User login
Are oral emergency contraceptives a safe & effective form of long-term birth control?
EVIDENCE SUMMARY
A systematic review of 22 trials (13 case series, 8 prospective, nonrandomized studies, and one randomized controlled trial; 12,407 patients) conducted in Europe, Asia, and the Americas evaluated the likelihood of pregnancy with repeated use of precoital and postcoital hormonal contraception.1 Some trials used more than one dose or medication. Many had inadequate reporting of research methods. Results were reported using the Pearl Index (PI)—the number of pregnancies per 100 woman-years.
In 11 studies (2700 patients), women took 750 mcg of levonorgestrel from 24 hours before to 24 hours after intercourse for an average duration of 5 cycles or months. Coital frequency varied from 1 to 15 times per month. The PI ranged from 0 to 18.6, with a pooled PI of 5.4 (95% confidence interval [CI], 4.1-7.0). Three of the trials (915 patients), with research methods reported as good, had a pooled PI of 8.9 (95% CI, 5.1-14.4). No serious adverse effects were reported in 10 of the 11 studies, but menstrual irregularity was commonly observed. In one of the largest studies (1315 patients), only 3% of women discontinued treatment because of adverse effects.
Six other trials (5785 patients) of levonorgestrel taken at doses ranging from 150 mcg to 1 mg for a mean duration of 9.2 cycles reported PIs of 0 to 9. Breakthrough bleeding was the most common adverse event. When all 17 studies of levonorgestrel were combined, the PI was 4.9 (95% CI, 4.3-5.5). The remaining studies in the systematic review described medicines not commonly used for emergency contraception or not available in the United States.
Other reported adverse effects: Headache, nausea, abdominal pain
A prospective, open-label study enrolled 321 women 18 to 45 years of age from Asia, Europe, and South America to evaluate the safety and efficacy of levonorgestrel 1.5 mg taken before or within 24 hours of intercourse as the exclusive means of contraception.2 Women who were lactating or recently postpartum were excluded; condoms were permitted for women who had concerns about risk of sexually transmitted illness. Data analysis included estimates of perfect use (consistent and correct use of levonorgestrel only) and typical use (use of other contraceptive methods in addition to levonorgestrel).
At baseline, weight, blood pressure, and hemoglobin were documented, and follow-up visits occurred at 2.5, 4.5, and 6.5 months. Pregnancy tests, blood pressure, and adverse effects were assessed at each visit; weight and hemoglobin were evaluated at the final visit. The primary outcome measure was the PI in women younger than 35 years who used only levonorgestrel for contraception.
In women younger than 35 years (208 patients), the PI was 11 (95% CI, 5.7-13.1) with perfect use and 10.3 (95% CI, 5.4-19.9) with typical use. In all ages 18 to 45 years, the PI was 7.1 (95% CI, 3.8-13.1) for typical use and 7.5 (95% CI, 4-13.9) for perfect use. Most women took 4 to 6 doses per month.
The most commonly reported adverse effects were headache (29%), nausea or abdominal pain (16%), influenza (11%), and acne or candidiasis (8%). Bleeding patterns varied with a tendency toward longer bleeding initially and lighter menstrual periods and less anemia in some patients at the end of the study.
RECOMMENDATIONS
The Office of Population Research at Princeton University suggests that moderate repeat use of emergency contraceptives is unlikely to cause serious harm, but estimates that women using progestin-only emergency contraception on a regular basis would have a 20% chance of pregnancy in a year.3
The American College of Obstetricians and Gynecologists states that long-term use of emergency contraception is less effective than other methods and may result in higher hormone levels and more adverse effects than other established means.4
The International Consortium for Emergency Contraception concluded that there is no basis for limiting the number of times that emergency contraceptives may be used in a menstrual cycle, that emergency contraceptives are safe, and that, although they are less effective than other forms of long-term contraception, using them repeatedly is more effective than using no method.5
The Society of Obstetricians and Gynecologists of Canada states that emergency contraception is intended for occasional use as a backup method.6 The Society also notes that repeat use isn’t as effective as regular use of other forms of contraception.
The Faculty of Sexual & Reproductive Healthcare of the (British) Royal College of Obstetricians and Gynaecologists says that use of levonorgestrel can be considered even if previously used one or more times in a menstrual cycle (SOR: D, based on non-analytical studies and expert opinion).7 The organization also recommends that emergency contraceptive providers share with patients that oral emergency contraceptive methods should not be used for long-term contraception (SOR: Good Practice Point, based on clinical experience of the guideline development group).
The Guttmacher Institute reports that without contraception, approximately 85% of sexually active women become pregnant each year.8 Long-acting reversible methods, such as implants and intrauterine devices, have annual pregnancy rates of 0.05% to 0.8%. With perfect (consistent and correct) use, combined oral contraceptives have a 0.3% annual pregnancy rate, but the rate rises to 9% with typical use. Condoms, when used perfectly, are associated with a 2% annual rate of pregnancy compared with an 18% rate with typical use.
1. Halpern V, Raymond EG, Lopez LM. Repeated use of pre-and postcoital hormonal contraception for the prevention of pregnancy. Cochrane Database Syst Rev. 2014 Sep 26;(9):CD007595.
2. Festin MPR, Bahamondes L, Nguyen TMH, et al. A prospective, open-label, single arm, multicentre study to evaluate efficacy, safety and acceptability of pericoital oral contraception using levonorgestrel 1.5mg. Hum Reprod. 2016;31:530-540.
3. Trussell J, Raymond EG, Cleland K. Emergency Contraception: A Last Chance to Prevent Unintended Pregnancy. Princeton, NJ: Office of Population Research & Association of Reproductive Health Professionals, June 2017. Available at: http://ec.princeton.edu/questions/ec-review.pdf. Accessed June 28, 2017.
4. American College of Obstetricians and Gynecologists. Emergency contraception. Obstet Gynecol. 2015;126:e1-e11.
5. International Consortium for Emergency Contraception. Repeated Use of Emergency Contraceptive Pills: The Facts. New York, NY: ICEC, October 2015. Available at: www.cecinfo.org/custom-content/uploads/2015/10/ICEC_Repeat-Use_Oct-2015.pdf. Accessed June 28, 2017.
6. Dunn S, Guilbert E, Burnett M, et al. Emergency contraception. J Obstet Can. 2012;34:870–878.
7. Faculty of Sexual & Reproductive Healthcare of the Royal College of Obstetricians and Gynaecologists. FSRH Guideline: Emergency Contraception. March 2017 (Updated May 29, 2017). Available at: https://www.fsrh.org/standards-and-guidance/documents/ceu-clinical-guidance-emergency-contraception-march-2017/. Accessed June 28, 2017.
8. Guttmacher Institute. Contraceptive Use in the United States. New York, NY: Guttmacher Institute, September 2016. Available at: www.guttmacher.org/fact-sheet/contraceptive-use-united-states. Accessed June 28, 2017.
EVIDENCE SUMMARY
A systematic review of 22 trials (13 case series, 8 prospective, nonrandomized studies, and one randomized controlled trial; 12,407 patients) conducted in Europe, Asia, and the Americas evaluated the likelihood of pregnancy with repeated use of precoital and postcoital hormonal contraception.1 Some trials used more than one dose or medication. Many had inadequate reporting of research methods. Results were reported using the Pearl Index (PI)—the number of pregnancies per 100 woman-years.
In 11 studies (2700 patients), women took 750 mcg of levonorgestrel from 24 hours before to 24 hours after intercourse for an average duration of 5 cycles or months. Coital frequency varied from 1 to 15 times per month. The PI ranged from 0 to 18.6, with a pooled PI of 5.4 (95% confidence interval [CI], 4.1-7.0). Three of the trials (915 patients), with research methods reported as good, had a pooled PI of 8.9 (95% CI, 5.1-14.4). No serious adverse effects were reported in 10 of the 11 studies, but menstrual irregularity was commonly observed. In one of the largest studies (1315 patients), only 3% of women discontinued treatment because of adverse effects.
Six other trials (5785 patients) of levonorgestrel taken at doses ranging from 150 mcg to 1 mg for a mean duration of 9.2 cycles reported PIs of 0 to 9. Breakthrough bleeding was the most common adverse event. When all 17 studies of levonorgestrel were combined, the PI was 4.9 (95% CI, 4.3-5.5). The remaining studies in the systematic review described medicines not commonly used for emergency contraception or not available in the United States.
Other reported adverse effects: Headache, nausea, abdominal pain
A prospective, open-label study enrolled 321 women 18 to 45 years of age from Asia, Europe, and South America to evaluate the safety and efficacy of levonorgestrel 1.5 mg taken before or within 24 hours of intercourse as the exclusive means of contraception.2 Women who were lactating or recently postpartum were excluded; condoms were permitted for women who had concerns about risk of sexually transmitted illness. Data analysis included estimates of perfect use (consistent and correct use of levonorgestrel only) and typical use (use of other contraceptive methods in addition to levonorgestrel).
At baseline, weight, blood pressure, and hemoglobin were documented, and follow-up visits occurred at 2.5, 4.5, and 6.5 months. Pregnancy tests, blood pressure, and adverse effects were assessed at each visit; weight and hemoglobin were evaluated at the final visit. The primary outcome measure was the PI in women younger than 35 years who used only levonorgestrel for contraception.
In women younger than 35 years (208 patients), the PI was 11 (95% CI, 5.7-13.1) with perfect use and 10.3 (95% CI, 5.4-19.9) with typical use. In all ages 18 to 45 years, the PI was 7.1 (95% CI, 3.8-13.1) for typical use and 7.5 (95% CI, 4-13.9) for perfect use. Most women took 4 to 6 doses per month.
The most commonly reported adverse effects were headache (29%), nausea or abdominal pain (16%), influenza (11%), and acne or candidiasis (8%). Bleeding patterns varied with a tendency toward longer bleeding initially and lighter menstrual periods and less anemia in some patients at the end of the study.
