User login
Suicidal, violent, and treatment-resistant
CASE Violent, then catatonic
Mr. T, age 52, has a long history of schizoaffective disorder, depressed type; several suicide attempts; and violent episodes. He is admitted to a mental health rehabilitation center under a forensic commitment.
Several years earlier, Mr. T had been charged with first-degree attempted murder, assault with a deadly weapon, and abuse of a dependent/geriatric adult after allegedly stabbing his mother in the upper chest and neck. At that time, Mr. T was not in psychiatric treatment and was drinking heavily. He had become obsessed with John F. Kennedy’s assassination and believed the Central Intelligence Agency (CIA), not Lee Harvey Oswald, was responsible. He feared the CIA wanted to kill him because of his knowledge, and he heard voices from his television he believed were threatening him. He acquired knives for self-protection. When his mother arrived at his apartment to take him to a psychiatric appointment, he believed she was conspiring with the CIA and attacked her. Mr. T’s mother survived her injuries. He was taken to the county jail, where psychiatric staff noted that Mr. T was psychotic.
The court found Mr. T incompetent to stand trial and sent him to a state hospital for psychiatric treatment and competency restoration. After 3 years, he was declared unable to be restored because of repeated decompensations, placed on a conservatorship, and sent back to county jail.
In the jail, Mr. T began to show signs of catatonia. He refused medications, food, and water, and became mute. He was admitted to a medical center after a 45-minute episode that appeared similar to a seizure; however, all laboratory evaluations were within normal limits, head CT was negative, and an EEG was unremarkable.
Mr. T’s catatonic state gradually resolved with increasing dosages of lorazepam, as well as clozapine. He showed improved mobility and oral intake. A month later, his train of thought was rambling and difficult to follow, circumstantial, and perseverating. However, at times he could be directed and respond to questions in a linear and logical fashion. Lorazepam was tapered, discontinued, and replaced with gabapentin because Mr. T viewed taking lorazepam as a threat to his sobriety.
Recently, Mr. T was transferred to our mental health rehabilitation center, where he expresses that he is grateful to be in a therapeutic environment. Upon admission, his medication regimen consists of clozapine, 300 mg by mouth at bedtime, duloxetine, 60 mg/d by mouth, gabapentin 600 mg by mouth 3 times a day, and docusate sodium, 250 mg/d by mouth. Our team has a discussion about the growing recognition of the pro-inflammatory state present in many patients who experience serious mental illness and the importance of augmenting standard evidence-based psychopharmacotherapy with agents that have neuroprotective properties.1,2 We offer Mr. T
[polldaddy:10375843]
The authors’ observations
Several studies have found that acute psychosis is associated with an inflammatory state, and interleukin-6 (IL-6) is a crucial biomarker. A recent meta-analysis of serum cytokines in patients with schizophrenia found that IL-6 levels were significantly increased among acutely ill patients compared with controls.3 IL-6 levels significantly decreased after treating acute episodes of schizophrenia.3 Further, levels of peripheral IL-6 mRNA levels in individuals with schizophrenia are directly correlated with severity of positive symptoms.4
Continue to: A meta-analyis reported...
A meta-analysis reported that tumor necrosis factor-alpha and IL-6 are elevated during acute psychosis3; however, IL-6 normalized with treatment, whereas tumor necrosis factor-alpha did not. This means that IL-6 is a more clinically meaningful biomarker to help gauge treatment response.
EVALUATION Elevated markers of inflammation
Laboratory testing reveals that Mr. T’s IL-6 level is 56.64 pg/mL, which is significantly elevated (reference range: 0.31 to 5.00 pg/mL). After reviewing the IL-6 results with Mr. T and explaining that there is “too much inflammation” in his brain, he agrees to take minocycline and complete follow-up IL-6 level tests to monitor his progress during treatment.
HISTORY Alcohol abuse, treatment resistance
According to Mr. T’s mother, he had met all developmental milestones and graduated from high school with plans to enter culinary school. At age 20, Mr. T began to experience psychotic symptoms, telling family members that he was being followed by FBI agents and was receiving messages from televisions. He began drinking heavily and was arrested twice for driving under the influence. In his mid-20s, he attempted suicide by overdose after his father died. Mr. T required inpatient hospitalization nearly every year thereafter. His mother, a registered nurse, was significantly involved in his care and carefully documented his treatment history.
Mr. T has had numerous medication trials, including oral and long-acting injectable risperidone, olanzapine, aripiprazole, ziprasidone, lithium, gabapentin, buspirone, quetiapine, trazodone, bupropion, and paroxetine. None of these medications were effective.
In his mid-40s, Mr. T attempted suicide by wandering into traffic and being struck by a motor vehicle. A year later, he attempted suicide by driving his car at high speed into a concrete highway median. Mr. T told first responders that he was “possessed,” and a demonic entity “forced” him to crash his car. He begged law enforcement officers at the scene to give him a gun so he could shoot himself.
Continue to: Mr. T entered an intensive outpatient treatment program...
Mr. T entered an intensive outpatient treatment program and was switched from long-acting injectable risperidone to oral aripiprazole. After taking aripiprazole for several weeks, he began to gamble compulsively at a nearby casino. Frustrated by the lack of response to psychotropic medications and his idiosyncratic response to aripiprazole, he stopped psychiatric treatment, relapsed to alcohol use, and isolated himself in his apartment shortly before stabbing his mother.
EVALUATION Pharmacogenomics testing
At the mental health rehabilitation center, Mr. T agrees to undergo pharmacogenomics testing, which suggests that he will have a normal response to selective serotonin reuptake inhibitors and is unlikely to experience adverse reactions. He does not carry the 2 alleles that place him at higher risk of serious dermatologic reactions when taking certain mood stabilizers. He is heterozygous for the C677T allele polymorphism in the MTHFR gene that is associated with reduced folic acid metabolism, moderately decreased serum folate levels, and moderately increased homocysteine levels. On the pharmacokinetic genes tested, Mr. T has the normal metabolism genotype on 5 of 6 cytochrome P450 (CYP) enzymes; he has the ultrarapid metabolizer genotype on CYP1A2. He also has normal activity and intermediate metabolizer phenotype on the 2 UGT enzymes tested, which are responsible for the glucuronidation process, a major part of phase II metabolism.
Based on these results, Mr. T’s clozapine dosage is decreased by 50% (from 300 to 150 mg/d) and he is started on fluvoxamine, 50 mg/d, because it is a strong inhibitor of CYP1A2. The reduced conversion of clozapine to norclozapine results in an average serum clozapine level of 527 ng/mL (a level of 350 ng/mL is usually therapeutic in patients with schizophrenia) and norclozapine level of 140 ng/mL (clozapine:norclozapine ratio = 3.8), which is to be expected because fluvoxamine can increase serum clozapine levels.
Due to accumulating evidence in the literature suggesting that latent infections in the CNS play a role in serious mental illnesses such as schizophrenia, Mr. T undergoes further laboratory testing.
[polldaddy:10375845]
The authors’ observations
Mr. T tested positive for TG and CMV and negative for HSV-1. We were aware of accumulating evidence that latent infections in the CNS play a role in serious mental illnesses such as schizophrenia, specifically TG5—a parasite transmitted by cats—and CMV and HSV-1,6 which are transmitted by humans. The theory that TG infection could be a factor in schizophrenia emerged in the 1990s but only in recent years received mainstream scientific attention. Toxoplasma gondii, the infectious parasite that causes toxoplasmosis, infects more than 30 million people in the United States; however, most individuals are asymptomatic because of the body’s immune response to the parasite.7
Continue to: A study of 162 individuals...
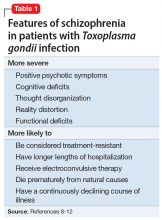
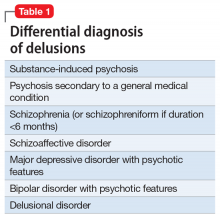
A study of 162 individuals with schizophrenia, bipolar disorder, or major depressive disorder found that this immunologic profile is associated with suicide attempts,8 which is consistent with Mr. T’s history. Research suggests that individuals with schizophrenia who have latent TG infection have a more severe form of the illness compared with patients without the infection.9-12 Many of these factors were present in Mr. T’s case (Table 18-12).
TREATMENT Improvement, then setback
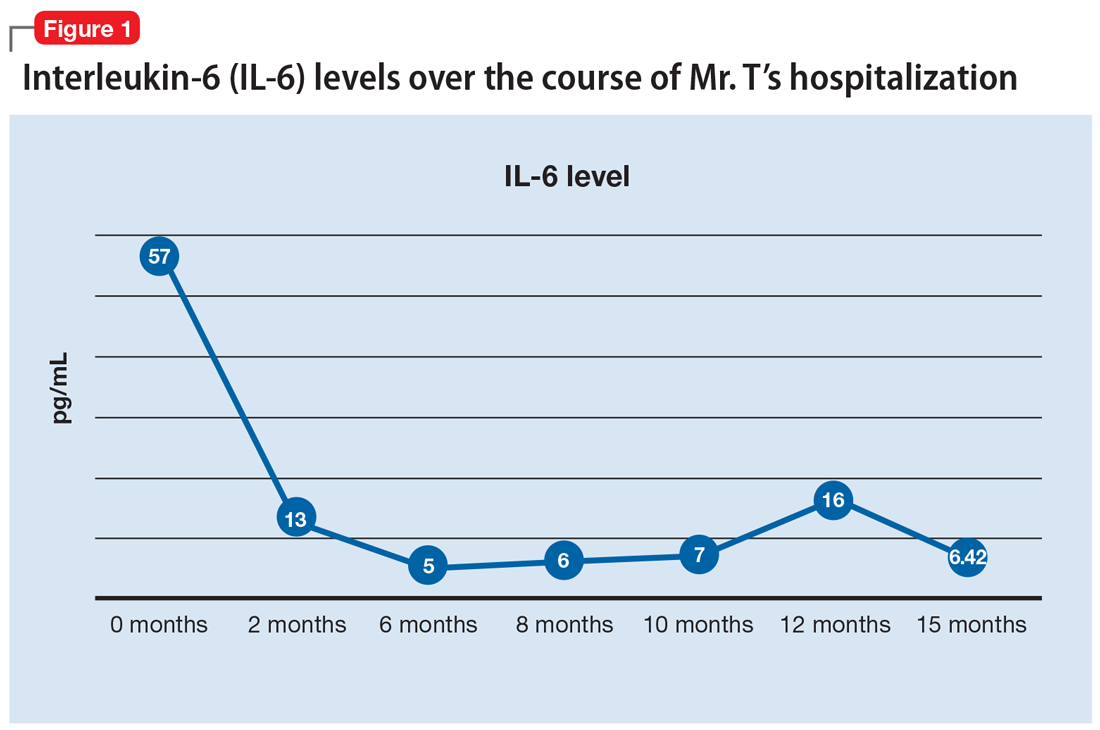
Mr. T’s medication regimen at the rehabilitation center includes clozapine, 100 mg/d; minocycline, 200 mg/d; fluvoxamine, 200 mg/d; and N-acetylcysteine, 1,200 mg/d. N-acetylcysteine is an antioxidant that could ease negative symptoms of schizophrenia by reducing oxidative stress caused by free radicals.13 Mr. T makes slow but steady improvement, and his IL-6 levels drop steadily (Figure 1).
After 6 months in the rehabilitation center, Mr. T no longer experiences catatonic symptoms and is able to participate in the therapeutic program. He is permitted to leave the facility on day passes with family members. However, approximately every 8 weeks, he continues to cycle through periods of intense anxiety, perseverates on topics, and exhibits fragmented thinking and speech. During these episodes, he has difficulty receiving and processing information.
During one of these periods, Mr. T eats 4 oleander leaves he gathered while on day pass outside of the facility. After he experiences stomach pain, nausea, and vomiting, he informs nursing staff that he ate oleander. He is brought to the emergency department, receives activated charcoal and a digoxin antidote, and is placed on continuous electrocardiogram monitoring. When asked why he made the suicide attempt, he said “I realized things will never be the same because of what happened. I felt trapped.” He later expresses regret and wants to return to the mental health rehabilitation center.
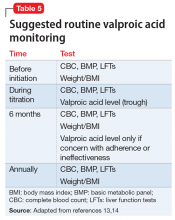
At the facility, Mr. T agrees to take 2 more agents—valproic acid and ginger root extract—that specifically target latent toxoplasmosis infection before pursuing electroconvulsive therapy. We offer valproic acid because it inhibits replication of TG in an in vitro model.14 Mr. T is started on extended-release valproic acid, 1,500 mg/d, which results in a therapeutic serum level of 74.8 µg/mL.
Continue to: Additionally, Mr. T expresses interest...
Additionally, Mr. T expresses interest in taking “natural” agents in addition to psychotropics. After reviewing the quality of available ginger root extract products, Mr. T is started on a supplement that contains 22.4 mg of gingerols and 6.7 mg of shogaols, titrated to 4 capsules twice daily.
The authors’ observations
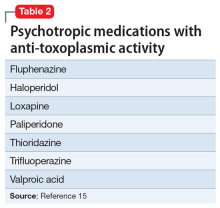
A retrospective cross-sectional analysis reported that patients with bipolar disorder who received medications with anti-toxoplasmic activity (Table 215), specifically valproic acid, had significantly fewer lifetime depressive episodes compared with patients who received medications without anti-toxoplasmic activity.15
Alternative medicine options
Research has demonstrated the beneficial effects of Chinese herbal plants for toxoplasmosis16,17 and ginger root extract has potent anti-toxoplasmic activity. A mouse model found that ginger root extract (Zingiber officinale) reduced the number of TG-infected cells by suppressing activation of apoptotic proteins the parasite induces, which prevents programmed cell death.18
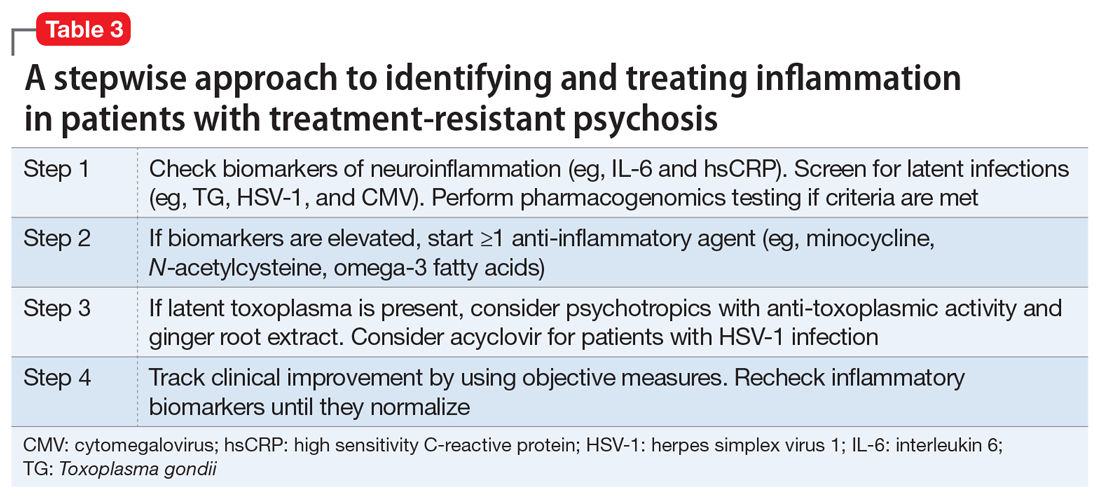
Table 3 presents a stepwise approach to identifying and treating inflammation in patients with treatment-resistant psychosis.
OUTCOME Immune response, improvement
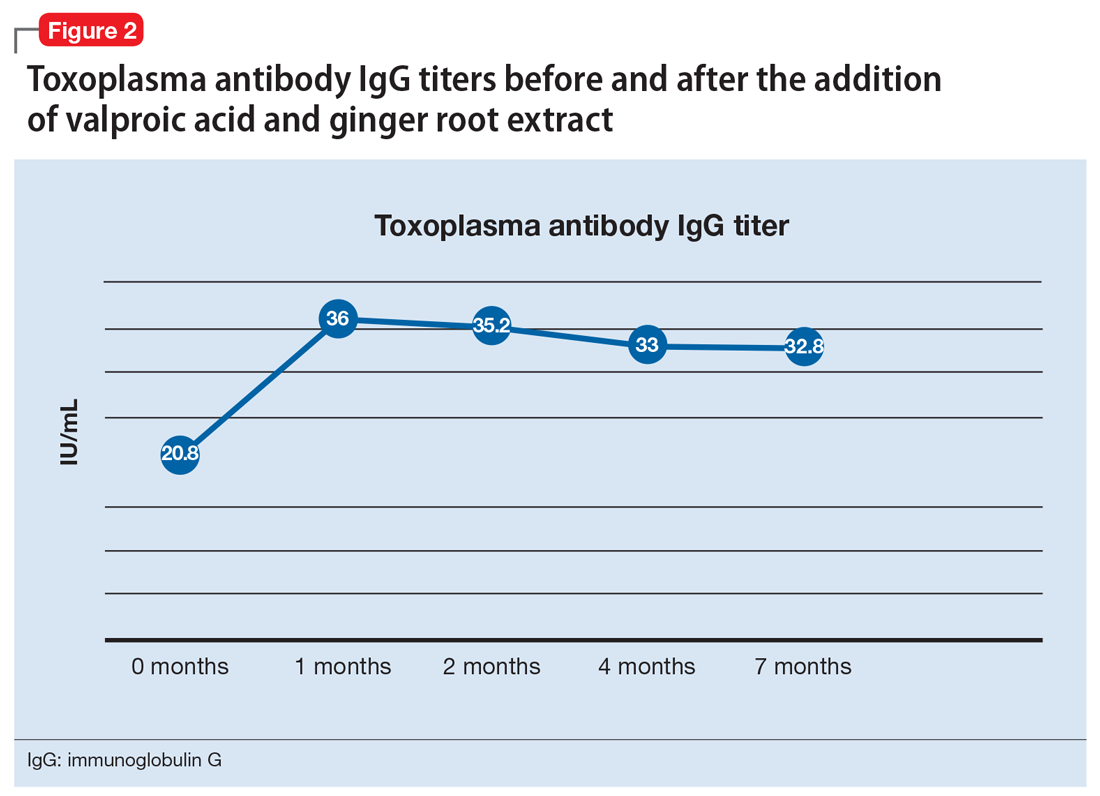
One month after the valproic acid and ginger root extract therapy is initiated, Mr. T’s toxoplasma antibody immunoglobulin G increases by 15.2 IU/mL, indicating that his immune system is mounting an enhanced response against the parasite (Figure 2). Mr. T continues to make progress while receiving the new regimen of clozapine, minocycline, valproic acid, and ginger root extract. He no longer cycles into periods of intense anxiety, perseverative thought, and fragmented thought and speech. He participates meaningfully in weekly psychotherapy and hopes to live independently and obtain gainful employment.
The District Attorney’s office dismisses his criminal charges, and Mr. T is discharged to a less restrictive level of care.
Continue to: Bottom Line
Bottom Line
Several studies have shown that neuroinflammation increases the severity of mental illness. Consider adjunct anti-inflammatory agents for patients who have elevated levels of inflammatory biomarkers and for whom standard treatment approaches do not adequately control psychiatric symptoms. Also consider testing for the presence of latent infections in the CNS, which could reveal the underlying cause of treatment resistance or the genesis of disabling psychiatric symptoms.
Related Resources
- Fond G, Macgregor A, Tamouza R, et al. Comparative analysis of anti-toxoplasmic activity of antipsychotic drugs and valproate. Eur Arch Psychiatry Clin Neurosci. 2014;264(2):179-183.
- Hamdani N, Daban-Huard C, Lajnef M, et al. Cognitive deterioration among bipolar disorder patients infected by Toxoplasma gondii is correlated to interleukin 6 levels. J Affect Disord. 2015;179:161-166.
- Monroe JM, Buckley PF, Miller BJ. Meta-analysis of antitoxoplasma gondii IgM antibodies in acute psychosis. Schizophr Bull. 2015;41(4):989-998.
Drug Brand Names
Acyclovir • Zovirax
Aripiprazole • Abilify
Bupropion • Wellbutrin
Buspirone • Buspar
Clozapine • Clozaril
Duloxetine • Cymbalta
Fluphenazine • Prolixin
Fluvoxamine • Luvox
Gabapentin • Neurontin
Haloperidol • Haldol
Lithium • Eskalith, Lithobid
Lorazepam • Ativan
Loxapine • Loxitane
Minocycline • Minocin
Olanzapine • Zyprexa
Paliperidone • Invega
Paroxetine • Paxil
Quetiapine • Seroquel
Risperidone • Risperdal, Risperdal Consta
Thioridazine • Mellaril
Trifluoperazine • Stelazine
Trazodone • Desyrel
Valproic acid • Depakote
Ziprasidone • Geodon
1. Koola MM, Raines JK, Hamilton RG, et al. Can anti-inflammatory medications improve symptoms and reduce mortality in schizophrenia? Current Psychiatry. 2016;15(5):52-57.
2. Nasrallah HA. Are you neuroprotecting your patients? 10 Adjunctive therapies to consider. Current Psychiatry. 2016;15(12):12-14.
3. Goldsmith DR, Rapaport MH, Miller BJ. A meta-analysis of blood cytokine network alterations in psychiatric patients: comparisons between schizophrenia, bipolar disorder and depression. Mol Psychiatry. 2016;21(12):1696-1709.
4. Chase KA, Cone JJ, Rosen C, et al. The value of interleukin 6 as a peripheral diagnostic marker in schizophrenia. BMC Psychiatry. 2016;16:152.
5. Torrey EF, Bartko JJ, Lun ZR, et al. Antibodies to Toxoplasma gondii in patients with schizophrenia: a meta-analysis. Schizophr Bull. 2007;33(3):729-736.
6. Shirts BH, Prasad KM, Pogue-Geile MF, et al. Antibodies to cytomegalovirus and herpes simplex virus 1 associated with cognitive function in schizophrenia. Schizophr Res. 2008;106(2-3):268-274.
7. Centers for Disease Control and Prevention. Parasites - Toxoplasmosis (Toxoplasma infection). https://www.cdc.gov/parasites/toxoplasmosis/index.html. Accessed February 26, 2019.
8. Dickerson F, Wilcox HC, Adamos M, et al. Suicide attempts and markers of immune response in individuals with serious mental illness. J Psychiatr Res. 2017;87:37-43.
9. Celik T, Kartalci S, Aytas O, et al. Association between latent toxoplasmosis and clinical course of schizophrenia - continuous course of the disease is characteristic for Toxoplasma gondii-infected patients. Folia Parasitol (Praha). 2015;62. doi: 10.14411/fp.2015.015.
10. Dickerson F, Boronow J, Stallings C, et al. Toxoplasma gondii in individuals with schizophrenia: association with clinical and demographic factors and with mortality. Schizophr Bull. 2007;33(3):737-740.
11. Esshili A, Thabet S, Jemli A, et al. Toxoplasma gondii infection in schizophrenia and associated clinical features. Psychiatry Res. 2016;245:327-332.
12. Holub D, Flegr J, Dragomirecka E, et al. Differences in onset of disease and severity of psychopathology between toxoplasmosis-related and toxoplasmosis-unrelated schizophrenia. Acta Psychiatr Scand. 2013;127(3):227-238.
13. Chen AT, Chibnall JT, Nasrallah HA. Placebo-controlled augmentation trials of the antioxidant NAC in schizophrenia: a review. Ann Clin Psychiatry. 2016;28(3):190-196.
14. Jones-Brando L, Torrey EF, Yolken R. Drugs used in the treatment of schizophrenia and bipolar disorder inhibit the replication of Toxoplasma gondii. Schizophr Res. 2003;62(3):237-244.
15. Fond G, Boyer L, Gaman A, et al. Treatment with anti-toxoplasmic activity (TATA) for toxoplasma positive patients with bipolar disorders or schizophrenia: a cross-sectional study. J Psychiatr Res. 2015;63:58-64.
16. Wei HX, Wei SS, Lindsay DS, et al. A systematic review and meta-analysis of the efficacy of anti-Toxoplasma gondii medicines in humans. PLoS One. 2015;10(9):e0138204.
17. Zhuo XH, Sun HC, Huang B, et al. Evaluation of potential anti-toxoplasmosis efficiency of combined traditional herbs in a mouse model. J Zhejiang Univ Sci B. 2017;18(6):453-461.
18. Choi WH, Jiang MH, Chu JP. Antiparasitic effects of Zingiber officinale (Ginger) extract against Toxoplasma gondii. Journal of Applied Biomedicine. 2013;11:15-26.
CASE Violent, then catatonic
Mr. T, age 52, has a long history of schizoaffective disorder, depressed type; several suicide attempts; and violent episodes. He is admitted to a mental health rehabilitation center under a forensic commitment.
Several years earlier, Mr. T had been charged with first-degree attempted murder, assault with a deadly weapon, and abuse of a dependent/geriatric adult after allegedly stabbing his mother in the upper chest and neck. At that time, Mr. T was not in psychiatric treatment and was drinking heavily. He had become obsessed with John F. Kennedy’s assassination and believed the Central Intelligence Agency (CIA), not Lee Harvey Oswald, was responsible. He feared the CIA wanted to kill him because of his knowledge, and he heard voices from his television he believed were threatening him. He acquired knives for self-protection. When his mother arrived at his apartment to take him to a psychiatric appointment, he believed she was conspiring with the CIA and attacked her. Mr. T’s mother survived her injuries. He was taken to the county jail, where psychiatric staff noted that Mr. T was psychotic.
The court found Mr. T incompetent to stand trial and sent him to a state hospital for psychiatric treatment and competency restoration. After 3 years, he was declared unable to be restored because of repeated decompensations, placed on a conservatorship, and sent back to county jail.
In the jail, Mr. T began to show signs of catatonia. He refused medications, food, and water, and became mute. He was admitted to a medical center after a 45-minute episode that appeared similar to a seizure; however, all laboratory evaluations were within normal limits, head CT was negative, and an EEG was unremarkable.
Mr. T’s catatonic state gradually resolved with increasing dosages of lorazepam, as well as clozapine. He showed improved mobility and oral intake. A month later, his train of thought was rambling and difficult to follow, circumstantial, and perseverating. However, at times he could be directed and respond to questions in a linear and logical fashion. Lorazepam was tapered, discontinued, and replaced with gabapentin because Mr. T viewed taking lorazepam as a threat to his sobriety.
Recently, Mr. T was transferred to our mental health rehabilitation center, where he expresses that he is grateful to be in a therapeutic environment. Upon admission, his medication regimen consists of clozapine, 300 mg by mouth at bedtime, duloxetine, 60 mg/d by mouth, gabapentin 600 mg by mouth 3 times a day, and docusate sodium, 250 mg/d by mouth. Our team has a discussion about the growing recognition of the pro-inflammatory state present in many patients who experience serious mental illness and the importance of augmenting standard evidence-based psychopharmacotherapy with agents that have neuroprotective properties.1,2 We offer Mr. T
[polldaddy:10375843]
The authors’ observations
Several studies have found that acute psychosis is associated with an inflammatory state, and interleukin-6 (IL-6) is a crucial biomarker. A recent meta-analysis of serum cytokines in patients with schizophrenia found that IL-6 levels were significantly increased among acutely ill patients compared with controls.3 IL-6 levels significantly decreased after treating acute episodes of schizophrenia.3 Further, levels of peripheral IL-6 mRNA levels in individuals with schizophrenia are directly correlated with severity of positive symptoms.4
Continue to: A meta-analyis reported...
A meta-analysis reported that tumor necrosis factor-alpha and IL-6 are elevated during acute psychosis3; however, IL-6 normalized with treatment, whereas tumor necrosis factor-alpha did not. This means that IL-6 is a more clinically meaningful biomarker to help gauge treatment response.
EVALUATION Elevated markers of inflammation
Laboratory testing reveals that Mr. T’s IL-6 level is 56.64 pg/mL, which is significantly elevated (reference range: 0.31 to 5.00 pg/mL). After reviewing the IL-6 results with Mr. T and explaining that there is “too much inflammation” in his brain, he agrees to take minocycline and complete follow-up IL-6 level tests to monitor his progress during treatment.
HISTORY Alcohol abuse, treatment resistance
According to Mr. T’s mother, he had met all developmental milestones and graduated from high school with plans to enter culinary school. At age 20, Mr. T began to experience psychotic symptoms, telling family members that he was being followed by FBI agents and was receiving messages from televisions. He began drinking heavily and was arrested twice for driving under the influence. In his mid-20s, he attempted suicide by overdose after his father died. Mr. T required inpatient hospitalization nearly every year thereafter. His mother, a registered nurse, was significantly involved in his care and carefully documented his treatment history.
Mr. T has had numerous medication trials, including oral and long-acting injectable risperidone, olanzapine, aripiprazole, ziprasidone, lithium, gabapentin, buspirone, quetiapine, trazodone, bupropion, and paroxetine. None of these medications were effective.
In his mid-40s, Mr. T attempted suicide by wandering into traffic and being struck by a motor vehicle. A year later, he attempted suicide by driving his car at high speed into a concrete highway median. Mr. T told first responders that he was “possessed,” and a demonic entity “forced” him to crash his car. He begged law enforcement officers at the scene to give him a gun so he could shoot himself.
Continue to: Mr. T entered an intensive outpatient treatment program...
Mr. T entered an intensive outpatient treatment program and was switched from long-acting injectable risperidone to oral aripiprazole. After taking aripiprazole for several weeks, he began to gamble compulsively at a nearby casino. Frustrated by the lack of response to psychotropic medications and his idiosyncratic response to aripiprazole, he stopped psychiatric treatment, relapsed to alcohol use, and isolated himself in his apartment shortly before stabbing his mother.
EVALUATION Pharmacogenomics testing
At the mental health rehabilitation center, Mr. T agrees to undergo pharmacogenomics testing, which suggests that he will have a normal response to selective serotonin reuptake inhibitors and is unlikely to experience adverse reactions. He does not carry the 2 alleles that place him at higher risk of serious dermatologic reactions when taking certain mood stabilizers. He is heterozygous for the C677T allele polymorphism in the MTHFR gene that is associated with reduced folic acid metabolism, moderately decreased serum folate levels, and moderately increased homocysteine levels. On the pharmacokinetic genes tested, Mr. T has the normal metabolism genotype on 5 of 6 cytochrome P450 (CYP) enzymes; he has the ultrarapid metabolizer genotype on CYP1A2. He also has normal activity and intermediate metabolizer phenotype on the 2 UGT enzymes tested, which are responsible for the glucuronidation process, a major part of phase II metabolism.
Based on these results, Mr. T’s clozapine dosage is decreased by 50% (from 300 to 150 mg/d) and he is started on fluvoxamine, 50 mg/d, because it is a strong inhibitor of CYP1A2. The reduced conversion of clozapine to norclozapine results in an average serum clozapine level of 527 ng/mL (a level of 350 ng/mL is usually therapeutic in patients with schizophrenia) and norclozapine level of 140 ng/mL (clozapine:norclozapine ratio = 3.8), which is to be expected because fluvoxamine can increase serum clozapine levels.
Due to accumulating evidence in the literature suggesting that latent infections in the CNS play a role in serious mental illnesses such as schizophrenia, Mr. T undergoes further laboratory testing.
[polldaddy:10375845]
The authors’ observations
Mr. T tested positive for TG and CMV and negative for HSV-1. We were aware of accumulating evidence that latent infections in the CNS play a role in serious mental illnesses such as schizophrenia, specifically TG5—a parasite transmitted by cats—and CMV and HSV-1,6 which are transmitted by humans. The theory that TG infection could be a factor in schizophrenia emerged in the 1990s but only in recent years received mainstream scientific attention. Toxoplasma gondii, the infectious parasite that causes toxoplasmosis, infects more than 30 million people in the United States; however, most individuals are asymptomatic because of the body’s immune response to the parasite.7
Continue to: A study of 162 individuals...
A study of 162 individuals with schizophrenia, bipolar disorder, or major depressive disorder found that this immunologic profile is associated with suicide attempts,8 which is consistent with Mr. T’s history. Research suggests that individuals with schizophrenia who have latent TG infection have a more severe form of the illness compared with patients without the infection.9-12 Many of these factors were present in Mr. T’s case (Table 18-12).
TREATMENT Improvement, then setback
Mr. T’s medication regimen at the rehabilitation center includes clozapine, 100 mg/d; minocycline, 200 mg/d; fluvoxamine, 200 mg/d; and N-acetylcysteine, 1,200 mg/d. N-acetylcysteine is an antioxidant that could ease negative symptoms of schizophrenia by reducing oxidative stress caused by free radicals.13 Mr. T makes slow but steady improvement, and his IL-6 levels drop steadily (Figure 1).

After 6 months in the rehabilitation center, Mr. T no longer experiences catatonic symptoms and is able to participate in the therapeutic program. He is permitted to leave the facility on day passes with family members. However, approximately every 8 weeks, he continues to cycle through periods of intense anxiety, perseverates on topics, and exhibits fragmented thinking and speech. During these episodes, he has difficulty receiving and processing information.
During one of these periods, Mr. T eats 4 oleander leaves he gathered while on day pass outside of the facility. After he experiences stomach pain, nausea, and vomiting, he informs nursing staff that he ate oleander. He is brought to the emergency department, receives activated charcoal and a digoxin antidote, and is placed on continuous electrocardiogram monitoring. When asked why he made the suicide attempt, he said “I realized things will never be the same because of what happened. I felt trapped.” He later expresses regret and wants to return to the mental health rehabilitation center.
At the facility, Mr. T agrees to take 2 more agents—valproic acid and ginger root extract—that specifically target latent toxoplasmosis infection before pursuing electroconvulsive therapy. We offer valproic acid because it inhibits replication of TG in an in vitro model.14 Mr. T is started on extended-release valproic acid, 1,500 mg/d, which results in a therapeutic serum level of 74.8 µg/mL.
Continue to: Additionally, Mr. T expresses interest...
Additionally, Mr. T expresses interest in taking “natural” agents in addition to psychotropics. After reviewing the quality of available ginger root extract products, Mr. T is started on a supplement that contains 22.4 mg of gingerols and 6.7 mg of shogaols, titrated to 4 capsules twice daily.
The authors’ observations
A retrospective cross-sectional analysis reported that patients with bipolar disorder who received medications with anti-toxoplasmic activity (Table 215), specifically valproic acid, had significantly fewer lifetime depressive episodes compared with patients who received medications without anti-toxoplasmic activity.15
Alternative medicine options
Research has demonstrated the beneficial effects of Chinese herbal plants for toxoplasmosis16,17 and ginger root extract has potent anti-toxoplasmic activity. A mouse model found that ginger root extract (Zingiber officinale) reduced the number of TG-infected cells by suppressing activation of apoptotic proteins the parasite induces, which prevents programmed cell death.18
Table 3 presents a stepwise approach to identifying and treating inflammation in patients with treatment-resistant psychosis.

OUTCOME Immune response, improvement
One month after the valproic acid and ginger root extract therapy is initiated, Mr. T’s toxoplasma antibody immunoglobulin G increases by 15.2 IU/mL, indicating that his immune system is mounting an enhanced response against the parasite (Figure 2). Mr. T continues to make progress while receiving the new regimen of clozapine, minocycline, valproic acid, and ginger root extract. He no longer cycles into periods of intense anxiety, perseverative thought, and fragmented thought and speech. He participates meaningfully in weekly psychotherapy and hopes to live independently and obtain gainful employment.

The District Attorney’s office dismisses his criminal charges, and Mr. T is discharged to a less restrictive level of care.
Continue to: Bottom Line
Bottom Line
Several studies have shown that neuroinflammation increases the severity of mental illness. Consider adjunct anti-inflammatory agents for patients who have elevated levels of inflammatory biomarkers and for whom standard treatment approaches do not adequately control psychiatric symptoms. Also consider testing for the presence of latent infections in the CNS, which could reveal the underlying cause of treatment resistance or the genesis of disabling psychiatric symptoms.
Related Resources
- Fond G, Macgregor A, Tamouza R, et al. Comparative analysis of anti-toxoplasmic activity of antipsychotic drugs and valproate. Eur Arch Psychiatry Clin Neurosci. 2014;264(2):179-183.
- Hamdani N, Daban-Huard C, Lajnef M, et al. Cognitive deterioration among bipolar disorder patients infected by Toxoplasma gondii is correlated to interleukin 6 levels. J Affect Disord. 2015;179:161-166.
- Monroe JM, Buckley PF, Miller BJ. Meta-analysis of antitoxoplasma gondii IgM antibodies in acute psychosis. Schizophr Bull. 2015;41(4):989-998.
Drug Brand Names
Acyclovir • Zovirax
Aripiprazole • Abilify
Bupropion • Wellbutrin
Buspirone • Buspar
Clozapine • Clozaril
Duloxetine • Cymbalta
Fluphenazine • Prolixin
Fluvoxamine • Luvox
Gabapentin • Neurontin
Haloperidol • Haldol
Lithium • Eskalith, Lithobid
Lorazepam • Ativan
Loxapine • Loxitane
Minocycline • Minocin
Olanzapine • Zyprexa
Paliperidone • Invega
Paroxetine • Paxil
Quetiapine • Seroquel
Risperidone • Risperdal, Risperdal Consta
Thioridazine • Mellaril
Trifluoperazine • Stelazine
Trazodone • Desyrel
Valproic acid • Depakote
Ziprasidone • Geodon
CASE Violent, then catatonic
Mr. T, age 52, has a long history of schizoaffective disorder, depressed type; several suicide attempts; and violent episodes. He is admitted to a mental health rehabilitation center under a forensic commitment.
Several years earlier, Mr. T had been charged with first-degree attempted murder, assault with a deadly weapon, and abuse of a dependent/geriatric adult after allegedly stabbing his mother in the upper chest and neck. At that time, Mr. T was not in psychiatric treatment and was drinking heavily. He had become obsessed with John F. Kennedy’s assassination and believed the Central Intelligence Agency (CIA), not Lee Harvey Oswald, was responsible. He feared the CIA wanted to kill him because of his knowledge, and he heard voices from his television he believed were threatening him. He acquired knives for self-protection. When his mother arrived at his apartment to take him to a psychiatric appointment, he believed she was conspiring with the CIA and attacked her. Mr. T’s mother survived her injuries. He was taken to the county jail, where psychiatric staff noted that Mr. T was psychotic.
The court found Mr. T incompetent to stand trial and sent him to a state hospital for psychiatric treatment and competency restoration. After 3 years, he was declared unable to be restored because of repeated decompensations, placed on a conservatorship, and sent back to county jail.
In the jail, Mr. T began to show signs of catatonia. He refused medications, food, and water, and became mute. He was admitted to a medical center after a 45-minute episode that appeared similar to a seizure; however, all laboratory evaluations were within normal limits, head CT was negative, and an EEG was unremarkable.
Mr. T’s catatonic state gradually resolved with increasing dosages of lorazepam, as well as clozapine. He showed improved mobility and oral intake. A month later, his train of thought was rambling and difficult to follow, circumstantial, and perseverating. However, at times he could be directed and respond to questions in a linear and logical fashion. Lorazepam was tapered, discontinued, and replaced with gabapentin because Mr. T viewed taking lorazepam as a threat to his sobriety.
Recently, Mr. T was transferred to our mental health rehabilitation center, where he expresses that he is grateful to be in a therapeutic environment. Upon admission, his medication regimen consists of clozapine, 300 mg by mouth at bedtime, duloxetine, 60 mg/d by mouth, gabapentin 600 mg by mouth 3 times a day, and docusate sodium, 250 mg/d by mouth. Our team has a discussion about the growing recognition of the pro-inflammatory state present in many patients who experience serious mental illness and the importance of augmenting standard evidence-based psychopharmacotherapy with agents that have neuroprotective properties.1,2 We offer Mr. T
[polldaddy:10375843]
The authors’ observations
Several studies have found that acute psychosis is associated with an inflammatory state, and interleukin-6 (IL-6) is a crucial biomarker. A recent meta-analysis of serum cytokines in patients with schizophrenia found that IL-6 levels were significantly increased among acutely ill patients compared with controls.3 IL-6 levels significantly decreased after treating acute episodes of schizophrenia.3 Further, levels of peripheral IL-6 mRNA levels in individuals with schizophrenia are directly correlated with severity of positive symptoms.4
Continue to: A meta-analyis reported...
A meta-analysis reported that tumor necrosis factor-alpha and IL-6 are elevated during acute psychosis3; however, IL-6 normalized with treatment, whereas tumor necrosis factor-alpha did not. This means that IL-6 is a more clinically meaningful biomarker to help gauge treatment response.
EVALUATION Elevated markers of inflammation
Laboratory testing reveals that Mr. T’s IL-6 level is 56.64 pg/mL, which is significantly elevated (reference range: 0.31 to 5.00 pg/mL). After reviewing the IL-6 results with Mr. T and explaining that there is “too much inflammation” in his brain, he agrees to take minocycline and complete follow-up IL-6 level tests to monitor his progress during treatment.
HISTORY Alcohol abuse, treatment resistance
According to Mr. T’s mother, he had met all developmental milestones and graduated from high school with plans to enter culinary school. At age 20, Mr. T began to experience psychotic symptoms, telling family members that he was being followed by FBI agents and was receiving messages from televisions. He began drinking heavily and was arrested twice for driving under the influence. In his mid-20s, he attempted suicide by overdose after his father died. Mr. T required inpatient hospitalization nearly every year thereafter. His mother, a registered nurse, was significantly involved in his care and carefully documented his treatment history.
Mr. T has had numerous medication trials, including oral and long-acting injectable risperidone, olanzapine, aripiprazole, ziprasidone, lithium, gabapentin, buspirone, quetiapine, trazodone, bupropion, and paroxetine. None of these medications were effective.
In his mid-40s, Mr. T attempted suicide by wandering into traffic and being struck by a motor vehicle. A year later, he attempted suicide by driving his car at high speed into a concrete highway median. Mr. T told first responders that he was “possessed,” and a demonic entity “forced” him to crash his car. He begged law enforcement officers at the scene to give him a gun so he could shoot himself.
Continue to: Mr. T entered an intensive outpatient treatment program...
Mr. T entered an intensive outpatient treatment program and was switched from long-acting injectable risperidone to oral aripiprazole. After taking aripiprazole for several weeks, he began to gamble compulsively at a nearby casino. Frustrated by the lack of response to psychotropic medications and his idiosyncratic response to aripiprazole, he stopped psychiatric treatment, relapsed to alcohol use, and isolated himself in his apartment shortly before stabbing his mother.
EVALUATION Pharmacogenomics testing
At the mental health rehabilitation center, Mr. T agrees to undergo pharmacogenomics testing, which suggests that he will have a normal response to selective serotonin reuptake inhibitors and is unlikely to experience adverse reactions. He does not carry the 2 alleles that place him at higher risk of serious dermatologic reactions when taking certain mood stabilizers. He is heterozygous for the C677T allele polymorphism in the MTHFR gene that is associated with reduced folic acid metabolism, moderately decreased serum folate levels, and moderately increased homocysteine levels. On the pharmacokinetic genes tested, Mr. T has the normal metabolism genotype on 5 of 6 cytochrome P450 (CYP) enzymes; he has the ultrarapid metabolizer genotype on CYP1A2. He also has normal activity and intermediate metabolizer phenotype on the 2 UGT enzymes tested, which are responsible for the glucuronidation process, a major part of phase II metabolism.
Based on these results, Mr. T’s clozapine dosage is decreased by 50% (from 300 to 150 mg/d) and he is started on fluvoxamine, 50 mg/d, because it is a strong inhibitor of CYP1A2. The reduced conversion of clozapine to norclozapine results in an average serum clozapine level of 527 ng/mL (a level of 350 ng/mL is usually therapeutic in patients with schizophrenia) and norclozapine level of 140 ng/mL (clozapine:norclozapine ratio = 3.8), which is to be expected because fluvoxamine can increase serum clozapine levels.
Due to accumulating evidence in the literature suggesting that latent infections in the CNS play a role in serious mental illnesses such as schizophrenia, Mr. T undergoes further laboratory testing.
[polldaddy:10375845]
The authors’ observations
Mr. T tested positive for TG and CMV and negative for HSV-1. We were aware of accumulating evidence that latent infections in the CNS play a role in serious mental illnesses such as schizophrenia, specifically TG5—a parasite transmitted by cats—and CMV and HSV-1,6 which are transmitted by humans. The theory that TG infection could be a factor in schizophrenia emerged in the 1990s but only in recent years received mainstream scientific attention. Toxoplasma gondii, the infectious parasite that causes toxoplasmosis, infects more than 30 million people in the United States; however, most individuals are asymptomatic because of the body’s immune response to the parasite.7
Continue to: A study of 162 individuals...
A study of 162 individuals with schizophrenia, bipolar disorder, or major depressive disorder found that this immunologic profile is associated with suicide attempts,8 which is consistent with Mr. T’s history. Research suggests that individuals with schizophrenia who have latent TG infection have a more severe form of the illness compared with patients without the infection.9-12 Many of these factors were present in Mr. T’s case (Table 18-12).
TREATMENT Improvement, then setback
Mr. T’s medication regimen at the rehabilitation center includes clozapine, 100 mg/d; minocycline, 200 mg/d; fluvoxamine, 200 mg/d; and N-acetylcysteine, 1,200 mg/d. N-acetylcysteine is an antioxidant that could ease negative symptoms of schizophrenia by reducing oxidative stress caused by free radicals.13 Mr. T makes slow but steady improvement, and his IL-6 levels drop steadily (Figure 1).

After 6 months in the rehabilitation center, Mr. T no longer experiences catatonic symptoms and is able to participate in the therapeutic program. He is permitted to leave the facility on day passes with family members. However, approximately every 8 weeks, he continues to cycle through periods of intense anxiety, perseverates on topics, and exhibits fragmented thinking and speech. During these episodes, he has difficulty receiving and processing information.
During one of these periods, Mr. T eats 4 oleander leaves he gathered while on day pass outside of the facility. After he experiences stomach pain, nausea, and vomiting, he informs nursing staff that he ate oleander. He is brought to the emergency department, receives activated charcoal and a digoxin antidote, and is placed on continuous electrocardiogram monitoring. When asked why he made the suicide attempt, he said “I realized things will never be the same because of what happened. I felt trapped.” He later expresses regret and wants to return to the mental health rehabilitation center.
At the facility, Mr. T agrees to take 2 more agents—valproic acid and ginger root extract—that specifically target latent toxoplasmosis infection before pursuing electroconvulsive therapy. We offer valproic acid because it inhibits replication of TG in an in vitro model.14 Mr. T is started on extended-release valproic acid, 1,500 mg/d, which results in a therapeutic serum level of 74.8 µg/mL.
Continue to: Additionally, Mr. T expresses interest...
Additionally, Mr. T expresses interest in taking “natural” agents in addition to psychotropics. After reviewing the quality of available ginger root extract products, Mr. T is started on a supplement that contains 22.4 mg of gingerols and 6.7 mg of shogaols, titrated to 4 capsules twice daily.
The authors’ observations
A retrospective cross-sectional analysis reported that patients with bipolar disorder who received medications with anti-toxoplasmic activity (Table 215), specifically valproic acid, had significantly fewer lifetime depressive episodes compared with patients who received medications without anti-toxoplasmic activity.15
Alternative medicine options
Research has demonstrated the beneficial effects of Chinese herbal plants for toxoplasmosis16,17 and ginger root extract has potent anti-toxoplasmic activity. A mouse model found that ginger root extract (Zingiber officinale) reduced the number of TG-infected cells by suppressing activation of apoptotic proteins the parasite induces, which prevents programmed cell death.18
Table 3 presents a stepwise approach to identifying and treating inflammation in patients with treatment-resistant psychosis.

OUTCOME Immune response, improvement
One month after the valproic acid and ginger root extract therapy is initiated, Mr. T’s toxoplasma antibody immunoglobulin G increases by 15.2 IU/mL, indicating that his immune system is mounting an enhanced response against the parasite (Figure 2). Mr. T continues to make progress while receiving the new regimen of clozapine, minocycline, valproic acid, and ginger root extract. He no longer cycles into periods of intense anxiety, perseverative thought, and fragmented thought and speech. He participates meaningfully in weekly psychotherapy and hopes to live independently and obtain gainful employment.

