User login
Functional Assessment in Veterans with Posttraumatic Stress Disorder
Heparin-Induced Thrombocytopenia and Thrombosis
Androgens in women: To replace or not?
Although women produce only one tenth the amount of androgen that men do, testosterone and related androgen metabolites are as important to women throughout the lifespan as is estrogen. Androgens modulate a feeling of well-being, increase energy, support bone metabolism, and improve sexual function in women.1-3 But too much androgen production, with elevated levels of testosterone and dehydroepiandrosterone (DHEA), can result in hirsutism, acne, and infertility in the setting of polycystic ovary syndrome (PCOS), all of which present clinical problems.
An equally complicated topic is androgen insufficiency in women. Not only is it difficult to diagnose, it is a major clinical issue to decide whether, when, and how to replace androgens in women. In this article, I look at androgen production throughout the female lifespan, particularly the relationship between estrogen and androgen. I also describe the evaluation of androgen insufficiency, which requires understanding of androgen physiology and ovarian function before and after menopause. These issues form the basis of the decision to replace androgen in women.
Androgen over the lifespan
In the premenopausal woman, androgen production is approximately equally divided between the adrenal gland and the ovaries. Androstenedione from both is converted to testosterone and then irreversibly to dihydrotestosterone (DHT). Androstenedione, testosterone, and even DHEA are secreted in equal quantities by the adrenals and ovaries. The only androgen that is predominantly adrenal is DHEAS, which is sulfated in the adrenal gland.
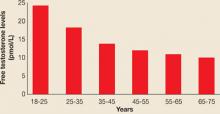
In premenopausal women, androstenedione is the precursor to testosterone, which is then metabolized to DHT, the androgen most active in hair follicles and implicated in hirsutism. It has been clear for many years that DHEA, although a weak androgen, is present in the greatest quantity in the circulation and is secreted during adrenarche, prior to menarche, beginning at ages 8 to 10. DHEAS peaks in young adulthood and begins to decline after age 40.4 The same is true for both total testosterone and free testosterone levels, which also decline in women after about age 25. Thus, peri- and postmenopausal women have approximately half the level of circulating androgens of women in their 20s (FIGURE 1, TABLE 1).5
FIGURE 1
Testosterone levels in women decline with aging
N=595
SOURCE: Davison S, et al5TABLE 1
How menopause affects plasma hormone levels
| HORMONE | MEAN PLASMA LEVEL | ||
|---|---|---|---|
| REPRODUCTIVE AGE* (N=15) | NATURALLY MENOPAUSAL (N=18) | OOPHORECTOMIZED (N=8) | |
| Estrone (pg/mL) | 58 | 49 | 48 |
| Estradiol (pg/mL) | 40 | 20† | 18 |
| Testosterone (ng/dL) | 44 | 30† | 12‡ |
| DHT (ng/dL) | 30 | 10† | <5‡ |
| Androstenedione (ng/dL) | 166 | 99† | 64‡ |
| DHEA (ng/dL) | 542 | 197† | 126§ |
| DHT=dihydrotestosterone, DHEA=dehydroepiandrosterone | |||
| * Mean value during early follicular phase | |||
| † P<.01 for comparison with reproductive age | |||
| ‡ P<.01 for comparison with naturally menopausal women | |||
| § P<.05 for comparison with naturally menopausal women | |||
| SOURCE: Vermeulen18 | |||
It matters how menopause happens
Circulating androgen levels are greatly influenced by menopause—how much depends on whether it occurs naturally with the ovaries intact, or by surgical removal of the ovaries. Not only does estradiol diminish significantly in naturally menopausal women, but all androgens do as well. In young oophorectomized women, estrogen levels are similar to levels in naturally menopausal women, but androgen levels—including testosterone, DHT, and androstenedione—are significantly lower than in naturally menopausal women, demonstrating that the circulating levels of androgen after natural menopause are still significantly greater than those in oophorectomized women.6 Thus, the postmenopausal ovary contributes significantly to circulating levels of androgen.
Androgen physiology
Both androgens and estrogens circulate in the bloodstream tightly bound to the protein sex hormone-binding globulin (SHBG), and more loosely bound to albumin. The SHBG-bound fraction is unavailable for biologic activity. Therefore, the amount of SHBG a woman produces is a key determinant of her level of androgen bioactivity. For this reason, it is crucial to measure circulating SHBG.
In a feedback mechanism, SHBG production is regulated by androgen and estrogen levels, with estrogen stimulating SHBG production and testosterone decreasing it.7 In the normal woman, about 65% of testosterone is bound to SHBG and 30% is bound to albumin, leaving only 0.5% to 2% free and bioactively available.8 In postmenopausal women taking hormone replacement therapy, SHBG increases, but the addition of methyltestosterone lowers the overall levels of SHBG, even in the presence of estrogen, increasing the amount of bioavailable testosterone simply by lowering SHBG levels. Postmenopausal replacement with estrogen alone decreases the amount of bioavailable testosterone because of higher SHBG levels.
SHBG is synthesized in the liver, whose metabolism is increased by exposure to steroids. Therefore, oral forms of estrogen replacement, which stimulate the liver because of the “first pass” effect, result in a greater increase in SHBG than do transdermal estrogen preparations.
Elevated androgen levels have both ill and good effects
As I stated earlier, an appropriate level of androgen is optimal for women as well as men. Elevated androgen levels are problematic, in that they are the hallmark of PCOS, usually resulting from increased ovarian production of androgen. This elevation can cause anovulation, infertility, hirsutism, and other androgen-mediated physiologic effects. Androgen is also associated with elevated low-density lipoprotein and decreased high-density lipoprotein cholesterol, implying a possible relationship with cardiovascular disease. At the same time, however, elevated testosterone has been correlated with increased bone density in both the hip and the femoral neck.9
It is clear that appropriate androgen secretion, which does not elicit the side effects described above, is best for both the health and well-being of the woman.
How androgen affects female sexual function
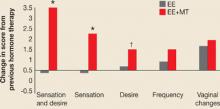
We have known for years that androgen—not estrogen—is associated with satisfactory sexual function. Although estrogen replacement increases vaginal lubrication, it is androgen, most commonly in the form of oral methyltestosterone or injectable testosterone, that increases frequency of intercourse, desire, and sexual sensation (FIGURE 2).10 The definition of androgen insufficiency has been hotly debated, and is currently “a pattern of clinical symptoms in the presence of decreased bioavailable testosterone and normal estrogen.”11
FIGURE 2
How estrogen plus androgen affects sexual function
*P<.01; †P=.05
EE=esterified estrogens; MT=methyltestosterone
SOURCE: Sarrel PM, et al19
Assessing androgen levels
Clinical signs and symptoms of androgen insufficiency are important in establishing the diagnosis. They include a diminished sense of well-being, unexplained fatigue, decreased sexual desire, and thinning and loss of pubic hair.11 Although it is possible to assess testosterone production and availability in women by measuring serum testosterone levels, a lack of consensus about the best measurement technique and interpretation of results makes it difficult to base the diagnosis of androgen insufficiency solely on serum levels.12 Therefore, the diagnosis of androgen insufficiency is primarily a clinical diagnosis of symptoms.11
Obtain serum samples between 8 and 10 AM after day 8 and before day 20 of the normal menstrual cycle because testosterone is subject to diurnal variation, peaking in the early morning, as well as cyclic variation, peaking around midcycle.
Because free testosterone is the only bioavailable steroid, total testosterone and either free testosterone or SHBG must be measured to assess how much androgen is actually available. From total testosterone and SHBG, one can assess the free testosterone index as a measure of bioavailable androgen (the free testosterone index is a ratio of the amount of total testosterone divided by the SHBG level).11 In fact, using the free testosterone index is preferable to the actual measurement of free testosterone because commercial assays lack the sensitivity and reliability to accurately measure the low levels of androgen found in women.
Several different testosterone assays exist, and the immunoassay for total testosterone is reasonably accurate. However, measurements of free testosterone are relatively inaccurate and poorly reproducible. Equilibrium dialysis is thought to be the gold standard for measuring free testosterone, but it is a difficult and time-consuming assay.12
Causes of low testosterone
In women, low testosterone secretion is usually the result of normal aging. Other conditions that alter testosterone production include oophorectomy, ovarian failure, adrenal insufficiency, hypopituitarism, and other forms of chronic illness.
Treatment with corticosteroids and estrogen therapy lowers active androgen levels in women.
What levels are cause for concern?
If androgen levels are at or below the 25th percentile of the normal range for reproductive-aged women, consider the possibility of androgen insufficiency and determine whether androgen replacement is in order.11
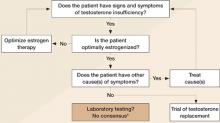
When the signs and symptoms of testosterone insufficiency are present, one must first assess estrogen levels by measuring serum estradiol, obtaining vaginal cytology, or both, and by determining whether symptoms of estrogen insufficiency are present, such as hot flashes, night sweats, and vaginal dryness. If the patient is estrogen-insufficient, the first step in resolving her symptoms is estrogen replacement. If estrogen levels are adequate and there is no other reason for the patient’s symptoms of fatigue, lack of sexual desire, or low energy, a trial of testosterone is reasonable.
Treating androgen insufficiency
Current therapies include oral methyltestosterone combined with estrogen, and intramuscular testosterone propionate, testosterone cypionate, and testosterone enanthate. Subcutaneous implants of testosterone propionate are also available, as are transdermal preparations (TABLE 2). However, the transdermal formulations are designed for androgen insufficiency in men, and therefore deliver approximately 10 times as much androgen as women normally produce. Testosterone gel preparations are available that can be applied in lower levels to achieve normal female androgen levels.
TABLE 2
Testosterone therapies available now—or in the pipeline
| Oral |
|
| Intramuscular |
|
| Subcutaneous (implant) |
|
| Transdermal |
|
| Other |
|
How long until relief?
It is clear from a number of studies13,14 that estrogen plus methyltestosterone oral replacement improves sexual desire in women after 12 to 16 weeks, and that this improvement is based on an increase in bioavailable testosterone. A testosterone patch under development delivers 300 μg per day. When used with conjugated equine estrogens, this patch has been shown to increase bioavailable testosterone in women without ovaries who have very low androgen levels.3
In a 2005 study,15 more than 500 women with hypoactive sexual desire who had undergone a total abdominal hysterectomy–bilateral salpingo-oophorectomy were randomized to placebo or a testosterone patch that delivered 300 μg per day for 24 weeks. Not only did serum testosterone levels increase, but satisfying sexual activity and the numbers of sexual interactions and orgasms increased (FIGURE 3). Side effects of therapy included increased facial hair and acne, but there was no increase in serious adverse effects, and no increase in withdrawal from the study because of side effects. Unfortunately, this patch is in development and unavailable commercially in the United States.
FIGURE 3
Assessing testosterone status in women
Adapted from Braunstein GD20
*Bachmann G, et al11
Watch for side effects, and follow closely
Testosterone therapy is most appropriate for women who have undergone surgical menopause and for postmenopausal women who are dissatisfied with estrogen therapy because of symptoms such as decreased libido and a diminished sense of well-being, including headaches and fatigue. Side effects of testosterone therapy include hirsutism, acne, alopecia, worsening lipoproteins, and, in the case of methyltestosterone, the possibility of liver toxicity, so women receiving testosterone should be followed frequently and carefully to detect any of these effects.
Androgen insufficiency in a nutshell
Androgens in women engender a general sense of well-being, which includes elevated energy and mood and increased libido. It is appropriate to consider androgen replacement using oral methyltestosterone, androgen implants, or transdermal androgen gels in women with a clinical diagnosis of androgen insufficiency.
Before initiating androgen therapy, however, it is important to measure total androgen level and assess clinical symptoms. Also, monitor the incidence of side effects to ensure that the patient does not exceed normal female androgen levels. It is hoped that additional forms of androgen replacement for women will become available in the near future.
The role of testosterone therapy in postmenopausal women: position statement of The North American Menopause Society. Menopause. 2005;12:497–511.
In 2005, the North American Menopause Society issued a comprehensive position statement on the role of testosterone therapy in postmenopausal women. Its purpose was to offer recommendations based on reliable evidence, and it reflects a thorough analysis of the data to date. Note that its findings, highlighted below, pertain to postmenopausal women only.
1. Endogenous testosterone levels have no clear link to sexual function
No definitive studies have established a relationship between endogenous testosterone levels and sexual function, and observational data have been mixed. Because of this lack of clarity, we do not have specific total or free testosterone values that indicate clinical testosterone deficiency.
Exogenous testosterone is a different story. Randomized controlled trials have demonstrated greater sexual desire and sexual responsiveness and more frequent sexual activity when exogenous testosterone is given. Almost all trials involved testosterone combined with estrogen or estrogen–progestogen therapy. The only trial that included a testosterone-alone arm found that testosterone added to estrogen therapy or given alone increased sexual desire, arousal, and frequency of sexual fantasies, compared with placebo or estrogen alone.14
2. Use the free testosterone index to determine testosterone bioavailability
Only 1% to 2% of circulating testosterone is free or bioavailable. The remainder binds tightly to sex hormone-binding globulin (SHBG, about 65%) or loosely to albumin (~30%). Because oral estrogen therapy increases SHBG levels, it lowers unbound testosterone. Conversely, obesity and hypothyroidism depress SHBG levels and increase free testosterone.
The simplest method to determine the amount of bioavailable testosterone is to measure total testosterone and SHBG, dividing total testosterone (ng/dL) by SHBG (nmol/L). Multiply this figure by 3.47 to obtain the free testosterone index. If the total testosterone value is reported in nmol/L, the multiplication factor is 100.
During the menopausal transition, free testosterone concentrations appear to remain fairly constant or increase slightly, probably because SHBG declines as ovarian estrogen production diminishes. One small study found little difference in total testosterone between younger premenopausal women (age 19–37 years) and older women (age 43–47), although the older age group lacked the midcycle rise in free testosterone and androstenedione.16
3. Causes of androgen insufficiency: Chronic illness, age, and oophorectomy, to name a few
Bilateral oophorectomy can lower testosterone levels by as much as 50%. Other contributors include increasing age, hypothalamic–pituitary–adrenal insufficiency, systemic glucocorticoids, hyperthyroidism and excessive thyroid medication, and chronic illness such as depression and advanced cancer. Both endogenous and exogenous estrogens lower testosterone levels by raising SHBG.
4. Sexual dysfunction is the only indication
Thus far, we lack sufficient data to justify use of testosterone for any other indication, including preserving bone mineral density, reducing hot flashes, and improving the patient’s overall sense of well-being.
5. A comprehensive clinical exam is mandatory
This includes a psychosexual and psychosocial history; a thorough medical history, including use of prescription and other drugs (such as selective serotonin reuptake inhibitors, which can reduce sexual desire); and a physical exam. It may also be appropriate to measure thyroid-stimulating hormone and prolactin and get a complete blood cell count. Consider the effects of other physical, psychological, and emotional complaints on sexual function, and ask about the relationship itself.
A study from Australia17 concluded that a postmenopausal woman’s previous level of sexual function, her feelings toward her partner, any change in partner status, and estradiol levels have the greatest influence on her sexual interest, arousal, and enjoyment. Declining levels of estradiol at menopause have a smaller impact than these psychological factors.
6. Non-oral forms of testosterone are preferred
To avoid the first-pass hepatic effects of oral administration, prescribe transdermal patches and topical gels and creams whenever possible, rather than oral testosterone.
Oral testosterone in combination with oral estrogen reduces high-density lipoprotein cholesterol and triglycerides in postmenopausal women, but non-oral testosterone has no significant effect on these parameters.
Extended use of high doses of oral testosterone can cause liver dysfunction in women.
7. Impact on fracture risk is unclear
Adding testosterone to estrogen therapy increases bone mineral density or reduces bone turnover, but no randomized trial has reported its effects on fracture risk in postmenopausal women.
8. No testosterone product is FDA-approved for sexual dysfunction in women
However, a few testosterone-containing prescription products are approved for use by women and men; some of these are used “off-label” to treat diminished sexual desire in women.
Be wary of custom-compounded prescription formulations because they do not undergo the same rigorous quality control as FDA-approved products.
A number of testosterone products are under development specifically for female sexual desire disorders, including an oral product, a cream, gels, a patch, a spray, and a vaginal ring.
9. Avoid testosterone in cancer and in heart and liver disease
Testosterone therapy is contraindicated in patients who have cancer of the breast or uterus, or cardiovascular or liver disease.
10. As with estrogen, use the lowest dosage for the shortest time possible
Once therapy meets treatment goals, it should be curtailed, if possible. And the dose should be kept as low as possible.
Most trials of testosterone therapy lasted 6 months or less, so we lack long-term data on safety and efficacy.
11. Supraphysiologic levels can cause adverse effects, some of them permanent
Risks include lowering of the voice (which may be permanent), enlargement of the clitoris, excess body hair, erythrocytosis, edema, and liver dysfunction. Psychological effects are also possible.
1. Sherwin BB. Hormones, mood, and cognitive functioning in postmenopausal women. Obstet Gynecol. 1996;87:20S-26S.
2. Raisz LG, Wiita B, Artis A, et al. Comparison of the effects of estrogen alone and estrogen plus androgen on biochemical markers of bone formation and resorption in postmenopausal women. J Clin Endocrinol Metab. 1996;81:37-43.
3. Shifren JL, Braunstein GD, Simon JA, et al. Transdermal testosterone treatment in women with impaired sexual function after oophorectomy. N Engl J Med. 2000;343:682-688.
4. Buster JE, et al. In: Lobo RA, ed. Treatment of the Postmenopausal Woman: Basic and Clinical Aspects. 2nd ed. Philadelphia: Lippincott, Williams & Wilkins; 1999:142.
5. Davison S, et al. Testosterone levels in women decline with aging. Abstract presented at the Endocrine Society Annual Meeting, held June 16–19, 2004, New Orleans.
6. Hughes CL, Jr, Wall LL, Creasman WT. Reproductive hormone levels in gynecologic oncology patients undergoing surgical castration after spontaneous menopause. Gynecol Oncol. 1991;40:42-45.
7. Selby C. Sex hormone-binding globulin. Ann Clin Biochem. 1990;27:532-541.
8. Simon JA. Estrogen replacement therapy: effects on the endogenous androgen milieu. Fertil Steril. 2002;77:S77-S82.
9. Miller KK, Biller BM, Hier J, Arena E, Klibanski A. Androgens and bone density in women with hypopituitarism. J Clin Endocrinol Metab. 2002;87:2770-2776.
10. Sarrel PM. Broadened spectrum of menopausal symptom relief. J Reprod Med. 1998;43:734-740.
11. Bachmann G, Bancroft J, Braunstein G, et al. Female androgen insufficiency: the Princeton consensus statement on definition, classification, and assessment. Fertil Steril. 2002;77:660-665.
12. Guay AT. Screening for androgen deficiency in women: methodological and interpretive issues. Fertil Steril. 2002;77:S83-S88.
13. Lobo RA, Rosen RC, Yang HM, Block B, Van Der Hoop RG. Comparative effects of oral and esterified estrogens with or without methyltestosterone on endocrine profiles on dimensions of sexual function in postmenopausal women with hypoactive sexual desire. Fertil Steril. 2003;79:1341-1352.
14. Sherwin BB, Gelfand MM, Brender W. Androgen enhances sexual motivation in females: a prospective, crossover study of sex steroid administration in the surgical menopause. Psychosom Med. 1985;47:339-351.
15. Buster JE, Kingsberg SA, Aguirre O, et al. Testosterone patch for low sexual desire in surgically menopausal women: a randomized trial. Obstet Gynecol. 2005;105:938-940.
16. Mushayandebvu T, Castracane VD, Gimpel T, Adel T, Santoro N. Evidence for diminished midcycle ovarian androgen production in older reproductive aged women. Fertil Steril. 1996;65:721-723.
17. Dennerstein L, Lehert P, Burger H. The relative effects of hormones and relationship factors on sexual function of women through the natural menopausal transition. Fertil Steril. 2005;84:174-180.
18. Vermeulen A. The hormonal activity of the postmenopausal ovary. J Clin Endocrinol Metab. 1976;42:247-253.
19. Sarrel P, Dobay B, Wiita B. Estrogen and estrogen–androgen replacement in postmenopausal women dissatisfied with estrogen-only therapy. Sexual behavior and neuroendocrine responses. J Reprod Med. 1998;43:847-856.
20. Braunstein GD. Androgen insufficiency in women: summary of critical issues. Fertil Steril. 2002;77(suppl 4):S94-S99.
Although women produce only one tenth the amount of androgen that men do, testosterone and related androgen metabolites are as important to women throughout the lifespan as is estrogen. Androgens modulate a feeling of well-being, increase energy, support bone metabolism, and improve sexual function in women.1-3 But too much androgen production, with elevated levels of testosterone and dehydroepiandrosterone (DHEA), can result in hirsutism, acne, and infertility in the setting of polycystic ovary syndrome (PCOS), all of which present clinical problems.
An equally complicated topic is androgen insufficiency in women. Not only is it difficult to diagnose, it is a major clinical issue to decide whether, when, and how to replace androgens in women. In this article, I look at androgen production throughout the female lifespan, particularly the relationship between estrogen and androgen. I also describe the evaluation of androgen insufficiency, which requires understanding of androgen physiology and ovarian function before and after menopause. These issues form the basis of the decision to replace androgen in women.
Androgen over the lifespan
In the premenopausal woman, androgen production is approximately equally divided between the adrenal gland and the ovaries. Androstenedione from both is converted to testosterone and then irreversibly to dihydrotestosterone (DHT). Androstenedione, testosterone, and even DHEA are secreted in equal quantities by the adrenals and ovaries. The only androgen that is predominantly adrenal is DHEAS, which is sulfated in the adrenal gland.
In premenopausal women, androstenedione is the precursor to testosterone, which is then metabolized to DHT, the androgen most active in hair follicles and implicated in hirsutism. It has been clear for many years that DHEA, although a weak androgen, is present in the greatest quantity in the circulation and is secreted during adrenarche, prior to menarche, beginning at ages 8 to 10. DHEAS peaks in young adulthood and begins to decline after age 40.4 The same is true for both total testosterone and free testosterone levels, which also decline in women after about age 25. Thus, peri- and postmenopausal women have approximately half the level of circulating androgens of women in their 20s (FIGURE 1, TABLE 1).5
FIGURE 1
Testosterone levels in women decline with aging
N=595
SOURCE: Davison S, et al5TABLE 1
How menopause affects plasma hormone levels
| HORMONE | MEAN PLASMA LEVEL | ||
|---|---|---|---|
| REPRODUCTIVE AGE* (N=15) | NATURALLY MENOPAUSAL (N=18) | OOPHORECTOMIZED (N=8) | |
| Estrone (pg/mL) | 58 | 49 | 48 |
| Estradiol (pg/mL) | 40 | 20† | 18 |
| Testosterone (ng/dL) | 44 | 30† | 12‡ |
| DHT (ng/dL) | 30 | 10† | <5‡ |
| Androstenedione (ng/dL) | 166 | 99† | 64‡ |
| DHEA (ng/dL) | 542 | 197† | 126§ |
| DHT=dihydrotestosterone, DHEA=dehydroepiandrosterone | |||
| * Mean value during early follicular phase | |||
| † P<.01 for comparison with reproductive age | |||
| ‡ P<.01 for comparison with naturally menopausal women | |||
| § P<.05 for comparison with naturally menopausal women | |||
| SOURCE: Vermeulen18 | |||
It matters how menopause happens
Circulating androgen levels are greatly influenced by menopause—how much depends on whether it occurs naturally with the ovaries intact, or by surgical removal of the ovaries. Not only does estradiol diminish significantly in naturally menopausal women, but all androgens do as well. In young oophorectomized women, estrogen levels are similar to levels in naturally menopausal women, but androgen levels—including testosterone, DHT, and androstenedione—are significantly lower than in naturally menopausal women, demonstrating that the circulating levels of androgen after natural menopause are still significantly greater than those in oophorectomized women.6 Thus, the postmenopausal ovary contributes significantly to circulating levels of androgen.
Androgen physiology
Both androgens and estrogens circulate in the bloodstream tightly bound to the protein sex hormone-binding globulin (SHBG), and more loosely bound to albumin. The SHBG-bound fraction is unavailable for biologic activity. Therefore, the amount of SHBG a woman produces is a key determinant of her level of androgen bioactivity. For this reason, it is crucial to measure circulating SHBG.
In a feedback mechanism, SHBG production is regulated by androgen and estrogen levels, with estrogen stimulating SHBG production and testosterone decreasing it.7 In the normal woman, about 65% of testosterone is bound to SHBG and 30% is bound to albumin, leaving only 0.5% to 2% free and bioactively available.8 In postmenopausal women taking hormone replacement therapy, SHBG increases, but the addition of methyltestosterone lowers the overall levels of SHBG, even in the presence of estrogen, increasing the amount of bioavailable testosterone simply by lowering SHBG levels. Postmenopausal replacement with estrogen alone decreases the amount of bioavailable testosterone because of higher SHBG levels.
SHBG is synthesized in the liver, whose metabolism is increased by exposure to steroids. Therefore, oral forms of estrogen replacement, which stimulate the liver because of the “first pass” effect, result in a greater increase in SHBG than do transdermal estrogen preparations.
Elevated androgen levels have both ill and good effects
As I stated earlier, an appropriate level of androgen is optimal for women as well as men. Elevated androgen levels are problematic, in that they are the hallmark of PCOS, usually resulting from increased ovarian production of androgen. This elevation can cause anovulation, infertility, hirsutism, and other androgen-mediated physiologic effects. Androgen is also associated with elevated low-density lipoprotein and decreased high-density lipoprotein cholesterol, implying a possible relationship with cardiovascular disease. At the same time, however, elevated testosterone has been correlated with increased bone density in both the hip and the femoral neck.9
It is clear that appropriate androgen secretion, which does not elicit the side effects described above, is best for both the health and well-being of the woman.
How androgen affects female sexual function
We have known for years that androgen—not estrogen—is associated with satisfactory sexual function. Although estrogen replacement increases vaginal lubrication, it is androgen, most commonly in the form of oral methyltestosterone or injectable testosterone, that increases frequency of intercourse, desire, and sexual sensation (FIGURE 2).10 The definition of androgen insufficiency has been hotly debated, and is currently “a pattern of clinical symptoms in the presence of decreased bioavailable testosterone and normal estrogen.”11
FIGURE 2
How estrogen plus androgen affects sexual function
*P<.01; †P=.05
EE=esterified estrogens; MT=methyltestosterone
SOURCE: Sarrel PM, et al19
Assessing androgen levels
Clinical signs and symptoms of androgen insufficiency are important in establishing the diagnosis. They include a diminished sense of well-being, unexplained fatigue, decreased sexual desire, and thinning and loss of pubic hair.11 Although it is possible to assess testosterone production and availability in women by measuring serum testosterone levels, a lack of consensus about the best measurement technique and interpretation of results makes it difficult to base the diagnosis of androgen insufficiency solely on serum levels.12 Therefore, the diagnosis of androgen insufficiency is primarily a clinical diagnosis of symptoms.11
Obtain serum samples between 8 and 10 AM after day 8 and before day 20 of the normal menstrual cycle because testosterone is subject to diurnal variation, peaking in the early morning, as well as cyclic variation, peaking around midcycle.
Because free testosterone is the only bioavailable steroid, total testosterone and either free testosterone or SHBG must be measured to assess how much androgen is actually available. From total testosterone and SHBG, one can assess the free testosterone index as a measure of bioavailable androgen (the free testosterone index is a ratio of the amount of total testosterone divided by the SHBG level).11 In fact, using the free testosterone index is preferable to the actual measurement of free testosterone because commercial assays lack the sensitivity and reliability to accurately measure the low levels of androgen found in women.
Several different testosterone assays exist, and the immunoassay for total testosterone is reasonably accurate. However, measurements of free testosterone are relatively inaccurate and poorly reproducible. Equilibrium dialysis is thought to be the gold standard for measuring free testosterone, but it is a difficult and time-consuming assay.12
Causes of low testosterone
In women, low testosterone secretion is usually the result of normal aging. Other conditions that alter testosterone production include oophorectomy, ovarian failure, adrenal insufficiency, hypopituitarism, and other forms of chronic illness.
Treatment with corticosteroids and estrogen therapy lowers active androgen levels in women.
What levels are cause for concern?
If androgen levels are at or below the 25th percentile of the normal range for reproductive-aged women, consider the possibility of androgen insufficiency and determine whether androgen replacement is in order.11
When the signs and symptoms of testosterone insufficiency are present, one must first assess estrogen levels by measuring serum estradiol, obtaining vaginal cytology, or both, and by determining whether symptoms of estrogen insufficiency are present, such as hot flashes, night sweats, and vaginal dryness. If the patient is estrogen-insufficient, the first step in resolving her symptoms is estrogen replacement. If estrogen levels are adequate and there is no other reason for the patient’s symptoms of fatigue, lack of sexual desire, or low energy, a trial of testosterone is reasonable.
Treating androgen insufficiency
Current therapies include oral methyltestosterone combined with estrogen, and intramuscular testosterone propionate, testosterone cypionate, and testosterone enanthate. Subcutaneous implants of testosterone propionate are also available, as are transdermal preparations (TABLE 2). However, the transdermal formulations are designed for androgen insufficiency in men, and therefore deliver approximately 10 times as much androgen as women normally produce. Testosterone gel preparations are available that can be applied in lower levels to achieve normal female androgen levels.
TABLE 2
Testosterone therapies available now—or in the pipeline
| Oral |
|
| Intramuscular |
|
| Subcutaneous (implant) |
|
| Transdermal |
|
| Other |
|
How long until relief?
It is clear from a number of studies13,14 that estrogen plus methyltestosterone oral replacement improves sexual desire in women after 12 to 16 weeks, and that this improvement is based on an increase in bioavailable testosterone. A testosterone patch under development delivers 300 μg per day. When used with conjugated equine estrogens, this patch has been shown to increase bioavailable testosterone in women without ovaries who have very low androgen levels.3
In a 2005 study,15 more than 500 women with hypoactive sexual desire who had undergone a total abdominal hysterectomy–bilateral salpingo-oophorectomy were randomized to placebo or a testosterone patch that delivered 300 μg per day for 24 weeks. Not only did serum testosterone levels increase, but satisfying sexual activity and the numbers of sexual interactions and orgasms increased (FIGURE 3). Side effects of therapy included increased facial hair and acne, but there was no increase in serious adverse effects, and no increase in withdrawal from the study because of side effects. Unfortunately, this patch is in development and unavailable commercially in the United States.
FIGURE 3
Assessing testosterone status in women
Adapted from Braunstein GD20
*Bachmann G, et al11
Watch for side effects, and follow closely
Testosterone therapy is most appropriate for women who have undergone surgical menopause and for postmenopausal women who are dissatisfied with estrogen therapy because of symptoms such as decreased libido and a diminished sense of well-being, including headaches and fatigue. Side effects of testosterone therapy include hirsutism, acne, alopecia, worsening lipoproteins, and, in the case of methyltestosterone, the possibility of liver toxicity, so women receiving testosterone should be followed frequently and carefully to detect any of these effects.
Androgen insufficiency in a nutshell
Androgens in women engender a general sense of well-being, which includes elevated energy and mood and increased libido. It is appropriate to consider androgen replacement using oral methyltestosterone, androgen implants, or transdermal androgen gels in women with a clinical diagnosis of androgen insufficiency.
Before initiating androgen therapy, however, it is important to measure total androgen level and assess clinical symptoms. Also, monitor the incidence of side effects to ensure that the patient does not exceed normal female androgen levels. It is hoped that additional forms of androgen replacement for women will become available in the near future.
The role of testosterone therapy in postmenopausal women: position statement of The North American Menopause Society. Menopause. 2005;12:497–511.
In 2005, the North American Menopause Society issued a comprehensive position statement on the role of testosterone therapy in postmenopausal women. Its purpose was to offer recommendations based on reliable evidence, and it reflects a thorough analysis of the data to date. Note that its findings, highlighted below, pertain to postmenopausal women only.
1. Endogenous testosterone levels have no clear link to sexual function
No definitive studies have established a relationship between endogenous testosterone levels and sexual function, and observational data have been mixed. Because of this lack of clarity, we do not have specific total or free testosterone values that indicate clinical testosterone deficiency.
Exogenous testosterone is a different story. Randomized controlled trials have demonstrated greater sexual desire and sexual responsiveness and more frequent sexual activity when exogenous testosterone is given. Almost all trials involved testosterone combined with estrogen or estrogen–progestogen therapy. The only trial that included a testosterone-alone arm found that testosterone added to estrogen therapy or given alone increased sexual desire, arousal, and frequency of sexual fantasies, compared with placebo or estrogen alone.14
2. Use the free testosterone index to determine testosterone bioavailability
Only 1% to 2% of circulating testosterone is free or bioavailable. The remainder binds tightly to sex hormone-binding globulin (SHBG, about 65%) or loosely to albumin (~30%). Because oral estrogen therapy increases SHBG levels, it lowers unbound testosterone. Conversely, obesity and hypothyroidism depress SHBG levels and increase free testosterone.
The simplest method to determine the amount of bioavailable testosterone is to measure total testosterone and SHBG, dividing total testosterone (ng/dL) by SHBG (nmol/L). Multiply this figure by 3.47 to obtain the free testosterone index. If the total testosterone value is reported in nmol/L, the multiplication factor is 100.
During the menopausal transition, free testosterone concentrations appear to remain fairly constant or increase slightly, probably because SHBG declines as ovarian estrogen production diminishes. One small study found little difference in total testosterone between younger premenopausal women (age 19–37 years) and older women (age 43–47), although the older age group lacked the midcycle rise in free testosterone and androstenedione.16
3. Causes of androgen insufficiency: Chronic illness, age, and oophorectomy, to name a few
Bilateral oophorectomy can lower testosterone levels by as much as 50%. Other contributors include increasing age, hypothalamic–pituitary–adrenal insufficiency, systemic glucocorticoids, hyperthyroidism and excessive thyroid medication, and chronic illness such as depression and advanced cancer. Both endogenous and exogenous estrogens lower testosterone levels by raising SHBG.
4. Sexual dysfunction is the only indication
Thus far, we lack sufficient data to justify use of testosterone for any other indication, including preserving bone mineral density, reducing hot flashes, and improving the patient’s overall sense of well-being.
5. A comprehensive clinical exam is mandatory
This includes a psychosexual and psychosocial history; a thorough medical history, including use of prescription and other drugs (such as selective serotonin reuptake inhibitors, which can reduce sexual desire); and a physical exam. It may also be appropriate to measure thyroid-stimulating hormone and prolactin and get a complete blood cell count. Consider the effects of other physical, psychological, and emotional complaints on sexual function, and ask about the relationship itself.
A study from Australia17 concluded that a postmenopausal woman’s previous level of sexual function, her feelings toward her partner, any change in partner status, and estradiol levels have the greatest influence on her sexual interest, arousal, and enjoyment. Declining levels of estradiol at menopause have a smaller impact than these psychological factors.
6. Non-oral forms of testosterone are preferred
To avoid the first-pass hepatic effects of oral administration, prescribe transdermal patches and topical gels and creams whenever possible, rather than oral testosterone.
Oral testosterone in combination with oral estrogen reduces high-density lipoprotein cholesterol and triglycerides in postmenopausal women, but non-oral testosterone has no significant effect on these parameters.
Extended use of high doses of oral testosterone can cause liver dysfunction in women.
7. Impact on fracture risk is unclear
Adding testosterone to estrogen therapy increases bone mineral density or reduces bone turnover, but no randomized trial has reported its effects on fracture risk in postmenopausal women.
8. No testosterone product is FDA-approved for sexual dysfunction in women
However, a few testosterone-containing prescription products are approved for use by women and men; some of these are used “off-label” to treat diminished sexual desire in women.
Be wary of custom-compounded prescription formulations because they do not undergo the same rigorous quality control as FDA-approved products.
A number of testosterone products are under development specifically for female sexual desire disorders, including an oral product, a cream, gels, a patch, a spray, and a vaginal ring.
9. Avoid testosterone in cancer and in heart and liver disease
Testosterone therapy is contraindicated in patients who have cancer of the breast or uterus, or cardiovascular or liver disease.
10. As with estrogen, use the lowest dosage for the shortest time possible
Once therapy meets treatment goals, it should be curtailed, if possible. And the dose should be kept as low as possible.
Most trials of testosterone therapy lasted 6 months or less, so we lack long-term data on safety and efficacy.
11. Supraphysiologic levels can cause adverse effects, some of them permanent
Risks include lowering of the voice (which may be permanent), enlargement of the clitoris, excess body hair, erythrocytosis, edema, and liver dysfunction. Psychological effects are also possible.
Although women produce only one tenth the amount of androgen that men do, testosterone and related androgen metabolites are as important to women throughout the lifespan as is estrogen. Androgens modulate a feeling of well-being, increase energy, support bone metabolism, and improve sexual function in women.1-3 But too much androgen production, with elevated levels of testosterone and dehydroepiandrosterone (DHEA), can result in hirsutism, acne, and infertility in the setting of polycystic ovary syndrome (PCOS), all of which present clinical problems.
An equally complicated topic is androgen insufficiency in women. Not only is it difficult to diagnose, it is a major clinical issue to decide whether, when, and how to replace androgens in women. In this article, I look at androgen production throughout the female lifespan, particularly the relationship between estrogen and androgen. I also describe the evaluation of androgen insufficiency, which requires understanding of androgen physiology and ovarian function before and after menopause. These issues form the basis of the decision to replace androgen in women.
Androgen over the lifespan
In the premenopausal woman, androgen production is approximately equally divided between the adrenal gland and the ovaries. Androstenedione from both is converted to testosterone and then irreversibly to dihydrotestosterone (DHT). Androstenedione, testosterone, and even DHEA are secreted in equal quantities by the adrenals and ovaries. The only androgen that is predominantly adrenal is DHEAS, which is sulfated in the adrenal gland.
In premenopausal women, androstenedione is the precursor to testosterone, which is then metabolized to DHT, the androgen most active in hair follicles and implicated in hirsutism. It has been clear for many years that DHEA, although a weak androgen, is present in the greatest quantity in the circulation and is secreted during adrenarche, prior to menarche, beginning at ages 8 to 10. DHEAS peaks in young adulthood and begins to decline after age 40.4 The same is true for both total testosterone and free testosterone levels, which also decline in women after about age 25. Thus, peri- and postmenopausal women have approximately half the level of circulating androgens of women in their 20s (FIGURE 1, TABLE 1).5
FIGURE 1
Testosterone levels in women decline with aging
N=595
SOURCE: Davison S, et al5TABLE 1
How menopause affects plasma hormone levels
| HORMONE | MEAN PLASMA LEVEL | ||
|---|---|---|---|
| REPRODUCTIVE AGE* (N=15) | NATURALLY MENOPAUSAL (N=18) | OOPHORECTOMIZED (N=8) | |
| Estrone (pg/mL) | 58 | 49 | 48 |
| Estradiol (pg/mL) | 40 | 20† | 18 |
| Testosterone (ng/dL) | 44 | 30† | 12‡ |
| DHT (ng/dL) | 30 | 10† | <5‡ |
| Androstenedione (ng/dL) | 166 | 99† | 64‡ |
| DHEA (ng/dL) | 542 | 197† | 126§ |
| DHT=dihydrotestosterone, DHEA=dehydroepiandrosterone | |||
| * Mean value during early follicular phase | |||
| † P<.01 for comparison with reproductive age | |||
| ‡ P<.01 for comparison with naturally menopausal women | |||
| § P<.05 for comparison with naturally menopausal women | |||
| SOURCE: Vermeulen18 | |||
It matters how menopause happens
Circulating androgen levels are greatly influenced by menopause—how much depends on whether it occurs naturally with the ovaries intact, or by surgical removal of the ovaries. Not only does estradiol diminish significantly in naturally menopausal women, but all androgens do as well. In young oophorectomized women, estrogen levels are similar to levels in naturally menopausal women, but androgen levels—including testosterone, DHT, and androstenedione—are significantly lower than in naturally menopausal women, demonstrating that the circulating levels of androgen after natural menopause are still significantly greater than those in oophorectomized women.6 Thus, the postmenopausal ovary contributes significantly to circulating levels of androgen.
Androgen physiology
Both androgens and estrogens circulate in the bloodstream tightly bound to the protein sex hormone-binding globulin (SHBG), and more loosely bound to albumin. The SHBG-bound fraction is unavailable for biologic activity. Therefore, the amount of SHBG a woman produces is a key determinant of her level of androgen bioactivity. For this reason, it is crucial to measure circulating SHBG.
In a feedback mechanism, SHBG production is regulated by androgen and estrogen levels, with estrogen stimulating SHBG production and testosterone decreasing it.7 In the normal woman, about 65% of testosterone is bound to SHBG and 30% is bound to albumin, leaving only 0.5% to 2% free and bioactively available.8 In postmenopausal women taking hormone replacement therapy, SHBG increases, but the addition of methyltestosterone lowers the overall levels of SHBG, even in the presence of estrogen, increasing the amount of bioavailable testosterone simply by lowering SHBG levels. Postmenopausal replacement with estrogen alone decreases the amount of bioavailable testosterone because of higher SHBG levels.
SHBG is synthesized in the liver, whose metabolism is increased by exposure to steroids. Therefore, oral forms of estrogen replacement, which stimulate the liver because of the “first pass” effect, result in a greater increase in SHBG than do transdermal estrogen preparations.
Elevated androgen levels have both ill and good effects
As I stated earlier, an appropriate level of androgen is optimal for women as well as men. Elevated androgen levels are problematic, in that they are the hallmark of PCOS, usually resulting from increased ovarian production of androgen. This elevation can cause anovulation, infertility, hirsutism, and other androgen-mediated physiologic effects. Androgen is also associated with elevated low-density lipoprotein and decreased high-density lipoprotein cholesterol, implying a possible relationship with cardiovascular disease. At the same time, however, elevated testosterone has been correlated with increased bone density in both the hip and the femoral neck.9
It is clear that appropriate androgen secretion, which does not elicit the side effects described above, is best for both the health and well-being of the woman.
How androgen affects female sexual function
We have known for years that androgen—not estrogen—is associated with satisfactory sexual function. Although estrogen replacement increases vaginal lubrication, it is androgen, most commonly in the form of oral methyltestosterone or injectable testosterone, that increases frequency of intercourse, desire, and sexual sensation (FIGURE 2).10 The definition of androgen insufficiency has been hotly debated, and is currently “a pattern of clinical symptoms in the presence of decreased bioavailable testosterone and normal estrogen.”11
FIGURE 2
How estrogen plus androgen affects sexual function
*P<.01; †P=.05
EE=esterified estrogens; MT=methyltestosterone
SOURCE: Sarrel PM, et al19
Assessing androgen levels
Clinical signs and symptoms of androgen insufficiency are important in establishing the diagnosis. They include a diminished sense of well-being, unexplained fatigue, decreased sexual desire, and thinning and loss of pubic hair.11 Although it is possible to assess testosterone production and availability in women by measuring serum testosterone levels, a lack of consensus about the best measurement technique and interpretation of results makes it difficult to base the diagnosis of androgen insufficiency solely on serum levels.12 Therefore, the diagnosis of androgen insufficiency is primarily a clinical diagnosis of symptoms.11
Obtain serum samples between 8 and 10 AM after day 8 and before day 20 of the normal menstrual cycle because testosterone is subject to diurnal variation, peaking in the early morning, as well as cyclic variation, peaking around midcycle.
Because free testosterone is the only bioavailable steroid, total testosterone and either free testosterone or SHBG must be measured to assess how much androgen is actually available. From total testosterone and SHBG, one can assess the free testosterone index as a measure of bioavailable androgen (the free testosterone index is a ratio of the amount of total testosterone divided by the SHBG level).11 In fact, using the free testosterone index is preferable to the actual measurement of free testosterone because commercial assays lack the sensitivity and reliability to accurately measure the low levels of androgen found in women.
Several different testosterone assays exist, and the immunoassay for total testosterone is reasonably accurate. However, measurements of free testosterone are relatively inaccurate and poorly reproducible. Equilibrium dialysis is thought to be the gold standard for measuring free testosterone, but it is a difficult and time-consuming assay.12
Causes of low testosterone
In women, low testosterone secretion is usually the result of normal aging. Other conditions that alter testosterone production include oophorectomy, ovarian failure, adrenal insufficiency, hypopituitarism, and other forms of chronic illness.
Treatment with corticosteroids and estrogen therapy lowers active androgen levels in women.
What levels are cause for concern?
If androgen levels are at or below the 25th percentile of the normal range for reproductive-aged women, consider the possibility of androgen insufficiency and determine whether androgen replacement is in order.11
When the signs and symptoms of testosterone insufficiency are present, one must first assess estrogen levels by measuring serum estradiol, obtaining vaginal cytology, or both, and by determining whether symptoms of estrogen insufficiency are present, such as hot flashes, night sweats, and vaginal dryness. If the patient is estrogen-insufficient, the first step in resolving her symptoms is estrogen replacement. If estrogen levels are adequate and there is no other reason for the patient’s symptoms of fatigue, lack of sexual desire, or low energy, a trial of testosterone is reasonable.
Treating androgen insufficiency
Current therapies include oral methyltestosterone combined with estrogen, and intramuscular testosterone propionate, testosterone cypionate, and testosterone enanthate. Subcutaneous implants of testosterone propionate are also available, as are transdermal preparations (TABLE 2). However, the transdermal formulations are designed for androgen insufficiency in men, and therefore deliver approximately 10 times as much androgen as women normally produce. Testosterone gel preparations are available that can be applied in lower levels to achieve normal female androgen levels.
TABLE 2
Testosterone therapies available now—or in the pipeline
| Oral |
|
| Intramuscular |
|
| Subcutaneous (implant) |
|
| Transdermal |
|
| Other |
|
How long until relief?
