User login
Botanical Briefs: Primula obconica Dermatitis
Etiology
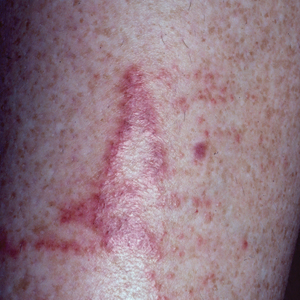
Calcareous soils of central and southwest China are home to Primula obconica1 (also known as German primrose and Libre Magenta).2 Primula obconica was introduced to Europe in the 1880s, where it became a popular ornamental and decorative household plant (Figure).3 It also is a frequent resident of greenhouses.

Primula obconica is a member of the family Primulaceae, which comprises semi-evergreen perennials. The genus name Primula is derived from Latin meaning “first”; obconica refers to the conelike shape of the plant’s vivid, cerise-red flowers.
Allergens From P obconica
The allergens primin (2-methoxy-6-pentyl-1,4-benzoquinone) and miconidin (2-methoxy-6-pentyl-1, 4-dihydroxybenzene) have been isolated from P obconica stems, leaves, and flowers. Allergies to P obconica are much more commonly detected in Europe than in the United States because the plant is part of standard allergen screening in dermatology clinics in Europe.4 In a British patch test study of 234 patients with hand dermatitis, 34 displayed immediate or delayed sensitization to P obconica allergens.5 However, in another study, researchers who surveyed the incidence of P obconica allergic contact dermatitis (CD) in the United Kingdom found a notable decline in the number of primin-positive patch tests from 1995 to 2000, which likely was attributable to a decrease in the number of plant retailers who stocked P obconica and the availability of primin-free varieties from 50% of suppliers.3 Furthermore, a study in the United States of 567 consecutive patch tests that included primin as part of standard screening found only 1 positive reaction, suggesting that routine patch testing for P obconica in the United States would have a low yield unless the patient has a relevant history.4
Cutaneous Presentation
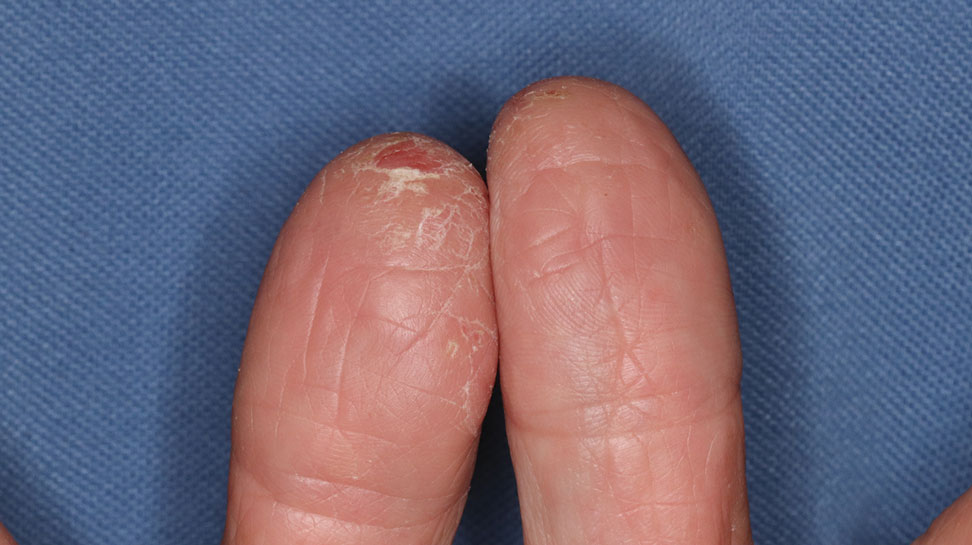
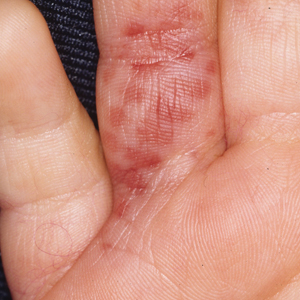
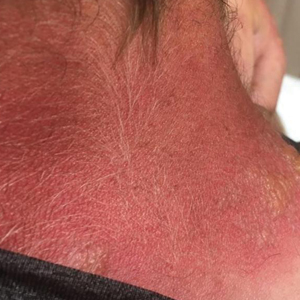
Clinical features of P obconica–induced dermatitis include fingertip dermatitis, as well as facial, hand, and forearm dermatitis.6 Patients typically present with lichenification and fissuring of the fingertips; fingertip vesicular dermatitis; or linear erythematous streaks, vesicles, and bullae on the forearms, hands, and face. Vesicles and bullae can be hemorrhagic in patients with pompholyxlike lesions.7
Some patients have been reported to present with facial angioedema; the clinical diagnosis of CD can be challenging when facial edema is more prominent than eczema.6 Furthermore, in a reported case of P obconica CD, the patient’s vesicular hand dermatitis became pustular and spread to the face.8
Allergy Testing
Patch testing is performed with synthetic primin to detect allergens of P obconica in patients who are sensitive to them, which can be useful because Primula dermatitis can have variable presentations and cases can be missed if patch testing is not performed.9 Diagnostic mimics—herpes simplex, pompholyx, seborrheic dermatitis, and scabies—should be considered before patch testing.7
Prevention and Treatment
Preventive Measures—Ideally, once CD occurs in response to P obconica, handling of and other exposure to the plant should be halted; thus, prevention becomes the mainstay of treatment. Alternatively, when exposure is a necessary occupational hazard, nitrile gloves should be worn; allergenicity can be decreased by overwatering or introducing more primin-free varieties.3,10
Cultivating the plant outdoors during the winter in milder climates can potentially decrease sensitivity because allergen production is lowest during cold months and highest during summer.11 Because P obconica is commonly grown indoors, allergenicity can persist year-round.
Pharmacotherapy—Drawing on experience treating CD caused by other plants, acute and chronic P obconica CD are primarily treated with a topical steroid or, if the face or genitals are affected, with a steroid-sparing agent, such as tacrolimus.12 A cool compress of water, saline, or Burow solution (aluminum acetate in water) can help decrease acute inflammation, especially in the setting of vesiculation.13
Mild CD also can be treated with a barrier cream and lipid-rich moisturizer. Their effectiveness likely is due to increased hydration and aiding impaired skin-barrier repair.14
Some success in treating chronic CD also has been reported with psoralen plus UVA and UVB light therapy, which function as local immunosuppressants, thus decreasing inflammation.15
Final Thoughts
Contact dermatitis caused by P obconica is common in Europe but less common in the United States and therefore often is underrecognized. Avoiding contact with the plant should be strongly recommended to allergic persons. Primula obconica allergic CD can be treated with a topical steroid.
- Nan P, Shi S, Peng S, et al. Genetic diversity in Primula obconica (Primulaceae) from Central and South‐west China as revealed by ISSR markers. Ann Bot. 2003;91:329-333. doi:10.1093/AOB/MCG018
- Primula obconica “Libre Magenta” (Ob). The Royal Horticultural Society. Accessed February 14, 2023. https://www.rhs.org.uk/plants/131697/i-primula-obconica-i-libre-magenta-(ob)/details
- Connolly M, McCune J, Dauncey E, et al. Primula obconica—is contact allergy on the decline? Contact Dermatitis. 2004;51:167-171. doi:10.1111/J.0105-1873.2004.00427.X
- Mowad C. Routine testing for Primula obconica: is it useful in the United States? Am J Contact Dermat. 1998;9:231-233.
- Agrup C, Fregert S, Rorsman H. Sensitization by routine patch testing with ether extract of Primula obconica. Br J Dermatol. 1969;81:897-898. doi:10.1111/J.1365-2133.1969.TB15970.X
- Lleonart Bellfill R, Casas Ramisa R, Nevot Falcó S. Primula dermatitis. Allergol Immunopathol (Madr). 1999;27:29-31.
- Thomson KF, Charles-Holmes R, Beck MH. Primula dermatitis mimicking herpes simplex. Contact Dermatitis. 1997;37:185-186. doi:10.1111/J.1600-0536.1997.TB00200.X
- Tabar AI, Quirce S, García BE, et al. Primula dermatitis: versatility in its clinical presentation and the advantages of patch tests with synthetic primin. Contact Dermatitis. 1994;30:47-48. doi:10.1111/J.1600-0536.1994.tb00734.X
- Apted JH. Primula obconica sensitivity and testing with primin. Australas J Dermatol. 1988;29:161-162. doi:10.1111/J.1440-0960.1988.TB00390.X
- Aplin CG, Lovell CR. Contact dermatitis due to hardy Primula species and their cultivars. Contact Dermatitis. 2001;44:23-29. doi:10.1034/J.1600-0536.2001.440105.X
- Christensen LP, Larsen E. Direct emission of the allergen primin from intact Primula obconica plants. Contact Dermatitis. 2000;42:149-153. doi:10.1034/J.1600-0536.2000.042003149.X
- Esser PR, Mueller S, Martin SF. Plant allergen-induced contact dermatitis. Planta Med. 2019;85:528-534. doi:10.1055/A-0873-1494
- Levin CY, Maibach HI. Do cool water or physiologic saline compresses enhance resolution of experimentally-induced irritant contact dermatitis? Contact Dermatitis. 2001;45:146-150. doi:10.1034/J.1600-0536.2001.045003146.X
- M, Lindberg M. The influence of a single application of different moisturizers on the skin capacitance. Acta Derm Venereol. 1991;71:79-82.
- Levin CY, Maibach HI. Irritant contact dermatitis: is there an immunologic component? Int Immunopharmacol. 2002;2:183-189. doi:10.1016/S1567-5769(01)00171-0
Etiology
Calcareous soils of central and southwest China are home to Primula obconica1 (also known as German primrose and Libre Magenta).2 Primula obconica was introduced to Europe in the 1880s, where it became a popular ornamental and decorative household plant (Figure).3 It also is a frequent resident of greenhouses.

Primula obconica is a member of the family Primulaceae, which comprises semi-evergreen perennials. The genus name Primula is derived from Latin meaning “first”; obconica refers to the conelike shape of the plant’s vivid, cerise-red flowers.
Allergens From P obconica
The allergens primin (2-methoxy-6-pentyl-1,4-benzoquinone) and miconidin (2-methoxy-6-pentyl-1, 4-dihydroxybenzene) have been isolated from P obconica stems, leaves, and flowers. Allergies to P obconica are much more commonly detected in Europe than in the United States because the plant is part of standard allergen screening in dermatology clinics in Europe.4 In a British patch test study of 234 patients with hand dermatitis, 34 displayed immediate or delayed sensitization to P obconica allergens.5 However, in another study, researchers who surveyed the incidence of P obconica allergic contact dermatitis (CD) in the United Kingdom found a notable decline in the number of primin-positive patch tests from 1995 to 2000, which likely was attributable to a decrease in the number of plant retailers who stocked P obconica and the availability of primin-free varieties from 50% of suppliers.3 Furthermore, a study in the United States of 567 consecutive patch tests that included primin as part of standard screening found only 1 positive reaction, suggesting that routine patch testing for P obconica in the United States would have a low yield unless the patient has a relevant history.4
Cutaneous Presentation
Clinical features of P obconica–induced dermatitis include fingertip dermatitis, as well as facial, hand, and forearm dermatitis.6 Patients typically present with lichenification and fissuring of the fingertips; fingertip vesicular dermatitis; or linear erythematous streaks, vesicles, and bullae on the forearms, hands, and face. Vesicles and bullae can be hemorrhagic in patients with pompholyxlike lesions.7
Some patients have been reported to present with facial angioedema; the clinical diagnosis of CD can be challenging when facial edema is more prominent than eczema.6 Furthermore, in a reported case of P obconica CD, the patient’s vesicular hand dermatitis became pustular and spread to the face.8
Allergy Testing
Patch testing is performed with synthetic primin to detect allergens of P obconica in patients who are sensitive to them, which can be useful because Primula dermatitis can have variable presentations and cases can be missed if patch testing is not performed.9 Diagnostic mimics—herpes simplex, pompholyx, seborrheic dermatitis, and scabies—should be considered before patch testing.7
Prevention and Treatment
Preventive Measures—Ideally, once CD occurs in response to P obconica, handling of and other exposure to the plant should be halted; thus, prevention becomes the mainstay of treatment. Alternatively, when exposure is a necessary occupational hazard, nitrile gloves should be worn; allergenicity can be decreased by overwatering or introducing more primin-free varieties.3,10
Cultivating the plant outdoors during the winter in milder climates can potentially decrease sensitivity because allergen production is lowest during cold months and highest during summer.11 Because P obconica is commonly grown indoors, allergenicity can persist year-round.
Pharmacotherapy—Drawing on experience treating CD caused by other plants, acute and chronic P obconica CD are primarily treated with a topical steroid or, if the face or genitals are affected, with a steroid-sparing agent, such as tacrolimus.12 A cool compress of water, saline, or Burow solution (aluminum acetate in water) can help decrease acute inflammation, especially in the setting of vesiculation.13
Mild CD also can be treated with a barrier cream and lipid-rich moisturizer. Their effectiveness likely is due to increased hydration and aiding impaired skin-barrier repair.14
Some success in treating chronic CD also has been reported with psoralen plus UVA and UVB light therapy, which function as local immunosuppressants, thus decreasing inflammation.15
Final Thoughts
Contact dermatitis caused by P obconica is common in Europe but less common in the United States and therefore often is underrecognized. Avoiding contact with the plant should be strongly recommended to allergic persons. Primula obconica allergic CD can be treated with a topical steroid.
Etiology
Calcareous soils of central and southwest China are home to Primula obconica1 (also known as German primrose and Libre Magenta).2 Primula obconica was introduced to Europe in the 1880s, where it became a popular ornamental and decorative household plant (Figure).3 It also is a frequent resident of greenhouses.