The District Attorney’s office dismisses his criminal charges, and Mr. T is discharged to a less restrictive level of care.
Continue to: Bottom Line
Bottom Line
Several studies have shown that neuroinflammation increases the severity of mental illness. Consider adjunct anti-inflammatory agents for patients who have elevated levels of inflammatory biomarkers and for whom standard treatment approaches do not adequately control psychiatric symptoms. Also consider testing for the presence of latent infections in the CNS, which could reveal the underlying cause of treatment resistance or the genesis of disabling psychiatric symptoms.
Related Resources
- Fond G, Macgregor A, Tamouza R, et al. Comparative analysis of anti-toxoplasmic activity of antipsychotic drugs and valproate. Eur Arch Psychiatry Clin Neurosci. 2014;264(2):179-183.
- Hamdani N, Daban-Huard C, Lajnef M, et al. Cognitive deterioration among bipolar disorder patients infected by Toxoplasma gondii is correlated to interleukin 6 levels. J Affect Disord. 2015;179:161-166.
- Monroe JM, Buckley PF, Miller BJ. Meta-analysis of antitoxoplasma gondii IgM antibodies in acute psychosis. Schizophr Bull. 2015;41(4):989-998.
Drug Brand Names
Acyclovir • Zovirax
Aripiprazole • Abilify
Bupropion • Wellbutrin
Buspirone • Buspar
Clozapine • Clozaril
Duloxetine • Cymbalta
Fluphenazine • Prolixin
Fluvoxamine • Luvox
Gabapentin • Neurontin
Haloperidol • Haldol
Lithium • Eskalith, Lithobid
Lorazepam • Ativan
Loxapine • Loxitane
Minocycline • Minocin
Olanzapine • Zyprexa
Paliperidone • Invega
Paroxetine • Paxil
Quetiapine • Seroquel
Risperidone • Risperdal, Risperdal Consta
Thioridazine • Mellaril
Trifluoperazine • Stelazine
Trazodone • Desyrel
Valproic acid • Depakote
Ziprasidone • Geodon
1. Koola MM, Raines JK, Hamilton RG, et al. Can anti-inflammatory medications improve symptoms and reduce mortality in schizophrenia? Current Psychiatry. 2016;15(5):52-57.
2. Nasrallah HA. Are you neuroprotecting your patients? 10 Adjunctive therapies to consider. Current Psychiatry. 2016;15(12):12-14.
3. Goldsmith DR, Rapaport MH, Miller BJ. A meta-analysis of blood cytokine network alterations in psychiatric patients: comparisons between schizophrenia, bipolar disorder and depression. Mol Psychiatry. 2016;21(12):1696-1709.
4. Chase KA, Cone JJ, Rosen C, et al. The value of interleukin 6 as a peripheral diagnostic marker in schizophrenia. BMC Psychiatry. 2016;16:152.
5. Torrey EF, Bartko JJ, Lun ZR, et al. Antibodies to Toxoplasma gondii in patients with schizophrenia: a meta-analysis. Schizophr Bull. 2007;33(3):729-736.
6. Shirts BH, Prasad KM, Pogue-Geile MF, et al. Antibodies to cytomegalovirus and herpes simplex virus 1 associated with cognitive function in schizophrenia. Schizophr Res. 2008;106(2-3):268-274.
7. Centers for Disease Control and Prevention. Parasites - Toxoplasmosis (Toxoplasma infection). https://www.cdc.gov/parasites/toxoplasmosis/index.html. Accessed February 26, 2019.
8. Dickerson F, Wilcox HC, Adamos M, et al. Suicide attempts and markers of immune response in individuals with serious mental illness. J Psychiatr Res. 2017;87:37-43.
9. Celik T, Kartalci S, Aytas O, et al. Association between latent toxoplasmosis and clinical course of schizophrenia - continuous course of the disease is characteristic for Toxoplasma gondii-infected patients. Folia Parasitol (Praha). 2015;62. doi: 10.14411/fp.2015.015.
10. Dickerson F, Boronow J, Stallings C, et al. Toxoplasma gondii in individuals with schizophrenia: association with clinical and demographic factors and with mortality. Schizophr Bull. 2007;33(3):737-740.
11. Esshili A, Thabet S, Jemli A, et al. Toxoplasma gondii infection in schizophrenia and associated clinical features. Psychiatry Res. 2016;245:327-332.
12. Holub D, Flegr J, Dragomirecka E, et al. Differences in onset of disease and severity of psychopathology between toxoplasmosis-related and toxoplasmosis-unrelated schizophrenia. Acta Psychiatr Scand. 2013;127(3):227-238.
13. Chen AT, Chibnall JT, Nasrallah HA. Placebo-controlled augmentation trials of the antioxidant NAC in schizophrenia: a review. Ann Clin Psychiatry. 2016;28(3):190-196.
14. Jones-Brando L, Torrey EF, Yolken R. Drugs used in the treatment of schizophrenia and bipolar disorder inhibit the replication of Toxoplasma gondii. Schizophr Res. 2003;62(3):237-244.
15. Fond G, Boyer L, Gaman A, et al. Treatment with anti-toxoplasmic activity (TATA) for toxoplasma positive patients with bipolar disorders or schizophrenia: a cross-sectional study. J Psychiatr Res. 2015;63:58-64.
16. Wei HX, Wei SS, Lindsay DS, et al. A systematic review and meta-analysis of the efficacy of anti-Toxoplasma gondii medicines in humans. PLoS One. 2015;10(9):e0138204.
17. Zhuo XH, Sun HC, Huang B, et al. Evaluation of potential anti-toxoplasmosis efficiency of combined traditional herbs in a mouse model. J Zhejiang Univ Sci B. 2017;18(6):453-461.
18. Choi WH, Jiang MH, Chu JP. Antiparasitic effects of Zingiber officinale (Ginger) extract against Toxoplasma gondii. Journal of Applied Biomedicine. 2013;11:15-26.
1. Koola MM, Raines JK, Hamilton RG, et al. Can anti-inflammatory medications improve symptoms and reduce mortality in schizophrenia? Current Psychiatry. 2016;15(5):52-57.
2. Nasrallah HA. Are you neuroprotecting your patients? 10 Adjunctive therapies to consider. Current Psychiatry. 2016;15(12):12-14.
3. Goldsmith DR, Rapaport MH, Miller BJ. A meta-analysis of blood cytokine network alterations in psychiatric patients: comparisons between schizophrenia, bipolar disorder and depression. Mol Psychiatry. 2016;21(12):1696-1709.
4. Chase KA, Cone JJ, Rosen C, et al. The value of interleukin 6 as a peripheral diagnostic marker in schizophrenia. BMC Psychiatry. 2016;16:152.
5. Torrey EF, Bartko JJ, Lun ZR, et al. Antibodies to Toxoplasma gondii in patients with schizophrenia: a meta-analysis. Schizophr Bull. 2007;33(3):729-736.
6. Shirts BH, Prasad KM, Pogue-Geile MF, et al. Antibodies to cytomegalovirus and herpes simplex virus 1 associated with cognitive function in schizophrenia. Schizophr Res. 2008;106(2-3):268-274.
7. Centers for Disease Control and Prevention. Parasites - Toxoplasmosis (Toxoplasma infection). https://www.cdc.gov/parasites/toxoplasmosis/index.html. Accessed February 26, 2019.
8. Dickerson F, Wilcox HC, Adamos M, et al. Suicide attempts and markers of immune response in individuals with serious mental illness. J Psychiatr Res. 2017;87:37-43.
9. Celik T, Kartalci S, Aytas O, et al. Association between latent toxoplasmosis and clinical course of schizophrenia - continuous course of the disease is characteristic for Toxoplasma gondii-infected patients. Folia Parasitol (Praha). 2015;62. doi: 10.14411/fp.2015.015.
10. Dickerson F, Boronow J, Stallings C, et al. Toxoplasma gondii in individuals with schizophrenia: association with clinical and demographic factors and with mortality. Schizophr Bull. 2007;33(3):737-740.
11. Esshili A, Thabet S, Jemli A, et al. Toxoplasma gondii infection in schizophrenia and associated clinical features. Psychiatry Res. 2016;245:327-332.
12. Holub D, Flegr J, Dragomirecka E, et al. Differences in onset of disease and severity of psychopathology between toxoplasmosis-related and toxoplasmosis-unrelated schizophrenia. Acta Psychiatr Scand. 2013;127(3):227-238.
13. Chen AT, Chibnall JT, Nasrallah HA. Placebo-controlled augmentation trials of the antioxidant NAC in schizophrenia: a review. Ann Clin Psychiatry. 2016;28(3):190-196.
14. Jones-Brando L, Torrey EF, Yolken R. Drugs used in the treatment of schizophrenia and bipolar disorder inhibit the replication of Toxoplasma gondii. Schizophr Res. 2003;62(3):237-244.
15. Fond G, Boyer L, Gaman A, et al. Treatment with anti-toxoplasmic activity (TATA) for toxoplasma positive patients with bipolar disorders or schizophrenia: a cross-sectional study. J Psychiatr Res. 2015;63:58-64.
16. Wei HX, Wei SS, Lindsay DS, et al. A systematic review and meta-analysis of the efficacy of anti-Toxoplasma gondii medicines in humans. PLoS One. 2015;10(9):e0138204.
17. Zhuo XH, Sun HC, Huang B, et al. Evaluation of potential anti-toxoplasmosis efficiency of combined traditional herbs in a mouse model. J Zhejiang Univ Sci B. 2017;18(6):453-461.
18. Choi WH, Jiang MH, Chu JP. Antiparasitic effects of Zingiber officinale (Ginger) extract against Toxoplasma gondii. Journal of Applied Biomedicine. 2013;11:15-26.
Seizure-like episodes, but is it really epilepsy?
CASE Increasingly frequent paroxysmal episodes
Ms. N, age 12, comes to the hospital for evaluation of paroxysmal episodes of pain, weakness, and muscle spasms. A neurologist who evaluated her as an outpatient had recommended a routine electroencephalogram (EEG); after those results were inconclusive, Ms. N’s mother brought her to the hospital for a 24-hour video EEG.
Ms. N has a history of asthma. She has no history of seizures or headache, but her mother has an unspecified seizure disorder that has been stable with antiepileptic medication for many years. Ms. N has no other family history of autoimmune or neurologic disorders.
Ms. N’s episodes began 6 months ago and have progressively increased in frequency from 5 to 12 episodes a day. She says that before she has an episode, she “ feels tingling in her fingers and mouth, and butterflies in her belly,” and then her “whole body clenches up.” She denies experiencing tongue biting, facial or extremity weakness, incontinence, or loss of consciousness during these episodes.
Shortly before her hospitalization, Ms. N had won a scholarship to attend an overnight art camp. Because her episodes were becoming more frequent and their etiology remained unclear, Ms. N and her mother decided it would be unsafe for her to attend, and that she should go to the hospital for evaluation instead.
EVALUATION Tough questions reveal answers
The pediatric team evaluates Ms. N. Her physical exam, laboratory values, and imaging are all within normal limits. Her neurologic exam demonstrates full strength, tone, and sensation in all extremities. All cranial nerves and reflexes are intact. No dysmorphic features or gait abnormalities are noted. All laboratory and imaging tests are normal, including complete blood cell count, electrolytes, calcium, magnesium, phosphorus, glucose, creatine kinase, liver enzymes, urine drug screen, human chorionic gonadotropin (hCG) urine test, and h
After the initial workup, the pediatric team consults the child and adolescent psychiatry team for a complete assessment of Ms. N due to concerns that a psychological component is contributing to her episodes. According to the psychosocial history obtained from Ms. N and her mother, Ms. N had experienced disrupted attachment, trauma, and loss. At age 5, Ms. N was temporarily removed from her mother’s custody after a fight between her mother and brother. At age 9, Ms. N’s stepfather, her primary father figure, died of a brain tumor.
Ms. N also has significant trauma stemming from her relationship with her biological father. Ms. N’s mother reports that her daughter was conceived during nonconsensual sexual intercourse. Ms. N did not have much contact with her biological father until 6 months ago, when he started picking her up at school and taking her to his home for several hours without permission or supervision. Afterwards, Ms. N confided to her mother and a teacher that her father sexually assaulted her during those visits.
Continue to: Ms. N and her mother...
Ms. N and her mother reported the assault to the police and were awaiting legal action.
During the interview with the psychiatry team, Ms. N denies that any thoughts or actions trigger the episodes and reports that she cannot control when they happen. Because she cannot anticipate the episodes, she says she is afraid to leave her house. She does not know why the episodes are happening and feels frustrated that they are getting worse. Ms. N says, “I have been feeling down lately,” but she denies hopelessness, worthlessness, suicidal ideation, homicidal ideation, delusions, or hallucinations.
In the hospital, when the psychiatry team asks Ms. N about her visits with her father, she says that they are “too painful to talk about,” and fears that discussing them will trigger an episode. However, her mother suggests that her daughter’s sexual trauma, as well as ongoing frustrations with the legal system, are influencing her mood; she has had low energy, poor appetite, and is spending more time in bed. Her mother also reports that Ms. N “avoids going out in the sun and spending time with her friends outside. She doesn’t seem to enjoy shopping and art like she used to.” Ms. N told her mother that she was having nightmares about the trauma and “could not stop thinking about some of the bad stuff that happened during the day.”
Ten minutes into the interview, while being questioned about her father, Ms. N experiences a spastic episode. She curls up in bed on her left side, clenches her entire body, and shuts her eyes. Her mother quickly runs to her bedside and counts the seconds until the end of the episode. After 25 seconds, Ms. N awakes with full recollection of the episode. On review of the video EEG during the episode, no ictal patterns are seen.
[polldaddy:10375873]
The authors’ observations
Paroxysmal episodes of weakness, numbness, and muscle spasms in a young female are suggestive of either epilepsy or nonepileptic seizure (NES).1,2 The negative EEG and physical features are inconsistent with epileptiform seizure, and Ms. N’s history and evaluation are suggestive of NES. Nonepileptic seizures are a type of a conversion disorder, or functional neurologic symptom disorder, in which a patient experiences weakness, abnormal movements, or seizure-like episodes that are inconsistent with organic neurologic disease.3 When a diagnosis of conversion disorder is suspected, a clinician must always consider other pathology that can explain the symptoms, such as migraine, vasovagal syncope, or intracranial mass. If a patient has focal neurologic deficits, head imaging should be pursued. Additionally, the clinician must screen for malingering and factitious disorder before establishing a definitive diagnosis. However, conversion disorder is not a diagnosis of exclusion. For example, a negative EEG does not rule out epilepsy, and patients can have both epilepsy and concomitant NES.
Continue to: Although NES is a common...
Although NES is a common type of conversion disorder, it is often difficult to diagnose, manage, and treat. Patients often receive antiepileptic medications but continue to have worsening events that are refractory to treatment. Various clinical features can suggest NES instead of epilepsy. Forced eye closure on video recording is a specific finding suggestive of NES, yet this feature is not sufficient to make the diagnosis.4 A video EEG must be performed to assess for epilepsy. The diagnosis of NES does not exclude the possibility that a patient has epilepsy, as NES can occur in up to 40% of patients with epilepsy.5 A video EEG without ictal patterns before, during, and after an observed episode is diagnostic of NES.6
[polldaddy:10375874]
The authors’ observations
Conversion disorders such as NES are a presentation of neurologic symptoms that cannot be readily accounted for by other conditions and are often associated with antecedent trauma. Multiple factors in Ms. N’s history increase her risk of NES, including loss of multiple loved ones, ongoing legal involvement, and alleged sexual abuse by her father.
Victims of sexual abuse are more likely than the general population to demonstrate symptoms of conversion disorder, especially NES.7,8 The onset of paroxysmal episodes after incestuous abuse in a teenage girl is characteristic of NES. Compared with patients with complex partial epilepsy (CPE), patients with NES are 3 times more likely to report sexual trauma.9,10 Children who report sexual abuse that precedes NES are more likely to have been victimized by a first-degree relative than patients with CPE who report sexual abuse.11 Risk factors for victims developing NES may be related to the severity of adversity, stress sensitivity, and decreased hippocampal volume.12,13
Ms. N endorsed many psychiatric symptoms that accompany her paroxysmal episodes; this is similar to findings in other patients with NES.14 One study found that depression is 3 times more prevalent and PTSD is 8 times more prevalent in patients with NES.12 During the evaluation, Ms. N’s mother said her daughter had low energy, poor appetite, lethargy, and anhedonia for the preceding 5 months, which is consistent with adjustment disorder.3 Her flashbacks, nightmares, difficulty sleeping, and agoraphobia, along with her trouble engaging with the people and activities that used to bring her joy, are symptoms of PTSD. Nonepileptic seizure is often associated with PTSD and can be viewed as an expression of a dissociated subtype.15
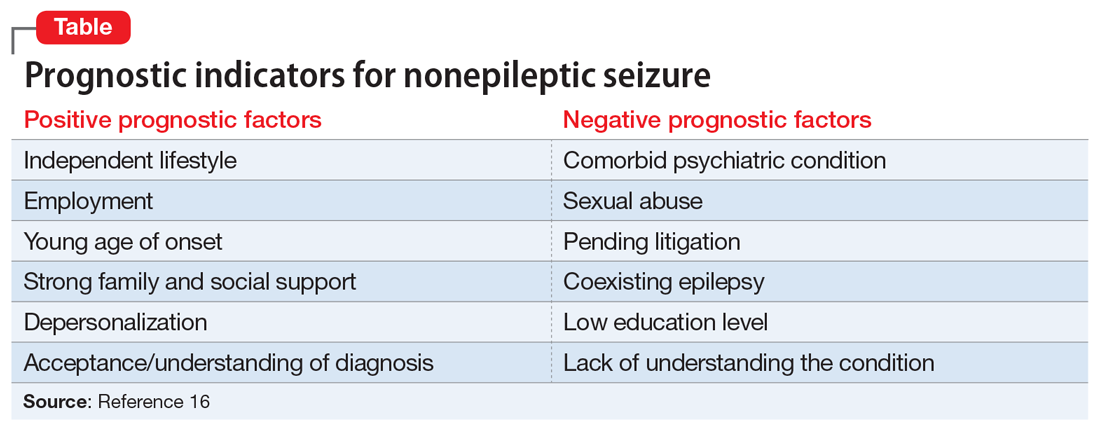
In a literature review, Durrant et al16 isolated prognostic indicators for NES (Table16). This study found that 70% of children and 40% of adults achieve remission from NES. Ms. N’s case has multiple concerning features, such as her comorbid psychiatric conditions, ongoing involvement in a legal case, and sexual trauma; this last factor is associated with the most severe symptoms and worse outcomes.16,17 Despite this somber reality, Ms. N has the support of her mother and is relatively young, which play a vital role in recovery.
Continue to: TREATMENT A strategy for minimizing the episodes
TREATMENT A strategy for minimizing the episodes
Ms. N’s medical workup remains unremarkable throughout the rest of her hospital stay. The psychiatry and pediatric teams discuss their assessments and agree that NES is the most likely diagnosis. The psychiatry team counsels Ms. N and her mother on the diagnosis and etiology of NES.
[polldaddy:10375876]
The authors’ observations
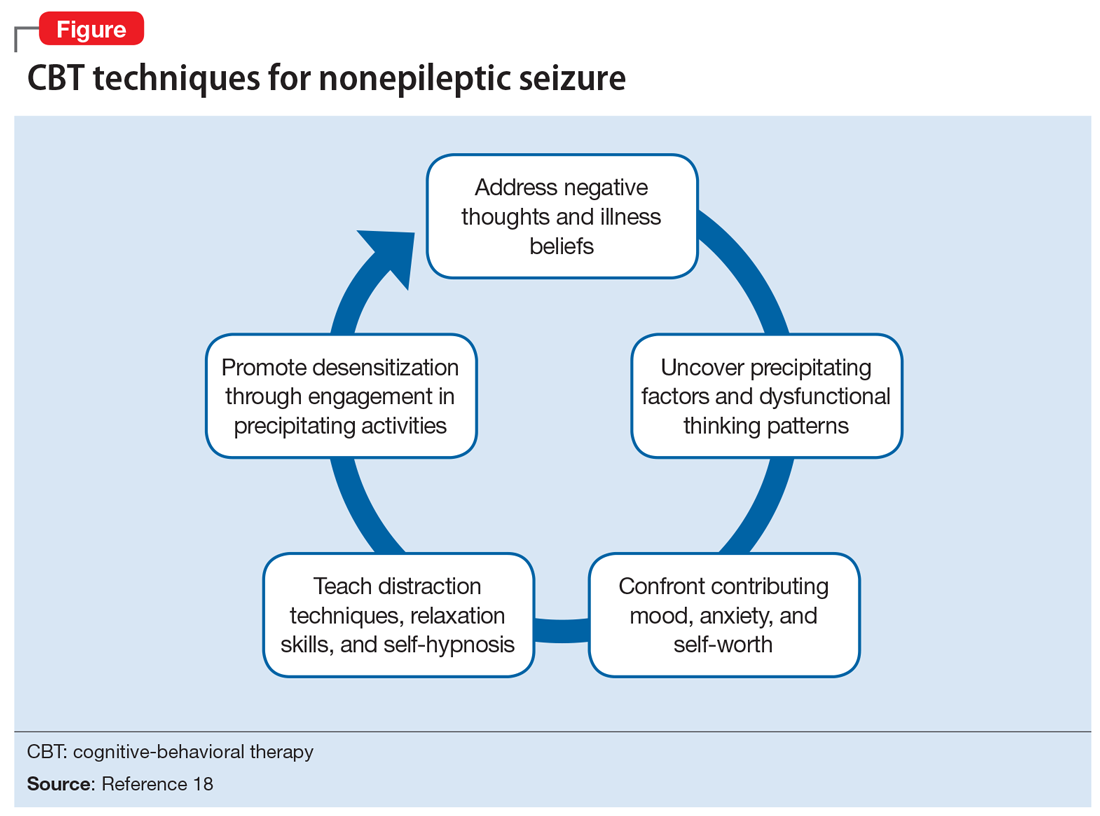
Cognitive-behavioral therapy is currently the treatment of choice for reducing seizure frequency in patients with NES.18,19 The use of CBT was suggested due to the theory that NES represents a dissociative response to trauma. Therapy focuses on changing a patient’s beliefs and perceptions associated with attacks.5 A randomized study of 66 patients with NES compared the use of CBT plus standard medical care with standard medical care alone.18 The standard medical care consisted of supportive treatment, an explanation of NES from a neuropsychiatrist, and supervised withdrawal of antiepileptic drugs. The CBT treatment group was offered weekly hour-long sessions for 12 weeks, accompanied by CBT homework and journaling the frequency and nature of seizure episodes (the CBT techniques are outlined in the Figure18). After 4 months, the CBT treatment group had fewer seizures, and after a 6-month follow-up, they were more likely to be seizure-free. However, in this study, CBT treatment did not improve mood or employment status.
A later investigation looked at using selective serotonin reuptake inhibitors to treat NES in adults.19 This study divided participants into 4 treatment groups: CBT with informed psychotherapy (CBT-ip), CBT-ip plus sertraline, sertraline alone, and treatment as usual. Sertraline was titrated up to a dose of 200 mg/d as tolerated. After 16 weeks of sertraline alone, seizure frequency did not decrease. Although both CBT groups showed a reduction in symptoms of up to 60%, the CBT-ip group reported fewer psychiatric symptoms with better social interactions, quality of life, and global functioning compared with patients treated with CBT-ip plus sertraline. The authors suggested that this may be due to the somatic adverse effects associated with sertraline. This study suggests that CBT without medication is the treatment of choice.
In addition to CBT, studies of psychodynamic psychotherapy for NES have had promising findings.20 Psychodynamic psychotherapy focuses on addressing conscious and unconscious anger, loss, feelings of isolation, and trauma. Through improving emotional processing, insight, coping skills and self-regulation, patients often benefit from an improvement in seizures, psychosocial functioning and health care utilization.
Metin et al21 found that group therapy alongside a family-centered approach elicited a strong and durable reduction in seizures in patients with NES. At enrollment, investigators distributed information on NES to patients and families. Psychoeducation and psychoanalysis with behavior modification techniques were provided in 90-minute weekly group sessions over 3 months. Participants also underwent monthly individualized sessions for standard psychiatric care for 9 months. During the group sessions, operant conditioning techniques were used to prevent secondary gain from seizure-like activity. Families met 4 times for 1 hour each to discuss seizures, receive psychoeducation on a subconscious etiology of NES, and learn behavior modification techniques. All 9 participants who completed group and individual therapy reported a significant and sustained reduction in seizure frequency by at least 50% at 12-month follow-up. Patients also demonstrated improvements in mood, anxiety, and quality of life.
Continue to: A meta-analysis...
A meta-analysis by Carlson and Perry22 that included 13 studies and 228 participants, examined different treatment modalities and their effectiveness for NES. They found that patients who received psychological intervention had a 47% remission rate and 82% improvement in seizure frequency compared with only 14% to 23% of those who did not receive therapy. They postulated that therapy for this illness must be flexible to properly address the socially, psychologically, and functionally heterogenous patient population. Although there are few randomized controlled trials for NES to determine the best evidence-based intervention, there is now consensus that NES has a favorable prognosis when barriers to psychological care are eliminated.
OUTCOME Referral for CBT
The treatment team advises Ms. N to engage in outpatient therapy after discharge from the hospital. Ms. N and her mother agree to the treatment plan, and leave the hospital with a referral for CBT the next day.
Bottom Line
Nonepileptic seizure (NES) is a type of conversion disorder characterized by seizure-like episodes without ictal qualities. Risk factors for NES include concomitant epilepsy, psychiatric disorders, unstable psychosocial situations, and antecedent trauma. Patients with a history of incestuous sexual abuse are most at risk for developing NES. A normal EEG that fully captures a seizure-like episode is diagnostic of NES. Cognitive-behavioral therapy can minimize seizure frequency and intensity.
Related Resources
- Marsh P, Benbadis S, Fernandez F. Psychogenic nonepileptic seizures: ways to win over skeptical patients. Current Psychiatry. 2008;7(1):21-24, 32-35.
- LaFrance WC Jr. Eye-opening behaviors help diagnose nonepileptic seizures. Current Psychiatry. 2006;5(11):121-122, 124, 130.
- LaFrance WC Jr, Kanner AM, Barry JJ. Treating patients with psychological nonepileptic seizures. In: Ettinger AB, Kanner AM, eds. Psychiatric issues in epilepsy: a practical guide to diagnosis and treatment. 2nd ed. Philadelphia, PA: Wolters Kluwer Health/ Lippincott Williams & Wilkins; 2007:461-488.
Drug Brand Name
Sertraline • Zoloft
1. Lesser R. Psychogenic seizures. Neurology. 1996;46(6):1499-1507.
2. Stone J, LaFrance W, Brown R, et al. Conversion disorder: current problems and potential solutions for DSM-5. J Psychosom Res. 2011;71(6):369-376.
3. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
4. Syed T, Arozullah A, Suciu G, et al. Do observer and self-reports of ictal eye closure predict psychogenic nonepileptic seizures? Epilepsia. 2008;49(5):898-904.
5. Vega-Zelaya L, Alvarez M, Ezquiaga E, et al. Psychogenic non-epileptic seizures in a surgical epilepsy unit: experience and a comprehensive review. Epilepsy Topics. 2014. doi: 10.5772/57439.
6. LaFrance W, Baker G, Duncan R, et al. Minimum requirements for the diagnosis of psychogenic nonepileptic seizures: a staged approach. Epilepsia. 2013;54(11):2005-2018.
7. Roeloes K, Pasman J. Stress, childhood trauma, and cognitive functions in functional neurologic disorders. In: Hallett M, Stone J, Carson A, eds. Handbook of clinical neurology: functional neurologic disorders. 3rd ed. New York, NY: Elsevier; 2017:139-155.
8. Paras M, Murad M, Chen L, et al. Sexual abuse and lifetime diagnosis of somatic disorders. JAMA. 2009;302(5):550.
9. Fiszman A, Alves-Leon SV, Nunes RG, et al. Traumatic events and posttraumatic stress disorder in patients with psychogenic nonepileptic seizures: a critical review. Epilepsy Behav. 2004;5(6):818-825.
10. Sharpe D, Faye C. Non-epileptic seizures and child sexual abuse: a critical review of the literature. Clin Psychol Rev. 2006;26(8):1020-1040.
11. Alper K, Devinsky O, Perrine K, et al. Nonepileptic seizures and childhood sexual and physical abuse. Neurology. 1993;43(10):1950-1953.
12. Plioplys S, Doss J, Siddarth P et al. A multisite controlled study of risk factors in pediatric psychogenic nonepileptic seizures. Epilepsia. 2014;55(11):1739-1747.
13. Andersen S, Tomada A, Vincow E, et al. Preliminary evidence for sensitive periods in the effect of childhood sexual abuse on regional brain development. J Neuropsychiatry Clin Neurosci. 2008;20(3):292-301.
14. Sar V. Childhood trauma, dissociation, and psychiatric comorbidity in patients with conversion disorder. Am J Psychiatry. 2004;161(12):2271-2276.
15. Rosenberg HJ, Rosenberg SD, Williamson PD, et al. A comparative study of trauma and posttraumatic stress disorder prevalence in epilepsy patients and psychogenic nonepileptic seizure patients. Epilepsia. 2000;41(4):447-452.
16. Durrant J, Rickards H, Cavanna A. Prognosis and outcome predictors in psychogenic nonepileptic seizures. Epilepsy Res Treat. 2011;2011:1-7.
17. Selkirk M, Duncan R, Oto M, et al. Clinical differences between patients with nonepileptic seizures who report antecedent sexual abuse and those who do not. Epilepsia. 2008;49(8):1446-1450.
18. Goldstein L, Chalder T, Chigwedere C, et al. Cognitive-behavioral therapy for psychogenic nonepileptic seizures: a pilot RCT. Neurology. 2010;74(24):1986-1994.
19. LaFrance W, Baird G, Barry J, et al. Multicenter pilot treatment trial for psychogenic nonepileptic seizures. JAMA Psychiatry. 2014;71(9):997.
20. Howlett S, Reuber M. An augmented model of brief psychodynamic interpersonal therapy for patients with nonepileptic seizures. Psychotherapy (Chic). 2009;46(1):125-138.
21. Metin SZ, Ozmen M, Metin B, et al. Treatment with group psychotherapy for chronic psychogenic nonepileptic seizures. Epilepsy Behav. 2013;28(1):91-94.
22. Carlson P, Perry KN. Psychological interventions for psychogenic non-epileptic seizures: a meta-analysis. Seizure. 2017;45:142-150.
CASE Increasingly frequent paroxysmal episodes
Ms. N, age 12, comes to the hospital for evaluation of paroxysmal episodes of pain, weakness, and muscle spasms. A neurologist who evaluated her as an outpatient had recommended a routine electroencephalogram (EEG); after those results were inconclusive, Ms. N’s mother brought her to the hospital for a 24-hour video EEG.
Ms. N has a history of asthma. She has no history of seizures or headache, but her mother has an unspecified seizure disorder that has been stable with antiepileptic medication for many years. Ms. N has no other family history of autoimmune or neurologic disorders.
Ms. N’s episodes began 6 months ago and have progressively increased in frequency from 5 to 12 episodes a day. She says that before she has an episode, she “ feels tingling in her fingers and mouth, and butterflies in her belly,” and then her “whole body clenches up.” She denies experiencing tongue biting, facial or extremity weakness, incontinence, or loss of consciousness during these episodes.
Shortly before her hospitalization, Ms. N had won a scholarship to attend an overnight art camp. Because her episodes were becoming more frequent and their etiology remained unclear, Ms. N and her mother decided it would be unsafe for her to attend, and that she should go to the hospital for evaluation instead.
EVALUATION Tough questions reveal answers
The pediatric team evaluates Ms. N. Her physical exam, laboratory values, and imaging are all within normal limits. Her neurologic exam demonstrates full strength, tone, and sensation in all extremities. All cranial nerves and reflexes are intact. No dysmorphic features or gait abnormalities are noted. All laboratory and imaging tests are normal, including complete blood cell count, electrolytes, calcium, magnesium, phosphorus, glucose, creatine kinase, liver enzymes, urine drug screen, human chorionic gonadotropin (hCG) urine test, and h
After the initial workup, the pediatric team consults the child and adolescent psychiatry team for a complete assessment of Ms. N due to concerns that a psychological component is contributing to her episodes. According to the psychosocial history obtained from Ms. N and her mother, Ms. N had experienced disrupted attachment, trauma, and loss. At age 5, Ms. N was temporarily removed from her mother’s custody after a fight between her mother and brother. At age 9, Ms. N’s stepfather, her primary father figure, died of a brain tumor.
Ms. N also has significant trauma stemming from her relationship with her biological father. Ms. N’s mother reports that her daughter was conceived during nonconsensual sexual intercourse. Ms. N did not have much contact with her biological father until 6 months ago, when he started picking her up at school and taking her to his home for several hours without permission or supervision. Afterwards, Ms. N confided to her mother and a teacher that her father sexually assaulted her during those visits.
Continue to: Ms. N and her mother...
Ms. N and her mother reported the assault to the police and were awaiting legal action.
During the interview with the psychiatry team, Ms. N denies that any thoughts or actions trigger the episodes and reports that she cannot control when they happen. Because she cannot anticipate the episodes, she says she is afraid to leave her house. She does not know why the episodes are happening and feels frustrated that they are getting worse. Ms. N says, “I have been feeling down lately,” but she denies hopelessness, worthlessness, suicidal ideation, homicidal ideation, delusions, or hallucinations.
In the hospital, when the psychiatry team asks Ms. N about her visits with her father, she says that they are “too painful to talk about,” and fears that discussing them will trigger an episode. However, her mother suggests that her daughter’s sexual trauma, as well as ongoing frustrations with the legal system, are influencing her mood; she has had low energy, poor appetite, and is spending more time in bed. Her mother also reports that Ms. N “avoids going out in the sun and spending time with her friends outside. She doesn’t seem to enjoy shopping and art like she used to.” Ms. N told her mother that she was having nightmares about the trauma and “could not stop thinking about some of the bad stuff that happened during the day.”
Ten minutes into the interview, while being questioned about her father, Ms. N experiences a spastic episode. She curls up in bed on her left side, clenches her entire body, and shuts her eyes. Her mother quickly runs to her bedside and counts the seconds until the end of the episode. After 25 seconds, Ms. N awakes with full recollection of the episode. On review of the video EEG during the episode, no ictal patterns are seen.
[polldaddy:10375873]
The authors’ observations
Paroxysmal episodes of weakness, numbness, and muscle spasms in a young female are suggestive of either epilepsy or nonepileptic seizure (NES).1,2 The negative EEG and physical features are inconsistent with epileptiform seizure, and Ms. N’s history and evaluation are suggestive of NES. Nonepileptic seizures are a type of a conversion disorder, or functional neurologic symptom disorder, in which a patient experiences weakness, abnormal movements, or seizure-like episodes that are inconsistent with organic neurologic disease.3 When a diagnosis of conversion disorder is suspected, a clinician must always consider other pathology that can explain the symptoms, such as migraine, vasovagal syncope, or intracranial mass. If a patient has focal neurologic deficits, head imaging should be pursued. Additionally, the clinician must screen for malingering and factitious disorder before establishing a definitive diagnosis. However, conversion disorder is not a diagnosis of exclusion. For example, a negative EEG does not rule out epilepsy, and patients can have both epilepsy and concomitant NES.
Continue to: Although NES is a common...
Although NES is a common type of conversion disorder, it is often difficult to diagnose, manage, and treat. Patients often receive antiepileptic medications but continue to have worsening events that are refractory to treatment. Various clinical features can suggest NES instead of epilepsy. Forced eye closure on video recording is a specific finding suggestive of NES, yet this feature is not sufficient to make the diagnosis.4 A video EEG must be performed to assess for epilepsy. The diagnosis of NES does not exclude the possibility that a patient has epilepsy, as NES can occur in up to 40% of patients with epilepsy.5 A video EEG without ictal patterns before, during, and after an observed episode is diagnostic of NES.6
[polldaddy:10375874]
The authors’ observations
Conversion disorders such as NES are a presentation of neurologic symptoms that cannot be readily accounted for by other conditions and are often associated with antecedent trauma. Multiple factors in Ms. N’s history increase her risk of NES, including loss of multiple loved ones, ongoing legal involvement, and alleged sexual abuse by her father.
Victims of sexual abuse are more likely than the general population to demonstrate symptoms of conversion disorder, especially NES.7,8 The onset of paroxysmal episodes after incestuous abuse in a teenage girl is characteristic of NES. Compared with patients with complex partial epilepsy (CPE), patients with NES are 3 times more likely to report sexual trauma.9,10 Children who report sexual abuse that precedes NES are more likely to have been victimized by a first-degree relative than patients with CPE who report sexual abuse.11 Risk factors for victims developing NES may be related to the severity of adversity, stress sensitivity, and decreased hippocampal volume.12,13
Ms. N endorsed many psychiatric symptoms that accompany her paroxysmal episodes; this is similar to findings in other patients with NES.14 One study found that depression is 3 times more prevalent and PTSD is 8 times more prevalent in patients with NES.12 During the evaluation, Ms. N’s mother said her daughter had low energy, poor appetite, lethargy, and anhedonia for the preceding 5 months, which is consistent with adjustment disorder.3 Her flashbacks, nightmares, difficulty sleeping, and agoraphobia, along with her trouble engaging with the people and activities that used to bring her joy, are symptoms of PTSD. Nonepileptic seizure is often associated with PTSD and can be viewed as an expression of a dissociated subtype.15
In a literature review, Durrant et al16 isolated prognostic indicators for NES (Table16). This study found that 70% of children and 40% of adults achieve remission from NES. Ms. N’s case has multiple concerning features, such as her comorbid psychiatric conditions, ongoing involvement in a legal case, and sexual trauma; this last factor is associated with the most severe symptoms and worse outcomes.16,17 Despite this somber reality, Ms. N has the support of her mother and is relatively young, which play a vital role in recovery.

Continue to: TREATMENT A strategy for minimizing the episodes
TREATMENT A strategy for minimizing the episodes
Ms. N’s medical workup remains unremarkable throughout the rest of her hospital stay. The psychiatry and pediatric teams discuss their assessments and agree that NES is the most likely diagnosis. The psychiatry team counsels Ms. N and her mother on the diagnosis and etiology of NES.
[polldaddy:10375876]
The authors’ observations
Cognitive-behavioral therapy is currently the treatment of choice for reducing seizure frequency in patients with NES.18,19 The use of CBT was suggested due to the theory that NES represents a dissociative response to trauma. Therapy focuses on changing a patient’s beliefs and perceptions associated with attacks.5 A randomized study of 66 patients with NES compared the use of CBT plus standard medical care with standard medical care alone.18 The standard medical care consisted of supportive treatment, an explanation of NES from a neuropsychiatrist, and supervised withdrawal of antiepileptic drugs. The CBT treatment group was offered weekly hour-long sessions for 12 weeks, accompanied by CBT homework and journaling the frequency and nature of seizure episodes (the CBT techniques are outlined in the Figure18). After 4 months, the CBT treatment group had fewer seizures, and after a 6-month follow-up, they were more likely to be seizure-free. However, in this study, CBT treatment did not improve mood or employment status.

A later investigation looked at using selective serotonin reuptake inhibitors to treat NES in adults.19 This study divided participants into 4 treatment groups: CBT with informed psychotherapy (CBT-ip), CBT-ip plus sertraline, sertraline alone, and treatment as usual. Sertraline was titrated up to a dose of 200 mg/d as tolerated. After 16 weeks of sertraline alone, seizure frequency did not decrease. Although both CBT groups showed a reduction in symptoms of up to 60%, the CBT-ip group reported fewer psychiatric symptoms with better social interactions, quality of life, and global functioning compared with patients treated with CBT-ip plus sertraline. The authors suggested that this may be due to the somatic adverse effects associated with sertraline. This study suggests that CBT without medication is the treatment of choice.
In addition to CBT, studies of psychodynamic psychotherapy for NES have had promising findings.20 Psychodynamic psychotherapy focuses on addressing conscious and unconscious anger, loss, feelings of isolation, and trauma. Through improving emotional processing, insight, coping skills and self-regulation, patients often benefit from an improvement in seizures, psychosocial functioning and health care utilization.
Metin et al21 found that group therapy alongside a family-centered approach elicited a strong and durable reduction in seizures in patients with NES. At enrollment, investigators distributed information on NES to patients and families. Psychoeducation and psychoanalysis with behavior modification techniques were provided in 90-minute weekly group sessions over 3 months. Participants also underwent monthly individualized sessions for standard psychiatric care for 9 months. During the group sessions, operant conditioning techniques were used to prevent secondary gain from seizure-like activity. Families met 4 times for 1 hour each to discuss seizures, receive psychoeducation on a subconscious etiology of NES, and learn behavior modification techniques. All 9 participants who completed group and individual therapy reported a significant and sustained reduction in seizure frequency by at least 50% at 12-month follow-up. Patients also demonstrated improvements in mood, anxiety, and quality of life.
Continue to: A meta-analysis...
A meta-analysis by Carlson and Perry22 that included 13 studies and 228 participants, examined different treatment modalities and their effectiveness for NES. They found that patients who received psychological intervention had a 47% remission rate and 82% improvement in seizure frequency compared with only 14% to 23% of those who did not receive therapy. They postulated that therapy for this illness must be flexible to properly address the socially, psychologically, and functionally heterogenous patient population. Although there are few randomized controlled trials for NES to determine the best evidence-based intervention, there is now consensus that NES has a favorable prognosis when barriers to psychological care are eliminated.
OUTCOME Referral for CBT
The treatment team advises Ms. N to engage in outpatient therapy after discharge from the hospital. Ms. N and her mother agree to the treatment plan, and leave the hospital with a referral for CBT the next day.
Bottom Line
Nonepileptic seizure (NES) is a type of conversion disorder characterized by seizure-like episodes without ictal qualities. Risk factors for NES include concomitant epilepsy, psychiatric disorders, unstable psychosocial situations, and antecedent trauma. Patients with a history of incestuous sexual abuse are most at risk for developing NES. A normal EEG that fully captures a seizure-like episode is diagnostic of NES. Cognitive-behavioral therapy can minimize seizure frequency and intensity.
Related Resources
- Marsh P, Benbadis S, Fernandez F. Psychogenic nonepileptic seizures: ways to win over skeptical patients. Current Psychiatry. 2008;7(1):21-24, 32-35.
- LaFrance WC Jr. Eye-opening behaviors help diagnose nonepileptic seizures. Current Psychiatry. 2006;5(11):121-122, 124, 130.
- LaFrance WC Jr, Kanner AM, Barry JJ. Treating patients with psychological nonepileptic seizures. In: Ettinger AB, Kanner AM, eds. Psychiatric issues in epilepsy: a practical guide to diagnosis and treatment. 2nd ed. Philadelphia, PA: Wolters Kluwer Health/ Lippincott Williams & Wilkins; 2007:461-488.
Drug Brand Name
Sertraline • Zoloft
CASE Increasingly frequent paroxysmal episodes
Ms. N, age 12, comes to the hospital for evaluation of paroxysmal episodes of pain, weakness, and muscle spasms. A neurologist who evaluated her as an outpatient had recommended a routine electroencephalogram (EEG); after those results were inconclusive, Ms. N’s mother brought her to the hospital for a 24-hour video EEG.
Ms. N has a history of asthma. She has no history of seizures or headache, but her mother has an unspecified seizure disorder that has been stable with antiepileptic medication for many years. Ms. N has no other family history of autoimmune or neurologic disorders.
Ms. N’s episodes began 6 months ago and have progressively increased in frequency from 5 to 12 episodes a day. She says that before she has an episode, she “ feels tingling in her fingers and mouth, and butterflies in her belly,” and then her “whole body clenches up.” She denies experiencing tongue biting, facial or extremity weakness, incontinence, or loss of consciousness during these episodes.
Shortly before her hospitalization, Ms. N had won a scholarship to attend an overnight art camp. Because her episodes were becoming more frequent and their etiology remained unclear, Ms. N and her mother decided it would be unsafe for her to attend, and that she should go to the hospital for evaluation instead.
EVALUATION Tough questions reveal answers
The pediatric team evaluates Ms. N. Her physical exam, laboratory values, and imaging are all within normal limits. Her neurologic exam demonstrates full strength, tone, and sensation in all extremities. All cranial nerves and reflexes are intact. No dysmorphic features or gait abnormalities are noted. All laboratory and imaging tests are normal, including complete blood cell count, electrolytes, calcium, magnesium, phosphorus, glucose, creatine kinase, liver enzymes, urine drug screen, human chorionic gonadotropin (hCG) urine test, and h
After the initial workup, the pediatric team consults the child and adolescent psychiatry team for a complete assessment of Ms. N due to concerns that a psychological component is contributing to her episodes. According to the psychosocial history obtained from Ms. N and her mother, Ms. N had experienced disrupted attachment, trauma, and loss. At age 5, Ms. N was temporarily removed from her mother’s custody after a fight between her mother and brother. At age 9, Ms. N’s stepfather, her primary father figure, died of a brain tumor.
Ms. N also has significant trauma stemming from her relationship with her biological father. Ms. N’s mother reports that her daughter was conceived during nonconsensual sexual intercourse. Ms. N did not have much contact with her biological father until 6 months ago, when he started picking her up at school and taking her to his home for several hours without permission or supervision. Afterwards, Ms. N confided to her mother and a teacher that her father sexually assaulted her during those visits.
Continue to: Ms. N and her mother...
Ms. N and her mother reported the assault to the police and were awaiting legal action.
During the interview with the psychiatry team, Ms. N denies that any thoughts or actions trigger the episodes and reports that she cannot control when they happen. Because she cannot anticipate the episodes, she says she is afraid to leave her house. She does not know why the episodes are happening and feels frustrated that they are getting worse. Ms. N says, “I have been feeling down lately,” but she denies hopelessness, worthlessness, suicidal ideation, homicidal ideation, delusions, or hallucinations.
In the hospital, when the psychiatry team asks Ms. N about her visits with her father, she says that they are “too painful to talk about,” and fears that discussing them will trigger an episode. However, her mother suggests that her daughter’s sexual trauma, as well as ongoing frustrations with the legal system, are influencing her mood; she has had low energy, poor appetite, and is spending more time in bed. Her mother also reports that Ms. N “avoids going out in the sun and spending time with her friends outside. She doesn’t seem to enjoy shopping and art like she used to.” Ms. N told her mother that she was having nightmares about the trauma and “could not stop thinking about some of the bad stuff that happened during the day.”
Ten minutes into the interview, while being questioned about her father, Ms. N experiences a spastic episode. She curls up in bed on her left side, clenches her entire body, and shuts her eyes. Her mother quickly runs to her bedside and counts the seconds until the end of the episode. After 25 seconds, Ms. N awakes with full recollection of the episode. On review of the video EEG during the episode, no ictal patterns are seen.
[polldaddy:10375873]
The authors’ observations
Paroxysmal episodes of weakness, numbness, and muscle spasms in a young female are suggestive of either epilepsy or nonepileptic seizure (NES).1,2 The negative EEG and physical features are inconsistent with epileptiform seizure, and Ms. N’s history and evaluation are suggestive of NES. Nonepileptic seizures are a type of a conversion disorder, or functional neurologic symptom disorder, in which a patient experiences weakness, abnormal movements, or seizure-like episodes that are inconsistent with organic neurologic disease.3 When a diagnosis of conversion disorder is suspected, a clinician must always consider other pathology that can explain the symptoms, such as migraine, vasovagal syncope, or intracranial mass. If a patient has focal neurologic deficits, head imaging should be pursued. Additionally, the clinician must screen for malingering and factitious disorder before establishing a definitive diagnosis. However, conversion disorder is not a diagnosis of exclusion. For example, a negative EEG does not rule out epilepsy, and patients can have both epilepsy and concomitant NES.
Continue to: Although NES is a common...
Although NES is a common type of conversion disorder, it is often difficult to diagnose, manage, and treat. Patients often receive antiepileptic medications but continue to have worsening events that are refractory to treatment. Various clinical features can suggest NES instead of epilepsy. Forced eye closure on video recording is a specific finding suggestive of NES, yet this feature is not sufficient to make the diagnosis.4 A video EEG must be performed to assess for epilepsy. The diagnosis of NES does not exclude the possibility that a patient has epilepsy, as NES can occur in up to 40% of patients with epilepsy.5 A video EEG without ictal patterns before, during, and after an observed episode is diagnostic of NES.6
[polldaddy:10375874]
The authors’ observations
Conversion disorders such as NES are a presentation of neurologic symptoms that cannot be readily accounted for by other conditions and are often associated with antecedent trauma. Multiple factors in Ms. N’s history increase her risk of NES, including loss of multiple loved ones, ongoing legal involvement, and alleged sexual abuse by her father.
Victims of sexual abuse are more likely than the general population to demonstrate symptoms of conversion disorder, especially NES.7,8 The onset of paroxysmal episodes after incestuous abuse in a teenage girl is characteristic of NES. Compared with patients with complex partial epilepsy (CPE), patients with NES are 3 times more likely to report sexual trauma.9,10 Children who report sexual abuse that precedes NES are more likely to have been victimized by a first-degree relative than patients with CPE who report sexual abuse.11 Risk factors for victims developing NES may be related to the severity of adversity, stress sensitivity, and decreased hippocampal volume.12,13
Ms. N endorsed many psychiatric symptoms that accompany her paroxysmal episodes; this is similar to findings in other patients with NES.14 One study found that depression is 3 times more prevalent and PTSD is 8 times more prevalent in patients with NES.12 During the evaluation, Ms. N’s mother said her daughter had low energy, poor appetite, lethargy, and anhedonia for the preceding 5 months, which is consistent with adjustment disorder.3 Her flashbacks, nightmares, difficulty sleeping, and agoraphobia, along with her trouble engaging with the people and activities that used to bring her joy, are symptoms of PTSD. Nonepileptic seizure is often associated with PTSD and can be viewed as an expression of a dissociated subtype.15
In a literature review, Durrant et al16 isolated prognostic indicators for NES (Table16). This study found that 70% of children and 40% of adults achieve remission from NES. Ms. N’s case has multiple concerning features, such as her comorbid psychiatric conditions, ongoing involvement in a legal case, and sexual trauma; this last factor is associated with the most severe symptoms and worse outcomes.16,17 Despite this somber reality, Ms. N has the support of her mother and is relatively young, which play a vital role in recovery.

Continue to: TREATMENT A strategy for minimizing the episodes
TREATMENT A strategy for minimizing the episodes
Ms. N’s medical workup remains unremarkable throughout the rest of her hospital stay. The psychiatry and pediatric teams discuss their assessments and agree that NES is the most likely diagnosis. The psychiatry team counsels Ms. N and her mother on the diagnosis and etiology of NES.
[polldaddy:10375876]
The authors’ observations
Cognitive-behavioral therapy is currently the treatment of choice for reducing seizure frequency in patients with NES.18,19 The use of CBT was suggested due to the theory that NES represents a dissociative response to trauma. Therapy focuses on changing a patient’s beliefs and perceptions associated with attacks.5 A randomized study of 66 patients with NES compared the use of CBT plus standard medical care with standard medical care alone.18 The standard medical care consisted of supportive treatment, an explanation of NES from a neuropsychiatrist, and supervised withdrawal of antiepileptic drugs. The CBT treatment group was offered weekly hour-long sessions for 12 weeks, accompanied by CBT homework and journaling the frequency and nature of seizure episodes (the CBT techniques are outlined in the Figure18). After 4 months, the CBT treatment group had fewer seizures, and after a 6-month follow-up, they were more likely to be seizure-free. However, in this study, CBT treatment did not improve mood or employment status.

