User login
Can mobile technology improve weight loss in overweight and obese patients?
EVIDENCE SUMMARY
A systematic review and meta-analysis of 84 moderate- to high-quality RCTs with 24,010 patients evaluated the use of “eHealth” interventions in preventing and treating overweight and obesity in adults 35 to 65 years of age (75% female).1 The studies included 183 active intervention arms with durations as long as 24 months (64% <6 months, 46% >6 months). The term eHealth included all forms of information technology used to deliver health care, but predominantly the Internet (Web site/Web-based), e-mail, and text messaging. Sixty percent (84) of eHealth interventional arms used one modality and 34% (47) used 2. Some intervention arms included non-eHealth modalities, such as paper-based measures and counseling.
The eHealth interventions were associated with significantly greater weight loss than minimal or no intervention (TABLE).1 Comparing eHealth interventions with no intervention showed significant differences by eHealth type (P=.05). The greatest weight loss accompanied interventions that combined Web-based measures with a non-eHealth intervention, (mean difference [MD]= −3.7 kg; 95% confidence interval [CI], −4.46 to −2.94), followed by mobile interventions alone (MD= −2.4 kg; 95% CI, −4.09 to −0.71) and Web-based interventions alone (MD= −2.2 kg; 95% CI, −2.98 to −1.44).
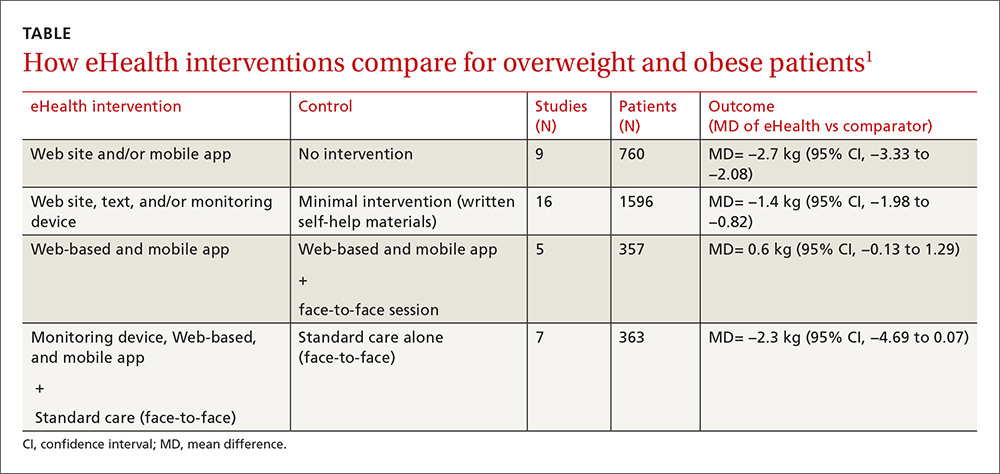
Similarly, comparing combined interventions (eHealth + eHealth or eHealth + non-eHealth) with a minimal intervention control showed a trend for difference by eHealth type (P=.005). Only a combination of eHealth with non-eHealth interventions resulted in significantly greater weight loss (Web site + non-eHealth: MD= −2.7 kg; 95% CI, −3.76 to −1.54; text + non-eHealth: MD= −1.8 kg; 95% CI, −2.49 to −1.12; computer + non-eHealth: MD=1.1 kg; 95% CI, −1.36 to −0.89).
Personal coaching plus smartphone monitoring beats interactive app
A 3-arm RCT of 385 overweight and obese participants (mean body mass index [BMI], 35 kg/m2) 18 to 35 years of age compared the effectiveness of weight loss interventions delivered by interactive smartphone application (CP [cell phone]), personal coaching enhanced by smartphone self-monitoring (PC), and usual care (control).2 The PC arm attended 6 weekly group sessions and received monthly phone calls. The usual care arm received 3 handouts on healthy eating and physical activity.
The CP arm showed the least amount of weight loss (−0.9 kg, −1.5 kg, and −1.0 kg at 6, 12, and 24 months, respectively) and no significant difference compared with controls at all measurement points. The PC arm had significantly greater weight loss than controls at 6 months (−1.9 kg; 95% CI, −3.17 to −0.67) and significantly greater weight loss than CP at 6 months (−2.2 kg; 95% CI, −3.42 to −0.97) and 12 months (−2.1 kg; 95% CI, −3.94 to −0.27). After 24 months, however, there was no significant difference in mean weight loss among treatment arms.
Automated behavioral program reduced weight and waist circumference
An RCT of 339 prediabetic, overweight, and obese patients 30 to 69 years old (mean BMI, 31 kg/m2) compared the effectiveness of Alive-PD, a fully automated, tailored, behavioral program, to usual care (control) for diabetes prevention.3 In addition to behavioral support, the program included weekly emails, Web-based tracking, a mobile phone app, and automated phone calls.
At 6 months, the intervention group had significantly greater mean weight loss (−3.4 kg vs −1.3 kg; P<.001), mean BMI (−1.1 kg/m2 vs −0.4 kg/m2; P<
Web-based program improves weight loss at 3 months, but not 12 months
An RCT of 65 overweight and obese participants (mean BMI, 32 kg/m2) with at least one cardiovascular risk factor compared the effect of a Web-based program with usual care on weight change at 3, 6, and 12 months.4 Participants in the intervention group were provided with Bluetooth-enabled scales and accelerometer activity bands to allow daily uploads. The Web-based program also provided weekly feedback based on the participant’s performance and a food diary.
The Web-based group had significantly greater weight loss at 3 months (mean= −3.4 kg [95% CI, −4.70 to −2.13] vs −0.5 kg [95% CI, −1.55 to 0.52]; P<.001) and 6 months (mean= −3.4 kg [95% CI, −4.95 to −1.98] vs −0.8 kg [95% CI, −2.23 to 0.61]; P=.02). At 12 months, however, the groups showed no significant difference (mean= −2.4 kg [95% CI, −3.48 to −0.97] vs −1.8 kg [95% CI, −3.15 to −0.44]; P=.77).
RECOMMENDATIONS
Guidelines from the American College of Cardiology, American Heart Association, and Obesity Society state that electronically delivered weight-loss programs may be prescribed, but may result in smaller weight loss than face-to-face interventions (SOR: B, moderate evidence from RCTs with some limitations or non-randomized trials).5
1. Hutchesson MJ, Rollo ME, Krukowski R, et al. eHealth interventions for the prevention and treatment of overweight and obesity in adults: a systematic review with meta-analysis. Obes Rev. 2015;16:376-392.
2. Svetkey LP, Batch BC, Lin P, et al. Cell phone intervention for you (CITY): A randomized, controlled trial of behavioral weight loss intervention for young adults using mobile technology. Obesity (Silver Spring). 2015;23:2133-2141.
3. Block G, Azar K, Romanelli R, et al. Diabetes prevention and weight loss with a fully automated behavioral intervention by email, web, and mobile phone: a randomized controlled trial among persons with prediabetes. J Med Internet Res. 2015;17:e240.
4. Watson S, Woodside J, Ware L, et al. Effect of a web-based behavior change program on weight loss and cardiovascular risk factors in overweight and obese adults at high risk of developing cardiovascular disease: randomized controlled trial. J Med Internet Res. 2015;17:e177.
5. Jensen MD, Ryan DH, Apovian CM, et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. Circulation. 2014;129:S102-S138.
EVIDENCE SUMMARY
A systematic review and meta-analysis of 84 moderate- to high-quality RCTs with 24,010 patients evaluated the use of “eHealth” interventions in preventing and treating overweight and obesity in adults 35 to 65 years of age (75% female).1 The studies included 183 active intervention arms with durations as long as 24 months (64% <6 months, 46% >6 months). The term eHealth included all forms of information technology used to deliver health care, but predominantly the Internet (Web site/Web-based), e-mail, and text messaging. Sixty percent (84) of eHealth interventional arms used one modality and 34% (47) used 2. Some intervention arms included non-eHealth modalities, such as paper-based measures and counseling.
The eHealth interventions were associated with significantly greater weight loss than minimal or no intervention (TABLE).1 Comparing eHealth interventions with no intervention showed significant differences by eHealth type (P=.05). The greatest weight loss accompanied interventions that combined Web-based measures with a non-eHealth intervention, (mean difference [MD]= −3.7 kg; 95% confidence interval [CI], −4.46 to −2.94), followed by mobile interventions alone (MD= −2.4 kg; 95% CI, −4.09 to −0.71) and Web-based interventions alone (MD= −2.2 kg; 95% CI, −2.98 to −1.44).

Similarly, comparing combined interventions (eHealth + eHealth or eHealth + non-eHealth) with a minimal intervention control showed a trend for difference by eHealth type (P=.005). Only a combination of eHealth with non-eHealth interventions resulted in significantly greater weight loss (Web site + non-eHealth: MD= −2.7 kg; 95% CI, −3.76 to −1.54; text + non-eHealth: MD= −1.8 kg; 95% CI, −2.49 to −1.12; computer + non-eHealth: MD=1.1 kg; 95% CI, −1.36 to −0.89).
Personal coaching plus smartphone monitoring beats interactive app
A 3-arm RCT of 385 overweight and obese participants (mean body mass index [BMI], 35 kg/m2) 18 to 35 years of age compared the effectiveness of weight loss interventions delivered by interactive smartphone application (CP [cell phone]), personal coaching enhanced by smartphone self-monitoring (PC), and usual care (control).2 The PC arm attended 6 weekly group sessions and received monthly phone calls. The usual care arm received 3 handouts on healthy eating and physical activity.
The CP arm showed the least amount of weight loss (−0.9 kg, −1.5 kg, and −1.0 kg at 6, 12, and 24 months, respectively) and no significant difference compared with controls at all measurement points. The PC arm had significantly greater weight loss than controls at 6 months (−1.9 kg; 95% CI, −3.17 to −0.67) and significantly greater weight loss than CP at 6 months (−2.2 kg; 95% CI, −3.42 to −0.97) and 12 months (−2.1 kg; 95% CI, −3.94 to −0.27). After 24 months, however, there was no significant difference in mean weight loss among treatment arms.
Automated behavioral program reduced weight and waist circumference
An RCT of 339 prediabetic, overweight, and obese patients 30 to 69 years old (mean BMI, 31 kg/m2) compared the effectiveness of Alive-PD, a fully automated, tailored, behavioral program, to usual care (control) for diabetes prevention.3 In addition to behavioral support, the program included weekly emails, Web-based tracking, a mobile phone app, and automated phone calls.
