User login
How can you best diagnose idiopathic normal pressure hydrocephalus?
Diagnose idiopathic normal pressure hydrocephalus (INPH) by clinical history, brain imaging, physical findings, and physiological criteria.
The clinical examination must show the characteristic gait disturbance and either impaired cognition or impaired urinary continence (strength of recommendation [SOR]: B, based on systematic review of small randomized controlled trial [RCT] and prospective trials).
The cerebrospinal fluid (CSF) opening pressure should be between 70 and 245 mm H2o (SOR: B, based on systematic review of small RCT and prospective trials). No single test has sufficient sensitivity to rule out the diagnosis of INPH (SOR: B, based on systematic review of small RCT and prospective trials).
Subtle clues help make the diagnosis
Sumathi Devarajan, MD
Oregon Health and Science University Family Medicine, Portland
Normal pressure hydrocephalus is primarily diagnosed clinically. The classic triad of gait instability, cognitive dysfunction, and urinary incontinence, however, seldom present together. The only promising diagnostic and therapeutic intervention is the response observed with a ventriculoperitoneal shunt. However, this intervention is invasive and not without risks. Neuroimaging plays a role, but only when the clinical suspicion is high.
Therefore, understanding the subtleties in the character of the gait, the time of onset, the progression of dementia, and the onset of urinary incontinence in relationship to one another helps in making the final diagnosis.
Evidence summary
Current uncertainty in diagnostic criteria makes estimates of the incidence of INPH unclear, but it is thought to cause fewer than 5% of cases of dementia.1
Two systematic reviews have looked at the question of diagnosing INPH.2,3 Unfortunately, there is no definitive test or physical finding for INPH. For patients over 40 years of age, INPH has an insidious onset, a progressive course, and lacks an identifiable antecedent cause. A brain imaging study reveals ventricular enlargement not attributable to other causes. Some suggest that the diagnosis be assessed as “probable,” “possible,” and “unlikely” based on the degree of fulfillment of a set of historical, imaging, clinical, and physiological criteria (TABLE).3
TABLE
Categorizing the likelihood of idiopathic normal pressure hydrocephalus3
| Probable INPH |
| HISTORY (MUST FULFILL ALL) |
|
| BRAIN IMAGING (MUST FULFILL ALL) |
|
| CLINICAL |
|
| Gait/balance should reveal at least 2 of the following 9 items: |
|
| Tests of cognition should show evidence of at least 2 of the following 7 characteristics that are not fully attributable to other conditions: |
|
| Symptoms of urinary incontinence not attributable to other primary urological disorders should be present: |
|
| PHYSIOLOGICAL |
|
| Possible INPH |
| HISTORY (MUST FULFILL ALL) |
|
| BRAIN IMAGING (MUST FULFILL ALL) |
|
| CLINICAL |
|
| PHYSIOLOGICAL |
|
Which patients will benefit from shunting?
Supplemental prognostic tests have been developed to help decide which patients are most likely to benefit from a ventriculoperitoneal shunt. Complicating comparisons between the various tests is the lack of a standard set of measures of function in gait, cognition, and urination; nor is there agreement on how long after shunting the clinician should make these measurements.
A systematic review4 of the most commonly used prognostic tests identified a response to a large-volume (40–50 mL) CSF tap test as having a positive predictive value (PPV) between 73% and 100% but a negative predictive value (NPV) of only 23% to 42%. Thus, observing an improvement of function after such a test is a good predictor of improvement after shunting, but many patients who do not respond to the test respond to shunting.
A variation of the CSF tap test is the extended lumbar drainage test, which involves placing a lumbar intrathecal catheter and allowing the drainage of 10 mL of CSF/hour for 72 hours. The PPV for this test ranges from 80% to 100%, and NPV from 66% to 100%.
A third possible test is the measurement of resistance to an infusion of saline into the lumbar subarachnoid space (CSF Ro test). This test has multiple variations of technique. Reported values for PPV are 75% to 92%, and for NPV of 27% to 92%.
Other tests, such as radionuclide cisternography or magnetic resonance imaging CSF flow void, have predictive values too low or have too few studies to be recommended.4
Recommendations from others
A recently published expert consensus statement proposes that the diagnosis of INPH be made using the history, clinical examination, and neuroimaging.4 Cases of probable INPH can proceed directly to ventriculoperitoneal shunt, or supplemental testing can be used to improve the certainty of a positive shunt response. A positive CSF tap test should lead to shunting.
Follow up negative tap tests with the extended lumbar drainage test or the CSF Ro test (or both). A positive response to any of the tests should lead to shunting; negative responses to all the tests indicates a low chance (<10%) of responding to a ventriculoperitoneal shunt.4
1. Hebb AO, Cusimano MD. Idiopathic normal pressure hydrocephalus: A systematic review of diagnosis and outcome. Neurosurgery 2001;49:1166-1184.
2. Marmarou A, Bergsneider M, Klinge P, Relkin N, Black PM. The value of supplemental prognostic tests for the preoperative assessment of idiopathic normal-pressure hydrocephalus. Neurosurgery 2005;57(3 suppl):S17-S28.
3. Relkin N, Marmarou A, Klinge P, Bergsneider M, Black PM. Diagnosing idiopathic normal-pressure hydrocephalus. Neurosurgery 2005;57(3 suppl):S4-S16;discussion ii-v.
4. Verrees M, Selman WR. Management of normal pressure hydrocephalus. Am Fam Physician 2004;70:1071-1078.
Diagnose idiopathic normal pressure hydrocephalus (INPH) by clinical history, brain imaging, physical findings, and physiological criteria.
The clinical examination must show the characteristic gait disturbance and either impaired cognition or impaired urinary continence (strength of recommendation [SOR]: B, based on systematic review of small randomized controlled trial [RCT] and prospective trials).
The cerebrospinal fluid (CSF) opening pressure should be between 70 and 245 mm H2o (SOR: B, based on systematic review of small RCT and prospective trials). No single test has sufficient sensitivity to rule out the diagnosis of INPH (SOR: B, based on systematic review of small RCT and prospective trials).
Subtle clues help make the diagnosis
Sumathi Devarajan, MD
Oregon Health and Science University Family Medicine, Portland
Normal pressure hydrocephalus is primarily diagnosed clinically. The classic triad of gait instability, cognitive dysfunction, and urinary incontinence, however, seldom present together. The only promising diagnostic and therapeutic intervention is the response observed with a ventriculoperitoneal shunt. However, this intervention is invasive and not without risks. Neuroimaging plays a role, but only when the clinical suspicion is high.
Therefore, understanding the subtleties in the character of the gait, the time of onset, the progression of dementia, and the onset of urinary incontinence in relationship to one another helps in making the final diagnosis.
Evidence summary
Current uncertainty in diagnostic criteria makes estimates of the incidence of INPH unclear, but it is thought to cause fewer than 5% of cases of dementia.1
Two systematic reviews have looked at the question of diagnosing INPH.2,3 Unfortunately, there is no definitive test or physical finding for INPH. For patients over 40 years of age, INPH has an insidious onset, a progressive course, and lacks an identifiable antecedent cause. A brain imaging study reveals ventricular enlargement not attributable to other causes. Some suggest that the diagnosis be assessed as “probable,” “possible,” and “unlikely” based on the degree of fulfillment of a set of historical, imaging, clinical, and physiological criteria (TABLE).3
TABLE
Categorizing the likelihood of idiopathic normal pressure hydrocephalus3
| Probable INPH |
| HISTORY (MUST FULFILL ALL) |
|
| BRAIN IMAGING (MUST FULFILL ALL) |
|
| CLINICAL |
|
| Gait/balance should reveal at least 2 of the following 9 items: |
|
| Tests of cognition should show evidence of at least 2 of the following 7 characteristics that are not fully attributable to other conditions: |
|
| Symptoms of urinary incontinence not attributable to other primary urological disorders should be present: |
|
| PHYSIOLOGICAL |
|
| Possible INPH |
| HISTORY (MUST FULFILL ALL) |
|
| BRAIN IMAGING (MUST FULFILL ALL) |
|
| CLINICAL |
|
| PHYSIOLOGICAL |
|
Which patients will benefit from shunting?
Supplemental prognostic tests have been developed to help decide which patients are most likely to benefit from a ventriculoperitoneal shunt. Complicating comparisons between the various tests is the lack of a standard set of measures of function in gait, cognition, and urination; nor is there agreement on how long after shunting the clinician should make these measurements.
A systematic review4 of the most commonly used prognostic tests identified a response to a large-volume (40–50 mL) CSF tap test as having a positive predictive value (PPV) between 73% and 100% but a negative predictive value (NPV) of only 23% to 42%. Thus, observing an improvement of function after such a test is a good predictor of improvement after shunting, but many patients who do not respond to the test respond to shunting.
A variation of the CSF tap test is the extended lumbar drainage test, which involves placing a lumbar intrathecal catheter and allowing the drainage of 10 mL of CSF/hour for 72 hours. The PPV for this test ranges from 80% to 100%, and NPV from 66% to 100%.
A third possible test is the measurement of resistance to an infusion of saline into the lumbar subarachnoid space (CSF Ro test). This test has multiple variations of technique. Reported values for PPV are 75% to 92%, and for NPV of 27% to 92%.
Other tests, such as radionuclide cisternography or magnetic resonance imaging CSF flow void, have predictive values too low or have too few studies to be recommended.4
Recommendations from others
A recently published expert consensus statement proposes that the diagnosis of INPH be made using the history, clinical examination, and neuroimaging.4 Cases of probable INPH can proceed directly to ventriculoperitoneal shunt, or supplemental testing can be used to improve the certainty of a positive shunt response. A positive CSF tap test should lead to shunting.
Follow up negative tap tests with the extended lumbar drainage test or the CSF Ro test (or both). A positive response to any of the tests should lead to shunting; negative responses to all the tests indicates a low chance (<10%) of responding to a ventriculoperitoneal shunt.4
Diagnose idiopathic normal pressure hydrocephalus (INPH) by clinical history, brain imaging, physical findings, and physiological criteria.
The clinical examination must show the characteristic gait disturbance and either impaired cognition or impaired urinary continence (strength of recommendation [SOR]: B, based on systematic review of small randomized controlled trial [RCT] and prospective trials).
The cerebrospinal fluid (CSF) opening pressure should be between 70 and 245 mm H2o (SOR: B, based on systematic review of small RCT and prospective trials). No single test has sufficient sensitivity to rule out the diagnosis of INPH (SOR: B, based on systematic review of small RCT and prospective trials).
Subtle clues help make the diagnosis
Sumathi Devarajan, MD
Oregon Health and Science University Family Medicine, Portland
Normal pressure hydrocephalus is primarily diagnosed clinically. The classic triad of gait instability, cognitive dysfunction, and urinary incontinence, however, seldom present together. The only promising diagnostic and therapeutic intervention is the response observed with a ventriculoperitoneal shunt. However, this intervention is invasive and not without risks. Neuroimaging plays a role, but only when the clinical suspicion is high.
Therefore, understanding the subtleties in the character of the gait, the time of onset, the progression of dementia, and the onset of urinary incontinence in relationship to one another helps in making the final diagnosis.
Evidence summary
Current uncertainty in diagnostic criteria makes estimates of the incidence of INPH unclear, but it is thought to cause fewer than 5% of cases of dementia.1
Two systematic reviews have looked at the question of diagnosing INPH.2,3 Unfortunately, there is no definitive test or physical finding for INPH. For patients over 40 years of age, INPH has an insidious onset, a progressive course, and lacks an identifiable antecedent cause. A brain imaging study reveals ventricular enlargement not attributable to other causes. Some suggest that the diagnosis be assessed as “probable,” “possible,” and “unlikely” based on the degree of fulfillment of a set of historical, imaging, clinical, and physiological criteria (TABLE).3
TABLE
Categorizing the likelihood of idiopathic normal pressure hydrocephalus3
| Probable INPH |
| HISTORY (MUST FULFILL ALL) |
|
| BRAIN IMAGING (MUST FULFILL ALL) |
|
| CLINICAL |
|
| Gait/balance should reveal at least 2 of the following 9 items: |
|
| Tests of cognition should show evidence of at least 2 of the following 7 characteristics that are not fully attributable to other conditions: |
|
| Symptoms of urinary incontinence not attributable to other primary urological disorders should be present: |
|
| PHYSIOLOGICAL |
|
| Possible INPH |
| HISTORY (MUST FULFILL ALL) |
|
| BRAIN IMAGING (MUST FULFILL ALL) |
|
| CLINICAL |
|
| PHYSIOLOGICAL |
|
Which patients will benefit from shunting?
Supplemental prognostic tests have been developed to help decide which patients are most likely to benefit from a ventriculoperitoneal shunt. Complicating comparisons between the various tests is the lack of a standard set of measures of function in gait, cognition, and urination; nor is there agreement on how long after shunting the clinician should make these measurements.
A systematic review4 of the most commonly used prognostic tests identified a response to a large-volume (40–50 mL) CSF tap test as having a positive predictive value (PPV) between 73% and 100% but a negative predictive value (NPV) of only 23% to 42%. Thus, observing an improvement of function after such a test is a good predictor of improvement after shunting, but many patients who do not respond to the test respond to shunting.
A variation of the CSF tap test is the extended lumbar drainage test, which involves placing a lumbar intrathecal catheter and allowing the drainage of 10 mL of CSF/hour for 72 hours. The PPV for this test ranges from 80% to 100%, and NPV from 66% to 100%.
A third possible test is the measurement of resistance to an infusion of saline into the lumbar subarachnoid space (CSF Ro test). This test has multiple variations of technique. Reported values for PPV are 75% to 92%, and for NPV of 27% to 92%.
Other tests, such as radionuclide cisternography or magnetic resonance imaging CSF flow void, have predictive values too low or have too few studies to be recommended.4
Recommendations from others
A recently published expert consensus statement proposes that the diagnosis of INPH be made using the history, clinical examination, and neuroimaging.4 Cases of probable INPH can proceed directly to ventriculoperitoneal shunt, or supplemental testing can be used to improve the certainty of a positive shunt response. A positive CSF tap test should lead to shunting.
Follow up negative tap tests with the extended lumbar drainage test or the CSF Ro test (or both). A positive response to any of the tests should lead to shunting; negative responses to all the tests indicates a low chance (<10%) of responding to a ventriculoperitoneal shunt.4
1. Hebb AO, Cusimano MD. Idiopathic normal pressure hydrocephalus: A systematic review of diagnosis and outcome. Neurosurgery 2001;49:1166-1184.
2. Marmarou A, Bergsneider M, Klinge P, Relkin N, Black PM. The value of supplemental prognostic tests for the preoperative assessment of idiopathic normal-pressure hydrocephalus. Neurosurgery 2005;57(3 suppl):S17-S28.
3. Relkin N, Marmarou A, Klinge P, Bergsneider M, Black PM. Diagnosing idiopathic normal-pressure hydrocephalus. Neurosurgery 2005;57(3 suppl):S4-S16;discussion ii-v.
4. Verrees M, Selman WR. Management of normal pressure hydrocephalus. Am Fam Physician 2004;70:1071-1078.
1. Hebb AO, Cusimano MD. Idiopathic normal pressure hydrocephalus: A systematic review of diagnosis and outcome. Neurosurgery 2001;49:1166-1184.
2. Marmarou A, Bergsneider M, Klinge P, Relkin N, Black PM. The value of supplemental prognostic tests for the preoperative assessment of idiopathic normal-pressure hydrocephalus. Neurosurgery 2005;57(3 suppl):S17-S28.
3. Relkin N, Marmarou A, Klinge P, Bergsneider M, Black PM. Diagnosing idiopathic normal-pressure hydrocephalus. Neurosurgery 2005;57(3 suppl):S4-S16;discussion ii-v.
4. Verrees M, Selman WR. Management of normal pressure hydrocephalus. Am Fam Physician 2004;70:1071-1078.
Evidence-based answers from the Family Physicians Inquiries Network
What steps can reduce morbidity and mortality caused by hip fractures?
Surgery within 24 hours of hip fracture is a critical step in reducing complications, and may decrease mortality compared with conservative care (strength of recommendation [sor]: B, cohort studies). Give patients heparin at the time of admission to prevent venous thromboembolism (VTE) (SOR: A, systematic reviews of RCTs). Anticoagulation should be continued in some form for 10 days or until the patient is fully ambulatory (SOR: A). Patients should also get prophylactic antibiotics in the 2 hours before surgery (SOR: A, meta-analysis of RCT). reduce the risk of postoperative delirium by avoiding certain medications, minimizing sleep disturbances, and providing adequate analgesia (SOR: B, systematic review of cohort studies). Aggressive pain control should also be top of mind—higher pain scores are associated with longer hospital stays, delayed ambulation, and long-term functional impairment (SOR: C, extrapolation from a single cohort study).
Ensure that proper treatment continues after discharge
Mary M. Stephens, MD, MPH
East Tennessee State University, Kingsport
As an FP working in a hospital, I am often asked to consult on cases of hip fracture. It’s important to maximize the patient’s condition quickly in order for the orthopedist to be able to proceed with surgical repair within the first 24 hours of the injury.
Many hospitals have anticoagulation protocols or standing orders for postop hip fracture management, which should make VTE prevention almost automatic. However, it’s important to ensure that these orders are initiated preoperatively if surgery is delayed, and that treatment gets continued for the appropriate length of time—even after the patient is discharged to their home or to a facility for rehabilitation.
As physicians, we worry about the short-term mortality from VTE and pulmonary embolism, but delirium can be devastating for family members to watch, and carries its own morbidities. I talk to the patient and family preoperatively or immediately postoperatively about this risk so they can be prepared.
Evidence summary
Although most patients undergo surgery for hip fracture, family physicians often serve as consultants for perioperative management and rehabilitation. Several interventions have been studied that influence outcomes for hip fracture patients.
Surgical interventions: Timing is critical
Most ambulatory, medically stable patients elect to have surgical repair of their fractures. A meta-analysis found few randomized trials comparing operative with nonoperative therapy; it concluded that surgical treatment seems to be associated with a reduced length of hospital stay and improved rehabilitation.1
The timing of surgery appears to be an important variable, particularly whether patients should undergo surgery within 24 hours of the fracture. Some studies found decreased mortality with earlier surgical intervention,2 while others have not.3 A 2006 observational study4 found that delay in operating was associated with an increased risk of death in the hospital, even after adjusting for comorbidities. For all deaths in the hospital, the odds ratio [OR] for delaying more than 1 day, relative to 1 day or less, was 1.27 (95% confidence interval [CI], 1.23–1.32). Despite the inconsistency regarding mortality rates, complication rates (such as decubitus ulcers) do increase with a delay in surgery.3
Unfractionated vs LMW heparin
After a hip fracture, patients are at very high risk for VTE. For untreated patients, the rate of deep vein thromboses (DVT) may be as high as 50%, with an associated fatal pulmonary embolism rate as high as 7.5%.5
The effectiveness of unfractionated and low-molecular-weight heparin was evaluated in a 2002 Cochrane systematic review.6 While evidence was insufficient to recommend 1 agent over another, both were found to significantly decrease the incidence of lower-extremity DVT over placebo (for unfractionated heparin, relative risk [RR]=0.59 [95% CI, 0.49–0.72]; for low-molecular-weight heparin, RR=0.60 [95% CI, 0.50–0.71]). Number needed to treat [NNT] with either agent was 7.
Reduce infections with antibiotic prophylaxis
Antibiotic prophylaxis has been supported by a Cochrane review, which concluded that single-dose antibiotic prophylaxis before surgery significantly reduced the risk of deep wound infections (RR=0.40; 95% CI, 0.24–0.67; NNT=55), as well as superficial wound, urinary, and respiratory tract infections.7
Patients should receive antibiotics less than 2 hours before surgery to reduce the risk of infection.8 Classen et al found that patients treated less than 2 hours before surgery had a 0.6% rate of infection (10/1208), compared with a 3.85% rate for those treated 2 to 24 hours ahead (14/369) (NNT=31).8 It is unclear whether multiple-dose therapy provides additional benefit when administered over the first 24 to 36 hours after surgery9 (OR=0.60; 95% CI, 0.18–2.02). First- or second-generation cephalosporins were used in most studies.
Delirium: A common but avoidable complication
Delirium is a common complication seen after hip fracture, affecting approximately 10% to 16% of patients.10,11 Delirium may increase the duration of hospitalization, and may be associated with an increased mortality at 1 year.12 Delirium can be avoided by looking at each patient’s risk factors.13 Studies suggest avoiding use of meperidine, benzodiazepines, and medications with anticholinergic side effects.11,13 Sleep deprivation, delayed mobility, and inadequate pain control are also associated with the development of delirium.13
One study showed that prophylaxis with haloperidol for hip fracture patients did not decrease the incidence of postoperative delirium but did reduce its duration and severity.14 Haloperidol prophylaxis was also associated with shorter hospital stays. Treatment with haloperidol or risperidone for the agitation of postoperative delirium has been recommended when behavioral interventions fail.13
Pain control improves recovery
Providing adequate analgesia is of the utmost importance. In a 2003 prospective cohort study, patients without sufficient analgesia had an increased risk of poor functional recovery and longer hospitalization.15 In another cohort study, those patients whose pain was inadequately controlled also had an increased risk for delirium (RR=9.0; 95% CI, 1.8–45.2).11 Meperidine use increased the risk for delirium compared with other opioid analgesics (RR=2.4; 95% CI, 1.3–4.5).11
Recommendations of others
The American College of Physicians provides a comprehensive evidence-based guideline for the management of hip fracture patients in their PIER series (Physicians’ Information and Education Resource) (TABLE).16
The American College of Chest Physicians has published evidence-based guidelines for the prevention of VTE.5 For patients undergoing hip fracture surgery, they recommend routine use of fondaparinux, low-molecular-weight heparin at high-risk dosing, adjusted-dose warfarin (at a target international normalized ratio [INR] of 2.5, range 2.0–3.0), or unfractionated heparin. They recommend against routine use of aspirin alone. If surgery must be delayed, physicians should initiate prophylaxis with unfractionated or low-molecular-weight heparin at the time of hospital admission. Anticoagulation should routinely continue for 10 days after surgery or until the patient is ambulatory. If anticoagulation is contraindicated, mechanical prophylaxis of VTE with foot and calf pumping devices is recommended.5,6
TABLE
6 steps for managing hip fracture from the American College of Physicians
|
| Source: PIER: Physicians’ Information and education resource, American College of Physicians, 2006.16 |
1. Parker MJ, Handoll HH, Bhargara A. Conservative versus operative treatment for hip fractures. Cochrane Database Syst Rev. 2000;(4):CD000337.
2. Dorotka R, Schoechtner H, Buchinger W. The influence of immediate surgical treatment of proximal femoral fractures on mortality and quality of life. Operation within six hours of the fracture versus later than six hours. J Bone Joint Surg Br. 2003;85:1107-1113.
3. Grimes JP, Gregory PM, Noveck H, Butler MS, Carson JL. The effects of time-to-surgery on mortality and morbidity in patients following hip fracture. Am J Med. 2002;112:702.
4. Bottle A, Aylin P. mortality associated with delay in operation after hip fracture: observational study. BMJ. 2006;332:947-951.
5. Geerts WH, Pineo GF, Heit JA, et al. Prevention of venous thromboembolism: the seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest. 2004;126(3 Suppl):S338-S400.
6. Handoll HH, Farrar MJ, Mcbirnie J, Tytherleighstrong G, Milne AA, Gillespie WJ. Heparin, low molecular weight heparin and physical methods for preventing deep vein thrombosis and pulmonary embolism following surgery for hip fractures. Cochrane Database Syst Rev. 2002;(4):CD000305
7. Gillespie WJ, Walenkamp G. Antibiotic prophylaxis for surgery for proximal femoral and other closed long bone fractures. Cochrane Database Syst Rev. 2001;(1):CD000244.
8. Classen DC, Evans RS, Pestotnik SL, Horn SD, Menlove RL, Burke JP. The timing of prophylactic administration of antibiotics and the risk of surgical wound infection. N Engl J Med. 1992;326:281-286.
9. March L, Chamberlain A, Cameron I, et al. Prevention, Treatment, and Rehabilitation of Fractured Neck of Femur. report from the Northern sydney Area Fractured Neck of Femur Health outcomes Project. St. Leonards, Australia: Public Health Unit, Northern Sydney Area Health Service; 1996. Available at: www.mja.com.au/public/issues/iprs2/march/fnof.pdf. Accessed on october 11, 2007.
10. Brauer C, Morrison RS, Silberzweig SB, Siu AL. The cause of delirium in patients with hip fracture. Arch Intern Med. 2000;160:1856-1860.
11. Morrison RS, Magaziner J, Gilbert M, et al. Relationship between pain and opioid analgesics on the development of delirium following hip fracture. J Gerontol A Biol Sci Med Sci. 2003;58:76-81.
12. Edelstein DM, Aharonoff GB, Karp A, Capla EL, Zuckerman JD, Koval KJ. Effect of postoperative delirium on outcome after hip fracture. Clin Orthop Relat Res. 2004;422:195-200.
13. Day H. Postoperative delirium. PIER: Physician’s Information and education resource, American College of Physicians. July 2006. Available at: pier.acponline.org/index.html. Accessed on October 11, 2007.
14. Kalisvaart KJ, de Jonghe JF, Bogaards MJ, et al. Haloperidol prophylaxis for elderly hip-surgery patients at risk for delirium: a randomized placebo-controlled study. J Am Geriatr Soc. 2005;53:1658-1666.
15. Morrison RS, Magaziner J, Mclaughlin MA, et al. The impact of post-operative pain on outcomes following hip fracture. Pain. 2003;103:303-311.
16. Christmas C. Hip fracture. PIER: Physicians’ Information and Education Resource, American College of Physicians. updated July 2006. Available at: pier.acponline.org/index.html. october 11, 2007.
Surgery within 24 hours of hip fracture is a critical step in reducing complications, and may decrease mortality compared with conservative care (strength of recommendation [sor]: B, cohort studies). Give patients heparin at the time of admission to prevent venous thromboembolism (VTE) (SOR: A, systematic reviews of RCTs). Anticoagulation should be continued in some form for 10 days or until the patient is fully ambulatory (SOR: A). Patients should also get prophylactic antibiotics in the 2 hours before surgery (SOR: A, meta-analysis of RCT). reduce the risk of postoperative delirium by avoiding certain medications, minimizing sleep disturbances, and providing adequate analgesia (SOR: B, systematic review of cohort studies). Aggressive pain control should also be top of mind—higher pain scores are associated with longer hospital stays, delayed ambulation, and long-term functional impairment (SOR: C, extrapolation from a single cohort study).
Ensure that proper treatment continues after discharge
Mary M. Stephens, MD, MPH
East Tennessee State University, Kingsport
As an FP working in a hospital, I am often asked to consult on cases of hip fracture. It’s important to maximize the patient’s condition quickly in order for the orthopedist to be able to proceed with surgical repair within the first 24 hours of the injury.
Many hospitals have anticoagulation protocols or standing orders for postop hip fracture management, which should make VTE prevention almost automatic. However, it’s important to ensure that these orders are initiated preoperatively if surgery is delayed, and that treatment gets continued for the appropriate length of time—even after the patient is discharged to their home or to a facility for rehabilitation.
As physicians, we worry about the short-term mortality from VTE and pulmonary embolism, but delirium can be devastating for family members to watch, and carries its own morbidities. I talk to the patient and family preoperatively or immediately postoperatively about this risk so they can be prepared.
Evidence summary
Although most patients undergo surgery for hip fracture, family physicians often serve as consultants for perioperative management and rehabilitation. Several interventions have been studied that influence outcomes for hip fracture patients.
