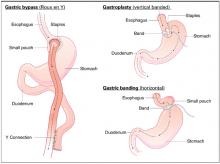
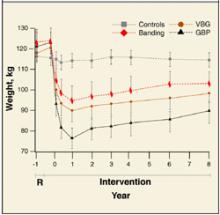
User login
What is the best way to evaluate and manage diarrhea in the febrile infant?
Routine infant diarrhea requires no lab work or cultures (strength of recommendation [SOR]: C); the degree of dehydration can be determined reliably by percent body weight change (SOR: B). However, bicarbonate may help rule out dehydration (SOR: B); electrolytes and blood urea nitrogen may be useful in evaluating complicated diarrhea with severe dehydration or when intravenous fluids are required; stool cultures are indicated for bloody or prolonged diarrhea, suspected food poisoning, or recent travel abroad (SOR: C).
Oral rehydrating solution is adequate fluid replacement for diarrhea associated with mild to moderate dehydration, followed by prompt refeeding with an age-appropriate diet (SOR: A); intravenous fluids are recommended for severe dehydration (SOR: C). Probiotics have been shown to safely reduce the duration and frequency of diarrhea (SOR: A).
Evidence summary
Evidence is summarized in the Table. Evaluation of an infant with diarrhea usually requires only a thorough history and physical exam. While no clinical trials have tested the impact of blood or stool testing on patient outcome, a recent systematic review suggested the only blood test reliable for ruling out dehydration is a serum bicarbonate greater than 15 to 17 mEq/L.1 Consensus reports have suggested laboratory studies are unnecessary unless dehydration is severe or IV fluids are required; stool cultures are necessary only for bloody or prolonged diarrhea, systemically ill infants, suspected food poisoning, or recent travel abroad.2,3
Effective management of infant diarrhea is based on the degree of dehydration, which can be estimated by percent body weight loss—the difference between the baseline and acute weights, divided by the baseline weight.3 If the baseline weight is not known, prolonged capillary refill time, abnormal skin turgor, and abnormal respiratory pattern are more reliable indicators of dehydration; other physical findings are less precise.1
Infants with diarrhea who are not dehydrated should continue age-appropriate nutrition.4 For infants with mild to moderate dehydration, however, rehydration using oral rehydrating solution is the initial therapy, followed by continued hydration to replace ongoing losses.2,3 A meta-analysis of randomized controlled trials (RCTs) in developed countries demonstrated equivalent efficacy of oral fluids compared with IV fluids, with an overall failure rate of only 3.6% for infants and children treated with oral rehydrating solution (95% confidence interval [CI], 1.4–5.8). There was no significant difference between oral rehydrating solution of varying sodium concentrations, and no increased risk of hypernatremia or hyponatremia compared with the IV treatment arm.5 Continued breastfeeding during the rehydrating phase significantly reduced dehydration (based on case control studies; odds ratio [OR]=5.23; 95% CI, 1.37–19.99; P=.016, limited by sample size).3,6 Breastfeeding also significantly reduced the number of diarrheal stools (found in a small-scale RCT).7 For obtunded or severely dehydrated infants, or those with an ileus or persistent vomiting, expert opinion suggested IV fluids.2,4
In a systematic review of RCTs comparing lower-concentration oral rehydrating solution with standard World Health Organization solution, lower-concentration solution showed superior efficacy. These resulted in fewer unscheduled infusions of IV fluids (OR=0.59; 95% CI, 0.45–0.79) and less stool output without increasing the incidence of hyponatremia.8
Unrestricted diets may reduce the duration of diarrhea compared with oral or IV fluids alone, and age-appropriate diets should be resumed immediately after hydration (based on a review of variable-quality RCTs and prospective trials or case series).2 No studies supported the effectiveness of BRAT (bananas, rice cereal, applesauce, toast) diets over the infant’s usual diet.2,4 A meta-analysis of variable-quality RCTs demonstrated no significant difference in stool frequency between lactose-containing and lactose-free diets.9 Comparisons of undiluted lactose-milk with diluted milk or delayed reintroduction of milk revealed no significant differences in treatment failure or duration of diarrhea, although stool output increased slightly with the undiluted diet. However, undiluted milk was superior for restoring body weight.9
Multiple RCTs showed that Lactobacillus supplementation shortened the duration of diarrhea for infants and young children10,11 and reduced the risk of diarrhea persisting more than 3 days (relative risk [RR]=0.43; 95% CI, 0.34–0.53; P<.001; number needed to treat [NNT]=4).11 This probiotic can be reconstituted in oral rehydrating solution and administered 1 to 8 times daily, depending on the formulation.
Antidiarrheal agents are not recommended (based on limited reviews and consensus reports).2-4,12
TABLE
Evaluative strategies and therapeutic interventions for infant diarrhea
| Routine diarrhea* | Complicated diarrhea† | SOR | ||||
|---|---|---|---|---|---|---|
| Recommended | Not recommended | Recommended | Not recommended | |||
| Evaluation | Serology | X | X | B1, C2-3 | ||
| Stool culture | X | X | C2-3 | |||
| Intervention | WHO ORS (Osm 311 mmol/L) | X | X‡ | A8 | ||
| ORS (Osm 250 mmol/L) | X | X | A8 | |||
| Age-appropriate diet after hydration | X | X | C2 | |||
| Continued breast-feeding | X | X | B6,7 | |||
| BRAT diet | X | X | C2,4 | |||
| Lactose-free or dilute lactose diet | X | X | B9 | |||
| Lactobacillus (probiotic) | X | X | A10,11 | |||
| Antidiarrheal agents | X | X | C2,4,12 | |||
| * Mild to moderate dehydration, diarrhea of short duration without bloody stools, severe systemic illness, suspected food poisoning, or recent foreign travel. | ||||||
| † Severe dehydration, prolonged diarrhea, or diarrhea with bloody stools, severe systemic illness, suspected food poisoning, or recent foreign travel. | ||||||
| ‡ Recommended for treatment of cholera. | ||||||
| ORS, oral rehydration solution; SOR, strength of recommendation; BRAT, bananas, rice cereal, applesauce, and toast. | ||||||
Recommendations from others
The Centers for Disease Control and Prevention (CDC) recommends oral rehydrating solution for mild to moderate dehydration, and boluses of normal saline or Lactated Ringer’s (20 cc/kg) for severe dehydration. For frail or malnourished infants, boluses of 10 cc/kg should be given until hydrated.
The CDC also recommended against nutrition containing simple sugars (soft drinks, juice, gelatin desserts) due to high osmotic loads, but noted that diets containing some fats may have a beneficial effect on intestinal motility. They also recommended age-appropriate use of complex carbohydrates, meats, yogurt, fruits and vegetables. Zinc supplementation may also be beneficial (SOR: C).12
Exam should note fever, weight loss, abdominal tenderness, blood in the stool
Lettie Carter, MD
North Shore University Hospital at Glen Cove, Glen Cove, NY
The evaluation and management of an infant with diarrhea as always, begins with history. The length and severity of the illness, sick contacts, oral intake, travel, and characteristics of the stool are all important factors to consider. The physical exam should note presence of fever, weight loss, abdominal tenderness, and blood in the stool. Laboratory studies such as electrolytes, stool culture, and Wright stain are really only indicated if the child is severely dehydrated, unable to maintain hydration with oral intake and requires IV fluids, or if the episode is unusually protracted or the stool bloody.
A regular age-appropriate diet is essential, but parents should be counseled to avoid adding too much juice to the diet in an effort to rehydrate.
1. Steiner MJ, DeWalt DA, Byerley JS. Is this child dehydrated? JAMA 2004;291:2746-2754.
2. Cincinnati Children’s Hospital Medical Center. Evidence based clinical practice guideline for children with acute gastroenteritis (AGE). Cincinnati, Ohio: Cincinnati Children’s Hospital Medical Center; 2001. Available at www.guideline.gov. Accessed on April 5, 2004.
3. Armon K, Stephenson T, MacFaul R, Eccleston P, Werneke U. An evidence and consensus based guideline for acute diarrhea management. Arch Dis Child 2001;85:132-142.
4. Practice parameter: the management of acute gastroenteritis in young children. American Academy of Pediatrics, Provisional Committee on Quality Improvement, Subcommittee on Acute Gastroenteritis. Pediatrics 1996;97:424-435.
5. Gavin N, Merrick N, Davidson B. Efficacy of glucose-based oral rehydration therapy. Pediatrics 1996;98:45-51.
6. Faruque AS, Mahalanabis D, Islam A, Hoque SS, Hasnat A. Breast feeding and oral rehydration at home during diarrhoea to prevent dehydration. Arch Dis Child 1992;67:1027-1029.
7. Khin MU, Nyunt NW, Myo K, Mu MK, Tin U, Thane T. Effect on clinical outcome of breast feeding during acute diarrhoea. Br Med J (Clin Res Ed) 1985;290:587-589.
8. Hahn S, Kim Y, Garner P. Reduced osmolarity oral rehydration for treating dehydration caused by acute diarrhoea in children (Cochrane Review). In: The Cochrane Library., Issue 4, 2004. Chichester, UK: John Wiley & Sons.
9. Brown KH, Peerson JM, Fontaine O. Use of nonhuman milks in the dietary management of young children with acute diarrhea: a meta-analysis of clinical trials. Pediatrics 1994;93:17-27.
10. Van Niel CW, Feudtner C, Garrison MM, Christakis D. Lactobacillus therapy for acute infectious diarrhea in children: a meta-analysis. Pediatrics 2002;109:678-684.
11. Szajewska H, Mrukowicz JZ. Probiotics in the treatment and prevention of acute infectious diarrhea in infants and children: a systematic review of published randomized, double-blind, placebo-controlled trials. J Pediatr Gastroenterol Nutr 2001;33(Suppl 2):S17-S25.
12. King CK, Glass R, Bresee JS, Duggan C. Centers for Disease Control and Prevention. Managing acute gastroenteritis among children: oral rehydration, maintenance, and nutritional therapy. MMWR Recomm Rep 2003;52(RR-16):1-16.
Routine infant diarrhea requires no lab work or cultures (strength of recommendation [SOR]: C); the degree of dehydration can be determined reliably by percent body weight change (SOR: B). However, bicarbonate may help rule out dehydration (SOR: B); electrolytes and blood urea nitrogen may be useful in evaluating complicated diarrhea with severe dehydration or when intravenous fluids are required; stool cultures are indicated for bloody or prolonged diarrhea, suspected food poisoning, or recent travel abroad (SOR: C).
Oral rehydrating solution is adequate fluid replacement for diarrhea associated with mild to moderate dehydration, followed by prompt refeeding with an age-appropriate diet (SOR: A); intravenous fluids are recommended for severe dehydration (SOR: C). Probiotics have been shown to safely reduce the duration and frequency of diarrhea (SOR: A).
Evidence summary
Evidence is summarized in the Table. Evaluation of an infant with diarrhea usually requires only a thorough history and physical exam. While no clinical trials have tested the impact of blood or stool testing on patient outcome, a recent systematic review suggested the only blood test reliable for ruling out dehydration is a serum bicarbonate greater than 15 to 17 mEq/L.1 Consensus reports have suggested laboratory studies are unnecessary unless dehydration is severe or IV fluids are required; stool cultures are necessary only for bloody or prolonged diarrhea, systemically ill infants, suspected food poisoning, or recent travel abroad.2,3
Effective management of infant diarrhea is based on the degree of dehydration, which can be estimated by percent body weight loss—the difference between the baseline and acute weights, divided by the baseline weight.3 If the baseline weight is not known, prolonged capillary refill time, abnormal skin turgor, and abnormal respiratory pattern are more reliable indicators of dehydration; other physical findings are less precise.1
Infants with diarrhea who are not dehydrated should continue age-appropriate nutrition.4 For infants with mild to moderate dehydration, however, rehydration using oral rehydrating solution is the initial therapy, followed by continued hydration to replace ongoing losses.2,3 A meta-analysis of randomized controlled trials (RCTs) in developed countries demonstrated equivalent efficacy of oral fluids compared with IV fluids, with an overall failure rate of only 3.6% for infants and children treated with oral rehydrating solution (95% confidence interval [CI], 1.4–5.8). There was no significant difference between oral rehydrating solution of varying sodium concentrations, and no increased risk of hypernatremia or hyponatremia compared with the IV treatment arm.5 Continued breastfeeding during the rehydrating phase significantly reduced dehydration (based on case control studies; odds ratio [OR]=5.23; 95% CI, 1.37–19.99; P=.016, limited by sample size).3,6 Breastfeeding also significantly reduced the number of diarrheal stools (found in a small-scale RCT).7 For obtunded or severely dehydrated infants, or those with an ileus or persistent vomiting, expert opinion suggested IV fluids.2,4
In a systematic review of RCTs comparing lower-concentration oral rehydrating solution with standard World Health Organization solution, lower-concentration solution showed superior efficacy. These resulted in fewer unscheduled infusions of IV fluids (OR=0.59; 95% CI, 0.45–0.79) and less stool output without increasing the incidence of hyponatremia.8
Unrestricted diets may reduce the duration of diarrhea compared with oral or IV fluids alone, and age-appropriate diets should be resumed immediately after hydration (based on a review of variable-quality RCTs and prospective trials or case series).2 No studies supported the effectiveness of BRAT (bananas, rice cereal, applesauce, toast) diets over the infant’s usual diet.2,4 A meta-analysis of variable-quality RCTs demonstrated no significant difference in stool frequency between lactose-containing and lactose-free diets.9 Comparisons of undiluted lactose-milk with diluted milk or delayed reintroduction of milk revealed no significant differences in treatment failure or duration of diarrhea, although stool output increased slightly with the undiluted diet. However, undiluted milk was superior for restoring body weight.9
Multiple RCTs showed that Lactobacillus supplementation shortened the duration of diarrhea for infants and young children10,11 and reduced the risk of diarrhea persisting more than 3 days (relative risk [RR]=0.43; 95% CI, 0.34–0.53; P<.001; number needed to treat [NNT]=4).11 This probiotic can be reconstituted in oral rehydrating solution and administered 1 to 8 times daily, depending on the formulation.
Antidiarrheal agents are not recommended (based on limited reviews and consensus reports).2-4,12
TABLE
Evaluative strategies and therapeutic interventions for infant diarrhea
| Routine diarrhea* | Complicated diarrhea† | SOR | ||||
|---|---|---|---|---|---|---|
| Recommended | Not recommended | Recommended | Not recommended | |||
| Evaluation | Serology | X | X | B1, C2-3 | ||
| Stool culture | X | X | C2-3 | |||
| Intervention | WHO ORS (Osm 311 mmol/L) | X | X‡ | A8 | ||
| ORS (Osm 250 mmol/L) | X | X | A8 | |||
| Age-appropriate diet after hydration | X | X | C2 | |||
| Continued breast-feeding | X | X | B6,7 | |||
| BRAT diet | X | X | C2,4 | |||
| Lactose-free or dilute lactose diet | X | X | B9 | |||
| Lactobacillus (probiotic) | X | X | A10,11 | |||
| Antidiarrheal agents | X | X | C2,4,12 | |||
| * Mild to moderate dehydration, diarrhea of short duration without bloody stools, severe systemic illness, suspected food poisoning, or recent foreign travel. | ||||||
| † Severe dehydration, prolonged diarrhea, or diarrhea with bloody stools, severe systemic illness, suspected food poisoning, or recent foreign travel. | ||||||
| ‡ Recommended for treatment of cholera. | ||||||
| ORS, oral rehydration solution; SOR, strength of recommendation; BRAT, bananas, rice cereal, applesauce, and toast. | ||||||
Recommendations from others
The Centers for Disease Control and Prevention (CDC) recommends oral rehydrating solution for mild to moderate dehydration, and boluses of normal saline or Lactated Ringer’s (20 cc/kg) for severe dehydration. For frail or malnourished infants, boluses of 10 cc/kg should be given until hydrated.
The CDC also recommended against nutrition containing simple sugars (soft drinks, juice, gelatin desserts) due to high osmotic loads, but noted that diets containing some fats may have a beneficial effect on intestinal motility. They also recommended age-appropriate use of complex carbohydrates, meats, yogurt, fruits and vegetables. Zinc supplementation may also be beneficial (SOR: C).12
Exam should note fever, weight loss, abdominal tenderness, blood in the stool
Lettie Carter, MD
North Shore University Hospital at Glen Cove, Glen Cove, NY
The evaluation and management of an infant with diarrhea as always, begins with history. The length and severity of the illness, sick contacts, oral intake, travel, and characteristics of the stool are all important factors to consider. The physical exam should note presence of fever, weight loss, abdominal tenderness, and blood in the stool. Laboratory studies such as electrolytes, stool culture, and Wright stain are really only indicated if the child is severely dehydrated, unable to maintain hydration with oral intake and requires IV fluids, or if the episode is unusually protracted or the stool bloody.
A regular age-appropriate diet is essential, but parents should be counseled to avoid adding too much juice to the diet in an effort to rehydrate.
Routine infant diarrhea requires no lab work or cultures (strength of recommendation [SOR]: C); the degree of dehydration can be determined reliably by percent body weight change (SOR: B). However, bicarbonate may help rule out dehydration (SOR: B); electrolytes and blood urea nitrogen may be useful in evaluating complicated diarrhea with severe dehydration or when intravenous fluids are required; stool cultures are indicated for bloody or prolonged diarrhea, suspected food poisoning, or recent travel abroad (SOR: C).
Oral rehydrating solution is adequate fluid replacement for diarrhea associated with mild to moderate dehydration, followed by prompt refeeding with an age-appropriate diet (SOR: A); intravenous fluids are recommended for severe dehydration (SOR: C). Probiotics have been shown to safely reduce the duration and frequency of diarrhea (SOR: A).
Evidence summary
Evidence is summarized in the Table. Evaluation of an infant with diarrhea usually requires only a thorough history and physical exam. While no clinical trials have tested the impact of blood or stool testing on patient outcome, a recent systematic review suggested the only blood test reliable for ruling out dehydration is a serum bicarbonate greater than 15 to 17 mEq/L.1 Consensus reports have suggested laboratory studies are unnecessary unless dehydration is severe or IV fluids are required; stool cultures are necessary only for bloody or prolonged diarrhea, systemically ill infants, suspected food poisoning, or recent travel abroad.2,3
Effective management of infant diarrhea is based on the degree of dehydration, which can be estimated by percent body weight loss—the difference between the baseline and acute weights, divided by the baseline weight.3 If the baseline weight is not known, prolonged capillary refill time, abnormal skin turgor, and abnormal respiratory pattern are more reliable indicators of dehydration; other physical findings are less precise.1
Infants with diarrhea who are not dehydrated should continue age-appropriate nutrition.4 For infants with mild to moderate dehydration, however, rehydration using oral rehydrating solution is the initial therapy, followed by continued hydration to replace ongoing losses.2,3 A meta-analysis of randomized controlled trials (RCTs) in developed countries demonstrated equivalent efficacy of oral fluids compared with IV fluids, with an overall failure rate of only 3.6% for infants and children treated with oral rehydrating solution (95% confidence interval [CI], 1.4–5.8). There was no significant difference between oral rehydrating solution of varying sodium concentrations, and no increased risk of hypernatremia or hyponatremia compared with the IV treatment arm.5 Continued breastfeeding during the rehydrating phase significantly reduced dehydration (based on case control studies; odds ratio [OR]=5.23; 95% CI, 1.37–19.99; P=.016, limited by sample size).3,6 Breastfeeding also significantly reduced the number of diarrheal stools (found in a small-scale RCT).7 For obtunded or severely dehydrated infants, or those with an ileus or persistent vomiting, expert opinion suggested IV fluids.2,4
In a systematic review of RCTs comparing lower-concentration oral rehydrating solution with standard World Health Organization solution, lower-concentration solution showed superior efficacy. These resulted in fewer unscheduled infusions of IV fluids (OR=0.59; 95% CI, 0.45–0.79) and less stool output without increasing the incidence of hyponatremia.8
Unrestricted diets may reduce the duration of diarrhea compared with oral or IV fluids alone, and age-appropriate diets should be resumed immediately after hydration (based on a review of variable-quality RCTs and prospective trials or case series).2 No studies supported the effectiveness of BRAT (bananas, rice cereal, applesauce, toast) diets over the infant’s usual diet.2,4 A meta-analysis of variable-quality RCTs demonstrated no significant difference in stool frequency between lactose-containing and lactose-free diets.9 Comparisons of undiluted lactose-milk with diluted milk or delayed reintroduction of milk revealed no significant differences in treatment failure or duration of diarrhea, although stool output increased slightly with the undiluted diet. However, undiluted milk was superior for restoring body weight.9
Multiple RCTs showed that Lactobacillus supplementation shortened the duration of diarrhea for infants and young children10,11 and reduced the risk of diarrhea persisting more than 3 days (relative risk [RR]=0.43; 95% CI, 0.34–0.53; P<.001; number needed to treat [NNT]=4).11 This probiotic can be reconstituted in oral rehydrating solution and administered 1 to 8 times daily, depending on the formulation.
Antidiarrheal agents are not recommended (based on limited reviews and consensus reports).2-4,12
TABLE
Evaluative strategies and therapeutic interventions for infant diarrhea
| Routine diarrhea* | Complicated diarrhea† | SOR | ||||
|---|---|---|---|---|---|---|
| Recommended | Not recommended | Recommended | Not recommended | |||
| Evaluation | Serology | X | X | B1, C2-3 | ||
| Stool culture | X | X | C2-3 | |||
| Intervention | WHO ORS (Osm 311 mmol/L) | X | X‡ | A8 | ||
| ORS (Osm 250 mmol/L) | X | X | A8 | |||
| Age-appropriate diet after hydration | X | X | C2 | |||
| Continued breast-feeding | X | X | B6,7 | |||
| BRAT diet | X | X | C2,4 | |||
| Lactose-free or dilute lactose diet | X | X | B9 | |||
| Lactobacillus (probiotic) | X | X | A10,11 | |||
| Antidiarrheal agents | X | X | C2,4,12 | |||
| * Mild to moderate dehydration, diarrhea of short duration without bloody stools, severe systemic illness, suspected food poisoning, or recent foreign travel. | ||||||
| † Severe dehydration, prolonged diarrhea, or diarrhea with bloody stools, severe systemic illness, suspected food poisoning, or recent foreign travel. | ||||||
| ‡ Recommended for treatment of cholera. | ||||||
| ORS, oral rehydration solution; SOR, strength of recommendation; BRAT, bananas, rice cereal, applesauce, and toast. | ||||||
Recommendations from others
The Centers for Disease Control and Prevention (CDC) recommends oral rehydrating solution for mild to moderate dehydration, and boluses of normal saline or Lactated Ringer’s (20 cc/kg) for severe dehydration. For frail or malnourished infants, boluses of 10 cc/kg should be given until hydrated.
The CDC also recommended against nutrition containing simple sugars (soft drinks, juice, gelatin desserts) due to high osmotic loads, but noted that diets containing some fats may have a beneficial effect on intestinal motility. They also recommended age-appropriate use of complex carbohydrates, meats, yogurt, fruits and vegetables. Zinc supplementation may also be beneficial (SOR: C).12
Exam should note fever, weight loss, abdominal tenderness, blood in the stool
Lettie Carter, MD
North Shore University Hospital at Glen Cove, Glen Cove, NY
The evaluation and management of an infant with diarrhea as always, begins with history. The length and severity of the illness, sick contacts, oral intake, travel, and characteristics of the stool are all important factors to consider. The physical exam should note presence of fever, weight loss, abdominal tenderness, and blood in the stool. Laboratory studies such as electrolytes, stool culture, and Wright stain are really only indicated if the child is severely dehydrated, unable to maintain hydration with oral intake and requires IV fluids, or if the episode is unusually protracted or the stool bloody.
A regular age-appropriate diet is essential, but parents should be counseled to avoid adding too much juice to the diet in an effort to rehydrate.
1. Steiner MJ, DeWalt DA, Byerley JS. Is this child dehydrated? JAMA 2004;291:2746-2754.
2. Cincinnati Children’s Hospital Medical Center. Evidence based clinical practice guideline for children with acute gastroenteritis (AGE). Cincinnati, Ohio: Cincinnati Children’s Hospital Medical Center; 2001. Available at www.guideline.gov. Accessed on April 5, 2004.
3. Armon K, Stephenson T, MacFaul R, Eccleston P, Werneke U. An evidence and consensus based guideline for acute diarrhea management. Arch Dis Child 2001;85:132-142.
4. Practice parameter: the management of acute gastroenteritis in young children. American Academy of Pediatrics, Provisional Committee on Quality Improvement, Subcommittee on Acute Gastroenteritis. Pediatrics 1996;97:424-435.
5. Gavin N, Merrick N, Davidson B. Efficacy of glucose-based oral rehydration therapy. Pediatrics 1996;98:45-51.
6. Faruque AS, Mahalanabis D, Islam A, Hoque SS, Hasnat A. Breast feeding and oral rehydration at home during diarrhoea to prevent dehydration. Arch Dis Child 1992;67:1027-1029.
7. Khin MU, Nyunt NW, Myo K, Mu MK, Tin U, Thane T. Effect on clinical outcome of breast feeding during acute diarrhoea. Br Med J (Clin Res Ed) 1985;290:587-589.
8. Hahn S, Kim Y, Garner P. Reduced osmolarity oral rehydration for treating dehydration caused by acute diarrhoea in children (Cochrane Review). In: The Cochrane Library., Issue 4, 2004. Chichester, UK: John Wiley & Sons.
9. Brown KH, Peerson JM, Fontaine O. Use of nonhuman milks in the dietary management of young children with acute diarrhea: a meta-analysis of clinical trials. Pediatrics 1994;93:17-27.
10. Van Niel CW, Feudtner C, Garrison MM, Christakis D. Lactobacillus therapy for acute infectious diarrhea in children: a meta-analysis. Pediatrics 2002;109:678-684.
11. Szajewska H, Mrukowicz JZ. Probiotics in the treatment and prevention of acute infectious diarrhea in infants and children: a systematic review of published randomized, double-blind, placebo-controlled trials. J Pediatr Gastroenterol Nutr 2001;33(Suppl 2):S17-S25.
12. King CK, Glass R, Bresee JS, Duggan C. Centers for Disease Control and Prevention. Managing acute gastroenteritis among children: oral rehydration, maintenance, and nutritional therapy. MMWR Recomm Rep 2003;52(RR-16):1-16.
1. Steiner MJ, DeWalt DA, Byerley JS. Is this child dehydrated? JAMA 2004;291:2746-2754.
2. Cincinnati Children’s Hospital Medical Center. Evidence based clinical practice guideline for children with acute gastroenteritis (AGE). Cincinnati, Ohio: Cincinnati Children’s Hospital Medical Center; 2001. Available at www.guideline.gov. Accessed on April 5, 2004.
3. Armon K, Stephenson T, MacFaul R, Eccleston P, Werneke U. An evidence and consensus based guideline for acute diarrhea management. Arch Dis Child 2001;85:132-142.
4. Practice parameter: the management of acute gastroenteritis in young children. American Academy of Pediatrics, Provisional Committee on Quality Improvement, Subcommittee on Acute Gastroenteritis. Pediatrics 1996;97:424-435.
5. Gavin N, Merrick N, Davidson B. Efficacy of glucose-based oral rehydration therapy. Pediatrics 1996;98:45-51.
6. Faruque AS, Mahalanabis D, Islam A, Hoque SS, Hasnat A. Breast feeding and oral rehydration at home during diarrhoea to prevent dehydration. Arch Dis Child 1992;67:1027-1029.
7. Khin MU, Nyunt NW, Myo K, Mu MK, Tin U, Thane T. Effect on clinical outcome of breast feeding during acute diarrhoea. Br Med J (Clin Res Ed) 1985;290:587-589.
8. Hahn S, Kim Y, Garner P. Reduced osmolarity oral rehydration for treating dehydration caused by acute diarrhoea in children (Cochrane Review). In: The Cochrane Library., Issue 4, 2004. Chichester, UK: John Wiley & Sons.
9. Brown KH, Peerson JM, Fontaine O. Use of nonhuman milks in the dietary management of young children with acute diarrhea: a meta-analysis of clinical trials. Pediatrics 1994;93:17-27.
10. Van Niel CW, Feudtner C, Garrison MM, Christakis D. Lactobacillus therapy for acute infectious diarrhea in children: a meta-analysis. Pediatrics 2002;109:678-684.
11. Szajewska H, Mrukowicz JZ. Probiotics in the treatment and prevention of acute infectious diarrhea in infants and children: a systematic review of published randomized, double-blind, placebo-controlled trials. J Pediatr Gastroenterol Nutr 2001;33(Suppl 2):S17-S25.
12. King CK, Glass R, Bresee JS, Duggan C. Centers for Disease Control and Prevention. Managing acute gastroenteritis among children: oral rehydration, maintenance, and nutritional therapy. MMWR Recomm Rep 2003;52(RR-16):1-16.
Evidence-based answers from the Family Physicians Inquiries Network
Are antibiotics effective in preventing pneumonia for nursing home patients?
Antibiotics should not be used for prophylaxis of pneumonia in nursing homes. We found no studies testing the effectiveness of antibiotics in preventing pneumonia in any population, including persons with predisposing conditions such as influenza. Three measures effectively prevent pneumonia in nursing home patients: influenza vaccination of residents (strength of recommendation [SOR]: B, based on systematic review of homogenous cohort observational studies); influenza vaccination of caregivers (SOR: B, based on individual randomized controlled trial); pneumococcal vaccination of residents (SOR: B, based on randomized, nonblinded clinical trials and consistent case-control studies).
