User login
Alendronate Therapy and Renal Insufficiency: A Prescription for Problems?
ENDOMETRIAL CANCER
ObGyns are the first to make the diagnosis and are frequently involved in the treatment of endometrial cancer. It is the most common gynecologic cancer—more common than ovarian cancer and cervical cancer combined.
There will be an estimated 41,200 cases and 7,350 deaths from uterine cancer in 2006.
This update reviews recent studies and practice guidelines that may affect how we manage this disease.
Anticipate cancer when the diagnosis is atypical hyperplasia
Trimble CL, Kauderer J, Zaino R, Silverberg S, Lim PC, Burke JJ II, Alberts D, Curtin J. Concurrent endometrial carcinoma in women with a biopsy diagnosis of atypical endometrial hyperplasia. Cancer. 2006;106:812–819.
When we see a diagnosis of atypical endometrial hyperplasia, we need to consider that there very well may be an endometrial cancer already present
ObGyns often manage women with a diagnosis of atypical endometrial hyperplasia, and we know that it is a precursor to endometrial cancer. A now-classic study1 found that 29% of women with complex atypical hyperplasia go on to develop endometrial cancer, and the standard recommendation for women with complex atypical hyperplasia is hysterectomy and bilateral salpingo-oophorectomy. (The exception to surgical management is for young women who wish to retain their ability to have children; in these cases, conservative management with progesterone therapy is often attempted.)
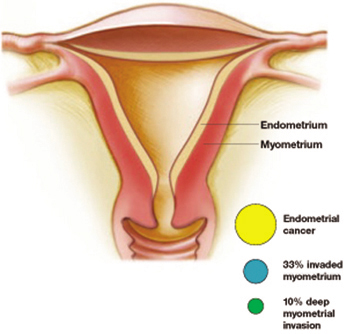
A considerably higher rate—42.6%—of concurrent endometrial carcinoma was found, in a large NIH-sponsored study conducted by Trimble and colleagues, from the Gynecologic Oncology Group member institutions. A panel of gynecologic pathologists analyzed the diagnostic biopsy specimens and the hysterectomy slides of 289 women who had a preoperative diagnosis of complex atypical hyperplasia. Of those who had concurrent cancer, about one third of the cancers invaded the myometrium, and about 10% involved deep myometrial invasion.
Atypical hyperplasia: A warning sign
More than 40% of women with a diagnosis of atypical hyperplasia had endometrial cancer. About a third of these cancers had invaded the myometrium—a tenth of them deeply. Trimble et al
Practice recommendations
I believe the findings of this study have two important implications for practice:
- When taking a patient with complex atypical hyperplasia to the operating room, an intraoperative frozen section is important. Understaging can occur if the surgeon is not prepared to perform staging.
- In counseling patients with complex atypical hyperplasia, it is important to inform them of the high risk of finding a concurrent cancer. Women who choose conservative medical management with progesterone due to their wish to retain childbearing potential should be informed of the risks. In addition, very close follow-up with serial endometrial biopsies or dilation and curettage should be considered.
No pre-op evidence of metastatic cancer? Don’t get too comfortable
Orr Jr J, Chamberlain D, Kilgore L, Naumann W. ACOG Practice Bulletin Number 65. Management of endometrial cancer. Obstet Gynecol. 2005;106:413–425.
ObGyns should have a consultant gynecologic oncologist available to perform full staging in the majority of endometrial cancer cases
The most significant and controversial aspect of the new ACOG Practice Bulletin is the strong recommendation that most women with endometrial cancer undergo full staging, including pelvic washings, bilateral pelvic and para-aortic lymphadenectomy, and complete resection of all disease. The exceptions include young or perimenopausal women with grade I endometrioid adenocarcinoma associated with atypical endometrial hyperplasia, and women at increased risk of mortality secondary to comorbidities. The bulletin acknowledges that the recommendations are based on limited or inconsistent evidence (Level B).
One of the reasons for full surgical staging for most endometrial cancers is the difficulty in accurately determining grade and depth of invasion intraoperatively. Because of this difficulty, gynecologic oncologists occasionally see patients who were understaged. This limits the oncologist’s ability to determine appropriate adjuvant therapy and accurately assess risk of recurrence.
Practice recommendations
Many ObGyns feel comfortable taking a patient with complex hyperplasia or grade I endometrial cancer to the operating room if there is no evidence of metastatic disease or deep myometrial invasion. These new guidelines mean that ObGyns should have a consultant gynecologic oncologist available to perform full staging in the majority of cases of endometrial cancer.
The bulletin also offers helpful guidelines for preoperative evaluation and postoperative adjuvant treatment, and discusses specific recommendations for women found to have endometrial cancer after a hysterectomy, progesterone therapy for early grade I disease, and management of endometrial cancer in patients with morbid obesity.
Estrogen therapy after hysterectomy for early-stage endometrial cancer
Barakat RR, Bundy BN, Spirtos NM, Bell J, Mannel RS. Randomized double-blind trial of estrogen replacement therapy versus placebo in stage I or II endometrial cancer: a gynecologic oncology group study. J Clin Oncol. 2006;24:587–592.
For women with problematic symptoms that are unresponsive to other drugs, short-term estrogen may be an option. Estrogen in this setting is unlikely to significantly increase the recurrence rate of endometrial cancer
This prospective, randomized, placebo-controlled study was initiated to examine whether estrogen replacement therapy had a deleterious effect on the risk of recurrence in patients with early-stage endometrial cancer. More than 1,236 women were randomized to either estrogen replacement therapy or placebo. Although the study did not complete accrual, and therefore definitive answers about the effect of estrogen replacement on survival cannot be made, some useful clinical information resulted.
The absolute recurrence rate in those taking estrogen therapy was 2.1%, which is quite low. This low rate did not differ significantly from the recurrence rate in the placebo group. It is unlikely that a randomized clinical trial will ever definitively answer the question of safety of estrogen replacement therapy in women with early-stage endometrial cancer. Therefore, the decision to use estrogen replacement therapy has to be individualized.
Estrogen replacement therapy will most likely be for the approximately one quarter of all women with endometrial cancer who are under the age of 50 and for whom surgical treatment of endometrial cancer will result in premature menopause.
Symptoms including hot flashes and night sweats can be addressed initially with agents such as venlafaxine, a serotonin and norepinephrine reuptake inhibitor.
For women whose problematic symptoms do not improve with these drugs, however, short-term estrogen may be an option. Estrogen in this setting was unlikely to significantly increase the recurrence rate of endometrial cancer, this study found.
Practice recommendations
The ACOG Committee Opinion for Hormone Replacement Therapy in Women Treated for Endometrial Cancer, Number 234, May 2000 (published before completion of this study) recommends individualization on the basis of potential benefit and risk to the patient.
It is a good recommendation, and now this study’s results can be included, as well, in discussions with patients about risks and benefits.
Surgery prevents Lynch syndrome cancers
Schmeler KM, Lynch HT, Chen L-M, Munsell MF, Soliman PT, Clark MB, Daniels MS, White KG, Boyd-Rogers SG, Conrad PG, Yang KY, Rubin MM, Sun CC, Slomovitz BM, Gershenson DM, Lu KH. Prophylactic surgery to reduce the risk of gynecologic cancers in the Lynch syndrome. N Engl J Med. 2006;354:261–269.
Lu K, Broaddus R. Gynecologic cancers in HNPCC. Familial Cancer. 2005;4:249–254.
Prophylactic hysterectomy with bilateral salpingo-oophorectomy is an effective strategy for prevention of endometrial and ovarian cancer in women with the Lynch syndrome
Although ObGyns are familiar with the Hereditary Breast/Ovarian Cancer syndrome and the BRCA1 and BRCA2 genes, few are familiar with the increased risk of endometrial cancer in the Lynch syndrome, also called hereditary nonpolyposis colorectal cancer syndrome (HNPCC).
The Lynch syndrome is an inherited cancer predisposition syndrome that increases risk for endometrial cancer, colon cancer, and ovarian cancer. There are also less common cancers associated with Lynch syndrome. The genes that are responsible for inherited cancer susceptibility in families with Lynch syndrome are MLH1, MSH2, and MSH6. These genes are part of a family of genes that are responsible for repairing DNA mistakes during DNA replication. Mutations in one of the genes occur in about 1 in 1,000 individuals, which is similar in frequency to mutations in BRCA1 and BRCA2.
Women with Lynch syndrome have a 40% to 60% lifetime risk of colon cancer and a 40% to 60% risk of endometrial cancer (compare this to the 5% lifetime risk of colon cancer and 3% lifetime risk of endometrial cancer in the general population).
ObGyns can:
- Identify women who may have Lynch syndrome
- Manage their endometrial and ovarian cancer risks
The New England Journal of Medicine report helps to further define prevention strategies. Of 315 women with documented germline mutations associated with the Lynch syndrome, 61 underwent prophylactic hysterectomy and were matched with 210 women who did not undergo hysterectomy.
Key results
- None of the women who underwent prophylactic hysterectomy developed endometrial cancer, whereas 69 women in the control group (33%) developed endometrial cancer.
- None of the women who underwent bilateral salpingo-oophorectomy developed ovarian cancer, whereas 12 women in the control group (5%) developed ovarian cancer.
Practice recommendations
- These findings suggest that prophylactic hysterectomy with bilateral salpingo-oopherectomy is an effective strategy for preventing endometrial and ovarian cancer in women with the Lynch syndrome.
- For endometrial and ovarian cancer screening, the available studies have shown that measurement of the endometrial stripe is unlikely to be effective.
- Current consensus group recommendations advise an annual endometrial biopsy and a transvaginal ultrasound to examine the ovaries.
- For colon cancer screening, a colonoscopy every 1 to 2 years is recommended.
1. Kurman R, Kaminski P, Norris H. The behavior of endometrial hyperplasia. A long-term study of “untreated” hyperplasia in 170 patients. Cancer. 1985;56:403-411.
The author reports no financial relationships relevant to this article.
ObGyns are the first to make the diagnosis and are frequently involved in the treatment of endometrial cancer. It is the most common gynecologic cancer—more common than ovarian cancer and cervical cancer combined.
There will be an estimated 41,200 cases and 7,350 deaths from uterine cancer in 2006.
This update reviews recent studies and practice guidelines that may affect how we manage this disease.
Anticipate cancer when the diagnosis is atypical hyperplasia
Trimble CL, Kauderer J, Zaino R, Silverberg S, Lim PC, Burke JJ II, Alberts D, Curtin J. Concurrent endometrial carcinoma in women with a biopsy diagnosis of atypical endometrial hyperplasia. Cancer. 2006;106:812–819.
When we see a diagnosis of atypical endometrial hyperplasia, we need to consider that there very well may be an endometrial cancer already present
ObGyns often manage women with a diagnosis of atypical endometrial hyperplasia, and we know that it is a precursor to endometrial cancer. A now-classic study1 found that 29% of women with complex atypical hyperplasia go on to develop endometrial cancer, and the standard recommendation for women with complex atypical hyperplasia is hysterectomy and bilateral salpingo-oophorectomy. (The exception to surgical management is for young women who wish to retain their ability to have children; in these cases, conservative management with progesterone therapy is often attempted.)
A considerably higher rate—42.6%—of concurrent endometrial carcinoma was found, in a large NIH-sponsored study conducted by Trimble and colleagues, from the Gynecologic Oncology Group member institutions. A panel of gynecologic pathologists analyzed the diagnostic biopsy specimens and the hysterectomy slides of 289 women who had a preoperative diagnosis of complex atypical hyperplasia. Of those who had concurrent cancer, about one third of the cancers invaded the myometrium, and about 10% involved deep myometrial invasion.

Atypical hyperplasia: A warning sign
More than 40% of women with a diagnosis of atypical hyperplasia had endometrial cancer. About a third of these cancers had invaded the myometrium—a tenth of them deeply. Trimble et al
Practice recommendations
I believe the findings of this study have two important implications for practice:
- When taking a patient with complex atypical hyperplasia to the operating room, an intraoperative frozen section is important. Understaging can occur if the surgeon is not prepared to perform staging.
- In counseling patients with complex atypical hyperplasia, it is important to inform them of the high risk of finding a concurrent cancer. Women who choose conservative medical management with progesterone due to their wish to retain childbearing potential should be informed of the risks. In addition, very close follow-up with serial endometrial biopsies or dilation and curettage should be considered.
No pre-op evidence of metastatic cancer? Don’t get too comfortable
Orr Jr J, Chamberlain D, Kilgore L, Naumann W. ACOG Practice Bulletin Number 65. Management of endometrial cancer. Obstet Gynecol. 2005;106:413–425.
ObGyns should have a consultant gynecologic oncologist available to perform full staging in the majority of endometrial cancer cases
The most significant and controversial aspect of the new ACOG Practice Bulletin is the strong recommendation that most women with endometrial cancer undergo full staging, including pelvic washings, bilateral pelvic and para-aortic lymphadenectomy, and complete resection of all disease. The exceptions include young or perimenopausal women with grade I endometrioid adenocarcinoma associated with atypical endometrial hyperplasia, and women at increased risk of mortality secondary to comorbidities. The bulletin acknowledges that the recommendations are based on limited or inconsistent evidence (Level B).
One of the reasons for full surgical staging for most endometrial cancers is the difficulty in accurately determining grade and depth of invasion intraoperatively. Because of this difficulty, gynecologic oncologists occasionally see patients who were understaged. This limits the oncologist’s ability to determine appropriate adjuvant therapy and accurately assess risk of recurrence.
Practice recommendations
Many ObGyns feel comfortable taking a patient with complex hyperplasia or grade I endometrial cancer to the operating room if there is no evidence of metastatic disease or deep myometrial invasion. These new guidelines mean that ObGyns should have a consultant gynecologic oncologist available to perform full staging in the majority of cases of endometrial cancer.
The bulletin also offers helpful guidelines for preoperative evaluation and postoperative adjuvant treatment, and discusses specific recommendations for women found to have endometrial cancer after a hysterectomy, progesterone therapy for early grade I disease, and management of endometrial cancer in patients with morbid obesity.
Estrogen therapy after hysterectomy for early-stage endometrial cancer
Barakat RR, Bundy BN, Spirtos NM, Bell J, Mannel RS. Randomized double-blind trial of estrogen replacement therapy versus placebo in stage I or II endometrial cancer: a gynecologic oncology group study. J Clin Oncol. 2006;24:587–592.
For women with problematic symptoms that are unresponsive to other drugs, short-term estrogen may be an option. Estrogen in this setting is unlikely to significantly increase the recurrence rate of endometrial cancer
This prospective, randomized, placebo-controlled study was initiated to examine whether estrogen replacement therapy had a deleterious effect on the risk of recurrence in patients with early-stage endometrial cancer. More than 1,236 women were randomized to either estrogen replacement therapy or placebo. Although the study did not complete accrual, and therefore definitive answers about the effect of estrogen replacement on survival cannot be made, some useful clinical information resulted.
The absolute recurrence rate in those taking estrogen therapy was 2.1%, which is quite low. This low rate did not differ significantly from the recurrence rate in the placebo group. It is unlikely that a randomized clinical trial will ever definitively answer the question of safety of estrogen replacement therapy in women with early-stage endometrial cancer. Therefore, the decision to use estrogen replacement therapy has to be individualized.
Estrogen replacement therapy will most likely be for the approximately one quarter of all women with endometrial cancer who are under the age of 50 and for whom surgical treatment of endometrial cancer will result in premature menopause.
Symptoms including hot flashes and night sweats can be addressed initially with agents such as venlafaxine, a serotonin and norepinephrine reuptake inhibitor.
For women whose problematic symptoms do not improve with these drugs, however, short-term estrogen may be an option. Estrogen in this setting was unlikely to significantly increase the recurrence rate of endometrial cancer, this study found.
Practice recommendations
The ACOG Committee Opinion for Hormone Replacement Therapy in Women Treated for Endometrial Cancer, Number 234, May 2000 (published before completion of this study) recommends individualization on the basis of potential benefit and risk to the patient.
It is a good recommendation, and now this study’s results can be included, as well, in discussions with patients about risks and benefits.
Surgery prevents Lynch syndrome cancers
Schmeler KM, Lynch HT, Chen L-M, Munsell MF, Soliman PT, Clark MB, Daniels MS, White KG, Boyd-Rogers SG, Conrad PG, Yang KY, Rubin MM, Sun CC, Slomovitz BM, Gershenson DM, Lu KH. Prophylactic surgery to reduce the risk of gynecologic cancers in the Lynch syndrome. N Engl J Med. 2006;354:261–269.
Lu K, Broaddus R. Gynecologic cancers in HNPCC. Familial Cancer. 2005;4:249–254.
Prophylactic hysterectomy with bilateral salpingo-oophorectomy is an effective strategy for prevention of endometrial and ovarian cancer in women with the Lynch syndrome
Although ObGyns are familiar with the Hereditary Breast/Ovarian Cancer syndrome and the BRCA1 and BRCA2 genes, few are familiar with the increased risk of endometrial cancer in the Lynch syndrome, also called hereditary nonpolyposis colorectal cancer syndrome (HNPCC).
The Lynch syndrome is an inherited cancer predisposition syndrome that increases risk for endometrial cancer, colon cancer, and ovarian cancer. There are also less common cancers associated with Lynch syndrome. The genes that are responsible for inherited cancer susceptibility in families with Lynch syndrome are MLH1, MSH2, and MSH6. These genes are part of a family of genes that are responsible for repairing DNA mistakes during DNA replication. Mutations in one of the genes occur in about 1 in 1,000 individuals, which is similar in frequency to mutations in BRCA1 and BRCA2.
Women with Lynch syndrome have a 40% to 60% lifetime risk of colon cancer and a 40% to 60% risk of endometrial cancer (compare this to the 5% lifetime risk of colon cancer and 3% lifetime risk of endometrial cancer in the general population).
ObGyns can:
- Identify women who may have Lynch syndrome
- Manage their endometrial and ovarian cancer risks
The New England Journal of Medicine report helps to further define prevention strategies. Of 315 women with documented germline mutations associated with the Lynch syndrome, 61 underwent prophylactic hysterectomy and were matched with 210 women who did not undergo hysterectomy.
Key results
- None of the women who underwent prophylactic hysterectomy developed endometrial cancer, whereas 69 women in the control group (33%) developed endometrial cancer.
- None of the women who underwent bilateral salpingo-oophorectomy developed ovarian cancer, whereas 12 women in the control group (5%) developed ovarian cancer.
Practice recommendations
- These findings suggest that prophylactic hysterectomy with bilateral salpingo-oopherectomy is an effective strategy for preventing endometrial and ovarian cancer in women with the Lynch syndrome.
- For endometrial and ovarian cancer screening, the available studies have shown that measurement of the endometrial stripe is unlikely to be effective.
- Current consensus group recommendations advise an annual endometrial biopsy and a transvaginal ultrasound to examine the ovaries.
- For colon cancer screening, a colonoscopy every 1 to 2 years is recommended.
ObGyns are the first to make the diagnosis and are frequently involved in the treatment of endometrial cancer. It is the most common gynecologic cancer—more common than ovarian cancer and cervical cancer combined.
There will be an estimated 41,200 cases and 7,350 deaths from uterine cancer in 2006.
This update reviews recent studies and practice guidelines that may affect how we manage this disease.
Anticipate cancer when the diagnosis is atypical hyperplasia
Trimble CL, Kauderer J, Zaino R, Silverberg S, Lim PC, Burke JJ II, Alberts D, Curtin J. Concurrent endometrial carcinoma in women with a biopsy diagnosis of atypical endometrial hyperplasia. Cancer. 2006;106:812–819.
When we see a diagnosis of atypical endometrial hyperplasia, we need to consider that there very well may be an endometrial cancer already present
ObGyns often manage women with a diagnosis of atypical endometrial hyperplasia, and we know that it is a precursor to endometrial cancer. A now-classic study1 found that 29% of women with complex atypical hyperplasia go on to develop endometrial cancer, and the standard recommendation for women with complex atypical hyperplasia is hysterectomy and bilateral salpingo-oophorectomy. (The exception to surgical management is for young women who wish to retain their ability to have children; in these cases, conservative management with progesterone therapy is often attempted.)
A considerably higher rate—42.6%—of concurrent endometrial carcinoma was found, in a large NIH-sponsored study conducted by Trimble and colleagues, from the Gynecologic Oncology Group member institutions. A panel of gynecologic pathologists analyzed the diagnostic biopsy specimens and the hysterectomy slides of 289 women who had a preoperative diagnosis of complex atypical hyperplasia. Of those who had concurrent cancer, about one third of the cancers invaded the myometrium, and about 10% involved deep myometrial invasion.

Atypical hyperplasia: A warning sign
More than 40% of women with a diagnosis of atypical hyperplasia had endometrial cancer. About a third of these cancers had invaded the myometrium—a tenth of them deeply. Trimble et al
Practice recommendations
I believe the findings of this study have two important implications for practice:
- When taking a patient with complex atypical hyperplasia to the operating room, an intraoperative frozen section is important. Understaging can occur if the surgeon is not prepared to perform staging.
- In counseling patients with complex atypical hyperplasia, it is important to inform them of the high risk of finding a concurrent cancer. Women who choose conservative medical management with progesterone due to their wish to retain childbearing potential should be informed of the risks. In addition, very close follow-up with serial endometrial biopsies or dilation and curettage should be considered.
No pre-op evidence of metastatic cancer? Don’t get too comfortable
Orr Jr J, Chamberlain D, Kilgore L, Naumann W. ACOG Practice Bulletin Number 65. Management of endometrial cancer. Obstet Gynecol. 2005;106:413–425.
ObGyns should have a consultant gynecologic oncologist available to perform full staging in the majority of endometrial cancer cases
The most significant and controversial aspect of the new ACOG Practice Bulletin is the strong recommendation that most women with endometrial cancer undergo full staging, including pelvic washings, bilateral pelvic and para-aortic lymphadenectomy, and complete resection of all disease. The exceptions include young or perimenopausal women with grade I endometrioid adenocarcinoma associated with atypical endometrial hyperplasia, and women at increased risk of mortality secondary to comorbidities. The bulletin acknowledges that the recommendations are based on limited or inconsistent evidence (Level B).
One of the reasons for full surgical staging for most endometrial cancers is the difficulty in accurately determining grade and depth of invasion intraoperatively. Because of this difficulty, gynecologic oncologists occasionally see patients who were understaged. This limits the oncologist’s ability to determine appropriate adjuvant therapy and accurately assess risk of recurrence.
Practice recommendations
Many ObGyns feel comfortable taking a patient with complex hyperplasia or grade I endometrial cancer to the operating room if there is no evidence of metastatic disease or deep myometrial invasion. These new guidelines mean that ObGyns should have a consultant gynecologic oncologist available to perform full staging in the majority of cases of endometrial cancer.
The bulletin also offers helpful guidelines for preoperative evaluation and postoperative adjuvant treatment, and discusses specific recommendations for women found to have endometrial cancer after a hysterectomy, progesterone therapy for early grade I disease, and management of endometrial cancer in patients with morbid obesity.
Estrogen therapy after hysterectomy for early-stage endometrial cancer
Barakat RR, Bundy BN, Spirtos NM, Bell J, Mannel RS. Randomized double-blind trial of estrogen replacement therapy versus placebo in stage I or II endometrial cancer: a gynecologic oncology group study. J Clin Oncol. 2006;24:587–592.
For women with problematic symptoms that are unresponsive to other drugs, short-term estrogen may be an option. Estrogen in this setting is unlikely to significantly increase the recurrence rate of endometrial cancer
This prospective, randomized, placebo-controlled study was initiated to examine whether estrogen replacement therapy had a deleterious effect on the risk of recurrence in patients with early-stage endometrial cancer. More than 1,236 women were randomized to either estrogen replacement therapy or placebo. Although the study did not complete accrual, and therefore definitive answers about the effect of estrogen replacement on survival cannot be made, some useful clinical information resulted.
The absolute recurrence rate in those taking estrogen therapy was 2.1%, which is quite low. This low rate did not differ significantly from the recurrence rate in the placebo group. It is unlikely that a randomized clinical trial will ever definitively answer the question of safety of estrogen replacement therapy in women with early-stage endometrial cancer. Therefore, the decision to use estrogen replacement therapy has to be individualized.
Estrogen replacement therapy will most likely be for the approximately one quarter of all women with endometrial cancer who are under the age of 50 and for whom surgical treatment of endometrial cancer will result in premature menopause.
Symptoms including hot flashes and night sweats can be addressed initially with agents such as venlafaxine, a serotonin and norepinephrine reuptake inhibitor.
