User login
FDA Medical Device Approval: Things You Didn't Learn in Medical School or Residency
5 Points on Rheumatoid Arthritis in the Cervical Spine: What You Need to Know
CONTRACEPTION
Greater access to Plan B leads to increased—and faster—use
Now that Plan B is available OTC to both men and women 18 years and older,1 several questions are in order:
- What are the effects of this change?
- Does OTC access or provision of the drug in advance reduce condom or oral contraceptive use?
- Does it increase the number of sexual partners or rate of sexually transmitted disease (STD)?
- Does it reduce unintended pregnancy?
Several randomized trials have found that advance provision of EC not only increases its utilization, but causes it to be used sooner.2-7 Most of the trials conducted so far have compared advance provision of EC with counseling about EC or a prescription for it. Only one trial has included a pharmacy-access arm, and it was conducted before FDA approval of OTC status.3 It found that pharmacy access did not increase use of EC, compared with standard access (ie, returning to the clinic when EC was needed). It is too early to tell what effect OTC availability will have on the usage rate, but data so far support the practice of giving the patient a supply of EC rather than just a prescription.
Increased access to EC does not affect regular contraceptive behavior
Multiple studies have shown that advance provision of EC has no significant effect on the use of regular contraception. Studies have examined the impact of EC on both baseline oral contraceptive usage and condom usage and found no significant change in either among women who used EC during the study.3-6
… nor does it cause promiscuity or increase the rate of STD
Multiple studies have demonstrated that advance provision of EC does not increase the number of sexual partners or rate of STD.3-6 The largest of these studies compared both pharmacy access without a prescription and advance provision of EC to standard access. That study included 2,117 sexually active young women and found no difference in the rate of STD or number of sex partners among the three study groups.3 Smaller studies comparing advance pro-vision of EC with standard access also found no significant difference in these variables.8,9
No evidence of fewer unintended pregnancies—yet
We know that progestin-only EC can reduce unintended pregnancy by almost 90%.10 However, studies have not yet demonstrated such a decrease in the general population. One reason may be that the two studies that considered unintended pregnancy as a primary outcome3,9 were too small to detect a difference in pregnancy rates, or it may be that EC was underutilized by women in the studies.
Prescribing information for levonorgestrel emergency contraception (EC) recommends ingestion of the first 0.75-mg tablet within 72 hours (3 days) of a single act of unprotected intercourse, with the second tablet taken 12 hours after the first.11 However, data show that levonorgestrel EC can prevent pregnancy up to 5 days after intercourse. In a World Health Organization multicenter randomized trial of various EC regimens, levonorgestrel EC prevented 79% to 84% of expected pregnancies when taken within 1 to 3 days, and 60% to 63% when taken 4 to 5 days after intercourse.12 Randomized trials have also found that taking both 0.75-mg levonorgestrel pills simultaneously prevents pregnancy as effectively as taking them 12 hours apart.
Levonorgestrel EC prevents or delays ovulation by inhibiting the luteinizing hormone surge during the follicular phase.13 Secondary mechanisms of contraceptive action include thickening of the cervical mucus; decreased pH level, which immobilizes sperm; and decreased recovery of sperm from the uterus.14
Levonorgestrel intrauterine system has benefits beyond contraception
The levonorgestrel intrauterine system (LNG-IUS) has been shown to significantly decrease blood loss and increase hemoglobin and serum ferritin levels in women with idiopathic menorrhagia.15 The LNGIUS reduces blood loss to a greater degree (as much as 96% after 1 year) than do placebo, nonsteroidal anti-inflammatory drugs, antifibrinolytic medication, and oral contraceptives.16 In one study,16 the LNG-IUS was the only treatment that reduced menstrual bleeding to less than 80 mL/day—the upper limit of normal.
LNG-IUS compares favorably to endometrial ablation
The LNG-IUS provides nonoperative, local, and minimally invasive treatment of menorrhagia, producing clinical results similar to those of different endometrial ablation methods for dysfunctional uterine bleeding or menorrhagia. The LNG-IUS is comparable to endometrial resection in its reduction of blood loss, patient satisfaction, rate of amenorrhea, and recurrent menorrhagia.17 It also is equivalent to thermal balloon ablation in its reduction of bleeding and increased quality of life and hemoglobin level.18,19 And it produces a higher amenorrhea rate than expectant management after endometrial resection in women with adenomyosis, and averts the need for further procedures, such as hysterectomy and repeat resection.20
In many women, LNG-IUS renders hysterectomy unnecessary
In a controlled trial involving 56 women on a waiting list for hysterectomy, 64% of those who received the LNG-IUS and 14% of those in a control group removed themselves from the list at the end of 6 months because they were satisfied with symptom control (P.001>21 In a trial involving 236 women with menorrhagia randomized to LNG-IUS or hysterectomy, the groups had similar quality-of-life scores at 1 and 5 years of follow-up—and costs associated with the LNG-IUS were significantly lower than those associated with hysterectomy, even after 50 women randomized to the LNG-IUS opted for and underwent hysterectomy.41
Consider the LNG-IUS a first-line therapy for symptomatic fibroids
The LNG-IUS continuously decreases fibroid and uterine volume and blood loss and increases ferritin levels over time among women with symptomatic fibroids.22 It should therefore be routinely considered a first-line therapy for women with fibroids who wish to preserve their childbearing potential.
Endometrial hyperplasia is reduced
The LNG-IUS can prevent and induce regression of endometrial hyperplasia.23,24 In addition, it reduces bleeding and spotting among women using hormone replacement therapy.25,26 Studies also suggest it may be beneficial in the treatment of stage I endometrial cancer, although further research into this effect is needed.27
Endometriosis-related pain is eased
In a randomized trial comparing the LNG-IUS with a gonadotropin-releasing hormone (GnRH) analogue among women with chronic pelvic pain due to endometriosis, both treatments reduced pain and improved psychological well-being to the same degree—but the LNG-IUS caused no systemic hypoestrogenic symptoms, unlike the GnRH analogue.28 In a randomized trial comparing the LNGIUS with expectant management among women who had undergone laparoscopic resection of endometriosis, women in the LNG-IUS arm had significantly decreased recurrent dysmenorrhea.29
In addition, the LNG-IUS is effective for as long as 5 years, can be used in conjunction with systemic estrogen, and is an effective contraceptive.
Continuous oral contraceptive regimens: 4 effective options
Oral contraceptives (OCs) can be prescribed for continuous use to achieve a number of different goals30:
- decrease the number of placebo days per cycle
- reduce the number of placebo weeks or withdrawal weeks per year
- eliminate withdrawal weeks from the cycle entirely
- reduce the incidence of breakthrough bleeding
Reduce the number of placebo days
Compared with the standard 28-day regimen (21 days of active pills followed by 7 days of placebo), extended regimens significantly reduce ovarian activity and produce smaller follicles and a lower estrogen level.31,32 Extended regimens may involve fewer days of placebo pills per cycle, or very small amounts of estrogen throughout the withdrawal week of the regimen. These modifications may translate into increased efficacy. In two randomized trials comparing extended regimens with a standard regimen, the extended regimens were highly effective, with a Pearl index of up to 1.29 (1.29 pregnancies for every 100 woman-years of use), and produced shorter withdrawal bleeds.33,34
Decrease the number of placebo or withdrawal weeks
The FDA approved the first OC to be packaged for extended use (Seasonale) in 2003. Each pack contains 84 active tablets of ethinyl estradiol (0.03 mg) and levonorgestrel (0.15 mg), followed by seven placebo pills. This highly effective regimen has a failure rate of 0.60 per 100 woman-years.35 Another extended-use OC (Seasonique) contains 7 days of ethinyl estradiol (10 μg) instead of placebo pills and may, therefore, suppress follicular development to an even greater degree during the withdrawal week.36
Extended cycles can be achieved with any monophasic OC in an off-label manner. Simply instruct the patient to take one active tablet for 42 consecutive days (known as “bicycling”) or for 63 consecutive days (“tricycling”), followed by 4 to 7 pill-free days.
Unscheduled bleeding with the 63-day regimen appears to be similar to the rate associated with the 21-day regimen.35 An extended-cycle regimen can be modified according to how often the user wants withdrawal bleeding.
Eliminate the withdrawal week
Perhaps the most radical extended-cycle regimen is continuous use of active pills with no placebo or withdrawal interval. This option is safe and acceptable to women, according to two small randomized trials and two prospective trials, but larger studies are needed to confirm these results.37-40 Continuous use for 1 year is associated with less bleeding, higher rates of amenorrhea, and similar side effects, compared with conventional regimens.37,38 Patient acceptance and satisfaction also are high,39 with most women choosing to keep taking the pill continuously. Lybrel, an OC designed for this purpose, contains 20 μg of ethinyl estradiol and 90 μg of levonorgestrel and is intended to eliminate menses through 1 year of continuous use.
Reduce breakthrough bleeding
For women who experience unscheduled bleeding while taking an OC continuously, one option is to stop taking pills when breakthrough bleeding occurs and initiate a hormone-free interval. This approach was studied in a randomized trial in which women were scheduled to take an OC continuously for 168 days.40 Women who had persistent unscheduled bleeding for longer than 7 days were randomized to a 3-day hormone-free interval or continuation of the active pills. Those who continued taking active pills had more bleeding over the long term, and a large percentage found it necessary to institute a delayed hormone-free interval.
This option may be particularly useful for women who experience persistent breakthrough bleeding on a continuous regimen.40
1. U.S. Food and Drug Administration. FDA approves over-the-counter access for Plan B for women 18 and older; prescription remains required for those 17 and under [August 24, 2006]. Available at: http://www.fda.gov/bbs/topics/NEWS/2006/NEW01436.html. Accessed July 11, 2007.
2. Harper CC, Cheong M, Rocca CH, Darney PD, Raine TR. The effect of increased access to emergency contraception among young adolescents. Obstet Gynecol. 2005;106:483-491.
3. Raine TR, Harper CC, Rocca CH, et al. Direct access to emergency contraception through pharmacies and effect on unintended pregnancy and STIs: a randomized controlled trial. JAMA. 2005;293:54-62.
4. Raymond EG, Trussell J, Polis CB. Population effect of increased access to emergency contraceptive pills: a systematic review. Obstet Gynecol. 2007;109:181-188.
5. Walsh TL, Frezieres RG. Patterns of emergency contraception use by age and ethnicity from a randomized trial comparing advance provision and information only. Contraception. 2006;74:110-117.
6. Jackson RA, Schwarz EB, Freedman L, Darney PD. Advance supply of emergency contraception. Effect on use and usual contraception—a randomized trial. Obstet Gynecol. 2003;102:8-16.
7. Hu X, Cheng L, Hua X, Glasier A. Advanced provision of emergency contraception to postnatal women in China makes no difference in abortion rates: a randomized controlled trial. Contraception. 2005;72:111-116.
8. Gold MA, Wolford JE, Smith KA, Parker AM. The effects of advance provision of emergency contraception on adolescent women’s sexual and contraceptive behaviors. J Pediatr Adolesc Gynecol. 2004;17:87-96.
9. Raymond EG, Stewart F, Weaver M, Monteith C, Van Der Pol B. Impact of increased access to emergency contraceptive pills: a randomized controlled trial. Obstet Gynecol. 2006;108:1098-1106.
10. Trussell J, Ellertson C, Stewart F. The effectiveness of the Yuzpe regimen of emergency contraception. Fam Plann Perspect. 1996;28:58-64, 87.
11. Plan B. [package insert] Pomona, NY: Duramed Pharmaceuticals Inc; 2006.
12. von Hertzen H, Piaggio G, Ding J, et al. Low dose mifepristone and two regimens of levonorgestrel for emergency contraception: a WHO multicentre randomised trial. Lancet. 2002;360:1803-1810.
13. Durand M, del Carmen Cravioto M, Raymond EG, et al. On the mechanisms of action of short-term levonorgestrel administration in emergency contraception. Contraception. 2001;64:227-234.
14. Croxatto HB, Devoto L, Durand M, et al. Mechanism of action of hormonal preparations used for emergency contraception: a review of the literature. Contraception. 2001;63:111-121.
15. Xiao B, Wu SC, Chong J, et al. Therapeutic effects of the levonorgestrel-releasing intrauterine system in treatment of idiopathic menorrhagia. Fertil Steril. 2003;79:963-969.
16. Milsom I, Andersson K, Andersch B, Rybo G. A comparison of flurbiprofen, tranexamic acid, and a levonorgestrel-releasing intrauterine contraceptive device in the treatment of idiopathic menorrhagia. Am J Obstet Gynecol. 1991;164:879-883.
17. Crosignani PG, Vercellini P, Mosconi P, et al. Levonorgestrel-releasing intrauterine device versus hysteroscopic endometrial resection in the treatment of dysfunctional uterine bleeding. Obstet Gynecol. 1997;90:257-263.
18. Soysal M, Soysal S, Ozer S. A randomized controlled trial of levonorgestrel releasing IUD and thermal balloon ablation in the treatment of menorrhagia. Zentralbl Gynakol. 2002;124:213-219.
19. Barrington JW, Arunkalaivanan AS, Abdel-Fattah M. Comparison between the levonorgestrel intrauterine system (LNG-IUS) and thermal balloon ablation in the treatment of menorrhagia. Eur J Obstet Gynecol Reprod Biol. 2003;108:72-74.
20. Maia H, Jr, Maltez A, Coelho G, et al. Insertion of Mirena after endometrial resection in patients with adenomyosis. J Am Assoc Gynecol Laparosc. 2003;10:512-516.
21. Lahteenmaki P, Haukkamaa M, Puolakka J, et al. Open randomised study of use of levonorgestrel releasing intrauterine system as alternative to hysterectomy. BMJ. 1998;316:1122-1126.
22. Grigorieva V, Chen-Mok M, Tarasova M, Mikhailov A. Use of a levonorgestrel-releasing intrauterine system to treat bleeding related to uterine leiomyomas. Fertil Steril. 2003;79:1194-1198.
23. Scarselli G, Tantini C, Colafranceschi M, et al. Levonorgestrel-nova-T and precancerous lesions of the endometrium. Eur J Gynaecol Oncol. 1988;9:284-286.
24. Perino A, Quartararo P, Catinella E, et al. Treatment of endometrial hyperplasia with levonorgestrel releasing intrauterine devices. Acta Eur Fertil. 1987;18:137-140.
25. Ettinger B, Pressman A, Silver P. Effect of age on reasons for initiation and discontinuation of hormone replacement therapy. Menopause. 1999;6:282-289.
26. Andersson K, Mattson LA, Rybo G, Stadberg E. Intrauterine release of levonorgestrel—a new way of adding progestogen in hormone replacement therapy. Obstet Gynecol. 1992;79:963-967.
27. Montz FJ, Bristow RE, Bovicelli A, et al. Intrauterine progesterone treatment of early endometrial cancer. Am J Obstet Gynecol. 2002;186:651-657.
28. Petta CA, Ferriani RA, Abrao MS, et al. Randomized clinical trial of a levonorgestrel-releasing intrauterine system and a depot GnRH analogue for the treatment of chronic pelvic pain in women with endometriosis. Hum Reprod. 2005;20:1993-1998.
29. Vercellini P, Frontino G, De Giorgi O, Aimi G, Zaina B, Crosignani PG. Comparison of a levonorgestrel-releasing intrauterine device versus expectant management after conservative surgery for symptomatic endometriosis: a pilot study. Fertil Steril. 2003;80:305-309.
30. Steinauer J, Autry AM. Extended cycle combined hormonal contraception. Obstet Gynecol Clin North Am. 2007;34:43-55, viii.
31. Spona J, Elstein M, Feichtinger W, et al. Shorter pill-free interval in combined oral contraceptives decreases follicular development. Contraception. 1996;54:71-77.
32. Sullivan H, Furniss H, Spona J, Elstein M. Effect of 21-day and 24-day oral contraceptive regimens containing gestodene (60 microg) and ethinyl estradiol (15 microg) on ovarian activity. Fertil Steril. 1999;72:115-120.
33. Bachmann G, Sulak PJ, Sampson-Landers C, Benda N, Marr J. Efficacy and safety of a low-dose 24-day combined oral contraceptive containing 20 micrograms ethinylestradiol and 3 mg drospirenone. Contraception. 2004;70:191-198.
34. Endrikat J, Cronin M, Gerlinger C, Ruebig A, Schmidt W, Düsterburg B. Open, multicenter comparison of efficacy, cycle control, and tolerability of a 23-day oral contraceptive regimen with 20 microg ethinyl estradiol and 75 microg gestodene and a 21-day regimen with 20 microg ethinyl estradiol and 150 microg desogestrel. Contraception. 2001;64:201-207.
35. Anderson FD, Hait H. A multicenter, randomized study of an extended cycle oral contraceptive. Contraception. 2003;68:89-96.
36. Anderson FD, Gibbons W, Portman D. Safety and efficacy of an extended-regimen oral contraceptive utilizing continuous low-dose ethinyl estradiol. Contraception. 2006;73:229-234.
37. Miller L, Hughes JP. Continuous combination oral contraceptive pills to eliminate withdrawal bleeding: a randomized trial. Obstet Gynecol. 2003;101:653-661.
38. Kwiecien M, Edelman A, Nichols MD, et al. Bleeding patterns and patient acceptability of standard or continuous dosing regimens of a low-dose oral contraceptive: a randomized trial. Contraception. 2003;67:9-13.
39. Foidart JM, Sulak PJ, Schellschmidt I, Zimmermann D. Yasmin Extended Regimen Study Group. The use of an oral contraceptive containing ethinylestradiol and drospirenone in an extended regimen over 126 days. Contraception. 2006;73:34-40.
40. Sulak PJ, Kuehl TJ, Coffee A, Willis S. Prospective analysis of occurrence and management of breakthrough bleeding during an extended oral contraceptive regimen. Am J Obstet Gynecol. 2006;195:935-941.
41. Hurskainen R, et al. Quality of life and cost-effectiveness of levonorgestrel-releasing intrauterine system versus hysterectomy for treatment of menorrhagia: a randomised trial. Lancet. 2001;357:273-277.
Dr. Newmann reports no financial relationship relevant to this article. Dr. Darney receives support from Organon, is a consultant to Organon and Bayer, and is a speaker for Organon and Bayer.
Greater access to Plan B leads to increased—and faster—use
Now that Plan B is available OTC to both men and women 18 years and older,1 several questions are in order:
- What are the effects of this change?
- Does OTC access or provision of the drug in advance reduce condom or oral contraceptive use?
- Does it increase the number of sexual partners or rate of sexually transmitted disease (STD)?
- Does it reduce unintended pregnancy?
Several randomized trials have found that advance provision of EC not only increases its utilization, but causes it to be used sooner.2-7 Most of the trials conducted so far have compared advance provision of EC with counseling about EC or a prescription for it. Only one trial has included a pharmacy-access arm, and it was conducted before FDA approval of OTC status.3 It found that pharmacy access did not increase use of EC, compared with standard access (ie, returning to the clinic when EC was needed). It is too early to tell what effect OTC availability will have on the usage rate, but data so far support the practice of giving the patient a supply of EC rather than just a prescription.
Increased access to EC does not affect regular contraceptive behavior
Multiple studies have shown that advance provision of EC has no significant effect on the use of regular contraception. Studies have examined the impact of EC on both baseline oral contraceptive usage and condom usage and found no significant change in either among women who used EC during the study.3-6
… nor does it cause promiscuity or increase the rate of STD
Multiple studies have demonstrated that advance provision of EC does not increase the number of sexual partners or rate of STD.3-6 The largest of these studies compared both pharmacy access without a prescription and advance provision of EC to standard access. That study included 2,117 sexually active young women and found no difference in the rate of STD or number of sex partners among the three study groups.3 Smaller studies comparing advance pro-vision of EC with standard access also found no significant difference in these variables.8,9
No evidence of fewer unintended pregnancies—yet
We know that progestin-only EC can reduce unintended pregnancy by almost 90%.10 However, studies have not yet demonstrated such a decrease in the general population. One reason may be that the two studies that considered unintended pregnancy as a primary outcome3,9 were too small to detect a difference in pregnancy rates, or it may be that EC was underutilized by women in the studies.
Prescribing information for levonorgestrel emergency contraception (EC) recommends ingestion of the first 0.75-mg tablet within 72 hours (3 days) of a single act of unprotected intercourse, with the second tablet taken 12 hours after the first.11 However, data show that levonorgestrel EC can prevent pregnancy up to 5 days after intercourse. In a World Health Organization multicenter randomized trial of various EC regimens, levonorgestrel EC prevented 79% to 84% of expected pregnancies when taken within 1 to 3 days, and 60% to 63% when taken 4 to 5 days after intercourse.12 Randomized trials have also found that taking both 0.75-mg levonorgestrel pills simultaneously prevents pregnancy as effectively as taking them 12 hours apart.
Levonorgestrel EC prevents or delays ovulation by inhibiting the luteinizing hormone surge during the follicular phase.13 Secondary mechanisms of contraceptive action include thickening of the cervical mucus; decreased pH level, which immobilizes sperm; and decreased recovery of sperm from the uterus.14
Levonorgestrel intrauterine system has benefits beyond contraception
The levonorgestrel intrauterine system (LNG-IUS) has been shown to significantly decrease blood loss and increase hemoglobin and serum ferritin levels in women with idiopathic menorrhagia.15 The LNGIUS reduces blood loss to a greater degree (as much as 96% after 1 year) than do placebo, nonsteroidal anti-inflammatory drugs, antifibrinolytic medication, and oral contraceptives.16 In one study,16 the LNG-IUS was the only treatment that reduced menstrual bleeding to less than 80 mL/day—the upper limit of normal.
LNG-IUS compares favorably to endometrial ablation
The LNG-IUS provides nonoperative, local, and minimally invasive treatment of menorrhagia, producing clinical results similar to those of different endometrial ablation methods for dysfunctional uterine bleeding or menorrhagia. The LNG-IUS is comparable to endometrial resection in its reduction of blood loss, patient satisfaction, rate of amenorrhea, and recurrent menorrhagia.17 It also is equivalent to thermal balloon ablation in its reduction of bleeding and increased quality of life and hemoglobin level.18,19 And it produces a higher amenorrhea rate than expectant management after endometrial resection in women with adenomyosis, and averts the need for further procedures, such as hysterectomy and repeat resection.20
In many women, LNG-IUS renders hysterectomy unnecessary
In a controlled trial involving 56 women on a waiting list for hysterectomy, 64% of those who received the LNG-IUS and 14% of those in a control group removed themselves from the list at the end of 6 months because they were satisfied with symptom control (P.001>21 In a trial involving 236 women with menorrhagia randomized to LNG-IUS or hysterectomy, the groups had similar quality-of-life scores at 1 and 5 years of follow-up—and costs associated with the LNG-IUS were significantly lower than those associated with hysterectomy, even after 50 women randomized to the LNG-IUS opted for and underwent hysterectomy.41
Consider the LNG-IUS a first-line therapy for symptomatic fibroids
The LNG-IUS continuously decreases fibroid and uterine volume and blood loss and increases ferritin levels over time among women with symptomatic fibroids.22 It should therefore be routinely considered a first-line therapy for women with fibroids who wish to preserve their childbearing potential.
Endometrial hyperplasia is reduced
The LNG-IUS can prevent and induce regression of endometrial hyperplasia.23,24 In addition, it reduces bleeding and spotting among women using hormone replacement therapy.25,26 Studies also suggest it may be beneficial in the treatment of stage I endometrial cancer, although further research into this effect is needed.27
Endometriosis-related pain is eased
In a randomized trial comparing the LNG-IUS with a gonadotropin-releasing hormone (GnRH) analogue among women with chronic pelvic pain due to endometriosis, both treatments reduced pain and improved psychological well-being to the same degree—but the LNG-IUS caused no systemic hypoestrogenic symptoms, unlike the GnRH analogue.28 In a randomized trial comparing the LNGIUS with expectant management among women who had undergone laparoscopic resection of endometriosis, women in the LNG-IUS arm had significantly decreased recurrent dysmenorrhea.29
In addition, the LNG-IUS is effective for as long as 5 years, can be used in conjunction with systemic estrogen, and is an effective contraceptive.
Continuous oral contraceptive regimens: 4 effective options
Oral contraceptives (OCs) can be prescribed for continuous use to achieve a number of different goals30:
- decrease the number of placebo days per cycle
- reduce the number of placebo weeks or withdrawal weeks per year
- eliminate withdrawal weeks from the cycle entirely
- reduce the incidence of breakthrough bleeding
Reduce the number of placebo days
Compared with the standard 28-day regimen (21 days of active pills followed by 7 days of placebo), extended regimens significantly reduce ovarian activity and produce smaller follicles and a lower estrogen level.31,32 Extended regimens may involve fewer days of placebo pills per cycle, or very small amounts of estrogen throughout the withdrawal week of the regimen. These modifications may translate into increased efficacy. In two randomized trials comparing extended regimens with a standard regimen, the extended regimens were highly effective, with a Pearl index of up to 1.29 (1.29 pregnancies for every 100 woman-years of use), and produced shorter withdrawal bleeds.33,34
Decrease the number of placebo or withdrawal weeks
The FDA approved the first OC to be packaged for extended use (Seasonale) in 2003. Each pack contains 84 active tablets of ethinyl estradiol (0.03 mg) and levonorgestrel (0.15 mg), followed by seven placebo pills. This highly effective regimen has a failure rate of 0.60 per 100 woman-years.35 Another extended-use OC (Seasonique) contains 7 days of ethinyl estradiol (10 μg) instead of placebo pills and may, therefore, suppress follicular development to an even greater degree during the withdrawal week.36
Extended cycles can be achieved with any monophasic OC in an off-label manner. Simply instruct the patient to take one active tablet for 42 consecutive days (known as “bicycling”) or for 63 consecutive days (“tricycling”), followed by 4 to 7 pill-free days.
Unscheduled bleeding with the 63-day regimen appears to be similar to the rate associated with the 21-day regimen.35 An extended-cycle regimen can be modified according to how often the user wants withdrawal bleeding.
Eliminate the withdrawal week
Perhaps the most radical extended-cycle regimen is continuous use of active pills with no placebo or withdrawal interval. This option is safe and acceptable to women, according to two small randomized trials and two prospective trials, but larger studies are needed to confirm these results.37-40 Continuous use for 1 year is associated with less bleeding, higher rates of amenorrhea, and similar side effects, compared with conventional regimens.37,38 Patient acceptance and satisfaction also are high,39 with most women choosing to keep taking the pill continuously. Lybrel, an OC designed for this purpose, contains 20 μg of ethinyl estradiol and 90 μg of levonorgestrel and is intended to eliminate menses through 1 year of continuous use.
Reduce breakthrough bleeding
For women who experience unscheduled bleeding while taking an OC continuously, one option is to stop taking pills when breakthrough bleeding occurs and initiate a hormone-free interval. This approach was studied in a randomized trial in which women were scheduled to take an OC continuously for 168 days.40 Women who had persistent unscheduled bleeding for longer than 7 days were randomized to a 3-day hormone-free interval or continuation of the active pills. Those who continued taking active pills had more bleeding over the long term, and a large percentage found it necessary to institute a delayed hormone-free interval.
This option may be particularly useful for women who experience persistent breakthrough bleeding on a continuous regimen.40
Greater access to Plan B leads to increased—and faster—use
Now that Plan B is available OTC to both men and women 18 years and older,1 several questions are in order:
- What are the effects of this change?
- Does OTC access or provision of the drug in advance reduce condom or oral contraceptive use?
- Does it increase the number of sexual partners or rate of sexually transmitted disease (STD)?
- Does it reduce unintended pregnancy?
Several randomized trials have found that advance provision of EC not only increases its utilization, but causes it to be used sooner.2-7 Most of the trials conducted so far have compared advance provision of EC with counseling about EC or a prescription for it. Only one trial has included a pharmacy-access arm, and it was conducted before FDA approval of OTC status.3 It found that pharmacy access did not increase use of EC, compared with standard access (ie, returning to the clinic when EC was needed). It is too early to tell what effect OTC availability will have on the usage rate, but data so far support the practice of giving the patient a supply of EC rather than just a prescription.
Increased access to EC does not affect regular contraceptive behavior
Multiple studies have shown that advance provision of EC has no significant effect on the use of regular contraception. Studies have examined the impact of EC on both baseline oral contraceptive usage and condom usage and found no significant change in either among women who used EC during the study.3-6
… nor does it cause promiscuity or increase the rate of STD
Multiple studies have demonstrated that advance provision of EC does not increase the number of sexual partners or rate of STD.3-6 The largest of these studies compared both pharmacy access without a prescription and advance provision of EC to standard access. That study included 2,117 sexually active young women and found no difference in the rate of STD or number of sex partners among the three study groups.3 Smaller studies comparing advance pro-vision of EC with standard access also found no significant difference in these variables.8,9
No evidence of fewer unintended pregnancies—yet
We know that progestin-only EC can reduce unintended pregnancy by almost 90%.10 However, studies have not yet demonstrated such a decrease in the general population. One reason may be that the two studies that considered unintended pregnancy as a primary outcome3,9 were too small to detect a difference in pregnancy rates, or it may be that EC was underutilized by women in the studies.
Prescribing information for levonorgestrel emergency contraception (EC) recommends ingestion of the first 0.75-mg tablet within 72 hours (3 days) of a single act of unprotected intercourse, with the second tablet taken 12 hours after the first.11 However, data show that levonorgestrel EC can prevent pregnancy up to 5 days after intercourse. In a World Health Organization multicenter randomized trial of various EC regimens, levonorgestrel EC prevented 79% to 84% of expected pregnancies when taken within 1 to 3 days, and 60% to 63% when taken 4 to 5 days after intercourse.12 Randomized trials have also found that taking both 0.75-mg levonorgestrel pills simultaneously prevents pregnancy as effectively as taking them 12 hours apart.
Levonorgestrel EC prevents or delays ovulation by inhibiting the luteinizing hormone surge during the follicular phase.13 Secondary mechanisms of contraceptive action include thickening of the cervical mucus; decreased pH level, which immobilizes sperm; and decreased recovery of sperm from the uterus.14
Levonorgestrel intrauterine system has benefits beyond contraception
The levonorgestrel intrauterine system (LNG-IUS) has been shown to significantly decrease blood loss and increase hemoglobin and serum ferritin levels in women with idiopathic menorrhagia.15 The LNGIUS reduces blood loss to a greater degree (as much as 96% after 1 year) than do placebo, nonsteroidal anti-inflammatory drugs, antifibrinolytic medication, and oral contraceptives.16 In one study,16 the LNG-IUS was the only treatment that reduced menstrual bleeding to less than 80 mL/day—the upper limit of normal.
LNG-IUS compares favorably to endometrial ablation
The LNG-IUS provides nonoperative, local, and minimally invasive treatment of menorrhagia, producing clinical results similar to those of different endometrial ablation methods for dysfunctional uterine bleeding or menorrhagia. The LNG-IUS is comparable to endometrial resection in its reduction of blood loss, patient satisfaction, rate of amenorrhea, and recurrent menorrhagia.17 It also is equivalent to thermal balloon ablation in its reduction of bleeding and increased quality of life and hemoglobin level.18,19 And it produces a higher amenorrhea rate than expectant management after endometrial resection in women with adenomyosis, and averts the need for further procedures, such as hysterectomy and repeat resection.20
In many women, LNG-IUS renders hysterectomy unnecessary
In a controlled trial involving 56 women on a waiting list for hysterectomy, 64% of those who received the LNG-IUS and 14% of those in a control group removed themselves from the list at the end of 6 months because they were satisfied with symptom control (P.001>21 In a trial involving 236 women with menorrhagia randomized to LNG-IUS or hysterectomy, the groups had similar quality-of-life scores at 1 and 5 years of follow-up—and costs associated with the LNG-IUS were significantly lower than those associated with hysterectomy, even after 50 women randomized to the LNG-IUS opted for and underwent hysterectomy.41
Consider the LNG-IUS a first-line therapy for symptomatic fibroids
The LNG-IUS continuously decreases fibroid and uterine volume and blood loss and increases ferritin levels over time among women with symptomatic fibroids.22 It should therefore be routinely considered a first-line therapy for women with fibroids who wish to preserve their childbearing potential.
Endometrial hyperplasia is reduced
The LNG-IUS can prevent and induce regression of endometrial hyperplasia.23,24 In addition, it reduces bleeding and spotting among women using hormone replacement therapy.25,26 Studies also suggest it may be beneficial in the treatment of stage I endometrial cancer, although further research into this effect is needed.27
Endometriosis-related pain is eased
In a randomized trial comparing the LNG-IUS with a gonadotropin-releasing hormone (GnRH) analogue among women with chronic pelvic pain due to endometriosis, both treatments reduced pain and improved psychological well-being to the same degree—but the LNG-IUS caused no systemic hypoestrogenic symptoms, unlike the GnRH analogue.28 In a randomized trial comparing the LNGIUS with expectant management among women who had undergone laparoscopic resection of endometriosis, women in the LNG-IUS arm had significantly decreased recurrent dysmenorrhea.29
In addition, the LNG-IUS is effective for as long as 5 years, can be used in conjunction with systemic estrogen, and is an effective contraceptive.
Continuous oral contraceptive regimens: 4 effective options
Oral contraceptives (OCs) can be prescribed for continuous use to achieve a number of different goals30:
- decrease the number of placebo days per cycle
- reduce the number of placebo weeks or withdrawal weeks per year
- eliminate withdrawal weeks from the cycle entirely
- reduce the incidence of breakthrough bleeding
Reduce the number of placebo days
Compared with the standard 28-day regimen (21 days of active pills followed by 7 days of placebo), extended regimens significantly reduce ovarian activity and produce smaller follicles and a lower estrogen level.31,32 Extended regimens may involve fewer days of placebo pills per cycle, or very small amounts of estrogen throughout the withdrawal week of the regimen. These modifications may translate into increased efficacy. In two randomized trials comparing extended regimens with a standard regimen, the extended regimens were highly effective, with a Pearl index of up to 1.29 (1.29 pregnancies for every 100 woman-years of use), and produced shorter withdrawal bleeds.33,34
Decrease the number of placebo or withdrawal weeks
The FDA approved the first OC to be packaged for extended use (Seasonale) in 2003. Each pack contains 84 active tablets of ethinyl estradiol (0.03 mg) and levonorgestrel (0.15 mg), followed by seven placebo pills. This highly effective regimen has a failure rate of 0.60 per 100 woman-years.35 Another extended-use OC (Seasonique) contains 7 days of ethinyl estradiol (10 μg) instead of placebo pills and may, therefore, suppress follicular development to an even greater degree during the withdrawal week.36
Extended cycles can be achieved with any monophasic OC in an off-label manner. Simply instruct the patient to take one active tablet for 42 consecutive days (known as “bicycling”) or for 63 consecutive days (“tricycling”), followed by 4 to 7 pill-free days.
Unscheduled bleeding with the 63-day regimen appears to be similar to the rate associated with the 21-day regimen.35 An extended-cycle regimen can be modified according to how often the user wants withdrawal bleeding.
Eliminate the withdrawal week
Perhaps the most radical extended-cycle regimen is continuous use of active pills with no placebo or withdrawal interval. This option is safe and acceptable to women, according to two small randomized trials and two prospective trials, but larger studies are needed to confirm these results.37-40 Continuous use for 1 year is associated with less bleeding, higher rates of amenorrhea, and similar side effects, compared with conventional regimens.37,38 Patient acceptance and satisfaction also are high,39 with most women choosing to keep taking the pill continuously. Lybrel, an OC designed for this purpose, contains 20 μg of ethinyl estradiol and 90 μg of levonorgestrel and is intended to eliminate menses through 1 year of continuous use.
Reduce breakthrough bleeding
For women who experience unscheduled bleeding while taking an OC continuously, one option is to stop taking pills when breakthrough bleeding occurs and initiate a hormone-free interval. This approach was studied in a randomized trial in which women were scheduled to take an OC continuously for 168 days.40 Women who had persistent unscheduled bleeding for longer than 7 days were randomized to a 3-day hormone-free interval or continuation of the active pills. Those who continued taking active pills had more bleeding over the long term, and a large percentage found it necessary to institute a delayed hormone-free interval.
This option may be particularly useful for women who experience persistent breakthrough bleeding on a continuous regimen.40
1. U.S. Food and Drug Administration. FDA approves over-the-counter access for Plan B for women 18 and older; prescription remains required for those 17 and under [August 24, 2006]. Available at: http://www.fda.gov/bbs/topics/NEWS/2006/NEW01436.html. Accessed July 11, 2007.
2. Harper CC, Cheong M, Rocca CH, Darney PD, Raine TR. The effect of increased access to emergency contraception among young adolescents. Obstet Gynecol. 2005;106:483-491.
3. Raine TR, Harper CC, Rocca CH, et al. Direct access to emergency contraception through pharmacies and effect on unintended pregnancy and STIs: a randomized controlled trial. JAMA. 2005;293:54-62.