A later investigation looked at using selective serotonin reuptake inhibitors to treat NES in adults.19 This study divided participants into 4 treatment groups: CBT with informed psychotherapy (CBT-ip), CBT-ip plus sertraline, sertraline alone, and treatment as usual. Sertraline was titrated up to a dose of 200 mg/d as tolerated. After 16 weeks of sertraline alone, seizure frequency did not decrease. Although both CBT groups showed a reduction in symptoms of up to 60%, the CBT-ip group reported fewer psychiatric symptoms with better social interactions, quality of life, and global functioning compared with patients treated with CBT-ip plus sertraline. The authors suggested that this may be due to the somatic adverse effects associated with sertraline. This study suggests that CBT without medication is the treatment of choice.
In addition to CBT, studies of psychodynamic psychotherapy for NES have had promising findings.20 Psychodynamic psychotherapy focuses on addressing conscious and unconscious anger, loss, feelings of isolation, and trauma. Through improving emotional processing, insight, coping skills and self-regulation, patients often benefit from an improvement in seizures, psychosocial functioning and health care utilization.
Metin et al21 found that group therapy alongside a family-centered approach elicited a strong and durable reduction in seizures in patients with NES. At enrollment, investigators distributed information on NES to patients and families. Psychoeducation and psychoanalysis with behavior modification techniques were provided in 90-minute weekly group sessions over 3 months. Participants also underwent monthly individualized sessions for standard psychiatric care for 9 months. During the group sessions, operant conditioning techniques were used to prevent secondary gain from seizure-like activity. Families met 4 times for 1 hour each to discuss seizures, receive psychoeducation on a subconscious etiology of NES, and learn behavior modification techniques. All 9 participants who completed group and individual therapy reported a significant and sustained reduction in seizure frequency by at least 50% at 12-month follow-up. Patients also demonstrated improvements in mood, anxiety, and quality of life.
Continue to: A meta-analysis...
A meta-analysis by Carlson and Perry22 that included 13 studies and 228 participants, examined different treatment modalities and their effectiveness for NES. They found that patients who received psychological intervention had a 47% remission rate and 82% improvement in seizure frequency compared with only 14% to 23% of those who did not receive therapy. They postulated that therapy for this illness must be flexible to properly address the socially, psychologically, and functionally heterogenous patient population. Although there are few randomized controlled trials for NES to determine the best evidence-based intervention, there is now consensus that NES has a favorable prognosis when barriers to psychological care are eliminated.
OUTCOME Referral for CBT
The treatment team advises Ms. N to engage in outpatient therapy after discharge from the hospital. Ms. N and her mother agree to the treatment plan, and leave the hospital with a referral for CBT the next day.
Bottom Line
Nonepileptic seizure (NES) is a type of conversion disorder characterized by seizure-like episodes without ictal qualities. Risk factors for NES include concomitant epilepsy, psychiatric disorders, unstable psychosocial situations, and antecedent trauma. Patients with a history of incestuous sexual abuse are most at risk for developing NES. A normal EEG that fully captures a seizure-like episode is diagnostic of NES. Cognitive-behavioral therapy can minimize seizure frequency and intensity.
Related Resources
- Marsh P, Benbadis S, Fernandez F. Psychogenic nonepileptic seizures: ways to win over skeptical patients. Current Psychiatry. 2008;7(1):21-24, 32-35.
- LaFrance WC Jr. Eye-opening behaviors help diagnose nonepileptic seizures. Current Psychiatry. 2006;5(11):121-122, 124, 130.
- LaFrance WC Jr, Kanner AM, Barry JJ. Treating patients with psychological nonepileptic seizures. In: Ettinger AB, Kanner AM, eds. Psychiatric issues in epilepsy: a practical guide to diagnosis and treatment. 2nd ed. Philadelphia, PA: Wolters Kluwer Health/ Lippincott Williams & Wilkins; 2007:461-488.
Drug Brand Name
Sertraline • Zoloft
1. Lesser R. Psychogenic seizures. Neurology. 1996;46(6):1499-1507.
2. Stone J, LaFrance W, Brown R, et al. Conversion disorder: current problems and potential solutions for DSM-5. J Psychosom Res. 2011;71(6):369-376.
3. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
4. Syed T, Arozullah A, Suciu G, et al. Do observer and self-reports of ictal eye closure predict psychogenic nonepileptic seizures? Epilepsia. 2008;49(5):898-904.
5. Vega-Zelaya L, Alvarez M, Ezquiaga E, et al. Psychogenic non-epileptic seizures in a surgical epilepsy unit: experience and a comprehensive review. Epilepsy Topics. 2014. doi: 10.5772/57439.
6. LaFrance W, Baker G, Duncan R, et al. Minimum requirements for the diagnosis of psychogenic nonepileptic seizures: a staged approach. Epilepsia. 2013;54(11):2005-2018.
7. Roeloes K, Pasman J. Stress, childhood trauma, and cognitive functions in functional neurologic disorders. In: Hallett M, Stone J, Carson A, eds. Handbook of clinical neurology: functional neurologic disorders. 3rd ed. New York, NY: Elsevier; 2017:139-155.
8. Paras M, Murad M, Chen L, et al. Sexual abuse and lifetime diagnosis of somatic disorders. JAMA. 2009;302(5):550.
9. Fiszman A, Alves-Leon SV, Nunes RG, et al. Traumatic events and posttraumatic stress disorder in patients with psychogenic nonepileptic seizures: a critical review. Epilepsy Behav. 2004;5(6):818-825.
10. Sharpe D, Faye C. Non-epileptic seizures and child sexual abuse: a critical review of the literature. Clin Psychol Rev. 2006;26(8):1020-1040.
11. Alper K, Devinsky O, Perrine K, et al. Nonepileptic seizures and childhood sexual and physical abuse. Neurology. 1993;43(10):1950-1953.
12. Plioplys S, Doss J, Siddarth P et al. A multisite controlled study of risk factors in pediatric psychogenic nonepileptic seizures. Epilepsia. 2014;55(11):1739-1747.
13. Andersen S, Tomada A, Vincow E, et al. Preliminary evidence for sensitive periods in the effect of childhood sexual abuse on regional brain development. J Neuropsychiatry Clin Neurosci. 2008;20(3):292-301.
14. Sar V. Childhood trauma, dissociation, and psychiatric comorbidity in patients with conversion disorder. Am J Psychiatry. 2004;161(12):2271-2276.
15. Rosenberg HJ, Rosenberg SD, Williamson PD, et al. A comparative study of trauma and posttraumatic stress disorder prevalence in epilepsy patients and psychogenic nonepileptic seizure patients. Epilepsia. 2000;41(4):447-452.
16. Durrant J, Rickards H, Cavanna A. Prognosis and outcome predictors in psychogenic nonepileptic seizures. Epilepsy Res Treat. 2011;2011:1-7.
17. Selkirk M, Duncan R, Oto M, et al. Clinical differences between patients with nonepileptic seizures who report antecedent sexual abuse and those who do not. Epilepsia. 2008;49(8):1446-1450.
18. Goldstein L, Chalder T, Chigwedere C, et al. Cognitive-behavioral therapy for psychogenic nonepileptic seizures: a pilot RCT. Neurology. 2010;74(24):1986-1994.
19. LaFrance W, Baird G, Barry J, et al. Multicenter pilot treatment trial for psychogenic nonepileptic seizures. JAMA Psychiatry. 2014;71(9):997.
20. Howlett S, Reuber M. An augmented model of brief psychodynamic interpersonal therapy for patients with nonepileptic seizures. Psychotherapy (Chic). 2009;46(1):125-138.
21. Metin SZ, Ozmen M, Metin B, et al. Treatment with group psychotherapy for chronic psychogenic nonepileptic seizures. Epilepsy Behav. 2013;28(1):91-94.
22. Carlson P, Perry KN. Psychological interventions for psychogenic non-epileptic seizures: a meta-analysis. Seizure. 2017;45:142-150.
1. Lesser R. Psychogenic seizures. Neurology. 1996;46(6):1499-1507.
2. Stone J, LaFrance W, Brown R, et al. Conversion disorder: current problems and potential solutions for DSM-5. J Psychosom Res. 2011;71(6):369-376.
3. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
4. Syed T, Arozullah A, Suciu G, et al. Do observer and self-reports of ictal eye closure predict psychogenic nonepileptic seizures? Epilepsia. 2008;49(5):898-904.
5. Vega-Zelaya L, Alvarez M, Ezquiaga E, et al. Psychogenic non-epileptic seizures in a surgical epilepsy unit: experience and a comprehensive review. Epilepsy Topics. 2014. doi: 10.5772/57439.
6. LaFrance W, Baker G, Duncan R, et al. Minimum requirements for the diagnosis of psychogenic nonepileptic seizures: a staged approach. Epilepsia. 2013;54(11):2005-2018.
7. Roeloes K, Pasman J. Stress, childhood trauma, and cognitive functions in functional neurologic disorders. In: Hallett M, Stone J, Carson A, eds. Handbook of clinical neurology: functional neurologic disorders. 3rd ed. New York, NY: Elsevier; 2017:139-155.
8. Paras M, Murad M, Chen L, et al. Sexual abuse and lifetime diagnosis of somatic disorders. JAMA. 2009;302(5):550.
9. Fiszman A, Alves-Leon SV, Nunes RG, et al. Traumatic events and posttraumatic stress disorder in patients with psychogenic nonepileptic seizures: a critical review. Epilepsy Behav. 2004;5(6):818-825.
10. Sharpe D, Faye C. Non-epileptic seizures and child sexual abuse: a critical review of the literature. Clin Psychol Rev. 2006;26(8):1020-1040.
11. Alper K, Devinsky O, Perrine K, et al. Nonepileptic seizures and childhood sexual and physical abuse. Neurology. 1993;43(10):1950-1953.
12. Plioplys S, Doss J, Siddarth P et al. A multisite controlled study of risk factors in pediatric psychogenic nonepileptic seizures. Epilepsia. 2014;55(11):1739-1747.
13. Andersen S, Tomada A, Vincow E, et al. Preliminary evidence for sensitive periods in the effect of childhood sexual abuse on regional brain development. J Neuropsychiatry Clin Neurosci. 2008;20(3):292-301.
14. Sar V. Childhood trauma, dissociation, and psychiatric comorbidity in patients with conversion disorder. Am J Psychiatry. 2004;161(12):2271-2276.
15. Rosenberg HJ, Rosenberg SD, Williamson PD, et al. A comparative study of trauma and posttraumatic stress disorder prevalence in epilepsy patients and psychogenic nonepileptic seizure patients. Epilepsia. 2000;41(4):447-452.
16. Durrant J, Rickards H, Cavanna A. Prognosis and outcome predictors in psychogenic nonepileptic seizures. Epilepsy Res Treat. 2011;2011:1-7.
17. Selkirk M, Duncan R, Oto M, et al. Clinical differences between patients with nonepileptic seizures who report antecedent sexual abuse and those who do not. Epilepsia. 2008;49(8):1446-1450.
18. Goldstein L, Chalder T, Chigwedere C, et al. Cognitive-behavioral therapy for psychogenic nonepileptic seizures: a pilot RCT. Neurology. 2010;74(24):1986-1994.
19. LaFrance W, Baird G, Barry J, et al. Multicenter pilot treatment trial for psychogenic nonepileptic seizures. JAMA Psychiatry. 2014;71(9):997.
20. Howlett S, Reuber M. An augmented model of brief psychodynamic interpersonal therapy for patients with nonepileptic seizures. Psychotherapy (Chic). 2009;46(1):125-138.
21. Metin SZ, Ozmen M, Metin B, et al. Treatment with group psychotherapy for chronic psychogenic nonepileptic seizures. Epilepsy Behav. 2013;28(1):91-94.
22. Carlson P, Perry KN. Psychological interventions for psychogenic non-epileptic seizures: a meta-analysis. Seizure. 2017;45:142-150.
The jealous insomniac
CASE Anxious and jealous
Mrs. H, age 28, presents to the emergency department (ED) with pressured speech, emotional lability, loose associations, and echolalia. On physical examination, Mrs. H is noted to have hand tremors. Mrs. H says she has not slept for the past 5 days and is experiencing anxiety and heart palpitations.
She also says that for the past 2 years she has believed that her husband is having an affair with her best friend. However, her current presentation—which she attributes to the alleged affair—began a week before she came to the ED. According to her husband, Mrs. H was “perfectly fine until a week ago” and her symptoms “appeared out of nowhere.” He reports that this has never happened before.
Mrs. H is admitted to the psychiatry unit. The nursing team reports that on the first night, Mrs. H was “running and screaming on the unit, out of control,” and was “tearful, manicky, and dysphoric.”
Mrs. H has no significant medical or psychiatric history. Her family history is significant for hyperthyroidism in her mother and maternal grandmother. Mrs. H says she smokes cigarettes (1 pack/d) but denies alcohol or illicit drug use.
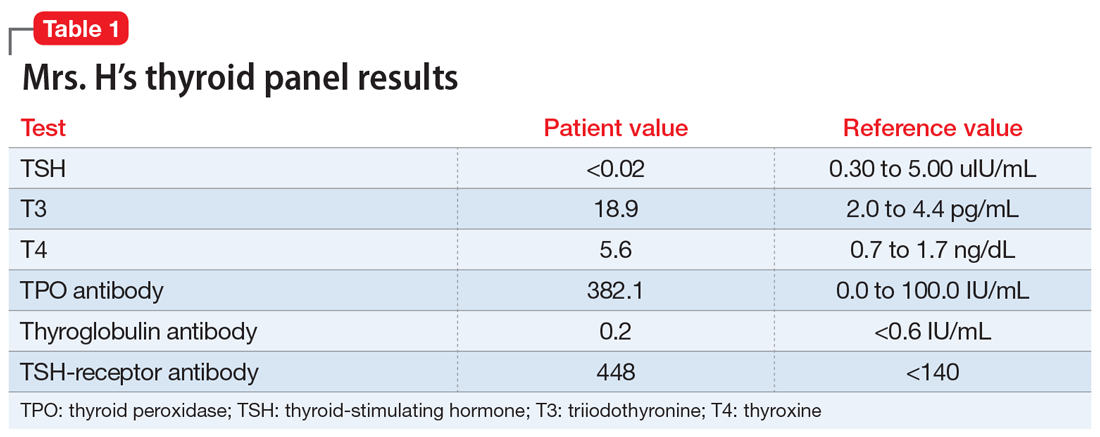
EVALUATION A telling thyroid panel
Mrs. H undergoes laboratory testing, including a complete blood count, comprehensive metabolic panel, and thyroid panel due to her family history of thyroid-related disorders. The thyroid panel shows the presence of the thyroid-stimulating hormone (TSH) receptor antibody; a low TSH level; elevated triiodothyronine (T3) and thyroxine (T4) levels, with T3 > T4; elevated thyroid peroxidase (TPO) antibody; and elevated thyroglobulin antibody (Table 1). A scan shows the thyroid gland to be normal/top-normal size and is read by radiology to be indicative of a resolving thyroiditis vs Graves’ disease. An electrocardiogram indicates a heart rate of 139 beats per minute.
[polldaddy:10352133]
The authors’ observations
Mrs. H fits the presentation of psychosis secondary to Graves’ disease. However, our differential consisted of thyroiditis, brief psychotic disorder, delusional disorder (jealous type), and bipolar mania.
Brief psychotic disorder, bipolar mania, and delusional disorder were better explained by Graves’ disease, and Mrs. H’s jealous delusion resulted in functional impairment, which eliminated delusional disorder. Her family history of hyperthyroidism, as well as her sex and history of tobacco use, supported the diagnosis of Graves’ disease. Although Mrs. H did not experience goiter, ophthalmopathy, or dermopathy, which are common signs and symptoms of Graves’ disease (Table 2), she did present with irritability, insomnia, tachycardia, and a hand tremor. Her psychiatric symptoms included anxiety, emotional lability and, most importantly, psychosis. Her laboratory results included the presence of the TSH-receptor antibody, a low TSH level, and elevated T3 and T4 levels (T3>T4), confirming the diagnosis of early-onset Graves’ disease.
Continue to: Graves' disease
Graves’ disease
Graves’ disease is the most common cause of hyperthyroidism, representing approximately 50% to 80% of cases.1 Graves’ disease occurs most often in women, smokers, and those with a personal or family history of autoimmune disease; although patients of any age may be affected, the peak incidence occurs between age 40 and 60.1
Graves’ disease results from the production of immunoglobulin G (IgG) antibodies that activate the TSH receptor on the surface of thyroid follicular cells.1 The presence of the TSH-receptor antibody, in addition to a low TSH and elevated T3 and T4 levels (T3>T4), are common laboratory findings in patients with this disease. A thyroid scan will also show increased radiotracer accumulation.
Patients with Graves’ disease, as well as those with hyperthyroidism, tend to report weight loss, increased appetite, heat intolerance, irritability, insomnia, and palpitations. In addition to the above symptoms, the identifying signs and symptoms of Graves’ disease include a goiter, ophthalmopathy, and dermopathy (Table 2). Rarely, patients with Graves’ disease can present with psychosis, which is often complicated by thyrotoxicosis.2
[polldaddy:10352135]
TREATMENT Antipsychotic and a beta blocker
Based on her signs, symptoms, and laboratory findings, Mrs. H receives risperidone, 1 mg twice daily, for psychosis, and atenolol, 25 mg twice daily, for heart palpitations. Over 4 days, her symptoms decrease; she experiences more linear thought and decreased flight-of-ideas, and becomes unsure about the truth of her husband’s alleged affair. Her impulsive behaviors and severe mood lability cease. Her tachycardia remains controlled with atenolol.
The authors’ observations
Rapid initiation of treatment is important when managing patients with Graves’ disease, because untreated patients have a higher risk of psychiatric illness, cardiac disease, arrhythmia, and sudden cardiac death.1 Patients with Graves’ disease typically are treated with thionamides, radioactive iodine, and/or surgery. When a patient presents with psychosis as a result of thyrotoxicosis, treatment focuses on improving the thyrotoxicosis through anti-thyroid medications and beta blockers (Table 33). Psychotropic medications, such as antipsychotics, are not indicated for primary treatment, but are given to patients who have severe psychosis until symptoms have resolved.3 For Mrs. H, the severity of her psychosis necessitated risperidone in addition to atenolol.
OUTCOME Continuous medical management; no ablation
Mrs. H is discharged with immediate outpatient follow-up with an endocrinology team to discuss the best long-term management of her thyroiditis. Mrs. H opts for continuous medical management (as opposed to ablation) and is administered methimazole, 15 mg/d, to treat Graves’ disease.
The authors’ observations
This case provides useful information regarding recognizing psychosis as the initial sign of Graves’ disease. Although Graves’ disease represents 50% to 80% of cases of hyperthyroidism,1 psychosis as the first clinical presentation of this disease is extremely rare. Several case reports, however, have described this phenomenon,2,3 and further studies would be helpful to determine its true prevalence.
Continue to: Bottom Line
Bottom Line
Although extremely rare, psychosis as the initial clinical presentation of Graves’ disease can occur. The early diagnosis of Graves’ disease is critical to prevent cardiovascular implications and death.
Related Resources
- Abraham P, Acharya S. Current and emerging treatment options for Graves’ hyperthyroidism. Ther Clin Risk Manag. 2010;6:29-40.
- Bunevicius R, Prange AJ Jr. Psychiatric manifestations of Graves’ hyperthyroidism: pathophysiology and treatment options. CNS Drugs. 2006;20(11):897-909.
- Ginsberg J. Diagnosis and management of Graves’ disease. CMAJ. 2003;168(5):575-585.
Drug Brand Names
Atenolol • Tenormin
Methimazole • Tapazole
Risperidone • Risperdal
1. Girgis C, Champion B, Wall J. Current concepts in Graves’ disease. Ther Adv Endocrinol Metab. 2011;2(3):135-144.
2. Urias-Uribe L, Valdez-Solis E, González-Milán C, et al. Psychosis crisis associated with thyrotoxicosis due to Graves’ disease. Case Rep Psychiatry. 2017;2017:6803682. doi: 10.1155/2017/6803682.
3. Ugwu ET, Maluze J, Onyebueke GC. Graves’ thyrotoxicosis presenting as schizophreniform psychosis: a case report and literature review. Int J Endocrinol Metab. 2017;15(1):e41977. doi: 10.5812/ijem.41977.
CASE Anxious and jealous
Mrs. H, age 28, presents to the emergency department (ED) with pressured speech, emotional lability, loose associations, and echolalia. On physical examination, Mrs. H is noted to have hand tremors. Mrs. H says she has not slept for the past 5 days and is experiencing anxiety and heart palpitations.
She also says that for the past 2 years she has believed that her husband is having an affair with her best friend. However, her current presentation—which she attributes to the alleged affair—began a week before she came to the ED. According to her husband, Mrs. H was “perfectly fine until a week ago” and her symptoms “appeared out of nowhere.” He reports that this has never happened before.
Mrs. H is admitted to the psychiatry unit. The nursing team reports that on the first night, Mrs. H was “running and screaming on the unit, out of control,” and was “tearful, manicky, and dysphoric.”
Mrs. H has no significant medical or psychiatric history. Her family history is significant for hyperthyroidism in her mother and maternal grandmother. Mrs. H says she smokes cigarettes (1 pack/d) but denies alcohol or illicit drug use.

EVALUATION A telling thyroid panel
Mrs. H undergoes laboratory testing, including a complete blood count, comprehensive metabolic panel, and thyroid panel due to her family history of thyroid-related disorders. The thyroid panel shows the presence of the thyroid-stimulating hormone (TSH) receptor antibody; a low TSH level; elevated triiodothyronine (T3) and thyroxine (T4) levels, with T3 > T4; elevated thyroid peroxidase (TPO) antibody; and elevated thyroglobulin antibody (Table 1). A scan shows the thyroid gland to be normal/top-normal size and is read by radiology to be indicative of a resolving thyroiditis vs Graves’ disease. An electrocardiogram indicates a heart rate of 139 beats per minute.
[polldaddy:10352133]
The authors’ observations
Mrs. H fits the presentation of psychosis secondary to Graves’ disease. However, our differential consisted of thyroiditis, brief psychotic disorder, delusional disorder (jealous type), and bipolar mania.
Brief psychotic disorder, bipolar mania, and delusional disorder were better explained by Graves’ disease, and Mrs. H’s jealous delusion resulted in functional impairment, which eliminated delusional disorder. Her family history of hyperthyroidism, as well as her sex and history of tobacco use, supported the diagnosis of Graves’ disease. Although Mrs. H did not experience goiter, ophthalmopathy, or dermopathy, which are common signs and symptoms of Graves’ disease (Table 2), she did present with irritability, insomnia, tachycardia, and a hand tremor. Her psychiatric symptoms included anxiety, emotional lability and, most importantly, psychosis. Her laboratory results included the presence of the TSH-receptor antibody, a low TSH level, and elevated T3 and T4 levels (T3>T4), confirming the diagnosis of early-onset Graves’ disease.
Continue to: Graves' disease
Graves’ disease
Graves’ disease is the most common cause of hyperthyroidism, representing approximately 50% to 80% of cases.1 Graves’ disease occurs most often in women, smokers, and those with a personal or family history of autoimmune disease; although patients of any age may be affected, the peak incidence occurs between age 40 and 60.1
Graves’ disease results from the production of immunoglobulin G (IgG) antibodies that activate the TSH receptor on the surface of thyroid follicular cells.1 The presence of the TSH-receptor antibody, in addition to a low TSH and elevated T3 and T4 levels (T3>T4), are common laboratory findings in patients with this disease. A thyroid scan will also show increased radiotracer accumulation.
Patients with Graves’ disease, as well as those with hyperthyroidism, tend to report weight loss, increased appetite, heat intolerance, irritability, insomnia, and palpitations. In addition to the above symptoms, the identifying signs and symptoms of Graves’ disease include a goiter, ophthalmopathy, and dermopathy (Table 2). Rarely, patients with Graves’ disease can present with psychosis, which is often complicated by thyrotoxicosis.2
[polldaddy:10352135]
TREATMENT Antipsychotic and a beta blocker
Based on her signs, symptoms, and laboratory findings, Mrs. H receives risperidone, 1 mg twice daily, for psychosis, and atenolol, 25 mg twice daily, for heart palpitations. Over 4 days, her symptoms decrease; she experiences more linear thought and decreased flight-of-ideas, and becomes unsure about the truth of her husband’s alleged affair. Her impulsive behaviors and severe mood lability cease. Her tachycardia remains controlled with atenolol.
The authors’ observations
Rapid initiation of treatment is important when managing patients with Graves’ disease, because untreated patients have a higher risk of psychiatric illness, cardiac disease, arrhythmia, and sudden cardiac death.1 Patients with Graves’ disease typically are treated with thionamides, radioactive iodine, and/or surgery. When a patient presents with psychosis as a result of thyrotoxicosis, treatment focuses on improving the thyrotoxicosis through anti-thyroid medications and beta blockers (Table 33). Psychotropic medications, such as antipsychotics, are not indicated for primary treatment, but are given to patients who have severe psychosis until symptoms have resolved.3 For Mrs. H, the severity of her psychosis necessitated risperidone in addition to atenolol.
OUTCOME Continuous medical management; no ablation
Mrs. H is discharged with immediate outpatient follow-up with an endocrinology team to discuss the best long-term management of her thyroiditis. Mrs. H opts for continuous medical management (as opposed to ablation) and is administered methimazole, 15 mg/d, to treat Graves’ disease.
The authors’ observations
This case provides useful information regarding recognizing psychosis as the initial sign of Graves’ disease. Although Graves’ disease represents 50% to 80% of cases of hyperthyroidism,1 psychosis as the first clinical presentation of this disease is extremely rare. Several case reports, however, have described this phenomenon,2,3 and further studies would be helpful to determine its true prevalence.
Continue to: Bottom Line
Bottom Line
Although extremely rare, psychosis as the initial clinical presentation of Graves’ disease can occur. The early diagnosis of Graves’ disease is critical to prevent cardiovascular implications and death.
Related Resources
- Abraham P, Acharya S. Current and emerging treatment options for Graves’ hyperthyroidism. Ther Clin Risk Manag. 2010;6:29-40.
- Bunevicius R, Prange AJ Jr. Psychiatric manifestations of Graves’ hyperthyroidism: pathophysiology and treatment options. CNS Drugs. 2006;20(11):897-909.
- Ginsberg J. Diagnosis and management of Graves’ disease. CMAJ. 2003;168(5):575-585.
Drug Brand Names
Atenolol • Tenormin
Methimazole • Tapazole
Risperidone • Risperdal
CASE Anxious and jealous
Mrs. H, age 28, presents to the emergency department (ED) with pressured speech, emotional lability, loose associations, and echolalia. On physical examination, Mrs. H is noted to have hand tremors. Mrs. H says she has not slept for the past 5 days and is experiencing anxiety and heart palpitations.
She also says that for the past 2 years she has believed that her husband is having an affair with her best friend. However, her current presentation—which she attributes to the alleged affair—began a week before she came to the ED. According to her husband, Mrs. H was “perfectly fine until a week ago” and her symptoms “appeared out of nowhere.” He reports that this has never happened before.
Mrs. H is admitted to the psychiatry unit. The nursing team reports that on the first night, Mrs. H was “running and screaming on the unit, out of control,” and was “tearful, manicky, and dysphoric.”
Mrs. H has no significant medical or psychiatric history. Her family history is significant for hyperthyroidism in her mother and maternal grandmother. Mrs. H says she smokes cigarettes (1 pack/d) but denies alcohol or illicit drug use.

EVALUATION A telling thyroid panel
Mrs. H undergoes laboratory testing, including a complete blood count, comprehensive metabolic panel, and thyroid panel due to her family history of thyroid-related disorders. The thyroid panel shows the presence of the thyroid-stimulating hormone (TSH) receptor antibody; a low TSH level; elevated triiodothyronine (T3) and thyroxine (T4) levels, with T3 > T4; elevated thyroid peroxidase (TPO) antibody; and elevated thyroglobulin antibody (Table 1). A scan shows the thyroid gland to be normal/top-normal size and is read by radiology to be indicative of a resolving thyroiditis vs Graves’ disease. An electrocardiogram indicates a heart rate of 139 beats per minute.
[polldaddy:10352133]
The authors’ observations
Mrs. H fits the presentation of psychosis secondary to Graves’ disease. However, our differential consisted of thyroiditis, brief psychotic disorder, delusional disorder (jealous type), and bipolar mania.
Brief psychotic disorder, bipolar mania, and delusional disorder were better explained by Graves’ disease, and Mrs. H’s jealous delusion resulted in functional impairment, which eliminated delusional disorder. Her family history of hyperthyroidism, as well as her sex and history of tobacco use, supported the diagnosis of Graves’ disease. Although Mrs. H did not experience goiter, ophthalmopathy, or dermopathy, which are common signs and symptoms of Graves’ disease (Table 2), she did present with irritability, insomnia, tachycardia, and a hand tremor. Her psychiatric symptoms included anxiety, emotional lability and, most importantly, psychosis. Her laboratory results included the presence of the TSH-receptor antibody, a low TSH level, and elevated T3 and T4 levels (T3>T4), confirming the diagnosis of early-onset Graves’ disease.
Continue to: Graves' disease
Graves’ disease
Graves’ disease is the most common cause of hyperthyroidism, representing approximately 50% to 80% of cases.1 Graves’ disease occurs most often in women, smokers, and those with a personal or family history of autoimmune disease; although patients of any age may be affected, the peak incidence occurs between age 40 and 60.1
Graves’ disease results from the production of immunoglobulin G (IgG) antibodies that activate the TSH receptor on the surface of thyroid follicular cells.1 The presence of the TSH-receptor antibody, in addition to a low TSH and elevated T3 and T4 levels (T3>T4), are common laboratory findings in patients with this disease. A thyroid scan will also show increased radiotracer accumulation.
Patients with Graves’ disease, as well as those with hyperthyroidism, tend to report weight loss, increased appetite, heat intolerance, irritability, insomnia, and palpitations. In addition to the above symptoms, the identifying signs and symptoms of Graves’ disease include a goiter, ophthalmopathy, and dermopathy (Table 2). Rarely, patients with Graves’ disease can present with psychosis, which is often complicated by thyrotoxicosis.2
[polldaddy:10352135]
TREATMENT Antipsychotic and a beta blocker
Based on her signs, symptoms, and laboratory findings, Mrs. H receives risperidone, 1 mg twice daily, for psychosis, and atenolol, 25 mg twice daily, for heart palpitations. Over 4 days, her symptoms decrease; she experiences more linear thought and decreased flight-of-ideas, and becomes unsure about the truth of her husband’s alleged affair. Her impulsive behaviors and severe mood lability cease. Her tachycardia remains controlled with atenolol.
The authors’ observations
Rapid initiation of treatment is important when managing patients with Graves’ disease, because untreated patients have a higher risk of psychiatric illness, cardiac disease, arrhythmia, and sudden cardiac death.1 Patients with Graves’ disease typically are treated with thionamides, radioactive iodine, and/or surgery. When a patient presents with psychosis as a result of thyrotoxicosis, treatment focuses on improving the thyrotoxicosis through anti-thyroid medications and beta blockers (Table 33). Psychotropic medications, such as antipsychotics, are not indicated for primary treatment, but are given to patients who have severe psychosis until symptoms have resolved.3 For Mrs. H, the severity of her psychosis necessitated risperidone in addition to atenolol.
OUTCOME Continuous medical management; no ablation
Mrs. H is discharged with immediate outpatient follow-up with an endocrinology team to discuss the best long-term management of her thyroiditis. Mrs. H opts for continuous medical management (as opposed to ablation) and is administered methimazole, 15 mg/d, to treat Graves’ disease.
The authors’ observations
This case provides useful information regarding recognizing psychosis as the initial sign of Graves’ disease. Although Graves’ disease represents 50% to 80% of cases of hyperthyroidism,1 psychosis as the first clinical presentation of this disease is extremely rare. Several case reports, however, have described this phenomenon,2,3 and further studies would be helpful to determine its true prevalence.
Continue to: Bottom Line
Bottom Line
Although extremely rare, psychosis as the initial clinical presentation of Graves’ disease can occur. The early diagnosis of Graves’ disease is critical to prevent cardiovascular implications and death.
Related Resources
- Abraham P, Acharya S. Current and emerging treatment options for Graves’ hyperthyroidism. Ther Clin Risk Manag. 2010;6:29-40.
- Bunevicius R, Prange AJ Jr. Psychiatric manifestations of Graves’ hyperthyroidism: pathophysiology and treatment options. CNS Drugs. 2006;20(11):897-909.
- Ginsberg J. Diagnosis and management of Graves’ disease. CMAJ. 2003;168(5):575-585.
Drug Brand Names
Atenolol • Tenormin
Methimazole • Tapazole
Risperidone • Risperdal
1. Girgis C, Champion B, Wall J. Current concepts in Graves’ disease. Ther Adv Endocrinol Metab. 2011;2(3):135-144.
2. Urias-Uribe L, Valdez-Solis E, González-Milán C, et al. Psychosis crisis associated with thyrotoxicosis due to Graves’ disease. Case Rep Psychiatry. 2017;2017:6803682. doi: 10.1155/2017/6803682.
3. Ugwu ET, Maluze J, Onyebueke GC. Graves’ thyrotoxicosis presenting as schizophreniform psychosis: a case report and literature review. Int J Endocrinol Metab. 2017;15(1):e41977. doi: 10.5812/ijem.41977.
1. Girgis C, Champion B, Wall J. Current concepts in Graves’ disease. Ther Adv Endocrinol Metab. 2011;2(3):135-144.
2. Urias-Uribe L, Valdez-Solis E, González-Milán C, et al. Psychosis crisis associated with thyrotoxicosis due to Graves’ disease. Case Rep Psychiatry. 2017;2017:6803682. doi: 10.1155/2017/6803682.
3. Ugwu ET, Maluze J, Onyebueke GC. Graves’ thyrotoxicosis presenting as schizophreniform psychosis: a case report and literature review. Int J Endocrinol Metab. 2017;15(1):e41977. doi: 10.5812/ijem.41977.
Between a rock and a hard place
CASE Irritable and short of breath
Mr. B, age 75, who lives alone, is brought to the emergency department (ED) for evaluation of shortness of breath. Mr. B is normally highly independent, and is able to drive, manage his own finances, attend to activities of daily living, and participate in social functions at church. On the day before he was taken to the ED, his home nurse had come to his home to dispense medications and found Mr. B was irritable, verbally rude, and repeatedly scratching the right side of his head. The nurse was unsure if Mr. B had taken his medications over the weekend. She called for emergency services, but Mr. B refused to go to the ED, and he was able to decline care because he was not in an acute medical emergency (95% oxygen on pulse oximetry).
The next day, when Mr. B’s nurse returned to his home, she found him to be tachypneic and verbigerating the phrase “I don’t know.” She contacted emergency services again, and Mr. B was taken to the ED.
In the ED, Mr. B has tachycardia, tachypnea, increased work of breathing, and diffuse rhonchi. He continues to repeat the phrase “I don’t know” and scratches the right side of his head repeatedly. The ED clinicians consult Psychiatry due to Mr. B’s confusion and because his nurse reports that his presentation is similar to a previous psychiatric hospitalization 9 years earlier.
[polldaddy:10332862]
EVALUATION Complex comorbidities
Mr. B has a lengthy history of schizophrenia, chronic right-sided heart failure secondary to pulmonary hypertension, moderate chronic obstructive pulmonary disease, hypertension, type 2 diabetes mellitus, and prostatic adenocarcinoma after external beam radiation therapy.
His symptoms of schizophrenia had been stable on his long-standing outpatient psychotropic regimen of haloperidol, 5 mg nightly; mirtazapine, 15 mg nightly, for appetite stimulation and insomnia; and trazodone, 100 mg nightly for insomnia. Mr. B has been receiving assertive community treatment (ACT) psychiatric services for schizophrenia; a nurse refills his pill box with his medications weekly. He does not have a history of medication nonadherence, and his nurse did not think he had missed any doses before the weekend.
He has acute changes in depressed mood, perseveration, and a Mini-Mental State Examination (MMSE) score of 26 (missing points for delayed recall and inability to construct a sentence), which indicates a cognitive assessment score on the low end of the normal range for people with at least an eighth grade education.
At the hospital, the psychiatrist diagnoses hypoactive delirium due to Mr. B’s fluctuating attention and disorientation. She also recommends that Mr. B continue his outpatient psychotropic regimen, and adds oral haloperidol, 5 mg, as needed for agitation (his QTc interval is 451 ms; reference range for men <430 ms, borderline prolonged 431 to 450 ms, prolonged >450 ms).
Continue to: An initial laboratory workup...
An initial laboratory workup and electrocardiogram reveal that Mr. B has an elevated troponin level (0.21 ng/mL; reference range <0.04; 0.04 to 0.39 ng/mL is elevated above the 99th percentile of a healthy population), non-ST-elevation myocardial infarction type II, Q waves in lead III, arteriovenous fistula with right axis deviation, acute on chronic kidney failure (creatinine level of 2.1 mg/dL, up from baseline of 1.4 mg/dL; reference range 0.84 to 1.21 mg/dL), elevated brain natriuretic peptide (111 pg/mL; reference range <125 pg/mL), and an elevated lactate level of 5.51 mmol/L (reference range 0.5 to 1 mmol/L). He also has a mixed respiratory alkalosis and metabolic acidosis with increased anion gap, transaminitis (aspartate aminotransferase 149 U/L; reference range 10 to 40 U/L), and elevated alkaline phosphatase (151 IU/L; reference range 44 to 147 IU/L). Urinalysis shows moderate ketones and is negative for nitrite or leukocyte esterase.
A brain CT rules out stroke. A chest X-ray shows subtle left basilar reticular opacity with a follow-up lateral view showing no consolidation and prominent pulmonary vasculature without overt edema.
In the ED, Mr. B is determined to have decision-making capacity and is able to authorize all treatment. Cardiology is also consulted, and Mr. B is admitted to the cardiac intensive care unit (CCU) for cardiogenic shock with close cardiac monitoring.
The Psychiatry and Cardiology teams discuss the risks and benefits of continuing antipsychotics. Due to the imminent risk of harm to Mr. B because of his significant agitation in the ED, which required treatment with one dose of IM haloperidol, 5 mg, and lorazepam, 2 mg, and close monitoring, the teams agree that the benefits of continuing haloperidol outweigh the risks.
On hospital Day 2, Mr. B’s repetitive scratching resolves. He is moved from the CCU to a general medical unit, where he begins to have episodes of mutism and negativism. By hospital Day 6, catatonia is suspected due to a MMSE of 6/30 and a Bush- Francis Catatonia Rating Scale (BFCRS) score of 14 for predominant stereotypy, perseveration, and withdrawal (Table 1). The teams determine that Mr. B lacks decisionmaking capacity due to his inability to rationally manipulate information. His brother is contacted and authorizes all treatment, deferring decision-making to the medical teams caring for Mr. B.
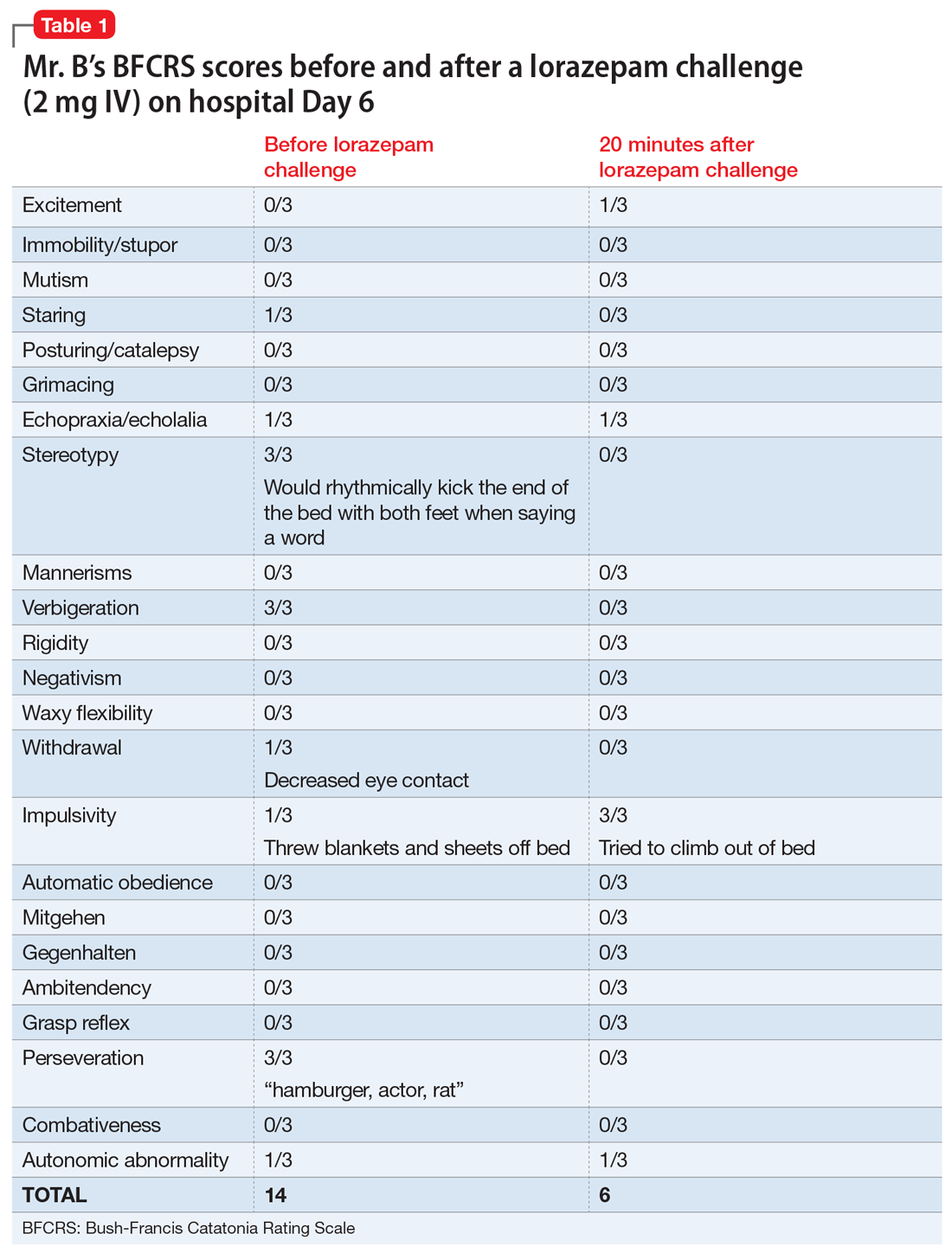
Continue to: Mr. B undergoes an EEG...
Mr. B undergoes an EEG, which rules out nonconvulsive status epilepticus and is consistent with encephalopathy/delirium. Neuroleptic malignant syndrome (NMS) is considered but is less likely because Mr. B had been receiving a stable dose of haloperidol for several years, is afebrile, has stable vital signs, has no muscle rigidity, and no evidence of leukocytosis, creatine kinase elevation, myoglobinuria, hyperkalemia, hyperphosphatemia, thrombocytosis, or hypocalcemia.
Based on these clinical findings, Mr. B is diagnosed with catatonia and delirium.
The authors’ observations
Delirium, characterized by inattention and changes in mental status, is a syndrome due to acute brain dysfunction. It can be subclassified as hyperactive or hypoactive based on the change of activity. Simple catatonia is characterized by changes in behavior, affect, and motor function (with hyper- or hypoactivity). It may arise from gammaaminobutyric acid hypoactivity, dopamine (D2) hypoactivity, and possibly glutamate N-methyl-d-aspartate (NMDA) hyperactivity.1 Malignant catatonia is simple catatonia combined with autonomic instability and hyperthermia, which is a life-threatening condition. The BFCRS is commonly used to assess symptoms.2
Both catatonia and delirium result in significant morbidity and mortality. The 2 conditions share signs and symptoms yet rarely are diagnosed at the same time. DSM-IV, DSM-IV-TR, and DSM-5 state that a diagnosis of catatonia due to another medical condition cannot be made exclusively in the presence of delirium.3,4 DSM-IV and DSM-IV-TR required at least 2 criteria from 5 areas, including motoric immobility, excessive motor activity, extreme negativism or mutism, peculiarities of voluntary movement, and echolalia or echopraxia. Instead of grouping symptoms into clusters, DSM-5 requires 3 criteria of 12 individual symptoms.3,4 A co-occurrence with a medical illness precludes using the DSM-5 “catatonia associated with another mental disorder (catatonia specifier)” with the “unspecified catatonia” diagnosis category.4
However, a growing body of literature suggests that delirium and catatonia can cooccur.5,6 In 2017, Wilson et al6 found that of 136 critically ill patients in the ICU, 43% (58 patients) had only delirium, 3% (4 patients) had only catatonia, 31% (42 patients) had both, and 24% (32 patients) had neither. In patients with both catatonia and delirium, the most common signs of catatonia were autonomic abnormalities (96%), immobility/ stupor (87%), staring (77%), mutism (60%), and posturing (60%).
Continue to: The differential diagnosis...
The differential diagnosis of catatonia is extensive and varied.3,4 The most common psychiatric causes are mood disorders (13% to 31%) and psychotic disorders (7% to 17%).7 Neuromedical etiologies account for 4% to 46% of cases.7 The most common medical and neurologic causes are seizure disorder, acute intermittent porphyria, systemic lupus erythematosus, and drugrelated adverse effects (particularly due to clozapine withdrawal, risperidone, and phencyclidine).7
A workup that includes physical examination, laboratory testing, and neuroimaging can be helpful to identify delirium and catatonia, but there is limited literature to guide identifying coexisting delirium and catatonia other than a blend of physical exam findings of delirium and catatonia. Electroencephalogram may be normal in primary catatonia or may show nonspecific changes in secondary catatonia.8 Additionally, discharges in the frontal lobes and anterior limbic systems with diffuse background slowing and dysrhythmic patterns may be seen.7 Neuroimaging with MRI can help to evaluate catatonia.9 Laboratory testing such as creatine phosphokinase levels can be high in simple catatonia and are often elevated in malignant catatonia.7 Considering the possible co-occurrence of delirium and catatonia is critical to providing good patient care because the 2 conditions are treated differently.
[polldaddy:10332867]
TREATMENT A balancing act
Over the next month, Mr. B alternates between appearing catatonic or delirious. When he appears more catatonic, the dose of lorazepam is increased, which results in increased impulsivity and agitation and leads to multiple interventions from the behavioral emergency response team. At times, the team must use restraints and haloperidol because Mr. B pulls out IV lines and is considered at high risk for falls. When Mr. B appears more delirious and the dose of lorazepam is decreased, he becomes more catatonic.
Following the diagnosis of catatonia on Day 6, oral haloperidol is discontinued to further mitigate Mr. B’s risk of developing NMS. On hospital Day 6, Mr. B improves significantly after a 2-mg IV lorazepam challenge, with a BFCRS score of 6. At this point, he is started on lorazepam, 1 mg IV 3 times a day.
On Day 7, based on the complicated nature of Mr. B’s medical and psychiatric comorbidities, the treatment team considers ECT to minimize medication adverse effects, but Mr. B’s medical condition is too tenuous.
Continue to: On Day 7...
On Day 7, lorazepam is decreased to 0.5 mg/0.5 mg/1 mg IV. On Day 9, it is further decreased to 0.5 mg IV 3 times a day because Mr. B appears to be more delirious. On Day 10, lorazepam is increased to 1 mg IV 3 times a day, and oral haloperidol, 2 mg as needed for agitation, is restarted after multiple nights when Mr. B had behavioral emergencies and was treated with IM haloperidol and lorazepam. On Day 11, lorazepam is decreased and switched from IV formulation to oral, 0.5 mg 3 times a day. On Day 13, oral haloperidol is increased to 2 mg twice a day because of overnight behavioral emergencies requiring treatment with IV haloperidol, 4 mg. On Day 17, oral haloperidol is increased to 2 mg in the morning and 3 mg every night at bedtime because Mr. B has increased morning agitation. On Day 19, oral lorazepam is increased to 1 mg 3 times a day because Mr. B appears more catatonic. On Day 21, oral haloperidol is consolidated to 5 mg every night at bedtime. On Day 31, oral lorazepam is increased to 2 mg/1 mg/1 mg because he appears more catatonic with increased stuttering and mannerisms. On Day 33, oral haloperidol is increased to 6 mg every night at bedtime because Mr. B has morning agitation.
Multiple lorazepam and haloperidol dose adjustments are needed to balance the situation: combating catatonia, addressing delirium, managing schizophrenia symptoms, and improving Mr. B’s cardiac status. Finally, Mr. B is stabilized on oral lorazepam, 2 mg every morning, 1 mg every day at noon, and 1 mg every day at bedtime, and oral haloperidol, 6 mg every day at bedtime. This regimen, Mr. B has a BFCRS score of 1 (Table 2) and returns to his baseline mental status.
The authors’ observations
Delirium and catatonia typically have different treatments. Delirium is routinely treated by addressing the underlying medical and environmental factors, and managing comorbid symptoms such as agitation and disturbing hallucinations by prescribing antipsychotics, restoring the sleep-wake cycle with melatonin, initiating nonpharmacologic behavioral management, and avoiding deliriogenic medications such as benzodiazepines, opioids, and steroids.10 Catatonia is managed by prescribing benzodiazepines (with or without ECT) and by avoiding dopamine antagonists such as antipsychotics and metoclopramide (which may worsen catatonia or precipitate malignant catatonia).
The first-line treatment for catatonia is benzodiazepines, with IV preferred over IM, sublingual, or oral formulations. Electroconvulsive therapy is commonly used with benzodiazepines and is effective in 85% to 90% of patients. For ECT, bitemporal placement and daily treatment with brief pulses are frequently used. It is also effective in 60% of patients who fail to respond to benzodiazepines. Thus, ECT should be considered within the first 48 to 72 hours of benzodiazepine failure.7
Amantadine, a NMDA antagonist, may be a possible treatment for catatonia. A case report published in 1986 described a patient who developed catatonia after the abrupt withdrawal of amantadine during neuroleptic therapy.11 Memantine also may serve as a treatment for catatonia through glutamate antagonism. A review identified 25 cases of patients with catatonia who were treated with amantadine or memantine.12 Oral amantadine was administered at 100 to 400 mg/d in divided doses, with lower doses for patients with diminished renal function.12 Memantine was administered at 5 to 20 mg/d.12 All patients showed improvement after 1 to 7 days of treatment.12 Thus, memantine may be considered for patients with catatonic schizophrenia or comorbid catatonia and delirium. Although memantine was not considered in Mr. B’s case, he would have been a good candidate for treatment with this agent.
Continue to: There are also case reports of...
There are also case reports of aripiprazole being used for catatonia in the context of psychosis or delirium in both adults and adolescents.13-15 Other medications used in case reports for treating catatonia include carbamazepine, valproate, and secondgeneration antipsychotics.7
Because most of the literature on pharmacotherapy for catatonia consists of case reports or small case series, further research on medication management of catatonia and delirium is needed to guide treatment.
OUTCOME Multiple rehospitalizations
On Day 57, Mr. B is discharged to a skilled nursing facility due to significant deconditioning. He is discharged with continued follow-up with his ACT psychiatrist and nurse. Mr. B’s catatonia remains resolved; however, he is unable to be safely managed at the skilled nursing facility.
During the next 7 months, he is readmitted to the ICU for acute on chronic hypoxic respiratory failure 5 times; his rehospitalizations are complicated by delirium due to cardiogenic shock and urosepsis. Mild hyperactive delirium re-emerges after worsening respiratory failure and contributes to falls in the skilled nursing facility.
Six months later, Mr. B continues to receive the initial hospital discharge lorazepam regimen of 2 mg every morning, 1 mg every day at noon, and 1 mg every night at bedtime. The Psychiatry team slowly tapers this to 0.5 mg twice daily.
Continue to: On Day 5...
On Day 5 of Mr. B’s fifth hospital readmission, based on his advance directive, Mr. B’s family implements the do-not-resuscitate and do-not-intubate orders. He is transitioned to comfort measures, and dies on Day 6 with his brother and the hospital chaplain present.
Bottom Line
Delirium and catatonia share signs and symptoms, yet rarely are diagnosed at the same time. Both conditions result in significant morbidity and mortality. An emerging literature supports the concurrence of these 2 syndromes and aids in their diagnosis and treatment. Comorbidity with other medical conditions, common with both delirium and catatonia, substantially complicates treatment; thus, additional research into new treatment approaches is critical.
Related Resources
- Wilson JE, Carlson R, Duggan MC, et al. Delirium and catatonia in critically ill patients: the delirium and catatonia prospective cohort investigation. Crit Care Med. 2017;45(11):1837-1844.
- Catatonia Information Center. Penn State University. http://catatonia.org/.
Drug Brand Names
Amantadine • Symmetrel
Aripiprazole • Abilify
Carbamazepine • Carbatrol, Tegretol
Clozapine • Clozaril
Haloperidol • Haldol
Lorazepam • Ativan
Memantine • Namenda
Metoclopramide • Reglan
Mirtazapine • Remeron
Risperidone • Risperdal
Topiramate • Topamax
Trazodone • Desyrel
Valproate • Depacon, Depakene, Depakote
1. Northoff G. What catatonia can tell us about “top-down modulation”: a neuropsychiatric hypothesis. Behav Brain Sci. 2002;25(5):555-577; discussion 578-604.
2. Bush G, Fink M, Petrides G, et al. Catatonia. I. Rating scale and standardized examination. Acta Psychiatr Scand. 1996;93(2):129-136.
3. Diagnostic and Statistical Manual of Mental Disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
4. Diagnostic and Statistical Manual of Mental Disorders, 4th ed, text rev. Washington, DC: American Psychiatric Association; 2000.
5. Oldham MA, Lee HB. Catatonia vis-à-vis delirium: the significance of recognizing catatonia in altered mental status. Gen Hosp Psychiatry. 2015;37(6):554-559.
6. Wilson JE, Carlson R, Duggan MC. Delirium and catatonia in critically ill patients: the delirium and catatonia prospective cohort investigation. Crit Care Med. 2017;45(11):1837-1844.
7. Fricchione GL, Gross AF, Huffman JC, et al. Chapter 21: Catatonia, neuroleptic malignant syndrome, and serotonin syndrome. In: Stern TA, Fricchione GL, Cassem NH, et al. Massachusetts General Hospital Handbook of General Hospital Psychiatry, 6th Ed. Philadelphia, PA: Saunders Elsevier; 2010:273-288.
8. Van der Kooi AW, Zaal IJ, Klijn FA, et al. Delirium detection using EEG: what and how to measure. Chest. 2015;147(1):94-101.
9. Wilson JE, Niu K, Nicolson SE, et al. The diagnostic criteria and structure of catatonia. Schizophr Res. 2015;164 (1-3):256-262.
10. Maldonado JR. Acute brain failure: pathophysiology, diagnosis, management, and sequelae of delirium. Crit Care Clin. 2017;33(3):461-519.
11. Brown CS, Wittkowsky AK, Bryant SG. Neurolepticinduced catatonia after abrupt withdrawal of amantadine during neuroleptic therapy. Pharmacotherapy. 1986;6(4):193-195.
12. Carroll BT, Goforth HW, Thomas C, et al. Review of adjunctive glutamate antagonist therapy in the treatment of catatonic syndromes. J Neuropsychiatry Clin Neurosci. 2007;19(4):406-412.
13. Huffman JC, Fricchione GL. Catatonia and psychosis in a patient with AIDS: treatment with lorazepam and aripiprazole. J Clin Psychopharmacol. 2005;25(5):508-510.
14. Roberto AJ, Pinnaka S, Mohan A, et al. Adolescent catatonia successfully treated with lorazepam and aripiprazole. Case Rep Psychiatry. 2014;2014:309517.
15. Voros V, Kovacs A, Herold R, et al. Effectiveness of intramuscular aripiprazole injection in patients with catatonia: report on three cases. Pharmacopsychiatry. 2009;42(6):286-287.
CASE Irritable and short of breath
Mr. B, age 75, who lives alone, is brought to the emergency department (ED) for evaluation of shortness of breath. Mr. B is normally highly independent, and is able to drive, manage his own finances, attend to activities of daily living, and participate in social functions at church. On the day before he was taken to the ED, his home nurse had come to his home to dispense medications and found Mr. B was irritable, verbally rude, and repeatedly scratching the right side of his head. The nurse was unsure if Mr. B had taken his medications over the weekend. She called for emergency services, but Mr. B refused to go to the ED, and he was able to decline care because he was not in an acute medical emergency (95% oxygen on pulse oximetry).
The next day, when Mr. B’s nurse returned to his home, she found him to be tachypneic and verbigerating the phrase “I don’t know.” She contacted emergency services again, and Mr. B was taken to the ED.
In the ED, Mr. B has tachycardia, tachypnea, increased work of breathing, and diffuse rhonchi. He continues to repeat the phrase “I don’t know” and scratches the right side of his head repeatedly. The ED clinicians consult Psychiatry due to Mr. B’s confusion and because his nurse reports that his presentation is similar to a previous psychiatric hospitalization 9 years earlier.
[polldaddy:10332862]
EVALUATION Complex comorbidities
Mr. B has a lengthy history of schizophrenia, chronic right-sided heart failure secondary to pulmonary hypertension, moderate chronic obstructive pulmonary disease, hypertension, type 2 diabetes mellitus, and prostatic adenocarcinoma after external beam radiation therapy.
His symptoms of schizophrenia had been stable on his long-standing outpatient psychotropic regimen of haloperidol, 5 mg nightly; mirtazapine, 15 mg nightly, for appetite stimulation and insomnia; and trazodone, 100 mg nightly for insomnia. Mr. B has been receiving assertive community treatment (ACT) psychiatric services for schizophrenia; a nurse refills his pill box with his medications weekly. He does not have a history of medication nonadherence, and his nurse did not think he had missed any doses before the weekend.
He has acute changes in depressed mood, perseveration, and a Mini-Mental State Examination (MMSE) score of 26 (missing points for delayed recall and inability to construct a sentence), which indicates a cognitive assessment score on the low end of the normal range for people with at least an eighth grade education.
At the hospital, the psychiatrist diagnoses hypoactive delirium due to Mr. B’s fluctuating attention and disorientation. She also recommends that Mr. B continue his outpatient psychotropic regimen, and adds oral haloperidol, 5 mg, as needed for agitation (his QTc interval is 451 ms; reference range for men <430 ms, borderline prolonged 431 to 450 ms, prolonged >450 ms).
Continue to: An initial laboratory workup...
An initial laboratory workup and electrocardiogram reveal that Mr. B has an elevated troponin level (0.21 ng/mL; reference range <0.04; 0.04 to 0.39 ng/mL is elevated above the 99th percentile of a healthy population), non-ST-elevation myocardial infarction type II, Q waves in lead III, arteriovenous fistula with right axis deviation, acute on chronic kidney failure (creatinine level of 2.1 mg/dL, up from baseline of 1.4 mg/dL; reference range 0.84 to 1.21 mg/dL), elevated brain natriuretic peptide (111 pg/mL; reference range <125 pg/mL), and an elevated lactate level of 5.51 mmol/L (reference range 0.5 to 1 mmol/L). He also has a mixed respiratory alkalosis and metabolic acidosis with increased anion gap, transaminitis (aspartate aminotransferase 149 U/L; reference range 10 to 40 U/L), and elevated alkaline phosphatase (151 IU/L; reference range 44 to 147 IU/L). Urinalysis shows moderate ketones and is negative for nitrite or leukocyte esterase.
A brain CT rules out stroke. A chest X-ray shows subtle left basilar reticular opacity with a follow-up lateral view showing no consolidation and prominent pulmonary vasculature without overt edema.
In the ED, Mr. B is determined to have decision-making capacity and is able to authorize all treatment. Cardiology is also consulted, and Mr. B is admitted to the cardiac intensive care unit (CCU) for cardiogenic shock with close cardiac monitoring.
The Psychiatry and Cardiology teams discuss the risks and benefits of continuing antipsychotics. Due to the imminent risk of harm to Mr. B because of his significant agitation in the ED, which required treatment with one dose of IM haloperidol, 5 mg, and lorazepam, 2 mg, and close monitoring, the teams agree that the benefits of continuing haloperidol outweigh the risks.
On hospital Day 2, Mr. B’s repetitive scratching resolves. He is moved from the CCU to a general medical unit, where he begins to have episodes of mutism and negativism. By hospital Day 6, catatonia is suspected due to a MMSE of 6/30 and a Bush- Francis Catatonia Rating Scale (BFCRS) score of 14 for predominant stereotypy, perseveration, and withdrawal (Table 1). The teams determine that Mr. B lacks decisionmaking capacity due to his inability to rationally manipulate information. His brother is contacted and authorizes all treatment, deferring decision-making to the medical teams caring for Mr. B.