At 6 months, the intervention group had significantly greater mean weight loss (−3.4 kg vs −1.3 kg; P<.001), mean BMI (−1.1 kg/m2 vs −0.4 kg/m2; P<
Web-based program improves weight loss at 3 months, but not 12 months
An RCT of 65 overweight and obese participants (mean BMI, 32 kg/m2) with at least one cardiovascular risk factor compared the effect of a Web-based program with usual care on weight change at 3, 6, and 12 months.4 Participants in the intervention group were provided with Bluetooth-enabled scales and accelerometer activity bands to allow daily uploads. The Web-based program also provided weekly feedback based on the participant’s performance and a food diary.
The Web-based group had significantly greater weight loss at 3 months (mean= −3.4 kg [95% CI, −4.70 to −2.13] vs −0.5 kg [95% CI, −1.55 to 0.52]; P<.001) and 6 months (mean= −3.4 kg [95% CI, −4.95 to −1.98] vs −0.8 kg [95% CI, −2.23 to 0.61]; P=.02). At 12 months, however, the groups showed no significant difference (mean= −2.4 kg [95% CI, −3.48 to −0.97] vs −1.8 kg [95% CI, −3.15 to −0.44]; P=.77).
RECOMMENDATIONS
Guidelines from the American College of Cardiology, American Heart Association, and Obesity Society state that electronically delivered weight-loss programs may be prescribed, but may result in smaller weight loss than face-to-face interventions (SOR: B, moderate evidence from RCTs with some limitations or non-randomized trials).5
EVIDENCE SUMMARY
A systematic review and meta-analysis of 84 moderate- to high-quality RCTs with 24,010 patients evaluated the use of “eHealth” interventions in preventing and treating overweight and obesity in adults 35 to 65 years of age (75% female).1 The studies included 183 active intervention arms with durations as long as 24 months (64% <6 months, 46% >6 months). The term eHealth included all forms of information technology used to deliver health care, but predominantly the Internet (Web site/Web-based), e-mail, and text messaging. Sixty percent (84) of eHealth interventional arms used one modality and 34% (47) used 2. Some intervention arms included non-eHealth modalities, such as paper-based measures and counseling.
The eHealth interventions were associated with significantly greater weight loss than minimal or no intervention (TABLE).1 Comparing eHealth interventions with no intervention showed significant differences by eHealth type (P=.05). The greatest weight loss accompanied interventions that combined Web-based measures with a non-eHealth intervention, (mean difference [MD]= −3.7 kg; 95% confidence interval [CI], −4.46 to −2.94), followed by mobile interventions alone (MD= −2.4 kg; 95% CI, −4.09 to −0.71) and Web-based interventions alone (MD= −2.2 kg; 95% CI, −2.98 to −1.44).

Similarly, comparing combined interventions (eHealth + eHealth or eHealth + non-eHealth) with a minimal intervention control showed a trend for difference by eHealth type (P=.005). Only a combination of eHealth with non-eHealth interventions resulted in significantly greater weight loss (Web site + non-eHealth: MD= −2.7 kg; 95% CI, −3.76 to −1.54; text + non-eHealth: MD= −1.8 kg; 95% CI, −2.49 to −1.12; computer + non-eHealth: MD=1.1 kg; 95% CI, −1.36 to −0.89).
Personal coaching plus smartphone monitoring beats interactive app
A 3-arm RCT of 385 overweight and obese participants (mean body mass index [BMI], 35 kg/m2) 18 to 35 years of age compared the effectiveness of weight loss interventions delivered by interactive smartphone application (CP [cell phone]), personal coaching enhanced by smartphone self-monitoring (PC), and usual care (control).2 The PC arm attended 6 weekly group sessions and received monthly phone calls. The usual care arm received 3 handouts on healthy eating and physical activity.
The CP arm showed the least amount of weight loss (−0.9 kg, −1.5 kg, and −1.0 kg at 6, 12, and 24 months, respectively) and no significant difference compared with controls at all measurement points. The PC arm had significantly greater weight loss than controls at 6 months (−1.9 kg; 95% CI, −3.17 to −0.67) and significantly greater weight loss than CP at 6 months (−2.2 kg; 95% CI, −3.42 to −0.97) and 12 months (−2.1 kg; 95% CI, −3.94 to −0.27). After 24 months, however, there was no significant difference in mean weight loss among treatment arms.
Automated behavioral program reduced weight and waist circumference
An RCT of 339 prediabetic, overweight, and obese patients 30 to 69 years old (mean BMI, 31 kg/m2) compared the effectiveness of Alive-PD, a fully automated, tailored, behavioral program, to usual care (control) for diabetes prevention.3 In addition to behavioral support, the program included weekly emails, Web-based tracking, a mobile phone app, and automated phone calls.
At 6 months, the intervention group had significantly greater mean weight loss (−3.4 kg vs −1.3 kg; P<.001), mean BMI (−1.1 kg/m2 vs −0.4 kg/m2; P<
Web-based program improves weight loss at 3 months, but not 12 months
An RCT of 65 overweight and obese participants (mean BMI, 32 kg/m2) with at least one cardiovascular risk factor compared the effect of a Web-based program with usual care on weight change at 3, 6, and 12 months.4 Participants in the intervention group were provided with Bluetooth-enabled scales and accelerometer activity bands to allow daily uploads. The Web-based program also provided weekly feedback based on the participant’s performance and a food diary.
The Web-based group had significantly greater weight loss at 3 months (mean= −3.4 kg [95% CI, −4.70 to −2.13] vs −0.5 kg [95% CI, −1.55 to 0.52]; P<.001) and 6 months (mean= −3.4 kg [95% CI, −4.95 to −1.98] vs −0.8 kg [95% CI, −2.23 to 0.61]; P=.02). At 12 months, however, the groups showed no significant difference (mean= −2.4 kg [95% CI, −3.48 to −0.97] vs −1.8 kg [95% CI, −3.15 to −0.44]; P=.77).
RECOMMENDATIONS
Guidelines from the American College of Cardiology, American Heart Association, and Obesity Society state that electronically delivered weight-loss programs may be prescribed, but may result in smaller weight loss than face-to-face interventions (SOR: B, moderate evidence from RCTs with some limitations or non-randomized trials).5
1. Hutchesson MJ, Rollo ME, Krukowski R, et al. eHealth interventions for the prevention and treatment of overweight and obesity in adults: a systematic review with meta-analysis. Obes Rev. 2015;16:376-392.
2. Svetkey LP, Batch BC, Lin P, et al. Cell phone intervention for you (CITY): A randomized, controlled trial of behavioral weight loss intervention for young adults using mobile technology. Obesity (Silver Spring). 2015;23:2133-2141.
3. Block G, Azar K, Romanelli R, et al. Diabetes prevention and weight loss with a fully automated behavioral intervention by email, web, and mobile phone: a randomized controlled trial among persons with prediabetes. J Med Internet Res. 2015;17:e240.
4. Watson S, Woodside J, Ware L, et al. Effect of a web-based behavior change program on weight loss and cardiovascular risk factors in overweight and obese adults at high risk of developing cardiovascular disease: randomized controlled trial. J Med Internet Res. 2015;17:e177.
5. Jensen MD, Ryan DH, Apovian CM, et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. Circulation. 2014;129:S102-S138.
1. Hutchesson MJ, Rollo ME, Krukowski R, et al. eHealth interventions for the prevention and treatment of overweight and obesity in adults: a systematic review with meta-analysis. Obes Rev. 2015;16:376-392.
2. Svetkey LP, Batch BC, Lin P, et al. Cell phone intervention for you (CITY): A randomized, controlled trial of behavioral weight loss intervention for young adults using mobile technology. Obesity (Silver Spring). 2015;23:2133-2141.
3. Block G, Azar K, Romanelli R, et al. Diabetes prevention and weight loss with a fully automated behavioral intervention by email, web, and mobile phone: a randomized controlled trial among persons with prediabetes. J Med Internet Res. 2015;17:e240.
4. Watson S, Woodside J, Ware L, et al. Effect of a web-based behavior change program on weight loss and cardiovascular risk factors in overweight and obese adults at high risk of developing cardiovascular disease: randomized controlled trial. J Med Internet Res. 2015;17:e177.
5. Jensen MD, Ryan DH, Apovian CM, et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. Circulation. 2014;129:S102-S138.
Evidence-based answers from the Family Physicians Inquiries Network
EVIDENCE-BASED ANSWER:
Yes, this technology can help in the short term. Mobile technology compared with minimal or no intervention increases short-term (<6 months) weight loss (1.4 to 2.7 kg) in overweight and obese patients (strength of recommendation [SOR]: A, meta-analysis of good-quality studies and randomized controlled trials [RCTs]).
Interventions that combine nonelectronic measures with mobile technology increase weight loss more effectively (3.7 kg) than no intervention (SOR: A, meta-analysis of good-quality studies and RCTs).
Using mobile technology shows no significant benefits for weight loss after 12 months (SOR: A, multiple good-quality RCTs).
Do pedometers increase activity and improve health outcomes?
EVIDENCE SUMMARY
A systematic review and meta-analysis identified 26 studies evaluating activity and health outcomes with the use of pedometers.1 The studies included 8 RCTs and 18 observational studies with 2767 patients (mean body mass index [BMI]: 30 kg/m2; mean age: 49 years; 85% women). The studies ranged from 3 to 104 weeks. From the RCT data, patients using pedometers had an increase of 2491 steps per day (about one mile) more than control group patients (8 trials, n=305; 95% confidence interval [CI], 1098-3885 steps/day; P<.001).
Across all of the observational studies, pedometer users had a 26.9% increase from their baseline physical activity (P=.001). When data from all of the studies were combined, the researchers found a decrease from baseline BMI (18 studies, n=562; mean difference [MD]=0.38 kg/m2; 95% CI, 0.05-0.72; P=.03) and a decrease in systolic BP (12 studies, n=468; MD=3.8 mm Hg; 95% CI, 1.7-5.9 mm Hg; P<.001). No statistically significant change was noted in cholesterol or fasting glucose levels. Weaknesses of this review include the heterogeneity of the interventions, relatively small study sizes, and short study durations.