4. Raymond EG, Trussell J, Polis CB. Population effect of increased access to emergency contraceptive pills: a systematic review. Obstet Gynecol. 2007;109:181-188.
5. Walsh TL, Frezieres RG. Patterns of emergency contraception use by age and ethnicity from a randomized trial comparing advance provision and information only. Contraception. 2006;74:110-117.
6. Jackson RA, Schwarz EB, Freedman L, Darney PD. Advance supply of emergency contraception. Effect on use and usual contraception—a randomized trial. Obstet Gynecol. 2003;102:8-16.
7. Hu X, Cheng L, Hua X, Glasier A. Advanced provision of emergency contraception to postnatal women in China makes no difference in abortion rates: a randomized controlled trial. Contraception. 2005;72:111-116.
8. Gold MA, Wolford JE, Smith KA, Parker AM. The effects of advance provision of emergency contraception on adolescent women’s sexual and contraceptive behaviors. J Pediatr Adolesc Gynecol. 2004;17:87-96.
9. Raymond EG, Stewart F, Weaver M, Monteith C, Van Der Pol B. Impact of increased access to emergency contraceptive pills: a randomized controlled trial. Obstet Gynecol. 2006;108:1098-1106.
10. Trussell J, Ellertson C, Stewart F. The effectiveness of the Yuzpe regimen of emergency contraception. Fam Plann Perspect. 1996;28:58-64, 87.
11. Plan B. [package insert] Pomona, NY: Duramed Pharmaceuticals Inc; 2006.
12. von Hertzen H, Piaggio G, Ding J, et al. Low dose mifepristone and two regimens of levonorgestrel for emergency contraception: a WHO multicentre randomised trial. Lancet. 2002;360:1803-1810.
13. Durand M, del Carmen Cravioto M, Raymond EG, et al. On the mechanisms of action of short-term levonorgestrel administration in emergency contraception. Contraception. 2001;64:227-234.
14. Croxatto HB, Devoto L, Durand M, et al. Mechanism of action of hormonal preparations used for emergency contraception: a review of the literature. Contraception. 2001;63:111-121.
15. Xiao B, Wu SC, Chong J, et al. Therapeutic effects of the levonorgestrel-releasing intrauterine system in treatment of idiopathic menorrhagia. Fertil Steril. 2003;79:963-969.
16. Milsom I, Andersson K, Andersch B, Rybo G. A comparison of flurbiprofen, tranexamic acid, and a levonorgestrel-releasing intrauterine contraceptive device in the treatment of idiopathic menorrhagia. Am J Obstet Gynecol. 1991;164:879-883.
17. Crosignani PG, Vercellini P, Mosconi P, et al. Levonorgestrel-releasing intrauterine device versus hysteroscopic endometrial resection in the treatment of dysfunctional uterine bleeding. Obstet Gynecol. 1997;90:257-263.
18. Soysal M, Soysal S, Ozer S. A randomized controlled trial of levonorgestrel releasing IUD and thermal balloon ablation in the treatment of menorrhagia. Zentralbl Gynakol. 2002;124:213-219.
19. Barrington JW, Arunkalaivanan AS, Abdel-Fattah M. Comparison between the levonorgestrel intrauterine system (LNG-IUS) and thermal balloon ablation in the treatment of menorrhagia. Eur J Obstet Gynecol Reprod Biol. 2003;108:72-74.
20. Maia H, Jr, Maltez A, Coelho G, et al. Insertion of Mirena after endometrial resection in patients with adenomyosis. J Am Assoc Gynecol Laparosc. 2003;10:512-516.
21. Lahteenmaki P, Haukkamaa M, Puolakka J, et al. Open randomised study of use of levonorgestrel releasing intrauterine system as alternative to hysterectomy. BMJ. 1998;316:1122-1126.
22. Grigorieva V, Chen-Mok M, Tarasova M, Mikhailov A. Use of a levonorgestrel-releasing intrauterine system to treat bleeding related to uterine leiomyomas. Fertil Steril. 2003;79:1194-1198.
23. Scarselli G, Tantini C, Colafranceschi M, et al. Levonorgestrel-nova-T and precancerous lesions of the endometrium. Eur J Gynaecol Oncol. 1988;9:284-286.
24. Perino A, Quartararo P, Catinella E, et al. Treatment of endometrial hyperplasia with levonorgestrel releasing intrauterine devices. Acta Eur Fertil. 1987;18:137-140.
25. Ettinger B, Pressman A, Silver P. Effect of age on reasons for initiation and discontinuation of hormone replacement therapy. Menopause. 1999;6:282-289.
26. Andersson K, Mattson LA, Rybo G, Stadberg E. Intrauterine release of levonorgestrel—a new way of adding progestogen in hormone replacement therapy. Obstet Gynecol. 1992;79:963-967.
27. Montz FJ, Bristow RE, Bovicelli A, et al. Intrauterine progesterone treatment of early endometrial cancer. Am J Obstet Gynecol. 2002;186:651-657.
28. Petta CA, Ferriani RA, Abrao MS, et al. Randomized clinical trial of a levonorgestrel-releasing intrauterine system and a depot GnRH analogue for the treatment of chronic pelvic pain in women with endometriosis. Hum Reprod. 2005;20:1993-1998.
29. Vercellini P, Frontino G, De Giorgi O, Aimi G, Zaina B, Crosignani PG. Comparison of a levonorgestrel-releasing intrauterine device versus expectant management after conservative surgery for symptomatic endometriosis: a pilot study. Fertil Steril. 2003;80:305-309.
30. Steinauer J, Autry AM. Extended cycle combined hormonal contraception. Obstet Gynecol Clin North Am. 2007;34:43-55, viii.
31. Spona J, Elstein M, Feichtinger W, et al. Shorter pill-free interval in combined oral contraceptives decreases follicular development. Contraception. 1996;54:71-77.
32. Sullivan H, Furniss H, Spona J, Elstein M. Effect of 21-day and 24-day oral contraceptive regimens containing gestodene (60 microg) and ethinyl estradiol (15 microg) on ovarian activity. Fertil Steril. 1999;72:115-120.
33. Bachmann G, Sulak PJ, Sampson-Landers C, Benda N, Marr J. Efficacy and safety of a low-dose 24-day combined oral contraceptive containing 20 micrograms ethinylestradiol and 3 mg drospirenone. Contraception. 2004;70:191-198.
34. Endrikat J, Cronin M, Gerlinger C, Ruebig A, Schmidt W, Düsterburg B. Open, multicenter comparison of efficacy, cycle control, and tolerability of a 23-day oral contraceptive regimen with 20 microg ethinyl estradiol and 75 microg gestodene and a 21-day regimen with 20 microg ethinyl estradiol and 150 microg desogestrel. Contraception. 2001;64:201-207.
35. Anderson FD, Hait H. A multicenter, randomized study of an extended cycle oral contraceptive. Contraception. 2003;68:89-96.
36. Anderson FD, Gibbons W, Portman D. Safety and efficacy of an extended-regimen oral contraceptive utilizing continuous low-dose ethinyl estradiol. Contraception. 2006;73:229-234.
37. Miller L, Hughes JP. Continuous combination oral contraceptive pills to eliminate withdrawal bleeding: a randomized trial. Obstet Gynecol. 2003;101:653-661.
38. Kwiecien M, Edelman A, Nichols MD, et al. Bleeding patterns and patient acceptability of standard or continuous dosing regimens of a low-dose oral contraceptive: a randomized trial. Contraception. 2003;67:9-13.
39. Foidart JM, Sulak PJ, Schellschmidt I, Zimmermann D. Yasmin Extended Regimen Study Group. The use of an oral contraceptive containing ethinylestradiol and drospirenone in an extended regimen over 126 days. Contraception. 2006;73:34-40.
40. Sulak PJ, Kuehl TJ, Coffee A, Willis S. Prospective analysis of occurrence and management of breakthrough bleeding during an extended oral contraceptive regimen. Am J Obstet Gynecol. 2006;195:935-941.
41. Hurskainen R, et al. Quality of life and cost-effectiveness of levonorgestrel-releasing intrauterine system versus hysterectomy for treatment of menorrhagia: a randomised trial. Lancet. 2001;357:273-277.
Dr. Newmann reports no financial relationship relevant to this article. Dr. Darney receives support from Organon, is a consultant to Organon and Bayer, and is a speaker for Organon and Bayer.
1. U.S. Food and Drug Administration. FDA approves over-the-counter access for Plan B for women 18 and older; prescription remains required for those 17 and under [August 24, 2006]. Available at: http://www.fda.gov/bbs/topics/NEWS/2006/NEW01436.html. Accessed July 11, 2007.
2. Harper CC, Cheong M, Rocca CH, Darney PD, Raine TR. The effect of increased access to emergency contraception among young adolescents. Obstet Gynecol. 2005;106:483-491.
3. Raine TR, Harper CC, Rocca CH, et al. Direct access to emergency contraception through pharmacies and effect on unintended pregnancy and STIs: a randomized controlled trial. JAMA. 2005;293:54-62.
4. Raymond EG, Trussell J, Polis CB. Population effect of increased access to emergency contraceptive pills: a systematic review. Obstet Gynecol. 2007;109:181-188.
5. Walsh TL, Frezieres RG. Patterns of emergency contraception use by age and ethnicity from a randomized trial comparing advance provision and information only. Contraception. 2006;74:110-117.
6. Jackson RA, Schwarz EB, Freedman L, Darney PD. Advance supply of emergency contraception. Effect on use and usual contraception—a randomized trial. Obstet Gynecol. 2003;102:8-16.
7. Hu X, Cheng L, Hua X, Glasier A. Advanced provision of emergency contraception to postnatal women in China makes no difference in abortion rates: a randomized controlled trial. Contraception. 2005;72:111-116.
8. Gold MA, Wolford JE, Smith KA, Parker AM. The effects of advance provision of emergency contraception on adolescent women’s sexual and contraceptive behaviors. J Pediatr Adolesc Gynecol. 2004;17:87-96.
9. Raymond EG, Stewart F, Weaver M, Monteith C, Van Der Pol B. Impact of increased access to emergency contraceptive pills: a randomized controlled trial. Obstet Gynecol. 2006;108:1098-1106.
10. Trussell J, Ellertson C, Stewart F. The effectiveness of the Yuzpe regimen of emergency contraception. Fam Plann Perspect. 1996;28:58-64, 87.
11. Plan B. [package insert] Pomona, NY: Duramed Pharmaceuticals Inc; 2006.
12. von Hertzen H, Piaggio G, Ding J, et al. Low dose mifepristone and two regimens of levonorgestrel for emergency contraception: a WHO multicentre randomised trial. Lancet. 2002;360:1803-1810.
13. Durand M, del Carmen Cravioto M, Raymond EG, et al. On the mechanisms of action of short-term levonorgestrel administration in emergency contraception. Contraception. 2001;64:227-234.
14. Croxatto HB, Devoto L, Durand M, et al. Mechanism of action of hormonal preparations used for emergency contraception: a review of the literature. Contraception. 2001;63:111-121.
15. Xiao B, Wu SC, Chong J, et al. Therapeutic effects of the levonorgestrel-releasing intrauterine system in treatment of idiopathic menorrhagia. Fertil Steril. 2003;79:963-969.
16. Milsom I, Andersson K, Andersch B, Rybo G. A comparison of flurbiprofen, tranexamic acid, and a levonorgestrel-releasing intrauterine contraceptive device in the treatment of idiopathic menorrhagia. Am J Obstet Gynecol. 1991;164:879-883.
17. Crosignani PG, Vercellini P, Mosconi P, et al. Levonorgestrel-releasing intrauterine device versus hysteroscopic endometrial resection in the treatment of dysfunctional uterine bleeding. Obstet Gynecol. 1997;90:257-263.
18. Soysal M, Soysal S, Ozer S. A randomized controlled trial of levonorgestrel releasing IUD and thermal balloon ablation in the treatment of menorrhagia. Zentralbl Gynakol. 2002;124:213-219.
19. Barrington JW, Arunkalaivanan AS, Abdel-Fattah M. Comparison between the levonorgestrel intrauterine system (LNG-IUS) and thermal balloon ablation in the treatment of menorrhagia. Eur J Obstet Gynecol Reprod Biol. 2003;108:72-74.
20. Maia H, Jr, Maltez A, Coelho G, et al. Insertion of Mirena after endometrial resection in patients with adenomyosis. J Am Assoc Gynecol Laparosc. 2003;10:512-516.
21. Lahteenmaki P, Haukkamaa M, Puolakka J, et al. Open randomised study of use of levonorgestrel releasing intrauterine system as alternative to hysterectomy. BMJ. 1998;316:1122-1126.
22. Grigorieva V, Chen-Mok M, Tarasova M, Mikhailov A. Use of a levonorgestrel-releasing intrauterine system to treat bleeding related to uterine leiomyomas. Fertil Steril. 2003;79:1194-1198.
23. Scarselli G, Tantini C, Colafranceschi M, et al. Levonorgestrel-nova-T and precancerous lesions of the endometrium. Eur J Gynaecol Oncol. 1988;9:284-286.
24. Perino A, Quartararo P, Catinella E, et al. Treatment of endometrial hyperplasia with levonorgestrel releasing intrauterine devices. Acta Eur Fertil. 1987;18:137-140.
25. Ettinger B, Pressman A, Silver P. Effect of age on reasons for initiation and discontinuation of hormone replacement therapy. Menopause. 1999;6:282-289.
26. Andersson K, Mattson LA, Rybo G, Stadberg E. Intrauterine release of levonorgestrel—a new way of adding progestogen in hormone replacement therapy. Obstet Gynecol. 1992;79:963-967.
27. Montz FJ, Bristow RE, Bovicelli A, et al. Intrauterine progesterone treatment of early endometrial cancer. Am J Obstet Gynecol. 2002;186:651-657.
28. Petta CA, Ferriani RA, Abrao MS, et al. Randomized clinical trial of a levonorgestrel-releasing intrauterine system and a depot GnRH analogue for the treatment of chronic pelvic pain in women with endometriosis. Hum Reprod. 2005;20:1993-1998.
29. Vercellini P, Frontino G, De Giorgi O, Aimi G, Zaina B, Crosignani PG. Comparison of a levonorgestrel-releasing intrauterine device versus expectant management after conservative surgery for symptomatic endometriosis: a pilot study. Fertil Steril. 2003;80:305-309.
30. Steinauer J, Autry AM. Extended cycle combined hormonal contraception. Obstet Gynecol Clin North Am. 2007;34:43-55, viii.
31. Spona J, Elstein M, Feichtinger W, et al. Shorter pill-free interval in combined oral contraceptives decreases follicular development. Contraception. 1996;54:71-77.
32. Sullivan H, Furniss H, Spona J, Elstein M. Effect of 21-day and 24-day oral contraceptive regimens containing gestodene (60 microg) and ethinyl estradiol (15 microg) on ovarian activity. Fertil Steril. 1999;72:115-120.
33. Bachmann G, Sulak PJ, Sampson-Landers C, Benda N, Marr J. Efficacy and safety of a low-dose 24-day combined oral contraceptive containing 20 micrograms ethinylestradiol and 3 mg drospirenone. Contraception. 2004;70:191-198.
34. Endrikat J, Cronin M, Gerlinger C, Ruebig A, Schmidt W, Düsterburg B. Open, multicenter comparison of efficacy, cycle control, and tolerability of a 23-day oral contraceptive regimen with 20 microg ethinyl estradiol and 75 microg gestodene and a 21-day regimen with 20 microg ethinyl estradiol and 150 microg desogestrel. Contraception. 2001;64:201-207.
35. Anderson FD, Hait H. A multicenter, randomized study of an extended cycle oral contraceptive. Contraception. 2003;68:89-96.
36. Anderson FD, Gibbons W, Portman D. Safety and efficacy of an extended-regimen oral contraceptive utilizing continuous low-dose ethinyl estradiol. Contraception. 2006;73:229-234.
37. Miller L, Hughes JP. Continuous combination oral contraceptive pills to eliminate withdrawal bleeding: a randomized trial. Obstet Gynecol. 2003;101:653-661.
38. Kwiecien M, Edelman A, Nichols MD, et al. Bleeding patterns and patient acceptability of standard or continuous dosing regimens of a low-dose oral contraceptive: a randomized trial. Contraception. 2003;67:9-13.
39. Foidart JM, Sulak PJ, Schellschmidt I, Zimmermann D. Yasmin Extended Regimen Study Group. The use of an oral contraceptive containing ethinylestradiol and drospirenone in an extended regimen over 126 days. Contraception. 2006;73:34-40.
40. Sulak PJ, Kuehl TJ, Coffee A, Willis S. Prospective analysis of occurrence and management of breakthrough bleeding during an extended oral contraceptive regimen. Am J Obstet Gynecol. 2006;195:935-941.
41. Hurskainen R, et al. Quality of life and cost-effectiveness of levonorgestrel-releasing intrauterine system versus hysterectomy for treatment of menorrhagia: a randomised trial. Lancet. 2001;357:273-277.
Dr. Newmann reports no financial relationship relevant to this article. Dr. Darney receives support from Organon, is a consultant to Organon and Bayer, and is a speaker for Organon and Bayer.
PROM dilemmas: Choosing a strategy, knowing when to call it quits
CASE: PROM at 22 weeks
J.S. is a 22-year-old woman at 22 weeks’ gestation in her second pregnancy. Her first gestation ended in spontaneous abortion at 10 weeks, followed by dilation and curettage. She has been referred to you by her midwife, who is concerned about J.S.’s complaints of loss of fluid over the past 2 weeks and who cannot document rupture of membranes by the usual means.
In your office, J.S. continues to complain of intermittent leakage of clear fluid. She says there has been no vaginal bleeding, foul-smelling discharge, fever, chills, or abdominal tenderness. You find a normal abdomen. A sterile speculum exam is equivocal, without evidence of pooling or ferning; a nitrazine test is positive, however. A complete blood count reveals no evidence of leukocytosis. Urinalysis is negative.
You suspect preterm premature rupture of membranes (PROM) when bedside ultrasonography (US) documents oligohydramnios with an amniotic fluid index of less than 5 cm. The kidneys, bladder, and stomach all appear normal.
What is the best way to confirm the diagnosis? What is the most appropriate management at this gestational age? And how do you counsel J.S. about the risk to her, and her baby, of continuing the pregnancy?
Given the very poor prognosis of many cases of early PROM, accurate diagnosis is critical to determine the best management strategy. The gold standard of diagnosis is sterile vaginal examination with a speculum to identify clear fluid leaking from the cervix or pooling in the posterior fornix. Use nitrazine paper to assess the fluid collected from the posterior fornix for alkaline pH; this method has a positive predictive value (PPV) of 99% and negative predictive value (NPV) of 96%.1 The appearance of “ferning”—a crystalline pattern that occurs when the saline amniotic fluid dries—carries a PPV of 98% to 99% and a NPV of 90% to 99%.2
One must also consider the patient’s history. When that history and the physical exam fail to render a clear result, use US to assess the amniotic fluid volume. A low volume in the presence of a convincing clinical history is very suspicious for PROM, as in the case just described.
Tinting the amniotic fluid may help
In equivocal cases, mix 1 to 3 mL of indigo carmine with 5 mL of sterile saline and insert it into the amniotic fluid under US guidance. This dye will make any leaking amniotic fluid obvious. Be aware, however, that instillation of the dye is very difficult in cases of severe oligohydramnios or anhydramnios. In this setting, amniocentesis can also cause contractions or vaginal bleeding.
New diagnostic tool on the horizon
Recent studies have focused on a new rapid test (AmniSure) that uses immunochromatography to detect trace amounts of placental α-microglobulin-1 protein.3 This protein is specific to amniotic fluid and present in vaginal secretions only when amniotic fluid is leaking through the cervix. One study of 203 patients suspected of having ruptured membranes found the AmniSure test to have a PPV of 100% and NPV of 99.1%.3 Although these findings are promising, further confirmatory studies are needed before this product can be recommended for widespread use.
CASE continued: Leakage of tinted fluid confirms PROM
Because the diagnostic steps taken so far have been inconclusive, J.S. undergoes amniocentesis with infusion of indigo carmine. Within 2 hours, blue dye is observed leaking from the cervix, confirming PROM. A sample of amniotic fluid obtained at the time of amniocentesis produces a negative gram stain and reveals a normal glucose level and leukocyte count. Amniotic fluid cultures are pending.
What is your next step?
Determining the best management strategy is next. The treatment plan should be based on gestational age, presence or absence of infection or labor, and fetal status. Therefore, the initial evaluation of a patient with PROM should focus on the collection of this clinical information.
I recommend these measures:
- Document the exact gestational age by careful review of available records and ultrasound biometry
- Identify indicators of infection, such as maternal fever and tachycardia, fundal tenderness, fetal tachycardia, and an elevated white blood cell count
- Amniocentesis may be required to rule out amnionitis in cases where the diagnosis is clinically unclear
- Document fetal presentation
- Initiate fetal heart rate (FHR) monitoring at the time of diagnosis and perform a biophysical profile (BPP).
Midtrimester PROM: 16 to 24 weeks’ gestation
Management differs for each gestational age.
Midtrimester PROM occurs in approximately 0.7% of all pregnancies and is a significant source of morbidity and mortality.4,5 It may be iatrogenic in nature when it follows an invasive procedure such as amniocentesis or fetoscopy. It also may occur spontaneously, with causes similar to those of PROM at later gestational ages. At this early gestational age, PROM is more likely to be associated with cervical incompetence and inflammation.6,7
Infection is a risk—and may be the underlying cause
Infection is associated with as many as 30% to 50% of cases of PROM.4,8-10 Half of the cases of intra-amniotic infection develop within 7 days after PROM. That’s because many cases of early PROM have infection or inflammation as their cause.
Intrauterine demise is common at early gestational ages
The risk of intrauterine fetal demise is inversely related to gestational age at the time of rupture. That is, the earlier the gestational age, the higher the rate of fetal death. One study found that the rate of intrauterine fetal demise was 33% when PROM occurred before 20 weeks’ gestation and 20% when it occurred between 20 and 24 weeks; it was rare after 25 weeks.10
Pulmonary hypoplasia is more common at this critical juncture
The midtrimester is a critical time for fetal lung development. During the canalicular stage (between 17 and 24 weeks’ gestation), the gas-exchanging acini and pulmonary capillaries are forming, so they are more susceptible to injury. The incidence of pulmonary hypoplasia is approximately 10% when PROM occurs earlier than 20 weeks’ gestation, although a wide range of rates has been reported.4,8-10 Pulmonary hypoplasia remains a significant cause of neonatal mortality and is found in as many as 77% of autopsies of infants from pregnancies complicated by midtrimester PROM.11
The incidence of pulmonary hypoplasia decreases by as much as 46% with each week of gestational age at the time of PROM.12 After 26 weeks, when the terminal sac stage of development occurs, the rate of pulmonary hypoplasia complicating PROM drops to less than 2%.12-14
The degree of oligohydramnios also affects the rate of pulmonary hypoplasia, which increases significantly when the amniotic fluid index is less than 5 cm.15
Limb deformity may be related to restricted movement
Although limb development occurs in the embryonic period, most limb growth takes place during the second and third trimesters.16 The restriction in movement and increased pressure associated with prolonged periods of oligohydramnios can lead to skeletal deformity in otherwise normal extremities.
The frequency of deformity varies widely among studies, but the mean incidence is 7%.4,8-11 A twofold higher incidence of skeletal abnormality occurs when midtrimester PROM is accompanied by severe oligohydramnios. In one study, the rate of skeletal abnormality was 54% when the deepest pocket of amniotic fluid was less than 1 cm, compared with 26% for matched pregnancies with a normal or mildly reduced volume.16
Maternal complications include retained placenta, endometritis
Maternal complications associated with very early PROM include a higher rate of cesarean section due to fetal malpresentation and FHR abnormalities, which often accompany oligohydramnios and intraamniotic infection.10 A classical incision is more likely in these cases due to the poorly developed lower uterine segment. Retained placenta necessitating postpartum curettage occurs in 9% to 18% of cases of PROM at less than 20 weeks’ gestation. In addition, postpartum endometritis complicates as many as 40% of cases of midtrimester PROM.4,8-11
General prognosis
The outcome of midtrimester PROM depends on the underlying cause. If it is iatrogenic, the outcome is usually favorable, with frequent resealing of the membranes; most cases end in a normal term delivery. The outcome of spontaneous PROM is more grim.
Midtrimester PROM has the same relatively short latency (approximately 17 days on average) as PROM that occurs later in pregnancy. Less than 50% of women with midtrimester PROM remain pregnant at the end of the first week, and as many as 75% of these women will have delivered by 28 days after PROM.11 These percentages indicate that most women with midtrimester PROM deliver before fetal viability can be attained, or in the risky periviable period.
Overall, midtrimester PROM is associated with significant fetal, neonatal, and maternal morbidity. The risks must be explained to the patient along with any management plan.
Given the very poor prognosis and small chance of prolonged latency, induction of labor and pregnancy termination are reasonable options at the time of presentation. The patient needs to know that expectant management can be associated with significant long-term morbidity and a higher rate of neonatal mortality.
CASE continued: Patient is apprised of the risks
After a frank discussion of the risks involved in continuing her pregnancy, J.S. chooses expectant management. Given the early gestational age and absence of any sign of infection, she is sent home for bed rest and instructed to check her temperature twice daily. She is told to return for evaluation if fever (>100°F) or symptoms of infection develop. Because of the very early gestational age, no steroid or antibiotic will be given until 24 weeks’ gestation, when she will be admitted for inpatient care.
PROM at 24 to 32 weeks’ gestation
Selecting a management strategy for a pregnancy at this gestational age means weighing the potential morbidity and mortality of immediate delivery against the morbidity and mortality of expectant management. At this gestational age, the principal source of fetal morbidity is prematurity itself. As many as 40% of infants delivered before 26 weeks’ gestation experience some type of long-term morbidity such as intraventricular hemorrhage (IVH), retinopathy of prematurity, necrotizing enterocolitis (NEC), or, most commonly, respiratory distress syndrome (RDS).16,17 It is true that fetal morbidity increases when chorioamnionitis is present, but the potential benefit of prolonging the pregnancy by 7 to 14 days is believed to outweigh the risk of infection at these gestational ages. Therefore, in the absence of contraindications, expectant management is the usual course of action.
Select patients carefully for expectant management
Consider expectant management only when fetal well-being can be documented, without evidence of infection. Abruption is a contraindication to expectant management, although the clinical nature of this diagnosis can make it difficult to identify. If abruption is diagnosed, aggressive management with labor augmentation or cesarean section and intravenous (IV) antibiotics is appropriate.
Repetitive fetal heart decelerations in the presence of active vaginal bleeding and uterine tenderness indicate placental insufficiency and are an indication for delivery.
More than 50% of patients deliver within the first week after PROM is diagnosed.18 At least 30% experience chorioamnionitis some time after the diagnosis of PROM, and 1% to 2% suffer cord prolapse.10,18,19 As many as 4% to 12% of cases of PROM will also be complicated by abruption,20,21 and a rate of intrauterine fetal demise as high as 1% has been documented.18 Therefore, if expectant management is selected, it should include close monitoring for these complications.
Hospitalization is warranted. Women who are stable and being managed expectantly should probably be hospitalized. One prospective trial comparing outcomes between women managed at home and women who were hospitalized found no significant difference in latency period or the rate of infection.22 However, the strict inclusion criteria for this study make it difficult to generalize the results. Only 18% of the 349 women screened for enrollment met these criteria.
The high rate of precipitous labor, frequent onset of infection, and need for frequent maternal and neonatal evaluation at this gestational age make hospitalization a prudent choice.
Fetal surveillance is mandatory
Most investigators would agree that a regular schedule of fetal surveillance is necessary during expectant management. But there is no clear evidence indicating which type, and what timing, of surveillance are best. It is clear that changes in the FHR pattern and BPP precede the onset of chorioamnionitis and intrauterine demise due to cord accidents.23-25 However, no studies have demonstrated a significant improvement in neonatal outcomes with daily or even twice-daily antenatal surveillance.
At our institution, we follow a regimen of daily surveillance, which consists of a nonstress test and/or BPP to confirm fetal well-being.
Tocolysis won’t prolong gestation beyond 48 hours…
There is no evidence that prolonged tocolysis with any therapy significantly increases long-term latency or improves any type of neonatal morbidity in pregnancies complicated by PROM. Tocolysis may prolong pregnancy over the short term (<48 hours),26,27 but its widespread use is not supported by the evidence.
Tocolysis is appropriate to achieve safe maternal transport or administer steroids.
…but corticosteroids are highly beneficial
Antenatal corticosteroids clearly improve neonatal outcomes when PROM occurs before 32 weeks’ gestation. Two large meta-analyses have found such benefits to be a decrease in the rates of RDS, IVH, NEC, and neonatal death.28,29 A recent prospective study confirmed these findings.30 The rate of RDS declined 26%—from 44% to 18%.
A consensus panel of the National Institutes of Health (NIH) recommended use of corticosteroids in cases of PROM between 24 and 32 weeks’ gestation in which there is no clinical evidence of infection.31 Any of the standard steroid regimens is appropriate. At our institution, we give an intramuscular injection of 12 mg of betamethasone and repeat this one time in 24 hours.
Are prophylactic antibiotics warranted?
The fact that infection is the most commonly identified cause of PROM prompts the question: Does treatment with IV antibiotics improve outcomes and prolong latency even in the absence of clinically apparent infection? Mercer and colleagues32 reported a significant reduction in chorioamnionitis, endometritis, and neonatal infection, including sepsis and pneumonia, in pregnancies treated with prophylactic antibiotics, compared with expectant management alone. In that study, latency also increased significantly following antibiotic therapy. Other meta-analyses confirm the benefits of prophylactic antibiotics, demonstrating a lower rate of neonatal sepsis and IVH following treatment.33,34
One large multicenter randomized trial found a reduced rate of IVH and RDS after treatment with IV erythromycin and ampicillin for 48 hours, followed by a 5-day course of amoxicillin and erythromycin.35 A Cochrane review of the use of prophylactic antibiotics in the setting of PROM included 19 studies with various antibiotic regimens.36 It concluded that antibiotic therapy prolongs latency (at both 48 hours and 7 days), decreases maternal infection, and reduces the incidence of neonatal complications, including infection, need for oxygen, IVH, and periventricular leukomalacia.
No superior regimen, but avoid amoxicillin-clavulanate. Although no single antibiotic regimen is clearly superior to the others, erythromycin has been associated with benefits most consistently. The most common dosage for erythromycin is 250 mg every 6 hours for a total of 48 hours and then an additional 5 days of oral treatment. Amoxicillin-clavulanate has been associated with an increased risk of NEC in at least two trials, and should probably be avoided. A Cochrane review confirms these conclusions.36
Choice of delivery route is flexible
Once the need for delivery arises, choose the route according to normal obstetric indications. In the setting of PROM with malpresentation, cesarean delivery is probably the best approach. However, in very-low-birth-weight infants, the best mode of delivery remains unclear.37 If the fetus is in cephalic presentation, an attempt at vaginal delivery does not appear to have a worse neonatal outcome.
If spontaneous labor does not occur or if induction is not indicated for maternal or fetal reasons, one may choose to deliver the patient at 32 weeks’ gestation or continue expectant management until 34 weeks’ gestation. This decision is discussed in more detail in the next section.
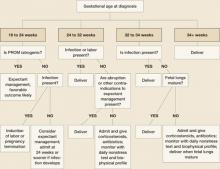
ALGORITHM
Management of PROM varies with gestational age
PROM at 32 to 34 weeks’ gestation
Although it is generally accepted that the fetus benefits from expectant management in pregnancies complicated by PROM before 32 weeks’ gestation, the management of PROM that arises between 32 and 34 weeks remains controversial and a focus of ongoing research. Because most neonatal morbidity is caused by prematurity, and the rate of prematurity-related complications decreases with increasing gestational age, some argue that the potential benefit of prolonging latency after 32 weeks’ gestation does not outweigh the risk of chorioamnionitis.
Continue the gestation? Or deliver?
Mercer and colleagues randomized 97 women with PROM between 32 and 36 weeks’ gestation and a mature lung profile to expectant management or immediate induction.38 Although expectant management did prolong pregnancy, no neonatal benefit was observed, and the rate of chorioamnionitis was higher with expectant management, with a longer hospital stay.
Cox and associates found a higher rate of chorioamnionitis among 68 women with PROM between 30 and 34 weeks’ gestation who were managed expectantly, compared with 61 women assigned to immediate induction.39 Neonatal morbidity was similar in both groups.
These studies suggest that expectant management after 32 weeks leads only to an increased rate of chorioamnionitis and longer maternal and neonatal hospitalization, without any demonstrable neonatal benefit. However, one significant limitation of these studies is the fact that patients managed expectantly received neither corticosteroids nor prophylactic antibiotics.
Are corticosteroids appropriate at this gestational age?
We lack sufficient evidence to support the routine use of corticosteroids after 32 weeks in pregnancies complicated by PROM. The NIH consensus panel suggested that they may be an option in patients without contraindications up to 34 weeks’ gestation.31
Some experts recommend testing for fetal lung maturity when PROM occurs between 32 and 34 weeks’ gestation. In this group, the rate of fetal lung maturity is between 50% and 60%.40,41 There is no clear benefit in prolonging a pregnancy when fetal lung maturity can be documented. However, in the setting of immature fetal lungs, expectant management may be appropriate following treatment with corticosteroids and a prophylactic antibiotic regimen. Patients who present at 34 weeks’ gestation or beyond are likely to benefit most from immediate delivery.
When expectant management is chosen between 32 and 34 weeks, inpatient hospitalization with daily monitoring is also recommended. The mode of delivery depends on the usual obstetric indications.
CASE resolved: Patient develops fever and spontaneous labor
Ten days after the documentation of PROM, J.S. reports a fever and abdominal tenderness, as well as frequent uterine contractions that began early in the day. She is admitted to the hospital. A physical examination confirms clinically apparent intra-amniotic infection and labor. The patient is started on IV antibiotics and, after a short labor, delivers a nonviable male infant weighing 500 g. Pathologic examination of the fetus and placenta reveals a normal, immature fetus with evidence of acute chorioamnionitis on placental sections.
1. Abe T. The detection of rupture of fetal membranes with the nitrazine indicator. Am J Obstet Gynecol. 1940;39:400.-
2. Davidson KM. Detection of premature rupture of the membranes. Clin Obstet Gynecol. 1991;34:715-722.
3. Cousins LM, Smok D, Lovett SM, Poeltler DM. AmniSure placental alpha microglobulin-1 rapid immunoassay versus standard diagnostic methods for detection of ruptured membranes. Am J Perinatol. 2005;22(6):317-320.
4. Taylor J, Garite TJ. Premature rupture of membranes before fetal viability. Obstet Gynecol. 1984;64:615-620.
5. Schucker JL, Mercer BM. Midtrimester premature rupture of the membranes. Semin Perinatol. 1996;20:389-400.
6. Hillier SL, Martius J, Krohn M, et al. A case-control study of chorioamnionic infection and histologic chorioamnionitis in prematurity. N Engl J Med. 1988;319:972-978.
7. Morales WJ. The effect of chorioamnionitis on the developmental outcome of preterm infants at one year. Obstet Gynecol. 1987;70:183-186.
8. Moretti M, Sibai BM. Maternal and perinatal outcome of expectant management of premature rupture of membranes in the midtrimester. Am J Obstet Gynecol. 1988;159:390-396.
9. Beydoun SN, Yasin SY. Premature rupture of the membranes before 28 weeks: conservative management. Am J Obstet Gynecol. 1986;155:471-479.
10. Major CA, Kitzmiller JL. Perinatal survival with expectant management of midtrimester rupture of membranes. Am J Obstet Gynecol. 1990;163:838-844.
11. Falk SJ, Campbell LJ, Lee-Parritz A, et al. Expectant management in spontaneous preterm premature rupture of membranes between 14 and 24 weeks’ gestation. J Perinatol. 2004;24:611-616.
12. Kilbride HW, Yeast J, Thibeault DW. Defining limits of survival: lethal pulmonary hypoplasia after midtrimester premature rupture of membranes. Am J Obstet Gynecol. 1996;175:675-681.
13. Rotschild A, Ling EW, Puterman ML, Farquharson D. Neonatal outcome after prolonged preterm rupture of the membranes. Am J Obstet Gynecol. 1990;162:46-52.
14. Farooqi A, Holmgren PA, Engberg S, Serenius F. Survival and 2-year outcome with expectant management of second-trimester rupture of membranes. Obstet Gynecol. 1998;92:895-901.
15. Vergani P, Ghidini A, Locatelli A, et al. Risk factors for pulmonary hypoplasia in second-trimester premature rupture of membranes. Am J Obstet Gynecol. 1994;170:1359-1364.
16. Blott M, Greenough A. Neonatal outcome after prolonged rupture of the membranes starting in the second trimester. Arch Dis Child. 1988;63:1146-1150.
