User login
Hormonal contraception in women with medical conditions
Decisive new data on risks and benefits of hormonal contraception will change how we manage many patients. These findings prompted the American College of Obstetricians and Gynecologists (ACOG) to update its practice bulletin (released in June) on hormonal contraception in women with coexisting medical conditions.1 Among the most notable areas of change:
- Family history of breast cancer or BRCA1 or 2 mutations. Combination oral contraceptives (OCs) do not appear to increase the risk of breast cancer in these women, and do help prevent ovarian cancer.
- Concomitant medications. More women are using enzyme-inducing anticonvulsants for conditions other than seizure disorders; some affect steroid levels.
- Obesity. Progestin-only and intrauterine methods may be better for obese women older than 35 years, who face an elevated baseline risk of venous thromboembolism.
- Lupus. Combination OCs are safe in women with stable, mild disease who are seronegative for antiphospholipid antibodies.
- Depot medroxyprogesterone acetate (DMPA). Although bone density declines in women using DMPA, it recovers within 3 years after the drug is discontinued.
- Patch and ring. Until method-specific data come in, assume that the patch and ring have the same contraindications as combination OCs.
For the fine points on these and other findings, we talked with Dr. Andrew M. Kaunitz, who assisted ACOG with preparation of the new bulletin.1
1. Breast cancer risk
OCs do not add to existing high risk
OBG Management: Women who have a family history of breast cancer have a higher-than-average risk of developing the cancer themselves, and it is widely assumed that estrogen further heightens that risk. Should these women avoid combination OCs?
KAUNITZ: No. Although these women have been reluctant to use hormonal contraceptives (as have many caregivers), we now have several lines of reassuring evidence.
Among the studies demonstrating safety of hormonal methods in this population is the 2002 Women’s CARE study, conducted by the Centers for Disease Control and Prevention and sponsored by the National Institute of Child Health and Human Development, which found no elevated risk of breast cancer in women currently or formerly using OCs, compared with women who had never used them.
This study compared 4,575 women who had breast cancer with 4,682 controls. The relative risk of breast cancer was 1.0 (95% confidence interval 0.8–1.3) among current OC users and 0.9 (0.8–1.0) among women who had previously used OCs. The relative risk did not increase consistently with higher doses or longer use.2 Nor did use of OCs add risk in women with a family history of breast cancer.2
OBG Management: An editorial accompanying that study said: “The importance of this finding for public health is enormous, because more than 75% of the women in the study had used oral contraceptives.”3
KAUNITZ: Yes, but this does not mean that women with a positive family history have no increased risk of breast cancer—they do. Rather, the use of hormonal contraceptives does not augment that risk further.
OBG Management: What about women with other high-risk factors, such as germline mutations? Do OCs increase their risk of breast cancer?
KAUNITZ: No. In the largest study to date of breast cancer risk associated with prior or current OC use in women 35 to 64 years of age with BRCA1 and 2 mutations, low-dose OC formulations did not increase it.4 In fact, OC use was associated with a significantly reduced risk of breast cancer in BRCA1 mutation carriers (odds ratio 0.22; 95% confidence interval 0.10–0.49).4
Pill reduces risk of ovarian cancer in BRCA carriers
KAUNITZ: It is also important to remember the higher risk of ovarian cancer in women with BRCA mutations. We now know that use of the Pill reduces ovarian cancer risk in BRCA-positive women, just as it does in the general population. In a study involving 451 women with BRCA1 or 2 mutations, who self-reported their lifetime history of OC use (or nonuse), the odds ratio for ovarian cancer associated with OC use for a minimum of 1 year was 0.85 (95% confidence interval 0.53–1.36) and declined by 5% (1%–9%) with each additional year of use (P for trend=.01). Use for 6 years or more carried an odds ratio of 0.62 (0.35–1.09).5
The bottom line: Women with a family history of breast cancer in general or BRCA1 or BRCA2 mutations more specifically, who have not completed childbearing or who want to avoid prophylactic mastectomy/oophorectomy, can use the Pill to prevent ovarian cancer without increasing their risk of breast cancer.5,6
2. Concomitant medications
Some drugs decrease steroid levels
Anticonvulsants
OBG Management: Many, perhaps most, women with medical conditions are already taking some kind of medication. What do ObGyns need to know about interactions between hormonal contraceptives and other medications, such as anticonvulsants and antibiotics?
KAUNITZ: We regularly encounter patients who are using anticonvulsants, both older and newer formulations.
Off-label use of anticonvulsants for indications other than seizure disorders (eg, bipolar disease) is increasing.
Levetiracetam and zonisamide. The revised practice bulletin includes pharmacokinetic data on 2 new anticonvulsants—levetiracetam7 and zonisamide.8 Fortunately, neither appears to reduce contraceptive steroid levels in women who are also taking combination OCs.
Which dosage, which method? Some widely used anticonvulsants do decrease steroid levels (TABLE 1); although some clinicians prescribe OCs containing 50 μg of ethinyl estradiol to offset the reduction, there is no evidence that this strategy is effective. The ObGyn may consider prescribing pills containing 30 to 35 μg of estradiol rather than lower doses, although again, we lack data to support this recommendation.
Another important point: Because serum steroid levels of women using progestin-only OCs and implants are lower than for combination OCs, low-dose progestin-only methods (progestin-only minipills and progestin implants) do not represent by themselves optimal contraceptives for women taking drugs (eg, anticonvulsants) that increase liver enzymes.9,10
This recommendation does not include the levonorgestrel-releasing intrauterine system. Contraceptive effects remain high with its use, even when anticonvulsants or other liver enzyme-inducing drugs are taken.11
DMPA, a high-dose progestin contraceptive, has not been formally studied in this regard; the efficacy of this injectable contraceptive does not appear to be reduced by concomitant use of enzyme inducers.30 Interestingly enough, DMPA has anticonvulsant effects itself and therefore may represent a particularly attractive contraceptive for women taking anticonvulsants.12
TABLE 1
Some anticonvulsants reduce steroid levels in women taking OCs, and some do not
| Interaction of anticonvulsants and combination OCs | |
|---|---|
| Anticonvulsants that decrease steroid levels in women taking oral contraceptives (OCs) | |
| Barbiturates (including phenobarbital and primidone) | |
| Carbamazepine and oxcarbazepine | |
| Felbamate | |
| Phenytoin | |
| Topiramate | |
| Vigabatrin | |
| Anticonvulsants that do not decrease steroid levels in women taking combination OCs | |
| Ethosuximide* | Tiagabine† |
| Gabapentin† | Valproic acid |
| Lamotrigine† | Zonisamide |
| Levetiracetam | |
| * No pharmacokinetic data available. | |
| †Pharmacokinetic study used anticonvulsant dose lower than that used in clinical practice. | |
| Source: American College of Obstetricians and Gynecologists.1 Reprinted by permission. | |
Antibiotics
KAUNITZ: As for antibiotics, we have often been taught that many drugs lower the efficacy of combination OCs, but in fact it is not clear that they do.
Rifampin. The only antibiotic for which we have pharmacokinetic evidence of substantially lower steroid levels is rifampin13 (although anecdotal reports of OC failure in women taking other antibiotics have been noted). Therefore, any woman who is taking rifampin should be advised that OCs (combination or progestin-only), transdermal or vaginal contraceptives, and hormonal implants are inadequate birth control (TABLE 2).
TABLE 2
Rifampin decreases steroid levels in women taking combination OCs; other anti-infectives do not
| Interaction of anti-infective agents and combination OCs | |
|---|---|
| Anti-infective that decreases steroid levels in women taking OCs | |
| Rifampin | |
| Anti-infectives that do not decrease steroid levels in women taking OCs | |
| Ampicillin | Miconazole* |
| Doxycycline | Quinolone antibiotics |
| Fluconazole | Tetracycline |
| Metronidazole | |
| *Vaginal administration does not lower steroid levels in women using the contraceptive vaginal ring. | |
| Source: American College of Obstetricians and Gynecologists.1 Reprinted by permission. | |
Antiretrovirals
KAUNITZ: Several small trials suggest that contraceptive steroid levels in OC users may be affected by antiretroviral medications (TABLE 3), but we lack clinical outcome studies.
TABLE 3
Antiretrovirals may affect steroid levels in women taking OCs
| Pharmacokinetic interactions between combination OCs and antiretroviral drugs | ||
|---|---|---|
| ANTIRETROVIRAL | CONTRACEPTIVE STEROID LEVELS | ANTIRETROVIRAL LEVELS |
| PROTEASE INHIBITORS | ||
| Nelfinavir | ↓ | No data |
| Ritonavir | ↓ | No data |
| Lopinavir/ritonavir | ↓ | No data |
| Atazanavir | ↑ | No data |
| Amprenavir | ↑ | ↓ |
| Indinavir | ↑ | No data |
| Saquinavir | No data | No change |
| NONNUCLEOSIDE REVERSE TRANSCRIPTASE INHIBITORS | ||
| Nevirapine | ↓ | No change |
| Efavirenz | ↑ | No change |
| Delavirdine | ?↑ | No data |
| Source: World Health Organization.29 Reprinted by permission. | ||
St. John’s wort
KAUNITZ: Another medication I want to mention is St. John’s wort, an over-the-counter hepatic enzyme inducer that many women take for depression. One clinical trial found elevated progestin and estrogen metabolism in women taking combination OCs and St. John’s wort concomitantly, as well as increased likelihood of breakthrough bleeding and ovulation.
St. John’s wort (300 mg thrice daily) was associated with a 13% to 15% reduction in the dose exposure of combination OCs containing 20 μg of ethinyl estradiol.14 So it is important to ask about St. John’s wort when counseling a woman about contraception.
St. John’s wort raised progestin and estrogen metabolism and increased breakthrough bleeding and ovulation in women taking OCs
3. Obesity
In obese women over 35, avoid combination OCs
OBG Management: ObGyns are seeing increasing numbers of overweight and obese women. Has selection of hormonal contraception for these women changed?
KAUNITZ: Yes. We now have a recognized obesity epidemic on our hands—and obesity, age over 35 years, and use of combination OCs all represent independent risk factors for thrombosis—prompting the question: Are there safer alternatives to combination OCs for obese women older than 35?
Tenfold risk for thromboembolism. Among the evidence addressing this question, a 2003 Dutch study found that women with a body mass index (BMI) greater than 25 who used combination OCs had 10 times the risk of venous thromboembolism of lean controls who did not use the Pill.15
Thus, progestin-only and intrauterine contraceptive methods may be more appropriate for older obese women. This language about obesity and combination contraceptives was not in the earlier version of the practice bulletin.
No need to rule out the patch
OBG Management: Aren’t some hormonal contraceptives less effective in obese women?
KAUNITZ: In a 2002 analysis of pooled data, women in the highest weight category (≥90 kg) who were using the contraceptive patch had a higher pregnancy rate than lower-weight women.16 However, this finding does not rule out use of the patch in overweight women who prefer it to less effective methods. Rather, it should be kept in mind when counseling these patients about their options.
Although the new ACOG bulletin cites data from Holt et al17 suggesting a higher failure rate in obese women using combination OCs, other OC clinical trials have not confirmed this association.18,19 In a study by Anderson and colleagues,18 which found no pregnancies among the heaviest women, the mean weight was 155.9 lb, but ranged from 91.0 to 360.0 lb, and the mean BMI was 26.0, but ranged from 15.2 to an extreme of 56.5!
What about DMPA? We also lack evidence of higher pregnancy rates among overweight women using DMPA (150-mg intramuscular or 104-mg subcutaneous formulations).20,21
4. Lupus
OCs are an option in some women with lupus
OBG Management: As The New England Journal of Medicine observed last year, there has been an “implicit moratorium” on prescribing combination hormonal contraceptives in women with systemic lupus erythematosus (SLE) because clinicians have feared that exogenous estrogens might exacerbate disease.22 This moratorium derives from data suggesting that estrogens worsen SLE, while androgens appear to protect against the condition.
The new practice bulletin now indicates that oral contraceptives are an option for this population. What is behind the change?
KAUNITZ: We now have data from 2 randomized clinical trials23,24 indicating that women can safely use combination OCs if they:
- have stable, mild disease
- are seronegative for antiphospholipid antibodies
- have no history of thrombosis
Disease remained stable. In the first trial,24 162 women with mild, stable SLE were randomized to combination OCs, progestin-only pills, or the copper IUD. Their disease level was established at baseline and over 12 months, using the Systemic Lupus Erythematosus Disease Activity Index. Disease remained stable in all 3 groups.
Estrogen did not increase severity of SLE. In the second trial,23 183 women with inactive or stable active SLE were randomized to combination OCs or placebo. The primary endpoint for this trial was severe lupus flare: 7 of 91 women (7.7%) in the OC group experienced a flare, compared with 7 of 92 women (7.6%) taking placebo. Thus, estrogen does not appear to increase the severity of SLE.
OBG Management: Isn’t another concern about patients with SLE the substantial risk of thrombosis?
KAUNITZ: Yes. In the first study,24 2 thromboses occurred in women taking OCs, and 2 occurred in women using the progestin-only pill. All the women with thromboses were seropositive for antiphospholipid antibodies. Hence, we need to exclude the presence of these antibodies in women with SLE prior to prescribing combination estrogen-progestin contraception. In women with lupus and a history of thrombosis, as with all women with a history of thrombosis, we should avoid combination hormonal contraception.
In the second study,23 which compared women on combination OCs to a placebo group, the OC group experienced 1 case of deep venous thrombosis (DVT) and 1 clotted graft. The placebo group experienced 1 case of DVT, 1 ocular thrombosis, 1 superficial thrombophlebitis, and 1 death (after the trial ended).
5. DMPA and bone density
DMPA does not appear to have long-term impact
OBG Management: There has been some furor over DMPA’s effect on bone mineral density (BMD). What are the latest findings in this area?
KAUNITZ: Studies assessing BMD in former DMPA users, including postmenopausal women, show that prior use of DMPA does not appear to have any long-term impact on BMD.25,26 In addition, more recent longitudinal data indicate that after DMPA is discontinued BMD fully recovers, which appears to take about 3 years in adults and as little as 1 year in teens.27,28
One population-based prospective cohort study followed 457 nongravid women aged 18 to 39 years. These women had bone density measured every 6 months for 3 years using dual-energy x-ray absorptiometry. Although bone density decreased among DMPA users at the spine and total hip compared with nonusers, there was no difference in bone density 30 months after discontinuation of birth control injections.27
In a study of 170 adolescent women 14 to 18 years of age, 80 were DMPA users and 90 were nonusers. Bone density was measured every 6 months for 24 or 36 months, and declined significantly at the hip and spine in DMPA users. Sixty-one women discontinued DMPA during the course of the study. After discontinuation, bone density increased at all anatomical sites, recovering completely over the course of 1 year.28
Although the US Food and Drug Administration issued a black box warning in the fall of 2004 that use of DMPA should be reconsidered after 2 years, particularly in teens, the studies I just mentioned suggest there should be no routine restrictions on the use of DMPA in terms of skeletal health, and that DMPA use by itself is not an indication for bone density measurement.
6. Patch and ring
Assume contraindications are the same as for OCs
OBG Management: What about newer forms of combination contraceptives such as the patch and ring?
KAUNITZ: The new practice bulletin contains information on the patch, the ring, and the levonorgestrel-releasing IUD, which were not available when the earlier bulletin was prepared. In that version we talked about combination pills only.
Even so, we continue to have much more data on the Pill than the ring or patch. For that reason, until more data become available, the patch and the ring should be assumed to have the same contraindications as combination OCs. We have very little data—if any—on the use of newer combination contraceptives in high-risk women or those with medical problems.
1. Use of Hormonal Contraception in Women With Coexisting Medical Conditions. ACOG Practice Bulletin #73. American College of Obstetricians and Gynecologists. Obstet Gynecol. 2006;107:1453-1472.
2. Marchbanks PA, McDonald JA, Wilson HG, et al. Oral contraceptives and the risk of breast cancer. N Engl J Med. 2002;346:2025-2032.
3. Davidson NE, Helzlsouer KJ. Good news about oral contraceptives. N Engl J Med. 2002;346:2078-2079.
4. Milne RL, Knight JA, John EM, et al. Oral contraceptive use and risk of early-onset breast cancer in carriers and non-carriers of BRCA1 and BRCA2 mutations. Cancer Epidemiol Biomarkers Prev. 2005;14:350-356.
5. Whittemore AS, Balise RR, Pharoah PD, et al. Oral contraceptive use and ovarian cancer risk among carriers of BRCA1 and BRCA2 mutations. Br J Cancer. 2004;91:1911-1915.
6. Narod SA, Risch H, Moslehi R, et al. Oral contraceptives and the risk of hereditary ovarian cancer. Hereditary Ovarian Cancer Clinical Study Group. N Engl J Med. 1998;339:424-428.
7. Ragueneau-Majlessi I, Levy RH, Janik F. Levetiracetam does not alter the pharmacokinetics of an oral contraceptive in healthy women. Epilepsia. 2002;43:697-702.
8. Griffith SG, Dai Y. Effect of zonisamide on the pharmacokinetics and pharmacodynamics of a combination ethinyl estradiol-norethindrone oral contraceptive in healthy women. Clin Ther. 2004;26:2056-2065.
9. McCann MF, Potter LS. Progestin-only oral contraception: a comprehensive review. Contraception. 1994;50(6 suppl 1):S1-S195.
10. Haukkamaa M. Contraception by Norplant subdermal capsules is not reliable in epileptic patients on anticonvulsant treatment. Contraception. 1986;33:559-565.
11. Bounds W, Guillebaud J. Observational series on women using the contraceptive Mirena concurrently with antiepileptic and other enzyme-inducing drugs. J Fam Plann Reprod Health Care. 2002;187:551-555.
12. Mattson RH, Rebar RW. Contraceptive methods for women with neurologic disorders. Am J Obstet Gynecol. 1993;168:2027-2032.
13. Back DJ, Breckenridge AM, Crawford F, et al. The effect of rifampicin on norethisterone pharmacokinetics. Eur J Clin Pharmacol. 1979;15:193-197.
14. Murphy PA, Kern SE, Stanczyk FZ, Westhoff CL. Interaction of St. John’s wort with oral contraceptives: effects on the pharmacokinetics of norethindrone and ethinyl estradiol, ovarian activity, and breakthrough bleeding. Contraception. 2005;71:402-408.
15. Abdollahi M, Cushman M, Rosendaal F. Obesity: risk of venous thrombosis and the interaction with coagulation factor levels and oral contraceptive use. Thromb Haemost. 2003;89:493-498.
16. Zieman M, Guillebaud J, et al. Contraceptive efficacy and cycle control with Ortho Evra/Evra transdermal system: the analysis of pooled data. Fertil Steril. 2002;77:S13-S18.
17. Holt VL, Scholes D, Wicklund KG, Cushing-Haugen KL, Daling JR. Body mass index, weight, and oral contraceptive failure risk. Obstet Gynecol. 2005;105:46-52.
18. Anderson FD. Hait H and the Seasonale-301 Study Group. A multicenter, randomized study of an extended cycle oral contraceptive. Contraception. 2003;68:89-96.
19. Anderson FD, Gibbons W, Portman D. Safety and efficacy of an extended-regimen oral contraceptive utilizing continuous low-dose ethinyl estradiol. Contraception. 2006;73:229-234.
20. Leiman G. Depo-medroxyprogesterone acetate as a contraceptive agent: its effect on weight and blood pressure. Am J Obstet Gynecol. 1972;114:97-102.
21. Jain J, Jakimiuk AJ, Bode FR, Ross D, Kaunitz AM. Contraceptive efficacy and safety of DMPA-SC. Contraception. 2004;70:269-275.
22. Bermas BL. Oral contraceptives in systemic lupus erythematosus—a tough pill to swallow? N Engl J Med. 2005;353:2602-2604.
23. Petri M, Kim MY, Kalunian KC, et al. Combined oral contraceptives in women with systemic lupus erythematosus. OC-SELENA Trial. N Engl J Med. 2005;353:2550-2558.
24. Sanchez-Guerrero, Urive AG, Jimenez-Santana L, et al. A trial of contraceptive methods in women with systemic lupus erythematosus. N Engl J Med. 2005;353:2539-2549.
25. Orr-Walker BJ, Evans MC, Ames RW, et al. The effect of past use of the injectable contraceptive depot medroxyprogesterone acetate on bone mineral density in normal postmenopausal women. Clin Endocrinol (Oxf). 1998;49:615-618.
26. Petitti DB, Piaggio G, Mehta S, Cravioto MC, Meirik O. Steroid hormone contraception and bone mineral density: a cross-sectional study in an international population. The WHO Study of Hormonal Contraception and Bone Health. Obstet Gynecol. 2000;95:736-744.
27. Scholes D, LaCroix AZ, Ichikawa LE, Barlow WE, Ott SM. Injectable hormone contraception and bone density: results from a prospective study [published erratum appears in Epidemiology. 2002;13:749]. Epidemiology. 2002;13:581-587.
28. Scholes D, LaCroix AZ, Ichikawa LE, et al. Change in bone mineral density among adolescent women using and discontinuing depot medroxyprogesterone acetate contraception. Arch Pediatr Adolesc Med. 2005;159:139-144.
29. World Health Organization. Medical eligibility criteria for contraceptive use. Annex 1. COCs and antiretroviral therapies. 3rd ed. Geneva: WHO; 2004.
30. Sapire KE. Depo-provera and carbamazapine. Br J Fam Plann. 1990;15:130.-
Dr. Kaunitz has received funding from Barr Laboratories, Berlex, Johnson & Johnson, and the National Institutes of Health. He is a speaker or consultant for the American College of Obstetricians and Gynecologists, the Association of Reproductive Health Professionals, Barr Laboratories, Berlex, Johnson & Johnson, Pfizer, and Procter & Gamble; and holds stock with Noven, Roche, and Sanofi-Aventis.
Decisive new data on risks and benefits of hormonal contraception will change how we manage many patients. These findings prompted the American College of Obstetricians and Gynecologists (ACOG) to update its practice bulletin (released in June) on hormonal contraception in women with coexisting medical conditions.1 Among the most notable areas of change:
- Family history of breast cancer or BRCA1 or 2 mutations. Combination oral contraceptives (OCs) do not appear to increase the risk of breast cancer in these women, and do help prevent ovarian cancer.
- Concomitant medications. More women are using enzyme-inducing anticonvulsants for conditions other than seizure disorders; some affect steroid levels.
- Obesity. Progestin-only and intrauterine methods may be better for obese women older than 35 years, who face an elevated baseline risk of venous thromboembolism.
- Lupus. Combination OCs are safe in women with stable, mild disease who are seronegative for antiphospholipid antibodies.
- Depot medroxyprogesterone acetate (DMPA). Although bone density declines in women using DMPA, it recovers within 3 years after the drug is discontinued.
- Patch and ring. Until method-specific data come in, assume that the patch and ring have the same contraindications as combination OCs.
For the fine points on these and other findings, we talked with Dr. Andrew M. Kaunitz, who assisted ACOG with preparation of the new bulletin.1
1. Breast cancer risk
OCs do not add to existing high risk
OBG Management: Women who have a family history of breast cancer have a higher-than-average risk of developing the cancer themselves, and it is widely assumed that estrogen further heightens that risk. Should these women avoid combination OCs?
KAUNITZ: No. Although these women have been reluctant to use hormonal contraceptives (as have many caregivers), we now have several lines of reassuring evidence.
Among the studies demonstrating safety of hormonal methods in this population is the 2002 Women’s CARE study, conducted by the Centers for Disease Control and Prevention and sponsored by the National Institute of Child Health and Human Development, which found no elevated risk of breast cancer in women currently or formerly using OCs, compared with women who had never used them.
This study compared 4,575 women who had breast cancer with 4,682 controls. The relative risk of breast cancer was 1.0 (95% confidence interval 0.8–1.3) among current OC users and 0.9 (0.8–1.0) among women who had previously used OCs. The relative risk did not increase consistently with higher doses or longer use.2 Nor did use of OCs add risk in women with a family history of breast cancer.2
OBG Management: An editorial accompanying that study said: “The importance of this finding for public health is enormous, because more than 75% of the women in the study had used oral contraceptives.”3
KAUNITZ: Yes, but this does not mean that women with a positive family history have no increased risk of breast cancer—they do. Rather, the use of hormonal contraceptives does not augment that risk further.
OBG Management: What about women with other high-risk factors, such as germline mutations? Do OCs increase their risk of breast cancer?
KAUNITZ: No. In the largest study to date of breast cancer risk associated with prior or current OC use in women 35 to 64 years of age with BRCA1 and 2 mutations, low-dose OC formulations did not increase it.4 In fact, OC use was associated with a significantly reduced risk of breast cancer in BRCA1 mutation carriers (odds ratio 0.22; 95% confidence interval 0.10–0.49).4
Pill reduces risk of ovarian cancer in BRCA carriers
KAUNITZ: It is also important to remember the higher risk of ovarian cancer in women with BRCA mutations. We now know that use of the Pill reduces ovarian cancer risk in BRCA-positive women, just as it does in the general population. In a study involving 451 women with BRCA1 or 2 mutations, who self-reported their lifetime history of OC use (or nonuse), the odds ratio for ovarian cancer associated with OC use for a minimum of 1 year was 0.85 (95% confidence interval 0.53–1.36) and declined by 5% (1%–9%) with each additional year of use (P for trend=.01). Use for 6 years or more carried an odds ratio of 0.62 (0.35–1.09).5
The bottom line: Women with a family history of breast cancer in general or BRCA1 or BRCA2 mutations more specifically, who have not completed childbearing or who want to avoid prophylactic mastectomy/oophorectomy, can use the Pill to prevent ovarian cancer without increasing their risk of breast cancer.5,6
2. Concomitant medications
Some drugs decrease steroid levels
Anticonvulsants
OBG Management: Many, perhaps most, women with medical conditions are already taking some kind of medication. What do ObGyns need to know about interactions between hormonal contraceptives and other medications, such as anticonvulsants and antibiotics?
KAUNITZ: We regularly encounter patients who are using anticonvulsants, both older and newer formulations.
Off-label use of anticonvulsants for indications other than seizure disorders (eg, bipolar disease) is increasing.
Levetiracetam and zonisamide. The revised practice bulletin includes pharmacokinetic data on 2 new anticonvulsants—levetiracetam7 and zonisamide.8 Fortunately, neither appears to reduce contraceptive steroid levels in women who are also taking combination OCs.
Which dosage, which method? Some widely used anticonvulsants do decrease steroid levels (TABLE 1); although some clinicians prescribe OCs containing 50 μg of ethinyl estradiol to offset the reduction, there is no evidence that this strategy is effective. The ObGyn may consider prescribing pills containing 30 to 35 μg of estradiol rather than lower doses, although again, we lack data to support this recommendation.
Another important point: Because serum steroid levels of women using progestin-only OCs and implants are lower than for combination OCs, low-dose progestin-only methods (progestin-only minipills and progestin implants) do not represent by themselves optimal contraceptives for women taking drugs (eg, anticonvulsants) that increase liver enzymes.9,10
This recommendation does not include the levonorgestrel-releasing intrauterine system. Contraceptive effects remain high with its use, even when anticonvulsants or other liver enzyme-inducing drugs are taken.11
DMPA, a high-dose progestin contraceptive, has not been formally studied in this regard; the efficacy of this injectable contraceptive does not appear to be reduced by concomitant use of enzyme inducers.30 Interestingly enough, DMPA has anticonvulsant effects itself and therefore may represent a particularly attractive contraceptive for women taking anticonvulsants.12
TABLE 1
Some anticonvulsants reduce steroid levels in women taking OCs, and some do not
| Interaction of anticonvulsants and combination OCs | |
|---|---|
| Anticonvulsants that decrease steroid levels in women taking oral contraceptives (OCs) | |
| Barbiturates (including phenobarbital and primidone) | |
| Carbamazepine and oxcarbazepine | |
| Felbamate | |
| Phenytoin | |
| Topiramate | |
| Vigabatrin | |
| Anticonvulsants that do not decrease steroid levels in women taking combination OCs | |
| Ethosuximide* | Tiagabine† |
| Gabapentin† | Valproic acid |
| Lamotrigine† | Zonisamide |
| Levetiracetam | |
| * No pharmacokinetic data available. | |
| †Pharmacokinetic study used anticonvulsant dose lower than that used in clinical practice. | |
| Source: American College of Obstetricians and Gynecologists.1 Reprinted by permission. | |
Antibiotics
KAUNITZ: As for antibiotics, we have often been taught that many drugs lower the efficacy of combination OCs, but in fact it is not clear that they do.
Rifampin. The only antibiotic for which we have pharmacokinetic evidence of substantially lower steroid levels is rifampin13 (although anecdotal reports of OC failure in women taking other antibiotics have been noted). Therefore, any woman who is taking rifampin should be advised that OCs (combination or progestin-only), transdermal or vaginal contraceptives, and hormonal implants are inadequate birth control (TABLE 2).
TABLE 2
Rifampin decreases steroid levels in women taking combination OCs; other anti-infectives do not
| Interaction of anti-infective agents and combination OCs | |
|---|---|
| Anti-infective that decreases steroid levels in women taking OCs | |
| Rifampin | |
| Anti-infectives that do not decrease steroid levels in women taking OCs | |
| Ampicillin | Miconazole* |
| Doxycycline | Quinolone antibiotics |
| Fluconazole | Tetracycline |
| Metronidazole | |
| *Vaginal administration does not lower steroid levels in women using the contraceptive vaginal ring. | |
| Source: American College of Obstetricians and Gynecologists.1 Reprinted by permission. | |
Antiretrovirals
KAUNITZ: Several small trials suggest that contraceptive steroid levels in OC users may be affected by antiretroviral medications (TABLE 3), but we lack clinical outcome studies.
TABLE 3
Antiretrovirals may affect steroid levels in women taking OCs
| Pharmacokinetic interactions between combination OCs and antiretroviral drugs | ||
|---|---|---|
| ANTIRETROVIRAL | CONTRACEPTIVE STEROID LEVELS | ANTIRETROVIRAL LEVELS |
| PROTEASE INHIBITORS | ||
| Nelfinavir | ↓ | No data |
| Ritonavir | ↓ | No data |
| Lopinavir/ritonavir | ↓ | No data |
| Atazanavir | ↑ | No data |
| Amprenavir | ↑ | ↓ |
| Indinavir | ↑ | No data |
| Saquinavir | No data | No change |
| NONNUCLEOSIDE REVERSE TRANSCRIPTASE INHIBITORS | ||
| Nevirapine | ↓ | No change |
| Efavirenz | ↑ | No change |
| Delavirdine | ?↑ | No data |
| Source: World Health Organization.29 Reprinted by permission. | ||
St. John’s wort
KAUNITZ: Another medication I want to mention is St. John’s wort, an over-the-counter hepatic enzyme inducer that many women take for depression. One clinical trial found elevated progestin and estrogen metabolism in women taking combination OCs and St. John’s wort concomitantly, as well as increased likelihood of breakthrough bleeding and ovulation.
St. John’s wort (300 mg thrice daily) was associated with a 13% to 15% reduction in the dose exposure of combination OCs containing 20 μg of ethinyl estradiol.14 So it is important to ask about St. John’s wort when counseling a woman about contraception.
St. John’s wort raised progestin and estrogen metabolism and increased breakthrough bleeding and ovulation in women taking OCs
3. Obesity
In obese women over 35, avoid combination OCs
OBG Management: ObGyns are seeing increasing numbers of overweight and obese women. Has selection of hormonal contraception for these women changed?
KAUNITZ: Yes. We now have a recognized obesity epidemic on our hands—and obesity, age over 35 years, and use of combination OCs all represent independent risk factors for thrombosis—prompting the question: Are there safer alternatives to combination OCs for obese women older than 35?
Tenfold risk for thromboembolism. Among the evidence addressing this question, a 2003 Dutch study found that women with a body mass index (BMI) greater than 25 who used combination OCs had 10 times the risk of venous thromboembolism of lean controls who did not use the Pill.15
Thus, progestin-only and intrauterine contraceptive methods may be more appropriate for older obese women. This language about obesity and combination contraceptives was not in the earlier version of the practice bulletin.
No need to rule out the patch
OBG Management: Aren’t some hormonal contraceptives less effective in obese women?
KAUNITZ: In a 2002 analysis of pooled data, women in the highest weight category (≥90 kg) who were using the contraceptive patch had a higher pregnancy rate than lower-weight women.16 However, this finding does not rule out use of the patch in overweight women who prefer it to less effective methods. Rather, it should be kept in mind when counseling these patients about their options.
Although the new ACOG bulletin cites data from Holt et al17 suggesting a higher failure rate in obese women using combination OCs, other OC clinical trials have not confirmed this association.18,19 In a study by Anderson and colleagues,18 which found no pregnancies among the heaviest women, the mean weight was 155.9 lb, but ranged from 91.0 to 360.0 lb, and the mean BMI was 26.0, but ranged from 15.2 to an extreme of 56.5!
What about DMPA? We also lack evidence of higher pregnancy rates among overweight women using DMPA (150-mg intramuscular or 104-mg subcutaneous formulations).20,21
4. Lupus
OCs are an option in some women with lupus
OBG Management: As The New England Journal of Medicine observed last year, there has been an “implicit moratorium” on prescribing combination hormonal contraceptives in women with systemic lupus erythematosus (SLE) because clinicians have feared that exogenous estrogens might exacerbate disease.22 This moratorium derives from data suggesting that estrogens worsen SLE, while androgens appear to protect against the condition.
The new practice bulletin now indicates that oral contraceptives are an option for this population. What is behind the change?
KAUNITZ: We now have data from 2 randomized clinical trials23,24 indicating that women can safely use combination OCs if they:
- have stable, mild disease
- are seronegative for antiphospholipid antibodies
- have no history of thrombosis
Disease remained stable. In the first trial,24 162 women with mild, stable SLE were randomized to combination OCs, progestin-only pills, or the copper IUD. Their disease level was established at baseline and over 12 months, using the Systemic Lupus Erythematosus Disease Activity Index. Disease remained stable in all 3 groups.
Estrogen did not increase severity of SLE. In the second trial,23 183 women with inactive or stable active SLE were randomized to combination OCs or placebo. The primary endpoint for this trial was severe lupus flare: 7 of 91 women (7.7%) in the OC group experienced a flare, compared with 7 of 92 women (7.6%) taking placebo. Thus, estrogen does not appear to increase the severity of SLE.
OBG Management: Isn’t another concern about patients with SLE the substantial risk of thrombosis?
KAUNITZ: Yes. In the first study,24 2 thromboses occurred in women taking OCs, and 2 occurred in women using the progestin-only pill. All the women with thromboses were seropositive for antiphospholipid antibodies. Hence, we need to exclude the presence of these antibodies in women with SLE prior to prescribing combination estrogen-progestin contraception. In women with lupus and a history of thrombosis, as with all women with a history of thrombosis, we should avoid combination hormonal contraception.
In the second study,23 which compared women on combination OCs to a placebo group, the OC group experienced 1 case of deep venous thrombosis (DVT) and 1 clotted graft. The placebo group experienced 1 case of DVT, 1 ocular thrombosis, 1 superficial thrombophlebitis, and 1 death (after the trial ended).
5. DMPA and bone density
DMPA does not appear to have long-term impact
OBG Management: There has been some furor over DMPA’s effect on bone mineral density (BMD). What are the latest findings in this area?
KAUNITZ: Studies assessing BMD in former DMPA users, including postmenopausal women, show that prior use of DMPA does not appear to have any long-term impact on BMD.25,26 In addition, more recent longitudinal data indicate that after DMPA is discontinued BMD fully recovers, which appears to take about 3 years in adults and as little as 1 year in teens.27,28
One population-based prospective cohort study followed 457 nongravid women aged 18 to 39 years. These women had bone density measured every 6 months for 3 years using dual-energy x-ray absorptiometry. Although bone density decreased among DMPA users at the spine and total hip compared with nonusers, there was no difference in bone density 30 months after discontinuation of birth control injections.27
In a study of 170 adolescent women 14 to 18 years of age, 80 were DMPA users and 90 were nonusers. Bone density was measured every 6 months for 24 or 36 months, and declined significantly at the hip and spine in DMPA users. Sixty-one women discontinued DMPA during the course of the study. After discontinuation, bone density increased at all anatomical sites, recovering completely over the course of 1 year.28
Although the US Food and Drug Administration issued a black box warning in the fall of 2004 that use of DMPA should be reconsidered after 2 years, particularly in teens, the studies I just mentioned suggest there should be no routine restrictions on the use of DMPA in terms of skeletal health, and that DMPA use by itself is not an indication for bone density measurement.
6. Patch and ring
Assume contraindications are the same as for OCs
OBG Management: What about newer forms of combination contraceptives such as the patch and ring?
KAUNITZ: The new practice bulletin contains information on the patch, the ring, and the levonorgestrel-releasing IUD, which were not available when the earlier bulletin was prepared. In that version we talked about combination pills only.
Even so, we continue to have much more data on the Pill than the ring or patch. For that reason, until more data become available, the patch and the ring should be assumed to have the same contraindications as combination OCs. We have very little data—if any—on the use of newer combination contraceptives in high-risk women or those with medical problems.
Decisive new data on risks and benefits of hormonal contraception will change how we manage many patients. These findings prompted the American College of Obstetricians and Gynecologists (ACOG) to update its practice bulletin (released in June) on hormonal contraception in women with coexisting medical conditions.1 Among the most notable areas of change:
- Family history of breast cancer or BRCA1 or 2 mutations. Combination oral contraceptives (OCs) do not appear to increase the risk of breast cancer in these women, and do help prevent ovarian cancer.
- Concomitant medications. More women are using enzyme-inducing anticonvulsants for conditions other than seizure disorders; some affect steroid levels.
- Obesity. Progestin-only and intrauterine methods may be better for obese women older than 35 years, who face an elevated baseline risk of venous thromboembolism.
- Lupus. Combination OCs are safe in women with stable, mild disease who are seronegative for antiphospholipid antibodies.
- Depot medroxyprogesterone acetate (DMPA). Although bone density declines in women using DMPA, it recovers within 3 years after the drug is discontinued.
- Patch and ring. Until method-specific data come in, assume that the patch and ring have the same contraindications as combination OCs.
For the fine points on these and other findings, we talked with Dr. Andrew M. Kaunitz, who assisted ACOG with preparation of the new bulletin.1
1. Breast cancer risk
OCs do not add to existing high risk
OBG Management: Women who have a family history of breast cancer have a higher-than-average risk of developing the cancer themselves, and it is widely assumed that estrogen further heightens that risk. Should these women avoid combination OCs?
KAUNITZ: No. Although these women have been reluctant to use hormonal contraceptives (as have many caregivers), we now have several lines of reassuring evidence.
Among the studies demonstrating safety of hormonal methods in this population is the 2002 Women’s CARE study, conducted by the Centers for Disease Control and Prevention and sponsored by the National Institute of Child Health and Human Development, which found no elevated risk of breast cancer in women currently or formerly using OCs, compared with women who had never used them.
This study compared 4,575 women who had breast cancer with 4,682 controls. The relative risk of breast cancer was 1.0 (95% confidence interval 0.8–1.3) among current OC users and 0.9 (0.8–1.0) among women who had previously used OCs. The relative risk did not increase consistently with higher doses or longer use.2 Nor did use of OCs add risk in women with a family history of breast cancer.2
OBG Management: An editorial accompanying that study said: “The importance of this finding for public health is enormous, because more than 75% of the women in the study had used oral contraceptives.”3
KAUNITZ: Yes, but this does not mean that women with a positive family history have no increased risk of breast cancer—they do. Rather, the use of hormonal contraceptives does not augment that risk further.
OBG Management: What about women with other high-risk factors, such as germline mutations? Do OCs increase their risk of breast cancer?
KAUNITZ: No. In the largest study to date of breast cancer risk associated with prior or current OC use in women 35 to 64 years of age with BRCA1 and 2 mutations, low-dose OC formulations did not increase it.4 In fact, OC use was associated with a significantly reduced risk of breast cancer in BRCA1 mutation carriers (odds ratio 0.22; 95% confidence interval 0.10–0.49).4
Pill reduces risk of ovarian cancer in BRCA carriers
KAUNITZ: It is also important to remember the higher risk of ovarian cancer in women with BRCA mutations. We now know that use of the Pill reduces ovarian cancer risk in BRCA-positive women, just as it does in the general population. In a study involving 451 women with BRCA1 or 2 mutations, who self-reported their lifetime history of OC use (or nonuse), the odds ratio for ovarian cancer associated with OC use for a minimum of 1 year was 0.85 (95% confidence interval 0.53–1.36) and declined by 5% (1%–9%) with each additional year of use (P for trend=.01). Use for 6 years or more carried an odds ratio of 0.62 (0.35–1.09).5
The bottom line: Women with a family history of breast cancer in general or BRCA1 or BRCA2 mutations more specifically, who have not completed childbearing or who want to avoid prophylactic mastectomy/oophorectomy, can use the Pill to prevent ovarian cancer without increasing their risk of breast cancer.5,6
2. Concomitant medications
Some drugs decrease steroid levels
Anticonvulsants
OBG Management: Many, perhaps most, women with medical conditions are already taking some kind of medication. What do ObGyns need to know about interactions between hormonal contraceptives and other medications, such as anticonvulsants and antibiotics?
KAUNITZ: We regularly encounter patients who are using anticonvulsants, both older and newer formulations.
Off-label use of anticonvulsants for indications other than seizure disorders (eg, bipolar disease) is increasing.
Levetiracetam and zonisamide. The revised practice bulletin includes pharmacokinetic data on 2 new anticonvulsants—levetiracetam7 and zonisamide.8 Fortunately, neither appears to reduce contraceptive steroid levels in women who are also taking combination OCs.
Which dosage, which method? Some widely used anticonvulsants do decrease steroid levels (TABLE 1); although some clinicians prescribe OCs containing 50 μg of ethinyl estradiol to offset the reduction, there is no evidence that this strategy is effective. The ObGyn may consider prescribing pills containing 30 to 35 μg of estradiol rather than lower doses, although again, we lack data to support this recommendation.
Another important point: Because serum steroid levels of women using progestin-only OCs and implants are lower than for combination OCs, low-dose progestin-only methods (progestin-only minipills and progestin implants) do not represent by themselves optimal contraceptives for women taking drugs (eg, anticonvulsants) that increase liver enzymes.9,10
This recommendation does not include the levonorgestrel-releasing intrauterine system. Contraceptive effects remain high with its use, even when anticonvulsants or other liver enzyme-inducing drugs are taken.11
DMPA, a high-dose progestin contraceptive, has not been formally studied in this regard; the efficacy of this injectable contraceptive does not appear to be reduced by concomitant use of enzyme inducers.30 Interestingly enough, DMPA has anticonvulsant effects itself and therefore may represent a particularly attractive contraceptive for women taking anticonvulsants.12
TABLE 1
Some anticonvulsants reduce steroid levels in women taking OCs, and some do not
| Interaction of anticonvulsants and combination OCs | |
|---|---|
| Anticonvulsants that decrease steroid levels in women taking oral contraceptives (OCs) | |
| Barbiturates (including phenobarbital and primidone) | |
| Carbamazepine and oxcarbazepine | |
| Felbamate | |
| Phenytoin | |
| Topiramate | |
| Vigabatrin | |
| Anticonvulsants that do not decrease steroid levels in women taking combination OCs | |
| Ethosuximide* | Tiagabine† |
| Gabapentin† | Valproic acid |
| Lamotrigine† | Zonisamide |
| Levetiracetam | |
| * No pharmacokinetic data available. | |
| †Pharmacokinetic study used anticonvulsant dose lower than that used in clinical practice. | |
| Source: American College of Obstetricians and Gynecologists.1 Reprinted by permission. | |
Antibiotics
KAUNITZ: As for antibiotics, we have often been taught that many drugs lower the efficacy of combination OCs, but in fact it is not clear that they do.