RECOMMENDATIONS
The Office of Population Research at Princeton University suggests that moderate repeat use of emergency contraceptives is unlikely to cause serious harm, but estimates that women using progestin-only emergency contraception on a regular basis would have a 20% chance of pregnancy in a year.3
The American College of Obstetricians and Gynecologists states that long-term use of emergency contraception is less effective than other methods and may result in higher hormone levels and more adverse effects than other established means.4
The International Consortium for Emergency Contraception concluded that there is no basis for limiting the number of times that emergency contraceptives may be used in a menstrual cycle, that emergency contraceptives are safe, and that, although they are less effective than other forms of long-term contraception, using them repeatedly is more effective than using no method.5
The Society of Obstetricians and Gynecologists of Canada states that emergency contraception is intended for occasional use as a backup method.6 The Society also notes that repeat use isn’t as effective as regular use of other forms of contraception.
The Faculty of Sexual & Reproductive Healthcare of the (British) Royal College of Obstetricians and Gynaecologists says that use of levonorgestrel can be considered even if previously used one or more times in a menstrual cycle (SOR: D, based on non-analytical studies and expert opinion).7 The organization also recommends that emergency contraceptive providers share with patients that oral emergency contraceptive methods should not be used for long-term contraception (SOR: Good Practice Point, based on clinical experience of the guideline development group).
The Guttmacher Institute reports that without contraception, approximately 85% of sexually active women become pregnant each year.8 Long-acting reversible methods, such as implants and intrauterine devices, have annual pregnancy rates of 0.05% to 0.8%. With perfect (consistent and correct) use, combined oral contraceptives have a 0.3% annual pregnancy rate, but the rate rises to 9% with typical use. Condoms, when used perfectly, are associated with a 2% annual rate of pregnancy compared with an 18% rate with typical use.
EVIDENCE SUMMARY
A systematic review of 22 trials (13 case series, 8 prospective, nonrandomized studies, and one randomized controlled trial; 12,407 patients) conducted in Europe, Asia, and the Americas evaluated the likelihood of pregnancy with repeated use of precoital and postcoital hormonal contraception.1 Some trials used more than one dose or medication. Many had inadequate reporting of research methods. Results were reported using the Pearl Index (PI)—the number of pregnancies per 100 woman-years.
In 11 studies (2700 patients), women took 750 mcg of levonorgestrel from 24 hours before to 24 hours after intercourse for an average duration of 5 cycles or months. Coital frequency varied from 1 to 15 times per month. The PI ranged from 0 to 18.6, with a pooled PI of 5.4 (95% confidence interval [CI], 4.1-7.0). Three of the trials (915 patients), with research methods reported as good, had a pooled PI of 8.9 (95% CI, 5.1-14.4). No serious adverse effects were reported in 10 of the 11 studies, but menstrual irregularity was commonly observed. In one of the largest studies (1315 patients), only 3% of women discontinued treatment because of adverse effects.
Six other trials (5785 patients) of levonorgestrel taken at doses ranging from 150 mcg to 1 mg for a mean duration of 9.2 cycles reported PIs of 0 to 9. Breakthrough bleeding was the most common adverse event. When all 17 studies of levonorgestrel were combined, the PI was 4.9 (95% CI, 4.3-5.5). The remaining studies in the systematic review described medicines not commonly used for emergency contraception or not available in the United States.
Other reported adverse effects: Headache, nausea, abdominal pain
A prospective, open-label study enrolled 321 women 18 to 45 years of age from Asia, Europe, and South America to evaluate the safety and efficacy of levonorgestrel 1.5 mg taken before or within 24 hours of intercourse as the exclusive means of contraception.2 Women who were lactating or recently postpartum were excluded; condoms were permitted for women who had concerns about risk of sexually transmitted illness. Data analysis included estimates of perfect use (consistent and correct use of levonorgestrel only) and typical use (use of other contraceptive methods in addition to levonorgestrel).
At baseline, weight, blood pressure, and hemoglobin were documented, and follow-up visits occurred at 2.5, 4.5, and 6.5 months. Pregnancy tests, blood pressure, and adverse effects were assessed at each visit; weight and hemoglobin were evaluated at the final visit. The primary outcome measure was the PI in women younger than 35 years who used only levonorgestrel for contraception.
In women younger than 35 years (208 patients), the PI was 11 (95% CI, 5.7-13.1) with perfect use and 10.3 (95% CI, 5.4-19.9) with typical use. In all ages 18 to 45 years, the PI was 7.1 (95% CI, 3.8-13.1) for typical use and 7.5 (95% CI, 4-13.9) for perfect use. Most women took 4 to 6 doses per month.
The most commonly reported adverse effects were headache (29%), nausea or abdominal pain (16%), influenza (11%), and acne or candidiasis (8%). Bleeding patterns varied with a tendency toward longer bleeding initially and lighter menstrual periods and less anemia in some patients at the end of the study.
RECOMMENDATIONS
The Office of Population Research at Princeton University suggests that moderate repeat use of emergency contraceptives is unlikely to cause serious harm, but estimates that women using progestin-only emergency contraception on a regular basis would have a 20% chance of pregnancy in a year.3
The American College of Obstetricians and Gynecologists states that long-term use of emergency contraception is less effective than other methods and may result in higher hormone levels and more adverse effects than other established means.4
The International Consortium for Emergency Contraception concluded that there is no basis for limiting the number of times that emergency contraceptives may be used in a menstrual cycle, that emergency contraceptives are safe, and that, although they are less effective than other forms of long-term contraception, using them repeatedly is more effective than using no method.5
The Society of Obstetricians and Gynecologists of Canada states that emergency contraception is intended for occasional use as a backup method.6 The Society also notes that repeat use isn’t as effective as regular use of other forms of contraception.
The Faculty of Sexual & Reproductive Healthcare of the (British) Royal College of Obstetricians and Gynaecologists says that use of levonorgestrel can be considered even if previously used one or more times in a menstrual cycle (SOR: D, based on non-analytical studies and expert opinion).7 The organization also recommends that emergency contraceptive providers share with patients that oral emergency contraceptive methods should not be used for long-term contraception (SOR: Good Practice Point, based on clinical experience of the guideline development group).
The Guttmacher Institute reports that without contraception, approximately 85% of sexually active women become pregnant each year.8 Long-acting reversible methods, such as implants and intrauterine devices, have annual pregnancy rates of 0.05% to 0.8%. With perfect (consistent and correct) use, combined oral contraceptives have a 0.3% annual pregnancy rate, but the rate rises to 9% with typical use. Condoms, when used perfectly, are associated with a 2% annual rate of pregnancy compared with an 18% rate with typical use.
1. Halpern V, Raymond EG, Lopez LM. Repeated use of pre-and postcoital hormonal contraception for the prevention of pregnancy. Cochrane Database Syst Rev. 2014 Sep 26;(9):CD007595.
2. Festin MPR, Bahamondes L, Nguyen TMH, et al. A prospective, open-label, single arm, multicentre study to evaluate efficacy, safety and acceptability of pericoital oral contraception using levonorgestrel 1.5mg. Hum Reprod. 2016;31:530-540.
3. Trussell J, Raymond EG, Cleland K. Emergency Contraception: A Last Chance to Prevent Unintended Pregnancy. Princeton, NJ: Office of Population Research & Association of Reproductive Health Professionals, June 2017. Available at: http://ec.princeton.edu/questions/ec-review.pdf. Accessed June 28, 2017.
4. American College of Obstetricians and Gynecologists. Emergency contraception. Obstet Gynecol. 2015;126:e1-e11.
5. International Consortium for Emergency Contraception. Repeated Use of Emergency Contraceptive Pills: The Facts. New York, NY: ICEC, October 2015. Available at: www.cecinfo.org/custom-content/uploads/2015/10/ICEC_Repeat-Use_Oct-2015.pdf. Accessed June 28, 2017.
6. Dunn S, Guilbert E, Burnett M, et al. Emergency contraception. J Obstet Can. 2012;34:870–878.
7. Faculty of Sexual & Reproductive Healthcare of the Royal College of Obstetricians and Gynaecologists. FSRH Guideline: Emergency Contraception. March 2017 (Updated May 29, 2017). Available at: https://www.fsrh.org/standards-and-guidance/documents/ceu-clinical-guidance-emergency-contraception-march-2017/. Accessed June 28, 2017.
8. Guttmacher Institute. Contraceptive Use in the United States. New York, NY: Guttmacher Institute, September 2016. Available at: www.guttmacher.org/fact-sheet/contraceptive-use-united-states. Accessed June 28, 2017.
1. Halpern V, Raymond EG, Lopez LM. Repeated use of pre-and postcoital hormonal contraception for the prevention of pregnancy. Cochrane Database Syst Rev. 2014 Sep 26;(9):CD007595.
2. Festin MPR, Bahamondes L, Nguyen TMH, et al. A prospective, open-label, single arm, multicentre study to evaluate efficacy, safety and acceptability of pericoital oral contraception using levonorgestrel 1.5mg. Hum Reprod. 2016;31:530-540.
3. Trussell J, Raymond EG, Cleland K. Emergency Contraception: A Last Chance to Prevent Unintended Pregnancy. Princeton, NJ: Office of Population Research & Association of Reproductive Health Professionals, June 2017. Available at: http://ec.princeton.edu/questions/ec-review.pdf. Accessed June 28, 2017.
4. American College of Obstetricians and Gynecologists. Emergency contraception. Obstet Gynecol. 2015;126:e1-e11.
5. International Consortium for Emergency Contraception. Repeated Use of Emergency Contraceptive Pills: The Facts. New York, NY: ICEC, October 2015. Available at: www.cecinfo.org/custom-content/uploads/2015/10/ICEC_Repeat-Use_Oct-2015.pdf. Accessed June 28, 2017.
6. Dunn S, Guilbert E, Burnett M, et al. Emergency contraception. J Obstet Can. 2012;34:870–878.
7. Faculty of Sexual & Reproductive Healthcare of the Royal College of Obstetricians and Gynaecologists. FSRH Guideline: Emergency Contraception. March 2017 (Updated May 29, 2017). Available at: https://www.fsrh.org/standards-and-guidance/documents/ceu-clinical-guidance-emergency-contraception-march-2017/. Accessed June 28, 2017.
8. Guttmacher Institute. Contraceptive Use in the United States. New York, NY: Guttmacher Institute, September 2016. Available at: www.guttmacher.org/fact-sheet/contraceptive-use-united-states. Accessed June 28, 2017.
Evidence-based answers from the Family Physicians Inquiries Network
EVIDENCE-BASED ANSWER:
Yes, but not as effective as some other methods. Annual pregnancy rates in women using pericoital levonorgestrel 150 mcg to 1 mg range from 4.9% to 8.9%; menstrual irregularity is the most common adverse effect (strength of recommendation [SOR]: B, Cochrane review of lower-quality trials).