17. Stevenson DK, Wright LL, Lemons JA, et al. Very low birth weight outcomes of the National Institute of Child Health and Human Development Neonatal Research Network, January 1993 through December 1994. Am J Obstet Gynecol. 1998;179:1632-1639.
18. Nelson LH, Anderson RL, O’Shea TM, Swain M. Expectant management of preterm premature rupture of membranes. Am J Obstet Gynecol. 1994;171:350-356.
19. Belady PH, Farkouh LJ, Gibbs RS. Intra-amniotic infection and premature rupture of the membranes. Clin Perinatol. 1997;24:43-57.
20. Ananth CV, Savitz DA, Williams MA. Placental abruption and its association with hypertension and prolonged rupture of membranes: a methodologic review and meta-analysis. Obstet Gynecol. 1996;88:309-318.
21. Gonen R, Hannah ME, Milligan JE. Does prolonged preterm premature rupture of the membranes predispose to abruptio placentae? Obstet Gynecol. 1989;74:347-350.
22. Carlan SJ, O’Brien WF, Parsons MT, Lense JJ. Preterm premature rupture of membranes: a randomized study of home versus hospital management. Obstet Gynecol. 1993;81:61-64.
23. Vintzileos AM, Campbell WA, Nochimson DJ, Weinbaum PJ. The use of the nonstress test in patients with premature rupture of the membranes. Am J Obstet Gynecol. 1986;155:149-153.
24. Smith CV, Greenspoon J, Phelan JP, Platt LD. Clinical utility of the nonstress test in the conservative management of women with preterm spontaneous premature rupture of the membranes. J Reprod Med. 1987;32:1-4.
25. Hanley ML, Vintzileos AM. Biophysical testing in premature rupture of the membranes. Semin Perinatol. 1996;20:418-425.
26. Levy DL, Warsof SL. Oral ritodrine and preterm premature rupture of membranes. Obstet Gynecol. 1985;66:621-623.
27. Weiner CP, Renk K, Klugman M. The therapeutic efficacy and cost-effectiveness of aggressive tocolysis for premature labor associated with premature rupture of the membranes. Am J Obstet Gynecol. 1988;159:216-222.
28. Ohlsson A. Treatments of preterm premature rupture of the membranes: a meta-analysis. Am J Obstet Gynecol. 1989;160:890-906.
29. Crowley PA. Antenatal corticosteroid therapy: a meta-analysis of the randomized trials, 1972 to 1994. Am J Obstet Gynecol. 1995;173:322-335.
30. Lewis DF, Brody K, Edwards MS, et al. Preterm premature ruptured membranes: a randomized trial of steroids after treatment with antibiotics. Obstet Gynecol. 1996;88:801-805.
31. National Institutes of Health. Effect of corticosteroids for fetal maturation on perinatal outcomes. NIH Consensus Development Panel on the Effect of Corticosteroids for Fetal Maturation on Perinatal Outcomes. JAMA. 1995;273:413-418.
32. Mercer BM, Miodovnik M, Thurnau GR, et al. Antibiotic therapy for reduction of infant morbidity after preterm premature rupture of the membranes. A randomized controlled trial. National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. JAMA. 1997;278:989-995.
33. Egarter C, Leitich H, Karas H, et al. Antibiotic treatment in preterm premature rupture of membranes and neonatal morbidity: a metaanalysis. Am J Obstet Gynecol. 1996;174:589-597.
34. Ananth CV, Guise JM, Thorp JM, Jr. Utility of antibiotic therapy in preterm premature rupture of membranes: a meta-analysis. Obstet Gynecol Surv. 1996;51:324-328.
35. Kenyon SL, Taylor DJ, Tarnow-Mordi W. Broad-spectrum antibiotics for preterm, prelabour rupture of fetal membranes: the ORACLE I randomised trial. ORACLE Collaborative Group. Lancet. 2001;357:979-988.
36. Kenyon S, Boulvain M, Neilson J. Antibiotics for preterm rupture of membranes. Cochrane Database Syst Rev. 2003;(2):CD001058.-
37. Bottoms SF, Paul RH, Iams JD, et al. Obstetric determinants of neonatal survival: influence of willingness to perform cesarean delivery on survival of extremely low-birth-weight infants. National Institute of Child Health and Human Development Network of Maternal-Fetal Medicine Units. Am J Obstet Gynecol. 1997;176:960-966.
38. Mercer BM, Crocker LG, Boe NM, Sibai BM. Induction versus expectant management in premature rupture of the membranes with mature amniotic fluid at 32 to 36 weeks: a randomized trial. Am J Obstet Gynecol. 1993;169:775-782.
39. Cox SM, Leveno KJ. Intentional delivery versus expectant management with preterm ruptured membranes at 30-34 weeks’ gestation. Obstet Gynecol. 1995;86:875-879.
40. Dudley J, Malcolm G, Ellwood D. Amniocentesis in the management of preterm premature rupture of the membranes. Aust N Z J Obstet Gynaecol. 1991;31:331-336.
41. Broekhuizen FF, Gilman M, Hamilton PR. Amniocentesis for gram stain and culture in preterm premature rupture of the membranes. Obstet Gynecol. 1985;66:316-321.
Dr. Esplin receives support from the National Institute of Child Health and Human Development and is a speaker for Adeza.
CASE: PROM at 22 weeks
J.S. is a 22-year-old woman at 22 weeks’ gestation in her second pregnancy. Her first gestation ended in spontaneous abortion at 10 weeks, followed by dilation and curettage. She has been referred to you by her midwife, who is concerned about J.S.’s complaints of loss of fluid over the past 2 weeks and who cannot document rupture of membranes by the usual means.
In your office, J.S. continues to complain of intermittent leakage of clear fluid. She says there has been no vaginal bleeding, foul-smelling discharge, fever, chills, or abdominal tenderness. You find a normal abdomen. A sterile speculum exam is equivocal, without evidence of pooling or ferning; a nitrazine test is positive, however. A complete blood count reveals no evidence of leukocytosis. Urinalysis is negative.
You suspect preterm premature rupture of membranes (PROM) when bedside ultrasonography (US) documents oligohydramnios with an amniotic fluid index of less than 5 cm. The kidneys, bladder, and stomach all appear normal.
What is the best way to confirm the diagnosis? What is the most appropriate management at this gestational age? And how do you counsel J.S. about the risk to her, and her baby, of continuing the pregnancy?
Given the very poor prognosis of many cases of early PROM, accurate diagnosis is critical to determine the best management strategy. The gold standard of diagnosis is sterile vaginal examination with a speculum to identify clear fluid leaking from the cervix or pooling in the posterior fornix. Use nitrazine paper to assess the fluid collected from the posterior fornix for alkaline pH; this method has a positive predictive value (PPV) of 99% and negative predictive value (NPV) of 96%.1 The appearance of “ferning”—a crystalline pattern that occurs when the saline amniotic fluid dries—carries a PPV of 98% to 99% and a NPV of 90% to 99%.2
One must also consider the patient’s history. When that history and the physical exam fail to render a clear result, use US to assess the amniotic fluid volume. A low volume in the presence of a convincing clinical history is very suspicious for PROM, as in the case just described.
Tinting the amniotic fluid may help
In equivocal cases, mix 1 to 3 mL of indigo carmine with 5 mL of sterile saline and insert it into the amniotic fluid under US guidance. This dye will make any leaking amniotic fluid obvious. Be aware, however, that instillation of the dye is very difficult in cases of severe oligohydramnios or anhydramnios. In this setting, amniocentesis can also cause contractions or vaginal bleeding.
New diagnostic tool on the horizon
Recent studies have focused on a new rapid test (AmniSure) that uses immunochromatography to detect trace amounts of placental α-microglobulin-1 protein.3 This protein is specific to amniotic fluid and present in vaginal secretions only when amniotic fluid is leaking through the cervix. One study of 203 patients suspected of having ruptured membranes found the AmniSure test to have a PPV of 100% and NPV of 99.1%.3 Although these findings are promising, further confirmatory studies are needed before this product can be recommended for widespread use.
CASE continued: Leakage of tinted fluid confirms PROM
Because the diagnostic steps taken so far have been inconclusive, J.S. undergoes amniocentesis with infusion of indigo carmine. Within 2 hours, blue dye is observed leaking from the cervix, confirming PROM. A sample of amniotic fluid obtained at the time of amniocentesis produces a negative gram stain and reveals a normal glucose level and leukocyte count. Amniotic fluid cultures are pending.
What is your next step?
Determining the best management strategy is next. The treatment plan should be based on gestational age, presence or absence of infection or labor, and fetal status. Therefore, the initial evaluation of a patient with PROM should focus on the collection of this clinical information.
I recommend these measures:
- Document the exact gestational age by careful review of available records and ultrasound biometry
- Identify indicators of infection, such as maternal fever and tachycardia, fundal tenderness, fetal tachycardia, and an elevated white blood cell count
- Amniocentesis may be required to rule out amnionitis in cases where the diagnosis is clinically unclear
- Document fetal presentation
- Initiate fetal heart rate (FHR) monitoring at the time of diagnosis and perform a biophysical profile (BPP).
Midtrimester PROM: 16 to 24 weeks’ gestation
Management differs for each gestational age.
Midtrimester PROM occurs in approximately 0.7% of all pregnancies and is a significant source of morbidity and mortality.4,5 It may be iatrogenic in nature when it follows an invasive procedure such as amniocentesis or fetoscopy. It also may occur spontaneously, with causes similar to those of PROM at later gestational ages. At this early gestational age, PROM is more likely to be associated with cervical incompetence and inflammation.6,7
Infection is a risk—and may be the underlying cause
Infection is associated with as many as 30% to 50% of cases of PROM.4,8-10 Half of the cases of intra-amniotic infection develop within 7 days after PROM. That’s because many cases of early PROM have infection or inflammation as their cause.
Intrauterine demise is common at early gestational ages
The risk of intrauterine fetal demise is inversely related to gestational age at the time of rupture. That is, the earlier the gestational age, the higher the rate of fetal death. One study found that the rate of intrauterine fetal demise was 33% when PROM occurred before 20 weeks’ gestation and 20% when it occurred between 20 and 24 weeks; it was rare after 25 weeks.10
Pulmonary hypoplasia is more common at this critical juncture
The midtrimester is a critical time for fetal lung development. During the canalicular stage (between 17 and 24 weeks’ gestation), the gas-exchanging acini and pulmonary capillaries are forming, so they are more susceptible to injury. The incidence of pulmonary hypoplasia is approximately 10% when PROM occurs earlier than 20 weeks’ gestation, although a wide range of rates has been reported.4,8-10 Pulmonary hypoplasia remains a significant cause of neonatal mortality and is found in as many as 77% of autopsies of infants from pregnancies complicated by midtrimester PROM.11
The incidence of pulmonary hypoplasia decreases by as much as 46% with each week of gestational age at the time of PROM.12 After 26 weeks, when the terminal sac stage of development occurs, the rate of pulmonary hypoplasia complicating PROM drops to less than 2%.12-14
The degree of oligohydramnios also affects the rate of pulmonary hypoplasia, which increases significantly when the amniotic fluid index is less than 5 cm.15
Limb deformity may be related to restricted movement
Although limb development occurs in the embryonic period, most limb growth takes place during the second and third trimesters.16 The restriction in movement and increased pressure associated with prolonged periods of oligohydramnios can lead to skeletal deformity in otherwise normal extremities.
The frequency of deformity varies widely among studies, but the mean incidence is 7%.4,8-11 A twofold higher incidence of skeletal abnormality occurs when midtrimester PROM is accompanied by severe oligohydramnios. In one study, the rate of skeletal abnormality was 54% when the deepest pocket of amniotic fluid was less than 1 cm, compared with 26% for matched pregnancies with a normal or mildly reduced volume.16
Maternal complications include retained placenta, endometritis
Maternal complications associated with very early PROM include a higher rate of cesarean section due to fetal malpresentation and FHR abnormalities, which often accompany oligohydramnios and intraamniotic infection.10 A classical incision is more likely in these cases due to the poorly developed lower uterine segment. Retained placenta necessitating postpartum curettage occurs in 9% to 18% of cases of PROM at less than 20 weeks’ gestation. In addition, postpartum endometritis complicates as many as 40% of cases of midtrimester PROM.4,8-11
General prognosis
The outcome of midtrimester PROM depends on the underlying cause. If it is iatrogenic, the outcome is usually favorable, with frequent resealing of the membranes; most cases end in a normal term delivery. The outcome of spontaneous PROM is more grim.
Midtrimester PROM has the same relatively short latency (approximately 17 days on average) as PROM that occurs later in pregnancy. Less than 50% of women with midtrimester PROM remain pregnant at the end of the first week, and as many as 75% of these women will have delivered by 28 days after PROM.11 These percentages indicate that most women with midtrimester PROM deliver before fetal viability can be attained, or in the risky periviable period.
Overall, midtrimester PROM is associated with significant fetal, neonatal, and maternal morbidity. The risks must be explained to the patient along with any management plan.
Given the very poor prognosis and small chance of prolonged latency, induction of labor and pregnancy termination are reasonable options at the time of presentation. The patient needs to know that expectant management can be associated with significant long-term morbidity and a higher rate of neonatal mortality.
CASE continued: Patient is apprised of the risks
After a frank discussion of the risks involved in continuing her pregnancy, J.S. chooses expectant management. Given the early gestational age and absence of any sign of infection, she is sent home for bed rest and instructed to check her temperature twice daily. She is told to return for evaluation if fever (>100°F) or symptoms of infection develop. Because of the very early gestational age, no steroid or antibiotic will be given until 24 weeks’ gestation, when she will be admitted for inpatient care.
PROM at 24 to 32 weeks’ gestation
Selecting a management strategy for a pregnancy at this gestational age means weighing the potential morbidity and mortality of immediate delivery against the morbidity and mortality of expectant management. At this gestational age, the principal source of fetal morbidity is prematurity itself. As many as 40% of infants delivered before 26 weeks’ gestation experience some type of long-term morbidity such as intraventricular hemorrhage (IVH), retinopathy of prematurity, necrotizing enterocolitis (NEC), or, most commonly, respiratory distress syndrome (RDS).16,17 It is true that fetal morbidity increases when chorioamnionitis is present, but the potential benefit of prolonging the pregnancy by 7 to 14 days is believed to outweigh the risk of infection at these gestational ages. Therefore, in the absence of contraindications, expectant management is the usual course of action.
Select patients carefully for expectant management
Consider expectant management only when fetal well-being can be documented, without evidence of infection. Abruption is a contraindication to expectant management, although the clinical nature of this diagnosis can make it difficult to identify. If abruption is diagnosed, aggressive management with labor augmentation or cesarean section and intravenous (IV) antibiotics is appropriate.
Repetitive fetal heart decelerations in the presence of active vaginal bleeding and uterine tenderness indicate placental insufficiency and are an indication for delivery.
More than 50% of patients deliver within the first week after PROM is diagnosed.18 At least 30% experience chorioamnionitis some time after the diagnosis of PROM, and 1% to 2% suffer cord prolapse.10,18,19 As many as 4% to 12% of cases of PROM will also be complicated by abruption,20,21 and a rate of intrauterine fetal demise as high as 1% has been documented.18 Therefore, if expectant management is selected, it should include close monitoring for these complications.
Hospitalization is warranted. Women who are stable and being managed expectantly should probably be hospitalized. One prospective trial comparing outcomes between women managed at home and women who were hospitalized found no significant difference in latency period or the rate of infection.22 However, the strict inclusion criteria for this study make it difficult to generalize the results. Only 18% of the 349 women screened for enrollment met these criteria.
The high rate of precipitous labor, frequent onset of infection, and need for frequent maternal and neonatal evaluation at this gestational age make hospitalization a prudent choice.
Fetal surveillance is mandatory
Most investigators would agree that a regular schedule of fetal surveillance is necessary during expectant management. But there is no clear evidence indicating which type, and what timing, of surveillance are best. It is clear that changes in the FHR pattern and BPP precede the onset of chorioamnionitis and intrauterine demise due to cord accidents.23-25 However, no studies have demonstrated a significant improvement in neonatal outcomes with daily or even twice-daily antenatal surveillance.
At our institution, we follow a regimen of daily surveillance, which consists of a nonstress test and/or BPP to confirm fetal well-being.
Tocolysis won’t prolong gestation beyond 48 hours…
There is no evidence that prolonged tocolysis with any therapy significantly increases long-term latency or improves any type of neonatal morbidity in pregnancies complicated by PROM. Tocolysis may prolong pregnancy over the short term (<48 hours),26,27 but its widespread use is not supported by the evidence.
Tocolysis is appropriate to achieve safe maternal transport or administer steroids.
…but corticosteroids are highly beneficial
Antenatal corticosteroids clearly improve neonatal outcomes when PROM occurs before 32 weeks’ gestation. Two large meta-analyses have found such benefits to be a decrease in the rates of RDS, IVH, NEC, and neonatal death.28,29 A recent prospective study confirmed these findings.30 The rate of RDS declined 26%—from 44% to 18%.
A consensus panel of the National Institutes of Health (NIH) recommended use of corticosteroids in cases of PROM between 24 and 32 weeks’ gestation in which there is no clinical evidence of infection.31 Any of the standard steroid regimens is appropriate. At our institution, we give an intramuscular injection of 12 mg of betamethasone and repeat this one time in 24 hours.
Are prophylactic antibiotics warranted?
The fact that infection is the most commonly identified cause of PROM prompts the question: Does treatment with IV antibiotics improve outcomes and prolong latency even in the absence of clinically apparent infection? Mercer and colleagues32 reported a significant reduction in chorioamnionitis, endometritis, and neonatal infection, including sepsis and pneumonia, in pregnancies treated with prophylactic antibiotics, compared with expectant management alone. In that study, latency also increased significantly following antibiotic therapy. Other meta-analyses confirm the benefits of prophylactic antibiotics, demonstrating a lower rate of neonatal sepsis and IVH following treatment.33,34
One large multicenter randomized trial found a reduced rate of IVH and RDS after treatment with IV erythromycin and ampicillin for 48 hours, followed by a 5-day course of amoxicillin and erythromycin.35 A Cochrane review of the use of prophylactic antibiotics in the setting of PROM included 19 studies with various antibiotic regimens.36 It concluded that antibiotic therapy prolongs latency (at both 48 hours and 7 days), decreases maternal infection, and reduces the incidence of neonatal complications, including infection, need for oxygen, IVH, and periventricular leukomalacia.
No superior regimen, but avoid amoxicillin-clavulanate. Although no single antibiotic regimen is clearly superior to the others, erythromycin has been associated with benefits most consistently. The most common dosage for erythromycin is 250 mg every 6 hours for a total of 48 hours and then an additional 5 days of oral treatment. Amoxicillin-clavulanate has been associated with an increased risk of NEC in at least two trials, and should probably be avoided. A Cochrane review confirms these conclusions.36
Choice of delivery route is flexible
Once the need for delivery arises, choose the route according to normal obstetric indications. In the setting of PROM with malpresentation, cesarean delivery is probably the best approach. However, in very-low-birth-weight infants, the best mode of delivery remains unclear.37 If the fetus is in cephalic presentation, an attempt at vaginal delivery does not appear to have a worse neonatal outcome.
If spontaneous labor does not occur or if induction is not indicated for maternal or fetal reasons, one may choose to deliver the patient at 32 weeks’ gestation or continue expectant management until 34 weeks’ gestation. This decision is discussed in more detail in the next section.
ALGORITHM
Management of PROM varies with gestational age
PROM at 32 to 34 weeks’ gestation
Although it is generally accepted that the fetus benefits from expectant management in pregnancies complicated by PROM before 32 weeks’ gestation, the management of PROM that arises between 32 and 34 weeks remains controversial and a focus of ongoing research. Because most neonatal morbidity is caused by prematurity, and the rate of prematurity-related complications decreases with increasing gestational age, some argue that the potential benefit of prolonging latency after 32 weeks’ gestation does not outweigh the risk of chorioamnionitis.
Continue the gestation? Or deliver?
Mercer and colleagues randomized 97 women with PROM between 32 and 36 weeks’ gestation and a mature lung profile to expectant management or immediate induction.38 Although expectant management did prolong pregnancy, no neonatal benefit was observed, and the rate of chorioamnionitis was higher with expectant management, with a longer hospital stay.
Cox and associates found a higher rate of chorioamnionitis among 68 women with PROM between 30 and 34 weeks’ gestation who were managed expectantly, compared with 61 women assigned to immediate induction.39 Neonatal morbidity was similar in both groups.
These studies suggest that expectant management after 32 weeks leads only to an increased rate of chorioamnionitis and longer maternal and neonatal hospitalization, without any demonstrable neonatal benefit. However, one significant limitation of these studies is the fact that patients managed expectantly received neither corticosteroids nor prophylactic antibiotics.
Are corticosteroids appropriate at this gestational age?
We lack sufficient evidence to support the routine use of corticosteroids after 32 weeks in pregnancies complicated by PROM. The NIH consensus panel suggested that they may be an option in patients without contraindications up to 34 weeks’ gestation.31
Some experts recommend testing for fetal lung maturity when PROM occurs between 32 and 34 weeks’ gestation. In this group, the rate of fetal lung maturity is between 50% and 60%.40,41 There is no clear benefit in prolonging a pregnancy when fetal lung maturity can be documented. However, in the setting of immature fetal lungs, expectant management may be appropriate following treatment with corticosteroids and a prophylactic antibiotic regimen. Patients who present at 34 weeks’ gestation or beyond are likely to benefit most from immediate delivery.
When expectant management is chosen between 32 and 34 weeks, inpatient hospitalization with daily monitoring is also recommended. The mode of delivery depends on the usual obstetric indications.
CASE resolved: Patient develops fever and spontaneous labor
Ten days after the documentation of PROM, J.S. reports a fever and abdominal tenderness, as well as frequent uterine contractions that began early in the day. She is admitted to the hospital. A physical examination confirms clinically apparent intra-amniotic infection and labor. The patient is started on IV antibiotics and, after a short labor, delivers a nonviable male infant weighing 500 g. Pathologic examination of the fetus and placenta reveals a normal, immature fetus with evidence of acute chorioamnionitis on placental sections.
CASE: PROM at 22 weeks
J.S. is a 22-year-old woman at 22 weeks’ gestation in her second pregnancy. Her first gestation ended in spontaneous abortion at 10 weeks, followed by dilation and curettage. She has been referred to you by her midwife, who is concerned about J.S.’s complaints of loss of fluid over the past 2 weeks and who cannot document rupture of membranes by the usual means.
In your office, J.S. continues to complain of intermittent leakage of clear fluid. She says there has been no vaginal bleeding, foul-smelling discharge, fever, chills, or abdominal tenderness. You find a normal abdomen. A sterile speculum exam is equivocal, without evidence of pooling or ferning; a nitrazine test is positive, however. A complete blood count reveals no evidence of leukocytosis. Urinalysis is negative.
You suspect preterm premature rupture of membranes (PROM) when bedside ultrasonography (US) documents oligohydramnios with an amniotic fluid index of less than 5 cm. The kidneys, bladder, and stomach all appear normal.
What is the best way to confirm the diagnosis? What is the most appropriate management at this gestational age? And how do you counsel J.S. about the risk to her, and her baby, of continuing the pregnancy?
Given the very poor prognosis of many cases of early PROM, accurate diagnosis is critical to determine the best management strategy. The gold standard of diagnosis is sterile vaginal examination with a speculum to identify clear fluid leaking from the cervix or pooling in the posterior fornix. Use nitrazine paper to assess the fluid collected from the posterior fornix for alkaline pH; this method has a positive predictive value (PPV) of 99% and negative predictive value (NPV) of 96%.1 The appearance of “ferning”—a crystalline pattern that occurs when the saline amniotic fluid dries—carries a PPV of 98% to 99% and a NPV of 90% to 99%.2
One must also consider the patient’s history. When that history and the physical exam fail to render a clear result, use US to assess the amniotic fluid volume. A low volume in the presence of a convincing clinical history is very suspicious for PROM, as in the case just described.
Tinting the amniotic fluid may help
In equivocal cases, mix 1 to 3 mL of indigo carmine with 5 mL of sterile saline and insert it into the amniotic fluid under US guidance. This dye will make any leaking amniotic fluid obvious. Be aware, however, that instillation of the dye is very difficult in cases of severe oligohydramnios or anhydramnios. In this setting, amniocentesis can also cause contractions or vaginal bleeding.
New diagnostic tool on the horizon
Recent studies have focused on a new rapid test (AmniSure) that uses immunochromatography to detect trace amounts of placental α-microglobulin-1 protein.3 This protein is specific to amniotic fluid and present in vaginal secretions only when amniotic fluid is leaking through the cervix. One study of 203 patients suspected of having ruptured membranes found the AmniSure test to have a PPV of 100% and NPV of 99.1%.3 Although these findings are promising, further confirmatory studies are needed before this product can be recommended for widespread use.
CASE continued: Leakage of tinted fluid confirms PROM
Because the diagnostic steps taken so far have been inconclusive, J.S. undergoes amniocentesis with infusion of indigo carmine. Within 2 hours, blue dye is observed leaking from the cervix, confirming PROM. A sample of amniotic fluid obtained at the time of amniocentesis produces a negative gram stain and reveals a normal glucose level and leukocyte count. Amniotic fluid cultures are pending.
What is your next step?
Determining the best management strategy is next. The treatment plan should be based on gestational age, presence or absence of infection or labor, and fetal status. Therefore, the initial evaluation of a patient with PROM should focus on the collection of this clinical information.
I recommend these measures:
- Document the exact gestational age by careful review of available records and ultrasound biometry
- Identify indicators of infection, such as maternal fever and tachycardia, fundal tenderness, fetal tachycardia, and an elevated white blood cell count
- Amniocentesis may be required to rule out amnionitis in cases where the diagnosis is clinically unclear
- Document fetal presentation
- Initiate fetal heart rate (FHR) monitoring at the time of diagnosis and perform a biophysical profile (BPP).
Midtrimester PROM: 16 to 24 weeks’ gestation
Management differs for each gestational age.
Midtrimester PROM occurs in approximately 0.7% of all pregnancies and is a significant source of morbidity and mortality.4,5 It may be iatrogenic in nature when it follows an invasive procedure such as amniocentesis or fetoscopy. It also may occur spontaneously, with causes similar to those of PROM at later gestational ages. At this early gestational age, PROM is more likely to be associated with cervical incompetence and inflammation.6,7
Infection is a risk—and may be the underlying cause
Infection is associated with as many as 30% to 50% of cases of PROM.4,8-10 Half of the cases of intra-amniotic infection develop within 7 days after PROM. That’s because many cases of early PROM have infection or inflammation as their cause.
Intrauterine demise is common at early gestational ages
The risk of intrauterine fetal demise is inversely related to gestational age at the time of rupture. That is, the earlier the gestational age, the higher the rate of fetal death. One study found that the rate of intrauterine fetal demise was 33% when PROM occurred before 20 weeks’ gestation and 20% when it occurred between 20 and 24 weeks; it was rare after 25 weeks.10
Pulmonary hypoplasia is more common at this critical juncture
The midtrimester is a critical time for fetal lung development. During the canalicular stage (between 17 and 24 weeks’ gestation), the gas-exchanging acini and pulmonary capillaries are forming, so they are more susceptible to injury. The incidence of pulmonary hypoplasia is approximately 10% when PROM occurs earlier than 20 weeks’ gestation, although a wide range of rates has been reported.4,8-10 Pulmonary hypoplasia remains a significant cause of neonatal mortality and is found in as many as 77% of autopsies of infants from pregnancies complicated by midtrimester PROM.11
The incidence of pulmonary hypoplasia decreases by as much as 46% with each week of gestational age at the time of PROM.12 After 26 weeks, when the terminal sac stage of development occurs, the rate of pulmonary hypoplasia complicating PROM drops to less than 2%.12-14
The degree of oligohydramnios also affects the rate of pulmonary hypoplasia, which increases significantly when the amniotic fluid index is less than 5 cm.15
Limb deformity may be related to restricted movement
Although limb development occurs in the embryonic period, most limb growth takes place during the second and third trimesters.16 The restriction in movement and increased pressure associated with prolonged periods of oligohydramnios can lead to skeletal deformity in otherwise normal extremities.
The frequency of deformity varies widely among studies, but the mean incidence is 7%.4,8-11 A twofold higher incidence of skeletal abnormality occurs when midtrimester PROM is accompanied by severe oligohydramnios. In one study, the rate of skeletal abnormality was 54% when the deepest pocket of amniotic fluid was less than 1 cm, compared with 26% for matched pregnancies with a normal or mildly reduced volume.16
Maternal complications include retained placenta, endometritis
Maternal complications associated with very early PROM include a higher rate of cesarean section due to fetal malpresentation and FHR abnormalities, which often accompany oligohydramnios and intraamniotic infection.10 A classical incision is more likely in these cases due to the poorly developed lower uterine segment. Retained placenta necessitating postpartum curettage occurs in 9% to 18% of cases of PROM at less than 20 weeks’ gestation. In addition, postpartum endometritis complicates as many as 40% of cases of midtrimester PROM.4,8-11
General prognosis
The outcome of midtrimester PROM depends on the underlying cause. If it is iatrogenic, the outcome is usually favorable, with frequent resealing of the membranes; most cases end in a normal term delivery. The outcome of spontaneous PROM is more grim.
Midtrimester PROM has the same relatively short latency (approximately 17 days on average) as PROM that occurs later in pregnancy. Less than 50% of women with midtrimester PROM remain pregnant at the end of the first week, and as many as 75% of these women will have delivered by 28 days after PROM.11 These percentages indicate that most women with midtrimester PROM deliver before fetal viability can be attained, or in the risky periviable period.
Overall, midtrimester PROM is associated with significant fetal, neonatal, and maternal morbidity. The risks must be explained to the patient along with any management plan.
Given the very poor prognosis and small chance of prolonged latency, induction of labor and pregnancy termination are reasonable options at the time of presentation. The patient needs to know that expectant management can be associated with significant long-term morbidity and a higher rate of neonatal mortality.
CASE continued: Patient is apprised of the risks
After a frank discussion of the risks involved in continuing her pregnancy, J.S. chooses expectant management. Given the early gestational age and absence of any sign of infection, she is sent home for bed rest and instructed to check her temperature twice daily. She is told to return for evaluation if fever (>100°F) or symptoms of infection develop. Because of the very early gestational age, no steroid or antibiotic will be given until 24 weeks’ gestation, when she will be admitted for inpatient care.
PROM at 24 to 32 weeks’ gestation
Selecting a management strategy for a pregnancy at this gestational age means weighing the potential morbidity and mortality of immediate delivery against the morbidity and mortality of expectant management. At this gestational age, the principal source of fetal morbidity is prematurity itself. As many as 40% of infants delivered before 26 weeks’ gestation experience some type of long-term morbidity such as intraventricular hemorrhage (IVH), retinopathy of prematurity, necrotizing enterocolitis (NEC), or, most commonly, respiratory distress syndrome (RDS).16,17 It is true that fetal morbidity increases when chorioamnionitis is present, but the potential benefit of prolonging the pregnancy by 7 to 14 days is believed to outweigh the risk of infection at these gestational ages. Therefore, in the absence of contraindications, expectant management is the usual course of action.
Select patients carefully for expectant management
Consider expectant management only when fetal well-being can be documented, without evidence of infection. Abruption is a contraindication to expectant management, although the clinical nature of this diagnosis can make it difficult to identify. If abruption is diagnosed, aggressive management with labor augmentation or cesarean section and intravenous (IV) antibiotics is appropriate.
Repetitive fetal heart decelerations in the presence of active vaginal bleeding and uterine tenderness indicate placental insufficiency and are an indication for delivery.
More than 50% of patients deliver within the first week after PROM is diagnosed.18 At least 30% experience chorioamnionitis some time after the diagnosis of PROM, and 1% to 2% suffer cord prolapse.10,18,19 As many as 4% to 12% of cases of PROM will also be complicated by abruption,20,21 and a rate of intrauterine fetal demise as high as 1% has been documented.18 Therefore, if expectant management is selected, it should include close monitoring for these complications.
Hospitalization is warranted. Women who are stable and being managed expectantly should probably be hospitalized. One prospective trial comparing outcomes between women managed at home and women who were hospitalized found no significant difference in latency period or the rate of infection.22 However, the strict inclusion criteria for this study make it difficult to generalize the results. Only 18% of the 349 women screened for enrollment met these criteria.
The high rate of precipitous labor, frequent onset of infection, and need for frequent maternal and neonatal evaluation at this gestational age make hospitalization a prudent choice.
Fetal surveillance is mandatory
Most investigators would agree that a regular schedule of fetal surveillance is necessary during expectant management. But there is no clear evidence indicating which type, and what timing, of surveillance are best. It is clear that changes in the FHR pattern and BPP precede the onset of chorioamnionitis and intrauterine demise due to cord accidents.23-25 However, no studies have demonstrated a significant improvement in neonatal outcomes with daily or even twice-daily antenatal surveillance.
At our institution, we follow a regimen of daily surveillance, which consists of a nonstress test and/or BPP to confirm fetal well-being.
Tocolysis won’t prolong gestation beyond 48 hours…
There is no evidence that prolonged tocolysis with any therapy significantly increases long-term latency or improves any type of neonatal morbidity in pregnancies complicated by PROM. Tocolysis may prolong pregnancy over the short term (<48 hours),26,27 but its widespread use is not supported by the evidence.
Tocolysis is appropriate to achieve safe maternal transport or administer steroids.
…but corticosteroids are highly beneficial
Antenatal corticosteroids clearly improve neonatal outcomes when PROM occurs before 32 weeks’ gestation. Two large meta-analyses have found such benefits to be a decrease in the rates of RDS, IVH, NEC, and neonatal death.28,29 A recent prospective study confirmed these findings.30 The rate of RDS declined 26%—from 44% to 18%.
A consensus panel of the National Institutes of Health (NIH) recommended use of corticosteroids in cases of PROM between 24 and 32 weeks’ gestation in which there is no clinical evidence of infection.31 Any of the standard steroid regimens is appropriate. At our institution, we give an intramuscular injection of 12 mg of betamethasone and repeat this one time in 24 hours.
Are prophylactic antibiotics warranted?
The fact that infection is the most commonly identified cause of PROM prompts the question: Does treatment with IV antibiotics improve outcomes and prolong latency even in the absence of clinically apparent infection? Mercer and colleagues32 reported a significant reduction in chorioamnionitis, endometritis, and neonatal infection, including sepsis and pneumonia, in pregnancies treated with prophylactic antibiotics, compared with expectant management alone. In that study, latency also increased significantly following antibiotic therapy. Other meta-analyses confirm the benefits of prophylactic antibiotics, demonstrating a lower rate of neonatal sepsis and IVH following treatment.33,34
One large multicenter randomized trial found a reduced rate of IVH and RDS after treatment with IV erythromycin and ampicillin for 48 hours, followed by a 5-day course of amoxicillin and erythromycin.35 A Cochrane review of the use of prophylactic antibiotics in the setting of PROM included 19 studies with various antibiotic regimens.36 It concluded that antibiotic therapy prolongs latency (at both 48 hours and 7 days), decreases maternal infection, and reduces the incidence of neonatal complications, including infection, need for oxygen, IVH, and periventricular leukomalacia.
No superior regimen, but avoid amoxicillin-clavulanate. Although no single antibiotic regimen is clearly superior to the others, erythromycin has been associated with benefits most consistently. The most common dosage for erythromycin is 250 mg every 6 hours for a total of 48 hours and then an additional 5 days of oral treatment. Amoxicillin-clavulanate has been associated with an increased risk of NEC in at least two trials, and should probably be avoided. A Cochrane review confirms these conclusions.36
Choice of delivery route is flexible
Once the need for delivery arises, choose the route according to normal obstetric indications. In the setting of PROM with malpresentation, cesarean delivery is probably the best approach. However, in very-low-birth-weight infants, the best mode of delivery remains unclear.37 If the fetus is in cephalic presentation, an attempt at vaginal delivery does not appear to have a worse neonatal outcome.
If spontaneous labor does not occur or if induction is not indicated for maternal or fetal reasons, one may choose to deliver the patient at 32 weeks’ gestation or continue expectant management until 34 weeks’ gestation. This decision is discussed in more detail in the next section.
ALGORITHM
Management of PROM varies with gestational age
PROM at 32 to 34 weeks’ gestation
Although it is generally accepted that the fetus benefits from expectant management in pregnancies complicated by PROM before 32 weeks’ gestation, the management of PROM that arises between 32 and 34 weeks remains controversial and a focus of ongoing research. Because most neonatal morbidity is caused by prematurity, and the rate of prematurity-related complications decreases with increasing gestational age, some argue that the potential benefit of prolonging latency after 32 weeks’ gestation does not outweigh the risk of chorioamnionitis.
Continue the gestation? Or deliver?
Mercer and colleagues randomized 97 women with PROM between 32 and 36 weeks’ gestation and a mature lung profile to expectant management or immediate induction.38 Although expectant management did prolong pregnancy, no neonatal benefit was observed, and the rate of chorioamnionitis was higher with expectant management, with a longer hospital stay.