Rifampin. The only antibiotic for which we have pharmacokinetic evidence of substantially lower steroid levels is rifampin13 (although anecdotal reports of OC failure in women taking other antibiotics have been noted). Therefore, any woman who is taking rifampin should be advised that OCs (combination or progestin-only), transdermal or vaginal contraceptives, and hormonal implants are inadequate birth control (TABLE 2).
TABLE 2
Rifampin decreases steroid levels in women taking combination OCs; other anti-infectives do not
| Interaction of anti-infective agents and combination OCs | |
|---|---|
| Anti-infective that decreases steroid levels in women taking OCs | |
| Rifampin | |
| Anti-infectives that do not decrease steroid levels in women taking OCs | |
| Ampicillin | Miconazole* |
| Doxycycline | Quinolone antibiotics |
| Fluconazole | Tetracycline |
| Metronidazole | |
| *Vaginal administration does not lower steroid levels in women using the contraceptive vaginal ring. | |
| Source: American College of Obstetricians and Gynecologists.1 Reprinted by permission. | |
Antiretrovirals
KAUNITZ: Several small trials suggest that contraceptive steroid levels in OC users may be affected by antiretroviral medications (TABLE 3), but we lack clinical outcome studies.
TABLE 3
Antiretrovirals may affect steroid levels in women taking OCs
| Pharmacokinetic interactions between combination OCs and antiretroviral drugs | ||
|---|---|---|
| ANTIRETROVIRAL | CONTRACEPTIVE STEROID LEVELS | ANTIRETROVIRAL LEVELS |
| PROTEASE INHIBITORS | ||
| Nelfinavir | ↓ | No data |
| Ritonavir | ↓ | No data |
| Lopinavir/ritonavir | ↓ | No data |
| Atazanavir | ↑ | No data |
| Amprenavir | ↑ | ↓ |
| Indinavir | ↑ | No data |
| Saquinavir | No data | No change |
| NONNUCLEOSIDE REVERSE TRANSCRIPTASE INHIBITORS | ||
| Nevirapine | ↓ | No change |
| Efavirenz | ↑ | No change |
| Delavirdine | ?↑ | No data |
| Source: World Health Organization.29 Reprinted by permission. | ||
St. John’s wort
KAUNITZ: Another medication I want to mention is St. John’s wort, an over-the-counter hepatic enzyme inducer that many women take for depression. One clinical trial found elevated progestin and estrogen metabolism in women taking combination OCs and St. John’s wort concomitantly, as well as increased likelihood of breakthrough bleeding and ovulation.
St. John’s wort (300 mg thrice daily) was associated with a 13% to 15% reduction in the dose exposure of combination OCs containing 20 μg of ethinyl estradiol.14 So it is important to ask about St. John’s wort when counseling a woman about contraception.
St. John’s wort raised progestin and estrogen metabolism and increased breakthrough bleeding and ovulation in women taking OCs
3. Obesity
In obese women over 35, avoid combination OCs
OBG Management: ObGyns are seeing increasing numbers of overweight and obese women. Has selection of hormonal contraception for these women changed?
KAUNITZ: Yes. We now have a recognized obesity epidemic on our hands—and obesity, age over 35 years, and use of combination OCs all represent independent risk factors for thrombosis—prompting the question: Are there safer alternatives to combination OCs for obese women older than 35?
Tenfold risk for thromboembolism. Among the evidence addressing this question, a 2003 Dutch study found that women with a body mass index (BMI) greater than 25 who used combination OCs had 10 times the risk of venous thromboembolism of lean controls who did not use the Pill.15
Thus, progestin-only and intrauterine contraceptive methods may be more appropriate for older obese women. This language about obesity and combination contraceptives was not in the earlier version of the practice bulletin.
No need to rule out the patch
OBG Management: Aren’t some hormonal contraceptives less effective in obese women?
KAUNITZ: In a 2002 analysis of pooled data, women in the highest weight category (≥90 kg) who were using the contraceptive patch had a higher pregnancy rate than lower-weight women.16 However, this finding does not rule out use of the patch in overweight women who prefer it to less effective methods. Rather, it should be kept in mind when counseling these patients about their options.
Although the new ACOG bulletin cites data from Holt et al17 suggesting a higher failure rate in obese women using combination OCs, other OC clinical trials have not confirmed this association.18,19 In a study by Anderson and colleagues,18 which found no pregnancies among the heaviest women, the mean weight was 155.9 lb, but ranged from 91.0 to 360.0 lb, and the mean BMI was 26.0, but ranged from 15.2 to an extreme of 56.5!
What about DMPA? We also lack evidence of higher pregnancy rates among overweight women using DMPA (150-mg intramuscular or 104-mg subcutaneous formulations).20,21
4. Lupus
OCs are an option in some women with lupus
OBG Management: As The New England Journal of Medicine observed last year, there has been an “implicit moratorium” on prescribing combination hormonal contraceptives in women with systemic lupus erythematosus (SLE) because clinicians have feared that exogenous estrogens might exacerbate disease.22 This moratorium derives from data suggesting that estrogens worsen SLE, while androgens appear to protect against the condition.
The new practice bulletin now indicates that oral contraceptives are an option for this population. What is behind the change?
KAUNITZ: We now have data from 2 randomized clinical trials23,24 indicating that women can safely use combination OCs if they:
- have stable, mild disease
- are seronegative for antiphospholipid antibodies
- have no history of thrombosis
Disease remained stable. In the first trial,24 162 women with mild, stable SLE were randomized to combination OCs, progestin-only pills, or the copper IUD. Their disease level was established at baseline and over 12 months, using the Systemic Lupus Erythematosus Disease Activity Index. Disease remained stable in all 3 groups.
Estrogen did not increase severity of SLE. In the second trial,23 183 women with inactive or stable active SLE were randomized to combination OCs or placebo. The primary endpoint for this trial was severe lupus flare: 7 of 91 women (7.7%) in the OC group experienced a flare, compared with 7 of 92 women (7.6%) taking placebo. Thus, estrogen does not appear to increase the severity of SLE.
OBG Management: Isn’t another concern about patients with SLE the substantial risk of thrombosis?
KAUNITZ: Yes. In the first study,24 2 thromboses occurred in women taking OCs, and 2 occurred in women using the progestin-only pill. All the women with thromboses were seropositive for antiphospholipid antibodies. Hence, we need to exclude the presence of these antibodies in women with SLE prior to prescribing combination estrogen-progestin contraception. In women with lupus and a history of thrombosis, as with all women with a history of thrombosis, we should avoid combination hormonal contraception.
In the second study,23 which compared women on combination OCs to a placebo group, the OC group experienced 1 case of deep venous thrombosis (DVT) and 1 clotted graft. The placebo group experienced 1 case of DVT, 1 ocular thrombosis, 1 superficial thrombophlebitis, and 1 death (after the trial ended).
5. DMPA and bone density
DMPA does not appear to have long-term impact
OBG Management: There has been some furor over DMPA’s effect on bone mineral density (BMD). What are the latest findings in this area?
KAUNITZ: Studies assessing BMD in former DMPA users, including postmenopausal women, show that prior use of DMPA does not appear to have any long-term impact on BMD.25,26 In addition, more recent longitudinal data indicate that after DMPA is discontinued BMD fully recovers, which appears to take about 3 years in adults and as little as 1 year in teens.27,28
One population-based prospective cohort study followed 457 nongravid women aged 18 to 39 years. These women had bone density measured every 6 months for 3 years using dual-energy x-ray absorptiometry. Although bone density decreased among DMPA users at the spine and total hip compared with nonusers, there was no difference in bone density 30 months after discontinuation of birth control injections.27
In a study of 170 adolescent women 14 to 18 years of age, 80 were DMPA users and 90 were nonusers. Bone density was measured every 6 months for 24 or 36 months, and declined significantly at the hip and spine in DMPA users. Sixty-one women discontinued DMPA during the course of the study. After discontinuation, bone density increased at all anatomical sites, recovering completely over the course of 1 year.28
Although the US Food and Drug Administration issued a black box warning in the fall of 2004 that use of DMPA should be reconsidered after 2 years, particularly in teens, the studies I just mentioned suggest there should be no routine restrictions on the use of DMPA in terms of skeletal health, and that DMPA use by itself is not an indication for bone density measurement.
6. Patch and ring
Assume contraindications are the same as for OCs
OBG Management: What about newer forms of combination contraceptives such as the patch and ring?
KAUNITZ: The new practice bulletin contains information on the patch, the ring, and the levonorgestrel-releasing IUD, which were not available when the earlier bulletin was prepared. In that version we talked about combination pills only.
Even so, we continue to have much more data on the Pill than the ring or patch. For that reason, until more data become available, the patch and the ring should be assumed to have the same contraindications as combination OCs. We have very little data—if any—on the use of newer combination contraceptives in high-risk women or those with medical problems.
1. Use of Hormonal Contraception in Women With Coexisting Medical Conditions. ACOG Practice Bulletin #73. American College of Obstetricians and Gynecologists. Obstet Gynecol. 2006;107:1453-1472.
2. Marchbanks PA, McDonald JA, Wilson HG, et al. Oral contraceptives and the risk of breast cancer. N Engl J Med. 2002;346:2025-2032.
3. Davidson NE, Helzlsouer KJ. Good news about oral contraceptives. N Engl J Med. 2002;346:2078-2079.
4. Milne RL, Knight JA, John EM, et al. Oral contraceptive use and risk of early-onset breast cancer in carriers and non-carriers of BRCA1 and BRCA2 mutations. Cancer Epidemiol Biomarkers Prev. 2005;14:350-356.
5. Whittemore AS, Balise RR, Pharoah PD, et al. Oral contraceptive use and ovarian cancer risk among carriers of BRCA1 and BRCA2 mutations. Br J Cancer. 2004;91:1911-1915.
6. Narod SA, Risch H, Moslehi R, et al. Oral contraceptives and the risk of hereditary ovarian cancer. Hereditary Ovarian Cancer Clinical Study Group. N Engl J Med. 1998;339:424-428.
7. Ragueneau-Majlessi I, Levy RH, Janik F. Levetiracetam does not alter the pharmacokinetics of an oral contraceptive in healthy women. Epilepsia. 2002;43:697-702.
8. Griffith SG, Dai Y. Effect of zonisamide on the pharmacokinetics and pharmacodynamics of a combination ethinyl estradiol-norethindrone oral contraceptive in healthy women. Clin Ther. 2004;26:2056-2065.
9. McCann MF, Potter LS. Progestin-only oral contraception: a comprehensive review. Contraception. 1994;50(6 suppl 1):S1-S195.
10. Haukkamaa M. Contraception by Norplant subdermal capsules is not reliable in epileptic patients on anticonvulsant treatment. Contraception. 1986;33:559-565.
11. Bounds W, Guillebaud J. Observational series on women using the contraceptive Mirena concurrently with antiepileptic and other enzyme-inducing drugs. J Fam Plann Reprod Health Care. 2002;187:551-555.
12. Mattson RH, Rebar RW. Contraceptive methods for women with neurologic disorders. Am J Obstet Gynecol. 1993;168:2027-2032.
13. Back DJ, Breckenridge AM, Crawford F, et al. The effect of rifampicin on norethisterone pharmacokinetics. Eur J Clin Pharmacol. 1979;15:193-197.
14. Murphy PA, Kern SE, Stanczyk FZ, Westhoff CL. Interaction of St. John’s wort with oral contraceptives: effects on the pharmacokinetics of norethindrone and ethinyl estradiol, ovarian activity, and breakthrough bleeding. Contraception. 2005;71:402-408.
15. Abdollahi M, Cushman M, Rosendaal F. Obesity: risk of venous thrombosis and the interaction with coagulation factor levels and oral contraceptive use. Thromb Haemost. 2003;89:493-498.
16. Zieman M, Guillebaud J, et al. Contraceptive efficacy and cycle control with Ortho Evra/Evra transdermal system: the analysis of pooled data. Fertil Steril. 2002;77:S13-S18.
17. Holt VL, Scholes D, Wicklund KG, Cushing-Haugen KL, Daling JR. Body mass index, weight, and oral contraceptive failure risk. Obstet Gynecol. 2005;105:46-52.
18. Anderson FD. Hait H and the Seasonale-301 Study Group. A multicenter, randomized study of an extended cycle oral contraceptive. Contraception. 2003;68:89-96.
19. Anderson FD, Gibbons W, Portman D. Safety and efficacy of an extended-regimen oral contraceptive utilizing continuous low-dose ethinyl estradiol. Contraception. 2006;73:229-234.
20. Leiman G. Depo-medroxyprogesterone acetate as a contraceptive agent: its effect on weight and blood pressure. Am J Obstet Gynecol. 1972;114:97-102.
21. Jain J, Jakimiuk AJ, Bode FR, Ross D, Kaunitz AM. Contraceptive efficacy and safety of DMPA-SC. Contraception. 2004;70:269-275.
22. Bermas BL. Oral contraceptives in systemic lupus erythematosus—a tough pill to swallow? N Engl J Med. 2005;353:2602-2604.
23. Petri M, Kim MY, Kalunian KC, et al. Combined oral contraceptives in women with systemic lupus erythematosus. OC-SELENA Trial. N Engl J Med. 2005;353:2550-2558.
24. Sanchez-Guerrero, Urive AG, Jimenez-Santana L, et al. A trial of contraceptive methods in women with systemic lupus erythematosus. N Engl J Med. 2005;353:2539-2549.
25. Orr-Walker BJ, Evans MC, Ames RW, et al. The effect of past use of the injectable contraceptive depot medroxyprogesterone acetate on bone mineral density in normal postmenopausal women. Clin Endocrinol (Oxf). 1998;49:615-618.
26. Petitti DB, Piaggio G, Mehta S, Cravioto MC, Meirik O. Steroid hormone contraception and bone mineral density: a cross-sectional study in an international population. The WHO Study of Hormonal Contraception and Bone Health. Obstet Gynecol. 2000;95:736-744.
27. Scholes D, LaCroix AZ, Ichikawa LE, Barlow WE, Ott SM. Injectable hormone contraception and bone density: results from a prospective study [published erratum appears in Epidemiology. 2002;13:749]. Epidemiology. 2002;13:581-587.
28. Scholes D, LaCroix AZ, Ichikawa LE, et al. Change in bone mineral density among adolescent women using and discontinuing depot medroxyprogesterone acetate contraception. Arch Pediatr Adolesc Med. 2005;159:139-144.
29. World Health Organization. Medical eligibility criteria for contraceptive use. Annex 1. COCs and antiretroviral therapies. 3rd ed. Geneva: WHO; 2004.
30. Sapire KE. Depo-provera and carbamazapine. Br J Fam Plann. 1990;15:130.-
Dr. Kaunitz has received funding from Barr Laboratories, Berlex, Johnson & Johnson, and the National Institutes of Health. He is a speaker or consultant for the American College of Obstetricians and Gynecologists, the Association of Reproductive Health Professionals, Barr Laboratories, Berlex, Johnson & Johnson, Pfizer, and Procter & Gamble; and holds stock with Noven, Roche, and Sanofi-Aventis.
1. Use of Hormonal Contraception in Women With Coexisting Medical Conditions. ACOG Practice Bulletin #73. American College of Obstetricians and Gynecologists. Obstet Gynecol. 2006;107:1453-1472.
2. Marchbanks PA, McDonald JA, Wilson HG, et al. Oral contraceptives and the risk of breast cancer. N Engl J Med. 2002;346:2025-2032.
3. Davidson NE, Helzlsouer KJ. Good news about oral contraceptives. N Engl J Med. 2002;346:2078-2079.
4. Milne RL, Knight JA, John EM, et al. Oral contraceptive use and risk of early-onset breast cancer in carriers and non-carriers of BRCA1 and BRCA2 mutations. Cancer Epidemiol Biomarkers Prev. 2005;14:350-356.
5. Whittemore AS, Balise RR, Pharoah PD, et al. Oral contraceptive use and ovarian cancer risk among carriers of BRCA1 and BRCA2 mutations. Br J Cancer. 2004;91:1911-1915.
6. Narod SA, Risch H, Moslehi R, et al. Oral contraceptives and the risk of hereditary ovarian cancer. Hereditary Ovarian Cancer Clinical Study Group. N Engl J Med. 1998;339:424-428.
7. Ragueneau-Majlessi I, Levy RH, Janik F. Levetiracetam does not alter the pharmacokinetics of an oral contraceptive in healthy women. Epilepsia. 2002;43:697-702.
8. Griffith SG, Dai Y. Effect of zonisamide on the pharmacokinetics and pharmacodynamics of a combination ethinyl estradiol-norethindrone oral contraceptive in healthy women. Clin Ther. 2004;26:2056-2065.
9. McCann MF, Potter LS. Progestin-only oral contraception: a comprehensive review. Contraception. 1994;50(6 suppl 1):S1-S195.
10. Haukkamaa M. Contraception by Norplant subdermal capsules is not reliable in epileptic patients on anticonvulsant treatment. Contraception. 1986;33:559-565.
11. Bounds W, Guillebaud J. Observational series on women using the contraceptive Mirena concurrently with antiepileptic and other enzyme-inducing drugs. J Fam Plann Reprod Health Care. 2002;187:551-555.
12. Mattson RH, Rebar RW. Contraceptive methods for women with neurologic disorders. Am J Obstet Gynecol. 1993;168:2027-2032.
13. Back DJ, Breckenridge AM, Crawford F, et al. The effect of rifampicin on norethisterone pharmacokinetics. Eur J Clin Pharmacol. 1979;15:193-197.
14. Murphy PA, Kern SE, Stanczyk FZ, Westhoff CL. Interaction of St. John’s wort with oral contraceptives: effects on the pharmacokinetics of norethindrone and ethinyl estradiol, ovarian activity, and breakthrough bleeding. Contraception. 2005;71:402-408.
15. Abdollahi M, Cushman M, Rosendaal F. Obesity: risk of venous thrombosis and the interaction with coagulation factor levels and oral contraceptive use. Thromb Haemost. 2003;89:493-498.
16. Zieman M, Guillebaud J, et al. Contraceptive efficacy and cycle control with Ortho Evra/Evra transdermal system: the analysis of pooled data. Fertil Steril. 2002;77:S13-S18.
17. Holt VL, Scholes D, Wicklund KG, Cushing-Haugen KL, Daling JR. Body mass index, weight, and oral contraceptive failure risk. Obstet Gynecol. 2005;105:46-52.
18. Anderson FD. Hait H and the Seasonale-301 Study Group. A multicenter, randomized study of an extended cycle oral contraceptive. Contraception. 2003;68:89-96.
19. Anderson FD, Gibbons W, Portman D. Safety and efficacy of an extended-regimen oral contraceptive utilizing continuous low-dose ethinyl estradiol. Contraception. 2006;73:229-234.
20. Leiman G. Depo-medroxyprogesterone acetate as a contraceptive agent: its effect on weight and blood pressure. Am J Obstet Gynecol. 1972;114:97-102.
21. Jain J, Jakimiuk AJ, Bode FR, Ross D, Kaunitz AM. Contraceptive efficacy and safety of DMPA-SC. Contraception. 2004;70:269-275.
22. Bermas BL. Oral contraceptives in systemic lupus erythematosus—a tough pill to swallow? N Engl J Med. 2005;353:2602-2604.
23. Petri M, Kim MY, Kalunian KC, et al. Combined oral contraceptives in women with systemic lupus erythematosus. OC-SELENA Trial. N Engl J Med. 2005;353:2550-2558.
24. Sanchez-Guerrero, Urive AG, Jimenez-Santana L, et al. A trial of contraceptive methods in women with systemic lupus erythematosus. N Engl J Med. 2005;353:2539-2549.
25. Orr-Walker BJ, Evans MC, Ames RW, et al. The effect of past use of the injectable contraceptive depot medroxyprogesterone acetate on bone mineral density in normal postmenopausal women. Clin Endocrinol (Oxf). 1998;49:615-618.
26. Petitti DB, Piaggio G, Mehta S, Cravioto MC, Meirik O. Steroid hormone contraception and bone mineral density: a cross-sectional study in an international population. The WHO Study of Hormonal Contraception and Bone Health. Obstet Gynecol. 2000;95:736-744.
27. Scholes D, LaCroix AZ, Ichikawa LE, Barlow WE, Ott SM. Injectable hormone contraception and bone density: results from a prospective study [published erratum appears in Epidemiology. 2002;13:749]. Epidemiology. 2002;13:581-587.
28. Scholes D, LaCroix AZ, Ichikawa LE, et al. Change in bone mineral density among adolescent women using and discontinuing depot medroxyprogesterone acetate contraception. Arch Pediatr Adolesc Med. 2005;159:139-144.
29. World Health Organization. Medical eligibility criteria for contraceptive use. Annex 1. COCs and antiretroviral therapies. 3rd ed. Geneva: WHO; 2004.
30. Sapire KE. Depo-provera and carbamazapine. Br J Fam Plann. 1990;15:130.-
Dr. Kaunitz has received funding from Barr Laboratories, Berlex, Johnson & Johnson, and the National Institutes of Health. He is a speaker or consultant for the American College of Obstetricians and Gynecologists, the Association of Reproductive Health Professionals, Barr Laboratories, Berlex, Johnson & Johnson, Pfizer, and Procter & Gamble; and holds stock with Noven, Roche, and Sanofi-Aventis.
HYSTEROSCOPIC STERILIZATION
With over 50,000 completed hysteroscopic sterilization procedures worldwide, and 5 years of data, what do we know so far about this innovation? It is now almost 4 years since the FDA approved Essure (Conceptus; San Carlos, Calif), the first hysteroscopic sterilization method available for use in the United States. Two other systems are in the works: Adiana (Adiana; Redwood City, Calif) has completed its Phase III clinical trial and Ovion (American Medical Systems; Minnetonka, Minn) is just beginning its clinical trial this year.
Comparison of the devices
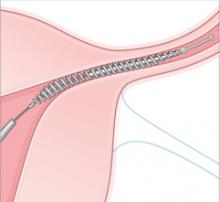
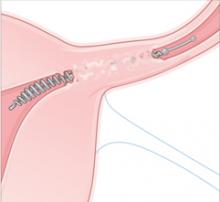
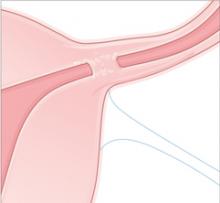
Essure is a disposable delivery system with polyethylene (PET) fibers wound in and around a stainless steel inner coil. An outer coil of nitinol, a superelastic titanium/nickel alloy, is deployed to anchor the device across the uterotubal junction. Wound down, the micro-insert is 0.8 mm in diameter. Once released, the coil expands to 1.5 to 2.0 mm to hold the inner coil and PET fibers in place at the uterine cornua.
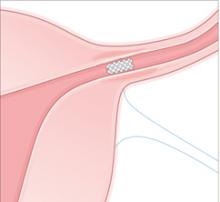
Over a period of about 3 months, the PET fibers elicit tissue ingrowth and proximal tubal occlusion. Women must use additional contraception during this time. Documentation of occlusion by a hysterosalpingogram about 3 months after device placement is required before patients may rely on the device and stop birth control.
Adiana (not yet available in the United States) uses a combination of controlled epithelial destruction and insertion of a porous biomatrix to induce vascularized tissue ingrowth. A catheter placed through the operating channel of a small hysteroscope delivers low-power bipolar electrosurgical energy to the tubal orifice (average less than 1 watt to the endosalpinx). A pushrod then delivers a small porous matrix of material into the tubal lumen. Ingrowth of healthy, vascularized tissue occurs over approximately 3 months, to occlude the tubes.
Retention of the matrix and tubal occlusion are documented by both transvaginal ultrasonography and hysterosalpingogram before patients may discontinue additional contraception.
Accessing the tubes
One of the greatest hurdles for occluding the fallopian tubes hysteroscopically is access to the tubes. Both the Adiana clinical trial (not yet published) and the post-market analysis of Essure (not yet published) have demonstrated excellent bilateral placement rates.
Technique is not hard to learn. Both types of devices are inserted through the operating channel of a small hysteroscope. Initial concerns about the ability to access the tubal ostia do not appear to be an issue—at least for those early-adopter physicians performing the procedures. Both clinical trials included gynecologists who were not experienced in operative hysteroscopy. These studies found that cannulation of the tubes is a technique that is easy to learn and rapidly accomplished in most circumstances.
Bilateral placement rates were similar for both devices in the pivotal trials: Adiana 95% (612/655); Essure 90% (464/518).
What are the contraindications?
Approximately 10% of patients have factors that preclude bilateral device placement:
Anatomic factors
- Blocked or stenotic tubes
- Intrauterine adhesions
- Visual field obstructed by polyps, fibroids, or shaggy endometrium
- Lateral tubes
Device or procedure failures due to
- Tubal spasm
- Patient pain/intolerance
- Device malfunction
A second procedure (after correcting the initial problems) will be successful in many women who have what appears to be a technical glitch.
Clinical outcomes, so far
Ultimately, of course, the success of these procedures and the benefits to our patients will be determined by the placement rates in the real world and the ability of a majority of women to rely on the devices for permanent sterilization.
What can we say so far?
Bilateral placement rates
Although the bilateral placement rates for Essure in the pivotal trial were 88% with one attempt, increasing to 92% of all patients enrolled with a second attempt, the data from the postmarket study is even more promising. After FDA approval in November 2002, the manufacturer trained gynecologists in the procedure, and then monitored clinical outcomes in the initial cases performed by these surgeons once they had completed their training and mandatory proctored cases (data not yet published).
Physicians who participated in the clinical trial were excluded from this analysis. The bilateral device placement rate for these women treated by novice users is over 94%. Adiana’s Phase III trial data demonstrate a similar bilateral success rate. It appears that despite the misgivings of many ObGyns, these systems are easy to learn.
I have incorporated the following tips and tricks into my office practice with great results. Patients are thrilled with their experience and leave ready to recruit their friends for the procedure.
- Pretreatment with a nonsteroidal anti-inflammatory drug to block prostaglandin release and uterotubal spasm
- Scheduling the procedure for the early follicular phase of the menstrual cycle to minimize shaggy endometrium, or
- Suppressing the endometrium with progestins from the first day of menses until the scheduled procedure
- Use of warm fluid for uterine distension to reduce spasm
- Placement of topical lidocaine gel into the uterus 10 to 15 minutes prior to the procedure
- Use of a pressure bag to assure adequate uterine distension
Patient safety
Few complications have been reported with either technique. There were the expected rare vasovagal reactions, as well as 2 cases of hypervolemia with Essure and 1 case of hyponatremia in the Adiana trial. Both of these situations should be avoidable with proper monitoring and limiting distension fluids to isotonic solutions. All patients recovered fully. There were no problems with persistent pain or changes in menstrual patterns at 1 year in the Essure trial.
Expulsion of the devices was associated with proximal positioning of the devices in all cases (3%). Patients had no symptoms, and most were able to have a second procedure with excellent placement and retention. Expulsions were identified at the postprocedure scout film or hysterosalpingogram.
Tubal perforation was noted in 0.9% of the patients. Predisposing factors were preexisting tubal occlusion or hydrosalpinx.
Perforations were asymptomatic, as well. Laparoscopic evaluation in 3 cases demonstrated no adhesions or reactions to the tiny perforation sites.
Delivery. An outer coil of nitinol, a superelastic titanium/nickel alloy is deployed to anchor the device across the uterotubal junction. Once released, the coil expands to 1.5 to 2.0 mm to hold the inner coil and PET fibers in place at the uterine cornua.
Occlusion. Over about 3 months, the PET fibers elicit tissue ingrowth and proximal tubal occlusion. Women must use additional contraception during this time. Documentation of occlusion by a hysterosalpingogram is required before patients may discontinue additional contraception.
Images: Rich Larocco
Anesthesia. Because hysteroscopic sterilization procedures may be performed without general anesthesia and by design avoid the need to access the abdominal cavity, they should be inherently safer for patients than the other available surgical sterilization methods. In the Essure and Adiana trials,1 more than 50% of the patients underwent their procedures under local anesthesia with no additional intravenous sedation. The others had local anesthesia with IV sedation. Only 1 patient (in the Essure trial) underwent general anesthesia.
Patient satisfaction and tolerance of the procedure were excellent; 88% of patients described their experience as good to excellent. Only 4% rated their procedure pain as severe. At discharge (approximately 40 minutes after conclusion of the procedure), 79% of patients had no pain and required no pain medication.
What are the potential complications?
The rare but devastating complications associated with laparoscopic sterilization should be avoidable with the hysteroscopic approach—at least for the 90% of patients for whom access to both tubes is feasible.
Ideal candidates. Hysteroscopic access seems to be the ideal approach for occlusion of the fallopian tubes in patients with medical conditions that may increase the risk of abdominal access, or for whom general anesthesia imposes added risk.
Problem conditions. The following clinical conditions and comorbidities all present significant challenges for the laparoscopic surgeon:
- Cardiac disease
- Thrombophilias
- Immune suppression
- Renal transplant
- Morbid obesity
- Previous abdominal surgery, especially bowel procedures
Delivery. A catheter placed through the operating channel of a small hysteroscope delivers low-power bipolar electrosurgical energy to the tubal orifice. A pushrod then delivers a small porous matrix of material into the tubal lumen.
Occlusion. Ingrowth of healthy, vascularized tissue occurs over approximately 3 months, to occlude the tubes. Retention of the matrix and tubal occlusion are documented by both transvaginal ultrasonography and hysterosalpingogram before patients may discontinue additional contraception.
Images: Rich Larocco
How many pregnancies?
There have been no pregnancies in Phase II and III trials of Essure, thus far. In July 2003, Cooper et al1 reported no pregnancies in 7,532 woman-months of use.
Conceptus is aware of 64 pregnancies among more than 50,000 procedures performed worldwide. None appeared to have occurred with proper demonstration of bilateral tubal occlusion after device placement. Most appear to be luteal phase pregnancies present at the time of the sterilization procedure, or failure of either the patient or the physician to assure tubal obstruction prior to stopping additional birth control methods.
There are no documented ectopic pregnancies, although 1 of the 64 reported cases may have been a very early tubal gestation. The patient was treated with methotrexate without firm documentation of the location of the pregnancy.
There are 2 pregnancies among the 605 patients with bilateral Adiana devices (6,860 woman-months of use as of September 2005). One resulted from an error in interpreting the hysterosalpingogram. The other did occur with a properly placed device and occlusion demonstrated on hysterosalpingogram.
It appears that hysteroscopic sterilization with Essure will have acceptable and preventable failure rates (longer term data and postmarket analysis are not yet available for the Adiana device). The calculated 5-year success rate is more than 99% for Essure; this compares favorably with all other surgical sterilization methods.
Do benefits outweigh costs?
The overall cost of hysteroscopic sterilization methods compares favorably with laparoscopic approaches. The expense of the disposable equipment is recouped by avoiding the costs of general anesthesia, and operating room and facility charges.
Advantages of the office setting
Payment for physicians is slightly more than the reimbursement for laparoscopic sterilization performed in a facility.
The real benefit to ObGyns, however, is in moving the procedure into the office environment. This allows us great flexibility in scheduling, and avoids the “down time” required for traveling to a facility, waiting for operating room turnover, anesthesia, and paperwork.
Benefit to healthcare systems. Researchers in closed healthcare systems have analyzed the expenses associated with Essure compared with laparoscopic tubal sterilization. When all costs associated with hysteroscopic sterilization are considered, including the need for additional procedures (when the tubes are not accessible or the procedure fails) and the 3-month hysterosalpingography, there remained a significant savings to the healthcare system for these procedures, compared with laparoscopic techniques.2
Ask women who are currently using contraceptive steroids about their menstrual cycles before they started hormonal birth control. Remind women who had menorrhagia or irregular cycles that no method of sterilization will manage their cycles.
The addition of endometrial ablation to the hysteroscopic sterilization procedure is an option.3 However, only 1 of the global ablation technologies currently has FDA approval for concomitant treatment with Essure: Thermachoice (Ethicon Women’s Health and Urology; Somerville, NJ).
Alternatives to permanent sterilization
In counseling women about permanent sterilization, it is important to cover the alternatives, as well.
IUDs. We should also consider the levonorgestrel-containing intrauterine device (IUD), Mirena (Berlex; Montville, NJ), for patients with menorrhagia who desire long-term contraception. Studies have demonstrated excellent patient satisfaction with this system and reduction in menstrual blood loss equivalent to endometrial ablation.4 Although not a permanent solution, the IUD does provide superb contraception, failure rates are similar to sterilization, and management of menorrhagia is excellent, and the cost is less than 10% of a combined endometrial ablation and sterilization procedure. The ParaGard copper-containing IUD is a good choice for women with normal or light flow, but not for those with heavy cycles.
Systemic hormone methods. For women willing to consider systemic hormones, Depo-Provera or the Implanon implantable rod provide excellent long-term contraception.
Vasectomy. Remember that vasectomy remains an option; however, many women want the assurance that they are in control.
Coding and insurance
The difference in payment for hysteroscopic sterilization can be considerable, depending on the site. When performed anywhere other than your office, payment for use of the facility, medications, personnel, and equipment goes to the facility, whether an ambulatory surgery center or a hospital. When we do these procedures in our offices, our reimbursement reflects the fact that we are using our office space, exam table, equipment, supplies, and personnel.
When considering where to perform hysteroscopic sterilization, remember that no one is paying for our space, personnel, and equipment when we are not in the office. Therefore, there is a great advantage in getting our overhead reimbursed when we perform procedures in the office.
The total relative value units payable to the physician for the new CPT code 58565 (hysteroscopy, surgical, with bilateral fallopian tube cannulation to induce occlusion by placement of permanent implants), which is the code for all systems currently under study:
- 12.12 if performed in a facility
- 57.91 if performed in the office
Most payers cover hysteroscopic sterilization when the policy covers sterilization. Determination of coverage by Medicaid has been secured in at least 36 states.
Past the tipping point
It is time to begin to adopt this technology into routine gynecology practice, for the benefits it offers patients, and practicing surgeons, as well. The data are accumulating on the safety and effectiveness of hysteroscopic sterilization techniques—more than 50,000 procedures have been performed worldwide, and we have 5 years of data.
An apt analogy. Although it is true that we might initiate this approach in up to 10% of women who may ultimately require laparoscopy, there appears to be little downside to the attempt. I would suggest the analogy of attempting an endometrial biopsy in the office in lieu of a D&C under anesthesia for postmenopausal women.
True, we sometimes fail, but for the vast majority of patients, it is clearly beneficial to attempt the office procedure and avoid anesthesia. Similarly, by avoiding abdominal access and general anesthesia for sterilization, we are providing a safer and more pleasant procedure with rapid recovery for our patients. Those few who require a different approach will have invested little time, energy, effort, or risk if we learn to perform hysteroscopic sterilization in the office setting.
Dr. Levy is a consultant to Conceptus, Inc.
1. Cooper JM, Carignan CS, Cher D, Kerin JF. Microinsert nonincisional hysteroscopic sterilization. Obstet Gynecol. 2003;102:59-67.
2. Levie MD, Chudnoff SG. Office hysteroscopic sterilization compared with laparoscopic sterilization: a critical cost analysis. J Min Invasive Gynecol. 2005;12:318-322.
3. Valle RF, Valdez J, Wright TC, Kenney M. Concomitant Essure tubal sterilization and Thermachoice endometrial ablation: feasibility and safety. Fertil Steril. 2006;86:152-158.
4. Busfield RA, Farquhar CM, Sowter MC, et al. A randomized trial comparing the levonorgestrel intrauterine system and thermal balloon ablation for heavy menstrual bleeding. BJOG. 2006;113:257-263.
With over 50,000 completed hysteroscopic sterilization procedures worldwide, and 5 years of data, what do we know so far about this innovation? It is now almost 4 years since the FDA approved Essure (Conceptus; San Carlos, Calif), the first hysteroscopic sterilization method available for use in the United States. Two other systems are in the works: Adiana (Adiana; Redwood City, Calif) has completed its Phase III clinical trial and Ovion (American Medical Systems; Minnetonka, Minn) is just beginning its clinical trial this year.
Comparison of the devices
Essure is a disposable delivery system with polyethylene (PET) fibers wound in and around a stainless steel inner coil. An outer coil of nitinol, a superelastic titanium/nickel alloy, is deployed to anchor the device across the uterotubal junction. Wound down, the micro-insert is 0.8 mm in diameter. Once released, the coil expands to 1.5 to 2.0 mm to hold the inner coil and PET fibers in place at the uterine cornua.
Over a period of about 3 months, the PET fibers elicit tissue ingrowth and proximal tubal occlusion. Women must use additional contraception during this time. Documentation of occlusion by a hysterosalpingogram about 3 months after device placement is required before patients may rely on the device and stop birth control.
Adiana (not yet available in the United States) uses a combination of controlled epithelial destruction and insertion of a porous biomatrix to induce vascularized tissue ingrowth. A catheter placed through the operating channel of a small hysteroscope delivers low-power bipolar electrosurgical energy to the tubal orifice (average less than 1 watt to the endosalpinx). A pushrod then delivers a small porous matrix of material into the tubal lumen. Ingrowth of healthy, vascularized tissue occurs over approximately 3 months, to occlude the tubes.
Retention of the matrix and tubal occlusion are documented by both transvaginal ultrasonography and hysterosalpingogram before patients may discontinue additional contraception.
Accessing the tubes
One of the greatest hurdles for occluding the fallopian tubes hysteroscopically is access to the tubes. Both the Adiana clinical trial (not yet published) and the post-market analysis of Essure (not yet published) have demonstrated excellent bilateral placement rates.
Technique is not hard to learn. Both types of devices are inserted through the operating channel of a small hysteroscope. Initial concerns about the ability to access the tubal ostia do not appear to be an issue—at least for those early-adopter physicians performing the procedures. Both clinical trials included gynecologists who were not experienced in operative hysteroscopy. These studies found that cannulation of the tubes is a technique that is easy to learn and rapidly accomplished in most circumstances.
Bilateral placement rates were similar for both devices in the pivotal trials: Adiana 95% (612/655); Essure 90% (464/518).
What are the contraindications?
Approximately 10% of patients have factors that preclude bilateral device placement:
Anatomic factors
- Blocked or stenotic tubes
- Intrauterine adhesions
- Visual field obstructed by polyps, fibroids, or shaggy endometrium
- Lateral tubes
Device or procedure failures due to
- Tubal spasm
- Patient pain/intolerance
- Device malfunction
A second procedure (after correcting the initial problems) will be successful in many women who have what appears to be a technical glitch.
Clinical outcomes, so far
Ultimately, of course, the success of these procedures and the benefits to our patients will be determined by the placement rates in the real world and the ability of a majority of women to rely on the devices for permanent sterilization.
What can we say so far?
Bilateral placement rates
Although the bilateral placement rates for Essure in the pivotal trial were 88% with one attempt, increasing to 92% of all patients enrolled with a second attempt, the data from the postmarket study is even more promising. After FDA approval in November 2002, the manufacturer trained gynecologists in the procedure, and then monitored clinical outcomes in the initial cases performed by these surgeons once they had completed their training and mandatory proctored cases (data not yet published).
Physicians who participated in the clinical trial were excluded from this analysis. The bilateral device placement rate for these women treated by novice users is over 94%. Adiana’s Phase III trial data demonstrate a similar bilateral success rate. It appears that despite the misgivings of many ObGyns, these systems are easy to learn.
I have incorporated the following tips and tricks into my office practice with great results. Patients are thrilled with their experience and leave ready to recruit their friends for the procedure.
- Pretreatment with a nonsteroidal anti-inflammatory drug to block prostaglandin release and uterotubal spasm
- Scheduling the procedure for the early follicular phase of the menstrual cycle to minimize shaggy endometrium, or
- Suppressing the endometrium with progestins from the first day of menses until the scheduled procedure
- Use of warm fluid for uterine distension to reduce spasm
- Placement of topical lidocaine gel into the uterus 10 to 15 minutes prior to the procedure
- Use of a pressure bag to assure adequate uterine distension
Patient safety
Few complications have been reported with either technique. There were the expected rare vasovagal reactions, as well as 2 cases of hypervolemia with Essure and 1 case of hyponatremia in the Adiana trial. Both of these situations should be avoidable with proper monitoring and limiting distension fluids to isotonic solutions. All patients recovered fully. There were no problems with persistent pain or changes in menstrual patterns at 1 year in the Essure trial.
Expulsion of the devices was associated with proximal positioning of the devices in all cases (3%). Patients had no symptoms, and most were able to have a second procedure with excellent placement and retention. Expulsions were identified at the postprocedure scout film or hysterosalpingogram.
Tubal perforation was noted in 0.9% of the patients. Predisposing factors were preexisting tubal occlusion or hydrosalpinx.
Perforations were asymptomatic, as well. Laparoscopic evaluation in 3 cases demonstrated no adhesions or reactions to the tiny perforation sites.
Delivery. An outer coil of nitinol, a superelastic titanium/nickel alloy is deployed to anchor the device across the uterotubal junction. Once released, the coil expands to 1.5 to 2.0 mm to hold the inner coil and PET fibers in place at the uterine cornua.
Occlusion. Over about 3 months, the PET fibers elicit tissue ingrowth and proximal tubal occlusion. Women must use additional contraception during this time. Documentation of occlusion by a hysterosalpingogram is required before patients may discontinue additional contraception.
Images: Rich Larocco
Anesthesia. Because hysteroscopic sterilization procedures may be performed without general anesthesia and by design avoid the need to access the abdominal cavity, they should be inherently safer for patients than the other available surgical sterilization methods. In the Essure and Adiana trials,1 more than 50% of the patients underwent their procedures under local anesthesia with no additional intravenous sedation. The others had local anesthesia with IV sedation. Only 1 patient (in the Essure trial) underwent general anesthesia.
Patient satisfaction and tolerance of the procedure were excellent; 88% of patients described their experience as good to excellent. Only 4% rated their procedure pain as severe. At discharge (approximately 40 minutes after conclusion of the procedure), 79% of patients had no pain and required no pain medication.
What are the potential complications?
The rare but devastating complications associated with laparoscopic sterilization should be avoidable with the hysteroscopic approach—at least for the 90% of patients for whom access to both tubes is feasible.
Ideal candidates. Hysteroscopic access seems to be the ideal approach for occlusion of the fallopian tubes in patients with medical conditions that may increase the risk of abdominal access, or for whom general anesthesia imposes added risk.