Primula obconica is a member of the family Primulaceae, which comprises semi-evergreen perennials. The genus name Primula is derived from Latin meaning “first”; obconica refers to the conelike shape of the plant’s vivid, cerise-red flowers.
Allergens From P obconica
The allergens primin (2-methoxy-6-pentyl-1,4-benzoquinone) and miconidin (2-methoxy-6-pentyl-1, 4-dihydroxybenzene) have been isolated from P obconica stems, leaves, and flowers. Allergies to P obconica are much more commonly detected in Europe than in the United States because the plant is part of standard allergen screening in dermatology clinics in Europe.4 In a British patch test study of 234 patients with hand dermatitis, 34 displayed immediate or delayed sensitization to P obconica allergens.5 However, in another study, researchers who surveyed the incidence of P obconica allergic contact dermatitis (CD) in the United Kingdom found a notable decline in the number of primin-positive patch tests from 1995 to 2000, which likely was attributable to a decrease in the number of plant retailers who stocked P obconica and the availability of primin-free varieties from 50% of suppliers.3 Furthermore, a study in the United States of 567 consecutive patch tests that included primin as part of standard screening found only 1 positive reaction, suggesting that routine patch testing for P obconica in the United States would have a low yield unless the patient has a relevant history.4
Cutaneous Presentation
Clinical features of P obconica–induced dermatitis include fingertip dermatitis, as well as facial, hand, and forearm dermatitis.6 Patients typically present with lichenification and fissuring of the fingertips; fingertip vesicular dermatitis; or linear erythematous streaks, vesicles, and bullae on the forearms, hands, and face. Vesicles and bullae can be hemorrhagic in patients with pompholyxlike lesions.7
Some patients have been reported to present with facial angioedema; the clinical diagnosis of CD can be challenging when facial edema is more prominent than eczema.6 Furthermore, in a reported case of P obconica CD, the patient’s vesicular hand dermatitis became pustular and spread to the face.8
Allergy Testing
Patch testing is performed with synthetic primin to detect allergens of P obconica in patients who are sensitive to them, which can be useful because Primula dermatitis can have variable presentations and cases can be missed if patch testing is not performed.9 Diagnostic mimics—herpes simplex, pompholyx, seborrheic dermatitis, and scabies—should be considered before patch testing.7
Prevention and Treatment
Preventive Measures—Ideally, once CD occurs in response to P obconica, handling of and other exposure to the plant should be halted; thus, prevention becomes the mainstay of treatment. Alternatively, when exposure is a necessary occupational hazard, nitrile gloves should be worn; allergenicity can be decreased by overwatering or introducing more primin-free varieties.3,10
Cultivating the plant outdoors during the winter in milder climates can potentially decrease sensitivity because allergen production is lowest during cold months and highest during summer.11 Because P obconica is commonly grown indoors, allergenicity can persist year-round.
Pharmacotherapy—Drawing on experience treating CD caused by other plants, acute and chronic P obconica CD are primarily treated with a topical steroid or, if the face or genitals are affected, with a steroid-sparing agent, such as tacrolimus.12 A cool compress of water, saline, or Burow solution (aluminum acetate in water) can help decrease acute inflammation, especially in the setting of vesiculation.13
Mild CD also can be treated with a barrier cream and lipid-rich moisturizer. Their effectiveness likely is due to increased hydration and aiding impaired skin-barrier repair.14
Some success in treating chronic CD also has been reported with psoralen plus UVA and UVB light therapy, which function as local immunosuppressants, thus decreasing inflammation.15
Final Thoughts
Contact dermatitis caused by P obconica is common in Europe but less common in the United States and therefore often is underrecognized. Avoiding contact with the plant should be strongly recommended to allergic persons. Primula obconica allergic CD can be treated with a topical steroid.
- Nan P, Shi S, Peng S, et al. Genetic diversity in Primula obconica (Primulaceae) from Central and South‐west China as revealed by ISSR markers. Ann Bot. 2003;91:329-333. doi:10.1093/AOB/MCG018
- Primula obconica “Libre Magenta” (Ob). The Royal Horticultural Society. Accessed February 14, 2023. https://www.rhs.org.uk/plants/131697/i-primula-obconica-i-libre-magenta-(ob)/details
- Connolly M, McCune J, Dauncey E, et al. Primula obconica—is contact allergy on the decline? Contact Dermatitis. 2004;51:167-171. doi:10.1111/J.0105-1873.2004.00427.X
- Mowad C. Routine testing for Primula obconica: is it useful in the United States? Am J Contact Dermat. 1998;9:231-233.
- Agrup C, Fregert S, Rorsman H. Sensitization by routine patch testing with ether extract of Primula obconica. Br J Dermatol. 1969;81:897-898. doi:10.1111/J.1365-2133.1969.TB15970.X
- Lleonart Bellfill R, Casas Ramisa R, Nevot Falcó S. Primula dermatitis. Allergol Immunopathol (Madr). 1999;27:29-31.
- Thomson KF, Charles-Holmes R, Beck MH. Primula dermatitis mimicking herpes simplex. Contact Dermatitis. 1997;37:185-186. doi:10.1111/J.1600-0536.1997.TB00200.X
- Tabar AI, Quirce S, García BE, et al. Primula dermatitis: versatility in its clinical presentation and the advantages of patch tests with synthetic primin. Contact Dermatitis. 1994;30:47-48. doi:10.1111/J.1600-0536.1994.tb00734.X
- Apted JH. Primula obconica sensitivity and testing with primin. Australas J Dermatol. 1988;29:161-162. doi:10.1111/J.1440-0960.1988.TB00390.X
- Aplin CG, Lovell CR. Contact dermatitis due to hardy Primula species and their cultivars. Contact Dermatitis. 2001;44:23-29. doi:10.1034/J.1600-0536.2001.440105.X
- Christensen LP, Larsen E. Direct emission of the allergen primin from intact Primula obconica plants. Contact Dermatitis. 2000;42:149-153. doi:10.1034/J.1600-0536.2000.042003149.X
- Esser PR, Mueller S, Martin SF. Plant allergen-induced contact dermatitis. Planta Med. 2019;85:528-534. doi:10.1055/A-0873-1494
- Levin CY, Maibach HI. Do cool water or physiologic saline compresses enhance resolution of experimentally-induced irritant contact dermatitis? Contact Dermatitis. 2001;45:146-150. doi:10.1034/J.1600-0536.2001.045003146.X
- M, Lindberg M. The influence of a single application of different moisturizers on the skin capacitance. Acta Derm Venereol. 1991;71:79-82.
- Levin CY, Maibach HI. Irritant contact dermatitis: is there an immunologic component? Int Immunopharmacol. 2002;2:183-189. doi:10.1016/S1567-5769(01)00171-0
- Nan P, Shi S, Peng S, et al. Genetic diversity in Primula obconica (Primulaceae) from Central and South‐west China as revealed by ISSR markers. Ann Bot. 2003;91:329-333. doi:10.1093/AOB/MCG018
- Primula obconica “Libre Magenta” (Ob). The Royal Horticultural Society. Accessed February 14, 2023. https://www.rhs.org.uk/plants/131697/i-primula-obconica-i-libre-magenta-(ob)/details
- Connolly M, McCune J, Dauncey E, et al. Primula obconica—is contact allergy on the decline? Contact Dermatitis. 2004;51:167-171. doi:10.1111/J.0105-1873.2004.00427.X
- Mowad C. Routine testing for Primula obconica: is it useful in the United States? Am J Contact Dermat. 1998;9:231-233.
- Agrup C, Fregert S, Rorsman H. Sensitization by routine patch testing with ether extract of Primula obconica. Br J Dermatol. 1969;81:897-898. doi:10.1111/J.1365-2133.1969.TB15970.X
- Lleonart Bellfill R, Casas Ramisa R, Nevot Falcó S. Primula dermatitis. Allergol Immunopathol (Madr). 1999;27:29-31.
- Thomson KF, Charles-Holmes R, Beck MH. Primula dermatitis mimicking herpes simplex. Contact Dermatitis. 1997;37:185-186. doi:10.1111/J.1600-0536.1997.TB00200.X
- Tabar AI, Quirce S, García BE, et al. Primula dermatitis: versatility in its clinical presentation and the advantages of patch tests with synthetic primin. Contact Dermatitis. 1994;30:47-48. doi:10.1111/J.1600-0536.1994.tb00734.X
- Apted JH. Primula obconica sensitivity and testing with primin. Australas J Dermatol. 1988;29:161-162. doi:10.1111/J.1440-0960.1988.TB00390.X
- Aplin CG, Lovell CR. Contact dermatitis due to hardy Primula species and their cultivars. Contact Dermatitis. 2001;44:23-29. doi:10.1034/J.1600-0536.2001.440105.X
- Christensen LP, Larsen E. Direct emission of the allergen primin from intact Primula obconica plants. Contact Dermatitis. 2000;42:149-153. doi:10.1034/J.1600-0536.2000.042003149.X
- Esser PR, Mueller S, Martin SF. Plant allergen-induced contact dermatitis. Planta Med. 2019;85:528-534. doi:10.1055/A-0873-1494
- Levin CY, Maibach HI. Do cool water or physiologic saline compresses enhance resolution of experimentally-induced irritant contact dermatitis? Contact Dermatitis. 2001;45:146-150. doi:10.1034/J.1600-0536.2001.045003146.X
- M, Lindberg M. The influence of a single application of different moisturizers on the skin capacitance. Acta Derm Venereol. 1991;71:79-82.
- Levin CY, Maibach HI. Irritant contact dermatitis: is there an immunologic component? Int Immunopharmacol. 2002;2:183-189. doi:10.1016/S1567-5769(01)00171-0
Practice Points
- Primula obconica is a household plant that can cause contact dermatitis (CD). Spent blossoms must be pinched off to keep the plant blooming, resulting in fingertip dermatitis.
- In the United States, P obconica is not a component of routine patch testing; therefore, it might be missed as the cause of an allergic reaction.
- Primin and miconidin are the principal allergens known to be responsible for causing P obconica dermatitis.
- Treatment of this condition is similar to the usual treatment of plant-induced CD: avoiding exposure to the plant and applying a topical steroid.
Botanical Briefs: Daffodils (Narcissus Species)
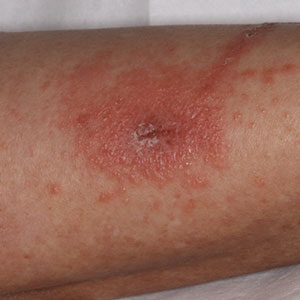
Contact dermatitis is a common problem in the floral bulb industry and is considered an occupational disease. Daffodils (Narcissus species)(Figure) are thought to be the most common cause of irritant contact dermatitis among florists.1
Clinical Importance
Picking daffodils can start as early as October, when the flowers are still closed. The picker’s hand slides down the stem to snap the stalk at the base. This potentially traumatic maneuver to the web of the fingers leads to abrasions, which are irritated by the sap and cause granulomatous sores and paronychia. An experienced picker can pick 20,000 flowers a day, leading to extensive contact with sap.2
Eczematous or granulomatous rash on the arms also is seen as the sap irritates the wrist and forearm. The pickers often hold the flowers until a bunch of 10 has been collected. The 10 flowers are held together by a rubber band and stacked along the arm, the chin, and the axilla, causing the rash to extend to those areas. Sap also can be transferred by the hand to other parts of the body, such as the face. In men, sap can be transferred to the genitalia as the men urinate in the field.
Narcissus also can cause poisoning if ingested by humans or animals. Researchers who analyzed calls made to the New Zealand Natural Poisons Centre between 2003 and 2010 determined that daffodil was the 11th most common call for plant-related poisoning.3
Although the severity of plant poisoning often is low due to the small amount of plant material usually consumed, more severe poisoning can occur when the plant is eaten for medicinal purposes or mistaken for an edible plant.3 Vomiting, respiratory symptoms, abdominal pain, diarrhea, trembling, and convulsions can occur when daffodils are ingested. Death has been reported due to ingestion of the bulbs.4
In February 2010, 10 children aged 10 and 11 years and their 22-year-old guide presented to an emergency department in Israel after ingesting Narcissus bulbs, which were mistakenly believed to be the bulbs of onions.4 Eight children and the guide vomited. One child and the guide reported abdominal pain. All were discharged in stable condition after 4 hours of observation.4
Clinical Manifestations
Daffodil rash or lily rash was first described in 1910.5 The typical rash presents as dryness, fissures, scaling, and erythema of the fingertips, hands, and forearms, often with subungual hyperkeratosis. Vesicles and pustules may be seen. The rash may extend to other areas of the body, including the face.6
Prevention and Treatment
Use of protective gloves and clothing to avoid contact with the plant is recommended.2 Treatment includes stopping contact with the irritant, eye irrigation, and supportive measures (airway, breathing, and circulation). Activated charcoal can be helpful if used within 1 hour after ingestion but is contraindicated in vomiting patients.4
Identifying Features
The genus Narcissus is in the family Amaryllidaceae and contains ornamental plants, including daffodil (trumpet Narcissus, Narcissus pseudonarcissus), jonquil (Narcissus jonquilla), and poet’s narcissus (Narcissus poeticus). Most species are perennial; the plant emerges from a bulb in spring. Leaves originate from the base of the plant and range from 5-cm to 1.2-meters long, depending on the species. The flowers span a range of shapes and colors—from a trumpet (the daffodil) to a ringlike cup (poet’s Narcissus) and in yellow, white, and pink.7
Distribution and Plant Facts
Distribution—There are approximately 80 to 100 wild Narcissus species, which are found in southwestern Europe, North Africa, the Balkan Peninsula, Italy, and France. There are more than 27,000 Narcissus cultivars registered in the International Daffodil Register.8
Plant Facts—The daffodil is the national flower of Wales. It also is often used to depict hope and joy and is the symbol of cancer charities in many countries.9
The name Narcissus is believed to have originated from Greek mythology. A handsome youth, Narcissus, fell in love with his own reflection, for which the gods punished him by turning him into a flower.10
Another theory states that Narcissus is derived from the Greek word narkao (to benumb) due to its narcotic properties. When an open wound is subjected to an extract of the bulb, numbness of the entire nervous system is said to occur as well as paralysis of the heart. This narcotic effect led Socrates to refer to the Narcissus plant as the “chaplet of the infernal gods.”11
Narcissus is an important flower in various ethnic rituals. The Greeks often planted daffodils near tombs. In Muslim culture, white is believed to be the symbol of good and purity; Narcissus was one of the most common white-flowered plants found in Muslim graveyards.12
Medicinal Qualities and Uses—Narcissus species have been used as medicinal plants for a variety of ailments. For example, Narcissus tazetta contains flavonoids, alkaloids, saponins, tannins, cardiac glycosides, oil, steroids, terpenoids, and anthraquinones that contribute to its antibacterial, antifungal, antiviral, antimalarial, anticancer, antioxidant, dermatologic, cardiovascular, immunomodulatory, and acetylcholinesterase inhibitory effects.13 In a study, chloroform extracts from N tazetta bulbs were found to be more active than doxorubicin against hepatocellular and colon cancer cell lines.14
More than 500 alkaloids have been isolated from the Narcissus genus.15 In 2001, the US Food and Drug Administration approved one of the alkaloids, galantamine, for the treatment of mild to moderate stages of Alzheimer disease.16 Galantamine selectively and reversibly inhibits acetylcholinesterase, the enzyme believed responsible for neurodegeneration seen in Alzheimer disease. Plants are the main source of galantamine, despite the ability of pharmaceutical companies to synthesize the compound. Galantamine hydrobromide is sold by prescription (Razadyne [Janssen Pharmaceuticals, Inc]); generic formulations approved by the US Food and Drug Administration have been produced by more than 15 pharmaceutical companies.17,18
Irritant and Allergen
Sap found in the bulbs and hollow stems of Narcissus contains calcium oxalate crystals, or raphides. The minute, needle-shaped calcium oxalate crystals are believed to be a waste product of cellular metabolism.19 When the plant structure is compromised by pickers snapping the stalk, the sharp crystals penetrate the skin to cause an irritant contact dermatitis.
Relevant Research—A study used electron microscopy to characterize the structure of raphides from various plants,2 though not from Narcissus species; the structure of each raphide was then compared to the degree of irritation it produced. The researchers concluded that more elongated crystals (those containing barbs) produce a greater degree of irritation. Narcissus species are known to cause varying degrees of skin irritation: For example, N tazetta rarely causes skin irritation, whereas N pseudonarcissi (daffodil) tends to cause remarkably more skin irritation.2
Allergic reactions to and strong toxicity from Narcissus species are not well understood. In a study, only 2 alkaloids—homolycorine and masonin—produced a weakly positive reaction in patch tests on sensitized guinea pigs, which correlates with the finding of a different study, in which only 2 of 12 patients whose findings were examined over 14 years had a positive patch test for Narcissus.20,21
However, IgE-mediated allergies indicative of an allergic response to Narcissus have been reported. A study isolated an allergenic protein, narcin, from bulbs of N tazetta. Narcin is a 13-kDa protein with potent allergenic effects capable of inducing production of proinflammatory cytokines and increasing IgE levels in mononuclear cells in peripheral blood.22
More research is required to find and understand the compounds responsible for causing an allergic reaction to Narcissus.
- Modi GM, Doherty CB, Katta R, et al. Irritant contact dermatitis from plants. Dermatitis. 2009;20:63-78. doi:10.2310/6620.2009.08051
- Julian CG, Bowers PW. The nature and distribution of daffodil pickers’ rash. Contact Dermatitis. 1997;37:259-262. doi:10.1111/j.1600-0536.1997.tb02461.x
- Slaughter RJ, Beasley DMG, Lambie BS, et al. Poisonous plants in New Zealand: a review of those that are most commonly enquired about to the National Poisons Centre. N Z Med J. 2012;125:87-118.
- Hussein A, Yassin A. Poisoning following ingestion of Narcissus tazetta bulbs by schoolchildren. Isr Med Assoc J. 2014;16:125-126.
- Hanks GR, ed. Narcissus and Daffodil: The Genus Narcissus. CRC Press; 2002. https://doi.org/10.1201/9780203219355
- McGovern TW. Botanical briefs: daffodils—Narcissus L. Cutis. 2000;65:130-132.
- The Editors of Encyclopaedia Britannica. Narcissus. Encyclopedia Britannica. Accessed December 13, 2022. https://www.britannica.com/plant/narcissus-plant
- M, A, D, et al. Alkaloids from Narcissus poeticus cv. Pink Parasol of various structural types and their biological activity. Arch Pharm Res. 2018;41:208-218. doi:10.1007/s12272-017-1000-4
- Crampton L. Beautiful daffodils: plant facts, toxicity, and a symbol of hope. Owlcation. April 19, 2022. Accessed December 13, 2022. https://owlcation.com/stem/Daffodils-Beautiful-Flowers-and-a-Symbol-of-Hope
- Rademaker M. Daffodil. DermNet. Published 1999. Accessed December 13, 2022. https://dermnetnz.org/topics/daffodil
- Grieve M. Narcissus. Accessed December 13, 2022. https://botanical.com/botanical/mgmh/n/narcis01.html
- Dafni A, Lev E, Beckmann S, et al. Ritual plants of Muslim graveyards in northern Israel. J Ethnobiolog Ethnomed. 2006;2:38. doi:10.1186/1746-4269-2-38
- Al-Snafi AE. Constituents and pharmacology of Narcissus tazetta. IOSR J Pharm. 2020;10:44-53.
- Shawky E, Abou-Donia AH, Darwish FA, et al. In vitro cytotoxicity of some Narcissus plants extracts. Nat Prod Res. 2015;29:363-365. doi:10.1080/14786419.2014.942302
- Havlasov J, M, Siatka T, et al. Chemical composition of bioactive alkaloid extracts from some Narcissus species and varieties and their biological activity. Nat Prod Commun. 2014;9:1151-1155.
- Pigni NB, S, V, et al. Alkaloids from Narcissus serotinus. J Nat Prod. 2012;75:1643-1647. doi:10.1021/np3003595
- Razadyne. Prescribing information. Janssen Pharmaceuticals, Inc; 2013. Accessed December 19, 2022. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/021169Orig1s032,021224Orig1s030,021615Orig1s023lbl.pdf
- Takos AM, Rook F. Towards a molecular understanding of the biosynthesis of amaryllidaceae alkaloids in support of their expanding medical use. Int J Mol Sci. 2013;14:11713-11741. doi:10.3390/ijms140611713
- Evans FJ, Schmidt RJ. Plants and plant products that induce contact dermatitis. Planta Med. 1980;38:289-316. doi:10.1055/s-2008-1074883
- Gude M, Hausen BM, Heitsch H, et al. An investigation of the irritant and allergenic properties of daffodils (Narcissus pseudonarcissus L., Amaryllidaceae). a review of daffodil dermatitis. Contact Dermatitis. 1988;19:1-10.
- Lamminpää A, Estlander T, Jolanki R, et al. Occupational allergic contact dermatitis caused by decorative plants. Contact Dermatitis. 1996;34:330-335.
- Sinha M, Singh A, Shokeen A, et al. Evidence of a novel allergenic protein Narcin in the bulbs of Narcissus tazetta. Int J Biochem Mol Biol. 2013;4:95-101.
Contact dermatitis is a common problem in the floral bulb industry and is considered an occupational disease. Daffodils (Narcissus species)(Figure) are thought to be the most common cause of irritant contact dermatitis among florists.1
Clinical Importance
Picking daffodils can start as early as October, when the flowers are still closed. The picker’s hand slides down the stem to snap the stalk at the base. This potentially traumatic maneuver to the web of the fingers leads to abrasions, which are irritated by the sap and cause granulomatous sores and paronychia. An experienced picker can pick 20,000 flowers a day, leading to extensive contact with sap.2
Eczematous or granulomatous rash on the arms also is seen as the sap irritates the wrist and forearm. The pickers often hold the flowers until a bunch of 10 has been collected. The 10 flowers are held together by a rubber band and stacked along the arm, the chin, and the axilla, causing the rash to extend to those areas. Sap also can be transferred by the hand to other parts of the body, such as the face. In men, sap can be transferred to the genitalia as the men urinate in the field.
Narcissus also can cause poisoning if ingested by humans or animals. Researchers who analyzed calls made to the New Zealand Natural Poisons Centre between 2003 and 2010 determined that daffodil was the 11th most common call for plant-related poisoning.3
Although the severity of plant poisoning often is low due to the small amount of plant material usually consumed, more severe poisoning can occur when the plant is eaten for medicinal purposes or mistaken for an edible plant.3 Vomiting, respiratory symptoms, abdominal pain, diarrhea, trembling, and convulsions can occur when daffodils are ingested. Death has been reported due to ingestion of the bulbs.4
In February 2010, 10 children aged 10 and 11 years and their 22-year-old guide presented to an emergency department in Israel after ingesting Narcissus bulbs, which were mistakenly believed to be the bulbs of onions.4 Eight children and the guide vomited. One child and the guide reported abdominal pain. All were discharged in stable condition after 4 hours of observation.4
Clinical Manifestations
Daffodil rash or lily rash was first described in 1910.5 The typical rash presents as dryness, fissures, scaling, and erythema of the fingertips, hands, and forearms, often with subungual hyperkeratosis. Vesicles and pustules may be seen. The rash may extend to other areas of the body, including the face.6
Prevention and Treatment
Use of protective gloves and clothing to avoid contact with the plant is recommended.2 Treatment includes stopping contact with the irritant, eye irrigation, and supportive measures (airway, breathing, and circulation). Activated charcoal can be helpful if used within 1 hour after ingestion but is contraindicated in vomiting patients.4
Identifying Features
The genus Narcissus is in the family Amaryllidaceae and contains ornamental plants, including daffodil (trumpet Narcissus, Narcissus pseudonarcissus), jonquil (Narcissus jonquilla), and poet’s narcissus (Narcissus poeticus). Most species are perennial; the plant emerges from a bulb in spring. Leaves originate from the base of the plant and range from 5-cm to 1.2-meters long, depending on the species. The flowers span a range of shapes and colors—from a trumpet (the daffodil) to a ringlike cup (poet’s Narcissus) and in yellow, white, and pink.7
Distribution and Plant Facts
Distribution—There are approximately 80 to 100 wild Narcissus species, which are found in southwestern Europe, North Africa, the Balkan Peninsula, Italy, and France. There are more than 27,000 Narcissus cultivars registered in the International Daffodil Register.8
Plant Facts—The daffodil is the national flower of Wales. It also is often used to depict hope and joy and is the symbol of cancer charities in many countries.9
The name Narcissus is believed to have originated from Greek mythology. A handsome youth, Narcissus, fell in love with his own reflection, for which the gods punished him by turning him into a flower.10
Another theory states that Narcissus is derived from the Greek word narkao (to benumb) due to its narcotic properties. When an open wound is subjected to an extract of the bulb, numbness of the entire nervous system is said to occur as well as paralysis of the heart. This narcotic effect led Socrates to refer to the Narcissus plant as the “chaplet of the infernal gods.”11
Narcissus is an important flower in various ethnic rituals. The Greeks often planted daffodils near tombs. In Muslim culture, white is believed to be the symbol of good and purity; Narcissus was one of the most common white-flowered plants found in Muslim graveyards.12
Medicinal Qualities and Uses—Narcissus species have been used as medicinal plants for a variety of ailments. For example, Narcissus tazetta contains flavonoids, alkaloids, saponins, tannins, cardiac glycosides, oil, steroids, terpenoids, and anthraquinones that contribute to its antibacterial, antifungal, antiviral, antimalarial, anticancer, antioxidant, dermatologic, cardiovascular, immunomodulatory, and acetylcholinesterase inhibitory effects.13 In a study, chloroform extracts from N tazetta bulbs were found to be more active than doxorubicin against hepatocellular and colon cancer cell lines.14
More than 500 alkaloids have been isolated from the Narcissus genus.15 In 2001, the US Food and Drug Administration approved one of the alkaloids, galantamine, for the treatment of mild to moderate stages of Alzheimer disease.16 Galantamine selectively and reversibly inhibits acetylcholinesterase, the enzyme believed responsible for neurodegeneration seen in Alzheimer disease. Plants are the main source of galantamine, despite the ability of pharmaceutical companies to synthesize the compound. Galantamine hydrobromide is sold by prescription (Razadyne [Janssen Pharmaceuticals, Inc]); generic formulations approved by the US Food and Drug Administration have been produced by more than 15 pharmaceutical companies.17,18
Irritant and Allergen
Sap found in the bulbs and hollow stems of Narcissus contains calcium oxalate crystals, or raphides. The minute, needle-shaped calcium oxalate crystals are believed to be a waste product of cellular metabolism.19 When the plant structure is compromised by pickers snapping the stalk, the sharp crystals penetrate the skin to cause an irritant contact dermatitis.
Relevant Research—A study used electron microscopy to characterize the structure of raphides from various plants,2 though not from Narcissus species; the structure of each raphide was then compared to the degree of irritation it produced. The researchers concluded that more elongated crystals (those containing barbs) produce a greater degree of irritation. Narcissus species are known to cause varying degrees of skin irritation: For example, N tazetta rarely causes skin irritation, whereas N pseudonarcissi (daffodil) tends to cause remarkably more skin irritation.2
Allergic reactions to and strong toxicity from Narcissus species are not well understood. In a study, only 2 alkaloids—homolycorine and masonin—produced a weakly positive reaction in patch tests on sensitized guinea pigs, which correlates with the finding of a different study, in which only 2 of 12 patients whose findings were examined over 14 years had a positive patch test for Narcissus.20,21
However, IgE-mediated allergies indicative of an allergic response to Narcissus have been reported. A study isolated an allergenic protein, narcin, from bulbs of N tazetta. Narcin is a 13-kDa protein with potent allergenic effects capable of inducing production of proinflammatory cytokines and increasing IgE levels in mononuclear cells in peripheral blood.22
More research is required to find and understand the compounds responsible for causing an allergic reaction to Narcissus.
Contact dermatitis is a common problem in the floral bulb industry and is considered an occupational disease. Daffodils (Narcissus species)(Figure) are thought to be the most common cause of irritant contact dermatitis among florists.1
Clinical Importance
Picking daffodils can start as early as October, when the flowers are still closed. The picker’s hand slides down the stem to snap the stalk at the base. This potentially traumatic maneuver to the web of the fingers leads to abrasions, which are irritated by the sap and cause granulomatous sores and paronychia. An experienced picker can pick 20,000 flowers a day, leading to extensive contact with sap.2
Eczematous or granulomatous rash on the arms also is seen as the sap irritates the wrist and forearm. The pickers often hold the flowers until a bunch of 10 has been collected. The 10 flowers are held together by a rubber band and stacked along the arm, the chin, and the axilla, causing the rash to extend to those areas. Sap also can be transferred by the hand to other parts of the body, such as the face. In men, sap can be transferred to the genitalia as the men urinate in the field.
Narcissus also can cause poisoning if ingested by humans or animals. Researchers who analyzed calls made to the New Zealand Natural Poisons Centre between 2003 and 2010 determined that daffodil was the 11th most common call for plant-related poisoning.3
Although the severity of plant poisoning often is low due to the small amount of plant material usually consumed, more severe poisoning can occur when the plant is eaten for medicinal purposes or mistaken for an edible plant.3 Vomiting, respiratory symptoms, abdominal pain, diarrhea, trembling, and convulsions can occur when daffodils are ingested. Death has been reported due to ingestion of the bulbs.4
In February 2010, 10 children aged 10 and 11 years and their 22-year-old guide presented to an emergency department in Israel after ingesting Narcissus bulbs, which were mistakenly believed to be the bulbs of onions.4 Eight children and the guide vomited. One child and the guide reported abdominal pain. All were discharged in stable condition after 4 hours of observation.4
Clinical Manifestations
Daffodil rash or lily rash was first described in 1910.5 The typical rash presents as dryness, fissures, scaling, and erythema of the fingertips, hands, and forearms, often with subungual hyperkeratosis. Vesicles and pustules may be seen. The rash may extend to other areas of the body, including the face.6
Prevention and Treatment
Use of protective gloves and clothing to avoid contact with the plant is recommended.2 Treatment includes stopping contact with the irritant, eye irrigation, and supportive measures (airway, breathing, and circulation). Activated charcoal can be helpful if used within 1 hour after ingestion but is contraindicated in vomiting patients.4
Identifying Features
The genus Narcissus is in the family Amaryllidaceae and contains ornamental plants, including daffodil (trumpet Narcissus, Narcissus pseudonarcissus), jonquil (Narcissus jonquilla), and poet’s narcissus (Narcissus poeticus). Most species are perennial; the plant emerges from a bulb in spring. Leaves originate from the base of the plant and range from 5-cm to 1.2-meters long, depending on the species. The flowers span a range of shapes and colors—from a trumpet (the daffodil) to a ringlike cup (poet’s Narcissus) and in yellow, white, and pink.7
Distribution and Plant Facts
Distribution—There are approximately 80 to 100 wild Narcissus species, which are found in southwestern Europe, North Africa, the Balkan Peninsula, Italy, and France. There are more than 27,000 Narcissus cultivars registered in the International Daffodil Register.8
Plant Facts—The daffodil is the national flower of Wales. It also is often used to depict hope and joy and is the symbol of cancer charities in many countries.9
The name Narcissus is believed to have originated from Greek mythology. A handsome youth, Narcissus, fell in love with his own reflection, for which the gods punished him by turning him into a flower.10
Another theory states that Narcissus is derived from the Greek word narkao (to benumb) due to its narcotic properties. When an open wound is subjected to an extract of the bulb, numbness of the entire nervous system is said to occur as well as paralysis of the heart. This narcotic effect led Socrates to refer to the Narcissus plant as the “chaplet of the infernal gods.”11
Narcissus is an important flower in various ethnic rituals. The Greeks often planted daffodils near tombs. In Muslim culture, white is believed to be the symbol of good and purity; Narcissus was one of the most common white-flowered plants found in Muslim graveyards.12
Medicinal Qualities and Uses—Narcissus species have been used as medicinal plants for a variety of ailments. For example, Narcissus tazetta contains flavonoids, alkaloids, saponins, tannins, cardiac glycosides, oil, steroids, terpenoids, and anthraquinones that contribute to its antibacterial, antifungal, antiviral, antimalarial, anticancer, antioxidant, dermatologic, cardiovascular, immunomodulatory, and acetylcholinesterase inhibitory effects.13 In a study, chloroform extracts from N tazetta bulbs were found to be more active than doxorubicin against hepatocellular and colon cancer cell lines.14
More than 500 alkaloids have been isolated from the Narcissus genus.15 In 2001, the US Food and Drug Administration approved one of the alkaloids, galantamine, for the treatment of mild to moderate stages of Alzheimer disease.16 Galantamine selectively and reversibly inhibits acetylcholinesterase, the enzyme believed responsible for neurodegeneration seen in Alzheimer disease. Plants are the main source of galantamine, despite the ability of pharmaceutical companies to synthesize the compound. Galantamine hydrobromide is sold by prescription (Razadyne [Janssen Pharmaceuticals, Inc]); generic formulations approved by the US Food and Drug Administration have been produced by more than 15 pharmaceutical companies.17,18
Irritant and Allergen
Sap found in the bulbs and hollow stems of Narcissus contains calcium oxalate crystals, or raphides. The minute, needle-shaped calcium oxalate crystals are believed to be a waste product of cellular metabolism.19 When the plant structure is compromised by pickers snapping the stalk, the sharp crystals penetrate the skin to cause an irritant contact dermatitis.
Relevant Research—A study used electron microscopy to characterize the structure of raphides from various plants,2 though not from Narcissus species; the structure of each raphide was then compared to the degree of irritation it produced. The researchers concluded that more elongated crystals (those containing barbs) produce a greater degree of irritation. Narcissus species are known to cause varying degrees of skin irritation: For example, N tazetta rarely causes skin irritation, whereas N pseudonarcissi (daffodil) tends to cause remarkably more skin irritation.2
Allergic reactions to and strong toxicity from Narcissus species are not well understood. In a study, only 2 alkaloids—homolycorine and masonin—produced a weakly positive reaction in patch tests on sensitized guinea pigs, which correlates with the finding of a different study, in which only 2 of 12 patients whose findings were examined over 14 years had a positive patch test for Narcissus.20,21
However, IgE-mediated allergies indicative of an allergic response to Narcissus have been reported. A study isolated an allergenic protein, narcin, from bulbs of N tazetta. Narcin is a 13-kDa protein with potent allergenic effects capable of inducing production of proinflammatory cytokines and increasing IgE levels in mononuclear cells in peripheral blood.22
More research is required to find and understand the compounds responsible for causing an allergic reaction to Narcissus.
- Modi GM, Doherty CB, Katta R, et al. Irritant contact dermatitis from plants. Dermatitis. 2009;20:63-78. doi:10.2310/6620.2009.08051
- Julian CG, Bowers PW. The nature and distribution of daffodil pickers’ rash. Contact Dermatitis. 1997;37:259-262. doi:10.1111/j.1600-0536.1997.tb02461.x
- Slaughter RJ, Beasley DMG, Lambie BS, et al. Poisonous plants in New Zealand: a review of those that are most commonly enquired about to the National Poisons Centre. N Z Med J. 2012;125:87-118.
- Hussein A, Yassin A. Poisoning following ingestion of Narcissus tazetta bulbs by schoolchildren. Isr Med Assoc J. 2014;16:125-126.
- Hanks GR, ed. Narcissus and Daffodil: The Genus Narcissus. CRC Press; 2002. https://doi.org/10.1201/9780203219355
- McGovern TW. Botanical briefs: daffodils—Narcissus L. Cutis. 2000;65:130-132.
- The Editors of Encyclopaedia Britannica. Narcissus. Encyclopedia Britannica. Accessed December 13, 2022. https://www.britannica.com/plant/narcissus-plant
- M, A, D, et al. Alkaloids from Narcissus poeticus cv. Pink Parasol of various structural types and their biological activity. Arch Pharm Res. 2018;41:208-218. doi:10.1007/s12272-017-1000-4
- Crampton L. Beautiful daffodils: plant facts, toxicity, and a symbol of hope. Owlcation. April 19, 2022. Accessed December 13, 2022. https://owlcation.com/stem/Daffodils-Beautiful-Flowers-and-a-Symbol-of-Hope
- Rademaker M. Daffodil. DermNet. Published 1999. Accessed December 13, 2022. https://dermnetnz.org/topics/daffodil
- Grieve M. Narcissus. Accessed December 13, 2022. https://botanical.com/botanical/mgmh/n/narcis01.html
- Dafni A, Lev E, Beckmann S, et al. Ritual plants of Muslim graveyards in northern Israel. J Ethnobiolog Ethnomed. 2006;2:38. doi:10.1186/1746-4269-2-38
- Al-Snafi AE. Constituents and pharmacology of Narcissus tazetta. IOSR J Pharm. 2020;10:44-53.
- Shawky E, Abou-Donia AH, Darwish FA, et al. In vitro cytotoxicity of some Narcissus plants extracts. Nat Prod Res. 2015;29:363-365. doi:10.1080/14786419.2014.942302
- Havlasov J, M, Siatka T, et al. Chemical composition of bioactive alkaloid extracts from some Narcissus species and varieties and their biological activity. Nat Prod Commun. 2014;9:1151-1155.
- Pigni NB, S, V, et al. Alkaloids from Narcissus serotinus. J Nat Prod. 2012;75:1643-1647. doi:10.1021/np3003595
- Razadyne. Prescribing information. Janssen Pharmaceuticals, Inc; 2013. Accessed December 19, 2022. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/021169Orig1s032,021224Orig1s030,021615Orig1s023lbl.pdf
- Takos AM, Rook F. Towards a molecular understanding of the biosynthesis of amaryllidaceae alkaloids in support of their expanding medical use. Int J Mol Sci. 2013;14:11713-11741. doi:10.3390/ijms140611713
- Evans FJ, Schmidt RJ. Plants and plant products that induce contact dermatitis. Planta Med. 1980;38:289-316. doi:10.1055/s-2008-1074883
- Gude M, Hausen BM, Heitsch H, et al. An investigation of the irritant and allergenic properties of daffodils (Narcissus pseudonarcissus L., Amaryllidaceae). a review of daffodil dermatitis. Contact Dermatitis. 1988;19:1-10.
- Lamminpää A, Estlander T, Jolanki R, et al. Occupational allergic contact dermatitis caused by decorative plants. Contact Dermatitis. 1996;34:330-335.
- Sinha M, Singh A, Shokeen A, et al. Evidence of a novel allergenic protein Narcin in the bulbs of Narcissus tazetta. Int J Biochem Mol Biol. 2013;4:95-101.
- Modi GM, Doherty CB, Katta R, et al. Irritant contact dermatitis from plants. Dermatitis. 2009;20:63-78. doi:10.2310/6620.2009.08051
- Julian CG, Bowers PW. The nature and distribution of daffodil pickers’ rash. Contact Dermatitis. 1997;37:259-262. doi:10.1111/j.1600-0536.1997.tb02461.x
- Slaughter RJ, Beasley DMG, Lambie BS, et al. Poisonous plants in New Zealand: a review of those that are most commonly enquired about to the National Poisons Centre. N Z Med J. 2012;125:87-118.
- Hussein A, Yassin A. Poisoning following ingestion of Narcissus tazetta bulbs by schoolchildren. Isr Med Assoc J. 2014;16:125-126.
- Hanks GR, ed. Narcissus and Daffodil: The Genus Narcissus. CRC Press; 2002. https://doi.org/10.1201/9780203219355
- McGovern TW. Botanical briefs: daffodils—Narcissus L. Cutis. 2000;65:130-132.
- The Editors of Encyclopaedia Britannica. Narcissus. Encyclopedia Britannica. Accessed December 13, 2022. https://www.britannica.com/plant/narcissus-plant
- M, A, D, et al. Alkaloids from Narcissus poeticus cv. Pink Parasol of various structural types and their biological activity. Arch Pharm Res. 2018;41:208-218. doi:10.1007/s12272-017-1000-4
- Crampton L. Beautiful daffodils: plant facts, toxicity, and a symbol of hope. Owlcation. April 19, 2022. Accessed December 13, 2022. https://owlcation.com/stem/Daffodils-Beautiful-Flowers-and-a-Symbol-of-Hope
- Rademaker M. Daffodil. DermNet. Published 1999. Accessed December 13, 2022. https://dermnetnz.org/topics/daffodil
- Grieve M. Narcissus. Accessed December 13, 2022. https://botanical.com/botanical/mgmh/n/narcis01.html
- Dafni A, Lev E, Beckmann S, et al. Ritual plants of Muslim graveyards in northern Israel. J Ethnobiolog Ethnomed. 2006;2:38. doi:10.1186/1746-4269-2-38
- Al-Snafi AE. Constituents and pharmacology of Narcissus tazetta. IOSR J Pharm. 2020;10:44-53.
- Shawky E, Abou-Donia AH, Darwish FA, et al. In vitro cytotoxicity of some Narcissus plants extracts. Nat Prod Res. 2015;29:363-365. doi:10.1080/14786419.2014.942302
- Havlasov J, M, Siatka T, et al. Chemical composition of bioactive alkaloid extracts from some Narcissus species and varieties and their biological activity. Nat Prod Commun. 2014;9:1151-1155.
- Pigni NB, S, V, et al. Alkaloids from Narcissus serotinus. J Nat Prod. 2012;75:1643-1647. doi:10.1021/np3003595
- Razadyne. Prescribing information. Janssen Pharmaceuticals, Inc; 2013. Accessed December 19, 2022. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/021169Orig1s032,021224Orig1s030,021615Orig1s023lbl.pdf
- Takos AM, Rook F. Towards a molecular understanding of the biosynthesis of amaryllidaceae alkaloids in support of their expanding medical use. Int J Mol Sci. 2013;14:11713-11741. doi:10.3390/ijms140611713
- Evans FJ, Schmidt RJ. Plants and plant products that induce contact dermatitis. Planta Med. 1980;38:289-316. doi:10.1055/s-2008-1074883
- Gude M, Hausen BM, Heitsch H, et al. An investigation of the irritant and allergenic properties of daffodils (Narcissus pseudonarcissus L., Amaryllidaceae). a review of daffodil dermatitis. Contact Dermatitis. 1988;19:1-10.
- Lamminpää A, Estlander T, Jolanki R, et al. Occupational allergic contact dermatitis caused by decorative plants. Contact Dermatitis. 1996;34:330-335.
- Sinha M, Singh A, Shokeen A, et al. Evidence of a novel allergenic protein Narcin in the bulbs of Narcissus tazetta. Int J Biochem Mol Biol. 2013;4:95-101.
Practice Points
- Narcissus species are thought to be the most common cause of irritant contact dermatitis among florists.
- Use of protective gloves and clothing to prevent Narcissus-induced contact dermatitis is recommended.
Botanical Briefs: Toxicodendron Dermatitis
Reactions to poison ivy, poison oak, and poison sumac, which affect 10 to 50 million Americans a year,1 are classified as Toxicodendron dermatitis; 50% to 75% of US adults are clinically sensitive to these plants.2 Furthermore, people of all ethnicities, skin types, and ages residing in most US geographical regions are at risk.3 Allergenicity is caused by urushiol, which is found in members of the Anacardiaceae family.4 Once absorbed, urushiol causes a type IV hypersensitivity reaction in those who are susceptible.5
Cutaneous Manifestations
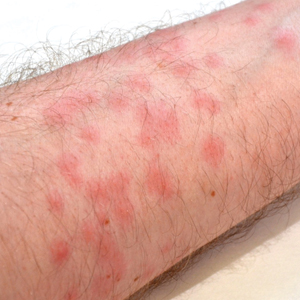
Toxicodendron dermatitis presents with an acute eczematous eruption characterized by streaks of intensely pruritic and erythematous papules and vesicles (Figure 1). Areas of involvement are characterized by sharp margins that follow the pattern of contact made by the plant’s leaves, berries, stems, and vines.6 The fluid content of the vesicles is not antigenic and cannot cause subsequent transmission to oneself or others.3 A person with prior contact to the plant who becomes sensitized develops an eruption 24 to 48 hours after subsequent contact with the plant; peak severity manifests 1 to 14 days later.7
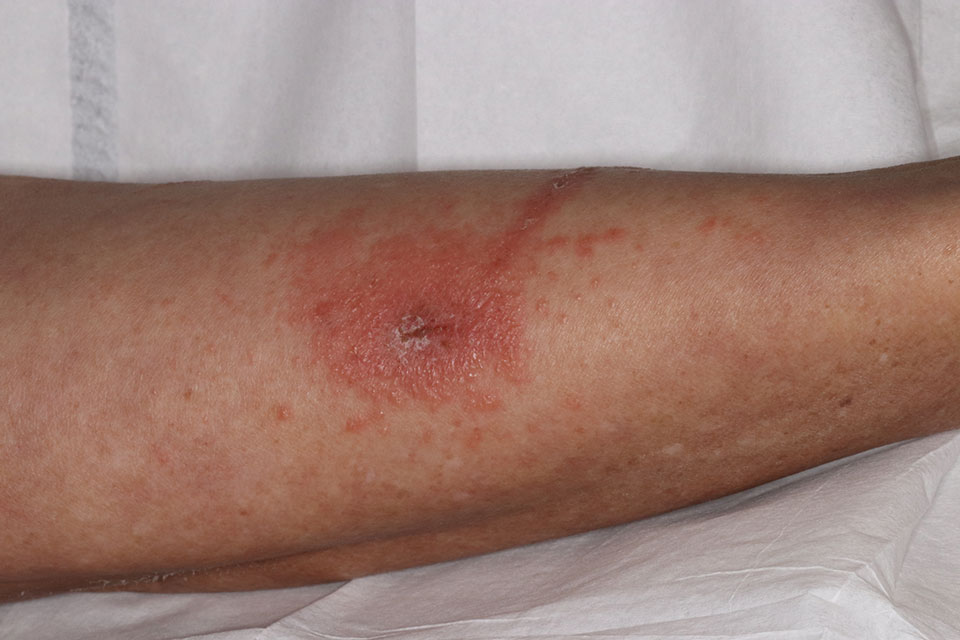
When left untreated, the eruption can last 3 weeks. If the plant is burned, urushiol can be aerosolized in smoke, causing respiratory tract inflammation and generalized dermatitis, which has been reported among wildland firefighters.2 Long-term complications from an outbreak are limited but can include postinflammatory hyperpigmentation and secondary bacterial infection.8 Rare reports of nephrotic syndrome also have appeared in the literature.9 Toxicodendron dermatitis can present distinctively as so-called black dot dermatitis.6
Nomenclature
Poison ivy, poison oak, and poison sumac are members of the family Anacardiaceae and genus Toxicodendron,6 derived from the Greek words toxikos (poison) and dendron (tree).10
Distribution
Toxicodendron plants characteristically are found in various regions of the United States. Poison ivy is the most common and is comprised of 2 species: Toxicodendron rydbergii and Toxicodendron radicans. Toxicodendron rydbergii is a nonclimbing dwarf shrub typically found in the northern and western United States. Toxicodendron radicans is a climbing vine found in the eastern United States. Poison oak also is comprised of 2 species—Toxicodendron toxicarium and Toxicodendron diversilobum—and is more common in the western United States. Poison sumac (also known as Toxicodendron vernix) is a small shrub that grows in moist swampy areas. It has a predilection for marshes of the eastern and southeastern United States.6,11
Identifying Features
Educating patients on how to identify poison ivy can play a key role in avoidance, which is the most important step in preventing Toxicodendron dermatitis. A challenge in identification of poison ivy is the plant’s variable appearance; it grows as a small shrub, low-lying vine, or vine that climbs other trees.
As the vine matures, it develops tiny, rough, “hairy” rootlets—hence the saying, “Hairy vine, no friend of mine!” Rootlets help the plant attach to trees growing near a water source. Vines can reach a diameter of 3 inches. From mature vines, solitary stems extend 1 to 2 inches with 3 characteristic leaves at the terminus (Figure 2), prompting another classic saying, “Leaves of 3, let it be!”12