Cox and associates found a higher rate of chorioamnionitis among 68 women with PROM between 30 and 34 weeks’ gestation who were managed expectantly, compared with 61 women assigned to immediate induction.39 Neonatal morbidity was similar in both groups.
These studies suggest that expectant management after 32 weeks leads only to an increased rate of chorioamnionitis and longer maternal and neonatal hospitalization, without any demonstrable neonatal benefit. However, one significant limitation of these studies is the fact that patients managed expectantly received neither corticosteroids nor prophylactic antibiotics.
Are corticosteroids appropriate at this gestational age?
We lack sufficient evidence to support the routine use of corticosteroids after 32 weeks in pregnancies complicated by PROM. The NIH consensus panel suggested that they may be an option in patients without contraindications up to 34 weeks’ gestation.31
Some experts recommend testing for fetal lung maturity when PROM occurs between 32 and 34 weeks’ gestation. In this group, the rate of fetal lung maturity is between 50% and 60%.40,41 There is no clear benefit in prolonging a pregnancy when fetal lung maturity can be documented. However, in the setting of immature fetal lungs, expectant management may be appropriate following treatment with corticosteroids and a prophylactic antibiotic regimen. Patients who present at 34 weeks’ gestation or beyond are likely to benefit most from immediate delivery.
When expectant management is chosen between 32 and 34 weeks, inpatient hospitalization with daily monitoring is also recommended. The mode of delivery depends on the usual obstetric indications.
CASE resolved: Patient develops fever and spontaneous labor
Ten days after the documentation of PROM, J.S. reports a fever and abdominal tenderness, as well as frequent uterine contractions that began early in the day. She is admitted to the hospital. A physical examination confirms clinically apparent intra-amniotic infection and labor. The patient is started on IV antibiotics and, after a short labor, delivers a nonviable male infant weighing 500 g. Pathologic examination of the fetus and placenta reveals a normal, immature fetus with evidence of acute chorioamnionitis on placental sections.
1. Abe T. The detection of rupture of fetal membranes with the nitrazine indicator. Am J Obstet Gynecol. 1940;39:400.-
2. Davidson KM. Detection of premature rupture of the membranes. Clin Obstet Gynecol. 1991;34:715-722.
3. Cousins LM, Smok D, Lovett SM, Poeltler DM. AmniSure placental alpha microglobulin-1 rapid immunoassay versus standard diagnostic methods for detection of ruptured membranes. Am J Perinatol. 2005;22(6):317-320.
4. Taylor J, Garite TJ. Premature rupture of membranes before fetal viability. Obstet Gynecol. 1984;64:615-620.
5. Schucker JL, Mercer BM. Midtrimester premature rupture of the membranes. Semin Perinatol. 1996;20:389-400.
6. Hillier SL, Martius J, Krohn M, et al. A case-control study of chorioamnionic infection and histologic chorioamnionitis in prematurity. N Engl J Med. 1988;319:972-978.
7. Morales WJ. The effect of chorioamnionitis on the developmental outcome of preterm infants at one year. Obstet Gynecol. 1987;70:183-186.
8. Moretti M, Sibai BM. Maternal and perinatal outcome of expectant management of premature rupture of membranes in the midtrimester. Am J Obstet Gynecol. 1988;159:390-396.
9. Beydoun SN, Yasin SY. Premature rupture of the membranes before 28 weeks: conservative management. Am J Obstet Gynecol. 1986;155:471-479.
10. Major CA, Kitzmiller JL. Perinatal survival with expectant management of midtrimester rupture of membranes. Am J Obstet Gynecol. 1990;163:838-844.
11. Falk SJ, Campbell LJ, Lee-Parritz A, et al. Expectant management in spontaneous preterm premature rupture of membranes between 14 and 24 weeks’ gestation. J Perinatol. 2004;24:611-616.
12. Kilbride HW, Yeast J, Thibeault DW. Defining limits of survival: lethal pulmonary hypoplasia after midtrimester premature rupture of membranes. Am J Obstet Gynecol. 1996;175:675-681.
13. Rotschild A, Ling EW, Puterman ML, Farquharson D. Neonatal outcome after prolonged preterm rupture of the membranes. Am J Obstet Gynecol. 1990;162:46-52.
14. Farooqi A, Holmgren PA, Engberg S, Serenius F. Survival and 2-year outcome with expectant management of second-trimester rupture of membranes. Obstet Gynecol. 1998;92:895-901.
15. Vergani P, Ghidini A, Locatelli A, et al. Risk factors for pulmonary hypoplasia in second-trimester premature rupture of membranes. Am J Obstet Gynecol. 1994;170:1359-1364.
16. Blott M, Greenough A. Neonatal outcome after prolonged rupture of the membranes starting in the second trimester. Arch Dis Child. 1988;63:1146-1150.
17. Stevenson DK, Wright LL, Lemons JA, et al. Very low birth weight outcomes of the National Institute of Child Health and Human Development Neonatal Research Network, January 1993 through December 1994. Am J Obstet Gynecol. 1998;179:1632-1639.
18. Nelson LH, Anderson RL, O’Shea TM, Swain M. Expectant management of preterm premature rupture of membranes. Am J Obstet Gynecol. 1994;171:350-356.
19. Belady PH, Farkouh LJ, Gibbs RS. Intra-amniotic infection and premature rupture of the membranes. Clin Perinatol. 1997;24:43-57.
20. Ananth CV, Savitz DA, Williams MA. Placental abruption and its association with hypertension and prolonged rupture of membranes: a methodologic review and meta-analysis. Obstet Gynecol. 1996;88:309-318.
21. Gonen R, Hannah ME, Milligan JE. Does prolonged preterm premature rupture of the membranes predispose to abruptio placentae? Obstet Gynecol. 1989;74:347-350.
22. Carlan SJ, O’Brien WF, Parsons MT, Lense JJ. Preterm premature rupture of membranes: a randomized study of home versus hospital management. Obstet Gynecol. 1993;81:61-64.
23. Vintzileos AM, Campbell WA, Nochimson DJ, Weinbaum PJ. The use of the nonstress test in patients with premature rupture of the membranes. Am J Obstet Gynecol. 1986;155:149-153.
24. Smith CV, Greenspoon J, Phelan JP, Platt LD. Clinical utility of the nonstress test in the conservative management of women with preterm spontaneous premature rupture of the membranes. J Reprod Med. 1987;32:1-4.
25. Hanley ML, Vintzileos AM. Biophysical testing in premature rupture of the membranes. Semin Perinatol. 1996;20:418-425.
26. Levy DL, Warsof SL. Oral ritodrine and preterm premature rupture of membranes. Obstet Gynecol. 1985;66:621-623.
27. Weiner CP, Renk K, Klugman M. The therapeutic efficacy and cost-effectiveness of aggressive tocolysis for premature labor associated with premature rupture of the membranes. Am J Obstet Gynecol. 1988;159:216-222.
28. Ohlsson A. Treatments of preterm premature rupture of the membranes: a meta-analysis. Am J Obstet Gynecol. 1989;160:890-906.
29. Crowley PA. Antenatal corticosteroid therapy: a meta-analysis of the randomized trials, 1972 to 1994. Am J Obstet Gynecol. 1995;173:322-335.
30. Lewis DF, Brody K, Edwards MS, et al. Preterm premature ruptured membranes: a randomized trial of steroids after treatment with antibiotics. Obstet Gynecol. 1996;88:801-805.
31. National Institutes of Health. Effect of corticosteroids for fetal maturation on perinatal outcomes. NIH Consensus Development Panel on the Effect of Corticosteroids for Fetal Maturation on Perinatal Outcomes. JAMA. 1995;273:413-418.
32. Mercer BM, Miodovnik M, Thurnau GR, et al. Antibiotic therapy for reduction of infant morbidity after preterm premature rupture of the membranes. A randomized controlled trial. National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. JAMA. 1997;278:989-995.
33. Egarter C, Leitich H, Karas H, et al. Antibiotic treatment in preterm premature rupture of membranes and neonatal morbidity: a metaanalysis. Am J Obstet Gynecol. 1996;174:589-597.
34. Ananth CV, Guise JM, Thorp JM, Jr. Utility of antibiotic therapy in preterm premature rupture of membranes: a meta-analysis. Obstet Gynecol Surv. 1996;51:324-328.
35. Kenyon SL, Taylor DJ, Tarnow-Mordi W. Broad-spectrum antibiotics for preterm, prelabour rupture of fetal membranes: the ORACLE I randomised trial. ORACLE Collaborative Group. Lancet. 2001;357:979-988.
36. Kenyon S, Boulvain M, Neilson J. Antibiotics for preterm rupture of membranes. Cochrane Database Syst Rev. 2003;(2):CD001058.-
37. Bottoms SF, Paul RH, Iams JD, et al. Obstetric determinants of neonatal survival: influence of willingness to perform cesarean delivery on survival of extremely low-birth-weight infants. National Institute of Child Health and Human Development Network of Maternal-Fetal Medicine Units. Am J Obstet Gynecol. 1997;176:960-966.
38. Mercer BM, Crocker LG, Boe NM, Sibai BM. Induction versus expectant management in premature rupture of the membranes with mature amniotic fluid at 32 to 36 weeks: a randomized trial. Am J Obstet Gynecol. 1993;169:775-782.
39. Cox SM, Leveno KJ. Intentional delivery versus expectant management with preterm ruptured membranes at 30-34 weeks’ gestation. Obstet Gynecol. 1995;86:875-879.
40. Dudley J, Malcolm G, Ellwood D. Amniocentesis in the management of preterm premature rupture of the membranes. Aust N Z J Obstet Gynaecol. 1991;31:331-336.
41. Broekhuizen FF, Gilman M, Hamilton PR. Amniocentesis for gram stain and culture in preterm premature rupture of the membranes. Obstet Gynecol. 1985;66:316-321.
Dr. Esplin receives support from the National Institute of Child Health and Human Development and is a speaker for Adeza.
1. Abe T. The detection of rupture of fetal membranes with the nitrazine indicator. Am J Obstet Gynecol. 1940;39:400.-
2. Davidson KM. Detection of premature rupture of the membranes. Clin Obstet Gynecol. 1991;34:715-722.
3. Cousins LM, Smok D, Lovett SM, Poeltler DM. AmniSure placental alpha microglobulin-1 rapid immunoassay versus standard diagnostic methods for detection of ruptured membranes. Am J Perinatol. 2005;22(6):317-320.
4. Taylor J, Garite TJ. Premature rupture of membranes before fetal viability. Obstet Gynecol. 1984;64:615-620.
5. Schucker JL, Mercer BM. Midtrimester premature rupture of the membranes. Semin Perinatol. 1996;20:389-400.
6. Hillier SL, Martius J, Krohn M, et al. A case-control study of chorioamnionic infection and histologic chorioamnionitis in prematurity. N Engl J Med. 1988;319:972-978.
7. Morales WJ. The effect of chorioamnionitis on the developmental outcome of preterm infants at one year. Obstet Gynecol. 1987;70:183-186.
8. Moretti M, Sibai BM. Maternal and perinatal outcome of expectant management of premature rupture of membranes in the midtrimester. Am J Obstet Gynecol. 1988;159:390-396.
9. Beydoun SN, Yasin SY. Premature rupture of the membranes before 28 weeks: conservative management. Am J Obstet Gynecol. 1986;155:471-479.
10. Major CA, Kitzmiller JL. Perinatal survival with expectant management of midtrimester rupture of membranes. Am J Obstet Gynecol. 1990;163:838-844.
11. Falk SJ, Campbell LJ, Lee-Parritz A, et al. Expectant management in spontaneous preterm premature rupture of membranes between 14 and 24 weeks’ gestation. J Perinatol. 2004;24:611-616.
12. Kilbride HW, Yeast J, Thibeault DW. Defining limits of survival: lethal pulmonary hypoplasia after midtrimester premature rupture of membranes. Am J Obstet Gynecol. 1996;175:675-681.
13. Rotschild A, Ling EW, Puterman ML, Farquharson D. Neonatal outcome after prolonged preterm rupture of the membranes. Am J Obstet Gynecol. 1990;162:46-52.
14. Farooqi A, Holmgren PA, Engberg S, Serenius F. Survival and 2-year outcome with expectant management of second-trimester rupture of membranes. Obstet Gynecol. 1998;92:895-901.
15. Vergani P, Ghidini A, Locatelli A, et al. Risk factors for pulmonary hypoplasia in second-trimester premature rupture of membranes. Am J Obstet Gynecol. 1994;170:1359-1364.
16. Blott M, Greenough A. Neonatal outcome after prolonged rupture of the membranes starting in the second trimester. Arch Dis Child. 1988;63:1146-1150.
17. Stevenson DK, Wright LL, Lemons JA, et al. Very low birth weight outcomes of the National Institute of Child Health and Human Development Neonatal Research Network, January 1993 through December 1994. Am J Obstet Gynecol. 1998;179:1632-1639.
18. Nelson LH, Anderson RL, O’Shea TM, Swain M. Expectant management of preterm premature rupture of membranes. Am J Obstet Gynecol. 1994;171:350-356.
19. Belady PH, Farkouh LJ, Gibbs RS. Intra-amniotic infection and premature rupture of the membranes. Clin Perinatol. 1997;24:43-57.
20. Ananth CV, Savitz DA, Williams MA. Placental abruption and its association with hypertension and prolonged rupture of membranes: a methodologic review and meta-analysis. Obstet Gynecol. 1996;88:309-318.
21. Gonen R, Hannah ME, Milligan JE. Does prolonged preterm premature rupture of the membranes predispose to abruptio placentae? Obstet Gynecol. 1989;74:347-350.
22. Carlan SJ, O’Brien WF, Parsons MT, Lense JJ. Preterm premature rupture of membranes: a randomized study of home versus hospital management. Obstet Gynecol. 1993;81:61-64.
23. Vintzileos AM, Campbell WA, Nochimson DJ, Weinbaum PJ. The use of the nonstress test in patients with premature rupture of the membranes. Am J Obstet Gynecol. 1986;155:149-153.
24. Smith CV, Greenspoon J, Phelan JP, Platt LD. Clinical utility of the nonstress test in the conservative management of women with preterm spontaneous premature rupture of the membranes. J Reprod Med. 1987;32:1-4.
25. Hanley ML, Vintzileos AM. Biophysical testing in premature rupture of the membranes. Semin Perinatol. 1996;20:418-425.
26. Levy DL, Warsof SL. Oral ritodrine and preterm premature rupture of membranes. Obstet Gynecol. 1985;66:621-623.
27. Weiner CP, Renk K, Klugman M. The therapeutic efficacy and cost-effectiveness of aggressive tocolysis for premature labor associated with premature rupture of the membranes. Am J Obstet Gynecol. 1988;159:216-222.
28. Ohlsson A. Treatments of preterm premature rupture of the membranes: a meta-analysis. Am J Obstet Gynecol. 1989;160:890-906.
29. Crowley PA. Antenatal corticosteroid therapy: a meta-analysis of the randomized trials, 1972 to 1994. Am J Obstet Gynecol. 1995;173:322-335.
30. Lewis DF, Brody K, Edwards MS, et al. Preterm premature ruptured membranes: a randomized trial of steroids after treatment with antibiotics. Obstet Gynecol. 1996;88:801-805.
31. National Institutes of Health. Effect of corticosteroids for fetal maturation on perinatal outcomes. NIH Consensus Development Panel on the Effect of Corticosteroids for Fetal Maturation on Perinatal Outcomes. JAMA. 1995;273:413-418.
32. Mercer BM, Miodovnik M, Thurnau GR, et al. Antibiotic therapy for reduction of infant morbidity after preterm premature rupture of the membranes. A randomized controlled trial. National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. JAMA. 1997;278:989-995.
33. Egarter C, Leitich H, Karas H, et al. Antibiotic treatment in preterm premature rupture of membranes and neonatal morbidity: a metaanalysis. Am J Obstet Gynecol. 1996;174:589-597.
34. Ananth CV, Guise JM, Thorp JM, Jr. Utility of antibiotic therapy in preterm premature rupture of membranes: a meta-analysis. Obstet Gynecol Surv. 1996;51:324-328.
35. Kenyon SL, Taylor DJ, Tarnow-Mordi W. Broad-spectrum antibiotics for preterm, prelabour rupture of fetal membranes: the ORACLE I randomised trial. ORACLE Collaborative Group. Lancet. 2001;357:979-988.
36. Kenyon S, Boulvain M, Neilson J. Antibiotics for preterm rupture of membranes. Cochrane Database Syst Rev. 2003;(2):CD001058.-
37. Bottoms SF, Paul RH, Iams JD, et al. Obstetric determinants of neonatal survival: influence of willingness to perform cesarean delivery on survival of extremely low-birth-weight infants. National Institute of Child Health and Human Development Network of Maternal-Fetal Medicine Units. Am J Obstet Gynecol. 1997;176:960-966.
38. Mercer BM, Crocker LG, Boe NM, Sibai BM. Induction versus expectant management in premature rupture of the membranes with mature amniotic fluid at 32 to 36 weeks: a randomized trial. Am J Obstet Gynecol. 1993;169:775-782.
39. Cox SM, Leveno KJ. Intentional delivery versus expectant management with preterm ruptured membranes at 30-34 weeks’ gestation. Obstet Gynecol. 1995;86:875-879.
40. Dudley J, Malcolm G, Ellwood D. Amniocentesis in the management of preterm premature rupture of the membranes. Aust N Z J Obstet Gynaecol. 1991;31:331-336.
41. Broekhuizen FF, Gilman M, Hamilton PR. Amniocentesis for gram stain and culture in preterm premature rupture of the membranes. Obstet Gynecol. 1985;66:316-321.
Dr. Esplin receives support from the National Institute of Child Health and Human Development and is a speaker for Adeza.
Averting complications of laparoscopy: Pearls from 5 patients
To view three clips of surgical pearls for laparoscopy, visit the Video Library.
<huc>Q.</huc>What is the only surgical procedure that is completely safe?
<huc>A.</huc>The surgical procedure that is not performed.
The unfortunate truth is that complications can occur during any operative procedure, despite our best efforts—and laparoscopy is no exception. Being vigilant for iatrogenic injuries, both during and after surgery, and ensuring that repairs are both thorough and timely, are two of our best weapons against major complications, along with meticulous technique and adequate experience.
This article features five cases that illustrate some of the most serious complications of laparoscopy—and how to prevent and manage them.
CASE 1: Surgical patient returns with signs of ureteral injury
A 42-year-old woman with a history of endometriosis undergoes laparoscopic hysterectomy and bilateral salpingo-oophorectomy. She is discharged 2 days later. Two days after that, she returns to the hospital complaining of fluid leaking from the vagina. She has no fever or any other significant complaint or physical findings other than abdominal tenderness, which is to be expected after surgery. A computed tomography (CT) scan with intravenous (IV) contrast reveals left ureteral obstruction near the bladder, with extravasation of contrast media into the abdominal cavity. Further investigation reveals a left ureteral transection.
Could this injury have been avoided? How should it be managed?
Postoperative diagnosis of ureteral injury can be challenging, in part because up to 50% of unilateral cases are asymptomatic. Be on the lookout for this complication in women who have undergone pelvic sidewall dissection or laparoscopic hysterectomy, such as the patient in the case just described. As the number of laparoscopic hysterectomies and retroperitoneal procedures has risen in recent years, so has the rate of ureteral injury, with an incidence of 0.3% to 2%.1,2
Ureteral injury can be caused by ligation, ischemia, resection, transection, crushing, or angulation. Three sites are particularly troublesome: the infundibulopelvic ligament, ovarian fossa, and ureteral tunnel.3,4 In Case 1, injury to the ureter was proximal to the bladder and probably occurred during transection of the uterosacral cardinal ligament complex.
What’s the best preventive strategy?
Meticulous technique is imperative to protect the ureters. This includes adequate visualization, intraperitoneal or retroperitoneal dissection, and early identification of the ureter. In a high-risk patient likely to have distorted anatomy due to severe endometriosis and fibrosis, retroperitoneal dissection of any adhesions or tumor and identification of the ureter are the best ways to avoid injury.
Intraperitoneal identification and dissection of the ureters can be enhanced by hydrodissection and resection of the affected peritoneum.3,4 To create a safe operating plane, make a small opening in the peritoneum below the ureter and inject 50 to 100 mL of lactated Ringer’s solution along the course of the ureter, which will displace it laterally.5
Although neither IV indigo carmine nor ureteral catheterization has been shown to reduce the risk of ureteral injury or identify ligation or thermal injury,3,6 both can help the surgeon identify intraoperative perforation of the ureter. Liberal use of cystoscopy with indigo carmine administration for identification of ureteral flow and ureteral catheterization can be used in potentially high-risk patients. If there is suspicion for devascularization or thermal injury, use prophylactic ureteral stents postoperatively for 2 to 4 weeks.
Don’t hesitate to consult a urologist
In Case 1, the surgeon sought immediate urologic consultation and the patient underwent laparotomy with ureteroneocystotomy without sequelae.
In general, management of ureteral injury depends on its severity and location, as well as the comfort level of the surgeon. Minor injuries are sometimes managed with cystoscopic stent placement, but more severe cases may require operative ureteral repair.
In cases like this one, where ureteral injury occurred in close proximity to the bladder, a ureteroneocystotomy is possible. However, in more cephalad injuries, there may be insufficient residual ureter to allow such a repair. In these cases, a Boari flap may be attempted to use bladder tissue to bridge the gap to the ureteral edge. Rarely, in high ureteral injuries, trans-ureteroureterostomy may be appropriate. This procedure carries the greatest risk, given that both kidneys are reliant on one ureter.
Is laparoscopic repair reasonable?
When surgical intervention is necessary, the choice between laparoscopy and laparotomy depends on the skill and comfort level of the surgeon and the availability of instruments and support team.6,7 That said, ureteral injury is usually treated via laparotomy.1 As operative laparoscopy becomes even more commonplace, reconstruction of the urinary system will increasingly be managed laparoscopically.
Depending on the size and location of the injury, reconstruction may involve ureteral reimplantation with or without a psoas hitch, Boari flap, or primary endtoend anastomosis.8-10
CASE 2: Postoperative symptoms lead to rehospitalization
A 35-year-old patient undergoes laparoscopic ovarian cystectomy and returns home the same day. She is readmitted 72 hours later because of lower abdominal tenderness, worsening nausea and vomiting, and urine-like drainage from her midline suprapubic trocar site. Analysis of the leaking fluid shows high creatinine levels consistent with urine. The patient has no fever and is hemodynamically stable. Examination reveals a moderately distended abdomen with decreased bowel sounds. Hematuria is evident on urine analysis.
Urologic consultation is obtained, and the patient undergoes simultaneous laparoscopy and cystoscopy, during which perforation of the bladder dome is discovered, apparently caused by the mid suprapubic trocar. The bladder is mobilized anteriorly, and both anterior and posterior aspects of the perforation are repaired in one layer laparoscopically.
After continuous drainage with a transurethral Foley catheter for 7 days, cystography shows complete healing of the bladder, and the Foley catheter is removed. The patient recovers completely.
Vesical injury sometimes occurs in patients who have a history of laparotomy, a full bladder at the time of surgery, or displaced anatomy due to pelvic adhesions.11 Although bladder injury is rare, laparoscopy increases the risk. Trocars, uterine manipulators, and blunt instruments can perforate or lacerate the bladder, and energy devices can cause thermal injury. The risk of bladder injury increases during laparoscopic hysterectomy.
Be vigilant about trocar placement and dissection techniques
Accessory trocars can injure a full bladder. Injury can also occur when distorted anatomy from a previous pelvic operation obscures bladder boundaries, making insertion of the midline trocar potentially perilous (FIGURE 1). The Veress needle and Rubin’s cannula can perforate the bladder.11-13 And in the anterior cul-de-sac, adhesiolysis, deep coagulation, laser ablation, or sharp excision of endometriosis implants can predispose a patient to bladder injury.
In women with severe endometriosis, lower-segment myoma, or a history of cesarean section, the bladder is vulnerable to laceration when blunt dissection is used during laparoscopic hysterectomy or laparoscopically assisted vaginal hysterectomy (LAVH). A vesical injury also can occur at the time of laparoscopic bladder-neck suspension upon entry into, and dissection of, the space of Retzius.
FIGURE 1 A bladder at risk
In this patient with a previous cesarean section, the bladder is adherent to the anterior abdominal wall. Needle mapping in the conventional midline trocar position indicates that the trocar must be relocated to avoid bladder injury.
Intraoperative findings that suggest bladder injury include air in the urinary catheter, hematuria, trocar site drainage of urine, or indigo carmine leakage. Postoperative signs and symptoms include leaking from incisional sites, a mass in the abdominal wall, and abdominal swelling.
Liberal use of cystoscopy or distension of the bladder with 300 to 500 mL of normal saline is recommended whenever there is a suspicion of bladder injury, especially during laparoscopic hysterectomy or LAVH. When a trocar causes the injury, look for both entry and exit punctures, both of which should be treated.
No matter how much care is taken, some bladder injuries, such as vesicovaginal fistulae, become apparent only postoperatively. More rarely, peritonitis or pseudoascites herald the injury. Retrograde cystography may aid identification.
Treatment of bladder injuries
Small perforations recognized intraoperatively may be conservatively managed by postoperative bladder drainage for 5 to 7 days. Most other bladder injuries require prompt intervention. For example, trocar injury to the bladder dome requires one- or two-layer closure followed by 5 to 7 days of urinary drainage. (Both closing and healing are promoted by drainage.)
Laparoscopy or laparotomy? Laparoscopic repair has become increasingly common, and bladder injury is a common complication of LAVH.13,14
CASE 3: Postop pain, tachycardia
A 41-year-old obese woman undergoes laparoscopic cystectomy for an 8-cm left ovarian mass. The abdomen is entered on the second attempt with a long Veress needle. The umbilical trocar is reinserted “several” times because of difficulty opening the peritoneum with the tip of the trocar sheath. The surgical procedure is completed within 2 hours, and the patient is discharged 23 hours later.
The next day, she experiences increasing abdominal pain and presents to the emergency room. Upon admission she reports intermittent chills, but denies nausea and vomiting. She is in mild distress, pale and tachycardic, with a temperature of 96.4°, pulse of 117, respiration rate of 20, blood pressure of 106/64 mm Hg, and oxygen saturation of 92%. She also has a diffusely tender abdomen but normal blood work. Abdominal and chest x-rays show a large right subphrenic air-fluid level that is consistent with free intraperitoneal air, unsurprising given her recent surgery. Bibasilar atelectasis and consolidation are noted on the initial chest x-ray.
During observation over the next 2 days, she remains afebrile and tachycardic, but her shortness of breath becomes progressively worse. Neither spiral CT nor lower-extremity Doppler suggests pulmonary embolism or deep venous thrombosis. Supplemental oxygen, aggressive pain management, albuterol, ipratropium, and acetylcysteine are initiated after pulmonary consultation.
The patient tolerates a regular diet on postoperative day 3 and has a bowel movement on day 5. However, the same day she begins vomiting and reports worsening abdominal pain. CT imaging of the abdomen and pelvis reveals free air in the abdomen and loculated fluid with air bubbles suspicious for intra-abdominal infection and perforated bowel.
Exploratory laparotomy reveals diffuse feculent peritonitis, as well as food particles and contrast media. There is a perforation in the antimesenteric side of the ileum approximately 1.5 feet proximal to the ileocecal valve. This perforation measures approximately 1 cm in diameter and is freely spilling intestinal contents. Small bowel resection is performed to treat the perforation.
Following the surgery, the patient recovers slowly.
Could the bowel perforation have been detected sooner?
Intestinal tract injury is a serious complication, particularly with postoperative diagnosis.15 Damage can occur during insertion of the Veress needle or trocar when the bowel is immobilized by adhesions, or during enterolysis.16 Unrecognized thermal injury can cause delayed bowel injury.
Small-bowel damage often occurs during uncontrolled insertion of the Veress needle or primary umbilical trocar. It also may result from sharp dissection or thermal injury.17,18 Abrasions and lacerations can occur if traction is exerted on the bowel using serrated graspers. When adhesions are dense and tissue planes poorly defined, the risk of laceration due to energy sources or sharp dissection increases.
Be cautious during bowel manipulation. Avoid blunt dissection. Be especially careful when the small bowel is adherent to the anterior abdominal wall (FIGURE 2A), particularly during evaluation of patients with a history of bowel resection, exploratory laparotomy for trauma-related peritonitis, or tumor debulking.
Remove the primary and ancillary cannulas under direct visualization with the laparoscope to prevent formation of a vacuum that can draw bowel into the incision and cause herniation.19
FIGURE 2 Adherent bowel, minor bleeding
A: Veress needle pressure measurements are persistently elevated before primary trocar insertion in this patient, raising the suspicion of adhesive disease from earlier surgery. As a result, the primary trocar is relocated to the left upper quadrant. Inspection confirms that small bowel is adherent to the anterior abdominal wall.
FIGURE 2 Adherent bowel, minor bleeding
B: After the small-bowel adhesions are dissected off the anterior abdominal wall via laparoscopy, a small hematoma is discovered, likely caused by the Veress needle. The patient is managed conservatively and recovers.
The value of open laparoscopy
In open laparoscopy, an abdominal incision is made into the peritoneal cavity so that the trocar can be placed under direct vision, after which the abdomen is insufflated. This approach can prevent bowel injury only when the adhesions and attachments are to the anterior abdominal wall and away from the entry site. When the attachment lies directly beneath the umbilicus, however, open laparoscopy is no guarantee against injury.
When bowel adhesions are severe, use alternative trocar sites such as the left upper quadrant (Palmer’s point) for the Veress needle and primary trocars.5,20,21
The likelihood of perforation can be reduced with preoperative bowel prep when there is a risk of bowel adhesions.
Identifying bowel injury
We recommend routine inspection of the structures beneath the primary trocar upon insertion of the laparoscope to look for injury to the bowel, mesentery, or vascular structures. If adhesions are found, evaluate the area carefully to rule out injury to the bowel or omentum. It may be necessary to change the position of the laparoscope to assess the patient.
Trauma to the intestinal tract can be mechanical or electrical in nature, and each type of trauma creates a distinctive, characteristic pattern. Thermal injury can be subtle and present as simple blanching or a distinct burn and charring. A small hole or obvious tear in the bowel wall can be the result of mechanical injury.22
Benign-appearing, superficial thermal bowel injuries may be managed conservatively.22 Minimal serosal burns (smaller than 5 mm in diameter) can be managed expectantly. Immediate surgical intervention is needed if the area of blanching on the intestinal serosa exceeds 5 mm in diameter or if the burn appears to involve more than the serosa.23
Small-bowel injuries that escape notice intraoperatively generally become apparent 2 to 4 days later, when the patient develops fever, nausea, lower abdominal pain, and anorexia. On postoperative day 5 or 6, the white blood cell (WBC) count rises and earlier symptoms may become worse. Radiography may reveal multiple air and fluid levels—another sign of bowel injury. Be aware that if the patient has clinical symptoms of gastrointestinal injury, even if the WBC count is normal, exploratory laparoscopy or laparotomy is necessary for accurate diagnosis.
Intraoperatively discovered injury
Careful inspection may reveal no leakage or bleeding in the affected area. Small punctures or superficial lacerations seal readily and may not require further treatment (FIGURE 2B), but larger perforations require repair. Straightforward repair is not always possible when the injury is extensive and considerable time has elapsed before it is discovered.
Inspect the intestine thoroughly at the conclusion of a procedure; obvious leakage requires intervention. Repair the small intestine in one or two layers, using the initial row of interrupted sutures to approximate the mucosa and muscularis.24 To lessen the risk of stenosis, close all lacerations transversely when they are smaller than one half the diameter of the bowel. If the laceration exceeds that size, segmental resection and anastomosis are necessary. Resection is prudent if the mesenteric blood supply is compromised.25
When performing one-layer repair of the small bowel, delayed absorbable suture (eg, Vicryl or PDS) or nonabsorbable suture (eg, silk) is recommended.26
At the conclusion of a repair, copiously irrigate the entire abdomen. Place a nasogastric tube only if ileus is anticipated; the tube can be removed when drainage diminishes and active bowel sounds and flatus appear. Do not give anything by mouth until the patient has return of bowel function and active peristalsis. Prescribe prophylactic antibiotics.
Note that peritonitis sometimes develops after repair of the bowel.25 This can be managed with prolonged bowel rest and peripheral or total parenteral nutrition.
Conservative management may be possible
Patients whose symptoms of bowel laceration become apparent after discharge can sometimes be managed conservatively. More than 50% of patients treated conservatively require no surgery.23 Inpatient management consists of monitoring the WBC count, providing hydration and IV antibiotics, and examining the patient every 6 hours, giving nothing by mouth.
When injury is discovered later
If conservative management with observation and bowel rest fails, or the patient complains of severe abdominal pain, vomiting, nausea, obstipation, or signs and symptoms of peritonitis, such as the patient in Case 3, immediate surgical intervention is necessary. When an injury is not detected until some time after initial surgery, resection of all necrotic tissue is mandatory. In most cases, the perforation is managed by segmental resection and reanastomosis. Evaluate the entire small and large bowel to rule out any other injury, and irrigate generously. Bowel rest, parenteral nutrition, and IV antibiotics also are indicated.
Of 36,928 procedures reported by members of the American Association of Gynecologic Laparoscopists, there were two deaths—both caused by unrecognized bowel injury.15
CASE 4: Large-bowel injury precipitates lengthy recovery
A surgeon performs a left laparoscopic salpingo-oophorectomy to remove an 8-cm ovarian endometrioma that is adherent to the rectosigmoid colon of a 40-year-old diabetic woman. Sharp and electrosurgical scissors are used to separate the adnexa from the rectosigmoid colon. No injury is observed, and she is discharged the same day. Four days later, she returns with severe abdominal pain, nausea, vomiting, and fatigue. Lab tests reveal a WBC count of 17,000; a CT scan shows pockets of air beneath the diaphragm, as well as fluid collection suggestive of a pelvic abscess.
Immediate laparotomy is performed, during which the surgeon discovers contamination of the abdominal viscera by bowel contents, as well as a 0.5-cm perforation of the rectosigmoid colon. The perforation is repaired in two layers after its edges are trimmed, and a diverting colostomy is performed. The patient is admitted to the ICU and requires antibiotic treatment, total parenteral nutrition, and bowel rest due to severe peritonitis. She gradually recovers and is discharged 3 weeks later. The diverting colostomy is reversed 3 months later.
Even small perforations in the large bowel can cause infection and abscess due to the high bacterial content of the colon. The most common cause of injury to the rectosigmoid colon is pelvic adhesiolysis during cul-de-sac dissection, treatment of pelvic endometriosis, and resection of adherent pelvic masses.
Sharp dissection with scissors or high-powered lasers is relatively safe near the bowel. When dissecting the cul-de-sac, identify the vagina and rectum by placing a probe or finger in each area. Begin dissection from the unaffected pararectal space, and proceed toward the obliterated cul-de-sac.27,28
Bowel prep is indicated before extensive pelvic surgery and when the history suggests endometriosis or significant pelvic adhesions. Some general surgeons base their decision to perform colostomy (or not) on whether the bowel was prepped preoperatively.29
If the large bowel is perforated by the Veress needle, the saline aspiration test will yield brownish fluid. When significant pelvic adhesiolysis or pelvic or endometriotic tumor resection is performed, inject air into the rectum afterward via a sigmoidoscope or bulb syringe and assess the submerged rectum and rectosigmoid colon for bubbling. The rectal wall may be weakened during these types of procedures, so instruct the patient to use oral stool softeners and avoid enemas.30
Delay in detection can have serious ramifications
When a large-bowel injury goes undetected at the time of operation, the patient generally presents on the third or fourth postoperative day with mild fever, occasionally sudden sharp epigastric pain, lower abdominal pain, slight nausea, and anorexia. By the fifth or sixth day, these symptoms have become more severe and are accompanied by peritonitis and an elevated WBC count.
Whenever a patient complains of abdominal pain and a deteriorating condition, assume that bowel injury is the cause until it is proved otherwise.
Intraoperative management
Repair small trocar wounds using primary suture closure. Copious lavage of the peritoneal cavity, drainage, and a broad-spectrum antibiotic minimize the risk of infection. Manage deep electrical injury to the right colon by resecting the injured segment and performing primary anastomosis. Primary closure or resection and reanastomosis may not be adequate when the vascular supply of the descending colon or rectum is compromised. In that case, perform a diverting colostomy or ileostomy, which can be reversed 6 to 12 weeks later.25,26
CASE 5: Vascular injury
A tall, thin, athletic 19-year-old undergoes diagnostic laparoscopy to rule out pelvic pathology after she complains of severe, monthly abdominal pain. Upon insertion of the laparoscope, the surgeon observes a large hematoma forming at the right pelvic sidewall. At the same time, the anesthesiologist reports a significant drop in blood pressure, and vascular injury is diagnosed. The surgeon attempts to control the bleeding using bipolar coagulation, but the problem only becomes worse. He decides to switch to laparotomy.