Two other suggested interventions have not been extensively tested: antiviral chemoprophylaxis during an influenza outbreak in the nursing home, and oral hygiene programs for nursing home residents.
Evidence summary
Overuse of antibiotics is already a problem in nursing homes. A large portion of bacterial pneumonia in the nursing home population results from aspiration of oropharyngeal bacteria, which is more likely to be drug-resistant if the resident has been on antibiotics.1 We found no studies that testing antibacterial agents for prevention of pneumonia in nursing home patients. However, 3 measures are clearly helpful in preventing pneumonia in nursing home patients:
- Influenza vaccination of residents: A meta-analysis of 20 cohort studies showed a 53% efficacy (95% confidence interval [CI], 35–66)—defined as 1 minus the odds ratio—for influenza immunization in preventing pneumonia.2
- Influenza vaccination of caregivers: A cluster randomized trial in British long-term care facilities demonstrated that influenza vaccination of health care workers (61% of 1078 workers) reduced the total nursing home mortality rate (odds ratio [OR]=0.56 [95% CI, 0.4–0.8]) for a drop in mortality rate from 17% to 10% (number needed to treat [NNT]=14.3).3
- Pneumococcal vaccination of residents: This evidence was reviewed in a prior Clinical Inquiry.4 The evidence comes primarily from 2 clinical trials in which the NNT to prevent 1 episode of pneumonia was about 35.
Two other proposed interventions require further study to evaluate their role in prophylaxis. Antiviral prophylaxis to prevent pneumonia during nursing home outbreaks of influenza has not been evaluated in controlled trials. Observational studies strongly suggest that amantadine, rimantadine, and oseltamivir are all effective in reducing spread of influenza during outbreaks in nursing homes (Table). Oseltamivir acts against influenza B as well as A and has fewer side effects, but it is more expensive.5,6 Presumably, decreasing the rate of influenza also reduces the rate of subsequent pneumonia.
Oral hygiene programs for nursing home residents may also reduce pneumonia. In a single study, 366 patients in 11 Japanese nursing homes were divided into controls (self-care) and those treated with rigorous oral care (by staff). The intervention group had a relative risk of 0.6 (95% CI, 0.36–0.99; NNT=12.5) for pneumonia over a 2-year period.7 The NNT for preventing a death by pneumonia was 11 (P<.01). This intriguing result merits follow up in larger groups in US nursing homes to see if this approach is feasible.
TABLE
Available treatment and prophylactic regimens for influenza
| Drug name | Regimen for treatment* | Regimen for prophylaxis† | Comments | Cost‡ |
|---|---|---|---|---|
| Oseltamivir (Tamiflu) | 75 mg orally twice daily for 5 days | 75 mg orally once daily for >7 days | Influenza A and B | 10 tabs $59.99 (no generic) |
| Rimantidine (Flumadine) | 100 mg orally twice daily (100 mg orally once daily in elderly) | 100 mg orally twice daily (100 mg orally once daily in elderly) | Influenza A only | 14 tabs $33.45 (no generic) |
| Amantadine (Symmetrel) | 100 mg orally twice daily (100 mg orally once daily in elderly) | 100 mg orally twice daily (100 mg orally once daily in elderly) | Influenza A only (consider lower doses in debilitated patients) | 60 tabs $75.58 (brand), $18.99 (generic) |
| Zanamivir (Relenza) | 2 inhalations (10 mg) every 12 hours for 5 days | Not indicated | Influenza A and B (inhalations may be difficult to administer to debilitated patients) | 20 inhalation doses $54.41 (no generic) |
| Source: Epocrates RX: Online and PDA-Based Reference, June 12, 2004. | ||||
| * Start treatment within 48 hours of onset of symptoms. | ||||
| † Start prophylaxis immediately or within 48 hours of exposure. | ||||
| ‡ Approximate retail price from www.drugstore.com, June 2004. | ||||
Recommendations from others
There are no recommendations about the use of antibiotic prophylaxis for pneumonia in either the nursing home or in the outpatient settings; however, there are clear recommendations against the overuse of antibiotics.8
The CDC Advisory Committee on Immunization Practices (ACIP) recommends:
- annual influenza vaccine for persons residing in nursing homes9
- annual influenza vaccine for health care workers in long-term care facilities9
- pneumococcal vaccine for persons residing in a nursing home (the schedule for an immunocompetent adult is a single dose, followed by a booster after age 65 if the first dose was before age 65, or after 5 years for persons <65 years with compromised immune status)10
- chemoprophylaxis for influenza outbreaks in nursing homes.11
Prevention is key for reducing pneumonia mortality
Jon O. Neher, MD
Valley Medical Center, Renton, Wash
Pneumonia is one of the most common causes of death for nursing home patients. While pneumonia can present with the classic fever, productive cough, and air hunger, it often presents with such nonspecific findings as altered mental status or mild tachypnea, which can significantly delay diagnosis. Additionally, many older adults poorly tolerate the metabolic demands of the disease and become critically ill very rapidly. Thus, prevention remains a key strategy for reducing mortality. Nursing home policies that facilitate vaccination and reduce disease transmission are critically important in this regard.
1. Yamaya M, Yanai M, Ohrui T, Arai H, Sasaki H. Interventions to prevent pneumonia among older adults. J Am Geriatr Soc 2001;49:85-90.
2. Gross PA, Hermogenes AW, Sacks HS, Lau J, Levandowski RA. The efficacy of influenza vaccine in elderly persons. A meta-analysis and review of the literature. Ann Intern Med 1995;123:518-527.
3. Potter J, Stott DJ, Roberts MA, et al. Influenza vaccination of health care workers in long-term-care hospitals reduces the mortality of elderly patients. J Infect Dis 1997;175:1-6.
4. McCormack O, Meza J, Martin S, Tatum P. Is pneumococcal vaccine effective in nursing home patients? J Fam Pract 2003;52:150-154.
5. Arden NH, Patriarca PA, Fasano MB, et al. The roles of vaccination and amantadine prophylaxis in controlling an outbreak of influenza A (H3N2) in a nursing home. Arch Intern Med 1988;148:865-868.
6. Parker R, Loewen N, Skowronski D. Experience with oseltamivir in the control of a nursing home influenza B outbreak. Can Commun Dis Rep 2001;27:37-40.
7. Yoneyama T, Yoshida M, Ohrui T, et al. Oral care reduces pneumonia in older patients in nursing homes. J Am Geriatr Soc 2002;50:430-433.
8. Strassbaugh LJ, Crossley KB, Nurse BA, Thrupp LD. Antimicrobial resistance in long-term care facilities. Infection Control and Hospital Epidemiology 1996;17:129-140.
9. Prevention and control of influenza: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 1999;48(RR-4):1-28.
10. Prevention of Pneumococcal Disease: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 1997;46(RR-8):1-24.
11. Bridges CB, Fukuda K, Uyeki TM, Cox NJ, Singleton JA. Centers for Disease Control and Prevention, Advisory Committee on Immunization Practices. Prevention and Control of Influenza. Recommendations of the Advisory Committee on Immunization Practices. MMWR Recomm Rep 2002;51(RR-3):1-31.
Antibiotics should not be used for prophylaxis of pneumonia in nursing homes. We found no studies testing the effectiveness of antibiotics in preventing pneumonia in any population, including persons with predisposing conditions such as influenza. Three measures effectively prevent pneumonia in nursing home patients: influenza vaccination of residents (strength of recommendation [SOR]: B, based on systematic review of homogenous cohort observational studies); influenza vaccination of caregivers (SOR: B, based on individual randomized controlled trial); pneumococcal vaccination of residents (SOR: B, based on randomized, nonblinded clinical trials and consistent case-control studies).
Two other suggested interventions have not been extensively tested: antiviral chemoprophylaxis during an influenza outbreak in the nursing home, and oral hygiene programs for nursing home residents.
Evidence summary
Overuse of antibiotics is already a problem in nursing homes. A large portion of bacterial pneumonia in the nursing home population results from aspiration of oropharyngeal bacteria, which is more likely to be drug-resistant if the resident has been on antibiotics.1 We found no studies that testing antibacterial agents for prevention of pneumonia in nursing home patients. However, 3 measures are clearly helpful in preventing pneumonia in nursing home patients:
- Influenza vaccination of residents: A meta-analysis of 20 cohort studies showed a 53% efficacy (95% confidence interval [CI], 35–66)—defined as 1 minus the odds ratio—for influenza immunization in preventing pneumonia.2
- Influenza vaccination of caregivers: A cluster randomized trial in British long-term care facilities demonstrated that influenza vaccination of health care workers (61% of 1078 workers) reduced the total nursing home mortality rate (odds ratio [OR]=0.56 [95% CI, 0.4–0.8]) for a drop in mortality rate from 17% to 10% (number needed to treat [NNT]=14.3).3
- Pneumococcal vaccination of residents: This evidence was reviewed in a prior Clinical Inquiry.4 The evidence comes primarily from 2 clinical trials in which the NNT to prevent 1 episode of pneumonia was about 35.
Two other proposed interventions require further study to evaluate their role in prophylaxis. Antiviral prophylaxis to prevent pneumonia during nursing home outbreaks of influenza has not been evaluated in controlled trials. Observational studies strongly suggest that amantadine, rimantadine, and oseltamivir are all effective in reducing spread of influenza during outbreaks in nursing homes (Table). Oseltamivir acts against influenza B as well as A and has fewer side effects, but it is more expensive.5,6 Presumably, decreasing the rate of influenza also reduces the rate of subsequent pneumonia.
Oral hygiene programs for nursing home residents may also reduce pneumonia. In a single study, 366 patients in 11 Japanese nursing homes were divided into controls (self-care) and those treated with rigorous oral care (by staff). The intervention group had a relative risk of 0.6 (95% CI, 0.36–0.99; NNT=12.5) for pneumonia over a 2-year period.7 The NNT for preventing a death by pneumonia was 11 (P<.01). This intriguing result merits follow up in larger groups in US nursing homes to see if this approach is feasible.
TABLE
Available treatment and prophylactic regimens for influenza
| Drug name | Regimen for treatment* | Regimen for prophylaxis† | Comments | Cost‡ |
|---|---|---|---|---|
| Oseltamivir (Tamiflu) | 75 mg orally twice daily for 5 days | 75 mg orally once daily for >7 days | Influenza A and B | 10 tabs $59.99 (no generic) |
| Rimantidine (Flumadine) | 100 mg orally twice daily (100 mg orally once daily in elderly) | 100 mg orally twice daily (100 mg orally once daily in elderly) | Influenza A only | 14 tabs $33.45 (no generic) |
| Amantadine (Symmetrel) | 100 mg orally twice daily (100 mg orally once daily in elderly) | 100 mg orally twice daily (100 mg orally once daily in elderly) | Influenza A only (consider lower doses in debilitated patients) | 60 tabs $75.58 (brand), $18.99 (generic) |
| Zanamivir (Relenza) | 2 inhalations (10 mg) every 12 hours for 5 days | Not indicated | Influenza A and B (inhalations may be difficult to administer to debilitated patients) | 20 inhalation doses $54.41 (no generic) |
| Source: Epocrates RX: Online and PDA-Based Reference, June 12, 2004. | ||||
| * Start treatment within 48 hours of onset of symptoms. | ||||
| † Start prophylaxis immediately or within 48 hours of exposure. | ||||
| ‡ Approximate retail price from www.drugstore.com, June 2004. | ||||
Recommendations from others
There are no recommendations about the use of antibiotic prophylaxis for pneumonia in either the nursing home or in the outpatient settings; however, there are clear recommendations against the overuse of antibiotics.8
The CDC Advisory Committee on Immunization Practices (ACIP) recommends:
- annual influenza vaccine for persons residing in nursing homes9
- annual influenza vaccine for health care workers in long-term care facilities9
- pneumococcal vaccine for persons residing in a nursing home (the schedule for an immunocompetent adult is a single dose, followed by a booster after age 65 if the first dose was before age 65, or after 5 years for persons <65 years with compromised immune status)10
- chemoprophylaxis for influenza outbreaks in nursing homes.11
Prevention is key for reducing pneumonia mortality
Jon O. Neher, MD
Valley Medical Center, Renton, Wash
Pneumonia is one of the most common causes of death for nursing home patients. While pneumonia can present with the classic fever, productive cough, and air hunger, it often presents with such nonspecific findings as altered mental status or mild tachypnea, which can significantly delay diagnosis. Additionally, many older adults poorly tolerate the metabolic demands of the disease and become critically ill very rapidly. Thus, prevention remains a key strategy for reducing mortality. Nursing home policies that facilitate vaccination and reduce disease transmission are critically important in this regard.
Antibiotics should not be used for prophylaxis of pneumonia in nursing homes. We found no studies testing the effectiveness of antibiotics in preventing pneumonia in any population, including persons with predisposing conditions such as influenza. Three measures effectively prevent pneumonia in nursing home patients: influenza vaccination of residents (strength of recommendation [SOR]: B, based on systematic review of homogenous cohort observational studies); influenza vaccination of caregivers (SOR: B, based on individual randomized controlled trial); pneumococcal vaccination of residents (SOR: B, based on randomized, nonblinded clinical trials and consistent case-control studies).
Two other suggested interventions have not been extensively tested: antiviral chemoprophylaxis during an influenza outbreak in the nursing home, and oral hygiene programs for nursing home residents.
Evidence summary
Overuse of antibiotics is already a problem in nursing homes. A large portion of bacterial pneumonia in the nursing home population results from aspiration of oropharyngeal bacteria, which is more likely to be drug-resistant if the resident has been on antibiotics.1 We found no studies that testing antibacterial agents for prevention of pneumonia in nursing home patients. However, 3 measures are clearly helpful in preventing pneumonia in nursing home patients:
- Influenza vaccination of residents: A meta-analysis of 20 cohort studies showed a 53% efficacy (95% confidence interval [CI], 35–66)—defined as 1 minus the odds ratio—for influenza immunization in preventing pneumonia.2
- Influenza vaccination of caregivers: A cluster randomized trial in British long-term care facilities demonstrated that influenza vaccination of health care workers (61% of 1078 workers) reduced the total nursing home mortality rate (odds ratio [OR]=0.56 [95% CI, 0.4–0.8]) for a drop in mortality rate from 17% to 10% (number needed to treat [NNT]=14.3).3
- Pneumococcal vaccination of residents: This evidence was reviewed in a prior Clinical Inquiry.4 The evidence comes primarily from 2 clinical trials in which the NNT to prevent 1 episode of pneumonia was about 35.
Two other proposed interventions require further study to evaluate their role in prophylaxis. Antiviral prophylaxis to prevent pneumonia during nursing home outbreaks of influenza has not been evaluated in controlled trials. Observational studies strongly suggest that amantadine, rimantadine, and oseltamivir are all effective in reducing spread of influenza during outbreaks in nursing homes (Table). Oseltamivir acts against influenza B as well as A and has fewer side effects, but it is more expensive.5,6 Presumably, decreasing the rate of influenza also reduces the rate of subsequent pneumonia.
Oral hygiene programs for nursing home residents may also reduce pneumonia. In a single study, 366 patients in 11 Japanese nursing homes were divided into controls (self-care) and those treated with rigorous oral care (by staff). The intervention group had a relative risk of 0.6 (95% CI, 0.36–0.99; NNT=12.5) for pneumonia over a 2-year period.7 The NNT for preventing a death by pneumonia was 11 (P<.01). This intriguing result merits follow up in larger groups in US nursing homes to see if this approach is feasible.
TABLE
Available treatment and prophylactic regimens for influenza
| Drug name | Regimen for treatment* | Regimen for prophylaxis† | Comments | Cost‡ |
|---|---|---|---|---|
| Oseltamivir (Tamiflu) | 75 mg orally twice daily for 5 days | 75 mg orally once daily for >7 days | Influenza A and B | 10 tabs $59.99 (no generic) |
| Rimantidine (Flumadine) | 100 mg orally twice daily (100 mg orally once daily in elderly) | 100 mg orally twice daily (100 mg orally once daily in elderly) | Influenza A only | 14 tabs $33.45 (no generic) |
| Amantadine (Symmetrel) | 100 mg orally twice daily (100 mg orally once daily in elderly) | 100 mg orally twice daily (100 mg orally once daily in elderly) | Influenza A only (consider lower doses in debilitated patients) | 60 tabs $75.58 (brand), $18.99 (generic) |
| Zanamivir (Relenza) | 2 inhalations (10 mg) every 12 hours for 5 days | Not indicated | Influenza A and B (inhalations may be difficult to administer to debilitated patients) | 20 inhalation doses $54.41 (no generic) |
| Source: Epocrates RX: Online and PDA-Based Reference, June 12, 2004. | ||||
| * Start treatment within 48 hours of onset of symptoms. | ||||
| † Start prophylaxis immediately or within 48 hours of exposure. | ||||
| ‡ Approximate retail price from www.drugstore.com, June 2004. | ||||
Recommendations from others
There are no recommendations about the use of antibiotic prophylaxis for pneumonia in either the nursing home or in the outpatient settings; however, there are clear recommendations against the overuse of antibiotics.8
The CDC Advisory Committee on Immunization Practices (ACIP) recommends:
- annual influenza vaccine for persons residing in nursing homes9
- annual influenza vaccine for health care workers in long-term care facilities9
- pneumococcal vaccine for persons residing in a nursing home (the schedule for an immunocompetent adult is a single dose, followed by a booster after age 65 if the first dose was before age 65, or after 5 years for persons <65 years with compromised immune status)10
- chemoprophylaxis for influenza outbreaks in nursing homes.11
Prevention is key for reducing pneumonia mortality
Jon O. Neher, MD
Valley Medical Center, Renton, Wash
Pneumonia is one of the most common causes of death for nursing home patients. While pneumonia can present with the classic fever, productive cough, and air hunger, it often presents with such nonspecific findings as altered mental status or mild tachypnea, which can significantly delay diagnosis. Additionally, many older adults poorly tolerate the metabolic demands of the disease and become critically ill very rapidly. Thus, prevention remains a key strategy for reducing mortality. Nursing home policies that facilitate vaccination and reduce disease transmission are critically important in this regard.
1. Yamaya M, Yanai M, Ohrui T, Arai H, Sasaki H. Interventions to prevent pneumonia among older adults. J Am Geriatr Soc 2001;49:85-90.
2. Gross PA, Hermogenes AW, Sacks HS, Lau J, Levandowski RA. The efficacy of influenza vaccine in elderly persons. A meta-analysis and review of the literature. Ann Intern Med 1995;123:518-527.
3. Potter J, Stott DJ, Roberts MA, et al. Influenza vaccination of health care workers in long-term-care hospitals reduces the mortality of elderly patients. J Infect Dis 1997;175:1-6.
4. McCormack O, Meza J, Martin S, Tatum P. Is pneumococcal vaccine effective in nursing home patients? J Fam Pract 2003;52:150-154.
5. Arden NH, Patriarca PA, Fasano MB, et al. The roles of vaccination and amantadine prophylaxis in controlling an outbreak of influenza A (H3N2) in a nursing home. Arch Intern Med 1988;148:865-868.
6. Parker R, Loewen N, Skowronski D. Experience with oseltamivir in the control of a nursing home influenza B outbreak. Can Commun Dis Rep 2001;27:37-40.
7. Yoneyama T, Yoshida M, Ohrui T, et al. Oral care reduces pneumonia in older patients in nursing homes. J Am Geriatr Soc 2002;50:430-433.
8. Strassbaugh LJ, Crossley KB, Nurse BA, Thrupp LD. Antimicrobial resistance in long-term care facilities. Infection Control and Hospital Epidemiology 1996;17:129-140.
9. Prevention and control of influenza: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 1999;48(RR-4):1-28.
10. Prevention of Pneumococcal Disease: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 1997;46(RR-8):1-24.
11. Bridges CB, Fukuda K, Uyeki TM, Cox NJ, Singleton JA. Centers for Disease Control and Prevention, Advisory Committee on Immunization Practices. Prevention and Control of Influenza. Recommendations of the Advisory Committee on Immunization Practices. MMWR Recomm Rep 2002;51(RR-3):1-31.
1. Yamaya M, Yanai M, Ohrui T, Arai H, Sasaki H. Interventions to prevent pneumonia among older adults. J Am Geriatr Soc 2001;49:85-90.
2. Gross PA, Hermogenes AW, Sacks HS, Lau J, Levandowski RA. The efficacy of influenza vaccine in elderly persons. A meta-analysis and review of the literature. Ann Intern Med 1995;123:518-527.
3. Potter J, Stott DJ, Roberts MA, et al. Influenza vaccination of health care workers in long-term-care hospitals reduces the mortality of elderly patients. J Infect Dis 1997;175:1-6.
4. McCormack O, Meza J, Martin S, Tatum P. Is pneumococcal vaccine effective in nursing home patients? J Fam Pract 2003;52:150-154.
5. Arden NH, Patriarca PA, Fasano MB, et al. The roles of vaccination and amantadine prophylaxis in controlling an outbreak of influenza A (H3N2) in a nursing home. Arch Intern Med 1988;148:865-868.
6. Parker R, Loewen N, Skowronski D. Experience with oseltamivir in the control of a nursing home influenza B outbreak. Can Commun Dis Rep 2001;27:37-40.
7. Yoneyama T, Yoshida M, Ohrui T, et al. Oral care reduces pneumonia in older patients in nursing homes. J Am Geriatr Soc 2002;50:430-433.
8. Strassbaugh LJ, Crossley KB, Nurse BA, Thrupp LD. Antimicrobial resistance in long-term care facilities. Infection Control and Hospital Epidemiology 1996;17:129-140.
9. Prevention and control of influenza: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 1999;48(RR-4):1-28.
10. Prevention of Pneumococcal Disease: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 1997;46(RR-8):1-24.
11. Bridges CB, Fukuda K, Uyeki TM, Cox NJ, Singleton JA. Centers for Disease Control and Prevention, Advisory Committee on Immunization Practices. Prevention and Control of Influenza. Recommendations of the Advisory Committee on Immunization Practices. MMWR Recomm Rep 2002;51(RR-3):1-31.
Evidence-based answers from the Family Physicians Inquiries Network
Is nedocromil effective in preventing asthmatic attacks in patients with asthma?
Nedocromil (Tilade) is effective for the treatment of mild persistent asthma. It has not been shown to be effective in more severe forms of asthma for both children and adults. Although no studies looked specifically at exacerbation rates, multiple clinical and biologic outcomes (symptom scores, quality of life measures, bronchodilator use, forced expiratory flow in 1 second [FEV1], and peak expiratory flow rate [PEFR]) improved with nedocromil use compared with placebo.
The most effective dose for preventing exacerbations appears to be 4 mg (2 puffs) 4 times a day (SOR: A, multiple randomized controlled trials [RCTs] and meta-analyses). More severe forms of asthma respond better to inhaled steroids than to nedocromil (SOR: A, multiple RCTs). Nedocromil may allow some patients with severe asthma to use lower doses of inhaled steroids (SOR: C, conflicting RCTs). Nedocromil is also effective for the treatment of exercise-induced asthma (SOR: A, multiple RCTs and meta-analyses).
In general, about 50% to 70% of patients respond to nedocromil (SOR: A, multiple RCTs and meta-analyses). Unfortunately, which patients respond is not predictable from clinical parameters.1 Nedocromil is worth trying in mild persistent asthma, particularly for children where the parents are worried about the growth issues associated with inhaled steroids. Side effects (sore throat, nausea, and headache) are mild and infrequent. Maximal efficacy is usually seen after 6 to 8 weeks.
Evidence summary
A systematic review encompassing 127 trial centers and 4723 patients concluded that inhaled nedocromil was effective for a variety of patients with asthma. Significant improvements were noted in FEV1, PEFR, use of bronchodilators, symptom scores, and quality of life scores. The reviewers found nedocromil to be most effective for patients with moderate disease already taking bronchodilators,2 corresponding to the “mild persistent asthma” category ( Table ).
A contemporaneous European RCT, not included in the review, compared 4 mg of inhaled nedocromil 4 times daily with inhaled placebo among 209 asthmatic children for 12 weeks.3 After 8 weeks, they found a statistically significant reduction in total daily asthma symptom scores (50% nedocromil vs 9% placebo; P<.01). The proportion of parents and children rating treatment as moderately or very effective was 78% in the treatment group and 59% in the placebo group (number needed to treat [NNT]=5.2; P<.01); clinicians’ ratings were 73% for nedocromil and 50% for placebo (NNT=4.3; P<.01). The frequency of side effects—including nausea, headache, and sleepiness—did not reach statistical significance; however, the nedocromil group reported up to a 20% incidence of sore throat. Most of the studies reported no dropouts due to side effects.
When patients are already using inhaled steroids, the evidence is less clear whether nedocromil confers additional benefits, such as fewer exacerbations or lower inhaled steroid doses. Two small studies of patients either already on inhaled steroids4 or considered to be steroid-resistant5 found nonsignificant trends towards reductions in bronchodilator use, increased PEFR, increased FEV1, and improved quality of life. Although both studies were underpowered, the study on steroid-resistant asthma did find a statistically significant 20% improvement in PEFR and decreased bronchodilator use for 50% of patients at 8 and 12 weeks.
The inherent waxing and waning nature of asthma makes demonstrating benefits difficult. Furthermore, nedocromil tends to have an all-ornothing effect rather than a dose-response gradient. Unfortunately, none of these trials found useful predictors to help clinicians determine which patients respond.1,5
In a Cochrane Review, 20 RCTs involving 280 participants showed that 4 mg (2 puffs) of nedocromil inhaled 15 to 60 minutes prior to exercise significantly reduced the severity and duration of exercise-induced asthma for both adults and children. The maximum percentage fall in FEV1 improved significantly compared with placebo, with a weighted mean difference of 15.5% (95% confidence interval, 13.2–18.1). In addition, the time to complete recovery was shortened from 30 minutes with placebo to 10 minutes with nedocromil.6
TABLE
Classification of asthma
| Classification | Symptom frequency | Spirometry findings |
|---|---|---|
| Severe persistent | Continual symptoms | PEFR <60% Variability >30% |
| Moderate persistent | Daily symptoms, more than 1 night per week | PEFR >60% but <80% Variability >30% |
| Mild persistent | More than twice per week but less than daily; more than 2 nights per month | PEFR >80% Variability 20%–30% |
| Mild intermittent | Less than once per week; less than or equal to 2 nights per month | PEFR >80% Variability <20% |
| Source: Global Initiative for Asthma, National Heart, Lung and Blood Institute 2003.7 | ||
Recommendations from others
The Global Initiative for Asthma and the National Heart, Lung and Blood Institute Expert Panel Report list nedocromil as an option for the treatment of exercise-induced asthma and mild persistent asthma for adults and children. However, it is listed as a second choice to the use of inhaled steroids in the case of mild persistent asthma. It is not recommended for moderate or severe persistent asthma, or for mild intermittent asthma.7
Nedocromil and cromolyn sodium are safe but many patients do not respond
Ron Baldwin, MD
University of Wyoming Family Practice Residency at Casper
Inhaled nedocromil and cromolyn sodium have long been recognized as agents with an excellent safety profile. Unfortunately, as pointed about above, many patients do not respond to these agents. In addition, 4-times-daily dosing makes compliance difficult. Clinicians and parents must weigh the theoretical risk of inhaled corticosteroid-induced growth retardation with this potential differential in effectiveness.
1. Parish RC, Miller LJ. Nedocromil sodium. Ann Pharmacother 1993;27:599-606.
2. Edwards AM, Stevens MT. The clinical efficacy of inhaled nedocromil sodium (Tilade) in the treatment of asthma. Eur Respir J 1993;6:35-41.
3. Armenio L, Baldini G, Baldare M, et al. Double blind, placebo controlled study of nedocromil sodium in asthma. Arch Dis Child 1993;68:193-197.
4. O’Hickey SP, Rees PJ. High dose nedocromil sodium as an addition to inhaled corticosteroids in the treatment of asthma. Respir Med 1994;88:499-502.
5. Marin JM, Carrizo SJ, Garcia R, Ejea MV. Effects of nedocromil sodium in steroid-resistant asthma: a randomized controlled trial. J Allergy Clin Immunol 1996;97:602-610.
6. Spooner CH, Saunders LD, Rowe BH. Nedocromil sodium for preventing exercise-induced bronchoconstriction (Cochrane Review). The Cochrane Library, Issue 4, 2003. Chichester, UK: John Wiley & Sons, Ltd.
7. Global Strategy for Asthma Management and Prevention. Bethesda, Md: Global Initiative for Asthma, National Heart, Lung and Blood Institute; 2003.
Nedocromil (Tilade) is effective for the treatment of mild persistent asthma. It has not been shown to be effective in more severe forms of asthma for both children and adults. Although no studies looked specifically at exacerbation rates, multiple clinical and biologic outcomes (symptom scores, quality of life measures, bronchodilator use, forced expiratory flow in 1 second [FEV1], and peak expiratory flow rate [PEFR]) improved with nedocromil use compared with placebo.
The most effective dose for preventing exacerbations appears to be 4 mg (2 puffs) 4 times a day (SOR: A, multiple randomized controlled trials [RCTs] and meta-analyses). More severe forms of asthma respond better to inhaled steroids than to nedocromil (SOR: A, multiple RCTs). Nedocromil may allow some patients with severe asthma to use lower doses of inhaled steroids (SOR: C, conflicting RCTs). Nedocromil is also effective for the treatment of exercise-induced asthma (SOR: A, multiple RCTs and meta-analyses).
