User login
Which nonhormonal treatments are effective for hot flashes?
Selective serotonin reuptake inhibitors (SSRIs [fluoxetine, sertraline, paroxetine]) and the selective norepinephrine reuptake inhibitor (SNRI) venlafaxine, as well as clonidine and gabapentin, reduce hot flashes by about 25% (approximately one per day) in women with and without a history of breast cancer. No studies compare medications against each other to determine a single best option (strength of recommendation [SOR]: A, systematic reviews and meta-analyses of randomized controlled trials [RCTs]). In comparison, estrogen reduces the frequency of hot flashes by about 75%, or 2.5 to 3 per day.
The phytoestrogens (soy isoflavones, red clover extract, black cohosh), vitamin E, and nonpharmacologic measures (relaxation therapy, exercise, acupuncture, homeopathy, magnet therapy) lack evidence of effectiveness (SOR: A, meta-analyses of RCTs, many of which were low quality).
EVIDENCE SUMMARY
A systematic review of 6 RCTs that evaluated SSRIs and SNRIs (fluoxetine, sertraline, paroxetine, venlafaxine) found them all to be effective for reducing hot flash frequency and symptom scores in women with previous breast cancer1 (TABLE1,2).
A 2006 meta-analysis combined the results of 7 RCTs (each evaluating a single SSRI [fluoxetine, paroxetine] or SNRI [venlafaxine]) and found that as a group, they reduced mean hot flash frequency (–1.13 hot flashes/d; 95% confidence interval [CI], –1.70 to –0.57) in women with and without breast cancer.2 No trial compared medications head to head, and the populations differed among studies, so that investigators couldn’t determine a single best agent.
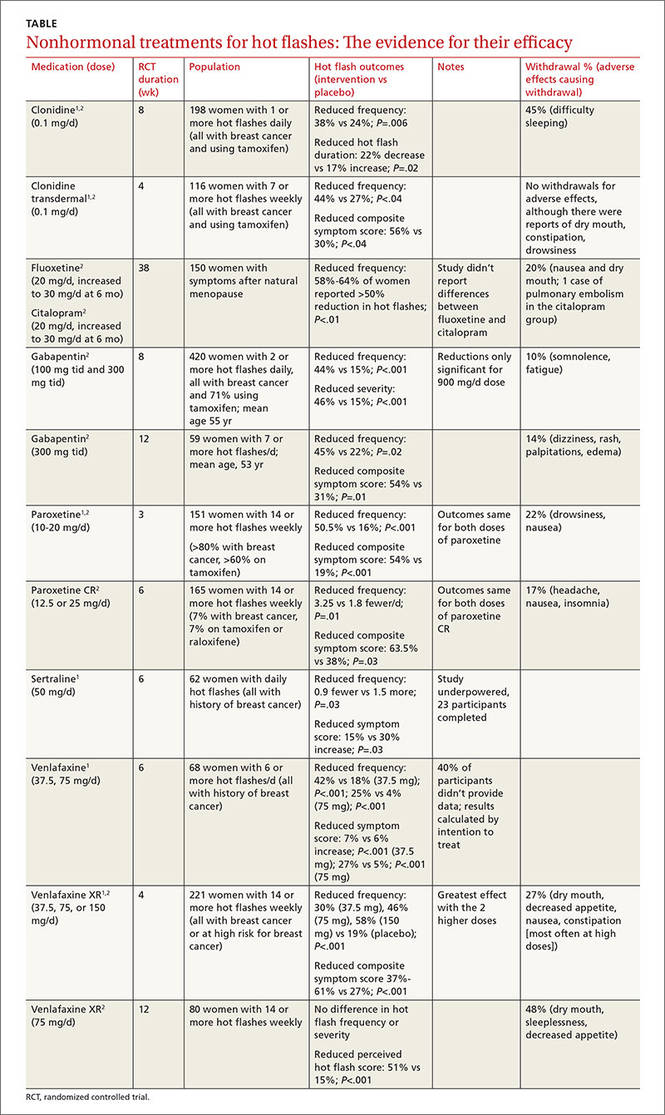
Clonidine and gabapentin decrease hot flash frequency
The 2006 meta-analysis also included 10 RCTs (743 patients) that studied clonidine in women with and without a history of breast cancer, and 2 RCTs (479 patients) that evaluated gabapentin in women with breast cancer.2 Both drugs reduced mean hot flash frequency (clonidine: –0.95 hot flashes/d, 95% CI, –1.44 to –0.47 at 4 weeks and –1.63 hot flashes/d, 95% CI, –2.76 to –0.05 at 8 weeks; gabapentin: –2.05 hot flashes/d; 95% CI, −2.80 to –1.30).
Phytoestrogens: The jury is still out
A meta-analysis of 43 RCTs (4364 patients) evaluated phytoestrogens that included dietary soy, soy extracts, red clover extracts, genistein extracts, and other types of phytoestrogens.3 The data from the only 5 RCTs (300 patients) that could be combined showed no effect from red clover extract on hot flash frequency. However, another 4 individual trials that couldn’t be combined each found that extracts with high levels of the phytoestrogen genistein (>30 mg/d) did reduce frequency. Investigators reported that many of the trials were small and had a high risk of bias.
A meta-analysis of 16 RCTs (2027 patients) that assessed black cohosh found that it didn’t reduce hot flash frequency (3 RCTs, 393 patients) or symptom severity scores (4 RCTs, 357 patients).4 Investigators reported high heterogeneity and recommended further research.
Nonpharmacologic therapies and vitamin E don’t help
Systematic reviews found that relaxation therapy (4 RCTs, 281 patients), exercise (3 RCTs, 454 patients), and acupuncture (8 RCTs, 414 patients) didn’t reduce hot flashes.5-7 In another review, vitamin E (1 RCT, 105 patients), homeopathy (2 RCTs, 124 patients), and magnetic devices (1 RCT, 11 patients) also produced no benefit.1
1. Rada G, Capurro D, Pantoja T, et al. Non-hormonal interventions for hot flushes in women with a history of breast cancer. Cochrane Database Syst Rev. 2010;(9):CD004923.
2. Nelson HD, Vesco KK, Haney E, et al. Nonhormonal therapies of menopausal hot flashes: systematic review and meta-analysis. JAMA. 2006;295:2057-2071.
3. Lethaby A, Marjoribanks J, Kronenberg F, et al. Phytoestrogens for menopausal vasomotor symptoms. Cochrane Database Syst Rev. 2013;(12):CD001395.
4. Leach MJ, Moore V. Black cohosh (Cimicifuga spp.) for menopausal symptoms. Cochrane Database Syst Rev. 2012;(9):CD007244.
5. Saensak S, Vutyavanich T, Somboonporn W, et al. Relaxation for perimenopausal and postmenopausal symptoms. Cochrane Database Syst Rev. 2014;(7):CD008582.
6. Daley A, Stokes-Lampard H, Thomas A, et al. Exercise for vasomotor menopausal symptoms. Cochrane Database Syst Rev. 2014:(11):CD006108.
7. Dodin S, Blanchet C, Marc I, et al. Acupuncture for menopausal hot flashes. Cochrane Database Syst Rev. 2013;(7):CD007410.
Selective serotonin reuptake inhibitors (SSRIs [fluoxetine, sertraline, paroxetine]) and the selective norepinephrine reuptake inhibitor (SNRI) venlafaxine, as well as clonidine and gabapentin, reduce hot flashes by about 25% (approximately one per day) in women with and without a history of breast cancer. No studies compare medications against each other to determine a single best option (strength of recommendation [SOR]: A, systematic reviews and meta-analyses of randomized controlled trials [RCTs]). In comparison, estrogen reduces the frequency of hot flashes by about 75%, or 2.5 to 3 per day.
The phytoestrogens (soy isoflavones, red clover extract, black cohosh), vitamin E, and nonpharmacologic measures (relaxation therapy, exercise, acupuncture, homeopathy, magnet therapy) lack evidence of effectiveness (SOR: A, meta-analyses of RCTs, many of which were low quality).
EVIDENCE SUMMARY
A systematic review of 6 RCTs that evaluated SSRIs and SNRIs (fluoxetine, sertraline, paroxetine, venlafaxine) found them all to be effective for reducing hot flash frequency and symptom scores in women with previous breast cancer1 (TABLE1,2).
A 2006 meta-analysis combined the results of 7 RCTs (each evaluating a single SSRI [fluoxetine, paroxetine] or SNRI [venlafaxine]) and found that as a group, they reduced mean hot flash frequency (–1.13 hot flashes/d; 95% confidence interval [CI], –1.70 to –0.57) in women with and without breast cancer.2 No trial compared medications head to head, and the populations differed among studies, so that investigators couldn’t determine a single best agent.

Clonidine and gabapentin decrease hot flash frequency
The 2006 meta-analysis also included 10 RCTs (743 patients) that studied clonidine in women with and without a history of breast cancer, and 2 RCTs (479 patients) that evaluated gabapentin in women with breast cancer.2 Both drugs reduced mean hot flash frequency (clonidine: –0.95 hot flashes/d, 95% CI, –1.44 to –0.47 at 4 weeks and –1.63 hot flashes/d, 95% CI, –2.76 to –0.05 at 8 weeks; gabapentin: –2.05 hot flashes/d; 95% CI, −2.80 to –1.30).
Phytoestrogens: The jury is still out
A meta-analysis of 43 RCTs (4364 patients) evaluated phytoestrogens that included dietary soy, soy extracts, red clover extracts, genistein extracts, and other types of phytoestrogens.3 The data from the only 5 RCTs (300 patients) that could be combined showed no effect from red clover extract on hot flash frequency. However, another 4 individual trials that couldn’t be combined each found that extracts with high levels of the phytoestrogen genistein (>30 mg/d) did reduce frequency. Investigators reported that many of the trials were small and had a high risk of bias.
A meta-analysis of 16 RCTs (2027 patients) that assessed black cohosh found that it didn’t reduce hot flash frequency (3 RCTs, 393 patients) or symptom severity scores (4 RCTs, 357 patients).4 Investigators reported high heterogeneity and recommended further research.