In women younger than 35 years who have sexual intercourse 6 or fewer times per month, correct and consistent use of pericoital levonorgestrel 1.5 mg results in an annual pregnancy rate of 11% (SOR: B, one large prospective, open-label trial).
Pericoital contraception is less effective than long-acting reversible contraceptives (annual pregnancy rates of 0.05%-0.8%) or perfect use of combined oral contraceptives (0.3% annual pregnancy rate), but similar to, or better than, typical use of combined oral contraception (9%) and condoms (18%).
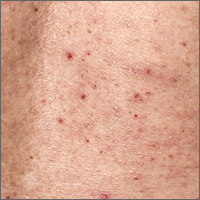
What is the most effective treatment for scabies?
EVIDENCE SUMMARY
A 2007 Cochrane review on scabies treatment identified 11 trials that evaluated permethrin for treating scabies.1 In 2 trials, 140 patients were randomized to receive either 200 mcg/kg of oral ivermectin or overnight application of 5% topical permethrin. Topical permethrin was superior to oral ivermectin with failure rates at 2 weeks of 8% and 39%, respectively (number needed to treat [NNT]=4; risk ratio [RR]=4.61; 95% confidence interval [CI], 2.07-10.26).
Two trials compared 5% topical permethrin with 10% topical crotamiton in 194 patients with follow-up at 28 days. Permethrin was superior to crotamiton with failure rates of 6% and 26%, respectively (NNT=6; RR=0.24; 95% CI, 0.10-0.55).
Five trials with 753 patients compared topical permethrin, 2.5% to 3.5%, with topical 1% lindane, but heterogeneity precluded pooling all the studies. In the 3 studies (554 patients) that were comparable, topical 3.5% permethrin was superior to lindane after a single application of each with failure rates of 9% and 15%, respectively (NNT=17; RR=0.59; 95% CI, 0.37-0.95).
Two trials that compared permethrin with topical benzyl benzoate (53 patients) and natural synergized pyrethrins (40 patients) showed no difference in treatment failures, but the trials were small and lacked sufficient statistical power.
Four additional studies included in the review compared crotamiton with lindane (100 patients), lindane with sulfur (68 patients), benzyl benzoate with sulfur (158 patients), and benzyl benzoate with natural synergized pyrethrins (240 patients). None demonstrated superiority, but all were small studies.1 A single small trial of 55 patients that compared oral ivermectin 200 mcg/kg with placebo showed failure rates at one week of 21% and 85%, respectively (NNT=2; RR=0.24; 95% CI, 0.12-0.51).1
Topical permethrin vs oral ivermectin
A 2014 systematic review of 5 studies included 2 new studies done after the 2007 Cochrane review.2 The new RCTs compared a single application of 5% topical permethrin with a single dose or 2 doses of oral ivermectin given 2 weeks apart. No statistically significant differences were found in these studies.2 Both underpowered studies favored topical permethrin, however.
The P value was .42 in one study of 242 adults and children, and this trial showed a clinical cure rate at 2 weeks of 93% using topical permethrin vs 86% using oral ivermectin.2
The other study of 120 adults and children didn’t report a P value or identify statistically significant differences between topical permethrin and oral ivermectin.2 This study reported a clinical cure rate of 87% with topical permethrin, 78% with a single dose of oral ivermectin, and 67% with 2 doses of oral ivermectin 2 weeks apart.2
Ivermectin may control endemic scabies better than permethrin
A 2015 randomized controlled trial with 2051 patients compared mass treatments in a scabies-endemic population in Fiji.3 The trial had 3 arms: a standard-care group treated with 5% topical permethrin if symptoms were present and retreated at 2 weeks if symptoms persisted; a permethrin group in which all participants, whether infected or not, received 5% permethrin followed by a second dose at 7 to 14 days if symptoms persisted; and an oral ivermectin group in which participants were treated with 200 mcg/kg, repeated in 7 to 14 days for those with baseline scabies.
At 12 months, the relative risk reductions were 94% (95% CI, 83%-100%) for the ivermectin group, 62% (95% CI, 49%-75%) for the permethrin group, and 49% (95% CI, 37%-60%) for the standard-care group.3 The study had multiple limitations, and all groups were permitted to receive standard care at any time during the 12-month follow-up period. Nevertheless, the findings suggest that endemic scabies control with ivermectin may be superior to topical permethrin.
RECOMMENDATIONS
The Centers for Disease Control and Prevention (CDC)4 and the European Guideline for the Management of Scabies5 both recommend topical permethrin as first-line therapy for classical scabies and note that oral ivermectin may be safe and effective but isn’t licensed for scabies treatment in most countries. Ivermectin isn’t approved by the United States Food and Drug Administration for treating scabies.
The CDC recommendations note that the safety of ivermectin in children weighing less than 15 kg and pregnant women hasn’t been established.4
1. Strong M, Johnstone P. Interventions for treating scabies. Cochrane Database Syst Rev. 2007;(3):CD000320.
2. Johnstone P, Strong M. Scabies. BMJ Clinical Evidence. 2014:1707.
3. Romani L, Whitfeld MJ, Koroivueta J, et al. Mass drug administration for scabies control in a population with endemic disease. N Engl J Med. 2015;373:2305-2313.
4. Centers for Disease Control and Prevention. Scabies. Treatment. Available at: www.cdc.gov/parasites/scabies/health_professionals/meds.html. Accessed February 26, 2016.
5. Scott G, Chosidow O. European guideline for the management of scabies, 2010. Int J STD AIDS. 2011;22:301-303.
EVIDENCE SUMMARY
A 2007 Cochrane review on scabies treatment identified 11 trials that evaluated permethrin for treating scabies.1 In 2 trials, 140 patients were randomized to receive either 200 mcg/kg of oral ivermectin or overnight application of 5% topical permethrin. Topical permethrin was superior to oral ivermectin with failure rates at 2 weeks of 8% and 39%, respectively (number needed to treat [NNT]=4; risk ratio [RR]=4.61; 95% confidence interval [CI], 2.07-10.26).
Two trials compared 5% topical permethrin with 10% topical crotamiton in 194 patients with follow-up at 28 days. Permethrin was superior to crotamiton with failure rates of 6% and 26%, respectively (NNT=6; RR=0.24; 95% CI, 0.10-0.55).
Five trials with 753 patients compared topical permethrin, 2.5% to 3.5%, with topical 1% lindane, but heterogeneity precluded pooling all the studies. In the 3 studies (554 patients) that were comparable, topical 3.5% permethrin was superior to lindane after a single application of each with failure rates of 9% and 15%, respectively (NNT=17; RR=0.59; 95% CI, 0.37-0.95).
Two trials that compared permethrin with topical benzyl benzoate (53 patients) and natural synergized pyrethrins (40 patients) showed no difference in treatment failures, but the trials were small and lacked sufficient statistical power.
Four additional studies included in the review compared crotamiton with lindane (100 patients), lindane with sulfur (68 patients), benzyl benzoate with sulfur (158 patients), and benzyl benzoate with natural synergized pyrethrins (240 patients). None demonstrated superiority, but all were small studies.1 A single small trial of 55 patients that compared oral ivermectin 200 mcg/kg with placebo showed failure rates at one week of 21% and 85%, respectively (NNT=2; RR=0.24; 95% CI, 0.12-0.51).1
Topical permethrin vs oral ivermectin
A 2014 systematic review of 5 studies included 2 new studies done after the 2007 Cochrane review.2 The new RCTs compared a single application of 5% topical permethrin with a single dose or 2 doses of oral ivermectin given 2 weeks apart. No statistically significant differences were found in these studies.2 Both underpowered studies favored topical permethrin, however.
The P value was .42 in one study of 242 adults and children, and this trial showed a clinical cure rate at 2 weeks of 93% using topical permethrin vs 86% using oral ivermectin.2
The other study of 120 adults and children didn’t report a P value or identify statistically significant differences between topical permethrin and oral ivermectin.2 This study reported a clinical cure rate of 87% with topical permethrin, 78% with a single dose of oral ivermectin, and 67% with 2 doses of oral ivermectin 2 weeks apart.2
Ivermectin may control endemic scabies better than permethrin
A 2015 randomized controlled trial with 2051 patients compared mass treatments in a scabies-endemic population in Fiji.3 The trial had 3 arms: a standard-care group treated with 5% topical permethrin if symptoms were present and retreated at 2 weeks if symptoms persisted; a permethrin group in which all participants, whether infected or not, received 5% permethrin followed by a second dose at 7 to 14 days if symptoms persisted; and an oral ivermectin group in which participants were treated with 200 mcg/kg, repeated in 7 to 14 days for those with baseline scabies.
At 12 months, the relative risk reductions were 94% (95% CI, 83%-100%) for the ivermectin group, 62% (95% CI, 49%-75%) for the permethrin group, and 49% (95% CI, 37%-60%) for the standard-care group.3 The study had multiple limitations, and all groups were permitted to receive standard care at any time during the 12-month follow-up period. Nevertheless, the findings suggest that endemic scabies control with ivermectin may be superior to topical permethrin.
RECOMMENDATIONS
The Centers for Disease Control and Prevention (CDC)4 and the European Guideline for the Management of Scabies5 both recommend topical permethrin as first-line therapy for classical scabies and note that oral ivermectin may be safe and effective but isn’t licensed for scabies treatment in most countries. Ivermectin isn’t approved by the United States Food and Drug Administration for treating scabies.
The CDC recommendations note that the safety of ivermectin in children weighing less than 15 kg and pregnant women hasn’t been established.4
EVIDENCE SUMMARY
A 2007 Cochrane review on scabies treatment identified 11 trials that evaluated permethrin for treating scabies.1 In 2 trials, 140 patients were randomized to receive either 200 mcg/kg of oral ivermectin or overnight application of 5% topical permethrin. Topical permethrin was superior to oral ivermectin with failure rates at 2 weeks of 8% and 39%, respectively (number needed to treat [NNT]=4; risk ratio [RR]=4.61; 95% confidence interval [CI], 2.07-10.26).