In general, about 50% to 70% of patients respond to nedocromil (SOR: A, multiple RCTs and meta-analyses). Unfortunately, which patients respond is not predictable from clinical parameters.1 Nedocromil is worth trying in mild persistent asthma, particularly for children where the parents are worried about the growth issues associated with inhaled steroids. Side effects (sore throat, nausea, and headache) are mild and infrequent. Maximal efficacy is usually seen after 6 to 8 weeks.
Evidence summary
A systematic review encompassing 127 trial centers and 4723 patients concluded that inhaled nedocromil was effective for a variety of patients with asthma. Significant improvements were noted in FEV1, PEFR, use of bronchodilators, symptom scores, and quality of life scores. The reviewers found nedocromil to be most effective for patients with moderate disease already taking bronchodilators,2 corresponding to the “mild persistent asthma” category ( Table ).
A contemporaneous European RCT, not included in the review, compared 4 mg of inhaled nedocromil 4 times daily with inhaled placebo among 209 asthmatic children for 12 weeks.3 After 8 weeks, they found a statistically significant reduction in total daily asthma symptom scores (50% nedocromil vs 9% placebo; P<.01). The proportion of parents and children rating treatment as moderately or very effective was 78% in the treatment group and 59% in the placebo group (number needed to treat [NNT]=5.2; P<.01); clinicians’ ratings were 73% for nedocromil and 50% for placebo (NNT=4.3; P<.01). The frequency of side effects—including nausea, headache, and sleepiness—did not reach statistical significance; however, the nedocromil group reported up to a 20% incidence of sore throat. Most of the studies reported no dropouts due to side effects.
When patients are already using inhaled steroids, the evidence is less clear whether nedocromil confers additional benefits, such as fewer exacerbations or lower inhaled steroid doses. Two small studies of patients either already on inhaled steroids4 or considered to be steroid-resistant5 found nonsignificant trends towards reductions in bronchodilator use, increased PEFR, increased FEV1, and improved quality of life. Although both studies were underpowered, the study on steroid-resistant asthma did find a statistically significant 20% improvement in PEFR and decreased bronchodilator use for 50% of patients at 8 and 12 weeks.
The inherent waxing and waning nature of asthma makes demonstrating benefits difficult. Furthermore, nedocromil tends to have an all-ornothing effect rather than a dose-response gradient. Unfortunately, none of these trials found useful predictors to help clinicians determine which patients respond.1,5
In a Cochrane Review, 20 RCTs involving 280 participants showed that 4 mg (2 puffs) of nedocromil inhaled 15 to 60 minutes prior to exercise significantly reduced the severity and duration of exercise-induced asthma for both adults and children. The maximum percentage fall in FEV1 improved significantly compared with placebo, with a weighted mean difference of 15.5% (95% confidence interval, 13.2–18.1). In addition, the time to complete recovery was shortened from 30 minutes with placebo to 10 minutes with nedocromil.6
TABLE
Classification of asthma
| Classification | Symptom frequency | Spirometry findings |
|---|---|---|
| Severe persistent | Continual symptoms | PEFR <60% Variability >30% |
| Moderate persistent | Daily symptoms, more than 1 night per week | PEFR >60% but <80% Variability >30% |
| Mild persistent | More than twice per week but less than daily; more than 2 nights per month | PEFR >80% Variability 20%–30% |
| Mild intermittent | Less than once per week; less than or equal to 2 nights per month | PEFR >80% Variability <20% |
| Source: Global Initiative for Asthma, National Heart, Lung and Blood Institute 2003.7 | ||
Recommendations from others
The Global Initiative for Asthma and the National Heart, Lung and Blood Institute Expert Panel Report list nedocromil as an option for the treatment of exercise-induced asthma and mild persistent asthma for adults and children. However, it is listed as a second choice to the use of inhaled steroids in the case of mild persistent asthma. It is not recommended for moderate or severe persistent asthma, or for mild intermittent asthma.7
Nedocromil and cromolyn sodium are safe but many patients do not respond
Ron Baldwin, MD
University of Wyoming Family Practice Residency at Casper
Inhaled nedocromil and cromolyn sodium have long been recognized as agents with an excellent safety profile. Unfortunately, as pointed about above, many patients do not respond to these agents. In addition, 4-times-daily dosing makes compliance difficult. Clinicians and parents must weigh the theoretical risk of inhaled corticosteroid-induced growth retardation with this potential differential in effectiveness.
Nedocromil (Tilade) is effective for the treatment of mild persistent asthma. It has not been shown to be effective in more severe forms of asthma for both children and adults. Although no studies looked specifically at exacerbation rates, multiple clinical and biologic outcomes (symptom scores, quality of life measures, bronchodilator use, forced expiratory flow in 1 second [FEV1], and peak expiratory flow rate [PEFR]) improved with nedocromil use compared with placebo.
The most effective dose for preventing exacerbations appears to be 4 mg (2 puffs) 4 times a day (SOR: A, multiple randomized controlled trials [RCTs] and meta-analyses). More severe forms of asthma respond better to inhaled steroids than to nedocromil (SOR: A, multiple RCTs). Nedocromil may allow some patients with severe asthma to use lower doses of inhaled steroids (SOR: C, conflicting RCTs). Nedocromil is also effective for the treatment of exercise-induced asthma (SOR: A, multiple RCTs and meta-analyses).
In general, about 50% to 70% of patients respond to nedocromil (SOR: A, multiple RCTs and meta-analyses). Unfortunately, which patients respond is not predictable from clinical parameters.1 Nedocromil is worth trying in mild persistent asthma, particularly for children where the parents are worried about the growth issues associated with inhaled steroids. Side effects (sore throat, nausea, and headache) are mild and infrequent. Maximal efficacy is usually seen after 6 to 8 weeks.
Evidence summary
A systematic review encompassing 127 trial centers and 4723 patients concluded that inhaled nedocromil was effective for a variety of patients with asthma. Significant improvements were noted in FEV1, PEFR, use of bronchodilators, symptom scores, and quality of life scores. The reviewers found nedocromil to be most effective for patients with moderate disease already taking bronchodilators,2 corresponding to the “mild persistent asthma” category ( Table ).
A contemporaneous European RCT, not included in the review, compared 4 mg of inhaled nedocromil 4 times daily with inhaled placebo among 209 asthmatic children for 12 weeks.3 After 8 weeks, they found a statistically significant reduction in total daily asthma symptom scores (50% nedocromil vs 9% placebo; P<.01). The proportion of parents and children rating treatment as moderately or very effective was 78% in the treatment group and 59% in the placebo group (number needed to treat [NNT]=5.2; P<.01); clinicians’ ratings were 73% for nedocromil and 50% for placebo (NNT=4.3; P<.01). The frequency of side effects—including nausea, headache, and sleepiness—did not reach statistical significance; however, the nedocromil group reported up to a 20% incidence of sore throat. Most of the studies reported no dropouts due to side effects.
When patients are already using inhaled steroids, the evidence is less clear whether nedocromil confers additional benefits, such as fewer exacerbations or lower inhaled steroid doses. Two small studies of patients either already on inhaled steroids4 or considered to be steroid-resistant5 found nonsignificant trends towards reductions in bronchodilator use, increased PEFR, increased FEV1, and improved quality of life. Although both studies were underpowered, the study on steroid-resistant asthma did find a statistically significant 20% improvement in PEFR and decreased bronchodilator use for 50% of patients at 8 and 12 weeks.
The inherent waxing and waning nature of asthma makes demonstrating benefits difficult. Furthermore, nedocromil tends to have an all-ornothing effect rather than a dose-response gradient. Unfortunately, none of these trials found useful predictors to help clinicians determine which patients respond.1,5
In a Cochrane Review, 20 RCTs involving 280 participants showed that 4 mg (2 puffs) of nedocromil inhaled 15 to 60 minutes prior to exercise significantly reduced the severity and duration of exercise-induced asthma for both adults and children. The maximum percentage fall in FEV1 improved significantly compared with placebo, with a weighted mean difference of 15.5% (95% confidence interval, 13.2–18.1). In addition, the time to complete recovery was shortened from 30 minutes with placebo to 10 minutes with nedocromil.6
TABLE
Classification of asthma
| Classification | Symptom frequency | Spirometry findings |
|---|---|---|
| Severe persistent | Continual symptoms | PEFR <60% Variability >30% |
| Moderate persistent | Daily symptoms, more than 1 night per week | PEFR >60% but <80% Variability >30% |
| Mild persistent | More than twice per week but less than daily; more than 2 nights per month | PEFR >80% Variability 20%–30% |
| Mild intermittent | Less than once per week; less than or equal to 2 nights per month | PEFR >80% Variability <20% |
| Source: Global Initiative for Asthma, National Heart, Lung and Blood Institute 2003.7 | ||
Recommendations from others
The Global Initiative for Asthma and the National Heart, Lung and Blood Institute Expert Panel Report list nedocromil as an option for the treatment of exercise-induced asthma and mild persistent asthma for adults and children. However, it is listed as a second choice to the use of inhaled steroids in the case of mild persistent asthma. It is not recommended for moderate or severe persistent asthma, or for mild intermittent asthma.7
Nedocromil and cromolyn sodium are safe but many patients do not respond
Ron Baldwin, MD
University of Wyoming Family Practice Residency at Casper
Inhaled nedocromil and cromolyn sodium have long been recognized as agents with an excellent safety profile. Unfortunately, as pointed about above, many patients do not respond to these agents. In addition, 4-times-daily dosing makes compliance difficult. Clinicians and parents must weigh the theoretical risk of inhaled corticosteroid-induced growth retardation with this potential differential in effectiveness.
1. Parish RC, Miller LJ. Nedocromil sodium. Ann Pharmacother 1993;27:599-606.
2. Edwards AM, Stevens MT. The clinical efficacy of inhaled nedocromil sodium (Tilade) in the treatment of asthma. Eur Respir J 1993;6:35-41.
3. Armenio L, Baldini G, Baldare M, et al. Double blind, placebo controlled study of nedocromil sodium in asthma. Arch Dis Child 1993;68:193-197.
4. O’Hickey SP, Rees PJ. High dose nedocromil sodium as an addition to inhaled corticosteroids in the treatment of asthma. Respir Med 1994;88:499-502.
5. Marin JM, Carrizo SJ, Garcia R, Ejea MV. Effects of nedocromil sodium in steroid-resistant asthma: a randomized controlled trial. J Allergy Clin Immunol 1996;97:602-610.
6. Spooner CH, Saunders LD, Rowe BH. Nedocromil sodium for preventing exercise-induced bronchoconstriction (Cochrane Review). The Cochrane Library, Issue 4, 2003. Chichester, UK: John Wiley & Sons, Ltd.
7. Global Strategy for Asthma Management and Prevention. Bethesda, Md: Global Initiative for Asthma, National Heart, Lung and Blood Institute; 2003.
1. Parish RC, Miller LJ. Nedocromil sodium. Ann Pharmacother 1993;27:599-606.
2. Edwards AM, Stevens MT. The clinical efficacy of inhaled nedocromil sodium (Tilade) in the treatment of asthma. Eur Respir J 1993;6:35-41.
3. Armenio L, Baldini G, Baldare M, et al. Double blind, placebo controlled study of nedocromil sodium in asthma. Arch Dis Child 1993;68:193-197.
4. O’Hickey SP, Rees PJ. High dose nedocromil sodium as an addition to inhaled corticosteroids in the treatment of asthma. Respir Med 1994;88:499-502.
5. Marin JM, Carrizo SJ, Garcia R, Ejea MV. Effects of nedocromil sodium in steroid-resistant asthma: a randomized controlled trial. J Allergy Clin Immunol 1996;97:602-610.
6. Spooner CH, Saunders LD, Rowe BH. Nedocromil sodium for preventing exercise-induced bronchoconstriction (Cochrane Review). The Cochrane Library, Issue 4, 2003. Chichester, UK: John Wiley & Sons, Ltd.
7. Global Strategy for Asthma Management and Prevention. Bethesda, Md: Global Initiative for Asthma, National Heart, Lung and Blood Institute; 2003.
Evidence-based answers from the Family Physicians Inquiries Network
How should thyroid replacement be initiated?
Levothyroxine (LT4) should be used alone as initial replacement for patients with hypothyroidism (strength of recommendation [SOR]: A). The optimal initial dose is 1.6 μg/kg/d for healthy people aged 60 years or younger (SOR: B). Patients aged more than 60 years may require less levothyroxine to achieve therapeutic serum thyroid hormone replacement, so initial replacement should be decreased to 25 to 50 μg daily (SOR: C).
Since patients with known heart disease may develop dysrhythmias, angina, and myocardial infarctions when started on full replacement doses, experts recommend starting 12.5 to 25 μg daily for this population (SOR: C). Brand-name (Synthroid, Levoxyl, etc) and generic LT4 are bioequivalent (SOR: B), although the US Food and Drug Administration (FDA) does not consider these products to be interchangeable until proven therapeutically equivalent.
Evidence summary
Initial thyroid replacement with synthetic LT4 is recommended because LT4 is safe, effective, reliably relieves symptoms, and normalizes lab tests for hypothyroid patients.1,2
Two recent randomized trials comparing LT4 alone or LT4 and LT3 together for a total of 86 adult hypothyroid patients found similar outcomes. One study, which enrolled patients with hypothy-roidism and mild depressive symptoms, assessed scores on the Symptom Check-List-90, the Comprehensive Epidemiological Screens for Depression, and the Medical Outcomes Study health status questionnaire at baseline and multiple times over the duration of the study. For these outcomes, no differences were found between the LT4 alone and combination LT4-LT3 treatment groups within 90% confidence intervals.3 A second study assessed changes in body weight, lipid profile, hypothyroid-specific health-related quality-of-life scores, and 13 neuropsychological measures pre- and posttreatment. This study detected no difference in body weight and serum lipids at baseline and after treatment. The hypothyroid-specific health-related quality-of-life scores similarly improved for both treatment groups. Twelve of 13 neuropsychological tests demonstrated no differences between treatment groups; the Grooved Peg Board Test of manual dexterity and fine visual-motor coordination demonstrated a slight improvement for the LT4 alone treatment group.4
The initial dose of LT4 can be based on the age and health status of the patient. The mean replacement dose of LT4 is 1.6 μg/kg/d for healthy patients aged ≤60 years.5 7 Patients aged >60 years should be started on 25 to 50 μg daily. An uncontrolled cohort study of 84 patients found that for patients aged >60 years, 25- to 50-μg doses of LT4 resulted in similar serum thyrotropin (TSH) levels as the higher (1.6 μg/kg/d) doses required for younger patients.7 Based on expert opinion, patients of any age with heart disease should be given lower doses of 12.5 to 25 μg daily as initial treatment.1,2
The choice of the LT4 preparation continues to be debated. In 1997, a bioequivalence study compared 2 generic brands to 2 name brands by having 22 women with hypothyroidism, who were euthyroid on replacement medication, take each preparation for 6 weeks.8 The area under the curve, peak serum concentration, and time to peak concentration for 3 indexes of thyroid function (thyroxine, triiodothyronine, and free T4 index) were not significantly different and met the FDA criterion for relative bioequivalence. However, they did not examine therapeutic equivalence and from a clinical perspective, some researchers and pharmaceutical companies felt that the authors could not comment on whether the products were interchangeable.8,9 The FDA now requires thyroxine bioavailability and bioequivalence studies to evaluate product substitution.10 The FDA lists Levothyroxine Sodium (Mylan) to be therapeutically equivalent and therefore interchangeable with Unithroid.11
Recommendations from others
The American Association of Clinical Endocrinologists Thyroid Task Force recommends the use of a high-quality brand preparation of LT4 rather than desiccated thyroid hormone, combinations of thyroid hormones, or LT3.12 It recommends a mean replacement dosage of LT4 of 1.6 μg/kg of body weight per day with initial dose ranging from 12.5 μg daily to a full replacement dosage based on the age, weight, and cardiac status of the patient.
UpToDate states that although LT4 products are standardized, subtle differences between preparations exist, and products should be interchanged only with sufficient monitoring after the change. In addition, they recommend generally avoiding generics because the pharmacy may interchange products without physicians being aware.1 . The Physicians’ Information and Education Resource from the American College of Physicians states “Name-brand LT4 products provide more consistent potency than generic preparations. The cost of brand-name LT4 products is only slightly more than that of generic preparations.”2
Instruct patients about the timing of levothyroxine and potential interactions
Santhi Penmetsa, MD
Department of Family and Community Medicine, Baylor College of Medicine
The starting dose of levothyroxine for hypothyroid patients is based on age, severity of the disease, duration of the disease, and existing comorbid conditions. For healthy adults 60 years of age or younger, the optimal starting dose is 1.6 μg/kg/d. For patients more than 60 years of age, the initial dose is 25 to 50 μg/d. To avoid cardiac complications among persons with known heart disease, the recommended initial levothyroxine dose is 12.5 μg/d. In my experience, these guidelines work well in initiating treatment for hypothyroidism.
Few of my patients have noted any difference between generic and brand-name thyroid supplements. Knowing what other medications the patient is taking is important, since medications such as estrogen can decrease the bioavailability of levothyroxine by increasing binding proteins. It is also important to instruct patients about the timing of levothyroxine intake, because some medications can affect absorption (eg, cholestyramine, calcium, or iron).
1. Ross DS. Treatment of hypothyroidism. UpToDate. Last update January 9, 2004. Available at: www.uptodate.com. Accessed on March 4, 2004.
2. McDermott MT. Hypothyroidism. ACP’s PIER: Physicians’ Information and Education Resource. Last update March 17, 2004. Available at: http://online.statref.com/document.aspx?fxid=50&docid=1 297. Accessed on May 10, 2004.
3. Sawka AM, Gerstein HC, Marriott MJ, MacQueen GM, Joffe RT. Does a combination regimen of thyroxine (T4) and 3,5,3’-triiodothyronine improve depressive symptoms better than T4 alone in patients with hypothyroidism? Results of a double-blind, randomized, controlled trial. J Clin Endocrinol Metab 2003;88:4551-4555.
4. Clyde PW, Harari AE, Getka EJ, Shakir KM. Combined levothyroxine plus liothyronine compared with levothyroxine alone in primary hypothyroidism: a randomized controlled trial. JAMA 2003;290:2952-2958.
5. Fish LH, Schwartz HL, Cavanaugh J, Steffes MW, Bantle JP, Oppenheimer JH. Replacement dose, metabolism, and bioavailability of levothyroxine in the treatment of hypothyroidism. Role of triiodothyronine in pituitary feedback in humans. N Engl J Med 1987;316:764-770.
6. Carr D, McLeod DT, Parry G, Thornes HM. Fine adjustment of thyroxine replacement dosage: comparison of the thyrotrophin releasing hormone test using a sensitive thyrotrophin assay with measurement of free thyroid hormones and clinical assessment. Clin Endocrinol (Oxf) 1988;28:325-333.
7. Sawin CT, Herman T, Molitch ME, London MH, Kramer SM. Aging and the thyroid: Decreased requirement for thyroid hormone in older hypothyroid patients. Am J Med 1983;75:206-209.
8. Dong BJ, Hauck WW, Gambertoglio JG, et al. Bioequivalence of generic and brand-name levothyroxine products in the treatment of hypothyroidism. JAMA 1997;277:1205-1213.
9. Rennie D. Thyroid storm. JAMA 1997;277:1238-1243.
10. Hennessey JV. Precise thyroxine dosing: Clinical requirements. Endocrinologist 2003;13:479-487.
11. FDA Center for Drug Evaluation and Research. Drugs@FDA: Levothyroxine Sodium (Generic Drug). Available at: http://www.accessdata.fda.gov/scripts/cder/drugsatfda/index.cfm. Accessed on June 3, 2004.
Levothyroxine (LT4) should be used alone as initial replacement for patients with hypothyroidism (strength of recommendation [SOR]: A). The optimal initial dose is 1.6 μg/kg/d for healthy people aged 60 years or younger (SOR: B). Patients aged more than 60 years may require less levothyroxine to achieve therapeutic serum thyroid hormone replacement, so initial replacement should be decreased to 25 to 50 μg daily (SOR: C).
Since patients with known heart disease may develop dysrhythmias, angina, and myocardial infarctions when started on full replacement doses, experts recommend starting 12.5 to 25 μg daily for this population (SOR: C). Brand-name (Synthroid, Levoxyl, etc) and generic LT4 are bioequivalent (SOR: B), although the US Food and Drug Administration (FDA) does not consider these products to be interchangeable until proven therapeutically equivalent.
Evidence summary
Initial thyroid replacement with synthetic LT4 is recommended because LT4 is safe, effective, reliably relieves symptoms, and normalizes lab tests for hypothyroid patients.1,2
Two recent randomized trials comparing LT4 alone or LT4 and LT3 together for a total of 86 adult hypothyroid patients found similar outcomes. One study, which enrolled patients with hypothy-roidism and mild depressive symptoms, assessed scores on the Symptom Check-List-90, the Comprehensive Epidemiological Screens for Depression, and the Medical Outcomes Study health status questionnaire at baseline and multiple times over the duration of the study. For these outcomes, no differences were found between the LT4 alone and combination LT4-LT3 treatment groups within 90% confidence intervals.3 A second study assessed changes in body weight, lipid profile, hypothyroid-specific health-related quality-of-life scores, and 13 neuropsychological measures pre- and posttreatment. This study detected no difference in body weight and serum lipids at baseline and after treatment. The hypothyroid-specific health-related quality-of-life scores similarly improved for both treatment groups. Twelve of 13 neuropsychological tests demonstrated no differences between treatment groups; the Grooved Peg Board Test of manual dexterity and fine visual-motor coordination demonstrated a slight improvement for the LT4 alone treatment group.4
The initial dose of LT4 can be based on the age and health status of the patient. The mean replacement dose of LT4 is 1.6 μg/kg/d for healthy patients aged ≤60 years.5 7 Patients aged >60 years should be started on 25 to 50 μg daily. An uncontrolled cohort study of 84 patients found that for patients aged >60 years, 25- to 50-μg doses of LT4 resulted in similar serum thyrotropin (TSH) levels as the higher (1.6 μg/kg/d) doses required for younger patients.7 Based on expert opinion, patients of any age with heart disease should be given lower doses of 12.5 to 25 μg daily as initial treatment.1,2
The choice of the LT4 preparation continues to be debated. In 1997, a bioequivalence study compared 2 generic brands to 2 name brands by having 22 women with hypothyroidism, who were euthyroid on replacement medication, take each preparation for 6 weeks.8 The area under the curve, peak serum concentration, and time to peak concentration for 3 indexes of thyroid function (thyroxine, triiodothyronine, and free T4 index) were not significantly different and met the FDA criterion for relative bioequivalence. However, they did not examine therapeutic equivalence and from a clinical perspective, some researchers and pharmaceutical companies felt that the authors could not comment on whether the products were interchangeable.8,9 The FDA now requires thyroxine bioavailability and bioequivalence studies to evaluate product substitution.10 The FDA lists Levothyroxine Sodium (Mylan) to be therapeutically equivalent and therefore interchangeable with Unithroid.11
Recommendations from others
The American Association of Clinical Endocrinologists Thyroid Task Force recommends the use of a high-quality brand preparation of LT4 rather than desiccated thyroid hormone, combinations of thyroid hormones, or LT3.12 It recommends a mean replacement dosage of LT4 of 1.6 μg/kg of body weight per day with initial dose ranging from 12.5 μg daily to a full replacement dosage based on the age, weight, and cardiac status of the patient.
UpToDate states that although LT4 products are standardized, subtle differences between preparations exist, and products should be interchanged only with sufficient monitoring after the change. In addition, they recommend generally avoiding generics because the pharmacy may interchange products without physicians being aware.1 . The Physicians’ Information and Education Resource from the American College of Physicians states “Name-brand LT4 products provide more consistent potency than generic preparations. The cost of brand-name LT4 products is only slightly more than that of generic preparations.”2
Instruct patients about the timing of levothyroxine and potential interactions
Santhi Penmetsa, MD
Department of Family and Community Medicine, Baylor College of Medicine
The starting dose of levothyroxine for hypothyroid patients is based on age, severity of the disease, duration of the disease, and existing comorbid conditions. For healthy adults 60 years of age or younger, the optimal starting dose is 1.6 μg/kg/d. For patients more than 60 years of age, the initial dose is 25 to 50 μg/d. To avoid cardiac complications among persons with known heart disease, the recommended initial levothyroxine dose is 12.5 μg/d. In my experience, these guidelines work well in initiating treatment for hypothyroidism.
Few of my patients have noted any difference between generic and brand-name thyroid supplements. Knowing what other medications the patient is taking is important, since medications such as estrogen can decrease the bioavailability of levothyroxine by increasing binding proteins. It is also important to instruct patients about the timing of levothyroxine intake, because some medications can affect absorption (eg, cholestyramine, calcium, or iron).
Levothyroxine (LT4) should be used alone as initial replacement for patients with hypothyroidism (strength of recommendation [SOR]: A). The optimal initial dose is 1.6 μg/kg/d for healthy people aged 60 years or younger (SOR: B). Patients aged more than 60 years may require less levothyroxine to achieve therapeutic serum thyroid hormone replacement, so initial replacement should be decreased to 25 to 50 μg daily (SOR: C).
Since patients with known heart disease may develop dysrhythmias, angina, and myocardial infarctions when started on full replacement doses, experts recommend starting 12.5 to 25 μg daily for this population (SOR: C). Brand-name (Synthroid, Levoxyl, etc) and generic LT4 are bioequivalent (SOR: B), although the US Food and Drug Administration (FDA) does not consider these products to be interchangeable until proven therapeutically equivalent.
Evidence summary
Initial thyroid replacement with synthetic LT4 is recommended because LT4 is safe, effective, reliably relieves symptoms, and normalizes lab tests for hypothyroid patients.1,2
Two recent randomized trials comparing LT4 alone or LT4 and LT3 together for a total of 86 adult hypothyroid patients found similar outcomes. One study, which enrolled patients with hypothy-roidism and mild depressive symptoms, assessed scores on the Symptom Check-List-90, the Comprehensive Epidemiological Screens for Depression, and the Medical Outcomes Study health status questionnaire at baseline and multiple times over the duration of the study. For these outcomes, no differences were found between the LT4 alone and combination LT4-LT3 treatment groups within 90% confidence intervals.3 A second study assessed changes in body weight, lipid profile, hypothyroid-specific health-related quality-of-life scores, and 13 neuropsychological measures pre- and posttreatment. This study detected no difference in body weight and serum lipids at baseline and after treatment. The hypothyroid-specific health-related quality-of-life scores similarly improved for both treatment groups. Twelve of 13 neuropsychological tests demonstrated no differences between treatment groups; the Grooved Peg Board Test of manual dexterity and fine visual-motor coordination demonstrated a slight improvement for the LT4 alone treatment group.4
The initial dose of LT4 can be based on the age and health status of the patient. The mean replacement dose of LT4 is 1.6 μg/kg/d for healthy patients aged ≤60 years.5 7 Patients aged >60 years should be started on 25 to 50 μg daily. An uncontrolled cohort study of 84 patients found that for patients aged >60 years, 25- to 50-μg doses of LT4 resulted in similar serum thyrotropin (TSH) levels as the higher (1.6 μg/kg/d) doses required for younger patients.7 Based on expert opinion, patients of any age with heart disease should be given lower doses of 12.5 to 25 μg daily as initial treatment.1,2
The choice of the LT4 preparation continues to be debated. In 1997, a bioequivalence study compared 2 generic brands to 2 name brands by having 22 women with hypothyroidism, who were euthyroid on replacement medication, take each preparation for 6 weeks.8 The area under the curve, peak serum concentration, and time to peak concentration for 3 indexes of thyroid function (thyroxine, triiodothyronine, and free T4 index) were not significantly different and met the FDA criterion for relative bioequivalence. However, they did not examine therapeutic equivalence and from a clinical perspective, some researchers and pharmaceutical companies felt that the authors could not comment on whether the products were interchangeable.8,9 The FDA now requires thyroxine bioavailability and bioequivalence studies to evaluate product substitution.10 The FDA lists Levothyroxine Sodium (Mylan) to be therapeutically equivalent and therefore interchangeable with Unithroid.11
Recommendations from others
The American Association of Clinical Endocrinologists Thyroid Task Force recommends the use of a high-quality brand preparation of LT4 rather than desiccated thyroid hormone, combinations of thyroid hormones, or LT3.12 It recommends a mean replacement dosage of LT4 of 1.6 μg/kg of body weight per day with initial dose ranging from 12.5 μg daily to a full replacement dosage based on the age, weight, and cardiac status of the patient.
UpToDate states that although LT4 products are standardized, subtle differences between preparations exist, and products should be interchanged only with sufficient monitoring after the change. In addition, they recommend generally avoiding generics because the pharmacy may interchange products without physicians being aware.1 . The Physicians’ Information and Education Resource from the American College of Physicians states “Name-brand LT4 products provide more consistent potency than generic preparations. The cost of brand-name LT4 products is only slightly more than that of generic preparations.”2
Instruct patients about the timing of levothyroxine and potential interactions
Santhi Penmetsa, MD
Department of Family and Community Medicine, Baylor College of Medicine
The starting dose of levothyroxine for hypothyroid patients is based on age, severity of the disease, duration of the disease, and existing comorbid conditions. For healthy adults 60 years of age or younger, the optimal starting dose is 1.6 μg/kg/d. For patients more than 60 years of age, the initial dose is 25 to 50 μg/d. To avoid cardiac complications among persons with known heart disease, the recommended initial levothyroxine dose is 12.5 μg/d. In my experience, these guidelines work well in initiating treatment for hypothyroidism.