For women whose problematic symptoms do not improve with these drugs, however, short-term estrogen may be an option. Estrogen in this setting was unlikely to significantly increase the recurrence rate of endometrial cancer, this study found.
Practice recommendations
The ACOG Committee Opinion for Hormone Replacement Therapy in Women Treated for Endometrial Cancer, Number 234, May 2000 (published before completion of this study) recommends individualization on the basis of potential benefit and risk to the patient.
It is a good recommendation, and now this study’s results can be included, as well, in discussions with patients about risks and benefits.
Surgery prevents Lynch syndrome cancers
Schmeler KM, Lynch HT, Chen L-M, Munsell MF, Soliman PT, Clark MB, Daniels MS, White KG, Boyd-Rogers SG, Conrad PG, Yang KY, Rubin MM, Sun CC, Slomovitz BM, Gershenson DM, Lu KH. Prophylactic surgery to reduce the risk of gynecologic cancers in the Lynch syndrome. N Engl J Med. 2006;354:261–269.
Lu K, Broaddus R. Gynecologic cancers in HNPCC. Familial Cancer. 2005;4:249–254.
Prophylactic hysterectomy with bilateral salpingo-oophorectomy is an effective strategy for prevention of endometrial and ovarian cancer in women with the Lynch syndrome
Although ObGyns are familiar with the Hereditary Breast/Ovarian Cancer syndrome and the BRCA1 and BRCA2 genes, few are familiar with the increased risk of endometrial cancer in the Lynch syndrome, also called hereditary nonpolyposis colorectal cancer syndrome (HNPCC).
The Lynch syndrome is an inherited cancer predisposition syndrome that increases risk for endometrial cancer, colon cancer, and ovarian cancer. There are also less common cancers associated with Lynch syndrome. The genes that are responsible for inherited cancer susceptibility in families with Lynch syndrome are MLH1, MSH2, and MSH6. These genes are part of a family of genes that are responsible for repairing DNA mistakes during DNA replication. Mutations in one of the genes occur in about 1 in 1,000 individuals, which is similar in frequency to mutations in BRCA1 and BRCA2.
Women with Lynch syndrome have a 40% to 60% lifetime risk of colon cancer and a 40% to 60% risk of endometrial cancer (compare this to the 5% lifetime risk of colon cancer and 3% lifetime risk of endometrial cancer in the general population).
ObGyns can:
- Identify women who may have Lynch syndrome
- Manage their endometrial and ovarian cancer risks
The New England Journal of Medicine report helps to further define prevention strategies. Of 315 women with documented germline mutations associated with the Lynch syndrome, 61 underwent prophylactic hysterectomy and were matched with 210 women who did not undergo hysterectomy.
Key results
- None of the women who underwent prophylactic hysterectomy developed endometrial cancer, whereas 69 women in the control group (33%) developed endometrial cancer.
- None of the women who underwent bilateral salpingo-oophorectomy developed ovarian cancer, whereas 12 women in the control group (5%) developed ovarian cancer.
Practice recommendations
- These findings suggest that prophylactic hysterectomy with bilateral salpingo-oopherectomy is an effective strategy for preventing endometrial and ovarian cancer in women with the Lynch syndrome.
- For endometrial and ovarian cancer screening, the available studies have shown that measurement of the endometrial stripe is unlikely to be effective.
- Current consensus group recommendations advise an annual endometrial biopsy and a transvaginal ultrasound to examine the ovaries.
- For colon cancer screening, a colonoscopy every 1 to 2 years is recommended.
1. Kurman R, Kaminski P, Norris H. The behavior of endometrial hyperplasia. A long-term study of “untreated” hyperplasia in 170 patients. Cancer. 1985;56:403-411.
The author reports no financial relationships relevant to this article.
1. Kurman R, Kaminski P, Norris H. The behavior of endometrial hyperplasia. A long-term study of “untreated” hyperplasia in 170 patients. Cancer. 1985;56:403-411.
The author reports no financial relationships relevant to this article.
How to divorce a difficult patient and live happily ever after
You need not despair if you’re confronted with a patient who disrupts your practice. You have every right to discharge her. But once a physician-patient relationship is established, you must terminate the relationship officially, to end your obligation. An orderly dismissal does not abandon your patient, and minimizes potential for legal problems.
Although difficult patients may be uncommon in your practice, it is unwise to give no thought to the possibility, and to have no plan to handle the situation. Protect yourself and your practice by following a consistent path with difficult patients, and seek legal counsel when faced with an unusual situation.
Difficult patients aren’t the only ones you may need to dismiss. You may have to dismiss patients because you are retiring or discontinuing your participation with an insurance company.
…chronically skip key appointments
You know that Kimberly means well, but she has a history of failing to keep her appointments. Your last encounter was a consult at the hospital, where she left against medical advice, as reported to you by her admitting physician. A recent positive test result has you concerned, particularly because she did not show up for 3 visits you’ve scheduled to discuss her care.
…never pay
You’ve delivered 2 of Julie’s babies, and now she’s on the schedule for her preventive gynecological care this afternoon. While you’re grabbing a bite of lunch, your manager comes in to let you know that Julie has never paid you a dime—for three years’ running, despite dozens of statements, phone calls and requests for payments at the front office.
…verbally abuse your staff
Sally has verbally abused your staff on multiple occasions since she became your patient several years ago. She often brings her partner, whom you’ve observed to be ill-tempered with staff. Although they’re pleasant to you when you walk into the room, their behavior is such that your staff refuses to provide nursing assistance any longer.
First, call your liability insurance carrier
Using your professional liability insurance carrier as an adviser is critical. In sensitive situations, such as a patient who displays disruptive behavior, and whom you believe may be litigious, consult the risk management department before a dismissal. Your carrier knows your state’s laws on terminating the patient-physician relationship.
Check insurance contracts
Although most health insurance contracts do not state dismissal terms, they can stipulate anything. HMOs often require specific procedures. If you’ve signed such a contract, you’ll need to be aware of and follow the rules before a situation comes up. Examples: Some payers require a period of time (eg, 90 days) before dismissal, and some require notification first, so that they can counsel the beneficiary.
In future contracting, negotiate for terms that are friendly to your practice, not just to the insurance company.
Warning signs
Difficult patients tend to:
- Fail to make payment arrangements according to normal practices
- Fail to comply with a recommended plan of care, including subsequent appointments
- Display disruptive or violent behavior in the practice (or the patient’s partner is disruptive or violent)
- Leave the hospital against medical advice
- Threaten lawsuits
- Abuse drugs or controlled substances
Put your policy in writing and practice it consistently
Your dismissal policy—and the reasons and protocols your practice follows—must be in writing. Decide what constitutes a reason for dismissal and make sure you apply your own rules consistently.
Do not discriminate or appear to discriminate
As a physician, you have the right to terminate a difficult patient from your practice. Exceptions are dismissals based on ethnicity, gender, religion, or age. If you apply your dismissal policy inconsistently, your actions could be considered discriminatory. For example, do not terminate one patient for failure to pay her $500 debt while ignoring another patient’s past-due balance.
Document, document, document
Document in the patient’s medical record any verbal or written communication to and from the patient. This is especially important in the case of a noncompliant patient. Record every instance, for example, of a patient who fails to show up for her appointment. Record your attempts to contact her to reschedule, and notate the consequences of her failure to keep the appointment.
No-shows are dangerous
Although no-show abuse is rampant for many practices, be careful about continuing to treat these patients without taking some action. Let’s say, for example, you notify a patient of a positive Pap smear and recommend a colposcopy. The patient cancels her first colposcopy and fails to show for the rescheduled procedure. You document your multiple attempts to contact the patient.
If the patient continues to schedule appointments and fails to show up for them, contact your malpractice carrier for advice. This patient will likely have a serious medical problem in the future, and you do not want to get tied up in a lawsuit claiming abandonment. Although it may be tedious, thorough documentation of your actions prior to dismissal will pay off in the long run. Include in the record when you communicated to the patient and what you told her regarding the consequences of her failure to present for her appointment.
Dismissal for nonpayment
In the case of nonpayment—the most common cause for dismissal—consider dismissal if the patient refuses to work out payment arrangements.
Consider offering the option of continued treatment on the basis of payment of past debts and future payment prior to service. It is standard practice to offer to allow the patient to return if she abides by this arrangement.
Threats and violence
Call local authorities immediately if a patient makes a threat or displays violent physical behavior. Stop short of broadcasting the situation to other area ObGyns, because that is the responsibility of the police.
When you have dealt with a violent or abusive patient, ask authorities to advise other physicians who may be affected.
You may immediately dismiss a patient when there is a threat or perceived threat of violence. Otherwise, follow the steps of a consistent process to terminate a patient.
Pre-dismissal strategy to avert allegations
The goal of the dismissal process is to terminate your patient-physician relationship while avoiding an allegation of abandonment or discrimination.
Consider alternatives
If the patient is in the course of treatment for a medical condition that requires physician supervision, such as an obstetrics patient or a postoperative gynecologic surgery patient, consider alternatives before dismissing her.
- Can you treat her during the course of the condition and dismiss her afterwards?
- Can you contact a community agency for assistance if, for example, the reason for her failure to show up for appointments is transportation or childcare challenges?
If you have no alternatives but still want to dismiss a patient during a specific course of treatment, seek counsel from your malpractice carrier.
Ask her about correcting the problem
Before dismissing a patient, attempt to communicate with her directly. Discuss your concerns. Document your conversation and her reactions. Proceed with the termination unless she assures you that she will alter her actions. This is optional, and certainly not recommended for a violent patient or one who has a violent partner. However, open communication is suggested because it can help avoid angering the patient. An angry patient may be more likely to sue, especially if she had been unaware of your intention or reasons to dismiss her.
If the cause for dismissal is noncompliance with your recommended treatment, document the recommendations and conversations in detail. Include the recommended treatment plan, her objections, and your statement of the consequences of her failure to comply. In the case of an adverse event, documentation may be crucial.
You may receive a transfer of medical records that includes a dismissal letter for a reason that causes you concern. You are not obligated to treat that patient if you have not yet established a relationship. The establishment normally occurs at the first appointment.
All the same, it’s wise to contact your malpractice carrier about any state laws that would impact the initiation of the patient-physician relationship and how you should handle refusing to treat a patient.
- The letter of dismissal (Sample letter) Outline in the letter the reason(s) for the dismissal. Be objective. Stating subjective reasons for dismissal may open the door for a case of abandonment on the basis of discrimination.
- How to handle the referral Include a copy of your medical records transfer request with your letter. When possible, include a referral source such as your medical society or hospital referral center. Do not refer her to a specific physician.
- How to handle the mailing Mail the letter with a return receipt requested. Patients may refuse a letter sent with receipt requested, so also send a copy by regular mail. Copy the letter and record the date and method by which the letters were sent. Keep the return receipt (or record of refusal) in the patient’s record.
- Consider 30-day continuance Most physicians allow 30 days to establish a relationship with another provider, and limit care during this time to acute needs only—but sensitivity to the patient’s situation is wise. Although 30 days typically suffice, check with your malpractice carrier if you are concerned about the patient’s condition or availability of other ObGyns.
- Specify termination of services In your written communication, outline what services you will provide until she locates another provider.
Be sure to indicate a specific termination date—30 days following the date of the dismissal letter is recommended.
A special thank you to Pam Hutcherson, RN, LNC, Risk Management Specialist, State Volunteer Mutual Insurance Company of Brentwood, Tennessee, who reviewed this article.
Atlanta-based Elizabeth W. Woodcock is a speaker, trainer, and author specializing in practice management. Among her recent books are Mastering Patient Flow.
You need not despair if you’re confronted with a patient who disrupts your practice. You have every right to discharge her. But once a physician-patient relationship is established, you must terminate the relationship officially, to end your obligation. An orderly dismissal does not abandon your patient, and minimizes potential for legal problems.
Although difficult patients may be uncommon in your practice, it is unwise to give no thought to the possibility, and to have no plan to handle the situation. Protect yourself and your practice by following a consistent path with difficult patients, and seek legal counsel when faced with an unusual situation.
Difficult patients aren’t the only ones you may need to dismiss. You may have to dismiss patients because you are retiring or discontinuing your participation with an insurance company.
…chronically skip key appointments
You know that Kimberly means well, but she has a history of failing to keep her appointments. Your last encounter was a consult at the hospital, where she left against medical advice, as reported to you by her admitting physician. A recent positive test result has you concerned, particularly because she did not show up for 3 visits you’ve scheduled to discuss her care.
…never pay
You’ve delivered 2 of Julie’s babies, and now she’s on the schedule for her preventive gynecological care this afternoon. While you’re grabbing a bite of lunch, your manager comes in to let you know that Julie has never paid you a dime—for three years’ running, despite dozens of statements, phone calls and requests for payments at the front office.
…verbally abuse your staff
Sally has verbally abused your staff on multiple occasions since she became your patient several years ago. She often brings her partner, whom you’ve observed to be ill-tempered with staff. Although they’re pleasant to you when you walk into the room, their behavior is such that your staff refuses to provide nursing assistance any longer.
First, call your liability insurance carrier
Using your professional liability insurance carrier as an adviser is critical. In sensitive situations, such as a patient who displays disruptive behavior, and whom you believe may be litigious, consult the risk management department before a dismissal. Your carrier knows your state’s laws on terminating the patient-physician relationship.
Check insurance contracts
Although most health insurance contracts do not state dismissal terms, they can stipulate anything. HMOs often require specific procedures. If you’ve signed such a contract, you’ll need to be aware of and follow the rules before a situation comes up. Examples: Some payers require a period of time (eg, 90 days) before dismissal, and some require notification first, so that they can counsel the beneficiary.
In future contracting, negotiate for terms that are friendly to your practice, not just to the insurance company.
Warning signs
Difficult patients tend to:
- Fail to make payment arrangements according to normal practices
- Fail to comply with a recommended plan of care, including subsequent appointments
- Display disruptive or violent behavior in the practice (or the patient’s partner is disruptive or violent)
- Leave the hospital against medical advice
- Threaten lawsuits
- Abuse drugs or controlled substances
Put your policy in writing and practice it consistently
Your dismissal policy—and the reasons and protocols your practice follows—must be in writing. Decide what constitutes a reason for dismissal and make sure you apply your own rules consistently.
Do not discriminate or appear to discriminate
As a physician, you have the right to terminate a difficult patient from your practice. Exceptions are dismissals based on ethnicity, gender, religion, or age. If you apply your dismissal policy inconsistently, your actions could be considered discriminatory. For example, do not terminate one patient for failure to pay her $500 debt while ignoring another patient’s past-due balance.
Document, document, document
Document in the patient’s medical record any verbal or written communication to and from the patient. This is especially important in the case of a noncompliant patient. Record every instance, for example, of a patient who fails to show up for her appointment. Record your attempts to contact her to reschedule, and notate the consequences of her failure to keep the appointment.
No-shows are dangerous
Although no-show abuse is rampant for many practices, be careful about continuing to treat these patients without taking some action. Let’s say, for example, you notify a patient of a positive Pap smear and recommend a colposcopy. The patient cancels her first colposcopy and fails to show for the rescheduled procedure. You document your multiple attempts to contact the patient.
If the patient continues to schedule appointments and fails to show up for them, contact your malpractice carrier for advice. This patient will likely have a serious medical problem in the future, and you do not want to get tied up in a lawsuit claiming abandonment. Although it may be tedious, thorough documentation of your actions prior to dismissal will pay off in the long run. Include in the record when you communicated to the patient and what you told her regarding the consequences of her failure to present for her appointment.
Dismissal for nonpayment
In the case of nonpayment—the most common cause for dismissal—consider dismissal if the patient refuses to work out payment arrangements.
Consider offering the option of continued treatment on the basis of payment of past debts and future payment prior to service. It is standard practice to offer to allow the patient to return if she abides by this arrangement.
Threats and violence
Call local authorities immediately if a patient makes a threat or displays violent physical behavior. Stop short of broadcasting the situation to other area ObGyns, because that is the responsibility of the police.
When you have dealt with a violent or abusive patient, ask authorities to advise other physicians who may be affected.
You may immediately dismiss a patient when there is a threat or perceived threat of violence. Otherwise, follow the steps of a consistent process to terminate a patient.
Pre-dismissal strategy to avert allegations
The goal of the dismissal process is to terminate your patient-physician relationship while avoiding an allegation of abandonment or discrimination.
Consider alternatives
If the patient is in the course of treatment for a medical condition that requires physician supervision, such as an obstetrics patient or a postoperative gynecologic surgery patient, consider alternatives before dismissing her.
- Can you treat her during the course of the condition and dismiss her afterwards?
- Can you contact a community agency for assistance if, for example, the reason for her failure to show up for appointments is transportation or childcare challenges?
If you have no alternatives but still want to dismiss a patient during a specific course of treatment, seek counsel from your malpractice carrier.
Ask her about correcting the problem
Before dismissing a patient, attempt to communicate with her directly. Discuss your concerns. Document your conversation and her reactions. Proceed with the termination unless she assures you that she will alter her actions. This is optional, and certainly not recommended for a violent patient or one who has a violent partner. However, open communication is suggested because it can help avoid angering the patient. An angry patient may be more likely to sue, especially if she had been unaware of your intention or reasons to dismiss her.
If the cause for dismissal is noncompliance with your recommended treatment, document the recommendations and conversations in detail. Include the recommended treatment plan, her objections, and your statement of the consequences of her failure to comply. In the case of an adverse event, documentation may be crucial.
You may receive a transfer of medical records that includes a dismissal letter for a reason that causes you concern. You are not obligated to treat that patient if you have not yet established a relationship. The establishment normally occurs at the first appointment.
All the same, it’s wise to contact your malpractice carrier about any state laws that would impact the initiation of the patient-physician relationship and how you should handle refusing to treat a patient.
- The letter of dismissal (Sample letter) Outline in the letter the reason(s) for the dismissal. Be objective. Stating subjective reasons for dismissal may open the door for a case of abandonment on the basis of discrimination.
- How to handle the referral Include a copy of your medical records transfer request with your letter. When possible, include a referral source such as your medical society or hospital referral center. Do not refer her to a specific physician.
- How to handle the mailing Mail the letter with a return receipt requested. Patients may refuse a letter sent with receipt requested, so also send a copy by regular mail. Copy the letter and record the date and method by which the letters were sent. Keep the return receipt (or record of refusal) in the patient’s record.
- Consider 30-day continuance Most physicians allow 30 days to establish a relationship with another provider, and limit care during this time to acute needs only—but sensitivity to the patient’s situation is wise. Although 30 days typically suffice, check with your malpractice carrier if you are concerned about the patient’s condition or availability of other ObGyns.
- Specify termination of services In your written communication, outline what services you will provide until she locates another provider.
Be sure to indicate a specific termination date—30 days following the date of the dismissal letter is recommended.
A special thank you to Pam Hutcherson, RN, LNC, Risk Management Specialist, State Volunteer Mutual Insurance Company of Brentwood, Tennessee, who reviewed this article.
You need not despair if you’re confronted with a patient who disrupts your practice. You have every right to discharge her. But once a physician-patient relationship is established, you must terminate the relationship officially, to end your obligation. An orderly dismissal does not abandon your patient, and minimizes potential for legal problems.
Although difficult patients may be uncommon in your practice, it is unwise to give no thought to the possibility, and to have no plan to handle the situation. Protect yourself and your practice by following a consistent path with difficult patients, and seek legal counsel when faced with an unusual situation.
Difficult patients aren’t the only ones you may need to dismiss. You may have to dismiss patients because you are retiring or discontinuing your participation with an insurance company.
…chronically skip key appointments
You know that Kimberly means well, but she has a history of failing to keep her appointments. Your last encounter was a consult at the hospital, where she left against medical advice, as reported to you by her admitting physician. A recent positive test result has you concerned, particularly because she did not show up for 3 visits you’ve scheduled to discuss her care.
…never pay
You’ve delivered 2 of Julie’s babies, and now she’s on the schedule for her preventive gynecological care this afternoon. While you’re grabbing a bite of lunch, your manager comes in to let you know that Julie has never paid you a dime—for three years’ running, despite dozens of statements, phone calls and requests for payments at the front office.
…verbally abuse your staff
Sally has verbally abused your staff on multiple occasions since she became your patient several years ago. She often brings her partner, whom you’ve observed to be ill-tempered with staff. Although they’re pleasant to you when you walk into the room, their behavior is such that your staff refuses to provide nursing assistance any longer.
First, call your liability insurance carrier
Using your professional liability insurance carrier as an adviser is critical. In sensitive situations, such as a patient who displays disruptive behavior, and whom you believe may be litigious, consult the risk management department before a dismissal. Your carrier knows your state’s laws on terminating the patient-physician relationship.
Check insurance contracts
Although most health insurance contracts do not state dismissal terms, they can stipulate anything. HMOs often require specific procedures. If you’ve signed such a contract, you’ll need to be aware of and follow the rules before a situation comes up. Examples: Some payers require a period of time (eg, 90 days) before dismissal, and some require notification first, so that they can counsel the beneficiary.
In future contracting, negotiate for terms that are friendly to your practice, not just to the insurance company.
Warning signs
Difficult patients tend to:
- Fail to make payment arrangements according to normal practices
- Fail to comply with a recommended plan of care, including subsequent appointments
- Display disruptive or violent behavior in the practice (or the patient’s partner is disruptive or violent)
- Leave the hospital against medical advice
- Threaten lawsuits
- Abuse drugs or controlled substances
Put your policy in writing and practice it consistently
Your dismissal policy—and the reasons and protocols your practice follows—must be in writing. Decide what constitutes a reason for dismissal and make sure you apply your own rules consistently.
Do not discriminate or appear to discriminate
As a physician, you have the right to terminate a difficult patient from your practice. Exceptions are dismissals based on ethnicity, gender, religion, or age. If you apply your dismissal policy inconsistently, your actions could be considered discriminatory. For example, do not terminate one patient for failure to pay her $500 debt while ignoring another patient’s past-due balance.
Document, document, document
Document in the patient’s medical record any verbal or written communication to and from the patient. This is especially important in the case of a noncompliant patient. Record every instance, for example, of a patient who fails to show up for her appointment. Record your attempts to contact her to reschedule, and notate the consequences of her failure to keep the appointment.
No-shows are dangerous
Although no-show abuse is rampant for many practices, be careful about continuing to treat these patients without taking some action. Let’s say, for example, you notify a patient of a positive Pap smear and recommend a colposcopy. The patient cancels her first colposcopy and fails to show for the rescheduled procedure. You document your multiple attempts to contact the patient.
If the patient continues to schedule appointments and fails to show up for them, contact your malpractice carrier for advice. This patient will likely have a serious medical problem in the future, and you do not want to get tied up in a lawsuit claiming abandonment. Although it may be tedious, thorough documentation of your actions prior to dismissal will pay off in the long run. Include in the record when you communicated to the patient and what you told her regarding the consequences of her failure to present for her appointment.
Dismissal for nonpayment
In the case of nonpayment—the most common cause for dismissal—consider dismissal if the patient refuses to work out payment arrangements.
Consider offering the option of continued treatment on the basis of payment of past debts and future payment prior to service. It is standard practice to offer to allow the patient to return if she abides by this arrangement.
Threats and violence
Call local authorities immediately if a patient makes a threat or displays violent physical behavior. Stop short of broadcasting the situation to other area ObGyns, because that is the responsibility of the police.
When you have dealt with a violent or abusive patient, ask authorities to advise other physicians who may be affected.
You may immediately dismiss a patient when there is a threat or perceived threat of violence. Otherwise, follow the steps of a consistent process to terminate a patient.
Pre-dismissal strategy to avert allegations
The goal of the dismissal process is to terminate your patient-physician relationship while avoiding an allegation of abandonment or discrimination.
Consider alternatives
If the patient is in the course of treatment for a medical condition that requires physician supervision, such as an obstetrics patient or a postoperative gynecologic surgery patient, consider alternatives before dismissing her.
- Can you treat her during the course of the condition and dismiss her afterwards?
- Can you contact a community agency for assistance if, for example, the reason for her failure to show up for appointments is transportation or childcare challenges?
If you have no alternatives but still want to dismiss a patient during a specific course of treatment, seek counsel from your malpractice carrier.
Ask her about correcting the problem
Before dismissing a patient, attempt to communicate with her directly. Discuss your concerns. Document your conversation and her reactions. Proceed with the termination unless she assures you that she will alter her actions. This is optional, and certainly not recommended for a violent patient or one who has a violent partner. However, open communication is suggested because it can help avoid angering the patient. An angry patient may be more likely to sue, especially if she had been unaware of your intention or reasons to dismiss her.
If the cause for dismissal is noncompliance with your recommended treatment, document the recommendations and conversations in detail. Include the recommended treatment plan, her objections, and your statement of the consequences of her failure to comply. In the case of an adverse event, documentation may be crucial.