A systematic review and meta-analysis of 11 RCTs (N=1258) evaluated pedometer effects in overweight patients with type 2 diabetes.2 (One RCT was included in the above meta-analysis.) Studies ran from 6 to 48 weeks, and mean enrollment BMI (where reported) was 30 kg/m2 or more in at least one treatment arm. Compared to controls, patients using pedometers had greater reductions in weight (weighted mean difference [WMD]= -0.65 kg; 95% CI, -1.12 to -0.17 kg) and BMI (WMD= -0.15 kg/m2; 95% CI, -0.29 to -0.02 kg/m2). The effect persisted in the subset of studies in which the intervention and control groups both received dietary counseling (WMD weight= -0.86 kg; 95% CI, -1.45 to -0.27 kg; WMD BMI= -0.30 kg/m2; 95% CI, -0.50 to -0.10 kg/m2). Study quality was low to moderate, and 5 studies used per-protocol analysis instead of intention-to-treat analysis.
Pedometer use benefits patients with musculoskeletal diseases, too
A systematic review and meta-analysis examined the use of pedometers in patients with musculoskeletal diseases.3 It included 7 RCTs lasting 4 weeks to one year with 484 adults, 40 to 82 years of age, with musculoskeletal disorders (eg, back pain, knee pain, hip pain). (One RCT was also included in the diabetes meta-analysis.) Pedometer use resulted in a mean increase in physical activity of 1950 steps per day above baseline (range=818-2829 steps/day; P<.05). The authors noted that 4 of the 7 studies also demonstrated significant improvement in pain scores and physical function. BMI data were not tracked in this review.
Pedometers increase walking in older patients
A RCT compared the effects of pedometer-based activity prescriptions with standard time-based activity prescriptions in 330 patients ≥65 years of age with baseline low activity levels.4 All patients received an initial physician visit followed by 3 telephone counseling sessions encouraging increased activity. The pedometer group was counseled on increasing steps (without specific targets), while the standard activity prescription group received time-related activity goals.
At one year, “leisure walking” had increased more for the pedometer group than for the standard group (mean 50 minutes/week vs 28 minutes/week; P=.03), although both groups equally increased their amount of “total activity.”
1. Bravata DM, Smith-Spangler C, Sundaram V, et al. Using pedometers to increase physical activity and improve health: a systematic review. JAMA. 2007;298:2296-2304.
2. Cai X, Qiu SH, Yin H, et al. Pedometer intervention and weight loss in overweight and obese adults with type 2 diabetes: a meta-analysis. Diabet Med. 2016;33:1035-1044.
3. Mansi S, Milosavljevic S, Baxter GD, et al. A systematic review of studies using pedometers as an intervention for musculoskeletal diseases. BMC Musculoskeletal Disorders. 2014;15:231.
4. Kolt GS, Schofield GM, Kerse N, et al. Healthy Steps trial: pedometer-based advice and physical activity for low-active older adults. Ann Fam Med. 2012;10:206-212.
EVIDENCE SUMMARY
A systematic review and meta-analysis identified 26 studies evaluating activity and health outcomes with the use of pedometers.1 The studies included 8 RCTs and 18 observational studies with 2767 patients (mean body mass index [BMI]: 30 kg/m2; mean age: 49 years; 85% women). The studies ranged from 3 to 104 weeks. From the RCT data, patients using pedometers had an increase of 2491 steps per day (about one mile) more than control group patients (8 trials, n=305; 95% confidence interval [CI], 1098-3885 steps/day; P<.001).
Across all of the observational studies, pedometer users had a 26.9% increase from their baseline physical activity (P=.001). When data from all of the studies were combined, the researchers found a decrease from baseline BMI (18 studies, n=562; mean difference [MD]=0.38 kg/m2; 95% CI, 0.05-0.72; P=.03) and a decrease in systolic BP (12 studies, n=468; MD=3.8 mm Hg; 95% CI, 1.7-5.9 mm Hg; P<.001). No statistically significant change was noted in cholesterol or fasting glucose levels. Weaknesses of this review include the heterogeneity of the interventions, relatively small study sizes, and short study durations.
A systematic review and meta-analysis of 11 RCTs (N=1258) evaluated pedometer effects in overweight patients with type 2 diabetes.2 (One RCT was included in the above meta-analysis.) Studies ran from 6 to 48 weeks, and mean enrollment BMI (where reported) was 30 kg/m2 or more in at least one treatment arm. Compared to controls, patients using pedometers had greater reductions in weight (weighted mean difference [WMD]= -0.65 kg; 95% CI, -1.12 to -0.17 kg) and BMI (WMD= -0.15 kg/m2; 95% CI, -0.29 to -0.02 kg/m2). The effect persisted in the subset of studies in which the intervention and control groups both received dietary counseling (WMD weight= -0.86 kg; 95% CI, -1.45 to -0.27 kg; WMD BMI= -0.30 kg/m2; 95% CI, -0.50 to -0.10 kg/m2). Study quality was low to moderate, and 5 studies used per-protocol analysis instead of intention-to-treat analysis.
Pedometer use benefits patients with musculoskeletal diseases, too
A systematic review and meta-analysis examined the use of pedometers in patients with musculoskeletal diseases.3 It included 7 RCTs lasting 4 weeks to one year with 484 adults, 40 to 82 years of age, with musculoskeletal disorders (eg, back pain, knee pain, hip pain). (One RCT was also included in the diabetes meta-analysis.) Pedometer use resulted in a mean increase in physical activity of 1950 steps per day above baseline (range=818-2829 steps/day; P<.05). The authors noted that 4 of the 7 studies also demonstrated significant improvement in pain scores and physical function. BMI data were not tracked in this review.
Pedometers increase walking in older patients
A RCT compared the effects of pedometer-based activity prescriptions with standard time-based activity prescriptions in 330 patients ≥65 years of age with baseline low activity levels.4 All patients received an initial physician visit followed by 3 telephone counseling sessions encouraging increased activity. The pedometer group was counseled on increasing steps (without specific targets), while the standard activity prescription group received time-related activity goals.
At one year, “leisure walking” had increased more for the pedometer group than for the standard group (mean 50 minutes/week vs 28 minutes/week; P=.03), although both groups equally increased their amount of “total activity.”
EVIDENCE SUMMARY
A systematic review and meta-analysis identified 26 studies evaluating activity and health outcomes with the use of pedometers.1 The studies included 8 RCTs and 18 observational studies with 2767 patients (mean body mass index [BMI]: 30 kg/m2; mean age: 49 years; 85% women). The studies ranged from 3 to 104 weeks. From the RCT data, patients using pedometers had an increase of 2491 steps per day (about one mile) more than control group patients (8 trials, n=305; 95% confidence interval [CI], 1098-3885 steps/day; P<.001).
Across all of the observational studies, pedometer users had a 26.9% increase from their baseline physical activity (P=.001). When data from all of the studies were combined, the researchers found a decrease from baseline BMI (18 studies, n=562; mean difference [MD]=0.38 kg/m2; 95% CI, 0.05-0.72; P=.03) and a decrease in systolic BP (12 studies, n=468; MD=3.8 mm Hg; 95% CI, 1.7-5.9 mm Hg; P<.001). No statistically significant change was noted in cholesterol or fasting glucose levels. Weaknesses of this review include the heterogeneity of the interventions, relatively small study sizes, and short study durations.
A systematic review and meta-analysis of 11 RCTs (N=1258) evaluated pedometer effects in overweight patients with type 2 diabetes.2 (One RCT was included in the above meta-analysis.) Studies ran from 6 to 48 weeks, and mean enrollment BMI (where reported) was 30 kg/m2 or more in at least one treatment arm. Compared to controls, patients using pedometers had greater reductions in weight (weighted mean difference [WMD]= -0.65 kg; 95% CI, -1.12 to -0.17 kg) and BMI (WMD= -0.15 kg/m2; 95% CI, -0.29 to -0.02 kg/m2). The effect persisted in the subset of studies in which the intervention and control groups both received dietary counseling (WMD weight= -0.86 kg; 95% CI, -1.45 to -0.27 kg; WMD BMI= -0.30 kg/m2; 95% CI, -0.50 to -0.10 kg/m2). Study quality was low to moderate, and 5 studies used per-protocol analysis instead of intention-to-treat analysis.
Pedometer use benefits patients with musculoskeletal diseases, too
A systematic review and meta-analysis examined the use of pedometers in patients with musculoskeletal diseases.3 It included 7 RCTs lasting 4 weeks to one year with 484 adults, 40 to 82 years of age, with musculoskeletal disorders (eg, back pain, knee pain, hip pain). (One RCT was also included in the diabetes meta-analysis.) Pedometer use resulted in a mean increase in physical activity of 1950 steps per day above baseline (range=818-2829 steps/day; P<.05). The authors noted that 4 of the 7 studies also demonstrated significant improvement in pain scores and physical function. BMI data were not tracked in this review.
Pedometers increase walking in older patients
A RCT compared the effects of pedometer-based activity prescriptions with standard time-based activity prescriptions in 330 patients ≥65 years of age with baseline low activity levels.4 All patients received an initial physician visit followed by 3 telephone counseling sessions encouraging increased activity. The pedometer group was counseled on increasing steps (without specific targets), while the standard activity prescription group received time-related activity goals.
At one year, “leisure walking” had increased more for the pedometer group than for the standard group (mean 50 minutes/week vs 28 minutes/week; P=.03), although both groups equally increased their amount of “total activity.”
1. Bravata DM, Smith-Spangler C, Sundaram V, et al. Using pedometers to increase physical activity and improve health: a systematic review. JAMA. 2007;298:2296-2304.
2. Cai X, Qiu SH, Yin H, et al. Pedometer intervention and weight loss in overweight and obese adults with type 2 diabetes: a meta-analysis. Diabet Med. 2016;33:1035-1044.
3. Mansi S, Milosavljevic S, Baxter GD, et al. A systematic review of studies using pedometers as an intervention for musculoskeletal diseases. BMC Musculoskeletal Disorders. 2014;15:231.
4. Kolt GS, Schofield GM, Kerse N, et al. Healthy Steps trial: pedometer-based advice and physical activity for low-active older adults. Ann Fam Med. 2012;10:206-212.
1. Bravata DM, Smith-Spangler C, Sundaram V, et al. Using pedometers to increase physical activity and improve health: a systematic review. JAMA. 2007;298:2296-2304.
2. Cai X, Qiu SH, Yin H, et al. Pedometer intervention and weight loss in overweight and obese adults with type 2 diabetes: a meta-analysis. Diabet Med. 2016;33:1035-1044.
