk of cancer recurrence. However, it can cause adverse effects, including depression, that might impair quality of life and treatment adherence. For patients with severe depression, stopping estrogen blockers may be warranted. Initiating an antidepressant that does not interfere with the metabolism of tamoxifen may help treat depression and vasomotor symptoms.
Related Resource
- Agarwala P. Tailoring depression treatment for women with breast cancer. Current Psychiatry. 2010;9(11):39-40,45-46,48-49.
Drug Brand Names
Agomelatine • Valdoxan
Bupropion • Wellbutrin, Zyban
Cyclophosphamide • Cytoxan
Doxorubicin • Adriamycin
Duloxetine • Cymbalta
Exemestane • Aromasin
Fluoxetine • Prozac
Leuprolide • Eligard, Lupron
Milnacipran • Savella
Paroxetine • Paxil
Sertraline • Zoloft
Tamoxifen • Soltamox
Trastuzumab • Herceptin
Venlafaxine • Effexor
1. Zabora J, BrintzenhofeSzoc K, Curbow B, et al. The prevalence of psychological distress by cancer site. Psychooncology. 2001;10(1):19-28.
2. Thompson DS, Spanier CA, Vogel VG. The relationship between tamoxifen, estrogen, and depressive symptoms. Breast J. 1999;5(6):375-382.
3. Halbreich U. Role of estrogen in postmenopausal depression. Neurology. 1997;48(5 suppl 7):S16-S19.
4. Schiller CE, Johnson SL, Abate AC, et al. Reproductive steroid regulation of mood and behavior. Compr Physiol. 2016;6(3):1135-1160.
5. Shariff S, Cumming CE, Lees A, et al. Mood disorder in women with early breast cancer taking tamoxifen, an estradiol receptor antagonist. An expected or unexpected effect? Ann N Y Acad Sci. 1995;761:365-368.
6. Duffy LS, Greenberg DB, Younger J, et al. Iatrogenic acute estrogen deficiency and psychiatric syndromes in breast cancer patients. Psychosomatics. 1999;40(4):304-308.
7. Cathcart CK, Jones SE, Pumroy CS, et al. Clinical recognition and management of depression in node negative breast cancer patients treated with tamoxifen. Breast Cancer Res Treat. 1993;27(3):277-281.
8. Lin J, Thompson DS. Case report: tamoxifen-induced depression. Primary Care Update for Ob/Gyns. 2001;8(5):207-208.
9. Pluss JL, DiBella NJ. Reversible central nervous system dysfunction due to tamoxifen in a patient with breast cancer. Ann Intern Med. 1984;101(5):652.
10. Bourque F, Karama S, Looper K, et al. Acute tamoxifen-induced depression and its prevention with venlafaxine. Psychosomatics. 2009;50(2):162-165.
11. De Berardis D, Brucchi M, Serroni N, et al. Successful use of agomelatine in the treatment of major depression in a woman taking tamoxifen: a case report. Clin Neuropharmacol. 2014;37(1):31-33.
12. Ito M, Baba H, Kawashima R, et al. A case of prolonged depression with tamoxifen. Japan Med Assoc J. 2006;49(4):167-172.
13. Anelli TF, Anelli A, Tran KN, et al. Tamoxifen administration is associated with a high rate of treatment-limiting symptoms in male breast cancer patients. Cancer. 1994;74(1):74-77.
14. Love RR, Cameron L, Connell BL, et al. Symptoms associated with tamoxifen treatment in postmenopausal women. Arch Intern Med. 1991;151(9):1842-1847.
15. Lee KC, Ray GT, Hunkeler EM, et al. Tamoxifen treatment and new-onset depression in breast cancer patients. Psychosomatics. 2007;48(3):205-210.
16. Day R, Ganz PA, Costantino JP. Tamoxifen and depression: more evidence from the National Surgical Adjuvant Breast and Bowel Project’s Breast Cancer Prevention (P-1) Randomized Study. J Natl Cancer Inst. 2001;93(21):1615-1623.