Nonpharmacologic therapies and vitamin E don’t help
Systematic reviews found that relaxation therapy (4 RCTs, 281 patients), exercise (3 RCTs, 454 patients), and acupuncture (8 RCTs, 414 patients) didn’t reduce hot flashes.5-7 In another review, vitamin E (1 RCT, 105 patients), homeopathy (2 RCTs, 124 patients), and magnetic devices (1 RCT, 11 patients) also produced no benefit.1
Selective serotonin reuptake inhibitors (SSRIs [fluoxetine, sertraline, paroxetine]) and the selective norepinephrine reuptake inhibitor (SNRI) venlafaxine, as well as clonidine and gabapentin, reduce hot flashes by about 25% (approximately one per day) in women with and without a history of breast cancer. No studies compare medications against each other to determine a single best option (strength of recommendation [SOR]: A, systematic reviews and meta-analyses of randomized controlled trials [RCTs]). In comparison, estrogen reduces the frequency of hot flashes by about 75%, or 2.5 to 3 per day.
The phytoestrogens (soy isoflavones, red clover extract, black cohosh), vitamin E, and nonpharmacologic measures (relaxation therapy, exercise, acupuncture, homeopathy, magnet therapy) lack evidence of effectiveness (SOR: A, meta-analyses of RCTs, many of which were low quality).
EVIDENCE SUMMARY
A systematic review of 6 RCTs that evaluated SSRIs and SNRIs (fluoxetine, sertraline, paroxetine, venlafaxine) found them all to be effective for reducing hot flash frequency and symptom scores in women with previous breast cancer1 (TABLE1,2).
A 2006 meta-analysis combined the results of 7 RCTs (each evaluating a single SSRI [fluoxetine, paroxetine] or SNRI [venlafaxine]) and found that as a group, they reduced mean hot flash frequency (–1.13 hot flashes/d; 95% confidence interval [CI], –1.70 to –0.57) in women with and without breast cancer.2 No trial compared medications head to head, and the populations differed among studies, so that investigators couldn’t determine a single best agent.

Clonidine and gabapentin decrease hot flash frequency
The 2006 meta-analysis also included 10 RCTs (743 patients) that studied clonidine in women with and without a history of breast cancer, and 2 RCTs (479 patients) that evaluated gabapentin in women with breast cancer.2 Both drugs reduced mean hot flash frequency (clonidine: –0.95 hot flashes/d, 95% CI, –1.44 to –0.47 at 4 weeks and –1.63 hot flashes/d, 95% CI, –2.76 to –0.05 at 8 weeks; gabapentin: –2.05 hot flashes/d; 95% CI, −2.80 to –1.30).
Phytoestrogens: The jury is still out
A meta-analysis of 43 RCTs (4364 patients) evaluated phytoestrogens that included dietary soy, soy extracts, red clover extracts, genistein extracts, and other types of phytoestrogens.3 The data from the only 5 RCTs (300 patients) that could be combined showed no effect from red clover extract on hot flash frequency. However, another 4 individual trials that couldn’t be combined each found that extracts with high levels of the phytoestrogen genistein (>30 mg/d) did reduce frequency. Investigators reported that many of the trials were small and had a high risk of bias.
A meta-analysis of 16 RCTs (2027 patients) that assessed black cohosh found that it didn’t reduce hot flash frequency (3 RCTs, 393 patients) or symptom severity scores (4 RCTs, 357 patients).4 Investigators reported high heterogeneity and recommended further research.
Nonpharmacologic therapies and vitamin E don’t help
Systematic reviews found that relaxation therapy (4 RCTs, 281 patients), exercise (3 RCTs, 454 patients), and acupuncture (8 RCTs, 414 patients) didn’t reduce hot flashes.5-7 In another review, vitamin E (1 RCT, 105 patients), homeopathy (2 RCTs, 124 patients), and magnetic devices (1 RCT, 11 patients) also produced no benefit.1
1. Rada G, Capurro D, Pantoja T, et al. Non-hormonal interventions for hot flushes in women with a history of breast cancer. Cochrane Database Syst Rev. 2010;(9):CD004923.
2. Nelson HD, Vesco KK, Haney E, et al. Nonhormonal therapies of menopausal hot flashes: systematic review and meta-analysis. JAMA. 2006;295:2057-2071.
3. Lethaby A, Marjoribanks J, Kronenberg F, et al. Phytoestrogens for menopausal vasomotor symptoms. Cochrane Database Syst Rev. 2013;(12):CD001395.
4. Leach MJ, Moore V. Black cohosh (Cimicifuga spp.) for menopausal symptoms. Cochrane Database Syst Rev. 2012;(9):CD007244.
5. Saensak S, Vutyavanich T, Somboonporn W, et al. Relaxation for perimenopausal and postmenopausal symptoms. Cochrane Database Syst Rev. 2014;(7):CD008582.
6. Daley A, Stokes-Lampard H, Thomas A, et al. Exercise for vasomotor menopausal symptoms. Cochrane Database Syst Rev. 2014:(11):CD006108.
7. Dodin S, Blanchet C, Marc I, et al. Acupuncture for menopausal hot flashes. Cochrane Database Syst Rev. 2013;(7):CD007410.
1. Rada G, Capurro D, Pantoja T, et al. Non-hormonal interventions for hot flushes in women with a history of breast cancer. Cochrane Database Syst Rev. 2010;(9):CD004923.
2. Nelson HD, Vesco KK, Haney E, et al. Nonhormonal therapies of menopausal hot flashes: systematic review and meta-analysis. JAMA. 2006;295:2057-2071.
3. Lethaby A, Marjoribanks J, Kronenberg F, et al. Phytoestrogens for menopausal vasomotor symptoms. Cochrane Database Syst Rev. 2013;(12):CD001395.
4. Leach MJ, Moore V. Black cohosh (Cimicifuga spp.) for menopausal symptoms. Cochrane Database Syst Rev. 2012;(9):CD007244.
5. Saensak S, Vutyavanich T, Somboonporn W, et al. Relaxation for perimenopausal and postmenopausal symptoms. Cochrane Database Syst Rev. 2014;(7):CD008582.
6. Daley A, Stokes-Lampard H, Thomas A, et al. Exercise for vasomotor menopausal symptoms. Cochrane Database Syst Rev. 2014:(11):CD006108.
7. Dodin S, Blanchet C, Marc I, et al. Acupuncture for menopausal hot flashes. Cochrane Database Syst Rev. 2013;(7):CD007410.
Evidence-based answers from the Family Physicians Inquiries Network
Does caffeine intake during pregnancy affect birth weight?
No. Reducing caffeinated coffee consumption by 180 mg of caffeine (the equivalent of 2 cups) per day after 16 weeks’ gestation doesn’t affect birth weight. Consuming more than 300 mg of caffeine per day is associated with a clinically trivial, and statistically insignificant (less than 1 ounce), reduction in birth weight, compared with consuming no caffeine (strength of recommendation: B, randomized controlled trial [RCT] and large prospective cohort study).
EVIDENCE SUMMARY
A Cochrane systematic review of the effects of caffeine on pregnancy identified 2 studies, only one of which addressed the question of maternal caffeine intake and infant birth weight.1 The double-blind RCT evaluating caffeine intake during pregnancy found no significant differences in birth weight or length of gestation between women who drank regular coffee and women who drank decaffeinated coffee.2
At 16 weeks’ gestation, investigators randomized 1207 pregnant women who reported daily intake of at least 3 cups of regular coffee to drink unlabeled instant coffee (which was either regular or decaffeinated) for the rest of their pregnancy. The women were allowed to request as much of their assigned instant coffee as they wanted.
Subjects were recruited from among all women with uncomplicated, singleton pregnancies who were expected to deliver at a Danish university hospital during the study period. Investigators interviewed the women at 20, 25, and 34 weeks to determine coffee consumption (including both coffee provided by the investigators and other coffee), consumption of other caffeinated beverages, and smoking status.
The difference in caffeine intake between the groups didn’t correspond to significant differences in birth weight (16 g lighter with caffeinated coffee; 95% confidence interval [CI], −40 g to 73 g; P=.48) or birth length (0.03 cm longer with caffeinated coffee; 95% CI, −0.29 to 0.22) among infants born to the 1150 women who completed the study.
Limitations of the study include randomizing women after 16 weeks’ gestation and the observation that many women correctly guessed which type of coffee they received (35% of women drinking caffeinated coffee and 49% of women drinking decaf).
A caffeine effect, but with study limitations
The Cochrane systematic review (described above) and a meta-analysis of 9 prospective cohort studies with a total of 90,000 patients that evaluated maternal caffeine intake found that it was associated with increased low birth weight, intrauterine growth restriction (IUGR), or small for gestational age (SGA) infants.3
Researchers assessed caffeine consumption from coffee or other sources either by questionnaire (5 studies) or interview (4 studies) at various times during pregnancy, mostly in the first or second trimester, and assigned subjects to 4 intake categories: none, low (50-149 mg/d), moderate (150-349 mg/d), and high (>350 mg/d).
Compared with no caffeine, all levels of caffeine intake were associated with increased rates of low birth weight, IUGR, or SGA (low intake: relative risk [RR]=1.13; 95% CI, 1.06-1.21; moderate intake: RR=1.38; 95% CI, 1.18-1.62; high intake: RR=1.60; 95% CI, 1.24-2.08).
A major limitation of the meta-analysis was that 8 of the included studies were identified by the reviewers as having quality problems. The reviewers also identified additional cohort studies, not included in the meta-analysis, which failed to show any association between caffeine intake and poor pregnancy outcomes.
Results of best-quality study prove clinically trivial
The best-quality prospective cohort study in the review described above was also the largest, comprising two-thirds of the total patients. It found a statistically significant, but clinically trivial, association between caffeine intake and birth weight.4
Investigators from Norway’s Institute of Public Health mailed surveys to 106,707 pregnant Norwegian women and recruited 59,123 with uncomplicated singleton pregnancies. The survey assessed diet and lifestyle at several stages of pregnancy and correlated caffeine intake with birth weight, gestational length, and SGA deliveries. Investigators calculated caffeine intake from coffee and other dietary sources (tea and chocolate).
Higher caffeine intake was associated with a small reduction in birth weight (8 g/100 mg/d of additional caffeine intake; 95% CI, −10 to −6 g/100 mg/d). Higher intake was also associated with increasing likelihood of SGA birth, a finding of borderline significance (odds ratio [OR]=1.18; 95% CI, 1.00-1.38, comparing intake <50 mg/d with 51-200 mg/d; OR=1.62; 95% CI, 1.26-2.29, comparing <50 mg/d with 201-300 mg/d; and OR=1.62; 95% CI, 1.15-2.29, comparing <50 mg/d with >300 mg/d).