Problem conditions. The following clinical conditions and comorbidities all present significant challenges for the laparoscopic surgeon:
- Cardiac disease
- Thrombophilias
- Immune suppression
- Renal transplant
- Morbid obesity
- Previous abdominal surgery, especially bowel procedures
Delivery. A catheter placed through the operating channel of a small hysteroscope delivers low-power bipolar electrosurgical energy to the tubal orifice. A pushrod then delivers a small porous matrix of material into the tubal lumen.
Occlusion. Ingrowth of healthy, vascularized tissue occurs over approximately 3 months, to occlude the tubes. Retention of the matrix and tubal occlusion are documented by both transvaginal ultrasonography and hysterosalpingogram before patients may discontinue additional contraception.
Images: Rich Larocco
How many pregnancies?
There have been no pregnancies in Phase II and III trials of Essure, thus far. In July 2003, Cooper et al1 reported no pregnancies in 7,532 woman-months of use.
Conceptus is aware of 64 pregnancies among more than 50,000 procedures performed worldwide. None appeared to have occurred with proper demonstration of bilateral tubal occlusion after device placement. Most appear to be luteal phase pregnancies present at the time of the sterilization procedure, or failure of either the patient or the physician to assure tubal obstruction prior to stopping additional birth control methods.
There are no documented ectopic pregnancies, although 1 of the 64 reported cases may have been a very early tubal gestation. The patient was treated with methotrexate without firm documentation of the location of the pregnancy.
There are 2 pregnancies among the 605 patients with bilateral Adiana devices (6,860 woman-months of use as of September 2005). One resulted from an error in interpreting the hysterosalpingogram. The other did occur with a properly placed device and occlusion demonstrated on hysterosalpingogram.
It appears that hysteroscopic sterilization with Essure will have acceptable and preventable failure rates (longer term data and postmarket analysis are not yet available for the Adiana device). The calculated 5-year success rate is more than 99% for Essure; this compares favorably with all other surgical sterilization methods.
Do benefits outweigh costs?
The overall cost of hysteroscopic sterilization methods compares favorably with laparoscopic approaches. The expense of the disposable equipment is recouped by avoiding the costs of general anesthesia, and operating room and facility charges.
Advantages of the office setting
Payment for physicians is slightly more than the reimbursement for laparoscopic sterilization performed in a facility.
The real benefit to ObGyns, however, is in moving the procedure into the office environment. This allows us great flexibility in scheduling, and avoids the “down time” required for traveling to a facility, waiting for operating room turnover, anesthesia, and paperwork.
Benefit to healthcare systems. Researchers in closed healthcare systems have analyzed the expenses associated with Essure compared with laparoscopic tubal sterilization. When all costs associated with hysteroscopic sterilization are considered, including the need for additional procedures (when the tubes are not accessible or the procedure fails) and the 3-month hysterosalpingography, there remained a significant savings to the healthcare system for these procedures, compared with laparoscopic techniques.2
Ask women who are currently using contraceptive steroids about their menstrual cycles before they started hormonal birth control. Remind women who had menorrhagia or irregular cycles that no method of sterilization will manage their cycles.
The addition of endometrial ablation to the hysteroscopic sterilization procedure is an option.3 However, only 1 of the global ablation technologies currently has FDA approval for concomitant treatment with Essure: Thermachoice (Ethicon Women’s Health and Urology; Somerville, NJ).
Alternatives to permanent sterilization
In counseling women about permanent sterilization, it is important to cover the alternatives, as well.
IUDs. We should also consider the levonorgestrel-containing intrauterine device (IUD), Mirena (Berlex; Montville, NJ), for patients with menorrhagia who desire long-term contraception. Studies have demonstrated excellent patient satisfaction with this system and reduction in menstrual blood loss equivalent to endometrial ablation.4 Although not a permanent solution, the IUD does provide superb contraception, failure rates are similar to sterilization, and management of menorrhagia is excellent, and the cost is less than 10% of a combined endometrial ablation and sterilization procedure. The ParaGard copper-containing IUD is a good choice for women with normal or light flow, but not for those with heavy cycles.
Systemic hormone methods. For women willing to consider systemic hormones, Depo-Provera or the Implanon implantable rod provide excellent long-term contraception.
Vasectomy. Remember that vasectomy remains an option; however, many women want the assurance that they are in control.
Coding and insurance
The difference in payment for hysteroscopic sterilization can be considerable, depending on the site. When performed anywhere other than your office, payment for use of the facility, medications, personnel, and equipment goes to the facility, whether an ambulatory surgery center or a hospital. When we do these procedures in our offices, our reimbursement reflects the fact that we are using our office space, exam table, equipment, supplies, and personnel.
When considering where to perform hysteroscopic sterilization, remember that no one is paying for our space, personnel, and equipment when we are not in the office. Therefore, there is a great advantage in getting our overhead reimbursed when we perform procedures in the office.
The total relative value units payable to the physician for the new CPT code 58565 (hysteroscopy, surgical, with bilateral fallopian tube cannulation to induce occlusion by placement of permanent implants), which is the code for all systems currently under study:
- 12.12 if performed in a facility
- 57.91 if performed in the office
Most payers cover hysteroscopic sterilization when the policy covers sterilization. Determination of coverage by Medicaid has been secured in at least 36 states.
Past the tipping point
It is time to begin to adopt this technology into routine gynecology practice, for the benefits it offers patients, and practicing surgeons, as well. The data are accumulating on the safety and effectiveness of hysteroscopic sterilization techniques—more than 50,000 procedures have been performed worldwide, and we have 5 years of data.
An apt analogy. Although it is true that we might initiate this approach in up to 10% of women who may ultimately require laparoscopy, there appears to be little downside to the attempt. I would suggest the analogy of attempting an endometrial biopsy in the office in lieu of a D&C under anesthesia for postmenopausal women.
True, we sometimes fail, but for the vast majority of patients, it is clearly beneficial to attempt the office procedure and avoid anesthesia. Similarly, by avoiding abdominal access and general anesthesia for sterilization, we are providing a safer and more pleasant procedure with rapid recovery for our patients. Those few who require a different approach will have invested little time, energy, effort, or risk if we learn to perform hysteroscopic sterilization in the office setting.
Dr. Levy is a consultant to Conceptus, Inc.
With over 50,000 completed hysteroscopic sterilization procedures worldwide, and 5 years of data, what do we know so far about this innovation? It is now almost 4 years since the FDA approved Essure (Conceptus; San Carlos, Calif), the first hysteroscopic sterilization method available for use in the United States. Two other systems are in the works: Adiana (Adiana; Redwood City, Calif) has completed its Phase III clinical trial and Ovion (American Medical Systems; Minnetonka, Minn) is just beginning its clinical trial this year.
Comparison of the devices
Essure is a disposable delivery system with polyethylene (PET) fibers wound in and around a stainless steel inner coil. An outer coil of nitinol, a superelastic titanium/nickel alloy, is deployed to anchor the device across the uterotubal junction. Wound down, the micro-insert is 0.8 mm in diameter. Once released, the coil expands to 1.5 to 2.0 mm to hold the inner coil and PET fibers in place at the uterine cornua.
Over a period of about 3 months, the PET fibers elicit tissue ingrowth and proximal tubal occlusion. Women must use additional contraception during this time. Documentation of occlusion by a hysterosalpingogram about 3 months after device placement is required before patients may rely on the device and stop birth control.
Adiana (not yet available in the United States) uses a combination of controlled epithelial destruction and insertion of a porous biomatrix to induce vascularized tissue ingrowth. A catheter placed through the operating channel of a small hysteroscope delivers low-power bipolar electrosurgical energy to the tubal orifice (average less than 1 watt to the endosalpinx). A pushrod then delivers a small porous matrix of material into the tubal lumen. Ingrowth of healthy, vascularized tissue occurs over approximately 3 months, to occlude the tubes.
Retention of the matrix and tubal occlusion are documented by both transvaginal ultrasonography and hysterosalpingogram before patients may discontinue additional contraception.
Accessing the tubes
One of the greatest hurdles for occluding the fallopian tubes hysteroscopically is access to the tubes. Both the Adiana clinical trial (not yet published) and the post-market analysis of Essure (not yet published) have demonstrated excellent bilateral placement rates.
Technique is not hard to learn. Both types of devices are inserted through the operating channel of a small hysteroscope. Initial concerns about the ability to access the tubal ostia do not appear to be an issue—at least for those early-adopter physicians performing the procedures. Both clinical trials included gynecologists who were not experienced in operative hysteroscopy. These studies found that cannulation of the tubes is a technique that is easy to learn and rapidly accomplished in most circumstances.
Bilateral placement rates were similar for both devices in the pivotal trials: Adiana 95% (612/655); Essure 90% (464/518).
What are the contraindications?
Approximately 10% of patients have factors that preclude bilateral device placement:
Anatomic factors
- Blocked or stenotic tubes
- Intrauterine adhesions
- Visual field obstructed by polyps, fibroids, or shaggy endometrium
- Lateral tubes
Device or procedure failures due to
- Tubal spasm
- Patient pain/intolerance
- Device malfunction
A second procedure (after correcting the initial problems) will be successful in many women who have what appears to be a technical glitch.
Clinical outcomes, so far
Ultimately, of course, the success of these procedures and the benefits to our patients will be determined by the placement rates in the real world and the ability of a majority of women to rely on the devices for permanent sterilization.
What can we say so far?
Bilateral placement rates
Although the bilateral placement rates for Essure in the pivotal trial were 88% with one attempt, increasing to 92% of all patients enrolled with a second attempt, the data from the postmarket study is even more promising. After FDA approval in November 2002, the manufacturer trained gynecologists in the procedure, and then monitored clinical outcomes in the initial cases performed by these surgeons once they had completed their training and mandatory proctored cases (data not yet published).
Physicians who participated in the clinical trial were excluded from this analysis. The bilateral device placement rate for these women treated by novice users is over 94%. Adiana’s Phase III trial data demonstrate a similar bilateral success rate. It appears that despite the misgivings of many ObGyns, these systems are easy to learn.
I have incorporated the following tips and tricks into my office practice with great results. Patients are thrilled with their experience and leave ready to recruit their friends for the procedure.
- Pretreatment with a nonsteroidal anti-inflammatory drug to block prostaglandin release and uterotubal spasm
- Scheduling the procedure for the early follicular phase of the menstrual cycle to minimize shaggy endometrium, or
- Suppressing the endometrium with progestins from the first day of menses until the scheduled procedure
- Use of warm fluid for uterine distension to reduce spasm
- Placement of topical lidocaine gel into the uterus 10 to 15 minutes prior to the procedure
- Use of a pressure bag to assure adequate uterine distension
Patient safety
Few complications have been reported with either technique. There were the expected rare vasovagal reactions, as well as 2 cases of hypervolemia with Essure and 1 case of hyponatremia in the Adiana trial. Both of these situations should be avoidable with proper monitoring and limiting distension fluids to isotonic solutions. All patients recovered fully. There were no problems with persistent pain or changes in menstrual patterns at 1 year in the Essure trial.
Expulsion of the devices was associated with proximal positioning of the devices in all cases (3%). Patients had no symptoms, and most were able to have a second procedure with excellent placement and retention. Expulsions were identified at the postprocedure scout film or hysterosalpingogram.
Tubal perforation was noted in 0.9% of the patients. Predisposing factors were preexisting tubal occlusion or hydrosalpinx.
Perforations were asymptomatic, as well. Laparoscopic evaluation in 3 cases demonstrated no adhesions or reactions to the tiny perforation sites.
Delivery. An outer coil of nitinol, a superelastic titanium/nickel alloy is deployed to anchor the device across the uterotubal junction. Once released, the coil expands to 1.5 to 2.0 mm to hold the inner coil and PET fibers in place at the uterine cornua.
Occlusion. Over about 3 months, the PET fibers elicit tissue ingrowth and proximal tubal occlusion. Women must use additional contraception during this time. Documentation of occlusion by a hysterosalpingogram is required before patients may discontinue additional contraception.
Images: Rich Larocco
Anesthesia. Because hysteroscopic sterilization procedures may be performed without general anesthesia and by design avoid the need to access the abdominal cavity, they should be inherently safer for patients than the other available surgical sterilization methods. In the Essure and Adiana trials,1 more than 50% of the patients underwent their procedures under local anesthesia with no additional intravenous sedation. The others had local anesthesia with IV sedation. Only 1 patient (in the Essure trial) underwent general anesthesia.
Patient satisfaction and tolerance of the procedure were excellent; 88% of patients described their experience as good to excellent. Only 4% rated their procedure pain as severe. At discharge (approximately 40 minutes after conclusion of the procedure), 79% of patients had no pain and required no pain medication.
What are the potential complications?
The rare but devastating complications associated with laparoscopic sterilization should be avoidable with the hysteroscopic approach—at least for the 90% of patients for whom access to both tubes is feasible.
Ideal candidates. Hysteroscopic access seems to be the ideal approach for occlusion of the fallopian tubes in patients with medical conditions that may increase the risk of abdominal access, or for whom general anesthesia imposes added risk.
Problem conditions. The following clinical conditions and comorbidities all present significant challenges for the laparoscopic surgeon:
- Cardiac disease
- Thrombophilias
- Immune suppression
- Renal transplant
- Morbid obesity
- Previous abdominal surgery, especially bowel procedures
Delivery. A catheter placed through the operating channel of a small hysteroscope delivers low-power bipolar electrosurgical energy to the tubal orifice. A pushrod then delivers a small porous matrix of material into the tubal lumen.
Occlusion. Ingrowth of healthy, vascularized tissue occurs over approximately 3 months, to occlude the tubes. Retention of the matrix and tubal occlusion are documented by both transvaginal ultrasonography and hysterosalpingogram before patients may discontinue additional contraception.
Images: Rich Larocco
How many pregnancies?
There have been no pregnancies in Phase II and III trials of Essure, thus far. In July 2003, Cooper et al1 reported no pregnancies in 7,532 woman-months of use.
Conceptus is aware of 64 pregnancies among more than 50,000 procedures performed worldwide. None appeared to have occurred with proper demonstration of bilateral tubal occlusion after device placement. Most appear to be luteal phase pregnancies present at the time of the sterilization procedure, or failure of either the patient or the physician to assure tubal obstruction prior to stopping additional birth control methods.
There are no documented ectopic pregnancies, although 1 of the 64 reported cases may have been a very early tubal gestation. The patient was treated with methotrexate without firm documentation of the location of the pregnancy.
There are 2 pregnancies among the 605 patients with bilateral Adiana devices (6,860 woman-months of use as of September 2005). One resulted from an error in interpreting the hysterosalpingogram. The other did occur with a properly placed device and occlusion demonstrated on hysterosalpingogram.
It appears that hysteroscopic sterilization with Essure will have acceptable and preventable failure rates (longer term data and postmarket analysis are not yet available for the Adiana device). The calculated 5-year success rate is more than 99% for Essure; this compares favorably with all other surgical sterilization methods.
Do benefits outweigh costs?
The overall cost of hysteroscopic sterilization methods compares favorably with laparoscopic approaches. The expense of the disposable equipment is recouped by avoiding the costs of general anesthesia, and operating room and facility charges.
Advantages of the office setting
Payment for physicians is slightly more than the reimbursement for laparoscopic sterilization performed in a facility.
The real benefit to ObGyns, however, is in moving the procedure into the office environment. This allows us great flexibility in scheduling, and avoids the “down time” required for traveling to a facility, waiting for operating room turnover, anesthesia, and paperwork.
Benefit to healthcare systems. Researchers in closed healthcare systems have analyzed the expenses associated with Essure compared with laparoscopic tubal sterilization. When all costs associated with hysteroscopic sterilization are considered, including the need for additional procedures (when the tubes are not accessible or the procedure fails) and the 3-month hysterosalpingography, there remained a significant savings to the healthcare system for these procedures, compared with laparoscopic techniques.2
Ask women who are currently using contraceptive steroids about their menstrual cycles before they started hormonal birth control. Remind women who had menorrhagia or irregular cycles that no method of sterilization will manage their cycles.
The addition of endometrial ablation to the hysteroscopic sterilization procedure is an option.3 However, only 1 of the global ablation technologies currently has FDA approval for concomitant treatment with Essure: Thermachoice (Ethicon Women’s Health and Urology; Somerville, NJ).
Alternatives to permanent sterilization
In counseling women about permanent sterilization, it is important to cover the alternatives, as well.
IUDs. We should also consider the levonorgestrel-containing intrauterine device (IUD), Mirena (Berlex; Montville, NJ), for patients with menorrhagia who desire long-term contraception. Studies have demonstrated excellent patient satisfaction with this system and reduction in menstrual blood loss equivalent to endometrial ablation.4 Although not a permanent solution, the IUD does provide superb contraception, failure rates are similar to sterilization, and management of menorrhagia is excellent, and the cost is less than 10% of a combined endometrial ablation and sterilization procedure. The ParaGard copper-containing IUD is a good choice for women with normal or light flow, but not for those with heavy cycles.
Systemic hormone methods. For women willing to consider systemic hormones, Depo-Provera or the Implanon implantable rod provide excellent long-term contraception.
Vasectomy. Remember that vasectomy remains an option; however, many women want the assurance that they are in control.
Coding and insurance
The difference in payment for hysteroscopic sterilization can be considerable, depending on the site. When performed anywhere other than your office, payment for use of the facility, medications, personnel, and equipment goes to the facility, whether an ambulatory surgery center or a hospital. When we do these procedures in our offices, our reimbursement reflects the fact that we are using our office space, exam table, equipment, supplies, and personnel.
When considering where to perform hysteroscopic sterilization, remember that no one is paying for our space, personnel, and equipment when we are not in the office. Therefore, there is a great advantage in getting our overhead reimbursed when we perform procedures in the office.
The total relative value units payable to the physician for the new CPT code 58565 (hysteroscopy, surgical, with bilateral fallopian tube cannulation to induce occlusion by placement of permanent implants), which is the code for all systems currently under study:
- 12.12 if performed in a facility
- 57.91 if performed in the office
Most payers cover hysteroscopic sterilization when the policy covers sterilization. Determination of coverage by Medicaid has been secured in at least 36 states.
Past the tipping point
It is time to begin to adopt this technology into routine gynecology practice, for the benefits it offers patients, and practicing surgeons, as well. The data are accumulating on the safety and effectiveness of hysteroscopic sterilization techniques—more than 50,000 procedures have been performed worldwide, and we have 5 years of data.
An apt analogy. Although it is true that we might initiate this approach in up to 10% of women who may ultimately require laparoscopy, there appears to be little downside to the attempt. I would suggest the analogy of attempting an endometrial biopsy in the office in lieu of a D&C under anesthesia for postmenopausal women.
True, we sometimes fail, but for the vast majority of patients, it is clearly beneficial to attempt the office procedure and avoid anesthesia. Similarly, by avoiding abdominal access and general anesthesia for sterilization, we are providing a safer and more pleasant procedure with rapid recovery for our patients. Those few who require a different approach will have invested little time, energy, effort, or risk if we learn to perform hysteroscopic sterilization in the office setting.
Dr. Levy is a consultant to Conceptus, Inc.
1. Cooper JM, Carignan CS, Cher D, Kerin JF. Microinsert nonincisional hysteroscopic sterilization. Obstet Gynecol. 2003;102:59-67.
2. Levie MD, Chudnoff SG. Office hysteroscopic sterilization compared with laparoscopic sterilization: a critical cost analysis. J Min Invasive Gynecol. 2005;12:318-322.
3. Valle RF, Valdez J, Wright TC, Kenney M. Concomitant Essure tubal sterilization and Thermachoice endometrial ablation: feasibility and safety. Fertil Steril. 2006;86:152-158.
4. Busfield RA, Farquhar CM, Sowter MC, et al. A randomized trial comparing the levonorgestrel intrauterine system and thermal balloon ablation for heavy menstrual bleeding. BJOG. 2006;113:257-263.
1. Cooper JM, Carignan CS, Cher D, Kerin JF. Microinsert nonincisional hysteroscopic sterilization. Obstet Gynecol. 2003;102:59-67.
2. Levie MD, Chudnoff SG. Office hysteroscopic sterilization compared with laparoscopic sterilization: a critical cost analysis. J Min Invasive Gynecol. 2005;12:318-322.
3. Valle RF, Valdez J, Wright TC, Kenney M. Concomitant Essure tubal sterilization and Thermachoice endometrial ablation: feasibility and safety. Fertil Steril. 2006;86:152-158.
4. Busfield RA, Farquhar CM, Sowter MC, et al. A randomized trial comparing the levonorgestrel intrauterine system and thermal balloon ablation for heavy menstrual bleeding. BJOG. 2006;113:257-263.
IN THIS ARTICLE
Membrane sweeping and GBS: A litigious combination?
Membrane stripping is a focus of controversy when it comes to the issue of group B streptococcus (GBS). This article looks at the literature on the subject and presents a recent legal case in which a woman colonized with GBS claimed membrane stripping was the proximate cause of her infant’s death. In the case, experts for the plaintiff testified that membrane sweeping in a women colonized with GBS is below the standard of care, despite evidence to the contrary. The case, which involved a 2-week jury trial, resulted in a defense verdict.
The legal case
A 22-year-old primigravida presented at just over 39 weeks’ gestation, reporting spontaneous rupture of membranes 1 hour earlier.
IUGR and Group B strep
Her antenatal course had been complicated by intrauterine growth restriction (IUGR), detected by ultrasound at 34 weeks’ gestation. Because of the IUGR, the fetus was being evaluated twice weekly with nonstress tests and amniotic fluid measurements. At 35 weeks, testing for GBS colonization was positive. At 37 weeks, the membranes were stripped to facilitate cervical ripening because of the diagnosed IUGR.
On admission, she was noted to be afebrile with stable vital signs. She was given antibiotics for the GBS and examined. The membranes were grossly ruptured, with clear fluid pooling in the vagina; the cervix was dilated 3 cm with 80% effacement; and the fetus was at –1 to –2 station.
Although the woman was noted to be contracting every 2 minutes, she was barely aware of the contractions. The fetal heart tracing was initially reassuring, with good variability and no decelerations. She was allowed to walk around for 30 minutes.
Sudden fetal bradycardia
Shortly after the patient was placed back on the fetal heart rate monitor, 52 minutes after her initial presentation and approximately 2 hours after rupture of membranes, a marked and sudden fetal bradycardia was noted.
Emergent cesarean section was performed with a low transverse incision. Eighteen minutes after the onset of the bradycardia, a male infant weighing 3,510 g was delivered, with Apgar scores of 0, 2, and 0, at 1, 5, and 10 minutes, respectively. The umbilical cord arterial pH was 6.97. Pediatricians tried to resuscitate the baby, but intubation revealed immediate return of bright red blood. Despite aggressive intervention, including CPR, respiratory support, antibiotics, and inotropic agents, the infant died at 1 hour of life.
Cause of death: GBS pneumonia
An autopsy revealed bilateral massive consolidation of the lungs due to hemorrhagic bronchopneumonia. Tissue and blood cultures of the spleen, lung, and placenta all grew GBS, as did umbilical cord blood cultures. The cause of death: respiratory failure due to overwhelming GBS pneumonia.
The mother’s postpartum course was complicated by a fever of 100.8°F on the second postoperative day, for which she was treated with intravenous ampicillin, gentamicin, and clindamycin. She was discharged home on the 4th postoperative day.
“Data insufficient” for or against
Many practitioners strip the membranes at term to keep patients from passing their due dates. When the membranes are stripped at 40 weeks’ gestation, two thirds of women enter spontaneous labor within 72 hours; without membrane stripping, only one third of women do.2 The strategy also decreases the chance that pregnancies will go past 42 weeks’ gestation.3
Even more important, studies have found membrane stripping to be safe.3-5 The risk of maternal and neonatal infections does not increase with the procedure, according to a Cochrane Review of 2,797 women in 22 different studies.5
The latest statement on the subject from the American College of Obstetricians and Gynecologists (ACOG) is a Committee Opinion published in December 2002—which came after the neonatal death in this case. It says the risks of membrane stripping in women colonized with GBS “have not been investigated in well-designed prospective studies. Therefore, data are insufficient to encourage or discourage this practice in women known to be GBS-colonized.”6
Expert testimony
Plaintiff
The main witnesses for the plaintiff were a perinatologist and an obstetrician who specializes in infectious diseases. They opined that the infant’s death was caused by the membrane stripping, given that the mother was known to be colonized with GBS.
The perinatologist said his opinion was based on the statements of the infectious disease specialist, who in turn cited a poster presentation at the Infectious Diseases Society for Obstetrics and Gynecology meeting in 20017—which occurred a year after the neonatal death. The poster presentation was a series of 8 cases of perinatal sepsis following membrane stripping; the cases occurred between 1993 and 2000 and were provided by a parents’ group with affected children, “The Jesse Cause.”
Only the perinatologist appeared at trial. When asked to identify a single piece of published, peer-reviewed literature documenting an increased risk of neonatal GBS with membrane stripping, he was unable to do so.
Defense
An expert testified that, although GBS colonization occurs in 20% of all pregnancies, there are no data—prospective, retrospective, or controlled—to suggest that membrane sweeping in GBS-positive patients is associated with GBS sepsis of the newborn, and that membrane sweeping was appropriate in a woman with a fetus affected by unexplained IUGR.
The jury returned a defense verdict after less than 1 day of deliberation. It was not appealed.
As this case demonstrates, expert witnesses sometimes testify on a plaintiff’s behalf despite clear data refuting their statements. ObGyns should be aware that even a practice with a long history, such as membrane stripping, may be proclaimed outside the standard of care by such witnesses. We consider this kind of testimony unethical.
Until we have more data confirming or refuting the association between membrane sweeping (in cases of GBS colonization) and neonatal sepsis, or the medicolegal system changes, obstetricians should proceed with caution. We counsel our patients thoroughly and document the discussion.
Suneet P. Chauhan, MD, Director of Maternal–Fetal Medicine Aurora Women’s Pavilion, Perinatal Assessment Center, West Allis, Wisc
Lower risk of postterm pregnancy
de Miranda E, van der Bom JG, Bonsel GJ, Bleker OP, Rosendaal FR. Membrane sweeping and prevention of post-term pregnancy in low-risk pregnancies: a randomized controlled trial. BJOG. APRIL 2006;113:402–408.
The conclusion of this herculean randomized controlled trial is unequivocal: Sweeping the membranes at 41 weeks’ gestation, regardless of parity, significantly reduces the likelihood a pregnancy will reach 42 weeks. Number needed to treat: 6.
Risks of postterm pregnancy are numerous: greater likelihood of longer labor, cesarean section or operative vaginal delivery, infection, postpartum hemorrhage, shoulder dystocia, stillbirth or neonatal death, and meconium aspiration, to name a few. So any strategy to prevent it—particularly one that is easy and inexpensive—is welcome. The only adverse effect of membrane sweeping is increased bleeding; otherwise, the rates of peripartum complications are similar in women with or without the intervention.
Until 2006, studies of membrane sweeping were not randomized. Empiric evidence has suggested that membrane sweeping is ineffective. As a result, many clinicians eschewed the practice. According to the American College of Obstetricians and Gynecologists (ACOG) practice bulletin on postterm pregnancy,8 management options at 41 weeks’ gestation are limited to labor induction or expectant management with antepartum surveillance.
The de Miranda study was conducted at 51 primary care midwifery practices in the Netherlands.
Strengths. In addition to the randomized, controlled design, the trial’s strengths are:
- Subanalysis of the data based on parity, on whether the gestational age was determined by ultrasound before 18 weeks, and whether the Bishop score was below 6 or at 6 or above
- Participation by several midwives reflected real clinical practice
In addition, almost 90% of patients who underwent the intervention said they would choose it again in the next pregnancy.
Weaknesses. The de Miranda study does have weaknesses:
- Patients randomized to the control group did not undergo a vaginal examination to determine whether they had a cervix favorable for labor induction. This omission seems unacceptable and contrary to ACOG recommendations.8
- The perinatal mortality rate (for all women in the study) was 5.4 per 1,000 births, which is higher than the 1.0 to 3.1 per 1,000 quoted in the ACOG practice bulletin.
There also is some question of which management strategy women prefer, because an earlier study by the Canadian Multicenter Postterm Pregnancy Trial Group9 reported that women assigned to induction were significantly more satisfied than those allocated to observation.
Tan PC, Jacob R, Omar SZ. Membrane sweeping at initiation of formal labor induction. A randomized controlled trial. Obstet Gynecol. MARCH 2006;107:569–577.
The Tan trial randomized 274 women scheduled for induction at term to membrane sweeping or no membrane sweeping at the initiation of induction. Although roughly 1 in 5 deliveries are induced, induction leads to spontaneous vaginal delivery much less often than does spontaneous labor. The Tan study sought to determine whether membrane sweeping increases the likelihood of spontaneous vaginal delivery. Swept women had:
- Higher spontaneous vaginal delivery rate (69% vs 56%, P=.041)
- Shorter induction-to-delivery interval (mean 14 vs 19 hours, P=.003)
- Fewer requirements for oxytocin (46% vs 59%, P=.037)
- Shorter duration of oxytocin infuson (mean 2.6 vs 4.3 hours, P=.001)
- Greater satisfaction with the birth process
Recommendations
These trials are sufficient reason to undertake membrane sweeping every 48 hours in women who strongly desire expectant management at 41 weeks’ gestation
Counsel patients about the risks of observation
Test fetal well-being twice weekly
Sweeping may ease labor induction
The authors report no financial relationships relevant to this article.
1. McColgin SW, Bennett WA, Roach H, et al. Parturitional factors associated with membrane stripping. Am J Obstet Gynecol. 1993;169:71-77.
2. Allott HA, Palmer CR. Sweeping the membranes: a valid procedure in stimulating the onset of labour? Br J Obstet Gynaecol. 1993;100:898-890.
3. McColgin SW, Hampton HL, McCaul JF, et al. Stripping of membranes at term: can it safely reduce the incidence of postterm pregnancy? Obstet Gynecol. 1990;76:678-680.
4. Netta D, Visintainer P, Bayliss P. Does cervical membrane stripping increase maternal colonization of group B streptococcus? Poster presented at: 23rd Annual Meeting of the Society for Maternal-Fetal Medicine; February 3-8, 2003; San Francisco, Calif.
5. Boulvain M, Stan C, Irion O. Membrane sweeping for induction of labour. The Cochrane Database of Systematic Reviews. 2005; Issue 1; Art No CD000451. DOI: 10.1002/14651858.
6. Prevention of Early-Onset Group B Streptococcal Disease in Newborns. ACOG Committee Opinion No. 279. American College of Obstetricians and Gynecologists. Washington, DC: ACOG; 2002.
7. Stamm CA, Bishop LA, McGregor JA, McFee JG, Perhach M. Cervical manipulation and membrane “stripping” associated with perinatal sepsis and death caused by group B streptococcus and other perinatal pathogens: cervical membrane disruption syndrome (CMDS). Poster presented at: Annual Meeting of the Infectious Diseases Society for Obstetrics and Gynecology; Aug 9-11, 2001; Quebec.
8. Management of Postterm Pregnancy. ACOG Practice Bulletin No. 55. American College of Obstetricians and Gynecologists. Washington, DC: ACOG; 2004.
9. Hannah ME, Hannah WJ, Hellmann J, et al. Induction of labor as compared with serial antenatal monitoring in post-term pregnancy. A randomized controlled trial. The Canadian Multicenter Post-term Pregnancy Trial Group. N Engl J Med. 1992;326:1587-1592.
Membrane stripping is a focus of controversy when it comes to the issue of group B streptococcus (GBS). This article looks at the literature on the subject and presents a recent legal case in which a woman colonized with GBS claimed membrane stripping was the proximate cause of her infant’s death. In the case, experts for the plaintiff testified that membrane sweeping in a women colonized with GBS is below the standard of care, despite evidence to the contrary. The case, which involved a 2-week jury trial, resulted in a defense verdict.
The legal case
A 22-year-old primigravida presented at just over 39 weeks’ gestation, reporting spontaneous rupture of membranes 1 hour earlier.
IUGR and Group B strep
Her antenatal course had been complicated by intrauterine growth restriction (IUGR), detected by ultrasound at 34 weeks’ gestation. Because of the IUGR, the fetus was being evaluated twice weekly with nonstress tests and amniotic fluid measurements. At 35 weeks, testing for GBS colonization was positive. At 37 weeks, the membranes were stripped to facilitate cervical ripening because of the diagnosed IUGR.
On admission, she was noted to be afebrile with stable vital signs. She was given antibiotics for the GBS and examined. The membranes were grossly ruptured, with clear fluid pooling in the vagina; the cervix was dilated 3 cm with 80% effacement; and the fetus was at –1 to –2 station.
Although the woman was noted to be contracting every 2 minutes, she was barely aware of the contractions. The fetal heart tracing was initially reassuring, with good variability and no decelerations. She was allowed to walk around for 30 minutes.
Sudden fetal bradycardia
Shortly after the patient was placed back on the fetal heart rate monitor, 52 minutes after her initial presentation and approximately 2 hours after rupture of membranes, a marked and sudden fetal bradycardia was noted.
Emergent cesarean section was performed with a low transverse incision. Eighteen minutes after the onset of the bradycardia, a male infant weighing 3,510 g was delivered, with Apgar scores of 0, 2, and 0, at 1, 5, and 10 minutes, respectively. The umbilical cord arterial pH was 6.97. Pediatricians tried to resuscitate the baby, but intubation revealed immediate return of bright red blood. Despite aggressive intervention, including CPR, respiratory support, antibiotics, and inotropic agents, the infant died at 1 hour of life.
Cause of death: GBS pneumonia
An autopsy revealed bilateral massive consolidation of the lungs due to hemorrhagic bronchopneumonia. Tissue and blood cultures of the spleen, lung, and placenta all grew GBS, as did umbilical cord blood cultures. The cause of death: respiratory failure due to overwhelming GBS pneumonia.
The mother’s postpartum course was complicated by a fever of 100.8°F on the second postoperative day, for which she was treated with intravenous ampicillin, gentamicin, and clindamycin. She was discharged home on the 4th postoperative day.
“Data insufficient” for or against
Many practitioners strip the membranes at term to keep patients from passing their due dates. When the membranes are stripped at 40 weeks’ gestation, two thirds of women enter spontaneous labor within 72 hours; without membrane stripping, only one third of women do.2 The strategy also decreases the chance that pregnancies will go past 42 weeks’ gestation.3
Even more important, studies have found membrane stripping to be safe.3-5 The risk of maternal and neonatal infections does not increase with the procedure, according to a Cochrane Review of 2,797 women in 22 different studies.5
The latest statement on the subject from the American College of Obstetricians and Gynecologists (ACOG) is a Committee Opinion published in December 2002—which came after the neonatal death in this case. It says the risks of membrane stripping in women colonized with GBS “have not been investigated in well-designed prospective studies. Therefore, data are insufficient to encourage or discourage this practice in women known to be GBS-colonized.”6
Expert testimony
Plaintiff
The main witnesses for the plaintiff were a perinatologist and an obstetrician who specializes in infectious diseases. They opined that the infant’s death was caused by the membrane stripping, given that the mother was known to be colonized with GBS.
The perinatologist said his opinion was based on the statements of the infectious disease specialist, who in turn cited a poster presentation at the Infectious Diseases Society for Obstetrics and Gynecology meeting in 20017—which occurred a year after the neonatal death. The poster presentation was a series of 8 cases of perinatal sepsis following membrane stripping; the cases occurred between 1993 and 2000 and were provided by a parents’ group with affected children, “The Jesse Cause.”
Only the perinatologist appeared at trial. When asked to identify a single piece of published, peer-reviewed literature documenting an increased risk of neonatal GBS with membrane stripping, he was unable to do so.
Defense
An expert testified that, although GBS colonization occurs in 20% of all pregnancies, there are no data—prospective, retrospective, or controlled—to suggest that membrane sweeping in GBS-positive patients is associated with GBS sepsis of the newborn, and that membrane sweeping was appropriate in a woman with a fetus affected by unexplained IUGR.
The jury returned a defense verdict after less than 1 day of deliberation. It was not appealed.
As this case demonstrates, expert witnesses sometimes testify on a plaintiff’s behalf despite clear data refuting their statements. ObGyns should be aware that even a practice with a long history, such as membrane stripping, may be proclaimed outside the standard of care by such witnesses. We consider this kind of testimony unethical.
Until we have more data confirming or refuting the association between membrane sweeping (in cases of GBS colonization) and neonatal sepsis, or the medicolegal system changes, obstetricians should proceed with caution. We counsel our patients thoroughly and document the discussion.
Suneet P. Chauhan, MD, Director of Maternal–Fetal Medicine Aurora Women’s Pavilion, Perinatal Assessment Center, West Allis, Wisc
Lower risk of postterm pregnancy
de Miranda E, van der Bom JG, Bonsel GJ, Bleker OP, Rosendaal FR. Membrane sweeping and prevention of post-term pregnancy in low-risk pregnancies: a randomized controlled trial. BJOG. APRIL 2006;113:402–408.
The conclusion of this herculean randomized controlled trial is unequivocal: Sweeping the membranes at 41 weeks’ gestation, regardless of parity, significantly reduces the likelihood a pregnancy will reach 42 weeks. Number needed to treat: 6.
Risks of postterm pregnancy are numerous: greater likelihood of longer labor, cesarean section or operative vaginal delivery, infection, postpartum hemorrhage, shoulder dystocia, stillbirth or neonatal death, and meconium aspiration, to name a few. So any strategy to prevent it—particularly one that is easy and inexpensive—is welcome. The only adverse effect of membrane sweeping is increased bleeding; otherwise, the rates of peripartum complications are similar in women with or without the intervention.
Until 2006, studies of membrane sweeping were not randomized. Empiric evidence has suggested that membrane sweeping is ineffective. As a result, many clinicians eschewed the practice. According to the American College of Obstetricians and Gynecologists (ACOG) practice bulletin on postterm pregnancy,8 management options at 41 weeks’ gestation are limited to labor induction or expectant management with antepartum surveillance.
The de Miranda study was conducted at 51 primary care midwifery practices in the Netherlands.
Strengths. In addition to the randomized, controlled design, the trial’s strengths are:
- Subanalysis of the data based on parity, on whether the gestational age was determined by ultrasound before 18 weeks, and whether the Bishop score was below 6 or at 6 or above
- Participation by several midwives reflected real clinical practice
In addition, almost 90% of patients who underwent the intervention said they would choose it again in the next pregnancy.
Weaknesses. The de Miranda study does have weaknesses:
- Patients randomized to the control group did not undergo a vaginal examination to determine whether they had a cervix favorable for labor induction. This omission seems unacceptable and contrary to ACOG recommendations.8
- The perinatal mortality rate (for all women in the study) was 5.4 per 1,000 births, which is higher than the 1.0 to 3.1 per 1,000 quoted in the ACOG practice bulletin.
There also is some question of which management strategy women prefer, because an earlier study by the Canadian Multicenter Postterm Pregnancy Trial Group9 reported that women assigned to induction were significantly more satisfied than those allocated to observation.
Tan PC, Jacob R, Omar SZ. Membrane sweeping at initiation of formal labor induction. A randomized controlled trial. Obstet Gynecol. MARCH 2006;107:569–577.
The Tan trial randomized 274 women scheduled for induction at term to membrane sweeping or no membrane sweeping at the initiation of induction. Although roughly 1 in 5 deliveries are induced, induction leads to spontaneous vaginal delivery much less often than does spontaneous labor. The Tan study sought to determine whether membrane sweeping increases the likelihood of spontaneous vaginal delivery. Swept women had:
- Higher spontaneous vaginal delivery rate (69% vs 56%, P=.041)
- Shorter induction-to-delivery interval (mean 14 vs 19 hours, P=.003)
- Fewer requirements for oxytocin (46% vs 59%, P=.037)
- Shorter duration of oxytocin infuson (mean 2.6 vs 4.3 hours, P=.001)
- Greater satisfaction with the birth process
Recommendations
These trials are sufficient reason to undertake membrane sweeping every 48 hours in women who strongly desire expectant management at 41 weeks’ gestation
Counsel patients about the risks of observation
Test fetal well-being twice weekly
Sweeping may ease labor induction
The authors report no financial relationships relevant to this article.
Membrane stripping is a focus of controversy when it comes to the issue of group B streptococcus (GBS). This article looks at the literature on the subject and presents a recent legal case in which a woman colonized with GBS claimed membrane stripping was the proximate cause of her infant’s death. In the case, experts for the plaintiff testified that membrane sweeping in a women colonized with GBS is below the standard of care, despite evidence to the contrary. The case, which involved a 2-week jury trial, resulted in a defense verdict.
The legal case
A 22-year-old primigravida presented at just over 39 weeks’ gestation, reporting spontaneous rupture of membranes 1 hour earlier.
IUGR and Group B strep
Her antenatal course had been complicated by intrauterine growth restriction (IUGR), detected by ultrasound at 34 weeks’ gestation. Because of the IUGR, the fetus was being evaluated twice weekly with nonstress tests and amniotic fluid measurements. At 35 weeks, testing for GBS colonization was positive. At 37 weeks, the membranes were stripped to facilitate cervical ripening because of the diagnosed IUGR.
On admission, she was noted to be afebrile with stable vital signs. She was given antibiotics for the GBS and examined. The membranes were grossly ruptured, with clear fluid pooling in the vagina; the cervix was dilated 3 cm with 80% effacement; and the fetus was at –1 to –2 station.
Although the woman was noted to be contracting every 2 minutes, she was barely aware of the contractions. The fetal heart tracing was initially reassuring, with good variability and no decelerations. She was allowed to walk around for 30 minutes.
Sudden fetal bradycardia
Shortly after the patient was placed back on the fetal heart rate monitor, 52 minutes after her initial presentation and approximately 2 hours after rupture of membranes, a marked and sudden fetal bradycardia was noted.
Emergent cesarean section was performed with a low transverse incision. Eighteen minutes after the onset of the bradycardia, a male infant weighing 3,510 g was delivered, with Apgar scores of 0, 2, and 0, at 1, 5, and 10 minutes, respectively. The umbilical cord arterial pH was 6.97. Pediatricians tried to resuscitate the baby, but intubation revealed immediate return of bright red blood. Despite aggressive intervention, including CPR, respiratory support, antibiotics, and inotropic agents, the infant died at 1 hour of life.
Cause of death: GBS pneumonia
An autopsy revealed bilateral massive consolidation of the lungs due to hemorrhagic bronchopneumonia. Tissue and blood cultures of the spleen, lung, and placenta all grew GBS, as did umbilical cord blood cultures. The cause of death: respiratory failure due to overwhelming GBS pneumonia.
The mother’s postpartum course was complicated by a fever of 100.8°F on the second postoperative day, for which she was treated with intravenous ampicillin, gentamicin, and clindamycin. She was discharged home on the 4th postoperative day.
