User login
Managing risk—to mother and fetuses—in a twin gestation
Multiple gestations are far more common today than they once were—up 70% since 1980.1 Today, every 1,000 live births include 32.3 sets of twins, a 2% increase in the rate of twin births since 2004. Why this phenomenon is occurring is not entirely understood but, certainly, the trend toward older maternal age and the emergence of assisted reproduction are both part of the explanation.
Multiple gestations are of particular concern to obstetricians because, even though they remain relatively rare, they are responsible for a significant percentage of perinatal morbidity and mortality.
The difficulties that twins encounter are often associated with preterm birth and occur most often in identical twins developing within a single gestational sac. Those difficulties include malformation, chromosomal abnormalities, learning disability, behavioral problems, chronic lung disease, neuromuscular developmental delay, cerebral palsy, and stillbirth. Women pregnant with twins are also at heightened risk, particularly of gestational hypertension, preeclampsia, and gestational diabetes.2
Your task is to manage these risks so that the outlook for mother and infant is as favorable as possible.
Determining chorionicity in the first trimester
Ultrasonographic determination of chorionicity should be the first step in the management of a twin gestation. The determination should be made as early as possible in the pregnancy because it has an immediate impact on counseling, risk of miscarriage, and efficacy of noninvasive screening. (See “Is there 1 sac, or more? Key to predicting risk.”)
The accuracy of ultrasonography (US) in determining chorionicity depends on gestational age. US predictors of dichorionicity include:
- gender discordance
- separate placentas
- the so-called twin-peak sign (also called the lambda sign) (FIGURE 1), in which the placenta appears to extend a short distance between the gestational sacs; compare this with FIGURE 2, showing monochorionic twins with the absence of an intervening placenta
- an intertwin membrane thicker than 1.5 mm to 2.0 mm.
US examination can accurately identify chorionicity at 10 to 14 weeks’ gestation, with overall sensitivity that is reported to be as high as 100%.3-5
FIGURE 1
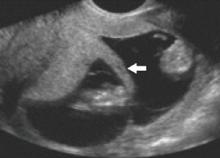
The twin-peak sign on US
This dichorionic–diamnionic twin gestation demonstrates the so-called twin peak, or lambda, sign (arrow), in which the placenta appears to extend a short distance between the gestational sacs.
FIGURE 2
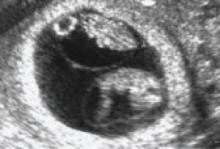
Monochorionic–diamnionic twin gestation
This US scan of a monochorionic twin gestation reveals the absence of an intervening placenta
Monochorionic twins
Twins who share a gestational sac are more likely than 2-sac twins to suffer spontaneous loss, congenital anomalies, growth restriction and discordancy, preterm delivery, and neurologic morbidity.
Spontaneous loss. In 1 comparative series, the risk of pregnancy loss at less than 24 weeks’ gestation was 12.2% for monochorionic twins, compared with 1.8% for dichorionic twins.6 Spontaneously conceived monochorionic twins may have the highest risk of loss.7 However, monochorionic twins occur more often in conceptions achieved by assisted reproductive technology—at a rate 3 to 10 times higher than the background rate of monochorionic twinning.8
- A multiple gestation involves a higher level of risk than a singleton pregnancy
- Chorionicity is the basis for determining risk. Twins within a single sac (monochorionic) are at higher risk of malformation, Down syndrome, and premature birth
- The risk of Down syndrome can be estimated by noninvasive screening in the first trimester and by chorionic villus sampling or amniocentesis later in the pregnancy
- A detailed anatomic survey at 18 to 20 weeks’ gestation should be done to detect possible malformations
- Assessment of cervical length, performed every 2 weeks from the 16th to the 28th week, may help predict premature delivery—but is not definitive
- Assessment of fetal growth every 4 weeks in dichorionic twins and every 2 weeks in monochorionic twins can alert you to potential problems. This is particularly important for detecting signs of twin-to-twin transfusion syndrome
Congenital anomalies. These occur 2 to 3 times as often in monochorionic twins, and have been reported in as many as 10% of such pregnancies. Reported anomalies include midline defects, cloacal abnormalities, neural tube defects, ventral wall defects, craniofacial abnormalities, conjoined twins, and acardiac twins.9-11 In light of these risks, a detailed anatomic survey is suggested for all twins.
Heart defects. The incidence of congenital heart defects is 4 times greater in monochorionic twins, even in the absence of twin-to-twin transfusion syndrome (TTTS).12 Cardiac malformations may occur secondary to abnormal lateralization during embryogenesis or result from an abnormal vascular distribution in the shared placenta.9,10 The presence of abnormal vascular communications may also cause limb reduction defects and the rare acardiac twin.
Long-term neurologic morbidity. In one series, the incidence of cerebral palsy was 8%, compared with 1% among dichorionic twins. In twins followed to 2 years of age, rates of minor neurologic morbidity were 15% in monochorionic twins and 3% in dichorionic twins. The overall rate of neurologic disorders in monochorionic twins was 23%, regardless of fetal weight.13 At 4 years, long-term neurologic morbidity was particularly high in single survivors of a monochorionic pair; the incidence of cerebral palsy has been reported to be as high as 50% in single survivors, compared with 14.3% in cases in which both twins survived.14
Twin-to-twin transfusion syndrome. In this condition, abnormal vascular connections arise in the shared placenta, allowing blood to be shunted from one fetus to the other. The syndrome is unique to monochorionic gestations and occurs in 15% to 20% of cases.15 A significant percentage of neurologic morbidity is probably the result of TTTS. To evaluate for TTTS, include a detailed anatomic survey and serial US every 2 weeks beginning in the second trimester as part of the surveillance of monochorionic twin gestations. (See “TTTS: Diagnosis, staging, treatment.”)
Twin-related morbidity and mortality are directly related to chorionicity. Twin embryos in a single chorion (monochorionic twins) have a higher rate of perinatal morbidity and mortality than do twins in separate sacs (dichorionic twins). To some extent, the higher risk faced by monochorionic twins—of twin-to-twin transfusion syndrome and certain structural and chromosomal abnormalities, for example—is the result of complications uniquely related to having a single placenta. But recent evidence also suggests that the higher risk of adverse outcomes is associated with monochorionicity itself, independent of the complications attributable to the single placenta.1
When twins develop in separate chorionic sacs, the risks are not as great. All fraternal twins (approximately 2/3 of all twins) are dichorionic and, therefore, at lower risk of an adverse outcome. The situation is more complex with identical (monozygotic) twins, however: Most (70%) are monochorionic, but approximately one third (30%) have separate chorionic sacs and are therefore dichorionic.
Reference
1. Leduc L, Takser L, Rinfret D. Persistence of adverse obstetric and neonatal outcomes in monochorionic twins after exclusion of disorders unique to monochorionic placentation. Am J Obstet Gynecol. 2005;193:1670-1675.
Down syndrome and other chromosomal abnormalities
Estimating odds
Assessing the likelihood of a chromosomal abnormality (aneuploidy) in a multiple gestation is complicated by differences in twinning mechanisms (chorionicity versus zygosity) and by the increasing rate of dizygotic twinning with advancing maternal age. The risk is greater in dizygotic twin gestations than in age-matched singleton gestations. The definition of advanced maternal age (AMA) in a twin pregnancy has ranged from 31 to 33 years of age in reports in the literature.2,16,17
The probability that a twin gestation contains a fetus with a chromosomal abnormality is directly related to zygosity. Each twin in a dizygotic gestation carries an independent risk, so the composite risk for the pregnancy is a summation of the independent risk for each fetus. For monozygotic twins, the risk is similar to the age-related risk in a singleton gestation. Presumptions about zygosity are based on chorionicity: Almost all (90%) dichorionic twins are dizygotic and all monochorionic twins are monozygotic.
What is the utility of noninvasive screening?
Multiple gestations can be screened for aneuploidy using maternal age, maternal serum markers, and nuchal translucency (NT) on US, or combinations of these assessments.
When first-trimester serum markers (free β-human chorionic gonadotropin and pregnancy-associated plasma protein A [PAPPA]) are combined with NT and maternal age, a pregnancy-specific risk can be calculated that includes the individual contribution of each fetus, thus yielding an improved detection rate. In monochorionic twins, the NTs are averaged to calculate a single risk for the entire pregnancy. In dichorionic twins, the risk for each fetus is calculated independently and then summed to establish a pregnancy-specific risk. The combined test has a reported detection rate of 84% for monochorionic twins and 70% for dichorionic twins, compared with detection rates of 85% to 87% for singletons at a 5% false-positive rate.18,19 The integrated test (combined test plus measurement of second-trimester serum analytes) has a 93% detection rate for monochorionic twins and a 78% detection rate for dichorionic twins, compared with 95% to 96% for singletons at the same 5% false-positive rate.18,19 Second-trimester screening has a lower detection rate in both singleton and twin gestations.
The diagnosis of twin-to-twin transfusion syndrome (TTTS) depends on the presence of a single monochorionic placenta and abnormalities in the volume of amniotic fluid (the polyhydramnios–oligohydramnios sequence). The syndrome may have an abrupt or gradual onset, heralded by discordancy and restriction in the growth of the 2 fetuses.
The natural history of the syndrome and treatment outcome are based on a staging system described by Quintero and colleagues1:
Stage I is characterized by polyhydramnios–oligohydramnios with the bladder still visible in the donor twin
Stage II The donor bladder is no longer visible
Stage III is defined by abnormal Doppler studies showing absent or reversed flow in the umbilical artery, reversed flow in the ductus venosus, or pulsatile umbilical venous flow
Stage IV is indicated by hydrops in either twin
Stage V One or both twins die.
The prognosis for TTTS grows poorer with increasing stage and is poor if the condition goes untreated, with a reported survival rate of only 25% to 50% for 1 twin when the diagnosis is made in the second trimester.2,3 Treatment options include removal of excess amniotic fluid through serial amniocenteses (amnioreduction), fetoscopic laser coagulation of communicating vessels, selective fetocide, and perforation of the membrane that separates the twins (septostomy).
Serial amnioreduction is the most common procedure for treating TTTS. When Senat and colleagues compared the efficacy of serial amnioreduction with fetoscopic laser occlusion in a randomized control trial, however, they found that the laser group had a significantly higher likelihood of survival of at least 1 twin (76%) than the amnioreduction group (56%).4
Septostomy. A recently published randomized trial in which amnioreduction was compared with septostomy found no difference in survival between the 2 treatments.5 Septostomy often has the advantage of requiring only 1 procedure to be successful, whereas repeated amniocenteses are necessary in serial amnioreduction. Septostomy does carry the risk of creating a single amnion, as the size of the membranous defect created by the perforation is difficult to control.
Selective fetocide using US-guided cord occlusion or radiofrequency ablation has been described when there is a coexisting fetal anomaly, growth restriction, or a chromosomal abnormality in 1 twin (heterokaryotypia).6,7 Use of bipolar coagulation in this setting has been associated with a liveborn in 83% of cases and intact neurologic survival in 70%.7 Radiofrequency ablation has also been described for selective fetal termination in monochorionic placentation with an abnormality in 1 twin.6 Data presented at the 2006 annual meeting for the Society for Maternal–Fetal Medicine showed no difference in the overall complication rate between these 2 techniques of selective fetocide.8
References
1. Quintero RA, Morales WJ, Allen MH, Bornick PW, Johnson PK, Kruger M. Staging of twin–twin transfusion syndrome. J Perinatol. 1999;19:550-555.
2. Berghella V, Kaufman M. Natural history of twin–twin transfusion syndrome. J Reprod Med. 2001;46:480-484.
3. Bromley B, Frigoletto FD, Setroff JA, Benacerraf BR. The natural history of oligohydramnios/polyhydramnios sequence in monochorionic diamniotic twins. Ultrasound Obstet Gynecol. 1992;2:317-320.
4. Senat MV, Deprest J, Boulvain M, Paupe A, Winer N, Ville Y. Endoscopic laser surgery versus serial amnioreduction for severe twin to twin transfusion syndrome. N Engl J Med. 2004;351:136-144.
5. Moise KJ, Dorman K, Lamvu G, et al. A randomized trial of amnioreduction versus septostomy in the treatment of twin–twin transfusion syndrome. Am J Obstet Gynecol. 2005;193:701-707.
6. Robyr R, Yamamoto M, Ville Y. Selective feticide in complicated monochorionic twin pregnancies using ultrasound-guided bipolar cord coagulation. BJOG. 2005;112:1344-1348.
7. Shevell T, Malone FD, Weintraub J, Harshwardhan MT, D’Alton ME. Radiofrequency ablation in a monochorionic twin discordant for fetal anomalies. Am J Obstet Gynecol. 2004;190:575-576.
8. Bebbington M, Danzer E, Johnson M, Wilson RD. RFA vs cord coagulation in complex monochorionic pregnancies. Am J Obstet Gynecol. 2006;195:S192.-
Prenatal diagnosis
Given the lower detection rate of aneuploidy in twin gestations and the associated increase in aneuploidy with advancing maternal age, many patients choose to undergo prenatal diagnosis rather than relying on screening. On the basis of maternal age alone, invasive prenatal diagnosis can be offered to women who will be 31 years or older at their estimated due date.
Available diagnostic options include chorionic villus sampling (CVS) or amniocentesis. CVS is performed at an earlier gestational age (10 to 13 weeks) than amniocentesis (15 to 20 weeks). Multiples pose specific technical considerations for either procedure, and accurate fetal mapping is essential. Successful sampling with CVS can be performed in more than 99% of cases; the rate of cross-contamination is less than 1%.
Is there a risk of miscarriage?
In counseling patients about the risk of fetal death that CVS or amniocentesis may entail, the place to begin is the background loss rate, which is greater in twin than in single gestations. The reported background loss rate of twins at 24 weeks’ gestation or less ranges from 5.8% to 7.2%.20,21 In women of advanced maternal age (35 years and older), a background rate as high as 17.6% has been described.21 Once parents are aware of this, they have a context for weighing the risk of miscarriage that prenatal testing may hold.
The twin loss rate following amniocentesis has been evaluated in several studies. (See “Integrating evidence and experience: Does invasive prenatal testing raise the risk of miscarriage in a twin gestation?”)
A greatly elevated risk of preterm birth
Multiple gestations are at extremely high risk for premature delivery, and—like all premature newborns—these infants are at risk for a wide range of disabilities. Risk factors for premature delivery include history of second trimester pregnancy loss, preterm birth at less than 35 weeks’ gestation, more than 2 previous curettage procedures, cone biopsy, müllerian anomaly, and diethylstilbestrol exposure. Unfortunately, current yardsticks for predicting premature delivery in multiple pregnancy have serious limitations, and available interventions have not been particularly successful.
Predictors
Measurement of cervical length has been evaluated as a predictor of preterm delivery in a number of twin studies that were looking for a cutoff point that can predict which twins are at greatest risk. No such cutoff has been found.22-25
In general, studies demonstrate a low risk of preterm delivery for women who have a cervical length measurement of more than 35 mm at 24 to 26 weeks. A shorter cervical length correlates with premature delivery, but specific cutoffs have proved not to be sensitive predictors.
In the largest published series, To and colleagues evaluated 1,163 sets of twins undergoing routine care with cervical length assessments at 22 to 24 weeks’ gestation. They demonstrated a direct correlation between cervical length and preterm delivery, but were unable to define a cutoff sufficiently sensitive to be useful.25 A shortened cervix may be predictive of prematurity in general, but it does not allow the obstetrician to predict with certainty which mothers will give birth prematurely or how long a particular mother will carry.
Fetal fibronectin. The presence of fetal fibronectin (ffN) in cervicovaginal secretions is widely used as an adjunct to other potential predictors of preterm delivery. In a multistudy review that included symptomatic women, a negative ffN had a 99% negative predictive value but a poor positive predictive value (13% to 30%) for delivery within 7 to 10 days.26
Use of ffN in conjunction with cervical length has also been investigated in twin gestations. Although the negative predictive value of ffN remained high, the addition of ffN to cervical length assessment did not improve the positive predictive value of cervical length alone.27,28
Interventions
Cerclage is often used in high-risk singleton pregnancies in which a shortened cervix is seen on a sonogram. The utility of cerclage in twins is less clear. Randomized controlled trials comparing women at risk of premature delivery treated with cerclage and controls not considered at risk found no difference in the rate of premature delivery in the 2 groups.29,30 Meta-analysis of 4 randomized controlled trials also found no benefit and, in fact, detected a possibility of actual harm. Cerclage twins were more likely to deliver early (at less than 35 weeks’ gestation) and had a 2.6 relative risk of perinatal mortality. The differences found in the meta-analysis were not statistically significant, however, and the overall sample size was small (n=48).31
The best available data seem to show that cerclage based on US indications of cervical shortening is not beneficial and may even be associated with worse outcome.
17-Hydroxyprogesterone caproate (17P) has been found to decrease the rate of recurrent preterm birth in singleton gestations by almost 35%.32 Although twins are at increased risk of preterm birth, the use of 17P has not, however, been shown to be of benefit.33
Bed rest. A Cochrane Database review of 6 randomized controlled trials compared 1) patients with a multiple gestation who were offered bed rest in the hospital with 2) patients hospitalized for complications of pregnancy. The review found that bed rest did not reduce the risk of preterm birth or of perinatal mortality in the routinely hospitalized women. There was, however, a tendency to a decreased number of low-birth-weight infants born to women given bed rest.34
INTEGRATING EVIDENCE AND EXPERIENCE
The evidence for amniocentesis
Toth-Pal and colleagues compared the twin loss rate after amniocentesis in 155 twin pairs; twins who had a structural anomaly or aneuploidy were excluded. The investigators found a 3.87% loss rate at 24 weeks or less, compared with a background loss rate of 2.39% in twins who did not undergo the procedure—an insignificant difference.1
Yukobowich and colleagues compared 476 diamniotic, dichorionic twin pairs that had undergone amniocentesis with 1) 477 twin pairs undergoing routine US examination and 2) 489 singleton amniocenteses. They found a 4-week postprocedure loss rate of 2.7% in the amniocentesis twins, compared with 0.63% in twin controls who had routine US and 0.6% in the amniocentesis singletons.2 The difference is significant, but the reported loss rate is still less than, or comparable to, the reported background twin loss rate at 24 weeks or less.
Chorionic villus sampling
Only a few studies of the loss rate in twins after CVS have been published, but those that are available report a loss rate lower than, or comparable to, the background rate for twins generally. In a series of 169 twin pairs undergoing CVS at an average gestational age of 10 weeks, the risk of loss at 20 weeks or more was 1.7%.3
CVS and amniocentesis, in tandem
Although CVS and amniocentesis are not directly comparable given the difference in the timing of procedure, a few series have compared the risk of loss for the 2 procedures. Eighty-one twin pairs that underwent amniocentesis were compared with 161 twins undergoing CVS. The rate of spontaneous delivery at less than 28 weeks was 2.9% for amniocentesis, compared with 3.2% following CVS.4
To sum up
Invasive testing does not appear to increase the risk of fetal loss above the background loss rate for twins overall. Prenatal diagnosis as early as 10 weeks is a feasible option in a twin gestation, given the limitations of screening in multiple gestations.
References
1. Toth-Pal E, Papp C, Beke A, Ban Z, Papp Z. Genetic amniocentesis in multiple pregnancy. Fetal Diagn Ther. 2004;19:138-144.
2. Yukobowich E, Anteby EY, Cohen SM, Lavy Y, Granat M, Yagel S. Risk of fetal loss in twin pregnancies undergoing second trimester amniocentesis. Obstet Gynecol. 2001;98:231-234.
3. Brambati B, Tului L, Guercilena S, Alberti E. Outcome of first-trimester chorionic villus sampling for genetic investigation in multiple pregnancy. Ultrasound Obstet Gynecol. 2001;17:209-216.
4. Wapner RJ, Johnson A, Davis G, Urban A, Morgan P, Jackson L. Prenatal diagnosis in twin gestations: a comparison between second trimester amniocentesis and first trimester chorionic villus sampling. Obstet Gynecol. 1993;82:49-56.
Some parents elect fetal reduction
Given the high level of risk in a multiple pregnancy, reducing the number of fetuses is an option that some patients choose. A recent trend toward reduction to singleton pregnancy seems to be related to:
- increasing maternal age
- single parenthood
- financial considerations
- the increased medical risk to mother and fetuses associated with twins.35
Evans and colleagues found that, although the overall rate of reduction from twins to a singleton was 3%, the percentage (76%) of women older than 35 years who opted for such a reduction was disproportionately high.36
In a series of 1,000 cases of multifetal pregnancy reduction, Stone et al found that the pregnancy loss rate was lowest (2.5%) when there was reduction to a singleton gestation.37 A comparative analysis of 2,000 cases of multifetal pregnancy reduction presented at the 2006 meeting of the Society for Maternal–Fetal Medicine found that the percentage of twin gestations undergoing reduction to a singleton has increased from 4% to 15.6% between 1999 and 2006, with an increase in the overall incidence of reduction to a singleton from 11.8% to 31.8%.38
You should discuss pregnancy reduction with patients at high risk of pregnancy-associated complications such as cervical incompetence, preterm delivery, severe maternal cardiac disease, hypertension, diabetes, and uterine anomalies, as well as with patients who are carrying higher-order multiple gestations, in which the fetuses are at risk of problems.
Wrap-up: The tasks facing you in a multiple gestation
Start your management of a multiple gestation by taking the essential first step of determining the chorionicity of the fetuses.
Once you have done that, explain the risks of twin pregnancy and the particular risks of a single-sac pregnancy. Parents will want to know their risk of having a child with an anomaly; pay particular attention to the likelihood of a Down syndrome child. Noninvasive screening for Down syndrome and other chromosomal anomalies may be sufficient, but an older mother may prefer more definitive answers from CVS or amniocentesis.
You must prepare parents of a twin gestation for the risk of premature delivery. Cervical length assessment in the second trimester to early-third trimester may provide some indications of what is to happen, but the predictive value of this procedure is limited, and a shortened cervical length should be interpreted with caution.
In a monochorionic pregnancy, fetal growth should be assessed at regular intervals to evaluate for possible growth restriction or TTTS. If you detect evidence of abnormal fetal growth or amniotic fluid, US surveillance is indicated. Routine antepartum US surveillance of twins is not, however, recommended.2
The authors report no financial relationships relevant to this article.
1. Martin JA, Hamilton BE, Sutton PD, Ventura SJ, Menacker F, Kirmeyer S. Births: final data 2004; Natl Vital Stat Rep. 2006;55:1-101.
2. Multiple gestation: Complicated twin, triplet and high-order multifetal pregnancy. ACOG Practice Bulletin; 2004. No. 56.
3. Sepulveda W, Sebire NJ, Hughes K, Odibo A, Nicolaides KH. The lambda sign at 10–14 weeks of gestation as a predictor of chorionicity in twin pregnancies. Ultrasound Obstet Gynecol. 1996;7:421-423.
4. Carroll SGM, Soothill PW, Abdel-Fattah SA, Porter H, Montague I, Kyle PM. Prediction of chorionicity in twin pregnancies at 10–14 weeks of gestation. Br J Obstet Gynaecol. 2002;109:182-186.
5. Stenhouse E, Hardwick C, Maharaj S, Webb J, Kelly T, Mackenzie FM. Chorionicity determination in twin pregnancies; how accurate are we? Ultrasound Obstet Gynecol. 2002;19:350-352.
6. Sebire NJ, Snijders RJ, Hughes K, Sepulveda W, Nicolaides KH. The hidden mortality of monochorionic twin pregnancies. Br J Obstet Gynaecol. 1997;104:1203-1207.
7. Sperling L, Kiil C, Larsen LU, et al. Naturally conceived twins with monochorionic placentation have the highest risk of fetal loss. Ultrasound Obstet Gynecol. 2006;28:644-652.
8. Trevett T, Johnson A. Monochorionic twin pregnancies. Clin Perinatol. 2005;32:475-494.
9. Rustico MA, Baietti MG, Coviello D, Orlandi E, Nicolini U. Managing twins discordant for fetal anomaly. Prenat Diagn. 2005;25:766-771.
10. Hall JG. Developmental biology IV. Lancet. 2003;362:735-743.
11. Mohammed SN, Swan MC, Wall SA, Wilkie AO. Monozygotic twins discordant for frontonasal malformation. Am J Med Genet A. 2004;130:384-388.
12. Karatza AA, Wolfenden JL, Taylor MJ, Wee L, Fisk NM, Gardiner HM. Influence of twin–twin transfusion syndrome on fetal cardiovascular structure and function; prospective case-control study of 136 monochorionic twin pregnancies. Heart. 2002;88:271-277.
13. Adegbite AL, Castille S, Ward S, Bajoria R. Neuromorbidity in preterm twins in relation to chorionicity and discordant birth weight. Am J Obstet Gynecol. 2004;190:156-163.
14. Lopriore E, Nagel HTC, Vandenbussche FPHA, Walther FJ. Long-term neurodevelopmental outcome in twin–twin transfusion syndrome. Am J Obstet Gynecol. 2003;189:1314-1319.
15. Bromley B, Frigoletto FD, Setroff JA, Benacerraf BR. The natural history of oligohydramnios/polyhydramnios sequence in monochorionic diamniotic twins. Ultrasound Obstet Gynecol. 1992;2:317-320.
16. Rodis JF, Egan JFX, Craffey A, Ciarleglio L, Greenstein RM, Scorza WE. Calculated risk of chromosomal abnormalities in twin gestations. Obstet Gynecol. 1990;76:1037-1041.
17. Meyers C, Adam R, Dungan J, Prenger V. Aneuploidy in twin gestations; when is maternal age advanced? Obstet Gynecol. 1997;89:248-251.
18. Wald J, Rish S. Prenatal screening for Down syndrome and neural tube defects in twin pregnancies. Prenat Diagn. 2005;25:740-745.
19. Malone FD, Canick JA, Ball RH, et al. The First- and Second-Trimester Evaluation of Risk (FASTER) Research Consortium. First trimester or second trimester screening, or both, for Down’s syndrome. N Engl J Med. 2005;353:2001-2011.
20. Yaron Y, Bryant-Greenwood PK, Dave N, et al. Multifetal pregnancy reduction of triplets to twins: comparison with nonreduced triplets and twins. Am J Obstet Gynecol. 1999;180:1268-1271.
21. La Sala GB, Nucera G, Gallinelli A, Nicoli A, Villani MT, Blickstein I. Spontaneous embryonic loss following in vitro fertilization: incidence and effect on outcomes. Am J Obstet Gynecol. 2004;191:741-746.
22. Imseis HM, Albert TA, Iams JD. Identifying twin gestations at low risk for preterm birth with a transvaginal ultrasonographic cervical measurement at 24 to 26 weeks’ gestation. Am J Obstet Gynecol. 1997;177:1149-1155.
23. Vayssiere C, Favre R, Audibert F, et al. Cervical length and funneling at 22 and 27 weeks to predict spontaneous birth before 32 weeks in twin pregnancies: a French prospective multicenter study. Am J Obstet Gynecol. 2002;187:1596-1604.
24. Guzman ER, Walters C, O’Reilly-Green C, et al. Use of cervical ultrasonography in prediction of spontaneous preterm birth in twin gestations. Am J Obstet Gynecol. 2000;183:1103-1107.
25. To MS, Fonseca EB, Molina FS, Cacho AM, Nicolaides KH. Maternal characteristics and cervical length in prediction of spontaneous early preterm delivery in twins. Am J Obstet Gynecol. 2006;194:1360-1365.
26. Honest H, Bachmann LM, Gupta JK, Kleijnen J, Khan KS. Accuracy of cervicovaginal fetal fibronectin test in predicting risk of spontaneous preterm birth: systematic review. BMJ. 2002;325:301.-
27. Goldenberg RL, Iams JD, Miodovnik M, et al. The preterm prediction study: risk factors in twin gestations. National Institute of Child Health and Human Development Maternal–Fetal Medicine Units Network. Am J Obstet Gynecol. 1996;175:1047-1053.
28. Gibson JL, Macara LM, Owen P, Young D, Macauley J, Mackenzie F. Prediction of preterm delivery in twin pregnancy: a prospective, observational study of cervical length and fetal fibronectin testing. Ultrasound Obstet Gynecol. 2004;23:561-566.
29. Berghella V, Odibo AO, Tolosa JE. Cerclage for prevention of preterm birth in women with a short cervix found on transvaginal ultrasound examination: a randomized trial. Am J Obstet Gynecol. 2004;191:1311-1317.
30. Rust OA, Atlas RO, Reed J, van Gaalen J, Baldussi J. Revisiting the short cervix detected by transvaginal ultrasound in the second trimester: why cerclage therapy may not help. Am J Obstet Gynecol. 2001;185:1098-1105.
31. Berghella V, Odibo AO, To MS, Rust OA, Althuisius SM. Cerclage for short cervix on ultrasonography: meta-analysis of trials using individual patient-level data. Obstet Gynecol. 2005;106:181-189.
32. Meis PJ, Klebanoff M, Thom E, et al. Prevention of recurrent preterm delivery by 17 alpha-hydroxyprogesterone caproate. N Engl J Med. 2003;348:2379-2385.
33. Caritis S, Rouse D. NICHD MFMU Network. A randomized controlled trial of 17-hydroxyprogesterone caproate for the prevention of preterm birth in twins. Am J Obstet Gynecol. 2006;195:S2.-
34. Crowther CA. Hospitalisation and bed rest for multiple pregnancy. Cochrane Database Syst Rev. 2001;(1):CD000110.-
35. Evans MI, Ciorica D, Britt DW, Fletcher JC. Update on selective reduction. Prenat Diagn. 2005;25:807-813.
36. Evans MI, Kaufman MI, Urban AJ, Britt DW, Fletcher JC. Fetal reduction from twins to a singleton: a reasonable consideration. Obstet Gynecol. 2004;104:102-109.
37. Stone J, Eddleman K, Lynch L, Berkowitz RL. A single center experience with 1000 consecutive cases of multifetal pregnancy reduction. Am J Obstet Gynecol. 2002;187:1163-1167.
38. Stone J, Matho A, Berkowitz R, Belogolovkin V, Eddleman K. Evolving trends in 2,000 cases of multifetal pregnancy reduction. Am J Obstet Gynecol. 2006;195:S184.-
Multiple gestations are far more common today than they once were—up 70% since 1980.1 Today, every 1,000 live births include 32.3 sets of twins, a 2% increase in the rate of twin births since 2004. Why this phenomenon is occurring is not entirely understood but, certainly, the trend toward older maternal age and the emergence of assisted reproduction are both part of the explanation.
Multiple gestations are of particular concern to obstetricians because, even though they remain relatively rare, they are responsible for a significant percentage of perinatal morbidity and mortality.
The difficulties that twins encounter are often associated with preterm birth and occur most often in identical twins developing within a single gestational sac. Those difficulties include malformation, chromosomal abnormalities, learning disability, behavioral problems, chronic lung disease, neuromuscular developmental delay, cerebral palsy, and stillbirth. Women pregnant with twins are also at heightened risk, particularly of gestational hypertension, preeclampsia, and gestational diabetes.2
Your task is to manage these risks so that the outlook for mother and infant is as favorable as possible.
Determining chorionicity in the first trimester
Ultrasonographic determination of chorionicity should be the first step in the management of a twin gestation. The determination should be made as early as possible in the pregnancy because it has an immediate impact on counseling, risk of miscarriage, and efficacy of noninvasive screening. (See “Is there 1 sac, or more? Key to predicting risk.”)
The accuracy of ultrasonography (US) in determining chorionicity depends on gestational age. US predictors of dichorionicity include:
- gender discordance
- separate placentas
- the so-called twin-peak sign (also called the lambda sign) (FIGURE 1), in which the placenta appears to extend a short distance between the gestational sacs; compare this with FIGURE 2, showing monochorionic twins with the absence of an intervening placenta
- an intertwin membrane thicker than 1.5 mm to 2.0 mm.
US examination can accurately identify chorionicity at 10 to 14 weeks’ gestation, with overall sensitivity that is reported to be as high as 100%.3-5
FIGURE 1
The twin-peak sign on US
This dichorionic–diamnionic twin gestation demonstrates the so-called twin peak, or lambda, sign (arrow), in which the placenta appears to extend a short distance between the gestational sacs.
FIGURE 2
Monochorionic–diamnionic twin gestation
This US scan of a monochorionic twin gestation reveals the absence of an intervening placenta
Monochorionic twins
Twins who share a gestational sac are more likely than 2-sac twins to suffer spontaneous loss, congenital anomalies, growth restriction and discordancy, preterm delivery, and neurologic morbidity.
Spontaneous loss. In 1 comparative series, the risk of pregnancy loss at less than 24 weeks’ gestation was 12.2% for monochorionic twins, compared with 1.8% for dichorionic twins.6 Spontaneously conceived monochorionic twins may have the highest risk of loss.7 However, monochorionic twins occur more often in conceptions achieved by assisted reproductive technology—at a rate 3 to 10 times higher than the background rate of monochorionic twinning.8
- A multiple gestation involves a higher level of risk than a singleton pregnancy
- Chorionicity is the basis for determining risk. Twins within a single sac (monochorionic) are at higher risk of malformation, Down syndrome, and premature birth
- The risk of Down syndrome can be estimated by noninvasive screening in the first trimester and by chorionic villus sampling or amniocentesis later in the pregnancy
- A detailed anatomic survey at 18 to 20 weeks’ gestation should be done to detect possible malformations
- Assessment of cervical length, performed every 2 weeks from the 16th to the 28th week, may help predict premature delivery—but is not definitive
- Assessment of fetal growth every 4 weeks in dichorionic twins and every 2 weeks in monochorionic twins can alert you to potential problems. This is particularly important for detecting signs of twin-to-twin transfusion syndrome
Congenital anomalies. These occur 2 to 3 times as often in monochorionic twins, and have been reported in as many as 10% of such pregnancies. Reported anomalies include midline defects, cloacal abnormalities, neural tube defects, ventral wall defects, craniofacial abnormalities, conjoined twins, and acardiac twins.9-11 In light of these risks, a detailed anatomic survey is suggested for all twins.
Heart defects. The incidence of congenital heart defects is 4 times greater in monochorionic twins, even in the absence of twin-to-twin transfusion syndrome (TTTS).12 Cardiac malformations may occur secondary to abnormal lateralization during embryogenesis or result from an abnormal vascular distribution in the shared placenta.9,10 The presence of abnormal vascular communications may also cause limb reduction defects and the rare acardiac twin.
Long-term neurologic morbidity. In one series, the incidence of cerebral palsy was 8%, compared with 1% among dichorionic twins. In twins followed to 2 years of age, rates of minor neurologic morbidity were 15% in monochorionic twins and 3% in dichorionic twins. The overall rate of neurologic disorders in monochorionic twins was 23%, regardless of fetal weight.13 At 4 years, long-term neurologic morbidity was particularly high in single survivors of a monochorionic pair; the incidence of cerebral palsy has been reported to be as high as 50% in single survivors, compared with 14.3% in cases in which both twins survived.14
Twin-to-twin transfusion syndrome. In this condition, abnormal vascular connections arise in the shared placenta, allowing blood to be shunted from one fetus to the other. The syndrome is unique to monochorionic gestations and occurs in 15% to 20% of cases.15 A significant percentage of neurologic morbidity is probably the result of TTTS. To evaluate for TTTS, include a detailed anatomic survey and serial US every 2 weeks beginning in the second trimester as part of the surveillance of monochorionic twin gestations. (See “TTTS: Diagnosis, staging, treatment.”)