1. Jahanfar S, Jaafar SH. Effects of restricted caffeine intake by mother on fetal, neonatal and pregnancy outcome. Cochrane Database Syst Rev. 2013;(2):CD006965.
2. Bech BH, Obel C, Henriksen TB, et al. Effect of reducing caffeine intake on birth weight and length of gestation: randomised controlled trial. BMJ. 2007;334:409.
3. Chen LW, Wu Y, Neelakantan N, et al. Maternal caffeine intake during pregnancy is associated with risk of low birth weight: a systematic review and dose-response meta-analysis. BMC Medicine. 2014;12:174-176.
4. Sengpiel V, Elind E, Bacelis J, et al. Maternal caffeine intake during pregnancy is associated with birth weight but not with gestational length: results form a large prospective observational cohort trial. BMC Medicine. 2013;11:42.
No. Reducing caffeinated coffee consumption by 180 mg of caffeine (the equivalent of 2 cups) per day after 16 weeks’ gestation doesn’t affect birth weight. Consuming more than 300 mg of caffeine per day is associated with a clinically trivial, and statistically insignificant (less than 1 ounce), reduction in birth weight, compared with consuming no caffeine (strength of recommendation: B, randomized controlled trial [RCT] and large prospective cohort study).
EVIDENCE SUMMARY
A Cochrane systematic review of the effects of caffeine on pregnancy identified 2 studies, only one of which addressed the question of maternal caffeine intake and infant birth weight.1 The double-blind RCT evaluating caffeine intake during pregnancy found no significant differences in birth weight or length of gestation between women who drank regular coffee and women who drank decaffeinated coffee.2
At 16 weeks’ gestation, investigators randomized 1207 pregnant women who reported daily intake of at least 3 cups of regular coffee to drink unlabeled instant coffee (which was either regular or decaffeinated) for the rest of their pregnancy. The women were allowed to request as much of their assigned instant coffee as they wanted.
Subjects were recruited from among all women with uncomplicated, singleton pregnancies who were expected to deliver at a Danish university hospital during the study period. Investigators interviewed the women at 20, 25, and 34 weeks to determine coffee consumption (including both coffee provided by the investigators and other coffee), consumption of other caffeinated beverages, and smoking status.
The difference in caffeine intake between the groups didn’t correspond to significant differences in birth weight (16 g lighter with caffeinated coffee; 95% confidence interval [CI], −40 g to 73 g; P=.48) or birth length (0.03 cm longer with caffeinated coffee; 95% CI, −0.29 to 0.22) among infants born to the 1150 women who completed the study.
Limitations of the study include randomizing women after 16 weeks’ gestation and the observation that many women correctly guessed which type of coffee they received (35% of women drinking caffeinated coffee and 49% of women drinking decaf).
A caffeine effect, but with study limitations
The Cochrane systematic review (described above) and a meta-analysis of 9 prospective cohort studies with a total of 90,000 patients that evaluated maternal caffeine intake found that it was associated with increased low birth weight, intrauterine growth restriction (IUGR), or small for gestational age (SGA) infants.3
Researchers assessed caffeine consumption from coffee or other sources either by questionnaire (5 studies) or interview (4 studies) at various times during pregnancy, mostly in the first or second trimester, and assigned subjects to 4 intake categories: none, low (50-149 mg/d), moderate (150-349 mg/d), and high (>350 mg/d).
Compared with no caffeine, all levels of caffeine intake were associated with increased rates of low birth weight, IUGR, or SGA (low intake: relative risk [RR]=1.13; 95% CI, 1.06-1.21; moderate intake: RR=1.38; 95% CI, 1.18-1.62; high intake: RR=1.60; 95% CI, 1.24-2.08).
A major limitation of the meta-analysis was that 8 of the included studies were identified by the reviewers as having quality problems. The reviewers also identified additional cohort studies, not included in the meta-analysis, which failed to show any association between caffeine intake and poor pregnancy outcomes.
Results of best-quality study prove clinically trivial
The best-quality prospective cohort study in the review described above was also the largest, comprising two-thirds of the total patients. It found a statistically significant, but clinically trivial, association between caffeine intake and birth weight.4
Investigators from Norway’s Institute of Public Health mailed surveys to 106,707 pregnant Norwegian women and recruited 59,123 with uncomplicated singleton pregnancies. The survey assessed diet and lifestyle at several stages of pregnancy and correlated caffeine intake with birth weight, gestational length, and SGA deliveries. Investigators calculated caffeine intake from coffee and other dietary sources (tea and chocolate).
Higher caffeine intake was associated with a small reduction in birth weight (8 g/100 mg/d of additional caffeine intake; 95% CI, −10 to −6 g/100 mg/d). Higher intake was also associated with increasing likelihood of SGA birth, a finding of borderline significance (odds ratio [OR]=1.18; 95% CI, 1.00-1.38, comparing intake <50 mg/d with 51-200 mg/d; OR=1.62; 95% CI, 1.26-2.29, comparing <50 mg/d with 201-300 mg/d; and OR=1.62; 95% CI, 1.15-2.29, comparing <50 mg/d with >300 mg/d).
No. Reducing caffeinated coffee consumption by 180 mg of caffeine (the equivalent of 2 cups) per day after 16 weeks’ gestation doesn’t affect birth weight. Consuming more than 300 mg of caffeine per day is associated with a clinically trivial, and statistically insignificant (less than 1 ounce), reduction in birth weight, compared with consuming no caffeine (strength of recommendation: B, randomized controlled trial [RCT] and large prospective cohort study).
EVIDENCE SUMMARY
A Cochrane systematic review of the effects of caffeine on pregnancy identified 2 studies, only one of which addressed the question of maternal caffeine intake and infant birth weight.1 The double-blind RCT evaluating caffeine intake during pregnancy found no significant differences in birth weight or length of gestation between women who drank regular coffee and women who drank decaffeinated coffee.2
At 16 weeks’ gestation, investigators randomized 1207 pregnant women who reported daily intake of at least 3 cups of regular coffee to drink unlabeled instant coffee (which was either regular or decaffeinated) for the rest of their pregnancy. The women were allowed to request as much of their assigned instant coffee as they wanted.
Subjects were recruited from among all women with uncomplicated, singleton pregnancies who were expected to deliver at a Danish university hospital during the study period. Investigators interviewed the women at 20, 25, and 34 weeks to determine coffee consumption (including both coffee provided by the investigators and other coffee), consumption of other caffeinated beverages, and smoking status.
The difference in caffeine intake between the groups didn’t correspond to significant differences in birth weight (16 g lighter with caffeinated coffee; 95% confidence interval [CI], −40 g to 73 g; P=.48) or birth length (0.03 cm longer with caffeinated coffee; 95% CI, −0.29 to 0.22) among infants born to the 1150 women who completed the study.
Limitations of the study include randomizing women after 16 weeks’ gestation and the observation that many women correctly guessed which type of coffee they received (35% of women drinking caffeinated coffee and 49% of women drinking decaf).
A caffeine effect, but with study limitations
The Cochrane systematic review (described above) and a meta-analysis of 9 prospective cohort studies with a total of 90,000 patients that evaluated maternal caffeine intake found that it was associated with increased low birth weight, intrauterine growth restriction (IUGR), or small for gestational age (SGA) infants.3
Researchers assessed caffeine consumption from coffee or other sources either by questionnaire (5 studies) or interview (4 studies) at various times during pregnancy, mostly in the first or second trimester, and assigned subjects to 4 intake categories: none, low (50-149 mg/d), moderate (150-349 mg/d), and high (>350 mg/d).
Compared with no caffeine, all levels of caffeine intake were associated with increased rates of low birth weight, IUGR, or SGA (low intake: relative risk [RR]=1.13; 95% CI, 1.06-1.21; moderate intake: RR=1.38; 95% CI, 1.18-1.62; high intake: RR=1.60; 95% CI, 1.24-2.08).
A major limitation of the meta-analysis was that 8 of the included studies were identified by the reviewers as having quality problems. The reviewers also identified additional cohort studies, not included in the meta-analysis, which failed to show any association between caffeine intake and poor pregnancy outcomes.
Results of best-quality study prove clinically trivial
The best-quality prospective cohort study in the review described above was also the largest, comprising two-thirds of the total patients. It found a statistically significant, but clinically trivial, association between caffeine intake and birth weight.4
Investigators from Norway’s Institute of Public Health mailed surveys to 106,707 pregnant Norwegian women and recruited 59,123 with uncomplicated singleton pregnancies. The survey assessed diet and lifestyle at several stages of pregnancy and correlated caffeine intake with birth weight, gestational length, and SGA deliveries. Investigators calculated caffeine intake from coffee and other dietary sources (tea and chocolate).
Higher caffeine intake was associated with a small reduction in birth weight (8 g/100 mg/d of additional caffeine intake; 95% CI, −10 to −6 g/100 mg/d). Higher intake was also associated with increasing likelihood of SGA birth, a finding of borderline significance (odds ratio [OR]=1.18; 95% CI, 1.00-1.38, comparing intake <50 mg/d with 51-200 mg/d; OR=1.62; 95% CI, 1.26-2.29, comparing <50 mg/d with 201-300 mg/d; and OR=1.62; 95% CI, 1.15-2.29, comparing <50 mg/d with >300 mg/d).
1. Jahanfar S, Jaafar SH. Effects of restricted caffeine intake by mother on fetal, neonatal and pregnancy outcome. Cochrane Database Syst Rev. 2013;(2):CD006965.
2. Bech BH, Obel C, Henriksen TB, et al. Effect of reducing caffeine intake on birth weight and length of gestation: randomised controlled trial. BMJ. 2007;334:409.
3. Chen LW, Wu Y, Neelakantan N, et al. Maternal caffeine intake during pregnancy is associated with risk of low birth weight: a systematic review and dose-response meta-analysis. BMC Medicine. 2014;12:174-176.
4. Sengpiel V, Elind E, Bacelis J, et al. Maternal caffeine intake during pregnancy is associated with birth weight but not with gestational length: results form a large prospective observational cohort trial. BMC Medicine. 2013;11:42.