3. Mansi S, Milosavljevic S, Baxter GD, et al. A systematic review of studies using pedometers as an intervention for musculoskeletal diseases. BMC Musculoskeletal Disorders. 2014;15:231.
4. Kolt GS, Schofield GM, Kerse N, et al. Healthy Steps trial: pedometer-based advice and physical activity for low-active older adults. Ann Fam Med. 2012;10:206-212.
Evidence-based answers from the Family Physicians Inquiries Network
EVIDENCE-BASED ANSWER:
Yes. In overweight and obese patients, exercise interventions using a pedometer increase steps by about a mile per day over the same interventions without access to pedometer information (strength of recommendation [SOR]: A, meta-analysis of randomized controlled trials [RCTs]) and are associated with a modest 4 mm Hg reduction in systolic blood pressure (BP) over baseline (SOR: B, meta-analysis of RCTs and cohort studies). In overweight patients with diabetes, pedometer use with nutritional counseling is associated with 0.86 kg greater weight loss than nutritional counseling alone (SOR: B, meta-analysis of lower quality RCTs).
Pedometers increase activity in patients with various musculoskeletal conditions and may help reduce pain (SOR: B, meta-analysis of RCTs with heterogeneous outcomes). In low-activity elderly patients, pedometers do not appear to increase total activity when added to an exercise program, but they do appear to increase walking (SOR: B, RCT).
There is no evidence concerning the impact of pedometers on cardiovascular outcomes.
Does vitamin D without calcium reduce fracture risk?
EVIDENCE SUMMARY
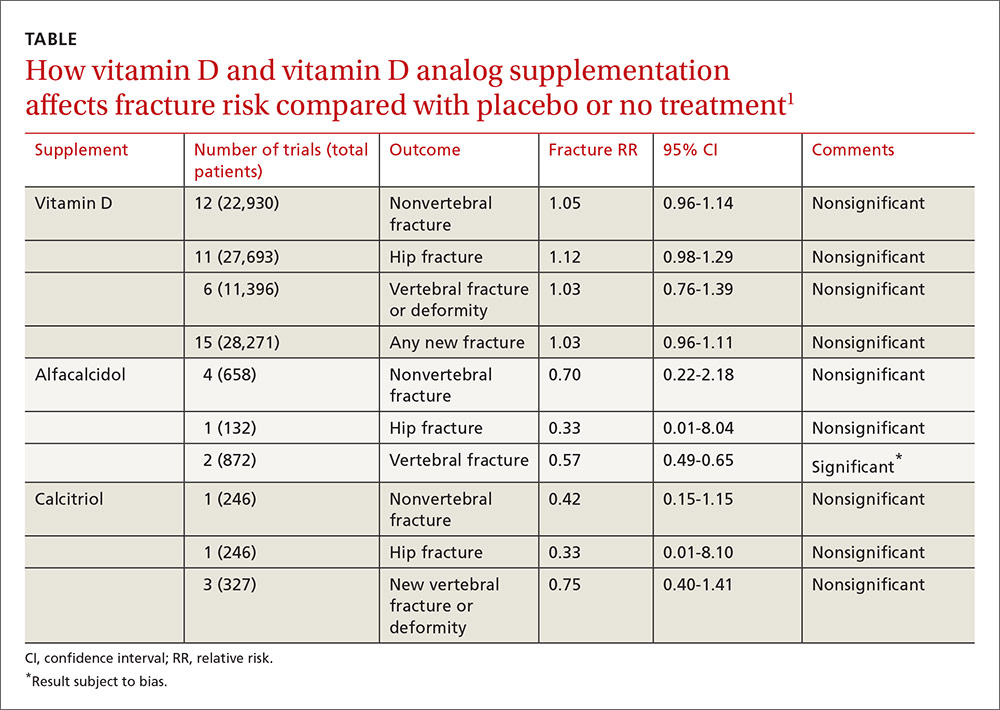
A 2014 meta-analysis of 15 trials (quasi-random and RCT) with a total of 28,271 patients that compared the effect of vitamin D on fracture risk with placebo or no treatment, found no benefit for vitamin D supplementation (TABLE).1 Patients lived in community and nursing home settings and ranged in age from 50 to 85 years; 24% to 100% were female.
Only 3 trials required patients to have had a previous fracture. Exclusions included: diseases affecting bone metabolism, cognitive impairment, drugs affecting bone metabolism (bisphosphonates, selective estrogen receptor modulators, and corticosteroids), renal failure, hypercalcemia, nephrolithiasis, and decreased mobility (recent stroke recovery and Parkinson’s disease).
Formulations of vitamin D included cholecalciferol (D3) 400 to 2000 IU/d for 4 months to 5 years or 100,000 to 500,000 IU every 3 to 12 months for 1 to 5 years; calcifediol (25(OH)D3) 600 IU/d for 4 years; and ergocalciferol (D2) 400 IU/d for 2 years or 3000 to 300,000 IU every 3 to 12 months for 10 months to 3 years.
Vitamin D analogs generally have no benefit either
The same meta-analysis compared vitamin D analogs to placebo or no treatment (8 trials, quasi-random and RCT, 1743 patients) on the risk of fracture, again finding no benefit in all but one case. Included patients were mostly by referral to tertiary or university hospitals and outpatient community settings.
Most of the studies included only a small number of patients (about 200), with the largest study having 740 patients. The age range was 50 to 77 years, and 50% to 100% were female. Most of the trials required patients to have osteoporosis or vitamin D deficiency with a previous vertebral deformity on imaging. Study exclusions included osteomalacia, malabsorption, hyperparathyroidism, active kidney stones, history of hypercalciuria, cancer, incurable disease, dementia, severe chronic illness (renal or liver failure), recent stroke or fracture, and drugs that affect bone metabolism.
Vitamin D analogs were given as alfacalcidol (1-alphahydroxyvitamin D3) 0.5 mcg twice daily or 1 mcg/d for 36 weeks to 2 years or calcitriol (1,25-dihydroxyvitamin D3) 0.25 to 1 mcg once or twice daily for one to 3 years. Researchers found a significant reduction in vertebral (but not nonvertebral or hip) fractures with alfacalcidol, but the finding occurred in a single trial that was assessed by the authors of the meta-analysis as subject to bias.
Supplementation doesn’t affect mortality, but does have some side effects
Patients taking vitamin D or an analog with or without calcium showed no difference in risk of death compared with patients taking placebo (29 trials, 71,032 patients; relative risk [RR]=0.97; 95% confidence interval [CI], 0.93-1.01).
Patients taking vitamin D or an analog were more likely than controls to have mild hypercalcemia, with an average increase of 2.7 mmol/L (21 trials, 17,124 patients; RR=2.28; 95% CI, 1.57-3.31). Patients taking calcitriol had the highest risk (4 trials, 988 patients; RR=4.41; 95% CI, 2.14-9.09).
Gastrointestinal adverse effects (4% increase) and renal calculi or mild renal insufficiency (16% increase) were more common with vitamin D and analogs than placebo (GI adverse effects: 15 trials, 47,761 patients; RR=1.04; 95% CI, 1.00-1.08; renal calculi or mild renal insufficiency: 11 trials, 46,548 patients; RR=1.16; 95% CI, 1.02-1.33).
RECOMMENDATIONS
There are no guidelines recommending vitamin D supplementation without calcium to prevent fracture.
1. Avenell A, Mak JC, O’Connell D. Vitamin D and vitamin D analogues for preventing fractures in post-menopausal women and older men. Cochrane Database Syst Rev. 2014;4:CD000227.
EVIDENCE SUMMARY
A 2014 meta-analysis of 15 trials (quasi-random and RCT) with a total of 28,271 patients that compared the effect of vitamin D on fracture risk with placebo or no treatment, found no benefit for vitamin D supplementation (TABLE).1 Patients lived in community and nursing home settings and ranged in age from 50 to 85 years; 24% to 100% were female.
Only 3 trials required patients to have had a previous fracture. Exclusions included: diseases affecting bone metabolism, cognitive impairment, drugs affecting bone metabolism (bisphosphonates, selective estrogen receptor modulators, and corticosteroids), renal failure, hypercalcemia, nephrolithiasis, and decreased mobility (recent stroke recovery and Parkinson’s disease).
Formulations of vitamin D included cholecalciferol (D3) 400 to 2000 IU/d for 4 months to 5 years or 100,000 to 500,000 IU every 3 to 12 months for 1 to 5 years; calcifediol (25(OH)D3) 600 IU/d for 4 years; and ergocalciferol (D2) 400 IU/d for 2 years or 3000 to 300,000 IU every 3 to 12 months for 10 months to 3 years.

Vitamin D analogs generally have no benefit either
The same meta-analysis compared vitamin D analogs to placebo or no treatment (8 trials, quasi-random and RCT, 1743 patients) on the risk of fracture, again finding no benefit in all but one case. Included patients were mostly by referral to tertiary or university hospitals and outpatient community settings.
Most of the studies included only a small number of patients (about 200), with the largest study having 740 patients. The age range was 50 to 77 years, and 50% to 100% were female. Most of the trials required patients to have osteoporosis or vitamin D deficiency with a previous vertebral deformity on imaging. Study exclusions included osteomalacia, malabsorption, hyperparathyroidism, active kidney stones, history of hypercalciuria, cancer, incurable disease, dementia, severe chronic illness (renal or liver failure), recent stroke or fracture, and drugs that affect bone metabolism.
Vitamin D analogs were given as alfacalcidol (1-alphahydroxyvitamin D3) 0.5 mcg twice daily or 1 mcg/d for 36 weeks to 2 years or calcitriol (1,25-dihydroxyvitamin D3) 0.25 to 1 mcg once or twice daily for one to 3 years. Researchers found a significant reduction in vertebral (but not nonvertebral or hip) fractures with alfacalcidol, but the finding occurred in a single trial that was assessed by the authors of the meta-analysis as subject to bias.
Supplementation doesn’t affect mortality, but does have some side effects
Patients taking vitamin D or an analog with or without calcium showed no difference in risk of death compared with patients taking placebo (29 trials, 71,032 patients; relative risk [RR]=0.97; 95% confidence interval [CI], 0.93-1.01).
Patients taking vitamin D or an analog were more likely than controls to have mild hypercalcemia, with an average increase of 2.7 mmol/L (21 trials, 17,124 patients; RR=2.28; 95% CI, 1.57-3.31). Patients taking calcitriol had the highest risk (4 trials, 988 patients; RR=4.41; 95% CI, 2.14-9.09).
