User login
Does low-dose aspirin reduce preeclampsia and other maternal-fetal complications?
Yes. The use of low-dose aspirin during pregnancy decreases the risk of preeclampsia for women considered at increased risk. The effect is smaller for women without risk factors (strength of recommendation [SOR]: A, based on randomized controlled trials [RCTs] and systematic reviews [SRs] of RCTs).
Rates of preterm delivery, perinatal death, and incidence of small-for-gestational age infants are decreased for women treated with low-dose aspirin (SOR: A, based on SRs and RCTs). A meta-analysis of RCTs has found no increased rates of harm from low-dose aspirin therapy, including placental abruption or other antepartum bleeding complications (SOR: A, based on SRs and RCTs).
I prescribe 81 mg/day of aspirin for women with previous severe preeclampsia
John Hill, DO
Department of Family Medicine, University of Colorado, Denver
Confused about when to use aspirin in pregnancy? You’re not alone. Over my 20 years of practice, I have reacted to disparate guidelines ranging from “never use aspirin in pregnancy” to “always use low-dose aspirin.” This review helps simplify my clinical practice.
With the benefit of evidence from multiple RCTs over the past 7 years, I now personally use 81 mg of aspirin each day in 2 groups of women: those who had severe preeclampsia in a prior pregnancy, and those who develop signs of preeclampsia or strong risk factors for it before the third trimester in their current pregnancy.
Evidence summary
Systematic reviews show aspirin lowers rates of preeclampsia
Four SRs published between 2001 and 20071-4 and a Cochrane Review updated in 20065 have demonstrated that low-dose aspirin helps to prevent preeclampsia, reduction in preterm delivery rates, and decreased perinatal mortality.
The 2001 SR by Duley1 included 39 trials and 30,563 patients. Patients were classified either as high-risk (previous severe preeclampsia, diabetes, chronic hypertension, renal disease, or autoimmune disease) or moderate-risk (remainder of subjects). Four individual studies (with a combined weight of 27%) did not support aspirin therapy. The largest trial not supporting aspirin therapy included 6275 subjects and had a relative risk of 1.14 (95% CI, 0.94–1.38).
Most studies in this review compared aspirin alone with placebo (28,802 subjects). However, 4 studies either compared combination therapy with aspirin or other thromboprophylaxis therapy (dipyridamole, heparin, or ozagrel). Although there were differences in risk stratification, variable doses of aspirin, and varied gestational age at trial entry, all studies reported an overall 15% reduction of preeclampsia (RR=0.85; 95% CI, 0.78–0.92).
The 2003 SR by Coomarasamy2 included 14 trials and 12,416 patients. The study exclusively evaluated high-risk pregnancies: women with history (or family history) of preeclampsia, chronic hypertension, gestational diabetes, or renal disease. The overall reduction in preeclampsia was 14% (relative risk [RR]=0.86; 95% confidence interval [CI], 0.76–0.96). Results were consistent across RCTs, and only 2 of the 14 studies (with a combined weight of 7.1%) did not support aspirin therapy.
TABLE
Low-dose aspirin reduces risk of preeclampsia, but how does it affect other maternal and fetal outcomes?
| STUDY (YEAR) | DEVELOPMENT OF PREECLAMPSIA | PRETERM DELIVERY | NEONATAL DEATH | SGA OR LOW BIRTH WEIGHT | RISK OF ABRUPTION & BLEEDING |
|---|---|---|---|---|---|
| Duley (2001)1 | Moderate-risk patients: 15% reduction High-risk patients: 15% reduction NNT=100 | 8% reduction NNT=72 | 14% reduction NNT= 250 | 8% reduction* | Not reported |
| Coomarasamy (2003)2 | 14% reduction | 14% reduction | 21% reduction | 215-g weight gain in aspirin group | No significant clinical difference in risk RR=0.98) |
| Ruano (2005)3 | Low-risk patients: no significant reduction High-risk patients: 13% reduction | Not reported | |||
| Askie (2007)4 | 10% reduction | 10% reduction | 9% reduction | 10% reduction | No significant clinical difference in risk (RR=0.90–1.15) |
| Cochrane (2007)5 | 19% reduction NNT=69 (overall), 118 (moderate risk), 18 (high-risk) | 7% reduction NNT=83 | 16% reduction NNT=227 | 8% reduction* | No significant clinical difference in risk (RR=1.06) |
| * Borderline for statistical significance (RR=0.92). | |||||
| SGA, small for gestational age; NNT, number needed to treat; RR, relative risk. | |||||
Ruano’s 2005 SR3 included 22 trials with 33,598 subjects and specifically compared low-risk vs high-risk patients. The authors concluded that there was no significant reduction in preeclampsia with the use of low-dose aspirin in the low-risk arm (RR=0.95; 95% CI, 0.81–1.11), and a 13% reduction among high-risk subjects (RR=0.87; 95% CI, 0.79–0.96).3
A 2007 meta-analysis by Askie4 included 31 trials with 32,217 women and their 32,819 infants. Main outcomes (regardless of initial maternal risks) were 1) onset of preeclampsia, 2) neonatal death, 3) preterm birth at <34 weeks gestation, 4) infant small for gestational age, and 5) pregnancy with serious adverse outcome. Results of these outcome measures consistently showed a relative risk reduction of 10% for subjects taking low-dose aspirin, except for neonatal deaths, which had a 9% reduction. This study also suggested that multiparous women and women with a history of hypertensive disorder of pregnancy may derive a larger benefit from low-dose aspirin.
A Cochrane Review5 updated in 2007 demonstrated that low-dose aspirin provided a moderate (19%) reduction in the overall risk of developing preeclampsia. New stratified analysis of the data indicates that in moderate-risk women, antiplatelet therapy is associated with a 15% reduction, and that high-risk women have a 27% reduction in the risk of developing preeclampsia. The effect on small-for-gestational-age infants revealed no overall clinically significant differences.
Aspirin dosing: One study recommends >75 mg/day
Studies varied in the aspirin dosage they used and duration of treatment. In all RCTs, the dose of aspirin ranged from 50 mg/day to 150 mg/day. Earlier trials used lower doses of aspirin (50–75 mg/day), while recent trials used 100 mg or more per day.
Early RCTs revealed no correlation between the dose of aspirin and the prevention of preeclampsia. However, Villar et al6 showed a greater effect among women treated with doses greater than 75 mg/day of aspirin (RR=0.49; 95% CI, 0.38–0.63).6
No evidence of harm from aspirin
There is no evidence of harm from low-dose aspirin therapy—including placental abruption, antenatal admissions, fetal intraventricular hemorrhage and other neonatal bleeding complications, admission to neonatal care unit, induction of labor, or caesarean delivery—regardless of initial risk stratification.7
Recommendations from others
The 2002 American College of Obstetricians and Gynecologists Practice Bulletin states that low-dose aspirin in women at low risk has not been shown to prevent preeclampsia and therefore is not recommended. They make no specific statement regarding the use of low-dose aspirin in moderate- to high-risk pregnancies.8
The Australasian Society for the Study of Hypertension in Pregnancy conclude that low-dose aspirin for prevention of preeclampsia is reasonable for the following conditions: 1) prior fetal loss after first trimester due to placental insufficiency or severe fetal growth retardation, and 2) women with severe early onset preeclampsia in previous pregnancy necessitating delivery ≤32 weeks gestation. Despite difficulties in predicting who will deliver preterm, consider women who have had severe early-onset preeclampsia in a previous pregnancy for low-dose aspirin therapy.9
The Canadian Hypertension Society Consensus Panel concludes low-dose aspirin therapy is effective in decreasing the incidence of preterm delivery and early-onset preeclampsia among women at risk of developing the syndrome.10
1. Duley L, Henderson-Smart DJ, Knight M, King JF. Antiplatelet drugs for prevention of pre-eclampsia and its consequences: systematic review. BMJ 2001;322:329-333.
2. Coomarasamy A, Papaioannou S, Gee H, Khan KS. Aspirin for prevention of pre-eclampsia in women with historical risk factors: a systematic review. Obstet Gynecol 2003;101:1319-1332.
3. Ruano R, Fontes RS, Zugaib M. Prevention of preeclampsia with low-dose aspirin—a systematic review and meta-analysis of the main randomized controlled trials. Clinics 2005;60:407-414.
4. Askie LM, Duley L, Henderson-Stewart DJ, et al. Antiplatelet agents for prevention of pre-eclampsia: a meta-analysis of individual patient data. Lancet 2007;369:1791-1798.
5. Duley L, Henderson-Smart DJ, Knight M, King JF. Antiplatelet agents for preventing pre-eclampsia and its complication (review). Cochrane Database Syst Rev 2003;(4):CD004659.-
6. Villar J, Abalos E, Nardin JM, et al. Strategies to prevent and treat pre-eclampsia: evidence from randomized controlled trials. Semin Nephrol 2004;24:607-615.
7. Coomarasamy A, Braunholtz D, Song F, et al. Individualizing use of aspirin to prevent pre-eclampsia: a framework for clinical decision making. BJOG 2003;110:882-888.
8. ACOG Committee on Obstetric Practice. Diagnosis and management of pre-eclampsia and eclampsia. ACOG Practice Bulletin, no 33. Int J Gynaecol Obstet 2002;77:67-75.
9. Brown MA, Brennecke SP, Crowther CA, et al. Aspirin and prevention of pre-eclampsia. Aust NZ J Obstet Gynaecol 1995;35:38-41.
10. Moutquin JM, Garner PR, Burrows RF, Rey E, et al. Report of the Canadian Hypertension Society Consensus Conference: 2. Nonpharmacologic management and prevention of hypertensive disorders in pregnancy. Can Med Assoc J 1997;57:907-919.
Yes. The use of low-dose aspirin during pregnancy decreases the risk of preeclampsia for women considered at increased risk. The effect is smaller for women without risk factors (strength of recommendation [SOR]: A, based on randomized controlled trials [RCTs] and systematic reviews [SRs] of RCTs).
Rates of preterm delivery, perinatal death, and incidence of small-for-gestational age infants are decreased for women treated with low-dose aspirin (SOR: A, based on SRs and RCTs). A meta-analysis of RCTs has found no increased rates of harm from low-dose aspirin therapy, including placental abruption or other antepartum bleeding complications (SOR: A, based on SRs and RCTs).
I prescribe 81 mg/day of aspirin for women with previous severe preeclampsia
John Hill, DO
Department of Family Medicine, University of Colorado, Denver
Confused about when to use aspirin in pregnancy? You’re not alone. Over my 20 years of practice, I have reacted to disparate guidelines ranging from “never use aspirin in pregnancy” to “always use low-dose aspirin.” This review helps simplify my clinical practice.
With the benefit of evidence from multiple RCTs over the past 7 years, I now personally use 81 mg of aspirin each day in 2 groups of women: those who had severe preeclampsia in a prior pregnancy, and those who develop signs of preeclampsia or strong risk factors for it before the third trimester in their current pregnancy.
Evidence summary
Systematic reviews show aspirin lowers rates of preeclampsia
Four SRs published between 2001 and 20071-4 and a Cochrane Review updated in 20065 have demonstrated that low-dose aspirin helps to prevent preeclampsia, reduction in preterm delivery rates, and decreased perinatal mortality.
The 2001 SR by Duley1 included 39 trials and 30,563 patients. Patients were classified either as high-risk (previous severe preeclampsia, diabetes, chronic hypertension, renal disease, or autoimmune disease) or moderate-risk (remainder of subjects). Four individual studies (with a combined weight of 27%) did not support aspirin therapy. The largest trial not supporting aspirin therapy included 6275 subjects and had a relative risk of 1.14 (95% CI, 0.94–1.38).
Most studies in this review compared aspirin alone with placebo (28,802 subjects). However, 4 studies either compared combination therapy with aspirin or other thromboprophylaxis therapy (dipyridamole, heparin, or ozagrel). Although there were differences in risk stratification, variable doses of aspirin, and varied gestational age at trial entry, all studies reported an overall 15% reduction of preeclampsia (RR=0.85; 95% CI, 0.78–0.92).
The 2003 SR by Coomarasamy2 included 14 trials and 12,416 patients. The study exclusively evaluated high-risk pregnancies: women with history (or family history) of preeclampsia, chronic hypertension, gestational diabetes, or renal disease. The overall reduction in preeclampsia was 14% (relative risk [RR]=0.86; 95% confidence interval [CI], 0.76–0.96). Results were consistent across RCTs, and only 2 of the 14 studies (with a combined weight of 7.1%) did not support aspirin therapy.
TABLE
Low-dose aspirin reduces risk of preeclampsia, but how does it affect other maternal and fetal outcomes?
| STUDY (YEAR) | DEVELOPMENT OF PREECLAMPSIA | PRETERM DELIVERY | NEONATAL DEATH | SGA OR LOW BIRTH WEIGHT | RISK OF ABRUPTION & BLEEDING |
|---|---|---|---|---|---|
| Duley (2001)1 | Moderate-risk patients: 15% reduction High-risk patients: 15% reduction NNT=100 | 8% reduction NNT=72 | 14% reduction NNT= 250 | 8% reduction* | Not reported |
| Coomarasamy (2003)2 | 14% reduction | 14% reduction | 21% reduction | 215-g weight gain in aspirin group | No significant clinical difference in risk RR=0.98) |
| Ruano (2005)3 | Low-risk patients: no significant reduction High-risk patients: 13% reduction | Not reported | |||
| Askie (2007)4 | 10% reduction | 10% reduction | 9% reduction | 10% reduction | No significant clinical difference in risk (RR=0.90–1.15) |
| Cochrane (2007)5 | 19% reduction NNT=69 (overall), 118 (moderate risk), 18 (high-risk) | 7% reduction NNT=83 | 16% reduction NNT=227 | 8% reduction* | No significant clinical difference in risk (RR=1.06) |
| * Borderline for statistical significance (RR=0.92). | |||||
| SGA, small for gestational age; NNT, number needed to treat; RR, relative risk. | |||||
Ruano’s 2005 SR3 included 22 trials with 33,598 subjects and specifically compared low-risk vs high-risk patients. The authors concluded that there was no significant reduction in preeclampsia with the use of low-dose aspirin in the low-risk arm (RR=0.95; 95% CI, 0.81–1.11), and a 13% reduction among high-risk subjects (RR=0.87; 95% CI, 0.79–0.96).3
A 2007 meta-analysis by Askie4 included 31 trials with 32,217 women and their 32,819 infants. Main outcomes (regardless of initial maternal risks) were 1) onset of preeclampsia, 2) neonatal death, 3) preterm birth at <34 weeks gestation, 4) infant small for gestational age, and 5) pregnancy with serious adverse outcome. Results of these outcome measures consistently showed a relative risk reduction of 10% for subjects taking low-dose aspirin, except for neonatal deaths, which had a 9% reduction. This study also suggested that multiparous women and women with a history of hypertensive disorder of pregnancy may derive a larger benefit from low-dose aspirin.
A Cochrane Review5 updated in 2007 demonstrated that low-dose aspirin provided a moderate (19%) reduction in the overall risk of developing preeclampsia. New stratified analysis of the data indicates that in moderate-risk women, antiplatelet therapy is associated with a 15% reduction, and that high-risk women have a 27% reduction in the risk of developing preeclampsia. The effect on small-for-gestational-age infants revealed no overall clinically significant differences.
Aspirin dosing: One study recommends >75 mg/day
Studies varied in the aspirin dosage they used and duration of treatment. In all RCTs, the dose of aspirin ranged from 50 mg/day to 150 mg/day. Earlier trials used lower doses of aspirin (50–75 mg/day), while recent trials used 100 mg or more per day.
Early RCTs revealed no correlation between the dose of aspirin and the prevention of preeclampsia. However, Villar et al6 showed a greater effect among women treated with doses greater than 75 mg/day of aspirin (RR=0.49; 95% CI, 0.38–0.63).6
No evidence of harm from aspirin
There is no evidence of harm from low-dose aspirin therapy—including placental abruption, antenatal admissions, fetal intraventricular hemorrhage and other neonatal bleeding complications, admission to neonatal care unit, induction of labor, or caesarean delivery—regardless of initial risk stratification.7
Recommendations from others
The 2002 American College of Obstetricians and Gynecologists Practice Bulletin states that low-dose aspirin in women at low risk has not been shown to prevent preeclampsia and therefore is not recommended. They make no specific statement regarding the use of low-dose aspirin in moderate- to high-risk pregnancies.8
The Australasian Society for the Study of Hypertension in Pregnancy conclude that low-dose aspirin for prevention of preeclampsia is reasonable for the following conditions: 1) prior fetal loss after first trimester due to placental insufficiency or severe fetal growth retardation, and 2) women with severe early onset preeclampsia in previous pregnancy necessitating delivery ≤32 weeks gestation. Despite difficulties in predicting who will deliver preterm, consider women who have had severe early-onset preeclampsia in a previous pregnancy for low-dose aspirin therapy.9
The Canadian Hypertension Society Consensus Panel concludes low-dose aspirin therapy is effective in decreasing the incidence of preterm delivery and early-onset preeclampsia among women at risk of developing the syndrome.10
Yes. The use of low-dose aspirin during pregnancy decreases the risk of preeclampsia for women considered at increased risk. The effect is smaller for women without risk factors (strength of recommendation [SOR]: A, based on randomized controlled trials [RCTs] and systematic reviews [SRs] of RCTs).
Rates of preterm delivery, perinatal death, and incidence of small-for-gestational age infants are decreased for women treated with low-dose aspirin (SOR: A, based on SRs and RCTs). A meta-analysis of RCTs has found no increased rates of harm from low-dose aspirin therapy, including placental abruption or other antepartum bleeding complications (SOR: A, based on SRs and RCTs).
I prescribe 81 mg/day of aspirin for women with previous severe preeclampsia
John Hill, DO
Department of Family Medicine, University of Colorado, Denver
Confused about when to use aspirin in pregnancy? You’re not alone. Over my 20 years of practice, I have reacted to disparate guidelines ranging from “never use aspirin in pregnancy” to “always use low-dose aspirin.” This review helps simplify my clinical practice.
With the benefit of evidence from multiple RCTs over the past 7 years, I now personally use 81 mg of aspirin each day in 2 groups of women: those who had severe preeclampsia in a prior pregnancy, and those who develop signs of preeclampsia or strong risk factors for it before the third trimester in their current pregnancy.
Evidence summary
Systematic reviews show aspirin lowers rates of preeclampsia
Four SRs published between 2001 and 20071-4 and a Cochrane Review updated in 20065 have demonstrated that low-dose aspirin helps to prevent preeclampsia, reduction in preterm delivery rates, and decreased perinatal mortality.
The 2001 SR by Duley1 included 39 trials and 30,563 patients. Patients were classified either as high-risk (previous severe preeclampsia, diabetes, chronic hypertension, renal disease, or autoimmune disease) or moderate-risk (remainder of subjects). Four individual studies (with a combined weight of 27%) did not support aspirin therapy. The largest trial not supporting aspirin therapy included 6275 subjects and had a relative risk of 1.14 (95% CI, 0.94–1.38).
Most studies in this review compared aspirin alone with placebo (28,802 subjects). However, 4 studies either compared combination therapy with aspirin or other thromboprophylaxis therapy (dipyridamole, heparin, or ozagrel). Although there were differences in risk stratification, variable doses of aspirin, and varied gestational age at trial entry, all studies reported an overall 15% reduction of preeclampsia (RR=0.85; 95% CI, 0.78–0.92).
The 2003 SR by Coomarasamy2 included 14 trials and 12,416 patients. The study exclusively evaluated high-risk pregnancies: women with history (or family history) of preeclampsia, chronic hypertension, gestational diabetes, or renal disease. The overall reduction in preeclampsia was 14% (relative risk [RR]=0.86; 95% confidence interval [CI], 0.76–0.96). Results were consistent across RCTs, and only 2 of the 14 studies (with a combined weight of 7.1%) did not support aspirin therapy.
TABLE
Low-dose aspirin reduces risk of preeclampsia, but how does it affect other maternal and fetal outcomes?
| STUDY (YEAR) | DEVELOPMENT OF PREECLAMPSIA | PRETERM DELIVERY | NEONATAL DEATH | SGA OR LOW BIRTH WEIGHT | RISK OF ABRUPTION & BLEEDING |
|---|---|---|---|---|---|
| Duley (2001)1 | Moderate-risk patients: 15% reduction High-risk patients: 15% reduction NNT=100 | 8% reduction NNT=72 | 14% reduction NNT= 250 | 8% reduction* | Not reported |
| Coomarasamy (2003)2 | 14% reduction | 14% reduction | 21% reduction | 215-g weight gain in aspirin group | No significant clinical difference in risk RR=0.98) |
| Ruano (2005)3 | Low-risk patients: no significant reduction High-risk patients: 13% reduction | Not reported | |||
| Askie (2007)4 | 10% reduction | 10% reduction | 9% reduction | 10% reduction | No significant clinical difference in risk (RR=0.90–1.15) |
| Cochrane (2007)5 | 19% reduction NNT=69 (overall), 118 (moderate risk), 18 (high-risk) | 7% reduction NNT=83 | 16% reduction NNT=227 | 8% reduction* | No significant clinical difference in risk (RR=1.06) |
| * Borderline for statistical significance (RR=0.92). | |||||
| SGA, small for gestational age; NNT, number needed to treat; RR, relative risk. | |||||
Ruano’s 2005 SR3 included 22 trials with 33,598 subjects and specifically compared low-risk vs high-risk patients. The authors concluded that there was no significant reduction in preeclampsia with the use of low-dose aspirin in the low-risk arm (RR=0.95; 95% CI, 0.81–1.11), and a 13% reduction among high-risk subjects (RR=0.87; 95% CI, 0.79–0.96).3
A 2007 meta-analysis by Askie4 included 31 trials with 32,217 women and their 32,819 infants. Main outcomes (regardless of initial maternal risks) were 1) onset of preeclampsia, 2) neonatal death, 3) preterm birth at <34 weeks gestation, 4) infant small for gestational age, and 5) pregnancy with serious adverse outcome. Results of these outcome measures consistently showed a relative risk reduction of 10% for subjects taking low-dose aspirin, except for neonatal deaths, which had a 9% reduction. This study also suggested that multiparous women and women with a history of hypertensive disorder of pregnancy may derive a larger benefit from low-dose aspirin.
A Cochrane Review5 updated in 2007 demonstrated that low-dose aspirin provided a moderate (19%) reduction in the overall risk of developing preeclampsia. New stratified analysis of the data indicates that in moderate-risk women, antiplatelet therapy is associated with a 15% reduction, and that high-risk women have a 27% reduction in the risk of developing preeclampsia. The effect on small-for-gestational-age infants revealed no overall clinically significant differences.
Aspirin dosing: One study recommends >75 mg/day
Studies varied in the aspirin dosage they used and duration of treatment. In all RCTs, the dose of aspirin ranged from 50 mg/day to 150 mg/day. Earlier trials used lower doses of aspirin (50–75 mg/day), while recent trials used 100 mg or more per day.
Early RCTs revealed no correlation between the dose of aspirin and the prevention of preeclampsia. However, Villar et al6 showed a greater effect among women treated with doses greater than 75 mg/day of aspirin (RR=0.49; 95% CI, 0.38–0.63).6
No evidence of harm from aspirin
There is no evidence of harm from low-dose aspirin therapy—including placental abruption, antenatal admissions, fetal intraventricular hemorrhage and other neonatal bleeding complications, admission to neonatal care unit, induction of labor, or caesarean delivery—regardless of initial risk stratification.7
Recommendations from others
The 2002 American College of Obstetricians and Gynecologists Practice Bulletin states that low-dose aspirin in women at low risk has not been shown to prevent preeclampsia and therefore is not recommended. They make no specific statement regarding the use of low-dose aspirin in moderate- to high-risk pregnancies.8
The Australasian Society for the Study of Hypertension in Pregnancy conclude that low-dose aspirin for prevention of preeclampsia is reasonable for the following conditions: 1) prior fetal loss after first trimester due to placental insufficiency or severe fetal growth retardation, and 2) women with severe early onset preeclampsia in previous pregnancy necessitating delivery ≤32 weeks gestation. Despite difficulties in predicting who will deliver preterm, consider women who have had severe early-onset preeclampsia in a previous pregnancy for low-dose aspirin therapy.9
The Canadian Hypertension Society Consensus Panel concludes low-dose aspirin therapy is effective in decreasing the incidence of preterm delivery and early-onset preeclampsia among women at risk of developing the syndrome.10
1. Duley L, Henderson-Smart DJ, Knight M, King JF. Antiplatelet drugs for prevention of pre-eclampsia and its consequences: systematic review. BMJ 2001;322:329-333.
2. Coomarasamy A, Papaioannou S, Gee H, Khan KS. Aspirin for prevention of pre-eclampsia in women with historical risk factors: a systematic review. Obstet Gynecol 2003;101:1319-1332.
3. Ruano R, Fontes RS, Zugaib M. Prevention of preeclampsia with low-dose aspirin—a systematic review and meta-analysis of the main randomized controlled trials. Clinics 2005;60:407-414.
4. Askie LM, Duley L, Henderson-Stewart DJ, et al. Antiplatelet agents for prevention of pre-eclampsia: a meta-analysis of individual patient data. Lancet 2007;369:1791-1798.
5. Duley L, Henderson-Smart DJ, Knight M, King JF. Antiplatelet agents for preventing pre-eclampsia and its complication (review). Cochrane Database Syst Rev 2003;(4):CD004659.-
6. Villar J, Abalos E, Nardin JM, et al. Strategies to prevent and treat pre-eclampsia: evidence from randomized controlled trials. Semin Nephrol 2004;24:607-615.
7. Coomarasamy A, Braunholtz D, Song F, et al. Individualizing use of aspirin to prevent pre-eclampsia: a framework for clinical decision making. BJOG 2003;110:882-888.
8. ACOG Committee on Obstetric Practice. Diagnosis and management of pre-eclampsia and eclampsia. ACOG Practice Bulletin, no 33. Int J Gynaecol Obstet 2002;77:67-75.
9. Brown MA, Brennecke SP, Crowther CA, et al. Aspirin and prevention of pre-eclampsia. Aust NZ J Obstet Gynaecol 1995;35:38-41.
10. Moutquin JM, Garner PR, Burrows RF, Rey E, et al. Report of the Canadian Hypertension Society Consensus Conference: 2. Nonpharmacologic management and prevention of hypertensive disorders in pregnancy. Can Med Assoc J 1997;57:907-919.
1. Duley L, Henderson-Smart DJ, Knight M, King JF. Antiplatelet drugs for prevention of pre-eclampsia and its consequences: systematic review. BMJ 2001;322:329-333.
2. Coomarasamy A, Papaioannou S, Gee H, Khan KS. Aspirin for prevention of pre-eclampsia in women with historical risk factors: a systematic review. Obstet Gynecol 2003;101:1319-1332.
3. Ruano R, Fontes RS, Zugaib M. Prevention of preeclampsia with low-dose aspirin—a systematic review and meta-analysis of the main randomized controlled trials. Clinics 2005;60:407-414.
4. Askie LM, Duley L, Henderson-Stewart DJ, et al. Antiplatelet agents for prevention of pre-eclampsia: a meta-analysis of individual patient data. Lancet 2007;369:1791-1798.
5. Duley L, Henderson-Smart DJ, Knight M, King JF. Antiplatelet agents for preventing pre-eclampsia and its complication (review). Cochrane Database Syst Rev 2003;(4):CD004659.-
6. Villar J, Abalos E, Nardin JM, et al. Strategies to prevent and treat pre-eclampsia: evidence from randomized controlled trials. Semin Nephrol 2004;24:607-615.
7. Coomarasamy A, Braunholtz D, Song F, et al. Individualizing use of aspirin to prevent pre-eclampsia: a framework for clinical decision making. BJOG 2003;110:882-888.
8. ACOG Committee on Obstetric Practice. Diagnosis and management of pre-eclampsia and eclampsia. ACOG Practice Bulletin, no 33. Int J Gynaecol Obstet 2002;77:67-75.
9. Brown MA, Brennecke SP, Crowther CA, et al. Aspirin and prevention of pre-eclampsia. Aust NZ J Obstet Gynaecol 1995;35:38-41.
10. Moutquin JM, Garner PR, Burrows RF, Rey E, et al. Report of the Canadian Hypertension Society Consensus Conference: 2. Nonpharmacologic management and prevention of hypertensive disorders in pregnancy. Can Med Assoc J 1997;57:907-919.
Evidence-based answers from the Family Physicians Inquiries Network
What is the most effective and safe malaria prophylaxis during pregnancy?
Chloroquine and mefloquine have superior safety profiles in pregnancy, though all antimalarials are effective for prophylaxis. Antimalarials will decrease the severity of maternal malaria infection and malaria-associated anemia, while decreasing the incidence of low birth weight and perinatal death in women having their first or second baby (strength of recommendation [SOR]: A, based on systematic review of consistent, good-quality patient-oriented evidence).
You can determine malaria risk and sensitivity of Plasmodium species by country at wwwn.cdc.gov/travel/destinationlist.aspx.1 Urge women to delay travel until after pregnancy if possible2 (SOR: C, based on patient-oriented expert opinion).