Surgical interventions: Timing is critical
Most ambulatory, medically stable patients elect to have surgical repair of their fractures. A meta-analysis found few randomized trials comparing operative with nonoperative therapy; it concluded that surgical treatment seems to be associated with a reduced length of hospital stay and improved rehabilitation.1
The timing of surgery appears to be an important variable, particularly whether patients should undergo surgery within 24 hours of the fracture. Some studies found decreased mortality with earlier surgical intervention,2 while others have not.3 A 2006 observational study4 found that delay in operating was associated with an increased risk of death in the hospital, even after adjusting for comorbidities. For all deaths in the hospital, the odds ratio [OR] for delaying more than 1 day, relative to 1 day or less, was 1.27 (95% confidence interval [CI], 1.23–1.32). Despite the inconsistency regarding mortality rates, complication rates (such as decubitus ulcers) do increase with a delay in surgery.3
Unfractionated vs LMW heparin
After a hip fracture, patients are at very high risk for VTE. For untreated patients, the rate of deep vein thromboses (DVT) may be as high as 50%, with an associated fatal pulmonary embolism rate as high as 7.5%.5
The effectiveness of unfractionated and low-molecular-weight heparin was evaluated in a 2002 Cochrane systematic review.6 While evidence was insufficient to recommend 1 agent over another, both were found to significantly decrease the incidence of lower-extremity DVT over placebo (for unfractionated heparin, relative risk [RR]=0.59 [95% CI, 0.49–0.72]; for low-molecular-weight heparin, RR=0.60 [95% CI, 0.50–0.71]). Number needed to treat [NNT] with either agent was 7.
Reduce infections with antibiotic prophylaxis
Antibiotic prophylaxis has been supported by a Cochrane review, which concluded that single-dose antibiotic prophylaxis before surgery significantly reduced the risk of deep wound infections (RR=0.40; 95% CI, 0.24–0.67; NNT=55), as well as superficial wound, urinary, and respiratory tract infections.7
Patients should receive antibiotics less than 2 hours before surgery to reduce the risk of infection.8 Classen et al found that patients treated less than 2 hours before surgery had a 0.6% rate of infection (10/1208), compared with a 3.85% rate for those treated 2 to 24 hours ahead (14/369) (NNT=31).8 It is unclear whether multiple-dose therapy provides additional benefit when administered over the first 24 to 36 hours after surgery9 (OR=0.60; 95% CI, 0.18–2.02). First- or second-generation cephalosporins were used in most studies.
Delirium: A common but avoidable complication
Delirium is a common complication seen after hip fracture, affecting approximately 10% to 16% of patients.10,11 Delirium may increase the duration of hospitalization, and may be associated with an increased mortality at 1 year.12 Delirium can be avoided by looking at each patient’s risk factors.13 Studies suggest avoiding use of meperidine, benzodiazepines, and medications with anticholinergic side effects.11,13 Sleep deprivation, delayed mobility, and inadequate pain control are also associated with the development of delirium.13
One study showed that prophylaxis with haloperidol for hip fracture patients did not decrease the incidence of postoperative delirium but did reduce its duration and severity.14 Haloperidol prophylaxis was also associated with shorter hospital stays. Treatment with haloperidol or risperidone for the agitation of postoperative delirium has been recommended when behavioral interventions fail.13
Pain control improves recovery
Providing adequate analgesia is of the utmost importance. In a 2003 prospective cohort study, patients without sufficient analgesia had an increased risk of poor functional recovery and longer hospitalization.15 In another cohort study, those patients whose pain was inadequately controlled also had an increased risk for delirium (RR=9.0; 95% CI, 1.8–45.2).11 Meperidine use increased the risk for delirium compared with other opioid analgesics (RR=2.4; 95% CI, 1.3–4.5).11
Recommendations of others
The American College of Physicians provides a comprehensive evidence-based guideline for the management of hip fracture patients in their PIER series (Physicians’ Information and Education Resource) (TABLE).16
The American College of Chest Physicians has published evidence-based guidelines for the prevention of VTE.5 For patients undergoing hip fracture surgery, they recommend routine use of fondaparinux, low-molecular-weight heparin at high-risk dosing, adjusted-dose warfarin (at a target international normalized ratio [INR] of 2.5, range 2.0–3.0), or unfractionated heparin. They recommend against routine use of aspirin alone. If surgery must be delayed, physicians should initiate prophylaxis with unfractionated or low-molecular-weight heparin at the time of hospital admission. Anticoagulation should routinely continue for 10 days after surgery or until the patient is ambulatory. If anticoagulation is contraindicated, mechanical prophylaxis of VTE with foot and calf pumping devices is recommended.5,6
TABLE
6 steps for managing hip fracture from the American College of Physicians
|
| Source: PIER: Physicians’ Information and education resource, American College of Physicians, 2006.16 |
Surgery within 24 hours of hip fracture is a critical step in reducing complications, and may decrease mortality compared with conservative care (strength of recommendation [sor]: B, cohort studies). Give patients heparin at the time of admission to prevent venous thromboembolism (VTE) (SOR: A, systematic reviews of RCTs). Anticoagulation should be continued in some form for 10 days or until the patient is fully ambulatory (SOR: A). Patients should also get prophylactic antibiotics in the 2 hours before surgery (SOR: A, meta-analysis of RCT). reduce the risk of postoperative delirium by avoiding certain medications, minimizing sleep disturbances, and providing adequate analgesia (SOR: B, systematic review of cohort studies). Aggressive pain control should also be top of mind—higher pain scores are associated with longer hospital stays, delayed ambulation, and long-term functional impairment (SOR: C, extrapolation from a single cohort study).
Ensure that proper treatment continues after discharge
Mary M. Stephens, MD, MPH
East Tennessee State University, Kingsport
As an FP working in a hospital, I am often asked to consult on cases of hip fracture. It’s important to maximize the patient’s condition quickly in order for the orthopedist to be able to proceed with surgical repair within the first 24 hours of the injury.
Many hospitals have anticoagulation protocols or standing orders for postop hip fracture management, which should make VTE prevention almost automatic. However, it’s important to ensure that these orders are initiated preoperatively if surgery is delayed, and that treatment gets continued for the appropriate length of time—even after the patient is discharged to their home or to a facility for rehabilitation.
As physicians, we worry about the short-term mortality from VTE and pulmonary embolism, but delirium can be devastating for family members to watch, and carries its own morbidities. I talk to the patient and family preoperatively or immediately postoperatively about this risk so they can be prepared.
Evidence summary
Although most patients undergo surgery for hip fracture, family physicians often serve as consultants for perioperative management and rehabilitation. Several interventions have been studied that influence outcomes for hip fracture patients.
Surgical interventions: Timing is critical
Most ambulatory, medically stable patients elect to have surgical repair of their fractures. A meta-analysis found few randomized trials comparing operative with nonoperative therapy; it concluded that surgical treatment seems to be associated with a reduced length of hospital stay and improved rehabilitation.1
The timing of surgery appears to be an important variable, particularly whether patients should undergo surgery within 24 hours of the fracture. Some studies found decreased mortality with earlier surgical intervention,2 while others have not.3 A 2006 observational study4 found that delay in operating was associated with an increased risk of death in the hospital, even after adjusting for comorbidities. For all deaths in the hospital, the odds ratio [OR] for delaying more than 1 day, relative to 1 day or less, was 1.27 (95% confidence interval [CI], 1.23–1.32). Despite the inconsistency regarding mortality rates, complication rates (such as decubitus ulcers) do increase with a delay in surgery.3
Unfractionated vs LMW heparin
After a hip fracture, patients are at very high risk for VTE. For untreated patients, the rate of deep vein thromboses (DVT) may be as high as 50%, with an associated fatal pulmonary embolism rate as high as 7.5%.5
The effectiveness of unfractionated and low-molecular-weight heparin was evaluated in a 2002 Cochrane systematic review.6 While evidence was insufficient to recommend 1 agent over another, both were found to significantly decrease the incidence of lower-extremity DVT over placebo (for unfractionated heparin, relative risk [RR]=0.59 [95% CI, 0.49–0.72]; for low-molecular-weight heparin, RR=0.60 [95% CI, 0.50–0.71]). Number needed to treat [NNT] with either agent was 7.
Reduce infections with antibiotic prophylaxis
Antibiotic prophylaxis has been supported by a Cochrane review, which concluded that single-dose antibiotic prophylaxis before surgery significantly reduced the risk of deep wound infections (RR=0.40; 95% CI, 0.24–0.67; NNT=55), as well as superficial wound, urinary, and respiratory tract infections.7
Patients should receive antibiotics less than 2 hours before surgery to reduce the risk of infection.8 Classen et al found that patients treated less than 2 hours before surgery had a 0.6% rate of infection (10/1208), compared with a 3.85% rate for those treated 2 to 24 hours ahead (14/369) (NNT=31).8 It is unclear whether multiple-dose therapy provides additional benefit when administered over the first 24 to 36 hours after surgery9 (OR=0.60; 95% CI, 0.18–2.02). First- or second-generation cephalosporins were used in most studies.
Delirium: A common but avoidable complication
Delirium is a common complication seen after hip fracture, affecting approximately 10% to 16% of patients.10,11 Delirium may increase the duration of hospitalization, and may be associated with an increased mortality at 1 year.12 Delirium can be avoided by looking at each patient’s risk factors.13 Studies suggest avoiding use of meperidine, benzodiazepines, and medications with anticholinergic side effects.11,13 Sleep deprivation, delayed mobility, and inadequate pain control are also associated with the development of delirium.13
One study showed that prophylaxis with haloperidol for hip fracture patients did not decrease the incidence of postoperative delirium but did reduce its duration and severity.14 Haloperidol prophylaxis was also associated with shorter hospital stays. Treatment with haloperidol or risperidone for the agitation of postoperative delirium has been recommended when behavioral interventions fail.13
Pain control improves recovery
Providing adequate analgesia is of the utmost importance. In a 2003 prospective cohort study, patients without sufficient analgesia had an increased risk of poor functional recovery and longer hospitalization.15 In another cohort study, those patients whose pain was inadequately controlled also had an increased risk for delirium (RR=9.0; 95% CI, 1.8–45.2).11 Meperidine use increased the risk for delirium compared with other opioid analgesics (RR=2.4; 95% CI, 1.3–4.5).11
Recommendations of others
The American College of Physicians provides a comprehensive evidence-based guideline for the management of hip fracture patients in their PIER series (Physicians’ Information and Education Resource) (TABLE).16
The American College of Chest Physicians has published evidence-based guidelines for the prevention of VTE.5 For patients undergoing hip fracture surgery, they recommend routine use of fondaparinux, low-molecular-weight heparin at high-risk dosing, adjusted-dose warfarin (at a target international normalized ratio [INR] of 2.5, range 2.0–3.0), or unfractionated heparin. They recommend against routine use of aspirin alone. If surgery must be delayed, physicians should initiate prophylaxis with unfractionated or low-molecular-weight heparin at the time of hospital admission. Anticoagulation should routinely continue for 10 days after surgery or until the patient is ambulatory. If anticoagulation is contraindicated, mechanical prophylaxis of VTE with foot and calf pumping devices is recommended.5,6
TABLE
6 steps for managing hip fracture from the American College of Physicians
|
| Source: PIER: Physicians’ Information and education resource, American College of Physicians, 2006.16 |
1. Parker MJ, Handoll HH, Bhargara A. Conservative versus operative treatment for hip fractures. Cochrane Database Syst Rev. 2000;(4):CD000337.
2. Dorotka R, Schoechtner H, Buchinger W. The influence of immediate surgical treatment of proximal femoral fractures on mortality and quality of life. Operation within six hours of the fracture versus later than six hours. J Bone Joint Surg Br. 2003;85:1107-1113.
3. Grimes JP, Gregory PM, Noveck H, Butler MS, Carson JL. The effects of time-to-surgery on mortality and morbidity in patients following hip fracture. Am J Med. 2002;112:702.
4. Bottle A, Aylin P. mortality associated with delay in operation after hip fracture: observational study. BMJ. 2006;332:947-951.
5. Geerts WH, Pineo GF, Heit JA, et al. Prevention of venous thromboembolism: the seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest. 2004;126(3 Suppl):S338-S400.
6. Handoll HH, Farrar MJ, Mcbirnie J, Tytherleighstrong G, Milne AA, Gillespie WJ. Heparin, low molecular weight heparin and physical methods for preventing deep vein thrombosis and pulmonary embolism following surgery for hip fractures. Cochrane Database Syst Rev. 2002;(4):CD000305
7. Gillespie WJ, Walenkamp G. Antibiotic prophylaxis for surgery for proximal femoral and other closed long bone fractures. Cochrane Database Syst Rev. 2001;(1):CD000244.
8. Classen DC, Evans RS, Pestotnik SL, Horn SD, Menlove RL, Burke JP. The timing of prophylactic administration of antibiotics and the risk of surgical wound infection. N Engl J Med. 1992;326:281-286.
9. March L, Chamberlain A, Cameron I, et al. Prevention, Treatment, and Rehabilitation of Fractured Neck of Femur. report from the Northern sydney Area Fractured Neck of Femur Health outcomes Project. St. Leonards, Australia: Public Health Unit, Northern Sydney Area Health Service; 1996. Available at: www.mja.com.au/public/issues/iprs2/march/fnof.pdf. Accessed on october 11, 2007.
10. Brauer C, Morrison RS, Silberzweig SB, Siu AL. The cause of delirium in patients with hip fracture. Arch Intern Med. 2000;160:1856-1860.
11. Morrison RS, Magaziner J, Gilbert M, et al. Relationship between pain and opioid analgesics on the development of delirium following hip fracture. J Gerontol A Biol Sci Med Sci. 2003;58:76-81.
12. Edelstein DM, Aharonoff GB, Karp A, Capla EL, Zuckerman JD, Koval KJ. Effect of postoperative delirium on outcome after hip fracture. Clin Orthop Relat Res. 2004;422:195-200.
13. Day H. Postoperative delirium. PIER: Physician’s Information and education resource, American College of Physicians. July 2006. Available at: pier.acponline.org/index.html. Accessed on October 11, 2007.
14. Kalisvaart KJ, de Jonghe JF, Bogaards MJ, et al. Haloperidol prophylaxis for elderly hip-surgery patients at risk for delirium: a randomized placebo-controlled study. J Am Geriatr Soc. 2005;53:1658-1666.
15. Morrison RS, Magaziner J, Mclaughlin MA, et al. The impact of post-operative pain on outcomes following hip fracture. Pain. 2003;103:303-311.
16. Christmas C. Hip fracture. PIER: Physicians’ Information and Education Resource, American College of Physicians. updated July 2006. Available at: pier.acponline.org/index.html. october 11, 2007.
1. Parker MJ, Handoll HH, Bhargara A. Conservative versus operative treatment for hip fractures. Cochrane Database Syst Rev. 2000;(4):CD000337.
2. Dorotka R, Schoechtner H, Buchinger W. The influence of immediate surgical treatment of proximal femoral fractures on mortality and quality of life. Operation within six hours of the fracture versus later than six hours. J Bone Joint Surg Br. 2003;85:1107-1113.
3. Grimes JP, Gregory PM, Noveck H, Butler MS, Carson JL. The effects of time-to-surgery on mortality and morbidity in patients following hip fracture. Am J Med. 2002;112:702.
4. Bottle A, Aylin P. mortality associated with delay in operation after hip fracture: observational study. BMJ. 2006;332:947-951.
5. Geerts WH, Pineo GF, Heit JA, et al. Prevention of venous thromboembolism: the seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest. 2004;126(3 Suppl):S338-S400.
6. Handoll HH, Farrar MJ, Mcbirnie J, Tytherleighstrong G, Milne AA, Gillespie WJ. Heparin, low molecular weight heparin and physical methods for preventing deep vein thrombosis and pulmonary embolism following surgery for hip fractures. Cochrane Database Syst Rev. 2002;(4):CD000305
7. Gillespie WJ, Walenkamp G. Antibiotic prophylaxis for surgery for proximal femoral and other closed long bone fractures. Cochrane Database Syst Rev. 2001;(1):CD000244.
8. Classen DC, Evans RS, Pestotnik SL, Horn SD, Menlove RL, Burke JP. The timing of prophylactic administration of antibiotics and the risk of surgical wound infection. N Engl J Med. 1992;326:281-286.
9. March L, Chamberlain A, Cameron I, et al. Prevention, Treatment, and Rehabilitation of Fractured Neck of Femur. report from the Northern sydney Area Fractured Neck of Femur Health outcomes Project. St. Leonards, Australia: Public Health Unit, Northern Sydney Area Health Service; 1996. Available at: www.mja.com.au/public/issues/iprs2/march/fnof.pdf. Accessed on october 11, 2007.
10. Brauer C, Morrison RS, Silberzweig SB, Siu AL. The cause of delirium in patients with hip fracture. Arch Intern Med. 2000;160:1856-1860.
11. Morrison RS, Magaziner J, Gilbert M, et al. Relationship between pain and opioid analgesics on the development of delirium following hip fracture. J Gerontol A Biol Sci Med Sci. 2003;58:76-81.
12. Edelstein DM, Aharonoff GB, Karp A, Capla EL, Zuckerman JD, Koval KJ. Effect of postoperative delirium on outcome after hip fracture. Clin Orthop Relat Res. 2004;422:195-200.
13. Day H. Postoperative delirium. PIER: Physician’s Information and education resource, American College of Physicians. July 2006. Available at: pier.acponline.org/index.html. Accessed on October 11, 2007.
14. Kalisvaart KJ, de Jonghe JF, Bogaards MJ, et al. Haloperidol prophylaxis for elderly hip-surgery patients at risk for delirium: a randomized placebo-controlled study. J Am Geriatr Soc. 2005;53:1658-1666.
15. Morrison RS, Magaziner J, Mclaughlin MA, et al. The impact of post-operative pain on outcomes following hip fracture. Pain. 2003;103:303-311.
16. Christmas C. Hip fracture. PIER: Physicians’ Information and Education Resource, American College of Physicians. updated July 2006. Available at: pier.acponline.org/index.html. october 11, 2007.
Evidence-based answers from the Family Physicians Inquiries Network
Does bed rest for preeclampsia improve neonatal outcomes?
No. strict bed rest in the hospital for pregnant women with preeclampsia does not appear to lower rates of perinatal mortality, neonatal mortality, or neonatal morbidity, including preterm birth, endotracheal intubations, or neonatal intensive care unit (NICU) admissions (strength of recommendation: B, based on 2 randomized controlled trials [RCT] and extrapolations from 2 RCTs of pregnant patients with nonproteinuric hypertension).
Ronald Januchowski, MD
Spartanburg Regional Medical Center, Spartanburg, SC
Changing long-standing practices is always a challenge We’ve said goodbye to magnesium for preterm labor, and now it looks like bed rest for preeclampsia is not far behind. Changing long-standing practices in response to stronger evidence-based information is always a challenge, especially when we’ve been relying on long-standing expert opinion or anecdotal evidence. Following these recommendations will be another challenge for us, even though we consider the relationship we have with our obstetrical nurses and physicians to be a good one.
Our plan to introduce these modifications will follow previous successful plans; the member of our group with the most “capital” in Obstetrics can bring others on board.
Evidence summary
Ten percent of preeclampsia occurs in pregnancies at less than 34 weeks of gestation. Traditionally, physicians often recommended bed rest to preterm, preeclamptic patients in the belief that it would improve neonatal outcomes.
RCTs find no difference between bed rest and ad lib movement
A single-center RCT investigated bed rest treatment for 105 patients with preeclampsia and gestational ages between 26 to 38 weeks. Patients were assigned to either strict bed rest with bathroom privileges in the hospital until delivery, or to bed rest with the ability to move freely around the hospital. Outcome assessors were not blinded to patient treatment allocation. There was no statistical difference between the 2 groups in perinatal or neonatal mortality, or in the neonatal morbidities of preterm births, endotracheal intubations, or NICU admissions.1
Similarly, a small, unblinded RCT of 40 preeclamptic patients treated in the hospital with strict bed confinement or without restrictions found no significant difference in fetal or perinatal mortality.2 No power calculations were reported for detecting differences in neonatal outcome rates in either of these studies.
Studies in nonproteinuric hypertension were no different
In addition to the studies in patients with preeclampsia, 2 RCTs measured neonatal outcomes with bed rest compared with normal activity in pregnancies complicated by nonproteinuric hypertension. These studies also found that bed rest did not improve neonatal outcomes.
The first trial was a multicenter RCT involving 218 patients between 28 to 38 weeks gestation with nonproteinuric hypertension (blood pressure >140/90 mm Hg). The patients were randomized to 2 groups: bed rest in the hospital but allowed to move around the ward, and normal activity at home without restrictions. The outcomes were measured by masked assessors. There were no statistical differences in perinatal or neonatal mortality, or in the neonatal morbidities of preterm birth, newborns small for their gestational age, or NICU admissions between the 2 groups.3
A second RCT of 135 nonproteinuric but hypertensive pregnant patients with diastolic blood pressures between 90 and 109 mm Hg also demonstrated no difference between patients treated with bed rest and sedation or normal activity in fetal or neonatal outcomes.4
Recommendations from others
An American College of Obstetrics and Gynecology practice bulletin on diagnosis and management of preeclampsia and eclampsia does not mention bed rest.5 The Canadian Hypertension Society Consensus Conference in 1997 stated that a “policy of hospital admission and strict bed rest is not advised for gestational hypertension with or without proteinuria.”6
1. Meher S, Abalos E, Carroli G. Bed rest with or without hospitalisation for hypertension during pregnancy. Cochrane Database Syst Rev 2005;CD(4):003-514.
2. Matthews DD, Agarwal V, Shuttleworth TP. A randomized controlled trial of complete bed rest versus ambulation in the management of proteinuric hypertension during pregnancy. Br J Obstet Gynecol 1982;89:128-131.
3. Crowther CA, Bouwmeester Am, Ashurst Hm. Does admission to hospital for bed rest prevent disease progression or improve fetal outcome in pregnancy complicated by non-proteinuric hyper-tension? Br J Obstet Gynecol 1992;99:13-17.
4. Matthews DD. A randomized controlled trial of bed rest and sedation or normal activity and non-sedation in the management of non-albuminuric hypertension in late pregnancy. Br J Obstet Gynecol 1977;84:108-114.
5. Diagnosis and management of preeclampsia and eclampsia. American College of obstetrics and Gynecology (ACoG) Practice bulletin, No. 33. Obstet Gynecol 2002;99:159-67.
6. Report of the Canadian Hypertension society Consensus Conference: Nonpharmacologic management and prevention of hypertensive disorders in pregnancy. Can Med Assoc J 1997;157:907-919.
No. strict bed rest in the hospital for pregnant women with preeclampsia does not appear to lower rates of perinatal mortality, neonatal mortality, or neonatal morbidity, including preterm birth, endotracheal intubations, or neonatal intensive care unit (NICU) admissions (strength of recommendation: B, based on 2 randomized controlled trials [RCT] and extrapolations from 2 RCTs of pregnant patients with nonproteinuric hypertension).
Ronald Januchowski, MD
Spartanburg Regional Medical Center, Spartanburg, SC
Changing long-standing practices is always a challenge We’ve said goodbye to magnesium for preterm labor, and now it looks like bed rest for preeclampsia is not far behind. Changing long-standing practices in response to stronger evidence-based information is always a challenge, especially when we’ve been relying on long-standing expert opinion or anecdotal evidence. Following these recommendations will be another challenge for us, even though we consider the relationship we have with our obstetrical nurses and physicians to be a good one.
Our plan to introduce these modifications will follow previous successful plans; the member of our group with the most “capital” in Obstetrics can bring others on board.
Evidence summary
Ten percent of preeclampsia occurs in pregnancies at less than 34 weeks of gestation. Traditionally, physicians often recommended bed rest to preterm, preeclamptic patients in the belief that it would improve neonatal outcomes.
RCTs find no difference between bed rest and ad lib movement
A single-center RCT investigated bed rest treatment for 105 patients with preeclampsia and gestational ages between 26 to 38 weeks. Patients were assigned to either strict bed rest with bathroom privileges in the hospital until delivery, or to bed rest with the ability to move freely around the hospital. Outcome assessors were not blinded to patient treatment allocation. There was no statistical difference between the 2 groups in perinatal or neonatal mortality, or in the neonatal morbidities of preterm births, endotracheal intubations, or NICU admissions.1
Similarly, a small, unblinded RCT of 40 preeclamptic patients treated in the hospital with strict bed confinement or without restrictions found no significant difference in fetal or perinatal mortality.2 No power calculations were reported for detecting differences in neonatal outcome rates in either of these studies.
Studies in nonproteinuric hypertension were no different
In addition to the studies in patients with preeclampsia, 2 RCTs measured neonatal outcomes with bed rest compared with normal activity in pregnancies complicated by nonproteinuric hypertension. These studies also found that bed rest did not improve neonatal outcomes.
The first trial was a multicenter RCT involving 218 patients between 28 to 38 weeks gestation with nonproteinuric hypertension (blood pressure >140/90 mm Hg). The patients were randomized to 2 groups: bed rest in the hospital but allowed to move around the ward, and normal activity at home without restrictions. The outcomes were measured by masked assessors. There were no statistical differences in perinatal or neonatal mortality, or in the neonatal morbidities of preterm birth, newborns small for their gestational age, or NICU admissions between the 2 groups.3
A second RCT of 135 nonproteinuric but hypertensive pregnant patients with diastolic blood pressures between 90 and 109 mm Hg also demonstrated no difference between patients treated with bed rest and sedation or normal activity in fetal or neonatal outcomes.4
Recommendations from others
An American College of Obstetrics and Gynecology practice bulletin on diagnosis and management of preeclampsia and eclampsia does not mention bed rest.5 The Canadian Hypertension Society Consensus Conference in 1997 stated that a “policy of hospital admission and strict bed rest is not advised for gestational hypertension with or without proteinuria.”6
No. strict bed rest in the hospital for pregnant women with preeclampsia does not appear to lower rates of perinatal mortality, neonatal mortality, or neonatal morbidity, including preterm birth, endotracheal intubations, or neonatal intensive care unit (NICU) admissions (strength of recommendation: B, based on 2 randomized controlled trials [RCT] and extrapolations from 2 RCTs of pregnant patients with nonproteinuric hypertension).
Ronald Januchowski, MD
Spartanburg Regional Medical Center, Spartanburg, SC
Changing long-standing practices is always a challenge We’ve said goodbye to magnesium for preterm labor, and now it looks like bed rest for preeclampsia is not far behind. Changing long-standing practices in response to stronger evidence-based information is always a challenge, especially when we’ve been relying on long-standing expert opinion or anecdotal evidence. Following these recommendations will be another challenge for us, even though we consider the relationship we have with our obstetrical nurses and physicians to be a good one.
Our plan to introduce these modifications will follow previous successful plans; the member of our group with the most “capital” in Obstetrics can bring others on board.
Evidence summary
Ten percent of preeclampsia occurs in pregnancies at less than 34 weeks of gestation. Traditionally, physicians often recommended bed rest to preterm, preeclamptic patients in the belief that it would improve neonatal outcomes.
RCTs find no difference between bed rest and ad lib movement
A single-center RCT investigated bed rest treatment for 105 patients with preeclampsia and gestational ages between 26 to 38 weeks. Patients were assigned to either strict bed rest with bathroom privileges in the hospital until delivery, or to bed rest with the ability to move freely around the hospital. Outcome assessors were not blinded to patient treatment allocation. There was no statistical difference between the 2 groups in perinatal or neonatal mortality, or in the neonatal morbidities of preterm births, endotracheal intubations, or NICU admissions.1
Similarly, a small, unblinded RCT of 40 preeclamptic patients treated in the hospital with strict bed confinement or without restrictions found no significant difference in fetal or perinatal mortality.2 No power calculations were reported for detecting differences in neonatal outcome rates in either of these studies.