You may receive a transfer of medical records that includes a dismissal letter for a reason that causes you concern. You are not obligated to treat that patient if you have not yet established a relationship. The establishment normally occurs at the first appointment.
All the same, it’s wise to contact your malpractice carrier about any state laws that would impact the initiation of the patient-physician relationship and how you should handle refusing to treat a patient.
- The letter of dismissal (Sample letter) Outline in the letter the reason(s) for the dismissal. Be objective. Stating subjective reasons for dismissal may open the door for a case of abandonment on the basis of discrimination.
- How to handle the referral Include a copy of your medical records transfer request with your letter. When possible, include a referral source such as your medical society or hospital referral center. Do not refer her to a specific physician.
- How to handle the mailing Mail the letter with a return receipt requested. Patients may refuse a letter sent with receipt requested, so also send a copy by regular mail. Copy the letter and record the date and method by which the letters were sent. Keep the return receipt (or record of refusal) in the patient’s record.
- Consider 30-day continuance Most physicians allow 30 days to establish a relationship with another provider, and limit care during this time to acute needs only—but sensitivity to the patient’s situation is wise. Although 30 days typically suffice, check with your malpractice carrier if you are concerned about the patient’s condition or availability of other ObGyns.
- Specify termination of services In your written communication, outline what services you will provide until she locates another provider.
Be sure to indicate a specific termination date—30 days following the date of the dismissal letter is recommended.
A special thank you to Pam Hutcherson, RN, LNC, Risk Management Specialist, State Volunteer Mutual Insurance Company of Brentwood, Tennessee, who reviewed this article.
Atlanta-based Elizabeth W. Woodcock is a speaker, trainer, and author specializing in practice management. Among her recent books are Mastering Patient Flow.
Atlanta-based Elizabeth W. Woodcock is a speaker, trainer, and author specializing in practice management. Among her recent books are Mastering Patient Flow.
IN THIS ARTICLE
Evaluating Hyperprolactinemia in Veterans
How Veterans Use Stroke Services in the VA and Beyond
INFECTIOUS DISEASES
For the 2006 Update, I have chosen to focus on 3 important new clinical reports that stand to improve patient care, and another development that necessitates a change in how we treat gonorrhea in pregnant women:
CMV vaccine. A new immunologic agent for the treatment and prevention of congenital cytomegalovirus (CMV) infection is extremely promising. Until now, no consistently effective therapy for this serious congenital infection has been identified.
- Recommended hygiene measures to prevent transmission—Page 64
Outpatient treatment of PID. Relatively inexpensive outpatient therapy for mild to moderately severe pelvic inflammatory disease was demonstrated to be equal to inpatient therapy in efficacy and safety.
- Whom to hospitalize—Page 68
Wound complications after cesarean delivery in the obese were reduced by use of subcutaneous closure and avoidance of surgical drains.
- Recommended technique—Page 70
2 antibiotics with unique application in the treatment of uncomplicated gonococcal infections in pregnant women—cefixime and spectinomycin—were recently withdrawn from the market. This unfortunate development is a special dilemma in pregnant women with allergy to beta-lactams.
- Alternative regimens, using other antibiotics—Page 75
A promising therapy for congenital CMV
For now, emphasize prevention
Nigro G, Adler SP, LaTorre R, Best AM. Passive immunization during pregnancy for congenital cytomegalovirus infection. N Engl J Med. 2005;353:1350–1362.
- Although anti-cytomegalovirus hyperimmune globulin appears to have great promise for prevention and treatment of congenital CMV infection, I propose that obstetricians avoid a rush to judgment and maintain their focus on simple measures to prevent horizontal transmission of CMV
Summary
Nigro and colleagues present a provocative report of a promising new treatment for congenital cytomegalovirus (CMV) infection. Their prospective cohort study at 8 Italian medical centers involved 157 pregnant women with confirmed primary CMV infection: 148 women were asymptomatic and were identified by routine serologic screening; 8 had symptomatic infections and 1 had ultrasound findings consistent with congenital CMV infection.
CMV was detected in the amniotic fluid of 45 women who had a primary infection more than 6 weeks before enrollment, and 31 of these women agreed to receive CMV-specific hyperimmune globulin (200 units per kilogram of maternal body weight). Nine of the 31 women received 1 or 2 additional infusions into either the amniotic fluid or umbilical cord because of persistent fetal abnormalities on ultrasound.
- Only 1 of the 31 treated women delivered an infected infant (adjusted odds ratio, 0.02; P<.001).
- In contrast, of the 14 women who declined treatment, 7 had infants who were symptomatically infected at birth.
There were 84 additional women who did not have an amniocentesis because their infection occurred within 6 weeks of enrollment, their gestational age was less than 20 weeks, or they declined the procedure. Of these, 37 agreed to treatment with 100 U of hyperimmune globulin per kilogram of maternal weight every month until delivery.
- 6 of these treated women delivered infected infants.
- In contrast, 19 of the untreated women (adjusted odds ratio 0.32; P=.04) delivered infected infants.
No adverse effects of hyperimmune globulin were noted in either treatment group.
Commentary
This study is remarkable because, until now, no consistently effective therapy for this serious congenital infection has been available. However, before we fully embrace the findings, 3 caveats should be considered.1
- Although the study was prospective, it was neither randomized nor controlled. The lack of strict randomization resulted in a curious blend of 2 cohorts—a treatment group and a prevention group. The dosage regimens were different both within and between the 2 groups.
- There are biological reasons to question the remarkable success rates reported by the authors. For example, administration of anti-HIV hyperimmune globulin has not protected neonates against perinatal transmission of HIV.2 Moreover, the presence of naturally acquired antibody against CMV does not fully protect a mother or her fetus against reactivation and subsequent perinatal transmission of CMV infection.1 This latter observation is particularly important in assessing the authors’ observations that major abnormalities identified by ultrasound, such as ascites, ventriculomegaly, intracerebral and intraabdominal echodensities, and intrauterine growth restriction apparently resolved completely in 14 fetuses after maternal treatment.
- The study did not address the financial and logistic issues of screening large obstetric populations for CMV infection, triaging patients with inevitable false-positive test results, performing targeted sonography and amniocentesis in affected women, and then treating at-risk women with hyperimmune globulin.
Recommendations
Hyperimmune globulin appears to be very safe and to have great promise for treatment and prevention of congenital CMV infection. However, additional investigations are needed to delineate the appropriate dose, method of administration, and timing of immunoprophylaxis and to define its precise level of effectiveness.
Meanwhile, focus on simple hygiene measures
Until confirmatory studies are reported, I propose that obstetricians avoid a rush to judgment and maintain their focus on simple measures to prevent horizontal transmission of CMV, such as:
- using CMV-negative blood products when transfusing pregnant women or fetuses
- encouraging expectant mothers to adopt safe sex practices
- encouraging expectant mothers to use careful handwashing techniques after handling infants’ diapers and toys.
Outpatient treatment of PID is effective, safe, and economical
Fertility and recurrence rates similar to inpatient therapy
Ness RB, Trautmann G, Richter HE, Randall H, Peipert JF, Nelson DB, et al. Effectiveness of treatment strategies of some women with pelvic inflammatory disease. Obstet Gynecol. 2005;106:573–580.
- Outpatient treatment is an effective and economically attractive alternative to inpatient therapy for women with mild to moderately severe pelvic inflammatory disease
Summary
Relatively inexpensive outpatient therapy for mild to moderately severe pelvic inflammatory disease (PID) proved effective and equivalent to inpatient treatment in key respects, in this long-term follow-up study.
Ness and colleagues describe 831 patients who had participated in a prospective, randomized, unblinded multicenter trial of outpatient versus inpatient treatment for mild-to-moderate PID.3 The patients were followed for a mean of 84 months (range 64–100 months).
- The inpatient treatment group received intravenous cefoxitin (2 grams every 6 hours) and either intravenous or oral doxycycline (100 mg twice daily) for at least 72 hours, followed by oral doxycycline (100 mg twice daily) to complete a 14-day course.
- The outpatient treatment group received a single 2-g intramuscular injection of cefoxitin plus a single 1-g oral dose of probenecid, followed by oral doxycycline (100 mg twice daily) for 14 days.
Equivalent outcomes
Outpatient treatment did not adversely affect subsequent fertility or increase the frequency of recurrent PID or chronic pelvic pain. The equivalence of outpatient compared with inpatient therapy extended to women of all races and to those with a history of PID; those colonized by Neisseria gonorrhoeae and/or Chlamydia trachomatis; and those with a high temperature, high white count, and high pelvic tenderness score.
Even in teenage women and women who had never had a live birth, outpatient and inpatient therapy were equivalent.
Risk of ectopic pregnancy was increased in outpatients (odds ratio 4.91); however, ectopic pregnancy was such a rare event that the 95% confidence interval was quite wide, ranging from 0.57 to 42.25.
Commentary
The initial encouraging results of the authors’ 2002 landmark Pelvic Inflammatory Disease Evaluation and Clinical Health (PEACH) Randomized Trial3 led to this long-term follow-up study. In the women who were treated as described above, the short-term clinical outcomes and markers of micro-biologic improvement were similar in the outpatient and inpatient groups. After a mean follow-up of 35 months, pregnancy rates were essentially equal (42%) in both groups. Moreover, the groups did not differ significantly in risk of recurrent PID, chronic pelvic pain, or ectopic pregnancy.
Extended follow-up is reassuring
PID, a common and potentially serious illness, is the single most common predisposing factor for ectopic pregnancy and one of the principal causes of infertility and chronic pelvic pain. The direct and indirect expenses of PID are enormous, and the PEACH trial provides great reassurance that women who are not seriously ill can be safely, effectively, and inexpensively treated as outpatients.
The additional 4 years of follow-up reassures us that outpatient treatment did not adversely affect long-term outcome. Moreover, outpatient therapy was not less effective in women who initially appeared to be at higher risk for adverse sequelae: teens, African-Americans, women with a history of PID, and women colonized with N gonorrhoeae and/or C trachomatis.
Cost comparison
A 14-day prescription for doxycycline should cost less than $25. The single 2-g dose of cefoxitin, combined with the administration charge, should not exceed $100. If cefotetan (2 g) were substituted for cefoxitin (the 2 drugs should be therapeutically equivalent in this clinical situation), the cost would be even less. Conservatively, the charges for a single day in the hospital combined with charges for intravenous antibiotics would be at least $300 to $400.
Beyond the issue of expense are considerations of patient and physician convenience, ease of management, and conservation of scarce resources.
Recommendations
In carefully selected patients, outpatient treatment makes good sense, economically and clinically.
Whom to hospitalize
Patients judged to be seriously ill, particularly those in whom a tubo-ovarian abscess is suspected, should be treated in the hospital. Even with modern antibiotics and sophisticated intensive care, mortalities still occur in women with severe PID complicated by a ruptured abscess.
In addition, patients should be hospitalized for treatment if they are judged to be at risk for noncompliance, lack a reliable support system at home, or have previously failed outpatient management.
A technique that reduces C-section wound complications in the obese
Closure method, but not surgical drains, lowers morbidity
Ramsey PS, White AM, Guinn D, et al. Subcutaneous tissue reapproximation, alone or in combination with drain, in obese women undergoing cesarean delivery. Obstet Gynecol. 2005;105:967–973.
- In obese women having cesarean delivery, closure of the subcutaneous layer reduces risk of wound complications such as seroma, hematoma, incisional abscess, and fascial dehiscence. Addition of a closed system drain did not improve outcome beyond that achieved with subcutaneous closure alone.
Summary
This prospective randomized trial at 5 medical centers assessed the role of 2 surgical techniques in decreasing the risk of wound complications after cesarean delivery in 280 obese women. Patients with subcutaneous thickness greater than or equal to 4 cm were randomized to either subcutaneous suture closure alone (149 women) or suture plus drain (131 women).
The primary study outcome was composite wound morbidity rate, defined by any of the following: subcutaneous tissue dehiscence, seroma, hematoma, incisional abscess, or fascial dehiscence.
Addition of drain did not improve wound morbidity
A running, nonlocking suture of 3-0 Vicryl was used for closure of the subcutaneous layer. The drain used was the Jackson-Pratt surgical drain (10 mm), and it was placed below the layer of subcutaneous suture and then connected to bulb suction. The drain was removed on the third postoperative day, or sooner, if drain output was less than 30 mL in 24 hours. The drain exited the wound via a separate stab site lateral to the incision. All of the skin incisions were closed with staples, which were removed 7 to 14 days after surgery. All patients received standard skin preparations and prophylactic antibiotics.
The composite wound morbidity rate was 17.4% in the suture group and 22.7% in the suture plus drain group (P=NS). Individual wound complication rates were similar in the 2 groups. The authors concluded that the surgical drain did not improve outcome beyond that achieved by closure of the subcutaneous layer.
Commentary
Endometritis and wound disruption are the most common complications of cesarean delivery. Wound complications clearly are the more serious, for they inevitably lead to persistent patient discomfort, prolonged hospitalization, and increased expense. Moreover, they may necessitate additional surgical intervention to drain a seroma, hematoma or abscess or to repair a fascial dehiscence.
Postcesarean wound complications are particularly likely in the obese, and, unfortunately, the prevalence of obesity is steadily increasing among obstetric patients.
In a landmark study of wound infections in many different types of surgery, Cruse and Foord4 demonstrated that sutures in the subcutaneous space actually increased the wound complication rate. DelValle and colleagues5 were among the first to challenge this observation and show that, at least in women having cesarean delivery, reapproximation of Camper’s fascia reduced risk of wound disruption.
Is thickness of subcutaneous layer a key determinant of wound morbidity?
Naumann et al6 and Vermillion and colleagues7 subsequently demonstrated that thickness of the subcutaneous layer was the key determinant of wound complications. Chelmow and colleagues8 recently published an excellent meta-analysis confirming that, in women with a subcutaneous layer greater than 2 cm, closure of the subcutaneous layer with suture significantly reduced the rate of wound disruption.
In the present study, the authors evaluated moderately to severely obese women who had a subcutaneous layer of 4 cm or greater. In light of the previous reports reviewed above, they were justified in omitting a treatment group in which no closure was done. The trial was well designed and included patients from varied populations. Not surprisingly, composite wound morbidity rates were high in both groups.
The addition of the surgical drain did not improve the morbidity rate, however. In fact, even though the drainage system was closed, women in the combined treatment group actually had slightly higher, although not statistically significant, rates of composite morbidity and individual morbidities.
Recommendations
When to omit drain
In view of the added time required to place the drain, greater patient discomfort, and the increased expense associated with the drain, this intervention should not be used in high-risk women having cesarean delivery.
DRUG THERAPYWe’ve lost 2 key weapons in our antibiotics arsenal
Use ceftriaxone or azithromycin for gonorrhea, now that cefixime and spectinomycin are unavailable
- Cefixime and spectinomycin, antibiotics with unique application for treatment of uncomplicated gonorrhea in pregnant women, were recently withdrawn from the market. In their absence, use ceftriaxone,125 mg intramuscularly in a single dose. Pregnant women who are allergic to beta-lactam antibiotics should be treated with a single 2-g oral dose of azithromycin.
Two antibiotics with unique application in treatment of uncomplicated gonococcal infections were recently withdrawn from the market. These drugs were not withdrawn because there were questions about their effectiveness or safety. Rather, the decisions to discontinue production appear to have been based on marketing and economic considerations.
- Cefixime, an oral cephalosporin that was highly effective in a single 400-mg dose against almost all strains of N gonorrhoeae.
- Spectinomycin, a parenteral agent (2 g, intramuscularly) that was the treatment of choice for uncomplicated gonorrhoeae in pregnant women allergic to beta-lactam antibiotics.
Recommendations
Nonpregnant women can be treated with either ceftriaxone, 125 mg IM in a single dose, or with a single oral dose of a quinolone antibiotic; for example, 500 mg ciprofloxacin, 400 mg ofloxacin, or 250 mg levofloxacin.
Pregnant women who are not allergic to beta-lactam antibiotics should be treated with ceftriaxone, 125 mg IM in a single dose.
Dilemma: Beta-lactam allergy in pregnant women
The dilemma is how best to treat pregnant patients who are allergic to beta-lactam antibiotics, now that spectinomycin is unavailable. Doxycycline and tetracycline provide reasonable coverage against N gonorrhoeae, but both are considered FDA pregnancy category D. Quinolone antibiotics have excellent activity against this organism, but they are considered FDA pregnancy category C because of concern about their effect on fetal cartilage.
Azithromycin is an acceptable alternative. For the pregnant patient who has a true life-threatening allergy to beta-lactams, I believe the most reasonable alternative is azithromycin. This drug is usually used in a single oral dose of 1 g to treat uncomplicated chlamydial infections. However, in a dose of 2 g, azithromycin does have acceptable activity against N gonorrhoeae. At this dosage, gastrointestinal effects are more likely, and cost may exceed $80.
UPDATE ON INFECTIOUS DISEASES
1. Duff P. Immunotherapy for congenital cytomegalovirus infection. N Engl J Med. 2005;353:1402-1404.
2. Watts DH. Management of human immunodeficient virus infection in pregnancy. N Engl J Med. 2002;346:1879-1891.
3. Ness RB, Soper DE, Holley RL, et al. Effectiveness of inpatient and outpatient treatment strategies for women with pelvic inflammatory disease: Results from the Pelvic Inflammatory Disease Evaluation and Clinical Health (PEACH) Randomized Trial. Am J Obstet Gynecol. 2002;186:929-937.
4. Cruse PJE, Foord R. A five-year prospective study of 23,649 surgical wounds. Arch Surg. 1973;107:206-210.
5. DelValle GO, Coombs P, Qualls C, Curet LB. Does closure of Camper fascia reduce the incidence of post-cesarean superficial wound disruption? Obstet Gynecol. 1992;80:1013-1016.
6. Naumann RW, Hauth JC, Owen J, Hodgkins PM, Lincoln T. Subcutaneous tissue approximation in relation to wound disruption after cesarean delivery in obese women. Obstet Gynecol. 1995;85:412-416.
7. Vermillion ST, Lamoutte C, Soper DE, Verdeja A. Wound infection after cesarean: effect of subcutaneous tissue thickness. Obstet Gynecol. 2000;95:923-926.
8. Chelmow D, Rodriguez EJ, Sabatini MM. Suture closure of subcutaneous fat and wound disruption after cesarean delivery: A meta analysis. Obstet Gynecol. 2004;103:974-980.
9. Sexually transmitted diseases treatment guidelines—2002 MMWR. 2002;51:1-79.
The author reports no financial relationships relevant to this article.
For the 2006 Update, I have chosen to focus on 3 important new clinical reports that stand to improve patient care, and another development that necessitates a change in how we treat gonorrhea in pregnant women:
CMV vaccine. A new immunologic agent for the treatment and prevention of congenital cytomegalovirus (CMV) infection is extremely promising. Until now, no consistently effective therapy for this serious congenital infection has been identified.
- Recommended hygiene measures to prevent transmission—Page 64
Outpatient treatment of PID. Relatively inexpensive outpatient therapy for mild to moderately severe pelvic inflammatory disease was demonstrated to be equal to inpatient therapy in efficacy and safety.
- Whom to hospitalize—Page 68
Wound complications after cesarean delivery in the obese were reduced by use of subcutaneous closure and avoidance of surgical drains.
- Recommended technique—Page 70
2 antibiotics with unique application in the treatment of uncomplicated gonococcal infections in pregnant women—cefixime and spectinomycin—were recently withdrawn from the market. This unfortunate development is a special dilemma in pregnant women with allergy to beta-lactams.
- Alternative regimens, using other antibiotics—Page 75
A promising therapy for congenital CMV
For now, emphasize prevention
Nigro G, Adler SP, LaTorre R, Best AM. Passive immunization during pregnancy for congenital cytomegalovirus infection. N Engl J Med. 2005;353:1350–1362.
- Although anti-cytomegalovirus hyperimmune globulin appears to have great promise for prevention and treatment of congenital CMV infection, I propose that obstetricians avoid a rush to judgment and maintain their focus on simple measures to prevent horizontal transmission of CMV
Summary
Nigro and colleagues present a provocative report of a promising new treatment for congenital cytomegalovirus (CMV) infection. Their prospective cohort study at 8 Italian medical centers involved 157 pregnant women with confirmed primary CMV infection: 148 women were asymptomatic and were identified by routine serologic screening; 8 had symptomatic infections and 1 had ultrasound findings consistent with congenital CMV infection.
CMV was detected in the amniotic fluid of 45 women who had a primary infection more than 6 weeks before enrollment, and 31 of these women agreed to receive CMV-specific hyperimmune globulin (200 units per kilogram of maternal body weight). Nine of the 31 women received 1 or 2 additional infusions into either the amniotic fluid or umbilical cord because of persistent fetal abnormalities on ultrasound.
- Only 1 of the 31 treated women delivered an infected infant (adjusted odds ratio, 0.02; P<.001).
- In contrast, of the 14 women who declined treatment, 7 had infants who were symptomatically infected at birth.
There were 84 additional women who did not have an amniocentesis because their infection occurred within 6 weeks of enrollment, their gestational age was less than 20 weeks, or they declined the procedure. Of these, 37 agreed to treatment with 100 U of hyperimmune globulin per kilogram of maternal weight every month until delivery.
- 6 of these treated women delivered infected infants.
- In contrast, 19 of the untreated women (adjusted odds ratio 0.32; P=.04) delivered infected infants.
No adverse effects of hyperimmune globulin were noted in either treatment group.
Commentary
This study is remarkable because, until now, no consistently effective therapy for this serious congenital infection has been available. However, before we fully embrace the findings, 3 caveats should be considered.1
- Although the study was prospective, it was neither randomized nor controlled. The lack of strict randomization resulted in a curious blend of 2 cohorts—a treatment group and a prevention group. The dosage regimens were different both within and between the 2 groups.
- There are biological reasons to question the remarkable success rates reported by the authors. For example, administration of anti-HIV hyperimmune globulin has not protected neonates against perinatal transmission of HIV.2 Moreover, the presence of naturally acquired antibody against CMV does not fully protect a mother or her fetus against reactivation and subsequent perinatal transmission of CMV infection.1 This latter observation is particularly important in assessing the authors’ observations that major abnormalities identified by ultrasound, such as ascites, ventriculomegaly, intracerebral and intraabdominal echodensities, and intrauterine growth restriction apparently resolved completely in 14 fetuses after maternal treatment.
- The study did not address the financial and logistic issues of screening large obstetric populations for CMV infection, triaging patients with inevitable false-positive test results, performing targeted sonography and amniocentesis in affected women, and then treating at-risk women with hyperimmune globulin.
Recommendations
Hyperimmune globulin appears to be very safe and to have great promise for treatment and prevention of congenital CMV infection. However, additional investigations are needed to delineate the appropriate dose, method of administration, and timing of immunoprophylaxis and to define its precise level of effectiveness.
Meanwhile, focus on simple hygiene measures
Until confirmatory studies are reported, I propose that obstetricians avoid a rush to judgment and maintain their focus on simple measures to prevent horizontal transmission of CMV, such as:
- using CMV-negative blood products when transfusing pregnant women or fetuses
- encouraging expectant mothers to adopt safe sex practices
- encouraging expectant mothers to use careful handwashing techniques after handling infants’ diapers and toys.
Outpatient treatment of PID is effective, safe, and economical
Fertility and recurrence rates similar to inpatient therapy
Ness RB, Trautmann G, Richter HE, Randall H, Peipert JF, Nelson DB, et al. Effectiveness of treatment strategies of some women with pelvic inflammatory disease. Obstet Gynecol. 2005;106:573–580.
- Outpatient treatment is an effective and economically attractive alternative to inpatient therapy for women with mild to moderately severe pelvic inflammatory disease
Summary
Relatively inexpensive outpatient therapy for mild to moderately severe pelvic inflammatory disease (PID) proved effective and equivalent to inpatient treatment in key respects, in this long-term follow-up study.
Ness and colleagues describe 831 patients who had participated in a prospective, randomized, unblinded multicenter trial of outpatient versus inpatient treatment for mild-to-moderate PID.3 The patients were followed for a mean of 84 months (range 64–100 months).
- The inpatient treatment group received intravenous cefoxitin (2 grams every 6 hours) and either intravenous or oral doxycycline (100 mg twice daily) for at least 72 hours, followed by oral doxycycline (100 mg twice daily) to complete a 14-day course.
- The outpatient treatment group received a single 2-g intramuscular injection of cefoxitin plus a single 1-g oral dose of probenecid, followed by oral doxycycline (100 mg twice daily) for 14 days.