It is clear from a number of studies13,14 that estrogen plus methyltestosterone oral replacement improves sexual desire in women after 12 to 16 weeks, and that this improvement is based on an increase in bioavailable testosterone. A testosterone patch under development delivers 300 μg per day. When used with conjugated equine estrogens, this patch has been shown to increase bioavailable testosterone in women without ovaries who have very low androgen levels.3
In a 2005 study,15 more than 500 women with hypoactive sexual desire who had undergone a total abdominal hysterectomy–bilateral salpingo-oophorectomy were randomized to placebo or a testosterone patch that delivered 300 μg per day for 24 weeks. Not only did serum testosterone levels increase, but satisfying sexual activity and the numbers of sexual interactions and orgasms increased (FIGURE 3). Side effects of therapy included increased facial hair and acne, but there was no increase in serious adverse effects, and no increase in withdrawal from the study because of side effects. Unfortunately, this patch is in development and unavailable commercially in the United States.
FIGURE 3
Assessing testosterone status in women
Adapted from Braunstein GD20
*Bachmann G, et al11
Watch for side effects, and follow closely
Testosterone therapy is most appropriate for women who have undergone surgical menopause and for postmenopausal women who are dissatisfied with estrogen therapy because of symptoms such as decreased libido and a diminished sense of well-being, including headaches and fatigue. Side effects of testosterone therapy include hirsutism, acne, alopecia, worsening lipoproteins, and, in the case of methyltestosterone, the possibility of liver toxicity, so women receiving testosterone should be followed frequently and carefully to detect any of these effects.
Androgen insufficiency in a nutshell
Androgens in women engender a general sense of well-being, which includes elevated energy and mood and increased libido. It is appropriate to consider androgen replacement using oral methyltestosterone, androgen implants, or transdermal androgen gels in women with a clinical diagnosis of androgen insufficiency.
Before initiating androgen therapy, however, it is important to measure total androgen level and assess clinical symptoms. Also, monitor the incidence of side effects to ensure that the patient does not exceed normal female androgen levels. It is hoped that additional forms of androgen replacement for women will become available in the near future.
The role of testosterone therapy in postmenopausal women: position statement of The North American Menopause Society. Menopause. 2005;12:497–511.
In 2005, the North American Menopause Society issued a comprehensive position statement on the role of testosterone therapy in postmenopausal women. Its purpose was to offer recommendations based on reliable evidence, and it reflects a thorough analysis of the data to date. Note that its findings, highlighted below, pertain to postmenopausal women only.
1. Endogenous testosterone levels have no clear link to sexual function
No definitive studies have established a relationship between endogenous testosterone levels and sexual function, and observational data have been mixed. Because of this lack of clarity, we do not have specific total or free testosterone values that indicate clinical testosterone deficiency.
Exogenous testosterone is a different story. Randomized controlled trials have demonstrated greater sexual desire and sexual responsiveness and more frequent sexual activity when exogenous testosterone is given. Almost all trials involved testosterone combined with estrogen or estrogen–progestogen therapy. The only trial that included a testosterone-alone arm found that testosterone added to estrogen therapy or given alone increased sexual desire, arousal, and frequency of sexual fantasies, compared with placebo or estrogen alone.14
2. Use the free testosterone index to determine testosterone bioavailability
Only 1% to 2% of circulating testosterone is free or bioavailable. The remainder binds tightly to sex hormone-binding globulin (SHBG, about 65%) or loosely to albumin (~30%). Because oral estrogen therapy increases SHBG levels, it lowers unbound testosterone. Conversely, obesity and hypothyroidism depress SHBG levels and increase free testosterone.
The simplest method to determine the amount of bioavailable testosterone is to measure total testosterone and SHBG, dividing total testosterone (ng/dL) by SHBG (nmol/L). Multiply this figure by 3.47 to obtain the free testosterone index. If the total testosterone value is reported in nmol/L, the multiplication factor is 100.
During the menopausal transition, free testosterone concentrations appear to remain fairly constant or increase slightly, probably because SHBG declines as ovarian estrogen production diminishes. One small study found little difference in total testosterone between younger premenopausal women (age 19–37 years) and older women (age 43–47), although the older age group lacked the midcycle rise in free testosterone and androstenedione.16
3. Causes of androgen insufficiency: Chronic illness, age, and oophorectomy, to name a few
Bilateral oophorectomy can lower testosterone levels by as much as 50%. Other contributors include increasing age, hypothalamic–pituitary–adrenal insufficiency, systemic glucocorticoids, hyperthyroidism and excessive thyroid medication, and chronic illness such as depression and advanced cancer. Both endogenous and exogenous estrogens lower testosterone levels by raising SHBG.
4. Sexual dysfunction is the only indication
Thus far, we lack sufficient data to justify use of testosterone for any other indication, including preserving bone mineral density, reducing hot flashes, and improving the patient’s overall sense of well-being.
5. A comprehensive clinical exam is mandatory
This includes a psychosexual and psychosocial history; a thorough medical history, including use of prescription and other drugs (such as selective serotonin reuptake inhibitors, which can reduce sexual desire); and a physical exam. It may also be appropriate to measure thyroid-stimulating hormone and prolactin and get a complete blood cell count. Consider the effects of other physical, psychological, and emotional complaints on sexual function, and ask about the relationship itself.
A study from Australia17 concluded that a postmenopausal woman’s previous level of sexual function, her feelings toward her partner, any change in partner status, and estradiol levels have the greatest influence on her sexual interest, arousal, and enjoyment. Declining levels of estradiol at menopause have a smaller impact than these psychological factors.
6. Non-oral forms of testosterone are preferred
To avoid the first-pass hepatic effects of oral administration, prescribe transdermal patches and topical gels and creams whenever possible, rather than oral testosterone.
Oral testosterone in combination with oral estrogen reduces high-density lipoprotein cholesterol and triglycerides in postmenopausal women, but non-oral testosterone has no significant effect on these parameters.
Extended use of high doses of oral testosterone can cause liver dysfunction in women.
7. Impact on fracture risk is unclear
Adding testosterone to estrogen therapy increases bone mineral density or reduces bone turnover, but no randomized trial has reported its effects on fracture risk in postmenopausal women.
8. No testosterone product is FDA-approved for sexual dysfunction in women
However, a few testosterone-containing prescription products are approved for use by women and men; some of these are used “off-label” to treat diminished sexual desire in women.
Be wary of custom-compounded prescription formulations because they do not undergo the same rigorous quality control as FDA-approved products.
A number of testosterone products are under development specifically for female sexual desire disorders, including an oral product, a cream, gels, a patch, a spray, and a vaginal ring.
9. Avoid testosterone in cancer and in heart and liver disease
Testosterone therapy is contraindicated in patients who have cancer of the breast or uterus, or cardiovascular or liver disease.
10. As with estrogen, use the lowest dosage for the shortest time possible
Once therapy meets treatment goals, it should be curtailed, if possible. And the dose should be kept as low as possible.
Most trials of testosterone therapy lasted 6 months or less, so we lack long-term data on safety and efficacy.
11. Supraphysiologic levels can cause adverse effects, some of them permanent
Risks include lowering of the voice (which may be permanent), enlargement of the clitoris, excess body hair, erythrocytosis, edema, and liver dysfunction. Psychological effects are also possible.
1. Sherwin BB. Hormones, mood, and cognitive functioning in postmenopausal women. Obstet Gynecol. 1996;87:20S-26S.
2. Raisz LG, Wiita B, Artis A, et al. Comparison of the effects of estrogen alone and estrogen plus androgen on biochemical markers of bone formation and resorption in postmenopausal women. J Clin Endocrinol Metab. 1996;81:37-43.
3. Shifren JL, Braunstein GD, Simon JA, et al. Transdermal testosterone treatment in women with impaired sexual function after oophorectomy. N Engl J Med. 2000;343:682-688.
4. Buster JE, et al. In: Lobo RA, ed. Treatment of the Postmenopausal Woman: Basic and Clinical Aspects. 2nd ed. Philadelphia: Lippincott, Williams & Wilkins; 1999:142.
5. Davison S, et al. Testosterone levels in women decline with aging. Abstract presented at the Endocrine Society Annual Meeting, held June 16–19, 2004, New Orleans.
6. Hughes CL, Jr, Wall LL, Creasman WT. Reproductive hormone levels in gynecologic oncology patients undergoing surgical castration after spontaneous menopause. Gynecol Oncol. 1991;40:42-45.
7. Selby C. Sex hormone-binding globulin. Ann Clin Biochem. 1990;27:532-541.
8. Simon JA. Estrogen replacement therapy: effects on the endogenous androgen milieu. Fertil Steril. 2002;77:S77-S82.
9. Miller KK, Biller BM, Hier J, Arena E, Klibanski A. Androgens and bone density in women with hypopituitarism. J Clin Endocrinol Metab. 2002;87:2770-2776.
10. Sarrel PM. Broadened spectrum of menopausal symptom relief. J Reprod Med. 1998;43:734-740.
11. Bachmann G, Bancroft J, Braunstein G, et al. Female androgen insufficiency: the Princeton consensus statement on definition, classification, and assessment. Fertil Steril. 2002;77:660-665.
12. Guay AT. Screening for androgen deficiency in women: methodological and interpretive issues. Fertil Steril. 2002;77:S83-S88.
13. Lobo RA, Rosen RC, Yang HM, Block B, Van Der Hoop RG. Comparative effects of oral and esterified estrogens with or without methyltestosterone on endocrine profiles on dimensions of sexual function in postmenopausal women with hypoactive sexual desire. Fertil Steril. 2003;79:1341-1352.
14. Sherwin BB, Gelfand MM, Brender W. Androgen enhances sexual motivation in females: a prospective, crossover study of sex steroid administration in the surgical menopause. Psychosom Med. 1985;47:339-351.
15. Buster JE, Kingsberg SA, Aguirre O, et al. Testosterone patch for low sexual desire in surgically menopausal women: a randomized trial. Obstet Gynecol. 2005;105:938-940.
16. Mushayandebvu T, Castracane VD, Gimpel T, Adel T, Santoro N. Evidence for diminished midcycle ovarian androgen production in older reproductive aged women. Fertil Steril. 1996;65:721-723.
17. Dennerstein L, Lehert P, Burger H. The relative effects of hormones and relationship factors on sexual function of women through the natural menopausal transition. Fertil Steril. 2005;84:174-180.
18. Vermeulen A. The hormonal activity of the postmenopausal ovary. J Clin Endocrinol Metab. 1976;42:247-253.
19. Sarrel P, Dobay B, Wiita B. Estrogen and estrogen–androgen replacement in postmenopausal women dissatisfied with estrogen-only therapy. Sexual behavior and neuroendocrine responses. J Reprod Med. 1998;43:847-856.
20. Braunstein GD. Androgen insufficiency in women: summary of critical issues. Fertil Steril. 2002;77(suppl 4):S94-S99.
1. Sherwin BB. Hormones, mood, and cognitive functioning in postmenopausal women. Obstet Gynecol. 1996;87:20S-26S.
2. Raisz LG, Wiita B, Artis A, et al. Comparison of the effects of estrogen alone and estrogen plus androgen on biochemical markers of bone formation and resorption in postmenopausal women. J Clin Endocrinol Metab. 1996;81:37-43.
3. Shifren JL, Braunstein GD, Simon JA, et al. Transdermal testosterone treatment in women with impaired sexual function after oophorectomy. N Engl J Med. 2000;343:682-688.
4. Buster JE, et al. In: Lobo RA, ed. Treatment of the Postmenopausal Woman: Basic and Clinical Aspects. 2nd ed. Philadelphia: Lippincott, Williams & Wilkins; 1999:142.
5. Davison S, et al. Testosterone levels in women decline with aging. Abstract presented at the Endocrine Society Annual Meeting, held June 16–19, 2004, New Orleans.
6. Hughes CL, Jr, Wall LL, Creasman WT. Reproductive hormone levels in gynecologic oncology patients undergoing surgical castration after spontaneous menopause. Gynecol Oncol. 1991;40:42-45.
7. Selby C. Sex hormone-binding globulin. Ann Clin Biochem. 1990;27:532-541.
8. Simon JA. Estrogen replacement therapy: effects on the endogenous androgen milieu. Fertil Steril. 2002;77:S77-S82.
9. Miller KK, Biller BM, Hier J, Arena E, Klibanski A. Androgens and bone density in women with hypopituitarism. J Clin Endocrinol Metab. 2002;87:2770-2776.
10. Sarrel PM. Broadened spectrum of menopausal symptom relief. J Reprod Med. 1998;43:734-740.
11. Bachmann G, Bancroft J, Braunstein G, et al. Female androgen insufficiency: the Princeton consensus statement on definition, classification, and assessment. Fertil Steril. 2002;77:660-665.
12. Guay AT. Screening for androgen deficiency in women: methodological and interpretive issues. Fertil Steril. 2002;77:S83-S88.
13. Lobo RA, Rosen RC, Yang HM, Block B, Van Der Hoop RG. Comparative effects of oral and esterified estrogens with or without methyltestosterone on endocrine profiles on dimensions of sexual function in postmenopausal women with hypoactive sexual desire. Fertil Steril. 2003;79:1341-1352.
14. Sherwin BB, Gelfand MM, Brender W. Androgen enhances sexual motivation in females: a prospective, crossover study of sex steroid administration in the surgical menopause. Psychosom Med. 1985;47:339-351.
15. Buster JE, Kingsberg SA, Aguirre O, et al. Testosterone patch for low sexual desire in surgically menopausal women: a randomized trial. Obstet Gynecol. 2005;105:938-940.
16. Mushayandebvu T, Castracane VD, Gimpel T, Adel T, Santoro N. Evidence for diminished midcycle ovarian androgen production in older reproductive aged women. Fertil Steril. 1996;65:721-723.
17. Dennerstein L, Lehert P, Burger H. The relative effects of hormones and relationship factors on sexual function of women through the natural menopausal transition. Fertil Steril. 2005;84:174-180.
18. Vermeulen A. The hormonal activity of the postmenopausal ovary. J Clin Endocrinol Metab. 1976;42:247-253.
19. Sarrel P, Dobay B, Wiita B. Estrogen and estrogen–androgen replacement in postmenopausal women dissatisfied with estrogen-only therapy. Sexual behavior and neuroendocrine responses. J Reprod Med. 1998;43:847-856.
20. Braunstein GD. Androgen insufficiency in women: summary of critical issues. Fertil Steril. 2002;77(suppl 4):S94-S99.
MENOPAUSE
I am delighted that Dr. Michael McClung, an internationally recognized expert in skeletal health, has agreed to review current evidence on the prevention of osteoporotic fractures in menopausal women in the latter part of this article.
New WHI analysis confirms safety of short-term combination HT
Anderson GL, Chlebowski RT, Rossouw JE, et al. Prior hormone therapy and breast cancer risk in the Women’s Health Initiative randomized trial of estrogen and progestin. Maturitas. 2006;55:103–115.
At the annual San Antonio Breast Cancer Symposium in December, investigators presented data showing that the incidence of breast cancer in US women decreased by 7% from 2002 to 2003, a striking decline that was most prominent among women aged 50 to 69 years. The presenters speculated that the plummeting rates of HT use following publication of the initial Women’s Health Initiative (WHI) findings in the summer of 2002 (in regard to the estrogen–progestin arm2) might be responsible for this decline.3
The major media attention that followed this presentation makes one thing clear: Concerns about developing breast cancer with HT use continue to fuel anxiety among women. Although secular trend data on the national breast cancer incidence can help generate hypotheses, they cannot explain the trends. What can shed light on the association between estrogen–progestin HT and breast cancer are important new data recently released by WHI investigators.
Women new to HT had no increased risk of breast cancer
In the 2006 subgroup analysis of WHI participants in the estrogen–progestin arm, investigators focused on HT use before enrollment in the trial. Recall that in this part of the WHI, 16,608 women with an intact uterus were randomized to conjugated equine estrogen plus medroxyprogesterone acetate or placebo. Use of the study medication was stopped after a mean follow-up of 5.6 years (mean exposure to HT: 4.4 years). Overall, the risk of invasive breast cancer was slightly higher with combination HT than placebo (hazard ratio [HR] 1.24; 95% confidence interval [CI] 1.01–1.54).2
In the 2006 report from the 2002 WHI study of estrogen–progestin HT versus placebo, investigators compared the risk of being diagnosed with breast cancer in 12,297 women who had not used HT prior to study enrollment with the risk in 4,311 participants who had previously used HT. Of the previous users, 42% reported less than 2 years of use prior to WHI enrollment, and 36% reported more than 4 years of HT prior to WHI enrollment.
The findings: Among WHI participants who had never before used HT, the use of estrogen–progestin HT in the study was not associated with an elevated risk of being diagnosed with breast cancer (HR 1.02; 95% CI 0.77–1.36). However, among previous HT users, the additional use of HT in the WHI study was associated with a risk nearly double that of placebo users (HR 1.96, 95% CI 1.17–3.27).
The reassuring results of this WHI subgroup analysis received little media attention in the United States, probably because the report appeared in a journal that has low readership in this country. WHI and other findings allow us to reassure women who have undergone hysterectomy that use of unopposed estrogen has little, if any, impact on breast cancer risk in menopausal women.4,5 This new WHI subgroup analysis, along with a recent review of European and North American data,6 allows ObGyns to counsel women with an intact uterus that up to 5 years of combination estrogen–progestin hormone therapy also has little, if any, impact on breast cancer risk.
Not much to recommend among nonhormonal therapies
Newton KM, Reed SD, LaCroix AZ, Grothaus LC, Ehrlich K, Guiltinan J. Treatment of vasomotor symptoms of menopause with black cohosh, multibotanicals, soy, hormone therapy or placebo. Ann Intern Med. 2006;145:869–879.
Grady D. Clinical practice. Management of menopausal symptoms. N Engl J Med. 2006;355:2338–2347.
Grady D, Cohen B, Tice J, et al. Ineffectiveness of sertraline for treatment of menopausal hot flushes: a randomized controlled trial. Obstet Gynecol. 2007;109:823–830.
Loprinzi CL, Kugler JW, Barton DL, et al. Phase III trial of gabapentin alone or in conjunction with an antidepressant in the management of hot flashes in women who have inadequate control with an antidepressant alone: NCCTG N03C5. J Clin Oncol. 2007;25:308–312.
Since publication of the initial WHI findings in 2002,2 interest in nonhormonal management of vasomotor symptoms has increased among menopausal women and their clinicians. The botanical black cohosh and “nutraceutical” soy or isoflavone supplements represent the nonprescription remedies most widely used for relief of hot flashes. Unfortunately, accumulating evidence does not support the efficacy of these popular remedies.
In a recent NIH-funded, randomized, double-blind, placebo-controlled clinical trial, Newton and colleagues compared the following interventions:
- black cohosh, 160 mg daily
- daily multibotanical supplement that included 200 mg of black cohosh and 9 other ingredients
- the multibotanical supplement plus counseling regarding dietary soy
- conjugated equine estrogen, 0.625 mg daily (with or without 2.5 mg of medroxyprogesterone acetate)
- placebo
The findings: At 3, 6, and 12 months, women allocated to estrogen (with or without progestin) had statistically significant relief of symptoms. In contrast, women allocated to botanical and/or herbal supplements experienced minimal relief, comparable to the effects of placebo.
The findings of this important study, as well as those of Grady, are discouraging: Black cohosh, botanicals, and encouraging increased soy intake are ineffective in the treatment of vasomotor symptoms.
Evidence on antidepressants is inconclusive
Selective serotonin reuptake inhibitors (SSRIs) and the antidepressant venlafaxine have been assessed for their effects on menopausal vasomotor symptoms, particularly in breast cancer survivors. In a recent review and also a randomized trial, Grady reports that the SSRIs citalopram and sertraline do not appear to be effective, and the findings in regard to the SSRI fluoxetine and venlafaxine have been inconsistent. Compared with placebo, the SSRI paroxetine has eased vasomotor symptoms to a modest degree in breast cancer survivors, but had little effect in women who have not had the disease.
Breast cancer survivors often take tamoxifen or aromatase inhibitors, medications that can induce or aggravate hot flashes. Breast cancer survivors also have a higher prevalence of mood disorders. These factors suggest that the experience and treatment of menopausal symptoms differ between breast cancer survivors and the general population.
Overall, Grady notes, for women with bothersome vasomotor symptoms who have no history of breast cancer, clinical trials of antidepressants have not been encouraging.
Gabapentin is more effective than antidepressants, but with a price
Clinical trials of gabapentin suggest that this anticonvulsant is moderately effective in the nonhormonal treatment of vasomotor symptoms, and the phase III trial by Loprinzi and colleagues finds it to be more effective therapy for vasomotor symptoms than antidepressants.
The drawback? This drug must be taken 2 or 3 times daily, and side effects (including fatigue) limit its attractiveness.
When deciding whom to treat, consider risk as well as BMD
Sanders KM, Nicholson GC, Watts JJ, et al. Half the burden of fragility fractures in the community occur in women without osteoporosis. When is fracture prevention cost-effective? Bone. 2006;38:694–700.
The diagnosis of osteoporosis in postmenopausal women is now based on a threshold bone mineral density (BMD) T-score of –2.5. However, BMD is only one of several important risk factors for fracture, and most patients who experience a fracture related to osteoporosis do not have BMD values in the range consistent with osteoporosis, as Sanders and colleagues observe. Therefore, clinicians are faced with this question: Which patients who do not have osteoporosis should be treated to prevent fracture?
The World Health Organization (WHO) task force on fracture risk assessment, under the leadership of Professor John Kanis, has developed an algorithm to estimate fracture probability in individual patients.7 This algorithm is based on a sophisticated analysis of almost all of the large epidemiological studies performed worldwide that have assessed relationships between clinical risk factors and fracture risk. By including the 3 major risk factors (age, BMD, and fracture history), as well as weaker risk factors (family history of hip fracture, current smoking, excess alcohol intake, and history of chronic glucocorticoid use), the absolute risk of developing a fracture of the spine, wrist, hip, or shoulder over the next 10 years will be estimated. This information will be the basis for revised guidelines by the National Osteoporosis Foundation (NOF) and other organizations. The new guidelines will include recommendations for treating patients at or above a certain threshold of fracture risk rather than a certain BMD threshold. The new treatment threshold will be based on a combination of cost- and clinical effectiveness.
The WHO algorithm and revised NOF guidelines are expected later this year.
New paradigm will shift focus to older women
This revised approach will shift the focus of therapy from young postmenopausal women at low fracture risk toward older women who do not have osteoporosis but do have an increased risk of fracture by virtue of their age and other factors.8 This will direct therapy more appropriately to patients who stand to gain the most and in whom therapy has been proven to reduce fracture risk.
Despite concerns, bisphosphonates appear to be safe for the long term
Bisphosphonates are the most extensively studied and widely used treatment for osteoporosis. Alendronate, the first bisphosphonate approved for the treatment of osteoporosis in the United States, has been available for more than 11 years. In general, all 3 of the currently approved bisphosphonates are well tolerated, and studies following patients for 7 to 10 years have not demonstrated significant adverse events or evidence of skeletal harm with long-term use.9-11 However, because the drugs accumulate in the skeleton, there is a theoretical concern that long-term use will lead to over-suppression of bone turnover.
Small series of patients receiving bisphosphonates have described unusual fractures, evidence of low formation, and poor fracture healing, suggesting skeletal harm in at least some patients.12 Bone biopsies performed in patients who received alendronate for 10 years or risedronate for 5 years showed evidence of bone remodeling in all the biopsy samples.11,13 There was no progressive inhibition of bone metabolism in those biopsies compared with biopsies taken from patients who had received shorter-term treatment.
These findings are consistent with bone-turnover marker data suggesting no progressive suppression of bone turnover with continued use.10,11,13 Biochemical indices of bone resorption are reduced to the lower half of the normal premenopausal range within about 3 months of beginning therapy, and values remain at that new level as long as patients receive the drug.
Risk of osteonecrosis of the jaw is low in general population
Woo SB, Hellstein JW, Kalmar JR. Narrative review: bisphosphonates and osteonecrosis of the jaws. Ann Intern Med. 2006;144:753–761.
Bilezikian JP. Osteonecrosis of the jaw—do bisphosphonates pose a risk? N Engl J Med. 2006;355:2278–2281.
An association between bisphosphonate therapy and nonhealing lesions of the jaw (so-called osteonecrosis of the jaw) has been observed, but primarily affects patients with cancer-related bone diseases who receive high doses of intravenous therapy in addition to chemotherapy. Patients receiving oral doses of bisphosphonates for osteoporosis in Paget’s disease have also had these lesions, as Woo and colleagues point out.
There is much that we do not know about this clinical problem, including its pathogenesis, whether the risk increases with longer-term use, and whether stopping therapy reduces the risk of developing lesions or improves the outcome of lesions already present. We do know that the incidence of exposed bone in the jaw in patients receiving bisphosphonate therapy for osteoporosis is low, estimated to range from 1 in 1,000 to 1 in 100,000 patients, according to Bilezikian.
Risk is very small, compared with potential benefits
It is important to put this risk in perspective. Based on data from the alendronate Fracture Intervention Trials (FIT), we have estimates of hip and spine fracture risk in certain types of patients. For example, for women age 68 with a femoral neck T-score of –2.5 or lower and no vertebral fractures, the likelihood of a clinical fracture over a mean treatment interval of 4.2 years was 19.6%.14 In women age 71 with a femoral neck T-score of –2.5 and 1 or more vertebral fractures, spine and hip fractures occurred in 15% and 2.2% of subjects, respectively, over 2.9 years.15 In these populations, alendronate reduced the risk of both hip and spine fracture by about 50%. For women without a vertebral fracture, the absolute reduction in the risk of clinical fracture over 4.2 years was 6.5% (number needed to treat [NNT]=15). In patients with a vertebral fracture, the absolute reduction in the incidence of further spine and hip fracture was 8.6% over 2.9 years (NNT=12).
This information argues strongly that the concern about osteonecrosis of the jaw does not justify withholding bisphosphonate therapy from patients with osteoporosis. The risk of such lesions in otherwise healthy patients with osteoporosis is very low (much lower than the risk of fracture), and most lesions heal spontaneously when treatment is stopped.
Clinical recommendations
For patients using or considering bisphosphonate therapy for osteoporosis, the following measures may be helpful:
- Have regular dental checkups and routine preventive dental care.
- If invasive dental procedures are planned, such as tooth extraction or implants, complete the dental work and allow the bone to heal before beginning bisphosphonate therapy.
- If a patient on bisphosphonate therapy plans invasive dental work, stop treatment for 3 months before the procedure and do not restart it until the jaw lesion is healed. Although there is no firm evidence that this strategy is helpful, it is certain that discontinuing bisphosphonate for a few months does not harm the skeleton.
- If a patient on bisphosphonate develops exposed bone, stop the drug and consult a dentist or oral surgeon experienced in the care of these lesions.
Some can take a holiday from bisphosphonate therapy
Black DM, Schwartz AV, Ensrud KE, et al, for the FLEX Research Group. Effects of continuing or stopping alendronate after 5 years of treatment: the Fracture Intervention Trial Long-Term Extension (FLEX): a randomized trial. JAMA. 2006;296:2927–2938.
There is evidence that bone metabolism continues to be affected for some time when alendronate is stopped after 2 to 5 years of treatment, as Black and colleagues found in the Fracture Intervention Trial Long-Term Extension (FLEX) and others have demonstrated.10 This raises the possibility that patients can take a “drug holiday” after several years of treatment. (This study was also reported in the March issue of OBG Management in “Examining the Evidence,” with a commentary by Steven R. Goldstein, MD.)
The FLEX trial attempted to determine whether it is better to continue or stop alendronate after several years’ exposure. One thousand ninety-nine women who had taken alendronate for 3 to 6 years in the FIT trials were randomly assigned to 5 or 10 mg of alendronate daily or placebo. All subjects received 500 mg of calcium and small vitamin D supplements and were followed for an additional 5 years.
The 2 alendronate groups were pooled for the analyses. Patients who switched to placebo for 5 years had declines in BMD at the total hip (2.4%) and spine (3.7%), compared with those who continued alendronate. However, values at the end of 5 years without therapy remained at or above pretreatment levels. Indices of bone turnover increased modestly when therapy was discontinued, but again the rates of bone turnover remained substantially lower than pretreatment values.
Fractures were collected as an exploratory endpoint. Compared with women who stopped treatment, women who continued alendronate reduced their risk of developing a clinical vertebral fracture by 55% (from 5.3% in the placebo group to 2.4% in the alendronate group). No difference was observed in the incidence of nonvertebral fractures between the 2 groups.
Who should take a holiday, and who can stay put?
Unfortunately, this study does not clearly answer the question. Patients at high risk for spine fracture, including those with a previous fracture, appeared to fare better if they continued treatment. Patients at lower risk did equally well whether they stopped or continued alendronate. This suggests that it would be appropriate to stop treatment in women who are not at high risk, including women who do not have osteoporosis by BMD criteria and have not experienced a fragility fracture since menopause.
The reason for stopping therapy in patients at low risk is because there was no added benefit observed with continued treatment, not because of concerns about risk.
When should the holiday end?
If treatment is stopped, the clinical question of whether and when to restart treatment becomes a challenge. The changes in bone density after treatment is stopped are too small to discern in individual patients. In theory, monitoring one or more bone-turnover markers is a more sensitive way to determine when the effects of bisphosphonates on skeletal remodeling are dissipating, but this approach is backed by very little clinical experience.
Another unresolved issue is whether the response after stopping treatment is the same in patients taking risedronate, ibandronate, or lower doses of alendronate.
1. Kaunitz AM. Update on menopause. OBG Management. 2006;18(5):45-54.
2. Rossouw JE, Anderson GL, Prentice RL, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women. Principal results from the Women’s Health Initiative. Writing Group for the Women’s Health Initiative. JAMA. 2002;288:321-333.
3. Berry D, et al. Presented at the 29th annual San Antonio Breast Cancer Symposium, December 14, 2006, San Antonio, Tex.
4. Anderson GL, Limacher M, Assaf AR, et al. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women’s Health Initiative randomized controlled trial. Women’s Health Initiative Steering Committee. JAMA. 2004;291:1701-1712.
5. Kaunitz AM. Hormone therapy and breast cancer risk—trumping fear with facts. Menopause. 2006;13:160-163.
6. Collins JA, Blake JM, Crosignani PE. Breast cancer risk with postmenopausal hormonal treatment. Hum Reprod Update. 2005;11:545-560.
7. de Laet C, Oden A, Johansson H, Johnell O, Jonsson B, Kanis JA. The impact of the use of multiple risk indicators for fracture on case-finding strategies: a mathematical approach. Osteoporosis Int. 2005;16:313-318.
8. McClung MR. Do current management strategies and guidelines adequately address fracture risk? Bone. 2006;38(Suppl 2):S13-S17.
9. Mellstrom DD, Sorensen OH, Goemaere S, Roux C, Johnson TD, Chines AA. Seven years of treatment with risedronate in women with postmenopausal osteoporosis. Calcif Tissue Int. 2004;75:462-468.
10. Bone HG, Hosking D, Devogelaer JP, et al. For the Alendronate Phase III Osteoporosis Treatment Study Group. Ten years’ experience with alendronate for osteoporosis in postmenopausal women. N Engl J Med. 2004;350:1189-1199.
11. Black DM, Schwartz AV, Ensrud KE, et al. For the FLEX Research Group. Effects of continuing or stopping alendronate after 5 years of treatment: the Fracture Intervension Trial Long-term Extension (FLEX): a randomized trial. JAMA. 2006;296:2927-2938.
12. Odvina CV, Zerwekh JE, Rao DS, Maalouf N, Gottschalk FA, Pak CY. Severely suppressed bone turnover: a potential complication of alendronate therapy. J Clin Edocrinol Metab. 2005;90:1294-1301.
13. Ste-Marie LG, Sod E, Johnson T, Chines A. Five years of treatment with risedronate and its effects on bone safety in women with postmenopausal osteoporosis. Calcif Tissue Int. 2004;75:469-476.
14. Cummings SR, Black DM, Thompson DE, et al. Effect of alendronate on risk of fracture in women with low bone density but without vertebral fractures: results from the Fracture Intervention Trial. JAMA. 1998;280:2077-2082.
15. Black DM, Cummings SR, Karpf DB, et al. Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Lancet. 1996;348:1535-1541.
Dr. Kaunitz has received funding from Barr Laboratories, Berlex, Medical Diagnostic Laboratories, Organon, and Warner Chilcott. He is a speaker or consultant for the American College of Obstetricians and Gynecologists, Barr Laboratories, Berlex, Johnson & Johnson, Merck, Noven Organon, and Warner-Chilcott. He holds stock with Barr, Johnson & Johnson, Procter & Gamble, Roche, and Sanofi-Aventis.
Dr. McClung receives grant/research support from and is a consultant to Amgen, Lilly, Merck, Novartis, Proctor & Gamble, Roche, and Sanofi-Aventis. He is a speaker for Lilly, Merck, Procter & Gamble, and Sanofi-Aventis.
I am delighted that Dr. Michael McClung, an internationally recognized expert in skeletal health, has agreed to review current evidence on the prevention of osteoporotic fractures in menopausal women in the latter part of this article.
New WHI analysis confirms safety of short-term combination HT
Anderson GL, Chlebowski RT, Rossouw JE, et al. Prior hormone therapy and breast cancer risk in the Women’s Health Initiative randomized trial of estrogen and progestin. Maturitas. 2006;55:103–115.
At the annual San Antonio Breast Cancer Symposium in December, investigators presented data showing that the incidence of breast cancer in US women decreased by 7% from 2002 to 2003, a striking decline that was most prominent among women aged 50 to 69 years. The presenters speculated that the plummeting rates of HT use following publication of the initial Women’s Health Initiative (WHI) findings in the summer of 2002 (in regard to the estrogen–progestin arm2) might be responsible for this decline.3
The major media attention that followed this presentation makes one thing clear: Concerns about developing breast cancer with HT use continue to fuel anxiety among women. Although secular trend data on the national breast cancer incidence can help generate hypotheses, they cannot explain the trends. What can shed light on the association between estrogen–progestin HT and breast cancer are important new data recently released by WHI investigators.
Women new to HT had no increased risk of breast cancer
In the 2006 subgroup analysis of WHI participants in the estrogen–progestin arm, investigators focused on HT use before enrollment in the trial. Recall that in this part of the WHI, 16,608 women with an intact uterus were randomized to conjugated equine estrogen plus medroxyprogesterone acetate or placebo. Use of the study medication was stopped after a mean follow-up of 5.6 years (mean exposure to HT: 4.4 years). Overall, the risk of invasive breast cancer was slightly higher with combination HT than placebo (hazard ratio [HR] 1.24; 95% confidence interval [CI] 1.01–1.54).2
In the 2006 report from the 2002 WHI study of estrogen–progestin HT versus placebo, investigators compared the risk of being diagnosed with breast cancer in 12,297 women who had not used HT prior to study enrollment with the risk in 4,311 participants who had previously used HT. Of the previous users, 42% reported less than 2 years of use prior to WHI enrollment, and 36% reported more than 4 years of HT prior to WHI enrollment.
The findings: Among WHI participants who had never before used HT, the use of estrogen–progestin HT in the study was not associated with an elevated risk of being diagnosed with breast cancer (HR 1.02; 95% CI 0.77–1.36). However, among previous HT users, the additional use of HT in the WHI study was associated with a risk nearly double that of placebo users (HR 1.96, 95% CI 1.17–3.27).
The reassuring results of this WHI subgroup analysis received little media attention in the United States, probably because the report appeared in a journal that has low readership in this country. WHI and other findings allow us to reassure women who have undergone hysterectomy that use of unopposed estrogen has little, if any, impact on breast cancer risk in menopausal women.4,5 This new WHI subgroup analysis, along with a recent review of European and North American data,6 allows ObGyns to counsel women with an intact uterus that up to 5 years of combination estrogen–progestin hormone therapy also has little, if any, impact on breast cancer risk.
Not much to recommend among nonhormonal therapies
Newton KM, Reed SD, LaCroix AZ, Grothaus LC, Ehrlich K, Guiltinan J. Treatment of vasomotor symptoms of menopause with black cohosh, multibotanicals, soy, hormone therapy or placebo. Ann Intern Med. 2006;145:869–879.
Grady D. Clinical practice. Management of menopausal symptoms. N Engl J Med. 2006;355:2338–2347.
Grady D, Cohen B, Tice J, et al. Ineffectiveness of sertraline for treatment of menopausal hot flushes: a randomized controlled trial. Obstet Gynecol. 2007;109:823–830.
Loprinzi CL, Kugler JW, Barton DL, et al. Phase III trial of gabapentin alone or in conjunction with an antidepressant in the management of hot flashes in women who have inadequate control with an antidepressant alone: NCCTG N03C5. J Clin Oncol. 2007;25:308–312.
Since publication of the initial WHI findings in 2002,2 interest in nonhormonal management of vasomotor symptoms has increased among menopausal women and their clinicians. The botanical black cohosh and “nutraceutical” soy or isoflavone supplements represent the nonprescription remedies most widely used for relief of hot flashes. Unfortunately, accumulating evidence does not support the efficacy of these popular remedies.
In a recent NIH-funded, randomized, double-blind, placebo-controlled clinical trial, Newton and colleagues compared the following interventions:
- black cohosh, 160 mg daily
- daily multibotanical supplement that included 200 mg of black cohosh and 9 other ingredients
- the multibotanical supplement plus counseling regarding dietary soy
- conjugated equine estrogen, 0.625 mg daily (with or without 2.5 mg of medroxyprogesterone acetate)
- placebo
The findings: At 3, 6, and 12 months, women allocated to estrogen (with or without progestin) had statistically significant relief of symptoms. In contrast, women allocated to botanical and/or herbal supplements experienced minimal relief, comparable to the effects of placebo.
The findings of this important study, as well as those of Grady, are discouraging: Black cohosh, botanicals, and encouraging increased soy intake are ineffective in the treatment of vasomotor symptoms.
Evidence on antidepressants is inconclusive
Selective serotonin reuptake inhibitors (SSRIs) and the antidepressant venlafaxine have been assessed for their effects on menopausal vasomotor symptoms, particularly in breast cancer survivors. In a recent review and also a randomized trial, Grady reports that the SSRIs citalopram and sertraline do not appear to be effective, and the findings in regard to the SSRI fluoxetine and venlafaxine have been inconsistent. Compared with placebo, the SSRI paroxetine has eased vasomotor symptoms to a modest degree in breast cancer survivors, but had little effect in women who have not had the disease.
Breast cancer survivors often take tamoxifen or aromatase inhibitors, medications that can induce or aggravate hot flashes. Breast cancer survivors also have a higher prevalence of mood disorders. These factors suggest that the experience and treatment of menopausal symptoms differ between breast cancer survivors and the general population.
Overall, Grady notes, for women with bothersome vasomotor symptoms who have no history of breast cancer, clinical trials of antidepressants have not been encouraging.
Gabapentin is more effective than antidepressants, but with a price
Clinical trials of gabapentin suggest that this anticonvulsant is moderately effective in the nonhormonal treatment of vasomotor symptoms, and the phase III trial by Loprinzi and colleagues finds it to be more effective therapy for vasomotor symptoms than antidepressants.
The drawback? This drug must be taken 2 or 3 times daily, and side effects (including fatigue) limit its attractiveness.
When deciding whom to treat, consider risk as well as BMD
Sanders KM, Nicholson GC, Watts JJ, et al. Half the burden of fragility fractures in the community occur in women without osteoporosis. When is fracture prevention cost-effective? Bone. 2006;38:694–700.
The diagnosis of osteoporosis in postmenopausal women is now based on a threshold bone mineral density (BMD) T-score of –2.5. However, BMD is only one of several important risk factors for fracture, and most patients who experience a fracture related to osteoporosis do not have BMD values in the range consistent with osteoporosis, as Sanders and colleagues observe. Therefore, clinicians are faced with this question: Which patients who do not have osteoporosis should be treated to prevent fracture?
The World Health Organization (WHO) task force on fracture risk assessment, under the leadership of Professor John Kanis, has developed an algorithm to estimate fracture probability in individual patients.7 This algorithm is based on a sophisticated analysis of almost all of the large epidemiological studies performed worldwide that have assessed relationships between clinical risk factors and fracture risk. By including the 3 major risk factors (age, BMD, and fracture history), as well as weaker risk factors (family history of hip fracture, current smoking, excess alcohol intake, and history of chronic glucocorticoid use), the absolute risk of developing a fracture of the spine, wrist, hip, or shoulder over the next 10 years will be estimated. This information will be the basis for revised guidelines by the National Osteoporosis Foundation (NOF) and other organizations. The new guidelines will include recommendations for treating patients at or above a certain threshold of fracture risk rather than a certain BMD threshold. The new treatment threshold will be based on a combination of cost- and clinical effectiveness.
The WHO algorithm and revised NOF guidelines are expected later this year.
New paradigm will shift focus to older women
This revised approach will shift the focus of therapy from young postmenopausal women at low fracture risk toward older women who do not have osteoporosis but do have an increased risk of fracture by virtue of their age and other factors.8 This will direct therapy more appropriately to patients who stand to gain the most and in whom therapy has been proven to reduce fracture risk.
Despite concerns, bisphosphonates appear to be safe for the long term
Bisphosphonates are the most extensively studied and widely used treatment for osteoporosis. Alendronate, the first bisphosphonate approved for the treatment of osteoporosis in the United States, has been available for more than 11 years. In general, all 3 of the currently approved bisphosphonates are well tolerated, and studies following patients for 7 to 10 years have not demonstrated significant adverse events or evidence of skeletal harm with long-term use.9-11 However, because the drugs accumulate in the skeleton, there is a theoretical concern that long-term use will lead to over-suppression of bone turnover.
Small series of patients receiving bisphosphonates have described unusual fractures, evidence of low formation, and poor fracture healing, suggesting skeletal harm in at least some patients.12 Bone biopsies performed in patients who received alendronate for 10 years or risedronate for 5 years showed evidence of bone remodeling in all the biopsy samples.11,13 There was no progressive inhibition of bone metabolism in those biopsies compared with biopsies taken from patients who had received shorter-term treatment.
These findings are consistent with bone-turnover marker data suggesting no progressive suppression of bone turnover with continued use.10,11,13 Biochemical indices of bone resorption are reduced to the lower half of the normal premenopausal range within about 3 months of beginning therapy, and values remain at that new level as long as patients receive the drug.
Risk of osteonecrosis of the jaw is low in general population
Woo SB, Hellstein JW, Kalmar JR. Narrative review: bisphosphonates and osteonecrosis of the jaws. Ann Intern Med. 2006;144:753–761.
Bilezikian JP. Osteonecrosis of the jaw—do bisphosphonates pose a risk? N Engl J Med. 2006;355:2278–2281.
An association between bisphosphonate therapy and nonhealing lesions of the jaw (so-called osteonecrosis of the jaw) has been observed, but primarily affects patients with cancer-related bone diseases who receive high doses of intravenous therapy in addition to chemotherapy. Patients receiving oral doses of bisphosphonates for osteoporosis in Paget’s disease have also had these lesions, as Woo and colleagues point out.
There is much that we do not know about this clinical problem, including its pathogenesis, whether the risk increases with longer-term use, and whether stopping therapy reduces the risk of developing lesions or improves the outcome of lesions already present. We do know that the incidence of exposed bone in the jaw in patients receiving bisphosphonate therapy for osteoporosis is low, estimated to range from 1 in 1,000 to 1 in 100,000 patients, according to Bilezikian.
Risk is very small, compared with potential benefits
It is important to put this risk in perspective. Based on data from the alendronate Fracture Intervention Trials (FIT), we have estimates of hip and spine fracture risk in certain types of patients. For example, for women age 68 with a femoral neck T-score of –2.5 or lower and no vertebral fractures, the likelihood of a clinical fracture over a mean treatment interval of 4.2 years was 19.6%.14 In women age 71 with a femoral neck T-score of –2.5 and 1 or more vertebral fractures, spine and hip fractures occurred in 15% and 2.2% of subjects, respectively, over 2.9 years.15 In these populations, alendronate reduced the risk of both hip and spine fracture by about 50%. For women without a vertebral fracture, the absolute reduction in the risk of clinical fracture over 4.2 years was 6.5% (number needed to treat [NNT]=15). In patients with a vertebral fracture, the absolute reduction in the incidence of further spine and hip fracture was 8.6% over 2.9 years (NNT=12).
This information argues strongly that the concern about osteonecrosis of the jaw does not justify withholding bisphosphonate therapy from patients with osteoporosis. The risk of such lesions in otherwise healthy patients with osteoporosis is very low (much lower than the risk of fracture), and most lesions heal spontaneously when treatment is stopped.
Clinical recommendations
For patients using or considering bisphosphonate therapy for osteoporosis, the following measures may be helpful:
- Have regular dental checkups and routine preventive dental care.
- If invasive dental procedures are planned, such as tooth extraction or implants, complete the dental work and allow the bone to heal before beginning bisphosphonate therapy.
- If a patient on bisphosphonate therapy plans invasive dental work, stop treatment for 3 months before the procedure and do not restart it until the jaw lesion is healed. Although there is no firm evidence that this strategy is helpful, it is certain that discontinuing bisphosphonate for a few months does not harm the skeleton.
- If a patient on bisphosphonate develops exposed bone, stop the drug and consult a dentist or oral surgeon experienced in the care of these lesions.
Some can take a holiday from bisphosphonate therapy
Black DM, Schwartz AV, Ensrud KE, et al, for the FLEX Research Group. Effects of continuing or stopping alendronate after 5 years of treatment: the Fracture Intervention Trial Long-Term Extension (FLEX): a randomized trial. JAMA. 2006;296:2927–2938.
There is evidence that bone metabolism continues to be affected for some time when alendronate is stopped after 2 to 5 years of treatment, as Black and colleagues found in the Fracture Intervention Trial Long-Term Extension (FLEX) and others have demonstrated.10 This raises the possibility that patients can take a “drug holiday” after several years of treatment. (This study was also reported in the March issue of OBG Management in “Examining the Evidence,” with a commentary by Steven R. Goldstein, MD.)
The FLEX trial attempted to determine whether it is better to continue or stop alendronate after several years’ exposure. One thousand ninety-nine women who had taken alendronate for 3 to 6 years in the FIT trials were randomly assigned to 5 or 10 mg of alendronate daily or placebo. All subjects received 500 mg of calcium and small vitamin D supplements and were followed for an additional 5 years.