Studies in nonproteinuric hypertension were no different
In addition to the studies in patients with preeclampsia, 2 RCTs measured neonatal outcomes with bed rest compared with normal activity in pregnancies complicated by nonproteinuric hypertension. These studies also found that bed rest did not improve neonatal outcomes.
The first trial was a multicenter RCT involving 218 patients between 28 to 38 weeks gestation with nonproteinuric hypertension (blood pressure >140/90 mm Hg). The patients were randomized to 2 groups: bed rest in the hospital but allowed to move around the ward, and normal activity at home without restrictions. The outcomes were measured by masked assessors. There were no statistical differences in perinatal or neonatal mortality, or in the neonatal morbidities of preterm birth, newborns small for their gestational age, or NICU admissions between the 2 groups.3
A second RCT of 135 nonproteinuric but hypertensive pregnant patients with diastolic blood pressures between 90 and 109 mm Hg also demonstrated no difference between patients treated with bed rest and sedation or normal activity in fetal or neonatal outcomes.4
Recommendations from others
An American College of Obstetrics and Gynecology practice bulletin on diagnosis and management of preeclampsia and eclampsia does not mention bed rest.5 The Canadian Hypertension Society Consensus Conference in 1997 stated that a “policy of hospital admission and strict bed rest is not advised for gestational hypertension with or without proteinuria.”6
1. Meher S, Abalos E, Carroli G. Bed rest with or without hospitalisation for hypertension during pregnancy. Cochrane Database Syst Rev 2005;CD(4):003-514.
2. Matthews DD, Agarwal V, Shuttleworth TP. A randomized controlled trial of complete bed rest versus ambulation in the management of proteinuric hypertension during pregnancy. Br J Obstet Gynecol 1982;89:128-131.
3. Crowther CA, Bouwmeester Am, Ashurst Hm. Does admission to hospital for bed rest prevent disease progression or improve fetal outcome in pregnancy complicated by non-proteinuric hyper-tension? Br J Obstet Gynecol 1992;99:13-17.
4. Matthews DD. A randomized controlled trial of bed rest and sedation or normal activity and non-sedation in the management of non-albuminuric hypertension in late pregnancy. Br J Obstet Gynecol 1977;84:108-114.
5. Diagnosis and management of preeclampsia and eclampsia. American College of obstetrics and Gynecology (ACoG) Practice bulletin, No. 33. Obstet Gynecol 2002;99:159-67.
6. Report of the Canadian Hypertension society Consensus Conference: Nonpharmacologic management and prevention of hypertensive disorders in pregnancy. Can Med Assoc J 1997;157:907-919.
1. Meher S, Abalos E, Carroli G. Bed rest with or without hospitalisation for hypertension during pregnancy. Cochrane Database Syst Rev 2005;CD(4):003-514.
2. Matthews DD, Agarwal V, Shuttleworth TP. A randomized controlled trial of complete bed rest versus ambulation in the management of proteinuric hypertension during pregnancy. Br J Obstet Gynecol 1982;89:128-131.
3. Crowther CA, Bouwmeester Am, Ashurst Hm. Does admission to hospital for bed rest prevent disease progression or improve fetal outcome in pregnancy complicated by non-proteinuric hyper-tension? Br J Obstet Gynecol 1992;99:13-17.
4. Matthews DD. A randomized controlled trial of bed rest and sedation or normal activity and non-sedation in the management of non-albuminuric hypertension in late pregnancy. Br J Obstet Gynecol 1977;84:108-114.
5. Diagnosis and management of preeclampsia and eclampsia. American College of obstetrics and Gynecology (ACoG) Practice bulletin, No. 33. Obstet Gynecol 2002;99:159-67.
6. Report of the Canadian Hypertension society Consensus Conference: Nonpharmacologic management and prevention of hypertensive disorders in pregnancy. Can Med Assoc J 1997;157:907-919.
Evidence-based answers from the Family Physicians Inquiries Network
Are DMARDs effective for rheumatologic diseases besides rheumatoid arthritis?
It’s unclear whether disease-modifying antirheumatic agents (DMARDs) as first-line therapy in nonrheumatoid rheumatologic diseases are effective because the question has not been studied. As second-line therapy, the use of some DmArDs appears to be beneficial for patients with psoriatic arthritis (strength of recommendation [SOR]: A, based on systematic reviews of good-quality randomized controlled trials) and ankylosing spondylitis (SOR: B, based on systematic reviews of moderate quality trials). Data on the safety and efficacy of DMARDs as second-line therapy for other arthritic conditions is limited (SOR: C, based on small prospective cohort trials).
There are many options, but remember the risks
Richard Hoffman, MD
Chesterfield Family Medicine Residency, Richmond, Va
Traditionally, nonsteroidal anti-inflammatory agents (NSAIDs) have been the mainstay of treatment for rheumatologic disorders other than rheumatoid arthritis. methotrexate has been used in psoriatic arthritis because it also controls the skin disorder; sulfasalazine has been used in arthritis associated with inflammatory bowel disease, as it helps the bowel disorder itself. However, little evidence shows a definitive benefit for the arthritis.
The advent of tumor necrosis factor (TNF) blockers has changed the direction of research in this area; these agents are being used more and more in inflammatory arthritides. While staying up to date on the TNF antagonists, it’s important to remember the complications associated with them—particularly the increased risk of infections and increased propensity for neoplastic disorders. Consider those on TNF blockers as relatively immunosuppressed (number needed to harm [NNH]=59 for infection and 154 for malignancy).1
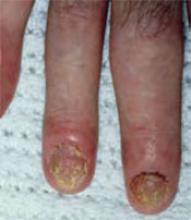
Psoriatic arthritis affecting the joints and nails
Evidence summary
The use of DMARDs has become standard of care for rheumatoid arthritis, for both therapy and prevention of progression of this debilitating disease. However, the use of DMARDs in nonrheumatoid rheumatologic disease is still under investigation, and at this point, the use of DMARDs as first-line therapy is not recommended; however, second-line therapy with DMARDs is common.
For psoriatic arthritis, DMARDs are beneficial as a second-line therapy
A Cochrane systematic review identified 13 randomized controlled trials enrolling a combined 1022 patients with psoriatic arthritis randomly assigned to receive a DMARD—methotrexate, sulfasalazine (Azulfidine), azathioprine (Imuran/ Azasan), or etretinate (Tegison; no longer available in the US)—compared with placebo.2 All agents were better than placebo; however, only 2 agents (parenteral high-dose methotrexate and sulfasalazine) had clinically important benefits for more than half the patients. The studies were too small to establish toxicity or to evaluate the other agents.
NSAIDs are still the preferred first-line therapy, concluded a recent publication on the treatment of psoriatic arthritis, which looked at 54 different studies; however, second-line therapy could include methotrexate, sulfasalazine, etanercept (Enbrel), infliximab (Remicade), cyclosporine, or combination therapy.3 Sulfasalazine appeared to be clinically beneficial for peripheral psoriatic arthritis.
Etanercept vs placebo. An initial study (60 patients) of etanercept vs placebo among patients who were permitted to stay on methotrexate or prednisone showed a response rate of 87% vs 23% (P<.0001; number needed to treat [NNT]=1.56).4
Infliximab vs placebo. A study of infliximab vs placebo involving 104 patients had similar results, with good response in 65% vs 10% (NNT=1.81) at 16 weeks; infliximab also inhibited radiographic progression by 22%.5
Cyclosporine. Although it is effective, reserve cyclosporine for patients who do not improve on other regimens, because of its nephrotoxicity.3
DMARDs show some benefit in treating ankylosing spondylitis
Two recent Cochrane systematic reviews on ankylosing spondylitis examined the use of sulfasalazine and methotrexate as second-line agents.6,7 Eleven trials were included in the sulfasalazine analysis, with a total of 895 patients. Sulfasalazine demonstrated some benefit in reducing erythrocyte sedimentation rates (ESRs) and morning stiffness, but there was no evidence that the drug reduced pain or improved physical function, spinal mobility, or rate of enthesitis. Sulfasalazine was well tolerated and may be useful in early mild disease for patients with peripheral arthritis and high ESRs. On the other hand, evidence was insufficient to determine whether methotrexate benefited patients with ankylosing spondylitis.
In other trials, infliximab and etanercept showed good potential for benefit in treating ankylosing spondylitis.
One study of infliximab vs placebo showed 61.2% vs 19.2% patients with good clinical benefit at 24 weeks and only mild or moderate adverse events (P<.001; NNT=2.38).8
Similarly, a smaller study (84 patients) showed that 60% of patients on etanercept vs 20% on placebo had good clinical benefit at only 12 weeks (P<.001, NNT=2.5).9
For other rheumatic diseases, studies are mixed
Due to cyclosporine’s toxicity, less toxic DMARDs are being evaluated to replace it for treatment of other rheumatic diseases. A recent randomized controlled trial of 100 patients with antineutrophil cytoplasmic antibody–associated systemic vasculitis showed methotrexate may be able to replace cyclosporine for both induction of remission (methotrexate=89.8% vs cyclosporine=93.5%; P=.041) and maintenance of remission (69.5% vs 46.5% at 18 months; P=.023).10
Initial trials on other rheumatic diseases have been small and have had varied results. There are mixed studies on the effectiveness of adding methotrexate to corticosteroids for giant cell arteritis.11,12
There has been no evidence of efficacy for the new TNF antagonists in either a small study on Sjögren’s syndrome (n=14)13 or a larger study on Wegener’s granulomatosis (n=180).14
The studies for use of DMARDs in lupus or scleroderma are of limited quality.
Recommendations from others
The Italian Society for Rheumatology consensus guidelines recommends TNF antagonists be considered in active psoriatic arthritis resistant to (a) NSAIDs, (b) at least 2 local steroid injections, and (c) at least 2 conventional DMARDs for patients with peripheral arthritis or enthesitis. They also recommend TNF antagonists be considered for psoriatic spondylitis resistant to NSAIDs.15
The Assessment in Ankylosing Spondylitis (ASAS) International Working Group and the European League Against Rheumatism (EULAR) recommendations for the treatment of ankylosing spondylitis, based on a systematic review of the literature and expert opinion, indicate that:
- There is good evidence for using NSAIDs and COX-2 inhibitors for symptomatic treatment.
- Conventional DMARDs are not well supported.
- TNF antagonists show a large benefit in both pain and function.
The ASAS/EULAR recommendation indicate that there is no evidence that any of these treatments actually modify the disease progression.16
Acknowledgments
The opinions and assertions contained herein are the private views of the authors and not to be construed as official, or as reflecting the views of the US Air Force medical service or the US Air Force at large.
1. Bongartz T, sutton AJ, Sweeting MJ, et al. Anti-TNF antibody therapy in rheumatoid arthritis and the risk of serious infections and malignancies: systematic review and meta-analysis of rare harmful effects in randomized controlled trials. JAMA 2006;295:2275-2285.
2. Jones G, Crotty M, Brooks P. Interventions for treating psoriatic arthritis. Cochrane Database Syst Rev 2000 (3):CD000212.
3. Manadan Am, Sequeira W, Block JA. The treatment of psoriatic arthritis. Am J Ther 2006;13:72-79.
4. Mease PJ, Goffe BS, Metz J, et al. etanercept in the treatment of psoriatic arthritis and psoriasis: a randomized trial. Lancet 2000;356:385-390.
5. Kavanaugh A, Antoni CE, Gladman D, et al. The Infliximab multinational Psoriatic Arthritis Controlled Trial (ImPACT): result of radiographic analyses after 1 year. Ann Rheum Dis 2006;65:1038-1043.
6. Chen J, Liu C. Sulfasalazine for ankylosing spondylitis. Cochrane Database Syst Rev 2005;(2):CD004800.-
7. Chen J, Liu C, Lin J. Methotrexate for ankylosing spondylitis. Cochrane Database Syst Rev 2006;(4):CD004524.-
8. Heijde D, Dijkmans B, Geusens P, et al. efficacy and safety of infliximab in patients with ankylosing spondylitis. Arthritis Rheum 2005;52:582-591.
9. Calin A, Dijkmans BA, Emery P, et al. Outcomes of a multicentre randomized clinical trial of etanercept to treat ankylosing spondylitis. Ann Rheum Dis 2004;63:1594-1600.
10. Grout K, Rasmussen N, Bacon P, et al. randomized trial of cyclophosphamide versus methotrexate for induction of remission in early systemic antineutrophil cytoplasmic antibody-associated vasculitis. Arthritis Rheum 2005;52:2461-2469.
11. Jover JA, Hernandez-Garcia C, Morado IC, et al. Combined treatment of giant-cell arteritis with methotrexate and prednisone. a randomized, double blinded, placebo-controlled trial. Ann Intern Med 2001;134:106-114.
12. Hoffman GS, Cid MC, Hellmann DB, et al. A multicenter, randomized, double-blind, placebo-controlled trial of adjuvant methotrexate treatment for giant cell arteritis. Arthritis Rheum 2002;46:1309-1318.
13. Sankar V, Brennan MT, Kok MR, et al. etanercept in sjögren’s syndrome: a twelve-week randomized, double-blind, placebo-controlled pilot clinical trial. Arthritis Rheum 2004;50:2240-2245.
14. Wegener’s Granulomatosis etanercept Trial (WGeT) research Group. etanercept plus standard therapy for Wegener’s granulomatosis. N Engl J Med 2005;352:351-361.
15. Salvarani C, Olivieri I, Pipitone N, et al. recommendations of the Italian society for rheumatology for the use of biologic (TNF-alpha blocking) agents in the treatment of psoriatic arthritis. Clin Exp Rheumatol 2006;24:70-78.
16. Zochling J, van der Heijde D, Dougados, et al. Current evidence for the management of ankylosing spondylitis: a systematic literature review for the AsAs/eulAr management recommendations in ankylosing spondylitis. Ann Rheum Dis 2006;65:442-452.
It’s unclear whether disease-modifying antirheumatic agents (DMARDs) as first-line therapy in nonrheumatoid rheumatologic diseases are effective because the question has not been studied. As second-line therapy, the use of some DmArDs appears to be beneficial for patients with psoriatic arthritis (strength of recommendation [SOR]: A, based on systematic reviews of good-quality randomized controlled trials) and ankylosing spondylitis (SOR: B, based on systematic reviews of moderate quality trials). Data on the safety and efficacy of DMARDs as second-line therapy for other arthritic conditions is limited (SOR: C, based on small prospective cohort trials).
There are many options, but remember the risks
Richard Hoffman, MD
Chesterfield Family Medicine Residency, Richmond, Va
Traditionally, nonsteroidal anti-inflammatory agents (NSAIDs) have been the mainstay of treatment for rheumatologic disorders other than rheumatoid arthritis. methotrexate has been used in psoriatic arthritis because it also controls the skin disorder; sulfasalazine has been used in arthritis associated with inflammatory bowel disease, as it helps the bowel disorder itself. However, little evidence shows a definitive benefit for the arthritis.
The advent of tumor necrosis factor (TNF) blockers has changed the direction of research in this area; these agents are being used more and more in inflammatory arthritides. While staying up to date on the TNF antagonists, it’s important to remember the complications associated with them—particularly the increased risk of infections and increased propensity for neoplastic disorders. Consider those on TNF blockers as relatively immunosuppressed (number needed to harm [NNH]=59 for infection and 154 for malignancy).1
Psoriatic arthritis affecting the joints and nails
Evidence summary
The use of DMARDs has become standard of care for rheumatoid arthritis, for both therapy and prevention of progression of this debilitating disease. However, the use of DMARDs in nonrheumatoid rheumatologic disease is still under investigation, and at this point, the use of DMARDs as first-line therapy is not recommended; however, second-line therapy with DMARDs is common.
For psoriatic arthritis, DMARDs are beneficial as a second-line therapy
A Cochrane systematic review identified 13 randomized controlled trials enrolling a combined 1022 patients with psoriatic arthritis randomly assigned to receive a DMARD—methotrexate, sulfasalazine (Azulfidine), azathioprine (Imuran/ Azasan), or etretinate (Tegison; no longer available in the US)—compared with placebo.2 All agents were better than placebo; however, only 2 agents (parenteral high-dose methotrexate and sulfasalazine) had clinically important benefits for more than half the patients. The studies were too small to establish toxicity or to evaluate the other agents.
NSAIDs are still the preferred first-line therapy, concluded a recent publication on the treatment of psoriatic arthritis, which looked at 54 different studies; however, second-line therapy could include methotrexate, sulfasalazine, etanercept (Enbrel), infliximab (Remicade), cyclosporine, or combination therapy.3 Sulfasalazine appeared to be clinically beneficial for peripheral psoriatic arthritis.
Etanercept vs placebo. An initial study (60 patients) of etanercept vs placebo among patients who were permitted to stay on methotrexate or prednisone showed a response rate of 87% vs 23% (P<.0001; number needed to treat [NNT]=1.56).4
Infliximab vs placebo. A study of infliximab vs placebo involving 104 patients had similar results, with good response in 65% vs 10% (NNT=1.81) at 16 weeks; infliximab also inhibited radiographic progression by 22%.5
Cyclosporine. Although it is effective, reserve cyclosporine for patients who do not improve on other regimens, because of its nephrotoxicity.3
DMARDs show some benefit in treating ankylosing spondylitis
Two recent Cochrane systematic reviews on ankylosing spondylitis examined the use of sulfasalazine and methotrexate as second-line agents.6,7 Eleven trials were included in the sulfasalazine analysis, with a total of 895 patients. Sulfasalazine demonstrated some benefit in reducing erythrocyte sedimentation rates (ESRs) and morning stiffness, but there was no evidence that the drug reduced pain or improved physical function, spinal mobility, or rate of enthesitis. Sulfasalazine was well tolerated and may be useful in early mild disease for patients with peripheral arthritis and high ESRs. On the other hand, evidence was insufficient to determine whether methotrexate benefited patients with ankylosing spondylitis.
In other trials, infliximab and etanercept showed good potential for benefit in treating ankylosing spondylitis.
One study of infliximab vs placebo showed 61.2% vs 19.2% patients with good clinical benefit at 24 weeks and only mild or moderate adverse events (P<.001; NNT=2.38).8
Similarly, a smaller study (84 patients) showed that 60% of patients on etanercept vs 20% on placebo had good clinical benefit at only 12 weeks (P<.001, NNT=2.5).9
For other rheumatic diseases, studies are mixed
Due to cyclosporine’s toxicity, less toxic DMARDs are being evaluated to replace it for treatment of other rheumatic diseases. A recent randomized controlled trial of 100 patients with antineutrophil cytoplasmic antibody–associated systemic vasculitis showed methotrexate may be able to replace cyclosporine for both induction of remission (methotrexate=89.8% vs cyclosporine=93.5%; P=.041) and maintenance of remission (69.5% vs 46.5% at 18 months; P=.023).10
Initial trials on other rheumatic diseases have been small and have had varied results. There are mixed studies on the effectiveness of adding methotrexate to corticosteroids for giant cell arteritis.11,12
There has been no evidence of efficacy for the new TNF antagonists in either a small study on Sjögren’s syndrome (n=14)13 or a larger study on Wegener’s granulomatosis (n=180).14
The studies for use of DMARDs in lupus or scleroderma are of limited quality.
Recommendations from others
The Italian Society for Rheumatology consensus guidelines recommends TNF antagonists be considered in active psoriatic arthritis resistant to (a) NSAIDs, (b) at least 2 local steroid injections, and (c) at least 2 conventional DMARDs for patients with peripheral arthritis or enthesitis. They also recommend TNF antagonists be considered for psoriatic spondylitis resistant to NSAIDs.15
The Assessment in Ankylosing Spondylitis (ASAS) International Working Group and the European League Against Rheumatism (EULAR) recommendations for the treatment of ankylosing spondylitis, based on a systematic review of the literature and expert opinion, indicate that:
- There is good evidence for using NSAIDs and COX-2 inhibitors for symptomatic treatment.
- Conventional DMARDs are not well supported.
- TNF antagonists show a large benefit in both pain and function.
The ASAS/EULAR recommendation indicate that there is no evidence that any of these treatments actually modify the disease progression.16
Acknowledgments
The opinions and assertions contained herein are the private views of the authors and not to be construed as official, or as reflecting the views of the US Air Force medical service or the US Air Force at large.
It’s unclear whether disease-modifying antirheumatic agents (DMARDs) as first-line therapy in nonrheumatoid rheumatologic diseases are effective because the question has not been studied. As second-line therapy, the use of some DmArDs appears to be beneficial for patients with psoriatic arthritis (strength of recommendation [SOR]: A, based on systematic reviews of good-quality randomized controlled trials) and ankylosing spondylitis (SOR: B, based on systematic reviews of moderate quality trials). Data on the safety and efficacy of DMARDs as second-line therapy for other arthritic conditions is limited (SOR: C, based on small prospective cohort trials).
There are many options, but remember the risks
Richard Hoffman, MD
Chesterfield Family Medicine Residency, Richmond, Va
Traditionally, nonsteroidal anti-inflammatory agents (NSAIDs) have been the mainstay of treatment for rheumatologic disorders other than rheumatoid arthritis. methotrexate has been used in psoriatic arthritis because it also controls the skin disorder; sulfasalazine has been used in arthritis associated with inflammatory bowel disease, as it helps the bowel disorder itself. However, little evidence shows a definitive benefit for the arthritis.
The advent of tumor necrosis factor (TNF) blockers has changed the direction of research in this area; these agents are being used more and more in inflammatory arthritides. While staying up to date on the TNF antagonists, it’s important to remember the complications associated with them—particularly the increased risk of infections and increased propensity for neoplastic disorders. Consider those on TNF blockers as relatively immunosuppressed (number needed to harm [NNH]=59 for infection and 154 for malignancy).1
Psoriatic arthritis affecting the joints and nails
Evidence summary
The use of DMARDs has become standard of care for rheumatoid arthritis, for both therapy and prevention of progression of this debilitating disease. However, the use of DMARDs in nonrheumatoid rheumatologic disease is still under investigation, and at this point, the use of DMARDs as first-line therapy is not recommended; however, second-line therapy with DMARDs is common.
For psoriatic arthritis, DMARDs are beneficial as a second-line therapy
A Cochrane systematic review identified 13 randomized controlled trials enrolling a combined 1022 patients with psoriatic arthritis randomly assigned to receive a DMARD—methotrexate, sulfasalazine (Azulfidine), azathioprine (Imuran/ Azasan), or etretinate (Tegison; no longer available in the US)—compared with placebo.2 All agents were better than placebo; however, only 2 agents (parenteral high-dose methotrexate and sulfasalazine) had clinically important benefits for more than half the patients. The studies were too small to establish toxicity or to evaluate the other agents.
NSAIDs are still the preferred first-line therapy, concluded a recent publication on the treatment of psoriatic arthritis, which looked at 54 different studies; however, second-line therapy could include methotrexate, sulfasalazine, etanercept (Enbrel), infliximab (Remicade), cyclosporine, or combination therapy.3 Sulfasalazine appeared to be clinically beneficial for peripheral psoriatic arthritis.
Etanercept vs placebo. An initial study (60 patients) of etanercept vs placebo among patients who were permitted to stay on methotrexate or prednisone showed a response rate of 87% vs 23% (P<.0001; number needed to treat [NNT]=1.56).4
Infliximab vs placebo. A study of infliximab vs placebo involving 104 patients had similar results, with good response in 65% vs 10% (NNT=1.81) at 16 weeks; infliximab also inhibited radiographic progression by 22%.5
Cyclosporine. Although it is effective, reserve cyclosporine for patients who do not improve on other regimens, because of its nephrotoxicity.3
DMARDs show some benefit in treating ankylosing spondylitis
Two recent Cochrane systematic reviews on ankylosing spondylitis examined the use of sulfasalazine and methotrexate as second-line agents.6,7 Eleven trials were included in the sulfasalazine analysis, with a total of 895 patients. Sulfasalazine demonstrated some benefit in reducing erythrocyte sedimentation rates (ESRs) and morning stiffness, but there was no evidence that the drug reduced pain or improved physical function, spinal mobility, or rate of enthesitis. Sulfasalazine was well tolerated and may be useful in early mild disease for patients with peripheral arthritis and high ESRs. On the other hand, evidence was insufficient to determine whether methotrexate benefited patients with ankylosing spondylitis.
In other trials, infliximab and etanercept showed good potential for benefit in treating ankylosing spondylitis.
One study of infliximab vs placebo showed 61.2% vs 19.2% patients with good clinical benefit at 24 weeks and only mild or moderate adverse events (P<.001; NNT=2.38).8
Similarly, a smaller study (84 patients) showed that 60% of patients on etanercept vs 20% on placebo had good clinical benefit at only 12 weeks (P<.001, NNT=2.5).9
For other rheumatic diseases, studies are mixed
Due to cyclosporine’s toxicity, less toxic DMARDs are being evaluated to replace it for treatment of other rheumatic diseases. A recent randomized controlled trial of 100 patients with antineutrophil cytoplasmic antibody–associated systemic vasculitis showed methotrexate may be able to replace cyclosporine for both induction of remission (methotrexate=89.8% vs cyclosporine=93.5%; P=.041) and maintenance of remission (69.5% vs 46.5% at 18 months; P=.023).10
Initial trials on other rheumatic diseases have been small and have had varied results. There are mixed studies on the effectiveness of adding methotrexate to corticosteroids for giant cell arteritis.11,12
There has been no evidence of efficacy for the new TNF antagonists in either a small study on Sjögren’s syndrome (n=14)13 or a larger study on Wegener’s granulomatosis (n=180).14
The studies for use of DMARDs in lupus or scleroderma are of limited quality.
Recommendations from others
The Italian Society for Rheumatology consensus guidelines recommends TNF antagonists be considered in active psoriatic arthritis resistant to (a) NSAIDs, (b) at least 2 local steroid injections, and (c) at least 2 conventional DMARDs for patients with peripheral arthritis or enthesitis. They also recommend TNF antagonists be considered for psoriatic spondylitis resistant to NSAIDs.15
The Assessment in Ankylosing Spondylitis (ASAS) International Working Group and the European League Against Rheumatism (EULAR) recommendations for the treatment of ankylosing spondylitis, based on a systematic review of the literature and expert opinion, indicate that:
- There is good evidence for using NSAIDs and COX-2 inhibitors for symptomatic treatment.
- Conventional DMARDs are not well supported.
- TNF antagonists show a large benefit in both pain and function.
The ASAS/EULAR recommendation indicate that there is no evidence that any of these treatments actually modify the disease progression.16
Acknowledgments
The opinions and assertions contained herein are the private views of the authors and not to be construed as official, or as reflecting the views of the US Air Force medical service or the US Air Force at large.
1. Bongartz T, sutton AJ, Sweeting MJ, et al. Anti-TNF antibody therapy in rheumatoid arthritis and the risk of serious infections and malignancies: systematic review and meta-analysis of rare harmful effects in randomized controlled trials. JAMA 2006;295:2275-2285.
2. Jones G, Crotty M, Brooks P. Interventions for treating psoriatic arthritis. Cochrane Database Syst Rev 2000 (3):CD000212.
3. Manadan Am, Sequeira W, Block JA. The treatment of psoriatic arthritis. Am J Ther 2006;13:72-79.
4. Mease PJ, Goffe BS, Metz J, et al. etanercept in the treatment of psoriatic arthritis and psoriasis: a randomized trial. Lancet 2000;356:385-390.