Equivalent outcomes
Outpatient treatment did not adversely affect subsequent fertility or increase the frequency of recurrent PID or chronic pelvic pain. The equivalence of outpatient compared with inpatient therapy extended to women of all races and to those with a history of PID; those colonized by Neisseria gonorrhoeae and/or Chlamydia trachomatis; and those with a high temperature, high white count, and high pelvic tenderness score.
Even in teenage women and women who had never had a live birth, outpatient and inpatient therapy were equivalent.
Risk of ectopic pregnancy was increased in outpatients (odds ratio 4.91); however, ectopic pregnancy was such a rare event that the 95% confidence interval was quite wide, ranging from 0.57 to 42.25.
Commentary
The initial encouraging results of the authors’ 2002 landmark Pelvic Inflammatory Disease Evaluation and Clinical Health (PEACH) Randomized Trial3 led to this long-term follow-up study. In the women who were treated as described above, the short-term clinical outcomes and markers of micro-biologic improvement were similar in the outpatient and inpatient groups. After a mean follow-up of 35 months, pregnancy rates were essentially equal (42%) in both groups. Moreover, the groups did not differ significantly in risk of recurrent PID, chronic pelvic pain, or ectopic pregnancy.
Extended follow-up is reassuring
PID, a common and potentially serious illness, is the single most common predisposing factor for ectopic pregnancy and one of the principal causes of infertility and chronic pelvic pain. The direct and indirect expenses of PID are enormous, and the PEACH trial provides great reassurance that women who are not seriously ill can be safely, effectively, and inexpensively treated as outpatients.
The additional 4 years of follow-up reassures us that outpatient treatment did not adversely affect long-term outcome. Moreover, outpatient therapy was not less effective in women who initially appeared to be at higher risk for adverse sequelae: teens, African-Americans, women with a history of PID, and women colonized with N gonorrhoeae and/or C trachomatis.
Cost comparison
A 14-day prescription for doxycycline should cost less than $25. The single 2-g dose of cefoxitin, combined with the administration charge, should not exceed $100. If cefotetan (2 g) were substituted for cefoxitin (the 2 drugs should be therapeutically equivalent in this clinical situation), the cost would be even less. Conservatively, the charges for a single day in the hospital combined with charges for intravenous antibiotics would be at least $300 to $400.
Beyond the issue of expense are considerations of patient and physician convenience, ease of management, and conservation of scarce resources.
Recommendations
In carefully selected patients, outpatient treatment makes good sense, economically and clinically.
Whom to hospitalize
Patients judged to be seriously ill, particularly those in whom a tubo-ovarian abscess is suspected, should be treated in the hospital. Even with modern antibiotics and sophisticated intensive care, mortalities still occur in women with severe PID complicated by a ruptured abscess.
In addition, patients should be hospitalized for treatment if they are judged to be at risk for noncompliance, lack a reliable support system at home, or have previously failed outpatient management.
A technique that reduces C-section wound complications in the obese
Closure method, but not surgical drains, lowers morbidity
Ramsey PS, White AM, Guinn D, et al. Subcutaneous tissue reapproximation, alone or in combination with drain, in obese women undergoing cesarean delivery. Obstet Gynecol. 2005;105:967–973.
- In obese women having cesarean delivery, closure of the subcutaneous layer reduces risk of wound complications such as seroma, hematoma, incisional abscess, and fascial dehiscence. Addition of a closed system drain did not improve outcome beyond that achieved with subcutaneous closure alone.
Summary
This prospective randomized trial at 5 medical centers assessed the role of 2 surgical techniques in decreasing the risk of wound complications after cesarean delivery in 280 obese women. Patients with subcutaneous thickness greater than or equal to 4 cm were randomized to either subcutaneous suture closure alone (149 women) or suture plus drain (131 women).
The primary study outcome was composite wound morbidity rate, defined by any of the following: subcutaneous tissue dehiscence, seroma, hematoma, incisional abscess, or fascial dehiscence.
Addition of drain did not improve wound morbidity
A running, nonlocking suture of 3-0 Vicryl was used for closure of the subcutaneous layer. The drain used was the Jackson-Pratt surgical drain (10 mm), and it was placed below the layer of subcutaneous suture and then connected to bulb suction. The drain was removed on the third postoperative day, or sooner, if drain output was less than 30 mL in 24 hours. The drain exited the wound via a separate stab site lateral to the incision. All of the skin incisions were closed with staples, which were removed 7 to 14 days after surgery. All patients received standard skin preparations and prophylactic antibiotics.
The composite wound morbidity rate was 17.4% in the suture group and 22.7% in the suture plus drain group (P=NS). Individual wound complication rates were similar in the 2 groups. The authors concluded that the surgical drain did not improve outcome beyond that achieved by closure of the subcutaneous layer.
Commentary
Endometritis and wound disruption are the most common complications of cesarean delivery. Wound complications clearly are the more serious, for they inevitably lead to persistent patient discomfort, prolonged hospitalization, and increased expense. Moreover, they may necessitate additional surgical intervention to drain a seroma, hematoma or abscess or to repair a fascial dehiscence.
Postcesarean wound complications are particularly likely in the obese, and, unfortunately, the prevalence of obesity is steadily increasing among obstetric patients.
In a landmark study of wound infections in many different types of surgery, Cruse and Foord4 demonstrated that sutures in the subcutaneous space actually increased the wound complication rate. DelValle and colleagues5 were among the first to challenge this observation and show that, at least in women having cesarean delivery, reapproximation of Camper’s fascia reduced risk of wound disruption.
Is thickness of subcutaneous layer a key determinant of wound morbidity?
Naumann et al6 and Vermillion and colleagues7 subsequently demonstrated that thickness of the subcutaneous layer was the key determinant of wound complications. Chelmow and colleagues8 recently published an excellent meta-analysis confirming that, in women with a subcutaneous layer greater than 2 cm, closure of the subcutaneous layer with suture significantly reduced the rate of wound disruption.
In the present study, the authors evaluated moderately to severely obese women who had a subcutaneous layer of 4 cm or greater. In light of the previous reports reviewed above, they were justified in omitting a treatment group in which no closure was done. The trial was well designed and included patients from varied populations. Not surprisingly, composite wound morbidity rates were high in both groups.
The addition of the surgical drain did not improve the morbidity rate, however. In fact, even though the drainage system was closed, women in the combined treatment group actually had slightly higher, although not statistically significant, rates of composite morbidity and individual morbidities.
Recommendations
When to omit drain
In view of the added time required to place the drain, greater patient discomfort, and the increased expense associated with the drain, this intervention should not be used in high-risk women having cesarean delivery.
DRUG THERAPYWe’ve lost 2 key weapons in our antibiotics arsenal
Use ceftriaxone or azithromycin for gonorrhea, now that cefixime and spectinomycin are unavailable
- Cefixime and spectinomycin, antibiotics with unique application for treatment of uncomplicated gonorrhea in pregnant women, were recently withdrawn from the market. In their absence, use ceftriaxone,125 mg intramuscularly in a single dose. Pregnant women who are allergic to beta-lactam antibiotics should be treated with a single 2-g oral dose of azithromycin.
Two antibiotics with unique application in treatment of uncomplicated gonococcal infections were recently withdrawn from the market. These drugs were not withdrawn because there were questions about their effectiveness or safety. Rather, the decisions to discontinue production appear to have been based on marketing and economic considerations.
- Cefixime, an oral cephalosporin that was highly effective in a single 400-mg dose against almost all strains of N gonorrhoeae.
- Spectinomycin, a parenteral agent (2 g, intramuscularly) that was the treatment of choice for uncomplicated gonorrhoeae in pregnant women allergic to beta-lactam antibiotics.
Recommendations
Nonpregnant women can be treated with either ceftriaxone, 125 mg IM in a single dose, or with a single oral dose of a quinolone antibiotic; for example, 500 mg ciprofloxacin, 400 mg ofloxacin, or 250 mg levofloxacin.
Pregnant women who are not allergic to beta-lactam antibiotics should be treated with ceftriaxone, 125 mg IM in a single dose.
Dilemma: Beta-lactam allergy in pregnant women
The dilemma is how best to treat pregnant patients who are allergic to beta-lactam antibiotics, now that spectinomycin is unavailable. Doxycycline and tetracycline provide reasonable coverage against N gonorrhoeae, but both are considered FDA pregnancy category D. Quinolone antibiotics have excellent activity against this organism, but they are considered FDA pregnancy category C because of concern about their effect on fetal cartilage.
Azithromycin is an acceptable alternative. For the pregnant patient who has a true life-threatening allergy to beta-lactams, I believe the most reasonable alternative is azithromycin. This drug is usually used in a single oral dose of 1 g to treat uncomplicated chlamydial infections. However, in a dose of 2 g, azithromycin does have acceptable activity against N gonorrhoeae. At this dosage, gastrointestinal effects are more likely, and cost may exceed $80.
For the 2006 Update, I have chosen to focus on 3 important new clinical reports that stand to improve patient care, and another development that necessitates a change in how we treat gonorrhea in pregnant women:
CMV vaccine. A new immunologic agent for the treatment and prevention of congenital cytomegalovirus (CMV) infection is extremely promising. Until now, no consistently effective therapy for this serious congenital infection has been identified.
- Recommended hygiene measures to prevent transmission—Page 64
Outpatient treatment of PID. Relatively inexpensive outpatient therapy for mild to moderately severe pelvic inflammatory disease was demonstrated to be equal to inpatient therapy in efficacy and safety.
- Whom to hospitalize—Page 68
Wound complications after cesarean delivery in the obese were reduced by use of subcutaneous closure and avoidance of surgical drains.
- Recommended technique—Page 70
2 antibiotics with unique application in the treatment of uncomplicated gonococcal infections in pregnant women—cefixime and spectinomycin—were recently withdrawn from the market. This unfortunate development is a special dilemma in pregnant women with allergy to beta-lactams.
- Alternative regimens, using other antibiotics—Page 75
A promising therapy for congenital CMV
For now, emphasize prevention
Nigro G, Adler SP, LaTorre R, Best AM. Passive immunization during pregnancy for congenital cytomegalovirus infection. N Engl J Med. 2005;353:1350–1362.
- Although anti-cytomegalovirus hyperimmune globulin appears to have great promise for prevention and treatment of congenital CMV infection, I propose that obstetricians avoid a rush to judgment and maintain their focus on simple measures to prevent horizontal transmission of CMV
Summary
Nigro and colleagues present a provocative report of a promising new treatment for congenital cytomegalovirus (CMV) infection. Their prospective cohort study at 8 Italian medical centers involved 157 pregnant women with confirmed primary CMV infection: 148 women were asymptomatic and were identified by routine serologic screening; 8 had symptomatic infections and 1 had ultrasound findings consistent with congenital CMV infection.
CMV was detected in the amniotic fluid of 45 women who had a primary infection more than 6 weeks before enrollment, and 31 of these women agreed to receive CMV-specific hyperimmune globulin (200 units per kilogram of maternal body weight). Nine of the 31 women received 1 or 2 additional infusions into either the amniotic fluid or umbilical cord because of persistent fetal abnormalities on ultrasound.
- Only 1 of the 31 treated women delivered an infected infant (adjusted odds ratio, 0.02; P<.001).
- In contrast, of the 14 women who declined treatment, 7 had infants who were symptomatically infected at birth.
There were 84 additional women who did not have an amniocentesis because their infection occurred within 6 weeks of enrollment, their gestational age was less than 20 weeks, or they declined the procedure. Of these, 37 agreed to treatment with 100 U of hyperimmune globulin per kilogram of maternal weight every month until delivery.
- 6 of these treated women delivered infected infants.
- In contrast, 19 of the untreated women (adjusted odds ratio 0.32; P=.04) delivered infected infants.
No adverse effects of hyperimmune globulin were noted in either treatment group.
Commentary
This study is remarkable because, until now, no consistently effective therapy for this serious congenital infection has been available. However, before we fully embrace the findings, 3 caveats should be considered.1
- Although the study was prospective, it was neither randomized nor controlled. The lack of strict randomization resulted in a curious blend of 2 cohorts—a treatment group and a prevention group. The dosage regimens were different both within and between the 2 groups.
- There are biological reasons to question the remarkable success rates reported by the authors. For example, administration of anti-HIV hyperimmune globulin has not protected neonates against perinatal transmission of HIV.2 Moreover, the presence of naturally acquired antibody against CMV does not fully protect a mother or her fetus against reactivation and subsequent perinatal transmission of CMV infection.1 This latter observation is particularly important in assessing the authors’ observations that major abnormalities identified by ultrasound, such as ascites, ventriculomegaly, intracerebral and intraabdominal echodensities, and intrauterine growth restriction apparently resolved completely in 14 fetuses after maternal treatment.
- The study did not address the financial and logistic issues of screening large obstetric populations for CMV infection, triaging patients with inevitable false-positive test results, performing targeted sonography and amniocentesis in affected women, and then treating at-risk women with hyperimmune globulin.
Recommendations
Hyperimmune globulin appears to be very safe and to have great promise for treatment and prevention of congenital CMV infection. However, additional investigations are needed to delineate the appropriate dose, method of administration, and timing of immunoprophylaxis and to define its precise level of effectiveness.
Meanwhile, focus on simple hygiene measures
Until confirmatory studies are reported, I propose that obstetricians avoid a rush to judgment and maintain their focus on simple measures to prevent horizontal transmission of CMV, such as:
- using CMV-negative blood products when transfusing pregnant women or fetuses
- encouraging expectant mothers to adopt safe sex practices
- encouraging expectant mothers to use careful handwashing techniques after handling infants’ diapers and toys.
Outpatient treatment of PID is effective, safe, and economical
Fertility and recurrence rates similar to inpatient therapy
Ness RB, Trautmann G, Richter HE, Randall H, Peipert JF, Nelson DB, et al. Effectiveness of treatment strategies of some women with pelvic inflammatory disease. Obstet Gynecol. 2005;106:573–580.
- Outpatient treatment is an effective and economically attractive alternative to inpatient therapy for women with mild to moderately severe pelvic inflammatory disease
Summary
Relatively inexpensive outpatient therapy for mild to moderately severe pelvic inflammatory disease (PID) proved effective and equivalent to inpatient treatment in key respects, in this long-term follow-up study.
Ness and colleagues describe 831 patients who had participated in a prospective, randomized, unblinded multicenter trial of outpatient versus inpatient treatment for mild-to-moderate PID.3 The patients were followed for a mean of 84 months (range 64–100 months).
- The inpatient treatment group received intravenous cefoxitin (2 grams every 6 hours) and either intravenous or oral doxycycline (100 mg twice daily) for at least 72 hours, followed by oral doxycycline (100 mg twice daily) to complete a 14-day course.
- The outpatient treatment group received a single 2-g intramuscular injection of cefoxitin plus a single 1-g oral dose of probenecid, followed by oral doxycycline (100 mg twice daily) for 14 days.
Equivalent outcomes
Outpatient treatment did not adversely affect subsequent fertility or increase the frequency of recurrent PID or chronic pelvic pain. The equivalence of outpatient compared with inpatient therapy extended to women of all races and to those with a history of PID; those colonized by Neisseria gonorrhoeae and/or Chlamydia trachomatis; and those with a high temperature, high white count, and high pelvic tenderness score.
Even in teenage women and women who had never had a live birth, outpatient and inpatient therapy were equivalent.
Risk of ectopic pregnancy was increased in outpatients (odds ratio 4.91); however, ectopic pregnancy was such a rare event that the 95% confidence interval was quite wide, ranging from 0.57 to 42.25.
Commentary
The initial encouraging results of the authors’ 2002 landmark Pelvic Inflammatory Disease Evaluation and Clinical Health (PEACH) Randomized Trial3 led to this long-term follow-up study. In the women who were treated as described above, the short-term clinical outcomes and markers of micro-biologic improvement were similar in the outpatient and inpatient groups. After a mean follow-up of 35 months, pregnancy rates were essentially equal (42%) in both groups. Moreover, the groups did not differ significantly in risk of recurrent PID, chronic pelvic pain, or ectopic pregnancy.
Extended follow-up is reassuring
PID, a common and potentially serious illness, is the single most common predisposing factor for ectopic pregnancy and one of the principal causes of infertility and chronic pelvic pain. The direct and indirect expenses of PID are enormous, and the PEACH trial provides great reassurance that women who are not seriously ill can be safely, effectively, and inexpensively treated as outpatients.
The additional 4 years of follow-up reassures us that outpatient treatment did not adversely affect long-term outcome. Moreover, outpatient therapy was not less effective in women who initially appeared to be at higher risk for adverse sequelae: teens, African-Americans, women with a history of PID, and women colonized with N gonorrhoeae and/or C trachomatis.
Cost comparison
A 14-day prescription for doxycycline should cost less than $25. The single 2-g dose of cefoxitin, combined with the administration charge, should not exceed $100. If cefotetan (2 g) were substituted for cefoxitin (the 2 drugs should be therapeutically equivalent in this clinical situation), the cost would be even less. Conservatively, the charges for a single day in the hospital combined with charges for intravenous antibiotics would be at least $300 to $400.
Beyond the issue of expense are considerations of patient and physician convenience, ease of management, and conservation of scarce resources.
Recommendations
In carefully selected patients, outpatient treatment makes good sense, economically and clinically.
Whom to hospitalize
Patients judged to be seriously ill, particularly those in whom a tubo-ovarian abscess is suspected, should be treated in the hospital. Even with modern antibiotics and sophisticated intensive care, mortalities still occur in women with severe PID complicated by a ruptured abscess.
In addition, patients should be hospitalized for treatment if they are judged to be at risk for noncompliance, lack a reliable support system at home, or have previously failed outpatient management.
A technique that reduces C-section wound complications in the obese
Closure method, but not surgical drains, lowers morbidity
Ramsey PS, White AM, Guinn D, et al. Subcutaneous tissue reapproximation, alone or in combination with drain, in obese women undergoing cesarean delivery. Obstet Gynecol. 2005;105:967–973.
- In obese women having cesarean delivery, closure of the subcutaneous layer reduces risk of wound complications such as seroma, hematoma, incisional abscess, and fascial dehiscence. Addition of a closed system drain did not improve outcome beyond that achieved with subcutaneous closure alone.
Summary
This prospective randomized trial at 5 medical centers assessed the role of 2 surgical techniques in decreasing the risk of wound complications after cesarean delivery in 280 obese women. Patients with subcutaneous thickness greater than or equal to 4 cm were randomized to either subcutaneous suture closure alone (149 women) or suture plus drain (131 women).
The primary study outcome was composite wound morbidity rate, defined by any of the following: subcutaneous tissue dehiscence, seroma, hematoma, incisional abscess, or fascial dehiscence.
Addition of drain did not improve wound morbidity
A running, nonlocking suture of 3-0 Vicryl was used for closure of the subcutaneous layer. The drain used was the Jackson-Pratt surgical drain (10 mm), and it was placed below the layer of subcutaneous suture and then connected to bulb suction. The drain was removed on the third postoperative day, or sooner, if drain output was less than 30 mL in 24 hours. The drain exited the wound via a separate stab site lateral to the incision. All of the skin incisions were closed with staples, which were removed 7 to 14 days after surgery. All patients received standard skin preparations and prophylactic antibiotics.
The composite wound morbidity rate was 17.4% in the suture group and 22.7% in the suture plus drain group (P=NS). Individual wound complication rates were similar in the 2 groups. The authors concluded that the surgical drain did not improve outcome beyond that achieved by closure of the subcutaneous layer.
Commentary
Endometritis and wound disruption are the most common complications of cesarean delivery. Wound complications clearly are the more serious, for they inevitably lead to persistent patient discomfort, prolonged hospitalization, and increased expense. Moreover, they may necessitate additional surgical intervention to drain a seroma, hematoma or abscess or to repair a fascial dehiscence.
Postcesarean wound complications are particularly likely in the obese, and, unfortunately, the prevalence of obesity is steadily increasing among obstetric patients.
In a landmark study of wound infections in many different types of surgery, Cruse and Foord4 demonstrated that sutures in the subcutaneous space actually increased the wound complication rate. DelValle and colleagues5 were among the first to challenge this observation and show that, at least in women having cesarean delivery, reapproximation of Camper’s fascia reduced risk of wound disruption.
Is thickness of subcutaneous layer a key determinant of wound morbidity?
Naumann et al6 and Vermillion and colleagues7 subsequently demonstrated that thickness of the subcutaneous layer was the key determinant of wound complications. Chelmow and colleagues8 recently published an excellent meta-analysis confirming that, in women with a subcutaneous layer greater than 2 cm, closure of the subcutaneous layer with suture significantly reduced the rate of wound disruption.
In the present study, the authors evaluated moderately to severely obese women who had a subcutaneous layer of 4 cm or greater. In light of the previous reports reviewed above, they were justified in omitting a treatment group in which no closure was done. The trial was well designed and included patients from varied populations. Not surprisingly, composite wound morbidity rates were high in both groups.
The addition of the surgical drain did not improve the morbidity rate, however. In fact, even though the drainage system was closed, women in the combined treatment group actually had slightly higher, although not statistically significant, rates of composite morbidity and individual morbidities.
Recommendations
When to omit drain
In view of the added time required to place the drain, greater patient discomfort, and the increased expense associated with the drain, this intervention should not be used in high-risk women having cesarean delivery.
DRUG THERAPYWe’ve lost 2 key weapons in our antibiotics arsenal
Use ceftriaxone or azithromycin for gonorrhea, now that cefixime and spectinomycin are unavailable
- Cefixime and spectinomycin, antibiotics with unique application for treatment of uncomplicated gonorrhea in pregnant women, were recently withdrawn from the market. In their absence, use ceftriaxone,125 mg intramuscularly in a single dose. Pregnant women who are allergic to beta-lactam antibiotics should be treated with a single 2-g oral dose of azithromycin.
Two antibiotics with unique application in treatment of uncomplicated gonococcal infections were recently withdrawn from the market. These drugs were not withdrawn because there were questions about their effectiveness or safety. Rather, the decisions to discontinue production appear to have been based on marketing and economic considerations.
- Cefixime, an oral cephalosporin that was highly effective in a single 400-mg dose against almost all strains of N gonorrhoeae.
- Spectinomycin, a parenteral agent (2 g, intramuscularly) that was the treatment of choice for uncomplicated gonorrhoeae in pregnant women allergic to beta-lactam antibiotics.
Recommendations
Nonpregnant women can be treated with either ceftriaxone, 125 mg IM in a single dose, or with a single oral dose of a quinolone antibiotic; for example, 500 mg ciprofloxacin, 400 mg ofloxacin, or 250 mg levofloxacin.
Pregnant women who are not allergic to beta-lactam antibiotics should be treated with ceftriaxone, 125 mg IM in a single dose.
Dilemma: Beta-lactam allergy in pregnant women
The dilemma is how best to treat pregnant patients who are allergic to beta-lactam antibiotics, now that spectinomycin is unavailable. Doxycycline and tetracycline provide reasonable coverage against N gonorrhoeae, but both are considered FDA pregnancy category D. Quinolone antibiotics have excellent activity against this organism, but they are considered FDA pregnancy category C because of concern about their effect on fetal cartilage.
Azithromycin is an acceptable alternative. For the pregnant patient who has a true life-threatening allergy to beta-lactams, I believe the most reasonable alternative is azithromycin. This drug is usually used in a single oral dose of 1 g to treat uncomplicated chlamydial infections. However, in a dose of 2 g, azithromycin does have acceptable activity against N gonorrhoeae. At this dosage, gastrointestinal effects are more likely, and cost may exceed $80.
UPDATE ON INFECTIOUS DISEASES
1. Duff P. Immunotherapy for congenital cytomegalovirus infection. N Engl J Med. 2005;353:1402-1404.
2. Watts DH. Management of human immunodeficient virus infection in pregnancy. N Engl J Med. 2002;346:1879-1891.
3. Ness RB, Soper DE, Holley RL, et al. Effectiveness of inpatient and outpatient treatment strategies for women with pelvic inflammatory disease: Results from the Pelvic Inflammatory Disease Evaluation and Clinical Health (PEACH) Randomized Trial. Am J Obstet Gynecol. 2002;186:929-937.
4. Cruse PJE, Foord R. A five-year prospective study of 23,649 surgical wounds. Arch Surg. 1973;107:206-210.
5. DelValle GO, Coombs P, Qualls C, Curet LB. Does closure of Camper fascia reduce the incidence of post-cesarean superficial wound disruption? Obstet Gynecol. 1992;80:1013-1016.
6. Naumann RW, Hauth JC, Owen J, Hodgkins PM, Lincoln T. Subcutaneous tissue approximation in relation to wound disruption after cesarean delivery in obese women. Obstet Gynecol. 1995;85:412-416.