Twin-related morbidity and mortality are directly related to chorionicity. Twin embryos in a single chorion (monochorionic twins) have a higher rate of perinatal morbidity and mortality than do twins in separate sacs (dichorionic twins). To some extent, the higher risk faced by monochorionic twins—of twin-to-twin transfusion syndrome and certain structural and chromosomal abnormalities, for example—is the result of complications uniquely related to having a single placenta. But recent evidence also suggests that the higher risk of adverse outcomes is associated with monochorionicity itself, independent of the complications attributable to the single placenta.1
When twins develop in separate chorionic sacs, the risks are not as great. All fraternal twins (approximately 2/3 of all twins) are dichorionic and, therefore, at lower risk of an adverse outcome. The situation is more complex with identical (monozygotic) twins, however: Most (70%) are monochorionic, but approximately one third (30%) have separate chorionic sacs and are therefore dichorionic.
Reference
1. Leduc L, Takser L, Rinfret D. Persistence of adverse obstetric and neonatal outcomes in monochorionic twins after exclusion of disorders unique to monochorionic placentation. Am J Obstet Gynecol. 2005;193:1670-1675.
Down syndrome and other chromosomal abnormalities
Estimating odds
Assessing the likelihood of a chromosomal abnormality (aneuploidy) in a multiple gestation is complicated by differences in twinning mechanisms (chorionicity versus zygosity) and by the increasing rate of dizygotic twinning with advancing maternal age. The risk is greater in dizygotic twin gestations than in age-matched singleton gestations. The definition of advanced maternal age (AMA) in a twin pregnancy has ranged from 31 to 33 years of age in reports in the literature.2,16,17
The probability that a twin gestation contains a fetus with a chromosomal abnormality is directly related to zygosity. Each twin in a dizygotic gestation carries an independent risk, so the composite risk for the pregnancy is a summation of the independent risk for each fetus. For monozygotic twins, the risk is similar to the age-related risk in a singleton gestation. Presumptions about zygosity are based on chorionicity: Almost all (90%) dichorionic twins are dizygotic and all monochorionic twins are monozygotic.
What is the utility of noninvasive screening?
Multiple gestations can be screened for aneuploidy using maternal age, maternal serum markers, and nuchal translucency (NT) on US, or combinations of these assessments.
When first-trimester serum markers (free β-human chorionic gonadotropin and pregnancy-associated plasma protein A [PAPPA]) are combined with NT and maternal age, a pregnancy-specific risk can be calculated that includes the individual contribution of each fetus, thus yielding an improved detection rate. In monochorionic twins, the NTs are averaged to calculate a single risk for the entire pregnancy. In dichorionic twins, the risk for each fetus is calculated independently and then summed to establish a pregnancy-specific risk. The combined test has a reported detection rate of 84% for monochorionic twins and 70% for dichorionic twins, compared with detection rates of 85% to 87% for singletons at a 5% false-positive rate.18,19 The integrated test (combined test plus measurement of second-trimester serum analytes) has a 93% detection rate for monochorionic twins and a 78% detection rate for dichorionic twins, compared with 95% to 96% for singletons at the same 5% false-positive rate.18,19 Second-trimester screening has a lower detection rate in both singleton and twin gestations.
The diagnosis of twin-to-twin transfusion syndrome (TTTS) depends on the presence of a single monochorionic placenta and abnormalities in the volume of amniotic fluid (the polyhydramnios–oligohydramnios sequence). The syndrome may have an abrupt or gradual onset, heralded by discordancy and restriction in the growth of the 2 fetuses.
The natural history of the syndrome and treatment outcome are based on a staging system described by Quintero and colleagues1:
Stage I is characterized by polyhydramnios–oligohydramnios with the bladder still visible in the donor twin
Stage II The donor bladder is no longer visible
Stage III is defined by abnormal Doppler studies showing absent or reversed flow in the umbilical artery, reversed flow in the ductus venosus, or pulsatile umbilical venous flow
Stage IV is indicated by hydrops in either twin
Stage V One or both twins die.
The prognosis for TTTS grows poorer with increasing stage and is poor if the condition goes untreated, with a reported survival rate of only 25% to 50% for 1 twin when the diagnosis is made in the second trimester.2,3 Treatment options include removal of excess amniotic fluid through serial amniocenteses (amnioreduction), fetoscopic laser coagulation of communicating vessels, selective fetocide, and perforation of the membrane that separates the twins (septostomy).
Serial amnioreduction is the most common procedure for treating TTTS. When Senat and colleagues compared the efficacy of serial amnioreduction with fetoscopic laser occlusion in a randomized control trial, however, they found that the laser group had a significantly higher likelihood of survival of at least 1 twin (76%) than the amnioreduction group (56%).4
Septostomy. A recently published randomized trial in which amnioreduction was compared with septostomy found no difference in survival between the 2 treatments.5 Septostomy often has the advantage of requiring only 1 procedure to be successful, whereas repeated amniocenteses are necessary in serial amnioreduction. Septostomy does carry the risk of creating a single amnion, as the size of the membranous defect created by the perforation is difficult to control.
Selective fetocide using US-guided cord occlusion or radiofrequency ablation has been described when there is a coexisting fetal anomaly, growth restriction, or a chromosomal abnormality in 1 twin (heterokaryotypia).6,7 Use of bipolar coagulation in this setting has been associated with a liveborn in 83% of cases and intact neurologic survival in 70%.7 Radiofrequency ablation has also been described for selective fetal termination in monochorionic placentation with an abnormality in 1 twin.6 Data presented at the 2006 annual meeting for the Society for Maternal–Fetal Medicine showed no difference in the overall complication rate between these 2 techniques of selective fetocide.8
References
1. Quintero RA, Morales WJ, Allen MH, Bornick PW, Johnson PK, Kruger M. Staging of twin–twin transfusion syndrome. J Perinatol. 1999;19:550-555.
2. Berghella V, Kaufman M. Natural history of twin–twin transfusion syndrome. J Reprod Med. 2001;46:480-484.
3. Bromley B, Frigoletto FD, Setroff JA, Benacerraf BR. The natural history of oligohydramnios/polyhydramnios sequence in monochorionic diamniotic twins. Ultrasound Obstet Gynecol. 1992;2:317-320.
4. Senat MV, Deprest J, Boulvain M, Paupe A, Winer N, Ville Y. Endoscopic laser surgery versus serial amnioreduction for severe twin to twin transfusion syndrome. N Engl J Med. 2004;351:136-144.
5. Moise KJ, Dorman K, Lamvu G, et al. A randomized trial of amnioreduction versus septostomy in the treatment of twin–twin transfusion syndrome. Am J Obstet Gynecol. 2005;193:701-707.
6. Robyr R, Yamamoto M, Ville Y. Selective feticide in complicated monochorionic twin pregnancies using ultrasound-guided bipolar cord coagulation. BJOG. 2005;112:1344-1348.
7. Shevell T, Malone FD, Weintraub J, Harshwardhan MT, D’Alton ME. Radiofrequency ablation in a monochorionic twin discordant for fetal anomalies. Am J Obstet Gynecol. 2004;190:575-576.
8. Bebbington M, Danzer E, Johnson M, Wilson RD. RFA vs cord coagulation in complex monochorionic pregnancies. Am J Obstet Gynecol. 2006;195:S192.-
Prenatal diagnosis
Given the lower detection rate of aneuploidy in twin gestations and the associated increase in aneuploidy with advancing maternal age, many patients choose to undergo prenatal diagnosis rather than relying on screening. On the basis of maternal age alone, invasive prenatal diagnosis can be offered to women who will be 31 years or older at their estimated due date.
Available diagnostic options include chorionic villus sampling (CVS) or amniocentesis. CVS is performed at an earlier gestational age (10 to 13 weeks) than amniocentesis (15 to 20 weeks). Multiples pose specific technical considerations for either procedure, and accurate fetal mapping is essential. Successful sampling with CVS can be performed in more than 99% of cases; the rate of cross-contamination is less than 1%.
Is there a risk of miscarriage?
In counseling patients about the risk of fetal death that CVS or amniocentesis may entail, the place to begin is the background loss rate, which is greater in twin than in single gestations. The reported background loss rate of twins at 24 weeks’ gestation or less ranges from 5.8% to 7.2%.20,21 In women of advanced maternal age (35 years and older), a background rate as high as 17.6% has been described.21 Once parents are aware of this, they have a context for weighing the risk of miscarriage that prenatal testing may hold.
The twin loss rate following amniocentesis has been evaluated in several studies. (See “Integrating evidence and experience: Does invasive prenatal testing raise the risk of miscarriage in a twin gestation?”)
A greatly elevated risk of preterm birth
Multiple gestations are at extremely high risk for premature delivery, and—like all premature newborns—these infants are at risk for a wide range of disabilities. Risk factors for premature delivery include history of second trimester pregnancy loss, preterm birth at less than 35 weeks’ gestation, more than 2 previous curettage procedures, cone biopsy, müllerian anomaly, and diethylstilbestrol exposure. Unfortunately, current yardsticks for predicting premature delivery in multiple pregnancy have serious limitations, and available interventions have not been particularly successful.
Predictors
Measurement of cervical length has been evaluated as a predictor of preterm delivery in a number of twin studies that were looking for a cutoff point that can predict which twins are at greatest risk. No such cutoff has been found.22-25
In general, studies demonstrate a low risk of preterm delivery for women who have a cervical length measurement of more than 35 mm at 24 to 26 weeks. A shorter cervical length correlates with premature delivery, but specific cutoffs have proved not to be sensitive predictors.
In the largest published series, To and colleagues evaluated 1,163 sets of twins undergoing routine care with cervical length assessments at 22 to 24 weeks’ gestation. They demonstrated a direct correlation between cervical length and preterm delivery, but were unable to define a cutoff sufficiently sensitive to be useful.25 A shortened cervix may be predictive of prematurity in general, but it does not allow the obstetrician to predict with certainty which mothers will give birth prematurely or how long a particular mother will carry.
Fetal fibronectin. The presence of fetal fibronectin (ffN) in cervicovaginal secretions is widely used as an adjunct to other potential predictors of preterm delivery. In a multistudy review that included symptomatic women, a negative ffN had a 99% negative predictive value but a poor positive predictive value (13% to 30%) for delivery within 7 to 10 days.26
Use of ffN in conjunction with cervical length has also been investigated in twin gestations. Although the negative predictive value of ffN remained high, the addition of ffN to cervical length assessment did not improve the positive predictive value of cervical length alone.27,28
Interventions
Cerclage is often used in high-risk singleton pregnancies in which a shortened cervix is seen on a sonogram. The utility of cerclage in twins is less clear. Randomized controlled trials comparing women at risk of premature delivery treated with cerclage and controls not considered at risk found no difference in the rate of premature delivery in the 2 groups.29,30 Meta-analysis of 4 randomized controlled trials also found no benefit and, in fact, detected a possibility of actual harm. Cerclage twins were more likely to deliver early (at less than 35 weeks’ gestation) and had a 2.6 relative risk of perinatal mortality. The differences found in the meta-analysis were not statistically significant, however, and the overall sample size was small (n=48).31
The best available data seem to show that cerclage based on US indications of cervical shortening is not beneficial and may even be associated with worse outcome.
17-Hydroxyprogesterone caproate (17P) has been found to decrease the rate of recurrent preterm birth in singleton gestations by almost 35%.32 Although twins are at increased risk of preterm birth, the use of 17P has not, however, been shown to be of benefit.33
Bed rest. A Cochrane Database review of 6 randomized controlled trials compared 1) patients with a multiple gestation who were offered bed rest in the hospital with 2) patients hospitalized for complications of pregnancy. The review found that bed rest did not reduce the risk of preterm birth or of perinatal mortality in the routinely hospitalized women. There was, however, a tendency to a decreased number of low-birth-weight infants born to women given bed rest.34
INTEGRATING EVIDENCE AND EXPERIENCE
The evidence for amniocentesis
Toth-Pal and colleagues compared the twin loss rate after amniocentesis in 155 twin pairs; twins who had a structural anomaly or aneuploidy were excluded. The investigators found a 3.87% loss rate at 24 weeks or less, compared with a background loss rate of 2.39% in twins who did not undergo the procedure—an insignificant difference.1
Yukobowich and colleagues compared 476 diamniotic, dichorionic twin pairs that had undergone amniocentesis with 1) 477 twin pairs undergoing routine US examination and 2) 489 singleton amniocenteses. They found a 4-week postprocedure loss rate of 2.7% in the amniocentesis twins, compared with 0.63% in twin controls who had routine US and 0.6% in the amniocentesis singletons.2 The difference is significant, but the reported loss rate is still less than, or comparable to, the reported background twin loss rate at 24 weeks or less.
Chorionic villus sampling
Only a few studies of the loss rate in twins after CVS have been published, but those that are available report a loss rate lower than, or comparable to, the background rate for twins generally. In a series of 169 twin pairs undergoing CVS at an average gestational age of 10 weeks, the risk of loss at 20 weeks or more was 1.7%.3
CVS and amniocentesis, in tandem
Although CVS and amniocentesis are not directly comparable given the difference in the timing of procedure, a few series have compared the risk of loss for the 2 procedures. Eighty-one twin pairs that underwent amniocentesis were compared with 161 twins undergoing CVS. The rate of spontaneous delivery at less than 28 weeks was 2.9% for amniocentesis, compared with 3.2% following CVS.4
To sum up
Invasive testing does not appear to increase the risk of fetal loss above the background loss rate for twins overall. Prenatal diagnosis as early as 10 weeks is a feasible option in a twin gestation, given the limitations of screening in multiple gestations.
References
1. Toth-Pal E, Papp C, Beke A, Ban Z, Papp Z. Genetic amniocentesis in multiple pregnancy. Fetal Diagn Ther. 2004;19:138-144.
2. Yukobowich E, Anteby EY, Cohen SM, Lavy Y, Granat M, Yagel S. Risk of fetal loss in twin pregnancies undergoing second trimester amniocentesis. Obstet Gynecol. 2001;98:231-234.
3. Brambati B, Tului L, Guercilena S, Alberti E. Outcome of first-trimester chorionic villus sampling for genetic investigation in multiple pregnancy. Ultrasound Obstet Gynecol. 2001;17:209-216.
4. Wapner RJ, Johnson A, Davis G, Urban A, Morgan P, Jackson L. Prenatal diagnosis in twin gestations: a comparison between second trimester amniocentesis and first trimester chorionic villus sampling. Obstet Gynecol. 1993;82:49-56.
Some parents elect fetal reduction
Given the high level of risk in a multiple pregnancy, reducing the number of fetuses is an option that some patients choose. A recent trend toward reduction to singleton pregnancy seems to be related to:
- increasing maternal age
- single parenthood
- financial considerations
- the increased medical risk to mother and fetuses associated with twins.35
Evans and colleagues found that, although the overall rate of reduction from twins to a singleton was 3%, the percentage (76%) of women older than 35 years who opted for such a reduction was disproportionately high.36
In a series of 1,000 cases of multifetal pregnancy reduction, Stone et al found that the pregnancy loss rate was lowest (2.5%) when there was reduction to a singleton gestation.37 A comparative analysis of 2,000 cases of multifetal pregnancy reduction presented at the 2006 meeting of the Society for Maternal–Fetal Medicine found that the percentage of twin gestations undergoing reduction to a singleton has increased from 4% to 15.6% between 1999 and 2006, with an increase in the overall incidence of reduction to a singleton from 11.8% to 31.8%.38
You should discuss pregnancy reduction with patients at high risk of pregnancy-associated complications such as cervical incompetence, preterm delivery, severe maternal cardiac disease, hypertension, diabetes, and uterine anomalies, as well as with patients who are carrying higher-order multiple gestations, in which the fetuses are at risk of problems.
Wrap-up: The tasks facing you in a multiple gestation
Start your management of a multiple gestation by taking the essential first step of determining the chorionicity of the fetuses.
Once you have done that, explain the risks of twin pregnancy and the particular risks of a single-sac pregnancy. Parents will want to know their risk of having a child with an anomaly; pay particular attention to the likelihood of a Down syndrome child. Noninvasive screening for Down syndrome and other chromosomal anomalies may be sufficient, but an older mother may prefer more definitive answers from CVS or amniocentesis.
You must prepare parents of a twin gestation for the risk of premature delivery. Cervical length assessment in the second trimester to early-third trimester may provide some indications of what is to happen, but the predictive value of this procedure is limited, and a shortened cervical length should be interpreted with caution.
In a monochorionic pregnancy, fetal growth should be assessed at regular intervals to evaluate for possible growth restriction or TTTS. If you detect evidence of abnormal fetal growth or amniotic fluid, US surveillance is indicated. Routine antepartum US surveillance of twins is not, however, recommended.2
The authors report no financial relationships relevant to this article.
Multiple gestations are far more common today than they once were—up 70% since 1980.1 Today, every 1,000 live births include 32.3 sets of twins, a 2% increase in the rate of twin births since 2004. Why this phenomenon is occurring is not entirely understood but, certainly, the trend toward older maternal age and the emergence of assisted reproduction are both part of the explanation.
Multiple gestations are of particular concern to obstetricians because, even though they remain relatively rare, they are responsible for a significant percentage of perinatal morbidity and mortality.
The difficulties that twins encounter are often associated with preterm birth and occur most often in identical twins developing within a single gestational sac. Those difficulties include malformation, chromosomal abnormalities, learning disability, behavioral problems, chronic lung disease, neuromuscular developmental delay, cerebral palsy, and stillbirth. Women pregnant with twins are also at heightened risk, particularly of gestational hypertension, preeclampsia, and gestational diabetes.2
Your task is to manage these risks so that the outlook for mother and infant is as favorable as possible.
Determining chorionicity in the first trimester
Ultrasonographic determination of chorionicity should be the first step in the management of a twin gestation. The determination should be made as early as possible in the pregnancy because it has an immediate impact on counseling, risk of miscarriage, and efficacy of noninvasive screening. (See “Is there 1 sac, or more? Key to predicting risk.”)
The accuracy of ultrasonography (US) in determining chorionicity depends on gestational age. US predictors of dichorionicity include:
- gender discordance
- separate placentas
- the so-called twin-peak sign (also called the lambda sign) (FIGURE 1), in which the placenta appears to extend a short distance between the gestational sacs; compare this with FIGURE 2, showing monochorionic twins with the absence of an intervening placenta
- an intertwin membrane thicker than 1.5 mm to 2.0 mm.
US examination can accurately identify chorionicity at 10 to 14 weeks’ gestation, with overall sensitivity that is reported to be as high as 100%.3-5
FIGURE 1
The twin-peak sign on US
This dichorionic–diamnionic twin gestation demonstrates the so-called twin peak, or lambda, sign (arrow), in which the placenta appears to extend a short distance between the gestational sacs.
FIGURE 2
Monochorionic–diamnionic twin gestation
This US scan of a monochorionic twin gestation reveals the absence of an intervening placenta
Monochorionic twins
Twins who share a gestational sac are more likely than 2-sac twins to suffer spontaneous loss, congenital anomalies, growth restriction and discordancy, preterm delivery, and neurologic morbidity.
Spontaneous loss. In 1 comparative series, the risk of pregnancy loss at less than 24 weeks’ gestation was 12.2% for monochorionic twins, compared with 1.8% for dichorionic twins.6 Spontaneously conceived monochorionic twins may have the highest risk of loss.7 However, monochorionic twins occur more often in conceptions achieved by assisted reproductive technology—at a rate 3 to 10 times higher than the background rate of monochorionic twinning.8
- A multiple gestation involves a higher level of risk than a singleton pregnancy
- Chorionicity is the basis for determining risk. Twins within a single sac (monochorionic) are at higher risk of malformation, Down syndrome, and premature birth
- The risk of Down syndrome can be estimated by noninvasive screening in the first trimester and by chorionic villus sampling or amniocentesis later in the pregnancy
- A detailed anatomic survey at 18 to 20 weeks’ gestation should be done to detect possible malformations
- Assessment of cervical length, performed every 2 weeks from the 16th to the 28th week, may help predict premature delivery—but is not definitive
- Assessment of fetal growth every 4 weeks in dichorionic twins and every 2 weeks in monochorionic twins can alert you to potential problems. This is particularly important for detecting signs of twin-to-twin transfusion syndrome
Congenital anomalies. These occur 2 to 3 times as often in monochorionic twins, and have been reported in as many as 10% of such pregnancies. Reported anomalies include midline defects, cloacal abnormalities, neural tube defects, ventral wall defects, craniofacial abnormalities, conjoined twins, and acardiac twins.9-11 In light of these risks, a detailed anatomic survey is suggested for all twins.
Heart defects. The incidence of congenital heart defects is 4 times greater in monochorionic twins, even in the absence of twin-to-twin transfusion syndrome (TTTS).12 Cardiac malformations may occur secondary to abnormal lateralization during embryogenesis or result from an abnormal vascular distribution in the shared placenta.9,10 The presence of abnormal vascular communications may also cause limb reduction defects and the rare acardiac twin.
Long-term neurologic morbidity. In one series, the incidence of cerebral palsy was 8%, compared with 1% among dichorionic twins. In twins followed to 2 years of age, rates of minor neurologic morbidity were 15% in monochorionic twins and 3% in dichorionic twins. The overall rate of neurologic disorders in monochorionic twins was 23%, regardless of fetal weight.13 At 4 years, long-term neurologic morbidity was particularly high in single survivors of a monochorionic pair; the incidence of cerebral palsy has been reported to be as high as 50% in single survivors, compared with 14.3% in cases in which both twins survived.14
Twin-to-twin transfusion syndrome. In this condition, abnormal vascular connections arise in the shared placenta, allowing blood to be shunted from one fetus to the other. The syndrome is unique to monochorionic gestations and occurs in 15% to 20% of cases.15 A significant percentage of neurologic morbidity is probably the result of TTTS. To evaluate for TTTS, include a detailed anatomic survey and serial US every 2 weeks beginning in the second trimester as part of the surveillance of monochorionic twin gestations. (See “TTTS: Diagnosis, staging, treatment.”)
Twin-related morbidity and mortality are directly related to chorionicity. Twin embryos in a single chorion (monochorionic twins) have a higher rate of perinatal morbidity and mortality than do twins in separate sacs (dichorionic twins). To some extent, the higher risk faced by monochorionic twins—of twin-to-twin transfusion syndrome and certain structural and chromosomal abnormalities, for example—is the result of complications uniquely related to having a single placenta. But recent evidence also suggests that the higher risk of adverse outcomes is associated with monochorionicity itself, independent of the complications attributable to the single placenta.1
When twins develop in separate chorionic sacs, the risks are not as great. All fraternal twins (approximately 2/3 of all twins) are dichorionic and, therefore, at lower risk of an adverse outcome. The situation is more complex with identical (monozygotic) twins, however: Most (70%) are monochorionic, but approximately one third (30%) have separate chorionic sacs and are therefore dichorionic.
Reference
1. Leduc L, Takser L, Rinfret D. Persistence of adverse obstetric and neonatal outcomes in monochorionic twins after exclusion of disorders unique to monochorionic placentation. Am J Obstet Gynecol. 2005;193:1670-1675.
Down syndrome and other chromosomal abnormalities
Estimating odds
Assessing the likelihood of a chromosomal abnormality (aneuploidy) in a multiple gestation is complicated by differences in twinning mechanisms (chorionicity versus zygosity) and by the increasing rate of dizygotic twinning with advancing maternal age. The risk is greater in dizygotic twin gestations than in age-matched singleton gestations. The definition of advanced maternal age (AMA) in a twin pregnancy has ranged from 31 to 33 years of age in reports in the literature.2,16,17
The probability that a twin gestation contains a fetus with a chromosomal abnormality is directly related to zygosity. Each twin in a dizygotic gestation carries an independent risk, so the composite risk for the pregnancy is a summation of the independent risk for each fetus. For monozygotic twins, the risk is similar to the age-related risk in a singleton gestation. Presumptions about zygosity are based on chorionicity: Almost all (90%) dichorionic twins are dizygotic and all monochorionic twins are monozygotic.
What is the utility of noninvasive screening?
Multiple gestations can be screened for aneuploidy using maternal age, maternal serum markers, and nuchal translucency (NT) on US, or combinations of these assessments.
When first-trimester serum markers (free β-human chorionic gonadotropin and pregnancy-associated plasma protein A [PAPPA]) are combined with NT and maternal age, a pregnancy-specific risk can be calculated that includes the individual contribution of each fetus, thus yielding an improved detection rate. In monochorionic twins, the NTs are averaged to calculate a single risk for the entire pregnancy. In dichorionic twins, the risk for each fetus is calculated independently and then summed to establish a pregnancy-specific risk. The combined test has a reported detection rate of 84% for monochorionic twins and 70% for dichorionic twins, compared with detection rates of 85% to 87% for singletons at a 5% false-positive rate.18,19 The integrated test (combined test plus measurement of second-trimester serum analytes) has a 93% detection rate for monochorionic twins and a 78% detection rate for dichorionic twins, compared with 95% to 96% for singletons at the same 5% false-positive rate.18,19 Second-trimester screening has a lower detection rate in both singleton and twin gestations.
The diagnosis of twin-to-twin transfusion syndrome (TTTS) depends on the presence of a single monochorionic placenta and abnormalities in the volume of amniotic fluid (the polyhydramnios–oligohydramnios sequence). The syndrome may have an abrupt or gradual onset, heralded by discordancy and restriction in the growth of the 2 fetuses.
The natural history of the syndrome and treatment outcome are based on a staging system described by Quintero and colleagues1:
Stage I is characterized by polyhydramnios–oligohydramnios with the bladder still visible in the donor twin
Stage II The donor bladder is no longer visible
Stage III is defined by abnormal Doppler studies showing absent or reversed flow in the umbilical artery, reversed flow in the ductus venosus, or pulsatile umbilical venous flow
Stage IV is indicated by hydrops in either twin
Stage V One or both twins die.
The prognosis for TTTS grows poorer with increasing stage and is poor if the condition goes untreated, with a reported survival rate of only 25% to 50% for 1 twin when the diagnosis is made in the second trimester.2,3 Treatment options include removal of excess amniotic fluid through serial amniocenteses (amnioreduction), fetoscopic laser coagulation of communicating vessels, selective fetocide, and perforation of the membrane that separates the twins (septostomy).
Serial amnioreduction is the most common procedure for treating TTTS. When Senat and colleagues compared the efficacy of serial amnioreduction with fetoscopic laser occlusion in a randomized control trial, however, they found that the laser group had a significantly higher likelihood of survival of at least 1 twin (76%) than the amnioreduction group (56%).4
Septostomy. A recently published randomized trial in which amnioreduction was compared with septostomy found no difference in survival between the 2 treatments.5 Septostomy often has the advantage of requiring only 1 procedure to be successful, whereas repeated amniocenteses are necessary in serial amnioreduction. Septostomy does carry the risk of creating a single amnion, as the size of the membranous defect created by the perforation is difficult to control.
Selective fetocide using US-guided cord occlusion or radiofrequency ablation has been described when there is a coexisting fetal anomaly, growth restriction, or a chromosomal abnormality in 1 twin (heterokaryotypia).6,7 Use of bipolar coagulation in this setting has been associated with a liveborn in 83% of cases and intact neurologic survival in 70%.7 Radiofrequency ablation has also been described for selective fetal termination in monochorionic placentation with an abnormality in 1 twin.6 Data presented at the 2006 annual meeting for the Society for Maternal–Fetal Medicine showed no difference in the overall complication rate between these 2 techniques of selective fetocide.8
References
1. Quintero RA, Morales WJ, Allen MH, Bornick PW, Johnson PK, Kruger M. Staging of twin–twin transfusion syndrome. J Perinatol. 1999;19:550-555.
2. Berghella V, Kaufman M. Natural history of twin–twin transfusion syndrome. J Reprod Med. 2001;46:480-484.
3. Bromley B, Frigoletto FD, Setroff JA, Benacerraf BR. The natural history of oligohydramnios/polyhydramnios sequence in monochorionic diamniotic twins. Ultrasound Obstet Gynecol. 1992;2:317-320.
4. Senat MV, Deprest J, Boulvain M, Paupe A, Winer N, Ville Y. Endoscopic laser surgery versus serial amnioreduction for severe twin to twin transfusion syndrome. N Engl J Med. 2004;351:136-144.
5. Moise KJ, Dorman K, Lamvu G, et al. A randomized trial of amnioreduction versus septostomy in the treatment of twin–twin transfusion syndrome. Am J Obstet Gynecol. 2005;193:701-707.
6. Robyr R, Yamamoto M, Ville Y. Selective feticide in complicated monochorionic twin pregnancies using ultrasound-guided bipolar cord coagulation. BJOG. 2005;112:1344-1348.
7. Shevell T, Malone FD, Weintraub J, Harshwardhan MT, D’Alton ME. Radiofrequency ablation in a monochorionic twin discordant for fetal anomalies. Am J Obstet Gynecol. 2004;190:575-576.
8. Bebbington M, Danzer E, Johnson M, Wilson RD. RFA vs cord coagulation in complex monochorionic pregnancies. Am J Obstet Gynecol. 2006;195:S192.-
Prenatal diagnosis
Given the lower detection rate of aneuploidy in twin gestations and the associated increase in aneuploidy with advancing maternal age, many patients choose to undergo prenatal diagnosis rather than relying on screening. On the basis of maternal age alone, invasive prenatal diagnosis can be offered to women who will be 31 years or older at their estimated due date.
Available diagnostic options include chorionic villus sampling (CVS) or amniocentesis. CVS is performed at an earlier gestational age (10 to 13 weeks) than amniocentesis (15 to 20 weeks). Multiples pose specific technical considerations for either procedure, and accurate fetal mapping is essential. Successful sampling with CVS can be performed in more than 99% of cases; the rate of cross-contamination is less than 1%.
Is there a risk of miscarriage?
In counseling patients about the risk of fetal death that CVS or amniocentesis may entail, the place to begin is the background loss rate, which is greater in twin than in single gestations. The reported background loss rate of twins at 24 weeks’ gestation or less ranges from 5.8% to 7.2%.20,21 In women of advanced maternal age (35 years and older), a background rate as high as 17.6% has been described.21 Once parents are aware of this, they have a context for weighing the risk of miscarriage that prenatal testing may hold.
The twin loss rate following amniocentesis has been evaluated in several studies. (See “Integrating evidence and experience: Does invasive prenatal testing raise the risk of miscarriage in a twin gestation?”)
A greatly elevated risk of preterm birth
Multiple gestations are at extremely high risk for premature delivery, and—like all premature newborns—these infants are at risk for a wide range of disabilities. Risk factors for premature delivery include history of second trimester pregnancy loss, preterm birth at less than 35 weeks’ gestation, more than 2 previous curettage procedures, cone biopsy, müllerian anomaly, and diethylstilbestrol exposure. Unfortunately, current yardsticks for predicting premature delivery in multiple pregnancy have serious limitations, and available interventions have not been particularly successful.
Predictors
Measurement of cervical length has been evaluated as a predictor of preterm delivery in a number of twin studies that were looking for a cutoff point that can predict which twins are at greatest risk. No such cutoff has been found.22-25
In general, studies demonstrate a low risk of preterm delivery for women who have a cervical length measurement of more than 35 mm at 24 to 26 weeks. A shorter cervical length correlates with premature delivery, but specific cutoffs have proved not to be sensitive predictors.
In the largest published series, To and colleagues evaluated 1,163 sets of twins undergoing routine care with cervical length assessments at 22 to 24 weeks’ gestation. They demonstrated a direct correlation between cervical length and preterm delivery, but were unable to define a cutoff sufficiently sensitive to be useful.25 A shortened cervix may be predictive of prematurity in general, but it does not allow the obstetrician to predict with certainty which mothers will give birth prematurely or how long a particular mother will carry.
Fetal fibronectin. The presence of fetal fibronectin (ffN) in cervicovaginal secretions is widely used as an adjunct to other potential predictors of preterm delivery. In a multistudy review that included symptomatic women, a negative ffN had a 99% negative predictive value but a poor positive predictive value (13% to 30%) for delivery within 7 to 10 days.26
Use of ffN in conjunction with cervical length has also been investigated in twin gestations. Although the negative predictive value of ffN remained high, the addition of ffN to cervical length assessment did not improve the positive predictive value of cervical length alone.27,28
Interventions
Cerclage is often used in high-risk singleton pregnancies in which a shortened cervix is seen on a sonogram. The utility of cerclage in twins is less clear. Randomized controlled trials comparing women at risk of premature delivery treated with cerclage and controls not considered at risk found no difference in the rate of premature delivery in the 2 groups.29,30 Meta-analysis of 4 randomized controlled trials also found no benefit and, in fact, detected a possibility of actual harm. Cerclage twins were more likely to deliver early (at less than 35 weeks’ gestation) and had a 2.6 relative risk of perinatal mortality. The differences found in the meta-analysis were not statistically significant, however, and the overall sample size was small (n=48).31
The best available data seem to show that cerclage based on US indications of cervical shortening is not beneficial and may even be associated with worse outcome.
17-Hydroxyprogesterone caproate (17P) has been found to decrease the rate of recurrent preterm birth in singleton gestations by almost 35%.32 Although twins are at increased risk of preterm birth, the use of 17P has not, however, been shown to be of benefit.33
Bed rest. A Cochrane Database review of 6 randomized controlled trials compared 1) patients with a multiple gestation who were offered bed rest in the hospital with 2) patients hospitalized for complications of pregnancy. The review found that bed rest did not reduce the risk of preterm birth or of perinatal mortality in the routinely hospitalized women. There was, however, a tendency to a decreased number of low-birth-weight infants born to women given bed rest.34
INTEGRATING EVIDENCE AND EXPERIENCE
The evidence for amniocentesis
Toth-Pal and colleagues compared the twin loss rate after amniocentesis in 155 twin pairs; twins who had a structural anomaly or aneuploidy were excluded. The investigators found a 3.87% loss rate at 24 weeks or less, compared with a background loss rate of 2.39% in twins who did not undergo the procedure—an insignificant difference.1
Yukobowich and colleagues compared 476 diamniotic, dichorionic twin pairs that had undergone amniocentesis with 1) 477 twin pairs undergoing routine US examination and 2) 489 singleton amniocenteses. They found a 4-week postprocedure loss rate of 2.7% in the amniocentesis twins, compared with 0.63% in twin controls who had routine US and 0.6% in the amniocentesis singletons.2 The difference is significant, but the reported loss rate is still less than, or comparable to, the reported background twin loss rate at 24 weeks or less.