Two trials compared 5% topical permethrin with 10% topical crotamiton in 194 patients with follow-up at 28 days. Permethrin was superior to crotamiton with failure rates of 6% and 26%, respectively (NNT=6; RR=0.24; 95% CI, 0.10-0.55).
Five trials with 753 patients compared topical permethrin, 2.5% to 3.5%, with topical 1% lindane, but heterogeneity precluded pooling all the studies. In the 3 studies (554 patients) that were comparable, topical 3.5% permethrin was superior to lindane after a single application of each with failure rates of 9% and 15%, respectively (NNT=17; RR=0.59; 95% CI, 0.37-0.95).
Two trials that compared permethrin with topical benzyl benzoate (53 patients) and natural synergized pyrethrins (40 patients) showed no difference in treatment failures, but the trials were small and lacked sufficient statistical power.
Four additional studies included in the review compared crotamiton with lindane (100 patients), lindane with sulfur (68 patients), benzyl benzoate with sulfur (158 patients), and benzyl benzoate with natural synergized pyrethrins (240 patients). None demonstrated superiority, but all were small studies.1 A single small trial of 55 patients that compared oral ivermectin 200 mcg/kg with placebo showed failure rates at one week of 21% and 85%, respectively (NNT=2; RR=0.24; 95% CI, 0.12-0.51).1
Topical permethrin vs oral ivermectin
A 2014 systematic review of 5 studies included 2 new studies done after the 2007 Cochrane review.2 The new RCTs compared a single application of 5% topical permethrin with a single dose or 2 doses of oral ivermectin given 2 weeks apart. No statistically significant differences were found in these studies.2 Both underpowered studies favored topical permethrin, however.
The P value was .42 in one study of 242 adults and children, and this trial showed a clinical cure rate at 2 weeks of 93% using topical permethrin vs 86% using oral ivermectin.2
The other study of 120 adults and children didn’t report a P value or identify statistically significant differences between topical permethrin and oral ivermectin.2 This study reported a clinical cure rate of 87% with topical permethrin, 78% with a single dose of oral ivermectin, and 67% with 2 doses of oral ivermectin 2 weeks apart.2
Ivermectin may control endemic scabies better than permethrin
A 2015 randomized controlled trial with 2051 patients compared mass treatments in a scabies-endemic population in Fiji.3 The trial had 3 arms: a standard-care group treated with 5% topical permethrin if symptoms were present and retreated at 2 weeks if symptoms persisted; a permethrin group in which all participants, whether infected or not, received 5% permethrin followed by a second dose at 7 to 14 days if symptoms persisted; and an oral ivermectin group in which participants were treated with 200 mcg/kg, repeated in 7 to 14 days for those with baseline scabies.
At 12 months, the relative risk reductions were 94% (95% CI, 83%-100%) for the ivermectin group, 62% (95% CI, 49%-75%) for the permethrin group, and 49% (95% CI, 37%-60%) for the standard-care group.3 The study had multiple limitations, and all groups were permitted to receive standard care at any time during the 12-month follow-up period. Nevertheless, the findings suggest that endemic scabies control with ivermectin may be superior to topical permethrin.
RECOMMENDATIONS
The Centers for Disease Control and Prevention (CDC)4 and the European Guideline for the Management of Scabies5 both recommend topical permethrin as first-line therapy for classical scabies and note that oral ivermectin may be safe and effective but isn’t licensed for scabies treatment in most countries. Ivermectin isn’t approved by the United States Food and Drug Administration for treating scabies.
The CDC recommendations note that the safety of ivermectin in children weighing less than 15 kg and pregnant women hasn’t been established.4
1. Strong M, Johnstone P. Interventions for treating scabies. Cochrane Database Syst Rev. 2007;(3):CD000320.
2. Johnstone P, Strong M. Scabies. BMJ Clinical Evidence. 2014:1707.
3. Romani L, Whitfeld MJ, Koroivueta J, et al. Mass drug administration for scabies control in a population with endemic disease. N Engl J Med. 2015;373:2305-2313.
4. Centers for Disease Control and Prevention. Scabies. Treatment. Available at: www.cdc.gov/parasites/scabies/health_professionals/meds.html. Accessed February 26, 2016.
5. Scott G, Chosidow O. European guideline for the management of scabies, 2010. Int J STD AIDS. 2011;22:301-303.
1. Strong M, Johnstone P. Interventions for treating scabies. Cochrane Database Syst Rev. 2007;(3):CD000320.
2. Johnstone P, Strong M. Scabies. BMJ Clinical Evidence. 2014:1707.
3. Romani L, Whitfeld MJ, Koroivueta J, et al. Mass drug administration for scabies control in a population with endemic disease. N Engl J Med. 2015;373:2305-2313.
4. Centers for Disease Control and Prevention. Scabies. Treatment. Available at: www.cdc.gov/parasites/scabies/health_professionals/meds.html. Accessed February 26, 2016.
5. Scott G, Chosidow O. European guideline for the management of scabies, 2010. Int J STD AIDS. 2011;22:301-303.
Evidence-based answers from the Family Physicians Inquiries Network
EVIDENCE-BASED ANSWER:
Topical permethrin is the most effective treatment for classic scabies (strength of recommendation [SOR]: A, meta-analyses with consistent results).
Topical lindane and crotamiton are inferior to permethrin but appear equivalent to each other and benzyl benzoate, sulfur, and natural synergized pyrethrins (SOR: B, limited randomized trials).
Although not as effective as topical permethrin, oral ivermectin is an effective treatment compared with placebo (SOR: B, a single small randomized trial).
Oral ivermectin may reduce the prevalence of scabies at one year in populations with endemic disease more than topical permethrin (SOR: B, a single randomized trial).
What effects—if any—does marijuana use during pregnancy have on the fetus or child?
EVIDENCE SUMMARY
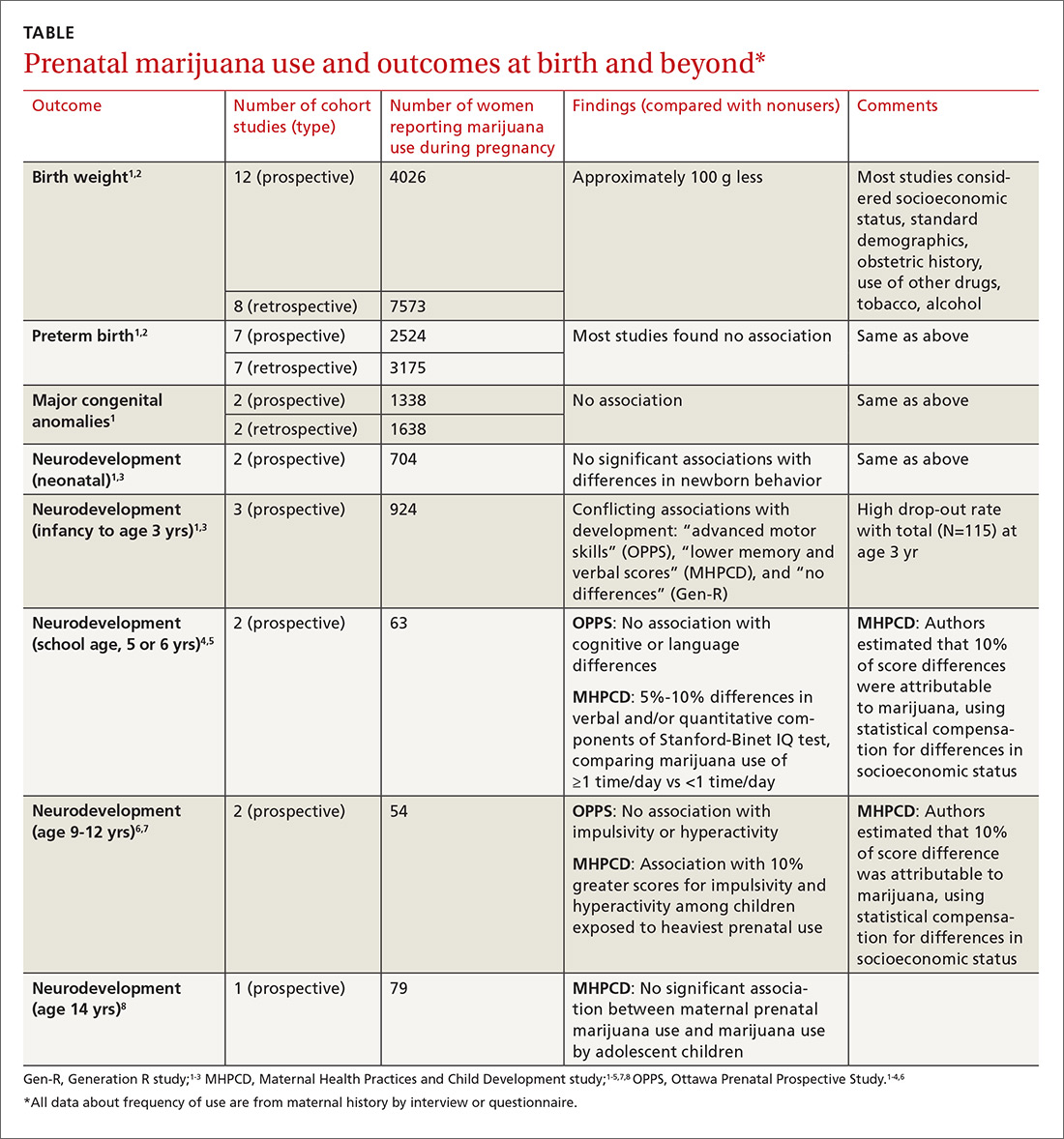
A large systematic review of prospective and retrospective cohort studies found little or no effect of maternal marijuana use on birth weight, stillbirths, preterm births, or congenital anomalies (TABLE1-8). Some studies found lower birth weights and some found higher birth weights. The authors couldn’t perform a meta-analysis because of heterogeneity, but estimated a clinically insignificant difference of 100 g. Most studies were limited by failure to account for concurrent maternal tobacco smoking.
Moreover, all studies used interview data to determine maternal prenatal marijuana use, which can be subject to large recall bias. A multicenter prospective study of 585 pregnant women that compared interview data with serum screening to identify tetrahydrocannabinol (THC) found poor correlation between history and laboratory validation, for example.1 Only 31% of pregnant women with positive THC testing self-reported marijuana use (31% sensitivity), and only 43% of women who reported marijuana use had a positive THC screen (43% specificity). Most studies didn’t quantify marijuana use well and didn’t associate use with trimester of exposure.