Few of my patients have noted any difference between generic and brand-name thyroid supplements. Knowing what other medications the patient is taking is important, since medications such as estrogen can decrease the bioavailability of levothyroxine by increasing binding proteins. It is also important to instruct patients about the timing of levothyroxine intake, because some medications can affect absorption (eg, cholestyramine, calcium, or iron).
1. Ross DS. Treatment of hypothyroidism. UpToDate. Last update January 9, 2004. Available at: www.uptodate.com. Accessed on March 4, 2004.
2. McDermott MT. Hypothyroidism. ACP’s PIER: Physicians’ Information and Education Resource. Last update March 17, 2004. Available at: http://online.statref.com/document.aspx?fxid=50&docid=1 297. Accessed on May 10, 2004.
3. Sawka AM, Gerstein HC, Marriott MJ, MacQueen GM, Joffe RT. Does a combination regimen of thyroxine (T4) and 3,5,3’-triiodothyronine improve depressive symptoms better than T4 alone in patients with hypothyroidism? Results of a double-blind, randomized, controlled trial. J Clin Endocrinol Metab 2003;88:4551-4555.
4. Clyde PW, Harari AE, Getka EJ, Shakir KM. Combined levothyroxine plus liothyronine compared with levothyroxine alone in primary hypothyroidism: a randomized controlled trial. JAMA 2003;290:2952-2958.
5. Fish LH, Schwartz HL, Cavanaugh J, Steffes MW, Bantle JP, Oppenheimer JH. Replacement dose, metabolism, and bioavailability of levothyroxine in the treatment of hypothyroidism. Role of triiodothyronine in pituitary feedback in humans. N Engl J Med 1987;316:764-770.
6. Carr D, McLeod DT, Parry G, Thornes HM. Fine adjustment of thyroxine replacement dosage: comparison of the thyrotrophin releasing hormone test using a sensitive thyrotrophin assay with measurement of free thyroid hormones and clinical assessment. Clin Endocrinol (Oxf) 1988;28:325-333.
7. Sawin CT, Herman T, Molitch ME, London MH, Kramer SM. Aging and the thyroid: Decreased requirement for thyroid hormone in older hypothyroid patients. Am J Med 1983;75:206-209.
8. Dong BJ, Hauck WW, Gambertoglio JG, et al. Bioequivalence of generic and brand-name levothyroxine products in the treatment of hypothyroidism. JAMA 1997;277:1205-1213.
9. Rennie D. Thyroid storm. JAMA 1997;277:1238-1243.
10. Hennessey JV. Precise thyroxine dosing: Clinical requirements. Endocrinologist 2003;13:479-487.
11. FDA Center for Drug Evaluation and Research. Drugs@FDA: Levothyroxine Sodium (Generic Drug). Available at: http://www.accessdata.fda.gov/scripts/cder/drugsatfda/index.cfm. Accessed on June 3, 2004.
1. Ross DS. Treatment of hypothyroidism. UpToDate. Last update January 9, 2004. Available at: www.uptodate.com. Accessed on March 4, 2004.
2. McDermott MT. Hypothyroidism. ACP’s PIER: Physicians’ Information and Education Resource. Last update March 17, 2004. Available at: http://online.statref.com/document.aspx?fxid=50&docid=1 297. Accessed on May 10, 2004.
3. Sawka AM, Gerstein HC, Marriott MJ, MacQueen GM, Joffe RT. Does a combination regimen of thyroxine (T4) and 3,5,3’-triiodothyronine improve depressive symptoms better than T4 alone in patients with hypothyroidism? Results of a double-blind, randomized, controlled trial. J Clin Endocrinol Metab 2003;88:4551-4555.
4. Clyde PW, Harari AE, Getka EJ, Shakir KM. Combined levothyroxine plus liothyronine compared with levothyroxine alone in primary hypothyroidism: a randomized controlled trial. JAMA 2003;290:2952-2958.
5. Fish LH, Schwartz HL, Cavanaugh J, Steffes MW, Bantle JP, Oppenheimer JH. Replacement dose, metabolism, and bioavailability of levothyroxine in the treatment of hypothyroidism. Role of triiodothyronine in pituitary feedback in humans. N Engl J Med 1987;316:764-770.
6. Carr D, McLeod DT, Parry G, Thornes HM. Fine adjustment of thyroxine replacement dosage: comparison of the thyrotrophin releasing hormone test using a sensitive thyrotrophin assay with measurement of free thyroid hormones and clinical assessment. Clin Endocrinol (Oxf) 1988;28:325-333.
7. Sawin CT, Herman T, Molitch ME, London MH, Kramer SM. Aging and the thyroid: Decreased requirement for thyroid hormone in older hypothyroid patients. Am J Med 1983;75:206-209.
8. Dong BJ, Hauck WW, Gambertoglio JG, et al. Bioequivalence of generic and brand-name levothyroxine products in the treatment of hypothyroidism. JAMA 1997;277:1205-1213.
9. Rennie D. Thyroid storm. JAMA 1997;277:1238-1243.
10. Hennessey JV. Precise thyroxine dosing: Clinical requirements. Endocrinologist 2003;13:479-487.
11. FDA Center for Drug Evaluation and Research. Drugs@FDA: Levothyroxine Sodium (Generic Drug). Available at: http://www.accessdata.fda.gov/scripts/cder/drugsatfda/index.cfm. Accessed on June 3, 2004.
Evidence-based answers from the Family Physicians Inquiries Network
Does acyclovir help herpes simplex virus cold sores if treatment is delayed?
When herpes simplex virus (HSV) type 1 lesions are in the papule or vesicle stage, there is no benefit to starting oral acyclovir (strength of recommendation [SOR]: C, based on expert opinion). However, topical acyclovir 5% cream applied 5 times a day decreases pain and the duration of hard crust (SOR: B, extrapolated from randomized controlled trials [RCTs]).
If started at the onset of symptoms (during the prodrome stage), acyclovir (400 mg 5 times daily for 5 days) decreases pain and healing time to loss of crust and valacyclovir (2 g twice daily for 1 day) reduces the lesion duration and time to healing and may prevent lesion development (SOR: A, based on RCTs).
Evidence summary
Cold sores, or herpes labialis, are caused by HSV. Recurrent lesions progress quickly through several stages (prodrome, erythema, papule, vesicle, ulcer, crust, residual swelling, healed).1 Because of the rapid development of the vesicle stage (<12 hours) and the rapid decrease in detectable virus after 48 hours, studies of antiviral therapy empirically require early treatment within the first several hours of signs or symptoms of a recurrence. For this reason, there are no controlled trials of oral medications given later than 12 hours after the onset of recurrent symptoms.
Although limited, the clearest indication of appropriate timing for HSV 1 treatment with acyclovir comes from a well-designed, double-blinded RCT of 174 adults with a history of culture confirmed HSV labialis who initiated self-treatment with acyclovir 400 mg or placebo 5 times a day for 5 days. Patients were asked to defer treatment until the next episode if they awoke with the lesion or first noticed them in the vesicle or ulcer stage. Ninety-seven percent of the patients started treatment within 1 hour of signs/symptoms of a recurrence. Of the 174 patients, 90 had lesions in the prodrome or erythema stage at the start of treatment and 84 had lesions in the papule or vesicle stage.
Overall, acyclovir did not effect lesion progression, size, or healing time to loss of hard crust or normal skin. However, the mean duration of pain for all patients significantly decreased (2.5 days vs 3.8 days for placebo, P=.01). For the subgroup of patients who started acyclovir treatment in the prodrome or erythema stage, the mean duration of pain significantly decreased (2.5 days vs 3.9 days for placebo, P=.02), as did healing time to loss of crust (5.8 days vs 7.9 days for placebo, P=.03). Among those who started acyclovir in the papular stage, the trend was toward drug benefit, but this was not statistically significant (mean pain duration: 2.5 vs. 3.6, P=.36; mean healing time to loss of crust: 8.0 vs. 7.2, P=.52).2 This evidence supports early (prodrome or erythema stage) but not late (macule, papule, vesicle, or crusted stage) treatment of HSV 1 with oral acyclovir.
Topical application of 5% acyclovir cream significantly decreases clinician-assessed duration of the episode and duration of patient-reported pain, based on 2 double-blind, multicenter RCTs that used a vehicle control. In these trials, 686 and 699 patients self-initiated treatment 5 times a day for 4 days beginning within 1 hour of the onset of a recurrent lesion. In the first study, the mean clinician-assessed duration of the episode with topical acyclovir was 4.3 vs 4.8 days for placebo (hazard ratio [HR]=1.23; 95% confidence interval [CI], 1.06–1.44), and the mean duration of patient-assessed pain was 2.9 vs 3.2 days (HR=1.20; 95% CI, 1.03–1.40). The second study showed a mean clinician-assessed duration with topical acyclovir of 4.6 vs 5.2 days for control (HR=1.24; 95% CI, 1.06–1.44), and the mean duration of patient-assessed pain was 3.1 vs 3.5 days (HR=1.21; 95% CI, 1.04–1.40). Benefits were seen regardless of whether treatment was initiated early (prodrome or erythema stage) or late (macule, papule, vesicle or crusted stage).3
Recent studies of valacyclovir (the L-valine ester of acyclovir, which has 3 to 5 times greater bioavailability) offer the most promise for effective self-initiated treatment of recurrent herpes labialis. In a report of 2 well-designed, multicenter RCTs, valacyclovir at the FDA-approved dosage of 2 g twice daily for 1 day at the onset of symptoms (before visible signs of a cold sore) significantly decreased the mean duration of the lesion and time to lesion healing. In the first study (n=603), episode duration was decreased by 1.1 days (5.0 days vs 6.1 days for placebo; 95% CI, –1.6 to –0.6) and in the second study (n=605) by 1.0 day (5.3 vs 6.3 days for placebo; 95% CI, –1.0 to –0.5). In the first study, the time to lesion healing was decreased by 1.3 days (4.8 vs 6.1 days for placebo; 95% CI, –1.9 to –0.7) and in the second study by 1.2 days (5.1 vs. 6.4 days; 95% CI, –1.8 to –0.7). There also was a trend towards preventing the development of lesions, but this was not statistically significant.4
Recommendations from others
The BMJ Clinical Evidence Guideline reiterates that no trials compare early vs late treatment, so no firm conclusions about the efficacy of delayed treatment can be drawn.5 UpToDate reports that HSV 1 studies take into account that acyclovir acts only during active viral replication, which largely precedes symptoms, and thus suggest that it has little effect if begun after the appearance of lesions.6
For late presenters, review local care and hygiene; for all patients, review management of recurrences
Owen McCormack, DO
Baylor College of Medicine, Houston, TX
Patients seek treatment for herpes labialis due to bothersome physical symptoms and psychosocial implications. Many patients can identify prodromal symptoms such as localized itching, burning, irritation, or pain. Diagnosis of the initial episode is frequently delayed as patients are evaluated after the time period when studies have shown the most benefit from antivirals. For the late presenters, I review local care and hygiene, and for all patients I review management of recurrences.
Patient-initiated treatment is effective for those who can recognize the earliest signs and symptoms and start treatment immediately with either a topical or systemic antiviral. Both formulations decrease the lesion time to healing and pain if started at the first onset of symptoms.
Cost is an important consideration when selecting a particular formulation. Approximate price for the regimens presented here are $12 for 5 days of oral acyclovir, $27 for 1 day of oral valacyclovir, and $37 for a 2-g tube of acyclovir cream, which can be used for more than 1 episode.7 Other factors to consider are pill burden, duration of treatment, patient preference, and lifestyle. Patients can keep a refill or medicine on-hand to manage recurrences with the advice to begin immediately with onset of signs or symptoms.
1. Spruance S, Overall J, Kern E, Krueger G, Pliam V, Miller W. The natural history of recurrent herpes simplex labialis. JAMA 1977;297:69-75.
2. Spruance S, Stewart J, Rowe N, McKoeugh M, Wenerstrom G, Freeman D. Treatment of recurrent herpes simplex labialis with oral acyclovir. J Infect Dis 1990;161:181-190.
3. Spruance S, Nett R, Marbury T, Wolff R, Johnson J, Spaulding T. Acyclovir cream for treatment of herpes simplex labialis: results of two randomized, double-blind, vehicle-controlled multicenter clinical trials. Antimicrobial Agents Chemother 2002;46:2238-2243.
4. Spruance S, Jones T, Blatter M, et al. High-dose, short-duration, early valacyclovir therapy for episodic treatment of cold sores: results of two randomized, placebo-controlled, multicenter studies. Antimicrobial Agents Chemother 2003;47:1072-1080.
5. Clin Evid [online] Issue 10 2003. London: BMJ Publishing Group, last updated December 2002. Available at: http://clinicalevidence.com. Accessed on May 19, 2004.
6. Klein R. Treatment and prevention of herpes simplex virus type 1 infection. UpToDate version 12.2, last updated February 25, 2004. Available at: UpToDate.com. Accessed on July 14, 2004.
7. Availableat: www. drugstore.com. Accessed on August 18, 2004.
When herpes simplex virus (HSV) type 1 lesions are in the papule or vesicle stage, there is no benefit to starting oral acyclovir (strength of recommendation [SOR]: C, based on expert opinion). However, topical acyclovir 5% cream applied 5 times a day decreases pain and the duration of hard crust (SOR: B, extrapolated from randomized controlled trials [RCTs]).
If started at the onset of symptoms (during the prodrome stage), acyclovir (400 mg 5 times daily for 5 days) decreases pain and healing time to loss of crust and valacyclovir (2 g twice daily for 1 day) reduces the lesion duration and time to healing and may prevent lesion development (SOR: A, based on RCTs).
Evidence summary
Cold sores, or herpes labialis, are caused by HSV. Recurrent lesions progress quickly through several stages (prodrome, erythema, papule, vesicle, ulcer, crust, residual swelling, healed).1 Because of the rapid development of the vesicle stage (<12 hours) and the rapid decrease in detectable virus after 48 hours, studies of antiviral therapy empirically require early treatment within the first several hours of signs or symptoms of a recurrence. For this reason, there are no controlled trials of oral medications given later than 12 hours after the onset of recurrent symptoms.
Although limited, the clearest indication of appropriate timing for HSV 1 treatment with acyclovir comes from a well-designed, double-blinded RCT of 174 adults with a history of culture confirmed HSV labialis who initiated self-treatment with acyclovir 400 mg or placebo 5 times a day for 5 days. Patients were asked to defer treatment until the next episode if they awoke with the lesion or first noticed them in the vesicle or ulcer stage. Ninety-seven percent of the patients started treatment within 1 hour of signs/symptoms of a recurrence. Of the 174 patients, 90 had lesions in the prodrome or erythema stage at the start of treatment and 84 had lesions in the papule or vesicle stage.
Overall, acyclovir did not effect lesion progression, size, or healing time to loss of hard crust or normal skin. However, the mean duration of pain for all patients significantly decreased (2.5 days vs 3.8 days for placebo, P=.01). For the subgroup of patients who started acyclovir treatment in the prodrome or erythema stage, the mean duration of pain significantly decreased (2.5 days vs 3.9 days for placebo, P=.02), as did healing time to loss of crust (5.8 days vs 7.9 days for placebo, P=.03). Among those who started acyclovir in the papular stage, the trend was toward drug benefit, but this was not statistically significant (mean pain duration: 2.5 vs. 3.6, P=.36; mean healing time to loss of crust: 8.0 vs. 7.2, P=.52).2 This evidence supports early (prodrome or erythema stage) but not late (macule, papule, vesicle, or crusted stage) treatment of HSV 1 with oral acyclovir.
Topical application of 5% acyclovir cream significantly decreases clinician-assessed duration of the episode and duration of patient-reported pain, based on 2 double-blind, multicenter RCTs that used a vehicle control. In these trials, 686 and 699 patients self-initiated treatment 5 times a day for 4 days beginning within 1 hour of the onset of a recurrent lesion. In the first study, the mean clinician-assessed duration of the episode with topical acyclovir was 4.3 vs 4.8 days for placebo (hazard ratio [HR]=1.23; 95% confidence interval [CI], 1.06–1.44), and the mean duration of patient-assessed pain was 2.9 vs 3.2 days (HR=1.20; 95% CI, 1.03–1.40). The second study showed a mean clinician-assessed duration with topical acyclovir of 4.6 vs 5.2 days for control (HR=1.24; 95% CI, 1.06–1.44), and the mean duration of patient-assessed pain was 3.1 vs 3.5 days (HR=1.21; 95% CI, 1.04–1.40). Benefits were seen regardless of whether treatment was initiated early (prodrome or erythema stage) or late (macule, papule, vesicle or crusted stage).3
Recent studies of valacyclovir (the L-valine ester of acyclovir, which has 3 to 5 times greater bioavailability) offer the most promise for effective self-initiated treatment of recurrent herpes labialis. In a report of 2 well-designed, multicenter RCTs, valacyclovir at the FDA-approved dosage of 2 g twice daily for 1 day at the onset of symptoms (before visible signs of a cold sore) significantly decreased the mean duration of the lesion and time to lesion healing. In the first study (n=603), episode duration was decreased by 1.1 days (5.0 days vs 6.1 days for placebo; 95% CI, –1.6 to –0.6) and in the second study (n=605) by 1.0 day (5.3 vs 6.3 days for placebo; 95% CI, –1.0 to –0.5). In the first study, the time to lesion healing was decreased by 1.3 days (4.8 vs 6.1 days for placebo; 95% CI, –1.9 to –0.7) and in the second study by 1.2 days (5.1 vs. 6.4 days; 95% CI, –1.8 to –0.7). There also was a trend towards preventing the development of lesions, but this was not statistically significant.4
Recommendations from others
The BMJ Clinical Evidence Guideline reiterates that no trials compare early vs late treatment, so no firm conclusions about the efficacy of delayed treatment can be drawn.5 UpToDate reports that HSV 1 studies take into account that acyclovir acts only during active viral replication, which largely precedes symptoms, and thus suggest that it has little effect if begun after the appearance of lesions.6
For late presenters, review local care and hygiene; for all patients, review management of recurrences
Owen McCormack, DO
Baylor College of Medicine, Houston, TX
Patients seek treatment for herpes labialis due to bothersome physical symptoms and psychosocial implications. Many patients can identify prodromal symptoms such as localized itching, burning, irritation, or pain. Diagnosis of the initial episode is frequently delayed as patients are evaluated after the time period when studies have shown the most benefit from antivirals. For the late presenters, I review local care and hygiene, and for all patients I review management of recurrences.
Patient-initiated treatment is effective for those who can recognize the earliest signs and symptoms and start treatment immediately with either a topical or systemic antiviral. Both formulations decrease the lesion time to healing and pain if started at the first onset of symptoms.
Cost is an important consideration when selecting a particular formulation. Approximate price for the regimens presented here are $12 for 5 days of oral acyclovir, $27 for 1 day of oral valacyclovir, and $37 for a 2-g tube of acyclovir cream, which can be used for more than 1 episode.7 Other factors to consider are pill burden, duration of treatment, patient preference, and lifestyle. Patients can keep a refill or medicine on-hand to manage recurrences with the advice to begin immediately with onset of signs or symptoms.
When herpes simplex virus (HSV) type 1 lesions are in the papule or vesicle stage, there is no benefit to starting oral acyclovir (strength of recommendation [SOR]: C, based on expert opinion). However, topical acyclovir 5% cream applied 5 times a day decreases pain and the duration of hard crust (SOR: B, extrapolated from randomized controlled trials [RCTs]).
If started at the onset of symptoms (during the prodrome stage), acyclovir (400 mg 5 times daily for 5 days) decreases pain and healing time to loss of crust and valacyclovir (2 g twice daily for 1 day) reduces the lesion duration and time to healing and may prevent lesion development (SOR: A, based on RCTs).
Evidence summary
Cold sores, or herpes labialis, are caused by HSV. Recurrent lesions progress quickly through several stages (prodrome, erythema, papule, vesicle, ulcer, crust, residual swelling, healed).1 Because of the rapid development of the vesicle stage (<12 hours) and the rapid decrease in detectable virus after 48 hours, studies of antiviral therapy empirically require early treatment within the first several hours of signs or symptoms of a recurrence. For this reason, there are no controlled trials of oral medications given later than 12 hours after the onset of recurrent symptoms.
Although limited, the clearest indication of appropriate timing for HSV 1 treatment with acyclovir comes from a well-designed, double-blinded RCT of 174 adults with a history of culture confirmed HSV labialis who initiated self-treatment with acyclovir 400 mg or placebo 5 times a day for 5 days. Patients were asked to defer treatment until the next episode if they awoke with the lesion or first noticed them in the vesicle or ulcer stage. Ninety-seven percent of the patients started treatment within 1 hour of signs/symptoms of a recurrence. Of the 174 patients, 90 had lesions in the prodrome or erythema stage at the start of treatment and 84 had lesions in the papule or vesicle stage.
Overall, acyclovir did not effect lesion progression, size, or healing time to loss of hard crust or normal skin. However, the mean duration of pain for all patients significantly decreased (2.5 days vs 3.8 days for placebo, P=.01). For the subgroup of patients who started acyclovir treatment in the prodrome or erythema stage, the mean duration of pain significantly decreased (2.5 days vs 3.9 days for placebo, P=.02), as did healing time to loss of crust (5.8 days vs 7.9 days for placebo, P=.03). Among those who started acyclovir in the papular stage, the trend was toward drug benefit, but this was not statistically significant (mean pain duration: 2.5 vs. 3.6, P=.36; mean healing time to loss of crust: 8.0 vs. 7.2, P=.52).2 This evidence supports early (prodrome or erythema stage) but not late (macule, papule, vesicle, or crusted stage) treatment of HSV 1 with oral acyclovir.
Topical application of 5% acyclovir cream significantly decreases clinician-assessed duration of the episode and duration of patient-reported pain, based on 2 double-blind, multicenter RCTs that used a vehicle control. In these trials, 686 and 699 patients self-initiated treatment 5 times a day for 4 days beginning within 1 hour of the onset of a recurrent lesion. In the first study, the mean clinician-assessed duration of the episode with topical acyclovir was 4.3 vs 4.8 days for placebo (hazard ratio [HR]=1.23; 95% confidence interval [CI], 1.06–1.44), and the mean duration of patient-assessed pain was 2.9 vs 3.2 days (HR=1.20; 95% CI, 1.03–1.40). The second study showed a mean clinician-assessed duration with topical acyclovir of 4.6 vs 5.2 days for control (HR=1.24; 95% CI, 1.06–1.44), and the mean duration of patient-assessed pain was 3.1 vs 3.5 days (HR=1.21; 95% CI, 1.04–1.40). Benefits were seen regardless of whether treatment was initiated early (prodrome or erythema stage) or late (macule, papule, vesicle or crusted stage).3
Recent studies of valacyclovir (the L-valine ester of acyclovir, which has 3 to 5 times greater bioavailability) offer the most promise for effective self-initiated treatment of recurrent herpes labialis. In a report of 2 well-designed, multicenter RCTs, valacyclovir at the FDA-approved dosage of 2 g twice daily for 1 day at the onset of symptoms (before visible signs of a cold sore) significantly decreased the mean duration of the lesion and time to lesion healing. In the first study (n=603), episode duration was decreased by 1.1 days (5.0 days vs 6.1 days for placebo; 95% CI, –1.6 to –0.6) and in the second study (n=605) by 1.0 day (5.3 vs 6.3 days for placebo; 95% CI, –1.0 to –0.5). In the first study, the time to lesion healing was decreased by 1.3 days (4.8 vs 6.1 days for placebo; 95% CI, –1.9 to –0.7) and in the second study by 1.2 days (5.1 vs. 6.4 days; 95% CI, –1.8 to –0.7). There also was a trend towards preventing the development of lesions, but this was not statistically significant.4
Recommendations from others
The BMJ Clinical Evidence Guideline reiterates that no trials compare early vs late treatment, so no firm conclusions about the efficacy of delayed treatment can be drawn.5 UpToDate reports that HSV 1 studies take into account that acyclovir acts only during active viral replication, which largely precedes symptoms, and thus suggest that it has little effect if begun after the appearance of lesions.6
For late presenters, review local care and hygiene; for all patients, review management of recurrences
Owen McCormack, DO
Baylor College of Medicine, Houston, TX
Patients seek treatment for herpes labialis due to bothersome physical symptoms and psychosocial implications. Many patients can identify prodromal symptoms such as localized itching, burning, irritation, or pain. Diagnosis of the initial episode is frequently delayed as patients are evaluated after the time period when studies have shown the most benefit from antivirals. For the late presenters, I review local care and hygiene, and for all patients I review management of recurrences.
Patient-initiated treatment is effective for those who can recognize the earliest signs and symptoms and start treatment immediately with either a topical or systemic antiviral. Both formulations decrease the lesion time to healing and pain if started at the first onset of symptoms.
Cost is an important consideration when selecting a particular formulation. Approximate price for the regimens presented here are $12 for 5 days of oral acyclovir, $27 for 1 day of oral valacyclovir, and $37 for a 2-g tube of acyclovir cream, which can be used for more than 1 episode.7 Other factors to consider are pill burden, duration of treatment, patient preference, and lifestyle. Patients can keep a refill or medicine on-hand to manage recurrences with the advice to begin immediately with onset of signs or symptoms.
1. Spruance S, Overall J, Kern E, Krueger G, Pliam V, Miller W. The natural history of recurrent herpes simplex labialis. JAMA 1977;297:69-75.
2. Spruance S, Stewart J, Rowe N, McKoeugh M, Wenerstrom G, Freeman D. Treatment of recurrent herpes simplex labialis with oral acyclovir. J Infect Dis 1990;161:181-190.
3. Spruance S, Nett R, Marbury T, Wolff R, Johnson J, Spaulding T. Acyclovir cream for treatment of herpes simplex labialis: results of two randomized, double-blind, vehicle-controlled multicenter clinical trials. Antimicrobial Agents Chemother 2002;46:2238-2243.
4. Spruance S, Jones T, Blatter M, et al. High-dose, short-duration, early valacyclovir therapy for episodic treatment of cold sores: results of two randomized, placebo-controlled, multicenter studies. Antimicrobial Agents Chemother 2003;47:1072-1080.
5. Clin Evid [online] Issue 10 2003. London: BMJ Publishing Group, last updated December 2002. Available at: http://clinicalevidence.com. Accessed on May 19, 2004.
6. Klein R. Treatment and prevention of herpes simplex virus type 1 infection. UpToDate version 12.2, last updated February 25, 2004. Available at: UpToDate.com. Accessed on July 14, 2004.
7. Availableat: www. drugstore.com. Accessed on August 18, 2004.
1. Spruance S, Overall J, Kern E, Krueger G, Pliam V, Miller W. The natural history of recurrent herpes simplex labialis. JAMA 1977;297:69-75.
2. Spruance S, Stewart J, Rowe N, McKoeugh M, Wenerstrom G, Freeman D. Treatment of recurrent herpes simplex labialis with oral acyclovir. J Infect Dis 1990;161:181-190.
3. Spruance S, Nett R, Marbury T, Wolff R, Johnson J, Spaulding T. Acyclovir cream for treatment of herpes simplex labialis: results of two randomized, double-blind, vehicle-controlled multicenter clinical trials. Antimicrobial Agents Chemother 2002;46:2238-2243.
4. Spruance S, Jones T, Blatter M, et al. High-dose, short-duration, early valacyclovir therapy for episodic treatment of cold sores: results of two randomized, placebo-controlled, multicenter studies. Antimicrobial Agents Chemother 2003;47:1072-1080.
5. Clin Evid [online] Issue 10 2003. London: BMJ Publishing Group, last updated December 2002. Available at: http://clinicalevidence.com. Accessed on May 19, 2004.
6. Klein R. Treatment and prevention of herpes simplex virus type 1 infection. UpToDate version 12.2, last updated February 25, 2004. Available at: UpToDate.com. Accessed on July 14, 2004.
7. Availableat: www. drugstore.com. Accessed on August 18, 2004.
Evidence-based answers from the Family Physicians Inquiries Network
Do steroid injections help with osteoarthritis of the knee?
Intra-articular steroid injections appear to provide 2 to 6 weeks of pain relief for patients with knee osteoarthritis (strength of recommendation [SOR]: A). Higher-dose steroids with or without joint lavage can provide pain relief up to 24 weeks (SOR: A). Steroid injections may be an appropriate adjunct in the treatment of osteoarthritis, which includes nonpharmacologic treatments (education, weight loss, physical therapy) and pharmacologic therapy (nonsteroidal anti-inflammatory drugs [NSAIDs], topical and opioid analgesics).1,2
Evidence summary
Osteoarthritis, also known as degenerative joint disease, is the most prevalent form of arthritis in the United States.3 For the elderly, it is a common cause of pain and disability, affecting patients’ ability to perform activities of daily living. Common causes of osteoarthritis include past and present biomechanical stresses affecting the articular cartilage, sub-chondral bone changes, and biochemical changes in the articular cartilage and synovial membrane.3
Treatment of patients with osteoarthritis of the knee should be individualized to the severity of symptoms for each patient. A treatment plan can include patient education, physical and occupational therapy, non-opioid oral and topical agents, NSAIDs, intra-articular corticosteroid injections, viscosupplementation injections, arthroscopic lavage, and total knee replacements.
