User login
What’s the best way to predict the success of a trial of labor after a previous C-section?
While 8 scoring tools predict success rates for a trial of labor after previous cesarean section (TOLAC), it’s unclear which is the best because no trials have compared prediction tools against each other, and each tool has a unique set of variables.
A “close-to-delivery” scoring nomogram predicting the success rate of TOLAC correlates well (90% accuracy) with actual outcomes (strength of recommendation [SOR]: B, prospective and retrospective cohort studies) and has been externally validated with multiple additional cohorts.
All other point-prediction scoring tools are accurate within 10% when predicting the success rate of TOLAC (SOR: B, prospective and retrospective cohort studies).
EVIDENCE SUMMARY
Seven validated prospective scoring systems, and one unvalidated system, predict a successful TOLAC based on a variety of clinical factors (TABLE1-11). The systems use different outcome statistics, so their predictive accuracy can’t be directly compared.12
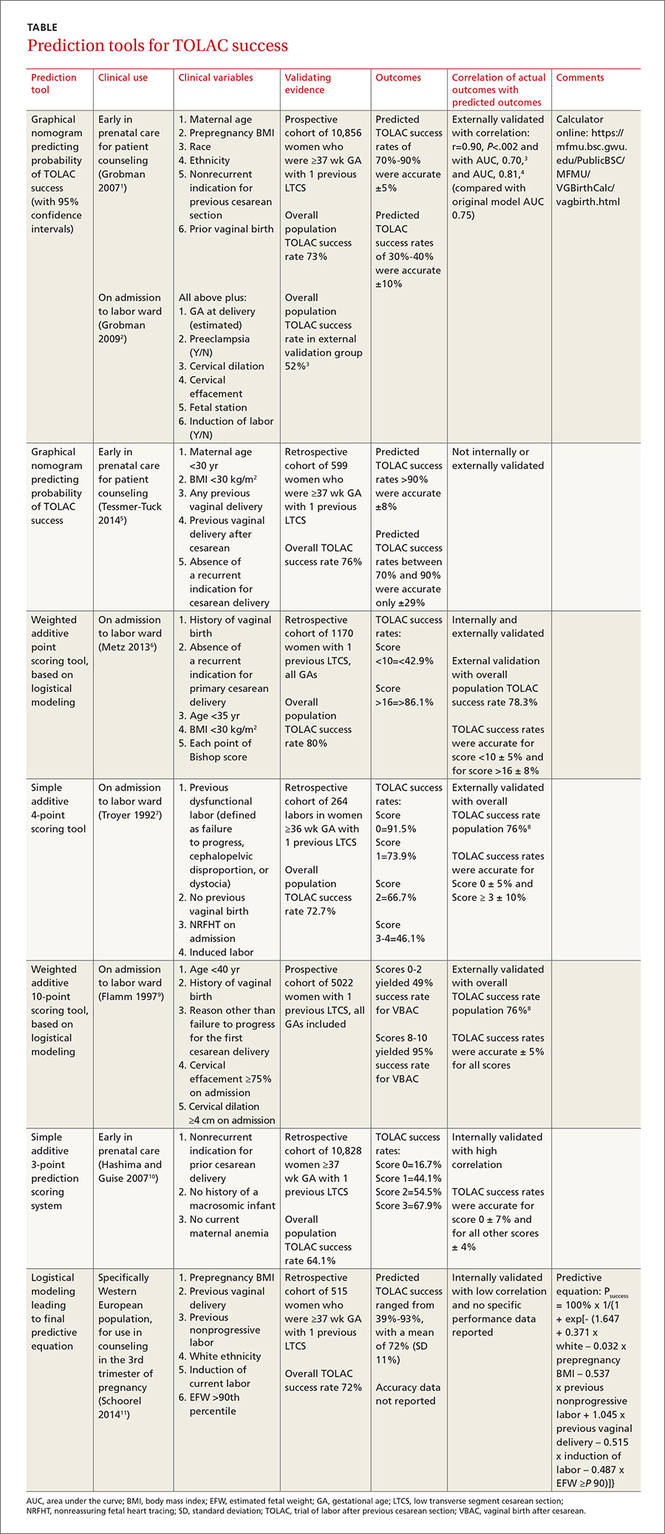
Grobman: Entry-to-care and close-to-delivery nomograms
Grobman et al created 2 prediction models, an “entry-to-care” model (used at the first prenatal visit), and a “close-to-delivery” model (used on admission to the labor ward).1,2 Both models display a graphic nomogram forecasting the probability of TOLAC success (with 95% confidence intervals [CIs]). The authors compared predicted TOLAC outcomes with actual TOLAC outcomes and found that the model predictions most successfully correlated with high-likelihood outcomes (70% to 90% chance of successful TOLAC, plus or minus approximately 5%). Both models were less accurate with low-likelihood outcomes (40% chance of successful TOLAC, plus or minus approximately 10%).
Many independent authors have validated the close-to-delivery model, comparing predicted with actual TOLAC success rates. In a retrospective cohort study of 490 women, Constantine et al found the correlation between the observed and predicted TOLAC rates to have an r of 0.90, P=.002, with an area under the curve (AUC) of 0.70.3 Yoki et al validated the model in a Japanese cohort of 729 women with an AUC of 0.81, consistent with the AUC of 0.75 reported in the development of the original model.4
Tessmer-Tuck: The close-to-delivery model without the race variable
Tessmer-Tuck et al developed a model similar to Grobman’s close-to-delivery model, but removed race/ethnicity as a variable and compared it to the accuracy of the Grobman nomogram.5 Variables considered in this model were maternal age <30 years (odds ratio [OR]=1.53; 95% CI, 1.00-2.36), body mass index (BMI) <30 kg/m2 (OR=1.82; 95% CI, 1.11-2.97), any previous vaginal delivery (OR=3.17; 95% CI, 1.50-6.80), previous vaginal delivery after cesarean (OR=2.24; 95% CI, 1.25-4.18), and absence of a recurrent indication for cesarean delivery (OR=1.81; 95% CI, 1.18-2.76).
The model provided a successful probability of vaginal birth after cesarean ranging from 38% to 98% with AUC of 0.723 (95% CI, 0.680-0.767). When compared with the Grobman model, the AUC for features in the Tessmer-Tuck model was 0.757 (95% CI, 0.713-0.801), similar to the AUC of 0.75 reported in the development of the original model. The predictive accuracy of TOLAC success between 70% and 90% was quite poor at only ±29%.
Metz: A 5-point scoring tool
Metz et al created a point scoring tool for use on admission to the labor ward, based on 5 variables weighted by degree of correlation with TOLAC success: a history of vaginal birth (OR=2.7; 95% CI, 1.8-4.1), absence of a recurrent indication for initial cesarean delivery (OR=2.0; 95% CI, 1.3-3.1), age <35 years (OR=2.0; 95% CI, 1.1-3.4), BMI <30 kg/m2 (OR=1.6; 95% CI, 1.1-2.4), and each point of Bishop score on admission (OR=1.3; 95% CI, 1.2-1.4).6
The authors internally validated this scoring tool with an AUC of 0.70 (95% CI, 0.67-0.74), then externally validated the tool with an independent cohort of 585 women and found an AUC of 0.80 (95% CI, 0.76-0.84). In the external validation cohort, TOLAC success rates were 37.4% (95% CI, 27.2-47.5) with a score <10 and 94.4% (95% CI, 90.9-97.8) with a score >16, performing within 8% of the prediction model.
Troyer: A simple 4-point tool
Troyer et al created a simple 4-point scoring tool for use on admission to the labor ward.7 The tool’s 4 variables—previous dysfunctional labor, no previous vaginal birth, nonreassuring fetal heart tracing (NRFHT) on admission, and induced labor—were found to reduce the success rate of a trial of labor (P<.05). Dinsmoor et al used this scoring tool in a group of 156 women with an overall TOLAC success rate of 76% (3% higher than Troyer’s group) and found that for labors with a favorable score (0), the tool performed within 5% and for labors with an unfavorable score (≥3), the tool performed within 10%.8
Flamm: 5 variables weighted by correlation with TOLAC success
Flamm et al also created a scoring tool for use on admission to the labor ward, based on 5 variables weighted according to degree of correlation with TOLAC success: age <40 years (OR=2.58; 95% CI, 1.55-4.3), history of a vaginal birth (OR=1.53-9.11 depending on where the vaginal birth fell in the woman’s reproductive history), reason other than failure to progress for the first cesarean delivery (OR=1.93; 95% CI, 1.58-2.35), cervical effacement ≥75% on admission (OR=2.72; 95% CI, 2.00-3.71), and cervical dilation ≥4 cm on admission (OR=2.16; 95% CI, 1.66-2.82).9 Dinsmoor validated this scoring tool as well in 156 women and found 100% TOLAC success for scores ≥7 (within 5% of the original tool) and 56% TOLAC success for scores ≤4 (compared with 49% for scores 0-2 in the original work).8
Hashima and Guise: A 3-point scoring tool
Hashima and Guise evaluated 16 variables and identified 7 associated with TOLAC outcome: indication for cesarean delivery (recurrent vs nonrecurrent), chorioamnionitis, macrosomicinfant, age, anemia, diabetes, and infant sex, from which they created a 3-point scoring tool using the variables most associated with TOLAC outcome. Each variable was assigned a score of 0 or 1, and the likelihood of TOLAC success was calculated.10
They found a relationship between score and TOLAC success. The original study population of 10,828 was randomly divided into a score development and validation group. TOLAC success percentages were most discordant between the tool development and internal validation groups for score 0 at 7%. Scores 1 to 3 were within 4% of each other.
Schoorel: A model designed for Western Europeann women
Finally, Schoorel et al developed and internally validated a prediction model for a Western European population, to be used during counseling in the third trimester of pregnancy.11 Six variables were identified and entered into the model calculations: prepregnancy BMI (entered as a continuous variable), (OR=0.96; 95% CI, 0.92-1.00); previous cesarean for nonprogressive labor (OR=0.50; 95% CI, 0.33-0.76); previous vaginal delivery (OR=3.81; 95% CI, 2.10-6.92); induction of labor (OR=0.52; 95% CI, 0.33-2.10); estimated fetal weight >90th percentile (OR=0.54; 95% CI, 0.14-2.02); and white ethnicity (OR=1.61; 95% CI, 0.97-2.66). The authors noted that the predicted probability of TOLAC success ranged from 39% to 93%, with a mean of 72% (standard deviation, 11%), and only noted the predicted probabilities were well calibrated from 65% upwards without additional data on specific performance.
RECOMMENDATIONS
The American College of Obstetricians and Gynecologists (ACOG) lists strong predictors of a successful vaginal birth after cesarean as previous vaginal birth and spontaneous labor. Factors associated with decreased probability of success are recurrent indication for initial cesarean delivery (labor dystocia), increased maternal age, nonwhite ethnicity, gestational age greater than 40 weeks, maternal obesity, preeclampsia, short interpregnancy interval, and increased neonatal birth weight. ACOG does not offer any weighted or risk-based scoring tools for predicting success.13
Neither the American Academy of Family Physicians nor the American College of Nurse Midwives recommend specific scoring tools or success predictors.
1. Grobman WA, Lai Y, Landon MB, et al; National Institute of Child Health and Human Development (NICHD) Maternal- Fetal Medicine Units Network (MFMU). Development of a nomogram for prediction of vaginal birth after cesarean delivery. Obstet Gynecol. 2007;109:806-812.
2. Grobman WA, Lai Y, Landon MB, et al. Does information available at admission for delivery improve prediction of vaginal birth after cesarean? Am J Perinatol. 2009;26:693-701.
3. Costantine MM, Fox KA, Pacheco LD, et al. Does information available at delivery improve the accuracy of predicting vaginal birth after cesarean? Validation of the published models in an independent patient cohort. Am J Perinatol. 2011;28:293-298.
4. Yoki A, Ishikawa K, Miyazaki K, et al. Validation of the prediction model for success of vaginal birth after cesarean delivery in Japanese women. Int J Med Sci. 2012;9:488-491.
5. Tessmer-Tuck JA, El-Nashar SA, Racek AR, et al. Predicting vaginal birth after cesarean section: a cohort study. Gynecol Obstet Invest. 2014;77:121-126.
6. Metz TD, Stoddard GJ, Henry E, et al. Simple, validated vaginal birth after cesarean delivery prediction model for use at the time of admission. Obstet Gynecol. 2013;122:571-578.
7. Troyer LR, Parisi VM. Obstetric parameters affecting success in a trial of labor: designation of a scoring system. Am J Obstet Gynecol. 1992;167(4 pt 1):1099-1104.
8. Dinsmoor MJ, Brock EL. Predicting failed trial of labor after primary cesarean delivery. Obstet Gynecol. 2004;103:282-286.
9. Flamm BL, Geiger AM. Vaginal birth after cesarean delivery: an admission scoring system. Obstet Gynecol. 1997;90:907-910.
10. Hashima JN, Guise JM. Vaginal birth after cesarean: a prenatal scoring tool. Am J Obstet Gynecol. 2007;196:e22-e23.
11. Schoorel ENC, van Kuijk SMJ, Melman S, et al. Vaginal birth after a caesarean section: the development of a Western European population-based prediction model for deliveries at term. BJOG. 2014;121:194-201.
12. Guise JM, Eden K, Emeis C, et al. Vaginal birth after cesarean: New insights. Evidence Report/Technology Assessment No. 191. AHRQ Publication No. 10-E003. Rockville, MD: Agency for Healthcare Research and Quality; 2010.
13. American College of Obstetricians and Gynecologists. ACOG Practice bulletin no. 115: Vaginal birth after previous cesarean delivery. Obstet Gynecol. 2010;116(2 pt 1):450-463.
While 8 scoring tools predict success rates for a trial of labor after previous cesarean section (TOLAC), it’s unclear which is the best because no trials have compared prediction tools against each other, and each tool has a unique set of variables.
A “close-to-delivery” scoring nomogram predicting the success rate of TOLAC correlates well (90% accuracy) with actual outcomes (strength of recommendation [SOR]: B, prospective and retrospective cohort studies) and has been externally validated with multiple additional cohorts.
All other point-prediction scoring tools are accurate within 10% when predicting the success rate of TOLAC (SOR: B, prospective and retrospective cohort studies).
EVIDENCE SUMMARY
Seven validated prospective scoring systems, and one unvalidated system, predict a successful TOLAC based on a variety of clinical factors (TABLE1-11). The systems use different outcome statistics, so their predictive accuracy can’t be directly compared.12

Grobman: Entry-to-care and close-to-delivery nomograms
Grobman et al created 2 prediction models, an “entry-to-care” model (used at the first prenatal visit), and a “close-to-delivery” model (used on admission to the labor ward).1,2 Both models display a graphic nomogram forecasting the probability of TOLAC success (with 95% confidence intervals [CIs]). The authors compared predicted TOLAC outcomes with actual TOLAC outcomes and found that the model predictions most successfully correlated with high-likelihood outcomes (70% to 90% chance of successful TOLAC, plus or minus approximately 5%). Both models were less accurate with low-likelihood outcomes (40% chance of successful TOLAC, plus or minus approximately 10%).
Many independent authors have validated the close-to-delivery model, comparing predicted with actual TOLAC success rates. In a retrospective cohort study of 490 women, Constantine et al found the correlation between the observed and predicted TOLAC rates to have an r of 0.90, P=.002, with an area under the curve (AUC) of 0.70.3 Yoki et al validated the model in a Japanese cohort of 729 women with an AUC of 0.81, consistent with the AUC of 0.75 reported in the development of the original model.4
Tessmer-Tuck: The close-to-delivery model without the race variable
Tessmer-Tuck et al developed a model similar to Grobman’s close-to-delivery model, but removed race/ethnicity as a variable and compared it to the accuracy of the Grobman nomogram.5 Variables considered in this model were maternal age <30 years (odds ratio [OR]=1.53; 95% CI, 1.00-2.36), body mass index (BMI) <30 kg/m2 (OR=1.82; 95% CI, 1.11-2.97), any previous vaginal delivery (OR=3.17; 95% CI, 1.50-6.80), previous vaginal delivery after cesarean (OR=2.24; 95% CI, 1.25-4.18), and absence of a recurrent indication for cesarean delivery (OR=1.81; 95% CI, 1.18-2.76).
The model provided a successful probability of vaginal birth after cesarean ranging from 38% to 98% with AUC of 0.723 (95% CI, 0.680-0.767). When compared with the Grobman model, the AUC for features in the Tessmer-Tuck model was 0.757 (95% CI, 0.713-0.801), similar to the AUC of 0.75 reported in the development of the original model. The predictive accuracy of TOLAC success between 70% and 90% was quite poor at only ±29%.
Metz: A 5-point scoring tool
Metz et al created a point scoring tool for use on admission to the labor ward, based on 5 variables weighted by degree of correlation with TOLAC success: a history of vaginal birth (OR=2.7; 95% CI, 1.8-4.1), absence of a recurrent indication for initial cesarean delivery (OR=2.0; 95% CI, 1.3-3.1), age <35 years (OR=2.0; 95% CI, 1.1-3.4), BMI <30 kg/m2 (OR=1.6; 95% CI, 1.1-2.4), and each point of Bishop score on admission (OR=1.3; 95% CI, 1.2-1.4).6
The authors internally validated this scoring tool with an AUC of 0.70 (95% CI, 0.67-0.74), then externally validated the tool with an independent cohort of 585 women and found an AUC of 0.80 (95% CI, 0.76-0.84). In the external validation cohort, TOLAC success rates were 37.4% (95% CI, 27.2-47.5) with a score <10 and 94.4% (95% CI, 90.9-97.8) with a score >16, performing within 8% of the prediction model.
Troyer: A simple 4-point tool
Troyer et al created a simple 4-point scoring tool for use on admission to the labor ward.7 The tool’s 4 variables—previous dysfunctional labor, no previous vaginal birth, nonreassuring fetal heart tracing (NRFHT) on admission, and induced labor—were found to reduce the success rate of a trial of labor (P<.05). Dinsmoor et al used this scoring tool in a group of 156 women with an overall TOLAC success rate of 76% (3% higher than Troyer’s group) and found that for labors with a favorable score (0), the tool performed within 5% and for labors with an unfavorable score (≥3), the tool performed within 10%.8
Flamm: 5 variables weighted by correlation with TOLAC success
Flamm et al also created a scoring tool for use on admission to the labor ward, based on 5 variables weighted according to degree of correlation with TOLAC success: age <40 years (OR=2.58; 95% CI, 1.55-4.3), history of a vaginal birth (OR=1.53-9.11 depending on where the vaginal birth fell in the woman’s reproductive history), reason other than failure to progress for the first cesarean delivery (OR=1.93; 95% CI, 1.58-2.35), cervical effacement ≥75% on admission (OR=2.72; 95% CI, 2.00-3.71), and cervical dilation ≥4 cm on admission (OR=2.16; 95% CI, 1.66-2.82).9 Dinsmoor validated this scoring tool as well in 156 women and found 100% TOLAC success for scores ≥7 (within 5% of the original tool) and 56% TOLAC success for scores ≤4 (compared with 49% for scores 0-2 in the original work).8
Hashima and Guise: A 3-point scoring tool
Hashima and Guise evaluated 16 variables and identified 7 associated with TOLAC outcome: indication for cesarean delivery (recurrent vs nonrecurrent), chorioamnionitis, macrosomicinfant, age, anemia, diabetes, and infant sex, from which they created a 3-point scoring tool using the variables most associated with TOLAC outcome. Each variable was assigned a score of 0 or 1, and the likelihood of TOLAC success was calculated.10
They found a relationship between score and TOLAC success. The original study population of 10,828 was randomly divided into a score development and validation group. TOLAC success percentages were most discordant between the tool development and internal validation groups for score 0 at 7%. Scores 1 to 3 were within 4% of each other.
Schoorel: A model designed for Western Europeann women
Finally, Schoorel et al developed and internally validated a prediction model for a Western European population, to be used during counseling in the third trimester of pregnancy.11 Six variables were identified and entered into the model calculations: prepregnancy BMI (entered as a continuous variable), (OR=0.96; 95% CI, 0.92-1.00); previous cesarean for nonprogressive labor (OR=0.50; 95% CI, 0.33-0.76); previous vaginal delivery (OR=3.81; 95% CI, 2.10-6.92); induction of labor (OR=0.52; 95% CI, 0.33-2.10); estimated fetal weight >90th percentile (OR=0.54; 95% CI, 0.14-2.02); and white ethnicity (OR=1.61; 95% CI, 0.97-2.66). The authors noted that the predicted probability of TOLAC success ranged from 39% to 93%, with a mean of 72% (standard deviation, 11%), and only noted the predicted probabilities were well calibrated from 65% upwards without additional data on specific performance.
RECOMMENDATIONS
The American College of Obstetricians and Gynecologists (ACOG) lists strong predictors of a successful vaginal birth after cesarean as previous vaginal birth and spontaneous labor. Factors associated with decreased probability of success are recurrent indication for initial cesarean delivery (labor dystocia), increased maternal age, nonwhite ethnicity, gestational age greater than 40 weeks, maternal obesity, preeclampsia, short interpregnancy interval, and increased neonatal birth weight. ACOG does not offer any weighted or risk-based scoring tools for predicting success.13
Neither the American Academy of Family Physicians nor the American College of Nurse Midwives recommend specific scoring tools or success predictors.
While 8 scoring tools predict success rates for a trial of labor after previous cesarean section (TOLAC), it’s unclear which is the best because no trials have compared prediction tools against each other, and each tool has a unique set of variables.
A “close-to-delivery” scoring nomogram predicting the success rate of TOLAC correlates well (90% accuracy) with actual outcomes (strength of recommendation [SOR]: B, prospective and retrospective cohort studies) and has been externally validated with multiple additional cohorts.
All other point-prediction scoring tools are accurate within 10% when predicting the success rate of TOLAC (SOR: B, prospective and retrospective cohort studies).
EVIDENCE SUMMARY
Seven validated prospective scoring systems, and one unvalidated system, predict a successful TOLAC based on a variety of clinical factors (TABLE1-11). The systems use different outcome statistics, so their predictive accuracy can’t be directly compared.12

Grobman: Entry-to-care and close-to-delivery nomograms
Grobman et al created 2 prediction models, an “entry-to-care” model (used at the first prenatal visit), and a “close-to-delivery” model (used on admission to the labor ward).1,2 Both models display a graphic nomogram forecasting the probability of TOLAC success (with 95% confidence intervals [CIs]). The authors compared predicted TOLAC outcomes with actual TOLAC outcomes and found that the model predictions most successfully correlated with high-likelihood outcomes (70% to 90% chance of successful TOLAC, plus or minus approximately 5%). Both models were less accurate with low-likelihood outcomes (40% chance of successful TOLAC, plus or minus approximately 10%).
Many independent authors have validated the close-to-delivery model, comparing predicted with actual TOLAC success rates. In a retrospective cohort study of 490 women, Constantine et al found the correlation between the observed and predicted TOLAC rates to have an r of 0.90, P=.002, with an area under the curve (AUC) of 0.70.3 Yoki et al validated the model in a Japanese cohort of 729 women with an AUC of 0.81, consistent with the AUC of 0.75 reported in the development of the original model.4
Tessmer-Tuck: The close-to-delivery model without the race variable
Tessmer-Tuck et al developed a model similar to Grobman’s close-to-delivery model, but removed race/ethnicity as a variable and compared it to the accuracy of the Grobman nomogram.5 Variables considered in this model were maternal age <30 years (odds ratio [OR]=1.53; 95% CI, 1.00-2.36), body mass index (BMI) <30 kg/m2 (OR=1.82; 95% CI, 1.11-2.97), any previous vaginal delivery (OR=3.17; 95% CI, 1.50-6.80), previous vaginal delivery after cesarean (OR=2.24; 95% CI, 1.25-4.18), and absence of a recurrent indication for cesarean delivery (OR=1.81; 95% CI, 1.18-2.76).