Continue to: Mr. B undergoes an EEG...
Mr. B undergoes an EEG, which rules out nonconvulsive status epilepticus and is consistent with encephalopathy/delirium. Neuroleptic malignant syndrome (NMS) is considered but is less likely because Mr. B had been receiving a stable dose of haloperidol for several years, is afebrile, has stable vital signs, has no muscle rigidity, and no evidence of leukocytosis, creatine kinase elevation, myoglobinuria, hyperkalemia, hyperphosphatemia, thrombocytosis, or hypocalcemia.
Based on these clinical findings, Mr. B is diagnosed with catatonia and delirium.
The authors’ observations
Delirium, characterized by inattention and changes in mental status, is a syndrome due to acute brain dysfunction. It can be subclassified as hyperactive or hypoactive based on the change of activity. Simple catatonia is characterized by changes in behavior, affect, and motor function (with hyper- or hypoactivity). It may arise from gammaaminobutyric acid hypoactivity, dopamine (D2) hypoactivity, and possibly glutamate N-methyl-d-aspartate (NMDA) hyperactivity.1 Malignant catatonia is simple catatonia combined with autonomic instability and hyperthermia, which is a life-threatening condition. The BFCRS is commonly used to assess symptoms.2
Both catatonia and delirium result in significant morbidity and mortality. The 2 conditions share signs and symptoms yet rarely are diagnosed at the same time. DSM-IV, DSM-IV-TR, and DSM-5 state that a diagnosis of catatonia due to another medical condition cannot be made exclusively in the presence of delirium.3,4 DSM-IV and DSM-IV-TR required at least 2 criteria from 5 areas, including motoric immobility, excessive motor activity, extreme negativism or mutism, peculiarities of voluntary movement, and echolalia or echopraxia. Instead of grouping symptoms into clusters, DSM-5 requires 3 criteria of 12 individual symptoms.3,4 A co-occurrence with a medical illness precludes using the DSM-5 “catatonia associated with another mental disorder (catatonia specifier)” with the “unspecified catatonia” diagnosis category.4
However, a growing body of literature suggests that delirium and catatonia can cooccur.5,6 In 2017, Wilson et al6 found that of 136 critically ill patients in the ICU, 43% (58 patients) had only delirium, 3% (4 patients) had only catatonia, 31% (42 patients) had both, and 24% (32 patients) had neither. In patients with both catatonia and delirium, the most common signs of catatonia were autonomic abnormalities (96%), immobility/ stupor (87%), staring (77%), mutism (60%), and posturing (60%).
Continue to: The differential diagnosis...
The differential diagnosis of catatonia is extensive and varied.3,4 The most common psychiatric causes are mood disorders (13% to 31%) and psychotic disorders (7% to 17%).7 Neuromedical etiologies account for 4% to 46% of cases.7 The most common medical and neurologic causes are seizure disorder, acute intermittent porphyria, systemic lupus erythematosus, and drugrelated adverse effects (particularly due to clozapine withdrawal, risperidone, and phencyclidine).7
A workup that includes physical examination, laboratory testing, and neuroimaging can be helpful to identify delirium and catatonia, but there is limited literature to guide identifying coexisting delirium and catatonia other than a blend of physical exam findings of delirium and catatonia. Electroencephalogram may be normal in primary catatonia or may show nonspecific changes in secondary catatonia.8 Additionally, discharges in the frontal lobes and anterior limbic systems with diffuse background slowing and dysrhythmic patterns may be seen.7 Neuroimaging with MRI can help to evaluate catatonia.9 Laboratory testing such as creatine phosphokinase levels can be high in simple catatonia and are often elevated in malignant catatonia.7 Considering the possible co-occurrence of delirium and catatonia is critical to providing good patient care because the 2 conditions are treated differently.
[polldaddy:10332867]
TREATMENT A balancing act
Over the next month, Mr. B alternates between appearing catatonic or delirious. When he appears more catatonic, the dose of lorazepam is increased, which results in increased impulsivity and agitation and leads to multiple interventions from the behavioral emergency response team. At times, the team must use restraints and haloperidol because Mr. B pulls out IV lines and is considered at high risk for falls. When Mr. B appears more delirious and the dose of lorazepam is decreased, he becomes more catatonic.
Following the diagnosis of catatonia on Day 6, oral haloperidol is discontinued to further mitigate Mr. B’s risk of developing NMS. On hospital Day 6, Mr. B improves significantly after a 2-mg IV lorazepam challenge, with a BFCRS score of 6. At this point, he is started on lorazepam, 1 mg IV 3 times a day.
On Day 7, based on the complicated nature of Mr. B’s medical and psychiatric comorbidities, the treatment team considers ECT to minimize medication adverse effects, but Mr. B’s medical condition is too tenuous.
Continue to: On Day 7...
On Day 7, lorazepam is decreased to 0.5 mg/0.5 mg/1 mg IV. On Day 9, it is further decreased to 0.5 mg IV 3 times a day because Mr. B appears to be more delirious. On Day 10, lorazepam is increased to 1 mg IV 3 times a day, and oral haloperidol, 2 mg as needed for agitation, is restarted after multiple nights when Mr. B had behavioral emergencies and was treated with IM haloperidol and lorazepam. On Day 11, lorazepam is decreased and switched from IV formulation to oral, 0.5 mg 3 times a day. On Day 13, oral haloperidol is increased to 2 mg twice a day because of overnight behavioral emergencies requiring treatment with IV haloperidol, 4 mg. On Day 17, oral haloperidol is increased to 2 mg in the morning and 3 mg every night at bedtime because Mr. B has increased morning agitation. On Day 19, oral lorazepam is increased to 1 mg 3 times a day because Mr. B appears more catatonic. On Day 21, oral haloperidol is consolidated to 5 mg every night at bedtime. On Day 31, oral lorazepam is increased to 2 mg/1 mg/1 mg because he appears more catatonic with increased stuttering and mannerisms. On Day 33, oral haloperidol is increased to 6 mg every night at bedtime because Mr. B has morning agitation.
Multiple lorazepam and haloperidol dose adjustments are needed to balance the situation: combating catatonia, addressing delirium, managing schizophrenia symptoms, and improving Mr. B’s cardiac status. Finally, Mr. B is stabilized on oral lorazepam, 2 mg every morning, 1 mg every day at noon, and 1 mg every day at bedtime, and oral haloperidol, 6 mg every day at bedtime. This regimen, Mr. B has a BFCRS score of 1 (Table 2) and returns to his baseline mental status.
The authors’ observations
Delirium and catatonia typically have different treatments. Delirium is routinely treated by addressing the underlying medical and environmental factors, and managing comorbid symptoms such as agitation and disturbing hallucinations by prescribing antipsychotics, restoring the sleep-wake cycle with melatonin, initiating nonpharmacologic behavioral management, and avoiding deliriogenic medications such as benzodiazepines, opioids, and steroids.10 Catatonia is managed by prescribing benzodiazepines (with or without ECT) and by avoiding dopamine antagonists such as antipsychotics and metoclopramide (which may worsen catatonia or precipitate malignant catatonia).
The first-line treatment for catatonia is benzodiazepines, with IV preferred over IM, sublingual, or oral formulations. Electroconvulsive therapy is commonly used with benzodiazepines and is effective in 85% to 90% of patients. For ECT, bitemporal placement and daily treatment with brief pulses are frequently used. It is also effective in 60% of patients who fail to respond to benzodiazepines. Thus, ECT should be considered within the first 48 to 72 hours of benzodiazepine failure.7
Amantadine, a NMDA antagonist, may be a possible treatment for catatonia. A case report published in 1986 described a patient who developed catatonia after the abrupt withdrawal of amantadine during neuroleptic therapy.11 Memantine also may serve as a treatment for catatonia through glutamate antagonism. A review identified 25 cases of patients with catatonia who were treated with amantadine or memantine.12 Oral amantadine was administered at 100 to 400 mg/d in divided doses, with lower doses for patients with diminished renal function.12 Memantine was administered at 5 to 20 mg/d.12 All patients showed improvement after 1 to 7 days of treatment.12 Thus, memantine may be considered for patients with catatonic schizophrenia or comorbid catatonia and delirium. Although memantine was not considered in Mr. B’s case, he would have been a good candidate for treatment with this agent.
Continue to: There are also case reports of...
There are also case reports of aripiprazole being used for catatonia in the context of psychosis or delirium in both adults and adolescents.13-15 Other medications used in case reports for treating catatonia include carbamazepine, valproate, and secondgeneration antipsychotics.7
Because most of the literature on pharmacotherapy for catatonia consists of case reports or small case series, further research on medication management of catatonia and delirium is needed to guide treatment.
OUTCOME Multiple rehospitalizations
On Day 57, Mr. B is discharged to a skilled nursing facility due to significant deconditioning. He is discharged with continued follow-up with his ACT psychiatrist and nurse. Mr. B’s catatonia remains resolved; however, he is unable to be safely managed at the skilled nursing facility.
During the next 7 months, he is readmitted to the ICU for acute on chronic hypoxic respiratory failure 5 times; his rehospitalizations are complicated by delirium due to cardiogenic shock and urosepsis. Mild hyperactive delirium re-emerges after worsening respiratory failure and contributes to falls in the skilled nursing facility.
Six months later, Mr. B continues to receive the initial hospital discharge lorazepam regimen of 2 mg every morning, 1 mg every day at noon, and 1 mg every night at bedtime. The Psychiatry team slowly tapers this to 0.5 mg twice daily.
Continue to: On Day 5...
On Day 5 of Mr. B’s fifth hospital readmission, based on his advance directive, Mr. B’s family implements the do-not-resuscitate and do-not-intubate orders. He is transitioned to comfort measures, and dies on Day 6 with his brother and the hospital chaplain present.
Bottom Line
Delirium and catatonia share signs and symptoms, yet rarely are diagnosed at the same time. Both conditions result in significant morbidity and mortality. An emerging literature supports the concurrence of these 2 syndromes and aids in their diagnosis and treatment. Comorbidity with other medical conditions, common with both delirium and catatonia, substantially complicates treatment; thus, additional research into new treatment approaches is critical.
Related Resources
- Wilson JE, Carlson R, Duggan MC, et al. Delirium and catatonia in critically ill patients: the delirium and catatonia prospective cohort investigation. Crit Care Med. 2017;45(11):1837-1844.
- Catatonia Information Center. Penn State University. http://catatonia.org/.
Drug Brand Names
Amantadine • Symmetrel
Aripiprazole • Abilify
Carbamazepine • Carbatrol, Tegretol
Clozapine • Clozaril
Haloperidol • Haldol
Lorazepam • Ativan
Memantine • Namenda
Metoclopramide • Reglan
Mirtazapine • Remeron
Risperidone • Risperdal
Topiramate • Topamax
Trazodone • Desyrel
Valproate • Depacon, Depakene, Depakote
CASE Irritable and short of breath
Mr. B, age 75, who lives alone, is brought to the emergency department (ED) for evaluation of shortness of breath. Mr. B is normally highly independent, and is able to drive, manage his own finances, attend to activities of daily living, and participate in social functions at church. On the day before he was taken to the ED, his home nurse had come to his home to dispense medications and found Mr. B was irritable, verbally rude, and repeatedly scratching the right side of his head. The nurse was unsure if Mr. B had taken his medications over the weekend. She called for emergency services, but Mr. B refused to go to the ED, and he was able to decline care because he was not in an acute medical emergency (95% oxygen on pulse oximetry).
The next day, when Mr. B’s nurse returned to his home, she found him to be tachypneic and verbigerating the phrase “I don’t know.” She contacted emergency services again, and Mr. B was taken to the ED.
In the ED, Mr. B has tachycardia, tachypnea, increased work of breathing, and diffuse rhonchi. He continues to repeat the phrase “I don’t know” and scratches the right side of his head repeatedly. The ED clinicians consult Psychiatry due to Mr. B’s confusion and because his nurse reports that his presentation is similar to a previous psychiatric hospitalization 9 years earlier.
[polldaddy:10332862]
EVALUATION Complex comorbidities
Mr. B has a lengthy history of schizophrenia, chronic right-sided heart failure secondary to pulmonary hypertension, moderate chronic obstructive pulmonary disease, hypertension, type 2 diabetes mellitus, and prostatic adenocarcinoma after external beam radiation therapy.
His symptoms of schizophrenia had been stable on his long-standing outpatient psychotropic regimen of haloperidol, 5 mg nightly; mirtazapine, 15 mg nightly, for appetite stimulation and insomnia; and trazodone, 100 mg nightly for insomnia. Mr. B has been receiving assertive community treatment (ACT) psychiatric services for schizophrenia; a nurse refills his pill box with his medications weekly. He does not have a history of medication nonadherence, and his nurse did not think he had missed any doses before the weekend.
He has acute changes in depressed mood, perseveration, and a Mini-Mental State Examination (MMSE) score of 26 (missing points for delayed recall and inability to construct a sentence), which indicates a cognitive assessment score on the low end of the normal range for people with at least an eighth grade education.
At the hospital, the psychiatrist diagnoses hypoactive delirium due to Mr. B’s fluctuating attention and disorientation. She also recommends that Mr. B continue his outpatient psychotropic regimen, and adds oral haloperidol, 5 mg, as needed for agitation (his QTc interval is 451 ms; reference range for men <430 ms, borderline prolonged 431 to 450 ms, prolonged >450 ms).
Continue to: An initial laboratory workup...
An initial laboratory workup and electrocardiogram reveal that Mr. B has an elevated troponin level (0.21 ng/mL; reference range <0.04; 0.04 to 0.39 ng/mL is elevated above the 99th percentile of a healthy population), non-ST-elevation myocardial infarction type II, Q waves in lead III, arteriovenous fistula with right axis deviation, acute on chronic kidney failure (creatinine level of 2.1 mg/dL, up from baseline of 1.4 mg/dL; reference range 0.84 to 1.21 mg/dL), elevated brain natriuretic peptide (111 pg/mL; reference range <125 pg/mL), and an elevated lactate level of 5.51 mmol/L (reference range 0.5 to 1 mmol/L). He also has a mixed respiratory alkalosis and metabolic acidosis with increased anion gap, transaminitis (aspartate aminotransferase 149 U/L; reference range 10 to 40 U/L), and elevated alkaline phosphatase (151 IU/L; reference range 44 to 147 IU/L). Urinalysis shows moderate ketones and is negative for nitrite or leukocyte esterase.
A brain CT rules out stroke. A chest X-ray shows subtle left basilar reticular opacity with a follow-up lateral view showing no consolidation and prominent pulmonary vasculature without overt edema.
In the ED, Mr. B is determined to have decision-making capacity and is able to authorize all treatment. Cardiology is also consulted, and Mr. B is admitted to the cardiac intensive care unit (CCU) for cardiogenic shock with close cardiac monitoring.
The Psychiatry and Cardiology teams discuss the risks and benefits of continuing antipsychotics. Due to the imminent risk of harm to Mr. B because of his significant agitation in the ED, which required treatment with one dose of IM haloperidol, 5 mg, and lorazepam, 2 mg, and close monitoring, the teams agree that the benefits of continuing haloperidol outweigh the risks.
On hospital Day 2, Mr. B’s repetitive scratching resolves. He is moved from the CCU to a general medical unit, where he begins to have episodes of mutism and negativism. By hospital Day 6, catatonia is suspected due to a MMSE of 6/30 and a Bush- Francis Catatonia Rating Scale (BFCRS) score of 14 for predominant stereotypy, perseveration, and withdrawal (Table 1). The teams determine that Mr. B lacks decisionmaking capacity due to his inability to rationally manipulate information. His brother is contacted and authorizes all treatment, deferring decision-making to the medical teams caring for Mr. B.

Continue to: Mr. B undergoes an EEG...
Mr. B undergoes an EEG, which rules out nonconvulsive status epilepticus and is consistent with encephalopathy/delirium. Neuroleptic malignant syndrome (NMS) is considered but is less likely because Mr. B had been receiving a stable dose of haloperidol for several years, is afebrile, has stable vital signs, has no muscle rigidity, and no evidence of leukocytosis, creatine kinase elevation, myoglobinuria, hyperkalemia, hyperphosphatemia, thrombocytosis, or hypocalcemia.
Based on these clinical findings, Mr. B is diagnosed with catatonia and delirium.
The authors’ observations
Delirium, characterized by inattention and changes in mental status, is a syndrome due to acute brain dysfunction. It can be subclassified as hyperactive or hypoactive based on the change of activity. Simple catatonia is characterized by changes in behavior, affect, and motor function (with hyper- or hypoactivity). It may arise from gammaaminobutyric acid hypoactivity, dopamine (D2) hypoactivity, and possibly glutamate N-methyl-d-aspartate (NMDA) hyperactivity.1 Malignant catatonia is simple catatonia combined with autonomic instability and hyperthermia, which is a life-threatening condition. The BFCRS is commonly used to assess symptoms.2
Both catatonia and delirium result in significant morbidity and mortality. The 2 conditions share signs and symptoms yet rarely are diagnosed at the same time. DSM-IV, DSM-IV-TR, and DSM-5 state that a diagnosis of catatonia due to another medical condition cannot be made exclusively in the presence of delirium.3,4 DSM-IV and DSM-IV-TR required at least 2 criteria from 5 areas, including motoric immobility, excessive motor activity, extreme negativism or mutism, peculiarities of voluntary movement, and echolalia or echopraxia. Instead of grouping symptoms into clusters, DSM-5 requires 3 criteria of 12 individual symptoms.3,4 A co-occurrence with a medical illness precludes using the DSM-5 “catatonia associated with another mental disorder (catatonia specifier)” with the “unspecified catatonia” diagnosis category.4
However, a growing body of literature suggests that delirium and catatonia can cooccur.5,6 In 2017, Wilson et al6 found that of 136 critically ill patients in the ICU, 43% (58 patients) had only delirium, 3% (4 patients) had only catatonia, 31% (42 patients) had both, and 24% (32 patients) had neither. In patients with both catatonia and delirium, the most common signs of catatonia were autonomic abnormalities (96%), immobility/ stupor (87%), staring (77%), mutism (60%), and posturing (60%).
Continue to: The differential diagnosis...
The differential diagnosis of catatonia is extensive and varied.3,4 The most common psychiatric causes are mood disorders (13% to 31%) and psychotic disorders (7% to 17%).7 Neuromedical etiologies account for 4% to 46% of cases.7 The most common medical and neurologic causes are seizure disorder, acute intermittent porphyria, systemic lupus erythematosus, and drugrelated adverse effects (particularly due to clozapine withdrawal, risperidone, and phencyclidine).7
A workup that includes physical examination, laboratory testing, and neuroimaging can be helpful to identify delirium and catatonia, but there is limited literature to guide identifying coexisting delirium and catatonia other than a blend of physical exam findings of delirium and catatonia. Electroencephalogram may be normal in primary catatonia or may show nonspecific changes in secondary catatonia.8 Additionally, discharges in the frontal lobes and anterior limbic systems with diffuse background slowing and dysrhythmic patterns may be seen.7 Neuroimaging with MRI can help to evaluate catatonia.9 Laboratory testing such as creatine phosphokinase levels can be high in simple catatonia and are often elevated in malignant catatonia.7 Considering the possible co-occurrence of delirium and catatonia is critical to providing good patient care because the 2 conditions are treated differently.
[polldaddy:10332867]
TREATMENT A balancing act
Over the next month, Mr. B alternates between appearing catatonic or delirious. When he appears more catatonic, the dose of lorazepam is increased, which results in increased impulsivity and agitation and leads to multiple interventions from the behavioral emergency response team. At times, the team must use restraints and haloperidol because Mr. B pulls out IV lines and is considered at high risk for falls. When Mr. B appears more delirious and the dose of lorazepam is decreased, he becomes more catatonic.
Following the diagnosis of catatonia on Day 6, oral haloperidol is discontinued to further mitigate Mr. B’s risk of developing NMS. On hospital Day 6, Mr. B improves significantly after a 2-mg IV lorazepam challenge, with a BFCRS score of 6. At this point, he is started on lorazepam, 1 mg IV 3 times a day.
On Day 7, based on the complicated nature of Mr. B’s medical and psychiatric comorbidities, the treatment team considers ECT to minimize medication adverse effects, but Mr. B’s medical condition is too tenuous.
Continue to: On Day 7...
On Day 7, lorazepam is decreased to 0.5 mg/0.5 mg/1 mg IV. On Day 9, it is further decreased to 0.5 mg IV 3 times a day because Mr. B appears to be more delirious. On Day 10, lorazepam is increased to 1 mg IV 3 times a day, and oral haloperidol, 2 mg as needed for agitation, is restarted after multiple nights when Mr. B had behavioral emergencies and was treated with IM haloperidol and lorazepam. On Day 11, lorazepam is decreased and switched from IV formulation to oral, 0.5 mg 3 times a day. On Day 13, oral haloperidol is increased to 2 mg twice a day because of overnight behavioral emergencies requiring treatment with IV haloperidol, 4 mg. On Day 17, oral haloperidol is increased to 2 mg in the morning and 3 mg every night at bedtime because Mr. B has increased morning agitation. On Day 19, oral lorazepam is increased to 1 mg 3 times a day because Mr. B appears more catatonic. On Day 21, oral haloperidol is consolidated to 5 mg every night at bedtime. On Day 31, oral lorazepam is increased to 2 mg/1 mg/1 mg because he appears more catatonic with increased stuttering and mannerisms. On Day 33, oral haloperidol is increased to 6 mg every night at bedtime because Mr. B has morning agitation.
Multiple lorazepam and haloperidol dose adjustments are needed to balance the situation: combating catatonia, addressing delirium, managing schizophrenia symptoms, and improving Mr. B’s cardiac status. Finally, Mr. B is stabilized on oral lorazepam, 2 mg every morning, 1 mg every day at noon, and 1 mg every day at bedtime, and oral haloperidol, 6 mg every day at bedtime. This regimen, Mr. B has a BFCRS score of 1 (Table 2) and returns to his baseline mental status.
The authors’ observations
Delirium and catatonia typically have different treatments. Delirium is routinely treated by addressing the underlying medical and environmental factors, and managing comorbid symptoms such as agitation and disturbing hallucinations by prescribing antipsychotics, restoring the sleep-wake cycle with melatonin, initiating nonpharmacologic behavioral management, and avoiding deliriogenic medications such as benzodiazepines, opioids, and steroids.10 Catatonia is managed by prescribing benzodiazepines (with or without ECT) and by avoiding dopamine antagonists such as antipsychotics and metoclopramide (which may worsen catatonia or precipitate malignant catatonia).
The first-line treatment for catatonia is benzodiazepines, with IV preferred over IM, sublingual, or oral formulations. Electroconvulsive therapy is commonly used with benzodiazepines and is effective in 85% to 90% of patients. For ECT, bitemporal placement and daily treatment with brief pulses are frequently used. It is also effective in 60% of patients who fail to respond to benzodiazepines. Thus, ECT should be considered within the first 48 to 72 hours of benzodiazepine failure.7
Amantadine, a NMDA antagonist, may be a possible treatment for catatonia. A case report published in 1986 described a patient who developed catatonia after the abrupt withdrawal of amantadine during neuroleptic therapy.11 Memantine also may serve as a treatment for catatonia through glutamate antagonism. A review identified 25 cases of patients with catatonia who were treated with amantadine or memantine.12 Oral amantadine was administered at 100 to 400 mg/d in divided doses, with lower doses for patients with diminished renal function.12 Memantine was administered at 5 to 20 mg/d.12 All patients showed improvement after 1 to 7 days of treatment.12 Thus, memantine may be considered for patients with catatonic schizophrenia or comorbid catatonia and delirium. Although memantine was not considered in Mr. B’s case, he would have been a good candidate for treatment with this agent.
Continue to: There are also case reports of...
There are also case reports of aripiprazole being used for catatonia in the context of psychosis or delirium in both adults and adolescents.13-15 Other medications used in case reports for treating catatonia include carbamazepine, valproate, and secondgeneration antipsychotics.7
Because most of the literature on pharmacotherapy for catatonia consists of case reports or small case series, further research on medication management of catatonia and delirium is needed to guide treatment.
OUTCOME Multiple rehospitalizations
On Day 57, Mr. B is discharged to a skilled nursing facility due to significant deconditioning. He is discharged with continued follow-up with his ACT psychiatrist and nurse. Mr. B’s catatonia remains resolved; however, he is unable to be safely managed at the skilled nursing facility.
During the next 7 months, he is readmitted to the ICU for acute on chronic hypoxic respiratory failure 5 times; his rehospitalizations are complicated by delirium due to cardiogenic shock and urosepsis. Mild hyperactive delirium re-emerges after worsening respiratory failure and contributes to falls in the skilled nursing facility.
Six months later, Mr. B continues to receive the initial hospital discharge lorazepam regimen of 2 mg every morning, 1 mg every day at noon, and 1 mg every night at bedtime. The Psychiatry team slowly tapers this to 0.5 mg twice daily.
Continue to: On Day 5...
On Day 5 of Mr. B’s fifth hospital readmission, based on his advance directive, Mr. B’s family implements the do-not-resuscitate and do-not-intubate orders. He is transitioned to comfort measures, and dies on Day 6 with his brother and the hospital chaplain present.
Bottom Line
Delirium and catatonia share signs and symptoms, yet rarely are diagnosed at the same time. Both conditions result in significant morbidity and mortality. An emerging literature supports the concurrence of these 2 syndromes and aids in their diagnosis and treatment. Comorbidity with other medical conditions, common with both delirium and catatonia, substantially complicates treatment; thus, additional research into new treatment approaches is critical.
Related Resources
- Wilson JE, Carlson R, Duggan MC, et al. Delirium and catatonia in critically ill patients: the delirium and catatonia prospective cohort investigation. Crit Care Med. 2017;45(11):1837-1844.
- Catatonia Information Center. Penn State University. http://catatonia.org/.
Drug Brand Names
Amantadine • Symmetrel
Aripiprazole • Abilify
Carbamazepine • Carbatrol, Tegretol
Clozapine • Clozaril
Haloperidol • Haldol
Lorazepam • Ativan
Memantine • Namenda
Metoclopramide • Reglan
Mirtazapine • Remeron
Risperidone • Risperdal
Topiramate • Topamax
Trazodone • Desyrel
Valproate • Depacon, Depakene, Depakote
1. Northoff G. What catatonia can tell us about “top-down modulation”: a neuropsychiatric hypothesis. Behav Brain Sci. 2002;25(5):555-577; discussion 578-604.
2. Bush G, Fink M, Petrides G, et al. Catatonia. I. Rating scale and standardized examination. Acta Psychiatr Scand. 1996;93(2):129-136.
3. Diagnostic and Statistical Manual of Mental Disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
4. Diagnostic and Statistical Manual of Mental Disorders, 4th ed, text rev. Washington, DC: American Psychiatric Association; 2000.
5. Oldham MA, Lee HB. Catatonia vis-à-vis delirium: the significance of recognizing catatonia in altered mental status. Gen Hosp Psychiatry. 2015;37(6):554-559.
6. Wilson JE, Carlson R, Duggan MC. Delirium and catatonia in critically ill patients: the delirium and catatonia prospective cohort investigation. Crit Care Med. 2017;45(11):1837-1844.
7. Fricchione GL, Gross AF, Huffman JC, et al. Chapter 21: Catatonia, neuroleptic malignant syndrome, and serotonin syndrome. In: Stern TA, Fricchione GL, Cassem NH, et al. Massachusetts General Hospital Handbook of General Hospital Psychiatry, 6th Ed. Philadelphia, PA: Saunders Elsevier; 2010:273-288.
8. Van der Kooi AW, Zaal IJ, Klijn FA, et al. Delirium detection using EEG: what and how to measure. Chest. 2015;147(1):94-101.
9. Wilson JE, Niu K, Nicolson SE, et al. The diagnostic criteria and structure of catatonia. Schizophr Res. 2015;164 (1-3):256-262.
10. Maldonado JR. Acute brain failure: pathophysiology, diagnosis, management, and sequelae of delirium. Crit Care Clin. 2017;33(3):461-519.
11. Brown CS, Wittkowsky AK, Bryant SG. Neurolepticinduced catatonia after abrupt withdrawal of amantadine during neuroleptic therapy. Pharmacotherapy. 1986;6(4):193-195.
12. Carroll BT, Goforth HW, Thomas C, et al. Review of adjunctive glutamate antagonist therapy in the treatment of catatonic syndromes. J Neuropsychiatry Clin Neurosci. 2007;19(4):406-412.
13. Huffman JC, Fricchione GL. Catatonia and psychosis in a patient with AIDS: treatment with lorazepam and aripiprazole. J Clin Psychopharmacol. 2005;25(5):508-510.
14. Roberto AJ, Pinnaka S, Mohan A, et al. Adolescent catatonia successfully treated with lorazepam and aripiprazole. Case Rep Psychiatry. 2014;2014:309517.
15. Voros V, Kovacs A, Herold R, et al. Effectiveness of intramuscular aripiprazole injection in patients with catatonia: report on three cases. Pharmacopsychiatry. 2009;42(6):286-287.
1. Northoff G. What catatonia can tell us about “top-down modulation”: a neuropsychiatric hypothesis. Behav Brain Sci. 2002;25(5):555-577; discussion 578-604.
2. Bush G, Fink M, Petrides G, et al. Catatonia. I. Rating scale and standardized examination. Acta Psychiatr Scand. 1996;93(2):129-136.
3. Diagnostic and Statistical Manual of Mental Disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
4. Diagnostic and Statistical Manual of Mental Disorders, 4th ed, text rev. Washington, DC: American Psychiatric Association; 2000.
5. Oldham MA, Lee HB. Catatonia vis-à-vis delirium: the significance of recognizing catatonia in altered mental status. Gen Hosp Psychiatry. 2015;37(6):554-559.
6. Wilson JE, Carlson R, Duggan MC. Delirium and catatonia in critically ill patients: the delirium and catatonia prospective cohort investigation. Crit Care Med. 2017;45(11):1837-1844.
7. Fricchione GL, Gross AF, Huffman JC, et al. Chapter 21: Catatonia, neuroleptic malignant syndrome, and serotonin syndrome. In: Stern TA, Fricchione GL, Cassem NH, et al. Massachusetts General Hospital Handbook of General Hospital Psychiatry, 6th Ed. Philadelphia, PA: Saunders Elsevier; 2010:273-288.
8. Van der Kooi AW, Zaal IJ, Klijn FA, et al. Delirium detection using EEG: what and how to measure. Chest. 2015;147(1):94-101.
9. Wilson JE, Niu K, Nicolson SE, et al. The diagnostic criteria and structure of catatonia. Schizophr Res. 2015;164 (1-3):256-262.
10. Maldonado JR. Acute brain failure: pathophysiology, diagnosis, management, and sequelae of delirium. Crit Care Clin. 2017;33(3):461-519.
11. Brown CS, Wittkowsky AK, Bryant SG. Neurolepticinduced catatonia after abrupt withdrawal of amantadine during neuroleptic therapy. Pharmacotherapy. 1986;6(4):193-195.
12. Carroll BT, Goforth HW, Thomas C, et al. Review of adjunctive glutamate antagonist therapy in the treatment of catatonic syndromes. J Neuropsychiatry Clin Neurosci. 2007;19(4):406-412.
13. Huffman JC, Fricchione GL. Catatonia and psychosis in a patient with AIDS: treatment with lorazepam and aripiprazole. J Clin Psychopharmacol. 2005;25(5):508-510.
14. Roberto AJ, Pinnaka S, Mohan A, et al. Adolescent catatonia successfully treated with lorazepam and aripiprazole. Case Rep Psychiatry. 2014;2014:309517.
15. Voros V, Kovacs A, Herold R, et al. Effectiveness of intramuscular aripiprazole injection in patients with catatonia: report on three cases. Pharmacopsychiatry. 2009;42(6):286-287.
From sweet to belligerent in the blink of an eye
CASE Combative and agitated
Ms. P, age 87, presents to the emergency department (ED) with her caregiver, who says Ms. P has new-onset altered mental status, agitation, and combativeness.
Ms. P resides at a long-term care (LTC) facility, where according to the nurses she normally is pleasant, well-oriented, and cooperative. Ms. P’s medical history includes major depressive disorder, generalized anxiety disorder, hypertension, chronic kidney disease (CKD) stage III, peptic ulcer disease, gastroesophageal reflux disease, coronary artery disease with 2 past myocardial infarctions requiring stents, chronic obstructive pulmonary disease, hyperlipidemia, bradycardia requiring a pacemaker, paroxysmal atrial fibrillation, asthma, aortic stenosis, peripheral vascular disease, esophageal stricture requiring dilation, deep vein thrombosis, and migraines.
Mr. P’s medication list includes acetaminophen, 650 mg every 6 hours; ipratropium/albuterol nebulized solution, 3 mL 4 times a day; aspirin, 81 mg/d; atorvastatin, 40 mg/d; calcitonin, 1 spray nasally at bedtime; clopidogrel, 75 mg/d; ezetimibe, 10 mg/d; fluoxetine, 20 mg/d; furosemide, 20 mg/d; isosorbide dinitrate, 120 mg/d; lisinopril, 15 mg/d; risperidone, 0.5 mg/d; magnesium oxide, 800 mg/d; pantoprazole, 40 mg/d; polyethylene glycol, 17 g/d; sotalol, 160 mg/d; olanzapine, 5 mg IM every 6 hours as needed for agitation; and tramadol, 50 mg every 8 hours as needed for headache.
Seven days before coming to the ED, Ms. P was started on ceftriaxone, 1 g/d, for suspected community-acquired pneumonia. At that time, the nursing staff noticed behavioral changes. Soon after, Ms. P began refusing all her medications. Two days before presenting to the ED, Ms. P was started on nitrofurantoin, 200 mg/d, for a suspected urinary tract infection, but it was discontinued because of an allergy.
Her caregiver reports that while at the LTC facility, Ms. P’s behavioral changes worsened. Ms. P claimed to be Jesus Christ and said she was talking to the devil; she chased other residents around the facility and slapped medications away from the nursing staff. According to caregivers, this behavior was out of character.
Shortly after arriving in the ED, Ms. P is admitted to the psychiatric unit.
[polldaddy:10332748]
The authors’ observations
Delirium is a complex, acute alteration in a patient’s mental status compared with his/her baseline functioning1 (Table 12). The onset of delirium is quick, happening within hours to days, with fluctuations in mental function. Patients might present with hyperactive, hypoactive, or mixed delirium.3 Patients with hyperactive delirium often have delusions and hallucinations; these patients might be agitated and could become violent with family and caregivers.3 Patients with hypoactive delirium are less likely to experience hallucinations and more likely to show symptoms of sedation.3 Patients with hypoactive delirium can be difficult to diagnose because it is challenging to interview them and understand what might be the cause of their sedated state. Patients also can exhibit a mixed delirium in which they fluctuate between periods of hyperactivity and hypoactivity.3
Continue to: Suspected delirium...
Suspected delirium should be considered a medical emergency because the outcome could be fatal.1 It is important to uncover and treat the underlying cause(s) of delirium rather than solely administering antipsychotics, which might mask the presenting symptoms. In an older study, Francis and Kapoor4 reported that 56% of geriatric patients with delirium had a single definite or probable etiology, while the other 44% had about 2.8 etiologies per patient on average. Delirium risk factors, causes, and factors to consider during patient evaluation are listed in Table 21,3,5-7 and Table 3.1,3,5-7