The 2 alendronate groups were pooled for the analyses. Patients who switched to placebo for 5 years had declines in BMD at the total hip (2.4%) and spine (3.7%), compared with those who continued alendronate. However, values at the end of 5 years without therapy remained at or above pretreatment levels. Indices of bone turnover increased modestly when therapy was discontinued, but again the rates of bone turnover remained substantially lower than pretreatment values.
Fractures were collected as an exploratory endpoint. Compared with women who stopped treatment, women who continued alendronate reduced their risk of developing a clinical vertebral fracture by 55% (from 5.3% in the placebo group to 2.4% in the alendronate group). No difference was observed in the incidence of nonvertebral fractures between the 2 groups.
Who should take a holiday, and who can stay put?
Unfortunately, this study does not clearly answer the question. Patients at high risk for spine fracture, including those with a previous fracture, appeared to fare better if they continued treatment. Patients at lower risk did equally well whether they stopped or continued alendronate. This suggests that it would be appropriate to stop treatment in women who are not at high risk, including women who do not have osteoporosis by BMD criteria and have not experienced a fragility fracture since menopause.
The reason for stopping therapy in patients at low risk is because there was no added benefit observed with continued treatment, not because of concerns about risk.
When should the holiday end?
If treatment is stopped, the clinical question of whether and when to restart treatment becomes a challenge. The changes in bone density after treatment is stopped are too small to discern in individual patients. In theory, monitoring one or more bone-turnover markers is a more sensitive way to determine when the effects of bisphosphonates on skeletal remodeling are dissipating, but this approach is backed by very little clinical experience.
Another unresolved issue is whether the response after stopping treatment is the same in patients taking risedronate, ibandronate, or lower doses of alendronate.
I am delighted that Dr. Michael McClung, an internationally recognized expert in skeletal health, has agreed to review current evidence on the prevention of osteoporotic fractures in menopausal women in the latter part of this article.
New WHI analysis confirms safety of short-term combination HT
Anderson GL, Chlebowski RT, Rossouw JE, et al. Prior hormone therapy and breast cancer risk in the Women’s Health Initiative randomized trial of estrogen and progestin. Maturitas. 2006;55:103–115.
At the annual San Antonio Breast Cancer Symposium in December, investigators presented data showing that the incidence of breast cancer in US women decreased by 7% from 2002 to 2003, a striking decline that was most prominent among women aged 50 to 69 years. The presenters speculated that the plummeting rates of HT use following publication of the initial Women’s Health Initiative (WHI) findings in the summer of 2002 (in regard to the estrogen–progestin arm2) might be responsible for this decline.3
The major media attention that followed this presentation makes one thing clear: Concerns about developing breast cancer with HT use continue to fuel anxiety among women. Although secular trend data on the national breast cancer incidence can help generate hypotheses, they cannot explain the trends. What can shed light on the association between estrogen–progestin HT and breast cancer are important new data recently released by WHI investigators.
Women new to HT had no increased risk of breast cancer
In the 2006 subgroup analysis of WHI participants in the estrogen–progestin arm, investigators focused on HT use before enrollment in the trial. Recall that in this part of the WHI, 16,608 women with an intact uterus were randomized to conjugated equine estrogen plus medroxyprogesterone acetate or placebo. Use of the study medication was stopped after a mean follow-up of 5.6 years (mean exposure to HT: 4.4 years). Overall, the risk of invasive breast cancer was slightly higher with combination HT than placebo (hazard ratio [HR] 1.24; 95% confidence interval [CI] 1.01–1.54).2
In the 2006 report from the 2002 WHI study of estrogen–progestin HT versus placebo, investigators compared the risk of being diagnosed with breast cancer in 12,297 women who had not used HT prior to study enrollment with the risk in 4,311 participants who had previously used HT. Of the previous users, 42% reported less than 2 years of use prior to WHI enrollment, and 36% reported more than 4 years of HT prior to WHI enrollment.
The findings: Among WHI participants who had never before used HT, the use of estrogen–progestin HT in the study was not associated with an elevated risk of being diagnosed with breast cancer (HR 1.02; 95% CI 0.77–1.36). However, among previous HT users, the additional use of HT in the WHI study was associated with a risk nearly double that of placebo users (HR 1.96, 95% CI 1.17–3.27).
The reassuring results of this WHI subgroup analysis received little media attention in the United States, probably because the report appeared in a journal that has low readership in this country. WHI and other findings allow us to reassure women who have undergone hysterectomy that use of unopposed estrogen has little, if any, impact on breast cancer risk in menopausal women.4,5 This new WHI subgroup analysis, along with a recent review of European and North American data,6 allows ObGyns to counsel women with an intact uterus that up to 5 years of combination estrogen–progestin hormone therapy also has little, if any, impact on breast cancer risk.
Not much to recommend among nonhormonal therapies
Newton KM, Reed SD, LaCroix AZ, Grothaus LC, Ehrlich K, Guiltinan J. Treatment of vasomotor symptoms of menopause with black cohosh, multibotanicals, soy, hormone therapy or placebo. Ann Intern Med. 2006;145:869–879.
Grady D. Clinical practice. Management of menopausal symptoms. N Engl J Med. 2006;355:2338–2347.
Grady D, Cohen B, Tice J, et al. Ineffectiveness of sertraline for treatment of menopausal hot flushes: a randomized controlled trial. Obstet Gynecol. 2007;109:823–830.
Loprinzi CL, Kugler JW, Barton DL, et al. Phase III trial of gabapentin alone or in conjunction with an antidepressant in the management of hot flashes in women who have inadequate control with an antidepressant alone: NCCTG N03C5. J Clin Oncol. 2007;25:308–312.
Since publication of the initial WHI findings in 2002,2 interest in nonhormonal management of vasomotor symptoms has increased among menopausal women and their clinicians. The botanical black cohosh and “nutraceutical” soy or isoflavone supplements represent the nonprescription remedies most widely used for relief of hot flashes. Unfortunately, accumulating evidence does not support the efficacy of these popular remedies.
In a recent NIH-funded, randomized, double-blind, placebo-controlled clinical trial, Newton and colleagues compared the following interventions:
- black cohosh, 160 mg daily
- daily multibotanical supplement that included 200 mg of black cohosh and 9 other ingredients
- the multibotanical supplement plus counseling regarding dietary soy
- conjugated equine estrogen, 0.625 mg daily (with or without 2.5 mg of medroxyprogesterone acetate)
- placebo
The findings: At 3, 6, and 12 months, women allocated to estrogen (with or without progestin) had statistically significant relief of symptoms. In contrast, women allocated to botanical and/or herbal supplements experienced minimal relief, comparable to the effects of placebo.
The findings of this important study, as well as those of Grady, are discouraging: Black cohosh, botanicals, and encouraging increased soy intake are ineffective in the treatment of vasomotor symptoms.
Evidence on antidepressants is inconclusive
Selective serotonin reuptake inhibitors (SSRIs) and the antidepressant venlafaxine have been assessed for their effects on menopausal vasomotor symptoms, particularly in breast cancer survivors. In a recent review and also a randomized trial, Grady reports that the SSRIs citalopram and sertraline do not appear to be effective, and the findings in regard to the SSRI fluoxetine and venlafaxine have been inconsistent. Compared with placebo, the SSRI paroxetine has eased vasomotor symptoms to a modest degree in breast cancer survivors, but had little effect in women who have not had the disease.
Breast cancer survivors often take tamoxifen or aromatase inhibitors, medications that can induce or aggravate hot flashes. Breast cancer survivors also have a higher prevalence of mood disorders. These factors suggest that the experience and treatment of menopausal symptoms differ between breast cancer survivors and the general population.
Overall, Grady notes, for women with bothersome vasomotor symptoms who have no history of breast cancer, clinical trials of antidepressants have not been encouraging.
Gabapentin is more effective than antidepressants, but with a price
Clinical trials of gabapentin suggest that this anticonvulsant is moderately effective in the nonhormonal treatment of vasomotor symptoms, and the phase III trial by Loprinzi and colleagues finds it to be more effective therapy for vasomotor symptoms than antidepressants.
The drawback? This drug must be taken 2 or 3 times daily, and side effects (including fatigue) limit its attractiveness.
When deciding whom to treat, consider risk as well as BMD
Sanders KM, Nicholson GC, Watts JJ, et al. Half the burden of fragility fractures in the community occur in women without osteoporosis. When is fracture prevention cost-effective? Bone. 2006;38:694–700.
The diagnosis of osteoporosis in postmenopausal women is now based on a threshold bone mineral density (BMD) T-score of –2.5. However, BMD is only one of several important risk factors for fracture, and most patients who experience a fracture related to osteoporosis do not have BMD values in the range consistent with osteoporosis, as Sanders and colleagues observe. Therefore, clinicians are faced with this question: Which patients who do not have osteoporosis should be treated to prevent fracture?
The World Health Organization (WHO) task force on fracture risk assessment, under the leadership of Professor John Kanis, has developed an algorithm to estimate fracture probability in individual patients.7 This algorithm is based on a sophisticated analysis of almost all of the large epidemiological studies performed worldwide that have assessed relationships between clinical risk factors and fracture risk. By including the 3 major risk factors (age, BMD, and fracture history), as well as weaker risk factors (family history of hip fracture, current smoking, excess alcohol intake, and history of chronic glucocorticoid use), the absolute risk of developing a fracture of the spine, wrist, hip, or shoulder over the next 10 years will be estimated. This information will be the basis for revised guidelines by the National Osteoporosis Foundation (NOF) and other organizations. The new guidelines will include recommendations for treating patients at or above a certain threshold of fracture risk rather than a certain BMD threshold. The new treatment threshold will be based on a combination of cost- and clinical effectiveness.
The WHO algorithm and revised NOF guidelines are expected later this year.
New paradigm will shift focus to older women
This revised approach will shift the focus of therapy from young postmenopausal women at low fracture risk toward older women who do not have osteoporosis but do have an increased risk of fracture by virtue of their age and other factors.8 This will direct therapy more appropriately to patients who stand to gain the most and in whom therapy has been proven to reduce fracture risk.
Despite concerns, bisphosphonates appear to be safe for the long term
Bisphosphonates are the most extensively studied and widely used treatment for osteoporosis. Alendronate, the first bisphosphonate approved for the treatment of osteoporosis in the United States, has been available for more than 11 years. In general, all 3 of the currently approved bisphosphonates are well tolerated, and studies following patients for 7 to 10 years have not demonstrated significant adverse events or evidence of skeletal harm with long-term use.9-11 However, because the drugs accumulate in the skeleton, there is a theoretical concern that long-term use will lead to over-suppression of bone turnover.
Small series of patients receiving bisphosphonates have described unusual fractures, evidence of low formation, and poor fracture healing, suggesting skeletal harm in at least some patients.12 Bone biopsies performed in patients who received alendronate for 10 years or risedronate for 5 years showed evidence of bone remodeling in all the biopsy samples.11,13 There was no progressive inhibition of bone metabolism in those biopsies compared with biopsies taken from patients who had received shorter-term treatment.
These findings are consistent with bone-turnover marker data suggesting no progressive suppression of bone turnover with continued use.10,11,13 Biochemical indices of bone resorption are reduced to the lower half of the normal premenopausal range within about 3 months of beginning therapy, and values remain at that new level as long as patients receive the drug.
Risk of osteonecrosis of the jaw is low in general population
Woo SB, Hellstein JW, Kalmar JR. Narrative review: bisphosphonates and osteonecrosis of the jaws. Ann Intern Med. 2006;144:753–761.
Bilezikian JP. Osteonecrosis of the jaw—do bisphosphonates pose a risk? N Engl J Med. 2006;355:2278–2281.
An association between bisphosphonate therapy and nonhealing lesions of the jaw (so-called osteonecrosis of the jaw) has been observed, but primarily affects patients with cancer-related bone diseases who receive high doses of intravenous therapy in addition to chemotherapy. Patients receiving oral doses of bisphosphonates for osteoporosis in Paget’s disease have also had these lesions, as Woo and colleagues point out.
There is much that we do not know about this clinical problem, including its pathogenesis, whether the risk increases with longer-term use, and whether stopping therapy reduces the risk of developing lesions or improves the outcome of lesions already present. We do know that the incidence of exposed bone in the jaw in patients receiving bisphosphonate therapy for osteoporosis is low, estimated to range from 1 in 1,000 to 1 in 100,000 patients, according to Bilezikian.
Risk is very small, compared with potential benefits
It is important to put this risk in perspective. Based on data from the alendronate Fracture Intervention Trials (FIT), we have estimates of hip and spine fracture risk in certain types of patients. For example, for women age 68 with a femoral neck T-score of –2.5 or lower and no vertebral fractures, the likelihood of a clinical fracture over a mean treatment interval of 4.2 years was 19.6%.14 In women age 71 with a femoral neck T-score of –2.5 and 1 or more vertebral fractures, spine and hip fractures occurred in 15% and 2.2% of subjects, respectively, over 2.9 years.15 In these populations, alendronate reduced the risk of both hip and spine fracture by about 50%. For women without a vertebral fracture, the absolute reduction in the risk of clinical fracture over 4.2 years was 6.5% (number needed to treat [NNT]=15). In patients with a vertebral fracture, the absolute reduction in the incidence of further spine and hip fracture was 8.6% over 2.9 years (NNT=12).
This information argues strongly that the concern about osteonecrosis of the jaw does not justify withholding bisphosphonate therapy from patients with osteoporosis. The risk of such lesions in otherwise healthy patients with osteoporosis is very low (much lower than the risk of fracture), and most lesions heal spontaneously when treatment is stopped.
Clinical recommendations
For patients using or considering bisphosphonate therapy for osteoporosis, the following measures may be helpful:
- Have regular dental checkups and routine preventive dental care.
- If invasive dental procedures are planned, such as tooth extraction or implants, complete the dental work and allow the bone to heal before beginning bisphosphonate therapy.
- If a patient on bisphosphonate therapy plans invasive dental work, stop treatment for 3 months before the procedure and do not restart it until the jaw lesion is healed. Although there is no firm evidence that this strategy is helpful, it is certain that discontinuing bisphosphonate for a few months does not harm the skeleton.
- If a patient on bisphosphonate develops exposed bone, stop the drug and consult a dentist or oral surgeon experienced in the care of these lesions.
Some can take a holiday from bisphosphonate therapy
Black DM, Schwartz AV, Ensrud KE, et al, for the FLEX Research Group. Effects of continuing or stopping alendronate after 5 years of treatment: the Fracture Intervention Trial Long-Term Extension (FLEX): a randomized trial. JAMA. 2006;296:2927–2938.
There is evidence that bone metabolism continues to be affected for some time when alendronate is stopped after 2 to 5 years of treatment, as Black and colleagues found in the Fracture Intervention Trial Long-Term Extension (FLEX) and others have demonstrated.10 This raises the possibility that patients can take a “drug holiday” after several years of treatment. (This study was also reported in the March issue of OBG Management in “Examining the Evidence,” with a commentary by Steven R. Goldstein, MD.)
The FLEX trial attempted to determine whether it is better to continue or stop alendronate after several years’ exposure. One thousand ninety-nine women who had taken alendronate for 3 to 6 years in the FIT trials were randomly assigned to 5 or 10 mg of alendronate daily or placebo. All subjects received 500 mg of calcium and small vitamin D supplements and were followed for an additional 5 years.
The 2 alendronate groups were pooled for the analyses. Patients who switched to placebo for 5 years had declines in BMD at the total hip (2.4%) and spine (3.7%), compared with those who continued alendronate. However, values at the end of 5 years without therapy remained at or above pretreatment levels. Indices of bone turnover increased modestly when therapy was discontinued, but again the rates of bone turnover remained substantially lower than pretreatment values.
Fractures were collected as an exploratory endpoint. Compared with women who stopped treatment, women who continued alendronate reduced their risk of developing a clinical vertebral fracture by 55% (from 5.3% in the placebo group to 2.4% in the alendronate group). No difference was observed in the incidence of nonvertebral fractures between the 2 groups.
Who should take a holiday, and who can stay put?
Unfortunately, this study does not clearly answer the question. Patients at high risk for spine fracture, including those with a previous fracture, appeared to fare better if they continued treatment. Patients at lower risk did equally well whether they stopped or continued alendronate. This suggests that it would be appropriate to stop treatment in women who are not at high risk, including women who do not have osteoporosis by BMD criteria and have not experienced a fragility fracture since menopause.
The reason for stopping therapy in patients at low risk is because there was no added benefit observed with continued treatment, not because of concerns about risk.
When should the holiday end?
If treatment is stopped, the clinical question of whether and when to restart treatment becomes a challenge. The changes in bone density after treatment is stopped are too small to discern in individual patients. In theory, monitoring one or more bone-turnover markers is a more sensitive way to determine when the effects of bisphosphonates on skeletal remodeling are dissipating, but this approach is backed by very little clinical experience.
Another unresolved issue is whether the response after stopping treatment is the same in patients taking risedronate, ibandronate, or lower doses of alendronate.
1. Kaunitz AM. Update on menopause. OBG Management. 2006;18(5):45-54.
2. Rossouw JE, Anderson GL, Prentice RL, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women. Principal results from the Women’s Health Initiative. Writing Group for the Women’s Health Initiative. JAMA. 2002;288:321-333.
3. Berry D, et al. Presented at the 29th annual San Antonio Breast Cancer Symposium, December 14, 2006, San Antonio, Tex.
4. Anderson GL, Limacher M, Assaf AR, et al. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women’s Health Initiative randomized controlled trial. Women’s Health Initiative Steering Committee. JAMA. 2004;291:1701-1712.
5. Kaunitz AM. Hormone therapy and breast cancer risk—trumping fear with facts. Menopause. 2006;13:160-163.
6. Collins JA, Blake JM, Crosignani PE. Breast cancer risk with postmenopausal hormonal treatment. Hum Reprod Update. 2005;11:545-560.
7. de Laet C, Oden A, Johansson H, Johnell O, Jonsson B, Kanis JA. The impact of the use of multiple risk indicators for fracture on case-finding strategies: a mathematical approach. Osteoporosis Int. 2005;16:313-318.
8. McClung MR. Do current management strategies and guidelines adequately address fracture risk? Bone. 2006;38(Suppl 2):S13-S17.
9. Mellstrom DD, Sorensen OH, Goemaere S, Roux C, Johnson TD, Chines AA. Seven years of treatment with risedronate in women with postmenopausal osteoporosis. Calcif Tissue Int. 2004;75:462-468.
10. Bone HG, Hosking D, Devogelaer JP, et al. For the Alendronate Phase III Osteoporosis Treatment Study Group. Ten years’ experience with alendronate for osteoporosis in postmenopausal women. N Engl J Med. 2004;350:1189-1199.
11. Black DM, Schwartz AV, Ensrud KE, et al. For the FLEX Research Group. Effects of continuing or stopping alendronate after 5 years of treatment: the Fracture Intervension Trial Long-term Extension (FLEX): a randomized trial. JAMA. 2006;296:2927-2938.
12. Odvina CV, Zerwekh JE, Rao DS, Maalouf N, Gottschalk FA, Pak CY. Severely suppressed bone turnover: a potential complication of alendronate therapy. J Clin Edocrinol Metab. 2005;90:1294-1301.
13. Ste-Marie LG, Sod E, Johnson T, Chines A. Five years of treatment with risedronate and its effects on bone safety in women with postmenopausal osteoporosis. Calcif Tissue Int. 2004;75:469-476.
14. Cummings SR, Black DM, Thompson DE, et al. Effect of alendronate on risk of fracture in women with low bone density but without vertebral fractures: results from the Fracture Intervention Trial. JAMA. 1998;280:2077-2082.
15. Black DM, Cummings SR, Karpf DB, et al. Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Lancet. 1996;348:1535-1541.
Dr. Kaunitz has received funding from Barr Laboratories, Berlex, Medical Diagnostic Laboratories, Organon, and Warner Chilcott. He is a speaker or consultant for the American College of Obstetricians and Gynecologists, Barr Laboratories, Berlex, Johnson & Johnson, Merck, Noven Organon, and Warner-Chilcott. He holds stock with Barr, Johnson & Johnson, Procter & Gamble, Roche, and Sanofi-Aventis.
Dr. McClung receives grant/research support from and is a consultant to Amgen, Lilly, Merck, Novartis, Proctor & Gamble, Roche, and Sanofi-Aventis. He is a speaker for Lilly, Merck, Procter & Gamble, and Sanofi-Aventis.
1. Kaunitz AM. Update on menopause. OBG Management. 2006;18(5):45-54.
2. Rossouw JE, Anderson GL, Prentice RL, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women. Principal results from the Women’s Health Initiative. Writing Group for the Women’s Health Initiative. JAMA. 2002;288:321-333.
3. Berry D, et al. Presented at the 29th annual San Antonio Breast Cancer Symposium, December 14, 2006, San Antonio, Tex.
4. Anderson GL, Limacher M, Assaf AR, et al. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women’s Health Initiative randomized controlled trial. Women’s Health Initiative Steering Committee. JAMA. 2004;291:1701-1712.
5. Kaunitz AM. Hormone therapy and breast cancer risk—trumping fear with facts. Menopause. 2006;13:160-163.
6. Collins JA, Blake JM, Crosignani PE. Breast cancer risk with postmenopausal hormonal treatment. Hum Reprod Update. 2005;11:545-560.
7. de Laet C, Oden A, Johansson H, Johnell O, Jonsson B, Kanis JA. The impact of the use of multiple risk indicators for fracture on case-finding strategies: a mathematical approach. Osteoporosis Int. 2005;16:313-318.
8. McClung MR. Do current management strategies and guidelines adequately address fracture risk? Bone. 2006;38(Suppl 2):S13-S17.
9. Mellstrom DD, Sorensen OH, Goemaere S, Roux C, Johnson TD, Chines AA. Seven years of treatment with risedronate in women with postmenopausal osteoporosis. Calcif Tissue Int. 2004;75:462-468.
10. Bone HG, Hosking D, Devogelaer JP, et al. For the Alendronate Phase III Osteoporosis Treatment Study Group. Ten years’ experience with alendronate for osteoporosis in postmenopausal women. N Engl J Med. 2004;350:1189-1199.
11. Black DM, Schwartz AV, Ensrud KE, et al. For the FLEX Research Group. Effects of continuing or stopping alendronate after 5 years of treatment: the Fracture Intervension Trial Long-term Extension (FLEX): a randomized trial. JAMA. 2006;296:2927-2938.
12. Odvina CV, Zerwekh JE, Rao DS, Maalouf N, Gottschalk FA, Pak CY. Severely suppressed bone turnover: a potential complication of alendronate therapy. J Clin Edocrinol Metab. 2005;90:1294-1301.
13. Ste-Marie LG, Sod E, Johnson T, Chines A. Five years of treatment with risedronate and its effects on bone safety in women with postmenopausal osteoporosis. Calcif Tissue Int. 2004;75:469-476.
14. Cummings SR, Black DM, Thompson DE, et al. Effect of alendronate on risk of fracture in women with low bone density but without vertebral fractures: results from the Fracture Intervention Trial. JAMA. 1998;280:2077-2082.
15. Black DM, Cummings SR, Karpf DB, et al. Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Lancet. 1996;348:1535-1541.
Dr. Kaunitz has received funding from Barr Laboratories, Berlex, Medical Diagnostic Laboratories, Organon, and Warner Chilcott. He is a speaker or consultant for the American College of Obstetricians and Gynecologists, Barr Laboratories, Berlex, Johnson & Johnson, Merck, Noven Organon, and Warner-Chilcott. He holds stock with Barr, Johnson & Johnson, Procter & Gamble, Roche, and Sanofi-Aventis.
Dr. McClung receives grant/research support from and is a consultant to Amgen, Lilly, Merck, Novartis, Proctor & Gamble, Roche, and Sanofi-Aventis. He is a speaker for Lilly, Merck, Procter & Gamble, and Sanofi-Aventis.
Case: Blisters after deliveries (but only with husband no. 2)
A 33-year-old Hispanic woman who was 5 months pregnant came to the hospital complaining of nausea and vomiting. She had a history of anticardiolipin antibody syndrome, diagnosed originally in 1993 after 2 spontaneous abortions. She had stopped taking warfarin (Coumadin) at the start of her pregnancy, and had been taking heparin for 3 months.
After 4 days of close monitoring, the patient had labor induced for severe life-threatening preeclampsia. One day after induction and delivery of a still-born fetus, she began to develop painful swelling of both hands and feet along with targetoid, urticarial, edematous, deep pink, slightly dusky papules and plaques on her hands, abdomen, lower extremities, and proximal thighs. Some of the edematous sites began to form vesicles and bullae (FIGURES 1 AND 2). When asked about this eruption, the patient mentioned having a similar rash after delivery of one of her children about 10 years before.
Interestingly, she noted that she only experienced these cutaneous findings during pregnancies with her second husband and not with her first. Biopsies were performed and showed prominent eosinophils in the dermis and a subepidermal vesicle.
FIGURE 1 Blisters on wrist…
Vesicles and bullae on the wrist after miscarriage.
FIGURE 2 …and abdomen
Similar bulla in the umbilicus.
What is your diagnosis?
Dx: Pemphigoid gestationis
The patient had pemphigoid gestationis, also known as herpes gestationis, a rare autoimmune bullous disease of pregnancy and the puerperium.1 Clinically and immunopathologically, pemphigoid gestationis is related to the pemphigoid disorders and is not virally mediated.2
In the United States, pemphigoid gestationis has an incidence of 1:10,000 to 1:50,000 pregnancies.3 Clinically, it manifests during the second or third trimester, with a sudden onset of extremely pruritic urticarial papules and plaques usually located around the umbilicus. These lesions often progress to tense vesicles and blisters and spread peripherally to the trunk, often sparing the face, palms, and soles.4 Worsening of the lesions at the time of delivery occurs in 75% of cases, and usually recurs with subsequent pregnancies.5 Occasionally, however, subsequent pregnancies are unaffected, so-called “skip pregnancies.”6 This occurs most often when there has been a change in paternity.7
The exact cause of pemphigoid gestationis is unknown. Investigative efforts led to the identification of an immunoglobulin G (IgG) autoantibody, which binds to bullous pemphigoid (BP) antigen 2, also called BP180, which is a protein associated with hemidesmosomes of basal keratinocytes.8-10 These hemidesmosomes form the central portion of the dermal–epidermal anchoring complex, whose function is to establish a connection between the basal keratinocytes and the upper dermis.11,12 This is critical for maintaining dermal–epidermal adhesion. It is hypothesized that binding of autoantibodies to BP180 initiates an inflammatory reaction, leading to blister formation at the dermal–epidermal junction.13
Autoimmune basis?
Histopathologic findings demonstrate subepidermal vesicles, spongiosis, and perivascular infiltrates of lymphocytes and histiocytes with a preponderance of eosinophils.3 The sine qua non of the disease, though, is demonstration through direct immunofluorescence of complement deposition and IgG in a linear band along the basement membrane.14
There appears to be a genetic predisposition toward the development of pemphigoid gestationis. Associations with human leukocyte antigens (HLAs) DR3 (61%–85%), DR4 (52%), or both (43%–50%) have been reported.3,15,16 Interestingly, 85% of persons with a history of pemphigoid gestationis were found to have anti-HLA antibodies, some of which were directed against paternal HLAs expressed in their placentae.17 These findings raised speculation about a possible immunologic insult against placental antigens during pregnancy. Evidence suggests that circulating auto-antibodies in patients with pemphigoid gestationis bind to the dermal–epidermal junction of skin and amnion in which BP180 antigen is also present.18-20
It has been demonstrated that in patients with pemphigoid gestationis the cells of the placenta stroma express abnormal major histocompatibility complex (MHC) class II molecules.21,22 This led to the proposition of 2 possible mechanisms for the initiation of an autoimmune response in pemphigoid gestationis. The first proposes that placental BP180 is presented to the maternal immune system in association with abnormal MHC molecules, which then trigger the production of autoantibodies that cross-react with the skin. Alternatively, the placental stromal cells may evoke an allogeneic reaction against the BP180 antigen presented by paternal MHC molecules of the placental stroma, which then cross-reacts with the skin.23 The latter theory supports the findings in this patient, who developed pemphigoid gestationis during the 2 pregnancies with her second husband and not during the pregnancies with her first husband.
Exploring the differential
It is important to differentiate the prebullous stage of pemphigoid gestationis from other pregnancy-related dermatoses.24 These include polymorphic eruption of pregnancy (PEP), pruritic urticarial papules and plaques of pregnancy (PUPPP), erythema multiforme, prurigo annularis, intrahepatic cholestasis of pregnancy, and impetigo herpetiformis. Impetigo herpetiformis is not related to bacterial or viral causes, but is rather a manifestation of pustular psoriasis during pregnancy. The target lesions that form in pemphigoid gestationis look just like the target lesions of erythema multiforme.
When there is no blister formation, it is impossible to distinguish pemphigoid gestationis from many of the other cutaneous eruptions of pregnancy. If uncertain, the clinician should perform punch biopsies of the involved skin, with one specimen sent for immunofluoresence studies. The biopsy should not pass directly through a bulla, due to risk of losing the overlying epidermis in the specimen. Do the punch biopsy at the edge of the bulla including some normal skin. Other important laboratory exams to perform would include liver function tests to look for an upward trend associated with intrahepatic cholestasis, and herpes simplex virus antibody testing for the association with erythema multiforme. The cutaneous findings and pertinent tests are listed in order of increasing potential as a life-threatening dermatosis (TABLE).
TABLE
Blisters in pregnancy: Six principal considerations
| DISEASE | ASSOCIATIONS | DIAGNOSIS | TREATMENT |
|---|---|---|---|
| Polymorphous eruption of pregnancy | Nonspecific pruritic eruption of pregnancy | Biopsy to differentiate from prebullous stage of pemphigoid (herpes) gestationis | Mild to mid-potency topical steroid; oral antihistamine |
| Pruritic urticarial papules and plaques of pregnancy | Occur in stretch marks, spare umbilicus; more common in primigravidas | Unless history is very clear, biopsy to differentiate from prebullous stage of pemphigoid gestationis | Emollient; pulse-dye laser during violaceous stage of striae; topical steroid; oral antihistamine |
| Erythema multiforme | Can involve mucous membranes; targetoid lesions; absence of pruritus; centripetal spread; favors palms/soles | Viral, bacterial, or drug-related eruption; most often with herpes simplex I or II virus; biopsy to differentiate from pemphigoid gestationis | Acyclovir, valacyclovir if HSV-related; treatment of bacterial infection; or removal of offending drug |
| Pemphigoid gestationis | Blistering, urticarial papules and plaques, pruritus | Biopsy for histologic diagnosis and immunofluorescence | Prednisone for short course, starting at 1 mg/kg, then tapering over 2–3 months; topical steroid |
| Intrahepatic cholestasis of pregnancy | With or without jaundice; otherwise, no cutaneous findings other than generalized pruritus; risk of preterm birth | Elevation of liver function tests, cholesterol, triglycerides; dark urine; right-upper-quadrant pain; nausea; greasy stools | Ursodeoxycholic acid; S-adenosyl-L-methionine |
| Impetigo herpetiformis (pustular psoriasis of pregnancy) | Extremely ill with fever, chills, nausea; vascular instability; pustules rather than vesicles | Biopsy if uncertain; pustules sterile; risk of hypocalcemia, hypoparathyroidism | High-dose oral steroid or cyclosporine |
Pemphigoid gestationis should resolve spontaneously within 2 to 3 months after delivery. Treatment is aimed at preventing new blisters and relieving pruritus, with topical corticosteroids and oral antihistamines in mild cases.2,25 In advanced lesions as seen in this case, 0.3 to 0.5 mg/kg prednisolone daily is usually sufficient.3,25 Alternative medications include sulfapyridine, dapsone, and cyclosporine, though disease response is variable and their safety is questionable.3
When the skin condition began, the patient was treated with oral antihistamines and topical steroids. On day 2, the diagnosis of pemphigoid gestationis was clear, and she was started on oral prednisone at 60 mg/day, which resulted in rapid symptom improvement in her lesions and swelling. New lesions stopped forming, and systemic steroids were tapered off over the 3 months after delivery. The skin lesions healed.
Summing up
Our patient had classic findings of pemphigoid gestationis with many characteristic lesions (including the umbilicus), making the diagnosis possible before biopsy confirmation. This was fortunate for her because her painful swelling responded quickly to the corticosteroids. When cases are less clinically obvious, biopsy for histopathology and immunofluorescence facilitates differentiation of pemphigoid gestationis from other dermatoses of pregnancy. Although it is interesting that our patient also had the anticardiolipin syndrome, most patients do not have both conditions.
The authors report no financial relationships relevant to this article.
1. Coupe RL. Herpes gestationis. Arch Dermatol. 1965;91:633-636.
2. Jenkins RE, Hern S, Black MM. Clinical features and management of 87 patients with pemphigoid gestationis. Clin Exp Dermatol. 1999;24:255-259.
3. Al-Fouzan AW, Galadari I, Oumeish I, et al. Herpes gestationis (Pemphigoid gestationis). Clin Dermatol. 2006;24:109-112.
4. Shornick JK. Herpes gestationis. J Am Acad Dermatol. 1987;17:539-556.
5. Holmes RC, Black MM, Dann J, et al. A comparative study of toxic erythema of pregnancy and herpes gestationis. Br J Dermatol. 1982;106:499-510.
6. Cozzani E, Basso M, Parodi A, Rebora A. Pemphigoid gestationis post partum after changing husband. Int J Dermatol. 2005;44:1057-1058.
7. Shornick JK, Black MM. Fetal risks in herpes gestationis. J Am Acad Dermatol. 1992;26:63-68.
8. Diaz LA, Ratrie H, III, Saunders WS, et al. Isolation of a human epidermal cDNA corresponding to the 180-kD autoantigen recognized by bullous pemphigoid and herpes gestationis sera. Immunolocalization of this protein to the hemidesmosome. J Clin Invest. 1990;86:1088-1094.
9. Giudice GJ, Emery DJ, Diaz LA. Cloning and primary structural analysis of the bullous pemphigoid autoantigen BP180. J Invest Dermatol. 1992;99:243-250.
10. Zillikens D, Giudice GJ. BP180/type XVIII collagen: its role in acquired and inherited disorders of the dermal epidermal junction. Arch Dermatol Res. 1999;91:187-194.
11. Borradori L, Sonnenberg A. Hemidesmosomes: roles in adhesion, signaling and human diseases. Curr Opin Cell Biol. 1996;8:647-656.
12. Zillikens D. Acquired skin disease of hemidesmosomes. J Dermatol Sci. 1999;20:134-154.
13. Schmidt E, Zillikens D. Autoimmune and inherited subepidermal blistering diseases: advances in the clinic and the laboratory. Adv Dermatol. 2000;16:113-157.
14. Shornick JD. Dermatoses of pregnancy. Semin Cutan Med Surg. 1998;17:172-181.
15. Holmes RC, Black MM, Jurecka W, et al. Clues to the aetiology and pathogenesis of herpes gestationis. Br J Dermatol. 1983;109:131-139.
16. Shornick JK, Stastny P, Gilliam JN. High frequency of histocompatibility antigens DR3 and DR4 in herpes gestationis. J Clin Invest. 1981;68:553-555.
17. Shornick JK, Stastny P, Gilliam JN. Paternal histo-compatibility (HLA) antigens and maternal anti-HLA antibodies in herpes gestationis. J Invest Dermatol. 1983;81:407-409.
18. Ortonne JP, Hsi BL, Verrando P, et al. Herpes gestationis factor reacts with the amniotic epithelial basement membrane. Br J Dermatol. 1987;117:147-154.
19. Kelly SE, Bhogal BS, Wojnarowska F, Black MM. Expression of a pemphigoid gestationis-related antigen by human placenta. Br J Dermatol. 1988;118:605-611.
20. Fairley JA, Heintz PW, Neuburg M, et al. Expression pattern of the bullous pemphigoid-180 antigen in normal and neoplastic epithelia. Br J Dermatol. 1995;133:385-391.
21. Kelly SE, Black MM, Fleming S. Antigen-presenting cells in the skin and placenta in pemphigoid gestationis. Br J Dermatol. 1990;122:593-599.
22. Borthwick GM, Holmes RC, Stirrat GM. Abnormal expression of class II MHC antigens in placentae from patients with pemphigoid gestationis. Placenta. 1988;9:81-94.
23. Kelly SE, Black MM, Fleming S. Pemphigoid gestationis: a unique mechanism of initiation of an autoimmune response by MHC class II molecules. J Pathol. 1989;158:81-82.
24. Borradori L, Saurat JH. Specific dermatoses of pregnancy. Toward a comprehensive view. Arch Dermatol. 1994;130:778-780.
25. Shimanovich I, Bröcker EB, Zillikens D. Pemphigoid gestationis: new insights into the pathogenesis lead to novel diagnostic tools. Br J Obstet Gynaecol. 2002;109:970-976.
A 33-year-old Hispanic woman who was 5 months pregnant came to the hospital complaining of nausea and vomiting. She had a history of anticardiolipin antibody syndrome, diagnosed originally in 1993 after 2 spontaneous abortions. She had stopped taking warfarin (Coumadin) at the start of her pregnancy, and had been taking heparin for 3 months.
After 4 days of close monitoring, the patient had labor induced for severe life-threatening preeclampsia. One day after induction and delivery of a still-born fetus, she began to develop painful swelling of both hands and feet along with targetoid, urticarial, edematous, deep pink, slightly dusky papules and plaques on her hands, abdomen, lower extremities, and proximal thighs. Some of the edematous sites began to form vesicles and bullae (FIGURES 1 AND 2). When asked about this eruption, the patient mentioned having a similar rash after delivery of one of her children about 10 years before.
Interestingly, she noted that she only experienced these cutaneous findings during pregnancies with her second husband and not with her first. Biopsies were performed and showed prominent eosinophils in the dermis and a subepidermal vesicle.
FIGURE 1 Blisters on wrist…
Vesicles and bullae on the wrist after miscarriage.
FIGURE 2 …and abdomen
Similar bulla in the umbilicus.
What is your diagnosis?
Dx: Pemphigoid gestationis
The patient had pemphigoid gestationis, also known as herpes gestationis, a rare autoimmune bullous disease of pregnancy and the puerperium.1 Clinically and immunopathologically, pemphigoid gestationis is related to the pemphigoid disorders and is not virally mediated.2
In the United States, pemphigoid gestationis has an incidence of 1:10,000 to 1:50,000 pregnancies.3 Clinically, it manifests during the second or third trimester, with a sudden onset of extremely pruritic urticarial papules and plaques usually located around the umbilicus. These lesions often progress to tense vesicles and blisters and spread peripherally to the trunk, often sparing the face, palms, and soles.4 Worsening of the lesions at the time of delivery occurs in 75% of cases, and usually recurs with subsequent pregnancies.5 Occasionally, however, subsequent pregnancies are unaffected, so-called “skip pregnancies.”6 This occurs most often when there has been a change in paternity.7
The exact cause of pemphigoid gestationis is unknown. Investigative efforts led to the identification of an immunoglobulin G (IgG) autoantibody, which binds to bullous pemphigoid (BP) antigen 2, also called BP180, which is a protein associated with hemidesmosomes of basal keratinocytes.8-10 These hemidesmosomes form the central portion of the dermal–epidermal anchoring complex, whose function is to establish a connection between the basal keratinocytes and the upper dermis.11,12 This is critical for maintaining dermal–epidermal adhesion. It is hypothesized that binding of autoantibodies to BP180 initiates an inflammatory reaction, leading to blister formation at the dermal–epidermal junction.13
Autoimmune basis?
Histopathologic findings demonstrate subepidermal vesicles, spongiosis, and perivascular infiltrates of lymphocytes and histiocytes with a preponderance of eosinophils.3 The sine qua non of the disease, though, is demonstration through direct immunofluorescence of complement deposition and IgG in a linear band along the basement membrane.14
There appears to be a genetic predisposition toward the development of pemphigoid gestationis. Associations with human leukocyte antigens (HLAs) DR3 (61%–85%), DR4 (52%), or both (43%–50%) have been reported.3,15,16 Interestingly, 85% of persons with a history of pemphigoid gestationis were found to have anti-HLA antibodies, some of which were directed against paternal HLAs expressed in their placentae.17 These findings raised speculation about a possible immunologic insult against placental antigens during pregnancy. Evidence suggests that circulating auto-antibodies in patients with pemphigoid gestationis bind to the dermal–epidermal junction of skin and amnion in which BP180 antigen is also present.18-20
It has been demonstrated that in patients with pemphigoid gestationis the cells of the placenta stroma express abnormal major histocompatibility complex (MHC) class II molecules.21,22 This led to the proposition of 2 possible mechanisms for the initiation of an autoimmune response in pemphigoid gestationis. The first proposes that placental BP180 is presented to the maternal immune system in association with abnormal MHC molecules, which then trigger the production of autoantibodies that cross-react with the skin. Alternatively, the placental stromal cells may evoke an allogeneic reaction against the BP180 antigen presented by paternal MHC molecules of the placental stroma, which then cross-reacts with the skin.23 The latter theory supports the findings in this patient, who developed pemphigoid gestationis during the 2 pregnancies with her second husband and not during the pregnancies with her first husband.
Exploring the differential
It is important to differentiate the prebullous stage of pemphigoid gestationis from other pregnancy-related dermatoses.24 These include polymorphic eruption of pregnancy (PEP), pruritic urticarial papules and plaques of pregnancy (PUPPP), erythema multiforme, prurigo annularis, intrahepatic cholestasis of pregnancy, and impetigo herpetiformis. Impetigo herpetiformis is not related to bacterial or viral causes, but is rather a manifestation of pustular psoriasis during pregnancy. The target lesions that form in pemphigoid gestationis look just like the target lesions of erythema multiforme.
When there is no blister formation, it is impossible to distinguish pemphigoid gestationis from many of the other cutaneous eruptions of pregnancy. If uncertain, the clinician should perform punch biopsies of the involved skin, with one specimen sent for immunofluoresence studies. The biopsy should not pass directly through a bulla, due to risk of losing the overlying epidermis in the specimen. Do the punch biopsy at the edge of the bulla including some normal skin. Other important laboratory exams to perform would include liver function tests to look for an upward trend associated with intrahepatic cholestasis, and herpes simplex virus antibody testing for the association with erythema multiforme. The cutaneous findings and pertinent tests are listed in order of increasing potential as a life-threatening dermatosis (TABLE).
TABLE
Blisters in pregnancy: Six principal considerations
| DISEASE | ASSOCIATIONS | DIAGNOSIS | TREATMENT |
|---|---|---|---|
| Polymorphous eruption of pregnancy | Nonspecific pruritic eruption of pregnancy | Biopsy to differentiate from prebullous stage of pemphigoid (herpes) gestationis | Mild to mid-potency topical steroid; oral antihistamine |
| Pruritic urticarial papules and plaques of pregnancy | Occur in stretch marks, spare umbilicus; more common in primigravidas | Unless history is very clear, biopsy to differentiate from prebullous stage of pemphigoid gestationis | Emollient; pulse-dye laser during violaceous stage of striae; topical steroid; oral antihistamine |
| Erythema multiforme | Can involve mucous membranes; targetoid lesions; absence of pruritus; centripetal spread; favors palms/soles | Viral, bacterial, or drug-related eruption; most often with herpes simplex I or II virus; biopsy to differentiate from pemphigoid gestationis | Acyclovir, valacyclovir if HSV-related; treatment of bacterial infection; or removal of offending drug |
| Pemphigoid gestationis | Blistering, urticarial papules and plaques, pruritus | Biopsy for histologic diagnosis and immunofluorescence | Prednisone for short course, starting at 1 mg/kg, then tapering over 2–3 months; topical steroid |
| Intrahepatic cholestasis of pregnancy | With or without jaundice; otherwise, no cutaneous findings other than generalized pruritus; risk of preterm birth | Elevation of liver function tests, cholesterol, triglycerides; dark urine; right-upper-quadrant pain; nausea; greasy stools | Ursodeoxycholic acid; S-adenosyl-L-methionine |
| Impetigo herpetiformis (pustular psoriasis of pregnancy) | Extremely ill with fever, chills, nausea; vascular instability; pustules rather than vesicles | Biopsy if uncertain; pustules sterile; risk of hypocalcemia, hypoparathyroidism | High-dose oral steroid or cyclosporine |
Pemphigoid gestationis should resolve spontaneously within 2 to 3 months after delivery. Treatment is aimed at preventing new blisters and relieving pruritus, with topical corticosteroids and oral antihistamines in mild cases.2,25 In advanced lesions as seen in this case, 0.3 to 0.5 mg/kg prednisolone daily is usually sufficient.3,25 Alternative medications include sulfapyridine, dapsone, and cyclosporine, though disease response is variable and their safety is questionable.3
When the skin condition began, the patient was treated with oral antihistamines and topical steroids. On day 2, the diagnosis of pemphigoid gestationis was clear, and she was started on oral prednisone at 60 mg/day, which resulted in rapid symptom improvement in her lesions and swelling. New lesions stopped forming, and systemic steroids were tapered off over the 3 months after delivery. The skin lesions healed.
Summing up
Our patient had classic findings of pemphigoid gestationis with many characteristic lesions (including the umbilicus), making the diagnosis possible before biopsy confirmation. This was fortunate for her because her painful swelling responded quickly to the corticosteroids. When cases are less clinically obvious, biopsy for histopathology and immunofluorescence facilitates differentiation of pemphigoid gestationis from other dermatoses of pregnancy. Although it is interesting that our patient also had the anticardiolipin syndrome, most patients do not have both conditions.
The authors report no financial relationships relevant to this article.
A 33-year-old Hispanic woman who was 5 months pregnant came to the hospital complaining of nausea and vomiting. She had a history of anticardiolipin antibody syndrome, diagnosed originally in 1993 after 2 spontaneous abortions. She had stopped taking warfarin (Coumadin) at the start of her pregnancy, and had been taking heparin for 3 months.
After 4 days of close monitoring, the patient had labor induced for severe life-threatening preeclampsia. One day after induction and delivery of a still-born fetus, she began to develop painful swelling of both hands and feet along with targetoid, urticarial, edematous, deep pink, slightly dusky papules and plaques on her hands, abdomen, lower extremities, and proximal thighs. Some of the edematous sites began to form vesicles and bullae (FIGURES 1 AND 2). When asked about this eruption, the patient mentioned having a similar rash after delivery of one of her children about 10 years before.