The model provided a successful probability of vaginal birth after cesarean ranging from 38% to 98% with AUC of 0.723 (95% CI, 0.680-0.767). When compared with the Grobman model, the AUC for features in the Tessmer-Tuck model was 0.757 (95% CI, 0.713-0.801), similar to the AUC of 0.75 reported in the development of the original model. The predictive accuracy of TOLAC success between 70% and 90% was quite poor at only ±29%.
Metz: A 5-point scoring tool
Metz et al created a point scoring tool for use on admission to the labor ward, based on 5 variables weighted by degree of correlation with TOLAC success: a history of vaginal birth (OR=2.7; 95% CI, 1.8-4.1), absence of a recurrent indication for initial cesarean delivery (OR=2.0; 95% CI, 1.3-3.1), age <35 years (OR=2.0; 95% CI, 1.1-3.4), BMI <30 kg/m2 (OR=1.6; 95% CI, 1.1-2.4), and each point of Bishop score on admission (OR=1.3; 95% CI, 1.2-1.4).6
The authors internally validated this scoring tool with an AUC of 0.70 (95% CI, 0.67-0.74), then externally validated the tool with an independent cohort of 585 women and found an AUC of 0.80 (95% CI, 0.76-0.84). In the external validation cohort, TOLAC success rates were 37.4% (95% CI, 27.2-47.5) with a score <10 and 94.4% (95% CI, 90.9-97.8) with a score >16, performing within 8% of the prediction model.
Troyer: A simple 4-point tool
Troyer et al created a simple 4-point scoring tool for use on admission to the labor ward.7 The tool’s 4 variables—previous dysfunctional labor, no previous vaginal birth, nonreassuring fetal heart tracing (NRFHT) on admission, and induced labor—were found to reduce the success rate of a trial of labor (P<.05). Dinsmoor et al used this scoring tool in a group of 156 women with an overall TOLAC success rate of 76% (3% higher than Troyer’s group) and found that for labors with a favorable score (0), the tool performed within 5% and for labors with an unfavorable score (≥3), the tool performed within 10%.8
Flamm: 5 variables weighted by correlation with TOLAC success
Flamm et al also created a scoring tool for use on admission to the labor ward, based on 5 variables weighted according to degree of correlation with TOLAC success: age <40 years (OR=2.58; 95% CI, 1.55-4.3), history of a vaginal birth (OR=1.53-9.11 depending on where the vaginal birth fell in the woman’s reproductive history), reason other than failure to progress for the first cesarean delivery (OR=1.93; 95% CI, 1.58-2.35), cervical effacement ≥75% on admission (OR=2.72; 95% CI, 2.00-3.71), and cervical dilation ≥4 cm on admission (OR=2.16; 95% CI, 1.66-2.82).9 Dinsmoor validated this scoring tool as well in 156 women and found 100% TOLAC success for scores ≥7 (within 5% of the original tool) and 56% TOLAC success for scores ≤4 (compared with 49% for scores 0-2 in the original work).8
Hashima and Guise: A 3-point scoring tool
Hashima and Guise evaluated 16 variables and identified 7 associated with TOLAC outcome: indication for cesarean delivery (recurrent vs nonrecurrent), chorioamnionitis, macrosomicinfant, age, anemia, diabetes, and infant sex, from which they created a 3-point scoring tool using the variables most associated with TOLAC outcome. Each variable was assigned a score of 0 or 1, and the likelihood of TOLAC success was calculated.10
They found a relationship between score and TOLAC success. The original study population of 10,828 was randomly divided into a score development and validation group. TOLAC success percentages were most discordant between the tool development and internal validation groups for score 0 at 7%. Scores 1 to 3 were within 4% of each other.
Schoorel: A model designed for Western Europeann women
Finally, Schoorel et al developed and internally validated a prediction model for a Western European population, to be used during counseling in the third trimester of pregnancy.11 Six variables were identified and entered into the model calculations: prepregnancy BMI (entered as a continuous variable), (OR=0.96; 95% CI, 0.92-1.00); previous cesarean for nonprogressive labor (OR=0.50; 95% CI, 0.33-0.76); previous vaginal delivery (OR=3.81; 95% CI, 2.10-6.92); induction of labor (OR=0.52; 95% CI, 0.33-2.10); estimated fetal weight >90th percentile (OR=0.54; 95% CI, 0.14-2.02); and white ethnicity (OR=1.61; 95% CI, 0.97-2.66). The authors noted that the predicted probability of TOLAC success ranged from 39% to 93%, with a mean of 72% (standard deviation, 11%), and only noted the predicted probabilities were well calibrated from 65% upwards without additional data on specific performance.
RECOMMENDATIONS
The American College of Obstetricians and Gynecologists (ACOG) lists strong predictors of a successful vaginal birth after cesarean as previous vaginal birth and spontaneous labor. Factors associated with decreased probability of success are recurrent indication for initial cesarean delivery (labor dystocia), increased maternal age, nonwhite ethnicity, gestational age greater than 40 weeks, maternal obesity, preeclampsia, short interpregnancy interval, and increased neonatal birth weight. ACOG does not offer any weighted or risk-based scoring tools for predicting success.13
Neither the American Academy of Family Physicians nor the American College of Nurse Midwives recommend specific scoring tools or success predictors.
1. Grobman WA, Lai Y, Landon MB, et al; National Institute of Child Health and Human Development (NICHD) Maternal- Fetal Medicine Units Network (MFMU). Development of a nomogram for prediction of vaginal birth after cesarean delivery. Obstet Gynecol. 2007;109:806-812.
2. Grobman WA, Lai Y, Landon MB, et al. Does information available at admission for delivery improve prediction of vaginal birth after cesarean? Am J Perinatol. 2009;26:693-701.
3. Costantine MM, Fox KA, Pacheco LD, et al. Does information available at delivery improve the accuracy of predicting vaginal birth after cesarean? Validation of the published models in an independent patient cohort. Am J Perinatol. 2011;28:293-298.
4. Yoki A, Ishikawa K, Miyazaki K, et al. Validation of the prediction model for success of vaginal birth after cesarean delivery in Japanese women. Int J Med Sci. 2012;9:488-491.
5. Tessmer-Tuck JA, El-Nashar SA, Racek AR, et al. Predicting vaginal birth after cesarean section: a cohort study. Gynecol Obstet Invest. 2014;77:121-126.
6. Metz TD, Stoddard GJ, Henry E, et al. Simple, validated vaginal birth after cesarean delivery prediction model for use at the time of admission. Obstet Gynecol. 2013;122:571-578.
7. Troyer LR, Parisi VM. Obstetric parameters affecting success in a trial of labor: designation of a scoring system. Am J Obstet Gynecol. 1992;167(4 pt 1):1099-1104.
8. Dinsmoor MJ, Brock EL. Predicting failed trial of labor after primary cesarean delivery. Obstet Gynecol. 2004;103:282-286.
9. Flamm BL, Geiger AM. Vaginal birth after cesarean delivery: an admission scoring system. Obstet Gynecol. 1997;90:907-910.
10. Hashima JN, Guise JM. Vaginal birth after cesarean: a prenatal scoring tool. Am J Obstet Gynecol. 2007;196:e22-e23.
11. Schoorel ENC, van Kuijk SMJ, Melman S, et al. Vaginal birth after a caesarean section: the development of a Western European population-based prediction model for deliveries at term. BJOG. 2014;121:194-201.
12. Guise JM, Eden K, Emeis C, et al. Vaginal birth after cesarean: New insights. Evidence Report/Technology Assessment No. 191. AHRQ Publication No. 10-E003. Rockville, MD: Agency for Healthcare Research and Quality; 2010.
13. American College of Obstetricians and Gynecologists. ACOG Practice bulletin no. 115: Vaginal birth after previous cesarean delivery. Obstet Gynecol. 2010;116(2 pt 1):450-463.
1. Grobman WA, Lai Y, Landon MB, et al; National Institute of Child Health and Human Development (NICHD) Maternal- Fetal Medicine Units Network (MFMU). Development of a nomogram for prediction of vaginal birth after cesarean delivery. Obstet Gynecol. 2007;109:806-812.
2. Grobman WA, Lai Y, Landon MB, et al. Does information available at admission for delivery improve prediction of vaginal birth after cesarean? Am J Perinatol. 2009;26:693-701.
3. Costantine MM, Fox KA, Pacheco LD, et al. Does information available at delivery improve the accuracy of predicting vaginal birth after cesarean? Validation of the published models in an independent patient cohort. Am J Perinatol. 2011;28:293-298.
4. Yoki A, Ishikawa K, Miyazaki K, et al. Validation of the prediction model for success of vaginal birth after cesarean delivery in Japanese women. Int J Med Sci. 2012;9:488-491.
5. Tessmer-Tuck JA, El-Nashar SA, Racek AR, et al. Predicting vaginal birth after cesarean section: a cohort study. Gynecol Obstet Invest. 2014;77:121-126.
6. Metz TD, Stoddard GJ, Henry E, et al. Simple, validated vaginal birth after cesarean delivery prediction model for use at the time of admission. Obstet Gynecol. 2013;122:571-578.
7. Troyer LR, Parisi VM. Obstetric parameters affecting success in a trial of labor: designation of a scoring system. Am J Obstet Gynecol. 1992;167(4 pt 1):1099-1104.
8. Dinsmoor MJ, Brock EL. Predicting failed trial of labor after primary cesarean delivery. Obstet Gynecol. 2004;103:282-286.
9. Flamm BL, Geiger AM. Vaginal birth after cesarean delivery: an admission scoring system. Obstet Gynecol. 1997;90:907-910.
10. Hashima JN, Guise JM. Vaginal birth after cesarean: a prenatal scoring tool. Am J Obstet Gynecol. 2007;196:e22-e23.
11. Schoorel ENC, van Kuijk SMJ, Melman S, et al. Vaginal birth after a caesarean section: the development of a Western European population-based prediction model for deliveries at term. BJOG. 2014;121:194-201.
12. Guise JM, Eden K, Emeis C, et al. Vaginal birth after cesarean: New insights. Evidence Report/Technology Assessment No. 191. AHRQ Publication No. 10-E003. Rockville, MD: Agency for Healthcare Research and Quality; 2010.
13. American College of Obstetricians and Gynecologists. ACOG Practice bulletin no. 115: Vaginal birth after previous cesarean delivery. Obstet Gynecol. 2010;116(2 pt 1):450-463.
Evidence-based answers from the Family Physicians Inquiries Network
Is arthroscopic subacromial decompression effective for shoulder impingement?
It’s impossible to say for certain in the absence of randomized controlled trials. However, in patients whose impingement symptoms don’t improve after 3 to 6 months, arthroscopic subacromial decompression (ASD) is associated with modest (about 10%) long-term improvement in pain and function compared with open acromioplasty or baseline (strength of recommendation [SOR]: B, cohort studies).
Patients older than 57 years may do better with surgery than physical therapy (SOR: B, single cohort study).
EVIDENCE SUMMARY
Six cohort studies found that patients who underwent ASD for subacromial impingement had improved pain and function scores at 4.5 to 12 years after surgery (TABLE1-7). Weaknesses of the overall data set include use of heterogeneous outcome measures across studies, lack of sham surgical controls, and lack of blinding.
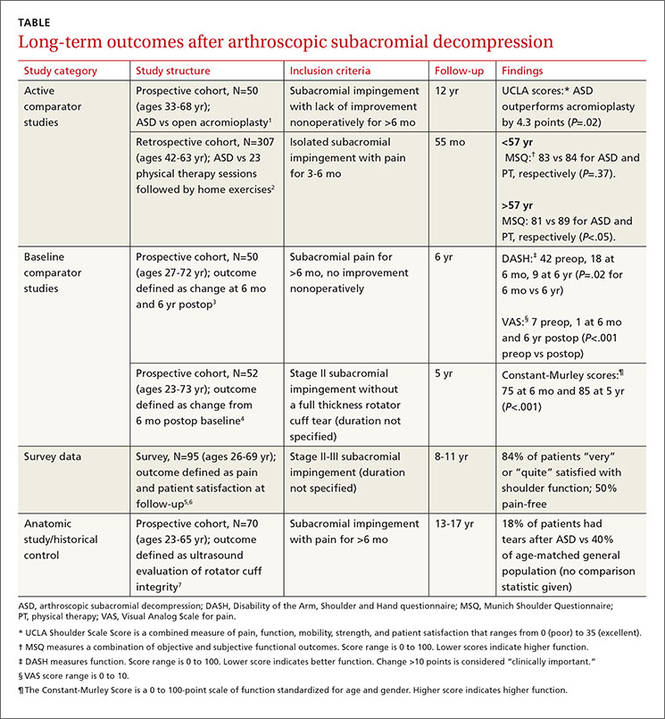
ASD improves pain and function slightly more than other treatments
One prospective and one retrospective cohort trial compared ASD with another intervention. In the prospective trial, ASD was associated with a 10% better combined pain and function score than open acromioplasty at 12 years.1 In the retrospective trial, ASD was also associated with a 10% better combined pain and function score than prolonged physical therapy in patients older than 57 years (the median age of study participants) but not patients younger than 57 years.2
Two other studies found improvements in pain and function
Two other prospective cohort studies didn’t use a comparison group but followed changes in standardized shoulder pain and function scores for 5 to 6 years after ASD. In one study, pain decreased 6 points on a 10-point visual analog scale by 6 months postop (P<.001).3 In both studies, a 9% to 10% improvement in function was seen between 6 months and 5 to 6 years after surgery.3,4
A third cohort study that asked patients about overall pain and satisfaction 8 to 11 years after ASD found that most were “very” or “quite” satisfied and half were pain-free.5,6
Rotator cuff tears found less likely with ASD
An anatomic study obtained ultrasounds of patients 13 to 17 years after ASD and compared the findings to rotator cuff ultrasounds of the general population.7 Patients who had ASD were 22% less likely to demonstrate rotator cuff tears at the end of the study (no statistics were reported to measure significance).
RECOMMENDATIONS
Guidelines from the Washington State Department of Labor and Industry state that patients who should undergo isolated subacromial decompression (with or without acromioplasty) need to have documented subacromial impingement syndrome with magnetic resonance imaging evidence of rotator cuff tendonopathy or tear, have undergone 12 weeks of conservative therapy (including at least active assisted range of motion and home-based exercises), and have had a subacromial injection with a local anesthetic that has provided documented relief of pain.8
No current guidelines are available from national or international orthopedic or sports medicine organizations.
1. Odenbring S, Wagner P, Atroshi I. Long-term outcomes of arthroscopic acromioplasty of chronic shoulder impingement syndrome: a prospective cohort study with a minimum of 12 years’ follow-up. Arthroscopy. 2008;24:1092–1098.
2. Biberthaler P, Beirer M, Kirchhoff S, et al. Significant benefit for older patients after arthroscopic subacromial decompression: a long-term follow-up study. Int Orthop. 2013;37:457–462.
3. Lunsjo K, Bengtsson M, Nordqvist A, et al. Patients with shoulder impingement remain satisfied 6 years after arthroscopic subacromial decompression. Acta Orthop. 2011;82:711–713.
4. Dom K, Van Glabbeek F, Van Riet RP, et al. Arthroscopic subacromial decompression for advanced (stage II) impingement syndrome: a study of 52 patients with 5 year follow-up. Acta Orthop Belg. 2003;69:13–17.
5. Klintberg IH, Karlsson J, Svantesson U. Health-related quality of life, patient satisfaction, and physical activity 8–11 years after arthroscopic subacromial decompression. J Shoulder Elbow Surg. 2011;20:598–608.
6. Klintberg IH, Svantesson U, Karlsson J. Long-term patient satisfaction and functional outcome 8-11 years after subacromial decompression. Knee Surg Sports Traumatol Arthrosc. 2010;18:394–403.
7. Bjornsson H, Norlin R, Knutsson A, et al. Fewer rotator cuff tears fifteen years after arthroscopic subacromial decompression. J Shoulder Elbow Surg. 2010;19:111–115.
8. Washington State Department of Labor and Industries. Shoulder Conditions Diagnosis and Treatment Guideline. Available at: http://www.lni.wa.gov/ClaimsIns/Files/OMD/MedTreat/FINALguidelineShoulderConditionsOct242013.pdf. Accessed October 20, 2015.
It’s impossible to say for certain in the absence of randomized controlled trials. However, in patients whose impingement symptoms don’t improve after 3 to 6 months, arthroscopic subacromial decompression (ASD) is associated with modest (about 10%) long-term improvement in pain and function compared with open acromioplasty or baseline (strength of recommendation [SOR]: B, cohort studies).
Patients older than 57 years may do better with surgery than physical therapy (SOR: B, single cohort study).
EVIDENCE SUMMARY
Six cohort studies found that patients who underwent ASD for subacromial impingement had improved pain and function scores at 4.5 to 12 years after surgery (TABLE1-7). Weaknesses of the overall data set include use of heterogeneous outcome measures across studies, lack of sham surgical controls, and lack of blinding.

ASD improves pain and function slightly more than other treatments
One prospective and one retrospective cohort trial compared ASD with another intervention. In the prospective trial, ASD was associated with a 10% better combined pain and function score than open acromioplasty at 12 years.1 In the retrospective trial, ASD was also associated with a 10% better combined pain and function score than prolonged physical therapy in patients older than 57 years (the median age of study participants) but not patients younger than 57 years.2
Two other studies found improvements in pain and function
Two other prospective cohort studies didn’t use a comparison group but followed changes in standardized shoulder pain and function scores for 5 to 6 years after ASD. In one study, pain decreased 6 points on a 10-point visual analog scale by 6 months postop (P<.001).3 In both studies, a 9% to 10% improvement in function was seen between 6 months and 5 to 6 years after surgery.3,4
A third cohort study that asked patients about overall pain and satisfaction 8 to 11 years after ASD found that most were “very” or “quite” satisfied and half were pain-free.5,6
Rotator cuff tears found less likely with ASD
An anatomic study obtained ultrasounds of patients 13 to 17 years after ASD and compared the findings to rotator cuff ultrasounds of the general population.7 Patients who had ASD were 22% less likely to demonstrate rotator cuff tears at the end of the study (no statistics were reported to measure significance).
RECOMMENDATIONS
Guidelines from the Washington State Department of Labor and Industry state that patients who should undergo isolated subacromial decompression (with or without acromioplasty) need to have documented subacromial impingement syndrome with magnetic resonance imaging evidence of rotator cuff tendonopathy or tear, have undergone 12 weeks of conservative therapy (including at least active assisted range of motion and home-based exercises), and have had a subacromial injection with a local anesthetic that has provided documented relief of pain.8
No current guidelines are available from national or international orthopedic or sports medicine organizations.
It’s impossible to say for certain in the absence of randomized controlled trials. However, in patients whose impingement symptoms don’t improve after 3 to 6 months, arthroscopic subacromial decompression (ASD) is associated with modest (about 10%) long-term improvement in pain and function compared with open acromioplasty or baseline (strength of recommendation [SOR]: B, cohort studies).
Patients older than 57 years may do better with surgery than physical therapy (SOR: B, single cohort study).
EVIDENCE SUMMARY
Six cohort studies found that patients who underwent ASD for subacromial impingement had improved pain and function scores at 4.5 to 12 years after surgery (TABLE1-7). Weaknesses of the overall data set include use of heterogeneous outcome measures across studies, lack of sham surgical controls, and lack of blinding.

ASD improves pain and function slightly more than other treatments
One prospective and one retrospective cohort trial compared ASD with another intervention. In the prospective trial, ASD was associated with a 10% better combined pain and function score than open acromioplasty at 12 years.1 In the retrospective trial, ASD was also associated with a 10% better combined pain and function score than prolonged physical therapy in patients older than 57 years (the median age of study participants) but not patients younger than 57 years.2
Two other studies found improvements in pain and function
Two other prospective cohort studies didn’t use a comparison group but followed changes in standardized shoulder pain and function scores for 5 to 6 years after ASD. In one study, pain decreased 6 points on a 10-point visual analog scale by 6 months postop (P<.001).3 In both studies, a 9% to 10% improvement in function was seen between 6 months and 5 to 6 years after surgery.3,4
A third cohort study that asked patients about overall pain and satisfaction 8 to 11 years after ASD found that most were “very” or “quite” satisfied and half were pain-free.5,6
Rotator cuff tears found less likely with ASD
An anatomic study obtained ultrasounds of patients 13 to 17 years after ASD and compared the findings to rotator cuff ultrasounds of the general population.7 Patients who had ASD were 22% less likely to demonstrate rotator cuff tears at the end of the study (no statistics were reported to measure significance).
RECOMMENDATIONS
Guidelines from the Washington State Department of Labor and Industry state that patients who should undergo isolated subacromial decompression (with or without acromioplasty) need to have documented subacromial impingement syndrome with magnetic resonance imaging evidence of rotator cuff tendonopathy or tear, have undergone 12 weeks of conservative therapy (including at least active assisted range of motion and home-based exercises), and have had a subacromial injection with a local anesthetic that has provided documented relief of pain.8
No current guidelines are available from national or international orthopedic or sports medicine organizations.
1. Odenbring S, Wagner P, Atroshi I. Long-term outcomes of arthroscopic acromioplasty of chronic shoulder impingement syndrome: a prospective cohort study with a minimum of 12 years’ follow-up. Arthroscopy. 2008;24:1092–1098.
2. Biberthaler P, Beirer M, Kirchhoff S, et al. Significant benefit for older patients after arthroscopic subacromial decompression: a long-term follow-up study. Int Orthop. 2013;37:457–462.
3. Lunsjo K, Bengtsson M, Nordqvist A, et al. Patients with shoulder impingement remain satisfied 6 years after arthroscopic subacromial decompression. Acta Orthop. 2011;82:711–713.
4. Dom K, Van Glabbeek F, Van Riet RP, et al. Arthroscopic subacromial decompression for advanced (stage II) impingement syndrome: a study of 52 patients with 5 year follow-up. Acta Orthop Belg. 2003;69:13–17.
5. Klintberg IH, Karlsson J, Svantesson U. Health-related quality of life, patient satisfaction, and physical activity 8–11 years after arthroscopic subacromial decompression. J Shoulder Elbow Surg. 2011;20:598–608.
6. Klintberg IH, Svantesson U, Karlsson J. Long-term patient satisfaction and functional outcome 8-11 years after subacromial decompression. Knee Surg Sports Traumatol Arthrosc. 2010;18:394–403.
7. Bjornsson H, Norlin R, Knutsson A, et al. Fewer rotator cuff tears fifteen years after arthroscopic subacromial decompression. J Shoulder Elbow Surg. 2010;19:111–115.
8. Washington State Department of Labor and Industries. Shoulder Conditions Diagnosis and Treatment Guideline. Available at: http://www.lni.wa.gov/ClaimsIns/Files/OMD/MedTreat/FINALguidelineShoulderConditionsOct242013.pdf. Accessed October 20, 2015.
1. Odenbring S, Wagner P, Atroshi I. Long-term outcomes of arthroscopic acromioplasty of chronic shoulder impingement syndrome: a prospective cohort study with a minimum of 12 years’ follow-up. Arthroscopy. 2008;24:1092–1098.
2. Biberthaler P, Beirer M, Kirchhoff S, et al. Significant benefit for older patients after arthroscopic subacromial decompression: a long-term follow-up study. Int Orthop. 2013;37:457–462.
3. Lunsjo K, Bengtsson M, Nordqvist A, et al. Patients with shoulder impingement remain satisfied 6 years after arthroscopic subacromial decompression. Acta Orthop. 2011;82:711–713.