Poison oak is characterized by 3 to 5 leaflets. Poison sumac has 7 to 13 pointed, smooth-edged leaves.6
Dermatitis-Inducing Plant Parts
The primary allergenic component of Toxicodendron plants is urushiol, a resinous sap found in stems, roots, leaves, and skins of the fruits. These components must be damaged or bruised to release the allergen; slight contact with an uninjured plant part might not lead to harm.2,13 Some common forms of transmission include skin contact, ingestion, inhalation of smoke from burning plants, and contact with skin through contaminated items, such as clothing, animals, and tools.14
Allergens
The catecholic ring and aliphatic chain of the urushiol molecule are allergenic.15 The degree of saturation and length of the side chains vary with different catechols. Urushiol displays cross-reactivity with poison ivy, poison oak, and poison sumac. Urushiol from these plants differs only slightly in structure; therefore, sensitization to one causes sensitization to all. There also is cross-reactivity between different members of the Anacardiaceae family, including Anacardium occidentale (tropical cashew nut), Mangifera indica (tropical mango tree), Ginkgo biloba (ginkgo tree), and Semecarpus anacardium (Indian marking nut tree).12
Poison ivy, poison oak, and poison sumac cause allergic contact dermatitis as a type IV hypersensitivity reaction. First, urushiol binds and penetrates the skin, where it is oxidized to quinone intermediates and bound to haptens. Then, the intermediates bind surface proteins on antigen-presenting cells, specifically Langerhans cells in the epidermis and dermis.5
Presentation of nonpeptide antigens, such as urushiol, to T cells requires expression of langerin (also known as CD207) and CD1a.16 Langerin is a C-type lectin that causes formation of Birbeck granules; CD1a is a major histocompatibility complex class I molecule found in Birbeck granules.5,17 After Langerhans cells internalize and process the urushiol self-hapten neoantigen, it is presented to CD4+ T cells.6 These cells then expand to form circulating activated T-effector and T-memory lymphocytes.18
The molecular link that occurs between the hapten and carrier protein determines the response. When linked by an amino nucleophile, selective induction of T-effector cells ensues, resulting in allergic contact dermatitis. When linked by a sulfhydryl bond, selective induction of suppressor cells occurs, resulting in a reduced allergic contact dermatitis response.19 In the case of activation of T-effector cells, a cell-mediated cytotoxic immune response is generated that destroys epidermal cells and dermal vasculature.2 The incidence and intensity of poison ivy sensitivity decline proportionally with age and the absence of continued exposure.20
Preventive Action—Patients should be counseled that if contact between plant and skin occurs, it is important to remove contaminated clothing or objects and wash them with soap to prevent additional exposure.14,21 Areas of the skin that made contact with the plant should be washed with water as soon as possible; after 30 minutes, urushiol has sufficiently penetrated to cause a reaction.2 Forceful unidirectional washing with a damp washcloth and liquid dishwashing soap is recommended.22
Several barrier creams are commercially available to help prevent absorption or to deactivate the urushiol antigen. These products are used widely by forestry workers and wildland firefighters.23 One such barrier cream is bentoquatam (sold as various trade names), an organoclay compound made of quaternium-18 bentonite that interferes with absorption of the allergen by acting as a physical blocker.24
Treatment
After Toxicodendron dermatitis develops, several treatments are available to help manage symptoms. Calamine lotion can be used to help dry weeping lesions.25,26 Topical steroids can be used to help control pruritus and alleviate inflammation. High-potency topical corticosteroids such as clobetasol and mid-potency steroids such as triamcinolone can be used. Topical anesthetics (eg, benzocaine, pramoxine, benzyl alcohol) might provide symptomatic relief.27,28
Oral antihistamines can allow for better sleep by providing sedation but do not target the pruritus of poison ivy dermatitis, which is not histamine mediated.29,30 Systemic corticosteroids usually are considered in more severe dermatitis—when 20% or more of the body surface area is involved; blistering and itching are severe; or the face, hands, or genitalia are involved.31,32
Clinical Uses
Therapeutic uses for poison ivy have been explored extensively. In 1892, Dakin33 reported that ingestion of leaves by Native Americans reduced the incidence and severity of skin lesions after contact with poison ivy. Consumption of poison ivy was further studied by Epstein and colleagues34 in 1974; they concluded that ingestion of a large amount of urushiol over a period of 3 months or longer may help with hyposensitization—but not complete desensitization—to contact with poison ivy. However, the risk for adverse effects is thought to outweigh benefits because ingestion can cause perianal dermatitis, mucocutaneous sequelae, and systemic contact dermatitis.2
Although the use of Toxicodendron plants in modern-day medicine is limited, development of a vaccine (immunotherapy) against Toxicodendron dermatitis offers an exciting opportunity for further research.
- Pariser DM, Ceilley RI, Lefkovits AM, et al. Poison ivy, oak and sumac. Derm Insights. 2003;4:26-28.
- Gladman AC. Toxicodendron dermatitis: poison ivy, oak, and sumac. Wilderness Environ Med. 2006;17:120-128. doi:10.1580/pr31-05.1
- Fisher AA. Poison ivy/oak/sumac. part II: specific features. Cutis. 1996;58:22-24.
- Cruse JM, Lewis RE. Atlas of Immunology. CRC Press; 2004.
- Valladeau J, Ravel O, Dezutter-Dambuyant C, et al. Langerin, a novel C-type lectin specific to Langerhans cells, is an endocytic receptor that induces the formation of Birbeck granules. Immunity. 2000;12:71-81. doi:10.1016/s1074-7613(00)80160-0
- Marks JG. Poison ivy and poison oak allergic contact dermatitis. J Allergy Clin Immunol. 1989;9:497-506.
- Williams JV, Light J, Marks JG Jr. Individual variations in allergic contact dermatitis from urushiol. Arch Dermatol. 1999;135:1002-1003. doi:10.1001/archderm.135.8.1002
- Brook I, Frazier EH, Yeager JK. Microbiology of infected poison ivy dermatitis. Br J Dermatol. 2000;142:943-946. doi:10.1046/j.1365-2133.2000.03475.x
- Rytand DA. Fatal anuria, the nephrotic syndrome and glomerular nephritis as sequels of the dermatitis of poison oak. Am J Med. 1948;5:548-560. doi:10.1016/0002-9343(48)90105-3
- Gledhill D. The Names of Plants. Cambridge University Press; 2008.
- American Academy of Dermatology Association. Poison ivy, oak, and sumac: how to treat the rash. Accessed October 19, 2022. https://www.aad.org/public/everyday-care/itchy-skin/poison-ivy/treat-rash
- Monroe J. Toxicodendron contact dermatitis: a case report and brief review. J Clin Aesthet Dermatol. 2020;13(9 suppl 1):S29-S34.
- Marks JG Jr, Anderson BE, DeLeo VA. Contact & Occupational Dermatology. 4th ed. Jaypee Brothers Medical Publishers; 2016.
- Fisher AA, Mitchell JC. Toxicodendron plants and spices. In: Rietschel RL, Fowler JF Jr, eds. Fisher’s Contact Dermatitis. 4th ed. Williams and Wilkins; 1995:461-523.
- Dawson CR. The chemistry of poison ivy. Trans N Y Acad Sci. 1956;18:427-443. doi:10.1111/j.2164-0947.1956.tb00465.x
- Hunger RE, Sieling PA, Ochoa MT, et al. Langerhans cells utilize CD1a and langerin to efficiently present nonpeptide antigens to T cells. J Clin Invest. 2004;113:701-708. doi:10.1172/JCI19655
- Hanau D, Fabre M, Schmitt DA, et al. Human epidermal Langerhans cells cointernalize by receptor-mediated endocytosis “non-classical” major histocompatibility complex class Imolecules (T6 antigens) and class II molecules (HLA-DR antigens). Proc Natl Acad Sci U S A. 1987;84:2901-2905. doi:10.1073/pnas.84.9.2901
- Gayer KD, Burnett JW. Toxicodendron dermatitis. Cutis. 1988;42:99-100.
- Dunn IS, Liberato DJ, Castagnoli N, et al. Contact sensitivity to urushiol: role of covalent bond formation. Cell Immunol. 1982;74:220-233. doi:10.1016/0008-8749(82)90023-5
- Kligman AM. Poison ivy (Rhus) dermatitis; an experimental study. AMA Arch Derm. 1958;77:149-180. doi:10.1001/archderm.1958.01560020001001
- Derraik JGB. Heracleum mantegazzianum and Toxicodendron succedaneum: plants of human health significance in New Zealand and the National Pest Plant Accord. N Z Med J. 2007;120:U2657.
- Neill BC, Neill JA, Brauker J, et al. Postexposure prevention of Toxicodendron dermatitis by early forceful unidirectional washing with liquid dishwashing soap. J Am Acad Dermatol. 2018;81:E25. doi:10.1016/j.jaad.2017.12.081
- Kim Y, Flamm A, ElSohly MA, et al. Poison ivy, oak, and sumac dermatitis: what is known and what is new? Dermatitis. 2019;30:183-190. doi:10.1097/DER.0000000000000472
- Marks JG Jr, Fowler JF Jr, Sheretz EF, et al. Prevention of poison ivy and poison oak allergic contact dermatitis by quaternium-18 bentonite. J Am Acad Dermatol. 1995;33:212-216. doi:10.1016/0190-9622(95)90237-6
- Baer RL. Poison ivy dermatitis. Cutis. 1990;46:34-36.
- Williford PM, Sheretz EF. Poison ivy dermatitis. nuances in treatment. Arch Fam Med. 1995;3:184.
- Amrol D, Keitel D, Hagaman D, et al. Topical pimecrolimus in the treatment of human allergic contact dermatitis. Ann Allergy Asthma Immunol. 2003;91:563-566. doi:10.1016/S1081-1206(10)61535-9
- Stephanides SL, Moore C. Toxicodendron poisoning treatment & management. Medscape. Updated June 13, 2022. Accessed October 19, 2022. https://emedicine.medscape.com/article/817671-treatment#d11
- Munday J, Bloomfield R, Goldman M, et al. Chlorpheniramine is no more effective than placebo in relieving the symptoms of childhood atopic dermatitis with a nocturnal itching and scratching component. Dermatology. 2002;205:40-45. doi:10.1159/000063138
- Yosipovitch G, Fleischer A. Itch associated with skin disease: advances in pathophysiology and emerging therapies. Am J Clin Dermatol. 2003;4:617-622. doi:10.2165/00128071-200304090-00004
- Li LY, Cruz PD Jr. Allergic contact dermatitis: pathophysiology applied to future therapy. Dermatol Ther. 2004;17:219-223. doi:10.1111/j.1396-0296.2004.04023.x
- Craig K, Meadows SE. What is the best duration of steroid therapy for contact dermatitis (Rhus)? J Fam Pract. 2006;55:166-167.
- Dakin R. Remarks on a cutaneous affection, produced by certain poisonous vegetables. Am J Med Sci. 1829;4:98-100.
- Epstein WL, Baer H, Dawson CR, et al. Poison oak hyposensitization. evaluation of purified urushiol. Arch Dermatol. 1974;109:356-360.
Reactions to poison ivy, poison oak, and poison sumac, which affect 10 to 50 million Americans a year,1 are classified as Toxicodendron dermatitis; 50% to 75% of US adults are clinically sensitive to these plants.2 Furthermore, people of all ethnicities, skin types, and ages residing in most US geographical regions are at risk.3 Allergenicity is caused by urushiol, which is found in members of the Anacardiaceae family.4 Once absorbed, urushiol causes a type IV hypersensitivity reaction in those who are susceptible.5
Cutaneous Manifestations
Toxicodendron dermatitis presents with an acute eczematous eruption characterized by streaks of intensely pruritic and erythematous papules and vesicles (Figure 1). Areas of involvement are characterized by sharp margins that follow the pattern of contact made by the plant’s leaves, berries, stems, and vines.6 The fluid content of the vesicles is not antigenic and cannot cause subsequent transmission to oneself or others.3 A person with prior contact to the plant who becomes sensitized develops an eruption 24 to 48 hours after subsequent contact with the plant; peak severity manifests 1 to 14 days later.7