Interestingly, she noted that she only experienced these cutaneous findings during pregnancies with her second husband and not with her first. Biopsies were performed and showed prominent eosinophils in the dermis and a subepidermal vesicle.
FIGURE 1 Blisters on wrist…
Vesicles and bullae on the wrist after miscarriage.
FIGURE 2 …and abdomen
Similar bulla in the umbilicus.
What is your diagnosis?
Dx: Pemphigoid gestationis
The patient had pemphigoid gestationis, also known as herpes gestationis, a rare autoimmune bullous disease of pregnancy and the puerperium.1 Clinically and immunopathologically, pemphigoid gestationis is related to the pemphigoid disorders and is not virally mediated.2
In the United States, pemphigoid gestationis has an incidence of 1:10,000 to 1:50,000 pregnancies.3 Clinically, it manifests during the second or third trimester, with a sudden onset of extremely pruritic urticarial papules and plaques usually located around the umbilicus. These lesions often progress to tense vesicles and blisters and spread peripherally to the trunk, often sparing the face, palms, and soles.4 Worsening of the lesions at the time of delivery occurs in 75% of cases, and usually recurs with subsequent pregnancies.5 Occasionally, however, subsequent pregnancies are unaffected, so-called “skip pregnancies.”6 This occurs most often when there has been a change in paternity.7
The exact cause of pemphigoid gestationis is unknown. Investigative efforts led to the identification of an immunoglobulin G (IgG) autoantibody, which binds to bullous pemphigoid (BP) antigen 2, also called BP180, which is a protein associated with hemidesmosomes of basal keratinocytes.8-10 These hemidesmosomes form the central portion of the dermal–epidermal anchoring complex, whose function is to establish a connection between the basal keratinocytes and the upper dermis.11,12 This is critical for maintaining dermal–epidermal adhesion. It is hypothesized that binding of autoantibodies to BP180 initiates an inflammatory reaction, leading to blister formation at the dermal–epidermal junction.13
Autoimmune basis?
Histopathologic findings demonstrate subepidermal vesicles, spongiosis, and perivascular infiltrates of lymphocytes and histiocytes with a preponderance of eosinophils.3 The sine qua non of the disease, though, is demonstration through direct immunofluorescence of complement deposition and IgG in a linear band along the basement membrane.14
There appears to be a genetic predisposition toward the development of pemphigoid gestationis. Associations with human leukocyte antigens (HLAs) DR3 (61%–85%), DR4 (52%), or both (43%–50%) have been reported.3,15,16 Interestingly, 85% of persons with a history of pemphigoid gestationis were found to have anti-HLA antibodies, some of which were directed against paternal HLAs expressed in their placentae.17 These findings raised speculation about a possible immunologic insult against placental antigens during pregnancy. Evidence suggests that circulating auto-antibodies in patients with pemphigoid gestationis bind to the dermal–epidermal junction of skin and amnion in which BP180 antigen is also present.18-20
It has been demonstrated that in patients with pemphigoid gestationis the cells of the placenta stroma express abnormal major histocompatibility complex (MHC) class II molecules.21,22 This led to the proposition of 2 possible mechanisms for the initiation of an autoimmune response in pemphigoid gestationis. The first proposes that placental BP180 is presented to the maternal immune system in association with abnormal MHC molecules, which then trigger the production of autoantibodies that cross-react with the skin. Alternatively, the placental stromal cells may evoke an allogeneic reaction against the BP180 antigen presented by paternal MHC molecules of the placental stroma, which then cross-reacts with the skin.23 The latter theory supports the findings in this patient, who developed pemphigoid gestationis during the 2 pregnancies with her second husband and not during the pregnancies with her first husband.
Exploring the differential
It is important to differentiate the prebullous stage of pemphigoid gestationis from other pregnancy-related dermatoses.24 These include polymorphic eruption of pregnancy (PEP), pruritic urticarial papules and plaques of pregnancy (PUPPP), erythema multiforme, prurigo annularis, intrahepatic cholestasis of pregnancy, and impetigo herpetiformis. Impetigo herpetiformis is not related to bacterial or viral causes, but is rather a manifestation of pustular psoriasis during pregnancy. The target lesions that form in pemphigoid gestationis look just like the target lesions of erythema multiforme.
When there is no blister formation, it is impossible to distinguish pemphigoid gestationis from many of the other cutaneous eruptions of pregnancy. If uncertain, the clinician should perform punch biopsies of the involved skin, with one specimen sent for immunofluoresence studies. The biopsy should not pass directly through a bulla, due to risk of losing the overlying epidermis in the specimen. Do the punch biopsy at the edge of the bulla including some normal skin. Other important laboratory exams to perform would include liver function tests to look for an upward trend associated with intrahepatic cholestasis, and herpes simplex virus antibody testing for the association with erythema multiforme. The cutaneous findings and pertinent tests are listed in order of increasing potential as a life-threatening dermatosis (TABLE).
TABLE
Blisters in pregnancy: Six principal considerations
| DISEASE | ASSOCIATIONS | DIAGNOSIS | TREATMENT |
|---|---|---|---|
| Polymorphous eruption of pregnancy | Nonspecific pruritic eruption of pregnancy | Biopsy to differentiate from prebullous stage of pemphigoid (herpes) gestationis | Mild to mid-potency topical steroid; oral antihistamine |
| Pruritic urticarial papules and plaques of pregnancy | Occur in stretch marks, spare umbilicus; more common in primigravidas | Unless history is very clear, biopsy to differentiate from prebullous stage of pemphigoid gestationis | Emollient; pulse-dye laser during violaceous stage of striae; topical steroid; oral antihistamine |
| Erythema multiforme | Can involve mucous membranes; targetoid lesions; absence of pruritus; centripetal spread; favors palms/soles | Viral, bacterial, or drug-related eruption; most often with herpes simplex I or II virus; biopsy to differentiate from pemphigoid gestationis | Acyclovir, valacyclovir if HSV-related; treatment of bacterial infection; or removal of offending drug |
| Pemphigoid gestationis | Blistering, urticarial papules and plaques, pruritus | Biopsy for histologic diagnosis and immunofluorescence | Prednisone for short course, starting at 1 mg/kg, then tapering over 2–3 months; topical steroid |
| Intrahepatic cholestasis of pregnancy | With or without jaundice; otherwise, no cutaneous findings other than generalized pruritus; risk of preterm birth | Elevation of liver function tests, cholesterol, triglycerides; dark urine; right-upper-quadrant pain; nausea; greasy stools | Ursodeoxycholic acid; S-adenosyl-L-methionine |
| Impetigo herpetiformis (pustular psoriasis of pregnancy) | Extremely ill with fever, chills, nausea; vascular instability; pustules rather than vesicles | Biopsy if uncertain; pustules sterile; risk of hypocalcemia, hypoparathyroidism | High-dose oral steroid or cyclosporine |
Pemphigoid gestationis should resolve spontaneously within 2 to 3 months after delivery. Treatment is aimed at preventing new blisters and relieving pruritus, with topical corticosteroids and oral antihistamines in mild cases.2,25 In advanced lesions as seen in this case, 0.3 to 0.5 mg/kg prednisolone daily is usually sufficient.3,25 Alternative medications include sulfapyridine, dapsone, and cyclosporine, though disease response is variable and their safety is questionable.3
When the skin condition began, the patient was treated with oral antihistamines and topical steroids. On day 2, the diagnosis of pemphigoid gestationis was clear, and she was started on oral prednisone at 60 mg/day, which resulted in rapid symptom improvement in her lesions and swelling. New lesions stopped forming, and systemic steroids were tapered off over the 3 months after delivery. The skin lesions healed.
Summing up
Our patient had classic findings of pemphigoid gestationis with many characteristic lesions (including the umbilicus), making the diagnosis possible before biopsy confirmation. This was fortunate for her because her painful swelling responded quickly to the corticosteroids. When cases are less clinically obvious, biopsy for histopathology and immunofluorescence facilitates differentiation of pemphigoid gestationis from other dermatoses of pregnancy. Although it is interesting that our patient also had the anticardiolipin syndrome, most patients do not have both conditions.
The authors report no financial relationships relevant to this article.
1. Coupe RL. Herpes gestationis. Arch Dermatol. 1965;91:633-636.
2. Jenkins RE, Hern S, Black MM. Clinical features and management of 87 patients with pemphigoid gestationis. Clin Exp Dermatol. 1999;24:255-259.
3. Al-Fouzan AW, Galadari I, Oumeish I, et al. Herpes gestationis (Pemphigoid gestationis). Clin Dermatol. 2006;24:109-112.
4. Shornick JK. Herpes gestationis. J Am Acad Dermatol. 1987;17:539-556.
5. Holmes RC, Black MM, Dann J, et al. A comparative study of toxic erythema of pregnancy and herpes gestationis. Br J Dermatol. 1982;106:499-510.
6. Cozzani E, Basso M, Parodi A, Rebora A. Pemphigoid gestationis post partum after changing husband. Int J Dermatol. 2005;44:1057-1058.
7. Shornick JK, Black MM. Fetal risks in herpes gestationis. J Am Acad Dermatol. 1992;26:63-68.
8. Diaz LA, Ratrie H, III, Saunders WS, et al. Isolation of a human epidermal cDNA corresponding to the 180-kD autoantigen recognized by bullous pemphigoid and herpes gestationis sera. Immunolocalization of this protein to the hemidesmosome. J Clin Invest. 1990;86:1088-1094.
9. Giudice GJ, Emery DJ, Diaz LA. Cloning and primary structural analysis of the bullous pemphigoid autoantigen BP180. J Invest Dermatol. 1992;99:243-250.
10. Zillikens D, Giudice GJ. BP180/type XVIII collagen: its role in acquired and inherited disorders of the dermal epidermal junction. Arch Dermatol Res. 1999;91:187-194.
11. Borradori L, Sonnenberg A. Hemidesmosomes: roles in adhesion, signaling and human diseases. Curr Opin Cell Biol. 1996;8:647-656.
12. Zillikens D. Acquired skin disease of hemidesmosomes. J Dermatol Sci. 1999;20:134-154.
13. Schmidt E, Zillikens D. Autoimmune and inherited subepidermal blistering diseases: advances in the clinic and the laboratory. Adv Dermatol. 2000;16:113-157.
14. Shornick JD. Dermatoses of pregnancy. Semin Cutan Med Surg. 1998;17:172-181.
15. Holmes RC, Black MM, Jurecka W, et al. Clues to the aetiology and pathogenesis of herpes gestationis. Br J Dermatol. 1983;109:131-139.
16. Shornick JK, Stastny P, Gilliam JN. High frequency of histocompatibility antigens DR3 and DR4 in herpes gestationis. J Clin Invest. 1981;68:553-555.
17. Shornick JK, Stastny P, Gilliam JN. Paternal histo-compatibility (HLA) antigens and maternal anti-HLA antibodies in herpes gestationis. J Invest Dermatol. 1983;81:407-409.
18. Ortonne JP, Hsi BL, Verrando P, et al. Herpes gestationis factor reacts with the amniotic epithelial basement membrane. Br J Dermatol. 1987;117:147-154.
19. Kelly SE, Bhogal BS, Wojnarowska F, Black MM. Expression of a pemphigoid gestationis-related antigen by human placenta. Br J Dermatol. 1988;118:605-611.
20. Fairley JA, Heintz PW, Neuburg M, et al. Expression pattern of the bullous pemphigoid-180 antigen in normal and neoplastic epithelia. Br J Dermatol. 1995;133:385-391.
21. Kelly SE, Black MM, Fleming S. Antigen-presenting cells in the skin and placenta in pemphigoid gestationis. Br J Dermatol. 1990;122:593-599.
22. Borthwick GM, Holmes RC, Stirrat GM. Abnormal expression of class II MHC antigens in placentae from patients with pemphigoid gestationis. Placenta. 1988;9:81-94.
23. Kelly SE, Black MM, Fleming S. Pemphigoid gestationis: a unique mechanism of initiation of an autoimmune response by MHC class II molecules. J Pathol. 1989;158:81-82.
24. Borradori L, Saurat JH. Specific dermatoses of pregnancy. Toward a comprehensive view. Arch Dermatol. 1994;130:778-780.
25. Shimanovich I, Bröcker EB, Zillikens D. Pemphigoid gestationis: new insights into the pathogenesis lead to novel diagnostic tools. Br J Obstet Gynaecol. 2002;109:970-976.
1. Coupe RL. Herpes gestationis. Arch Dermatol. 1965;91:633-636.
2. Jenkins RE, Hern S, Black MM. Clinical features and management of 87 patients with pemphigoid gestationis. Clin Exp Dermatol. 1999;24:255-259.
3. Al-Fouzan AW, Galadari I, Oumeish I, et al. Herpes gestationis (Pemphigoid gestationis). Clin Dermatol. 2006;24:109-112.
4. Shornick JK. Herpes gestationis. J Am Acad Dermatol. 1987;17:539-556.
5. Holmes RC, Black MM, Dann J, et al. A comparative study of toxic erythema of pregnancy and herpes gestationis. Br J Dermatol. 1982;106:499-510.
6. Cozzani E, Basso M, Parodi A, Rebora A. Pemphigoid gestationis post partum after changing husband. Int J Dermatol. 2005;44:1057-1058.
7. Shornick JK, Black MM. Fetal risks in herpes gestationis. J Am Acad Dermatol. 1992;26:63-68.
8. Diaz LA, Ratrie H, III, Saunders WS, et al. Isolation of a human epidermal cDNA corresponding to the 180-kD autoantigen recognized by bullous pemphigoid and herpes gestationis sera. Immunolocalization of this protein to the hemidesmosome. J Clin Invest. 1990;86:1088-1094.
9. Giudice GJ, Emery DJ, Diaz LA. Cloning and primary structural analysis of the bullous pemphigoid autoantigen BP180. J Invest Dermatol. 1992;99:243-250.
10. Zillikens D, Giudice GJ. BP180/type XVIII collagen: its role in acquired and inherited disorders of the dermal epidermal junction. Arch Dermatol Res. 1999;91:187-194.
11. Borradori L, Sonnenberg A. Hemidesmosomes: roles in adhesion, signaling and human diseases. Curr Opin Cell Biol. 1996;8:647-656.
12. Zillikens D. Acquired skin disease of hemidesmosomes. J Dermatol Sci. 1999;20:134-154.
13. Schmidt E, Zillikens D. Autoimmune and inherited subepidermal blistering diseases: advances in the clinic and the laboratory. Adv Dermatol. 2000;16:113-157.
14. Shornick JD. Dermatoses of pregnancy. Semin Cutan Med Surg. 1998;17:172-181.
15. Holmes RC, Black MM, Jurecka W, et al. Clues to the aetiology and pathogenesis of herpes gestationis. Br J Dermatol. 1983;109:131-139.
16. Shornick JK, Stastny P, Gilliam JN. High frequency of histocompatibility antigens DR3 and DR4 in herpes gestationis. J Clin Invest. 1981;68:553-555.
17. Shornick JK, Stastny P, Gilliam JN. Paternal histo-compatibility (HLA) antigens and maternal anti-HLA antibodies in herpes gestationis. J Invest Dermatol. 1983;81:407-409.
18. Ortonne JP, Hsi BL, Verrando P, et al. Herpes gestationis factor reacts with the amniotic epithelial basement membrane. Br J Dermatol. 1987;117:147-154.
19. Kelly SE, Bhogal BS, Wojnarowska F, Black MM. Expression of a pemphigoid gestationis-related antigen by human placenta. Br J Dermatol. 1988;118:605-611.
20. Fairley JA, Heintz PW, Neuburg M, et al. Expression pattern of the bullous pemphigoid-180 antigen in normal and neoplastic epithelia. Br J Dermatol. 1995;133:385-391.
21. Kelly SE, Black MM, Fleming S. Antigen-presenting cells in the skin and placenta in pemphigoid gestationis. Br J Dermatol. 1990;122:593-599.
22. Borthwick GM, Holmes RC, Stirrat GM. Abnormal expression of class II MHC antigens in placentae from patients with pemphigoid gestationis. Placenta. 1988;9:81-94.
23. Kelly SE, Black MM, Fleming S. Pemphigoid gestationis: a unique mechanism of initiation of an autoimmune response by MHC class II molecules. J Pathol. 1989;158:81-82.
24. Borradori L, Saurat JH. Specific dermatoses of pregnancy. Toward a comprehensive view. Arch Dermatol. 1994;130:778-780.
25. Shimanovich I, Bröcker EB, Zillikens D. Pemphigoid gestationis: new insights into the pathogenesis lead to novel diagnostic tools. Br J Obstet Gynaecol. 2002;109:970-976.
IN THIS ARTICLE
What you need to know about thyroid disorders in pregnancy
Until recently, thyroid dysfunction was thought to have little influence on pregnancy as long as it was treated, and management was straightforward. That was before case-control studies in prominent journals suggested an association between even subclinical hypothyroidism and impaired neonatal neurodevelopment.1-4
The risk associated with hyperthyroidism in pregnancy is less clear. Currently, it is believed to cause no adverse effects; the low thyroid-stimulating hormone (TSH) resolves in most women within 4 to 12 weeks.
As for the nonpregnant state, there is no agreement between the American College of Physicians and its British counterpart as to whether isolated, subclinical hyperthyroidism leads to morbidity or mortality, although some investigators have found an excess risk of atrial fibrillation and possibly increased bone loss in postmenopausal women. Treatment of hyperthyroidism in non-pregnant women is recommended only if low TSH persists after 4 to 12 weeks and the level is less than 0.1 mIU/L.5
This article discusses the detection and management of thyroid disease in pregnancy, concentrating on 2 representative cases. (See TABLE 1, for a list of the full spectrum of thyroid disorders.)
TABLE 1
The spectrum of thyroid disorders is wide
| Hypothyroidism |
| Hashimoto’s or subacute thyroiditis |
| Subclinical hypothyroidism |
| Subclinical hypothyroxemia |
| Postpartum thyroiditis |
| Secondary hypothyroidism |
|
| Hyperthyroidism |
| Grave’s disease |
| Subclinical hyperthyroidism |
| Thyroid storm |
| Secondary hyperthyroidism |
|
CASE 1: History of Graves’ disease
S.H., 32, is 6 weeks’ pregnant with her first child. She has a history of Graves’ disease, and underwent radioactive iodine treatment 10 years ago. She then became hypothyroid and has been on levothyroxine replacement for the past 9 years. She visits her endocrinologist annually and reports good control on 125 μg daily of oral levothyroxine sodium.
How should her pregnancy be managed?
When the mother has a history of Graves’ disease, regardless of her current thyroid state, 1% to 5% of newborns develop hyperthyroidism due to transplacental passage of thyroid-stimulating immunoglobulins (TSI). Fetal or neonatal hyperthyroidism is associated with fetal tachycardia (heart rate >160 bpm), poor growth, goiter, craniosynostosis, and advanced bone age. Therefore, fetal growth and heart rate should be monitored throughout pregnancy in these women. Investigators have published monograms on fetal thyroid measurement,6 and even argued that Doppler ultrasonography can differentiate between fetal hypo- and hyperthyroidism caused by drugs or disease processes.7 However, measurement of TSI levels (poor predictive value) and ultrasonography for fetal goiter (low yield) are controversial.
Another important consideration: The requirement for thyroxine hormone increases by approximately 30% in women on thyroid supplementation during pregnancy.8 This has been demonstrated in more than 9 studies, with athyrotic women experiencing greater increases than women with autoimmune hypothyroidism.9 The need for thyroxine increases as early as 5 weeks’ gestation and plateaus by 16 weeks.
Because hypothyroxemia or hypothyroidism (clinical or subclinical) may be associated with adverse neurodevelopment in the newborn, I recommend increasing the dosage of levothyroxine at the first encounter with this patient to 150 μg/day (<25% dosage increase). I also suggest measuring the baseline TSH level, if no reading is available from the past 3 months. If baseline TSH is less than 2.5 mIU/L, the dosage increase is probably adequate. If the TSH exceeds 2.5 mIU/L, however, I would ask the patient to take 1 extra pill (125 μg) on 2 days of the week (>30% dosage increase) and measure TSH again 4 to 6 weeks later (thyroxine takes 5 weeks to equilibrate after a change in dosage). Once the dosage has been adequately adjusted, I would monitor TSH every 6 to 8 weeks until delivery. At that time, the dosage should be reduced to the prepregnancy level, with TSH measured again in 4 to 6 weeks to confirm that the dosage is adequate.
Levothyroxine absorption is hampered by ferrous sulfate, aluminum hydroxide antacids, proton-pump inhibitors, and cholestyramine. Levothyroxine should be ingested at least 4 hours before or after the prenatal vitamin. The metabolism of levothyroxine is altered by phenytoin, carbamazepine, and rifampin.
Subclinical hypothyroidism can progress to overt disease
The majority of women with hypothyroidism are asymptomatic, with only 20% to 30% having any complaints, usually nonspecific (TABLE 2). Women with 1 or 2 symptoms are no more likely to have abnormal thyroid function tests than are asymptomatic women.
Overt hypothyroidism is primarily diagnosed with laboratory tests—specifically, low free thyroxine (FT4) or free triiodothyronine (FT3), or both, resulting in elevated TSH levels.
If untreated, overt hypothyroidism is associated with significant morbidity in both the nonpregnant and pregnant states (TABLE 3). Levothyroxine is easily administered and well tolerated, with no to few adverse effects with appropriate follow-up.10
In women with subclinical hypothyroidism, only 1 of the thyroid function tests is elevated—either elevated TSH with normal free thyroid hormone levels (mild thyroid failure) or normal TSH with low FT4 levels (hypothyroxemia). Most cases of mild thyroid failure are thought to be related to thyroid dysfunction, whereas hypothyroxemia is usually associated with a deficiency of iodine.
Subclinical hypothyroidism can occur in women with a history of thyroid disease, after surgery or radioactive iodine therapy for toxic goiter, or as the result of an inadequate dosage of thyroid medication. It can also occur in women with no history of thyroid dysfunction, detected in routine testing in women with no symptoms or with nonspecific complaints that could be related to thyroid disease. Experts agree that women with secondary subclinical disease should be treated to achieve a euthyroid state because approximately 5% per year will develop overt disease. Considerable controversy clouds management of women with primary subclinical hypothyroidism.
Subclinical hypothyroidism is more common among white women (~67%) than among black women.
TABLE 2
Know these signs and symptoms of thyroid dysfunction
| HYPOTHYROIDISM | HYPERTHYROIDISM |
|---|---|
| Fatigue | Resting tremors |
| Constipation | Hyperdefecation |
| Somnolence | Insomnia |
| Cold intolerance | Heat intolerance |
| Hair loss | Diaphoresis |
| Depression | Nervousness |
| Decreased libido | Palpitations |
| Menstrual irregularities | |
| Weight gain despite poor appetite | Weight loss |
| Dry skin | Warm, moist skin |
| Deafness | Ophthalmopathy |
| Hoarseness | Sinus tachycardia |
| Paresthesia | |
| Carpal tunnel syndrome | |
| Periorbital puffiness | |
| Slow cerebration or movement | |
| Slowing ankle jerk | Hyperreflexia |
| Goiter | Thyromegaly |
TABLE 3
Consequences of untreated thyroid dysfunction are significant
| HYPOTHYROIDISM | HYPERTHYROIDISM |
|---|---|
| Nonpregnant state | |
| Hyperlipidemia | Atrial fibrillation |
| Atherosclerosis | Congestive heart failure |
| Osteoporosis | |
| Neuropsychiatric disorders | Neuropsychiatric disorders with or without dementia/Alzheimer’s disease |
| Reduced functional status and quality of life | Reduced functional status and quality of life |
| Pregnancy | |
| Spontaneous abortion | Spontaneous abortion |
| Preterm delivery <32 weeks | Preterm labor |
| Low birth weight | Low birth weight |
| Perinatal morbidity and mortality | Stillbirth |
| Preeclampsia/gestational hypertension | Preeclampsia |
| Anovulation | |
| Cesarean delivery | |
| Postpartum hemorrhage | |
| Placental abruption | |
| Nonreassuring fetal heart rate tracing | |
| Impaired neurodevelopment | |
| Subclinical disease | |
| Risk factor for overt disease | Risk factor for overt disease |
Subclinical hyperthyroidism is more elusive
Overt hyperthyroidism can be detected through symptom-based screening (TABLE 2).
Subclinical hyperthyroidism is defined as low TSH with normal thyroid hormone levels. The pituitary appears to be more sensitive to the presence of thyroid hormones than to their absence. Subclinical hyperthyroidism is most common in black women and smokers. Approximately 50% of women with subclinical disease will have normal TSH levels several weeks to 1 year later.
Because subclinical hyperthyroidism can occur in up to 20% of women on thyroid replacement therapy, the dosage should be adjusted to achieve a euthyroid state.
CASE 2 Diabetes, with a family history of hypothyroidism
M.H., 30, is 12 weeks’ pregnant with her second child and reports a 12-year history of diabetes. Before she became pregnant, she was taking insulin, with HbA1C=8.5%. Her history includes a mother with hypothyroidism.
How should she be managed?
Besides the obvious need for good diabetes control, this case merits screening for thyroid dysfunction, as the patient has 2 risk factors (TABLE 4).
One prominent controversy of the 21st century is whether all pregnant women should undergo routine screening for hypothyroidism. The controversy extends to screening all women of childbearing age.
TABLE 4
Risk factors for hypothyroidism include other autoimmune disorders
| Family history of thyroid disease |
| More than 3 symptoms |
| History of postpartum thyroid disease |
| Type 1 diabetes mellitus |
| Recurrent spontaneous abortions |
| Unexplained intrauterine fetal demise |
| Other autoimmune disorders |
|
$64,000 question: Should all women be screened?
Screening would involve testing thyroid function in women with no history and few or no signs and symptoms of thyroid dysfunction. Such screening could be population-based (using special methods to recruit, contact, and follow patients) or case-finding (performed on patients who present for unrelated reasons). The decision to screen a woman who is pregnant or planning to conceive should be based on many factors, most notably whether treatment prevents impaired neonatal neurodevelopment and preterm delivery.
A mother’s elevated TSH level can have lasting effects in the child
Haddow and colleagues1 measured the IQ of 47 children, ages 7 to 9 years, whose mothers had had an elevated serum TSH concentration in the second trimester, 15 children whose mothers had high serum TSH values in combination with low thyroxine levels in the second trimester, and 124 children whose mothers had normal TSH values. None of these children had hypothyroidism at birth. The children of the women with an elevated TSH concentration had lower IQs. Interestingly, the group with hypothyroxemia was not evaluated at the time, and the mean FT4 level was low in the entire group, suggesting overt hypothyroidism rather than subclinical disease.
In a study from the Netherlands, Pop and associates2 found impaired psychomotor function in 22 infants (age 10 months) whose mothers had had FT4 below the 10th percentile at 12 weeks of gestation, compared with 194 infants whose mothers had normal readings. When these children were reevaluated at 2 years, no neurodevelopmental delay was found in the infants whose mothers had a spontaneously increased free thyroxine level after the first trimester.
There is much speculation about precisely when thyroid hormone is critical for fetal brain development. The study by Pop and associates2 would suggest it is important after the first trimester. That study also recommends exogenous thyroxine for FT4 values below 0.96 ng/mL (12 pmol/L).
In Italy, Vermiglio and coworkers11 conducted behavioral and neuropsychological testing in 27 children at ages 18 to 36 months and again at 8 to 10 years. Mothers of 16 of these children were from a moderately iodine-deficient area (group A), and the mothers of 11 children were from a marginally iodine-sufficient area and were monitored with thyroid function tests in the first trimester (group B). Attention-deficit and hyperactivity disorders were more prevalent in group A.
Two studies published in 2006 also suggest that maternal free thyroxine levels in the first trimester of pregnancy correlate with impaired neonatal behavior at 3 months, and impaired mental development at ages 6, 9, and 12 months.3,4
Thyroid disorders affect approximately 5% of the general population, two thirds of them women.17 Subclinical hypothyroidism occurs in an additional 4.3%, and subclinical hyperthyroidism in 0.7%.
In pregnancy, subclinical disease is present in 3.6% of women; overt hypothyroidism, in 2.5%; and overt hyperthyroidism, in 0.2%. In addition, thyroid disease affects 5% to 9% of postpartum women.14
No consensus on whom to test or what test is best
There is no clear agreement about which population should be targeted for screening or what test to use. Most medical societies do not recommend routine screening, including the American College of Obstetricians and Gynecologists, which recommends TSH testing only in women with a history of thyroid disease and in women with “symptoms” (but does not specify which symptoms or how many symptoms warrant testing). A majority of organizations agree that all high-risk women should be tested when pregnancy is planned or as soon as pregnancy is confirmed.
TABLE 5
Comparison of screening recommendations highlights lack of consensus (and, in pregnancy, the absence of guidance)
| YEAR | ORGANIZATION | NONPREGNANT STATE | PREGNANCY |
|---|---|---|---|
| 1994 | American Association of Clinical Endocrinologists (AACE), American Academy of Family Physicians | Periodic assessment via thyroid function tests in older women | No recommendation |
| 1998 | American College of Physicians | Office screening of women >50 years of age | No recommendation |
| 2000 | American Thyroid Association (ATA) | Measure TSH every 5 years in women age 35 and older (probably men also) | No recommendation |
| 2002 | American College of Obstetricians and Gynecologists | Measure TSH every 5 years in women age 65 and older | No screening recommended |
| 2003 | Institute of Medicine | Screening is not cost-effective in Medicare population | No recommendation |
| 2004 | United States Preventive Services Task Force | Routine screening of children and adults is not recommended | No recommendation |
| 2004 | AACE, ATA, the Endocrine Society Consensus Group | No population-based screening, but “aggressive case finding” in women at high risk and those over age 60 | Do not support routine testing; recommend “aggressive case finding” and screening pregnant women at high risk |
Proponents of routine screening argue that it may limit health risks to children and save money in the long run, and they point out that thyroid disease is easy to treat with pills. Opponents note that no cost-benefit analysis has been performed, the benefits of treating mild disease are unclear, and screening a large population could be a significant expense ($40–100 per person) and would necessitate a lifelong commitment to daily medication in asymptomatic patients.
As a diagnostic test, the TSH immunoassay has 98% sensitivity and 92% specificity, and the current third-generation test lacks biases between methods and does not require method-specific reference ranges. However, it has low predictive value as a screening test (7–25%), possibly because of multiple confounding variables. Despite being the “gold standard,” it can lead to falsely positive results.
TABLE 6
What makes a TSH measurement falsely high or low?
| ELEVATED TSH | LOW TSH |
|---|---|
| Recovery from nonthyroidal illness | Euthyroid sick syndrome |
| Late evening TSH surge | Recovery from normal pregnancy |
| Assay variability | |
| Adrenal insufficiency | |
| Drugs: metoclopramide, amiodarone, cholecystographic dye (sodium ipodate) | Drugs: glucocorticoids, dopamine |
As for the value of FT4 alone as a screening test, we lack sufficient data on its utility. Another problem is that equilibrium dialysis, the most accurate and reliable laboratory method to measure FT4, is too technically complex and expensive for routine use. The most widely used 2-step radioimmunoassay is automated, but different methods are used by different commercial laboratories, cutoffs vary for every laboratory, and the results are sensitive to abnormal binding-protein states such as pregnancy in a method-specific manner. Tandem mass spectrometry is as reliable as equilibrium dialysis, but is not yet readily available.12
Another consideration: The physiologic changes in pregnancy render the cutoffs for the nongravid state inapplicable. TSH is lower in pregnancy, whereas the FT4 level is probably slightly increased or unchanged (TT4 is 1.5 times the prepregnancy value).
The normal reference values in each trimester of pregnancy from iodine-sufficient, autoimmune thyroid antibody-negative women are becoming available for TSH,12 as are nomograms that adjust for fetal number and gestational age.12 The measurement of FT4 still needs to be standardized across laboratories (method-specific, trimester-specific, and, possibly, population-specific reference ranges) for pregnancy.
How to manage subclinical thyroid disorders
In the nonpregnant state, subclinical hyperthyroidism should be treated in the following groups if the abnormal thyroid levels persist beyond 4 to 12 weeks and the TSH level is less than 0.1 mIU/L:
- High-risk women: postmenopausal or over age 60
- Low-risk women with cardiac disease, low bone density, or nodular thyroid disease.
If the TSH level is between 0.1 and 0.5 mIU/L, treatment is recommended for high-risk women with cardiac disease, low bone density, or nodular thyroid disease.
Subclinical hypothyroidism with a TSH level of 4.5 to 10 mIU/L need not be treated even in an elderly woman or a patient with a high antibody titer. Treatment of any woman is beneficial when the TSH level exceeds 10 mIU/L because it can ease symptoms, reduce low-density lipoprotein cholesterol, and prevent progression to overt disease. However, treatment may not lower morbidity and mortality and carries a roughly 20% risk of causing subclinical hyperthyroidism. It also involves a lifelong commitment to daily medication.
How to treat subclinical hypothyroidism in pregnancy
It is clear that overt hypothyroidism warrants treatment in both the pregnant and nonpregnant states, but the management of subclinical disease remains controversial. No trials have assessed the benefits of thyroid hormone replacement on the neuropsychological development of the newborn. Expert opinion suggests that women be treated if they are planning a pregnancy, are already pregnant, or have high TSH or low FT4.
Until we have more data, pregnant women and those planning a pregnancy should be treated with levothyroxine (starting at 2 μg/kg/day) if they are found to have elevated TSH or low FT4.
Two studies will answer questions about effects in pregnancy
The Controlled Antenatal Thyroid Screening (CATS) study will be completed in 2009, and the National Institute of Child Health and Human Development Maternal–Fetal Medicine Units (NICHD-MFMU) study will conclude in 2014. The CATS study screened 22,000 pregnant women in the United Kingdom before 16 weeks’ gestation. Half these women were treated with levothyroxine in pregnancy if they had a TSH measurement above the 97.5th percentile or FT4 below the 2.5th percentile, and half had their blood samples stored and tested only after delivery.14 Cutoff values were derived from previously obtained antenatal sera from well-dated pregnancies, and were adjusted after every 2,000 to 3,000 samples.
The CATS study was conducted in an iodine-sufficient area with a median urinary iodine excretion of 100 μg/L (range: 11–240 μg/L). Each group contained 400 women with subclinical hypothyroidism (52% had low FT4, 45% had high TSH, and 3% had both). Antithyroid peroxidase antibodies were present in 50% of women with elevated TSH but in only 10% of women with low FT4. Neuropsychological development in their children is being tested at 3 years of age.
The NICHD-MFMU study plans to screen 110,000 women at 14 centers over 2 years, and will randomize roughly 1,000 women to thyroxine treatment or placebo. They plan to assess intellectual development of the infants yearly for 5 years, and test the mothers for postpartum thyroid dysfunction and follow them at 1 and 5 years to detect the rate of progression to overt hypothyroidism.
Postpartum dysfunction can be transient or permanent
Postpartum thyroid dysfunction (PPTD) is an autoimmune disorder that occurs at 13 to 19 weeks postpartum, affects 1 in 12 women worldwide, and is usually associated with psychiatric symptomatology.15
PPTD also is strongly associated with antithyroid peroxidase antibodies (TPOAbs).16 Premawardhana and colleagues found that 10% of women are TPOAbs-positive in the first trimester; of these, 50% develop PPTD. Of the women with PPTD, 20% to 30% develop permanent hypothyroidism, and an additional 30% to 40% develop it by 7 years. In contrast, only 5% of women without PPTD progress to overt disease by 7 years. These findings have generated considerable controversy about routine screening for PPTD. Proponents argue that PPTD is highly prevalent, linked to considerable morbidity, is easily diagnosed with relatively inexpensive tests, and is easy to treat effectively. Critics note the lack of consensus on the best screening test (thyroid function test versus TPOAbs), optimal timing of screening (early pregnancy or postpartum), and lack of high-quality, prospective cost-benefit analyses. The NICHD-MFMU hopes to resolve these controversies.
The author reports no financial relationships relevant to this article.
1. Haddow JE, Palomaki GE, Allan WC, et al. Maternal thyroid deficiency during pregnancy and subsequent neuropsychological development of the child [see comment]. N Engl J Med. 1999;341:549-555.
2. Pop VJ, Kuijpens JL, van Baar AL, et al. Low maternal free thyroxine concentrations during early pregnancy are associated with impaired psychomotor development in infancy [see comment]. Clin Endocrinol. 1999;50:149-155.
3. Kasatkina EP, Samsonova LN, Ivakhnenko VN, et al. Gestational hypothyroxinemia and cognitive function in offspring. Neurosci Behav Physiol. 2006;36:619-624.
4. Kooistra L, Crawford S, van Baar AL, Brouwers EP, Pop VJ. Neonatal effects of maternal hypothyroxinemia during early pregnancy. Pediatrics. 2006;117:161-167.
5. Surks MI, Ortiz E, Daniels GH, et al. Subclinical thyroid disease: scientific review and guidelines for diagnosis and management [see comment]. JAMA. 2004;291:228-238.
6. Ranzini AC, Ananth CV, Smulian JC, Kung M, Limbachia A, Vintzileos AM. Ultrasonography of the fetal thyroid: nomograms based on biparietal diameter and gestational age. J Ultrasound Med. 2001;20:613-7.
7. Polak M, Le Gac I, Vuillard E, et al. Fetal and neonatal thyroid function in relation to maternal Graves’ disease. Best Practice & Research Clin Endocrinol Metab. 2004;18:289-302.
8. Alexander EK, Marqusee E, Lawrence J, Jarolim P, Fischer GA, Larsen PR. Timing and magnitude of increases in levothyroxine requirements during pregnancy in women with hypothyroidism [see comment]. N Engl J Med. 2004;351:241-249.
9. Mandel SJ, Spencer CA, Hollowell JG. Are detection and treatment of thyroid insufficiency in pregnancy feasible? Thyroid. 2005;15:44-53.
10. Negro R, Formoso G, Mangieri T, Pezzarossa A, Dazzi D, Hassan H. Levothyroxine treatment in euthyroid pregnant women with autoimmune thyroid disease: effects on obstetrical complications [see comment]. J Clin Endocrinol Metab. 2006;91:2587-2591.
11. Vermiglio F, Lo Presti VP, Moleti M, et al. Attention deficit and hyperactivity disorders in the offspring of mothers exposed to mild-moderate iodine deficiency: a possible novel iodine deficiency disorder in developed countries. J Clin Endocrinol Metab. 2004;89:6054-6060.
12. Soldin OP, Tractenberg RE, Hollowell JG, Jonklaas J, Janicic N, Soldin SJ. Trimester-specific changes in maternal thyroid hormone, thyrotropin, and thyroglobulin concentrations during gestation: trends and associations across trimesters in iodine sufficiency. Thyroid. 2004;14:1084-1090.
13. Dashe JS, Casey BM, Wells CE, et al. Thyroid-stimulating hormone in singleton and twin pregnancy: importance of gestational age-specific reference ranges. Obstet Gynecol. 2005;106:753-757.
14. Lazarus JH, Premawardhana LDKE. Screening for thyroid disease in pregnancy. J Clin Pathol. 2005;58:449-452.
15. Nicholson WK, Robinson KA, Smallridge RC, Ladenson PW, Powe NR. Prevalence of postpartum thyroid dysfunction: a quantitative review. Thyroid. 2006;16:573-582.
16. Premawardhana LDKE, Parkes AB, John R, Harris B, Lazarus JH. Thyroid peroxidase antibodies in early pregnancy: utility for prediction of postpartum thyroid dysfunction and implications for screening. Thyroid. 2004;14:610-615.
17. Hollowell JG, Staehling NW, Flanders WD, et al. Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III) [see comment]. J Clin Endocrinol Metab. 2002;87:489-499.
Until recently, thyroid dysfunction was thought to have little influence on pregnancy as long as it was treated, and management was straightforward. That was before case-control studies in prominent journals suggested an association between even subclinical hypothyroidism and impaired neonatal neurodevelopment.1-4
The risk associated with hyperthyroidism in pregnancy is less clear. Currently, it is believed to cause no adverse effects; the low thyroid-stimulating hormone (TSH) resolves in most women within 4 to 12 weeks.
As for the nonpregnant state, there is no agreement between the American College of Physicians and its British counterpart as to whether isolated, subclinical hyperthyroidism leads to morbidity or mortality, although some investigators have found an excess risk of atrial fibrillation and possibly increased bone loss in postmenopausal women. Treatment of hyperthyroidism in non-pregnant women is recommended only if low TSH persists after 4 to 12 weeks and the level is less than 0.1 mIU/L.5
This article discusses the detection and management of thyroid disease in pregnancy, concentrating on 2 representative cases. (See TABLE 1, for a list of the full spectrum of thyroid disorders.)
TABLE 1
The spectrum of thyroid disorders is wide
| Hypothyroidism |
| Hashimoto’s or subacute thyroiditis |
| Subclinical hypothyroidism |
| Subclinical hypothyroxemia |
| Postpartum thyroiditis |
| Secondary hypothyroidism |
|
| Hyperthyroidism |
| Grave’s disease |
| Subclinical hyperthyroidism |
| Thyroid storm |
| Secondary hyperthyroidism |
|
CASE 1: History of Graves’ disease
S.H., 32, is 6 weeks’ pregnant with her first child. She has a history of Graves’ disease, and underwent radioactive iodine treatment 10 years ago. She then became hypothyroid and has been on levothyroxine replacement for the past 9 years. She visits her endocrinologist annually and reports good control on 125 μg daily of oral levothyroxine sodium.
How should her pregnancy be managed?
When the mother has a history of Graves’ disease, regardless of her current thyroid state, 1% to 5% of newborns develop hyperthyroidism due to transplacental passage of thyroid-stimulating immunoglobulins (TSI). Fetal or neonatal hyperthyroidism is associated with fetal tachycardia (heart rate >160 bpm), poor growth, goiter, craniosynostosis, and advanced bone age. Therefore, fetal growth and heart rate should be monitored throughout pregnancy in these women. Investigators have published monograms on fetal thyroid measurement,6 and even argued that Doppler ultrasonography can differentiate between fetal hypo- and hyperthyroidism caused by drugs or disease processes.7 However, measurement of TSI levels (poor predictive value) and ultrasonography for fetal goiter (low yield) are controversial.
Another important consideration: The requirement for thyroxine hormone increases by approximately 30% in women on thyroid supplementation during pregnancy.8 This has been demonstrated in more than 9 studies, with athyrotic women experiencing greater increases than women with autoimmune hypothyroidism.9 The need for thyroxine increases as early as 5 weeks’ gestation and plateaus by 16 weeks.
Because hypothyroxemia or hypothyroidism (clinical or subclinical) may be associated with adverse neurodevelopment in the newborn, I recommend increasing the dosage of levothyroxine at the first encounter with this patient to 150 μg/day (<25% dosage increase). I also suggest measuring the baseline TSH level, if no reading is available from the past 3 months. If baseline TSH is less than 2.5 mIU/L, the dosage increase is probably adequate. If the TSH exceeds 2.5 mIU/L, however, I would ask the patient to take 1 extra pill (125 μg) on 2 days of the week (>30% dosage increase) and measure TSH again 4 to 6 weeks later (thyroxine takes 5 weeks to equilibrate after a change in dosage). Once the dosage has been adequately adjusted, I would monitor TSH every 6 to 8 weeks until delivery. At that time, the dosage should be reduced to the prepregnancy level, with TSH measured again in 4 to 6 weeks to confirm that the dosage is adequate.
Levothyroxine absorption is hampered by ferrous sulfate, aluminum hydroxide antacids, proton-pump inhibitors, and cholestyramine. Levothyroxine should be ingested at least 4 hours before or after the prenatal vitamin. The metabolism of levothyroxine is altered by phenytoin, carbamazepine, and rifampin.
Subclinical hypothyroidism can progress to overt disease
The majority of women with hypothyroidism are asymptomatic, with only 20% to 30% having any complaints, usually nonspecific (TABLE 2). Women with 1 or 2 symptoms are no more likely to have abnormal thyroid function tests than are asymptomatic women.
Overt hypothyroidism is primarily diagnosed with laboratory tests—specifically, low free thyroxine (FT4) or free triiodothyronine (FT3), or both, resulting in elevated TSH levels.
If untreated, overt hypothyroidism is associated with significant morbidity in both the nonpregnant and pregnant states (TABLE 3). Levothyroxine is easily administered and well tolerated, with no to few adverse effects with appropriate follow-up.10
In women with subclinical hypothyroidism, only 1 of the thyroid function tests is elevated—either elevated TSH with normal free thyroid hormone levels (mild thyroid failure) or normal TSH with low FT4 levels (hypothyroxemia). Most cases of mild thyroid failure are thought to be related to thyroid dysfunction, whereas hypothyroxemia is usually associated with a deficiency of iodine.
Subclinical hypothyroidism can occur in women with a history of thyroid disease, after surgery or radioactive iodine therapy for toxic goiter, or as the result of an inadequate dosage of thyroid medication. It can also occur in women with no history of thyroid dysfunction, detected in routine testing in women with no symptoms or with nonspecific complaints that could be related to thyroid disease. Experts agree that women with secondary subclinical disease should be treated to achieve a euthyroid state because approximately 5% per year will develop overt disease. Considerable controversy clouds management of women with primary subclinical hypothyroidism.
Subclinical hypothyroidism is more common among white women (~67%) than among black women.