Gastrointestinal adverse effects (4% increase) and renal calculi or mild renal insufficiency (16% increase) were more common with vitamin D and analogs than placebo (GI adverse effects: 15 trials, 47,761 patients; RR=1.04; 95% CI, 1.00-1.08; renal calculi or mild renal insufficiency: 11 trials, 46,548 patients; RR=1.16; 95% CI, 1.02-1.33).
RECOMMENDATIONS
There are no guidelines recommending vitamin D supplementation without calcium to prevent fracture.
EVIDENCE SUMMARY
A 2014 meta-analysis of 15 trials (quasi-random and RCT) with a total of 28,271 patients that compared the effect of vitamin D on fracture risk with placebo or no treatment, found no benefit for vitamin D supplementation (TABLE).1 Patients lived in community and nursing home settings and ranged in age from 50 to 85 years; 24% to 100% were female.
Only 3 trials required patients to have had a previous fracture. Exclusions included: diseases affecting bone metabolism, cognitive impairment, drugs affecting bone metabolism (bisphosphonates, selective estrogen receptor modulators, and corticosteroids), renal failure, hypercalcemia, nephrolithiasis, and decreased mobility (recent stroke recovery and Parkinson’s disease).
Formulations of vitamin D included cholecalciferol (D3) 400 to 2000 IU/d for 4 months to 5 years or 100,000 to 500,000 IU every 3 to 12 months for 1 to 5 years; calcifediol (25(OH)D3) 600 IU/d for 4 years; and ergocalciferol (D2) 400 IU/d for 2 years or 3000 to 300,000 IU every 3 to 12 months for 10 months to 3 years.

Vitamin D analogs generally have no benefit either
The same meta-analysis compared vitamin D analogs to placebo or no treatment (8 trials, quasi-random and RCT, 1743 patients) on the risk of fracture, again finding no benefit in all but one case. Included patients were mostly by referral to tertiary or university hospitals and outpatient community settings.
Most of the studies included only a small number of patients (about 200), with the largest study having 740 patients. The age range was 50 to 77 years, and 50% to 100% were female. Most of the trials required patients to have osteoporosis or vitamin D deficiency with a previous vertebral deformity on imaging. Study exclusions included osteomalacia, malabsorption, hyperparathyroidism, active kidney stones, history of hypercalciuria, cancer, incurable disease, dementia, severe chronic illness (renal or liver failure), recent stroke or fracture, and drugs that affect bone metabolism.
Vitamin D analogs were given as alfacalcidol (1-alphahydroxyvitamin D3) 0.5 mcg twice daily or 1 mcg/d for 36 weeks to 2 years or calcitriol (1,25-dihydroxyvitamin D3) 0.25 to 1 mcg once or twice daily for one to 3 years. Researchers found a significant reduction in vertebral (but not nonvertebral or hip) fractures with alfacalcidol, but the finding occurred in a single trial that was assessed by the authors of the meta-analysis as subject to bias.
Supplementation doesn’t affect mortality, but does have some side effects
Patients taking vitamin D or an analog with or without calcium showed no difference in risk of death compared with patients taking placebo (29 trials, 71,032 patients; relative risk [RR]=0.97; 95% confidence interval [CI], 0.93-1.01).
Patients taking vitamin D or an analog were more likely than controls to have mild hypercalcemia, with an average increase of 2.7 mmol/L (21 trials, 17,124 patients; RR=2.28; 95% CI, 1.57-3.31). Patients taking calcitriol had the highest risk (4 trials, 988 patients; RR=4.41; 95% CI, 2.14-9.09).
Gastrointestinal adverse effects (4% increase) and renal calculi or mild renal insufficiency (16% increase) were more common with vitamin D and analogs than placebo (GI adverse effects: 15 trials, 47,761 patients; RR=1.04; 95% CI, 1.00-1.08; renal calculi or mild renal insufficiency: 11 trials, 46,548 patients; RR=1.16; 95% CI, 1.02-1.33).
RECOMMENDATIONS
There are no guidelines recommending vitamin D supplementation without calcium to prevent fracture.
1. Avenell A, Mak JC, O’Connell D. Vitamin D and vitamin D analogues for preventing fractures in post-menopausal women and older men. Cochrane Database Syst Rev. 2014;4:CD000227.
1. Avenell A, Mak JC, O’Connell D. Vitamin D and vitamin D analogues for preventing fractures in post-menopausal women and older men. Cochrane Database Syst Rev. 2014;4:CD000227.
Evidence-based answers from the Family Physicians Inquiries Network
EVIDENCE-BASED ANSWER:
No. Supplemental vitamin D without calcium—in doses averaging as much as 800 IU per day—doesn’t reduce the risk of hip, vertebral, or nonvertebral fractures in postmenopausal women and older men (strength of recommendation [SOR]: A, large, high-quality meta-analysis of randomized or quasi-randomized placebo-controlled trials).
The vitamin D analogs alfacalcidol and calcitriol also don’t reduce hip or nonvertebral fractures (SOR: A, multiple randomized, controlled trials [RCTs]), although alfacalcidol (but not calcitriol) does reduce vertebral fractures by 43% (SOR: B, one RCT and one quasi-randomized trial with potential for bias)
Vitamin D supplementation, with or without calcium, doesn’t affect mortality. It does double the risk of mild hypercalcemia (about 2.7 mmol/L increase), raise the risk of renal calculi or mild renal insufficiency by 16%, and slightly increase (4%) gastrointestinal adverse effects (SOR: A, meta-analysis of RCTs or quasi-randomized trials).
Does breastfeeding affect the risk of childhood obesity?
EVIDENCE SUMMARY
A systematic review and meta-analysis of prospective cohort studies evaluating infant risk factors for childhood obesity found that breastfeeding was associated with a lower risk of obesity.1 The authors identified 10 trials (primarily from the United States and Europe) with more than 76,000 infants that compared the effect of some breastfeeding in the first year to no breastfeeding. Follow-up ranged from 2 to 14 years (median 6 years).
Having ever breastfed decreased the odds of future overweight (BMI >85th percentile) or obesity (BMI >95th percentile) by 15% (adjusted odds ratio [AOR]=0.85; 95% confidence interval [CI], 0.74-0.99).
Subsequent studies suggest increased risk with formula feeding
Three large, prospective, longitudinal cohort studies have been published since the meta-analysis. One, which followed 43,367 term infants in Japan, found that formula feeding before 6 months was associated with increased risk of obesity compared with continuous breastfeeding for 6 months.2 Researchers evaluated weight at 7 years and adjusted for child and maternal factors associated with weight gain (AOR for obesity, formula-fed infants=1.8; 95% CI, 1.3-2.6).
A similar prospective longitudinal cohort study of 2868 infants in Australia analyzed maternal breastfeeding diaries and followed children’s weight to age 20 years.3 Introducing a milk other than breast milk before 6 months of age was linked to increased risk of obesity at age 20 (odds ratio [OR]=1.5; 95% CI, 1.1-1.9).
Finally, in a prospective cohort of 568 children in India, 17% of children who breastfed for fewer than 6 months were above the 90th percentile for weight at age 5 years, compared with 10% of children who were breastfed for at least 18 months.4 The result didn’t reach statistical significance, however (P=.08).
Interventions that increase breastfeeding don’t seem to have an impact
An RCT of an intervention to promote breastfeeding didn’t find any effect on subsequent obesity rates. Researchers in Belarus randomized 17,046 mother-infant pairs to breastfeeding promotion, modeled on the UNICEF Baby-Friendly Hospital Initiative, or usual care. The intervention increased the prevalence of exclusive breastfeeding (at 3 months, 43% vs 6%; at 6 months, 7% vs 0.6%; P values not given).
When researchers evaluated 13,879 children at 11 or 12 years by intention-to-treat analysis, however, they found no difference in mean BMI between the children whose mothers received the intervention and those whose mothers didn’t (BMI difference=0.16; 95% CI, -0.02 to 0.35).5
Introduction of solid foods: Later is better
A systematic review investigated the association between the timing of introducing complementary (solid) foods and childhood obesity in 23 primarily cross-sectional and cohort studies (17 from the United States, Canada, and Europe) with more than 33,000 patients. Follow-up ranged from 4 to 19 years.
Eight of the 21 studies that used BMI as an outcome found that early introduction of complementary foods was associated with a higher childhood BMI. In the largest study (a cohort of 17,561 infants), introducing complementary foods before 3 months was associated with higher risk of obesity at age 5 years than introducing them thereafter (OR=1.3; 95% CI, 1.1-1.6).6 Introduction of solids after 4 months was not associated with childhood obesity.
A systematic review of 10 primarily cross-sectional and cohort studies with more than 3000 infants evaluated associations between the types of complementary foods given and the development of childhood obesity.7 Six of the 10 studies were from Europe and none were from the United States. Follow-up ages ranged from 4 to 11 years.
Outcomes were heterogeneous, and no meta-analysis could be performed. The authors cited 3 studies (total 1174 infants) that found various positive associations between total caloric intake during complementary feeding and childhood obesity. No consistent evidence pointed to increased risk from specific foods or food groups.
Scheduled feeding is linked to rapid infant weight gain
A cohort study evaluated the baseline data of an Australian RCT (on an intervention to promote proper nutrition) in 612 infants, mean age 4.3 months.8 Researchers looked at the relationship between feeding on demand vs scheduled feeding (assessed by parental report) and weight gain in infancy. “Rapid weight gain” was defined as >0.67 change in weight-for-age Z-score between birth and enrollment.
Scheduled feeding was associated with rapid weight gain at a higher rate than feeding on demand (OR=2.3; 95% CI, 1.1-4.6). This study didn’t use childhood obesity as an outcome.
1. Weng SF, Redsell SA, Swift JA, et al. Systematic review and meta-analyses of risk factors for childhood overweight identifiable during infancy. Arch Dis Child. 2012;97:1019-1026.
2. Yamakawa M, Yorifuji T, Inoue S, et al. Breastfeeding and obesity among schoolchildren: a national longitudinal survey in Japan. JAMA Pediatr. 2013;167:919-925.
3. Oddy WH, Mari TA, Huang RC, et al. Early infant feeding and adiposity risk: from infancy to adulthood. Ann Nutr Metab. 2014;64:262-270.