A synergistic relationship between comorbidities, environment, and medications can induce delirium.5 Identifying irreversible and reversible causes is the key to treating delirium. After the cause has been identified, it can be addressed and the patient could return to his/her previous level of functioning. If the delirium is the result of multiple irreversible causes, it could become chronic.
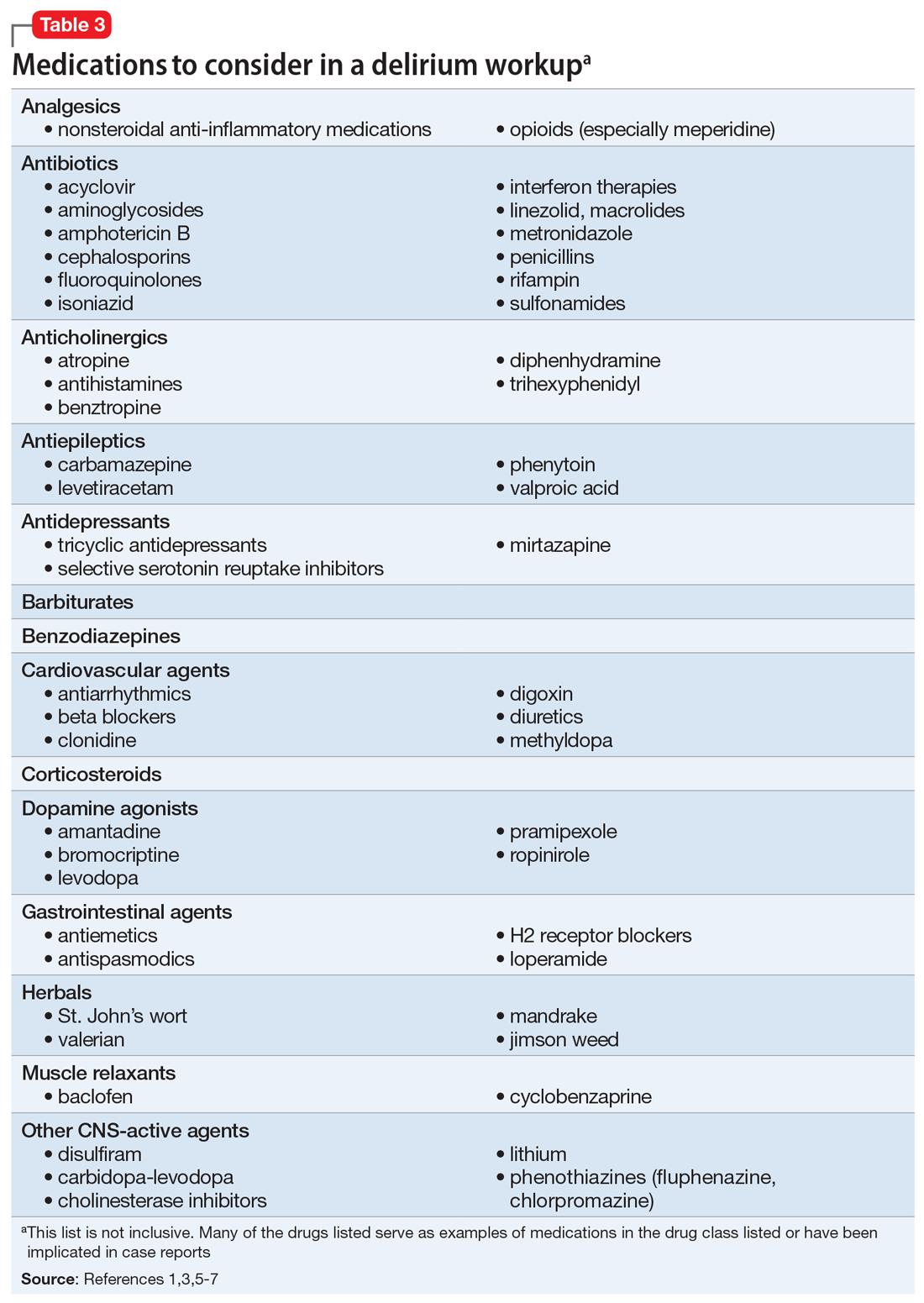
[polldaddy:10332749]
EVALUATION Cardiac dysfunction
Ms. P undergoes laboratory testing. The results include: white blood cell count, 5.9/µL; hemoglobin, 13.6 g/dL; hematocrit, 42.6%; platelets, 304 × 103/µL; sodium,143 mEq/L; potassium, 3.2 mEq/L; chloride, 96 mEq/L; carbon dioxide, 23 mEq/L; blood glucose, 87 mg/dL; creatinine, 1.2 mg/dL; estimated creatinine clearance (eCrCl) level of 33 mL/min/1.73 m2; calcium, 9.5 mg/dL; albumin, 3.6 g/dL; liver enzymes within normal limits; thyroid-stimulating hormone, 0.78 mIU/L; vitamin B12, 995 pg/mL; folic acid, 16.6 ng/mL; vitamin D, 31 pg/mL; and rapid plasma reagin: nonreactive. Urinalysis is unremarkable, and no culture is performed. Urine drug screening/toxicology is positive for the benzodiazepines that she received in the ED (oral alprazolam 0.25 mg given once and oral lorazepam 0.5 mg given once).
Electrocardiogram (ECG) shows atrial flutter/tachycardia with rapid ventricular response, marked left axis deviation, nonspecific ST- and T-wave abnormality, QT/QTC of 301/387 ms, and ventricular rate 151 beats per minute. A CT scan of the head and brain without contrast shows mild atrophy and chronic white matter changes and no acute intracranial abnormality. A two-view chest radiography shows no acute cardiopulmonary findings. Her temperature is 98.4°F; heart rate is 122 beats per minute; respiratory rate is 20 breaths per minute; blood pressure is 161/98 mm Hg; and oxygen saturation is 86% on room air.
Based on this data, Ms. P’s cardiac condition seems to be worsening, which is thought to be caused by her refusal of furosemide, lisinopril, isosorbide, sotalol, clopidogrel, and aspirin. The treatment team plans to work on compliance to resolve these cardiac issues and places Ms. P on 1:1 observation with a sitter and music in attempt to calm her.
Continue to: The authors' observations
The authors’ observations
Many factors can contribute to behavioral or cognitive changes in geriatric patients. Often, a major change noted in an older patient can be attributed to new-onset dementia, dementia with behavioral disturbances, delirium, depression, or acute psychosis. These potential causes should be considered and ruled out in a step-by-step progression. Because patients are unreliable historians during acute distress, a complete history from family or caregivers and exhaustive workup is paramount.
TREATMENT Medication adjustments
In an attempt to resolve Ms. P’s disruptive behaviors, her risperidone dosage is changed to 0.5 mg twice daily. Ms. P is encouraged to use the provided oxygen to raise her saturation level.
On hospital Day 3, a loose stool prompts a Clostridium difficile test as a possible source of delirium; however, the results are negative.
On hospital Day 4, Ms. P is confused and irritable overnight, yelling profanities at staff, refusing care, inappropriately disrobing, and having difficulty falling asleep and staying asleep. Risperidone is discontinued because it appears to have had little or no effect on Ms. P’s disruptive behaviors. Olanzapine, 10 mg/d, is initiated with mirtazapine, 7.5 mg/d, to help with mood, appetite, and sleep. Fluoxetine is also discontinued because of a possible interaction with clopidogrel.
On hospital Days 6 to 8, Ms. P remains upset and unable to follow instructions. Melatonin is initiated to improve her sleep cycle. On Day 9, she continues to decline and is cursing at hospital staff; haloperidol is initiated at 5 mg every morning, 10 mg at bedtime, and 5 mg IM as needed for agitation. Her sleep improves with melatonin and mirtazapine. IV hydration also is initiated. Ms. P has a slight improvement in medication compliance. On Day 11, haloperidol is increased to 5 mg in the morning, 5 mg in the afternoon, and 10 mg at bedtime. On Day 12, haloperidol is changed to 7.5 mg twice daily; a slight improvement in Ms. P’s behavior is noted.
Continue to: On hospital Day 13...
On hospital Day 13, Ms. P’s behavior declines again. She screams profanities at staff and does not recognize the clinicians who have been providing care to her. The physician initiates valproic acid, 125 mg, 3 times a day, to target Ms. P’s behavioral disturbances. A pharmacist notes that the patient’s sotalol could be contributing to Ms. P’s psychiatric presentation, and that based on her eCrCl level of 33 mL/min/1.73 m2, a dosage adjustment or medication change might be warranted.
On Day 14, Ms. P displays erratic behavior and intermittent tachycardia. A cardiac consultation is ordered. A repeat ECG reveals atrial fibrillation with rapid rate and a QT/QTc of 409/432 ms. Ms. P is transferred to the telemetry unit, where the cardiologist discontinues sotalol because the dosage is not properly renally adjusted. Sotalol hydrochloride has been associated with life-threatening ventricular tachycardia.8 Diltiazem, 30 mg every 6 hours is initiated to replace sotalol.
By Day 16, the treatment team notes improved cognition and behavior. On Day 17, the cardiologist reports that Ms. P’s atrial fibrillation is controlled. An ECG reveals mild left ventricular hypertrophy, an ejection fraction of 50% to 55%, no stenosis in the mitral or tricuspid valves, no valvular pulmonic stenosis, and moderate aortic sclerosis. Cardiac markers also are evaluated (creatinine phosphokinase: 105 U/L; creatinine kinase–MB fraction: 2.6 ng/mL; troponin: 0.01 ng/mL; pro-B-type natriuretic peptide: 2,073 pg/mL); and myocardial infarction is ruled out.
On Day 19, Ms. P’s diltiazem is consolidated to a controlled-delivery formulation, 180 mg/d, along with the addition of metoprolol, 12.5 mg twice daily. Ms. P is transferred back to the psychiatric unit.
OUTCOME Gradual improvement
On Days 20 to 23, Ms. P shows remarkable progress, and her mental status, cognition, and behavior slowly return to baseline. Haloperidol and valproic acid are tapered and discontinued. Ms. P is observed to be healthy and oriented to person, place, and time.
Continue to: On Day 25...
On Day 25, she is discharged from the hospital, and returns to the LTC facility.
The authors’ observations
Ms. P’s delirium was a combination of her older age, non-renally adjusted sotalol, and CKD. At admission, the hospital treatment team first thought that pneumonia or antibiotic use could have caused delirium. However, Ms. P’s condition did not improve after antibiotics were stopped. In addition, several chest radiographs found no evidence of pneumonia. It is important to check for any source of infection because infection is a common source of delirium in older patients.1 Urine samples revealed no pathogens, a C. difficile test was negative, and the patient’s white blood cell counts remained within normal limits. Physicians began looking elsewhere for potential causes of Ms. P’s delirium.
Ms. P’s vital signs ruled out a temperature irregularity or hypertension as the cause of her delirium. She has a slightly low oxygen saturation when she first presented, but this quickly returned to normal with administration of oxygen, which ruled out hypoxemia. Laboratory results concluded that Ms. P’s glucose levels were within a normal range and she had no electrolyte imbalances. A head CT scan showed slight atrophy of white matter that is consistent with Ms. P’s age. The head CT scan also showed that Ms. P had no acute condition or head trauma.
In terms of organ function, Ms. P was in relatively healthy condition other than paroxysmal atrial fibrillation and CKD. Chronic kidney disease can interrupt the normal pharmacokinetics of medications. Reviewing Ms. P’s medication list, several agents could have induced delirium, including antidepressants, antipsychotics, cardiovascular medications (beta blocker/antiarrhythmic [sotalol]), and opioid analgesics such as tramadol.5 Ms. P’s condition did not improve after discontinuing fluoxetine, risperidone, or olanzapine, although haloperidol was started in their place. Ms. P scored an 8 on the Naranjo Adverse Drug Reaction Probability Scale, indicating this event was a probable adverse drug reaction.9
Identifying a cause
This was a unique case where sotalol was identified as the culprit for inducing Ms. P’s delirium, because her age and CKD are irreversible. It is important to note that antiarrhythmics can induce arrhythmias when present in high concentrations or administered without appropriate renal dose adjustments. Although Ms. P’s serum levels of sotalol were not evaluated, because of her renal impairment, it is possible that toxic levels of sotalol accumulated and lead to arrhythmias and delirium. Of note, a cardiologist was consulted to safely change Ms. P to a calcium channel blocker so she could undergo cardiac monitoring. With the addition of diltiazem and metoprolol, the patient’s delirium subsided and her arrhythmia was controlled. Once the source of Ms. P’s delirium had been identified, antipsychotics were no longer needed.
Continue to: Bottom Line
Bottom Line
Delirium is a complex disorder that often has multiple causes, both reversible and irreversible. A “process of elimination” approach should be used to accurately identify and manage delirium. If a patient with delirium has little to no response to antipsychotic medications, the underlying cause or causes likely has not yet been addressed, and the evaluation should continue.
Related Resources
- Marcantonio ER. Delirium in hospitalized older adults. N Engl J Med. 2017;377:1456-1466.
- Inouye SK, Westendorp RGJ, Saczynski JS. Delirium in elderly people. Lancet. 2014;383(9920):911-922.
Drug Brand Names
Acyclovir • Zovirax
Alprazolam • Niravam, Xanax
Amantadine • Symmetrel
Amphotericin B • Abelcet
Atorvastatin • Lipitor
Atropine • Atropen
Baclofen • EnovaRX-Baclofen
Benztropine • Cogentin
Bromocriptine • Cycloset
Calcitonin • Miacalcin
Carbamazepine • Tegretol
Carbidopa-levodopa • Duopa
Ceftriaxone • Rocephin
Chlorpromazine • Thorazine
Clonidine • Catapres
Clopidogrel • Plavix
Cyclobenzaprine • Amrix
Digoxin • Lanoxin
Diltiazem • Cardizem
Disulfiram • Antabuse
Ezetimibe • Zetia
Fluoxetine • Prozac
Fluphenazine • Prolixin
Furosemide • Lasix
Haloperidol • Haldol
Ipratropium/albuterol nebulized solution • Combivent Respimat
Isoniazid • Isotamine
Isosorbide nitrate • Dilatrate
Levetiracetam • Keppra
Levodopa • Stalevo
Linezolid • Zyvox
Lisinopril • Zestril
Lithium • Eskalith, Lithobid
Lorazepam • Ativan
Magnesium Oxide • Mag-200
Meperidine • Demerol
Methyldopa • Aldomet
Metoprolol • Lopressor
Metronidazole • Flagyl
Mirtazapine • Remeron
Nitrofurantoin • Macrobid
Olanzapine • Zyprexa
Pantoprazole • Protonix
Phenytoin • Dilantin
Pramipexole • Mirapex
Rifampin • Rifadin
Risperidone • Risperdal
Ropinirole • Requip
Sotalol hydrochloride • Betapace AF
Tramadol • Ultram
Trihexyphenidyl • Trihexane
Valproic acid • Depakote
1. Fong TG, Tulebaev SR, Inouye SK. Delirium in elderly adults: diagnosis, prevention, and treatment. Nat Rev Neurol. 2009;5(4):210-220.
2. Diagnostic and statistical manual of mental disorders, fifth edition. Washington, DC: American Psychiatric Association; 2013.
3. American Psychiatric Association. Practice guideline for the treatment of patients with delirium. Am J Psychiatry. 1999;156(suppl 5):1-20.
4. Francis J, Kapoor WN. Delirium in hospitalized elderly. J Gen Intern Med. 1990;5(1):65-79.
5. Alagiakrishnan K, Wiens CA. An approach to drug induced delirium in the elderly. Postgrad Med J. 2004;80(945):388-393.
6. Cook IA. Guideline watch: practice guideline for the treatment of patients with delirium. Arlington, VA: American Psychiatric Publishing; 2004.
7. Bourgeois J, Ategan A, Losier B. Delirium in the hospital: emphasis on the management of geriatric patients. Current Psychiatry. 2014;13(8):29,36-42.
8. Betapace AF [package insert]. Zug, Switzerland: Covis Pharma; 2016.
9. Naranjo CA, Busto U, Sellers EM, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30(2):239-245.
CASE Combative and agitated
Ms. P, age 87, presents to the emergency department (ED) with her caregiver, who says Ms. P has new-onset altered mental status, agitation, and combativeness.
Ms. P resides at a long-term care (LTC) facility, where according to the nurses she normally is pleasant, well-oriented, and cooperative. Ms. P’s medical history includes major depressive disorder, generalized anxiety disorder, hypertension, chronic kidney disease (CKD) stage III, peptic ulcer disease, gastroesophageal reflux disease, coronary artery disease with 2 past myocardial infarctions requiring stents, chronic obstructive pulmonary disease, hyperlipidemia, bradycardia requiring a pacemaker, paroxysmal atrial fibrillation, asthma, aortic stenosis, peripheral vascular disease, esophageal stricture requiring dilation, deep vein thrombosis, and migraines.
Mr. P’s medication list includes acetaminophen, 650 mg every 6 hours; ipratropium/albuterol nebulized solution, 3 mL 4 times a day; aspirin, 81 mg/d; atorvastatin, 40 mg/d; calcitonin, 1 spray nasally at bedtime; clopidogrel, 75 mg/d; ezetimibe, 10 mg/d; fluoxetine, 20 mg/d; furosemide, 20 mg/d; isosorbide dinitrate, 120 mg/d; lisinopril, 15 mg/d; risperidone, 0.5 mg/d; magnesium oxide, 800 mg/d; pantoprazole, 40 mg/d; polyethylene glycol, 17 g/d; sotalol, 160 mg/d; olanzapine, 5 mg IM every 6 hours as needed for agitation; and tramadol, 50 mg every 8 hours as needed for headache.
Seven days before coming to the ED, Ms. P was started on ceftriaxone, 1 g/d, for suspected community-acquired pneumonia. At that time, the nursing staff noticed behavioral changes. Soon after, Ms. P began refusing all her medications. Two days before presenting to the ED, Ms. P was started on nitrofurantoin, 200 mg/d, for a suspected urinary tract infection, but it was discontinued because of an allergy.
Her caregiver reports that while at the LTC facility, Ms. P’s behavioral changes worsened. Ms. P claimed to be Jesus Christ and said she was talking to the devil; she chased other residents around the facility and slapped medications away from the nursing staff. According to caregivers, this behavior was out of character.
Shortly after arriving in the ED, Ms. P is admitted to the psychiatric unit.
[polldaddy:10332748]
The authors’ observations
Delirium is a complex, acute alteration in a patient’s mental status compared with his/her baseline functioning1 (Table 12). The onset of delirium is quick, happening within hours to days, with fluctuations in mental function. Patients might present with hyperactive, hypoactive, or mixed delirium.3 Patients with hyperactive delirium often have delusions and hallucinations; these patients might be agitated and could become violent with family and caregivers.3 Patients with hypoactive delirium are less likely to experience hallucinations and more likely to show symptoms of sedation.3 Patients with hypoactive delirium can be difficult to diagnose because it is challenging to interview them and understand what might be the cause of their sedated state. Patients also can exhibit a mixed delirium in which they fluctuate between periods of hyperactivity and hypoactivity.3
Continue to: Suspected delirium...
Suspected delirium should be considered a medical emergency because the outcome could be fatal.1 It is important to uncover and treat the underlying cause(s) of delirium rather than solely administering antipsychotics, which might mask the presenting symptoms. In an older study, Francis and Kapoor4 reported that 56% of geriatric patients with delirium had a single definite or probable etiology, while the other 44% had about 2.8 etiologies per patient on average. Delirium risk factors, causes, and factors to consider during patient evaluation are listed in Table 21,3,5-7 and Table 3.1,3,5-7

A synergistic relationship between comorbidities, environment, and medications can induce delirium.5 Identifying irreversible and reversible causes is the key to treating delirium. After the cause has been identified, it can be addressed and the patient could return to his/her previous level of functioning. If the delirium is the result of multiple irreversible causes, it could become chronic.

[polldaddy:10332749]
EVALUATION Cardiac dysfunction
Ms. P undergoes laboratory testing. The results include: white blood cell count, 5.9/µL; hemoglobin, 13.6 g/dL; hematocrit, 42.6%; platelets, 304 × 103/µL; sodium,143 mEq/L; potassium, 3.2 mEq/L; chloride, 96 mEq/L; carbon dioxide, 23 mEq/L; blood glucose, 87 mg/dL; creatinine, 1.2 mg/dL; estimated creatinine clearance (eCrCl) level of 33 mL/min/1.73 m2; calcium, 9.5 mg/dL; albumin, 3.6 g/dL; liver enzymes within normal limits; thyroid-stimulating hormone, 0.78 mIU/L; vitamin B12, 995 pg/mL; folic acid, 16.6 ng/mL; vitamin D, 31 pg/mL; and rapid plasma reagin: nonreactive. Urinalysis is unremarkable, and no culture is performed. Urine drug screening/toxicology is positive for the benzodiazepines that she received in the ED (oral alprazolam 0.25 mg given once and oral lorazepam 0.5 mg given once).
Electrocardiogram (ECG) shows atrial flutter/tachycardia with rapid ventricular response, marked left axis deviation, nonspecific ST- and T-wave abnormality, QT/QTC of 301/387 ms, and ventricular rate 151 beats per minute. A CT scan of the head and brain without contrast shows mild atrophy and chronic white matter changes and no acute intracranial abnormality. A two-view chest radiography shows no acute cardiopulmonary findings. Her temperature is 98.4°F; heart rate is 122 beats per minute; respiratory rate is 20 breaths per minute; blood pressure is 161/98 mm Hg; and oxygen saturation is 86% on room air.
Based on this data, Ms. P’s cardiac condition seems to be worsening, which is thought to be caused by her refusal of furosemide, lisinopril, isosorbide, sotalol, clopidogrel, and aspirin. The treatment team plans to work on compliance to resolve these cardiac issues and places Ms. P on 1:1 observation with a sitter and music in attempt to calm her.
Continue to: The authors' observations
The authors’ observations
Many factors can contribute to behavioral or cognitive changes in geriatric patients. Often, a major change noted in an older patient can be attributed to new-onset dementia, dementia with behavioral disturbances, delirium, depression, or acute psychosis. These potential causes should be considered and ruled out in a step-by-step progression. Because patients are unreliable historians during acute distress, a complete history from family or caregivers and exhaustive workup is paramount.
TREATMENT Medication adjustments
In an attempt to resolve Ms. P’s disruptive behaviors, her risperidone dosage is changed to 0.5 mg twice daily. Ms. P is encouraged to use the provided oxygen to raise her saturation level.
On hospital Day 3, a loose stool prompts a Clostridium difficile test as a possible source of delirium; however, the results are negative.
On hospital Day 4, Ms. P is confused and irritable overnight, yelling profanities at staff, refusing care, inappropriately disrobing, and having difficulty falling asleep and staying asleep. Risperidone is discontinued because it appears to have had little or no effect on Ms. P’s disruptive behaviors. Olanzapine, 10 mg/d, is initiated with mirtazapine, 7.5 mg/d, to help with mood, appetite, and sleep. Fluoxetine is also discontinued because of a possible interaction with clopidogrel.
On hospital Days 6 to 8, Ms. P remains upset and unable to follow instructions. Melatonin is initiated to improve her sleep cycle. On Day 9, she continues to decline and is cursing at hospital staff; haloperidol is initiated at 5 mg every morning, 10 mg at bedtime, and 5 mg IM as needed for agitation. Her sleep improves with melatonin and mirtazapine. IV hydration also is initiated. Ms. P has a slight improvement in medication compliance. On Day 11, haloperidol is increased to 5 mg in the morning, 5 mg in the afternoon, and 10 mg at bedtime. On Day 12, haloperidol is changed to 7.5 mg twice daily; a slight improvement in Ms. P’s behavior is noted.
Continue to: On hospital Day 13...
On hospital Day 13, Ms. P’s behavior declines again. She screams profanities at staff and does not recognize the clinicians who have been providing care to her. The physician initiates valproic acid, 125 mg, 3 times a day, to target Ms. P’s behavioral disturbances. A pharmacist notes that the patient’s sotalol could be contributing to Ms. P’s psychiatric presentation, and that based on her eCrCl level of 33 mL/min/1.73 m2, a dosage adjustment or medication change might be warranted.
On Day 14, Ms. P displays erratic behavior and intermittent tachycardia. A cardiac consultation is ordered. A repeat ECG reveals atrial fibrillation with rapid rate and a QT/QTc of 409/432 ms. Ms. P is transferred to the telemetry unit, where the cardiologist discontinues sotalol because the dosage is not properly renally adjusted. Sotalol hydrochloride has been associated with life-threatening ventricular tachycardia.8 Diltiazem, 30 mg every 6 hours is initiated to replace sotalol.
By Day 16, the treatment team notes improved cognition and behavior. On Day 17, the cardiologist reports that Ms. P’s atrial fibrillation is controlled. An ECG reveals mild left ventricular hypertrophy, an ejection fraction of 50% to 55%, no stenosis in the mitral or tricuspid valves, no valvular pulmonic stenosis, and moderate aortic sclerosis. Cardiac markers also are evaluated (creatinine phosphokinase: 105 U/L; creatinine kinase–MB fraction: 2.6 ng/mL; troponin: 0.01 ng/mL; pro-B-type natriuretic peptide: 2,073 pg/mL); and myocardial infarction is ruled out.
On Day 19, Ms. P’s diltiazem is consolidated to a controlled-delivery formulation, 180 mg/d, along with the addition of metoprolol, 12.5 mg twice daily. Ms. P is transferred back to the psychiatric unit.
OUTCOME Gradual improvement
On Days 20 to 23, Ms. P shows remarkable progress, and her mental status, cognition, and behavior slowly return to baseline. Haloperidol and valproic acid are tapered and discontinued. Ms. P is observed to be healthy and oriented to person, place, and time.
Continue to: On Day 25...
On Day 25, she is discharged from the hospital, and returns to the LTC facility.
The authors’ observations
Ms. P’s delirium was a combination of her older age, non-renally adjusted sotalol, and CKD. At admission, the hospital treatment team first thought that pneumonia or antibiotic use could have caused delirium. However, Ms. P’s condition did not improve after antibiotics were stopped. In addition, several chest radiographs found no evidence of pneumonia. It is important to check for any source of infection because infection is a common source of delirium in older patients.1 Urine samples revealed no pathogens, a C. difficile test was negative, and the patient’s white blood cell counts remained within normal limits. Physicians began looking elsewhere for potential causes of Ms. P’s delirium.
Ms. P’s vital signs ruled out a temperature irregularity or hypertension as the cause of her delirium. She has a slightly low oxygen saturation when she first presented, but this quickly returned to normal with administration of oxygen, which ruled out hypoxemia. Laboratory results concluded that Ms. P’s glucose levels were within a normal range and she had no electrolyte imbalances. A head CT scan showed slight atrophy of white matter that is consistent with Ms. P’s age. The head CT scan also showed that Ms. P had no acute condition or head trauma.
In terms of organ function, Ms. P was in relatively healthy condition other than paroxysmal atrial fibrillation and CKD. Chronic kidney disease can interrupt the normal pharmacokinetics of medications. Reviewing Ms. P’s medication list, several agents could have induced delirium, including antidepressants, antipsychotics, cardiovascular medications (beta blocker/antiarrhythmic [sotalol]), and opioid analgesics such as tramadol.5 Ms. P’s condition did not improve after discontinuing fluoxetine, risperidone, or olanzapine, although haloperidol was started in their place. Ms. P scored an 8 on the Naranjo Adverse Drug Reaction Probability Scale, indicating this event was a probable adverse drug reaction.9
Identifying a cause
This was a unique case where sotalol was identified as the culprit for inducing Ms. P’s delirium, because her age and CKD are irreversible. It is important to note that antiarrhythmics can induce arrhythmias when present in high concentrations or administered without appropriate renal dose adjustments. Although Ms. P’s serum levels of sotalol were not evaluated, because of her renal impairment, it is possible that toxic levels of sotalol accumulated and lead to arrhythmias and delirium. Of note, a cardiologist was consulted to safely change Ms. P to a calcium channel blocker so she could undergo cardiac monitoring. With the addition of diltiazem and metoprolol, the patient’s delirium subsided and her arrhythmia was controlled. Once the source of Ms. P’s delirium had been identified, antipsychotics were no longer needed.
Continue to: Bottom Line
Bottom Line
Delirium is a complex disorder that often has multiple causes, both reversible and irreversible. A “process of elimination” approach should be used to accurately identify and manage delirium. If a patient with delirium has little to no response to antipsychotic medications, the underlying cause or causes likely has not yet been addressed, and the evaluation should continue.
Related Resources
- Marcantonio ER. Delirium in hospitalized older adults. N Engl J Med. 2017;377:1456-1466.
- Inouye SK, Westendorp RGJ, Saczynski JS. Delirium in elderly people. Lancet. 2014;383(9920):911-922.
Drug Brand Names
Acyclovir • Zovirax
Alprazolam • Niravam, Xanax
Amantadine • Symmetrel
Amphotericin B • Abelcet
Atorvastatin • Lipitor
Atropine • Atropen
Baclofen • EnovaRX-Baclofen
Benztropine • Cogentin
Bromocriptine • Cycloset
Calcitonin • Miacalcin
Carbamazepine • Tegretol
Carbidopa-levodopa • Duopa
Ceftriaxone • Rocephin
Chlorpromazine • Thorazine
Clonidine • Catapres
Clopidogrel • Plavix
Cyclobenzaprine • Amrix
Digoxin • Lanoxin
Diltiazem • Cardizem
Disulfiram • Antabuse
Ezetimibe • Zetia
Fluoxetine • Prozac
Fluphenazine • Prolixin
Furosemide • Lasix
Haloperidol • Haldol
Ipratropium/albuterol nebulized solution • Combivent Respimat
Isoniazid • Isotamine
Isosorbide nitrate • Dilatrate
Levetiracetam • Keppra
Levodopa • Stalevo
Linezolid • Zyvox
Lisinopril • Zestril
Lithium • Eskalith, Lithobid
Lorazepam • Ativan
Magnesium Oxide • Mag-200
Meperidine • Demerol
Methyldopa • Aldomet
Metoprolol • Lopressor
Metronidazole • Flagyl
Mirtazapine • Remeron
Nitrofurantoin • Macrobid
Olanzapine • Zyprexa
Pantoprazole • Protonix
Phenytoin • Dilantin
Pramipexole • Mirapex
Rifampin • Rifadin
Risperidone • Risperdal
Ropinirole • Requip
Sotalol hydrochloride • Betapace AF
Tramadol • Ultram
Trihexyphenidyl • Trihexane
Valproic acid • Depakote
CASE Combative and agitated
Ms. P, age 87, presents to the emergency department (ED) with her caregiver, who says Ms. P has new-onset altered mental status, agitation, and combativeness.
Ms. P resides at a long-term care (LTC) facility, where according to the nurses she normally is pleasant, well-oriented, and cooperative. Ms. P’s medical history includes major depressive disorder, generalized anxiety disorder, hypertension, chronic kidney disease (CKD) stage III, peptic ulcer disease, gastroesophageal reflux disease, coronary artery disease with 2 past myocardial infarctions requiring stents, chronic obstructive pulmonary disease, hyperlipidemia, bradycardia requiring a pacemaker, paroxysmal atrial fibrillation, asthma, aortic stenosis, peripheral vascular disease, esophageal stricture requiring dilation, deep vein thrombosis, and migraines.
Mr. P’s medication list includes acetaminophen, 650 mg every 6 hours; ipratropium/albuterol nebulized solution, 3 mL 4 times a day; aspirin, 81 mg/d; atorvastatin, 40 mg/d; calcitonin, 1 spray nasally at bedtime; clopidogrel, 75 mg/d; ezetimibe, 10 mg/d; fluoxetine, 20 mg/d; furosemide, 20 mg/d; isosorbide dinitrate, 120 mg/d; lisinopril, 15 mg/d; risperidone, 0.5 mg/d; magnesium oxide, 800 mg/d; pantoprazole, 40 mg/d; polyethylene glycol, 17 g/d; sotalol, 160 mg/d; olanzapine, 5 mg IM every 6 hours as needed for agitation; and tramadol, 50 mg every 8 hours as needed for headache.
Seven days before coming to the ED, Ms. P was started on ceftriaxone, 1 g/d, for suspected community-acquired pneumonia. At that time, the nursing staff noticed behavioral changes. Soon after, Ms. P began refusing all her medications. Two days before presenting to the ED, Ms. P was started on nitrofurantoin, 200 mg/d, for a suspected urinary tract infection, but it was discontinued because of an allergy.
Her caregiver reports that while at the LTC facility, Ms. P’s behavioral changes worsened. Ms. P claimed to be Jesus Christ and said she was talking to the devil; she chased other residents around the facility and slapped medications away from the nursing staff. According to caregivers, this behavior was out of character.
Shortly after arriving in the ED, Ms. P is admitted to the psychiatric unit.
[polldaddy:10332748]
The authors’ observations
Delirium is a complex, acute alteration in a patient’s mental status compared with his/her baseline functioning1 (Table 12). The onset of delirium is quick, happening within hours to days, with fluctuations in mental function. Patients might present with hyperactive, hypoactive, or mixed delirium.3 Patients with hyperactive delirium often have delusions and hallucinations; these patients might be agitated and could become violent with family and caregivers.3 Patients with hypoactive delirium are less likely to experience hallucinations and more likely to show symptoms of sedation.3 Patients with hypoactive delirium can be difficult to diagnose because it is challenging to interview them and understand what might be the cause of their sedated state. Patients also can exhibit a mixed delirium in which they fluctuate between periods of hyperactivity and hypoactivity.3
Continue to: Suspected delirium...
Suspected delirium should be considered a medical emergency because the outcome could be fatal.1 It is important to uncover and treat the underlying cause(s) of delirium rather than solely administering antipsychotics, which might mask the presenting symptoms. In an older study, Francis and Kapoor4 reported that 56% of geriatric patients with delirium had a single definite or probable etiology, while the other 44% had about 2.8 etiologies per patient on average. Delirium risk factors, causes, and factors to consider during patient evaluation are listed in Table 21,3,5-7 and Table 3.1,3,5-7

A synergistic relationship between comorbidities, environment, and medications can induce delirium.5 Identifying irreversible and reversible causes is the key to treating delirium. After the cause has been identified, it can be addressed and the patient could return to his/her previous level of functioning. If the delirium is the result of multiple irreversible causes, it could become chronic.

[polldaddy:10332749]
EVALUATION Cardiac dysfunction
Ms. P undergoes laboratory testing. The results include: white blood cell count, 5.9/µL; hemoglobin, 13.6 g/dL; hematocrit, 42.6%; platelets, 304 × 103/µL; sodium,143 mEq/L; potassium, 3.2 mEq/L; chloride, 96 mEq/L; carbon dioxide, 23 mEq/L; blood glucose, 87 mg/dL; creatinine, 1.2 mg/dL; estimated creatinine clearance (eCrCl) level of 33 mL/min/1.73 m2; calcium, 9.5 mg/dL; albumin, 3.6 g/dL; liver enzymes within normal limits; thyroid-stimulating hormone, 0.78 mIU/L; vitamin B12, 995 pg/mL; folic acid, 16.6 ng/mL; vitamin D, 31 pg/mL; and rapid plasma reagin: nonreactive. Urinalysis is unremarkable, and no culture is performed. Urine drug screening/toxicology is positive for the benzodiazepines that she received in the ED (oral alprazolam 0.25 mg given once and oral lorazepam 0.5 mg given once).
Electrocardiogram (ECG) shows atrial flutter/tachycardia with rapid ventricular response, marked left axis deviation, nonspecific ST- and T-wave abnormality, QT/QTC of 301/387 ms, and ventricular rate 151 beats per minute. A CT scan of the head and brain without contrast shows mild atrophy and chronic white matter changes and no acute intracranial abnormality. A two-view chest radiography shows no acute cardiopulmonary findings. Her temperature is 98.4°F; heart rate is 122 beats per minute; respiratory rate is 20 breaths per minute; blood pressure is 161/98 mm Hg; and oxygen saturation is 86% on room air.
Based on this data, Ms. P’s cardiac condition seems to be worsening, which is thought to be caused by her refusal of furosemide, lisinopril, isosorbide, sotalol, clopidogrel, and aspirin. The treatment team plans to work on compliance to resolve these cardiac issues and places Ms. P on 1:1 observation with a sitter and music in attempt to calm her.
Continue to: The authors' observations
The authors’ observations
Many factors can contribute to behavioral or cognitive changes in geriatric patients. Often, a major change noted in an older patient can be attributed to new-onset dementia, dementia with behavioral disturbances, delirium, depression, or acute psychosis. These potential causes should be considered and ruled out in a step-by-step progression. Because patients are unreliable historians during acute distress, a complete history from family or caregivers and exhaustive workup is paramount.
TREATMENT Medication adjustments
In an attempt to resolve Ms. P’s disruptive behaviors, her risperidone dosage is changed to 0.5 mg twice daily. Ms. P is encouraged to use the provided oxygen to raise her saturation level.
On hospital Day 3, a loose stool prompts a Clostridium difficile test as a possible source of delirium; however, the results are negative.
On hospital Day 4, Ms. P is confused and irritable overnight, yelling profanities at staff, refusing care, inappropriately disrobing, and having difficulty falling asleep and staying asleep. Risperidone is discontinued because it appears to have had little or no effect on Ms. P’s disruptive behaviors. Olanzapine, 10 mg/d, is initiated with mirtazapine, 7.5 mg/d, to help with mood, appetite, and sleep. Fluoxetine is also discontinued because of a possible interaction with clopidogrel.
On hospital Days 6 to 8, Ms. P remains upset and unable to follow instructions. Melatonin is initiated to improve her sleep cycle. On Day 9, she continues to decline and is cursing at hospital staff; haloperidol is initiated at 5 mg every morning, 10 mg at bedtime, and 5 mg IM as needed for agitation. Her sleep improves with melatonin and mirtazapine. IV hydration also is initiated. Ms. P has a slight improvement in medication compliance. On Day 11, haloperidol is increased to 5 mg in the morning, 5 mg in the afternoon, and 10 mg at bedtime. On Day 12, haloperidol is changed to 7.5 mg twice daily; a slight improvement in Ms. P’s behavior is noted.
Continue to: On hospital Day 13...
On hospital Day 13, Ms. P’s behavior declines again. She screams profanities at staff and does not recognize the clinicians who have been providing care to her. The physician initiates valproic acid, 125 mg, 3 times a day, to target Ms. P’s behavioral disturbances. A pharmacist notes that the patient’s sotalol could be contributing to Ms. P’s psychiatric presentation, and that based on her eCrCl level of 33 mL/min/1.73 m2, a dosage adjustment or medication change might be warranted.
On Day 14, Ms. P displays erratic behavior and intermittent tachycardia. A cardiac consultation is ordered. A repeat ECG reveals atrial fibrillation with rapid rate and a QT/QTc of 409/432 ms. Ms. P is transferred to the telemetry unit, where the cardiologist discontinues sotalol because the dosage is not properly renally adjusted. Sotalol hydrochloride has been associated with life-threatening ventricular tachycardia.8 Diltiazem, 30 mg every 6 hours is initiated to replace sotalol.
By Day 16, the treatment team notes improved cognition and behavior. On Day 17, the cardiologist reports that Ms. P’s atrial fibrillation is controlled. An ECG reveals mild left ventricular hypertrophy, an ejection fraction of 50% to 55%, no stenosis in the mitral or tricuspid valves, no valvular pulmonic stenosis, and moderate aortic sclerosis. Cardiac markers also are evaluated (creatinine phosphokinase: 105 U/L; creatinine kinase–MB fraction: 2.6 ng/mL; troponin: 0.01 ng/mL; pro-B-type natriuretic peptide: 2,073 pg/mL); and myocardial infarction is ruled out.
On Day 19, Ms. P’s diltiazem is consolidated to a controlled-delivery formulation, 180 mg/d, along with the addition of metoprolol, 12.5 mg twice daily. Ms. P is transferred back to the psychiatric unit.
OUTCOME Gradual improvement
On Days 20 to 23, Ms. P shows remarkable progress, and her mental status, cognition, and behavior slowly return to baseline. Haloperidol and valproic acid are tapered and discontinued. Ms. P is observed to be healthy and oriented to person, place, and time.
Continue to: On Day 25...
On Day 25, she is discharged from the hospital, and returns to the LTC facility.
The authors’ observations
Ms. P’s delirium was a combination of her older age, non-renally adjusted sotalol, and CKD. At admission, the hospital treatment team first thought that pneumonia or antibiotic use could have caused delirium. However, Ms. P’s condition did not improve after antibiotics were stopped. In addition, several chest radiographs found no evidence of pneumonia. It is important to check for any source of infection because infection is a common source of delirium in older patients.1 Urine samples revealed no pathogens, a C. difficile test was negative, and the patient’s white blood cell counts remained within normal limits. Physicians began looking elsewhere for potential causes of Ms. P’s delirium.
Ms. P’s vital signs ruled out a temperature irregularity or hypertension as the cause of her delirium. She has a slightly low oxygen saturation when she first presented, but this quickly returned to normal with administration of oxygen, which ruled out hypoxemia. Laboratory results concluded that Ms. P’s glucose levels were within a normal range and she had no electrolyte imbalances. A head CT scan showed slight atrophy of white matter that is consistent with Ms. P’s age. The head CT scan also showed that Ms. P had no acute condition or head trauma.
In terms of organ function, Ms. P was in relatively healthy condition other than paroxysmal atrial fibrillation and CKD. Chronic kidney disease can interrupt the normal pharmacokinetics of medications. Reviewing Ms. P’s medication list, several agents could have induced delirium, including antidepressants, antipsychotics, cardiovascular medications (beta blocker/antiarrhythmic [sotalol]), and opioid analgesics such as tramadol.5 Ms. P’s condition did not improve after discontinuing fluoxetine, risperidone, or olanzapine, although haloperidol was started in their place. Ms. P scored an 8 on the Naranjo Adverse Drug Reaction Probability Scale, indicating this event was a probable adverse drug reaction.9
Identifying a cause
This was a unique case where sotalol was identified as the culprit for inducing Ms. P’s delirium, because her age and CKD are irreversible. It is important to note that antiarrhythmics can induce arrhythmias when present in high concentrations or administered without appropriate renal dose adjustments. Although Ms. P’s serum levels of sotalol were not evaluated, because of her renal impairment, it is possible that toxic levels of sotalol accumulated and lead to arrhythmias and delirium. Of note, a cardiologist was consulted to safely change Ms. P to a calcium channel blocker so she could undergo cardiac monitoring. With the addition of diltiazem and metoprolol, the patient’s delirium subsided and her arrhythmia was controlled. Once the source of Ms. P’s delirium had been identified, antipsychotics were no longer needed.
Continue to: Bottom Line
Bottom Line
Delirium is a complex disorder that often has multiple causes, both reversible and irreversible. A “process of elimination” approach should be used to accurately identify and manage delirium. If a patient with delirium has little to no response to antipsychotic medications, the underlying cause or causes likely has not yet been addressed, and the evaluation should continue.
Related Resources
- Marcantonio ER. Delirium in hospitalized older adults. N Engl J Med. 2017;377:1456-1466.
- Inouye SK, Westendorp RGJ, Saczynski JS. Delirium in elderly people. Lancet. 2014;383(9920):911-922.
Drug Brand Names
Acyclovir • Zovirax
Alprazolam • Niravam, Xanax
Amantadine • Symmetrel
Amphotericin B • Abelcet
Atorvastatin • Lipitor
Atropine • Atropen
Baclofen • EnovaRX-Baclofen
Benztropine • Cogentin
Bromocriptine • Cycloset
Calcitonin • Miacalcin
Carbamazepine • Tegretol
Carbidopa-levodopa • Duopa
Ceftriaxone • Rocephin
Chlorpromazine • Thorazine
Clonidine • Catapres
Clopidogrel • Plavix
Cyclobenzaprine • Amrix
Digoxin • Lanoxin
Diltiazem • Cardizem
Disulfiram • Antabuse
Ezetimibe • Zetia
Fluoxetine • Prozac
Fluphenazine • Prolixin
Furosemide • Lasix
Haloperidol • Haldol
Ipratropium/albuterol nebulized solution • Combivent Respimat
Isoniazid • Isotamine
Isosorbide nitrate • Dilatrate
Levetiracetam • Keppra
Levodopa • Stalevo
Linezolid • Zyvox
Lisinopril • Zestril
Lithium • Eskalith, Lithobid
Lorazepam • Ativan
Magnesium Oxide • Mag-200
Meperidine • Demerol
Methyldopa • Aldomet
Metoprolol • Lopressor
Metronidazole • Flagyl
Mirtazapine • Remeron
Nitrofurantoin • Macrobid
Olanzapine • Zyprexa
Pantoprazole • Protonix
Phenytoin • Dilantin
Pramipexole • Mirapex
Rifampin • Rifadin
Risperidone • Risperdal
Ropinirole • Requip
Sotalol hydrochloride • Betapace AF
Tramadol • Ultram
Trihexyphenidyl • Trihexane
Valproic acid • Depakote
1. Fong TG, Tulebaev SR, Inouye SK. Delirium in elderly adults: diagnosis, prevention, and treatment. Nat Rev Neurol. 2009;5(4):210-220.
2. Diagnostic and statistical manual of mental disorders, fifth edition. Washington, DC: American Psychiatric Association; 2013.
3. American Psychiatric Association. Practice guideline for the treatment of patients with delirium. Am J Psychiatry. 1999;156(suppl 5):1-20.
4. Francis J, Kapoor WN. Delirium in hospitalized elderly. J Gen Intern Med. 1990;5(1):65-79.
5. Alagiakrishnan K, Wiens CA. An approach to drug induced delirium in the elderly. Postgrad Med J. 2004;80(945):388-393.
6. Cook IA. Guideline watch: practice guideline for the treatment of patients with delirium. Arlington, VA: American Psychiatric Publishing; 2004.
7. Bourgeois J, Ategan A, Losier B. Delirium in the hospital: emphasis on the management of geriatric patients. Current Psychiatry. 2014;13(8):29,36-42.
8. Betapace AF [package insert]. Zug, Switzerland: Covis Pharma; 2016.
9. Naranjo CA, Busto U, Sellers EM, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30(2):239-245.
1. Fong TG, Tulebaev SR, Inouye SK. Delirium in elderly adults: diagnosis, prevention, and treatment. Nat Rev Neurol. 2009;5(4):210-220.
2. Diagnostic and statistical manual of mental disorders, fifth edition. Washington, DC: American Psychiatric Association; 2013.
3. American Psychiatric Association. Practice guideline for the treatment of patients with delirium. Am J Psychiatry. 1999;156(suppl 5):1-20.
4. Francis J, Kapoor WN. Delirium in hospitalized elderly. J Gen Intern Med. 1990;5(1):65-79.
5. Alagiakrishnan K, Wiens CA. An approach to drug induced delirium in the elderly. Postgrad Med J. 2004;80(945):388-393.
6. Cook IA. Guideline watch: practice guideline for the treatment of patients with delirium. Arlington, VA: American Psychiatric Publishing; 2004.
7. Bourgeois J, Ategan A, Losier B. Delirium in the hospital: emphasis on the management of geriatric patients. Current Psychiatry. 2014;13(8):29,36-42.
8. Betapace AF [package insert]. Zug, Switzerland: Covis Pharma; 2016.
9. Naranjo CA, Busto U, Sellers EM, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30(2):239-245.
An insidious onset of symptoms
CASE Tremors, increasing anxiety
Ms. S, age 56, has a history of depression and anxiety.
The authors’ observations
The incidence of serotonin syndrome has increased because of increasing use of serotonergic agents.1-3 Although the severity could range from benign to life-threatening, the potential lethality combined with difficulty of diagnosis makes this condition of continued interest. Stimulation of the 5-hydroxytryptamine (5-HT) receptor subtypes, specifically 5-HT1A and 5-HT2, are implicated in this syndrome.4,5
Serotonin syndrome is a clinical diagnosis with a triad of symptoms that includes mental status changes, autonomic hyperactivity, and neuromuscular abnormalities.1,2 However, because of the varied presentation and similarity to other syndromes such as NMS, serotonin syndrome often is undiagnosed.5
TREATMENT Discontinue fluoxetine
Several months after the fluoxetine increase, Ms. S's physical symptoms emerge. Several weeks later, she notices significant hypertension of 162/102 mm Hg by checking her blood pressure at home. She had no history of hypertension before taking fluoxetine. She then tells her psychiatrist she has been experiencing confusion, shakiness, loss of balance, forgetfulness, joint pain, sweating, and fatigue, along with worsening anxiety. The treatment team makes a diagnosis of serotonin syndrome and recommends discontinuing fluoxetine and starting cyproheptadine, 4 mg initially, and then repeating the cyproheptadine dose in several hours if her symptoms do not resolve. Approximately, 2.5 months after the serotonin syndrome reaction, Ms. S receives hydroxyzine, 10 mg every 8 hours, as needed for anxiety.
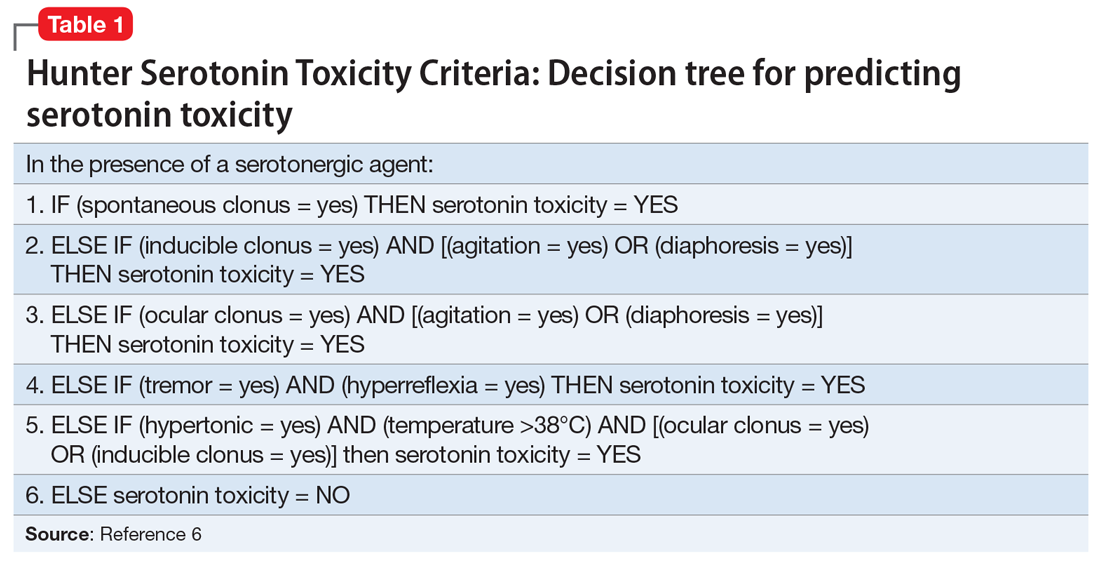
The authors’ observations
The diagnosis of serotonin syndrome is most accurately made using Hunter Serotonin Toxicity Criteria (Table 16). Because Ms. S had an insidious onset of symptoms, and treatment was initiated before full evaluation, it is unknown if she met Hunter criteria. To meet these criteria, a patient must have ≥1 of the following6:
- spontaneous clonus
- inducible clonus plus agitation or diaphoresis
- ocular clonus plus agitation or diaphoresis
- tremor plus hyperreflexia
- hypertonia plus temperature >38°C plus ocular or inducible clonus.
The Sternbach diagnostic criteria for serotonin syndrome (Table 23) is another commonly used tool.3 These criteria include the addition or increase of a serotonin agent and absence of substances or metabolic derangements that could account for symptoms and at least 3 of the following 10 symptoms3:
- mental status changes (confusion, hypomania)
- agitation
- myoclonus
- hyperreflexia
- diaphoresis
- shivering
- tremor
- diarrhea
- incoordination
- fever.
Continue to: Ms. S met the Sternbach diagnostic criteria...
Ms. S met the Sternbach diagnostic criteria for serotonin syndrome.
Ms. S was taking a single serotonin agent and initially had mild symptoms. More commonly, a patient who presents with serotonin syndrome has been receiving ≥2 serotonergic agents or toxic levels of a single agent, and these agents usually include a psychotropic medication such as a monoamine oxidase inhibitor, tricyclic antidepressant, or SSRI, as well as a medication from a different class, such as dextromethorphan, linezolid, tramadol, methylene blue, and/or St. John’s wort.1,7-13 However, in this case, Ms. S also was taking bupropion, a known inhibitor of cytochrome P450 2D6. Bupropion might have increased Ms. S’s fluoxetine levels.
Ms. S was a healthy, middle-age patient who took no medications other than those listed, had no medical comorbidities, and had a straightforward psychiatric history, which makes the diagnosis of serotonin syndrome clearer. However, other potential differential diagnoses, such as NMS, delirium tremens, and anticholinergic toxicity, might cloud the clinical picture. When differentiating NMS and serotonin syndrome, it is helpful to note whether a patient shows tremor, diarrhea, and myoclonus present in the absence of muscular, “lead-pipe” rigidity, which suggests a diagnosis of serotonin syndrome.2,3,5
[polldaddy:10279173]
The authors’ observations
Treating serotonin syndrome includes supportive care, discontinuing offending agents, administering benzodiazepines, and using a serotonin antagonist as an antidote for patients with moderate-to-severe cases. Cyproheptadine is an antihistaminergic medication with non-specific 5-HT1A and 5-HT2 antagonism. It is FDA-approved for specific allergic reactions, urticaria, and anaphylaxis adjunctive therapy, but not for serotonin syndrome. Case series support the use of cyproheptadine for acute management of serotonin syndrome, with rapid symptom improvement.4,7,14-18 We observed a similar outcome with Ms. S. Her significant autonomic symptoms resolved rapidly, although she experienced some residual, mild symptoms that took weeks to resolve.
Continue to: Because serotonergic agents...
Because serotonergic agents are used frequently and readily by primary care clinicians as well as psychiatrists, the ability to properly diagnose this syndrome is vital, particularly because severe cases can rapidly deteriorate.1,9,16,17 This presentation of a single serotonergic agent causing significant symptoms that worsened over months is not typical, but important to recognize as a patient begins to experience autonomic instability. As was the case with Ms. S, it is important to remain vigilant when changing dosages or adding medications. Symptoms of serotonin syndrome might be vague and difficult to diagnose, especially if the clinician is not aware of the variability of presentation of this syndrome. Cyproheptadine can be used safely and rapidly and should be considered a treatment option for serotonin syndrome.
OUTCOME Hypertension resolves
After her first dose of cyproheptadine, Ms. S’s blood pressure drops to 146/86 mm Hg. Three hours later, she repeats the cyproheptadine dose and her blood pressure drops to 106/60 mm Hg. She reports that her anxiety has lessened, although she is still tremulous. Overall, she says she feels better. She experiences improvement of her condition with a pharmacologic regimen of bupropion, gabapentin, and hydroxyzine.
Several weeks later, her health returns to baseline, with complete resolution of hypertension.
Bottom Line
Although serotonin syndrome is most commonly associated with co-administered serotonergic medications, symptoms can emerge with a single, moderately dosed agent. Treatment includes withdrawing the offending agent, and administering a serotonin antagonist. Mild cases of serotonin syndrome usually resolve.
Related Resources
- Turner AH, Kim JJ, McCarron RM. Differentiating serotonin syndrome and neuroleptic malignant syndrome. Current Psychiatry. 2019;18(2):30-36.
- Iqbal MM, Basil MJ, Kaplan J, et al. Overview of serotonin syndrome. Ann Clin Psychiatry. 2012;24(4):310-318.
Drug Brand Names
Bupropion • Wellbutrin, Zyban
Cyproheptadine • Periactin
Dextromethorphan • Benylin
Duloxetine • Cymbalta
Fluoxetine • Prozac
Gabapentin • Neurontin
Hydroxyzine • Atarax
Linezolid • Zyvox
Tramadol • Ultram, Ryzolt
1. Mason PJ, Morris VA, Balcezak TJ. Serotonin syndrome: presentation of 2 cases and review of literature. Medicine (Baltimore). 2000;79(4):201-209.
2. Boyer EW, Shannon M. The serotonin syndrome. N Engl J Med. 2005;352(11):1112-1120.
3. Sternbach H. The serotonin syndrome. Am J Psychiatry. 1991;148(6):705-713.
4. Graudins A, Stearman A, Chan B. Treatment of the serotonin syndrome with cyproheptadine. J Emerg Med. 1998;16(4):615-619.
5. Mills KC. Serotonin syndrome a clinical update. Crit Care Clin. 1997;13(4):763-783.
6. Dunkley EJ, Isbister GK, Sibbritt D, et al. The Hunter Serotonin Toxicity Criteria: simple and accurate diagnostic decision rules for serotonin toxicity. QJM. 2003;96:635-642.
7. Horowitz BZ, Mullins ME. Cyproheptadine for serotonin syndrome in an accidental pediatric sertraline ingestion. Pediatr Emerg Care. 1999;15(5):325-327.
8. Kolecki P. Isolated venlafaxine-induced serotonin syndrome. J Emerg Med. 1996;15:491-493.
9. Pan J, Shen W. Serotonin syndrome induced by low-dose venlafaxine. Ann Pharmacother. 2003;37(2):209-211.
10. Hernández JL, Ramos FJ, Infante J, et al. Severe serotonin syndrome induced by mirtazapine monotherapy. Ann Pharmacother. 2002;36(4):641-643.
11. Patel DD, Galarneau D. Serotonin syndrome with fluoxetine: two case reports. Ochsner J. 2016;16(4):554-557.
12. Frank C. Recognition and treatment of serotonin syndrome. Can Fam Physician. 2008;54(7):988-992.
13. Zuschlag ZD, Warren MW, K Schultz S. Serotonin toxicity and urinary analgesics: a case report and systematic literature review of methylene blue-induced serotonin syndrome. Psychosomatics. 2018;59(6):539-546.
14. Kapur S, Zipursky RB, Jones C, et al. Cyproheptadine: a potent in vivo serotonin antagonist. Am J Psychiatry. 1997;154(6):884.
15. Baigel GD. Cyproheptadine and the treatment of an unconscious patient with the serotonin syndrome. Eur J Anaesthesiol. 2003;20(7):586-588.
16. Lappin RI, Auchincloss EL. Treatment of the serotonin syndrome with cyproheptadine. N Engl J Med. 1994;331:1021-1022.
17. McDaniel WW. Serotonin syndrome: early management with cyproheptadine. Ann Pharmacother. 2001;35(7-8):870-873.
18. Kolecki P. Venlafaxine induced serotonin syndrome occurring after abstinence from phenelzine for more than two weeks. J Toxicol Clin Toxicol. 1997;35(2):211-212.
CASE Tremors, increasing anxiety
Ms. S, age 56, has a history of depression and anxiety.
The authors’ observations
The incidence of serotonin syndrome has increased because of increasing use of serotonergic agents.1-3 Although the severity could range from benign to life-threatening, the potential lethality combined with difficulty of diagnosis makes this condition of continued interest. Stimulation of the 5-hydroxytryptamine (5-HT) receptor subtypes, specifically 5-HT1A and 5-HT2, are implicated in this syndrome.4,5
Serotonin syndrome is a clinical diagnosis with a triad of symptoms that includes mental status changes, autonomic hyperactivity, and neuromuscular abnormalities.1,2 However, because of the varied presentation and similarity to other syndromes such as NMS, serotonin syndrome often is undiagnosed.5
TREATMENT Discontinue fluoxetine
Several months after the fluoxetine increase, Ms. S's physical symptoms emerge. Several weeks later, she notices significant hypertension of 162/102 mm Hg by checking her blood pressure at home. She had no history of hypertension before taking fluoxetine. She then tells her psychiatrist she has been experiencing confusion, shakiness, loss of balance, forgetfulness, joint pain, sweating, and fatigue, along with worsening anxiety. The treatment team makes a diagnosis of serotonin syndrome and recommends discontinuing fluoxetine and starting cyproheptadine, 4 mg initially, and then repeating the cyproheptadine dose in several hours if her symptoms do not resolve. Approximately, 2.5 months after the serotonin syndrome reaction, Ms. S receives hydroxyzine, 10 mg every 8 hours, as needed for anxiety.