When left untreated, the eruption can last 3 weeks. If the plant is burned, urushiol can be aerosolized in smoke, causing respiratory tract inflammation and generalized dermatitis, which has been reported among wildland firefighters.2 Long-term complications from an outbreak are limited but can include postinflammatory hyperpigmentation and secondary bacterial infection.8 Rare reports of nephrotic syndrome also have appeared in the literature.9 Toxicodendron dermatitis can present distinctively as so-called black dot dermatitis.6
Nomenclature
Poison ivy, poison oak, and poison sumac are members of the family Anacardiaceae and genus Toxicodendron,6 derived from the Greek words toxikos (poison) and dendron (tree).10
Distribution
Toxicodendron plants characteristically are found in various regions of the United States. Poison ivy is the most common and is comprised of 2 species: Toxicodendron rydbergii and Toxicodendron radicans. Toxicodendron rydbergii is a nonclimbing dwarf shrub typically found in the northern and western United States. Toxicodendron radicans is a climbing vine found in the eastern United States. Poison oak also is comprised of 2 species—Toxicodendron toxicarium and Toxicodendron diversilobum—and is more common in the western United States. Poison sumac (also known as Toxicodendron vernix) is a small shrub that grows in moist swampy areas. It has a predilection for marshes of the eastern and southeastern United States.6,11
Identifying Features
Educating patients on how to identify poison ivy can play a key role in avoidance, which is the most important step in preventing Toxicodendron dermatitis. A challenge in identification of poison ivy is the plant’s variable appearance; it grows as a small shrub, low-lying vine, or vine that climbs other trees.
As the vine matures, it develops tiny, rough, “hairy” rootlets—hence the saying, “Hairy vine, no friend of mine!” Rootlets help the plant attach to trees growing near a water source. Vines can reach a diameter of 3 inches. From mature vines, solitary stems extend 1 to 2 inches with 3 characteristic leaves at the terminus (Figure 2), prompting another classic saying, “Leaves of 3, let it be!”12