4. Caleyachetty A, Krishnaveni GV, Veena SR, et al. Breast-feeding duration, age of starting solids, and high BMI risk and adiposity in Indian children. Matern Child Nutr. 2013;9:199-216.
5. Martin RM, Patel, R, Kramer MS, et al. Effects of promoting longer-term and exclusive breastfeeding on adiposity and insulin-like growth factor-I at age 11.5 years: a randomized trial. JAMA. 2013;309:1005-1013.
6. Pearce J, Taylor MA, Langley-Evans SC. Timing of the introduction of complementary feeding and risk of childhood obesity: a systematic review. Int J Obes (Lond). 2013;37:1295-1306.
7. Pearce J, Langley-Evans. The types of food introduced during complementary feeding and risk of childhood obesity: a systematic review. Int J Obes (Lond). 2013;37:477-485.
8. Mihrshahi S, Battistutta D, Magarey A, et al. Determinants of rapid weight gain during infancy: baseline results from the NOURISH randomised controlled trial. BMC Pediatr. 2011;11:99.
EVIDENCE SUMMARY
A systematic review and meta-analysis of prospective cohort studies evaluating infant risk factors for childhood obesity found that breastfeeding was associated with a lower risk of obesity.1 The authors identified 10 trials (primarily from the United States and Europe) with more than 76,000 infants that compared the effect of some breastfeeding in the first year to no breastfeeding. Follow-up ranged from 2 to 14 years (median 6 years).
Having ever breastfed decreased the odds of future overweight (BMI >85th percentile) or obesity (BMI >95th percentile) by 15% (adjusted odds ratio [AOR]=0.85; 95% confidence interval [CI], 0.74-0.99).
Subsequent studies suggest increased risk with formula feeding
Three large, prospective, longitudinal cohort studies have been published since the meta-analysis. One, which followed 43,367 term infants in Japan, found that formula feeding before 6 months was associated with increased risk of obesity compared with continuous breastfeeding for 6 months.2 Researchers evaluated weight at 7 years and adjusted for child and maternal factors associated with weight gain (AOR for obesity, formula-fed infants=1.8; 95% CI, 1.3-2.6).
A similar prospective longitudinal cohort study of 2868 infants in Australia analyzed maternal breastfeeding diaries and followed children’s weight to age 20 years.3 Introducing a milk other than breast milk before 6 months of age was linked to increased risk of obesity at age 20 (odds ratio [OR]=1.5; 95% CI, 1.1-1.9).
Finally, in a prospective cohort of 568 children in India, 17% of children who breastfed for fewer than 6 months were above the 90th percentile for weight at age 5 years, compared with 10% of children who were breastfed for at least 18 months.4 The result didn’t reach statistical significance, however (P=.08).
Interventions that increase breastfeeding don’t seem to have an impact
An RCT of an intervention to promote breastfeeding didn’t find any effect on subsequent obesity rates. Researchers in Belarus randomized 17,046 mother-infant pairs to breastfeeding promotion, modeled on the UNICEF Baby-Friendly Hospital Initiative, or usual care. The intervention increased the prevalence of exclusive breastfeeding (at 3 months, 43% vs 6%; at 6 months, 7% vs 0.6%; P values not given).
When researchers evaluated 13,879 children at 11 or 12 years by intention-to-treat analysis, however, they found no difference in mean BMI between the children whose mothers received the intervention and those whose mothers didn’t (BMI difference=0.16; 95% CI, -0.02 to 0.35).5
Introduction of solid foods: Later is better
A systematic review investigated the association between the timing of introducing complementary (solid) foods and childhood obesity in 23 primarily cross-sectional and cohort studies (17 from the United States, Canada, and Europe) with more than 33,000 patients. Follow-up ranged from 4 to 19 years.
Eight of the 21 studies that used BMI as an outcome found that early introduction of complementary foods was associated with a higher childhood BMI. In the largest study (a cohort of 17,561 infants), introducing complementary foods before 3 months was associated with higher risk of obesity at age 5 years than introducing them thereafter (OR=1.3; 95% CI, 1.1-1.6).6 Introduction of solids after 4 months was not associated with childhood obesity.
A systematic review of 10 primarily cross-sectional and cohort studies with more than 3000 infants evaluated associations between the types of complementary foods given and the development of childhood obesity.7 Six of the 10 studies were from Europe and none were from the United States. Follow-up ages ranged from 4 to 11 years.
Outcomes were heterogeneous, and no meta-analysis could be performed. The authors cited 3 studies (total 1174 infants) that found various positive associations between total caloric intake during complementary feeding and childhood obesity. No consistent evidence pointed to increased risk from specific foods or food groups.
Scheduled feeding is linked to rapid infant weight gain
A cohort study evaluated the baseline data of an Australian RCT (on an intervention to promote proper nutrition) in 612 infants, mean age 4.3 months.8 Researchers looked at the relationship between feeding on demand vs scheduled feeding (assessed by parental report) and weight gain in infancy. “Rapid weight gain” was defined as >0.67 change in weight-for-age Z-score between birth and enrollment.
Scheduled feeding was associated with rapid weight gain at a higher rate than feeding on demand (OR=2.3; 95% CI, 1.1-4.6). This study didn’t use childhood obesity as an outcome.
EVIDENCE SUMMARY
A systematic review and meta-analysis of prospective cohort studies evaluating infant risk factors for childhood obesity found that breastfeeding was associated with a lower risk of obesity.1 The authors identified 10 trials (primarily from the United States and Europe) with more than 76,000 infants that compared the effect of some breastfeeding in the first year to no breastfeeding. Follow-up ranged from 2 to 14 years (median 6 years).
Having ever breastfed decreased the odds of future overweight (BMI >85th percentile) or obesity (BMI >95th percentile) by 15% (adjusted odds ratio [AOR]=0.85; 95% confidence interval [CI], 0.74-0.99).
Subsequent studies suggest increased risk with formula feeding
Three large, prospective, longitudinal cohort studies have been published since the meta-analysis. One, which followed 43,367 term infants in Japan, found that formula feeding before 6 months was associated with increased risk of obesity compared with continuous breastfeeding for 6 months.2 Researchers evaluated weight at 7 years and adjusted for child and maternal factors associated with weight gain (AOR for obesity, formula-fed infants=1.8; 95% CI, 1.3-2.6).
A similar prospective longitudinal cohort study of 2868 infants in Australia analyzed maternal breastfeeding diaries and followed children’s weight to age 20 years.3 Introducing a milk other than breast milk before 6 months of age was linked to increased risk of obesity at age 20 (odds ratio [OR]=1.5; 95% CI, 1.1-1.9).
Finally, in a prospective cohort of 568 children in India, 17% of children who breastfed for fewer than 6 months were above the 90th percentile for weight at age 5 years, compared with 10% of children who were breastfed for at least 18 months.4 The result didn’t reach statistical significance, however (P=.08).
Interventions that increase breastfeeding don’t seem to have an impact
An RCT of an intervention to promote breastfeeding didn’t find any effect on subsequent obesity rates. Researchers in Belarus randomized 17,046 mother-infant pairs to breastfeeding promotion, modeled on the UNICEF Baby-Friendly Hospital Initiative, or usual care. The intervention increased the prevalence of exclusive breastfeeding (at 3 months, 43% vs 6%; at 6 months, 7% vs 0.6%; P values not given).
When researchers evaluated 13,879 children at 11 or 12 years by intention-to-treat analysis, however, they found no difference in mean BMI between the children whose mothers received the intervention and those whose mothers didn’t (BMI difference=0.16; 95% CI, -0.02 to 0.35).5
Introduction of solid foods: Later is better
A systematic review investigated the association between the timing of introducing complementary (solid) foods and childhood obesity in 23 primarily cross-sectional and cohort studies (17 from the United States, Canada, and Europe) with more than 33,000 patients. Follow-up ranged from 4 to 19 years.
Eight of the 21 studies that used BMI as an outcome found that early introduction of complementary foods was associated with a higher childhood BMI. In the largest study (a cohort of 17,561 infants), introducing complementary foods before 3 months was associated with higher risk of obesity at age 5 years than introducing them thereafter (OR=1.3; 95% CI, 1.1-1.6).6 Introduction of solids after 4 months was not associated with childhood obesity.
A systematic review of 10 primarily cross-sectional and cohort studies with more than 3000 infants evaluated associations between the types of complementary foods given and the development of childhood obesity.7 Six of the 10 studies were from Europe and none were from the United States. Follow-up ages ranged from 4 to 11 years.
Outcomes were heterogeneous, and no meta-analysis could be performed. The authors cited 3 studies (total 1174 infants) that found various positive associations between total caloric intake during complementary feeding and childhood obesity. No consistent evidence pointed to increased risk from specific foods or food groups.
Scheduled feeding is linked to rapid infant weight gain
A cohort study evaluated the baseline data of an Australian RCT (on an intervention to promote proper nutrition) in 612 infants, mean age 4.3 months.8 Researchers looked at the relationship between feeding on demand vs scheduled feeding (assessed by parental report) and weight gain in infancy. “Rapid weight gain” was defined as >0.67 change in weight-for-age Z-score between birth and enrollment.
Scheduled feeding was associated with rapid weight gain at a higher rate than feeding on demand (OR=2.3; 95% CI, 1.1-4.6). This study didn’t use childhood obesity as an outcome.
1. Weng SF, Redsell SA, Swift JA, et al. Systematic review and meta-analyses of risk factors for childhood overweight identifiable during infancy. Arch Dis Child. 2012;97:1019-1026.
2. Yamakawa M, Yorifuji T, Inoue S, et al. Breastfeeding and obesity among schoolchildren: a national longitudinal survey in Japan. JAMA Pediatr. 2013;167:919-925.
3. Oddy WH, Mari TA, Huang RC, et al. Early infant feeding and adiposity risk: from infancy to adulthood. Ann Nutr Metab. 2014;64:262-270.
4. Caleyachetty A, Krishnaveni GV, Veena SR, et al. Breast-feeding duration, age of starting solids, and high BMI risk and adiposity in Indian children. Matern Child Nutr. 2013;9:199-216.
5. Martin RM, Patel, R, Kramer MS, et al. Effects of promoting longer-term and exclusive breastfeeding on adiposity and insulin-like growth factor-I at age 11.5 years: a randomized trial. JAMA. 2013;309:1005-1013.
6. Pearce J, Taylor MA, Langley-Evans SC. Timing of the introduction of complementary feeding and risk of childhood obesity: a systematic review. Int J Obes (Lond). 2013;37:1295-1306.