Chorionic villus sampling
Only a few studies of the loss rate in twins after CVS have been published, but those that are available report a loss rate lower than, or comparable to, the background rate for twins generally. In a series of 169 twin pairs undergoing CVS at an average gestational age of 10 weeks, the risk of loss at 20 weeks or more was 1.7%.3
CVS and amniocentesis, in tandem
Although CVS and amniocentesis are not directly comparable given the difference in the timing of procedure, a few series have compared the risk of loss for the 2 procedures. Eighty-one twin pairs that underwent amniocentesis were compared with 161 twins undergoing CVS. The rate of spontaneous delivery at less than 28 weeks was 2.9% for amniocentesis, compared with 3.2% following CVS.4
To sum up
Invasive testing does not appear to increase the risk of fetal loss above the background loss rate for twins overall. Prenatal diagnosis as early as 10 weeks is a feasible option in a twin gestation, given the limitations of screening in multiple gestations.
References
1. Toth-Pal E, Papp C, Beke A, Ban Z, Papp Z. Genetic amniocentesis in multiple pregnancy. Fetal Diagn Ther. 2004;19:138-144.
2. Yukobowich E, Anteby EY, Cohen SM, Lavy Y, Granat M, Yagel S. Risk of fetal loss in twin pregnancies undergoing second trimester amniocentesis. Obstet Gynecol. 2001;98:231-234.
3. Brambati B, Tului L, Guercilena S, Alberti E. Outcome of first-trimester chorionic villus sampling for genetic investigation in multiple pregnancy. Ultrasound Obstet Gynecol. 2001;17:209-216.
4. Wapner RJ, Johnson A, Davis G, Urban A, Morgan P, Jackson L. Prenatal diagnosis in twin gestations: a comparison between second trimester amniocentesis and first trimester chorionic villus sampling. Obstet Gynecol. 1993;82:49-56.
Some parents elect fetal reduction
Given the high level of risk in a multiple pregnancy, reducing the number of fetuses is an option that some patients choose. A recent trend toward reduction to singleton pregnancy seems to be related to:
- increasing maternal age
- single parenthood
- financial considerations
- the increased medical risk to mother and fetuses associated with twins.35
Evans and colleagues found that, although the overall rate of reduction from twins to a singleton was 3%, the percentage (76%) of women older than 35 years who opted for such a reduction was disproportionately high.36
In a series of 1,000 cases of multifetal pregnancy reduction, Stone et al found that the pregnancy loss rate was lowest (2.5%) when there was reduction to a singleton gestation.37 A comparative analysis of 2,000 cases of multifetal pregnancy reduction presented at the 2006 meeting of the Society for Maternal–Fetal Medicine found that the percentage of twin gestations undergoing reduction to a singleton has increased from 4% to 15.6% between 1999 and 2006, with an increase in the overall incidence of reduction to a singleton from 11.8% to 31.8%.38
You should discuss pregnancy reduction with patients at high risk of pregnancy-associated complications such as cervical incompetence, preterm delivery, severe maternal cardiac disease, hypertension, diabetes, and uterine anomalies, as well as with patients who are carrying higher-order multiple gestations, in which the fetuses are at risk of problems.
Wrap-up: The tasks facing you in a multiple gestation
Start your management of a multiple gestation by taking the essential first step of determining the chorionicity of the fetuses.
Once you have done that, explain the risks of twin pregnancy and the particular risks of a single-sac pregnancy. Parents will want to know their risk of having a child with an anomaly; pay particular attention to the likelihood of a Down syndrome child. Noninvasive screening for Down syndrome and other chromosomal anomalies may be sufficient, but an older mother may prefer more definitive answers from CVS or amniocentesis.
You must prepare parents of a twin gestation for the risk of premature delivery. Cervical length assessment in the second trimester to early-third trimester may provide some indications of what is to happen, but the predictive value of this procedure is limited, and a shortened cervical length should be interpreted with caution.
In a monochorionic pregnancy, fetal growth should be assessed at regular intervals to evaluate for possible growth restriction or TTTS. If you detect evidence of abnormal fetal growth or amniotic fluid, US surveillance is indicated. Routine antepartum US surveillance of twins is not, however, recommended.2
The authors report no financial relationships relevant to this article.
1. Martin JA, Hamilton BE, Sutton PD, Ventura SJ, Menacker F, Kirmeyer S. Births: final data 2004; Natl Vital Stat Rep. 2006;55:1-101.
2. Multiple gestation: Complicated twin, triplet and high-order multifetal pregnancy. ACOG Practice Bulletin; 2004. No. 56.
3. Sepulveda W, Sebire NJ, Hughes K, Odibo A, Nicolaides KH. The lambda sign at 10–14 weeks of gestation as a predictor of chorionicity in twin pregnancies. Ultrasound Obstet Gynecol. 1996;7:421-423.
4. Carroll SGM, Soothill PW, Abdel-Fattah SA, Porter H, Montague I, Kyle PM. Prediction of chorionicity in twin pregnancies at 10–14 weeks of gestation. Br J Obstet Gynaecol. 2002;109:182-186.
5. Stenhouse E, Hardwick C, Maharaj S, Webb J, Kelly T, Mackenzie FM. Chorionicity determination in twin pregnancies; how accurate are we? Ultrasound Obstet Gynecol. 2002;19:350-352.
6. Sebire NJ, Snijders RJ, Hughes K, Sepulveda W, Nicolaides KH. The hidden mortality of monochorionic twin pregnancies. Br J Obstet Gynaecol. 1997;104:1203-1207.
7. Sperling L, Kiil C, Larsen LU, et al. Naturally conceived twins with monochorionic placentation have the highest risk of fetal loss. Ultrasound Obstet Gynecol. 2006;28:644-652.
8. Trevett T, Johnson A. Monochorionic twin pregnancies. Clin Perinatol. 2005;32:475-494.
9. Rustico MA, Baietti MG, Coviello D, Orlandi E, Nicolini U. Managing twins discordant for fetal anomaly. Prenat Diagn. 2005;25:766-771.
10. Hall JG. Developmental biology IV. Lancet. 2003;362:735-743.
11. Mohammed SN, Swan MC, Wall SA, Wilkie AO. Monozygotic twins discordant for frontonasal malformation. Am J Med Genet A. 2004;130:384-388.
12. Karatza AA, Wolfenden JL, Taylor MJ, Wee L, Fisk NM, Gardiner HM. Influence of twin–twin transfusion syndrome on fetal cardiovascular structure and function; prospective case-control study of 136 monochorionic twin pregnancies. Heart. 2002;88:271-277.
13. Adegbite AL, Castille S, Ward S, Bajoria R. Neuromorbidity in preterm twins in relation to chorionicity and discordant birth weight. Am J Obstet Gynecol. 2004;190:156-163.
14. Lopriore E, Nagel HTC, Vandenbussche FPHA, Walther FJ. Long-term neurodevelopmental outcome in twin–twin transfusion syndrome. Am J Obstet Gynecol. 2003;189:1314-1319.
15. Bromley B, Frigoletto FD, Setroff JA, Benacerraf BR. The natural history of oligohydramnios/polyhydramnios sequence in monochorionic diamniotic twins. Ultrasound Obstet Gynecol. 1992;2:317-320.
16. Rodis JF, Egan JFX, Craffey A, Ciarleglio L, Greenstein RM, Scorza WE. Calculated risk of chromosomal abnormalities in twin gestations. Obstet Gynecol. 1990;76:1037-1041.
17. Meyers C, Adam R, Dungan J, Prenger V. Aneuploidy in twin gestations; when is maternal age advanced? Obstet Gynecol. 1997;89:248-251.
18. Wald J, Rish S. Prenatal screening for Down syndrome and neural tube defects in twin pregnancies. Prenat Diagn. 2005;25:740-745.
19. Malone FD, Canick JA, Ball RH, et al. The First- and Second-Trimester Evaluation of Risk (FASTER) Research Consortium. First trimester or second trimester screening, or both, for Down’s syndrome. N Engl J Med. 2005;353:2001-2011.
20. Yaron Y, Bryant-Greenwood PK, Dave N, et al. Multifetal pregnancy reduction of triplets to twins: comparison with nonreduced triplets and twins. Am J Obstet Gynecol. 1999;180:1268-1271.
21. La Sala GB, Nucera G, Gallinelli A, Nicoli A, Villani MT, Blickstein I. Spontaneous embryonic loss following in vitro fertilization: incidence and effect on outcomes. Am J Obstet Gynecol. 2004;191:741-746.
22. Imseis HM, Albert TA, Iams JD. Identifying twin gestations at low risk for preterm birth with a transvaginal ultrasonographic cervical measurement at 24 to 26 weeks’ gestation. Am J Obstet Gynecol. 1997;177:1149-1155.
23. Vayssiere C, Favre R, Audibert F, et al. Cervical length and funneling at 22 and 27 weeks to predict spontaneous birth before 32 weeks in twin pregnancies: a French prospective multicenter study. Am J Obstet Gynecol. 2002;187:1596-1604.
24. Guzman ER, Walters C, O’Reilly-Green C, et al. Use of cervical ultrasonography in prediction of spontaneous preterm birth in twin gestations. Am J Obstet Gynecol. 2000;183:1103-1107.
25. To MS, Fonseca EB, Molina FS, Cacho AM, Nicolaides KH. Maternal characteristics and cervical length in prediction of spontaneous early preterm delivery in twins. Am J Obstet Gynecol. 2006;194:1360-1365.
26. Honest H, Bachmann LM, Gupta JK, Kleijnen J, Khan KS. Accuracy of cervicovaginal fetal fibronectin test in predicting risk of spontaneous preterm birth: systematic review. BMJ. 2002;325:301.-
27. Goldenberg RL, Iams JD, Miodovnik M, et al. The preterm prediction study: risk factors in twin gestations. National Institute of Child Health and Human Development Maternal–Fetal Medicine Units Network. Am J Obstet Gynecol. 1996;175:1047-1053.
28. Gibson JL, Macara LM, Owen P, Young D, Macauley J, Mackenzie F. Prediction of preterm delivery in twin pregnancy: a prospective, observational study of cervical length and fetal fibronectin testing. Ultrasound Obstet Gynecol. 2004;23:561-566.
29. Berghella V, Odibo AO, Tolosa JE. Cerclage for prevention of preterm birth in women with a short cervix found on transvaginal ultrasound examination: a randomized trial. Am J Obstet Gynecol. 2004;191:1311-1317.
30. Rust OA, Atlas RO, Reed J, van Gaalen J, Baldussi J. Revisiting the short cervix detected by transvaginal ultrasound in the second trimester: why cerclage therapy may not help. Am J Obstet Gynecol. 2001;185:1098-1105.
31. Berghella V, Odibo AO, To MS, Rust OA, Althuisius SM. Cerclage for short cervix on ultrasonography: meta-analysis of trials using individual patient-level data. Obstet Gynecol. 2005;106:181-189.
32. Meis PJ, Klebanoff M, Thom E, et al. Prevention of recurrent preterm delivery by 17 alpha-hydroxyprogesterone caproate. N Engl J Med. 2003;348:2379-2385.
33. Caritis S, Rouse D. NICHD MFMU Network. A randomized controlled trial of 17-hydroxyprogesterone caproate for the prevention of preterm birth in twins. Am J Obstet Gynecol. 2006;195:S2.-
34. Crowther CA. Hospitalisation and bed rest for multiple pregnancy. Cochrane Database Syst Rev. 2001;(1):CD000110.-
35. Evans MI, Ciorica D, Britt DW, Fletcher JC. Update on selective reduction. Prenat Diagn. 2005;25:807-813.
36. Evans MI, Kaufman MI, Urban AJ, Britt DW, Fletcher JC. Fetal reduction from twins to a singleton: a reasonable consideration. Obstet Gynecol. 2004;104:102-109.
37. Stone J, Eddleman K, Lynch L, Berkowitz RL. A single center experience with 1000 consecutive cases of multifetal pregnancy reduction. Am J Obstet Gynecol. 2002;187:1163-1167.
38. Stone J, Matho A, Berkowitz R, Belogolovkin V, Eddleman K. Evolving trends in 2,000 cases of multifetal pregnancy reduction. Am J Obstet Gynecol. 2006;195:S184.-
1. Martin JA, Hamilton BE, Sutton PD, Ventura SJ, Menacker F, Kirmeyer S. Births: final data 2004; Natl Vital Stat Rep. 2006;55:1-101.
2. Multiple gestation: Complicated twin, triplet and high-order multifetal pregnancy. ACOG Practice Bulletin; 2004. No. 56.
3. Sepulveda W, Sebire NJ, Hughes K, Odibo A, Nicolaides KH. The lambda sign at 10–14 weeks of gestation as a predictor of chorionicity in twin pregnancies. Ultrasound Obstet Gynecol. 1996;7:421-423.
4. Carroll SGM, Soothill PW, Abdel-Fattah SA, Porter H, Montague I, Kyle PM. Prediction of chorionicity in twin pregnancies at 10–14 weeks of gestation. Br J Obstet Gynaecol. 2002;109:182-186.
5. Stenhouse E, Hardwick C, Maharaj S, Webb J, Kelly T, Mackenzie FM. Chorionicity determination in twin pregnancies; how accurate are we? Ultrasound Obstet Gynecol. 2002;19:350-352.
6. Sebire NJ, Snijders RJ, Hughes K, Sepulveda W, Nicolaides KH. The hidden mortality of monochorionic twin pregnancies. Br J Obstet Gynaecol. 1997;104:1203-1207.
7. Sperling L, Kiil C, Larsen LU, et al. Naturally conceived twins with monochorionic placentation have the highest risk of fetal loss. Ultrasound Obstet Gynecol. 2006;28:644-652.
8. Trevett T, Johnson A. Monochorionic twin pregnancies. Clin Perinatol. 2005;32:475-494.
9. Rustico MA, Baietti MG, Coviello D, Orlandi E, Nicolini U. Managing twins discordant for fetal anomaly. Prenat Diagn. 2005;25:766-771.
10. Hall JG. Developmental biology IV. Lancet. 2003;362:735-743.
11. Mohammed SN, Swan MC, Wall SA, Wilkie AO. Monozygotic twins discordant for frontonasal malformation. Am J Med Genet A. 2004;130:384-388.
12. Karatza AA, Wolfenden JL, Taylor MJ, Wee L, Fisk NM, Gardiner HM. Influence of twin–twin transfusion syndrome on fetal cardiovascular structure and function; prospective case-control study of 136 monochorionic twin pregnancies. Heart. 2002;88:271-277.
13. Adegbite AL, Castille S, Ward S, Bajoria R. Neuromorbidity in preterm twins in relation to chorionicity and discordant birth weight. Am J Obstet Gynecol. 2004;190:156-163.
14. Lopriore E, Nagel HTC, Vandenbussche FPHA, Walther FJ. Long-term neurodevelopmental outcome in twin–twin transfusion syndrome. Am J Obstet Gynecol. 2003;189:1314-1319.
15. Bromley B, Frigoletto FD, Setroff JA, Benacerraf BR. The natural history of oligohydramnios/polyhydramnios sequence in monochorionic diamniotic twins. Ultrasound Obstet Gynecol. 1992;2:317-320.
16. Rodis JF, Egan JFX, Craffey A, Ciarleglio L, Greenstein RM, Scorza WE. Calculated risk of chromosomal abnormalities in twin gestations. Obstet Gynecol. 1990;76:1037-1041.
17. Meyers C, Adam R, Dungan J, Prenger V. Aneuploidy in twin gestations; when is maternal age advanced? Obstet Gynecol. 1997;89:248-251.
18. Wald J, Rish S. Prenatal screening for Down syndrome and neural tube defects in twin pregnancies. Prenat Diagn. 2005;25:740-745.
19. Malone FD, Canick JA, Ball RH, et al. The First- and Second-Trimester Evaluation of Risk (FASTER) Research Consortium. First trimester or second trimester screening, or both, for Down’s syndrome. N Engl J Med. 2005;353:2001-2011.
20. Yaron Y, Bryant-Greenwood PK, Dave N, et al. Multifetal pregnancy reduction of triplets to twins: comparison with nonreduced triplets and twins. Am J Obstet Gynecol. 1999;180:1268-1271.
21. La Sala GB, Nucera G, Gallinelli A, Nicoli A, Villani MT, Blickstein I. Spontaneous embryonic loss following in vitro fertilization: incidence and effect on outcomes. Am J Obstet Gynecol. 2004;191:741-746.
22. Imseis HM, Albert TA, Iams JD. Identifying twin gestations at low risk for preterm birth with a transvaginal ultrasonographic cervical measurement at 24 to 26 weeks’ gestation. Am J Obstet Gynecol. 1997;177:1149-1155.
23. Vayssiere C, Favre R, Audibert F, et al. Cervical length and funneling at 22 and 27 weeks to predict spontaneous birth before 32 weeks in twin pregnancies: a French prospective multicenter study. Am J Obstet Gynecol. 2002;187:1596-1604.
24. Guzman ER, Walters C, O’Reilly-Green C, et al. Use of cervical ultrasonography in prediction of spontaneous preterm birth in twin gestations. Am J Obstet Gynecol. 2000;183:1103-1107.
25. To MS, Fonseca EB, Molina FS, Cacho AM, Nicolaides KH. Maternal characteristics and cervical length in prediction of spontaneous early preterm delivery in twins. Am J Obstet Gynecol. 2006;194:1360-1365.
26. Honest H, Bachmann LM, Gupta JK, Kleijnen J, Khan KS. Accuracy of cervicovaginal fetal fibronectin test in predicting risk of spontaneous preterm birth: systematic review. BMJ. 2002;325:301.-
27. Goldenberg RL, Iams JD, Miodovnik M, et al. The preterm prediction study: risk factors in twin gestations. National Institute of Child Health and Human Development Maternal–Fetal Medicine Units Network. Am J Obstet Gynecol. 1996;175:1047-1053.
28. Gibson JL, Macara LM, Owen P, Young D, Macauley J, Mackenzie F. Prediction of preterm delivery in twin pregnancy: a prospective, observational study of cervical length and fetal fibronectin testing. Ultrasound Obstet Gynecol. 2004;23:561-566.
29. Berghella V, Odibo AO, Tolosa JE. Cerclage for prevention of preterm birth in women with a short cervix found on transvaginal ultrasound examination: a randomized trial. Am J Obstet Gynecol. 2004;191:1311-1317.
30. Rust OA, Atlas RO, Reed J, van Gaalen J, Baldussi J. Revisiting the short cervix detected by transvaginal ultrasound in the second trimester: why cerclage therapy may not help. Am J Obstet Gynecol. 2001;185:1098-1105.
31. Berghella V, Odibo AO, To MS, Rust OA, Althuisius SM. Cerclage for short cervix on ultrasonography: meta-analysis of trials using individual patient-level data. Obstet Gynecol. 2005;106:181-189.
32. Meis PJ, Klebanoff M, Thom E, et al. Prevention of recurrent preterm delivery by 17 alpha-hydroxyprogesterone caproate. N Engl J Med. 2003;348:2379-2385.
33. Caritis S, Rouse D. NICHD MFMU Network. A randomized controlled trial of 17-hydroxyprogesterone caproate for the prevention of preterm birth in twins. Am J Obstet Gynecol. 2006;195:S2.-
34. Crowther CA. Hospitalisation and bed rest for multiple pregnancy. Cochrane Database Syst Rev. 2001;(1):CD000110.-
35. Evans MI, Ciorica D, Britt DW, Fletcher JC. Update on selective reduction. Prenat Diagn. 2005;25:807-813.
36. Evans MI, Kaufman MI, Urban AJ, Britt DW, Fletcher JC. Fetal reduction from twins to a singleton: a reasonable consideration. Obstet Gynecol. 2004;104:102-109.
37. Stone J, Eddleman K, Lynch L, Berkowitz RL. A single center experience with 1000 consecutive cases of multifetal pregnancy reduction. Am J Obstet Gynecol. 2002;187:1163-1167.
38. Stone J, Matho A, Berkowitz R, Belogolovkin V, Eddleman K. Evolving trends in 2,000 cases of multifetal pregnancy reduction. Am J Obstet Gynecol. 2006;195:S184.-
Evaluating a Primary Care Tobacco Cessation Program [Published correction appears in: Fed Pract. 2007;24(8):45.]
Ectopic Ureter in Adulthood
The Process of Progress in Medicine, in Sports Medicine, and in Baseball Medicine
5 Points on Arthroscopic Double-Row and "Transosseous-Equivalent" Rotator Cuff Repair
It’s time to re-tool the annual exam: Here’s how
Where does this shift in the surveillance strategy for cervical cancer leave us? Implementing new screening intervals gives us a wonderful opportunity to reevaluate the annual exam, and to educate ourselves and patients about interventions that make an impact on health.
Eliminate the annual exam?
Do we still need routine encounters with our patients? In this article, I address 2 topics that can help answer the question: I review the evidence that supports annual “well-woman” visits and outline the interventions that have proven benefit.
Time to retire a time-honored tradition
The utility of an annual health visit—ie, a comprehensive head-to-toe physical exam coupled with a battery of tests for early identification of disease and intervention—came into question with the rise of evidence-based medicine in the mid-1970s and, eventually, became unsupportable. In 1979, the Canadian Task Force on the Periodic Health Examination concluded that the value of only a few preventive interventions was supported by data. In 1989, Oboler and colleagues concluded that “comprehensive annual exams in asymptomatic adults have little screening value…”1
The American College of Physicians, American Medical Association, US Preventive Services Task Force (USPSTF), and US Public Health Service all concur: The routine, annual, comprehensive physical exam is unnecessary. Instead, physicians should institute a selective approach to identifying and preventing health problems in all patients—one based on gender, age, health history, and risk factors.
Some interventions have helped
The incidence of, and mortality from, cervical cancer dropped strikingly in the United States with the advent of annual screening with the Pap smear. Mammography has recently been proved to increase the early detection rate of breast cancer and to reduce the rate of death from breast cancer. The challenge we face, therefore, is to determine which screening tests and interventions are valuable and will translate into improved health outcomes. The USPSTF has set out broad recommendations on 10 areas of screening for women:
- monitor blood pressure
- screen for cervical and colorectal cancers, depression, diabetes, and osteoporosis
- test for chlamydial infection
- measure the cholesterol level
- perform mammography.
New tool helps you develop an exam
Available for you is an excellent online resource developed by the Agency for Healthcare Research and Quality (AHRQ) for adopting the USPSTF screening recommendations. AHRQ has created the “electronic preventive services select” (or ePSS) Web site (http://epss.ahrq.gov), which is searchable by patient sex, age, and behavioral risk factors. The evidence for various preventive services is graded, guiding you on both interventions that are strongly recommended and those that should not be offered routinely because they lack data to support utility.
Make the transition with a systematic approach
We can capitalize on the habit that patients have established and have them come in annually for appropriate, evidence-based services. How do we make the change from the typical ObGyn visit—one that includes a breast and pelvic examination, cervical cancer screening, and mammography—to an evidence-based, annual well-woman visit that can be rapidly implemented and easily documented, using a paper or an electronic medical record?
I recommend creating templates for the annual well-woman visit that are age-specific and include check boxes for the age-appropriate history, physical exam, testing, and counseling that you’ll provide. You can create a distinct form for each of the various age and risk groups or, more simply, devise a single form that includes all guidelines for screening, from which you choose the appropriate areas based, again, on age and risk status.
What should you include on the template that you create? Here are possible items, based on what I use in my practice:
History. Document the patient’s age, allergies, medications, contraceptive method, and risk factors (eg, smoking, a history of infection with high-risk HPV types, and a significant family history of colon, breast, and ovarian cancer and of heart disease and diabetes). Develop a problem list of concerns that the patient, and you, have. Note: I ask the patient to complete a checklist review of systems at every annual visit; doing so helps identify specific health concerns she may want to discuss.
Physical exam. Measure height, weight, body mass index, and blood pressure. Check off items included in the examination of breast, abdominal, and pelvic structures, and elaborate on abnormal findings in a space provided. Include an area on the form for noting “other” concerns, such as findings of skin, musculoskeletal, upper respiratory, and cardiac assessments—any of which is performed as indicated.
Lab testing. Document routine testing with 1) a check box to indicate which tests have been ordered and 2) a line on which to note the tests that were identified as appropriate but were not performed or were deemed inappropriate—and why. Such documentation is helpful when coding pay-for-performance measures.
Counseling. Develop a list that includes smoking cessation, weight loss, exercise, contraception, and prevention of osteoporosis and sexually transmitted infection. The list helps you recall, and discuss, essential areas (TABLE 1).
The goal in developing and using a template? It provides a single, easy-to-use form that is flexible and applicable to all women, and that encourages consistent adherence to guidelines for screening and prevention.
TABLE 1
Remember to provide lifestyle counseling!
| Don’t smoke |
| Drink alcohol in moderation |
| Eat healthy—ie, high-fiber, low-fat foods, including fruits and vegetables |
| Exercise often—ie, aerobic, weight-bearing, and balance activities |
| Maintain healthy weight* |
| Use a condom during sexual intercourse |
| Use a contraceptive |
| *Be prepared to provide strategies for effective, sustainable weight loss to your patients |
With a format in place, screen in 7 areas
What do guidelines recommend that we embrace as interventions to make a difference in patients’ long-term health? Research and consensus have established that the annual well-woman visit be organized around clinical areas of concern, comprising 7 primary intervention areas and 3 optional areas of general health (TABLE 2). In addition, ObGyns are well-positioned to add several areas of counseling, support, and intervention:
- lifelong contraception management and planning
- pre-pregnancy counseling
- prevention of sexually transmitted infection
- identification of sexual concerns
- management of menopause.
Chlamydial infection. “Grade-A” evidence supports annual screening for Chlamydia trachomatis for 1) all sexually active women 25 years and younger and 2) older women who engage in high-risk behavior (eg, more than one sex partner).
Pap smear and HPV typing. ACOG and the American Cancer Society recommend annual Pap smear testing beginning 3 years after the onset of sexual activity and continuing until 30 years of age. Routine testing for high-risk HPV subtypes may be undertaken with the Pap smear for women older than 30 years.
For most women who test negative for HPV and who have negative Pap smear cytology, Pap smear testing should be repeated no more often than every 3 years. Women who are positive for a high-risk HPV type despite a negative Pap smear should continue to be screened annually with cytology and HPV testing.
Breast health. Many groups recommend training women to perform monthly breast self-examination (BSE), although the USPSTF states that there is “insufficient evidence to recommend for or against” BSE. All groups do, however, advise an annual breast examination by a clinician, along with annual or biennial mammography beginning at 40 years of age and annual mammography beginning at 50 years.
Although many women do detect a breast lump when performing a BSE, it is unclear whether BSE improves survival from breast cancer. That’s because many lumps that women discover are benign.
Generally, therefore, I tell patients to pay attention to their breasts as they would other body parts: Don’t ignore an obvious change but don’t feel it is necessary to perform a standardized examination of the breasts monthly; evidence just does not support such a need.
Cardiovascular health. Assess blood pressure in every patient at every visit. Persistently high readings (>130/80 mm Hg) should prompt action—whether lifestyle modification or medication. Many physicians are slow to treat young women with so-called labile or borderline hypertension because the onset of cardiovascular disease is generally at an older age in women, but evidence shows that women suffer from proportionately more strokes at a young age than men do. Aggressive management of persistent hypertension may improve outcome.
- Aspirin therapy is recommended for prevention of stroke in women 45 to 65 years who are at risk. Do not recommend aspirin routinely, however, for women younger than 65 years as a means of preventing myocardial infarction.
- Perform a baseline lipid profile on all women older than 45 years. A woman who has a risk factor for cardiovascular disease—smoking, hypertension, obesity or overweight, a family history of early-onset cardiovascular disease—should be screened at any age.
- Screening may be performed as a random lipid profile to eliminate the barrier of returning after an 8-hour fast. Only women who have a significant abnormality need to return for repeat testing after an overnight fast.
- I usually intervene with lifestyle modification recommendations first—more exercise, weight loss, more monounsaturated fats and omega-3 fats in the diet—and have the patient return for a fasting lipid profile after 3 to 6 months.
Although quality evidence is lacking on the benefit of counseling about weight reduction and exercise, my experience is that providing a message to patients consistently about a healthy lifestyle is more effective than almost any other medical intervention. To have an impact on cardiovascular health, however, it is imperative that we have basic knowledge about nutrition and exercise physiology—which were not taught in medical school.
It is, clearly, not useful to simply tell a patient to lose weight. Evidence does support sustained weight loss when a person participates in an organized program, such as Weight Watchers. Even moderate weight loss is associated with a reduction in the risk of hypertension, an improvement in lipid levels, and a substantial reduction in the risk of breast cancer.
I find that this last statistic—namely, that lifetime physical activity and maintenance of normal body weight is associated with a 20% to 40% reduction in the risk of breast cancer compared with the risk in women who do not exercise or who gain 10 kg or more above their high school weight—is a huge motivator. Why? It’s well-known that women are more concerned about breast cancer than about cardiovascular disease—even though statistics demonstrate that heart disease is the leading cause of death among women.
Diabetes. Women who have a history of gestational diabetes also have a markedly increased risk of type II diabetes within 5 years of the pregnancy. Clearly, these women, as well as those who are obese, have a strong family history of diabetes, or have abnormal lipid levels, should be screened with a random glucose measurement. Women who suffer chronic monilial infection should also be assessed for diabetes.
Colorectal cancer. The second leading cause of cancer death and the fourth most common cancer in the United States carries the same risk for women as it does for men. Polyps and cancers are more likely to present on the right (ascending) side of the colon in women, however, making screening with flexible sigmoidoscopy potentially less useful.
Sixty-five percent of the US population has not been adequately screened for colorectal cancer. This is regrettable, because good-quality data support an association between screening and a reduction in mortality—even simple screening with annual fecal occult blood testing. Ideally, colon cancer testing in people of average risk should begin at 50 years with either
- colonoscopy every 10 years
- flexible sigmoidoscopy every 5 years with or without annual fecal occult blood testing
- dual-contrast barium enema every 5 years
- fecal occult blood testing annually or
- perhaps, virtual colonoscopy or stool-based DNA testing for patients who decline traditional evaluation.
Osteoporosis. For most women, screening for osteoporosis should begin at 65 years with a test of bone mineral density. Younger women who have a significant risk factor (weight, less than 127 pounds; hyperthyroidism; steroid use; a strong family history) might benefit from screening at an earlier age. All women who take more than 7.5 mg of prednisone daily or who have sustained a nontraumatic fracture should be treated to prevent osteoporosis regardless of findings on a dual energy x-ray absortiometry (DEXA) scan.
(Note: It is vital for you to provide osteoporosis screening to Medicare patients because this is 1 of only 2 office-based performance measures in the voluntary Medicare pay-for-performance list for 2007 that are applicable to gynecology practice; the other is screening for incontinence.)
Depression. We know that depression is more common, and tends to present with more physical complaints, in women than in men. Any patient who has vague somatic symptoms, chronic pain, fatigue, decreased libido, and sleep disturbances, or such “hormonal” complaints as premenstrual syndrome and hot flashes, should be screened for depression.
I have found that the Beck Depression Inventory is easy and quick to administer if indicated. This screening instrument can be downloaded from several Web sites (search the terms Beck/Depression/Inventory). For patients who screen positive, provide a resource sheet that includes a listing of specialist referrals and local depression hotline numbers.
TABLE 2
The pillars of an annual primary screening program
| ESSENTIAL | |
|
|
| OPTIONAL | |
|
|
Plus 3 at your discretion
The USPSTF has listed 3 optional areas for annual assessment: thyroid disease, bladder health, and domestic violence.
Thyroid. Because thyroid abnormalities are more common in women and because they may have an impact on the regularity of the menstrual cycle and on weight and hair loss, it seems sensible and appropriate to screen on a selected basis with a test of thyroid-stimulating hormone.
Incontinence. You should definitely include this problem in the review-of-systems questionnaire. Doing so will not only help the patient identify an embarrassing problem that she may be reluctant to bring up, but will also help drive additional services in your practice—such as urodynamic evaluation and surgery.
Build a relationship
The annual visit should reinforce the physician–patient relationship by educating women about the appropriate screening tests and supporting them as active participants in their health care. Take a consistent, balanced approach that complies with guidelines but that also addresses the patient’s concerns by incorporating education and appropriate interventions.
1. Oboler SK, LaForce FM. The periodic physical examination in asymptomatic adults. Ann Intern Med. 1989;110:214-226.
The author reports no financial relationships relevant to this article.
Where does this shift in the surveillance strategy for cervical cancer leave us? Implementing new screening intervals gives us a wonderful opportunity to reevaluate the annual exam, and to educate ourselves and patients about interventions that make an impact on health.
Eliminate the annual exam?
Do we still need routine encounters with our patients? In this article, I address 2 topics that can help answer the question: I review the evidence that supports annual “well-woman” visits and outline the interventions that have proven benefit.
Time to retire a time-honored tradition
The utility of an annual health visit—ie, a comprehensive head-to-toe physical exam coupled with a battery of tests for early identification of disease and intervention—came into question with the rise of evidence-based medicine in the mid-1970s and, eventually, became unsupportable. In 1979, the Canadian Task Force on the Periodic Health Examination concluded that the value of only a few preventive interventions was supported by data. In 1989, Oboler and colleagues concluded that “comprehensive annual exams in asymptomatic adults have little screening value…”1
The American College of Physicians, American Medical Association, US Preventive Services Task Force (USPSTF), and US Public Health Service all concur: The routine, annual, comprehensive physical exam is unnecessary. Instead, physicians should institute a selective approach to identifying and preventing health problems in all patients—one based on gender, age, health history, and risk factors.
Some interventions have helped
The incidence of, and mortality from, cervical cancer dropped strikingly in the United States with the advent of annual screening with the Pap smear. Mammography has recently been proved to increase the early detection rate of breast cancer and to reduce the rate of death from breast cancer. The challenge we face, therefore, is to determine which screening tests and interventions are valuable and will translate into improved health outcomes. The USPSTF has set out broad recommendations on 10 areas of screening for women:
- monitor blood pressure
- screen for cervical and colorectal cancers, depression, diabetes, and osteoporosis
- test for chlamydial infection
- measure the cholesterol level
- perform mammography.
New tool helps you develop an exam
Available for you is an excellent online resource developed by the Agency for Healthcare Research and Quality (AHRQ) for adopting the USPSTF screening recommendations. AHRQ has created the “electronic preventive services select” (or ePSS) Web site (http://epss.ahrq.gov), which is searchable by patient sex, age, and behavioral risk factors. The evidence for various preventive services is graded, guiding you on both interventions that are strongly recommended and those that should not be offered routinely because they lack data to support utility.
Make the transition with a systematic approach
We can capitalize on the habit that patients have established and have them come in annually for appropriate, evidence-based services. How do we make the change from the typical ObGyn visit—one that includes a breast and pelvic examination, cervical cancer screening, and mammography—to an evidence-based, annual well-woman visit that can be rapidly implemented and easily documented, using a paper or an electronic medical record?
I recommend creating templates for the annual well-woman visit that are age-specific and include check boxes for the age-appropriate history, physical exam, testing, and counseling that you’ll provide. You can create a distinct form for each of the various age and risk groups or, more simply, devise a single form that includes all guidelines for screening, from which you choose the appropriate areas based, again, on age and risk status.