5. Kavanaugh A, Antoni CE, Gladman D, et al. The Infliximab multinational Psoriatic Arthritis Controlled Trial (ImPACT): result of radiographic analyses after 1 year. Ann Rheum Dis 2006;65:1038-1043.
6. Chen J, Liu C. Sulfasalazine for ankylosing spondylitis. Cochrane Database Syst Rev 2005;(2):CD004800.-
7. Chen J, Liu C, Lin J. Methotrexate for ankylosing spondylitis. Cochrane Database Syst Rev 2006;(4):CD004524.-
8. Heijde D, Dijkmans B, Geusens P, et al. efficacy and safety of infliximab in patients with ankylosing spondylitis. Arthritis Rheum 2005;52:582-591.
9. Calin A, Dijkmans BA, Emery P, et al. Outcomes of a multicentre randomized clinical trial of etanercept to treat ankylosing spondylitis. Ann Rheum Dis 2004;63:1594-1600.
10. Grout K, Rasmussen N, Bacon P, et al. randomized trial of cyclophosphamide versus methotrexate for induction of remission in early systemic antineutrophil cytoplasmic antibody-associated vasculitis. Arthritis Rheum 2005;52:2461-2469.
11. Jover JA, Hernandez-Garcia C, Morado IC, et al. Combined treatment of giant-cell arteritis with methotrexate and prednisone. a randomized, double blinded, placebo-controlled trial. Ann Intern Med 2001;134:106-114.
12. Hoffman GS, Cid MC, Hellmann DB, et al. A multicenter, randomized, double-blind, placebo-controlled trial of adjuvant methotrexate treatment for giant cell arteritis. Arthritis Rheum 2002;46:1309-1318.
13. Sankar V, Brennan MT, Kok MR, et al. etanercept in sjögren’s syndrome: a twelve-week randomized, double-blind, placebo-controlled pilot clinical trial. Arthritis Rheum 2004;50:2240-2245.
14. Wegener’s Granulomatosis etanercept Trial (WGeT) research Group. etanercept plus standard therapy for Wegener’s granulomatosis. N Engl J Med 2005;352:351-361.
15. Salvarani C, Olivieri I, Pipitone N, et al. recommendations of the Italian society for rheumatology for the use of biologic (TNF-alpha blocking) agents in the treatment of psoriatic arthritis. Clin Exp Rheumatol 2006;24:70-78.
16. Zochling J, van der Heijde D, Dougados, et al. Current evidence for the management of ankylosing spondylitis: a systematic literature review for the AsAs/eulAr management recommendations in ankylosing spondylitis. Ann Rheum Dis 2006;65:442-452.
1. Bongartz T, sutton AJ, Sweeting MJ, et al. Anti-TNF antibody therapy in rheumatoid arthritis and the risk of serious infections and malignancies: systematic review and meta-analysis of rare harmful effects in randomized controlled trials. JAMA 2006;295:2275-2285.
2. Jones G, Crotty M, Brooks P. Interventions for treating psoriatic arthritis. Cochrane Database Syst Rev 2000 (3):CD000212.
3. Manadan Am, Sequeira W, Block JA. The treatment of psoriatic arthritis. Am J Ther 2006;13:72-79.
4. Mease PJ, Goffe BS, Metz J, et al. etanercept in the treatment of psoriatic arthritis and psoriasis: a randomized trial. Lancet 2000;356:385-390.
5. Kavanaugh A, Antoni CE, Gladman D, et al. The Infliximab multinational Psoriatic Arthritis Controlled Trial (ImPACT): result of radiographic analyses after 1 year. Ann Rheum Dis 2006;65:1038-1043.
6. Chen J, Liu C. Sulfasalazine for ankylosing spondylitis. Cochrane Database Syst Rev 2005;(2):CD004800.-
7. Chen J, Liu C, Lin J. Methotrexate for ankylosing spondylitis. Cochrane Database Syst Rev 2006;(4):CD004524.-
8. Heijde D, Dijkmans B, Geusens P, et al. efficacy and safety of infliximab in patients with ankylosing spondylitis. Arthritis Rheum 2005;52:582-591.
9. Calin A, Dijkmans BA, Emery P, et al. Outcomes of a multicentre randomized clinical trial of etanercept to treat ankylosing spondylitis. Ann Rheum Dis 2004;63:1594-1600.
10. Grout K, Rasmussen N, Bacon P, et al. randomized trial of cyclophosphamide versus methotrexate for induction of remission in early systemic antineutrophil cytoplasmic antibody-associated vasculitis. Arthritis Rheum 2005;52:2461-2469.
11. Jover JA, Hernandez-Garcia C, Morado IC, et al. Combined treatment of giant-cell arteritis with methotrexate and prednisone. a randomized, double blinded, placebo-controlled trial. Ann Intern Med 2001;134:106-114.
12. Hoffman GS, Cid MC, Hellmann DB, et al. A multicenter, randomized, double-blind, placebo-controlled trial of adjuvant methotrexate treatment for giant cell arteritis. Arthritis Rheum 2002;46:1309-1318.
13. Sankar V, Brennan MT, Kok MR, et al. etanercept in sjögren’s syndrome: a twelve-week randomized, double-blind, placebo-controlled pilot clinical trial. Arthritis Rheum 2004;50:2240-2245.
14. Wegener’s Granulomatosis etanercept Trial (WGeT) research Group. etanercept plus standard therapy for Wegener’s granulomatosis. N Engl J Med 2005;352:351-361.
15. Salvarani C, Olivieri I, Pipitone N, et al. recommendations of the Italian society for rheumatology for the use of biologic (TNF-alpha blocking) agents in the treatment of psoriatic arthritis. Clin Exp Rheumatol 2006;24:70-78.
16. Zochling J, van der Heijde D, Dougados, et al. Current evidence for the management of ankylosing spondylitis: a systematic literature review for the AsAs/eulAr management recommendations in ankylosing spondylitis. Ann Rheum Dis 2006;65:442-452.
Evidence-based answers from the Family Physicians Inquiries Network
How should you further evaluate an adult with a testicular mass?
Perform a scrotal ultrasonography immediately to determine whether emergency surgery is necessary for patients with an exam or history that suggests testicular torsion or rupture (strength of recommendation [SOR]: B, based on cohort trials of patient oriented outcomes). In less urgent cases, ultrasound is also useful for verifying diagnoses made by physical exam, and to exclude conditions such as neoplasm, for which further workup is indicated (SOR: C, based on expert opinion).
In those cases in which ultrasound and clinical exam are inconclusive or conflicting, magnetic resonance imaging (MRI) can provide additional information to improve management and decrease unnecessary surgery (SOR: B, based on cohort trials of patient-oriented outcomes).
Acutely painful testicle? Involve a radiologist and urologist early on
Peter C. Smith, MD
Rose Family Medicine Residency, University of Colorado Health Sciences Center, Denver
One of the keys to managing testicular masses is to differentiate normal anatomical structures and benign peritesticular pathology (such as varicoceles and spermatoceles) from true testicular masses. Early in my career, after I counseled men to do testicular self-exams, they occasionally made return visits concerned about a mass. These were almost always the testicular appendix, the epididymis, or scrotal inclusion cysts. I now describe these findings as a routine part of my counseling. Given the devastating consequences of a missed or delayed diagnosis of torsion, infarction, and cancer, I always make 2 phone calls early on when a patient has an acutely painful testicle or a true testicular mass: I call the radiologist and the urologist. These 2 phone calls can substantially reduce the risk of diagnostic delay.
Evidence summary
A wide variety of conditions can cause scrotal masses (see TABLE 1 for a list of causes of acute scrotal swelling and TABLE 2 for causes of nonacute swelling).1,2 Many just require that you reassure the patient; however, some conditions do need diagnostic testing to determine appropriate treatment.
TABLE 1
Causes of acute scrotal swelling1,2
| CONDITION | CLINICAL PRESENTATION | PHYSICAL EXAM/CLINICAL COMMENTS |
|---|---|---|
| Epididymitis | • Severe swelling and pain | • Edema, tenderness, erythema • Positive urinalysis because it’s often associated with urinary tract infection or prostatitis • Can result in abscess formation |
| Testicular torsion | • Severe pain sudden in onset (except in neonates) | • Usually occurs in post-pubertal and neonatal age group • Often presents with an asymmetric high riding testis or transverse orientation of affected testis • Cremasteric reflex usually absent • Not relieved with elevation • Surgical emergency |
| Trauma | • Associated with wide spectrum of injuries | • May result in testicular rupture or torsion, which are surgical emergencies |
| Torsion of appendix testis | • Gradual onset of pain | • Usually pre-pubertal age group • Cremasteric reflex preserved • Tenderness often localized to anterosuperior testes • Surgery not required in majority of cases |
| Inguinal hernia | • Pain and swelling | • May hear bowel sounds on affected side |
TABLE 2
Causes of nonacute scrotal swelling1
| CONDITION | CLINICAL PRESENTATION | PHYSICAL EXAM/CLINICAL COMMENTS |
|---|---|---|
| Hydrocele | • Painless mass that may increase in size throughout the day | • Can be transilluminated • Reactive hydrocele may be associated with testicular neoplasm, epididymitis, orchitis, or torsion |
| Testicular cyst | • None | • Benign incidental finding • Nonpalpable |
| Varicocele | • Scrotal swelling secondary to dilation of spermatic veins • May present as infertility • May present with pain if intratesticular | • Usually left-sided • Described as a bag of worms superior to the testicle • Noticeable when standing or with Valsalva maneuver |
| Spermatocele | • If painful, relieved with elevation | • Often an incidental finding on exam • Freely mobile • Usually located in epididymal head |
| Epidermoid cyst | • Painless mass | • Found anywhere in epididymis • Often surgically removed because it may be difficult to differentiate from malignancy |
| Primary testicular tumor1,9 | • Solid mass • Classically painless but may produce testicular discomfort | • 10% present acutely with hemorrhage • Most common malignancy in males between ages 18 and 40 |
| Metastatic tumor | • Painless mass | • Possible primary cancers include leukemia lymphoma, melanoma, lung, prostate, kidney, GI tract |
Ultrasound is the best initial test
Testicular torsion and acute epididymoorchitis are the most common causes of an acute scrotum.3 Patients with an acute scrotum require an urgent ultrasound to exclude pathology that requires immediate surgery (TABLES 1 AND 2).1 Although clinical exam identifies almost all cases of torsion, a few cases are missed.4 In a study of 209 emergency scrotal explorations, clinical exam by general practitioners and surgeons correctly diagnosed only 92.5% and 94% of testicular torsion cases, respectively, compared with the surgical diagnosis.4
In another study, which used surgery as the diagnostic gold standard, color Doppler ultrasound had a sensitivity of 93.5% for the diagnosis of testicular torsion;5 this has led some to say the combination of both clinical exam and ultrasound should be used to determine the need for surgery.1 However, this combination has not been thoroughly evaluated by researchers, and the best evidence shows that physician exam is essentially the same as color Doppler ultrasound for diagnosing testicular torsion. If torsion cannot be reliably excluded, emergent surgical exploration is mandatory.4
For patients who have a nonacute scrotal mass, ultrasound is often indicated to distinguish intratesticular from extratesticular masses.1 Although testicular neoplasm is relatively rare, it is a concern for patients with non-painful masses. Fortunately, false-negative scrotal ultrasounds are rare. In a small study comparing clinical exam with ultrasound for diagnosis of testicular tumor, the negative predictive value of ultrasound was 100%.6
Although ultrasound has high sensitivity for detection of testicular neoplasm, it cannot differentiate benign from malignant tumors.2 Additionally, ultrasound sometimes fails to differentiate a neoplastic process from a complication of an infection such as an abscess. In those instances, a repeat ultrasound is suggested after antibiotic administration to ensure resolution of the mass.2
When ultrasound is inconclusive, MRI may be helpful
When clinical and ultrasound findings are inconclusive, MRI may help deter-mine a diagnosis. For example, MRI can help distinguish inflammation or abscess from neoplasm, thus preventing a patient from undergoing unnecessary surgical intervention.2,7 If testicular neoplasm cannot be excluded based on clinical and radiographic findings, surgery is indicated.1
Recommendations from others
Few current evidence-based recommendations exist on the approach to patients with scrotal masses. The National Collaborating Centre for Primary Care (UK) suggests an urgent ultrasound when a scrotal mass does not transilluminate or when the examiner cannot distinguish the body of the testis.8
1. Micallef M, Torreggiani WC, Hurley M, Dinsmore WW, Hogan B. The ultrasound investigation of scrotal swelling. Int J STD AIDS 2000;11:297-302.
2. Eyre RC. Evaluation of nonacute scrotal pathology in adults. In: Rose BD, ed. UpToDate [online database]. Version 14.1. Waltham, Mass: UpToDate; 2006.
3. Akin EA, Khati NJ, Hill MC. Ultrasound of the scrotum. Ultrasound Q 2004;20:181-200.
4. Watkin NA, Reiger NA, Moisey CU. Is the conservative management of the acute scrotum justified on clinical grounds? Br J Urol 1996;78:623-627.
5. Andipa E, Liberopoulos K, Asvestis C. Magnetic resonance imaging and ultrasound evaluation of penile and testicular masses. World J Urol 2004;22:382-391.
6. van Dijk R, Doesburg WH, Verbeek AL, van der Schouw YT, Debruyne FM, Rosenbusch G. Ultrasonography versus clinical examination in evaluation of testicular tumors. J Clin Ultrasound 1994;22:179-182.
7. Muglia V, Tucci S, Jr, Elias J, Jr, Trad CS, Bilbey J, Cooperberg PL. Magnetic resonance imaging of scrotal diseases: when it makes the difference. Urology 2002;59:419-423.
8. National Collaborating Centre for Primary Care Referral guidelines for suspected cancer. London: National Institute for Health and Clinical Excellence; June 2005. NICE Clinical Guideline 27. Available at www.nice.org.uk/page.aspx?o=261649. Accessed on August 29, 2007.
Perform a scrotal ultrasonography immediately to determine whether emergency surgery is necessary for patients with an exam or history that suggests testicular torsion or rupture (strength of recommendation [SOR]: B, based on cohort trials of patient oriented outcomes). In less urgent cases, ultrasound is also useful for verifying diagnoses made by physical exam, and to exclude conditions such as neoplasm, for which further workup is indicated (SOR: C, based on expert opinion).
In those cases in which ultrasound and clinical exam are inconclusive or conflicting, magnetic resonance imaging (MRI) can provide additional information to improve management and decrease unnecessary surgery (SOR: B, based on cohort trials of patient-oriented outcomes).
Acutely painful testicle? Involve a radiologist and urologist early on
Peter C. Smith, MD
Rose Family Medicine Residency, University of Colorado Health Sciences Center, Denver
One of the keys to managing testicular masses is to differentiate normal anatomical structures and benign peritesticular pathology (such as varicoceles and spermatoceles) from true testicular masses. Early in my career, after I counseled men to do testicular self-exams, they occasionally made return visits concerned about a mass. These were almost always the testicular appendix, the epididymis, or scrotal inclusion cysts. I now describe these findings as a routine part of my counseling. Given the devastating consequences of a missed or delayed diagnosis of torsion, infarction, and cancer, I always make 2 phone calls early on when a patient has an acutely painful testicle or a true testicular mass: I call the radiologist and the urologist. These 2 phone calls can substantially reduce the risk of diagnostic delay.
Evidence summary
A wide variety of conditions can cause scrotal masses (see TABLE 1 for a list of causes of acute scrotal swelling and TABLE 2 for causes of nonacute swelling).1,2 Many just require that you reassure the patient; however, some conditions do need diagnostic testing to determine appropriate treatment.
TABLE 1
Causes of acute scrotal swelling1,2
| CONDITION | CLINICAL PRESENTATION | PHYSICAL EXAM/CLINICAL COMMENTS |
|---|---|---|
| Epididymitis | • Severe swelling and pain | • Edema, tenderness, erythema • Positive urinalysis because it’s often associated with urinary tract infection or prostatitis • Can result in abscess formation |
| Testicular torsion | • Severe pain sudden in onset (except in neonates) | • Usually occurs in post-pubertal and neonatal age group • Often presents with an asymmetric high riding testis or transverse orientation of affected testis • Cremasteric reflex usually absent • Not relieved with elevation • Surgical emergency |
| Trauma | • Associated with wide spectrum of injuries | • May result in testicular rupture or torsion, which are surgical emergencies |
| Torsion of appendix testis | • Gradual onset of pain | • Usually pre-pubertal age group • Cremasteric reflex preserved • Tenderness often localized to anterosuperior testes • Surgery not required in majority of cases |
| Inguinal hernia | • Pain and swelling | • May hear bowel sounds on affected side |
TABLE 2
Causes of nonacute scrotal swelling1
| CONDITION | CLINICAL PRESENTATION | PHYSICAL EXAM/CLINICAL COMMENTS |
|---|---|---|
| Hydrocele | • Painless mass that may increase in size throughout the day | • Can be transilluminated • Reactive hydrocele may be associated with testicular neoplasm, epididymitis, orchitis, or torsion |
| Testicular cyst | • None | • Benign incidental finding • Nonpalpable |
| Varicocele | • Scrotal swelling secondary to dilation of spermatic veins • May present as infertility • May present with pain if intratesticular | • Usually left-sided • Described as a bag of worms superior to the testicle • Noticeable when standing or with Valsalva maneuver |
| Spermatocele | • If painful, relieved with elevation | • Often an incidental finding on exam • Freely mobile • Usually located in epididymal head |
| Epidermoid cyst | • Painless mass | • Found anywhere in epididymis • Often surgically removed because it may be difficult to differentiate from malignancy |
| Primary testicular tumor1,9 | • Solid mass • Classically painless but may produce testicular discomfort | • 10% present acutely with hemorrhage • Most common malignancy in males between ages 18 and 40 |
| Metastatic tumor | • Painless mass | • Possible primary cancers include leukemia lymphoma, melanoma, lung, prostate, kidney, GI tract |
Ultrasound is the best initial test
Testicular torsion and acute epididymoorchitis are the most common causes of an acute scrotum.3 Patients with an acute scrotum require an urgent ultrasound to exclude pathology that requires immediate surgery (TABLES 1 AND 2).1 Although clinical exam identifies almost all cases of torsion, a few cases are missed.4 In a study of 209 emergency scrotal explorations, clinical exam by general practitioners and surgeons correctly diagnosed only 92.5% and 94% of testicular torsion cases, respectively, compared with the surgical diagnosis.4
In another study, which used surgery as the diagnostic gold standard, color Doppler ultrasound had a sensitivity of 93.5% for the diagnosis of testicular torsion;5 this has led some to say the combination of both clinical exam and ultrasound should be used to determine the need for surgery.1 However, this combination has not been thoroughly evaluated by researchers, and the best evidence shows that physician exam is essentially the same as color Doppler ultrasound for diagnosing testicular torsion. If torsion cannot be reliably excluded, emergent surgical exploration is mandatory.4
For patients who have a nonacute scrotal mass, ultrasound is often indicated to distinguish intratesticular from extratesticular masses.1 Although testicular neoplasm is relatively rare, it is a concern for patients with non-painful masses. Fortunately, false-negative scrotal ultrasounds are rare. In a small study comparing clinical exam with ultrasound for diagnosis of testicular tumor, the negative predictive value of ultrasound was 100%.6
Although ultrasound has high sensitivity for detection of testicular neoplasm, it cannot differentiate benign from malignant tumors.2 Additionally, ultrasound sometimes fails to differentiate a neoplastic process from a complication of an infection such as an abscess. In those instances, a repeat ultrasound is suggested after antibiotic administration to ensure resolution of the mass.2
When ultrasound is inconclusive, MRI may be helpful
When clinical and ultrasound findings are inconclusive, MRI may help deter-mine a diagnosis. For example, MRI can help distinguish inflammation or abscess from neoplasm, thus preventing a patient from undergoing unnecessary surgical intervention.2,7 If testicular neoplasm cannot be excluded based on clinical and radiographic findings, surgery is indicated.1
Recommendations from others
Few current evidence-based recommendations exist on the approach to patients with scrotal masses. The National Collaborating Centre for Primary Care (UK) suggests an urgent ultrasound when a scrotal mass does not transilluminate or when the examiner cannot distinguish the body of the testis.8
Perform a scrotal ultrasonography immediately to determine whether emergency surgery is necessary for patients with an exam or history that suggests testicular torsion or rupture (strength of recommendation [SOR]: B, based on cohort trials of patient oriented outcomes). In less urgent cases, ultrasound is also useful for verifying diagnoses made by physical exam, and to exclude conditions such as neoplasm, for which further workup is indicated (SOR: C, based on expert opinion).
In those cases in which ultrasound and clinical exam are inconclusive or conflicting, magnetic resonance imaging (MRI) can provide additional information to improve management and decrease unnecessary surgery (SOR: B, based on cohort trials of patient-oriented outcomes).
Acutely painful testicle? Involve a radiologist and urologist early on
Peter C. Smith, MD
Rose Family Medicine Residency, University of Colorado Health Sciences Center, Denver
One of the keys to managing testicular masses is to differentiate normal anatomical structures and benign peritesticular pathology (such as varicoceles and spermatoceles) from true testicular masses. Early in my career, after I counseled men to do testicular self-exams, they occasionally made return visits concerned about a mass. These were almost always the testicular appendix, the epididymis, or scrotal inclusion cysts. I now describe these findings as a routine part of my counseling. Given the devastating consequences of a missed or delayed diagnosis of torsion, infarction, and cancer, I always make 2 phone calls early on when a patient has an acutely painful testicle or a true testicular mass: I call the radiologist and the urologist. These 2 phone calls can substantially reduce the risk of diagnostic delay.
Evidence summary
A wide variety of conditions can cause scrotal masses (see TABLE 1 for a list of causes of acute scrotal swelling and TABLE 2 for causes of nonacute swelling).1,2 Many just require that you reassure the patient; however, some conditions do need diagnostic testing to determine appropriate treatment.
TABLE 1
Causes of acute scrotal swelling1,2
| CONDITION | CLINICAL PRESENTATION | PHYSICAL EXAM/CLINICAL COMMENTS |
|---|---|---|
| Epididymitis | • Severe swelling and pain | • Edema, tenderness, erythema • Positive urinalysis because it’s often associated with urinary tract infection or prostatitis • Can result in abscess formation |
| Testicular torsion | • Severe pain sudden in onset (except in neonates) | • Usually occurs in post-pubertal and neonatal age group • Often presents with an asymmetric high riding testis or transverse orientation of affected testis • Cremasteric reflex usually absent • Not relieved with elevation • Surgical emergency |
| Trauma | • Associated with wide spectrum of injuries | • May result in testicular rupture or torsion, which are surgical emergencies |
| Torsion of appendix testis | • Gradual onset of pain | • Usually pre-pubertal age group • Cremasteric reflex preserved • Tenderness often localized to anterosuperior testes • Surgery not required in majority of cases |
| Inguinal hernia | • Pain and swelling | • May hear bowel sounds on affected side |
TABLE 2
Causes of nonacute scrotal swelling1
| CONDITION | CLINICAL PRESENTATION | PHYSICAL EXAM/CLINICAL COMMENTS |
|---|---|---|
| Hydrocele | • Painless mass that may increase in size throughout the day | • Can be transilluminated • Reactive hydrocele may be associated with testicular neoplasm, epididymitis, orchitis, or torsion |
| Testicular cyst | • None | • Benign incidental finding • Nonpalpable |
| Varicocele | • Scrotal swelling secondary to dilation of spermatic veins • May present as infertility • May present with pain if intratesticular | • Usually left-sided • Described as a bag of worms superior to the testicle • Noticeable when standing or with Valsalva maneuver |
| Spermatocele | • If painful, relieved with elevation | • Often an incidental finding on exam • Freely mobile • Usually located in epididymal head |
| Epidermoid cyst | • Painless mass | • Found anywhere in epididymis • Often surgically removed because it may be difficult to differentiate from malignancy |
| Primary testicular tumor1,9 | • Solid mass • Classically painless but may produce testicular discomfort | • 10% present acutely with hemorrhage • Most common malignancy in males between ages 18 and 40 |
| Metastatic tumor | • Painless mass | • Possible primary cancers include leukemia lymphoma, melanoma, lung, prostate, kidney, GI tract |
Ultrasound is the best initial test
Testicular torsion and acute epididymoorchitis are the most common causes of an acute scrotum.3 Patients with an acute scrotum require an urgent ultrasound to exclude pathology that requires immediate surgery (TABLES 1 AND 2).1 Although clinical exam identifies almost all cases of torsion, a few cases are missed.4 In a study of 209 emergency scrotal explorations, clinical exam by general practitioners and surgeons correctly diagnosed only 92.5% and 94% of testicular torsion cases, respectively, compared with the surgical diagnosis.4
In another study, which used surgery as the diagnostic gold standard, color Doppler ultrasound had a sensitivity of 93.5% for the diagnosis of testicular torsion;5 this has led some to say the combination of both clinical exam and ultrasound should be used to determine the need for surgery.1 However, this combination has not been thoroughly evaluated by researchers, and the best evidence shows that physician exam is essentially the same as color Doppler ultrasound for diagnosing testicular torsion. If torsion cannot be reliably excluded, emergent surgical exploration is mandatory.4
For patients who have a nonacute scrotal mass, ultrasound is often indicated to distinguish intratesticular from extratesticular masses.1 Although testicular neoplasm is relatively rare, it is a concern for patients with non-painful masses. Fortunately, false-negative scrotal ultrasounds are rare. In a small study comparing clinical exam with ultrasound for diagnosis of testicular tumor, the negative predictive value of ultrasound was 100%.6
Although ultrasound has high sensitivity for detection of testicular neoplasm, it cannot differentiate benign from malignant tumors.2 Additionally, ultrasound sometimes fails to differentiate a neoplastic process from a complication of an infection such as an abscess. In those instances, a repeat ultrasound is suggested after antibiotic administration to ensure resolution of the mass.2
When ultrasound is inconclusive, MRI may be helpful
When clinical and ultrasound findings are inconclusive, MRI may help deter-mine a diagnosis. For example, MRI can help distinguish inflammation or abscess from neoplasm, thus preventing a patient from undergoing unnecessary surgical intervention.2,7 If testicular neoplasm cannot be excluded based on clinical and radiographic findings, surgery is indicated.1
Recommendations from others
Few current evidence-based recommendations exist on the approach to patients with scrotal masses. The National Collaborating Centre for Primary Care (UK) suggests an urgent ultrasound when a scrotal mass does not transilluminate or when the examiner cannot distinguish the body of the testis.8
1. Micallef M, Torreggiani WC, Hurley M, Dinsmore WW, Hogan B. The ultrasound investigation of scrotal swelling. Int J STD AIDS 2000;11:297-302.
2. Eyre RC. Evaluation of nonacute scrotal pathology in adults. In: Rose BD, ed. UpToDate [online database]. Version 14.1. Waltham, Mass: UpToDate; 2006.
3. Akin EA, Khati NJ, Hill MC. Ultrasound of the scrotum. Ultrasound Q 2004;20:181-200.
4. Watkin NA, Reiger NA, Moisey CU. Is the conservative management of the acute scrotum justified on clinical grounds? Br J Urol 1996;78:623-627.
5. Andipa E, Liberopoulos K, Asvestis C. Magnetic resonance imaging and ultrasound evaluation of penile and testicular masses. World J Urol 2004;22:382-391.
6. van Dijk R, Doesburg WH, Verbeek AL, van der Schouw YT, Debruyne FM, Rosenbusch G. Ultrasonography versus clinical examination in evaluation of testicular tumors. J Clin Ultrasound 1994;22:179-182.
7. Muglia V, Tucci S, Jr, Elias J, Jr, Trad CS, Bilbey J, Cooperberg PL. Magnetic resonance imaging of scrotal diseases: when it makes the difference. Urology 2002;59:419-423.