Poison oak is characterized by 3 to 5 leaflets. Poison sumac has 7 to 13 pointed, smooth-edged leaves.6
Dermatitis-Inducing Plant Parts
The primary allergenic component of Toxicodendron plants is urushiol, a resinous sap found in stems, roots, leaves, and skins of the fruits. These components must be damaged or bruised to release the allergen; slight contact with an uninjured plant part might not lead to harm.2,13 Some common forms of transmission include skin contact, ingestion, inhalation of smoke from burning plants, and contact with skin through contaminated items, such as clothing, animals, and tools.14
Allergens
The catecholic ring and aliphatic chain of the urushiol molecule are allergenic.15 The degree of saturation and length of the side chains vary with different catechols. Urushiol displays cross-reactivity with poison ivy, poison oak, and poison sumac. Urushiol from these plants differs only slightly in structure; therefore, sensitization to one causes sensitization to all. There also is cross-reactivity between different members of the Anacardiaceae family, including Anacardium occidentale (tropical cashew nut), Mangifera indica (tropical mango tree), Ginkgo biloba (ginkgo tree), and Semecarpus anacardium (Indian marking nut tree).12
Poison ivy, poison oak, and poison sumac cause allergic contact dermatitis as a type IV hypersensitivity reaction. First, urushiol binds and penetrates the skin, where it is oxidized to quinone intermediates and bound to haptens. Then, the intermediates bind surface proteins on antigen-presenting cells, specifically Langerhans cells in the epidermis and dermis.5
Presentation of nonpeptide antigens, such as urushiol, to T cells requires expression of langerin (also known as CD207) and CD1a.16 Langerin is a C-type lectin that causes formation of Birbeck granules; CD1a is a major histocompatibility complex class I molecule found in Birbeck granules.5,17 After Langerhans cells internalize and process the urushiol self-hapten neoantigen, it is presented to CD4+ T cells.6 These cells then expand to form circulating activated T-effector and T-memory lymphocytes.18
The molecular link that occurs between the hapten and carrier protein determines the response. When linked by an amino nucleophile, selective induction of T-effector cells ensues, resulting in allergic contact dermatitis. When linked by a sulfhydryl bond, selective induction of suppressor cells occurs, resulting in a reduced allergic contact dermatitis response.19 In the case of activation of T-effector cells, a cell-mediated cytotoxic immune response is generated that destroys epidermal cells and dermal vasculature.2 The incidence and intensity of poison ivy sensitivity decline proportionally with age and the absence of continued exposure.20
Preventive Action—Patients should be counseled that if contact between plant and skin occurs, it is important to remove contaminated clothing or objects and wash them with soap to prevent additional exposure.14,21 Areas of the skin that made contact with the plant should be washed with water as soon as possible; after 30 minutes, urushiol has sufficiently penetrated to cause a reaction.2 Forceful unidirectional washing with a damp washcloth and liquid dishwashing soap is recommended.22
Several barrier creams are commercially available to help prevent absorption or to deactivate the urushiol antigen. These products are used widely by forestry workers and wildland firefighters.23 One such barrier cream is bentoquatam (sold as various trade names), an organoclay compound made of quaternium-18 bentonite that interferes with absorption of the allergen by acting as a physical blocker.24
Treatment
After Toxicodendron dermatitis develops, several treatments are available to help manage symptoms. Calamine lotion can be used to help dry weeping lesions.25,26 Topical steroids can be used to help control pruritus and alleviate inflammation. High-potency topical corticosteroids such as clobetasol and mid-potency steroids such as triamcinolone can be used. Topical anesthetics (eg, benzocaine, pramoxine, benzyl alcohol) might provide symptomatic relief.27,28
Oral antihistamines can allow for better sleep by providing sedation but do not target the pruritus of poison ivy dermatitis, which is not histamine mediated.29,30 Systemic corticosteroids usually are considered in more severe dermatitis—when 20% or more of the body surface area is involved; blistering and itching are severe; or the face, hands, or genitalia are involved.31,32
Clinical Uses
Therapeutic uses for poison ivy have been explored extensively. In 1892, Dakin33 reported that ingestion of leaves by Native Americans reduced the incidence and severity of skin lesions after contact with poison ivy. Consumption of poison ivy was further studied by Epstein and colleagues34 in 1974; they concluded that ingestion of a large amount of urushiol over a period of 3 months or longer may help with hyposensitization—but not complete desensitization—to contact with poison ivy. However, the risk for adverse effects is thought to outweigh benefits because ingestion can cause perianal dermatitis, mucocutaneous sequelae, and systemic contact dermatitis.2
Although the use of Toxicodendron plants in modern-day medicine is limited, development of a vaccine (immunotherapy) against Toxicodendron dermatitis offers an exciting opportunity for further research.
Reactions to poison ivy, poison oak, and poison sumac, which affect 10 to 50 million Americans a year,1 are classified as Toxicodendron dermatitis; 50% to 75% of US adults are clinically sensitive to these plants.2 Furthermore, people of all ethnicities, skin types, and ages residing in most US geographical regions are at risk.3 Allergenicity is caused by urushiol, which is found in members of the Anacardiaceae family.4 Once absorbed, urushiol causes a type IV hypersensitivity reaction in those who are susceptible.5
Cutaneous Manifestations
Toxicodendron dermatitis presents with an acute eczematous eruption characterized by streaks of intensely pruritic and erythematous papules and vesicles (Figure 1). Areas of involvement are characterized by sharp margins that follow the pattern of contact made by the plant’s leaves, berries, stems, and vines.6 The fluid content of the vesicles is not antigenic and cannot cause subsequent transmission to oneself or others.3 A person with prior contact to the plant who becomes sensitized develops an eruption 24 to 48 hours after subsequent contact with the plant; peak severity manifests 1 to 14 days later.7

When left untreated, the eruption can last 3 weeks. If the plant is burned, urushiol can be aerosolized in smoke, causing respiratory tract inflammation and generalized dermatitis, which has been reported among wildland firefighters.2 Long-term complications from an outbreak are limited but can include postinflammatory hyperpigmentation and secondary bacterial infection.8 Rare reports of nephrotic syndrome also have appeared in the literature.9 Toxicodendron dermatitis can present distinctively as so-called black dot dermatitis.6
Nomenclature
Poison ivy, poison oak, and poison sumac are members of the family Anacardiaceae and genus Toxicodendron,6 derived from the Greek words toxikos (poison) and dendron (tree).10
Distribution
Toxicodendron plants characteristically are found in various regions of the United States. Poison ivy is the most common and is comprised of 2 species: Toxicodendron rydbergii and Toxicodendron radicans. Toxicodendron rydbergii is a nonclimbing dwarf shrub typically found in the northern and western United States. Toxicodendron radicans is a climbing vine found in the eastern United States. Poison oak also is comprised of 2 species—Toxicodendron toxicarium and Toxicodendron diversilobum—and is more common in the western United States. Poison sumac (also known as Toxicodendron vernix) is a small shrub that grows in moist swampy areas. It has a predilection for marshes of the eastern and southeastern United States.6,11
Identifying Features
Educating patients on how to identify poison ivy can play a key role in avoidance, which is the most important step in preventing Toxicodendron dermatitis. A challenge in identification of poison ivy is the plant’s variable appearance; it grows as a small shrub, low-lying vine, or vine that climbs other trees.
As the vine matures, it develops tiny, rough, “hairy” rootlets—hence the saying, “Hairy vine, no friend of mine!” Rootlets help the plant attach to trees growing near a water source. Vines can reach a diameter of 3 inches. From mature vines, solitary stems extend 1 to 2 inches with 3 characteristic leaves at the terminus (Figure 2), prompting another classic saying, “Leaves of 3, let it be!”12