A vascular surgeon is called in, and injury to the right common iliac artery and vein—apparently caused during insertion of the primary umbilical trocar—is repaired. The patient is given 5 U of red blood cells. She goes home 10 days later, but returns with thrombophlebitis and rejection of the graft. After several surgeries, she finally recovers, with some sequelae, such as unilateral leg edema.
Management of vascular injury depends on the source and type of injury. On major vessels, electrocoagulation is contraindicated. After immediate atraumatic compression with tamponade to control bleeding, vascular repair, in consultation with a vascular surgeon, is indicated. At times, a vascular graft may be required.
Smaller vessels, such as the infundibulopelvic ligament or uterine vessels, can be managed by clips, suture, or loop ligatures. If thermal energy is used in the repair, be careful to avoid injury to surrounding structures.
Most emergency laparotomies are performed for uncontrolled bleeding.30,31 Lack of control or a wrong angle at insertion of the Veress needle and trocars is a major cause of large-vessel injury. Sharp dissection of adhesions, uterosacral ablation, transection of vascular pedicles without adequate dessication, and rough handling of tissues can all cause bleeding. Distorted anatomy is a main cause of vascular injury and can compound injury in areas more prone to bleeding, such as the oviduct, infundibulopelvic ligament, mesosalpinx, and pelvic sidewall vessels.
The return of pressure gradients to normal levels at the end of a procedure can be accompanied by bleeding into the retroperitoneal space, so evaluate the patient in a supine position after intra-abdominal pressure is reduced.
A vascular surgeon may be required
Depending on the type of vessel, size and location of the injury, and degree of bleeding, you may use unipolar or bipolar electrocoagulation, suture, clips, vasopressin, or loop ligatures to control bleeding. Although diluted vasopressin (10 U in 60 mL of lactated Ringer’s saline) can decrease oozing from raw peritoneal areas, injury to a major vessel, such as the iliac vessels, vena cava, or aorta, needs immediate control and proper repair. The decision to perform laparoscopy or laparotomy depends on your preference and experience. In any case, a vascular surgeon may be consulted for major vascular injuries.32
If a major vessel is injured, do not crush-clamp it. If possible (and if your laparoscopic skills are advanced), insert a sponge via a 10-mm trocar and apply pressure to the vessel to minimize bleeding and enhance visualization. The decision to repair the injury laparoscopically or by laparotomy should be made judiciously and promptly. n
The authors acknowledge the editorial contributions of Kristina Petrasek and Barbara Page, of the University of California, Berkeley, to the manuscript of this article.
1. Ostrzenski A, Radolinski B, Ostrzenska K. A review of laparoscopic ureteral injury in pelvic surgery. Obstet Gynecol Surv. 2003;58:794-799.
2. Kabalin J. Chapter 1—Surgical anatomy of the retroperitoneum, kidneys, and ureters. In: Walsh P, Retik A, Wein A, eds. Campbell’s Urology. 8th ed. Philadelphia: Saunders; 2002 36-40.
3. Chan J, Morrow J, Manetta A. Prevention of ureteral injuries in gynecologic surgery. Am J Obstet Gynecol. 2003;188:1273-1277.
4. Grainger DA, Soderstrom RM, Schiff SF, Glickman MG, DeCherney AH, Diamond MP. Ureteral injuries at laparoscopy: insights into diagnosis, management, and prevention. Obstet Gynecol. 1990;75:839-843.
5. Nezhat C. Chapter 20. Operative Gynecologic Laparoscopy: Principles and Techniques. 2nd ed. New York: McGraw–Hill; 2000.
6. Ou CS, Huang IA, Rowbotham R. Laparoscopic ureteroureteral anastomosis for repair of ureteral injury involving stricture. Int Urogynecol J Pelvic Floor Dysfunct. 2005;16:155-157.
7. Modi P, Goel R, Dodiya S. Laparoscopic ureteroneocystostomy for distal ureteral injuries. Urology. 2005;66:751-753.
8. Nezhat C, Nezhat F, Nezhat CH, et al. Urinary tract endometriosis treated by laparoscopy. Fertil Steril. 1996;66:920-924.
9. Nezhat C, Nezhat F. Laparoscopic repair of ureter resected during operative laparoscopy. Obstet Gynecol. 1992;80:543-544.
10. Nezhat CH, Nezhat FR, Freiha F, Nezhat CR. Laparoscopic vesicopsoas hitch for infiltrative ureteral endometriosis. Fertil Steril. 1999;71:376-379.
11. Georgy FM, Fettman HH, Chefetz MD. Complications of laparoscopy: two cases of perforated urinary bladder. Am J Obstet Gynecol. 1974;120:1121-1122.
12. Sherer DM. Inadvertent transvaginal cystotomy during laparoscopy. Int J Gynaecol Obstet. 1990;32:77-79.
13. Nezhat CH, Seidman DS, Nezhat F, et al. Laparoscopic management of internal and unintentional cystotomy. J Urol. 1996;156:1400-1402.
14. Lee CL, Lai YM, Soong YK. Management of urinary bladder injuries in laparoscopic assisted vaginal hysterectomy. Acta Obstet Gynecol Scand. 1996;75:174-177.
15. Peterson HB, et al. American Association of Gynecologic Laparoscopists’ 1988 membership survey on operative laparoscopy. J Reprod Med. 1990;35:587-589.
16. Phillips JM, Hulka JF, Hulka B, et al. 1978 AAGL membership survey. J Reprod Med. 1981;26:529-533.
17. Chapron C, Pierre F, et al. Gastrointestinal injuries during laparoscopy. Hum Reprod. 1999;14:333-337.
18. Schrenk P, Woisetschlager R, Rieger R, et al. Mechanism, management, and prevention of laparoscopic bowel injuries. Gastrointest Endosc. 1996;43:572-574.
19. Sauer M, Jarrett JC. Small bowel obstruction following diagnostic laparoscopy. Fertil Steril. 1984;42:653-654.
20. Penfield AJ. How to prevent complications of open laparoscopy. J Reprod Med. 1985;30:660-663.
21. Brill A, Nezhat F, Nezhat CH, Nezhat C. The incidence of adhesions after prior laparatomy: a laparoscopic appraisal. Obstet Gynecol. 1995;85:269-272.
22. Levy BS, Soderstrom RM, Dail DH. Bowel injuries during laparoscopy: gross anatomy and histology. J Reprod Med. 1985;30:168-172.
23. Wheeless CR. Gastrointestinal injuries associated with laparoscopy. In: Phillips JM, ed. Endoscopy in Gynecology. Santa Fe Springs, Calif: American Association of Gynecologic Laparoscopists; 1978.
24. Borton M. Laparoscopic Complications: Prevention and Management. Philadelphia: Decker; 1986.
25. DeCherney AH. Laparoscopy with unexpected viscus penetration. In: Nichols DH, ed. Clinical Problems, Injuries, and Complications of Gynecologic Surgery. Baltimore: Williams & Wilkins; 1988.
26. Nezhat C, Nezhat F, Ambroze W, Pennington E. Laparoscopic repair of small bowel and colon: a report of 26 cases. Surg Endosc. 1993;7:88-89.
27. Redwine D. Laparoscopic en bloc resection for treatment of the obliterated cul-de-sac in endometriosis. J Reprod Med. 1992;37:696-698.
28. Nezhat C, Nezhat F, et al. Laparoscopic treatment of infiltrative rectosigmoid colon and rectovaginal septum endometriosis by the technique of videolaseroscopy and the CO2 laser. Br J Obstet Gynaecol. 1992;99:664-667.
29. Nezhat C, Seidman D, Nezhat F, et al. The role of intraoperative proctosigmoidoscopy in laparoscopic pelvic surgery. J Am Assoc Gynecol Laparosc. 2004;11:47-49.
30. Chapron CM, et al. Major vascular injuries during gynecologic laparoscopy. J Am Coll Surg. 1997;185:461-465.
31. Geers J, Holden C. Major vascular injury as a complication of laparoscopic surgery: a report of three cases and review of the literature. Am Surg. 1996;62:377-379.
32. Nezhat C, Childers J, Nezhat F, et al. Major retroperitoneal vascular injury during laparoscopic surgery. Hum Reprod. 1997;12:480-483.
Dr. Farr Nezhat reports no relevant financial relationships. Dr. Ceana Nezhat is a speaker and consultant for Karl Storz, Johnson & Johnson, Valleylab, US Surgical, and Viking. Dr. Camran Nezhat is a speaker for or receives educational support from Karl Storz, Stryker, Johnson & Johnson, Valleylab, and Baxter.
To view three clips of surgical pearls for laparoscopy, visit the Video Library.
<huc>Q.</huc>What is the only surgical procedure that is completely safe?
<huc>A.</huc>The surgical procedure that is not performed.
The unfortunate truth is that complications can occur during any operative procedure, despite our best efforts—and laparoscopy is no exception. Being vigilant for iatrogenic injuries, both during and after surgery, and ensuring that repairs are both thorough and timely, are two of our best weapons against major complications, along with meticulous technique and adequate experience.
This article features five cases that illustrate some of the most serious complications of laparoscopy—and how to prevent and manage them.
CASE 1: Surgical patient returns with signs of ureteral injury
A 42-year-old woman with a history of endometriosis undergoes laparoscopic hysterectomy and bilateral salpingo-oophorectomy. She is discharged 2 days later. Two days after that, she returns to the hospital complaining of fluid leaking from the vagina. She has no fever or any other significant complaint or physical findings other than abdominal tenderness, which is to be expected after surgery. A computed tomography (CT) scan with intravenous (IV) contrast reveals left ureteral obstruction near the bladder, with extravasation of contrast media into the abdominal cavity. Further investigation reveals a left ureteral transection.
Could this injury have been avoided? How should it be managed?
Postoperative diagnosis of ureteral injury can be challenging, in part because up to 50% of unilateral cases are asymptomatic. Be on the lookout for this complication in women who have undergone pelvic sidewall dissection or laparoscopic hysterectomy, such as the patient in the case just described. As the number of laparoscopic hysterectomies and retroperitoneal procedures has risen in recent years, so has the rate of ureteral injury, with an incidence of 0.3% to 2%.1,2
Ureteral injury can be caused by ligation, ischemia, resection, transection, crushing, or angulation. Three sites are particularly troublesome: the infundibulopelvic ligament, ovarian fossa, and ureteral tunnel.3,4 In Case 1, injury to the ureter was proximal to the bladder and probably occurred during transection of the uterosacral cardinal ligament complex.
What’s the best preventive strategy?
Meticulous technique is imperative to protect the ureters. This includes adequate visualization, intraperitoneal or retroperitoneal dissection, and early identification of the ureter. In a high-risk patient likely to have distorted anatomy due to severe endometriosis and fibrosis, retroperitoneal dissection of any adhesions or tumor and identification of the ureter are the best ways to avoid injury.
Intraperitoneal identification and dissection of the ureters can be enhanced by hydrodissection and resection of the affected peritoneum.3,4 To create a safe operating plane, make a small opening in the peritoneum below the ureter and inject 50 to 100 mL of lactated Ringer’s solution along the course of the ureter, which will displace it laterally.5
Although neither IV indigo carmine nor ureteral catheterization has been shown to reduce the risk of ureteral injury or identify ligation or thermal injury,3,6 both can help the surgeon identify intraoperative perforation of the ureter. Liberal use of cystoscopy with indigo carmine administration for identification of ureteral flow and ureteral catheterization can be used in potentially high-risk patients. If there is suspicion for devascularization or thermal injury, use prophylactic ureteral stents postoperatively for 2 to 4 weeks.
Don’t hesitate to consult a urologist
In Case 1, the surgeon sought immediate urologic consultation and the patient underwent laparotomy with ureteroneocystotomy without sequelae.
In general, management of ureteral injury depends on its severity and location, as well as the comfort level of the surgeon. Minor injuries are sometimes managed with cystoscopic stent placement, but more severe cases may require operative ureteral repair.
In cases like this one, where ureteral injury occurred in close proximity to the bladder, a ureteroneocystotomy is possible. However, in more cephalad injuries, there may be insufficient residual ureter to allow such a repair. In these cases, a Boari flap may be attempted to use bladder tissue to bridge the gap to the ureteral edge. Rarely, in high ureteral injuries, trans-ureteroureterostomy may be appropriate. This procedure carries the greatest risk, given that both kidneys are reliant on one ureter.
Is laparoscopic repair reasonable?
When surgical intervention is necessary, the choice between laparoscopy and laparotomy depends on the skill and comfort level of the surgeon and the availability of instruments and support team.6,7 That said, ureteral injury is usually treated via laparotomy.1 As operative laparoscopy becomes even more commonplace, reconstruction of the urinary system will increasingly be managed laparoscopically.
Depending on the size and location of the injury, reconstruction may involve ureteral reimplantation with or without a psoas hitch, Boari flap, or primary endtoend anastomosis.8-10
CASE 2: Postoperative symptoms lead to rehospitalization
A 35-year-old patient undergoes laparoscopic ovarian cystectomy and returns home the same day. She is readmitted 72 hours later because of lower abdominal tenderness, worsening nausea and vomiting, and urine-like drainage from her midline suprapubic trocar site. Analysis of the leaking fluid shows high creatinine levels consistent with urine. The patient has no fever and is hemodynamically stable. Examination reveals a moderately distended abdomen with decreased bowel sounds. Hematuria is evident on urine analysis.
Urologic consultation is obtained, and the patient undergoes simultaneous laparoscopy and cystoscopy, during which perforation of the bladder dome is discovered, apparently caused by the mid suprapubic trocar. The bladder is mobilized anteriorly, and both anterior and posterior aspects of the perforation are repaired in one layer laparoscopically.
After continuous drainage with a transurethral Foley catheter for 7 days, cystography shows complete healing of the bladder, and the Foley catheter is removed. The patient recovers completely.
Vesical injury sometimes occurs in patients who have a history of laparotomy, a full bladder at the time of surgery, or displaced anatomy due to pelvic adhesions.11 Although bladder injury is rare, laparoscopy increases the risk. Trocars, uterine manipulators, and blunt instruments can perforate or lacerate the bladder, and energy devices can cause thermal injury. The risk of bladder injury increases during laparoscopic hysterectomy.
Be vigilant about trocar placement and dissection techniques
Accessory trocars can injure a full bladder. Injury can also occur when distorted anatomy from a previous pelvic operation obscures bladder boundaries, making insertion of the midline trocar potentially perilous (FIGURE 1). The Veress needle and Rubin’s cannula can perforate the bladder.11-13 And in the anterior cul-de-sac, adhesiolysis, deep coagulation, laser ablation, or sharp excision of endometriosis implants can predispose a patient to bladder injury.
In women with severe endometriosis, lower-segment myoma, or a history of cesarean section, the bladder is vulnerable to laceration when blunt dissection is used during laparoscopic hysterectomy or laparoscopically assisted vaginal hysterectomy (LAVH). A vesical injury also can occur at the time of laparoscopic bladder-neck suspension upon entry into, and dissection of, the space of Retzius.
FIGURE 1 A bladder at risk
In this patient with a previous cesarean section, the bladder is adherent to the anterior abdominal wall. Needle mapping in the conventional midline trocar position indicates that the trocar must be relocated to avoid bladder injury.
Intraoperative findings that suggest bladder injury include air in the urinary catheter, hematuria, trocar site drainage of urine, or indigo carmine leakage. Postoperative signs and symptoms include leaking from incisional sites, a mass in the abdominal wall, and abdominal swelling.
Liberal use of cystoscopy or distension of the bladder with 300 to 500 mL of normal saline is recommended whenever there is a suspicion of bladder injury, especially during laparoscopic hysterectomy or LAVH. When a trocar causes the injury, look for both entry and exit punctures, both of which should be treated.
No matter how much care is taken, some bladder injuries, such as vesicovaginal fistulae, become apparent only postoperatively. More rarely, peritonitis or pseudoascites herald the injury. Retrograde cystography may aid identification.
Treatment of bladder injuries
Small perforations recognized intraoperatively may be conservatively managed by postoperative bladder drainage for 5 to 7 days. Most other bladder injuries require prompt intervention. For example, trocar injury to the bladder dome requires one- or two-layer closure followed by 5 to 7 days of urinary drainage. (Both closing and healing are promoted by drainage.)
Laparoscopy or laparotomy? Laparoscopic repair has become increasingly common, and bladder injury is a common complication of LAVH.13,14
CASE 3: Postop pain, tachycardia
A 41-year-old obese woman undergoes laparoscopic cystectomy for an 8-cm left ovarian mass. The abdomen is entered on the second attempt with a long Veress needle. The umbilical trocar is reinserted “several” times because of difficulty opening the peritoneum with the tip of the trocar sheath. The surgical procedure is completed within 2 hours, and the patient is discharged 23 hours later.
The next day, she experiences increasing abdominal pain and presents to the emergency room. Upon admission she reports intermittent chills, but denies nausea and vomiting. She is in mild distress, pale and tachycardic, with a temperature of 96.4°, pulse of 117, respiration rate of 20, blood pressure of 106/64 mm Hg, and oxygen saturation of 92%. She also has a diffusely tender abdomen but normal blood work. Abdominal and chest x-rays show a large right subphrenic air-fluid level that is consistent with free intraperitoneal air, unsurprising given her recent surgery. Bibasilar atelectasis and consolidation are noted on the initial chest x-ray.
During observation over the next 2 days, she remains afebrile and tachycardic, but her shortness of breath becomes progressively worse. Neither spiral CT nor lower-extremity Doppler suggests pulmonary embolism or deep venous thrombosis. Supplemental oxygen, aggressive pain management, albuterol, ipratropium, and acetylcysteine are initiated after pulmonary consultation.
The patient tolerates a regular diet on postoperative day 3 and has a bowel movement on day 5. However, the same day she begins vomiting and reports worsening abdominal pain. CT imaging of the abdomen and pelvis reveals free air in the abdomen and loculated fluid with air bubbles suspicious for intra-abdominal infection and perforated bowel.
Exploratory laparotomy reveals diffuse feculent peritonitis, as well as food particles and contrast media. There is a perforation in the antimesenteric side of the ileum approximately 1.5 feet proximal to the ileocecal valve. This perforation measures approximately 1 cm in diameter and is freely spilling intestinal contents. Small bowel resection is performed to treat the perforation.
Following the surgery, the patient recovers slowly.
Could the bowel perforation have been detected sooner?
Intestinal tract injury is a serious complication, particularly with postoperative diagnosis.15 Damage can occur during insertion of the Veress needle or trocar when the bowel is immobilized by adhesions, or during enterolysis.16 Unrecognized thermal injury can cause delayed bowel injury.
Small-bowel damage often occurs during uncontrolled insertion of the Veress needle or primary umbilical trocar. It also may result from sharp dissection or thermal injury.17,18 Abrasions and lacerations can occur if traction is exerted on the bowel using serrated graspers. When adhesions are dense and tissue planes poorly defined, the risk of laceration due to energy sources or sharp dissection increases.
Be cautious during bowel manipulation. Avoid blunt dissection. Be especially careful when the small bowel is adherent to the anterior abdominal wall (FIGURE 2A), particularly during evaluation of patients with a history of bowel resection, exploratory laparotomy for trauma-related peritonitis, or tumor debulking.
Remove the primary and ancillary cannulas under direct visualization with the laparoscope to prevent formation of a vacuum that can draw bowel into the incision and cause herniation.19
FIGURE 2 Adherent bowel, minor bleeding
A: Veress needle pressure measurements are persistently elevated before primary trocar insertion in this patient, raising the suspicion of adhesive disease from earlier surgery. As a result, the primary trocar is relocated to the left upper quadrant. Inspection confirms that small bowel is adherent to the anterior abdominal wall.
FIGURE 2 Adherent bowel, minor bleeding
B: After the small-bowel adhesions are dissected off the anterior abdominal wall via laparoscopy, a small hematoma is discovered, likely caused by the Veress needle. The patient is managed conservatively and recovers.
The value of open laparoscopy
In open laparoscopy, an abdominal incision is made into the peritoneal cavity so that the trocar can be placed under direct vision, after which the abdomen is insufflated. This approach can prevent bowel injury only when the adhesions and attachments are to the anterior abdominal wall and away from the entry site. When the attachment lies directly beneath the umbilicus, however, open laparoscopy is no guarantee against injury.
When bowel adhesions are severe, use alternative trocar sites such as the left upper quadrant (Palmer’s point) for the Veress needle and primary trocars.5,20,21
The likelihood of perforation can be reduced with preoperative bowel prep when there is a risk of bowel adhesions.
Identifying bowel injury
We recommend routine inspection of the structures beneath the primary trocar upon insertion of the laparoscope to look for injury to the bowel, mesentery, or vascular structures. If adhesions are found, evaluate the area carefully to rule out injury to the bowel or omentum. It may be necessary to change the position of the laparoscope to assess the patient.
Trauma to the intestinal tract can be mechanical or electrical in nature, and each type of trauma creates a distinctive, characteristic pattern. Thermal injury can be subtle and present as simple blanching or a distinct burn and charring. A small hole or obvious tear in the bowel wall can be the result of mechanical injury.22
Benign-appearing, superficial thermal bowel injuries may be managed conservatively.22 Minimal serosal burns (smaller than 5 mm in diameter) can be managed expectantly. Immediate surgical intervention is needed if the area of blanching on the intestinal serosa exceeds 5 mm in diameter or if the burn appears to involve more than the serosa.23
Small-bowel injuries that escape notice intraoperatively generally become apparent 2 to 4 days later, when the patient develops fever, nausea, lower abdominal pain, and anorexia. On postoperative day 5 or 6, the white blood cell (WBC) count rises and earlier symptoms may become worse. Radiography may reveal multiple air and fluid levels—another sign of bowel injury. Be aware that if the patient has clinical symptoms of gastrointestinal injury, even if the WBC count is normal, exploratory laparoscopy or laparotomy is necessary for accurate diagnosis.
Intraoperatively discovered injury
Careful inspection may reveal no leakage or bleeding in the affected area. Small punctures or superficial lacerations seal readily and may not require further treatment (FIGURE 2B), but larger perforations require repair. Straightforward repair is not always possible when the injury is extensive and considerable time has elapsed before it is discovered.
Inspect the intestine thoroughly at the conclusion of a procedure; obvious leakage requires intervention. Repair the small intestine in one or two layers, using the initial row of interrupted sutures to approximate the mucosa and muscularis.24 To lessen the risk of stenosis, close all lacerations transversely when they are smaller than one half the diameter of the bowel. If the laceration exceeds that size, segmental resection and anastomosis are necessary. Resection is prudent if the mesenteric blood supply is compromised.25
When performing one-layer repair of the small bowel, delayed absorbable suture (eg, Vicryl or PDS) or nonabsorbable suture (eg, silk) is recommended.26
At the conclusion of a repair, copiously irrigate the entire abdomen. Place a nasogastric tube only if ileus is anticipated; the tube can be removed when drainage diminishes and active bowel sounds and flatus appear. Do not give anything by mouth until the patient has return of bowel function and active peristalsis. Prescribe prophylactic antibiotics.
Note that peritonitis sometimes develops after repair of the bowel.25 This can be managed with prolonged bowel rest and peripheral or total parenteral nutrition.
Conservative management may be possible
Patients whose symptoms of bowel laceration become apparent after discharge can sometimes be managed conservatively. More than 50% of patients treated conservatively require no surgery.23 Inpatient management consists of monitoring the WBC count, providing hydration and IV antibiotics, and examining the patient every 6 hours, giving nothing by mouth.
When injury is discovered later
If conservative management with observation and bowel rest fails, or the patient complains of severe abdominal pain, vomiting, nausea, obstipation, or signs and symptoms of peritonitis, such as the patient in Case 3, immediate surgical intervention is necessary. When an injury is not detected until some time after initial surgery, resection of all necrotic tissue is mandatory. In most cases, the perforation is managed by segmental resection and reanastomosis. Evaluate the entire small and large bowel to rule out any other injury, and irrigate generously. Bowel rest, parenteral nutrition, and IV antibiotics also are indicated.
Of 36,928 procedures reported by members of the American Association of Gynecologic Laparoscopists, there were two deaths—both caused by unrecognized bowel injury.15
CASE 4: Large-bowel injury precipitates lengthy recovery
A surgeon performs a left laparoscopic salpingo-oophorectomy to remove an 8-cm ovarian endometrioma that is adherent to the rectosigmoid colon of a 40-year-old diabetic woman. Sharp and electrosurgical scissors are used to separate the adnexa from the rectosigmoid colon. No injury is observed, and she is discharged the same day. Four days later, she returns with severe abdominal pain, nausea, vomiting, and fatigue. Lab tests reveal a WBC count of 17,000; a CT scan shows pockets of air beneath the diaphragm, as well as fluid collection suggestive of a pelvic abscess.
Immediate laparotomy is performed, during which the surgeon discovers contamination of the abdominal viscera by bowel contents, as well as a 0.5-cm perforation of the rectosigmoid colon. The perforation is repaired in two layers after its edges are trimmed, and a diverting colostomy is performed. The patient is admitted to the ICU and requires antibiotic treatment, total parenteral nutrition, and bowel rest due to severe peritonitis. She gradually recovers and is discharged 3 weeks later. The diverting colostomy is reversed 3 months later.
Even small perforations in the large bowel can cause infection and abscess due to the high bacterial content of the colon. The most common cause of injury to the rectosigmoid colon is pelvic adhesiolysis during cul-de-sac dissection, treatment of pelvic endometriosis, and resection of adherent pelvic masses.
Sharp dissection with scissors or high-powered lasers is relatively safe near the bowel. When dissecting the cul-de-sac, identify the vagina and rectum by placing a probe or finger in each area. Begin dissection from the unaffected pararectal space, and proceed toward the obliterated cul-de-sac.27,28
Bowel prep is indicated before extensive pelvic surgery and when the history suggests endometriosis or significant pelvic adhesions. Some general surgeons base their decision to perform colostomy (or not) on whether the bowel was prepped preoperatively.29
If the large bowel is perforated by the Veress needle, the saline aspiration test will yield brownish fluid. When significant pelvic adhesiolysis or pelvic or endometriotic tumor resection is performed, inject air into the rectum afterward via a sigmoidoscope or bulb syringe and assess the submerged rectum and rectosigmoid colon for bubbling. The rectal wall may be weakened during these types of procedures, so instruct the patient to use oral stool softeners and avoid enemas.30
Delay in detection can have serious ramifications
When a large-bowel injury goes undetected at the time of operation, the patient generally presents on the third or fourth postoperative day with mild fever, occasionally sudden sharp epigastric pain, lower abdominal pain, slight nausea, and anorexia. By the fifth or sixth day, these symptoms have become more severe and are accompanied by peritonitis and an elevated WBC count.
Whenever a patient complains of abdominal pain and a deteriorating condition, assume that bowel injury is the cause until it is proved otherwise.
Intraoperative management
Repair small trocar wounds using primary suture closure. Copious lavage of the peritoneal cavity, drainage, and a broad-spectrum antibiotic minimize the risk of infection. Manage deep electrical injury to the right colon by resecting the injured segment and performing primary anastomosis. Primary closure or resection and reanastomosis may not be adequate when the vascular supply of the descending colon or rectum is compromised. In that case, perform a diverting colostomy or ileostomy, which can be reversed 6 to 12 weeks later.25,26
CASE 5: Vascular injury
A tall, thin, athletic 19-year-old undergoes diagnostic laparoscopy to rule out pelvic pathology after she complains of severe, monthly abdominal pain. Upon insertion of the laparoscope, the surgeon observes a large hematoma forming at the right pelvic sidewall. At the same time, the anesthesiologist reports a significant drop in blood pressure, and vascular injury is diagnosed. The surgeon attempts to control the bleeding using bipolar coagulation, but the problem only becomes worse. He decides to switch to laparotomy.
A vascular surgeon is called in, and injury to the right common iliac artery and vein—apparently caused during insertion of the primary umbilical trocar—is repaired. The patient is given 5 U of red blood cells. She goes home 10 days later, but returns with thrombophlebitis and rejection of the graft. After several surgeries, she finally recovers, with some sequelae, such as unilateral leg edema.
Management of vascular injury depends on the source and type of injury. On major vessels, electrocoagulation is contraindicated. After immediate atraumatic compression with tamponade to control bleeding, vascular repair, in consultation with a vascular surgeon, is indicated. At times, a vascular graft may be required.
Smaller vessels, such as the infundibulopelvic ligament or uterine vessels, can be managed by clips, suture, or loop ligatures. If thermal energy is used in the repair, be careful to avoid injury to surrounding structures.
Most emergency laparotomies are performed for uncontrolled bleeding.30,31 Lack of control or a wrong angle at insertion of the Veress needle and trocars is a major cause of large-vessel injury. Sharp dissection of adhesions, uterosacral ablation, transection of vascular pedicles without adequate dessication, and rough handling of tissues can all cause bleeding. Distorted anatomy is a main cause of vascular injury and can compound injury in areas more prone to bleeding, such as the oviduct, infundibulopelvic ligament, mesosalpinx, and pelvic sidewall vessels.
The return of pressure gradients to normal levels at the end of a procedure can be accompanied by bleeding into the retroperitoneal space, so evaluate the patient in a supine position after intra-abdominal pressure is reduced.
A vascular surgeon may be required
Depending on the type of vessel, size and location of the injury, and degree of bleeding, you may use unipolar or bipolar electrocoagulation, suture, clips, vasopressin, or loop ligatures to control bleeding. Although diluted vasopressin (10 U in 60 mL of lactated Ringer’s saline) can decrease oozing from raw peritoneal areas, injury to a major vessel, such as the iliac vessels, vena cava, or aorta, needs immediate control and proper repair. The decision to perform laparoscopy or laparotomy depends on your preference and experience. In any case, a vascular surgeon may be consulted for major vascular injuries.32
If a major vessel is injured, do not crush-clamp it. If possible (and if your laparoscopic skills are advanced), insert a sponge via a 10-mm trocar and apply pressure to the vessel to minimize bleeding and enhance visualization. The decision to repair the injury laparoscopically or by laparotomy should be made judiciously and promptly. n
The authors acknowledge the editorial contributions of Kristina Petrasek and Barbara Page, of the University of California, Berkeley, to the manuscript of this article.
To view three clips of surgical pearls for laparoscopy, visit the Video Library.
<huc>Q.</huc>What is the only surgical procedure that is completely safe?
<huc>A.</huc>The surgical procedure that is not performed.
The unfortunate truth is that complications can occur during any operative procedure, despite our best efforts—and laparoscopy is no exception. Being vigilant for iatrogenic injuries, both during and after surgery, and ensuring that repairs are both thorough and timely, are two of our best weapons against major complications, along with meticulous technique and adequate experience.
This article features five cases that illustrate some of the most serious complications of laparoscopy—and how to prevent and manage them.
CASE 1: Surgical patient returns with signs of ureteral injury
A 42-year-old woman with a history of endometriosis undergoes laparoscopic hysterectomy and bilateral salpingo-oophorectomy. She is discharged 2 days later. Two days after that, she returns to the hospital complaining of fluid leaking from the vagina. She has no fever or any other significant complaint or physical findings other than abdominal tenderness, which is to be expected after surgery. A computed tomography (CT) scan with intravenous (IV) contrast reveals left ureteral obstruction near the bladder, with extravasation of contrast media into the abdominal cavity. Further investigation reveals a left ureteral transection.
Could this injury have been avoided? How should it be managed?
Postoperative diagnosis of ureteral injury can be challenging, in part because up to 50% of unilateral cases are asymptomatic. Be on the lookout for this complication in women who have undergone pelvic sidewall dissection or laparoscopic hysterectomy, such as the patient in the case just described. As the number of laparoscopic hysterectomies and retroperitoneal procedures has risen in recent years, so has the rate of ureteral injury, with an incidence of 0.3% to 2%.1,2
Ureteral injury can be caused by ligation, ischemia, resection, transection, crushing, or angulation. Three sites are particularly troublesome: the infundibulopelvic ligament, ovarian fossa, and ureteral tunnel.3,4 In Case 1, injury to the ureter was proximal to the bladder and probably occurred during transection of the uterosacral cardinal ligament complex.
What’s the best preventive strategy?
Meticulous technique is imperative to protect the ureters. This includes adequate visualization, intraperitoneal or retroperitoneal dissection, and early identification of the ureter. In a high-risk patient likely to have distorted anatomy due to severe endometriosis and fibrosis, retroperitoneal dissection of any adhesions or tumor and identification of the ureter are the best ways to avoid injury.
Intraperitoneal identification and dissection of the ureters can be enhanced by hydrodissection and resection of the affected peritoneum.3,4 To create a safe operating plane, make a small opening in the peritoneum below the ureter and inject 50 to 100 mL of lactated Ringer’s solution along the course of the ureter, which will displace it laterally.5
Although neither IV indigo carmine nor ureteral catheterization has been shown to reduce the risk of ureteral injury or identify ligation or thermal injury,3,6 both can help the surgeon identify intraoperative perforation of the ureter. Liberal use of cystoscopy with indigo carmine administration for identification of ureteral flow and ureteral catheterization can be used in potentially high-risk patients. If there is suspicion for devascularization or thermal injury, use prophylactic ureteral stents postoperatively for 2 to 4 weeks.
Don’t hesitate to consult a urologist
In Case 1, the surgeon sought immediate urologic consultation and the patient underwent laparotomy with ureteroneocystotomy without sequelae.
In general, management of ureteral injury depends on its severity and location, as well as the comfort level of the surgeon. Minor injuries are sometimes managed with cystoscopic stent placement, but more severe cases may require operative ureteral repair.
In cases like this one, where ureteral injury occurred in close proximity to the bladder, a ureteroneocystotomy is possible. However, in more cephalad injuries, there may be insufficient residual ureter to allow such a repair. In these cases, a Boari flap may be attempted to use bladder tissue to bridge the gap to the ureteral edge. Rarely, in high ureteral injuries, trans-ureteroureterostomy may be appropriate. This procedure carries the greatest risk, given that both kidneys are reliant on one ureter.
Is laparoscopic repair reasonable?
When surgical intervention is necessary, the choice between laparoscopy and laparotomy depends on the skill and comfort level of the surgeon and the availability of instruments and support team.6,7 That said, ureteral injury is usually treated via laparotomy.1 As operative laparoscopy becomes even more commonplace, reconstruction of the urinary system will increasingly be managed laparoscopically.
Depending on the size and location of the injury, reconstruction may involve ureteral reimplantation with or without a psoas hitch, Boari flap, or primary endtoend anastomosis.8-10
CASE 2: Postoperative symptoms lead to rehospitalization
A 35-year-old patient undergoes laparoscopic ovarian cystectomy and returns home the same day. She is readmitted 72 hours later because of lower abdominal tenderness, worsening nausea and vomiting, and urine-like drainage from her midline suprapubic trocar site. Analysis of the leaking fluid shows high creatinine levels consistent with urine. The patient has no fever and is hemodynamically stable. Examination reveals a moderately distended abdomen with decreased bowel sounds. Hematuria is evident on urine analysis.
Urologic consultation is obtained, and the patient undergoes simultaneous laparoscopy and cystoscopy, during which perforation of the bladder dome is discovered, apparently caused by the mid suprapubic trocar. The bladder is mobilized anteriorly, and both anterior and posterior aspects of the perforation are repaired in one layer laparoscopically.
After continuous drainage with a transurethral Foley catheter for 7 days, cystography shows complete healing of the bladder, and the Foley catheter is removed. The patient recovers completely.
Vesical injury sometimes occurs in patients who have a history of laparotomy, a full bladder at the time of surgery, or displaced anatomy due to pelvic adhesions.11 Although bladder injury is rare, laparoscopy increases the risk. Trocars, uterine manipulators, and blunt instruments can perforate or lacerate the bladder, and energy devices can cause thermal injury. The risk of bladder injury increases during laparoscopic hysterectomy.
Be vigilant about trocar placement and dissection techniques
Accessory trocars can injure a full bladder. Injury can also occur when distorted anatomy from a previous pelvic operation obscures bladder boundaries, making insertion of the midline trocar potentially perilous (FIGURE 1). The Veress needle and Rubin’s cannula can perforate the bladder.11-13 And in the anterior cul-de-sac, adhesiolysis, deep coagulation, laser ablation, or sharp excision of endometriosis implants can predispose a patient to bladder injury.
In women with severe endometriosis, lower-segment myoma, or a history of cesarean section, the bladder is vulnerable to laceration when blunt dissection is used during laparoscopic hysterectomy or laparoscopically assisted vaginal hysterectomy (LAVH). A vesical injury also can occur at the time of laparoscopic bladder-neck suspension upon entry into, and dissection of, the space of Retzius.
FIGURE 1 A bladder at risk
In this patient with a previous cesarean section, the bladder is adherent to the anterior abdominal wall. Needle mapping in the conventional midline trocar position indicates that the trocar must be relocated to avoid bladder injury.
Intraoperative findings that suggest bladder injury include air in the urinary catheter, hematuria, trocar site drainage of urine, or indigo carmine leakage. Postoperative signs and symptoms include leaking from incisional sites, a mass in the abdominal wall, and abdominal swelling.