TABLE 2
Know these signs and symptoms of thyroid dysfunction
| HYPOTHYROIDISM | HYPERTHYROIDISM |
|---|---|
| Fatigue | Resting tremors |
| Constipation | Hyperdefecation |
| Somnolence | Insomnia |
| Cold intolerance | Heat intolerance |
| Hair loss | Diaphoresis |
| Depression | Nervousness |
| Decreased libido | Palpitations |
| Menstrual irregularities | |
| Weight gain despite poor appetite | Weight loss |
| Dry skin | Warm, moist skin |
| Deafness | Ophthalmopathy |
| Hoarseness | Sinus tachycardia |
| Paresthesia | |
| Carpal tunnel syndrome | |
| Periorbital puffiness | |
| Slow cerebration or movement | |
| Slowing ankle jerk | Hyperreflexia |
| Goiter | Thyromegaly |
TABLE 3
Consequences of untreated thyroid dysfunction are significant
| HYPOTHYROIDISM | HYPERTHYROIDISM |
|---|---|
| Nonpregnant state | |
| Hyperlipidemia | Atrial fibrillation |
| Atherosclerosis | Congestive heart failure |
| Osteoporosis | |
| Neuropsychiatric disorders | Neuropsychiatric disorders with or without dementia/Alzheimer’s disease |
| Reduced functional status and quality of life | Reduced functional status and quality of life |
| Pregnancy | |
| Spontaneous abortion | Spontaneous abortion |
| Preterm delivery <32 weeks | Preterm labor |
| Low birth weight | Low birth weight |
| Perinatal morbidity and mortality | Stillbirth |
| Preeclampsia/gestational hypertension | Preeclampsia |
| Anovulation | |
| Cesarean delivery | |
| Postpartum hemorrhage | |
| Placental abruption | |
| Nonreassuring fetal heart rate tracing | |
| Impaired neurodevelopment | |
| Subclinical disease | |
| Risk factor for overt disease | Risk factor for overt disease |
Subclinical hyperthyroidism is more elusive
Overt hyperthyroidism can be detected through symptom-based screening (TABLE 2).
Subclinical hyperthyroidism is defined as low TSH with normal thyroid hormone levels. The pituitary appears to be more sensitive to the presence of thyroid hormones than to their absence. Subclinical hyperthyroidism is most common in black women and smokers. Approximately 50% of women with subclinical disease will have normal TSH levels several weeks to 1 year later.
Because subclinical hyperthyroidism can occur in up to 20% of women on thyroid replacement therapy, the dosage should be adjusted to achieve a euthyroid state.
CASE 2 Diabetes, with a family history of hypothyroidism
M.H., 30, is 12 weeks’ pregnant with her second child and reports a 12-year history of diabetes. Before she became pregnant, she was taking insulin, with HbA1C=8.5%. Her history includes a mother with hypothyroidism.
How should she be managed?
Besides the obvious need for good diabetes control, this case merits screening for thyroid dysfunction, as the patient has 2 risk factors (TABLE 4).
One prominent controversy of the 21st century is whether all pregnant women should undergo routine screening for hypothyroidism. The controversy extends to screening all women of childbearing age.
TABLE 4
Risk factors for hypothyroidism include other autoimmune disorders
| Family history of thyroid disease |
| More than 3 symptoms |
| History of postpartum thyroid disease |
| Type 1 diabetes mellitus |
| Recurrent spontaneous abortions |
| Unexplained intrauterine fetal demise |
| Other autoimmune disorders |
|
$64,000 question: Should all women be screened?
Screening would involve testing thyroid function in women with no history and few or no signs and symptoms of thyroid dysfunction. Such screening could be population-based (using special methods to recruit, contact, and follow patients) or case-finding (performed on patients who present for unrelated reasons). The decision to screen a woman who is pregnant or planning to conceive should be based on many factors, most notably whether treatment prevents impaired neonatal neurodevelopment and preterm delivery.
A mother’s elevated TSH level can have lasting effects in the child
Haddow and colleagues1 measured the IQ of 47 children, ages 7 to 9 years, whose mothers had had an elevated serum TSH concentration in the second trimester, 15 children whose mothers had high serum TSH values in combination with low thyroxine levels in the second trimester, and 124 children whose mothers had normal TSH values. None of these children had hypothyroidism at birth. The children of the women with an elevated TSH concentration had lower IQs. Interestingly, the group with hypothyroxemia was not evaluated at the time, and the mean FT4 level was low in the entire group, suggesting overt hypothyroidism rather than subclinical disease.
In a study from the Netherlands, Pop and associates2 found impaired psychomotor function in 22 infants (age 10 months) whose mothers had had FT4 below the 10th percentile at 12 weeks of gestation, compared with 194 infants whose mothers had normal readings. When these children were reevaluated at 2 years, no neurodevelopmental delay was found in the infants whose mothers had a spontaneously increased free thyroxine level after the first trimester.
There is much speculation about precisely when thyroid hormone is critical for fetal brain development. The study by Pop and associates2 would suggest it is important after the first trimester. That study also recommends exogenous thyroxine for FT4 values below 0.96 ng/mL (12 pmol/L).
In Italy, Vermiglio and coworkers11 conducted behavioral and neuropsychological testing in 27 children at ages 18 to 36 months and again at 8 to 10 years. Mothers of 16 of these children were from a moderately iodine-deficient area (group A), and the mothers of 11 children were from a marginally iodine-sufficient area and were monitored with thyroid function tests in the first trimester (group B). Attention-deficit and hyperactivity disorders were more prevalent in group A.
Two studies published in 2006 also suggest that maternal free thyroxine levels in the first trimester of pregnancy correlate with impaired neonatal behavior at 3 months, and impaired mental development at ages 6, 9, and 12 months.3,4
Thyroid disorders affect approximately 5% of the general population, two thirds of them women.17 Subclinical hypothyroidism occurs in an additional 4.3%, and subclinical hyperthyroidism in 0.7%.
In pregnancy, subclinical disease is present in 3.6% of women; overt hypothyroidism, in 2.5%; and overt hyperthyroidism, in 0.2%. In addition, thyroid disease affects 5% to 9% of postpartum women.14
No consensus on whom to test or what test is best
There is no clear agreement about which population should be targeted for screening or what test to use. Most medical societies do not recommend routine screening, including the American College of Obstetricians and Gynecologists, which recommends TSH testing only in women with a history of thyroid disease and in women with “symptoms” (but does not specify which symptoms or how many symptoms warrant testing). A majority of organizations agree that all high-risk women should be tested when pregnancy is planned or as soon as pregnancy is confirmed.
TABLE 5
Comparison of screening recommendations highlights lack of consensus (and, in pregnancy, the absence of guidance)
| YEAR | ORGANIZATION | NONPREGNANT STATE | PREGNANCY |
|---|---|---|---|
| 1994 | American Association of Clinical Endocrinologists (AACE), American Academy of Family Physicians | Periodic assessment via thyroid function tests in older women | No recommendation |
| 1998 | American College of Physicians | Office screening of women >50 years of age | No recommendation |
| 2000 | American Thyroid Association (ATA) | Measure TSH every 5 years in women age 35 and older (probably men also) | No recommendation |
| 2002 | American College of Obstetricians and Gynecologists | Measure TSH every 5 years in women age 65 and older | No screening recommended |
| 2003 | Institute of Medicine | Screening is not cost-effective in Medicare population | No recommendation |
| 2004 | United States Preventive Services Task Force | Routine screening of children and adults is not recommended | No recommendation |
| 2004 | AACE, ATA, the Endocrine Society Consensus Group | No population-based screening, but “aggressive case finding” in women at high risk and those over age 60 | Do not support routine testing; recommend “aggressive case finding” and screening pregnant women at high risk |
Proponents of routine screening argue that it may limit health risks to children and save money in the long run, and they point out that thyroid disease is easy to treat with pills. Opponents note that no cost-benefit analysis has been performed, the benefits of treating mild disease are unclear, and screening a large population could be a significant expense ($40–100 per person) and would necessitate a lifelong commitment to daily medication in asymptomatic patients.
As a diagnostic test, the TSH immunoassay has 98% sensitivity and 92% specificity, and the current third-generation test lacks biases between methods and does not require method-specific reference ranges. However, it has low predictive value as a screening test (7–25%), possibly because of multiple confounding variables. Despite being the “gold standard,” it can lead to falsely positive results.
TABLE 6
What makes a TSH measurement falsely high or low?
| ELEVATED TSH | LOW TSH |
|---|---|
| Recovery from nonthyroidal illness | Euthyroid sick syndrome |
| Late evening TSH surge | Recovery from normal pregnancy |
| Assay variability | |
| Adrenal insufficiency | |
| Drugs: metoclopramide, amiodarone, cholecystographic dye (sodium ipodate) | Drugs: glucocorticoids, dopamine |
As for the value of FT4 alone as a screening test, we lack sufficient data on its utility. Another problem is that equilibrium dialysis, the most accurate and reliable laboratory method to measure FT4, is too technically complex and expensive for routine use. The most widely used 2-step radioimmunoassay is automated, but different methods are used by different commercial laboratories, cutoffs vary for every laboratory, and the results are sensitive to abnormal binding-protein states such as pregnancy in a method-specific manner. Tandem mass spectrometry is as reliable as equilibrium dialysis, but is not yet readily available.12
Another consideration: The physiologic changes in pregnancy render the cutoffs for the nongravid state inapplicable. TSH is lower in pregnancy, whereas the FT4 level is probably slightly increased or unchanged (TT4 is 1.5 times the prepregnancy value).
The normal reference values in each trimester of pregnancy from iodine-sufficient, autoimmune thyroid antibody-negative women are becoming available for TSH,12 as are nomograms that adjust for fetal number and gestational age.12 The measurement of FT4 still needs to be standardized across laboratories (method-specific, trimester-specific, and, possibly, population-specific reference ranges) for pregnancy.
How to manage subclinical thyroid disorders
In the nonpregnant state, subclinical hyperthyroidism should be treated in the following groups if the abnormal thyroid levels persist beyond 4 to 12 weeks and the TSH level is less than 0.1 mIU/L:
- High-risk women: postmenopausal or over age 60
- Low-risk women with cardiac disease, low bone density, or nodular thyroid disease.
If the TSH level is between 0.1 and 0.5 mIU/L, treatment is recommended for high-risk women with cardiac disease, low bone density, or nodular thyroid disease.
Subclinical hypothyroidism with a TSH level of 4.5 to 10 mIU/L need not be treated even in an elderly woman or a patient with a high antibody titer. Treatment of any woman is beneficial when the TSH level exceeds 10 mIU/L because it can ease symptoms, reduce low-density lipoprotein cholesterol, and prevent progression to overt disease. However, treatment may not lower morbidity and mortality and carries a roughly 20% risk of causing subclinical hyperthyroidism. It also involves a lifelong commitment to daily medication.
How to treat subclinical hypothyroidism in pregnancy
It is clear that overt hypothyroidism warrants treatment in both the pregnant and nonpregnant states, but the management of subclinical disease remains controversial. No trials have assessed the benefits of thyroid hormone replacement on the neuropsychological development of the newborn. Expert opinion suggests that women be treated if they are planning a pregnancy, are already pregnant, or have high TSH or low FT4.
Until we have more data, pregnant women and those planning a pregnancy should be treated with levothyroxine (starting at 2 μg/kg/day) if they are found to have elevated TSH or low FT4.
Two studies will answer questions about effects in pregnancy
The Controlled Antenatal Thyroid Screening (CATS) study will be completed in 2009, and the National Institute of Child Health and Human Development Maternal–Fetal Medicine Units (NICHD-MFMU) study will conclude in 2014. The CATS study screened 22,000 pregnant women in the United Kingdom before 16 weeks’ gestation. Half these women were treated with levothyroxine in pregnancy if they had a TSH measurement above the 97.5th percentile or FT4 below the 2.5th percentile, and half had their blood samples stored and tested only after delivery.14 Cutoff values were derived from previously obtained antenatal sera from well-dated pregnancies, and were adjusted after every 2,000 to 3,000 samples.
The CATS study was conducted in an iodine-sufficient area with a median urinary iodine excretion of 100 μg/L (range: 11–240 μg/L). Each group contained 400 women with subclinical hypothyroidism (52% had low FT4, 45% had high TSH, and 3% had both). Antithyroid peroxidase antibodies were present in 50% of women with elevated TSH but in only 10% of women with low FT4. Neuropsychological development in their children is being tested at 3 years of age.
The NICHD-MFMU study plans to screen 110,000 women at 14 centers over 2 years, and will randomize roughly 1,000 women to thyroxine treatment or placebo. They plan to assess intellectual development of the infants yearly for 5 years, and test the mothers for postpartum thyroid dysfunction and follow them at 1 and 5 years to detect the rate of progression to overt hypothyroidism.
Postpartum dysfunction can be transient or permanent
Postpartum thyroid dysfunction (PPTD) is an autoimmune disorder that occurs at 13 to 19 weeks postpartum, affects 1 in 12 women worldwide, and is usually associated with psychiatric symptomatology.15
PPTD also is strongly associated with antithyroid peroxidase antibodies (TPOAbs).16 Premawardhana and colleagues found that 10% of women are TPOAbs-positive in the first trimester; of these, 50% develop PPTD. Of the women with PPTD, 20% to 30% develop permanent hypothyroidism, and an additional 30% to 40% develop it by 7 years. In contrast, only 5% of women without PPTD progress to overt disease by 7 years. These findings have generated considerable controversy about routine screening for PPTD. Proponents argue that PPTD is highly prevalent, linked to considerable morbidity, is easily diagnosed with relatively inexpensive tests, and is easy to treat effectively. Critics note the lack of consensus on the best screening test (thyroid function test versus TPOAbs), optimal timing of screening (early pregnancy or postpartum), and lack of high-quality, prospective cost-benefit analyses. The NICHD-MFMU hopes to resolve these controversies.
The author reports no financial relationships relevant to this article.
Until recently, thyroid dysfunction was thought to have little influence on pregnancy as long as it was treated, and management was straightforward. That was before case-control studies in prominent journals suggested an association between even subclinical hypothyroidism and impaired neonatal neurodevelopment.1-4
The risk associated with hyperthyroidism in pregnancy is less clear. Currently, it is believed to cause no adverse effects; the low thyroid-stimulating hormone (TSH) resolves in most women within 4 to 12 weeks.
As for the nonpregnant state, there is no agreement between the American College of Physicians and its British counterpart as to whether isolated, subclinical hyperthyroidism leads to morbidity or mortality, although some investigators have found an excess risk of atrial fibrillation and possibly increased bone loss in postmenopausal women. Treatment of hyperthyroidism in non-pregnant women is recommended only if low TSH persists after 4 to 12 weeks and the level is less than 0.1 mIU/L.5
This article discusses the detection and management of thyroid disease in pregnancy, concentrating on 2 representative cases. (See TABLE 1, for a list of the full spectrum of thyroid disorders.)
TABLE 1
The spectrum of thyroid disorders is wide
| Hypothyroidism |
| Hashimoto’s or subacute thyroiditis |
| Subclinical hypothyroidism |
| Subclinical hypothyroxemia |
| Postpartum thyroiditis |
| Secondary hypothyroidism |
|
| Hyperthyroidism |
| Grave’s disease |
| Subclinical hyperthyroidism |
| Thyroid storm |
| Secondary hyperthyroidism |
|
CASE 1: History of Graves’ disease
S.H., 32, is 6 weeks’ pregnant with her first child. She has a history of Graves’ disease, and underwent radioactive iodine treatment 10 years ago. She then became hypothyroid and has been on levothyroxine replacement for the past 9 years. She visits her endocrinologist annually and reports good control on 125 μg daily of oral levothyroxine sodium.
How should her pregnancy be managed?
When the mother has a history of Graves’ disease, regardless of her current thyroid state, 1% to 5% of newborns develop hyperthyroidism due to transplacental passage of thyroid-stimulating immunoglobulins (TSI). Fetal or neonatal hyperthyroidism is associated with fetal tachycardia (heart rate >160 bpm), poor growth, goiter, craniosynostosis, and advanced bone age. Therefore, fetal growth and heart rate should be monitored throughout pregnancy in these women. Investigators have published monograms on fetal thyroid measurement,6 and even argued that Doppler ultrasonography can differentiate between fetal hypo- and hyperthyroidism caused by drugs or disease processes.7 However, measurement of TSI levels (poor predictive value) and ultrasonography for fetal goiter (low yield) are controversial.
Another important consideration: The requirement for thyroxine hormone increases by approximately 30% in women on thyroid supplementation during pregnancy.8 This has been demonstrated in more than 9 studies, with athyrotic women experiencing greater increases than women with autoimmune hypothyroidism.9 The need for thyroxine increases as early as 5 weeks’ gestation and plateaus by 16 weeks.
Because hypothyroxemia or hypothyroidism (clinical or subclinical) may be associated with adverse neurodevelopment in the newborn, I recommend increasing the dosage of levothyroxine at the first encounter with this patient to 150 μg/day (<25% dosage increase). I also suggest measuring the baseline TSH level, if no reading is available from the past 3 months. If baseline TSH is less than 2.5 mIU/L, the dosage increase is probably adequate. If the TSH exceeds 2.5 mIU/L, however, I would ask the patient to take 1 extra pill (125 μg) on 2 days of the week (>30% dosage increase) and measure TSH again 4 to 6 weeks later (thyroxine takes 5 weeks to equilibrate after a change in dosage). Once the dosage has been adequately adjusted, I would monitor TSH every 6 to 8 weeks until delivery. At that time, the dosage should be reduced to the prepregnancy level, with TSH measured again in 4 to 6 weeks to confirm that the dosage is adequate.
Levothyroxine absorption is hampered by ferrous sulfate, aluminum hydroxide antacids, proton-pump inhibitors, and cholestyramine. Levothyroxine should be ingested at least 4 hours before or after the prenatal vitamin. The metabolism of levothyroxine is altered by phenytoin, carbamazepine, and rifampin.
Subclinical hypothyroidism can progress to overt disease
The majority of women with hypothyroidism are asymptomatic, with only 20% to 30% having any complaints, usually nonspecific (TABLE 2). Women with 1 or 2 symptoms are no more likely to have abnormal thyroid function tests than are asymptomatic women.
Overt hypothyroidism is primarily diagnosed with laboratory tests—specifically, low free thyroxine (FT4) or free triiodothyronine (FT3), or both, resulting in elevated TSH levels.
If untreated, overt hypothyroidism is associated with significant morbidity in both the nonpregnant and pregnant states (TABLE 3). Levothyroxine is easily administered and well tolerated, with no to few adverse effects with appropriate follow-up.10
In women with subclinical hypothyroidism, only 1 of the thyroid function tests is elevated—either elevated TSH with normal free thyroid hormone levels (mild thyroid failure) or normal TSH with low FT4 levels (hypothyroxemia). Most cases of mild thyroid failure are thought to be related to thyroid dysfunction, whereas hypothyroxemia is usually associated with a deficiency of iodine.
Subclinical hypothyroidism can occur in women with a history of thyroid disease, after surgery or radioactive iodine therapy for toxic goiter, or as the result of an inadequate dosage of thyroid medication. It can also occur in women with no history of thyroid dysfunction, detected in routine testing in women with no symptoms or with nonspecific complaints that could be related to thyroid disease. Experts agree that women with secondary subclinical disease should be treated to achieve a euthyroid state because approximately 5% per year will develop overt disease. Considerable controversy clouds management of women with primary subclinical hypothyroidism.
Subclinical hypothyroidism is more common among white women (~67%) than among black women.
TABLE 2
Know these signs and symptoms of thyroid dysfunction
| HYPOTHYROIDISM | HYPERTHYROIDISM |
|---|---|
| Fatigue | Resting tremors |
| Constipation | Hyperdefecation |
| Somnolence | Insomnia |
| Cold intolerance | Heat intolerance |
| Hair loss | Diaphoresis |
| Depression | Nervousness |
| Decreased libido | Palpitations |
| Menstrual irregularities | |
| Weight gain despite poor appetite | Weight loss |
| Dry skin | Warm, moist skin |
| Deafness | Ophthalmopathy |
| Hoarseness | Sinus tachycardia |
| Paresthesia | |
| Carpal tunnel syndrome | |
| Periorbital puffiness | |
| Slow cerebration or movement | |
| Slowing ankle jerk | Hyperreflexia |
| Goiter | Thyromegaly |
TABLE 3
Consequences of untreated thyroid dysfunction are significant
| HYPOTHYROIDISM | HYPERTHYROIDISM |
|---|---|
| Nonpregnant state | |
| Hyperlipidemia | Atrial fibrillation |
| Atherosclerosis | Congestive heart failure |
| Osteoporosis | |
| Neuropsychiatric disorders | Neuropsychiatric disorders with or without dementia/Alzheimer’s disease |
| Reduced functional status and quality of life | Reduced functional status and quality of life |
| Pregnancy | |
| Spontaneous abortion | Spontaneous abortion |
| Preterm delivery <32 weeks | Preterm labor |
| Low birth weight | Low birth weight |
| Perinatal morbidity and mortality | Stillbirth |
| Preeclampsia/gestational hypertension | Preeclampsia |
| Anovulation | |
| Cesarean delivery | |
| Postpartum hemorrhage | |
| Placental abruption | |
| Nonreassuring fetal heart rate tracing | |
| Impaired neurodevelopment | |
| Subclinical disease | |
| Risk factor for overt disease | Risk factor for overt disease |
Subclinical hyperthyroidism is more elusive
Overt hyperthyroidism can be detected through symptom-based screening (TABLE 2).
Subclinical hyperthyroidism is defined as low TSH with normal thyroid hormone levels. The pituitary appears to be more sensitive to the presence of thyroid hormones than to their absence. Subclinical hyperthyroidism is most common in black women and smokers. Approximately 50% of women with subclinical disease will have normal TSH levels several weeks to 1 year later.
Because subclinical hyperthyroidism can occur in up to 20% of women on thyroid replacement therapy, the dosage should be adjusted to achieve a euthyroid state.
CASE 2 Diabetes, with a family history of hypothyroidism
M.H., 30, is 12 weeks’ pregnant with her second child and reports a 12-year history of diabetes. Before she became pregnant, she was taking insulin, with HbA1C=8.5%. Her history includes a mother with hypothyroidism.
How should she be managed?
Besides the obvious need for good diabetes control, this case merits screening for thyroid dysfunction, as the patient has 2 risk factors (TABLE 4).
One prominent controversy of the 21st century is whether all pregnant women should undergo routine screening for hypothyroidism. The controversy extends to screening all women of childbearing age.
TABLE 4
Risk factors for hypothyroidism include other autoimmune disorders
| Family history of thyroid disease |
| More than 3 symptoms |
| History of postpartum thyroid disease |
| Type 1 diabetes mellitus |
| Recurrent spontaneous abortions |
| Unexplained intrauterine fetal demise |
| Other autoimmune disorders |
|
$64,000 question: Should all women be screened?
Screening would involve testing thyroid function in women with no history and few or no signs and symptoms of thyroid dysfunction. Such screening could be population-based (using special methods to recruit, contact, and follow patients) or case-finding (performed on patients who present for unrelated reasons). The decision to screen a woman who is pregnant or planning to conceive should be based on many factors, most notably whether treatment prevents impaired neonatal neurodevelopment and preterm delivery.
A mother’s elevated TSH level can have lasting effects in the child
Haddow and colleagues1 measured the IQ of 47 children, ages 7 to 9 years, whose mothers had had an elevated serum TSH concentration in the second trimester, 15 children whose mothers had high serum TSH values in combination with low thyroxine levels in the second trimester, and 124 children whose mothers had normal TSH values. None of these children had hypothyroidism at birth. The children of the women with an elevated TSH concentration had lower IQs. Interestingly, the group with hypothyroxemia was not evaluated at the time, and the mean FT4 level was low in the entire group, suggesting overt hypothyroidism rather than subclinical disease.
In a study from the Netherlands, Pop and associates2 found impaired psychomotor function in 22 infants (age 10 months) whose mothers had had FT4 below the 10th percentile at 12 weeks of gestation, compared with 194 infants whose mothers had normal readings. When these children were reevaluated at 2 years, no neurodevelopmental delay was found in the infants whose mothers had a spontaneously increased free thyroxine level after the first trimester.
There is much speculation about precisely when thyroid hormone is critical for fetal brain development. The study by Pop and associates2 would suggest it is important after the first trimester. That study also recommends exogenous thyroxine for FT4 values below 0.96 ng/mL (12 pmol/L).
In Italy, Vermiglio and coworkers11 conducted behavioral and neuropsychological testing in 27 children at ages 18 to 36 months and again at 8 to 10 years. Mothers of 16 of these children were from a moderately iodine-deficient area (group A), and the mothers of 11 children were from a marginally iodine-sufficient area and were monitored with thyroid function tests in the first trimester (group B). Attention-deficit and hyperactivity disorders were more prevalent in group A.
Two studies published in 2006 also suggest that maternal free thyroxine levels in the first trimester of pregnancy correlate with impaired neonatal behavior at 3 months, and impaired mental development at ages 6, 9, and 12 months.3,4
Thyroid disorders affect approximately 5% of the general population, two thirds of them women.17 Subclinical hypothyroidism occurs in an additional 4.3%, and subclinical hyperthyroidism in 0.7%.
In pregnancy, subclinical disease is present in 3.6% of women; overt hypothyroidism, in 2.5%; and overt hyperthyroidism, in 0.2%. In addition, thyroid disease affects 5% to 9% of postpartum women.14
No consensus on whom to test or what test is best
There is no clear agreement about which population should be targeted for screening or what test to use. Most medical societies do not recommend routine screening, including the American College of Obstetricians and Gynecologists, which recommends TSH testing only in women with a history of thyroid disease and in women with “symptoms” (but does not specify which symptoms or how many symptoms warrant testing). A majority of organizations agree that all high-risk women should be tested when pregnancy is planned or as soon as pregnancy is confirmed.
TABLE 5
Comparison of screening recommendations highlights lack of consensus (and, in pregnancy, the absence of guidance)
| YEAR | ORGANIZATION | NONPREGNANT STATE | PREGNANCY |
|---|---|---|---|
| 1994 | American Association of Clinical Endocrinologists (AACE), American Academy of Family Physicians | Periodic assessment via thyroid function tests in older women | No recommendation |
| 1998 | American College of Physicians | Office screening of women >50 years of age | No recommendation |
| 2000 | American Thyroid Association (ATA) | Measure TSH every 5 years in women age 35 and older (probably men also) | No recommendation |
| 2002 | American College of Obstetricians and Gynecologists | Measure TSH every 5 years in women age 65 and older | No screening recommended |
| 2003 | Institute of Medicine | Screening is not cost-effective in Medicare population | No recommendation |
| 2004 | United States Preventive Services Task Force | Routine screening of children and adults is not recommended | No recommendation |
| 2004 | AACE, ATA, the Endocrine Society Consensus Group | No population-based screening, but “aggressive case finding” in women at high risk and those over age 60 | Do not support routine testing; recommend “aggressive case finding” and screening pregnant women at high risk |
Proponents of routine screening argue that it may limit health risks to children and save money in the long run, and they point out that thyroid disease is easy to treat with pills. Opponents note that no cost-benefit analysis has been performed, the benefits of treating mild disease are unclear, and screening a large population could be a significant expense ($40–100 per person) and would necessitate a lifelong commitment to daily medication in asymptomatic patients.
As a diagnostic test, the TSH immunoassay has 98% sensitivity and 92% specificity, and the current third-generation test lacks biases between methods and does not require method-specific reference ranges. However, it has low predictive value as a screening test (7–25%), possibly because of multiple confounding variables. Despite being the “gold standard,” it can lead to falsely positive results.
TABLE 6
What makes a TSH measurement falsely high or low?
| ELEVATED TSH | LOW TSH |
|---|---|
| Recovery from nonthyroidal illness | Euthyroid sick syndrome |
| Late evening TSH surge | Recovery from normal pregnancy |
| Assay variability | |
| Adrenal insufficiency | |
| Drugs: metoclopramide, amiodarone, cholecystographic dye (sodium ipodate) | Drugs: glucocorticoids, dopamine |
As for the value of FT4 alone as a screening test, we lack sufficient data on its utility. Another problem is that equilibrium dialysis, the most accurate and reliable laboratory method to measure FT4, is too technically complex and expensive for routine use. The most widely used 2-step radioimmunoassay is automated, but different methods are used by different commercial laboratories, cutoffs vary for every laboratory, and the results are sensitive to abnormal binding-protein states such as pregnancy in a method-specific manner. Tandem mass spectrometry is as reliable as equilibrium dialysis, but is not yet readily available.12
Another consideration: The physiologic changes in pregnancy render the cutoffs for the nongravid state inapplicable. TSH is lower in pregnancy, whereas the FT4 level is probably slightly increased or unchanged (TT4 is 1.5 times the prepregnancy value).
The normal reference values in each trimester of pregnancy from iodine-sufficient, autoimmune thyroid antibody-negative women are becoming available for TSH,12 as are nomograms that adjust for fetal number and gestational age.12 The measurement of FT4 still needs to be standardized across laboratories (method-specific, trimester-specific, and, possibly, population-specific reference ranges) for pregnancy.
How to manage subclinical thyroid disorders
In the nonpregnant state, subclinical hyperthyroidism should be treated in the following groups if the abnormal thyroid levels persist beyond 4 to 12 weeks and the TSH level is less than 0.1 mIU/L:
- High-risk women: postmenopausal or over age 60
- Low-risk women with cardiac disease, low bone density, or nodular thyroid disease.
If the TSH level is between 0.1 and 0.5 mIU/L, treatment is recommended for high-risk women with cardiac disease, low bone density, or nodular thyroid disease.
Subclinical hypothyroidism with a TSH level of 4.5 to 10 mIU/L need not be treated even in an elderly woman or a patient with a high antibody titer. Treatment of any woman is beneficial when the TSH level exceeds 10 mIU/L because it can ease symptoms, reduce low-density lipoprotein cholesterol, and prevent progression to overt disease. However, treatment may not lower morbidity and mortality and carries a roughly 20% risk of causing subclinical hyperthyroidism. It also involves a lifelong commitment to daily medication.
How to treat subclinical hypothyroidism in pregnancy
It is clear that overt hypothyroidism warrants treatment in both the pregnant and nonpregnant states, but the management of subclinical disease remains controversial. No trials have assessed the benefits of thyroid hormone replacement on the neuropsychological development of the newborn. Expert opinion suggests that women be treated if they are planning a pregnancy, are already pregnant, or have high TSH or low FT4.
Until we have more data, pregnant women and those planning a pregnancy should be treated with levothyroxine (starting at 2 μg/kg/day) if they are found to have elevated TSH or low FT4.
Two studies will answer questions about effects in pregnancy
The Controlled Antenatal Thyroid Screening (CATS) study will be completed in 2009, and the National Institute of Child Health and Human Development Maternal–Fetal Medicine Units (NICHD-MFMU) study will conclude in 2014. The CATS study screened 22,000 pregnant women in the United Kingdom before 16 weeks’ gestation. Half these women were treated with levothyroxine in pregnancy if they had a TSH measurement above the 97.5th percentile or FT4 below the 2.5th percentile, and half had their blood samples stored and tested only after delivery.14 Cutoff values were derived from previously obtained antenatal sera from well-dated pregnancies, and were adjusted after every 2,000 to 3,000 samples.
The CATS study was conducted in an iodine-sufficient area with a median urinary iodine excretion of 100 μg/L (range: 11–240 μg/L). Each group contained 400 women with subclinical hypothyroidism (52% had low FT4, 45% had high TSH, and 3% had both). Antithyroid peroxidase antibodies were present in 50% of women with elevated TSH but in only 10% of women with low FT4. Neuropsychological development in their children is being tested at 3 years of age.
The NICHD-MFMU study plans to screen 110,000 women at 14 centers over 2 years, and will randomize roughly 1,000 women to thyroxine treatment or placebo. They plan to assess intellectual development of the infants yearly for 5 years, and test the mothers for postpartum thyroid dysfunction and follow them at 1 and 5 years to detect the rate of progression to overt hypothyroidism.
Postpartum dysfunction can be transient or permanent
Postpartum thyroid dysfunction (PPTD) is an autoimmune disorder that occurs at 13 to 19 weeks postpartum, affects 1 in 12 women worldwide, and is usually associated with psychiatric symptomatology.15
PPTD also is strongly associated with antithyroid peroxidase antibodies (TPOAbs).16 Premawardhana and colleagues found that 10% of women are TPOAbs-positive in the first trimester; of these, 50% develop PPTD. Of the women with PPTD, 20% to 30% develop permanent hypothyroidism, and an additional 30% to 40% develop it by 7 years. In contrast, only 5% of women without PPTD progress to overt disease by 7 years. These findings have generated considerable controversy about routine screening for PPTD. Proponents argue that PPTD is highly prevalent, linked to considerable morbidity, is easily diagnosed with relatively inexpensive tests, and is easy to treat effectively. Critics note the lack of consensus on the best screening test (thyroid function test versus TPOAbs), optimal timing of screening (early pregnancy or postpartum), and lack of high-quality, prospective cost-benefit analyses. The NICHD-MFMU hopes to resolve these controversies.
The author reports no financial relationships relevant to this article.
1. Haddow JE, Palomaki GE, Allan WC, et al. Maternal thyroid deficiency during pregnancy and subsequent neuropsychological development of the child [see comment]. N Engl J Med. 1999;341:549-555.
2. Pop VJ, Kuijpens JL, van Baar AL, et al. Low maternal free thyroxine concentrations during early pregnancy are associated with impaired psychomotor development in infancy [see comment]. Clin Endocrinol. 1999;50:149-155.
3. Kasatkina EP, Samsonova LN, Ivakhnenko VN, et al. Gestational hypothyroxinemia and cognitive function in offspring. Neurosci Behav Physiol. 2006;36:619-624.
4. Kooistra L, Crawford S, van Baar AL, Brouwers EP, Pop VJ. Neonatal effects of maternal hypothyroxinemia during early pregnancy. Pediatrics. 2006;117:161-167.
5. Surks MI, Ortiz E, Daniels GH, et al. Subclinical thyroid disease: scientific review and guidelines for diagnosis and management [see comment]. JAMA. 2004;291:228-238.
6. Ranzini AC, Ananth CV, Smulian JC, Kung M, Limbachia A, Vintzileos AM. Ultrasonography of the fetal thyroid: nomograms based on biparietal diameter and gestational age. J Ultrasound Med. 2001;20:613-7.
7. Polak M, Le Gac I, Vuillard E, et al. Fetal and neonatal thyroid function in relation to maternal Graves’ disease. Best Practice & Research Clin Endocrinol Metab. 2004;18:289-302.
8. Alexander EK, Marqusee E, Lawrence J, Jarolim P, Fischer GA, Larsen PR. Timing and magnitude of increases in levothyroxine requirements during pregnancy in women with hypothyroidism [see comment]. N Engl J Med. 2004;351:241-249.
9. Mandel SJ, Spencer CA, Hollowell JG. Are detection and treatment of thyroid insufficiency in pregnancy feasible? Thyroid. 2005;15:44-53.
10. Negro R, Formoso G, Mangieri T, Pezzarossa A, Dazzi D, Hassan H. Levothyroxine treatment in euthyroid pregnant women with autoimmune thyroid disease: effects on obstetrical complications [see comment]. J Clin Endocrinol Metab. 2006;91:2587-2591.
11. Vermiglio F, Lo Presti VP, Moleti M, et al. Attention deficit and hyperactivity disorders in the offspring of mothers exposed to mild-moderate iodine deficiency: a possible novel iodine deficiency disorder in developed countries. J Clin Endocrinol Metab. 2004;89:6054-6060.
12. Soldin OP, Tractenberg RE, Hollowell JG, Jonklaas J, Janicic N, Soldin SJ. Trimester-specific changes in maternal thyroid hormone, thyrotropin, and thyroglobulin concentrations during gestation: trends and associations across trimesters in iodine sufficiency. Thyroid. 2004;14:1084-1090.
13. Dashe JS, Casey BM, Wells CE, et al. Thyroid-stimulating hormone in singleton and twin pregnancy: importance of gestational age-specific reference ranges. Obstet Gynecol. 2005;106:753-757.
14. Lazarus JH, Premawardhana LDKE. Screening for thyroid disease in pregnancy. J Clin Pathol. 2005;58:449-452.
15. Nicholson WK, Robinson KA, Smallridge RC, Ladenson PW, Powe NR. Prevalence of postpartum thyroid dysfunction: a quantitative review. Thyroid. 2006;16:573-582.
16. Premawardhana LDKE, Parkes AB, John R, Harris B, Lazarus JH. Thyroid peroxidase antibodies in early pregnancy: utility for prediction of postpartum thyroid dysfunction and implications for screening. Thyroid. 2004;14:610-615.
17. Hollowell JG, Staehling NW, Flanders WD, et al. Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III) [see comment]. J Clin Endocrinol Metab. 2002;87:489-499.
1. Haddow JE, Palomaki GE, Allan WC, et al. Maternal thyroid deficiency during pregnancy and subsequent neuropsychological development of the child [see comment]. N Engl J Med. 1999;341:549-555.
2. Pop VJ, Kuijpens JL, van Baar AL, et al. Low maternal free thyroxine concentrations during early pregnancy are associated with impaired psychomotor development in infancy [see comment]. Clin Endocrinol. 1999;50:149-155.
3. Kasatkina EP, Samsonova LN, Ivakhnenko VN, et al. Gestational hypothyroxinemia and cognitive function in offspring. Neurosci Behav Physiol. 2006;36:619-624.
4. Kooistra L, Crawford S, van Baar AL, Brouwers EP, Pop VJ. Neonatal effects of maternal hypothyroxinemia during early pregnancy. Pediatrics. 2006;117:161-167.
5. Surks MI, Ortiz E, Daniels GH, et al. Subclinical thyroid disease: scientific review and guidelines for diagnosis and management [see comment]. JAMA. 2004;291:228-238.
6. Ranzini AC, Ananth CV, Smulian JC, Kung M, Limbachia A, Vintzileos AM. Ultrasonography of the fetal thyroid: nomograms based on biparietal diameter and gestational age. J Ultrasound Med. 2001;20:613-7.
7. Polak M, Le Gac I, Vuillard E, et al. Fetal and neonatal thyroid function in relation to maternal Graves’ disease. Best Practice & Research Clin Endocrinol Metab. 2004;18:289-302.
8. Alexander EK, Marqusee E, Lawrence J, Jarolim P, Fischer GA, Larsen PR. Timing and magnitude of increases in levothyroxine requirements during pregnancy in women with hypothyroidism [see comment]. N Engl J Med. 2004;351:241-249.
9. Mandel SJ, Spencer CA, Hollowell JG. Are detection and treatment of thyroid insufficiency in pregnancy feasible? Thyroid. 2005;15:44-53.
10. Negro R, Formoso G, Mangieri T, Pezzarossa A, Dazzi D, Hassan H. Levothyroxine treatment in euthyroid pregnant women with autoimmune thyroid disease: effects on obstetrical complications [see comment]. J Clin Endocrinol Metab. 2006;91:2587-2591.
11. Vermiglio F, Lo Presti VP, Moleti M, et al. Attention deficit and hyperactivity disorders in the offspring of mothers exposed to mild-moderate iodine deficiency: a possible novel iodine deficiency disorder in developed countries. J Clin Endocrinol Metab. 2004;89:6054-6060.
12. Soldin OP, Tractenberg RE, Hollowell JG, Jonklaas J, Janicic N, Soldin SJ. Trimester-specific changes in maternal thyroid hormone, thyrotropin, and thyroglobulin concentrations during gestation: trends and associations across trimesters in iodine sufficiency. Thyroid. 2004;14:1084-1090.
13. Dashe JS, Casey BM, Wells CE, et al. Thyroid-stimulating hormone in singleton and twin pregnancy: importance of gestational age-specific reference ranges. Obstet Gynecol. 2005;106:753-757.
14. Lazarus JH, Premawardhana LDKE. Screening for thyroid disease in pregnancy. J Clin Pathol. 2005;58:449-452.
15. Nicholson WK, Robinson KA, Smallridge RC, Ladenson PW, Powe NR. Prevalence of postpartum thyroid dysfunction: a quantitative review. Thyroid. 2006;16:573-582.
16. Premawardhana LDKE, Parkes AB, John R, Harris B, Lazarus JH. Thyroid peroxidase antibodies in early pregnancy: utility for prediction of postpartum thyroid dysfunction and implications for screening. Thyroid. 2004;14:610-615.
17. Hollowell JG, Staehling NW, Flanders WD, et al. Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III) [see comment]. J Clin Endocrinol Metab. 2002;87:489-499.
Averting adhesions: Surgical techniques and tools
CASE Could bowel obstruction have been prevented?
B.H., 34, undergoes laparotomy for removal of an 8-cm myoma and a left ovarian cyst, which is found to be an endometrioma. Now she has come to the emergency department complaining of abdominal distension, pain, vomiting, and an inability to defecate. Small-bowel obstruction is diagnosed. Another laparotomy reveals that the obstructed bowel is adhered to the prior surgical incision.
Could this scenario have been avoided?
Adhesions need no introduction. Every surgeon is familiar with them; they are so ubiquitous they sometimes seem to be a given. Nevertheless, there are steps you can take to reduce the incidence of postoperative adhesions. In this article we describe surgical techniques, barriers, and peritoneal instillates that can help.
Why worry?
Intra-abdominal adhesions can cause pain, infertility, and bowel obstruction, and complicate future surgeries.1-3 Most studies suggest that more than 50% of women with adhesion-related small bowel obstruction have a history of gynecologic or obstetric operations. Within 1 year of laparotomy, adhesions cause intestinal obstruction in 1% of patients. After even a single previous abdominal operation, 93% of patients develop adhesions, compared with only 10.4% of patients who have never undergone laparotomy (FIGURE 1).1-3
We recently found an incidence of adhesion-related intestinal obstruction after operation for a benign gynecologic indication of 8 cases per 1,000 operations.4 Total abdominal hysterectomy (TAH) was the most common cause of small bowel obstruction (13.6 cases per 1,000 surgeries).
FIGURE 1 Adhesions through the laparoscope
Severe adhesions between the intestines and omentum to the uterus.
Omental adhesions to the anterior abdominal wall.
Adhesion between the intestine (lower left hand corner) and the anterior abdominal wall.
Surgical technique
Basic strategies
Tissue desiccation, necrosis, and the use of reactive suture material can predispose the patient to adhesion formation. Many studies in animal models have demonstrated an association between adhesions and these parameters. The following practices can help:
- Continuously irrigate the operative field during laparotomy
- Use nonreactive suture material such as polyglycolic acid (Dexon), polyglactin (Vicryl), or polydioxanone (PDS). Using reactive material, such as catgut, is discouraged
- Use powder-free gloves and prevent foreign-body infiltration (eg, powder, gauze, lint) of the wound.
No single parameter is as important as good surgical technique, attention to microsurgical principles, and precise hemostasis (TABLE 1).
TABLE 1
Surgical principles to reduce adhesion formation
| Take a laparoscopic approach when feasible |
| Minimize tissue necrosis |
| Provide meticulous hemostasis |
| Liberally irrigate the abdominal cavity |
| Use nonreactive suture materials |
When performing laparotomy
|
Peritoneal closure is unnecessary
Several randomized trials have demonstrated that closure of the parietal or visceral peritoneum is unnecessary. This practice is associated with slightly longer operating times and greater postoperative pain and may cause more adhesions.5 In 1 study, the rate of adhesion formation after laparotomy with peritoneal closure was 22.2%, compared with 16% without closure.6
Ellis7 noted an increasing number of medicolegal claims arising from adhesion-related complications, and recommended that “peritoneal defects and the pelvic floor should be left open, since they rapidly reperitonealized.”
Laparoscopy is more protective than open surgery
Abdominal surgeries injure tissues more severely than laparoscopy, and are associated with a greater degree of adhesion formation in up to 94% of patients, although laparoscopy can also cause adhesions.2 Laparoscopy is more protective because it involves minimal handling of tissue and little manipulation of the internal organs. Surgery is performed in a closed environment, tissue moistness is maintained, and contamination with glove powders or lint does not occur. In addition, the tamponade effect of carbon dioxide pneumoperitoneum facilitates hemostasis. Laparoscopy is also associated with a lower incidence of infection.
Adhesion-reducing substances
Many adhesion-reducing products have been evaluated in human and animal models. A basic assumption behind these substances is that surgically injured tissues heal without forming adhesions if the traumatized surfaces in apposition are separated to allow each to heal independently.
The ideal substance is resorbable, adherent to the traumatized surface, applicable through the laparoscope, and inexpensive, with high biocompatibility. So far, no substance or material has proved to be unequivocally effective.
Adhesion barriers are widely studied
The following products are among the most widely investigated substances (TABLE 2).
Expanded polytetrafluoroethylene, or ePTFE. Gore-Tex surgical membrane, constructed of ePTFE (Preclude, WL Gore), is nonabsorbable and produced in thin sheets (0.1 mm), with an average pore size of less than 1 μm. It is sutured to the tissue so that it overlaps the incision by at least 1 cm. It prevents adhesion formation—and reformation—independent of the type of injury. It is also effective in the presence of blood.
In a randomized trial, ePTFE decreased postmyomectomy and pelvic sidewall adhesions.8,9 In our experience, this is the most effective adhesion-reducing substance available. It is not widely used, however, because it is nonabsorbable and has to be fixed to the tissue.
Combined hyaluronic acid (HA) and carboxymethylcellulose (CMC). Known most widely by its trade name, Seprafilm (Genzyme Corp), this bioresorbable product is composed of sodium HA and CMC, a combination that produces a transparent and absorbable membrane that lasts for 7 days after application.10,11
In a study of 259 patients undergoing laparotomy for bowel resection or enterolysis, the incidence of repeat bowel obstruction was similar in the group treated with Seprafilm and the historical control group.11 However, 9 of 12 bowel obstructions in the treated group resolved without surgery, compared with 5 of 12 in the control group. The enterolysis rate in the treated group was 1.5%, compared with 3.9% in the control group.
Because of its stickiness, Seprafilm is not ideal for laparoscopy. However, it can be rolled and passed through the trocar, with the film separated from its paper backing inside the abdominal cavity.
Oxidized regenerated cellulose. Known under the brand name Interceed (TC7), this absorbable adhesion barrier (Johnson & Johnson) is the most widely studied product available today. Several randomized trials have shown that it reduces postoperative formation of adhesions on the pelvic sidewalls and near the adnexa.12-14
The efficacy of Interceed is reduced in the presence of blood. It is the easiest adhesion barrier to use at laparoscopy.
Newer agents in development include CMC and polyethylene oxide (PEO) composite gel (Oxiplex/AP, FrizoMed) and polylactide (PLa): copolymer of 70:30 Poly (L-lactide-co-D,L-lactide) film (SurgiWrap, Mast Biosurgery).