4. Dom K, Van Glabbeek F, Van Riet RP, et al. Arthroscopic subacromial decompression for advanced (stage II) impingement syndrome: a study of 52 patients with 5 year follow-up. Acta Orthop Belg. 2003;69:13–17.
5. Klintberg IH, Karlsson J, Svantesson U. Health-related quality of life, patient satisfaction, and physical activity 8–11 years after arthroscopic subacromial decompression. J Shoulder Elbow Surg. 2011;20:598–608.
6. Klintberg IH, Svantesson U, Karlsson J. Long-term patient satisfaction and functional outcome 8-11 years after subacromial decompression. Knee Surg Sports Traumatol Arthrosc. 2010;18:394–403.
7. Bjornsson H, Norlin R, Knutsson A, et al. Fewer rotator cuff tears fifteen years after arthroscopic subacromial decompression. J Shoulder Elbow Surg. 2010;19:111–115.
8. Washington State Department of Labor and Industries. Shoulder Conditions Diagnosis and Treatment Guideline. Available at: http://www.lni.wa.gov/ClaimsIns/Files/OMD/MedTreat/FINALguidelineShoulderConditionsOct242013.pdf. Accessed October 20, 2015.
Evidence-based answers from the Family Physicians Inquiries Network
Does high dietary soy intake affect a woman’s risk of primary or recurrent breast cancer?
No, it doesn’t affect the risk of primary breast cancer, but it does (favorably) affect the risk of cancer recurrence.
Compared with diets low in soy, high dietary intake of soy protein or soy isoflavones isn’t associated with any alteration in the risk of developing primary breast cancer (strength of recommendation [SOR]: B, systematic review of prospective cohort studies). In patients with breast cancer, however, consuming a diet high in soy is associated with a 25% decrease in cancer recurrence and a 15% decrease in mortality (SOR: B, prospective cohort studies).
EVIDENCE SUMMARY
A large systematic review evaluated the relationship between dietary soy intake and risk of a primary breast cancer diagnosis. It included 7 prospective cohort studies, which comprised the best quality evidence available (numerous other reviewed studies were of lower quality). The review found no significant association between dietary soy intake and primary breast cancer (TABLE1-6).
Investigators either surveyed women for intake of soy isoflavones or soy foods or products (tofu, soybeans, lentils, miso) or measured urinary or plasma levels of soy isoflavones. They adjusted for age, alcohol use, smoking status, body mass index, caloric intake, and hormone replacement therapy, then followed subjects for 7 to 23 years, comparing the risk of breast cancer for the lowest and highest levels of soy intake.
Six of the prospective cohort studies found no association between soy intake and breast cancer risk; one study, comprising 4% of the total population, found a lower risk with higher soy intake (effect size=0.44; 95% confidence interval [CI], 0.26-0.73; an effect size of 0.2 is considered small, 0.6 medium, and 1.2 large). The authors didn’t do a meta-analysis of the prospective cohort studies.
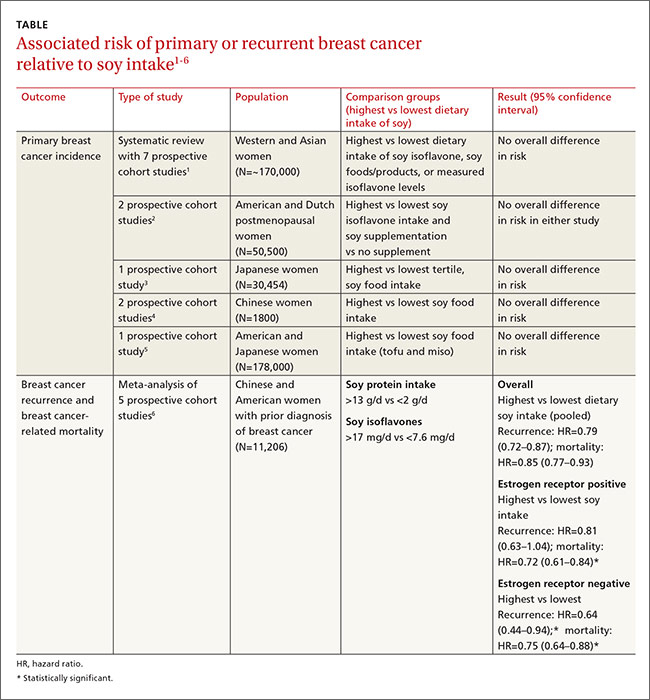
Other cohort studies yield similar findings
Four other large systematic reviews evaluating soy intake and breast cancer risk incorporated a total of 6 individual prospective cohort studies that weren’t included in the previously described review (again, these studies comprised the best quality evidence within the reviews). The 6 studies found no association between soy intake and breast cancer risk.
In 2 of the studies, investigators surveyed postmenopausal women and followed them for 4 to 8 years.2 Investigators in another study adjusted for age, family and gynecologic history, hormone and medication use, exercise, and other factors.3 In 2 other studies, investigators evaluated population subsets that consumed the most vs the fewest servings per week or kilograms per year of soy foods.4 The sixth study compared low with high intake of soy foods and miso.5
Soy intake after breast cancer diagnosis reduces recurrence risk in most studies
Most prospective cohort studies evaluating the association between dietary soy intake after breast cancer diagnosis found an overall 21% decrease in recurrence with high soy intake and a 15% reduction in mortality (TABLE1-6).
Investigators in a meta-analysis of 5 studies that followed women for 4 to 7 years after first breast cancer diagnosis found that higher soy intake was associated with lower mortality but not less recurrence in women who were estrogen receptor positive. Both recurrence and mortality were decreased in estrogen receptor negative women.6
The study also found lower recurrence and mortality in premenopausal women with higher soy intake (recurrence hazard ratio [HR]=0.91; 95% CI, 0.72-1.14; mortality HR=0.78; 95% CI, 0.69-0.88). In postmenopausal women, higher intake was likewise associated with improvement of both outcomes (recurrence HR=0.67; 95% CI, 0.56-0.80; mortality HR=0.81; 95% CI, 0.73-0.91).
An earlier meta-analysis of 4 prospective cohort studies, 2 of which were not included above, also found reduced risk of breast cancer recurrence in groups with high vs low soy isoflavone intake (HR=0.84; 95% CI, 0.70-0.99).7 Women taking tamoxifen showed no difference in mortality or recurrence risk associated with soy intake.
An additional small prospective cohort study (n=256) found similar reductions in recurrence and mortality associated with higher consumption of soy protein.8
1. Chen M, Rao Y, Zheng Y, et al. Association between soy isoflavone intake and breast cancer risk for pre- and post-menopausal women: a meta-analysis of epidemiological studies. PLoS One. 2014;9:e89288.
2. Fritz H, Seely D, Flower G, et al. Soy, red clover, and isoflavones and breast cancer: a systematic review. PLoS One. 2013;8:e81968.
3. Nagata C, Mizoue T, Tanaka K, et al. Soy intake and breast cancer risk: an evaluation based on a systematic review of epidemiologic evidence among the Japanese population. Jpn J Clin Oncol. 2014;44:282–295.
4. Liu XO, Huang YB, Gao Y, et al. Association between dietary factors and breast cancer risk among Chinese females: systematic review and meta-analysis. Asian Pac J Cancer Prev. 2014;15:1291–1298.
5. Qin LQ, Xu JY, Wang PY, et al. Soyfood intake in the prevention of breast cancer risk in women: a meta-analysis of observational epidemiological studies. J Nutr Sci Vitaminol (Tokyo). 2006;52:428–436.
6. Chi F, Wu R, Zeng YC, et al. Post-diagnosis soy food intake and breast cancer survival: a meta-analysis of cohort studies. Asian Pac J Cancer Prev. 2013;14:2407–2412.
7. Dong JY, Qin LQ. Soy isoflavones consumption and risk of breast cancer incidence or recurrence: a meta-analysis of prospective studies. Breast Cancer Res Treat. 2011;125:315-323.
8. Kang HB, Zhang YF, Yang JD, et al. Study on soy isoflavone consumption and risk of breast cancer and survival. Asian Pac J Cancer Prev. 2012;13:995–998.
No, it doesn’t affect the risk of primary breast cancer, but it does (favorably) affect the risk of cancer recurrence.
Compared with diets low in soy, high dietary intake of soy protein or soy isoflavones isn’t associated with any alteration in the risk of developing primary breast cancer (strength of recommendation [SOR]: B, systematic review of prospective cohort studies). In patients with breast cancer, however, consuming a diet high in soy is associated with a 25% decrease in cancer recurrence and a 15% decrease in mortality (SOR: B, prospective cohort studies).
EVIDENCE SUMMARY
A large systematic review evaluated the relationship between dietary soy intake and risk of a primary breast cancer diagnosis. It included 7 prospective cohort studies, which comprised the best quality evidence available (numerous other reviewed studies were of lower quality). The review found no significant association between dietary soy intake and primary breast cancer (TABLE1-6).
Investigators either surveyed women for intake of soy isoflavones or soy foods or products (tofu, soybeans, lentils, miso) or measured urinary or plasma levels of soy isoflavones. They adjusted for age, alcohol use, smoking status, body mass index, caloric intake, and hormone replacement therapy, then followed subjects for 7 to 23 years, comparing the risk of breast cancer for the lowest and highest levels of soy intake.
Six of the prospective cohort studies found no association between soy intake and breast cancer risk; one study, comprising 4% of the total population, found a lower risk with higher soy intake (effect size=0.44; 95% confidence interval [CI], 0.26-0.73; an effect size of 0.2 is considered small, 0.6 medium, and 1.2 large). The authors didn’t do a meta-analysis of the prospective cohort studies.

Other cohort studies yield similar findings
Four other large systematic reviews evaluating soy intake and breast cancer risk incorporated a total of 6 individual prospective cohort studies that weren’t included in the previously described review (again, these studies comprised the best quality evidence within the reviews). The 6 studies found no association between soy intake and breast cancer risk.
In 2 of the studies, investigators surveyed postmenopausal women and followed them for 4 to 8 years.2 Investigators in another study adjusted for age, family and gynecologic history, hormone and medication use, exercise, and other factors.3 In 2 other studies, investigators evaluated population subsets that consumed the most vs the fewest servings per week or kilograms per year of soy foods.4 The sixth study compared low with high intake of soy foods and miso.5
Soy intake after breast cancer diagnosis reduces recurrence risk in most studies
Most prospective cohort studies evaluating the association between dietary soy intake after breast cancer diagnosis found an overall 21% decrease in recurrence with high soy intake and a 15% reduction in mortality (TABLE1-6).
Investigators in a meta-analysis of 5 studies that followed women for 4 to 7 years after first breast cancer diagnosis found that higher soy intake was associated with lower mortality but not less recurrence in women who were estrogen receptor positive. Both recurrence and mortality were decreased in estrogen receptor negative women.6
The study also found lower recurrence and mortality in premenopausal women with higher soy intake (recurrence hazard ratio [HR]=0.91; 95% CI, 0.72-1.14; mortality HR=0.78; 95% CI, 0.69-0.88). In postmenopausal women, higher intake was likewise associated with improvement of both outcomes (recurrence HR=0.67; 95% CI, 0.56-0.80; mortality HR=0.81; 95% CI, 0.73-0.91).
An earlier meta-analysis of 4 prospective cohort studies, 2 of which were not included above, also found reduced risk of breast cancer recurrence in groups with high vs low soy isoflavone intake (HR=0.84; 95% CI, 0.70-0.99).7 Women taking tamoxifen showed no difference in mortality or recurrence risk associated with soy intake.
An additional small prospective cohort study (n=256) found similar reductions in recurrence and mortality associated with higher consumption of soy protein.8
No, it doesn’t affect the risk of primary breast cancer, but it does (favorably) affect the risk of cancer recurrence.
Compared with diets low in soy, high dietary intake of soy protein or soy isoflavones isn’t associated with any alteration in the risk of developing primary breast cancer (strength of recommendation [SOR]: B, systematic review of prospective cohort studies). In patients with breast cancer, however, consuming a diet high in soy is associated with a 25% decrease in cancer recurrence and a 15% decrease in mortality (SOR: B, prospective cohort studies).
EVIDENCE SUMMARY
A large systematic review evaluated the relationship between dietary soy intake and risk of a primary breast cancer diagnosis. It included 7 prospective cohort studies, which comprised the best quality evidence available (numerous other reviewed studies were of lower quality). The review found no significant association between dietary soy intake and primary breast cancer (TABLE1-6).
Investigators either surveyed women for intake of soy isoflavones or soy foods or products (tofu, soybeans, lentils, miso) or measured urinary or plasma levels of soy isoflavones. They adjusted for age, alcohol use, smoking status, body mass index, caloric intake, and hormone replacement therapy, then followed subjects for 7 to 23 years, comparing the risk of breast cancer for the lowest and highest levels of soy intake.
Six of the prospective cohort studies found no association between soy intake and breast cancer risk; one study, comprising 4% of the total population, found a lower risk with higher soy intake (effect size=0.44; 95% confidence interval [CI], 0.26-0.73; an effect size of 0.2 is considered small, 0.6 medium, and 1.2 large). The authors didn’t do a meta-analysis of the prospective cohort studies.

Other cohort studies yield similar findings
Four other large systematic reviews evaluating soy intake and breast cancer risk incorporated a total of 6 individual prospective cohort studies that weren’t included in the previously described review (again, these studies comprised the best quality evidence within the reviews). The 6 studies found no association between soy intake and breast cancer risk.
In 2 of the studies, investigators surveyed postmenopausal women and followed them for 4 to 8 years.2 Investigators in another study adjusted for age, family and gynecologic history, hormone and medication use, exercise, and other factors.3 In 2 other studies, investigators evaluated population subsets that consumed the most vs the fewest servings per week or kilograms per year of soy foods.4 The sixth study compared low with high intake of soy foods and miso.5
Soy intake after breast cancer diagnosis reduces recurrence risk in most studies
Most prospective cohort studies evaluating the association between dietary soy intake after breast cancer diagnosis found an overall 21% decrease in recurrence with high soy intake and a 15% reduction in mortality (TABLE1-6).
Investigators in a meta-analysis of 5 studies that followed women for 4 to 7 years after first breast cancer diagnosis found that higher soy intake was associated with lower mortality but not less recurrence in women who were estrogen receptor positive. Both recurrence and mortality were decreased in estrogen receptor negative women.6
The study also found lower recurrence and mortality in premenopausal women with higher soy intake (recurrence hazard ratio [HR]=0.91; 95% CI, 0.72-1.14; mortality HR=0.78; 95% CI, 0.69-0.88). In postmenopausal women, higher intake was likewise associated with improvement of both outcomes (recurrence HR=0.67; 95% CI, 0.56-0.80; mortality HR=0.81; 95% CI, 0.73-0.91).
An earlier meta-analysis of 4 prospective cohort studies, 2 of which were not included above, also found reduced risk of breast cancer recurrence in groups with high vs low soy isoflavone intake (HR=0.84; 95% CI, 0.70-0.99).7 Women taking tamoxifen showed no difference in mortality or recurrence risk associated with soy intake.
An additional small prospective cohort study (n=256) found similar reductions in recurrence and mortality associated with higher consumption of soy protein.8
1. Chen M, Rao Y, Zheng Y, et al. Association between soy isoflavone intake and breast cancer risk for pre- and post-menopausal women: a meta-analysis of epidemiological studies. PLoS One. 2014;9:e89288.
2. Fritz H, Seely D, Flower G, et al. Soy, red clover, and isoflavones and breast cancer: a systematic review. PLoS One. 2013;8:e81968.
3. Nagata C, Mizoue T, Tanaka K, et al. Soy intake and breast cancer risk: an evaluation based on a systematic review of epidemiologic evidence among the Japanese population. Jpn J Clin Oncol. 2014;44:282–295.
4. Liu XO, Huang YB, Gao Y, et al. Association between dietary factors and breast cancer risk among Chinese females: systematic review and meta-analysis. Asian Pac J Cancer Prev. 2014;15:1291–1298.
5. Qin LQ, Xu JY, Wang PY, et al. Soyfood intake in the prevention of breast cancer risk in women: a meta-analysis of observational epidemiological studies. J Nutr Sci Vitaminol (Tokyo). 2006;52:428–436.
6. Chi F, Wu R, Zeng YC, et al. Post-diagnosis soy food intake and breast cancer survival: a meta-analysis of cohort studies. Asian Pac J Cancer Prev. 2013;14:2407–2412.
7. Dong JY, Qin LQ. Soy isoflavones consumption and risk of breast cancer incidence or recurrence: a meta-analysis of prospective studies. Breast Cancer Res Treat. 2011;125:315-323.
8. Kang HB, Zhang YF, Yang JD, et al. Study on soy isoflavone consumption and risk of breast cancer and survival. Asian Pac J Cancer Prev. 2012;13:995–998.
1. Chen M, Rao Y, Zheng Y, et al. Association between soy isoflavone intake and breast cancer risk for pre- and post-menopausal women: a meta-analysis of epidemiological studies. PLoS One. 2014;9:e89288.
2. Fritz H, Seely D, Flower G, et al. Soy, red clover, and isoflavones and breast cancer: a systematic review. PLoS One. 2013;8:e81968.
3. Nagata C, Mizoue T, Tanaka K, et al. Soy intake and breast cancer risk: an evaluation based on a systematic review of epidemiologic evidence among the Japanese population. Jpn J Clin Oncol. 2014;44:282–295.
4. Liu XO, Huang YB, Gao Y, et al. Association between dietary factors and breast cancer risk among Chinese females: systematic review and meta-analysis. Asian Pac J Cancer Prev. 2014;15:1291–1298.
5. Qin LQ, Xu JY, Wang PY, et al. Soyfood intake in the prevention of breast cancer risk in women: a meta-analysis of observational epidemiological studies. J Nutr Sci Vitaminol (Tokyo). 2006;52:428–436.
6. Chi F, Wu R, Zeng YC, et al. Post-diagnosis soy food intake and breast cancer survival: a meta-analysis of cohort studies. Asian Pac J Cancer Prev. 2013;14:2407–2412.
7. Dong JY, Qin LQ. Soy isoflavones consumption and risk of breast cancer incidence or recurrence: a meta-analysis of prospective studies. Breast Cancer Res Treat. 2011;125:315-323.
8. Kang HB, Zhang YF, Yang JD, et al. Study on soy isoflavone consumption and risk of breast cancer and survival. Asian Pac J Cancer Prev. 2012;13:995–998.
Evidence-based answers from the Family Physicians Inquiries Network
Which interventions can increase breastfeeding duration?
Breastfeeding support, beyond standard care, from lay people or professionals increases both short- and long-term breastfeeding duration (strength of recommendation: B, meta-analyses of randomized controlled trials [RCTs] with demonstrated heterogeneity).
EVIDENCE SUMMARY
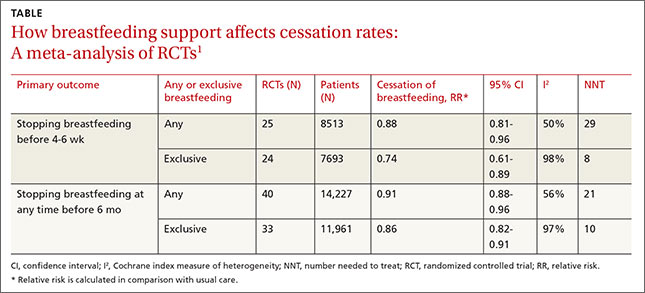
A 2012 Cochrane review of 52 studies (44 RCTs and 8 cluster-randomized trials; N=56,451) assessed the overall effectiveness of multiple supportive measures on decreasing cessation of “any” (partial and exclusive) and “exclusive” breastfeeding compared with usual care.1 Participants were healthy breastfeeding mothers of healthy term babies. Support interventions were defined broadly but included individual and group interactions, as well as contact in person or over the phone by professionals or lay volunteers. Patients were approached proactively or reactively upon request, and the interventions occurred one or more times.
The interventions reduced discontinuation rates among both “exclusive” and “any” breastfeeding mothers (TABLE1). The review found lay and professional support to be equally effective at promoting continuation of breastfeeding. Limitations include a moderate to high amount of heterogeneity, as well as the inherent difficulty of blinding subjects in the studies.
Lay support can make a significant difference in the short term
A 2008 systematic review of 38 RCTs (N=29,020) compared any counseling or behavioral intervention initiated from a clinician’s practice (office or hospital) with usual care.2 The review excluded community and peer-initiated interventions. The reviewers defined breastfeeding duration as follows: initiation (up to 2 weeks), short-term (one to 3 months), intermediate-term (4 to 5 months), long-term (6 to 8 months), and prolonged (9 or more months). Investigators also analyzed breastfeeding rates by “exclusive” and “nonexclusive” (formula supplementation) regimens.
For nonexclusive breastfeeding, the review found interventions to promote breastfeeding improved rates only at initiation (18 RCTs, N=7688; relative risk [RR] for cessation of breastfeeding=1.04; 95% confidence interval [CI], 1.0-1.08; number needed to treat [NNT]=38) and in the short term (18 RCTs, N= 19,358; RR=1.10; 95% CI, 1.02-1.19; NNT=7). For exclusive breastfeeding, interventions improved rates only in the short term (17 RCTs, N=20,552; RR=1.72; 95% CI, 1.0-2.97; NNT=3).
The review found that lay support (defined as counseling or social support from peers) but not professional support was significantly associated with improving rates of both “nonexclusive” and “exclusive’ breastfeeding, but only over the short term (5 RCTs, N not provided; RR=1.22; 95% CI, 1.08-1.37; and 4 RCTs, N not provided; RR=1.65; 95% CI, 1.03-2.63; respectively). As with the Cochrane review, the results for all study groups demonstrated moderate to significant heterogeneity.
RECOMMENDATIONS
The Surgeon General, the American Academy of Family Physicians, the American Academy of Pediatrics, and the American College of Obstetricians and Gynecologists all recommend that women be educated about the benefits of breastfeeding and receive supportive interventions before and after delivery.3-6
1. Renfrew MJ, McCormick FM, Wade A, et al. Support for healthy breastfeeding mothers with healthy term babies. Cochrane Database Syst Rev. 2012;5:CD001141.
2. Chung M, Raman G, Trikalinos T, et al. Interventions in primary care to promote breastfeeding: an evidence review for the U.S. Preventive Services Task Force. Ann Intern Med. 2008;149:565-582.
3. United States Department of Health and Human Services. The Surgeon General’s Call to Action to Support Breastfeeding. US Department of Health and Human Services, Office of the Surgeon General Web site. Available at: http://www.surgeongeneral.gov/library/calls/breastfeeding/. Accessed January 19, 2015.
4. American Academy of Family Physicians. Breastfeeding, Family Physicians Supporting (Position Paper). American Academy of Family Physicians Web site. Available at: http://www.aafp.org/about/policies/all/breastfeeding-support.html (updated Nov. 4, 2014). Accessed January 19, 2015.
5. Johnson M, Landers S, Noble L, et al. American Academy of Pediatrics, Section on Breastfeeding. Policy Statement: Breastfeeding and the use of human milk. Pediatrics. 2012;129:e827–e841.
6. Committee on Health Care for Underserved Women, American College of Obstetricians and Gynecologists. ACOG Committee Opinion No. 361: Breastfeeding: maternal and infant aspects. Obstet Gynecol. 2007;109(2 Pt 1):479-480.
Breastfeeding support, beyond standard care, from lay people or professionals increases both short- and long-term breastfeeding duration (strength of recommendation: B, meta-analyses of randomized controlled trials [RCTs] with demonstrated heterogeneity).