8. National Collaborating Centre for Primary Care Referral guidelines for suspected cancer. London: National Institute for Health and Clinical Excellence; June 2005. NICE Clinical Guideline 27. Available at www.nice.org.uk/page.aspx?o=261649. Accessed on August 29, 2007.
1. Micallef M, Torreggiani WC, Hurley M, Dinsmore WW, Hogan B. The ultrasound investigation of scrotal swelling. Int J STD AIDS 2000;11:297-302.
2. Eyre RC. Evaluation of nonacute scrotal pathology in adults. In: Rose BD, ed. UpToDate [online database]. Version 14.1. Waltham, Mass: UpToDate; 2006.
3. Akin EA, Khati NJ, Hill MC. Ultrasound of the scrotum. Ultrasound Q 2004;20:181-200.
4. Watkin NA, Reiger NA, Moisey CU. Is the conservative management of the acute scrotum justified on clinical grounds? Br J Urol 1996;78:623-627.
5. Andipa E, Liberopoulos K, Asvestis C. Magnetic resonance imaging and ultrasound evaluation of penile and testicular masses. World J Urol 2004;22:382-391.
6. van Dijk R, Doesburg WH, Verbeek AL, van der Schouw YT, Debruyne FM, Rosenbusch G. Ultrasonography versus clinical examination in evaluation of testicular tumors. J Clin Ultrasound 1994;22:179-182.
7. Muglia V, Tucci S, Jr, Elias J, Jr, Trad CS, Bilbey J, Cooperberg PL. Magnetic resonance imaging of scrotal diseases: when it makes the difference. Urology 2002;59:419-423.
8. National Collaborating Centre for Primary Care Referral guidelines for suspected cancer. London: National Institute for Health and Clinical Excellence; June 2005. NICE Clinical Guideline 27. Available at www.nice.org.uk/page.aspx?o=261649. Accessed on August 29, 2007.
Evidence-based answers from the Family Physicians Inquiries Network
What is the best approach to a solitary pulmonary nodule identified by chest x-ray?
Your initial risk assessment should include the patient’s smoking history, advancing age, cancer history, and chest radiography features (strength of recommendation [SOR]: A, based on a validated clinical decision rule). You’ll also need to review old chest radiographs (SOR: C, based on expert opinion). A solitary pulmonary nodule unchanged for >2 years on chest radiograph or containing benign central calcifications requires no further work-up (SOR: B, based on historical cohort studies).
While radiologists’ interpretations of a nodule’s calcification on chest radiograph and malignancy on computed tomography (CT) are incorrect in a substantial portion of cases (SOR: B, based on limited-quality diagnostic cohort studies), spiral CT with contrast is still diagnostically useful in making decisions regarding watchful waiting, needle biopsy, or surgery (SOR: B, based on a decision analysis study).
18-fluorodeoxyglucose positron emission tomography (FDG PET) is useful for assessing malignancy risk (SOR: B, based on decision analysis study), but not for solitary pulmonary nodules <1 cm (SOR: C, based on expert opinion).
Direct more costly, invasive tests to those with higher risk of malignancy
Parul Harsora, MD
Rhesa Sanni-Thomas, DO
UT Southwestern Medical Center, Dallas, Tex
Risk stratification of a solitary pulmonary nodule allows the clinician to direct more costly and invasive testing to patients with a higher probability of malignancy. Historical factors such as previous cancer, advanced age, and smoking increase suspicion for malignancy, but CT is generally warranted in all new solitary pulmonary nodules found on chest radiographs. It’s important to obtain a thorough history regarding symptoms (cough, night sweats, weight loss), occupational exposure (asbestos, bird droppings, decaying wood), travel, and comorbid conditions (especially immunocompromised states); this is likely to prove helpful in the workup.
Evidence summary
A solitary pulmonary nodule, or “coin lesion,” is an intraparenchymal finding on chest radiograph or CT that is less than 3 to 4 cm in diameter and not associated with atelectasis or adenopathy. Malignancy rates range from 15% to 75%, depending on the population studied.1 Although early detection of malignancy portends a major improvement in survival (up to 75% at 5 years following surgical resection of stage IA disease), most lung cancers progress asymptomatically until quite advanced.2
The presumed benign nature of lesions that are either unchanged over 2 years or have central calcifications is based on 3 retrospective studies from the 1950s.3-6 However, these should not be considered absolutes. A recent study revisiting the original data calculated the predictive value of benign nature based on no growth to be only 65% (95% confidence interval [CI], 47%–83%).7 Also, a study assessing the accuracy of radiologists’ assessment of calcification in solitary pulmonary nodules compared with thin-section CT found that 7% of “definitely calcified” nodules on chest radiograph lacked calcification on thin-section CT.8
Which clinical variables best predict malignancy?
The best available clinical decision rule was derived and validated from a single split population of patients with solitary pulmonary nodules.9 The outcome variable was defined as malignancy based on histologic tissue analysis or benignity by radiographic stability or resolution over 2 years. The authors did not report whether those determining outcomes and predictors were appropriately blinded.
The authors found that 3 clinical variables (age, smoking history, and cancer history) plus 3 radiographic variables (diameter, spiculation, and nodule location in the upper lobes) were independent predictors of malignancy. An online calculator using this prediction model is available at www.chestx-ray.com/SPN/ SPNProb.html.10
CT or PET?
Three comparative studies observed 8 to 12 radiologists’ readings of high-resolution CT images of 28 to 56 patients with solitary pulmonary nodules (established diagnoses by either histology or stability over time).11-13 Approximately half the nodules represented malignant lesions.
Radiologists assigned a level of confidence to their assessment of each case as benign or malignant. At a minimum, they were informed of each patient’s age and gender, and in 2 studies they also knew other information, such as the patient’s smoking and cancer histories. The study showed that the radiologists would have correctly diagnosed a pair of solitary pulmonary nodule cases, one malignant and one benign, between 75% and 83% of the time. Conversely, 17% to 25% of the time they would have diagnosed the case pair incorrectly.
A meta-analysis of 40 studies of FDG PET scanning for solitary pulmonary nodules yielded a maximum joint sensitivity and specificity of 90% (95% CI, 86.4%– 92.7%).14 The methodological quality of studies included in the meta-analysis was fair, with small sample sizes (inclusion criteria were for a minimum of 10 patients with pulmonary nodules and malignant prevalence of at least 0.5); masking was frequently incomplete.
Sensitivity of histologic/cytologic tests varies
A recent systematic review of studies evaluating patients with suspected lung cancer looked into the diagnostic sensitivity of various methods of histologic and cytologic tests.15 Researchers compared the evaluated test results to a reference standard of pathology/histology, definitive cytology, or at least 1-year radiographic follow-up.
Transbronchial needle aspiration showed a sensitivity of 67% (95% CI, 64%–70%) for peripheral lung malignancy of any size; however, only 5 studies met study criteria and their sample sizes varied greatly (n=20 to n=480). Eight studies looking at bronchoscopy (including brush or biopsy) for peripheral lung lesions <2 cm in diameter yielded a sensitivity of only 33% (95% CI, 28%–38%). In the same systematic review, 61 studies of transthoracic needle aspiration for localized pulmonary lesions of any size had a pooled sensitivity of 90% (95% CI, 88%–92%). The prevalence of malignancy in the studies ranged from 0.58 to 0.93.15 Factors affecting heterogeneity between studies included the wide range in study dates, imaging technology used, and study sizes.
What test is most cost-effective?
CT appears cost-effective when the pretest probability of malignancy is <90%; therefore, consider it on virtually all new cases of solitary pulmonary nodules.1 Also, when CT and pretest risk-assessments are discordant (eg, a patient has a low pretest probability of malignancy but his CT is suggestive of malignancy), the FDG PET scan is the most economically feasible at less than $20,000 per quality-adjusted life year.
Recommendations from others
The American College of Chest Physicians (ACCP)2 suggests pursuing no further evaluation if a nodule is unchanged for >2 years or has benign central calcifications. They recommend that physicians perform CT on every patient with a new nodule to characterize the nodule, its location, and the mediastinum. They do not recommend PET scans for nodules <1 cm. Patients who are marginal surgical candidates and have a negative PET scan should have a repeat CT scan in 3 months; serial CTs at 3, 6, 12, and 24 months are suggested, too, if prior chest radiographs are negative.
The ACCP states that transthoracic needle aspiration is not indicated in surgical candidates unless they decline surgery; then transthoracic needle aspiration or a transbronchial approach are the preferred procedure. Transthoracic needle aspiration may also be useful in establishing a diagnosis for patients who are not surgical candidates or who have a high surgical risk.
ACCP expert consensus favors the reference standard of video-assisted thoracoscopic surgery with wedge resection as the ideal method for obtaining tissue diagnosis in consenting, operable patients with solitary pulmonary nodules. Objective evidence is lacking on follow-up monitoring methods for patients with a nodule who do not have a tissue diagnosis and observation alone is chosen. ACCP expert consensus favors a 2-year follow-up with CT scanning at 3, 6, 12, and 24 months to monitor for nodule growth.2
1. Gould MK, Sanders GD, Barnett PG, et al. Cost-effectiveness of alternative management strategies for patients with solitary pulmonary nodules. Ann Intern Med 2003;138:724-735.
2. Tan BB, Flaherty KR, Kazerooni EA, Iannettoni MD. The solitary pulmonary nodule. Chest 2003;123(1 suppl):89S-96S.
3. Hood RT, Good CA, Clagett OT, McDonald JR. Solitary circumscribed lesions of lung: study of 156 cases in which resection was performed. JAMA 1953;152:1175-1181.
4. Good CA, Hood RT, McDonald JR. Significance of solitary mass in lung. AJR Am J Roentgenol 1953;70:543-554.
5. Good CA. Management of patient with solitary mass in lung. Chic Med Soc Bull 1953;55:893-896.
6. Good CA, Wilson TW. The solitary circumscribed pulmonary nodule: study of 705 cases encountered roentgenologically in a period of three and one-half years. JAMA 1958;166:210-215.
7. Yankelevitz DF, Henschke CI. Does 2-year stability imply that pulmonary nodules are benign? AJR Am J Roentgenol 1997;168:325-328.
8. Berger WG, Erly WK, Krupinski EA, Standen JR, Stern RG. The solitary pulmonary nodule on chest radiography: can we really tell if the nodule is calcified? AJR Am J Roentgenol 2001;176:201-204.
9. Swensen SG, Silverstein MD, Ilstrup DM, Schleck CD, Edell ES. The probability of malignancy in solitary pulmonary nodules: application to small radiographically intermediate nodules. Arch Intern Med 1997;157:849-855.
10. Gurney JW. Probability of malignancy in SPN [Web page]. Available at: www.chestx-ray.com/SPN/ SPNProb.html. Accessed on September 7, 2007.
11. Li F, Aoyama M, Shiraishi J, et al. Radiologists’ performance for differentiating benign from malignant lung nodules on high-resolution CT using computer-estimated likelihood of malignancy. AJR Am J Roentgenol 2004;183:1209-1215.
12. Shah SK, McNitt-Gray MF, De Zoysa KR, et al. Solitary pulmonary nodule diagnosis on CT: results of an observer study. Acad Radiol 2005;12:496-501.
13. Matsuki Y, Nakamura K, Watanabe H, Aoki T, et al. Usefulness of an artificial neural network for differentiating benign from malignant pulmonary nodules on high-resolution CT: evaluation with receiver operating characteristic analysis. AJR Am J Roentgenol 2002;178:657-663.
14. Gould MK, Maclean CC, Kuschner WG, Rydzak CE, Owens DK. Accuracy of positron emission tomography for diagnosis of pulmonary nodules and mass lesions: a meta-analysis. JAMA 2001;285:914-924.
15. Schreiber G, McCrory DC. Performance characteristics of different modalities for diagnosis of suspected lung cancer. Chest 2003;123:115S-128S.
Your initial risk assessment should include the patient’s smoking history, advancing age, cancer history, and chest radiography features (strength of recommendation [SOR]: A, based on a validated clinical decision rule). You’ll also need to review old chest radiographs (SOR: C, based on expert opinion). A solitary pulmonary nodule unchanged for >2 years on chest radiograph or containing benign central calcifications requires no further work-up (SOR: B, based on historical cohort studies).
While radiologists’ interpretations of a nodule’s calcification on chest radiograph and malignancy on computed tomography (CT) are incorrect in a substantial portion of cases (SOR: B, based on limited-quality diagnostic cohort studies), spiral CT with contrast is still diagnostically useful in making decisions regarding watchful waiting, needle biopsy, or surgery (SOR: B, based on a decision analysis study).
18-fluorodeoxyglucose positron emission tomography (FDG PET) is useful for assessing malignancy risk (SOR: B, based on decision analysis study), but not for solitary pulmonary nodules <1 cm (SOR: C, based on expert opinion).
Direct more costly, invasive tests to those with higher risk of malignancy
Parul Harsora, MD
Rhesa Sanni-Thomas, DO
UT Southwestern Medical Center, Dallas, Tex
Risk stratification of a solitary pulmonary nodule allows the clinician to direct more costly and invasive testing to patients with a higher probability of malignancy. Historical factors such as previous cancer, advanced age, and smoking increase suspicion for malignancy, but CT is generally warranted in all new solitary pulmonary nodules found on chest radiographs. It’s important to obtain a thorough history regarding symptoms (cough, night sweats, weight loss), occupational exposure (asbestos, bird droppings, decaying wood), travel, and comorbid conditions (especially immunocompromised states); this is likely to prove helpful in the workup.
Evidence summary
A solitary pulmonary nodule, or “coin lesion,” is an intraparenchymal finding on chest radiograph or CT that is less than 3 to 4 cm in diameter and not associated with atelectasis or adenopathy. Malignancy rates range from 15% to 75%, depending on the population studied.1 Although early detection of malignancy portends a major improvement in survival (up to 75% at 5 years following surgical resection of stage IA disease), most lung cancers progress asymptomatically until quite advanced.2
The presumed benign nature of lesions that are either unchanged over 2 years or have central calcifications is based on 3 retrospective studies from the 1950s.3-6 However, these should not be considered absolutes. A recent study revisiting the original data calculated the predictive value of benign nature based on no growth to be only 65% (95% confidence interval [CI], 47%–83%).7 Also, a study assessing the accuracy of radiologists’ assessment of calcification in solitary pulmonary nodules compared with thin-section CT found that 7% of “definitely calcified” nodules on chest radiograph lacked calcification on thin-section CT.8
Which clinical variables best predict malignancy?
The best available clinical decision rule was derived and validated from a single split population of patients with solitary pulmonary nodules.9 The outcome variable was defined as malignancy based on histologic tissue analysis or benignity by radiographic stability or resolution over 2 years. The authors did not report whether those determining outcomes and predictors were appropriately blinded.
The authors found that 3 clinical variables (age, smoking history, and cancer history) plus 3 radiographic variables (diameter, spiculation, and nodule location in the upper lobes) were independent predictors of malignancy. An online calculator using this prediction model is available at www.chestx-ray.com/SPN/ SPNProb.html.10
CT or PET?
Three comparative studies observed 8 to 12 radiologists’ readings of high-resolution CT images of 28 to 56 patients with solitary pulmonary nodules (established diagnoses by either histology or stability over time).11-13 Approximately half the nodules represented malignant lesions.
Radiologists assigned a level of confidence to their assessment of each case as benign or malignant. At a minimum, they were informed of each patient’s age and gender, and in 2 studies they also knew other information, such as the patient’s smoking and cancer histories. The study showed that the radiologists would have correctly diagnosed a pair of solitary pulmonary nodule cases, one malignant and one benign, between 75% and 83% of the time. Conversely, 17% to 25% of the time they would have diagnosed the case pair incorrectly.
A meta-analysis of 40 studies of FDG PET scanning for solitary pulmonary nodules yielded a maximum joint sensitivity and specificity of 90% (95% CI, 86.4%– 92.7%).14 The methodological quality of studies included in the meta-analysis was fair, with small sample sizes (inclusion criteria were for a minimum of 10 patients with pulmonary nodules and malignant prevalence of at least 0.5); masking was frequently incomplete.
Sensitivity of histologic/cytologic tests varies
A recent systematic review of studies evaluating patients with suspected lung cancer looked into the diagnostic sensitivity of various methods of histologic and cytologic tests.15 Researchers compared the evaluated test results to a reference standard of pathology/histology, definitive cytology, or at least 1-year radiographic follow-up.
Transbronchial needle aspiration showed a sensitivity of 67% (95% CI, 64%–70%) for peripheral lung malignancy of any size; however, only 5 studies met study criteria and their sample sizes varied greatly (n=20 to n=480). Eight studies looking at bronchoscopy (including brush or biopsy) for peripheral lung lesions <2 cm in diameter yielded a sensitivity of only 33% (95% CI, 28%–38%). In the same systematic review, 61 studies of transthoracic needle aspiration for localized pulmonary lesions of any size had a pooled sensitivity of 90% (95% CI, 88%–92%). The prevalence of malignancy in the studies ranged from 0.58 to 0.93.15 Factors affecting heterogeneity between studies included the wide range in study dates, imaging technology used, and study sizes.
What test is most cost-effective?
CT appears cost-effective when the pretest probability of malignancy is <90%; therefore, consider it on virtually all new cases of solitary pulmonary nodules.1 Also, when CT and pretest risk-assessments are discordant (eg, a patient has a low pretest probability of malignancy but his CT is suggestive of malignancy), the FDG PET scan is the most economically feasible at less than $20,000 per quality-adjusted life year.
Recommendations from others
The American College of Chest Physicians (ACCP)2 suggests pursuing no further evaluation if a nodule is unchanged for >2 years or has benign central calcifications. They recommend that physicians perform CT on every patient with a new nodule to characterize the nodule, its location, and the mediastinum. They do not recommend PET scans for nodules <1 cm. Patients who are marginal surgical candidates and have a negative PET scan should have a repeat CT scan in 3 months; serial CTs at 3, 6, 12, and 24 months are suggested, too, if prior chest radiographs are negative.
The ACCP states that transthoracic needle aspiration is not indicated in surgical candidates unless they decline surgery; then transthoracic needle aspiration or a transbronchial approach are the preferred procedure. Transthoracic needle aspiration may also be useful in establishing a diagnosis for patients who are not surgical candidates or who have a high surgical risk.
ACCP expert consensus favors the reference standard of video-assisted thoracoscopic surgery with wedge resection as the ideal method for obtaining tissue diagnosis in consenting, operable patients with solitary pulmonary nodules. Objective evidence is lacking on follow-up monitoring methods for patients with a nodule who do not have a tissue diagnosis and observation alone is chosen. ACCP expert consensus favors a 2-year follow-up with CT scanning at 3, 6, 12, and 24 months to monitor for nodule growth.2
Your initial risk assessment should include the patient’s smoking history, advancing age, cancer history, and chest radiography features (strength of recommendation [SOR]: A, based on a validated clinical decision rule). You’ll also need to review old chest radiographs (SOR: C, based on expert opinion). A solitary pulmonary nodule unchanged for >2 years on chest radiograph or containing benign central calcifications requires no further work-up (SOR: B, based on historical cohort studies).
While radiologists’ interpretations of a nodule’s calcification on chest radiograph and malignancy on computed tomography (CT) are incorrect in a substantial portion of cases (SOR: B, based on limited-quality diagnostic cohort studies), spiral CT with contrast is still diagnostically useful in making decisions regarding watchful waiting, needle biopsy, or surgery (SOR: B, based on a decision analysis study).
18-fluorodeoxyglucose positron emission tomography (FDG PET) is useful for assessing malignancy risk (SOR: B, based on decision analysis study), but not for solitary pulmonary nodules <1 cm (SOR: C, based on expert opinion).
Direct more costly, invasive tests to those with higher risk of malignancy
Parul Harsora, MD
Rhesa Sanni-Thomas, DO
UT Southwestern Medical Center, Dallas, Tex
Risk stratification of a solitary pulmonary nodule allows the clinician to direct more costly and invasive testing to patients with a higher probability of malignancy. Historical factors such as previous cancer, advanced age, and smoking increase suspicion for malignancy, but CT is generally warranted in all new solitary pulmonary nodules found on chest radiographs. It’s important to obtain a thorough history regarding symptoms (cough, night sweats, weight loss), occupational exposure (asbestos, bird droppings, decaying wood), travel, and comorbid conditions (especially immunocompromised states); this is likely to prove helpful in the workup.
Evidence summary
A solitary pulmonary nodule, or “coin lesion,” is an intraparenchymal finding on chest radiograph or CT that is less than 3 to 4 cm in diameter and not associated with atelectasis or adenopathy. Malignancy rates range from 15% to 75%, depending on the population studied.1 Although early detection of malignancy portends a major improvement in survival (up to 75% at 5 years following surgical resection of stage IA disease), most lung cancers progress asymptomatically until quite advanced.2
The presumed benign nature of lesions that are either unchanged over 2 years or have central calcifications is based on 3 retrospective studies from the 1950s.3-6 However, these should not be considered absolutes. A recent study revisiting the original data calculated the predictive value of benign nature based on no growth to be only 65% (95% confidence interval [CI], 47%–83%).7 Also, a study assessing the accuracy of radiologists’ assessment of calcification in solitary pulmonary nodules compared with thin-section CT found that 7% of “definitely calcified” nodules on chest radiograph lacked calcification on thin-section CT.8
Which clinical variables best predict malignancy?
The best available clinical decision rule was derived and validated from a single split population of patients with solitary pulmonary nodules.9 The outcome variable was defined as malignancy based on histologic tissue analysis or benignity by radiographic stability or resolution over 2 years. The authors did not report whether those determining outcomes and predictors were appropriately blinded.
The authors found that 3 clinical variables (age, smoking history, and cancer history) plus 3 radiographic variables (diameter, spiculation, and nodule location in the upper lobes) were independent predictors of malignancy. An online calculator using this prediction model is available at www.chestx-ray.com/SPN/ SPNProb.html.10
CT or PET?
Three comparative studies observed 8 to 12 radiologists’ readings of high-resolution CT images of 28 to 56 patients with solitary pulmonary nodules (established diagnoses by either histology or stability over time).11-13 Approximately half the nodules represented malignant lesions.
Radiologists assigned a level of confidence to their assessment of each case as benign or malignant. At a minimum, they were informed of each patient’s age and gender, and in 2 studies they also knew other information, such as the patient’s smoking and cancer histories. The study showed that the radiologists would have correctly diagnosed a pair of solitary pulmonary nodule cases, one malignant and one benign, between 75% and 83% of the time. Conversely, 17% to 25% of the time they would have diagnosed the case pair incorrectly.
A meta-analysis of 40 studies of FDG PET scanning for solitary pulmonary nodules yielded a maximum joint sensitivity and specificity of 90% (95% CI, 86.4%– 92.7%).14 The methodological quality of studies included in the meta-analysis was fair, with small sample sizes (inclusion criteria were for a minimum of 10 patients with pulmonary nodules and malignant prevalence of at least 0.5); masking was frequently incomplete.
Sensitivity of histologic/cytologic tests varies
A recent systematic review of studies evaluating patients with suspected lung cancer looked into the diagnostic sensitivity of various methods of histologic and cytologic tests.15 Researchers compared the evaluated test results to a reference standard of pathology/histology, definitive cytology, or at least 1-year radiographic follow-up.
Transbronchial needle aspiration showed a sensitivity of 67% (95% CI, 64%–70%) for peripheral lung malignancy of any size; however, only 5 studies met study criteria and their sample sizes varied greatly (n=20 to n=480). Eight studies looking at bronchoscopy (including brush or biopsy) for peripheral lung lesions <2 cm in diameter yielded a sensitivity of only 33% (95% CI, 28%–38%). In the same systematic review, 61 studies of transthoracic needle aspiration for localized pulmonary lesions of any size had a pooled sensitivity of 90% (95% CI, 88%–92%). The prevalence of malignancy in the studies ranged from 0.58 to 0.93.15 Factors affecting heterogeneity between studies included the wide range in study dates, imaging technology used, and study sizes.
What test is most cost-effective?
CT appears cost-effective when the pretest probability of malignancy is <90%; therefore, consider it on virtually all new cases of solitary pulmonary nodules.1 Also, when CT and pretest risk-assessments are discordant (eg, a patient has a low pretest probability of malignancy but his CT is suggestive of malignancy), the FDG PET scan is the most economically feasible at less than $20,000 per quality-adjusted life year.
Recommendations from others
The American College of Chest Physicians (ACCP)2 suggests pursuing no further evaluation if a nodule is unchanged for >2 years or has benign central calcifications. They recommend that physicians perform CT on every patient with a new nodule to characterize the nodule, its location, and the mediastinum. They do not recommend PET scans for nodules <1 cm. Patients who are marginal surgical candidates and have a negative PET scan should have a repeat CT scan in 3 months; serial CTs at 3, 6, 12, and 24 months are suggested, too, if prior chest radiographs are negative.
The ACCP states that transthoracic needle aspiration is not indicated in surgical candidates unless they decline surgery; then transthoracic needle aspiration or a transbronchial approach are the preferred procedure. Transthoracic needle aspiration may also be useful in establishing a diagnosis for patients who are not surgical candidates or who have a high surgical risk.
ACCP expert consensus favors the reference standard of video-assisted thoracoscopic surgery with wedge resection as the ideal method for obtaining tissue diagnosis in consenting, operable patients with solitary pulmonary nodules. Objective evidence is lacking on follow-up monitoring methods for patients with a nodule who do not have a tissue diagnosis and observation alone is chosen. ACCP expert consensus favors a 2-year follow-up with CT scanning at 3, 6, 12, and 24 months to monitor for nodule growth.2
1. Gould MK, Sanders GD, Barnett PG, et al. Cost-effectiveness of alternative management strategies for patients with solitary pulmonary nodules. Ann Intern Med 2003;138:724-735.
2. Tan BB, Flaherty KR, Kazerooni EA, Iannettoni MD. The solitary pulmonary nodule. Chest 2003;123(1 suppl):89S-96S.
3. Hood RT, Good CA, Clagett OT, McDonald JR. Solitary circumscribed lesions of lung: study of 156 cases in which resection was performed. JAMA 1953;152:1175-1181.
4. Good CA, Hood RT, McDonald JR. Significance of solitary mass in lung. AJR Am J Roentgenol 1953;70:543-554.
5. Good CA. Management of patient with solitary mass in lung. Chic Med Soc Bull 1953;55:893-896.
6. Good CA, Wilson TW. The solitary circumscribed pulmonary nodule: study of 705 cases encountered roentgenologically in a period of three and one-half years. JAMA 1958;166:210-215.
7. Yankelevitz DF, Henschke CI. Does 2-year stability imply that pulmonary nodules are benign? AJR Am J Roentgenol 1997;168:325-328.
8. Berger WG, Erly WK, Krupinski EA, Standen JR, Stern RG. The solitary pulmonary nodule on chest radiography: can we really tell if the nodule is calcified? AJR Am J Roentgenol 2001;176:201-204.
9. Swensen SG, Silverstein MD, Ilstrup DM, Schleck CD, Edell ES. The probability of malignancy in solitary pulmonary nodules: application to small radiographically intermediate nodules. Arch Intern Med 1997;157:849-855.
10. Gurney JW. Probability of malignancy in SPN [Web page]. Available at: www.chestx-ray.com/SPN/ SPNProb.html. Accessed on September 7, 2007.
11. Li F, Aoyama M, Shiraishi J, et al. Radiologists’ performance for differentiating benign from malignant lung nodules on high-resolution CT using computer-estimated likelihood of malignancy. AJR Am J Roentgenol 2004;183:1209-1215.