“Data insufficient” for or against
Many practitioners strip the membranes at term to keep patients from passing their due dates. When the membranes are stripped at 40 weeks’ gestation, two thirds of women enter spontaneous labor within 72 hours; without membrane stripping, only one third of women do.2 The strategy also decreases the chance that pregnancies will go past 42 weeks’ gestation.3
Even more important, studies have found membrane stripping to be safe.3-5 The risk of maternal and neonatal infections does not increase with the procedure, according to a Cochrane Review of 2,797 women in 22 different studies.5
The latest statement on the subject from the American College of Obstetricians and Gynecologists (ACOG) is a Committee Opinion published in December 2002—which came after the neonatal death in this case. It says the risks of membrane stripping in women colonized with GBS “have not been investigated in well-designed prospective studies. Therefore, data are insufficient to encourage or discourage this practice in women known to be GBS-colonized.”6
Expert testimony
Plaintiff
The main witnesses for the plaintiff were a perinatologist and an obstetrician who specializes in infectious diseases. They opined that the infant’s death was caused by the membrane stripping, given that the mother was known to be colonized with GBS.
The perinatologist said his opinion was based on the statements of the infectious disease specialist, who in turn cited a poster presentation at the Infectious Diseases Society for Obstetrics and Gynecology meeting in 20017—which occurred a year after the neonatal death. The poster presentation was a series of 8 cases of perinatal sepsis following membrane stripping; the cases occurred between 1993 and 2000 and were provided by a parents’ group with affected children, “The Jesse Cause.”
Only the perinatologist appeared at trial. When asked to identify a single piece of published, peer-reviewed literature documenting an increased risk of neonatal GBS with membrane stripping, he was unable to do so.
Defense
An expert testified that, although GBS colonization occurs in 20% of all pregnancies, there are no data—prospective, retrospective, or controlled—to suggest that membrane sweeping in GBS-positive patients is associated with GBS sepsis of the newborn, and that membrane sweeping was appropriate in a woman with a fetus affected by unexplained IUGR.
The jury returned a defense verdict after less than 1 day of deliberation. It was not appealed.
As this case demonstrates, expert witnesses sometimes testify on a plaintiff’s behalf despite clear data refuting their statements. ObGyns should be aware that even a practice with a long history, such as membrane stripping, may be proclaimed outside the standard of care by such witnesses. We consider this kind of testimony unethical.
Until we have more data confirming or refuting the association between membrane sweeping (in cases of GBS colonization) and neonatal sepsis, or the medicolegal system changes, obstetricians should proceed with caution. We counsel our patients thoroughly and document the discussion.
Suneet P. Chauhan, MD, Director of Maternal–Fetal Medicine Aurora Women’s Pavilion, Perinatal Assessment Center, West Allis, Wisc
Lower risk of postterm pregnancy
de Miranda E, van der Bom JG, Bonsel GJ, Bleker OP, Rosendaal FR. Membrane sweeping and prevention of post-term pregnancy in low-risk pregnancies: a randomized controlled trial. BJOG. APRIL 2006;113:402–408.
The conclusion of this herculean randomized controlled trial is unequivocal: Sweeping the membranes at 41 weeks’ gestation, regardless of parity, significantly reduces the likelihood a pregnancy will reach 42 weeks. Number needed to treat: 6.
Risks of postterm pregnancy are numerous: greater likelihood of longer labor, cesarean section or operative vaginal delivery, infection, postpartum hemorrhage, shoulder dystocia, stillbirth or neonatal death, and meconium aspiration, to name a few. So any strategy to prevent it—particularly one that is easy and inexpensive—is welcome. The only adverse effect of membrane sweeping is increased bleeding; otherwise, the rates of peripartum complications are similar in women with or without the intervention.
Until 2006, studies of membrane sweeping were not randomized. Empiric evidence has suggested that membrane sweeping is ineffective. As a result, many clinicians eschewed the practice. According to the American College of Obstetricians and Gynecologists (ACOG) practice bulletin on postterm pregnancy,8 management options at 41 weeks’ gestation are limited to labor induction or expectant management with antepartum surveillance.
The de Miranda study was conducted at 51 primary care midwifery practices in the Netherlands.
Strengths. In addition to the randomized, controlled design, the trial’s strengths are:
- Subanalysis of the data based on parity, on whether the gestational age was determined by ultrasound before 18 weeks, and whether the Bishop score was below 6 or at 6 or above
- Participation by several midwives reflected real clinical practice
In addition, almost 90% of patients who underwent the intervention said they would choose it again in the next pregnancy.
Weaknesses. The de Miranda study does have weaknesses:
- Patients randomized to the control group did not undergo a vaginal examination to determine whether they had a cervix favorable for labor induction. This omission seems unacceptable and contrary to ACOG recommendations.8
- The perinatal mortality rate (for all women in the study) was 5.4 per 1,000 births, which is higher than the 1.0 to 3.1 per 1,000 quoted in the ACOG practice bulletin.
There also is some question of which management strategy women prefer, because an earlier study by the Canadian Multicenter Postterm Pregnancy Trial Group9 reported that women assigned to induction were significantly more satisfied than those allocated to observation.
Tan PC, Jacob R, Omar SZ. Membrane sweeping at initiation of formal labor induction. A randomized controlled trial. Obstet Gynecol. MARCH 2006;107:569–577.
The Tan trial randomized 274 women scheduled for induction at term to membrane sweeping or no membrane sweeping at the initiation of induction. Although roughly 1 in 5 deliveries are induced, induction leads to spontaneous vaginal delivery much less often than does spontaneous labor. The Tan study sought to determine whether membrane sweeping increases the likelihood of spontaneous vaginal delivery. Swept women had:
- Higher spontaneous vaginal delivery rate (69% vs 56%, P=.041)
- Shorter induction-to-delivery interval (mean 14 vs 19 hours, P=.003)
- Fewer requirements for oxytocin (46% vs 59%, P=.037)
- Shorter duration of oxytocin infuson (mean 2.6 vs 4.3 hours, P=.001)
- Greater satisfaction with the birth process
Recommendations
These trials are sufficient reason to undertake membrane sweeping every 48 hours in women who strongly desire expectant management at 41 weeks’ gestation
Counsel patients about the risks of observation
Test fetal well-being twice weekly
Sweeping may ease labor induction
The authors report no financial relationships relevant to this article.
1. McColgin SW, Bennett WA, Roach H, et al. Parturitional factors associated with membrane stripping. Am J Obstet Gynecol. 1993;169:71-77.
2. Allott HA, Palmer CR. Sweeping the membranes: a valid procedure in stimulating the onset of labour? Br J Obstet Gynaecol. 1993;100:898-890.
3. McColgin SW, Hampton HL, McCaul JF, et al. Stripping of membranes at term: can it safely reduce the incidence of postterm pregnancy? Obstet Gynecol. 1990;76:678-680.
4. Netta D, Visintainer P, Bayliss P. Does cervical membrane stripping increase maternal colonization of group B streptococcus? Poster presented at: 23rd Annual Meeting of the Society for Maternal-Fetal Medicine; February 3-8, 2003; San Francisco, Calif.
5. Boulvain M, Stan C, Irion O. Membrane sweeping for induction of labour. The Cochrane Database of Systematic Reviews. 2005; Issue 1; Art No CD000451. DOI: 10.1002/14651858.
6. Prevention of Early-Onset Group B Streptococcal Disease in Newborns. ACOG Committee Opinion No. 279. American College of Obstetricians and Gynecologists. Washington, DC: ACOG; 2002.
7. Stamm CA, Bishop LA, McGregor JA, McFee JG, Perhach M. Cervical manipulation and membrane “stripping” associated with perinatal sepsis and death caused by group B streptococcus and other perinatal pathogens: cervical membrane disruption syndrome (CMDS). Poster presented at: Annual Meeting of the Infectious Diseases Society for Obstetrics and Gynecology; Aug 9-11, 2001; Quebec.
8. Management of Postterm Pregnancy. ACOG Practice Bulletin No. 55. American College of Obstetricians and Gynecologists. Washington, DC: ACOG; 2004.
9. Hannah ME, Hannah WJ, Hellmann J, et al. Induction of labor as compared with serial antenatal monitoring in post-term pregnancy. A randomized controlled trial. The Canadian Multicenter Post-term Pregnancy Trial Group. N Engl J Med. 1992;326:1587-1592.
1. McColgin SW, Bennett WA, Roach H, et al. Parturitional factors associated with membrane stripping. Am J Obstet Gynecol. 1993;169:71-77.
2. Allott HA, Palmer CR. Sweeping the membranes: a valid procedure in stimulating the onset of labour? Br J Obstet Gynaecol. 1993;100:898-890.
3. McColgin SW, Hampton HL, McCaul JF, et al. Stripping of membranes at term: can it safely reduce the incidence of postterm pregnancy? Obstet Gynecol. 1990;76:678-680.
4. Netta D, Visintainer P, Bayliss P. Does cervical membrane stripping increase maternal colonization of group B streptococcus? Poster presented at: 23rd Annual Meeting of the Society for Maternal-Fetal Medicine; February 3-8, 2003; San Francisco, Calif.
5. Boulvain M, Stan C, Irion O. Membrane sweeping for induction of labour. The Cochrane Database of Systematic Reviews. 2005; Issue 1; Art No CD000451. DOI: 10.1002/14651858.
6. Prevention of Early-Onset Group B Streptococcal Disease in Newborns. ACOG Committee Opinion No. 279. American College of Obstetricians and Gynecologists. Washington, DC: ACOG; 2002.
7. Stamm CA, Bishop LA, McGregor JA, McFee JG, Perhach M. Cervical manipulation and membrane “stripping” associated with perinatal sepsis and death caused by group B streptococcus and other perinatal pathogens: cervical membrane disruption syndrome (CMDS). Poster presented at: Annual Meeting of the Infectious Diseases Society for Obstetrics and Gynecology; Aug 9-11, 2001; Quebec.
8. Management of Postterm Pregnancy. ACOG Practice Bulletin No. 55. American College of Obstetricians and Gynecologists. Washington, DC: ACOG; 2004.
9. Hannah ME, Hannah WJ, Hellmann J, et al. Induction of labor as compared with serial antenatal monitoring in post-term pregnancy. A randomized controlled trial. The Canadian Multicenter Post-term Pregnancy Trial Group. N Engl J Med. 1992;326:1587-1592.
OB DILEMMAS: Is this induction necessary?
What would you do?
Membrane sweeping and group B strep: A litigious combination?
We present 3 scenarios and our recommendations for each. In each case, we cite the supporting evidence to date on the critical questions that lead to an appropriate decision.
| CONDITION | 0 | 1 | 2 | 3 |
|---|---|---|---|---|
| Dilation (cm) | Closed | 1–2 | 3–4 | ≥5 |
| Effacement (%) | 0–30 | 40–50 | 60–70 | ≥80 |
| Station | –3 | –2 | –1,0 | +1,+2 |
| Consistency | Firm | Medium | Soft | — |
| Position | Posterior | Midposition | Anterior | — |
| Source: Bishop EH.4 Reprinted by permission | ||||
CASE 1: Primigravida at 42 weeks
G.C. is a 24-year-old gravida 1 para 0 at 42 0/7 weeks’ gestation, according to her last menstrual period and an ultrasound at 18 weeks. She has had twice-weekly fetal testing since 41weeks’ gestation, with adequate amniotic fluid noted and reassuring results. A nonstress test today was reactive, and the amniotic fluid index is 10.2. Her cervix is closed, firm, and 50% effaced. The fetus is at -3 station and vertex. Estimated fetal weight is 3,500 g. What are the options?
Critical questions
Is she postterm?
Yes. Because the perinatal mortality rate for postterm pregnancies (defined as 42 or more weeks3) is twice that of term gestations, there is evidence to support labor induction after 41 completed weeks. Induction would appear to be justified in this woman’s case.
Is her Bishop score less than 5?
Yes. This patient has an “unfavorable” cervix, according to her Bishop score of 1. (A score of less than 5 is considered unfavorable.) The Bishop scoring system is now generally used to predict the likelihood of successful labor induction, although it was originally used to prevent iatrogenic prematurity in women undergoing elective induction of labor. It is based on clinical findings at the cervical examination: degree of cervical dilation, effacement, consistency, and the position and station of the fetal presenting part.4,5
Is she nulliparous?
Yes. Elective induction should be strongly discouraged in nulliparous patients. Among nulliparous women, an unfavorable Bishop score is associated with almost twice the risk of cesarean delivery when labor is induced rather than spontaneous.6 However, if induction is indicated, cervical ripening may help. Cervical ripening prepares the cervix by promoting dilation and effacement,7 using pharmacologic or mechanical means.
Our recommendations
Although G.C. is nulliparous with an unfavorable cervical examination, her gestational age of 42 weeks provides reason to proceed with induction of labor.
Prostaglandins for cervical ripening
These agents dissolve collagen bundles and increase the submucosal water content of the cervix.8
Off-label but evidence-based. Our prostaglandin of choice is misoprostol (Cytotec), a synthetic prostaglandin E1 analog. Although its use for this purpose is off-label,9 an extensive body of literature attests its safety and efficacy for cervical ripening, provided it is properly administered.10 Misoprostol appears to be more effective than prostaglandin E2 at achieving vaginal delivery within 24 hours.11
Misoprostol is also cheaper and requires no special handling, in contrast to prostaglandin E2.9,12
Caveats. Uterine hyperstimulation and meconium-stained amniotic fluid appear to be more common with misoprostol, although these risks can be minimized by using a dose of 25 μg (1/4 of a 100-μg tablet) at an interval of 3 to 6 hours, with oxytocin given no later than 4 hours after the last dose of misoprostol.11
Prostaglandin administration is associated with increased risk of uterine rupture in women with a prior cesarean delivery or other uterine surgery (SEE CASE 2).13-16 Thus misoprostol and other prostaglandins should be avoided in these women.
Administer prostaglandins in or close to the labor and delivery unit, and where uterine activity and fetal heart rate can be continuously monitored.1 The patient should remain supine for 30 minutes.
Mechanical means of cervical ripening
The 16F Foley catheter is placed transcervically into the extra-amniotic space. The balloon is then inflated with 30 mL of saline and pulled back so that it rests against the internal cervical os.
Low cost. This method of cervical ripening is low in cost and carries less risk of hyperstimulation.17 Thus, it is especially beneficial when the patient needs cervical ripening but is contracting too frequently for safe administration of prostaglandins.
Limitations. In some women it is impossible to place the catheter into the cervical canal because of discomfort or unfavorable position or consistency of the cervix. Also, the catheter may increase the risk of infection or cause disruption of a low-lying placenta.
Combined pharmacologic and mechanical methods?
Although the combination would appear to have greater potential for success, it has not proven to be more effective.7
Sequential cervical ripening on an outpatient basis also has been suggested, but further studies are needed before it can be recommended.18
After G.C. is counseled about the risks of postterm pregnancy, as well as the risk of cesarean delivery with induction of labor, she decides to proceed with labor induction. Cervical ripening with misoprostol is begun. After 2 doses, the patient is dilated 4 cm, and oxytocin is initiated along with artificial rupture of membranes. Labor ultimately arrests at 7 cm dilation, and a healthy male infant is delivered by cesarean.
CASE 2: Mild preeclampsia at 37 weeks
M.A. is a 35-year-old gravida 4 para 2012, who complains of a headache at 37 5/7 weeks’ gestation. Her blood pressure is 157/97, and she has 1+ proteinuria on dipstick urinalysis. Her laboratory tests are unremarkable, including normal serum creatinine, liver function tests, and platelets.
The fetus appears to be appropriately grown with normal amniotic fluid. Antepartum fetal heart rate testing is reactive and reassuring. The patient is having intermittent, mild uterine contractions, and her cervix is dilated 3 cm. She is given acetaminophen for the headache, which brings relief.
You diagnose mild preeclampsia at term, for which induction of labor is clearly indicated. However, M.A.’s pregnancy history is notable for a term vaginal delivery followed by a low transverse cesarean section for a term breech infant after a failed external cephalic version. She strongly desires vaginal delivery.
Do you accede to her wish for a trial of labor?
Critical questions
Is her pregnancy history favorable?
No. This patient’s previous deliveries have a bearing on the current pregnancy.15 Specifically, the patient should have had no more than 1 low transverse cesarean delivery, no other uterine scars, no previous uterine rupture, and she should have a clinically adequate pelvis.
Are facilities and staff adequate?
Obviously the answer to this question is unique to the site. It is necessary to have immediate availability of the obstetrician throughout active labor, and to have adequate personnel to perform an emergency cesarean if necessary.
If these criteria are met, is induction of labor appropriate?
Perhaps. Recent data suggest that women with a uterine scar who undergo induction with prostaglandins have a risk of uterine rupture 5 times that of women who enter spontaneous labor (24.5 per 1,000 versus 5.2 per 1,000).16 For this reason, do not use prostaglandins for labor induction in women with viable gestations who have a prior low transverse incision.
Oxytocin. Augmentation of labor with oxytocin does not appear to increase the risk of rupture, compared with spontaneous labor.14 Induction of labor with oxytocin has been associated with a slightly higher risk of uterine rupture, compared with spontaneous labor, although both types of labor have a rupture rate under 1%.14
Our recommendations
If M.A. still desires a trial of labor after she has been counseled about the risks and benefits of vaginal birth after cesarean delivery, induction should be considered. She has a reasonable likelihood of success, because she has given birth vaginally in the past, her previous cesarean delivery was for a nonrecurring indication (breech presentation), and she does not require any cervical ripening agents, as this has occurred naturally.15
Amniotomy may help reduce time to delivery
One option for induction of labor is amniotomy with oxytocin augmentation as needed. Amniotomy, or the artificial rupture of membranes, has been shown to be an effective method of labor induction in women with a favorable cervix.19
The combination of amniotomy and oxytocin administration is particularly effective, resulting in a shorter induction to delivery time, compared with women undergoing oxytocin induction alone.20
Sweeping or stripping the fetal membranes
Digital separation of the chorioamniotic membrane from the walls of the cervix and lower uterine segment during sterile vaginal examination could also be incorporated into M.A.’s induction process.21
Membrane sweeping causes the release of endogenous prostaglandins and can lead to labor without the need for induction agents or amniotomy. Although membrane sweeping is generally performed without admission to the hospital, M.A. would require hospitalization because of her diagnosis of preeclampsia.
If the cervix is unfavorable
If this patient had an unfavorable cervix, would induction of labor be contraindicated? Certainly the use of prostaglandins for preinduction cervical ripening would be contraindicated, given the existing evidence, although use of a transcervical Foley catheter would be acceptable.13-16 Published studies suggest that transcervical Foley catheter induction does not appreciably increase the risk of uterine rupture, although these studies have relatively small sample sizes and are not randomized.22,23
Although transcervical Foley catheter induction is often begun on an outpatient basis, it should probably be limited to hospital use in a woman with a previous uterine scar.24
M.A. receives magnesium sulfate for seizure prophylaxis. Labor is successfully induced with membrane sweeping and subsequent amniotomy. Labor is augmented with oxytocin, with continuous fetal heart rate and uterine activity monitoring throughout labor. She successfully delivers a healthy female infant.
CASE 3: Preeclampsia remote from term
L.A. is a 19-year-old gravida 1 para 0 who was hospitalized with preeclampsia at 28 weeks’ gestation. At that time, she was given betamethasone and monitored on inpatient bed rest. At 30 1/7 weeks’ gestation, she begins complaining of a headache. Her blood pressure is 168/110, and she has 2+ proteinuria on a dipstick. Her platelet count is 110,000, serum creatinine is 1.0, and she has slightly elevated liver transaminases in the 40s. Her cervix is closed, long, high, firm, and posterior. What are your choices?
Critical questions
Does preeclampsia rule out induction?
No. In a woman with severe preeclampsia remote from term, labor induction is not contraindicated.25-27 In fact, it may be a particularly reasonable option in a patient who is stable. Even eclampsia is not a contraindication to labor induction. However, rapidly evolving disease may preclude a prolonged labor induction, because delivery is the key to resolution of preeclampsia.
Are any clinical features in her favor?
Yes. The best predictors of success are a favorable Bishop score and a gestational age greater than 28 weeks.25-27
Our recommendations
Although L.A.’s Bishop score is unfavorable, her relatively stable clinical status and her gestational age suggest that labor induction should not be ruled out.
Preinduction cervical ripening
If cervical ripening is necessary, the transcervical Foley catheter may be the best choice, particularly among pregnancies affected by intrauterine growth restriction, as hyperstimulation is unlikely to occur with this method.
L.A. undergoes cervical ripening with the transcervical Foley catheter and subsequent amniotomy and oxytocin infusion. She is given magnesium sulfate for seizure prophylaxis throughout the cervical ripening and induction processes, and her clinical status is closely monitored, including blood pressure, urine output, and laboratory values. Her platelet count, serum creatinine, and liver transaminases remain stable, and she has a successful vaginal delivery.
1. Induction of labor. ACOG Practice Bulletin No 10. Washington, DC: ACOG; November 1999.
2. Zhang J, Yancey MK, et al. US national trends in labor induction,1989-1998. J Reprod Med. 2002;47:120-124.
3. Management of postterm pregnancy. ACOG Practice Bulletin No 55. Washington, DC: ACOG; September 2004.
4. Bishop EH. Pelvic scoring for elective induction. Obstet Gynecol. 1964;24:266-268.
5. Calder AA, Brennand JE. Labor and normal delivery: induction of labor. Curr Opin Obstet Gynecol. 1997;3:764-768.
6. Johnson DP, et al. Risk of cesarean delivery after induction of labor at term in nulliparous women with an unfavorable cervix. Am J Obstet Gynecol. 2003;188:1565-1569.
7. Chung JH, Huang WH, Rumney PJ, Garite TJ, Nageotte MP. A prospective randomized controlled trial that compared misoprostol, Foley catheter and combination misoprostol-Foley catheter for labor induction. Am J Obstet Gynecol. 2003;189:1031-1035.
8. Rayburn WF, Lightfoot SA, Newland JR, Smith CV, Christensen HD. A model for investigating microscopic changes induced by prostaglandin E2 in the term cervix. J Matern Fetal Invest. 1994;4:137.-
9. New US Food and Drug Administration labeling on Cytotec (misoprostol) use and pregnancy. ACOG Committee Opinion No 283. Washington, DC: ACOG; May 2003.
10. Wing DA. Labor induction with misoprostol. Am J Obstet Gynecol. 1999;181:339-345.
11. Response to Searle’s drug warning on misoprostol. ACOG Committee Opinion No 248. Washington, DC: ACOG; December 2000.
12. Wing DA. A benefit-risk assessment of misoprostol for cervical ripening and labour induction. Drug Safety. 2002;25:665-676.
13. Wing DA, et al. Disruption of prior uterine incision following misoprostol for labor induction in women with previous cesarean delivery. Obstet Gynecol. 1998;91:828-830.
14. Lydon-Rochelle M, Holt VL, Easterling TR, Martin DP. Risk of uterine rupture during labor among women with a prior cesarean delivery. N Engl J Med. 2001;345:3-8.
15. Vaginal birth after previous cesarean delivery. ACOG Practice Bulletin No 54. Washington, DC: ACOG; July 2004.
16. Induction of labor for vaginal birth after cesarean delivery. ACOG Committee Opinion No 271. Washington, DC: ACOG; April 2002.
17. Boulvain M, Kelly A, Lohse C, Stan C, Irion O. Mechanical methods for induction of labor. Cochrane Database Syst Rev. 2001;(4):CD001233.-
18. Sawai SK, Williams MC, O’Brien WF, et al. Sequential outpatient application of intravaginal E2 gel in the management of postdates pregnancies. Obstet Gynecol. 1991;78:19-23.
19. Booth JH, Kurdyak VB. Elective induction of labour: a controlled study. Can Med Assoc J. 1970;103:245-248.
20. Moldin PG, Sundell G. Induction of labour: a randomised clinical trial of amniotomy versus amniotomy with oxytocin infusion. Br J Obstet Gynaecol. 1996;103:306-312.
21. Boulvain M, Stan C, Irion O. Membrane sweeping for induction of labor. Cochrane Database Syst Rev. 2005;CD000451.-
22. Ravasia D, Wood SL, Pollard JK. Uterine rupture during induced labor among women with previous cesarean delivery. Am J Obstet Gynecol. 2000;183:1176-1179.
23. Bujold E, Blackwell SC, Gauthier RJ. Cervical ripening with transcervical Foley catheter and the risk of uterine rupture. Obstet Gynecol. 2004;103:18-23.
24. Sciscione AC, Muench M, et al. Transcervical Foley catheter for preinduction cervical ripening in an outpatient versus inpatient setting. Obstet Gynecol. 2001;98:751-756.
25. Nassar AH, Adra AM, Chaktoura N, et al. Severe preeclampsia remote from term: labor induction or elective cesarean delivery? Am J Obstet Gynecol. 1998;179:1210-1213.
26. Blackwell SC, Redman ME, Tomlinson M, et al. Labor induction for the preterm severe preeclamptic patient: is it worth the effort? J Matern Fetal Med. 2001;10:305-311.
27. Alexander JM, Bloom SL, et al. Severe preeclampsia and the very low birth weight infant: is induction of labor harmful? Obstet Gynecol. 1999;93:485-488.
What would you do?
Membrane sweeping and group B strep: A litigious combination?
We present 3 scenarios and our recommendations for each. In each case, we cite the supporting evidence to date on the critical questions that lead to an appropriate decision.
| CONDITION | 0 | 1 | 2 | 3 |
|---|---|---|---|---|
| Dilation (cm) | Closed | 1–2 | 3–4 | ≥5 |
| Effacement (%) | 0–30 | 40–50 | 60–70 | ≥80 |
| Station | –3 | –2 | –1,0 | +1,+2 |
| Consistency | Firm | Medium | Soft | — |
| Position | Posterior | Midposition | Anterior | — |
| Source: Bishop EH.4 Reprinted by permission | ||||
CASE 1: Primigravida at 42 weeks
G.C. is a 24-year-old gravida 1 para 0 at 42 0/7 weeks’ gestation, according to her last menstrual period and an ultrasound at 18 weeks. She has had twice-weekly fetal testing since 41weeks’ gestation, with adequate amniotic fluid noted and reassuring results. A nonstress test today was reactive, and the amniotic fluid index is 10.2. Her cervix is closed, firm, and 50% effaced. The fetus is at -3 station and vertex. Estimated fetal weight is 3,500 g. What are the options?
Critical questions
Is she postterm?
Yes. Because the perinatal mortality rate for postterm pregnancies (defined as 42 or more weeks3) is twice that of term gestations, there is evidence to support labor induction after 41 completed weeks. Induction would appear to be justified in this woman’s case.
Is her Bishop score less than 5?
Yes. This patient has an “unfavorable” cervix, according to her Bishop score of 1. (A score of less than 5 is considered unfavorable.) The Bishop scoring system is now generally used to predict the likelihood of successful labor induction, although it was originally used to prevent iatrogenic prematurity in women undergoing elective induction of labor. It is based on clinical findings at the cervical examination: degree of cervical dilation, effacement, consistency, and the position and station of the fetal presenting part.4,5
Is she nulliparous?
Yes. Elective induction should be strongly discouraged in nulliparous patients. Among nulliparous women, an unfavorable Bishop score is associated with almost twice the risk of cesarean delivery when labor is induced rather than spontaneous.6 However, if induction is indicated, cervical ripening may help. Cervical ripening prepares the cervix by promoting dilation and effacement,7 using pharmacologic or mechanical means.
Our recommendations
Although G.C. is nulliparous with an unfavorable cervical examination, her gestational age of 42 weeks provides reason to proceed with induction of labor.
Prostaglandins for cervical ripening
These agents dissolve collagen bundles and increase the submucosal water content of the cervix.8
Off-label but evidence-based. Our prostaglandin of choice is misoprostol (Cytotec), a synthetic prostaglandin E1 analog. Although its use for this purpose is off-label,9 an extensive body of literature attests its safety and efficacy for cervical ripening, provided it is properly administered.10 Misoprostol appears to be more effective than prostaglandin E2 at achieving vaginal delivery within 24 hours.11
Misoprostol is also cheaper and requires no special handling, in contrast to prostaglandin E2.9,12
Caveats. Uterine hyperstimulation and meconium-stained amniotic fluid appear to be more common with misoprostol, although these risks can be minimized by using a dose of 25 μg (1/4 of a 100-μg tablet) at an interval of 3 to 6 hours, with oxytocin given no later than 4 hours after the last dose of misoprostol.11
Prostaglandin administration is associated with increased risk of uterine rupture in women with a prior cesarean delivery or other uterine surgery (SEE CASE 2).13-16 Thus misoprostol and other prostaglandins should be avoided in these women.
Administer prostaglandins in or close to the labor and delivery unit, and where uterine activity and fetal heart rate can be continuously monitored.1 The patient should remain supine for 30 minutes.
Mechanical means of cervical ripening
The 16F Foley catheter is placed transcervically into the extra-amniotic space. The balloon is then inflated with 30 mL of saline and pulled back so that it rests against the internal cervical os.
Low cost. This method of cervical ripening is low in cost and carries less risk of hyperstimulation.17 Thus, it is especially beneficial when the patient needs cervical ripening but is contracting too frequently for safe administration of prostaglandins.
Limitations. In some women it is impossible to place the catheter into the cervical canal because of discomfort or unfavorable position or consistency of the cervix. Also, the catheter may increase the risk of infection or cause disruption of a low-lying placenta.
Combined pharmacologic and mechanical methods?
Although the combination would appear to have greater potential for success, it has not proven to be more effective.7
Sequential cervical ripening on an outpatient basis also has been suggested, but further studies are needed before it can be recommended.18
After G.C. is counseled about the risks of postterm pregnancy, as well as the risk of cesarean delivery with induction of labor, she decides to proceed with labor induction. Cervical ripening with misoprostol is begun. After 2 doses, the patient is dilated 4 cm, and oxytocin is initiated along with artificial rupture of membranes. Labor ultimately arrests at 7 cm dilation, and a healthy male infant is delivered by cesarean.
CASE 2: Mild preeclampsia at 37 weeks
M.A. is a 35-year-old gravida 4 para 2012, who complains of a headache at 37 5/7 weeks’ gestation. Her blood pressure is 157/97, and she has 1+ proteinuria on dipstick urinalysis. Her laboratory tests are unremarkable, including normal serum creatinine, liver function tests, and platelets.
The fetus appears to be appropriately grown with normal amniotic fluid. Antepartum fetal heart rate testing is reactive and reassuring. The patient is having intermittent, mild uterine contractions, and her cervix is dilated 3 cm. She is given acetaminophen for the headache, which brings relief.
You diagnose mild preeclampsia at term, for which induction of labor is clearly indicated. However, M.A.’s pregnancy history is notable for a term vaginal delivery followed by a low transverse cesarean section for a term breech infant after a failed external cephalic version. She strongly desires vaginal delivery.
Do you accede to her wish for a trial of labor?
Critical questions
Is her pregnancy history favorable?
No. This patient’s previous deliveries have a bearing on the current pregnancy.15 Specifically, the patient should have had no more than 1 low transverse cesarean delivery, no other uterine scars, no previous uterine rupture, and she should have a clinically adequate pelvis.
Are facilities and staff adequate?
Obviously the answer to this question is unique to the site. It is necessary to have immediate availability of the obstetrician throughout active labor, and to have adequate personnel to perform an emergency cesarean if necessary.
If these criteria are met, is induction of labor appropriate?
Perhaps. Recent data suggest that women with a uterine scar who undergo induction with prostaglandins have a risk of uterine rupture 5 times that of women who enter spontaneous labor (24.5 per 1,000 versus 5.2 per 1,000).16 For this reason, do not use prostaglandins for labor induction in women with viable gestations who have a prior low transverse incision.
Oxytocin. Augmentation of labor with oxytocin does not appear to increase the risk of rupture, compared with spontaneous labor.14 Induction of labor with oxytocin has been associated with a slightly higher risk of uterine rupture, compared with spontaneous labor, although both types of labor have a rupture rate under 1%.14
Our recommendations
If M.A. still desires a trial of labor after she has been counseled about the risks and benefits of vaginal birth after cesarean delivery, induction should be considered. She has a reasonable likelihood of success, because she has given birth vaginally in the past, her previous cesarean delivery was for a nonrecurring indication (breech presentation), and she does not require any cervical ripening agents, as this has occurred naturally.15
Amniotomy may help reduce time to delivery
One option for induction of labor is amniotomy with oxytocin augmentation as needed. Amniotomy, or the artificial rupture of membranes, has been shown to be an effective method of labor induction in women with a favorable cervix.19
The combination of amniotomy and oxytocin administration is particularly effective, resulting in a shorter induction to delivery time, compared with women undergoing oxytocin induction alone.20
Sweeping or stripping the fetal membranes
Digital separation of the chorioamniotic membrane from the walls of the cervix and lower uterine segment during sterile vaginal examination could also be incorporated into M.A.’s induction process.21
Membrane sweeping causes the release of endogenous prostaglandins and can lead to labor without the need for induction agents or amniotomy. Although membrane sweeping is generally performed without admission to the hospital, M.A. would require hospitalization because of her diagnosis of preeclampsia.
If the cervix is unfavorable
If this patient had an unfavorable cervix, would induction of labor be contraindicated? Certainly the use of prostaglandins for preinduction cervical ripening would be contraindicated, given the existing evidence, although use of a transcervical Foley catheter would be acceptable.13-16 Published studies suggest that transcervical Foley catheter induction does not appreciably increase the risk of uterine rupture, although these studies have relatively small sample sizes and are not randomized.22,23
Although transcervical Foley catheter induction is often begun on an outpatient basis, it should probably be limited to hospital use in a woman with a previous uterine scar.24
M.A. receives magnesium sulfate for seizure prophylaxis. Labor is successfully induced with membrane sweeping and subsequent amniotomy. Labor is augmented with oxytocin, with continuous fetal heart rate and uterine activity monitoring throughout labor. She successfully delivers a healthy female infant.
CASE 3: Preeclampsia remote from term
L.A. is a 19-year-old gravida 1 para 0 who was hospitalized with preeclampsia at 28 weeks’ gestation. At that time, she was given betamethasone and monitored on inpatient bed rest. At 30 1/7 weeks’ gestation, she begins complaining of a headache. Her blood pressure is 168/110, and she has 2+ proteinuria on a dipstick. Her platelet count is 110,000, serum creatinine is 1.0, and she has slightly elevated liver transaminases in the 40s. Her cervix is closed, long, high, firm, and posterior. What are your choices?
Critical questions
Does preeclampsia rule out induction?
No. In a woman with severe preeclampsia remote from term, labor induction is not contraindicated.25-27 In fact, it may be a particularly reasonable option in a patient who is stable. Even eclampsia is not a contraindication to labor induction. However, rapidly evolving disease may preclude a prolonged labor induction, because delivery is the key to resolution of preeclampsia.
Are any clinical features in her favor?
Yes. The best predictors of success are a favorable Bishop score and a gestational age greater than 28 weeks.25-27
Our recommendations
Although L.A.’s Bishop score is unfavorable, her relatively stable clinical status and her gestational age suggest that labor induction should not be ruled out.
Preinduction cervical ripening
If cervical ripening is necessary, the transcervical Foley catheter may be the best choice, particularly among pregnancies affected by intrauterine growth restriction, as hyperstimulation is unlikely to occur with this method.
L.A. undergoes cervical ripening with the transcervical Foley catheter and subsequent amniotomy and oxytocin infusion. She is given magnesium sulfate for seizure prophylaxis throughout the cervical ripening and induction processes, and her clinical status is closely monitored, including blood pressure, urine output, and laboratory values. Her platelet count, serum creatinine, and liver transaminases remain stable, and she has a successful vaginal delivery.
What would you do?
Membrane sweeping and group B strep: A litigious combination?
We present 3 scenarios and our recommendations for each. In each case, we cite the supporting evidence to date on the critical questions that lead to an appropriate decision.
| CONDITION | 0 | 1 | 2 | 3 |
|---|---|---|---|---|
| Dilation (cm) | Closed | 1–2 | 3–4 | ≥5 |
| Effacement (%) | 0–30 | 40–50 | 60–70 | ≥80 |
| Station | –3 | –2 | –1,0 | +1,+2 |
| Consistency | Firm | Medium | Soft | — |
| Position | Posterior | Midposition | Anterior | — |
| Source: Bishop EH.4 Reprinted by permission | ||||
CASE 1: Primigravida at 42 weeks
G.C. is a 24-year-old gravida 1 para 0 at 42 0/7 weeks’ gestation, according to her last menstrual period and an ultrasound at 18 weeks. She has had twice-weekly fetal testing since 41weeks’ gestation, with adequate amniotic fluid noted and reassuring results. A nonstress test today was reactive, and the amniotic fluid index is 10.2. Her cervix is closed, firm, and 50% effaced. The fetus is at -3 station and vertex. Estimated fetal weight is 3,500 g. What are the options?
Critical questions
Is she postterm?
Yes. Because the perinatal mortality rate for postterm pregnancies (defined as 42 or more weeks3) is twice that of term gestations, there is evidence to support labor induction after 41 completed weeks. Induction would appear to be justified in this woman’s case.
Is her Bishop score less than 5?
Yes. This patient has an “unfavorable” cervix, according to her Bishop score of 1. (A score of less than 5 is considered unfavorable.) The Bishop scoring system is now generally used to predict the likelihood of successful labor induction, although it was originally used to prevent iatrogenic prematurity in women undergoing elective induction of labor. It is based on clinical findings at the cervical examination: degree of cervical dilation, effacement, consistency, and the position and station of the fetal presenting part.4,5
Is she nulliparous?
Yes. Elective induction should be strongly discouraged in nulliparous patients. Among nulliparous women, an unfavorable Bishop score is associated with almost twice the risk of cesarean delivery when labor is induced rather than spontaneous.6 However, if induction is indicated, cervical ripening may help. Cervical ripening prepares the cervix by promoting dilation and effacement,7 using pharmacologic or mechanical means.
Our recommendations
Although G.C. is nulliparous with an unfavorable cervical examination, her gestational age of 42 weeks provides reason to proceed with induction of labor.
Prostaglandins for cervical ripening
These agents dissolve collagen bundles and increase the submucosal water content of the cervix.8
Off-label but evidence-based. Our prostaglandin of choice is misoprostol (Cytotec), a synthetic prostaglandin E1 analog. Although its use for this purpose is off-label,9 an extensive body of literature attests its safety and efficacy for cervical ripening, provided it is properly administered.10 Misoprostol appears to be more effective than prostaglandin E2 at achieving vaginal delivery within 24 hours.11
Misoprostol is also cheaper and requires no special handling, in contrast to prostaglandin E2.9,12
Caveats. Uterine hyperstimulation and meconium-stained amniotic fluid appear to be more common with misoprostol, although these risks can be minimized by using a dose of 25 μg (1/4 of a 100-μg tablet) at an interval of 3 to 6 hours, with oxytocin given no later than 4 hours after the last dose of misoprostol.11
Prostaglandin administration is associated with increased risk of uterine rupture in women with a prior cesarean delivery or other uterine surgery (SEE CASE 2).13-16 Thus misoprostol and other prostaglandins should be avoided in these women.
Administer prostaglandins in or close to the labor and delivery unit, and where uterine activity and fetal heart rate can be continuously monitored.1 The patient should remain supine for 30 minutes.
Mechanical means of cervical ripening
The 16F Foley catheter is placed transcervically into the extra-amniotic space. The balloon is then inflated with 30 mL of saline and pulled back so that it rests against the internal cervical os.
Low cost. This method of cervical ripening is low in cost and carries less risk of hyperstimulation.17 Thus, it is especially beneficial when the patient needs cervical ripening but is contracting too frequently for safe administration of prostaglandins.
Limitations. In some women it is impossible to place the catheter into the cervical canal because of discomfort or unfavorable position or consistency of the cervix. Also, the catheter may increase the risk of infection or cause disruption of a low-lying placenta.
Combined pharmacologic and mechanical methods?
Although the combination would appear to have greater potential for success, it has not proven to be more effective.7
Sequential cervical ripening on an outpatient basis also has been suggested, but further studies are needed before it can be recommended.18
After G.C. is counseled about the risks of postterm pregnancy, as well as the risk of cesarean delivery with induction of labor, she decides to proceed with labor induction. Cervical ripening with misoprostol is begun. After 2 doses, the patient is dilated 4 cm, and oxytocin is initiated along with artificial rupture of membranes. Labor ultimately arrests at 7 cm dilation, and a healthy male infant is delivered by cesarean.
CASE 2: Mild preeclampsia at 37 weeks
M.A. is a 35-year-old gravida 4 para 2012, who complains of a headache at 37 5/7 weeks’ gestation. Her blood pressure is 157/97, and she has 1+ proteinuria on dipstick urinalysis. Her laboratory tests are unremarkable, including normal serum creatinine, liver function tests, and platelets.
The fetus appears to be appropriately grown with normal amniotic fluid. Antepartum fetal heart rate testing is reactive and reassuring. The patient is having intermittent, mild uterine contractions, and her cervix is dilated 3 cm. She is given acetaminophen for the headache, which brings relief.
You diagnose mild preeclampsia at term, for which induction of labor is clearly indicated. However, M.A.’s pregnancy history is notable for a term vaginal delivery followed by a low transverse cesarean section for a term breech infant after a failed external cephalic version. She strongly desires vaginal delivery.
Do you accede to her wish for a trial of labor?
Critical questions
Is her pregnancy history favorable?
No. This patient’s previous deliveries have a bearing on the current pregnancy.15 Specifically, the patient should have had no more than 1 low transverse cesarean delivery, no other uterine scars, no previous uterine rupture, and she should have a clinically adequate pelvis.
Are facilities and staff adequate?
Obviously the answer to this question is unique to the site. It is necessary to have immediate availability of the obstetrician throughout active labor, and to have adequate personnel to perform an emergency cesarean if necessary.
If these criteria are met, is induction of labor appropriate?
Perhaps. Recent data suggest that women with a uterine scar who undergo induction with prostaglandins have a risk of uterine rupture 5 times that of women who enter spontaneous labor (24.5 per 1,000 versus 5.2 per 1,000).16 For this reason, do not use prostaglandins for labor induction in women with viable gestations who have a prior low transverse incision.
Oxytocin. Augmentation of labor with oxytocin does not appear to increase the risk of rupture, compared with spontaneous labor.14 Induction of labor with oxytocin has been associated with a slightly higher risk of uterine rupture, compared with spontaneous labor, although both types of labor have a rupture rate under 1%.14
Our recommendations
If M.A. still desires a trial of labor after she has been counseled about the risks and benefits of vaginal birth after cesarean delivery, induction should be considered. She has a reasonable likelihood of success, because she has given birth vaginally in the past, her previous cesarean delivery was for a nonrecurring indication (breech presentation), and she does not require any cervical ripening agents, as this has occurred naturally.15
Amniotomy may help reduce time to delivery
One option for induction of labor is amniotomy with oxytocin augmentation as needed. Amniotomy, or the artificial rupture of membranes, has been shown to be an effective method of labor induction in women with a favorable cervix.19
The combination of amniotomy and oxytocin administration is particularly effective, resulting in a shorter induction to delivery time, compared with women undergoing oxytocin induction alone.20
Sweeping or stripping the fetal membranes
Digital separation of the chorioamniotic membrane from the walls of the cervix and lower uterine segment during sterile vaginal examination could also be incorporated into M.A.’s induction process.21
Membrane sweeping causes the release of endogenous prostaglandins and can lead to labor without the need for induction agents or amniotomy. Although membrane sweeping is generally performed without admission to the hospital, M.A. would require hospitalization because of her diagnosis of preeclampsia.