1. Jahanfar S, Jaafar SH. Effects of restricted caffeine intake by mother on fetal, neonatal and pregnancy outcome. Cochrane Database Syst Rev. 2013;(2):CD006965.
2. Bech BH, Obel C, Henriksen TB, et al. Effect of reducing caffeine intake on birth weight and length of gestation: randomised controlled trial. BMJ. 2007;334:409.
3. Chen LW, Wu Y, Neelakantan N, et al. Maternal caffeine intake during pregnancy is associated with risk of low birth weight: a systematic review and dose-response meta-analysis. BMC Medicine. 2014;12:174-176.
4. Sengpiel V, Elind E, Bacelis J, et al. Maternal caffeine intake during pregnancy is associated with birth weight but not with gestational length: results form a large prospective observational cohort trial. BMC Medicine. 2013;11:42.
Evidence-based answers from the Family Physicians Inquiries Network
Is lower BP worth it in higher-risk patients with diabetes or coronary disease?
There is no simple answer; the risk/benefit picture is complicated. Controlling blood pressure to a target of 130/80 mm Hg or lower produces mixed results in patients with diabetes and coronary disease equivalents (chronic kidney disease [CKD], coronary artery disease, peripheral arterial disease, and previous stroke).
No evidence indicates that patients with diabetes or most patients with CKD have better outcomes if their blood pressure is controlled below 140/90 mm Hg. Patients with diabetes controlled to lower systolic blood pressure targets (below 120 mm Hg) have fewer strokes, but more serious adverse events. Achieving diastolic blood pressure targets below 80 mm Hg doesn’t reduce mortality, strokes, myocardial infarction, or congestive heart failure (strength of recommendation [SOR]: A, systematic review of randomized controlled trials [RCTs]).
Tight blood pressure control (approximately 130/80 mm Hg or lower) reduces the risk of kidney failure by 27% in CKD patients with proteinuria at baseline. In patients without proteinuria, it doesn’t add benefit over standard blood pressure control (140/90 mm Hg) for reducing kidney failure, mortality, or cardiovascular events (SOR: A, meta-analysis of RCTs).
Controlling hypertension to 130/80 mm Hg or lower in patients with coronary artery disease reduces heart failure (27%) and stroke (18%) but increases the incidence of hypotensive episodes (220%) when compared with standard 140/90 mm Hg target blood pressure. Lower target pressures don’t affect total or cardiovascular mortality, myocardial infarction, or angina, but do increase the need for revascularization in 6% of patients (SOR: A, meta-analysis of RCTs).
Controlling systolic blood pressure to a target of 120 mm Hg, compared with the standard target of 140 mm Hg, reduces a composite outcome (myocardial infarction, acute coronary syndrome, stroke, congestive heart failure, or cardiovascular death) by 25% and a secondary outcome of all-cause mortality by 27% in patients ages 50 and older with cardiovascular risk factors (but not diabetes or previous stroke).
However, intensive control doesn’t significantly improve the composite outcome in patients who are female, black, or younger than 75 years, or who have systolic blood pressures above 132 mm Hg, previous CKD, or previous cardiovascular disease. Intensive control causes more hypotension, syncope, and electrolyte abnormalities, but not falls resulting in injuries (SOR: B, large RCT).
No evidence-based studies exist to guide BP control in patients with peripheral artery disease or previous stroke. Current guidelines recommend treating hypertension to a target of 140/90 mm Hg in these patients.
EVIDENCE SUMMARY
A Cochrane systematic review of 5 RCTs with a total of 7314 patients evaluated cardiovascular outcomes after 4.7 years follow-up in patients with diabetes who were treated for hypertension to either “lower” or “standard” target blood pressures.1 One trial in the review (ACCORD, 4734 patients) compared outcomes from significantly lower and standard systolic blood pressures (119/64 mm Hg vs 134/71 mm Hg; P<.0001) in patients with diabetes and either cardiovascular disease or 2 risk factors for cardiovascular disease. The authors evaluated outcomes based on achieved systolic blood pressures rather than intention to treat.
They found a reduced incidence of stroke (risk ratio [RR]=0.58; 95% confidence interval [CI], 0.39-0.88; P=.009; number needed to treat [NNT]=91) but no change in mortality (RR=1.05; 95% CI, 0.84-1.30) at lower blood pressures. Achieving the lower systolic blood pressure increased the number of serious adverse effects, however (RR=2.58; 95% CI, 1.70-3.91; P<.0001; absolute risk increase=2%; number needed to harm=50).
Four RCTs (2580 patients) in the systematic review compared clinical outcomes produced by achieving significantly lower or standard diastolic blood pressure targets (128/76 mm Hg vs 135/83 mm Hg; P<.0001). The trials found no significant difference in total mortality (RR=0.73; 95% CI, 0.53-1.01), stroke (RR=0.67; 95% CI, 0.42-1.05), myocardial infarction (RR=0.95; 95% CI, 0.64-1.40), or congestive heart failure (RR=1.06; 95% CI, 0.58-1.92). Sensitivity analysis of trials comparing diastolic blood pressure targets below 80 mm Hg and below 90 mm Hg showed similar results.
The 4 RCTs didn’t report end-stage renal failure or total serious adverse events. The authors stated that there was a high risk of selection bias in favor of lower blood pressure targets.
Patients with CKD
A systematic review and meta-analysis of 11 RCTs (9287 patients) compared outcomes of achieving lower blood pressure targets or standard targets in patients with CKD. Intensive blood pressure treatment reduced the risk of kidney failure only in patients with proteinuria at baseline (hazard ratio [HR]=0.73; 95% CI, 0.62-0.86; 5 trials, 1703 patients).2 Investigators didn’t report the degree of proteinuria for all the trials, but in one trial, patients had proteinuria of 1 to 3 g/d.
Achieved blood pressures in the intensive therapy group averaged 7.7/4.9 mm Hg lower, with pressures typically ranging from 75 to 80 mm Hg diastolic and 125 to 135 mm Hg systolic. Intensive blood pressure lowering didn’t reduce kidney failure in patients without baseline proteinuria (HR=1.12; 95% CI, 0.67-1.87; 3 trials, 1218 patients). Nor did it reduce death (RR=0.94; 95% CI, 0.84-1.05; 10 trials, 6788 patients) or major cardiovascular outcomes (RR=1.09; 95% CI, 0.83-1.42; 5 trials, 5308 patients).
Patients with coronary artery disease
A meta-analysis of 15 RCTs (66,504 patients) that evaluated tight control of hypertension (≤130/80 mm Hg) compared with standard control (<140/90 mm Hg) in patients with coronary artery disease found reduced rates of heart failure (RR=0.73; 95% CI, 0.64-0.84; 10 trials, 37,990 patients) and stroke (RR=0.82; 95% CI, 0.69-0.98; 9 trials, 8344 patients) but increased rates of hypotension (RR=2.19; 95% CI, 1.80-2.66; 6 trials, 17,836 patients).3
Achieving lower blood pressure targets didn’t reduce all-cause mortality (RR=0.96; 95% CI, 0.89-1.04; 13 trials, 39,262 patients), cardiovascular mortality (RR=0.96; 95% CI, 0.86-1.07; 11 trials, 38,452 patients), myocardial infarction (RR=0.92; 95% CI, 0.85-1.00; 14 trials, 39,696 patients), or angina (RR=0.92; 95% CI, 0.84-1.0; 11 trials, 28,007 patients). But it slightly increased the need for revascularization (RR=1.06; 95% CI, 1.01-1.12; 11 trials, 38,450 patients).
The SPRINT trial: Promising results for intensive treatment of some patients
The Systolic Blood Pressure Intervention Trial (SPRINT), a large RCT, found that targeting systolic blood pressures below 120 mm Hg (compared with a target below 140 mm Hg) in middle-aged and older patients with increased cardiovascular risk reduced a composite outcome that included cardiovascular death by 25%.4
Researchers recruited 9361 patients older than 50 years (mean age 68 years; >28% older than 75 years) with systolic blood pressure between 130 and 180 mm Hg and increased cardiovascular risk defined by one or more of the following: preexisting cardiovascular disease, CKD with estimated glomerular filtration rate between 20 and 60 mL/min/1.73 m2, age >75 years, and Framingham 10-year risk of 15% or more. They excluded patients with diabetes or previous stroke.
Patients were randomized to intensive treatment (target systolic BP <120; mean achieved 121.4) or standard treatment (target systolic BP <140; mean achieved 136.2). Treatment typically comprised 3 (intensive) or 2 (standard) agents. The primary outcome was a composite of myocardial infarction, acute coronary syndrome, stroke, congestive heart failure, or cardiovascular death.
The study, which was originally intended to run for 5 years, was stopped at 3.26 years based on positive results. Intensive treatment improved the primary composite outcome overall (1.65% vs 2.19%; HR=0.75; 95% CI, 0.64-0.89; P<.001; NNT=61 over 3.26 years), all-cause mortality (HR=0.73; 95% CI, 0.60-0.90; P=.003; NNT=90), and cardiovascular death (HR=0.57; 95% CI, 0.38-0.85; P=.005; NNT=172).
However, intensive treatment didn’t significantly improve the primary composite outcome in these subgroups:
- female patients (HR=0.84; 95% CI, 0.62-1.14)
- black patients (HR=0.77; 95% CI, 0.55-1.06)
- patients with preexisting CKD (HR=0.82; 95% CI, 0.63-1.07) or cardiovascular disease (HR=0.83; 95% CI, 0.62-1.09)
- patients younger than 75 years (HR=0.80; 95% CI, 0.64-1.00)
- patients with systolic blood pressures higher than 132 mm Hg (BP >132 to <145 mm Hg, HR=0.77; 95% CI, 0.57-1.03; BP ≥145 mm Hg, HR=0.83; 95% CI, 0.63-1.09).
Intensive treatment also produced more net serious adverse events (HR=1.88; 4.7% vs 2.5%; P<.001), including: ≥30% decrease of glomerular filtration rates to values below 60 mL/min/1.73 m2 (HR=3.49; 95% CI, 2.44-5.10; P<.001), syncope (HR=1.44; 3.5% vs 2.4%; P=.003), hypotension (HR=1.70; 3.4% vs 2.0%; P<.001), and electrolyte abnormalities (HR=1.38; 3.8% vs 2.8%; P=.006). It didn’t cause injurious falls (HR=1.00; P=.97) or orthostatic hypotension in clinic (HR=0.88; 16.6% vs 18.3%; P=.01).