The authors’ observations
The diagnosis of serotonin syndrome is most accurately made using Hunter Serotonin Toxicity Criteria (Table 16). Because Ms. S had an insidious onset of symptoms, and treatment was initiated before full evaluation, it is unknown if she met Hunter criteria. To meet these criteria, a patient must have ≥1 of the following6:
- spontaneous clonus
- inducible clonus plus agitation or diaphoresis
- ocular clonus plus agitation or diaphoresis
- tremor plus hyperreflexia
- hypertonia plus temperature >38°C plus ocular or inducible clonus.
The Sternbach diagnostic criteria for serotonin syndrome (Table 23) is another commonly used tool.3 These criteria include the addition or increase of a serotonin agent and absence of substances or metabolic derangements that could account for symptoms and at least 3 of the following 10 symptoms3:
- mental status changes (confusion, hypomania)
- agitation
- myoclonus
- hyperreflexia
- diaphoresis
- shivering
- tremor
- diarrhea
- incoordination
- fever.
Continue to: Ms. S met the Sternbach diagnostic criteria...
Ms. S met the Sternbach diagnostic criteria for serotonin syndrome.
Ms. S was taking a single serotonin agent and initially had mild symptoms. More commonly, a patient who presents with serotonin syndrome has been receiving ≥2 serotonergic agents or toxic levels of a single agent, and these agents usually include a psychotropic medication such as a monoamine oxidase inhibitor, tricyclic antidepressant, or SSRI, as well as a medication from a different class, such as dextromethorphan, linezolid, tramadol, methylene blue, and/or St. John’s wort.1,7-13 However, in this case, Ms. S also was taking bupropion, a known inhibitor of cytochrome P450 2D6. Bupropion might have increased Ms. S’s fluoxetine levels.
Ms. S was a healthy, middle-age patient who took no medications other than those listed, had no medical comorbidities, and had a straightforward psychiatric history, which makes the diagnosis of serotonin syndrome clearer. However, other potential differential diagnoses, such as NMS, delirium tremens, and anticholinergic toxicity, might cloud the clinical picture. When differentiating NMS and serotonin syndrome, it is helpful to note whether a patient shows tremor, diarrhea, and myoclonus present in the absence of muscular, “lead-pipe” rigidity, which suggests a diagnosis of serotonin syndrome.2,3,5
[polldaddy:10279173]
The authors’ observations
Treating serotonin syndrome includes supportive care, discontinuing offending agents, administering benzodiazepines, and using a serotonin antagonist as an antidote for patients with moderate-to-severe cases. Cyproheptadine is an antihistaminergic medication with non-specific 5-HT1A and 5-HT2 antagonism. It is FDA-approved for specific allergic reactions, urticaria, and anaphylaxis adjunctive therapy, but not for serotonin syndrome. Case series support the use of cyproheptadine for acute management of serotonin syndrome, with rapid symptom improvement.4,7,14-18 We observed a similar outcome with Ms. S. Her significant autonomic symptoms resolved rapidly, although she experienced some residual, mild symptoms that took weeks to resolve.
Continue to: Because serotonergic agents...
Because serotonergic agents are used frequently and readily by primary care clinicians as well as psychiatrists, the ability to properly diagnose this syndrome is vital, particularly because severe cases can rapidly deteriorate.1,9,16,17 This presentation of a single serotonergic agent causing significant symptoms that worsened over months is not typical, but important to recognize as a patient begins to experience autonomic instability. As was the case with Ms. S, it is important to remain vigilant when changing dosages or adding medications. Symptoms of serotonin syndrome might be vague and difficult to diagnose, especially if the clinician is not aware of the variability of presentation of this syndrome. Cyproheptadine can be used safely and rapidly and should be considered a treatment option for serotonin syndrome.
OUTCOME Hypertension resolves
After her first dose of cyproheptadine, Ms. S’s blood pressure drops to 146/86 mm Hg. Three hours later, she repeats the cyproheptadine dose and her blood pressure drops to 106/60 mm Hg. She reports that her anxiety has lessened, although she is still tremulous. Overall, she says she feels better. She experiences improvement of her condition with a pharmacologic regimen of bupropion, gabapentin, and hydroxyzine.
Several weeks later, her health returns to baseline, with complete resolution of hypertension.
Bottom Line
Although serotonin syndrome is most commonly associated with co-administered serotonergic medications, symptoms can emerge with a single, moderately dosed agent. Treatment includes withdrawing the offending agent, and administering a serotonin antagonist. Mild cases of serotonin syndrome usually resolve.
Related Resources
- Turner AH, Kim JJ, McCarron RM. Differentiating serotonin syndrome and neuroleptic malignant syndrome. Current Psychiatry. 2019;18(2):30-36.
- Iqbal MM, Basil MJ, Kaplan J, et al. Overview of serotonin syndrome. Ann Clin Psychiatry. 2012;24(4):310-318.
Drug Brand Names
Bupropion • Wellbutrin, Zyban
Cyproheptadine • Periactin
Dextromethorphan • Benylin
Duloxetine • Cymbalta
Fluoxetine • Prozac
Gabapentin • Neurontin
Hydroxyzine • Atarax
Linezolid • Zyvox
Tramadol • Ultram, Ryzolt
CASE Tremors, increasing anxiety
Ms. S, age 56, has a history of depression and anxiety.
The authors’ observations
The incidence of serotonin syndrome has increased because of increasing use of serotonergic agents.1-3 Although the severity could range from benign to life-threatening, the potential lethality combined with difficulty of diagnosis makes this condition of continued interest. Stimulation of the 5-hydroxytryptamine (5-HT) receptor subtypes, specifically 5-HT1A and 5-HT2, are implicated in this syndrome.4,5
Serotonin syndrome is a clinical diagnosis with a triad of symptoms that includes mental status changes, autonomic hyperactivity, and neuromuscular abnormalities.1,2 However, because of the varied presentation and similarity to other syndromes such as NMS, serotonin syndrome often is undiagnosed.5
TREATMENT Discontinue fluoxetine
Several months after the fluoxetine increase, Ms. S's physical symptoms emerge. Several weeks later, she notices significant hypertension of 162/102 mm Hg by checking her blood pressure at home. She had no history of hypertension before taking fluoxetine. She then tells her psychiatrist she has been experiencing confusion, shakiness, loss of balance, forgetfulness, joint pain, sweating, and fatigue, along with worsening anxiety. The treatment team makes a diagnosis of serotonin syndrome and recommends discontinuing fluoxetine and starting cyproheptadine, 4 mg initially, and then repeating the cyproheptadine dose in several hours if her symptoms do not resolve. Approximately, 2.5 months after the serotonin syndrome reaction, Ms. S receives hydroxyzine, 10 mg every 8 hours, as needed for anxiety.

The authors’ observations
The diagnosis of serotonin syndrome is most accurately made using Hunter Serotonin Toxicity Criteria (Table 16). Because Ms. S had an insidious onset of symptoms, and treatment was initiated before full evaluation, it is unknown if she met Hunter criteria. To meet these criteria, a patient must have ≥1 of the following6:
- spontaneous clonus
- inducible clonus plus agitation or diaphoresis
- ocular clonus plus agitation or diaphoresis
- tremor plus hyperreflexia
- hypertonia plus temperature >38°C plus ocular or inducible clonus.
The Sternbach diagnostic criteria for serotonin syndrome (Table 23) is another commonly used tool.3 These criteria include the addition or increase of a serotonin agent and absence of substances or metabolic derangements that could account for symptoms and at least 3 of the following 10 symptoms3:
- mental status changes (confusion, hypomania)
- agitation
- myoclonus
- hyperreflexia
- diaphoresis
- shivering
- tremor
- diarrhea
- incoordination
- fever.
Continue to: Ms. S met the Sternbach diagnostic criteria...
Ms. S met the Sternbach diagnostic criteria for serotonin syndrome.
Ms. S was taking a single serotonin agent and initially had mild symptoms. More commonly, a patient who presents with serotonin syndrome has been receiving ≥2 serotonergic agents or toxic levels of a single agent, and these agents usually include a psychotropic medication such as a monoamine oxidase inhibitor, tricyclic antidepressant, or SSRI, as well as a medication from a different class, such as dextromethorphan, linezolid, tramadol, methylene blue, and/or St. John’s wort.1,7-13 However, in this case, Ms. S also was taking bupropion, a known inhibitor of cytochrome P450 2D6. Bupropion might have increased Ms. S’s fluoxetine levels.
Ms. S was a healthy, middle-age patient who took no medications other than those listed, had no medical comorbidities, and had a straightforward psychiatric history, which makes the diagnosis of serotonin syndrome clearer. However, other potential differential diagnoses, such as NMS, delirium tremens, and anticholinergic toxicity, might cloud the clinical picture. When differentiating NMS and serotonin syndrome, it is helpful to note whether a patient shows tremor, diarrhea, and myoclonus present in the absence of muscular, “lead-pipe” rigidity, which suggests a diagnosis of serotonin syndrome.2,3,5
[polldaddy:10279173]
The authors’ observations
Treating serotonin syndrome includes supportive care, discontinuing offending agents, administering benzodiazepines, and using a serotonin antagonist as an antidote for patients with moderate-to-severe cases. Cyproheptadine is an antihistaminergic medication with non-specific 5-HT1A and 5-HT2 antagonism. It is FDA-approved for specific allergic reactions, urticaria, and anaphylaxis adjunctive therapy, but not for serotonin syndrome. Case series support the use of cyproheptadine for acute management of serotonin syndrome, with rapid symptom improvement.4,7,14-18 We observed a similar outcome with Ms. S. Her significant autonomic symptoms resolved rapidly, although she experienced some residual, mild symptoms that took weeks to resolve.
Continue to: Because serotonergic agents...
Because serotonergic agents are used frequently and readily by primary care clinicians as well as psychiatrists, the ability to properly diagnose this syndrome is vital, particularly because severe cases can rapidly deteriorate.1,9,16,17 This presentation of a single serotonergic agent causing significant symptoms that worsened over months is not typical, but important to recognize as a patient begins to experience autonomic instability. As was the case with Ms. S, it is important to remain vigilant when changing dosages or adding medications. Symptoms of serotonin syndrome might be vague and difficult to diagnose, especially if the clinician is not aware of the variability of presentation of this syndrome. Cyproheptadine can be used safely and rapidly and should be considered a treatment option for serotonin syndrome.
OUTCOME Hypertension resolves
After her first dose of cyproheptadine, Ms. S’s blood pressure drops to 146/86 mm Hg. Three hours later, she repeats the cyproheptadine dose and her blood pressure drops to 106/60 mm Hg. She reports that her anxiety has lessened, although she is still tremulous. Overall, she says she feels better. She experiences improvement of her condition with a pharmacologic regimen of bupropion, gabapentin, and hydroxyzine.
Several weeks later, her health returns to baseline, with complete resolution of hypertension.
Bottom Line
Although serotonin syndrome is most commonly associated with co-administered serotonergic medications, symptoms can emerge with a single, moderately dosed agent. Treatment includes withdrawing the offending agent, and administering a serotonin antagonist. Mild cases of serotonin syndrome usually resolve.
Related Resources
- Turner AH, Kim JJ, McCarron RM. Differentiating serotonin syndrome and neuroleptic malignant syndrome. Current Psychiatry. 2019;18(2):30-36.
- Iqbal MM, Basil MJ, Kaplan J, et al. Overview of serotonin syndrome. Ann Clin Psychiatry. 2012;24(4):310-318.
Drug Brand Names
Bupropion • Wellbutrin, Zyban
Cyproheptadine • Periactin
Dextromethorphan • Benylin
Duloxetine • Cymbalta
Fluoxetine • Prozac
Gabapentin • Neurontin
Hydroxyzine • Atarax
Linezolid • Zyvox
Tramadol • Ultram, Ryzolt
1. Mason PJ, Morris VA, Balcezak TJ. Serotonin syndrome: presentation of 2 cases and review of literature. Medicine (Baltimore). 2000;79(4):201-209.
2. Boyer EW, Shannon M. The serotonin syndrome. N Engl J Med. 2005;352(11):1112-1120.
3. Sternbach H. The serotonin syndrome. Am J Psychiatry. 1991;148(6):705-713.
4. Graudins A, Stearman A, Chan B. Treatment of the serotonin syndrome with cyproheptadine. J Emerg Med. 1998;16(4):615-619.
5. Mills KC. Serotonin syndrome a clinical update. Crit Care Clin. 1997;13(4):763-783.
6. Dunkley EJ, Isbister GK, Sibbritt D, et al. The Hunter Serotonin Toxicity Criteria: simple and accurate diagnostic decision rules for serotonin toxicity. QJM. 2003;96:635-642.
7. Horowitz BZ, Mullins ME. Cyproheptadine for serotonin syndrome in an accidental pediatric sertraline ingestion. Pediatr Emerg Care. 1999;15(5):325-327.
8. Kolecki P. Isolated venlafaxine-induced serotonin syndrome. J Emerg Med. 1996;15:491-493.
9. Pan J, Shen W. Serotonin syndrome induced by low-dose venlafaxine. Ann Pharmacother. 2003;37(2):209-211.
10. Hernández JL, Ramos FJ, Infante J, et al. Severe serotonin syndrome induced by mirtazapine monotherapy. Ann Pharmacother. 2002;36(4):641-643.
11. Patel DD, Galarneau D. Serotonin syndrome with fluoxetine: two case reports. Ochsner J. 2016;16(4):554-557.
12. Frank C. Recognition and treatment of serotonin syndrome. Can Fam Physician. 2008;54(7):988-992.
13. Zuschlag ZD, Warren MW, K Schultz S. Serotonin toxicity and urinary analgesics: a case report and systematic literature review of methylene blue-induced serotonin syndrome. Psychosomatics. 2018;59(6):539-546.
14. Kapur S, Zipursky RB, Jones C, et al. Cyproheptadine: a potent in vivo serotonin antagonist. Am J Psychiatry. 1997;154(6):884.
15. Baigel GD. Cyproheptadine and the treatment of an unconscious patient with the serotonin syndrome. Eur J Anaesthesiol. 2003;20(7):586-588.
16. Lappin RI, Auchincloss EL. Treatment of the serotonin syndrome with cyproheptadine. N Engl J Med. 1994;331:1021-1022.
17. McDaniel WW. Serotonin syndrome: early management with cyproheptadine. Ann Pharmacother. 2001;35(7-8):870-873.
18. Kolecki P. Venlafaxine induced serotonin syndrome occurring after abstinence from phenelzine for more than two weeks. J Toxicol Clin Toxicol. 1997;35(2):211-212.
1. Mason PJ, Morris VA, Balcezak TJ. Serotonin syndrome: presentation of 2 cases and review of literature. Medicine (Baltimore). 2000;79(4):201-209.
2. Boyer EW, Shannon M. The serotonin syndrome. N Engl J Med. 2005;352(11):1112-1120.
3. Sternbach H. The serotonin syndrome. Am J Psychiatry. 1991;148(6):705-713.
4. Graudins A, Stearman A, Chan B. Treatment of the serotonin syndrome with cyproheptadine. J Emerg Med. 1998;16(4):615-619.
5. Mills KC. Serotonin syndrome a clinical update. Crit Care Clin. 1997;13(4):763-783.
6. Dunkley EJ, Isbister GK, Sibbritt D, et al. The Hunter Serotonin Toxicity Criteria: simple and accurate diagnostic decision rules for serotonin toxicity. QJM. 2003;96:635-642.
7. Horowitz BZ, Mullins ME. Cyproheptadine for serotonin syndrome in an accidental pediatric sertraline ingestion. Pediatr Emerg Care. 1999;15(5):325-327.
8. Kolecki P. Isolated venlafaxine-induced serotonin syndrome. J Emerg Med. 1996;15:491-493.
9. Pan J, Shen W. Serotonin syndrome induced by low-dose venlafaxine. Ann Pharmacother. 2003;37(2):209-211.
10. Hernández JL, Ramos FJ, Infante J, et al. Severe serotonin syndrome induced by mirtazapine monotherapy. Ann Pharmacother. 2002;36(4):641-643.
11. Patel DD, Galarneau D. Serotonin syndrome with fluoxetine: two case reports. Ochsner J. 2016;16(4):554-557.
12. Frank C. Recognition and treatment of serotonin syndrome. Can Fam Physician. 2008;54(7):988-992.
13. Zuschlag ZD, Warren MW, K Schultz S. Serotonin toxicity and urinary analgesics: a case report and systematic literature review of methylene blue-induced serotonin syndrome. Psychosomatics. 2018;59(6):539-546.
14. Kapur S, Zipursky RB, Jones C, et al. Cyproheptadine: a potent in vivo serotonin antagonist. Am J Psychiatry. 1997;154(6):884.
15. Baigel GD. Cyproheptadine and the treatment of an unconscious patient with the serotonin syndrome. Eur J Anaesthesiol. 2003;20(7):586-588.
16. Lappin RI, Auchincloss EL. Treatment of the serotonin syndrome with cyproheptadine. N Engl J Med. 1994;331:1021-1022.
17. McDaniel WW. Serotonin syndrome: early management with cyproheptadine. Ann Pharmacother. 2001;35(7-8):870-873.
18. Kolecki P. Venlafaxine induced serotonin syndrome occurring after abstinence from phenelzine for more than two weeks. J Toxicol Clin Toxicol. 1997;35(2):211-212.
Young, angry, and in need of a liver transplant
CASE Rash, fever, extreme lethargy; multiple hospital visits
Ms. L, age 21, a single woman with a history of major depressive disorder (MDD), is directly admitted from an outside community hospital to our tertiary care academic hospital with acute liver failure.
One month earlier, Ms. L had an argument with her family and punched a wall, fracturing her hand. Following the episode, Ms. L’s primary care physician (PCP) prescribed valproic acid, 500 mg/d, to address “mood swings,” which included angry outbursts and irritability. According to her PCP, no baseline laboratory tests were ordered for Ms. L when she started valproic acid because she was young and otherwise healthy.
After Ms. L had been taking valproic acid for approximately 2 weeks, her mother noticed she became extremely lethargic and took her to the emergency department (ED) of a community hospital (Visit 1) (Table 1). At this time, her laboratory results were notable for an aspartate aminotransferase (AST) level of 303 IU/L (reference range: 8 to 40 IU/L) and an alanine aminotransferase (ALT) level of 241 IU/L (reference range: 20 to 60 IU/L). She also underwent a liver ultrasound, urine toxicology screen, blood alcohol level, and acetaminophen level; the results of all of these tests were unremarkable. Her valproic acid level was within therapeutic limits, consistent with patient adherence; her ammonia level was within normal limits. At Visit 1, Ms. L’s transaminitis was presumed to be secondary to valproic acid. The ED clinicians told her to stop taking valproic acid and discharged her. Her PCP did not give her any follow-up instructions for further laboratory tests or any other recommendations.
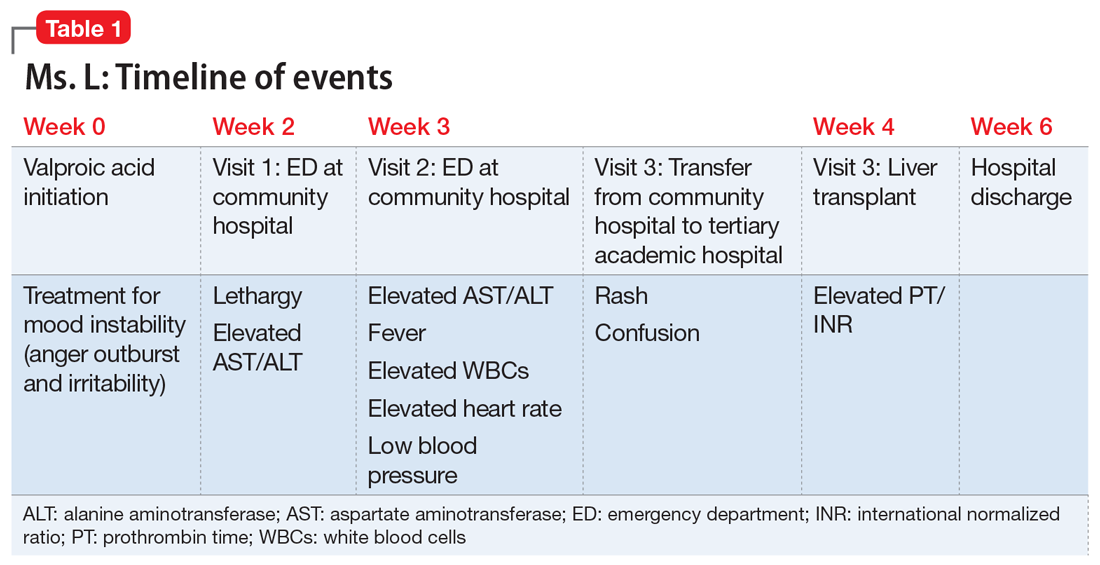
During the next week, even though she stopped taking the valproic acid as instructed, Ms. L developed a rash and fever, and continued to have lethargy and general malaise. When she returned to the ED (Visit 2) (Table 1), she was febrile, tachycardic, and hypotensive, with an elevated white blood cell count, eosinophilia, low platelets, and elevated liver function tests. At Visit 2, she was alert and oriented to person, place, time, and situation. Ms. L insisted that she had not overdosed on any medications, or used illicit drugs or alcohol. A test for hepatitis C was negative. Her ammonia level was 58 µmol/L (reference range: 11 to 32 µmol/L). Ms. L received N-acetylcysteine (NAC), prednisone, diphenhydramine, famotidine, and ibuprofen before she was transferred to our tertiary care hospital.
When she arrives at our facility (Visit 3) (Table 1), Ms. L is admitted with acute liver failure. She has an ALT level of 4,091 IU/L, and an AST level of 2,049 IU/L. Ms. L’s mother says that her daughter had been taking sertraline for depression for “some time” with no adverse effects, although she is not clear on the dose or frequency. Her mother says that Ms. L generally likes to spend most of her time at home, and does not believe her daughter is a danger to herself or others. Ms. L’s mother could not describe any episodes of mania or recurrent, dangerous anger episodes. Ms. L has no other medical history and has otherwise been healthy.
On hospital Day 2, Ms. L’s ammonia level is 72 µmol/L, which is slightly elevated. The hepatology team confirms that Ms. L may require a liver transplantation. The primary team consults the inpatient psychiatry consultation-liaison (C-L) team for a pre-transplant psychiatric evaluation.
[polldaddy:10307646]
The authors’ observations
The differential diagnosis for Ms. L was broad and included both accidental and intentional medication overdose. The primary team consulted the inpatient psychiatry C-L team not only for a pre-transplant evaluation, but also to assess for possible overdose.
Continue to: A review of the records...
A review of the records from Visit 1 and Visit 2 at the outside hospital found no acetaminophen in Ms. L’s system and verified that there was no evidence of a current valproic acid overdose. Ms. L had stated that she had not overdosed on any other medications or used any illicit drugs or alcohol. Ms. L’s complex symptoms—namely fever, acute liver failure, and rash—were more consistent with an adverse effect of valproic acid or possibly an inherent autoimmune process.
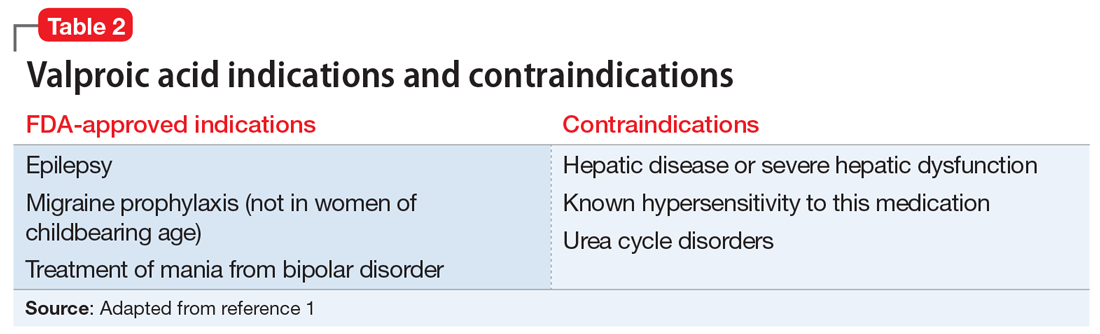
Liver damage from valproic acid
Valproic acid is FDA-approved for treating bipolar disorder, epilepsy, and migraine headaches1 (Table 21). Common adverse effects include nausea, vomiting, sleepiness, and dry mouth. Rarely, valproic acid can impair liver function. While receiving valproic acid, 5% to 10% of patients develop elevated ALT levels, but most are asymptomatic and resolve with time, even if the patient continues taking valproic acid.2 Valproic acid hepatotoxicity resulting in liver transplantation for a healthy patient is extremely rare (Table 31). Liver failure, both fatal and non-fatal, is more prevalent in patients concurrently taking other medications, such as antiepileptics, benzodiazepines, and antipsychotics, as compared with patients receiving only valproic acid.3
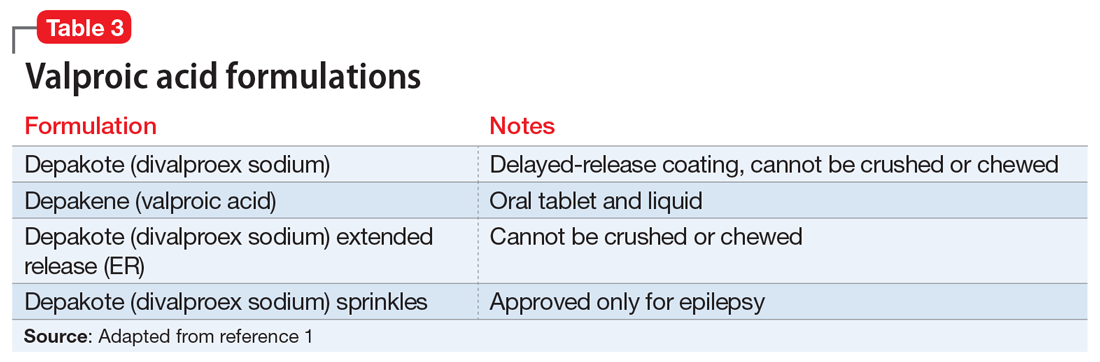
There are 3 clinically distinguishable forms of hepatotoxicity due to valproic acid2:
- hyperammonemia
- acute liver failure and jaundice
- Myriad ProReye-like syndrome, which is generally seen in children.
In case reports of hyperammonemia due to valproic acid, previously healthy patients experience confusion, lethargy, and eventual coma in the context of elevated serum ammonia levels; these symptoms resolved upon discontinuing valproic acid.4,5 Liver function remained normal, with normal to near-normal liver enzymes and bilirubin.3 Hyperammonemia and resulting encephalopathy generally occurred within 1 to 3 weeks after initiation of valproate therapy, with resolution of hyperammonemia and resulting symptoms within a few days after stopping valproic acid.2-4
At Visit 2, Ms. L’s presentation was not initially consistent with hepatic encephalopathy. She was alert and oriented to person, place, time, and situation. Additionally, Ms. L’s presenting problem was elevated liver function tests, not elevated ammonia levels. At Visit 2, her ammonia level was 58 µmol/L; on Day 2 (Visit 3) of her hospital stay, her ammonia level was 72 µmol/L (slightly elevated).
Continue to: At Visit 2 in the ED...
At Visit 2 in the ED, Ms. L was started on NAC because the team suspected she was experiencing drug rash with eosinophilia and systemic symptoms (DRESS) syndrome. This syndrome is characterized by extensive rash, fever, and involvement of at least 1 internal organ. It is a variation of a drug-induced hypersensitivity syndrome. Ms. L’s unremarkable valproic acid levels combined with the psychiatry assessment ruled out valproic hepatotoxicity due to overdose, either intentional or accidental.
In case reports, patients who developed acute liver failure due to valproic acid typically had a hepatitis-like syndrome consisting of moderate elevation in liver enzymes, jaundice, and liver failure necessitating transplantation after at least 1 month of treatment with valproic acid.2 In addition to the typical hepatitis-like syndrome resulting from valproic acid, case reports have also linked treatment with valproic acid to DRESS syndrome.2 This syndrome is known to occur with anticonvulsants such as phenobarbital, lamotrigine, and phenytoin, but there are only a few reported cases of DRESS syndrome due to valproic acid therapy alone.6 Drug rash with eosinophilia and systemic symptoms syndrome differs from other acute liver failure cases in that patients also develop lymphadenopathy, fever, and rash.2,6,7 Patients with DRESS syndrome typically respond to corticosteroid therapy and discontinuation of valproic acid, and the liver damage resolves after several weeks, without a need for transplantation.2,6,7
Ms. L seemed to have similarities to DRESS syndrome. However, the severity of her liver damage, which might require transplantation even after only 2 weeks of valproic acid therapy, initially led the hepatology and C-L teams to consider her presentation similar to severe hepatitis-like cases.
EVALUATION Consent for transplantation
As an inpatient, Ms. L undergoes further laboratory testing. Her hepatic function panel demonstrates a total protein level of 4.8 g/dL, an albumin level of 2.0 g/dL, total bilirubin level of 12.2 mg/dL, and alkaline phosphatase of 166 IU/L. Her laboratory results indicate a prothrombin time (PT) of 77.4 seconds, partial thromboplastin time of 61.5 seconds, and PT international normalized ratio (INR) of 9.6. Ms. L’s basic metabolic panel is within normal limits except for a blood urea nitrogen level of 6 mg/dL, glucose level of 136 mg/dL, and calcium level of 7.0 mg/dL. Her complete blood count indicates a white blood cell count of 12.1, hemoglobin of 10.3 g/dL, hematocrit of 30.4%, mean corpuscular volume of 85.9 fL, and platelet count of 84. Her lipase level is normal at 49 U/L. Her serum acetaminophen concentration is <3.0 mcg/mL, valproic acid level was <2 µg/mL, and she is negative for hepatitis A, B, and C. A urine toxicology screen and testing for herpes simplex, rapid plasma reagin, and human immunodeficiency virus are all negative. Results from several auto-antibodies tests are negative and within normal limits, except filamentous actin (F-actin) antibody, which is slightly higher than normal at 21.4 ELISA units. Based on these results, Ms. L’s liver failure seemed most likely secondary to a reaction to valproic acid.
During her pre-transplant psychiatric evaluation, Ms. L is found to be a poor historian with minimal speech production, flat affect, and clouded sensorium. She denies overdosing on her prescribed valproic acid or sertraline, reports no current suicidal ideation, and does not want to die. She accurately recalls her correct daily dosing of each medication, and verifies that she stopped taking valproic acid 2 weeks ago after being advised to do so by the ED clinicians at Visit 2. She continued to take sertraline until Visit 2. She denied any past or present episodes consistent with mania, which was consistent with her mother’s report.
Continue to: Ms. L becomes agitated...
Ms. L becomes agitated upon further questioning, and requests immediate discharge so that she can return to her family. The evaluation is postponed briefly.
When they reconvene, the C-L team performs a decision-making capacity evaluation, which reveals that Ms. L’s mood and affect are consistent with fear of her impending liver transplant and being alone and approximately 2 hours from her family. This is likely complicated by delirium due to hepatotoxicity. Further discussion between Ms. L and the multidisciplinary team focuses on the risks, benefits, adverse effects of, and alternatives to her current treatment; the possibility of needing a liver transplantation; and how to help her family with transportation to the hospital. Following the discussion, Ms. L is fully cooperative with further treatment, and the pre-transplant psychiatric evaluation is completed.
On physical examination, Ms. L is noted to have a widespread morbilliform rash covering 50% to 60% of her body.
[polldaddy:10307651]
The authors’ observations
L-carnitine supplementation
Multiple studies have shown that supplementation with L-carnitine may increase survival from severe hepatotoxicity due to valproic acid.8,9 Valproic acid may contribute to carnitine deficiency due to its inhibition of carnitine biosynthesis via a decrease in alpha-ketoglutarate concentration.8 Hepatotoxicity or hyperammonemia due to valproic acid may be potentiated by a carnitine deficiency, either pre-existing or resulting from valproic acid.8 L-carnitine supplementation has hastened the decrease of valproic acid–induced ammonemia in a dose-dependent manner,10 and it is currently recommended in cases of valproic acid toxicity, especially in children.8 Children at high risk for developing carnitine deficiency who need to receive valproic acid can be given carnitine supplementation.11 It is not known whether L-carnitine is clinically effective in protecting the liver or hastening liver recovery,8 but it is believed that it might prevent adverse effects of hepatotoxicity and hyperammonemia, especially in patients who receive long-term valproic acid therapy.12
TREATMENT Decompensation and transplantation
Ms. L’s treatment regimen includes NAC, lactulose, and L-carnitine supplementation. During the course of Ms. L’s hospital stay, her liver enzymes begin to trend downward, but her INR and PT remain elevated.
Continue to: On hospital Day 6...
On hospital Day 6, she develops more severe symptoms of hepatic encephalopathy, with significant altered mental status and inattention. Ms. L is transferred to the ICU, intubated, and placed on the liver transplant list.
On hospital Day 9, she undergoes a liver transplantation.
[polldaddy:10307652]
The authors’ observations
Baseline laboratory testing should have been conducted prior to initiating valproic acid. As Ms. L’s symptoms worsened, better communication with her PCP and closer monitoring after starting valproic acid might have resulted in more immediate care. Early recognition of her symptoms and decompensation may have triggered earlier inpatient admission and/or transfer to a tertiary care facility for observation and treatment. Additionally, repeat laboratory testing and instructions on when to return to the ED should have been provided at Visit 1.
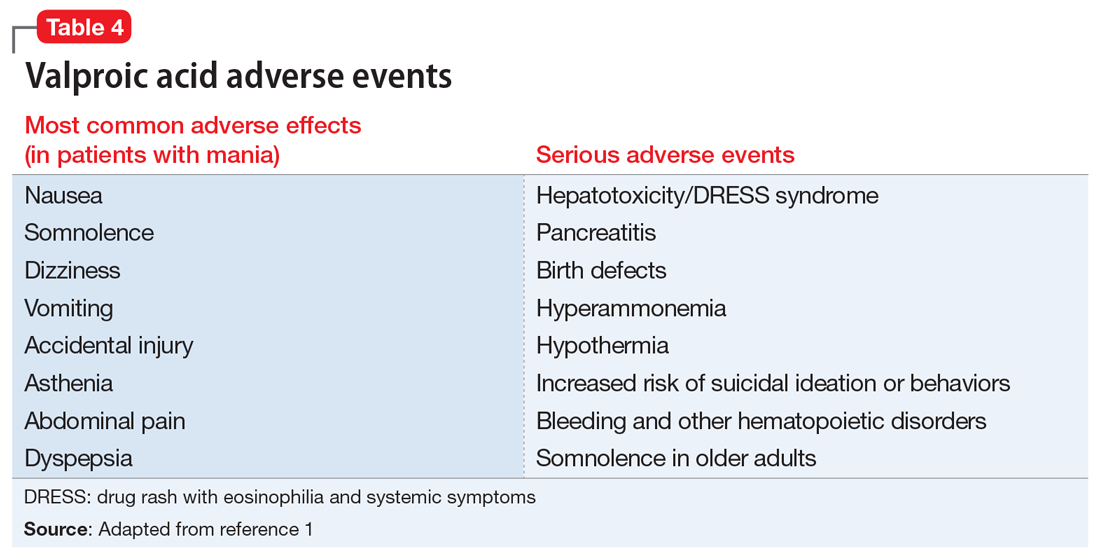
This case demonstrates the need for all clinicians who prescribe valproic acid to remain diligent about the accurate diagnosis of mood and behavioral symptoms, knowing when psychotropic medications are indicated, and carefully considering and discussing even rare, potentially life-threatening adverse effects of all medications with patients.
Although rare, after starting valproic acid, a patient may experience a rapid decompensation and life-threatening illness. Ideally, clinicians should closely monitor patients after initiating valproic acid (Table 41). Clinicians must have a clear knowledge of the recommended monitoring and indications for hospitalization and treatment when they note adverse effects such as elevated liver enzymes or transaminitis (Table 513,14). Even after stopping valproic acid, patients who have experienced adverse events should be closely monitored to ensure complete resolution.
Continue to: Consider patient-specific factors
Consider patient-specific factors
Consider the mental state, intellectual capacity, and social support of each patient before initiating valproic acid. Its use as a mood stabilizer for “mood swings” outside of the context of bipolar disorder is questionable. Valproic acid is FDA-approved for treating bipolar disorder and seizures, but not for anger outbursts/irritability. Prior to starting valproic acid, Ms. L may have benefited from alternative nonpharmacologic treatments, such as psychotherapy, for her anger outbursts and poor coping skills. Therapeutic techniques that focused on helping her acquire better coping mechanisms may have been useful, especially because her mood symptoms did not meet criteria for bipolar disorder, and her depression had long been controlled with sertraline monotherapy.
OUTCOME Discharged after 20 days
Ms. L stays in the hospital for 10 days after receiving her liver transplant. She has low appetite and some difficulty with sleep after the transplant; therefore, the C-L team recommends mirtazapine, 15 mg/d. She has no behavioral problems during her stay, and is set up with home health, case management, and psychiatry follow-up. On hospital Day 20, she is discharged.
Bottom Line
Use caution when prescribing valproic acid, even in young, otherwise healthy patients. Although rare, some patients may experience a rapid decompensation and life-threatening illness after starting valproic acid. When prescribing valproic acid, ensure close follow-up after initiation, including mental status examinations, physical examinations, and laboratory testing.
Related Resource
- Doroudgar S, Chou TI. How to modify psychotropic therapy for patients who have liver dysfunction. Current Psychiatry. 2014;13(12):46-49.
Drug Brand Names
Diphenhydramine • Benadryl
Famotidine • Fluxid, Pepcid
Lamotrigine • Lamictal
Mirtazapine • Remeron
N-acetylcysteine • Mucomyst
Phenobarbital • Luminal
Phenytoin • Dilantin
Prednisone • Cortan, Deltasone
Sertraline • Zoloft
Valproic acid • Depakene
1. Depakote [package insert]. North Chicago, IL: AbbVie, Inc.; 2019.
2. National Institutes of Health. U.S. Department of Health and Human Services. Drug Record: Valproate. https://livertox.nlm.nih.gov/Valproate.htm. Updated October 30, 2018. Accessed March 21, 2019.
3. Schmid MM, Freudenmann RW, Keller F, et al. Non-fatal and fatal liver failure associated with valproic acid. Pharmacopsychiatry. 2013;46(2):63-68.
4. Patel N, Landry KB, Fargason RE, et al. Reversible encephalopathy due to valproic acid induced hyperammonemia in a patient with Bipolar I disorder: a cautionary report. Psychopharmacol Bull. 2017;47(1):40-44.
5. Eze E, Workman M, Donley B. Hyperammonemia and coma developed by a woman treated with valproic acid for affective disorder. Psychiatr Serv. 1998;49(10):1358-1359.
6. Darban M and Bagheri B. Drug reaction with eosinophilia and systemic symptoms induced by valproic acid: a case report. Iran Red Crescent Med J. 2016;18(9): e35825.
7. van Zoelen MA, de Graaf M, van Dijk MR, et al. Valproic acid-induced DRESS syndrome with acute liver failure. Neth J Med. 2012;70(3):155.
8. Lheureux PE, Hantson P. Carnitine in the treatment of valproic acid-induced toxicity. Clin Toxicol (Phila). 2009;47(2):101-111.
9. Bohan TP, Helton E, McDonald I, et al. Effect of L-carnitine treatment for valproate-induced hepatotoxicity. Neurology. 2001;56(10):1405-1409.
10. Böhles H, Sewell AC, Wenzel D. The effect of carnitine supplementation in valproate-induced hyperammonaemia. Acta Paediatr. 1996;85(4):446-449.
11. Raskind JY, El-Chaar GM. The role of carnitine supplementation during valproic acid therapy. Ann Pharmacother. 2000;34(5):630-638.
12. Romero-Falcón A, de la Santa-Belda E, García-Contreras R, et al. A case of valproate-associated hepatotoxicity treated with L-carnitine. Eur J Intern Med. 2003;14(5):338-340.
13. National Institute for Health and Clinical Excellence. Bipolar disorder: the management of bipolar disorder in adults, children, and adolescents, in primary and secondary care. https://www.nice.org.uk/guidance/cg185. Updated April 2018. Accessed March 21, 2019.
14 . Hirschfeld RMA, Bowden CL, Gitlin MJ, et al. Practice guideline for the treatment of patients with biopolar disorder: second edition. American Psychiatric Association. https://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/bipolar.pdf. Published 2002. Accessed March 21, 2019.
CASE Rash, fever, extreme lethargy; multiple hospital visits
Ms. L, age 21, a single woman with a history of major depressive disorder (MDD), is directly admitted from an outside community hospital to our tertiary care academic hospital with acute liver failure.
One month earlier, Ms. L had an argument with her family and punched a wall, fracturing her hand. Following the episode, Ms. L’s primary care physician (PCP) prescribed valproic acid, 500 mg/d, to address “mood swings,” which included angry outbursts and irritability. According to her PCP, no baseline laboratory tests were ordered for Ms. L when she started valproic acid because she was young and otherwise healthy.
After Ms. L had been taking valproic acid for approximately 2 weeks, her mother noticed she became extremely lethargic and took her to the emergency department (ED) of a community hospital (Visit 1) (Table 1). At this time, her laboratory results were notable for an aspartate aminotransferase (AST) level of 303 IU/L (reference range: 8 to 40 IU/L) and an alanine aminotransferase (ALT) level of 241 IU/L (reference range: 20 to 60 IU/L). She also underwent a liver ultrasound, urine toxicology screen, blood alcohol level, and acetaminophen level; the results of all of these tests were unremarkable. Her valproic acid level was within therapeutic limits, consistent with patient adherence; her ammonia level was within normal limits. At Visit 1, Ms. L’s transaminitis was presumed to be secondary to valproic acid. The ED clinicians told her to stop taking valproic acid and discharged her. Her PCP did not give her any follow-up instructions for further laboratory tests or any other recommendations.

During the next week, even though she stopped taking the valproic acid as instructed, Ms. L developed a rash and fever, and continued to have lethargy and general malaise. When she returned to the ED (Visit 2) (Table 1), she was febrile, tachycardic, and hypotensive, with an elevated white blood cell count, eosinophilia, low platelets, and elevated liver function tests. At Visit 2, she was alert and oriented to person, place, time, and situation. Ms. L insisted that she had not overdosed on any medications, or used illicit drugs or alcohol. A test for hepatitis C was negative. Her ammonia level was 58 µmol/L (reference range: 11 to 32 µmol/L). Ms. L received N-acetylcysteine (NAC), prednisone, diphenhydramine, famotidine, and ibuprofen before she was transferred to our tertiary care hospital.
When she arrives at our facility (Visit 3) (Table 1), Ms. L is admitted with acute liver failure. She has an ALT level of 4,091 IU/L, and an AST level of 2,049 IU/L. Ms. L’s mother says that her daughter had been taking sertraline for depression for “some time” with no adverse effects, although she is not clear on the dose or frequency. Her mother says that Ms. L generally likes to spend most of her time at home, and does not believe her daughter is a danger to herself or others. Ms. L’s mother could not describe any episodes of mania or recurrent, dangerous anger episodes. Ms. L has no other medical history and has otherwise been healthy.
On hospital Day 2, Ms. L’s ammonia level is 72 µmol/L, which is slightly elevated. The hepatology team confirms that Ms. L may require a liver transplantation. The primary team consults the inpatient psychiatry consultation-liaison (C-L) team for a pre-transplant psychiatric evaluation.
[polldaddy:10307646]
The authors’ observations
The differential diagnosis for Ms. L was broad and included both accidental and intentional medication overdose. The primary team consulted the inpatient psychiatry C-L team not only for a pre-transplant evaluation, but also to assess for possible overdose.
Continue to: A review of the records...
A review of the records from Visit 1 and Visit 2 at the outside hospital found no acetaminophen in Ms. L’s system and verified that there was no evidence of a current valproic acid overdose. Ms. L had stated that she had not overdosed on any other medications or used any illicit drugs or alcohol. Ms. L’s complex symptoms—namely fever, acute liver failure, and rash—were more consistent with an adverse effect of valproic acid or possibly an inherent autoimmune process.

Liver damage from valproic acid
Valproic acid is FDA-approved for treating bipolar disorder, epilepsy, and migraine headaches1 (Table 21). Common adverse effects include nausea, vomiting, sleepiness, and dry mouth. Rarely, valproic acid can impair liver function. While receiving valproic acid, 5% to 10% of patients develop elevated ALT levels, but most are asymptomatic and resolve with time, even if the patient continues taking valproic acid.2 Valproic acid hepatotoxicity resulting in liver transplantation for a healthy patient is extremely rare (Table 31). Liver failure, both fatal and non-fatal, is more prevalent in patients concurrently taking other medications, such as antiepileptics, benzodiazepines, and antipsychotics, as compared with patients receiving only valproic acid.3

There are 3 clinically distinguishable forms of hepatotoxicity due to valproic acid2:
- hyperammonemia
- acute liver failure and jaundice
- Myriad ProReye-like syndrome, which is generally seen in children.
In case reports of hyperammonemia due to valproic acid, previously healthy patients experience confusion, lethargy, and eventual coma in the context of elevated serum ammonia levels; these symptoms resolved upon discontinuing valproic acid.4,5 Liver function remained normal, with normal to near-normal liver enzymes and bilirubin.3 Hyperammonemia and resulting encephalopathy generally occurred within 1 to 3 weeks after initiation of valproate therapy, with resolution of hyperammonemia and resulting symptoms within a few days after stopping valproic acid.2-4
At Visit 2, Ms. L’s presentation was not initially consistent with hepatic encephalopathy. She was alert and oriented to person, place, time, and situation. Additionally, Ms. L’s presenting problem was elevated liver function tests, not elevated ammonia levels. At Visit 2, her ammonia level was 58 µmol/L; on Day 2 (Visit 3) of her hospital stay, her ammonia level was 72 µmol/L (slightly elevated).
Continue to: At Visit 2 in the ED...
At Visit 2 in the ED, Ms. L was started on NAC because the team suspected she was experiencing drug rash with eosinophilia and systemic symptoms (DRESS) syndrome. This syndrome is characterized by extensive rash, fever, and involvement of at least 1 internal organ. It is a variation of a drug-induced hypersensitivity syndrome. Ms. L’s unremarkable valproic acid levels combined with the psychiatry assessment ruled out valproic hepatotoxicity due to overdose, either intentional or accidental.
In case reports, patients who developed acute liver failure due to valproic acid typically had a hepatitis-like syndrome consisting of moderate elevation in liver enzymes, jaundice, and liver failure necessitating transplantation after at least 1 month of treatment with valproic acid.2 In addition to the typical hepatitis-like syndrome resulting from valproic acid, case reports have also linked treatment with valproic acid to DRESS syndrome.2 This syndrome is known to occur with anticonvulsants such as phenobarbital, lamotrigine, and phenytoin, but there are only a few reported cases of DRESS syndrome due to valproic acid therapy alone.6 Drug rash with eosinophilia and systemic symptoms syndrome differs from other acute liver failure cases in that patients also develop lymphadenopathy, fever, and rash.2,6,7 Patients with DRESS syndrome typically respond to corticosteroid therapy and discontinuation of valproic acid, and the liver damage resolves after several weeks, without a need for transplantation.2,6,7
Ms. L seemed to have similarities to DRESS syndrome. However, the severity of her liver damage, which might require transplantation even after only 2 weeks of valproic acid therapy, initially led the hepatology and C-L teams to consider her presentation similar to severe hepatitis-like cases.
EVALUATION Consent for transplantation
As an inpatient, Ms. L undergoes further laboratory testing. Her hepatic function panel demonstrates a total protein level of 4.8 g/dL, an albumin level of 2.0 g/dL, total bilirubin level of 12.2 mg/dL, and alkaline phosphatase of 166 IU/L. Her laboratory results indicate a prothrombin time (PT) of 77.4 seconds, partial thromboplastin time of 61.5 seconds, and PT international normalized ratio (INR) of 9.6. Ms. L’s basic metabolic panel is within normal limits except for a blood urea nitrogen level of 6 mg/dL, glucose level of 136 mg/dL, and calcium level of 7.0 mg/dL. Her complete blood count indicates a white blood cell count of 12.1, hemoglobin of 10.3 g/dL, hematocrit of 30.4%, mean corpuscular volume of 85.9 fL, and platelet count of 84. Her lipase level is normal at 49 U/L. Her serum acetaminophen concentration is <3.0 mcg/mL, valproic acid level was <2 µg/mL, and she is negative for hepatitis A, B, and C. A urine toxicology screen and testing for herpes simplex, rapid plasma reagin, and human immunodeficiency virus are all negative. Results from several auto-antibodies tests are negative and within normal limits, except filamentous actin (F-actin) antibody, which is slightly higher than normal at 21.4 ELISA units. Based on these results, Ms. L’s liver failure seemed most likely secondary to a reaction to valproic acid.
During her pre-transplant psychiatric evaluation, Ms. L is found to be a poor historian with minimal speech production, flat affect, and clouded sensorium. She denies overdosing on her prescribed valproic acid or sertraline, reports no current suicidal ideation, and does not want to die. She accurately recalls her correct daily dosing of each medication, and verifies that she stopped taking valproic acid 2 weeks ago after being advised to do so by the ED clinicians at Visit 2. She continued to take sertraline until Visit 2. She denied any past or present episodes consistent with mania, which was consistent with her mother’s report.
Continue to: Ms. L becomes agitated...
Ms. L becomes agitated upon further questioning, and requests immediate discharge so that she can return to her family. The evaluation is postponed briefly.
When they reconvene, the C-L team performs a decision-making capacity evaluation, which reveals that Ms. L’s mood and affect are consistent with fear of her impending liver transplant and being alone and approximately 2 hours from her family. This is likely complicated by delirium due to hepatotoxicity. Further discussion between Ms. L and the multidisciplinary team focuses on the risks, benefits, adverse effects of, and alternatives to her current treatment; the possibility of needing a liver transplantation; and how to help her family with transportation to the hospital. Following the discussion, Ms. L is fully cooperative with further treatment, and the pre-transplant psychiatric evaluation is completed.
On physical examination, Ms. L is noted to have a widespread morbilliform rash covering 50% to 60% of her body.
[polldaddy:10307651]
The authors’ observations
L-carnitine supplementation
Multiple studies have shown that supplementation with L-carnitine may increase survival from severe hepatotoxicity due to valproic acid.8,9 Valproic acid may contribute to carnitine deficiency due to its inhibition of carnitine biosynthesis via a decrease in alpha-ketoglutarate concentration.8 Hepatotoxicity or hyperammonemia due to valproic acid may be potentiated by a carnitine deficiency, either pre-existing or resulting from valproic acid.8 L-carnitine supplementation has hastened the decrease of valproic acid–induced ammonemia in a dose-dependent manner,10 and it is currently recommended in cases of valproic acid toxicity, especially in children.8 Children at high risk for developing carnitine deficiency who need to receive valproic acid can be given carnitine supplementation.11 It is not known whether L-carnitine is clinically effective in protecting the liver or hastening liver recovery,8 but it is believed that it might prevent adverse effects of hepatotoxicity and hyperammonemia, especially in patients who receive long-term valproic acid therapy.12
TREATMENT Decompensation and transplantation
Ms. L’s treatment regimen includes NAC, lactulose, and L-carnitine supplementation. During the course of Ms. L’s hospital stay, her liver enzymes begin to trend downward, but her INR and PT remain elevated.
Continue to: On hospital Day 6...
On hospital Day 6, she develops more severe symptoms of hepatic encephalopathy, with significant altered mental status and inattention. Ms. L is transferred to the ICU, intubated, and placed on the liver transplant list.
On hospital Day 9, she undergoes a liver transplantation.
[polldaddy:10307652]
The authors’ observations
Baseline laboratory testing should have been conducted prior to initiating valproic acid. As Ms. L’s symptoms worsened, better communication with her PCP and closer monitoring after starting valproic acid might have resulted in more immediate care. Early recognition of her symptoms and decompensation may have triggered earlier inpatient admission and/or transfer to a tertiary care facility for observation and treatment. Additionally, repeat laboratory testing and instructions on when to return to the ED should have been provided at Visit 1.