What should you include on the template that you create? Here are possible items, based on what I use in my practice:
History. Document the patient’s age, allergies, medications, contraceptive method, and risk factors (eg, smoking, a history of infection with high-risk HPV types, and a significant family history of colon, breast, and ovarian cancer and of heart disease and diabetes). Develop a problem list of concerns that the patient, and you, have. Note: I ask the patient to complete a checklist review of systems at every annual visit; doing so helps identify specific health concerns she may want to discuss.
Physical exam. Measure height, weight, body mass index, and blood pressure. Check off items included in the examination of breast, abdominal, and pelvic structures, and elaborate on abnormal findings in a space provided. Include an area on the form for noting “other” concerns, such as findings of skin, musculoskeletal, upper respiratory, and cardiac assessments—any of which is performed as indicated.
Lab testing. Document routine testing with 1) a check box to indicate which tests have been ordered and 2) a line on which to note the tests that were identified as appropriate but were not performed or were deemed inappropriate—and why. Such documentation is helpful when coding pay-for-performance measures.
Counseling. Develop a list that includes smoking cessation, weight loss, exercise, contraception, and prevention of osteoporosis and sexually transmitted infection. The list helps you recall, and discuss, essential areas (TABLE 1).
The goal in developing and using a template? It provides a single, easy-to-use form that is flexible and applicable to all women, and that encourages consistent adherence to guidelines for screening and prevention.
TABLE 1
Remember to provide lifestyle counseling!
| Don’t smoke |
| Drink alcohol in moderation |
| Eat healthy—ie, high-fiber, low-fat foods, including fruits and vegetables |
| Exercise often—ie, aerobic, weight-bearing, and balance activities |
| Maintain healthy weight* |
| Use a condom during sexual intercourse |
| Use a contraceptive |
| *Be prepared to provide strategies for effective, sustainable weight loss to your patients |
With a format in place, screen in 7 areas
What do guidelines recommend that we embrace as interventions to make a difference in patients’ long-term health? Research and consensus have established that the annual well-woman visit be organized around clinical areas of concern, comprising 7 primary intervention areas and 3 optional areas of general health (TABLE 2). In addition, ObGyns are well-positioned to add several areas of counseling, support, and intervention:
- lifelong contraception management and planning
- pre-pregnancy counseling
- prevention of sexually transmitted infection
- identification of sexual concerns
- management of menopause.
Chlamydial infection. “Grade-A” evidence supports annual screening for Chlamydia trachomatis for 1) all sexually active women 25 years and younger and 2) older women who engage in high-risk behavior (eg, more than one sex partner).
Pap smear and HPV typing. ACOG and the American Cancer Society recommend annual Pap smear testing beginning 3 years after the onset of sexual activity and continuing until 30 years of age. Routine testing for high-risk HPV subtypes may be undertaken with the Pap smear for women older than 30 years.
For most women who test negative for HPV and who have negative Pap smear cytology, Pap smear testing should be repeated no more often than every 3 years. Women who are positive for a high-risk HPV type despite a negative Pap smear should continue to be screened annually with cytology and HPV testing.
Breast health. Many groups recommend training women to perform monthly breast self-examination (BSE), although the USPSTF states that there is “insufficient evidence to recommend for or against” BSE. All groups do, however, advise an annual breast examination by a clinician, along with annual or biennial mammography beginning at 40 years of age and annual mammography beginning at 50 years.
Although many women do detect a breast lump when performing a BSE, it is unclear whether BSE improves survival from breast cancer. That’s because many lumps that women discover are benign.
Generally, therefore, I tell patients to pay attention to their breasts as they would other body parts: Don’t ignore an obvious change but don’t feel it is necessary to perform a standardized examination of the breasts monthly; evidence just does not support such a need.
Cardiovascular health. Assess blood pressure in every patient at every visit. Persistently high readings (>130/80 mm Hg) should prompt action—whether lifestyle modification or medication. Many physicians are slow to treat young women with so-called labile or borderline hypertension because the onset of cardiovascular disease is generally at an older age in women, but evidence shows that women suffer from proportionately more strokes at a young age than men do. Aggressive management of persistent hypertension may improve outcome.
- Aspirin therapy is recommended for prevention of stroke in women 45 to 65 years who are at risk. Do not recommend aspirin routinely, however, for women younger than 65 years as a means of preventing myocardial infarction.
- Perform a baseline lipid profile on all women older than 45 years. A woman who has a risk factor for cardiovascular disease—smoking, hypertension, obesity or overweight, a family history of early-onset cardiovascular disease—should be screened at any age.
- Screening may be performed as a random lipid profile to eliminate the barrier of returning after an 8-hour fast. Only women who have a significant abnormality need to return for repeat testing after an overnight fast.
- I usually intervene with lifestyle modification recommendations first—more exercise, weight loss, more monounsaturated fats and omega-3 fats in the diet—and have the patient return for a fasting lipid profile after 3 to 6 months.
Although quality evidence is lacking on the benefit of counseling about weight reduction and exercise, my experience is that providing a message to patients consistently about a healthy lifestyle is more effective than almost any other medical intervention. To have an impact on cardiovascular health, however, it is imperative that we have basic knowledge about nutrition and exercise physiology—which were not taught in medical school.
It is, clearly, not useful to simply tell a patient to lose weight. Evidence does support sustained weight loss when a person participates in an organized program, such as Weight Watchers. Even moderate weight loss is associated with a reduction in the risk of hypertension, an improvement in lipid levels, and a substantial reduction in the risk of breast cancer.
I find that this last statistic—namely, that lifetime physical activity and maintenance of normal body weight is associated with a 20% to 40% reduction in the risk of breast cancer compared with the risk in women who do not exercise or who gain 10 kg or more above their high school weight—is a huge motivator. Why? It’s well-known that women are more concerned about breast cancer than about cardiovascular disease—even though statistics demonstrate that heart disease is the leading cause of death among women.
Diabetes. Women who have a history of gestational diabetes also have a markedly increased risk of type II diabetes within 5 years of the pregnancy. Clearly, these women, as well as those who are obese, have a strong family history of diabetes, or have abnormal lipid levels, should be screened with a random glucose measurement. Women who suffer chronic monilial infection should also be assessed for diabetes.
Colorectal cancer. The second leading cause of cancer death and the fourth most common cancer in the United States carries the same risk for women as it does for men. Polyps and cancers are more likely to present on the right (ascending) side of the colon in women, however, making screening with flexible sigmoidoscopy potentially less useful.
Sixty-five percent of the US population has not been adequately screened for colorectal cancer. This is regrettable, because good-quality data support an association between screening and a reduction in mortality—even simple screening with annual fecal occult blood testing. Ideally, colon cancer testing in people of average risk should begin at 50 years with either
- colonoscopy every 10 years
- flexible sigmoidoscopy every 5 years with or without annual fecal occult blood testing
- dual-contrast barium enema every 5 years
- fecal occult blood testing annually or
- perhaps, virtual colonoscopy or stool-based DNA testing for patients who decline traditional evaluation.
Osteoporosis. For most women, screening for osteoporosis should begin at 65 years with a test of bone mineral density. Younger women who have a significant risk factor (weight, less than 127 pounds; hyperthyroidism; steroid use; a strong family history) might benefit from screening at an earlier age. All women who take more than 7.5 mg of prednisone daily or who have sustained a nontraumatic fracture should be treated to prevent osteoporosis regardless of findings on a dual energy x-ray absortiometry (DEXA) scan.
(Note: It is vital for you to provide osteoporosis screening to Medicare patients because this is 1 of only 2 office-based performance measures in the voluntary Medicare pay-for-performance list for 2007 that are applicable to gynecology practice; the other is screening for incontinence.)
Depression. We know that depression is more common, and tends to present with more physical complaints, in women than in men. Any patient who has vague somatic symptoms, chronic pain, fatigue, decreased libido, and sleep disturbances, or such “hormonal” complaints as premenstrual syndrome and hot flashes, should be screened for depression.
I have found that the Beck Depression Inventory is easy and quick to administer if indicated. This screening instrument can be downloaded from several Web sites (search the terms Beck/Depression/Inventory). For patients who screen positive, provide a resource sheet that includes a listing of specialist referrals and local depression hotline numbers.
TABLE 2
The pillars of an annual primary screening program
| ESSENTIAL | |
|
|
| OPTIONAL | |
|
|
Plus 3 at your discretion
The USPSTF has listed 3 optional areas for annual assessment: thyroid disease, bladder health, and domestic violence.
Thyroid. Because thyroid abnormalities are more common in women and because they may have an impact on the regularity of the menstrual cycle and on weight and hair loss, it seems sensible and appropriate to screen on a selected basis with a test of thyroid-stimulating hormone.
Incontinence. You should definitely include this problem in the review-of-systems questionnaire. Doing so will not only help the patient identify an embarrassing problem that she may be reluctant to bring up, but will also help drive additional services in your practice—such as urodynamic evaluation and surgery.
Build a relationship
The annual visit should reinforce the physician–patient relationship by educating women about the appropriate screening tests and supporting them as active participants in their health care. Take a consistent, balanced approach that complies with guidelines but that also addresses the patient’s concerns by incorporating education and appropriate interventions.
Where does this shift in the surveillance strategy for cervical cancer leave us? Implementing new screening intervals gives us a wonderful opportunity to reevaluate the annual exam, and to educate ourselves and patients about interventions that make an impact on health.
Eliminate the annual exam?
Do we still need routine encounters with our patients? In this article, I address 2 topics that can help answer the question: I review the evidence that supports annual “well-woman” visits and outline the interventions that have proven benefit.
Time to retire a time-honored tradition
The utility of an annual health visit—ie, a comprehensive head-to-toe physical exam coupled with a battery of tests for early identification of disease and intervention—came into question with the rise of evidence-based medicine in the mid-1970s and, eventually, became unsupportable. In 1979, the Canadian Task Force on the Periodic Health Examination concluded that the value of only a few preventive interventions was supported by data. In 1989, Oboler and colleagues concluded that “comprehensive annual exams in asymptomatic adults have little screening value…”1
The American College of Physicians, American Medical Association, US Preventive Services Task Force (USPSTF), and US Public Health Service all concur: The routine, annual, comprehensive physical exam is unnecessary. Instead, physicians should institute a selective approach to identifying and preventing health problems in all patients—one based on gender, age, health history, and risk factors.
Some interventions have helped
The incidence of, and mortality from, cervical cancer dropped strikingly in the United States with the advent of annual screening with the Pap smear. Mammography has recently been proved to increase the early detection rate of breast cancer and to reduce the rate of death from breast cancer. The challenge we face, therefore, is to determine which screening tests and interventions are valuable and will translate into improved health outcomes. The USPSTF has set out broad recommendations on 10 areas of screening for women:
- monitor blood pressure
- screen for cervical and colorectal cancers, depression, diabetes, and osteoporosis
- test for chlamydial infection
- measure the cholesterol level
- perform mammography.
New tool helps you develop an exam
Available for you is an excellent online resource developed by the Agency for Healthcare Research and Quality (AHRQ) for adopting the USPSTF screening recommendations. AHRQ has created the “electronic preventive services select” (or ePSS) Web site (http://epss.ahrq.gov), which is searchable by patient sex, age, and behavioral risk factors. The evidence for various preventive services is graded, guiding you on both interventions that are strongly recommended and those that should not be offered routinely because they lack data to support utility.
Make the transition with a systematic approach
We can capitalize on the habit that patients have established and have them come in annually for appropriate, evidence-based services. How do we make the change from the typical ObGyn visit—one that includes a breast and pelvic examination, cervical cancer screening, and mammography—to an evidence-based, annual well-woman visit that can be rapidly implemented and easily documented, using a paper or an electronic medical record?
I recommend creating templates for the annual well-woman visit that are age-specific and include check boxes for the age-appropriate history, physical exam, testing, and counseling that you’ll provide. You can create a distinct form for each of the various age and risk groups or, more simply, devise a single form that includes all guidelines for screening, from which you choose the appropriate areas based, again, on age and risk status.
What should you include on the template that you create? Here are possible items, based on what I use in my practice:
History. Document the patient’s age, allergies, medications, contraceptive method, and risk factors (eg, smoking, a history of infection with high-risk HPV types, and a significant family history of colon, breast, and ovarian cancer and of heart disease and diabetes). Develop a problem list of concerns that the patient, and you, have. Note: I ask the patient to complete a checklist review of systems at every annual visit; doing so helps identify specific health concerns she may want to discuss.
Physical exam. Measure height, weight, body mass index, and blood pressure. Check off items included in the examination of breast, abdominal, and pelvic structures, and elaborate on abnormal findings in a space provided. Include an area on the form for noting “other” concerns, such as findings of skin, musculoskeletal, upper respiratory, and cardiac assessments—any of which is performed as indicated.
Lab testing. Document routine testing with 1) a check box to indicate which tests have been ordered and 2) a line on which to note the tests that were identified as appropriate but were not performed or were deemed inappropriate—and why. Such documentation is helpful when coding pay-for-performance measures.
Counseling. Develop a list that includes smoking cessation, weight loss, exercise, contraception, and prevention of osteoporosis and sexually transmitted infection. The list helps you recall, and discuss, essential areas (TABLE 1).
The goal in developing and using a template? It provides a single, easy-to-use form that is flexible and applicable to all women, and that encourages consistent adherence to guidelines for screening and prevention.
TABLE 1
Remember to provide lifestyle counseling!
| Don’t smoke |
| Drink alcohol in moderation |
| Eat healthy—ie, high-fiber, low-fat foods, including fruits and vegetables |
| Exercise often—ie, aerobic, weight-bearing, and balance activities |
| Maintain healthy weight* |
| Use a condom during sexual intercourse |
| Use a contraceptive |
| *Be prepared to provide strategies for effective, sustainable weight loss to your patients |
With a format in place, screen in 7 areas
What do guidelines recommend that we embrace as interventions to make a difference in patients’ long-term health? Research and consensus have established that the annual well-woman visit be organized around clinical areas of concern, comprising 7 primary intervention areas and 3 optional areas of general health (TABLE 2). In addition, ObGyns are well-positioned to add several areas of counseling, support, and intervention:
- lifelong contraception management and planning
- pre-pregnancy counseling
- prevention of sexually transmitted infection
- identification of sexual concerns
- management of menopause.
Chlamydial infection. “Grade-A” evidence supports annual screening for Chlamydia trachomatis for 1) all sexually active women 25 years and younger and 2) older women who engage in high-risk behavior (eg, more than one sex partner).
Pap smear and HPV typing. ACOG and the American Cancer Society recommend annual Pap smear testing beginning 3 years after the onset of sexual activity and continuing until 30 years of age. Routine testing for high-risk HPV subtypes may be undertaken with the Pap smear for women older than 30 years.
For most women who test negative for HPV and who have negative Pap smear cytology, Pap smear testing should be repeated no more often than every 3 years. Women who are positive for a high-risk HPV type despite a negative Pap smear should continue to be screened annually with cytology and HPV testing.
Breast health. Many groups recommend training women to perform monthly breast self-examination (BSE), although the USPSTF states that there is “insufficient evidence to recommend for or against” BSE. All groups do, however, advise an annual breast examination by a clinician, along with annual or biennial mammography beginning at 40 years of age and annual mammography beginning at 50 years.
Although many women do detect a breast lump when performing a BSE, it is unclear whether BSE improves survival from breast cancer. That’s because many lumps that women discover are benign.
Generally, therefore, I tell patients to pay attention to their breasts as they would other body parts: Don’t ignore an obvious change but don’t feel it is necessary to perform a standardized examination of the breasts monthly; evidence just does not support such a need.
Cardiovascular health. Assess blood pressure in every patient at every visit. Persistently high readings (>130/80 mm Hg) should prompt action—whether lifestyle modification or medication. Many physicians are slow to treat young women with so-called labile or borderline hypertension because the onset of cardiovascular disease is generally at an older age in women, but evidence shows that women suffer from proportionately more strokes at a young age than men do. Aggressive management of persistent hypertension may improve outcome.
- Aspirin therapy is recommended for prevention of stroke in women 45 to 65 years who are at risk. Do not recommend aspirin routinely, however, for women younger than 65 years as a means of preventing myocardial infarction.
- Perform a baseline lipid profile on all women older than 45 years. A woman who has a risk factor for cardiovascular disease—smoking, hypertension, obesity or overweight, a family history of early-onset cardiovascular disease—should be screened at any age.
- Screening may be performed as a random lipid profile to eliminate the barrier of returning after an 8-hour fast. Only women who have a significant abnormality need to return for repeat testing after an overnight fast.
- I usually intervene with lifestyle modification recommendations first—more exercise, weight loss, more monounsaturated fats and omega-3 fats in the diet—and have the patient return for a fasting lipid profile after 3 to 6 months.
Although quality evidence is lacking on the benefit of counseling about weight reduction and exercise, my experience is that providing a message to patients consistently about a healthy lifestyle is more effective than almost any other medical intervention. To have an impact on cardiovascular health, however, it is imperative that we have basic knowledge about nutrition and exercise physiology—which were not taught in medical school.
It is, clearly, not useful to simply tell a patient to lose weight. Evidence does support sustained weight loss when a person participates in an organized program, such as Weight Watchers. Even moderate weight loss is associated with a reduction in the risk of hypertension, an improvement in lipid levels, and a substantial reduction in the risk of breast cancer.
I find that this last statistic—namely, that lifetime physical activity and maintenance of normal body weight is associated with a 20% to 40% reduction in the risk of breast cancer compared with the risk in women who do not exercise or who gain 10 kg or more above their high school weight—is a huge motivator. Why? It’s well-known that women are more concerned about breast cancer than about cardiovascular disease—even though statistics demonstrate that heart disease is the leading cause of death among women.
Diabetes. Women who have a history of gestational diabetes also have a markedly increased risk of type II diabetes within 5 years of the pregnancy. Clearly, these women, as well as those who are obese, have a strong family history of diabetes, or have abnormal lipid levels, should be screened with a random glucose measurement. Women who suffer chronic monilial infection should also be assessed for diabetes.
Colorectal cancer. The second leading cause of cancer death and the fourth most common cancer in the United States carries the same risk for women as it does for men. Polyps and cancers are more likely to present on the right (ascending) side of the colon in women, however, making screening with flexible sigmoidoscopy potentially less useful.
Sixty-five percent of the US population has not been adequately screened for colorectal cancer. This is regrettable, because good-quality data support an association between screening and a reduction in mortality—even simple screening with annual fecal occult blood testing. Ideally, colon cancer testing in people of average risk should begin at 50 years with either
- colonoscopy every 10 years
- flexible sigmoidoscopy every 5 years with or without annual fecal occult blood testing
- dual-contrast barium enema every 5 years
- fecal occult blood testing annually or
- perhaps, virtual colonoscopy or stool-based DNA testing for patients who decline traditional evaluation.
Osteoporosis. For most women, screening for osteoporosis should begin at 65 years with a test of bone mineral density. Younger women who have a significant risk factor (weight, less than 127 pounds; hyperthyroidism; steroid use; a strong family history) might benefit from screening at an earlier age. All women who take more than 7.5 mg of prednisone daily or who have sustained a nontraumatic fracture should be treated to prevent osteoporosis regardless of findings on a dual energy x-ray absortiometry (DEXA) scan.
(Note: It is vital for you to provide osteoporosis screening to Medicare patients because this is 1 of only 2 office-based performance measures in the voluntary Medicare pay-for-performance list for 2007 that are applicable to gynecology practice; the other is screening for incontinence.)
Depression. We know that depression is more common, and tends to present with more physical complaints, in women than in men. Any patient who has vague somatic symptoms, chronic pain, fatigue, decreased libido, and sleep disturbances, or such “hormonal” complaints as premenstrual syndrome and hot flashes, should be screened for depression.
I have found that the Beck Depression Inventory is easy and quick to administer if indicated. This screening instrument can be downloaded from several Web sites (search the terms Beck/Depression/Inventory). For patients who screen positive, provide a resource sheet that includes a listing of specialist referrals and local depression hotline numbers.
TABLE 2
The pillars of an annual primary screening program
| ESSENTIAL | |
|
|
| OPTIONAL | |
|
|
Plus 3 at your discretion
The USPSTF has listed 3 optional areas for annual assessment: thyroid disease, bladder health, and domestic violence.
Thyroid. Because thyroid abnormalities are more common in women and because they may have an impact on the regularity of the menstrual cycle and on weight and hair loss, it seems sensible and appropriate to screen on a selected basis with a test of thyroid-stimulating hormone.
Incontinence. You should definitely include this problem in the review-of-systems questionnaire. Doing so will not only help the patient identify an embarrassing problem that she may be reluctant to bring up, but will also help drive additional services in your practice—such as urodynamic evaluation and surgery.
Build a relationship
The annual visit should reinforce the physician–patient relationship by educating women about the appropriate screening tests and supporting them as active participants in their health care. Take a consistent, balanced approach that complies with guidelines but that also addresses the patient’s concerns by incorporating education and appropriate interventions.
1. Oboler SK, LaForce FM. The periodic physical examination in asymptomatic adults. Ann Intern Med. 1989;110:214-226.
The author reports no financial relationships relevant to this article.
1. Oboler SK, LaForce FM. The periodic physical examination in asymptomatic adults. Ann Intern Med. 1989;110:214-226.
The author reports no financial relationships relevant to this article.
INFECTIOUS DISEASE
Four studies caught my eye this past year. The first describes the use of systematic methodology to confirm the diagnosis of primary cytomegalovirus (CMV) infection in pregnancy and lower the rate of unnecessary pregnancy termination. Investigators were able to reclassify approximately 70% of women who had been diagnosed with CMV infection and reduce the number of pregnancy terminations by 73%.
Two other studies help define the emerging problem of community-acquired methicillin-resistant Staphylococcus aureus (MRSA) infection, when to look for it, and how to treat it. In the first, researchers isolated S. aureus from the wounds of 320 patients with community-acquired infection and tested the samples for methicillin resistance, finding a prevalence of 59%. In the second study, investigators analyzed culture specimens from pregnant women for the presence of group B streptococci and S. aureus colonization. They found colonization with group B streptococci to be significantly associated with S. aureus colonization, with a prevalence odds ratio of 2.1.
The fourth study concerns the human papillomavirus (HPV) vaccine. Women given an HPV-16 L1 virus-like particle vaccine and followed for 4 years remained 100% free of cervical intraepithelial neoplasia (CIN) grades 2 and 3, unlike women who received placebo.
I believe these 4 studies represent the most significant developments of the past year in the field of infectious disease.
Don’t rush a diagnosis of CMV infection in pregnancy
Guerra B, Simonazzi G, Banfi A, et al. Impact of diagnostic and confirmatory tests and prenatal counseling on the rate of pregnancy termination among women with positive cytomegalovirus immunoglobulin M antibody titers. Am J Obstet Gynecol. 2007;196:221.e1–6.
CMV infection is a common and important perinatal pathogen. Each year in the United States, approximately 1% of gravidas acquire primary infection. Of these, about 40% transmit infection to the fetus. The rate of transmission is highest when maternal infection occurs in the third trimester, but the risk of serious fetal injury is greatest when maternal infection occurs in the first trimester. Ten percent to 20% of congenitally infected infants are acutely symptomatic at birth. Approximately 20% of these newborns die; most survivors have serious long-term complications. In contrast, CMV infection that recurs during pregnancy poses only minimal risk to the baby.1
Many women choose to have their pregnancy terminated when they learn they have a primary CMV infection.
Details of the study
This retrospective study was designed to determine whether a systematic diagnostic algorithm reduces the rate of unnecessary abortion in women who have apparent acute CMV infection during pregnancy. Guerra and colleagues evaluated 1,857 consecutive patients in practices in Italy who had a positive anti-CMV immunoglobulin M (IgM) antibody assay in the first or second trimester and were referred to a tertiary care facility for further diagnostic testing. Universal screening for CMV is now common among practitioners in Italy, and virtually all of these patients were completely asymptomatic.
At the tertiary facility, investigators tested again for CMV-specific IgM, as well as IgG, by enzyme immunoassay. They also tested for IgM by immunoblot and determined the avidity of anti-CMV IgG. Women who had IgG of low or moderate avidity with confirmed IgM, and those who clearly seroconverted to IgG were assumed to have a primary infection.
Women who were positive for IgM with high-avidity IgG were assumed to have nonprimary infection. Women who were seronegative for both antibodies were classified as uninfected. Those who were IgM-negative with high-avidity IgG were classified as previously infected. Women with an acute infection were then counseled by a specialist and offered amniocentesis and targeted ultrasonography.
Only 11.9% of women with primary infection chose abortion
Of the 1,857 women in this study, 445 were classified as having primary infection (group 1); 53 (11.9%) women elected to terminate their pregnancy. At autopsy, 38 of the 53 fetuses were found to be infected. In the other 15 cases, the pregnancy was terminated in the first trimester, and postmortem examination was not performed.
In the 1,205 women found to have nonprimary infection or previous infection (group 2), only 5 (0.4%) had the pregnancy terminated in the first trimester, and no postmortem examinations were performed. The difference in the observed rates of abortion between groups 1 and 2 was highly significant (P<.001).
Given their observations in group 1, the authors estimated that, on the basis of the initial screening tests at the referring institutions, approximately 196 (11.9%) of all patients in groups 1 and 2 would have elected abortion. By using confirmatory tests combined with counseling by a specialist, the authors were able to reduce the number of abortions from 196 to 53, a 73% decrease.
Always confirm an initial diagnosis
Given the ominous prognosis for congenital CMV infection and the major psychological implications and sobering finality of abortion, it is imperative that clinicians confirm the diagnosis of primary CMV infection. Because most cases of CMV infection in immunocompetent adults are asymptomatic, the diagnosis is typically confirmed by serology. Unfortunately, the serologic tests for CMV are not as straight-forward and reliable as tests for other viral infections such as rubella. Commercially available tests for anti-CMV IgM often have false-positive and false-negative results. In addition, IgM antibody may be detected as long as 9 months after a primary infection and may subsequently re-appear during reactivation of a latent infection or reinfection.2,3
Be selective, on the basis of risk factors and clinical manifestations, when screening pregnant women for cytomegalovirus infection.
Routine screening is not necessary
The authors’ findings vividly illustrate the potential errors that can occur when a large number of asymptomatic patients are routinely screened for CMV. Because of these pitfalls, I do not recommend routine screening. Rather, screening should be selective, directed at women who:
- have clinical manifestations of CMV infection
- are immunosuppressed
- have small children in daycare or work in daycare themselves or
- have documented exposure to someone with CMV infection.
If the initial immunoassay for CMV IgM is positive, a confirmatory immunoblot test for IgM should be performed, as well as avidity testing for IgG.
If primary infection is confirmed, the patient should undergo targeted ultrasonography and amniocentesis to assess for manifestations of congenital infection and to detect CMV in amniotic fluid by culture or polymerase chain reaction (PCR) testing. If the sonogram shows signs of fetal injury, or the PCR test is positive, the woman should be counseled about the options, which include experimental immunotherapy with hyperimmune anti-CMV globulin4 and pregnancy termination.
The study by Guerra and colleagues is a welcome addition to the obstetric literature. By using a systematic diagnostic algorithm that included an enzyme-linked immunosorbent assay and an immunoblot assay for IgM antibody and avidity testing for IgG antibody, the authors were able to reclassify approximately 70% of patients as either uninfected or previously infected. As a result, they reduced the number of pregnancy terminations by 73%, an objective end-point that clearly has great social, economic, and medical impact.
Most community S. aureus infections are methicillin-resistant
Moran GJ, Krishnadasan A, Gorwitz RJ, et al. Methicillin-resistant S. aureus infections among patients in the emergency department. N Engl J Med. 2006;355:666–674.
Moran and colleagues reviewed the records of 422 adults with acute purulent and soft-tissue infections who were evaluated in 11 university-affiliated emergency departments in August 2004. Wounds were routinely cultured. When S. aureus was isolated, the organisms were tested for antimicrobial susceptibility to identify those that were methicillin-resistant. The PCR test was used to identify genes for staphylococcal enterotoxins A through E and H, toxic shock syndrome toxin, and Panton–Valentin leukocidin. The same methodology was used to identify the gene complex staphylococcal cassette chromosome mec (SCCmec). This complex contains the mecA gene that confers methicillin resistance.
Of the 422 patients, 320 (76%) had S. aureus isolated from their wound. The prevalence of methicillin resistance was 59%. Ninety-seven percent of MRSA isolates were pulsed-field type USA 300. SCCmec type IV and the Panton–Valentin leukocidin gene were detected in 98% of MRSA isolates. Other toxin genes were rare.
Only 2 drugs were 100% effective
Among MRSA isolates, 100% were susceptible to rifampin and trimethoprim-sulfamethoxazole (TMP-SMX), 95% were susceptible to clindamycin, and 92% were sensitive to tetracycline. Only 60% were sensitive to fluoroquinolones, and only 6% were sensitive to erythromycin. Only 43% of patients received initial empiric therapy with antibiotics to which their organisms were sensitive.
Reason to worry
S. aureus is an important pathogen in obstetric patients. It is the causative organism of toxic shock syndrome and the dominant pathogen in patients with puerperal mastitis, as well as one of the key causes of postoperative wound infection. When penicillin was developed in 1941, all strains of S. aureus were sensitive to the drug. Within a few short years, however, most hospital-acquired strains became resistant.
Methicillin was introduced in 1961 to treat these resistant staphylococcal species. Unfortunately, by the mid-1960s, methicillin-resistant S. aureus (MRSA) infections began to appear. By the 1990s, MRSA infections were common in hospitalized patients, particularly in intensive care units. Hospital-acquired MRSA isolates are often sensitive to only a few select antibiotics such as vancomycin, linezolid, and quinupristin/dalfopristin.5
In the late 1990s and early 2000s, MRSA began to appear in community-acquired infections in both adults and children. Most of these isolates have been implicated in skin and soft-tissue infections, but some have been responsible for invasive infection, bacteremia, and even death.6 Compared with hospital-acquired MRSA, these community isolates are more likely to be sensitive to commonly used antibiotics.
Always culture an infected wound
Knowledge of these sensitivity patterns is of great importance. Regrettably, as noted by Moran and associates, more than half of the patients (57%) were initially treated with antibiotics to which their infecting organism was resistant.
The clinical implications are clear:
- We must be aware that many community-acquired soft-tissue infections will be caused by drug-resistant staphylococci.
- Because antibiotic resistance is so prevalent, a culture of the infected wound should be obtained routinely so that antimicrobial therapy can be modified if the patient fails to respond to initial treatment.
- Antibiotic therapy alone is rarely sufficient for abscesses in the soft tissue and skin; adequate surgical drainage is essential.
- Fundamental infection-control measures, such as careful handwashing, adequate skin preparation prior to surgery, and local wound care, are of greater importance than ever.
Most cases of community-acquired MRSA have been isolated from skin and soft tissue; surgical drainage is necessary when infection advances to abscess in those sites.
In gravidas with group B strep, look for S. aureus
Chen KT, Huard RC, Della-Latta P, Saiman L. Prevalence of methicillin-sensitive and methicillin-resistant Staphylococcus aureus in pregnant women. Obstet Gynecol. 2006;108:482–487.
To assess the prevalence of methicillin-sensitive and community-acquired methicillin-resistant S. aureus colonization in pregnant women, Chen and colleagues evaluated de-identified culture specimens that had originally been submitted to the microbiology laboratory for identification of group B streptococcal infection. As opposed to hospital-associated MRSA isolates, community-associated methicillin-resistant strains were defined as those possessing the type IV or V staphylococcal chromosomal cassette mec element and lacking a multi-drug-resistant phenotype.
Of the 2,963 culture specimens in the prospective surveillance study, 743 (25%) were positive for group B streptococci, and 507 (17%) were positive for S. aureus. Group B streptococcal colonization was significantly associated with S. aureus colonization; the prevalence odds ratio was 2.1. Fourteen of the 507 S. aureus isolates were methicillin-resistant (2.8%; 95% confidence interval [CI] 1.4–4.2%). Thirteen of the 14 strains (93%) were community-acquired.
S. aureus may cause sepsis, wound infection, bacteremia, and other ills
The unique feature of this study is the observation that vaginal colonization with group B streptococci was significantly associated with colonization with S. aureus—one of the possible causative pathogens in chorioamnionitis, endometritis, wound infection, bacteremia, puerperal mastitis, and toxic shock syndrome. The organism also may cause serious neonatal infection, particularly sepsis.
The prevalence of group B streptococcal colonization in this study (25.1%, 95% CI 23.5–26.7%) is comparable to data reported from several other investigators.7 Colonized women are at increased risk for chorioamnionitis and puerperal endometritis, and their infants are at increased risk of sepsis, pneumonia, and meningitis. Fortunately, intrapartum antibiotic prophylaxis significantly reduces the risk of both maternal and neonatal group B streptococcal infection.8
As I noted earlier in this update, the antimicrobial susceptibility of S. aureus has become increasingly limited, particularly in light of the recent increase in both hospital- and community-acquired methicillin-resistant strains. In this study by Chen and colleagues, 2.8% of S. aureus isolates were methicillin-resistant. Of these, all but one were community-acquired.
Clinical suggestions
These findings certainly do not indicate the need for routine cultures for S. aureus vaginal colonization in all pregnant women. Nor are cultures needed in women who test positive for group B streptococci at 35 to 37 weeks. However, clinicians should be alert for possible staphylococcal infections, such as wound abscess, furuncle, carbuncle, or mastitis, in these women. If such an infection appears, obtain a culture of the purulent collection. Pending the result, treat the patient empirically with a drug that is likely to be effective against community-acquired MRSA. One hundred percent of these strains are sensitive to rifampin and TMP-SMX, and 90% to 95% are sensitive to tetracycline.9
Univalent HPV vaccine is 100% effective against CIN grades 2, 3
Mao C, Koutsky LA, Ault KA, et al. Efficacy of human papillomavirus-16 vaccine to prevent cervical intraepithelial neoplasia. Obstet Gynecol. 2006;107:18–27.
Mao and colleagues set out to assess the long-term protection of a univalent HPV vaccine against CIN grades 2, 3. Their prospective, randomized, double-blind, placebo-controlled trial involved 2,391 women, aged 16 to 23 years, who received either 40 μg of HPV-16 L1 virus-like particle vaccine or placebo intramuscularly at day 1, month 2, and month 6. Genital samples for HPV-16 DNA and cervical cytology specimens were collected at day 1, month 7, and then every 6 months for 48 months. A radioimmunoassay was used to assess antibody titers to HPV-16.
Of the 750 women who received placebo, 6 developed HPV-16–related CIN 2, and 6 developed CIN 3. Among the 755 vaccinated women, no cases of CIN occurred. Thus, the vaccine was 100% effective in this trial (95% CI 65–100%).
Among women who received placebo, 111 cases of persistent HPV-16 infection occurred, compared with 7 cases in vaccinated women (vaccine efficacy 94%; 95% CI 88–98%).