Guidelines for patients with peripheral artery disease, previous stroke
A national guideline by an expert panel recommended treating patients with hypertension who have peripheral artery disease or previous stroke to standard values for the general population: <140/90 mm Hg if ages 60 years or younger, <150/90 mm Hg if older than 60 years.5
1. Arguedas JA, Leiva V, Wright JM. Blood pressure targets for hypertension in people with diabetes mellitus. Cochrane Database Syst Rev. 2013;(10):CD008277.
2. Lv J, Ehteshami P, Sarnak M, et al. Effects of intensive blood pressure lowering on the progression of chronic kidney disease: a systematic review and meta-analysis. CMAJ. 2013;185:949-957.
3. Bangalore S, Kumar S, Volodarskiy A, et al. Blood pressure targets in patients with coronary artery disease: observations from traditional and Bayesian random effects meta-analysis of randomised trials. Heart. 2013;99:601-613.
4. SPRINT Research Group, Wright JT, Williamson JD, Whelton PK, et al. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med. 2015;373:2103-2116.
5. James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the eighth Joint National Committee (JNC 8). JAMA. 2014;311:507-520.
There is no simple answer; the risk/benefit picture is complicated. Controlling blood pressure to a target of 130/80 mm Hg or lower produces mixed results in patients with diabetes and coronary disease equivalents (chronic kidney disease [CKD], coronary artery disease, peripheral arterial disease, and previous stroke).
No evidence indicates that patients with diabetes or most patients with CKD have better outcomes if their blood pressure is controlled below 140/90 mm Hg. Patients with diabetes controlled to lower systolic blood pressure targets (below 120 mm Hg) have fewer strokes, but more serious adverse events. Achieving diastolic blood pressure targets below 80 mm Hg doesn’t reduce mortality, strokes, myocardial infarction, or congestive heart failure (strength of recommendation [SOR]: A, systematic review of randomized controlled trials [RCTs]).
Tight blood pressure control (approximately 130/80 mm Hg or lower) reduces the risk of kidney failure by 27% in CKD patients with proteinuria at baseline. In patients without proteinuria, it doesn’t add benefit over standard blood pressure control (140/90 mm Hg) for reducing kidney failure, mortality, or cardiovascular events (SOR: A, meta-analysis of RCTs).
Controlling hypertension to 130/80 mm Hg or lower in patients with coronary artery disease reduces heart failure (27%) and stroke (18%) but increases the incidence of hypotensive episodes (220%) when compared with standard 140/90 mm Hg target blood pressure. Lower target pressures don’t affect total or cardiovascular mortality, myocardial infarction, or angina, but do increase the need for revascularization in 6% of patients (SOR: A, meta-analysis of RCTs).
Controlling systolic blood pressure to a target of 120 mm Hg, compared with the standard target of 140 mm Hg, reduces a composite outcome (myocardial infarction, acute coronary syndrome, stroke, congestive heart failure, or cardiovascular death) by 25% and a secondary outcome of all-cause mortality by 27% in patients ages 50 and older with cardiovascular risk factors (but not diabetes or previous stroke).
However, intensive control doesn’t significantly improve the composite outcome in patients who are female, black, or younger than 75 years, or who have systolic blood pressures above 132 mm Hg, previous CKD, or previous cardiovascular disease. Intensive control causes more hypotension, syncope, and electrolyte abnormalities, but not falls resulting in injuries (SOR: B, large RCT).
No evidence-based studies exist to guide BP control in patients with peripheral artery disease or previous stroke. Current guidelines recommend treating hypertension to a target of 140/90 mm Hg in these patients.
EVIDENCE SUMMARY
A Cochrane systematic review of 5 RCTs with a total of 7314 patients evaluated cardiovascular outcomes after 4.7 years follow-up in patients with diabetes who were treated for hypertension to either “lower” or “standard” target blood pressures.1 One trial in the review (ACCORD, 4734 patients) compared outcomes from significantly lower and standard systolic blood pressures (119/64 mm Hg vs 134/71 mm Hg; P<.0001) in patients with diabetes and either cardiovascular disease or 2 risk factors for cardiovascular disease. The authors evaluated outcomes based on achieved systolic blood pressures rather than intention to treat.
They found a reduced incidence of stroke (risk ratio [RR]=0.58; 95% confidence interval [CI], 0.39-0.88; P=.009; number needed to treat [NNT]=91) but no change in mortality (RR=1.05; 95% CI, 0.84-1.30) at lower blood pressures. Achieving the lower systolic blood pressure increased the number of serious adverse effects, however (RR=2.58; 95% CI, 1.70-3.91; P<.0001; absolute risk increase=2%; number needed to harm=50).
Four RCTs (2580 patients) in the systematic review compared clinical outcomes produced by achieving significantly lower or standard diastolic blood pressure targets (128/76 mm Hg vs 135/83 mm Hg; P<.0001). The trials found no significant difference in total mortality (RR=0.73; 95% CI, 0.53-1.01), stroke (RR=0.67; 95% CI, 0.42-1.05), myocardial infarction (RR=0.95; 95% CI, 0.64-1.40), or congestive heart failure (RR=1.06; 95% CI, 0.58-1.92). Sensitivity analysis of trials comparing diastolic blood pressure targets below 80 mm Hg and below 90 mm Hg showed similar results.
The 4 RCTs didn’t report end-stage renal failure or total serious adverse events. The authors stated that there was a high risk of selection bias in favor of lower blood pressure targets.
Patients with CKD
A systematic review and meta-analysis of 11 RCTs (9287 patients) compared outcomes of achieving lower blood pressure targets or standard targets in patients with CKD. Intensive blood pressure treatment reduced the risk of kidney failure only in patients with proteinuria at baseline (hazard ratio [HR]=0.73; 95% CI, 0.62-0.86; 5 trials, 1703 patients).2 Investigators didn’t report the degree of proteinuria for all the trials, but in one trial, patients had proteinuria of 1 to 3 g/d.
Achieved blood pressures in the intensive therapy group averaged 7.7/4.9 mm Hg lower, with pressures typically ranging from 75 to 80 mm Hg diastolic and 125 to 135 mm Hg systolic. Intensive blood pressure lowering didn’t reduce kidney failure in patients without baseline proteinuria (HR=1.12; 95% CI, 0.67-1.87; 3 trials, 1218 patients). Nor did it reduce death (RR=0.94; 95% CI, 0.84-1.05; 10 trials, 6788 patients) or major cardiovascular outcomes (RR=1.09; 95% CI, 0.83-1.42; 5 trials, 5308 patients).
Patients with coronary artery disease
A meta-analysis of 15 RCTs (66,504 patients) that evaluated tight control of hypertension (≤130/80 mm Hg) compared with standard control (<140/90 mm Hg) in patients with coronary artery disease found reduced rates of heart failure (RR=0.73; 95% CI, 0.64-0.84; 10 trials, 37,990 patients) and stroke (RR=0.82; 95% CI, 0.69-0.98; 9 trials, 8344 patients) but increased rates of hypotension (RR=2.19; 95% CI, 1.80-2.66; 6 trials, 17,836 patients).3
Achieving lower blood pressure targets didn’t reduce all-cause mortality (RR=0.96; 95% CI, 0.89-1.04; 13 trials, 39,262 patients), cardiovascular mortality (RR=0.96; 95% CI, 0.86-1.07; 11 trials, 38,452 patients), myocardial infarction (RR=0.92; 95% CI, 0.85-1.00; 14 trials, 39,696 patients), or angina (RR=0.92; 95% CI, 0.84-1.0; 11 trials, 28,007 patients). But it slightly increased the need for revascularization (RR=1.06; 95% CI, 1.01-1.12; 11 trials, 38,450 patients).
The SPRINT trial: Promising results for intensive treatment of some patients
The Systolic Blood Pressure Intervention Trial (SPRINT), a large RCT, found that targeting systolic blood pressures below 120 mm Hg (compared with a target below 140 mm Hg) in middle-aged and older patients with increased cardiovascular risk reduced a composite outcome that included cardiovascular death by 25%.4
Researchers recruited 9361 patients older than 50 years (mean age 68 years; >28% older than 75 years) with systolic blood pressure between 130 and 180 mm Hg and increased cardiovascular risk defined by one or more of the following: preexisting cardiovascular disease, CKD with estimated glomerular filtration rate between 20 and 60 mL/min/1.73 m2, age >75 years, and Framingham 10-year risk of 15% or more. They excluded patients with diabetes or previous stroke.
Patients were randomized to intensive treatment (target systolic BP <120; mean achieved 121.4) or standard treatment (target systolic BP <140; mean achieved 136.2). Treatment typically comprised 3 (intensive) or 2 (standard) agents. The primary outcome was a composite of myocardial infarction, acute coronary syndrome, stroke, congestive heart failure, or cardiovascular death.
The study, which was originally intended to run for 5 years, was stopped at 3.26 years based on positive results. Intensive treatment improved the primary composite outcome overall (1.65% vs 2.19%; HR=0.75; 95% CI, 0.64-0.89; P<.001; NNT=61 over 3.26 years), all-cause mortality (HR=0.73; 95% CI, 0.60-0.90; P=.003; NNT=90), and cardiovascular death (HR=0.57; 95% CI, 0.38-0.85; P=.005; NNT=172).
However, intensive treatment didn’t significantly improve the primary composite outcome in these subgroups:
- female patients (HR=0.84; 95% CI, 0.62-1.14)
- black patients (HR=0.77; 95% CI, 0.55-1.06)
- patients with preexisting CKD (HR=0.82; 95% CI, 0.63-1.07) or cardiovascular disease (HR=0.83; 95% CI, 0.62-1.09)
- patients younger than 75 years (HR=0.80; 95% CI, 0.64-1.00)
- patients with systolic blood pressures higher than 132 mm Hg (BP >132 to <145 mm Hg, HR=0.77; 95% CI, 0.57-1.03; BP ≥145 mm Hg, HR=0.83; 95% CI, 0.63-1.09).