Liberal use of cystoscopy or distension of the bladder with 300 to 500 mL of normal saline is recommended whenever there is a suspicion of bladder injury, especially during laparoscopic hysterectomy or LAVH. When a trocar causes the injury, look for both entry and exit punctures, both of which should be treated.
No matter how much care is taken, some bladder injuries, such as vesicovaginal fistulae, become apparent only postoperatively. More rarely, peritonitis or pseudoascites herald the injury. Retrograde cystography may aid identification.
Treatment of bladder injuries
Small perforations recognized intraoperatively may be conservatively managed by postoperative bladder drainage for 5 to 7 days. Most other bladder injuries require prompt intervention. For example, trocar injury to the bladder dome requires one- or two-layer closure followed by 5 to 7 days of urinary drainage. (Both closing and healing are promoted by drainage.)
Laparoscopy or laparotomy? Laparoscopic repair has become increasingly common, and bladder injury is a common complication of LAVH.13,14
CASE 3: Postop pain, tachycardia
A 41-year-old obese woman undergoes laparoscopic cystectomy for an 8-cm left ovarian mass. The abdomen is entered on the second attempt with a long Veress needle. The umbilical trocar is reinserted “several” times because of difficulty opening the peritoneum with the tip of the trocar sheath. The surgical procedure is completed within 2 hours, and the patient is discharged 23 hours later.
The next day, she experiences increasing abdominal pain and presents to the emergency room. Upon admission she reports intermittent chills, but denies nausea and vomiting. She is in mild distress, pale and tachycardic, with a temperature of 96.4°, pulse of 117, respiration rate of 20, blood pressure of 106/64 mm Hg, and oxygen saturation of 92%. She also has a diffusely tender abdomen but normal blood work. Abdominal and chest x-rays show a large right subphrenic air-fluid level that is consistent with free intraperitoneal air, unsurprising given her recent surgery. Bibasilar atelectasis and consolidation are noted on the initial chest x-ray.
During observation over the next 2 days, she remains afebrile and tachycardic, but her shortness of breath becomes progressively worse. Neither spiral CT nor lower-extremity Doppler suggests pulmonary embolism or deep venous thrombosis. Supplemental oxygen, aggressive pain management, albuterol, ipratropium, and acetylcysteine are initiated after pulmonary consultation.
The patient tolerates a regular diet on postoperative day 3 and has a bowel movement on day 5. However, the same day she begins vomiting and reports worsening abdominal pain. CT imaging of the abdomen and pelvis reveals free air in the abdomen and loculated fluid with air bubbles suspicious for intra-abdominal infection and perforated bowel.
Exploratory laparotomy reveals diffuse feculent peritonitis, as well as food particles and contrast media. There is a perforation in the antimesenteric side of the ileum approximately 1.5 feet proximal to the ileocecal valve. This perforation measures approximately 1 cm in diameter and is freely spilling intestinal contents. Small bowel resection is performed to treat the perforation.
Following the surgery, the patient recovers slowly.
Could the bowel perforation have been detected sooner?
Intestinal tract injury is a serious complication, particularly with postoperative diagnosis.15 Damage can occur during insertion of the Veress needle or trocar when the bowel is immobilized by adhesions, or during enterolysis.16 Unrecognized thermal injury can cause delayed bowel injury.
Small-bowel damage often occurs during uncontrolled insertion of the Veress needle or primary umbilical trocar. It also may result from sharp dissection or thermal injury.17,18 Abrasions and lacerations can occur if traction is exerted on the bowel using serrated graspers. When adhesions are dense and tissue planes poorly defined, the risk of laceration due to energy sources or sharp dissection increases.
Be cautious during bowel manipulation. Avoid blunt dissection. Be especially careful when the small bowel is adherent to the anterior abdominal wall (FIGURE 2A), particularly during evaluation of patients with a history of bowel resection, exploratory laparotomy for trauma-related peritonitis, or tumor debulking.
Remove the primary and ancillary cannulas under direct visualization with the laparoscope to prevent formation of a vacuum that can draw bowel into the incision and cause herniation.19
FIGURE 2 Adherent bowel, minor bleeding
A: Veress needle pressure measurements are persistently elevated before primary trocar insertion in this patient, raising the suspicion of adhesive disease from earlier surgery. As a result, the primary trocar is relocated to the left upper quadrant. Inspection confirms that small bowel is adherent to the anterior abdominal wall.
FIGURE 2 Adherent bowel, minor bleeding
B: After the small-bowel adhesions are dissected off the anterior abdominal wall via laparoscopy, a small hematoma is discovered, likely caused by the Veress needle. The patient is managed conservatively and recovers.
The value of open laparoscopy
In open laparoscopy, an abdominal incision is made into the peritoneal cavity so that the trocar can be placed under direct vision, after which the abdomen is insufflated. This approach can prevent bowel injury only when the adhesions and attachments are to the anterior abdominal wall and away from the entry site. When the attachment lies directly beneath the umbilicus, however, open laparoscopy is no guarantee against injury.
When bowel adhesions are severe, use alternative trocar sites such as the left upper quadrant (Palmer’s point) for the Veress needle and primary trocars.5,20,21
The likelihood of perforation can be reduced with preoperative bowel prep when there is a risk of bowel adhesions.
Identifying bowel injury
We recommend routine inspection of the structures beneath the primary trocar upon insertion of the laparoscope to look for injury to the bowel, mesentery, or vascular structures. If adhesions are found, evaluate the area carefully to rule out injury to the bowel or omentum. It may be necessary to change the position of the laparoscope to assess the patient.
Trauma to the intestinal tract can be mechanical or electrical in nature, and each type of trauma creates a distinctive, characteristic pattern. Thermal injury can be subtle and present as simple blanching or a distinct burn and charring. A small hole or obvious tear in the bowel wall can be the result of mechanical injury.22
Benign-appearing, superficial thermal bowel injuries may be managed conservatively.22 Minimal serosal burns (smaller than 5 mm in diameter) can be managed expectantly. Immediate surgical intervention is needed if the area of blanching on the intestinal serosa exceeds 5 mm in diameter or if the burn appears to involve more than the serosa.23
Small-bowel injuries that escape notice intraoperatively generally become apparent 2 to 4 days later, when the patient develops fever, nausea, lower abdominal pain, and anorexia. On postoperative day 5 or 6, the white blood cell (WBC) count rises and earlier symptoms may become worse. Radiography may reveal multiple air and fluid levels—another sign of bowel injury. Be aware that if the patient has clinical symptoms of gastrointestinal injury, even if the WBC count is normal, exploratory laparoscopy or laparotomy is necessary for accurate diagnosis.
Intraoperatively discovered injury
Careful inspection may reveal no leakage or bleeding in the affected area. Small punctures or superficial lacerations seal readily and may not require further treatment (FIGURE 2B), but larger perforations require repair. Straightforward repair is not always possible when the injury is extensive and considerable time has elapsed before it is discovered.
Inspect the intestine thoroughly at the conclusion of a procedure; obvious leakage requires intervention. Repair the small intestine in one or two layers, using the initial row of interrupted sutures to approximate the mucosa and muscularis.24 To lessen the risk of stenosis, close all lacerations transversely when they are smaller than one half the diameter of the bowel. If the laceration exceeds that size, segmental resection and anastomosis are necessary. Resection is prudent if the mesenteric blood supply is compromised.25
When performing one-layer repair of the small bowel, delayed absorbable suture (eg, Vicryl or PDS) or nonabsorbable suture (eg, silk) is recommended.26
At the conclusion of a repair, copiously irrigate the entire abdomen. Place a nasogastric tube only if ileus is anticipated; the tube can be removed when drainage diminishes and active bowel sounds and flatus appear. Do not give anything by mouth until the patient has return of bowel function and active peristalsis. Prescribe prophylactic antibiotics.
Note that peritonitis sometimes develops after repair of the bowel.25 This can be managed with prolonged bowel rest and peripheral or total parenteral nutrition.
Conservative management may be possible
Patients whose symptoms of bowel laceration become apparent after discharge can sometimes be managed conservatively. More than 50% of patients treated conservatively require no surgery.23 Inpatient management consists of monitoring the WBC count, providing hydration and IV antibiotics, and examining the patient every 6 hours, giving nothing by mouth.
When injury is discovered later
If conservative management with observation and bowel rest fails, or the patient complains of severe abdominal pain, vomiting, nausea, obstipation, or signs and symptoms of peritonitis, such as the patient in Case 3, immediate surgical intervention is necessary. When an injury is not detected until some time after initial surgery, resection of all necrotic tissue is mandatory. In most cases, the perforation is managed by segmental resection and reanastomosis. Evaluate the entire small and large bowel to rule out any other injury, and irrigate generously. Bowel rest, parenteral nutrition, and IV antibiotics also are indicated.
Of 36,928 procedures reported by members of the American Association of Gynecologic Laparoscopists, there were two deaths—both caused by unrecognized bowel injury.15
CASE 4: Large-bowel injury precipitates lengthy recovery
A surgeon performs a left laparoscopic salpingo-oophorectomy to remove an 8-cm ovarian endometrioma that is adherent to the rectosigmoid colon of a 40-year-old diabetic woman. Sharp and electrosurgical scissors are used to separate the adnexa from the rectosigmoid colon. No injury is observed, and she is discharged the same day. Four days later, she returns with severe abdominal pain, nausea, vomiting, and fatigue. Lab tests reveal a WBC count of 17,000; a CT scan shows pockets of air beneath the diaphragm, as well as fluid collection suggestive of a pelvic abscess.
Immediate laparotomy is performed, during which the surgeon discovers contamination of the abdominal viscera by bowel contents, as well as a 0.5-cm perforation of the rectosigmoid colon. The perforation is repaired in two layers after its edges are trimmed, and a diverting colostomy is performed. The patient is admitted to the ICU and requires antibiotic treatment, total parenteral nutrition, and bowel rest due to severe peritonitis. She gradually recovers and is discharged 3 weeks later. The diverting colostomy is reversed 3 months later.
Even small perforations in the large bowel can cause infection and abscess due to the high bacterial content of the colon. The most common cause of injury to the rectosigmoid colon is pelvic adhesiolysis during cul-de-sac dissection, treatment of pelvic endometriosis, and resection of adherent pelvic masses.
Sharp dissection with scissors or high-powered lasers is relatively safe near the bowel. When dissecting the cul-de-sac, identify the vagina and rectum by placing a probe or finger in each area. Begin dissection from the unaffected pararectal space, and proceed toward the obliterated cul-de-sac.27,28
Bowel prep is indicated before extensive pelvic surgery and when the history suggests endometriosis or significant pelvic adhesions. Some general surgeons base their decision to perform colostomy (or not) on whether the bowel was prepped preoperatively.29
If the large bowel is perforated by the Veress needle, the saline aspiration test will yield brownish fluid. When significant pelvic adhesiolysis or pelvic or endometriotic tumor resection is performed, inject air into the rectum afterward via a sigmoidoscope or bulb syringe and assess the submerged rectum and rectosigmoid colon for bubbling. The rectal wall may be weakened during these types of procedures, so instruct the patient to use oral stool softeners and avoid enemas.30
Delay in detection can have serious ramifications
When a large-bowel injury goes undetected at the time of operation, the patient generally presents on the third or fourth postoperative day with mild fever, occasionally sudden sharp epigastric pain, lower abdominal pain, slight nausea, and anorexia. By the fifth or sixth day, these symptoms have become more severe and are accompanied by peritonitis and an elevated WBC count.
Whenever a patient complains of abdominal pain and a deteriorating condition, assume that bowel injury is the cause until it is proved otherwise.
Intraoperative management
Repair small trocar wounds using primary suture closure. Copious lavage of the peritoneal cavity, drainage, and a broad-spectrum antibiotic minimize the risk of infection. Manage deep electrical injury to the right colon by resecting the injured segment and performing primary anastomosis. Primary closure or resection and reanastomosis may not be adequate when the vascular supply of the descending colon or rectum is compromised. In that case, perform a diverting colostomy or ileostomy, which can be reversed 6 to 12 weeks later.25,26
CASE 5: Vascular injury
A tall, thin, athletic 19-year-old undergoes diagnostic laparoscopy to rule out pelvic pathology after she complains of severe, monthly abdominal pain. Upon insertion of the laparoscope, the surgeon observes a large hematoma forming at the right pelvic sidewall. At the same time, the anesthesiologist reports a significant drop in blood pressure, and vascular injury is diagnosed. The surgeon attempts to control the bleeding using bipolar coagulation, but the problem only becomes worse. He decides to switch to laparotomy.
A vascular surgeon is called in, and injury to the right common iliac artery and vein—apparently caused during insertion of the primary umbilical trocar—is repaired. The patient is given 5 U of red blood cells. She goes home 10 days later, but returns with thrombophlebitis and rejection of the graft. After several surgeries, she finally recovers, with some sequelae, such as unilateral leg edema.
Management of vascular injury depends on the source and type of injury. On major vessels, electrocoagulation is contraindicated. After immediate atraumatic compression with tamponade to control bleeding, vascular repair, in consultation with a vascular surgeon, is indicated. At times, a vascular graft may be required.
Smaller vessels, such as the infundibulopelvic ligament or uterine vessels, can be managed by clips, suture, or loop ligatures. If thermal energy is used in the repair, be careful to avoid injury to surrounding structures.
Most emergency laparotomies are performed for uncontrolled bleeding.30,31 Lack of control or a wrong angle at insertion of the Veress needle and trocars is a major cause of large-vessel injury. Sharp dissection of adhesions, uterosacral ablation, transection of vascular pedicles without adequate dessication, and rough handling of tissues can all cause bleeding. Distorted anatomy is a main cause of vascular injury and can compound injury in areas more prone to bleeding, such as the oviduct, infundibulopelvic ligament, mesosalpinx, and pelvic sidewall vessels.
The return of pressure gradients to normal levels at the end of a procedure can be accompanied by bleeding into the retroperitoneal space, so evaluate the patient in a supine position after intra-abdominal pressure is reduced.
A vascular surgeon may be required
Depending on the type of vessel, size and location of the injury, and degree of bleeding, you may use unipolar or bipolar electrocoagulation, suture, clips, vasopressin, or loop ligatures to control bleeding. Although diluted vasopressin (10 U in 60 mL of lactated Ringer’s saline) can decrease oozing from raw peritoneal areas, injury to a major vessel, such as the iliac vessels, vena cava, or aorta, needs immediate control and proper repair. The decision to perform laparoscopy or laparotomy depends on your preference and experience. In any case, a vascular surgeon may be consulted for major vascular injuries.32
If a major vessel is injured, do not crush-clamp it. If possible (and if your laparoscopic skills are advanced), insert a sponge via a 10-mm trocar and apply pressure to the vessel to minimize bleeding and enhance visualization. The decision to repair the injury laparoscopically or by laparotomy should be made judiciously and promptly. n
The authors acknowledge the editorial contributions of Kristina Petrasek and Barbara Page, of the University of California, Berkeley, to the manuscript of this article.
1. Ostrzenski A, Radolinski B, Ostrzenska K. A review of laparoscopic ureteral injury in pelvic surgery. Obstet Gynecol Surv. 2003;58:794-799.
2. Kabalin J. Chapter 1—Surgical anatomy of the retroperitoneum, kidneys, and ureters. In: Walsh P, Retik A, Wein A, eds. Campbell’s Urology. 8th ed. Philadelphia: Saunders; 2002 36-40.
3. Chan J, Morrow J, Manetta A. Prevention of ureteral injuries in gynecologic surgery. Am J Obstet Gynecol. 2003;188:1273-1277.
4. Grainger DA, Soderstrom RM, Schiff SF, Glickman MG, DeCherney AH, Diamond MP. Ureteral injuries at laparoscopy: insights into diagnosis, management, and prevention. Obstet Gynecol. 1990;75:839-843.
5. Nezhat C. Chapter 20. Operative Gynecologic Laparoscopy: Principles and Techniques. 2nd ed. New York: McGraw–Hill; 2000.
6. Ou CS, Huang IA, Rowbotham R. Laparoscopic ureteroureteral anastomosis for repair of ureteral injury involving stricture. Int Urogynecol J Pelvic Floor Dysfunct. 2005;16:155-157.
7. Modi P, Goel R, Dodiya S. Laparoscopic ureteroneocystostomy for distal ureteral injuries. Urology. 2005;66:751-753.
8. Nezhat C, Nezhat F, Nezhat CH, et al. Urinary tract endometriosis treated by laparoscopy. Fertil Steril. 1996;66:920-924.
9. Nezhat C, Nezhat F. Laparoscopic repair of ureter resected during operative laparoscopy. Obstet Gynecol. 1992;80:543-544.
10. Nezhat CH, Nezhat FR, Freiha F, Nezhat CR. Laparoscopic vesicopsoas hitch for infiltrative ureteral endometriosis. Fertil Steril. 1999;71:376-379.
11. Georgy FM, Fettman HH, Chefetz MD. Complications of laparoscopy: two cases of perforated urinary bladder. Am J Obstet Gynecol. 1974;120:1121-1122.
12. Sherer DM. Inadvertent transvaginal cystotomy during laparoscopy. Int J Gynaecol Obstet. 1990;32:77-79.
13. Nezhat CH, Seidman DS, Nezhat F, et al. Laparoscopic management of internal and unintentional cystotomy. J Urol. 1996;156:1400-1402.
14. Lee CL, Lai YM, Soong YK. Management of urinary bladder injuries in laparoscopic assisted vaginal hysterectomy. Acta Obstet Gynecol Scand. 1996;75:174-177.
15. Peterson HB, et al. American Association of Gynecologic Laparoscopists’ 1988 membership survey on operative laparoscopy. J Reprod Med. 1990;35:587-589.
16. Phillips JM, Hulka JF, Hulka B, et al. 1978 AAGL membership survey. J Reprod Med. 1981;26:529-533.
17. Chapron C, Pierre F, et al. Gastrointestinal injuries during laparoscopy. Hum Reprod. 1999;14:333-337.
18. Schrenk P, Woisetschlager R, Rieger R, et al. Mechanism, management, and prevention of laparoscopic bowel injuries. Gastrointest Endosc. 1996;43:572-574.
19. Sauer M, Jarrett JC. Small bowel obstruction following diagnostic laparoscopy. Fertil Steril. 1984;42:653-654.
20. Penfield AJ. How to prevent complications of open laparoscopy. J Reprod Med. 1985;30:660-663.
21. Brill A, Nezhat F, Nezhat CH, Nezhat C. The incidence of adhesions after prior laparatomy: a laparoscopic appraisal. Obstet Gynecol. 1995;85:269-272.
22. Levy BS, Soderstrom RM, Dail DH. Bowel injuries during laparoscopy: gross anatomy and histology. J Reprod Med. 1985;30:168-172.
23. Wheeless CR. Gastrointestinal injuries associated with laparoscopy. In: Phillips JM, ed. Endoscopy in Gynecology. Santa Fe Springs, Calif: American Association of Gynecologic Laparoscopists; 1978.
24. Borton M. Laparoscopic Complications: Prevention and Management. Philadelphia: Decker; 1986.
25. DeCherney AH. Laparoscopy with unexpected viscus penetration. In: Nichols DH, ed. Clinical Problems, Injuries, and Complications of Gynecologic Surgery. Baltimore: Williams & Wilkins; 1988.
26. Nezhat C, Nezhat F, Ambroze W, Pennington E. Laparoscopic repair of small bowel and colon: a report of 26 cases. Surg Endosc. 1993;7:88-89.
27. Redwine D. Laparoscopic en bloc resection for treatment of the obliterated cul-de-sac in endometriosis. J Reprod Med. 1992;37:696-698.
28. Nezhat C, Nezhat F, et al. Laparoscopic treatment of infiltrative rectosigmoid colon and rectovaginal septum endometriosis by the technique of videolaseroscopy and the CO2 laser. Br J Obstet Gynaecol. 1992;99:664-667.
29. Nezhat C, Seidman D, Nezhat F, et al. The role of intraoperative proctosigmoidoscopy in laparoscopic pelvic surgery. J Am Assoc Gynecol Laparosc. 2004;11:47-49.
30. Chapron CM, et al. Major vascular injuries during gynecologic laparoscopy. J Am Coll Surg. 1997;185:461-465.
31. Geers J, Holden C. Major vascular injury as a complication of laparoscopic surgery: a report of three cases and review of the literature. Am Surg. 1996;62:377-379.
32. Nezhat C, Childers J, Nezhat F, et al. Major retroperitoneal vascular injury during laparoscopic surgery. Hum Reprod. 1997;12:480-483.
Dr. Farr Nezhat reports no relevant financial relationships. Dr. Ceana Nezhat is a speaker and consultant for Karl Storz, Johnson & Johnson, Valleylab, US Surgical, and Viking. Dr. Camran Nezhat is a speaker for or receives educational support from Karl Storz, Stryker, Johnson & Johnson, Valleylab, and Baxter.
1. Ostrzenski A, Radolinski B, Ostrzenska K. A review of laparoscopic ureteral injury in pelvic surgery. Obstet Gynecol Surv. 2003;58:794-799.
2. Kabalin J. Chapter 1—Surgical anatomy of the retroperitoneum, kidneys, and ureters. In: Walsh P, Retik A, Wein A, eds. Campbell’s Urology. 8th ed. Philadelphia: Saunders; 2002 36-40.
3. Chan J, Morrow J, Manetta A. Prevention of ureteral injuries in gynecologic surgery. Am J Obstet Gynecol. 2003;188:1273-1277.
4. Grainger DA, Soderstrom RM, Schiff SF, Glickman MG, DeCherney AH, Diamond MP. Ureteral injuries at laparoscopy: insights into diagnosis, management, and prevention. Obstet Gynecol. 1990;75:839-843.
5. Nezhat C. Chapter 20. Operative Gynecologic Laparoscopy: Principles and Techniques. 2nd ed. New York: McGraw–Hill; 2000.
6. Ou CS, Huang IA, Rowbotham R. Laparoscopic ureteroureteral anastomosis for repair of ureteral injury involving stricture. Int Urogynecol J Pelvic Floor Dysfunct. 2005;16:155-157.
7. Modi P, Goel R, Dodiya S. Laparoscopic ureteroneocystostomy for distal ureteral injuries. Urology. 2005;66:751-753.
8. Nezhat C, Nezhat F, Nezhat CH, et al. Urinary tract endometriosis treated by laparoscopy. Fertil Steril. 1996;66:920-924.
9. Nezhat C, Nezhat F. Laparoscopic repair of ureter resected during operative laparoscopy. Obstet Gynecol. 1992;80:543-544.
10. Nezhat CH, Nezhat FR, Freiha F, Nezhat CR. Laparoscopic vesicopsoas hitch for infiltrative ureteral endometriosis. Fertil Steril. 1999;71:376-379.
11. Georgy FM, Fettman HH, Chefetz MD. Complications of laparoscopy: two cases of perforated urinary bladder. Am J Obstet Gynecol. 1974;120:1121-1122.
12. Sherer DM. Inadvertent transvaginal cystotomy during laparoscopy. Int J Gynaecol Obstet. 1990;32:77-79.
13. Nezhat CH, Seidman DS, Nezhat F, et al. Laparoscopic management of internal and unintentional cystotomy. J Urol. 1996;156:1400-1402.
14. Lee CL, Lai YM, Soong YK. Management of urinary bladder injuries in laparoscopic assisted vaginal hysterectomy. Acta Obstet Gynecol Scand. 1996;75:174-177.
15. Peterson HB, et al. American Association of Gynecologic Laparoscopists’ 1988 membership survey on operative laparoscopy. J Reprod Med. 1990;35:587-589.
16. Phillips JM, Hulka JF, Hulka B, et al. 1978 AAGL membership survey. J Reprod Med. 1981;26:529-533.
17. Chapron C, Pierre F, et al. Gastrointestinal injuries during laparoscopy. Hum Reprod. 1999;14:333-337.
18. Schrenk P, Woisetschlager R, Rieger R, et al. Mechanism, management, and prevention of laparoscopic bowel injuries. Gastrointest Endosc. 1996;43:572-574.
19. Sauer M, Jarrett JC. Small bowel obstruction following diagnostic laparoscopy. Fertil Steril. 1984;42:653-654.
20. Penfield AJ. How to prevent complications of open laparoscopy. J Reprod Med. 1985;30:660-663.
21. Brill A, Nezhat F, Nezhat CH, Nezhat C. The incidence of adhesions after prior laparatomy: a laparoscopic appraisal. Obstet Gynecol. 1995;85:269-272.
22. Levy BS, Soderstrom RM, Dail DH. Bowel injuries during laparoscopy: gross anatomy and histology. J Reprod Med. 1985;30:168-172.
23. Wheeless CR. Gastrointestinal injuries associated with laparoscopy. In: Phillips JM, ed. Endoscopy in Gynecology. Santa Fe Springs, Calif: American Association of Gynecologic Laparoscopists; 1978.
24. Borton M. Laparoscopic Complications: Prevention and Management. Philadelphia: Decker; 1986.
25. DeCherney AH. Laparoscopy with unexpected viscus penetration. In: Nichols DH, ed. Clinical Problems, Injuries, and Complications of Gynecologic Surgery. Baltimore: Williams & Wilkins; 1988.
26. Nezhat C, Nezhat F, Ambroze W, Pennington E. Laparoscopic repair of small bowel and colon: a report of 26 cases. Surg Endosc. 1993;7:88-89.
27. Redwine D. Laparoscopic en bloc resection for treatment of the obliterated cul-de-sac in endometriosis. J Reprod Med. 1992;37:696-698.
28. Nezhat C, Nezhat F, et al. Laparoscopic treatment of infiltrative rectosigmoid colon and rectovaginal septum endometriosis by the technique of videolaseroscopy and the CO2 laser. Br J Obstet Gynaecol. 1992;99:664-667.
29. Nezhat C, Seidman D, Nezhat F, et al. The role of intraoperative proctosigmoidoscopy in laparoscopic pelvic surgery. J Am Assoc Gynecol Laparosc. 2004;11:47-49.
30. Chapron CM, et al. Major vascular injuries during gynecologic laparoscopy. J Am Coll Surg. 1997;185:461-465.
31. Geers J, Holden C. Major vascular injury as a complication of laparoscopic surgery: a report of three cases and review of the literature. Am Surg. 1996;62:377-379.
32. Nezhat C, Childers J, Nezhat F, et al. Major retroperitoneal vascular injury during laparoscopic surgery. Hum Reprod. 1997;12:480-483.
Dr. Farr Nezhat reports no relevant financial relationships. Dr. Ceana Nezhat is a speaker and consultant for Karl Storz, Johnson & Johnson, Valleylab, US Surgical, and Viking. Dr. Camran Nezhat is a speaker for or receives educational support from Karl Storz, Stryker, Johnson & Johnson, Valleylab, and Baxter.
Evaluating Primary Care Providers' Responses to Serum Alanine Aminotransferase Elevations
Initial Steps in Treating the Male Patient
A Woman With Unilateral Knee Pain in the Absence of Arthritis or Trauma
Your questions and concerns addressed: Is it time for electronic medical records in your practice?
An ObGyn reported the following signs of a problem to a colleague: “Our practice was literally drowning in paperwork. An exam room was recently converted to hold more charts, and 2 warehouses held our overflow. Employees were constantly searching for records, and telephone messages were delayed for hours or days until the chart could be reviewed. Notoriously bad handwriting and incomplete documentation hampered good communication and good medical care. Transcription costs were out of control. Forms helped but added to the ongoing costs and storage problems.”
What are the treatment options?
Electronic medical records (EMR) have progressed from arcane, slow, cumbersome documentation systems to sophisticated, complex, comprehensive ones. These modern systems hold the potential to reduce administrative and management costs by 30% or more, improve clinical workflow, reduce medical errors, facilitate communication between patient and physician, and enable analysis of data for best practice methods, best outcomes and identifying risks and complications.
For practices like the one described in the preceding paragraph—not a fictional account but actual testimony provided by an ObGyn—EMR offer a powerful potential solution to the problems that result from an overwhelming amount of paper documentation, correspondence, charting, claims, and financial transactions. In this article, I offer a general introduction to EMR; in the next (August) issue of OBG Management, I’ll speak with a group of ObGyns and medical practice managers about their experiences—and inexperience—with EMR.
Progress and paradox
Physicians and scientists have made substantial progress over the past 25 years in pharmacotherapeutics, diagnostic technology, procedures, and treatment protocols. In obstetrics and gynecology alone, consider the array of technologies—3-dimensional ultrasonography, minimally invasive surgery, receptor-specific drugs, in vitro fertilization, long-acting reversible contraceptives—that have advanced the quality and effectiveness of care. Yet little progress has been made in the process of caring for patients.
The fact is that physicians, and other health-care providers, are rooted in paper-based processes that sustain inefficiencies, increase costs, and defy the gains that other industries have made by adopting electronic technologies for handling information. Why are we so stuck?
The state of EMR
EMR—of varying functionality—have been available for longer than 20 years. Early models were developed by physicians who had an interest in software coding and design, and were of limited functionality, arcane, and difficult to use in a clinical setting. Some of those early models, and even a few commercial systems in use today, rely on scanning paper documents into computer files. Such systems may eliminate some paper and facilitate document retrieval, but they do nothing to ease management of the complex transactions of health care, and they do not address handwriting illegibility.
Development of complex EMR systems was limited by primitive technology, inadequate distribution channels, and programming that was cumbersome and expensive to maintain. But these barriers have been overcome with fast processors, inexpensive and abundant memory, broadband Internet connectivity, and programming languages that facilitate automated software development.
Modern EMR systems are not simply data repositories: They also support workflow from the beginning to the end of a patient’s consultation with a health-care provider—an event that generates multiple transactions with multiple recipients. A single consultation may, for example, generate orders for lab tests, imaging studies, a surgical procedure, consultation with other physicians, prescriptions, and counseling, and record the subsequent financial transaction. EMR systems by necessity interact with multiple organizations, institutions, instruments, and other software systems. To software developers, and to the clinicians who use their systems, the challenge is to deftly navigate the complexities of health care.
Forces accelerating adoption
Momentum from the Executive Office. In 2004, President George W. Bush set a goal: nationwide adoption of EMR—to include all medical practices—within a decade. In a speech that year at Vanderbilt University Medical Center, the President said: “One of the amazing discrepancies in American society today is we’re literally changing how medicine is delivered in incredibly positive ways, and yet docs are still spending a lot of time writing things on paper.”1
Certifying body arises from the private sector. Subsequently, the US Department of Health and Human Services (HHS) established the Office of the National Coordinator for Health Information Technology and the American Health Information Community. The sweeping goal of these bodies? Better health care by application of information technology and creation of standards for certifying EMR systems that provide core functionality.
In response, 3 private sector health information management groups jointly formed the Certification Commission for Healthcare Information Technology (CCHIT; www.cchit.org). In 2005, this private-sector entity entered into a contract with HHS, to, in the commission’s words, “develop and evaluate certification criteria and create an inspection process for healthcare information technology” in 3 areas:
- Ambulatory EMR for offices
- Inpatient EMR for hospitals and health systems
- The network components through which EMR share information.
Pay-for-performance pushes the issue. Today, insurers—federal and private— are mandating adherence to standards of care for maximal reimbursement of services. These reimbursement schemes, called pay-for-performance, or P4P, are based on providers delivering documentation that specific protocols are followed and outcomes are monitored. The point is that it will be nearly impossible for physicians to comply with insurers’ P4P requirements unless that documentation is in an electronic format.
The market speaks—loudly. Other forces are bringing clinicians to a reckoning with EMR:
- Some malpractice carriers offer a discount on premiums to physicians who document work using EMR
- Patients are asking for electronic access to their providers by way of Web sites and e-mail
- More and more requests for documentation from multiple interested parties to a patient’s care increase overhead costs and place greater demands on paper-based systems.
Reticence has been the watchword
Despite the external and internal forces that are driving adoption, physicians have, as a whole, been reticent to adopt EMR. The nonprofit Healthcare Information and Management Systems Society (HIMSS) reports that 26% of ambulatory practices have adopted EMR, but this penetration is predominantly in multi-specialty clinics and hospital-owned practices.2 Few data exist on the penetration of EMR in single-specialty ObGyn practices; anecdotally, vendors estimate a penetration of 10% to 15%.
Why this slow pace toward something broadly acknowledged as key to the well-being of health care?
It means a change. Adopting EMR represents change; well-designed EMR systems streamline workflow in a practice by automating many functions, eliminating duplications of effort, and shifting roles from moving paper to managing digital information. Fear of change and resistance to change are the most common reasons that single-specialty ObGyn practices have not adopted EMR.
It costs. Expense is often cited as the reason why a practice has not adopted an EMR. True: Upfront hardware costs, software costs (license fees, subscription fees), implementation fees, and training costs add up. But a well-designed EMR system should provide a substantial return on investment (ROI) based on savings and on an increase in revenue.
It may be awkward. Some physicians cannot type well. They do not adopt EMR, therefore, because they fear embarrassment using a computer to enter clinical documentation in the consultation room in front of a patient.
On the plus side
On the other side of the coin, the advantages of EMR to physicians are several:
Documentation. EMR facilitate complete documentation of a patient’s visit, current needs and care plan, and record—thereby reducing the clinician’s liability and the risk of medical error. Functions include order entry, prescribing, accurate coding based on work-effort, tracking of outstanding lab tests, and notification.
No chart pulls. With EMR, patient chart pulls are almost nonexistent. A chart is available anywhere a computer is located, any time it is needed.
Decision-making. Probably most importantly, EMR provide clinical decision support by means of alerts (drug interactions, allergies) and reminders (need for follow-up, test orders).
Portal to the patient. Internet-accessed portals that are part of EMR systems facilitate asynchronous communication with patients. A patient can make an appointment, refill a prescription, and request educational materials through such a Web portal. Once an appointment is scheduled, the patient can enter her medical history so that it is specific to the appointment—a feature that is particularly useful when a woman knows the reason that she is visiting the ObGyn (“I’m pregnant,” “My cycle has changed”).
Such a patient-entered history can populate the EMR and contribute elements for appropriate coding. Furthermore, a Web portal in an EMR system enables the physician to reply to a patient with secure messages 1) about lab results, reminders, and appointments and 2) that deliver educational materials.
Keys to embarking on a successful transition
Because EMR are still used by only a small minority of practices, those that seek to move away from the paper record are almost always doing so for the first time. Uncertainty about the adoption adds to anxiety. There are, however, simple steps to take to maintain control over the adoption process and methodically manage it to a successful outcome.
Begin with the end in mind. The goal of adoption is not to purchase an EMR system; EMR are only a tool. The goal of the practice should be to transform its existing workflows to make significant improvements over the status quo. Before ever looking at an EMR system, you (and your colleagues, when applicable) must answer several key questions:
- What are we trying to accomplish?
- What is it about the status quo that we want to change?
- How will we measure success a year after completing the transition?
- Do you know whether they are ready for change?
- Do they understand change?
- Are they threatened by it?
- Is there broad and vocal leadership backing the impending changes?
- Has the impact of the change been discussed with all people involved so they have a clear understanding of its impact on their personal future?
- And is the practice, as a team, prepared to go through the turmoil of change as a necessary step on a path to transformation?
A medical practice that addresses these 3 initial tasks sets itself up for a successful transition from paper to EMR. A good plan—in which goals are well defined and a sense of urgency is consistently communicated and supported by the practice’s leadership—has an excellent chance of resulting in the best possible selection and implementation of an EMR system and accomplishing the goals set for the practice.
In contrast, a transition from paper that begins with such a vague notion as “I guess we need an EMR eventually, so we might as well start now” is much more likely to spark turmoil among staff. The staff then embarks on a selection and implementation process that is heavily influenced by emotion and interpractice politics. They face a diminished opportunity for completing the transition efficiently and successfully.
Throughout this process, the staff should always bear in mind that this is a transformation that they plan, control, and execute. EMR are simply a means to an end—not the end itself.
Two types of systems, various material needs
There are 2 primary configurations of EMR systems: client-server applications and remote-hosted systems. The latter operate through an Application Service Provider (ASP). (See “What are the 2 types of EMR?”)
In addition to type of system, keep these material needs in mind as you plan:
Connectivity. Most medical practices rely on the Internet for a variety of functions; truly, the Internet has become a vital link in health-care information technology.
An ASP system depends on the Internet, whereas client-server applications require a dial-up modem connection or other Internet connectivity to obtain information from outside sources. I recommend purchase of broadband Internet connectivity because it facilitates transmission of large files, such as images and data-rich documents.
Hardware. All EMR require computers for data entry. One attractive option for a medical practice is the TabletPC, which is available from several manufacturers and which uses the Windows XP Tablet PC Edition operating system. Combined with a secure wireless network for moving from room to room, the TabletPC is a technological breakthrough for physicians to document information in a clinical setting. It permits cursive data entry using a special electronic pen, voice recognition entry, and keyboard entry. Whereas a desktop computer places a barrier—the monitor or screen—between physician and patient, a TabletPC emulates the flat, horizontal surface of a paper chart or clipboard.