The authors also point out that marijuana potency has increased substantially since the 1980s when many of the studies were done (THC content was 3.2% in 1983 and 13% in 2008); prenatal marijuana use in the present day may expose the fetus to larger amounts of THC.1
A 2016 retrospective cohort study of 56 mothers who reported prenatal marijuana use found no differences in preterm birth, low birth weight, or Apgar scores.2
Neurodevelopmental effects on infants, long-term effects on children, teens
Three prospective cohort studies evaluated neurodevelopmental outcomes in neonates and infants, and 2 studies continued to follow children into adolescence.1,3 All found essentially no differences associated with prenatal marijuana at birth, throughout infancy, and through age 3 years. The studies had the same limitations as those described previously (potential recall bias for identifying which children were exposed to marijuana prenatally and poorly quantified marijuana use not well-associated with trimester of exposure).
The Ottawa Prenatal Prospective Study (OPPS) examined 140 low-risk pregnancies in white women of higher socioeconomic status who used marijuana during pregnancy.1,3-7 Investigators considered: socioeconomic status, standard demographics, obstetric history, and use of other drugs, tobacco, and alcohol. Using a standardized newborn assessment scale, they found subtle behavioral differences at one week but not 9 days. Investigators evaluated children again at 3 years of age, school entry (5 or 6 years), and 9 to 12 years.
The Maternal Health Practices and Child Development study (MHPCD) of 564 high-risk pregnancies in predominantly minority women of low socioeconomic status followed infants from birth through 14 years of age.1,3-5,7,8 It found some small differences in outcomes among children exposed to marijuana prenatally. Of note, when investigators evaluated marijuana use at age 14 years, they compared adolescent self-report history with urine THC testing (specificity 78%).
The MHPCD study was limited because, compared with the nonusing group, mothers who used marijuana were also 20% to 25% more likely to be single and poor, to live in poorer quality homes, and to use alcohol, tobacco, and other drugs. Investigators used statistical modeling to account for these environmental differences and estimated that 10% of the difference in outcomes was attributable to prenatal marijuana exposure.
The Generation R study (Gen R) enrolled 220 lower-risk pregnancies in multiethnic European women of higher socioeconomic status, followed children to 3 years of age, and found no marijuana-associated differences in any parameter.1,3,4 The final assessment included only 51 children.
RECOMMENDATIONS
The American College of Obstetricians and Gynecologists (ACOG) recommends screening all women for tobacco, alcohol, and drug use (including marijuana) during early pregnancy.9 Women who report marijuana use should be counseled regarding potential adverse consequences to fetal health and be encouraged to discontinue use.
ACOG says that insufficient data exist to evaluate the effects of marijuana use on infants during lactation and breastfeeding and recommends against it.
The American Society of Addiction Medicine also recommends screening pregnant women for drug use and making appropriate referrals for substance use treatment.10
1. Metz TD, Stickrath EH. Marijuana use in pregnancy and lactation: a review of the evidence. Am J Obstet Gynecol. 2015;213:761-778.
2. Chabarria KC, Racusin DA, Antony KM, et al. Marijuana use and its effects in pregnancy. Am J Obstet Gynecol. 2016;215:506.e1-e7.
3. Warner TD, Roussos-Ross D, Behnke M. It’s not your mother’s marijuana: effects on maternal-fetal health and the developing child. Clinical Perinatology. 2014;41:877-894.
4. Huizink AC. Prenatal cannabis exposure and infant outcomes: overview of studies. Prog Neuro-Psychopharmacol Biol Psychiatry. 2014;52:45-52.
5. Goldschmidt L, Richardson GA, Willford J, et al. Prenatal marijuana exposure and intelligence test performance at age 6. J Am Acad Child Adolesc Psychiatry. 2008;47:254-263.
6. Fried PA. The Ottawa Prenatal Prospective Study (OPPS): methodological issues and findings—it’s easy to throw the baby out with the bath water. Life Sci. 1995;56:2159-2168.
7. Goldschmidt L, Day NL, Richardson GA. Effects of prenatal marijuana exposure on child behavior problems at age 10. Neurotoxicol Teratol. 2000;22:325-336.
8. Day NL, Goldschmidt L, Thomas CA. Prenatal marijuana exposure contributes to the prediction of marijuana use at age 14. Addiction. 2006;101:1313-1322.
9. American College of Obstetricians and Gynecologists Committee on Obstetric Practice. Committee Opinion No. 637: Marijuana use during pregnancy and lactation. Obstet Gynecol. 2015;126:234-238.
10. American Society of Addiction Medicine. Public policy statement on women, alcohol and other drugs, and pregnancy. Chevy Chase MD: American Society of Addiction Medicine; 2011. Available at: http://www.asam.org/docs/default-source/public-policy-statements/1womenandpregnancy_7-11.pdf. Accessed July 5, 2016.
EVIDENCE SUMMARY
A large systematic review of prospective and retrospective cohort studies found little or no effect of maternal marijuana use on birth weight, stillbirths, preterm births, or congenital anomalies (TABLE1-8). Some studies found lower birth weights and some found higher birth weights. The authors couldn’t perform a meta-analysis because of heterogeneity, but estimated a clinically insignificant difference of 100 g. Most studies were limited by failure to account for concurrent maternal tobacco smoking.

Moreover, all studies used interview data to determine maternal prenatal marijuana use, which can be subject to large recall bias. A multicenter prospective study of 585 pregnant women that compared interview data with serum screening to identify tetrahydrocannabinol (THC) found poor correlation between history and laboratory validation, for example.1 Only 31% of pregnant women with positive THC testing self-reported marijuana use (31% sensitivity), and only 43% of women who reported marijuana use had a positive THC screen (43% specificity). Most studies didn’t quantify marijuana use well and didn’t associate use with trimester of exposure.
The authors also point out that marijuana potency has increased substantially since the 1980s when many of the studies were done (THC content was 3.2% in 1983 and 13% in 2008); prenatal marijuana use in the present day may expose the fetus to larger amounts of THC.1
A 2016 retrospective cohort study of 56 mothers who reported prenatal marijuana use found no differences in preterm birth, low birth weight, or Apgar scores.2
Neurodevelopmental effects on infants, long-term effects on children, teens
Three prospective cohort studies evaluated neurodevelopmental outcomes in neonates and infants, and 2 studies continued to follow children into adolescence.1,3 All found essentially no differences associated with prenatal marijuana at birth, throughout infancy, and through age 3 years. The studies had the same limitations as those described previously (potential recall bias for identifying which children were exposed to marijuana prenatally and poorly quantified marijuana use not well-associated with trimester of exposure).
The Ottawa Prenatal Prospective Study (OPPS) examined 140 low-risk pregnancies in white women of higher socioeconomic status who used marijuana during pregnancy.1,3-7 Investigators considered: socioeconomic status, standard demographics, obstetric history, and use of other drugs, tobacco, and alcohol. Using a standardized newborn assessment scale, they found subtle behavioral differences at one week but not 9 days. Investigators evaluated children again at 3 years of age, school entry (5 or 6 years), and 9 to 12 years.
The Maternal Health Practices and Child Development study (MHPCD) of 564 high-risk pregnancies in predominantly minority women of low socioeconomic status followed infants from birth through 14 years of age.1,3-5,7,8 It found some small differences in outcomes among children exposed to marijuana prenatally. Of note, when investigators evaluated marijuana use at age 14 years, they compared adolescent self-report history with urine THC testing (specificity 78%).
The MHPCD study was limited because, compared with the nonusing group, mothers who used marijuana were also 20% to 25% more likely to be single and poor, to live in poorer quality homes, and to use alcohol, tobacco, and other drugs. Investigators used statistical modeling to account for these environmental differences and estimated that 10% of the difference in outcomes was attributable to prenatal marijuana exposure.
The Generation R study (Gen R) enrolled 220 lower-risk pregnancies in multiethnic European women of higher socioeconomic status, followed children to 3 years of age, and found no marijuana-associated differences in any parameter.1,3,4 The final assessment included only 51 children.
RECOMMENDATIONS
The American College of Obstetricians and Gynecologists (ACOG) recommends screening all women for tobacco, alcohol, and drug use (including marijuana) during early pregnancy.9 Women who report marijuana use should be counseled regarding potential adverse consequences to fetal health and be encouraged to discontinue use.
ACOG says that insufficient data exist to evaluate the effects of marijuana use on infants during lactation and breastfeeding and recommends against it.
The American Society of Addiction Medicine also recommends screening pregnant women for drug use and making appropriate referrals for substance use treatment.10
EVIDENCE SUMMARY
A large systematic review of prospective and retrospective cohort studies found little or no effect of maternal marijuana use on birth weight, stillbirths, preterm births, or congenital anomalies (TABLE1-8). Some studies found lower birth weights and some found higher birth weights. The authors couldn’t perform a meta-analysis because of heterogeneity, but estimated a clinically insignificant difference of 100 g. Most studies were limited by failure to account for concurrent maternal tobacco smoking.

Moreover, all studies used interview data to determine maternal prenatal marijuana use, which can be subject to large recall bias. A multicenter prospective study of 585 pregnant women that compared interview data with serum screening to identify tetrahydrocannabinol (THC) found poor correlation between history and laboratory validation, for example.1 Only 31% of pregnant women with positive THC testing self-reported marijuana use (31% sensitivity), and only 43% of women who reported marijuana use had a positive THC screen (43% specificity). Most studies didn’t quantify marijuana use well and didn’t associate use with trimester of exposure.
The authors also point out that marijuana potency has increased substantially since the 1980s when many of the studies were done (THC content was 3.2% in 1983 and 13% in 2008); prenatal marijuana use in the present day may expose the fetus to larger amounts of THC.1
A 2016 retrospective cohort study of 56 mothers who reported prenatal marijuana use found no differences in preterm birth, low birth weight, or Apgar scores.2
Neurodevelopmental effects on infants, long-term effects on children, teens
Three prospective cohort studies evaluated neurodevelopmental outcomes in neonates and infants, and 2 studies continued to follow children into adolescence.1,3 All found essentially no differences associated with prenatal marijuana at birth, throughout infancy, and through age 3 years. The studies had the same limitations as those described previously (potential recall bias for identifying which children were exposed to marijuana prenatally and poorly quantified marijuana use not well-associated with trimester of exposure).