TABLE 2
The array of selected adhesion barriers and peritoneal instillates, and how they work
| PRODUCT | SPECIAL FEATURES |
|---|---|
| Barriers | |
| Expanded polytetrafluoroethylene (Preclude [Gore-Tex surgical membrane]) |
|
| Hyaluronic acid and carboxymethylcellulose (Seprafilm) |
|
| Oxidized regenerated cellulose (Interceed [TC7]) |
|
| Instillates | |
| 4% icodextrin (Adept) |
|
| Hyaluronic acid and ferric ion (InterGel) |
|
| HAL-C bioresorbable membrane (Sepracoat) |
|
| Hydrogel (SprayGel) to form membrane |
|
| Hydrogel (Adhibit) |
|
| Fibrin sealant (Tissucol) |
|
Peritoneal instillates
The newest peritoneal instillate is 4% icodextrin solution (Adept, Baxter BioSurgery). It is FDA-approved for the reduction of adhesion reformation after laparoscopic adhesiolysis. In a randomized study, the authors found that instillation of 4% icodextrin solution decreased adhesion formation and reformation after laparoscopic gynecologic surgery.15
Hyaluronic acid. Intergel (Lifecore, Johnson & Johnson Gynecare) is a cross-linked HA with ferric ion. It effectively reduces the number, severity, and extent of adhesions after abdominal operation.16 However, the product was withdrawn from the market after several reports of late-onset postoperative pain requiring surgery.
Sepracoat. This product (HAL-C Bioresorbable Membrane, Genzyme Corp) is a modification of Seprafilm. It coats serosal surfaces and is absorbed from the peritoneal cavity within 7 days. Its mechanism of action includes the reduction of tissue desiccation. Preliminary data show it to be effective in reducing postoperative adhesions.17 However, it did not receive FDA approval for clinical use, and was withdrawn from the market in 1997.
Hydrogel. A novel technique of substance delivery into the abdominal cavity is by combining 2 streams of liquid polymers, delivered via catheter to target tissue. When combined, the 2 streams produce a solid polymer within minutes. Sprayable hydrogel (SprayGel, Confluent Surgical) can be easily applied at laparoscopy. The solid polymer acts as an adhesion barrier and can potentially serve as a vehicle for localized delivery of drugs.
In a randomized study, Mettler et al18 evaluated 66 women who underwent myomectomy with or without SprayGel application. Second-look laparoscopy was performed in 40 women. Seven of 22 patients (31.8%) in the SprayGel group and 2 of 18 patients (11.1%) in the control group remained free of adhesions. However, the power of this study is small, and the authors did not break the women into subgroups based on whether they underwent surgery via laparoscopy or laparotomy. In the United States, the pivotal study of SprayGel was stopped prior to completion.
A similar product is a sprayable selfpolymerizing gel called Adhibit (Angiotech). An unpublished study from Europe showed it to be promising.
Fibrin sealant. Fibrin glue (Tissucol, Baxter) has been used as an adhesion-reducing substance, although clinical data on this application are scarce. This product is not approved by the FDA.
Which operations are the biggest culprits?
Myomectomy
Myomectomy performed through a laparotomy incision usually causes adhesions, so women who undergo this operation are good candidates for adhesion-reducing substances. The rate of adhesion formation after abdominal myomectomy is more than 90%—and it is 70% by laparoscopy.
Two helpful preventive strategies:
- Use a laparoscopic approach when feasible, and
- apply a barrier, such as the Gore-Tex ePTFE membrane, Seprafilm, or, if the myomectomy incision is not oozing, Interceed. Instillation of 1 L of 4% icodextrin may also be useful.
Hysterectomy
Most small-bowel obstruction follows abdominal hysterectomy, although a considerable period of time may pass before the problem occurs. When it does, a general surgeon usually manages the patient, and the treating gynecologist is unaware of this serious complication.
We recently found an incidence of adhesion-related small-bowel obstruction of 14 cases per 1,000 total abdominal hysterectomies and 1 case per 1,000 vaginal hysterectomies (P<.001).4 We did not encounter any small-bowel obstruction among 303 cases of laparoscopic supracervical hysterectomy.
Application of an adhesion-reducing substance to the vaginal vault or cervical stump may prevent small-bowel obstruction. Most adhesions implicated in small-bowel obstruction involve the vaginal vault. Appropriate products include Interceed, Preclude, Seprafilm, or perhaps Adept.
Fertility-promoting surgery
No adhesion-reducing substance has proved to be effective in increasing the pregnancy rate after a fertility-promoting procedure such as reconstructive tubal surgery or surgery for endometriosis.
CASE Recommendations
B.H., the patient described at the beginning of this article, should have had her initial surgery performed by an experienced laparoscopist, with minimal coagulation, meticulous hemostasis, “layered” repair of the myomectomy incision using nonreactive sutures, and liberal irrigation of the abdominal cavity. At the conclusion of the operation, the incision could have been covered with Gore-Tex surgical membrane or Seprafilm (or Interceed if there was no oozing) at least 1 cm beyond the incision. Instillation of Adept might have been useful as well.
The second operation also should have involved a laparoscopic route, which is associated with a lower rate of adhesions and could have reduced her risk of further bowel obstruction.
The authors report no financial relationships relevant to this article.
1. Diamond MP. Incidence of postsurgical adhesions. In: diZerega G, ed. Peritoneal Surgery. New York: Springer-Verlag; 2000;217:223.-
2. Tulandi T, Al-Shahrani A. Adhesion prevention in gynecologic surgery. Curr Opin Obstet Gynecol. 2005;17:395-398.
3. Al-Took S, Platt R, Tulandi T. Adhesion-related small bowel obstruction after gynecologic operations. Am J Obstet Gynecol. 1999;180:313-315.
4. Al-Sunaidi M, Tulandi T. Adhesion-related bowel obstruction after hysterectomy for benign conditions. Obstet Gynecol. 2006;108:1162-1166.
5. Tulandi T, Al-Jaroudi D. Non-closure of peritoneum: a reappraisal. Am J Obstet Gynecol. 2003;189:609-612.
6. Tulandi T, Hum HS, Gelfand MM. Closure of laparotomy incisions with or without peritoneal suturing and second-look laparoscopy. Am J Obstet Gynecol. 1988;158:536-537.
7. Ellis H. Medicolegal consequences of postoperative intra-abdominal adhesions. J R Soc Med. 2001;94:331-332.
8. Franklin R, Haney A, Kettel L, et al. An expanded polytetrafluoroethylene barrier (Gore-Tex surgical membrane) reduces post-myomectomy adhesion formation. Myomectomy Adhesion Multicenter Study Group. Fertil Steril. 1995;63:491-493.
9. Haney A, Hesla J, Hurst B, et al. Expanded polytetrafluororethylene (Gore-Tex surgical membrane) is superior to oxidized regenerated cellulose (Interceed TC7) in preventing adhesions. Fertil Steril. 1995;63:1021-1026.
10. Diamond MP. and the Seprafilm Adhesion Study Group. Reduction of adhesions after uterine myomectomy by Seprafilm membrane (HAL-F): a blinded, prospective, randomized, multicenter clinical study. Fertil Steril. 1996;66:904-910.
11. Vrijland WW, Tseng LN, Eijkman HJ, et al. Fewer intraperitoneal adhesions with use of hyaluronic acid-carboxymethylcellulose membrane: a randomized clinical trial. Ann Surg. 2002;235:193-199.
12. Sekiba K. The use of Interceed (TC7) absorbable adhesion barrier to reduce postoperative adhesion reformation in infertility and endometriosis surgery. Obstetrics and Gynecology Adhesion Prevention Committee. Obstet Gynecol. 1992;79:518-522
13. Azziz R. and the Interceed (TC7) Adhesion Barrier Study Group II. Microsurgery alone or with Interceed absorbable adhesion barrier for pelvic side wall adhesion reformation. Surg Gynecol Obstet. 1993;77:135-139.
14. Nordic Adhesion Prevention Study Group. The efficacy of Interceed (TC7) for prevention of reformation of postoperative adhesions on ovaries, fallopian tubes, and fimbriae in microsurgical operations for fertility: a multicenter study. Fertil Steril. 1995;63:709-714.
15. DiZerega GS, Verco SJ, Young P, et al. A randomized, controlled pilot study of the safety and efficacy of 4% icodextrin solution in the reduction of adhesions following laparoscopic gynaecological surgery. Human Reprod. 2002;17:1031-1038.
16. Hill-West JL, Dunn RC, Hubbell JA. Local release of fibrinolytic agents for adhesion prevention. J Surg Res. 1995;59:759-763.
17. Diamond MP. Reduction of de novo postsurgical adhesions by intraoperative precoating with Sepracoat (HAL-C) solution: a prospective, randomized, blinded, placebo-controlled multicenter study. The Sepracoat Adhesion Study Group. Fertil Steril. 1998;69:1067-1074.
18. Mettler L, Audebert A, Lehmann-Willenbrock E, Schive-Peterhansl K, Jacobs VR. A randomized, prospective, controlled, multicenter clinical trial of a sprayable, site-specific adhesion barrier system in patients undergoing myomectomy. Fertil Steril. 2004;82:398-404.
CASE Could bowel obstruction have been prevented?
B.H., 34, undergoes laparotomy for removal of an 8-cm myoma and a left ovarian cyst, which is found to be an endometrioma. Now she has come to the emergency department complaining of abdominal distension, pain, vomiting, and an inability to defecate. Small-bowel obstruction is diagnosed. Another laparotomy reveals that the obstructed bowel is adhered to the prior surgical incision.
Could this scenario have been avoided?
Adhesions need no introduction. Every surgeon is familiar with them; they are so ubiquitous they sometimes seem to be a given. Nevertheless, there are steps you can take to reduce the incidence of postoperative adhesions. In this article we describe surgical techniques, barriers, and peritoneal instillates that can help.
Why worry?
Intra-abdominal adhesions can cause pain, infertility, and bowel obstruction, and complicate future surgeries.1-3 Most studies suggest that more than 50% of women with adhesion-related small bowel obstruction have a history of gynecologic or obstetric operations. Within 1 year of laparotomy, adhesions cause intestinal obstruction in 1% of patients. After even a single previous abdominal operation, 93% of patients develop adhesions, compared with only 10.4% of patients who have never undergone laparotomy (FIGURE 1).1-3
We recently found an incidence of adhesion-related intestinal obstruction after operation for a benign gynecologic indication of 8 cases per 1,000 operations.4 Total abdominal hysterectomy (TAH) was the most common cause of small bowel obstruction (13.6 cases per 1,000 surgeries).
FIGURE 1 Adhesions through the laparoscope
Severe adhesions between the intestines and omentum to the uterus.
Omental adhesions to the anterior abdominal wall.
Adhesion between the intestine (lower left hand corner) and the anterior abdominal wall.
Surgical technique
Basic strategies
Tissue desiccation, necrosis, and the use of reactive suture material can predispose the patient to adhesion formation. Many studies in animal models have demonstrated an association between adhesions and these parameters. The following practices can help:
- Continuously irrigate the operative field during laparotomy
- Use nonreactive suture material such as polyglycolic acid (Dexon), polyglactin (Vicryl), or polydioxanone (PDS). Using reactive material, such as catgut, is discouraged
- Use powder-free gloves and prevent foreign-body infiltration (eg, powder, gauze, lint) of the wound.
No single parameter is as important as good surgical technique, attention to microsurgical principles, and precise hemostasis (TABLE 1).
TABLE 1
Surgical principles to reduce adhesion formation
| Take a laparoscopic approach when feasible |
| Minimize tissue necrosis |
| Provide meticulous hemostasis |
| Liberally irrigate the abdominal cavity |
| Use nonreactive suture materials |
When performing laparotomy
|
Peritoneal closure is unnecessary
Several randomized trials have demonstrated that closure of the parietal or visceral peritoneum is unnecessary. This practice is associated with slightly longer operating times and greater postoperative pain and may cause more adhesions.5 In 1 study, the rate of adhesion formation after laparotomy with peritoneal closure was 22.2%, compared with 16% without closure.6
Ellis7 noted an increasing number of medicolegal claims arising from adhesion-related complications, and recommended that “peritoneal defects and the pelvic floor should be left open, since they rapidly reperitonealized.”
Laparoscopy is more protective than open surgery
Abdominal surgeries injure tissues more severely than laparoscopy, and are associated with a greater degree of adhesion formation in up to 94% of patients, although laparoscopy can also cause adhesions.2 Laparoscopy is more protective because it involves minimal handling of tissue and little manipulation of the internal organs. Surgery is performed in a closed environment, tissue moistness is maintained, and contamination with glove powders or lint does not occur. In addition, the tamponade effect of carbon dioxide pneumoperitoneum facilitates hemostasis. Laparoscopy is also associated with a lower incidence of infection.
Adhesion-reducing substances
Many adhesion-reducing products have been evaluated in human and animal models. A basic assumption behind these substances is that surgically injured tissues heal without forming adhesions if the traumatized surfaces in apposition are separated to allow each to heal independently.
The ideal substance is resorbable, adherent to the traumatized surface, applicable through the laparoscope, and inexpensive, with high biocompatibility. So far, no substance or material has proved to be unequivocally effective.
Adhesion barriers are widely studied
The following products are among the most widely investigated substances (TABLE 2).
Expanded polytetrafluoroethylene, or ePTFE. Gore-Tex surgical membrane, constructed of ePTFE (Preclude, WL Gore), is nonabsorbable and produced in thin sheets (0.1 mm), with an average pore size of less than 1 μm. It is sutured to the tissue so that it overlaps the incision by at least 1 cm. It prevents adhesion formation—and reformation—independent of the type of injury. It is also effective in the presence of blood.
In a randomized trial, ePTFE decreased postmyomectomy and pelvic sidewall adhesions.8,9 In our experience, this is the most effective adhesion-reducing substance available. It is not widely used, however, because it is nonabsorbable and has to be fixed to the tissue.
Combined hyaluronic acid (HA) and carboxymethylcellulose (CMC). Known most widely by its trade name, Seprafilm (Genzyme Corp), this bioresorbable product is composed of sodium HA and CMC, a combination that produces a transparent and absorbable membrane that lasts for 7 days after application.10,11
In a study of 259 patients undergoing laparotomy for bowel resection or enterolysis, the incidence of repeat bowel obstruction was similar in the group treated with Seprafilm and the historical control group.11 However, 9 of 12 bowel obstructions in the treated group resolved without surgery, compared with 5 of 12 in the control group. The enterolysis rate in the treated group was 1.5%, compared with 3.9% in the control group.
Because of its stickiness, Seprafilm is not ideal for laparoscopy. However, it can be rolled and passed through the trocar, with the film separated from its paper backing inside the abdominal cavity.
Oxidized regenerated cellulose. Known under the brand name Interceed (TC7), this absorbable adhesion barrier (Johnson & Johnson) is the most widely studied product available today. Several randomized trials have shown that it reduces postoperative formation of adhesions on the pelvic sidewalls and near the adnexa.12-14
The efficacy of Interceed is reduced in the presence of blood. It is the easiest adhesion barrier to use at laparoscopy.
Newer agents in development include CMC and polyethylene oxide (PEO) composite gel (Oxiplex/AP, FrizoMed) and polylactide (PLa): copolymer of 70:30 Poly (L-lactide-co-D,L-lactide) film (SurgiWrap, Mast Biosurgery).
TABLE 2
The array of selected adhesion barriers and peritoneal instillates, and how they work
| PRODUCT | SPECIAL FEATURES |
|---|---|
| Barriers | |
| Expanded polytetrafluoroethylene (Preclude [Gore-Tex surgical membrane]) |
|
| Hyaluronic acid and carboxymethylcellulose (Seprafilm) |
|
| Oxidized regenerated cellulose (Interceed [TC7]) |
|
| Instillates | |
| 4% icodextrin (Adept) |
|
| Hyaluronic acid and ferric ion (InterGel) |
|
| HAL-C bioresorbable membrane (Sepracoat) |
|
| Hydrogel (SprayGel) to form membrane |
|
| Hydrogel (Adhibit) |
|
| Fibrin sealant (Tissucol) |
|
Peritoneal instillates
The newest peritoneal instillate is 4% icodextrin solution (Adept, Baxter BioSurgery). It is FDA-approved for the reduction of adhesion reformation after laparoscopic adhesiolysis. In a randomized study, the authors found that instillation of 4% icodextrin solution decreased adhesion formation and reformation after laparoscopic gynecologic surgery.15
Hyaluronic acid. Intergel (Lifecore, Johnson & Johnson Gynecare) is a cross-linked HA with ferric ion. It effectively reduces the number, severity, and extent of adhesions after abdominal operation.16 However, the product was withdrawn from the market after several reports of late-onset postoperative pain requiring surgery.
Sepracoat. This product (HAL-C Bioresorbable Membrane, Genzyme Corp) is a modification of Seprafilm. It coats serosal surfaces and is absorbed from the peritoneal cavity within 7 days. Its mechanism of action includes the reduction of tissue desiccation. Preliminary data show it to be effective in reducing postoperative adhesions.17 However, it did not receive FDA approval for clinical use, and was withdrawn from the market in 1997.
Hydrogel. A novel technique of substance delivery into the abdominal cavity is by combining 2 streams of liquid polymers, delivered via catheter to target tissue. When combined, the 2 streams produce a solid polymer within minutes. Sprayable hydrogel (SprayGel, Confluent Surgical) can be easily applied at laparoscopy. The solid polymer acts as an adhesion barrier and can potentially serve as a vehicle for localized delivery of drugs.
In a randomized study, Mettler et al18 evaluated 66 women who underwent myomectomy with or without SprayGel application. Second-look laparoscopy was performed in 40 women. Seven of 22 patients (31.8%) in the SprayGel group and 2 of 18 patients (11.1%) in the control group remained free of adhesions. However, the power of this study is small, and the authors did not break the women into subgroups based on whether they underwent surgery via laparoscopy or laparotomy. In the United States, the pivotal study of SprayGel was stopped prior to completion.
A similar product is a sprayable selfpolymerizing gel called Adhibit (Angiotech). An unpublished study from Europe showed it to be promising.
Fibrin sealant. Fibrin glue (Tissucol, Baxter) has been used as an adhesion-reducing substance, although clinical data on this application are scarce. This product is not approved by the FDA.
Which operations are the biggest culprits?
Myomectomy
Myomectomy performed through a laparotomy incision usually causes adhesions, so women who undergo this operation are good candidates for adhesion-reducing substances. The rate of adhesion formation after abdominal myomectomy is more than 90%—and it is 70% by laparoscopy.
Two helpful preventive strategies:
- Use a laparoscopic approach when feasible, and
- apply a barrier, such as the Gore-Tex ePTFE membrane, Seprafilm, or, if the myomectomy incision is not oozing, Interceed. Instillation of 1 L of 4% icodextrin may also be useful.
Hysterectomy
Most small-bowel obstruction follows abdominal hysterectomy, although a considerable period of time may pass before the problem occurs. When it does, a general surgeon usually manages the patient, and the treating gynecologist is unaware of this serious complication.
We recently found an incidence of adhesion-related small-bowel obstruction of 14 cases per 1,000 total abdominal hysterectomies and 1 case per 1,000 vaginal hysterectomies (P<.001).4 We did not encounter any small-bowel obstruction among 303 cases of laparoscopic supracervical hysterectomy.
Application of an adhesion-reducing substance to the vaginal vault or cervical stump may prevent small-bowel obstruction. Most adhesions implicated in small-bowel obstruction involve the vaginal vault. Appropriate products include Interceed, Preclude, Seprafilm, or perhaps Adept.
Fertility-promoting surgery
No adhesion-reducing substance has proved to be effective in increasing the pregnancy rate after a fertility-promoting procedure such as reconstructive tubal surgery or surgery for endometriosis.
CASE Recommendations
B.H., the patient described at the beginning of this article, should have had her initial surgery performed by an experienced laparoscopist, with minimal coagulation, meticulous hemostasis, “layered” repair of the myomectomy incision using nonreactive sutures, and liberal irrigation of the abdominal cavity. At the conclusion of the operation, the incision could have been covered with Gore-Tex surgical membrane or Seprafilm (or Interceed if there was no oozing) at least 1 cm beyond the incision. Instillation of Adept might have been useful as well.
The second operation also should have involved a laparoscopic route, which is associated with a lower rate of adhesions and could have reduced her risk of further bowel obstruction.
The authors report no financial relationships relevant to this article.
CASE Could bowel obstruction have been prevented?
B.H., 34, undergoes laparotomy for removal of an 8-cm myoma and a left ovarian cyst, which is found to be an endometrioma. Now she has come to the emergency department complaining of abdominal distension, pain, vomiting, and an inability to defecate. Small-bowel obstruction is diagnosed. Another laparotomy reveals that the obstructed bowel is adhered to the prior surgical incision.
Could this scenario have been avoided?
Adhesions need no introduction. Every surgeon is familiar with them; they are so ubiquitous they sometimes seem to be a given. Nevertheless, there are steps you can take to reduce the incidence of postoperative adhesions. In this article we describe surgical techniques, barriers, and peritoneal instillates that can help.
Why worry?
Intra-abdominal adhesions can cause pain, infertility, and bowel obstruction, and complicate future surgeries.1-3 Most studies suggest that more than 50% of women with adhesion-related small bowel obstruction have a history of gynecologic or obstetric operations. Within 1 year of laparotomy, adhesions cause intestinal obstruction in 1% of patients. After even a single previous abdominal operation, 93% of patients develop adhesions, compared with only 10.4% of patients who have never undergone laparotomy (FIGURE 1).1-3
We recently found an incidence of adhesion-related intestinal obstruction after operation for a benign gynecologic indication of 8 cases per 1,000 operations.4 Total abdominal hysterectomy (TAH) was the most common cause of small bowel obstruction (13.6 cases per 1,000 surgeries).
FIGURE 1 Adhesions through the laparoscope
Severe adhesions between the intestines and omentum to the uterus.
Omental adhesions to the anterior abdominal wall.
Adhesion between the intestine (lower left hand corner) and the anterior abdominal wall.
Surgical technique
Basic strategies
Tissue desiccation, necrosis, and the use of reactive suture material can predispose the patient to adhesion formation. Many studies in animal models have demonstrated an association between adhesions and these parameters. The following practices can help:
- Continuously irrigate the operative field during laparotomy
- Use nonreactive suture material such as polyglycolic acid (Dexon), polyglactin (Vicryl), or polydioxanone (PDS). Using reactive material, such as catgut, is discouraged
- Use powder-free gloves and prevent foreign-body infiltration (eg, powder, gauze, lint) of the wound.
No single parameter is as important as good surgical technique, attention to microsurgical principles, and precise hemostasis (TABLE 1).
TABLE 1
Surgical principles to reduce adhesion formation
| Take a laparoscopic approach when feasible |
| Minimize tissue necrosis |
| Provide meticulous hemostasis |
| Liberally irrigate the abdominal cavity |
| Use nonreactive suture materials |
When performing laparotomy
|
Peritoneal closure is unnecessary
Several randomized trials have demonstrated that closure of the parietal or visceral peritoneum is unnecessary. This practice is associated with slightly longer operating times and greater postoperative pain and may cause more adhesions.5 In 1 study, the rate of adhesion formation after laparotomy with peritoneal closure was 22.2%, compared with 16% without closure.6
Ellis7 noted an increasing number of medicolegal claims arising from adhesion-related complications, and recommended that “peritoneal defects and the pelvic floor should be left open, since they rapidly reperitonealized.”
Laparoscopy is more protective than open surgery
Abdominal surgeries injure tissues more severely than laparoscopy, and are associated with a greater degree of adhesion formation in up to 94% of patients, although laparoscopy can also cause adhesions.2 Laparoscopy is more protective because it involves minimal handling of tissue and little manipulation of the internal organs. Surgery is performed in a closed environment, tissue moistness is maintained, and contamination with glove powders or lint does not occur. In addition, the tamponade effect of carbon dioxide pneumoperitoneum facilitates hemostasis. Laparoscopy is also associated with a lower incidence of infection.
Adhesion-reducing substances
Many adhesion-reducing products have been evaluated in human and animal models. A basic assumption behind these substances is that surgically injured tissues heal without forming adhesions if the traumatized surfaces in apposition are separated to allow each to heal independently.
The ideal substance is resorbable, adherent to the traumatized surface, applicable through the laparoscope, and inexpensive, with high biocompatibility. So far, no substance or material has proved to be unequivocally effective.
Adhesion barriers are widely studied
The following products are among the most widely investigated substances (TABLE 2).
Expanded polytetrafluoroethylene, or ePTFE. Gore-Tex surgical membrane, constructed of ePTFE (Preclude, WL Gore), is nonabsorbable and produced in thin sheets (0.1 mm), with an average pore size of less than 1 μm. It is sutured to the tissue so that it overlaps the incision by at least 1 cm. It prevents adhesion formation—and reformation—independent of the type of injury. It is also effective in the presence of blood.
In a randomized trial, ePTFE decreased postmyomectomy and pelvic sidewall adhesions.8,9 In our experience, this is the most effective adhesion-reducing substance available. It is not widely used, however, because it is nonabsorbable and has to be fixed to the tissue.
Combined hyaluronic acid (HA) and carboxymethylcellulose (CMC). Known most widely by its trade name, Seprafilm (Genzyme Corp), this bioresorbable product is composed of sodium HA and CMC, a combination that produces a transparent and absorbable membrane that lasts for 7 days after application.10,11
In a study of 259 patients undergoing laparotomy for bowel resection or enterolysis, the incidence of repeat bowel obstruction was similar in the group treated with Seprafilm and the historical control group.11 However, 9 of 12 bowel obstructions in the treated group resolved without surgery, compared with 5 of 12 in the control group. The enterolysis rate in the treated group was 1.5%, compared with 3.9% in the control group.
Because of its stickiness, Seprafilm is not ideal for laparoscopy. However, it can be rolled and passed through the trocar, with the film separated from its paper backing inside the abdominal cavity.
Oxidized regenerated cellulose. Known under the brand name Interceed (TC7), this absorbable adhesion barrier (Johnson & Johnson) is the most widely studied product available today. Several randomized trials have shown that it reduces postoperative formation of adhesions on the pelvic sidewalls and near the adnexa.12-14
The efficacy of Interceed is reduced in the presence of blood. It is the easiest adhesion barrier to use at laparoscopy.
Newer agents in development include CMC and polyethylene oxide (PEO) composite gel (Oxiplex/AP, FrizoMed) and polylactide (PLa): copolymer of 70:30 Poly (L-lactide-co-D,L-lactide) film (SurgiWrap, Mast Biosurgery).
TABLE 2
The array of selected adhesion barriers and peritoneal instillates, and how they work
| PRODUCT | SPECIAL FEATURES |
|---|---|
| Barriers | |
| Expanded polytetrafluoroethylene (Preclude [Gore-Tex surgical membrane]) |
|
| Hyaluronic acid and carboxymethylcellulose (Seprafilm) |
|
| Oxidized regenerated cellulose (Interceed [TC7]) |
|
| Instillates | |
| 4% icodextrin (Adept) |
|
| Hyaluronic acid and ferric ion (InterGel) |
|
| HAL-C bioresorbable membrane (Sepracoat) |
|
| Hydrogel (SprayGel) to form membrane |
|
| Hydrogel (Adhibit) |
|
| Fibrin sealant (Tissucol) |
|
Peritoneal instillates
The newest peritoneal instillate is 4% icodextrin solution (Adept, Baxter BioSurgery). It is FDA-approved for the reduction of adhesion reformation after laparoscopic adhesiolysis. In a randomized study, the authors found that instillation of 4% icodextrin solution decreased adhesion formation and reformation after laparoscopic gynecologic surgery.15
Hyaluronic acid. Intergel (Lifecore, Johnson & Johnson Gynecare) is a cross-linked HA with ferric ion. It effectively reduces the number, severity, and extent of adhesions after abdominal operation.16 However, the product was withdrawn from the market after several reports of late-onset postoperative pain requiring surgery.
Sepracoat. This product (HAL-C Bioresorbable Membrane, Genzyme Corp) is a modification of Seprafilm. It coats serosal surfaces and is absorbed from the peritoneal cavity within 7 days. Its mechanism of action includes the reduction of tissue desiccation. Preliminary data show it to be effective in reducing postoperative adhesions.17 However, it did not receive FDA approval for clinical use, and was withdrawn from the market in 1997.
Hydrogel. A novel technique of substance delivery into the abdominal cavity is by combining 2 streams of liquid polymers, delivered via catheter to target tissue. When combined, the 2 streams produce a solid polymer within minutes. Sprayable hydrogel (SprayGel, Confluent Surgical) can be easily applied at laparoscopy. The solid polymer acts as an adhesion barrier and can potentially serve as a vehicle for localized delivery of drugs.
In a randomized study, Mettler et al18 evaluated 66 women who underwent myomectomy with or without SprayGel application. Second-look laparoscopy was performed in 40 women. Seven of 22 patients (31.8%) in the SprayGel group and 2 of 18 patients (11.1%) in the control group remained free of adhesions. However, the power of this study is small, and the authors did not break the women into subgroups based on whether they underwent surgery via laparoscopy or laparotomy. In the United States, the pivotal study of SprayGel was stopped prior to completion.
A similar product is a sprayable selfpolymerizing gel called Adhibit (Angiotech). An unpublished study from Europe showed it to be promising.
Fibrin sealant. Fibrin glue (Tissucol, Baxter) has been used as an adhesion-reducing substance, although clinical data on this application are scarce. This product is not approved by the FDA.
Which operations are the biggest culprits?
Myomectomy
Myomectomy performed through a laparotomy incision usually causes adhesions, so women who undergo this operation are good candidates for adhesion-reducing substances. The rate of adhesion formation after abdominal myomectomy is more than 90%—and it is 70% by laparoscopy.
Two helpful preventive strategies:
- Use a laparoscopic approach when feasible, and
- apply a barrier, such as the Gore-Tex ePTFE membrane, Seprafilm, or, if the myomectomy incision is not oozing, Interceed. Instillation of 1 L of 4% icodextrin may also be useful.
Hysterectomy
Most small-bowel obstruction follows abdominal hysterectomy, although a considerable period of time may pass before the problem occurs. When it does, a general surgeon usually manages the patient, and the treating gynecologist is unaware of this serious complication.
We recently found an incidence of adhesion-related small-bowel obstruction of 14 cases per 1,000 total abdominal hysterectomies and 1 case per 1,000 vaginal hysterectomies (P<.001).4 We did not encounter any small-bowel obstruction among 303 cases of laparoscopic supracervical hysterectomy.
Application of an adhesion-reducing substance to the vaginal vault or cervical stump may prevent small-bowel obstruction. Most adhesions implicated in small-bowel obstruction involve the vaginal vault. Appropriate products include Interceed, Preclude, Seprafilm, or perhaps Adept.
Fertility-promoting surgery
No adhesion-reducing substance has proved to be effective in increasing the pregnancy rate after a fertility-promoting procedure such as reconstructive tubal surgery or surgery for endometriosis.
CASE Recommendations
B.H., the patient described at the beginning of this article, should have had her initial surgery performed by an experienced laparoscopist, with minimal coagulation, meticulous hemostasis, “layered” repair of the myomectomy incision using nonreactive sutures, and liberal irrigation of the abdominal cavity. At the conclusion of the operation, the incision could have been covered with Gore-Tex surgical membrane or Seprafilm (or Interceed if there was no oozing) at least 1 cm beyond the incision. Instillation of Adept might have been useful as well.
The second operation also should have involved a laparoscopic route, which is associated with a lower rate of adhesions and could have reduced her risk of further bowel obstruction.
The authors report no financial relationships relevant to this article.
1. Diamond MP. Incidence of postsurgical adhesions. In: diZerega G, ed. Peritoneal Surgery. New York: Springer-Verlag; 2000;217:223.-
2. Tulandi T, Al-Shahrani A. Adhesion prevention in gynecologic surgery. Curr Opin Obstet Gynecol. 2005;17:395-398.
3. Al-Took S, Platt R, Tulandi T. Adhesion-related small bowel obstruction after gynecologic operations. Am J Obstet Gynecol. 1999;180:313-315.
4. Al-Sunaidi M, Tulandi T. Adhesion-related bowel obstruction after hysterectomy for benign conditions. Obstet Gynecol. 2006;108:1162-1166.
5. Tulandi T, Al-Jaroudi D. Non-closure of peritoneum: a reappraisal. Am J Obstet Gynecol. 2003;189:609-612.
6. Tulandi T, Hum HS, Gelfand MM. Closure of laparotomy incisions with or without peritoneal suturing and second-look laparoscopy. Am J Obstet Gynecol. 1988;158:536-537.
7. Ellis H. Medicolegal consequences of postoperative intra-abdominal adhesions. J R Soc Med. 2001;94:331-332.
8. Franklin R, Haney A, Kettel L, et al. An expanded polytetrafluoroethylene barrier (Gore-Tex surgical membrane) reduces post-myomectomy adhesion formation. Myomectomy Adhesion Multicenter Study Group. Fertil Steril. 1995;63:491-493.
9. Haney A, Hesla J, Hurst B, et al. Expanded polytetrafluororethylene (Gore-Tex surgical membrane) is superior to oxidized regenerated cellulose (Interceed TC7) in preventing adhesions. Fertil Steril. 1995;63:1021-1026.
10. Diamond MP. and the Seprafilm Adhesion Study Group. Reduction of adhesions after uterine myomectomy by Seprafilm membrane (HAL-F): a blinded, prospective, randomized, multicenter clinical study. Fertil Steril. 1996;66:904-910.
11. Vrijland WW, Tseng LN, Eijkman HJ, et al. Fewer intraperitoneal adhesions with use of hyaluronic acid-carboxymethylcellulose membrane: a randomized clinical trial. Ann Surg. 2002;235:193-199.
12. Sekiba K. The use of Interceed (TC7) absorbable adhesion barrier to reduce postoperative adhesion reformation in infertility and endometriosis surgery. Obstetrics and Gynecology Adhesion Prevention Committee. Obstet Gynecol. 1992;79:518-522
13. Azziz R. and the Interceed (TC7) Adhesion Barrier Study Group II. Microsurgery alone or with Interceed absorbable adhesion barrier for pelvic side wall adhesion reformation. Surg Gynecol Obstet. 1993;77:135-139.
14. Nordic Adhesion Prevention Study Group. The efficacy of Interceed (TC7) for prevention of reformation of postoperative adhesions on ovaries, fallopian tubes, and fimbriae in microsurgical operations for fertility: a multicenter study. Fertil Steril. 1995;63:709-714.
15. DiZerega GS, Verco SJ, Young P, et al. A randomized, controlled pilot study of the safety and efficacy of 4% icodextrin solution in the reduction of adhesions following laparoscopic gynaecological surgery. Human Reprod. 2002;17:1031-1038.
16. Hill-West JL, Dunn RC, Hubbell JA. Local release of fibrinolytic agents for adhesion prevention. J Surg Res. 1995;59:759-763.
17. Diamond MP. Reduction of de novo postsurgical adhesions by intraoperative precoating with Sepracoat (HAL-C) solution: a prospective, randomized, blinded, placebo-controlled multicenter study. The Sepracoat Adhesion Study Group. Fertil Steril. 1998;69:1067-1074.
18. Mettler L, Audebert A, Lehmann-Willenbrock E, Schive-Peterhansl K, Jacobs VR. A randomized, prospective, controlled, multicenter clinical trial of a sprayable, site-specific adhesion barrier system in patients undergoing myomectomy. Fertil Steril. 2004;82:398-404.
1. Diamond MP. Incidence of postsurgical adhesions. In: diZerega G, ed. Peritoneal Surgery. New York: Springer-Verlag; 2000;217:223.-
2. Tulandi T, Al-Shahrani A. Adhesion prevention in gynecologic surgery. Curr Opin Obstet Gynecol. 2005;17:395-398.
3. Al-Took S, Platt R, Tulandi T. Adhesion-related small bowel obstruction after gynecologic operations. Am J Obstet Gynecol. 1999;180:313-315.
4. Al-Sunaidi M, Tulandi T. Adhesion-related bowel obstruction after hysterectomy for benign conditions. Obstet Gynecol. 2006;108:1162-1166.
5. Tulandi T, Al-Jaroudi D. Non-closure of peritoneum: a reappraisal. Am J Obstet Gynecol. 2003;189:609-612.
6. Tulandi T, Hum HS, Gelfand MM. Closure of laparotomy incisions with or without peritoneal suturing and second-look laparoscopy. Am J Obstet Gynecol. 1988;158:536-537.
7. Ellis H. Medicolegal consequences of postoperative intra-abdominal adhesions. J R Soc Med. 2001;94:331-332.
8. Franklin R, Haney A, Kettel L, et al. An expanded polytetrafluoroethylene barrier (Gore-Tex surgical membrane) reduces post-myomectomy adhesion formation. Myomectomy Adhesion Multicenter Study Group. Fertil Steril. 1995;63:491-493.
9. Haney A, Hesla J, Hurst B, et al. Expanded polytetrafluororethylene (Gore-Tex surgical membrane) is superior to oxidized regenerated cellulose (Interceed TC7) in preventing adhesions. Fertil Steril. 1995;63:1021-1026.
10. Diamond MP. and the Seprafilm Adhesion Study Group. Reduction of adhesions after uterine myomectomy by Seprafilm membrane (HAL-F): a blinded, prospective, randomized, multicenter clinical study. Fertil Steril. 1996;66:904-910.
11. Vrijland WW, Tseng LN, Eijkman HJ, et al. Fewer intraperitoneal adhesions with use of hyaluronic acid-carboxymethylcellulose membrane: a randomized clinical trial. Ann Surg. 2002;235:193-199.
12. Sekiba K. The use of Interceed (TC7) absorbable adhesion barrier to reduce postoperative adhesion reformation in infertility and endometriosis surgery. Obstetrics and Gynecology Adhesion Prevention Committee. Obstet Gynecol. 1992;79:518-522
13. Azziz R. and the Interceed (TC7) Adhesion Barrier Study Group II. Microsurgery alone or with Interceed absorbable adhesion barrier for pelvic side wall adhesion reformation. Surg Gynecol Obstet. 1993;77:135-139.
14. Nordic Adhesion Prevention Study Group. The efficacy of Interceed (TC7) for prevention of reformation of postoperative adhesions on ovaries, fallopian tubes, and fimbriae in microsurgical operations for fertility: a multicenter study. Fertil Steril. 1995;63:709-714.
15. DiZerega GS, Verco SJ, Young P, et al. A randomized, controlled pilot study of the safety and efficacy of 4% icodextrin solution in the reduction of adhesions following laparoscopic gynaecological surgery. Human Reprod. 2002;17:1031-1038.
16. Hill-West JL, Dunn RC, Hubbell JA. Local release of fibrinolytic agents for adhesion prevention. J Surg Res. 1995;59:759-763.
17. Diamond MP. Reduction of de novo postsurgical adhesions by intraoperative precoating with Sepracoat (HAL-C) solution: a prospective, randomized, blinded, placebo-controlled multicenter study. The Sepracoat Adhesion Study Group. Fertil Steril. 1998;69:1067-1074.
18. Mettler L, Audebert A, Lehmann-Willenbrock E, Schive-Peterhansl K, Jacobs VR. A randomized, prospective, controlled, multicenter clinical trial of a sprayable, site-specific adhesion barrier system in patients undergoing myomectomy. Fertil Steril. 2004;82:398-404.
The Senior Screening Health Assessment and Preventive Education Program
Is Critical Incident Stress Management Effective?
OCs, breakthrough bleeding, and patients’ need to know
- Lack of adherence is a common cause of breakthrough bleeding. Focus counseling on ensuring that patients understand and can follow pill-taking instructions before switching pills or contraceptive method
- If breakthrough bleeding extends beyond 4 cycles and a woman wishes to continue using an oral contraceptive, consider switching to a pill with a higher ethinyl estradiol:progestin ratio, either by increasing the estradiol dose or by decreasing the relative progestin dose
- Breakthrough bleeding may be due to progestin type; switching from an estrane to a gonane may reduce it
- Women who have breakthrough bleeding after having well-controlled menstrual cycles on an oral contraceptive should be assessed for causes not related to their birth control pills, such as pregnancy, cervicitis, smoking, or interactions with medications.
In 1982, more than 20% of women surveyed in a nationally representative sample had discontinued oral contraceptives (OCs) on their own or at the recommendation of their physician due to bleeding or spotting.1 Sadly, the percentage today has not decreased much.
Understandable concern, embarrassment, and annoyance lead these women to abandon OCs.1,2 What they often don’t know, though, is that breakthrough bleeding generally is greatest in the first 3 to 4 months after starting OCs,3 and it steadily declines and stabilizes by the end of the fourth cycle.4 Timely counsel could enable many of these women to cope with the bleeding and stick with an effective contraceptive method. Additional incentives are noncontraceptive benefits of OCs: improved menstrual regularity and decreased menstrual blood loss, dysmenorrhea, and risk of ovarian and endometrial cancer.
Women who discontinue OCs on their own switch to less effective methods of birth control or use no method.1,2 Consequences may be unexpected pregnancies and an increased abortion rate.5 With patients who are using an OC, it would be appropriate to ask periodically whether they are satisfied with OC use.
In this review, we discuss the mechanisms and management of breakthrough bleeding in women taking OCs, and provide tips for counseling that may help decrease the risk of discontinuation due to menstrual abnormalities in the initial months of use.
Breakthrough bleeding in this review refers to either unplanned spotting or bleeding, regardless of requirement for protection—unless defined otherwise by a specific study under discussion.
For the purpose of performing studies, unplanned bleeding is classified by the World Health Organization into 2 categories:
- breakthrough bleeding, which requires sanitary protection, and
- spotting, which does not require sanitary protection.6 Despite this formal classification, trials have varied in their terminology and method of recording menstrual irregularities, making comparisons between studies difficult. In addition, there is wide variation among women in tolerance to bleeding abnormalities, perceptions of heavy vs light bleeding, as well as the need for protection.3
Nevertheless, menstrual abnormalities are consistently cited as a common reason for discontinuing OCs. A prospective US study of 1,657 women performed in the 1990s reported that 37% of OC users had stopped taking OCs by 6 months after starting a new prescription because of side effects.2 Irregular bleeding was the most common cause, cited by 12% of women, followed by nausea, weight gain, and mood changes, which ranged from 5% to 7%.
Breakthrough bleeding may be due to any the following variables:
- physiologic effects of OCs on the endometrium,
- OC-related parameters, including dose, formulation, and regimen,
- patient behavior (including compliance, using concomitant medications, and smoking),
- benign or malignant pathology.
OCs and the endometrium: Estrogen-progestin balance significant
Progestin and estrogen in combination OCs have profound effects on the endometrium that, although not contributing to contraception, do lead to a predictable pattern of bleeding or such problems as breakthrough bleeding or lack of withdrawal bleed.
Normally, estrogen causes the endometrium to proliferate. Progesterone stabilizes the growing uterine lining. Since the introduction of OCs in 1960, the trend in formulation has been to use the least amount of hormone necessary to inhibit ovulation. Given that the progestin is primarily responsible for the contraceptive efficacy of OCs, the risk of pregnancy is not altered with decreases in the estrogen component. However, significantly lowering the estrogen in OCs may account for breakthrough bleeding. Unplanned bleeding, though, is not dependent solely on the estrogen component, as variations in the progestin can contribute to breakthrough bleeding.7
Most OC users in the US take low-dose formulations, so designated because the estrogen component is 8 Studies that have compared OCs containing 20 μg ethinyl estradiol (EE) with those containing 30 μg or 35 μg EE have not been very useful for judging breakthrough bleeding rates because the products often also vary in the phasing and type of progestin. Some studies show more breakthrough bleeding with 20 μg EE pills,9-11 but others show equal or improved cycle control with the lower EE dose.
Estrogen-progestin balance is more important than absolute level of estrogen.
Endrikat et al12 conducted a study to compare two 20 μg EE pills containing different progestins, and to compare 2 levonorgestrel-based formulations with differing EE amounts. An OC of 20 μg EE/100 μg levonorgestrel was compared with a preparation of 20 μg EE/500 μg norethisterone. A 30 μg EE/150 μg levonorgestrel pill was used as a standard reference preparation.
Overall, the 30 μg EE preparation showed a lower cumulative incidence of breakthrough bleeding compared with the 20 μg EE/100 μg levonorgestrel and 20 μg EE/500 μg norethisterone pills over 13 cycles (1.0% vs 4.1% and 11.7%, respectively). However, the 20 μg EE/500 μg norethisterone pills consistently had a higher breakthrough bleeding rate than the 20 μg EE/100 μg levonorgestrel pill. This suggests that, although the higher EE component in the 30 μg pill was important when comparing 2 formulations with the same progestin, the difference in progestins of the two 20 μg EE pills was most likely responsible for the differing rates of breakthrough bleeding.
This study highlights the ability to achieve greater cycle control by titrating the EE component of an OC in a balanced ratio with the same progestin, but suggests that the absolute quantity of EE in a given pill may be less important than maintaining a balance between the 2 hormones or less important than the impact of different progestins on breakthrough bleeding rates.
The delicate balance between estrogen and progesterone supplementation required for contraception may also lead to progestin-induced decidualization and endometrial atrophy, which can result in asynchronous, erratic bleeding.7,13 This has been primarily studied in long-acting progestin-only contraceptives such as implants. Alterations in angiogenic factors14 may play a role. Hysteroscopic studies have shown abnormalities in superficial endometrial blood vessels in terms of size, proliferation, and fragility in women using norplant.13,15,16 Abnormalities in endothelial cells and extracellular matrix proteins,17 tissue factor,18 and endometrial lymphoid cells19 may contribute to breakthrough bleeding in progestin-dominant environments.
OC formulations, doses, regimens
More than 30 formulations of combination OCs are available in the US, with different doses and types of estrogen and progestin (TABLE 1).20 Approved OCs have been studied in clinical trials to assess contraceptive efficacy and cycle control; however, comparisons between studies regarding bleeding phenomena are impaired by inconsistent terminology.3
Whereas some studies describe breakthrough bleeding and spotting according to their recognized definitions, others simply refer to intermenstrual bleeding or use spotting to refer to any unexpected bleeding. In addition, cycle control studies of OC users frequently do not account for the effects of missed pills, use of concomitant medications, or smoking. The percentage of women who experience breakthrough bleeding in a given cycle varies widely even in different trials of the same formulation.