EVIDENCE SUMMARY
A 2012 Cochrane review of 52 studies (44 RCTs and 8 cluster-randomized trials; N=56,451) assessed the overall effectiveness of multiple supportive measures on decreasing cessation of “any” (partial and exclusive) and “exclusive” breastfeeding compared with usual care.1 Participants were healthy breastfeeding mothers of healthy term babies. Support interventions were defined broadly but included individual and group interactions, as well as contact in person or over the phone by professionals or lay volunteers. Patients were approached proactively or reactively upon request, and the interventions occurred one or more times.
The interventions reduced discontinuation rates among both “exclusive” and “any” breastfeeding mothers (TABLE1). The review found lay and professional support to be equally effective at promoting continuation of breastfeeding. Limitations include a moderate to high amount of heterogeneity, as well as the inherent difficulty of blinding subjects in the studies.
Lay support can make a significant difference in the short term
A 2008 systematic review of 38 RCTs (N=29,020) compared any counseling or behavioral intervention initiated from a clinician’s practice (office or hospital) with usual care.2 The review excluded community and peer-initiated interventions. The reviewers defined breastfeeding duration as follows: initiation (up to 2 weeks), short-term (one to 3 months), intermediate-term (4 to 5 months), long-term (6 to 8 months), and prolonged (9 or more months). Investigators also analyzed breastfeeding rates by “exclusive” and “nonexclusive” (formula supplementation) regimens.
For nonexclusive breastfeeding, the review found interventions to promote breastfeeding improved rates only at initiation (18 RCTs, N=7688; relative risk [RR] for cessation of breastfeeding=1.04; 95% confidence interval [CI], 1.0-1.08; number needed to treat [NNT]=38) and in the short term (18 RCTs, N= 19,358; RR=1.10; 95% CI, 1.02-1.19; NNT=7). For exclusive breastfeeding, interventions improved rates only in the short term (17 RCTs, N=20,552; RR=1.72; 95% CI, 1.0-2.97; NNT=3).
The review found that lay support (defined as counseling or social support from peers) but not professional support was significantly associated with improving rates of both “nonexclusive” and “exclusive’ breastfeeding, but only over the short term (5 RCTs, N not provided; RR=1.22; 95% CI, 1.08-1.37; and 4 RCTs, N not provided; RR=1.65; 95% CI, 1.03-2.63; respectively). As with the Cochrane review, the results for all study groups demonstrated moderate to significant heterogeneity.

RECOMMENDATIONS
The Surgeon General, the American Academy of Family Physicians, the American Academy of Pediatrics, and the American College of Obstetricians and Gynecologists all recommend that women be educated about the benefits of breastfeeding and receive supportive interventions before and after delivery.3-6
Breastfeeding support, beyond standard care, from lay people or professionals increases both short- and long-term breastfeeding duration (strength of recommendation: B, meta-analyses of randomized controlled trials [RCTs] with demonstrated heterogeneity).
EVIDENCE SUMMARY
A 2012 Cochrane review of 52 studies (44 RCTs and 8 cluster-randomized trials; N=56,451) assessed the overall effectiveness of multiple supportive measures on decreasing cessation of “any” (partial and exclusive) and “exclusive” breastfeeding compared with usual care.1 Participants were healthy breastfeeding mothers of healthy term babies. Support interventions were defined broadly but included individual and group interactions, as well as contact in person or over the phone by professionals or lay volunteers. Patients were approached proactively or reactively upon request, and the interventions occurred one or more times.
The interventions reduced discontinuation rates among both “exclusive” and “any” breastfeeding mothers (TABLE1). The review found lay and professional support to be equally effective at promoting continuation of breastfeeding. Limitations include a moderate to high amount of heterogeneity, as well as the inherent difficulty of blinding subjects in the studies.
Lay support can make a significant difference in the short term
A 2008 systematic review of 38 RCTs (N=29,020) compared any counseling or behavioral intervention initiated from a clinician’s practice (office or hospital) with usual care.2 The review excluded community and peer-initiated interventions. The reviewers defined breastfeeding duration as follows: initiation (up to 2 weeks), short-term (one to 3 months), intermediate-term (4 to 5 months), long-term (6 to 8 months), and prolonged (9 or more months). Investigators also analyzed breastfeeding rates by “exclusive” and “nonexclusive” (formula supplementation) regimens.
For nonexclusive breastfeeding, the review found interventions to promote breastfeeding improved rates only at initiation (18 RCTs, N=7688; relative risk [RR] for cessation of breastfeeding=1.04; 95% confidence interval [CI], 1.0-1.08; number needed to treat [NNT]=38) and in the short term (18 RCTs, N= 19,358; RR=1.10; 95% CI, 1.02-1.19; NNT=7). For exclusive breastfeeding, interventions improved rates only in the short term (17 RCTs, N=20,552; RR=1.72; 95% CI, 1.0-2.97; NNT=3).
The review found that lay support (defined as counseling or social support from peers) but not professional support was significantly associated with improving rates of both “nonexclusive” and “exclusive’ breastfeeding, but only over the short term (5 RCTs, N not provided; RR=1.22; 95% CI, 1.08-1.37; and 4 RCTs, N not provided; RR=1.65; 95% CI, 1.03-2.63; respectively). As with the Cochrane review, the results for all study groups demonstrated moderate to significant heterogeneity.

RECOMMENDATIONS
The Surgeon General, the American Academy of Family Physicians, the American Academy of Pediatrics, and the American College of Obstetricians and Gynecologists all recommend that women be educated about the benefits of breastfeeding and receive supportive interventions before and after delivery.3-6
1. Renfrew MJ, McCormick FM, Wade A, et al. Support for healthy breastfeeding mothers with healthy term babies. Cochrane Database Syst Rev. 2012;5:CD001141.
2. Chung M, Raman G, Trikalinos T, et al. Interventions in primary care to promote breastfeeding: an evidence review for the U.S. Preventive Services Task Force. Ann Intern Med. 2008;149:565-582.
3. United States Department of Health and Human Services. The Surgeon General’s Call to Action to Support Breastfeeding. US Department of Health and Human Services, Office of the Surgeon General Web site. Available at: http://www.surgeongeneral.gov/library/calls/breastfeeding/. Accessed January 19, 2015.
4. American Academy of Family Physicians. Breastfeeding, Family Physicians Supporting (Position Paper). American Academy of Family Physicians Web site. Available at: http://www.aafp.org/about/policies/all/breastfeeding-support.html (updated Nov. 4, 2014). Accessed January 19, 2015.
5. Johnson M, Landers S, Noble L, et al. American Academy of Pediatrics, Section on Breastfeeding. Policy Statement: Breastfeeding and the use of human milk. Pediatrics. 2012;129:e827–e841.
6. Committee on Health Care for Underserved Women, American College of Obstetricians and Gynecologists. ACOG Committee Opinion No. 361: Breastfeeding: maternal and infant aspects. Obstet Gynecol. 2007;109(2 Pt 1):479-480.
1. Renfrew MJ, McCormick FM, Wade A, et al. Support for healthy breastfeeding mothers with healthy term babies. Cochrane Database Syst Rev. 2012;5:CD001141.
2. Chung M, Raman G, Trikalinos T, et al. Interventions in primary care to promote breastfeeding: an evidence review for the U.S. Preventive Services Task Force. Ann Intern Med. 2008;149:565-582.
3. United States Department of Health and Human Services. The Surgeon General’s Call to Action to Support Breastfeeding. US Department of Health and Human Services, Office of the Surgeon General Web site. Available at: http://www.surgeongeneral.gov/library/calls/breastfeeding/. Accessed January 19, 2015.
4. American Academy of Family Physicians. Breastfeeding, Family Physicians Supporting (Position Paper). American Academy of Family Physicians Web site. Available at: http://www.aafp.org/about/policies/all/breastfeeding-support.html (updated Nov. 4, 2014). Accessed January 19, 2015.
5. Johnson M, Landers S, Noble L, et al. American Academy of Pediatrics, Section on Breastfeeding. Policy Statement: Breastfeeding and the use of human milk. Pediatrics. 2012;129:e827–e841.
6. Committee on Health Care for Underserved Women, American College of Obstetricians and Gynecologists. ACOG Committee Opinion No. 361: Breastfeeding: maternal and infant aspects. Obstet Gynecol. 2007;109(2 Pt 1):479-480.
Evidence-based answers from the Family Physicians Inquiries Network
How effective are opioids for chronic low back pain?
Short-term (<4 months) treatment with opioids provides modest relief of chronic low back pain, but only minimal improvement in function compared with placebo (strength of recommendation [SOR]: B, systematic review of lower-quality randomized controlled trials [RCTs]).
Tramadol isn’t superior to nonsteroidal anti-inflammatory drugs (NSAIDs) for pain relief (SOR: A, consistent results from RCTs). In addition, oxycodone with titrated morphine isn’t better than naproxen for relieving pain or improving function (SOR: C, a low-quality RCT).
Although no long-term RCTs have been done, cohort studies have shown that 6 to 12 months of opioid use is associated with a small decrease in pain and either very minimal improvement in, or worsening of, disability (SOR: B, prospective cohort trials).
EVIDENCE SUMMARY
A systematic review and meta-analysis of 15 RCTs with a total enrollment of 5540 assessed the efficacy of opioids in adults with chronic low back pain of at least 12 weeks’ duration.1 Five low-quality studies (1378 patients) that compared tramadol with placebo found tramadol to be moderately superior to placebo for relieving pain (standard mean difference [SMD]= -0.55; 95% confidence interval [CI], -0.66 to -0.44) but only modestly better for improving function (SMD= −0.18; 95% CI, -0.29 to -0.07).
Six trials with 1887 patients compared strong opioids (morphine, hydromorphone, oxycodone, oxymorphone, and tapentadol) with placebo. The opioids were better than placebo for improving pain (SMD= -0.43; 95% CI, -0.52 to -0.33) and function (SMD= -0.26; 95% CI, -0.37 to -0.15). The general interpretation of SMD effect size is 0.2=small, 0.5=medium, 0.8=large. In this case, larger negative numbers correlate with greater improvement.
How opioids stack up against NSAIDs
Two separate double-blind, double-dummy studies randomized adults with low back pain of at least 12 weeks’ duration to receive celecoxib 200 mg twice daily (404 and 398 patients, respectively) or tramadol 50 mg 4 times daily (392 and 404 patients, respectively) for 6 weeks.2 The primary outcome measure was at least a 30% improvement in pain using a 0 (no pain) to 10 (worst possible pain) scale. In both studies, more patients taking celecoxib had positive responses than patients taking tramadol (63% vs 50%, P<.001, and 64% vs 55%, P<.008, respectively).
A small RCT (36 patients who had suffered low back pain for more than 6 months) randomized patients to one of 3 treatment groups for 16 weeks: oxycodone as much as 20 mg/d (13 patients); naproxen as much as 1 g/d (12 patients); or oxycodone and sustained-release morphine (titrated up to 200 mg morphine equivalent/d (11 patients).3 After 16 weeks, patients receiving oxycodone or naproxen were treated with oxycodone and sustained-release morphine for another 16 weeks, as were patients already receiving this therapy. Pain was assessed on a 0 (none) to 100 (worst possible pain) scale.
Both opioid groups had significantly less pain on average (59.8 for oxycodone, 54.9 for titrated morphine) than the naproxen group (65.5; F=16.07; P<.001) but no significant difference in activity level. However, an independent analysis of the naproxen group and titrated morphine group found no significant difference in either pain relief (SMD= -0.58; 95% CI, -1.42 to 0.26) or disability (SMD= -0.06; 95% CI, -0.88 to 0.76) between the 2 groups.4
How does long-term opioid use affect pain and function?
Two prospective cohort studies have evaluated long-term opioid use. The first (715 patients) used a Roland-Morris Disability Questionnaire (RMDQ) to assess disability at 6 months in patients taking opioids compared with patients not taking opioids.5 Patients using opioids showed an increase in RMDQ score of 1.18 units (95% CI, 0.17-2.19) on a 0 to 24 scale, with 24 representing greatest disability.
The second study evaluated pain and function in 1843 adults with acute back injuries taking opioids for a year.6 Pain, rated on a 0 to 10 scale, decreased from 7.7 at baseline to 6.8 at one year (no P value). At the end of the first quarter, the RMDQ score decreased from 18.8 at baseline (the end of the first quarter) to 17.5 at one year (no P value). Clinically meaningful improvement in pain and function (30% or more) occurred in 26% (95% CI, 18%-36%) and 16% (95% CI, 10%-25%) of patients, respectively.
RECOMMENDATIONS
The 2007 clinical practice guideline on low back pain from The American College of Physicians and American Pain Society recommends opioids, including tramadol, for patients with severe back pain who don’t get adequate relief from acetaminophen or NSAIDs.7
The 2009 National Institute for Health and Care Excellence (NICE) guidelines for early management of persistent, nonspecific low back pain recommend considering strong opioids (buprenorphine, fentanyl, and oxycodone) for short-term use in severe pain and referral to a specialist for patients requiring prolonged use of strong opioids.8
The 2013 British Pain Society guidelines for low back and radicular pain recommend tight restrictions on the use of strong opioids. They also recommend giving the lowest possible dose of opioids for the shortest time possible.9
1. Chaparro LE, Furlan AD, Deshpande A, et al. Opioids compared with placebo or other treatments for chronic low back pain: an update of the Cochrane Review. Spine (Phila Pa 1976). 2014;39:556-563.
2. O’Donnell JB, Ekman EF, Spalding WM, et al. The effectiveness of a weak opioid medication versus a cyclo-oxygenase-2 (COX-2) selective non-steroidal anti-inflammatory drug in treating flare-up of chronic low-back pain: results from two randomized, double-blind, 6-week studies. J Int Med Res. 2009;37:1789-1802.
3. Jamison RN, Raymond SA, Slawsby EA, et al. Opioid therapy for chronic noncancer back pain. A randomized prospective study. Spine (Phila Pa 1976). 1998;23:2591-2600.
4. White AP, Arnold PM, Norvell DC, et al. Pharmacologic management of chronic low back pain: synthesis of the evidence. Spine (Phila Pa 1976). 2011;36(21 Suppl):S131-S43.
5. Ashworth J, Green DJ, Dunn KM, et al. Opioid use among low back pain patients in primary care: Is opioid prescription associated with disability at 6-month follow-up? Pain. 2013;154:1038-1044.
6. Franklin GM, Rahman EA, Turner JA, et al. Opioid use for chronic low back pain: A prospective, population-based study among injured workers in Washington state, 2002-2005. Clin J Pain. 2009;25:743-751.
7. Chou R, Qaseem A, Snow V, et al; Clinical Efficacy Assessment Subcommittee of the American College of Physicians; American College of Physicians; American Pain Society Low Back Pain Guidelines Panel. Diagnosis and treatment of low back pain: a joint clinical practice guideline from the American College of Physicians and the American Pain Society. Ann Intern Med. 2007;147:478-491.
8. National Institute for Health and Care Excellence. Low back pain: early management of persistent non-specific low back pain. National Institute for Health and Care Excellence Web site. Available at: http://guidance.nice.org.uk/CG88. Accessed April 1, 2015.
9. Lee J, Gupta S, Price C, et al; British Pain Society. Low back and radicular pain: a pathway for care developed by the British Pain Society. Br J Anaesth. 2013;111:112-120.
Short-term (<4 months) treatment with opioids provides modest relief of chronic low back pain, but only minimal improvement in function compared with placebo (strength of recommendation [SOR]: B, systematic review of lower-quality randomized controlled trials [RCTs]).
Tramadol isn’t superior to nonsteroidal anti-inflammatory drugs (NSAIDs) for pain relief (SOR: A, consistent results from RCTs). In addition, oxycodone with titrated morphine isn’t better than naproxen for relieving pain or improving function (SOR: C, a low-quality RCT).
Although no long-term RCTs have been done, cohort studies have shown that 6 to 12 months of opioid use is associated with a small decrease in pain and either very minimal improvement in, or worsening of, disability (SOR: B, prospective cohort trials).
EVIDENCE SUMMARY
A systematic review and meta-analysis of 15 RCTs with a total enrollment of 5540 assessed the efficacy of opioids in adults with chronic low back pain of at least 12 weeks’ duration.1 Five low-quality studies (1378 patients) that compared tramadol with placebo found tramadol to be moderately superior to placebo for relieving pain (standard mean difference [SMD]= -0.55; 95% confidence interval [CI], -0.66 to -0.44) but only modestly better for improving function (SMD= −0.18; 95% CI, -0.29 to -0.07).
Six trials with 1887 patients compared strong opioids (morphine, hydromorphone, oxycodone, oxymorphone, and tapentadol) with placebo. The opioids were better than placebo for improving pain (SMD= -0.43; 95% CI, -0.52 to -0.33) and function (SMD= -0.26; 95% CI, -0.37 to -0.15). The general interpretation of SMD effect size is 0.2=small, 0.5=medium, 0.8=large. In this case, larger negative numbers correlate with greater improvement.
How opioids stack up against NSAIDs
Two separate double-blind, double-dummy studies randomized adults with low back pain of at least 12 weeks’ duration to receive celecoxib 200 mg twice daily (404 and 398 patients, respectively) or tramadol 50 mg 4 times daily (392 and 404 patients, respectively) for 6 weeks.2 The primary outcome measure was at least a 30% improvement in pain using a 0 (no pain) to 10 (worst possible pain) scale. In both studies, more patients taking celecoxib had positive responses than patients taking tramadol (63% vs 50%, P<.001, and 64% vs 55%, P<.008, respectively).
A small RCT (36 patients who had suffered low back pain for more than 6 months) randomized patients to one of 3 treatment groups for 16 weeks: oxycodone as much as 20 mg/d (13 patients); naproxen as much as 1 g/d (12 patients); or oxycodone and sustained-release morphine (titrated up to 200 mg morphine equivalent/d (11 patients).3 After 16 weeks, patients receiving oxycodone or naproxen were treated with oxycodone and sustained-release morphine for another 16 weeks, as were patients already receiving this therapy. Pain was assessed on a 0 (none) to 100 (worst possible pain) scale.
Both opioid groups had significantly less pain on average (59.8 for oxycodone, 54.9 for titrated morphine) than the naproxen group (65.5; F=16.07; P<.001) but no significant difference in activity level. However, an independent analysis of the naproxen group and titrated morphine group found no significant difference in either pain relief (SMD= -0.58; 95% CI, -1.42 to 0.26) or disability (SMD= -0.06; 95% CI, -0.88 to 0.76) between the 2 groups.4
How does long-term opioid use affect pain and function?
Two prospective cohort studies have evaluated long-term opioid use. The first (715 patients) used a Roland-Morris Disability Questionnaire (RMDQ) to assess disability at 6 months in patients taking opioids compared with patients not taking opioids.5 Patients using opioids showed an increase in RMDQ score of 1.18 units (95% CI, 0.17-2.19) on a 0 to 24 scale, with 24 representing greatest disability.
The second study evaluated pain and function in 1843 adults with acute back injuries taking opioids for a year.6 Pain, rated on a 0 to 10 scale, decreased from 7.7 at baseline to 6.8 at one year (no P value). At the end of the first quarter, the RMDQ score decreased from 18.8 at baseline (the end of the first quarter) to 17.5 at one year (no P value). Clinically meaningful improvement in pain and function (30% or more) occurred in 26% (95% CI, 18%-36%) and 16% (95% CI, 10%-25%) of patients, respectively.
RECOMMENDATIONS
The 2007 clinical practice guideline on low back pain from The American College of Physicians and American Pain Society recommends opioids, including tramadol, for patients with severe back pain who don’t get adequate relief from acetaminophen or NSAIDs.7
The 2009 National Institute for Health and Care Excellence (NICE) guidelines for early management of persistent, nonspecific low back pain recommend considering strong opioids (buprenorphine, fentanyl, and oxycodone) for short-term use in severe pain and referral to a specialist for patients requiring prolonged use of strong opioids.8
The 2013 British Pain Society guidelines for low back and radicular pain recommend tight restrictions on the use of strong opioids. They also recommend giving the lowest possible dose of opioids for the shortest time possible.9
Short-term (<4 months) treatment with opioids provides modest relief of chronic low back pain, but only minimal improvement in function compared with placebo (strength of recommendation [SOR]: B, systematic review of lower-quality randomized controlled trials [RCTs]).
Tramadol isn’t superior to nonsteroidal anti-inflammatory drugs (NSAIDs) for pain relief (SOR: A, consistent results from RCTs). In addition, oxycodone with titrated morphine isn’t better than naproxen for relieving pain or improving function (SOR: C, a low-quality RCT).
Although no long-term RCTs have been done, cohort studies have shown that 6 to 12 months of opioid use is associated with a small decrease in pain and either very minimal improvement in, or worsening of, disability (SOR: B, prospective cohort trials).
EVIDENCE SUMMARY
A systematic review and meta-analysis of 15 RCTs with a total enrollment of 5540 assessed the efficacy of opioids in adults with chronic low back pain of at least 12 weeks’ duration.1 Five low-quality studies (1378 patients) that compared tramadol with placebo found tramadol to be moderately superior to placebo for relieving pain (standard mean difference [SMD]= -0.55; 95% confidence interval [CI], -0.66 to -0.44) but only modestly better for improving function (SMD= −0.18; 95% CI, -0.29 to -0.07).
Six trials with 1887 patients compared strong opioids (morphine, hydromorphone, oxycodone, oxymorphone, and tapentadol) with placebo. The opioids were better than placebo for improving pain (SMD= -0.43; 95% CI, -0.52 to -0.33) and function (SMD= -0.26; 95% CI, -0.37 to -0.15). The general interpretation of SMD effect size is 0.2=small, 0.5=medium, 0.8=large. In this case, larger negative numbers correlate with greater improvement.
How opioids stack up against NSAIDs
Two separate double-blind, double-dummy studies randomized adults with low back pain of at least 12 weeks’ duration to receive celecoxib 200 mg twice daily (404 and 398 patients, respectively) or tramadol 50 mg 4 times daily (392 and 404 patients, respectively) for 6 weeks.2 The primary outcome measure was at least a 30% improvement in pain using a 0 (no pain) to 10 (worst possible pain) scale. In both studies, more patients taking celecoxib had positive responses than patients taking tramadol (63% vs 50%, P<.001, and 64% vs 55%, P<.008, respectively).
A small RCT (36 patients who had suffered low back pain for more than 6 months) randomized patients to one of 3 treatment groups for 16 weeks: oxycodone as much as 20 mg/d (13 patients); naproxen as much as 1 g/d (12 patients); or oxycodone and sustained-release morphine (titrated up to 200 mg morphine equivalent/d (11 patients).3 After 16 weeks, patients receiving oxycodone or naproxen were treated with oxycodone and sustained-release morphine for another 16 weeks, as were patients already receiving this therapy. Pain was assessed on a 0 (none) to 100 (worst possible pain) scale.
Both opioid groups had significantly less pain on average (59.8 for oxycodone, 54.9 for titrated morphine) than the naproxen group (65.5; F=16.07; P<.001) but no significant difference in activity level. However, an independent analysis of the naproxen group and titrated morphine group found no significant difference in either pain relief (SMD= -0.58; 95% CI, -1.42 to 0.26) or disability (SMD= -0.06; 95% CI, -0.88 to 0.76) between the 2 groups.4
How does long-term opioid use affect pain and function?
Two prospective cohort studies have evaluated long-term opioid use. The first (715 patients) used a Roland-Morris Disability Questionnaire (RMDQ) to assess disability at 6 months in patients taking opioids compared with patients not taking opioids.5 Patients using opioids showed an increase in RMDQ score of 1.18 units (95% CI, 0.17-2.19) on a 0 to 24 scale, with 24 representing greatest disability.