12. Shah SK, McNitt-Gray MF, De Zoysa KR, et al. Solitary pulmonary nodule diagnosis on CT: results of an observer study. Acad Radiol 2005;12:496-501.
13. Matsuki Y, Nakamura K, Watanabe H, Aoki T, et al. Usefulness of an artificial neural network for differentiating benign from malignant pulmonary nodules on high-resolution CT: evaluation with receiver operating characteristic analysis. AJR Am J Roentgenol 2002;178:657-663.
14. Gould MK, Maclean CC, Kuschner WG, Rydzak CE, Owens DK. Accuracy of positron emission tomography for diagnosis of pulmonary nodules and mass lesions: a meta-analysis. JAMA 2001;285:914-924.
15. Schreiber G, McCrory DC. Performance characteristics of different modalities for diagnosis of suspected lung cancer. Chest 2003;123:115S-128S.
1. Gould MK, Sanders GD, Barnett PG, et al. Cost-effectiveness of alternative management strategies for patients with solitary pulmonary nodules. Ann Intern Med 2003;138:724-735.
2. Tan BB, Flaherty KR, Kazerooni EA, Iannettoni MD. The solitary pulmonary nodule. Chest 2003;123(1 suppl):89S-96S.
3. Hood RT, Good CA, Clagett OT, McDonald JR. Solitary circumscribed lesions of lung: study of 156 cases in which resection was performed. JAMA 1953;152:1175-1181.
4. Good CA, Hood RT, McDonald JR. Significance of solitary mass in lung. AJR Am J Roentgenol 1953;70:543-554.
5. Good CA. Management of patient with solitary mass in lung. Chic Med Soc Bull 1953;55:893-896.
6. Good CA, Wilson TW. The solitary circumscribed pulmonary nodule: study of 705 cases encountered roentgenologically in a period of three and one-half years. JAMA 1958;166:210-215.
7. Yankelevitz DF, Henschke CI. Does 2-year stability imply that pulmonary nodules are benign? AJR Am J Roentgenol 1997;168:325-328.
8. Berger WG, Erly WK, Krupinski EA, Standen JR, Stern RG. The solitary pulmonary nodule on chest radiography: can we really tell if the nodule is calcified? AJR Am J Roentgenol 2001;176:201-204.
9. Swensen SG, Silverstein MD, Ilstrup DM, Schleck CD, Edell ES. The probability of malignancy in solitary pulmonary nodules: application to small radiographically intermediate nodules. Arch Intern Med 1997;157:849-855.
10. Gurney JW. Probability of malignancy in SPN [Web page]. Available at: www.chestx-ray.com/SPN/ SPNProb.html. Accessed on September 7, 2007.
11. Li F, Aoyama M, Shiraishi J, et al. Radiologists’ performance for differentiating benign from malignant lung nodules on high-resolution CT using computer-estimated likelihood of malignancy. AJR Am J Roentgenol 2004;183:1209-1215.
12. Shah SK, McNitt-Gray MF, De Zoysa KR, et al. Solitary pulmonary nodule diagnosis on CT: results of an observer study. Acad Radiol 2005;12:496-501.
13. Matsuki Y, Nakamura K, Watanabe H, Aoki T, et al. Usefulness of an artificial neural network for differentiating benign from malignant pulmonary nodules on high-resolution CT: evaluation with receiver operating characteristic analysis. AJR Am J Roentgenol 2002;178:657-663.
14. Gould MK, Maclean CC, Kuschner WG, Rydzak CE, Owens DK. Accuracy of positron emission tomography for diagnosis of pulmonary nodules and mass lesions: a meta-analysis. JAMA 2001;285:914-924.
15. Schreiber G, McCrory DC. Performance characteristics of different modalities for diagnosis of suspected lung cancer. Chest 2003;123:115S-128S.
Evidence-based answers from the Family Physicians Inquiries Network
How do exercise and diet compare for weight loss?
Exercise alone produces short-term weight loss that is comparable with that induced by diet, after which a plateau in weight loss appears to occur (strength of recommendation [SOR]: B). Exercise in combination with diet promotes maintenance of weight loss above either intervention alone in both obese and overweight men and women (SOR: A). Exercise-induced weight loss has been shown to preferentially reduce abdominal fat and increase lean skeletal muscle compared with that induced by diet (SOR: B).
Multiple short bouts of exercise per day are as effective as a single long bout in producing weight loss (SOR: B). Adherence improves when exercise can be completed at home or home equipment is used (SOR: B).
The real challenge: Motivating patients to exercise
Henry Domke, MD
St. Mary’s Health Center, Jefferson City, Missouri
The evidence is pretty clear. The real challenge is motivating patients to start and maintain an exercise plan. The key points I make with my patients are: Aim for 5 to 7 times each week. Start slowly (10 minutes per session) and gradually build (at least to 20 minutes within a few months). Walking is often preferred, but do what you enjoy. Having a “buddy” work out with you may help you stick with it.
Evidence summary
Exercise vs diet: Some conflicting results
Studies comparing the effectiveness of exercise and diet in weight reduction have yielded conflicting results. Earlier studies, including a meta-analysis and randomized (noncontrolled) study, favored interventions that included caloric restriction (diet alone or diet plus exercise).1,2
However, subjects on caloric restriction regained a significant amount of weight over time (0.9 kg±7.7 at 2-year follow-up). Subjects who did aerobic exercise but did not diet lost less weight initially (0.7 kg±2.8) but maintained their weight loss better than those who dieted or dieted with exercise.
These earlier studies failed to control for the confounding variable of energy balance—that is, ensuring the amount of calories reduced was comparable with the amount of calories burned through exercise between groups. A more recent randomized controlled trial suggests that aerobic exercise and caloric restriction are equally beneficial in reducing weight for obese men when controlling for negative energy balance.3 However, those who exercised experienced greater fat reduction and maintenance of skeletal muscle mass than those who only restricted calories. Similar findings regarding fat reduction have been reported elsewhere.4
Combining diet and exercise appears to be superior to diet alone, based on the results of a recent meta-analysis of randomized controlled trials.5 However, this meta-analysis did not specify type of exercise, so it is unclear whether outcomes varied by activity.
Exercise: Is there a dose-response relationship?
Several studies have looked at the relationship between duration and intensity for exercise and weight loss. A dose-response relationship has been observed between the amount of time spent in aerobic exercise per week and the amount of weight lost for overweight women.6,7
There appears to be no significant difference in weight loss based on duration of a single aerobic exercise episode; rather, weight loss is similar whether completed in short or long bouts.7,8 One study found that at 12 months, individuals exercising more than 200 minutes per week lost 7.8 kg more (P<.01) than those exercising less than 150 minutes per week.7 Another study noted that at 18 months, subjects exercising more than 200 minutes per week lost 9.6 kg more than subjects exercising less than 150 minutes per week (P<.05).6
Studies with energy expenditure, rather than time spent exercising, as the independent variable had similar results. At 18 months, individuals with higher energy expenditure (2500 kcal/week) lost 6.7 kg±8.1 compared with a mean loss of 4.1±8.3 in subjects with lower energy expenditure (maximum of 1000 kcal/week).9
Recommendations from others
The National Institutes of Health’s National Heart, Lung and Blood Institute,10 the US Department of Health and Human Services,11 the Centers for Disease Control and Prevention’s Healthy People 201012 recommend between 30 to 90 minutes of daily moderate physical activity, and that this activity be done at least 5 days a week—or even 7 days per week—depending on whether a person’s goal is weight maintenance or weight loss.
Another option, offered by the CDC, is that people do 20 minutes of vigorous activity 3 days or more per week. All of the groups recommend staying within caloric intake requirements (TABLE).
TABLE
How much exercise is best? Government agencies weigh in
| AGENCY | PHYSICAL ACTIVITY LEVEL | ACTIVITIES | DURATION | FREQUENCY | NOTES |
|---|---|---|---|---|---|
| CDC12 | Moderate | Bicycling 5–9 mph, level terrain or with a few hills, brisk walking, golf, mowing lawn, recreational swimming, scrubbing floors/washing windows, tennis (doubles), weight lifting/Nautilus machines/free weights | 30 min | 5 or more days/week | All adults |
| CDC12 (alternative) | Vigorous | Bicycling more than 10 mph (alternative) or on steep uphill terrain, circuit training, moving/pushing furniture, mowing lawn (hand mower), racewalking, jogging, running, swimming laps, tennis (singles) | 20 min | 3 or more days/week | All adults |
| DHHS11 | Moderate | Bicycling (<10 mph), dancing, golf, hiking, light gardening/yard work stretching, walking (3.5 mph), weight lifting (general light workout) | 60–90 min | Daily | All adults attempting to lose weight |
| NHLBI10 | Moderate | Basketball, bicycling 5 miles/30 min, gardening, running 10 min/mile, social dancing, swimming laps, walking 15–20 min/mile | 30 min | Daily | All adult |
1. Miller WC, Koceja DM, Hamilton EJ. A meta-analysis of the past 25 years of weight loss research using diet, exercise or diet plus exercise intervention. Int J Obes 1997;21:941-947.
2. Skender M, Goodrick G, Del Junco D. Comparison of 2 year weight loss trends in behavioral treatments of obesity: Diet, Exercise, and Combination interventions. J Amer Diet Assoc 1996;96:342-346.
3. Ross R, Freeman JA, Janssen I. Exercise alone is an effective strategy for reducing obesity and related comorbidities. Exerc Sport Sci Rev 2000;28:165-170.
4. Tsai A, Sandretto A, Chung Y. Dieting is more effective in reducing weight but exercise is more effective in reducing fat during the early phase of a weight-reducing program in healthy humans. J Nut Biochem 2003;14:541-549.
5. Curioni C, Lourenco P. Long-term weight loss after diet and exercise: a systematic review. Internat J Obes 2005;29:1168-1174.
6. Jakicic J, Marcus B, Gallagher K, Napolitano M, Lang W. Effect of exercise duration and intensity on weight loss in overweight, sedentary women, a randomized trial. JAMA 2003;290:1323-1330.
7. Jakicic J, Winters C, Lang W, Wing R. Effects of intermittent exercise and use of home exercise equipment on adherence, weight loss, and fitness in overweight women—a randomized trial. JAMA 1999;282:1554-1560.
8. Schmidt W, Biwer C, Kalscheuer L. Effects of long versus short bout exercise on fitness and weight loss in over-weight females. J Am Coll Nutr 2001;20:494-501.
9. Jeffrey RW, Wing RR, Sherwood NE, Tate DF. Physical activity and weight loss: does prescribing higher physical activity goals improve outcome? Am J Clin Nutr 2003;78:684-689.
10. Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults: The Evidence Report. Obes Res 1998;6:6-26.
11. Department of Health and Human Services Dietary guidelines for Americans 2005 [Internet monograph]. Washington, DC: Department of Health and Human Services; 2005. Available at: www.health.gov/dietaryguidelines/dga2005/document/html/chapter4.htm. Accessed on September 4, 2007
12. Healthy People 2010 [Web site] Rockville, Md: Office of Disease Prevention and Health Promotion, US Department of Health and Human Services; 2002. Available at: www.healthypeople.gov/document/html/volume2/22physical.htm. Accessed on September 4, 2007.
Exercise alone produces short-term weight loss that is comparable with that induced by diet, after which a plateau in weight loss appears to occur (strength of recommendation [SOR]: B). Exercise in combination with diet promotes maintenance of weight loss above either intervention alone in both obese and overweight men and women (SOR: A). Exercise-induced weight loss has been shown to preferentially reduce abdominal fat and increase lean skeletal muscle compared with that induced by diet (SOR: B).
Multiple short bouts of exercise per day are as effective as a single long bout in producing weight loss (SOR: B). Adherence improves when exercise can be completed at home or home equipment is used (SOR: B).
The real challenge: Motivating patients to exercise
Henry Domke, MD
St. Mary’s Health Center, Jefferson City, Missouri
The evidence is pretty clear. The real challenge is motivating patients to start and maintain an exercise plan. The key points I make with my patients are: Aim for 5 to 7 times each week. Start slowly (10 minutes per session) and gradually build (at least to 20 minutes within a few months). Walking is often preferred, but do what you enjoy. Having a “buddy” work out with you may help you stick with it.
Evidence summary
Exercise vs diet: Some conflicting results
Studies comparing the effectiveness of exercise and diet in weight reduction have yielded conflicting results. Earlier studies, including a meta-analysis and randomized (noncontrolled) study, favored interventions that included caloric restriction (diet alone or diet plus exercise).1,2
However, subjects on caloric restriction regained a significant amount of weight over time (0.9 kg±7.7 at 2-year follow-up). Subjects who did aerobic exercise but did not diet lost less weight initially (0.7 kg±2.8) but maintained their weight loss better than those who dieted or dieted with exercise.
These earlier studies failed to control for the confounding variable of energy balance—that is, ensuring the amount of calories reduced was comparable with the amount of calories burned through exercise between groups. A more recent randomized controlled trial suggests that aerobic exercise and caloric restriction are equally beneficial in reducing weight for obese men when controlling for negative energy balance.3 However, those who exercised experienced greater fat reduction and maintenance of skeletal muscle mass than those who only restricted calories. Similar findings regarding fat reduction have been reported elsewhere.4
Combining diet and exercise appears to be superior to diet alone, based on the results of a recent meta-analysis of randomized controlled trials.5 However, this meta-analysis did not specify type of exercise, so it is unclear whether outcomes varied by activity.
Exercise: Is there a dose-response relationship?
Several studies have looked at the relationship between duration and intensity for exercise and weight loss. A dose-response relationship has been observed between the amount of time spent in aerobic exercise per week and the amount of weight lost for overweight women.6,7
There appears to be no significant difference in weight loss based on duration of a single aerobic exercise episode; rather, weight loss is similar whether completed in short or long bouts.7,8 One study found that at 12 months, individuals exercising more than 200 minutes per week lost 7.8 kg more (P<.01) than those exercising less than 150 minutes per week.7 Another study noted that at 18 months, subjects exercising more than 200 minutes per week lost 9.6 kg more than subjects exercising less than 150 minutes per week (P<.05).6
Studies with energy expenditure, rather than time spent exercising, as the independent variable had similar results. At 18 months, individuals with higher energy expenditure (2500 kcal/week) lost 6.7 kg±8.1 compared with a mean loss of 4.1±8.3 in subjects with lower energy expenditure (maximum of 1000 kcal/week).9
Recommendations from others
The National Institutes of Health’s National Heart, Lung and Blood Institute,10 the US Department of Health and Human Services,11 the Centers for Disease Control and Prevention’s Healthy People 201012 recommend between 30 to 90 minutes of daily moderate physical activity, and that this activity be done at least 5 days a week—or even 7 days per week—depending on whether a person’s goal is weight maintenance or weight loss.
Another option, offered by the CDC, is that people do 20 minutes of vigorous activity 3 days or more per week. All of the groups recommend staying within caloric intake requirements (TABLE).
TABLE
How much exercise is best? Government agencies weigh in
| AGENCY | PHYSICAL ACTIVITY LEVEL | ACTIVITIES | DURATION | FREQUENCY | NOTES |
|---|---|---|---|---|---|
| CDC12 | Moderate | Bicycling 5–9 mph, level terrain or with a few hills, brisk walking, golf, mowing lawn, recreational swimming, scrubbing floors/washing windows, tennis (doubles), weight lifting/Nautilus machines/free weights | 30 min | 5 or more days/week | All adults |
| CDC12 (alternative) | Vigorous | Bicycling more than 10 mph (alternative) or on steep uphill terrain, circuit training, moving/pushing furniture, mowing lawn (hand mower), racewalking, jogging, running, swimming laps, tennis (singles) | 20 min | 3 or more days/week | All adults |
| DHHS11 | Moderate | Bicycling (<10 mph), dancing, golf, hiking, light gardening/yard work stretching, walking (3.5 mph), weight lifting (general light workout) | 60–90 min | Daily | All adults attempting to lose weight |
| NHLBI10 | Moderate | Basketball, bicycling 5 miles/30 min, gardening, running 10 min/mile, social dancing, swimming laps, walking 15–20 min/mile | 30 min | Daily | All adult |
Exercise alone produces short-term weight loss that is comparable with that induced by diet, after which a plateau in weight loss appears to occur (strength of recommendation [SOR]: B). Exercise in combination with diet promotes maintenance of weight loss above either intervention alone in both obese and overweight men and women (SOR: A). Exercise-induced weight loss has been shown to preferentially reduce abdominal fat and increase lean skeletal muscle compared with that induced by diet (SOR: B).
Multiple short bouts of exercise per day are as effective as a single long bout in producing weight loss (SOR: B). Adherence improves when exercise can be completed at home or home equipment is used (SOR: B).
The real challenge: Motivating patients to exercise
Henry Domke, MD
St. Mary’s Health Center, Jefferson City, Missouri
The evidence is pretty clear. The real challenge is motivating patients to start and maintain an exercise plan. The key points I make with my patients are: Aim for 5 to 7 times each week. Start slowly (10 minutes per session) and gradually build (at least to 20 minutes within a few months). Walking is often preferred, but do what you enjoy. Having a “buddy” work out with you may help you stick with it.
Evidence summary
Exercise vs diet: Some conflicting results
Studies comparing the effectiveness of exercise and diet in weight reduction have yielded conflicting results. Earlier studies, including a meta-analysis and randomized (noncontrolled) study, favored interventions that included caloric restriction (diet alone or diet plus exercise).1,2
However, subjects on caloric restriction regained a significant amount of weight over time (0.9 kg±7.7 at 2-year follow-up). Subjects who did aerobic exercise but did not diet lost less weight initially (0.7 kg±2.8) but maintained their weight loss better than those who dieted or dieted with exercise.
These earlier studies failed to control for the confounding variable of energy balance—that is, ensuring the amount of calories reduced was comparable with the amount of calories burned through exercise between groups. A more recent randomized controlled trial suggests that aerobic exercise and caloric restriction are equally beneficial in reducing weight for obese men when controlling for negative energy balance.3 However, those who exercised experienced greater fat reduction and maintenance of skeletal muscle mass than those who only restricted calories. Similar findings regarding fat reduction have been reported elsewhere.4
Combining diet and exercise appears to be superior to diet alone, based on the results of a recent meta-analysis of randomized controlled trials.5 However, this meta-analysis did not specify type of exercise, so it is unclear whether outcomes varied by activity.
Exercise: Is there a dose-response relationship?
Several studies have looked at the relationship between duration and intensity for exercise and weight loss. A dose-response relationship has been observed between the amount of time spent in aerobic exercise per week and the amount of weight lost for overweight women.6,7
There appears to be no significant difference in weight loss based on duration of a single aerobic exercise episode; rather, weight loss is similar whether completed in short or long bouts.7,8 One study found that at 12 months, individuals exercising more than 200 minutes per week lost 7.8 kg more (P<.01) than those exercising less than 150 minutes per week.7 Another study noted that at 18 months, subjects exercising more than 200 minutes per week lost 9.6 kg more than subjects exercising less than 150 minutes per week (P<.05).6
Studies with energy expenditure, rather than time spent exercising, as the independent variable had similar results. At 18 months, individuals with higher energy expenditure (2500 kcal/week) lost 6.7 kg±8.1 compared with a mean loss of 4.1±8.3 in subjects with lower energy expenditure (maximum of 1000 kcal/week).9
Recommendations from others
The National Institutes of Health’s National Heart, Lung and Blood Institute,10 the US Department of Health and Human Services,11 the Centers for Disease Control and Prevention’s Healthy People 201012 recommend between 30 to 90 minutes of daily moderate physical activity, and that this activity be done at least 5 days a week—or even 7 days per week—depending on whether a person’s goal is weight maintenance or weight loss.
Another option, offered by the CDC, is that people do 20 minutes of vigorous activity 3 days or more per week. All of the groups recommend staying within caloric intake requirements (TABLE).
TABLE
How much exercise is best? Government agencies weigh in
| AGENCY | PHYSICAL ACTIVITY LEVEL | ACTIVITIES | DURATION | FREQUENCY | NOTES |
|---|---|---|---|---|---|
| CDC12 | Moderate | Bicycling 5–9 mph, level terrain or with a few hills, brisk walking, golf, mowing lawn, recreational swimming, scrubbing floors/washing windows, tennis (doubles), weight lifting/Nautilus machines/free weights | 30 min | 5 or more days/week | All adults |
| CDC12 (alternative) | Vigorous | Bicycling more than 10 mph (alternative) or on steep uphill terrain, circuit training, moving/pushing furniture, mowing lawn (hand mower), racewalking, jogging, running, swimming laps, tennis (singles) | 20 min | 3 or more days/week | All adults |
| DHHS11 | Moderate | Bicycling (<10 mph), dancing, golf, hiking, light gardening/yard work stretching, walking (3.5 mph), weight lifting (general light workout) | 60–90 min | Daily | All adults attempting to lose weight |
| NHLBI10 | Moderate | Basketball, bicycling 5 miles/30 min, gardening, running 10 min/mile, social dancing, swimming laps, walking 15–20 min/mile | 30 min | Daily | All adult |
1. Miller WC, Koceja DM, Hamilton EJ. A meta-analysis of the past 25 years of weight loss research using diet, exercise or diet plus exercise intervention. Int J Obes 1997;21:941-947.
2. Skender M, Goodrick G, Del Junco D. Comparison of 2 year weight loss trends in behavioral treatments of obesity: Diet, Exercise, and Combination interventions. J Amer Diet Assoc 1996;96:342-346.
3. Ross R, Freeman JA, Janssen I. Exercise alone is an effective strategy for reducing obesity and related comorbidities. Exerc Sport Sci Rev 2000;28:165-170.
4. Tsai A, Sandretto A, Chung Y. Dieting is more effective in reducing weight but exercise is more effective in reducing fat during the early phase of a weight-reducing program in healthy humans. J Nut Biochem 2003;14:541-549.
5. Curioni C, Lourenco P. Long-term weight loss after diet and exercise: a systematic review. Internat J Obes 2005;29:1168-1174.
6. Jakicic J, Marcus B, Gallagher K, Napolitano M, Lang W. Effect of exercise duration and intensity on weight loss in overweight, sedentary women, a randomized trial. JAMA 2003;290:1323-1330.
7. Jakicic J, Winters C, Lang W, Wing R. Effects of intermittent exercise and use of home exercise equipment on adherence, weight loss, and fitness in overweight women—a randomized trial. JAMA 1999;282:1554-1560.
8. Schmidt W, Biwer C, Kalscheuer L. Effects of long versus short bout exercise on fitness and weight loss in over-weight females. J Am Coll Nutr 2001;20:494-501.
9. Jeffrey RW, Wing RR, Sherwood NE, Tate DF. Physical activity and weight loss: does prescribing higher physical activity goals improve outcome? Am J Clin Nutr 2003;78:684-689.
10. Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults: The Evidence Report. Obes Res 1998;6:6-26.
11. Department of Health and Human Services Dietary guidelines for Americans 2005 [Internet monograph]. Washington, DC: Department of Health and Human Services; 2005. Available at: www.health.gov/dietaryguidelines/dga2005/document/html/chapter4.htm. Accessed on September 4, 2007
12. Healthy People 2010 [Web site] Rockville, Md: Office of Disease Prevention and Health Promotion, US Department of Health and Human Services; 2002. Available at: www.healthypeople.gov/document/html/volume2/22physical.htm. Accessed on September 4, 2007.
1. Miller WC, Koceja DM, Hamilton EJ. A meta-analysis of the past 25 years of weight loss research using diet, exercise or diet plus exercise intervention. Int J Obes 1997;21:941-947.
2. Skender M, Goodrick G, Del Junco D. Comparison of 2 year weight loss trends in behavioral treatments of obesity: Diet, Exercise, and Combination interventions. J Amer Diet Assoc 1996;96:342-346.
3. Ross R, Freeman JA, Janssen I. Exercise alone is an effective strategy for reducing obesity and related comorbidities. Exerc Sport Sci Rev 2000;28:165-170.
4. Tsai A, Sandretto A, Chung Y. Dieting is more effective in reducing weight but exercise is more effective in reducing fat during the early phase of a weight-reducing program in healthy humans. J Nut Biochem 2003;14:541-549.
5. Curioni C, Lourenco P. Long-term weight loss after diet and exercise: a systematic review. Internat J Obes 2005;29:1168-1174.
6. Jakicic J, Marcus B, Gallagher K, Napolitano M, Lang W. Effect of exercise duration and intensity on weight loss in overweight, sedentary women, a randomized trial. JAMA 2003;290:1323-1330.
7. Jakicic J, Winters C, Lang W, Wing R. Effects of intermittent exercise and use of home exercise equipment on adherence, weight loss, and fitness in overweight women—a randomized trial. JAMA 1999;282:1554-1560.
8. Schmidt W, Biwer C, Kalscheuer L. Effects of long versus short bout exercise on fitness and weight loss in over-weight females. J Am Coll Nutr 2001;20:494-501.
9. Jeffrey RW, Wing RR, Sherwood NE, Tate DF. Physical activity and weight loss: does prescribing higher physical activity goals improve outcome? Am J Clin Nutr 2003;78:684-689.
10. Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults: The Evidence Report. Obes Res 1998;6:6-26.
11. Department of Health and Human Services Dietary guidelines for Americans 2005 [Internet monograph]. Washington, DC: Department of Health and Human Services; 2005. Available at: www.health.gov/dietaryguidelines/dga2005/document/html/chapter4.htm. Accessed on September 4, 2007
12. Healthy People 2010 [Web site] Rockville, Md: Office of Disease Prevention and Health Promotion, US Department of Health and Human Services; 2002. Available at: www.healthypeople.gov/document/html/volume2/22physical.htm. Accessed on September 4, 2007.
Evidence-based answers from the Family Physicians Inquiries Network
Which tool is most useful in diagnosing bipolar disorder in children?
No single, well-validated screening instrument for clinical diagnosis of bipolar disorder in children exists. That said, the Kiddie Schedule for Affective Disorders and Schizophrenia (KSADS), a semi-structured interview, along with clinical evaluation by a childhood mental health specialist, is used most frequently in major research studies (strength of recommendation [SOR]: C).
As a screening tool in the primary care setting, family history of bipolar disorder in either biologic parent increases the odds of diagnosis (SOR: A). High or low scores on parent-reported screening tests (Parent Young Mania Rating Scale [P-YMRS], Parent General Behavior Inventory [P-GBI], and Child Behavior Checklist [CBCL]) also significantly increase or decrease the likelihood of diagnosis (SOR: B).
Make sure it’s not ADHD
Adam J. Zolotor, MD, MPH
University of North Carolina at Chapel Hill
When evaluating a child for mental health, behavioral, or academic concerns, I always begin with an assessment targeting potential attention deficit hyperactivity disorder (ADHD). Distinguishing mania from hyperactivity and impulsivity is difficult. The most useful clue is family history. Suspicion of bipolar disorder (based on mood cycling or family history) would prompt me to refer to a child mental health specialist. Also, when I’m treating a child with ADHD, I consider alternate or comorbid conditions when he or she fails to achieve behavioral goals.
Of the rating scales reviewed above, I consider the P-GBI and the P-YMRS useful in risk stratification. However, screening instruments are less useful when a disease is rare (as with childhood bipolar disorder). Children with hyperactivity and impulsivity may have a range of conditions from hyperthyroidism to anxiety disorders, but we must listen to the history, observe the patient, and proceed with an evaluation based on the likelihood of disease.