Don’t forget to discuss mosquito netting and insect repellant
Meg Hayes, MD
Department of Family Medicine, Oregon Health and Science University, Portland
Adverse outcomes associated with malaria during pregnancy include restricted fetal growth, low birth weight, preterm delivery, congenital infection, spontaneous abortion, and perinatal death. You should counsel travelers to avoid travel to areas where malaria is endemic during pregnancy.
For those who are unable to avoid travel, or who reside in malaria-endemic areas during pregnancy, physicians should focus not only on chemoprophylaxis, but provide verbal and written counsel regarding malaria personal protection measures. Because mosquitoes usually feed at night, travelers should remain within screened areas after dusk, use permethrin-treated bed nets, wear protective clothing, and apply insect repellant. Advise patients who travel to malaria-endemic areas to quickly report febrile illnesses and to disclose their travel histories to healthcare providers.
Evidence summary
Malaria is a parasitic infection that causes significant morbidity and mortality worldwide, with more than 500 million people becoming severely ill every year.2 For pregnant women, malarial infection can be severe, with high fevers, chills, and anemia leading to increased risk of poor maternal and fetal outcomes—including death. Pregnant women are also more likely to become infected and to develop more severe disease—they attract twice as many mosquitoes as nonpregnant women and have a relative immuno-suppression.3
TABLE
Antimalarials for prophylaxis: Chloroquine, mefloquine are best choices during pregnancy
| DRUG | EFFICACY | SAFETY | PREGNANCY CLASS* | AVAILABILITY |
|---|---|---|---|---|
| Chloroquine | Good | Excellent | C | Worldwide |
| Chloroquine/proguanil | Good | Excellent | C | Worldwide |
| Mefloquine | Excellent | Good | C | Worldwide |
| Quinine | Excellent | Good | C† | Worldwide |
| Atovaquone/proguanil | Excellent | Good | C (poorly studied) | Worldwide |
| Artesunate | Excellent | Good | N/A | Asia, Africa, limited in UK, not in US |
| Primaquine | Good | Fair | C‡ | Worldwide |
| Doxycycline | Excellent | Fair | D (teratogenic) | Worldwide |
| Sulfadoxine/pyrimethamine | Fair | Poor | C | Worldwide, but restricted in US |
| Note: Prescribers and patients are urged to refer to the CDC reference about pregnancy in malaria (wwwn.cdc.gov/travel/contentMalariaPregnantPublic.aspx) and to specific country information regarding sensitivities of malaria (wwwn.cdc.gov/travel/destinationList.aspx). | ||||
| * Pregnancy class C: Animal reproduction studies have shown an adverse effect on the fetus and there are no adequate and well-controlled studies in humans, but potential benefits may warrant use of the drug in pregnant women, despite potential risks. | ||||
| * Pregnancy class D: There is positive evidence of human fetal risk based on adverse reaction data from investigational or marketing experience or studies in humans, but potential benefits may warrant use of the drug in pregnant women, despite potential risks. | ||||
| † Monitor patients for maternal hypoglycemia. | ||||
| ‡ There is no evidence of teratogenicity, but primaquine is associated with fetal intravascular hemolysis. | ||||
Chemoprophylaxis lowers rates of maternal infection
Although prophylaxis for pregnant patients traveling to malarial regions is a public concern, data for decision-making must be extrapolated from the available evidence, which is based primarily on women living in endemic areas. In a Cochrane systematic review, antimalarials were found to decrease the incidence of maternal infections (relative risk [RR]=0.27; 95% confidence interval [CI], 0.17–0.44) and reduce maternal anemia (RR=0.62; 95% CI, 0.50–0.78) in low-parity women—ie, during a first or second pregnancy.3
In low-parity women, these drugs were also found to decrease perinatal death (RR=0.73; 95% CI, 0.53–0.99) and low birth weight (RR=0.57; 95% CI, 0.46–0.72) associated with malarial infection. When used in all parity groups, antimalarials were somewhat less effective, yet still reduced maternal infections (RR=0.53; 95% CI, 0.33–0.86); the effects were similar with all antimalarials tested.3,4
Chloroquine, mefloquine are safe in pregnancy, doxycycline is not
While chemoprophylaxis in pregnancy appears efficacious, a major question remains—which agents are safest for both the woman and fetus? Some drugs routinely used in nonpregnant individuals should not be offered to pregnant women because of known direct effects on the fetus. Doxycycline is teratogenic, and primaquine poses a significant risk of fetal intravascular hemolysis in G6PD-deficient fetuses.5 Other drugs, such as atovaquone/proguanil and artesunate, are not well studied in pregnancy, and therefore are not recommended for use unless other options are not available.2,6
Among drugs that are well studied and without known direct fetal-damaging effects, adverse drug reaction profiles can guide use based on disease prevalence and drug-resistance patterns.
- Chloroquine is widely used because it is inexpensive and well tolerated, with only pruritus, mouth ulcers, and gastrointestinal upset as the most common adverse effects.
- Mefloquine is usually well tolerated, but can cause dose-related neuropsychiatric effects; it is contraindicated in those with a history of epilepsy or psychiatric disease.
- Sulfadoxine and pyrimethamine are not normally used as prophylaxis for any patient, due to the risk of toxic epidermal necrolysis and Stevens-Johnson syndrome, and the possible risk of jaundice and kernicterus if used in the third trimester of pregnancy.
- Quinine, which can be used for treatment or prophylaxis, may cause hypoglycemia, an effect that is more pronounced during pregnancy and requires close monitoring of blood glucose levels.5,7
Given these reaction profiles, chloroquine or mefloquine are usually the best choice with their superior safety and efficacy.
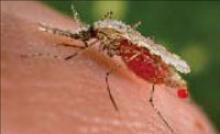
Figure
Best protection: Avoidance
Chloroquine and mefloquine are the safest antimalarials for use in pregnant women, but personal protection measures are also critical. Above, an Anopheles stephensi mosquito expelling a droplet of blood from its abdomen after having engorged itself on its human host’s blood. (Source: CDC.)
Recommendations from others
The World Health Organization (WHO) recommends pregnant women avoid travel to malarial regions. If travel is required, WHO recommends chloroquine as first-line prophylaxis in pregnancy (plus proguanil if the region exhibits emerging chloroquine resistance). In areas with proven chloroquine resistance, mefloquine is the drug of choice. Other antimalarials—such as quinine, pyrimethamine, sulfadoxine, and artesunate—should not be withheld if the preferred drugs are not available, or if the infection is life-threatening.2
The Centers for Disease Control and Prevention (CDC) also recommends avoiding travel to malaria-endemic regions during pregnancy, but if travel is necessary, the CDC advises use of chloroquine (or mefloquine in regions with chloroquine resistance). The CDC discourages the use of atovaquone/proguanil, doxycycline, and primaquine, due to known adverse fetal effects or inadequate experience in pregnancy.6
Acknowledgments
The opinions and assertions contained herein are the private views of the authors and not to be construed as official, or as reflecting the views of the US Air Force Medical Service or the US Air Force at large.
1. Centers for Disease Control and Prevention Web site. Destinations: CDC Traveler’s Health. Available at: wwwn.cdc.gov/travel/destinationlist.aspx. Accessed on December 7, 2007.
2. Malaria. In: International Travel and Health. Geneva, Switzerland: World Health Organization; 2007. Available at: whqlibdoc.who.int/publications/2005/9241580364_chap7.pdf. Accessed on December 7, 2007.
3. Orton L, Garner P. Drugs for treating uncomplicated malaria in pregnant women. Cochrane Database Syst Rev 2005;(3):CD004912.-
4. Garner P, Gülmezoglu AM. Drugs for preventing malaria in pregnant women. Cochrane Database Syst Rev 2006;(4):CD000169.-
5. Phillips-Howard PA, Wood D. The safety of antimalarial drugs in pregnancy. Drug Saf 1996;14:131-145.
6. Centers for Disease Control and Prevention Web site. Diseases: Malaria: Prevention, Pregnant Women, Public Info. Available at: wwwn.cdc.gov/travel/ contentMalariaPregnantPublic.aspx. Accessed on December 7, 2007.
7. Taylor WR, White NJ. Antimalarial drug toxicity: a review. Drug Saf 2004;27:25-61.
Chloroquine and mefloquine have superior safety profiles in pregnancy, though all antimalarials are effective for prophylaxis. Antimalarials will decrease the severity of maternal malaria infection and malaria-associated anemia, while decreasing the incidence of low birth weight and perinatal death in women having their first or second baby (strength of recommendation [SOR]: A, based on systematic review of consistent, good-quality patient-oriented evidence).
You can determine malaria risk and sensitivity of Plasmodium species by country at wwwn.cdc.gov/travel/destinationlist.aspx.1 Urge women to delay travel until after pregnancy if possible2 (SOR: C, based on patient-oriented expert opinion).
Don’t forget to discuss mosquito netting and insect repellant
Meg Hayes, MD
Department of Family Medicine, Oregon Health and Science University, Portland
Adverse outcomes associated with malaria during pregnancy include restricted fetal growth, low birth weight, preterm delivery, congenital infection, spontaneous abortion, and perinatal death. You should counsel travelers to avoid travel to areas where malaria is endemic during pregnancy.
For those who are unable to avoid travel, or who reside in malaria-endemic areas during pregnancy, physicians should focus not only on chemoprophylaxis, but provide verbal and written counsel regarding malaria personal protection measures. Because mosquitoes usually feed at night, travelers should remain within screened areas after dusk, use permethrin-treated bed nets, wear protective clothing, and apply insect repellant. Advise patients who travel to malaria-endemic areas to quickly report febrile illnesses and to disclose their travel histories to healthcare providers.
Evidence summary
Malaria is a parasitic infection that causes significant morbidity and mortality worldwide, with more than 500 million people becoming severely ill every year.2 For pregnant women, malarial infection can be severe, with high fevers, chills, and anemia leading to increased risk of poor maternal and fetal outcomes—including death. Pregnant women are also more likely to become infected and to develop more severe disease—they attract twice as many mosquitoes as nonpregnant women and have a relative immuno-suppression.3
TABLE
Antimalarials for prophylaxis: Chloroquine, mefloquine are best choices during pregnancy
| DRUG | EFFICACY | SAFETY | PREGNANCY CLASS* | AVAILABILITY |
|---|---|---|---|---|
| Chloroquine | Good | Excellent | C | Worldwide |
| Chloroquine/proguanil | Good | Excellent | C | Worldwide |
| Mefloquine | Excellent | Good | C | Worldwide |
| Quinine | Excellent | Good | C† | Worldwide |
| Atovaquone/proguanil | Excellent | Good | C (poorly studied) | Worldwide |
| Artesunate | Excellent | Good | N/A | Asia, Africa, limited in UK, not in US |
| Primaquine | Good | Fair | C‡ | Worldwide |
| Doxycycline | Excellent | Fair | D (teratogenic) | Worldwide |
| Sulfadoxine/pyrimethamine | Fair | Poor | C | Worldwide, but restricted in US |
| Note: Prescribers and patients are urged to refer to the CDC reference about pregnancy in malaria (wwwn.cdc.gov/travel/contentMalariaPregnantPublic.aspx) and to specific country information regarding sensitivities of malaria (wwwn.cdc.gov/travel/destinationList.aspx). | ||||
| * Pregnancy class C: Animal reproduction studies have shown an adverse effect on the fetus and there are no adequate and well-controlled studies in humans, but potential benefits may warrant use of the drug in pregnant women, despite potential risks. | ||||
| * Pregnancy class D: There is positive evidence of human fetal risk based on adverse reaction data from investigational or marketing experience or studies in humans, but potential benefits may warrant use of the drug in pregnant women, despite potential risks. | ||||
| † Monitor patients for maternal hypoglycemia. | ||||
| ‡ There is no evidence of teratogenicity, but primaquine is associated with fetal intravascular hemolysis. | ||||
Chemoprophylaxis lowers rates of maternal infection
Although prophylaxis for pregnant patients traveling to malarial regions is a public concern, data for decision-making must be extrapolated from the available evidence, which is based primarily on women living in endemic areas. In a Cochrane systematic review, antimalarials were found to decrease the incidence of maternal infections (relative risk [RR]=0.27; 95% confidence interval [CI], 0.17–0.44) and reduce maternal anemia (RR=0.62; 95% CI, 0.50–0.78) in low-parity women—ie, during a first or second pregnancy.3
In low-parity women, these drugs were also found to decrease perinatal death (RR=0.73; 95% CI, 0.53–0.99) and low birth weight (RR=0.57; 95% CI, 0.46–0.72) associated with malarial infection. When used in all parity groups, antimalarials were somewhat less effective, yet still reduced maternal infections (RR=0.53; 95% CI, 0.33–0.86); the effects were similar with all antimalarials tested.3,4
Chloroquine, mefloquine are safe in pregnancy, doxycycline is not
While chemoprophylaxis in pregnancy appears efficacious, a major question remains—which agents are safest for both the woman and fetus? Some drugs routinely used in nonpregnant individuals should not be offered to pregnant women because of known direct effects on the fetus. Doxycycline is teratogenic, and primaquine poses a significant risk of fetal intravascular hemolysis in G6PD-deficient fetuses.5 Other drugs, such as atovaquone/proguanil and artesunate, are not well studied in pregnancy, and therefore are not recommended for use unless other options are not available.2,6
Among drugs that are well studied and without known direct fetal-damaging effects, adverse drug reaction profiles can guide use based on disease prevalence and drug-resistance patterns.
- Chloroquine is widely used because it is inexpensive and well tolerated, with only pruritus, mouth ulcers, and gastrointestinal upset as the most common adverse effects.
- Mefloquine is usually well tolerated, but can cause dose-related neuropsychiatric effects; it is contraindicated in those with a history of epilepsy or psychiatric disease.
- Sulfadoxine and pyrimethamine are not normally used as prophylaxis for any patient, due to the risk of toxic epidermal necrolysis and Stevens-Johnson syndrome, and the possible risk of jaundice and kernicterus if used in the third trimester of pregnancy.
- Quinine, which can be used for treatment or prophylaxis, may cause hypoglycemia, an effect that is more pronounced during pregnancy and requires close monitoring of blood glucose levels.5,7
Given these reaction profiles, chloroquine or mefloquine are usually the best choice with their superior safety and efficacy.
Figure
Best protection: Avoidance
Chloroquine and mefloquine are the safest antimalarials for use in pregnant women, but personal protection measures are also critical. Above, an Anopheles stephensi mosquito expelling a droplet of blood from its abdomen after having engorged itself on its human host’s blood. (Source: CDC.)
Recommendations from others
The World Health Organization (WHO) recommends pregnant women avoid travel to malarial regions. If travel is required, WHO recommends chloroquine as first-line prophylaxis in pregnancy (plus proguanil if the region exhibits emerging chloroquine resistance). In areas with proven chloroquine resistance, mefloquine is the drug of choice. Other antimalarials—such as quinine, pyrimethamine, sulfadoxine, and artesunate—should not be withheld if the preferred drugs are not available, or if the infection is life-threatening.2
The Centers for Disease Control and Prevention (CDC) also recommends avoiding travel to malaria-endemic regions during pregnancy, but if travel is necessary, the CDC advises use of chloroquine (or mefloquine in regions with chloroquine resistance). The CDC discourages the use of atovaquone/proguanil, doxycycline, and primaquine, due to known adverse fetal effects or inadequate experience in pregnancy.6
Acknowledgments
The opinions and assertions contained herein are the private views of the authors and not to be construed as official, or as reflecting the views of the US Air Force Medical Service or the US Air Force at large.
Chloroquine and mefloquine have superior safety profiles in pregnancy, though all antimalarials are effective for prophylaxis. Antimalarials will decrease the severity of maternal malaria infection and malaria-associated anemia, while decreasing the incidence of low birth weight and perinatal death in women having their first or second baby (strength of recommendation [SOR]: A, based on systematic review of consistent, good-quality patient-oriented evidence).
You can determine malaria risk and sensitivity of Plasmodium species by country at wwwn.cdc.gov/travel/destinationlist.aspx.1 Urge women to delay travel until after pregnancy if possible2 (SOR: C, based on patient-oriented expert opinion).
Don’t forget to discuss mosquito netting and insect repellant
Meg Hayes, MD
Department of Family Medicine, Oregon Health and Science University, Portland
Adverse outcomes associated with malaria during pregnancy include restricted fetal growth, low birth weight, preterm delivery, congenital infection, spontaneous abortion, and perinatal death. You should counsel travelers to avoid travel to areas where malaria is endemic during pregnancy.
For those who are unable to avoid travel, or who reside in malaria-endemic areas during pregnancy, physicians should focus not only on chemoprophylaxis, but provide verbal and written counsel regarding malaria personal protection measures. Because mosquitoes usually feed at night, travelers should remain within screened areas after dusk, use permethrin-treated bed nets, wear protective clothing, and apply insect repellant. Advise patients who travel to malaria-endemic areas to quickly report febrile illnesses and to disclose their travel histories to healthcare providers.
Evidence summary
Malaria is a parasitic infection that causes significant morbidity and mortality worldwide, with more than 500 million people becoming severely ill every year.2 For pregnant women, malarial infection can be severe, with high fevers, chills, and anemia leading to increased risk of poor maternal and fetal outcomes—including death. Pregnant women are also more likely to become infected and to develop more severe disease—they attract twice as many mosquitoes as nonpregnant women and have a relative immuno-suppression.3
TABLE
Antimalarials for prophylaxis: Chloroquine, mefloquine are best choices during pregnancy
| DRUG | EFFICACY | SAFETY | PREGNANCY CLASS* | AVAILABILITY |
|---|---|---|---|---|
| Chloroquine | Good | Excellent | C | Worldwide |
| Chloroquine/proguanil | Good | Excellent | C | Worldwide |
| Mefloquine | Excellent | Good | C | Worldwide |
| Quinine | Excellent | Good | C† | Worldwide |
| Atovaquone/proguanil | Excellent | Good | C (poorly studied) | Worldwide |
| Artesunate | Excellent | Good | N/A | Asia, Africa, limited in UK, not in US |
| Primaquine | Good | Fair | C‡ | Worldwide |
| Doxycycline | Excellent | Fair | D (teratogenic) | Worldwide |
| Sulfadoxine/pyrimethamine | Fair | Poor | C | Worldwide, but restricted in US |
| Note: Prescribers and patients are urged to refer to the CDC reference about pregnancy in malaria (wwwn.cdc.gov/travel/contentMalariaPregnantPublic.aspx) and to specific country information regarding sensitivities of malaria (wwwn.cdc.gov/travel/destinationList.aspx). | ||||
| * Pregnancy class C: Animal reproduction studies have shown an adverse effect on the fetus and there are no adequate and well-controlled studies in humans, but potential benefits may warrant use of the drug in pregnant women, despite potential risks. | ||||
| * Pregnancy class D: There is positive evidence of human fetal risk based on adverse reaction data from investigational or marketing experience or studies in humans, but potential benefits may warrant use of the drug in pregnant women, despite potential risks. | ||||
| † Monitor patients for maternal hypoglycemia. | ||||
| ‡ There is no evidence of teratogenicity, but primaquine is associated with fetal intravascular hemolysis. | ||||
Chemoprophylaxis lowers rates of maternal infection
Although prophylaxis for pregnant patients traveling to malarial regions is a public concern, data for decision-making must be extrapolated from the available evidence, which is based primarily on women living in endemic areas. In a Cochrane systematic review, antimalarials were found to decrease the incidence of maternal infections (relative risk [RR]=0.27; 95% confidence interval [CI], 0.17–0.44) and reduce maternal anemia (RR=0.62; 95% CI, 0.50–0.78) in low-parity women—ie, during a first or second pregnancy.3
In low-parity women, these drugs were also found to decrease perinatal death (RR=0.73; 95% CI, 0.53–0.99) and low birth weight (RR=0.57; 95% CI, 0.46–0.72) associated with malarial infection. When used in all parity groups, antimalarials were somewhat less effective, yet still reduced maternal infections (RR=0.53; 95% CI, 0.33–0.86); the effects were similar with all antimalarials tested.3,4
Chloroquine, mefloquine are safe in pregnancy, doxycycline is not
While chemoprophylaxis in pregnancy appears efficacious, a major question remains—which agents are safest for both the woman and fetus? Some drugs routinely used in nonpregnant individuals should not be offered to pregnant women because of known direct effects on the fetus. Doxycycline is teratogenic, and primaquine poses a significant risk of fetal intravascular hemolysis in G6PD-deficient fetuses.5 Other drugs, such as atovaquone/proguanil and artesunate, are not well studied in pregnancy, and therefore are not recommended for use unless other options are not available.2,6
Among drugs that are well studied and without known direct fetal-damaging effects, adverse drug reaction profiles can guide use based on disease prevalence and drug-resistance patterns.
- Chloroquine is widely used because it is inexpensive and well tolerated, with only pruritus, mouth ulcers, and gastrointestinal upset as the most common adverse effects.
- Mefloquine is usually well tolerated, but can cause dose-related neuropsychiatric effects; it is contraindicated in those with a history of epilepsy or psychiatric disease.
- Sulfadoxine and pyrimethamine are not normally used as prophylaxis for any patient, due to the risk of toxic epidermal necrolysis and Stevens-Johnson syndrome, and the possible risk of jaundice and kernicterus if used in the third trimester of pregnancy.
- Quinine, which can be used for treatment or prophylaxis, may cause hypoglycemia, an effect that is more pronounced during pregnancy and requires close monitoring of blood glucose levels.5,7
Given these reaction profiles, chloroquine or mefloquine are usually the best choice with their superior safety and efficacy.
Figure
Best protection: Avoidance
Chloroquine and mefloquine are the safest antimalarials for use in pregnant women, but personal protection measures are also critical. Above, an Anopheles stephensi mosquito expelling a droplet of blood from its abdomen after having engorged itself on its human host’s blood. (Source: CDC.)
Recommendations from others
The World Health Organization (WHO) recommends pregnant women avoid travel to malarial regions. If travel is required, WHO recommends chloroquine as first-line prophylaxis in pregnancy (plus proguanil if the region exhibits emerging chloroquine resistance). In areas with proven chloroquine resistance, mefloquine is the drug of choice. Other antimalarials—such as quinine, pyrimethamine, sulfadoxine, and artesunate—should not be withheld if the preferred drugs are not available, or if the infection is life-threatening.2
The Centers for Disease Control and Prevention (CDC) also recommends avoiding travel to malaria-endemic regions during pregnancy, but if travel is necessary, the CDC advises use of chloroquine (or mefloquine in regions with chloroquine resistance). The CDC discourages the use of atovaquone/proguanil, doxycycline, and primaquine, due to known adverse fetal effects or inadequate experience in pregnancy.6
Acknowledgments
The opinions and assertions contained herein are the private views of the authors and not to be construed as official, or as reflecting the views of the US Air Force Medical Service or the US Air Force at large.
1. Centers for Disease Control and Prevention Web site. Destinations: CDC Traveler’s Health. Available at: wwwn.cdc.gov/travel/destinationlist.aspx. Accessed on December 7, 2007.
2. Malaria. In: International Travel and Health. Geneva, Switzerland: World Health Organization; 2007. Available at: whqlibdoc.who.int/publications/2005/9241580364_chap7.pdf. Accessed on December 7, 2007.
3. Orton L, Garner P. Drugs for treating uncomplicated malaria in pregnant women. Cochrane Database Syst Rev 2005;(3):CD004912.-
4. Garner P, Gülmezoglu AM. Drugs for preventing malaria in pregnant women. Cochrane Database Syst Rev 2006;(4):CD000169.-
5. Phillips-Howard PA, Wood D. The safety of antimalarial drugs in pregnancy. Drug Saf 1996;14:131-145.
6. Centers for Disease Control and Prevention Web site. Diseases: Malaria: Prevention, Pregnant Women, Public Info. Available at: wwwn.cdc.gov/travel/ contentMalariaPregnantPublic.aspx. Accessed on December 7, 2007.
7. Taylor WR, White NJ. Antimalarial drug toxicity: a review. Drug Saf 2004;27:25-61.
1. Centers for Disease Control and Prevention Web site. Destinations: CDC Traveler’s Health. Available at: wwwn.cdc.gov/travel/destinationlist.aspx. Accessed on December 7, 2007.
2. Malaria. In: International Travel and Health. Geneva, Switzerland: World Health Organization; 2007. Available at: whqlibdoc.who.int/publications/2005/9241580364_chap7.pdf. Accessed on December 7, 2007.
3. Orton L, Garner P. Drugs for treating uncomplicated malaria in pregnant women. Cochrane Database Syst Rev 2005;(3):CD004912.-
4. Garner P, Gülmezoglu AM. Drugs for preventing malaria in pregnant women. Cochrane Database Syst Rev 2006;(4):CD000169.-
5. Phillips-Howard PA, Wood D. The safety of antimalarial drugs in pregnancy. Drug Saf 1996;14:131-145.
6. Centers for Disease Control and Prevention Web site. Diseases: Malaria: Prevention, Pregnant Women, Public Info. Available at: wwwn.cdc.gov/travel/ contentMalariaPregnantPublic.aspx. Accessed on December 7, 2007.
7. Taylor WR, White NJ. Antimalarial drug toxicity: a review. Drug Saf 2004;27:25-61.
Evidence-based answers from the Family Physicians Inquiries Network
Does screening reduce lung cancer mortality?
It’s not clear. Neither routine chest x-ray (with or without sputum cytology) nor low-dose computed tomography (CT) have been proven to reduce mortality when used for lung cancer screening, although low-dose CT screening does identify lung cancer at an early stage in high-risk patients (strength of recommendation: B, based on heterogeneous cohort studies). Large studies of both imaging approaches are ongoing.
Let’s prevent lung cancer so we don’t have to worry about screening
Tim Huber, MD
Oroville Hospital, Oroville, Calif
While some trials suggest possibly useful screening tools, and myriad other trials are underway, one point often gets short shrift: the importance of preventing cancer from occurring in the first place. Most family physicians already screen for smoking and offer counseling and pharmacologic assistance to smokers. We should also be aggressively counseling our adolescent and young adult patients against starting to smoke. Ideally, we would help people reduce their exposure to secondhand smoke, as well. When a teachable moment comes along, we should take the time to educate our patients about their specific risk factors and how they can be modified. Preventing the problem before it starts is our patients’ best defense against lung cancer.
Evidence summary
Chest x-ray and cytology: A trend toward reduced mortality
A Cochrane review1 identified 6 randomized controlled trials (RCTs) and 1 non-RCT (with a total of 245,610 patients) that screened patients with serial chest x-rays, with or without sputum cytology. Most patients were current or ex-smokers or had significant exposure to industrial smoke. No studies included an unscreened control group, and only 1 included women.
There was a trend toward reduced mortality with the combination of annual chest x-ray and sputum cytology compared with annual x-ray alone, but it was not statistically significant (relative risk [RR]=0.88; 95% confidence interval [CI], 0.74–1.03). However, more frequent screening with chest x-rays (2 or 3 times/ year) was associated with an 11% increase in mortality compared with less frequent x-rays (RR=1.11; 95% CI, 1.00–1.23). The authors concluded that there was insufficient evidence to support screening with chest x-ray or sputum cytology.
Low-dose CT: Studies reach different conclusions
A 2006 study followed a cohort of at-risk patients using low-dose CT screening.2 There were 31,567 patients evaluated initially, of which 27,456 had an annual repeat screening. Most patients were current or former smokers (83%); patients with exposure to occupational and secondhand smoke were also included. A positive initial screen was defined as a solid or partly solid noncalcified nodule ≥5 mm in diameter; a nonsolid, noncalcified nodule ≥8 mm in diameter; or a solid endobronchial nodule. A positive screen during follow-up was defined as any new noncalcified nodule, regardless of size.
Positive tests occurred in 13% of baseline screens and 5% of annual screens. Biopsies were performed according to a study protocol based on a nodule’s size and behavior over time. Out of a total of 5646 positive screens, there were 535 biopsies, and a diagnosis of cancer in 492 patients. Of those with cancer, 412 (84%) had clinical stage I lung cancer; the authors estimated their 10-year survival rate was 88% (95% CI, 84%–91%). If patients with stage I disease underwent surgical resection within 1 month of diagnosis, their estimated 10-year survival increased to 92% (95% CI, 88%–95%).
However, a cohort study using annual CT scanning to screen 3246 patients for lung cancer came to a different conclusion.3 The authors compared the observed number of lung cancer cases, resections, advanced lung cancer diagnoses, and deaths in screened patients with the expected rates based on validated prediction models. Lung cancer was diagnosed in 144 patients compared with 44 expected cases (RR=3.2; 95% CI, 2.7–3.8). Subsequently, 109 patients underwent lung resection compared with 11 expected (RR=10.0; 95% CI, 8.2–11.9). However, there was no decline in advanced cancers (42 actual vs 33 expected; P=.14) and no difference in deaths due to lung cancer (38 actual vs 38.8 expected; P=.9). The 81 patients diagnosed in this study with stage I disease who underwent surgical resection had 4-year estimated survival rates of 94% (95% CI, 85%–97%), matching the prior low-dose CT study.