This case demonstrates the need for all clinicians who prescribe valproic acid to remain diligent about the accurate diagnosis of mood and behavioral symptoms, knowing when psychotropic medications are indicated, and carefully considering and discussing even rare, potentially life-threatening adverse effects of all medications with patients.
Although rare, after starting valproic acid, a patient may experience a rapid decompensation and life-threatening illness. Ideally, clinicians should closely monitor patients after initiating valproic acid (Table 41). Clinicians must have a clear knowledge of the recommended monitoring and indications for hospitalization and treatment when they note adverse effects such as elevated liver enzymes or transaminitis (Table 513,14). Even after stopping valproic acid, patients who have experienced adverse events should be closely monitored to ensure complete resolution.
Continue to: Consider patient-specific factors
Consider patient-specific factors
Consider the mental state, intellectual capacity, and social support of each patient before initiating valproic acid. Its use as a mood stabilizer for “mood swings” outside of the context of bipolar disorder is questionable. Valproic acid is FDA-approved for treating bipolar disorder and seizures, but not for anger outbursts/irritability. Prior to starting valproic acid, Ms. L may have benefited from alternative nonpharmacologic treatments, such as psychotherapy, for her anger outbursts and poor coping skills. Therapeutic techniques that focused on helping her acquire better coping mechanisms may have been useful, especially because her mood symptoms did not meet criteria for bipolar disorder, and her depression had long been controlled with sertraline monotherapy.
OUTCOME Discharged after 20 days
Ms. L stays in the hospital for 10 days after receiving her liver transplant. She has low appetite and some difficulty with sleep after the transplant; therefore, the C-L team recommends mirtazapine, 15 mg/d. She has no behavioral problems during her stay, and is set up with home health, case management, and psychiatry follow-up. On hospital Day 20, she is discharged.
Bottom Line
Use caution when prescribing valproic acid, even in young, otherwise healthy patients. Although rare, some patients may experience a rapid decompensation and life-threatening illness after starting valproic acid. When prescribing valproic acid, ensure close follow-up after initiation, including mental status examinations, physical examinations, and laboratory testing.
Related Resource
- Doroudgar S, Chou TI. How to modify psychotropic therapy for patients who have liver dysfunction. Current Psychiatry. 2014;13(12):46-49.
Drug Brand Names
Diphenhydramine • Benadryl
Famotidine • Fluxid, Pepcid
Lamotrigine • Lamictal
Mirtazapine • Remeron
N-acetylcysteine • Mucomyst
Phenobarbital • Luminal
Phenytoin • Dilantin
Prednisone • Cortan, Deltasone
Sertraline • Zoloft
Valproic acid • Depakene
CASE Rash, fever, extreme lethargy; multiple hospital visits
Ms. L, age 21, a single woman with a history of major depressive disorder (MDD), is directly admitted from an outside community hospital to our tertiary care academic hospital with acute liver failure.
One month earlier, Ms. L had an argument with her family and punched a wall, fracturing her hand. Following the episode, Ms. L’s primary care physician (PCP) prescribed valproic acid, 500 mg/d, to address “mood swings,” which included angry outbursts and irritability. According to her PCP, no baseline laboratory tests were ordered for Ms. L when she started valproic acid because she was young and otherwise healthy.
After Ms. L had been taking valproic acid for approximately 2 weeks, her mother noticed she became extremely lethargic and took her to the emergency department (ED) of a community hospital (Visit 1) (Table 1). At this time, her laboratory results were notable for an aspartate aminotransferase (AST) level of 303 IU/L (reference range: 8 to 40 IU/L) and an alanine aminotransferase (ALT) level of 241 IU/L (reference range: 20 to 60 IU/L). She also underwent a liver ultrasound, urine toxicology screen, blood alcohol level, and acetaminophen level; the results of all of these tests were unremarkable. Her valproic acid level was within therapeutic limits, consistent with patient adherence; her ammonia level was within normal limits. At Visit 1, Ms. L’s transaminitis was presumed to be secondary to valproic acid. The ED clinicians told her to stop taking valproic acid and discharged her. Her PCP did not give her any follow-up instructions for further laboratory tests or any other recommendations.

During the next week, even though she stopped taking the valproic acid as instructed, Ms. L developed a rash and fever, and continued to have lethargy and general malaise. When she returned to the ED (Visit 2) (Table 1), she was febrile, tachycardic, and hypotensive, with an elevated white blood cell count, eosinophilia, low platelets, and elevated liver function tests. At Visit 2, she was alert and oriented to person, place, time, and situation. Ms. L insisted that she had not overdosed on any medications, or used illicit drugs or alcohol. A test for hepatitis C was negative. Her ammonia level was 58 µmol/L (reference range: 11 to 32 µmol/L). Ms. L received N-acetylcysteine (NAC), prednisone, diphenhydramine, famotidine, and ibuprofen before she was transferred to our tertiary care hospital.
When she arrives at our facility (Visit 3) (Table 1), Ms. L is admitted with acute liver failure. She has an ALT level of 4,091 IU/L, and an AST level of 2,049 IU/L. Ms. L’s mother says that her daughter had been taking sertraline for depression for “some time” with no adverse effects, although she is not clear on the dose or frequency. Her mother says that Ms. L generally likes to spend most of her time at home, and does not believe her daughter is a danger to herself or others. Ms. L’s mother could not describe any episodes of mania or recurrent, dangerous anger episodes. Ms. L has no other medical history and has otherwise been healthy.
On hospital Day 2, Ms. L’s ammonia level is 72 µmol/L, which is slightly elevated. The hepatology team confirms that Ms. L may require a liver transplantation. The primary team consults the inpatient psychiatry consultation-liaison (C-L) team for a pre-transplant psychiatric evaluation.
[polldaddy:10307646]
The authors’ observations
The differential diagnosis for Ms. L was broad and included both accidental and intentional medication overdose. The primary team consulted the inpatient psychiatry C-L team not only for a pre-transplant evaluation, but also to assess for possible overdose.
Continue to: A review of the records...
A review of the records from Visit 1 and Visit 2 at the outside hospital found no acetaminophen in Ms. L’s system and verified that there was no evidence of a current valproic acid overdose. Ms. L had stated that she had not overdosed on any other medications or used any illicit drugs or alcohol. Ms. L’s complex symptoms—namely fever, acute liver failure, and rash—were more consistent with an adverse effect of valproic acid or possibly an inherent autoimmune process.

Liver damage from valproic acid
Valproic acid is FDA-approved for treating bipolar disorder, epilepsy, and migraine headaches1 (Table 21). Common adverse effects include nausea, vomiting, sleepiness, and dry mouth. Rarely, valproic acid can impair liver function. While receiving valproic acid, 5% to 10% of patients develop elevated ALT levels, but most are asymptomatic and resolve with time, even if the patient continues taking valproic acid.2 Valproic acid hepatotoxicity resulting in liver transplantation for a healthy patient is extremely rare (Table 31). Liver failure, both fatal and non-fatal, is more prevalent in patients concurrently taking other medications, such as antiepileptics, benzodiazepines, and antipsychotics, as compared with patients receiving only valproic acid.3

There are 3 clinically distinguishable forms of hepatotoxicity due to valproic acid2:
- hyperammonemia
- acute liver failure and jaundice
- Myriad ProReye-like syndrome, which is generally seen in children.
In case reports of hyperammonemia due to valproic acid, previously healthy patients experience confusion, lethargy, and eventual coma in the context of elevated serum ammonia levels; these symptoms resolved upon discontinuing valproic acid.4,5 Liver function remained normal, with normal to near-normal liver enzymes and bilirubin.3 Hyperammonemia and resulting encephalopathy generally occurred within 1 to 3 weeks after initiation of valproate therapy, with resolution of hyperammonemia and resulting symptoms within a few days after stopping valproic acid.2-4
At Visit 2, Ms. L’s presentation was not initially consistent with hepatic encephalopathy. She was alert and oriented to person, place, time, and situation. Additionally, Ms. L’s presenting problem was elevated liver function tests, not elevated ammonia levels. At Visit 2, her ammonia level was 58 µmol/L; on Day 2 (Visit 3) of her hospital stay, her ammonia level was 72 µmol/L (slightly elevated).
Continue to: At Visit 2 in the ED...
At Visit 2 in the ED, Ms. L was started on NAC because the team suspected she was experiencing drug rash with eosinophilia and systemic symptoms (DRESS) syndrome. This syndrome is characterized by extensive rash, fever, and involvement of at least 1 internal organ. It is a variation of a drug-induced hypersensitivity syndrome. Ms. L’s unremarkable valproic acid levels combined with the psychiatry assessment ruled out valproic hepatotoxicity due to overdose, either intentional or accidental.
In case reports, patients who developed acute liver failure due to valproic acid typically had a hepatitis-like syndrome consisting of moderate elevation in liver enzymes, jaundice, and liver failure necessitating transplantation after at least 1 month of treatment with valproic acid.2 In addition to the typical hepatitis-like syndrome resulting from valproic acid, case reports have also linked treatment with valproic acid to DRESS syndrome.2 This syndrome is known to occur with anticonvulsants such as phenobarbital, lamotrigine, and phenytoin, but there are only a few reported cases of DRESS syndrome due to valproic acid therapy alone.6 Drug rash with eosinophilia and systemic symptoms syndrome differs from other acute liver failure cases in that patients also develop lymphadenopathy, fever, and rash.2,6,7 Patients with DRESS syndrome typically respond to corticosteroid therapy and discontinuation of valproic acid, and the liver damage resolves after several weeks, without a need for transplantation.2,6,7
Ms. L seemed to have similarities to DRESS syndrome. However, the severity of her liver damage, which might require transplantation even after only 2 weeks of valproic acid therapy, initially led the hepatology and C-L teams to consider her presentation similar to severe hepatitis-like cases.
EVALUATION Consent for transplantation
As an inpatient, Ms. L undergoes further laboratory testing. Her hepatic function panel demonstrates a total protein level of 4.8 g/dL, an albumin level of 2.0 g/dL, total bilirubin level of 12.2 mg/dL, and alkaline phosphatase of 166 IU/L. Her laboratory results indicate a prothrombin time (PT) of 77.4 seconds, partial thromboplastin time of 61.5 seconds, and PT international normalized ratio (INR) of 9.6. Ms. L’s basic metabolic panel is within normal limits except for a blood urea nitrogen level of 6 mg/dL, glucose level of 136 mg/dL, and calcium level of 7.0 mg/dL. Her complete blood count indicates a white blood cell count of 12.1, hemoglobin of 10.3 g/dL, hematocrit of 30.4%, mean corpuscular volume of 85.9 fL, and platelet count of 84. Her lipase level is normal at 49 U/L. Her serum acetaminophen concentration is <3.0 mcg/mL, valproic acid level was <2 µg/mL, and she is negative for hepatitis A, B, and C. A urine toxicology screen and testing for herpes simplex, rapid plasma reagin, and human immunodeficiency virus are all negative. Results from several auto-antibodies tests are negative and within normal limits, except filamentous actin (F-actin) antibody, which is slightly higher than normal at 21.4 ELISA units. Based on these results, Ms. L’s liver failure seemed most likely secondary to a reaction to valproic acid.
During her pre-transplant psychiatric evaluation, Ms. L is found to be a poor historian with minimal speech production, flat affect, and clouded sensorium. She denies overdosing on her prescribed valproic acid or sertraline, reports no current suicidal ideation, and does not want to die. She accurately recalls her correct daily dosing of each medication, and verifies that she stopped taking valproic acid 2 weeks ago after being advised to do so by the ED clinicians at Visit 2. She continued to take sertraline until Visit 2. She denied any past or present episodes consistent with mania, which was consistent with her mother’s report.
Continue to: Ms. L becomes agitated...
Ms. L becomes agitated upon further questioning, and requests immediate discharge so that she can return to her family. The evaluation is postponed briefly.
When they reconvene, the C-L team performs a decision-making capacity evaluation, which reveals that Ms. L’s mood and affect are consistent with fear of her impending liver transplant and being alone and approximately 2 hours from her family. This is likely complicated by delirium due to hepatotoxicity. Further discussion between Ms. L and the multidisciplinary team focuses on the risks, benefits, adverse effects of, and alternatives to her current treatment; the possibility of needing a liver transplantation; and how to help her family with transportation to the hospital. Following the discussion, Ms. L is fully cooperative with further treatment, and the pre-transplant psychiatric evaluation is completed.
On physical examination, Ms. L is noted to have a widespread morbilliform rash covering 50% to 60% of her body.
[polldaddy:10307651]
The authors’ observations
L-carnitine supplementation
Multiple studies have shown that supplementation with L-carnitine may increase survival from severe hepatotoxicity due to valproic acid.8,9 Valproic acid may contribute to carnitine deficiency due to its inhibition of carnitine biosynthesis via a decrease in alpha-ketoglutarate concentration.8 Hepatotoxicity or hyperammonemia due to valproic acid may be potentiated by a carnitine deficiency, either pre-existing or resulting from valproic acid.8 L-carnitine supplementation has hastened the decrease of valproic acid–induced ammonemia in a dose-dependent manner,10 and it is currently recommended in cases of valproic acid toxicity, especially in children.8 Children at high risk for developing carnitine deficiency who need to receive valproic acid can be given carnitine supplementation.11 It is not known whether L-carnitine is clinically effective in protecting the liver or hastening liver recovery,8 but it is believed that it might prevent adverse effects of hepatotoxicity and hyperammonemia, especially in patients who receive long-term valproic acid therapy.12
TREATMENT Decompensation and transplantation
Ms. L’s treatment regimen includes NAC, lactulose, and L-carnitine supplementation. During the course of Ms. L’s hospital stay, her liver enzymes begin to trend downward, but her INR and PT remain elevated.
Continue to: On hospital Day 6...
On hospital Day 6, she develops more severe symptoms of hepatic encephalopathy, with significant altered mental status and inattention. Ms. L is transferred to the ICU, intubated, and placed on the liver transplant list.
On hospital Day 9, she undergoes a liver transplantation.
[polldaddy:10307652]
The authors’ observations
Baseline laboratory testing should have been conducted prior to initiating valproic acid. As Ms. L’s symptoms worsened, better communication with her PCP and closer monitoring after starting valproic acid might have resulted in more immediate care. Early recognition of her symptoms and decompensation may have triggered earlier inpatient admission and/or transfer to a tertiary care facility for observation and treatment. Additionally, repeat laboratory testing and instructions on when to return to the ED should have been provided at Visit 1.

This case demonstrates the need for all clinicians who prescribe valproic acid to remain diligent about the accurate diagnosis of mood and behavioral symptoms, knowing when psychotropic medications are indicated, and carefully considering and discussing even rare, potentially life-threatening adverse effects of all medications with patients.
Although rare, after starting valproic acid, a patient may experience a rapid decompensation and life-threatening illness. Ideally, clinicians should closely monitor patients after initiating valproic acid (Table 41). Clinicians must have a clear knowledge of the recommended monitoring and indications for hospitalization and treatment when they note adverse effects such as elevated liver enzymes or transaminitis (Table 513,14). Even after stopping valproic acid, patients who have experienced adverse events should be closely monitored to ensure complete resolution.
Continue to: Consider patient-specific factors
Consider patient-specific factors
Consider the mental state, intellectual capacity, and social support of each patient before initiating valproic acid. Its use as a mood stabilizer for “mood swings” outside of the context of bipolar disorder is questionable. Valproic acid is FDA-approved for treating bipolar disorder and seizures, but not for anger outbursts/irritability. Prior to starting valproic acid, Ms. L may have benefited from alternative nonpharmacologic treatments, such as psychotherapy, for her anger outbursts and poor coping skills. Therapeutic techniques that focused on helping her acquire better coping mechanisms may have been useful, especially because her mood symptoms did not meet criteria for bipolar disorder, and her depression had long been controlled with sertraline monotherapy.
OUTCOME Discharged after 20 days
Ms. L stays in the hospital for 10 days after receiving her liver transplant. She has low appetite and some difficulty with sleep after the transplant; therefore, the C-L team recommends mirtazapine, 15 mg/d. She has no behavioral problems during her stay, and is set up with home health, case management, and psychiatry follow-up. On hospital Day 20, she is discharged.
Bottom Line
Use caution when prescribing valproic acid, even in young, otherwise healthy patients. Although rare, some patients may experience a rapid decompensation and life-threatening illness after starting valproic acid. When prescribing valproic acid, ensure close follow-up after initiation, including mental status examinations, physical examinations, and laboratory testing.
Related Resource
- Doroudgar S, Chou TI. How to modify psychotropic therapy for patients who have liver dysfunction. Current Psychiatry. 2014;13(12):46-49.
Drug Brand Names
Diphenhydramine • Benadryl
Famotidine • Fluxid, Pepcid
Lamotrigine • Lamictal
Mirtazapine • Remeron
N-acetylcysteine • Mucomyst
Phenobarbital • Luminal
Phenytoin • Dilantin
Prednisone • Cortan, Deltasone
Sertraline • Zoloft
Valproic acid • Depakene
1. Depakote [package insert]. North Chicago, IL: AbbVie, Inc.; 2019.
2. National Institutes of Health. U.S. Department of Health and Human Services. Drug Record: Valproate. https://livertox.nlm.nih.gov/Valproate.htm. Updated October 30, 2018. Accessed March 21, 2019.
3. Schmid MM, Freudenmann RW, Keller F, et al. Non-fatal and fatal liver failure associated with valproic acid. Pharmacopsychiatry. 2013;46(2):63-68.
4. Patel N, Landry KB, Fargason RE, et al. Reversible encephalopathy due to valproic acid induced hyperammonemia in a patient with Bipolar I disorder: a cautionary report. Psychopharmacol Bull. 2017;47(1):40-44.
5. Eze E, Workman M, Donley B. Hyperammonemia and coma developed by a woman treated with valproic acid for affective disorder. Psychiatr Serv. 1998;49(10):1358-1359.
6. Darban M and Bagheri B. Drug reaction with eosinophilia and systemic symptoms induced by valproic acid: a case report. Iran Red Crescent Med J. 2016;18(9): e35825.
7. van Zoelen MA, de Graaf M, van Dijk MR, et al. Valproic acid-induced DRESS syndrome with acute liver failure. Neth J Med. 2012;70(3):155.
8. Lheureux PE, Hantson P. Carnitine in the treatment of valproic acid-induced toxicity. Clin Toxicol (Phila). 2009;47(2):101-111.
9. Bohan TP, Helton E, McDonald I, et al. Effect of L-carnitine treatment for valproate-induced hepatotoxicity. Neurology. 2001;56(10):1405-1409.
10. Böhles H, Sewell AC, Wenzel D. The effect of carnitine supplementation in valproate-induced hyperammonaemia. Acta Paediatr. 1996;85(4):446-449.
11. Raskind JY, El-Chaar GM. The role of carnitine supplementation during valproic acid therapy. Ann Pharmacother. 2000;34(5):630-638.
12. Romero-Falcón A, de la Santa-Belda E, García-Contreras R, et al. A case of valproate-associated hepatotoxicity treated with L-carnitine. Eur J Intern Med. 2003;14(5):338-340.
13. National Institute for Health and Clinical Excellence. Bipolar disorder: the management of bipolar disorder in adults, children, and adolescents, in primary and secondary care. https://www.nice.org.uk/guidance/cg185. Updated April 2018. Accessed March 21, 2019.
14 . Hirschfeld RMA, Bowden CL, Gitlin MJ, et al. Practice guideline for the treatment of patients with biopolar disorder: second edition. American Psychiatric Association. https://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/bipolar.pdf. Published 2002. Accessed March 21, 2019.
1. Depakote [package insert]. North Chicago, IL: AbbVie, Inc.; 2019.
2. National Institutes of Health. U.S. Department of Health and Human Services. Drug Record: Valproate. https://livertox.nlm.nih.gov/Valproate.htm. Updated October 30, 2018. Accessed March 21, 2019.
3. Schmid MM, Freudenmann RW, Keller F, et al. Non-fatal and fatal liver failure associated with valproic acid. Pharmacopsychiatry. 2013;46(2):63-68.
4. Patel N, Landry KB, Fargason RE, et al. Reversible encephalopathy due to valproic acid induced hyperammonemia in a patient with Bipolar I disorder: a cautionary report. Psychopharmacol Bull. 2017;47(1):40-44.
5. Eze E, Workman M, Donley B. Hyperammonemia and coma developed by a woman treated with valproic acid for affective disorder. Psychiatr Serv. 1998;49(10):1358-1359.
6. Darban M and Bagheri B. Drug reaction with eosinophilia and systemic symptoms induced by valproic acid: a case report. Iran Red Crescent Med J. 2016;18(9): e35825.
7. van Zoelen MA, de Graaf M, van Dijk MR, et al. Valproic acid-induced DRESS syndrome with acute liver failure. Neth J Med. 2012;70(3):155.
8. Lheureux PE, Hantson P. Carnitine in the treatment of valproic acid-induced toxicity. Clin Toxicol (Phila). 2009;47(2):101-111.
9. Bohan TP, Helton E, McDonald I, et al. Effect of L-carnitine treatment for valproate-induced hepatotoxicity. Neurology. 2001;56(10):1405-1409.
10. Böhles H, Sewell AC, Wenzel D. The effect of carnitine supplementation in valproate-induced hyperammonaemia. Acta Paediatr. 1996;85(4):446-449.
11. Raskind JY, El-Chaar GM. The role of carnitine supplementation during valproic acid therapy. Ann Pharmacother. 2000;34(5):630-638.
12. Romero-Falcón A, de la Santa-Belda E, García-Contreras R, et al. A case of valproate-associated hepatotoxicity treated with L-carnitine. Eur J Intern Med. 2003;14(5):338-340.
13. National Institute for Health and Clinical Excellence. Bipolar disorder: the management of bipolar disorder in adults, children, and adolescents, in primary and secondary care. https://www.nice.org.uk/guidance/cg185. Updated April 2018. Accessed March 21, 2019.
14 . Hirschfeld RMA, Bowden CL, Gitlin MJ, et al. Practice guideline for the treatment of patients with biopolar disorder: second edition. American Psychiatric Association. https://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/bipolar.pdf. Published 2002. Accessed March 21, 2019.
The angry disciple
CASE Disorganized thoughts and grandiose delusions
Mr. J, age 54, presents to the psychiatric emergency department (ED) with agitation and disruptive behavior. He claims that he is “the son of Jesus Christ” and has to travel to the Middle East to be baptized. Mr. J is irritable, shouting, and threatening staff members. He receives
The next day, he is calm and cooperative, but continues to express the same religious delusions. Mr. J is admitted to the psychiatric inpatient unit for further evaluation.
On the unit, Mr. J is pleasant and cooperative, but tangential in thought process. He reports he was “saved” by God 4 years ago, and that God communicates with him through music. Despite this, he denies having auditory or visual hallucinations.
Approximately 3 months earlier, Mr. J had stopped working and left his home and family in another state to pursue his “mission” of being baptized in the Middle East. Mr. J has been homeless since then. Despite that, he reports that his mood is “great” and denies any recent changes in mood, sleep, appetite, energy level, or psychomotor agitation. Although no formal cognitive testing is performed, Mr. J is alert and oriented to person, place, and time with intact remote and recent memory, no language deficits, and no lapses in concentration or attention throughout interview.
Mr. J says he has been drinking alcohol regularly throughout his adult life, often a few times per week, up to “a case and a half” of beer at times. He claims he’s had multiple periods of sobriety but denies having experienced withdrawal symptoms during those times. Mr. J reports 1 prior psychiatric hospitalization 25 years ago after attempting suicide by overdose following the loss of a loved one. At that time, he was diagnosed with posttraumatic stress disorder (PTSD). During this admission, he denies having any symptoms of PTSD or periods of mania or depression, and he has not undergone psychiatric treatment since he had been diagnosed with PTSD. He denies any family history of psychiatric illness as well as any medical comorbidities or medication use.
[polldaddy:10279202]
The authors’ observations
Mr. J’s presentation had a wide differential diagnosis (Table 1). The initial agitation Mr. J displayed in the psychiatric ED was likely secondary to acute alcohol intoxication, given that he was subsequently pleasant, calm, and cooperative after the alcohol was metabolized. Despite this, Mr. J continued to demonstrate delusions of a religious and somewhat grandiose nature with tangential thought processes, which made substance-induced psychosis less likely to be the sole diagnosis. Although it is possible to develop psychotic symptoms due to severe alcohol withdrawal (alcoholic hallucinosis), Mr. J’s vital signs remained stable, and he demonstrated no other signs or symptoms of withdrawal throughout his hospitalization. His presentation also did not fit that of delirium tremens because he was not confused or disoriented, and did not demonstrate perceptual disturbance.
While delusions were the most prominent feature of Mr. J’s apparent psychosis, the presence of disorganized thought processes and impaired functioning, as evidenced by Mr. J’s unemployment and recent homelessness, were more consistent with a primary psychotic disorder than a delusional disorder.1
Continue to: Mr. J began to exhibit...
Mr. J began to exhibit these psychotic symptoms in his early 50s; because the average age of onset of schizophrenia for males is approximately age 20 to 25, the likelihood of his presentation being the result of a primary psychotic disorder was low.1 Although less common, it was possible that Mr. J had developed late-onset schizophrenia, where the first episode typically occurs after approximately age 40 to 45. Mr. J also described that he was in a “great” mood but had grandiose delusions and had made recent impulsive decisions, which suggests there was a possible mood component to his presentation and a potential diagnosis of schizoaffective disorder or bipolar disorder with psychotic symptoms. However, before any of these diagnoses could be made, a medical or neurologic condition that could cause his symptoms needed to be investigated and ruled out. Further collateral information regarding Mr. J’s history and timeline of symptoms was required.
EVALUATION Family history reveals clues
All laboratory studies completed during Mr. J’s hospitalization are unremarkable, including complete blood count, basic metabolic panel, hepatic function panel, gamma-glutamyl transferase test, magnesium, phosphate, thyroid-stimulating hormone, vitamin B12, thiamine, folate, urinalysis, and urine drug screen. Mr. J does not undergo any head imaging.
Mr. J has not been in touch with his family since leaving his home approximately 3 months before he presented to the ED, and he gives consent for the inpatient team to attempt to contact them. One week into hospitalization, Mr. J’s sibling informs the team of a family history of genetically confirmed Huntington’s disease (HD), with psychiatric symptoms preceding the onset of motor symptoms in multiple first-degree relatives. His family says that before Mr. J first developed delusions 4 years ago, he had not exhibited any psychotic symptoms during periods of alcohol use or sobriety.
Mr. J does not demonstrate any overt movement symptoms on the unit and denies noting any rigidity, change in gait, or abnormal/uncontrolled movements. The inpatient psychiatric team consults neurology and a full neurologic evaluation is performed. The results are unremarkable outside of his psychiatric symptoms; specifically, Mr. J does not demonstrate even subtle motor signs or cognitive impairment. Given Mr. J’s family history, unremarkable lab findings, and age at presentation, the neurology team and inpatient psychiatry team suspect that his psychosis is likely an early presentation of HD.
[polldaddy:10279212]
The authors’ observations
Genetics of Huntington’s disease
Huntington’s disease is an autosomal dominant neurodegenerative disorder caused by expansion of cytosine-adenine-guanine (CAG) trinucleotide repeats within the Huntingtin (HTT) gene on chromosome 4, which codes for the huntingtin protein.2,3 While the function of “normal” huntingtin protein is not fully understood, it is known that CAG repeat expansion in the HTT gene of >35 repeats codes for a mutant huntingtin protein.2,3 The mutant huntingtin protein causes progressive neuronal loss in the basal ganglia and striatum, resulting in the clinical Huntington’s phenotype.3 Notably, the patient’s age at disease onset is inversely correlated with the number of repeats. For example, expansions of approximately 40 to 50 CAG repeats often result in adult-onset HD, while expansions of >60 repeats are typically associated with juvenile-onset HD (before age 20). CAG repeat lengths of approximately 36 to 39 demonstrate reduced penetrance, with some individuals developing symptomatic HD while others do not.2 Instability of the CAG repeat expansion can result in genetic “anticipation,” wherein repeat length increases between generations, causing earlier age of onset in affected offspring. Genetic anticipation in HD occurs more frequently in paternal transmission—approximately 80% to 90% of juvenile HD cases are inherited paternally, at times with the number of CAG repeats exceeding 200.3
Continue to: Psychiatric manifestations of Huntington's disease
Psychiatric manifestations of Huntington’s disease
Huntington’s disease is characterized by motor, cognitive, and behavioral disturbances (Table 22,4). Motor symptoms include a characteristic and well-recognized chorea, often predominating earlier in HD, that progresses to rigidity, spasticity, and bradykinesia later in the disease course.2 Cognitive impairments develop in a similar progressive manner and can often precede the onset of motor symptoms, beginning with early executive dysfunction. Thinking often becomes more rigid and less efficient, causing difficulty with multi-tasking and concentration, and often progressing to subcortical dementia.2
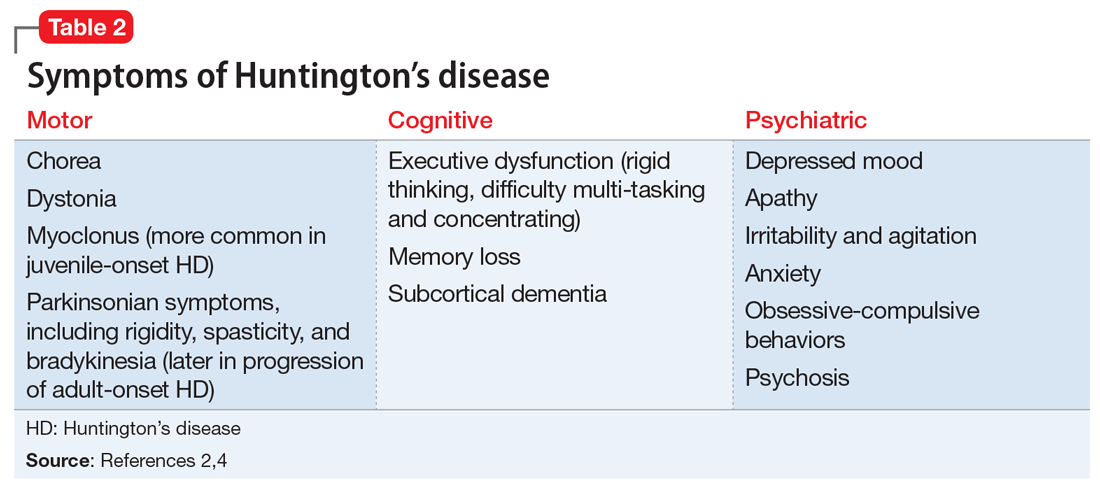
Psychiatric symptoms have long been recognized as a feature of HD; the estimated lifetime prevalence in patients with HD ranges from approximately 33% to 76%.4 Depressed mood, anxiety, irritability, and apathy are the most commonly reported symptoms, while a smaller percentage of patients with HD can experience obsessive-compulsive disorder (10% to 52%) or psychotic symptoms (3% to 11%).4 A more specific schizophrenia-like psychosis occurs in approximately 3% to 6% of patients, and often is a paranoid type.5,6 Positive psychotic symptoms, such as hallucinations and delusions, typically become less overt as HD progresses and cognitive impairments worsen.7
Although the onset of motor symptoms leads to diagnosis in the majority of patients with HD, many patients present with psychiatric symptoms—most commonly depression—prior to motor symptoms.8 An increasing body of literature details instances of psychosis preceding motor symptom onset by up to 10 years.6,9-12 In many of these cases, the patient has a family history of HD-associated psychosis. Family history is a major risk factor for HD-associated psychosis, as is early-onset HD.7,9
TREATMENT Antipsychotics result in some improvement
On Day 1 or 2, Mr. J is started on risperidone, 1 mg twice daily, to manage his symptoms. He shows incremental improvement in thought organization. Although his religious and grandiose delusions persist, they become less fixed, and he is able to take the team’s suggestion that he reconnect with his family.
Mr. J is aware of his family history of HD and acknowledges that multiple relatives had early psychiatric manifestations of HD. Despite this, he still has difficulty recognizing any connection between other family members’ presentation and his own. The psychiatry and neurology teams discuss the process, ethics, and implications of genetic testing for HD with Mr. J; however, he is ambivalent regarding genetic testing, and states he would consider it after discussing it with his family.
Continue to: The neurology team recommends...
The neurology team recommends against imaging for Mr. J because HD-related changes are not typically seen until later in the disease progression. On Day 9, they recommend changing from risperidone to quetiapine (50 mg every night at bedtime) due to evidence of its effectiveness specifically for treating behavioral symptoms of HD.13
While receiving quetiapine, Mr. J experiences significant drowsiness. Because he had experienced improvement in thought organization while he was receiving risperidone, he is switched back to risperidone.
[polldaddy:10279220]
The authors’ observations
Currently, no treatments are available to prevent the development or progression of HD. However, symptomatic treatment of motor and behavioral disturbances can lead to functional improvement and improved quality of life for individuals affected by HD.
There are no extensive clinical trials to date, but multiple case reports and studies have shown second-generation antipsychotics (SGAs), including quetiapine, olanzapine,
OUTCOME Discharge despite persistent delusions
Mr. J’s religious and grandiose delusions continue throughout hospitalization despite treatment with antipsychotics. However, because he remains calm and cooperative and demonstrates improvement in thought organization, he is deemed safe for discharge and instructed to continue risperidone. The team coordinates with Mr. J’s family to arrange transportation home and outpatient neurology follow-up.
Bottom Line
Psychiatric manifestations, including psychosis, are prominent symptoms of Huntington’s disease (HD) and may precede the onset of more readily recognized motor symptoms. This poses a diagnostic challenge, and clinicians should remain cognizant of this possibility, especially in patients with a family history of HD-associated psychosis.
Related Resources
- Huntington’s Disease Society of America. http://hdsa.org.
- National Institute of Neurological Disorders and Stroke. Huntington’s disease information page: What research is being done? https://www.ninds.nih.gov/Disorders/All-Disorders/Huntingtons-Disease-Information-Page.
- Scher LM. How to target psychiatric symptoms of Huntington’s disease. Current Psychiatry. 2012;11(9):34-39.
Drug Brand Names
Aripiprazole • Abilify
Clozapine • Clozaril
Haloperidol • Haldol
Olanzapine • Zyprexa
Quetiapine • Seroquel
Risperidone • Risperdal
1. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Publishing; 2013.
2. Novak MJ, Tabrizi SJ. Huntington’s disease: clinical presentation and treatment. Int Rev Neurobiol. 2011;98:297-323.
3. Reiner A, Dragatsis I, Dietrich P. Genetics and neuropathology of Huntington’s disease. Int Rev Neurobiol. 2011;98:325-372.
4. van Duijn E, Kingma EM, Van der mast RC. Psychopathology in verified Huntington’s disease gene carriers. J Neuropsychiatry Clin Neurosci. 2007;19(4):441-448.
5. Naarding P, Kremer HP, Zitman FG. Huntington’s disease: a review of the literature on prevalence and treatment of neuropsychiatric phenomena. Eur Psychiatry. 2001;16(8):439-445.
6. Xu C, Yogaratnam J, Tan N, et al. Psychosis, treatment emergent extrapyramidal events, and subsequent onset of Huntington’s disease: a case report and review of the literature. Clin Psychopharmacol Neurosci. 2016;14(3):302-304.
7. Mendez MF. Huntington’s disease: update and review of neuropsychiatric aspects. Int J Psychiatry Med. 1994;24(3):189-208.
8. Di Maio L, Squitieri F, Napolitano G, et al. Onset symptoms in 510 patients with Huntington’s disease. J Med Genet. 1993;30(4):289-292.
9. Jauhar S, Ritchie S. Psychiatric and behavioural manifestations of Huntington’s disease. Adv Psychiatr Treat. 2010;16(3):168-175.
10. Nagel M, Rumpf HJ, Kasten M. Acute psychosis in a verified Huntington disease gene carrier with subtle motor signs: psychiatric criteria should be considered for the diagnosis. Gen Hosp Psychiatry. 2014;36(3):361.e3-e4. doi: 10.1016/j.genhosppsych.2014.01.008.
11. Corrêa BB, Xavier M, Guimarães J. Association of Huntington’s disease and schizophrenia-like psychosis in a Huntington’s disease pedigree. Clin Pract Epidemiol Ment Health. 2006;2:1.
12. Ding J, Gadit AM. Psychosis with Huntington’s disease: role of antipsychotic medications. BMJ Case Rep. 2014: bcr2013202625. doi: 10.1136/bcr-2013-202625.
13. Alpay M, Koroshetz WJ. Quetiapine in the treatment of behavioral disturbances in patients with Huntington’s disease. Psychosomatics. 2006;47(1):70-72.
14. Duff K, Beglinger LJ, O’Rourke ME, et al. Risperidone and the treatment of psychiatric, motor, and cognitive symptoms in Huntington’s disease. Ann Clin Psychiatry. 2008;20(1):1-3.
15. Paleacu D, Anca M, Giladi N. Olanzapine in Huntington’s disease. Acta Neurol Scand. 2002;105(6):441-444.
16. Lin W, Chou Y. Aripiprazole effects on psychosis and chorea in a patient with Huntington’s disease. Am J Psychiatry. 2008;165(9):1207-1208.
17. van Vugt JP, Siesling S, Vergeer M, et al. Clozapine versus placebo in Huntington’s disease: a double blind randomized comparative study. J Neurol Neurosurg Psychiatr. 1997;63(1):35-39.
CASE Disorganized thoughts and grandiose delusions
Mr. J, age 54, presents to the psychiatric emergency department (ED) with agitation and disruptive behavior. He claims that he is “the son of Jesus Christ” and has to travel to the Middle East to be baptized. Mr. J is irritable, shouting, and threatening staff members. He receives
The next day, he is calm and cooperative, but continues to express the same religious delusions. Mr. J is admitted to the psychiatric inpatient unit for further evaluation.
On the unit, Mr. J is pleasant and cooperative, but tangential in thought process. He reports he was “saved” by God 4 years ago, and that God communicates with him through music. Despite this, he denies having auditory or visual hallucinations.
Approximately 3 months earlier, Mr. J had stopped working and left his home and family in another state to pursue his “mission” of being baptized in the Middle East. Mr. J has been homeless since then. Despite that, he reports that his mood is “great” and denies any recent changes in mood, sleep, appetite, energy level, or psychomotor agitation. Although no formal cognitive testing is performed, Mr. J is alert and oriented to person, place, and time with intact remote and recent memory, no language deficits, and no lapses in concentration or attention throughout interview.
Mr. J says he has been drinking alcohol regularly throughout his adult life, often a few times per week, up to “a case and a half” of beer at times. He claims he’s had multiple periods of sobriety but denies having experienced withdrawal symptoms during those times. Mr. J reports 1 prior psychiatric hospitalization 25 years ago after attempting suicide by overdose following the loss of a loved one. At that time, he was diagnosed with posttraumatic stress disorder (PTSD). During this admission, he denies having any symptoms of PTSD or periods of mania or depression, and he has not undergone psychiatric treatment since he had been diagnosed with PTSD. He denies any family history of psychiatric illness as well as any medical comorbidities or medication use.
[polldaddy:10279202]
The authors’ observations
Mr. J’s presentation had a wide differential diagnosis (Table 1). The initial agitation Mr. J displayed in the psychiatric ED was likely secondary to acute alcohol intoxication, given that he was subsequently pleasant, calm, and cooperative after the alcohol was metabolized. Despite this, Mr. J continued to demonstrate delusions of a religious and somewhat grandiose nature with tangential thought processes, which made substance-induced psychosis less likely to be the sole diagnosis. Although it is possible to develop psychotic symptoms due to severe alcohol withdrawal (alcoholic hallucinosis), Mr. J’s vital signs remained stable, and he demonstrated no other signs or symptoms of withdrawal throughout his hospitalization. His presentation also did not fit that of delirium tremens because he was not confused or disoriented, and did not demonstrate perceptual disturbance.
While delusions were the most prominent feature of Mr. J’s apparent psychosis, the presence of disorganized thought processes and impaired functioning, as evidenced by Mr. J’s unemployment and recent homelessness, were more consistent with a primary psychotic disorder than a delusional disorder.1
Continue to: Mr. J began to exhibit...
Mr. J began to exhibit these psychotic symptoms in his early 50s; because the average age of onset of schizophrenia for males is approximately age 20 to 25, the likelihood of his presentation being the result of a primary psychotic disorder was low.1 Although less common, it was possible that Mr. J had developed late-onset schizophrenia, where the first episode typically occurs after approximately age 40 to 45. Mr. J also described that he was in a “great” mood but had grandiose delusions and had made recent impulsive decisions, which suggests there was a possible mood component to his presentation and a potential diagnosis of schizoaffective disorder or bipolar disorder with psychotic symptoms. However, before any of these diagnoses could be made, a medical or neurologic condition that could cause his symptoms needed to be investigated and ruled out. Further collateral information regarding Mr. J’s history and timeline of symptoms was required.
EVALUATION Family history reveals clues
All laboratory studies completed during Mr. J’s hospitalization are unremarkable, including complete blood count, basic metabolic panel, hepatic function panel, gamma-glutamyl transferase test, magnesium, phosphate, thyroid-stimulating hormone, vitamin B12, thiamine, folate, urinalysis, and urine drug screen. Mr. J does not undergo any head imaging.
Mr. J has not been in touch with his family since leaving his home approximately 3 months before he presented to the ED, and he gives consent for the inpatient team to attempt to contact them. One week into hospitalization, Mr. J’s sibling informs the team of a family history of genetically confirmed Huntington’s disease (HD), with psychiatric symptoms preceding the onset of motor symptoms in multiple first-degree relatives. His family says that before Mr. J first developed delusions 4 years ago, he had not exhibited any psychotic symptoms during periods of alcohol use or sobriety.
Mr. J does not demonstrate any overt movement symptoms on the unit and denies noting any rigidity, change in gait, or abnormal/uncontrolled movements. The inpatient psychiatric team consults neurology and a full neurologic evaluation is performed. The results are unremarkable outside of his psychiatric symptoms; specifically, Mr. J does not demonstrate even subtle motor signs or cognitive impairment. Given Mr. J’s family history, unremarkable lab findings, and age at presentation, the neurology team and inpatient psychiatry team suspect that his psychosis is likely an early presentation of HD.
[polldaddy:10279212]
The authors’ observations
Genetics of Huntington’s disease
Huntington’s disease is an autosomal dominant neurodegenerative disorder caused by expansion of cytosine-adenine-guanine (CAG) trinucleotide repeats within the Huntingtin (HTT) gene on chromosome 4, which codes for the huntingtin protein.2,3 While the function of “normal” huntingtin protein is not fully understood, it is known that CAG repeat expansion in the HTT gene of >35 repeats codes for a mutant huntingtin protein.2,3 The mutant huntingtin protein causes progressive neuronal loss in the basal ganglia and striatum, resulting in the clinical Huntington’s phenotype.3 Notably, the patient’s age at disease onset is inversely correlated with the number of repeats. For example, expansions of approximately 40 to 50 CAG repeats often result in adult-onset HD, while expansions of >60 repeats are typically associated with juvenile-onset HD (before age 20). CAG repeat lengths of approximately 36 to 39 demonstrate reduced penetrance, with some individuals developing symptomatic HD while others do not.2 Instability of the CAG repeat expansion can result in genetic “anticipation,” wherein repeat length increases between generations, causing earlier age of onset in affected offspring. Genetic anticipation in HD occurs more frequently in paternal transmission—approximately 80% to 90% of juvenile HD cases are inherited paternally, at times with the number of CAG repeats exceeding 200.3
Continue to: Psychiatric manifestations of Huntington's disease
Psychiatric manifestations of Huntington’s disease
Huntington’s disease is characterized by motor, cognitive, and behavioral disturbances (Table 22,4). Motor symptoms include a characteristic and well-recognized chorea, often predominating earlier in HD, that progresses to rigidity, spasticity, and bradykinesia later in the disease course.2 Cognitive impairments develop in a similar progressive manner and can often precede the onset of motor symptoms, beginning with early executive dysfunction. Thinking often becomes more rigid and less efficient, causing difficulty with multi-tasking and concentration, and often progressing to subcortical dementia.2