The second study evaluated pain and function in 1843 adults with acute back injuries taking opioids for a year.6 Pain, rated on a 0 to 10 scale, decreased from 7.7 at baseline to 6.8 at one year (no P value). At the end of the first quarter, the RMDQ score decreased from 18.8 at baseline (the end of the first quarter) to 17.5 at one year (no P value). Clinically meaningful improvement in pain and function (30% or more) occurred in 26% (95% CI, 18%-36%) and 16% (95% CI, 10%-25%) of patients, respectively.
RECOMMENDATIONS
The 2007 clinical practice guideline on low back pain from The American College of Physicians and American Pain Society recommends opioids, including tramadol, for patients with severe back pain who don’t get adequate relief from acetaminophen or NSAIDs.7
The 2009 National Institute for Health and Care Excellence (NICE) guidelines for early management of persistent, nonspecific low back pain recommend considering strong opioids (buprenorphine, fentanyl, and oxycodone) for short-term use in severe pain and referral to a specialist for patients requiring prolonged use of strong opioids.8
The 2013 British Pain Society guidelines for low back and radicular pain recommend tight restrictions on the use of strong opioids. They also recommend giving the lowest possible dose of opioids for the shortest time possible.9
1. Chaparro LE, Furlan AD, Deshpande A, et al. Opioids compared with placebo or other treatments for chronic low back pain: an update of the Cochrane Review. Spine (Phila Pa 1976). 2014;39:556-563.
2. O’Donnell JB, Ekman EF, Spalding WM, et al. The effectiveness of a weak opioid medication versus a cyclo-oxygenase-2 (COX-2) selective non-steroidal anti-inflammatory drug in treating flare-up of chronic low-back pain: results from two randomized, double-blind, 6-week studies. J Int Med Res. 2009;37:1789-1802.
3. Jamison RN, Raymond SA, Slawsby EA, et al. Opioid therapy for chronic noncancer back pain. A randomized prospective study. Spine (Phila Pa 1976). 1998;23:2591-2600.
4. White AP, Arnold PM, Norvell DC, et al. Pharmacologic management of chronic low back pain: synthesis of the evidence. Spine (Phila Pa 1976). 2011;36(21 Suppl):S131-S43.
5. Ashworth J, Green DJ, Dunn KM, et al. Opioid use among low back pain patients in primary care: Is opioid prescription associated with disability at 6-month follow-up? Pain. 2013;154:1038-1044.
6. Franklin GM, Rahman EA, Turner JA, et al. Opioid use for chronic low back pain: A prospective, population-based study among injured workers in Washington state, 2002-2005. Clin J Pain. 2009;25:743-751.
7. Chou R, Qaseem A, Snow V, et al; Clinical Efficacy Assessment Subcommittee of the American College of Physicians; American College of Physicians; American Pain Society Low Back Pain Guidelines Panel. Diagnosis and treatment of low back pain: a joint clinical practice guideline from the American College of Physicians and the American Pain Society. Ann Intern Med. 2007;147:478-491.
8. National Institute for Health and Care Excellence. Low back pain: early management of persistent non-specific low back pain. National Institute for Health and Care Excellence Web site. Available at: http://guidance.nice.org.uk/CG88. Accessed April 1, 2015.
9. Lee J, Gupta S, Price C, et al; British Pain Society. Low back and radicular pain: a pathway for care developed by the British Pain Society. Br J Anaesth. 2013;111:112-120.
1. Chaparro LE, Furlan AD, Deshpande A, et al. Opioids compared with placebo or other treatments for chronic low back pain: an update of the Cochrane Review. Spine (Phila Pa 1976). 2014;39:556-563.
2. O’Donnell JB, Ekman EF, Spalding WM, et al. The effectiveness of a weak opioid medication versus a cyclo-oxygenase-2 (COX-2) selective non-steroidal anti-inflammatory drug in treating flare-up of chronic low-back pain: results from two randomized, double-blind, 6-week studies. J Int Med Res. 2009;37:1789-1802.
3. Jamison RN, Raymond SA, Slawsby EA, et al. Opioid therapy for chronic noncancer back pain. A randomized prospective study. Spine (Phila Pa 1976). 1998;23:2591-2600.
4. White AP, Arnold PM, Norvell DC, et al. Pharmacologic management of chronic low back pain: synthesis of the evidence. Spine (Phila Pa 1976). 2011;36(21 Suppl):S131-S43.
5. Ashworth J, Green DJ, Dunn KM, et al. Opioid use among low back pain patients in primary care: Is opioid prescription associated with disability at 6-month follow-up? Pain. 2013;154:1038-1044.
6. Franklin GM, Rahman EA, Turner JA, et al. Opioid use for chronic low back pain: A prospective, population-based study among injured workers in Washington state, 2002-2005. Clin J Pain. 2009;25:743-751.
7. Chou R, Qaseem A, Snow V, et al; Clinical Efficacy Assessment Subcommittee of the American College of Physicians; American College of Physicians; American Pain Society Low Back Pain Guidelines Panel. Diagnosis and treatment of low back pain: a joint clinical practice guideline from the American College of Physicians and the American Pain Society. Ann Intern Med. 2007;147:478-491.
8. National Institute for Health and Care Excellence. Low back pain: early management of persistent non-specific low back pain. National Institute for Health and Care Excellence Web site. Available at: http://guidance.nice.org.uk/CG88. Accessed April 1, 2015.
9. Lee J, Gupta S, Price C, et al; British Pain Society. Low back and radicular pain: a pathway for care developed by the British Pain Society. Br J Anaesth. 2013;111:112-120.
Evidence-based answers from the Family Physicians Inquiries Network
Does qHPV vaccine prevent anal intraepithelial neoplasia and condylomata in men?
Yes. Quadrivalent human papillomavirus (qHPV) vaccine reduces rates of anal intraepithelial neoplasia (AIN) by 50% to 54%, and persistent anal infection by 59%, associated with the 4 types of HPV in the vaccine (6, 11, 16, and 18) in young men who have sex with men (MSM); it also reduces external genital lesions by 66%, and persistent HPV infection associated with the same 4 HPV types by 48 to 59% in all young men, heterosexual men,and MSM (strength of recommendation [SOR]: B, randomized, placebo-controlled trials [RCTs]).
In addition, the vaccine is associated with a 50% to 55% decrease in recurrent high-grade AIN and anogenital condylomatain older MSM (SOR: B, cohort studies).
EVIDENCE SUMMARY
Two RCTs that evaluated qHPV in young men for preventing outcomes associated with the 4 HPV subtypes in the vaccine (6, 11, 16, and 18) found that it reduced them by 50% to 66% using an intention-to-treat protocol (TABLE1-4).
Vaccination reduces AIN and persistent infection in MSM
The first RCT evaluated a subset of 602 MSM from the second, larger RCT for preventing AIN and persistent HPV infection.1 The intention-to-treat population included men with 5 or fewer lifetime sexual partners who had engaged in insertive or receptive anal intercourse or oral sex within the last year, were not necessarily HPV-negative at enrollment, and received at least one dose of vaccine (or placebo).
The vaccine reduced AIN associated with the 4 HPV types (6.3 vs 12.6 events per 100 person-years; relative risk reduction [RRR]=50.3%; 95% confidence interval [CI], 25.7-67.2; number needed to treat [NNT]=16 to prevent one AIN case per year) and with HPV of any type (13 vs 17.5 events per 100 person-years; RRR=25.7%; 95% CI, -1.1 to 45.6). It also reduced the rate of persistent HPV infection with the 4 HPV vaccine subtypes (8.8 vs 21.6 events per 100 person-years; RRR=59.4%; 95% CI, 43%-71%; NNT=8 to prevent one persistent HPV infection per year).
Investigators in the study also evaluated vaccine efficacy in a smaller subset (194 men) using per-protocol analysis and found higher prevention rates (78% for AIN due to HPV types 6, 11, 16, and 18). Investigators followed these subjects every 6 months for 36 months with polymerase chain reaction testing for HPV DNA, high-resolution anoscopy with anal cytology, and anal biopsy and histology if there were atypia.
The vaccine decreases persistent HPV infection and external genital lesions
The second RCT, including both MSM and heterosexual men, found that qHPV vaccine reduced rates of persistent HPV infection by 48%, and external genital lesions (condylomata or intraepithelial neoplasia involving the penis, perineum, or perianal area) by 66% associated with HPV types 6, 11, 16, and 18 using the intention-to-treat protocol.2
Investigators used the same protocols used in the first RCT, and the per-protocol population again had higher prevention rates (84% for any HPV type, 90% against the 4 vaccine types). The only adverse effect of the vaccine was injection site pain (57% vs 51% with placebo; P<.001).
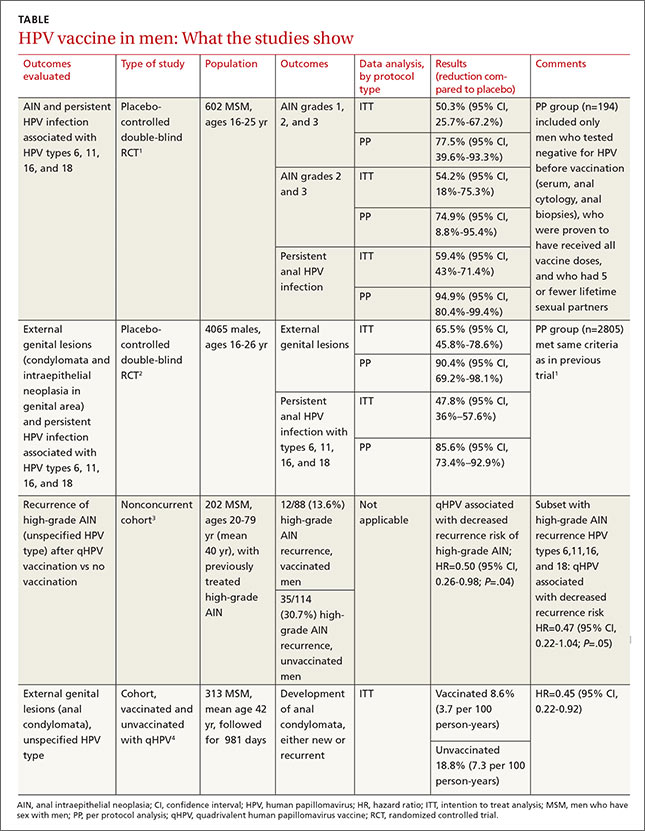
The vaccine also helps older MSM
A nonconcurrent cohort study that evaluated qHPV vaccination among older MSM with previously treated high-grade AIN found a 50% decrease in recurrence rates in the 2 years after vaccination.3 Investigators recruited HIV-negative men, some of whom chose vaccination (not randomized), and followed them for 2 years. Study limitations included using medical records for data collection and the predominance of white, nonsmoking men with private insurance.
A post-hoc analysis of older men without previous anal condylomata (210 men) or with treated condylomata and no recurrence in the year before vaccination (103 men) found that qHPV vaccination was associated with 55% lower rates of anal condylomata.4
RECOMMENDATIONS
The Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices recommends routine use of qHPV vaccine in males ages 11 through 21 years, and optional use in unvaccinated men as old as 26 years.5
1. Palefsky JM, Giuliano AR, Goldstone S, et al. HPV vaccine against anal HPV infection and anal intraepithelial neoplasia. N Engl J Med. 2011;365:1576-1585.
2. Giuliano AR, Palefsky JM, Goldstone S, et al. Efficacy of quadrivalent HPV vaccine against HPV infection and disease in males. N Engl J Med. 2011;364:401-411.
3. Swedish KA, Factor SH, Goldstone SE. Prevention of recurrent high-grade anal neoplasia with quadrivalent human papillomavirus vaccination of men who have sex with men: a nonconcurrent cohort study. Clin Infect Dis. 2012;54:891-898.
4. Swedish KA, Goldstone SE. Prevention of anal condyloma with quadrivalent human papillomavirus vaccination of older men who have sex with men. PLoS One. 2014;9:e93393.
5. Markowitz LE, Dunne EF, Saraiya M, et al. Human papillomavirus vaccination: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2014;63(RR-05):1-30.
Yes. Quadrivalent human papillomavirus (qHPV) vaccine reduces rates of anal intraepithelial neoplasia (AIN) by 50% to 54%, and persistent anal infection by 59%, associated with the 4 types of HPV in the vaccine (6, 11, 16, and 18) in young men who have sex with men (MSM); it also reduces external genital lesions by 66%, and persistent HPV infection associated with the same 4 HPV types by 48 to 59% in all young men, heterosexual men,and MSM (strength of recommendation [SOR]: B, randomized, placebo-controlled trials [RCTs]).
In addition, the vaccine is associated with a 50% to 55% decrease in recurrent high-grade AIN and anogenital condylomatain older MSM (SOR: B, cohort studies).
EVIDENCE SUMMARY
Two RCTs that evaluated qHPV in young men for preventing outcomes associated with the 4 HPV subtypes in the vaccine (6, 11, 16, and 18) found that it reduced them by 50% to 66% using an intention-to-treat protocol (TABLE1-4).
Vaccination reduces AIN and persistent infection in MSM
The first RCT evaluated a subset of 602 MSM from the second, larger RCT for preventing AIN and persistent HPV infection.1 The intention-to-treat population included men with 5 or fewer lifetime sexual partners who had engaged in insertive or receptive anal intercourse or oral sex within the last year, were not necessarily HPV-negative at enrollment, and received at least one dose of vaccine (or placebo).
The vaccine reduced AIN associated with the 4 HPV types (6.3 vs 12.6 events per 100 person-years; relative risk reduction [RRR]=50.3%; 95% confidence interval [CI], 25.7-67.2; number needed to treat [NNT]=16 to prevent one AIN case per year) and with HPV of any type (13 vs 17.5 events per 100 person-years; RRR=25.7%; 95% CI, -1.1 to 45.6). It also reduced the rate of persistent HPV infection with the 4 HPV vaccine subtypes (8.8 vs 21.6 events per 100 person-years; RRR=59.4%; 95% CI, 43%-71%; NNT=8 to prevent one persistent HPV infection per year).
Investigators in the study also evaluated vaccine efficacy in a smaller subset (194 men) using per-protocol analysis and found higher prevention rates (78% for AIN due to HPV types 6, 11, 16, and 18). Investigators followed these subjects every 6 months for 36 months with polymerase chain reaction testing for HPV DNA, high-resolution anoscopy with anal cytology, and anal biopsy and histology if there were atypia.
The vaccine decreases persistent HPV infection and external genital lesions
The second RCT, including both MSM and heterosexual men, found that qHPV vaccine reduced rates of persistent HPV infection by 48%, and external genital lesions (condylomata or intraepithelial neoplasia involving the penis, perineum, or perianal area) by 66% associated with HPV types 6, 11, 16, and 18 using the intention-to-treat protocol.2
Investigators used the same protocols used in the first RCT, and the per-protocol population again had higher prevention rates (84% for any HPV type, 90% against the 4 vaccine types). The only adverse effect of the vaccine was injection site pain (57% vs 51% with placebo; P<.001).

The vaccine also helps older MSM
A nonconcurrent cohort study that evaluated qHPV vaccination among older MSM with previously treated high-grade AIN found a 50% decrease in recurrence rates in the 2 years after vaccination.3 Investigators recruited HIV-negative men, some of whom chose vaccination (not randomized), and followed them for 2 years. Study limitations included using medical records for data collection and the predominance of white, nonsmoking men with private insurance.
A post-hoc analysis of older men without previous anal condylomata (210 men) or with treated condylomata and no recurrence in the year before vaccination (103 men) found that qHPV vaccination was associated with 55% lower rates of anal condylomata.4
RECOMMENDATIONS
The Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices recommends routine use of qHPV vaccine in males ages 11 through 21 years, and optional use in unvaccinated men as old as 26 years.5
Yes. Quadrivalent human papillomavirus (qHPV) vaccine reduces rates of anal intraepithelial neoplasia (AIN) by 50% to 54%, and persistent anal infection by 59%, associated with the 4 types of HPV in the vaccine (6, 11, 16, and 18) in young men who have sex with men (MSM); it also reduces external genital lesions by 66%, and persistent HPV infection associated with the same 4 HPV types by 48 to 59% in all young men, heterosexual men,and MSM (strength of recommendation [SOR]: B, randomized, placebo-controlled trials [RCTs]).
In addition, the vaccine is associated with a 50% to 55% decrease in recurrent high-grade AIN and anogenital condylomatain older MSM (SOR: B, cohort studies).
EVIDENCE SUMMARY
Two RCTs that evaluated qHPV in young men for preventing outcomes associated with the 4 HPV subtypes in the vaccine (6, 11, 16, and 18) found that it reduced them by 50% to 66% using an intention-to-treat protocol (TABLE1-4).
Vaccination reduces AIN and persistent infection in MSM
The first RCT evaluated a subset of 602 MSM from the second, larger RCT for preventing AIN and persistent HPV infection.1 The intention-to-treat population included men with 5 or fewer lifetime sexual partners who had engaged in insertive or receptive anal intercourse or oral sex within the last year, were not necessarily HPV-negative at enrollment, and received at least one dose of vaccine (or placebo).
The vaccine reduced AIN associated with the 4 HPV types (6.3 vs 12.6 events per 100 person-years; relative risk reduction [RRR]=50.3%; 95% confidence interval [CI], 25.7-67.2; number needed to treat [NNT]=16 to prevent one AIN case per year) and with HPV of any type (13 vs 17.5 events per 100 person-years; RRR=25.7%; 95% CI, -1.1 to 45.6). It also reduced the rate of persistent HPV infection with the 4 HPV vaccine subtypes (8.8 vs 21.6 events per 100 person-years; RRR=59.4%; 95% CI, 43%-71%; NNT=8 to prevent one persistent HPV infection per year).
Investigators in the study also evaluated vaccine efficacy in a smaller subset (194 men) using per-protocol analysis and found higher prevention rates (78% for AIN due to HPV types 6, 11, 16, and 18). Investigators followed these subjects every 6 months for 36 months with polymerase chain reaction testing for HPV DNA, high-resolution anoscopy with anal cytology, and anal biopsy and histology if there were atypia.
The vaccine decreases persistent HPV infection and external genital lesions
The second RCT, including both MSM and heterosexual men, found that qHPV vaccine reduced rates of persistent HPV infection by 48%, and external genital lesions (condylomata or intraepithelial neoplasia involving the penis, perineum, or perianal area) by 66% associated with HPV types 6, 11, 16, and 18 using the intention-to-treat protocol.2
Investigators used the same protocols used in the first RCT, and the per-protocol population again had higher prevention rates (84% for any HPV type, 90% against the 4 vaccine types). The only adverse effect of the vaccine was injection site pain (57% vs 51% with placebo; P<.001).

The vaccine also helps older MSM
A nonconcurrent cohort study that evaluated qHPV vaccination among older MSM with previously treated high-grade AIN found a 50% decrease in recurrence rates in the 2 years after vaccination.3 Investigators recruited HIV-negative men, some of whom chose vaccination (not randomized), and followed them for 2 years. Study limitations included using medical records for data collection and the predominance of white, nonsmoking men with private insurance.
A post-hoc analysis of older men without previous anal condylomata (210 men) or with treated condylomata and no recurrence in the year before vaccination (103 men) found that qHPV vaccination was associated with 55% lower rates of anal condylomata.4
RECOMMENDATIONS
The Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices recommends routine use of qHPV vaccine in males ages 11 through 21 years, and optional use in unvaccinated men as old as 26 years.5
1. Palefsky JM, Giuliano AR, Goldstone S, et al. HPV vaccine against anal HPV infection and anal intraepithelial neoplasia. N Engl J Med. 2011;365:1576-1585.
2. Giuliano AR, Palefsky JM, Goldstone S, et al. Efficacy of quadrivalent HPV vaccine against HPV infection and disease in males. N Engl J Med. 2011;364:401-411.
3. Swedish KA, Factor SH, Goldstone SE. Prevention of recurrent high-grade anal neoplasia with quadrivalent human papillomavirus vaccination of men who have sex with men: a nonconcurrent cohort study. Clin Infect Dis. 2012;54:891-898.
4. Swedish KA, Goldstone SE. Prevention of anal condyloma with quadrivalent human papillomavirus vaccination of older men who have sex with men. PLoS One. 2014;9:e93393.
5. Markowitz LE, Dunne EF, Saraiya M, et al. Human papillomavirus vaccination: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2014;63(RR-05):1-30.
1. Palefsky JM, Giuliano AR, Goldstone S, et al. HPV vaccine against anal HPV infection and anal intraepithelial neoplasia. N Engl J Med. 2011;365:1576-1585.
2. Giuliano AR, Palefsky JM, Goldstone S, et al. Efficacy of quadrivalent HPV vaccine against HPV infection and disease in males. N Engl J Med. 2011;364:401-411.
3. Swedish KA, Factor SH, Goldstone SE. Prevention of recurrent high-grade anal neoplasia with quadrivalent human papillomavirus vaccination of men who have sex with men: a nonconcurrent cohort study. Clin Infect Dis. 2012;54:891-898.
4. Swedish KA, Goldstone SE. Prevention of anal condyloma with quadrivalent human papillomavirus vaccination of older men who have sex with men. PLoS One. 2014;9:e93393.
5. Markowitz LE, Dunne EF, Saraiya M, et al. Human papillomavirus vaccination: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2014;63(RR-05):1-30.
Evidence-based answers from the Family Physicians Inquiries Network
What’s the best test for underlying osteomyelitis in patients with diabetic foot ulcers?
Magnetic resonance imaging (MRI) has a higher sensitivity and specificity (90% and 79%) than plain radiography (54% and 68%) for diagnosing diabetic foot osteomyelitis. MRI performs somewhat better than any of several common tests—probe to bone (PTB), erythrocyte sedimentation rate (ESR) >70 mm/hr, C-reactive protein (CRP) >14 mg/L, procalcitonin >0.3 ng/mL, and ulcer size >2 cm2—although PTB has the highest specificity of any test and is commonly used together with MRI. No studies have directly compared MRI with a combination of these tests, which may assist in diagnosis (strength of recommendation [SOR]: B, meta-analysis of cohort trials and individual cohort and case control trial).
Experts recommend obtaining plain films when considering diabetic foot ulcers to evaluate for bony abnormalities, soft tissue gas, and foreign body; MRI should be considered in most situations when infection is suspected (SOR: B, evidence-based guidelines).
EVIDENCE SUMMARY
One-fifth of patients with diabetes who have foot ulcerations will develop osteomyelitis.1,2 Most cases of diabetic foot osteomyelitis result from the spread of a foot infection to underlying bone.2
MRI has highest sensitivity, probe to bone test is most specific
A meta-analysis3 of 9 cohort trials (8 prospective, 1 retrospective) of 612 patients with diabetes and a foot ulcer examined the accuracy of diagnostic methods for osteomyelitis (TABLE3,4). MRI had the highest sensitivity (90%), followed by bone scan (81%). Bone scan was the least specific (28%), however. Plain film radiography had the lowest sensitivity (54%). A PTB test was highly specific (91%) but had moderate sensitivity (60%). (PTB involves inserting a sterile, blunt stainless steel probe into an ulcerated lesion. If the probe comes to a hard stop, considered to be bone, the test is positive.)
A meta-analysis of 21 prospective and retrospective trials with 1027 diabetic patients with foot ulcers or suspected osteomyelitis found that ulcer size >2 cm2, PTB, and ESR >70 mm/hr were helpful in making the diagnosis.4
Combining ESR with ulcer size increases specificity
A prospective trial of 46 diabetic patients hospitalized with a foot infection examined the accuracy of a combination of clinical and laboratory diagnostic features in patients with diabetic foot osteomyelitis that had been diagnosed by MRI or histopathology.5 (Twenty-four patients had osteomyelitis, and 22 didn’t.)