Major studies of both methods are ongoing
Other major studies are in progress. There is an RCT involving 154,942 male and female patients using annual chest x-ray screening vs no screening that will involve 14 years of follow-up.4 Another RCT is evaluating annual low-dose CT vs annual chest x-ray for 3 years in 50,000 at-risk men and women.5 Finally, investigators are conducting an RCT with 4000 at-risk patients comparing annual CT screening with no screening.6
Recommendations from others
The US Preventive Services Task Force gives an “I” recommendation (data insufficient) to screening for lung cancer with cytology, chest x-ray, or CT scanning.7 The American College of Chest Physicians stated in 2003 that the early studies of low-dose CT appeared promising; however, they recommended that individuals should only be screened with low-dose CT in the context of well- designed clinical trials.8
1. Manser RL, Irving LB, Stone C, Byrnes G, Abramson M, Campbell D. Screening for lung cancer. Cochrane Database System Rev. 2004;(1):CD001991.-
2. The International Early Lung Cancer Action Program Investigators; Henschke CI Yankelevitz DF Libby DM Pasmantier MW Smith JP, Miettinen OS. Survival of patients with stage I lung cancer detected on CT Screening. N Engl J Med. 2006;355:1763–1771.-
3. Bach PB, Jett JR, Pastorino U, Tockman MS, Swensen SJ, Begg CB. Computed tomography screening and Lung cancer outcomes. JAMA. 2008;279:953-961.
4. Oken MM, Marcus PM, Hu P, et al. PLCO Project Team. Baseline chest radiograph for lung cancer detection in the randomized Prostate, lung, Colorectal, and ovarian Cancer screening Trial. J Natl Cancer Inst. 2005;97:1832-1839.
5. National Lung Screening Trial (NLST) National Cancer Institute Web site. Available at: www.cancer.gov/nlst. Accessed on December 7, 2008.
6. Van Iersel CA, de Koning HJ, Draisma G, et al. Risk-based selection from the general population in a screening trial: selection criteria, recruitment and power for the Dutch-Belgian randomised lung cancer multi-slice CT screening trial (nelson). Int J Cancer 2008;120:868-874.
7. Recommendation statement lung cancer screening. US Preventive services Task Force Web site. Available at: www.ahrq.gov/clinic/3rduspstf/lungcancer/lungcanrs.htm. Accessed on December 7, 2008.
8. Bach PB, Niewoehner, Black WC. Screening for lung cancer: the guidelines. Chest 2003;123:83-88.
It’s not clear. Neither routine chest x-ray (with or without sputum cytology) nor low-dose computed tomography (CT) have been proven to reduce mortality when used for lung cancer screening, although low-dose CT screening does identify lung cancer at an early stage in high-risk patients (strength of recommendation: B, based on heterogeneous cohort studies). Large studies of both imaging approaches are ongoing.
Let’s prevent lung cancer so we don’t have to worry about screening
Tim Huber, MD
Oroville Hospital, Oroville, Calif
While some trials suggest possibly useful screening tools, and myriad other trials are underway, one point often gets short shrift: the importance of preventing cancer from occurring in the first place. Most family physicians already screen for smoking and offer counseling and pharmacologic assistance to smokers. We should also be aggressively counseling our adolescent and young adult patients against starting to smoke. Ideally, we would help people reduce their exposure to secondhand smoke, as well. When a teachable moment comes along, we should take the time to educate our patients about their specific risk factors and how they can be modified. Preventing the problem before it starts is our patients’ best defense against lung cancer.
Evidence summary
Chest x-ray and cytology: A trend toward reduced mortality
A Cochrane review1 identified 6 randomized controlled trials (RCTs) and 1 non-RCT (with a total of 245,610 patients) that screened patients with serial chest x-rays, with or without sputum cytology. Most patients were current or ex-smokers or had significant exposure to industrial smoke. No studies included an unscreened control group, and only 1 included women.
There was a trend toward reduced mortality with the combination of annual chest x-ray and sputum cytology compared with annual x-ray alone, but it was not statistically significant (relative risk [RR]=0.88; 95% confidence interval [CI], 0.74–1.03). However, more frequent screening with chest x-rays (2 or 3 times/ year) was associated with an 11% increase in mortality compared with less frequent x-rays (RR=1.11; 95% CI, 1.00–1.23). The authors concluded that there was insufficient evidence to support screening with chest x-ray or sputum cytology.
Low-dose CT: Studies reach different conclusions
A 2006 study followed a cohort of at-risk patients using low-dose CT screening.2 There were 31,567 patients evaluated initially, of which 27,456 had an annual repeat screening. Most patients were current or former smokers (83%); patients with exposure to occupational and secondhand smoke were also included. A positive initial screen was defined as a solid or partly solid noncalcified nodule ≥5 mm in diameter; a nonsolid, noncalcified nodule ≥8 mm in diameter; or a solid endobronchial nodule. A positive screen during follow-up was defined as any new noncalcified nodule, regardless of size.
Positive tests occurred in 13% of baseline screens and 5% of annual screens. Biopsies were performed according to a study protocol based on a nodule’s size and behavior over time. Out of a total of 5646 positive screens, there were 535 biopsies, and a diagnosis of cancer in 492 patients. Of those with cancer, 412 (84%) had clinical stage I lung cancer; the authors estimated their 10-year survival rate was 88% (95% CI, 84%–91%). If patients with stage I disease underwent surgical resection within 1 month of diagnosis, their estimated 10-year survival increased to 92% (95% CI, 88%–95%).
However, a cohort study using annual CT scanning to screen 3246 patients for lung cancer came to a different conclusion.3 The authors compared the observed number of lung cancer cases, resections, advanced lung cancer diagnoses, and deaths in screened patients with the expected rates based on validated prediction models. Lung cancer was diagnosed in 144 patients compared with 44 expected cases (RR=3.2; 95% CI, 2.7–3.8). Subsequently, 109 patients underwent lung resection compared with 11 expected (RR=10.0; 95% CI, 8.2–11.9). However, there was no decline in advanced cancers (42 actual vs 33 expected; P=.14) and no difference in deaths due to lung cancer (38 actual vs 38.8 expected; P=.9). The 81 patients diagnosed in this study with stage I disease who underwent surgical resection had 4-year estimated survival rates of 94% (95% CI, 85%–97%), matching the prior low-dose CT study.
Major studies of both methods are ongoing
Other major studies are in progress. There is an RCT involving 154,942 male and female patients using annual chest x-ray screening vs no screening that will involve 14 years of follow-up.4 Another RCT is evaluating annual low-dose CT vs annual chest x-ray for 3 years in 50,000 at-risk men and women.5 Finally, investigators are conducting an RCT with 4000 at-risk patients comparing annual CT screening with no screening.6
Recommendations from others
The US Preventive Services Task Force gives an “I” recommendation (data insufficient) to screening for lung cancer with cytology, chest x-ray, or CT scanning.7 The American College of Chest Physicians stated in 2003 that the early studies of low-dose CT appeared promising; however, they recommended that individuals should only be screened with low-dose CT in the context of well- designed clinical trials.8
It’s not clear. Neither routine chest x-ray (with or without sputum cytology) nor low-dose computed tomography (CT) have been proven to reduce mortality when used for lung cancer screening, although low-dose CT screening does identify lung cancer at an early stage in high-risk patients (strength of recommendation: B, based on heterogeneous cohort studies). Large studies of both imaging approaches are ongoing.
Let’s prevent lung cancer so we don’t have to worry about screening
Tim Huber, MD
Oroville Hospital, Oroville, Calif
While some trials suggest possibly useful screening tools, and myriad other trials are underway, one point often gets short shrift: the importance of preventing cancer from occurring in the first place. Most family physicians already screen for smoking and offer counseling and pharmacologic assistance to smokers. We should also be aggressively counseling our adolescent and young adult patients against starting to smoke. Ideally, we would help people reduce their exposure to secondhand smoke, as well. When a teachable moment comes along, we should take the time to educate our patients about their specific risk factors and how they can be modified. Preventing the problem before it starts is our patients’ best defense against lung cancer.
Evidence summary
Chest x-ray and cytology: A trend toward reduced mortality
A Cochrane review1 identified 6 randomized controlled trials (RCTs) and 1 non-RCT (with a total of 245,610 patients) that screened patients with serial chest x-rays, with or without sputum cytology. Most patients were current or ex-smokers or had significant exposure to industrial smoke. No studies included an unscreened control group, and only 1 included women.
There was a trend toward reduced mortality with the combination of annual chest x-ray and sputum cytology compared with annual x-ray alone, but it was not statistically significant (relative risk [RR]=0.88; 95% confidence interval [CI], 0.74–1.03). However, more frequent screening with chest x-rays (2 or 3 times/ year) was associated with an 11% increase in mortality compared with less frequent x-rays (RR=1.11; 95% CI, 1.00–1.23). The authors concluded that there was insufficient evidence to support screening with chest x-ray or sputum cytology.
Low-dose CT: Studies reach different conclusions
A 2006 study followed a cohort of at-risk patients using low-dose CT screening.2 There were 31,567 patients evaluated initially, of which 27,456 had an annual repeat screening. Most patients were current or former smokers (83%); patients with exposure to occupational and secondhand smoke were also included. A positive initial screen was defined as a solid or partly solid noncalcified nodule ≥5 mm in diameter; a nonsolid, noncalcified nodule ≥8 mm in diameter; or a solid endobronchial nodule. A positive screen during follow-up was defined as any new noncalcified nodule, regardless of size.
Positive tests occurred in 13% of baseline screens and 5% of annual screens. Biopsies were performed according to a study protocol based on a nodule’s size and behavior over time. Out of a total of 5646 positive screens, there were 535 biopsies, and a diagnosis of cancer in 492 patients. Of those with cancer, 412 (84%) had clinical stage I lung cancer; the authors estimated their 10-year survival rate was 88% (95% CI, 84%–91%). If patients with stage I disease underwent surgical resection within 1 month of diagnosis, their estimated 10-year survival increased to 92% (95% CI, 88%–95%).
However, a cohort study using annual CT scanning to screen 3246 patients for lung cancer came to a different conclusion.3 The authors compared the observed number of lung cancer cases, resections, advanced lung cancer diagnoses, and deaths in screened patients with the expected rates based on validated prediction models. Lung cancer was diagnosed in 144 patients compared with 44 expected cases (RR=3.2; 95% CI, 2.7–3.8). Subsequently, 109 patients underwent lung resection compared with 11 expected (RR=10.0; 95% CI, 8.2–11.9). However, there was no decline in advanced cancers (42 actual vs 33 expected; P=.14) and no difference in deaths due to lung cancer (38 actual vs 38.8 expected; P=.9). The 81 patients diagnosed in this study with stage I disease who underwent surgical resection had 4-year estimated survival rates of 94% (95% CI, 85%–97%), matching the prior low-dose CT study.
Major studies of both methods are ongoing
Other major studies are in progress. There is an RCT involving 154,942 male and female patients using annual chest x-ray screening vs no screening that will involve 14 years of follow-up.4 Another RCT is evaluating annual low-dose CT vs annual chest x-ray for 3 years in 50,000 at-risk men and women.5 Finally, investigators are conducting an RCT with 4000 at-risk patients comparing annual CT screening with no screening.6
Recommendations from others
The US Preventive Services Task Force gives an “I” recommendation (data insufficient) to screening for lung cancer with cytology, chest x-ray, or CT scanning.7 The American College of Chest Physicians stated in 2003 that the early studies of low-dose CT appeared promising; however, they recommended that individuals should only be screened with low-dose CT in the context of well- designed clinical trials.8
1. Manser RL, Irving LB, Stone C, Byrnes G, Abramson M, Campbell D. Screening for lung cancer. Cochrane Database System Rev. 2004;(1):CD001991.-
2. The International Early Lung Cancer Action Program Investigators; Henschke CI Yankelevitz DF Libby DM Pasmantier MW Smith JP, Miettinen OS. Survival of patients with stage I lung cancer detected on CT Screening. N Engl J Med. 2006;355:1763–1771.-
3. Bach PB, Jett JR, Pastorino U, Tockman MS, Swensen SJ, Begg CB. Computed tomography screening and Lung cancer outcomes. JAMA. 2008;279:953-961.
4. Oken MM, Marcus PM, Hu P, et al. PLCO Project Team. Baseline chest radiograph for lung cancer detection in the randomized Prostate, lung, Colorectal, and ovarian Cancer screening Trial. J Natl Cancer Inst. 2005;97:1832-1839.
5. National Lung Screening Trial (NLST) National Cancer Institute Web site. Available at: www.cancer.gov/nlst. Accessed on December 7, 2008.
6. Van Iersel CA, de Koning HJ, Draisma G, et al. Risk-based selection from the general population in a screening trial: selection criteria, recruitment and power for the Dutch-Belgian randomised lung cancer multi-slice CT screening trial (nelson). Int J Cancer 2008;120:868-874.
7. Recommendation statement lung cancer screening. US Preventive services Task Force Web site. Available at: www.ahrq.gov/clinic/3rduspstf/lungcancer/lungcanrs.htm. Accessed on December 7, 2008.
8. Bach PB, Niewoehner, Black WC. Screening for lung cancer: the guidelines. Chest 2003;123:83-88.
1. Manser RL, Irving LB, Stone C, Byrnes G, Abramson M, Campbell D. Screening for lung cancer. Cochrane Database System Rev. 2004;(1):CD001991.-
2. The International Early Lung Cancer Action Program Investigators; Henschke CI Yankelevitz DF Libby DM Pasmantier MW Smith JP, Miettinen OS. Survival of patients with stage I lung cancer detected on CT Screening. N Engl J Med. 2006;355:1763–1771.-
3. Bach PB, Jett JR, Pastorino U, Tockman MS, Swensen SJ, Begg CB. Computed tomography screening and Lung cancer outcomes. JAMA. 2008;279:953-961.
4. Oken MM, Marcus PM, Hu P, et al. PLCO Project Team. Baseline chest radiograph for lung cancer detection in the randomized Prostate, lung, Colorectal, and ovarian Cancer screening Trial. J Natl Cancer Inst. 2005;97:1832-1839.
5. National Lung Screening Trial (NLST) National Cancer Institute Web site. Available at: www.cancer.gov/nlst. Accessed on December 7, 2008.
6. Van Iersel CA, de Koning HJ, Draisma G, et al. Risk-based selection from the general population in a screening trial: selection criteria, recruitment and power for the Dutch-Belgian randomised lung cancer multi-slice CT screening trial (nelson). Int J Cancer 2008;120:868-874.
7. Recommendation statement lung cancer screening. US Preventive services Task Force Web site. Available at: www.ahrq.gov/clinic/3rduspstf/lungcancer/lungcanrs.htm. Accessed on December 7, 2008.
8. Bach PB, Niewoehner, Black WC. Screening for lung cancer: the guidelines. Chest 2003;123:83-88.
Evidence-based answers from the Family Physicians Inquiries Network
What is the best portable method of purifying water to prevent infectious disease?
There isn’t a single best method, but there are 5 that adequately purify water according to environmental Protection agency (EPA) standards. These include 1) boiling for 1 minute if below 2000 m (6562 feet) and 3 minutes if above, 2) chlorine dioxide tablets, 3) MIoX purifier, 4) ultraviolet light (steriPEN), and 5) portable filtration with a absolute pore size <1 micrometer combined with halogenation or charcoal filtration (strength of recommendation [SOR]: C, based on expert opinion and microbiological testing). Halogenation alone (ie, chlorine and iodine) is not effective against Cryptosporidium (SOR: C, based on microbiological testing).
Why boil water when there are so many other options?
Timothy Mott, MD, FAAFP
US Naval Hospital, Sigonella, Italy
These days, “boil it, peel it, or forget it” only goes so far with the unencumbered traveler. Experience tells me that most hear “Boil it” and instantly go right to “Forget it!” Fortunately, there is an excellent resource to assist patients in choosing a personally acceptable portable water purification system. It’s called the Water Purification Database at usachppm.apgea.army.mil/WPD/CompareDevices.aspx.1
This outstanding database was developed by an impartial third-party for the US Army and gives clear, well-organized guidance on over 60 purifiers. For each purifier, the guide covers efficacy against primary pathogens, purification mechanism, links to manufacturers, and an advantages/ disadvantages breakdown (such as weight, cost, and ease of use). Add this site to your Internet “favorites” folder.
Evidence summary
With the rise in international travel and adventure sports, individuals are at increased risk of acquiring infections by drinking water from impure water sources. Common waterborne infections that back-country and international travelers may contract include bacterial diarrhea, viruses, protozoa (such as Giardia and Cryptosporidium), and parasites (such as schistosoma). The risk of infection varies based on travel location.
To prevent illness, travelers may seek medical guidance regarding safe water practice. In one study, 36% of travelers sought advice from a physician prior to international travel.2 Preventing waterborne infections should be a component of traveler education, in addition to other standard advice, such as mosquito avoidance and immunizations.3 (For more on travel safety, see these Clinical Inquiries: “When should travelers begin malaria prophylaxis?” in the November 2007 Journal of Family Practice, pages 950–952, and “What is the most effective and safe malaria prophylaxis during pregnancy?” on page 51 of this issue.)
Which devices meet EPA standards?
The EPA has established a “minimal microbiological hazard” allowed for a portable water purification system to be considered safe. Water purifiers must reduce bacteria by 99.9999%, viruses by 99.99%, and protozoa (such as Cryptosporidium parvum) by 99.9% to receive an EPA certification number.4
There are no head-to-head trials comparing the effectiveness of different methods of purification to prevent infectious disease. The majority of the evidence is based on data provided by manufacturers to the EPA, with some independent studies and expert opinion (TABLE).
Expert opinion recommends bringing water to a rapid boil for at least 3 minutes and letting it cool as an effective means of water purification.5 Chlorine dioxide tablets, the MIOX purifier, and UV light (SteriPEN) have all met EPA standards for lower pathogen counts under ideal conditions. Halogenation does not reduce Cryptosporidium below the microbiological hazard of 99.9%, but it is generally accepted to effectively treat viruses, bacteria, and other protozoa after filtering through a cloth to remove large particles.6
Filtration with an absolute pore size of <0.1 micrometer (10 times smaller than the EPA standard) has been generally accepted as effective against protozoa and bacteria, but it is not effective against viruses because of their small size.7 When combined with either halogenation or charcoal filters, filtration can be effective against all pathogens.8
TABLE
Portable water purification: How do these 6 methods compare?
| METHOD | EFFECTIVENESS | ADVANTAGES | DISADVANTAGES |
|---|---|---|---|
| Boiling with cooling* | Kills viruses, bacteria, protozoa, and parasites | simple, universally accepted, no special equipment required | Time-consuming, may require large amounts of fuel |
chlorine dioxide*
| Kills bacteria, viruses, protozoa, and parasites | same as chlorine/iodine treatment but also treats Cryptosporidium, good palatability | Must wait up to 4 hours to treat Cryptosporidium, costs more than iodine/chlorine ($13 for 30 tabs) |
Chlorine/iodine
| Kills bacteria, viruses, protozoa (not Cryptosporidium), and parasites | Inexpensive, easy, lightweight, treats large quantities | Does not kill Cryptosporidium, poor taste, must wait for water to be treated; contraindicated in pregnancy, thyroid disease; not recommended beyond few weeks of use |
Filtration †
| Removes parasites, Giardia, Cryptosporidium, and bacteria | Able to use water immediately, removes sediment, many have combination of activated carbon, chemical disinfectant, or both | Can potentially be expensive, filters may clog easily, heavy, not effective against small particle viruses, therefore should supplement with chlorine or iodine |
MIOX Purifier*
| Kills bacteria, viruses, protozoa, and parasites | light (8 oz), sturdy, treats large quantities; requires camera batteries and salt | Cost $130, must wait for 4 hours and treat with higher strength to treat Cryptosporidium; requires 30 minutes to treat viruses, bacteria, and Giardia |

UV light (steriPEN) ‡ | Kills bacteria, viruses, protozoa, parasites in clear water | Light (8 oz), quick (treats 16 oz of water in 1 minute) | Cost $100, does not work in turbid conditions |
| * Meets EPA standards. | |||
| † some filtration systems meet EPA standards. See chppm-www.apgea.army.mil/WPD/CompareDevices.aspx for testing results of individual filters.1 | |||
| ‡ Meets EPA standards in clear water. | |||
Recommendations from others
The US Army Center for Health Promotion and Preventive Medicine (USACHPPM) published a report in 2006 on the efficacy of commercial off-the-shelf individual water purifiers.8 Using National Sanitation Foundation Protocol P248 and applying it to “real-world” emergency military operational conditions, USACHPPM found that no device scored high on every attribute, and that overall scores for most devices were in the moderate range. The top score for any device was 79 (out of 100).8
The overall top 3 scoring products were: 1) the SweetWater Purifier from Mountain Safety Research; 2) the Micropur MP 1 tablets from Katadyn North America, Inc; and 3) the First Need Deluxe water purifier from General Ecology, Inc.
Acknowledgments
The opinions and assertions contained herein are the private views of the authors and not to be construed as official, or as reflecting the views of the US Air Force Medical Service or the US air Force at large.
1. Commercially available individual water purifiers. Water Purification Database, US Army Center for Health Promotion and Preventive Medicine Web site. Available at: usachppm.apgea.army.mil/WPD/CompareDevices.aspx. Accessed on December 7, 2007.
2. Hamer DH, Connor Ba. Travel health knowledge, attitudes and practices among united states travelers. J Travel Med 2004;11:23-26.
3. Hill DR, Ericsson CD, Pearson RD, et al. The Practice of Travel Medicine: Guidelines by the Infectious Diseases Society of America. Clin Infect Dis 2006;43:1499-1539.
4. US Environmental Protection agency: Guide standard and Protocol for Testing Microbiological Water Purifiers: Report to Task Force. Cincinnati, OH: US environmental Protection agency; 1987.
5. Centers for Disease Control and Prevention Water treatment methods. Available at: wwwn.cdc.gov/travel/contentWaterTreatment.aspx. Accessed on December 7, 2007.
6. Gerba CP, Johnson DC, Hasan MN. efficacy of iodine water purification tablets against Cryptosporidium oocysts and Giardia cysts. Wilderness Environ Med 1997;8:96-100.
7. Centers for Disease Control and Prevention Division of Parasitic Diseases Preventing Cryptosporidiosis: a guide to water filters and bottled water. Available at: www.cdc.gov/ncidod/dpd/parasites/cryptosporidiosis/factsht_crypto_prevent_water.htm. Accessed on December 7, 2007.
8. Water supply Management Program Project No 31-EC-03E0. Performance and health risk assessment of commercial-off-the-shelf individual water purifiers. Aberdeen Proving Ground, MD: US Army Center for Health Promotion and Preventive Medicine; 2006. Available at: usachppm.apgea.army.mil/WPD/PDFDocs/Finalreport.pdf. Accessed on December 7, 2007.
There isn’t a single best method, but there are 5 that adequately purify water according to environmental Protection agency (EPA) standards. These include 1) boiling for 1 minute if below 2000 m (6562 feet) and 3 minutes if above, 2) chlorine dioxide tablets, 3) MIoX purifier, 4) ultraviolet light (steriPEN), and 5) portable filtration with a absolute pore size <1 micrometer combined with halogenation or charcoal filtration (strength of recommendation [SOR]: C, based on expert opinion and microbiological testing). Halogenation alone (ie, chlorine and iodine) is not effective against Cryptosporidium (SOR: C, based on microbiological testing).
Why boil water when there are so many other options?
Timothy Mott, MD, FAAFP
US Naval Hospital, Sigonella, Italy
These days, “boil it, peel it, or forget it” only goes so far with the unencumbered traveler. Experience tells me that most hear “Boil it” and instantly go right to “Forget it!” Fortunately, there is an excellent resource to assist patients in choosing a personally acceptable portable water purification system. It’s called the Water Purification Database at usachppm.apgea.army.mil/WPD/CompareDevices.aspx.1
This outstanding database was developed by an impartial third-party for the US Army and gives clear, well-organized guidance on over 60 purifiers. For each purifier, the guide covers efficacy against primary pathogens, purification mechanism, links to manufacturers, and an advantages/ disadvantages breakdown (such as weight, cost, and ease of use). Add this site to your Internet “favorites” folder.
Evidence summary
With the rise in international travel and adventure sports, individuals are at increased risk of acquiring infections by drinking water from impure water sources. Common waterborne infections that back-country and international travelers may contract include bacterial diarrhea, viruses, protozoa (such as Giardia and Cryptosporidium), and parasites (such as schistosoma). The risk of infection varies based on travel location.
To prevent illness, travelers may seek medical guidance regarding safe water practice. In one study, 36% of travelers sought advice from a physician prior to international travel.2 Preventing waterborne infections should be a component of traveler education, in addition to other standard advice, such as mosquito avoidance and immunizations.3 (For more on travel safety, see these Clinical Inquiries: “When should travelers begin malaria prophylaxis?” in the November 2007 Journal of Family Practice, pages 950–952, and “What is the most effective and safe malaria prophylaxis during pregnancy?” on page 51 of this issue.)
Which devices meet EPA standards?
The EPA has established a “minimal microbiological hazard” allowed for a portable water purification system to be considered safe. Water purifiers must reduce bacteria by 99.9999%, viruses by 99.99%, and protozoa (such as Cryptosporidium parvum) by 99.9% to receive an EPA certification number.4
There are no head-to-head trials comparing the effectiveness of different methods of purification to prevent infectious disease. The majority of the evidence is based on data provided by manufacturers to the EPA, with some independent studies and expert opinion (TABLE).
Expert opinion recommends bringing water to a rapid boil for at least 3 minutes and letting it cool as an effective means of water purification.5 Chlorine dioxide tablets, the MIOX purifier, and UV light (SteriPEN) have all met EPA standards for lower pathogen counts under ideal conditions. Halogenation does not reduce Cryptosporidium below the microbiological hazard of 99.9%, but it is generally accepted to effectively treat viruses, bacteria, and other protozoa after filtering through a cloth to remove large particles.6
Filtration with an absolute pore size of <0.1 micrometer (10 times smaller than the EPA standard) has been generally accepted as effective against protozoa and bacteria, but it is not effective against viruses because of their small size.7 When combined with either halogenation or charcoal filters, filtration can be effective against all pathogens.8
TABLE
Portable water purification: How do these 6 methods compare?
| METHOD | EFFECTIVENESS | ADVANTAGES | DISADVANTAGES |
|---|---|---|---|
| Boiling with cooling* | Kills viruses, bacteria, protozoa, and parasites | simple, universally accepted, no special equipment required | Time-consuming, may require large amounts of fuel |
chlorine dioxide*
| Kills bacteria, viruses, protozoa, and parasites | same as chlorine/iodine treatment but also treats Cryptosporidium, good palatability | Must wait up to 4 hours to treat Cryptosporidium, costs more than iodine/chlorine ($13 for 30 tabs) |
Chlorine/iodine
| Kills bacteria, viruses, protozoa (not Cryptosporidium), and parasites | Inexpensive, easy, lightweight, treats large quantities | Does not kill Cryptosporidium, poor taste, must wait for water to be treated; contraindicated in pregnancy, thyroid disease; not recommended beyond few weeks of use |
Filtration †
| Removes parasites, Giardia, Cryptosporidium, and bacteria | Able to use water immediately, removes sediment, many have combination of activated carbon, chemical disinfectant, or both | Can potentially be expensive, filters may clog easily, heavy, not effective against small particle viruses, therefore should supplement with chlorine or iodine |
MIOX Purifier*
| Kills bacteria, viruses, protozoa, and parasites | light (8 oz), sturdy, treats large quantities; requires camera batteries and salt | Cost $130, must wait for 4 hours and treat with higher strength to treat Cryptosporidium; requires 30 minutes to treat viruses, bacteria, and Giardia |

UV light (steriPEN) ‡ | Kills bacteria, viruses, protozoa, parasites in clear water | Light (8 oz), quick (treats 16 oz of water in 1 minute) | Cost $100, does not work in turbid conditions |
| * Meets EPA standards. | |||
| † some filtration systems meet EPA standards. See chppm-www.apgea.army.mil/WPD/CompareDevices.aspx for testing results of individual filters.1 | |||
| ‡ Meets EPA standards in clear water. | |||
Recommendations from others
The US Army Center for Health Promotion and Preventive Medicine (USACHPPM) published a report in 2006 on the efficacy of commercial off-the-shelf individual water purifiers.8 Using National Sanitation Foundation Protocol P248 and applying it to “real-world” emergency military operational conditions, USACHPPM found that no device scored high on every attribute, and that overall scores for most devices were in the moderate range. The top score for any device was 79 (out of 100).8
The overall top 3 scoring products were: 1) the SweetWater Purifier from Mountain Safety Research; 2) the Micropur MP 1 tablets from Katadyn North America, Inc; and 3) the First Need Deluxe water purifier from General Ecology, Inc.
Acknowledgments
The opinions and assertions contained herein are the private views of the authors and not to be construed as official, or as reflecting the views of the US Air Force Medical Service or the US air Force at large.
There isn’t a single best method, but there are 5 that adequately purify water according to environmental Protection agency (EPA) standards. These include 1) boiling for 1 minute if below 2000 m (6562 feet) and 3 minutes if above, 2) chlorine dioxide tablets, 3) MIoX purifier, 4) ultraviolet light (steriPEN), and 5) portable filtration with a absolute pore size <1 micrometer combined with halogenation or charcoal filtration (strength of recommendation [SOR]: C, based on expert opinion and microbiological testing). Halogenation alone (ie, chlorine and iodine) is not effective against Cryptosporidium (SOR: C, based on microbiological testing).