If the cervix is unfavorable
If this patient had an unfavorable cervix, would induction of labor be contraindicated? Certainly the use of prostaglandins for preinduction cervical ripening would be contraindicated, given the existing evidence, although use of a transcervical Foley catheter would be acceptable.13-16 Published studies suggest that transcervical Foley catheter induction does not appreciably increase the risk of uterine rupture, although these studies have relatively small sample sizes and are not randomized.22,23
Although transcervical Foley catheter induction is often begun on an outpatient basis, it should probably be limited to hospital use in a woman with a previous uterine scar.24
M.A. receives magnesium sulfate for seizure prophylaxis. Labor is successfully induced with membrane sweeping and subsequent amniotomy. Labor is augmented with oxytocin, with continuous fetal heart rate and uterine activity monitoring throughout labor. She successfully delivers a healthy female infant.
CASE 3: Preeclampsia remote from term
L.A. is a 19-year-old gravida 1 para 0 who was hospitalized with preeclampsia at 28 weeks’ gestation. At that time, she was given betamethasone and monitored on inpatient bed rest. At 30 1/7 weeks’ gestation, she begins complaining of a headache. Her blood pressure is 168/110, and she has 2+ proteinuria on a dipstick. Her platelet count is 110,000, serum creatinine is 1.0, and she has slightly elevated liver transaminases in the 40s. Her cervix is closed, long, high, firm, and posterior. What are your choices?
Critical questions
Does preeclampsia rule out induction?
No. In a woman with severe preeclampsia remote from term, labor induction is not contraindicated.25-27 In fact, it may be a particularly reasonable option in a patient who is stable. Even eclampsia is not a contraindication to labor induction. However, rapidly evolving disease may preclude a prolonged labor induction, because delivery is the key to resolution of preeclampsia.
Are any clinical features in her favor?
Yes. The best predictors of success are a favorable Bishop score and a gestational age greater than 28 weeks.25-27
Our recommendations
Although L.A.’s Bishop score is unfavorable, her relatively stable clinical status and her gestational age suggest that labor induction should not be ruled out.
Preinduction cervical ripening
If cervical ripening is necessary, the transcervical Foley catheter may be the best choice, particularly among pregnancies affected by intrauterine growth restriction, as hyperstimulation is unlikely to occur with this method.
L.A. undergoes cervical ripening with the transcervical Foley catheter and subsequent amniotomy and oxytocin infusion. She is given magnesium sulfate for seizure prophylaxis throughout the cervical ripening and induction processes, and her clinical status is closely monitored, including blood pressure, urine output, and laboratory values. Her platelet count, serum creatinine, and liver transaminases remain stable, and she has a successful vaginal delivery.
1. Induction of labor. ACOG Practice Bulletin No 10. Washington, DC: ACOG; November 1999.
2. Zhang J, Yancey MK, et al. US national trends in labor induction,1989-1998. J Reprod Med. 2002;47:120-124.
3. Management of postterm pregnancy. ACOG Practice Bulletin No 55. Washington, DC: ACOG; September 2004.
4. Bishop EH. Pelvic scoring for elective induction. Obstet Gynecol. 1964;24:266-268.
5. Calder AA, Brennand JE. Labor and normal delivery: induction of labor. Curr Opin Obstet Gynecol. 1997;3:764-768.
6. Johnson DP, et al. Risk of cesarean delivery after induction of labor at term in nulliparous women with an unfavorable cervix. Am J Obstet Gynecol. 2003;188:1565-1569.
7. Chung JH, Huang WH, Rumney PJ, Garite TJ, Nageotte MP. A prospective randomized controlled trial that compared misoprostol, Foley catheter and combination misoprostol-Foley catheter for labor induction. Am J Obstet Gynecol. 2003;189:1031-1035.
8. Rayburn WF, Lightfoot SA, Newland JR, Smith CV, Christensen HD. A model for investigating microscopic changes induced by prostaglandin E2 in the term cervix. J Matern Fetal Invest. 1994;4:137.-
9. New US Food and Drug Administration labeling on Cytotec (misoprostol) use and pregnancy. ACOG Committee Opinion No 283. Washington, DC: ACOG; May 2003.
10. Wing DA. Labor induction with misoprostol. Am J Obstet Gynecol. 1999;181:339-345.
11. Response to Searle’s drug warning on misoprostol. ACOG Committee Opinion No 248. Washington, DC: ACOG; December 2000.
12. Wing DA. A benefit-risk assessment of misoprostol for cervical ripening and labour induction. Drug Safety. 2002;25:665-676.
13. Wing DA, et al. Disruption of prior uterine incision following misoprostol for labor induction in women with previous cesarean delivery. Obstet Gynecol. 1998;91:828-830.
14. Lydon-Rochelle M, Holt VL, Easterling TR, Martin DP. Risk of uterine rupture during labor among women with a prior cesarean delivery. N Engl J Med. 2001;345:3-8.
15. Vaginal birth after previous cesarean delivery. ACOG Practice Bulletin No 54. Washington, DC: ACOG; July 2004.
16. Induction of labor for vaginal birth after cesarean delivery. ACOG Committee Opinion No 271. Washington, DC: ACOG; April 2002.
17. Boulvain M, Kelly A, Lohse C, Stan C, Irion O. Mechanical methods for induction of labor. Cochrane Database Syst Rev. 2001;(4):CD001233.-
18. Sawai SK, Williams MC, O’Brien WF, et al. Sequential outpatient application of intravaginal E2 gel in the management of postdates pregnancies. Obstet Gynecol. 1991;78:19-23.
19. Booth JH, Kurdyak VB. Elective induction of labour: a controlled study. Can Med Assoc J. 1970;103:245-248.
20. Moldin PG, Sundell G. Induction of labour: a randomised clinical trial of amniotomy versus amniotomy with oxytocin infusion. Br J Obstet Gynaecol. 1996;103:306-312.
21. Boulvain M, Stan C, Irion O. Membrane sweeping for induction of labor. Cochrane Database Syst Rev. 2005;CD000451.-
22. Ravasia D, Wood SL, Pollard JK. Uterine rupture during induced labor among women with previous cesarean delivery. Am J Obstet Gynecol. 2000;183:1176-1179.
23. Bujold E, Blackwell SC, Gauthier RJ. Cervical ripening with transcervical Foley catheter and the risk of uterine rupture. Obstet Gynecol. 2004;103:18-23.
24. Sciscione AC, Muench M, et al. Transcervical Foley catheter for preinduction cervical ripening in an outpatient versus inpatient setting. Obstet Gynecol. 2001;98:751-756.
25. Nassar AH, Adra AM, Chaktoura N, et al. Severe preeclampsia remote from term: labor induction or elective cesarean delivery? Am J Obstet Gynecol. 1998;179:1210-1213.
26. Blackwell SC, Redman ME, Tomlinson M, et al. Labor induction for the preterm severe preeclamptic patient: is it worth the effort? J Matern Fetal Med. 2001;10:305-311.
27. Alexander JM, Bloom SL, et al. Severe preeclampsia and the very low birth weight infant: is induction of labor harmful? Obstet Gynecol. 1999;93:485-488.
1. Induction of labor. ACOG Practice Bulletin No 10. Washington, DC: ACOG; November 1999.
2. Zhang J, Yancey MK, et al. US national trends in labor induction,1989-1998. J Reprod Med. 2002;47:120-124.
3. Management of postterm pregnancy. ACOG Practice Bulletin No 55. Washington, DC: ACOG; September 2004.
4. Bishop EH. Pelvic scoring for elective induction. Obstet Gynecol. 1964;24:266-268.
5. Calder AA, Brennand JE. Labor and normal delivery: induction of labor. Curr Opin Obstet Gynecol. 1997;3:764-768.
6. Johnson DP, et al. Risk of cesarean delivery after induction of labor at term in nulliparous women with an unfavorable cervix. Am J Obstet Gynecol. 2003;188:1565-1569.
7. Chung JH, Huang WH, Rumney PJ, Garite TJ, Nageotte MP. A prospective randomized controlled trial that compared misoprostol, Foley catheter and combination misoprostol-Foley catheter for labor induction. Am J Obstet Gynecol. 2003;189:1031-1035.
8. Rayburn WF, Lightfoot SA, Newland JR, Smith CV, Christensen HD. A model for investigating microscopic changes induced by prostaglandin E2 in the term cervix. J Matern Fetal Invest. 1994;4:137.-
9. New US Food and Drug Administration labeling on Cytotec (misoprostol) use and pregnancy. ACOG Committee Opinion No 283. Washington, DC: ACOG; May 2003.
10. Wing DA. Labor induction with misoprostol. Am J Obstet Gynecol. 1999;181:339-345.
11. Response to Searle’s drug warning on misoprostol. ACOG Committee Opinion No 248. Washington, DC: ACOG; December 2000.
12. Wing DA. A benefit-risk assessment of misoprostol for cervical ripening and labour induction. Drug Safety. 2002;25:665-676.
13. Wing DA, et al. Disruption of prior uterine incision following misoprostol for labor induction in women with previous cesarean delivery. Obstet Gynecol. 1998;91:828-830.
14. Lydon-Rochelle M, Holt VL, Easterling TR, Martin DP. Risk of uterine rupture during labor among women with a prior cesarean delivery. N Engl J Med. 2001;345:3-8.
15. Vaginal birth after previous cesarean delivery. ACOG Practice Bulletin No 54. Washington, DC: ACOG; July 2004.
16. Induction of labor for vaginal birth after cesarean delivery. ACOG Committee Opinion No 271. Washington, DC: ACOG; April 2002.
17. Boulvain M, Kelly A, Lohse C, Stan C, Irion O. Mechanical methods for induction of labor. Cochrane Database Syst Rev. 2001;(4):CD001233.-
18. Sawai SK, Williams MC, O’Brien WF, et al. Sequential outpatient application of intravaginal E2 gel in the management of postdates pregnancies. Obstet Gynecol. 1991;78:19-23.
19. Booth JH, Kurdyak VB. Elective induction of labour: a controlled study. Can Med Assoc J. 1970;103:245-248.
20. Moldin PG, Sundell G. Induction of labour: a randomised clinical trial of amniotomy versus amniotomy with oxytocin infusion. Br J Obstet Gynaecol. 1996;103:306-312.
21. Boulvain M, Stan C, Irion O. Membrane sweeping for induction of labor. Cochrane Database Syst Rev. 2005;CD000451.-
22. Ravasia D, Wood SL, Pollard JK. Uterine rupture during induced labor among women with previous cesarean delivery. Am J Obstet Gynecol. 2000;183:1176-1179.
23. Bujold E, Blackwell SC, Gauthier RJ. Cervical ripening with transcervical Foley catheter and the risk of uterine rupture. Obstet Gynecol. 2004;103:18-23.
24. Sciscione AC, Muench M, et al. Transcervical Foley catheter for preinduction cervical ripening in an outpatient versus inpatient setting. Obstet Gynecol. 2001;98:751-756.
25. Nassar AH, Adra AM, Chaktoura N, et al. Severe preeclampsia remote from term: labor induction or elective cesarean delivery? Am J Obstet Gynecol. 1998;179:1210-1213.
26. Blackwell SC, Redman ME, Tomlinson M, et al. Labor induction for the preterm severe preeclamptic patient: is it worth the effort? J Matern Fetal Med. 2001;10:305-311.
27. Alexander JM, Bloom SL, et al. Severe preeclampsia and the very low birth weight infant: is induction of labor harmful? Obstet Gynecol. 1999;93:485-488.
The Trouble with Treponemes
Clinical Decision Making in Hypertension
Drug therapy for incontinence: New agents, new applications
Cost vs side effects for overactive bladder drugs
All of the long-acting preparations for overactive bladder have proven more effective than placebo. However, undesirable side effects have led to high rates of discontinuation over the long term.
The new preparations have a better side-effect profile than older medications such as oxybutynin. Now that oxybutynin is available in generic form, however, the new drugs are usually at least 3 times more expensive than short-acting oxybutynin. Although “none of these drugs are as effective as advertisements to the public have suggested” (The Medical Letter11), nonetheless, pharmacologic therapy is a useful option for women with symptoms of overactive bladder. Most women on these medications for a long time tend to take the drugs intermittently, depending on symptoms, or discontinue them because of side effects.
Use non-drug tactics, too
I recommend a bladder behavioral modification program with fluid management and timed voiding or bladder drills, in addition to the drug regimen, for maximum therapeutic benefit.
Treatment of stress incontinence
Stress incontinence is widespread among women of all ages, due to the vulnerability of the anatomical supports of the female urethra and bladder neck. It occurs when the force to which the sphincter mechanism is subjected during moments of exertion exceeds the sphincter’s ability to remain closed (“sphincter strength”).
Physical therapy sometimes suffices
A wide variety of treatments have been used for this problem. Because urethral closure depends largely on coordinated contractions of the pelvic floor in synchrony with increased intra-abdominal pressure, rehabilitation of the pelvic muscles through structured, supervised programs of physical therapy improves or cures many women.13
How drugs affect the urethra
The urethra and bladder neck contain alpha-adrenergic receptors, stimulation of which can increase urethral tone. Conversely, blockade of these receptors can lead to urinary stress incontinence by reducing urethral outlet resistance.14
Alpha-agonist drugs are common components of many over-the-counter cold remedies (eg, pseudoephedrine, ephedrine, phenylpropanolamine, etc) and have been readily available. Besides increasing urethral tone, however, alpha-agonists can also raise blood pressure by constricting arteriolar smooth muscle.
In 2000, an epidemiological study15 of phenylpropanolamine found that use of this medication raised the risk for stroke, even in young women, and the drug was later removed from the market by the FDA. These events led to a decline in use of such medications for stress incontinence, even for preparations that remain on the market.
How duloxetine works
It is a selective serotonin and norepinephrine reuptake inhibitor that is FDA-approved for major depressive disorder in adults, and for diabetic peripheral neuropathic pain. Besides inhibiting serotonin and norepinephrine reuptake in the brain, duloxetine inhibits reuptake in the sacral spinal cord, where the drug exerts an interesting effect on Onuf’s nucleus, which regulates tone of the urethral striated muscle sphincter through the pudendal nerve.16 The accumulation of serotonin and norepinephrine at Onuf’s nucleus (by reuptake blockade) increases efferent activity to the urethra, improving urethral tone. This is thought to have a therapeutic effect.
Dosage. The usual dose for depression is 20 to 30 mg twice daily or 60 mg once daily.
Contraindications include severe renal impairment and hepatic disease.
Side effects include nausea, dry mouth, constipation, dizziness, fatigue, increased sweating, and somnolence.17
Performance in clinical trials. Van Kerrebroeck and colleagues18 conducted a multicenter, randomized, double-blind, placebo-controlled trial of duloxetine in the treatment of stress incontinence. The trial involved 494 women from 6 European nations and Canada. Episodes of stress incontinence decreased 50% in women taking the drug (40 mg twice daily), compared with 29% among women taking placebo.
Nausea was the main side effect noted in the study and tended to be moderate and transient, rather than progressive. However, 22% of women taking duloxetine discontinued it because of side effects, compared with 5% of the women who were taking placebo.
A multicenter, randomized, double-blind, placebo-controlled trial involving 683 women from the United States and Canada, who took 40 mg of duloxetine twice daily, found a decrease in episodes of stress incontinence similar to that demonstrated by van Kerrebroeck et al,18 with comparable discontinuation rates.19
Cardozo and colleagues20 compared duloxetine with placebo in patients with stress incontinence symptoms severe enough that they had been placed on a waiting list for surgery. After taking duloxetine, 20% of these women were no longer interested in surgery, compared with none of the women in the placebo group.
Duloxetine may avert surgery. Taken together, these studies indicate that duloxetine is effective and may improve incontinence enough to render surgery unnecessary.
INTEGRATING EVIDENCE AND EXPERIENCE
Zinner N, Gittleman M, Harris R, et al. Trospium chloride improves overactive bladder symptoms: a multicenter phase III trial. J Urol. 2004;171:2311–2315
What goes into a drug approval? In weighing the merits of trospium chloride (Sanctura), one of the studies the US Food and Drug Administration considered was a 12-week multicenter, double-blind, parallel, placebo-controlled trial that compared 20 mg of the drug (twice daily) with placebo.
Trospium significantly reduced the frequency of toilet voids and urge incontinence episodes, compared with placebo. It also increased the average volume per void and decreased urge severity and daytime frequency.
However, several factors limited this study’s application to “real-life” women with overactive bladder.
1. Most participants were older white women
A total of 523 patients were enrolled, more than 70% of them women. The mean age was over 60 years, and about 80% of participants were white. More than half had already used a drug for overactive bladder.
2. Brief duration did not reflect real life
Most patients with overactive bladder need treatment for much longer than 12 weeks, so this study does not address the long-term efficacy or tolerability of trospium chloride.
3. Placebo response was substantial
Patients in the placebo arm had less frequent urination, decreased nocturia, fewer episodes of urge incontinence in 24 hours, and reduced urgency with voiding. Although these outcomes were statistically significant in the treatment group, were they clinically meaningful?
A difference of 2 tablespoons. The mean increase in average voided volume in the trospium group was 32.1 mL, compared with an increase of 7.7 mL in the placebo group. Although the P value was outstanding (P≤0.0001), one could argue that an increase in bladder capacity of less than 2 tablespoons is clinically meaningless.
Try non-drug measures first
In this study, 54% of patients taking placebo reduced the number of incontinence episodes in 24 hours, compared with 71% on the active drug. In addition, 10% of patients taking placebo became completely dry, compared with 21% on the active drug.
Because the placebo effect in these studies is always strong, focused behavioral therapy (“bladder drill” or bladder retraining) should be the first line of treatment for overactive bladder, reserving drugs for those in whom behavioral treatment is not effective, or as an initial “crutch” to help advance the behavioral program, weaning patients off medications whenever possible.
1. Wall LL. Diagnosis and management of urinary incontinence due to detrusor instability. Obstet Gynecol Surv. 1990;45:1S-47S.
2. Guay DRP. Clinical pharmacokinetics of drugs used to treat urge incontinence. Clin Pharmacol. 2003;42:1243-1285.
3. Kreder K, Mayne C, Jonas U. Long-term safety, tolerability and efficacy of extended-release tolterodine in the treatment of overactive bladder. Eur Urol. 2002;41:588-595.
4. Diokno AC, Appell RA, Sand PK, et al. Prospective, randomized, double-blind study of the efficacy and tolerability of the extended-release formulations of oxybutynin and tolterodine for overactive bladder: results of the OPERA trial. Mayo Clin Proc. 2003;78:687-695.
5. Cardozo L, Lisec M, Millard R, et al. Randomized, double-blind placebo-controlled trial of the once daily antimuscarinic agent solifenacin succinate in patients with overactive bladder. J Urol. 2004;172:1919-1924.
6. Chapple CR, Rechberge T, Al-Shukri S, et al. Randomized, double-blind placebo- and tolterodine-controlled trial of the once-daily antimuscarinic agent solifenacin in patients with symptomatic overactive bladder. BJU International. 2004;93:303-310.
7. Haab F, Stewart L, Dwyer P. Darifenacin, an M3 selective receptor antagonist, is an effective and well-tolerated once-daily treatment for overactive bladder. Eur Urol. 2004;45:420-429.
8. Cardozo L, Dixon A. Increased warning time with darifenacin: a new concept in the management of urinary urgency. J Urol. 2005;173:1214-1218.
9. Angel M. The Truth About the Drug Companies: How They Deceive Us and What to Do About It. New York: Random House; 2004.
10. Solifenacin and darifenacin for overactive bladder. Med Lett Drugs Ther. 2005;14:23-24.
11. Trospium chloride (Sanctura): another anticholinergic for overactive bladder. Med Lett Drugs Ther. 2004;46:63-64.
12. Hofner K, Oelke M, Machtens S, et al. Trospium chloride: an effective drug in the treatment of overactive bladder and detrusor hyperreflexia. World J Urol. 2001;19:336-343.
13. Nygaard IE, Heit M. Stress urinary incontinence. Obstet Gynecol. 2004;104:607-620.
14. Wall LL, Addison WA. Prazosin-induced stress incontinence. Obstet Gynecol. 1990;75:558-560.
15. Kernan WN, Viscoli CM, Brass LM, et al. Phenylpropanolamine and the risk of hemorrhagic stroke. N Engl J Med. 2000;343:1826-1832.
16. Zinner NR, Koke SC, Viktrup L. Pharmacotherapy for stress urinary incontinence: present and future options. Drugs. 2004;14:1503-1516.
17. Duloxetine (Cymbalta): a new SNRI for depression Med Lett Drugs Ther. 2004;46:81-82.
18. van Kerrebroeck P, Abrams P, Lange R, et al. Duloxetine versus placebo in the treatment of European and Canadian women with stress urinary incontinence. BJOG. 2004;111:249-257.
19. Dmochowski RR, Mikos JR, Norton PA, et al. Duloxetine versus placebo for the treatment of North American women with stress urinary incontinence. J Urol. 2003;170:1259-1263.
20. Cardozo L, Drutz HP, Baygani SK, Bump RC. Pharmacological treatment of women awaiting surgery for stress urinary incontinence. Obstet Gynecol. 2004;104:511-519.
Dr. Wall reports no financial relationship with any company whose products are mentioned in this article.
Cost vs side effects for overactive bladder drugs
All of the long-acting preparations for overactive bladder have proven more effective than placebo. However, undesirable side effects have led to high rates of discontinuation over the long term.
The new preparations have a better side-effect profile than older medications such as oxybutynin. Now that oxybutynin is available in generic form, however, the new drugs are usually at least 3 times more expensive than short-acting oxybutynin. Although “none of these drugs are as effective as advertisements to the public have suggested” (The Medical Letter11), nonetheless, pharmacologic therapy is a useful option for women with symptoms of overactive bladder. Most women on these medications for a long time tend to take the drugs intermittently, depending on symptoms, or discontinue them because of side effects.
Use non-drug tactics, too
I recommend a bladder behavioral modification program with fluid management and timed voiding or bladder drills, in addition to the drug regimen, for maximum therapeutic benefit.
Treatment of stress incontinence
Stress incontinence is widespread among women of all ages, due to the vulnerability of the anatomical supports of the female urethra and bladder neck. It occurs when the force to which the sphincter mechanism is subjected during moments of exertion exceeds the sphincter’s ability to remain closed (“sphincter strength”).
Physical therapy sometimes suffices
A wide variety of treatments have been used for this problem. Because urethral closure depends largely on coordinated contractions of the pelvic floor in synchrony with increased intra-abdominal pressure, rehabilitation of the pelvic muscles through structured, supervised programs of physical therapy improves or cures many women.13
How drugs affect the urethra
The urethra and bladder neck contain alpha-adrenergic receptors, stimulation of which can increase urethral tone. Conversely, blockade of these receptors can lead to urinary stress incontinence by reducing urethral outlet resistance.14
Alpha-agonist drugs are common components of many over-the-counter cold remedies (eg, pseudoephedrine, ephedrine, phenylpropanolamine, etc) and have been readily available. Besides increasing urethral tone, however, alpha-agonists can also raise blood pressure by constricting arteriolar smooth muscle.
In 2000, an epidemiological study15 of phenylpropanolamine found that use of this medication raised the risk for stroke, even in young women, and the drug was later removed from the market by the FDA. These events led to a decline in use of such medications for stress incontinence, even for preparations that remain on the market.
How duloxetine works
It is a selective serotonin and norepinephrine reuptake inhibitor that is FDA-approved for major depressive disorder in adults, and for diabetic peripheral neuropathic pain. Besides inhibiting serotonin and norepinephrine reuptake in the brain, duloxetine inhibits reuptake in the sacral spinal cord, where the drug exerts an interesting effect on Onuf’s nucleus, which regulates tone of the urethral striated muscle sphincter through the pudendal nerve.16 The accumulation of serotonin and norepinephrine at Onuf’s nucleus (by reuptake blockade) increases efferent activity to the urethra, improving urethral tone. This is thought to have a therapeutic effect.
Dosage. The usual dose for depression is 20 to 30 mg twice daily or 60 mg once daily.
Contraindications include severe renal impairment and hepatic disease.
Side effects include nausea, dry mouth, constipation, dizziness, fatigue, increased sweating, and somnolence.17
Performance in clinical trials. Van Kerrebroeck and colleagues18 conducted a multicenter, randomized, double-blind, placebo-controlled trial of duloxetine in the treatment of stress incontinence. The trial involved 494 women from 6 European nations and Canada. Episodes of stress incontinence decreased 50% in women taking the drug (40 mg twice daily), compared with 29% among women taking placebo.
Nausea was the main side effect noted in the study and tended to be moderate and transient, rather than progressive. However, 22% of women taking duloxetine discontinued it because of side effects, compared with 5% of the women who were taking placebo.
A multicenter, randomized, double-blind, placebo-controlled trial involving 683 women from the United States and Canada, who took 40 mg of duloxetine twice daily, found a decrease in episodes of stress incontinence similar to that demonstrated by van Kerrebroeck et al,18 with comparable discontinuation rates.19
Cardozo and colleagues20 compared duloxetine with placebo in patients with stress incontinence symptoms severe enough that they had been placed on a waiting list for surgery. After taking duloxetine, 20% of these women were no longer interested in surgery, compared with none of the women in the placebo group.
Duloxetine may avert surgery. Taken together, these studies indicate that duloxetine is effective and may improve incontinence enough to render surgery unnecessary.
INTEGRATING EVIDENCE AND EXPERIENCE
Zinner N, Gittleman M, Harris R, et al. Trospium chloride improves overactive bladder symptoms: a multicenter phase III trial. J Urol. 2004;171:2311–2315
What goes into a drug approval? In weighing the merits of trospium chloride (Sanctura), one of the studies the US Food and Drug Administration considered was a 12-week multicenter, double-blind, parallel, placebo-controlled trial that compared 20 mg of the drug (twice daily) with placebo.
Trospium significantly reduced the frequency of toilet voids and urge incontinence episodes, compared with placebo. It also increased the average volume per void and decreased urge severity and daytime frequency.
However, several factors limited this study’s application to “real-life” women with overactive bladder.
1. Most participants were older white women
A total of 523 patients were enrolled, more than 70% of them women. The mean age was over 60 years, and about 80% of participants were white. More than half had already used a drug for overactive bladder.
2. Brief duration did not reflect real life
Most patients with overactive bladder need treatment for much longer than 12 weeks, so this study does not address the long-term efficacy or tolerability of trospium chloride.
3. Placebo response was substantial
Patients in the placebo arm had less frequent urination, decreased nocturia, fewer episodes of urge incontinence in 24 hours, and reduced urgency with voiding. Although these outcomes were statistically significant in the treatment group, were they clinically meaningful?
A difference of 2 tablespoons. The mean increase in average voided volume in the trospium group was 32.1 mL, compared with an increase of 7.7 mL in the placebo group. Although the P value was outstanding (P≤0.0001), one could argue that an increase in bladder capacity of less than 2 tablespoons is clinically meaningless.
Try non-drug measures first
In this study, 54% of patients taking placebo reduced the number of incontinence episodes in 24 hours, compared with 71% on the active drug. In addition, 10% of patients taking placebo became completely dry, compared with 21% on the active drug.
Because the placebo effect in these studies is always strong, focused behavioral therapy (“bladder drill” or bladder retraining) should be the first line of treatment for overactive bladder, reserving drugs for those in whom behavioral treatment is not effective, or as an initial “crutch” to help advance the behavioral program, weaning patients off medications whenever possible.
Cost vs side effects for overactive bladder drugs
All of the long-acting preparations for overactive bladder have proven more effective than placebo. However, undesirable side effects have led to high rates of discontinuation over the long term.
The new preparations have a better side-effect profile than older medications such as oxybutynin. Now that oxybutynin is available in generic form, however, the new drugs are usually at least 3 times more expensive than short-acting oxybutynin. Although “none of these drugs are as effective as advertisements to the public have suggested” (The Medical Letter11), nonetheless, pharmacologic therapy is a useful option for women with symptoms of overactive bladder. Most women on these medications for a long time tend to take the drugs intermittently, depending on symptoms, or discontinue them because of side effects.
Use non-drug tactics, too
I recommend a bladder behavioral modification program with fluid management and timed voiding or bladder drills, in addition to the drug regimen, for maximum therapeutic benefit.
Treatment of stress incontinence
Stress incontinence is widespread among women of all ages, due to the vulnerability of the anatomical supports of the female urethra and bladder neck. It occurs when the force to which the sphincter mechanism is subjected during moments of exertion exceeds the sphincter’s ability to remain closed (“sphincter strength”).
Physical therapy sometimes suffices
A wide variety of treatments have been used for this problem. Because urethral closure depends largely on coordinated contractions of the pelvic floor in synchrony with increased intra-abdominal pressure, rehabilitation of the pelvic muscles through structured, supervised programs of physical therapy improves or cures many women.13
How drugs affect the urethra
The urethra and bladder neck contain alpha-adrenergic receptors, stimulation of which can increase urethral tone. Conversely, blockade of these receptors can lead to urinary stress incontinence by reducing urethral outlet resistance.14
Alpha-agonist drugs are common components of many over-the-counter cold remedies (eg, pseudoephedrine, ephedrine, phenylpropanolamine, etc) and have been readily available. Besides increasing urethral tone, however, alpha-agonists can also raise blood pressure by constricting arteriolar smooth muscle.
In 2000, an epidemiological study15 of phenylpropanolamine found that use of this medication raised the risk for stroke, even in young women, and the drug was later removed from the market by the FDA. These events led to a decline in use of such medications for stress incontinence, even for preparations that remain on the market.
How duloxetine works
It is a selective serotonin and norepinephrine reuptake inhibitor that is FDA-approved for major depressive disorder in adults, and for diabetic peripheral neuropathic pain. Besides inhibiting serotonin and norepinephrine reuptake in the brain, duloxetine inhibits reuptake in the sacral spinal cord, where the drug exerts an interesting effect on Onuf’s nucleus, which regulates tone of the urethral striated muscle sphincter through the pudendal nerve.16 The accumulation of serotonin and norepinephrine at Onuf’s nucleus (by reuptake blockade) increases efferent activity to the urethra, improving urethral tone. This is thought to have a therapeutic effect.
Dosage. The usual dose for depression is 20 to 30 mg twice daily or 60 mg once daily.
Contraindications include severe renal impairment and hepatic disease.
Side effects include nausea, dry mouth, constipation, dizziness, fatigue, increased sweating, and somnolence.17
Performance in clinical trials. Van Kerrebroeck and colleagues18 conducted a multicenter, randomized, double-blind, placebo-controlled trial of duloxetine in the treatment of stress incontinence. The trial involved 494 women from 6 European nations and Canada. Episodes of stress incontinence decreased 50% in women taking the drug (40 mg twice daily), compared with 29% among women taking placebo.
Nausea was the main side effect noted in the study and tended to be moderate and transient, rather than progressive. However, 22% of women taking duloxetine discontinued it because of side effects, compared with 5% of the women who were taking placebo.
A multicenter, randomized, double-blind, placebo-controlled trial involving 683 women from the United States and Canada, who took 40 mg of duloxetine twice daily, found a decrease in episodes of stress incontinence similar to that demonstrated by van Kerrebroeck et al,18 with comparable discontinuation rates.19
Cardozo and colleagues20 compared duloxetine with placebo in patients with stress incontinence symptoms severe enough that they had been placed on a waiting list for surgery. After taking duloxetine, 20% of these women were no longer interested in surgery, compared with none of the women in the placebo group.
Duloxetine may avert surgery. Taken together, these studies indicate that duloxetine is effective and may improve incontinence enough to render surgery unnecessary.
INTEGRATING EVIDENCE AND EXPERIENCE
Zinner N, Gittleman M, Harris R, et al. Trospium chloride improves overactive bladder symptoms: a multicenter phase III trial. J Urol. 2004;171:2311–2315
What goes into a drug approval? In weighing the merits of trospium chloride (Sanctura), one of the studies the US Food and Drug Administration considered was a 12-week multicenter, double-blind, parallel, placebo-controlled trial that compared 20 mg of the drug (twice daily) with placebo.
Trospium significantly reduced the frequency of toilet voids and urge incontinence episodes, compared with placebo. It also increased the average volume per void and decreased urge severity and daytime frequency.
However, several factors limited this study’s application to “real-life” women with overactive bladder.
1. Most participants were older white women
A total of 523 patients were enrolled, more than 70% of them women. The mean age was over 60 years, and about 80% of participants were white. More than half had already used a drug for overactive bladder.
2. Brief duration did not reflect real life
Most patients with overactive bladder need treatment for much longer than 12 weeks, so this study does not address the long-term efficacy or tolerability of trospium chloride.
3. Placebo response was substantial
Patients in the placebo arm had less frequent urination, decreased nocturia, fewer episodes of urge incontinence in 24 hours, and reduced urgency with voiding. Although these outcomes were statistically significant in the treatment group, were they clinically meaningful?
A difference of 2 tablespoons. The mean increase in average voided volume in the trospium group was 32.1 mL, compared with an increase of 7.7 mL in the placebo group. Although the P value was outstanding (P≤0.0001), one could argue that an increase in bladder capacity of less than 2 tablespoons is clinically meaningless.
Try non-drug measures first
In this study, 54% of patients taking placebo reduced the number of incontinence episodes in 24 hours, compared with 71% on the active drug. In addition, 10% of patients taking placebo became completely dry, compared with 21% on the active drug.
Because the placebo effect in these studies is always strong, focused behavioral therapy (“bladder drill” or bladder retraining) should be the first line of treatment for overactive bladder, reserving drugs for those in whom behavioral treatment is not effective, or as an initial “crutch” to help advance the behavioral program, weaning patients off medications whenever possible.
1. Wall LL. Diagnosis and management of urinary incontinence due to detrusor instability. Obstet Gynecol Surv. 1990;45:1S-47S.
2. Guay DRP. Clinical pharmacokinetics of drugs used to treat urge incontinence. Clin Pharmacol. 2003;42:1243-1285.
3. Kreder K, Mayne C, Jonas U. Long-term safety, tolerability and efficacy of extended-release tolterodine in the treatment of overactive bladder. Eur Urol. 2002;41:588-595.
4. Diokno AC, Appell RA, Sand PK, et al. Prospective, randomized, double-blind study of the efficacy and tolerability of the extended-release formulations of oxybutynin and tolterodine for overactive bladder: results of the OPERA trial. Mayo Clin Proc. 2003;78:687-695.
5. Cardozo L, Lisec M, Millard R, et al. Randomized, double-blind placebo-controlled trial of the once daily antimuscarinic agent solifenacin succinate in patients with overactive bladder. J Urol. 2004;172:1919-1924.
6. Chapple CR, Rechberge T, Al-Shukri S, et al. Randomized, double-blind placebo- and tolterodine-controlled trial of the once-daily antimuscarinic agent solifenacin in patients with symptomatic overactive bladder. BJU International. 2004;93:303-310.
7. Haab F, Stewart L, Dwyer P. Darifenacin, an M3 selective receptor antagonist, is an effective and well-tolerated once-daily treatment for overactive bladder. Eur Urol. 2004;45:420-429.
8. Cardozo L, Dixon A. Increased warning time with darifenacin: a new concept in the management of urinary urgency. J Urol. 2005;173:1214-1218.
9. Angel M. The Truth About the Drug Companies: How They Deceive Us and What to Do About It. New York: Random House; 2004.
10. Solifenacin and darifenacin for overactive bladder. Med Lett Drugs Ther. 2005;14:23-24.
11. Trospium chloride (Sanctura): another anticholinergic for overactive bladder. Med Lett Drugs Ther. 2004;46:63-64.
12. Hofner K, Oelke M, Machtens S, et al. Trospium chloride: an effective drug in the treatment of overactive bladder and detrusor hyperreflexia. World J Urol. 2001;19:336-343.
13. Nygaard IE, Heit M. Stress urinary incontinence. Obstet Gynecol. 2004;104:607-620.
14. Wall LL, Addison WA. Prazosin-induced stress incontinence. Obstet Gynecol. 1990;75:558-560.
15. Kernan WN, Viscoli CM, Brass LM, et al. Phenylpropanolamine and the risk of hemorrhagic stroke. N Engl J Med. 2000;343:1826-1832.
16. Zinner NR, Koke SC, Viktrup L. Pharmacotherapy for stress urinary incontinence: present and future options. Drugs. 2004;14:1503-1516.
17. Duloxetine (Cymbalta): a new SNRI for depression Med Lett Drugs Ther. 2004;46:81-82.
18. van Kerrebroeck P, Abrams P, Lange R, et al. Duloxetine versus placebo in the treatment of European and Canadian women with stress urinary incontinence. BJOG. 2004;111:249-257.
19. Dmochowski RR, Mikos JR, Norton PA, et al. Duloxetine versus placebo for the treatment of North American women with stress urinary incontinence. J Urol. 2003;170:1259-1263.
20. Cardozo L, Drutz HP, Baygani SK, Bump RC. Pharmacological treatment of women awaiting surgery for stress urinary incontinence. Obstet Gynecol. 2004;104:511-519.
Dr. Wall reports no financial relationship with any company whose products are mentioned in this article.
1. Wall LL. Diagnosis and management of urinary incontinence due to detrusor instability. Obstet Gynecol Surv. 1990;45:1S-47S.
2. Guay DRP. Clinical pharmacokinetics of drugs used to treat urge incontinence. Clin Pharmacol. 2003;42:1243-1285.
3. Kreder K, Mayne C, Jonas U. Long-term safety, tolerability and efficacy of extended-release tolterodine in the treatment of overactive bladder. Eur Urol. 2002;41:588-595.
4. Diokno AC, Appell RA, Sand PK, et al. Prospective, randomized, double-blind study of the efficacy and tolerability of the extended-release formulations of oxybutynin and tolterodine for overactive bladder: results of the OPERA trial. Mayo Clin Proc. 2003;78:687-695.
5. Cardozo L, Lisec M, Millard R, et al. Randomized, double-blind placebo-controlled trial of the once daily antimuscarinic agent solifenacin succinate in patients with overactive bladder. J Urol. 2004;172:1919-1924.
6. Chapple CR, Rechberge T, Al-Shukri S, et al. Randomized, double-blind placebo- and tolterodine-controlled trial of the once-daily antimuscarinic agent solifenacin in patients with symptomatic overactive bladder. BJU International. 2004;93:303-310.
7. Haab F, Stewart L, Dwyer P. Darifenacin, an M3 selective receptor antagonist, is an effective and well-tolerated once-daily treatment for overactive bladder. Eur Urol. 2004;45:420-429.
8. Cardozo L, Dixon A. Increased warning time with darifenacin: a new concept in the management of urinary urgency. J Urol. 2005;173:1214-1218.
9. Angel M. The Truth About the Drug Companies: How They Deceive Us and What to Do About It. New York: Random House; 2004.
10. Solifenacin and darifenacin for overactive bladder. Med Lett Drugs Ther. 2005;14:23-24.
11. Trospium chloride (Sanctura): another anticholinergic for overactive bladder. Med Lett Drugs Ther. 2004;46:63-64.
12. Hofner K, Oelke M, Machtens S, et al. Trospium chloride: an effective drug in the treatment of overactive bladder and detrusor hyperreflexia. World J Urol. 2001;19:336-343.
13. Nygaard IE, Heit M. Stress urinary incontinence. Obstet Gynecol. 2004;104:607-620.
14. Wall LL, Addison WA. Prazosin-induced stress incontinence. Obstet Gynecol. 1990;75:558-560.
15. Kernan WN, Viscoli CM, Brass LM, et al. Phenylpropanolamine and the risk of hemorrhagic stroke. N Engl J Med. 2000;343:1826-1832.
16. Zinner NR, Koke SC, Viktrup L. Pharmacotherapy for stress urinary incontinence: present and future options. Drugs. 2004;14:1503-1516.
17. Duloxetine (Cymbalta): a new SNRI for depression Med Lett Drugs Ther. 2004;46:81-82.
18. van Kerrebroeck P, Abrams P, Lange R, et al. Duloxetine versus placebo in the treatment of European and Canadian women with stress urinary incontinence. BJOG. 2004;111:249-257.
19. Dmochowski RR, Mikos JR, Norton PA, et al. Duloxetine versus placebo for the treatment of North American women with stress urinary incontinence. J Urol. 2003;170:1259-1263.
20. Cardozo L, Drutz HP, Baygani SK, Bump RC. Pharmacological treatment of women awaiting surgery for stress urinary incontinence. Obstet Gynecol. 2004;104:511-519.
Dr. Wall reports no financial relationship with any company whose products are mentioned in this article.
IN THIS ARTICLE
- A simple questionnaire to differentiate urge and stress incontinence
CONTRACEPTION
Three new developments this year stand to make a difference in the high rate of unintended pregnancies in the United States. Increased use of highly effective, long-acting, user-independent methods is an effective way to lower the rate of unintended pregnancies in couples using contraception. Two such methods are the contraceptive implant and the intrauterine contraceptive. Emergency contraception is an effective way to reduce the risk of unintended pregnancy after failure of a contraceptive method or unprotected or forced sex. A randomized trial showed that direct access to EC does not increase high-risk behavior.
Half of pregnancies are unintended
- The United States has one of the highest rates of unintended births among industrialized countries.
- Of the 6 million pregnancies each year in the US, nearly 3 million are unintended, resulting in 1.4 million unintended births and 1.3 million abortions.
- Half of these unintended pregnancies are due to failure or incorrect or inconsistent use of a contraceptive method.1
REFERENCE
1. Contraception Counts: Ranking State Efforts. New York: Guttmacher Institute; February 2006.
Implanon essentials: How it works and what to tell patients
The single-rod implant (Implanon) is a new, highly effective, long-acting, rapidly reversible contraceptive, approved by the FDA, July 17, 2006.
A new single-rod implant that provides highly effective contraception for up to 3 years is expected to be widely available in the United States in 2007. Once inserted, Implanon is independent of user compliance and is rapidly effective and reversible. It is in use worldwide in more than 30 countries since 1998.
The new device is a nonbiodegradable 40 × 2.0 mm rod of 40% ethylene vinyl acetate (EVA) and 60% etonogestrel (ENG) covered with a rate-controlling EVA membrane.
The rod contains 68 mg of ENG, initially absorbed by the body at a rate of 60 μg/day, slowly declining to 30 μg/day after 3 years of use.2 Steady release of ENG into the circulation avoids first-pass effects on the liver.
Manufacturer-sponsored training: Call 1-877-IMPLANON
Before clinicians can order the implant, they must undergo training sponsored by the manufacturer, Organon. To take part in the training, which is set to begin in August, call 1-877-IMPLANON.
How the implant works
Ovarian and cervical mechanisms, which function prior to fertilization, provide high contraceptive efficacy.
Ovulation is suppressed. The ENG implant, unlike previous levonorgestrel-containing implants, works primarily by suppressing ovulation.3 ENG alters the hypothalamic–pituitary–ovarian axis and down-regulates the luteinizing hormone surge, which is required to support the production, growth, and maturation of ovarian follicles.
Ovulation returns rapidly after removal of the implant.3
Cervical mechanisms also prevent fertilization. Anti-estrogenic actions of ENG make the cervical mucus viscous, scanty, and impenetrable by sperm.