The Ottawa Prenatal Prospective Study (OPPS) examined 140 low-risk pregnancies in white women of higher socioeconomic status who used marijuana during pregnancy.1,3-7 Investigators considered: socioeconomic status, standard demographics, obstetric history, and use of other drugs, tobacco, and alcohol. Using a standardized newborn assessment scale, they found subtle behavioral differences at one week but not 9 days. Investigators evaluated children again at 3 years of age, school entry (5 or 6 years), and 9 to 12 years.
The Maternal Health Practices and Child Development study (MHPCD) of 564 high-risk pregnancies in predominantly minority women of low socioeconomic status followed infants from birth through 14 years of age.1,3-5,7,8 It found some small differences in outcomes among children exposed to marijuana prenatally. Of note, when investigators evaluated marijuana use at age 14 years, they compared adolescent self-report history with urine THC testing (specificity 78%).
The MHPCD study was limited because, compared with the nonusing group, mothers who used marijuana were also 20% to 25% more likely to be single and poor, to live in poorer quality homes, and to use alcohol, tobacco, and other drugs. Investigators used statistical modeling to account for these environmental differences and estimated that 10% of the difference in outcomes was attributable to prenatal marijuana exposure.
The Generation R study (Gen R) enrolled 220 lower-risk pregnancies in multiethnic European women of higher socioeconomic status, followed children to 3 years of age, and found no marijuana-associated differences in any parameter.1,3,4 The final assessment included only 51 children.
RECOMMENDATIONS
The American College of Obstetricians and Gynecologists (ACOG) recommends screening all women for tobacco, alcohol, and drug use (including marijuana) during early pregnancy.9 Women who report marijuana use should be counseled regarding potential adverse consequences to fetal health and be encouraged to discontinue use.
ACOG says that insufficient data exist to evaluate the effects of marijuana use on infants during lactation and breastfeeding and recommends against it.
The American Society of Addiction Medicine also recommends screening pregnant women for drug use and making appropriate referrals for substance use treatment.10
1. Metz TD, Stickrath EH. Marijuana use in pregnancy and lactation: a review of the evidence. Am J Obstet Gynecol. 2015;213:761-778.
2. Chabarria KC, Racusin DA, Antony KM, et al. Marijuana use and its effects in pregnancy. Am J Obstet Gynecol. 2016;215:506.e1-e7.
3. Warner TD, Roussos-Ross D, Behnke M. It’s not your mother’s marijuana: effects on maternal-fetal health and the developing child. Clinical Perinatology. 2014;41:877-894.
4. Huizink AC. Prenatal cannabis exposure and infant outcomes: overview of studies. Prog Neuro-Psychopharmacol Biol Psychiatry. 2014;52:45-52.
5. Goldschmidt L, Richardson GA, Willford J, et al. Prenatal marijuana exposure and intelligence test performance at age 6. J Am Acad Child Adolesc Psychiatry. 2008;47:254-263.
6. Fried PA. The Ottawa Prenatal Prospective Study (OPPS): methodological issues and findings—it’s easy to throw the baby out with the bath water. Life Sci. 1995;56:2159-2168.
7. Goldschmidt L, Day NL, Richardson GA. Effects of prenatal marijuana exposure on child behavior problems at age 10. Neurotoxicol Teratol. 2000;22:325-336.
8. Day NL, Goldschmidt L, Thomas CA. Prenatal marijuana exposure contributes to the prediction of marijuana use at age 14. Addiction. 2006;101:1313-1322.
9. American College of Obstetricians and Gynecologists Committee on Obstetric Practice. Committee Opinion No. 637: Marijuana use during pregnancy and lactation. Obstet Gynecol. 2015;126:234-238.
10. American Society of Addiction Medicine. Public policy statement on women, alcohol and other drugs, and pregnancy. Chevy Chase MD: American Society of Addiction Medicine; 2011. Available at: http://www.asam.org/docs/default-source/public-policy-statements/1womenandpregnancy_7-11.pdf. Accessed July 5, 2016.
1. Metz TD, Stickrath EH. Marijuana use in pregnancy and lactation: a review of the evidence. Am J Obstet Gynecol. 2015;213:761-778.
2. Chabarria KC, Racusin DA, Antony KM, et al. Marijuana use and its effects in pregnancy. Am J Obstet Gynecol. 2016;215:506.e1-e7.
3. Warner TD, Roussos-Ross D, Behnke M. It’s not your mother’s marijuana: effects on maternal-fetal health and the developing child. Clinical Perinatology. 2014;41:877-894.
4. Huizink AC. Prenatal cannabis exposure and infant outcomes: overview of studies. Prog Neuro-Psychopharmacol Biol Psychiatry. 2014;52:45-52.
5. Goldschmidt L, Richardson GA, Willford J, et al. Prenatal marijuana exposure and intelligence test performance at age 6. J Am Acad Child Adolesc Psychiatry. 2008;47:254-263.
6. Fried PA. The Ottawa Prenatal Prospective Study (OPPS): methodological issues and findings—it’s easy to throw the baby out with the bath water. Life Sci. 1995;56:2159-2168.
7. Goldschmidt L, Day NL, Richardson GA. Effects of prenatal marijuana exposure on child behavior problems at age 10. Neurotoxicol Teratol. 2000;22:325-336.
8. Day NL, Goldschmidt L, Thomas CA. Prenatal marijuana exposure contributes to the prediction of marijuana use at age 14. Addiction. 2006;101:1313-1322.
9. American College of Obstetricians and Gynecologists Committee on Obstetric Practice. Committee Opinion No. 637: Marijuana use during pregnancy and lactation. Obstet Gynecol. 2015;126:234-238.
10. American Society of Addiction Medicine. Public policy statement on women, alcohol and other drugs, and pregnancy. Chevy Chase MD: American Society of Addiction Medicine; 2011. Available at: http://www.asam.org/docs/default-source/public-policy-statements/1womenandpregnancy_7-11.pdf. Accessed July 5, 2016.
Evidence-based answers from the Family Physicians Inquiries Network
EVIDENCE-BASED ANSWER:
The effects are unclear. Marijuana use during pregnancy is associated with clinically unimportant lower birth weights (growth differences of approximately 100 g), but no differences in preterm births or congenital anomalies (strength of recommendation [SOR]: B, prospective and retrospective cohort studies with methodologic flaws).
Similarly, prenatal marijuana use isn’t associated with differences in neurodevelopmental outcomes (behavior problems, intellect, visual perception, language, or sustained attention and memory tasks) at birth, in the neonatal period, or in childhood through age 3 years. However, it may be associated with minimally lower verbal/quantitative IQ scores (1%) at age 6 years and increased impulsivity and hyperactivity (1%) at 10 years. Prenatal use isn’t linked to increased substance use at age 14 years (SOR: B, conflicting long-term prospective and retrospective cohort studies with methodologic flaws).
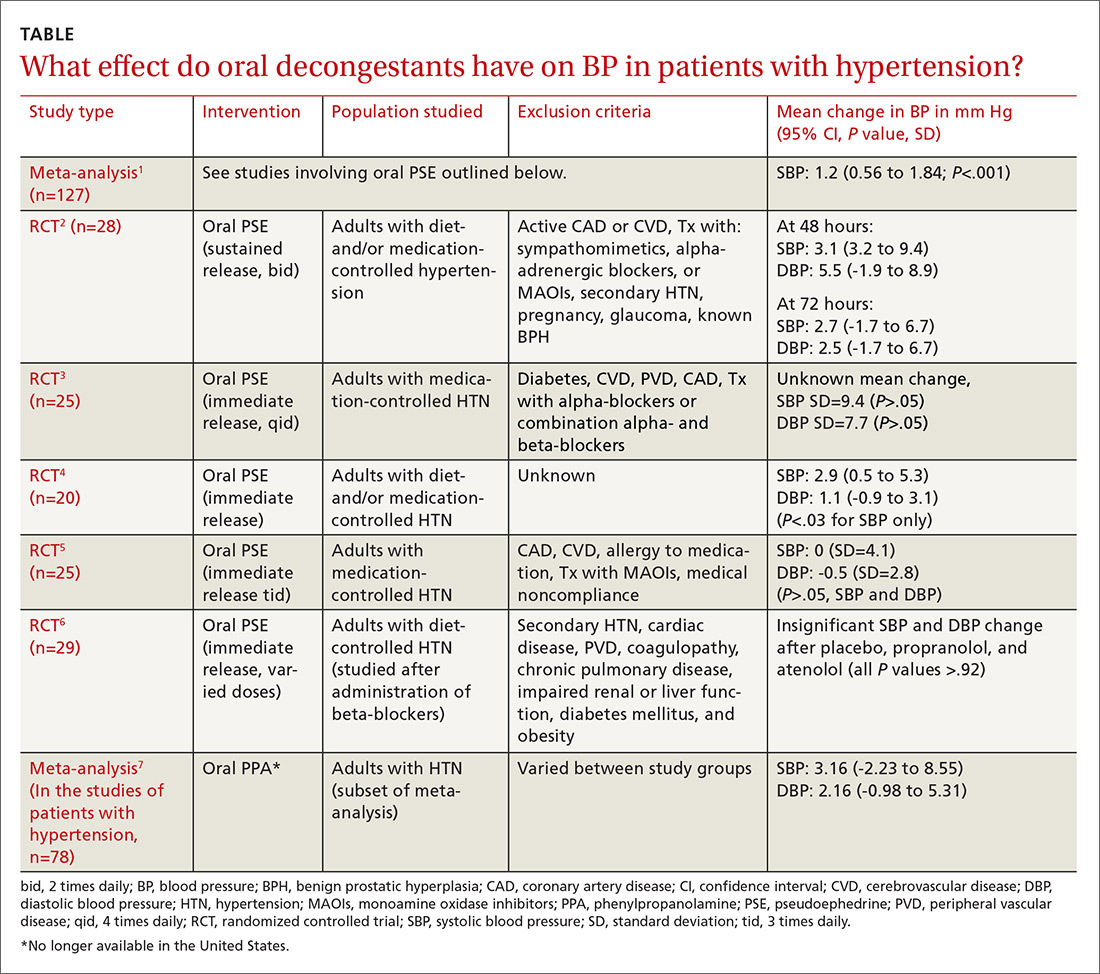
Do oral decongestants have a clinically significant effect on BP in patients with hypertension?