Poison oak is characterized by 3 to 5 leaflets. Poison sumac has 7 to 13 pointed, smooth-edged leaves.6
Dermatitis-Inducing Plant Parts
The primary allergenic component of Toxicodendron plants is urushiol, a resinous sap found in stems, roots, leaves, and skins of the fruits. These components must be damaged or bruised to release the allergen; slight contact with an uninjured plant part might not lead to harm.2,13 Some common forms of transmission include skin contact, ingestion, inhalation of smoke from burning plants, and contact with skin through contaminated items, such as clothing, animals, and tools.14
Allergens
The catecholic ring and aliphatic chain of the urushiol molecule are allergenic.15 The degree of saturation and length of the side chains vary with different catechols. Urushiol displays cross-reactivity with poison ivy, poison oak, and poison sumac. Urushiol from these plants differs only slightly in structure; therefore, sensitization to one causes sensitization to all. There also is cross-reactivity between different members of the Anacardiaceae family, including Anacardium occidentale (tropical cashew nut), Mangifera indica (tropical mango tree), Ginkgo biloba (ginkgo tree), and Semecarpus anacardium (Indian marking nut tree).12
Poison ivy, poison oak, and poison sumac cause allergic contact dermatitis as a type IV hypersensitivity reaction. First, urushiol binds and penetrates the skin, where it is oxidized to quinone intermediates and bound to haptens. Then, the intermediates bind surface proteins on antigen-presenting cells, specifically Langerhans cells in the epidermis and dermis.5
Presentation of nonpeptide antigens, such as urushiol, to T cells requires expression of langerin (also known as CD207) and CD1a.16 Langerin is a C-type lectin that causes formation of Birbeck granules; CD1a is a major histocompatibility complex class I molecule found in Birbeck granules.5,17 After Langerhans cells internalize and process the urushiol self-hapten neoantigen, it is presented to CD4+ T cells.6 These cells then expand to form circulating activated T-effector and T-memory lymphocytes.18
The molecular link that occurs between the hapten and carrier protein determines the response. When linked by an amino nucleophile, selective induction of T-effector cells ensues, resulting in allergic contact dermatitis. When linked by a sulfhydryl bond, selective induction of suppressor cells occurs, resulting in a reduced allergic contact dermatitis response.19 In the case of activation of T-effector cells, a cell-mediated cytotoxic immune response is generated that destroys epidermal cells and dermal vasculature.2 The incidence and intensity of poison ivy sensitivity decline proportionally with age and the absence of continued exposure.20
Preventive Action—Patients should be counseled that if contact between plant and skin occurs, it is important to remove contaminated clothing or objects and wash them with soap to prevent additional exposure.14,21 Areas of the skin that made contact with the plant should be washed with water as soon as possible; after 30 minutes, urushiol has sufficiently penetrated to cause a reaction.2 Forceful unidirectional washing with a damp washcloth and liquid dishwashing soap is recommended.22
Several barrier creams are commercially available to help prevent absorption or to deactivate the urushiol antigen. These products are used widely by forestry workers and wildland firefighters.23 One such barrier cream is bentoquatam (sold as various trade names), an organoclay compound made of quaternium-18 bentonite that interferes with absorption of the allergen by acting as a physical blocker.24
Treatment
After Toxicodendron dermatitis develops, several treatments are available to help manage symptoms. Calamine lotion can be used to help dry weeping lesions.25,26 Topical steroids can be used to help control pruritus and alleviate inflammation. High-potency topical corticosteroids such as clobetasol and mid-potency steroids such as triamcinolone can be used. Topical anesthetics (eg, benzocaine, pramoxine, benzyl alcohol) might provide symptomatic relief.27,28
Oral antihistamines can allow for better sleep by providing sedation but do not target the pruritus of poison ivy dermatitis, which is not histamine mediated.29,30 Systemic corticosteroids usually are considered in more severe dermatitis—when 20% or more of the body surface area is involved; blistering and itching are severe; or the face, hands, or genitalia are involved.31,32
Clinical Uses
Therapeutic uses for poison ivy have been explored extensively. In 1892, Dakin33 reported that ingestion of leaves by Native Americans reduced the incidence and severity of skin lesions after contact with poison ivy. Consumption of poison ivy was further studied by Epstein and colleagues34 in 1974; they concluded that ingestion of a large amount of urushiol over a period of 3 months or longer may help with hyposensitization—but not complete desensitization—to contact with poison ivy. However, the risk for adverse effects is thought to outweigh benefits because ingestion can cause perianal dermatitis, mucocutaneous sequelae, and systemic contact dermatitis.2
Although the use of Toxicodendron plants in modern-day medicine is limited, development of a vaccine (immunotherapy) against Toxicodendron dermatitis offers an exciting opportunity for further research.
- Pariser DM, Ceilley RI, Lefkovits AM, et al. Poison ivy, oak and sumac. Derm Insights. 2003;4:26-28.
- Gladman AC. Toxicodendron dermatitis: poison ivy, oak, and sumac. Wilderness Environ Med. 2006;17:120-128. doi:10.1580/pr31-05.1
- Fisher AA. Poison ivy/oak/sumac. part II: specific features. Cutis. 1996;58:22-24.
- Cruse JM, Lewis RE. Atlas of Immunology. CRC Press; 2004.
- Valladeau J, Ravel O, Dezutter-Dambuyant C, et al. Langerin, a novel C-type lectin specific to Langerhans cells, is an endocytic receptor that induces the formation of Birbeck granules. Immunity. 2000;12:71-81. doi:10.1016/s1074-7613(00)80160-0
- Marks JG. Poison ivy and poison oak allergic contact dermatitis. J Allergy Clin Immunol. 1989;9:497-506.
- Williams JV, Light J, Marks JG Jr. Individual variations in allergic contact dermatitis from urushiol. Arch Dermatol. 1999;135:1002-1003. doi:10.1001/archderm.135.8.1002
- Brook I, Frazier EH, Yeager JK. Microbiology of infected poison ivy dermatitis. Br J Dermatol. 2000;142:943-946. doi:10.1046/j.1365-2133.2000.03475.x
- Rytand DA. Fatal anuria, the nephrotic syndrome and glomerular nephritis as sequels of the dermatitis of poison oak. Am J Med. 1948;5:548-560. doi:10.1016/0002-9343(48)90105-3
- Gledhill D. The Names of Plants. Cambridge University Press; 2008.
- American Academy of Dermatology Association. Poison ivy, oak, and sumac: how to treat the rash. Accessed October 19, 2022. https://www.aad.org/public/everyday-care/itchy-skin/poison-ivy/treat-rash
- Monroe J. Toxicodendron contact dermatitis: a case report and brief review. J Clin Aesthet Dermatol. 2020;13(9 suppl 1):S29-S34.
- Marks JG Jr, Anderson BE, DeLeo VA. Contact & Occupational Dermatology. 4th ed. Jaypee Brothers Medical Publishers; 2016.
- Fisher AA, Mitchell JC. Toxicodendron plants and spices. In: Rietschel RL, Fowler JF Jr, eds. Fisher’s Contact Dermatitis. 4th ed. Williams and Wilkins; 1995:461-523.
- Dawson CR. The chemistry of poison ivy. Trans N Y Acad Sci. 1956;18:427-443. doi:10.1111/j.2164-0947.1956.tb00465.x
- Hunger RE, Sieling PA, Ochoa MT, et al. Langerhans cells utilize CD1a and langerin to efficiently present nonpeptide antigens to T cells. J Clin Invest. 2004;113:701-708. doi:10.1172/JCI19655
- Hanau D, Fabre M, Schmitt DA, et al. Human epidermal Langerhans cells cointernalize by receptor-mediated endocytosis “non-classical” major histocompatibility complex class Imolecules (T6 antigens) and class II molecules (HLA-DR antigens). Proc Natl Acad Sci U S A. 1987;84:2901-2905. doi:10.1073/pnas.84.9.2901
- Gayer KD, Burnett JW. Toxicodendron dermatitis. Cutis. 1988;42:99-100.
- Dunn IS, Liberato DJ, Castagnoli N, et al. Contact sensitivity to urushiol: role of covalent bond formation. Cell Immunol. 1982;74:220-233. doi:10.1016/0008-8749(82)90023-5
- Kligman AM. Poison ivy (Rhus) dermatitis; an experimental study. AMA Arch Derm. 1958;77:149-180. doi:10.1001/archderm.1958.01560020001001
- Derraik JGB. Heracleum mantegazzianum and Toxicodendron succedaneum: plants of human health significance in New Zealand and the National Pest Plant Accord. N Z Med J. 2007;120:U2657.
- Neill BC, Neill JA, Brauker J, et al. Postexposure prevention of Toxicodendron dermatitis by early forceful unidirectional washing with liquid dishwashing soap. J Am Acad Dermatol. 2018;81:E25. doi:10.1016/j.jaad.2017.12.081
- Kim Y, Flamm A, ElSohly MA, et al. Poison ivy, oak, and sumac dermatitis: what is known and what is new? Dermatitis. 2019;30:183-190. doi:10.1097/DER.0000000000000472
- Marks JG Jr, Fowler JF Jr, Sheretz EF, et al. Prevention of poison ivy and poison oak allergic contact dermatitis by quaternium-18 bentonite. J Am Acad Dermatol. 1995;33:212-216. doi:10.1016/0190-9622(95)90237-6
- Baer RL. Poison ivy dermatitis. Cutis. 1990;46:34-36.
- Williford PM, Sheretz EF. Poison ivy dermatitis. nuances in treatment. Arch Fam Med. 1995;3:184.
- Amrol D, Keitel D, Hagaman D, et al. Topical pimecrolimus in the treatment of human allergic contact dermatitis. Ann Allergy Asthma Immunol. 2003;91:563-566. doi:10.1016/S1081-1206(10)61535-9
- Stephanides SL, Moore C. Toxicodendron poisoning treatment & management. Medscape. Updated June 13, 2022. Accessed October 19, 2022. https://emedicine.medscape.com/article/817671-treatment#d11
- Munday J, Bloomfield R, Goldman M, et al. Chlorpheniramine is no more effective than placebo in relieving the symptoms of childhood atopic dermatitis with a nocturnal itching and scratching component. Dermatology. 2002;205:40-45. doi:10.1159/000063138
- Yosipovitch G, Fleischer A. Itch associated with skin disease: advances in pathophysiology and emerging therapies. Am J Clin Dermatol. 2003;4:617-622. doi:10.2165/00128071-200304090-00004
- Li LY, Cruz PD Jr. Allergic contact dermatitis: pathophysiology applied to future therapy. Dermatol Ther. 2004;17:219-223. doi:10.1111/j.1396-0296.2004.04023.x
- Craig K, Meadows SE. What is the best duration of steroid therapy for contact dermatitis (Rhus)? J Fam Pract. 2006;55:166-167.
- Dakin R. Remarks on a cutaneous affection, produced by certain poisonous vegetables. Am J Med Sci. 1829;4:98-100.
- Epstein WL, Baer H, Dawson CR, et al. Poison oak hyposensitization. evaluation of purified urushiol. Arch Dermatol. 1974;109:356-360.
- Pariser DM, Ceilley RI, Lefkovits AM, et al. Poison ivy, oak and sumac. Derm Insights. 2003;4:26-28.
- Gladman AC. Toxicodendron dermatitis: poison ivy, oak, and sumac. Wilderness Environ Med. 2006;17:120-128. doi:10.1580/pr31-05.1
- Fisher AA. Poison ivy/oak/sumac. part II: specific features. Cutis. 1996;58:22-24.
- Cruse JM, Lewis RE. Atlas of Immunology. CRC Press; 2004.
- Valladeau J, Ravel O, Dezutter-Dambuyant C, et al. Langerin, a novel C-type lectin specific to Langerhans cells, is an endocytic receptor that induces the formation of Birbeck granules. Immunity. 2000;12:71-81. doi:10.1016/s1074-7613(00)80160-0
- Marks JG. Poison ivy and poison oak allergic contact dermatitis. J Allergy Clin Immunol. 1989;9:497-506.
- Williams JV, Light J, Marks JG Jr. Individual variations in allergic contact dermatitis from urushiol. Arch Dermatol. 1999;135:1002-1003. doi:10.1001/archderm.135.8.1002
- Brook I, Frazier EH, Yeager JK. Microbiology of infected poison ivy dermatitis. Br J Dermatol. 2000;142:943-946. doi:10.1046/j.1365-2133.2000.03475.x
- Rytand DA. Fatal anuria, the nephrotic syndrome and glomerular nephritis as sequels of the dermatitis of poison oak. Am J Med. 1948;5:548-560. doi:10.1016/0002-9343(48)90105-3
- Gledhill D. The Names of Plants. Cambridge University Press; 2008.
- American Academy of Dermatology Association. Poison ivy, oak, and sumac: how to treat the rash. Accessed October 19, 2022. https://www.aad.org/public/everyday-care/itchy-skin/poison-ivy/treat-rash
- Monroe J. Toxicodendron contact dermatitis: a case report and brief review. J Clin Aesthet Dermatol. 2020;13(9 suppl 1):S29-S34.
- Marks JG Jr, Anderson BE, DeLeo VA. Contact & Occupational Dermatology. 4th ed. Jaypee Brothers Medical Publishers; 2016.
- Fisher AA, Mitchell JC. Toxicodendron plants and spices. In: Rietschel RL, Fowler JF Jr, eds. Fisher’s Contact Dermatitis. 4th ed. Williams and Wilkins; 1995:461-523.
- Dawson CR. The chemistry of poison ivy. Trans N Y Acad Sci. 1956;18:427-443. doi:10.1111/j.2164-0947.1956.tb00465.x
- Hunger RE, Sieling PA, Ochoa MT, et al. Langerhans cells utilize CD1a and langerin to efficiently present nonpeptide antigens to T cells. J Clin Invest. 2004;113:701-708. doi:10.1172/JCI19655
- Hanau D, Fabre M, Schmitt DA, et al. Human epidermal Langerhans cells cointernalize by receptor-mediated endocytosis “non-classical” major histocompatibility complex class Imolecules (T6 antigens) and class II molecules (HLA-DR antigens). Proc Natl Acad Sci U S A. 1987;84:2901-2905. doi:10.1073/pnas.84.9.2901
- Gayer KD, Burnett JW. Toxicodendron dermatitis. Cutis. 1988;42:99-100.
- Dunn IS, Liberato DJ, Castagnoli N, et al. Contact sensitivity to urushiol: role of covalent bond formation. Cell Immunol. 1982;74:220-233. doi:10.1016/0008-8749(82)90023-5
- Kligman AM. Poison ivy (Rhus) dermatitis; an experimental study. AMA Arch Derm. 1958;77:149-180. doi:10.1001/archderm.1958.01560020001001
- Derraik JGB. Heracleum mantegazzianum and Toxicodendron succedaneum: plants of human health significance in New Zealand and the National Pest Plant Accord. N Z Med J. 2007;120:U2657.
- Neill BC, Neill JA, Brauker J, et al. Postexposure prevention of Toxicodendron dermatitis by early forceful unidirectional washing with liquid dishwashing soap. J Am Acad Dermatol. 2018;81:E25. doi:10.1016/j.jaad.2017.12.081
- Kim Y, Flamm A, ElSohly MA, et al. Poison ivy, oak, and sumac dermatitis: what is known and what is new? Dermatitis. 2019;30:183-190. doi:10.1097/DER.0000000000000472
- Marks JG Jr, Fowler JF Jr, Sheretz EF, et al. Prevention of poison ivy and poison oak allergic contact dermatitis by quaternium-18 bentonite. J Am Acad Dermatol. 1995;33:212-216. doi:10.1016/0190-9622(95)90237-6
- Baer RL. Poison ivy dermatitis. Cutis. 1990;46:34-36.
- Williford PM, Sheretz EF. Poison ivy dermatitis. nuances in treatment. Arch Fam Med. 1995;3:184.
- Amrol D, Keitel D, Hagaman D, et al. Topical pimecrolimus in the treatment of human allergic contact dermatitis. Ann Allergy Asthma Immunol. 2003;91:563-566. doi:10.1016/S1081-1206(10)61535-9
- Stephanides SL, Moore C. Toxicodendron poisoning treatment & management. Medscape. Updated June 13, 2022. Accessed October 19, 2022. https://emedicine.medscape.com/article/817671-treatment#d11
- Munday J, Bloomfield R, Goldman M, et al. Chlorpheniramine is no more effective than placebo in relieving the symptoms of childhood atopic dermatitis with a nocturnal itching and scratching component. Dermatology. 2002;205:40-45. doi:10.1159/000063138
- Yosipovitch G, Fleischer A. Itch associated with skin disease: advances in pathophysiology and emerging therapies. Am J Clin Dermatol. 2003;4:617-622. doi:10.2165/00128071-200304090-00004
- Li LY, Cruz PD Jr. Allergic contact dermatitis: pathophysiology applied to future therapy. Dermatol Ther. 2004;17:219-223. doi:10.1111/j.1396-0296.2004.04023.x
- Craig K, Meadows SE. What is the best duration of steroid therapy for contact dermatitis (Rhus)? J Fam Pract. 2006;55:166-167.
- Dakin R. Remarks on a cutaneous affection, produced by certain poisonous vegetables. Am J Med Sci. 1829;4:98-100.
- Epstein WL, Baer H, Dawson CR, et al. Poison oak hyposensitization. evaluation of purified urushiol. Arch Dermatol. 1974;109:356-360.
Practice Points
- Toxicodendron dermatitis is a pruritic vesicular eruption in areas of contact with the plant.
- Identification and avoidance are primary methods of preventing Toxicodendron dermatitis.
Botanical Briefs: Tulipalin A
Cutaneous Manifestations
Contact dermatitis is a common problem for individuals who work in the floral industry. Hand dermatitis has been reported in as many as 26% of floral employees.1Tulipa species have been identified as one of the most common causes of hand dermatitis. Tulipalin A (α-methylene-γ-butyrolactone) is the main sensitizer in tulips (Figure 1) and its precursor tuliposide A also occurs both in tulips and the Peruvian lily (Alstroemeria).

In a 1996 study, 18% (9/51) of tulip workers were found to be allergic to tulipalin A.2 In a more recent study of 164 tulip workers, 48 (29.3%) had clinical evidence of contact dermatitis and subsequently underwent patch testing; 17 (35.4%) showed a positive reaction to either tulipalin A or to tulip-bulb extract.3 Itching was the most common symptom (39 workers [81.3%]) and hand eczema at the tip of the thumb and index finger was the most common finding. In 9 (18.8%) workers, eczema had spread to other body parts including the forearm, face, legs, and abdomen.3
Peruvian lily is widely used in floral arrangements and has become a leading cause of hand dermatitis in florists (Figure 2). Large amounts of free tulipalin A are present in bulb scales of tulips, along with a small amount of tuliposide A. In young developing shoots, the situation is reversed: Both compounds are found in all parts of the plant to some degree, though tulipalin A is the major allergen, and more mature parts of the plant and bulb are most allergenic.

Cultural Considerations
In traditional Kurdish cuisine, raw herbs are part of snacking or are served as a side dish (sawza). Snacks often are consumed raw on the spot. Tulipa montana, Tulipa armena, and possibly other Tulipa species are consumed as a snack.4 Traditionally, Tulipa systola is consumed by the Kurds as an anti-inflammatory medicine and for pain relief. It also has been proposed that T systola has antioxidant properties.5 Cooked tulip also has been consumed in time of famine in Europe, though none of these dietary practices are recommended.4
Clinical Presentation
“Tulip fingers” describes the most common presentation of contact dermatitis caused by tulip bulbs. Erythematous scaling plaques are seen in the periungual skin and first and second fingertips of the dominant hand. Other manifestations include diffuse dry dermatitis of the hand; paronychia; pulpitis; and secondary spread to the face, neck, arms, and genitalia, with eczematous papules and plaques.6 Clinical signs include erythema, vesicles, hyperkeratosis, and exfoliation of the fingertips. The allergen also can cause airborne contact dermatitis and manifest as conjunctivitis, rhinitis, and asthma.2 A considerable number of tulip workers develop paresthesia and tenderness in the fingertips within several hours after working with tulip bulbs, known as “tulip fire.”7
Plant Facts
There are approximately 250 genera of bulbous plants. Tulips are members of the genus Tulipa and family Liliaceae. Tulips often are thought of as native to southwest central Asia and Turkey8; however, Tulipa sylvestris is native to Portugal, Spain, and North Africa.
Etymology and Symbolism—The word tulip is derived from the Turkish word türbent meaning a turban, possibly because the flower is compared to turbans worn by men of the Ottoman Empire in the 16th century. In Turkish culture, the tulip is a symbol of paradise on earth and can have divine status. In the Netherlands, on the other hand, the tulip represents the briefness of life.
History—By 1562, tulip bulbs had already been introduced to Holland by merchants. However, the first shipment of tulip bulbs was mistaken by the Dutch for onions and were either roasted over a fire or perished when planted in gardens with vegetables. Carolus Clusius—botanist, director of the imperial medical garden in Vienna and recipient of many plants through diplomatic channels—was particularly fond of flower bulbs and contributed to the popularity of the tulip in Europe by sending bulbs and seeds to other European countries.
In the early 17th century, the tulip craze began in France, fueled by a viral disease of tulips that produced variegated color patterns on the petals; entire properties were sold in exchange for a single tulip bulb. The tulip craze drifted from France to Holland in 1634 for 3 years before the tulip market collapsed.
More recently, in 2003 investors started a multimillion-euro tulip fund in the Netherlands to develop new varieties of tulip. Tulip bulbs were used to create money with high percentages over the selling price. With exorbitant pricing and ever-changing ownership of bulbs—bulbs were bought and sold as many as 10 times—the tulip fund collapsed 1 year later and investors lost their money. Bulb speculators then took their profit abroad. In 2006, bulb owners were charged with fraud; the tulip craze often is cited as one of the early major stock market collapses.
Tulips continue to grow in popularity. Today, nearly 6000 cultivars are registered, with 40 new cultivars registered every 5 years.9
Identifying Features
At the base of the erect tulip plant is a cluster of 2 or 3 thick bluish-green leaves. Three petals and 3 sepals make up the solitary bell-shaped flower. Many tulips can propagate only by means of their scaly bulbs. The flowers arise from the tips of stems in different solid colors, except true blue—from pure white to all shades of yellow, red, and a deep purple that is almost black. Solid-color tulips are called “self-colored.” So-called broken tulips are individual flowers with multiple colors, a condition caused by a viral disease transmitted by aphids.10
Tulip Allergen
Tuliposide A is found in many species of the genera Tulipa, Alstroemeria, and Erythronium.6 So far, 7 analogs have been identified: 1-tuliposide A and B; 6-tuliposide A and B; and tuliposides D, E, and F. 6-Tuliposide A and B are the principal tuliposides found in tulip cultivars.11 With trauma and maturation, tuliposides A and B are hydrolyzed to tulipalin A and tulipalin B, respectively.
Tulipalin A and tulipalin B have antimicrobial properties against bacteria and fungi; tulipalin A is mostly an antifungal agent, and tulipalin B has mostly bacteriostatic characteristics.12 The highest concentration of tulipalin A is found in the outer layer of the bulb, followed by (in descending order) the stem, leaves, and petals.13
The prevalence of tulipalin A allergy led the German Federal Institute for Risk Assessment to assign tuliposide A and tulipalin A to category B, which is a “solid-based indication for contact allergenic effects”; both chemicals also are considered skin sensitizers, defined by the Globally Harmonized System of Classification and Labelling of Chemicals of the United Nations as a substance that will induce an allergic response following skin contact.14 Patients who are allergic to tulips have cross-sensitivity to Alstroemeria because tuliposide A and its metabolites are found in both plants.15
Symptoms of an allergic response to tulipalin A can be immediate or delayed.14 The most common allergic contact dermatitis caused by tulip bulbs is type IV hypersensitivity, though type I reactions can occur. Symptoms of a type I reaction including contact urticaria, rhinitis, hoarseness, and dyspnea have been reported.14
The variety of tulip handled also contributes to the severity of dermatitis. Handling bulbs of Rose Copeland variety tulips and cutting the flowers of Preludium tulips have been associated with more severe allergic dermatitis presentations, whereas the Red Emperor tulip was found to have less tuliposide A and thus provoke a weaker patch-test reaction.7
A Word About Garlic—Garlic is in the subfamily Allioideae (formerly Alliaceae) taxonomically related to the tulip family (Liliaceae). Garlic also can cause hand dermatitis in cooks, with a similar clinical appearance as tulip fingers. Gas chromatography has shown that garlic contains predominantly tuliposide B, which has been found to be much less allergenic than tuliposide A.7,16
Prevention of Tulipa Dermatitis
Tuliposide A and its metabolites can be found in storehouses and trucks used to transport tulips, in clothing, and in any other place where dust containing the allergen has settled. The best prevention against contact dermatitis is to avoid the inciting plants. Gloves may prevent contact dermatitis due to tuliposide A, which penetrates vinyl but not nitrile gloves. Barrier creams have been proposed, but data are scant.1
- Thiboutot DM, Hamory BH, Marks JG Jr. Dermatoses among floral shop workers. J Am Acad Dermatol. 1990;22:54-58. doi: 10.1016/0190-9622(90)70007-5
- Bruze M, Bjorkner B, Hellstrom AC. Occupational dermatoses in nursery workers. Am J Contact Dermat. 1996;7:100-103.
- Hassan I, Rasool F, Akhtar S, et al. Contact dermatitis caused by tulips: identification of contact sensitizers in tulip works of Kashmir Valley in North India. Contact Dermatitis. 2018;78:64-69. doi:10.1111/cod.12870
- Pieroni A, Zahir H, Amin HI, et al. Where tulips and crocuses are popular food snacks: Kurdish traditional foraging reveals traces of mobile pastoralism in Southern Iraqi Kurdistan. J Ethnobiol Ethnomed. 2019;15:59. doi:10.1186/s13002-019-0341-0
- Amin HIM, Ibrahim MF, Hussain FHS, et al. Phytochemistry and ethnopharmacology of some medicine plants used in the Kurdistan region of Iraq. Nat Prod Commun. 2016;11:291-296.
- Crawford GH. Botanical dermatology [Plant identification – other families: Liliaceae]. Medscape. Updated June 10, 2021. Accessed August 18, 2022. https://emedicine.medscape.com/article/1090097-overview#a3
- Gette MT, Marks JE Jr. Tulip fingers. Arch Dermatol. 1990;126:203-205.
- Bruynzeel DP. Bulb dermatitis: dermatological problems in the flower bulb industries. Contact Dermatitis. 1997;37:70-77. doi:10.1111/j.1600-0536.1997.tb00042.x
- Christenhusz MJ, Govaerts RHA, David J, et al. Tiptoe through the tulips—cultural history, molecular phylogenetics and classification of Tulipa (Liliaceae). Bot J Linn Soc. 2013;172:280-328. doi:10.1111/boj.12061
- The Editors of Encyclopaedia Britannica. Tulip. Encyclopedia Britannica. Updated July 4, 2022. Accessed August 18, 2022. https://www.britannica.com/plant/tulip
- Hausen BM. Airborne contact dermatitis caused by tulip bulbs. J Am Acad Dermatol. 1982;7:500-503. doi:10.1016/s0190-9622(82)70132-x
- Nomura T, Ogita S, Kato Y. A novel lactone-forming carboxylesterase: molecular identification of a tuliposide A-converting enzyme in tulip. Plant Physiol. 2012;159:565-578. doi:10.1104/pp.112.195388
- Khalid MM, Greenberg MI. Tulip finger. Clin Toxicol (Phila). 2018; 56:860. doi:10.1080/15563650.2018.1440588
- McCluskey J, Bourgeois M, Harbison R. Tulipalin A induced phytotoxicity. Int J Crit Illn Inj Sci. 2014;4:181-183. doi:10.4103/2229-5151.134187
- Marks JG Jr. Allergic contact dermatitis to Alstroemeria. Arch Dermatol. 1988;124:914-916.
- Sasseville D. Clinical patterns of phytodermatitis. Dermatol Clin. 2009;27:299-308. doi:10.1016/j.det.2009.05.010
Cutaneous Manifestations
Contact dermatitis is a common problem for individuals who work in the floral industry. Hand dermatitis has been reported in as many as 26% of floral employees.1Tulipa species have been identified as one of the most common causes of hand dermatitis. Tulipalin A (α-methylene-γ-butyrolactone) is the main sensitizer in tulips (Figure 1) and its precursor tuliposide A also occurs both in tulips and the Peruvian lily (Alstroemeria).