Cost-effectiveness depends on long-term use; early removal negates this benefit. At press time, the manufacturer had not released the price of Implanon.
Lack of protection against sexually transmitted infections is a disadvantage of the ENG implant, as well as all nonbarrier contraceptive methods.
Discontinuation rates have varied by region, but are usually due to bleeding pattern changes.
- In an international multicenter trial, 31% discontinued by 2 years and only 6% discontinued in the third year.4 Again, the most common reason was irregular bleeding.
- In a US series, 49% discontinued by 2 years. The most common reason was bleeding pattern changes (13%).5 The rate of discontinuation was highest during the first 8 months.
What to tell patients. To improve continuation, counseling should strongly stress the expected change in bleeding patterns.
Clinical trials
Outstanding efficacy. In an international multicenter trial, there were no intrauterine or ectopic pregnancies in a total of 1,200 woman-years (15,000 cycles of exposure, 2,000 of which were in the third year of use).4 The Pearl index was 0 (95% CI 0.0–0.2).4 In the US series, after a total exposure of 474 woman-years (6,186 cycles), no intrauterine or ectopic pregnancies were observed.5 It should be noted that phase III data from Indonesia were retracted by the manufacturer in 2004.6 The 2 trials noted above included a total of 965 women and were not included in this retraction.
Reasons for failures. Pregnancies were noted from postmarketing data in Australia; most of these pregnancies resulted from either incorrect timing at the initial insertion or failure to insert the implant. Based on the Australian phase IV data, with 204,486 devices inserted, the failure of the method itself was estimated to be 1 per 1,000 insertions.7 Implanon may be less effective in obese women or, as the Australian experience showed, with concomitant use of drugs that stimulate the liver’s cytochrome metabolism of steroids, such as some antibiotics (eg, rifampin) or anticonvulsants (eg, phenytoin).
Side effects
Infrequent bleeding. The main side effect is a change in bleeding patterns. In the US series, amenorrhea occurred in 14% to 20% of women. In the same series, women experienced infrequent bleeding (<3 episodes in 90 days) in 30% to 40% of the 90-day reference periods, making it the most common pattern experienced. Prolonged bleeding (>14 days of bleeding in one episode) varied from 14% to 36%, and frequent bleeding (>5 episodes in 90 days) varied from 7% to 14%.5 Anemia was not observed in the US series despite the irregular bleeding; in fact, hemoglobins rise.
Although the ENG implant is designed to facilitate rapid and simple insertion and removal, clinicians must first be trained. The manufacturer, Organon, announced last month that it would begin training doctors in August.
Insertion Average time: 1 minute4
The single-rod implant is preloaded in a disposable applicator. Insertion is done in the office using local anesthesia.
Place the 1.5-inch long implant on the inner aspect of the nondominant arm. Position the applicator needle subdermally and withdraw the cannula, leaving the implant rod in place.
After insertion, the implant may not be visible but should be palpable.
Removal Average time: 4 minutes4
Removal requires a 2- to 3-mm incision at the distal tip of the implant. Push the other end of the rod until it pops out.
Timing the insertion
- Between days 1 and 5 of menses, in women who either have not been using a contraceptive method or have been using a nonhormonal method
- During a hormone-free week, in women changing from a combination or progestin-only oral contraceptive, or from intrauterine contraception
- The day on which the next injection is scheduled, in women changing from injectable contraception
No backup contraceptive is necessary if timing of insertion occurs as detailed.
In all cases, exclude pregnancy before insertion.
Timing the removal
The ENG implant can be removed at any time, but must be removed after 3 years.
Return to ovulation is rapid following removal, so women still desiring contraception should begin another method immediately or have a new rod inserted through the removal incision.
Unpredictable bleeding pattern. Unlike with Norplant, there was no trend over time toward a particular bleeding pattern. Implanon patterns are irregular and unpredictable and vary from one 90-day reference period to the next. Similar results were noted in the multicenter international trial.4
Possible therapies include estrogen supplementation, nonsteroidal anti-inflammatory drugs, oral contraceptive pills, and observation.
Other side effects. In the US series, the most frequent nonmenstrual adverse effects possibly related to the ENG implant were acne (14.5%), headache (12.7%), weight gain (12.1%), and emotional lability (14.2%).
Contraindications
The ENG implant should not be placed in women with undiagnosed abnormal genital bleeding, known or suspected pregnancy, or hypersensitivity to any of the components in the ENG implant.
REFERENCES
Why the FDA removed ParaGard’s parity restriction
- An FDA labeling change for the ParaGard intrauterine device confirms what the evidence has long supported: The risk of pelvic infection is more related to sexual behavior than to age, contraceptive choice, or parity
- Evidence supports a link between cervical infection—but not IUD use—and pelvic inflammatory disease and infertility
The Food and Drug Administration has approved a less restrictive label for the ParaGard T380A copper intrauterine contraceptive. Evidence has long supported the conclusion that risk of pelvic infection is related more to a woman’s and her partner’s sexual behavior than to her age, contraceptive choice, or parity.
A woman with at least one child and in a mutually monogamous relationship is no longer listed as the recommended patient profile. Nor is ParaGard contraindicated for a woman with a history of sexually transmitted disease or pelvic inflammatory disease (PID), unless she has current acute PID or engages in sexual behavior suggesting a high risk for PID.
Why was the label restrictive to begin with?
Early studies8,9 that showed an increased risk of PID and infertility in intrauterine contraceptive users have been re-analyzed; most of the increased risk was associated with a single type of intrauterine contraceptive that is no longer on the market (Dalkon Shield), and with high-risk sexual behaviors.10-12 In most analyses of these studies, the increased risk of PID was present only in the first 20 days after insertion, indicating undiagnosed cervical infection at the time of insertion.
Furthermore, many studies had methodological flaws that introduced bias into the results, such as comparing intrauterine contraceptive users with users of combination oral contraceptives (who have a decreased risk of PID compared with nonusers).13
These early studies also equated nulliparity with high-risk sexual behavior. As young women are more likely to acquire sexually transmitted cervical infections, and because young age is associated with nulliparity, many studies erroneously concluded that the increased risk of PID and infertility was attributed to nulliparity.
A case-control study in nulliparous Mexican women who were seeking treatment for primary infertility found no association between tubal infertility and past copper IUD use. In this study, 358 women with primary infertility and documented tubal occlusion (cases) were compared with two sets of controls: 953 nulliparous women with primary infertility and no tubal occlusion, and 584 primigravid women. Past use of a copper IUD was not associated with tubal occlusion, compared with either infertile women without tubal occlusion or primigravid controls (P values 1.0 and 0.9, respectively).14 However, tubal infertility was associated with a past infection with Chlamydia (as evidenced by Chlamydia antibodies). This study further supports an association between PID and infertility and cervical infection—not IUD use.
Protective effect of progestin
The levonorgestrel-releasing intrauterine system (LNG-IUS) may even protect against PID. One of the primary physiologic effects of progestin contraception is thickening of the cervical mucus, which protects against ascending genital tract infection. This protective effect results in a decreased incidence of PID in women who use combination oral contraceptive pills, progestin implants, and progestin injectables.15 A randomized controlled trial found that the cumulative 36-month rate of PID was lower in users of a LNG-IUS contraceptive than in users of a copper IUD (Nova-T) (0.5 and 2.0, respectively; P< 0.013), in both parous and nulliparous women.16 This finding was more marked in women under the age of 25.
Prescribing IUDs in young women
When considering an intrauterine contraceptive for a young woman, it is therefore important to assess her risk of a STI, based on her and her partner’s sexual behavior, and not on parity or age. It is important to screen for STIs at the time of or prior to insertion of an intrauterine contraceptive, and to treat cervicitis prior to insertion.
Nulliparous women who are at low risk of STIs can be offered the intrauterine contraceptive as an effective, long-term, user-independent contraception.
The labeling for levonorgestrel intrauterine contraceptives should also reflect the evidence that the risk of pelvic infection is more related to a patient’s and/or her partner’s sexual behavior than to her age, contraceptive choice, or parity.
REFERENCES
Direct access to Plan B does not promote high-risk behavior
Raine TR, Harper CC, Rocca CH, et al. Direct access to emergency contraception through pharmacies and effect on unintended pregnancy and STIs: a randomized controlled trial. JAMA. 2005;293:54–62.
- Advance provision of emergency contraception results in increased usage of emergency contraception without an associated change in risky sexual behavior, sexually transmitted diseases, or use of long-term contraception
- Clinicians should provide emergency contraception in advance of need to ensure timely and appropriate emergency contraceptive use
Emergency contraception could significantly reduce the risk of unintended pregnancy after contraceptive method failure, or unprotected or forced sex. The newer progestin-only emergency contraceptive pills have now largely replaced the older combined (estrogen and progestin) pills because they are more effective and have fewer side effects.
Various emergency contraception regimens are effective
The only dedicated progestin-only emergency contraception pill product in the United States is Plan B, which contains 2 tablets of 0.75 mg of levonorgestrel. Although the recommended treatment schedule is an initial dose within 72 hours of unprotected intercourse and a second dose 12 hours later, a single dose of 1.5 mg of levonorgestrel is as effective as and causes no more side effects than 2 tablets of 0.75-mg doses 12 hours apart.17,18
The sooner the better?
Emergency contraception pills are more effective the sooner after sex that they are initiated. Both combination oral contraceptive pills and progestin-only regimens are moderately effective even if initiated more than 72 hours after unprotected intercourse. 18-20 No data are available on the efficacy of emergency contraception pills taken more than 120 hours (5 days) after unprotected intercourse.
Randomized trial
“It seems unreasonable to restrict access”
Raine et al21 added significantly to our knowledge of emergency contraception. This is the first randomized trial addressing the question of the effect of access on emergency contraceptive usage. A total of 2,117 young women (age 15 to 24 years) were randomly assigned to these 3 groups:
- Pharmacy access (without consulting a physician)
- Advance provision of 3 packs of Plan B
- Clinic access (ie, usual care, which required a clinic visit to obtain emergency contraception)
The study concluded: “While removing the requirement to go through pharmacists or clinics to obtain emergency contraception increases use, the public health impact may be negligible because of high rates of unprotected intercourse and relative underutilization of the method. Given that there is clear evidence that neither pharmacy access nor advance provision compromises contraceptive or sexual behavior, it seems unreasonable to restrict access to emergency contraception to clinics.” The conclusion reflected the following several outcomes, which were assessed after 6 months.
4 key outcomes
- Use of emergency contraception
- The advance provision group used emergency contraception at nearly twice the rate (37.4%) of the clinic access group (21.0%).
- Usage rates were similar in the pharmacy access (24.2%) and the clinic access group (21.0%).
- New sexually transmitted infection rates were similar in all groups
Levels of STIs, such as Chlamydia, were similar across all groups, and changes in HSV-2 serology were similar across all groups. - Many did not use the EC, even with advance provision
In this study, advance provision of emergency contraceptives did not lower pregnancy rates. This finding is disappointing; the likely explanation is that women at highest risk do not use emergency contraception often enough or at all. Thus, the overall pregnancy rate is unchanged. Nearly half (45%) of the women in the study who reported having unprotected sex did not use emergency contraception during the study period, even when they received it in advance. - High-risk sexual behavior did not increase in any group
Women who had increased access to emergency contraception did not have sex more frequently. Receiving emergency contraceptives in advance did not affect the number of sex partners, with most women having only one partner. Data on teens in the same study found that teens did not take more sexual risks than women aged 20 to 24.22
A concern with placing emergency contraception directly in the hands of women has been the theory that it would result in increased high-risk behavior and lower use of regular contraception.
This trial found:
- That women with pharmacy access and women given 3 packs of emergency contraceptives in advance were no more likely to change their regular contraceptive method than women who could obtain emergency contraception only via a clinic visit.
- That women with increased access to emergency contraceptives use their routine contraception with the same consistency as women without increased access.
No downside to easier, wider access to Plan B
This trial adds to the argument for wider and easier access to emergency contraception for women. There is no apparent downside from wide access to the current progestin-only emergency contraception regimen. The latest World Health Organization medical eligibility criteria describe no situation in which the risks of emergency contraception outweigh the benefits.23 The study by Raine et al21 provides evidence against concerns about the potential for increased high-risk sexual behavior.
Eight states (Alaska, California, Hawaii, Maine, Massachusetts, New Hampshire, New Mexico, Washington) have passed legislation allowing pharmacists to prescribe emergency contraceptives without a prescription. Norway, Sweden, India, and the Netherlands allow emergency contraceptive availability over-the-counter.
17. Arowojolu AO, Okewole IA, Adekunle AO. Comparative evaluation of the effectiveness and safety of two regimens of levonorgestrel for emergency contraception in Nigerians. Contraception. 2002;66:269-273.
18. von Hertzen H, Piaggio G, Ding J, et al. Low dose mifepristone and two regimens of levonorgestrel for emergency contraception: a WHO multicentre randomised trial. Lancet. 2002;360:1803-1810.
19. Ellertson C, Evans M, Ferden S, et al. Extending the time limit for starting the Yuzpe regimen of emergency contraception to 120 hours. Obstet Gynecol. 2003;101:1168-1171.
20. Rodrigues I, Grou F, Joly J. Effectiveness of emergency contraceptive pills between 72 and 120 hours after unprotected sexual intercourse. Am J Obstet Gynecol. 2001;184:531-537.
21. Raine TR, Harper CC, Rocca CH, et al. Direct access to emergency contraception through pharmacies and effect on unintended pregnancy and STIs: a randomized controlled trial. JAMA. 2005;293:54-62.
22. Harper CC, Cheong M, Rocca CH, Darney PD, Raine TR. The effect of increased access to emergency contraception among young adolescents. Obstet Gynecol. 2005;106:483-491.
23. Medical Eligibilty Criteria for Contraceptive Use. Geneva: World Health Organisation; 2004
Dr. Parvataneni is a consultant to Organon. Dr. Darney is a consultant to Organon and is a speaker for Berlex and Organon.
Three new developments this year stand to make a difference in the high rate of unintended pregnancies in the United States. Increased use of highly effective, long-acting, user-independent methods is an effective way to lower the rate of unintended pregnancies in couples using contraception. Two such methods are the contraceptive implant and the intrauterine contraceptive. Emergency contraception is an effective way to reduce the risk of unintended pregnancy after failure of a contraceptive method or unprotected or forced sex. A randomized trial showed that direct access to EC does not increase high-risk behavior.
Half of pregnancies are unintended
- The United States has one of the highest rates of unintended births among industrialized countries.
- Of the 6 million pregnancies each year in the US, nearly 3 million are unintended, resulting in 1.4 million unintended births and 1.3 million abortions.
- Half of these unintended pregnancies are due to failure or incorrect or inconsistent use of a contraceptive method.1
REFERENCE
1. Contraception Counts: Ranking State Efforts. New York: Guttmacher Institute; February 2006.
Implanon essentials: How it works and what to tell patients
The single-rod implant (Implanon) is a new, highly effective, long-acting, rapidly reversible contraceptive, approved by the FDA, July 17, 2006.
A new single-rod implant that provides highly effective contraception for up to 3 years is expected to be widely available in the United States in 2007. Once inserted, Implanon is independent of user compliance and is rapidly effective and reversible. It is in use worldwide in more than 30 countries since 1998.
The new device is a nonbiodegradable 40 × 2.0 mm rod of 40% ethylene vinyl acetate (EVA) and 60% etonogestrel (ENG) covered with a rate-controlling EVA membrane.
The rod contains 68 mg of ENG, initially absorbed by the body at a rate of 60 μg/day, slowly declining to 30 μg/day after 3 years of use.2 Steady release of ENG into the circulation avoids first-pass effects on the liver.
Manufacturer-sponsored training: Call 1-877-IMPLANON
Before clinicians can order the implant, they must undergo training sponsored by the manufacturer, Organon. To take part in the training, which is set to begin in August, call 1-877-IMPLANON.
How the implant works
Ovarian and cervical mechanisms, which function prior to fertilization, provide high contraceptive efficacy.
Ovulation is suppressed. The ENG implant, unlike previous levonorgestrel-containing implants, works primarily by suppressing ovulation.3 ENG alters the hypothalamic–pituitary–ovarian axis and down-regulates the luteinizing hormone surge, which is required to support the production, growth, and maturation of ovarian follicles.
Ovulation returns rapidly after removal of the implant.3
Cervical mechanisms also prevent fertilization. Anti-estrogenic actions of ENG make the cervical mucus viscous, scanty, and impenetrable by sperm.
Cost-effectiveness depends on long-term use; early removal negates this benefit. At press time, the manufacturer had not released the price of Implanon.
Lack of protection against sexually transmitted infections is a disadvantage of the ENG implant, as well as all nonbarrier contraceptive methods.
Discontinuation rates have varied by region, but are usually due to bleeding pattern changes.
- In an international multicenter trial, 31% discontinued by 2 years and only 6% discontinued in the third year.4 Again, the most common reason was irregular bleeding.
- In a US series, 49% discontinued by 2 years. The most common reason was bleeding pattern changes (13%).5 The rate of discontinuation was highest during the first 8 months.
What to tell patients. To improve continuation, counseling should strongly stress the expected change in bleeding patterns.
Clinical trials
Outstanding efficacy. In an international multicenter trial, there were no intrauterine or ectopic pregnancies in a total of 1,200 woman-years (15,000 cycles of exposure, 2,000 of which were in the third year of use).4 The Pearl index was 0 (95% CI 0.0–0.2).4 In the US series, after a total exposure of 474 woman-years (6,186 cycles), no intrauterine or ectopic pregnancies were observed.5 It should be noted that phase III data from Indonesia were retracted by the manufacturer in 2004.6 The 2 trials noted above included a total of 965 women and were not included in this retraction.
Reasons for failures. Pregnancies were noted from postmarketing data in Australia; most of these pregnancies resulted from either incorrect timing at the initial insertion or failure to insert the implant. Based on the Australian phase IV data, with 204,486 devices inserted, the failure of the method itself was estimated to be 1 per 1,000 insertions.7 Implanon may be less effective in obese women or, as the Australian experience showed, with concomitant use of drugs that stimulate the liver’s cytochrome metabolism of steroids, such as some antibiotics (eg, rifampin) or anticonvulsants (eg, phenytoin).
Side effects
Infrequent bleeding. The main side effect is a change in bleeding patterns. In the US series, amenorrhea occurred in 14% to 20% of women. In the same series, women experienced infrequent bleeding (<3 episodes in 90 days) in 30% to 40% of the 90-day reference periods, making it the most common pattern experienced. Prolonged bleeding (>14 days of bleeding in one episode) varied from 14% to 36%, and frequent bleeding (>5 episodes in 90 days) varied from 7% to 14%.5 Anemia was not observed in the US series despite the irregular bleeding; in fact, hemoglobins rise.
Although the ENG implant is designed to facilitate rapid and simple insertion and removal, clinicians must first be trained. The manufacturer, Organon, announced last month that it would begin training doctors in August.
Insertion Average time: 1 minute4
The single-rod implant is preloaded in a disposable applicator. Insertion is done in the office using local anesthesia.
Place the 1.5-inch long implant on the inner aspect of the nondominant arm. Position the applicator needle subdermally and withdraw the cannula, leaving the implant rod in place.
After insertion, the implant may not be visible but should be palpable.
Removal Average time: 4 minutes4
Removal requires a 2- to 3-mm incision at the distal tip of the implant. Push the other end of the rod until it pops out.
Timing the insertion
- Between days 1 and 5 of menses, in women who either have not been using a contraceptive method or have been using a nonhormonal method
- During a hormone-free week, in women changing from a combination or progestin-only oral contraceptive, or from intrauterine contraception
- The day on which the next injection is scheduled, in women changing from injectable contraception
No backup contraceptive is necessary if timing of insertion occurs as detailed.
In all cases, exclude pregnancy before insertion.
Timing the removal
The ENG implant can be removed at any time, but must be removed after 3 years.
Return to ovulation is rapid following removal, so women still desiring contraception should begin another method immediately or have a new rod inserted through the removal incision.
Unpredictable bleeding pattern. Unlike with Norplant, there was no trend over time toward a particular bleeding pattern. Implanon patterns are irregular and unpredictable and vary from one 90-day reference period to the next. Similar results were noted in the multicenter international trial.4
Possible therapies include estrogen supplementation, nonsteroidal anti-inflammatory drugs, oral contraceptive pills, and observation.
Other side effects. In the US series, the most frequent nonmenstrual adverse effects possibly related to the ENG implant were acne (14.5%), headache (12.7%), weight gain (12.1%), and emotional lability (14.2%).
Contraindications
The ENG implant should not be placed in women with undiagnosed abnormal genital bleeding, known or suspected pregnancy, or hypersensitivity to any of the components in the ENG implant.
REFERENCES
Why the FDA removed ParaGard’s parity restriction
- An FDA labeling change for the ParaGard intrauterine device confirms what the evidence has long supported: The risk of pelvic infection is more related to sexual behavior than to age, contraceptive choice, or parity
- Evidence supports a link between cervical infection—but not IUD use—and pelvic inflammatory disease and infertility
The Food and Drug Administration has approved a less restrictive label for the ParaGard T380A copper intrauterine contraceptive. Evidence has long supported the conclusion that risk of pelvic infection is related more to a woman’s and her partner’s sexual behavior than to her age, contraceptive choice, or parity.
A woman with at least one child and in a mutually monogamous relationship is no longer listed as the recommended patient profile. Nor is ParaGard contraindicated for a woman with a history of sexually transmitted disease or pelvic inflammatory disease (PID), unless she has current acute PID or engages in sexual behavior suggesting a high risk for PID.
Why was the label restrictive to begin with?
Early studies8,9 that showed an increased risk of PID and infertility in intrauterine contraceptive users have been re-analyzed; most of the increased risk was associated with a single type of intrauterine contraceptive that is no longer on the market (Dalkon Shield), and with high-risk sexual behaviors.10-12 In most analyses of these studies, the increased risk of PID was present only in the first 20 days after insertion, indicating undiagnosed cervical infection at the time of insertion.
Furthermore, many studies had methodological flaws that introduced bias into the results, such as comparing intrauterine contraceptive users with users of combination oral contraceptives (who have a decreased risk of PID compared with nonusers).13
These early studies also equated nulliparity with high-risk sexual behavior. As young women are more likely to acquire sexually transmitted cervical infections, and because young age is associated with nulliparity, many studies erroneously concluded that the increased risk of PID and infertility was attributed to nulliparity.
A case-control study in nulliparous Mexican women who were seeking treatment for primary infertility found no association between tubal infertility and past copper IUD use. In this study, 358 women with primary infertility and documented tubal occlusion (cases) were compared with two sets of controls: 953 nulliparous women with primary infertility and no tubal occlusion, and 584 primigravid women. Past use of a copper IUD was not associated with tubal occlusion, compared with either infertile women without tubal occlusion or primigravid controls (P values 1.0 and 0.9, respectively).14 However, tubal infertility was associated with a past infection with Chlamydia (as evidenced by Chlamydia antibodies). This study further supports an association between PID and infertility and cervical infection—not IUD use.
Protective effect of progestin
The levonorgestrel-releasing intrauterine system (LNG-IUS) may even protect against PID. One of the primary physiologic effects of progestin contraception is thickening of the cervical mucus, which protects against ascending genital tract infection. This protective effect results in a decreased incidence of PID in women who use combination oral contraceptive pills, progestin implants, and progestin injectables.15 A randomized controlled trial found that the cumulative 36-month rate of PID was lower in users of a LNG-IUS contraceptive than in users of a copper IUD (Nova-T) (0.5 and 2.0, respectively; P< 0.013), in both parous and nulliparous women.16 This finding was more marked in women under the age of 25.
Prescribing IUDs in young women
When considering an intrauterine contraceptive for a young woman, it is therefore important to assess her risk of a STI, based on her and her partner’s sexual behavior, and not on parity or age. It is important to screen for STIs at the time of or prior to insertion of an intrauterine contraceptive, and to treat cervicitis prior to insertion.
Nulliparous women who are at low risk of STIs can be offered the intrauterine contraceptive as an effective, long-term, user-independent contraception.
The labeling for levonorgestrel intrauterine contraceptives should also reflect the evidence that the risk of pelvic infection is more related to a patient’s and/or her partner’s sexual behavior than to her age, contraceptive choice, or parity.
REFERENCES
Direct access to Plan B does not promote high-risk behavior
Raine TR, Harper CC, Rocca CH, et al. Direct access to emergency contraception through pharmacies and effect on unintended pregnancy and STIs: a randomized controlled trial. JAMA. 2005;293:54–62.
- Advance provision of emergency contraception results in increased usage of emergency contraception without an associated change in risky sexual behavior, sexually transmitted diseases, or use of long-term contraception
- Clinicians should provide emergency contraception in advance of need to ensure timely and appropriate emergency contraceptive use
Emergency contraception could significantly reduce the risk of unintended pregnancy after contraceptive method failure, or unprotected or forced sex. The newer progestin-only emergency contraceptive pills have now largely replaced the older combined (estrogen and progestin) pills because they are more effective and have fewer side effects.
Various emergency contraception regimens are effective
The only dedicated progestin-only emergency contraception pill product in the United States is Plan B, which contains 2 tablets of 0.75 mg of levonorgestrel. Although the recommended treatment schedule is an initial dose within 72 hours of unprotected intercourse and a second dose 12 hours later, a single dose of 1.5 mg of levonorgestrel is as effective as and causes no more side effects than 2 tablets of 0.75-mg doses 12 hours apart.17,18
The sooner the better?
Emergency contraception pills are more effective the sooner after sex that they are initiated. Both combination oral contraceptive pills and progestin-only regimens are moderately effective even if initiated more than 72 hours after unprotected intercourse. 18-20 No data are available on the efficacy of emergency contraception pills taken more than 120 hours (5 days) after unprotected intercourse.
Randomized trial
“It seems unreasonable to restrict access”
Raine et al21 added significantly to our knowledge of emergency contraception. This is the first randomized trial addressing the question of the effect of access on emergency contraceptive usage. A total of 2,117 young women (age 15 to 24 years) were randomly assigned to these 3 groups:
- Pharmacy access (without consulting a physician)
- Advance provision of 3 packs of Plan B
- Clinic access (ie, usual care, which required a clinic visit to obtain emergency contraception)
The study concluded: “While removing the requirement to go through pharmacists or clinics to obtain emergency contraception increases use, the public health impact may be negligible because of high rates of unprotected intercourse and relative underutilization of the method. Given that there is clear evidence that neither pharmacy access nor advance provision compromises contraceptive or sexual behavior, it seems unreasonable to restrict access to emergency contraception to clinics.” The conclusion reflected the following several outcomes, which were assessed after 6 months.
4 key outcomes
- Use of emergency contraception
- The advance provision group used emergency contraception at nearly twice the rate (37.4%) of the clinic access group (21.0%).
- Usage rates were similar in the pharmacy access (24.2%) and the clinic access group (21.0%).
- New sexually transmitted infection rates were similar in all groups
Levels of STIs, such as Chlamydia, were similar across all groups, and changes in HSV-2 serology were similar across all groups. - Many did not use the EC, even with advance provision
In this study, advance provision of emergency contraceptives did not lower pregnancy rates. This finding is disappointing; the likely explanation is that women at highest risk do not use emergency contraception often enough or at all. Thus, the overall pregnancy rate is unchanged. Nearly half (45%) of the women in the study who reported having unprotected sex did not use emergency contraception during the study period, even when they received it in advance. - High-risk sexual behavior did not increase in any group
Women who had increased access to emergency contraception did not have sex more frequently. Receiving emergency contraceptives in advance did not affect the number of sex partners, with most women having only one partner. Data on teens in the same study found that teens did not take more sexual risks than women aged 20 to 24.22
A concern with placing emergency contraception directly in the hands of women has been the theory that it would result in increased high-risk behavior and lower use of regular contraception.
This trial found:
- That women with pharmacy access and women given 3 packs of emergency contraceptives in advance were no more likely to change their regular contraceptive method than women who could obtain emergency contraception only via a clinic visit.
- That women with increased access to emergency contraceptives use their routine contraception with the same consistency as women without increased access.
No downside to easier, wider access to Plan B
This trial adds to the argument for wider and easier access to emergency contraception for women. There is no apparent downside from wide access to the current progestin-only emergency contraception regimen. The latest World Health Organization medical eligibility criteria describe no situation in which the risks of emergency contraception outweigh the benefits.23 The study by Raine et al21 provides evidence against concerns about the potential for increased high-risk sexual behavior.
Eight states (Alaska, California, Hawaii, Maine, Massachusetts, New Hampshire, New Mexico, Washington) have passed legislation allowing pharmacists to prescribe emergency contraceptives without a prescription. Norway, Sweden, India, and the Netherlands allow emergency contraceptive availability over-the-counter.
Three new developments this year stand to make a difference in the high rate of unintended pregnancies in the United States. Increased use of highly effective, long-acting, user-independent methods is an effective way to lower the rate of unintended pregnancies in couples using contraception. Two such methods are the contraceptive implant and the intrauterine contraceptive. Emergency contraception is an effective way to reduce the risk of unintended pregnancy after failure of a contraceptive method or unprotected or forced sex. A randomized trial showed that direct access to EC does not increase high-risk behavior.
Half of pregnancies are unintended
- The United States has one of the highest rates of unintended births among industrialized countries.
- Of the 6 million pregnancies each year in the US, nearly 3 million are unintended, resulting in 1.4 million unintended births and 1.3 million abortions.
- Half of these unintended pregnancies are due to failure or incorrect or inconsistent use of a contraceptive method.1
REFERENCE
1. Contraception Counts: Ranking State Efforts. New York: Guttmacher Institute; February 2006.
Implanon essentials: How it works and what to tell patients
The single-rod implant (Implanon) is a new, highly effective, long-acting, rapidly reversible contraceptive, approved by the FDA, July 17, 2006.
A new single-rod implant that provides highly effective contraception for up to 3 years is expected to be widely available in the United States in 2007. Once inserted, Implanon is independent of user compliance and is rapidly effective and reversible. It is in use worldwide in more than 30 countries since 1998.
The new device is a nonbiodegradable 40 × 2.0 mm rod of 40% ethylene vinyl acetate (EVA) and 60% etonogestrel (ENG) covered with a rate-controlling EVA membrane.
The rod contains 68 mg of ENG, initially absorbed by the body at a rate of 60 μg/day, slowly declining to 30 μg/day after 3 years of use.2 Steady release of ENG into the circulation avoids first-pass effects on the liver.
Manufacturer-sponsored training: Call 1-877-IMPLANON
Before clinicians can order the implant, they must undergo training sponsored by the manufacturer, Organon. To take part in the training, which is set to begin in August, call 1-877-IMPLANON.
How the implant works
Ovarian and cervical mechanisms, which function prior to fertilization, provide high contraceptive efficacy.
Ovulation is suppressed. The ENG implant, unlike previous levonorgestrel-containing implants, works primarily by suppressing ovulation.3 ENG alters the hypothalamic–pituitary–ovarian axis and down-regulates the luteinizing hormone surge, which is required to support the production, growth, and maturation of ovarian follicles.
Ovulation returns rapidly after removal of the implant.3
Cervical mechanisms also prevent fertilization. Anti-estrogenic actions of ENG make the cervical mucus viscous, scanty, and impenetrable by sperm.
Cost-effectiveness depends on long-term use; early removal negates this benefit. At press time, the manufacturer had not released the price of Implanon.
Lack of protection against sexually transmitted infections is a disadvantage of the ENG implant, as well as all nonbarrier contraceptive methods.
Discontinuation rates have varied by region, but are usually due to bleeding pattern changes.
- In an international multicenter trial, 31% discontinued by 2 years and only 6% discontinued in the third year.4 Again, the most common reason was irregular bleeding.
- In a US series, 49% discontinued by 2 years. The most common reason was bleeding pattern changes (13%).5 The rate of discontinuation was highest during the first 8 months.
What to tell patients. To improve continuation, counseling should strongly stress the expected change in bleeding patterns.
Clinical trials
Outstanding efficacy. In an international multicenter trial, there were no intrauterine or ectopic pregnancies in a total of 1,200 woman-years (15,000 cycles of exposure, 2,000 of which were in the third year of use).4 The Pearl index was 0 (95% CI 0.0–0.2).4 In the US series, after a total exposure of 474 woman-years (6,186 cycles), no intrauterine or ectopic pregnancies were observed.5 It should be noted that phase III data from Indonesia were retracted by the manufacturer in 2004.6 The 2 trials noted above included a total of 965 women and were not included in this retraction.
Reasons for failures. Pregnancies were noted from postmarketing data in Australia; most of these pregnancies resulted from either incorrect timing at the initial insertion or failure to insert the implant. Based on the Australian phase IV data, with 204,486 devices inserted, the failure of the method itself was estimated to be 1 per 1,000 insertions.7 Implanon may be less effective in obese women or, as the Australian experience showed, with concomitant use of drugs that stimulate the liver’s cytochrome metabolism of steroids, such as some antibiotics (eg, rifampin) or anticonvulsants (eg, phenytoin).
Side effects
Infrequent bleeding. The main side effect is a change in bleeding patterns. In the US series, amenorrhea occurred in 14% to 20% of women. In the same series, women experienced infrequent bleeding (<3 episodes in 90 days) in 30% to 40% of the 90-day reference periods, making it the most common pattern experienced. Prolonged bleeding (>14 days of bleeding in one episode) varied from 14% to 36%, and frequent bleeding (>5 episodes in 90 days) varied from 7% to 14%.5 Anemia was not observed in the US series despite the irregular bleeding; in fact, hemoglobins rise.
Although the ENG implant is designed to facilitate rapid and simple insertion and removal, clinicians must first be trained. The manufacturer, Organon, announced last month that it would begin training doctors in August.
Insertion Average time: 1 minute4
The single-rod implant is preloaded in a disposable applicator. Insertion is done in the office using local anesthesia.
Place the 1.5-inch long implant on the inner aspect of the nondominant arm. Position the applicator needle subdermally and withdraw the cannula, leaving the implant rod in place.
After insertion, the implant may not be visible but should be palpable.
Removal Average time: 4 minutes4
Removal requires a 2- to 3-mm incision at the distal tip of the implant. Push the other end of the rod until it pops out.
Timing the insertion
- Between days 1 and 5 of menses, in women who either have not been using a contraceptive method or have been using a nonhormonal method
- During a hormone-free week, in women changing from a combination or progestin-only oral contraceptive, or from intrauterine contraception
- The day on which the next injection is scheduled, in women changing from injectable contraception
No backup contraceptive is necessary if timing of insertion occurs as detailed.
In all cases, exclude pregnancy before insertion.
Timing the removal
The ENG implant can be removed at any time, but must be removed after 3 years.
Return to ovulation is rapid following removal, so women still desiring contraception should begin another method immediately or have a new rod inserted through the removal incision.
Unpredictable bleeding pattern. Unlike with Norplant, there was no trend over time toward a particular bleeding pattern. Implanon patterns are irregular and unpredictable and vary from one 90-day reference period to the next. Similar results were noted in the multicenter international trial.4
Possible therapies include estrogen supplementation, nonsteroidal anti-inflammatory drugs, oral contraceptive pills, and observation.
Other side effects. In the US series, the most frequent nonmenstrual adverse effects possibly related to the ENG implant were acne (14.5%), headache (12.7%), weight gain (12.1%), and emotional lability (14.2%).
Contraindications
The ENG implant should not be placed in women with undiagnosed abnormal genital bleeding, known or suspected pregnancy, or hypersensitivity to any of the components in the ENG implant.
REFERENCES
Why the FDA removed ParaGard’s parity restriction
- An FDA labeling change for the ParaGard intrauterine device confirms what the evidence has long supported: The risk of pelvic infection is more related to sexual behavior than to age, contraceptive choice, or parity
- Evidence supports a link between cervical infection—but not IUD use—and pelvic inflammatory disease and infertility
The Food and Drug Administration has approved a less restrictive label for the ParaGard T380A copper intrauterine contraceptive. Evidence has long supported the conclusion that risk of pelvic infection is related more to a woman’s and her partner’s sexual behavior than to her age, contraceptive choice, or parity.
A woman with at least one child and in a mutually monogamous relationship is no longer listed as the recommended patient profile. Nor is ParaGard contraindicated for a woman with a history of sexually transmitted disease or pelvic inflammatory disease (PID), unless she has current acute PID or engages in sexual behavior suggesting a high risk for PID.
Why was the label restrictive to begin with?
Early studies8,9 that showed an increased risk of PID and infertility in intrauterine contraceptive users have been re-analyzed; most of the increased risk was associated with a single type of intrauterine contraceptive that is no longer on the market (Dalkon Shield), and with high-risk sexual behaviors.10-12 In most analyses of these studies, the increased risk of PID was present only in the first 20 days after insertion, indicating undiagnosed cervical infection at the time of insertion.
Furthermore, many studies had methodological flaws that introduced bias into the results, such as comparing intrauterine contraceptive users with users of combination oral contraceptives (who have a decreased risk of PID compared with nonusers).13
These early studies also equated nulliparity with high-risk sexual behavior. As young women are more likely to acquire sexually transmitted cervical infections, and because young age is associated with nulliparity, many studies erroneously concluded that the increased risk of PID and infertility was attributed to nulliparity.
A case-control study in nulliparous Mexican women who were seeking treatment for primary infertility found no association between tubal infertility and past copper IUD use. In this study, 358 women with primary infertility and documented tubal occlusion (cases) were compared with two sets of controls: 953 nulliparous women with primary infertility and no tubal occlusion, and 584 primigravid women. Past use of a copper IUD was not associated with tubal occlusion, compared with either infertile women without tubal occlusion or primigravid controls (P values 1.0 and 0.9, respectively).14 However, tubal infertility was associated with a past infection with Chlamydia (as evidenced by Chlamydia antibodies). This study further supports an association between PID and infertility and cervical infection—not IUD use.
Protective effect of progestin
The levonorgestrel-releasing intrauterine system (LNG-IUS) may even protect against PID. One of the primary physiologic effects of progestin contraception is thickening of the cervical mucus, which protects against ascending genital tract infection. This protective effect results in a decreased incidence of PID in women who use combination oral contraceptive pills, progestin implants, and progestin injectables.15 A randomized controlled trial found that the cumulative 36-month rate of PID was lower in users of a LNG-IUS contraceptive than in users of a copper IUD (Nova-T) (0.5 and 2.0, respectively; P< 0.013), in both parous and nulliparous women.16 This finding was more marked in women under the age of 25.
Prescribing IUDs in young women
When considering an intrauterine contraceptive for a young woman, it is therefore important to assess her risk of a STI, based on her and her partner’s sexual behavior, and not on parity or age. It is important to screen for STIs at the time of or prior to insertion of an intrauterine contraceptive, and to treat cervicitis prior to insertion.
Nulliparous women who are at low risk of STIs can be offered the intrauterine contraceptive as an effective, long-term, user-independent contraception.
The labeling for levonorgestrel intrauterine contraceptives should also reflect the evidence that the risk of pelvic infection is more related to a patient’s and/or her partner’s sexual behavior than to her age, contraceptive choice, or parity.
REFERENCES
Direct access to Plan B does not promote high-risk behavior
Raine TR, Harper CC, Rocca CH, et al. Direct access to emergency contraception through pharmacies and effect on unintended pregnancy and STIs: a randomized controlled trial. JAMA. 2005;293:54–62.
- Advance provision of emergency contraception results in increased usage of emergency contraception without an associated change in risky sexual behavior, sexually transmitted diseases, or use of long-term contraception
- Clinicians should provide emergency contraception in advance of need to ensure timely and appropriate emergency contraceptive use
Emergency contraception could significantly reduce the risk of unintended pregnancy after contraceptive method failure, or unprotected or forced sex. The newer progestin-only emergency contraceptive pills have now largely replaced the older combined (estrogen and progestin) pills because they are more effective and have fewer side effects.
Various emergency contraception regimens are effective
The only dedicated progestin-only emergency contraception pill product in the United States is Plan B, which contains 2 tablets of 0.75 mg of levonorgestrel. Although the recommended treatment schedule is an initial dose within 72 hours of unprotected intercourse and a second dose 12 hours later, a single dose of 1.5 mg of levonorgestrel is as effective as and causes no more side effects than 2 tablets of 0.75-mg doses 12 hours apart.17,18
The sooner the better?
Emergency contraception pills are more effective the sooner after sex that they are initiated. Both combination oral contraceptive pills and progestin-only regimens are moderately effective even if initiated more than 72 hours after unprotected intercourse. 18-20 No data are available on the efficacy of emergency contraception pills taken more than 120 hours (5 days) after unprotected intercourse.
Randomized trial
“It seems unreasonable to restrict access”
Raine et al21 added significantly to our knowledge of emergency contraception. This is the first randomized trial addressing the question of the effect of access on emergency contraceptive usage. A total of 2,117 young women (age 15 to 24 years) were randomly assigned to these 3 groups:
- Pharmacy access (without consulting a physician)
- Advance provision of 3 packs of Plan B
- Clinic access (ie, usual care, which required a clinic visit to obtain emergency contraception)
The study concluded: “While removing the requirement to go through pharmacists or clinics to obtain emergency contraception increases use, the public health impact may be negligible because of high rates of unprotected intercourse and relative underutilization of the method. Given that there is clear evidence that neither pharmacy access nor advance provision compromises contraceptive or sexual behavior, it seems unreasonable to restrict access to emergency contraception to clinics.” The conclusion reflected the following several outcomes, which were assessed after 6 months.
4 key outcomes
- Use of emergency contraception
- The advance provision group used emergency contraception at nearly twice the rate (37.4%) of the clinic access group (21.0%).
- Usage rates were similar in the pharmacy access (24.2%) and the clinic access group (21.0%).
- New sexually transmitted infection rates were similar in all groups
Levels of STIs, such as Chlamydia, were similar across all groups, and changes in HSV-2 serology were similar across all groups. - Many did not use the EC, even with advance provision
In this study, advance provision of emergency contraceptives did not lower pregnancy rates. This finding is disappointing; the likely explanation is that women at highest risk do not use emergency contraception often enough or at all. Thus, the overall pregnancy rate is unchanged. Nearly half (45%) of the women in the study who reported having unprotected sex did not use emergency contraception during the study period, even when they received it in advance. - High-risk sexual behavior did not increase in any group
Women who had increased access to emergency contraception did not have sex more frequently. Receiving emergency contraceptives in advance did not affect the number of sex partners, with most women having only one partner. Data on teens in the same study found that teens did not take more sexual risks than women aged 20 to 24.22
A concern with placing emergency contraception directly in the hands of women has been the theory that it would result in increased high-risk behavior and lower use of regular contraception.
This trial found:
- That women with pharmacy access and women given 3 packs of emergency contraceptives in advance were no more likely to change their regular contraceptive method than women who could obtain emergency contraception only via a clinic visit.
- That women with increased access to emergency contraceptives use their routine contraception with the same consistency as women without increased access.
No downside to easier, wider access to Plan B
This trial adds to the argument for wider and easier access to emergency contraception for women. There is no apparent downside from wide access to the current progestin-only emergency contraception regimen. The latest World Health Organization medical eligibility criteria describe no situation in which the risks of emergency contraception outweigh the benefits.23 The study by Raine et al21 provides evidence against concerns about the potential for increased high-risk sexual behavior.