EVIDENCE SUMMARY
A meta-analysis of 24 RCTs examined the effect of pseudoephedrine on BP and heart rate.1 Just 5 of the 24 studies specifically included hypertensive patients. In the population of patients with hypertension, the meta-analysis showed a small (1.2 mm Hg) rise in systolic BP with pseudoephedrine that was statistically significant (95% confidence interval [CI], 0.56-1.84 mm Hg), but the slight changes in diastolic BP and heart rate were not significant. No patient-oriented outcomes were measured.
The highest quality study within this group was a randomized, double-blind, placebo-controlled crossover study with 28 patients given sustained-release pseudoephedrine 120 mg twice daily for 72 hours, with BP measurements taken at 48 and 72 hours.2 The study was powered to identify an increase in systolic BP of 11 mm Hg, but the results showed just a 3.1 mm Hg rise in systolic BP at 48 hours (see TABLE1-7 for CI and other data).
In another double-blind, placebo-controlled RCT of 29 adults with hypertension (only 25 were included in the data analysis), there was no significant elevation in BP when oral pseudoephedrine was administered over the course of 3 days.3
Across the 5 studies in the meta-analysis, immediate-release and sustained-release forms of pseudoephedrine were included, hypertension was described as controlled but definitions of control were not always specified, and study length varied from 2 hours to 4 weeks.2-6 Patients on antihypertensive medications were included in some of the studies; patients who had active cardiovascular disease, peripheral vascular disease, and/or cerebrovascular disease were excluded.
One study specifically looked at the effects of a single dose of pseudoephedrine on BP in patients treated with 2 different beta-blockers and found no significant change from baseline, but this study was not powered to show differences less than 5 mm Hg.6 The study did show a change of 1 to 2 mm Hg in systolic BP, but this was not statistically significant.
An absence of information on older patients
There is a paucity of literature on treating older adults and medically complex patients (eg, those with uncontrolled or secondary causes of hypertension, cerebrovascular disease, coronary artery disease) with decongestants, as they were excluded in all studies. And the available evidence does not include reports of adverse events other than changes in BP.
1. Salerno SM, Jackson JL, Berbano EP. Effect of oral pseudoephedrine on blood pressure and heart rate. Arch Intern Med. 2005;165:1686-1694.
2. Beck RA, Mercado DL, Seguin SM, et al. Cardiovascular effects of pseudoephedrine in medically controlled hypertensive patients. Arch Int Med. 1992;152:1242-1245.
3. Bradley JG, Kallail KJ, Dorsch JN, et al. The effects of pseudoephedrine on blood pressure in patients with controlled, uncomplicated hypertension: a randomized, double-blind, placebo-controlled trial. J Am Board Fam Pract. 1991;4:201-206.
4. Chua SS, Benrimoj SI, Gordon RD, et al. A controlled clinical trial on the cardiovascular effects of single doses of pseudoephedrine in hypertensive patients. Br J Clin Pharmacol. 1989;28:369-372.
5. Coates ML, Rembold CM, Farr BM. Does pseudoephedrine increase blood pressure in patients with controlled hypertension? J Fam Pract. 1995;40:22-26.
6. Mores N, Campia U, Navarra P, et al. No cardiovascular effects of single-dose pseudoephedrine in patients with essential hypertension treated with beta-blockers. Eur J Clin Pharmacol. 1999;55:251-254.
7. Salerno SM, Jackson JL, Berbano EP. The impact of oral phenylpropanolamine on blood pressure: a meta-analysis and review of the literature. J Hum Hypertens. 2005;19:643-652.
EVIDENCE SUMMARY
A meta-analysis of 24 RCTs examined the effect of pseudoephedrine on BP and heart rate.1 Just 5 of the 24 studies specifically included hypertensive patients. In the population of patients with hypertension, the meta-analysis showed a small (1.2 mm Hg) rise in systolic BP with pseudoephedrine that was statistically significant (95% confidence interval [CI], 0.56-1.84 mm Hg), but the slight changes in diastolic BP and heart rate were not significant. No patient-oriented outcomes were measured.
The highest quality study within this group was a randomized, double-blind, placebo-controlled crossover study with 28 patients given sustained-release pseudoephedrine 120 mg twice daily for 72 hours, with BP measurements taken at 48 and 72 hours.2 The study was powered to identify an increase in systolic BP of 11 mm Hg, but the results showed just a 3.1 mm Hg rise in systolic BP at 48 hours (see TABLE1-7 for CI and other data).
In another double-blind, placebo-controlled RCT of 29 adults with hypertension (only 25 were included in the data analysis), there was no significant elevation in BP when oral pseudoephedrine was administered over the course of 3 days.3
Across the 5 studies in the meta-analysis, immediate-release and sustained-release forms of pseudoephedrine were included, hypertension was described as controlled but definitions of control were not always specified, and study length varied from 2 hours to 4 weeks.2-6 Patients on antihypertensive medications were included in some of the studies; patients who had active cardiovascular disease, peripheral vascular disease, and/or cerebrovascular disease were excluded.
One study specifically looked at the effects of a single dose of pseudoephedrine on BP in patients treated with 2 different beta-blockers and found no significant change from baseline, but this study was not powered to show differences less than 5 mm Hg.6 The study did show a change of 1 to 2 mm Hg in systolic BP, but this was not statistically significant.
An absence of information on older patients
There is a paucity of literature on treating older adults and medically complex patients (eg, those with uncontrolled or secondary causes of hypertension, cerebrovascular disease, coronary artery disease) with decongestants, as they were excluded in all studies. And the available evidence does not include reports of adverse events other than changes in BP.
EVIDENCE SUMMARY
A meta-analysis of 24 RCTs examined the effect of pseudoephedrine on BP and heart rate.1 Just 5 of the 24 studies specifically included hypertensive patients. In the population of patients with hypertension, the meta-analysis showed a small (1.2 mm Hg) rise in systolic BP with pseudoephedrine that was statistically significant (95% confidence interval [CI], 0.56-1.84 mm Hg), but the slight changes in diastolic BP and heart rate were not significant. No patient-oriented outcomes were measured.
The highest quality study within this group was a randomized, double-blind, placebo-controlled crossover study with 28 patients given sustained-release pseudoephedrine 120 mg twice daily for 72 hours, with BP measurements taken at 48 and 72 hours.2 The study was powered to identify an increase in systolic BP of 11 mm Hg, but the results showed just a 3.1 mm Hg rise in systolic BP at 48 hours (see TABLE1-7 for CI and other data).
In another double-blind, placebo-controlled RCT of 29 adults with hypertension (only 25 were included in the data analysis), there was no significant elevation in BP when oral pseudoephedrine was administered over the course of 3 days.3
Across the 5 studies in the meta-analysis, immediate-release and sustained-release forms of pseudoephedrine were included, hypertension was described as controlled but definitions of control were not always specified, and study length varied from 2 hours to 4 weeks.2-6 Patients on antihypertensive medications were included in some of the studies; patients who had active cardiovascular disease, peripheral vascular disease, and/or cerebrovascular disease were excluded.
One study specifically looked at the effects of a single dose of pseudoephedrine on BP in patients treated with 2 different beta-blockers and found no significant change from baseline, but this study was not powered to show differences less than 5 mm Hg.6 The study did show a change of 1 to 2 mm Hg in systolic BP, but this was not statistically significant.
An absence of information on older patients
There is a paucity of literature on treating older adults and medically complex patients (eg, those with uncontrolled or secondary causes of hypertension, cerebrovascular disease, coronary artery disease) with decongestants, as they were excluded in all studies. And the available evidence does not include reports of adverse events other than changes in BP.
1. Salerno SM, Jackson JL, Berbano EP. Effect of oral pseudoephedrine on blood pressure and heart rate. Arch Intern Med. 2005;165:1686-1694.
2. Beck RA, Mercado DL, Seguin SM, et al. Cardiovascular effects of pseudoephedrine in medically controlled hypertensive patients. Arch Int Med. 1992;152:1242-1245.
3. Bradley JG, Kallail KJ, Dorsch JN, et al. The effects of pseudoephedrine on blood pressure in patients with controlled, uncomplicated hypertension: a randomized, double-blind, placebo-controlled trial. J Am Board Fam Pract. 1991;4:201-206.
4. Chua SS, Benrimoj SI, Gordon RD, et al. A controlled clinical trial on the cardiovascular effects of single doses of pseudoephedrine in hypertensive patients. Br J Clin Pharmacol. 1989;28:369-372.
5. Coates ML, Rembold CM, Farr BM. Does pseudoephedrine increase blood pressure in patients with controlled hypertension? J Fam Pract. 1995;40:22-26.
6. Mores N, Campia U, Navarra P, et al. No cardiovascular effects of single-dose pseudoephedrine in patients with essential hypertension treated with beta-blockers. Eur J Clin Pharmacol. 1999;55:251-254.
7. Salerno SM, Jackson JL, Berbano EP. The impact of oral phenylpropanolamine on blood pressure: a meta-analysis and review of the literature. J Hum Hypertens. 2005;19:643-652.
1. Salerno SM, Jackson JL, Berbano EP. Effect of oral pseudoephedrine on blood pressure and heart rate. Arch Intern Med. 2005;165:1686-1694.
2. Beck RA, Mercado DL, Seguin SM, et al. Cardiovascular effects of pseudoephedrine in medically controlled hypertensive patients. Arch Int Med. 1992;152:1242-1245.
3. Bradley JG, Kallail KJ, Dorsch JN, et al. The effects of pseudoephedrine on blood pressure in patients with controlled, uncomplicated hypertension: a randomized, double-blind, placebo-controlled trial. J Am Board Fam Pract. 1991;4:201-206.
4. Chua SS, Benrimoj SI, Gordon RD, et al. A controlled clinical trial on the cardiovascular effects of single doses of pseudoephedrine in hypertensive patients. Br J Clin Pharmacol. 1989;28:369-372.
5. Coates ML, Rembold CM, Farr BM. Does pseudoephedrine increase blood pressure in patients with controlled hypertension? J Fam Pract. 1995;40:22-26.
6. Mores N, Campia U, Navarra P, et al. No cardiovascular effects of single-dose pseudoephedrine in patients with essential hypertension treated with beta-blockers. Eur J Clin Pharmacol. 1999;55:251-254.