Why boil water when there are so many other options?
Timothy Mott, MD, FAAFP
US Naval Hospital, Sigonella, Italy
These days, “boil it, peel it, or forget it” only goes so far with the unencumbered traveler. Experience tells me that most hear “Boil it” and instantly go right to “Forget it!” Fortunately, there is an excellent resource to assist patients in choosing a personally acceptable portable water purification system. It’s called the Water Purification Database at usachppm.apgea.army.mil/WPD/CompareDevices.aspx.1
This outstanding database was developed by an impartial third-party for the US Army and gives clear, well-organized guidance on over 60 purifiers. For each purifier, the guide covers efficacy against primary pathogens, purification mechanism, links to manufacturers, and an advantages/ disadvantages breakdown (such as weight, cost, and ease of use). Add this site to your Internet “favorites” folder.
Evidence summary
With the rise in international travel and adventure sports, individuals are at increased risk of acquiring infections by drinking water from impure water sources. Common waterborne infections that back-country and international travelers may contract include bacterial diarrhea, viruses, protozoa (such as Giardia and Cryptosporidium), and parasites (such as schistosoma). The risk of infection varies based on travel location.
To prevent illness, travelers may seek medical guidance regarding safe water practice. In one study, 36% of travelers sought advice from a physician prior to international travel.2 Preventing waterborne infections should be a component of traveler education, in addition to other standard advice, such as mosquito avoidance and immunizations.3 (For more on travel safety, see these Clinical Inquiries: “When should travelers begin malaria prophylaxis?” in the November 2007 Journal of Family Practice, pages 950–952, and “What is the most effective and safe malaria prophylaxis during pregnancy?” on page 51 of this issue.)
Which devices meet EPA standards?
The EPA has established a “minimal microbiological hazard” allowed for a portable water purification system to be considered safe. Water purifiers must reduce bacteria by 99.9999%, viruses by 99.99%, and protozoa (such as Cryptosporidium parvum) by 99.9% to receive an EPA certification number.4
There are no head-to-head trials comparing the effectiveness of different methods of purification to prevent infectious disease. The majority of the evidence is based on data provided by manufacturers to the EPA, with some independent studies and expert opinion (TABLE).
Expert opinion recommends bringing water to a rapid boil for at least 3 minutes and letting it cool as an effective means of water purification.5 Chlorine dioxide tablets, the MIOX purifier, and UV light (SteriPEN) have all met EPA standards for lower pathogen counts under ideal conditions. Halogenation does not reduce Cryptosporidium below the microbiological hazard of 99.9%, but it is generally accepted to effectively treat viruses, bacteria, and other protozoa after filtering through a cloth to remove large particles.6
Filtration with an absolute pore size of <0.1 micrometer (10 times smaller than the EPA standard) has been generally accepted as effective against protozoa and bacteria, but it is not effective against viruses because of their small size.7 When combined with either halogenation or charcoal filters, filtration can be effective against all pathogens.8
TABLE
Portable water purification: How do these 6 methods compare?
| METHOD | EFFECTIVENESS | ADVANTAGES | DISADVANTAGES |
|---|---|---|---|
| Boiling with cooling* | Kills viruses, bacteria, protozoa, and parasites | simple, universally accepted, no special equipment required | Time-consuming, may require large amounts of fuel |
chlorine dioxide*
| Kills bacteria, viruses, protozoa, and parasites | same as chlorine/iodine treatment but also treats Cryptosporidium, good palatability | Must wait up to 4 hours to treat Cryptosporidium, costs more than iodine/chlorine ($13 for 30 tabs) |
Chlorine/iodine
| Kills bacteria, viruses, protozoa (not Cryptosporidium), and parasites | Inexpensive, easy, lightweight, treats large quantities | Does not kill Cryptosporidium, poor taste, must wait for water to be treated; contraindicated in pregnancy, thyroid disease; not recommended beyond few weeks of use |
Filtration †
| Removes parasites, Giardia, Cryptosporidium, and bacteria | Able to use water immediately, removes sediment, many have combination of activated carbon, chemical disinfectant, or both | Can potentially be expensive, filters may clog easily, heavy, not effective against small particle viruses, therefore should supplement with chlorine or iodine |
MIOX Purifier*
| Kills bacteria, viruses, protozoa, and parasites | light (8 oz), sturdy, treats large quantities; requires camera batteries and salt | Cost $130, must wait for 4 hours and treat with higher strength to treat Cryptosporidium; requires 30 minutes to treat viruses, bacteria, and Giardia |

UV light (steriPEN) ‡ | Kills bacteria, viruses, protozoa, parasites in clear water | Light (8 oz), quick (treats 16 oz of water in 1 minute) | Cost $100, does not work in turbid conditions |
| * Meets EPA standards. | |||
| † some filtration systems meet EPA standards. See chppm-www.apgea.army.mil/WPD/CompareDevices.aspx for testing results of individual filters.1 | |||
| ‡ Meets EPA standards in clear water. | |||
Recommendations from others
The US Army Center for Health Promotion and Preventive Medicine (USACHPPM) published a report in 2006 on the efficacy of commercial off-the-shelf individual water purifiers.8 Using National Sanitation Foundation Protocol P248 and applying it to “real-world” emergency military operational conditions, USACHPPM found that no device scored high on every attribute, and that overall scores for most devices were in the moderate range. The top score for any device was 79 (out of 100).8
The overall top 3 scoring products were: 1) the SweetWater Purifier from Mountain Safety Research; 2) the Micropur MP 1 tablets from Katadyn North America, Inc; and 3) the First Need Deluxe water purifier from General Ecology, Inc.
Acknowledgments
The opinions and assertions contained herein are the private views of the authors and not to be construed as official, or as reflecting the views of the US Air Force Medical Service or the US air Force at large.
1. Commercially available individual water purifiers. Water Purification Database, US Army Center for Health Promotion and Preventive Medicine Web site. Available at: usachppm.apgea.army.mil/WPD/CompareDevices.aspx. Accessed on December 7, 2007.
2. Hamer DH, Connor Ba. Travel health knowledge, attitudes and practices among united states travelers. J Travel Med 2004;11:23-26.
3. Hill DR, Ericsson CD, Pearson RD, et al. The Practice of Travel Medicine: Guidelines by the Infectious Diseases Society of America. Clin Infect Dis 2006;43:1499-1539.
4. US Environmental Protection agency: Guide standard and Protocol for Testing Microbiological Water Purifiers: Report to Task Force. Cincinnati, OH: US environmental Protection agency; 1987.
5. Centers for Disease Control and Prevention Water treatment methods. Available at: wwwn.cdc.gov/travel/contentWaterTreatment.aspx. Accessed on December 7, 2007.
6. Gerba CP, Johnson DC, Hasan MN. efficacy of iodine water purification tablets against Cryptosporidium oocysts and Giardia cysts. Wilderness Environ Med 1997;8:96-100.
7. Centers for Disease Control and Prevention Division of Parasitic Diseases Preventing Cryptosporidiosis: a guide to water filters and bottled water. Available at: www.cdc.gov/ncidod/dpd/parasites/cryptosporidiosis/factsht_crypto_prevent_water.htm. Accessed on December 7, 2007.
8. Water supply Management Program Project No 31-EC-03E0. Performance and health risk assessment of commercial-off-the-shelf individual water purifiers. Aberdeen Proving Ground, MD: US Army Center for Health Promotion and Preventive Medicine; 2006. Available at: usachppm.apgea.army.mil/WPD/PDFDocs/Finalreport.pdf. Accessed on December 7, 2007.
1. Commercially available individual water purifiers. Water Purification Database, US Army Center for Health Promotion and Preventive Medicine Web site. Available at: usachppm.apgea.army.mil/WPD/CompareDevices.aspx. Accessed on December 7, 2007.
2. Hamer DH, Connor Ba. Travel health knowledge, attitudes and practices among united states travelers. J Travel Med 2004;11:23-26.
3. Hill DR, Ericsson CD, Pearson RD, et al. The Practice of Travel Medicine: Guidelines by the Infectious Diseases Society of America. Clin Infect Dis 2006;43:1499-1539.
4. US Environmental Protection agency: Guide standard and Protocol for Testing Microbiological Water Purifiers: Report to Task Force. Cincinnati, OH: US environmental Protection agency; 1987.
5. Centers for Disease Control and Prevention Water treatment methods. Available at: wwwn.cdc.gov/travel/contentWaterTreatment.aspx. Accessed on December 7, 2007.
6. Gerba CP, Johnson DC, Hasan MN. efficacy of iodine water purification tablets against Cryptosporidium oocysts and Giardia cysts. Wilderness Environ Med 1997;8:96-100.
7. Centers for Disease Control and Prevention Division of Parasitic Diseases Preventing Cryptosporidiosis: a guide to water filters and bottled water. Available at: www.cdc.gov/ncidod/dpd/parasites/cryptosporidiosis/factsht_crypto_prevent_water.htm. Accessed on December 7, 2007.
8. Water supply Management Program Project No 31-EC-03E0. Performance and health risk assessment of commercial-off-the-shelf individual water purifiers. Aberdeen Proving Ground, MD: US Army Center for Health Promotion and Preventive Medicine; 2006. Available at: usachppm.apgea.army.mil/WPD/PDFDocs/Finalreport.pdf. Accessed on December 7, 2007.
Evidence-based answers from the Family Physicians Inquiries Network
What is the best treatment for chronic constipation in the elderly?
There is no one best evidence-based treatment for chronic constipation in the elderly. While the most common first-line treatments are dietary fiber and exercise, the evidence is insufficient to support this approach in the geriatric population (strength of recommendation [SOR]: for dietary fiber: A, based on a systematic review; for exercise: SOR: B, based on 1 good- and 1 fair-quality randomized controlled trial [RCT]).
Herbal supplements (such as aloe), alternative treatments (biofeedback), lubricants (mineral oil), and combination laxatives sold in the US have not been sufficiently studied in controlled trials to make a recommendation (SOR: A, based on systematic review).
An abdominal kneading device can be used to treat chronic constipation, but the evidence is limited (SOR: B, based on 1 cohort study.)
Polyethylene glycol has not been studied in the elderly. A newer agent, lubiprostone (Amitiza), appears to be effective for the treatment of chronic constipation for elderly patients (SOR: B, based on subgroup analysis of RCTs.)
Is the patient truly constipated?
Mandi Sehgal, MD
Department of Family Medicine/Geriatrics, University of Cincinnati
Many older people feel that if they do not have a bowel movement every day they are constipated. However, constipation is defined as fewer than 3 bowel movements per week. So, the first thing we must do is to confirm that the patient is truly constipated.
Before I start my patients on any medicine, I suggest a trial of increased daily water and fiber intake along with exercise, followed by a trial of stool softeners and stimulant laxatives, if needed. If all of these methods fail, I consider trying polyethylene glycol, which can be titrated to effect. As with all medication use by the elderly, it is important to titrate cautiously (“start low and go slow”) and add other medications only when necessary.
Evidence summary
Few well-designed studies have focused on constipation treatment among the elderly. Our search located 1 systematic review of pharmacologic management, a systematic review of fiber management, 2 RCTs on the effect of exercise, and 1 before-after cohort study on abdominal massage. These studies were all conducted among geriatric patients with constipation. Two high-quality systematic reviews regarding chronic constipation management for adults of all ages included management options not studied in exclusively geriatric populations, such as herbal supplements, biofeedback, tegaserod, and polyethylene glycol.
Laxatives, fiber, and exercise: Studies are inconclusive
Two good-quality systematic reviews looked at 10 RCTs comparing laxatives with placebo, and 10 RCTs comparing 1 laxative with another.1,2 The studies generally had few participants, were of short duration, and were conducted in institutional settings. Most lacked power to make valid conclusions. These studies varied in the reported outcome measures, including stool frequency, stool consistency, straining, decrease in laxative use, and symptom scores. The reviews concluded that the best pharmacologic treatment for chronic constipation in the elderly has not been established.
Five of the higher-quality studies attained statistical significance. They showed a small but significant improvement in bowel movement frequency with a laxative when compared with placebo or another laxative (TABLE). The authors noted that multiple poor-quality studies have shown nonsignificant trends for improved constipation symptoms with laxatives compared with placebo.
Inconsistent findings on fiber. A good-quality systematic review3 of dietary fiber in the treatment of constipation for older patients located 8 moderate- to high-quality studies (6 RCTs and 2 blinded before-after studies), with 269 study participants in institutional settings. Results among studies were inconsistent, casting doubt on the efficacy of fiber treatment for constipation in the institutionalized elder.
Two RCTs4,5 investigating the effect of exercise on 246 institutionalized older patients showed no improvement in constipation. One study was of good quality, reporting adequate power and used an intention-to-treat analysis. The other was of fair quality.
Alternative TXs not well studied
A high-quality systematic review6 of constipation management among adults of all ages in North America found a lack of quality RCTs examining herbal supplement treatment. Biofeedback has been studied in adult populations, but no RCTs with placebo or sham-controls have been published.
One before-after cohort study7 investigated an external kneading mechanical device (Free-Lax) that was applied to the abdomen for 20 minutes once daily in 30 randomly selected chronically constipated nursing home residents. Researchers found significant improvements in bowel movement frequency, stool consistency and volume, and colonic transit time without side effects (TABLE).
A look beyond geriatric patients
Polyethylene glycol, tegaserod, and lubiprostone have not been studied in trials of exclusively geriatric populations. Two high-quality systematic reviews,6,8 including medium- to high-quality RCTs of pharmacologic management of chronic constipation, found good evidence to support treatment with polyethylene glycol and tegaserod in adults of all ages. Of the 8 RCTs looking at polyethylene glycol, only 1 of the studies—a high-quality crossover comparison of polyethylene glycol vs placebo with 37 out-patient subjects—included a population with a mean age >60 years (mean age 62, range 42–89 years).
TABLE
How well do these interventions work for older patients with chronic constipation?
| INTERVENTION VS COMPARISON | STOOL FREQUENCY (STOOLS PER WEEK) | NNT‡ |
|---|---|---|
| Agiolax* vs lactulose | 4.5 vs 2.2 | 43 |
| Agiolax* vs lactulose | 5.6 vs 4.2 | 71 |
| Lactitol† vs placebo | 4.9 vs 3.6 | 77 |
| Lactitol† vs lactulose | 5.5 vs 4.9 | 160 |
| Lactulose vs sorbitol† | 7.0 vs 6.7 | 330 |
| External abdominal kneading (before-after) | 3.9 vs 1.4 | 40 |
| * Agiolax is a combination bulk and stimulant laxative not readily found in the United States. | ||
| † Lactitol and sorbitol are sugar alcohols used as replacement sweeteners and approved by the FDA as food additives. | ||
| ‡ Number needed to treat (NNT) for 1 person to have 1 more stool per week. | ||
A subgroup analysis9 of 331 elderly patients enrolled in 2 RCTs of tegaserod found no difference in outcomes between treatment with tegaserod and placebo, although this analysis was limited by inadequate power.
Tegaserod linked to ischemic events. A recent analysis of clinical trials found a statistically significant increase in cardiovascular ischemic events associated with tegaserod. The manufacturer took the product off the market in compliance with an FDA request in March 2007.
Lubiprostone offers promise. Lubiprostone, a chloride channel activator approved by the FDA for the treatment of chronic idiopathic constipation, has been studied in 6 placebo-controlled, double-blind, randomized Phase II and III clinical trials. In 2 unpublished pooled analyses of 3 of the trials, lubiprostone was found to be effective in a total of 220 elderly patients 65 years of age and older.10,11
Recommendations from others
The American College of Gastroenterology Chronic Constipation Task Force evidence-based guidelines make no reference to age, but state that evidence is best for treatment with psyllium, tegaserod, polyethylene glycol, and lactulose.12 They found insufficient evidence to support use of stimulants, stool softeners, lubricants, herbal supplements, biofeedback, and alternative treatments.
The American Gastroenterological Association guidelines on constipation are primarily based on expert opinion.13 Age is not specified in their recommendations. Dietary and exercise modifications are recommended as first-line treatments, followed by laxatives. Laxatives are recommended based on cost, in order from the least to most expensive agents. Suppositories, enemas, biofeedback, and (in refractory cases) surgery are recommended for patients with pelvic floor dysfunction.
The Registered Nurses Association of Ontario guidelines for constipation prevention in the older adult population recommend fluid and dietary fiber, regular exercise, and consistent toileting.14
1. Petticrew M, Watt I, Brand M. What’s the “best buy” for treatment of constipation? Results of a systematic review of the efficacy and comparative efficacy of laxatives in the elderly. Br J Gen Pract 1999;49:387-393.
2. Petticrew M, Watt I, Sheldon T. Systematic review of the effectiveness of laxatives in the elderly. Health Technol Assess 1997;1:i–iv-1–52.
3. Kenny KA, Skelly JM. Dietary fiber for constipation in older adults: a systematic review. Clinical Effectiveness in Nursing 2001;5:120-128.
4. Chin APMJ, van Poppel MN, van Mechelen W. Effects of resistance and functional-skills training on habitual activity and constipation among older adults living in long-term care facilities: a randomized controlled trial. BMC Geriatr 2006;6:9.-
5. Simmons SF, Schnelle JF. Effects of an exercise and scheduled-toileting intervention on appetite and constipation in nursing home residents. J Nutr Health Aging 2004;8:116-121.
6. Brandt LJ, Prather CM, Quigley EM, Schiller LR, Schoenfeld P, Talley NJ. Systematic review on the management of chronic constipation in North America. Am J Gastroenterol 2005;100 suppl 1:S5-S21.
7. Mimidis K, Galinsky D, Rimon E, Papadopoulos V, Zicherman Y, Oreopoulos D. Use of a device that applies external kneading-like force on the abdomen for treatment of constipation. World J Gastroenterol 2005;11:1971-1975.
8. Ramkumar D, Rao SS. Efficacy and safety of traditional medical therapies for chronic constipation: systematic review. Am J Gastroenterol 2005;100:936-971.
9. Baun RF, Levy HB. Tegaserod for treating chronic constipation in elderly patients. Ann Pharmacother 2007;41:309-313.
10. Ueno R, Joswick TR, Wahle A, et al. Efficacy and safety of lubiprostone for the treatment of chronic constipation in elderly vs non-elderly subjects. Gastroenterology 2006;130(suppl 2):A189.-
11. Ueno R, Panas R, Wahle A, et al. Long-term safety and efficacy of lubiprostone for the treatment of chronic constipation in elderly subjects. Gastroenterology 2006;130(suppl 2):A188.-
12. American College of Gastroenterology Chronic Constipation Task Force. An evidence-based approach to the management of chronic constipation in North America. Am J Gastroenterol 2005;100 Suppl 1:S1-S4.
13. Locke GR, 3rd, Pemberton JH, Phillips SF. American Gastroenterological Association Medical Position Statement: guidelines on constipation. Gastroenterology 2000;119:1761-1766.
14. Registered Nurses Association of Ontario (RNAO). Prevention of constipation in the older adult population. Toronto, Ontario: RNAO; 2005. Available at: www.guideline.gov/summary/summary.aspx?ss=15&doc_id=7004&nbr=4213. Accessed on November 8, 2007.
There is no one best evidence-based treatment for chronic constipation in the elderly. While the most common first-line treatments are dietary fiber and exercise, the evidence is insufficient to support this approach in the geriatric population (strength of recommendation [SOR]: for dietary fiber: A, based on a systematic review; for exercise: SOR: B, based on 1 good- and 1 fair-quality randomized controlled trial [RCT]).
Herbal supplements (such as aloe), alternative treatments (biofeedback), lubricants (mineral oil), and combination laxatives sold in the US have not been sufficiently studied in controlled trials to make a recommendation (SOR: A, based on systematic review).
An abdominal kneading device can be used to treat chronic constipation, but the evidence is limited (SOR: B, based on 1 cohort study.)
Polyethylene glycol has not been studied in the elderly. A newer agent, lubiprostone (Amitiza), appears to be effective for the treatment of chronic constipation for elderly patients (SOR: B, based on subgroup analysis of RCTs.)
Is the patient truly constipated?
Mandi Sehgal, MD
Department of Family Medicine/Geriatrics, University of Cincinnati
Many older people feel that if they do not have a bowel movement every day they are constipated. However, constipation is defined as fewer than 3 bowel movements per week. So, the first thing we must do is to confirm that the patient is truly constipated.
Before I start my patients on any medicine, I suggest a trial of increased daily water and fiber intake along with exercise, followed by a trial of stool softeners and stimulant laxatives, if needed. If all of these methods fail, I consider trying polyethylene glycol, which can be titrated to effect. As with all medication use by the elderly, it is important to titrate cautiously (“start low and go slow”) and add other medications only when necessary.
Evidence summary
Few well-designed studies have focused on constipation treatment among the elderly. Our search located 1 systematic review of pharmacologic management, a systematic review of fiber management, 2 RCTs on the effect of exercise, and 1 before-after cohort study on abdominal massage. These studies were all conducted among geriatric patients with constipation. Two high-quality systematic reviews regarding chronic constipation management for adults of all ages included management options not studied in exclusively geriatric populations, such as herbal supplements, biofeedback, tegaserod, and polyethylene glycol.
Laxatives, fiber, and exercise: Studies are inconclusive
Two good-quality systematic reviews looked at 10 RCTs comparing laxatives with placebo, and 10 RCTs comparing 1 laxative with another.1,2 The studies generally had few participants, were of short duration, and were conducted in institutional settings. Most lacked power to make valid conclusions. These studies varied in the reported outcome measures, including stool frequency, stool consistency, straining, decrease in laxative use, and symptom scores. The reviews concluded that the best pharmacologic treatment for chronic constipation in the elderly has not been established.
Five of the higher-quality studies attained statistical significance. They showed a small but significant improvement in bowel movement frequency with a laxative when compared with placebo or another laxative (TABLE). The authors noted that multiple poor-quality studies have shown nonsignificant trends for improved constipation symptoms with laxatives compared with placebo.
Inconsistent findings on fiber. A good-quality systematic review3 of dietary fiber in the treatment of constipation for older patients located 8 moderate- to high-quality studies (6 RCTs and 2 blinded before-after studies), with 269 study participants in institutional settings. Results among studies were inconsistent, casting doubt on the efficacy of fiber treatment for constipation in the institutionalized elder.
Two RCTs4,5 investigating the effect of exercise on 246 institutionalized older patients showed no improvement in constipation. One study was of good quality, reporting adequate power and used an intention-to-treat analysis. The other was of fair quality.
Alternative TXs not well studied
A high-quality systematic review6 of constipation management among adults of all ages in North America found a lack of quality RCTs examining herbal supplement treatment. Biofeedback has been studied in adult populations, but no RCTs with placebo or sham-controls have been published.
One before-after cohort study7 investigated an external kneading mechanical device (Free-Lax) that was applied to the abdomen for 20 minutes once daily in 30 randomly selected chronically constipated nursing home residents. Researchers found significant improvements in bowel movement frequency, stool consistency and volume, and colonic transit time without side effects (TABLE).
A look beyond geriatric patients
Polyethylene glycol, tegaserod, and lubiprostone have not been studied in trials of exclusively geriatric populations. Two high-quality systematic reviews,6,8 including medium- to high-quality RCTs of pharmacologic management of chronic constipation, found good evidence to support treatment with polyethylene glycol and tegaserod in adults of all ages. Of the 8 RCTs looking at polyethylene glycol, only 1 of the studies—a high-quality crossover comparison of polyethylene glycol vs placebo with 37 out-patient subjects—included a population with a mean age >60 years (mean age 62, range 42–89 years).
TABLE
How well do these interventions work for older patients with chronic constipation?
| INTERVENTION VS COMPARISON | STOOL FREQUENCY (STOOLS PER WEEK) | NNT‡ |
|---|---|---|
| Agiolax* vs lactulose | 4.5 vs 2.2 | 43 |
| Agiolax* vs lactulose | 5.6 vs 4.2 | 71 |
| Lactitol† vs placebo | 4.9 vs 3.6 | 77 |
| Lactitol† vs lactulose | 5.5 vs 4.9 | 160 |
| Lactulose vs sorbitol† | 7.0 vs 6.7 | 330 |
| External abdominal kneading (before-after) | 3.9 vs 1.4 | 40 |
| * Agiolax is a combination bulk and stimulant laxative not readily found in the United States. | ||
| † Lactitol and sorbitol are sugar alcohols used as replacement sweeteners and approved by the FDA as food additives. | ||
| ‡ Number needed to treat (NNT) for 1 person to have 1 more stool per week. | ||
A subgroup analysis9 of 331 elderly patients enrolled in 2 RCTs of tegaserod found no difference in outcomes between treatment with tegaserod and placebo, although this analysis was limited by inadequate power.
Tegaserod linked to ischemic events. A recent analysis of clinical trials found a statistically significant increase in cardiovascular ischemic events associated with tegaserod. The manufacturer took the product off the market in compliance with an FDA request in March 2007.
Lubiprostone offers promise. Lubiprostone, a chloride channel activator approved by the FDA for the treatment of chronic idiopathic constipation, has been studied in 6 placebo-controlled, double-blind, randomized Phase II and III clinical trials. In 2 unpublished pooled analyses of 3 of the trials, lubiprostone was found to be effective in a total of 220 elderly patients 65 years of age and older.10,11
Recommendations from others
The American College of Gastroenterology Chronic Constipation Task Force evidence-based guidelines make no reference to age, but state that evidence is best for treatment with psyllium, tegaserod, polyethylene glycol, and lactulose.12 They found insufficient evidence to support use of stimulants, stool softeners, lubricants, herbal supplements, biofeedback, and alternative treatments.
The American Gastroenterological Association guidelines on constipation are primarily based on expert opinion.13 Age is not specified in their recommendations. Dietary and exercise modifications are recommended as first-line treatments, followed by laxatives. Laxatives are recommended based on cost, in order from the least to most expensive agents. Suppositories, enemas, biofeedback, and (in refractory cases) surgery are recommended for patients with pelvic floor dysfunction.
The Registered Nurses Association of Ontario guidelines for constipation prevention in the older adult population recommend fluid and dietary fiber, regular exercise, and consistent toileting.14
There is no one best evidence-based treatment for chronic constipation in the elderly. While the most common first-line treatments are dietary fiber and exercise, the evidence is insufficient to support this approach in the geriatric population (strength of recommendation [SOR]: for dietary fiber: A, based on a systematic review; for exercise: SOR: B, based on 1 good- and 1 fair-quality randomized controlled trial [RCT]).
Herbal supplements (such as aloe), alternative treatments (biofeedback), lubricants (mineral oil), and combination laxatives sold in the US have not been sufficiently studied in controlled trials to make a recommendation (SOR: A, based on systematic review).
An abdominal kneading device can be used to treat chronic constipation, but the evidence is limited (SOR: B, based on 1 cohort study.)
Polyethylene glycol has not been studied in the elderly. A newer agent, lubiprostone (Amitiza), appears to be effective for the treatment of chronic constipation for elderly patients (SOR: B, based on subgroup analysis of RCTs.)
Is the patient truly constipated?
Mandi Sehgal, MD
Department of Family Medicine/Geriatrics, University of Cincinnati
Many older people feel that if they do not have a bowel movement every day they are constipated. However, constipation is defined as fewer than 3 bowel movements per week. So, the first thing we must do is to confirm that the patient is truly constipated.
Before I start my patients on any medicine, I suggest a trial of increased daily water and fiber intake along with exercise, followed by a trial of stool softeners and stimulant laxatives, if needed. If all of these methods fail, I consider trying polyethylene glycol, which can be titrated to effect. As with all medication use by the elderly, it is important to titrate cautiously (“start low and go slow”) and add other medications only when necessary.
Evidence summary
Few well-designed studies have focused on constipation treatment among the elderly. Our search located 1 systematic review of pharmacologic management, a systematic review of fiber management, 2 RCTs on the effect of exercise, and 1 before-after cohort study on abdominal massage. These studies were all conducted among geriatric patients with constipation. Two high-quality systematic reviews regarding chronic constipation management for adults of all ages included management options not studied in exclusively geriatric populations, such as herbal supplements, biofeedback, tegaserod, and polyethylene glycol.
Laxatives, fiber, and exercise: Studies are inconclusive
Two good-quality systematic reviews looked at 10 RCTs comparing laxatives with placebo, and 10 RCTs comparing 1 laxative with another.1,2 The studies generally had few participants, were of short duration, and were conducted in institutional settings. Most lacked power to make valid conclusions. These studies varied in the reported outcome measures, including stool frequency, stool consistency, straining, decrease in laxative use, and symptom scores. The reviews concluded that the best pharmacologic treatment for chronic constipation in the elderly has not been established.
Five of the higher-quality studies attained statistical significance. They showed a small but significant improvement in bowel movement frequency with a laxative when compared with placebo or another laxative (TABLE). The authors noted that multiple poor-quality studies have shown nonsignificant trends for improved constipation symptoms with laxatives compared with placebo.
Inconsistent findings on fiber. A good-quality systematic review3 of dietary fiber in the treatment of constipation for older patients located 8 moderate- to high-quality studies (6 RCTs and 2 blinded before-after studies), with 269 study participants in institutional settings. Results among studies were inconsistent, casting doubt on the efficacy of fiber treatment for constipation in the institutionalized elder.