Eight states (Alaska, California, Hawaii, Maine, Massachusetts, New Hampshire, New Mexico, Washington) have passed legislation allowing pharmacists to prescribe emergency contraceptives without a prescription. Norway, Sweden, India, and the Netherlands allow emergency contraceptive availability over-the-counter.
17. Arowojolu AO, Okewole IA, Adekunle AO. Comparative evaluation of the effectiveness and safety of two regimens of levonorgestrel for emergency contraception in Nigerians. Contraception. 2002;66:269-273.
18. von Hertzen H, Piaggio G, Ding J, et al. Low dose mifepristone and two regimens of levonorgestrel for emergency contraception: a WHO multicentre randomised trial. Lancet. 2002;360:1803-1810.
19. Ellertson C, Evans M, Ferden S, et al. Extending the time limit for starting the Yuzpe regimen of emergency contraception to 120 hours. Obstet Gynecol. 2003;101:1168-1171.
20. Rodrigues I, Grou F, Joly J. Effectiveness of emergency contraceptive pills between 72 and 120 hours after unprotected sexual intercourse. Am J Obstet Gynecol. 2001;184:531-537.
21. Raine TR, Harper CC, Rocca CH, et al. Direct access to emergency contraception through pharmacies and effect on unintended pregnancy and STIs: a randomized controlled trial. JAMA. 2005;293:54-62.
22. Harper CC, Cheong M, Rocca CH, Darney PD, Raine TR. The effect of increased access to emergency contraception among young adolescents. Obstet Gynecol. 2005;106:483-491.
23. Medical Eligibilty Criteria for Contraceptive Use. Geneva: World Health Organisation; 2004
Dr. Parvataneni is a consultant to Organon. Dr. Darney is a consultant to Organon and is a speaker for Berlex and Organon.
17. Arowojolu AO, Okewole IA, Adekunle AO. Comparative evaluation of the effectiveness and safety of two regimens of levonorgestrel for emergency contraception in Nigerians. Contraception. 2002;66:269-273.
18. von Hertzen H, Piaggio G, Ding J, et al. Low dose mifepristone and two regimens of levonorgestrel for emergency contraception: a WHO multicentre randomised trial. Lancet. 2002;360:1803-1810.
19. Ellertson C, Evans M, Ferden S, et al. Extending the time limit for starting the Yuzpe regimen of emergency contraception to 120 hours. Obstet Gynecol. 2003;101:1168-1171.
20. Rodrigues I, Grou F, Joly J. Effectiveness of emergency contraceptive pills between 72 and 120 hours after unprotected sexual intercourse. Am J Obstet Gynecol. 2001;184:531-537.
21. Raine TR, Harper CC, Rocca CH, et al. Direct access to emergency contraception through pharmacies and effect on unintended pregnancy and STIs: a randomized controlled trial. JAMA. 2005;293:54-62.
22. Harper CC, Cheong M, Rocca CH, Darney PD, Raine TR. The effect of increased access to emergency contraception among young adolescents. Obstet Gynecol. 2005;106:483-491.
23. Medical Eligibilty Criteria for Contraceptive Use. Geneva: World Health Organisation; 2004
Dr. Parvataneni is a consultant to Organon. Dr. Darney is a consultant to Organon and is a speaker for Berlex and Organon.
Shoulder dystocia: What is the legal standard of care?
If permanent injury occurs after shoulder dystocia, it can also trigger a lawsuit that can last for years and end in a large jury verdict—even if you handled the case with textbook perfection. Lawsuits involving brachial plexus injuries following shoulder dystocia are now the second most common type of lawsuit in obstetrics, exceeded only by those due to neurologic damage from birth asphyxia.1 Brachial plexus injury is often difficult to defend in court and results in scores of millions of dollars in damages each year. The plaintiff is usually a lovely child with an obvious and permanent injury, and the defense is typically an undocumented claim that the obstetrician applied no undue force at delivery.(Sidebar)
Given the difficulties of knowing when shoulder dystocia will occur, how best to resolve it, and whether a claim is likely, how can we prepare for this event? What is the accepted standard of care? This article answers these questions by surveying the evidence on these aspects of management:
- risk factors for shoulder dystocia
- how to choose mode of delivery
- specific labor-management practices
- the 4 most widely used maneuvers to resolve shoulder dystocia
- what information the documentation should include.
No single “standard of care”
In many states, the term “standard of care” has a specific legal meaning, but in most of the United States—and to most physicians— the term means care that would be rendered by the majority of well-trained individuals. Complicating this definition is the fact that medicine often offers no single “right way.” Thus, it may be more appropriate to speak of “standards of care”: the range of therapeutic choices a reasonable practitioner might decide to use.
Traction is the most used and abused of terms in shoulder dystocia lawsuits. Many plaintiff expert witnesses claim that traction should never be applied to a baby’s head during delivery. Other “experts” claim only “gentle” traction is warranted. These statements are designed to support the most frequent contention against obstetricians when permanent brachial plexus injury occurs: As there is an injury, it must have been caused by a doctor or midwife who used “excessive traction” to deliver the baby. This statement is usually made without defining “excessive” and without evidence that more force than necessary was used.
“Excessive” vs “minimum necessary” traction
Routine or “moderate” traction is used in most deliveries. The birth attendant almost always depresses the fetal head and applies a moderate amount of traction to it to help the baby’s anterior shoulder slide beneath the mother’s pubic bone.38 The only time traction is unnecessary is when the expulsive forces of the mother are so strong or uncontrolled that she pushes the baby out entirely on her own.
There is ambiguity—often contrived—about what exactly constitutes mild, moderate, routine, and “excessive” traction. No study has ever been published that accurately and unambiguously quantifies the amount of force used in actual deliveries.
Once shoulder dystocia is diagnosed, further attempts at routine traction without the use of other maneuvers should be avoided. At best these attempts are unavailing. At worst they serve only to keep the anterior shoulder lodged behind the maternal symphysis.
Much misinformation surrounds the role of traction during the McRoberts maneuver and other efforts to resolve dystocia. The reality is simple: An obstetrician cannot determine whether a maneuver has released the anterior shoulder unless moderate traction is applied after the maneuver to see if the baby can be delivered. Although extreme force at this or any point is not appropriate, moderate traction is entirely appropriate.
“Excessive traction” is an oxymoron, although plaintiff lawyers often use the term. An obstetrician uses a given amount of force in attempting to free a stuck shoulder. Once the shoulder is freed, no more force is applied. Thus, by definition, “excessive force”—more force than is necessary to deliver the baby—is never used. The proper term to describe the amount of force applied by a physician to resolve shoulder dystocia is “minimum necessary traction.”
Injury can follow a traction-free delivery
For many years, obstetricians familiar with shoulder dystocia have claimed that brachial plexus injuries can occur even in the absence of significant traction—either in utero or as a result of the natural forces of labor. Yet plaintiff attorneys and expert witnesses have contended that all brachial plexus injuries are the result of someone pulling “too hard.”
A recent case reported by Allen and Gurewitsch39 settled this question once and for all. They describe a delivery in which a patient requested no intervention of any kind. Despite no hand having touched the baby during delivery—thus, no “excessive traction” having been applied —the baby suffered a brachial plexus injury. This case proved that brachial plexus injuries can occur spontaneously and are not necessarily caused by traction.
Why dystocia cannot be predicted
…despite known risk factors
The risk of shoulder dystocia is higher in women with diabetes,2-5 a macrosomic fetus,2,6-8 obesity,5,8 or a previous shoulder dystocia.9-11 The problem: The predictive value of these factors is so low and their false-positive rate so high they cannot be used reliably in clinical decision-making.11-13
Prevention is impossible
Even if prediction were possible, the only preventive option is elective cesarean section. After all, this is the only intervention that might potentially avoid the infrequent but dreaded outcomes of asphyxia and permanent brachial plexus injury. But as the literature shows, even this is not an absolute guarantee.14,15 Moreover, the strategy of inducing labor several weeks prior to the due date to prevent a baby from becoming “too big” has been shown in many studies to be ineffective in lowering the shoulder dystocia rate.16-18
Risk factors are not clinically useful
The American College of Obstetricians and Gynecologists (ACOG) and Williams Obstetrics concur that risk factors for shoulder dystocia cannot be applied in a clinically useful way to prevent brachial plexus injury. As the ACOG practice bulletin on shoulder dystocia19 observes:
- “Shoulder dystocia cannot be predicted or prevented because accurate methods for identifying which fetuses will experience this complication do not exist.”
- “Elective induction of labor or elective cesarean delivery for all women suspected of carrying a fetus with macrosomia is not appropriate.”
Identify highest risk
Nevertheless, there are generally accepted guidelines for attempting to ascertain which patients are at the absolute highest risk for shoulder dystocia:
- Any woman with gestational diabetes. For any given week of gestation in the third trimester, the ratio of thorax and shoulder size to head volume is larger in babies of diabetic mothers.20 Thus, in these women, it is important to estimate fetal weight near term to determine whether a trial of vaginal delivery makes sense.
- If, for any reason, the fetus appears to be larger than average. Indications of size may come from palpation of the maternal abdomen, fundal height measurements significantly greater than dates, ultrasound estimation of large fetal weight, or maternal perception. In these cases, ultrasound imaging is advisable near term to estimate fetal weight. This estimate can be factored into the selection of delivery mode.
How big is “too big”?
There are 2 problems with using estimates of fetal weight in determining mothers and babies at highest risk:
- How is “too big” defined?
- What action should one take if a baby is thought to be “too big”?
As for what to do if a fetus is estimated to be in this size range, ACOG states: “Planned cesarean delivery to prevent shoulder dystocia may be considered [emphasis added] for suspected fetal macrosomia within the above weight parameters.”19 The decision as to whether to recommend or perform a cesarean section in these circumstances is intentionally left up to the physician and the patient.
The problem, of course, is that all our data are from measurements of babies after delivery—information obstetricians do not have at the time they must decide on the mode of delivery.
TABLE
How fetal weight affects the rate of dystocia
| ESTIMATED FETAL WEIGHT | RATE OF SHOULDER DYSTOCIA (%) | |
|---|---|---|
| NONDIABETIC MOTHERS | DIABETIC MOTHERS | |
| 1.1 | 3.7 | |
| 4,000–4,499 g | 10 | 23.1 |
| >5,000 g | 22.6 | 50 |
| Source: Acker D et al2 | ||
Choosing a mode of delivery: Not so simple
The obstetrician must determine whether the risk of shoulder dystocia is high enough to outweigh the risks to a mother of elective cesarean section. This is far from simple. Although it is true that women at the highest risk for dystocia—those with gestational diabetes and suspected macrosomia— have a risk for shoulder dystocia somewhere between 25% and 50%, this is not the main concern.
The main concern is this: What percentage of even these high-risk patients will have a shoulder dystocia that results in a permanent brachial plexus injury? The answer: Permanent injury is rare, even in highest-risk cases.
Only 10% to 20% of infants born after shoulder dystocia suffer brachial plexus injuries.16,21-23 Of these, only 10% to 15% are permanently injured.5,24,25 Thus, even in women at highest risk, the odds of having an infant with permanent brachial plexus injury are roughly 1 in 450.14 In women at lower risk for shoulder dystocia, the odds of permanent brachial plexus injury are much lower: somewhere between 1 in 2,500 and 1 in 10,000.
When is cesarean section warranted?
In deciding the answer to this question, the obstetrician must consider that cesarean section is not without its own risks: excessive bleeding, infection, injury to bowel or bladder, deep venous thrombosis, and the need for hysterectomy.
These adverse events occur much more frequently than does permanent brachial plexus injury.26 And the risks are higher yet for the very same patients at greatest risk for shoulder dystocia—diabetic and obese women.
Prevent “I didn’t know” accusations
This is the point at which the patient’s input becomes vital. It is important to convey to her in readily understandable terms the risks—to both her and her child—of cesarean section versus attempted vaginal delivery. Plaintiff attorneys often claim that, had their client known there was a 1 in 450 chance of her baby having a permanent injury, she would have opted for cesarean section. The truth of this claim is, of course, open to question. However, from a medicolegal perspective, it is extremely important that the woman be informed of the degree of risk to herself and her baby so that her decision is truly informed—even if it is not the choice the obstetrician would have made.
The consensus in surgery is that the patient should be informed when the threshold of risk for an adverse event reaches 1% or higher. Although it is an informal teaching, this threshold is documented in the medical literature.27
The option of cesarean section should be discussed and possibly recommended for all women whose infants are estimated to weigh more than 5,000 g in the absence of diabetes and 4,500 g or more in women with diabetes.
Often a mother will voice concern about whether she will be able to deliver her baby safely vaginally. She may feel that her infant is too big, that she is too small, or that her obesity will make her delivery more difficult. Do not blithely ignore such concerns or provide blanket reassurances that everything will be OK.
Instead, review with her any risk factors she may have for shoulder dystocia and discuss the specific odds of injury to her baby should dystocia arise. Then discuss the risks to her and the discomfort she will experience if she elects a cesarean section.
Patients have a right to know the risks
Although it is appropriate to be reassuring when there are no significant risk factors, patients deserve to know what risks they run and to have these risks put into perspective. For example, if the mother has diabetes and her baby is estimated to weigh over 4,500 g, the risk of permanent brachial plexus injury approaches 1 in 450. The same is true if she is nondiabetic but has an estimated fetal weight of 5,000 g or more.
In high-risk cases such as these, you should discuss the risks with the patient and have her participate in the decisionmaking. You should also clearly document this discussion in the medical record.
Labor management
Prolonged second stage and instrumental delivery
Although the literature is not clear on this point, there is a trend toward increased rates of shoulder dystocia with a prolonged second stage of labor2,3,28 and with instrumental deliveries.6,12,29,30 Most experts believe this trend merely reflects the fact that bigger babies—the known major risk factor for shoulder dystocia—encounter these sorts of labor problems more frequently than do smaller babies. Whatever the reason, it warrants attention. An obstetrician’s care of any laboring woman should follow standard practices regarding arrest of labor and descent or a prolonged second stage.
Plaintiffs are quick to condemn vacuum and forceps
The same applies to intervention with forceps or vacuum. Only in women at highest risk for shoulder dystocia—those with diabetes or with suspected macrosomic fetuses—should standard management be modified.
Given the potential for shoulder dystocia in such high-risk circumstances, not to mention our inability to predict dystocia, prudence dictates that we avoid aggressive management and the use of forceps or vacuum in these cases.
These practices are often condemned in court by plaintiff lawyers and their expert witnesses.
Oxytocin is OK
In cases of arrest of labor and descent, the use of oxytocin is appropriate. A laboring woman should be given adequate time to deliver on her own, especially if a regional anesthetic has been used.
…but prepare to act quickly. In high-risk cases, be prepared to move more quickly than normal to cesarean section.
Is your team prepared? 4 standards of care
Although it is true that an obstetrician must be prepared for the possibility of shoulder dystocia in any delivery, to act as though it will occur in all deliveries is simply not reasonable, given that the rate of dystocia is 0.5% to 1.5%, or 1 in 67 to 200 deliveries.12,21,25,29
Nevertheless, 4 specific standards apply to all delivery facilities:
- The entire labor and delivery staff should know what to do and what each person’s role is when shoulder dystocia is diagnosed.
- Labor and delivery nurses should know how and when to initiate McRoberts maneuver and apply suprapubic pressure.
- The team should immediately obtain the assistance of another obstetrician, a pediatrician, and an anesthesiologist, even though they are not likely to arrive before the dystocia is resolved.
- The obstetrician should be mentally prepared for the possibility of shoulder dystocia. This requires the ability to quickly recognize it, familiarity with the various techniques for resolving it, and avoidance of unnecessary traction. It also is vital for the obstetrician to remain composed and in charge, as the obstetrician becomes the leader of the medical team when this emergency arises.
How to recognize shoulder dystocia
There are 2 ways to diagnose dystocia.
- “Turtle sign.” The first is recognizing the pathognomonic “turtle sign,” in which, after delivery of the baby’s head, the head immediately retracts back up against the mother’s perineum, causing the baby’s cheeks to bulge.
- The second diagnostic sign is when, after delivery of the head, the moderate amount of traction usually used does not suffice to deliver the anterior shoulder. Cease attempts at routine traction as soon as shoulder dystocia is diagnosed.
The 4 main maneuvers
The 4 maneuvers generally used by obstetricians to resolve shoulder dystocia are considered the standard of care:
- McRoberts maneuver
- Suprapubic pressure
- Woods screw maneuver
- Delivery of the posterior arm
McRoberts maneuver is often the only one needed
In this maneuver, the laboring woman’s thighs are hyperflexed against her abdomen.31 This hyperflexion does not increase the diameter of the pelvis, as is sometimes claimed. Rather, it flattens the sacrum and changes the angle of the symphysis pubis in relation to the baby’s anterior shoulder, often freeing it. It is an extremely effective way to resolve shoulder dystocia and is often the only maneuver necessary.
Family members can assist—contrary to plaintiff attorney contentions. This maneuver can be performed by nurses or family members if they are properly instructed. Plaintiff attorneys will sometimes argue that the use of family members in this situation is inappropriate, but they are wrong. Family members are sometimes instructed to hold a mother’s legs in a certain position while she is pushing; they can certainly be instructed to hold the legs against the maternal abdomen during attempts to resolve a shoulder dystocia.
Suprapubic pressure with or without McRoberts
In this maneuver, a nurse or other attendant places direct pressure with an open hand or fist just above the mother’s symphysis pubis. The pressure can be directed straight down or to the left or right. Wherever it is directed, the aim of the pressure is to push the baby’s anterior shoulder out of its position behind the mother’s pubic bone.
The combination of McRoberts maneuver and suprapubic pressure can resolve shoulder dystocia in as many as 58% of cases.22
Woods screw maneuver attempts to “spin” the baby
If the McRoberts maneuver and suprapubic pressure do not resolve the shoulder dystocia, the Woods screw maneuver is usually implemented next.32 In this maneuver, the obstetrician inserts a hand into the posterior vagina and pushes the front of the baby’s posterior shoulder in a spiral direction (clockwise or counterclockwise). The goal is to “unjam” the anterior shoulder from its trapped position behind the symphysis pubis.
The Woods screw maneuver is very effective. After it has been used, it is appropriate to apply moderate traction to the baby’s head to determine whether the baby can be delivered.
Variant: Rubens maneuver. In this maneuver, the obstetrician pushes on the posterior aspect of the posterior shoulder. In addition to spinning the shoulders, as in the Woods screw maneuver, the Rubens maneuver causes shoulder abduction, thus decreasing the biacromial diameter that has to pass through the pelvic outlet.
Attempts to deliver the posterior arm
If shoulder dystocia still persists, the next strategy is usually an attempt to deliver the baby’s posterior arm. This is done by placing a hand deep into the posterior aspect of the vagina, grabbing the baby’s posterior arm, sweeping that arm across the baby’s chest, and delivering it. Once the posterior arm and shoulder are delivered, it is almost always possible to deliver the baby directly from this position or to move the baby in a spiral direction (clockwise or counterclockwise) to free the anterior shoulder.
Other maneuvers
Two other maneuvers are occasionally used, though neither is considered mainstream.
Gaskin or “all fours” maneuver. This technique is frequently advocated by the midwife community.33 It involves moving the laboring woman from the standard lithotomy pushing position to her hands and knees to free the stuck anterior shoulder. However, many have questioned the practicality of turning a fatigued, laboring woman rapidly enough to deliver a baby within the 4 to 6 minutes available, particularly when an epidural has been given or other maneuvers have already used up much of the allotted time.
Zavanelli maneuver if all else fails. This maneuver should be attempted only when all other efforts have failed.34 It involves flexing the fetal head and attempting to push the baby’s head back into the vagina, followed by emergency cesarean section.
Although case reports have described successful use of this maneuver, there also have been reports of fetal death, fractured spines, and other severe fetal damage. Thus, this maneuver should be the absolute last resort in desperate emergencies.35
What not to do
Traction
Do not continue to apply traction to the fetal head if the shoulder does not come. Once shoulder dystocia is diagnosed, cease all attempts to deliver the baby by continued pulling. Carefully but expeditiously use the various maneuvers you were trained to do, applying moderate traction after each one to see if the shoulder has been freed.
Fundal pressure
Do not apply fundal pressure. It never helps resolve shoulder dystocia, but only further jams the stuck shoulder against the maternal pubic bone. It also can cause injury to the fetus or even rupture the uterus.
Fundal pressure is often cited in court as a definite standard of care violation.
Theory vs evidence
A 3-member team is adequate
Shoulder dystocia occurs unexpectedly. Once it does occur, the obstetrician has 4 to 6 minutes to resolve it before the threat of central neurologic damage to the baby becomes significant. Although it would be very helpful for additional personnel to be available, it is not always possible to assemble this team quickly enough.
In reality, the only personnel truly necessary to resolve a shoulder dystocia are:
- The delivering doctor or midwife
- A medically trained assistant familiar with McRoberts maneuver and suprapubic pressure
- Any other available person, including a family member, who can be drafted to help and instructed to participate in the McRoberts maneuver by flexing one of the mother’s thighs
Drills are not an absolute necessity
It is sometimes claimed that formal shoulder dystocia drills should be conducted in labor and delivery units at fixed intervals. Although this may be a useful and reasonable educational practice, it is more important that each individual on the labor and delivery team know what his or her role is during such an emergency. Whether this is achieved through a practice drill or didactic instruction does not matter.
In short, there is nothing about the concept of a drill that is “standard of care.” What is standard of care is that every team member knows what to do, how to do it, when to do it, and how to document it.
Episiotomy is often superfluous
Multiple studies have shown that episiotomy is not necessary to resolve shoulder dystocia, although many textbooks and other published protocols still recommend it.36 The obstructing factor in shoulder dystocia is not the soft tissue of the perineum but the symphysis pubis. The only time episiotomy helps is when more room is needed for the obstetrician’s hand to enter the posterior aspect of the vagina to perform a shoulder dystocia maneuver. If you can perform all necessary maneuvers without episiotomy, it is superfluous.
Document early and always
Because shoulder dystocia often leads to litigation, it is extremely important to document what happened during delivery as soon as feasible and in as much detail as possible. Standardized forms are now available. (FORM)
At minimum, you should record:
- how shoulder dystocia was diagnosed
- which shoulder was anterior and which was posterior
- quantification of the force applied initially and in subsequent traction attempts, using terms such as “mild,” “moderate,” or “significant”
- duration of attempts to resolve the dystocia
- maneuvers performed
- approximate length of time each maneuver was tried
- condition of the baby at delivery, including Apgar scores, a description of all injuries and bruises, and cord pH, if obtained
- time from delivery of the fetal head to delivery of the body
- documentation of the discussion with the patient following delivery
Given that the most frequent criticism of obstetricians in the courtroom in brachial plexus injury lawsuits is that they pulled too hard, the best defense consists of careful, complete, and contemporaneous documentation of one’s actions at delivery.
Lawsuits happen
Even when everything is done correctly, there is a very high likelihood that a lawsuit will be filed when there is a permanent brachial plexus injury.
The 2 claims generally made against obstetricians are:
- The obstetrician should have known or predicted that the risk of shoulder dystocia was high, and should have performed a cesarean section or at least offered the mother that choice.
- As the baby has a permanent brachial plexus injury, the obstetrician must have pulled too hard at delivery.
The best defense
The best defense is, as always, to have practiced good medicine and to have documented it. You must be able to demonstrate from your records—years after a delivery that you no longer remember—that you:
- made appropriate prenatal judgments and were aware of risk factors
- informed the mother of such risk factors when they are significant
- provided proper obstetrical care
- documented in the medical record that you knew what you were doing and did it correctly
Some good news is on the horizon. Recent research has produced a mathematical tool that appears to be able to predict 50% to 75% of all women destined to have shoulder dystocia, with a false-positive rate of only 2% to 3%.37 If this model holds up under further investigation, it may become possible to avoid most shoulder dystocia deliveries and, with them, permanent brachial plexus injuries.
Meanwhile, what is an obstetrician to do about shoulder dystocia? As always, give the best care you can. Know the risk factors. When possible, consider alternatives to vaginal delivery and be less aggressive in the management of labor. Know the techniques for resolving shoulder dystocia and have a preestablished plan for what to do.
Document, document, document.You can give the best care in the world, but if you cannot demonstrate on paper years down the road that you did so, our current liability system will make it seem as if you did not.
1. Professional Insurance Association of America risk management data, 2005.
2. Acker D, Sachs B, Friedman E. Risk factors for shoulder dystocia. Obstet Gynecol. 1985;66:762-768.
3. Al-Najashi S, Al-Suleiman S, El-Yahia A, Rahman M, Rahman J. Shoulder dystocia—a clinical study of 56 cases. Aust N Z J Obstet Gynaecol. 1989;29:129.-
4. Casey BM, Lucas MJ, McIntire DD, Leveno KJ. Pregnancy outcomes in women with gestational diabetes compared with the general obstetric population. Obstet Gynecol. 1997;90:869-873.
5. Sandmire HF, O’Halloin TJ. Shoulder dystocia: its incidence and associated risk factors. Int J Obstet Gynaecol. 1988;26:65-73.
6. Nesbitt TS, Gilbert WM, Herrchen B. Shoulder dystocia and associated risk factors with macrosomic infants born in California. Am J Obstet Gynecol. 1998;179:47-480.
7. Kolderup LB, Laros RK, Jr, Musci TJ. Incidence of persistent birth injury in macrosomic infants: association with mode of delivery. Am J Obstet Gynecol. 1997;177:37-41.
8. Emerson R. Obesity and its association with the complications of pregnancy. Br Med J. 1962;2:516-519.
9. Smith RB, Lane C, Pearson JF. Shoulder dystocia: what happens at the next delivery? Br J Obstet Gynaecol. 1994;101:713-715.
10. Ginsberg NA, Moisidis C. How to predict recurrent shoulder dystocia. Am J Obstet Gynecol. 2001;184:1427-1430.
11. Gherman RB. Shoulder dystocia: an evidence-based evaluation of the obstetrical nightmare. Clin Obstet Gynecol. 2002;45:345-361.
12. Baskett TF, Allen AC. Perinatal implications of shoulder dystocia. Obstet Gynecol. 1995;86:14-17.
13. Lewis DF, Edwards MS, Asrat T, et al. Can shoulder dystocia be predicted? Preconceptual and prenatal factors. J Reprod Med. 1998;43:654-658.
14. Rouse DJ, Owen J, Goldenberg RL, Cliver SP. The effectiveness and costs of elective cesarean delivery for fetal macrosomia diagnosed by ultrasound. JAMA. 1996;276:1480-1486.
15. Gherman RB, Ouzounian JG, Goodwin TM. Brachial plexus palsy: an in utero injury? Am J Obstet Gynecol. 1999;180:1303-1307.
16. Delpapa DH, Mueller-Heubach E. Pregnancy outcome following ultrasound diagnosis of macrosomia. Obstet Gynecol. 1991;78:340-343.
17. Gonen O, Rosen DJ, Dolfin Z, et al. Induction of labor versus expectant management in macrosomia: a randomized study. Obstet Gynecol. 1997;89:913-917.
18. Leaphart WL, Meyer MC, Capeless EL. Labor induction with a prenatal diagnosis of fetal macrosomia. J Matern Fetal Med. 1997;6:99-102.
19. American College of Obstetricians and Gynecologists. ACOG Practice Bulletin #40: Shoulder Dystocia. Washington, DC: ACOG; November 2002.
20. Elliott JP, Garite TJ, Freeman RK, McQuown DS, Patel JM. Ultrasonic prediction of fetal macrosomia in diabetic patients. Obstet Gynecol. 1982;60:159-162.
21. Gherman RB. Persistent brachial plexus injury: the outcome of concern among patients with suspected fetal macrosomia. Am J Obstet Gynecol. 1998;178:195.-
22. McFarland MB, Langer O, Piper JM, Berkus MD. Perinatal outcome and the type and number of maneuvers in shoulder dystocia. Int J Obstet Gynaecol. 1996;55:219-224.
23. Bofill JA, Rust OA, Devidas M, et al. Shoulder dystocia and operative vaginal delivery. J Matern Fetal Med. 1997;6:220-224.
24. Johnson NR. Shoulder dystocia: a study of 47 cases. Aust N Z J Obstet Gynaecol. 1979;19:28-31.
25. Nocon JJ, Weisbrod L. Shoulder dystocia. Chapter 14. In: O’Grady JP, Gimovsky M, eds. Operative Obstetrics. Philadelphia: Williams & Wilkins; 1995:339-353.
26. Creasy RK, Resnik R. Maternal-Fetal Medicine. 5th ed. Philadelphia: Saunders; 2004:690-691.
27. Nichols DH, DeLancey JO, eds. Clinical Problems, Injuries and Complications of Gynecologic and Obstetric Surgery. Baltimore: Williams & Wilkins; 1995:447.
28. Hopewood HG. Shoulder dystocia: fifteen years’ experience in a community hospital. Am J Obstet Gynecol. 1982;144:162-166.
29. Benedetti TJ, Gabbe SG. Shoulder dystocia: a complication of fetal macrosomia and prolonged second stage of labor with midpelvic delivery. Obstet Gynecol. 1978;52:526-529.
30. McFarland LV, Raskin M, Daling JR, Benedetti TJ. Erb/Duchenne’s palsy: a consequence of fetal macrosomia and method of delivery. Obstet Gynecol. 1986;68:784-788.
31. Gonik B, Stringer CA, Held B. An alternate mechanism for management of shoulder dystocia. Am J Obstet Gynecol. 1983;145:882-884.
32. Woods CE. A principle of physics as applicable to shoulder delivery. Am J Obstet Gynecol. 1943;45:796-804.
33. Bruner JP, Drummond SB, Meenan AL, Gaskin IM. All-fours maneuver for reducing shoulder dystocia during labor. J Reprod Med. 1998;43:439-443.
34. Sandberg EC. The Zavanelli maneuver: a potentially revolutionary method for the resolution of shoulder dystocia. Am J Obstet Gynecol. 1985;152:479.-
35. Sandberg EC. The Zavanelli maneuver: 12 years of recorded experience. Obstet Gynecol. 1999;93:312-317.
36. Gurewitsch ED, Donithan M, Stalllings SP, et al. Episiotomy versus fetal manipulation in managing severe shoulder dystocia: a comparison of outcomes. Am J Obstet Gynecol. 2004;191:911-916.
37. Dyachenko A, Ciampi A, Fahey J, et al. Prediction of risk for shoulder dystocia with neonatal injury. Am J Obstet Gynecol. 2006 Jul 14 [Epub ahead of print].
38. DeCherney AH, Pernoll ML, eds. Lange Obstetric and Gynecologic Diagnosis and Treatment. 8th ed. Norwalk, Conn: Appleton & Lange; 1994:219.
39. Allen RH, Gurewitsch ED. Temporary Erb-Duchenne palsy without shoulder dystocia or traction to the fetal head. Obstet Gynecol. 2005;105:1210-1212.
Dr. Lerner is a consultant for LMS Medical Services.
If permanent injury occurs after shoulder dystocia, it can also trigger a lawsuit that can last for years and end in a large jury verdict—even if you handled the case with textbook perfection. Lawsuits involving brachial plexus injuries following shoulder dystocia are now the second most common type of lawsuit in obstetrics, exceeded only by those due to neurologic damage from birth asphyxia.1 Brachial plexus injury is often difficult to defend in court and results in scores of millions of dollars in damages each year. The plaintiff is usually a lovely child with an obvious and permanent injury, and the defense is typically an undocumented claim that the obstetrician applied no undue force at delivery.(Sidebar)
Given the difficulties of knowing when shoulder dystocia will occur, how best to resolve it, and whether a claim is likely, how can we prepare for this event? What is the accepted standard of care? This article answers these questions by surveying the evidence on these aspects of management:
- risk factors for shoulder dystocia
- how to choose mode of delivery
- specific labor-management practices
- the 4 most widely used maneuvers to resolve shoulder dystocia
- what information the documentation should include.
No single “standard of care”
In many states, the term “standard of care” has a specific legal meaning, but in most of the United States—and to most physicians— the term means care that would be rendered by the majority of well-trained individuals. Complicating this definition is the fact that medicine often offers no single “right way.” Thus, it may be more appropriate to speak of “standards of care”: the range of therapeutic choices a reasonable practitioner might decide to use.
Traction is the most used and abused of terms in shoulder dystocia lawsuits. Many plaintiff expert witnesses claim that traction should never be applied to a baby’s head during delivery. Other “experts” claim only “gentle” traction is warranted. These statements are designed to support the most frequent contention against obstetricians when permanent brachial plexus injury occurs: As there is an injury, it must have been caused by a doctor or midwife who used “excessive traction” to deliver the baby. This statement is usually made without defining “excessive” and without evidence that more force than necessary was used.
“Excessive” vs “minimum necessary” traction
Routine or “moderate” traction is used in most deliveries. The birth attendant almost always depresses the fetal head and applies a moderate amount of traction to it to help the baby’s anterior shoulder slide beneath the mother’s pubic bone.38 The only time traction is unnecessary is when the expulsive forces of the mother are so strong or uncontrolled that she pushes the baby out entirely on her own.
There is ambiguity—often contrived—about what exactly constitutes mild, moderate, routine, and “excessive” traction. No study has ever been published that accurately and unambiguously quantifies the amount of force used in actual deliveries.
Once shoulder dystocia is diagnosed, further attempts at routine traction without the use of other maneuvers should be avoided. At best these attempts are unavailing. At worst they serve only to keep the anterior shoulder lodged behind the maternal symphysis.
Much misinformation surrounds the role of traction during the McRoberts maneuver and other efforts to resolve dystocia. The reality is simple: An obstetrician cannot determine whether a maneuver has released the anterior shoulder unless moderate traction is applied after the maneuver to see if the baby can be delivered. Although extreme force at this or any point is not appropriate, moderate traction is entirely appropriate.
“Excessive traction” is an oxymoron, although plaintiff lawyers often use the term. An obstetrician uses a given amount of force in attempting to free a stuck shoulder. Once the shoulder is freed, no more force is applied. Thus, by definition, “excessive force”—more force than is necessary to deliver the baby—is never used. The proper term to describe the amount of force applied by a physician to resolve shoulder dystocia is “minimum necessary traction.”
Injury can follow a traction-free delivery
For many years, obstetricians familiar with shoulder dystocia have claimed that brachial plexus injuries can occur even in the absence of significant traction—either in utero or as a result of the natural forces of labor. Yet plaintiff attorneys and expert witnesses have contended that all brachial plexus injuries are the result of someone pulling “too hard.”
A recent case reported by Allen and Gurewitsch39 settled this question once and for all. They describe a delivery in which a patient requested no intervention of any kind. Despite no hand having touched the baby during delivery—thus, no “excessive traction” having been applied —the baby suffered a brachial plexus injury. This case proved that brachial plexus injuries can occur spontaneously and are not necessarily caused by traction.
Why dystocia cannot be predicted
…despite known risk factors
The risk of shoulder dystocia is higher in women with diabetes,2-5 a macrosomic fetus,2,6-8 obesity,5,8 or a previous shoulder dystocia.9-11 The problem: The predictive value of these factors is so low and their false-positive rate so high they cannot be used reliably in clinical decision-making.11-13
Prevention is impossible
Even if prediction were possible, the only preventive option is elective cesarean section. After all, this is the only intervention that might potentially avoid the infrequent but dreaded outcomes of asphyxia and permanent brachial plexus injury. But as the literature shows, even this is not an absolute guarantee.14,15 Moreover, the strategy of inducing labor several weeks prior to the due date to prevent a baby from becoming “too big” has been shown in many studies to be ineffective in lowering the shoulder dystocia rate.16-18
Risk factors are not clinically useful
The American College of Obstetricians and Gynecologists (ACOG) and Williams Obstetrics concur that risk factors for shoulder dystocia cannot be applied in a clinically useful way to prevent brachial plexus injury. As the ACOG practice bulletin on shoulder dystocia19 observes:
- “Shoulder dystocia cannot be predicted or prevented because accurate methods for identifying which fetuses will experience this complication do not exist.”
- “Elective induction of labor or elective cesarean delivery for all women suspected of carrying a fetus with macrosomia is not appropriate.”
Identify highest risk
Nevertheless, there are generally accepted guidelines for attempting to ascertain which patients are at the absolute highest risk for shoulder dystocia:
- Any woman with gestational diabetes. For any given week of gestation in the third trimester, the ratio of thorax and shoulder size to head volume is larger in babies of diabetic mothers.20 Thus, in these women, it is important to estimate fetal weight near term to determine whether a trial of vaginal delivery makes sense.
- If, for any reason, the fetus appears to be larger than average. Indications of size may come from palpation of the maternal abdomen, fundal height measurements significantly greater than dates, ultrasound estimation of large fetal weight, or maternal perception. In these cases, ultrasound imaging is advisable near term to estimate fetal weight. This estimate can be factored into the selection of delivery mode.
How big is “too big”?
There are 2 problems with using estimates of fetal weight in determining mothers and babies at highest risk:
- How is “too big” defined?
- What action should one take if a baby is thought to be “too big”?
As for what to do if a fetus is estimated to be in this size range, ACOG states: “Planned cesarean delivery to prevent shoulder dystocia may be considered [emphasis added] for suspected fetal macrosomia within the above weight parameters.”19 The decision as to whether to recommend or perform a cesarean section in these circumstances is intentionally left up to the physician and the patient.
The problem, of course, is that all our data are from measurements of babies after delivery—information obstetricians do not have at the time they must decide on the mode of delivery.
TABLE
How fetal weight affects the rate of dystocia
| ESTIMATED FETAL WEIGHT | RATE OF SHOULDER DYSTOCIA (%) | |
|---|---|---|
| NONDIABETIC MOTHERS | DIABETIC MOTHERS | |
| 1.1 | 3.7 | |
| 4,000–4,499 g | 10 | 23.1 |
| >5,000 g | 22.6 | 50 |
| Source: Acker D et al2 | ||
Choosing a mode of delivery: Not so simple
The obstetrician must determine whether the risk of shoulder dystocia is high enough to outweigh the risks to a mother of elective cesarean section. This is far from simple. Although it is true that women at the highest risk for dystocia—those with gestational diabetes and suspected macrosomia— have a risk for shoulder dystocia somewhere between 25% and 50%, this is not the main concern.
The main concern is this: What percentage of even these high-risk patients will have a shoulder dystocia that results in a permanent brachial plexus injury? The answer: Permanent injury is rare, even in highest-risk cases.
Only 10% to 20% of infants born after shoulder dystocia suffer brachial plexus injuries.16,21-23 Of these, only 10% to 15% are permanently injured.5,24,25 Thus, even in women at highest risk, the odds of having an infant with permanent brachial plexus injury are roughly 1 in 450.14 In women at lower risk for shoulder dystocia, the odds of permanent brachial plexus injury are much lower: somewhere between 1 in 2,500 and 1 in 10,000.
When is cesarean section warranted?
In deciding the answer to this question, the obstetrician must consider that cesarean section is not without its own risks: excessive bleeding, infection, injury to bowel or bladder, deep venous thrombosis, and the need for hysterectomy.
These adverse events occur much more frequently than does permanent brachial plexus injury.26 And the risks are higher yet for the very same patients at greatest risk for shoulder dystocia—diabetic and obese women.
Prevent “I didn’t know” accusations
This is the point at which the patient’s input becomes vital. It is important to convey to her in readily understandable terms the risks—to both her and her child—of cesarean section versus attempted vaginal delivery. Plaintiff attorneys often claim that, had their client known there was a 1 in 450 chance of her baby having a permanent injury, she would have opted for cesarean section. The truth of this claim is, of course, open to question. However, from a medicolegal perspective, it is extremely important that the woman be informed of the degree of risk to herself and her baby so that her decision is truly informed—even if it is not the choice the obstetrician would have made.
The consensus in surgery is that the patient should be informed when the threshold of risk for an adverse event reaches 1% or higher. Although it is an informal teaching, this threshold is documented in the medical literature.27
The option of cesarean section should be discussed and possibly recommended for all women whose infants are estimated to weigh more than 5,000 g in the absence of diabetes and 4,500 g or more in women with diabetes.
Often a mother will voice concern about whether she will be able to deliver her baby safely vaginally. She may feel that her infant is too big, that she is too small, or that her obesity will make her delivery more difficult. Do not blithely ignore such concerns or provide blanket reassurances that everything will be OK.
Instead, review with her any risk factors she may have for shoulder dystocia and discuss the specific odds of injury to her baby should dystocia arise. Then discuss the risks to her and the discomfort she will experience if she elects a cesarean section.
Patients have a right to know the risks
Although it is appropriate to be reassuring when there are no significant risk factors, patients deserve to know what risks they run and to have these risks put into perspective. For example, if the mother has diabetes and her baby is estimated to weigh over 4,500 g, the risk of permanent brachial plexus injury approaches 1 in 450. The same is true if she is nondiabetic but has an estimated fetal weight of 5,000 g or more.
In high-risk cases such as these, you should discuss the risks with the patient and have her participate in the decisionmaking. You should also clearly document this discussion in the medical record.
Labor management
Prolonged second stage and instrumental delivery
Although the literature is not clear on this point, there is a trend toward increased rates of shoulder dystocia with a prolonged second stage of labor2,3,28 and with instrumental deliveries.6,12,29,30 Most experts believe this trend merely reflects the fact that bigger babies—the known major risk factor for shoulder dystocia—encounter these sorts of labor problems more frequently than do smaller babies. Whatever the reason, it warrants attention. An obstetrician’s care of any laboring woman should follow standard practices regarding arrest of labor and descent or a prolonged second stage.
Plaintiffs are quick to condemn vacuum and forceps
The same applies to intervention with forceps or vacuum. Only in women at highest risk for shoulder dystocia—those with diabetes or with suspected macrosomic fetuses—should standard management be modified.
Given the potential for shoulder dystocia in such high-risk circumstances, not to mention our inability to predict dystocia, prudence dictates that we avoid aggressive management and the use of forceps or vacuum in these cases.
These practices are often condemned in court by plaintiff lawyers and their expert witnesses.
Oxytocin is OK
In cases of arrest of labor and descent, the use of oxytocin is appropriate. A laboring woman should be given adequate time to deliver on her own, especially if a regional anesthetic has been used.
…but prepare to act quickly. In high-risk cases, be prepared to move more quickly than normal to cesarean section.
Is your team prepared? 4 standards of care
Although it is true that an obstetrician must be prepared for the possibility of shoulder dystocia in any delivery, to act as though it will occur in all deliveries is simply not reasonable, given that the rate of dystocia is 0.5% to 1.5%, or 1 in 67 to 200 deliveries.12,21,25,29
Nevertheless, 4 specific standards apply to all delivery facilities:
- The entire labor and delivery staff should know what to do and what each person’s role is when shoulder dystocia is diagnosed.
- Labor and delivery nurses should know how and when to initiate McRoberts maneuver and apply suprapubic pressure.
- The team should immediately obtain the assistance of another obstetrician, a pediatrician, and an anesthesiologist, even though they are not likely to arrive before the dystocia is resolved.