Evidence summary
Retrospective analysis of 2 large cohort studies of adults with bipolar disorder indicated that at least 50% of these patients had an onset of illness before age 19, establishing support for the presence of bipolar disorder among children and adolescents.1 The criteria in the 4th edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV) cannot be easily applied to most children and adolescents with bipolar disorder because most do not meet the criteria for Bipolar I or II, but fall into the less well-defined category Bipolar NOS (not otherwise specified).2,3
Compared with adults, children and adolescents are more difficult to diagnose because they are less likely to have discrete episodes of mania, and instead present with severe irritability, rapid cycling, or mixed mania.2,4 In laddition, symptoms progress and evolve as children and adolescents grow.1 Comorbid disorders such as ADHD, oppositional defiant disorder, conduct disorder, and learning disorders are common in this population, further complicating diagnosis.2
Screening instruments are imperfect
Different versions of the KSADS have been used in most research studies on this disorder.2 Despite this, concerns about the validity of the instrument still exist because of lack of sufficient testing, vagueness of the diagnostic criteria, and the subjective nature of the test.5,6 Because specialized training is required to administer the test and testing can last a full day, its use in most office settings is impractical. It is also not meant as a stand-alone test, but to be used in conjunction with a clinical evaluation by a trained mental health professional.7
In a general clinical setting, family history and selected screening instruments may help to increase or decrease clinical suspicion for the disorder and guide referral for more specialized evaluation by a child mental health provider. In addition, a meta-analysis found that children or adolescents who have a biologic parent with bipolar disorder have 2 to 10 times the odds of being diagnosed with bipolar disorder.7
Three screening tests (CBCL, P-GBI, and P-YMRS) available for the office setting use parent-reported scores, and perform best when compared with KSADS as the standard.3 These instruments were associated with likelihood ratios that significantly improved the odds of diagnosis and could allow clinicians to stratify patients as high or low risk (TABLE).3
TABLE
Likelihood ratios for 3 screening tools you can use in the office
| For ages 5–10* | For ages 11–17† | |||||||
| IF THE SCORE IS… | IF THE SCORE IS… | |||||||
| LOW | MOD. LOW | HIGH | VERY HIGH | LOW | MOD. LOW | HIGH | VERY HIGH | |
| THEN THE LR FOR THE INSTRUMENT IS… | THEN THE LR FOR THE INSTRUMENT IS… | |||||||
| P-YMRS | 0.08 | 0.48 | 6.94 | 8.92 | 0.20 | 0 .32 | 4.07 | 7.41 |
| P-GBI | 0.10 | 0.48 | 4.90 | 6.29 | 0.06 | 0.25 | 4.82 | 9.21 |
| CBCL | 0.07 | 0.47 | 3.15 | 3.52 | 0.04 | 0.53 | 2.65 | 4.29 |
| * Population studied had a 50.3% prevalence of bipolar disorder. | † Population studied had a 40.7% prevalence of bipolar disorder. | |||||||
Recommendations from others
Two consensus conferences, a Canadian guideline, and a National Institute of Mental Health round-table all concluded that there is currently no ideal test for the diagnosis of child and adolescent bipolar disorder, but that such an instrument needed to be developed.2,5,6,8 One consensus conference further concluded that the diagnosis is best made by childhood mental health specialists based on multiple informants, such as the child and parents, with symptoms present in at least 2 settings or by direct observation.6
A Canadian consensus conference proposed screening patients with depressive symptoms for a history of hypomanic or manic symptoms, and consider an underlying mood disorder in those with vague or nonspecific somatic symptoms or reverse vegetative symptoms (eg, hypersomnia and hyperphagia). Their recommendations also emphasized screening for family history of bipolar disorder when there were clinical concerns.8
1. Post R, Kowatch R. The health care crisis of childhood-onset bipolar illness: some recommendations for its amelioration. J Clin Psychiatry 2006;67:115-125.
2. National Institute of Mental Health research round-table on prepubertal bipolar disorder J Am Acad Chld Adolesc Psychiatry 2001;40:871-878.
3. Youngstrom E, Findling R, Calabrese J, et al. Comparing the diagnostic accuracy of six potential screening instruments for bipolar disorder in youths aged 5 to 17 years. J Am Acad Child Adolesc Psychiatry 2004;43:847-858.
4. Weckerly J. Pediatric bipolar mood disorder. J Dev Behav Pediatr 2002;23:42-56.
5. Coyle J, Pine D, Charney D, et al. Depression and bipolar support alliance consensus statement on the unmet needs in diagnosis and treatment of mood disorders in children and adolescents. J Am Acad Child Adolesc Psychiatry 2003;42:1494-1503.
6. Carlson G, Jensen P, Findling R, et al. Methodological Issues and Controversies in Clinical Trials with Child and Adolescent Patients with Bipolar Disorder: Report of a Consensus Conference. J Child Adolesc Psychopharamcol 2003;13:13-27.
7. Youngstrom E, Findling R, Youngstrom J, Calabrese J. Toward an evidence-based assessment of pediatric bipolar disorder. J Clin Child Adolesc Psychol 2005;34:433-448.
8. Yatham L, Kennedy S, O’Donovan C, et al. Canadian Network for Mood and Anxiety Treatments (CAN-MAT) guidelines for the management of patients with bipolar disorder: consensus and controversies. Bipolar Disord 2005;7(Suppl 3):5-69.
No single, well-validated screening instrument for clinical diagnosis of bipolar disorder in children exists. That said, the Kiddie Schedule for Affective Disorders and Schizophrenia (KSADS), a semi-structured interview, along with clinical evaluation by a childhood mental health specialist, is used most frequently in major research studies (strength of recommendation [SOR]: C).
As a screening tool in the primary care setting, family history of bipolar disorder in either biologic parent increases the odds of diagnosis (SOR: A). High or low scores on parent-reported screening tests (Parent Young Mania Rating Scale [P-YMRS], Parent General Behavior Inventory [P-GBI], and Child Behavior Checklist [CBCL]) also significantly increase or decrease the likelihood of diagnosis (SOR: B).
Make sure it’s not ADHD
Adam J. Zolotor, MD, MPH
University of North Carolina at Chapel Hill
When evaluating a child for mental health, behavioral, or academic concerns, I always begin with an assessment targeting potential attention deficit hyperactivity disorder (ADHD). Distinguishing mania from hyperactivity and impulsivity is difficult. The most useful clue is family history. Suspicion of bipolar disorder (based on mood cycling or family history) would prompt me to refer to a child mental health specialist. Also, when I’m treating a child with ADHD, I consider alternate or comorbid conditions when he or she fails to achieve behavioral goals.
Of the rating scales reviewed above, I consider the P-GBI and the P-YMRS useful in risk stratification. However, screening instruments are less useful when a disease is rare (as with childhood bipolar disorder). Children with hyperactivity and impulsivity may have a range of conditions from hyperthyroidism to anxiety disorders, but we must listen to the history, observe the patient, and proceed with an evaluation based on the likelihood of disease.
Evidence summary
Retrospective analysis of 2 large cohort studies of adults with bipolar disorder indicated that at least 50% of these patients had an onset of illness before age 19, establishing support for the presence of bipolar disorder among children and adolescents.1 The criteria in the 4th edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV) cannot be easily applied to most children and adolescents with bipolar disorder because most do not meet the criteria for Bipolar I or II, but fall into the less well-defined category Bipolar NOS (not otherwise specified).2,3
Compared with adults, children and adolescents are more difficult to diagnose because they are less likely to have discrete episodes of mania, and instead present with severe irritability, rapid cycling, or mixed mania.2,4 In laddition, symptoms progress and evolve as children and adolescents grow.1 Comorbid disorders such as ADHD, oppositional defiant disorder, conduct disorder, and learning disorders are common in this population, further complicating diagnosis.2
Screening instruments are imperfect
Different versions of the KSADS have been used in most research studies on this disorder.2 Despite this, concerns about the validity of the instrument still exist because of lack of sufficient testing, vagueness of the diagnostic criteria, and the subjective nature of the test.5,6 Because specialized training is required to administer the test and testing can last a full day, its use in most office settings is impractical. It is also not meant as a stand-alone test, but to be used in conjunction with a clinical evaluation by a trained mental health professional.7
In a general clinical setting, family history and selected screening instruments may help to increase or decrease clinical suspicion for the disorder and guide referral for more specialized evaluation by a child mental health provider. In addition, a meta-analysis found that children or adolescents who have a biologic parent with bipolar disorder have 2 to 10 times the odds of being diagnosed with bipolar disorder.7
Three screening tests (CBCL, P-GBI, and P-YMRS) available for the office setting use parent-reported scores, and perform best when compared with KSADS as the standard.3 These instruments were associated with likelihood ratios that significantly improved the odds of diagnosis and could allow clinicians to stratify patients as high or low risk (TABLE).3
TABLE
Likelihood ratios for 3 screening tools you can use in the office
| For ages 5–10* | For ages 11–17† | |||||||
| IF THE SCORE IS… | IF THE SCORE IS… | |||||||
| LOW | MOD. LOW | HIGH | VERY HIGH | LOW | MOD. LOW | HIGH | VERY HIGH | |
| THEN THE LR FOR THE INSTRUMENT IS… | THEN THE LR FOR THE INSTRUMENT IS… | |||||||
| P-YMRS | 0.08 | 0.48 | 6.94 | 8.92 | 0.20 | 0 .32 | 4.07 | 7.41 |
| P-GBI | 0.10 | 0.48 | 4.90 | 6.29 | 0.06 | 0.25 | 4.82 | 9.21 |
| CBCL | 0.07 | 0.47 | 3.15 | 3.52 | 0.04 | 0.53 | 2.65 | 4.29 |
| * Population studied had a 50.3% prevalence of bipolar disorder. | † Population studied had a 40.7% prevalence of bipolar disorder. | |||||||
Recommendations from others
Two consensus conferences, a Canadian guideline, and a National Institute of Mental Health round-table all concluded that there is currently no ideal test for the diagnosis of child and adolescent bipolar disorder, but that such an instrument needed to be developed.2,5,6,8 One consensus conference further concluded that the diagnosis is best made by childhood mental health specialists based on multiple informants, such as the child and parents, with symptoms present in at least 2 settings or by direct observation.6
A Canadian consensus conference proposed screening patients with depressive symptoms for a history of hypomanic or manic symptoms, and consider an underlying mood disorder in those with vague or nonspecific somatic symptoms or reverse vegetative symptoms (eg, hypersomnia and hyperphagia). Their recommendations also emphasized screening for family history of bipolar disorder when there were clinical concerns.8
No single, well-validated screening instrument for clinical diagnosis of bipolar disorder in children exists. That said, the Kiddie Schedule for Affective Disorders and Schizophrenia (KSADS), a semi-structured interview, along with clinical evaluation by a childhood mental health specialist, is used most frequently in major research studies (strength of recommendation [SOR]: C).
As a screening tool in the primary care setting, family history of bipolar disorder in either biologic parent increases the odds of diagnosis (SOR: A). High or low scores on parent-reported screening tests (Parent Young Mania Rating Scale [P-YMRS], Parent General Behavior Inventory [P-GBI], and Child Behavior Checklist [CBCL]) also significantly increase or decrease the likelihood of diagnosis (SOR: B).
Make sure it’s not ADHD
Adam J. Zolotor, MD, MPH
University of North Carolina at Chapel Hill
When evaluating a child for mental health, behavioral, or academic concerns, I always begin with an assessment targeting potential attention deficit hyperactivity disorder (ADHD). Distinguishing mania from hyperactivity and impulsivity is difficult. The most useful clue is family history. Suspicion of bipolar disorder (based on mood cycling or family history) would prompt me to refer to a child mental health specialist. Also, when I’m treating a child with ADHD, I consider alternate or comorbid conditions when he or she fails to achieve behavioral goals.
Of the rating scales reviewed above, I consider the P-GBI and the P-YMRS useful in risk stratification. However, screening instruments are less useful when a disease is rare (as with childhood bipolar disorder). Children with hyperactivity and impulsivity may have a range of conditions from hyperthyroidism to anxiety disorders, but we must listen to the history, observe the patient, and proceed with an evaluation based on the likelihood of disease.
Evidence summary
Retrospective analysis of 2 large cohort studies of adults with bipolar disorder indicated that at least 50% of these patients had an onset of illness before age 19, establishing support for the presence of bipolar disorder among children and adolescents.1 The criteria in the 4th edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV) cannot be easily applied to most children and adolescents with bipolar disorder because most do not meet the criteria for Bipolar I or II, but fall into the less well-defined category Bipolar NOS (not otherwise specified).2,3
Compared with adults, children and adolescents are more difficult to diagnose because they are less likely to have discrete episodes of mania, and instead present with severe irritability, rapid cycling, or mixed mania.2,4 In laddition, symptoms progress and evolve as children and adolescents grow.1 Comorbid disorders such as ADHD, oppositional defiant disorder, conduct disorder, and learning disorders are common in this population, further complicating diagnosis.2
Screening instruments are imperfect
Different versions of the KSADS have been used in most research studies on this disorder.2 Despite this, concerns about the validity of the instrument still exist because of lack of sufficient testing, vagueness of the diagnostic criteria, and the subjective nature of the test.5,6 Because specialized training is required to administer the test and testing can last a full day, its use in most office settings is impractical. It is also not meant as a stand-alone test, but to be used in conjunction with a clinical evaluation by a trained mental health professional.7
In a general clinical setting, family history and selected screening instruments may help to increase or decrease clinical suspicion for the disorder and guide referral for more specialized evaluation by a child mental health provider. In addition, a meta-analysis found that children or adolescents who have a biologic parent with bipolar disorder have 2 to 10 times the odds of being diagnosed with bipolar disorder.7
Three screening tests (CBCL, P-GBI, and P-YMRS) available for the office setting use parent-reported scores, and perform best when compared with KSADS as the standard.3 These instruments were associated with likelihood ratios that significantly improved the odds of diagnosis and could allow clinicians to stratify patients as high or low risk (TABLE).3
TABLE
Likelihood ratios for 3 screening tools you can use in the office
| For ages 5–10* | For ages 11–17† | |||||||
| IF THE SCORE IS… | IF THE SCORE IS… | |||||||
| LOW | MOD. LOW | HIGH | VERY HIGH | LOW | MOD. LOW | HIGH | VERY HIGH | |
| THEN THE LR FOR THE INSTRUMENT IS… | THEN THE LR FOR THE INSTRUMENT IS… | |||||||
| P-YMRS | 0.08 | 0.48 | 6.94 | 8.92 | 0.20 | 0 .32 | 4.07 | 7.41 |
| P-GBI | 0.10 | 0.48 | 4.90 | 6.29 | 0.06 | 0.25 | 4.82 | 9.21 |
| CBCL | 0.07 | 0.47 | 3.15 | 3.52 | 0.04 | 0.53 | 2.65 | 4.29 |
| * Population studied had a 50.3% prevalence of bipolar disorder. | † Population studied had a 40.7% prevalence of bipolar disorder. | |||||||
Recommendations from others
Two consensus conferences, a Canadian guideline, and a National Institute of Mental Health round-table all concluded that there is currently no ideal test for the diagnosis of child and adolescent bipolar disorder, but that such an instrument needed to be developed.2,5,6,8 One consensus conference further concluded that the diagnosis is best made by childhood mental health specialists based on multiple informants, such as the child and parents, with symptoms present in at least 2 settings or by direct observation.6
A Canadian consensus conference proposed screening patients with depressive symptoms for a history of hypomanic or manic symptoms, and consider an underlying mood disorder in those with vague or nonspecific somatic symptoms or reverse vegetative symptoms (eg, hypersomnia and hyperphagia). Their recommendations also emphasized screening for family history of bipolar disorder when there were clinical concerns.8
1. Post R, Kowatch R. The health care crisis of childhood-onset bipolar illness: some recommendations for its amelioration. J Clin Psychiatry 2006;67:115-125.
2. National Institute of Mental Health research round-table on prepubertal bipolar disorder J Am Acad Chld Adolesc Psychiatry 2001;40:871-878.
3. Youngstrom E, Findling R, Calabrese J, et al. Comparing the diagnostic accuracy of six potential screening instruments for bipolar disorder in youths aged 5 to 17 years. J Am Acad Child Adolesc Psychiatry 2004;43:847-858.
4. Weckerly J. Pediatric bipolar mood disorder. J Dev Behav Pediatr 2002;23:42-56.
5. Coyle J, Pine D, Charney D, et al. Depression and bipolar support alliance consensus statement on the unmet needs in diagnosis and treatment of mood disorders in children and adolescents. J Am Acad Child Adolesc Psychiatry 2003;42:1494-1503.
6. Carlson G, Jensen P, Findling R, et al. Methodological Issues and Controversies in Clinical Trials with Child and Adolescent Patients with Bipolar Disorder: Report of a Consensus Conference. J Child Adolesc Psychopharamcol 2003;13:13-27.
7. Youngstrom E, Findling R, Youngstrom J, Calabrese J. Toward an evidence-based assessment of pediatric bipolar disorder. J Clin Child Adolesc Psychol 2005;34:433-448.
8. Yatham L, Kennedy S, O’Donovan C, et al. Canadian Network for Mood and Anxiety Treatments (CAN-MAT) guidelines for the management of patients with bipolar disorder: consensus and controversies. Bipolar Disord 2005;7(Suppl 3):5-69.
1. Post R, Kowatch R. The health care crisis of childhood-onset bipolar illness: some recommendations for its amelioration. J Clin Psychiatry 2006;67:115-125.
2. National Institute of Mental Health research round-table on prepubertal bipolar disorder J Am Acad Chld Adolesc Psychiatry 2001;40:871-878.
3. Youngstrom E, Findling R, Calabrese J, et al. Comparing the diagnostic accuracy of six potential screening instruments for bipolar disorder in youths aged 5 to 17 years. J Am Acad Child Adolesc Psychiatry 2004;43:847-858.
4. Weckerly J. Pediatric bipolar mood disorder. J Dev Behav Pediatr 2002;23:42-56.
5. Coyle J, Pine D, Charney D, et al. Depression and bipolar support alliance consensus statement on the unmet needs in diagnosis and treatment of mood disorders in children and adolescents. J Am Acad Child Adolesc Psychiatry 2003;42:1494-1503.
6. Carlson G, Jensen P, Findling R, et al. Methodological Issues and Controversies in Clinical Trials with Child and Adolescent Patients with Bipolar Disorder: Report of a Consensus Conference. J Child Adolesc Psychopharamcol 2003;13:13-27.
7. Youngstrom E, Findling R, Youngstrom J, Calabrese J. Toward an evidence-based assessment of pediatric bipolar disorder. J Clin Child Adolesc Psychol 2005;34:433-448.
8. Yatham L, Kennedy S, O’Donovan C, et al. Canadian Network for Mood and Anxiety Treatments (CAN-MAT) guidelines for the management of patients with bipolar disorder: consensus and controversies. Bipolar Disord 2005;7(Suppl 3):5-69.
Evidence-based answers from the Family Physicians Inquiries Network
Which nondrug alternatives can help with insomnia?
Cognitive behavioral therapy (CBT) interventions—particularly stimulus control and sleep hygiene—are well-validated, effective treatments for chronic insomnia that are equivalent or superior to pharmacological interventions (strength of recommendation: A, based on systematic reviews). The long-term efficacy of CBT interventions, and their successful implementation by primary care physicians (as compared with behavioral science providers), is unclear.
Can I provide these interventions without a referral?
John D Hallgren, Lt Col, USAF, MC
Uniformed Services University of the Health Sciences, RAF Menwith Hill, UK
A large proportion of people in my patient population are shift workers, so chronic insomnia plays a large role in my daily workload, both directly and indirectly. This summary tells me that I have a proven and equally efficacious alternative to drugs for these sufferers—which is great.
However, I was disappointed to see that none of the CBT interventions were performed by family physicians in the office. So the good news is that I have a nondrug intervention for insomnia; the bad news is I don’t know if it’s something I can provide without a referral. Maybe it’s time for some practice-based research to see if that is possible.
Evidence summary
Approximately 10% to 15% of adults complain of chronic insomnia, best defined as difficulty initiating or maintaining sleep 3 or more nights per week for 6 months or longer, with secondary impairments in daytime functioning, including fatigue and disturbed mood.1-3
Behavioral and psychological treatments have emerged as increasingly popular adjunctive interventions to pharmacotherapy and as independent interventions for chronic insomnia. No evidence exists that behavioral treatments have adverse effects.1
Sleep hygiene, relaxation training, and cognitive therapy improve sleep
CBT interventions are based on the notion that distorted thoughts about sleep and learned behavior patterns hyperarouse the central nervous system and deregulate sleep cycles, resulting in chronic insomnia.4 CBT interventions combine empirically tested behavioral, cognitive, and educational procedures to alter faulty beliefs and attitudes, modify sleep habits, and regulate sleep-wake schedules.3
These interventions include stimulus control, sleep hygiene, sleep restriction, relaxation training, and cognitive therapy.5 These methods can be used separately; however, they are increasingly being used together to treat the complexities of individual patients.5
Five recent high-quality randomized control trials (RCTs) confirmed findings from earlier RCTs that CBT methods improve sleep.5 Compared with those given a placebo or placed on a waiting list, CBT-treated patients in these RCTs reported clinically significant improvements in sleep onset latency, sleep efficiency, time awake after sleep onset, and total sleep time. In one RCT, 64% of CBT patients had improvements in sleep efficiency and time awake after sleep onset, compared with 8% who improved with a placebo intervention (number needed to treat [NNT]=1.8).5 Further, sleep onset latency for primary care patients with chronic insomnia was decreased from 61 to 28 minutes, compared with 74 to 70 minutes for a waiting-list group.5 The maintenance of sleep gains from CBT beyond 1 year is unknown since no published RCT clinical trials to date have lasted longer than 12 months.1
An important related meta-analysis of 21 studies validated behavior therapy, and revealed CBT reduced sleep onset latency by an additional 8.8 minutes over medication (95% confidence interval, 0.17–1.04 minutes).6 Although not superior on other outcomes, behavior therapy produced similar short-term results to pharmacotherapy across all other sleep measures, without attendant medication side effects.
Stimulus control is the most effective CBT intervention
A recent systematic review with meta-analysis of 37 clinical investigations determined that stimulus control was the most effective CBT intervention.3 Stimulus control consists of 5 basic instructions (TABLE) designed to help the patient reassociate sleep stimuli (ie, bed/bedroom) with falling asleep and establishing consistent sleep-wake schedules. These 5 instructions are frequently used in combination with CBT sleep hygiene techniques (TABLE) and can be easily integrated into the office setting.3,4
Among the CBT techniques, stimulus control and sleep hygiene are the least time-consuming and may be more easily applied in the primary care setting; however, minimal research has been done into the specific incorporation of CBT into primary care settings.
Researchers conducting a single-blind randomized group study in a Veterans Affairs primary care clinic concluded that an abbreviated CBT approach with two 25-minute sessions effectively improved participant sleep onset latency, and time awake after sleep onset.7 Researchers reviewed participants’ sleep logs and a behavioral health provider offered patients a condensed education on sleep hygiene, stimulus control, and sleep restrictions strategies. The study was limited because of small sample size (<25). Generalizability to practice is restricted because sessions were conducted by a behavioral health provider, not a family physician.
TABLE
Patient needs a good night’s sleep? Offer this advice
| STIMULUS CONTROL INSTRUCTIONS3 |
|
| SLEEP HYGIENE INSTRUCTIONS4 |
|
Recommendations from others
The Agency for Healthcare Research and Quality recommends CBT as an effective treatment in the management of chronic .8 It also recommends that further large-scale RCTs be conducted to establish CBT’s effectiveness across subsets of the population of individuals with chronic (ie, gender, age, shift workers, and those with psychiatric illnesses).
The American Psychological Association (APA) recommends CBT as the “treatment of choice” for chronic , with 70% to 80% of patients showing a treatment response.9
Acknowledgments
The opinions and assertions contained herein are the private views of the authors and not to be construed as official, or as reflecting the views of the US Air Force Medical Service or the US Air Force at large.
1. NIH State-of-the-Science Conference Statement on Manifestations and Management of Chronic Insomnia in Adults. NIH Consensus Science Statement. 2005; 22(2). Available at: consensus.nih.gov/2005/2005InsomniaSOS026main.htm. Accessed on September 4, 2007.
2. Ohayon M. Epidemiology of insomnia: What we know and what we still need to learn. Sleep Med Rev 2002;6:97-111.
3. Morin CM. Cognitive-behavioral approaches to the treatment of insomnia. J Clin Psychiatry 2004;65 Suppl 16:33-40.
4. Smith MT, Neubauer DN. Cognitive behavioral therapy for chronic insomnia. Clinical Cornerstone 2003;5:1-9.
5. Morin CM, Bootzin RR, Buysse DJ, Edinger JD, Espie CA. Psychological and behavioral treatment of insomnia: Update of the recent evidence (1998–2004). Sleep 2006;29:1398-1413.
6. Smith MT, Perlis ML, Park A, et al. Comparative meta-analysis of pharmacotherapy and behavior therapy for persistent insomnia. Am J Psychiatry 2002;159:5-11.
7. Edinger JD, Sampson WS. A primary care “friendly” cognitive behavioral insomnia therapy. Sleep 2003;26:177-182.
8. Buscemi N, Vandermeer B, Friesen C, et al. Manifestations of chronic insomnia in adults. Evidence report/technology assessment No. 125. (Prepared by the University of Alberta Evidence-based Practice Center, under Contract N. C400000021.) AHRQ Publication No. 05-E021-1. Rockville, Md: Agency for Healthcare Research and Quality. June 2005. Available at: www.ahrq.gov/downloads/pub/evidence/pdf/insomnia/insomnia.pdf. Accessed on September 4, 2007.
9. American Psychological Association Web site. Getting a good night’s sleep with the help of psychology. Available at: www.psychologymatters.org/insomnia.html. Accessed on September 4, 2007.
Cognitive behavioral therapy (CBT) interventions—particularly stimulus control and sleep hygiene—are well-validated, effective treatments for chronic insomnia that are equivalent or superior to pharmacological interventions (strength of recommendation: A, based on systematic reviews). The long-term efficacy of CBT interventions, and their successful implementation by primary care physicians (as compared with behavioral science providers), is unclear.
Can I provide these interventions without a referral?
John D Hallgren, Lt Col, USAF, MC
Uniformed Services University of the Health Sciences, RAF Menwith Hill, UK
A large proportion of people in my patient population are shift workers, so chronic insomnia plays a large role in my daily workload, both directly and indirectly. This summary tells me that I have a proven and equally efficacious alternative to drugs for these sufferers—which is great.
However, I was disappointed to see that none of the CBT interventions were performed by family physicians in the office. So the good news is that I have a nondrug intervention for insomnia; the bad news is I don’t know if it’s something I can provide without a referral. Maybe it’s time for some practice-based research to see if that is possible.