Our knowledge of the long-term safety and efficacy of intra-articular knee corticosteroid injection is based on limited data. In a randomized, double-blind, placebo-controlled crossover study, investigators randomized 59 patients aged 51 to 89 years to receive either an intra-articular injection of 1 mL of 40 mg methylprednisolone or 1 mL of 0.9% saline. After 3 weeks, patients receiving steroid injection had a minimal change in baseline visual analogue score for pain compared with those receiving saline (median change: –2.0 mm vs 0 mm on a 100-mm scale).4
A randomized, single-blinded study involving 84 patients demonstrated significant self-report-ed “overall improvement” for patients given intra-articular triamcinolone hexacetonide (78%) compared with placebo (49%) after 1 week (P<.05).5 It also confirmed reports that visual analogue score for pain and distance walked in 1 minute improves significantly for both steroid- and placebo-treated groups up to 6 weeks. Only the steroid-treated patients exhibited improved walking distance at 1 week compared with baseline (P<.001).
A recent randomized, double-blind, placebo-controlled trial studied the long-term safety and efficacy of treatment of knee osteoarthritis with repeated steroid injections.6 These investigators studied 66 patients aged 40 to 80 years recruited from rheumatology clinics. One half (n=33) received injections of triamcinolone acetonide 40 mg, and the other half received saline injections every 3 months for 2 years. At 1- and 2-year interval follow-ups, no statistically significant difference was seen between the 2 groups in loss of joint space and no progression of degenerative disease, as demonstrated by measurements of joint space widths by standardized fluoroscopically guided radiographs. Although the primary outcome measure of this study was to assess radiologic joint space narrowing with repeated injections, knee pain and stiffness appeared to improve after 2 years, although these results were not well quantified.
A limitation of most studies testing intraarticular therapy has been sample size. Combining studies may allow the ability to detect levels of pain relief not found in individual studies. A recent meta-analysis of 6 randomized controlled trials using intra-articular corticosteroid knee injections found short-term relief of pain for 2 weeks (relative risk [RR]=1.66; 95% confidence interval [CI], 1.37–2.01).7 The number needed to treat (NNT) range for these studies is 1.3 to 3.5. Two additional studies included in this study using higher-dose steroids (prednisone equivalent dose of 37.5 to 80 mg), with or without joint lavage, assessed improvement at 16 to 24 weeks. Although neither individual study showed statistically significant differences, the pooled data from the 2 studies favored symptom improvement at 16 to 24 weeks (RR=2.09; 95% CI, 1.2–3.7; NNT=4.4).7
Recommendations from others
Guidelines for the treatment of knee osteoarthritis were outlined by a task force for the European League Against Rheumatism (EULAR) Standing Committee for Clinical Trials. The task force recommended intra-articular steroid injection for acute exacerbation of knee pain. This task force performed an evidence-based review and concluded at least 1 randomized control trial recommended intraarticular steroid for patients with osteoarthritis. It was noted that intra-articular steroid injections were effective for only short-term pain relief and that there are no predictors of success of treatment, such as the presence or absence of such factors as joint effusion, degree of radio-logic change, age, or obesity.2
The American College of Rheumatology Subcommittee on Osteoarthritis Guidelines developed both nonpharmacological and pharmacological recommendations for the treatment of osteoarthritis of the knee.8 These recommendations include: use of intra-articular steroid injection for patients with acute exacerbations who had evidence for joint inflammation, and joint aspiration accompanying the intra-articular injection for “short-term relief.”
Intra-articular steroids provide extra relief for patients with acute exacerbations
Wail Malaty, MD
Hendersonville Family Practice Residency Program, Hendersonville, NC
This well-constructed review demonstrates that intra-articular steroid injections provide up to 3 weeks of pain relief for patients with osteoarthritis of the knee. While this may not seem like much, in practice it can be quite helpful in some situations. It provides supplemental pain relief for patients with acute exacerbations of their disease. It is also useful as a temporizing measure for patients who are candidates for total knee replacement but are not quite ready for it psychologically.
1. Rozental TD, Sculco TP. Intra-articular corticosteroids: an updated overview. Am J Orthop 2000;29:18-23.
2. Pendleton A, Arden N, Dougados M, et al. EULAR recommendations for the management of knee osteoarthritis: report of a task force of the Standing Committee for International Clinical Studies Including Therapeutic Trials (ESCISIT). Ann Rheum Dis 2000;59:936-944.
3. Hochberg MC, Altman RD, Brandt KD, et al. Guidelines for the medical management of osteoarthritis. Part II. Osteoarthritis of the knee. American College of Rheumatology. Arthritis Rheum 1995;38:1541-1546.
4. Jones A, Doherty M. Intra-articular corticosteroids are effective in osteoarthritis but there are no clinical predictors of response. Ann Rheum Dis 1996;55:829-832.
5. Gaffney K, Ledingham J, Perry JD. Intra-articular triamcinolone hexacetonide in knee osteoarthritis: factors influencing the clinical response. Ann Rheum Dis 1995;54:379-381.
6. Raynauld JP, Buckland-Wright C, Ward R, et al. Safety and efficacy of long-term intraarticular steroid injections in osteoarthritis of the knee: a randomized, double-blind, placebo-controlled trial. Arthritis Rheum 2003;48:370-377.
7. Arroll B, Goodyear-Smith F. Corticosteroid injections for osteoarthritis of the knee: meta-analysis. BMJ 2004;328:869-870.
8. Altman RD, Hochberg HC, Moskowitz RW, Schnitzer TJ. Recommendations for the medical management of osteoarthritis of the hip and knee 2000 update: American College of Rheumatology Subcommittee on Osteoarthritis Guidelines. Arthritis Rheum 2000;43:1905-1915.
Intra-articular steroid injections appear to provide 2 to 6 weeks of pain relief for patients with knee osteoarthritis (strength of recommendation [SOR]: A). Higher-dose steroids with or without joint lavage can provide pain relief up to 24 weeks (SOR: A). Steroid injections may be an appropriate adjunct in the treatment of osteoarthritis, which includes nonpharmacologic treatments (education, weight loss, physical therapy) and pharmacologic therapy (nonsteroidal anti-inflammatory drugs [NSAIDs], topical and opioid analgesics).1,2
Evidence summary
Osteoarthritis, also known as degenerative joint disease, is the most prevalent form of arthritis in the United States.3 For the elderly, it is a common cause of pain and disability, affecting patients’ ability to perform activities of daily living. Common causes of osteoarthritis include past and present biomechanical stresses affecting the articular cartilage, sub-chondral bone changes, and biochemical changes in the articular cartilage and synovial membrane.3
Treatment of patients with osteoarthritis of the knee should be individualized to the severity of symptoms for each patient. A treatment plan can include patient education, physical and occupational therapy, non-opioid oral and topical agents, NSAIDs, intra-articular corticosteroid injections, viscosupplementation injections, arthroscopic lavage, and total knee replacements.
Our knowledge of the long-term safety and efficacy of intra-articular knee corticosteroid injection is based on limited data. In a randomized, double-blind, placebo-controlled crossover study, investigators randomized 59 patients aged 51 to 89 years to receive either an intra-articular injection of 1 mL of 40 mg methylprednisolone or 1 mL of 0.9% saline. After 3 weeks, patients receiving steroid injection had a minimal change in baseline visual analogue score for pain compared with those receiving saline (median change: –2.0 mm vs 0 mm on a 100-mm scale).4
A randomized, single-blinded study involving 84 patients demonstrated significant self-report-ed “overall improvement” for patients given intra-articular triamcinolone hexacetonide (78%) compared with placebo (49%) after 1 week (P<.05).5 It also confirmed reports that visual analogue score for pain and distance walked in 1 minute improves significantly for both steroid- and placebo-treated groups up to 6 weeks. Only the steroid-treated patients exhibited improved walking distance at 1 week compared with baseline (P<.001).
A recent randomized, double-blind, placebo-controlled trial studied the long-term safety and efficacy of treatment of knee osteoarthritis with repeated steroid injections.6 These investigators studied 66 patients aged 40 to 80 years recruited from rheumatology clinics. One half (n=33) received injections of triamcinolone acetonide 40 mg, and the other half received saline injections every 3 months for 2 years. At 1- and 2-year interval follow-ups, no statistically significant difference was seen between the 2 groups in loss of joint space and no progression of degenerative disease, as demonstrated by measurements of joint space widths by standardized fluoroscopically guided radiographs. Although the primary outcome measure of this study was to assess radiologic joint space narrowing with repeated injections, knee pain and stiffness appeared to improve after 2 years, although these results were not well quantified.
A limitation of most studies testing intraarticular therapy has been sample size. Combining studies may allow the ability to detect levels of pain relief not found in individual studies. A recent meta-analysis of 6 randomized controlled trials using intra-articular corticosteroid knee injections found short-term relief of pain for 2 weeks (relative risk [RR]=1.66; 95% confidence interval [CI], 1.37–2.01).7 The number needed to treat (NNT) range for these studies is 1.3 to 3.5. Two additional studies included in this study using higher-dose steroids (prednisone equivalent dose of 37.5 to 80 mg), with or without joint lavage, assessed improvement at 16 to 24 weeks. Although neither individual study showed statistically significant differences, the pooled data from the 2 studies favored symptom improvement at 16 to 24 weeks (RR=2.09; 95% CI, 1.2–3.7; NNT=4.4).7
Recommendations from others
Guidelines for the treatment of knee osteoarthritis were outlined by a task force for the European League Against Rheumatism (EULAR) Standing Committee for Clinical Trials. The task force recommended intra-articular steroid injection for acute exacerbation of knee pain. This task force performed an evidence-based review and concluded at least 1 randomized control trial recommended intraarticular steroid for patients with osteoarthritis. It was noted that intra-articular steroid injections were effective for only short-term pain relief and that there are no predictors of success of treatment, such as the presence or absence of such factors as joint effusion, degree of radio-logic change, age, or obesity.2
The American College of Rheumatology Subcommittee on Osteoarthritis Guidelines developed both nonpharmacological and pharmacological recommendations for the treatment of osteoarthritis of the knee.8 These recommendations include: use of intra-articular steroid injection for patients with acute exacerbations who had evidence for joint inflammation, and joint aspiration accompanying the intra-articular injection for “short-term relief.”
Intra-articular steroids provide extra relief for patients with acute exacerbations
Wail Malaty, MD
Hendersonville Family Practice Residency Program, Hendersonville, NC
This well-constructed review demonstrates that intra-articular steroid injections provide up to 3 weeks of pain relief for patients with osteoarthritis of the knee. While this may not seem like much, in practice it can be quite helpful in some situations. It provides supplemental pain relief for patients with acute exacerbations of their disease. It is also useful as a temporizing measure for patients who are candidates for total knee replacement but are not quite ready for it psychologically.
Intra-articular steroid injections appear to provide 2 to 6 weeks of pain relief for patients with knee osteoarthritis (strength of recommendation [SOR]: A). Higher-dose steroids with or without joint lavage can provide pain relief up to 24 weeks (SOR: A). Steroid injections may be an appropriate adjunct in the treatment of osteoarthritis, which includes nonpharmacologic treatments (education, weight loss, physical therapy) and pharmacologic therapy (nonsteroidal anti-inflammatory drugs [NSAIDs], topical and opioid analgesics).1,2
Evidence summary
Osteoarthritis, also known as degenerative joint disease, is the most prevalent form of arthritis in the United States.3 For the elderly, it is a common cause of pain and disability, affecting patients’ ability to perform activities of daily living. Common causes of osteoarthritis include past and present biomechanical stresses affecting the articular cartilage, sub-chondral bone changes, and biochemical changes in the articular cartilage and synovial membrane.3
Treatment of patients with osteoarthritis of the knee should be individualized to the severity of symptoms for each patient. A treatment plan can include patient education, physical and occupational therapy, non-opioid oral and topical agents, NSAIDs, intra-articular corticosteroid injections, viscosupplementation injections, arthroscopic lavage, and total knee replacements.
Our knowledge of the long-term safety and efficacy of intra-articular knee corticosteroid injection is based on limited data. In a randomized, double-blind, placebo-controlled crossover study, investigators randomized 59 patients aged 51 to 89 years to receive either an intra-articular injection of 1 mL of 40 mg methylprednisolone or 1 mL of 0.9% saline. After 3 weeks, patients receiving steroid injection had a minimal change in baseline visual analogue score for pain compared with those receiving saline (median change: –2.0 mm vs 0 mm on a 100-mm scale).4
A randomized, single-blinded study involving 84 patients demonstrated significant self-report-ed “overall improvement” for patients given intra-articular triamcinolone hexacetonide (78%) compared with placebo (49%) after 1 week (P<.05).5 It also confirmed reports that visual analogue score for pain and distance walked in 1 minute improves significantly for both steroid- and placebo-treated groups up to 6 weeks. Only the steroid-treated patients exhibited improved walking distance at 1 week compared with baseline (P<.001).
A recent randomized, double-blind, placebo-controlled trial studied the long-term safety and efficacy of treatment of knee osteoarthritis with repeated steroid injections.6 These investigators studied 66 patients aged 40 to 80 years recruited from rheumatology clinics. One half (n=33) received injections of triamcinolone acetonide 40 mg, and the other half received saline injections every 3 months for 2 years. At 1- and 2-year interval follow-ups, no statistically significant difference was seen between the 2 groups in loss of joint space and no progression of degenerative disease, as demonstrated by measurements of joint space widths by standardized fluoroscopically guided radiographs. Although the primary outcome measure of this study was to assess radiologic joint space narrowing with repeated injections, knee pain and stiffness appeared to improve after 2 years, although these results were not well quantified.
A limitation of most studies testing intraarticular therapy has been sample size. Combining studies may allow the ability to detect levels of pain relief not found in individual studies. A recent meta-analysis of 6 randomized controlled trials using intra-articular corticosteroid knee injections found short-term relief of pain for 2 weeks (relative risk [RR]=1.66; 95% confidence interval [CI], 1.37–2.01).7 The number needed to treat (NNT) range for these studies is 1.3 to 3.5. Two additional studies included in this study using higher-dose steroids (prednisone equivalent dose of 37.5 to 80 mg), with or without joint lavage, assessed improvement at 16 to 24 weeks. Although neither individual study showed statistically significant differences, the pooled data from the 2 studies favored symptom improvement at 16 to 24 weeks (RR=2.09; 95% CI, 1.2–3.7; NNT=4.4).7
Recommendations from others
Guidelines for the treatment of knee osteoarthritis were outlined by a task force for the European League Against Rheumatism (EULAR) Standing Committee for Clinical Trials. The task force recommended intra-articular steroid injection for acute exacerbation of knee pain. This task force performed an evidence-based review and concluded at least 1 randomized control trial recommended intraarticular steroid for patients with osteoarthritis. It was noted that intra-articular steroid injections were effective for only short-term pain relief and that there are no predictors of success of treatment, such as the presence or absence of such factors as joint effusion, degree of radio-logic change, age, or obesity.2
The American College of Rheumatology Subcommittee on Osteoarthritis Guidelines developed both nonpharmacological and pharmacological recommendations for the treatment of osteoarthritis of the knee.8 These recommendations include: use of intra-articular steroid injection for patients with acute exacerbations who had evidence for joint inflammation, and joint aspiration accompanying the intra-articular injection for “short-term relief.”
Intra-articular steroids provide extra relief for patients with acute exacerbations
Wail Malaty, MD
Hendersonville Family Practice Residency Program, Hendersonville, NC
This well-constructed review demonstrates that intra-articular steroid injections provide up to 3 weeks of pain relief for patients with osteoarthritis of the knee. While this may not seem like much, in practice it can be quite helpful in some situations. It provides supplemental pain relief for patients with acute exacerbations of their disease. It is also useful as a temporizing measure for patients who are candidates for total knee replacement but are not quite ready for it psychologically.
1. Rozental TD, Sculco TP. Intra-articular corticosteroids: an updated overview. Am J Orthop 2000;29:18-23.
2. Pendleton A, Arden N, Dougados M, et al. EULAR recommendations for the management of knee osteoarthritis: report of a task force of the Standing Committee for International Clinical Studies Including Therapeutic Trials (ESCISIT). Ann Rheum Dis 2000;59:936-944.
3. Hochberg MC, Altman RD, Brandt KD, et al. Guidelines for the medical management of osteoarthritis. Part II. Osteoarthritis of the knee. American College of Rheumatology. Arthritis Rheum 1995;38:1541-1546.
4. Jones A, Doherty M. Intra-articular corticosteroids are effective in osteoarthritis but there are no clinical predictors of response. Ann Rheum Dis 1996;55:829-832.
5. Gaffney K, Ledingham J, Perry JD. Intra-articular triamcinolone hexacetonide in knee osteoarthritis: factors influencing the clinical response. Ann Rheum Dis 1995;54:379-381.
6. Raynauld JP, Buckland-Wright C, Ward R, et al. Safety and efficacy of long-term intraarticular steroid injections in osteoarthritis of the knee: a randomized, double-blind, placebo-controlled trial. Arthritis Rheum 2003;48:370-377.
7. Arroll B, Goodyear-Smith F. Corticosteroid injections for osteoarthritis of the knee: meta-analysis. BMJ 2004;328:869-870.
8. Altman RD, Hochberg HC, Moskowitz RW, Schnitzer TJ. Recommendations for the medical management of osteoarthritis of the hip and knee 2000 update: American College of Rheumatology Subcommittee on Osteoarthritis Guidelines. Arthritis Rheum 2000;43:1905-1915.
1. Rozental TD, Sculco TP. Intra-articular corticosteroids: an updated overview. Am J Orthop 2000;29:18-23.
2. Pendleton A, Arden N, Dougados M, et al. EULAR recommendations for the management of knee osteoarthritis: report of a task force of the Standing Committee for International Clinical Studies Including Therapeutic Trials (ESCISIT). Ann Rheum Dis 2000;59:936-944.
3. Hochberg MC, Altman RD, Brandt KD, et al. Guidelines for the medical management of osteoarthritis. Part II. Osteoarthritis of the knee. American College of Rheumatology. Arthritis Rheum 1995;38:1541-1546.
4. Jones A, Doherty M. Intra-articular corticosteroids are effective in osteoarthritis but there are no clinical predictors of response. Ann Rheum Dis 1996;55:829-832.
5. Gaffney K, Ledingham J, Perry JD. Intra-articular triamcinolone hexacetonide in knee osteoarthritis: factors influencing the clinical response. Ann Rheum Dis 1995;54:379-381.
6. Raynauld JP, Buckland-Wright C, Ward R, et al. Safety and efficacy of long-term intraarticular steroid injections in osteoarthritis of the knee: a randomized, double-blind, placebo-controlled trial. Arthritis Rheum 2003;48:370-377.
7. Arroll B, Goodyear-Smith F. Corticosteroid injections for osteoarthritis of the knee: meta-analysis. BMJ 2004;328:869-870.
8. Altman RD, Hochberg HC, Moskowitz RW, Schnitzer TJ. Recommendations for the medical management of osteoarthritis of the hip and knee 2000 update: American College of Rheumatology Subcommittee on Osteoarthritis Guidelines. Arthritis Rheum 2000;43:1905-1915.
Evidence-based answers from the Family Physicians Inquiries Network
For knee pain, how predictive is physical examination for meniscal injury?
No single clinical examination element, or combination of such elements, reliably detects meniscal injury. The McMurray test is best for ruling in meniscal pathology. Assuming a 9% prevalence of meniscal tears among all knee injuries (a rate reflecting national primary care data), the posttest probability that a patient with McMurray’s sign has a meniscal injury ranges from <30% to 63% (strength of recommendation [SOR]: B). In contrast, the absence of any positive physical examination findings effectively rules out meniscal pathology, yielding a posttest probability of 0.8% for lateral meniscus injury, 1.0% for medial meniscus injury, and 3.8% for any meniscal injury among primary care populations (SOR: B).
Evidence summary
The accuracy of physical examination findings for meniscal injury varies widely among meta-analyses. In a meta-analysis of 13 studies, no physical examination test—including assessment for joint effusion, McMurray test, joint line tenderness, or the Apley compression test—yielded clinically significant positive or negative likelihood ratios for a meniscal tear ( Table ). The McMurray test performed best, but at 9% to 11% pretest probability of JFP_1104_CI.final 10/18/04 11:06 AM Page 918 meniscal lesions, based on prevalence estimates among primary care/specialist populations,2 the posttest probability of a positive exam is still <30%.
A meta-analysis of 4 studies by Jackson compared the utility of the McMurray test and joint line tenderness.3 For detecting meniscal tears, the McMurray test had a clinically and statistically significant positive likelihood ratio of 17.33, corresponding to a posttest probability of nearly 61%. Negative likelihood ratios for the McMurray test and joint line tenderness (0.5 and 0.8) were not clinically significant, indicating that absence of the McMurray sign or joint line tenderness alone is of little benefit in ruling out meniscal injury.
In another meta-analysis including 9 studies of meniscal injury diagnosis,4 individual tests for joint line tenderness, joint effusion, the medial-lateral grind test, and the McMurray test failed to yield statistically significant likelihood ratios for the presence or absence of meniscal tears ( Table footnotes). Positive and negative likelihood ratios for aggregate physical examination were 2.7 (95% confidence interval [CI], 1.4–5.1) and 0.4 (95% CI, 0.2–0.7), which are statistically, but not clinically, significant values for ruling meniscal lesions in or out.
Jackson’s meta-analysis also calculated the posttest probability of injury for a composite meniscal examination. Based on the positive likelihood ratio of 3.1 (95% CI, 0.54–5.7) and negative likelihood ratio of 0.19 (95% CI, 0.11–0.77), the posttest probability of a medial meniscal tear was 17% in the setting of composite physical exam findings and 1% in the absence of physical exam findings. For a lateral meniscal tear, based on the positive likelihood ratio of 11 (95% CI, 1.8–20.2), and negative likelihood ratio of 0.13 (95% CI, 0.0–0.25), the posttest probability of injury with a positive exam was 41% and with a negative exam 0.8%.
Authors of all meta-analyses noted the lack of standardization in physical examination maneuvers (especially the McMurray test)5 and, in some cases, no specification of how physical examination tests were performed. Authors analyzed the utility of the aggregate and composite knee examinations without specifying what constituted such an exam. No study included in the meta-analyses used control subjects without meniscal pathology, and few studies were blinded. Lack of blinding may have introduced verification bias; use of specialty patients in all studies made referral bias likely. Studies were heterogeneous and results were associated with wide confidence intervals, introducing an element of random error into the processes of combining and interpreting data.
TABLE
Physical exams for meniscal tear
| Summary characteristics | Solomon et al 4 | Scholten et al 1 | Jackson et al 3 |
| 9 studies 1018 patients Specialist population Specialist examiners | 13 studies 2231 patients Specialist population Specialist examiners | 4 studies 424 patients Specialist population Specialist examiners | |
| McMurray | Positive likelihood ratio (95% CI) | ||
| 1.3 (0.9–1.7) | 1.5–9.5 | 17.3 (2.7–68) | |
| Joint line tenderness | 0.9 (0.8–1.0) | 0.8–14.9 | 1.1 (0.7–1.6) |
| Aggregate exam | 2.7 (1.4–5.1) | — | — |
| Aggregate exam, medial meniscus tears | — | — | 3.1 (0.54–5.7) |
| Aggregate exam, lateral meniscus tears | — | — | 11 (1.8–20.2) |
| McMurray | Negative likelihood ratio (95% CI) | ||
| 0.8 (0.6–1.1) | 0.4–0.9 | 0.5 (0.3–0.8) | |
| Joint line tenderness | 1.1 (1.0–1.3) | 0.2–2.1 | 0.8 (0.3–3.5) |
| Aggregate exam | 0.4 (0.2–0.7) | — | — |
| Aggregate exam, medial meniscus tears | — | — | 0.19 (0.11–0.77) |
| Aggregate exam, lateral meniscus tears | — | — | 0.13 (0–0.25) |
| Note: The results are presented as likelihood ratios, which represent the change in the odds of a diagnosis, based on the outcome of the test. For example, given a positive likelihood ratio of 2, if a test result is positive, the odds of the disease being present is doubled. A positive likelihood ratio >10 provides strong evidence that the disorder is present. A negative likelihood ratio <0.1 provides strong evidence that the disorder is not present. Scores between 0.5 and 2.0 are neutral. In Scholten’s meta-analysis, likelihood ratios are given in ranges (no composite value given). | |||
Recommendations from others
The American Academy of Orthopaedic Surgeons’ clinical guideline on the evaluation and treatment of knee injuries lists the following findings as associated with a meniscal tear: delayed swelling of the knee, twisting injury, painful popping and catching, effusion, joint line tenderness, positive McMurray’s test, and negative radiography.6 The guideline fails to list the strength and type of supporting evidence for these associations.
The American College of Radiology’s Appropriateness Criteria for Acute Trauma to the Knee states that decision rules for meniscal tears and other soft tissue injuries to the knee are being investigated, but it fails to mention specific evaluation strategies for meniscal tears.7
Meniscus injury likely with suggestive history, joint line tenderness, and an inability to squat because of pain
Roy Henderson, MD
Director, Sports Medicine Fellowship, MacNeal Family Practice Residency Program, Chicago, Ill
I often suspect meniscal injuries as a cause of knee pain but am rarely certain based on physical examination alone. I look for a history of joint line pain, locking, or popping with movement. If the patient lacks joint line tenderness, a meniscal injury is unlikely. The McMurray test is usually negative. In the absence of another explanation for the patient’s symptoms, a meniscus injury is high on my list in the presence of a suggestive history, joint line tenderness, and an inability to squat because of pain. When my suspicion is high I usually resort to an MRI.
1. Scholten RJ, Deville WL, Opstelten W, Bijl D, van der Plas CG, Bouter LM. The accuracy of physical diagnostic tests for assessing meniscal lesions of the knee: a meta-analysis. J Fam Pract 2001;50:938-944.
2. National Ambulatory Medical Care Survey 1996. Available at: ftp://ftp.cdc.gov/pub/Health-Statistics/NCHS/Datasets/NAMCS/. Accessed on August 18, 2004.
3. Jackson JL, O’Malley PG, Kroenke K. Evaluation of acute knee pain in primary care. Ann Intern Med 2003;139:575-588.
4. Solomon DH, Simel DL, Bates DW, Katz JN, Schaffer JL. The rational clinical examination. Does this patient have a torn meniscus or ligament of the knee?. JAMA 2001;286:1610-1620.
5. Stratford PW, Binkley J. A review of the McMurray test: definition, interpretation, and clinical usefulness. J Orthop Sports Phys Ther 1995;22:116-120.
6. American Academy of Orthopaedic Surgeons. AAOS Clinical Guideline on Knee Injury: Support Document. Last updated February 26, 2002. Rosemont, Ill: American Academy of Orthopaedic Surgeons; 2001. Available at: http://www.guidelines.gov. Accessed on September 30, 2004.
7. American College of Radiology (ACR) Expert. Panel on Musculoskeletal Imaging ACR Appropriateness Criteria for Acute Trauma to the Knee. Updated October 1, 2002. Reston, Va: American College of Radiology; 2001. Available at: http://www.acr.org/cgi-bin/fr?tmpl:appcrit,pdf:0365374_acute_trauma_to_knee.ac.p df. Accessed on September 30, 2004.
No single clinical examination element, or combination of such elements, reliably detects meniscal injury. The McMurray test is best for ruling in meniscal pathology. Assuming a 9% prevalence of meniscal tears among all knee injuries (a rate reflecting national primary care data), the posttest probability that a patient with McMurray’s sign has a meniscal injury ranges from <30% to 63% (strength of recommendation [SOR]: B). In contrast, the absence of any positive physical examination findings effectively rules out meniscal pathology, yielding a posttest probability of 0.8% for lateral meniscus injury, 1.0% for medial meniscus injury, and 3.8% for any meniscal injury among primary care populations (SOR: B).
Evidence summary
The accuracy of physical examination findings for meniscal injury varies widely among meta-analyses. In a meta-analysis of 13 studies, no physical examination test—including assessment for joint effusion, McMurray test, joint line tenderness, or the Apley compression test—yielded clinically significant positive or negative likelihood ratios for a meniscal tear ( Table ). The McMurray test performed best, but at 9% to 11% pretest probability of JFP_1104_CI.final 10/18/04 11:06 AM Page 918 meniscal lesions, based on prevalence estimates among primary care/specialist populations,2 the posttest probability of a positive exam is still <30%.
A meta-analysis of 4 studies by Jackson compared the utility of the McMurray test and joint line tenderness.3 For detecting meniscal tears, the McMurray test had a clinically and statistically significant positive likelihood ratio of 17.33, corresponding to a posttest probability of nearly 61%. Negative likelihood ratios for the McMurray test and joint line tenderness (0.5 and 0.8) were not clinically significant, indicating that absence of the McMurray sign or joint line tenderness alone is of little benefit in ruling out meniscal injury.
In another meta-analysis including 9 studies of meniscal injury diagnosis,4 individual tests for joint line tenderness, joint effusion, the medial-lateral grind test, and the McMurray test failed to yield statistically significant likelihood ratios for the presence or absence of meniscal tears ( Table footnotes). Positive and negative likelihood ratios for aggregate physical examination were 2.7 (95% confidence interval [CI], 1.4–5.1) and 0.4 (95% CI, 0.2–0.7), which are statistically, but not clinically, significant values for ruling meniscal lesions in or out.