7. Vermillion ST, Lamoutte C, Soper DE, Verdeja A. Wound infection after cesarean: effect of subcutaneous tissue thickness. Obstet Gynecol. 2000;95:923-926.
8. Chelmow D, Rodriguez EJ, Sabatini MM. Suture closure of subcutaneous fat and wound disruption after cesarean delivery: A meta analysis. Obstet Gynecol. 2004;103:974-980.
9. Sexually transmitted diseases treatment guidelines—2002 MMWR. 2002;51:1-79.
The author reports no financial relationships relevant to this article.
UPDATE ON INFECTIOUS DISEASES
1. Duff P. Immunotherapy for congenital cytomegalovirus infection. N Engl J Med. 2005;353:1402-1404.
2. Watts DH. Management of human immunodeficient virus infection in pregnancy. N Engl J Med. 2002;346:1879-1891.
3. Ness RB, Soper DE, Holley RL, et al. Effectiveness of inpatient and outpatient treatment strategies for women with pelvic inflammatory disease: Results from the Pelvic Inflammatory Disease Evaluation and Clinical Health (PEACH) Randomized Trial. Am J Obstet Gynecol. 2002;186:929-937.
4. Cruse PJE, Foord R. A five-year prospective study of 23,649 surgical wounds. Arch Surg. 1973;107:206-210.
5. DelValle GO, Coombs P, Qualls C, Curet LB. Does closure of Camper fascia reduce the incidence of post-cesarean superficial wound disruption? Obstet Gynecol. 1992;80:1013-1016.
6. Naumann RW, Hauth JC, Owen J, Hodgkins PM, Lincoln T. Subcutaneous tissue approximation in relation to wound disruption after cesarean delivery in obese women. Obstet Gynecol. 1995;85:412-416.
7. Vermillion ST, Lamoutte C, Soper DE, Verdeja A. Wound infection after cesarean: effect of subcutaneous tissue thickness. Obstet Gynecol. 2000;95:923-926.
8. Chelmow D, Rodriguez EJ, Sabatini MM. Suture closure of subcutaneous fat and wound disruption after cesarean delivery: A meta analysis. Obstet Gynecol. 2004;103:974-980.
9. Sexually transmitted diseases treatment guidelines—2002 MMWR. 2002;51:1-79.
The author reports no financial relationships relevant to this article.
Postmenopausal HRT: What is fact, what is fiction?
HRT stops vaginal atrophy, hot flashes, and bone loss
Three applications form the basis for HRT in postmenopausal women:
- Hot flashes subside. Hot flashes occur with varying intensity in about 85% of women, and are effectively treated with estrogen, whether given orally, transdermally, or vaginally.1,2 As long as an appropriate blood level of the hormone is reached, hot flashes diminish.3-5 This reduction is dose-related.
- Measurable improvements in vaginal atrophy. Estrogen’s efficacy in relieving dryness, itching, burning, and dyspareunia is well demonstrated, regardless of the route of administration.3,6,7 A fall in vaginal pH from 6.0 to 5.0 after estrogen administration has been documented,8 as has the increase in the number of superficial cells of the vagina with exogenous estrogen.9
- HRT maintains or increases bone mineral density (BMD). Most estrogen preparations on the US market have been shown to improve BMD.10-15 “Improvement” means no significant loss, or an increase, in BMD. In the WHI, both vertebral and nonvertebral fractures diminished unequivocally in women using estrogen—alone or with a progestin.16,17 Other clinical trials also have shown increased BMD, as well as decreased urinary and serum markers of bone turnover.
Do new data link progestin to cancer?
Although compelling evidence supports the use of progestational agents in addition to estrogen to prevent endometrial hyperplasia and endometrial cancer,18 a 2005 report19 suggests that chronic, long-term use of estrogen with a progestin may increase the risk of endometrial carcinoma. Because this is the only study in which this risk has been found, corroboration is required.
Until then, give progestin at a sufficient dose and duration to inhibit endometrial hyperplasia.20-25
Effects on heart disease may be age-related
With notable exceptions, the overall conclusion of clinical trials and observational studies to date is that estrogen helps prevent coronary heart disease (CHD).26-30 This finding was first observed in the late 1980s with evidence that estrogen increases high-density lipoprotein (HDL) cholesterol and reduces total and low-density lipoprotein (LDL) cholesterol.31
Some experts argue that these observational trials are biased because many of the women taking estrogen had modified their lifestyles to maintain their weight, control their diet, and exercise regularly.32 Indeed, the randomized, placebo-controlled Heart and Estrogen Replacement Study (HERS) and both arms of the WHI trial found no evidence for a significant increase or decrease in CHD events.33-35
Time from menopause to HRT may be key
Both the HERS and WHI trials enrolled older women who had entered menopause a few months to several years before starting HRT.36 In addition, the estrogen-progestin arm of the WHI trial lacked sufficient power to detect a significant difference in CHD outcomes.37
The WHI findings contrast those of the large, ongoing, observational Nurses Health Study, which has shown a consistent decrease in CHD incidence in women who began HRT with the onset of menopausal symptoms.27-30 The most recent data suggest that the interval between menopause and the start of HRT may explain the different findings in randomized, controlled trials and observational studies.38 The WHI data support this theory: CHD was lower in women who began taking HRT within 5 years of menopause, compared with women who initiated HRT more than 5 years afterward.36 In addition, data from the estrogen-only arm of the WHI show fewer CHD events in women younger than 60.34
Several other studies support this hypothesis:
- The surgically postmenopausal cynomolgus macaque had a lower rate of atherosclerotic plaque development when estrogen was given, with or without a progestin.39,40
- In the Rancho Bernardo study, women who had used HRT had less cardiac calcification documented by computed tomography, compared with nonusers.41
- Estrogen has been shown, by measurement of carotid intimal medial thickness, to inhibit atherosclerotic plaque in humans.42
- Older women with established atherosclerosis do not undergo any significant change in plaque size with the use of exogenous estrogen.43
Although these findings support the use of estrogen or estrogen-progestin early after menopause as a way of preventing CHD, further clinical trials are needed.44
Stroke risk is small but real
Both arms of the WHI found an increased incidence of stroke in women using hormones, compared with nonusers.16,36 The exact mechanisms underlying this increased risk are unclear.
The actual attributable risk was an increase of 0.7 cases of stroke per 1,000 women per year over placebo in the estrogen-progestin arm,36 and 1.2 cases per 1,000 in the estrogen-only arm.16 The relative hazards were 1.31 (95% confidence interval [CI] 1.02–1.68) and 1.30 (95% CI 1.10–1.77), respectively.
Note that women in the estrogen-only arm had a greater incidence of hypertension and diabetes mellitus—known risk factors for stroke—than did women in the estrogen-progestin arm.16,36
VTE risk is twice as high in HRT users
Postmenopausal women who take estrogen have a higher risk of venous thromboembolism (VTE) than those who do not. This risk translated into a relative hazard of 2.06 (1.57–2.70) in the WHI estrogen-progestin arm, or an attributable risk of 3.6 cases per 1,000 women, compared with 1.8 cases per thousand in the control group.36
The absolute increased risk is 1.8 cases per 1,000 women, or, as expressed in the study itself, 18 cases per 10,000 women per year.
I have deliberately reduced the attributable risk to the number of cases per thousand because I believe this number is more easily understood by the patient and accurately demonstrates the low risk.
In the estrogen-only arm of the WHI, the hazard ratio for VTE was 1.33 (0.99–1.79), or an absolute increased risk of 0.7 cases per thousand—although this finding was not significant. The attributable risk was 2.7 cases per 1,000 women, compared with 2.0 cases per thousand among controls.16
Like stroke, the risk of VTE may be confounded by other factors besides use of exogenous estrogen.
No cause and effect for HRT and breast cancer
Nothing frightens women as much as breast cancer, and articles focusing on the relationship between breast cancer and HRT have drawn widespread attention. However, despite voluminous literature, the etiology of breast cancer remains elusive—and there is no evidence that either estrogen or progestins cause the disease.45,46 Rather, there is only an association between the use of estrogen, progestin, and breast cancer. Linking the finding of an increased risk with an implication of causality would be inappropriate.
Breast cancer risk with HRT is not consistently elevated, in studies
In fact, a qualitative review of observational studies from 1975 to 2000 found no significant increase or decrease in the risk of breast cancer with estrogen or estrogen-progestin in 80% of the reports.47
Risk factors for breast cancer (TABLE 1) include family history, obesity, late childbirth, and hormone therapy—but obesity and family history have higher relative risks than the use of HRT.48
TABLE 1
Relative risk of breast cancer
| CHARACTERISTIC | RELATIVE RISK |
|---|---|
| 2 family members with breast cancer | 14 |
| 1 family member with breast cancer | 2.2 |
| Obesity | 1.8 |
| Young age at menarche | 1.6 |
| Hormone therapy | 1.3 |
| >30 years of age at birth of first child | 1.3 |
| Menopause | 0.7 |
WHI arms find different risks
In the widely publicized WHI, women in the estrogen-progestin arm had an overall relative hazard for breast cancer of 1.24 (95% CI 1.01–1.54), but there was no increased risk in women who had never before used hormones.36 Women who had previously used hormones for 5 years or more did have an increased risk.36 The incidence of breast cancer in the study population was 3 cases per 1,000 women, and the excess number was 0.7 more cases with the use of estrogen-progestin (TABLE 2).
Conversely, in the estrogen-only arm of the WHI,16 the relative hazard for breast cancer was 0.77 (95% CI 0.59–1.01), and the reduction in risk was almost statistically significant. There are at least 2 potential explanations for the lower incidence of breast cancer in this arm:
- Without a progestin, estrogen increases breast density only minimally, allowing for easier mammographic interpretation.
- Women susceptible to breast cancer because of their previous use of estrogen may not have been present in the at-risk population in sufficient numbers to cause an increase.
TABLE 2
Extra cases of breast cancer, by risk factor
| RISK FACTOR | BREAST CANCERS DIAGNOSED OVER 20 YEARS FROM AGES 50 TO 70 (PER 1,000) | EXTRA BREAST CANCERS (PER 1,000) |
|---|---|---|
| Never used HRT | 45 | - |
| >5 years HRT | 47 | 2 |
| >10 years HRT | 51 | 6 |
| >15 years HRT | 57 | 12 |
| Late menopause (age 60) | 59 | 14 |
| Alcohol (2 drinks/day) | 72 | 27 |
| No daily exercise | 72 | 27 |
| Weight gain (>20 kg) | 90 | 45 |
| Reprinted from THE LANCET, Vol. 350: 1047–1059, Collaborative Group on Hormonal Factors in Breast Cancer, Breast cancer and hormone replacement therapy: collaborative reanalysis of data from 51 epidemiological studies of 52,705 women with breast cancer and 108,411 women without breast cancer. Copyright 1997, with permission from Elsevier | ||
HRT may promote, rather than induce, breast cancer
The role of hormones in the etiology of breast cancer is difficult to assess. The Million Women Study49 found that the elevated risk of breast cancer disappeared within 1 year of stopping HRT. This finding implies that hormones may be a promoter, rather than inducer, of neoplasms in the breast.
Breast cancer may be present in many women, but apparent in few
When autopsies were performed on women in their 40s who had died from other diseases, the incidence of breast cancer was 39%, but the clinical detection rate was only 1% for this population.50 This discrepancy suggests that neoplastic cells may be present in the body at any time, but become clinically apparent only under certain conditions.51
More recent data suggest that undifferentiated stem cells in the breast become dysfunctional and result in cancer.52 This theory is supported by the various histologic types of cancer found in the breast.
A weak link
Although it may be compelling to link hormone use with breast cancer, the association is weak and the incidence is lower than in other known relationships such as obesity. At present, the cause of breast neoplasia appears to be multifactorial.
1. Freedman RR. Pathophysiology and treatment of menopausal hot flashes. Semin Reprod Med. 2005;23:11-25.
2. Utian WH. Psychosocial and socioeconomic burden of vasomotor symptoms in menopause: a comprehensive review. Health Qual Life Outcomes. 2005;3:47.-
3. Archer DF. Percutaneous 17beta-estradiol gel for the treatment of vasomotor symptoms in postmenopausal women. Menopause. 2003;10:516-521.
4. Archer DF. Low-dose hormone therapy for postmenopausal women. Clin Obstet Gynecol. 2003;46:317-324.
5. Nelson HD. Commonly used types of postmenopausal estrogen for treatment of hot flashes: scientific review. JAMA. 2004;291:1610-1620.
6. Ballagh SA. Vaginal rings for menopausal symptom relief. Drugs Aging. 2004;21:757-766.
7. Speroff L. Efficacy and tolerability of a novel estradiol vaginal ring for relief of menopausal symptoms. Obstet Gynecol. 2003;102:823-834.
8. Notelovitz M. Urogenital atrophy and low-dose vaginal estrogen therapy. Menopause. 2000;7:140-142.
9. Utian WH, Burry KA, Archer DF, et al. Efficacy and safety of low, standard, and high dosages of an estradiol transdermal system (Esclim) compared with placebo on vasomotor symptoms in highly symptomatic menopausal patients. The Esclim Study Group. Am J Obstet Gynecol. 1999;181:71-79.
10. Christiansen C. Effects of drospirenone/estrogen combinations on bone metabolism. Climacteric. 2005;8(suppl 3):35-41.
11. Delmas PD, Confavreux E, Garnero P, et al. A combination of low doses of 17 beta-estradiol and norethisterone acetate prevents bone loss and normalizes bone turnover in postmenopausal women. Osteoporos Int. 2000;11:177-187.
12. Lindsay R, Gallagher JC, Kleerekoper M, Pickar JH. Effect of lower doses of conjugated equine estrogens with and without medroxyprogesterone acetate on bone in early postmenopausal women. JAMA. 2002;287:2668-2676.
13. Lindsay R, Gallagher JC, Kleerekoper M, Pickar JH. Bone response to treatment with lower doses of conjugated estrogens with and without medroxyprogesterone acetate in early postmenopausal women. Osteoporos Int. 2005;16:372-379.
14. Ravn P, Bidstrup M, Wasnich RD, et al. Alendronate and estrogen-progestin in the long-term prevention of bone loss: four-year results from the early postmenopausal intervention cohort study. A randomized, controlled trial. Ann Intern Med. 1999;131:935-942.
15. Recker RR, Davies KM, Dowd RM, Heaney RP. The effect of low-dose continuous estrogen and progesterone therapy with calcium and vitamin D on bone in elderly women. A randomized, controlled trial. Ann Intern Med. 1999;130:897-904.
16. Anderson GL, Limacher M, Assaf AR, et al. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women’s Health Initiative randomized controlled trial. JAMA. 2004;291:1701-1712.
17. Cauley JA, Robbins J, Chen Z, et al. Effects of estrogen plus progestin on risk of fracture and bone mineral density: the Women’s Health Initiative randomized trial. JAMA. 2003;290:1729-1738.
18. Archer DF. The effect of the duration of progestin use on the occurrence of endometrial cancer in postmenopausal women. Menopause. 2001;8:245-251.
19. Lacey JV, Jr, Brinton LA, Lubin JH, Sherman ME, Schatzkin A, Schairer C. Endometrial carcinoma risks among menopausal estrogen plus progestin and unopposed estrogen users in a cohort of postmenopausal women. Cancer Epidemiol Biomarkers Prev. 2005;14:1724-1731.
20. Archer DF, Furst K, Tipping D, Dain MP, Vandepol C. A randomized comparison of continuous combined transdermal delivery of estradiol-norethindrone acetate and estradiol alone for menopause. CombiPatch Study Group. Obstet Gynecol. 1999;94:498-503.
21. Bouchard P, De Cicco-Nardone F, Spielmann D, Garcea N. Bleeding profile and endometrial safety of continuous combined regimens 1mg 17beta-estradiol/trimegestone versus 1or 2 mg 17beta-estradiol/norethisterone acetate in postmenopausal women. Gynecol Endocrinol. 2005;21:142-148.
22. Kurman RJ, Felix JC, Archer DF, Nanavati N, Arce J, Moyer DL. Norethindrone acetate and estradiol-induced endometrial hyperplasia. Obstet Gynecol. 2000;96:373-379.
23. Speroff L, Rowan J, Symons J, Genant H, Wilborn W. The comparative effect on bone density, endometrium, and lipids of continuous hormones as replacement therapy (CHART study). A randomized controlled trial. JAMA. 1996;276:1397-1403.
24. Sturdee DW, Ulrich LG, Barlow DH, et al. The endometrial response to sequential and continuous combined oestrogen-progestogen replacement therapy. BJOG. 2000;107:1392-1400.
25. Ylikorkala O, Wahlstrom T, Caubel P, Lane R. Intermittent progestin administration as part of hormone replacement therapy: long-term comparison between estradiol 1mg combined with intermittent norgestimate and estradiol 2 mg combined with constant norethisterone acetate. Acta Obstet Gynecol Scand. 2002;81:654-660.
26. Espeland MA, Bush TL, Mebane-Sims I, et al. Rationale, design, and conduct of the PEPI Trial. Postmenopausal Estrogen/Progestin Interventions. Control Clin Trials. 1995;16(suppl):3S-19S.
27. Grodstein F, Stampfer MJ, Manson JE, et al. Postmenopausal estrogen and progestin use and the risk of cardiovascular disease. N Engl J Med. 1996;335:453-461.
28. Stampfer MJ, Colditz GA, Willett WC, et al. Postmenopausal estrogen therapy and cardiovascular disease. Ten-year follow-up from the nurses’ health study. N Engl J Med. 1991;325:756-762.
29. Grodstein F, Manson JE, Colditz GA, Willett WC, Speizer FE, Stampfer MJ. A prospective, observational study of postmenopausal hormone therapy and primary prevention of cardiovascular disease. Ann Intern Med. 2000;133:933-941.
30. Grodstein F, Manson JE, Stampfer MJ. Postmenopausal hormone use and secondary prevention of coronary events in the nurses’ health study. a prospective, observational study. Ann Intern Med. 2001;135:1-8.
31. Bush TL, Cowan LD, Barrett-Connor E, et al. Estrogen use and all-cause mortality. Preliminary results from the Lipid Research Clinics Program Follow-Up Study. JAMA. 1983;249:903-906.
32. Barrett-Connor E, Grady D. Hormone replacement therapy, heart disease, and other considerations. Annu Rev Public Health. 1998;19:55-72.
33. Grady D, Herrington D, Bittner V, et al. Cardiovascular disease outcomes during 6.8 years of hormone therapy: Heart and Estrogen/progestin Replacement Study follow-up (HERS II). JAMA. 2002;288:49-57.
34. Hsia J, Langer RD, Manson JE, et al. Conjugated equine estrogens and coronary heart disease: the Women’s Health Initiative. Arch Intern Med. 2006;166:357-365.
35. Manson JE, Hsia J, Johnson KC, et al. Estrogen plus progestin and the risk of coronary heart disease. N Engl J Med. 2003;349:523-534.
36. Rossouw JE, Anderson GL, Prentice RL, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288:321-333.
37. Naftolin F, Taylor HS, Karas R, et al. The Women’s Health Initiative could not have detected cardioprotective effects of starting hormone therapy during the menopausal transition. Fertil Steril. 2004;81:1498-1501.
38. Grodstein F, Manson JE, Stampfer MJ. Hormone therapy and coronary heart disease: the role of time since menopause and age at hormone initiation. J Womens Health (Larchmt). 2006;15:35-44.
39. Clarkson TB, Appt SE. Controversies about HRT-lessons from monkey models. Maturitas. 2005;51:64-74.
40. Wagner JD, Clarkson TB. The applicability of hormonal effects on atherosclerosis in animals to heart disease in postmenopausal women. Semin Reprod Med. 2005;23:149-156.
41. Barrett-Connor E, Laughlin GA. Hormone therapy and coronary artery calcification in symptomatic postmenopausal women: the Rancho Bernardo Study. Menopause. 2005;12:40-48.
42. Hodis HN, Mack WJ, Lobo RA, et al. Estrogen in the prevention of atherosclerosis. A randomized, double-blind, placebo-controlled trial. Ann Intern Med. 2001;135:939-953.
43. Herrington DM, Reboussin DM, Brosnihan KB, et al. Effects of estrogen replacement on the progression of coronary-artery atherosclerosis. N Engl J Med. 2000;343:522-529.
44. Harman SM, Brinton EA, Cedars M, et al. KEEPS: the Kronos Early Estrogen Prevention Study. Climacteric. 2005;8:3-12.
45. Clemons M, Goss P. Estrogen and the risk of breast cancer. N Engl J Med. 2001;344:276-285.
46. Yager JD, Davidson NE. Estrogen carcinogenesis in breast cancer. N Engl J Med. 2006;354:270-282.
47. Bush TL, Whiteman M, Flaws JA. Hormone replacement therapy and breast cancer: a qualitative review. Obstet Gynecol. 2001;98:498-508.
48. Morimoto LM, White E, Chen Z, et al. Obesity, body size, and risk of postmenopausal breast cancer: the Women’s Health Initiative (United States). Cancer Causes Control. 2002;13:741-751.
49. Beral V. Breast cancer and hormone-replacement therapy in the Million Women Study. Lancet. 2003;362:419-427.
50. Black WC, Welch HG. Advances in diagnostic imaging and overestimations of disease prevalence and the benefits of therapy. N Engl J Med. 1993;328:1237-1243.
51. Folkman J, Kalluri R. Cancer without disease. Nature. 2004;427:787.-
52. Dontu G, Al-Hajj M, Abdallah WM, Clarke MF, Wicha MS. Stem cells in normal breast development and breast cancer. Cell Prolif. 2003;36 Suppl 1:59-72.
HRT stops vaginal atrophy, hot flashes, and bone loss
Three applications form the basis for HRT in postmenopausal women:
- Hot flashes subside. Hot flashes occur with varying intensity in about 85% of women, and are effectively treated with estrogen, whether given orally, transdermally, or vaginally.1,2 As long as an appropriate blood level of the hormone is reached, hot flashes diminish.3-5 This reduction is dose-related.
- Measurable improvements in vaginal atrophy. Estrogen’s efficacy in relieving dryness, itching, burning, and dyspareunia is well demonstrated, regardless of the route of administration.3,6,7 A fall in vaginal pH from 6.0 to 5.0 after estrogen administration has been documented,8 as has the increase in the number of superficial cells of the vagina with exogenous estrogen.9
- HRT maintains or increases bone mineral density (BMD). Most estrogen preparations on the US market have been shown to improve BMD.10-15 “Improvement” means no significant loss, or an increase, in BMD. In the WHI, both vertebral and nonvertebral fractures diminished unequivocally in women using estrogen—alone or with a progestin.16,17 Other clinical trials also have shown increased BMD, as well as decreased urinary and serum markers of bone turnover.
Do new data link progestin to cancer?
Although compelling evidence supports the use of progestational agents in addition to estrogen to prevent endometrial hyperplasia and endometrial cancer,18 a 2005 report19 suggests that chronic, long-term use of estrogen with a progestin may increase the risk of endometrial carcinoma. Because this is the only study in which this risk has been found, corroboration is required.
Until then, give progestin at a sufficient dose and duration to inhibit endometrial hyperplasia.20-25
Effects on heart disease may be age-related
With notable exceptions, the overall conclusion of clinical trials and observational studies to date is that estrogen helps prevent coronary heart disease (CHD).26-30 This finding was first observed in the late 1980s with evidence that estrogen increases high-density lipoprotein (HDL) cholesterol and reduces total and low-density lipoprotein (LDL) cholesterol.31
Some experts argue that these observational trials are biased because many of the women taking estrogen had modified their lifestyles to maintain their weight, control their diet, and exercise regularly.32 Indeed, the randomized, placebo-controlled Heart and Estrogen Replacement Study (HERS) and both arms of the WHI trial found no evidence for a significant increase or decrease in CHD events.33-35
Time from menopause to HRT may be key
Both the HERS and WHI trials enrolled older women who had entered menopause a few months to several years before starting HRT.36 In addition, the estrogen-progestin arm of the WHI trial lacked sufficient power to detect a significant difference in CHD outcomes.37
The WHI findings contrast those of the large, ongoing, observational Nurses Health Study, which has shown a consistent decrease in CHD incidence in women who began HRT with the onset of menopausal symptoms.27-30 The most recent data suggest that the interval between menopause and the start of HRT may explain the different findings in randomized, controlled trials and observational studies.38 The WHI data support this theory: CHD was lower in women who began taking HRT within 5 years of menopause, compared with women who initiated HRT more than 5 years afterward.36 In addition, data from the estrogen-only arm of the WHI show fewer CHD events in women younger than 60.34
Several other studies support this hypothesis:
- The surgically postmenopausal cynomolgus macaque had a lower rate of atherosclerotic plaque development when estrogen was given, with or without a progestin.39,40
- In the Rancho Bernardo study, women who had used HRT had less cardiac calcification documented by computed tomography, compared with nonusers.41
- Estrogen has been shown, by measurement of carotid intimal medial thickness, to inhibit atherosclerotic plaque in humans.42
- Older women with established atherosclerosis do not undergo any significant change in plaque size with the use of exogenous estrogen.43
Although these findings support the use of estrogen or estrogen-progestin early after menopause as a way of preventing CHD, further clinical trials are needed.44
Stroke risk is small but real
Both arms of the WHI found an increased incidence of stroke in women using hormones, compared with nonusers.16,36 The exact mechanisms underlying this increased risk are unclear.