ESR >70 mm/hr had a sensitivity of 83% and specificity of 77% (positive likelihood ratio [LR+]=3.6; negative likelihood ratio [LR−]=0.22). Ulcer size >2 cm2 had a sensitivity of 88% and specificity of 77% (LR+=3.8; LR−=0.16). Combined, an ESR >70 mm/hr and ulcer size >2cm2 had a slightly better specificity than either finding alone, 82%, but a lower sensitivity of 79% (LR+=4.4; LR−= 0.26).
Serum markers accurately distinguish osteomyelitis from infection
An individual prospective cohort trial of 61 adult patients with diabetes and a foot infection, published after the meta-analysis4 described previously, examined the accuracy of serum markers (ESR, CRP, procalcitonin) for diagnosing osteomyelitis.6 A positive PTB test and imaging study (plain film, MRI, or nuclear scintigraphy) were used as the diagnostic gold standard.
Thirty-four patients had a soft tissue infection and 27 had osteomyelitis. All markers were higher in patients with osteomyelitis than in patients with a soft tissue infection (ESR=76 mm/hr vs 66 mm/hr; P<.001; CRP=25 mg/L vs 8.7 mg/L; P<.001; procalcitonin=2.4 ng/mL vs 0.71 ng/mL; P<.001). The sensitivity and specificity for each marker at its optimum points were: ESR >67 mm/hr (sensitivity 84%; specificity 75%; LR+=3.4; LR−=0.21); CRP >14 mg/L (sensitivity 85%; specificity 83%; LR+=5; LR−=0.18); and procalcitonin >0.3 ng/mL (sensitivity 81%; specificity 71%; LR+=2.8; LR−=0.27).
RECOMMENDATIONS
The Infectious Diseases Society of America (IDSA) recommends performing the PTB test on any diabetic foot infection with an open wound (level of evidence: strong moderate).7 It also recommends performing plain radiography on all patients presenting with a new infection to evaluate for bony abnormalities, soft tissue gas, and foreign bodies (level of evidence: strong moderate).
The IDSA, the American College of Radiology diagnostic imaging expert panel, and the National Institute for Health and Clinical Excellence recommend using MRI in most clinical scenarios when osteomyelitis is suspected (level of evidence: strong moderate).8,9
1. Gemechu FW, Seemant F, Curley CA. Diabetic foot infections. Am Fam Physician. 2013;88:177-184.
2. Lavery LA, Armstrong DG, Peters EJ, et al. Probe-to-bone test for diagnosing diabetic foot osteomyelitis: reliable or relic? Diabetes Care. 2007;30:270-274.
3. Dinh MT, Abad CL, Safdar N. Diagnostic accuracy of the physical examination and imaging tests for osteomyelitis underlying diabetic foot ulcers: meta-analysis. Clin Infect Dis. 2008;47:519-527.
4. Butalia S, Palda VA, Sargeant RJ, et al. Does this patient with diabetes have osteomyelitis of the lower extremity? JAMA. 2008;299:806-813.
5. Ertugrul BM, Savk O, Ozturk B, et al. The diagnosis of diabetic foot osteomyelitis: examination findings and laboratory values. Med Sci Monit. 2009;15:CR307-CR312.
6. Michail M, Jude E, Liaskos C, et al. The performance of serum inflammatory markers for the diagnosis and follow-up of patients with osteomyelitis. Int J Low Extrem Wounds. 2013;12:94-99.
7. Lipsky BA, Berendt AR, Cornia PB, et al. 2012 Infectious Diseases Society of America clinical practice guideline for the diagnosis and treatment of diabetic foot infections. Clin Infect Dis. 2012;54:e132-e173.
8. Schweitzer ME, Daffner RH, Weissman BN, et al. ACR Appropriateness Criteria on suspected osteomyelitis in patients with diabetes mellitus. J Am Coll Radiol. 2008;5:881-886.
9. Tan T, Shaw EJ, Siddiqui F, et al; Guideline Development Group. Inpatient management of diabetic foot problems: summary of NICE guidance. BMJ. 2011;342:d1280.
Magnetic resonance imaging (MRI) has a higher sensitivity and specificity (90% and 79%) than plain radiography (54% and 68%) for diagnosing diabetic foot osteomyelitis. MRI performs somewhat better than any of several common tests—probe to bone (PTB), erythrocyte sedimentation rate (ESR) >70 mm/hr, C-reactive protein (CRP) >14 mg/L, procalcitonin >0.3 ng/mL, and ulcer size >2 cm2—although PTB has the highest specificity of any test and is commonly used together with MRI. No studies have directly compared MRI with a combination of these tests, which may assist in diagnosis (strength of recommendation [SOR]: B, meta-analysis of cohort trials and individual cohort and case control trial).
Experts recommend obtaining plain films when considering diabetic foot ulcers to evaluate for bony abnormalities, soft tissue gas, and foreign body; MRI should be considered in most situations when infection is suspected (SOR: B, evidence-based guidelines).
EVIDENCE SUMMARY
One-fifth of patients with diabetes who have foot ulcerations will develop osteomyelitis.1,2 Most cases of diabetic foot osteomyelitis result from the spread of a foot infection to underlying bone.2
MRI has highest sensitivity, probe to bone test is most specific
A meta-analysis3 of 9 cohort trials (8 prospective, 1 retrospective) of 612 patients with diabetes and a foot ulcer examined the accuracy of diagnostic methods for osteomyelitis (TABLE3,4). MRI had the highest sensitivity (90%), followed by bone scan (81%). Bone scan was the least specific (28%), however. Plain film radiography had the lowest sensitivity (54%). A PTB test was highly specific (91%) but had moderate sensitivity (60%). (PTB involves inserting a sterile, blunt stainless steel probe into an ulcerated lesion. If the probe comes to a hard stop, considered to be bone, the test is positive.)

A meta-analysis of 21 prospective and retrospective trials with 1027 diabetic patients with foot ulcers or suspected osteomyelitis found that ulcer size >2 cm2, PTB, and ESR >70 mm/hr were helpful in making the diagnosis.4
Combining ESR with ulcer size increases specificity
A prospective trial of 46 diabetic patients hospitalized with a foot infection examined the accuracy of a combination of clinical and laboratory diagnostic features in patients with diabetic foot osteomyelitis that had been diagnosed by MRI or histopathology.5 (Twenty-four patients had osteomyelitis, and 22 didn’t.)
ESR >70 mm/hr had a sensitivity of 83% and specificity of 77% (positive likelihood ratio [LR+]=3.6; negative likelihood ratio [LR−]=0.22). Ulcer size >2 cm2 had a sensitivity of 88% and specificity of 77% (LR+=3.8; LR−=0.16). Combined, an ESR >70 mm/hr and ulcer size >2cm2 had a slightly better specificity than either finding alone, 82%, but a lower sensitivity of 79% (LR+=4.4; LR−= 0.26).
Serum markers accurately distinguish osteomyelitis from infection
An individual prospective cohort trial of 61 adult patients with diabetes and a foot infection, published after the meta-analysis4 described previously, examined the accuracy of serum markers (ESR, CRP, procalcitonin) for diagnosing osteomyelitis.6 A positive PTB test and imaging study (plain film, MRI, or nuclear scintigraphy) were used as the diagnostic gold standard.
Thirty-four patients had a soft tissue infection and 27 had osteomyelitis. All markers were higher in patients with osteomyelitis than in patients with a soft tissue infection (ESR=76 mm/hr vs 66 mm/hr; P<.001; CRP=25 mg/L vs 8.7 mg/L; P<.001; procalcitonin=2.4 ng/mL vs 0.71 ng/mL; P<.001). The sensitivity and specificity for each marker at its optimum points were: ESR >67 mm/hr (sensitivity 84%; specificity 75%; LR+=3.4; LR−=0.21); CRP >14 mg/L (sensitivity 85%; specificity 83%; LR+=5; LR−=0.18); and procalcitonin >0.3 ng/mL (sensitivity 81%; specificity 71%; LR+=2.8; LR−=0.27).
RECOMMENDATIONS
The Infectious Diseases Society of America (IDSA) recommends performing the PTB test on any diabetic foot infection with an open wound (level of evidence: strong moderate).7 It also recommends performing plain radiography on all patients presenting with a new infection to evaluate for bony abnormalities, soft tissue gas, and foreign bodies (level of evidence: strong moderate).
The IDSA, the American College of Radiology diagnostic imaging expert panel, and the National Institute for Health and Clinical Excellence recommend using MRI in most clinical scenarios when osteomyelitis is suspected (level of evidence: strong moderate).8,9
Magnetic resonance imaging (MRI) has a higher sensitivity and specificity (90% and 79%) than plain radiography (54% and 68%) for diagnosing diabetic foot osteomyelitis. MRI performs somewhat better than any of several common tests—probe to bone (PTB), erythrocyte sedimentation rate (ESR) >70 mm/hr, C-reactive protein (CRP) >14 mg/L, procalcitonin >0.3 ng/mL, and ulcer size >2 cm2—although PTB has the highest specificity of any test and is commonly used together with MRI. No studies have directly compared MRI with a combination of these tests, which may assist in diagnosis (strength of recommendation [SOR]: B, meta-analysis of cohort trials and individual cohort and case control trial).
Experts recommend obtaining plain films when considering diabetic foot ulcers to evaluate for bony abnormalities, soft tissue gas, and foreign body; MRI should be considered in most situations when infection is suspected (SOR: B, evidence-based guidelines).
EVIDENCE SUMMARY
One-fifth of patients with diabetes who have foot ulcerations will develop osteomyelitis.1,2 Most cases of diabetic foot osteomyelitis result from the spread of a foot infection to underlying bone.2
MRI has highest sensitivity, probe to bone test is most specific
A meta-analysis3 of 9 cohort trials (8 prospective, 1 retrospective) of 612 patients with diabetes and a foot ulcer examined the accuracy of diagnostic methods for osteomyelitis (TABLE3,4). MRI had the highest sensitivity (90%), followed by bone scan (81%). Bone scan was the least specific (28%), however. Plain film radiography had the lowest sensitivity (54%). A PTB test was highly specific (91%) but had moderate sensitivity (60%). (PTB involves inserting a sterile, blunt stainless steel probe into an ulcerated lesion. If the probe comes to a hard stop, considered to be bone, the test is positive.)

A meta-analysis of 21 prospective and retrospective trials with 1027 diabetic patients with foot ulcers or suspected osteomyelitis found that ulcer size >2 cm2, PTB, and ESR >70 mm/hr were helpful in making the diagnosis.4
Combining ESR with ulcer size increases specificity
A prospective trial of 46 diabetic patients hospitalized with a foot infection examined the accuracy of a combination of clinical and laboratory diagnostic features in patients with diabetic foot osteomyelitis that had been diagnosed by MRI or histopathology.5 (Twenty-four patients had osteomyelitis, and 22 didn’t.)
ESR >70 mm/hr had a sensitivity of 83% and specificity of 77% (positive likelihood ratio [LR+]=3.6; negative likelihood ratio [LR−]=0.22). Ulcer size >2 cm2 had a sensitivity of 88% and specificity of 77% (LR+=3.8; LR−=0.16). Combined, an ESR >70 mm/hr and ulcer size >2cm2 had a slightly better specificity than either finding alone, 82%, but a lower sensitivity of 79% (LR+=4.4; LR−= 0.26).
Serum markers accurately distinguish osteomyelitis from infection
An individual prospective cohort trial of 61 adult patients with diabetes and a foot infection, published after the meta-analysis4 described previously, examined the accuracy of serum markers (ESR, CRP, procalcitonin) for diagnosing osteomyelitis.6 A positive PTB test and imaging study (plain film, MRI, or nuclear scintigraphy) were used as the diagnostic gold standard.
Thirty-four patients had a soft tissue infection and 27 had osteomyelitis. All markers were higher in patients with osteomyelitis than in patients with a soft tissue infection (ESR=76 mm/hr vs 66 mm/hr; P<.001; CRP=25 mg/L vs 8.7 mg/L; P<.001; procalcitonin=2.4 ng/mL vs 0.71 ng/mL; P<.001). The sensitivity and specificity for each marker at its optimum points were: ESR >67 mm/hr (sensitivity 84%; specificity 75%; LR+=3.4; LR−=0.21); CRP >14 mg/L (sensitivity 85%; specificity 83%; LR+=5; LR−=0.18); and procalcitonin >0.3 ng/mL (sensitivity 81%; specificity 71%; LR+=2.8; LR−=0.27).
RECOMMENDATIONS
The Infectious Diseases Society of America (IDSA) recommends performing the PTB test on any diabetic foot infection with an open wound (level of evidence: strong moderate).7 It also recommends performing plain radiography on all patients presenting with a new infection to evaluate for bony abnormalities, soft tissue gas, and foreign bodies (level of evidence: strong moderate).
The IDSA, the American College of Radiology diagnostic imaging expert panel, and the National Institute for Health and Clinical Excellence recommend using MRI in most clinical scenarios when osteomyelitis is suspected (level of evidence: strong moderate).8,9
1. Gemechu FW, Seemant F, Curley CA. Diabetic foot infections. Am Fam Physician. 2013;88:177-184.
2. Lavery LA, Armstrong DG, Peters EJ, et al. Probe-to-bone test for diagnosing diabetic foot osteomyelitis: reliable or relic? Diabetes Care. 2007;30:270-274.
3. Dinh MT, Abad CL, Safdar N. Diagnostic accuracy of the physical examination and imaging tests for osteomyelitis underlying diabetic foot ulcers: meta-analysis. Clin Infect Dis. 2008;47:519-527.
4. Butalia S, Palda VA, Sargeant RJ, et al. Does this patient with diabetes have osteomyelitis of the lower extremity? JAMA. 2008;299:806-813.
5. Ertugrul BM, Savk O, Ozturk B, et al. The diagnosis of diabetic foot osteomyelitis: examination findings and laboratory values. Med Sci Monit. 2009;15:CR307-CR312.
6. Michail M, Jude E, Liaskos C, et al. The performance of serum inflammatory markers for the diagnosis and follow-up of patients with osteomyelitis. Int J Low Extrem Wounds. 2013;12:94-99.
7. Lipsky BA, Berendt AR, Cornia PB, et al. 2012 Infectious Diseases Society of America clinical practice guideline for the diagnosis and treatment of diabetic foot infections. Clin Infect Dis. 2012;54:e132-e173.
8. Schweitzer ME, Daffner RH, Weissman BN, et al. ACR Appropriateness Criteria on suspected osteomyelitis in patients with diabetes mellitus. J Am Coll Radiol. 2008;5:881-886.
9. Tan T, Shaw EJ, Siddiqui F, et al; Guideline Development Group. Inpatient management of diabetic foot problems: summary of NICE guidance. BMJ. 2011;342:d1280.
1. Gemechu FW, Seemant F, Curley CA. Diabetic foot infections. Am Fam Physician. 2013;88:177-184.
2. Lavery LA, Armstrong DG, Peters EJ, et al. Probe-to-bone test for diagnosing diabetic foot osteomyelitis: reliable or relic? Diabetes Care. 2007;30:270-274.
3. Dinh MT, Abad CL, Safdar N. Diagnostic accuracy of the physical examination and imaging tests for osteomyelitis underlying diabetic foot ulcers: meta-analysis. Clin Infect Dis. 2008;47:519-527.
4. Butalia S, Palda VA, Sargeant RJ, et al. Does this patient with diabetes have osteomyelitis of the lower extremity? JAMA. 2008;299:806-813.
5. Ertugrul BM, Savk O, Ozturk B, et al. The diagnosis of diabetic foot osteomyelitis: examination findings and laboratory values. Med Sci Monit. 2009;15:CR307-CR312.
6. Michail M, Jude E, Liaskos C, et al. The performance of serum inflammatory markers for the diagnosis and follow-up of patients with osteomyelitis. Int J Low Extrem Wounds. 2013;12:94-99.
7. Lipsky BA, Berendt AR, Cornia PB, et al. 2012 Infectious Diseases Society of America clinical practice guideline for the diagnosis and treatment of diabetic foot infections. Clin Infect Dis. 2012;54:e132-e173.
8. Schweitzer ME, Daffner RH, Weissman BN, et al. ACR Appropriateness Criteria on suspected osteomyelitis in patients with diabetes mellitus. J Am Coll Radiol. 2008;5:881-886.
9. Tan T, Shaw EJ, Siddiqui F, et al; Guideline Development Group. Inpatient management of diabetic foot problems: summary of NICE guidance. BMJ. 2011;342:d1280.
Evidence-based answers from the Family Physicians Inquiries Network
Do statins increase the risk of developing diabetes?
Yes. Statin therapy produces a small increase in the incidence of diabetes: one additional case per 255 patients taking statins over 4 years (strength of recommendation [SOR]: A, meta-analysis). Intensive statin therapy, compared with moderate therapy, produces an additional 2 cases of diabetes per 1000 patient years (SOR: B, meta-analysis with significant heterogeneity among trials).
EVIDENCE SUMMARY
A meta-analysis of 13 randomized, placebo or standard of care-controlled statin trials (113,148 patients, 81% without diabetes at enrollment, mean ages 55-76 years) found that statin therapy increased the incidence of diabetes by 9% over 4 years (odds ratio [OR]=1.09; 95% confidence interval [CI], 1.02-1.17), or one additional case per 255 patients.1 The increased risk was similar for lipophilic (pravastatin, rosuvastatin) and hydrophilic (atorvastatin, simvastatin, lovastatin) statins, although the analysis wasn’t adjusted for doses used.
In a meta-regression analysis, baseline body mass index or percentage change in low-density lipoprotein cholesterol didn’t appear to confer additional risk. The risk of diabetes with statins was generally higher in studies with older patients (data given graphically).
Higher statin doses mean higher risk
A meta-analysis of 5 placebo and standard-of-care randomized controlled trials (39,612 patients, 83% without diabetes at enrollment, mean age 58-64 years) found that the risk of diabetes was higher with higher-dose statins.2 Therapy with atorvastatin 80 mg or simvastatin 40 to 80 mg was defined as intensive. Treatment with simvastatin 20 to 40 mg, atorvastatin 10 mg, or pravastatin 40 mg was defined as moderate.
At a mean follow-up of 4.9 years, intensive statin therapy was associated with a higher risk of developing diabetes than moderate therapy (OR=1.12; 95% CI, 1.04-1.22) with 2 additional cases of diabetes per 1000 patient-years in the intensive therapy group. The authors noted significant heterogeneity between trials with regard to major cardiovascular events.
Similar results were found in a subsequent population-based cohort study of 471,250 nondiabetic patients older than 66 years who were newly prescribed a statin.3 The study authors used the incidence of new diabetes in patients taking pravastatin as the baseline, since it had been associated with reduced rates of diabetes in a large cardiovascular prevention trial.4 Without adjusting for dose, patients were at significantly higher risk of diabetes if prescribed atorvastatin (hazard ratio [HR]=1.22; 95% CI, 1.15-1.29), rosuvastatin (HR=1.18; 95% CI, 1.10-1.26), or simvastatin (HR=1.10; 95% CI, 1.04-1.17) compared with pravastatin. The risk with fluvastatin and lovastatin was similar to pravastatin.
A subanalysis that compared moderate- and high-dose statin therapy with low-dose therapy (atorvastatin <20 mg, rosuvastatin <10 mg, simvastatin <80 mg, or any dose of fluvastatin, lovastatin, or pravastatin) found a 22% increased risk of diabetes (HR=1.22; 95% CI, 1.19-1.26) for moderate-dose therapy (atorvastatin 20-79 mg, rosuvastatin 10-39 mg, or simvastatin >80 mg) and a 30% increased risk (HR=1.3; 95% CI, 1.2-1.4) for high-dose therapy (atorvastatin ≥80 mg or rosuvastatin ≥40 mg).
A cohort trial also shows increased diabetes risk
A smaller subsequent cohort trial based on data from Taiwan National Health Insurance records compared 8412 nondiabetic adult patients (mean age 63 years) taking statins with 33,648 age- and risk-matched controls not taking statins over a mean duration of 7.2 years.5 Statin use was associated with a 15% higher risk of developing diabetes (HR=1.15; 95% CI, 1.08-1.22).
RECOMMENDATIONS
The 2013 American College of Cardiology/American Heart Association guidelines for lipid-lowering therapy recommend that patients taking statins be screened for diabetes according to current screening recommendations.6 The guidelines advise encouraging patients who develop diabetes while on statin therapy to adhere to a heart-healthy dietary pattern, engage in physical activity, achieve and maintain a healthy body weight, cease tobacco use, and continue statin therapy to reduce the risk of cardiovascular events.
1. Sattar N, Preiss D, Murray HM, et al. Statins and risk of incident diabetes: a collaborative meta-analysis of randomised statin trials. Lancet. 2010;375:735-742.
2. Preiss D, Seshasai SR, Welsh P, et al. Risk of incident diabetes with intensive-dose compared with moderatedose statin therapy: a meta-analysis. JAMA. 2011;305:2556-2564.
3. Carter AA, Gomes T, Camacho X, et al. Risk of incident diabetes among patients treated with statins: population-based study. BMJ. 2013;346:f2610.
4. Freeman DJ, Morrie J, Sattar N, et al. Pravastatin and the development of diabetes mellitus: evidence for a protective treatment effect in the West of Scotland Coronary Prevention Study. Circulation. 2001;103:357-362.
5. Wang KL, Liu CJ, Chao TF, et al. Statins, risk of diabetes and implications on outcomes in the general population. J Am Coll Cardiol. 2012;60:1231-1238.
6. Stone NJ, Robinson JG, Lichtenstein AH, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129:S1-S45.
Yes. Statin therapy produces a small increase in the incidence of diabetes: one additional case per 255 patients taking statins over 4 years (strength of recommendation [SOR]: A, meta-analysis). Intensive statin therapy, compared with moderate therapy, produces an additional 2 cases of diabetes per 1000 patient years (SOR: B, meta-analysis with significant heterogeneity among trials).
EVIDENCE SUMMARY
A meta-analysis of 13 randomized, placebo or standard of care-controlled statin trials (113,148 patients, 81% without diabetes at enrollment, mean ages 55-76 years) found that statin therapy increased the incidence of diabetes by 9% over 4 years (odds ratio [OR]=1.09; 95% confidence interval [CI], 1.02-1.17), or one additional case per 255 patients.1 The increased risk was similar for lipophilic (pravastatin, rosuvastatin) and hydrophilic (atorvastatin, simvastatin, lovastatin) statins, although the analysis wasn’t adjusted for doses used.
In a meta-regression analysis, baseline body mass index or percentage change in low-density lipoprotein cholesterol didn’t appear to confer additional risk. The risk of diabetes with statins was generally higher in studies with older patients (data given graphically).
Higher statin doses mean higher risk
A meta-analysis of 5 placebo and standard-of-care randomized controlled trials (39,612 patients, 83% without diabetes at enrollment, mean age 58-64 years) found that the risk of diabetes was higher with higher-dose statins.2 Therapy with atorvastatin 80 mg or simvastatin 40 to 80 mg was defined as intensive. Treatment with simvastatin 20 to 40 mg, atorvastatin 10 mg, or pravastatin 40 mg was defined as moderate.
At a mean follow-up of 4.9 years, intensive statin therapy was associated with a higher risk of developing diabetes than moderate therapy (OR=1.12; 95% CI, 1.04-1.22) with 2 additional cases of diabetes per 1000 patient-years in the intensive therapy group. The authors noted significant heterogeneity between trials with regard to major cardiovascular events.
Similar results were found in a subsequent population-based cohort study of 471,250 nondiabetic patients older than 66 years who were newly prescribed a statin.3 The study authors used the incidence of new diabetes in patients taking pravastatin as the baseline, since it had been associated with reduced rates of diabetes in a large cardiovascular prevention trial.4 Without adjusting for dose, patients were at significantly higher risk of diabetes if prescribed atorvastatin (hazard ratio [HR]=1.22; 95% CI, 1.15-1.29), rosuvastatin (HR=1.18; 95% CI, 1.10-1.26), or simvastatin (HR=1.10; 95% CI, 1.04-1.17) compared with pravastatin. The risk with fluvastatin and lovastatin was similar to pravastatin.