Psychiatric symptoms have long been recognized as a feature of HD; the estimated lifetime prevalence in patients with HD ranges from approximately 33% to 76%.4 Depressed mood, anxiety, irritability, and apathy are the most commonly reported symptoms, while a smaller percentage of patients with HD can experience obsessive-compulsive disorder (10% to 52%) or psychotic symptoms (3% to 11%).4 A more specific schizophrenia-like psychosis occurs in approximately 3% to 6% of patients, and often is a paranoid type.5,6 Positive psychotic symptoms, such as hallucinations and delusions, typically become less overt as HD progresses and cognitive impairments worsen.7
Although the onset of motor symptoms leads to diagnosis in the majority of patients with HD, many patients present with psychiatric symptoms—most commonly depression—prior to motor symptoms.8 An increasing body of literature details instances of psychosis preceding motor symptom onset by up to 10 years.6,9-12 In many of these cases, the patient has a family history of HD-associated psychosis. Family history is a major risk factor for HD-associated psychosis, as is early-onset HD.7,9
TREATMENT Antipsychotics result in some improvement
On Day 1 or 2, Mr. J is started on risperidone, 1 mg twice daily, to manage his symptoms. He shows incremental improvement in thought organization. Although his religious and grandiose delusions persist, they become less fixed, and he is able to take the team’s suggestion that he reconnect with his family.
Mr. J is aware of his family history of HD and acknowledges that multiple relatives had early psychiatric manifestations of HD. Despite this, he still has difficulty recognizing any connection between other family members’ presentation and his own. The psychiatry and neurology teams discuss the process, ethics, and implications of genetic testing for HD with Mr. J; however, he is ambivalent regarding genetic testing, and states he would consider it after discussing it with his family.
Continue to: The neurology team recommends...
The neurology team recommends against imaging for Mr. J because HD-related changes are not typically seen until later in the disease progression. On Day 9, they recommend changing from risperidone to quetiapine (50 mg every night at bedtime) due to evidence of its effectiveness specifically for treating behavioral symptoms of HD.13
While receiving quetiapine, Mr. J experiences significant drowsiness. Because he had experienced improvement in thought organization while he was receiving risperidone, he is switched back to risperidone.
[polldaddy:10279220]
The authors’ observations
Currently, no treatments are available to prevent the development or progression of HD. However, symptomatic treatment of motor and behavioral disturbances can lead to functional improvement and improved quality of life for individuals affected by HD.
There are no extensive clinical trials to date, but multiple case reports and studies have shown second-generation antipsychotics (SGAs), including quetiapine, olanzapine,
OUTCOME Discharge despite persistent delusions
Mr. J’s religious and grandiose delusions continue throughout hospitalization despite treatment with antipsychotics. However, because he remains calm and cooperative and demonstrates improvement in thought organization, he is deemed safe for discharge and instructed to continue risperidone. The team coordinates with Mr. J’s family to arrange transportation home and outpatient neurology follow-up.
Bottom Line
Psychiatric manifestations, including psychosis, are prominent symptoms of Huntington’s disease (HD) and may precede the onset of more readily recognized motor symptoms. This poses a diagnostic challenge, and clinicians should remain cognizant of this possibility, especially in patients with a family history of HD-associated psychosis.
Related Resources
- Huntington’s Disease Society of America. http://hdsa.org.
- National Institute of Neurological Disorders and Stroke. Huntington’s disease information page: What research is being done? https://www.ninds.nih.gov/Disorders/All-Disorders/Huntingtons-Disease-Information-Page.
- Scher LM. How to target psychiatric symptoms of Huntington’s disease. Current Psychiatry. 2012;11(9):34-39.
Drug Brand Names
Aripiprazole • Abilify
Clozapine • Clozaril
Haloperidol • Haldol
Olanzapine • Zyprexa
Quetiapine • Seroquel
Risperidone • Risperdal
CASE Disorganized thoughts and grandiose delusions
Mr. J, age 54, presents to the psychiatric emergency department (ED) with agitation and disruptive behavior. He claims that he is “the son of Jesus Christ” and has to travel to the Middle East to be baptized. Mr. J is irritable, shouting, and threatening staff members. He receives
The next day, he is calm and cooperative, but continues to express the same religious delusions. Mr. J is admitted to the psychiatric inpatient unit for further evaluation.
On the unit, Mr. J is pleasant and cooperative, but tangential in thought process. He reports he was “saved” by God 4 years ago, and that God communicates with him through music. Despite this, he denies having auditory or visual hallucinations.
Approximately 3 months earlier, Mr. J had stopped working and left his home and family in another state to pursue his “mission” of being baptized in the Middle East. Mr. J has been homeless since then. Despite that, he reports that his mood is “great” and denies any recent changes in mood, sleep, appetite, energy level, or psychomotor agitation. Although no formal cognitive testing is performed, Mr. J is alert and oriented to person, place, and time with intact remote and recent memory, no language deficits, and no lapses in concentration or attention throughout interview.
Mr. J says he has been drinking alcohol regularly throughout his adult life, often a few times per week, up to “a case and a half” of beer at times. He claims he’s had multiple periods of sobriety but denies having experienced withdrawal symptoms during those times. Mr. J reports 1 prior psychiatric hospitalization 25 years ago after attempting suicide by overdose following the loss of a loved one. At that time, he was diagnosed with posttraumatic stress disorder (PTSD). During this admission, he denies having any symptoms of PTSD or periods of mania or depression, and he has not undergone psychiatric treatment since he had been diagnosed with PTSD. He denies any family history of psychiatric illness as well as any medical comorbidities or medication use.
[polldaddy:10279202]
The authors’ observations
Mr. J’s presentation had a wide differential diagnosis (Table 1). The initial agitation Mr. J displayed in the psychiatric ED was likely secondary to acute alcohol intoxication, given that he was subsequently pleasant, calm, and cooperative after the alcohol was metabolized. Despite this, Mr. J continued to demonstrate delusions of a religious and somewhat grandiose nature with tangential thought processes, which made substance-induced psychosis less likely to be the sole diagnosis. Although it is possible to develop psychotic symptoms due to severe alcohol withdrawal (alcoholic hallucinosis), Mr. J’s vital signs remained stable, and he demonstrated no other signs or symptoms of withdrawal throughout his hospitalization. His presentation also did not fit that of delirium tremens because he was not confused or disoriented, and did not demonstrate perceptual disturbance.
While delusions were the most prominent feature of Mr. J’s apparent psychosis, the presence of disorganized thought processes and impaired functioning, as evidenced by Mr. J’s unemployment and recent homelessness, were more consistent with a primary psychotic disorder than a delusional disorder.1
Continue to: Mr. J began to exhibit...
Mr. J began to exhibit these psychotic symptoms in his early 50s; because the average age of onset of schizophrenia for males is approximately age 20 to 25, the likelihood of his presentation being the result of a primary psychotic disorder was low.1 Although less common, it was possible that Mr. J had developed late-onset schizophrenia, where the first episode typically occurs after approximately age 40 to 45. Mr. J also described that he was in a “great” mood but had grandiose delusions and had made recent impulsive decisions, which suggests there was a possible mood component to his presentation and a potential diagnosis of schizoaffective disorder or bipolar disorder with psychotic symptoms. However, before any of these diagnoses could be made, a medical or neurologic condition that could cause his symptoms needed to be investigated and ruled out. Further collateral information regarding Mr. J’s history and timeline of symptoms was required.
EVALUATION Family history reveals clues
All laboratory studies completed during Mr. J’s hospitalization are unremarkable, including complete blood count, basic metabolic panel, hepatic function panel, gamma-glutamyl transferase test, magnesium, phosphate, thyroid-stimulating hormone, vitamin B12, thiamine, folate, urinalysis, and urine drug screen. Mr. J does not undergo any head imaging.
Mr. J has not been in touch with his family since leaving his home approximately 3 months before he presented to the ED, and he gives consent for the inpatient team to attempt to contact them. One week into hospitalization, Mr. J’s sibling informs the team of a family history of genetically confirmed Huntington’s disease (HD), with psychiatric symptoms preceding the onset of motor symptoms in multiple first-degree relatives. His family says that before Mr. J first developed delusions 4 years ago, he had not exhibited any psychotic symptoms during periods of alcohol use or sobriety.
Mr. J does not demonstrate any overt movement symptoms on the unit and denies noting any rigidity, change in gait, or abnormal/uncontrolled movements. The inpatient psychiatric team consults neurology and a full neurologic evaluation is performed. The results are unremarkable outside of his psychiatric symptoms; specifically, Mr. J does not demonstrate even subtle motor signs or cognitive impairment. Given Mr. J’s family history, unremarkable lab findings, and age at presentation, the neurology team and inpatient psychiatry team suspect that his psychosis is likely an early presentation of HD.
[polldaddy:10279212]
The authors’ observations
Genetics of Huntington’s disease
Huntington’s disease is an autosomal dominant neurodegenerative disorder caused by expansion of cytosine-adenine-guanine (CAG) trinucleotide repeats within the Huntingtin (HTT) gene on chromosome 4, which codes for the huntingtin protein.2,3 While the function of “normal” huntingtin protein is not fully understood, it is known that CAG repeat expansion in the HTT gene of >35 repeats codes for a mutant huntingtin protein.2,3 The mutant huntingtin protein causes progressive neuronal loss in the basal ganglia and striatum, resulting in the clinical Huntington’s phenotype.3 Notably, the patient’s age at disease onset is inversely correlated with the number of repeats. For example, expansions of approximately 40 to 50 CAG repeats often result in adult-onset HD, while expansions of >60 repeats are typically associated with juvenile-onset HD (before age 20). CAG repeat lengths of approximately 36 to 39 demonstrate reduced penetrance, with some individuals developing symptomatic HD while others do not.2 Instability of the CAG repeat expansion can result in genetic “anticipation,” wherein repeat length increases between generations, causing earlier age of onset in affected offspring. Genetic anticipation in HD occurs more frequently in paternal transmission—approximately 80% to 90% of juvenile HD cases are inherited paternally, at times with the number of CAG repeats exceeding 200.3
Continue to: Psychiatric manifestations of Huntington's disease
Psychiatric manifestations of Huntington’s disease
Huntington’s disease is characterized by motor, cognitive, and behavioral disturbances (Table 22,4). Motor symptoms include a characteristic and well-recognized chorea, often predominating earlier in HD, that progresses to rigidity, spasticity, and bradykinesia later in the disease course.2 Cognitive impairments develop in a similar progressive manner and can often precede the onset of motor symptoms, beginning with early executive dysfunction. Thinking often becomes more rigid and less efficient, causing difficulty with multi-tasking and concentration, and often progressing to subcortical dementia.2

Psychiatric symptoms have long been recognized as a feature of HD; the estimated lifetime prevalence in patients with HD ranges from approximately 33% to 76%.4 Depressed mood, anxiety, irritability, and apathy are the most commonly reported symptoms, while a smaller percentage of patients with HD can experience obsessive-compulsive disorder (10% to 52%) or psychotic symptoms (3% to 11%).4 A more specific schizophrenia-like psychosis occurs in approximately 3% to 6% of patients, and often is a paranoid type.5,6 Positive psychotic symptoms, such as hallucinations and delusions, typically become less overt as HD progresses and cognitive impairments worsen.7
Although the onset of motor symptoms leads to diagnosis in the majority of patients with HD, many patients present with psychiatric symptoms—most commonly depression—prior to motor symptoms.8 An increasing body of literature details instances of psychosis preceding motor symptom onset by up to 10 years.6,9-12 In many of these cases, the patient has a family history of HD-associated psychosis. Family history is a major risk factor for HD-associated psychosis, as is early-onset HD.7,9
TREATMENT Antipsychotics result in some improvement
On Day 1 or 2, Mr. J is started on risperidone, 1 mg twice daily, to manage his symptoms. He shows incremental improvement in thought organization. Although his religious and grandiose delusions persist, they become less fixed, and he is able to take the team’s suggestion that he reconnect with his family.
Mr. J is aware of his family history of HD and acknowledges that multiple relatives had early psychiatric manifestations of HD. Despite this, he still has difficulty recognizing any connection between other family members’ presentation and his own. The psychiatry and neurology teams discuss the process, ethics, and implications of genetic testing for HD with Mr. J; however, he is ambivalent regarding genetic testing, and states he would consider it after discussing it with his family.
Continue to: The neurology team recommends...
The neurology team recommends against imaging for Mr. J because HD-related changes are not typically seen until later in the disease progression. On Day 9, they recommend changing from risperidone to quetiapine (50 mg every night at bedtime) due to evidence of its effectiveness specifically for treating behavioral symptoms of HD.13
While receiving quetiapine, Mr. J experiences significant drowsiness. Because he had experienced improvement in thought organization while he was receiving risperidone, he is switched back to risperidone.
[polldaddy:10279220]
The authors’ observations
Currently, no treatments are available to prevent the development or progression of HD. However, symptomatic treatment of motor and behavioral disturbances can lead to functional improvement and improved quality of life for individuals affected by HD.
There are no extensive clinical trials to date, but multiple case reports and studies have shown second-generation antipsychotics (SGAs), including quetiapine, olanzapine,
OUTCOME Discharge despite persistent delusions
Mr. J’s religious and grandiose delusions continue throughout hospitalization despite treatment with antipsychotics. However, because he remains calm and cooperative and demonstrates improvement in thought organization, he is deemed safe for discharge and instructed to continue risperidone. The team coordinates with Mr. J’s family to arrange transportation home and outpatient neurology follow-up.
Bottom Line
Psychiatric manifestations, including psychosis, are prominent symptoms of Huntington’s disease (HD) and may precede the onset of more readily recognized motor symptoms. This poses a diagnostic challenge, and clinicians should remain cognizant of this possibility, especially in patients with a family history of HD-associated psychosis.
Related Resources
- Huntington’s Disease Society of America. http://hdsa.org.
- National Institute of Neurological Disorders and Stroke. Huntington’s disease information page: What research is being done? https://www.ninds.nih.gov/Disorders/All-Disorders/Huntingtons-Disease-Information-Page.
- Scher LM. How to target psychiatric symptoms of Huntington’s disease. Current Psychiatry. 2012;11(9):34-39.
Drug Brand Names
Aripiprazole • Abilify
Clozapine • Clozaril
Haloperidol • Haldol
Olanzapine • Zyprexa
Quetiapine • Seroquel
Risperidone • Risperdal
1. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Publishing; 2013.
2. Novak MJ, Tabrizi SJ. Huntington’s disease: clinical presentation and treatment. Int Rev Neurobiol. 2011;98:297-323.
3. Reiner A, Dragatsis I, Dietrich P. Genetics and neuropathology of Huntington’s disease. Int Rev Neurobiol. 2011;98:325-372.
4. van Duijn E, Kingma EM, Van der mast RC. Psychopathology in verified Huntington’s disease gene carriers. J Neuropsychiatry Clin Neurosci. 2007;19(4):441-448.
5. Naarding P, Kremer HP, Zitman FG. Huntington’s disease: a review of the literature on prevalence and treatment of neuropsychiatric phenomena. Eur Psychiatry. 2001;16(8):439-445.
6. Xu C, Yogaratnam J, Tan N, et al. Psychosis, treatment emergent extrapyramidal events, and subsequent onset of Huntington’s disease: a case report and review of the literature. Clin Psychopharmacol Neurosci. 2016;14(3):302-304.
7. Mendez MF. Huntington’s disease: update and review of neuropsychiatric aspects. Int J Psychiatry Med. 1994;24(3):189-208.
8. Di Maio L, Squitieri F, Napolitano G, et al. Onset symptoms in 510 patients with Huntington’s disease. J Med Genet. 1993;30(4):289-292.
9. Jauhar S, Ritchie S. Psychiatric and behavioural manifestations of Huntington’s disease. Adv Psychiatr Treat. 2010;16(3):168-175.
10. Nagel M, Rumpf HJ, Kasten M. Acute psychosis in a verified Huntington disease gene carrier with subtle motor signs: psychiatric criteria should be considered for the diagnosis. Gen Hosp Psychiatry. 2014;36(3):361.e3-e4. doi: 10.1016/j.genhosppsych.2014.01.008.
11. Corrêa BB, Xavier M, Guimarães J. Association of Huntington’s disease and schizophrenia-like psychosis in a Huntington’s disease pedigree. Clin Pract Epidemiol Ment Health. 2006;2:1.
12. Ding J, Gadit AM. Psychosis with Huntington’s disease: role of antipsychotic medications. BMJ Case Rep. 2014: bcr2013202625. doi: 10.1136/bcr-2013-202625.
13. Alpay M, Koroshetz WJ. Quetiapine in the treatment of behavioral disturbances in patients with Huntington’s disease. Psychosomatics. 2006;47(1):70-72.
14. Duff K, Beglinger LJ, O’Rourke ME, et al. Risperidone and the treatment of psychiatric, motor, and cognitive symptoms in Huntington’s disease. Ann Clin Psychiatry. 2008;20(1):1-3.
15. Paleacu D, Anca M, Giladi N. Olanzapine in Huntington’s disease. Acta Neurol Scand. 2002;105(6):441-444.
16. Lin W, Chou Y. Aripiprazole effects on psychosis and chorea in a patient with Huntington’s disease. Am J Psychiatry. 2008;165(9):1207-1208.
17. van Vugt JP, Siesling S, Vergeer M, et al. Clozapine versus placebo in Huntington’s disease: a double blind randomized comparative study. J Neurol Neurosurg Psychiatr. 1997;63(1):35-39.
1. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Publishing; 2013.
2. Novak MJ, Tabrizi SJ. Huntington’s disease: clinical presentation and treatment. Int Rev Neurobiol. 2011;98:297-323.
3. Reiner A, Dragatsis I, Dietrich P. Genetics and neuropathology of Huntington’s disease. Int Rev Neurobiol. 2011;98:325-372.
4. van Duijn E, Kingma EM, Van der mast RC. Psychopathology in verified Huntington’s disease gene carriers. J Neuropsychiatry Clin Neurosci. 2007;19(4):441-448.
5. Naarding P, Kremer HP, Zitman FG. Huntington’s disease: a review of the literature on prevalence and treatment of neuropsychiatric phenomena. Eur Psychiatry. 2001;16(8):439-445.
6. Xu C, Yogaratnam J, Tan N, et al. Psychosis, treatment emergent extrapyramidal events, and subsequent onset of Huntington’s disease: a case report and review of the literature. Clin Psychopharmacol Neurosci. 2016;14(3):302-304.
7. Mendez MF. Huntington’s disease: update and review of neuropsychiatric aspects. Int J Psychiatry Med. 1994;24(3):189-208.
8. Di Maio L, Squitieri F, Napolitano G, et al. Onset symptoms in 510 patients with Huntington’s disease. J Med Genet. 1993;30(4):289-292.
9. Jauhar S, Ritchie S. Psychiatric and behavioural manifestations of Huntington’s disease. Adv Psychiatr Treat. 2010;16(3):168-175.
10. Nagel M, Rumpf HJ, Kasten M. Acute psychosis in a verified Huntington disease gene carrier with subtle motor signs: psychiatric criteria should be considered for the diagnosis. Gen Hosp Psychiatry. 2014;36(3):361.e3-e4. doi: 10.1016/j.genhosppsych.2014.01.008.
11. Corrêa BB, Xavier M, Guimarães J. Association of Huntington’s disease and schizophrenia-like psychosis in a Huntington’s disease pedigree. Clin Pract Epidemiol Ment Health. 2006;2:1.
12. Ding J, Gadit AM. Psychosis with Huntington’s disease: role of antipsychotic medications. BMJ Case Rep. 2014: bcr2013202625. doi: 10.1136/bcr-2013-202625.
13. Alpay M, Koroshetz WJ. Quetiapine in the treatment of behavioral disturbances in patients with Huntington’s disease. Psychosomatics. 2006;47(1):70-72.
14. Duff K, Beglinger LJ, O’Rourke ME, et al. Risperidone and the treatment of psychiatric, motor, and cognitive symptoms in Huntington’s disease. Ann Clin Psychiatry. 2008;20(1):1-3.
15. Paleacu D, Anca M, Giladi N. Olanzapine in Huntington’s disease. Acta Neurol Scand. 2002;105(6):441-444.
16. Lin W, Chou Y. Aripiprazole effects on psychosis and chorea in a patient with Huntington’s disease. Am J Psychiatry. 2008;165(9):1207-1208.
17. van Vugt JP, Siesling S, Vergeer M, et al. Clozapine versus placebo in Huntington’s disease: a double blind randomized comparative study. J Neurol Neurosurg Psychiatr. 1997;63(1):35-39.
A suicide attempt, or something else?
CASE Unexplained hypoglycemia
Ms. A, age 12, is brought to the emergency department (ED) via ambulance with altered mentation and life-threatening hypoglycemia for management of a hypoglycemic seizure. Earlier that day, Ms. A’s parents had found her unresponsive and incontinent of urine. In the ED, Ms. A is minimally responsive. Her blood glucose level measurements are in the range of 30 to 39 mg/dL (reference range: 70 to 99 mg/dL), despite having received IV dextrose first from paramedics, and then in the ED. Ms. A has no history of hypoglycemia or diabetes. Her parents say that the night before coming to the ED, Ms. A had experienced flu-like symptoms, including nausea, vomiting, and diarrhea, that continued overnight and resulted in minimal food intake for 24 hours (Table 1).
A physical exam demonstrates left-sided weakness of face, arm, and leg, rightward gaze, and left-sided neglect. However, the results of CT angiography and an MRI of the brain rule out a stroke. An EEG shows right hemispheric slowing consistent with postictal paralysis, but no ongoing seizure activity. Ms. A is transferred to the pediatric intensive care unit (PICU).
Although Ms. A has no psychiatric diagnoses, she has a history of depressive symptoms, self-harm by cutting, and a suicide attempt by ingestion of an over-the-counter (OTC) medication 1 year ago. She had reported the suicide attempt to her parents several months after the fact, and asked them to find her a therapist, which her parents arranged. She also has a history of asthma, which is well-controlled with montelukast, 5 mg/d.
EVALUATION Elevated insulin levels
Subsequent investigations for organic causes of hypoglycemia are negative for adrenal insufficiency, fatty acid oxidation defect, and sepsis. Blood results demonstrate significantly elevated insulin levels of 92.4 mcIU/mL (reference range: 2.6 to 24.9 mcIU/mL) and a C-peptide level of 9.5 ng/mL (reference range: 1.1 to 4.4 ng/mL).
On Day 1 of admission to the PICU, Ms. A’s blood glucose level normalizes, and her mentation improves. Her parents report that one of them has diabetes and takes oral hypoglycemic agents at home, including glipizide immediate release (IR) tablets, 10 mg, and long-acting insulin glargine. The treatment team suspects that Ms. A may have ingested one or both of these agents, and orders a toxicologic screening for oral hypoglycemic agents.
On Day 2, the toxicology results are returned and are positive for glipizide, which Ms. A had not been prescribed. Ms. A states that she had taken only her montelukast tablet on the day of admission and adamantly denies deliberately ingesting her parent’s diabetes medications. Her parents check the home medications and state there are no missing glipizide IR tablets or insulin vials. They also report that Ms. A had no access to extended-release glipizide.
The treatment team discuss Ms. A’s clinical condition and toxicology results with the pediatric endocrinology team. The endocrinology team states that with no history of hypoglycemic episodes, it is unlikely that Ms. A had an endogenous etiology that would present so catastrophically. In their experience, inexplicable hypoglycemia in a healthy individual who lives in a household with someone who has diabetes is due to ingestion of a hypoglycemic agent until proven otherwise.
[polldaddy:10252689]
Continue to: The authors' observations
The authors’ observations
In the context of Ms. A’s prior suicide attempt and history of self-harm, the pediatric team was concerned that her presentation was consistent with a suicide attempt and consulted the psychiatry service.
Glipizide is a second-generation sulfonylurea used to treat type 2 diabetes. It lowers blood glucose by stimulating pancreatic insulin secretion. It is a rare drug of overdose.1 Although pediatric glipizide overdoses have been documented, there are currently no pediatric or adolescent glipizide pharmacokinetic studies in the literature.1-4 In adults, the immediate-release formulation has 100% oral bioavailability, with a maximum plasma concentration (Tmax) of approximately 2 hours.5 The half-life typically ranges from 4 to 6 hours in adults.6 Patients who do not have diabetes are much more susceptible to the hypoglycemic effects of glipizide because the medication simulates their fully functional pancreas to produce a vigorous insulin response.
Ms. A’s significantly elevated insulin was consistent with normal glipizide effects in a healthy child, while the elevated C-peptide was consistent with insulin being endogenously produced, which ruled out ingestion of her parent’s insulin. Importantly, the pediatric endocrinology team noted that, in their experience, a single 5- to 10-mg dose of glipizide IR was sufficient to lower blood glucose levels to the low 30s mg/dL in the context of a functional pancreas, which suggested that Ms. A might have accidentally ingested a single glipizide IR tablet, and might be telling the truth when she denies deliberately ingesting it to hurt herself.
The clinical value of pharmacokinetics
The screen of Ms. A’s toxicology sample detected glipizide. The laboratory used a semi-quantitative serum screen of several hypoglycemic agents. A positive result for each agent is based on a quantitative cut-off value, which is 3 ng/mL for glipizide. The clinical chemist on call was asked to assist in interpreting the results. The serum specimen collected on Day 1 had a significantly positive glipizide result of 86 to 130 ng/mL. The maximum effective glipizide concentration for adult patients with diabetes is 100 ng/mL.7 Thus, the glipizide level of 86 to 130 ng/mL (20.5 hours after initial symptoms) is consistent with the clinical presentation of persistent hypoglycemia requiring ongoing glucose replacement therapy.
Due to the lack of pediatric pharmacokinetic data for glipizide and only a single serum measurement, it is not possible to estimate the glipizide concentration at the time of maximal symptoms (loss of consciousness at 2:30
Continue to: Clinicians need to be aware that...
Clinicians need to be aware that although hypoglycemia usually presents rapidly, in children glipizide IR can rarely cause delayed hypoglycemia up to 16 hours after ingestion,2 and a delay of 45 hours was reported in a case of ingestion of extended-release glipizide.8 Hypoglycemia can last up to nearly 24 hours and is exacerbated if the patient has not eaten.1,2 Importantly, Ms. A’s parents reported that she had no access to extended-release glipizide. When detailed pharmacokinetic data are not available, the information provided by the patient and parents becomes extremely important, especially in distinguishing between single and multiple overdoses prior to presentation, or co-ingestions, or decreased food intake that could exacerbate hypoglycemia.
EVALUATION Safety assessment
On Day 2, Ms. A and her parents are interviewed separately, and they all are consistent in their recollection that Ms. A had been feverish with flu-like symptoms throughout the night before coming to the ED, and had still seemed mildly confused on the morning of admission.
During the interview, her parents wonder when Ms. A took her daily dose of a single montelukast tablet for asthma, and whether she had accidentally confused it with their glipizide. They report that on the morning of admission, both the glipizide and montelukast medication vials were in the same room. The vials are the same color, the same size, and labeled from the same pharmacy, and contain white, scored, round tablets that look very similar.
During the interview, Ms. A consistently denies having thoughts of hurting or killing herself on the day of admission or before that.
[polldaddy:10252690]
Continue to: The authors' observations
The authors’ observations
This case was ultimately an accidental ingestion of glipizide, rather than a suicide attempt. The initial suspicion for a suicide attempt had been reasonable in the context of Ms. A’s depressive symptoms, remote history of a prior suicide attempt by ingesting an OTC medication, and toxicologic evidence of ingesting a drug not prescribed to her. Additionally, because of the life-threatening presentation, it was easy to make the erroneous assumption that the ingestion of glipizide must have involved many tablets, and thus must have been deliberate. However, through multidisciplinary teamwork, we were able to demonstrate that this was likely an accidental ingestion by a patient who had an acute febrile illness. Her illness had caused confusion, which contributed to the accidental ingestion, and also caused reduced food intake, which enhanced the hypoglycemic effects of glipizide. Additionally, a lack of awareness of medication safety in the home had facilitated the confusion between the two medication vials.
A single tablet of glipizide IR is sufficient to produce profound clinical effects that could be mistaken by medical and psychiatric teams for a much larger and/or deliberate overdose, especially in patients with a psychiatric history. The inappropriate psychiatric hospitalization of a patient, especially a child, who has been mistakenly diagnosed as having attempted suicide, can have negative therapeutic consequences (Table 2). A psychiatric admission would have been misguided if it attempted to address safety and reduce suicidality when no such concerns were present. Additionally, it could have damaged relationships with the patient and the family, especially in a child who had historically not sought psychiatric care despite depressive symptoms and a previous suicide attempt. When assessing for suicidality, consider accidental ingestion in the differential and use specialty expertise and confirmatory testing in the evaluation, taking the pharmacokinetics of the suspected agent into account.
OUTCOME Outpatient treatment
Ms. A’s neurologic symptoms resolve within 24 hours of admission. She is offered psychiatric inpatient hospitalization to address her depressive symptoms; however, her parents prefer that she receive outpatient care. Ms. A’s parents also state that after Ms. A’s admission, they locked up all household medications and will be more mindful with medication in the home. Because her parents are arranging appropriate outpatient treatment for Ms. A’s depression and maintenance of her safety, an involuntary hospitalization is not deemed necessary.
On Day 2, Ms. A is eating normally, her blood glucose levels remain stable, and she is discharged home.
Bottom Line
Oral hypoglycemic agents can cause life-threatening syndromes in healthy patients and can clinically mimic large, intentional overdoses. Clinicians must be aware of the differential of accidental ingestion when assessing for suicidality, and can use toxicology results in their assessment.
Related Resources
- Kidemergencies.com. Emergencies: One pill can kill. http://kidemergencies.com/onepill1.html.
- Safe Kids Worldwide. Medication safety. https://www.safekids.org/medicinesafety.
- American Association of Poison Control Centers. http://www.aapcc.org/.
Drug Brand Names
Glipizide • Glucotrol
Insulin glargine • Lantus
Montelukast • Singulair
1. Spiller HA, Villalobos D, Krenzelok EP, et al. Prospective multicenter study of sulfonylurea ingestion in children. J Pediatr. 1997;131(1):141-146.
2. Quadrani DA, Spiller HA, Widder P. Five year retrospective evaluation of sulfonylurea ingestion in children. J Toxicol Clin Toxicol. 1996;34(3):267-270.
3. Borowski H, Caraccio T, Mofenson H. Sulfonylurea ingestion in children: is an 8-hour observation period sufficient? J Pediatr. 1998;133(4):584-585.
4. Little GL, Boniface KS. Are one or two dangerous? Sulfony-lurea exposure in toddlers. J Emerg Med. 2005;28(3):305-310.
5. Huupponen R, Seppala P, Iisalo E. Glipizide pharma-cokinetics and response in diabetics. Int J Clin Pharmacol Ther Toxicol. 1982;20(9):417-422.
6. Baselt RC. Disposition of toxic drugs and chemicals in man. 10th ed. Seal Beach, California: Biomedical Publications; 2014.
7. Simonson DC, Kourides IA, Feinglos M, et al; the Glipizide Gastrointestinal Therapeutic System Study Group. Efficacy, safety, and dose-response characteristics of glipizide gastrointestinal therapeutic system on glycemic control and insulin secretion in NIDDM. Results of two multicenter, randomized, placebo-controlled clinical trials. Diabetes Care. 1997;20(4):597-606.
8. Pelavin PI, Abramson E, Pon S, et al. Extended-release glipizide overdose presenting with delayed hypoglycemia and treated with subcutaneous octreotide. J Pediatr Endocrinol Metab. 2009;22(2):171-175.
CASE Unexplained hypoglycemia
Ms. A, age 12, is brought to the emergency department (ED) via ambulance with altered mentation and life-threatening hypoglycemia for management of a hypoglycemic seizure. Earlier that day, Ms. A’s parents had found her unresponsive and incontinent of urine. In the ED, Ms. A is minimally responsive. Her blood glucose level measurements are in the range of 30 to 39 mg/dL (reference range: 70 to 99 mg/dL), despite having received IV dextrose first from paramedics, and then in the ED. Ms. A has no history of hypoglycemia or diabetes. Her parents say that the night before coming to the ED, Ms. A had experienced flu-like symptoms, including nausea, vomiting, and diarrhea, that continued overnight and resulted in minimal food intake for 24 hours (Table 1).

A physical exam demonstrates left-sided weakness of face, arm, and leg, rightward gaze, and left-sided neglect. However, the results of CT angiography and an MRI of the brain rule out a stroke. An EEG shows right hemispheric slowing consistent with postictal paralysis, but no ongoing seizure activity. Ms. A is transferred to the pediatric intensive care unit (PICU).
Although Ms. A has no psychiatric diagnoses, she has a history of depressive symptoms, self-harm by cutting, and a suicide attempt by ingestion of an over-the-counter (OTC) medication 1 year ago. She had reported the suicide attempt to her parents several months after the fact, and asked them to find her a therapist, which her parents arranged. She also has a history of asthma, which is well-controlled with montelukast, 5 mg/d.
EVALUATION Elevated insulin levels
Subsequent investigations for organic causes of hypoglycemia are negative for adrenal insufficiency, fatty acid oxidation defect, and sepsis. Blood results demonstrate significantly elevated insulin levels of 92.4 mcIU/mL (reference range: 2.6 to 24.9 mcIU/mL) and a C-peptide level of 9.5 ng/mL (reference range: 1.1 to 4.4 ng/mL).
On Day 1 of admission to the PICU, Ms. A’s blood glucose level normalizes, and her mentation improves. Her parents report that one of them has diabetes and takes oral hypoglycemic agents at home, including glipizide immediate release (IR) tablets, 10 mg, and long-acting insulin glargine. The treatment team suspects that Ms. A may have ingested one or both of these agents, and orders a toxicologic screening for oral hypoglycemic agents.
On Day 2, the toxicology results are returned and are positive for glipizide, which Ms. A had not been prescribed. Ms. A states that she had taken only her montelukast tablet on the day of admission and adamantly denies deliberately ingesting her parent’s diabetes medications. Her parents check the home medications and state there are no missing glipizide IR tablets or insulin vials. They also report that Ms. A had no access to extended-release glipizide.
The treatment team discuss Ms. A’s clinical condition and toxicology results with the pediatric endocrinology team. The endocrinology team states that with no history of hypoglycemic episodes, it is unlikely that Ms. A had an endogenous etiology that would present so catastrophically. In their experience, inexplicable hypoglycemia in a healthy individual who lives in a household with someone who has diabetes is due to ingestion of a hypoglycemic agent until proven otherwise.
[polldaddy:10252689]
Continue to: The authors' observations
The authors’ observations
In the context of Ms. A’s prior suicide attempt and history of self-harm, the pediatric team was concerned that her presentation was consistent with a suicide attempt and consulted the psychiatry service.
Glipizide is a second-generation sulfonylurea used to treat type 2 diabetes. It lowers blood glucose by stimulating pancreatic insulin secretion. It is a rare drug of overdose.1 Although pediatric glipizide overdoses have been documented, there are currently no pediatric or adolescent glipizide pharmacokinetic studies in the literature.1-4 In adults, the immediate-release formulation has 100% oral bioavailability, with a maximum plasma concentration (Tmax) of approximately 2 hours.5 The half-life typically ranges from 4 to 6 hours in adults.6 Patients who do not have diabetes are much more susceptible to the hypoglycemic effects of glipizide because the medication simulates their fully functional pancreas to produce a vigorous insulin response.
Ms. A’s significantly elevated insulin was consistent with normal glipizide effects in a healthy child, while the elevated C-peptide was consistent with insulin being endogenously produced, which ruled out ingestion of her parent’s insulin. Importantly, the pediatric endocrinology team noted that, in their experience, a single 5- to 10-mg dose of glipizide IR was sufficient to lower blood glucose levels to the low 30s mg/dL in the context of a functional pancreas, which suggested that Ms. A might have accidentally ingested a single glipizide IR tablet, and might be telling the truth when she denies deliberately ingesting it to hurt herself.
The clinical value of pharmacokinetics
The screen of Ms. A’s toxicology sample detected glipizide. The laboratory used a semi-quantitative serum screen of several hypoglycemic agents. A positive result for each agent is based on a quantitative cut-off value, which is 3 ng/mL for glipizide. The clinical chemist on call was asked to assist in interpreting the results. The serum specimen collected on Day 1 had a significantly positive glipizide result of 86 to 130 ng/mL. The maximum effective glipizide concentration for adult patients with diabetes is 100 ng/mL.7 Thus, the glipizide level of 86 to 130 ng/mL (20.5 hours after initial symptoms) is consistent with the clinical presentation of persistent hypoglycemia requiring ongoing glucose replacement therapy.
Due to the lack of pediatric pharmacokinetic data for glipizide and only a single serum measurement, it is not possible to estimate the glipizide concentration at the time of maximal symptoms (loss of consciousness at 2:30
Continue to: Clinicians need to be aware that...
Clinicians need to be aware that although hypoglycemia usually presents rapidly, in children glipizide IR can rarely cause delayed hypoglycemia up to 16 hours after ingestion,2 and a delay of 45 hours was reported in a case of ingestion of extended-release glipizide.8 Hypoglycemia can last up to nearly 24 hours and is exacerbated if the patient has not eaten.1,2 Importantly, Ms. A’s parents reported that she had no access to extended-release glipizide. When detailed pharmacokinetic data are not available, the information provided by the patient and parents becomes extremely important, especially in distinguishing between single and multiple overdoses prior to presentation, or co-ingestions, or decreased food intake that could exacerbate hypoglycemia.
EVALUATION Safety assessment
On Day 2, Ms. A and her parents are interviewed separately, and they all are consistent in their recollection that Ms. A had been feverish with flu-like symptoms throughout the night before coming to the ED, and had still seemed mildly confused on the morning of admission.
During the interview, her parents wonder when Ms. A took her daily dose of a single montelukast tablet for asthma, and whether she had accidentally confused it with their glipizide. They report that on the morning of admission, both the glipizide and montelukast medication vials were in the same room. The vials are the same color, the same size, and labeled from the same pharmacy, and contain white, scored, round tablets that look very similar.
During the interview, Ms. A consistently denies having thoughts of hurting or killing herself on the day of admission or before that.
[polldaddy:10252690]
Continue to: The authors' observations
The authors’ observations
This case was ultimately an accidental ingestion of glipizide, rather than a suicide attempt. The initial suspicion for a suicide attempt had been reasonable in the context of Ms. A’s depressive symptoms, remote history of a prior suicide attempt by ingesting an OTC medication, and toxicologic evidence of ingesting a drug not prescribed to her. Additionally, because of the life-threatening presentation, it was easy to make the erroneous assumption that the ingestion of glipizide must have involved many tablets, and thus must have been deliberate. However, through multidisciplinary teamwork, we were able to demonstrate that this was likely an accidental ingestion by a patient who had an acute febrile illness. Her illness had caused confusion, which contributed to the accidental ingestion, and also caused reduced food intake, which enhanced the hypoglycemic effects of glipizide. Additionally, a lack of awareness of medication safety in the home had facilitated the confusion between the two medication vials.
A single tablet of glipizide IR is sufficient to produce profound clinical effects that could be mistaken by medical and psychiatric teams for a much larger and/or deliberate overdose, especially in patients with a psychiatric history. The inappropriate psychiatric hospitalization of a patient, especially a child, who has been mistakenly diagnosed as having attempted suicide, can have negative therapeutic consequences (Table 2). A psychiatric admission would have been misguided if it attempted to address safety and reduce suicidality when no such concerns were present. Additionally, it could have damaged relationships with the patient and the family, especially in a child who had historically not sought psychiatric care despite depressive symptoms and a previous suicide attempt. When assessing for suicidality, consider accidental ingestion in the differential and use specialty expertise and confirmatory testing in the evaluation, taking the pharmacokinetics of the suspected agent into account.
OUTCOME Outpatient treatment
Ms. A’s neurologic symptoms resolve within 24 hours of admission. She is offered psychiatric inpatient hospitalization to address her depressive symptoms; however, her parents prefer that she receive outpatient care. Ms. A’s parents also state that after Ms. A’s admission, they locked up all household medications and will be more mindful with medication in the home. Because her parents are arranging appropriate outpatient treatment for Ms. A’s depression and maintenance of her safety, an involuntary hospitalization is not deemed necessary.
On Day 2, Ms. A is eating normally, her blood glucose levels remain stable, and she is discharged home.
Bottom Line
Oral hypoglycemic agents can cause life-threatening syndromes in healthy patients and can clinically mimic large, intentional overdoses. Clinicians must be aware of the differential of accidental ingestion when assessing for suicidality, and can use toxicology results in their assessment.
Related Resources
- Kidemergencies.com. Emergencies: One pill can kill. http://kidemergencies.com/onepill1.html.
- Safe Kids Worldwide. Medication safety. https://www.safekids.org/medicinesafety.
- American Association of Poison Control Centers. http://www.aapcc.org/.
Drug Brand Names
Glipizide • Glucotrol
Insulin glargine • Lantus
Montelukast • Singulair
CASE Unexplained hypoglycemia
Ms. A, age 12, is brought to the emergency department (ED) via ambulance with altered mentation and life-threatening hypoglycemia for management of a hypoglycemic seizure. Earlier that day, Ms. A’s parents had found her unresponsive and incontinent of urine. In the ED, Ms. A is minimally responsive. Her blood glucose level measurements are in the range of 30 to 39 mg/dL (reference range: 70 to 99 mg/dL), despite having received IV dextrose first from paramedics, and then in the ED. Ms. A has no history of hypoglycemia or diabetes. Her parents say that the night before coming to the ED, Ms. A had experienced flu-like symptoms, including nausea, vomiting, and diarrhea, that continued overnight and resulted in minimal food intake for 24 hours (Table 1).

A physical exam demonstrates left-sided weakness of face, arm, and leg, rightward gaze, and left-sided neglect. However, the results of CT angiography and an MRI of the brain rule out a stroke. An EEG shows right hemispheric slowing consistent with postictal paralysis, but no ongoing seizure activity. Ms. A is transferred to the pediatric intensive care unit (PICU).
Although Ms. A has no psychiatric diagnoses, she has a history of depressive symptoms, self-harm by cutting, and a suicide attempt by ingestion of an over-the-counter (OTC) medication 1 year ago. She had reported the suicide attempt to her parents several months after the fact, and asked them to find her a therapist, which her parents arranged. She also has a history of asthma, which is well-controlled with montelukast, 5 mg/d.
EVALUATION Elevated insulin levels
Subsequent investigations for organic causes of hypoglycemia are negative for adrenal insufficiency, fatty acid oxidation defect, and sepsis. Blood results demonstrate significantly elevated insulin levels of 92.4 mcIU/mL (reference range: 2.6 to 24.9 mcIU/mL) and a C-peptide level of 9.5 ng/mL (reference range: 1.1 to 4.4 ng/mL).
On Day 1 of admission to the PICU, Ms. A’s blood glucose level normalizes, and her mentation improves. Her parents report that one of them has diabetes and takes oral hypoglycemic agents at home, including glipizide immediate release (IR) tablets, 10 mg, and long-acting insulin glargine. The treatment team suspects that Ms. A may have ingested one or both of these agents, and orders a toxicologic screening for oral hypoglycemic agents.
On Day 2, the toxicology results are returned and are positive for glipizide, which Ms. A had not been prescribed. Ms. A states that she had taken only her montelukast tablet on the day of admission and adamantly denies deliberately ingesting her parent’s diabetes medications. Her parents check the home medications and state there are no missing glipizide IR tablets or insulin vials. They also report that Ms. A had no access to extended-release glipizide.
The treatment team discuss Ms. A’s clinical condition and toxicology results with the pediatric endocrinology team. The endocrinology team states that with no history of hypoglycemic episodes, it is unlikely that Ms. A had an endogenous etiology that would present so catastrophically. In their experience, inexplicable hypoglycemia in a healthy individual who lives in a household with someone who has diabetes is due to ingestion of a hypoglycemic agent until proven otherwise.
[polldaddy:10252689]
Continue to: The authors' observations
The authors’ observations
In the context of Ms. A’s prior suicide attempt and history of self-harm, the pediatric team was concerned that her presentation was consistent with a suicide attempt and consulted the psychiatry service.
Glipizide is a second-generation sulfonylurea used to treat type 2 diabetes. It lowers blood glucose by stimulating pancreatic insulin secretion. It is a rare drug of overdose.1 Although pediatric glipizide overdoses have been documented, there are currently no pediatric or adolescent glipizide pharmacokinetic studies in the literature.1-4 In adults, the immediate-release formulation has 100% oral bioavailability, with a maximum plasma concentration (Tmax) of approximately 2 hours.5 The half-life typically ranges from 4 to 6 hours in adults.6 Patients who do not have diabetes are much more susceptible to the hypoglycemic effects of glipizide because the medication simulates their fully functional pancreas to produce a vigorous insulin response.
Ms. A’s significantly elevated insulin was consistent with normal glipizide effects in a healthy child, while the elevated C-peptide was consistent with insulin being endogenously produced, which ruled out ingestion of her parent’s insulin. Importantly, the pediatric endocrinology team noted that, in their experience, a single 5- to 10-mg dose of glipizide IR was sufficient to lower blood glucose levels to the low 30s mg/dL in the context of a functional pancreas, which suggested that Ms. A might have accidentally ingested a single glipizide IR tablet, and might be telling the truth when she denies deliberately ingesting it to hurt herself.
The clinical value of pharmacokinetics
The screen of Ms. A’s toxicology sample detected glipizide. The laboratory used a semi-quantitative serum screen of several hypoglycemic agents. A positive result for each agent is based on a quantitative cut-off value, which is 3 ng/mL for glipizide. The clinical chemist on call was asked to assist in interpreting the results. The serum specimen collected on Day 1 had a significantly positive glipizide result of 86 to 130 ng/mL. The maximum effective glipizide concentration for adult patients with diabetes is 100 ng/mL.7 Thus, the glipizide level of 86 to 130 ng/mL (20.5 hours after initial symptoms) is consistent with the clinical presentation of persistent hypoglycemia requiring ongoing glucose replacement therapy.
Due to the lack of pediatric pharmacokinetic data for glipizide and only a single serum measurement, it is not possible to estimate the glipizide concentration at the time of maximal symptoms (loss of consciousness at 2:30
Continue to: Clinicians need to be aware that...
Clinicians need to be aware that although hypoglycemia usually presents rapidly, in children glipizide IR can rarely cause delayed hypoglycemia up to 16 hours after ingestion,2 and a delay of 45 hours was reported in a case of ingestion of extended-release glipizide.8 Hypoglycemia can last up to nearly 24 hours and is exacerbated if the patient has not eaten.1,2 Importantly, Ms. A’s parents reported that she had no access to extended-release glipizide. When detailed pharmacokinetic data are not available, the information provided by the patient and parents becomes extremely important, especially in distinguishing between single and multiple overdoses prior to presentation, or co-ingestions, or decreased food intake that could exacerbate hypoglycemia.
EVALUATION Safety assessment
On Day 2, Ms. A and her parents are interviewed separately, and they all are consistent in their recollection that Ms. A had been feverish with flu-like symptoms throughout the night before coming to the ED, and had still seemed mildly confused on the morning of admission.
During the interview, her parents wonder when Ms. A took her daily dose of a single montelukast tablet for asthma, and whether she had accidentally confused it with their glipizide. They report that on the morning of admission, both the glipizide and montelukast medication vials were in the same room. The vials are the same color, the same size, and labeled from the same pharmacy, and contain white, scored, round tablets that look very similar.
During the interview, Ms. A consistently denies having thoughts of hurting or killing herself on the day of admission or before that.
[polldaddy:10252690]
Continue to: The authors' observations
The authors’ observations
This case was ultimately an accidental ingestion of glipizide, rather than a suicide attempt. The initial suspicion for a suicide attempt had been reasonable in the context of Ms. A’s depressive symptoms, remote history of a prior suicide attempt by ingesting an OTC medication, and toxicologic evidence of ingesting a drug not prescribed to her. Additionally, because of the life-threatening presentation, it was easy to make the erroneous assumption that the ingestion of glipizide must have involved many tablets, and thus must have been deliberate. However, through multidisciplinary teamwork, we were able to demonstrate that this was likely an accidental ingestion by a patient who had an acute febrile illness. Her illness had caused confusion, which contributed to the accidental ingestion, and also caused reduced food intake, which enhanced the hypoglycemic effects of glipizide. Additionally, a lack of awareness of medication safety in the home had facilitated the confusion between the two medication vials.
A single tablet of glipizide IR is sufficient to produce profound clinical effects that could be mistaken by medical and psychiatric teams for a much larger and/or deliberate overdose, especially in patients with a psychiatric history. The inappropriate psychiatric hospitalization of a patient, especially a child, who has been mistakenly diagnosed as having attempted suicide, can have negative therapeutic consequences (Table 2). A psychiatric admission would have been misguided if it attempted to address safety and reduce suicidality when no such concerns were present. Additionally, it could have damaged relationships with the patient and the family, especially in a child who had historically not sought psychiatric care despite depressive symptoms and a previous suicide attempt. When assessing for suicidality, consider accidental ingestion in the differential and use specialty expertise and confirmatory testing in the evaluation, taking the pharmacokinetics of the suspected agent into account.
OUTCOME Outpatient treatment
Ms. A’s neurologic symptoms resolve within 24 hours of admission. She is offered psychiatric inpatient hospitalization to address her depressive symptoms; however, her parents prefer that she receive outpatient care. Ms. A’s parents also state that after Ms. A’s admission, they locked up all household medications and will be more mindful with medication in the home. Because her parents are arranging appropriate outpatient treatment for Ms. A’s depression and maintenance of her safety, an involuntary hospitalization is not deemed necessary.
On Day 2, Ms. A is eating normally, her blood glucose levels remain stable, and she is discharged home.
Bottom Line
Oral hypoglycemic agents can cause life-threatening syndromes in healthy patients and can clinically mimic large, intentional overdoses. Clinicians must be aware of the differential of accidental ingestion when assessing for suicidality, and can use toxicology results in their assessment.
Related Resources
- Kidemergencies.com. Emergencies: One pill can kill. http://kidemergencies.com/onepill1.html.
- Safe Kids Worldwide. Medication safety. https://www.safekids.org/medicinesafety.
- American Association of Poison Control Centers. http://www.aapcc.org/.
Drug Brand Names
Glipizide • Glucotrol
Insulin glargine • Lantus
Montelukast • Singulair
1. Spiller HA, Villalobos D, Krenzelok EP, et al. Prospective multicenter study of sulfonylurea ingestion in children. J Pediatr. 1997;131(1):141-146.
2. Quadrani DA, Spiller HA, Widder P. Five year retrospective evaluation of sulfonylurea ingestion in children. J Toxicol Clin Toxicol. 1996;34(3):267-270.
3. Borowski H, Caraccio T, Mofenson H. Sulfonylurea ingestion in children: is an 8-hour observation period sufficient? J Pediatr. 1998;133(4):584-585.
4. Little GL, Boniface KS. Are one or two dangerous? Sulfony-lurea exposure in toddlers. J Emerg Med. 2005;28(3):305-310.
5. Huupponen R, Seppala P, Iisalo E. Glipizide pharma-cokinetics and response in diabetics. Int J Clin Pharmacol Ther Toxicol. 1982;20(9):417-422.
6. Baselt RC. Disposition of toxic drugs and chemicals in man. 10th ed. Seal Beach, California: Biomedical Publications; 2014.
7. Simonson DC, Kourides IA, Feinglos M, et al; the Glipizide Gastrointestinal Therapeutic System Study Group. Efficacy, safety, and dose-response characteristics of glipizide gastrointestinal therapeutic system on glycemic control and insulin secretion in NIDDM. Results of two multicenter, randomized, placebo-controlled clinical trials. Diabetes Care. 1997;20(4):597-606.
8. Pelavin PI, Abramson E, Pon S, et al. Extended-release glipizide overdose presenting with delayed hypoglycemia and treated with subcutaneous octreotide. J Pediatr Endocrinol Metab. 2009;22(2):171-175.
1. Spiller HA, Villalobos D, Krenzelok EP, et al. Prospective multicenter study of sulfonylurea ingestion in children. J Pediatr. 1997;131(1):141-146.
2. Quadrani DA, Spiller HA, Widder P. Five year retrospective evaluation of sulfonylurea ingestion in children. J Toxicol Clin Toxicol. 1996;34(3):267-270.
3. Borowski H, Caraccio T, Mofenson H. Sulfonylurea ingestion in children: is an 8-hour observation period sufficient? J Pediatr. 1998;133(4):584-585.
4. Little GL, Boniface KS. Are one or two dangerous? Sulfony-lurea exposure in toddlers. J Emerg Med. 2005;28(3):305-310.
5. Huupponen R, Seppala P, Iisalo E. Glipizide pharma-cokinetics and response in diabetics. Int J Clin Pharmacol Ther Toxicol. 1982;20(9):417-422.
6. Baselt RC. Disposition of toxic drugs and chemicals in man. 10th ed. Seal Beach, California: Biomedical Publications; 2014.
7. Simonson DC, Kourides IA, Feinglos M, et al; the Glipizide Gastrointestinal Therapeutic System Study Group. Efficacy, safety, and dose-response characteristics of glipizide gastrointestinal therapeutic system on glycemic control and insulin secretion in NIDDM. Results of two multicenter, randomized, placebo-controlled clinical trials. Diabetes Care. 1997;20(4):597-606.
8. Pelavin PI, Abramson E, Pon S, et al. Extended-release glipizide overdose presenting with delayed hypoglycemia and treated with subcutaneous octreotide. J Pediatr Endocrinol Metab. 2009;22(2):171-175.