The actual attributable risk was an increase of 0.7 cases of stroke per 1,000 women per year over placebo in the estrogen-progestin arm,36 and 1.2 cases per 1,000 in the estrogen-only arm.16 The relative hazards were 1.31 (95% confidence interval [CI] 1.02–1.68) and 1.30 (95% CI 1.10–1.77), respectively.
Note that women in the estrogen-only arm had a greater incidence of hypertension and diabetes mellitus—known risk factors for stroke—than did women in the estrogen-progestin arm.16,36
VTE risk is twice as high in HRT users
Postmenopausal women who take estrogen have a higher risk of venous thromboembolism (VTE) than those who do not. This risk translated into a relative hazard of 2.06 (1.57–2.70) in the WHI estrogen-progestin arm, or an attributable risk of 3.6 cases per 1,000 women, compared with 1.8 cases per thousand in the control group.36
The absolute increased risk is 1.8 cases per 1,000 women, or, as expressed in the study itself, 18 cases per 10,000 women per year.
I have deliberately reduced the attributable risk to the number of cases per thousand because I believe this number is more easily understood by the patient and accurately demonstrates the low risk.
In the estrogen-only arm of the WHI, the hazard ratio for VTE was 1.33 (0.99–1.79), or an absolute increased risk of 0.7 cases per thousand—although this finding was not significant. The attributable risk was 2.7 cases per 1,000 women, compared with 2.0 cases per thousand among controls.16
Like stroke, the risk of VTE may be confounded by other factors besides use of exogenous estrogen.
No cause and effect for HRT and breast cancer
Nothing frightens women as much as breast cancer, and articles focusing on the relationship between breast cancer and HRT have drawn widespread attention. However, despite voluminous literature, the etiology of breast cancer remains elusive—and there is no evidence that either estrogen or progestins cause the disease.45,46 Rather, there is only an association between the use of estrogen, progestin, and breast cancer. Linking the finding of an increased risk with an implication of causality would be inappropriate.
Breast cancer risk with HRT is not consistently elevated, in studies
In fact, a qualitative review of observational studies from 1975 to 2000 found no significant increase or decrease in the risk of breast cancer with estrogen or estrogen-progestin in 80% of the reports.47
Risk factors for breast cancer (TABLE 1) include family history, obesity, late childbirth, and hormone therapy—but obesity and family history have higher relative risks than the use of HRT.48
TABLE 1
Relative risk of breast cancer
| CHARACTERISTIC | RELATIVE RISK |
|---|---|
| 2 family members with breast cancer | 14 |
| 1 family member with breast cancer | 2.2 |
| Obesity | 1.8 |
| Young age at menarche | 1.6 |
| Hormone therapy | 1.3 |
| >30 years of age at birth of first child | 1.3 |
| Menopause | 0.7 |
WHI arms find different risks
In the widely publicized WHI, women in the estrogen-progestin arm had an overall relative hazard for breast cancer of 1.24 (95% CI 1.01–1.54), but there was no increased risk in women who had never before used hormones.36 Women who had previously used hormones for 5 years or more did have an increased risk.36 The incidence of breast cancer in the study population was 3 cases per 1,000 women, and the excess number was 0.7 more cases with the use of estrogen-progestin (TABLE 2).
Conversely, in the estrogen-only arm of the WHI,16 the relative hazard for breast cancer was 0.77 (95% CI 0.59–1.01), and the reduction in risk was almost statistically significant. There are at least 2 potential explanations for the lower incidence of breast cancer in this arm:
- Without a progestin, estrogen increases breast density only minimally, allowing for easier mammographic interpretation.
- Women susceptible to breast cancer because of their previous use of estrogen may not have been present in the at-risk population in sufficient numbers to cause an increase.
TABLE 2
Extra cases of breast cancer, by risk factor
| RISK FACTOR | BREAST CANCERS DIAGNOSED OVER 20 YEARS FROM AGES 50 TO 70 (PER 1,000) | EXTRA BREAST CANCERS (PER 1,000) |
|---|---|---|
| Never used HRT | 45 | - |
| >5 years HRT | 47 | 2 |
| >10 years HRT | 51 | 6 |
| >15 years HRT | 57 | 12 |
| Late menopause (age 60) | 59 | 14 |
| Alcohol (2 drinks/day) | 72 | 27 |
| No daily exercise | 72 | 27 |
| Weight gain (>20 kg) | 90 | 45 |
| Reprinted from THE LANCET, Vol. 350: 1047–1059, Collaborative Group on Hormonal Factors in Breast Cancer, Breast cancer and hormone replacement therapy: collaborative reanalysis of data from 51 epidemiological studies of 52,705 women with breast cancer and 108,411 women without breast cancer. Copyright 1997, with permission from Elsevier | ||
HRT may promote, rather than induce, breast cancer
The role of hormones in the etiology of breast cancer is difficult to assess. The Million Women Study49 found that the elevated risk of breast cancer disappeared within 1 year of stopping HRT. This finding implies that hormones may be a promoter, rather than inducer, of neoplasms in the breast.
Breast cancer may be present in many women, but apparent in few
When autopsies were performed on women in their 40s who had died from other diseases, the incidence of breast cancer was 39%, but the clinical detection rate was only 1% for this population.50 This discrepancy suggests that neoplastic cells may be present in the body at any time, but become clinically apparent only under certain conditions.51
More recent data suggest that undifferentiated stem cells in the breast become dysfunctional and result in cancer.52 This theory is supported by the various histologic types of cancer found in the breast.
A weak link
Although it may be compelling to link hormone use with breast cancer, the association is weak and the incidence is lower than in other known relationships such as obesity. At present, the cause of breast neoplasia appears to be multifactorial.
HRT stops vaginal atrophy, hot flashes, and bone loss
Three applications form the basis for HRT in postmenopausal women:
- Hot flashes subside. Hot flashes occur with varying intensity in about 85% of women, and are effectively treated with estrogen, whether given orally, transdermally, or vaginally.1,2 As long as an appropriate blood level of the hormone is reached, hot flashes diminish.3-5 This reduction is dose-related.
- Measurable improvements in vaginal atrophy. Estrogen’s efficacy in relieving dryness, itching, burning, and dyspareunia is well demonstrated, regardless of the route of administration.3,6,7 A fall in vaginal pH from 6.0 to 5.0 after estrogen administration has been documented,8 as has the increase in the number of superficial cells of the vagina with exogenous estrogen.9
- HRT maintains or increases bone mineral density (BMD). Most estrogen preparations on the US market have been shown to improve BMD.10-15 “Improvement” means no significant loss, or an increase, in BMD. In the WHI, both vertebral and nonvertebral fractures diminished unequivocally in women using estrogen—alone or with a progestin.16,17 Other clinical trials also have shown increased BMD, as well as decreased urinary and serum markers of bone turnover.
Do new data link progestin to cancer?
Although compelling evidence supports the use of progestational agents in addition to estrogen to prevent endometrial hyperplasia and endometrial cancer,18 a 2005 report19 suggests that chronic, long-term use of estrogen with a progestin may increase the risk of endometrial carcinoma. Because this is the only study in which this risk has been found, corroboration is required.
Until then, give progestin at a sufficient dose and duration to inhibit endometrial hyperplasia.20-25
Effects on heart disease may be age-related
With notable exceptions, the overall conclusion of clinical trials and observational studies to date is that estrogen helps prevent coronary heart disease (CHD).26-30 This finding was first observed in the late 1980s with evidence that estrogen increases high-density lipoprotein (HDL) cholesterol and reduces total and low-density lipoprotein (LDL) cholesterol.31
Some experts argue that these observational trials are biased because many of the women taking estrogen had modified their lifestyles to maintain their weight, control their diet, and exercise regularly.32 Indeed, the randomized, placebo-controlled Heart and Estrogen Replacement Study (HERS) and both arms of the WHI trial found no evidence for a significant increase or decrease in CHD events.33-35
Time from menopause to HRT may be key
Both the HERS and WHI trials enrolled older women who had entered menopause a few months to several years before starting HRT.36 In addition, the estrogen-progestin arm of the WHI trial lacked sufficient power to detect a significant difference in CHD outcomes.37
The WHI findings contrast those of the large, ongoing, observational Nurses Health Study, which has shown a consistent decrease in CHD incidence in women who began HRT with the onset of menopausal symptoms.27-30 The most recent data suggest that the interval between menopause and the start of HRT may explain the different findings in randomized, controlled trials and observational studies.38 The WHI data support this theory: CHD was lower in women who began taking HRT within 5 years of menopause, compared with women who initiated HRT more than 5 years afterward.36 In addition, data from the estrogen-only arm of the WHI show fewer CHD events in women younger than 60.34
Several other studies support this hypothesis:
- The surgically postmenopausal cynomolgus macaque had a lower rate of atherosclerotic plaque development when estrogen was given, with or without a progestin.39,40
- In the Rancho Bernardo study, women who had used HRT had less cardiac calcification documented by computed tomography, compared with nonusers.41
- Estrogen has been shown, by measurement of carotid intimal medial thickness, to inhibit atherosclerotic plaque in humans.42
- Older women with established atherosclerosis do not undergo any significant change in plaque size with the use of exogenous estrogen.43
Although these findings support the use of estrogen or estrogen-progestin early after menopause as a way of preventing CHD, further clinical trials are needed.44
Stroke risk is small but real
Both arms of the WHI found an increased incidence of stroke in women using hormones, compared with nonusers.16,36 The exact mechanisms underlying this increased risk are unclear.
The actual attributable risk was an increase of 0.7 cases of stroke per 1,000 women per year over placebo in the estrogen-progestin arm,36 and 1.2 cases per 1,000 in the estrogen-only arm.16 The relative hazards were 1.31 (95% confidence interval [CI] 1.02–1.68) and 1.30 (95% CI 1.10–1.77), respectively.
Note that women in the estrogen-only arm had a greater incidence of hypertension and diabetes mellitus—known risk factors for stroke—than did women in the estrogen-progestin arm.16,36
VTE risk is twice as high in HRT users
Postmenopausal women who take estrogen have a higher risk of venous thromboembolism (VTE) than those who do not. This risk translated into a relative hazard of 2.06 (1.57–2.70) in the WHI estrogen-progestin arm, or an attributable risk of 3.6 cases per 1,000 women, compared with 1.8 cases per thousand in the control group.36
The absolute increased risk is 1.8 cases per 1,000 women, or, as expressed in the study itself, 18 cases per 10,000 women per year.
I have deliberately reduced the attributable risk to the number of cases per thousand because I believe this number is more easily understood by the patient and accurately demonstrates the low risk.
In the estrogen-only arm of the WHI, the hazard ratio for VTE was 1.33 (0.99–1.79), or an absolute increased risk of 0.7 cases per thousand—although this finding was not significant. The attributable risk was 2.7 cases per 1,000 women, compared with 2.0 cases per thousand among controls.16
Like stroke, the risk of VTE may be confounded by other factors besides use of exogenous estrogen.
No cause and effect for HRT and breast cancer
Nothing frightens women as much as breast cancer, and articles focusing on the relationship between breast cancer and HRT have drawn widespread attention. However, despite voluminous literature, the etiology of breast cancer remains elusive—and there is no evidence that either estrogen or progestins cause the disease.45,46 Rather, there is only an association between the use of estrogen, progestin, and breast cancer. Linking the finding of an increased risk with an implication of causality would be inappropriate.
Breast cancer risk with HRT is not consistently elevated, in studies
In fact, a qualitative review of observational studies from 1975 to 2000 found no significant increase or decrease in the risk of breast cancer with estrogen or estrogen-progestin in 80% of the reports.47
Risk factors for breast cancer (TABLE 1) include family history, obesity, late childbirth, and hormone therapy—but obesity and family history have higher relative risks than the use of HRT.48
TABLE 1
Relative risk of breast cancer
| CHARACTERISTIC | RELATIVE RISK |
|---|---|
| 2 family members with breast cancer | 14 |
| 1 family member with breast cancer | 2.2 |
| Obesity | 1.8 |
| Young age at menarche | 1.6 |
| Hormone therapy | 1.3 |
| >30 years of age at birth of first child | 1.3 |
| Menopause | 0.7 |
WHI arms find different risks
In the widely publicized WHI, women in the estrogen-progestin arm had an overall relative hazard for breast cancer of 1.24 (95% CI 1.01–1.54), but there was no increased risk in women who had never before used hormones.36 Women who had previously used hormones for 5 years or more did have an increased risk.36 The incidence of breast cancer in the study population was 3 cases per 1,000 women, and the excess number was 0.7 more cases with the use of estrogen-progestin (TABLE 2).
Conversely, in the estrogen-only arm of the WHI,16 the relative hazard for breast cancer was 0.77 (95% CI 0.59–1.01), and the reduction in risk was almost statistically significant. There are at least 2 potential explanations for the lower incidence of breast cancer in this arm:
- Without a progestin, estrogen increases breast density only minimally, allowing for easier mammographic interpretation.
- Women susceptible to breast cancer because of their previous use of estrogen may not have been present in the at-risk population in sufficient numbers to cause an increase.
TABLE 2
Extra cases of breast cancer, by risk factor
| RISK FACTOR | BREAST CANCERS DIAGNOSED OVER 20 YEARS FROM AGES 50 TO 70 (PER 1,000) | EXTRA BREAST CANCERS (PER 1,000) |
|---|---|---|
| Never used HRT | 45 | - |
| >5 years HRT | 47 | 2 |
| >10 years HRT | 51 | 6 |
| >15 years HRT | 57 | 12 |
| Late menopause (age 60) | 59 | 14 |
| Alcohol (2 drinks/day) | 72 | 27 |
| No daily exercise | 72 | 27 |
| Weight gain (>20 kg) | 90 | 45 |
| Reprinted from THE LANCET, Vol. 350: 1047–1059, Collaborative Group on Hormonal Factors in Breast Cancer, Breast cancer and hormone replacement therapy: collaborative reanalysis of data from 51 epidemiological studies of 52,705 women with breast cancer and 108,411 women without breast cancer. Copyright 1997, with permission from Elsevier | ||
HRT may promote, rather than induce, breast cancer
The role of hormones in the etiology of breast cancer is difficult to assess. The Million Women Study49 found that the elevated risk of breast cancer disappeared within 1 year of stopping HRT. This finding implies that hormones may be a promoter, rather than inducer, of neoplasms in the breast.
Breast cancer may be present in many women, but apparent in few
When autopsies were performed on women in their 40s who had died from other diseases, the incidence of breast cancer was 39%, but the clinical detection rate was only 1% for this population.50 This discrepancy suggests that neoplastic cells may be present in the body at any time, but become clinically apparent only under certain conditions.51
More recent data suggest that undifferentiated stem cells in the breast become dysfunctional and result in cancer.52 This theory is supported by the various histologic types of cancer found in the breast.
A weak link
Although it may be compelling to link hormone use with breast cancer, the association is weak and the incidence is lower than in other known relationships such as obesity. At present, the cause of breast neoplasia appears to be multifactorial.
1. Freedman RR. Pathophysiology and treatment of menopausal hot flashes. Semin Reprod Med. 2005;23:11-25.
2. Utian WH. Psychosocial and socioeconomic burden of vasomotor symptoms in menopause: a comprehensive review. Health Qual Life Outcomes. 2005;3:47.-
3. Archer DF. Percutaneous 17beta-estradiol gel for the treatment of vasomotor symptoms in postmenopausal women. Menopause. 2003;10:516-521.
4. Archer DF. Low-dose hormone therapy for postmenopausal women. Clin Obstet Gynecol. 2003;46:317-324.
5. Nelson HD. Commonly used types of postmenopausal estrogen for treatment of hot flashes: scientific review. JAMA. 2004;291:1610-1620.
6. Ballagh SA. Vaginal rings for menopausal symptom relief. Drugs Aging. 2004;21:757-766.
7. Speroff L. Efficacy and tolerability of a novel estradiol vaginal ring for relief of menopausal symptoms. Obstet Gynecol. 2003;102:823-834.
8. Notelovitz M. Urogenital atrophy and low-dose vaginal estrogen therapy. Menopause. 2000;7:140-142.
9. Utian WH, Burry KA, Archer DF, et al. Efficacy and safety of low, standard, and high dosages of an estradiol transdermal system (Esclim) compared with placebo on vasomotor symptoms in highly symptomatic menopausal patients. The Esclim Study Group. Am J Obstet Gynecol. 1999;181:71-79.
10. Christiansen C. Effects of drospirenone/estrogen combinations on bone metabolism. Climacteric. 2005;8(suppl 3):35-41.
11. Delmas PD, Confavreux E, Garnero P, et al. A combination of low doses of 17 beta-estradiol and norethisterone acetate prevents bone loss and normalizes bone turnover in postmenopausal women. Osteoporos Int. 2000;11:177-187.
12. Lindsay R, Gallagher JC, Kleerekoper M, Pickar JH. Effect of lower doses of conjugated equine estrogens with and without medroxyprogesterone acetate on bone in early postmenopausal women. JAMA. 2002;287:2668-2676.
13. Lindsay R, Gallagher JC, Kleerekoper M, Pickar JH. Bone response to treatment with lower doses of conjugated estrogens with and without medroxyprogesterone acetate in early postmenopausal women. Osteoporos Int. 2005;16:372-379.
14. Ravn P, Bidstrup M, Wasnich RD, et al. Alendronate and estrogen-progestin in the long-term prevention of bone loss: four-year results from the early postmenopausal intervention cohort study. A randomized, controlled trial. Ann Intern Med. 1999;131:935-942.
15. Recker RR, Davies KM, Dowd RM, Heaney RP. The effect of low-dose continuous estrogen and progesterone therapy with calcium and vitamin D on bone in elderly women. A randomized, controlled trial. Ann Intern Med. 1999;130:897-904.
16. Anderson GL, Limacher M, Assaf AR, et al. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women’s Health Initiative randomized controlled trial. JAMA. 2004;291:1701-1712.
17. Cauley JA, Robbins J, Chen Z, et al. Effects of estrogen plus progestin on risk of fracture and bone mineral density: the Women’s Health Initiative randomized trial. JAMA. 2003;290:1729-1738.
18. Archer DF. The effect of the duration of progestin use on the occurrence of endometrial cancer in postmenopausal women. Menopause. 2001;8:245-251.
19. Lacey JV, Jr, Brinton LA, Lubin JH, Sherman ME, Schatzkin A, Schairer C. Endometrial carcinoma risks among menopausal estrogen plus progestin and unopposed estrogen users in a cohort of postmenopausal women. Cancer Epidemiol Biomarkers Prev. 2005;14:1724-1731.
20. Archer DF, Furst K, Tipping D, Dain MP, Vandepol C. A randomized comparison of continuous combined transdermal delivery of estradiol-norethindrone acetate and estradiol alone for menopause. CombiPatch Study Group. Obstet Gynecol. 1999;94:498-503.
21. Bouchard P, De Cicco-Nardone F, Spielmann D, Garcea N. Bleeding profile and endometrial safety of continuous combined regimens 1mg 17beta-estradiol/trimegestone versus 1or 2 mg 17beta-estradiol/norethisterone acetate in postmenopausal women. Gynecol Endocrinol. 2005;21:142-148.
22. Kurman RJ, Felix JC, Archer DF, Nanavati N, Arce J, Moyer DL. Norethindrone acetate and estradiol-induced endometrial hyperplasia. Obstet Gynecol. 2000;96:373-379.
23. Speroff L, Rowan J, Symons J, Genant H, Wilborn W. The comparative effect on bone density, endometrium, and lipids of continuous hormones as replacement therapy (CHART study). A randomized controlled trial. JAMA. 1996;276:1397-1403.
24. Sturdee DW, Ulrich LG, Barlow DH, et al. The endometrial response to sequential and continuous combined oestrogen-progestogen replacement therapy. BJOG. 2000;107:1392-1400.
25. Ylikorkala O, Wahlstrom T, Caubel P, Lane R. Intermittent progestin administration as part of hormone replacement therapy: long-term comparison between estradiol 1mg combined with intermittent norgestimate and estradiol 2 mg combined with constant norethisterone acetate. Acta Obstet Gynecol Scand. 2002;81:654-660.
26. Espeland MA, Bush TL, Mebane-Sims I, et al. Rationale, design, and conduct of the PEPI Trial. Postmenopausal Estrogen/Progestin Interventions. Control Clin Trials. 1995;16(suppl):3S-19S.
27. Grodstein F, Stampfer MJ, Manson JE, et al. Postmenopausal estrogen and progestin use and the risk of cardiovascular disease. N Engl J Med. 1996;335:453-461.
28. Stampfer MJ, Colditz GA, Willett WC, et al. Postmenopausal estrogen therapy and cardiovascular disease. Ten-year follow-up from the nurses’ health study. N Engl J Med. 1991;325:756-762.
29. Grodstein F, Manson JE, Colditz GA, Willett WC, Speizer FE, Stampfer MJ. A prospective, observational study of postmenopausal hormone therapy and primary prevention of cardiovascular disease. Ann Intern Med. 2000;133:933-941.
30. Grodstein F, Manson JE, Stampfer MJ. Postmenopausal hormone use and secondary prevention of coronary events in the nurses’ health study. a prospective, observational study. Ann Intern Med. 2001;135:1-8.
31. Bush TL, Cowan LD, Barrett-Connor E, et al. Estrogen use and all-cause mortality. Preliminary results from the Lipid Research Clinics Program Follow-Up Study. JAMA. 1983;249:903-906.
32. Barrett-Connor E, Grady D. Hormone replacement therapy, heart disease, and other considerations. Annu Rev Public Health. 1998;19:55-72.
33. Grady D, Herrington D, Bittner V, et al. Cardiovascular disease outcomes during 6.8 years of hormone therapy: Heart and Estrogen/progestin Replacement Study follow-up (HERS II). JAMA. 2002;288:49-57.
34. Hsia J, Langer RD, Manson JE, et al. Conjugated equine estrogens and coronary heart disease: the Women’s Health Initiative. Arch Intern Med. 2006;166:357-365.
35. Manson JE, Hsia J, Johnson KC, et al. Estrogen plus progestin and the risk of coronary heart disease. N Engl J Med. 2003;349:523-534.
36. Rossouw JE, Anderson GL, Prentice RL, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288:321-333.
37. Naftolin F, Taylor HS, Karas R, et al. The Women’s Health Initiative could not have detected cardioprotective effects of starting hormone therapy during the menopausal transition. Fertil Steril. 2004;81:1498-1501.
38. Grodstein F, Manson JE, Stampfer MJ. Hormone therapy and coronary heart disease: the role of time since menopause and age at hormone initiation. J Womens Health (Larchmt). 2006;15:35-44.
39. Clarkson TB, Appt SE. Controversies about HRT-lessons from monkey models. Maturitas. 2005;51:64-74.
40. Wagner JD, Clarkson TB. The applicability of hormonal effects on atherosclerosis in animals to heart disease in postmenopausal women. Semin Reprod Med. 2005;23:149-156.
41. Barrett-Connor E, Laughlin GA. Hormone therapy and coronary artery calcification in symptomatic postmenopausal women: the Rancho Bernardo Study. Menopause. 2005;12:40-48.
42. Hodis HN, Mack WJ, Lobo RA, et al. Estrogen in the prevention of atherosclerosis. A randomized, double-blind, placebo-controlled trial. Ann Intern Med. 2001;135:939-953.
43. Herrington DM, Reboussin DM, Brosnihan KB, et al. Effects of estrogen replacement on the progression of coronary-artery atherosclerosis. N Engl J Med. 2000;343:522-529.
44. Harman SM, Brinton EA, Cedars M, et al. KEEPS: the Kronos Early Estrogen Prevention Study. Climacteric. 2005;8:3-12.
45. Clemons M, Goss P. Estrogen and the risk of breast cancer. N Engl J Med. 2001;344:276-285.
46. Yager JD, Davidson NE. Estrogen carcinogenesis in breast cancer. N Engl J Med. 2006;354:270-282.
47. Bush TL, Whiteman M, Flaws JA. Hormone replacement therapy and breast cancer: a qualitative review. Obstet Gynecol. 2001;98:498-508.