Jackson’s meta-analysis also calculated the posttest probability of injury for a composite meniscal examination. Based on the positive likelihood ratio of 3.1 (95% CI, 0.54–5.7) and negative likelihood ratio of 0.19 (95% CI, 0.11–0.77), the posttest probability of a medial meniscal tear was 17% in the setting of composite physical exam findings and 1% in the absence of physical exam findings. For a lateral meniscal tear, based on the positive likelihood ratio of 11 (95% CI, 1.8–20.2), and negative likelihood ratio of 0.13 (95% CI, 0.0–0.25), the posttest probability of injury with a positive exam was 41% and with a negative exam 0.8%.
Authors of all meta-analyses noted the lack of standardization in physical examination maneuvers (especially the McMurray test)5 and, in some cases, no specification of how physical examination tests were performed. Authors analyzed the utility of the aggregate and composite knee examinations without specifying what constituted such an exam. No study included in the meta-analyses used control subjects without meniscal pathology, and few studies were blinded. Lack of blinding may have introduced verification bias; use of specialty patients in all studies made referral bias likely. Studies were heterogeneous and results were associated with wide confidence intervals, introducing an element of random error into the processes of combining and interpreting data.
TABLE
Physical exams for meniscal tear
| Summary characteristics | Solomon et al 4 | Scholten et al 1 | Jackson et al 3 |
| 9 studies 1018 patients Specialist population Specialist examiners | 13 studies 2231 patients Specialist population Specialist examiners | 4 studies 424 patients Specialist population Specialist examiners | |
| McMurray | Positive likelihood ratio (95% CI) | ||
| 1.3 (0.9–1.7) | 1.5–9.5 | 17.3 (2.7–68) | |
| Joint line tenderness | 0.9 (0.8–1.0) | 0.8–14.9 | 1.1 (0.7–1.6) |
| Aggregate exam | 2.7 (1.4–5.1) | — | — |
| Aggregate exam, medial meniscus tears | — | — | 3.1 (0.54–5.7) |
| Aggregate exam, lateral meniscus tears | — | — | 11 (1.8–20.2) |
| McMurray | Negative likelihood ratio (95% CI) | ||
| 0.8 (0.6–1.1) | 0.4–0.9 | 0.5 (0.3–0.8) | |
| Joint line tenderness | 1.1 (1.0–1.3) | 0.2–2.1 | 0.8 (0.3–3.5) |
| Aggregate exam | 0.4 (0.2–0.7) | — | — |
| Aggregate exam, medial meniscus tears | — | — | 0.19 (0.11–0.77) |
| Aggregate exam, lateral meniscus tears | — | — | 0.13 (0–0.25) |
| Note: The results are presented as likelihood ratios, which represent the change in the odds of a diagnosis, based on the outcome of the test. For example, given a positive likelihood ratio of 2, if a test result is positive, the odds of the disease being present is doubled. A positive likelihood ratio >10 provides strong evidence that the disorder is present. A negative likelihood ratio <0.1 provides strong evidence that the disorder is not present. Scores between 0.5 and 2.0 are neutral. In Scholten’s meta-analysis, likelihood ratios are given in ranges (no composite value given). | |||
Recommendations from others
The American Academy of Orthopaedic Surgeons’ clinical guideline on the evaluation and treatment of knee injuries lists the following findings as associated with a meniscal tear: delayed swelling of the knee, twisting injury, painful popping and catching, effusion, joint line tenderness, positive McMurray’s test, and negative radiography.6 The guideline fails to list the strength and type of supporting evidence for these associations.
The American College of Radiology’s Appropriateness Criteria for Acute Trauma to the Knee states that decision rules for meniscal tears and other soft tissue injuries to the knee are being investigated, but it fails to mention specific evaluation strategies for meniscal tears.7
Meniscus injury likely with suggestive history, joint line tenderness, and an inability to squat because of pain
Roy Henderson, MD
Director, Sports Medicine Fellowship, MacNeal Family Practice Residency Program, Chicago, Ill
I often suspect meniscal injuries as a cause of knee pain but am rarely certain based on physical examination alone. I look for a history of joint line pain, locking, or popping with movement. If the patient lacks joint line tenderness, a meniscal injury is unlikely. The McMurray test is usually negative. In the absence of another explanation for the patient’s symptoms, a meniscus injury is high on my list in the presence of a suggestive history, joint line tenderness, and an inability to squat because of pain. When my suspicion is high I usually resort to an MRI.
No single clinical examination element, or combination of such elements, reliably detects meniscal injury. The McMurray test is best for ruling in meniscal pathology. Assuming a 9% prevalence of meniscal tears among all knee injuries (a rate reflecting national primary care data), the posttest probability that a patient with McMurray’s sign has a meniscal injury ranges from <30% to 63% (strength of recommendation [SOR]: B). In contrast, the absence of any positive physical examination findings effectively rules out meniscal pathology, yielding a posttest probability of 0.8% for lateral meniscus injury, 1.0% for medial meniscus injury, and 3.8% for any meniscal injury among primary care populations (SOR: B).
Evidence summary
The accuracy of physical examination findings for meniscal injury varies widely among meta-analyses. In a meta-analysis of 13 studies, no physical examination test—including assessment for joint effusion, McMurray test, joint line tenderness, or the Apley compression test—yielded clinically significant positive or negative likelihood ratios for a meniscal tear ( Table ). The McMurray test performed best, but at 9% to 11% pretest probability of JFP_1104_CI.final 10/18/04 11:06 AM Page 918 meniscal lesions, based on prevalence estimates among primary care/specialist populations,2 the posttest probability of a positive exam is still <30%.
A meta-analysis of 4 studies by Jackson compared the utility of the McMurray test and joint line tenderness.3 For detecting meniscal tears, the McMurray test had a clinically and statistically significant positive likelihood ratio of 17.33, corresponding to a posttest probability of nearly 61%. Negative likelihood ratios for the McMurray test and joint line tenderness (0.5 and 0.8) were not clinically significant, indicating that absence of the McMurray sign or joint line tenderness alone is of little benefit in ruling out meniscal injury.
In another meta-analysis including 9 studies of meniscal injury diagnosis,4 individual tests for joint line tenderness, joint effusion, the medial-lateral grind test, and the McMurray test failed to yield statistically significant likelihood ratios for the presence or absence of meniscal tears ( Table footnotes). Positive and negative likelihood ratios for aggregate physical examination were 2.7 (95% confidence interval [CI], 1.4–5.1) and 0.4 (95% CI, 0.2–0.7), which are statistically, but not clinically, significant values for ruling meniscal lesions in or out.
Jackson’s meta-analysis also calculated the posttest probability of injury for a composite meniscal examination. Based on the positive likelihood ratio of 3.1 (95% CI, 0.54–5.7) and negative likelihood ratio of 0.19 (95% CI, 0.11–0.77), the posttest probability of a medial meniscal tear was 17% in the setting of composite physical exam findings and 1% in the absence of physical exam findings. For a lateral meniscal tear, based on the positive likelihood ratio of 11 (95% CI, 1.8–20.2), and negative likelihood ratio of 0.13 (95% CI, 0.0–0.25), the posttest probability of injury with a positive exam was 41% and with a negative exam 0.8%.
Authors of all meta-analyses noted the lack of standardization in physical examination maneuvers (especially the McMurray test)5 and, in some cases, no specification of how physical examination tests were performed. Authors analyzed the utility of the aggregate and composite knee examinations without specifying what constituted such an exam. No study included in the meta-analyses used control subjects without meniscal pathology, and few studies were blinded. Lack of blinding may have introduced verification bias; use of specialty patients in all studies made referral bias likely. Studies were heterogeneous and results were associated with wide confidence intervals, introducing an element of random error into the processes of combining and interpreting data.
TABLE
Physical exams for meniscal tear
| Summary characteristics | Solomon et al 4 | Scholten et al 1 | Jackson et al 3 |
| 9 studies 1018 patients Specialist population Specialist examiners | 13 studies 2231 patients Specialist population Specialist examiners | 4 studies 424 patients Specialist population Specialist examiners | |
| McMurray | Positive likelihood ratio (95% CI) | ||
| 1.3 (0.9–1.7) | 1.5–9.5 | 17.3 (2.7–68) | |
| Joint line tenderness | 0.9 (0.8–1.0) | 0.8–14.9 | 1.1 (0.7–1.6) |
| Aggregate exam | 2.7 (1.4–5.1) | — | — |
| Aggregate exam, medial meniscus tears | — | — | 3.1 (0.54–5.7) |
| Aggregate exam, lateral meniscus tears | — | — | 11 (1.8–20.2) |
| McMurray | Negative likelihood ratio (95% CI) | ||
| 0.8 (0.6–1.1) | 0.4–0.9 | 0.5 (0.3–0.8) | |
| Joint line tenderness | 1.1 (1.0–1.3) | 0.2–2.1 | 0.8 (0.3–3.5) |
| Aggregate exam | 0.4 (0.2–0.7) | — | — |
| Aggregate exam, medial meniscus tears | — | — | 0.19 (0.11–0.77) |
| Aggregate exam, lateral meniscus tears | — | — | 0.13 (0–0.25) |
| Note: The results are presented as likelihood ratios, which represent the change in the odds of a diagnosis, based on the outcome of the test. For example, given a positive likelihood ratio of 2, if a test result is positive, the odds of the disease being present is doubled. A positive likelihood ratio >10 provides strong evidence that the disorder is present. A negative likelihood ratio <0.1 provides strong evidence that the disorder is not present. Scores between 0.5 and 2.0 are neutral. In Scholten’s meta-analysis, likelihood ratios are given in ranges (no composite value given). | |||
Recommendations from others
The American Academy of Orthopaedic Surgeons’ clinical guideline on the evaluation and treatment of knee injuries lists the following findings as associated with a meniscal tear: delayed swelling of the knee, twisting injury, painful popping and catching, effusion, joint line tenderness, positive McMurray’s test, and negative radiography.6 The guideline fails to list the strength and type of supporting evidence for these associations.
The American College of Radiology’s Appropriateness Criteria for Acute Trauma to the Knee states that decision rules for meniscal tears and other soft tissue injuries to the knee are being investigated, but it fails to mention specific evaluation strategies for meniscal tears.7
Meniscus injury likely with suggestive history, joint line tenderness, and an inability to squat because of pain
Roy Henderson, MD
Director, Sports Medicine Fellowship, MacNeal Family Practice Residency Program, Chicago, Ill
I often suspect meniscal injuries as a cause of knee pain but am rarely certain based on physical examination alone. I look for a history of joint line pain, locking, or popping with movement. If the patient lacks joint line tenderness, a meniscal injury is unlikely. The McMurray test is usually negative. In the absence of another explanation for the patient’s symptoms, a meniscus injury is high on my list in the presence of a suggestive history, joint line tenderness, and an inability to squat because of pain. When my suspicion is high I usually resort to an MRI.
1. Scholten RJ, Deville WL, Opstelten W, Bijl D, van der Plas CG, Bouter LM. The accuracy of physical diagnostic tests for assessing meniscal lesions of the knee: a meta-analysis. J Fam Pract 2001;50:938-944.
2. National Ambulatory Medical Care Survey 1996. Available at: ftp://ftp.cdc.gov/pub/Health-Statistics/NCHS/Datasets/NAMCS/. Accessed on August 18, 2004.
3. Jackson JL, O’Malley PG, Kroenke K. Evaluation of acute knee pain in primary care. Ann Intern Med 2003;139:575-588.
4. Solomon DH, Simel DL, Bates DW, Katz JN, Schaffer JL. The rational clinical examination. Does this patient have a torn meniscus or ligament of the knee?. JAMA 2001;286:1610-1620.
5. Stratford PW, Binkley J. A review of the McMurray test: definition, interpretation, and clinical usefulness. J Orthop Sports Phys Ther 1995;22:116-120.
6. American Academy of Orthopaedic Surgeons. AAOS Clinical Guideline on Knee Injury: Support Document. Last updated February 26, 2002. Rosemont, Ill: American Academy of Orthopaedic Surgeons; 2001. Available at: http://www.guidelines.gov. Accessed on September 30, 2004.
7. American College of Radiology (ACR) Expert. Panel on Musculoskeletal Imaging ACR Appropriateness Criteria for Acute Trauma to the Knee. Updated October 1, 2002. Reston, Va: American College of Radiology; 2001. Available at: http://www.acr.org/cgi-bin/fr?tmpl:appcrit,pdf:0365374_acute_trauma_to_knee.ac.p df. Accessed on September 30, 2004.
1. Scholten RJ, Deville WL, Opstelten W, Bijl D, van der Plas CG, Bouter LM. The accuracy of physical diagnostic tests for assessing meniscal lesions of the knee: a meta-analysis. J Fam Pract 2001;50:938-944.
2. National Ambulatory Medical Care Survey 1996. Available at: ftp://ftp.cdc.gov/pub/Health-Statistics/NCHS/Datasets/NAMCS/. Accessed on August 18, 2004.
3. Jackson JL, O’Malley PG, Kroenke K. Evaluation of acute knee pain in primary care. Ann Intern Med 2003;139:575-588.
4. Solomon DH, Simel DL, Bates DW, Katz JN, Schaffer JL. The rational clinical examination. Does this patient have a torn meniscus or ligament of the knee?. JAMA 2001;286:1610-1620.
5. Stratford PW, Binkley J. A review of the McMurray test: definition, interpretation, and clinical usefulness. J Orthop Sports Phys Ther 1995;22:116-120.
6. American Academy of Orthopaedic Surgeons. AAOS Clinical Guideline on Knee Injury: Support Document. Last updated February 26, 2002. Rosemont, Ill: American Academy of Orthopaedic Surgeons; 2001. Available at: http://www.guidelines.gov. Accessed on September 30, 2004.
7. American College of Radiology (ACR) Expert. Panel on Musculoskeletal Imaging ACR Appropriateness Criteria for Acute Trauma to the Knee. Updated October 1, 2002. Reston, Va: American College of Radiology; 2001. Available at: http://www.acr.org/cgi-bin/fr?tmpl:appcrit,pdf:0365374_acute_trauma_to_knee.ac.p df. Accessed on September 30, 2004.
Evidence-based answers from the Family Physicians Inquiries Network
How effective is gastric bypass for weight loss?
Gastric bypass results in weight loss of approximately 33% at 2 years and 25% at 8 years (strength of recommendation [SOR]: B, based on a cohort study). Gastric bypass is one type of bariatric surgery, which also includes gastroplasty and gastric banding procedures ( Figure 1 ). These procedures all can produce enough weight loss to measurably improve health, but they differ in the amount of long-term weight loss, as well as side effects, which can be serious.
Gastric bypass is more effective than gastroplasty for weight loss and is associated with fewer revisions, but it has more side effects (SOR: A, based on a systematic review). Limited evidence suggests that gastric bypass produces more weight loss than gastric banding (SOR: B, based on a cohort study).
Bariatric surgery, including gastric bypass, improves conditions comorbid with obesity, including diabetes, abnormal lipid profiles, and low quality-of-life scores. It decreases the incidence of hypertension at 2 years after surgery, but whether this effect is sustained is unclear (SOR: B, based on a cohort study and multiple case series). Bariatric surgery also improves obstructive sleep apnea, obesity hypoventilation syndrome, menstrual irregularity, and female urinary stress incontinence (SOR: C, based on multiple case series). Bariatric surgery has a complication rate of 13% and a mortality rate of 0.2% (SOR: B, based on 1 cohort study).
FIGURE 1
Bariatric surgical techniques for weight loss
Evidence summary
A systematic review comparing bariatric surgery with conventional medical therapy for obesity included 1 randomized controlled trial and the Swedish Obesity Study, a large cohort study with matched controls. Surgery produced 23 to 28 kg more weight loss at 2 years.1 The study demonstrated 33% ± 10% weight loss for gastric bypass and 0% for medical therapy (not described) at 2 years,2 and 25% ± 6% loss vs 0.9% gain at 8 years.3 Among bariatric surgical techniques, patients undergoing gastric bypass lost more weight than those with gastroplasty (using staples to partition the stomach, either horizontally or vertically ( Figure 1 ) (P=.057, not significant) or gastric banding (placing a constricting ring around the stomach) (P<.05) at 8 years.3
The same systematic review assessed multiple randomized controlled trials comparing gastric bypass with gastroplasty and found greater weight loss, fewer revisions, and more side effects from gastric bypass ( Figure 2 ).1 Five trials comparing gastric bypass with horizontal gastroplasty demonstrated significantly greater weight loss from gastric bypass. Five other trials comparing weight loss from gastric bypass with vertical gastroplasty produced mixed results, with 3 trials favoring gastric bypass and 2 showing no difference.1 Fewer patients required revision after gastric bypass (0%–4%) compared with vertical gastroplasty (9%) or horizontal gastroplasty (19%–40%). One included trial found that postoperative dumping syndrome (28% vs 0%, P<0.05) and heartburn (59% vs 32%, P<.05) were more common with gastric bypass than with gastroplasty.1
Bariatric surgery, including gastric bypass, improves a variety of obesity-related comorbid conditions. Diabetes prevalence decreased among gastric bypass patients at 2 years (0.0% vs 4.7%, P<0.005) and 8 years (3.6% vs 18.5%, P<.0005) compared with those receiving medical therapy.2,3 In a case series involving 154 diabetic gastric bypass patients, diabetes resolved for 83% by 1 year, and for 86% at 5 to 7 years.4 In several case series, most patients became euglycemic and discontinued insulin or oral agents.
In the Swedish Obesity Study, hypertriglyceridemia decreased postoperatively but hypercholesterolemia did not.5 In a case series, bariatric surgery reduced triglycerides (50%) as well as total cholesterol (15%) (P<.05 for both) at 6 months and significantly increased high-density lipoprotein cholesterol levels at 1 and 5 years.6
Bariatric surgery significantly lowered the incidence of hypertension at 2 years (3.2%) compared with conventional treatment (9.9%), but after 8 years this difference disappeared.2,3,5 However, in multiple large case series with morbidly obese patients, hypertension resolved or improved. The largest study showed resolution of hypertension for 69% at 1 to 2 years (91% follow-up), 66% at 5 to 7 years (50% follow-up), and 51% at 10 to 12 years (37% follow-up).4
Bariatric surgery improved obstructive sleep apnea and obesity hypoventilation syndrome in 2 case series. In one, Epworth Sleepiness Scale scores, minimum O2 saturation, and other measures improved significantly (P<.001) by 3 to 21 months after surgery.7
In another case series, menstrual irregularities decreased from 40.4% to 4.6% following surgery (P<.001) among women who lost 50% of their excess weight.8 The incidence of urinary stress incontinence also decreased significantly (61.2% to 11.6%, P<.001 in this study8 ). The Swedish Obesity Study found significant improvements in Health-Related Quality of Life scores at 2 years with surgery vs conventional treatment.9
Bariatric surgery, including gastric bypass, has significant postoperative morbidity and mortality. Thirteen percent of patients in the Swedish Obesity Study experienced peri-operative complications, including pulmonary symptoms (6.2%), abdominal infection (2.1%), wound complications (1.8%), bleeding (0.9%), thromboembolic events (0.8%), and other miscellaneous complications (4.8%). Postoperative complications required reoperation for 2.2% of surgical patients, and there were 4 postoperative deaths (0.2% of the operative patients; 3 due to leakage, and 1 due to a technical laparoscopic error).2
Nutritional and vitamin deficiencies are common following gastric bypass, including deficiencies of vitamin B12, iron, folate, and calcium. Lifelong nutritional supplementation is generally necessary following this procedure.10
FIGURE 2
Long-term weight loss with bariatric surgery
Long-term weight loss with bariatric surgery: comparison of controls, horizontal gastric banding (Banding), vertical band-ed gastroplasty (VPG), and gastric bypass (GBP). Source: Sjostrom et al 2000. 3
Recommendations from others
A 1991 National Institutes of Health consensus conference suggested consideration of obesity surgery for patients with a body-mass index ≥40, or ≥35 plus severe obesity-related medical comorbidities (such as severe sleep apnea, obesity hypoventilation syndrome, obesity-related cardiomyopathy, or severe diabetes) who have not been successfully treated with non-surgical attempts at weight reduction.
Selected patients should be well-informed and motivated, with acceptable operative risk. A multidisciplinary team with medical, surgical, psychiatric, and nutritional expertise should evaluate patients who are candidates for surgery. An experienced surgeon, working in a clinical setting with adequate support for all aspects of management and assessment, should perform the surgery.
Lifelong medical surveillance is necessary after surgery, and patients should be selected who are likely to comply with this.11
Bariatric surgery is an important option for select patients
Tim Mott, MD
Family Practice Staff, Navy Hospital, Pensacola, Fla
The lack of successful interventions for obesity is frustrating. This is accentuated as obesity is increasingly recognized as the proverbial forest in which we find ourselves hacking at the “trees” of diabetes, hypertension, dyslipidemia, and many other diseases. As we focus on this, the second-leading preventable cause of death, we find ourselves uniquely skilled as family physicians to offer balanced advice and advocacy.12
Bariatric surgery is an important option for select patients. For such a patient, I continuously advocate for lifestyle changes, document all non-surgical measures pursued (important for third-party review), discuss realistic expectations and risks, and direct the patient to a trusted bariatric surgery center. For the postsurgical patient, I reinforce the lifestyle commitments, ensure ongoing vitamin and mineral supplementation, and help monitor for possible complications.
1. Colquitt J, Clegg A, Sidhu M, Royle P. Surgery for morbid obesity (Cochrane Review). In: The Cochrane Library, Issue 4, 2003; Chichester, UK: John Wiley & Sons, Ltd.
2. Torgerson JS, Sjostrom L. The Swedish Obese Subjects (SOS) study—rationale and results. Int J Obes Relat Metab Disord 2001;25 Supp1:S2-S4.
3. Sjostrom CD, Peltonen M, Wedel H, Sjostrom L. Differentiated long-term effects of intentional weight loss on diabetes and hypertension. Hypertension 2000;36:20-25.
4. Sugerman HJ, Wolfe LG, Sica DA, Clore JN. Diabetes and hypertension in severe obesity and effects of gastric bypass-induced weight loss. Ann Surg 2003;237:751-758.
5. Sjostrom CD, Lissner L, Wedel H, Sjostrom L. Reduction in incidence of diabetes, hypertension, and lipid disturbances after intentional weight loss induced by bariatric surgery: the SOS Intervention Study. Obes Res 1999;7:477-484.
6. Brolin RE, Bradley LJ, Wilson AC, Cody RP. Lipid risk profile and weight stability after gastric restrictive operations for morbid obesity. J Gastrointest Surg 2000;4:464-469.
7. Rasheid S, Banasiak M, Gallagher SF, et al. Gastric bypass is an effective treatment for obstructive sleep apnea in patients with clinically significant obesity. Obes Surg 2003;13:58-61.
8. Deitel M, Stone E, Kassam HA, Wilk EJ, Sutherland DJ. Gynecologic-obstetric changes after loss of massive excess weight following bariatric surgery. J Am Coll Nutr 1988;7:147-153.
9. Karlsson J, Sjostrom L, Sullivan M. Swedish obese subjects (SOS)- an intervention study of obesity. Two-year follow-up of health-related quality of life (HRQL) and eating behavior after gastric surgery for severe obesity. Int J Obes Relat Metab Disord 1998;22:113-126.
10. Kushner R. Managing the obese patient after bariatric surgery: A case report of severe malnutrition and review of the literature. J Parenteral Enteral Nutrition 2000;24:126-132.
11. NIH conference: Gastrointestinal surgery for severe obesity. Consensus Development Conference Panel. Ann Intern Med 1991;115:956-961.
12. Flegal K, Carroll M, Ogden C, et al. Prevalence trends in obesity among US adults, 1999–2000. JAMA 2002;288:1723-1727.
Gastric bypass results in weight loss of approximately 33% at 2 years and 25% at 8 years (strength of recommendation [SOR]: B, based on a cohort study). Gastric bypass is one type of bariatric surgery, which also includes gastroplasty and gastric banding procedures ( Figure 1 ). These procedures all can produce enough weight loss to measurably improve health, but they differ in the amount of long-term weight loss, as well as side effects, which can be serious.
Gastric bypass is more effective than gastroplasty for weight loss and is associated with fewer revisions, but it has more side effects (SOR: A, based on a systematic review). Limited evidence suggests that gastric bypass produces more weight loss than gastric banding (SOR: B, based on a cohort study).
Bariatric surgery, including gastric bypass, improves conditions comorbid with obesity, including diabetes, abnormal lipid profiles, and low quality-of-life scores. It decreases the incidence of hypertension at 2 years after surgery, but whether this effect is sustained is unclear (SOR: B, based on a cohort study and multiple case series). Bariatric surgery also improves obstructive sleep apnea, obesity hypoventilation syndrome, menstrual irregularity, and female urinary stress incontinence (SOR: C, based on multiple case series). Bariatric surgery has a complication rate of 13% and a mortality rate of 0.2% (SOR: B, based on 1 cohort study).
FIGURE 1
Bariatric surgical techniques for weight loss
Evidence summary
A systematic review comparing bariatric surgery with conventional medical therapy for obesity included 1 randomized controlled trial and the Swedish Obesity Study, a large cohort study with matched controls. Surgery produced 23 to 28 kg more weight loss at 2 years.1 The study demonstrated 33% ± 10% weight loss for gastric bypass and 0% for medical therapy (not described) at 2 years,2 and 25% ± 6% loss vs 0.9% gain at 8 years.3 Among bariatric surgical techniques, patients undergoing gastric bypass lost more weight than those with gastroplasty (using staples to partition the stomach, either horizontally or vertically ( Figure 1 ) (P=.057, not significant) or gastric banding (placing a constricting ring around the stomach) (P<.05) at 8 years.3
The same systematic review assessed multiple randomized controlled trials comparing gastric bypass with gastroplasty and found greater weight loss, fewer revisions, and more side effects from gastric bypass ( Figure 2 ).1 Five trials comparing gastric bypass with horizontal gastroplasty demonstrated significantly greater weight loss from gastric bypass. Five other trials comparing weight loss from gastric bypass with vertical gastroplasty produced mixed results, with 3 trials favoring gastric bypass and 2 showing no difference.1 Fewer patients required revision after gastric bypass (0%–4%) compared with vertical gastroplasty (9%) or horizontal gastroplasty (19%–40%). One included trial found that postoperative dumping syndrome (28% vs 0%, P<0.05) and heartburn (59% vs 32%, P<.05) were more common with gastric bypass than with gastroplasty.1
Bariatric surgery, including gastric bypass, improves a variety of obesity-related comorbid conditions. Diabetes prevalence decreased among gastric bypass patients at 2 years (0.0% vs 4.7%, P<0.005) and 8 years (3.6% vs 18.5%, P<.0005) compared with those receiving medical therapy.2,3 In a case series involving 154 diabetic gastric bypass patients, diabetes resolved for 83% by 1 year, and for 86% at 5 to 7 years.4 In several case series, most patients became euglycemic and discontinued insulin or oral agents.
In the Swedish Obesity Study, hypertriglyceridemia decreased postoperatively but hypercholesterolemia did not.5 In a case series, bariatric surgery reduced triglycerides (50%) as well as total cholesterol (15%) (P<.05 for both) at 6 months and significantly increased high-density lipoprotein cholesterol levels at 1 and 5 years.6
Bariatric surgery significantly lowered the incidence of hypertension at 2 years (3.2%) compared with conventional treatment (9.9%), but after 8 years this difference disappeared.2,3,5 However, in multiple large case series with morbidly obese patients, hypertension resolved or improved. The largest study showed resolution of hypertension for 69% at 1 to 2 years (91% follow-up), 66% at 5 to 7 years (50% follow-up), and 51% at 10 to 12 years (37% follow-up).4
Bariatric surgery improved obstructive sleep apnea and obesity hypoventilation syndrome in 2 case series. In one, Epworth Sleepiness Scale scores, minimum O2 saturation, and other measures improved significantly (P<.001) by 3 to 21 months after surgery.7
In another case series, menstrual irregularities decreased from 40.4% to 4.6% following surgery (P<.001) among women who lost 50% of their excess weight.8 The incidence of urinary stress incontinence also decreased significantly (61.2% to 11.6%, P<.001 in this study8 ). The Swedish Obesity Study found significant improvements in Health-Related Quality of Life scores at 2 years with surgery vs conventional treatment.9
Bariatric surgery, including gastric bypass, has significant postoperative morbidity and mortality. Thirteen percent of patients in the Swedish Obesity Study experienced peri-operative complications, including pulmonary symptoms (6.2%), abdominal infection (2.1%), wound complications (1.8%), bleeding (0.9%), thromboembolic events (0.8%), and other miscellaneous complications (4.8%). Postoperative complications required reoperation for 2.2% of surgical patients, and there were 4 postoperative deaths (0.2% of the operative patients; 3 due to leakage, and 1 due to a technical laparoscopic error).2
Nutritional and vitamin deficiencies are common following gastric bypass, including deficiencies of vitamin B12, iron, folate, and calcium. Lifelong nutritional supplementation is generally necessary following this procedure.10
FIGURE 2
Long-term weight loss with bariatric surgery
Long-term weight loss with bariatric surgery: comparison of controls, horizontal gastric banding (Banding), vertical band-ed gastroplasty (VPG), and gastric bypass (GBP). Source: Sjostrom et al 2000. 3
Recommendations from others
A 1991 National Institutes of Health consensus conference suggested consideration of obesity surgery for patients with a body-mass index ≥40, or ≥35 plus severe obesity-related medical comorbidities (such as severe sleep apnea, obesity hypoventilation syndrome, obesity-related cardiomyopathy, or severe diabetes) who have not been successfully treated with non-surgical attempts at weight reduction.