Importantly, a TabletPC has all the functionality of a desktop computer. Although workflow varies from practice to practice, it can be said generally that most clinical personnel work best with a TabletPC because of its mobility and most clerical personnel work easily with a desktop computer.
The price of a TabletPC? Two to 4 times that of a desktop computer.
Infrastructure. Other devices—printers, scanners, wireless networks, digital cameras—are required to operate an EMR system. A practice that uses a client-server application must purchase a data server. One with a system that operates by remote access either requires a virtual private network (VPN) for secure Internet connection or must install an emulator (such as Citrix).
People. Physicians and staff in the practice are always the key to success when implementing an EMR system. Consider a vendor’s ability to assist with the human variables of change management when you assess systems. Even the best EMR cannot save a practice from a poorly planned and executed transformation.
The 2 primary configurations of an electronic medical records system reflect the way that the system holds, handles, and delivers data.
Client-server application. This type of EMR resides on-site. The medical practice owns the software and hardware and is responsible for data backup, disaster recovery, database maintenance, security, Internet distribution for remote access, and information technology (IT) support. The practice is responsible for loading maintenance and upgrade updates into computers.
Client-server applications are usually sold as an upfront purchase with annual upgrade and maintenance fees that are 18% to 22% of initial cost.
Because the cost of EMR downtime is so high to a practice, you must budget for professional IT staff to establish and maintain high-availability (redundant) servers, Internet access, business continuity plans, network and database administration, security and intrusion detection plans, and data backup.
Remote-hosted system. An Application Service Provider (ASP) system is hosted from a remote data center and distributed through the Internet. Upgrades are deployed regularly to subscribers by the vendor, also by way of the Internet, without need for the practice to install disks or make changes to the server. Data are stored at data centers and backed up in real time. Data backups are maintained at remote sites for disaster recovery. An ASP system enables physicians to have access to patient records at any location that has Internet availability.
ASP systems are usually sold by monthly subscription, which includes fees for upgrades, support, maintenance, security, data backup, and data storage. They are especially attractive to small or medium-sized practices (as many as 30 physicians).
Going shopping
Many physicians want an EMR system to support the conventional process in their practice. This is a prescription for failure! Instead, evaluate the design of an EMR system for its ability to facilitate change in the process and in roles, and to eliminate manual functions and analysis of data.
One EMR system may appear to be the same as the next, but differences are revealed in the way that they optimize workflow. Generic systems may support several primary care medical specialties well, but may impose inefficiencies in other specialties—particularly in niche specialties. Similarly, a specialty-specific system works well for the specialty for which it was designed but is inefficient in another specialty. Approximately 80% of clinical workflow needs may be met by a generic EMR system, but the 20% that are specialty-specific can make the difference between success and failure.
Ensure that the sales presentations you attend address:
- the needs of your practice
- the criteria for success that you defined during planning.
When you arrive at a choice of an EMR system, assess the learning curve that you’ll have to climb for the system to become fully functional. Remember that salesperson? He, or she, gave a slick presentation but you didn’t settle for a dry description; you were sure to try the system live to discover how easily you can learn to navigate its functions.
At last: Implementation and training
EMR vendors develop implementation and training programs to establish their system in a practice and bring it live. These programs are based on vendors’ experience with their product.
As you prepare for your EMR system, keep this in mind: One that’s been well-designed is more than a repository of data. By design, it also reengineers workflow for optimal practice efficiency, safety, and financial management.
Pearls for welcoming a system
Use EMR incrementally. Do not change from paper to electronic abruptly while you, your colleagues, and the staff are learning the system. Vendors of EMR systems design pathways by which physicians ease-in, so to speak, to EMR in a way that minimizes any drop in productivity and loss of revenue. It’s wise to ask vendors about their plan for implementation and training before you sign a contract to purchase.
Expect that it will take 12 to 24 months to convert the practice completely. The time from paper to electronic records depends on the size and age of a practice. In my experience, the half-life of converting an ObGyn practice is approximately 15 months; by 24 months, the records of approximately 95% of active patients will be entered into EMR.
Don’t scan all paper records into EMR. Scanning indiscriminately is expensive, disruptive, and doesn’t contribute to ongoing clinical excellence. As part of the conversion process, vendors have methods to enter critical clinical information into the electronic system for uninterrupted use. Instead of wholesale scanning, therefore, be selective and scan only clinically relevant materials—the past several Pap smear results, mammogram reports, operative reports, consultant letters, and similar predefined clinical documents. This usually suffices for ongoing clinical care and avoids excessive expenditure of time, energy, and money.
The promise we talk about needs to become actual
As I noted at the outset, fewer than 25% of physicians have EMR, and estimates are that no more than 10% to 15% of ObGyns have adopted a system. Yet experience has demonstrated: A well-designed EMR offers physicians streamlined workflow, the ability to provide better care, and more time for leisure.
To move the flow and utility of medical information properly into this century, the next step, I urge, is for physicians to recognize the value of EMR, set goals for implementing a system, and reengineer their practice for maximal clinical efficiency, patient safety, and financial gain.
CASE REVISITED: Good outcome; no recurrence
One year later, the ObGyn whose practice was in disarray told a different story: “The ‘Patient Portal’ section of our EMR system is a great time saver. We were amazed at the acceptance and rapid adoption—even our octogenarians love it. The universal access to data is of incalculable value. One of our physicians loves to go home early, have dinner, and then review his charts from home. The EMR improves my recordkeeping, makes encounter documentation more complete, and helps me avoid medication errors. Our billing staff loves the thorough documentation.”
1. USA Today On-Line. http://www.usatoday.com/news/politicselections/nation/president/2004-05-27-bush-medical-records_x.htm. May 27, 2004. Accessed June 15, 2007.
2. Healthcare Information and Management Systems Society. Ambulatory Care: Latest trends. http://www.himss.org/ASP/topics_FocusDynamic.asp?ffilename="1907OBGM_Article4" aid=190. Accessed June 19, 2007.
An ObGyn reported the following signs of a problem to a colleague: “Our practice was literally drowning in paperwork. An exam room was recently converted to hold more charts, and 2 warehouses held our overflow. Employees were constantly searching for records, and telephone messages were delayed for hours or days until the chart could be reviewed. Notoriously bad handwriting and incomplete documentation hampered good communication and good medical care. Transcription costs were out of control. Forms helped but added to the ongoing costs and storage problems.”
What are the treatment options?
Electronic medical records (EMR) have progressed from arcane, slow, cumbersome documentation systems to sophisticated, complex, comprehensive ones. These modern systems hold the potential to reduce administrative and management costs by 30% or more, improve clinical workflow, reduce medical errors, facilitate communication between patient and physician, and enable analysis of data for best practice methods, best outcomes and identifying risks and complications.
For practices like the one described in the preceding paragraph—not a fictional account but actual testimony provided by an ObGyn—EMR offer a powerful potential solution to the problems that result from an overwhelming amount of paper documentation, correspondence, charting, claims, and financial transactions. In this article, I offer a general introduction to EMR; in the next (August) issue of OBG Management, I’ll speak with a group of ObGyns and medical practice managers about their experiences—and inexperience—with EMR.
Progress and paradox
Physicians and scientists have made substantial progress over the past 25 years in pharmacotherapeutics, diagnostic technology, procedures, and treatment protocols. In obstetrics and gynecology alone, consider the array of technologies—3-dimensional ultrasonography, minimally invasive surgery, receptor-specific drugs, in vitro fertilization, long-acting reversible contraceptives—that have advanced the quality and effectiveness of care. Yet little progress has been made in the process of caring for patients.
The fact is that physicians, and other health-care providers, are rooted in paper-based processes that sustain inefficiencies, increase costs, and defy the gains that other industries have made by adopting electronic technologies for handling information. Why are we so stuck?
The state of EMR
EMR—of varying functionality—have been available for longer than 20 years. Early models were developed by physicians who had an interest in software coding and design, and were of limited functionality, arcane, and difficult to use in a clinical setting. Some of those early models, and even a few commercial systems in use today, rely on scanning paper documents into computer files. Such systems may eliminate some paper and facilitate document retrieval, but they do nothing to ease management of the complex transactions of health care, and they do not address handwriting illegibility.
Development of complex EMR systems was limited by primitive technology, inadequate distribution channels, and programming that was cumbersome and expensive to maintain. But these barriers have been overcome with fast processors, inexpensive and abundant memory, broadband Internet connectivity, and programming languages that facilitate automated software development.
Modern EMR systems are not simply data repositories: They also support workflow from the beginning to the end of a patient’s consultation with a health-care provider—an event that generates multiple transactions with multiple recipients. A single consultation may, for example, generate orders for lab tests, imaging studies, a surgical procedure, consultation with other physicians, prescriptions, and counseling, and record the subsequent financial transaction. EMR systems by necessity interact with multiple organizations, institutions, instruments, and other software systems. To software developers, and to the clinicians who use their systems, the challenge is to deftly navigate the complexities of health care.
Forces accelerating adoption
Momentum from the Executive Office. In 2004, President George W. Bush set a goal: nationwide adoption of EMR—to include all medical practices—within a decade. In a speech that year at Vanderbilt University Medical Center, the President said: “One of the amazing discrepancies in American society today is we’re literally changing how medicine is delivered in incredibly positive ways, and yet docs are still spending a lot of time writing things on paper.”1
Certifying body arises from the private sector. Subsequently, the US Department of Health and Human Services (HHS) established the Office of the National Coordinator for Health Information Technology and the American Health Information Community. The sweeping goal of these bodies? Better health care by application of information technology and creation of standards for certifying EMR systems that provide core functionality.
In response, 3 private sector health information management groups jointly formed the Certification Commission for Healthcare Information Technology (CCHIT; www.cchit.org). In 2005, this private-sector entity entered into a contract with HHS, to, in the commission’s words, “develop and evaluate certification criteria and create an inspection process for healthcare information technology” in 3 areas:
- Ambulatory EMR for offices
- Inpatient EMR for hospitals and health systems
- The network components through which EMR share information.
Pay-for-performance pushes the issue. Today, insurers—federal and private— are mandating adherence to standards of care for maximal reimbursement of services. These reimbursement schemes, called pay-for-performance, or P4P, are based on providers delivering documentation that specific protocols are followed and outcomes are monitored. The point is that it will be nearly impossible for physicians to comply with insurers’ P4P requirements unless that documentation is in an electronic format.
The market speaks—loudly. Other forces are bringing clinicians to a reckoning with EMR:
- Some malpractice carriers offer a discount on premiums to physicians who document work using EMR
- Patients are asking for electronic access to their providers by way of Web sites and e-mail
- More and more requests for documentation from multiple interested parties to a patient’s care increase overhead costs and place greater demands on paper-based systems.
Reticence has been the watchword
Despite the external and internal forces that are driving adoption, physicians have, as a whole, been reticent to adopt EMR. The nonprofit Healthcare Information and Management Systems Society (HIMSS) reports that 26% of ambulatory practices have adopted EMR, but this penetration is predominantly in multi-specialty clinics and hospital-owned practices.2 Few data exist on the penetration of EMR in single-specialty ObGyn practices; anecdotally, vendors estimate a penetration of 10% to 15%.
Why this slow pace toward something broadly acknowledged as key to the well-being of health care?
It means a change. Adopting EMR represents change; well-designed EMR systems streamline workflow in a practice by automating many functions, eliminating duplications of effort, and shifting roles from moving paper to managing digital information. Fear of change and resistance to change are the most common reasons that single-specialty ObGyn practices have not adopted EMR.
It costs. Expense is often cited as the reason why a practice has not adopted an EMR. True: Upfront hardware costs, software costs (license fees, subscription fees), implementation fees, and training costs add up. But a well-designed EMR system should provide a substantial return on investment (ROI) based on savings and on an increase in revenue.
It may be awkward. Some physicians cannot type well. They do not adopt EMR, therefore, because they fear embarrassment using a computer to enter clinical documentation in the consultation room in front of a patient.
On the plus side
On the other side of the coin, the advantages of EMR to physicians are several:
Documentation. EMR facilitate complete documentation of a patient’s visit, current needs and care plan, and record—thereby reducing the clinician’s liability and the risk of medical error. Functions include order entry, prescribing, accurate coding based on work-effort, tracking of outstanding lab tests, and notification.
No chart pulls. With EMR, patient chart pulls are almost nonexistent. A chart is available anywhere a computer is located, any time it is needed.
Decision-making. Probably most importantly, EMR provide clinical decision support by means of alerts (drug interactions, allergies) and reminders (need for follow-up, test orders).
Portal to the patient. Internet-accessed portals that are part of EMR systems facilitate asynchronous communication with patients. A patient can make an appointment, refill a prescription, and request educational materials through such a Web portal. Once an appointment is scheduled, the patient can enter her medical history so that it is specific to the appointment—a feature that is particularly useful when a woman knows the reason that she is visiting the ObGyn (“I’m pregnant,” “My cycle has changed”).
Such a patient-entered history can populate the EMR and contribute elements for appropriate coding. Furthermore, a Web portal in an EMR system enables the physician to reply to a patient with secure messages 1) about lab results, reminders, and appointments and 2) that deliver educational materials.
Keys to embarking on a successful transition
Because EMR are still used by only a small minority of practices, those that seek to move away from the paper record are almost always doing so for the first time. Uncertainty about the adoption adds to anxiety. There are, however, simple steps to take to maintain control over the adoption process and methodically manage it to a successful outcome.
Begin with the end in mind. The goal of adoption is not to purchase an EMR system; EMR are only a tool. The goal of the practice should be to transform its existing workflows to make significant improvements over the status quo. Before ever looking at an EMR system, you (and your colleagues, when applicable) must answer several key questions:
- What are we trying to accomplish?
- What is it about the status quo that we want to change?
- How will we measure success a year after completing the transition?
- Do you know whether they are ready for change?
- Do they understand change?
- Are they threatened by it?
- Is there broad and vocal leadership backing the impending changes?
- Has the impact of the change been discussed with all people involved so they have a clear understanding of its impact on their personal future?
- And is the practice, as a team, prepared to go through the turmoil of change as a necessary step on a path to transformation?
A medical practice that addresses these 3 initial tasks sets itself up for a successful transition from paper to EMR. A good plan—in which goals are well defined and a sense of urgency is consistently communicated and supported by the practice’s leadership—has an excellent chance of resulting in the best possible selection and implementation of an EMR system and accomplishing the goals set for the practice.
In contrast, a transition from paper that begins with such a vague notion as “I guess we need an EMR eventually, so we might as well start now” is much more likely to spark turmoil among staff. The staff then embarks on a selection and implementation process that is heavily influenced by emotion and interpractice politics. They face a diminished opportunity for completing the transition efficiently and successfully.
Throughout this process, the staff should always bear in mind that this is a transformation that they plan, control, and execute. EMR are simply a means to an end—not the end itself.
Two types of systems, various material needs
There are 2 primary configurations of EMR systems: client-server applications and remote-hosted systems. The latter operate through an Application Service Provider (ASP). (See “What are the 2 types of EMR?”)
In addition to type of system, keep these material needs in mind as you plan:
Connectivity. Most medical practices rely on the Internet for a variety of functions; truly, the Internet has become a vital link in health-care information technology.
An ASP system depends on the Internet, whereas client-server applications require a dial-up modem connection or other Internet connectivity to obtain information from outside sources. I recommend purchase of broadband Internet connectivity because it facilitates transmission of large files, such as images and data-rich documents.
Hardware. All EMR require computers for data entry. One attractive option for a medical practice is the TabletPC, which is available from several manufacturers and which uses the Windows XP Tablet PC Edition operating system. Combined with a secure wireless network for moving from room to room, the TabletPC is a technological breakthrough for physicians to document information in a clinical setting. It permits cursive data entry using a special electronic pen, voice recognition entry, and keyboard entry. Whereas a desktop computer places a barrier—the monitor or screen—between physician and patient, a TabletPC emulates the flat, horizontal surface of a paper chart or clipboard.
Importantly, a TabletPC has all the functionality of a desktop computer. Although workflow varies from practice to practice, it can be said generally that most clinical personnel work best with a TabletPC because of its mobility and most clerical personnel work easily with a desktop computer.
The price of a TabletPC? Two to 4 times that of a desktop computer.
Infrastructure. Other devices—printers, scanners, wireless networks, digital cameras—are required to operate an EMR system. A practice that uses a client-server application must purchase a data server. One with a system that operates by remote access either requires a virtual private network (VPN) for secure Internet connection or must install an emulator (such as Citrix).
People. Physicians and staff in the practice are always the key to success when implementing an EMR system. Consider a vendor’s ability to assist with the human variables of change management when you assess systems. Even the best EMR cannot save a practice from a poorly planned and executed transformation.
The 2 primary configurations of an electronic medical records system reflect the way that the system holds, handles, and delivers data.
Client-server application. This type of EMR resides on-site. The medical practice owns the software and hardware and is responsible for data backup, disaster recovery, database maintenance, security, Internet distribution for remote access, and information technology (IT) support. The practice is responsible for loading maintenance and upgrade updates into computers.
Client-server applications are usually sold as an upfront purchase with annual upgrade and maintenance fees that are 18% to 22% of initial cost.
Because the cost of EMR downtime is so high to a practice, you must budget for professional IT staff to establish and maintain high-availability (redundant) servers, Internet access, business continuity plans, network and database administration, security and intrusion detection plans, and data backup.
Remote-hosted system. An Application Service Provider (ASP) system is hosted from a remote data center and distributed through the Internet. Upgrades are deployed regularly to subscribers by the vendor, also by way of the Internet, without need for the practice to install disks or make changes to the server. Data are stored at data centers and backed up in real time. Data backups are maintained at remote sites for disaster recovery. An ASP system enables physicians to have access to patient records at any location that has Internet availability.
ASP systems are usually sold by monthly subscription, which includes fees for upgrades, support, maintenance, security, data backup, and data storage. They are especially attractive to small or medium-sized practices (as many as 30 physicians).
Going shopping
Many physicians want an EMR system to support the conventional process in their practice. This is a prescription for failure! Instead, evaluate the design of an EMR system for its ability to facilitate change in the process and in roles, and to eliminate manual functions and analysis of data.
One EMR system may appear to be the same as the next, but differences are revealed in the way that they optimize workflow. Generic systems may support several primary care medical specialties well, but may impose inefficiencies in other specialties—particularly in niche specialties. Similarly, a specialty-specific system works well for the specialty for which it was designed but is inefficient in another specialty. Approximately 80% of clinical workflow needs may be met by a generic EMR system, but the 20% that are specialty-specific can make the difference between success and failure.
Ensure that the sales presentations you attend address:
- the needs of your practice
- the criteria for success that you defined during planning.
When you arrive at a choice of an EMR system, assess the learning curve that you’ll have to climb for the system to become fully functional. Remember that salesperson? He, or she, gave a slick presentation but you didn’t settle for a dry description; you were sure to try the system live to discover how easily you can learn to navigate its functions.
At last: Implementation and training
EMR vendors develop implementation and training programs to establish their system in a practice and bring it live. These programs are based on vendors’ experience with their product.
As you prepare for your EMR system, keep this in mind: One that’s been well-designed is more than a repository of data. By design, it also reengineers workflow for optimal practice efficiency, safety, and financial management.
Pearls for welcoming a system
Use EMR incrementally. Do not change from paper to electronic abruptly while you, your colleagues, and the staff are learning the system. Vendors of EMR systems design pathways by which physicians ease-in, so to speak, to EMR in a way that minimizes any drop in productivity and loss of revenue. It’s wise to ask vendors about their plan for implementation and training before you sign a contract to purchase.
Expect that it will take 12 to 24 months to convert the practice completely. The time from paper to electronic records depends on the size and age of a practice. In my experience, the half-life of converting an ObGyn practice is approximately 15 months; by 24 months, the records of approximately 95% of active patients will be entered into EMR.
Don’t scan all paper records into EMR. Scanning indiscriminately is expensive, disruptive, and doesn’t contribute to ongoing clinical excellence. As part of the conversion process, vendors have methods to enter critical clinical information into the electronic system for uninterrupted use. Instead of wholesale scanning, therefore, be selective and scan only clinically relevant materials—the past several Pap smear results, mammogram reports, operative reports, consultant letters, and similar predefined clinical documents. This usually suffices for ongoing clinical care and avoids excessive expenditure of time, energy, and money.
The promise we talk about needs to become actual
As I noted at the outset, fewer than 25% of physicians have EMR, and estimates are that no more than 10% to 15% of ObGyns have adopted a system. Yet experience has demonstrated: A well-designed EMR offers physicians streamlined workflow, the ability to provide better care, and more time for leisure.
To move the flow and utility of medical information properly into this century, the next step, I urge, is for physicians to recognize the value of EMR, set goals for implementing a system, and reengineer their practice for maximal clinical efficiency, patient safety, and financial gain.
CASE REVISITED: Good outcome; no recurrence
One year later, the ObGyn whose practice was in disarray told a different story: “The ‘Patient Portal’ section of our EMR system is a great time saver. We were amazed at the acceptance and rapid adoption—even our octogenarians love it. The universal access to data is of incalculable value. One of our physicians loves to go home early, have dinner, and then review his charts from home. The EMR improves my recordkeeping, makes encounter documentation more complete, and helps me avoid medication errors. Our billing staff loves the thorough documentation.”
An ObGyn reported the following signs of a problem to a colleague: “Our practice was literally drowning in paperwork. An exam room was recently converted to hold more charts, and 2 warehouses held our overflow. Employees were constantly searching for records, and telephone messages were delayed for hours or days until the chart could be reviewed. Notoriously bad handwriting and incomplete documentation hampered good communication and good medical care. Transcription costs were out of control. Forms helped but added to the ongoing costs and storage problems.”
What are the treatment options?
Electronic medical records (EMR) have progressed from arcane, slow, cumbersome documentation systems to sophisticated, complex, comprehensive ones. These modern systems hold the potential to reduce administrative and management costs by 30% or more, improve clinical workflow, reduce medical errors, facilitate communication between patient and physician, and enable analysis of data for best practice methods, best outcomes and identifying risks and complications.
For practices like the one described in the preceding paragraph—not a fictional account but actual testimony provided by an ObGyn—EMR offer a powerful potential solution to the problems that result from an overwhelming amount of paper documentation, correspondence, charting, claims, and financial transactions. In this article, I offer a general introduction to EMR; in the next (August) issue of OBG Management, I’ll speak with a group of ObGyns and medical practice managers about their experiences—and inexperience—with EMR.
Progress and paradox
Physicians and scientists have made substantial progress over the past 25 years in pharmacotherapeutics, diagnostic technology, procedures, and treatment protocols. In obstetrics and gynecology alone, consider the array of technologies—3-dimensional ultrasonography, minimally invasive surgery, receptor-specific drugs, in vitro fertilization, long-acting reversible contraceptives—that have advanced the quality and effectiveness of care. Yet little progress has been made in the process of caring for patients.
The fact is that physicians, and other health-care providers, are rooted in paper-based processes that sustain inefficiencies, increase costs, and defy the gains that other industries have made by adopting electronic technologies for handling information. Why are we so stuck?
The state of EMR
EMR—of varying functionality—have been available for longer than 20 years. Early models were developed by physicians who had an interest in software coding and design, and were of limited functionality, arcane, and difficult to use in a clinical setting. Some of those early models, and even a few commercial systems in use today, rely on scanning paper documents into computer files. Such systems may eliminate some paper and facilitate document retrieval, but they do nothing to ease management of the complex transactions of health care, and they do not address handwriting illegibility.
Development of complex EMR systems was limited by primitive technology, inadequate distribution channels, and programming that was cumbersome and expensive to maintain. But these barriers have been overcome with fast processors, inexpensive and abundant memory, broadband Internet connectivity, and programming languages that facilitate automated software development.
Modern EMR systems are not simply data repositories: They also support workflow from the beginning to the end of a patient’s consultation with a health-care provider—an event that generates multiple transactions with multiple recipients. A single consultation may, for example, generate orders for lab tests, imaging studies, a surgical procedure, consultation with other physicians, prescriptions, and counseling, and record the subsequent financial transaction. EMR systems by necessity interact with multiple organizations, institutions, instruments, and other software systems. To software developers, and to the clinicians who use their systems, the challenge is to deftly navigate the complexities of health care.
Forces accelerating adoption
Momentum from the Executive Office. In 2004, President George W. Bush set a goal: nationwide adoption of EMR—to include all medical practices—within a decade. In a speech that year at Vanderbilt University Medical Center, the President said: “One of the amazing discrepancies in American society today is we’re literally changing how medicine is delivered in incredibly positive ways, and yet docs are still spending a lot of time writing things on paper.”1
Certifying body arises from the private sector. Subsequently, the US Department of Health and Human Services (HHS) established the Office of the National Coordinator for Health Information Technology and the American Health Information Community. The sweeping goal of these bodies? Better health care by application of information technology and creation of standards for certifying EMR systems that provide core functionality.
In response, 3 private sector health information management groups jointly formed the Certification Commission for Healthcare Information Technology (CCHIT; www.cchit.org). In 2005, this private-sector entity entered into a contract with HHS, to, in the commission’s words, “develop and evaluate certification criteria and create an inspection process for healthcare information technology” in 3 areas:
- Ambulatory EMR for offices
- Inpatient EMR for hospitals and health systems
- The network components through which EMR share information.
Pay-for-performance pushes the issue. Today, insurers—federal and private— are mandating adherence to standards of care for maximal reimbursement of services. These reimbursement schemes, called pay-for-performance, or P4P, are based on providers delivering documentation that specific protocols are followed and outcomes are monitored. The point is that it will be nearly impossible for physicians to comply with insurers’ P4P requirements unless that documentation is in an electronic format.
The market speaks—loudly. Other forces are bringing clinicians to a reckoning with EMR:
- Some malpractice carriers offer a discount on premiums to physicians who document work using EMR
- Patients are asking for electronic access to their providers by way of Web sites and e-mail
- More and more requests for documentation from multiple interested parties to a patient’s care increase overhead costs and place greater demands on paper-based systems.
Reticence has been the watchword
Despite the external and internal forces that are driving adoption, physicians have, as a whole, been reticent to adopt EMR. The nonprofit Healthcare Information and Management Systems Society (HIMSS) reports that 26% of ambulatory practices have adopted EMR, but this penetration is predominantly in multi-specialty clinics and hospital-owned practices.2 Few data exist on the penetration of EMR in single-specialty ObGyn practices; anecdotally, vendors estimate a penetration of 10% to 15%.
Why this slow pace toward something broadly acknowledged as key to the well-being of health care?
It means a change. Adopting EMR represents change; well-designed EMR systems streamline workflow in a practice by automating many functions, eliminating duplications of effort, and shifting roles from moving paper to managing digital information. Fear of change and resistance to change are the most common reasons that single-specialty ObGyn practices have not adopted EMR.
It costs. Expense is often cited as the reason why a practice has not adopted an EMR. True: Upfront hardware costs, software costs (license fees, subscription fees), implementation fees, and training costs add up. But a well-designed EMR system should provide a substantial return on investment (ROI) based on savings and on an increase in revenue.
It may be awkward. Some physicians cannot type well. They do not adopt EMR, therefore, because they fear embarrassment using a computer to enter clinical documentation in the consultation room in front of a patient.
On the plus side
On the other side of the coin, the advantages of EMR to physicians are several:
Documentation. EMR facilitate complete documentation of a patient’s visit, current needs and care plan, and record—thereby reducing the clinician’s liability and the risk of medical error. Functions include order entry, prescribing, accurate coding based on work-effort, tracking of outstanding lab tests, and notification.
No chart pulls. With EMR, patient chart pulls are almost nonexistent. A chart is available anywhere a computer is located, any time it is needed.
Decision-making. Probably most importantly, EMR provide clinical decision support by means of alerts (drug interactions, allergies) and reminders (need for follow-up, test orders).
Portal to the patient. Internet-accessed portals that are part of EMR systems facilitate asynchronous communication with patients. A patient can make an appointment, refill a prescription, and request educational materials through such a Web portal. Once an appointment is scheduled, the patient can enter her medical history so that it is specific to the appointment—a feature that is particularly useful when a woman knows the reason that she is visiting the ObGyn (“I’m pregnant,” “My cycle has changed”).
Such a patient-entered history can populate the EMR and contribute elements for appropriate coding. Furthermore, a Web portal in an EMR system enables the physician to reply to a patient with secure messages 1) about lab results, reminders, and appointments and 2) that deliver educational materials.
Keys to embarking on a successful transition
Because EMR are still used by only a small minority of practices, those that seek to move away from the paper record are almost always doing so for the first time. Uncertainty about the adoption adds to anxiety. There are, however, simple steps to take to maintain control over the adoption process and methodically manage it to a successful outcome.
Begin with the end in mind. The goal of adoption is not to purchase an EMR system; EMR are only a tool. The goal of the practice should be to transform its existing workflows to make significant improvements over the status quo. Before ever looking at an EMR system, you (and your colleagues, when applicable) must answer several key questions:
- What are we trying to accomplish?
- What is it about the status quo that we want to change?
- How will we measure success a year after completing the transition?
- Do you know whether they are ready for change?
- Do they understand change?
- Are they threatened by it?
- Is there broad and vocal leadership backing the impending changes?
- Has the impact of the change been discussed with all people involved so they have a clear understanding of its impact on their personal future?
- And is the practice, as a team, prepared to go through the turmoil of change as a necessary step on a path to transformation?
A medical practice that addresses these 3 initial tasks sets itself up for a successful transition from paper to EMR. A good plan—in which goals are well defined and a sense of urgency is consistently communicated and supported by the practice’s leadership—has an excellent chance of resulting in the best possible selection and implementation of an EMR system and accomplishing the goals set for the practice.
In contrast, a transition from paper that begins with such a vague notion as “I guess we need an EMR eventually, so we might as well start now” is much more likely to spark turmoil among staff. The staff then embarks on a selection and implementation process that is heavily influenced by emotion and interpractice politics. They face a diminished opportunity for completing the transition efficiently and successfully.
Throughout this process, the staff should always bear in mind that this is a transformation that they plan, control, and execute. EMR are simply a means to an end—not the end itself.
Two types of systems, various material needs
There are 2 primary configurations of EMR systems: client-server applications and remote-hosted systems. The latter operate through an Application Service Provider (ASP). (See “What are the 2 types of EMR?”)
In addition to type of system, keep these material needs in mind as you plan:
Connectivity. Most medical practices rely on the Internet for a variety of functions; truly, the Internet has become a vital link in health-care information technology.
An ASP system depends on the Internet, whereas client-server applications require a dial-up modem connection or other Internet connectivity to obtain information from outside sources. I recommend purchase of broadband Internet connectivity because it facilitates transmission of large files, such as images and data-rich documents.
Hardware. All EMR require computers for data entry. One attractive option for a medical practice is the TabletPC, which is available from several manufacturers and which uses the Windows XP Tablet PC Edition operating system. Combined with a secure wireless network for moving from room to room, the TabletPC is a technological breakthrough for physicians to document information in a clinical setting. It permits cursive data entry using a special electronic pen, voice recognition entry, and keyboard entry. Whereas a desktop computer places a barrier—the monitor or screen—between physician and patient, a TabletPC emulates the flat, horizontal surface of a paper chart or clipboard.
Importantly, a TabletPC has all the functionality of a desktop computer. Although workflow varies from practice to practice, it can be said generally that most clinical personnel work best with a TabletPC because of its mobility and most clerical personnel work easily with a desktop computer.
The price of a TabletPC? Two to 4 times that of a desktop computer.
Infrastructure. Other devices—printers, scanners, wireless networks, digital cameras—are required to operate an EMR system. A practice that uses a client-server application must purchase a data server. One with a system that operates by remote access either requires a virtual private network (VPN) for secure Internet connection or must install an emulator (such as Citrix).
People. Physicians and staff in the practice are always the key to success when implementing an EMR system. Consider a vendor’s ability to assist with the human variables of change management when you assess systems. Even the best EMR cannot save a practice from a poorly planned and executed transformation.
The 2 primary configurations of an electronic medical records system reflect the way that the system holds, handles, and delivers data.
Client-server application. This type of EMR resides on-site. The medical practice owns the software and hardware and is responsible for data backup, disaster recovery, database maintenance, security, Internet distribution for remote access, and information technology (IT) support. The practice is responsible for loading maintenance and upgrade updates into computers.
Client-server applications are usually sold as an upfront purchase with annual upgrade and maintenance fees that are 18% to 22% of initial cost.
Because the cost of EMR downtime is so high to a practice, you must budget for professional IT staff to establish and maintain high-availability (redundant) servers, Internet access, business continuity plans, network and database administration, security and intrusion detection plans, and data backup.
Remote-hosted system. An Application Service Provider (ASP) system is hosted from a remote data center and distributed through the Internet. Upgrades are deployed regularly to subscribers by the vendor, also by way of the Internet, without need for the practice to install disks or make changes to the server. Data are stored at data centers and backed up in real time. Data backups are maintained at remote sites for disaster recovery. An ASP system enables physicians to have access to patient records at any location that has Internet availability.
ASP systems are usually sold by monthly subscription, which includes fees for upgrades, support, maintenance, security, data backup, and data storage. They are especially attractive to small or medium-sized practices (as many as 30 physicians).
Going shopping
Many physicians want an EMR system to support the conventional process in their practice. This is a prescription for failure! Instead, evaluate the design of an EMR system for its ability to facilitate change in the process and in roles, and to eliminate manual functions and analysis of data.
One EMR system may appear to be the same as the next, but differences are revealed in the way that they optimize workflow. Generic systems may support several primary care medical specialties well, but may impose inefficiencies in other specialties—particularly in niche specialties. Similarly, a specialty-specific system works well for the specialty for which it was designed but is inefficient in another specialty. Approximately 80% of clinical workflow needs may be met by a generic EMR system, but the 20% that are specialty-specific can make the difference between success and failure.
Ensure that the sales presentations you attend address:
- the needs of your practice
- the criteria for success that you defined during planning.
When you arrive at a choice of an EMR system, assess the learning curve that you’ll have to climb for the system to become fully functional. Remember that salesperson? He, or she, gave a slick presentation but you didn’t settle for a dry description; you were sure to try the system live to discover how easily you can learn to navigate its functions.
At last: Implementation and training
EMR vendors develop implementation and training programs to establish their system in a practice and bring it live. These programs are based on vendors’ experience with their product.
As you prepare for your EMR system, keep this in mind: One that’s been well-designed is more than a repository of data. By design, it also reengineers workflow for optimal practice efficiency, safety, and financial management.
Pearls for welcoming a system
Use EMR incrementally. Do not change from paper to electronic abruptly while you, your colleagues, and the staff are learning the system. Vendors of EMR systems design pathways by which physicians ease-in, so to speak, to EMR in a way that minimizes any drop in productivity and loss of revenue. It’s wise to ask vendors about their plan for implementation and training before you sign a contract to purchase.
Expect that it will take 12 to 24 months to convert the practice completely. The time from paper to electronic records depends on the size and age of a practice. In my experience, the half-life of converting an ObGyn practice is approximately 15 months; by 24 months, the records of approximately 95% of active patients will be entered into EMR.
Don’t scan all paper records into EMR. Scanning indiscriminately is expensive, disruptive, and doesn’t contribute to ongoing clinical excellence. As part of the conversion process, vendors have methods to enter critical clinical information into the electronic system for uninterrupted use. Instead of wholesale scanning, therefore, be selective and scan only clinically relevant materials—the past several Pap smear results, mammogram reports, operative reports, consultant letters, and similar predefined clinical documents. This usually suffices for ongoing clinical care and avoids excessive expenditure of time, energy, and money.
The promise we talk about needs to become actual
As I noted at the outset, fewer than 25% of physicians have EMR, and estimates are that no more than 10% to 15% of ObGyns have adopted a system. Yet experience has demonstrated: A well-designed EMR offers physicians streamlined workflow, the ability to provide better care, and more time for leisure.
To move the flow and utility of medical information properly into this century, the next step, I urge, is for physicians to recognize the value of EMR, set goals for implementing a system, and reengineer their practice for maximal clinical efficiency, patient safety, and financial gain.
CASE REVISITED: Good outcome; no recurrence
One year later, the ObGyn whose practice was in disarray told a different story: “The ‘Patient Portal’ section of our EMR system is a great time saver. We were amazed at the acceptance and rapid adoption—even our octogenarians love it. The universal access to data is of incalculable value. One of our physicians loves to go home early, have dinner, and then review his charts from home. The EMR improves my recordkeeping, makes encounter documentation more complete, and helps me avoid medication errors. Our billing staff loves the thorough documentation.”
1. USA Today On-Line. http://www.usatoday.com/news/politicselections/nation/president/2004-05-27-bush-medical-records_x.htm. May 27, 2004. Accessed June 15, 2007.
2. Healthcare Information and Management Systems Society. Ambulatory Care: Latest trends. http://www.himss.org/ASP/topics_FocusDynamic.asp?ffilename="1907OBGM_Article4" aid=190. Accessed June 19, 2007.