48. Morimoto LM, White E, Chen Z, et al. Obesity, body size, and risk of postmenopausal breast cancer: the Women’s Health Initiative (United States). Cancer Causes Control. 2002;13:741-751.
49. Beral V. Breast cancer and hormone-replacement therapy in the Million Women Study. Lancet. 2003;362:419-427.
50. Black WC, Welch HG. Advances in diagnostic imaging and overestimations of disease prevalence and the benefits of therapy. N Engl J Med. 1993;328:1237-1243.
51. Folkman J, Kalluri R. Cancer without disease. Nature. 2004;427:787.-
52. Dontu G, Al-Hajj M, Abdallah WM, Clarke MF, Wicha MS. Stem cells in normal breast development and breast cancer. Cell Prolif. 2003;36 Suppl 1:59-72.
1. Freedman RR. Pathophysiology and treatment of menopausal hot flashes. Semin Reprod Med. 2005;23:11-25.
2. Utian WH. Psychosocial and socioeconomic burden of vasomotor symptoms in menopause: a comprehensive review. Health Qual Life Outcomes. 2005;3:47.-
3. Archer DF. Percutaneous 17beta-estradiol gel for the treatment of vasomotor symptoms in postmenopausal women. Menopause. 2003;10:516-521.
4. Archer DF. Low-dose hormone therapy for postmenopausal women. Clin Obstet Gynecol. 2003;46:317-324.
5. Nelson HD. Commonly used types of postmenopausal estrogen for treatment of hot flashes: scientific review. JAMA. 2004;291:1610-1620.
6. Ballagh SA. Vaginal rings for menopausal symptom relief. Drugs Aging. 2004;21:757-766.
7. Speroff L. Efficacy and tolerability of a novel estradiol vaginal ring for relief of menopausal symptoms. Obstet Gynecol. 2003;102:823-834.
8. Notelovitz M. Urogenital atrophy and low-dose vaginal estrogen therapy. Menopause. 2000;7:140-142.
9. Utian WH, Burry KA, Archer DF, et al. Efficacy and safety of low, standard, and high dosages of an estradiol transdermal system (Esclim) compared with placebo on vasomotor symptoms in highly symptomatic menopausal patients. The Esclim Study Group. Am J Obstet Gynecol. 1999;181:71-79.
10. Christiansen C. Effects of drospirenone/estrogen combinations on bone metabolism. Climacteric. 2005;8(suppl 3):35-41.
11. Delmas PD, Confavreux E, Garnero P, et al. A combination of low doses of 17 beta-estradiol and norethisterone acetate prevents bone loss and normalizes bone turnover in postmenopausal women. Osteoporos Int. 2000;11:177-187.
12. Lindsay R, Gallagher JC, Kleerekoper M, Pickar JH. Effect of lower doses of conjugated equine estrogens with and without medroxyprogesterone acetate on bone in early postmenopausal women. JAMA. 2002;287:2668-2676.
13. Lindsay R, Gallagher JC, Kleerekoper M, Pickar JH. Bone response to treatment with lower doses of conjugated estrogens with and without medroxyprogesterone acetate in early postmenopausal women. Osteoporos Int. 2005;16:372-379.
14. Ravn P, Bidstrup M, Wasnich RD, et al. Alendronate and estrogen-progestin in the long-term prevention of bone loss: four-year results from the early postmenopausal intervention cohort study. A randomized, controlled trial. Ann Intern Med. 1999;131:935-942.
15. Recker RR, Davies KM, Dowd RM, Heaney RP. The effect of low-dose continuous estrogen and progesterone therapy with calcium and vitamin D on bone in elderly women. A randomized, controlled trial. Ann Intern Med. 1999;130:897-904.
16. Anderson GL, Limacher M, Assaf AR, et al. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women’s Health Initiative randomized controlled trial. JAMA. 2004;291:1701-1712.
17. Cauley JA, Robbins J, Chen Z, et al. Effects of estrogen plus progestin on risk of fracture and bone mineral density: the Women’s Health Initiative randomized trial. JAMA. 2003;290:1729-1738.
18. Archer DF. The effect of the duration of progestin use on the occurrence of endometrial cancer in postmenopausal women. Menopause. 2001;8:245-251.
19. Lacey JV, Jr, Brinton LA, Lubin JH, Sherman ME, Schatzkin A, Schairer C. Endometrial carcinoma risks among menopausal estrogen plus progestin and unopposed estrogen users in a cohort of postmenopausal women. Cancer Epidemiol Biomarkers Prev. 2005;14:1724-1731.
20. Archer DF, Furst K, Tipping D, Dain MP, Vandepol C. A randomized comparison of continuous combined transdermal delivery of estradiol-norethindrone acetate and estradiol alone for menopause. CombiPatch Study Group. Obstet Gynecol. 1999;94:498-503.
21. Bouchard P, De Cicco-Nardone F, Spielmann D, Garcea N. Bleeding profile and endometrial safety of continuous combined regimens 1mg 17beta-estradiol/trimegestone versus 1or 2 mg 17beta-estradiol/norethisterone acetate in postmenopausal women. Gynecol Endocrinol. 2005;21:142-148.
22. Kurman RJ, Felix JC, Archer DF, Nanavati N, Arce J, Moyer DL. Norethindrone acetate and estradiol-induced endometrial hyperplasia. Obstet Gynecol. 2000;96:373-379.
23. Speroff L, Rowan J, Symons J, Genant H, Wilborn W. The comparative effect on bone density, endometrium, and lipids of continuous hormones as replacement therapy (CHART study). A randomized controlled trial. JAMA. 1996;276:1397-1403.
24. Sturdee DW, Ulrich LG, Barlow DH, et al. The endometrial response to sequential and continuous combined oestrogen-progestogen replacement therapy. BJOG. 2000;107:1392-1400.
25. Ylikorkala O, Wahlstrom T, Caubel P, Lane R. Intermittent progestin administration as part of hormone replacement therapy: long-term comparison between estradiol 1mg combined with intermittent norgestimate and estradiol 2 mg combined with constant norethisterone acetate. Acta Obstet Gynecol Scand. 2002;81:654-660.
26. Espeland MA, Bush TL, Mebane-Sims I, et al. Rationale, design, and conduct of the PEPI Trial. Postmenopausal Estrogen/Progestin Interventions. Control Clin Trials. 1995;16(suppl):3S-19S.
27. Grodstein F, Stampfer MJ, Manson JE, et al. Postmenopausal estrogen and progestin use and the risk of cardiovascular disease. N Engl J Med. 1996;335:453-461.
28. Stampfer MJ, Colditz GA, Willett WC, et al. Postmenopausal estrogen therapy and cardiovascular disease. Ten-year follow-up from the nurses’ health study. N Engl J Med. 1991;325:756-762.
29. Grodstein F, Manson JE, Colditz GA, Willett WC, Speizer FE, Stampfer MJ. A prospective, observational study of postmenopausal hormone therapy and primary prevention of cardiovascular disease. Ann Intern Med. 2000;133:933-941.
30. Grodstein F, Manson JE, Stampfer MJ. Postmenopausal hormone use and secondary prevention of coronary events in the nurses’ health study. a prospective, observational study. Ann Intern Med. 2001;135:1-8.
31. Bush TL, Cowan LD, Barrett-Connor E, et al. Estrogen use and all-cause mortality. Preliminary results from the Lipid Research Clinics Program Follow-Up Study. JAMA. 1983;249:903-906.
32. Barrett-Connor E, Grady D. Hormone replacement therapy, heart disease, and other considerations. Annu Rev Public Health. 1998;19:55-72.
33. Grady D, Herrington D, Bittner V, et al. Cardiovascular disease outcomes during 6.8 years of hormone therapy: Heart and Estrogen/progestin Replacement Study follow-up (HERS II). JAMA. 2002;288:49-57.
34. Hsia J, Langer RD, Manson JE, et al. Conjugated equine estrogens and coronary heart disease: the Women’s Health Initiative. Arch Intern Med. 2006;166:357-365.
35. Manson JE, Hsia J, Johnson KC, et al. Estrogen plus progestin and the risk of coronary heart disease. N Engl J Med. 2003;349:523-534.
36. Rossouw JE, Anderson GL, Prentice RL, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288:321-333.
37. Naftolin F, Taylor HS, Karas R, et al. The Women’s Health Initiative could not have detected cardioprotective effects of starting hormone therapy during the menopausal transition. Fertil Steril. 2004;81:1498-1501.
38. Grodstein F, Manson JE, Stampfer MJ. Hormone therapy and coronary heart disease: the role of time since menopause and age at hormone initiation. J Womens Health (Larchmt). 2006;15:35-44.
39. Clarkson TB, Appt SE. Controversies about HRT-lessons from monkey models. Maturitas. 2005;51:64-74.
40. Wagner JD, Clarkson TB. The applicability of hormonal effects on atherosclerosis in animals to heart disease in postmenopausal women. Semin Reprod Med. 2005;23:149-156.
41. Barrett-Connor E, Laughlin GA. Hormone therapy and coronary artery calcification in symptomatic postmenopausal women: the Rancho Bernardo Study. Menopause. 2005;12:40-48.
42. Hodis HN, Mack WJ, Lobo RA, et al. Estrogen in the prevention of atherosclerosis. A randomized, double-blind, placebo-controlled trial. Ann Intern Med. 2001;135:939-953.
43. Herrington DM, Reboussin DM, Brosnihan KB, et al. Effects of estrogen replacement on the progression of coronary-artery atherosclerosis. N Engl J Med. 2000;343:522-529.
44. Harman SM, Brinton EA, Cedars M, et al. KEEPS: the Kronos Early Estrogen Prevention Study. Climacteric. 2005;8:3-12.
45. Clemons M, Goss P. Estrogen and the risk of breast cancer. N Engl J Med. 2001;344:276-285.
46. Yager JD, Davidson NE. Estrogen carcinogenesis in breast cancer. N Engl J Med. 2006;354:270-282.
47. Bush TL, Whiteman M, Flaws JA. Hormone replacement therapy and breast cancer: a qualitative review. Obstet Gynecol. 2001;98:498-508.
48. Morimoto LM, White E, Chen Z, et al. Obesity, body size, and risk of postmenopausal breast cancer: the Women’s Health Initiative (United States). Cancer Causes Control. 2002;13:741-751.
49. Beral V. Breast cancer and hormone-replacement therapy in the Million Women Study. Lancet. 2003;362:419-427.
50. Black WC, Welch HG. Advances in diagnostic imaging and overestimations of disease prevalence and the benefits of therapy. N Engl J Med. 1993;328:1237-1243.
51. Folkman J, Kalluri R. Cancer without disease. Nature. 2004;427:787.-
52. Dontu G, Al-Hajj M, Abdallah WM, Clarke MF, Wicha MS. Stem cells in normal breast development and breast cancer. Cell Prolif. 2003;36 Suppl 1:59-72.
Unattended Cardiopulmonary Sleep Studies to Diagnose Obstructive Sleep Apnea
Adult Kawasaki Disease
MENOPAUSE
The pendulum set in motion during the summer of 2002 continues its return swing toward a balanced perspective on hormone therapy.1,2 Now we have the opportunity to help our menopausal patients make decisions in a less emotional environment. Nonetheless, explaining the findings of the initial Women’s Health Initiative report—added to the findings of many subsequent WHI reports—is tricky even for the statistics experts.
How can we confidently counsel our patients?
American women fear breast cancer more than any other disease.3 That is understandable. Recent large studies, including WHI and the British Million Women Study, have been remarkably consistent in finding that combination hormone therapy (HT) modestly elevates the risk of being diagnosed with invasive breast cancer (relative risks or hazard ratios >1.0 and <2.0).2,4,5
Our challenge is to understand what the relative risk (RR) and hazard ratios mean as we guide our patients in making decisions about HT. To place these risks in a meaningful context, let’s compare them with risk factors for other cancers, other risk factors for breast cancer, and translate these relative risks into absolute risks, which patients understand much better.
We might start with lung cancer as a comparison. While the relative risk for breast cancer is less than 2 for menopausal women using combination HT, the relative risk of lung cancer is 15 to 30 for cigarette smokers.6
Other breast cancer risk factors of magnitudes similar to that of combination HT (RR <2.0) include menarche prior to age 12, high socioeconomic status, nulli-parity, never having nursed an infant, first full-term pregnancy after age 30, and alcohol consumption.7
Relative risk vs attributable risk
If our patients understand how the relative risks used in clinical trials translate into absolute or attributable risks, they will be better prepared to make sound choices regarding HT. Too often, however, relative risks are confused with attributable risk, which in this context is the incidence of an outcome (breast cancer) in women exposed to HT, minus the incidence in those not exposed.
The WHI trial of combination HT found an RR of 1.26 for breast cancer, meaning that HT users were 26% more likely to be diagnosed with this disease than were participants randomized to the placebo arm. Applying this RR to the absolute incidence of breast cancer observed in participants, the study’s authors noted that the attributable risk associated with use of combination HT was “low”: 8 additional breast tumors were diagnosed annually per 10,000 women (0.08% or ~0.1%) in the combination HT arm compared with the placebo arm.2 The annual breast cancer incidence in the HT arm (38 of 10,000 participants) is indeed some 26% higher than in the placebo arm (30 of 10,000 participants).
Keep in mind that WHI participants’ mean age at screening was 63 years and mean duration of HT use was 5.2 years. Because the incidence of breast cancer rises with age, and risk at baseline relates to attributable risk, the attributable risk associated with use of HT by younger menopausal women (those most likely to be seeking treatment for bothersome vasomotor symptoms) would be substantially lower than the 0.08% additional risk noted by the WHI investigators.
Most physicians misinterpret WHI—except ObGyns
- The findings of the Women’s Health Initiative are misunderstood by most primary care specialists, although ObGyns have a better understanding of the risks and benefits compared to other specialties.8
- We hypothesize that physicians who overestimated the increase or decrease in risk were making the error of confusing relative risk with absolute risk difference. There is a great need for physician education about the attributable risks and benefits of HRT.8
A survey of physicians underscored the difficulty of trying to translate relative risks into attributable risks, and thereby helps us understand how readily our patients may overestimate their own risk. In Williams and colleagues’ survey8 of Florida physicians, conducted in 2004, prior to publication of the results of the WHI estrogen-only trial, all respondents correctly indicated that HT was associated with an elevated risk of breast cancer.
When asked to characterize the attributable risk of breast cancer associated with HT (the choices were 0.1%, 3%, 10%, and 30%), fewer than half of physicians answered correctly that the attributable risk is 0.1%. More than half of physicians picked one of the wrong choices—all of which were higher than the correct attributable risk of breast cancer associated with HT.
Breast cancer risk: Estrogen-only vs combined HT
In 2004, results of the WHI clinical trial of women with hysterectomy indicated that estrogen was not associated with an increased risk of breast cancer,9 consistent with a number of large observational studies conducted in the United States10,11 and Sweden.12 Although the British Million Women study found a minimally elevated risk of breast cancer with use of estrogen alone, this risk (RR 1.3) was substantially lower than the risk associated with combination HT (RR 2.0).5 Other studies have also found that estrogen-only therapy, compared with combination HT, is associated with either less increased risk or no increased risk.13
Confident counseling
Overall, this body of evidence allows us to confidently counsel menopausal patients who have had a hysterectomy and are contemplating use of HT that use of estrogen-only HT is associated with no increased risk or a minimally increased risk of breast cancer.4
How does HT affect mammograms?
- Seven statistical models showed that both screening mammography and treatment have helped reduce the rate of death from breast cancer in the United States.14
- The overall diagnostic accuracy of digital and film mammography as a means of screening for breast cancer is similar, but digital mammography is more accurate in women younger than 50 years, women with radiographically dense breasts, and premenopausal or perimenopausal women.15
- Use of estrogen plus progestin is associated with increases in mammographic density.17
Mammography has contributed much to the detection and treatment of breast cancer, as well as its decline in mortality in the United States since the mid-1970s,14 reminding us of the importance of this screening test. Increased breast density reduces the sensitivity of mammograms,15 however, and use of HT (particularly combination HT) increases mammographic breast density.16,17 In the WHI trial of combination HT, women assigned to HT were more likely to have abnormal mammograms requiring recall.18
HT may become an indication for digital mammographic technique
In contrast with the findings of many observational studies, the breast tumors found in combination-HT users in the WHI trial were also larger, and disease stage was more advanced at diagnosis.18 As the WHI authors speculated, these sobering observations suggest that combination HT may have the dual impact of stimulating growth in existing tumors and delaying mammographic diagnosis.18 Speroff has suggested that the differences between findings of observational studies and the WHI reflect that the WHI participants were older postmenopausal women, who were more likely to have preexisting tumors, and therefore the results may be of less relevance to younger postmenopausal women using HT.16
For women with dense breast tissue, use of digital as opposed to film mammography enhances accuracy.15 Accordingly, as digital mammography becomes more available, use of HT may become an indication for use of digital mammographic technique among postmenopausal women.
Use digital if it’s available
In practice settings where digital mammography is available, its use should be considered in preference to film mammography for women using menopausal HT.
Evidence-based answers to 3 top concerns of patients
- Armed with a balanced perspective based on evidence rather than fear, our patients can make sound decisions on use of menopausal HT. We can advise our patients to consider the following evidence-based lines of reasoning:
How does HT affect risk of breast cancer?
- Combination hormone therapy vs estrogen only. Women considering whether to start HT, as well as those deciding whether to continue, need to understand the small but real risk of breast cancer attributable to combination HT, and that this risk is lower (if present at all) with estrogen-only therapy if they have had a hysterectomy.
- It may help to place the risks associated with combination HT in perspective with other breast cancer risk factors and risk factors for other cancers.
- Symptomatic women in their 50s contemplating initiation or ongoing use of HT should also recognize that any increased relative risk of breast cancer associated with use of combination HT translates into an attributable risk substantially lower than that faced by older menopausal women (the WHI population).
- Risk increases with longer duration of combination HT. A consistent finding of recent large studies is that the risk of breast cancer increased with longer durations of combination HT use.4 This observation supports clinical strategies that attempt to minimize the duration of combination HT use.
Does HT affect coronary risk?
- Timing of HT initiation in relation to menopause onset or to age might influence coronary risk, with users under age 60 possibly experiencing cardioprotection, concluded a Nurses Health Study report. This study provides reassurance for younger menopausal women (in their 50s) with respect to coronary artery disease risk associated with HT use.19
What is the right duration?
- Not indefinitely. Consistent with the guidelines of The North American Menopause Society and the American College of Obstetricians and Gynecologists,20,21 HT should not be prescribed indefinitely, but should be tailored to a woman’s need for treatment of bothersome menopausal symptoms.4
What if hot flashes reheat?
- More than half of the women with vasomotor symptoms at randomization to active conjugated equine estrogen+medroxyprogesterone acetate also reported these symptoms after discontinuing use of the study pills, concluded a study of symptom experience after stopping HT.22
Because we cannot predict in an individual woman how long menopausal symptoms will persist, and such symptoms often return after HT is discontinued,22 women with bothersome menopausal symptoms and their clinicians should collaboratively decide on use of HT based on an understanding of all the risks and benefits of this therapy.21
Dr. Kauntiz has received funding from Barr Laboratories, Berlex, Johnson & Johnson, and the National Institutes of Health. He is a speaker or consultant for the American College of Obstetricians and Gynecologists, the Association of Reproductive Health Professionals, Barr Laboratories, Berlex, Johnson & Johnson, Pfizer, and Procter & Gamble; and holds stock with Noven, Roche, and Sanofi-Aventis.
1. Utian WH. Update on menopause. OBG Management. 2005;17(5):51-63.
2. Writing Group for the Women’s Health Initiative. Risks and benefits of estrogen plus progestin in healthy postmenopausal women. Principal results from the Women’s Health Initiative Randomized Controlled Trial. JAMA. 2002;288:321-323.
3. Society for Women’s Health Research. Women’s fear of heart disease has almost doubled in three years, but breast cancer remains most feared disease. July 7, 2005. Available at: http://www.womenshealthresearch.org/press/releases/070705.htm. Accessed October 20, 2005.
4. Kaunitz AM. Hormone therapy and breast cancer risk-trumping fear with facts. Menopause. 2006; in press.
5. Million Women Study Collaborators. Breast cancer and hormone-replacement therapy in the Million Women Study. Lancet. 2003;362:419-427.
6. Sasco AJ, Secretan MB, Straif K. Tobacco smoking and cancer: a brief review of recent epidemiological evidence. Lung Cancer. 2004;45(suppl):S3-S9.
7. American Cancer Society. Breast cancer facts and figures 2005–2006. Atlanta, Ga: American Cancer Society, Inc. Available at: http://www.cancer.org/downloads/STT/CAFF2005BrF.pdf. Accessed October 20, 2005.
8. Williams RS, Christie D, Sistrom C. Assessment of the understanding of the risks and benefits of hormone replacement therapy (HRT) in primary care physicians. Am J Obstet Gynecol. 2005;193:551-558.
9. The Women’s Health Initiative Steering Committee. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy. The Women’s Health Initiative Randomized Controlled Trial. JAMA. 2004;291:1701-1712.
10. Weiss LK, Burkman RT, Cushing-Haugen KL, et al. Hormone replacement therapy regimens and breast cancer risk. Obstet Gynecol. 2002;100:1148-1158.
11. Li CI, Malone KE, Porter PL, et al. Relationship between long durations and different regimens of hormone therapy and risk of breast cancer. JAMA. 2003;289:3254-3263.
12. Olsson HL, Ingvar C, Bladström A. Hormone replacement therapy containing progestins and given continuously increases breast carcinoma risk in Sweden. Cancer. 2003;97:1387-1392.
13. Li CI. Postmenopausal hormone therapy and the risk of breast cancer: the view of an epidemiologist. Maturitas. 2004;49:44-50.
14. Berry DA, Cronin KA, Plevritis SK, et al, for the Cancer Intervention and Surveillance Modeling Network (CISNET) Collaborators. Effect of screening and adjuvant therapy on mortality from breast cancer. N Engl J Med. 2005;353:1784-1792.
15. Pisano ED, Gatsonis C, Hendrick E, et al, for the Digital Mammographic Imaging Screening Trial (DMIST) Investigators Group. Diagnostic performance of digital versus film mammography for breast-cancer screening. N Engl J Med. 2005;353:1773-1783.
16. Speroff L. Postmenopausal hormone therapy and breast cancer. Endocrine. 2004;24:211-216.
17. McTiernan A, Martin CF, Peck JD, et al, for the Women’s Health Initiative Mammogram Density Study Investigators. Estrogen-plus-progestin use and mammographic density in postmenopausal women: Women’s Health Initiative randomized trial. J Natl Cancer Inst. 2005;97:1366-1376.
18. Chlebowski RT, Hendrix SL, Langer RD, et al, for the WHI Investigators. Influence of estrogen plus progestin on breast cancer and mammography in healthy postmenopausal women: the Women’s Health Initiative randomized trial. JAMA. 2003;289:3243-3253.
19. Grodstein F, Manson JE, Stampfer MJ. Hormone therapy and coronary heart disease: the role of time since menopause and age at hormone initiation. J Womens Health (Larchmt). 2006;15:35-44.
20. North American Menopause Society. Recommendations for estrogen and progestin use in peri- and postmenopausal women: October 2004 position statement of The North American Menopause Society. Menopause. 2004;11:589-600.
21. American College of Obstetricians and Gynecologists. Hormone therapy. Obstet Gynecol. 2004;104(suppl):1S-4S.
22. Ockene JK, Barad DH, Cochrane BB, et al. Symptom experience after discontinuing use of estrogen plus progestin. JAMA. 2005;294:183-193.
The pendulum set in motion during the summer of 2002 continues its return swing toward a balanced perspective on hormone therapy.1,2 Now we have the opportunity to help our menopausal patients make decisions in a less emotional environment. Nonetheless, explaining the findings of the initial Women’s Health Initiative report—added to the findings of many subsequent WHI reports—is tricky even for the statistics experts.
How can we confidently counsel our patients?
American women fear breast cancer more than any other disease.3 That is understandable. Recent large studies, including WHI and the British Million Women Study, have been remarkably consistent in finding that combination hormone therapy (HT) modestly elevates the risk of being diagnosed with invasive breast cancer (relative risks or hazard ratios >1.0 and <2.0).2,4,5
Our challenge is to understand what the relative risk (RR) and hazard ratios mean as we guide our patients in making decisions about HT. To place these risks in a meaningful context, let’s compare them with risk factors for other cancers, other risk factors for breast cancer, and translate these relative risks into absolute risks, which patients understand much better.