Two RCTs4,5 investigating the effect of exercise on 246 institutionalized older patients showed no improvement in constipation. One study was of good quality, reporting adequate power and used an intention-to-treat analysis. The other was of fair quality.
Alternative TXs not well studied
A high-quality systematic review6 of constipation management among adults of all ages in North America found a lack of quality RCTs examining herbal supplement treatment. Biofeedback has been studied in adult populations, but no RCTs with placebo or sham-controls have been published.
One before-after cohort study7 investigated an external kneading mechanical device (Free-Lax) that was applied to the abdomen for 20 minutes once daily in 30 randomly selected chronically constipated nursing home residents. Researchers found significant improvements in bowel movement frequency, stool consistency and volume, and colonic transit time without side effects (TABLE).
A look beyond geriatric patients
Polyethylene glycol, tegaserod, and lubiprostone have not been studied in trials of exclusively geriatric populations. Two high-quality systematic reviews,6,8 including medium- to high-quality RCTs of pharmacologic management of chronic constipation, found good evidence to support treatment with polyethylene glycol and tegaserod in adults of all ages. Of the 8 RCTs looking at polyethylene glycol, only 1 of the studies—a high-quality crossover comparison of polyethylene glycol vs placebo with 37 out-patient subjects—included a population with a mean age >60 years (mean age 62, range 42–89 years).
TABLE
How well do these interventions work for older patients with chronic constipation?
| INTERVENTION VS COMPARISON | STOOL FREQUENCY (STOOLS PER WEEK) | NNT‡ |
|---|---|---|
| Agiolax* vs lactulose | 4.5 vs 2.2 | 43 |
| Agiolax* vs lactulose | 5.6 vs 4.2 | 71 |
| Lactitol† vs placebo | 4.9 vs 3.6 | 77 |
| Lactitol† vs lactulose | 5.5 vs 4.9 | 160 |
| Lactulose vs sorbitol† | 7.0 vs 6.7 | 330 |
| External abdominal kneading (before-after) | 3.9 vs 1.4 | 40 |
| * Agiolax is a combination bulk and stimulant laxative not readily found in the United States. | ||
| † Lactitol and sorbitol are sugar alcohols used as replacement sweeteners and approved by the FDA as food additives. | ||
| ‡ Number needed to treat (NNT) for 1 person to have 1 more stool per week. | ||
A subgroup analysis9 of 331 elderly patients enrolled in 2 RCTs of tegaserod found no difference in outcomes between treatment with tegaserod and placebo, although this analysis was limited by inadequate power.
Tegaserod linked to ischemic events. A recent analysis of clinical trials found a statistically significant increase in cardiovascular ischemic events associated with tegaserod. The manufacturer took the product off the market in compliance with an FDA request in March 2007.
Lubiprostone offers promise. Lubiprostone, a chloride channel activator approved by the FDA for the treatment of chronic idiopathic constipation, has been studied in 6 placebo-controlled, double-blind, randomized Phase II and III clinical trials. In 2 unpublished pooled analyses of 3 of the trials, lubiprostone was found to be effective in a total of 220 elderly patients 65 years of age and older.10,11
Recommendations from others
The American College of Gastroenterology Chronic Constipation Task Force evidence-based guidelines make no reference to age, but state that evidence is best for treatment with psyllium, tegaserod, polyethylene glycol, and lactulose.12 They found insufficient evidence to support use of stimulants, stool softeners, lubricants, herbal supplements, biofeedback, and alternative treatments.
The American Gastroenterological Association guidelines on constipation are primarily based on expert opinion.13 Age is not specified in their recommendations. Dietary and exercise modifications are recommended as first-line treatments, followed by laxatives. Laxatives are recommended based on cost, in order from the least to most expensive agents. Suppositories, enemas, biofeedback, and (in refractory cases) surgery are recommended for patients with pelvic floor dysfunction.
The Registered Nurses Association of Ontario guidelines for constipation prevention in the older adult population recommend fluid and dietary fiber, regular exercise, and consistent toileting.14
1. Petticrew M, Watt I, Brand M. What’s the “best buy” for treatment of constipation? Results of a systematic review of the efficacy and comparative efficacy of laxatives in the elderly. Br J Gen Pract 1999;49:387-393.
2. Petticrew M, Watt I, Sheldon T. Systematic review of the effectiveness of laxatives in the elderly. Health Technol Assess 1997;1:i–iv-1–52.
3. Kenny KA, Skelly JM. Dietary fiber for constipation in older adults: a systematic review. Clinical Effectiveness in Nursing 2001;5:120-128.
4. Chin APMJ, van Poppel MN, van Mechelen W. Effects of resistance and functional-skills training on habitual activity and constipation among older adults living in long-term care facilities: a randomized controlled trial. BMC Geriatr 2006;6:9.-
5. Simmons SF, Schnelle JF. Effects of an exercise and scheduled-toileting intervention on appetite and constipation in nursing home residents. J Nutr Health Aging 2004;8:116-121.
6. Brandt LJ, Prather CM, Quigley EM, Schiller LR, Schoenfeld P, Talley NJ. Systematic review on the management of chronic constipation in North America. Am J Gastroenterol 2005;100 suppl 1:S5-S21.
7. Mimidis K, Galinsky D, Rimon E, Papadopoulos V, Zicherman Y, Oreopoulos D. Use of a device that applies external kneading-like force on the abdomen for treatment of constipation. World J Gastroenterol 2005;11:1971-1975.
8. Ramkumar D, Rao SS. Efficacy and safety of traditional medical therapies for chronic constipation: systematic review. Am J Gastroenterol 2005;100:936-971.
9. Baun RF, Levy HB. Tegaserod for treating chronic constipation in elderly patients. Ann Pharmacother 2007;41:309-313.
10. Ueno R, Joswick TR, Wahle A, et al. Efficacy and safety of lubiprostone for the treatment of chronic constipation in elderly vs non-elderly subjects. Gastroenterology 2006;130(suppl 2):A189.-
11. Ueno R, Panas R, Wahle A, et al. Long-term safety and efficacy of lubiprostone for the treatment of chronic constipation in elderly subjects. Gastroenterology 2006;130(suppl 2):A188.-
12. American College of Gastroenterology Chronic Constipation Task Force. An evidence-based approach to the management of chronic constipation in North America. Am J Gastroenterol 2005;100 Suppl 1:S1-S4.
13. Locke GR, 3rd, Pemberton JH, Phillips SF. American Gastroenterological Association Medical Position Statement: guidelines on constipation. Gastroenterology 2000;119:1761-1766.
14. Registered Nurses Association of Ontario (RNAO). Prevention of constipation in the older adult population. Toronto, Ontario: RNAO; 2005. Available at: www.guideline.gov/summary/summary.aspx?ss=15&doc_id=7004&nbr=4213. Accessed on November 8, 2007.
1. Petticrew M, Watt I, Brand M. What’s the “best buy” for treatment of constipation? Results of a systematic review of the efficacy and comparative efficacy of laxatives in the elderly. Br J Gen Pract 1999;49:387-393.
2. Petticrew M, Watt I, Sheldon T. Systematic review of the effectiveness of laxatives in the elderly. Health Technol Assess 1997;1:i–iv-1–52.
3. Kenny KA, Skelly JM. Dietary fiber for constipation in older adults: a systematic review. Clinical Effectiveness in Nursing 2001;5:120-128.
4. Chin APMJ, van Poppel MN, van Mechelen W. Effects of resistance and functional-skills training on habitual activity and constipation among older adults living in long-term care facilities: a randomized controlled trial. BMC Geriatr 2006;6:9.-
5. Simmons SF, Schnelle JF. Effects of an exercise and scheduled-toileting intervention on appetite and constipation in nursing home residents. J Nutr Health Aging 2004;8:116-121.
6. Brandt LJ, Prather CM, Quigley EM, Schiller LR, Schoenfeld P, Talley NJ. Systematic review on the management of chronic constipation in North America. Am J Gastroenterol 2005;100 suppl 1:S5-S21.
7. Mimidis K, Galinsky D, Rimon E, Papadopoulos V, Zicherman Y, Oreopoulos D. Use of a device that applies external kneading-like force on the abdomen for treatment of constipation. World J Gastroenterol 2005;11:1971-1975.
8. Ramkumar D, Rao SS. Efficacy and safety of traditional medical therapies for chronic constipation: systematic review. Am J Gastroenterol 2005;100:936-971.
9. Baun RF, Levy HB. Tegaserod for treating chronic constipation in elderly patients. Ann Pharmacother 2007;41:309-313.
10. Ueno R, Joswick TR, Wahle A, et al. Efficacy and safety of lubiprostone for the treatment of chronic constipation in elderly vs non-elderly subjects. Gastroenterology 2006;130(suppl 2):A189.-
11. Ueno R, Panas R, Wahle A, et al. Long-term safety and efficacy of lubiprostone for the treatment of chronic constipation in elderly subjects. Gastroenterology 2006;130(suppl 2):A188.-
12. American College of Gastroenterology Chronic Constipation Task Force. An evidence-based approach to the management of chronic constipation in North America. Am J Gastroenterol 2005;100 Suppl 1:S1-S4.
13. Locke GR, 3rd, Pemberton JH, Phillips SF. American Gastroenterological Association Medical Position Statement: guidelines on constipation. Gastroenterology 2000;119:1761-1766.
14. Registered Nurses Association of Ontario (RNAO). Prevention of constipation in the older adult population. Toronto, Ontario: RNAO; 2005. Available at: www.guideline.gov/summary/summary.aspx?ss=15&doc_id=7004&nbr=4213. Accessed on November 8, 2007.
Evidence-based answers from the Family Physicians Inquiries Network
How should you document a patient’s refusal to undergo a necessary intervention?
Your documentation of a patient’s refusal to undergo a test or intervention should include: an assessment of the patient’s competence to make decisions, a statement indicating a lack of coercion; a description of your discussion with him (or her) regarding the need for the treatment, alternatives to treatment, possible risks of treatment, and potential consequences of refusal; and a summary of the patient’s reasons for refusal (strength of recommendation [SOR]: C, based on expert opinion and case series).
Keep the dialogue going (and this form may help)
Timothy E. Huber, MD
Oroville, Calif
We all have (or will) come across patients who refuse a clearly indicated intervention. Some are well informed, some are misinformed, and some have no desire to be informed. All, however, need education before they can make a reasoned, competent decision.
An “Against Medical Advice” sheet provides little education and sets up barriers between the 2 sides. An “Informed Refusal of Care” sheet should be used in the same manner as “Informed Consent for Care.” It can properly educate the uninformed or misinformed patient, and spark a discussion with the well-informed patient regarding the nature of their choice. The point of an “Informed Refusal of Care” sheet is to be a summary of the dialogue between 2 people about the care that one person can provide and the care that one person wishes to receive. When this occurs, both people can depart knowing that they gave—and received—relevant information about the situation.
Evidence summary
The law of informed consent defines the right to informed refusal. Thus, each case must establish:
- that the patient or decision maker is competent,
- that the decision is voluntary, and
- that the physician disclosed the risks of the choice to the patient, including a discussion of risks and alternatives to treatment, and potential consequences of treatment refusal, including jeopardy to health or life.1
The general standard of disclosure has evolved to what an ordinary, reasonable patient would wish to know.2 To understand the patient’s perspective,3 reasons for the refusal should be explored4 and documented.5
Medical records that clearly reflect the decision-making process can be pivotal in the success or failure of legal claims.6 In addition to the discussion with the patient, the medical record should describe any involvement of family or other third parties. If imminently or potentially serious consequences are likely to result from patient refusal, health care providers might consider having the refusal signed and witnessed.7
Not all AMA forms afford protection. There are samples of refusal of consent forms,8 but a study of annotated case law revealed that the “discharge against medical advice” forms used by some hospitals might provide little legal protection.9 Documenting what specific advice was given to the patient is most important.
Recommendations from others
The American College of Obstetricians and Gynecologists addresses this issue explicitly in a committee opinion on Informed Refusal.2 They advocate documenting the explanation of the need for the proposed treatment, the patient’s refusal to consent, the patient’s reasons, and the possible consequences of refusal.
Guidelines on vaccination refusal from the Advisory Committee on Immunization Practices and the American Academy of Family Physicians encourage physicians to enter into a thorough discussion of the risks and benefits of immunization, and document such discussions clearly in the medical record.10
The American Academy of Pediatrics has published a “Refusal to Vaccinate” form,11 though they warn that it does not substitute for good communication.12
The Renal Physicians Association and the American Society of Nephrology guideline on dialysis promotes the concepts of patient autonomy, informed consent or refusal, and the necessity of documenting physician-patient discussions.13
Likewise, the American Academy of Pediatrics addresses similar issues in its guidelines on forgoing life-sustaining medical treatment.14
1. Moskop JC. Informed consent and refusal of treatment: challenges for emergency physicians. Emerg Med Clin North Am 2006;24:605-618.
2. ACOG, Committee on Professional Liability. Informed refusal. Obstet Gynecol 2004;104:1465-1466.
3. Carrese JA. Refusal of care: patients’ well-being and physicians’ ethical obligations. JAMA 2006;296:691-695.
4. Parker MH, Tobin B. Refusal of treatment. Med J Aust 2001;174:531-532.
5. Wettstein RM. The right to refuse psychiatric treatment. Psychiatr Clin North Am 1999;22:173-182.
6. Roach WH, Jr, Hoban RG, Broccolo BM, Roth AB, Blanchard TP. Medical Records and the Law (4th ed). American Health Information management Association. Sudbury, Mass: Jones and Bartlett Publishers, 2006: 98.
7. Siegel DM. Consent and refusal of treatment. Emerg Med Clin North Am 1993;11:833-840.
8. Johnson LJ. Malpractice Consult: documenting refusal to consent. Med Econ 2002;79:143.-
9. Devitt PJ, Devitt AC, Dewan M. An examination of whether discharging patients against medical advice protects physicians from malpractice charges. Psychiatr Serv 2000;51:899-902.
10. Kroger AT, Atkinson WL, Marcuse EK, Pickering LK. Advisory Committee on Immunization Practices (ACIP) Centers for Disease Control and Prevention (CDC). General recommendations on immunization: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2006;55(RR-15):1-48.Erratum in: MMWR Morb Mortal Wkly Rep.2006;55:1303.
11. CISP: Childhood Immunization Support Program Web site. American Academy of Pediatrics. Available at: www.cispimmunize.org/pro/pdf/refusaltovaccinate_revised%204-11-06.pdf. Accessed on November 8, 2007.
12. Diekema DS. American Academy of Pediatrics, Committee on Bioethics. Responding to parental refusals of immunization of children. Pediatrics 2005;115:1428-1431.
13. Galla JH. Clinical practice guideline on shared decision-making in the appropriate initiation of and withdrawal from dialysis. The Renal Physicians’ Association and the American Society of Nephrology. J Am Soc Nephrol. 2000;11:1340-1342.Corrected and republished in J Am Soc Nephrol 2000;11: 2 p. following 1788.
14. American Academy of Pediatrics, Committee on Bioethics: Guidelines on foregoing life-sustaining medical treatment. Pediatrics 1994;93:532-536.
Your documentation of a patient’s refusal to undergo a test or intervention should include: an assessment of the patient’s competence to make decisions, a statement indicating a lack of coercion; a description of your discussion with him (or her) regarding the need for the treatment, alternatives to treatment, possible risks of treatment, and potential consequences of refusal; and a summary of the patient’s reasons for refusal (strength of recommendation [SOR]: C, based on expert opinion and case series).
Keep the dialogue going (and this form may help)
Timothy E. Huber, MD
Oroville, Calif
We all have (or will) come across patients who refuse a clearly indicated intervention. Some are well informed, some are misinformed, and some have no desire to be informed. All, however, need education before they can make a reasoned, competent decision.
An “Against Medical Advice” sheet provides little education and sets up barriers between the 2 sides. An “Informed Refusal of Care” sheet should be used in the same manner as “Informed Consent for Care.” It can properly educate the uninformed or misinformed patient, and spark a discussion with the well-informed patient regarding the nature of their choice. The point of an “Informed Refusal of Care” sheet is to be a summary of the dialogue between 2 people about the care that one person can provide and the care that one person wishes to receive. When this occurs, both people can depart knowing that they gave—and received—relevant information about the situation.
Evidence summary
The law of informed consent defines the right to informed refusal. Thus, each case must establish:
- that the patient or decision maker is competent,
- that the decision is voluntary, and
- that the physician disclosed the risks of the choice to the patient, including a discussion of risks and alternatives to treatment, and potential consequences of treatment refusal, including jeopardy to health or life.1
The general standard of disclosure has evolved to what an ordinary, reasonable patient would wish to know.2 To understand the patient’s perspective,3 reasons for the refusal should be explored4 and documented.5
Medical records that clearly reflect the decision-making process can be pivotal in the success or failure of legal claims.6 In addition to the discussion with the patient, the medical record should describe any involvement of family or other third parties. If imminently or potentially serious consequences are likely to result from patient refusal, health care providers might consider having the refusal signed and witnessed.7
Not all AMA forms afford protection. There are samples of refusal of consent forms,8 but a study of annotated case law revealed that the “discharge against medical advice” forms used by some hospitals might provide little legal protection.9 Documenting what specific advice was given to the patient is most important.
Recommendations from others
The American College of Obstetricians and Gynecologists addresses this issue explicitly in a committee opinion on Informed Refusal.2 They advocate documenting the explanation of the need for the proposed treatment, the patient’s refusal to consent, the patient’s reasons, and the possible consequences of refusal.
Guidelines on vaccination refusal from the Advisory Committee on Immunization Practices and the American Academy of Family Physicians encourage physicians to enter into a thorough discussion of the risks and benefits of immunization, and document such discussions clearly in the medical record.10
The American Academy of Pediatrics has published a “Refusal to Vaccinate” form,11 though they warn that it does not substitute for good communication.12
The Renal Physicians Association and the American Society of Nephrology guideline on dialysis promotes the concepts of patient autonomy, informed consent or refusal, and the necessity of documenting physician-patient discussions.13
Likewise, the American Academy of Pediatrics addresses similar issues in its guidelines on forgoing life-sustaining medical treatment.14
Your documentation of a patient’s refusal to undergo a test or intervention should include: an assessment of the patient’s competence to make decisions, a statement indicating a lack of coercion; a description of your discussion with him (or her) regarding the need for the treatment, alternatives to treatment, possible risks of treatment, and potential consequences of refusal; and a summary of the patient’s reasons for refusal (strength of recommendation [SOR]: C, based on expert opinion and case series).
Keep the dialogue going (and this form may help)
Timothy E. Huber, MD
Oroville, Calif
We all have (or will) come across patients who refuse a clearly indicated intervention. Some are well informed, some are misinformed, and some have no desire to be informed. All, however, need education before they can make a reasoned, competent decision.
An “Against Medical Advice” sheet provides little education and sets up barriers between the 2 sides. An “Informed Refusal of Care” sheet should be used in the same manner as “Informed Consent for Care.” It can properly educate the uninformed or misinformed patient, and spark a discussion with the well-informed patient regarding the nature of their choice. The point of an “Informed Refusal of Care” sheet is to be a summary of the dialogue between 2 people about the care that one person can provide and the care that one person wishes to receive. When this occurs, both people can depart knowing that they gave—and received—relevant information about the situation.
Evidence summary
The law of informed consent defines the right to informed refusal. Thus, each case must establish:
- that the patient or decision maker is competent,
- that the decision is voluntary, and
- that the physician disclosed the risks of the choice to the patient, including a discussion of risks and alternatives to treatment, and potential consequences of treatment refusal, including jeopardy to health or life.1
The general standard of disclosure has evolved to what an ordinary, reasonable patient would wish to know.2 To understand the patient’s perspective,3 reasons for the refusal should be explored4 and documented.5
Medical records that clearly reflect the decision-making process can be pivotal in the success or failure of legal claims.6 In addition to the discussion with the patient, the medical record should describe any involvement of family or other third parties. If imminently or potentially serious consequences are likely to result from patient refusal, health care providers might consider having the refusal signed and witnessed.7
Not all AMA forms afford protection. There are samples of refusal of consent forms,8 but a study of annotated case law revealed that the “discharge against medical advice” forms used by some hospitals might provide little legal protection.9 Documenting what specific advice was given to the patient is most important.
Recommendations from others
The American College of Obstetricians and Gynecologists addresses this issue explicitly in a committee opinion on Informed Refusal.2 They advocate documenting the explanation of the need for the proposed treatment, the patient’s refusal to consent, the patient’s reasons, and the possible consequences of refusal.
Guidelines on vaccination refusal from the Advisory Committee on Immunization Practices and the American Academy of Family Physicians encourage physicians to enter into a thorough discussion of the risks and benefits of immunization, and document such discussions clearly in the medical record.10
The American Academy of Pediatrics has published a “Refusal to Vaccinate” form,11 though they warn that it does not substitute for good communication.12
The Renal Physicians Association and the American Society of Nephrology guideline on dialysis promotes the concepts of patient autonomy, informed consent or refusal, and the necessity of documenting physician-patient discussions.13
Likewise, the American Academy of Pediatrics addresses similar issues in its guidelines on forgoing life-sustaining medical treatment.14
1. Moskop JC. Informed consent and refusal of treatment: challenges for emergency physicians. Emerg Med Clin North Am 2006;24:605-618.
2. ACOG, Committee on Professional Liability. Informed refusal. Obstet Gynecol 2004;104:1465-1466.
3. Carrese JA. Refusal of care: patients’ well-being and physicians’ ethical obligations. JAMA 2006;296:691-695.
4. Parker MH, Tobin B. Refusal of treatment. Med J Aust 2001;174:531-532.
5. Wettstein RM. The right to refuse psychiatric treatment. Psychiatr Clin North Am 1999;22:173-182.
6. Roach WH, Jr, Hoban RG, Broccolo BM, Roth AB, Blanchard TP. Medical Records and the Law (4th ed). American Health Information management Association. Sudbury, Mass: Jones and Bartlett Publishers, 2006: 98.
7. Siegel DM. Consent and refusal of treatment. Emerg Med Clin North Am 1993;11:833-840.
8. Johnson LJ. Malpractice Consult: documenting refusal to consent. Med Econ 2002;79:143.-
9. Devitt PJ, Devitt AC, Dewan M. An examination of whether discharging patients against medical advice protects physicians from malpractice charges. Psychiatr Serv 2000;51:899-902.
10. Kroger AT, Atkinson WL, Marcuse EK, Pickering LK. Advisory Committee on Immunization Practices (ACIP) Centers for Disease Control and Prevention (CDC). General recommendations on immunization: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2006;55(RR-15):1-48.Erratum in: MMWR Morb Mortal Wkly Rep.2006;55:1303.
11. CISP: Childhood Immunization Support Program Web site. American Academy of Pediatrics. Available at: www.cispimmunize.org/pro/pdf/refusaltovaccinate_revised%204-11-06.pdf. Accessed on November 8, 2007.
12. Diekema DS. American Academy of Pediatrics, Committee on Bioethics. Responding to parental refusals of immunization of children. Pediatrics 2005;115:1428-1431.
13. Galla JH. Clinical practice guideline on shared decision-making in the appropriate initiation of and withdrawal from dialysis. The Renal Physicians’ Association and the American Society of Nephrology. J Am Soc Nephrol. 2000;11:1340-1342.Corrected and republished in J Am Soc Nephrol 2000;11: 2 p. following 1788.
14. American Academy of Pediatrics, Committee on Bioethics: Guidelines on foregoing life-sustaining medical treatment. Pediatrics 1994;93:532-536.
1. Moskop JC. Informed consent and refusal of treatment: challenges for emergency physicians. Emerg Med Clin North Am 2006;24:605-618.
2. ACOG, Committee on Professional Liability. Informed refusal. Obstet Gynecol 2004;104:1465-1466.
3. Carrese JA. Refusal of care: patients’ well-being and physicians’ ethical obligations. JAMA 2006;296:691-695.
4. Parker MH, Tobin B. Refusal of treatment. Med J Aust 2001;174:531-532.
5. Wettstein RM. The right to refuse psychiatric treatment. Psychiatr Clin North Am 1999;22:173-182.
6. Roach WH, Jr, Hoban RG, Broccolo BM, Roth AB, Blanchard TP. Medical Records and the Law (4th ed). American Health Information management Association. Sudbury, Mass: Jones and Bartlett Publishers, 2006: 98.
7. Siegel DM. Consent and refusal of treatment. Emerg Med Clin North Am 1993;11:833-840.
8. Johnson LJ. Malpractice Consult: documenting refusal to consent. Med Econ 2002;79:143.-
9. Devitt PJ, Devitt AC, Dewan M. An examination of whether discharging patients against medical advice protects physicians from malpractice charges. Psychiatr Serv 2000;51:899-902.
10. Kroger AT, Atkinson WL, Marcuse EK, Pickering LK. Advisory Committee on Immunization Practices (ACIP) Centers for Disease Control and Prevention (CDC). General recommendations on immunization: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2006;55(RR-15):1-48.Erratum in: MMWR Morb Mortal Wkly Rep.2006;55:1303.
11. CISP: Childhood Immunization Support Program Web site. American Academy of Pediatrics. Available at: www.cispimmunize.org/pro/pdf/refusaltovaccinate_revised%204-11-06.pdf. Accessed on November 8, 2007.
12. Diekema DS. American Academy of Pediatrics, Committee on Bioethics. Responding to parental refusals of immunization of children. Pediatrics 2005;115:1428-1431.
13. Galla JH. Clinical practice guideline on shared decision-making in the appropriate initiation of and withdrawal from dialysis. The Renal Physicians’ Association and the American Society of Nephrology. J Am Soc Nephrol. 2000;11:1340-1342.Corrected and republished in J Am Soc Nephrol 2000;11: 2 p. following 1788.
14. American Academy of Pediatrics, Committee on Bioethics: Guidelines on foregoing life-sustaining medical treatment. Pediatrics 1994;93:532-536.
Evidence-based answers from the Family Physicians Inquiries Network
Are steroid injections effective for tenosynovitis of the hand?
Yes. Steroid injections are an effective first-line therapy for flexor tenosynovitis of the hand, with a number needed to treat [NNT] of 2.3 for injection of steroids and lidocaine (strength of recommendation [SOR]: B, based on 1 prospective RCT and 2 low-quality studies). Injection into the tendon sheath may not be critical to a successful outcome (SOR: B, based on 1 prospective uncontrolled trial).
For de quervain’s tenosynovitis, steroid injections without splinting are more effective than injection plus splinting or splinting alone. The cure rates are 83% (steroid alone), 61% (steroid plus splinting), and 14% (splinting alone) (SOR: B, based on a systematic review of descriptive noncontrolled studies). Injecting into the tendon compartments was more effective than injecting into the surrounding soft tissues (SOR: B, based on 1 prospective controlled trial).
Steroids are helpful, especially for a quick return to function
Robert Gauer, MD
Fort Bragg, NC
I often see flexor and de Quervain’s tenosynovitis in my practice, particularly among patients whose occupations require repetitive hand use. Acute treatment for these conditions typically consists of immobilization with buddy taping or finger/thumb spica splinting. For those who do not improve in 4 weeks, or require a quick return to function, I’ve found that corticosteroid injection using a 25- to 30-gauge needle can be very effective.
De Quervain’s tenosynovitis must be injected into the sheath between the abductor longus and extensor pollicis brevis; flexor tenosynovitis is injected in the nodule. I typically have excellent results, with patients returning to function within 72 hours. Depigmentation and atrophy can occur with injections, especially in small-statured or dark-pigmented patients. surgical release is rarely required for either condition.
Infectious tenosynovitis must be recognized early; it is typically due to lacerations or puncture wounds. In these cases, I immediately refer to orthopedic surgery for further treatment and evaluation.
Evidence summary
Flexor tenosynovitis and de Quervain’s tenosynovitis are the 2 most common types of tenosynovitis of the hand. While the term “tenosynovitis” implies an inflammatory condition, pathoanatomically it is better described as a friction overuse injury, resulting in fibrosis of the surrounding tissue and subsequent narrowing of the synovium.1
“Trigger finger” due to tenosynovitis
Flexor tenosynovitis: Steroid injections provided relief
A prospective, double-blinded RCT2 published by an orthopedic group in 1995 compared 24 patients with primary flexor tenosynovitis. Patients were injected with either 1 cc of betamethasone (Celestone 6 mg) and 3 cc of 1% lidocaine, or 4 cc of 1% lidocaine alone. A successful outcome was defined as absence of triggering and pain, both subjectively and on examination. Follow-up examination was completed for all patients at 3 weeks and 4 months after injection.
The treatment group had success outcomes for 10 of 14 patients (71%) at 3 weeks and 9 of 14 patients (64%) at 4 months, compared with 2 of 10 (20%) at both 3 weeks and 4 months in the control group (NNT=2.3; P<.05 at 4 months). No significant side effects were noted. It is unclear how the study blinded the white, thick consistency of betamethasone compared with the clear nature of lidocaine alone.