7. Pearce J, Langley-Evans. The types of food introduced during complementary feeding and risk of childhood obesity: a systematic review. Int J Obes (Lond). 2013;37:477-485.
8. Mihrshahi S, Battistutta D, Magarey A, et al. Determinants of rapid weight gain during infancy: baseline results from the NOURISH randomised controlled trial. BMC Pediatr. 2011;11:99.
1. Weng SF, Redsell SA, Swift JA, et al. Systematic review and meta-analyses of risk factors for childhood overweight identifiable during infancy. Arch Dis Child. 2012;97:1019-1026.
2. Yamakawa M, Yorifuji T, Inoue S, et al. Breastfeeding and obesity among schoolchildren: a national longitudinal survey in Japan. JAMA Pediatr. 2013;167:919-925.
3. Oddy WH, Mari TA, Huang RC, et al. Early infant feeding and adiposity risk: from infancy to adulthood. Ann Nutr Metab. 2014;64:262-270.
4. Caleyachetty A, Krishnaveni GV, Veena SR, et al. Breast-feeding duration, age of starting solids, and high BMI risk and adiposity in Indian children. Matern Child Nutr. 2013;9:199-216.
5. Martin RM, Patel, R, Kramer MS, et al. Effects of promoting longer-term and exclusive breastfeeding on adiposity and insulin-like growth factor-I at age 11.5 years: a randomized trial. JAMA. 2013;309:1005-1013.
6. Pearce J, Taylor MA, Langley-Evans SC. Timing of the introduction of complementary feeding and risk of childhood obesity: a systematic review. Int J Obes (Lond). 2013;37:1295-1306.
7. Pearce J, Langley-Evans. The types of food introduced during complementary feeding and risk of childhood obesity: a systematic review. Int J Obes (Lond). 2013;37:477-485.
8. Mihrshahi S, Battistutta D, Magarey A, et al. Determinants of rapid weight gain during infancy: baseline results from the NOURISH randomised controlled trial. BMC Pediatr. 2011;11:99.
Evidence-based answers from the Family Physicians Inquiries Network
EVIDENCE-BASED ANSWER:
Yes. Ever having breastfed during the first year of life is associated with a 15% lower risk of overweight or obesity over the next 2 to 14 years compared with never having breastfed. Breastfeeding exclusively for 6 months is associated with a 30% to 50% reduction in risk (strength of recommendation [SOR]: B, meta-analysis of cohort studies and subsequent cohort studies). However, interventions that increase breastfeeding rates during the first 3 to 6 months of life don’t appear to alter body mass index (BMI) at 11 to 12 years of age (SOR: B, randomized clinical trial [RCT]).
Introducing complementary (solid) foods before 3 months is associated with a 30% greater risk of childhood obesity than later introduction; starting solid foods after 4 months isn’t linked to increased obesity. High caloric density of complementary feedings may be associated with greater childhood obesity (SOR: C, systematic reviews of heterogeneous cohort studies).
Scheduled feeding doubles the risk of rapid infant weight gain compared with on-demand feeding, although it’s unclear whether a direct relationship exists between rapid infant weight gain and childhood obesity (SOR: B, cohort study).
Which treatments are safe and effective for chronic sinusitis?
EVIDENCE-BASED ANSWER:
For adults with chronic rhinosinusitis (CRS), intranasal steroid (INS) therapy is more likely than placebo to improve symptoms (50% vs 32%; strength of recommendation [SOR]: A, systematic reviews).
Nasal saline irrigation (SI) alleviates symptoms better than no therapy (SOR: A, systematic reviews), but it’s probably not as effective as INS treatment (SOR: B, randomized controlled trial [RCT] with wide confidence interval).
Long-term (12 weeks) macrolide therapy doesn’t alter patient-oriented quality-of-life measures (SOR: A, systematic reviews).
Endoscopic sinus surgery improves CRS symptoms—nasal obstruction, discharge, and facial pain—over baseline (SOR: A, systematic reviews). Surgery and medical therapy appear about equivalent in terms of symptom improvement and quality-of-life measures (SOR: B, systematic reviews of low-quality RCTs).
EVIDENCE SUMMARY
The TABLE1-4 shows the major results of the meta-analyses for the various medical therapy trials.
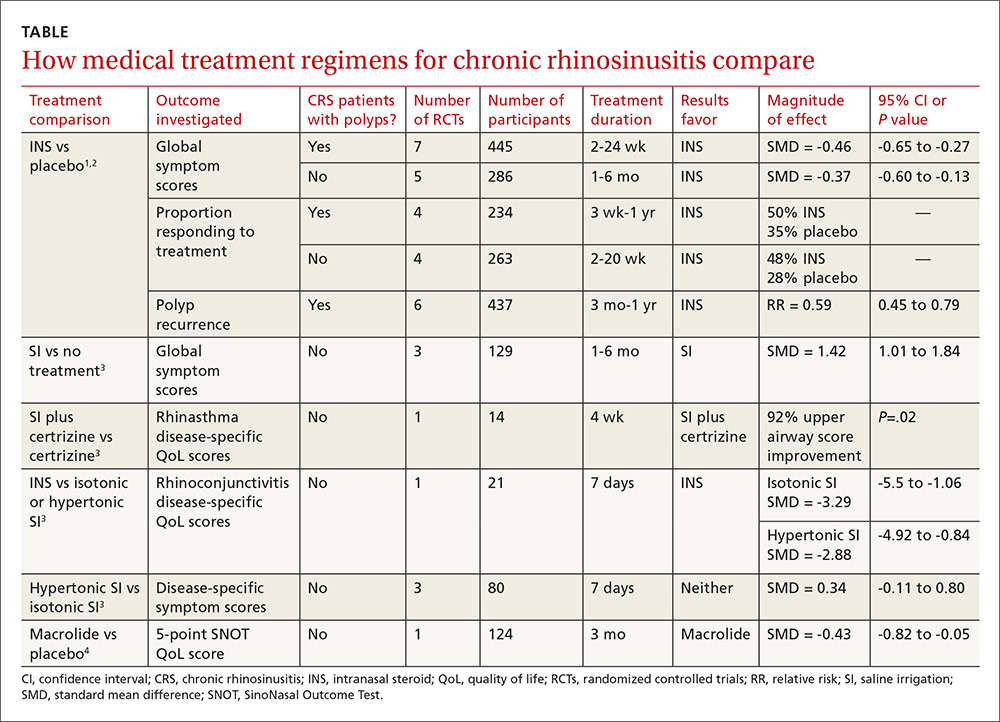
Two systematic reviews with meta-analyses evaluated treatment with INS for CRS with nasal polyps (40 RCTs; 3624 patients, mean age 48 years, 64% male) and without polyps (10 RCTs; 590 patients, mean age 39 years, 51% male).1,2 Trials reported sinonasal symptom outcomes differently and couldn’t be combined. In addition to reducing rate of polyp occurrence, for both CRS with and without polyps, key findings were:
- Global symptom scores were better for INS than placebo.
- Proportion of patients responding was greater for INS than with placebo.
There was no significant difference between adverse event rates with INS and placebo.
A systematic review and meta-analysis (8 RCTs, 389 patients) compared different SI regimens for CRS.3 The standardized mean difference was used to combine trials using various symptom outcomes. Key findings included the following:
- SI was better than no treatment.
- SI adjunctive therapy (with an antihistamine) improved disease-specific quality-of-life scores.
- SI was less effective than INS therapy for symptom improvement.
Hypertonic and isotonic saline yielded similar symptom scores. No adverse effects were reported.
One meta-analysis evaluated patient-reported outcomes with 12 weeks of macrolide therapy compared to placebo using the results of the SinoNasal Outcome Test (SNOT). The SNOT is a quality-of-life questionnaire that lists symptoms and the social-emotional consequences of CRS; a negative change in the SNOT score, on a 0 to 5 scale, indicates improvement. Overall the SNOT score improved 8% with macrolide therapy—statistically significant, but of uncertain clinical importance.4
Surgery improves nasal obstruction, pain, and postnasal discharge
A systematic review of 21 studies (prospective RCTs, prospective controlled clinical trials, cohort studies, case series, and retrospective record reviews) with a total of 2070 patients analyzed the effectiveness of endoscopic sinus surgery alone for improving CRS symptoms.5 Mean duration of post-operative follow-up was 14 months. Meta-analysis was performed separately for each symptom and the standard mean difference of the symptom severity score before and after surgery was reported as the effect size (ES) for the outcome measure (an ES of 0.2 is considered small; 0.6, moderate; 1.2, large; and 2, very large).
All symptoms improved compared to their preoperative severity scores. Nasal obstruction improved the most (ES=1.73; 95% CI, 1.45-2.02). Large symptom improvement was also observed for facial pain (ES=1.13; 95% CI, 0.96-1.31) and postnasal discharge (ES=1.19; 95% CI, 0.96-1.43).
Surgery and medical therapy may provide comparable symptom relief
A recent Cochrane review of 4 low-quality RCTs including 378 patients compared surgical with medical interventions for CRS with nasal polyps. Study heterogeneity and selective outcome reporting prevented meta-analysis.
The 3 comparison groups were endoscopic sinus surgery vs systemic steroids + INS; polypectomy vs systemic steroid + INS; and endoscopic surgery + INS vs antibiotic + “high-dose” INS. Overall, neither surgery nor medical therapy was superior in terms of patient-reported symptom scores or quality-of-life measures.6
1. Kalish L, Snidvongs K, Sivasubramaniam R, et al. Topical steroids for nasal polyps. Cochrane Database Syst Rev. 2012;(12):CD006549.
2. Snidvongs K, Kalish L, Sacks R, et al. Topical steroids for chronic rhinosinusitis without polyps. Cochrane Database Syst Rev. 2011;(8):CD009274.
3. Harvey R, Hannan SA, Badia L, et al. Nasal saline irrigation for the symptoms of chronic rhinosinusitis. Cochrane Database Syst Rev. 2007;(3):CD006394.
4. Pynnonen MA, Venkatraman G, Davis GE. Macrolide therapy for chronic rhinosinusitis: a meta-analysis. Otolaryngol Head Neck Surg. 2013;148:366-373.
5. Chester AC, Antisdel JL, Sindwani R. Symptom-specific outcomes of endoscopic sinus surgery: a systematic review. Otolaryngol Head Neck Surg. 2009;140:633-639.