In a 1996 study, 18% (9/51) of tulip workers were found to be allergic to tulipalin A.2 In a more recent study of 164 tulip workers, 48 (29.3%) had clinical evidence of contact dermatitis and subsequently underwent patch testing; 17 (35.4%) showed a positive reaction to either tulipalin A or to tulip-bulb extract.3 Itching was the most common symptom (39 workers [81.3%]) and hand eczema at the tip of the thumb and index finger was the most common finding. In 9 (18.8%) workers, eczema had spread to other body parts including the forearm, face, legs, and abdomen.3
Peruvian lily is widely used in floral arrangements and has become a leading cause of hand dermatitis in florists (Figure 2). Large amounts of free tulipalin A are present in bulb scales of tulips, along with a small amount of tuliposide A. In young developing shoots, the situation is reversed: Both compounds are found in all parts of the plant to some degree, though tulipalin A is the major allergen, and more mature parts of the plant and bulb are most allergenic.

Cultural Considerations
In traditional Kurdish cuisine, raw herbs are part of snacking or are served as a side dish (sawza). Snacks often are consumed raw on the spot. Tulipa montana, Tulipa armena, and possibly other Tulipa species are consumed as a snack.4 Traditionally, Tulipa systola is consumed by the Kurds as an anti-inflammatory medicine and for pain relief. It also has been proposed that T systola has antioxidant properties.5 Cooked tulip also has been consumed in time of famine in Europe, though none of these dietary practices are recommended.4
Clinical Presentation
“Tulip fingers” describes the most common presentation of contact dermatitis caused by tulip bulbs. Erythematous scaling plaques are seen in the periungual skin and first and second fingertips of the dominant hand. Other manifestations include diffuse dry dermatitis of the hand; paronychia; pulpitis; and secondary spread to the face, neck, arms, and genitalia, with eczematous papules and plaques.6 Clinical signs include erythema, vesicles, hyperkeratosis, and exfoliation of the fingertips. The allergen also can cause airborne contact dermatitis and manifest as conjunctivitis, rhinitis, and asthma.2 A considerable number of tulip workers develop paresthesia and tenderness in the fingertips within several hours after working with tulip bulbs, known as “tulip fire.”7
Plant Facts
There are approximately 250 genera of bulbous plants. Tulips are members of the genus Tulipa and family Liliaceae. Tulips often are thought of as native to southwest central Asia and Turkey8; however, Tulipa sylvestris is native to Portugal, Spain, and North Africa.
Etymology and Symbolism—The word tulip is derived from the Turkish word türbent meaning a turban, possibly because the flower is compared to turbans worn by men of the Ottoman Empire in the 16th century. In Turkish culture, the tulip is a symbol of paradise on earth and can have divine status. In the Netherlands, on the other hand, the tulip represents the briefness of life.
History—By 1562, tulip bulbs had already been introduced to Holland by merchants. However, the first shipment of tulip bulbs was mistaken by the Dutch for onions and were either roasted over a fire or perished when planted in gardens with vegetables. Carolus Clusius—botanist, director of the imperial medical garden in Vienna and recipient of many plants through diplomatic channels—was particularly fond of flower bulbs and contributed to the popularity of the tulip in Europe by sending bulbs and seeds to other European countries.
In the early 17th century, the tulip craze began in France, fueled by a viral disease of tulips that produced variegated color patterns on the petals; entire properties were sold in exchange for a single tulip bulb. The tulip craze drifted from France to Holland in 1634 for 3 years before the tulip market collapsed.
More recently, in 2003 investors started a multimillion-euro tulip fund in the Netherlands to develop new varieties of tulip. Tulip bulbs were used to create money with high percentages over the selling price. With exorbitant pricing and ever-changing ownership of bulbs—bulbs were bought and sold as many as 10 times—the tulip fund collapsed 1 year later and investors lost their money. Bulb speculators then took their profit abroad. In 2006, bulb owners were charged with fraud; the tulip craze often is cited as one of the early major stock market collapses.
Tulips continue to grow in popularity. Today, nearly 6000 cultivars are registered, with 40 new cultivars registered every 5 years.9
Identifying Features
At the base of the erect tulip plant is a cluster of 2 or 3 thick bluish-green leaves. Three petals and 3 sepals make up the solitary bell-shaped flower. Many tulips can propagate only by means of their scaly bulbs. The flowers arise from the tips of stems in different solid colors, except true blue—from pure white to all shades of yellow, red, and a deep purple that is almost black. Solid-color tulips are called “self-colored.” So-called broken tulips are individual flowers with multiple colors, a condition caused by a viral disease transmitted by aphids.10
Tulip Allergen
Tuliposide A is found in many species of the genera Tulipa, Alstroemeria, and Erythronium.6 So far, 7 analogs have been identified: 1-tuliposide A and B; 6-tuliposide A and B; and tuliposides D, E, and F. 6-Tuliposide A and B are the principal tuliposides found in tulip cultivars.11 With trauma and maturation, tuliposides A and B are hydrolyzed to tulipalin A and tulipalin B, respectively.
Tulipalin A and tulipalin B have antimicrobial properties against bacteria and fungi; tulipalin A is mostly an antifungal agent, and tulipalin B has mostly bacteriostatic characteristics.12 The highest concentration of tulipalin A is found in the outer layer of the bulb, followed by (in descending order) the stem, leaves, and petals.13
The prevalence of tulipalin A allergy led the German Federal Institute for Risk Assessment to assign tuliposide A and tulipalin A to category B, which is a “solid-based indication for contact allergenic effects”; both chemicals also are considered skin sensitizers, defined by the Globally Harmonized System of Classification and Labelling of Chemicals of the United Nations as a substance that will induce an allergic response following skin contact.14 Patients who are allergic to tulips have cross-sensitivity to Alstroemeria because tuliposide A and its metabolites are found in both plants.15
Symptoms of an allergic response to tulipalin A can be immediate or delayed.14 The most common allergic contact dermatitis caused by tulip bulbs is type IV hypersensitivity, though type I reactions can occur. Symptoms of a type I reaction including contact urticaria, rhinitis, hoarseness, and dyspnea have been reported.14
The variety of tulip handled also contributes to the severity of dermatitis. Handling bulbs of Rose Copeland variety tulips and cutting the flowers of Preludium tulips have been associated with more severe allergic dermatitis presentations, whereas the Red Emperor tulip was found to have less tuliposide A and thus provoke a weaker patch-test reaction.7
A Word About Garlic—Garlic is in the subfamily Allioideae (formerly Alliaceae) taxonomically related to the tulip family (Liliaceae). Garlic also can cause hand dermatitis in cooks, with a similar clinical appearance as tulip fingers. Gas chromatography has shown that garlic contains predominantly tuliposide B, which has been found to be much less allergenic than tuliposide A.7,16
Prevention of Tulipa Dermatitis
Tuliposide A and its metabolites can be found in storehouses and trucks used to transport tulips, in clothing, and in any other place where dust containing the allergen has settled. The best prevention against contact dermatitis is to avoid the inciting plants. Gloves may prevent contact dermatitis due to tuliposide A, which penetrates vinyl but not nitrile gloves. Barrier creams have been proposed, but data are scant.1
Cutaneous Manifestations
Contact dermatitis is a common problem for individuals who work in the floral industry. Hand dermatitis has been reported in as many as 26% of floral employees.1Tulipa species have been identified as one of the most common causes of hand dermatitis. Tulipalin A (α-methylene-γ-butyrolactone) is the main sensitizer in tulips (Figure 1) and its precursor tuliposide A also occurs both in tulips and the Peruvian lily (Alstroemeria).

In a 1996 study, 18% (9/51) of tulip workers were found to be allergic to tulipalin A.2 In a more recent study of 164 tulip workers, 48 (29.3%) had clinical evidence of contact dermatitis and subsequently underwent patch testing; 17 (35.4%) showed a positive reaction to either tulipalin A or to tulip-bulb extract.3 Itching was the most common symptom (39 workers [81.3%]) and hand eczema at the tip of the thumb and index finger was the most common finding. In 9 (18.8%) workers, eczema had spread to other body parts including the forearm, face, legs, and abdomen.3
Peruvian lily is widely used in floral arrangements and has become a leading cause of hand dermatitis in florists (Figure 2). Large amounts of free tulipalin A are present in bulb scales of tulips, along with a small amount of tuliposide A. In young developing shoots, the situation is reversed: Both compounds are found in all parts of the plant to some degree, though tulipalin A is the major allergen, and more mature parts of the plant and bulb are most allergenic.

Cultural Considerations
In traditional Kurdish cuisine, raw herbs are part of snacking or are served as a side dish (sawza). Snacks often are consumed raw on the spot. Tulipa montana, Tulipa armena, and possibly other Tulipa species are consumed as a snack.4 Traditionally, Tulipa systola is consumed by the Kurds as an anti-inflammatory medicine and for pain relief. It also has been proposed that T systola has antioxidant properties.5 Cooked tulip also has been consumed in time of famine in Europe, though none of these dietary practices are recommended.4
Clinical Presentation
“Tulip fingers” describes the most common presentation of contact dermatitis caused by tulip bulbs. Erythematous scaling plaques are seen in the periungual skin and first and second fingertips of the dominant hand. Other manifestations include diffuse dry dermatitis of the hand; paronychia; pulpitis; and secondary spread to the face, neck, arms, and genitalia, with eczematous papules and plaques.6 Clinical signs include erythema, vesicles, hyperkeratosis, and exfoliation of the fingertips. The allergen also can cause airborne contact dermatitis and manifest as conjunctivitis, rhinitis, and asthma.2 A considerable number of tulip workers develop paresthesia and tenderness in the fingertips within several hours after working with tulip bulbs, known as “tulip fire.”7
Plant Facts
There are approximately 250 genera of bulbous plants. Tulips are members of the genus Tulipa and family Liliaceae. Tulips often are thought of as native to southwest central Asia and Turkey8; however, Tulipa sylvestris is native to Portugal, Spain, and North Africa.
Etymology and Symbolism—The word tulip is derived from the Turkish word türbent meaning a turban, possibly because the flower is compared to turbans worn by men of the Ottoman Empire in the 16th century. In Turkish culture, the tulip is a symbol of paradise on earth and can have divine status. In the Netherlands, on the other hand, the tulip represents the briefness of life.
History—By 1562, tulip bulbs had already been introduced to Holland by merchants. However, the first shipment of tulip bulbs was mistaken by the Dutch for onions and were either roasted over a fire or perished when planted in gardens with vegetables. Carolus Clusius—botanist, director of the imperial medical garden in Vienna and recipient of many plants through diplomatic channels—was particularly fond of flower bulbs and contributed to the popularity of the tulip in Europe by sending bulbs and seeds to other European countries.
In the early 17th century, the tulip craze began in France, fueled by a viral disease of tulips that produced variegated color patterns on the petals; entire properties were sold in exchange for a single tulip bulb. The tulip craze drifted from France to Holland in 1634 for 3 years before the tulip market collapsed.
More recently, in 2003 investors started a multimillion-euro tulip fund in the Netherlands to develop new varieties of tulip. Tulip bulbs were used to create money with high percentages over the selling price. With exorbitant pricing and ever-changing ownership of bulbs—bulbs were bought and sold as many as 10 times—the tulip fund collapsed 1 year later and investors lost their money. Bulb speculators then took their profit abroad. In 2006, bulb owners were charged with fraud; the tulip craze often is cited as one of the early major stock market collapses.
Tulips continue to grow in popularity. Today, nearly 6000 cultivars are registered, with 40 new cultivars registered every 5 years.9
Identifying Features
At the base of the erect tulip plant is a cluster of 2 or 3 thick bluish-green leaves. Three petals and 3 sepals make up the solitary bell-shaped flower. Many tulips can propagate only by means of their scaly bulbs. The flowers arise from the tips of stems in different solid colors, except true blue—from pure white to all shades of yellow, red, and a deep purple that is almost black. Solid-color tulips are called “self-colored.” So-called broken tulips are individual flowers with multiple colors, a condition caused by a viral disease transmitted by aphids.10
Tulip Allergen
Tuliposide A is found in many species of the genera Tulipa, Alstroemeria, and Erythronium.6 So far, 7 analogs have been identified: 1-tuliposide A and B; 6-tuliposide A and B; and tuliposides D, E, and F. 6-Tuliposide A and B are the principal tuliposides found in tulip cultivars.11 With trauma and maturation, tuliposides A and B are hydrolyzed to tulipalin A and tulipalin B, respectively.
Tulipalin A and tulipalin B have antimicrobial properties against bacteria and fungi; tulipalin A is mostly an antifungal agent, and tulipalin B has mostly bacteriostatic characteristics.12 The highest concentration of tulipalin A is found in the outer layer of the bulb, followed by (in descending order) the stem, leaves, and petals.13
The prevalence of tulipalin A allergy led the German Federal Institute for Risk Assessment to assign tuliposide A and tulipalin A to category B, which is a “solid-based indication for contact allergenic effects”; both chemicals also are considered skin sensitizers, defined by the Globally Harmonized System of Classification and Labelling of Chemicals of the United Nations as a substance that will induce an allergic response following skin contact.14 Patients who are allergic to tulips have cross-sensitivity to Alstroemeria because tuliposide A and its metabolites are found in both plants.15
Symptoms of an allergic response to tulipalin A can be immediate or delayed.14 The most common allergic contact dermatitis caused by tulip bulbs is type IV hypersensitivity, though type I reactions can occur. Symptoms of a type I reaction including contact urticaria, rhinitis, hoarseness, and dyspnea have been reported.14
The variety of tulip handled also contributes to the severity of dermatitis. Handling bulbs of Rose Copeland variety tulips and cutting the flowers of Preludium tulips have been associated with more severe allergic dermatitis presentations, whereas the Red Emperor tulip was found to have less tuliposide A and thus provoke a weaker patch-test reaction.7
A Word About Garlic—Garlic is in the subfamily Allioideae (formerly Alliaceae) taxonomically related to the tulip family (Liliaceae). Garlic also can cause hand dermatitis in cooks, with a similar clinical appearance as tulip fingers. Gas chromatography has shown that garlic contains predominantly tuliposide B, which has been found to be much less allergenic than tuliposide A.7,16
Prevention of Tulipa Dermatitis
Tuliposide A and its metabolites can be found in storehouses and trucks used to transport tulips, in clothing, and in any other place where dust containing the allergen has settled. The best prevention against contact dermatitis is to avoid the inciting plants. Gloves may prevent contact dermatitis due to tuliposide A, which penetrates vinyl but not nitrile gloves. Barrier creams have been proposed, but data are scant.1
- Thiboutot DM, Hamory BH, Marks JG Jr. Dermatoses among floral shop workers. J Am Acad Dermatol. 1990;22:54-58. doi: 10.1016/0190-9622(90)70007-5
- Bruze M, Bjorkner B, Hellstrom AC. Occupational dermatoses in nursery workers. Am J Contact Dermat. 1996;7:100-103.
- Hassan I, Rasool F, Akhtar S, et al. Contact dermatitis caused by tulips: identification of contact sensitizers in tulip works of Kashmir Valley in North India. Contact Dermatitis. 2018;78:64-69. doi:10.1111/cod.12870
- Pieroni A, Zahir H, Amin HI, et al. Where tulips and crocuses are popular food snacks: Kurdish traditional foraging reveals traces of mobile pastoralism in Southern Iraqi Kurdistan. J Ethnobiol Ethnomed. 2019;15:59. doi:10.1186/s13002-019-0341-0
- Amin HIM, Ibrahim MF, Hussain FHS, et al. Phytochemistry and ethnopharmacology of some medicine plants used in the Kurdistan region of Iraq. Nat Prod Commun. 2016;11:291-296.
- Crawford GH. Botanical dermatology [Plant identification – other families: Liliaceae]. Medscape. Updated June 10, 2021. Accessed August 18, 2022. https://emedicine.medscape.com/article/1090097-overview#a3
- Gette MT, Marks JE Jr. Tulip fingers. Arch Dermatol. 1990;126:203-205.
- Bruynzeel DP. Bulb dermatitis: dermatological problems in the flower bulb industries. Contact Dermatitis. 1997;37:70-77. doi:10.1111/j.1600-0536.1997.tb00042.x
- Christenhusz MJ, Govaerts RHA, David J, et al. Tiptoe through the tulips—cultural history, molecular phylogenetics and classification of Tulipa (Liliaceae). Bot J Linn Soc. 2013;172:280-328. doi:10.1111/boj.12061
- The Editors of Encyclopaedia Britannica. Tulip. Encyclopedia Britannica. Updated July 4, 2022. Accessed August 18, 2022. https://www.britannica.com/plant/tulip
- Hausen BM. Airborne contact dermatitis caused by tulip bulbs. J Am Acad Dermatol. 1982;7:500-503. doi:10.1016/s0190-9622(82)70132-x
- Nomura T, Ogita S, Kato Y. A novel lactone-forming carboxylesterase: molecular identification of a tuliposide A-converting enzyme in tulip. Plant Physiol. 2012;159:565-578. doi:10.1104/pp.112.195388
- Khalid MM, Greenberg MI. Tulip finger. Clin Toxicol (Phila). 2018; 56:860. doi:10.1080/15563650.2018.1440588
- McCluskey J, Bourgeois M, Harbison R. Tulipalin A induced phytotoxicity. Int J Crit Illn Inj Sci. 2014;4:181-183. doi:10.4103/2229-5151.134187
- Marks JG Jr. Allergic contact dermatitis to Alstroemeria. Arch Dermatol. 1988;124:914-916.
- Sasseville D. Clinical patterns of phytodermatitis. Dermatol Clin. 2009;27:299-308. doi:10.1016/j.det.2009.05.010
- Thiboutot DM, Hamory BH, Marks JG Jr. Dermatoses among floral shop workers. J Am Acad Dermatol. 1990;22:54-58. doi: 10.1016/0190-9622(90)70007-5
- Bruze M, Bjorkner B, Hellstrom AC. Occupational dermatoses in nursery workers. Am J Contact Dermat. 1996;7:100-103.
- Hassan I, Rasool F, Akhtar S, et al. Contact dermatitis caused by tulips: identification of contact sensitizers in tulip works of Kashmir Valley in North India. Contact Dermatitis. 2018;78:64-69. doi:10.1111/cod.12870
- Pieroni A, Zahir H, Amin HI, et al. Where tulips and crocuses are popular food snacks: Kurdish traditional foraging reveals traces of mobile pastoralism in Southern Iraqi Kurdistan. J Ethnobiol Ethnomed. 2019;15:59. doi:10.1186/s13002-019-0341-0
- Amin HIM, Ibrahim MF, Hussain FHS, et al. Phytochemistry and ethnopharmacology of some medicine plants used in the Kurdistan region of Iraq. Nat Prod Commun. 2016;11:291-296.
- Crawford GH. Botanical dermatology [Plant identification – other families: Liliaceae]. Medscape. Updated June 10, 2021. Accessed August 18, 2022. https://emedicine.medscape.com/article/1090097-overview#a3
- Gette MT, Marks JE Jr. Tulip fingers. Arch Dermatol. 1990;126:203-205.
- Bruynzeel DP. Bulb dermatitis: dermatological problems in the flower bulb industries. Contact Dermatitis. 1997;37:70-77. doi:10.1111/j.1600-0536.1997.tb00042.x
- Christenhusz MJ, Govaerts RHA, David J, et al. Tiptoe through the tulips—cultural history, molecular phylogenetics and classification of Tulipa (Liliaceae). Bot J Linn Soc. 2013;172:280-328. doi:10.1111/boj.12061
- The Editors of Encyclopaedia Britannica. Tulip. Encyclopedia Britannica. Updated July 4, 2022. Accessed August 18, 2022. https://www.britannica.com/plant/tulip
- Hausen BM. Airborne contact dermatitis caused by tulip bulbs. J Am Acad Dermatol. 1982;7:500-503. doi:10.1016/s0190-9622(82)70132-x
- Nomura T, Ogita S, Kato Y. A novel lactone-forming carboxylesterase: molecular identification of a tuliposide A-converting enzyme in tulip. Plant Physiol. 2012;159:565-578. doi:10.1104/pp.112.195388
- Khalid MM, Greenberg MI. Tulip finger. Clin Toxicol (Phila). 2018; 56:860. doi:10.1080/15563650.2018.1440588
- McCluskey J, Bourgeois M, Harbison R. Tulipalin A induced phytotoxicity. Int J Crit Illn Inj Sci. 2014;4:181-183. doi:10.4103/2229-5151.134187
- Marks JG Jr. Allergic contact dermatitis to Alstroemeria. Arch Dermatol. 1988;124:914-916.
- Sasseville D. Clinical patterns of phytodermatitis. Dermatol Clin. 2009;27:299-308. doi:10.1016/j.det.2009.05.010
Practice Points
- Tulips are a common cause of contact dermatitis among floral workers.
- Tulipalin A is the primary sensitizer in tulips causing allergic contact dermatitis.
- The best preventative for tulip contact dermatitis is avoiding the inciting plants.
Botanical Briefs: Ginkgo (Ginkgo biloba)
An ancient tree of the Ginkgoaceae family, Ginkgo biloba is known as a living fossil because its genome has been identified in fossils older than 200 million years.1 An individual tree can live longer than 1000 years. Originating in China, G biloba (here, “ginkgo”) is cultivated worldwide for its attractive foliage (Figure 1). Ginkgo extract has long been used in traditional Chinese medicine; however, contact with the plant proper can provoke allergic contact dermatitis.