Injection site may not matter. In another prospective, uncontrolled trial, 107 patients with flexor tenosynovitis were injected with 1 cc of betamethasone, 0.5 cc of 1% lidocaine, and 0.5 cc of radio opaque dye.3 Some patients got their injections in the tendon sheath at the A1 pulley site, others got their injections in the subcutaneous tissue surrounding the pulley, and a third group got injections in both sites. Patients graded their relief subjectively as either good (total alleviation of symptoms), fair (lasting improvement), or poor (only transient improvement or none at all).
Those who received an intrasheath injection reported good results 47% of the time at 2 weeks follow-up, compared with 70% and 50% for patients in the subcutaneous and mixed groups, respectively. There was no statistically significant difference between the groups, suggesting that the exact location of injection may not be important.
de Quervain’s tenosynovitis: Steroids better than splinting
A pooled quantitative literature search concerning the treatment of de Quervain’s tenosynovitis compared 7 studies (a total of 459 wrists) with identical diagnostic and success criteria.4 Average follow-up was 9.6 months (range, 1 week to 7 years). There were no control groups in the studies, and none of the studies were randomized. Of the 226 cases treated with steroid injection alone, 83% were cured, though 30 of these needed a second injection. Sixty-one percent of those treated with injection and splint were cured, while 14% treated with splint alone reported cure.
Steroids outperform splinting and NSAIDs. A retrospective study not included in the above review compared steroid injection with splinting and nonsteroidal anti-inflammatory drugs (NSAIDs).5 Researchers stratified subjects into minimal, mild, or moderate-to-severe, based on their severity of disease and limitation on their activities of daily living. Mean follow-up was 2.3 years.
Of those cases treated with splinting and NSAIDs, 15 of 17 in the minimal group had resolution of symptoms, but only 4 of 20 in the mild group and 2 of 8 in the moderate-to-severe group had symptoms resolve. The injection group obtained better results, with 100% of cases in the minimal and mild groups resolving and 76% of those in the more severe group resolving completely, with an additional 7% reporting improvement.
Injection site appears to matter with de Quervain’s tenosynovitis. In 1 small, controlled, prospective, double-blinded study, the authors attempted to correlate clinical relief of de Quervain’s tenosynovitis with accuracy of injection into the first dorsal compartment.6 The researchers enrolled 19 patients. The same hand surgeon injected 3 cc of 1% lidocaine, 1 cc betamethasone, and 1 cc Omnipaque 300 dye into the abductor pollicis longus sheath and then attempted, with ulnar deviation of the needle, to fill the extensor pollicis brevis sheath.
Patients were followed-up at 1 month and 3 months postinjection. Success—defined as a negative Finkelstein’s test, absence of pain, and normal activities of daily living—was noted in 11 of 19 patients at 3 months. In a radiographic check, 4 of 5 of the patients with dye in both compartments were asymptomatic, while the 3 who had no dye in either compartment remained symptomatic. This suggests that the location of injection may be important in de Quervain’s tenosynovitis.
Recommendations from others
The Brigham and Women’s Hospital guidelines for treatment of de Quervain’s tenosynovitis state that corticosteroid injections “may be very helpful,” and that they should be considered if symptoms persist beyond 6 weeks of conservative treatment.7DeLee and Drez’s Orthopedic Sports Medicine text recommends corticosteroid injection for de Quervain’s tenosynovitis after 2 weeks of conservative treatment have failed.8
UpToDate recommends steroid injection for de Quervain’s tenosynovitis if pain persists for more than 2 to 6 weeks despite splinting, icing, and NSAID therapy.9 For flexor tenosynovitis, UpToDate recommends local injection when symptoms persist for 4 to 6 weeks despite splinting.10
1. Zingas C, Failla JM, Van Holsbeeck M. Injection accuracy and clinical relief of de Quervain’s tendinitis. J Hand Surgery (Am) 1998;23:89-96.
2. Murphy D, Failla JM, Koniuch MP. Steroid versus placebo injection for trigger finger. J Hand Surg (Am) 1995;20:628-631.
3. Taras JS, Raphael JS, Pan WT, et al. Corticosteroid injections for trigger digits: is intrasheath injection necessary? J Hand Surg (Am) 1998;23:717-22.
4. Richie CA, 3rd, Briner WW, Jr. Corticosteroid injection for treatment of de Quervain’s tenosynovitis: a pooled quantitative literature evaluation. J Am Board Fam Prac 2003;16:102-106.
5. Lane LB, Boretz RS, Stuchin SA. Treatment of de Quervain’s disease: role of conservative management. J Hand Surgery (Br) 2001;26:258-260.
6. Zingas C, Failla JM, Van Holsbeeck M. Injection accuracy and clinical relief of de Quervain’s tendinitis. J Hand Surgery (Am) 1998;23:89-96.
7. Brigham and Women’s Hospital. Upper Extremity Musculoskeletal Disorders: A Guide to Prevention, Diagnosis and Treatment. Boston, Mass: Brigham and Women’s Hospital; 2003:9.
8. DeLee J, Drez D, Miller M. DeLee and Drez’s Orthopaedic Sports Medicine: Principles And Practice. 2nd ed. Philadelphia, Pa: Saunders; 2003.
9. Anderson BC, Sheon RP. de Quervain’s tenosynovitis. UpToDate [online database]. Updated May 7, 2004. Waltham, Mass: UpToDate; 2004.
10. Anderson BC. Trigger finger (flexor tenosynovitis). UpToDate [online database]. Updated December 26, 2000. Waltham, Mass: UpToDate; 2000.
Yes. Steroid injections are an effective first-line therapy for flexor tenosynovitis of the hand, with a number needed to treat [NNT] of 2.3 for injection of steroids and lidocaine (strength of recommendation [SOR]: B, based on 1 prospective RCT and 2 low-quality studies). Injection into the tendon sheath may not be critical to a successful outcome (SOR: B, based on 1 prospective uncontrolled trial).
For de quervain’s tenosynovitis, steroid injections without splinting are more effective than injection plus splinting or splinting alone. The cure rates are 83% (steroid alone), 61% (steroid plus splinting), and 14% (splinting alone) (SOR: B, based on a systematic review of descriptive noncontrolled studies). Injecting into the tendon compartments was more effective than injecting into the surrounding soft tissues (SOR: B, based on 1 prospective controlled trial).
Steroids are helpful, especially for a quick return to function
Robert Gauer, MD
Fort Bragg, NC
I often see flexor and de Quervain’s tenosynovitis in my practice, particularly among patients whose occupations require repetitive hand use. Acute treatment for these conditions typically consists of immobilization with buddy taping or finger/thumb spica splinting. For those who do not improve in 4 weeks, or require a quick return to function, I’ve found that corticosteroid injection using a 25- to 30-gauge needle can be very effective.
De Quervain’s tenosynovitis must be injected into the sheath between the abductor longus and extensor pollicis brevis; flexor tenosynovitis is injected in the nodule. I typically have excellent results, with patients returning to function within 72 hours. Depigmentation and atrophy can occur with injections, especially in small-statured or dark-pigmented patients. surgical release is rarely required for either condition.
Infectious tenosynovitis must be recognized early; it is typically due to lacerations or puncture wounds. In these cases, I immediately refer to orthopedic surgery for further treatment and evaluation.
Evidence summary
Flexor tenosynovitis and de Quervain’s tenosynovitis are the 2 most common types of tenosynovitis of the hand. While the term “tenosynovitis” implies an inflammatory condition, pathoanatomically it is better described as a friction overuse injury, resulting in fibrosis of the surrounding tissue and subsequent narrowing of the synovium.1
“Trigger finger” due to tenosynovitis
Flexor tenosynovitis: Steroid injections provided relief
A prospective, double-blinded RCT2 published by an orthopedic group in 1995 compared 24 patients with primary flexor tenosynovitis. Patients were injected with either 1 cc of betamethasone (Celestone 6 mg) and 3 cc of 1% lidocaine, or 4 cc of 1% lidocaine alone. A successful outcome was defined as absence of triggering and pain, both subjectively and on examination. Follow-up examination was completed for all patients at 3 weeks and 4 months after injection.
The treatment group had success outcomes for 10 of 14 patients (71%) at 3 weeks and 9 of 14 patients (64%) at 4 months, compared with 2 of 10 (20%) at both 3 weeks and 4 months in the control group (NNT=2.3; P<.05 at 4 months). No significant side effects were noted. It is unclear how the study blinded the white, thick consistency of betamethasone compared with the clear nature of lidocaine alone.
Injection site may not matter. In another prospective, uncontrolled trial, 107 patients with flexor tenosynovitis were injected with 1 cc of betamethasone, 0.5 cc of 1% lidocaine, and 0.5 cc of radio opaque dye.3 Some patients got their injections in the tendon sheath at the A1 pulley site, others got their injections in the subcutaneous tissue surrounding the pulley, and a third group got injections in both sites. Patients graded their relief subjectively as either good (total alleviation of symptoms), fair (lasting improvement), or poor (only transient improvement or none at all).
Those who received an intrasheath injection reported good results 47% of the time at 2 weeks follow-up, compared with 70% and 50% for patients in the subcutaneous and mixed groups, respectively. There was no statistically significant difference between the groups, suggesting that the exact location of injection may not be important.
de Quervain’s tenosynovitis: Steroids better than splinting
A pooled quantitative literature search concerning the treatment of de Quervain’s tenosynovitis compared 7 studies (a total of 459 wrists) with identical diagnostic and success criteria.4 Average follow-up was 9.6 months (range, 1 week to 7 years). There were no control groups in the studies, and none of the studies were randomized. Of the 226 cases treated with steroid injection alone, 83% were cured, though 30 of these needed a second injection. Sixty-one percent of those treated with injection and splint were cured, while 14% treated with splint alone reported cure.
Steroids outperform splinting and NSAIDs. A retrospective study not included in the above review compared steroid injection with splinting and nonsteroidal anti-inflammatory drugs (NSAIDs).5 Researchers stratified subjects into minimal, mild, or moderate-to-severe, based on their severity of disease and limitation on their activities of daily living. Mean follow-up was 2.3 years.
Of those cases treated with splinting and NSAIDs, 15 of 17 in the minimal group had resolution of symptoms, but only 4 of 20 in the mild group and 2 of 8 in the moderate-to-severe group had symptoms resolve. The injection group obtained better results, with 100% of cases in the minimal and mild groups resolving and 76% of those in the more severe group resolving completely, with an additional 7% reporting improvement.
Injection site appears to matter with de Quervain’s tenosynovitis. In 1 small, controlled, prospective, double-blinded study, the authors attempted to correlate clinical relief of de Quervain’s tenosynovitis with accuracy of injection into the first dorsal compartment.6 The researchers enrolled 19 patients. The same hand surgeon injected 3 cc of 1% lidocaine, 1 cc betamethasone, and 1 cc Omnipaque 300 dye into the abductor pollicis longus sheath and then attempted, with ulnar deviation of the needle, to fill the extensor pollicis brevis sheath.
Patients were followed-up at 1 month and 3 months postinjection. Success—defined as a negative Finkelstein’s test, absence of pain, and normal activities of daily living—was noted in 11 of 19 patients at 3 months. In a radiographic check, 4 of 5 of the patients with dye in both compartments were asymptomatic, while the 3 who had no dye in either compartment remained symptomatic. This suggests that the location of injection may be important in de Quervain’s tenosynovitis.
Recommendations from others
The Brigham and Women’s Hospital guidelines for treatment of de Quervain’s tenosynovitis state that corticosteroid injections “may be very helpful,” and that they should be considered if symptoms persist beyond 6 weeks of conservative treatment.7DeLee and Drez’s Orthopedic Sports Medicine text recommends corticosteroid injection for de Quervain’s tenosynovitis after 2 weeks of conservative treatment have failed.8
UpToDate recommends steroid injection for de Quervain’s tenosynovitis if pain persists for more than 2 to 6 weeks despite splinting, icing, and NSAID therapy.9 For flexor tenosynovitis, UpToDate recommends local injection when symptoms persist for 4 to 6 weeks despite splinting.10
Yes. Steroid injections are an effective first-line therapy for flexor tenosynovitis of the hand, with a number needed to treat [NNT] of 2.3 for injection of steroids and lidocaine (strength of recommendation [SOR]: B, based on 1 prospective RCT and 2 low-quality studies). Injection into the tendon sheath may not be critical to a successful outcome (SOR: B, based on 1 prospective uncontrolled trial).
For de quervain’s tenosynovitis, steroid injections without splinting are more effective than injection plus splinting or splinting alone. The cure rates are 83% (steroid alone), 61% (steroid plus splinting), and 14% (splinting alone) (SOR: B, based on a systematic review of descriptive noncontrolled studies). Injecting into the tendon compartments was more effective than injecting into the surrounding soft tissues (SOR: B, based on 1 prospective controlled trial).
Steroids are helpful, especially for a quick return to function
Robert Gauer, MD
Fort Bragg, NC
I often see flexor and de Quervain’s tenosynovitis in my practice, particularly among patients whose occupations require repetitive hand use. Acute treatment for these conditions typically consists of immobilization with buddy taping or finger/thumb spica splinting. For those who do not improve in 4 weeks, or require a quick return to function, I’ve found that corticosteroid injection using a 25- to 30-gauge needle can be very effective.
De Quervain’s tenosynovitis must be injected into the sheath between the abductor longus and extensor pollicis brevis; flexor tenosynovitis is injected in the nodule. I typically have excellent results, with patients returning to function within 72 hours. Depigmentation and atrophy can occur with injections, especially in small-statured or dark-pigmented patients. surgical release is rarely required for either condition.
Infectious tenosynovitis must be recognized early; it is typically due to lacerations or puncture wounds. In these cases, I immediately refer to orthopedic surgery for further treatment and evaluation.
Evidence summary
Flexor tenosynovitis and de Quervain’s tenosynovitis are the 2 most common types of tenosynovitis of the hand. While the term “tenosynovitis” implies an inflammatory condition, pathoanatomically it is better described as a friction overuse injury, resulting in fibrosis of the surrounding tissue and subsequent narrowing of the synovium.1
“Trigger finger” due to tenosynovitis
Flexor tenosynovitis: Steroid injections provided relief
A prospective, double-blinded RCT2 published by an orthopedic group in 1995 compared 24 patients with primary flexor tenosynovitis. Patients were injected with either 1 cc of betamethasone (Celestone 6 mg) and 3 cc of 1% lidocaine, or 4 cc of 1% lidocaine alone. A successful outcome was defined as absence of triggering and pain, both subjectively and on examination. Follow-up examination was completed for all patients at 3 weeks and 4 months after injection.
The treatment group had success outcomes for 10 of 14 patients (71%) at 3 weeks and 9 of 14 patients (64%) at 4 months, compared with 2 of 10 (20%) at both 3 weeks and 4 months in the control group (NNT=2.3; P<.05 at 4 months). No significant side effects were noted. It is unclear how the study blinded the white, thick consistency of betamethasone compared with the clear nature of lidocaine alone.
Injection site may not matter. In another prospective, uncontrolled trial, 107 patients with flexor tenosynovitis were injected with 1 cc of betamethasone, 0.5 cc of 1% lidocaine, and 0.5 cc of radio opaque dye.3 Some patients got their injections in the tendon sheath at the A1 pulley site, others got their injections in the subcutaneous tissue surrounding the pulley, and a third group got injections in both sites. Patients graded their relief subjectively as either good (total alleviation of symptoms), fair (lasting improvement), or poor (only transient improvement or none at all).
Those who received an intrasheath injection reported good results 47% of the time at 2 weeks follow-up, compared with 70% and 50% for patients in the subcutaneous and mixed groups, respectively. There was no statistically significant difference between the groups, suggesting that the exact location of injection may not be important.
de Quervain’s tenosynovitis: Steroids better than splinting
A pooled quantitative literature search concerning the treatment of de Quervain’s tenosynovitis compared 7 studies (a total of 459 wrists) with identical diagnostic and success criteria.4 Average follow-up was 9.6 months (range, 1 week to 7 years). There were no control groups in the studies, and none of the studies were randomized. Of the 226 cases treated with steroid injection alone, 83% were cured, though 30 of these needed a second injection. Sixty-one percent of those treated with injection and splint were cured, while 14% treated with splint alone reported cure.
Steroids outperform splinting and NSAIDs. A retrospective study not included in the above review compared steroid injection with splinting and nonsteroidal anti-inflammatory drugs (NSAIDs).5 Researchers stratified subjects into minimal, mild, or moderate-to-severe, based on their severity of disease and limitation on their activities of daily living. Mean follow-up was 2.3 years.
Of those cases treated with splinting and NSAIDs, 15 of 17 in the minimal group had resolution of symptoms, but only 4 of 20 in the mild group and 2 of 8 in the moderate-to-severe group had symptoms resolve. The injection group obtained better results, with 100% of cases in the minimal and mild groups resolving and 76% of those in the more severe group resolving completely, with an additional 7% reporting improvement.
Injection site appears to matter with de Quervain’s tenosynovitis. In 1 small, controlled, prospective, double-blinded study, the authors attempted to correlate clinical relief of de Quervain’s tenosynovitis with accuracy of injection into the first dorsal compartment.6 The researchers enrolled 19 patients. The same hand surgeon injected 3 cc of 1% lidocaine, 1 cc betamethasone, and 1 cc Omnipaque 300 dye into the abductor pollicis longus sheath and then attempted, with ulnar deviation of the needle, to fill the extensor pollicis brevis sheath.
Patients were followed-up at 1 month and 3 months postinjection. Success—defined as a negative Finkelstein’s test, absence of pain, and normal activities of daily living—was noted in 11 of 19 patients at 3 months. In a radiographic check, 4 of 5 of the patients with dye in both compartments were asymptomatic, while the 3 who had no dye in either compartment remained symptomatic. This suggests that the location of injection may be important in de Quervain’s tenosynovitis.
Recommendations from others
The Brigham and Women’s Hospital guidelines for treatment of de Quervain’s tenosynovitis state that corticosteroid injections “may be very helpful,” and that they should be considered if symptoms persist beyond 6 weeks of conservative treatment.7DeLee and Drez’s Orthopedic Sports Medicine text recommends corticosteroid injection for de Quervain’s tenosynovitis after 2 weeks of conservative treatment have failed.8
UpToDate recommends steroid injection for de Quervain’s tenosynovitis if pain persists for more than 2 to 6 weeks despite splinting, icing, and NSAID therapy.9 For flexor tenosynovitis, UpToDate recommends local injection when symptoms persist for 4 to 6 weeks despite splinting.10
1. Zingas C, Failla JM, Van Holsbeeck M. Injection accuracy and clinical relief of de Quervain’s tendinitis. J Hand Surgery (Am) 1998;23:89-96.
2. Murphy D, Failla JM, Koniuch MP. Steroid versus placebo injection for trigger finger. J Hand Surg (Am) 1995;20:628-631.
3. Taras JS, Raphael JS, Pan WT, et al. Corticosteroid injections for trigger digits: is intrasheath injection necessary? J Hand Surg (Am) 1998;23:717-22.
4. Richie CA, 3rd, Briner WW, Jr. Corticosteroid injection for treatment of de Quervain’s tenosynovitis: a pooled quantitative literature evaluation. J Am Board Fam Prac 2003;16:102-106.
5. Lane LB, Boretz RS, Stuchin SA. Treatment of de Quervain’s disease: role of conservative management. J Hand Surgery (Br) 2001;26:258-260.
6. Zingas C, Failla JM, Van Holsbeeck M. Injection accuracy and clinical relief of de Quervain’s tendinitis. J Hand Surgery (Am) 1998;23:89-96.
7. Brigham and Women’s Hospital. Upper Extremity Musculoskeletal Disorders: A Guide to Prevention, Diagnosis and Treatment. Boston, Mass: Brigham and Women’s Hospital; 2003:9.
8. DeLee J, Drez D, Miller M. DeLee and Drez’s Orthopaedic Sports Medicine: Principles And Practice. 2nd ed. Philadelphia, Pa: Saunders; 2003.
9. Anderson BC, Sheon RP. de Quervain’s tenosynovitis. UpToDate [online database]. Updated May 7, 2004. Waltham, Mass: UpToDate; 2004.
10. Anderson BC. Trigger finger (flexor tenosynovitis). UpToDate [online database]. Updated December 26, 2000. Waltham, Mass: UpToDate; 2000.
1. Zingas C, Failla JM, Van Holsbeeck M. Injection accuracy and clinical relief of de Quervain’s tendinitis. J Hand Surgery (Am) 1998;23:89-96.
2. Murphy D, Failla JM, Koniuch MP. Steroid versus placebo injection for trigger finger. J Hand Surg (Am) 1995;20:628-631.
3. Taras JS, Raphael JS, Pan WT, et al. Corticosteroid injections for trigger digits: is intrasheath injection necessary? J Hand Surg (Am) 1998;23:717-22.
4. Richie CA, 3rd, Briner WW, Jr. Corticosteroid injection for treatment of de Quervain’s tenosynovitis: a pooled quantitative literature evaluation. J Am Board Fam Prac 2003;16:102-106.
5. Lane LB, Boretz RS, Stuchin SA. Treatment of de Quervain’s disease: role of conservative management. J Hand Surgery (Br) 2001;26:258-260.
6. Zingas C, Failla JM, Van Holsbeeck M. Injection accuracy and clinical relief of de Quervain’s tendinitis. J Hand Surgery (Am) 1998;23:89-96.
7. Brigham and Women’s Hospital. Upper Extremity Musculoskeletal Disorders: A Guide to Prevention, Diagnosis and Treatment. Boston, Mass: Brigham and Women’s Hospital; 2003:9.
8. DeLee J, Drez D, Miller M. DeLee and Drez’s Orthopaedic Sports Medicine: Principles And Practice. 2nd ed. Philadelphia, Pa: Saunders; 2003.
9. Anderson BC, Sheon RP. de Quervain’s tenosynovitis. UpToDate [online database]. Updated May 7, 2004. Waltham, Mass: UpToDate; 2004.
10. Anderson BC. Trigger finger (flexor tenosynovitis). UpToDate [online database]. Updated December 26, 2000. Waltham, Mass: UpToDate; 2000.
Evidence-based answers from the Family Physicians Inquiries Network
What is the risk of bowel strangulation in an adult with an untreated inguinal hernia?
The risk of bowel strangulation is estimated to be small—less than 1% per year (strength of recommendation [SOR]: B, based on small cohort studies with short follow-up). Experts recommend repair for patients with risk factors for poor outcomes after potential strangulation. These risk factors include advanced age, limited access to emergency care, significant concomitant illness, inability to recognize symptoms of bowel incarceration, and poor operative risk (American society of Anesthesiologists class III and IV) (SOR: C, based on expert opinion and case series). It is reasonable to offer elective surgery or watchful waiting to low-risk patients who understand the risks of strangulation (SOR: C, based on expert opinion and case series).
Watchful waiting, yes, but not for high-risk seniors
Michael K. Park, MD
University of Colorado Health Sciences Center, Rose Family Medicine Residency, Denver
The evidence reinforces “watchful waiting” as a reasonable management approach. However, certain patients—say, a 66-year-old diabetic farmer—should probably undergo elective herniorrhaphy to preempt the increased risk of complications with emergent repair.
shared decision-making is an essential process in accounting for individual preferences. In addition to knowing the risks of strangulation, patients opting for surgery also need to be aware of the differences between open and laparoscopic techniques. The former may be done under local anesthesia; the latter decreases postoperative pain and recovery time, but requires general anesthesia and increases the rates of serious complications.
Evidence summary
In 2 randomized controlled trials (RCTs) comparing elective repair of inguinal hernias with watchful waiting, the cohorts who made up the control groups experienced strangulation rates of 1.8 per thousand (0.18%) and 7.9 per thousand (0.79%) occurrences per patient-year.1,2 In the first of these 2 trials,1 with 364 control group patients, median follow-up was only 3.2 years (maximum 4.5 years), and by 4 years 31% of patients had crossed over to the treatment group for elective repair. The mean follow-up time in the second trial,2 which had 80 control group participants, was 1.6 years; 29% of patients eventually crossed over for repair.
Spanish study may have overestimated the risk. A retrospective study3 of 70 patients with incarcerated inguinal hernias presenting for emergency surgery in Northern Spain reported a cumulative 2.8% probability of strangulation at 3 months, rising to 4.5% after 2 years. This study did not include patients presenting for elective repair of hernias, and therefore it likely overestimated the rate of strangulation among patients in a primary care setting.
When to repair inguinal hernia
Experts recommend repair of an inguinal hernia in patients with risk factors for poor outcomes after potential strangulation. Risk factors include advanced age and significant concomitant illness.
In 2001, a prospective study4 of 669 patients presenting for elective hernia repair in London found that only 0.3% of patients required resection of bowel or omentum.
Risk appears to be <1% a year. Collectively, these studies suggest that the risk of strangulation is less than 1% per year (0.18% to 0.79%) among all patients with inguinal hernias, at least in the first few years of the onset of the hernia. As you’d expect, the risk of strangulation is higher (2.8% to 4.5%) among patients presenting for emergency repair of incarcerated hernias. We found no prospective studies that followed patients for more than 4.5 years.
Age factors into poor outcomes
A number of studies5,6 have examined risk factors for increased rates of strangulation and poor outcomes. Older age increases the risk of a poor outcome, peaking in the seventh decade. Patient comorbidity and late hospitalization also make emergent repair more risky.3,5
Retrospective studies3,5,7 of the temporal duration and the natural history of inguinal hernias, as well as operative complication rates, have shown conflicting results.
70- and 80-year olds have greater risk. A Turkish study5 of patients needing emergent surgical repair found morbidity to be significantly related to American Society of Anesthesiologists (ASA) class, with mortality rates of 3% and 14% for ASA class III and IV patients, respectively. This was a retrospective chart review that analyzed factors responsible for unfavorable outcomes; it found increased complications in hernia patients who had coexisting disease, hernias of longer duration, as well as higher ASA class. This study5 and another retrospective study6 found the need for emergent repair peaked for patients 70 to 80 years of age.
longer history of herniation may more postop complications. The Spanish retrospective review3 of emergent surgical repair of incarcerated hernias (noted earlier) reported a 3.4% postoperative mortality rate. All deaths were among patients over 65 years of age and ASA class III or IV. This review also found more postoperative complications and a higher mortality for hernias present for more than 10 years.
Another study raises questions. A retrospective study from Israel8 also showed that patients who underwent emergency repair were older, had a longer history of herniation than those undergoing elective repair, and had higher ASA scores. However, a case-control study7 and a chart review9 found that the risk of strangulation was higher for hernias of shorter duration.
We found no studies addressing potential exacerbating conditions of inguinal hernia, such as chronic cough, bladder outlet obstruction with straining, constipation, obesity, or bilateral hernias.
Recommendations from others
All the textbooks and guidelines we identified acknowledge that many patients forego operation and remain minimally symptomatic for long periods of time, and that operations themselves have risks and complications.10–12 The avoidable risks of strangulation and emergent operation lead most experts to favor operative treatment.
In ACS Surgery: Principles & Practice 2007,10 the authors lament the difficulty of obtaining accurate studies of the natural history of inguinal hernia because surgeons have been taught that it is best to operate at diagnosis, making it hard to find an adequate population to study. The authors acknowledge that while many primary care physicians advise their patients to delay operations if the hernia is minimally asymptomatic, they do not share this belief.
The American College of Physicians’ PIER: The Physicians’ Information and Education Resource11 recommends assessing the hernia and the patient on a case-by-case basis. They recommend deferring an operation for poor-risk patients with minimal symptoms if the hernia is easily reducible and is unquestionably an inguinal hernia, if there are no past episodes of obstruction, and if the risks of untreated hernia are fully understood by the patient. Sabiston Textbook of Surgery makes virtually the same points and recommendations.12
1. Fitzgibbons RJ, Giobbie-Hurder A, Gibbs JO, et al. watchful waiting vs repair of inguinal hernia in minimally symptomatic men: a randomized clinical trial. JAMA 2006;295:285-292.
2. O’Dwyer PJ, Norrie J, Alani A, walker A, Duffy F, Horgan P. Observation or operation for patients with an asymptomatic inguinal hernia: A randomised clinical trial. Ann Surg 2006;244:167-173.
3. Alvarez JA, Baldonedo RF, Bear IG, Solis JAS, Alvarez A, Alvarez JI. Incarcerated groin hernias in adults: Presentation and outcome. Hernia 2004;8:121-126.
4. Hair A, Paterson C, Wright D, Baxter JN, O’Dwyer PJ. What effect does the duration of an inguinal hernia have on patient symptoms? J Am Coll Surg 2001;193:125-129.
5. Kulah B, Duzgun AP, Moran M, Kulacoglu IH, Ozmen MM, Coskun F. Emergency hernia repairs in elderly patients. Am J Surg 2001;182:455-459.
6. McEntee G, O’carroll A, Mooney B, Egan TJ, Delaney PV. Timing of strangulation in adult hernias. Br J Surg 1989;76:725-726.
7. Rai S, Chandra SS, Smile SR. A study of the risk of strangulation and obstruction in groin hernias. Aust N Z J Surg 1998;68:650-654.
8. Ohana manevwitch I, Weil R, et al. Inguinal hernia: challenging the traditional indication for surgery in asymptomatic patients. Hernia 2004;8:117-120.