Evidence summary
Approximately 10% to 15% of adults complain of chronic insomnia, best defined as difficulty initiating or maintaining sleep 3 or more nights per week for 6 months or longer, with secondary impairments in daytime functioning, including fatigue and disturbed mood.1-3
Behavioral and psychological treatments have emerged as increasingly popular adjunctive interventions to pharmacotherapy and as independent interventions for chronic insomnia. No evidence exists that behavioral treatments have adverse effects.1
Sleep hygiene, relaxation training, and cognitive therapy improve sleep
CBT interventions are based on the notion that distorted thoughts about sleep and learned behavior patterns hyperarouse the central nervous system and deregulate sleep cycles, resulting in chronic insomnia.4 CBT interventions combine empirically tested behavioral, cognitive, and educational procedures to alter faulty beliefs and attitudes, modify sleep habits, and regulate sleep-wake schedules.3
These interventions include stimulus control, sleep hygiene, sleep restriction, relaxation training, and cognitive therapy.5 These methods can be used separately; however, they are increasingly being used together to treat the complexities of individual patients.5
Five recent high-quality randomized control trials (RCTs) confirmed findings from earlier RCTs that CBT methods improve sleep.5 Compared with those given a placebo or placed on a waiting list, CBT-treated patients in these RCTs reported clinically significant improvements in sleep onset latency, sleep efficiency, time awake after sleep onset, and total sleep time. In one RCT, 64% of CBT patients had improvements in sleep efficiency and time awake after sleep onset, compared with 8% who improved with a placebo intervention (number needed to treat [NNT]=1.8).5 Further, sleep onset latency for primary care patients with chronic insomnia was decreased from 61 to 28 minutes, compared with 74 to 70 minutes for a waiting-list group.5 The maintenance of sleep gains from CBT beyond 1 year is unknown since no published RCT clinical trials to date have lasted longer than 12 months.1
An important related meta-analysis of 21 studies validated behavior therapy, and revealed CBT reduced sleep onset latency by an additional 8.8 minutes over medication (95% confidence interval, 0.17–1.04 minutes).6 Although not superior on other outcomes, behavior therapy produced similar short-term results to pharmacotherapy across all other sleep measures, without attendant medication side effects.
Stimulus control is the most effective CBT intervention
A recent systematic review with meta-analysis of 37 clinical investigations determined that stimulus control was the most effective CBT intervention.3 Stimulus control consists of 5 basic instructions (TABLE) designed to help the patient reassociate sleep stimuli (ie, bed/bedroom) with falling asleep and establishing consistent sleep-wake schedules. These 5 instructions are frequently used in combination with CBT sleep hygiene techniques (TABLE) and can be easily integrated into the office setting.3,4
Among the CBT techniques, stimulus control and sleep hygiene are the least time-consuming and may be more easily applied in the primary care setting; however, minimal research has been done into the specific incorporation of CBT into primary care settings.
Researchers conducting a single-blind randomized group study in a Veterans Affairs primary care clinic concluded that an abbreviated CBT approach with two 25-minute sessions effectively improved participant sleep onset latency, and time awake after sleep onset.7 Researchers reviewed participants’ sleep logs and a behavioral health provider offered patients a condensed education on sleep hygiene, stimulus control, and sleep restrictions strategies. The study was limited because of small sample size (<25). Generalizability to practice is restricted because sessions were conducted by a behavioral health provider, not a family physician.
TABLE
Patient needs a good night’s sleep? Offer this advice
| STIMULUS CONTROL INSTRUCTIONS3 |
|
| SLEEP HYGIENE INSTRUCTIONS4 |
|
Recommendations from others
The Agency for Healthcare Research and Quality recommends CBT as an effective treatment in the management of chronic .8 It also recommends that further large-scale RCTs be conducted to establish CBT’s effectiveness across subsets of the population of individuals with chronic (ie, gender, age, shift workers, and those with psychiatric illnesses).
The American Psychological Association (APA) recommends CBT as the “treatment of choice” for chronic , with 70% to 80% of patients showing a treatment response.9
Acknowledgments
The opinions and assertions contained herein are the private views of the authors and not to be construed as official, or as reflecting the views of the US Air Force Medical Service or the US Air Force at large.
Cognitive behavioral therapy (CBT) interventions—particularly stimulus control and sleep hygiene—are well-validated, effective treatments for chronic insomnia that are equivalent or superior to pharmacological interventions (strength of recommendation: A, based on systematic reviews). The long-term efficacy of CBT interventions, and their successful implementation by primary care physicians (as compared with behavioral science providers), is unclear.
Can I provide these interventions without a referral?
John D Hallgren, Lt Col, USAF, MC
Uniformed Services University of the Health Sciences, RAF Menwith Hill, UK
A large proportion of people in my patient population are shift workers, so chronic insomnia plays a large role in my daily workload, both directly and indirectly. This summary tells me that I have a proven and equally efficacious alternative to drugs for these sufferers—which is great.
However, I was disappointed to see that none of the CBT interventions were performed by family physicians in the office. So the good news is that I have a nondrug intervention for insomnia; the bad news is I don’t know if it’s something I can provide without a referral. Maybe it’s time for some practice-based research to see if that is possible.
Evidence summary
Approximately 10% to 15% of adults complain of chronic insomnia, best defined as difficulty initiating or maintaining sleep 3 or more nights per week for 6 months or longer, with secondary impairments in daytime functioning, including fatigue and disturbed mood.1-3
Behavioral and psychological treatments have emerged as increasingly popular adjunctive interventions to pharmacotherapy and as independent interventions for chronic insomnia. No evidence exists that behavioral treatments have adverse effects.1
Sleep hygiene, relaxation training, and cognitive therapy improve sleep
CBT interventions are based on the notion that distorted thoughts about sleep and learned behavior patterns hyperarouse the central nervous system and deregulate sleep cycles, resulting in chronic insomnia.4 CBT interventions combine empirically tested behavioral, cognitive, and educational procedures to alter faulty beliefs and attitudes, modify sleep habits, and regulate sleep-wake schedules.3
These interventions include stimulus control, sleep hygiene, sleep restriction, relaxation training, and cognitive therapy.5 These methods can be used separately; however, they are increasingly being used together to treat the complexities of individual patients.5
Five recent high-quality randomized control trials (RCTs) confirmed findings from earlier RCTs that CBT methods improve sleep.5 Compared with those given a placebo or placed on a waiting list, CBT-treated patients in these RCTs reported clinically significant improvements in sleep onset latency, sleep efficiency, time awake after sleep onset, and total sleep time. In one RCT, 64% of CBT patients had improvements in sleep efficiency and time awake after sleep onset, compared with 8% who improved with a placebo intervention (number needed to treat [NNT]=1.8).5 Further, sleep onset latency for primary care patients with chronic insomnia was decreased from 61 to 28 minutes, compared with 74 to 70 minutes for a waiting-list group.5 The maintenance of sleep gains from CBT beyond 1 year is unknown since no published RCT clinical trials to date have lasted longer than 12 months.1
An important related meta-analysis of 21 studies validated behavior therapy, and revealed CBT reduced sleep onset latency by an additional 8.8 minutes over medication (95% confidence interval, 0.17–1.04 minutes).6 Although not superior on other outcomes, behavior therapy produced similar short-term results to pharmacotherapy across all other sleep measures, without attendant medication side effects.
Stimulus control is the most effective CBT intervention
A recent systematic review with meta-analysis of 37 clinical investigations determined that stimulus control was the most effective CBT intervention.3 Stimulus control consists of 5 basic instructions (TABLE) designed to help the patient reassociate sleep stimuli (ie, bed/bedroom) with falling asleep and establishing consistent sleep-wake schedules. These 5 instructions are frequently used in combination with CBT sleep hygiene techniques (TABLE) and can be easily integrated into the office setting.3,4
Among the CBT techniques, stimulus control and sleep hygiene are the least time-consuming and may be more easily applied in the primary care setting; however, minimal research has been done into the specific incorporation of CBT into primary care settings.
Researchers conducting a single-blind randomized group study in a Veterans Affairs primary care clinic concluded that an abbreviated CBT approach with two 25-minute sessions effectively improved participant sleep onset latency, and time awake after sleep onset.7 Researchers reviewed participants’ sleep logs and a behavioral health provider offered patients a condensed education on sleep hygiene, stimulus control, and sleep restrictions strategies. The study was limited because of small sample size (<25). Generalizability to practice is restricted because sessions were conducted by a behavioral health provider, not a family physician.
TABLE
Patient needs a good night’s sleep? Offer this advice
| STIMULUS CONTROL INSTRUCTIONS3 |
|
| SLEEP HYGIENE INSTRUCTIONS4 |
|
Recommendations from others
The Agency for Healthcare Research and Quality recommends CBT as an effective treatment in the management of chronic .8 It also recommends that further large-scale RCTs be conducted to establish CBT’s effectiveness across subsets of the population of individuals with chronic (ie, gender, age, shift workers, and those with psychiatric illnesses).
The American Psychological Association (APA) recommends CBT as the “treatment of choice” for chronic , with 70% to 80% of patients showing a treatment response.9
Acknowledgments
The opinions and assertions contained herein are the private views of the authors and not to be construed as official, or as reflecting the views of the US Air Force Medical Service or the US Air Force at large.
1. NIH State-of-the-Science Conference Statement on Manifestations and Management of Chronic Insomnia in Adults. NIH Consensus Science Statement. 2005; 22(2). Available at: consensus.nih.gov/2005/2005InsomniaSOS026main.htm. Accessed on September 4, 2007.
2. Ohayon M. Epidemiology of insomnia: What we know and what we still need to learn. Sleep Med Rev 2002;6:97-111.
3. Morin CM. Cognitive-behavioral approaches to the treatment of insomnia. J Clin Psychiatry 2004;65 Suppl 16:33-40.
4. Smith MT, Neubauer DN. Cognitive behavioral therapy for chronic insomnia. Clinical Cornerstone 2003;5:1-9.
5. Morin CM, Bootzin RR, Buysse DJ, Edinger JD, Espie CA. Psychological and behavioral treatment of insomnia: Update of the recent evidence (1998–2004). Sleep 2006;29:1398-1413.
6. Smith MT, Perlis ML, Park A, et al. Comparative meta-analysis of pharmacotherapy and behavior therapy for persistent insomnia. Am J Psychiatry 2002;159:5-11.
7. Edinger JD, Sampson WS. A primary care “friendly” cognitive behavioral insomnia therapy. Sleep 2003;26:177-182.
8. Buscemi N, Vandermeer B, Friesen C, et al. Manifestations of chronic insomnia in adults. Evidence report/technology assessment No. 125. (Prepared by the University of Alberta Evidence-based Practice Center, under Contract N. C400000021.) AHRQ Publication No. 05-E021-1. Rockville, Md: Agency for Healthcare Research and Quality. June 2005. Available at: www.ahrq.gov/downloads/pub/evidence/pdf/insomnia/insomnia.pdf. Accessed on September 4, 2007.
9. American Psychological Association Web site. Getting a good night’s sleep with the help of psychology. Available at: www.psychologymatters.org/insomnia.html. Accessed on September 4, 2007.
1. NIH State-of-the-Science Conference Statement on Manifestations and Management of Chronic Insomnia in Adults. NIH Consensus Science Statement. 2005; 22(2). Available at: consensus.nih.gov/2005/2005InsomniaSOS026main.htm. Accessed on September 4, 2007.
2. Ohayon M. Epidemiology of insomnia: What we know and what we still need to learn. Sleep Med Rev 2002;6:97-111.
3. Morin CM. Cognitive-behavioral approaches to the treatment of insomnia. J Clin Psychiatry 2004;65 Suppl 16:33-40.
4. Smith MT, Neubauer DN. Cognitive behavioral therapy for chronic insomnia. Clinical Cornerstone 2003;5:1-9.
5. Morin CM, Bootzin RR, Buysse DJ, Edinger JD, Espie CA. Psychological and behavioral treatment of insomnia: Update of the recent evidence (1998–2004). Sleep 2006;29:1398-1413.
6. Smith MT, Perlis ML, Park A, et al. Comparative meta-analysis of pharmacotherapy and behavior therapy for persistent insomnia. Am J Psychiatry 2002;159:5-11.
7. Edinger JD, Sampson WS. A primary care “friendly” cognitive behavioral insomnia therapy. Sleep 2003;26:177-182.
8. Buscemi N, Vandermeer B, Friesen C, et al. Manifestations of chronic insomnia in adults. Evidence report/technology assessment No. 125. (Prepared by the University of Alberta Evidence-based Practice Center, under Contract N. C400000021.) AHRQ Publication No. 05-E021-1. Rockville, Md: Agency for Healthcare Research and Quality. June 2005. Available at: www.ahrq.gov/downloads/pub/evidence/pdf/insomnia/insomnia.pdf. Accessed on September 4, 2007.
9. American Psychological Association Web site. Getting a good night’s sleep with the help of psychology. Available at: www.psychologymatters.org/insomnia.html. Accessed on September 4, 2007.
Evidence-based answers from the Family Physicians Inquiries Network
Should we use appetite stimulants for malnourished elderly patients?
Probably not. Only 1 appetite stimulate, megestrol acetate oral suspension (Megace) at 400 mg or 800 mg daily, has been studied in this population. The data show only limited benefit, mixed outcomes, and potential harm (strength of recommendation: B, based on small, randomized, controlled trials).
Good advice for a common problem
Kayleen P. Papin, MD
Medical College of Wisconsin, Milwaukee
This question hits home for me. I recently sat down with the husband, and main caregiver, of a woman with advanced dementia. The woman eats very little and is losing weight despite her husband’s great efforts at encouraging her to eat. Under the care of another physician, she had been given megestrol acetate and there had been some improvement. Her visit to my office was an opportunity to continue an ongoing conversation with her husband about his wife’s overall decline, her advancing dementia, and the sorrow he was feeling over her failing health.
Should we use appetite stimulants in malnourished elderly patients? “probably not.” that is a good place to start to avoid harm to our most frail, declining, elderly patients for whom we care. That leaves open flexibility to patient, family, and caregiver preferences, but reminds us that the most important part of caring for these patients and their families is clear, compassionate communication regarding goals and expectations.
Evidence summary
Although a number of studies have evaluated various appetite stimulants—megestrol, dronabinol (Marinol), cyproheptadine (Periactin), thalidomide (Thalomid), pentoxifylline (Pentoxil/Trental), nandrolone decanoate (DecaDurabolin), oxandrolone (Oxandrin), and corticosteroids—in patients with AIDS, anorexia cachexia syndrome, and advanced cancer, only megestrol has been studied in malnourished elderly patients.
Two studies, mixed results
One placebo-controlled randomized clinical trial studied 45 malnourished patients who were recently discharged from an acute care hospital to a nursing home. The patients (predominately female, with a mean age of 83) were randomized into 4 treatment arms (placebo or megestrol 200 mg, 400 mg, or 800 mg daily) and followed for 63 days.
Only those receiving megestrol (400 mg or 800 mg daily) demonstrated a statistically significant increase in patient appetite and a dose-responsive increase in prealbumin level at the 20 day interim analysis (7.5 and 9.0 mg/dL, respectfully). But at the final assessment (63 days), only the 400-mg dose maintained a statistically significant increase in prealbumin over placebo. However, there was no significant improvement in serum albumin or clinical endpoints (weight, functional status, or health-related quality of life).1
In contrast, an earlier Veterans Administration (and predominantly male) study showed 13/21 of those treated with megestrol (800 mg daily for 12 weeks) noted weight gain (≥4 lb sustained at 3 months post-treatment), compared with 5/23 of those receiving placebo (number needed to treat [NNT]=2.5).2 Of note, only 9/26 patients had sustained weight gain in the megestrol group at the 12-month endpoint post-treatment, comparable with 7/25 in the placebo group.
Some small, but statistically significant, score improvements were noted during the treatment period in appetite and enjoyment of life; however, no differences were noted in scores on the more widely accepted Geriatric Depression Scale.
Adverse effects
As in all therapeutic interventions, benefit must be balanced against risk. The Megace ES package insert notes the following potential adverse effects: diarrhea, cardiomyopathy, palpitation, hepatomegaly, leukopenia, edema, paresthesia, confusion, convulsion, depression, neuropathy, hypesthesia and abnormal thinking, thrombophlebitis, pulmonary embolism, and glucose intolerance.3
To date, the prevalence rates of these potential adverse effects have only been studied in patients with AIDS. No data reflecting potential rates in elderly patients have been published.
Recommendations from others
The American Geriatric Society4 made 3 comments on appetite stimulation:
- There are no FDA-approved drugs available for the promotion of weight gain in older adults.
- A minority of patients receiving mirtazapine report appetite stimulation and weight gain.
- All drugs used for appetite have substantial potential adverse events.
We found only 1 national guideline on this topic: Unintentional Weight Loss in the Elderly from the University of Texas School of Nursing.5 The guideline indicates that drugs should not be used as first-line intervention in the elderly, as there has been inadequate testing in this population. Benefits are restricted to small weight gains without indication of decreased morbidity or mortality, improved quality of life, or improved functional ability.
Acknowledgments
The opinions and assertions contained herein are the private views of the author and not to be construed as official, or as reflecting the views of the US Air Force Medical Service or the US Air Force at large.
1. Reuben DB, Hirsch SH, Zhou K, Greendale GA. The effects of megestrol acetate suspension for elderly patients with reduced appetite after hospitalization: a phase II randomized clinical trial. J Am Geriatr Soc 2005;53:970-975.
2. Yeh SS, Wu SY, Lee TP, et al. Improvement in quality-of-life measures and stimulation of weight gain after treatment with megestrol acetate or suspension in geriatric cachexia: results of a double-blind, placebo controlled study. J Am Geriatr Soc 2001;48:485-492.
3. Megace Physicians’ Desk Reference 61st ed. Montvale, NJ: Thomson; 2007:2461-2463.
4. Malnutrition. Geriatrics at Your Fingertips [website]. Available at: www.geriatricsatyourfingertips.org/ebook/gayf_20.asp. Accessed August 6, 2007.
5. University of Texas, School of Nursing. Unintentional Weight Loss in the Elderly. Austin, Tex: University of Texas, School of Nursing; 2006. Available at: www.guideline.gov/summary/summary.aspx?doc_id=9435. Accessed August 6, 2007.
Probably not. Only 1 appetite stimulate, megestrol acetate oral suspension (Megace) at 400 mg or 800 mg daily, has been studied in this population. The data show only limited benefit, mixed outcomes, and potential harm (strength of recommendation: B, based on small, randomized, controlled trials).
Good advice for a common problem
Kayleen P. Papin, MD
Medical College of Wisconsin, Milwaukee
This question hits home for me. I recently sat down with the husband, and main caregiver, of a woman with advanced dementia. The woman eats very little and is losing weight despite her husband’s great efforts at encouraging her to eat. Under the care of another physician, she had been given megestrol acetate and there had been some improvement. Her visit to my office was an opportunity to continue an ongoing conversation with her husband about his wife’s overall decline, her advancing dementia, and the sorrow he was feeling over her failing health.
Should we use appetite stimulants in malnourished elderly patients? “probably not.” that is a good place to start to avoid harm to our most frail, declining, elderly patients for whom we care. That leaves open flexibility to patient, family, and caregiver preferences, but reminds us that the most important part of caring for these patients and their families is clear, compassionate communication regarding goals and expectations.
Evidence summary
Although a number of studies have evaluated various appetite stimulants—megestrol, dronabinol (Marinol), cyproheptadine (Periactin), thalidomide (Thalomid), pentoxifylline (Pentoxil/Trental), nandrolone decanoate (DecaDurabolin), oxandrolone (Oxandrin), and corticosteroids—in patients with AIDS, anorexia cachexia syndrome, and advanced cancer, only megestrol has been studied in malnourished elderly patients.
Two studies, mixed results
One placebo-controlled randomized clinical trial studied 45 malnourished patients who were recently discharged from an acute care hospital to a nursing home. The patients (predominately female, with a mean age of 83) were randomized into 4 treatment arms (placebo or megestrol 200 mg, 400 mg, or 800 mg daily) and followed for 63 days.
Only those receiving megestrol (400 mg or 800 mg daily) demonstrated a statistically significant increase in patient appetite and a dose-responsive increase in prealbumin level at the 20 day interim analysis (7.5 and 9.0 mg/dL, respectfully). But at the final assessment (63 days), only the 400-mg dose maintained a statistically significant increase in prealbumin over placebo. However, there was no significant improvement in serum albumin or clinical endpoints (weight, functional status, or health-related quality of life).1
In contrast, an earlier Veterans Administration (and predominantly male) study showed 13/21 of those treated with megestrol (800 mg daily for 12 weeks) noted weight gain (≥4 lb sustained at 3 months post-treatment), compared with 5/23 of those receiving placebo (number needed to treat [NNT]=2.5).2 Of note, only 9/26 patients had sustained weight gain in the megestrol group at the 12-month endpoint post-treatment, comparable with 7/25 in the placebo group.
Some small, but statistically significant, score improvements were noted during the treatment period in appetite and enjoyment of life; however, no differences were noted in scores on the more widely accepted Geriatric Depression Scale.
Adverse effects
As in all therapeutic interventions, benefit must be balanced against risk. The Megace ES package insert notes the following potential adverse effects: diarrhea, cardiomyopathy, palpitation, hepatomegaly, leukopenia, edema, paresthesia, confusion, convulsion, depression, neuropathy, hypesthesia and abnormal thinking, thrombophlebitis, pulmonary embolism, and glucose intolerance.3
To date, the prevalence rates of these potential adverse effects have only been studied in patients with AIDS. No data reflecting potential rates in elderly patients have been published.
Recommendations from others
The American Geriatric Society4 made 3 comments on appetite stimulation:
- There are no FDA-approved drugs available for the promotion of weight gain in older adults.
- A minority of patients receiving mirtazapine report appetite stimulation and weight gain.
- All drugs used for appetite have substantial potential adverse events.
We found only 1 national guideline on this topic: Unintentional Weight Loss in the Elderly from the University of Texas School of Nursing.5 The guideline indicates that drugs should not be used as first-line intervention in the elderly, as there has been inadequate testing in this population. Benefits are restricted to small weight gains without indication of decreased morbidity or mortality, improved quality of life, or improved functional ability.
Acknowledgments
The opinions and assertions contained herein are the private views of the author and not to be construed as official, or as reflecting the views of the US Air Force Medical Service or the US Air Force at large.
Probably not. Only 1 appetite stimulate, megestrol acetate oral suspension (Megace) at 400 mg or 800 mg daily, has been studied in this population. The data show only limited benefit, mixed outcomes, and potential harm (strength of recommendation: B, based on small, randomized, controlled trials).
Good advice for a common problem
Kayleen P. Papin, MD
Medical College of Wisconsin, Milwaukee
This question hits home for me. I recently sat down with the husband, and main caregiver, of a woman with advanced dementia. The woman eats very little and is losing weight despite her husband’s great efforts at encouraging her to eat. Under the care of another physician, she had been given megestrol acetate and there had been some improvement. Her visit to my office was an opportunity to continue an ongoing conversation with her husband about his wife’s overall decline, her advancing dementia, and the sorrow he was feeling over her failing health.
Should we use appetite stimulants in malnourished elderly patients? “probably not.” that is a good place to start to avoid harm to our most frail, declining, elderly patients for whom we care. That leaves open flexibility to patient, family, and caregiver preferences, but reminds us that the most important part of caring for these patients and their families is clear, compassionate communication regarding goals and expectations.
Evidence summary
Although a number of studies have evaluated various appetite stimulants—megestrol, dronabinol (Marinol), cyproheptadine (Periactin), thalidomide (Thalomid), pentoxifylline (Pentoxil/Trental), nandrolone decanoate (DecaDurabolin), oxandrolone (Oxandrin), and corticosteroids—in patients with AIDS, anorexia cachexia syndrome, and advanced cancer, only megestrol has been studied in malnourished elderly patients.
Two studies, mixed results
One placebo-controlled randomized clinical trial studied 45 malnourished patients who were recently discharged from an acute care hospital to a nursing home. The patients (predominately female, with a mean age of 83) were randomized into 4 treatment arms (placebo or megestrol 200 mg, 400 mg, or 800 mg daily) and followed for 63 days.
Only those receiving megestrol (400 mg or 800 mg daily) demonstrated a statistically significant increase in patient appetite and a dose-responsive increase in prealbumin level at the 20 day interim analysis (7.5 and 9.0 mg/dL, respectfully). But at the final assessment (63 days), only the 400-mg dose maintained a statistically significant increase in prealbumin over placebo. However, there was no significant improvement in serum albumin or clinical endpoints (weight, functional status, or health-related quality of life).1
In contrast, an earlier Veterans Administration (and predominantly male) study showed 13/21 of those treated with megestrol (800 mg daily for 12 weeks) noted weight gain (≥4 lb sustained at 3 months post-treatment), compared with 5/23 of those receiving placebo (number needed to treat [NNT]=2.5).2 Of note, only 9/26 patients had sustained weight gain in the megestrol group at the 12-month endpoint post-treatment, comparable with 7/25 in the placebo group.
Some small, but statistically significant, score improvements were noted during the treatment period in appetite and enjoyment of life; however, no differences were noted in scores on the more widely accepted Geriatric Depression Scale.
Adverse effects
As in all therapeutic interventions, benefit must be balanced against risk. The Megace ES package insert notes the following potential adverse effects: diarrhea, cardiomyopathy, palpitation, hepatomegaly, leukopenia, edema, paresthesia, confusion, convulsion, depression, neuropathy, hypesthesia and abnormal thinking, thrombophlebitis, pulmonary embolism, and glucose intolerance.3
To date, the prevalence rates of these potential adverse effects have only been studied in patients with AIDS. No data reflecting potential rates in elderly patients have been published.
Recommendations from others
The American Geriatric Society4 made 3 comments on appetite stimulation:
- There are no FDA-approved drugs available for the promotion of weight gain in older adults.
- A minority of patients receiving mirtazapine report appetite stimulation and weight gain.
- All drugs used for appetite have substantial potential adverse events.
We found only 1 national guideline on this topic: Unintentional Weight Loss in the Elderly from the University of Texas School of Nursing.5 The guideline indicates that drugs should not be used as first-line intervention in the elderly, as there has been inadequate testing in this population. Benefits are restricted to small weight gains without indication of decreased morbidity or mortality, improved quality of life, or improved functional ability.
Acknowledgments
The opinions and assertions contained herein are the private views of the author and not to be construed as official, or as reflecting the views of the US Air Force Medical Service or the US Air Force at large.
1. Reuben DB, Hirsch SH, Zhou K, Greendale GA. The effects of megestrol acetate suspension for elderly patients with reduced appetite after hospitalization: a phase II randomized clinical trial. J Am Geriatr Soc 2005;53:970-975.
2. Yeh SS, Wu SY, Lee TP, et al. Improvement in quality-of-life measures and stimulation of weight gain after treatment with megestrol acetate or suspension in geriatric cachexia: results of a double-blind, placebo controlled study. J Am Geriatr Soc 2001;48:485-492.
3. Megace Physicians’ Desk Reference 61st ed. Montvale, NJ: Thomson; 2007:2461-2463.
4. Malnutrition. Geriatrics at Your Fingertips [website]. Available at: www.geriatricsatyourfingertips.org/ebook/gayf_20.asp. Accessed August 6, 2007.
5. University of Texas, School of Nursing. Unintentional Weight Loss in the Elderly. Austin, Tex: University of Texas, School of Nursing; 2006. Available at: www.guideline.gov/summary/summary.aspx?doc_id=9435. Accessed August 6, 2007.
1. Reuben DB, Hirsch SH, Zhou K, Greendale GA. The effects of megestrol acetate suspension for elderly patients with reduced appetite after hospitalization: a phase II randomized clinical trial. J Am Geriatr Soc 2005;53:970-975.
2. Yeh SS, Wu SY, Lee TP, et al. Improvement in quality-of-life measures and stimulation of weight gain after treatment with megestrol acetate or suspension in geriatric cachexia: results of a double-blind, placebo controlled study. J Am Geriatr Soc 2001;48:485-492.
3. Megace Physicians’ Desk Reference 61st ed. Montvale, NJ: Thomson; 2007:2461-2463.
4. Malnutrition. Geriatrics at Your Fingertips [website]. Available at: www.geriatricsatyourfingertips.org/ebook/gayf_20.asp. Accessed August 6, 2007.
5. University of Texas, School of Nursing. Unintentional Weight Loss in the Elderly. Austin, Tex: University of Texas, School of Nursing; 2006. Available at: www.guideline.gov/summary/summary.aspx?doc_id=9435. Accessed August 6, 2007.
Evidence-based answers from the Family Physicians Inquiries Network