- The obstetrician should be mentally prepared for the possibility of shoulder dystocia. This requires the ability to quickly recognize it, familiarity with the various techniques for resolving it, and avoidance of unnecessary traction. It also is vital for the obstetrician to remain composed and in charge, as the obstetrician becomes the leader of the medical team when this emergency arises.
How to recognize shoulder dystocia
There are 2 ways to diagnose dystocia.
- “Turtle sign.” The first is recognizing the pathognomonic “turtle sign,” in which, after delivery of the baby’s head, the head immediately retracts back up against the mother’s perineum, causing the baby’s cheeks to bulge.
- The second diagnostic sign is when, after delivery of the head, the moderate amount of traction usually used does not suffice to deliver the anterior shoulder. Cease attempts at routine traction as soon as shoulder dystocia is diagnosed.
The 4 main maneuvers
The 4 maneuvers generally used by obstetricians to resolve shoulder dystocia are considered the standard of care:
- McRoberts maneuver
- Suprapubic pressure
- Woods screw maneuver
- Delivery of the posterior arm
McRoberts maneuver is often the only one needed
In this maneuver, the laboring woman’s thighs are hyperflexed against her abdomen.31 This hyperflexion does not increase the diameter of the pelvis, as is sometimes claimed. Rather, it flattens the sacrum and changes the angle of the symphysis pubis in relation to the baby’s anterior shoulder, often freeing it. It is an extremely effective way to resolve shoulder dystocia and is often the only maneuver necessary.
Family members can assist—contrary to plaintiff attorney contentions. This maneuver can be performed by nurses or family members if they are properly instructed. Plaintiff attorneys will sometimes argue that the use of family members in this situation is inappropriate, but they are wrong. Family members are sometimes instructed to hold a mother’s legs in a certain position while she is pushing; they can certainly be instructed to hold the legs against the maternal abdomen during attempts to resolve a shoulder dystocia.
Suprapubic pressure with or without McRoberts
In this maneuver, a nurse or other attendant places direct pressure with an open hand or fist just above the mother’s symphysis pubis. The pressure can be directed straight down or to the left or right. Wherever it is directed, the aim of the pressure is to push the baby’s anterior shoulder out of its position behind the mother’s pubic bone.
The combination of McRoberts maneuver and suprapubic pressure can resolve shoulder dystocia in as many as 58% of cases.22
Woods screw maneuver attempts to “spin” the baby
If the McRoberts maneuver and suprapubic pressure do not resolve the shoulder dystocia, the Woods screw maneuver is usually implemented next.32 In this maneuver, the obstetrician inserts a hand into the posterior vagina and pushes the front of the baby’s posterior shoulder in a spiral direction (clockwise or counterclockwise). The goal is to “unjam” the anterior shoulder from its trapped position behind the symphysis pubis.
The Woods screw maneuver is very effective. After it has been used, it is appropriate to apply moderate traction to the baby’s head to determine whether the baby can be delivered.
Variant: Rubens maneuver. In this maneuver, the obstetrician pushes on the posterior aspect of the posterior shoulder. In addition to spinning the shoulders, as in the Woods screw maneuver, the Rubens maneuver causes shoulder abduction, thus decreasing the biacromial diameter that has to pass through the pelvic outlet.
Attempts to deliver the posterior arm
If shoulder dystocia still persists, the next strategy is usually an attempt to deliver the baby’s posterior arm. This is done by placing a hand deep into the posterior aspect of the vagina, grabbing the baby’s posterior arm, sweeping that arm across the baby’s chest, and delivering it. Once the posterior arm and shoulder are delivered, it is almost always possible to deliver the baby directly from this position or to move the baby in a spiral direction (clockwise or counterclockwise) to free the anterior shoulder.
Other maneuvers
Two other maneuvers are occasionally used, though neither is considered mainstream.
Gaskin or “all fours” maneuver. This technique is frequently advocated by the midwife community.33 It involves moving the laboring woman from the standard lithotomy pushing position to her hands and knees to free the stuck anterior shoulder. However, many have questioned the practicality of turning a fatigued, laboring woman rapidly enough to deliver a baby within the 4 to 6 minutes available, particularly when an epidural has been given or other maneuvers have already used up much of the allotted time.
Zavanelli maneuver if all else fails. This maneuver should be attempted only when all other efforts have failed.34 It involves flexing the fetal head and attempting to push the baby’s head back into the vagina, followed by emergency cesarean section.
Although case reports have described successful use of this maneuver, there also have been reports of fetal death, fractured spines, and other severe fetal damage. Thus, this maneuver should be the absolute last resort in desperate emergencies.35
What not to do
Traction
Do not continue to apply traction to the fetal head if the shoulder does not come. Once shoulder dystocia is diagnosed, cease all attempts to deliver the baby by continued pulling. Carefully but expeditiously use the various maneuvers you were trained to do, applying moderate traction after each one to see if the shoulder has been freed.
Fundal pressure
Do not apply fundal pressure. It never helps resolve shoulder dystocia, but only further jams the stuck shoulder against the maternal pubic bone. It also can cause injury to the fetus or even rupture the uterus.
Fundal pressure is often cited in court as a definite standard of care violation.
Theory vs evidence
A 3-member team is adequate
Shoulder dystocia occurs unexpectedly. Once it does occur, the obstetrician has 4 to 6 minutes to resolve it before the threat of central neurologic damage to the baby becomes significant. Although it would be very helpful for additional personnel to be available, it is not always possible to assemble this team quickly enough.
In reality, the only personnel truly necessary to resolve a shoulder dystocia are:
- The delivering doctor or midwife
- A medically trained assistant familiar with McRoberts maneuver and suprapubic pressure
- Any other available person, including a family member, who can be drafted to help and instructed to participate in the McRoberts maneuver by flexing one of the mother’s thighs
Drills are not an absolute necessity
It is sometimes claimed that formal shoulder dystocia drills should be conducted in labor and delivery units at fixed intervals. Although this may be a useful and reasonable educational practice, it is more important that each individual on the labor and delivery team know what his or her role is during such an emergency. Whether this is achieved through a practice drill or didactic instruction does not matter.
In short, there is nothing about the concept of a drill that is “standard of care.” What is standard of care is that every team member knows what to do, how to do it, when to do it, and how to document it.
Episiotomy is often superfluous
Multiple studies have shown that episiotomy is not necessary to resolve shoulder dystocia, although many textbooks and other published protocols still recommend it.36 The obstructing factor in shoulder dystocia is not the soft tissue of the perineum but the symphysis pubis. The only time episiotomy helps is when more room is needed for the obstetrician’s hand to enter the posterior aspect of the vagina to perform a shoulder dystocia maneuver. If you can perform all necessary maneuvers without episiotomy, it is superfluous.
Document early and always
Because shoulder dystocia often leads to litigation, it is extremely important to document what happened during delivery as soon as feasible and in as much detail as possible. Standardized forms are now available. (FORM)
At minimum, you should record:
- how shoulder dystocia was diagnosed
- which shoulder was anterior and which was posterior
- quantification of the force applied initially and in subsequent traction attempts, using terms such as “mild,” “moderate,” or “significant”
- duration of attempts to resolve the dystocia
- maneuvers performed
- approximate length of time each maneuver was tried
- condition of the baby at delivery, including Apgar scores, a description of all injuries and bruises, and cord pH, if obtained
- time from delivery of the fetal head to delivery of the body
- documentation of the discussion with the patient following delivery
Given that the most frequent criticism of obstetricians in the courtroom in brachial plexus injury lawsuits is that they pulled too hard, the best defense consists of careful, complete, and contemporaneous documentation of one’s actions at delivery.
Lawsuits happen
Even when everything is done correctly, there is a very high likelihood that a lawsuit will be filed when there is a permanent brachial plexus injury.
The 2 claims generally made against obstetricians are:
- The obstetrician should have known or predicted that the risk of shoulder dystocia was high, and should have performed a cesarean section or at least offered the mother that choice.
- As the baby has a permanent brachial plexus injury, the obstetrician must have pulled too hard at delivery.
The best defense
The best defense is, as always, to have practiced good medicine and to have documented it. You must be able to demonstrate from your records—years after a delivery that you no longer remember—that you:
- made appropriate prenatal judgments and were aware of risk factors
- informed the mother of such risk factors when they are significant
- provided proper obstetrical care
- documented in the medical record that you knew what you were doing and did it correctly
Some good news is on the horizon. Recent research has produced a mathematical tool that appears to be able to predict 50% to 75% of all women destined to have shoulder dystocia, with a false-positive rate of only 2% to 3%.37 If this model holds up under further investigation, it may become possible to avoid most shoulder dystocia deliveries and, with them, permanent brachial plexus injuries.
Meanwhile, what is an obstetrician to do about shoulder dystocia? As always, give the best care you can. Know the risk factors. When possible, consider alternatives to vaginal delivery and be less aggressive in the management of labor. Know the techniques for resolving shoulder dystocia and have a preestablished plan for what to do.
Document, document, document.You can give the best care in the world, but if you cannot demonstrate on paper years down the road that you did so, our current liability system will make it seem as if you did not.
If permanent injury occurs after shoulder dystocia, it can also trigger a lawsuit that can last for years and end in a large jury verdict—even if you handled the case with textbook perfection. Lawsuits involving brachial plexus injuries following shoulder dystocia are now the second most common type of lawsuit in obstetrics, exceeded only by those due to neurologic damage from birth asphyxia.1 Brachial plexus injury is often difficult to defend in court and results in scores of millions of dollars in damages each year. The plaintiff is usually a lovely child with an obvious and permanent injury, and the defense is typically an undocumented claim that the obstetrician applied no undue force at delivery.(Sidebar)
Given the difficulties of knowing when shoulder dystocia will occur, how best to resolve it, and whether a claim is likely, how can we prepare for this event? What is the accepted standard of care? This article answers these questions by surveying the evidence on these aspects of management:
- risk factors for shoulder dystocia
- how to choose mode of delivery
- specific labor-management practices
- the 4 most widely used maneuvers to resolve shoulder dystocia
- what information the documentation should include.
No single “standard of care”
In many states, the term “standard of care” has a specific legal meaning, but in most of the United States—and to most physicians— the term means care that would be rendered by the majority of well-trained individuals. Complicating this definition is the fact that medicine often offers no single “right way.” Thus, it may be more appropriate to speak of “standards of care”: the range of therapeutic choices a reasonable practitioner might decide to use.
Traction is the most used and abused of terms in shoulder dystocia lawsuits. Many plaintiff expert witnesses claim that traction should never be applied to a baby’s head during delivery. Other “experts” claim only “gentle” traction is warranted. These statements are designed to support the most frequent contention against obstetricians when permanent brachial plexus injury occurs: As there is an injury, it must have been caused by a doctor or midwife who used “excessive traction” to deliver the baby. This statement is usually made without defining “excessive” and without evidence that more force than necessary was used.
“Excessive” vs “minimum necessary” traction
Routine or “moderate” traction is used in most deliveries. The birth attendant almost always depresses the fetal head and applies a moderate amount of traction to it to help the baby’s anterior shoulder slide beneath the mother’s pubic bone.38 The only time traction is unnecessary is when the expulsive forces of the mother are so strong or uncontrolled that she pushes the baby out entirely on her own.
There is ambiguity—often contrived—about what exactly constitutes mild, moderate, routine, and “excessive” traction. No study has ever been published that accurately and unambiguously quantifies the amount of force used in actual deliveries.
Once shoulder dystocia is diagnosed, further attempts at routine traction without the use of other maneuvers should be avoided. At best these attempts are unavailing. At worst they serve only to keep the anterior shoulder lodged behind the maternal symphysis.
Much misinformation surrounds the role of traction during the McRoberts maneuver and other efforts to resolve dystocia. The reality is simple: An obstetrician cannot determine whether a maneuver has released the anterior shoulder unless moderate traction is applied after the maneuver to see if the baby can be delivered. Although extreme force at this or any point is not appropriate, moderate traction is entirely appropriate.
“Excessive traction” is an oxymoron, although plaintiff lawyers often use the term. An obstetrician uses a given amount of force in attempting to free a stuck shoulder. Once the shoulder is freed, no more force is applied. Thus, by definition, “excessive force”—more force than is necessary to deliver the baby—is never used. The proper term to describe the amount of force applied by a physician to resolve shoulder dystocia is “minimum necessary traction.”
Injury can follow a traction-free delivery
For many years, obstetricians familiar with shoulder dystocia have claimed that brachial plexus injuries can occur even in the absence of significant traction—either in utero or as a result of the natural forces of labor. Yet plaintiff attorneys and expert witnesses have contended that all brachial plexus injuries are the result of someone pulling “too hard.”
A recent case reported by Allen and Gurewitsch39 settled this question once and for all. They describe a delivery in which a patient requested no intervention of any kind. Despite no hand having touched the baby during delivery—thus, no “excessive traction” having been applied —the baby suffered a brachial plexus injury. This case proved that brachial plexus injuries can occur spontaneously and are not necessarily caused by traction.
Why dystocia cannot be predicted
…despite known risk factors
The risk of shoulder dystocia is higher in women with diabetes,2-5 a macrosomic fetus,2,6-8 obesity,5,8 or a previous shoulder dystocia.9-11 The problem: The predictive value of these factors is so low and their false-positive rate so high they cannot be used reliably in clinical decision-making.11-13
Prevention is impossible
Even if prediction were possible, the only preventive option is elective cesarean section. After all, this is the only intervention that might potentially avoid the infrequent but dreaded outcomes of asphyxia and permanent brachial plexus injury. But as the literature shows, even this is not an absolute guarantee.14,15 Moreover, the strategy of inducing labor several weeks prior to the due date to prevent a baby from becoming “too big” has been shown in many studies to be ineffective in lowering the shoulder dystocia rate.16-18
Risk factors are not clinically useful
The American College of Obstetricians and Gynecologists (ACOG) and Williams Obstetrics concur that risk factors for shoulder dystocia cannot be applied in a clinically useful way to prevent brachial plexus injury. As the ACOG practice bulletin on shoulder dystocia19 observes:
- “Shoulder dystocia cannot be predicted or prevented because accurate methods for identifying which fetuses will experience this complication do not exist.”
- “Elective induction of labor or elective cesarean delivery for all women suspected of carrying a fetus with macrosomia is not appropriate.”
Identify highest risk
Nevertheless, there are generally accepted guidelines for attempting to ascertain which patients are at the absolute highest risk for shoulder dystocia:
- Any woman with gestational diabetes. For any given week of gestation in the third trimester, the ratio of thorax and shoulder size to head volume is larger in babies of diabetic mothers.20 Thus, in these women, it is important to estimate fetal weight near term to determine whether a trial of vaginal delivery makes sense.
- If, for any reason, the fetus appears to be larger than average. Indications of size may come from palpation of the maternal abdomen, fundal height measurements significantly greater than dates, ultrasound estimation of large fetal weight, or maternal perception. In these cases, ultrasound imaging is advisable near term to estimate fetal weight. This estimate can be factored into the selection of delivery mode.
How big is “too big”?
There are 2 problems with using estimates of fetal weight in determining mothers and babies at highest risk:
- How is “too big” defined?
- What action should one take if a baby is thought to be “too big”?
As for what to do if a fetus is estimated to be in this size range, ACOG states: “Planned cesarean delivery to prevent shoulder dystocia may be considered [emphasis added] for suspected fetal macrosomia within the above weight parameters.”19 The decision as to whether to recommend or perform a cesarean section in these circumstances is intentionally left up to the physician and the patient.
The problem, of course, is that all our data are from measurements of babies after delivery—information obstetricians do not have at the time they must decide on the mode of delivery.
TABLE
How fetal weight affects the rate of dystocia
| ESTIMATED FETAL WEIGHT | RATE OF SHOULDER DYSTOCIA (%) | |
|---|---|---|
| NONDIABETIC MOTHERS | DIABETIC MOTHERS | |
| 1.1 | 3.7 | |
| 4,000–4,499 g | 10 | 23.1 |
| >5,000 g | 22.6 | 50 |
| Source: Acker D et al2 | ||
Choosing a mode of delivery: Not so simple
The obstetrician must determine whether the risk of shoulder dystocia is high enough to outweigh the risks to a mother of elective cesarean section. This is far from simple. Although it is true that women at the highest risk for dystocia—those with gestational diabetes and suspected macrosomia— have a risk for shoulder dystocia somewhere between 25% and 50%, this is not the main concern.
The main concern is this: What percentage of even these high-risk patients will have a shoulder dystocia that results in a permanent brachial plexus injury? The answer: Permanent injury is rare, even in highest-risk cases.
Only 10% to 20% of infants born after shoulder dystocia suffer brachial plexus injuries.16,21-23 Of these, only 10% to 15% are permanently injured.5,24,25 Thus, even in women at highest risk, the odds of having an infant with permanent brachial plexus injury are roughly 1 in 450.14 In women at lower risk for shoulder dystocia, the odds of permanent brachial plexus injury are much lower: somewhere between 1 in 2,500 and 1 in 10,000.
When is cesarean section warranted?
In deciding the answer to this question, the obstetrician must consider that cesarean section is not without its own risks: excessive bleeding, infection, injury to bowel or bladder, deep venous thrombosis, and the need for hysterectomy.
These adverse events occur much more frequently than does permanent brachial plexus injury.26 And the risks are higher yet for the very same patients at greatest risk for shoulder dystocia—diabetic and obese women.
Prevent “I didn’t know” accusations
This is the point at which the patient’s input becomes vital. It is important to convey to her in readily understandable terms the risks—to both her and her child—of cesarean section versus attempted vaginal delivery. Plaintiff attorneys often claim that, had their client known there was a 1 in 450 chance of her baby having a permanent injury, she would have opted for cesarean section. The truth of this claim is, of course, open to question. However, from a medicolegal perspective, it is extremely important that the woman be informed of the degree of risk to herself and her baby so that her decision is truly informed—even if it is not the choice the obstetrician would have made.
The consensus in surgery is that the patient should be informed when the threshold of risk for an adverse event reaches 1% or higher. Although it is an informal teaching, this threshold is documented in the medical literature.27
The option of cesarean section should be discussed and possibly recommended for all women whose infants are estimated to weigh more than 5,000 g in the absence of diabetes and 4,500 g or more in women with diabetes.
Often a mother will voice concern about whether she will be able to deliver her baby safely vaginally. She may feel that her infant is too big, that she is too small, or that her obesity will make her delivery more difficult. Do not blithely ignore such concerns or provide blanket reassurances that everything will be OK.
Instead, review with her any risk factors she may have for shoulder dystocia and discuss the specific odds of injury to her baby should dystocia arise. Then discuss the risks to her and the discomfort she will experience if she elects a cesarean section.
Patients have a right to know the risks
Although it is appropriate to be reassuring when there are no significant risk factors, patients deserve to know what risks they run and to have these risks put into perspective. For example, if the mother has diabetes and her baby is estimated to weigh over 4,500 g, the risk of permanent brachial plexus injury approaches 1 in 450. The same is true if she is nondiabetic but has an estimated fetal weight of 5,000 g or more.
In high-risk cases such as these, you should discuss the risks with the patient and have her participate in the decisionmaking. You should also clearly document this discussion in the medical record.
Labor management
Prolonged second stage and instrumental delivery
Although the literature is not clear on this point, there is a trend toward increased rates of shoulder dystocia with a prolonged second stage of labor2,3,28 and with instrumental deliveries.6,12,29,30 Most experts believe this trend merely reflects the fact that bigger babies—the known major risk factor for shoulder dystocia—encounter these sorts of labor problems more frequently than do smaller babies. Whatever the reason, it warrants attention. An obstetrician’s care of any laboring woman should follow standard practices regarding arrest of labor and descent or a prolonged second stage.
Plaintiffs are quick to condemn vacuum and forceps
The same applies to intervention with forceps or vacuum. Only in women at highest risk for shoulder dystocia—those with diabetes or with suspected macrosomic fetuses—should standard management be modified.
Given the potential for shoulder dystocia in such high-risk circumstances, not to mention our inability to predict dystocia, prudence dictates that we avoid aggressive management and the use of forceps or vacuum in these cases.
These practices are often condemned in court by plaintiff lawyers and their expert witnesses.
Oxytocin is OK
In cases of arrest of labor and descent, the use of oxytocin is appropriate. A laboring woman should be given adequate time to deliver on her own, especially if a regional anesthetic has been used.
…but prepare to act quickly. In high-risk cases, be prepared to move more quickly than normal to cesarean section.
Is your team prepared? 4 standards of care
Although it is true that an obstetrician must be prepared for the possibility of shoulder dystocia in any delivery, to act as though it will occur in all deliveries is simply not reasonable, given that the rate of dystocia is 0.5% to 1.5%, or 1 in 67 to 200 deliveries.12,21,25,29
Nevertheless, 4 specific standards apply to all delivery facilities:
- The entire labor and delivery staff should know what to do and what each person’s role is when shoulder dystocia is diagnosed.
- Labor and delivery nurses should know how and when to initiate McRoberts maneuver and apply suprapubic pressure.
- The team should immediately obtain the assistance of another obstetrician, a pediatrician, and an anesthesiologist, even though they are not likely to arrive before the dystocia is resolved.
- The obstetrician should be mentally prepared for the possibility of shoulder dystocia. This requires the ability to quickly recognize it, familiarity with the various techniques for resolving it, and avoidance of unnecessary traction. It also is vital for the obstetrician to remain composed and in charge, as the obstetrician becomes the leader of the medical team when this emergency arises.
How to recognize shoulder dystocia
There are 2 ways to diagnose dystocia.
- “Turtle sign.” The first is recognizing the pathognomonic “turtle sign,” in which, after delivery of the baby’s head, the head immediately retracts back up against the mother’s perineum, causing the baby’s cheeks to bulge.
- The second diagnostic sign is when, after delivery of the head, the moderate amount of traction usually used does not suffice to deliver the anterior shoulder. Cease attempts at routine traction as soon as shoulder dystocia is diagnosed.
The 4 main maneuvers
The 4 maneuvers generally used by obstetricians to resolve shoulder dystocia are considered the standard of care:
- McRoberts maneuver
- Suprapubic pressure
- Woods screw maneuver
- Delivery of the posterior arm
McRoberts maneuver is often the only one needed
In this maneuver, the laboring woman’s thighs are hyperflexed against her abdomen.31 This hyperflexion does not increase the diameter of the pelvis, as is sometimes claimed. Rather, it flattens the sacrum and changes the angle of the symphysis pubis in relation to the baby’s anterior shoulder, often freeing it. It is an extremely effective way to resolve shoulder dystocia and is often the only maneuver necessary.
Family members can assist—contrary to plaintiff attorney contentions. This maneuver can be performed by nurses or family members if they are properly instructed. Plaintiff attorneys will sometimes argue that the use of family members in this situation is inappropriate, but they are wrong. Family members are sometimes instructed to hold a mother’s legs in a certain position while she is pushing; they can certainly be instructed to hold the legs against the maternal abdomen during attempts to resolve a shoulder dystocia.
Suprapubic pressure with or without McRoberts
In this maneuver, a nurse or other attendant places direct pressure with an open hand or fist just above the mother’s symphysis pubis. The pressure can be directed straight down or to the left or right. Wherever it is directed, the aim of the pressure is to push the baby’s anterior shoulder out of its position behind the mother’s pubic bone.
The combination of McRoberts maneuver and suprapubic pressure can resolve shoulder dystocia in as many as 58% of cases.22
Woods screw maneuver attempts to “spin” the baby
If the McRoberts maneuver and suprapubic pressure do not resolve the shoulder dystocia, the Woods screw maneuver is usually implemented next.32 In this maneuver, the obstetrician inserts a hand into the posterior vagina and pushes the front of the baby’s posterior shoulder in a spiral direction (clockwise or counterclockwise). The goal is to “unjam” the anterior shoulder from its trapped position behind the symphysis pubis.
The Woods screw maneuver is very effective. After it has been used, it is appropriate to apply moderate traction to the baby’s head to determine whether the baby can be delivered.
Variant: Rubens maneuver. In this maneuver, the obstetrician pushes on the posterior aspect of the posterior shoulder. In addition to spinning the shoulders, as in the Woods screw maneuver, the Rubens maneuver causes shoulder abduction, thus decreasing the biacromial diameter that has to pass through the pelvic outlet.
Attempts to deliver the posterior arm
If shoulder dystocia still persists, the next strategy is usually an attempt to deliver the baby’s posterior arm. This is done by placing a hand deep into the posterior aspect of the vagina, grabbing the baby’s posterior arm, sweeping that arm across the baby’s chest, and delivering it. Once the posterior arm and shoulder are delivered, it is almost always possible to deliver the baby directly from this position or to move the baby in a spiral direction (clockwise or counterclockwise) to free the anterior shoulder.
Other maneuvers
Two other maneuvers are occasionally used, though neither is considered mainstream.
Gaskin or “all fours” maneuver. This technique is frequently advocated by the midwife community.33 It involves moving the laboring woman from the standard lithotomy pushing position to her hands and knees to free the stuck anterior shoulder. However, many have questioned the practicality of turning a fatigued, laboring woman rapidly enough to deliver a baby within the 4 to 6 minutes available, particularly when an epidural has been given or other maneuvers have already used up much of the allotted time.
Zavanelli maneuver if all else fails. This maneuver should be attempted only when all other efforts have failed.34 It involves flexing the fetal head and attempting to push the baby’s head back into the vagina, followed by emergency cesarean section.
Although case reports have described successful use of this maneuver, there also have been reports of fetal death, fractured spines, and other severe fetal damage. Thus, this maneuver should be the absolute last resort in desperate emergencies.35
What not to do
Traction
Do not continue to apply traction to the fetal head if the shoulder does not come. Once shoulder dystocia is diagnosed, cease all attempts to deliver the baby by continued pulling. Carefully but expeditiously use the various maneuvers you were trained to do, applying moderate traction after each one to see if the shoulder has been freed.
Fundal pressure
Do not apply fundal pressure. It never helps resolve shoulder dystocia, but only further jams the stuck shoulder against the maternal pubic bone. It also can cause injury to the fetus or even rupture the uterus.
Fundal pressure is often cited in court as a definite standard of care violation.
Theory vs evidence
A 3-member team is adequate
Shoulder dystocia occurs unexpectedly. Once it does occur, the obstetrician has 4 to 6 minutes to resolve it before the threat of central neurologic damage to the baby becomes significant. Although it would be very helpful for additional personnel to be available, it is not always possible to assemble this team quickly enough.
In reality, the only personnel truly necessary to resolve a shoulder dystocia are:
- The delivering doctor or midwife
- A medically trained assistant familiar with McRoberts maneuver and suprapubic pressure
- Any other available person, including a family member, who can be drafted to help and instructed to participate in the McRoberts maneuver by flexing one of the mother’s thighs
Drills are not an absolute necessity
It is sometimes claimed that formal shoulder dystocia drills should be conducted in labor and delivery units at fixed intervals. Although this may be a useful and reasonable educational practice, it is more important that each individual on the labor and delivery team know what his or her role is during such an emergency. Whether this is achieved through a practice drill or didactic instruction does not matter.
In short, there is nothing about the concept of a drill that is “standard of care.” What is standard of care is that every team member knows what to do, how to do it, when to do it, and how to document it.
Episiotomy is often superfluous
Multiple studies have shown that episiotomy is not necessary to resolve shoulder dystocia, although many textbooks and other published protocols still recommend it.36 The obstructing factor in shoulder dystocia is not the soft tissue of the perineum but the symphysis pubis. The only time episiotomy helps is when more room is needed for the obstetrician’s hand to enter the posterior aspect of the vagina to perform a shoulder dystocia maneuver. If you can perform all necessary maneuvers without episiotomy, it is superfluous.
Document early and always
Because shoulder dystocia often leads to litigation, it is extremely important to document what happened during delivery as soon as feasible and in as much detail as possible. Standardized forms are now available. (FORM)
At minimum, you should record:
- how shoulder dystocia was diagnosed
- which shoulder was anterior and which was posterior
- quantification of the force applied initially and in subsequent traction attempts, using terms such as “mild,” “moderate,” or “significant”
- duration of attempts to resolve the dystocia
- maneuvers performed
- approximate length of time each maneuver was tried
- condition of the baby at delivery, including Apgar scores, a description of all injuries and bruises, and cord pH, if obtained
- time from delivery of the fetal head to delivery of the body
- documentation of the discussion with the patient following delivery
Given that the most frequent criticism of obstetricians in the courtroom in brachial plexus injury lawsuits is that they pulled too hard, the best defense consists of careful, complete, and contemporaneous documentation of one’s actions at delivery.
Lawsuits happen
Even when everything is done correctly, there is a very high likelihood that a lawsuit will be filed when there is a permanent brachial plexus injury.
The 2 claims generally made against obstetricians are:
- The obstetrician should have known or predicted that the risk of shoulder dystocia was high, and should have performed a cesarean section or at least offered the mother that choice.
- As the baby has a permanent brachial plexus injury, the obstetrician must have pulled too hard at delivery.
The best defense
The best defense is, as always, to have practiced good medicine and to have documented it. You must be able to demonstrate from your records—years after a delivery that you no longer remember—that you:
- made appropriate prenatal judgments and were aware of risk factors
- informed the mother of such risk factors when they are significant
- provided proper obstetrical care
- documented in the medical record that you knew what you were doing and did it correctly
Some good news is on the horizon. Recent research has produced a mathematical tool that appears to be able to predict 50% to 75% of all women destined to have shoulder dystocia, with a false-positive rate of only 2% to 3%.37 If this model holds up under further investigation, it may become possible to avoid most shoulder dystocia deliveries and, with them, permanent brachial plexus injuries.
Meanwhile, what is an obstetrician to do about shoulder dystocia? As always, give the best care you can. Know the risk factors. When possible, consider alternatives to vaginal delivery and be less aggressive in the management of labor. Know the techniques for resolving shoulder dystocia and have a preestablished plan for what to do.
Document, document, document.You can give the best care in the world, but if you cannot demonstrate on paper years down the road that you did so, our current liability system will make it seem as if you did not.
1. Professional Insurance Association of America risk management data, 2005.
2. Acker D, Sachs B, Friedman E. Risk factors for shoulder dystocia. Obstet Gynecol. 1985;66:762-768.
3. Al-Najashi S, Al-Suleiman S, El-Yahia A, Rahman M, Rahman J. Shoulder dystocia—a clinical study of 56 cases. Aust N Z J Obstet Gynaecol. 1989;29:129.-
4. Casey BM, Lucas MJ, McIntire DD, Leveno KJ. Pregnancy outcomes in women with gestational diabetes compared with the general obstetric population. Obstet Gynecol. 1997;90:869-873.
5. Sandmire HF, O’Halloin TJ. Shoulder dystocia: its incidence and associated risk factors. Int J Obstet Gynaecol. 1988;26:65-73.
6. Nesbitt TS, Gilbert WM, Herrchen B. Shoulder dystocia and associated risk factors with macrosomic infants born in California. Am J Obstet Gynecol. 1998;179:47-480.
7. Kolderup LB, Laros RK, Jr, Musci TJ. Incidence of persistent birth injury in macrosomic infants: association with mode of delivery. Am J Obstet Gynecol. 1997;177:37-41.
8. Emerson R. Obesity and its association with the complications of pregnancy. Br Med J. 1962;2:516-519.
9. Smith RB, Lane C, Pearson JF. Shoulder dystocia: what happens at the next delivery? Br J Obstet Gynaecol. 1994;101:713-715.
10. Ginsberg NA, Moisidis C. How to predict recurrent shoulder dystocia. Am J Obstet Gynecol. 2001;184:1427-1430.
11. Gherman RB. Shoulder dystocia: an evidence-based evaluation of the obstetrical nightmare. Clin Obstet Gynecol. 2002;45:345-361.
12. Baskett TF, Allen AC. Perinatal implications of shoulder dystocia. Obstet Gynecol. 1995;86:14-17.
13. Lewis DF, Edwards MS, Asrat T, et al. Can shoulder dystocia be predicted? Preconceptual and prenatal factors. J Reprod Med. 1998;43:654-658.
14. Rouse DJ, Owen J, Goldenberg RL, Cliver SP. The effectiveness and costs of elective cesarean delivery for fetal macrosomia diagnosed by ultrasound. JAMA. 1996;276:1480-1486.
15. Gherman RB, Ouzounian JG, Goodwin TM. Brachial plexus palsy: an in utero injury? Am J Obstet Gynecol. 1999;180:1303-1307.
16. Delpapa DH, Mueller-Heubach E. Pregnancy outcome following ultrasound diagnosis of macrosomia. Obstet Gynecol. 1991;78:340-343.
17. Gonen O, Rosen DJ, Dolfin Z, et al. Induction of labor versus expectant management in macrosomia: a randomized study. Obstet Gynecol. 1997;89:913-917.
18. Leaphart WL, Meyer MC, Capeless EL. Labor induction with a prenatal diagnosis of fetal macrosomia. J Matern Fetal Med. 1997;6:99-102.
19. American College of Obstetricians and Gynecologists. ACOG Practice Bulletin #40: Shoulder Dystocia. Washington, DC: ACOG; November 2002.
20. Elliott JP, Garite TJ, Freeman RK, McQuown DS, Patel JM. Ultrasonic prediction of fetal macrosomia in diabetic patients. Obstet Gynecol. 1982;60:159-162.
21. Gherman RB. Persistent brachial plexus injury: the outcome of concern among patients with suspected fetal macrosomia. Am J Obstet Gynecol. 1998;178:195.-
22. McFarland MB, Langer O, Piper JM, Berkus MD. Perinatal outcome and the type and number of maneuvers in shoulder dystocia. Int J Obstet Gynaecol. 1996;55:219-224.
23. Bofill JA, Rust OA, Devidas M, et al. Shoulder dystocia and operative vaginal delivery. J Matern Fetal Med. 1997;6:220-224.
24. Johnson NR. Shoulder dystocia: a study of 47 cases. Aust N Z J Obstet Gynaecol. 1979;19:28-31.
25. Nocon JJ, Weisbrod L. Shoulder dystocia. Chapter 14. In: O’Grady JP, Gimovsky M, eds. Operative Obstetrics. Philadelphia: Williams & Wilkins; 1995:339-353.
26. Creasy RK, Resnik R. Maternal-Fetal Medicine. 5th ed. Philadelphia: Saunders; 2004:690-691.
27. Nichols DH, DeLancey JO, eds. Clinical Problems, Injuries and Complications of Gynecologic and Obstetric Surgery. Baltimore: Williams & Wilkins; 1995:447.
28. Hopewood HG. Shoulder dystocia: fifteen years’ experience in a community hospital. Am J Obstet Gynecol. 1982;144:162-166.
29. Benedetti TJ, Gabbe SG. Shoulder dystocia: a complication of fetal macrosomia and prolonged second stage of labor with midpelvic delivery. Obstet Gynecol. 1978;52:526-529.
30. McFarland LV, Raskin M, Daling JR, Benedetti TJ. Erb/Duchenne’s palsy: a consequence of fetal macrosomia and method of delivery. Obstet Gynecol. 1986;68:784-788.
31. Gonik B, Stringer CA, Held B. An alternate mechanism for management of shoulder dystocia. Am J Obstet Gynecol. 1983;145:882-884.
32. Woods CE. A principle of physics as applicable to shoulder delivery. Am J Obstet Gynecol. 1943;45:796-804.
33. Bruner JP, Drummond SB, Meenan AL, Gaskin IM. All-fours maneuver for reducing shoulder dystocia during labor. J Reprod Med. 1998;43:439-443.
34. Sandberg EC. The Zavanelli maneuver: a potentially revolutionary method for the resolution of shoulder dystocia. Am J Obstet Gynecol. 1985;152:479.-
35. Sandberg EC. The Zavanelli maneuver: 12 years of recorded experience. Obstet Gynecol. 1999;93:312-317.
36. Gurewitsch ED, Donithan M, Stalllings SP, et al. Episiotomy versus fetal manipulation in managing severe shoulder dystocia: a comparison of outcomes. Am J Obstet Gynecol. 2004;191:911-916.
37. Dyachenko A, Ciampi A, Fahey J, et al. Prediction of risk for shoulder dystocia with neonatal injury. Am J Obstet Gynecol. 2006 Jul 14 [Epub ahead of print].
38. DeCherney AH, Pernoll ML, eds. Lange Obstetric and Gynecologic Diagnosis and Treatment. 8th ed. Norwalk, Conn: Appleton & Lange; 1994:219.
39. Allen RH, Gurewitsch ED. Temporary Erb-Duchenne palsy without shoulder dystocia or traction to the fetal head. Obstet Gynecol. 2005;105:1210-1212.
Dr. Lerner is a consultant for LMS Medical Services.
1. Professional Insurance Association of America risk management data, 2005.
2. Acker D, Sachs B, Friedman E. Risk factors for shoulder dystocia. Obstet Gynecol. 1985;66:762-768.
3. Al-Najashi S, Al-Suleiman S, El-Yahia A, Rahman M, Rahman J. Shoulder dystocia—a clinical study of 56 cases. Aust N Z J Obstet Gynaecol. 1989;29:129.-
4. Casey BM, Lucas MJ, McIntire DD, Leveno KJ. Pregnancy outcomes in women with gestational diabetes compared with the general obstetric population. Obstet Gynecol. 1997;90:869-873.
5. Sandmire HF, O’Halloin TJ. Shoulder dystocia: its incidence and associated risk factors. Int J Obstet Gynaecol. 1988;26:65-73.
6. Nesbitt TS, Gilbert WM, Herrchen B. Shoulder dystocia and associated risk factors with macrosomic infants born in California. Am J Obstet Gynecol. 1998;179:47-480.
7. Kolderup LB, Laros RK, Jr, Musci TJ. Incidence of persistent birth injury in macrosomic infants: association with mode of delivery. Am J Obstet Gynecol. 1997;177:37-41.
8. Emerson R. Obesity and its association with the complications of pregnancy. Br Med J. 1962;2:516-519.
9. Smith RB, Lane C, Pearson JF. Shoulder dystocia: what happens at the next delivery? Br J Obstet Gynaecol. 1994;101:713-715.
10. Ginsberg NA, Moisidis C. How to predict recurrent shoulder dystocia. Am J Obstet Gynecol. 2001;184:1427-1430.
11. Gherman RB. Shoulder dystocia: an evidence-based evaluation of the obstetrical nightmare. Clin Obstet Gynecol. 2002;45:345-361.
12. Baskett TF, Allen AC. Perinatal implications of shoulder dystocia. Obstet Gynecol. 1995;86:14-17.
13. Lewis DF, Edwards MS, Asrat T, et al. Can shoulder dystocia be predicted? Preconceptual and prenatal factors. J Reprod Med. 1998;43:654-658.
14. Rouse DJ, Owen J, Goldenberg RL, Cliver SP. The effectiveness and costs of elective cesarean delivery for fetal macrosomia diagnosed by ultrasound. JAMA. 1996;276:1480-1486.
15. Gherman RB, Ouzounian JG, Goodwin TM. Brachial plexus palsy: an in utero injury? Am J Obstet Gynecol. 1999;180:1303-1307.
16. Delpapa DH, Mueller-Heubach E. Pregnancy outcome following ultrasound diagnosis of macrosomia. Obstet Gynecol. 1991;78:340-343.
17. Gonen O, Rosen DJ, Dolfin Z, et al. Induction of labor versus expectant management in macrosomia: a randomized study. Obstet Gynecol. 1997;89:913-917.
18. Leaphart WL, Meyer MC, Capeless EL. Labor induction with a prenatal diagnosis of fetal macrosomia. J Matern Fetal Med. 1997;6:99-102.
19. American College of Obstetricians and Gynecologists. ACOG Practice Bulletin #40: Shoulder Dystocia. Washington, DC: ACOG; November 2002.
20. Elliott JP, Garite TJ, Freeman RK, McQuown DS, Patel JM. Ultrasonic prediction of fetal macrosomia in diabetic patients. Obstet Gynecol. 1982;60:159-162.
21. Gherman RB. Persistent brachial plexus injury: the outcome of concern among patients with suspected fetal macrosomia. Am J Obstet Gynecol. 1998;178:195.-
22. McFarland MB, Langer O, Piper JM, Berkus MD. Perinatal outcome and the type and number of maneuvers in shoulder dystocia. Int J Obstet Gynaecol. 1996;55:219-224.
23. Bofill JA, Rust OA, Devidas M, et al. Shoulder dystocia and operative vaginal delivery. J Matern Fetal Med. 1997;6:220-224.
24. Johnson NR. Shoulder dystocia: a study of 47 cases. Aust N Z J Obstet Gynaecol. 1979;19:28-31.
25. Nocon JJ, Weisbrod L. Shoulder dystocia. Chapter 14. In: O’Grady JP, Gimovsky M, eds. Operative Obstetrics. Philadelphia: Williams & Wilkins; 1995:339-353.
26. Creasy RK, Resnik R. Maternal-Fetal Medicine. 5th ed. Philadelphia: Saunders; 2004:690-691.
27. Nichols DH, DeLancey JO, eds. Clinical Problems, Injuries and Complications of Gynecologic and Obstetric Surgery. Baltimore: Williams & Wilkins; 1995:447.
28. Hopewood HG. Shoulder dystocia: fifteen years’ experience in a community hospital. Am J Obstet Gynecol. 1982;144:162-166.
29. Benedetti TJ, Gabbe SG. Shoulder dystocia: a complication of fetal macrosomia and prolonged second stage of labor with midpelvic delivery. Obstet Gynecol. 1978;52:526-529.
30. McFarland LV, Raskin M, Daling JR, Benedetti TJ. Erb/Duchenne’s palsy: a consequence of fetal macrosomia and method of delivery. Obstet Gynecol. 1986;68:784-788.
31. Gonik B, Stringer CA, Held B. An alternate mechanism for management of shoulder dystocia. Am J Obstet Gynecol. 1983;145:882-884.
32. Woods CE. A principle of physics as applicable to shoulder delivery. Am J Obstet Gynecol. 1943;45:796-804.
33. Bruner JP, Drummond SB, Meenan AL, Gaskin IM. All-fours maneuver for reducing shoulder dystocia during labor. J Reprod Med. 1998;43:439-443.
34. Sandberg EC. The Zavanelli maneuver: a potentially revolutionary method for the resolution of shoulder dystocia. Am J Obstet Gynecol. 1985;152:479.-
35. Sandberg EC. The Zavanelli maneuver: 12 years of recorded experience. Obstet Gynecol. 1999;93:312-317.
36. Gurewitsch ED, Donithan M, Stalllings SP, et al. Episiotomy versus fetal manipulation in managing severe shoulder dystocia: a comparison of outcomes. Am J Obstet Gynecol. 2004;191:911-916.
37. Dyachenko A, Ciampi A, Fahey J, et al. Prediction of risk for shoulder dystocia with neonatal injury. Am J Obstet Gynecol. 2006 Jul 14 [Epub ahead of print].
38. DeCherney AH, Pernoll ML, eds. Lange Obstetric and Gynecologic Diagnosis and Treatment. 8th ed. Norwalk, Conn: Appleton & Lange; 1994:219.
39. Allen RH, Gurewitsch ED. Temporary Erb-Duchenne palsy without shoulder dystocia or traction to the fetal head. Obstet Gynecol. 2005;105:1210-1212.
Dr. Lerner is a consultant for LMS Medical Services.