9. Gallegos NC, Dawson J, Jarvis M, Hobsley M. Risk of strangulation in groin hernias. Br J Surg 1991;78:1171-1173.
10. Fitzgibbons RJ, Richards AT, Quinn TH. Open hernia repair. In: Souba WW, Wilmore DW, Fink MP, et al, eds.ACS Surgery: Principles and Practice 2007.New York, NY: webMD Professional Publishing; 2007. Available at: www.acssurgery.com. Accessed on June 22, 2007.
11. Kingsnorth AN, Khan JA. Hernia. In: PIER—The Physicians’ Information and Education Resource [database online]. Philadelphia, Pa: American College of Physicians; 2007.
12. Malangoni MA, Gagliardi RJ. Hernias. In: Townsend CM, Beauchamp RD, Evers BM, Mattox KL, eds. Sabiston Textbook of Surgery. 17th ed. Philadelphia, Pa: Saunders; 2004:1199–1217. Available at: www.mdconsult.com/das/book/0/view/1235/394.html. Accessed on November 8, 2007.
The risk of bowel strangulation is estimated to be small—less than 1% per year (strength of recommendation [SOR]: B, based on small cohort studies with short follow-up). Experts recommend repair for patients with risk factors for poor outcomes after potential strangulation. These risk factors include advanced age, limited access to emergency care, significant concomitant illness, inability to recognize symptoms of bowel incarceration, and poor operative risk (American society of Anesthesiologists class III and IV) (SOR: C, based on expert opinion and case series). It is reasonable to offer elective surgery or watchful waiting to low-risk patients who understand the risks of strangulation (SOR: C, based on expert opinion and case series).
Watchful waiting, yes, but not for high-risk seniors
Michael K. Park, MD
University of Colorado Health Sciences Center, Rose Family Medicine Residency, Denver
The evidence reinforces “watchful waiting” as a reasonable management approach. However, certain patients—say, a 66-year-old diabetic farmer—should probably undergo elective herniorrhaphy to preempt the increased risk of complications with emergent repair.
shared decision-making is an essential process in accounting for individual preferences. In addition to knowing the risks of strangulation, patients opting for surgery also need to be aware of the differences between open and laparoscopic techniques. The former may be done under local anesthesia; the latter decreases postoperative pain and recovery time, but requires general anesthesia and increases the rates of serious complications.
Evidence summary
In 2 randomized controlled trials (RCTs) comparing elective repair of inguinal hernias with watchful waiting, the cohorts who made up the control groups experienced strangulation rates of 1.8 per thousand (0.18%) and 7.9 per thousand (0.79%) occurrences per patient-year.1,2 In the first of these 2 trials,1 with 364 control group patients, median follow-up was only 3.2 years (maximum 4.5 years), and by 4 years 31% of patients had crossed over to the treatment group for elective repair. The mean follow-up time in the second trial,2 which had 80 control group participants, was 1.6 years; 29% of patients eventually crossed over for repair.
Spanish study may have overestimated the risk. A retrospective study3 of 70 patients with incarcerated inguinal hernias presenting for emergency surgery in Northern Spain reported a cumulative 2.8% probability of strangulation at 3 months, rising to 4.5% after 2 years. This study did not include patients presenting for elective repair of hernias, and therefore it likely overestimated the rate of strangulation among patients in a primary care setting.
When to repair inguinal hernia
Experts recommend repair of an inguinal hernia in patients with risk factors for poor outcomes after potential strangulation. Risk factors include advanced age and significant concomitant illness.
In 2001, a prospective study4 of 669 patients presenting for elective hernia repair in London found that only 0.3% of patients required resection of bowel or omentum.
Risk appears to be <1% a year. Collectively, these studies suggest that the risk of strangulation is less than 1% per year (0.18% to 0.79%) among all patients with inguinal hernias, at least in the first few years of the onset of the hernia. As you’d expect, the risk of strangulation is higher (2.8% to 4.5%) among patients presenting for emergency repair of incarcerated hernias. We found no prospective studies that followed patients for more than 4.5 years.
Age factors into poor outcomes
A number of studies5,6 have examined risk factors for increased rates of strangulation and poor outcomes. Older age increases the risk of a poor outcome, peaking in the seventh decade. Patient comorbidity and late hospitalization also make emergent repair more risky.3,5
Retrospective studies3,5,7 of the temporal duration and the natural history of inguinal hernias, as well as operative complication rates, have shown conflicting results.
70- and 80-year olds have greater risk. A Turkish study5 of patients needing emergent surgical repair found morbidity to be significantly related to American Society of Anesthesiologists (ASA) class, with mortality rates of 3% and 14% for ASA class III and IV patients, respectively. This was a retrospective chart review that analyzed factors responsible for unfavorable outcomes; it found increased complications in hernia patients who had coexisting disease, hernias of longer duration, as well as higher ASA class. This study5 and another retrospective study6 found the need for emergent repair peaked for patients 70 to 80 years of age.
longer history of herniation may more postop complications. The Spanish retrospective review3 of emergent surgical repair of incarcerated hernias (noted earlier) reported a 3.4% postoperative mortality rate. All deaths were among patients over 65 years of age and ASA class III or IV. This review also found more postoperative complications and a higher mortality for hernias present for more than 10 years.
Another study raises questions. A retrospective study from Israel8 also showed that patients who underwent emergency repair were older, had a longer history of herniation than those undergoing elective repair, and had higher ASA scores. However, a case-control study7 and a chart review9 found that the risk of strangulation was higher for hernias of shorter duration.
We found no studies addressing potential exacerbating conditions of inguinal hernia, such as chronic cough, bladder outlet obstruction with straining, constipation, obesity, or bilateral hernias.
Recommendations from others
All the textbooks and guidelines we identified acknowledge that many patients forego operation and remain minimally symptomatic for long periods of time, and that operations themselves have risks and complications.10–12 The avoidable risks of strangulation and emergent operation lead most experts to favor operative treatment.
In ACS Surgery: Principles & Practice 2007,10 the authors lament the difficulty of obtaining accurate studies of the natural history of inguinal hernia because surgeons have been taught that it is best to operate at diagnosis, making it hard to find an adequate population to study. The authors acknowledge that while many primary care physicians advise their patients to delay operations if the hernia is minimally asymptomatic, they do not share this belief.
The American College of Physicians’ PIER: The Physicians’ Information and Education Resource11 recommends assessing the hernia and the patient on a case-by-case basis. They recommend deferring an operation for poor-risk patients with minimal symptoms if the hernia is easily reducible and is unquestionably an inguinal hernia, if there are no past episodes of obstruction, and if the risks of untreated hernia are fully understood by the patient. Sabiston Textbook of Surgery makes virtually the same points and recommendations.12
The risk of bowel strangulation is estimated to be small—less than 1% per year (strength of recommendation [SOR]: B, based on small cohort studies with short follow-up). Experts recommend repair for patients with risk factors for poor outcomes after potential strangulation. These risk factors include advanced age, limited access to emergency care, significant concomitant illness, inability to recognize symptoms of bowel incarceration, and poor operative risk (American society of Anesthesiologists class III and IV) (SOR: C, based on expert opinion and case series). It is reasonable to offer elective surgery or watchful waiting to low-risk patients who understand the risks of strangulation (SOR: C, based on expert opinion and case series).
Watchful waiting, yes, but not for high-risk seniors
Michael K. Park, MD
University of Colorado Health Sciences Center, Rose Family Medicine Residency, Denver
The evidence reinforces “watchful waiting” as a reasonable management approach. However, certain patients—say, a 66-year-old diabetic farmer—should probably undergo elective herniorrhaphy to preempt the increased risk of complications with emergent repair.
shared decision-making is an essential process in accounting for individual preferences. In addition to knowing the risks of strangulation, patients opting for surgery also need to be aware of the differences between open and laparoscopic techniques. The former may be done under local anesthesia; the latter decreases postoperative pain and recovery time, but requires general anesthesia and increases the rates of serious complications.
Evidence summary
In 2 randomized controlled trials (RCTs) comparing elective repair of inguinal hernias with watchful waiting, the cohorts who made up the control groups experienced strangulation rates of 1.8 per thousand (0.18%) and 7.9 per thousand (0.79%) occurrences per patient-year.1,2 In the first of these 2 trials,1 with 364 control group patients, median follow-up was only 3.2 years (maximum 4.5 years), and by 4 years 31% of patients had crossed over to the treatment group for elective repair. The mean follow-up time in the second trial,2 which had 80 control group participants, was 1.6 years; 29% of patients eventually crossed over for repair.
Spanish study may have overestimated the risk. A retrospective study3 of 70 patients with incarcerated inguinal hernias presenting for emergency surgery in Northern Spain reported a cumulative 2.8% probability of strangulation at 3 months, rising to 4.5% after 2 years. This study did not include patients presenting for elective repair of hernias, and therefore it likely overestimated the rate of strangulation among patients in a primary care setting.
When to repair inguinal hernia
Experts recommend repair of an inguinal hernia in patients with risk factors for poor outcomes after potential strangulation. Risk factors include advanced age and significant concomitant illness.
In 2001, a prospective study4 of 669 patients presenting for elective hernia repair in London found that only 0.3% of patients required resection of bowel or omentum.
Risk appears to be <1% a year. Collectively, these studies suggest that the risk of strangulation is less than 1% per year (0.18% to 0.79%) among all patients with inguinal hernias, at least in the first few years of the onset of the hernia. As you’d expect, the risk of strangulation is higher (2.8% to 4.5%) among patients presenting for emergency repair of incarcerated hernias. We found no prospective studies that followed patients for more than 4.5 years.
Age factors into poor outcomes
A number of studies5,6 have examined risk factors for increased rates of strangulation and poor outcomes. Older age increases the risk of a poor outcome, peaking in the seventh decade. Patient comorbidity and late hospitalization also make emergent repair more risky.3,5
Retrospective studies3,5,7 of the temporal duration and the natural history of inguinal hernias, as well as operative complication rates, have shown conflicting results.
70- and 80-year olds have greater risk. A Turkish study5 of patients needing emergent surgical repair found morbidity to be significantly related to American Society of Anesthesiologists (ASA) class, with mortality rates of 3% and 14% for ASA class III and IV patients, respectively. This was a retrospective chart review that analyzed factors responsible for unfavorable outcomes; it found increased complications in hernia patients who had coexisting disease, hernias of longer duration, as well as higher ASA class. This study5 and another retrospective study6 found the need for emergent repair peaked for patients 70 to 80 years of age.
longer history of herniation may more postop complications. The Spanish retrospective review3 of emergent surgical repair of incarcerated hernias (noted earlier) reported a 3.4% postoperative mortality rate. All deaths were among patients over 65 years of age and ASA class III or IV. This review also found more postoperative complications and a higher mortality for hernias present for more than 10 years.
Another study raises questions. A retrospective study from Israel8 also showed that patients who underwent emergency repair were older, had a longer history of herniation than those undergoing elective repair, and had higher ASA scores. However, a case-control study7 and a chart review9 found that the risk of strangulation was higher for hernias of shorter duration.
We found no studies addressing potential exacerbating conditions of inguinal hernia, such as chronic cough, bladder outlet obstruction with straining, constipation, obesity, or bilateral hernias.
Recommendations from others
All the textbooks and guidelines we identified acknowledge that many patients forego operation and remain minimally symptomatic for long periods of time, and that operations themselves have risks and complications.10–12 The avoidable risks of strangulation and emergent operation lead most experts to favor operative treatment.
In ACS Surgery: Principles & Practice 2007,10 the authors lament the difficulty of obtaining accurate studies of the natural history of inguinal hernia because surgeons have been taught that it is best to operate at diagnosis, making it hard to find an adequate population to study. The authors acknowledge that while many primary care physicians advise their patients to delay operations if the hernia is minimally asymptomatic, they do not share this belief.
The American College of Physicians’ PIER: The Physicians’ Information and Education Resource11 recommends assessing the hernia and the patient on a case-by-case basis. They recommend deferring an operation for poor-risk patients with minimal symptoms if the hernia is easily reducible and is unquestionably an inguinal hernia, if there are no past episodes of obstruction, and if the risks of untreated hernia are fully understood by the patient. Sabiston Textbook of Surgery makes virtually the same points and recommendations.12
1. Fitzgibbons RJ, Giobbie-Hurder A, Gibbs JO, et al. watchful waiting vs repair of inguinal hernia in minimally symptomatic men: a randomized clinical trial. JAMA 2006;295:285-292.
2. O’Dwyer PJ, Norrie J, Alani A, walker A, Duffy F, Horgan P. Observation or operation for patients with an asymptomatic inguinal hernia: A randomised clinical trial. Ann Surg 2006;244:167-173.
3. Alvarez JA, Baldonedo RF, Bear IG, Solis JAS, Alvarez A, Alvarez JI. Incarcerated groin hernias in adults: Presentation and outcome. Hernia 2004;8:121-126.
4. Hair A, Paterson C, Wright D, Baxter JN, O’Dwyer PJ. What effect does the duration of an inguinal hernia have on patient symptoms? J Am Coll Surg 2001;193:125-129.
5. Kulah B, Duzgun AP, Moran M, Kulacoglu IH, Ozmen MM, Coskun F. Emergency hernia repairs in elderly patients. Am J Surg 2001;182:455-459.
6. McEntee G, O’carroll A, Mooney B, Egan TJ, Delaney PV. Timing of strangulation in adult hernias. Br J Surg 1989;76:725-726.
7. Rai S, Chandra SS, Smile SR. A study of the risk of strangulation and obstruction in groin hernias. Aust N Z J Surg 1998;68:650-654.
8. Ohana manevwitch I, Weil R, et al. Inguinal hernia: challenging the traditional indication for surgery in asymptomatic patients. Hernia 2004;8:117-120.
9. Gallegos NC, Dawson J, Jarvis M, Hobsley M. Risk of strangulation in groin hernias. Br J Surg 1991;78:1171-1173.
10. Fitzgibbons RJ, Richards AT, Quinn TH. Open hernia repair. In: Souba WW, Wilmore DW, Fink MP, et al, eds.ACS Surgery: Principles and Practice 2007.New York, NY: webMD Professional Publishing; 2007. Available at: www.acssurgery.com. Accessed on June 22, 2007.
11. Kingsnorth AN, Khan JA. Hernia. In: PIER—The Physicians’ Information and Education Resource [database online]. Philadelphia, Pa: American College of Physicians; 2007.
12. Malangoni MA, Gagliardi RJ. Hernias. In: Townsend CM, Beauchamp RD, Evers BM, Mattox KL, eds. Sabiston Textbook of Surgery. 17th ed. Philadelphia, Pa: Saunders; 2004:1199–1217. Available at: www.mdconsult.com/das/book/0/view/1235/394.html. Accessed on November 8, 2007.
1. Fitzgibbons RJ, Giobbie-Hurder A, Gibbs JO, et al. watchful waiting vs repair of inguinal hernia in minimally symptomatic men: a randomized clinical trial. JAMA 2006;295:285-292.
2. O’Dwyer PJ, Norrie J, Alani A, walker A, Duffy F, Horgan P. Observation or operation for patients with an asymptomatic inguinal hernia: A randomised clinical trial. Ann Surg 2006;244:167-173.
3. Alvarez JA, Baldonedo RF, Bear IG, Solis JAS, Alvarez A, Alvarez JI. Incarcerated groin hernias in adults: Presentation and outcome. Hernia 2004;8:121-126.
4. Hair A, Paterson C, Wright D, Baxter JN, O’Dwyer PJ. What effect does the duration of an inguinal hernia have on patient symptoms? J Am Coll Surg 2001;193:125-129.
5. Kulah B, Duzgun AP, Moran M, Kulacoglu IH, Ozmen MM, Coskun F. Emergency hernia repairs in elderly patients. Am J Surg 2001;182:455-459.
6. McEntee G, O’carroll A, Mooney B, Egan TJ, Delaney PV. Timing of strangulation in adult hernias. Br J Surg 1989;76:725-726.
7. Rai S, Chandra SS, Smile SR. A study of the risk of strangulation and obstruction in groin hernias. Aust N Z J Surg 1998;68:650-654.
8. Ohana manevwitch I, Weil R, et al. Inguinal hernia: challenging the traditional indication for surgery in asymptomatic patients. Hernia 2004;8:117-120.
9. Gallegos NC, Dawson J, Jarvis M, Hobsley M. Risk of strangulation in groin hernias. Br J Surg 1991;78:1171-1173.
10. Fitzgibbons RJ, Richards AT, Quinn TH. Open hernia repair. In: Souba WW, Wilmore DW, Fink MP, et al, eds.ACS Surgery: Principles and Practice 2007.New York, NY: webMD Professional Publishing; 2007. Available at: www.acssurgery.com. Accessed on June 22, 2007.
11. Kingsnorth AN, Khan JA. Hernia. In: PIER—The Physicians’ Information and Education Resource [database online]. Philadelphia, Pa: American College of Physicians; 2007.
12. Malangoni MA, Gagliardi RJ. Hernias. In: Townsend CM, Beauchamp RD, Evers BM, Mattox KL, eds. Sabiston Textbook of Surgery. 17th ed. Philadelphia, Pa: Saunders; 2004:1199–1217. Available at: www.mdconsult.com/das/book/0/view/1235/394.html. Accessed on November 8, 2007.
Evidence-based answers from the Family Physicians Inquiries Network
Do COX-2 inhibitors worsen renal function?
No, COX-2 inhibitors, as a class, do not worsen renal function for those without renal disease. Celecoxib is the only COX-2 inhibitor available, and it is associated with a lower risk of renal dysfunction and hypertension when compared with controls. Available data do not allow for adjusted risk assessment for patients with preexisting renal disease on COX-2 inhibitors (strength of recommendation [SOR]: A, based on meta-analysis).
Use celecoxib cautiously in patients at risk of serious complications
Vincent LO, MD
San Joaquin Family Medicine Residency, French Camp, Calif
Recent studies have raised concerns about the safety of this class of medication. For example, rofecoxib was linked with increased cardiovascular events, leading to it being pulled from the market.1 The claim of decreased gastrointestinal bleeding with long-term use of COX-2 inhibitors has also been questioned.2
Although this Clinical Inquiry concludes that celecoxib does not appear to worsen renal function, it should still be used with caution for patients who are elderly, hospitalized, or at risk of developing serious complications such as acute renal failure, heart failure, and gastrointestinal bleeding.
Evidence summary
A 2006 meta-analysis, including 114 trials and 116,094 patients randomized to either cyclooxygenase-2 (COX-2) inhibitor or control (placebo, nonsteroidal anti-inflammatory drug [NSAID], or mixed), indicated that the COX-2 inhibitors, as a class, had no effect on renal endpoints.3 Trials were reviewed for data on renal endpoints, including peripheral edema, hypertension, and renal dysfunction (defined as significant worsening of serum urea or creatinine, or clinical evidence of kidney disease and renal failure).
When viewed separately, rofecoxib (Vioxx) was associated with a composite relative risk (RR) of 1.53 (95% confidence interval [CI], 1.33–1.76) for all renal endpoints compared with controls. In contrast, the composite RR for the same endpoints among patients taking celecoxib (Celebrex) was 0.97 (95% CI, 0.84–1.12), indicating no effect on renal function. In fact, for the specific outcomes of hypertension and renal dysfunction, celecoxib was associated with a decreased risk compared with controls (TABLE).3
Stratified analysis by type of control (placebo, alternate NSAID, or mixed) yielded consistent results; rofecoxib was uniquely associated with adverse renal outcomes. No effect on renal function was noted for celecoxib compared with the same controls: the RR for adverse renal effects was 0.87 (95% CI, 0.55–1.38), 0.93 (95% CI, 0.70–1.23), and 1.26 (95% CI, 0.94–1.69) for celecoxib vs placebo, NSAID, and mixed controls, respectively. Statistical analysis for heterogeneity showed that the variation in effects on renal function among the COX-2 inhibitors was more likely due to actual differences than due to chance (heterogeneity [I2]=57%; P<.001).
Data were not available to assess the effect of COX-2 agents on patients with pre-existing renal disease, primarily because trials reporting abnormal renal function at baseline were excluded from this meta-analysis.
A recent randomized controlled trial compared standard dosing of diclofenac (75 mg twice daily) and ibuprofen (800 mg 3 times daily) with high-dose celecoxib (400 mg twice daily) for patients with normal kidney function being treated for osteoarthritis and rheumatoid arthritis.4 The mean increase in serum creatinine in the celecoxib arm was less than that noted in the diclofenac controls (0.009 mg/dL vs 0.027 mg/dL; P<.05; number needed to harm [NNH]=56). No difference in mean serum creatinine was seen among those patients using ibuprofen (800 mg 3 times daily) compared with those using high-dose celecoxib.
This evidence further supports the safety of celecoxib vs standard NSAIDs with respect to renal dysfunction.
Recommendations from others The American Pain Society 2002 guideline recommends acetaminophen for mild pain from osteoarthritis.5 For moderate to severe pain and inflammation, a COX-2 inhibitor was the first choice, unless there is significant risk of hypertension or kidney disorder. For active rheumatoid arthritis, the addition of a COX-2 agent to disease-modifying anti-rheumatic drugs (DMARDs) is advised unless there is uncontrolled hypertension or renal disease.6 However, these recommendations came out before the data on the cardiovascular effects of some COX-2 inhibitors.
The American College of rheumatology recommends the use of a COX-2 agent for osteoarthritis or pain unresponsive to acetaminophen. Their 2000 guidelines warn that due to potential renal toxicity, COX-2 inhibitors should not be used for patients with severe renal insufficiency, and used with caution in cases of mild to moderate renal insufficiency.
In 2005, these guidelines were amended to include the recommendation that patients with increased cardiovascular risk be cautioned about the risks associated with COX-2 inhibitor use.7
TABLE
Celecoxib is associated with a decreased risk of hypertension and renal dysfunction
| CELECOXIB | ROFECOXIB | |
|---|---|---|
| Hypertension | 0.83 (95% CI, 0.71–0.97) | 1.55 (95% CI, 1.29–1.85) |
| Peripheral edema | 1.09 (95% CI, 0.91–1.31) | 1.43 (95% CI, 1.23–1.66) |
| Renal dysfunction | 0.61 (95% CI, 0.40–0.94) | 2.31 (95% CI, 1.05–5.07) |
| Source: Zhang J, Ding EL, Song Y, JAMA 2006.3 | ||
1. Bresalier RS, Sandler RS, Quan H, et al. Cardiovascular events associated with rofecoxib in a colorectal adenoma chemoprevention trial. N Engl J Med 2005;352:1092-1102.
2. Hippisley-Cox J, Coupland C, Logan R. Risk of adverse gastrointestinal outcomes in patients taking cyclo-oxygenase-2 inhibitors or conventional non-steroidal anti-inflammatory drugs: population based nested case-control analysis. BMJ 2005;331:1310-1316.
3. Zhang J, Ding EL, Song Y. Adverse effects of cyclooxygenase 2 inhibitors on renal and arrhythmia events. JAMA 2006;296:1619-1632.
4. Whelton A, Lefkowith JL, West CR, Verburg KM. Cardiorenal effects of celecoxib as compared with the nonsteroidal anti-inflammatory drugs diclofenac and ibuprofen. Kidney Intl. 2006;70:1495-1502.
5. Simon LS, Lipman AG, Jacox AK, et al. Pain in Osteoarthritis, Rheumatoid Arthritis and Juvenile Chronic Arthritis. 2nd ed. Glenview, Ill: American Pain society; 2002.
6. American College of Rheumatology (ACR) sub-committee on Osteoarthritis Guidelines. recommendations for the medical management of osteoarthritis of the hip and knee: 2000 update. American College of Rheumatology Subcommittee on osteoarthritis Guidelines. Arthritis Rheum. 2000;43:1905-1915.
7. American College of Rheumatology Subcommittee on rheumatoid Arthritis Guidelines. Guidelines for the management of rheumatoid arthritis: 2002 update. Arthritis Rheum. 2002;46:328-346.
No, COX-2 inhibitors, as a class, do not worsen renal function for those without renal disease. Celecoxib is the only COX-2 inhibitor available, and it is associated with a lower risk of renal dysfunction and hypertension when compared with controls. Available data do not allow for adjusted risk assessment for patients with preexisting renal disease on COX-2 inhibitors (strength of recommendation [SOR]: A, based on meta-analysis).
Use celecoxib cautiously in patients at risk of serious complications
Vincent LO, MD
San Joaquin Family Medicine Residency, French Camp, Calif
Recent studies have raised concerns about the safety of this class of medication. For example, rofecoxib was linked with increased cardiovascular events, leading to it being pulled from the market.1 The claim of decreased gastrointestinal bleeding with long-term use of COX-2 inhibitors has also been questioned.2
Although this Clinical Inquiry concludes that celecoxib does not appear to worsen renal function, it should still be used with caution for patients who are elderly, hospitalized, or at risk of developing serious complications such as acute renal failure, heart failure, and gastrointestinal bleeding.
Evidence summary
A 2006 meta-analysis, including 114 trials and 116,094 patients randomized to either cyclooxygenase-2 (COX-2) inhibitor or control (placebo, nonsteroidal anti-inflammatory drug [NSAID], or mixed), indicated that the COX-2 inhibitors, as a class, had no effect on renal endpoints.3 Trials were reviewed for data on renal endpoints, including peripheral edema, hypertension, and renal dysfunction (defined as significant worsening of serum urea or creatinine, or clinical evidence of kidney disease and renal failure).
When viewed separately, rofecoxib (Vioxx) was associated with a composite relative risk (RR) of 1.53 (95% confidence interval [CI], 1.33–1.76) for all renal endpoints compared with controls. In contrast, the composite RR for the same endpoints among patients taking celecoxib (Celebrex) was 0.97 (95% CI, 0.84–1.12), indicating no effect on renal function. In fact, for the specific outcomes of hypertension and renal dysfunction, celecoxib was associated with a decreased risk compared with controls (TABLE).3
Stratified analysis by type of control (placebo, alternate NSAID, or mixed) yielded consistent results; rofecoxib was uniquely associated with adverse renal outcomes. No effect on renal function was noted for celecoxib compared with the same controls: the RR for adverse renal effects was 0.87 (95% CI, 0.55–1.38), 0.93 (95% CI, 0.70–1.23), and 1.26 (95% CI, 0.94–1.69) for celecoxib vs placebo, NSAID, and mixed controls, respectively. Statistical analysis for heterogeneity showed that the variation in effects on renal function among the COX-2 inhibitors was more likely due to actual differences than due to chance (heterogeneity [I2]=57%; P<.001).
Data were not available to assess the effect of COX-2 agents on patients with pre-existing renal disease, primarily because trials reporting abnormal renal function at baseline were excluded from this meta-analysis.
A recent randomized controlled trial compared standard dosing of diclofenac (75 mg twice daily) and ibuprofen (800 mg 3 times daily) with high-dose celecoxib (400 mg twice daily) for patients with normal kidney function being treated for osteoarthritis and rheumatoid arthritis.4 The mean increase in serum creatinine in the celecoxib arm was less than that noted in the diclofenac controls (0.009 mg/dL vs 0.027 mg/dL; P<.05; number needed to harm [NNH]=56). No difference in mean serum creatinine was seen among those patients using ibuprofen (800 mg 3 times daily) compared with those using high-dose celecoxib.
This evidence further supports the safety of celecoxib vs standard NSAIDs with respect to renal dysfunction.
Recommendations from others The American Pain Society 2002 guideline recommends acetaminophen for mild pain from osteoarthritis.5 For moderate to severe pain and inflammation, a COX-2 inhibitor was the first choice, unless there is significant risk of hypertension or kidney disorder. For active rheumatoid arthritis, the addition of a COX-2 agent to disease-modifying anti-rheumatic drugs (DMARDs) is advised unless there is uncontrolled hypertension or renal disease.6 However, these recommendations came out before the data on the cardiovascular effects of some COX-2 inhibitors.
The American College of rheumatology recommends the use of a COX-2 agent for osteoarthritis or pain unresponsive to acetaminophen. Their 2000 guidelines warn that due to potential renal toxicity, COX-2 inhibitors should not be used for patients with severe renal insufficiency, and used with caution in cases of mild to moderate renal insufficiency.
In 2005, these guidelines were amended to include the recommendation that patients with increased cardiovascular risk be cautioned about the risks associated with COX-2 inhibitor use.7
TABLE
Celecoxib is associated with a decreased risk of hypertension and renal dysfunction
| CELECOXIB | ROFECOXIB | |
|---|---|---|
| Hypertension | 0.83 (95% CI, 0.71–0.97) | 1.55 (95% CI, 1.29–1.85) |
| Peripheral edema | 1.09 (95% CI, 0.91–1.31) | 1.43 (95% CI, 1.23–1.66) |
| Renal dysfunction | 0.61 (95% CI, 0.40–0.94) | 2.31 (95% CI, 1.05–5.07) |
| Source: Zhang J, Ding EL, Song Y, JAMA 2006.3 | ||
No, COX-2 inhibitors, as a class, do not worsen renal function for those without renal disease. Celecoxib is the only COX-2 inhibitor available, and it is associated with a lower risk of renal dysfunction and hypertension when compared with controls. Available data do not allow for adjusted risk assessment for patients with preexisting renal disease on COX-2 inhibitors (strength of recommendation [SOR]: A, based on meta-analysis).