6. Rimmer J, Fokkens W, Chong LY, et al. Surgical versus medical interventions for chronic rhinosinusitis with nasal polyps. Cochrane Database Syst Rev. 2014;(12):CD0069991.
EVIDENCE-BASED ANSWER:
For adults with chronic rhinosinusitis (CRS), intranasal steroid (INS) therapy is more likely than placebo to improve symptoms (50% vs 32%; strength of recommendation [SOR]: A, systematic reviews).
Nasal saline irrigation (SI) alleviates symptoms better than no therapy (SOR: A, systematic reviews), but it’s probably not as effective as INS treatment (SOR: B, randomized controlled trial [RCT] with wide confidence interval).
Long-term (12 weeks) macrolide therapy doesn’t alter patient-oriented quality-of-life measures (SOR: A, systematic reviews).
Endoscopic sinus surgery improves CRS symptoms—nasal obstruction, discharge, and facial pain—over baseline (SOR: A, systematic reviews). Surgery and medical therapy appear about equivalent in terms of symptom improvement and quality-of-life measures (SOR: B, systematic reviews of low-quality RCTs).
EVIDENCE SUMMARY
The TABLE1-4 shows the major results of the meta-analyses for the various medical therapy trials.

Two systematic reviews with meta-analyses evaluated treatment with INS for CRS with nasal polyps (40 RCTs; 3624 patients, mean age 48 years, 64% male) and without polyps (10 RCTs; 590 patients, mean age 39 years, 51% male).1,2 Trials reported sinonasal symptom outcomes differently and couldn’t be combined. In addition to reducing rate of polyp occurrence, for both CRS with and without polyps, key findings were:
- Global symptom scores were better for INS than placebo.
- Proportion of patients responding was greater for INS than with placebo.
There was no significant difference between adverse event rates with INS and placebo.
A systematic review and meta-analysis (8 RCTs, 389 patients) compared different SI regimens for CRS.3 The standardized mean difference was used to combine trials using various symptom outcomes. Key findings included the following:
- SI was better than no treatment.
- SI adjunctive therapy (with an antihistamine) improved disease-specific quality-of-life scores.
- SI was less effective than INS therapy for symptom improvement.
Hypertonic and isotonic saline yielded similar symptom scores. No adverse effects were reported.
One meta-analysis evaluated patient-reported outcomes with 12 weeks of macrolide therapy compared to placebo using the results of the SinoNasal Outcome Test (SNOT). The SNOT is a quality-of-life questionnaire that lists symptoms and the social-emotional consequences of CRS; a negative change in the SNOT score, on a 0 to 5 scale, indicates improvement. Overall the SNOT score improved 8% with macrolide therapy—statistically significant, but of uncertain clinical importance.4
Surgery improves nasal obstruction, pain, and postnasal discharge
A systematic review of 21 studies (prospective RCTs, prospective controlled clinical trials, cohort studies, case series, and retrospective record reviews) with a total of 2070 patients analyzed the effectiveness of endoscopic sinus surgery alone for improving CRS symptoms.5 Mean duration of post-operative follow-up was 14 months. Meta-analysis was performed separately for each symptom and the standard mean difference of the symptom severity score before and after surgery was reported as the effect size (ES) for the outcome measure (an ES of 0.2 is considered small; 0.6, moderate; 1.2, large; and 2, very large).
All symptoms improved compared to their preoperative severity scores. Nasal obstruction improved the most (ES=1.73; 95% CI, 1.45-2.02). Large symptom improvement was also observed for facial pain (ES=1.13; 95% CI, 0.96-1.31) and postnasal discharge (ES=1.19; 95% CI, 0.96-1.43).
Surgery and medical therapy may provide comparable symptom relief
A recent Cochrane review of 4 low-quality RCTs including 378 patients compared surgical with medical interventions for CRS with nasal polyps. Study heterogeneity and selective outcome reporting prevented meta-analysis.
The 3 comparison groups were endoscopic sinus surgery vs systemic steroids + INS; polypectomy vs systemic steroid + INS; and endoscopic surgery + INS vs antibiotic + “high-dose” INS. Overall, neither surgery nor medical therapy was superior in terms of patient-reported symptom scores or quality-of-life measures.6
EVIDENCE-BASED ANSWER:
For adults with chronic rhinosinusitis (CRS), intranasal steroid (INS) therapy is more likely than placebo to improve symptoms (50% vs 32%; strength of recommendation [SOR]: A, systematic reviews).
Nasal saline irrigation (SI) alleviates symptoms better than no therapy (SOR: A, systematic reviews), but it’s probably not as effective as INS treatment (SOR: B, randomized controlled trial [RCT] with wide confidence interval).
Long-term (12 weeks) macrolide therapy doesn’t alter patient-oriented quality-of-life measures (SOR: A, systematic reviews).
Endoscopic sinus surgery improves CRS symptoms—nasal obstruction, discharge, and facial pain—over baseline (SOR: A, systematic reviews). Surgery and medical therapy appear about equivalent in terms of symptom improvement and quality-of-life measures (SOR: B, systematic reviews of low-quality RCTs).
EVIDENCE SUMMARY
The TABLE1-4 shows the major results of the meta-analyses for the various medical therapy trials.

Two systematic reviews with meta-analyses evaluated treatment with INS for CRS with nasal polyps (40 RCTs; 3624 patients, mean age 48 years, 64% male) and without polyps (10 RCTs; 590 patients, mean age 39 years, 51% male).1,2 Trials reported sinonasal symptom outcomes differently and couldn’t be combined. In addition to reducing rate of polyp occurrence, for both CRS with and without polyps, key findings were:
- Global symptom scores were better for INS than placebo.
- Proportion of patients responding was greater for INS than with placebo.
There was no significant difference between adverse event rates with INS and placebo.
A systematic review and meta-analysis (8 RCTs, 389 patients) compared different SI regimens for CRS.3 The standardized mean difference was used to combine trials using various symptom outcomes. Key findings included the following:
- SI was better than no treatment.
- SI adjunctive therapy (with an antihistamine) improved disease-specific quality-of-life scores.
- SI was less effective than INS therapy for symptom improvement.
Hypertonic and isotonic saline yielded similar symptom scores. No adverse effects were reported.
One meta-analysis evaluated patient-reported outcomes with 12 weeks of macrolide therapy compared to placebo using the results of the SinoNasal Outcome Test (SNOT). The SNOT is a quality-of-life questionnaire that lists symptoms and the social-emotional consequences of CRS; a negative change in the SNOT score, on a 0 to 5 scale, indicates improvement. Overall the SNOT score improved 8% with macrolide therapy—statistically significant, but of uncertain clinical importance.4
Surgery improves nasal obstruction, pain, and postnasal discharge
A systematic review of 21 studies (prospective RCTs, prospective controlled clinical trials, cohort studies, case series, and retrospective record reviews) with a total of 2070 patients analyzed the effectiveness of endoscopic sinus surgery alone for improving CRS symptoms.5 Mean duration of post-operative follow-up was 14 months. Meta-analysis was performed separately for each symptom and the standard mean difference of the symptom severity score before and after surgery was reported as the effect size (ES) for the outcome measure (an ES of 0.2 is considered small; 0.6, moderate; 1.2, large; and 2, very large).
All symptoms improved compared to their preoperative severity scores. Nasal obstruction improved the most (ES=1.73; 95% CI, 1.45-2.02). Large symptom improvement was also observed for facial pain (ES=1.13; 95% CI, 0.96-1.31) and postnasal discharge (ES=1.19; 95% CI, 0.96-1.43).
Surgery and medical therapy may provide comparable symptom relief
A recent Cochrane review of 4 low-quality RCTs including 378 patients compared surgical with medical interventions for CRS with nasal polyps. Study heterogeneity and selective outcome reporting prevented meta-analysis.
The 3 comparison groups were endoscopic sinus surgery vs systemic steroids + INS; polypectomy vs systemic steroid + INS; and endoscopic surgery + INS vs antibiotic + “high-dose” INS. Overall, neither surgery nor medical therapy was superior in terms of patient-reported symptom scores or quality-of-life measures.6
1. Kalish L, Snidvongs K, Sivasubramaniam R, et al. Topical steroids for nasal polyps. Cochrane Database Syst Rev. 2012;(12):CD006549.
2. Snidvongs K, Kalish L, Sacks R, et al. Topical steroids for chronic rhinosinusitis without polyps. Cochrane Database Syst Rev. 2011;(8):CD009274.
3. Harvey R, Hannan SA, Badia L, et al. Nasal saline irrigation for the symptoms of chronic rhinosinusitis. Cochrane Database Syst Rev. 2007;(3):CD006394.
4. Pynnonen MA, Venkatraman G, Davis GE. Macrolide therapy for chronic rhinosinusitis: a meta-analysis. Otolaryngol Head Neck Surg. 2013;148:366-373.
5. Chester AC, Antisdel JL, Sindwani R. Symptom-specific outcomes of endoscopic sinus surgery: a systematic review. Otolaryngol Head Neck Surg. 2009;140:633-639.
6. Rimmer J, Fokkens W, Chong LY, et al. Surgical versus medical interventions for chronic rhinosinusitis with nasal polyps. Cochrane Database Syst Rev. 2014;(12):CD0069991.
1. Kalish L, Snidvongs K, Sivasubramaniam R, et al. Topical steroids for nasal polyps. Cochrane Database Syst Rev. 2012;(12):CD006549.
2. Snidvongs K, Kalish L, Sacks R, et al. Topical steroids for chronic rhinosinusitis without polyps. Cochrane Database Syst Rev. 2011;(8):CD009274.
3. Harvey R, Hannan SA, Badia L, et al. Nasal saline irrigation for the symptoms of chronic rhinosinusitis. Cochrane Database Syst Rev. 2007;(3):CD006394.
4. Pynnonen MA, Venkatraman G, Davis GE. Macrolide therapy for chronic rhinosinusitis: a meta-analysis. Otolaryngol Head Neck Surg. 2013;148:366-373.
5. Chester AC, Antisdel JL, Sindwani R. Symptom-specific outcomes of endoscopic sinus surgery: a systematic review. Otolaryngol Head Neck Surg. 2009;140:633-639.
6. Rimmer J, Fokkens W, Chong LY, et al. Surgical versus medical interventions for chronic rhinosinusitis with nasal polyps. Cochrane Database Syst Rev. 2014;(12):CD0069991.
Evidence-based answers from the Family Physicians Inquiries Network