1. USA Today On-Line. http://www.usatoday.com/news/politicselections/nation/president/2004-05-27-bush-medical-records_x.htm. May 27, 2004. Accessed June 15, 2007.
2. Healthcare Information and Management Systems Society. Ambulatory Care: Latest trends. http://www.himss.org/ASP/topics_FocusDynamic.asp?ffilename="1907OBGM_Article4" aid=190. Accessed June 19, 2007.
GYNECOLOGIC ONCOLOGY
ObGyns perform most of the screening for cancers of the ovary and breast. The first cancer is especially lethal, though rare, and the second is especially feared among women. This update reviews screening guidelines and recent studies that may affect how we detect and prevent ovarian and breast cancers.
Among the findings:
- In the only multicenter, prospective, randomized, controlled study to date to look at the use of CA-125 and transvaginal ultrasound screening in a low-risk population of postmenopausal women in the United States, researchers found no evidence to suggest a need to revise the present (1996) ovarian cancer screening guidelines of the US Preventive Services Task Force.
- Using a Markov decision-analysis model, investigators explored the health effects of prophylactic bilateral salpingo-oophorectomy in women at average risk of ovarian cancer undergoing hysterectomy. They found that removing the ovaries may decrease overall survival.
- Investigators found the opposite to be true in women with BRCA1 or BRCA2 mutations. Prophylactic bilateral salpingo-oophorectomy greatly reduced the overall mortality rate, as well as the risk of ovarian and breast cancer.
- In a prospective cohort study of BRCA mutation carriers with no history of breast cancer who underwent prophylactic oophorectomy, researchers found the short-term use of hormone replacement therapy to be safe, with no loss of protection against breast cancer.
No need to revise screening guidelines for ovarian cancer
Buys S, Partridge E, Greene M, et al; for the PLCO Project Team. Ovarian cancer screening in the Prostate, Lung, Colorectal and Ovarian (PLCO) cancer screening trial: findings from the initial screen of a randomized trial. Am J Obstet Gynecol. 2005;193:1630–1639.
The need to identify a marker for the early detection of ovarian cancer is especially urgent, given that approximately 75% of women with the cancer present with late-stage disease. Because the disease is rare, finding a cost-effective screening test with good sensitivity and very high specificity (to decrease too many false-positive results) will be challenging.
So far, no prospective, randomized studies of any ovarian cancer screening modality have demonstrated a decrease in mortality—the gold standard of efficacy for any screening test. Therefore, the Prostate, Lung, Colorectal, and Ovarian (PLCO) cancer trial is a critical study—it is the only multicenter, prospective, randomized, controlled study in the United States to tackle the question of whether CA-125 and transvaginal ultrasonographic (US) screening will be effective in a low-risk population of postmenopausal women aged 55 to 74.
In this large study, 1 arm underwent ovarian cancer screening with both modalities and the other arm underwent no such screening.
This study reports on baseline, or ‘prevalent,’ cancers
This preliminary report does not comment on the efficacy of ovarian cancer screening; data on the effect of repeated annual screens on detection rates and mortality will become available over the next several years.
Rather, the purpose of this preliminary report was to detail the baseline ovarian cancer screening tests of the 39,115 women randomized to the intervention arm from November 15, 1993, to December 13, 2001. These results describe “prevalent” cancers—that is, cancers that are present on the first screen. The more important information about efficacy of screening will come over the next several years, as “incident” cancers develop.
Roughly 6% of women had at least 1 abnormal finding at baseline
Among 28,506 women with results for both baseline tests, 1,706 had at least 1 abnormal finding:
- 1,338 had an abnormal transvaginal US scan
- 402 had an abnormal level on the CA-125 test
- 34 had abnormalities in both tests
- 29 malignant neoplasms were identified in this population, 20 of them invasive.
When combined, CA-125 and transvaginal ultrasonography had good positive predictive value
In general, a positive predictive value (PPV) of more than 10% (ie, 10 surgeries to detect 1 cancer) is considered reasonable justification for a screening test. In the PLCO trial, the PPV was 4% for CA-125 alone (16 neoplasms in 402 positive screens), 1.6% for transvaginal ultrasonography alone (22 neoplasms in 1,338 positive screens), and 26.5% if both tests were abnormal (9 neoplasms in 34 positive screens).
When tumors of low malignant potential were excluded, the PPV was 3.7% for an abnormal CA-125, 1.0% for an abnormal transvaginal sonogram, and 23.5% if both tests were abnormal. A PPV of 23.5% for both tests is fairly good (ie, approximately 4 surgeries to detect 1 cancer). However, if only women in whom both screening tests were abnormal went to surgery, 12 of 20 invasive cancers would be missed.
Bottom line: Routine screening still not justified
Nothing in the findings reported here suggests that we need to revise the current (from 1996) ovarian cancer screening guidelines of the US Preventive Services Task Force,1 which state that “routine screening for ovarian cancer by US, the measurement of serum tumor markers, or pelvic examination is not recommended.”
We will need to wait until the PLCO trial results come in to see the effect of repeated annual ovarian cancer screens on detection rates and mortality.
Consider ovarian conservation in hysterectomy for benign disease
Parker W, Broder MS, Liu Z, Shoupe D, Farquhar C, Berek JS. Ovarian conservation at the time of hysterectomy for benign disease. Obstet Gynecol. 2005;106:219–226.
As discussed earlier, we have no good screening tool for the early detection of ovarian cancer. Although rare, ovarian cancer is a lethal, scary disease, and most ObGyns prophylactically remove the ovaries at the time of hysterectomy in most postmenopausal and many perimenopausal women.
The downside to this strategy seems low among postmenopausal women, and the upside, in terms of not having to worry about ovarian cancer, seems high. The study by Parker and colleagues, while having definite limitations, asks us to question this routine practice pattern. The authors found that prophylactic oophorectomy may be associated with decreased overall survival.
Model used SEER data, Nurses’ Health Study to predict survival
Parker and colleagues used a Markov decision-analysis model (a hypothetical mathematical model that uses published data to create cohorts of patients to estimate risk of morbidity or mortality, or both, over time) to evaluate the risks and benefits of ovarian conservation at the time of hysterectomy for benign disease. Age-specific mortality estimates for ovarian cancer were based on Surveillance, Epidemiology and End Results (SEER) statistics.
For women at average risk of ovarian cancer, the probability of surviving to 80 years of age after hysterectomy between 50 and 54 years varied, and was 62.8% and 62.5% for ovarian conservation with and without estrogen therapy, respectively, compared with 62.2% and 53.9% for oophorectomy with and without estrogen therapy. The main reason that the model found decreased overall survival with prophylactic oophorectomy was an increase in coronary artery disease after oophorectomy—a finding that was based on data from the Nurses’ Health Study.
At the very least, think hard about the decision to remove the ovaries
This report estimated that about 300,000 prophylactic oophorectomies are carried out annually in the United States. Although this study has limitations, we believe it encourages debate and reexamination of the benefit of prophylactic oophorectomy for benign indications in young, low-risk patients.
The most important finding from the study is that oophorectomy conferred no survival advantage. Given the rarity of ovarian cancer among the general population, this effect is not that surprising.
For now, careful risk assessment remains a fundamental component of management, so that women who are at increased risk of ovarian cancer can undergo prophylactic salpingo-oophorectomy.
For low-risk women, who constitute the majority of patients, the data to support removing ovaries at the time of hysterectomy are less clear.
Make sure the patient understands the low risk of cancer and possible cardiac benefits of preservation
- Conduct a thorough discussion with the patient about the pros and cons of oophorectomy for benign disease.
- The risk of ovarian cancer in the general population is low; patients should understand that they may derive cardiac protection from postmenopausal ovarian function.
Do consider oophorectomy among carriers of a BRCA mutation
Domchek SM, Friebel TM, Neuhausen SL, et al. Mortality after bilateral salpingo-oophorectomy in BRCA1 and BRCA2 mutation carriers: a prospective cohort study. Lancet Oncol. 2006;7:223–229.
Women known to have BRCA1 or BRCA2 mutations are now managed by means of surveillance or with prophylactic bilateral salpingo-oophorectomy. Although bilateral salpingo-oophorectomy has been shown to reduce the risk of ovarian cancer by 90% and the risk of breast cancer by 50%, until this study few data shed light on the effect of the procedure on overall mortality among women with BRCA mutations.
This prospective cohort study identified 155 patients with BRCA1 or BRCA2 mutations who elected to undergo bilateral salpingo-oophorectomy and matched them by age with a control group of 271. All women were followed until death by any cause or by breast, ovarian, or primary peritoneal cancer. The women were followed for a mean of 3.1 years in the bilateral salpingo-oophorectomy group and 2.1 years in the control group.
Overall and cancer-specific survival improved with oophorectomy
Among BRCA mutation carriers, women who chose prophylactic bilateral salpingo-oophorectomy had improved overall and cancer-specific survival, compared with women who did not undergo the surgery.
In the analysis of the matched BRCA mutation carriers, women who chose to undergo the procedure had a decreased risk of overall mortality (hazard ratio [HR]=0.24; 95% confidence interval [CI], 0.08–0.71); they also had a decreased risk of mortality due to both breast cancer (HR=0.1; 95% CI, 0.02–0.71) and ovarian cancer (HR=0.05; 95% CI, 0.01–0.46).
Practice recommendations
Apparently, unlike women at average risk of ovarian cancer (for whom prophylactic oophorectomy in conjunction with hysterectomy for benign disease may be associated with decreased overall survival; see the review of the study by Parker and colleagues), women with BRCA mutations may benefit from oophorectomy.
Advantages of prophylactic bilateral salpingo-oophorectomy in this patient population should be discussed with potential surgical candidates, because:
- Women who have a BRCA mutation and who have had bilateral salpingo-oophorectomy were shown to have improved overall and cancer-specific survival.
- In this specific group of BRCA mutation carriers, this study did not demonstrate an increased risk of mortality from cardiovascular disease, osteoporosis, or other causes associated with premature menopause from bilateral salpingo-oophorectomy.
In BRCA carriers, HRT after oophorectomy does not raise breast cancer risk
Rebbeck TR, Friebel T, Wagner T, et al; for the PROSE Study Group. Effect of short-term hormone replacement therapy on breast cancer risk reduction after bilateral prophylactic oophorectomy in BRCA1 and BRCA2 mutation carriers: the PROSE Study Group. J Clin Oncol. 2005;23:7804–7810.
In women with BRCA1 or BRCA2 mutations, the risk of ovarian cancer is staggering (20% to 40% for BRCA1 mutations, 15% to 25% for BRCA2 mutations), and prophylactic bilateral salpingo-oophorectomy is the only strategy proven to significantly reduce risk. A second important benefit for bilateral salpingo-oophorectomy among premenopausal mutation carriers is that the procedure decreases the risk of breast cancer by 50%. In women who have a lifetime risk of breast cancer that is as high as 80%, this benefit is extremely welcome. Current recommendations are for women with BRCA1 or BRCA2 mutations to undergo bilateral salpingo-oophorectomy at age 35 to 40, or when child-bearing is complete.
Yet, for many women in this age group, quality of life is substantially altered when premature menopause kicks in after the surgery. Most ObGyns feel comfortable giving short-term hormone replacement therapy (HRT) after prophylactic bilateral salpingo-oophorectomy to premenopausal women who do not have a history of breast cancer. However, until this study, no data were available that addressed the question of whether short-term HRT affects breast cancer risk.
In a prospective cohort of 462 women, of whom 155 underwent bilateral salpingo-oophorectomy, Rebbeck and colleagues evaluated the risk of developing breast cancer over an average of 3.6 years based on exposure to any type of HRT. In this multicenter study conducted at 13 different institutions in the United States and Europe, they found that women who underwent bilateral salpingo-oophorectomy for a BRCA1 or BRCA2 mutation were more likely to be older, have had children, and were more likely to use HRT. Compared with women who did not have bilateral salpingo-oophorectomy, women who had undergone the procedure and used any short-term HRT (including estrogen, progesterone, or a combination) still had a substantial decrease in breast cancer risk (HR=0.37; 95% CI, 0.14–0.96).
Practice recommendations
We can now reassure young women who must decide whether to undergo prophylactic bilateral salpingo-oophorectomy to reduce their staggering risks of breast and ovarian cancer: Short-term HRT to address the hot flashes, night sweats, and vaginal dryness associated with premature surgical menopause, first, is clinically reasonable and, second, will not substantially reduce the benefits of bilateral salpingo-oophorectomy for breast cancer risk.
1. US Preventive Services Task Force. Guide to Clinical Preventive Services. 2nd ed. Baltimore: Williams & Wilkins; 1996. Available at: http://odphp.osophs.dhhs.gov/pubs/guidecps/. Accessed June 4, 2007.
The authors report no financial relationships relevant to this article
ObGyns perform most of the screening for cancers of the ovary and breast. The first cancer is especially lethal, though rare, and the second is especially feared among women. This update reviews screening guidelines and recent studies that may affect how we detect and prevent ovarian and breast cancers.
Among the findings:
- In the only multicenter, prospective, randomized, controlled study to date to look at the use of CA-125 and transvaginal ultrasound screening in a low-risk population of postmenopausal women in the United States, researchers found no evidence to suggest a need to revise the present (1996) ovarian cancer screening guidelines of the US Preventive Services Task Force.
- Using a Markov decision-analysis model, investigators explored the health effects of prophylactic bilateral salpingo-oophorectomy in women at average risk of ovarian cancer undergoing hysterectomy. They found that removing the ovaries may decrease overall survival.
- Investigators found the opposite to be true in women with BRCA1 or BRCA2 mutations. Prophylactic bilateral salpingo-oophorectomy greatly reduced the overall mortality rate, as well as the risk of ovarian and breast cancer.
- In a prospective cohort study of BRCA mutation carriers with no history of breast cancer who underwent prophylactic oophorectomy, researchers found the short-term use of hormone replacement therapy to be safe, with no loss of protection against breast cancer.
No need to revise screening guidelines for ovarian cancer
Buys S, Partridge E, Greene M, et al; for the PLCO Project Team. Ovarian cancer screening in the Prostate, Lung, Colorectal and Ovarian (PLCO) cancer screening trial: findings from the initial screen of a randomized trial. Am J Obstet Gynecol. 2005;193:1630–1639.
The need to identify a marker for the early detection of ovarian cancer is especially urgent, given that approximately 75% of women with the cancer present with late-stage disease. Because the disease is rare, finding a cost-effective screening test with good sensitivity and very high specificity (to decrease too many false-positive results) will be challenging.
So far, no prospective, randomized studies of any ovarian cancer screening modality have demonstrated a decrease in mortality—the gold standard of efficacy for any screening test. Therefore, the Prostate, Lung, Colorectal, and Ovarian (PLCO) cancer trial is a critical study—it is the only multicenter, prospective, randomized, controlled study in the United States to tackle the question of whether CA-125 and transvaginal ultrasonographic (US) screening will be effective in a low-risk population of postmenopausal women aged 55 to 74.
In this large study, 1 arm underwent ovarian cancer screening with both modalities and the other arm underwent no such screening.
This study reports on baseline, or ‘prevalent,’ cancers
This preliminary report does not comment on the efficacy of ovarian cancer screening; data on the effect of repeated annual screens on detection rates and mortality will become available over the next several years.
Rather, the purpose of this preliminary report was to detail the baseline ovarian cancer screening tests of the 39,115 women randomized to the intervention arm from November 15, 1993, to December 13, 2001. These results describe “prevalent” cancers—that is, cancers that are present on the first screen. The more important information about efficacy of screening will come over the next several years, as “incident” cancers develop.
Roughly 6% of women had at least 1 abnormal finding at baseline
Among 28,506 women with results for both baseline tests, 1,706 had at least 1 abnormal finding:
- 1,338 had an abnormal transvaginal US scan
- 402 had an abnormal level on the CA-125 test
- 34 had abnormalities in both tests
- 29 malignant neoplasms were identified in this population, 20 of them invasive.
When combined, CA-125 and transvaginal ultrasonography had good positive predictive value
In general, a positive predictive value (PPV) of more than 10% (ie, 10 surgeries to detect 1 cancer) is considered reasonable justification for a screening test. In the PLCO trial, the PPV was 4% for CA-125 alone (16 neoplasms in 402 positive screens), 1.6% for transvaginal ultrasonography alone (22 neoplasms in 1,338 positive screens), and 26.5% if both tests were abnormal (9 neoplasms in 34 positive screens).
When tumors of low malignant potential were excluded, the PPV was 3.7% for an abnormal CA-125, 1.0% for an abnormal transvaginal sonogram, and 23.5% if both tests were abnormal. A PPV of 23.5% for both tests is fairly good (ie, approximately 4 surgeries to detect 1 cancer). However, if only women in whom both screening tests were abnormal went to surgery, 12 of 20 invasive cancers would be missed.
Bottom line: Routine screening still not justified
Nothing in the findings reported here suggests that we need to revise the current (from 1996) ovarian cancer screening guidelines of the US Preventive Services Task Force,1 which state that “routine screening for ovarian cancer by US, the measurement of serum tumor markers, or pelvic examination is not recommended.”
We will need to wait until the PLCO trial results come in to see the effect of repeated annual ovarian cancer screens on detection rates and mortality.
Consider ovarian conservation in hysterectomy for benign disease
Parker W, Broder MS, Liu Z, Shoupe D, Farquhar C, Berek JS. Ovarian conservation at the time of hysterectomy for benign disease. Obstet Gynecol. 2005;106:219–226.
As discussed earlier, we have no good screening tool for the early detection of ovarian cancer. Although rare, ovarian cancer is a lethal, scary disease, and most ObGyns prophylactically remove the ovaries at the time of hysterectomy in most postmenopausal and many perimenopausal women.
The downside to this strategy seems low among postmenopausal women, and the upside, in terms of not having to worry about ovarian cancer, seems high. The study by Parker and colleagues, while having definite limitations, asks us to question this routine practice pattern. The authors found that prophylactic oophorectomy may be associated with decreased overall survival.
Model used SEER data, Nurses’ Health Study to predict survival
Parker and colleagues used a Markov decision-analysis model (a hypothetical mathematical model that uses published data to create cohorts of patients to estimate risk of morbidity or mortality, or both, over time) to evaluate the risks and benefits of ovarian conservation at the time of hysterectomy for benign disease. Age-specific mortality estimates for ovarian cancer were based on Surveillance, Epidemiology and End Results (SEER) statistics.
For women at average risk of ovarian cancer, the probability of surviving to 80 years of age after hysterectomy between 50 and 54 years varied, and was 62.8% and 62.5% for ovarian conservation with and without estrogen therapy, respectively, compared with 62.2% and 53.9% for oophorectomy with and without estrogen therapy. The main reason that the model found decreased overall survival with prophylactic oophorectomy was an increase in coronary artery disease after oophorectomy—a finding that was based on data from the Nurses’ Health Study.
At the very least, think hard about the decision to remove the ovaries
This report estimated that about 300,000 prophylactic oophorectomies are carried out annually in the United States. Although this study has limitations, we believe it encourages debate and reexamination of the benefit of prophylactic oophorectomy for benign indications in young, low-risk patients.
The most important finding from the study is that oophorectomy conferred no survival advantage. Given the rarity of ovarian cancer among the general population, this effect is not that surprising.
For now, careful risk assessment remains a fundamental component of management, so that women who are at increased risk of ovarian cancer can undergo prophylactic salpingo-oophorectomy.
For low-risk women, who constitute the majority of patients, the data to support removing ovaries at the time of hysterectomy are less clear.
Make sure the patient understands the low risk of cancer and possible cardiac benefits of preservation
- Conduct a thorough discussion with the patient about the pros and cons of oophorectomy for benign disease.
- The risk of ovarian cancer in the general population is low; patients should understand that they may derive cardiac protection from postmenopausal ovarian function.
Do consider oophorectomy among carriers of a BRCA mutation
Domchek SM, Friebel TM, Neuhausen SL, et al. Mortality after bilateral salpingo-oophorectomy in BRCA1 and BRCA2 mutation carriers: a prospective cohort study. Lancet Oncol. 2006;7:223–229.
Women known to have BRCA1 or BRCA2 mutations are now managed by means of surveillance or with prophylactic bilateral salpingo-oophorectomy. Although bilateral salpingo-oophorectomy has been shown to reduce the risk of ovarian cancer by 90% and the risk of breast cancer by 50%, until this study few data shed light on the effect of the procedure on overall mortality among women with BRCA mutations.
This prospective cohort study identified 155 patients with BRCA1 or BRCA2 mutations who elected to undergo bilateral salpingo-oophorectomy and matched them by age with a control group of 271. All women were followed until death by any cause or by breast, ovarian, or primary peritoneal cancer. The women were followed for a mean of 3.1 years in the bilateral salpingo-oophorectomy group and 2.1 years in the control group.
Overall and cancer-specific survival improved with oophorectomy
Among BRCA mutation carriers, women who chose prophylactic bilateral salpingo-oophorectomy had improved overall and cancer-specific survival, compared with women who did not undergo the surgery.
In the analysis of the matched BRCA mutation carriers, women who chose to undergo the procedure had a decreased risk of overall mortality (hazard ratio [HR]=0.24; 95% confidence interval [CI], 0.08–0.71); they also had a decreased risk of mortality due to both breast cancer (HR=0.1; 95% CI, 0.02–0.71) and ovarian cancer (HR=0.05; 95% CI, 0.01–0.46).
Practice recommendations
Apparently, unlike women at average risk of ovarian cancer (for whom prophylactic oophorectomy in conjunction with hysterectomy for benign disease may be associated with decreased overall survival; see the review of the study by Parker and colleagues), women with BRCA mutations may benefit from oophorectomy.
Advantages of prophylactic bilateral salpingo-oophorectomy in this patient population should be discussed with potential surgical candidates, because:
- Women who have a BRCA mutation and who have had bilateral salpingo-oophorectomy were shown to have improved overall and cancer-specific survival.
- In this specific group of BRCA mutation carriers, this study did not demonstrate an increased risk of mortality from cardiovascular disease, osteoporosis, or other causes associated with premature menopause from bilateral salpingo-oophorectomy.
In BRCA carriers, HRT after oophorectomy does not raise breast cancer risk
Rebbeck TR, Friebel T, Wagner T, et al; for the PROSE Study Group. Effect of short-term hormone replacement therapy on breast cancer risk reduction after bilateral prophylactic oophorectomy in BRCA1 and BRCA2 mutation carriers: the PROSE Study Group. J Clin Oncol. 2005;23:7804–7810.
In women with BRCA1 or BRCA2 mutations, the risk of ovarian cancer is staggering (20% to 40% for BRCA1 mutations, 15% to 25% for BRCA2 mutations), and prophylactic bilateral salpingo-oophorectomy is the only strategy proven to significantly reduce risk. A second important benefit for bilateral salpingo-oophorectomy among premenopausal mutation carriers is that the procedure decreases the risk of breast cancer by 50%. In women who have a lifetime risk of breast cancer that is as high as 80%, this benefit is extremely welcome. Current recommendations are for women with BRCA1 or BRCA2 mutations to undergo bilateral salpingo-oophorectomy at age 35 to 40, or when child-bearing is complete.
Yet, for many women in this age group, quality of life is substantially altered when premature menopause kicks in after the surgery. Most ObGyns feel comfortable giving short-term hormone replacement therapy (HRT) after prophylactic bilateral salpingo-oophorectomy to premenopausal women who do not have a history of breast cancer. However, until this study, no data were available that addressed the question of whether short-term HRT affects breast cancer risk.
In a prospective cohort of 462 women, of whom 155 underwent bilateral salpingo-oophorectomy, Rebbeck and colleagues evaluated the risk of developing breast cancer over an average of 3.6 years based on exposure to any type of HRT. In this multicenter study conducted at 13 different institutions in the United States and Europe, they found that women who underwent bilateral salpingo-oophorectomy for a BRCA1 or BRCA2 mutation were more likely to be older, have had children, and were more likely to use HRT. Compared with women who did not have bilateral salpingo-oophorectomy, women who had undergone the procedure and used any short-term HRT (including estrogen, progesterone, or a combination) still had a substantial decrease in breast cancer risk (HR=0.37; 95% CI, 0.14–0.96).
Practice recommendations
We can now reassure young women who must decide whether to undergo prophylactic bilateral salpingo-oophorectomy to reduce their staggering risks of breast and ovarian cancer: Short-term HRT to address the hot flashes, night sweats, and vaginal dryness associated with premature surgical menopause, first, is clinically reasonable and, second, will not substantially reduce the benefits of bilateral salpingo-oophorectomy for breast cancer risk.
ObGyns perform most of the screening for cancers of the ovary and breast. The first cancer is especially lethal, though rare, and the second is especially feared among women. This update reviews screening guidelines and recent studies that may affect how we detect and prevent ovarian and breast cancers.
Among the findings:
- In the only multicenter, prospective, randomized, controlled study to date to look at the use of CA-125 and transvaginal ultrasound screening in a low-risk population of postmenopausal women in the United States, researchers found no evidence to suggest a need to revise the present (1996) ovarian cancer screening guidelines of the US Preventive Services Task Force.
- Using a Markov decision-analysis model, investigators explored the health effects of prophylactic bilateral salpingo-oophorectomy in women at average risk of ovarian cancer undergoing hysterectomy. They found that removing the ovaries may decrease overall survival.
- Investigators found the opposite to be true in women with BRCA1 or BRCA2 mutations. Prophylactic bilateral salpingo-oophorectomy greatly reduced the overall mortality rate, as well as the risk of ovarian and breast cancer.
- In a prospective cohort study of BRCA mutation carriers with no history of breast cancer who underwent prophylactic oophorectomy, researchers found the short-term use of hormone replacement therapy to be safe, with no loss of protection against breast cancer.
No need to revise screening guidelines for ovarian cancer
Buys S, Partridge E, Greene M, et al; for the PLCO Project Team. Ovarian cancer screening in the Prostate, Lung, Colorectal and Ovarian (PLCO) cancer screening trial: findings from the initial screen of a randomized trial. Am J Obstet Gynecol. 2005;193:1630–1639.
The need to identify a marker for the early detection of ovarian cancer is especially urgent, given that approximately 75% of women with the cancer present with late-stage disease. Because the disease is rare, finding a cost-effective screening test with good sensitivity and very high specificity (to decrease too many false-positive results) will be challenging.
So far, no prospective, randomized studies of any ovarian cancer screening modality have demonstrated a decrease in mortality—the gold standard of efficacy for any screening test. Therefore, the Prostate, Lung, Colorectal, and Ovarian (PLCO) cancer trial is a critical study—it is the only multicenter, prospective, randomized, controlled study in the United States to tackle the question of whether CA-125 and transvaginal ultrasonographic (US) screening will be effective in a low-risk population of postmenopausal women aged 55 to 74.
In this large study, 1 arm underwent ovarian cancer screening with both modalities and the other arm underwent no such screening.
This study reports on baseline, or ‘prevalent,’ cancers
This preliminary report does not comment on the efficacy of ovarian cancer screening; data on the effect of repeated annual screens on detection rates and mortality will become available over the next several years.
Rather, the purpose of this preliminary report was to detail the baseline ovarian cancer screening tests of the 39,115 women randomized to the intervention arm from November 15, 1993, to December 13, 2001. These results describe “prevalent” cancers—that is, cancers that are present on the first screen. The more important information about efficacy of screening will come over the next several years, as “incident” cancers develop.
Roughly 6% of women had at least 1 abnormal finding at baseline
Among 28,506 women with results for both baseline tests, 1,706 had at least 1 abnormal finding:
- 1,338 had an abnormal transvaginal US scan
- 402 had an abnormal level on the CA-125 test
- 34 had abnormalities in both tests
- 29 malignant neoplasms were identified in this population, 20 of them invasive.
When combined, CA-125 and transvaginal ultrasonography had good positive predictive value
In general, a positive predictive value (PPV) of more than 10% (ie, 10 surgeries to detect 1 cancer) is considered reasonable justification for a screening test. In the PLCO trial, the PPV was 4% for CA-125 alone (16 neoplasms in 402 positive screens), 1.6% for transvaginal ultrasonography alone (22 neoplasms in 1,338 positive screens), and 26.5% if both tests were abnormal (9 neoplasms in 34 positive screens).
When tumors of low malignant potential were excluded, the PPV was 3.7% for an abnormal CA-125, 1.0% for an abnormal transvaginal sonogram, and 23.5% if both tests were abnormal. A PPV of 23.5% for both tests is fairly good (ie, approximately 4 surgeries to detect 1 cancer). However, if only women in whom both screening tests were abnormal went to surgery, 12 of 20 invasive cancers would be missed.
Bottom line: Routine screening still not justified
Nothing in the findings reported here suggests that we need to revise the current (from 1996) ovarian cancer screening guidelines of the US Preventive Services Task Force,1 which state that “routine screening for ovarian cancer by US, the measurement of serum tumor markers, or pelvic examination is not recommended.”
We will need to wait until the PLCO trial results come in to see the effect of repeated annual ovarian cancer screens on detection rates and mortality.
Consider ovarian conservation in hysterectomy for benign disease
Parker W, Broder MS, Liu Z, Shoupe D, Farquhar C, Berek JS. Ovarian conservation at the time of hysterectomy for benign disease. Obstet Gynecol. 2005;106:219–226.
As discussed earlier, we have no good screening tool for the early detection of ovarian cancer. Although rare, ovarian cancer is a lethal, scary disease, and most ObGyns prophylactically remove the ovaries at the time of hysterectomy in most postmenopausal and many perimenopausal women.
The downside to this strategy seems low among postmenopausal women, and the upside, in terms of not having to worry about ovarian cancer, seems high. The study by Parker and colleagues, while having definite limitations, asks us to question this routine practice pattern. The authors found that prophylactic oophorectomy may be associated with decreased overall survival.
Model used SEER data, Nurses’ Health Study to predict survival
Parker and colleagues used a Markov decision-analysis model (a hypothetical mathematical model that uses published data to create cohorts of patients to estimate risk of morbidity or mortality, or both, over time) to evaluate the risks and benefits of ovarian conservation at the time of hysterectomy for benign disease. Age-specific mortality estimates for ovarian cancer were based on Surveillance, Epidemiology and End Results (SEER) statistics.
For women at average risk of ovarian cancer, the probability of surviving to 80 years of age after hysterectomy between 50 and 54 years varied, and was 62.8% and 62.5% for ovarian conservation with and without estrogen therapy, respectively, compared with 62.2% and 53.9% for oophorectomy with and without estrogen therapy. The main reason that the model found decreased overall survival with prophylactic oophorectomy was an increase in coronary artery disease after oophorectomy—a finding that was based on data from the Nurses’ Health Study.
At the very least, think hard about the decision to remove the ovaries
This report estimated that about 300,000 prophylactic oophorectomies are carried out annually in the United States. Although this study has limitations, we believe it encourages debate and reexamination of the benefit of prophylactic oophorectomy for benign indications in young, low-risk patients.
The most important finding from the study is that oophorectomy conferred no survival advantage. Given the rarity of ovarian cancer among the general population, this effect is not that surprising.
For now, careful risk assessment remains a fundamental component of management, so that women who are at increased risk of ovarian cancer can undergo prophylactic salpingo-oophorectomy.
For low-risk women, who constitute the majority of patients, the data to support removing ovaries at the time of hysterectomy are less clear.
Make sure the patient understands the low risk of cancer and possible cardiac benefits of preservation
- Conduct a thorough discussion with the patient about the pros and cons of oophorectomy for benign disease.
- The risk of ovarian cancer in the general population is low; patients should understand that they may derive cardiac protection from postmenopausal ovarian function.
Do consider oophorectomy among carriers of a BRCA mutation
Domchek SM, Friebel TM, Neuhausen SL, et al. Mortality after bilateral salpingo-oophorectomy in BRCA1 and BRCA2 mutation carriers: a prospective cohort study. Lancet Oncol. 2006;7:223–229.
Women known to have BRCA1 or BRCA2 mutations are now managed by means of surveillance or with prophylactic bilateral salpingo-oophorectomy. Although bilateral salpingo-oophorectomy has been shown to reduce the risk of ovarian cancer by 90% and the risk of breast cancer by 50%, until this study few data shed light on the effect of the procedure on overall mortality among women with BRCA mutations.
This prospective cohort study identified 155 patients with BRCA1 or BRCA2 mutations who elected to undergo bilateral salpingo-oophorectomy and matched them by age with a control group of 271. All women were followed until death by any cause or by breast, ovarian, or primary peritoneal cancer. The women were followed for a mean of 3.1 years in the bilateral salpingo-oophorectomy group and 2.1 years in the control group.
Overall and cancer-specific survival improved with oophorectomy
Among BRCA mutation carriers, women who chose prophylactic bilateral salpingo-oophorectomy had improved overall and cancer-specific survival, compared with women who did not undergo the surgery.
In the analysis of the matched BRCA mutation carriers, women who chose to undergo the procedure had a decreased risk of overall mortality (hazard ratio [HR]=0.24; 95% confidence interval [CI], 0.08–0.71); they also had a decreased risk of mortality due to both breast cancer (HR=0.1; 95% CI, 0.02–0.71) and ovarian cancer (HR=0.05; 95% CI, 0.01–0.46).
Practice recommendations
Apparently, unlike women at average risk of ovarian cancer (for whom prophylactic oophorectomy in conjunction with hysterectomy for benign disease may be associated with decreased overall survival; see the review of the study by Parker and colleagues), women with BRCA mutations may benefit from oophorectomy.
Advantages of prophylactic bilateral salpingo-oophorectomy in this patient population should be discussed with potential surgical candidates, because:
- Women who have a BRCA mutation and who have had bilateral salpingo-oophorectomy were shown to have improved overall and cancer-specific survival.
- In this specific group of BRCA mutation carriers, this study did not demonstrate an increased risk of mortality from cardiovascular disease, osteoporosis, or other causes associated with premature menopause from bilateral salpingo-oophorectomy.
In BRCA carriers, HRT after oophorectomy does not raise breast cancer risk
Rebbeck TR, Friebel T, Wagner T, et al; for the PROSE Study Group. Effect of short-term hormone replacement therapy on breast cancer risk reduction after bilateral prophylactic oophorectomy in BRCA1 and BRCA2 mutation carriers: the PROSE Study Group. J Clin Oncol. 2005;23:7804–7810.
In women with BRCA1 or BRCA2 mutations, the risk of ovarian cancer is staggering (20% to 40% for BRCA1 mutations, 15% to 25% for BRCA2 mutations), and prophylactic bilateral salpingo-oophorectomy is the only strategy proven to significantly reduce risk. A second important benefit for bilateral salpingo-oophorectomy among premenopausal mutation carriers is that the procedure decreases the risk of breast cancer by 50%. In women who have a lifetime risk of breast cancer that is as high as 80%, this benefit is extremely welcome. Current recommendations are for women with BRCA1 or BRCA2 mutations to undergo bilateral salpingo-oophorectomy at age 35 to 40, or when child-bearing is complete.
Yet, for many women in this age group, quality of life is substantially altered when premature menopause kicks in after the surgery. Most ObGyns feel comfortable giving short-term hormone replacement therapy (HRT) after prophylactic bilateral salpingo-oophorectomy to premenopausal women who do not have a history of breast cancer. However, until this study, no data were available that addressed the question of whether short-term HRT affects breast cancer risk.
In a prospective cohort of 462 women, of whom 155 underwent bilateral salpingo-oophorectomy, Rebbeck and colleagues evaluated the risk of developing breast cancer over an average of 3.6 years based on exposure to any type of HRT. In this multicenter study conducted at 13 different institutions in the United States and Europe, they found that women who underwent bilateral salpingo-oophorectomy for a BRCA1 or BRCA2 mutation were more likely to be older, have had children, and were more likely to use HRT. Compared with women who did not have bilateral salpingo-oophorectomy, women who had undergone the procedure and used any short-term HRT (including estrogen, progesterone, or a combination) still had a substantial decrease in breast cancer risk (HR=0.37; 95% CI, 0.14–0.96).
Practice recommendations
We can now reassure young women who must decide whether to undergo prophylactic bilateral salpingo-oophorectomy to reduce their staggering risks of breast and ovarian cancer: Short-term HRT to address the hot flashes, night sweats, and vaginal dryness associated with premature surgical menopause, first, is clinically reasonable and, second, will not substantially reduce the benefits of bilateral salpingo-oophorectomy for breast cancer risk.
1. US Preventive Services Task Force. Guide to Clinical Preventive Services. 2nd ed. Baltimore: Williams & Wilkins; 1996. Available at: http://odphp.osophs.dhhs.gov/pubs/guidecps/. Accessed June 4, 2007.
The authors report no financial relationships relevant to this article
1. US Preventive Services Task Force. Guide to Clinical Preventive Services. 2nd ed. Baltimore: Williams & Wilkins; 1996. Available at: http://odphp.osophs.dhhs.gov/pubs/guidecps/. Accessed June 4, 2007.
The authors report no financial relationships relevant to this article