Use celecoxib cautiously in patients at risk of serious complications
Vincent LO, MD
San Joaquin Family Medicine Residency, French Camp, Calif
Recent studies have raised concerns about the safety of this class of medication. For example, rofecoxib was linked with increased cardiovascular events, leading to it being pulled from the market.1 The claim of decreased gastrointestinal bleeding with long-term use of COX-2 inhibitors has also been questioned.2
Although this Clinical Inquiry concludes that celecoxib does not appear to worsen renal function, it should still be used with caution for patients who are elderly, hospitalized, or at risk of developing serious complications such as acute renal failure, heart failure, and gastrointestinal bleeding.
Evidence summary
A 2006 meta-analysis, including 114 trials and 116,094 patients randomized to either cyclooxygenase-2 (COX-2) inhibitor or control (placebo, nonsteroidal anti-inflammatory drug [NSAID], or mixed), indicated that the COX-2 inhibitors, as a class, had no effect on renal endpoints.3 Trials were reviewed for data on renal endpoints, including peripheral edema, hypertension, and renal dysfunction (defined as significant worsening of serum urea or creatinine, or clinical evidence of kidney disease and renal failure).
When viewed separately, rofecoxib (Vioxx) was associated with a composite relative risk (RR) of 1.53 (95% confidence interval [CI], 1.33–1.76) for all renal endpoints compared with controls. In contrast, the composite RR for the same endpoints among patients taking celecoxib (Celebrex) was 0.97 (95% CI, 0.84–1.12), indicating no effect on renal function. In fact, for the specific outcomes of hypertension and renal dysfunction, celecoxib was associated with a decreased risk compared with controls (TABLE).3
Stratified analysis by type of control (placebo, alternate NSAID, or mixed) yielded consistent results; rofecoxib was uniquely associated with adverse renal outcomes. No effect on renal function was noted for celecoxib compared with the same controls: the RR for adverse renal effects was 0.87 (95% CI, 0.55–1.38), 0.93 (95% CI, 0.70–1.23), and 1.26 (95% CI, 0.94–1.69) for celecoxib vs placebo, NSAID, and mixed controls, respectively. Statistical analysis for heterogeneity showed that the variation in effects on renal function among the COX-2 inhibitors was more likely due to actual differences than due to chance (heterogeneity [I2]=57%; P<.001).
Data were not available to assess the effect of COX-2 agents on patients with pre-existing renal disease, primarily because trials reporting abnormal renal function at baseline were excluded from this meta-analysis.
A recent randomized controlled trial compared standard dosing of diclofenac (75 mg twice daily) and ibuprofen (800 mg 3 times daily) with high-dose celecoxib (400 mg twice daily) for patients with normal kidney function being treated for osteoarthritis and rheumatoid arthritis.4 The mean increase in serum creatinine in the celecoxib arm was less than that noted in the diclofenac controls (0.009 mg/dL vs 0.027 mg/dL; P<.05; number needed to harm [NNH]=56). No difference in mean serum creatinine was seen among those patients using ibuprofen (800 mg 3 times daily) compared with those using high-dose celecoxib.
This evidence further supports the safety of celecoxib vs standard NSAIDs with respect to renal dysfunction.
Recommendations from others The American Pain Society 2002 guideline recommends acetaminophen for mild pain from osteoarthritis.5 For moderate to severe pain and inflammation, a COX-2 inhibitor was the first choice, unless there is significant risk of hypertension or kidney disorder. For active rheumatoid arthritis, the addition of a COX-2 agent to disease-modifying anti-rheumatic drugs (DMARDs) is advised unless there is uncontrolled hypertension or renal disease.6 However, these recommendations came out before the data on the cardiovascular effects of some COX-2 inhibitors.
The American College of rheumatology recommends the use of a COX-2 agent for osteoarthritis or pain unresponsive to acetaminophen. Their 2000 guidelines warn that due to potential renal toxicity, COX-2 inhibitors should not be used for patients with severe renal insufficiency, and used with caution in cases of mild to moderate renal insufficiency.
In 2005, these guidelines were amended to include the recommendation that patients with increased cardiovascular risk be cautioned about the risks associated with COX-2 inhibitor use.7
TABLE
Celecoxib is associated with a decreased risk of hypertension and renal dysfunction
| CELECOXIB | ROFECOXIB | |
|---|---|---|
| Hypertension | 0.83 (95% CI, 0.71–0.97) | 1.55 (95% CI, 1.29–1.85) |
| Peripheral edema | 1.09 (95% CI, 0.91–1.31) | 1.43 (95% CI, 1.23–1.66) |
| Renal dysfunction | 0.61 (95% CI, 0.40–0.94) | 2.31 (95% CI, 1.05–5.07) |
| Source: Zhang J, Ding EL, Song Y, JAMA 2006.3 | ||
1. Bresalier RS, Sandler RS, Quan H, et al. Cardiovascular events associated with rofecoxib in a colorectal adenoma chemoprevention trial. N Engl J Med 2005;352:1092-1102.
2. Hippisley-Cox J, Coupland C, Logan R. Risk of adverse gastrointestinal outcomes in patients taking cyclo-oxygenase-2 inhibitors or conventional non-steroidal anti-inflammatory drugs: population based nested case-control analysis. BMJ 2005;331:1310-1316.
3. Zhang J, Ding EL, Song Y. Adverse effects of cyclooxygenase 2 inhibitors on renal and arrhythmia events. JAMA 2006;296:1619-1632.
4. Whelton A, Lefkowith JL, West CR, Verburg KM. Cardiorenal effects of celecoxib as compared with the nonsteroidal anti-inflammatory drugs diclofenac and ibuprofen. Kidney Intl. 2006;70:1495-1502.
5. Simon LS, Lipman AG, Jacox AK, et al. Pain in Osteoarthritis, Rheumatoid Arthritis and Juvenile Chronic Arthritis. 2nd ed. Glenview, Ill: American Pain society; 2002.
6. American College of Rheumatology (ACR) sub-committee on Osteoarthritis Guidelines. recommendations for the medical management of osteoarthritis of the hip and knee: 2000 update. American College of Rheumatology Subcommittee on osteoarthritis Guidelines. Arthritis Rheum. 2000;43:1905-1915.
7. American College of Rheumatology Subcommittee on rheumatoid Arthritis Guidelines. Guidelines for the management of rheumatoid arthritis: 2002 update. Arthritis Rheum. 2002;46:328-346.
1. Bresalier RS, Sandler RS, Quan H, et al. Cardiovascular events associated with rofecoxib in a colorectal adenoma chemoprevention trial. N Engl J Med 2005;352:1092-1102.
2. Hippisley-Cox J, Coupland C, Logan R. Risk of adverse gastrointestinal outcomes in patients taking cyclo-oxygenase-2 inhibitors or conventional non-steroidal anti-inflammatory drugs: population based nested case-control analysis. BMJ 2005;331:1310-1316.
3. Zhang J, Ding EL, Song Y. Adverse effects of cyclooxygenase 2 inhibitors on renal and arrhythmia events. JAMA 2006;296:1619-1632.
4. Whelton A, Lefkowith JL, West CR, Verburg KM. Cardiorenal effects of celecoxib as compared with the nonsteroidal anti-inflammatory drugs diclofenac and ibuprofen. Kidney Intl. 2006;70:1495-1502.
5. Simon LS, Lipman AG, Jacox AK, et al. Pain in Osteoarthritis, Rheumatoid Arthritis and Juvenile Chronic Arthritis. 2nd ed. Glenview, Ill: American Pain society; 2002.
6. American College of Rheumatology (ACR) sub-committee on Osteoarthritis Guidelines. recommendations for the medical management of osteoarthritis of the hip and knee: 2000 update. American College of Rheumatology Subcommittee on osteoarthritis Guidelines. Arthritis Rheum. 2000;43:1905-1915.
7. American College of Rheumatology Subcommittee on rheumatoid Arthritis Guidelines. Guidelines for the management of rheumatoid arthritis: 2002 update. Arthritis Rheum. 2002;46:328-346.
Evidence-based answers from the Family Physicians Inquiries Network
When should travelers begin malaria prophylaxis?
Travelers should start on chloroquine 1 to 2 weeks before entering an area without chloroquine resistance (strength of recommendation [SOR]: C, based on expert opinion). In areas with chloroquine-resistant Plasmodium falciparum, travelers will need to take atovaquone/proguanil, doxycycline, or primaquine 1 day before entering the area, or mefloquine 2 to 7 weeks before travel (SOR: B, based on prospective patient-oriented outcomes and expert opinion).
Before prescribing medications, determine malaria risk and sensitivity of Plasmodium species by country at wwwn.cdc.gov/travel/yellowBookCh5MalariaYellowFeverTable.aspx (SOR: C, based on patient-oriented expert opinion).
5 tips to help travelers avoid malaria
Brian V. Reamy, MD
Uniformed Services University, Bethesda, Md
Despite our best efforts, more than 10,000 American and European travelers contract malaria each year. Five clinical pointers are helpful in prescribing malaria prophylaxis and preventing malaria in travelers.
1. Advise patients that they’ll need to get their antimalarials before they leave for their trip. The CDC recommends against the purchase of antimalarials while overseas because of concerns about product quality.
2. Encourage patients to plan ahead. Most local community pharmacies do not routinely stock antimalarials and must special order them. If a patient mentions an upcoming trip, advise them that they’ll need to allow an extra 2 weeks to obtain their medications.
3. Consult 1 of 2 continuously updated Web sites prior to selecting a medication for malaria prophylaxis: wwwn.cdc.gov/travel/destinationList.aspx or www.who.int/ith/en.
Start times vary from 1 day to several weeks prior to travel based on the medication selected.
4. Encourage patients to spray clothing with permethrin prior to travel. Permethrin remains effective as a repellent even after months of clothing use and multiple washes.
5. Encourage travelers to finish their medication after they return and to report unexplained fevers for up to 1 year after travel.
Evidence summary
Travelers to malaria-endemic areas should avoid mosquito bites by using netting and repellents, and use chemoprophylaxis to prevent infection.
Although no drug regimen guarantees protection against malaria, physicians should prescribe 1 of several options based on the location of travel, the susceptibility of indigenous P falciparum, and the side-effect profile.1
Timing and dosage of prophylactic drugs
Prophylactic medications must be started at different times before travel, but for some medications the optimal time to initiate treatment is unclear. Evidence-based recommendations2,3 with consideration for side-effect profiles are given in the TABLE.
In contrast to the pretreatment times for all other malarial prophylaxes, the generally accepted pretreatment time for mefloquine is 1 to 2 weeks before entering a risk area. However, this may still be inadequate due to the drug’s long half-life, which results in a long delay in reaching therapeutic blood levels.4 The evidence indicates that mefloquine should be started at least 2, and as many as 7, weeks before travel.
The standard recommended dose of 250 mg/week of mefloquine “produces maximum steady-state plasma concentrations of 1000 to 2000 mcg/L, which are reached only after 7 to 10 weeks.”4 One study of 293 children under the age of 5 years in Malawi found that plasma concentrations of mefloquine were below prophylactic level (500 mcg/mL) against P falciparum until the fourth to seventh week of once-weekly dosing (P<.0003).5
One way of reaching prophylactic levels earlier would be to give mefloquine 250 mg daily for 3 days followed by 250 mg weekly.4 A safety study of 157 healthy US Marine volunteers showed that preloading achieves prophylactic blood levels of mefloquine by the third day while weekly mefloquine is subprophylactic until the fifth week.4
While a study of the long-term use of mefloquine in 421 healthy Peace Corps volunteers has shown it to be safe,6 clinical trials and case reports indicate that a loading dose of mefloquine is associated with adverse drug events, which include neuropsychiatric and gastrointestinal symptoms.4,7
TABLE
Evidence-based recommendations for prevention of malaria2-3,8
| DRUG | USAGE | ADULT DOSE | TREATMENT SCHEDULE |
|---|---|---|---|
| Atovaquone/proguanil Contraindicated in pregnancy | Prophylaxis in areas with chloroquine-resistant or mefloquine-resistant P falciparum | 1 tablet orally each day 250 mg atovaquone and 100 mg proguanil hydrochloride) | Daily from 1 day prior to entry until 7 days after leaving |
| Chloroquine | Prophylaxis only in areas with chloroquine-sensitive P falciparum | 300 mg base (500 mg salt) orally, once/week | Weekly from 2 weeks prior to entry until 4 weeks after leaving (take on the same day of the week) |
| Doxycycline Contraindicated in children <8 years of age and pregnant women | Prophylaxis in areas with chloroquine-resistant or mefloquine-resistant P falciparum | 100 mg orally, daily | Daily from 1 day prior to entry until 4 weeks after leaving |
| Mefloquine | Prophylaxis in areas with chloroquine-resistant P falciparum | 228 mg base (250 mg salt) orally, once/week | Weekly from 2–7 weeks before entry until 4 weeks after leaving (take on the same day of the week) |
| Primaquine | An option for prophylaxis in special circumstances | 30 mg base (52.6 mg salt) orally, daily | Daily from 1 day prior to entry until 7 days after leaving |
Recommendations from others
The World Health Organization (WHO) states that “weekly mefloquine should be started at least 1 week, but preferably 2–3 weeks before departure, to achieve higher pre-travel blood levels and to allow side effects to be detected before travel so that possible alternatives can be considered.”8
Centers for Disease Control and Prevention recommendations integrate recommendations from WHO and Cochrane.
Acknowledgments
The opinions and assertions contained herein are the private views of the authors and not to be construed as official, or as reflecting the views of the US Air Force Medical Service or the US Air Force at large.
1. Chen LH, Keystone JS. New strategies for the prevention of malaria in travelers. Infect Dis Clin North Am 2005;19:185-210.
2. Physicians’ Desk Reference. 61st ed. Montvale, NJ: Thomson; 2007:2786.
3. Parise M, Barber A, Mali S. Prevention of specific infectious diseases—malaria. In Arguin PM, Kozarsky PE, Navin AW (eds), Health Information for International Travel 2005-2006. Atlanta, Ga: US Department of Health and Human Services, Public Health Service, Centers for Disease Control and Prevention; 2007. Available at: wwwn.cdc.gov/travel/yellowBookCh4-Malaria.aspx. Accessed on October 11, 2007.
4. Boudreau E, Schuster B, Sanchez J, et al. Tolerability of prophylactic Lariam regimens. Trop Med Parasitol 1993;44:257-265.
5. Slutsker LM, Khoromana CO, Payne D, et al. Mefloquine therapy for Plasmodium falciparum. malaria in children under 5 years of age in Malawi: in vivo/in vitro efficacy and correlation of drug concentration with parasitological outcome. Bull World Health Organ 1990;68:53-59.
6. Lobel HO, Miani M, Eng T, et al. Long-term malaria prophylaxis with weekly mefloquine. Lancet 1993;341:848-51.
7. Schlagenhauf P. Mefloquine for malaria chemoprophylaxis 1992-1998: a review. J Travel Med 1999;6:122-133.
8. International Travel and Health 2005. Chapter 7: Malaria. Geneva: World Health Organization; 2005. Available at: whqlibdoc.who.int/publications/2005/9241580364_chap7.pdf. Accessed on October 11, 2007.
Travelers should start on chloroquine 1 to 2 weeks before entering an area without chloroquine resistance (strength of recommendation [SOR]: C, based on expert opinion). In areas with chloroquine-resistant Plasmodium falciparum, travelers will need to take atovaquone/proguanil, doxycycline, or primaquine 1 day before entering the area, or mefloquine 2 to 7 weeks before travel (SOR: B, based on prospective patient-oriented outcomes and expert opinion).
Before prescribing medications, determine malaria risk and sensitivity of Plasmodium species by country at wwwn.cdc.gov/travel/yellowBookCh5MalariaYellowFeverTable.aspx (SOR: C, based on patient-oriented expert opinion).
5 tips to help travelers avoid malaria
Brian V. Reamy, MD
Uniformed Services University, Bethesda, Md
Despite our best efforts, more than 10,000 American and European travelers contract malaria each year. Five clinical pointers are helpful in prescribing malaria prophylaxis and preventing malaria in travelers.
1. Advise patients that they’ll need to get their antimalarials before they leave for their trip. The CDC recommends against the purchase of antimalarials while overseas because of concerns about product quality.
2. Encourage patients to plan ahead. Most local community pharmacies do not routinely stock antimalarials and must special order them. If a patient mentions an upcoming trip, advise them that they’ll need to allow an extra 2 weeks to obtain their medications.
3. Consult 1 of 2 continuously updated Web sites prior to selecting a medication for malaria prophylaxis: wwwn.cdc.gov/travel/destinationList.aspx or www.who.int/ith/en.
Start times vary from 1 day to several weeks prior to travel based on the medication selected.
4. Encourage patients to spray clothing with permethrin prior to travel. Permethrin remains effective as a repellent even after months of clothing use and multiple washes.
5. Encourage travelers to finish their medication after they return and to report unexplained fevers for up to 1 year after travel.
Evidence summary
Travelers to malaria-endemic areas should avoid mosquito bites by using netting and repellents, and use chemoprophylaxis to prevent infection.
Although no drug regimen guarantees protection against malaria, physicians should prescribe 1 of several options based on the location of travel, the susceptibility of indigenous P falciparum, and the side-effect profile.1
Timing and dosage of prophylactic drugs
Prophylactic medications must be started at different times before travel, but for some medications the optimal time to initiate treatment is unclear. Evidence-based recommendations2,3 with consideration for side-effect profiles are given in the TABLE.
In contrast to the pretreatment times for all other malarial prophylaxes, the generally accepted pretreatment time for mefloquine is 1 to 2 weeks before entering a risk area. However, this may still be inadequate due to the drug’s long half-life, which results in a long delay in reaching therapeutic blood levels.4 The evidence indicates that mefloquine should be started at least 2, and as many as 7, weeks before travel.
The standard recommended dose of 250 mg/week of mefloquine “produces maximum steady-state plasma concentrations of 1000 to 2000 mcg/L, which are reached only after 7 to 10 weeks.”4 One study of 293 children under the age of 5 years in Malawi found that plasma concentrations of mefloquine were below prophylactic level (500 mcg/mL) against P falciparum until the fourth to seventh week of once-weekly dosing (P<.0003).5
One way of reaching prophylactic levels earlier would be to give mefloquine 250 mg daily for 3 days followed by 250 mg weekly.4 A safety study of 157 healthy US Marine volunteers showed that preloading achieves prophylactic blood levels of mefloquine by the third day while weekly mefloquine is subprophylactic until the fifth week.4
While a study of the long-term use of mefloquine in 421 healthy Peace Corps volunteers has shown it to be safe,6 clinical trials and case reports indicate that a loading dose of mefloquine is associated with adverse drug events, which include neuropsychiatric and gastrointestinal symptoms.4,7
TABLE
Evidence-based recommendations for prevention of malaria2-3,8
| DRUG | USAGE | ADULT DOSE | TREATMENT SCHEDULE |
|---|---|---|---|
| Atovaquone/proguanil Contraindicated in pregnancy | Prophylaxis in areas with chloroquine-resistant or mefloquine-resistant P falciparum | 1 tablet orally each day 250 mg atovaquone and 100 mg proguanil hydrochloride) | Daily from 1 day prior to entry until 7 days after leaving |
| Chloroquine | Prophylaxis only in areas with chloroquine-sensitive P falciparum | 300 mg base (500 mg salt) orally, once/week | Weekly from 2 weeks prior to entry until 4 weeks after leaving (take on the same day of the week) |
| Doxycycline Contraindicated in children <8 years of age and pregnant women | Prophylaxis in areas with chloroquine-resistant or mefloquine-resistant P falciparum | 100 mg orally, daily | Daily from 1 day prior to entry until 4 weeks after leaving |
| Mefloquine | Prophylaxis in areas with chloroquine-resistant P falciparum | 228 mg base (250 mg salt) orally, once/week | Weekly from 2–7 weeks before entry until 4 weeks after leaving (take on the same day of the week) |
| Primaquine | An option for prophylaxis in special circumstances | 30 mg base (52.6 mg salt) orally, daily | Daily from 1 day prior to entry until 7 days after leaving |
Recommendations from others
The World Health Organization (WHO) states that “weekly mefloquine should be started at least 1 week, but preferably 2–3 weeks before departure, to achieve higher pre-travel blood levels and to allow side effects to be detected before travel so that possible alternatives can be considered.”8
Centers for Disease Control and Prevention recommendations integrate recommendations from WHO and Cochrane.
Acknowledgments
The opinions and assertions contained herein are the private views of the authors and not to be construed as official, or as reflecting the views of the US Air Force Medical Service or the US Air Force at large.
Travelers should start on chloroquine 1 to 2 weeks before entering an area without chloroquine resistance (strength of recommendation [SOR]: C, based on expert opinion). In areas with chloroquine-resistant Plasmodium falciparum, travelers will need to take atovaquone/proguanil, doxycycline, or primaquine 1 day before entering the area, or mefloquine 2 to 7 weeks before travel (SOR: B, based on prospective patient-oriented outcomes and expert opinion).
Before prescribing medications, determine malaria risk and sensitivity of Plasmodium species by country at wwwn.cdc.gov/travel/yellowBookCh5MalariaYellowFeverTable.aspx (SOR: C, based on patient-oriented expert opinion).
5 tips to help travelers avoid malaria
Brian V. Reamy, MD
Uniformed Services University, Bethesda, Md
Despite our best efforts, more than 10,000 American and European travelers contract malaria each year. Five clinical pointers are helpful in prescribing malaria prophylaxis and preventing malaria in travelers.
1. Advise patients that they’ll need to get their antimalarials before they leave for their trip. The CDC recommends against the purchase of antimalarials while overseas because of concerns about product quality.
2. Encourage patients to plan ahead. Most local community pharmacies do not routinely stock antimalarials and must special order them. If a patient mentions an upcoming trip, advise them that they’ll need to allow an extra 2 weeks to obtain their medications.
3. Consult 1 of 2 continuously updated Web sites prior to selecting a medication for malaria prophylaxis: wwwn.cdc.gov/travel/destinationList.aspx or www.who.int/ith/en.
Start times vary from 1 day to several weeks prior to travel based on the medication selected.
4. Encourage patients to spray clothing with permethrin prior to travel. Permethrin remains effective as a repellent even after months of clothing use and multiple washes.
5. Encourage travelers to finish their medication after they return and to report unexplained fevers for up to 1 year after travel.
Evidence summary
Travelers to malaria-endemic areas should avoid mosquito bites by using netting and repellents, and use chemoprophylaxis to prevent infection.
Although no drug regimen guarantees protection against malaria, physicians should prescribe 1 of several options based on the location of travel, the susceptibility of indigenous P falciparum, and the side-effect profile.1
Timing and dosage of prophylactic drugs
Prophylactic medications must be started at different times before travel, but for some medications the optimal time to initiate treatment is unclear. Evidence-based recommendations2,3 with consideration for side-effect profiles are given in the TABLE.
In contrast to the pretreatment times for all other malarial prophylaxes, the generally accepted pretreatment time for mefloquine is 1 to 2 weeks before entering a risk area. However, this may still be inadequate due to the drug’s long half-life, which results in a long delay in reaching therapeutic blood levels.4 The evidence indicates that mefloquine should be started at least 2, and as many as 7, weeks before travel.
The standard recommended dose of 250 mg/week of mefloquine “produces maximum steady-state plasma concentrations of 1000 to 2000 mcg/L, which are reached only after 7 to 10 weeks.”4 One study of 293 children under the age of 5 years in Malawi found that plasma concentrations of mefloquine were below prophylactic level (500 mcg/mL) against P falciparum until the fourth to seventh week of once-weekly dosing (P<.0003).5
One way of reaching prophylactic levels earlier would be to give mefloquine 250 mg daily for 3 days followed by 250 mg weekly.4 A safety study of 157 healthy US Marine volunteers showed that preloading achieves prophylactic blood levels of mefloquine by the third day while weekly mefloquine is subprophylactic until the fifth week.4
While a study of the long-term use of mefloquine in 421 healthy Peace Corps volunteers has shown it to be safe,6 clinical trials and case reports indicate that a loading dose of mefloquine is associated with adverse drug events, which include neuropsychiatric and gastrointestinal symptoms.4,7
TABLE
Evidence-based recommendations for prevention of malaria2-3,8
| DRUG | USAGE | ADULT DOSE | TREATMENT SCHEDULE |
|---|---|---|---|
| Atovaquone/proguanil Contraindicated in pregnancy | Prophylaxis in areas with chloroquine-resistant or mefloquine-resistant P falciparum | 1 tablet orally each day 250 mg atovaquone and 100 mg proguanil hydrochloride) | Daily from 1 day prior to entry until 7 days after leaving |
| Chloroquine | Prophylaxis only in areas with chloroquine-sensitive P falciparum | 300 mg base (500 mg salt) orally, once/week | Weekly from 2 weeks prior to entry until 4 weeks after leaving (take on the same day of the week) |
| Doxycycline Contraindicated in children <8 years of age and pregnant women | Prophylaxis in areas with chloroquine-resistant or mefloquine-resistant P falciparum | 100 mg orally, daily | Daily from 1 day prior to entry until 4 weeks after leaving |
| Mefloquine | Prophylaxis in areas with chloroquine-resistant P falciparum | 228 mg base (250 mg salt) orally, once/week | Weekly from 2–7 weeks before entry until 4 weeks after leaving (take on the same day of the week) |
| Primaquine | An option for prophylaxis in special circumstances | 30 mg base (52.6 mg salt) orally, daily | Daily from 1 day prior to entry until 7 days after leaving |
Recommendations from others
The World Health Organization (WHO) states that “weekly mefloquine should be started at least 1 week, but preferably 2–3 weeks before departure, to achieve higher pre-travel blood levels and to allow side effects to be detected before travel so that possible alternatives can be considered.”8
Centers for Disease Control and Prevention recommendations integrate recommendations from WHO and Cochrane.
Acknowledgments
The opinions and assertions contained herein are the private views of the authors and not to be construed as official, or as reflecting the views of the US Air Force Medical Service or the US Air Force at large.
1. Chen LH, Keystone JS. New strategies for the prevention of malaria in travelers. Infect Dis Clin North Am 2005;19:185-210.
2. Physicians’ Desk Reference. 61st ed. Montvale, NJ: Thomson; 2007:2786.
3. Parise M, Barber A, Mali S. Prevention of specific infectious diseases—malaria. In Arguin PM, Kozarsky PE, Navin AW (eds), Health Information for International Travel 2005-2006. Atlanta, Ga: US Department of Health and Human Services, Public Health Service, Centers for Disease Control and Prevention; 2007. Available at: wwwn.cdc.gov/travel/yellowBookCh4-Malaria.aspx. Accessed on October 11, 2007.
4. Boudreau E, Schuster B, Sanchez J, et al. Tolerability of prophylactic Lariam regimens. Trop Med Parasitol 1993;44:257-265.
5. Slutsker LM, Khoromana CO, Payne D, et al. Mefloquine therapy for Plasmodium falciparum. malaria in children under 5 years of age in Malawi: in vivo/in vitro efficacy and correlation of drug concentration with parasitological outcome. Bull World Health Organ 1990;68:53-59.
6. Lobel HO, Miani M, Eng T, et al. Long-term malaria prophylaxis with weekly mefloquine. Lancet 1993;341:848-51.
7. Schlagenhauf P. Mefloquine for malaria chemoprophylaxis 1992-1998: a review. J Travel Med 1999;6:122-133.
8. International Travel and Health 2005. Chapter 7: Malaria. Geneva: World Health Organization; 2005. Available at: whqlibdoc.who.int/publications/2005/9241580364_chap7.pdf. Accessed on October 11, 2007.
1. Chen LH, Keystone JS. New strategies for the prevention of malaria in travelers. Infect Dis Clin North Am 2005;19:185-210.
2. Physicians’ Desk Reference. 61st ed. Montvale, NJ: Thomson; 2007:2786.
3. Parise M, Barber A, Mali S. Prevention of specific infectious diseases—malaria. In Arguin PM, Kozarsky PE, Navin AW (eds), Health Information for International Travel 2005-2006. Atlanta, Ga: US Department of Health and Human Services, Public Health Service, Centers for Disease Control and Prevention; 2007. Available at: wwwn.cdc.gov/travel/yellowBookCh4-Malaria.aspx. Accessed on October 11, 2007.
4. Boudreau E, Schuster B, Sanchez J, et al. Tolerability of prophylactic Lariam regimens. Trop Med Parasitol 1993;44:257-265.
5. Slutsker LM, Khoromana CO, Payne D, et al. Mefloquine therapy for Plasmodium falciparum. malaria in children under 5 years of age in Malawi: in vivo/in vitro efficacy and correlation of drug concentration with parasitological outcome. Bull World Health Organ 1990;68:53-59.
6. Lobel HO, Miani M, Eng T, et al. Long-term malaria prophylaxis with weekly mefloquine. Lancet 1993;341:848-51.
7. Schlagenhauf P. Mefloquine for malaria chemoprophylaxis 1992-1998: a review. J Travel Med 1999;6:122-133.
8. International Travel and Health 2005. Chapter 7: Malaria. Geneva: World Health Organization; 2005. Available at: whqlibdoc.who.int/publications/2005/9241580364_chap7.pdf. Accessed on October 11, 2007.
Evidence-based answers from the Family Physicians Inquiries Network