Following immunization, antibody to HPV-16 peaked at month 7, declined through month 18, and remained stable between months 30 and 48.
Any effective vaccine is important
Because 3,500 to 4,000 women still die from cervical cancer each year in the United States, and almost 274,000 die worldwide, the development of any HPV vaccine that provides lasting protection against CIN is important.
The vaccine evaluated by Mao and colleagues targeted a single strain of HPV, genotype 16. The recently approved quadrivalent vaccine, Gardasil, targets types 6, 11, 16, and 18. Of the more than 100 genotypes of HPV that have been discovered, approximately 30 are present in the mucosa of the genital tract, and 15 of these 30 are associated with cervical cancer. However, 2 HPV strains—types 16 and 18—are responsible for about two thirds of all cases of cervical cancer; 90% of genital warts cases result from infection with types 6 and 11.10
Emphasize to patients that preexisting cytologic abnormalities and genital warts don’t respond to vaccination against human papillomavirus.The Advisory Committee on Immunization Practices recommends that the quadrivalent vaccine be given to girls at age 11 or 12 years, prior to the onset of sexual activity, to be maximally effective against all 4 genotypes included in the vaccine.10
If a woman is infected with HPV prior to vaccination, she may develop abnormal cervical cytology related to the genotypes in the vaccine, as well as genotypes not included. Nevertheless, ACOG recommends that the vaccine be considered in all females ages 9 to 26.11 HPV genotyping is not recommended before giving the vaccine because any type of routine screening reduces the cost-effectiveness of the vaccination program.10
Fundamentals of vaccination
The quadrivalent vaccine must be administered intramuscularly (0.5 mL) in 3 doses on day 1 and at 2 and 6 months. The principal adverse effect is a local reaction such as pain, swelling, or pruritus at the injection site. Low-grade fever occurs in approximately 10% of patients.
Although the vaccine is classified by the FDA as pregnancy category B, the manufacturer recommends against its use during pregnancy. It may be administered to lactating women, however. The approximate cost of the 3-dose series, including administration fees, is $400 to $500.
It’s a vaccine, not a treatment
Patients need to understand that vaccination is not a treatment for preexisting cytologic abnormalities or genital warts. Nor can it be expected to be perfectly protective over a person’s lifetime against infection caused by genotypes 6, 11, 16, and 18. Women must continue to have regular cytologic screening. No reliable scientific data suggest that vaccination of young girls will increase sexual promiscuity in the adolescent population.10
The author reports no financial relationships relevant to this article.
1. Duff P. Immunotherapy for congenital cytomegalovirus infection. N Engl J Med. 2005;353:1402-1404.
2. Munro SC, Hall B, Whybin LR, et al. Diagnosis of and screening for cytomegalovirus infection in pregnant women. J Clin Microbiol. 2005;431:4713-4718.
3. Lazzarotto T, Gabrielli L, Lanari M, et al. Congenital cytomegalovirus infection: recent advances in the diagnosis of maternal infection. Hum Immunol. 2004;65:410-415.
4. Nigro G, Adler SP, LaTorre R, Best AM. Passive immunization during pregnancy for congenital cytomegalovirus infection. N Engl J Med. 2005;353:1350-1362.
5. Gibbs RS. Emerging infections in obstetric and gynecologic practice. Obstet Gynecol. 2006;108:480-481.
6. Laible VR, Sheffield JS, Roberts S, McIntire DD, Trevino S, Wendel GD. Clinical presentation of community-acquired methicillin-resistant Staphylococcus aureus in pregnancy. Obstet Gynecol. 2005;106:461-465.
7. Edwards RK, Clark P, Duff P. Intrapartum antibiotic prophylaxis 2: positive predictive value of antenatal group B streptococcal cultures and antibiotic susceptibility of clinical isolates. Obstet Gynecol. 2002;100:590-594.
8. Locksmith GJ, Clark P, Duff P. Maternal and neonatal infection rates with three different protocols for prevention of group B streptococcal disease. Am J Obstet Gynecol. 1999;180:416-422.
9. Moran GJ, Krisnadasan A, Gorwitz RJ, et al. Methicillin-resistant S. aureus infections among patients in the emergency department. N Engl J Med. 2006;355:666-674.
10. Monk BJ, Wiley DJ. Will human papillomavirus prophylactic vaccination change sexual practices of adolescent and young adult women in America? Obstet Gynecol. 2006;108:420-424.
11. Human papillomavirus vaccination. ACOG Committee Opinion #344. Washington, DC: American College of Obstetricians and Gynecologists; September 2006.
Four studies caught my eye this past year. The first describes the use of systematic methodology to confirm the diagnosis of primary cytomegalovirus (CMV) infection in pregnancy and lower the rate of unnecessary pregnancy termination. Investigators were able to reclassify approximately 70% of women who had been diagnosed with CMV infection and reduce the number of pregnancy terminations by 73%.
Two other studies help define the emerging problem of community-acquired methicillin-resistant Staphylococcus aureus (MRSA) infection, when to look for it, and how to treat it. In the first, researchers isolated S. aureus from the wounds of 320 patients with community-acquired infection and tested the samples for methicillin resistance, finding a prevalence of 59%. In the second study, investigators analyzed culture specimens from pregnant women for the presence of group B streptococci and S. aureus colonization. They found colonization with group B streptococci to be significantly associated with S. aureus colonization, with a prevalence odds ratio of 2.1.
The fourth study concerns the human papillomavirus (HPV) vaccine. Women given an HPV-16 L1 virus-like particle vaccine and followed for 4 years remained 100% free of cervical intraepithelial neoplasia (CIN) grades 2 and 3, unlike women who received placebo.
I believe these 4 studies represent the most significant developments of the past year in the field of infectious disease.
Don’t rush a diagnosis of CMV infection in pregnancy
Guerra B, Simonazzi G, Banfi A, et al. Impact of diagnostic and confirmatory tests and prenatal counseling on the rate of pregnancy termination among women with positive cytomegalovirus immunoglobulin M antibody titers. Am J Obstet Gynecol. 2007;196:221.e1–6.
CMV infection is a common and important perinatal pathogen. Each year in the United States, approximately 1% of gravidas acquire primary infection. Of these, about 40% transmit infection to the fetus. The rate of transmission is highest when maternal infection occurs in the third trimester, but the risk of serious fetal injury is greatest when maternal infection occurs in the first trimester. Ten percent to 20% of congenitally infected infants are acutely symptomatic at birth. Approximately 20% of these newborns die; most survivors have serious long-term complications. In contrast, CMV infection that recurs during pregnancy poses only minimal risk to the baby.1
Many women choose to have their pregnancy terminated when they learn they have a primary CMV infection.
Details of the study
This retrospective study was designed to determine whether a systematic diagnostic algorithm reduces the rate of unnecessary abortion in women who have apparent acute CMV infection during pregnancy. Guerra and colleagues evaluated 1,857 consecutive patients in practices in Italy who had a positive anti-CMV immunoglobulin M (IgM) antibody assay in the first or second trimester and were referred to a tertiary care facility for further diagnostic testing. Universal screening for CMV is now common among practitioners in Italy, and virtually all of these patients were completely asymptomatic.
At the tertiary facility, investigators tested again for CMV-specific IgM, as well as IgG, by enzyme immunoassay. They also tested for IgM by immunoblot and determined the avidity of anti-CMV IgG. Women who had IgG of low or moderate avidity with confirmed IgM, and those who clearly seroconverted to IgG were assumed to have a primary infection.
Women who were positive for IgM with high-avidity IgG were assumed to have nonprimary infection. Women who were seronegative for both antibodies were classified as uninfected. Those who were IgM-negative with high-avidity IgG were classified as previously infected. Women with an acute infection were then counseled by a specialist and offered amniocentesis and targeted ultrasonography.
Only 11.9% of women with primary infection chose abortion
Of the 1,857 women in this study, 445 were classified as having primary infection (group 1); 53 (11.9%) women elected to terminate their pregnancy. At autopsy, 38 of the 53 fetuses were found to be infected. In the other 15 cases, the pregnancy was terminated in the first trimester, and postmortem examination was not performed.
In the 1,205 women found to have nonprimary infection or previous infection (group 2), only 5 (0.4%) had the pregnancy terminated in the first trimester, and no postmortem examinations were performed. The difference in the observed rates of abortion between groups 1 and 2 was highly significant (P<.001).
Given their observations in group 1, the authors estimated that, on the basis of the initial screening tests at the referring institutions, approximately 196 (11.9%) of all patients in groups 1 and 2 would have elected abortion. By using confirmatory tests combined with counseling by a specialist, the authors were able to reduce the number of abortions from 196 to 53, a 73% decrease.
Always confirm an initial diagnosis
Given the ominous prognosis for congenital CMV infection and the major psychological implications and sobering finality of abortion, it is imperative that clinicians confirm the diagnosis of primary CMV infection. Because most cases of CMV infection in immunocompetent adults are asymptomatic, the diagnosis is typically confirmed by serology. Unfortunately, the serologic tests for CMV are not as straight-forward and reliable as tests for other viral infections such as rubella. Commercially available tests for anti-CMV IgM often have false-positive and false-negative results. In addition, IgM antibody may be detected as long as 9 months after a primary infection and may subsequently re-appear during reactivation of a latent infection or reinfection.2,3
Be selective, on the basis of risk factors and clinical manifestations, when screening pregnant women for cytomegalovirus infection.
Routine screening is not necessary
The authors’ findings vividly illustrate the potential errors that can occur when a large number of asymptomatic patients are routinely screened for CMV. Because of these pitfalls, I do not recommend routine screening. Rather, screening should be selective, directed at women who:
- have clinical manifestations of CMV infection
- are immunosuppressed
- have small children in daycare or work in daycare themselves or
- have documented exposure to someone with CMV infection.
If the initial immunoassay for CMV IgM is positive, a confirmatory immunoblot test for IgM should be performed, as well as avidity testing for IgG.
If primary infection is confirmed, the patient should undergo targeted ultrasonography and amniocentesis to assess for manifestations of congenital infection and to detect CMV in amniotic fluid by culture or polymerase chain reaction (PCR) testing. If the sonogram shows signs of fetal injury, or the PCR test is positive, the woman should be counseled about the options, which include experimental immunotherapy with hyperimmune anti-CMV globulin4 and pregnancy termination.
The study by Guerra and colleagues is a welcome addition to the obstetric literature. By using a systematic diagnostic algorithm that included an enzyme-linked immunosorbent assay and an immunoblot assay for IgM antibody and avidity testing for IgG antibody, the authors were able to reclassify approximately 70% of patients as either uninfected or previously infected. As a result, they reduced the number of pregnancy terminations by 73%, an objective end-point that clearly has great social, economic, and medical impact.
Most community S. aureus infections are methicillin-resistant
Moran GJ, Krishnadasan A, Gorwitz RJ, et al. Methicillin-resistant S. aureus infections among patients in the emergency department. N Engl J Med. 2006;355:666–674.
Moran and colleagues reviewed the records of 422 adults with acute purulent and soft-tissue infections who were evaluated in 11 university-affiliated emergency departments in August 2004. Wounds were routinely cultured. When S. aureus was isolated, the organisms were tested for antimicrobial susceptibility to identify those that were methicillin-resistant. The PCR test was used to identify genes for staphylococcal enterotoxins A through E and H, toxic shock syndrome toxin, and Panton–Valentin leukocidin. The same methodology was used to identify the gene complex staphylococcal cassette chromosome mec (SCCmec). This complex contains the mecA gene that confers methicillin resistance.
Of the 422 patients, 320 (76%) had S. aureus isolated from their wound. The prevalence of methicillin resistance was 59%. Ninety-seven percent of MRSA isolates were pulsed-field type USA 300. SCCmec type IV and the Panton–Valentin leukocidin gene were detected in 98% of MRSA isolates. Other toxin genes were rare.
Only 2 drugs were 100% effective
Among MRSA isolates, 100% were susceptible to rifampin and trimethoprim-sulfamethoxazole (TMP-SMX), 95% were susceptible to clindamycin, and 92% were sensitive to tetracycline. Only 60% were sensitive to fluoroquinolones, and only 6% were sensitive to erythromycin. Only 43% of patients received initial empiric therapy with antibiotics to which their organisms were sensitive.
Reason to worry
S. aureus is an important pathogen in obstetric patients. It is the causative organism of toxic shock syndrome and the dominant pathogen in patients with puerperal mastitis, as well as one of the key causes of postoperative wound infection. When penicillin was developed in 1941, all strains of S. aureus were sensitive to the drug. Within a few short years, however, most hospital-acquired strains became resistant.
Methicillin was introduced in 1961 to treat these resistant staphylococcal species. Unfortunately, by the mid-1960s, methicillin-resistant S. aureus (MRSA) infections began to appear. By the 1990s, MRSA infections were common in hospitalized patients, particularly in intensive care units. Hospital-acquired MRSA isolates are often sensitive to only a few select antibiotics such as vancomycin, linezolid, and quinupristin/dalfopristin.5
In the late 1990s and early 2000s, MRSA began to appear in community-acquired infections in both adults and children. Most of these isolates have been implicated in skin and soft-tissue infections, but some have been responsible for invasive infection, bacteremia, and even death.6 Compared with hospital-acquired MRSA, these community isolates are more likely to be sensitive to commonly used antibiotics.
Always culture an infected wound
Knowledge of these sensitivity patterns is of great importance. Regrettably, as noted by Moran and associates, more than half of the patients (57%) were initially treated with antibiotics to which their infecting organism was resistant.
The clinical implications are clear:
- We must be aware that many community-acquired soft-tissue infections will be caused by drug-resistant staphylococci.
- Because antibiotic resistance is so prevalent, a culture of the infected wound should be obtained routinely so that antimicrobial therapy can be modified if the patient fails to respond to initial treatment.
- Antibiotic therapy alone is rarely sufficient for abscesses in the soft tissue and skin; adequate surgical drainage is essential.
- Fundamental infection-control measures, such as careful handwashing, adequate skin preparation prior to surgery, and local wound care, are of greater importance than ever.
Most cases of community-acquired MRSA have been isolated from skin and soft tissue; surgical drainage is necessary when infection advances to abscess in those sites.
In gravidas with group B strep, look for S. aureus
Chen KT, Huard RC, Della-Latta P, Saiman L. Prevalence of methicillin-sensitive and methicillin-resistant Staphylococcus aureus in pregnant women. Obstet Gynecol. 2006;108:482–487.
To assess the prevalence of methicillin-sensitive and community-acquired methicillin-resistant S. aureus colonization in pregnant women, Chen and colleagues evaluated de-identified culture specimens that had originally been submitted to the microbiology laboratory for identification of group B streptococcal infection. As opposed to hospital-associated MRSA isolates, community-associated methicillin-resistant strains were defined as those possessing the type IV or V staphylococcal chromosomal cassette mec element and lacking a multi-drug-resistant phenotype.
Of the 2,963 culture specimens in the prospective surveillance study, 743 (25%) were positive for group B streptococci, and 507 (17%) were positive for S. aureus. Group B streptococcal colonization was significantly associated with S. aureus colonization; the prevalence odds ratio was 2.1. Fourteen of the 507 S. aureus isolates were methicillin-resistant (2.8%; 95% confidence interval [CI] 1.4–4.2%). Thirteen of the 14 strains (93%) were community-acquired.
S. aureus may cause sepsis, wound infection, bacteremia, and other ills
The unique feature of this study is the observation that vaginal colonization with group B streptococci was significantly associated with colonization with S. aureus—one of the possible causative pathogens in chorioamnionitis, endometritis, wound infection, bacteremia, puerperal mastitis, and toxic shock syndrome. The organism also may cause serious neonatal infection, particularly sepsis.
The prevalence of group B streptococcal colonization in this study (25.1%, 95% CI 23.5–26.7%) is comparable to data reported from several other investigators.7 Colonized women are at increased risk for chorioamnionitis and puerperal endometritis, and their infants are at increased risk of sepsis, pneumonia, and meningitis. Fortunately, intrapartum antibiotic prophylaxis significantly reduces the risk of both maternal and neonatal group B streptococcal infection.8
As I noted earlier in this update, the antimicrobial susceptibility of S. aureus has become increasingly limited, particularly in light of the recent increase in both hospital- and community-acquired methicillin-resistant strains. In this study by Chen and colleagues, 2.8% of S. aureus isolates were methicillin-resistant. Of these, all but one were community-acquired.
Clinical suggestions
These findings certainly do not indicate the need for routine cultures for S. aureus vaginal colonization in all pregnant women. Nor are cultures needed in women who test positive for group B streptococci at 35 to 37 weeks. However, clinicians should be alert for possible staphylococcal infections, such as wound abscess, furuncle, carbuncle, or mastitis, in these women. If such an infection appears, obtain a culture of the purulent collection. Pending the result, treat the patient empirically with a drug that is likely to be effective against community-acquired MRSA. One hundred percent of these strains are sensitive to rifampin and TMP-SMX, and 90% to 95% are sensitive to tetracycline.9
Univalent HPV vaccine is 100% effective against CIN grades 2, 3
Mao C, Koutsky LA, Ault KA, et al. Efficacy of human papillomavirus-16 vaccine to prevent cervical intraepithelial neoplasia. Obstet Gynecol. 2006;107:18–27.
Mao and colleagues set out to assess the long-term protection of a univalent HPV vaccine against CIN grades 2, 3. Their prospective, randomized, double-blind, placebo-controlled trial involved 2,391 women, aged 16 to 23 years, who received either 40 μg of HPV-16 L1 virus-like particle vaccine or placebo intramuscularly at day 1, month 2, and month 6. Genital samples for HPV-16 DNA and cervical cytology specimens were collected at day 1, month 7, and then every 6 months for 48 months. A radioimmunoassay was used to assess antibody titers to HPV-16.
Of the 750 women who received placebo, 6 developed HPV-16–related CIN 2, and 6 developed CIN 3. Among the 755 vaccinated women, no cases of CIN occurred. Thus, the vaccine was 100% effective in this trial (95% CI 65–100%).
Among women who received placebo, 111 cases of persistent HPV-16 infection occurred, compared with 7 cases in vaccinated women (vaccine efficacy 94%; 95% CI 88–98%).
Following immunization, antibody to HPV-16 peaked at month 7, declined through month 18, and remained stable between months 30 and 48.
Any effective vaccine is important
Because 3,500 to 4,000 women still die from cervical cancer each year in the United States, and almost 274,000 die worldwide, the development of any HPV vaccine that provides lasting protection against CIN is important.
The vaccine evaluated by Mao and colleagues targeted a single strain of HPV, genotype 16. The recently approved quadrivalent vaccine, Gardasil, targets types 6, 11, 16, and 18. Of the more than 100 genotypes of HPV that have been discovered, approximately 30 are present in the mucosa of the genital tract, and 15 of these 30 are associated with cervical cancer. However, 2 HPV strains—types 16 and 18—are responsible for about two thirds of all cases of cervical cancer; 90% of genital warts cases result from infection with types 6 and 11.10
Emphasize to patients that preexisting cytologic abnormalities and genital warts don’t respond to vaccination against human papillomavirus.The Advisory Committee on Immunization Practices recommends that the quadrivalent vaccine be given to girls at age 11 or 12 years, prior to the onset of sexual activity, to be maximally effective against all 4 genotypes included in the vaccine.10
If a woman is infected with HPV prior to vaccination, she may develop abnormal cervical cytology related to the genotypes in the vaccine, as well as genotypes not included. Nevertheless, ACOG recommends that the vaccine be considered in all females ages 9 to 26.11 HPV genotyping is not recommended before giving the vaccine because any type of routine screening reduces the cost-effectiveness of the vaccination program.10
Fundamentals of vaccination
The quadrivalent vaccine must be administered intramuscularly (0.5 mL) in 3 doses on day 1 and at 2 and 6 months. The principal adverse effect is a local reaction such as pain, swelling, or pruritus at the injection site. Low-grade fever occurs in approximately 10% of patients.
Although the vaccine is classified by the FDA as pregnancy category B, the manufacturer recommends against its use during pregnancy. It may be administered to lactating women, however. The approximate cost of the 3-dose series, including administration fees, is $400 to $500.
It’s a vaccine, not a treatment
Patients need to understand that vaccination is not a treatment for preexisting cytologic abnormalities or genital warts. Nor can it be expected to be perfectly protective over a person’s lifetime against infection caused by genotypes 6, 11, 16, and 18. Women must continue to have regular cytologic screening. No reliable scientific data suggest that vaccination of young girls will increase sexual promiscuity in the adolescent population.10
The author reports no financial relationships relevant to this article.
Four studies caught my eye this past year. The first describes the use of systematic methodology to confirm the diagnosis of primary cytomegalovirus (CMV) infection in pregnancy and lower the rate of unnecessary pregnancy termination. Investigators were able to reclassify approximately 70% of women who had been diagnosed with CMV infection and reduce the number of pregnancy terminations by 73%.
Two other studies help define the emerging problem of community-acquired methicillin-resistant Staphylococcus aureus (MRSA) infection, when to look for it, and how to treat it. In the first, researchers isolated S. aureus from the wounds of 320 patients with community-acquired infection and tested the samples for methicillin resistance, finding a prevalence of 59%. In the second study, investigators analyzed culture specimens from pregnant women for the presence of group B streptococci and S. aureus colonization. They found colonization with group B streptococci to be significantly associated with S. aureus colonization, with a prevalence odds ratio of 2.1.
The fourth study concerns the human papillomavirus (HPV) vaccine. Women given an HPV-16 L1 virus-like particle vaccine and followed for 4 years remained 100% free of cervical intraepithelial neoplasia (CIN) grades 2 and 3, unlike women who received placebo.
I believe these 4 studies represent the most significant developments of the past year in the field of infectious disease.
Don’t rush a diagnosis of CMV infection in pregnancy
Guerra B, Simonazzi G, Banfi A, et al. Impact of diagnostic and confirmatory tests and prenatal counseling on the rate of pregnancy termination among women with positive cytomegalovirus immunoglobulin M antibody titers. Am J Obstet Gynecol. 2007;196:221.e1–6.
CMV infection is a common and important perinatal pathogen. Each year in the United States, approximately 1% of gravidas acquire primary infection. Of these, about 40% transmit infection to the fetus. The rate of transmission is highest when maternal infection occurs in the third trimester, but the risk of serious fetal injury is greatest when maternal infection occurs in the first trimester. Ten percent to 20% of congenitally infected infants are acutely symptomatic at birth. Approximately 20% of these newborns die; most survivors have serious long-term complications. In contrast, CMV infection that recurs during pregnancy poses only minimal risk to the baby.1
Many women choose to have their pregnancy terminated when they learn they have a primary CMV infection.
Details of the study
This retrospective study was designed to determine whether a systematic diagnostic algorithm reduces the rate of unnecessary abortion in women who have apparent acute CMV infection during pregnancy. Guerra and colleagues evaluated 1,857 consecutive patients in practices in Italy who had a positive anti-CMV immunoglobulin M (IgM) antibody assay in the first or second trimester and were referred to a tertiary care facility for further diagnostic testing. Universal screening for CMV is now common among practitioners in Italy, and virtually all of these patients were completely asymptomatic.
At the tertiary facility, investigators tested again for CMV-specific IgM, as well as IgG, by enzyme immunoassay. They also tested for IgM by immunoblot and determined the avidity of anti-CMV IgG. Women who had IgG of low or moderate avidity with confirmed IgM, and those who clearly seroconverted to IgG were assumed to have a primary infection.
Women who were positive for IgM with high-avidity IgG were assumed to have nonprimary infection. Women who were seronegative for both antibodies were classified as uninfected. Those who were IgM-negative with high-avidity IgG were classified as previously infected. Women with an acute infection were then counseled by a specialist and offered amniocentesis and targeted ultrasonography.
Only 11.9% of women with primary infection chose abortion
Of the 1,857 women in this study, 445 were classified as having primary infection (group 1); 53 (11.9%) women elected to terminate their pregnancy. At autopsy, 38 of the 53 fetuses were found to be infected. In the other 15 cases, the pregnancy was terminated in the first trimester, and postmortem examination was not performed.
In the 1,205 women found to have nonprimary infection or previous infection (group 2), only 5 (0.4%) had the pregnancy terminated in the first trimester, and no postmortem examinations were performed. The difference in the observed rates of abortion between groups 1 and 2 was highly significant (P<.001).
Given their observations in group 1, the authors estimated that, on the basis of the initial screening tests at the referring institutions, approximately 196 (11.9%) of all patients in groups 1 and 2 would have elected abortion. By using confirmatory tests combined with counseling by a specialist, the authors were able to reduce the number of abortions from 196 to 53, a 73% decrease.
Always confirm an initial diagnosis
Given the ominous prognosis for congenital CMV infection and the major psychological implications and sobering finality of abortion, it is imperative that clinicians confirm the diagnosis of primary CMV infection. Because most cases of CMV infection in immunocompetent adults are asymptomatic, the diagnosis is typically confirmed by serology. Unfortunately, the serologic tests for CMV are not as straight-forward and reliable as tests for other viral infections such as rubella. Commercially available tests for anti-CMV IgM often have false-positive and false-negative results. In addition, IgM antibody may be detected as long as 9 months after a primary infection and may subsequently re-appear during reactivation of a latent infection or reinfection.2,3
Be selective, on the basis of risk factors and clinical manifestations, when screening pregnant women for cytomegalovirus infection.
Routine screening is not necessary
The authors’ findings vividly illustrate the potential errors that can occur when a large number of asymptomatic patients are routinely screened for CMV. Because of these pitfalls, I do not recommend routine screening. Rather, screening should be selective, directed at women who:
- have clinical manifestations of CMV infection
- are immunosuppressed
- have small children in daycare or work in daycare themselves or
- have documented exposure to someone with CMV infection.
If the initial immunoassay for CMV IgM is positive, a confirmatory immunoblot test for IgM should be performed, as well as avidity testing for IgG.
If primary infection is confirmed, the patient should undergo targeted ultrasonography and amniocentesis to assess for manifestations of congenital infection and to detect CMV in amniotic fluid by culture or polymerase chain reaction (PCR) testing. If the sonogram shows signs of fetal injury, or the PCR test is positive, the woman should be counseled about the options, which include experimental immunotherapy with hyperimmune anti-CMV globulin4 and pregnancy termination.
The study by Guerra and colleagues is a welcome addition to the obstetric literature. By using a systematic diagnostic algorithm that included an enzyme-linked immunosorbent assay and an immunoblot assay for IgM antibody and avidity testing for IgG antibody, the authors were able to reclassify approximately 70% of patients as either uninfected or previously infected. As a result, they reduced the number of pregnancy terminations by 73%, an objective end-point that clearly has great social, economic, and medical impact.
Most community S. aureus infections are methicillin-resistant
Moran GJ, Krishnadasan A, Gorwitz RJ, et al. Methicillin-resistant S. aureus infections among patients in the emergency department. N Engl J Med. 2006;355:666–674.
Moran and colleagues reviewed the records of 422 adults with acute purulent and soft-tissue infections who were evaluated in 11 university-affiliated emergency departments in August 2004. Wounds were routinely cultured. When S. aureus was isolated, the organisms were tested for antimicrobial susceptibility to identify those that were methicillin-resistant. The PCR test was used to identify genes for staphylococcal enterotoxins A through E and H, toxic shock syndrome toxin, and Panton–Valentin leukocidin. The same methodology was used to identify the gene complex staphylococcal cassette chromosome mec (SCCmec). This complex contains the mecA gene that confers methicillin resistance.
Of the 422 patients, 320 (76%) had S. aureus isolated from their wound. The prevalence of methicillin resistance was 59%. Ninety-seven percent of MRSA isolates were pulsed-field type USA 300. SCCmec type IV and the Panton–Valentin leukocidin gene were detected in 98% of MRSA isolates. Other toxin genes were rare.
Only 2 drugs were 100% effective
Among MRSA isolates, 100% were susceptible to rifampin and trimethoprim-sulfamethoxazole (TMP-SMX), 95% were susceptible to clindamycin, and 92% were sensitive to tetracycline. Only 60% were sensitive to fluoroquinolones, and only 6% were sensitive to erythromycin. Only 43% of patients received initial empiric therapy with antibiotics to which their organisms were sensitive.
Reason to worry
S. aureus is an important pathogen in obstetric patients. It is the causative organism of toxic shock syndrome and the dominant pathogen in patients with puerperal mastitis, as well as one of the key causes of postoperative wound infection. When penicillin was developed in 1941, all strains of S. aureus were sensitive to the drug. Within a few short years, however, most hospital-acquired strains became resistant.
Methicillin was introduced in 1961 to treat these resistant staphylococcal species. Unfortunately, by the mid-1960s, methicillin-resistant S. aureus (MRSA) infections began to appear. By the 1990s, MRSA infections were common in hospitalized patients, particularly in intensive care units. Hospital-acquired MRSA isolates are often sensitive to only a few select antibiotics such as vancomycin, linezolid, and quinupristin/dalfopristin.5
In the late 1990s and early 2000s, MRSA began to appear in community-acquired infections in both adults and children. Most of these isolates have been implicated in skin and soft-tissue infections, but some have been responsible for invasive infection, bacteremia, and even death.6 Compared with hospital-acquired MRSA, these community isolates are more likely to be sensitive to commonly used antibiotics.
Always culture an infected wound
Knowledge of these sensitivity patterns is of great importance. Regrettably, as noted by Moran and associates, more than half of the patients (57%) were initially treated with antibiotics to which their infecting organism was resistant.
The clinical implications are clear:
- We must be aware that many community-acquired soft-tissue infections will be caused by drug-resistant staphylococci.
- Because antibiotic resistance is so prevalent, a culture of the infected wound should be obtained routinely so that antimicrobial therapy can be modified if the patient fails to respond to initial treatment.
- Antibiotic therapy alone is rarely sufficient for abscesses in the soft tissue and skin; adequate surgical drainage is essential.
- Fundamental infection-control measures, such as careful handwashing, adequate skin preparation prior to surgery, and local wound care, are of greater importance than ever.
Most cases of community-acquired MRSA have been isolated from skin and soft tissue; surgical drainage is necessary when infection advances to abscess in those sites.
In gravidas with group B strep, look for S. aureus
Chen KT, Huard RC, Della-Latta P, Saiman L. Prevalence of methicillin-sensitive and methicillin-resistant Staphylococcus aureus in pregnant women. Obstet Gynecol. 2006;108:482–487.
To assess the prevalence of methicillin-sensitive and community-acquired methicillin-resistant S. aureus colonization in pregnant women, Chen and colleagues evaluated de-identified culture specimens that had originally been submitted to the microbiology laboratory for identification of group B streptococcal infection. As opposed to hospital-associated MRSA isolates, community-associated methicillin-resistant strains were defined as those possessing the type IV or V staphylococcal chromosomal cassette mec element and lacking a multi-drug-resistant phenotype.
Of the 2,963 culture specimens in the prospective surveillance study, 743 (25%) were positive for group B streptococci, and 507 (17%) were positive for S. aureus. Group B streptococcal colonization was significantly associated with S. aureus colonization; the prevalence odds ratio was 2.1. Fourteen of the 507 S. aureus isolates were methicillin-resistant (2.8%; 95% confidence interval [CI] 1.4–4.2%). Thirteen of the 14 strains (93%) were community-acquired.
S. aureus may cause sepsis, wound infection, bacteremia, and other ills
The unique feature of this study is the observation that vaginal colonization with group B streptococci was significantly associated with colonization with S. aureus—one of the possible causative pathogens in chorioamnionitis, endometritis, wound infection, bacteremia, puerperal mastitis, and toxic shock syndrome. The organism also may cause serious neonatal infection, particularly sepsis.
The prevalence of group B streptococcal colonization in this study (25.1%, 95% CI 23.5–26.7%) is comparable to data reported from several other investigators.7 Colonized women are at increased risk for chorioamnionitis and puerperal endometritis, and their infants are at increased risk of sepsis, pneumonia, and meningitis. Fortunately, intrapartum antibiotic prophylaxis significantly reduces the risk of both maternal and neonatal group B streptococcal infection.8
As I noted earlier in this update, the antimicrobial susceptibility of S. aureus has become increasingly limited, particularly in light of the recent increase in both hospital- and community-acquired methicillin-resistant strains. In this study by Chen and colleagues, 2.8% of S. aureus isolates were methicillin-resistant. Of these, all but one were community-acquired.
Clinical suggestions
These findings certainly do not indicate the need for routine cultures for S. aureus vaginal colonization in all pregnant women. Nor are cultures needed in women who test positive for group B streptococci at 35 to 37 weeks. However, clinicians should be alert for possible staphylococcal infections, such as wound abscess, furuncle, carbuncle, or mastitis, in these women. If such an infection appears, obtain a culture of the purulent collection. Pending the result, treat the patient empirically with a drug that is likely to be effective against community-acquired MRSA. One hundred percent of these strains are sensitive to rifampin and TMP-SMX, and 90% to 95% are sensitive to tetracycline.9
Univalent HPV vaccine is 100% effective against CIN grades 2, 3
Mao C, Koutsky LA, Ault KA, et al. Efficacy of human papillomavirus-16 vaccine to prevent cervical intraepithelial neoplasia. Obstet Gynecol. 2006;107:18–27.
Mao and colleagues set out to assess the long-term protection of a univalent HPV vaccine against CIN grades 2, 3. Their prospective, randomized, double-blind, placebo-controlled trial involved 2,391 women, aged 16 to 23 years, who received either 40 μg of HPV-16 L1 virus-like particle vaccine or placebo intramuscularly at day 1, month 2, and month 6. Genital samples for HPV-16 DNA and cervical cytology specimens were collected at day 1, month 7, and then every 6 months for 48 months. A radioimmunoassay was used to assess antibody titers to HPV-16.
Of the 750 women who received placebo, 6 developed HPV-16–related CIN 2, and 6 developed CIN 3. Among the 755 vaccinated women, no cases of CIN occurred. Thus, the vaccine was 100% effective in this trial (95% CI 65–100%).
Among women who received placebo, 111 cases of persistent HPV-16 infection occurred, compared with 7 cases in vaccinated women (vaccine efficacy 94%; 95% CI 88–98%).
Following immunization, antibody to HPV-16 peaked at month 7, declined through month 18, and remained stable between months 30 and 48.
Any effective vaccine is important
Because 3,500 to 4,000 women still die from cervical cancer each year in the United States, and almost 274,000 die worldwide, the development of any HPV vaccine that provides lasting protection against CIN is important.