Intensive treatment also produced more net serious adverse events (HR=1.88; 4.7% vs 2.5%; P<.001), including: ≥30% decrease of glomerular filtration rates to values below 60 mL/min/1.73 m2 (HR=3.49; 95% CI, 2.44-5.10; P<.001), syncope (HR=1.44; 3.5% vs 2.4%; P=.003), hypotension (HR=1.70; 3.4% vs 2.0%; P<.001), and electrolyte abnormalities (HR=1.38; 3.8% vs 2.8%; P=.006). It didn’t cause injurious falls (HR=1.00; P=.97) or orthostatic hypotension in clinic (HR=0.88; 16.6% vs 18.3%; P=.01).
Guidelines for patients with peripheral artery disease, previous stroke
A national guideline by an expert panel recommended treating patients with hypertension who have peripheral artery disease or previous stroke to standard values for the general population: <140/90 mm Hg if ages 60 years or younger, <150/90 mm Hg if older than 60 years.5
There is no simple answer; the risk/benefit picture is complicated. Controlling blood pressure to a target of 130/80 mm Hg or lower produces mixed results in patients with diabetes and coronary disease equivalents (chronic kidney disease [CKD], coronary artery disease, peripheral arterial disease, and previous stroke).
No evidence indicates that patients with diabetes or most patients with CKD have better outcomes if their blood pressure is controlled below 140/90 mm Hg. Patients with diabetes controlled to lower systolic blood pressure targets (below 120 mm Hg) have fewer strokes, but more serious adverse events. Achieving diastolic blood pressure targets below 80 mm Hg doesn’t reduce mortality, strokes, myocardial infarction, or congestive heart failure (strength of recommendation [SOR]: A, systematic review of randomized controlled trials [RCTs]).
Tight blood pressure control (approximately 130/80 mm Hg or lower) reduces the risk of kidney failure by 27% in CKD patients with proteinuria at baseline. In patients without proteinuria, it doesn’t add benefit over standard blood pressure control (140/90 mm Hg) for reducing kidney failure, mortality, or cardiovascular events (SOR: A, meta-analysis of RCTs).
Controlling hypertension to 130/80 mm Hg or lower in patients with coronary artery disease reduces heart failure (27%) and stroke (18%) but increases the incidence of hypotensive episodes (220%) when compared with standard 140/90 mm Hg target blood pressure. Lower target pressures don’t affect total or cardiovascular mortality, myocardial infarction, or angina, but do increase the need for revascularization in 6% of patients (SOR: A, meta-analysis of RCTs).
Controlling systolic blood pressure to a target of 120 mm Hg, compared with the standard target of 140 mm Hg, reduces a composite outcome (myocardial infarction, acute coronary syndrome, stroke, congestive heart failure, or cardiovascular death) by 25% and a secondary outcome of all-cause mortality by 27% in patients ages 50 and older with cardiovascular risk factors (but not diabetes or previous stroke).
However, intensive control doesn’t significantly improve the composite outcome in patients who are female, black, or younger than 75 years, or who have systolic blood pressures above 132 mm Hg, previous CKD, or previous cardiovascular disease. Intensive control causes more hypotension, syncope, and electrolyte abnormalities, but not falls resulting in injuries (SOR: B, large RCT).
No evidence-based studies exist to guide BP control in patients with peripheral artery disease or previous stroke. Current guidelines recommend treating hypertension to a target of 140/90 mm Hg in these patients.
EVIDENCE SUMMARY
A Cochrane systematic review of 5 RCTs with a total of 7314 patients evaluated cardiovascular outcomes after 4.7 years follow-up in patients with diabetes who were treated for hypertension to either “lower” or “standard” target blood pressures.1 One trial in the review (ACCORD, 4734 patients) compared outcomes from significantly lower and standard systolic blood pressures (119/64 mm Hg vs 134/71 mm Hg; P<.0001) in patients with diabetes and either cardiovascular disease or 2 risk factors for cardiovascular disease. The authors evaluated outcomes based on achieved systolic blood pressures rather than intention to treat.
They found a reduced incidence of stroke (risk ratio [RR]=0.58; 95% confidence interval [CI], 0.39-0.88; P=.009; number needed to treat [NNT]=91) but no change in mortality (RR=1.05; 95% CI, 0.84-1.30) at lower blood pressures. Achieving the lower systolic blood pressure increased the number of serious adverse effects, however (RR=2.58; 95% CI, 1.70-3.91; P<.0001; absolute risk increase=2%; number needed to harm=50).
Four RCTs (2580 patients) in the systematic review compared clinical outcomes produced by achieving significantly lower or standard diastolic blood pressure targets (128/76 mm Hg vs 135/83 mm Hg; P<.0001). The trials found no significant difference in total mortality (RR=0.73; 95% CI, 0.53-1.01), stroke (RR=0.67; 95% CI, 0.42-1.05), myocardial infarction (RR=0.95; 95% CI, 0.64-1.40), or congestive heart failure (RR=1.06; 95% CI, 0.58-1.92). Sensitivity analysis of trials comparing diastolic blood pressure targets below 80 mm Hg and below 90 mm Hg showed similar results.
The 4 RCTs didn’t report end-stage renal failure or total serious adverse events. The authors stated that there was a high risk of selection bias in favor of lower blood pressure targets.
Patients with CKD
A systematic review and meta-analysis of 11 RCTs (9287 patients) compared outcomes of achieving lower blood pressure targets or standard targets in patients with CKD. Intensive blood pressure treatment reduced the risk of kidney failure only in patients with proteinuria at baseline (hazard ratio [HR]=0.73; 95% CI, 0.62-0.86; 5 trials, 1703 patients).2 Investigators didn’t report the degree of proteinuria for all the trials, but in one trial, patients had proteinuria of 1 to 3 g/d.
Achieved blood pressures in the intensive therapy group averaged 7.7/4.9 mm Hg lower, with pressures typically ranging from 75 to 80 mm Hg diastolic and 125 to 135 mm Hg systolic. Intensive blood pressure lowering didn’t reduce kidney failure in patients without baseline proteinuria (HR=1.12; 95% CI, 0.67-1.87; 3 trials, 1218 patients). Nor did it reduce death (RR=0.94; 95% CI, 0.84-1.05; 10 trials, 6788 patients) or major cardiovascular outcomes (RR=1.09; 95% CI, 0.83-1.42; 5 trials, 5308 patients).
Patients with coronary artery disease
A meta-analysis of 15 RCTs (66,504 patients) that evaluated tight control of hypertension (≤130/80 mm Hg) compared with standard control (<140/90 mm Hg) in patients with coronary artery disease found reduced rates of heart failure (RR=0.73; 95% CI, 0.64-0.84; 10 trials, 37,990 patients) and stroke (RR=0.82; 95% CI, 0.69-0.98; 9 trials, 8344 patients) but increased rates of hypotension (RR=2.19; 95% CI, 1.80-2.66; 6 trials, 17,836 patients).3
Achieving lower blood pressure targets didn’t reduce all-cause mortality (RR=0.96; 95% CI, 0.89-1.04; 13 trials, 39,262 patients), cardiovascular mortality (RR=0.96; 95% CI, 0.86-1.07; 11 trials, 38,452 patients), myocardial infarction (RR=0.92; 95% CI, 0.85-1.00; 14 trials, 39,696 patients), or angina (RR=0.92; 95% CI, 0.84-1.0; 11 trials, 28,007 patients). But it slightly increased the need for revascularization (RR=1.06; 95% CI, 1.01-1.12; 11 trials, 38,450 patients).
The SPRINT trial: Promising results for intensive treatment of some patients
The Systolic Blood Pressure Intervention Trial (SPRINT), a large RCT, found that targeting systolic blood pressures below 120 mm Hg (compared with a target below 140 mm Hg) in middle-aged and older patients with increased cardiovascular risk reduced a composite outcome that included cardiovascular death by 25%.4
Researchers recruited 9361 patients older than 50 years (mean age 68 years; >28% older than 75 years) with systolic blood pressure between 130 and 180 mm Hg and increased cardiovascular risk defined by one or more of the following: preexisting cardiovascular disease, CKD with estimated glomerular filtration rate between 20 and 60 mL/min/1.73 m2, age >75 years, and Framingham 10-year risk of 15% or more. They excluded patients with diabetes or previous stroke.
Patients were randomized to intensive treatment (target systolic BP <120; mean achieved 121.4) or standard treatment (target systolic BP <140; mean achieved 136.2). Treatment typically comprised 3 (intensive) or 2 (standard) agents. The primary outcome was a composite of myocardial infarction, acute coronary syndrome, stroke, congestive heart failure, or cardiovascular death.
The study, which was originally intended to run for 5 years, was stopped at 3.26 years based on positive results. Intensive treatment improved the primary composite outcome overall (1.65% vs 2.19%; HR=0.75; 95% CI, 0.64-0.89; P<.001; NNT=61 over 3.26 years), all-cause mortality (HR=0.73; 95% CI, 0.60-0.90; P=.003; NNT=90), and cardiovascular death (HR=0.57; 95% CI, 0.38-0.85; P=.005; NNT=172).
However, intensive treatment didn’t significantly improve the primary composite outcome in these subgroups:
- female patients (HR=0.84; 95% CI, 0.62-1.14)
- black patients (HR=0.77; 95% CI, 0.55-1.06)
- patients with preexisting CKD (HR=0.82; 95% CI, 0.63-1.07) or cardiovascular disease (HR=0.83; 95% CI, 0.62-1.09)
- patients younger than 75 years (HR=0.80; 95% CI, 0.64-1.00)
- patients with systolic blood pressures higher than 132 mm Hg (BP >132 to <145 mm Hg, HR=0.77; 95% CI, 0.57-1.03; BP ≥145 mm Hg, HR=0.83; 95% CI, 0.63-1.09).
Intensive treatment also produced more net serious adverse events (HR=1.88; 4.7% vs 2.5%; P<.001), including: ≥30% decrease of glomerular filtration rates to values below 60 mL/min/1.73 m2 (HR=3.49; 95% CI, 2.44-5.10; P<.001), syncope (HR=1.44; 3.5% vs 2.4%; P=.003), hypotension (HR=1.70; 3.4% vs 2.0%; P<.001), and electrolyte abnormalities (HR=1.38; 3.8% vs 2.8%; P=.006). It didn’t cause injurious falls (HR=1.00; P=.97) or orthostatic hypotension in clinic (HR=0.88; 16.6% vs 18.3%; P=.01).