Pay attention to progestin level. Conventional wisdom holds that OCs with the lowest doses of EE (≤20 μg) are associated with more breakthrough bleeding.11 However, even moderately low doses of either EE or progestin can increase the incidence of breakthrough bleeding. For example, when 3 pills with the same estrogen and progestin (50 μg EE/100 μg norethindrone; 35 μg EE/100 μg norethindrone; and 35 μg EE/50 μg norethindrone) were compared in 192 women over 8 cycles, the pill containing the lowest amount of norethindrone (35 μg EE/50 μg norethindrone) caused the highest rates of breakthrough bleeding (decreasing to approximately 50% by cycle 8 as compared with 35% in the 35 μg EE/100 μg norethindrone pill and 25% in the 50 μg EE/100 μg norethindrone pill).21
In addition, the number of intermenstrual bleeding days plateaued more slowly as the amount of both hormones in the OC formulations decreased. This underscores the importance of the relative proportion of estrogen and progestin contained in combination OCs and its impact on breakthrough bleeding.
Similarly, a large comprehensive study in 1,991 women compared 7 different formulations of combination OCs containing different dose combinations of EE and norgestimate—20/250, 50/250, 35/125, 20/60, 50/60, 30/90, 25/125.22 Total intermenstrual bleeding was more frequent at lower doses of either estrogen or progestin. However, as long as a similar estrogen-progestin ratio was maintained, bleeding rates were considered acceptable (approximately 10% of days per cycle with bleeding). the authors also noted that in the low-dose range of OCs, small changes in the absolute amount of either EE or norgestimate might result in noticeable changes in bleeding
TABLE 1
Available OCs by formulation and regimen
| TRADE NAME | GENERIC NAME(S) | ESTROGEN (DOSE) | PROGESTIN (DOSE) |
|---|---|---|---|
| MONOPHASIC | |||
| Alesse, Levlite | Aviane, Lessina | Ethinyl estradiol (20 μg) | Levonorgestrel (0.1 mg) |
| Mircette | Kariva | Ethinyl estradiol (20 μg × 21 days + 10 μg × 5 days during placebo week) | Desogestrel (0.15 mg) |
| Loestrin FE | Microgestin FE 1/20, June FE 1/20 | Ethinyl estradiol (20 μg) | Norethindrone acetate (1 mg) |
| Yaz | Ethinyl estradiol (20 μg) | Drospirenone (3 mg) | |
| Levlen, Nordette | Levora, Portia | Ethinyl estradiol (30 μg) | Levonorgestrel (0.15 mg) |
| Lo/Ovral | Low-ogestrel, Cryselle | Ethinyl estradiol (30 μg) | Norgestrel (0.3 mg) |
| Desogen, Ortho-cept | Apri | Ethinyl estradiol (30 μg) | Desogestrel (0.15 mg) |
| Loestrin 21 1/5/30 | Microgestin, Junel Fe | Ethinyl estradiol (30 μg) | Norethindrone acetate (1.5 mg) |
| Yasmin | Ethinyl estradiol (30 μg) | Drospirenone (3 mg) | |
| Ovcon 35 | Ethinyl estradiol (35 μg) | Norethindrone (0.4 mg) | |
| Ortho-Cyclen | Mononesessa, Sprintec | Ethinyl estradiol (35 μg) | Norgestimate (0.25 mg) |
| Brevicon, Modicon | Nortrel, Necon 0.5/35 | Ethinyl estradiol (35 μg) | Norethindrone (0.5 mg) |
| Demulen 1/35 | Zovia 1/35 | Ethinyl estradiol (35 μg) | Ethynodiol diacetate (1 mg) |
| Ortho-Novum 1/35, Norinyl 1+35 | Necon 1/35, Nortrel | Ethinyl estradiol (35 μg) | Norethindrone (1 mg) |
| Ortho-Novum 1/50 | Necon 1/50 | Ethinyl estradiol (50 μg) | Norethindrone (1 mg) |
| Ovral | Ogestrel | Ethinyl estradiol (50 μg) | Norgestrel (0.5 mg) |
| Ovcon 50 | Ethinyl estradiol (50 μg) | Norethindrone (1 mg) | |
| Demulen 1/50 | Zovia 1/50 | Ethinyl estradiol (50 μg) | Ethynodiol diacetate (1 mg) |
| Norinyl 1/50 | Mestranol (50 μg) | Norethindrone (1 mg) | |
| BIPHASIC | |||
| Ortho-Novum 10/11, Jenest | Necon 10/11, Nelova 10/11 | Ethinyl estradiol (35 μg) | Norethindrone (0.5 mg × 10 days, 1 mg × 11 days) |
| TRIPHASIC | |||
| Ortho Tri-Cyclen Lo | Ethinyl estradiol (25 μg) | Norgestimate (0.18 mg × 7 days, 0.215 mg × 7 days, 0.25 mg × 7 days) | |
| Cyclessa | Velivet | Ethinyl estradiol (25 μg) | Desogestrel (0.1 mg × 7 days, 0.125 mg × 7 days, 0.15 mg × 7 days) |
| Triphasil, Tri-Levlen | Trivora, Enpresse | Ethinyl estradiol (30 μg × 6 days, 40 μg × 5 days, 30 μg × 10 days) | Levonorgestrel (0.05 mg × 6 days, 0.075 mg × 5 days, 0.125 mg × 10 days) |
| Tri-Norinyl | Ethinyl estradiol (35 μg) | Norethindrone (0.5 mg × 7 days, 1 mg × 9 days, 0.5 mg × 5 days) | |
| Ortho Tri-Cyclen | Tri-Sprintec, TriNessa | Ethinyl estradiol (35 μg) | Norgestimate (0.18 mg × 7 days, 0.215 mg × 7 days, 0.25 mg × 7 days) |
| Ortho-Novum 7/7/7 | Nortrel 7/7/7, Necon 7/7/7 | Ethinyl estradiol (35 μg) | Norethindrone (0.5 mg × 7 days, 0.75 mg × 7 days, 1 mg × 7 days) |
| Estrostep FE | Ethinyl estradiol (20 μg × 5 days, 30 μg × 7 days, 35 μg × 9 days) | Norethindrone acetate (1 mg) | |
| EXTENDED CYCLE | |||
| Seasonale | Ethinyl estradiol (30 μg × 84 days followed by 7 placebo pills) | Levonorgestrel (0.15 mg) | |
| Seasonique | Ethinyl estradiol (30 μg × 84 days followed by 10 μg × 7 days) | Levonorgestrel (0.15 mg) | |
Type of progestin may affect breakthrough bleeding.
All combination OCs contain either EE or mestranol. However, a variety of progestins have come into use. The 2 most common contraceptive progestins are derived from 19-nortestosterone, and are classified as gonanes or estranes.23
Estranes include norethindrone and its derivatives, norethindrone acetate and ethinyodiol diacetate. Gonanes include levonorgestrel, norgestrel, desogestrel, gestodene, and norgestimate.
Each progestin differs in half-life, estrogenic, progestogenic, and androgenic properties, and these variations may explain differing rates of breakthrough bleeding among formulations.4 As shown by Endrikat et al,12 pills with the same quantity of EE but different progestins can have marked differences in breakthrough bleeding rates.
Although gonanes have greater progestational activity, no trial has determined which progestin has the best bleeding profile. A recent Cochrane review comparing different progestins did find that, compared with pills containing levonorgestrel, those containing gestodene may be associated with less intermenstrual bleeding.24
Regardless of the progestin used or the quantity of EE, breakthrough bleeding generally decreases with each successive cycle. One study that compared 2 combination OCs composed of EE/norgestimate and EE/norgestrel demonstrated bleeding rates of 11.3% and 10.6% during the first 6 cycles, which decreased to 5.1% and 6.3% in cycles 13 to 24, respectively.25 Additionally, all women using OCs can experience some cycles without a withdrawal bleed—a menstrual abnormality that may be concerning to those who desire a menstrual period as confirmation that they are not pregnant.
Comparing regimens. OC regimens are available as biphasic, triphasic, extended-cycle, and continuous use. Women using extended-cycle contraceptives may experience more breakthrough bleeding than those using a standard 28-day pill. However, in a 3-month cycle, there are only 7 days of planned bleeding. This is in contrast to 28-day cycles during 3 months in which there are 21 days of planned bleeding.
Though women on extended-cycle regimens may initially experience more breakthrough bleeding than women using 28-day regimens, the total number of planned and unplanned bleeding days may still decrease. Women using a 3-month cycle OC (30 μg EE/150 μg levonorgestrel) experienced more unscheduled bleeding than women using a standard 28-day cycle OC of the same formulation and dose.26 The number of bleeding days decreased with each cycle. Another study examined continuous OC use (20 μg EE/100 μg levonorgestrel) over a period of 1 year, and reported a decreasing number of bleeding days over time.27 In the case of continuous use, all bleeding is unscheduled, and any bleeding is considered breakthrough bleeding.
Multiphasic OC regimens were developed with the intention of decreasing breakthrough bleeding by mimicking the rising and falling pattern of estrogen and progesterone in the normal menstrual cycle.28 After the introduction of the biphasic pill, an increase in breakthrough bleeding was noted, which led to the development of the triphasic pill.29 Though the multiphasic hypothesis is physiologically plausible, recent reviews of the literature have found the evidence for its efficacy too limited and methodologically flawed to draw any definitive conclusions about a decrease in breakthrough bleeding.30,31
Patient behaviors are contributory
Skipping a pill is a common cause of breakthrough bleeding.5 Compliance with any OC regimen is crucial to achieving a regular and predictable bleeding pattern. Of 6,676 women surveyed retrospectively, 19% reported missing 1 or more pills per cycle, and 10% reported missing 2 or more pills per cycle.32 Prospective studies have found even higher rates of inconsistent use.
TABLE 2
What to review with patients who are starting a combination OC
|
Other side effects also undermine adherence. For example, women experiencing nausea may skip pills, which leads to breakthrough bleeding and, ultimately, discontinuation.34 Patients need to understand the impact of skipping pills. Women who report irregular bleeding are 1.6 to 1.7 times more likely than those not reporting this side effect to miss 2 or more pills per cycle.5 Even 1 missed pill can increase the risk of bleeding irregularities.35
Failure to take the pill at the same time every day and poor comprehension of pill-taking instructions are other strong predictors of inconsistent use and breakthrough bleeding.32
Taking some prescription and over-the-counter medications, as well as herbal supplements, may interfere with the activity of OCs to alter bleeding patterns and contraceptive efficacy.36 Medications that induce the cytochrome P-450 system (CYP450) in the liver increase the metabolism of OCs. Anticonvulsants, the antituberculosis agent rifampin, and antifungals such as griseofulvin can increase the clearance of steroid hormones and thus lead to breakthrough bleeding. the herbal supplement St. John’s wort, commonly used for mild or moderate depression, is associated with CYP450 induction. It has been shown to increase the incidence of breakthrough bleeding and probably ovulation in women taking an OC.37
Smoking is associated with such anti-estrogenic effects as early menopause, osteoporosis, and menstrual abnormalities.38 these effects may be related to induction of hepatic estrogen and progesterone metabolism by smoking.39,40
Before receiving OCs, women are made aware of the relationship between smoking, OCs, and an increased risk of myocardial infarction, stroke, and venous thromboembolism.41 They should also understand that the anti-estrogenic effect of smoking may lower estrogen levels and lead to breakthrough bleeding, even in women who are reliable pill-takers.42,43
Smoking appears to have a dose-response relationship with breakthrough bleeding. Increasing levels of smoking have been associated with an increased risk of spotting or bleeding in each cycle.44 The difference in cycle control between smokers and nonsmokers appears to be more pronounced with each cycle. Smokers demonstrate a 30% elevation in the risk of bleeding irregularities compared with nonsmokers in the first cycle of use, which rises to an 86% increased risk by the sixth cycle.
Reports conflict regarding the relationship between smoking and contraceptive efficacy, suggesting that confounding factors like compliance may be more important than the antihormonal effect of cigarettes.45 Nevertheless, women who smoke should be informed of this potential complicating factor to OC use and as yet another reason to encourage smoking cessation.
Bleeding is sometimes pathologic
When a woman experiences difficult cycle control after the first 3 to 4 months of OC use, consider the possibility of benign and malignant growths, including endometrial polyps, submucous myomas, and cervical or endometrial cancer.46 Additionally, contraceptive failure must always be a consideration, and what appears to be breakthrough bleeding may actually represent bleeding in early pregnancy.
Cervicitis is an important but largely unrecognized source of unplanned bleeding in women using OCs. Causative organisms include Chlamydia trachomatis Neisseria gonorrhoeae, and Trichomonas vaginalis.22 Intermenstrual bleeding in women previously well controlled on OCs is particularly suggestive of asymptomatic chlamydial cervicitis.
Krettek et al47 found that 29.2% of women who had been taking OCs for more than 3 months and presented with intermenstrual spotting had a positive test for C. trachomatis. By comparison, chlamydial cervicitis was found in 10.7% of matched controls taking OCs without spotting who were screened for symptoms of vaginitis or high-risk sexual behavior, and in just 6.1% of women undergoing routine screening before the initiation of contraception.
Three-pronged management
Managing breakthrough bleeding involves effective pretreatment; ongoing counseling and reassurance; and timely and appropriate testing (TABLE 2). In some cases, pill-switching or other forms of medical management may be helpful, but these options are largely unproven.
Counseling reduces anxiety, improves satisfaction, adherence
In a recent survey, 649 Canadian women who were picking up prescriptions for OCs were asked to complete a questionnaire at the pharmacy while they waited.48 Over one third (34.5%) reported they had not received counseling from their healthcare provider about breakthrough bleeding. Furthermore, only 28.3% of women who were counseled, and 26.1% of women who were not counseled, gave the optimal response to breakthrough bleeding as defined in this study (“continue taking pill and not call my doctor”).
Lack of counseling can lead to poor method satisfaction and significant cost expenditures because of visits and phone calls by women experiencing unexpected bothersome side effects.5 Compared with women who reported the highest satisfaction with the care they received from their provider, those reporting the lowest scores were 1.6 to 2.2 times as likely to be dissatisfied with the pill.
Inform women that breakthrough bleeding is common in the first 3 or 4 cycles of OC use, that bleeding irregularities tend to decline with each successive cycle, and that they should not discontinue pill use without discussing their concerns with you. Remind women to keep sanitary protection with them during the first few months.
The impact of poor counseling was underscored in a study of women enrolled in clinical trials of OCs, contraceptive vaginal rings, and Depo-Provera. Women taking an OC were the least likely to have been warned of menstrual irregularities and thus tended to stop using that method more often than those using a ring or Depo-Provera.49 Of women who discontinue OCs, 47% use a less effective method and 19% use no method at all.1
Give specific instructions for specific regimens. Given the array of OC regimens available, make sure women know how to take them properly. This will help ensure contraceptive efficacy and cycle control. Women who do not understand pill-package instructions are up to 2.8 times more likely to miss pills, which increases the risk of breakthrough bleeding and impacts contraceptive efficacy.5 Among women who were counseled about the consequences of missed pills, 76% reported knowing what to do in response (“use another form of birth control that month”). Of women who received no such counseling, only 48% gave the appropriate response (P.001>48
To improve adherence, advise women to establish a routine for pill-taking: taking the pill at the same time each day or linking pill ingestion with another daily activity, such as tooth brushing. Women without an established routine were 3.6 times more likely to miss 2 or more pills per cycle than women with a routine.5
Reassurance regarding efficacy
Reassure users who take their pills routinely that breakthrough bleeding and contraceptive efficacy are not linked.50 Breakthrough bleeding is not a sign that OCs are not working.4 On the other hand, approximately 1 million unintended pregnancies in the United States each year are associated with misuse or discontinuation of OCs.51
When to consider diagnostic testing
For OC users who continue to experience breakthrough bleeding beyond 3 to 4 cycles, other potential causes must be ruled out using appropriate diagnostic tests. A pregnancy test, appropriate testing for cervical infection, pelvic ultrasonography, Pap smear, or endometrial biopsy may be warranted, depending on clinical circumstances.
Fall-back options
If breakthrough bleeding continues beyond 3 months, and other reasons, including poor adherence and pathologic processes, are excluded, one option would be to provide the patient with estrogen or switch her to a different pill, though no clinical trials support definitive recommendations.
Aside from changing from a multiphasic to a monophasic formulation, altering the progestin component is often a first step in trying to control breakthrough bleeding.46 An OC with a gonane rather than an estrane progestin may be beneficial as this class of progestins may provide more consistent hormonal effects on the endometrium.
Choosing an OC with a higher quantity of ee may also help, particularly for women using 20 μg pills. When possible, the same progestin should be used.
You may want to start a trial of conjugated estrogen, 1.25 mg, or estradiol, 2 mg, administered for 7 days when bleeding occurs. This can be repeated if necessary; however, if breakthrough bleeding continues despite this treatment, consideration of a different pill or method should be undertaken.
1. Pratt WF, Bachrach CA. What do women use when they stop using the pill? Fam Plann Perspect. 1987;19:257-266.
2. Rosenberg MJ, Waugh MS. Oral contraceptive discontinuation: A prospective evaluation of frequency and reasons. Am J Obstet Gynecol. 1998;179:577-582.
3. Thorneycroft IH. Cycle control with oral contraceptives: A review of the literature. Am J Obstet Gynecol. 1999;180:S280-S287.
4. Speroff L, Darney PD. A Clinical Guide for Contraception. 3rd ed. Philadelphia, Pa: Lippincott Williams & Wilkins; 2001.
5. Rosenberg MJ, Waugh MS, Burnhill MS. Compliance, counseling, and satisfaction with oral contraceptives: A prospective evaluation. Fam Plann Perspect. 1998;30:89-92-104.
6. Belsey EM, Machin D, d’Arcangues C. The analysis of vaginal bleeding patterns induced by fertility regulating methods. World Health Organization Special Programme of Research, Development and Research Training in Human Reproduction. Contraception. 1986;34:253-260.
7. ESHRE Capri Workshop Group. Ovarian and endometrial function during hormonal contraception. Hum Reprod. 2001;16:1527-1535.
8. Kaunitz AM. Oral contraceptive estrogen dose considerations. Contraception. 1998;58(Suppl):15S-21S.
9. Preston SN. A report of a collaborative dose-response clinical study using decreasing doses of combination oral contraceptives. Contraception. 1972;6:17-35.
10. Akerlund M, Rode A, Westergaard J. Comparative profiles of reliability, cycle control and side effects of two oral contraceptive formulations containing 150 mcg desogestrel and either 30 mcg or 20 mcg ethinyl oestradiol. Br J Obstet Gynaecol. 1993;100:832-838.
11. Gallo MF, Nanda K, Grimes DA, Schulz KF. Twenty micrograms vs >20 microg estrogen oral contraceptives for contraception: a systematic review of randomized controlled trials. Contraception. 2005;71:162-169.
12. Endrikat J, Hite R, Bannemerschult R, Gerlinger C, Schmidt W. Multicenter, comparative study of cycle control, efficacy and tolerability of two low-dose oral contraceptives containing 20 μg ethinyl estradiol/100 μg levonorgestrel and 20 μg ethinyl estradiol/50 μg noresthisterone. Contraception. 2001;64:3-10.
13. Hickey M, Fraser I, Dwarte D, Graham S. Endometrial vasculature in Norplant users: preliminary results from a hysteroscopic study. Hum Reprod. 1996;11(Suppl 2):35-44.
14. Smith SK. Steroids and endometrial breakthrough bleeding: future directions for research. Hum Reprod. 2000;15(Suppl 3):197-202.
15. Song JY, Markham R, Russell P, Wong T, Young L, Fraser IS. The effect of high-dose medium- and long-term progestogen exposure on endometrial vessels. Hum Reprod. 1995;10:797-800.
16. Hickey M, Dwarte D, Fraser IS. Superficial endometrial vascular fragility in Norplant users and in women with ovulatory dysfunctional uterine bleeding. Hum Reprod. 2000;15:1509-1514.
17. Rodriguez-Manzaneque JC, Graubert M, Iruela-Arispe ML. Endothelial cell dysfunction following prolonged activation of progesterone receptor. Hum Reprod. 2000;15(Suppl 3):39-47.
18. Lockwood CJ, Runic R, Wan L, Krikun G, Demopolous R, Schatz F. The role of tissue factor in regulating endometrial haemostsis: Implications for progestin-only contraception. Hum Reprod. 2000;15(Suppl 3):144-151.
19. Clark DA, Wang S, Rogers P, Vince G, Affandi B. Endometrial lymphoid cells in abnormal uterine bleeding due to levonorgestrel (Norplant). Hum Reprod. 1996;11:1438-1444.
20. Hatcher RA, Trussell J, Stewart FH. Contraceptive Technology. 18th ed. New York: Ardent Media; 2004.
21. Saleh WA, Burkman RT, Zacur HA, Kimball AW, Kwieterovich P, Bell W. A randomized trial of three oral contraceptives: comparison of bleeding patterns by contraceptive types and steroid levels. Am J Obstet Gynecol. 1993;168:1740-1747.
22. Lawson JS, Yuliano SE, Pasquale SA, Osterman JJ. Optimum dosing of an oral contraceptive. A report from the study of seven combinations of norgestimate and ethinyl estradiol. Am J Obstet Gynecol. 1979;134:315-320.
23. Stenchever MA, Ling FW. Comprehensive Gynecology. 4th ed. St Louis, Mo: Mosby; 2001.
24. Maitra N, Kulier R, Bloemenkamp KW, Helmerhorst FM, Gulmezoglu AM. Progestogens in combined oral contraceptives for contraception. Cochrane Database Syst Rev. 2004;(3):CD004861.-
25. Corson SL. Efficacy and clinical profile of a new oral contraceptive containing norgestimate. US clinical trials. Acta Obstet Gynecol Scand Suppl. 1990;152:25-31.
26. Anderson FD, Hait H. A multicenter, randomized study of an extended cycle oral contraceptive. Contraception. 2003;68:89-96.
27. Miller L, Hughes JP. Continuous combined oral contraceptive pills to eliminate withdrawal bleeding: a randomized trial. Obstet Gynecol. 2003;101:653-661.
28. Upton GV. The phasic approach to oral contraception: the triphasic concept and its clinical application. Int J Fertil Steril. 1983;28:121-140.
29. Mishell DR, Jr. Oral contraception: past, present, and future perspectives. Int J Fertil Steril. 1991;37(Suppl):s7-s18.
30. Van Vliet H, Grimes D, Helmerhorst F, Schulz K. Biphasic versus triphasic oral contraceptives for contraception. Cochrane Database Syst Rev. 2006;3:CD003283.-
31. Van Vliet H, Grimes D, Helmerhorst F, Schulz K. Biphasic versus monophasic oral contraceptives for contraception. Cochrane Database Syst Rev. 2006;3:CD002032.-
32. Rosenberg MJ, Waugh MS, Meehan TE. Use and misuse of oral contraceptives: Risk indicators for poor pill taking and discontinuation. Contraception. 1995;51:283-288.
33. Potter L, Oakley D, de Leon-Wong E, Canamar R. Measuring compliance among oral contraceptive users. Fam Plann Perspect. 1996;28:154-158.
34. Stubblefield PG. Menstrual impact of contraception. Am J Obstet Gynecol. 1994;170:513-1522.
35. Talwar PP, Dingfelder JR, Ravenholt RT. Increased risk of breakthrough bleeding when one oral-contraceptive tablet is missed. N Engl J Med. 1977;296:1236-1237.
36. Wallach M, Grimes DA. Modern Oral Contraception: Updates from the Contraceptive Report. Totowa, NJ: Emron; 2000.
37. Murphy PA, Kern SE, Stanczyk FZ, Westhoff CL. Interaction of St. John’s Wort with oral contraceptives: effects on the pharmacokinectics of norethindrone and ethinyl estradiol, ovarian activity and breakthrough bleeding. Contraception. 2005;71:402-408.
38. Baron JA, Greenberg ER. Cigarette smoking and estrogen related disease in women. In: Smoking and Reproductive Health, ed. Rosenberg MJ. Boston: PSG; .1987:149–160.
39. Jusko WJ. Influence of cigarette smoking on drug metabolism in man. Drug Metab Rev. 1979;9:221-236.
40. Basu J, Mikhail MS, Palan PR, Thysen B, Bloch E, Romney SL. Endogenous estradiol and progesterone concentrations in smokers on oral contraceptives. Gynecol Obstet Invest. 1992;33:224-227.
41. Faculty of Family Planning and Reproductive Health Care Clinical Effectiveness Unit FFPRHC Guidance (October 2003): First prescription of combined oral contraception. J Fam Plann Reprod Health Care. 2003;29:209-222.
42. Sparrow MJ. Pregnancies in reliable pill takers. NZ Med J. 1989;102:575-577.
43. Kanarkowski R, Tornatore KM, D’Ambrosio R, Gardner MJ, Jusko WJ. Pharmacokinetics of single and multiple doses of ethinyl estradiol and levonorgestrel in relation to smoking. Clin Pharmacol Ther. 1988;43:23-31.
44. Rosenberg MJ, Waugh MS, Stevens CM. Smoking and cycle control among oral contraceptive users. Am J Obstet Gynecol. 1996;174:628-632.
45. Vessey MP, Villard-Makintosh L, Jacobs HS. Anti-estrogenic effect of cigarette smoking. N Engl J Med. 1987;317:769-770.
46. Darney PD. OC practice guidelines: minimizing side effects. Int J Fertil Womens Med. 1997;42(suppl 1):158-169.
47. Krettek JE, Arkin SI, Chaisilwattana P, Monif GR. Chlamydia trachomatis in patients who used oral contraceptives and had intermenstrual spotting. Obstet Gynecol. 1993;81:728-731.
48. Gaudet LM, Kives S, Hahn PM, Reid RL. What women believe about oral contraceptives and the effect of counseling. Contraception. 2004;69:31-36.
49. Belsey EM. The association between vaginal bleeding patterns and reasons for discontinuation of contraceptive use. Contraception. 1988;38:207-225.
50. Jung-Hoffman C, Kuhl H. Intra- and interindividual variations in contraceptive steroid levels during 12 treatment cycles: no relation to irregular bleedings. Contraception. 1990;(42):423-438.
51. Rosenberg MJ, Waugh MS, Long S. Unintended pregnancies and use, misuse and discontinuation of oral contraceptives. J Reprod Med. 1995;40:355-360.
- Lack of adherence is a common cause of breakthrough bleeding. Focus counseling on ensuring that patients understand and can follow pill-taking instructions before switching pills or contraceptive method
- If breakthrough bleeding extends beyond 4 cycles and a woman wishes to continue using an oral contraceptive, consider switching to a pill with a higher ethinyl estradiol:progestin ratio, either by increasing the estradiol dose or by decreasing the relative progestin dose
- Breakthrough bleeding may be due to progestin type; switching from an estrane to a gonane may reduce it
- Women who have breakthrough bleeding after having well-controlled menstrual cycles on an oral contraceptive should be assessed for causes not related to their birth control pills, such as pregnancy, cervicitis, smoking, or interactions with medications.
In 1982, more than 20% of women surveyed in a nationally representative sample had discontinued oral contraceptives (OCs) on their own or at the recommendation of their physician due to bleeding or spotting.1 Sadly, the percentage today has not decreased much.
Understandable concern, embarrassment, and annoyance lead these women to abandon OCs.1,2 What they often don’t know, though, is that breakthrough bleeding generally is greatest in the first 3 to 4 months after starting OCs,3 and it steadily declines and stabilizes by the end of the fourth cycle.4 Timely counsel could enable many of these women to cope with the bleeding and stick with an effective contraceptive method. Additional incentives are noncontraceptive benefits of OCs: improved menstrual regularity and decreased menstrual blood loss, dysmenorrhea, and risk of ovarian and endometrial cancer.
Women who discontinue OCs on their own switch to less effective methods of birth control or use no method.1,2 Consequences may be unexpected pregnancies and an increased abortion rate.5 With patients who are using an OC, it would be appropriate to ask periodically whether they are satisfied with OC use.
In this review, we discuss the mechanisms and management of breakthrough bleeding in women taking OCs, and provide tips for counseling that may help decrease the risk of discontinuation due to menstrual abnormalities in the initial months of use.
Breakthrough bleeding in this review refers to either unplanned spotting or bleeding, regardless of requirement for protection—unless defined otherwise by a specific study under discussion.
For the purpose of performing studies, unplanned bleeding is classified by the World Health Organization into 2 categories:
- breakthrough bleeding, which requires sanitary protection, and
- spotting, which does not require sanitary protection.6 Despite this formal classification, trials have varied in their terminology and method of recording menstrual irregularities, making comparisons between studies difficult. In addition, there is wide variation among women in tolerance to bleeding abnormalities, perceptions of heavy vs light bleeding, as well as the need for protection.3
Nevertheless, menstrual abnormalities are consistently cited as a common reason for discontinuing OCs. A prospective US study of 1,657 women performed in the 1990s reported that 37% of OC users had stopped taking OCs by 6 months after starting a new prescription because of side effects.2 Irregular bleeding was the most common cause, cited by 12% of women, followed by nausea, weight gain, and mood changes, which ranged from 5% to 7%.
Breakthrough bleeding may be due to any the following variables:
- physiologic effects of OCs on the endometrium,
- OC-related parameters, including dose, formulation, and regimen,
- patient behavior (including compliance, using concomitant medications, and smoking),
- benign or malignant pathology.
OCs and the endometrium: Estrogen-progestin balance significant
Progestin and estrogen in combination OCs have profound effects on the endometrium that, although not contributing to contraception, do lead to a predictable pattern of bleeding or such problems as breakthrough bleeding or lack of withdrawal bleed.
Normally, estrogen causes the endometrium to proliferate. Progesterone stabilizes the growing uterine lining. Since the introduction of OCs in 1960, the trend in formulation has been to use the least amount of hormone necessary to inhibit ovulation. Given that the progestin is primarily responsible for the contraceptive efficacy of OCs, the risk of pregnancy is not altered with decreases in the estrogen component. However, significantly lowering the estrogen in OCs may account for breakthrough bleeding. Unplanned bleeding, though, is not dependent solely on the estrogen component, as variations in the progestin can contribute to breakthrough bleeding.7
Most OC users in the US take low-dose formulations, so designated because the estrogen component is 8 Studies that have compared OCs containing 20 μg ethinyl estradiol (EE) with those containing 30 μg or 35 μg EE have not been very useful for judging breakthrough bleeding rates because the products often also vary in the phasing and type of progestin. Some studies show more breakthrough bleeding with 20 μg EE pills,9-11 but others show equal or improved cycle control with the lower EE dose.
Estrogen-progestin balance is more important than absolute level of estrogen.
Endrikat et al12 conducted a study to compare two 20 μg EE pills containing different progestins, and to compare 2 levonorgestrel-based formulations with differing EE amounts. An OC of 20 μg EE/100 μg levonorgestrel was compared with a preparation of 20 μg EE/500 μg norethisterone. A 30 μg EE/150 μg levonorgestrel pill was used as a standard reference preparation.
Overall, the 30 μg EE preparation showed a lower cumulative incidence of breakthrough bleeding compared with the 20 μg EE/100 μg levonorgestrel and 20 μg EE/500 μg norethisterone pills over 13 cycles (1.0% vs 4.1% and 11.7%, respectively). However, the 20 μg EE/500 μg norethisterone pills consistently had a higher breakthrough bleeding rate than the 20 μg EE/100 μg levonorgestrel pill. This suggests that, although the higher EE component in the 30 μg pill was important when comparing 2 formulations with the same progestin, the difference in progestins of the two 20 μg EE pills was most likely responsible for the differing rates of breakthrough bleeding.
This study highlights the ability to achieve greater cycle control by titrating the EE component of an OC in a balanced ratio with the same progestin, but suggests that the absolute quantity of EE in a given pill may be less important than maintaining a balance between the 2 hormones or less important than the impact of different progestins on breakthrough bleeding rates.
The delicate balance between estrogen and progesterone supplementation required for contraception may also lead to progestin-induced decidualization and endometrial atrophy, which can result in asynchronous, erratic bleeding.7,13 This has been primarily studied in long-acting progestin-only contraceptives such as implants. Alterations in angiogenic factors14 may play a role. Hysteroscopic studies have shown abnormalities in superficial endometrial blood vessels in terms of size, proliferation, and fragility in women using norplant.13,15,16 Abnormalities in endothelial cells and extracellular matrix proteins,17 tissue factor,18 and endometrial lymphoid cells19 may contribute to breakthrough bleeding in progestin-dominant environments.
OC formulations, doses, regimens
More than 30 formulations of combination OCs are available in the US, with different doses and types of estrogen and progestin (TABLE 1).20 Approved OCs have been studied in clinical trials to assess contraceptive efficacy and cycle control; however, comparisons between studies regarding bleeding phenomena are impaired by inconsistent terminology.3
Whereas some studies describe breakthrough bleeding and spotting according to their recognized definitions, others simply refer to intermenstrual bleeding or use spotting to refer to any unexpected bleeding. In addition, cycle control studies of OC users frequently do not account for the effects of missed pills, use of concomitant medications, or smoking. The percentage of women who experience breakthrough bleeding in a given cycle varies widely even in different trials of the same formulation.
Pay attention to progestin level. Conventional wisdom holds that OCs with the lowest doses of EE (≤20 μg) are associated with more breakthrough bleeding.11 However, even moderately low doses of either EE or progestin can increase the incidence of breakthrough bleeding. For example, when 3 pills with the same estrogen and progestin (50 μg EE/100 μg norethindrone; 35 μg EE/100 μg norethindrone; and 35 μg EE/50 μg norethindrone) were compared in 192 women over 8 cycles, the pill containing the lowest amount of norethindrone (35 μg EE/50 μg norethindrone) caused the highest rates of breakthrough bleeding (decreasing to approximately 50% by cycle 8 as compared with 35% in the 35 μg EE/100 μg norethindrone pill and 25% in the 50 μg EE/100 μg norethindrone pill).21
In addition, the number of intermenstrual bleeding days plateaued more slowly as the amount of both hormones in the OC formulations decreased. This underscores the importance of the relative proportion of estrogen and progestin contained in combination OCs and its impact on breakthrough bleeding.
Similarly, a large comprehensive study in 1,991 women compared 7 different formulations of combination OCs containing different dose combinations of EE and norgestimate—20/250, 50/250, 35/125, 20/60, 50/60, 30/90, 25/125.22 Total intermenstrual bleeding was more frequent at lower doses of either estrogen or progestin. However, as long as a similar estrogen-progestin ratio was maintained, bleeding rates were considered acceptable (approximately 10% of days per cycle with bleeding). the authors also noted that in the low-dose range of OCs, small changes in the absolute amount of either EE or norgestimate might result in noticeable changes in bleeding
TABLE 1
Available OCs by formulation and regimen
| TRADE NAME | GENERIC NAME(S) | ESTROGEN (DOSE) | PROGESTIN (DOSE) |
|---|---|---|---|
| MONOPHASIC | |||
| Alesse, Levlite | Aviane, Lessina | Ethinyl estradiol (20 μg) | Levonorgestrel (0.1 mg) |
| Mircette | Kariva | Ethinyl estradiol (20 μg × 21 days + 10 μg × 5 days during placebo week) | Desogestrel (0.15 mg) |
| Loestrin FE | Microgestin FE 1/20, June FE 1/20 | Ethinyl estradiol (20 μg) | Norethindrone acetate (1 mg) |
| Yaz | Ethinyl estradiol (20 μg) | Drospirenone (3 mg) | |
| Levlen, Nordette | Levora, Portia | Ethinyl estradiol (30 μg) | Levonorgestrel (0.15 mg) |
| Lo/Ovral | Low-ogestrel, Cryselle | Ethinyl estradiol (30 μg) | Norgestrel (0.3 mg) |
| Desogen, Ortho-cept | Apri | Ethinyl estradiol (30 μg) | Desogestrel (0.15 mg) |
| Loestrin 21 1/5/30 | Microgestin, Junel Fe | Ethinyl estradiol (30 μg) | Norethindrone acetate (1.5 mg) |
| Yasmin | Ethinyl estradiol (30 μg) | Drospirenone (3 mg) | |
| Ovcon 35 | Ethinyl estradiol (35 μg) | Norethindrone (0.4 mg) | |
| Ortho-Cyclen | Mononesessa, Sprintec | Ethinyl estradiol (35 μg) | Norgestimate (0.25 mg) |
| Brevicon, Modicon | Nortrel, Necon 0.5/35 | Ethinyl estradiol (35 μg) | Norethindrone (0.5 mg) |
| Demulen 1/35 | Zovia 1/35 | Ethinyl estradiol (35 μg) | Ethynodiol diacetate (1 mg) |
| Ortho-Novum 1/35, Norinyl 1+35 | Necon 1/35, Nortrel | Ethinyl estradiol (35 μg) | Norethindrone (1 mg) |
| Ortho-Novum 1/50 | Necon 1/50 | Ethinyl estradiol (50 μg) | Norethindrone (1 mg) |
| Ovral | Ogestrel | Ethinyl estradiol (50 μg) | Norgestrel (0.5 mg) |
| Ovcon 50 | Ethinyl estradiol (50 μg) | Norethindrone (1 mg) | |
| Demulen 1/50 | Zovia 1/50 | Ethinyl estradiol (50 μg) | Ethynodiol diacetate (1 mg) |
| Norinyl 1/50 | Mestranol (50 μg) | Norethindrone (1 mg) | |
| BIPHASIC | |||
| Ortho-Novum 10/11, Jenest | Necon 10/11, Nelova 10/11 | Ethinyl estradiol (35 μg) | Norethindrone (0.5 mg × 10 days, 1 mg × 11 days) |
| TRIPHASIC | |||
| Ortho Tri-Cyclen Lo | Ethinyl estradiol (25 μg) | Norgestimate (0.18 mg × 7 days, 0.215 mg × 7 days, 0.25 mg × 7 days) | |
| Cyclessa | Velivet | Ethinyl estradiol (25 μg) | Desogestrel (0.1 mg × 7 days, 0.125 mg × 7 days, 0.15 mg × 7 days) |
| Triphasil, Tri-Levlen | Trivora, Enpresse | Ethinyl estradiol (30 μg × 6 days, 40 μg × 5 days, 30 μg × 10 days) | Levonorgestrel (0.05 mg × 6 days, 0.075 mg × 5 days, 0.125 mg × 10 days) |
| Tri-Norinyl | Ethinyl estradiol (35 μg) | Norethindrone (0.5 mg × 7 days, 1 mg × 9 days, 0.5 mg × 5 days) | |
| Ortho Tri-Cyclen | Tri-Sprintec, TriNessa | Ethinyl estradiol (35 μg) | Norgestimate (0.18 mg × 7 days, 0.215 mg × 7 days, 0.25 mg × 7 days) |
| Ortho-Novum 7/7/7 | Nortrel 7/7/7, Necon 7/7/7 | Ethinyl estradiol (35 μg) | Norethindrone (0.5 mg × 7 days, 0.75 mg × 7 days, 1 mg × 7 days) |
| Estrostep FE | Ethinyl estradiol (20 μg × 5 days, 30 μg × 7 days, 35 μg × 9 days) | Norethindrone acetate (1 mg) | |
| EXTENDED CYCLE | |||
| Seasonale | Ethinyl estradiol (30 μg × 84 days followed by 7 placebo pills) | Levonorgestrel (0.15 mg) | |
| Seasonique | Ethinyl estradiol (30 μg × 84 days followed by 10 μg × 7 days) | Levonorgestrel (0.15 mg) | |
Type of progestin may affect breakthrough bleeding.
All combination OCs contain either EE or mestranol. However, a variety of progestins have come into use. The 2 most common contraceptive progestins are derived from 19-nortestosterone, and are classified as gonanes or estranes.23
Estranes include norethindrone and its derivatives, norethindrone acetate and ethinyodiol diacetate. Gonanes include levonorgestrel, norgestrel, desogestrel, gestodene, and norgestimate.
Each progestin differs in half-life, estrogenic, progestogenic, and androgenic properties, and these variations may explain differing rates of breakthrough bleeding among formulations.4 As shown by Endrikat et al,12 pills with the same quantity of EE but different progestins can have marked differences in breakthrough bleeding rates.
Although gonanes have greater progestational activity, no trial has determined which progestin has the best bleeding profile. A recent Cochrane review comparing different progestins did find that, compared with pills containing levonorgestrel, those containing gestodene may be associated with less intermenstrual bleeding.24
Regardless of the progestin used or the quantity of EE, breakthrough bleeding generally decreases with each successive cycle. One study that compared 2 combination OCs composed of EE/norgestimate and EE/norgestrel demonstrated bleeding rates of 11.3% and 10.6% during the first 6 cycles, which decreased to 5.1% and 6.3% in cycles 13 to 24, respectively.25 Additionally, all women using OCs can experience some cycles without a withdrawal bleed—a menstrual abnormality that may be concerning to those who desire a menstrual period as confirmation that they are not pregnant.
Comparing regimens. OC regimens are available as biphasic, triphasic, extended-cycle, and continuous use. Women using extended-cycle contraceptives may experience more breakthrough bleeding than those using a standard 28-day pill. However, in a 3-month cycle, there are only 7 days of planned bleeding. This is in contrast to 28-day cycles during 3 months in which there are 21 days of planned bleeding.
Though women on extended-cycle regimens may initially experience more breakthrough bleeding than women using 28-day regimens, the total number of planned and unplanned bleeding days may still decrease. Women using a 3-month cycle OC (30 μg EE/150 μg levonorgestrel) experienced more unscheduled bleeding than women using a standard 28-day cycle OC of the same formulation and dose.26 The number of bleeding days decreased with each cycle. Another study examined continuous OC use (20 μg EE/100 μg levonorgestrel) over a period of 1 year, and reported a decreasing number of bleeding days over time.27 In the case of continuous use, all bleeding is unscheduled, and any bleeding is considered breakthrough bleeding.
Multiphasic OC regimens were developed with the intention of decreasing breakthrough bleeding by mimicking the rising and falling pattern of estrogen and progesterone in the normal menstrual cycle.28 After the introduction of the biphasic pill, an increase in breakthrough bleeding was noted, which led to the development of the triphasic pill.29 Though the multiphasic hypothesis is physiologically plausible, recent reviews of the literature have found the evidence for its efficacy too limited and methodologically flawed to draw any definitive conclusions about a decrease in breakthrough bleeding.30,31
Patient behaviors are contributory
Skipping a pill is a common cause of breakthrough bleeding.5 Compliance with any OC regimen is crucial to achieving a regular and predictable bleeding pattern. Of 6,676 women surveyed retrospectively, 19% reported missing 1 or more pills per cycle, and 10% reported missing 2 or more pills per cycle.32 Prospective studies have found even higher rates of inconsistent use.
TABLE 2
What to review with patients who are starting a combination OC
|
Other side effects also undermine adherence. For example, women experiencing nausea may skip pills, which leads to breakthrough bleeding and, ultimately, discontinuation.34 Patients need to understand the impact of skipping pills. Women who report irregular bleeding are 1.6 to 1.7 times more likely than those not reporting this side effect to miss 2 or more pills per cycle.5 Even 1 missed pill can increase the risk of bleeding irregularities.35
Failure to take the pill at the same time every day and poor comprehension of pill-taking instructions are other strong predictors of inconsistent use and breakthrough bleeding.32
Taking some prescription and over-the-counter medications, as well as herbal supplements, may interfere with the activity of OCs to alter bleeding patterns and contraceptive efficacy.36 Medications that induce the cytochrome P-450 system (CYP450) in the liver increase the metabolism of OCs. Anticonvulsants, the antituberculosis agent rifampin, and antifungals such as griseofulvin can increase the clearance of steroid hormones and thus lead to breakthrough bleeding. the herbal supplement St. John’s wort, commonly used for mild or moderate depression, is associated with CYP450 induction. It has been shown to increase the incidence of breakthrough bleeding and probably ovulation in women taking an OC.37
Smoking is associated with such anti-estrogenic effects as early menopause, osteoporosis, and menstrual abnormalities.38 these effects may be related to induction of hepatic estrogen and progesterone metabolism by smoking.39,40
Before receiving OCs, women are made aware of the relationship between smoking, OCs, and an increased risk of myocardial infarction, stroke, and venous thromboembolism.41 They should also understand that the anti-estrogenic effect of smoking may lower estrogen levels and lead to breakthrough bleeding, even in women who are reliable pill-takers.42,43
Smoking appears to have a dose-response relationship with breakthrough bleeding. Increasing levels of smoking have been associated with an increased risk of spotting or bleeding in each cycle.44 The difference in cycle control between smokers and nonsmokers appears to be more pronounced with each cycle. Smokers demonstrate a 30% elevation in the risk of bleeding irregularities compared with nonsmokers in the first cycle of use, which rises to an 86% increased risk by the sixth cycle.
Reports conflict regarding the relationship between smoking and contraceptive efficacy, suggesting that confounding factors like compliance may be more important than the antihormonal effect of cigarettes.45 Nevertheless, women who smoke should be informed of this potential complicating factor to OC use and as yet another reason to encourage smoking cessation.
Bleeding is sometimes pathologic
When a woman experiences difficult cycle control after the first 3 to 4 months of OC use, consider the possibility of benign and malignant growths, including endometrial polyps, submucous myomas, and cervical or endometrial cancer.46 Additionally, contraceptive failure must always be a consideration, and what appears to be breakthrough bleeding may actually represent bleeding in early pregnancy.
Cervicitis is an important but largely unrecognized source of unplanned bleeding in women using OCs. Causative organisms include Chlamydia trachomatis Neisseria gonorrhoeae, and Trichomonas vaginalis.22 Intermenstrual bleeding in women previously well controlled on OCs is particularly suggestive of asymptomatic chlamydial cervicitis.
Krettek et al47 found that 29.2% of women who had been taking OCs for more than 3 months and presented with intermenstrual spotting had a positive test for C. trachomatis. By comparison, chlamydial cervicitis was found in 10.7% of matched controls taking OCs without spotting who were screened for symptoms of vaginitis or high-risk sexual behavior, and in just 6.1% of women undergoing routine screening before the initiation of contraception.
Three-pronged management
Managing breakthrough bleeding involves effective pretreatment; ongoing counseling and reassurance; and timely and appropriate testing (TABLE 2). In some cases, pill-switching or other forms of medical management may be helpful, but these options are largely unproven.