The vaccine evaluated by Mao and colleagues targeted a single strain of HPV, genotype 16. The recently approved quadrivalent vaccine, Gardasil, targets types 6, 11, 16, and 18. Of the more than 100 genotypes of HPV that have been discovered, approximately 30 are present in the mucosa of the genital tract, and 15 of these 30 are associated with cervical cancer. However, 2 HPV strains—types 16 and 18—are responsible for about two thirds of all cases of cervical cancer; 90% of genital warts cases result from infection with types 6 and 11.10
Emphasize to patients that preexisting cytologic abnormalities and genital warts don’t respond to vaccination against human papillomavirus.The Advisory Committee on Immunization Practices recommends that the quadrivalent vaccine be given to girls at age 11 or 12 years, prior to the onset of sexual activity, to be maximally effective against all 4 genotypes included in the vaccine.10
If a woman is infected with HPV prior to vaccination, she may develop abnormal cervical cytology related to the genotypes in the vaccine, as well as genotypes not included. Nevertheless, ACOG recommends that the vaccine be considered in all females ages 9 to 26.11 HPV genotyping is not recommended before giving the vaccine because any type of routine screening reduces the cost-effectiveness of the vaccination program.10
Fundamentals of vaccination
The quadrivalent vaccine must be administered intramuscularly (0.5 mL) in 3 doses on day 1 and at 2 and 6 months. The principal adverse effect is a local reaction such as pain, swelling, or pruritus at the injection site. Low-grade fever occurs in approximately 10% of patients.
Although the vaccine is classified by the FDA as pregnancy category B, the manufacturer recommends against its use during pregnancy. It may be administered to lactating women, however. The approximate cost of the 3-dose series, including administration fees, is $400 to $500.
It’s a vaccine, not a treatment
Patients need to understand that vaccination is not a treatment for preexisting cytologic abnormalities or genital warts. Nor can it be expected to be perfectly protective over a person’s lifetime against infection caused by genotypes 6, 11, 16, and 18. Women must continue to have regular cytologic screening. No reliable scientific data suggest that vaccination of young girls will increase sexual promiscuity in the adolescent population.10
The author reports no financial relationships relevant to this article.
1. Duff P. Immunotherapy for congenital cytomegalovirus infection. N Engl J Med. 2005;353:1402-1404.
2. Munro SC, Hall B, Whybin LR, et al. Diagnosis of and screening for cytomegalovirus infection in pregnant women. J Clin Microbiol. 2005;431:4713-4718.
3. Lazzarotto T, Gabrielli L, Lanari M, et al. Congenital cytomegalovirus infection: recent advances in the diagnosis of maternal infection. Hum Immunol. 2004;65:410-415.
4. Nigro G, Adler SP, LaTorre R, Best AM. Passive immunization during pregnancy for congenital cytomegalovirus infection. N Engl J Med. 2005;353:1350-1362.
5. Gibbs RS. Emerging infections in obstetric and gynecologic practice. Obstet Gynecol. 2006;108:480-481.
6. Laible VR, Sheffield JS, Roberts S, McIntire DD, Trevino S, Wendel GD. Clinical presentation of community-acquired methicillin-resistant Staphylococcus aureus in pregnancy. Obstet Gynecol. 2005;106:461-465.
7. Edwards RK, Clark P, Duff P. Intrapartum antibiotic prophylaxis 2: positive predictive value of antenatal group B streptococcal cultures and antibiotic susceptibility of clinical isolates. Obstet Gynecol. 2002;100:590-594.
8. Locksmith GJ, Clark P, Duff P. Maternal and neonatal infection rates with three different protocols for prevention of group B streptococcal disease. Am J Obstet Gynecol. 1999;180:416-422.
9. Moran GJ, Krisnadasan A, Gorwitz RJ, et al. Methicillin-resistant S. aureus infections among patients in the emergency department. N Engl J Med. 2006;355:666-674.
10. Monk BJ, Wiley DJ. Will human papillomavirus prophylactic vaccination change sexual practices of adolescent and young adult women in America? Obstet Gynecol. 2006;108:420-424.
11. Human papillomavirus vaccination. ACOG Committee Opinion #344. Washington, DC: American College of Obstetricians and Gynecologists; September 2006.
1. Duff P. Immunotherapy for congenital cytomegalovirus infection. N Engl J Med. 2005;353:1402-1404.
2. Munro SC, Hall B, Whybin LR, et al. Diagnosis of and screening for cytomegalovirus infection in pregnant women. J Clin Microbiol. 2005;431:4713-4718.
3. Lazzarotto T, Gabrielli L, Lanari M, et al. Congenital cytomegalovirus infection: recent advances in the diagnosis of maternal infection. Hum Immunol. 2004;65:410-415.
4. Nigro G, Adler SP, LaTorre R, Best AM. Passive immunization during pregnancy for congenital cytomegalovirus infection. N Engl J Med. 2005;353:1350-1362.
5. Gibbs RS. Emerging infections in obstetric and gynecologic practice. Obstet Gynecol. 2006;108:480-481.
6. Laible VR, Sheffield JS, Roberts S, McIntire DD, Trevino S, Wendel GD. Clinical presentation of community-acquired methicillin-resistant Staphylococcus aureus in pregnancy. Obstet Gynecol. 2005;106:461-465.
7. Edwards RK, Clark P, Duff P. Intrapartum antibiotic prophylaxis 2: positive predictive value of antenatal group B streptococcal cultures and antibiotic susceptibility of clinical isolates. Obstet Gynecol. 2002;100:590-594.
8. Locksmith GJ, Clark P, Duff P. Maternal and neonatal infection rates with three different protocols for prevention of group B streptococcal disease. Am J Obstet Gynecol. 1999;180:416-422.
9. Moran GJ, Krisnadasan A, Gorwitz RJ, et al. Methicillin-resistant S. aureus infections among patients in the emergency department. N Engl J Med. 2006;355:666-674.
10. Monk BJ, Wiley DJ. Will human papillomavirus prophylactic vaccination change sexual practices of adolescent and young adult women in America? Obstet Gynecol. 2006;108:420-424.
11. Human papillomavirus vaccination. ACOG Committee Opinion #344. Washington, DC: American College of Obstetricians and Gynecologists; September 2006.
Opinion: A time for change Laparoscopic hysterectomy: Learn it—or get left behind!
Human beings are master adapters. Thrust into a hostile environment, or subjected to other overwhelming forces, we quickly adapt to new demands, however harsh they may be. Then we maintain our new skill set with impressive devotion.
And that is the problem: We embrace our skills long after their usefulness has passed.
Gynecologic surgeons are guilty of the same failing. Although we know the vaginal route to be safer, quicker, cheaper, and easier on the patient, 65% to 70% of us still perform hysterectomy using the abdominal approach.1,2
The reason? That was the way we were taught, back in the sometimes hostile years of residency, and no compelling force since has caused us to update our behavior.
Let us not cling to abdominal hysterectomy when a less invasive alternative would be better for the patient. Like the vaginal approach, the laparoscopic route has much to offer. Although some surgical teachers have successfully integrated laparoscopic surgery into their residency training programs, many more opportunities are needed. Applications for laparoscopic fellowships continue to increase in number, largely because young physicians feel their training is deficient in this area.
The time has come to refocus our attention on the alternatives to abdominal hysterectomy, and to learn and perform the least invasive surgical approach whenever possible. This article explores in brief the indications, goals, and basic technique for laparoscopic hysterectomy, and the technological developments that have made it timely and safe.
Indications
As always, a thorough pelvic–rectal examination and evaluation of uterine mobility and vaginal accessibility remain the standard of care for deciding the route of hysterectomy. We believe—as many surgeons do—that the size of the uterus is usually irrelevant when determining the surgical approach.
Laparoscopic-assisted vaginal hysterectomy is indicated when the surgeon needs to remove the uterus and cervix vaginally at the time of other laparoscopic procedures, such as excision of endometriosis, appendectomy, or salpingo-oophorectomy.
Total laparoscopic hysterectomy is warranted when vaginal exposure is inadequate, a large uterus would make the vaginal approach too difficult, the patient has undergone multiple surgeries, or an adnexal mass is suspicious for malignancy.
Supracervical laparoscopic hysterectomy is appropriate when there is normal pelvic support without dyspareunia or cervical abnormalities.
Goals of laparoscopic hysterectomy
For both total and supracervical hysterectomy, the first goal is to secure the uterine vessels (FIGURE 1). This goal can be achieved using a number of tools:
- Electrosurgery with bipolar cautery
- Harmonic energy
- Vascular clips
- Ligating suture
Our preference is to clamp and coagulate the uterine vascular bundle using curved ultrasonic shears (Harmonic Ace).3
Secure the uterine vessels at the ascending branches rather than where they enter the lower uterus, as the latter area is in close proximity to the ureter (FIGURE 2). To ensure hemostasis when using the ultrasonic shears, relax tissue tension and activate the device using minimum power.
FIGURE 1 Secure the uterine vessels
The left ascending uterine vessels are secured using the curved, ultrasonic shears.
FIGURE 2 Proximity of key structures
Because the ureter (no. 1) and uterine vessels (no. 2) are in close proximity, it is advisable to secure the vessels at the ascending branches (no. 3).
Secondary goal: Identify tissue structures
To identify the 3 levels of tissue structures in the lower pelvis, it is necessary to manipulate the uterus. We recommend learning to use a laparoscopic uterine tissue manipulator instead of a cervical–vaginal manipulator. The former makes it possible to maintain visualization throughout the procedure, obtain adequate exposure, and control tissue tension.
The 3 levels of tissue to be identified are (FIGURE 3):
- Level 1—ascending uterine vascular bundle
- Level 2—junction of the uterosacral–cardinal ligaments
- Level 3—junction of the cervix and vagina
If the uterus is large enough to interfere with visualization of the uterosacral–cardinal ligaments or the cervical–vaginal junction, or both, in situ tissue morcellation is warranted. This debulking should eventually allow visualization of the lower tissue structures.
FIGURE 3 Three levels of tissue
Level 1 corresponds to the ascending uterine vessels, level 2 to the uterosacral–cardinal ligament junction, and level 3 to the cervical–vaginal junction.
How tissue levels come into play
Total hysterectomy. Level 3 is the end-point. Once the uterine vessels are secured and the levels are identified, perform anterior and posterior colpotomy (FIGURE 4). Using traction and counter-traction, coagulate and divide the broad ligament, starting at level 1 and ending at level 3. Perform this step bilaterally.
Remove the cervix, uterus, and adnexa (if planned) via the vagina. Close the vaginal cuff using laparoscopic suturing for appropriate cuff support.4
Supracervical hysterectomy. Level 2 is the endpoint. Begin at level 1 using reverse cone drilling (FIGURE 5). This will enable you to reach level 2. Then extract the uterus using the tissue morcellator.
Laparoscopic-assisted vaginal hysterectomy. Clamp, cut, and ligate the uterine vessels vaginally.
FIGURE 4 Anterior and posterior colpotomy
After the uterine vessels are secured and the 3 tissue levels have been identified, perform anterior (A) and posterior (B) colpotomy.
FIGURE 5 Supracervical hysterectomy
Begin the procedure at tissue level 1 using reverse cone drilling to reach the level 2 endpoint.
Always locate the ureter
Regardless of the type of hysterectomy being performed, it is critical to observe the ureter and ensure that it is out of harm’s way before securing the uterine vessels and identifying the tissue levels.
At the end of the procedure, always reduce intra-abdominal pelvic pressure to 5 mm Hg and check all tissue sites for hemostasis.
Not so long ago in the mid-1980s, we had fewer trocar options, laparoscopic suturing was limited, unipolar cautery was popular, endocutters could not guarantee hemostasis across staple lines, laparoscopes were large, images were unpredictable, monitors and cameras were nonexistent, and gas insufflators were bulky and slow. Despite these shortcomings, many surgeons and nurses believed minimally invasive surgery conferred advantages worth pursuing.
Then Semm pelviscopy (Kiel, Germany) reached the United States and intrigued American surgeons, both general and gynecologic.5 The ability to suture laparoscopically was crucial to the success of advanced operative laparoscopy.6 Laparoscopic cholecystectomy emerged, hastening further improvements in equipment and instrumentation.
Beginning in the late 1990s, laparoscopic surgeons witnessed even bigger changes in operating room technologies. The Internet increased the patient’s understanding of her options, and this new awareness motivated hospitals, industry, and physicians to upgrade women’s surgery. One result was specialized gynecologic OR nurse directors with telesurgery/telemedicine integrated into the suites. Digital platform cameras; smaller, clearer laparoscopes; and voice-activated lighting soon followed, as did better insufflators, image capturing, and other advances.
Today we rely on safer electrosurgery units (bipolar and tripolar) and “harmonic” energy.7 Tissue extractors enable us to remove large volumes of tissue quickly and safely. And all these developments have led to proven, enhanced outcomes for the patient.3
A promising future
The future for advanced operative laparoscopy is bright. As patients continue to press for minimally invasive procedures, the range of surgical options available to them will expand. To keep up, we will have little choice but to acquire expertise in minimally invasive applications.
Dr. Steven D. McCarus is a consultant to Ethicon Endo-Surgery and Gynecare.
Dr. Tamberly F. McCarus has no financial relationships relevant to this article.
1. Farquhar CM, Steiner CA. Hysterectomy Rates in the United States 1990-1997. Obstet Gynecol. 2002;99:229-234.
2. Kovac SR, et al. Key Clinical Decision: Determining the Route of Hysterectomy. Cincinnati: Ethicon Endosurgery, Center for Clinical Decision Support; 1999.
3. McCarus SD. Harmonic ultrasonic energy in gynecologic surgery: hysterectomy with the Harmonic Ace and the McCarus technique. A supplement to OBG Management. 2006;18(4).-
4. McCarus SD. Laparoscopic suturing. OBG Management. 2000;12(10).-
5. Semm K. Operative Manual for Endoscopic Abdominal Surgery. Chicago: Year Book Publishers; 1987.
6. Hay DL, Levine RL, von Fraunhofer JA, Masterson BJ. Chromic gut pelviscopic loop ligature: effect of the number of pulls on the tensile strength. J Reprod Med. 1990;35:260-262.
7. McCarus SD. Physiologic mechanism of the ultrasonically activated scalpel. J Am Assoc Gynecol Laparosc. 1996;3:601-608.
Human beings are master adapters. Thrust into a hostile environment, or subjected to other overwhelming forces, we quickly adapt to new demands, however harsh they may be. Then we maintain our new skill set with impressive devotion.
And that is the problem: We embrace our skills long after their usefulness has passed.
Gynecologic surgeons are guilty of the same failing. Although we know the vaginal route to be safer, quicker, cheaper, and easier on the patient, 65% to 70% of us still perform hysterectomy using the abdominal approach.1,2
The reason? That was the way we were taught, back in the sometimes hostile years of residency, and no compelling force since has caused us to update our behavior.
Let us not cling to abdominal hysterectomy when a less invasive alternative would be better for the patient. Like the vaginal approach, the laparoscopic route has much to offer. Although some surgical teachers have successfully integrated laparoscopic surgery into their residency training programs, many more opportunities are needed. Applications for laparoscopic fellowships continue to increase in number, largely because young physicians feel their training is deficient in this area.
The time has come to refocus our attention on the alternatives to abdominal hysterectomy, and to learn and perform the least invasive surgical approach whenever possible. This article explores in brief the indications, goals, and basic technique for laparoscopic hysterectomy, and the technological developments that have made it timely and safe.
Indications
As always, a thorough pelvic–rectal examination and evaluation of uterine mobility and vaginal accessibility remain the standard of care for deciding the route of hysterectomy. We believe—as many surgeons do—that the size of the uterus is usually irrelevant when determining the surgical approach.
Laparoscopic-assisted vaginal hysterectomy is indicated when the surgeon needs to remove the uterus and cervix vaginally at the time of other laparoscopic procedures, such as excision of endometriosis, appendectomy, or salpingo-oophorectomy.
Total laparoscopic hysterectomy is warranted when vaginal exposure is inadequate, a large uterus would make the vaginal approach too difficult, the patient has undergone multiple surgeries, or an adnexal mass is suspicious for malignancy.
Supracervical laparoscopic hysterectomy is appropriate when there is normal pelvic support without dyspareunia or cervical abnormalities.
Goals of laparoscopic hysterectomy
For both total and supracervical hysterectomy, the first goal is to secure the uterine vessels (FIGURE 1). This goal can be achieved using a number of tools:
- Electrosurgery with bipolar cautery
- Harmonic energy
- Vascular clips
- Ligating suture
Our preference is to clamp and coagulate the uterine vascular bundle using curved ultrasonic shears (Harmonic Ace).3
Secure the uterine vessels at the ascending branches rather than where they enter the lower uterus, as the latter area is in close proximity to the ureter (FIGURE 2). To ensure hemostasis when using the ultrasonic shears, relax tissue tension and activate the device using minimum power.
FIGURE 1 Secure the uterine vessels
The left ascending uterine vessels are secured using the curved, ultrasonic shears.
FIGURE 2 Proximity of key structures
Because the ureter (no. 1) and uterine vessels (no. 2) are in close proximity, it is advisable to secure the vessels at the ascending branches (no. 3).
Secondary goal: Identify tissue structures
To identify the 3 levels of tissue structures in the lower pelvis, it is necessary to manipulate the uterus. We recommend learning to use a laparoscopic uterine tissue manipulator instead of a cervical–vaginal manipulator. The former makes it possible to maintain visualization throughout the procedure, obtain adequate exposure, and control tissue tension.
The 3 levels of tissue to be identified are (FIGURE 3):
- Level 1—ascending uterine vascular bundle
- Level 2—junction of the uterosacral–cardinal ligaments
- Level 3—junction of the cervix and vagina
If the uterus is large enough to interfere with visualization of the uterosacral–cardinal ligaments or the cervical–vaginal junction, or both, in situ tissue morcellation is warranted. This debulking should eventually allow visualization of the lower tissue structures.
FIGURE 3 Three levels of tissue
Level 1 corresponds to the ascending uterine vessels, level 2 to the uterosacral–cardinal ligament junction, and level 3 to the cervical–vaginal junction.
How tissue levels come into play
Total hysterectomy. Level 3 is the end-point. Once the uterine vessels are secured and the levels are identified, perform anterior and posterior colpotomy (FIGURE 4). Using traction and counter-traction, coagulate and divide the broad ligament, starting at level 1 and ending at level 3. Perform this step bilaterally.
Remove the cervix, uterus, and adnexa (if planned) via the vagina. Close the vaginal cuff using laparoscopic suturing for appropriate cuff support.4
Supracervical hysterectomy. Level 2 is the endpoint. Begin at level 1 using reverse cone drilling (FIGURE 5). This will enable you to reach level 2. Then extract the uterus using the tissue morcellator.
Laparoscopic-assisted vaginal hysterectomy. Clamp, cut, and ligate the uterine vessels vaginally.
FIGURE 4 Anterior and posterior colpotomy
After the uterine vessels are secured and the 3 tissue levels have been identified, perform anterior (A) and posterior (B) colpotomy.
FIGURE 5 Supracervical hysterectomy
Begin the procedure at tissue level 1 using reverse cone drilling to reach the level 2 endpoint.
Always locate the ureter
Regardless of the type of hysterectomy being performed, it is critical to observe the ureter and ensure that it is out of harm’s way before securing the uterine vessels and identifying the tissue levels.
At the end of the procedure, always reduce intra-abdominal pelvic pressure to 5 mm Hg and check all tissue sites for hemostasis.
Not so long ago in the mid-1980s, we had fewer trocar options, laparoscopic suturing was limited, unipolar cautery was popular, endocutters could not guarantee hemostasis across staple lines, laparoscopes were large, images were unpredictable, monitors and cameras were nonexistent, and gas insufflators were bulky and slow. Despite these shortcomings, many surgeons and nurses believed minimally invasive surgery conferred advantages worth pursuing.
Then Semm pelviscopy (Kiel, Germany) reached the United States and intrigued American surgeons, both general and gynecologic.5 The ability to suture laparoscopically was crucial to the success of advanced operative laparoscopy.6 Laparoscopic cholecystectomy emerged, hastening further improvements in equipment and instrumentation.
Beginning in the late 1990s, laparoscopic surgeons witnessed even bigger changes in operating room technologies. The Internet increased the patient’s understanding of her options, and this new awareness motivated hospitals, industry, and physicians to upgrade women’s surgery. One result was specialized gynecologic OR nurse directors with telesurgery/telemedicine integrated into the suites. Digital platform cameras; smaller, clearer laparoscopes; and voice-activated lighting soon followed, as did better insufflators, image capturing, and other advances.
Today we rely on safer electrosurgery units (bipolar and tripolar) and “harmonic” energy.7 Tissue extractors enable us to remove large volumes of tissue quickly and safely. And all these developments have led to proven, enhanced outcomes for the patient.3
A promising future
The future for advanced operative laparoscopy is bright. As patients continue to press for minimally invasive procedures, the range of surgical options available to them will expand. To keep up, we will have little choice but to acquire expertise in minimally invasive applications.
Dr. Steven D. McCarus is a consultant to Ethicon Endo-Surgery and Gynecare.
Dr. Tamberly F. McCarus has no financial relationships relevant to this article.
Human beings are master adapters. Thrust into a hostile environment, or subjected to other overwhelming forces, we quickly adapt to new demands, however harsh they may be. Then we maintain our new skill set with impressive devotion.
And that is the problem: We embrace our skills long after their usefulness has passed.
Gynecologic surgeons are guilty of the same failing. Although we know the vaginal route to be safer, quicker, cheaper, and easier on the patient, 65% to 70% of us still perform hysterectomy using the abdominal approach.1,2
The reason? That was the way we were taught, back in the sometimes hostile years of residency, and no compelling force since has caused us to update our behavior.
Let us not cling to abdominal hysterectomy when a less invasive alternative would be better for the patient. Like the vaginal approach, the laparoscopic route has much to offer. Although some surgical teachers have successfully integrated laparoscopic surgery into their residency training programs, many more opportunities are needed. Applications for laparoscopic fellowships continue to increase in number, largely because young physicians feel their training is deficient in this area.
The time has come to refocus our attention on the alternatives to abdominal hysterectomy, and to learn and perform the least invasive surgical approach whenever possible. This article explores in brief the indications, goals, and basic technique for laparoscopic hysterectomy, and the technological developments that have made it timely and safe.
Indications
As always, a thorough pelvic–rectal examination and evaluation of uterine mobility and vaginal accessibility remain the standard of care for deciding the route of hysterectomy. We believe—as many surgeons do—that the size of the uterus is usually irrelevant when determining the surgical approach.
Laparoscopic-assisted vaginal hysterectomy is indicated when the surgeon needs to remove the uterus and cervix vaginally at the time of other laparoscopic procedures, such as excision of endometriosis, appendectomy, or salpingo-oophorectomy.
Total laparoscopic hysterectomy is warranted when vaginal exposure is inadequate, a large uterus would make the vaginal approach too difficult, the patient has undergone multiple surgeries, or an adnexal mass is suspicious for malignancy.
Supracervical laparoscopic hysterectomy is appropriate when there is normal pelvic support without dyspareunia or cervical abnormalities.
Goals of laparoscopic hysterectomy
For both total and supracervical hysterectomy, the first goal is to secure the uterine vessels (FIGURE 1). This goal can be achieved using a number of tools:
- Electrosurgery with bipolar cautery
- Harmonic energy
- Vascular clips
- Ligating suture
Our preference is to clamp and coagulate the uterine vascular bundle using curved ultrasonic shears (Harmonic Ace).3
Secure the uterine vessels at the ascending branches rather than where they enter the lower uterus, as the latter area is in close proximity to the ureter (FIGURE 2). To ensure hemostasis when using the ultrasonic shears, relax tissue tension and activate the device using minimum power.
FIGURE 1 Secure the uterine vessels
The left ascending uterine vessels are secured using the curved, ultrasonic shears.
FIGURE 2 Proximity of key structures
Because the ureter (no. 1) and uterine vessels (no. 2) are in close proximity, it is advisable to secure the vessels at the ascending branches (no. 3).
Secondary goal: Identify tissue structures
To identify the 3 levels of tissue structures in the lower pelvis, it is necessary to manipulate the uterus. We recommend learning to use a laparoscopic uterine tissue manipulator instead of a cervical–vaginal manipulator. The former makes it possible to maintain visualization throughout the procedure, obtain adequate exposure, and control tissue tension.
The 3 levels of tissue to be identified are (FIGURE 3):
- Level 1—ascending uterine vascular bundle
- Level 2—junction of the uterosacral–cardinal ligaments
- Level 3—junction of the cervix and vagina
If the uterus is large enough to interfere with visualization of the uterosacral–cardinal ligaments or the cervical–vaginal junction, or both, in situ tissue morcellation is warranted. This debulking should eventually allow visualization of the lower tissue structures.
FIGURE 3 Three levels of tissue
Level 1 corresponds to the ascending uterine vessels, level 2 to the uterosacral–cardinal ligament junction, and level 3 to the cervical–vaginal junction.
How tissue levels come into play
Total hysterectomy. Level 3 is the end-point. Once the uterine vessels are secured and the levels are identified, perform anterior and posterior colpotomy (FIGURE 4). Using traction and counter-traction, coagulate and divide the broad ligament, starting at level 1 and ending at level 3. Perform this step bilaterally.
Remove the cervix, uterus, and adnexa (if planned) via the vagina. Close the vaginal cuff using laparoscopic suturing for appropriate cuff support.4
Supracervical hysterectomy. Level 2 is the endpoint. Begin at level 1 using reverse cone drilling (FIGURE 5). This will enable you to reach level 2. Then extract the uterus using the tissue morcellator.
Laparoscopic-assisted vaginal hysterectomy. Clamp, cut, and ligate the uterine vessels vaginally.
FIGURE 4 Anterior and posterior colpotomy
After the uterine vessels are secured and the 3 tissue levels have been identified, perform anterior (A) and posterior (B) colpotomy.
FIGURE 5 Supracervical hysterectomy
Begin the procedure at tissue level 1 using reverse cone drilling to reach the level 2 endpoint.
Always locate the ureter
Regardless of the type of hysterectomy being performed, it is critical to observe the ureter and ensure that it is out of harm’s way before securing the uterine vessels and identifying the tissue levels.
At the end of the procedure, always reduce intra-abdominal pelvic pressure to 5 mm Hg and check all tissue sites for hemostasis.
Not so long ago in the mid-1980s, we had fewer trocar options, laparoscopic suturing was limited, unipolar cautery was popular, endocutters could not guarantee hemostasis across staple lines, laparoscopes were large, images were unpredictable, monitors and cameras were nonexistent, and gas insufflators were bulky and slow. Despite these shortcomings, many surgeons and nurses believed minimally invasive surgery conferred advantages worth pursuing.
Then Semm pelviscopy (Kiel, Germany) reached the United States and intrigued American surgeons, both general and gynecologic.5 The ability to suture laparoscopically was crucial to the success of advanced operative laparoscopy.6 Laparoscopic cholecystectomy emerged, hastening further improvements in equipment and instrumentation.
Beginning in the late 1990s, laparoscopic surgeons witnessed even bigger changes in operating room technologies. The Internet increased the patient’s understanding of her options, and this new awareness motivated hospitals, industry, and physicians to upgrade women’s surgery. One result was specialized gynecologic OR nurse directors with telesurgery/telemedicine integrated into the suites. Digital platform cameras; smaller, clearer laparoscopes; and voice-activated lighting soon followed, as did better insufflators, image capturing, and other advances.
Today we rely on safer electrosurgery units (bipolar and tripolar) and “harmonic” energy.7 Tissue extractors enable us to remove large volumes of tissue quickly and safely. And all these developments have led to proven, enhanced outcomes for the patient.3
A promising future
The future for advanced operative laparoscopy is bright. As patients continue to press for minimally invasive procedures, the range of surgical options available to them will expand. To keep up, we will have little choice but to acquire expertise in minimally invasive applications.
Dr. Steven D. McCarus is a consultant to Ethicon Endo-Surgery and Gynecare.
Dr. Tamberly F. McCarus has no financial relationships relevant to this article.
1. Farquhar CM, Steiner CA. Hysterectomy Rates in the United States 1990-1997. Obstet Gynecol. 2002;99:229-234.
2. Kovac SR, et al. Key Clinical Decision: Determining the Route of Hysterectomy. Cincinnati: Ethicon Endosurgery, Center for Clinical Decision Support; 1999.
3. McCarus SD. Harmonic ultrasonic energy in gynecologic surgery: hysterectomy with the Harmonic Ace and the McCarus technique. A supplement to OBG Management. 2006;18(4).-
4. McCarus SD. Laparoscopic suturing. OBG Management. 2000;12(10).-
5. Semm K. Operative Manual for Endoscopic Abdominal Surgery. Chicago: Year Book Publishers; 1987.
6. Hay DL, Levine RL, von Fraunhofer JA, Masterson BJ. Chromic gut pelviscopic loop ligature: effect of the number of pulls on the tensile strength. J Reprod Med. 1990;35:260-262.
7. McCarus SD. Physiologic mechanism of the ultrasonically activated scalpel. J Am Assoc Gynecol Laparosc. 1996;3:601-608.
1. Farquhar CM, Steiner CA. Hysterectomy Rates in the United States 1990-1997. Obstet Gynecol. 2002;99:229-234.
2. Kovac SR, et al. Key Clinical Decision: Determining the Route of Hysterectomy. Cincinnati: Ethicon Endosurgery, Center for Clinical Decision Support; 1999.
3. McCarus SD. Harmonic ultrasonic energy in gynecologic surgery: hysterectomy with the Harmonic Ace and the McCarus technique. A supplement to OBG Management. 2006;18(4).-
4. McCarus SD. Laparoscopic suturing. OBG Management. 2000;12(10).-
5. Semm K. Operative Manual for Endoscopic Abdominal Surgery. Chicago: Year Book Publishers; 1987.
6. Hay DL, Levine RL, von Fraunhofer JA, Masterson BJ. Chromic gut pelviscopic loop ligature: effect of the number of pulls on the tensile strength. J Reprod Med. 1990;35:260-262.
7. McCarus SD. Physiologic mechanism of the ultrasonically activated scalpel. J Am Assoc Gynecol Laparosc. 1996;3:601-608.
Reducing the medicolegal risk of vacuum extraction
CASE Three hours of pushing
C.A., age 29 years, is 40 weeks’ pregnant with her first child. After an unremarkable pregnancy, she arrives at the hospital for cervical ripening and induction of labor. Oxytocin is given, and labor progresses uneventfully. When C.A.’s cervix is dilated 8 cm, however, labor stalls. The physician orders placement of a pressure catheter and increases the dosage of oxytocin, and the cervix dilates fully. Although C.A. pushes well, the vertex descends only from +1 to +2 station (of 5 stations) after 3 hours.
How would you manage this delivery?
One option in C.A.’s case is operative vaginal delivery using the vacuum extractor, which has replaced the forceps as the most commonly used approach for operative vaginal delivery. Like the forceps, the vacuum extractor has vociferous detractors as well as supporters. Liberal use of cesarean section and questions regarding the safety of operative vaginal delivery vis-à-vis cesarean section have fueled the debate over its role in obstetric practice.
Among the benefits of vacuum extraction are its cost-effectiveness and shorter hospital stay (TABLE 1). It also obviates the need for cesarean section, including repeat cesarean. Risks include an increased incidence of genital tract trauma and a greater risk of fetal subgaleal hemorrhage.
We review 4 critical spheres of concern in regard to vacuum extraction:
- Patient selection
- Informed consent
- Technique
- Documentation
Increased understanding of these aspects of vacuum extraction will improve outcomes for the patient and limit medicolegal risk.
In the case of C.A., the physician offered 3 options:
- Continue maternal expulsive efforts to allow descent
- Attempt delivery by vacuum extraction
- Proceed to cesarean section on the basis of protracted descent.
Risks and benefits were reviewed with the patient, who chose to deliver by cesarean section. A 3,780-g infant in occiput posterior position was delivered safely.
TABLE 1
Delicate balance: Risks and benefits of operative vaginal delivery
| WHO? | BENEFIT | RISK |
|---|---|---|
| Mother | Cost-effective Less blood loss Lower risk of febrile morbidity Maternal preference No need for cesarean section or repeat cesarean Shorter hospitalization and convalescence | Increased incidence of genital tract trauma Possible damage to pelvic floor, with urinary and anal incontinence |
| Fetus | Fewer respiratory difficulties at birth | Increased risk of subgaleal hemorrhage Association with shoulder dystocia |
1. Patient selection: Maternal and fetal indications
Vacuum extraction may be justified for maternal or fetal indications.1,2 Maternal indications include prolongation or arrest of the second stage of labor, or the need to shorten the second stage, for reasons such as maternal cardiac disease, complex congenital cardiovascular disorders, and maternal exhaustion.
No definitive time limit for the second stage of labor
There is more flexibility today than in the past about what constitutes a “safe” length of the second stage. Recommendations concerning when the mother should begin pushing—and for how long—have evolved from a strict time limit to a focus on progression. If the fetal heart rate (FHR) tracing is reassuring, the second stage no longer needs to be limited to 2 or 3 hours. On the contrary, if the patient is still able and willing to push, changes in positioning and further expectant management remain acceptable in contemporary practice.3 Otherwise, a trial of vacuum extraction may be appropriate.
Vacuum extraction is particularly useful when the mother has difficulty pushing because of exhaustion and the fetal head has descended enough that it distends the labia between contractions, as in outlet deliveries.
Fetal indications
Fetal indications for operative vaginal delivery include distress, jeopardy, or a “nonreassuring” FHR tracing. Such a tracing may include late and prolonged decelerations, baseline bradycardia or tachycardia with or without variable decelerations, or, occasionally, a normal baseline rate with diminished variability.
Use vacuum or forceps?
The choice depends on which device would achieve delivery in the safest manner with the lowest risk of fetal injury. With the vacuum, force is exerted directly on the fetal scalp and only secondarily on the fetal skull. This puts fetal vessels that traverse the subgaleal space at risk for injury (FIGURE). With forceps, force is exerted directly on the fetal skull and mitigated by the petrous bone. Little or no force is exerted on the fetal scalp, lessening the risk of traumatic injury such as potentially fatal subgaleal hemorrhage.
Indications and contraindications for vacuum extraction are similar, but not identical, to those for forceps delivery (TABLE 2).2,3 The most important determinant for either device is the experience of the operator. You must be familiar with the instrument and technique before making any attempt to assist delivery. An inability to accurately assess fetal position or station, fetopelvic proportion, adequacy of labor, engagement of the fetal head, or any degree of malpresentation (including minor degrees of deflexion) is a contraindication to a trial of operative vaginal delivery.
Vacuum extraction should be reserved for fetuses at more than 34 weeks’ gestation because of the increased risk of intracranial hemorrhage associated with prematurity.