Selected patients should be well-informed and motivated, with acceptable operative risk. A multidisciplinary team with medical, surgical, psychiatric, and nutritional expertise should evaluate patients who are candidates for surgery. An experienced surgeon, working in a clinical setting with adequate support for all aspects of management and assessment, should perform the surgery.
Lifelong medical surveillance is necessary after surgery, and patients should be selected who are likely to comply with this.11
Bariatric surgery is an important option for select patients
Tim Mott, MD
Family Practice Staff, Navy Hospital, Pensacola, Fla
The lack of successful interventions for obesity is frustrating. This is accentuated as obesity is increasingly recognized as the proverbial forest in which we find ourselves hacking at the “trees” of diabetes, hypertension, dyslipidemia, and many other diseases. As we focus on this, the second-leading preventable cause of death, we find ourselves uniquely skilled as family physicians to offer balanced advice and advocacy.12
Bariatric surgery is an important option for select patients. For such a patient, I continuously advocate for lifestyle changes, document all non-surgical measures pursued (important for third-party review), discuss realistic expectations and risks, and direct the patient to a trusted bariatric surgery center. For the postsurgical patient, I reinforce the lifestyle commitments, ensure ongoing vitamin and mineral supplementation, and help monitor for possible complications.
Gastric bypass results in weight loss of approximately 33% at 2 years and 25% at 8 years (strength of recommendation [SOR]: B, based on a cohort study). Gastric bypass is one type of bariatric surgery, which also includes gastroplasty and gastric banding procedures ( Figure 1 ). These procedures all can produce enough weight loss to measurably improve health, but they differ in the amount of long-term weight loss, as well as side effects, which can be serious.
Gastric bypass is more effective than gastroplasty for weight loss and is associated with fewer revisions, but it has more side effects (SOR: A, based on a systematic review). Limited evidence suggests that gastric bypass produces more weight loss than gastric banding (SOR: B, based on a cohort study).
Bariatric surgery, including gastric bypass, improves conditions comorbid with obesity, including diabetes, abnormal lipid profiles, and low quality-of-life scores. It decreases the incidence of hypertension at 2 years after surgery, but whether this effect is sustained is unclear (SOR: B, based on a cohort study and multiple case series). Bariatric surgery also improves obstructive sleep apnea, obesity hypoventilation syndrome, menstrual irregularity, and female urinary stress incontinence (SOR: C, based on multiple case series). Bariatric surgery has a complication rate of 13% and a mortality rate of 0.2% (SOR: B, based on 1 cohort study).
FIGURE 1
Bariatric surgical techniques for weight loss
Evidence summary
A systematic review comparing bariatric surgery with conventional medical therapy for obesity included 1 randomized controlled trial and the Swedish Obesity Study, a large cohort study with matched controls. Surgery produced 23 to 28 kg more weight loss at 2 years.1 The study demonstrated 33% ± 10% weight loss for gastric bypass and 0% for medical therapy (not described) at 2 years,2 and 25% ± 6% loss vs 0.9% gain at 8 years.3 Among bariatric surgical techniques, patients undergoing gastric bypass lost more weight than those with gastroplasty (using staples to partition the stomach, either horizontally or vertically ( Figure 1 ) (P=.057, not significant) or gastric banding (placing a constricting ring around the stomach) (P<.05) at 8 years.3
The same systematic review assessed multiple randomized controlled trials comparing gastric bypass with gastroplasty and found greater weight loss, fewer revisions, and more side effects from gastric bypass ( Figure 2 ).1 Five trials comparing gastric bypass with horizontal gastroplasty demonstrated significantly greater weight loss from gastric bypass. Five other trials comparing weight loss from gastric bypass with vertical gastroplasty produced mixed results, with 3 trials favoring gastric bypass and 2 showing no difference.1 Fewer patients required revision after gastric bypass (0%–4%) compared with vertical gastroplasty (9%) or horizontal gastroplasty (19%–40%). One included trial found that postoperative dumping syndrome (28% vs 0%, P<0.05) and heartburn (59% vs 32%, P<.05) were more common with gastric bypass than with gastroplasty.1
Bariatric surgery, including gastric bypass, improves a variety of obesity-related comorbid conditions. Diabetes prevalence decreased among gastric bypass patients at 2 years (0.0% vs 4.7%, P<0.005) and 8 years (3.6% vs 18.5%, P<.0005) compared with those receiving medical therapy.2,3 In a case series involving 154 diabetic gastric bypass patients, diabetes resolved for 83% by 1 year, and for 86% at 5 to 7 years.4 In several case series, most patients became euglycemic and discontinued insulin or oral agents.
In the Swedish Obesity Study, hypertriglyceridemia decreased postoperatively but hypercholesterolemia did not.5 In a case series, bariatric surgery reduced triglycerides (50%) as well as total cholesterol (15%) (P<.05 for both) at 6 months and significantly increased high-density lipoprotein cholesterol levels at 1 and 5 years.6
Bariatric surgery significantly lowered the incidence of hypertension at 2 years (3.2%) compared with conventional treatment (9.9%), but after 8 years this difference disappeared.2,3,5 However, in multiple large case series with morbidly obese patients, hypertension resolved or improved. The largest study showed resolution of hypertension for 69% at 1 to 2 years (91% follow-up), 66% at 5 to 7 years (50% follow-up), and 51% at 10 to 12 years (37% follow-up).4
Bariatric surgery improved obstructive sleep apnea and obesity hypoventilation syndrome in 2 case series. In one, Epworth Sleepiness Scale scores, minimum O2 saturation, and other measures improved significantly (P<.001) by 3 to 21 months after surgery.7
In another case series, menstrual irregularities decreased from 40.4% to 4.6% following surgery (P<.001) among women who lost 50% of their excess weight.8 The incidence of urinary stress incontinence also decreased significantly (61.2% to 11.6%, P<.001 in this study8 ). The Swedish Obesity Study found significant improvements in Health-Related Quality of Life scores at 2 years with surgery vs conventional treatment.9
Bariatric surgery, including gastric bypass, has significant postoperative morbidity and mortality. Thirteen percent of patients in the Swedish Obesity Study experienced peri-operative complications, including pulmonary symptoms (6.2%), abdominal infection (2.1%), wound complications (1.8%), bleeding (0.9%), thromboembolic events (0.8%), and other miscellaneous complications (4.8%). Postoperative complications required reoperation for 2.2% of surgical patients, and there were 4 postoperative deaths (0.2% of the operative patients; 3 due to leakage, and 1 due to a technical laparoscopic error).2
Nutritional and vitamin deficiencies are common following gastric bypass, including deficiencies of vitamin B12, iron, folate, and calcium. Lifelong nutritional supplementation is generally necessary following this procedure.10
FIGURE 2
Long-term weight loss with bariatric surgery
Long-term weight loss with bariatric surgery: comparison of controls, horizontal gastric banding (Banding), vertical band-ed gastroplasty (VPG), and gastric bypass (GBP). Source: Sjostrom et al 2000. 3
Recommendations from others
A 1991 National Institutes of Health consensus conference suggested consideration of obesity surgery for patients with a body-mass index ≥40, or ≥35 plus severe obesity-related medical comorbidities (such as severe sleep apnea, obesity hypoventilation syndrome, obesity-related cardiomyopathy, or severe diabetes) who have not been successfully treated with non-surgical attempts at weight reduction.
Selected patients should be well-informed and motivated, with acceptable operative risk. A multidisciplinary team with medical, surgical, psychiatric, and nutritional expertise should evaluate patients who are candidates for surgery. An experienced surgeon, working in a clinical setting with adequate support for all aspects of management and assessment, should perform the surgery.
Lifelong medical surveillance is necessary after surgery, and patients should be selected who are likely to comply with this.11
Bariatric surgery is an important option for select patients
Tim Mott, MD
Family Practice Staff, Navy Hospital, Pensacola, Fla
The lack of successful interventions for obesity is frustrating. This is accentuated as obesity is increasingly recognized as the proverbial forest in which we find ourselves hacking at the “trees” of diabetes, hypertension, dyslipidemia, and many other diseases. As we focus on this, the second-leading preventable cause of death, we find ourselves uniquely skilled as family physicians to offer balanced advice and advocacy.12
Bariatric surgery is an important option for select patients. For such a patient, I continuously advocate for lifestyle changes, document all non-surgical measures pursued (important for third-party review), discuss realistic expectations and risks, and direct the patient to a trusted bariatric surgery center. For the postsurgical patient, I reinforce the lifestyle commitments, ensure ongoing vitamin and mineral supplementation, and help monitor for possible complications.
1. Colquitt J, Clegg A, Sidhu M, Royle P. Surgery for morbid obesity (Cochrane Review). In: The Cochrane Library, Issue 4, 2003; Chichester, UK: John Wiley & Sons, Ltd.
2. Torgerson JS, Sjostrom L. The Swedish Obese Subjects (SOS) study—rationale and results. Int J Obes Relat Metab Disord 2001;25 Supp1:S2-S4.
3. Sjostrom CD, Peltonen M, Wedel H, Sjostrom L. Differentiated long-term effects of intentional weight loss on diabetes and hypertension. Hypertension 2000;36:20-25.
4. Sugerman HJ, Wolfe LG, Sica DA, Clore JN. Diabetes and hypertension in severe obesity and effects of gastric bypass-induced weight loss. Ann Surg 2003;237:751-758.
5. Sjostrom CD, Lissner L, Wedel H, Sjostrom L. Reduction in incidence of diabetes, hypertension, and lipid disturbances after intentional weight loss induced by bariatric surgery: the SOS Intervention Study. Obes Res 1999;7:477-484.
6. Brolin RE, Bradley LJ, Wilson AC, Cody RP. Lipid risk profile and weight stability after gastric restrictive operations for morbid obesity. J Gastrointest Surg 2000;4:464-469.
7. Rasheid S, Banasiak M, Gallagher SF, et al. Gastric bypass is an effective treatment for obstructive sleep apnea in patients with clinically significant obesity. Obes Surg 2003;13:58-61.
8. Deitel M, Stone E, Kassam HA, Wilk EJ, Sutherland DJ. Gynecologic-obstetric changes after loss of massive excess weight following bariatric surgery. J Am Coll Nutr 1988;7:147-153.
9. Karlsson J, Sjostrom L, Sullivan M. Swedish obese subjects (SOS)- an intervention study of obesity. Two-year follow-up of health-related quality of life (HRQL) and eating behavior after gastric surgery for severe obesity. Int J Obes Relat Metab Disord 1998;22:113-126.
10. Kushner R. Managing the obese patient after bariatric surgery: A case report of severe malnutrition and review of the literature. J Parenteral Enteral Nutrition 2000;24:126-132.
11. NIH conference: Gastrointestinal surgery for severe obesity. Consensus Development Conference Panel. Ann Intern Med 1991;115:956-961.
12. Flegal K, Carroll M, Ogden C, et al. Prevalence trends in obesity among US adults, 1999–2000. JAMA 2002;288:1723-1727.
1. Colquitt J, Clegg A, Sidhu M, Royle P. Surgery for morbid obesity (Cochrane Review). In: The Cochrane Library, Issue 4, 2003; Chichester, UK: John Wiley & Sons, Ltd.
2. Torgerson JS, Sjostrom L. The Swedish Obese Subjects (SOS) study—rationale and results. Int J Obes Relat Metab Disord 2001;25 Supp1:S2-S4.
3. Sjostrom CD, Peltonen M, Wedel H, Sjostrom L. Differentiated long-term effects of intentional weight loss on diabetes and hypertension. Hypertension 2000;36:20-25.
4. Sugerman HJ, Wolfe LG, Sica DA, Clore JN. Diabetes and hypertension in severe obesity and effects of gastric bypass-induced weight loss. Ann Surg 2003;237:751-758.
5. Sjostrom CD, Lissner L, Wedel H, Sjostrom L. Reduction in incidence of diabetes, hypertension, and lipid disturbances after intentional weight loss induced by bariatric surgery: the SOS Intervention Study. Obes Res 1999;7:477-484.
6. Brolin RE, Bradley LJ, Wilson AC, Cody RP. Lipid risk profile and weight stability after gastric restrictive operations for morbid obesity. J Gastrointest Surg 2000;4:464-469.
7. Rasheid S, Banasiak M, Gallagher SF, et al. Gastric bypass is an effective treatment for obstructive sleep apnea in patients with clinically significant obesity. Obes Surg 2003;13:58-61.
8. Deitel M, Stone E, Kassam HA, Wilk EJ, Sutherland DJ. Gynecologic-obstetric changes after loss of massive excess weight following bariatric surgery. J Am Coll Nutr 1988;7:147-153.
9. Karlsson J, Sjostrom L, Sullivan M. Swedish obese subjects (SOS)- an intervention study of obesity. Two-year follow-up of health-related quality of life (HRQL) and eating behavior after gastric surgery for severe obesity. Int J Obes Relat Metab Disord 1998;22:113-126.
10. Kushner R. Managing the obese patient after bariatric surgery: A case report of severe malnutrition and review of the literature. J Parenteral Enteral Nutrition 2000;24:126-132.
11. NIH conference: Gastrointestinal surgery for severe obesity. Consensus Development Conference Panel. Ann Intern Med 1991;115:956-961.
12. Flegal K, Carroll M, Ogden C, et al. Prevalence trends in obesity among US adults, 1999–2000. JAMA 2002;288:1723-1727.
Evidence-based answers from the Family Physicians Inquiries Network
Does tight control of blood glucose in pregnant women with diabetes improve neonatal outcomes?
In pregnant women with preexisting type 1 diabetes mellitus, maintaining near-normal blood glucose levels decreases the rate of major congenital anomalies (defined as those causing death or a serious handicap necessitating surgical correction or medical treatment). Prolonged preconception control of blood sugar to near normal levels reduces the rate of major congenital anomalies close to those seen in women without diabetes (strength of recommendation [SOR]: A, based on prospective cohort studies and randomized controlled trial [RCT]).
Intensive management reduces the risk of congenital anomalies more than conventional therapy, and lowers the risk of neonatal hypoglycemia (SOR: B, based on RCT). Very tight control does not reduce clinically significant neonatal morbidity but does increase the risk of maternal hypoglycemia (SOR: B, based on a systematic review). Evidence is insufficient about whether or not these statements hold true for women with type 2 diabetes.
In women with impaired glucose tolerance, dietary control reduces neonatal hypoglycemia. To date, studies have not found statistically significant reductions in admission rates to the special care nursery or birth weights above the 90th percentile (SOR: B, systematic review). Evidence is insufficient to suggest improved outcomes with therapy in women with gestational diabetes. Standard recommendations typically recommend tight control in this population as well.
Evidence summary
Two studies show that in type 1 diabetes mellitus, elevated blood glucose levels in early pregnancy (HbA1c=6%–8%) are associated with a threefold increase in fetal malformations.1,2 Maintaining preconception and early pregnancy blood glucose levels in the normal range can reduce this risk. A meta-analysis comparing 16 studies of women with pregestational diabetes—13 of which included only women with type 1 diabetes—found that women receiving preconception care had lower early first trimester HbA1c levels than those who did not (7.9% vs 9.6%) and delivered fewer infants with major congenital anomalies (relative risk [RR]=0.36; 95% confidence interval [CI], 0.22–0.59).2 One limitation of this study was that preconception care was not consistently defined among the included studies.
A 10-year RCT evaluated the outcomes of 270 pregnancies in women who had received either intensive (SQ infusion or multiple daily injections) or conventional insulin regimens prior to pregnancy. Women were advised to use intensive therapy when they were trying to conceive, and all were changed to intensive therapy if pregnancy was confirmed. Women in the intensive therapy group had normal HbA1c levels for an average of 40 months before conception. Women receiving intensive therapy had lower mean HbA1c levels at conception (7.4 ± 1.3 SD vs 8.1 ± 1.7 SD) and fewer major congenital anomalies (0.7% vs 5.9%; number needed to treat=19) than did women in the conventional group. When infants with genetic malformations were excluded from the analysis, rates of congenital malformations were similar in women switched to intensive therapy either before or after conception (3.8% vs 3.6%). No differences were seen between neonatal mortality, spontaneous abortion rates, birth weights, Apgar scores, and hypocalcemia or hypoglycemia rates.3
When tight and very tight control of glucose in pregnant women with pregestational diabetes were compared in a Cochrane systematic review, rates of maternal hypoglycemia in the very tightly controlled group were higher (odds ratio [OR]=25.96; 95% CI, 4.91–137.26).5 An RCT of 118 women with pregestational diabetes compared 4-times-daily vs twice-daily doses of insulin. Infants born to women receiving 4-times-daily insulin had significantly lower rates of neonatal hypoglycemia (RR=0.17; 95% CI, 0.04–0.74). While the trend was toward improved neonatal metabolic effects in the trials, the clinical significance of these findings is not clear.
Whether or not treatment of gestational diabetes improves outcomes is uncertain. A Cochrane systematic review evaluating a small number of trials, with variable quality and inconsistent outcome measures, compared dietary management to routine care in gestational diabetics. While fewer infants with birth weights >4000 g were delivered in the diet therapy group (OR=0.78; 95% CI, 0.45–1.35), the results were not statistically significant. No other important clinical differences were found.6
Another Cochrane systematic review evaluated the effects of dietary treatment of women with impaired glucose tolerance and gestational diabetes. Three trials with a total of 223 women with impaired glucose tolerance found a significant reduction in the rate of neonatal hypoglycemia (RR=0.25; 95% CI, 0.07–0.86). There was no significant change in the rates of cesarean section (RR=0.86; 95% CI, 0.51–1.45), admission to the special care nursery (RR=0.49; 95% CI, 0.19–1.24), or birth weights greater than the 90th percentile (RR=0.55; 95% CI, 0.19–1.61). Inadequate power may well account for the failure to reach significance in these outcomes.7
Recommendations from others
The American College of Obstetrics and Gynecology (ACOG) recommends that women with pregestational diabetes maintain fasting plasma glucose levels between 60–90 mg/dL and 2-hour postprandial levels <120 mg/dL.8 For women with gestational diabetes who are not controlled within these targets on dietary therapy alone, ACOG recommends the additional of insulin therapy.9
The American Diabetes Association recommends that women with pregestational diabetes maintain capillary plasma glucose levels of 80–110 mg/dL before and <155 mg/dL 2 hours after meals before pregnancy and while trying to conceive.10 The ADA does not list target glucose levels for women with pregestational diabetes once they become pregnant. The ADA recommends the use of diet and insulin therapy to maintain preprandial plasma glucose levels of <105 mg/dL and 2-hour postprandial levels below <130 mg/dL in gestational diabetes.11
Glucose control makes a difference for pregnancy outcomes in type I diabetes
Linda French, MD
Michigan State University, East Lansing
It is well accepted that glucose control makes a difference for pregnancy outcomes in women with type 1 diabetes. Since similar studies have not been done in women with preexisting type 2 diabetes, we have to assume that the risk is also high for them. Preconception counseling about glucose control is so important for women with diabetes. Fortunately, because they generally have routine visits for their chronic care, we have an opportunity to initiate discussion of glucose control in relationship to pregnancy planning. Routine diabetes care visits also give us the opportunity to discuss other important preconception topics.
- Allopurinol • Lopurin, Zyloprim
- Amitriptyline • Elavil, Endep
- Benzbromarone • Urinorm
- Botulinim toxin A • Botox
- Clindamycin • Cleocin
- Fluoxetine • Prozac
- Fluticasone • Flovent
- Gabapentin • Neurontin
- Metronidazole (intravaginal) • MetroGel
- Probenecid • Benemid, Probalan
- Sumatriptan • Imitrex
- Tizanidine • Zanaflex
- Triamcinolone • Azmacort
- Valproate • Depacon
1. Vaarasmaki MS, Hartikainen A, Anttila M, Pramila S, Koivisto M. Factors predicting peri- and neonatal outcome in diabetic pregnancy. Early Hum Dev 2000;59:61-70.
2. Ray JG, O’Brien TE, Chan WS. Preconception care and the risk of congenital anomalies in the offspring of women with diabetes mellitus: a meta-analysis. QJM 2001;94:435-444.
3. Pregnancy outcomes in the Diabetes Control and Complications Trial. Am J Obstet Gynecol 1996;174:1343-1353.
4. Nachum Z, Ben-Shlomo I, Weiner E, Shalev E. Twice daily versus four times daily insulin dose regimens for diabetes in pregnancy. BMJ 1999;319:1223-1227.
5. Walkinshaw SA. Very tight versus tight control for diabetes in pregnancy (Cochrane Review). In: The Cochrane Library, Issue 4, 2003. Chichester, UK: John Wiley & Sons, Ltd. Last updated 2-15-1999. Accessed on January 4, 2004.
6. Walkinshaw SA. Dietary regulation for ‘gestational diabetes’ (Cochrane Review). In: The Cochrane Library, Issue 4, 2003. Chichester, UK: John Wiley & Sons, Ltd. Last updated 2-25-1999. Accessed on January 4, 2004.
7. West J, Walkinshaw SA. Treatments for gestational diabetes and impaired glucose tolerance in pregnancy (Cochrane Review). In: The Cochrane Library, Issue 4, 2003. Chichester, UK: John Wiley & Sons, Ltd. Last updated 9-12-2002. Accessed on January 4, 2004.
8. ACOG technical bulletin Diabetes and pregnancy. Number 200—December 1994 (replaces No. 92, May 1986). Committee on Technical Bulletins of the American College of Obstetricians and Gynecologists. Int J Gynaecol Obstet 1995;48:331-339.
9. Gestational Diabetes. ACOG Pract Bull No. 30. American College of Obstetricians and Gynecologists. Obstet Gynecol 2001;98:525-538.
10. Preconception care of women with diabetes. Diabetes Care 2004;27 Suppl 1:S76-S78.Available at: care.diabetesjournals.org/cgi/content/full/27/suppl_1/s76. Accessed on January 4, 2004.
11. Gestational diabetes mellitus. Diabetes Care 2003;26 Suppl 1:S103-S105.Available at: care.diabetesjournals.org/cgi/content/full/26/suppl_1/s103. Accessed on January 4, 2004.
In pregnant women with preexisting type 1 diabetes mellitus, maintaining near-normal blood glucose levels decreases the rate of major congenital anomalies (defined as those causing death or a serious handicap necessitating surgical correction or medical treatment). Prolonged preconception control of blood sugar to near normal levels reduces the rate of major congenital anomalies close to those seen in women without diabetes (strength of recommendation [SOR]: A, based on prospective cohort studies and randomized controlled trial [RCT]).
Intensive management reduces the risk of congenital anomalies more than conventional therapy, and lowers the risk of neonatal hypoglycemia (SOR: B, based on RCT). Very tight control does not reduce clinically significant neonatal morbidity but does increase the risk of maternal hypoglycemia (SOR: B, based on a systematic review). Evidence is insufficient about whether or not these statements hold true for women with type 2 diabetes.
In women with impaired glucose tolerance, dietary control reduces neonatal hypoglycemia. To date, studies have not found statistically significant reductions in admission rates to the special care nursery or birth weights above the 90th percentile (SOR: B, systematic review). Evidence is insufficient to suggest improved outcomes with therapy in women with gestational diabetes. Standard recommendations typically recommend tight control in this population as well.
Evidence summary
Two studies show that in type 1 diabetes mellitus, elevated blood glucose levels in early pregnancy (HbA1c=6%–8%) are associated with a threefold increase in fetal malformations.1,2 Maintaining preconception and early pregnancy blood glucose levels in the normal range can reduce this risk. A meta-analysis comparing 16 studies of women with pregestational diabetes—13 of which included only women with type 1 diabetes—found that women receiving preconception care had lower early first trimester HbA1c levels than those who did not (7.9% vs 9.6%) and delivered fewer infants with major congenital anomalies (relative risk [RR]=0.36; 95% confidence interval [CI], 0.22–0.59).2 One limitation of this study was that preconception care was not consistently defined among the included studies.
A 10-year RCT evaluated the outcomes of 270 pregnancies in women who had received either intensive (SQ infusion or multiple daily injections) or conventional insulin regimens prior to pregnancy. Women were advised to use intensive therapy when they were trying to conceive, and all were changed to intensive therapy if pregnancy was confirmed. Women in the intensive therapy group had normal HbA1c levels for an average of 40 months before conception. Women receiving intensive therapy had lower mean HbA1c levels at conception (7.4 ± 1.3 SD vs 8.1 ± 1.7 SD) and fewer major congenital anomalies (0.7% vs 5.9%; number needed to treat=19) than did women in the conventional group. When infants with genetic malformations were excluded from the analysis, rates of congenital malformations were similar in women switched to intensive therapy either before or after conception (3.8% vs 3.6%). No differences were seen between neonatal mortality, spontaneous abortion rates, birth weights, Apgar scores, and hypocalcemia or hypoglycemia rates.3
When tight and very tight control of glucose in pregnant women with pregestational diabetes were compared in a Cochrane systematic review, rates of maternal hypoglycemia in the very tightly controlled group were higher (odds ratio [OR]=25.96; 95% CI, 4.91–137.26).5 An RCT of 118 women with pregestational diabetes compared 4-times-daily vs twice-daily doses of insulin. Infants born to women receiving 4-times-daily insulin had significantly lower rates of neonatal hypoglycemia (RR=0.17; 95% CI, 0.04–0.74). While the trend was toward improved neonatal metabolic effects in the trials, the clinical significance of these findings is not clear.
Whether or not treatment of gestational diabetes improves outcomes is uncertain. A Cochrane systematic review evaluating a small number of trials, with variable quality and inconsistent outcome measures, compared dietary management to routine care in gestational diabetics. While fewer infants with birth weights >4000 g were delivered in the diet therapy group (OR=0.78; 95% CI, 0.45–1.35), the results were not statistically significant. No other important clinical differences were found.6
Another Cochrane systematic review evaluated the effects of dietary treatment of women with impaired glucose tolerance and gestational diabetes. Three trials with a total of 223 women with impaired glucose tolerance found a significant reduction in the rate of neonatal hypoglycemia (RR=0.25; 95% CI, 0.07–0.86). There was no significant change in the rates of cesarean section (RR=0.86; 95% CI, 0.51–1.45), admission to the special care nursery (RR=0.49; 95% CI, 0.19–1.24), or birth weights greater than the 90th percentile (RR=0.55; 95% CI, 0.19–1.61). Inadequate power may well account for the failure to reach significance in these outcomes.7
Recommendations from others
The American College of Obstetrics and Gynecology (ACOG) recommends that women with pregestational diabetes maintain fasting plasma glucose levels between 60–90 mg/dL and 2-hour postprandial levels <120 mg/dL.8 For women with gestational diabetes who are not controlled within these targets on dietary therapy alone, ACOG recommends the additional of insulin therapy.9
The American Diabetes Association recommends that women with pregestational diabetes maintain capillary plasma glucose levels of 80–110 mg/dL before and <155 mg/dL 2 hours after meals before pregnancy and while trying to conceive.10 The ADA does not list target glucose levels for women with pregestational diabetes once they become pregnant. The ADA recommends the use of diet and insulin therapy to maintain preprandial plasma glucose levels of <105 mg/dL and 2-hour postprandial levels below <130 mg/dL in gestational diabetes.11
Glucose control makes a difference for pregnancy outcomes in type I diabetes
Linda French, MD
Michigan State University, East Lansing
It is well accepted that glucose control makes a difference for pregnancy outcomes in women with type 1 diabetes. Since similar studies have not been done in women with preexisting type 2 diabetes, we have to assume that the risk is also high for them. Preconception counseling about glucose control is so important for women with diabetes. Fortunately, because they generally have routine visits for their chronic care, we have an opportunity to initiate discussion of glucose control in relationship to pregnancy planning. Routine diabetes care visits also give us the opportunity to discuss other important preconception topics.
- Allopurinol • Lopurin, Zyloprim
- Amitriptyline • Elavil, Endep
- Benzbromarone • Urinorm
- Botulinim toxin A • Botox
- Clindamycin • Cleocin
- Fluoxetine • Prozac
- Fluticasone • Flovent
- Gabapentin • Neurontin
- Metronidazole (intravaginal) • MetroGel
- Probenecid • Benemid, Probalan
- Sumatriptan • Imitrex
- Tizanidine • Zanaflex
- Triamcinolone • Azmacort
- Valproate • Depacon
In pregnant women with preexisting type 1 diabetes mellitus, maintaining near-normal blood glucose levels decreases the rate of major congenital anomalies (defined as those causing death or a serious handicap necessitating surgical correction or medical treatment). Prolonged preconception control of blood sugar to near normal levels reduces the rate of major congenital anomalies close to those seen in women without diabetes (strength of recommendation [SOR]: A, based on prospective cohort studies and randomized controlled trial [RCT]).
Intensive management reduces the risk of congenital anomalies more than conventional therapy, and lowers the risk of neonatal hypoglycemia (SOR: B, based on RCT). Very tight control does not reduce clinically significant neonatal morbidity but does increase the risk of maternal hypoglycemia (SOR: B, based on a systematic review). Evidence is insufficient about whether or not these statements hold true for women with type 2 diabetes.
In women with impaired glucose tolerance, dietary control reduces neonatal hypoglycemia. To date, studies have not found statistically significant reductions in admission rates to the special care nursery or birth weights above the 90th percentile (SOR: B, systematic review). Evidence is insufficient to suggest improved outcomes with therapy in women with gestational diabetes. Standard recommendations typically recommend tight control in this population as well.