17. Juurlink D. Revisiting the drug interaction between tamoxifen and SSRI antidepressants. BMJ. 2016;354:i5309.
18. Davies C, Godwin J, Gray R, et al. Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of randomised trials. Lancet. 2011;378(9793):771-784.
19. Buijs C, de Vries EGE, Mourits MJE, et al. The influence of endocrine treatments for breast cancer on health-related quality of life. Cancer Treat Rev. 2008;34(7):640-655.
1. Zabora J, BrintzenhofeSzoc K, Curbow B, et al. The prevalence of psychological distress by cancer site. Psychooncology. 2001;10(1):19-28.
2. Thompson DS, Spanier CA, Vogel VG. The relationship between tamoxifen, estrogen, and depressive symptoms. Breast J. 1999;5(6):375-382.
3. Halbreich U. Role of estrogen in postmenopausal depression. Neurology. 1997;48(5 suppl 7):S16-S19.
4. Schiller CE, Johnson SL, Abate AC, et al. Reproductive steroid regulation of mood and behavior. Compr Physiol. 2016;6(3):1135-1160.
5. Shariff S, Cumming CE, Lees A, et al. Mood disorder in women with early breast cancer taking tamoxifen, an estradiol receptor antagonist. An expected or unexpected effect? Ann N Y Acad Sci. 1995;761:365-368.
6. Duffy LS, Greenberg DB, Younger J, et al. Iatrogenic acute estrogen deficiency and psychiatric syndromes in breast cancer patients. Psychosomatics. 1999;40(4):304-308.
7. Cathcart CK, Jones SE, Pumroy CS, et al. Clinical recognition and management of depression in node negative breast cancer patients treated with tamoxifen. Breast Cancer Res Treat. 1993;27(3):277-281.
8. Lin J, Thompson DS. Case report: tamoxifen-induced depression. Primary Care Update for Ob/Gyns. 2001;8(5):207-208.
9. Pluss JL, DiBella NJ. Reversible central nervous system dysfunction due to tamoxifen in a patient with breast cancer. Ann Intern Med. 1984;101(5):652.
10. Bourque F, Karama S, Looper K, et al. Acute tamoxifen-induced depression and its prevention with venlafaxine. Psychosomatics. 2009;50(2):162-165.
11. De Berardis D, Brucchi M, Serroni N, et al. Successful use of agomelatine in the treatment of major depression in a woman taking tamoxifen: a case report. Clin Neuropharmacol. 2014;37(1):31-33.
12. Ito M, Baba H, Kawashima R, et al. A case of prolonged depression with tamoxifen. Japan Med Assoc J. 2006;49(4):167-172.
13. Anelli TF, Anelli A, Tran KN, et al. Tamoxifen administration is associated with a high rate of treatment-limiting symptoms in male breast cancer patients. Cancer. 1994;74(1):74-77.
14. Love RR, Cameron L, Connell BL, et al. Symptoms associated with tamoxifen treatment in postmenopausal women. Arch Intern Med. 1991;151(9):1842-1847.
15. Lee KC, Ray GT, Hunkeler EM, et al. Tamoxifen treatment and new-onset depression in breast cancer patients. Psychosomatics. 2007;48(3):205-210.
16. Day R, Ganz PA, Costantino JP. Tamoxifen and depression: more evidence from the National Surgical Adjuvant Breast and Bowel Project’s Breast Cancer Prevention (P-1) Randomized Study. J Natl Cancer Inst. 2001;93(21):1615-1623.
17. Juurlink D. Revisiting the drug interaction between tamoxifen and SSRI antidepressants. BMJ. 2016;354:i5309.
18. Davies C, Godwin J, Gray R, et al. Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of randomised trials. Lancet. 2011;378(9793):771-784.
19. Buijs C, de Vries EGE, Mourits MJE, et al. The influence of endocrine treatments for breast cancer on health-related quality of life. Cancer Treat Rev. 2008;34(7):640-655.