All decisions involving vacuum extraction should be made with caution. The adequacy of the pelvis, estimated fetal size, and any suggestions of fetopelvic disproportion are of particular significance.3
FIGURE
Subgaleal hemorrhage, a deadly complication
Blood can accumulate in a large potential space between the galea aponeurotica and the periosteum of the cranial bones after vacuum extraction. An infant with subgaleal hemorrhage will exhibit a boggy scalp, with swelling that crosses the suture lines and expands head circumferenceTABLE 2
Factors that predict success—or failure—of vacuum extraction
| When a woman fits overlapping categories, the decision to use vacuum extraction—or not—may be a judgment call* |
| GOOD CANDIDACY |
| Multiparous |
| Term pregnancy |
| Occiput anterior position, well-flexed |
| Wide subpubic arch |
| Compliant |
| MARGINAL CANDIDACY |
| Primiparous |
| Post-term |
| Occiput posterior position |
| Average subpubic arch |
| Gestational diabetes |
| Arrest disorders in second stage |
| POOR CANDIDACY |
| Protraction disorders in second stage |
| Narrow subpubic arch |
| Uncertain position of fetal head |
| Deflexion or asynclitism |
| Anticipated large-for-gestational-age infant |
| Poor maternal compliance |
| * When faced with a good indication in a marginal candidate, we recommend delivery in a “double setup” situation in which preparations are made for both vacuum extraction and cesarean section. If the vacuum can be properly applied, the first application of traction is crucial. We will only proceed if significant descent is achieved. If the fetal head (not the scalp) can be advanced a full station, then we proceed cautiously. If not, ready access to cesarean section allows for completion of the delivery in a timely manner. |
2. Informed consent: Elicit the patient’s desires
Thorough discussion with the patient and her family—to explain the reasoning behind the clinical decision to use the vacuum extractor and delineate the alternatives—is paramount. Moreover, the patient should be encouraged to actively participate in this discussion.
Among the alternatives to vacuum extraction are expectant observation and expedited delivery by cesarean section. Because patients increasingly are requesting elective cesarean section in the absence of obvious obstetric indications, this option should receive extra attention.
Most women still consider vaginal delivery an important milestone of female adulthood. When safety concerns arise and the situation makes vaginal delivery unwise, many women experience disappointment and postpartum depression over their “failed” attempt at vaginal delivery. These perceptions need to be addressed in discussions with the patient.
The risk–benefit equation
Vacuum extraction lessens the risk of maternal lacerations, either of the lower genital tract in the case of obstetric forceps, or of the cervix and lower uterine segment in the case of cesarean section. In addition, vacuum extraction can be performed comfortably in the absence of regional anesthesia.
Avoiding cesarean section can produce multiple benefits
Another maternal benefit of vacuum extraction is the decreased need for cesarean section. A reduction in the primary cesarean rate also lowers the need for repeat cesarean section, which can be more technically challenging than primary C-section due to the presence of dense scar tissue and intra-abdominal adhesions. Cesarean section also increases the risk of placenta accreta, increta, or percreta in subsequent pregnancies. These complications increase the likelihood of emergency hysterectomy, massive blood loss, and serious maternal morbidity and mortality.
Even in the absence of placenta accreta, both primary and repeat cesarean sections raise the risk of hemorrhage and febrile morbidity, prolong convalescence, and increase cost, compared with vaginal delivery. For these reasons, avoiding primary cesarean section can obviate the need for multiple surgical procedures and their attendant risks. The degree to which these factors favor vaginal delivery over cesarean section is subject to debate.
Maternal risks include pelvic floor trauma
Both vacuum extraction and forceps delivery increase the risk of anal sphincter injury and can impair fecal continence.4 Both methods also appear to increase trauma to the genital tract in comparison with spontaneous delivery and may predispose the woman to pelvic floor dysfunction, including urinary and anal incontinence.5-10 However, anal sphincter trauma was less frequent after vacuum extraction than after forceps delivery.1
Other maternal injuries associated with vacuum extraction include perineal lacerations and injuries to the vulva, vagina, and cervix. Vacuum extraction also has been implicated as a significant risk factor for postpartum hemorrhage11 and genital-tract infection.1
Fewer neonatal respiratory problems with vaginal delivery
Compared with cesarean section, vaginal delivery is thought to diminish the risk of intrapartum aspiration and respiratory problems in the newborn. It also may facilitate the transition from fetal to neonatal circulation and reduce the need for immediate resuscitation at birth.
Neonatal risks include soft-tissue injury and potential hemorrhage
Infants delivered by vacuum extraction have a significantly higher rate of intracranial hemorrhage, brachial plexus injuries, convulsions, central nervous system depression, and the need for mechanical ventilation, compared with spontaneously delivered infants (TABLE 3).12,13
Although vacuum extraction is associated with a wide range of soft tissue injuries, they are often less serious than the fetal scalp injuries associated with obstetric forceps. Cup marks, bruising, and minor lacerations of the scalp and caput succedaneum are common fetal injuries, although the majority resolve without apparent sequelae.14
Subgaleal hemorrhage is the most serious neonatal complication of vacuum extraction, occurring in 1% to 3.8% of vacuum extractions (FIGURE).15 It coexists with neonatal coagulopathy in 19% to 29% of newborns16 and increases the risk of progression to hemorrhagic shock and death. Subgaleal hemorrhage has a mortality rate ranging from 2.7% to 22.8%.15-17
Cephalhematoma is another complication associated with vacuum extraction. It involves an accumulation of blood beneath the periosteum of a cranial bone (usually the parietal bone), and it almost always resolves spontaneously. The incidence of cephalhematoma varies. It is significantly more common in deliveries involving vacuum extraction (9.8%) than in forceps deliveries (4.1%).18 Its incidence increases with the length of time the vacuum cup is applied and with paramedian application.18
Intracranial hemorrhage occurs in 1 of 860 vacuum extractions, 1 of 664 forceps deliveries, 1 of 954 cesarean deliveries, and 1 of 1,900 spontaneous deliveries.12 Subdural hemorrhage is the most common form of intracranial hemorrhage and is almost invariably the result of birth trauma. However, asymptomatic subdural hematoma occurs in up to 6.1% of uncomplicated vaginal deliveries.19
Other, less common types of intracranial hemorrhage, such as subarachnoid, intraventricular, and intraparenchymal hemorrhage, have a more complex etiology, which includes birth asphyxia, hemorrhagic diathesis, infection, and vascular abnormalities.20
Retinal hemorrhage also may occur after vacuum extraction, with an incidence of 49% to 77.8%, compared with 30.3% after forceps delivery, 30.4% after normal vaginal delivery, and 8.3% after cesarean delivery.21 It generally resolves spontaneously without any permanent damage.22
TABLE 3
Vacuum extraction can injure the fetus
| DIRECT INJURY |
| Cephalhematoma |
| Intracranial hemorrhage (parenchymal, subdural, intraventricular, subarachnoid) |
| Nerve injury |
| Scalp laceration, abrasion, ecchymoses, necrosis |
| Skull fracture |
| Subgaleal hemorrhage |
| INDIRECT INJURY |
| Anemia, hyperbilirubinemia |
| Brachial plexus injury |
| Scalp infection or abscess |
| SOURCE: O’Grady et al31 |
Shoulder dystocia and brachial plexus palsy
Vacuum extraction also is associated with shoulder dystocia and brachial plexus palsy, although the primary risk factor for these complications is thought to be increased fetal size.23-25 The incidence of shoulder dystocia with vacuum extraction is 3.5%, compared with 1.5% for forceps delivery.25
The risk of brachial plexus palsy also increases with vacuum extraction, especially as the duration of the procedure increases.25
Less common complications associated with vacuum extraction are skull fractures, fetal hemorrhage from bleeding at the site of scalp electrodes, sepsis originating from infected scalp trauma, and corneal injury.
No long-term impairment
Long-term outcome studies of children delivered by vacuum extraction show no differences in physical or cognitive functioning or intelligence scores, compared with other modes of delivery.26
3. Technique: Create conditions that ensure success
Certain prerequisites to vacuum extraction can assure successful application and strict adherence to protocol. These prerequisites include having an appropriate indication, thorough informed consent, proper maternal positioning, adequate anesthesia, and knowledge of fetal position and station (TABLE 4).1 These objectives can be accomplished in the following steps:
- After an informed consent discussion, assess maternal positioning and repeat the pelvic exam. Also ascertain the adequacy of anesthesia. Insert a bladder catheter.
- Perform a “ghost” trial of vacuum extraction to visualize the procedure before the actual attempt.
- Test the function of the vacuum.
- Lubricate the vacuum cup with surgical soap or gel, insert it into the vagina, and maneuver it onto the fetal head. Place the vacuum extractor over the sagittal suture about 6 cm distal to the anterior fontanel and 2 cm proximal to the posterior fontanel. (The illustration on page 74 demonstrates positioning.) Apply a small degree of vacuum (approximately 20 mm Hg). Double-check application.
- Gradually apply full vacuum (550–600 mm Hg, depending on cup size), allowing the scalp to mold to the extractor cup.
- Apply 2-handed traction in concert with uterine contractions and supplemented by maternal pushing. Assuming there is no loss of vacuum (“pop-off” of the cup), the initial traction effort should produce a gain in station. If a “pop-off” occurs, a single additional attempt at delivery may be warranted.
- As the head crowns, perform episiotomy as needed and slowly deliver the fetal head. Remove the vacuum cup.
- After delivery of the placenta, inspect the vagina, cervix, and perineum closely.
- Dictate a full operative note and annotate the delivery in the chart. See the section on documentation, below.
Vacuum extraction may fail for a number of reasons (TABLE 5).
TABLE 4
Perform these predelivery checks before applying traction
| Is anesthesia adequate? Is maternal positioning correct? |
| Is the bladder empty? |
| Is the fetus in the proper attitude (flexion)? |
| Is fetal status reassuring? |
Is the vacuum properly applied?
|
| Has the patient been instructed on when and how long to push? |
| Are the proposed maneuvers appropriate? |
TABLE 5
Why might vacuum extraction fail?
| INSTRUMENT-RELATED |
| Pump failure |
| Vacuum leak |
| TECHNIQUE-RELATED |
| Failure to encourage maternal valsalva with traction efforts |
| Inappropriate intensity of traction |
| Incorrect axis of traction |
| Maternal tissue trapped beneath vacuum cup |
| Poor cup position |
| OBSTETRIC CONDITIONS |
Congenital anomaly
|
| Fetal macrosomia |
| Incomplete cervical dilation |
Position and attitude problems
|
| Unappreciated cephalopelvic disproportion |
| SOURCE: Modified from Plauche et al32 |
Most important variable: Cup placement
The single most critical step in vacuum extraction is placement of the cup. It should be applied at the point of maximum fetal cranial flexion, which is proximal to the leading edge of the posterior fontanel.
Once full vacuum is achieved, encourage the mother to push with the next contraction, and apply steady traction in concert with her efforts.
The initial application of traction should be directed to maintain proper flexion of the fetal head, and should bring about descent of the fetal head. If there is no descent with the first application of traction, and correct technique and cup placement have been applied, abandon operative vaginal delivery (TABLE 6).
Do not make a further attempt to deliver the child using forceps, as the risk of intracranial hemorrhage appears to be highest in infants delivered using a combination of vacuum extraction and forceps.
TABLE 6
Repeat traction efforts reap a diminishing return
| NUMBER OF TRACTION EFFORTS | SUCCESS RATE | |
|---|---|---|
| VACUUM EXTRACTION (N=433) | FORCEPS (N=555) | |
| 1 or 2 | 68.4% | 38.4% |
| 3 or 4 | 24.9% | 48.6% |
| 5 or more | 6.7% | 12.9% |
| Adapted from Sjostedt33 | ||
4. Documentation: The chart is the most important witness
The value of complete and contemporaneous notation cannot be overstated. The patient’s chart is the permanent repository of the record of delivery. It is without doubt the most important witness to the event and should be treated as such. Include a dictated operative note as well as notation in the chart itself. Notes should be legible and properly dated, with the time of day indicated.
When operative vaginal delivery is performed, record the following:
- indication for the procedure
- course of labor
- anesthesia
- personnel present
- instruments used
- position and station of the fetal head
- force and duration of traction
- complications, including how they were recognized and managed
- immediate condition of the newborn and all steps taken in resuscitation.
Operative vaginal delivery is no newcomer to obstetrics. Hindu writings from about 1000 BC, and Hippocrates’ own musings from the fifth century BC, describe instruments and techniques to combat arrested labor and salvage the lives of both mother and child.27 Crude forceps were described by the Muslim physician Albucasis in the 11th century.27
Before the advent of safe cesarean section, many maternal lives were no doubt saved by these instruments and techniques. Unfortunately, destruction of the fetus and maternal death were frequent outcomes of operative vaginal delivery by forceps before the 20th century.28
As for vacuum extraction in particular, the idea of attaching a device to the fetal head to aid in delivery is credited to Arnett, a 19th century surgeon and inventor, who envisioned the “pneumatic tractor.”29
In 1957, Malmstrom reintroduced the vacuum as an aid in delivery, designing a rigid cup that was connected by rubber tubing to a vacuum source.30 This allowed the separation of the pump mechanism from the cup and made for easier application.
Most recently, Kobayashi developed the soft-cup design, a low-cost flexible plastic alternative that allows for a disposable instrument.31
Minimizing medicolegal risk
The best way to prevent an accusation of medical malpractice is to develop strong clinical and interpersonal skills. These simple, intuitive suggestions may help:
- Understand the role of operative vaginal delivery in current practice.
- Develop a simple and interactive discussion model for use in labor and delivery with the patient and her family.
- Consider a woman’s preferences for delivery.
- Know the indications and contraindications for vacuum extraction.
- Use the checks and safeguards listed under 3. Technique: Create conditions that ensure success.
- Perform vacuum extraction in the cesarean section room. Stop the procedure at once if any problem arises, and proceed to cesarean delivery.
- Make all chart notations completely legible, and add dictated notes.
If you are a new physician or lack significant experience with vacuum extraction, ask for input, supervision, and education from more experienced clinicians. Also make it a point to ask about department guidelines and review the credentialing process. Once you become adept at vacuum extraction, mentor more junior colleagues.
Two critical concerns
When contemplating vacuum-assisted delivery, 2 risks are paramount:
- failure of the vacuum extractor to achieve delivery
- the potential for fetal and maternal injury.
Training must ensure appropriate case selection and technique. Vacuum extraction must be performed with the same precision and care used with forceps. If application of the device is incorrect, or if there is a wrong direction of traction, excessive traction, or traction in the presence of disproportion, the cup will slip or pop off, and vacuum delivery will fail, with the potential for traumatic fetal injury.
All risks must be discussed with the patient to fulfill informed consent, and the risks and benefits of alternative treatments should be part of the discussion. Active participation, in considering how best to approach delivery, is required of all parties concerned.
The vacuum extractor can be a useful adjunct in certain circumstances, and its use has become widespread in American delivery suites. As with the obstetric forceps, which largely antedated its use, the vacuum extractor can lessen the overall risks of childbirth for both mother and infant.
The authors report no financial relationships relevant to this article.
1. O’Grady JP, Gimovsky ML, McIlhargie CJ, eds. Operative Obstetrics. Pearl River, NY: Parthenon Publishing; 1995.
2. Johanson RB, Menon BKV. Vacuum extraction versus forceps for assisted vaginal delivery. Cochrane Database Syst Rev. 2000;(2):CD000224.-
3. Bloom SL, Casey BM, Schaffer JL, et al. Pushing in the second stage of labor. Am J Obstet Gynecol 2006;194:10-13.
4. Operative vaginal delivery. ACOG Practice Bulletin #17. Washington, DC: American College of Obstetricians and Gynecologists; June 2000.
5. Power D, Fitzpatrick M, O’Herlihy C. Obstetric anal sphincter injury: how to avoid, how to repair: a literature review. J Fam Pract 2006;55:193-200.
6. Chaliha C, Kalia V, Stanton S, et al. Antenatal prediction of postpartum urinary and fecal incontinence. Obstet Gynecol 1999;94:689-694.
7. Bofill JA, Rust OA, Schorr SJ, et al. A randomized prospective trial of the obstetric forceps versus the M-cup vacuum extractor. Am J Obstet Gynecol 1996;175:1325-1330.
8. Salamalekis E, Loghis C, Pyrgiotis E, et al. Soft cup vacuum extractor versus forceps delivery. J Obstet Gynecol. 1995;15:245-246.
9. Zetterstrom JP, Lopez A, Anzen B, et al. Anal incontinence after vaginal delivery: a prospective study in primiparous women. Br J Obstet Gynaecol. 1999;106:324-330.
10. Johanson RB, Heycock E, Carter J, et al. Maternal and child health after assisted vaginal delivery: five-year follow up of a randomized controlled study comparing forceps and ventouse. Br J Obstet Gynaecol. 1999;106:544-549
11. Faltin DL, Otero M, Petignat P, et al. Women’s health 18 years after rupture of the anal sphincter during childbirth: I. Fecal incontinence. Am J Obstet Gynecol. 2006;194:1255-1259.
12. Plauche WC. Fetal cranial injuries related to delivery with the Malmsträm vacuum extractor. Obstet Gynecol. 1979;53:750-757.
13. Towner D, Castro MA, Eby-Wilkens E, et al. Effect of mode of delivery in nulliparous women on neonatal intracranial injury. N Engl J Med. 1999;341:1709-1714.
14. Sheiner E, Sarid L, Levy A, et al. Obstetric risk factors and outcome of pregnancies complicated with early postpartum hemorrhage: a population-based study. J Matern Fetal Neonatal Med. 2005;18:149-154.
15. Johanson R. Choice of instrument for vaginal delivery. Curr Opin Obstet Gynecol. 1997;9:361-365.
16. Chadwick LM, Pemberton PJ, Kurinczuk JJ. Neonatal subgaleal hematoma: associated risk factors, complications, and outcome. J Pediatr Child Health. 1996;32:228-232.
17. Ng PC, Siu YK, Lewindon PJ. Subaponeurotic hemorrhage in the 1990s: a 3-year surveillance. Acta Pediatr. 1995;84:1065-1069
18. Bofill JA, Rust OA, Devidas M, et al. Neonatal cephalohematoma from vacuum extraction. J Reprod Med. 1997;42:565-569.
19. Doumouchtsis SK, Arulkumaran S. Head injuries after instrumental vaginal deliveries. Curr Opin Obstet Gynecol. 2006;18:129-134.
20. Govaert P. Cranial Hemorrhage in the Term Newborn Infant. London: Mac Keith Press; 1993.
21. Hughes LA, May K, Talbot JF, Parsons MA. Incidence, distribution, and duration of birth-related retinal hemorrhages: a prospective study. J AAPOS. 2006;10:102-106.
22. Sheiner E, Levy A, Hershkovitz R, et al. Determining factors associated with shoulder dystocia: a population-based study. Eur J Obstet Gynecol Reprod Biol. 2006;126:11-15.
23. Baskett TF, Allen AC. Perinatal implications of shoulder dystocia. Obstet Gynecol. 1995;86:15-18.
24. Mollberg M, Hagerg H, Bager B, et al. Risk factors for obstetric brachial plexus palsy among neonates delivered by vacuum extraction. Obstet Gynecol. 2005;106:913-918.
25. Caughey AB, Sandberg PL, Alantnik MG, et al. Forceps compared with vacuum: rates of neonatal and maternal morbidity. Obstet Gynecol. 2005;106:908-912.
26. Ngan HYS, Miu P, Ko L, et al. Long-term neurological sequelae following vacuum extractor delivery. Aust NZ J Obstet Gynecol. 1990;30:111-114.
27. Lyons AS, Petrucelli RJ. Medicine: An Illustrated History. New York: Harry N Abrams; 1978.
28. Speert H. Obstetric and Gynecologic Milestones Illustrated. Pearl River, NY: Parthenon Publishing; 1996.
29. Arnett N. Elements of Physics or Natural Philosophy, General and Medical, Explained Independently of Technical Mathematics and Containing New Disquisitions and Practical Suggestions. 2nd ed. Philadelphia: Carney and Lea; 1831.
30. Malmstrom T. The vacuum extractor, an obstetrical instrument. I. Acta Obstet Gynecol Scand. 1957;36(suppl 3):5-50.
31. O’Grady JP, Gimovsky ML, McIlhargie CJ. Vacuum Extraction in Modern Obstetric Practice. Pearl River, NY: Parthenon Publishing; 1995.
32. Plauche WC, Morrison JC, O’Sullivan MJ. Surgical Obstetrics. Philadelphia: WB Saunders; 1992.
33. Sjostedt JE. The vacuum extractor and forceps in obstetrics: a clinical study. Acta Obstet Gynecol Scand. 1967;48:638-639.
CASE Three hours of pushing
C.A., age 29 years, is 40 weeks’ pregnant with her first child. After an unremarkable pregnancy, she arrives at the hospital for cervical ripening and induction of labor. Oxytocin is given, and labor progresses uneventfully. When C.A.’s cervix is dilated 8 cm, however, labor stalls. The physician orders placement of a pressure catheter and increases the dosage of oxytocin, and the cervix dilates fully. Although C.A. pushes well, the vertex descends only from +1 to +2 station (of 5 stations) after 3 hours.
How would you manage this delivery?
One option in C.A.’s case is operative vaginal delivery using the vacuum extractor, which has replaced the forceps as the most commonly used approach for operative vaginal delivery. Like the forceps, the vacuum extractor has vociferous detractors as well as supporters. Liberal use of cesarean section and questions regarding the safety of operative vaginal delivery vis-à-vis cesarean section have fueled the debate over its role in obstetric practice.
Among the benefits of vacuum extraction are its cost-effectiveness and shorter hospital stay (TABLE 1). It also obviates the need for cesarean section, including repeat cesarean. Risks include an increased incidence of genital tract trauma and a greater risk of fetal subgaleal hemorrhage.
We review 4 critical spheres of concern in regard to vacuum extraction:
- Patient selection
- Informed consent
- Technique
- Documentation
Increased understanding of these aspects of vacuum extraction will improve outcomes for the patient and limit medicolegal risk.
In the case of C.A., the physician offered 3 options:
- Continue maternal expulsive efforts to allow descent
- Attempt delivery by vacuum extraction
- Proceed to cesarean section on the basis of protracted descent.
Risks and benefits were reviewed with the patient, who chose to deliver by cesarean section. A 3,780-g infant in occiput posterior position was delivered safely.
TABLE 1
Delicate balance: Risks and benefits of operative vaginal delivery
| WHO? | BENEFIT | RISK |
|---|---|---|
| Mother | Cost-effective Less blood loss Lower risk of febrile morbidity Maternal preference No need for cesarean section or repeat cesarean Shorter hospitalization and convalescence | Increased incidence of genital tract trauma Possible damage to pelvic floor, with urinary and anal incontinence |
| Fetus | Fewer respiratory difficulties at birth | Increased risk of subgaleal hemorrhage Association with shoulder dystocia |
1. Patient selection: Maternal and fetal indications
Vacuum extraction may be justified for maternal or fetal indications.1,2 Maternal indications include prolongation or arrest of the second stage of labor, or the need to shorten the second stage, for reasons such as maternal cardiac disease, complex congenital cardiovascular disorders, and maternal exhaustion.
No definitive time limit for the second stage of labor
There is more flexibility today than in the past about what constitutes a “safe” length of the second stage. Recommendations concerning when the mother should begin pushing—and for how long—have evolved from a strict time limit to a focus on progression. If the fetal heart rate (FHR) tracing is reassuring, the second stage no longer needs to be limited to 2 or 3 hours. On the contrary, if the patient is still able and willing to push, changes in positioning and further expectant management remain acceptable in contemporary practice.3 Otherwise, a trial of vacuum extraction may be appropriate.
Vacuum extraction is particularly useful when the mother has difficulty pushing because of exhaustion and the fetal head has descended enough that it distends the labia between contractions, as in outlet deliveries.
Fetal indications
Fetal indications for operative vaginal delivery include distress, jeopardy, or a “nonreassuring” FHR tracing. Such a tracing may include late and prolonged decelerations, baseline bradycardia or tachycardia with or without variable decelerations, or, occasionally, a normal baseline rate with diminished variability.
Use vacuum or forceps?
The choice depends on which device would achieve delivery in the safest manner with the lowest risk of fetal injury. With the vacuum, force is exerted directly on the fetal scalp and only secondarily on the fetal skull. This puts fetal vessels that traverse the subgaleal space at risk for injury (FIGURE). With forceps, force is exerted directly on the fetal skull and mitigated by the petrous bone. Little or no force is exerted on the fetal scalp, lessening the risk of traumatic injury such as potentially fatal subgaleal hemorrhage.
Indications and contraindications for vacuum extraction are similar, but not identical, to those for forceps delivery (TABLE 2).2,3 The most important determinant for either device is the experience of the operator. You must be familiar with the instrument and technique before making any attempt to assist delivery. An inability to accurately assess fetal position or station, fetopelvic proportion, adequacy of labor, engagement of the fetal head, or any degree of malpresentation (including minor degrees of deflexion) is a contraindication to a trial of operative vaginal delivery.
Vacuum extraction should be reserved for fetuses at more than 34 weeks’ gestation because of the increased risk of intracranial hemorrhage associated with prematurity.
All decisions involving vacuum extraction should be made with caution. The adequacy of the pelvis, estimated fetal size, and any suggestions of fetopelvic disproportion are of particular significance.3
FIGURE
Subgaleal hemorrhage, a deadly complication
Blood can accumulate in a large potential space between the galea aponeurotica and the periosteum of the cranial bones after vacuum extraction. An infant with subgaleal hemorrhage will exhibit a boggy scalp, with swelling that crosses the suture lines and expands head circumferenceTABLE 2
Factors that predict success—or failure—of vacuum extraction
| When a woman fits overlapping categories, the decision to use vacuum extraction—or not—may be a judgment call* |
| GOOD CANDIDACY |
| Multiparous |
| Term pregnancy |
| Occiput anterior position, well-flexed |
| Wide subpubic arch |
| Compliant |
| MARGINAL CANDIDACY |
| Primiparous |
| Post-term |
| Occiput posterior position |
| Average subpubic arch |
| Gestational diabetes |
| Arrest disorders in second stage |
| POOR CANDIDACY |
| Protraction disorders in second stage |
| Narrow subpubic arch |
| Uncertain position of fetal head |
| Deflexion or asynclitism |
| Anticipated large-for-gestational-age infant |
| Poor maternal compliance |
| * When faced with a good indication in a marginal candidate, we recommend delivery in a “double setup” situation in which preparations are made for both vacuum extraction and cesarean section. If the vacuum can be properly applied, the first application of traction is crucial. We will only proceed if significant descent is achieved. If the fetal head (not the scalp) can be advanced a full station, then we proceed cautiously. If not, ready access to cesarean section allows for completion of the delivery in a timely manner. |
2. Informed consent: Elicit the patient’s desires
Thorough discussion with the patient and her family—to explain the reasoning behind the clinical decision to use the vacuum extractor and delineate the alternatives—is paramount. Moreover, the patient should be encouraged to actively participate in this discussion.
Among the alternatives to vacuum extraction are expectant observation and expedited delivery by cesarean section. Because patients increasingly are requesting elective cesarean section in the absence of obvious obstetric indications, this option should receive extra attention.
Most women still consider vaginal delivery an important milestone of female adulthood. When safety concerns arise and the situation makes vaginal delivery unwise, many women experience disappointment and postpartum depression over their “failed” attempt at vaginal delivery. These perceptions need to be addressed in discussions with the patient.
The risk–benefit equation
Vacuum extraction lessens the risk of maternal lacerations, either of the lower genital tract in the case of obstetric forceps, or of the cervix and lower uterine segment in the case of cesarean section. In addition, vacuum extraction can be performed comfortably in the absence of regional anesthesia.
Avoiding cesarean section can produce multiple benefits
Another maternal benefit of vacuum extraction is the decreased need for cesarean section. A reduction in the primary cesarean rate also lowers the need for repeat cesarean section, which can be more technically challenging than primary C-section due to the presence of dense scar tissue and intra-abdominal adhesions. Cesarean section also increases the risk of placenta accreta, increta, or percreta in subsequent pregnancies. These complications increase the likelihood of emergency hysterectomy, massive blood loss, and serious maternal morbidity and mortality.
Even in the absence of placenta accreta, both primary and repeat cesarean sections raise the risk of hemorrhage and febrile morbidity, prolong convalescence, and increase cost, compared with vaginal delivery. For these reasons, avoiding primary cesarean section can obviate the need for multiple surgical procedures and their attendant risks. The degree to which these factors favor vaginal delivery over cesarean section is subject to debate.
Maternal risks include pelvic floor trauma
Both vacuum extraction and forceps delivery increase the risk of anal sphincter injury and can impair fecal continence.4 Both methods also appear to increase trauma to the genital tract in comparison with spontaneous delivery and may predispose the woman to pelvic floor dysfunction, including urinary and anal incontinence.5-10 However, anal sphincter trauma was less frequent after vacuum extraction than after forceps delivery.1
Other maternal injuries associated with vacuum extraction include perineal lacerations and injuries to the vulva, vagina, and cervix. Vacuum extraction also has been implicated as a significant risk factor for postpartum hemorrhage11 and genital-tract infection.1
Fewer neonatal respiratory problems with vaginal delivery
Compared with cesarean section, vaginal delivery is thought to diminish the risk of intrapartum aspiration and respiratory problems in the newborn. It also may facilitate the transition from fetal to neonatal circulation and reduce the need for immediate resuscitation at birth.
Neonatal risks include soft-tissue injury and potential hemorrhage
Infants delivered by vacuum extraction have a significantly higher rate of intracranial hemorrhage, brachial plexus injuries, convulsions, central nervous system depression, and the need for mechanical ventilation, compared with spontaneously delivered infants (TABLE 3).12,13
Although vacuum extraction is associated with a wide range of soft tissue injuries, they are often less serious than the fetal scalp injuries associated with obstetric forceps. Cup marks, bruising, and minor lacerations of the scalp and caput succedaneum are common fetal injuries, although the majority resolve without apparent sequelae.14
Subgaleal hemorrhage is the most serious neonatal complication of vacuum extraction, occurring in 1% to 3.8% of vacuum extractions (FIGURE).15 It coexists with neonatal coagulopathy in 19% to 29% of newborns16 and increases the risk of progression to hemorrhagic shock and death. Subgaleal hemorrhage has a mortality rate ranging from 2.7% to 22.8%.15-17
Cephalhematoma is another complication associated with vacuum extraction. It involves an accumulation of blood beneath the periosteum of a cranial bone (usually the parietal bone), and it almost always resolves spontaneously. The incidence of cephalhematoma varies. It is significantly more common in deliveries involving vacuum extraction (9.8%) than in forceps deliveries (4.1%).18 Its incidence increases with the length of time the vacuum cup is applied and with paramedian application.18
Intracranial hemorrhage occurs in 1 of 860 vacuum extractions, 1 of 664 forceps deliveries, 1 of 954 cesarean deliveries, and 1 of 1,900 spontaneous deliveries.12 Subdural hemorrhage is the most common form of intracranial hemorrhage and is almost invariably the result of birth trauma. However, asymptomatic subdural hematoma occurs in up to 6.1% of uncomplicated vaginal deliveries.19
Other, less common types of intracranial hemorrhage, such as subarachnoid, intraventricular, and intraparenchymal hemorrhage, have a more complex etiology, which includes birth asphyxia, hemorrhagic diathesis, infection, and vascular abnormalities.20
Retinal hemorrhage also may occur after vacuum extraction, with an incidence of 49% to 77.8%, compared with 30.3% after forceps delivery, 30.4% after normal vaginal delivery, and 8.3% after cesarean delivery.21 It generally resolves spontaneously without any permanent damage.22
TABLE 3
Vacuum extraction can injure the fetus
| DIRECT INJURY |
| Cephalhematoma |
| Intracranial hemorrhage (parenchymal, subdural, intraventricular, subarachnoid) |
| Nerve injury |
| Scalp laceration, abrasion, ecchymoses, necrosis |
| Skull fracture |
| Subgaleal hemorrhage |
| INDIRECT INJURY |
| Anemia, hyperbilirubinemia |
| Brachial plexus injury |
| Scalp infection or abscess |
| SOURCE: O’Grady et al31 |
Shoulder dystocia and brachial plexus palsy
Vacuum extraction also is associated with shoulder dystocia and brachial plexus palsy, although the primary risk factor for these complications is thought to be increased fetal size.23-25 The incidence of shoulder dystocia with vacuum extraction is 3.5%, compared with 1.5% for forceps delivery.25
The risk of brachial plexus palsy also increases with vacuum extraction, especially as the duration of the procedure increases.25
Less common complications associated with vacuum extraction are skull fractures, fetal hemorrhage from bleeding at the site of scalp electrodes, sepsis originating from infected scalp trauma, and corneal injury.
No long-term impairment
Long-term outcome studies of children delivered by vacuum extraction show no differences in physical or cognitive functioning or intelligence scores, compared with other modes of delivery.26
3. Technique: Create conditions that ensure success
Certain prerequisites to vacuum extraction can assure successful application and strict adherence to protocol. These prerequisites include having an appropriate indication, thorough informed consent, proper maternal positioning, adequate anesthesia, and knowledge of fetal position and station (TABLE 4).1 These objectives can be accomplished in the following steps:
- After an informed consent discussion, assess maternal positioning and repeat the pelvic exam. Also ascertain the adequacy of anesthesia. Insert a bladder catheter.
- Perform a “ghost” trial of vacuum extraction to visualize the procedure before the actual attempt.
- Test the function of the vacuum.
- Lubricate the vacuum cup with surgical soap or gel, insert it into the vagina, and maneuver it onto the fetal head. Place the vacuum extractor over the sagittal suture about 6 cm distal to the anterior fontanel and 2 cm proximal to the posterior fontanel. (The illustration on page 74 demonstrates positioning.) Apply a small degree of vacuum (approximately 20 mm Hg). Double-check application.
- Gradually apply full vacuum (550–600 mm Hg, depending on cup size), allowing the scalp to mold to the extractor cup.
- Apply 2-handed traction in concert with uterine contractions and supplemented by maternal pushing. Assuming there is no loss of vacuum (“pop-off” of the cup), the initial traction effort should produce a gain in station. If a “pop-off” occurs, a single additional attempt at delivery may be warranted.
- As the head crowns, perform episiotomy as needed and slowly deliver the fetal head. Remove the vacuum cup.
- After delivery of the placenta, inspect the vagina, cervix, and perineum closely.
- Dictate a full operative note and annotate the delivery in the chart. See the section on documentation, below.
Vacuum extraction may fail for a number of reasons (TABLE 5).
TABLE 4
Perform these predelivery checks before applying traction
| Is anesthesia adequate? Is maternal positioning correct? |
| Is the bladder empty? |
| Is the fetus in the proper attitude (flexion)? |
| Is fetal status reassuring? |
Is the vacuum properly applied?
|
| Has the patient been instructed on when and how long to push? |
| Are the proposed maneuvers appropriate? |
TABLE 5
Why might vacuum extraction fail?
| INSTRUMENT-RELATED |
| Pump failure |
| Vacuum leak |
| TECHNIQUE-RELATED |
| Failure to encourage maternal valsalva with traction efforts |
| Inappropriate intensity of traction |
| Incorrect axis of traction |
| Maternal tissue trapped beneath vacuum cup |
| Poor cup position |
| OBSTETRIC CONDITIONS |
Congenital anomaly
|
| Fetal macrosomia |
| Incomplete cervical dilation |
Position and attitude problems
|
| Unappreciated cephalopelvic disproportion |
| SOURCE: Modified from Plauche et al32 |
Most important variable: Cup placement
The single most critical step in vacuum extraction is placement of the cup. It should be applied at the point of maximum fetal cranial flexion, which is proximal to the leading edge of the posterior fontanel.
Once full vacuum is achieved, encourage the mother to push with the next contraction, and apply steady traction in concert with her efforts.