A subanalysis that compared moderate- and high-dose statin therapy with low-dose therapy (atorvastatin <20 mg, rosuvastatin <10 mg, simvastatin <80 mg, or any dose of fluvastatin, lovastatin, or pravastatin) found a 22% increased risk of diabetes (HR=1.22; 95% CI, 1.19-1.26) for moderate-dose therapy (atorvastatin 20-79 mg, rosuvastatin 10-39 mg, or simvastatin >80 mg) and a 30% increased risk (HR=1.3; 95% CI, 1.2-1.4) for high-dose therapy (atorvastatin ≥80 mg or rosuvastatin ≥40 mg).
A cohort trial also shows increased diabetes risk
A smaller subsequent cohort trial based on data from Taiwan National Health Insurance records compared 8412 nondiabetic adult patients (mean age 63 years) taking statins with 33,648 age- and risk-matched controls not taking statins over a mean duration of 7.2 years.5 Statin use was associated with a 15% higher risk of developing diabetes (HR=1.15; 95% CI, 1.08-1.22).
RECOMMENDATIONS
The 2013 American College of Cardiology/American Heart Association guidelines for lipid-lowering therapy recommend that patients taking statins be screened for diabetes according to current screening recommendations.6 The guidelines advise encouraging patients who develop diabetes while on statin therapy to adhere to a heart-healthy dietary pattern, engage in physical activity, achieve and maintain a healthy body weight, cease tobacco use, and continue statin therapy to reduce the risk of cardiovascular events.
Yes. Statin therapy produces a small increase in the incidence of diabetes: one additional case per 255 patients taking statins over 4 years (strength of recommendation [SOR]: A, meta-analysis). Intensive statin therapy, compared with moderate therapy, produces an additional 2 cases of diabetes per 1000 patient years (SOR: B, meta-analysis with significant heterogeneity among trials).
EVIDENCE SUMMARY
A meta-analysis of 13 randomized, placebo or standard of care-controlled statin trials (113,148 patients, 81% without diabetes at enrollment, mean ages 55-76 years) found that statin therapy increased the incidence of diabetes by 9% over 4 years (odds ratio [OR]=1.09; 95% confidence interval [CI], 1.02-1.17), or one additional case per 255 patients.1 The increased risk was similar for lipophilic (pravastatin, rosuvastatin) and hydrophilic (atorvastatin, simvastatin, lovastatin) statins, although the analysis wasn’t adjusted for doses used.
In a meta-regression analysis, baseline body mass index or percentage change in low-density lipoprotein cholesterol didn’t appear to confer additional risk. The risk of diabetes with statins was generally higher in studies with older patients (data given graphically).
Higher statin doses mean higher risk
A meta-analysis of 5 placebo and standard-of-care randomized controlled trials (39,612 patients, 83% without diabetes at enrollment, mean age 58-64 years) found that the risk of diabetes was higher with higher-dose statins.2 Therapy with atorvastatin 80 mg or simvastatin 40 to 80 mg was defined as intensive. Treatment with simvastatin 20 to 40 mg, atorvastatin 10 mg, or pravastatin 40 mg was defined as moderate.
At a mean follow-up of 4.9 years, intensive statin therapy was associated with a higher risk of developing diabetes than moderate therapy (OR=1.12; 95% CI, 1.04-1.22) with 2 additional cases of diabetes per 1000 patient-years in the intensive therapy group. The authors noted significant heterogeneity between trials with regard to major cardiovascular events.
Similar results were found in a subsequent population-based cohort study of 471,250 nondiabetic patients older than 66 years who were newly prescribed a statin.3 The study authors used the incidence of new diabetes in patients taking pravastatin as the baseline, since it had been associated with reduced rates of diabetes in a large cardiovascular prevention trial.4 Without adjusting for dose, patients were at significantly higher risk of diabetes if prescribed atorvastatin (hazard ratio [HR]=1.22; 95% CI, 1.15-1.29), rosuvastatin (HR=1.18; 95% CI, 1.10-1.26), or simvastatin (HR=1.10; 95% CI, 1.04-1.17) compared with pravastatin. The risk with fluvastatin and lovastatin was similar to pravastatin.
A subanalysis that compared moderate- and high-dose statin therapy with low-dose therapy (atorvastatin <20 mg, rosuvastatin <10 mg, simvastatin <80 mg, or any dose of fluvastatin, lovastatin, or pravastatin) found a 22% increased risk of diabetes (HR=1.22; 95% CI, 1.19-1.26) for moderate-dose therapy (atorvastatin 20-79 mg, rosuvastatin 10-39 mg, or simvastatin >80 mg) and a 30% increased risk (HR=1.3; 95% CI, 1.2-1.4) for high-dose therapy (atorvastatin ≥80 mg or rosuvastatin ≥40 mg).
A cohort trial also shows increased diabetes risk
A smaller subsequent cohort trial based on data from Taiwan National Health Insurance records compared 8412 nondiabetic adult patients (mean age 63 years) taking statins with 33,648 age- and risk-matched controls not taking statins over a mean duration of 7.2 years.5 Statin use was associated with a 15% higher risk of developing diabetes (HR=1.15; 95% CI, 1.08-1.22).
RECOMMENDATIONS
The 2013 American College of Cardiology/American Heart Association guidelines for lipid-lowering therapy recommend that patients taking statins be screened for diabetes according to current screening recommendations.6 The guidelines advise encouraging patients who develop diabetes while on statin therapy to adhere to a heart-healthy dietary pattern, engage in physical activity, achieve and maintain a healthy body weight, cease tobacco use, and continue statin therapy to reduce the risk of cardiovascular events.
1. Sattar N, Preiss D, Murray HM, et al. Statins and risk of incident diabetes: a collaborative meta-analysis of randomised statin trials. Lancet. 2010;375:735-742.
2. Preiss D, Seshasai SR, Welsh P, et al. Risk of incident diabetes with intensive-dose compared with moderatedose statin therapy: a meta-analysis. JAMA. 2011;305:2556-2564.
3. Carter AA, Gomes T, Camacho X, et al. Risk of incident diabetes among patients treated with statins: population-based study. BMJ. 2013;346:f2610.
4. Freeman DJ, Morrie J, Sattar N, et al. Pravastatin and the development of diabetes mellitus: evidence for a protective treatment effect in the West of Scotland Coronary Prevention Study. Circulation. 2001;103:357-362.
5. Wang KL, Liu CJ, Chao TF, et al. Statins, risk of diabetes and implications on outcomes in the general population. J Am Coll Cardiol. 2012;60:1231-1238.
6. Stone NJ, Robinson JG, Lichtenstein AH, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129:S1-S45.
1. Sattar N, Preiss D, Murray HM, et al. Statins and risk of incident diabetes: a collaborative meta-analysis of randomised statin trials. Lancet. 2010;375:735-742.
2. Preiss D, Seshasai SR, Welsh P, et al. Risk of incident diabetes with intensive-dose compared with moderatedose statin therapy: a meta-analysis. JAMA. 2011;305:2556-2564.
3. Carter AA, Gomes T, Camacho X, et al. Risk of incident diabetes among patients treated with statins: population-based study. BMJ. 2013;346:f2610.
4. Freeman DJ, Morrie J, Sattar N, et al. Pravastatin and the development of diabetes mellitus: evidence for a protective treatment effect in the West of Scotland Coronary Prevention Study. Circulation. 2001;103:357-362.
5. Wang KL, Liu CJ, Chao TF, et al. Statins, risk of diabetes and implications on outcomes in the general population. J Am Coll Cardiol. 2012;60:1231-1238.
6. Stone NJ, Robinson JG, Lichtenstein AH, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129:S1-S45.
Evidence-based answers from the Family Physicians Inquiries Network
What therapies alleviate symptoms of polycystic ovary syndrome?
Treatment of polycystic ovary syndrome (PCOS) in women not actively seeking to become pregnant is symptom-specific. Lifestyle modification (LSM) reduces body weight by 3.5 kg (strength of recommendation [SOR]: A, meta-analysis) and metformin reduces it by 3 kg (SOR B, cohort trial).
LSM may be better tolerated; adding metformin to LSM doesn’t lead to additional weight loss (SOR: B, randomized controlled trial [RCT]).
Spironolactone improves hirsutism scores by an absolute 8% to 22% (SOR: A, multiple RCTs); adding metformin to spironolactone improves Ferriman-Gallwey (FG) hirsutism scores an additional absolute 1.4% (SOR: B, RCT). Oral contraceptive pills (OCPs) are 12 times more likely to result in complete menstrual regularity than metformin (SOR: A, meta-analysis). Combining OCPs with metformin improves hirsutism scores by 8% over using an OCP alone (SOR: A, meta-analysis).
Statin medications don’t alter weight, hirsutism, or menstruation (SOR: B, small meta-analysis).
EVIDENCE SUMMARY
Women with PCOS who are not seeking pregnancy commonly have symptoms such as excessive weight, hirsutism, and menstrual irregularities. This review focuses on interventions to manage those symptoms. The TABLE summarizes the results of the interventions.
Lifestyle modification improves symptoms; no benefit to adding metformin
A Cochrane meta-analysis of 6 RCTs with 164 patients compared LSM (with diet and exercise) and no or minimal intervention. LSM reduced weight more than minimal intervention (mean difference [MD]=-3.5 kg; 95% confidence interval [CI], -4.5 to -2.0).1 It also improved hirsutism, assessed with the 36-point FG score, where a lower score corresponds to less hirsutism (MD=-1.2 points, 95% CI, -2.4 to -0.1). No data were available on menstrual regularity.
A double-blind RCT comparing LSM alone with LSM plus metformin in 114 patients with PCOS found no difference in mean weight reduction (-2- to -3 kg, data from graph), ovulation rate, or androgen levels at 6 months.2 Six patients dropped out of the LSM-with-metformin group, whereas no patients dropped out of the LSM-alone group.
Metformin decreases BMI more than thiazolidinediones
In a meta-analysis of 10 RCTs (459 patients) comparing the effects of metformin and thiazolidinediones (TZDs), metformin reduced body mass index (BMI) more than TZDs at 3 months (weighted mean difference [WMD]=-2.5 kg/m2; 95% CI, -3.3 to -.6) and 6 months (WMD=-0.70 kg/m2; 95% CI, -0.76 to -0.65).3
In a prospective cohort dose-comparison study, 201 women with PCOS received either metformin 1000 mg or 1500 to 1700 mg daily for 6 months. Patients were asked not to modify their diet or exercise routines. In both dosage groups, patients lost weight from baseline (-3 kg; P<.01), and the number of menstrual cycles increased (0.7 per 6 months; P<.001).4 No clear dose-response relationship was observed.
Spironolactone can significantly reduce hirsutism
A systematic review identified 4 studies (132 patients) of antiandrogen therapy for hirsutism in PCOS. The 3 studies that used the FG score as an outcome all showed significant reductions in hirsutism after 6 to 12 months of treatment with spironolactone.5
A 6-month RCT of 198 patients with PCOS compared outcomes for spironolactone (50 mg/d), metformin (1000 mg/d), or both. Combined therapy was marginally better than either agent alone for reducing the FG score (end score for combined therapy 9.1 vs 9.6 for spironolactone and 9.7 for metformin, an absolute difference for combined therapy vs spironolactone of -0.5 FG points or -1.4%; P<.05).6
OCPs normalize menstrual cycles and reduce hirsutism
A Cochrane review evaluating the effects of OCPs on patients with PCOS included 4 RCTs (104 patients) that compared OCPs with metformin (1500-2000 mg/d) and 2 RCTs (70 patients) that compared the combination of an OCP and metformin with the OCP alone. Use of an OCP was much more likely to normalize menstrual cycling than metformin alone (2 trials, N=35; odds ratio [OR]=12; 95% CI, 2.2-100). Combining an OCP with metformin resulted in slightly better FG scores than an OCP alone (1 trial, N=40; WMD=-2.8 points; 95% CI, -5.4 to -0.17).7 There was no difference in the final BMI between patients taking an OCP alone, metformin alone, or both.
An RCT of 35 patients compared the effect on insulin levels of an OCP with rosiglitazone 4 mg/d and also looked at menstrual cycling as a secondary outcome. The study found no difference in effect on insulin levels in the 2 groups. All patients taking the OCP reported regular menstrual cycles at the end of the study compared with 75% of the patients taking rosiglitazone (P=.7).8 The study was underpowered to find a difference, however.
Statins alone don’t affect hirsutism, menstruation, or BMI
A Cochrane review identified 4 RCTs (244 women, ages 18-39 years) that compared a statin alone with placebo, another agent, or another agent plus a statin.9 One RCT of 48 patients found that a statin combined with an OCP improved hirsutism compared with a statin alone. Two RCTs (85 patients) found that statins didn’t lead to resumption of regular menstrual cycles. Statins also didn’t alter BMI in 3 studies of 105 patients.
Trials report no adverse effects, but VTE may be a concern with OCPs
A meta-analysis evaluated the safety of metformin, OCPs, and antiandrogens in 22 clinical trials with 1335 patients, primarily PCOS patients. The trials reported no cases of lactic acidosis with metformin, no drug-induced liver injury with antiandrogens, and no venous thromboembolism (VTE) with OCPs. The meta-analysis authors noted, however, that in a cohort trial of 1.6 million Danish women followed for 15 years, OCPs were associated with a 2- to 3-fold increase in risk of VTE, with higher risks linked to higher ethinyl estradiol content.10
RECOMMENDATIONS
A 2009 practice bulletin from The American College of Obestetrics and Gynecology (ACOG) recommends OCPs, progestin, metformin, and TZDs for anovulation and amenorrhea in patients with PCOS. OCPs, antiandrogens, metformin, eflornithine, and mechanical hair removal are recommended for hirsutism. ACOG advocates LSM, insulin-sensitizing agents (such as metformin), and statins to prevent cardiovascular disease and diabetes.11
1. Moran LJ, Hutchison SK, Norman RJ, et al. Lifestyle changes in women with polycystic ovary syndrome. Cochrane Database Syst Rev. 2011;(2):CD007506.
2. Ladson G, Dodson WC, Sweet SD, et al. The effects of metformin with lifestyle therapy in polycystic ovary syndrome: a randomized double-blind study. Fertil Steril. 2011;95:1059-1066.
3. Li XJ, Yu YX, Liu CQ, et al. Metformin vs thiazolidinediones for treatment of clinical, hormonal and metabolic characteristics of polycystic ovary syndrome: a meta-analysis. Clin Endocrinol (Oxf). 2011;74:332-339.
4. Fulghesu AM, Romualdi D, Di Florio C, et al. Is there a doseresponse relationship of metformin treatment in patients with polycystic ovary syndrome? Results from a multicentric study. Hum Reprod. 2012; 27:3057-3066.
5. Christy NA, Franks AS, Cross LB. Spironolactone for hirsutism in polycystic ovary syndrome. Ann Pharmacother. 2005;39:1517-1521.
6. Ganie MA, Khurana ML, Nisar S, et al. Improved efficacy of low-dose spironolactone and metformin combination than either drug alone in the management of women with polycystic ovary syndrome (PCOS): a six-month, open-label randomized study. J Clin Endocrinol Metab. 2013;98:3599-3607.
7. Costello M, Shrestha B, Eden J, et al. Insulin-sensitising drugs versus the combined oral contraceptive pill for hirsutism, acne and risk of diabetes, cardiovascular disease, and endometrial cancer in polycystic ovary syndrome. Cochrane Database Syst Rev. 2007;(1):CD005552.
8. Tfayli H, Ulnach JW, Lee S, et al. Drospirenone/ethinyl estradiol versus rosiglitazone treatment in overweight adolescents with polycystic ovary syndrome: comparison of metabolic, hormonal, and cardiovascular risk factors. J Clin Endocrinol Metab. 2011;96:1311-1319.
9. Raval AD, Hunter T, Stuckey B, et al. Statins for women with polycystic ovary syndrome not actively trying to conceive. Cochrane Database Syst Rev. 2011;(10):CD008565.
10. Domecq JP, Prutsky G, Mullan RJ, et al. Adverse effects of the common treatments for polycystic ovary syndrome: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2013;98:4646-4654.
11. ACOG Committee on Practice Bulletins—Gynecology. ACOG Practice Bulletin No. 108: Polycystic ovary syndrome. Obstet Gynecol. 2009;114:936-949.
Treatment of polycystic ovary syndrome (PCOS) in women not actively seeking to become pregnant is symptom-specific. Lifestyle modification (LSM) reduces body weight by 3.5 kg (strength of recommendation [SOR]: A, meta-analysis) and metformin reduces it by 3 kg (SOR B, cohort trial).
LSM may be better tolerated; adding metformin to LSM doesn’t lead to additional weight loss (SOR: B, randomized controlled trial [RCT]).
Spironolactone improves hirsutism scores by an absolute 8% to 22% (SOR: A, multiple RCTs); adding metformin to spironolactone improves Ferriman-Gallwey (FG) hirsutism scores an additional absolute 1.4% (SOR: B, RCT). Oral contraceptive pills (OCPs) are 12 times more likely to result in complete menstrual regularity than metformin (SOR: A, meta-analysis). Combining OCPs with metformin improves hirsutism scores by 8% over using an OCP alone (SOR: A, meta-analysis).
Statin medications don’t alter weight, hirsutism, or menstruation (SOR: B, small meta-analysis).
EVIDENCE SUMMARY
Women with PCOS who are not seeking pregnancy commonly have symptoms such as excessive weight, hirsutism, and menstrual irregularities. This review focuses on interventions to manage those symptoms. The TABLE summarizes the results of the interventions.
Lifestyle modification improves symptoms; no benefit to adding metformin
A Cochrane meta-analysis of 6 RCTs with 164 patients compared LSM (with diet and exercise) and no or minimal intervention. LSM reduced weight more than minimal intervention (mean difference [MD]=-3.5 kg; 95% confidence interval [CI], -4.5 to -2.0).1 It also improved hirsutism, assessed with the 36-point FG score, where a lower score corresponds to less hirsutism (MD=-1.2 points, 95% CI, -2.4 to -0.1). No data were available on menstrual regularity.
A double-blind RCT comparing LSM alone with LSM plus metformin in 114 patients with PCOS found no difference in mean weight reduction (-2- to -3 kg, data from graph), ovulation rate, or androgen levels at 6 months.2 Six patients dropped out of the LSM-with-metformin group, whereas no patients dropped out of the LSM-alone group.

Metformin decreases BMI more than thiazolidinediones
In a meta-analysis of 10 RCTs (459 patients) comparing the effects of metformin and thiazolidinediones (TZDs), metformin reduced body mass index (BMI) more than TZDs at 3 months (weighted mean difference [WMD]=-2.5 kg/m2; 95% CI, -3.3 to -.6) and 6 months (WMD=-0.70 kg/m2; 95% CI, -0.76 to -0.65).3
In a prospective cohort dose-comparison study, 201 women with PCOS received either metformin 1000 mg or 1500 to 1700 mg daily for 6 months. Patients were asked not to modify their diet or exercise routines. In both dosage groups, patients lost weight from baseline (-3 kg; P<.01), and the number of menstrual cycles increased (0.7 per 6 months; P<.001).4 No clear dose-response relationship was observed.
Spironolactone can significantly reduce hirsutism
A systematic review identified 4 studies (132 patients) of antiandrogen therapy for hirsutism in PCOS. The 3 studies that used the FG score as an outcome all showed significant reductions in hirsutism after 6 to 12 months of treatment with spironolactone.5
A 6-month RCT of 198 patients with PCOS compared outcomes for spironolactone (50 mg/d), metformin (1000 mg/d), or both. Combined therapy was marginally better than either agent alone for reducing the FG score (end score for combined therapy 9.1 vs 9.6 for spironolactone and 9.7 for metformin, an absolute difference for combined therapy vs spironolactone of -0.5 FG points or -1.4%; P<.05).6
OCPs normalize menstrual cycles and reduce hirsutism
A Cochrane review evaluating the effects of OCPs on patients with PCOS included 4 RCTs (104 patients) that compared OCPs with metformin (1500-2000 mg/d) and 2 RCTs (70 patients) that compared the combination of an OCP and metformin with the OCP alone. Use of an OCP was much more likely to normalize menstrual cycling than metformin alone (2 trials, N=35; odds ratio [OR]=12; 95% CI, 2.2-100). Combining an OCP with metformin resulted in slightly better FG scores than an OCP alone (1 trial, N=40; WMD=-2.8 points; 95% CI, -5.4 to -0.17).7 There was no difference in the final BMI between patients taking an OCP alone, metformin alone, or both.
An RCT of 35 patients compared the effect on insulin levels of an OCP with rosiglitazone 4 mg/d and also looked at menstrual cycling as a secondary outcome. The study found no difference in effect on insulin levels in the 2 groups. All patients taking the OCP reported regular menstrual cycles at the end of the study compared with 75% of the patients taking rosiglitazone (P=.7).8 The study was underpowered to find a difference, however.
Statins alone don’t affect hirsutism, menstruation, or BMI
A Cochrane review identified 4 RCTs (244 women, ages 18-39 years) that compared a statin alone with placebo, another agent, or another agent plus a statin.9 One RCT of 48 patients found that a statin combined with an OCP improved hirsutism compared with a statin alone. Two RCTs (85 patients) found that statins didn’t lead to resumption of regular menstrual cycles. Statins also didn’t alter BMI in 3 studies of 105 patients.
Trials report no adverse effects, but VTE may be a concern with OCPs
A meta-analysis evaluated the safety of metformin, OCPs, and antiandrogens in 22 clinical trials with 1335 patients, primarily PCOS patients. The trials reported no cases of lactic acidosis with metformin, no drug-induced liver injury with antiandrogens, and no venous thromboembolism (VTE) with OCPs. The meta-analysis authors noted, however, that in a cohort trial of 1.6 million Danish women followed for 15 years, OCPs were associated with a 2- to 3-fold increase in risk of VTE, with higher risks linked to higher ethinyl estradiol content.10
RECOMMENDATIONS
A 2009 practice bulletin from The American College of Obestetrics and Gynecology (ACOG) recommends OCPs, progestin, metformin, and TZDs for anovulation and amenorrhea in patients with PCOS. OCPs, antiandrogens, metformin, eflornithine, and mechanical hair removal are recommended for hirsutism. ACOG advocates LSM, insulin-sensitizing agents (such as metformin), and statins to prevent cardiovascular disease and diabetes.11
Treatment of polycystic ovary syndrome (PCOS) in women not actively seeking to become pregnant is symptom-specific. Lifestyle modification (LSM) reduces body weight by 3.5 kg (strength of recommendation [SOR]: A, meta-analysis) and metformin reduces it by 3 kg (SOR B, cohort trial).
LSM may be better tolerated; adding metformin to LSM doesn’t lead to additional weight loss (SOR: B, randomized controlled trial [RCT]).
Spironolactone improves hirsutism scores by an absolute 8% to 22% (SOR: A, multiple RCTs); adding metformin to spironolactone improves Ferriman-Gallwey (FG) hirsutism scores an additional absolute 1.4% (SOR: B, RCT). Oral contraceptive pills (OCPs) are 12 times more likely to result in complete menstrual regularity than metformin (SOR: A, meta-analysis). Combining OCPs with metformin improves hirsutism scores by 8% over using an OCP alone (SOR: A, meta-analysis).
Statin medications don’t alter weight, hirsutism, or menstruation (SOR: B, small meta-analysis).
EVIDENCE SUMMARY
Women with PCOS who are not seeking pregnancy commonly have symptoms such as excessive weight, hirsutism, and menstrual irregularities. This review focuses on interventions to manage those symptoms. The TABLE summarizes the results of the interventions.