Evidence summary
Two studies show that in type 1 diabetes mellitus, elevated blood glucose levels in early pregnancy (HbA1c=6%–8%) are associated with a threefold increase in fetal malformations.1,2 Maintaining preconception and early pregnancy blood glucose levels in the normal range can reduce this risk. A meta-analysis comparing 16 studies of women with pregestational diabetes—13 of which included only women with type 1 diabetes—found that women receiving preconception care had lower early first trimester HbA1c levels than those who did not (7.9% vs 9.6%) and delivered fewer infants with major congenital anomalies (relative risk [RR]=0.36; 95% confidence interval [CI], 0.22–0.59).2 One limitation of this study was that preconception care was not consistently defined among the included studies.
A 10-year RCT evaluated the outcomes of 270 pregnancies in women who had received either intensive (SQ infusion or multiple daily injections) or conventional insulin regimens prior to pregnancy. Women were advised to use intensive therapy when they were trying to conceive, and all were changed to intensive therapy if pregnancy was confirmed. Women in the intensive therapy group had normal HbA1c levels for an average of 40 months before conception. Women receiving intensive therapy had lower mean HbA1c levels at conception (7.4 ± 1.3 SD vs 8.1 ± 1.7 SD) and fewer major congenital anomalies (0.7% vs 5.9%; number needed to treat=19) than did women in the conventional group. When infants with genetic malformations were excluded from the analysis, rates of congenital malformations were similar in women switched to intensive therapy either before or after conception (3.8% vs 3.6%). No differences were seen between neonatal mortality, spontaneous abortion rates, birth weights, Apgar scores, and hypocalcemia or hypoglycemia rates.3
When tight and very tight control of glucose in pregnant women with pregestational diabetes were compared in a Cochrane systematic review, rates of maternal hypoglycemia in the very tightly controlled group were higher (odds ratio [OR]=25.96; 95% CI, 4.91–137.26).5 An RCT of 118 women with pregestational diabetes compared 4-times-daily vs twice-daily doses of insulin. Infants born to women receiving 4-times-daily insulin had significantly lower rates of neonatal hypoglycemia (RR=0.17; 95% CI, 0.04–0.74). While the trend was toward improved neonatal metabolic effects in the trials, the clinical significance of these findings is not clear.
Whether or not treatment of gestational diabetes improves outcomes is uncertain. A Cochrane systematic review evaluating a small number of trials, with variable quality and inconsistent outcome measures, compared dietary management to routine care in gestational diabetics. While fewer infants with birth weights >4000 g were delivered in the diet therapy group (OR=0.78; 95% CI, 0.45–1.35), the results were not statistically significant. No other important clinical differences were found.6
Another Cochrane systematic review evaluated the effects of dietary treatment of women with impaired glucose tolerance and gestational diabetes. Three trials with a total of 223 women with impaired glucose tolerance found a significant reduction in the rate of neonatal hypoglycemia (RR=0.25; 95% CI, 0.07–0.86). There was no significant change in the rates of cesarean section (RR=0.86; 95% CI, 0.51–1.45), admission to the special care nursery (RR=0.49; 95% CI, 0.19–1.24), or birth weights greater than the 90th percentile (RR=0.55; 95% CI, 0.19–1.61). Inadequate power may well account for the failure to reach significance in these outcomes.7
Recommendations from others
The American College of Obstetrics and Gynecology (ACOG) recommends that women with pregestational diabetes maintain fasting plasma glucose levels between 60–90 mg/dL and 2-hour postprandial levels <120 mg/dL.8 For women with gestational diabetes who are not controlled within these targets on dietary therapy alone, ACOG recommends the additional of insulin therapy.9
The American Diabetes Association recommends that women with pregestational diabetes maintain capillary plasma glucose levels of 80–110 mg/dL before and <155 mg/dL 2 hours after meals before pregnancy and while trying to conceive.10 The ADA does not list target glucose levels for women with pregestational diabetes once they become pregnant. The ADA recommends the use of diet and insulin therapy to maintain preprandial plasma glucose levels of <105 mg/dL and 2-hour postprandial levels below <130 mg/dL in gestational diabetes.11
Glucose control makes a difference for pregnancy outcomes in type I diabetes
Linda French, MD
Michigan State University, East Lansing
It is well accepted that glucose control makes a difference for pregnancy outcomes in women with type 1 diabetes. Since similar studies have not been done in women with preexisting type 2 diabetes, we have to assume that the risk is also high for them. Preconception counseling about glucose control is so important for women with diabetes. Fortunately, because they generally have routine visits for their chronic care, we have an opportunity to initiate discussion of glucose control in relationship to pregnancy planning. Routine diabetes care visits also give us the opportunity to discuss other important preconception topics.
- Allopurinol • Lopurin, Zyloprim
- Amitriptyline • Elavil, Endep
- Benzbromarone • Urinorm
- Botulinim toxin A • Botox
- Clindamycin • Cleocin
- Fluoxetine • Prozac
- Fluticasone • Flovent
- Gabapentin • Neurontin
- Metronidazole (intravaginal) • MetroGel
- Probenecid • Benemid, Probalan
- Sumatriptan • Imitrex
- Tizanidine • Zanaflex
- Triamcinolone • Azmacort
- Valproate • Depacon
1. Vaarasmaki MS, Hartikainen A, Anttila M, Pramila S, Koivisto M. Factors predicting peri- and neonatal outcome in diabetic pregnancy. Early Hum Dev 2000;59:61-70.
2. Ray JG, O’Brien TE, Chan WS. Preconception care and the risk of congenital anomalies in the offspring of women with diabetes mellitus: a meta-analysis. QJM 2001;94:435-444.
3. Pregnancy outcomes in the Diabetes Control and Complications Trial. Am J Obstet Gynecol 1996;174:1343-1353.
4. Nachum Z, Ben-Shlomo I, Weiner E, Shalev E. Twice daily versus four times daily insulin dose regimens for diabetes in pregnancy. BMJ 1999;319:1223-1227.
5. Walkinshaw SA. Very tight versus tight control for diabetes in pregnancy (Cochrane Review). In: The Cochrane Library, Issue 4, 2003. Chichester, UK: John Wiley & Sons, Ltd. Last updated 2-15-1999. Accessed on January 4, 2004.
6. Walkinshaw SA. Dietary regulation for ‘gestational diabetes’ (Cochrane Review). In: The Cochrane Library, Issue 4, 2003. Chichester, UK: John Wiley & Sons, Ltd. Last updated 2-25-1999. Accessed on January 4, 2004.
7. West J, Walkinshaw SA. Treatments for gestational diabetes and impaired glucose tolerance in pregnancy (Cochrane Review). In: The Cochrane Library, Issue 4, 2003. Chichester, UK: John Wiley & Sons, Ltd. Last updated 9-12-2002. Accessed on January 4, 2004.
8. ACOG technical bulletin Diabetes and pregnancy. Number 200—December 1994 (replaces No. 92, May 1986). Committee on Technical Bulletins of the American College of Obstetricians and Gynecologists. Int J Gynaecol Obstet 1995;48:331-339.
9. Gestational Diabetes. ACOG Pract Bull No. 30. American College of Obstetricians and Gynecologists. Obstet Gynecol 2001;98:525-538.
10. Preconception care of women with diabetes. Diabetes Care 2004;27 Suppl 1:S76-S78.Available at: care.diabetesjournals.org/cgi/content/full/27/suppl_1/s76. Accessed on January 4, 2004.
11. Gestational diabetes mellitus. Diabetes Care 2003;26 Suppl 1:S103-S105.Available at: care.diabetesjournals.org/cgi/content/full/26/suppl_1/s103. Accessed on January 4, 2004.
1. Vaarasmaki MS, Hartikainen A, Anttila M, Pramila S, Koivisto M. Factors predicting peri- and neonatal outcome in diabetic pregnancy. Early Hum Dev 2000;59:61-70.
2. Ray JG, O’Brien TE, Chan WS. Preconception care and the risk of congenital anomalies in the offspring of women with diabetes mellitus: a meta-analysis. QJM 2001;94:435-444.
3. Pregnancy outcomes in the Diabetes Control and Complications Trial. Am J Obstet Gynecol 1996;174:1343-1353.
4. Nachum Z, Ben-Shlomo I, Weiner E, Shalev E. Twice daily versus four times daily insulin dose regimens for diabetes in pregnancy. BMJ 1999;319:1223-1227.
5. Walkinshaw SA. Very tight versus tight control for diabetes in pregnancy (Cochrane Review). In: The Cochrane Library, Issue 4, 2003. Chichester, UK: John Wiley & Sons, Ltd. Last updated 2-15-1999. Accessed on January 4, 2004.
6. Walkinshaw SA. Dietary regulation for ‘gestational diabetes’ (Cochrane Review). In: The Cochrane Library, Issue 4, 2003. Chichester, UK: John Wiley & Sons, Ltd. Last updated 2-25-1999. Accessed on January 4, 2004.
7. West J, Walkinshaw SA. Treatments for gestational diabetes and impaired glucose tolerance in pregnancy (Cochrane Review). In: The Cochrane Library, Issue 4, 2003. Chichester, UK: John Wiley & Sons, Ltd. Last updated 9-12-2002. Accessed on January 4, 2004.
8. ACOG technical bulletin Diabetes and pregnancy. Number 200—December 1994 (replaces No. 92, May 1986). Committee on Technical Bulletins of the American College of Obstetricians and Gynecologists. Int J Gynaecol Obstet 1995;48:331-339.
9. Gestational Diabetes. ACOG Pract Bull No. 30. American College of Obstetricians and Gynecologists. Obstet Gynecol 2001;98:525-538.
10. Preconception care of women with diabetes. Diabetes Care 2004;27 Suppl 1:S76-S78.Available at: care.diabetesjournals.org/cgi/content/full/27/suppl_1/s76. Accessed on January 4, 2004.
11. Gestational diabetes mellitus. Diabetes Care 2003;26 Suppl 1:S103-S105.Available at: care.diabetesjournals.org/cgi/content/full/26/suppl_1/s103. Accessed on January 4, 2004.
Evidence-based answers from the Family Physicians Inquiries Network
How effective is prophylactic therapy for gout in people with prior attacks?
Colchicine (strength of recommendation [SOR]: B, based on 1 double-blind crossover study), allopurinol (SOR: B, based on 2 cohort studies), and weight loss (SOR: B, based on 1 small cohort study) have been shown to reduce symptomatic recurrences of gout, although the data to support their use is limited. Some evidence suggests that despite their serum uric acid–lowering effects, uricosurics (such as probenecid) fail to reduce gout attacks (SOR: B, based on 2 cohort studies). We were unable to find any double-blind, placebo-controlled long-term outcome studies addressing this problem.
Evidence summary
The majority of gout sufferers are uric acid undersecretors rather than overproducers; however, many patients will have a combination of these 2 processes, as well as caloric or purine overindulgence. Efforts to limit the frequency and intensity of gout attacks have focused on reducing the uric acid load or reducing the inflammatory response to intra-articular crystal deposition. Pharmacologic therapies include 1) uricosurics, such as probenecid, sulfinpyrazone and benzbromarone (used mostly in Europe), which increase the renal clearance of uric acid, 2) xanthine oxidase inhibitors such as allopurinol, which limit the formation of uric acid to yield a more water soluble chemical, and 3) anti-inflammatory medications, including nonsteroidal anti-inflammatory drugs (NSAIDs) and colchicine. Obesity and insulin resistance are associated with elevated uric acid, suggesting that weight loss may also help reduce episodes of gout.
A double-blinded crossover study of 38 veteran men with recurrent gout found that the addition of daily colchicine to uricosurics reduced the frequency of attacks by nearly two thirds in 6 months of follow-up.1 A cohort study of 208 men with confirmed gout who used either daily colchicine alone or colchicine with uricosurics for 2 to 10 years found marked improvements in attack frequency in both groups, yet there was no difference between the intervention groups.2 An additional study followed 734 patients (including some of the subjects in the first cohort study) and reported similar outcomes.3
Allopurinol was studied in 46 patients using prophylactic colchicine with an average follow-up of 12 months.4 Attack rates were unchanged for the first several weeks followed by a decline in the attack rate and a regression of tophi. When allopurinol was added to uricosurics in 48 patients, tophi were reduced.5
An average weight loss of 7.7 kg had a beneficial effect on serum uric acid levels and gout attack rates in 13 nondiabetic men, who were placed on a carefully controlled 1600-calorie diet with 40% of calories from complex carbohydrates.6
In a small study, the addition of uricosurics did not reduce the gout attack rate in 14 patients with nontophaceous gout.7 Patients were followed over 12 to 15 months in a crossover study of colchicine and placebo versus colchicine and sulfinpyrazone. Although this study had limited power, a larger cohort study had similar findings over a longer follow-up period.3
We were unable to find any applicable studies of daily NSAID use, dietary purine control, or alcohol reduction for the secondary prevention of gout. A prospective study of primary gout involving 47,150 men followed over 12 years noted a relative risk (RR) of gout 1.41 (95% confidence interval [CI], 1.07–1.86) in the highest quintile of meat eaters, a RR of 1.51 (95% CI, 1.17–1.95) in the highest quintile of seafood eaters, and an inverse relationship of dairy intake with gout risk.8 Thiazide diuretics appear to increase the likelihood of a gout diagnosis and if used, could be discontinued, although no studies have investigated this intervention. Most of the gout studies were performed in the 1960s using simple cohort designs and limited statistical analysis; some used combinations of medications and variable dosing. Only allopurinol appears effective in resorbing tophi5 and may have greater utility for patients with severe tophaceous gout, in those intolerant to uricosurics, in gross overproduction of uric acid, for patients with uric acid stones, or for those with renal impairment.
Recommendations from others
An expert panel, recruited by the Agency for Healthcare Research and Quality, recently published a summary combining evidence and expert opinion, which suggested that colchicine is a good prophylactic therapy and that uric acid lowering drugs (allopurinol, probenecid, and sulfinpyrazone) are effective in decreasing attack frequency in those with more than 2 attacks per year.9 Weight loss and alcohol reduction were also encouraged. A Cochrane review of this topic is scheduled for completion in 2004.
Prophylactic therapy is recommended for frequent attacks
Thuy Hanh Trinh, MD
Baylor College of Medicine, Houston, Tex
Long-term therapy is recommended when frequent gouty attacks occur. Care is warranted in the use of colchicine with erythromycin, simvastatin, and cyclosporine, since these drugs modify the excretion of colchicine, which may lead to toxic doses.10 Uric acid–lowering agents, such as allopurinol and probenecid, should be avoided in acute attacks of gout, due to potential worsening of inflammation.
Nonadherence with long-term prophylactic therapy for gout can lead to acute attacks, but patients who adhere to prophylactic therapy can still experience occasional acute breakthroughs of gout. The Cochrane review in progress may shed more insight into the prevention of acute gouty inflammation..
1. Paulus HE, Schlosstein LH, Godfrey RG, Klinenberg JR, Bluestone R. Prophylactic colchicine therapy of intercritical gout. A placebo-controlled study of probenecid-treated patients. Arthritis Rheum 1974;17:609-614.
2. Yu TF, Gutman AB. Efficacy of colchicine prophylaxis in gout. Prevention of recurrent gouty arthritis over a mean period of five years in 208 gouty subjects. Ann Intern Med 1961;55:179-192.
3. Gutman AB. Treatment of primary gout: the present status. Arthritis Rheum 1965;8:911-920.
4. Rundles RW, Metz EN, Silberman HR. Allopurinol in the treatment of gout. Ann Int Med 1966;64:229-258.
5. Kuzell WC, Seebach LM, Glover RP, Jackman AE. Treatment of gout with allopurinol and sulphinpyrazone in combination and with allopurinol alone. Ann Rheum Dis 1966;25:634-642.
6. Dessein PH, Shipton EA, Stanwix AE, Joffe BI, Ramokgadi J. Beneficial effects of weight loss associated with moderate calorie/carbohydrate restriction, and increased proportional intake of protein and unsaturated fat on serum urate and lipoprotein levels in gout: a pilot study. Ann Rheum Dis 2000;59:539-543.
7. Gaines LM, Shulman LE. The failure of uricosuric drugs to reduce the attack rate in primary non-tophaceous gout. Arthritis Rheum 1969;12:663.-
8. Choi HK, Atkinson K, Karlson EW, Willett W, Curhan G. Purine-rich foods, dairy and protein intake, and the risk of gout in men. N Engl J Med 2004;350:1093-1103.
9. Mikuls TR, MacLean CH, Olivieri J, et al. Quality of care indicators for gout management. Arthritis Rheum 2004;50:937-943.
10. Terkeltaub R. Clinical practice. Gout. N Engl J Med 2003;349:1647-1655.
Colchicine (strength of recommendation [SOR]: B, based on 1 double-blind crossover study), allopurinol (SOR: B, based on 2 cohort studies), and weight loss (SOR: B, based on 1 small cohort study) have been shown to reduce symptomatic recurrences of gout, although the data to support their use is limited. Some evidence suggests that despite their serum uric acid–lowering effects, uricosurics (such as probenecid) fail to reduce gout attacks (SOR: B, based on 2 cohort studies). We were unable to find any double-blind, placebo-controlled long-term outcome studies addressing this problem.
Evidence summary
The majority of gout sufferers are uric acid undersecretors rather than overproducers; however, many patients will have a combination of these 2 processes, as well as caloric or purine overindulgence. Efforts to limit the frequency and intensity of gout attacks have focused on reducing the uric acid load or reducing the inflammatory response to intra-articular crystal deposition. Pharmacologic therapies include 1) uricosurics, such as probenecid, sulfinpyrazone and benzbromarone (used mostly in Europe), which increase the renal clearance of uric acid, 2) xanthine oxidase inhibitors such as allopurinol, which limit the formation of uric acid to yield a more water soluble chemical, and 3) anti-inflammatory medications, including nonsteroidal anti-inflammatory drugs (NSAIDs) and colchicine. Obesity and insulin resistance are associated with elevated uric acid, suggesting that weight loss may also help reduce episodes of gout.
A double-blinded crossover study of 38 veteran men with recurrent gout found that the addition of daily colchicine to uricosurics reduced the frequency of attacks by nearly two thirds in 6 months of follow-up.1 A cohort study of 208 men with confirmed gout who used either daily colchicine alone or colchicine with uricosurics for 2 to 10 years found marked improvements in attack frequency in both groups, yet there was no difference between the intervention groups.2 An additional study followed 734 patients (including some of the subjects in the first cohort study) and reported similar outcomes.3
Allopurinol was studied in 46 patients using prophylactic colchicine with an average follow-up of 12 months.4 Attack rates were unchanged for the first several weeks followed by a decline in the attack rate and a regression of tophi. When allopurinol was added to uricosurics in 48 patients, tophi were reduced.5
An average weight loss of 7.7 kg had a beneficial effect on serum uric acid levels and gout attack rates in 13 nondiabetic men, who were placed on a carefully controlled 1600-calorie diet with 40% of calories from complex carbohydrates.6
In a small study, the addition of uricosurics did not reduce the gout attack rate in 14 patients with nontophaceous gout.7 Patients were followed over 12 to 15 months in a crossover study of colchicine and placebo versus colchicine and sulfinpyrazone. Although this study had limited power, a larger cohort study had similar findings over a longer follow-up period.3
We were unable to find any applicable studies of daily NSAID use, dietary purine control, or alcohol reduction for the secondary prevention of gout. A prospective study of primary gout involving 47,150 men followed over 12 years noted a relative risk (RR) of gout 1.41 (95% confidence interval [CI], 1.07–1.86) in the highest quintile of meat eaters, a RR of 1.51 (95% CI, 1.17–1.95) in the highest quintile of seafood eaters, and an inverse relationship of dairy intake with gout risk.8 Thiazide diuretics appear to increase the likelihood of a gout diagnosis and if used, could be discontinued, although no studies have investigated this intervention. Most of the gout studies were performed in the 1960s using simple cohort designs and limited statistical analysis; some used combinations of medications and variable dosing. Only allopurinol appears effective in resorbing tophi5 and may have greater utility for patients with severe tophaceous gout, in those intolerant to uricosurics, in gross overproduction of uric acid, for patients with uric acid stones, or for those with renal impairment.
Recommendations from others
An expert panel, recruited by the Agency for Healthcare Research and Quality, recently published a summary combining evidence and expert opinion, which suggested that colchicine is a good prophylactic therapy and that uric acid lowering drugs (allopurinol, probenecid, and sulfinpyrazone) are effective in decreasing attack frequency in those with more than 2 attacks per year.9 Weight loss and alcohol reduction were also encouraged. A Cochrane review of this topic is scheduled for completion in 2004.
Prophylactic therapy is recommended for frequent attacks
Thuy Hanh Trinh, MD
Baylor College of Medicine, Houston, Tex
Long-term therapy is recommended when frequent gouty attacks occur. Care is warranted in the use of colchicine with erythromycin, simvastatin, and cyclosporine, since these drugs modify the excretion of colchicine, which may lead to toxic doses.10 Uric acid–lowering agents, such as allopurinol and probenecid, should be avoided in acute attacks of gout, due to potential worsening of inflammation.
Nonadherence with long-term prophylactic therapy for gout can lead to acute attacks, but patients who adhere to prophylactic therapy can still experience occasional acute breakthroughs of gout. The Cochrane review in progress may shed more insight into the prevention of acute gouty inflammation..
Colchicine (strength of recommendation [SOR]: B, based on 1 double-blind crossover study), allopurinol (SOR: B, based on 2 cohort studies), and weight loss (SOR: B, based on 1 small cohort study) have been shown to reduce symptomatic recurrences of gout, although the data to support their use is limited. Some evidence suggests that despite their serum uric acid–lowering effects, uricosurics (such as probenecid) fail to reduce gout attacks (SOR: B, based on 2 cohort studies). We were unable to find any double-blind, placebo-controlled long-term outcome studies addressing this problem.
Evidence summary
The majority of gout sufferers are uric acid undersecretors rather than overproducers; however, many patients will have a combination of these 2 processes, as well as caloric or purine overindulgence. Efforts to limit the frequency and intensity of gout attacks have focused on reducing the uric acid load or reducing the inflammatory response to intra-articular crystal deposition. Pharmacologic therapies include 1) uricosurics, such as probenecid, sulfinpyrazone and benzbromarone (used mostly in Europe), which increase the renal clearance of uric acid, 2) xanthine oxidase inhibitors such as allopurinol, which limit the formation of uric acid to yield a more water soluble chemical, and 3) anti-inflammatory medications, including nonsteroidal anti-inflammatory drugs (NSAIDs) and colchicine. Obesity and insulin resistance are associated with elevated uric acid, suggesting that weight loss may also help reduce episodes of gout.
A double-blinded crossover study of 38 veteran men with recurrent gout found that the addition of daily colchicine to uricosurics reduced the frequency of attacks by nearly two thirds in 6 months of follow-up.1 A cohort study of 208 men with confirmed gout who used either daily colchicine alone or colchicine with uricosurics for 2 to 10 years found marked improvements in attack frequency in both groups, yet there was no difference between the intervention groups.2 An additional study followed 734 patients (including some of the subjects in the first cohort study) and reported similar outcomes.3
Allopurinol was studied in 46 patients using prophylactic colchicine with an average follow-up of 12 months.4 Attack rates were unchanged for the first several weeks followed by a decline in the attack rate and a regression of tophi. When allopurinol was added to uricosurics in 48 patients, tophi were reduced.5
An average weight loss of 7.7 kg had a beneficial effect on serum uric acid levels and gout attack rates in 13 nondiabetic men, who were placed on a carefully controlled 1600-calorie diet with 40% of calories from complex carbohydrates.6
In a small study, the addition of uricosurics did not reduce the gout attack rate in 14 patients with nontophaceous gout.7 Patients were followed over 12 to 15 months in a crossover study of colchicine and placebo versus colchicine and sulfinpyrazone. Although this study had limited power, a larger cohort study had similar findings over a longer follow-up period.3
We were unable to find any applicable studies of daily NSAID use, dietary purine control, or alcohol reduction for the secondary prevention of gout. A prospective study of primary gout involving 47,150 men followed over 12 years noted a relative risk (RR) of gout 1.41 (95% confidence interval [CI], 1.07–1.86) in the highest quintile of meat eaters, a RR of 1.51 (95% CI, 1.17–1.95) in the highest quintile of seafood eaters, and an inverse relationship of dairy intake with gout risk.8 Thiazide diuretics appear to increase the likelihood of a gout diagnosis and if used, could be discontinued, although no studies have investigated this intervention. Most of the gout studies were performed in the 1960s using simple cohort designs and limited statistical analysis; some used combinations of medications and variable dosing. Only allopurinol appears effective in resorbing tophi5 and may have greater utility for patients with severe tophaceous gout, in those intolerant to uricosurics, in gross overproduction of uric acid, for patients with uric acid stones, or for those with renal impairment.
Recommendations from others
An expert panel, recruited by the Agency for Healthcare Research and Quality, recently published a summary combining evidence and expert opinion, which suggested that colchicine is a good prophylactic therapy and that uric acid lowering drugs (allopurinol, probenecid, and sulfinpyrazone) are effective in decreasing attack frequency in those with more than 2 attacks per year.9 Weight loss and alcohol reduction were also encouraged. A Cochrane review of this topic is scheduled for completion in 2004.
Prophylactic therapy is recommended for frequent attacks
Thuy Hanh Trinh, MD
Baylor College of Medicine, Houston, Tex
Long-term therapy is recommended when frequent gouty attacks occur. Care is warranted in the use of colchicine with erythromycin, simvastatin, and cyclosporine, since these drugs modify the excretion of colchicine, which may lead to toxic doses.10 Uric acid–lowering agents, such as allopurinol and probenecid, should be avoided in acute attacks of gout, due to potential worsening of inflammation.
Nonadherence with long-term prophylactic therapy for gout can lead to acute attacks, but patients who adhere to prophylactic therapy can still experience occasional acute breakthroughs of gout. The Cochrane review in progress may shed more insight into the prevention of acute gouty inflammation..
1. Paulus HE, Schlosstein LH, Godfrey RG, Klinenberg JR, Bluestone R. Prophylactic colchicine therapy of intercritical gout. A placebo-controlled study of probenecid-treated patients. Arthritis Rheum 1974;17:609-614.
2. Yu TF, Gutman AB. Efficacy of colchicine prophylaxis in gout. Prevention of recurrent gouty arthritis over a mean period of five years in 208 gouty subjects. Ann Intern Med 1961;55:179-192.
3. Gutman AB. Treatment of primary gout: the present status. Arthritis Rheum 1965;8:911-920.
4. Rundles RW, Metz EN, Silberman HR. Allopurinol in the treatment of gout. Ann Int Med 1966;64:229-258.
5. Kuzell WC, Seebach LM, Glover RP, Jackman AE. Treatment of gout with allopurinol and sulphinpyrazone in combination and with allopurinol alone. Ann Rheum Dis 1966;25:634-642.
6. Dessein PH, Shipton EA, Stanwix AE, Joffe BI, Ramokgadi J. Beneficial effects of weight loss associated with moderate calorie/carbohydrate restriction, and increased proportional intake of protein and unsaturated fat on serum urate and lipoprotein levels in gout: a pilot study. Ann Rheum Dis 2000;59:539-543.
7. Gaines LM, Shulman LE. The failure of uricosuric drugs to reduce the attack rate in primary non-tophaceous gout. Arthritis Rheum 1969;12:663.-
8. Choi HK, Atkinson K, Karlson EW, Willett W, Curhan G. Purine-rich foods, dairy and protein intake, and the risk of gout in men. N Engl J Med 2004;350:1093-1103.
9. Mikuls TR, MacLean CH, Olivieri J, et al. Quality of care indicators for gout management. Arthritis Rheum 2004;50:937-943.
10. Terkeltaub R. Clinical practice. Gout. N Engl J Med 2003;349:1647-1655.
1. Paulus HE, Schlosstein LH, Godfrey RG, Klinenberg JR, Bluestone R. Prophylactic colchicine therapy of intercritical gout. A placebo-controlled study of probenecid-treated patients. Arthritis Rheum 1974;17:609-614.
2. Yu TF, Gutman AB. Efficacy of colchicine prophylaxis in gout. Prevention of recurrent gouty arthritis over a mean period of five years in 208 gouty subjects. Ann Intern Med 1961;55:179-192.
3. Gutman AB. Treatment of primary gout: the present status. Arthritis Rheum 1965;8:911-920.
4. Rundles RW, Metz EN, Silberman HR. Allopurinol in the treatment of gout. Ann Int Med 1966;64:229-258.
5. Kuzell WC, Seebach LM, Glover RP, Jackman AE. Treatment of gout with allopurinol and sulphinpyrazone in combination and with allopurinol alone. Ann Rheum Dis 1966;25:634-642.
6. Dessein PH, Shipton EA, Stanwix AE, Joffe BI, Ramokgadi J. Beneficial effects of weight loss associated with moderate calorie/carbohydrate restriction, and increased proportional intake of protein and unsaturated fat on serum urate and lipoprotein levels in gout: a pilot study. Ann Rheum Dis 2000;59:539-543.
7. Gaines LM, Shulman LE. The failure of uricosuric drugs to reduce the attack rate in primary non-tophaceous gout. Arthritis Rheum 1969;12:663.-
8. Choi HK, Atkinson K, Karlson EW, Willett W, Curhan G. Purine-rich foods, dairy and protein intake, and the risk of gout in men. N Engl J Med 2004;350:1093-1103.
9. Mikuls TR, MacLean CH, Olivieri J, et al. Quality of care indicators for gout management. Arthritis Rheum 2004;50:937-943.
10. Terkeltaub R. Clinical practice. Gout. N Engl J Med 2003;349:1647-1655.
Evidence-based answers from the Family Physicians Inquiries Network