The initial application of traction should be directed to maintain proper flexion of the fetal head, and should bring about descent of the fetal head. If there is no descent with the first application of traction, and correct technique and cup placement have been applied, abandon operative vaginal delivery (TABLE 6).
Do not make a further attempt to deliver the child using forceps, as the risk of intracranial hemorrhage appears to be highest in infants delivered using a combination of vacuum extraction and forceps.
TABLE 6
Repeat traction efforts reap a diminishing return
| NUMBER OF TRACTION EFFORTS | SUCCESS RATE | |
|---|---|---|
| VACUUM EXTRACTION (N=433) | FORCEPS (N=555) | |
| 1 or 2 | 68.4% | 38.4% |
| 3 or 4 | 24.9% | 48.6% |
| 5 or more | 6.7% | 12.9% |
| Adapted from Sjostedt33 | ||
4. Documentation: The chart is the most important witness
The value of complete and contemporaneous notation cannot be overstated. The patient’s chart is the permanent repository of the record of delivery. It is without doubt the most important witness to the event and should be treated as such. Include a dictated operative note as well as notation in the chart itself. Notes should be legible and properly dated, with the time of day indicated.
When operative vaginal delivery is performed, record the following:
- indication for the procedure
- course of labor
- anesthesia
- personnel present
- instruments used
- position and station of the fetal head
- force and duration of traction
- complications, including how they were recognized and managed
- immediate condition of the newborn and all steps taken in resuscitation.
Operative vaginal delivery is no newcomer to obstetrics. Hindu writings from about 1000 BC, and Hippocrates’ own musings from the fifth century BC, describe instruments and techniques to combat arrested labor and salvage the lives of both mother and child.27 Crude forceps were described by the Muslim physician Albucasis in the 11th century.27
Before the advent of safe cesarean section, many maternal lives were no doubt saved by these instruments and techniques. Unfortunately, destruction of the fetus and maternal death were frequent outcomes of operative vaginal delivery by forceps before the 20th century.28
As for vacuum extraction in particular, the idea of attaching a device to the fetal head to aid in delivery is credited to Arnett, a 19th century surgeon and inventor, who envisioned the “pneumatic tractor.”29
In 1957, Malmstrom reintroduced the vacuum as an aid in delivery, designing a rigid cup that was connected by rubber tubing to a vacuum source.30 This allowed the separation of the pump mechanism from the cup and made for easier application.
Most recently, Kobayashi developed the soft-cup design, a low-cost flexible plastic alternative that allows for a disposable instrument.31
Minimizing medicolegal risk
The best way to prevent an accusation of medical malpractice is to develop strong clinical and interpersonal skills. These simple, intuitive suggestions may help:
- Understand the role of operative vaginal delivery in current practice.
- Develop a simple and interactive discussion model for use in labor and delivery with the patient and her family.
- Consider a woman’s preferences for delivery.
- Know the indications and contraindications for vacuum extraction.
- Use the checks and safeguards listed under 3. Technique: Create conditions that ensure success.
- Perform vacuum extraction in the cesarean section room. Stop the procedure at once if any problem arises, and proceed to cesarean delivery.
- Make all chart notations completely legible, and add dictated notes.
If you are a new physician or lack significant experience with vacuum extraction, ask for input, supervision, and education from more experienced clinicians. Also make it a point to ask about department guidelines and review the credentialing process. Once you become adept at vacuum extraction, mentor more junior colleagues.
Two critical concerns
When contemplating vacuum-assisted delivery, 2 risks are paramount:
- failure of the vacuum extractor to achieve delivery
- the potential for fetal and maternal injury.
Training must ensure appropriate case selection and technique. Vacuum extraction must be performed with the same precision and care used with forceps. If application of the device is incorrect, or if there is a wrong direction of traction, excessive traction, or traction in the presence of disproportion, the cup will slip or pop off, and vacuum delivery will fail, with the potential for traumatic fetal injury.
All risks must be discussed with the patient to fulfill informed consent, and the risks and benefits of alternative treatments should be part of the discussion. Active participation, in considering how best to approach delivery, is required of all parties concerned.
The vacuum extractor can be a useful adjunct in certain circumstances, and its use has become widespread in American delivery suites. As with the obstetric forceps, which largely antedated its use, the vacuum extractor can lessen the overall risks of childbirth for both mother and infant.
The authors report no financial relationships relevant to this article.
CASE Three hours of pushing
C.A., age 29 years, is 40 weeks’ pregnant with her first child. After an unremarkable pregnancy, she arrives at the hospital for cervical ripening and induction of labor. Oxytocin is given, and labor progresses uneventfully. When C.A.’s cervix is dilated 8 cm, however, labor stalls. The physician orders placement of a pressure catheter and increases the dosage of oxytocin, and the cervix dilates fully. Although C.A. pushes well, the vertex descends only from +1 to +2 station (of 5 stations) after 3 hours.
How would you manage this delivery?
One option in C.A.’s case is operative vaginal delivery using the vacuum extractor, which has replaced the forceps as the most commonly used approach for operative vaginal delivery. Like the forceps, the vacuum extractor has vociferous detractors as well as supporters. Liberal use of cesarean section and questions regarding the safety of operative vaginal delivery vis-à-vis cesarean section have fueled the debate over its role in obstetric practice.
Among the benefits of vacuum extraction are its cost-effectiveness and shorter hospital stay (TABLE 1). It also obviates the need for cesarean section, including repeat cesarean. Risks include an increased incidence of genital tract trauma and a greater risk of fetal subgaleal hemorrhage.
We review 4 critical spheres of concern in regard to vacuum extraction:
- Patient selection
- Informed consent
- Technique
- Documentation
Increased understanding of these aspects of vacuum extraction will improve outcomes for the patient and limit medicolegal risk.
In the case of C.A., the physician offered 3 options:
- Continue maternal expulsive efforts to allow descent
- Attempt delivery by vacuum extraction
- Proceed to cesarean section on the basis of protracted descent.
Risks and benefits were reviewed with the patient, who chose to deliver by cesarean section. A 3,780-g infant in occiput posterior position was delivered safely.
TABLE 1
Delicate balance: Risks and benefits of operative vaginal delivery
| WHO? | BENEFIT | RISK |
|---|---|---|
| Mother | Cost-effective Less blood loss Lower risk of febrile morbidity Maternal preference No need for cesarean section or repeat cesarean Shorter hospitalization and convalescence | Increased incidence of genital tract trauma Possible damage to pelvic floor, with urinary and anal incontinence |
| Fetus | Fewer respiratory difficulties at birth | Increased risk of subgaleal hemorrhage Association with shoulder dystocia |
1. Patient selection: Maternal and fetal indications
Vacuum extraction may be justified for maternal or fetal indications.1,2 Maternal indications include prolongation or arrest of the second stage of labor, or the need to shorten the second stage, for reasons such as maternal cardiac disease, complex congenital cardiovascular disorders, and maternal exhaustion.
No definitive time limit for the second stage of labor
There is more flexibility today than in the past about what constitutes a “safe” length of the second stage. Recommendations concerning when the mother should begin pushing—and for how long—have evolved from a strict time limit to a focus on progression. If the fetal heart rate (FHR) tracing is reassuring, the second stage no longer needs to be limited to 2 or 3 hours. On the contrary, if the patient is still able and willing to push, changes in positioning and further expectant management remain acceptable in contemporary practice.3 Otherwise, a trial of vacuum extraction may be appropriate.
Vacuum extraction is particularly useful when the mother has difficulty pushing because of exhaustion and the fetal head has descended enough that it distends the labia between contractions, as in outlet deliveries.
Fetal indications
Fetal indications for operative vaginal delivery include distress, jeopardy, or a “nonreassuring” FHR tracing. Such a tracing may include late and prolonged decelerations, baseline bradycardia or tachycardia with or without variable decelerations, or, occasionally, a normal baseline rate with diminished variability.
Use vacuum or forceps?
The choice depends on which device would achieve delivery in the safest manner with the lowest risk of fetal injury. With the vacuum, force is exerted directly on the fetal scalp and only secondarily on the fetal skull. This puts fetal vessels that traverse the subgaleal space at risk for injury (FIGURE). With forceps, force is exerted directly on the fetal skull and mitigated by the petrous bone. Little or no force is exerted on the fetal scalp, lessening the risk of traumatic injury such as potentially fatal subgaleal hemorrhage.
Indications and contraindications for vacuum extraction are similar, but not identical, to those for forceps delivery (TABLE 2).2,3 The most important determinant for either device is the experience of the operator. You must be familiar with the instrument and technique before making any attempt to assist delivery. An inability to accurately assess fetal position or station, fetopelvic proportion, adequacy of labor, engagement of the fetal head, or any degree of malpresentation (including minor degrees of deflexion) is a contraindication to a trial of operative vaginal delivery.
Vacuum extraction should be reserved for fetuses at more than 34 weeks’ gestation because of the increased risk of intracranial hemorrhage associated with prematurity.
All decisions involving vacuum extraction should be made with caution. The adequacy of the pelvis, estimated fetal size, and any suggestions of fetopelvic disproportion are of particular significance.3
FIGURE
Subgaleal hemorrhage, a deadly complication
Blood can accumulate in a large potential space between the galea aponeurotica and the periosteum of the cranial bones after vacuum extraction. An infant with subgaleal hemorrhage will exhibit a boggy scalp, with swelling that crosses the suture lines and expands head circumferenceTABLE 2
Factors that predict success—or failure—of vacuum extraction
| When a woman fits overlapping categories, the decision to use vacuum extraction—or not—may be a judgment call* |
| GOOD CANDIDACY |
| Multiparous |
| Term pregnancy |
| Occiput anterior position, well-flexed |
| Wide subpubic arch |
| Compliant |
| MARGINAL CANDIDACY |
| Primiparous |
| Post-term |
| Occiput posterior position |
| Average subpubic arch |
| Gestational diabetes |
| Arrest disorders in second stage |
| POOR CANDIDACY |
| Protraction disorders in second stage |
| Narrow subpubic arch |
| Uncertain position of fetal head |
| Deflexion or asynclitism |
| Anticipated large-for-gestational-age infant |
| Poor maternal compliance |
| * When faced with a good indication in a marginal candidate, we recommend delivery in a “double setup” situation in which preparations are made for both vacuum extraction and cesarean section. If the vacuum can be properly applied, the first application of traction is crucial. We will only proceed if significant descent is achieved. If the fetal head (not the scalp) can be advanced a full station, then we proceed cautiously. If not, ready access to cesarean section allows for completion of the delivery in a timely manner. |
2. Informed consent: Elicit the patient’s desires
Thorough discussion with the patient and her family—to explain the reasoning behind the clinical decision to use the vacuum extractor and delineate the alternatives—is paramount. Moreover, the patient should be encouraged to actively participate in this discussion.
Among the alternatives to vacuum extraction are expectant observation and expedited delivery by cesarean section. Because patients increasingly are requesting elective cesarean section in the absence of obvious obstetric indications, this option should receive extra attention.
Most women still consider vaginal delivery an important milestone of female adulthood. When safety concerns arise and the situation makes vaginal delivery unwise, many women experience disappointment and postpartum depression over their “failed” attempt at vaginal delivery. These perceptions need to be addressed in discussions with the patient.
The risk–benefit equation
Vacuum extraction lessens the risk of maternal lacerations, either of the lower genital tract in the case of obstetric forceps, or of the cervix and lower uterine segment in the case of cesarean section. In addition, vacuum extraction can be performed comfortably in the absence of regional anesthesia.
Avoiding cesarean section can produce multiple benefits
Another maternal benefit of vacuum extraction is the decreased need for cesarean section. A reduction in the primary cesarean rate also lowers the need for repeat cesarean section, which can be more technically challenging than primary C-section due to the presence of dense scar tissue and intra-abdominal adhesions. Cesarean section also increases the risk of placenta accreta, increta, or percreta in subsequent pregnancies. These complications increase the likelihood of emergency hysterectomy, massive blood loss, and serious maternal morbidity and mortality.
Even in the absence of placenta accreta, both primary and repeat cesarean sections raise the risk of hemorrhage and febrile morbidity, prolong convalescence, and increase cost, compared with vaginal delivery. For these reasons, avoiding primary cesarean section can obviate the need for multiple surgical procedures and their attendant risks. The degree to which these factors favor vaginal delivery over cesarean section is subject to debate.
Maternal risks include pelvic floor trauma
Both vacuum extraction and forceps delivery increase the risk of anal sphincter injury and can impair fecal continence.4 Both methods also appear to increase trauma to the genital tract in comparison with spontaneous delivery and may predispose the woman to pelvic floor dysfunction, including urinary and anal incontinence.5-10 However, anal sphincter trauma was less frequent after vacuum extraction than after forceps delivery.1
Other maternal injuries associated with vacuum extraction include perineal lacerations and injuries to the vulva, vagina, and cervix. Vacuum extraction also has been implicated as a significant risk factor for postpartum hemorrhage11 and genital-tract infection.1
Fewer neonatal respiratory problems with vaginal delivery
Compared with cesarean section, vaginal delivery is thought to diminish the risk of intrapartum aspiration and respiratory problems in the newborn. It also may facilitate the transition from fetal to neonatal circulation and reduce the need for immediate resuscitation at birth.
Neonatal risks include soft-tissue injury and potential hemorrhage
Infants delivered by vacuum extraction have a significantly higher rate of intracranial hemorrhage, brachial plexus injuries, convulsions, central nervous system depression, and the need for mechanical ventilation, compared with spontaneously delivered infants (TABLE 3).12,13
Although vacuum extraction is associated with a wide range of soft tissue injuries, they are often less serious than the fetal scalp injuries associated with obstetric forceps. Cup marks, bruising, and minor lacerations of the scalp and caput succedaneum are common fetal injuries, although the majority resolve without apparent sequelae.14
Subgaleal hemorrhage is the most serious neonatal complication of vacuum extraction, occurring in 1% to 3.8% of vacuum extractions (FIGURE).15 It coexists with neonatal coagulopathy in 19% to 29% of newborns16 and increases the risk of progression to hemorrhagic shock and death. Subgaleal hemorrhage has a mortality rate ranging from 2.7% to 22.8%.15-17
Cephalhematoma is another complication associated with vacuum extraction. It involves an accumulation of blood beneath the periosteum of a cranial bone (usually the parietal bone), and it almost always resolves spontaneously. The incidence of cephalhematoma varies. It is significantly more common in deliveries involving vacuum extraction (9.8%) than in forceps deliveries (4.1%).18 Its incidence increases with the length of time the vacuum cup is applied and with paramedian application.18
Intracranial hemorrhage occurs in 1 of 860 vacuum extractions, 1 of 664 forceps deliveries, 1 of 954 cesarean deliveries, and 1 of 1,900 spontaneous deliveries.12 Subdural hemorrhage is the most common form of intracranial hemorrhage and is almost invariably the result of birth trauma. However, asymptomatic subdural hematoma occurs in up to 6.1% of uncomplicated vaginal deliveries.19
Other, less common types of intracranial hemorrhage, such as subarachnoid, intraventricular, and intraparenchymal hemorrhage, have a more complex etiology, which includes birth asphyxia, hemorrhagic diathesis, infection, and vascular abnormalities.20
Retinal hemorrhage also may occur after vacuum extraction, with an incidence of 49% to 77.8%, compared with 30.3% after forceps delivery, 30.4% after normal vaginal delivery, and 8.3% after cesarean delivery.21 It generally resolves spontaneously without any permanent damage.22
TABLE 3
Vacuum extraction can injure the fetus
| DIRECT INJURY |
| Cephalhematoma |
| Intracranial hemorrhage (parenchymal, subdural, intraventricular, subarachnoid) |
| Nerve injury |
| Scalp laceration, abrasion, ecchymoses, necrosis |
| Skull fracture |
| Subgaleal hemorrhage |
| INDIRECT INJURY |
| Anemia, hyperbilirubinemia |
| Brachial plexus injury |
| Scalp infection or abscess |
| SOURCE: O’Grady et al31 |
Shoulder dystocia and brachial plexus palsy
Vacuum extraction also is associated with shoulder dystocia and brachial plexus palsy, although the primary risk factor for these complications is thought to be increased fetal size.23-25 The incidence of shoulder dystocia with vacuum extraction is 3.5%, compared with 1.5% for forceps delivery.25
The risk of brachial plexus palsy also increases with vacuum extraction, especially as the duration of the procedure increases.25
Less common complications associated with vacuum extraction are skull fractures, fetal hemorrhage from bleeding at the site of scalp electrodes, sepsis originating from infected scalp trauma, and corneal injury.
No long-term impairment
Long-term outcome studies of children delivered by vacuum extraction show no differences in physical or cognitive functioning or intelligence scores, compared with other modes of delivery.26
3. Technique: Create conditions that ensure success
Certain prerequisites to vacuum extraction can assure successful application and strict adherence to protocol. These prerequisites include having an appropriate indication, thorough informed consent, proper maternal positioning, adequate anesthesia, and knowledge of fetal position and station (TABLE 4).1 These objectives can be accomplished in the following steps:
- After an informed consent discussion, assess maternal positioning and repeat the pelvic exam. Also ascertain the adequacy of anesthesia. Insert a bladder catheter.
- Perform a “ghost” trial of vacuum extraction to visualize the procedure before the actual attempt.
- Test the function of the vacuum.
- Lubricate the vacuum cup with surgical soap or gel, insert it into the vagina, and maneuver it onto the fetal head. Place the vacuum extractor over the sagittal suture about 6 cm distal to the anterior fontanel and 2 cm proximal to the posterior fontanel. (The illustration on page 74 demonstrates positioning.) Apply a small degree of vacuum (approximately 20 mm Hg). Double-check application.
- Gradually apply full vacuum (550–600 mm Hg, depending on cup size), allowing the scalp to mold to the extractor cup.
- Apply 2-handed traction in concert with uterine contractions and supplemented by maternal pushing. Assuming there is no loss of vacuum (“pop-off” of the cup), the initial traction effort should produce a gain in station. If a “pop-off” occurs, a single additional attempt at delivery may be warranted.
- As the head crowns, perform episiotomy as needed and slowly deliver the fetal head. Remove the vacuum cup.
- After delivery of the placenta, inspect the vagina, cervix, and perineum closely.
- Dictate a full operative note and annotate the delivery in the chart. See the section on documentation, below.
Vacuum extraction may fail for a number of reasons (TABLE 5).
TABLE 4
Perform these predelivery checks before applying traction
| Is anesthesia adequate? Is maternal positioning correct? |
| Is the bladder empty? |
| Is the fetus in the proper attitude (flexion)? |
| Is fetal status reassuring? |
Is the vacuum properly applied?
|
| Has the patient been instructed on when and how long to push? |
| Are the proposed maneuvers appropriate? |
TABLE 5
Why might vacuum extraction fail?
| INSTRUMENT-RELATED |
| Pump failure |
| Vacuum leak |
| TECHNIQUE-RELATED |
| Failure to encourage maternal valsalva with traction efforts |
| Inappropriate intensity of traction |
| Incorrect axis of traction |
| Maternal tissue trapped beneath vacuum cup |
| Poor cup position |
| OBSTETRIC CONDITIONS |
Congenital anomaly
|
| Fetal macrosomia |
| Incomplete cervical dilation |
Position and attitude problems
|
| Unappreciated cephalopelvic disproportion |
| SOURCE: Modified from Plauche et al32 |
Most important variable: Cup placement
The single most critical step in vacuum extraction is placement of the cup. It should be applied at the point of maximum fetal cranial flexion, which is proximal to the leading edge of the posterior fontanel.
Once full vacuum is achieved, encourage the mother to push with the next contraction, and apply steady traction in concert with her efforts.
The initial application of traction should be directed to maintain proper flexion of the fetal head, and should bring about descent of the fetal head. If there is no descent with the first application of traction, and correct technique and cup placement have been applied, abandon operative vaginal delivery (TABLE 6).
Do not make a further attempt to deliver the child using forceps, as the risk of intracranial hemorrhage appears to be highest in infants delivered using a combination of vacuum extraction and forceps.
TABLE 6
Repeat traction efforts reap a diminishing return
| NUMBER OF TRACTION EFFORTS | SUCCESS RATE | |
|---|---|---|
| VACUUM EXTRACTION (N=433) | FORCEPS (N=555) | |
| 1 or 2 | 68.4% | 38.4% |
| 3 or 4 | 24.9% | 48.6% |
| 5 or more | 6.7% | 12.9% |
| Adapted from Sjostedt33 | ||
4. Documentation: The chart is the most important witness
The value of complete and contemporaneous notation cannot be overstated. The patient’s chart is the permanent repository of the record of delivery. It is without doubt the most important witness to the event and should be treated as such. Include a dictated operative note as well as notation in the chart itself. Notes should be legible and properly dated, with the time of day indicated.
When operative vaginal delivery is performed, record the following:
- indication for the procedure
- course of labor
- anesthesia
- personnel present
- instruments used
- position and station of the fetal head
- force and duration of traction
- complications, including how they were recognized and managed
- immediate condition of the newborn and all steps taken in resuscitation.
Operative vaginal delivery is no newcomer to obstetrics. Hindu writings from about 1000 BC, and Hippocrates’ own musings from the fifth century BC, describe instruments and techniques to combat arrested labor and salvage the lives of both mother and child.27 Crude forceps were described by the Muslim physician Albucasis in the 11th century.27
Before the advent of safe cesarean section, many maternal lives were no doubt saved by these instruments and techniques. Unfortunately, destruction of the fetus and maternal death were frequent outcomes of operative vaginal delivery by forceps before the 20th century.28
As for vacuum extraction in particular, the idea of attaching a device to the fetal head to aid in delivery is credited to Arnett, a 19th century surgeon and inventor, who envisioned the “pneumatic tractor.”29
In 1957, Malmstrom reintroduced the vacuum as an aid in delivery, designing a rigid cup that was connected by rubber tubing to a vacuum source.30 This allowed the separation of the pump mechanism from the cup and made for easier application.
Most recently, Kobayashi developed the soft-cup design, a low-cost flexible plastic alternative that allows for a disposable instrument.31
Minimizing medicolegal risk
The best way to prevent an accusation of medical malpractice is to develop strong clinical and interpersonal skills. These simple, intuitive suggestions may help:
- Understand the role of operative vaginal delivery in current practice.
- Develop a simple and interactive discussion model for use in labor and delivery with the patient and her family.
- Consider a woman’s preferences for delivery.
- Know the indications and contraindications for vacuum extraction.
- Use the checks and safeguards listed under 3. Technique: Create conditions that ensure success.
- Perform vacuum extraction in the cesarean section room. Stop the procedure at once if any problem arises, and proceed to cesarean delivery.
- Make all chart notations completely legible, and add dictated notes.
If you are a new physician or lack significant experience with vacuum extraction, ask for input, supervision, and education from more experienced clinicians. Also make it a point to ask about department guidelines and review the credentialing process. Once you become adept at vacuum extraction, mentor more junior colleagues.
Two critical concerns
When contemplating vacuum-assisted delivery, 2 risks are paramount:
- failure of the vacuum extractor to achieve delivery
- the potential for fetal and maternal injury.
Training must ensure appropriate case selection and technique. Vacuum extraction must be performed with the same precision and care used with forceps. If application of the device is incorrect, or if there is a wrong direction of traction, excessive traction, or traction in the presence of disproportion, the cup will slip or pop off, and vacuum delivery will fail, with the potential for traumatic fetal injury.
All risks must be discussed with the patient to fulfill informed consent, and the risks and benefits of alternative treatments should be part of the discussion. Active participation, in considering how best to approach delivery, is required of all parties concerned.
The vacuum extractor can be a useful adjunct in certain circumstances, and its use has become widespread in American delivery suites. As with the obstetric forceps, which largely antedated its use, the vacuum extractor can lessen the overall risks of childbirth for both mother and infant.
The authors report no financial relationships relevant to this article.
1. O’Grady JP, Gimovsky ML, McIlhargie CJ, eds. Operative Obstetrics. Pearl River, NY: Parthenon Publishing; 1995.
2. Johanson RB, Menon BKV. Vacuum extraction versus forceps for assisted vaginal delivery. Cochrane Database Syst Rev. 2000;(2):CD000224.-
3. Bloom SL, Casey BM, Schaffer JL, et al. Pushing in the second stage of labor. Am J Obstet Gynecol 2006;194:10-13.
4. Operative vaginal delivery. ACOG Practice Bulletin #17. Washington, DC: American College of Obstetricians and Gynecologists; June 2000.
5. Power D, Fitzpatrick M, O’Herlihy C. Obstetric anal sphincter injury: how to avoid, how to repair: a literature review. J Fam Pract 2006;55:193-200.
6. Chaliha C, Kalia V, Stanton S, et al. Antenatal prediction of postpartum urinary and fecal incontinence. Obstet Gynecol 1999;94:689-694.
7. Bofill JA, Rust OA, Schorr SJ, et al. A randomized prospective trial of the obstetric forceps versus the M-cup vacuum extractor. Am J Obstet Gynecol 1996;175:1325-1330.
8. Salamalekis E, Loghis C, Pyrgiotis E, et al. Soft cup vacuum extractor versus forceps delivery. J Obstet Gynecol. 1995;15:245-246.
9. Zetterstrom JP, Lopez A, Anzen B, et al. Anal incontinence after vaginal delivery: a prospective study in primiparous women. Br J Obstet Gynaecol. 1999;106:324-330.
10. Johanson RB, Heycock E, Carter J, et al. Maternal and child health after assisted vaginal delivery: five-year follow up of a randomized controlled study comparing forceps and ventouse. Br J Obstet Gynaecol. 1999;106:544-549
11. Faltin DL, Otero M, Petignat P, et al. Women’s health 18 years after rupture of the anal sphincter during childbirth: I. Fecal incontinence. Am J Obstet Gynecol. 2006;194:1255-1259.
12. Plauche WC. Fetal cranial injuries related to delivery with the Malmsträm vacuum extractor. Obstet Gynecol. 1979;53:750-757.
13. Towner D, Castro MA, Eby-Wilkens E, et al. Effect of mode of delivery in nulliparous women on neonatal intracranial injury. N Engl J Med. 1999;341:1709-1714.
14. Sheiner E, Sarid L, Levy A, et al. Obstetric risk factors and outcome of pregnancies complicated with early postpartum hemorrhage: a population-based study. J Matern Fetal Neonatal Med. 2005;18:149-154.
15. Johanson R. Choice of instrument for vaginal delivery. Curr Opin Obstet Gynecol. 1997;9:361-365.
16. Chadwick LM, Pemberton PJ, Kurinczuk JJ. Neonatal subgaleal hematoma: associated risk factors, complications, and outcome. J Pediatr Child Health. 1996;32:228-232.
17. Ng PC, Siu YK, Lewindon PJ. Subaponeurotic hemorrhage in the 1990s: a 3-year surveillance. Acta Pediatr. 1995;84:1065-1069
18. Bofill JA, Rust OA, Devidas M, et al. Neonatal cephalohematoma from vacuum extraction. J Reprod Med. 1997;42:565-569.
19. Doumouchtsis SK, Arulkumaran S. Head injuries after instrumental vaginal deliveries. Curr Opin Obstet Gynecol. 2006;18:129-134.
20. Govaert P. Cranial Hemorrhage in the Term Newborn Infant. London: Mac Keith Press; 1993.
21. Hughes LA, May K, Talbot JF, Parsons MA. Incidence, distribution, and duration of birth-related retinal hemorrhages: a prospective study. J AAPOS. 2006;10:102-106.
22. Sheiner E, Levy A, Hershkovitz R, et al. Determining factors associated with shoulder dystocia: a population-based study. Eur J Obstet Gynecol Reprod Biol. 2006;126:11-15.
23. Baskett TF, Allen AC. Perinatal implications of shoulder dystocia. Obstet Gynecol. 1995;86:15-18.
24. Mollberg M, Hagerg H, Bager B, et al. Risk factors for obstetric brachial plexus palsy among neonates delivered by vacuum extraction. Obstet Gynecol. 2005;106:913-918.
25. Caughey AB, Sandberg PL, Alantnik MG, et al. Forceps compared with vacuum: rates of neonatal and maternal morbidity. Obstet Gynecol. 2005;106:908-912.
26. Ngan HYS, Miu P, Ko L, et al. Long-term neurological sequelae following vacuum extractor delivery. Aust NZ J Obstet Gynecol. 1990;30:111-114.
27. Lyons AS, Petrucelli RJ. Medicine: An Illustrated History. New York: Harry N Abrams; 1978.
28. Speert H. Obstetric and Gynecologic Milestones Illustrated. Pearl River, NY: Parthenon Publishing; 1996.
29. Arnett N. Elements of Physics or Natural Philosophy, General and Medical, Explained Independently of Technical Mathematics and Containing New Disquisitions and Practical Suggestions. 2nd ed. Philadelphia: Carney and Lea; 1831.
30. Malmstrom T. The vacuum extractor, an obstetrical instrument. I. Acta Obstet Gynecol Scand. 1957;36(suppl 3):5-50.
31. O’Grady JP, Gimovsky ML, McIlhargie CJ. Vacuum Extraction in Modern Obstetric Practice. Pearl River, NY: Parthenon Publishing; 1995.
32. Plauche WC, Morrison JC, O’Sullivan MJ. Surgical Obstetrics. Philadelphia: WB Saunders; 1992.
33. Sjostedt JE. The vacuum extractor and forceps in obstetrics: a clinical study. Acta Obstet Gynecol Scand. 1967;48:638-639.
1. O’Grady JP, Gimovsky ML, McIlhargie CJ, eds. Operative Obstetrics. Pearl River, NY: Parthenon Publishing; 1995.
2. Johanson RB, Menon BKV. Vacuum extraction versus forceps for assisted vaginal delivery. Cochrane Database Syst Rev. 2000;(2):CD000224.-
3. Bloom SL, Casey BM, Schaffer JL, et al. Pushing in the second stage of labor. Am J Obstet Gynecol 2006;194:10-13.
4. Operative vaginal delivery. ACOG Practice Bulletin #17. Washington, DC: American College of Obstetricians and Gynecologists; June 2000.
5. Power D, Fitzpatrick M, O’Herlihy C. Obstetric anal sphincter injury: how to avoid, how to repair: a literature review. J Fam Pract 2006;55:193-200.
6. Chaliha C, Kalia V, Stanton S, et al. Antenatal prediction of postpartum urinary and fecal incontinence. Obstet Gynecol 1999;94:689-694.
7. Bofill JA, Rust OA, Schorr SJ, et al. A randomized prospective trial of the obstetric forceps versus the M-cup vacuum extractor. Am J Obstet Gynecol 1996;175:1325-1330.
8. Salamalekis E, Loghis C, Pyrgiotis E, et al. Soft cup vacuum extractor versus forceps delivery. J Obstet Gynecol. 1995;15:245-246.
9. Zetterstrom JP, Lopez A, Anzen B, et al. Anal incontinence after vaginal delivery: a prospective study in primiparous women. Br J Obstet Gynaecol. 1999;106:324-330.
10. Johanson RB, Heycock E, Carter J, et al. Maternal and child health after assisted vaginal delivery: five-year follow up of a randomized controlled study comparing forceps and ventouse. Br J Obstet Gynaecol. 1999;106:544-549
11. Faltin DL, Otero M, Petignat P, et al. Women’s health 18 years after rupture of the anal sphincter during childbirth: I. Fecal incontinence. Am J Obstet Gynecol. 2006;194:1255-1259.
12. Plauche WC. Fetal cranial injuries related to delivery with the Malmsträm vacuum extractor. Obstet Gynecol. 1979;53:750-757.
13. Towner D, Castro MA, Eby-Wilkens E, et al. Effect of mode of delivery in nulliparous women on neonatal intracranial injury. N Engl J Med. 1999;341:1709-1714.
14. Sheiner E, Sarid L, Levy A, et al. Obstetric risk factors and outcome of pregnancies complicated with early postpartum hemorrhage: a population-based study. J Matern Fetal Neonatal Med. 2005;18:149-154.
15. Johanson R. Choice of instrument for vaginal delivery. Curr Opin Obstet Gynecol. 1997;9:361-365.
16. Chadwick LM, Pemberton PJ, Kurinczuk JJ. Neonatal subgaleal hematoma: associated risk factors, complications, and outcome. J Pediatr Child Health. 1996;32:228-232.
17. Ng PC, Siu YK, Lewindon PJ. Subaponeurotic hemorrhage in the 1990s: a 3-year surveillance. Acta Pediatr. 1995;84:1065-1069
18. Bofill JA, Rust OA, Devidas M, et al. Neonatal cephalohematoma from vacuum extraction. J Reprod Med. 1997;42:565-569.
19. Doumouchtsis SK, Arulkumaran S. Head injuries after instrumental vaginal deliveries. Curr Opin Obstet Gynecol. 2006;18:129-134.
20. Govaert P. Cranial Hemorrhage in the Term Newborn Infant. London: Mac Keith Press; 1993.
21. Hughes LA, May K, Talbot JF, Parsons MA. Incidence, distribution, and duration of birth-related retinal hemorrhages: a prospective study. J AAPOS. 2006;10:102-106.
22. Sheiner E, Levy A, Hershkovitz R, et al. Determining factors associated with shoulder dystocia: a population-based study. Eur J Obstet Gynecol Reprod Biol. 2006;126:11-15.
23. Baskett TF, Allen AC. Perinatal implications of shoulder dystocia. Obstet Gynecol. 1995;86:15-18.
24. Mollberg M, Hagerg H, Bager B, et al. Risk factors for obstetric brachial plexus palsy among neonates delivered by vacuum extraction. Obstet Gynecol. 2005;106:913-918.
25. Caughey AB, Sandberg PL, Alantnik MG, et al. Forceps compared with vacuum: rates of neonatal and maternal morbidity. Obstet Gynecol. 2005;106:908-912.
26. Ngan HYS, Miu P, Ko L, et al. Long-term neurological sequelae following vacuum extractor delivery. Aust NZ J Obstet Gynecol. 1990;30:111-114.
27. Lyons AS, Petrucelli RJ. Medicine: An Illustrated History. New York: Harry N Abrams; 1978.
28. Speert H. Obstetric and Gynecologic Milestones Illustrated. Pearl River, NY: Parthenon Publishing; 1996.
29. Arnett N. Elements of Physics or Natural Philosophy, General and Medical, Explained Independently of Technical Mathematics and Containing New Disquisitions and Practical Suggestions. 2nd ed. Philadelphia: Carney and Lea; 1831.
30. Malmstrom T. The vacuum extractor, an obstetrical instrument. I. Acta Obstet Gynecol Scand. 1957;36(suppl 3):5-50.
31. O’Grady JP, Gimovsky ML, McIlhargie CJ. Vacuum Extraction in Modern Obstetric Practice. Pearl River, NY: Parthenon Publishing; 1995.
32. Plauche WC, Morrison JC, O’Sullivan MJ. Surgical Obstetrics. Philadelphia: WB Saunders; 1992.
33. Sjostedt JE. The vacuum extractor and forceps in obstetrics: a clinical study. Acta Obstet Gynecol Scand. 1967;48:638-639.