Guidelines for patients with peripheral artery disease, previous stroke
A national guideline by an expert panel recommended treating patients with hypertension who have peripheral artery disease or previous stroke to standard values for the general population: <140/90 mm Hg if ages 60 years or younger, <150/90 mm Hg if older than 60 years.5
1. Arguedas JA, Leiva V, Wright JM. Blood pressure targets for hypertension in people with diabetes mellitus. Cochrane Database Syst Rev. 2013;(10):CD008277.
2. Lv J, Ehteshami P, Sarnak M, et al. Effects of intensive blood pressure lowering on the progression of chronic kidney disease: a systematic review and meta-analysis. CMAJ. 2013;185:949-957.
3. Bangalore S, Kumar S, Volodarskiy A, et al. Blood pressure targets in patients with coronary artery disease: observations from traditional and Bayesian random effects meta-analysis of randomised trials. Heart. 2013;99:601-613.
4. SPRINT Research Group, Wright JT, Williamson JD, Whelton PK, et al. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med. 2015;373:2103-2116.
5. James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the eighth Joint National Committee (JNC 8). JAMA. 2014;311:507-520.
1. Arguedas JA, Leiva V, Wright JM. Blood pressure targets for hypertension in people with diabetes mellitus. Cochrane Database Syst Rev. 2013;(10):CD008277.
2. Lv J, Ehteshami P, Sarnak M, et al. Effects of intensive blood pressure lowering on the progression of chronic kidney disease: a systematic review and meta-analysis. CMAJ. 2013;185:949-957.
3. Bangalore S, Kumar S, Volodarskiy A, et al. Blood pressure targets in patients with coronary artery disease: observations from traditional and Bayesian random effects meta-analysis of randomised trials. Heart. 2013;99:601-613.
4. SPRINT Research Group, Wright JT, Williamson JD, Whelton PK, et al. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med. 2015;373:2103-2116.
5. James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the eighth Joint National Committee (JNC 8). JAMA. 2014;311:507-520.
Evidence-based answers from the Family Physicians Inquiries Network
Do corticosteroid injections improve carpal tunnel syndrome symptoms?
Yes. Injected corticosteroids reduce symptoms of carpal tunnel syndrome (CTS) more effectively than placebo or systemic steroids, but no better than anti-inflammatory medication and splinting, from one to 12 weeks after therapy (strength of recommendation [SOR]: A, meta-analysis of randomized controlled trials [RCTs] and consistent RCT).
A 40-mg injection of methylprednisolone reduces symptoms as effectively as an 80-mg injection for as long as 10 weeks, but the 80-mg dose reduces progression to surgery at one year (SOR: B, RCT). Long-term effects of injections decrease by 12 months (SOR: B, RCT).
After corticosteroid injections, 14% of patients proceed to surgery at one year, and 33% proceed to surgery at 5 years (SOR: B, cohort trial).
EVIDENCE SUMMARY
A 2007 Cochrane review of 12 RCTs with 671 patients compared the efficacy of corticosteroid injections for CTS with placebo injections or other nonsurgical interventions.1 Patients who received corticosteroid injections showed clinical improvement at one month or less compared with placebo (2 trials, 141 patients; 73% corticosteroids vs 28% placebo; relative risk [RR]=2.58; 95% confidence interval [CI], 1.72-3.87; number needed to treat [NNT]=2).
Compared with systemic corticosteroids, corticosteroid injections didn’t improve symptoms on a Global Symptom Score (scale of 0-50, with 50 indicating the most severe symptoms) at 2 weeks (one trial, 60 patients; mean difference [MD]= −4.2; 95% CI, −8.7 to 0.26), but did improve symptoms at 8 weeks (MD= −7.16; 95% CI, −11.5 to −2.86) and 12 weeks (MD= −7.1; 95% CI, −11.7 to −2.52).
Patients showed no difference in scores between corticosteroid injection and oral anti-inflammatory medication with neutral angle wrist splints on the Symptom Severity Scale (1 to 5, with 5 indicating the most severe symptoms) at 2 weeks (1 trial, 23 patients [37 wrists]; MD=0.0; 95% CI, −0.64 to 0.64) or 8 weeks (MD=0.1; 95% CI, −0.33 to 0.53).
Higher corticosteroid dose reduces surgery at one year
A 2013 high-quality RCT with 111 patients assessed pain relief and rates of surgery at one year with local corticosteroid injections for CTS.2 This trial had 3 arms with 37 patients in each: 80-mg methylprednisolone injection, 40-mg methylprednisolone injection, or placebo injection.
Both corticosteroid groups showed greater improvement on the Symptom Severity Scale at 10 weeks compared with placebo (40-mg methylprednisolone group: MD= −0.88; 95% CI, −1.3 to −0.46; 80-mg methylprednisolone group: MD= −0.64; 95% CI, −1.06 to −0.21). There was no difference between the methylprednisolone groups.
The incidence of surgery at one year was lower in the 80-mg methylprednisolone group compared with placebo (73% vs 92%; RR=0.79; 95% CI, 0.64-0.99; NNT=5) but not in the 40-mg methylprednisolone group compared with placebo (81% vs 92%; RR=0.88; 95% CI, 0.73-1.06).
Corticosteroids improve symptoms and disability, but effects wear off
A randomized double-blind, placebo-controlled trial conducted in 2010 examined the effectiveness of corticosteroid injections given by general practitioners to 69 patients with CTS.3 Patients were randomized to receive 10 mg of either triamcinolone or saline. They were reassessed after one week, and patients in the saline injection group who had inadequate symptom relief received a triamcinolone injection as bail-out treatment. Follow-up by patient questionnaire was done at 1, 3, 6, and 12 months.
Investigators assessed symptoms and disability using the Symptom Severity Scale and Functional Disability Scale, which are part of the Boston Carpal Tunnel Questionnaire. Like the Symptom Severity Scale, the Functional Disability Scale is scored from 1 to 5, with higher scores indicating more severe disability.
One week after treatment, the corticosteroid group showed greater improvement in symptom severity and functional disability than the saline group (symptom severity decreased from 2.9 to 1.9 with triamcinolone vs 2.8 to 2.5 with saline; MD=0.64; 95% CI, 0.32-0.96; functional disability decreased from 2.5 to 1.9 with triamcinolone but remained at 2.4 with saline; MD=0.59; 95% CI, 0.23-0.94).
Long-term follow-up of 35 patients who responded to corticosteroid injections found that the effects wore off over 12 months when assessed using the Symptom Severity Scale (mean score 1.5 at 1 month, 2.0 at 12 months; P=.08).
Surgery rates at one and 5 years
A 2012 prospective cohort study examined the 5-year rate of surgical intervention after a 20-mg methylprednisolone injection in 824 patients diagnosed with CTS who had failed conservative treatment.4 A total of 500 patients had a relapse of symptoms, and 372 of them elected to have a second injection. A Kaplan-Meier survivorship analysis determined rates of surgical intervention to be 14.5% (95% CI, 11.9-17) at one year and 33.2% (95% CI, 28.7-37.8) at 5 years.
RECOMMENDATION
A 2010 American Academy of Orthopaedic Surgeons evidence-based practice guideline on the treatment of CTS recommends corticosteroid injection before considering surgery (Grade B, Level 1 suggested recommendation with good evidence).5
1. Marshall S, Tardif G, Ashworth N. Local corticosteroid injection for carpal tunnel syndrome. Cochrane Database Syst Rev. 2007;(2):CD001554.
2. Atroshi I, Flondell M, Hofer M, et al. Methylprednisolone injections for the carpal tunnel syndrome: a randomized, placebo-controlled trial. Ann Intern Med. 2013;159:309-317.
3. Peters-Veluthamaningal C, Winters JC, Gronier KH, et al. Randomised controlled trial of local corticosteroid injections for carpal tunnel syndrome in general practice. BMC Fam Pract. 2010;11:54.
4. Jenkins PJ, Duckworth AD, Watts AC, et al. Corticosteroid injection for carpal tunnel syndrome: a 5-year survivorship analysis. Hand. 2012;7:151-156.
5. Keith MW, Masear V, Chung KC, et al. American Academy of Orthopaedic Surgeons clinical practice guideline on the treatment of carpal tunnel syndrome. J Bone Joint Surg Am. 2010;92:218-219.
Yes. Injected corticosteroids reduce symptoms of carpal tunnel syndrome (CTS) more effectively than placebo or systemic steroids, but no better than anti-inflammatory medication and splinting, from one to 12 weeks after therapy (strength of recommendation [SOR]: A, meta-analysis of randomized controlled trials [RCTs] and consistent RCT).
A 40-mg injection of methylprednisolone reduces symptoms as effectively as an 80-mg injection for as long as 10 weeks, but the 80-mg dose reduces progression to surgery at one year (SOR: B, RCT). Long-term effects of injections decrease by 12 months (SOR: B, RCT).
After corticosteroid injections, 14% of patients proceed to surgery at one year, and 33% proceed to surgery at 5 years (SOR: B, cohort trial).
EVIDENCE SUMMARY
A 2007 Cochrane review of 12 RCTs with 671 patients compared the efficacy of corticosteroid injections for CTS with placebo injections or other nonsurgical interventions.1 Patients who received corticosteroid injections showed clinical improvement at one month or less compared with placebo (2 trials, 141 patients; 73% corticosteroids vs 28% placebo; relative risk [RR]=2.58; 95% confidence interval [CI], 1.72-3.87; number needed to treat [NNT]=2).
Compared with systemic corticosteroids, corticosteroid injections didn’t improve symptoms on a Global Symptom Score (scale of 0-50, with 50 indicating the most severe symptoms) at 2 weeks (one trial, 60 patients; mean difference [MD]= −4.2; 95% CI, −8.7 to 0.26), but did improve symptoms at 8 weeks (MD= −7.16; 95% CI, −11.5 to −2.86) and 12 weeks (MD= −7.1; 95% CI, −11.7 to −2.52).
Patients showed no difference in scores between corticosteroid injection and oral anti-inflammatory medication with neutral angle wrist splints on the Symptom Severity Scale (1 to 5, with 5 indicating the most severe symptoms) at 2 weeks (1 trial, 23 patients [37 wrists]; MD=0.0; 95% CI, −0.64 to 0.64) or 8 weeks (MD=0.1; 95% CI, −0.33 to 0.53).