We might start with lung cancer as a comparison. While the relative risk for breast cancer is less than 2 for menopausal women using combination HT, the relative risk of lung cancer is 15 to 30 for cigarette smokers.6
Other breast cancer risk factors of magnitudes similar to that of combination HT (RR <2.0) include menarche prior to age 12, high socioeconomic status, nulli-parity, never having nursed an infant, first full-term pregnancy after age 30, and alcohol consumption.7
Relative risk vs attributable risk
If our patients understand how the relative risks used in clinical trials translate into absolute or attributable risks, they will be better prepared to make sound choices regarding HT. Too often, however, relative risks are confused with attributable risk, which in this context is the incidence of an outcome (breast cancer) in women exposed to HT, minus the incidence in those not exposed.
The WHI trial of combination HT found an RR of 1.26 for breast cancer, meaning that HT users were 26% more likely to be diagnosed with this disease than were participants randomized to the placebo arm. Applying this RR to the absolute incidence of breast cancer observed in participants, the study’s authors noted that the attributable risk associated with use of combination HT was “low”: 8 additional breast tumors were diagnosed annually per 10,000 women (0.08% or ~0.1%) in the combination HT arm compared with the placebo arm.2 The annual breast cancer incidence in the HT arm (38 of 10,000 participants) is indeed some 26% higher than in the placebo arm (30 of 10,000 participants).
Keep in mind that WHI participants’ mean age at screening was 63 years and mean duration of HT use was 5.2 years. Because the incidence of breast cancer rises with age, and risk at baseline relates to attributable risk, the attributable risk associated with use of HT by younger menopausal women (those most likely to be seeking treatment for bothersome vasomotor symptoms) would be substantially lower than the 0.08% additional risk noted by the WHI investigators.
Most physicians misinterpret WHI—except ObGyns
- The findings of the Women’s Health Initiative are misunderstood by most primary care specialists, although ObGyns have a better understanding of the risks and benefits compared to other specialties.8
- We hypothesize that physicians who overestimated the increase or decrease in risk were making the error of confusing relative risk with absolute risk difference. There is a great need for physician education about the attributable risks and benefits of HRT.8
A survey of physicians underscored the difficulty of trying to translate relative risks into attributable risks, and thereby helps us understand how readily our patients may overestimate their own risk. In Williams and colleagues’ survey8 of Florida physicians, conducted in 2004, prior to publication of the results of the WHI estrogen-only trial, all respondents correctly indicated that HT was associated with an elevated risk of breast cancer.
When asked to characterize the attributable risk of breast cancer associated with HT (the choices were 0.1%, 3%, 10%, and 30%), fewer than half of physicians answered correctly that the attributable risk is 0.1%. More than half of physicians picked one of the wrong choices—all of which were higher than the correct attributable risk of breast cancer associated with HT.
Breast cancer risk: Estrogen-only vs combined HT
In 2004, results of the WHI clinical trial of women with hysterectomy indicated that estrogen was not associated with an increased risk of breast cancer,9 consistent with a number of large observational studies conducted in the United States10,11 and Sweden.12 Although the British Million Women study found a minimally elevated risk of breast cancer with use of estrogen alone, this risk (RR 1.3) was substantially lower than the risk associated with combination HT (RR 2.0).5 Other studies have also found that estrogen-only therapy, compared with combination HT, is associated with either less increased risk or no increased risk.13
Confident counseling
Overall, this body of evidence allows us to confidently counsel menopausal patients who have had a hysterectomy and are contemplating use of HT that use of estrogen-only HT is associated with no increased risk or a minimally increased risk of breast cancer.4
How does HT affect mammograms?
- Seven statistical models showed that both screening mammography and treatment have helped reduce the rate of death from breast cancer in the United States.14
- The overall diagnostic accuracy of digital and film mammography as a means of screening for breast cancer is similar, but digital mammography is more accurate in women younger than 50 years, women with radiographically dense breasts, and premenopausal or perimenopausal women.15
- Use of estrogen plus progestin is associated with increases in mammographic density.17
Mammography has contributed much to the detection and treatment of breast cancer, as well as its decline in mortality in the United States since the mid-1970s,14 reminding us of the importance of this screening test. Increased breast density reduces the sensitivity of mammograms,15 however, and use of HT (particularly combination HT) increases mammographic breast density.16,17 In the WHI trial of combination HT, women assigned to HT were more likely to have abnormal mammograms requiring recall.18
HT may become an indication for digital mammographic technique
In contrast with the findings of many observational studies, the breast tumors found in combination-HT users in the WHI trial were also larger, and disease stage was more advanced at diagnosis.18 As the WHI authors speculated, these sobering observations suggest that combination HT may have the dual impact of stimulating growth in existing tumors and delaying mammographic diagnosis.18 Speroff has suggested that the differences between findings of observational studies and the WHI reflect that the WHI participants were older postmenopausal women, who were more likely to have preexisting tumors, and therefore the results may be of less relevance to younger postmenopausal women using HT.16
For women with dense breast tissue, use of digital as opposed to film mammography enhances accuracy.15 Accordingly, as digital mammography becomes more available, use of HT may become an indication for use of digital mammographic technique among postmenopausal women.
Use digital if it’s available
In practice settings where digital mammography is available, its use should be considered in preference to film mammography for women using menopausal HT.
Evidence-based answers to 3 top concerns of patients
- Armed with a balanced perspective based on evidence rather than fear, our patients can make sound decisions on use of menopausal HT. We can advise our patients to consider the following evidence-based lines of reasoning:
How does HT affect risk of breast cancer?
- Combination hormone therapy vs estrogen only. Women considering whether to start HT, as well as those deciding whether to continue, need to understand the small but real risk of breast cancer attributable to combination HT, and that this risk is lower (if present at all) with estrogen-only therapy if they have had a hysterectomy.
- It may help to place the risks associated with combination HT in perspective with other breast cancer risk factors and risk factors for other cancers.
- Symptomatic women in their 50s contemplating initiation or ongoing use of HT should also recognize that any increased relative risk of breast cancer associated with use of combination HT translates into an attributable risk substantially lower than that faced by older menopausal women (the WHI population).
- Risk increases with longer duration of combination HT. A consistent finding of recent large studies is that the risk of breast cancer increased with longer durations of combination HT use.4 This observation supports clinical strategies that attempt to minimize the duration of combination HT use.
Does HT affect coronary risk?
- Timing of HT initiation in relation to menopause onset or to age might influence coronary risk, with users under age 60 possibly experiencing cardioprotection, concluded a Nurses Health Study report. This study provides reassurance for younger menopausal women (in their 50s) with respect to coronary artery disease risk associated with HT use.19
What is the right duration?
- Not indefinitely. Consistent with the guidelines of The North American Menopause Society and the American College of Obstetricians and Gynecologists,20,21 HT should not be prescribed indefinitely, but should be tailored to a woman’s need for treatment of bothersome menopausal symptoms.4
What if hot flashes reheat?
- More than half of the women with vasomotor symptoms at randomization to active conjugated equine estrogen+medroxyprogesterone acetate also reported these symptoms after discontinuing use of the study pills, concluded a study of symptom experience after stopping HT.22
Because we cannot predict in an individual woman how long menopausal symptoms will persist, and such symptoms often return after HT is discontinued,22 women with bothersome menopausal symptoms and their clinicians should collaboratively decide on use of HT based on an understanding of all the risks and benefits of this therapy.21
Dr. Kauntiz has received funding from Barr Laboratories, Berlex, Johnson & Johnson, and the National Institutes of Health. He is a speaker or consultant for the American College of Obstetricians and Gynecologists, the Association of Reproductive Health Professionals, Barr Laboratories, Berlex, Johnson & Johnson, Pfizer, and Procter & Gamble; and holds stock with Noven, Roche, and Sanofi-Aventis.
The pendulum set in motion during the summer of 2002 continues its return swing toward a balanced perspective on hormone therapy.1,2 Now we have the opportunity to help our menopausal patients make decisions in a less emotional environment. Nonetheless, explaining the findings of the initial Women’s Health Initiative report—added to the findings of many subsequent WHI reports—is tricky even for the statistics experts.
How can we confidently counsel our patients?
American women fear breast cancer more than any other disease.3 That is understandable. Recent large studies, including WHI and the British Million Women Study, have been remarkably consistent in finding that combination hormone therapy (HT) modestly elevates the risk of being diagnosed with invasive breast cancer (relative risks or hazard ratios >1.0 and <2.0).2,4,5
Our challenge is to understand what the relative risk (RR) and hazard ratios mean as we guide our patients in making decisions about HT. To place these risks in a meaningful context, let’s compare them with risk factors for other cancers, other risk factors for breast cancer, and translate these relative risks into absolute risks, which patients understand much better.
We might start with lung cancer as a comparison. While the relative risk for breast cancer is less than 2 for menopausal women using combination HT, the relative risk of lung cancer is 15 to 30 for cigarette smokers.6
Other breast cancer risk factors of magnitudes similar to that of combination HT (RR <2.0) include menarche prior to age 12, high socioeconomic status, nulli-parity, never having nursed an infant, first full-term pregnancy after age 30, and alcohol consumption.7
Relative risk vs attributable risk
If our patients understand how the relative risks used in clinical trials translate into absolute or attributable risks, they will be better prepared to make sound choices regarding HT. Too often, however, relative risks are confused with attributable risk, which in this context is the incidence of an outcome (breast cancer) in women exposed to HT, minus the incidence in those not exposed.
The WHI trial of combination HT found an RR of 1.26 for breast cancer, meaning that HT users were 26% more likely to be diagnosed with this disease than were participants randomized to the placebo arm. Applying this RR to the absolute incidence of breast cancer observed in participants, the study’s authors noted that the attributable risk associated with use of combination HT was “low”: 8 additional breast tumors were diagnosed annually per 10,000 women (0.08% or ~0.1%) in the combination HT arm compared with the placebo arm.2 The annual breast cancer incidence in the HT arm (38 of 10,000 participants) is indeed some 26% higher than in the placebo arm (30 of 10,000 participants).
Keep in mind that WHI participants’ mean age at screening was 63 years and mean duration of HT use was 5.2 years. Because the incidence of breast cancer rises with age, and risk at baseline relates to attributable risk, the attributable risk associated with use of HT by younger menopausal women (those most likely to be seeking treatment for bothersome vasomotor symptoms) would be substantially lower than the 0.08% additional risk noted by the WHI investigators.
Most physicians misinterpret WHI—except ObGyns
- The findings of the Women’s Health Initiative are misunderstood by most primary care specialists, although ObGyns have a better understanding of the risks and benefits compared to other specialties.8
- We hypothesize that physicians who overestimated the increase or decrease in risk were making the error of confusing relative risk with absolute risk difference. There is a great need for physician education about the attributable risks and benefits of HRT.8
A survey of physicians underscored the difficulty of trying to translate relative risks into attributable risks, and thereby helps us understand how readily our patients may overestimate their own risk. In Williams and colleagues’ survey8 of Florida physicians, conducted in 2004, prior to publication of the results of the WHI estrogen-only trial, all respondents correctly indicated that HT was associated with an elevated risk of breast cancer.
When asked to characterize the attributable risk of breast cancer associated with HT (the choices were 0.1%, 3%, 10%, and 30%), fewer than half of physicians answered correctly that the attributable risk is 0.1%. More than half of physicians picked one of the wrong choices—all of which were higher than the correct attributable risk of breast cancer associated with HT.
Breast cancer risk: Estrogen-only vs combined HT
In 2004, results of the WHI clinical trial of women with hysterectomy indicated that estrogen was not associated with an increased risk of breast cancer,9 consistent with a number of large observational studies conducted in the United States10,11 and Sweden.12 Although the British Million Women study found a minimally elevated risk of breast cancer with use of estrogen alone, this risk (RR 1.3) was substantially lower than the risk associated with combination HT (RR 2.0).5 Other studies have also found that estrogen-only therapy, compared with combination HT, is associated with either less increased risk or no increased risk.13
Confident counseling
Overall, this body of evidence allows us to confidently counsel menopausal patients who have had a hysterectomy and are contemplating use of HT that use of estrogen-only HT is associated with no increased risk or a minimally increased risk of breast cancer.4
How does HT affect mammograms?
- Seven statistical models showed that both screening mammography and treatment have helped reduce the rate of death from breast cancer in the United States.14
- The overall diagnostic accuracy of digital and film mammography as a means of screening for breast cancer is similar, but digital mammography is more accurate in women younger than 50 years, women with radiographically dense breasts, and premenopausal or perimenopausal women.15
- Use of estrogen plus progestin is associated with increases in mammographic density.17
Mammography has contributed much to the detection and treatment of breast cancer, as well as its decline in mortality in the United States since the mid-1970s,14 reminding us of the importance of this screening test. Increased breast density reduces the sensitivity of mammograms,15 however, and use of HT (particularly combination HT) increases mammographic breast density.16,17 In the WHI trial of combination HT, women assigned to HT were more likely to have abnormal mammograms requiring recall.18
HT may become an indication for digital mammographic technique
In contrast with the findings of many observational studies, the breast tumors found in combination-HT users in the WHI trial were also larger, and disease stage was more advanced at diagnosis.18 As the WHI authors speculated, these sobering observations suggest that combination HT may have the dual impact of stimulating growth in existing tumors and delaying mammographic diagnosis.18 Speroff has suggested that the differences between findings of observational studies and the WHI reflect that the WHI participants were older postmenopausal women, who were more likely to have preexisting tumors, and therefore the results may be of less relevance to younger postmenopausal women using HT.16
For women with dense breast tissue, use of digital as opposed to film mammography enhances accuracy.15 Accordingly, as digital mammography becomes more available, use of HT may become an indication for use of digital mammographic technique among postmenopausal women.
Use digital if it’s available
In practice settings where digital mammography is available, its use should be considered in preference to film mammography for women using menopausal HT.
Evidence-based answers to 3 top concerns of patients
- Armed with a balanced perspective based on evidence rather than fear, our patients can make sound decisions on use of menopausal HT. We can advise our patients to consider the following evidence-based lines of reasoning:
How does HT affect risk of breast cancer?
- Combination hormone therapy vs estrogen only. Women considering whether to start HT, as well as those deciding whether to continue, need to understand the small but real risk of breast cancer attributable to combination HT, and that this risk is lower (if present at all) with estrogen-only therapy if they have had a hysterectomy.
- It may help to place the risks associated with combination HT in perspective with other breast cancer risk factors and risk factors for other cancers.
- Symptomatic women in their 50s contemplating initiation or ongoing use of HT should also recognize that any increased relative risk of breast cancer associated with use of combination HT translates into an attributable risk substantially lower than that faced by older menopausal women (the WHI population).
- Risk increases with longer duration of combination HT. A consistent finding of recent large studies is that the risk of breast cancer increased with longer durations of combination HT use.4 This observation supports clinical strategies that attempt to minimize the duration of combination HT use.
Does HT affect coronary risk?
- Timing of HT initiation in relation to menopause onset or to age might influence coronary risk, with users under age 60 possibly experiencing cardioprotection, concluded a Nurses Health Study report. This study provides reassurance for younger menopausal women (in their 50s) with respect to coronary artery disease risk associated with HT use.19
What is the right duration?
- Not indefinitely. Consistent with the guidelines of The North American Menopause Society and the American College of Obstetricians and Gynecologists,20,21 HT should not be prescribed indefinitely, but should be tailored to a woman’s need for treatment of bothersome menopausal symptoms.4
What if hot flashes reheat?
- More than half of the women with vasomotor symptoms at randomization to active conjugated equine estrogen+medroxyprogesterone acetate also reported these symptoms after discontinuing use of the study pills, concluded a study of symptom experience after stopping HT.22
Because we cannot predict in an individual woman how long menopausal symptoms will persist, and such symptoms often return after HT is discontinued,22 women with bothersome menopausal symptoms and their clinicians should collaboratively decide on use of HT based on an understanding of all the risks and benefits of this therapy.21
Dr. Kauntiz has received funding from Barr Laboratories, Berlex, Johnson & Johnson, and the National Institutes of Health. He is a speaker or consultant for the American College of Obstetricians and Gynecologists, the Association of Reproductive Health Professionals, Barr Laboratories, Berlex, Johnson & Johnson, Pfizer, and Procter & Gamble; and holds stock with Noven, Roche, and Sanofi-Aventis.
1. Utian WH. Update on menopause. OBG Management. 2005;17(5):51-63.
2. Writing Group for the Women’s Health Initiative. Risks and benefits of estrogen plus progestin in healthy postmenopausal women. Principal results from the Women’s Health Initiative Randomized Controlled Trial. JAMA. 2002;288:321-323.
3. Society for Women’s Health Research. Women’s fear of heart disease has almost doubled in three years, but breast cancer remains most feared disease. July 7, 2005. Available at: http://www.womenshealthresearch.org/press/releases/070705.htm. Accessed October 20, 2005.
4. Kaunitz AM. Hormone therapy and breast cancer risk-trumping fear with facts. Menopause. 2006; in press.
5. Million Women Study Collaborators. Breast cancer and hormone-replacement therapy in the Million Women Study. Lancet. 2003;362:419-427.
6. Sasco AJ, Secretan MB, Straif K. Tobacco smoking and cancer: a brief review of recent epidemiological evidence. Lung Cancer. 2004;45(suppl):S3-S9.
7. American Cancer Society. Breast cancer facts and figures 2005–2006. Atlanta, Ga: American Cancer Society, Inc. Available at: http://www.cancer.org/downloads/STT/CAFF2005BrF.pdf. Accessed October 20, 2005.
8. Williams RS, Christie D, Sistrom C. Assessment of the understanding of the risks and benefits of hormone replacement therapy (HRT) in primary care physicians. Am J Obstet Gynecol. 2005;193:551-558.
9. The Women’s Health Initiative Steering Committee. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy. The Women’s Health Initiative Randomized Controlled Trial. JAMA. 2004;291:1701-1712.
10. Weiss LK, Burkman RT, Cushing-Haugen KL, et al. Hormone replacement therapy regimens and breast cancer risk. Obstet Gynecol. 2002;100:1148-1158.
11. Li CI, Malone KE, Porter PL, et al. Relationship between long durations and different regimens of hormone therapy and risk of breast cancer. JAMA. 2003;289:3254-3263.
12. Olsson HL, Ingvar C, Bladström A. Hormone replacement therapy containing progestins and given continuously increases breast carcinoma risk in Sweden. Cancer. 2003;97:1387-1392.
13. Li CI. Postmenopausal hormone therapy and the risk of breast cancer: the view of an epidemiologist. Maturitas. 2004;49:44-50.
14. Berry DA, Cronin KA, Plevritis SK, et al, for the Cancer Intervention and Surveillance Modeling Network (CISNET) Collaborators. Effect of screening and adjuvant therapy on mortality from breast cancer. N Engl J Med. 2005;353:1784-1792.
15. Pisano ED, Gatsonis C, Hendrick E, et al, for the Digital Mammographic Imaging Screening Trial (DMIST) Investigators Group. Diagnostic performance of digital versus film mammography for breast-cancer screening. N Engl J Med. 2005;353:1773-1783.
16. Speroff L. Postmenopausal hormone therapy and breast cancer. Endocrine. 2004;24:211-216.
17. McTiernan A, Martin CF, Peck JD, et al, for the Women’s Health Initiative Mammogram Density Study Investigators. Estrogen-plus-progestin use and mammographic density in postmenopausal women: Women’s Health Initiative randomized trial. J Natl Cancer Inst. 2005;97:1366-1376.
18. Chlebowski RT, Hendrix SL, Langer RD, et al, for the WHI Investigators. Influence of estrogen plus progestin on breast cancer and mammography in healthy postmenopausal women: the Women’s Health Initiative randomized trial. JAMA. 2003;289:3243-3253.
19. Grodstein F, Manson JE, Stampfer MJ. Hormone therapy and coronary heart disease: the role of time since menopause and age at hormone initiation. J Womens Health (Larchmt). 2006;15:35-44.
20. North American Menopause Society. Recommendations for estrogen and progestin use in peri- and postmenopausal women: October 2004 position statement of The North American Menopause Society. Menopause. 2004;11:589-600.
21. American College of Obstetricians and Gynecologists. Hormone therapy. Obstet Gynecol. 2004;104(suppl):1S-4S.
22. Ockene JK, Barad DH, Cochrane BB, et al. Symptom experience after discontinuing use of estrogen plus progestin. JAMA. 2005;294:183-193.
1. Utian WH. Update on menopause. OBG Management. 2005;17(5):51-63.
2. Writing Group for the Women’s Health Initiative. Risks and benefits of estrogen plus progestin in healthy postmenopausal women. Principal results from the Women’s Health Initiative Randomized Controlled Trial. JAMA. 2002;288:321-323.
3. Society for Women’s Health Research. Women’s fear of heart disease has almost doubled in three years, but breast cancer remains most feared disease. July 7, 2005. Available at: http://www.womenshealthresearch.org/press/releases/070705.htm. Accessed October 20, 2005.
4. Kaunitz AM. Hormone therapy and breast cancer risk-trumping fear with facts. Menopause. 2006; in press.
5. Million Women Study Collaborators. Breast cancer and hormone-replacement therapy in the Million Women Study. Lancet. 2003;362:419-427.
6. Sasco AJ, Secretan MB, Straif K. Tobacco smoking and cancer: a brief review of recent epidemiological evidence. Lung Cancer. 2004;45(suppl):S3-S9.
7. American Cancer Society. Breast cancer facts and figures 2005–2006. Atlanta, Ga: American Cancer Society, Inc. Available at: http://www.cancer.org/downloads/STT/CAFF2005BrF.pdf. Accessed October 20, 2005.
8. Williams RS, Christie D, Sistrom C. Assessment of the understanding of the risks and benefits of hormone replacement therapy (HRT) in primary care physicians. Am J Obstet Gynecol. 2005;193:551-558.
9. The Women’s Health Initiative Steering Committee. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy. The Women’s Health Initiative Randomized Controlled Trial. JAMA. 2004;291:1701-1712.
10. Weiss LK, Burkman RT, Cushing-Haugen KL, et al. Hormone replacement therapy regimens and breast cancer risk. Obstet Gynecol. 2002;100:1148-1158.
11. Li CI, Malone KE, Porter PL, et al. Relationship between long durations and different regimens of hormone therapy and risk of breast cancer. JAMA. 2003;289:3254-3263.
12. Olsson HL, Ingvar C, Bladström A. Hormone replacement therapy containing progestins and given continuously increases breast carcinoma risk in Sweden. Cancer. 2003;97:1387-1392.
13. Li CI. Postmenopausal hormone therapy and the risk of breast cancer: the view of an epidemiologist. Maturitas. 2004;49:44-50.
14. Berry DA, Cronin KA, Plevritis SK, et al, for the Cancer Intervention and Surveillance Modeling Network (CISNET) Collaborators. Effect of screening and adjuvant therapy on mortality from breast cancer. N Engl J Med. 2005;353:1784-1792.
15. Pisano ED, Gatsonis C, Hendrick E, et al, for the Digital Mammographic Imaging Screening Trial (DMIST) Investigators Group. Diagnostic performance of digital versus film mammography for breast-cancer screening. N Engl J Med. 2005;353:1773-1783.
16. Speroff L. Postmenopausal hormone therapy and breast cancer. Endocrine. 2004;24:211-216.
17. McTiernan A, Martin CF, Peck JD, et al, for the Women’s Health Initiative Mammogram Density Study Investigators. Estrogen-plus-progestin use and mammographic density in postmenopausal women: Women’s Health Initiative randomized trial. J Natl Cancer Inst. 2005;97:1366-1376.
18. Chlebowski RT, Hendrix SL, Langer RD, et al, for the WHI Investigators. Influence of estrogen plus progestin on breast cancer and mammography in healthy postmenopausal women: the Women’s Health Initiative randomized trial. JAMA. 2003;289:3243-3253.
19. Grodstein F, Manson JE, Stampfer MJ. Hormone therapy and coronary heart disease: the role of time since menopause and age at hormone initiation. J Womens Health (Larchmt). 2006;15:35-44.
20. North American Menopause Society. Recommendations for estrogen and progestin use in peri- and postmenopausal women: October 2004 position statement of The North American Menopause Society. Menopause. 2004;11:589-600.
21. American College of Obstetricians and Gynecologists. Hormone therapy. Obstet Gynecol. 2004;104(suppl):1S-4S.
22. Ockene JK, Barad DH, Cochrane BB, et al. Symptom experience after discontinuing use of estrogen plus progestin. JAMA. 2005;294:183-193.