Counseling reduces anxiety, improves satisfaction, adherence
In a recent survey, 649 Canadian women who were picking up prescriptions for OCs were asked to complete a questionnaire at the pharmacy while they waited.48 Over one third (34.5%) reported they had not received counseling from their healthcare provider about breakthrough bleeding. Furthermore, only 28.3% of women who were counseled, and 26.1% of women who were not counseled, gave the optimal response to breakthrough bleeding as defined in this study (“continue taking pill and not call my doctor”).
Lack of counseling can lead to poor method satisfaction and significant cost expenditures because of visits and phone calls by women experiencing unexpected bothersome side effects.5 Compared with women who reported the highest satisfaction with the care they received from their provider, those reporting the lowest scores were 1.6 to 2.2 times as likely to be dissatisfied with the pill.
Inform women that breakthrough bleeding is common in the first 3 or 4 cycles of OC use, that bleeding irregularities tend to decline with each successive cycle, and that they should not discontinue pill use without discussing their concerns with you. Remind women to keep sanitary protection with them during the first few months.
The impact of poor counseling was underscored in a study of women enrolled in clinical trials of OCs, contraceptive vaginal rings, and Depo-Provera. Women taking an OC were the least likely to have been warned of menstrual irregularities and thus tended to stop using that method more often than those using a ring or Depo-Provera.49 Of women who discontinue OCs, 47% use a less effective method and 19% use no method at all.1
Give specific instructions for specific regimens. Given the array of OC regimens available, make sure women know how to take them properly. This will help ensure contraceptive efficacy and cycle control. Women who do not understand pill-package instructions are up to 2.8 times more likely to miss pills, which increases the risk of breakthrough bleeding and impacts contraceptive efficacy.5 Among women who were counseled about the consequences of missed pills, 76% reported knowing what to do in response (“use another form of birth control that month”). Of women who received no such counseling, only 48% gave the appropriate response (P.001>48
To improve adherence, advise women to establish a routine for pill-taking: taking the pill at the same time each day or linking pill ingestion with another daily activity, such as tooth brushing. Women without an established routine were 3.6 times more likely to miss 2 or more pills per cycle than women with a routine.5
Reassurance regarding efficacy
Reassure users who take their pills routinely that breakthrough bleeding and contraceptive efficacy are not linked.50 Breakthrough bleeding is not a sign that OCs are not working.4 On the other hand, approximately 1 million unintended pregnancies in the United States each year are associated with misuse or discontinuation of OCs.51
When to consider diagnostic testing
For OC users who continue to experience breakthrough bleeding beyond 3 to 4 cycles, other potential causes must be ruled out using appropriate diagnostic tests. A pregnancy test, appropriate testing for cervical infection, pelvic ultrasonography, Pap smear, or endometrial biopsy may be warranted, depending on clinical circumstances.
Fall-back options
If breakthrough bleeding continues beyond 3 months, and other reasons, including poor adherence and pathologic processes, are excluded, one option would be to provide the patient with estrogen or switch her to a different pill, though no clinical trials support definitive recommendations.
Aside from changing from a multiphasic to a monophasic formulation, altering the progestin component is often a first step in trying to control breakthrough bleeding.46 An OC with a gonane rather than an estrane progestin may be beneficial as this class of progestins may provide more consistent hormonal effects on the endometrium.
Choosing an OC with a higher quantity of ee may also help, particularly for women using 20 μg pills. When possible, the same progestin should be used.
You may want to start a trial of conjugated estrogen, 1.25 mg, or estradiol, 2 mg, administered for 7 days when bleeding occurs. This can be repeated if necessary; however, if breakthrough bleeding continues despite this treatment, consideration of a different pill or method should be undertaken.
- Lack of adherence is a common cause of breakthrough bleeding. Focus counseling on ensuring that patients understand and can follow pill-taking instructions before switching pills or contraceptive method
- If breakthrough bleeding extends beyond 4 cycles and a woman wishes to continue using an oral contraceptive, consider switching to a pill with a higher ethinyl estradiol:progestin ratio, either by increasing the estradiol dose or by decreasing the relative progestin dose
- Breakthrough bleeding may be due to progestin type; switching from an estrane to a gonane may reduce it
- Women who have breakthrough bleeding after having well-controlled menstrual cycles on an oral contraceptive should be assessed for causes not related to their birth control pills, such as pregnancy, cervicitis, smoking, or interactions with medications.
In 1982, more than 20% of women surveyed in a nationally representative sample had discontinued oral contraceptives (OCs) on their own or at the recommendation of their physician due to bleeding or spotting.1 Sadly, the percentage today has not decreased much.
Understandable concern, embarrassment, and annoyance lead these women to abandon OCs.1,2 What they often don’t know, though, is that breakthrough bleeding generally is greatest in the first 3 to 4 months after starting OCs,3 and it steadily declines and stabilizes by the end of the fourth cycle.4 Timely counsel could enable many of these women to cope with the bleeding and stick with an effective contraceptive method. Additional incentives are noncontraceptive benefits of OCs: improved menstrual regularity and decreased menstrual blood loss, dysmenorrhea, and risk of ovarian and endometrial cancer.
Women who discontinue OCs on their own switch to less effective methods of birth control or use no method.1,2 Consequences may be unexpected pregnancies and an increased abortion rate.5 With patients who are using an OC, it would be appropriate to ask periodically whether they are satisfied with OC use.
In this review, we discuss the mechanisms and management of breakthrough bleeding in women taking OCs, and provide tips for counseling that may help decrease the risk of discontinuation due to menstrual abnormalities in the initial months of use.
Breakthrough bleeding in this review refers to either unplanned spotting or bleeding, regardless of requirement for protection—unless defined otherwise by a specific study under discussion.
For the purpose of performing studies, unplanned bleeding is classified by the World Health Organization into 2 categories:
- breakthrough bleeding, which requires sanitary protection, and
- spotting, which does not require sanitary protection.6 Despite this formal classification, trials have varied in their terminology and method of recording menstrual irregularities, making comparisons between studies difficult. In addition, there is wide variation among women in tolerance to bleeding abnormalities, perceptions of heavy vs light bleeding, as well as the need for protection.3
Nevertheless, menstrual abnormalities are consistently cited as a common reason for discontinuing OCs. A prospective US study of 1,657 women performed in the 1990s reported that 37% of OC users had stopped taking OCs by 6 months after starting a new prescription because of side effects.2 Irregular bleeding was the most common cause, cited by 12% of women, followed by nausea, weight gain, and mood changes, which ranged from 5% to 7%.
Breakthrough bleeding may be due to any the following variables:
- physiologic effects of OCs on the endometrium,
- OC-related parameters, including dose, formulation, and regimen,
- patient behavior (including compliance, using concomitant medications, and smoking),
- benign or malignant pathology.
OCs and the endometrium: Estrogen-progestin balance significant
Progestin and estrogen in combination OCs have profound effects on the endometrium that, although not contributing to contraception, do lead to a predictable pattern of bleeding or such problems as breakthrough bleeding or lack of withdrawal bleed.
Normally, estrogen causes the endometrium to proliferate. Progesterone stabilizes the growing uterine lining. Since the introduction of OCs in 1960, the trend in formulation has been to use the least amount of hormone necessary to inhibit ovulation. Given that the progestin is primarily responsible for the contraceptive efficacy of OCs, the risk of pregnancy is not altered with decreases in the estrogen component. However, significantly lowering the estrogen in OCs may account for breakthrough bleeding. Unplanned bleeding, though, is not dependent solely on the estrogen component, as variations in the progestin can contribute to breakthrough bleeding.7
Most OC users in the US take low-dose formulations, so designated because the estrogen component is 8 Studies that have compared OCs containing 20 μg ethinyl estradiol (EE) with those containing 30 μg or 35 μg EE have not been very useful for judging breakthrough bleeding rates because the products often also vary in the phasing and type of progestin. Some studies show more breakthrough bleeding with 20 μg EE pills,9-11 but others show equal or improved cycle control with the lower EE dose.
Estrogen-progestin balance is more important than absolute level of estrogen.
Endrikat et al12 conducted a study to compare two 20 μg EE pills containing different progestins, and to compare 2 levonorgestrel-based formulations with differing EE amounts. An OC of 20 μg EE/100 μg levonorgestrel was compared with a preparation of 20 μg EE/500 μg norethisterone. A 30 μg EE/150 μg levonorgestrel pill was used as a standard reference preparation.
Overall, the 30 μg EE preparation showed a lower cumulative incidence of breakthrough bleeding compared with the 20 μg EE/100 μg levonorgestrel and 20 μg EE/500 μg norethisterone pills over 13 cycles (1.0% vs 4.1% and 11.7%, respectively). However, the 20 μg EE/500 μg norethisterone pills consistently had a higher breakthrough bleeding rate than the 20 μg EE/100 μg levonorgestrel pill. This suggests that, although the higher EE component in the 30 μg pill was important when comparing 2 formulations with the same progestin, the difference in progestins of the two 20 μg EE pills was most likely responsible for the differing rates of breakthrough bleeding.
This study highlights the ability to achieve greater cycle control by titrating the EE component of an OC in a balanced ratio with the same progestin, but suggests that the absolute quantity of EE in a given pill may be less important than maintaining a balance between the 2 hormones or less important than the impact of different progestins on breakthrough bleeding rates.
The delicate balance between estrogen and progesterone supplementation required for contraception may also lead to progestin-induced decidualization and endometrial atrophy, which can result in asynchronous, erratic bleeding.7,13 This has been primarily studied in long-acting progestin-only contraceptives such as implants. Alterations in angiogenic factors14 may play a role. Hysteroscopic studies have shown abnormalities in superficial endometrial blood vessels in terms of size, proliferation, and fragility in women using norplant.13,15,16 Abnormalities in endothelial cells and extracellular matrix proteins,17 tissue factor,18 and endometrial lymphoid cells19 may contribute to breakthrough bleeding in progestin-dominant environments.
OC formulations, doses, regimens
More than 30 formulations of combination OCs are available in the US, with different doses and types of estrogen and progestin (TABLE 1).20 Approved OCs have been studied in clinical trials to assess contraceptive efficacy and cycle control; however, comparisons between studies regarding bleeding phenomena are impaired by inconsistent terminology.3
Whereas some studies describe breakthrough bleeding and spotting according to their recognized definitions, others simply refer to intermenstrual bleeding or use spotting to refer to any unexpected bleeding. In addition, cycle control studies of OC users frequently do not account for the effects of missed pills, use of concomitant medications, or smoking. The percentage of women who experience breakthrough bleeding in a given cycle varies widely even in different trials of the same formulation.
Pay attention to progestin level. Conventional wisdom holds that OCs with the lowest doses of EE (≤20 μg) are associated with more breakthrough bleeding.11 However, even moderately low doses of either EE or progestin can increase the incidence of breakthrough bleeding. For example, when 3 pills with the same estrogen and progestin (50 μg EE/100 μg norethindrone; 35 μg EE/100 μg norethindrone; and 35 μg EE/50 μg norethindrone) were compared in 192 women over 8 cycles, the pill containing the lowest amount of norethindrone (35 μg EE/50 μg norethindrone) caused the highest rates of breakthrough bleeding (decreasing to approximately 50% by cycle 8 as compared with 35% in the 35 μg EE/100 μg norethindrone pill and 25% in the 50 μg EE/100 μg norethindrone pill).21
In addition, the number of intermenstrual bleeding days plateaued more slowly as the amount of both hormones in the OC formulations decreased. This underscores the importance of the relative proportion of estrogen and progestin contained in combination OCs and its impact on breakthrough bleeding.
Similarly, a large comprehensive study in 1,991 women compared 7 different formulations of combination OCs containing different dose combinations of EE and norgestimate—20/250, 50/250, 35/125, 20/60, 50/60, 30/90, 25/125.22 Total intermenstrual bleeding was more frequent at lower doses of either estrogen or progestin. However, as long as a similar estrogen-progestin ratio was maintained, bleeding rates were considered acceptable (approximately 10% of days per cycle with bleeding). the authors also noted that in the low-dose range of OCs, small changes in the absolute amount of either EE or norgestimate might result in noticeable changes in bleeding
TABLE 1
Available OCs by formulation and regimen
| TRADE NAME | GENERIC NAME(S) | ESTROGEN (DOSE) | PROGESTIN (DOSE) |
|---|---|---|---|
| MONOPHASIC | |||
| Alesse, Levlite | Aviane, Lessina | Ethinyl estradiol (20 μg) | Levonorgestrel (0.1 mg) |
| Mircette | Kariva | Ethinyl estradiol (20 μg × 21 days + 10 μg × 5 days during placebo week) | Desogestrel (0.15 mg) |
| Loestrin FE | Microgestin FE 1/20, June FE 1/20 | Ethinyl estradiol (20 μg) | Norethindrone acetate (1 mg) |
| Yaz | Ethinyl estradiol (20 μg) | Drospirenone (3 mg) | |
| Levlen, Nordette | Levora, Portia | Ethinyl estradiol (30 μg) | Levonorgestrel (0.15 mg) |
| Lo/Ovral | Low-ogestrel, Cryselle | Ethinyl estradiol (30 μg) | Norgestrel (0.3 mg) |
| Desogen, Ortho-cept | Apri | Ethinyl estradiol (30 μg) | Desogestrel (0.15 mg) |
| Loestrin 21 1/5/30 | Microgestin, Junel Fe | Ethinyl estradiol (30 μg) | Norethindrone acetate (1.5 mg) |
| Yasmin | Ethinyl estradiol (30 μg) | Drospirenone (3 mg) | |
| Ovcon 35 | Ethinyl estradiol (35 μg) | Norethindrone (0.4 mg) | |
| Ortho-Cyclen | Mononesessa, Sprintec | Ethinyl estradiol (35 μg) | Norgestimate (0.25 mg) |
| Brevicon, Modicon | Nortrel, Necon 0.5/35 | Ethinyl estradiol (35 μg) | Norethindrone (0.5 mg) |
| Demulen 1/35 | Zovia 1/35 | Ethinyl estradiol (35 μg) | Ethynodiol diacetate (1 mg) |
| Ortho-Novum 1/35, Norinyl 1+35 | Necon 1/35, Nortrel | Ethinyl estradiol (35 μg) | Norethindrone (1 mg) |
| Ortho-Novum 1/50 | Necon 1/50 | Ethinyl estradiol (50 μg) | Norethindrone (1 mg) |
| Ovral | Ogestrel | Ethinyl estradiol (50 μg) | Norgestrel (0.5 mg) |
| Ovcon 50 | Ethinyl estradiol (50 μg) | Norethindrone (1 mg) | |
| Demulen 1/50 | Zovia 1/50 | Ethinyl estradiol (50 μg) | Ethynodiol diacetate (1 mg) |
| Norinyl 1/50 | Mestranol (50 μg) | Norethindrone (1 mg) | |
| BIPHASIC | |||
| Ortho-Novum 10/11, Jenest | Necon 10/11, Nelova 10/11 | Ethinyl estradiol (35 μg) | Norethindrone (0.5 mg × 10 days, 1 mg × 11 days) |
| TRIPHASIC | |||
| Ortho Tri-Cyclen Lo | Ethinyl estradiol (25 μg) | Norgestimate (0.18 mg × 7 days, 0.215 mg × 7 days, 0.25 mg × 7 days) | |
| Cyclessa | Velivet | Ethinyl estradiol (25 μg) | Desogestrel (0.1 mg × 7 days, 0.125 mg × 7 days, 0.15 mg × 7 days) |
| Triphasil, Tri-Levlen | Trivora, Enpresse | Ethinyl estradiol (30 μg × 6 days, 40 μg × 5 days, 30 μg × 10 days) | Levonorgestrel (0.05 mg × 6 days, 0.075 mg × 5 days, 0.125 mg × 10 days) |
| Tri-Norinyl | Ethinyl estradiol (35 μg) | Norethindrone (0.5 mg × 7 days, 1 mg × 9 days, 0.5 mg × 5 days) | |
| Ortho Tri-Cyclen | Tri-Sprintec, TriNessa | Ethinyl estradiol (35 μg) | Norgestimate (0.18 mg × 7 days, 0.215 mg × 7 days, 0.25 mg × 7 days) |
| Ortho-Novum 7/7/7 | Nortrel 7/7/7, Necon 7/7/7 | Ethinyl estradiol (35 μg) | Norethindrone (0.5 mg × 7 days, 0.75 mg × 7 days, 1 mg × 7 days) |
| Estrostep FE | Ethinyl estradiol (20 μg × 5 days, 30 μg × 7 days, 35 μg × 9 days) | Norethindrone acetate (1 mg) | |
| EXTENDED CYCLE | |||
| Seasonale | Ethinyl estradiol (30 μg × 84 days followed by 7 placebo pills) | Levonorgestrel (0.15 mg) | |
| Seasonique | Ethinyl estradiol (30 μg × 84 days followed by 10 μg × 7 days) | Levonorgestrel (0.15 mg) | |
Type of progestin may affect breakthrough bleeding.
All combination OCs contain either EE or mestranol. However, a variety of progestins have come into use. The 2 most common contraceptive progestins are derived from 19-nortestosterone, and are classified as gonanes or estranes.23
Estranes include norethindrone and its derivatives, norethindrone acetate and ethinyodiol diacetate. Gonanes include levonorgestrel, norgestrel, desogestrel, gestodene, and norgestimate.
Each progestin differs in half-life, estrogenic, progestogenic, and androgenic properties, and these variations may explain differing rates of breakthrough bleeding among formulations.4 As shown by Endrikat et al,12 pills with the same quantity of EE but different progestins can have marked differences in breakthrough bleeding rates.
Although gonanes have greater progestational activity, no trial has determined which progestin has the best bleeding profile. A recent Cochrane review comparing different progestins did find that, compared with pills containing levonorgestrel, those containing gestodene may be associated with less intermenstrual bleeding.24
Regardless of the progestin used or the quantity of EE, breakthrough bleeding generally decreases with each successive cycle. One study that compared 2 combination OCs composed of EE/norgestimate and EE/norgestrel demonstrated bleeding rates of 11.3% and 10.6% during the first 6 cycles, which decreased to 5.1% and 6.3% in cycles 13 to 24, respectively.25 Additionally, all women using OCs can experience some cycles without a withdrawal bleed—a menstrual abnormality that may be concerning to those who desire a menstrual period as confirmation that they are not pregnant.
Comparing regimens. OC regimens are available as biphasic, triphasic, extended-cycle, and continuous use. Women using extended-cycle contraceptives may experience more breakthrough bleeding than those using a standard 28-day pill. However, in a 3-month cycle, there are only 7 days of planned bleeding. This is in contrast to 28-day cycles during 3 months in which there are 21 days of planned bleeding.
Though women on extended-cycle regimens may initially experience more breakthrough bleeding than women using 28-day regimens, the total number of planned and unplanned bleeding days may still decrease. Women using a 3-month cycle OC (30 μg EE/150 μg levonorgestrel) experienced more unscheduled bleeding than women using a standard 28-day cycle OC of the same formulation and dose.26 The number of bleeding days decreased with each cycle. Another study examined continuous OC use (20 μg EE/100 μg levonorgestrel) over a period of 1 year, and reported a decreasing number of bleeding days over time.27 In the case of continuous use, all bleeding is unscheduled, and any bleeding is considered breakthrough bleeding.
Multiphasic OC regimens were developed with the intention of decreasing breakthrough bleeding by mimicking the rising and falling pattern of estrogen and progesterone in the normal menstrual cycle.28 After the introduction of the biphasic pill, an increase in breakthrough bleeding was noted, which led to the development of the triphasic pill.29 Though the multiphasic hypothesis is physiologically plausible, recent reviews of the literature have found the evidence for its efficacy too limited and methodologically flawed to draw any definitive conclusions about a decrease in breakthrough bleeding.30,31
Patient behaviors are contributory
Skipping a pill is a common cause of breakthrough bleeding.5 Compliance with any OC regimen is crucial to achieving a regular and predictable bleeding pattern. Of 6,676 women surveyed retrospectively, 19% reported missing 1 or more pills per cycle, and 10% reported missing 2 or more pills per cycle.32 Prospective studies have found even higher rates of inconsistent use.
TABLE 2
What to review with patients who are starting a combination OC
|
Other side effects also undermine adherence. For example, women experiencing nausea may skip pills, which leads to breakthrough bleeding and, ultimately, discontinuation.34 Patients need to understand the impact of skipping pills. Women who report irregular bleeding are 1.6 to 1.7 times more likely than those not reporting this side effect to miss 2 or more pills per cycle.5 Even 1 missed pill can increase the risk of bleeding irregularities.35
Failure to take the pill at the same time every day and poor comprehension of pill-taking instructions are other strong predictors of inconsistent use and breakthrough bleeding.32
Taking some prescription and over-the-counter medications, as well as herbal supplements, may interfere with the activity of OCs to alter bleeding patterns and contraceptive efficacy.36 Medications that induce the cytochrome P-450 system (CYP450) in the liver increase the metabolism of OCs. Anticonvulsants, the antituberculosis agent rifampin, and antifungals such as griseofulvin can increase the clearance of steroid hormones and thus lead to breakthrough bleeding. the herbal supplement St. John’s wort, commonly used for mild or moderate depression, is associated with CYP450 induction. It has been shown to increase the incidence of breakthrough bleeding and probably ovulation in women taking an OC.37
Smoking is associated with such anti-estrogenic effects as early menopause, osteoporosis, and menstrual abnormalities.38 these effects may be related to induction of hepatic estrogen and progesterone metabolism by smoking.39,40
Before receiving OCs, women are made aware of the relationship between smoking, OCs, and an increased risk of myocardial infarction, stroke, and venous thromboembolism.41 They should also understand that the anti-estrogenic effect of smoking may lower estrogen levels and lead to breakthrough bleeding, even in women who are reliable pill-takers.42,43
Smoking appears to have a dose-response relationship with breakthrough bleeding. Increasing levels of smoking have been associated with an increased risk of spotting or bleeding in each cycle.44 The difference in cycle control between smokers and nonsmokers appears to be more pronounced with each cycle. Smokers demonstrate a 30% elevation in the risk of bleeding irregularities compared with nonsmokers in the first cycle of use, which rises to an 86% increased risk by the sixth cycle.
Reports conflict regarding the relationship between smoking and contraceptive efficacy, suggesting that confounding factors like compliance may be more important than the antihormonal effect of cigarettes.45 Nevertheless, women who smoke should be informed of this potential complicating factor to OC use and as yet another reason to encourage smoking cessation.
Bleeding is sometimes pathologic
When a woman experiences difficult cycle control after the first 3 to 4 months of OC use, consider the possibility of benign and malignant growths, including endometrial polyps, submucous myomas, and cervical or endometrial cancer.46 Additionally, contraceptive failure must always be a consideration, and what appears to be breakthrough bleeding may actually represent bleeding in early pregnancy.
Cervicitis is an important but largely unrecognized source of unplanned bleeding in women using OCs. Causative organisms include Chlamydia trachomatis Neisseria gonorrhoeae, and Trichomonas vaginalis.22 Intermenstrual bleeding in women previously well controlled on OCs is particularly suggestive of asymptomatic chlamydial cervicitis.
Krettek et al47 found that 29.2% of women who had been taking OCs for more than 3 months and presented with intermenstrual spotting had a positive test for C. trachomatis. By comparison, chlamydial cervicitis was found in 10.7% of matched controls taking OCs without spotting who were screened for symptoms of vaginitis or high-risk sexual behavior, and in just 6.1% of women undergoing routine screening before the initiation of contraception.
Three-pronged management
Managing breakthrough bleeding involves effective pretreatment; ongoing counseling and reassurance; and timely and appropriate testing (TABLE 2). In some cases, pill-switching or other forms of medical management may be helpful, but these options are largely unproven.
Counseling reduces anxiety, improves satisfaction, adherence
In a recent survey, 649 Canadian women who were picking up prescriptions for OCs were asked to complete a questionnaire at the pharmacy while they waited.48 Over one third (34.5%) reported they had not received counseling from their healthcare provider about breakthrough bleeding. Furthermore, only 28.3% of women who were counseled, and 26.1% of women who were not counseled, gave the optimal response to breakthrough bleeding as defined in this study (“continue taking pill and not call my doctor”).
Lack of counseling can lead to poor method satisfaction and significant cost expenditures because of visits and phone calls by women experiencing unexpected bothersome side effects.5 Compared with women who reported the highest satisfaction with the care they received from their provider, those reporting the lowest scores were 1.6 to 2.2 times as likely to be dissatisfied with the pill.
Inform women that breakthrough bleeding is common in the first 3 or 4 cycles of OC use, that bleeding irregularities tend to decline with each successive cycle, and that they should not discontinue pill use without discussing their concerns with you. Remind women to keep sanitary protection with them during the first few months.
The impact of poor counseling was underscored in a study of women enrolled in clinical trials of OCs, contraceptive vaginal rings, and Depo-Provera. Women taking an OC were the least likely to have been warned of menstrual irregularities and thus tended to stop using that method more often than those using a ring or Depo-Provera.49 Of women who discontinue OCs, 47% use a less effective method and 19% use no method at all.1
Give specific instructions for specific regimens. Given the array of OC regimens available, make sure women know how to take them properly. This will help ensure contraceptive efficacy and cycle control. Women who do not understand pill-package instructions are up to 2.8 times more likely to miss pills, which increases the risk of breakthrough bleeding and impacts contraceptive efficacy.5 Among women who were counseled about the consequences of missed pills, 76% reported knowing what to do in response (“use another form of birth control that month”). Of women who received no such counseling, only 48% gave the appropriate response (P.001>48
To improve adherence, advise women to establish a routine for pill-taking: taking the pill at the same time each day or linking pill ingestion with another daily activity, such as tooth brushing. Women without an established routine were 3.6 times more likely to miss 2 or more pills per cycle than women with a routine.5
Reassurance regarding efficacy
Reassure users who take their pills routinely that breakthrough bleeding and contraceptive efficacy are not linked.50 Breakthrough bleeding is not a sign that OCs are not working.4 On the other hand, approximately 1 million unintended pregnancies in the United States each year are associated with misuse or discontinuation of OCs.51
When to consider diagnostic testing
For OC users who continue to experience breakthrough bleeding beyond 3 to 4 cycles, other potential causes must be ruled out using appropriate diagnostic tests. A pregnancy test, appropriate testing for cervical infection, pelvic ultrasonography, Pap smear, or endometrial biopsy may be warranted, depending on clinical circumstances.
Fall-back options
If breakthrough bleeding continues beyond 3 months, and other reasons, including poor adherence and pathologic processes, are excluded, one option would be to provide the patient with estrogen or switch her to a different pill, though no clinical trials support definitive recommendations.
Aside from changing from a multiphasic to a monophasic formulation, altering the progestin component is often a first step in trying to control breakthrough bleeding.46 An OC with a gonane rather than an estrane progestin may be beneficial as this class of progestins may provide more consistent hormonal effects on the endometrium.
Choosing an OC with a higher quantity of ee may also help, particularly for women using 20 μg pills. When possible, the same progestin should be used.
You may want to start a trial of conjugated estrogen, 1.25 mg, or estradiol, 2 mg, administered for 7 days when bleeding occurs. This can be repeated if necessary; however, if breakthrough bleeding continues despite this treatment, consideration of a different pill or method should be undertaken.
1. Pratt WF, Bachrach CA. What do women use when they stop using the pill? Fam Plann Perspect. 1987;19:257-266.
2. Rosenberg MJ, Waugh MS. Oral contraceptive discontinuation: A prospective evaluation of frequency and reasons. Am J Obstet Gynecol. 1998;179:577-582.
3. Thorneycroft IH. Cycle control with oral contraceptives: A review of the literature. Am J Obstet Gynecol. 1999;180:S280-S287.
4. Speroff L, Darney PD. A Clinical Guide for Contraception. 3rd ed. Philadelphia, Pa: Lippincott Williams & Wilkins; 2001.
5. Rosenberg MJ, Waugh MS, Burnhill MS. Compliance, counseling, and satisfaction with oral contraceptives: A prospective evaluation. Fam Plann Perspect. 1998;30:89-92-104.
6. Belsey EM, Machin D, d’Arcangues C. The analysis of vaginal bleeding patterns induced by fertility regulating methods. World Health Organization Special Programme of Research, Development and Research Training in Human Reproduction. Contraception. 1986;34:253-260.
7. ESHRE Capri Workshop Group. Ovarian and endometrial function during hormonal contraception. Hum Reprod. 2001;16:1527-1535.
8. Kaunitz AM. Oral contraceptive estrogen dose considerations. Contraception. 1998;58(Suppl):15S-21S.
9. Preston SN. A report of a collaborative dose-response clinical study using decreasing doses of combination oral contraceptives. Contraception. 1972;6:17-35.
10. Akerlund M, Rode A, Westergaard J. Comparative profiles of reliability, cycle control and side effects of two oral contraceptive formulations containing 150 mcg desogestrel and either 30 mcg or 20 mcg ethinyl oestradiol. Br J Obstet Gynaecol. 1993;100:832-838.
11. Gallo MF, Nanda K, Grimes DA, Schulz KF. Twenty micrograms vs >20 microg estrogen oral contraceptives for contraception: a systematic review of randomized controlled trials. Contraception. 2005;71:162-169.
12. Endrikat J, Hite R, Bannemerschult R, Gerlinger C, Schmidt W. Multicenter, comparative study of cycle control, efficacy and tolerability of two low-dose oral contraceptives containing 20 μg ethinyl estradiol/100 μg levonorgestrel and 20 μg ethinyl estradiol/50 μg noresthisterone. Contraception. 2001;64:3-10.
13. Hickey M, Fraser I, Dwarte D, Graham S. Endometrial vasculature in Norplant users: preliminary results from a hysteroscopic study. Hum Reprod. 1996;11(Suppl 2):35-44.
14. Smith SK. Steroids and endometrial breakthrough bleeding: future directions for research. Hum Reprod. 2000;15(Suppl 3):197-202.
15. Song JY, Markham R, Russell P, Wong T, Young L, Fraser IS. The effect of high-dose medium- and long-term progestogen exposure on endometrial vessels. Hum Reprod. 1995;10:797-800.
16. Hickey M, Dwarte D, Fraser IS. Superficial endometrial vascular fragility in Norplant users and in women with ovulatory dysfunctional uterine bleeding. Hum Reprod. 2000;15:1509-1514.
17. Rodriguez-Manzaneque JC, Graubert M, Iruela-Arispe ML. Endothelial cell dysfunction following prolonged activation of progesterone receptor. Hum Reprod. 2000;15(Suppl 3):39-47.
18. Lockwood CJ, Runic R, Wan L, Krikun G, Demopolous R, Schatz F. The role of tissue factor in regulating endometrial haemostsis: Implications for progestin-only contraception. Hum Reprod. 2000;15(Suppl 3):144-151.
19. Clark DA, Wang S, Rogers P, Vince G, Affandi B. Endometrial lymphoid cells in abnormal uterine bleeding due to levonorgestrel (Norplant). Hum Reprod. 1996;11:1438-1444.
20. Hatcher RA, Trussell J, Stewart FH. Contraceptive Technology. 18th ed. New York: Ardent Media; 2004.
21. Saleh WA, Burkman RT, Zacur HA, Kimball AW, Kwieterovich P, Bell W. A randomized trial of three oral contraceptives: comparison of bleeding patterns by contraceptive types and steroid levels. Am J Obstet Gynecol. 1993;168:1740-1747.
22. Lawson JS, Yuliano SE, Pasquale SA, Osterman JJ. Optimum dosing of an oral contraceptive. A report from the study of seven combinations of norgestimate and ethinyl estradiol. Am J Obstet Gynecol. 1979;134:315-320.
23. Stenchever MA, Ling FW. Comprehensive Gynecology. 4th ed. St Louis, Mo: Mosby; 2001.
24. Maitra N, Kulier R, Bloemenkamp KW, Helmerhorst FM, Gulmezoglu AM. Progestogens in combined oral contraceptives for contraception. Cochrane Database Syst Rev. 2004;(3):CD004861.-
25. Corson SL. Efficacy and clinical profile of a new oral contraceptive containing norgestimate. US clinical trials. Acta Obstet Gynecol Scand Suppl. 1990;152:25-31.
26. Anderson FD, Hait H. A multicenter, randomized study of an extended cycle oral contraceptive. Contraception. 2003;68:89-96.
27. Miller L, Hughes JP. Continuous combined oral contraceptive pills to eliminate withdrawal bleeding: a randomized trial. Obstet Gynecol. 2003;101:653-661.
28. Upton GV. The phasic approach to oral contraception: the triphasic concept and its clinical application. Int J Fertil Steril. 1983;28:121-140.
29. Mishell DR, Jr. Oral contraception: past, present, and future perspectives. Int J Fertil Steril. 1991;37(Suppl):s7-s18.
30. Van Vliet H, Grimes D, Helmerhorst F, Schulz K. Biphasic versus triphasic oral contraceptives for contraception. Cochrane Database Syst Rev. 2006;3:CD003283.-
31. Van Vliet H, Grimes D, Helmerhorst F, Schulz K. Biphasic versus monophasic oral contraceptives for contraception. Cochrane Database Syst Rev. 2006;3:CD002032.-
32. Rosenberg MJ, Waugh MS, Meehan TE. Use and misuse of oral contraceptives: Risk indicators for poor pill taking and discontinuation. Contraception. 1995;51:283-288.
33. Potter L, Oakley D, de Leon-Wong E, Canamar R. Measuring compliance among oral contraceptive users. Fam Plann Perspect. 1996;28:154-158.
34. Stubblefield PG. Menstrual impact of contraception. Am J Obstet Gynecol. 1994;170:513-1522.
35. Talwar PP, Dingfelder JR, Ravenholt RT. Increased risk of breakthrough bleeding when one oral-contraceptive tablet is missed. N Engl J Med. 1977;296:1236-1237.
36. Wallach M, Grimes DA. Modern Oral Contraception: Updates from the Contraceptive Report. Totowa, NJ: Emron; 2000.
37. Murphy PA, Kern SE, Stanczyk FZ, Westhoff CL. Interaction of St. John’s Wort with oral contraceptives: effects on the pharmacokinectics of norethindrone and ethinyl estradiol, ovarian activity and breakthrough bleeding. Contraception. 2005;71:402-408.
38. Baron JA, Greenberg ER. Cigarette smoking and estrogen related disease in women. In: Smoking and Reproductive Health, ed. Rosenberg MJ. Boston: PSG; .1987:149–160.
39. Jusko WJ. Influence of cigarette smoking on drug metabolism in man. Drug Metab Rev. 1979;9:221-236.
40. Basu J, Mikhail MS, Palan PR, Thysen B, Bloch E, Romney SL. Endogenous estradiol and progesterone concentrations in smokers on oral contraceptives. Gynecol Obstet Invest. 1992;33:224-227.
41. Faculty of Family Planning and Reproductive Health Care Clinical Effectiveness Unit FFPRHC Guidance (October 2003): First prescription of combined oral contraception. J Fam Plann Reprod Health Care. 2003;29:209-222.
42. Sparrow MJ. Pregnancies in reliable pill takers. NZ Med J. 1989;102:575-577.
43. Kanarkowski R, Tornatore KM, D’Ambrosio R, Gardner MJ, Jusko WJ. Pharmacokinetics of single and multiple doses of ethinyl estradiol and levonorgestrel in relation to smoking. Clin Pharmacol Ther. 1988;43:23-31.
44. Rosenberg MJ, Waugh MS, Stevens CM. Smoking and cycle control among oral contraceptive users. Am J Obstet Gynecol. 1996;174:628-632.
45. Vessey MP, Villard-Makintosh L, Jacobs HS. Anti-estrogenic effect of cigarette smoking. N Engl J Med. 1987;317:769-770.
46. Darney PD. OC practice guidelines: minimizing side effects. Int J Fertil Womens Med. 1997;42(suppl 1):158-169.
47. Krettek JE, Arkin SI, Chaisilwattana P, Monif GR. Chlamydia trachomatis in patients who used oral contraceptives and had intermenstrual spotting. Obstet Gynecol. 1993;81:728-731.
48. Gaudet LM, Kives S, Hahn PM, Reid RL. What women believe about oral contraceptives and the effect of counseling. Contraception. 2004;69:31-36.
49. Belsey EM. The association between vaginal bleeding patterns and reasons for discontinuation of contraceptive use. Contraception. 1988;38:207-225.
50. Jung-Hoffman C, Kuhl H. Intra- and interindividual variations in contraceptive steroid levels during 12 treatment cycles: no relation to irregular bleedings. Contraception. 1990;(42):423-438.
51. Rosenberg MJ, Waugh MS, Long S. Unintended pregnancies and use, misuse and discontinuation of oral contraceptives. J Reprod Med. 1995;40:355-360.
1. Pratt WF, Bachrach CA. What do women use when they stop using the pill? Fam Plann Perspect. 1987;19:257-266.
2. Rosenberg MJ, Waugh MS. Oral contraceptive discontinuation: A prospective evaluation of frequency and reasons. Am J Obstet Gynecol. 1998;179:577-582.
3. Thorneycroft IH. Cycle control with oral contraceptives: A review of the literature. Am J Obstet Gynecol. 1999;180:S280-S287.
4. Speroff L, Darney PD. A Clinical Guide for Contraception. 3rd ed. Philadelphia, Pa: Lippincott Williams & Wilkins; 2001.
5. Rosenberg MJ, Waugh MS, Burnhill MS. Compliance, counseling, and satisfaction with oral contraceptives: A prospective evaluation. Fam Plann Perspect. 1998;30:89-92-104.
6. Belsey EM, Machin D, d’Arcangues C. The analysis of vaginal bleeding patterns induced by fertility regulating methods. World Health Organization Special Programme of Research, Development and Research Training in Human Reproduction. Contraception. 1986;34:253-260.
7. ESHRE Capri Workshop Group. Ovarian and endometrial function during hormonal contraception. Hum Reprod. 2001;16:1527-1535.
8. Kaunitz AM. Oral contraceptive estrogen dose considerations. Contraception. 1998;58(Suppl):15S-21S.
9. Preston SN. A report of a collaborative dose-response clinical study using decreasing doses of combination oral contraceptives. Contraception. 1972;6:17-35.
10. Akerlund M, Rode A, Westergaard J. Comparative profiles of reliability, cycle control and side effects of two oral contraceptive formulations containing 150 mcg desogestrel and either 30 mcg or 20 mcg ethinyl oestradiol. Br J Obstet Gynaecol. 1993;100:832-838.
11. Gallo MF, Nanda K, Grimes DA, Schulz KF. Twenty micrograms vs >20 microg estrogen oral contraceptives for contraception: a systematic review of randomized controlled trials. Contraception. 2005;71:162-169.
12. Endrikat J, Hite R, Bannemerschult R, Gerlinger C, Schmidt W. Multicenter, comparative study of cycle control, efficacy and tolerability of two low-dose oral contraceptives containing 20 μg ethinyl estradiol/100 μg levonorgestrel and 20 μg ethinyl estradiol/50 μg noresthisterone. Contraception. 2001;64:3-10.
13. Hickey M, Fraser I, Dwarte D, Graham S. Endometrial vasculature in Norplant users: preliminary results from a hysteroscopic study. Hum Reprod. 1996;11(Suppl 2):35-44.
14. Smith SK. Steroids and endometrial breakthrough bleeding: future directions for research. Hum Reprod. 2000;15(Suppl 3):197-202.
15. Song JY, Markham R, Russell P, Wong T, Young L, Fraser IS. The effect of high-dose medium- and long-term progestogen exposure on endometrial vessels. Hum Reprod. 1995;10:797-800.
16. Hickey M, Dwarte D, Fraser IS. Superficial endometrial vascular fragility in Norplant users and in women with ovulatory dysfunctional uterine bleeding. Hum Reprod. 2000;15:1509-1514.
17. Rodriguez-Manzaneque JC, Graubert M, Iruela-Arispe ML. Endothelial cell dysfunction following prolonged activation of progesterone receptor. Hum Reprod. 2000;15(Suppl 3):39-47.
18. Lockwood CJ, Runic R, Wan L, Krikun G, Demopolous R, Schatz F. The role of tissue factor in regulating endometrial haemostsis: Implications for progestin-only contraception. Hum Reprod. 2000;15(Suppl 3):144-151.
19. Clark DA, Wang S, Rogers P, Vince G, Affandi B. Endometrial lymphoid cells in abnormal uterine bleeding due to levonorgestrel (Norplant). Hum Reprod. 1996;11:1438-1444.
20. Hatcher RA, Trussell J, Stewart FH. Contraceptive Technology. 18th ed. New York: Ardent Media; 2004.
21. Saleh WA, Burkman RT, Zacur HA, Kimball AW, Kwieterovich P, Bell W. A randomized trial of three oral contraceptives: comparison of bleeding patterns by contraceptive types and steroid levels. Am J Obstet Gynecol. 1993;168:1740-1747.
22. Lawson JS, Yuliano SE, Pasquale SA, Osterman JJ. Optimum dosing of an oral contraceptive. A report from the study of seven combinations of norgestimate and ethinyl estradiol. Am J Obstet Gynecol. 1979;134:315-320.
23. Stenchever MA, Ling FW. Comprehensive Gynecology. 4th ed. St Louis, Mo: Mosby; 2001.
24. Maitra N, Kulier R, Bloemenkamp KW, Helmerhorst FM, Gulmezoglu AM. Progestogens in combined oral contraceptives for contraception. Cochrane Database Syst Rev. 2004;(3):CD004861.-
25. Corson SL. Efficacy and clinical profile of a new oral contraceptive containing norgestimate. US clinical trials. Acta Obstet Gynecol Scand Suppl. 1990;152:25-31.
26. Anderson FD, Hait H. A multicenter, randomized study of an extended cycle oral contraceptive. Contraception. 2003;68:89-96.
27. Miller L, Hughes JP. Continuous combined oral contraceptive pills to eliminate withdrawal bleeding: a randomized trial. Obstet Gynecol. 2003;101:653-661.
28. Upton GV. The phasic approach to oral contraception: the triphasic concept and its clinical application. Int J Fertil Steril. 1983;28:121-140.
29. Mishell DR, Jr. Oral contraception: past, present, and future perspectives. Int J Fertil Steril. 1991;37(Suppl):s7-s18.
30. Van Vliet H, Grimes D, Helmerhorst F, Schulz K. Biphasic versus triphasic oral contraceptives for contraception. Cochrane Database Syst Rev. 2006;3:CD003283.-
31. Van Vliet H, Grimes D, Helmerhorst F, Schulz K. Biphasic versus monophasic oral contraceptives for contraception. Cochrane Database Syst Rev. 2006;3:CD002032.-
32. Rosenberg MJ, Waugh MS, Meehan TE. Use and misuse of oral contraceptives: Risk indicators for poor pill taking and discontinuation. Contraception. 1995;51:283-288.
33. Potter L, Oakley D, de Leon-Wong E, Canamar R. Measuring compliance among oral contraceptive users. Fam Plann Perspect. 1996;28:154-158.
34. Stubblefield PG. Menstrual impact of contraception. Am J Obstet Gynecol. 1994;170:513-1522.
35. Talwar PP, Dingfelder JR, Ravenholt RT. Increased risk of breakthrough bleeding when one oral-contraceptive tablet is missed. N Engl J Med. 1977;296:1236-1237.
36. Wallach M, Grimes DA. Modern Oral Contraception: Updates from the Contraceptive Report. Totowa, NJ: Emron; 2000.
37. Murphy PA, Kern SE, Stanczyk FZ, Westhoff CL. Interaction of St. John’s Wort with oral contraceptives: effects on the pharmacokinectics of norethindrone and ethinyl estradiol, ovarian activity and breakthrough bleeding. Contraception. 2005;71:402-408.
38. Baron JA, Greenberg ER. Cigarette smoking and estrogen related disease in women. In: Smoking and Reproductive Health, ed. Rosenberg MJ. Boston: PSG; .1987:149–160.
39. Jusko WJ. Influence of cigarette smoking on drug metabolism in man. Drug Metab Rev. 1979;9:221-236.
40. Basu J, Mikhail MS, Palan PR, Thysen B, Bloch E, Romney SL. Endogenous estradiol and progesterone concentrations in smokers on oral contraceptives. Gynecol Obstet Invest. 1992;33:224-227.
41. Faculty of Family Planning and Reproductive Health Care Clinical Effectiveness Unit FFPRHC Guidance (October 2003): First prescription of combined oral contraception. J Fam Plann Reprod Health Care. 2003;29:209-222.
42. Sparrow MJ. Pregnancies in reliable pill takers. NZ Med J. 1989;102:575-577.
43. Kanarkowski R, Tornatore KM, D’Ambrosio R, Gardner MJ, Jusko WJ. Pharmacokinetics of single and multiple doses of ethinyl estradiol and levonorgestrel in relation to smoking. Clin Pharmacol Ther. 1988;43:23-31.
44. Rosenberg MJ, Waugh MS, Stevens CM. Smoking and cycle control among oral contraceptive users. Am J Obstet Gynecol. 1996;174:628-632.
45. Vessey MP, Villard-Makintosh L, Jacobs HS. Anti-estrogenic effect of cigarette smoking. N Engl J Med. 1987;317:769-770.
46. Darney PD. OC practice guidelines: minimizing side effects. Int J Fertil Womens Med. 1997;42(suppl 1):158-169.
47. Krettek JE, Arkin SI, Chaisilwattana P, Monif GR. Chlamydia trachomatis in patients who used oral contraceptives and had intermenstrual spotting. Obstet Gynecol. 1993;81:728-731.
48. Gaudet LM, Kives S, Hahn PM, Reid RL. What women believe about oral contraceptives and the effect of counseling. Contraception. 2004;69:31-36.
49. Belsey EM. The association between vaginal bleeding patterns and reasons for discontinuation of contraceptive use. Contraception. 1988;38:207-225.
50. Jung-Hoffman C, Kuhl H. Intra- and interindividual variations in contraceptive steroid levels during 12 treatment cycles: no relation to irregular bleedings. Contraception. 1990;(42):423-438.
51. Rosenberg MJ, Waugh MS, Long S. Unintended pregnancies and use, misuse and discontinuation of oral contraceptives. J Reprod Med. 1995;40:355-360.