Higher corticosteroid dose reduces surgery at one year
A 2013 high-quality RCT with 111 patients assessed pain relief and rates of surgery at one year with local corticosteroid injections for CTS.2 This trial had 3 arms with 37 patients in each: 80-mg methylprednisolone injection, 40-mg methylprednisolone injection, or placebo injection.
Both corticosteroid groups showed greater improvement on the Symptom Severity Scale at 10 weeks compared with placebo (40-mg methylprednisolone group: MD= −0.88; 95% CI, −1.3 to −0.46; 80-mg methylprednisolone group: MD= −0.64; 95% CI, −1.06 to −0.21). There was no difference between the methylprednisolone groups.
The incidence of surgery at one year was lower in the 80-mg methylprednisolone group compared with placebo (73% vs 92%; RR=0.79; 95% CI, 0.64-0.99; NNT=5) but not in the 40-mg methylprednisolone group compared with placebo (81% vs 92%; RR=0.88; 95% CI, 0.73-1.06).
Corticosteroids improve symptoms and disability, but effects wear off
A randomized double-blind, placebo-controlled trial conducted in 2010 examined the effectiveness of corticosteroid injections given by general practitioners to 69 patients with CTS.3 Patients were randomized to receive 10 mg of either triamcinolone or saline. They were reassessed after one week, and patients in the saline injection group who had inadequate symptom relief received a triamcinolone injection as bail-out treatment. Follow-up by patient questionnaire was done at 1, 3, 6, and 12 months.
Investigators assessed symptoms and disability using the Symptom Severity Scale and Functional Disability Scale, which are part of the Boston Carpal Tunnel Questionnaire. Like the Symptom Severity Scale, the Functional Disability Scale is scored from 1 to 5, with higher scores indicating more severe disability.
One week after treatment, the corticosteroid group showed greater improvement in symptom severity and functional disability than the saline group (symptom severity decreased from 2.9 to 1.9 with triamcinolone vs 2.8 to 2.5 with saline; MD=0.64; 95% CI, 0.32-0.96; functional disability decreased from 2.5 to 1.9 with triamcinolone but remained at 2.4 with saline; MD=0.59; 95% CI, 0.23-0.94).
Long-term follow-up of 35 patients who responded to corticosteroid injections found that the effects wore off over 12 months when assessed using the Symptom Severity Scale (mean score 1.5 at 1 month, 2.0 at 12 months; P=.08).
Surgery rates at one and 5 years
A 2012 prospective cohort study examined the 5-year rate of surgical intervention after a 20-mg methylprednisolone injection in 824 patients diagnosed with CTS who had failed conservative treatment.4 A total of 500 patients had a relapse of symptoms, and 372 of them elected to have a second injection. A Kaplan-Meier survivorship analysis determined rates of surgical intervention to be 14.5% (95% CI, 11.9-17) at one year and 33.2% (95% CI, 28.7-37.8) at 5 years.
RECOMMENDATION
A 2010 American Academy of Orthopaedic Surgeons evidence-based practice guideline on the treatment of CTS recommends corticosteroid injection before considering surgery (Grade B, Level 1 suggested recommendation with good evidence).5
Yes. Injected corticosteroids reduce symptoms of carpal tunnel syndrome (CTS) more effectively than placebo or systemic steroids, but no better than anti-inflammatory medication and splinting, from one to 12 weeks after therapy (strength of recommendation [SOR]: A, meta-analysis of randomized controlled trials [RCTs] and consistent RCT).
A 40-mg injection of methylprednisolone reduces symptoms as effectively as an 80-mg injection for as long as 10 weeks, but the 80-mg dose reduces progression to surgery at one year (SOR: B, RCT). Long-term effects of injections decrease by 12 months (SOR: B, RCT).
After corticosteroid injections, 14% of patients proceed to surgery at one year, and 33% proceed to surgery at 5 years (SOR: B, cohort trial).
EVIDENCE SUMMARY
A 2007 Cochrane review of 12 RCTs with 671 patients compared the efficacy of corticosteroid injections for CTS with placebo injections or other nonsurgical interventions.1 Patients who received corticosteroid injections showed clinical improvement at one month or less compared with placebo (2 trials, 141 patients; 73% corticosteroids vs 28% placebo; relative risk [RR]=2.58; 95% confidence interval [CI], 1.72-3.87; number needed to treat [NNT]=2).
Compared with systemic corticosteroids, corticosteroid injections didn’t improve symptoms on a Global Symptom Score (scale of 0-50, with 50 indicating the most severe symptoms) at 2 weeks (one trial, 60 patients; mean difference [MD]= −4.2; 95% CI, −8.7 to 0.26), but did improve symptoms at 8 weeks (MD= −7.16; 95% CI, −11.5 to −2.86) and 12 weeks (MD= −7.1; 95% CI, −11.7 to −2.52).
Patients showed no difference in scores between corticosteroid injection and oral anti-inflammatory medication with neutral angle wrist splints on the Symptom Severity Scale (1 to 5, with 5 indicating the most severe symptoms) at 2 weeks (1 trial, 23 patients [37 wrists]; MD=0.0; 95% CI, −0.64 to 0.64) or 8 weeks (MD=0.1; 95% CI, −0.33 to 0.53).
Higher corticosteroid dose reduces surgery at one year
A 2013 high-quality RCT with 111 patients assessed pain relief and rates of surgery at one year with local corticosteroid injections for CTS.2 This trial had 3 arms with 37 patients in each: 80-mg methylprednisolone injection, 40-mg methylprednisolone injection, or placebo injection.
Both corticosteroid groups showed greater improvement on the Symptom Severity Scale at 10 weeks compared with placebo (40-mg methylprednisolone group: MD= −0.88; 95% CI, −1.3 to −0.46; 80-mg methylprednisolone group: MD= −0.64; 95% CI, −1.06 to −0.21). There was no difference between the methylprednisolone groups.
The incidence of surgery at one year was lower in the 80-mg methylprednisolone group compared with placebo (73% vs 92%; RR=0.79; 95% CI, 0.64-0.99; NNT=5) but not in the 40-mg methylprednisolone group compared with placebo (81% vs 92%; RR=0.88; 95% CI, 0.73-1.06).
Corticosteroids improve symptoms and disability, but effects wear off
A randomized double-blind, placebo-controlled trial conducted in 2010 examined the effectiveness of corticosteroid injections given by general practitioners to 69 patients with CTS.3 Patients were randomized to receive 10 mg of either triamcinolone or saline. They were reassessed after one week, and patients in the saline injection group who had inadequate symptom relief received a triamcinolone injection as bail-out treatment. Follow-up by patient questionnaire was done at 1, 3, 6, and 12 months.
Investigators assessed symptoms and disability using the Symptom Severity Scale and Functional Disability Scale, which are part of the Boston Carpal Tunnel Questionnaire. Like the Symptom Severity Scale, the Functional Disability Scale is scored from 1 to 5, with higher scores indicating more severe disability.
One week after treatment, the corticosteroid group showed greater improvement in symptom severity and functional disability than the saline group (symptom severity decreased from 2.9 to 1.9 with triamcinolone vs 2.8 to 2.5 with saline; MD=0.64; 95% CI, 0.32-0.96; functional disability decreased from 2.5 to 1.9 with triamcinolone but remained at 2.4 with saline; MD=0.59; 95% CI, 0.23-0.94).
Long-term follow-up of 35 patients who responded to corticosteroid injections found that the effects wore off over 12 months when assessed using the Symptom Severity Scale (mean score 1.5 at 1 month, 2.0 at 12 months; P=.08).
Surgery rates at one and 5 years
A 2012 prospective cohort study examined the 5-year rate of surgical intervention after a 20-mg methylprednisolone injection in 824 patients diagnosed with CTS who had failed conservative treatment.4 A total of 500 patients had a relapse of symptoms, and 372 of them elected to have a second injection. A Kaplan-Meier survivorship analysis determined rates of surgical intervention to be 14.5% (95% CI, 11.9-17) at one year and 33.2% (95% CI, 28.7-37.8) at 5 years.
RECOMMENDATION
A 2010 American Academy of Orthopaedic Surgeons evidence-based practice guideline on the treatment of CTS recommends corticosteroid injection before considering surgery (Grade B, Level 1 suggested recommendation with good evidence).5
1. Marshall S, Tardif G, Ashworth N. Local corticosteroid injection for carpal tunnel syndrome. Cochrane Database Syst Rev. 2007;(2):CD001554.
2. Atroshi I, Flondell M, Hofer M, et al. Methylprednisolone injections for the carpal tunnel syndrome: a randomized, placebo-controlled trial. Ann Intern Med. 2013;159:309-317.
3. Peters-Veluthamaningal C, Winters JC, Gronier KH, et al. Randomised controlled trial of local corticosteroid injections for carpal tunnel syndrome in general practice. BMC Fam Pract. 2010;11:54.
4. Jenkins PJ, Duckworth AD, Watts AC, et al. Corticosteroid injection for carpal tunnel syndrome: a 5-year survivorship analysis. Hand. 2012;7:151-156.
5. Keith MW, Masear V, Chung KC, et al. American Academy of Orthopaedic Surgeons clinical practice guideline on the treatment of carpal tunnel syndrome. J Bone Joint Surg Am. 2010;92:218-219.
1. Marshall S, Tardif G, Ashworth N. Local corticosteroid injection for carpal tunnel syndrome. Cochrane Database Syst Rev. 2007;(2):CD001554.
2. Atroshi I, Flondell M, Hofer M, et al. Methylprednisolone injections for the carpal tunnel syndrome: a randomized, placebo-controlled trial. Ann Intern Med. 2013;159:309-317.
3. Peters-Veluthamaningal C, Winters JC, Gronier KH, et al. Randomised controlled trial of local corticosteroid injections for carpal tunnel syndrome in general practice. BMC Fam Pract. 2010;11:54.
4. Jenkins PJ, Duckworth AD, Watts AC, et al. Corticosteroid injection for carpal tunnel syndrome: a 5-year survivorship analysis. Hand. 2012;7:151-156.
5. Keith MW, Masear V, Chung KC, et al. American Academy of Orthopaedic Surgeons clinical practice guideline on the treatment of carpal tunnel syndrome. J Bone Joint Surg Am. 2010;92:218-219.
Evidence-based answers from the Family Physicians Inquiries Network