Lifestyle modification improves symptoms; no benefit to adding metformin
A Cochrane meta-analysis of 6 RCTs with 164 patients compared LSM (with diet and exercise) and no or minimal intervention. LSM reduced weight more than minimal intervention (mean difference [MD]=-3.5 kg; 95% confidence interval [CI], -4.5 to -2.0).1 It also improved hirsutism, assessed with the 36-point FG score, where a lower score corresponds to less hirsutism (MD=-1.2 points, 95% CI, -2.4 to -0.1). No data were available on menstrual regularity.
A double-blind RCT comparing LSM alone with LSM plus metformin in 114 patients with PCOS found no difference in mean weight reduction (-2- to -3 kg, data from graph), ovulation rate, or androgen levels at 6 months.2 Six patients dropped out of the LSM-with-metformin group, whereas no patients dropped out of the LSM-alone group.

Metformin decreases BMI more than thiazolidinediones
In a meta-analysis of 10 RCTs (459 patients) comparing the effects of metformin and thiazolidinediones (TZDs), metformin reduced body mass index (BMI) more than TZDs at 3 months (weighted mean difference [WMD]=-2.5 kg/m2; 95% CI, -3.3 to -.6) and 6 months (WMD=-0.70 kg/m2; 95% CI, -0.76 to -0.65).3
In a prospective cohort dose-comparison study, 201 women with PCOS received either metformin 1000 mg or 1500 to 1700 mg daily for 6 months. Patients were asked not to modify their diet or exercise routines. In both dosage groups, patients lost weight from baseline (-3 kg; P<.01), and the number of menstrual cycles increased (0.7 per 6 months; P<.001).4 No clear dose-response relationship was observed.
Spironolactone can significantly reduce hirsutism
A systematic review identified 4 studies (132 patients) of antiandrogen therapy for hirsutism in PCOS. The 3 studies that used the FG score as an outcome all showed significant reductions in hirsutism after 6 to 12 months of treatment with spironolactone.5
A 6-month RCT of 198 patients with PCOS compared outcomes for spironolactone (50 mg/d), metformin (1000 mg/d), or both. Combined therapy was marginally better than either agent alone for reducing the FG score (end score for combined therapy 9.1 vs 9.6 for spironolactone and 9.7 for metformin, an absolute difference for combined therapy vs spironolactone of -0.5 FG points or -1.4%; P<.05).6
OCPs normalize menstrual cycles and reduce hirsutism
A Cochrane review evaluating the effects of OCPs on patients with PCOS included 4 RCTs (104 patients) that compared OCPs with metformin (1500-2000 mg/d) and 2 RCTs (70 patients) that compared the combination of an OCP and metformin with the OCP alone. Use of an OCP was much more likely to normalize menstrual cycling than metformin alone (2 trials, N=35; odds ratio [OR]=12; 95% CI, 2.2-100). Combining an OCP with metformin resulted in slightly better FG scores than an OCP alone (1 trial, N=40; WMD=-2.8 points; 95% CI, -5.4 to -0.17).7 There was no difference in the final BMI between patients taking an OCP alone, metformin alone, or both.
An RCT of 35 patients compared the effect on insulin levels of an OCP with rosiglitazone 4 mg/d and also looked at menstrual cycling as a secondary outcome. The study found no difference in effect on insulin levels in the 2 groups. All patients taking the OCP reported regular menstrual cycles at the end of the study compared with 75% of the patients taking rosiglitazone (P=.7).8 The study was underpowered to find a difference, however.
Statins alone don’t affect hirsutism, menstruation, or BMI
A Cochrane review identified 4 RCTs (244 women, ages 18-39 years) that compared a statin alone with placebo, another agent, or another agent plus a statin.9 One RCT of 48 patients found that a statin combined with an OCP improved hirsutism compared with a statin alone. Two RCTs (85 patients) found that statins didn’t lead to resumption of regular menstrual cycles. Statins also didn’t alter BMI in 3 studies of 105 patients.
Trials report no adverse effects, but VTE may be a concern with OCPs
A meta-analysis evaluated the safety of metformin, OCPs, and antiandrogens in 22 clinical trials with 1335 patients, primarily PCOS patients. The trials reported no cases of lactic acidosis with metformin, no drug-induced liver injury with antiandrogens, and no venous thromboembolism (VTE) with OCPs. The meta-analysis authors noted, however, that in a cohort trial of 1.6 million Danish women followed for 15 years, OCPs were associated with a 2- to 3-fold increase in risk of VTE, with higher risks linked to higher ethinyl estradiol content.10
RECOMMENDATIONS
A 2009 practice bulletin from The American College of Obestetrics and Gynecology (ACOG) recommends OCPs, progestin, metformin, and TZDs for anovulation and amenorrhea in patients with PCOS. OCPs, antiandrogens, metformin, eflornithine, and mechanical hair removal are recommended for hirsutism. ACOG advocates LSM, insulin-sensitizing agents (such as metformin), and statins to prevent cardiovascular disease and diabetes.11
1. Moran LJ, Hutchison SK, Norman RJ, et al. Lifestyle changes in women with polycystic ovary syndrome. Cochrane Database Syst Rev. 2011;(2):CD007506.
2. Ladson G, Dodson WC, Sweet SD, et al. The effects of metformin with lifestyle therapy in polycystic ovary syndrome: a randomized double-blind study. Fertil Steril. 2011;95:1059-1066.
3. Li XJ, Yu YX, Liu CQ, et al. Metformin vs thiazolidinediones for treatment of clinical, hormonal and metabolic characteristics of polycystic ovary syndrome: a meta-analysis. Clin Endocrinol (Oxf). 2011;74:332-339.
4. Fulghesu AM, Romualdi D, Di Florio C, et al. Is there a doseresponse relationship of metformin treatment in patients with polycystic ovary syndrome? Results from a multicentric study. Hum Reprod. 2012; 27:3057-3066.
5. Christy NA, Franks AS, Cross LB. Spironolactone for hirsutism in polycystic ovary syndrome. Ann Pharmacother. 2005;39:1517-1521.
6. Ganie MA, Khurana ML, Nisar S, et al. Improved efficacy of low-dose spironolactone and metformin combination than either drug alone in the management of women with polycystic ovary syndrome (PCOS): a six-month, open-label randomized study. J Clin Endocrinol Metab. 2013;98:3599-3607.
7. Costello M, Shrestha B, Eden J, et al. Insulin-sensitising drugs versus the combined oral contraceptive pill for hirsutism, acne and risk of diabetes, cardiovascular disease, and endometrial cancer in polycystic ovary syndrome. Cochrane Database Syst Rev. 2007;(1):CD005552.
8. Tfayli H, Ulnach JW, Lee S, et al. Drospirenone/ethinyl estradiol versus rosiglitazone treatment in overweight adolescents with polycystic ovary syndrome: comparison of metabolic, hormonal, and cardiovascular risk factors. J Clin Endocrinol Metab. 2011;96:1311-1319.
9. Raval AD, Hunter T, Stuckey B, et al. Statins for women with polycystic ovary syndrome not actively trying to conceive. Cochrane Database Syst Rev. 2011;(10):CD008565.
10. Domecq JP, Prutsky G, Mullan RJ, et al. Adverse effects of the common treatments for polycystic ovary syndrome: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2013;98:4646-4654.
11. ACOG Committee on Practice Bulletins—Gynecology. ACOG Practice Bulletin No. 108: Polycystic ovary syndrome. Obstet Gynecol. 2009;114:936-949.
1. Moran LJ, Hutchison SK, Norman RJ, et al. Lifestyle changes in women with polycystic ovary syndrome. Cochrane Database Syst Rev. 2011;(2):CD007506.
2. Ladson G, Dodson WC, Sweet SD, et al. The effects of metformin with lifestyle therapy in polycystic ovary syndrome: a randomized double-blind study. Fertil Steril. 2011;95:1059-1066.
3. Li XJ, Yu YX, Liu CQ, et al. Metformin vs thiazolidinediones for treatment of clinical, hormonal and metabolic characteristics of polycystic ovary syndrome: a meta-analysis. Clin Endocrinol (Oxf). 2011;74:332-339.
4. Fulghesu AM, Romualdi D, Di Florio C, et al. Is there a doseresponse relationship of metformin treatment in patients with polycystic ovary syndrome? Results from a multicentric study. Hum Reprod. 2012; 27:3057-3066.
5. Christy NA, Franks AS, Cross LB. Spironolactone for hirsutism in polycystic ovary syndrome. Ann Pharmacother. 2005;39:1517-1521.
6. Ganie MA, Khurana ML, Nisar S, et al. Improved efficacy of low-dose spironolactone and metformin combination than either drug alone in the management of women with polycystic ovary syndrome (PCOS): a six-month, open-label randomized study. J Clin Endocrinol Metab. 2013;98:3599-3607.
7. Costello M, Shrestha B, Eden J, et al. Insulin-sensitising drugs versus the combined oral contraceptive pill for hirsutism, acne and risk of diabetes, cardiovascular disease, and endometrial cancer in polycystic ovary syndrome. Cochrane Database Syst Rev. 2007;(1):CD005552.
8. Tfayli H, Ulnach JW, Lee S, et al. Drospirenone/ethinyl estradiol versus rosiglitazone treatment in overweight adolescents with polycystic ovary syndrome: comparison of metabolic, hormonal, and cardiovascular risk factors. J Clin Endocrinol Metab. 2011;96:1311-1319.
9. Raval AD, Hunter T, Stuckey B, et al. Statins for women with polycystic ovary syndrome not actively trying to conceive. Cochrane Database Syst Rev. 2011;(10):CD008565.
10. Domecq JP, Prutsky G, Mullan RJ, et al. Adverse effects of the common treatments for polycystic ovary syndrome: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2013;98:4646-4654.
11. ACOG Committee on Practice Bulletins—Gynecology. ACOG Practice Bulletin No. 108: Polycystic ovary syndrome. Obstet Gynecol. 2009;114:936-949.
Evidence-based answers from the Family Physicians Inquiries Network
Does primary nocturnal enuresis affect childrens’ self-esteem?
Yes. Children with primary nocturnal enuresis often, but not always, score about 10% lower on standardized rating scales for self-esteem, or scores for symptoms similar to low self-esteem (sadness, anxiety, social fears, distress) than children without enuresis (strength of recommendation [SOR]: B, systematic review of cohort and case-control studies with some heterogenous results).
Enuretic children 8 to 9 years of age are less likely to have lower self-esteem than older children, ages 10 to 12 years (SOR: B, case-control study).
Successful treatment of primary nocturnal enuresis improves self-esteem ratings, probably to normal (SOR: B, randomized, controlled trial, prospective cohort, and case-control studies).
EVIDENCE SUMMARY
A systematic review including 4 case-control and 3 cohort studies of the impact of nocturnal enuresis on children and young people found that bedwetting was often, but not always, associated with lower self-esteem scores (or scores for symptoms similar to lower self-esteem) on standardized questionnaires.1 The studies defined self-esteem in various ways and used a variety of questionnaires to measure it, so direct comparisons weren’t possible.
The first case-control study in the review found that enuretic older children (10-12 years) and girls had lower self-esteem scores than younger children (8-9 years) and boys. The second case-control study reported lower self-esteem scores on only 1 of 3 assessment instruments.
The third case-control study, which compared self-esteem scores in enuretic children with scores for children who had asthma and heart disease, found that enuresis was associated with the lowest self-esteem. The final case-control study reported that young adolescents with enuresis were more likely to suffer “angry distress.”
The first cohort study in the systematic review found a significantly higher incidence of sadness, anxiety, and social fears in children with enuresis than in children without and reported that 65% were “not happy” about having enuresis.
In the second cohort study, children with more severe enuresis, and girls, had significantly worse self-esteem scores than children with mild enuresis or boys (actual scores and some statistics not supplied), although these findings weren’t replicated on the second standardized scale that the investigators used.
The third cohort study reported that 37% of approximately 800 children with enuresis rated it “really difficult,” on a 4-point Likert scale.
How enuresis treatment affects self-esteem
The same systematic review, plus 2 additional studies, demonstrated that successful treatment of enuresis improves self-esteem scores, likely to normal.1-3 A randomized controlled trial found that treatment improved self-esteem scores by about 5%; children with the greatest treatment success showed the largest improvement (no statistics supplied).2
In a prospective cohort study, treated children demonstrated about a 30% improvement in scores measuring anxiety, depression, and internal distress.3 A case-control study in the systematic review also found about a 30% improvement in self-esteem scores among successfully treated children (both boys and girls) and a return to nonenuretic norms.1 Scores for unsuccessfully treated children didn’t improve.
RECOMMENDATIONS
A guideline on the management of bedwetting from the National Institute for Health and Clinical Excellence (now called the National Institute for Health and Care Excellence) says that enuresis can have a deep impact on a child’s behavior and emotional well-being and that treatment has a positive effect on self-esteem.4
The Evidence-Based Medicine guidelines for enuresis in a child5 say that enuresis as such does not indicate a psychological disturbance and that psychotherapy may be useful when enuresis is associated with significant problems of self-esteem or behavior.
The American Academy of Child and Adolescent Psychiatry practice parameter for children with enuresis states that the psychological consequences of enuresis must be recognized and addressed with sensitivity during evaluation and management.6
1. National Clinical Guideline Centre (UK). Impact of bedwetting on children and young people and their families. In: Nocturnal Enuresis: The Management of Bedwetting in Children and Young People. London, UK: Royal College of Physicians; 2010. Available at: www.ncbi.nlm.nih.gov/books/NBK62729/. Accessed January 24, 2014.
2. Moffatt ME, Kato C, Pless IB. Improvements in self-concept after treatment of nocturnal enuresis: randomized controlled trial. J Pediatr. 1987;110:647-652.
3. HiraSing RA, van Leerdam FJ, Bolk-Bennink LF, et al. Effect of dry bed training on behavioural problems in enuretic children. Acta Paediatr. 2002; 91:960-964.
4. Nunes VD, O’Flynn N, Evans J, et al; Guideline Development Group. Management of bedwetting in children and young people: summary of NICE guidance. BMJ. 2010;341:c5399.
5. Enuresis in a child. Evidence-Based Medicine Guidelines. Essential Evidence Plus [online database]. Available at: www.essentialevidenceplus.com/content/ebmg_ebm/633. Accessed January 24, 2014.
6. Fritz G, Rockney R; American Academy of Child and Adolescent Psychiatry Work Group on Quality Issues. Summary of the practice parameter for the assessment and treatment of children and adolescents with enuresis. J Am Acad Child Adolesc Psychiatry. 2004;43:123-125.
Yes. Children with primary nocturnal enuresis often, but not always, score about 10% lower on standardized rating scales for self-esteem, or scores for symptoms similar to low self-esteem (sadness, anxiety, social fears, distress) than children without enuresis (strength of recommendation [SOR]: B, systematic review of cohort and case-control studies with some heterogenous results).
Enuretic children 8 to 9 years of age are less likely to have lower self-esteem than older children, ages 10 to 12 years (SOR: B, case-control study).
Successful treatment of primary nocturnal enuresis improves self-esteem ratings, probably to normal (SOR: B, randomized, controlled trial, prospective cohort, and case-control studies).
EVIDENCE SUMMARY
A systematic review including 4 case-control and 3 cohort studies of the impact of nocturnal enuresis on children and young people found that bedwetting was often, but not always, associated with lower self-esteem scores (or scores for symptoms similar to lower self-esteem) on standardized questionnaires.1 The studies defined self-esteem in various ways and used a variety of questionnaires to measure it, so direct comparisons weren’t possible.
The first case-control study in the review found that enuretic older children (10-12 years) and girls had lower self-esteem scores than younger children (8-9 years) and boys. The second case-control study reported lower self-esteem scores on only 1 of 3 assessment instruments.
The third case-control study, which compared self-esteem scores in enuretic children with scores for children who had asthma and heart disease, found that enuresis was associated with the lowest self-esteem. The final case-control study reported that young adolescents with enuresis were more likely to suffer “angry distress.”
The first cohort study in the systematic review found a significantly higher incidence of sadness, anxiety, and social fears in children with enuresis than in children without and reported that 65% were “not happy” about having enuresis.
In the second cohort study, children with more severe enuresis, and girls, had significantly worse self-esteem scores than children with mild enuresis or boys (actual scores and some statistics not supplied), although these findings weren’t replicated on the second standardized scale that the investigators used.
The third cohort study reported that 37% of approximately 800 children with enuresis rated it “really difficult,” on a 4-point Likert scale.
How enuresis treatment affects self-esteem
The same systematic review, plus 2 additional studies, demonstrated that successful treatment of enuresis improves self-esteem scores, likely to normal.1-3 A randomized controlled trial found that treatment improved self-esteem scores by about 5%; children with the greatest treatment success showed the largest improvement (no statistics supplied).2
In a prospective cohort study, treated children demonstrated about a 30% improvement in scores measuring anxiety, depression, and internal distress.3 A case-control study in the systematic review also found about a 30% improvement in self-esteem scores among successfully treated children (both boys and girls) and a return to nonenuretic norms.1 Scores for unsuccessfully treated children didn’t improve.
RECOMMENDATIONS
A guideline on the management of bedwetting from the National Institute for Health and Clinical Excellence (now called the National Institute for Health and Care Excellence) says that enuresis can have a deep impact on a child’s behavior and emotional well-being and that treatment has a positive effect on self-esteem.4
The Evidence-Based Medicine guidelines for enuresis in a child5 say that enuresis as such does not indicate a psychological disturbance and that psychotherapy may be useful when enuresis is associated with significant problems of self-esteem or behavior.
The American Academy of Child and Adolescent Psychiatry practice parameter for children with enuresis states that the psychological consequences of enuresis must be recognized and addressed with sensitivity during evaluation and management.6
Yes. Children with primary nocturnal enuresis often, but not always, score about 10% lower on standardized rating scales for self-esteem, or scores for symptoms similar to low self-esteem (sadness, anxiety, social fears, distress) than children without enuresis (strength of recommendation [SOR]: B, systematic review of cohort and case-control studies with some heterogenous results).
Enuretic children 8 to 9 years of age are less likely to have lower self-esteem than older children, ages 10 to 12 years (SOR: B, case-control study).
Successful treatment of primary nocturnal enuresis improves self-esteem ratings, probably to normal (SOR: B, randomized, controlled trial, prospective cohort, and case-control studies).
EVIDENCE SUMMARY
A systematic review including 4 case-control and 3 cohort studies of the impact of nocturnal enuresis on children and young people found that bedwetting was often, but not always, associated with lower self-esteem scores (or scores for symptoms similar to lower self-esteem) on standardized questionnaires.1 The studies defined self-esteem in various ways and used a variety of questionnaires to measure it, so direct comparisons weren’t possible.
The first case-control study in the review found that enuretic older children (10-12 years) and girls had lower self-esteem scores than younger children (8-9 years) and boys. The second case-control study reported lower self-esteem scores on only 1 of 3 assessment instruments.
The third case-control study, which compared self-esteem scores in enuretic children with scores for children who had asthma and heart disease, found that enuresis was associated with the lowest self-esteem. The final case-control study reported that young adolescents with enuresis were more likely to suffer “angry distress.”
The first cohort study in the systematic review found a significantly higher incidence of sadness, anxiety, and social fears in children with enuresis than in children without and reported that 65% were “not happy” about having enuresis.
In the second cohort study, children with more severe enuresis, and girls, had significantly worse self-esteem scores than children with mild enuresis or boys (actual scores and some statistics not supplied), although these findings weren’t replicated on the second standardized scale that the investigators used.
The third cohort study reported that 37% of approximately 800 children with enuresis rated it “really difficult,” on a 4-point Likert scale.
How enuresis treatment affects self-esteem
The same systematic review, plus 2 additional studies, demonstrated that successful treatment of enuresis improves self-esteem scores, likely to normal.1-3 A randomized controlled trial found that treatment improved self-esteem scores by about 5%; children with the greatest treatment success showed the largest improvement (no statistics supplied).2
In a prospective cohort study, treated children demonstrated about a 30% improvement in scores measuring anxiety, depression, and internal distress.3 A case-control study in the systematic review also found about a 30% improvement in self-esteem scores among successfully treated children (both boys and girls) and a return to nonenuretic norms.1 Scores for unsuccessfully treated children didn’t improve.
RECOMMENDATIONS
A guideline on the management of bedwetting from the National Institute for Health and Clinical Excellence (now called the National Institute for Health and Care Excellence) says that enuresis can have a deep impact on a child’s behavior and emotional well-being and that treatment has a positive effect on self-esteem.4
The Evidence-Based Medicine guidelines for enuresis in a child5 say that enuresis as such does not indicate a psychological disturbance and that psychotherapy may be useful when enuresis is associated with significant problems of self-esteem or behavior.
The American Academy of Child and Adolescent Psychiatry practice parameter for children with enuresis states that the psychological consequences of enuresis must be recognized and addressed with sensitivity during evaluation and management.6
1. National Clinical Guideline Centre (UK). Impact of bedwetting on children and young people and their families. In: Nocturnal Enuresis: The Management of Bedwetting in Children and Young People. London, UK: Royal College of Physicians; 2010. Available at: www.ncbi.nlm.nih.gov/books/NBK62729/. Accessed January 24, 2014.
2. Moffatt ME, Kato C, Pless IB. Improvements in self-concept after treatment of nocturnal enuresis: randomized controlled trial. J Pediatr. 1987;110:647-652.
3. HiraSing RA, van Leerdam FJ, Bolk-Bennink LF, et al. Effect of dry bed training on behavioural problems in enuretic children. Acta Paediatr. 2002; 91:960-964.
4. Nunes VD, O’Flynn N, Evans J, et al; Guideline Development Group. Management of bedwetting in children and young people: summary of NICE guidance. BMJ. 2010;341:c5399.
5. Enuresis in a child. Evidence-Based Medicine Guidelines. Essential Evidence Plus [online database]. Available at: www.essentialevidenceplus.com/content/ebmg_ebm/633. Accessed January 24, 2014.
6. Fritz G, Rockney R; American Academy of Child and Adolescent Psychiatry Work Group on Quality Issues. Summary of the practice parameter for the assessment and treatment of children and adolescents with enuresis. J Am Acad Child Adolesc Psychiatry. 2004;43:123-125.
1. National Clinical Guideline Centre (UK). Impact of bedwetting on children and young people and their families. In: Nocturnal Enuresis: The Management of Bedwetting in Children and Young People. London, UK: Royal College of Physicians; 2010. Available at: www.ncbi.nlm.nih.gov/books/NBK62729/. Accessed January 24, 2014.
2. Moffatt ME, Kato C, Pless IB. Improvements in self-concept after treatment of nocturnal enuresis: randomized controlled trial. J Pediatr. 1987;110:647-652.
3. HiraSing RA, van Leerdam FJ, Bolk-Bennink LF, et al. Effect of dry bed training on behavioural problems in enuretic children. Acta Paediatr. 2002; 91:960-964.
4. Nunes VD, O’Flynn N, Evans J, et al; Guideline Development Group. Management of bedwetting in children and young people: summary of NICE guidance. BMJ. 2010;341:c5399.
5. Enuresis in a child. Evidence-Based Medicine Guidelines. Essential Evidence Plus [online database]. Available at: www.essentialevidenceplus.com/content/ebmg_ebm/633. Accessed January 24, 2014.
6. Fritz G, Rockney R; American Academy of Child and Adolescent Psychiatry Work Group on Quality Issues. Summary of the practice parameter for the assessment and treatment of children and adolescents with enuresis. J Am Acad Child Adolesc Psychiatry. 2004;43:123-125.
Evidence-based answers from the Family Physicians Inquiries Network