7. Salerno SM, Jackson JL, Berbano EP. The impact of oral phenylpropanolamine on blood pressure: a meta-analysis and review of the literature. J Hum Hypertens. 2005;19:643-652.
Evidence-based answers from the Family Physicians Inquiries Network
EVIDENCE-BASED ANSWER:
It is unclear. Pseudoephedrine causes an average increase of 1.2 mm Hg in systolic blood pressure (BP) in patients with controlled hypertension. However, the studies are not adequately powered to provide evidence about whether this rise in systolic BP is linked to patient-oriented outcomes (strength of recommendation [SOR]: C, multiple randomized controlled trials [RCTs] supporting disease-oriented evidence). Significant variations in BP are defined differently among studies (TABLE1-7). In addition, we do not have data on chronic use of oral decongestants; the longest time on medication in these trials was 4 weeks.
Do ACE inhibitors or ARBs help prevent kidney disease in patients with diabetes and normal BP?
EVIDENCE SUMMARY
A 2011 meta-analysis of 5 RCTs (total 2975 patients) that compared ACE inhibitor therapy with placebo in diabetic patients without hypertension and albuminuria found that ACE inhibitors reduced the risk of new-onset microalbuminuria or macroalbuminuria by 18% (relative risk [RR]=0.82; 95% confidence interval [CI], 0.73-0.92).1 Normal albuminuria was defined in all included studies as an albumin excretion rate of <30 mg/d on a timed specimen confirmed with 3 serial measurements.
The RCTs included patients treated with lisinopril, enalapril, and perindopril. All but one examined patients with type 1 diabetes (2781 patients). The study that evaluated type 2 diabetes (194 patients) assessed patients with hypertension who used other antihypertensives to achieve normal blood pressure targets before ACE inhibitor initiation, a potential limitation.
Compared with placebo or no treatment, ACE inhibitor therapy reduced the risk of death from any cause (6 studies; 11,350 patients; RR=0.84; 95% CI, 0.73-0.97).1 Patient populations across pooled RCTs were heterogeneous, including subjects with type 1 and type 2 diabetes, with or without hypertension, and with or without albuminuria.
ACE inhibitors increase risk of cough
Patients taking an ACE inhibitor have an increased risk of cough (6 studies; 11,791 patients; RR=1.84; 95% CI, 1.24-2.72).1 ACE inhibitor therapy doesn’t increase the risk of headache or hyperkalemia.
ARBs don’t help prevent diabetic kidney disease in normotensive patients
The 2011 meta-analysis also included 5 RCTs (4604 patients, approximately 3000 with type 2 diabetes and more than 1000 with type 1 diabetes) that compared ARBs with placebo in patients without hypertension.1 Unlike ACE inhibitor therapy, ARB treatment didn’t significantly affect new-onset microalbuminuria or macroalbuminuria (RR=1.06; 95% CI, 0.67-1.69).
The trials evaluated losartan, candesartan, olmesartan, and valsartan. One study used other antihypertensives to achieve target blood pressure, and another included patients of any albuminuria status.
Compared with placebo or no treatment, ARBs didn’t reduce the risk of death (5 studies; 7653 patients; RR=1.12; 95% CI, 0.88-1.41).1 All 5 RCTs assessed normoalbuminuric patients. Three of the 5 studies examined normotensive patients; one evaluated only hypertensive patients, and another assessed mostly hypertensive patients.
ARBs usually don’t produce significant adverse effects
Within the meta-analysis, ARBs didn’t increase risk of cough, headache, or hyperkalemia.1
1. Lv J, Perkovic V, Foote CV, et al. Antihypertensive agents for preventing diabetic kidney disease. Cochrane Database Syst Rev. 2012;(12):CD004136.
EVIDENCE SUMMARY
A 2011 meta-analysis of 5 RCTs (total 2975 patients) that compared ACE inhibitor therapy with placebo in diabetic patients without hypertension and albuminuria found that ACE inhibitors reduced the risk of new-onset microalbuminuria or macroalbuminuria by 18% (relative risk [RR]=0.82; 95% confidence interval [CI], 0.73-0.92).1 Normal albuminuria was defined in all included studies as an albumin excretion rate of <30 mg/d on a timed specimen confirmed with 3 serial measurements.
The RCTs included patients treated with lisinopril, enalapril, and perindopril. All but one examined patients with type 1 diabetes (2781 patients). The study that evaluated type 2 diabetes (194 patients) assessed patients with hypertension who used other antihypertensives to achieve normal blood pressure targets before ACE inhibitor initiation, a potential limitation.
Compared with placebo or no treatment, ACE inhibitor therapy reduced the risk of death from any cause (6 studies; 11,350 patients; RR=0.84; 95% CI, 0.73-0.97).1 Patient populations across pooled RCTs were heterogeneous, including subjects with type 1 and type 2 diabetes, with or without hypertension, and with or without albuminuria.
ACE inhibitors increase risk of cough
Patients taking an ACE inhibitor have an increased risk of cough (6 studies; 11,791 patients; RR=1.84; 95% CI, 1.24-2.72).1 ACE inhibitor therapy doesn’t increase the risk of headache or hyperkalemia.
ARBs don’t help prevent diabetic kidney disease in normotensive patients
The 2011 meta-analysis also included 5 RCTs (4604 patients, approximately 3000 with type 2 diabetes and more than 1000 with type 1 diabetes) that compared ARBs with placebo in patients without hypertension.1 Unlike ACE inhibitor therapy, ARB treatment didn’t significantly affect new-onset microalbuminuria or macroalbuminuria (RR=1.06; 95% CI, 0.67-1.69).
The trials evaluated losartan, candesartan, olmesartan, and valsartan. One study used other antihypertensives to achieve target blood pressure, and another included patients of any albuminuria status.
Compared with placebo or no treatment, ARBs didn’t reduce the risk of death (5 studies; 7653 patients; RR=1.12; 95% CI, 0.88-1.41).1 All 5 RCTs assessed normoalbuminuric patients. Three of the 5 studies examined normotensive patients; one evaluated only hypertensive patients, and another assessed mostly hypertensive patients.
ARBs usually don’t produce significant adverse effects
Within the meta-analysis, ARBs didn’t increase risk of cough, headache, or hyperkalemia.1
EVIDENCE SUMMARY
A 2011 meta-analysis of 5 RCTs (total 2975 patients) that compared ACE inhibitor therapy with placebo in diabetic patients without hypertension and albuminuria found that ACE inhibitors reduced the risk of new-onset microalbuminuria or macroalbuminuria by 18% (relative risk [RR]=0.82; 95% confidence interval [CI], 0.73-0.92).1 Normal albuminuria was defined in all included studies as an albumin excretion rate of <30 mg/d on a timed specimen confirmed with 3 serial measurements.
The RCTs included patients treated with lisinopril, enalapril, and perindopril. All but one examined patients with type 1 diabetes (2781 patients). The study that evaluated type 2 diabetes (194 patients) assessed patients with hypertension who used other antihypertensives to achieve normal blood pressure targets before ACE inhibitor initiation, a potential limitation.
Compared with placebo or no treatment, ACE inhibitor therapy reduced the risk of death from any cause (6 studies; 11,350 patients; RR=0.84; 95% CI, 0.73-0.97).1 Patient populations across pooled RCTs were heterogeneous, including subjects with type 1 and type 2 diabetes, with or without hypertension, and with or without albuminuria.
ACE inhibitors increase risk of cough
Patients taking an ACE inhibitor have an increased risk of cough (6 studies; 11,791 patients; RR=1.84; 95% CI, 1.24-2.72).1 ACE inhibitor therapy doesn’t increase the risk of headache or hyperkalemia.
ARBs don’t help prevent diabetic kidney disease in normotensive patients
The 2011 meta-analysis also included 5 RCTs (4604 patients, approximately 3000 with type 2 diabetes and more than 1000 with type 1 diabetes) that compared ARBs with placebo in patients without hypertension.1 Unlike ACE inhibitor therapy, ARB treatment didn’t significantly affect new-onset microalbuminuria or macroalbuminuria (RR=1.06; 95% CI, 0.67-1.69).
The trials evaluated losartan, candesartan, olmesartan, and valsartan. One study used other antihypertensives to achieve target blood pressure, and another included patients of any albuminuria status.
Compared with placebo or no treatment, ARBs didn’t reduce the risk of death (5 studies; 7653 patients; RR=1.12; 95% CI, 0.88-1.41).1 All 5 RCTs assessed normoalbuminuric patients. Three of the 5 studies examined normotensive patients; one evaluated only hypertensive patients, and another assessed mostly hypertensive patients.
ARBs usually don’t produce significant adverse effects
Within the meta-analysis, ARBs didn’t increase risk of cough, headache, or hyperkalemia.1
1. Lv J, Perkovic V, Foote CV, et al. Antihypertensive agents for preventing diabetic kidney disease. Cochrane Database Syst Rev. 2012;(12):CD004136.
1. Lv J, Perkovic V, Foote CV, et al. Antihypertensive agents for preventing diabetic kidney disease. Cochrane Database Syst Rev. 2012;(12):CD004136.
Evidence-based answers from the Family Physicians Inquiries Network
EVIDENCE-BASED ANSWER:
Yes for angiotensin-converting enzyme (ACE) inhibitors, no for angiotensin receptor blockers (ARBs).
In normotensive patients with type 1 and type 2 diabetes, ACE inhibitor therapy reduces the risk of developing diabetic kidney disease, defined as new-onset microalbuminuria or macroalbuminuria, by 18% (strength of recommendation [SOR]: C, meta-analysis of randomized controlled trials [RCTs], disease-oriented evidence).
ACE inhibitor treatment improves all-cause mortality by 16% in patients with diabetes, including patients with and without hypertension. Patients on ACE inhibitor therapy are at increased risk of cough (SOR: A, meta-analysis of RCTs).
ARB therapy doesn’t lower the risk of developing kidney disease in normotensive patients with type 2 diabetes (SOR: C, meta-analysis of RCTs, disease-oriented evidence); nor does it reduce all-cause mortality in patients with or without hypertension (SOR: A, meta-analysis of RCTs). ARBs aren’t associated with significant adverse events (SOR: A, meta-analysis of RCTs).