Dermatitis-Inducing Components
The allergenic component of the ginkgo tree is ginkgolic acid, which is structurally similar to urushiol and anacardic acid.2,3 This compound can cause a cross-reaction in a person previously sensitized by contact with other plants. Urushiol is found in poison ivy(Toxicodendron radicans); anacardic acid is found in the cashew tree (Anacardium occidentale). Both plants belong to the family Anacardiaceae, commonly known as the cashew family.
Members of Anacardiaceae are the most common causes of plant-induced allergic contact dermatitis and include the cashew tree, mango tree, poison ivy, poison oak, and poison sumac. These plants can cross-react to cause contact dermatitis (Table).3 Patch tests have revealed that some individuals who are sensitive to components of the ginkgo tree also demonstrate sensitivity to poison ivy and poison sumac4,5; countering this finding, Lepoittevin and colleagues6 demonstrated in animal studies that there was no cross-reactivity between ginkgo and urushiol, suggesting that patients with a reported cross-reaction might truly have been previously sensitized to both plants. In general, patients who have a history of a reaction to any Anacardiaceae plant should take precautions when handling them.
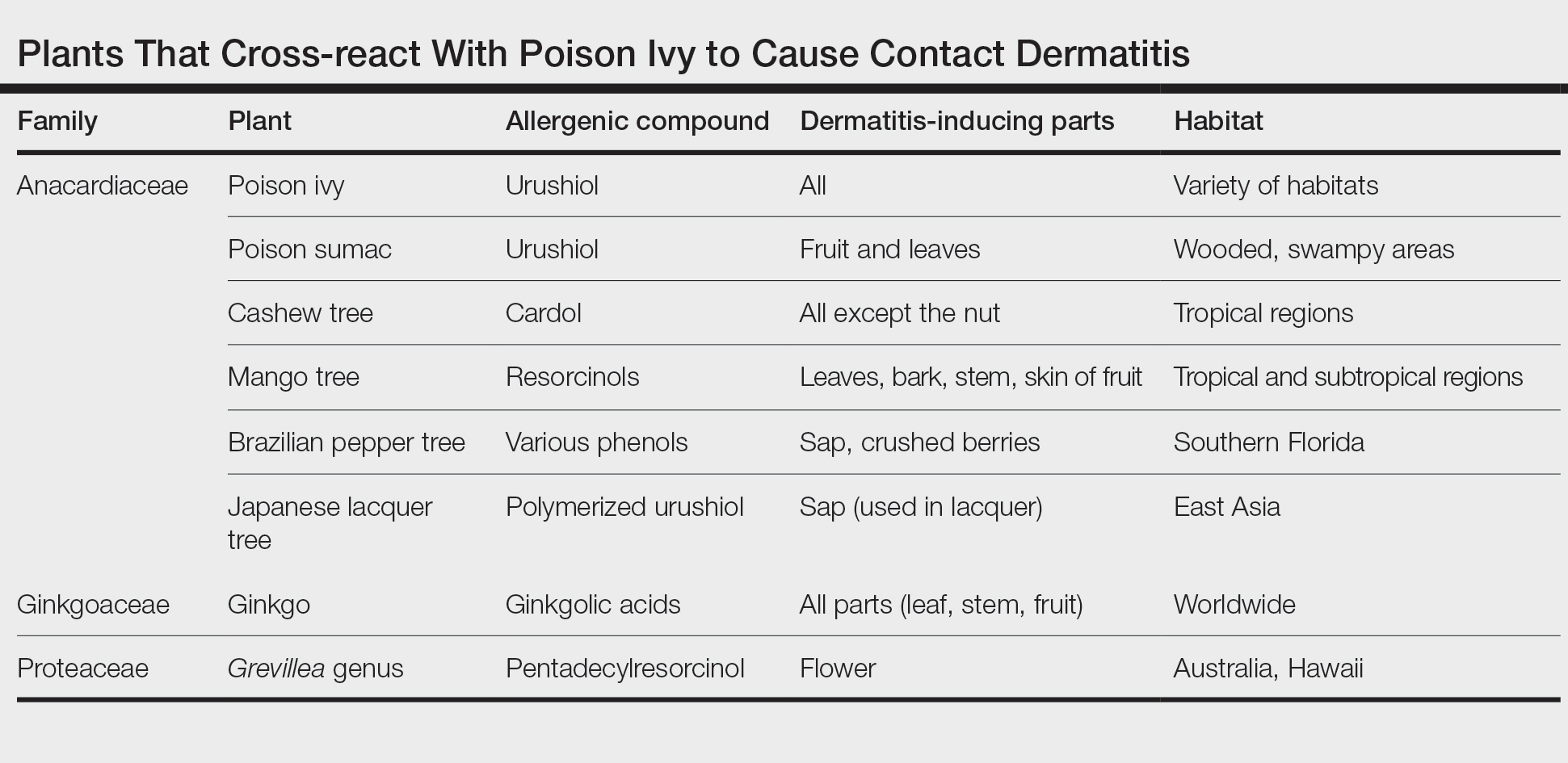
Therapeutic Benefit of Ginkgo
Ginkgo extract is sold as the herbal supplement EGB761, which acts as an antioxidant.7 In France, Germany, and China, it is a commonly prescribed herbal medicine.8 It is purported to support memory and attention; studies have shown improvement in cognition and in involvement with activities of daily living for patients with dementia.9,10 Ginkgo extract might lessen peripheral vascular disease and cerebral circulatory disease, having been shown in vitro and in animal models to prevent platelet aggregation induced by platelet-activating factor and to stimulate vasodilation by increasing production of nitric oxide.11,12
Furthermore, purified ginkgo extract might have beneficial effects on skin. A study in rats showed that when intraperitoneal ginkgo extract was given prior to radiation therapy, 100% of rats receiving placebo developed radiation dermatitis vs 13% of those that received ginkgo extract (P<.0001). An excisional skin biopsy showed a decrease in markers of oxidative stress in rats that received ginkgo extract prior to radiation.7
A randomized, double-blind clinical trial showed a significant reduction in disease progression in vitiligo patients assigned to receive ginkgo extract orally compared to placebo (P=.006).13 Research for many possible uses of ginkgo extract is ongoing.
Cutaneous Manifestations
Contact with the fruit of the ginkgo tree can induce allergic contact dermatitis,14 most often as erythematous papules, vesicles, and in some cases edema.5,15
Exposures While Picking Berries—In 1939, Bolus15 reported the case of a patient who presented with edema, erythema, and vesicular lesions involving the hands and face after picking berries from a ginkgo tree. Later, patch testing on this patient, using ginkgo fruit, resulted in burning and stinging that necessitated removal of the patch, suggesting an irritant reaction. This was followed by a vesicular reaction that then developed within 24 hours, which was more consistent with allergy. Similarly, in 1988, a case series of contact dermatitis was reported in 3 patients after gathering ginkgo fruit.5
Incidental Exposure While Walking—In 1965, dermatitis broke out in 35 high school students, mainly affecting exposed portions of the leg, after ginkgo fruit fell and its pulp was exposed on a path at their school.4 Subsequently, patch testing was performed on 29 volunteers—some who had been exposed to ginkgo on that path, others without prior exposure. It was established that testing with ginkgo pulp directly caused an irritant reaction in all students, regardless of prior ginkgo exposure, but all prior ginkgo-exposed students in this study reacted positively to an acetone extract of ginkgo pulp and either poison ivy extract or pentadecylcatechol.4
Systemic Contact After Eating Fruit—An illustrative case of dermatitis, stomatitis, and proctitis was reported in a man with history of poison oak contact dermatitis who had eaten fruit from a ginkgo tree, suggesting systemic contact dermatitis. Weeks after resolution of symptoms, he reacted positively to ginkgo fruit and poison ivy extracts on patch testing.16
Ginkgo dermatitis tends to resolve upon removal of the inciting agent and application of a topical steroid.8,17 Although many reported cases involve the fruit, allergic contact dermatitis can result from exposure to any part of the plant. In a reported case, a woman developed airborne contact dermatitis from working with sarcotesta of the ginkgo plant.18 Despite wearing rubber gloves, she broke out 1 week after exposure with erythema on the face and arms and severe facial edema.
Ginkgo leaves also can cause allergic contact dermatitis.19 Precautions should be taken when handling any component of the ginkgo tree.
Oral ginkgo supplementation has been implicated in a variety of other cutaneous reactions—from benign to life-threatening. When the ginkgo allergen concentration is too high within the supplement, as has been noted in some formulations, patients have presented with a diffuse morbilliform eruption within 1 or 2 weeks after taking ginkgo.20 One patient—who was not taking any other medication—experienced an episode of acute generalized exanthematous pustulosis 48 hours after taking ginkgo.21 Ingestion of ginkgo extract also has been associated with Stevens-Johnson syndrome.22-24
Other Adverse Reactions
The adverse effects of ginkgo supplement vary widely. In addition to dermatitis, ginkgo supplement can cause headaches, palpitations, tachycardia, vasculitis, nausea, and other symptoms.14
Metabolic Disturbance—One patient taking ginkgo who died after a seizure was found to have subtherapeutic levels of valproate and phenytoin,25 which could be due to ginkgo’s effect on cytochrome p450 enzyme CYP2C19.26 Ginkgo interactions with many cytochrome enzymes have been studied for potential drug interactions. Any other direct effects remain variable and controversial.27,28
Hemorrhage—Another serious effect associated with taking ginkgo supplements is hemorrhage, often in conjunction with warfarin14; however, a meta-analysis indicated that ginkgo generally does not increase the risk of bleeding.29 Other studies have shown that taking ginkgo with warfarin showed no difference in clotting status, and ginkgo with aspirin resulted in no clinically significant difference in bruising, bleeding, or platelet function in an analysis over a period of 1 month.30,31 These findings notwithstanding, pregnant women, surgical patients, and those taking a blood thinner are advised as a general precaution not to take ginkgo extract.
Carcinogenesis—Ginkgo extract has antioxidant properties, but there is evidence that it might act as a carcinogen. An animal study reported by the US National Toxicology Program found that ginkgo induced mutagenic activity in the liver, thyroid, and nose of mice and rats. Over time, rodent liver underwent changes consistent with hepatic enzyme induction.32 More research is needed to clarify the role of ginkgo in this process.
Toxicity by Ingestion—Ginkgo seeds can cause food poisoning due to the compound 4’-O-methylpyridoxine (also known as ginkgotoxin).33 Because methylpyridoxine can cause depletion of pyridoxal phosphate (a form of vitamin B6 necessary for the synthesis of γ-aminobutyric acid), overconsumption of ginkgo seeds, even when fully cooked, might result in convulsions and even death.33
Nomenclature and Distribution of Plants
Gingko biloba belongs to the Ginkgoaceae family (class Ginkgophytes). The tree originated in China but might no longer exist in a truly wild form. It is grown worldwide for its beauty and longevity. The female ginkgo tree is a gymnosperm, producing fruit with seeds that are not coated by an ovary wall15; male (nonfruiting) trees are preferentially planted because the fruit is surrounded by a pulp that, when dropped, emits a sour smell described variously as rancid butter, vomit, or excrement.5
Identifying Features and Plant Facts
The deciduous ginkgo tree has unique fan-shaped leaves and is cultivated for its beauty and resistance to disease (Figure 2).4,34 It is nicknamed the maidenhair tree because the leaves are similar to the pinnae of the maidenhair fern.34 Because G biloba is resistant to pollution, it often is planted along city streets.17 The leaf—5- to 8-cm wide and a symbol of the city of Tokyo, Japan34—grows in clusters (Figure 3)5 and is green but turns yellow before it falls in autumn.34 Leaf veins branch out into the blade without anastomosing.34

Male flowers grow in a catkinlike pattern; female flowers grow on long stems.5 The fruit is small, dark, and shriveled, with a hint of silver4; it typically is 2 to 2.5 cm in diameter and contains the ginkgo nut or seed. The kernel of the ginkgo nut is edible when roasted and is used in traditional Chinese and Japanese cuisine as a dish served on special occasions in autumn.33

Final Thoughts
Given that G biloba is a beautiful, commonly planted ornamental tree, gardeners and landscapers should be aware of the risk for allergic contact dermatitis and use proper protection. Dermatologists should be aware of its cross-reactivity with other common plants such as poison ivy and poison oak to help patients identify the cause of their reactions and avoid the inciting agent. Because ginkgo extract also can cause a cutaneous reaction or interact with other medications, providers should remember to take a thorough medication history that includes herbal medicines and supplements.
- Lyu J. Ginkgo history told by genomes. Nat Plants. 2019;5:1029. doi:10.1038/s41477-019-0529-2
- ElSohly MA, Adawadkar PD, Benigni DA, et al. Analogues of poison ivy urushiol. Synthesis and biological activity of disubstituted n-alkylbenzenes. J Med Chem. 1986;29:606-611. doi:10.1021/jm00155a003
- He X, Bernart MW, Nolan GS, et al. High-performance liquid chromatography–electrospray ionization-mass spectrometry study of ginkgolic acid in the leaves and fruits of the ginkgo tree (Ginkgo biloba). J Chromatogr Sci. 2000;38:169-173. doi:10.1093/chromsci/38.4.169
- Sowers WF, Weary PE, Collins OD, et al. Ginkgo-tree dermatitis. Arch Dermatol. 1965;91:452-456. doi:10.1001/archderm.1965.01600110038009
- Tomb RR, Foussereau J, Sell Y. Mini-epidemic of contact dermatitis from ginkgo tree fruit (Ginkgo biloba L.). Contact Dermatitis. 1988;19:281-283. doi:10.1111/j.1600-0536.1988.tb02928.x
- Lepoittevin J-P, Benezra C, Asakawa Y. Allergic contact dermatitis to Ginkgo biloba L.: relationship with urushiol. Arch Dermatol Res. 1989;281:227-230. doi:10.1007/BF00431055
- Yirmibesoglu E, Karahacioglu E, Kilic D, et al. The protective effects of Ginkgo biloba extract (EGb-761) on radiation-induced dermatitis: an experimental study. Clin Exp Dermatol. 2012;37:387-394. doi:10.1111/j.1365-2230.2011.04253.x
- Jiang L, Su L, Cui H, et al. Ginkgo biloba extract for dementia: a systematic review. Shanghai Arch Psychiatry. 2013;25:10-21. doi:10.3969/j.issn.1002-0829.2013.01.005
- Oken BS, Storzbach DM, Kaye JA. The efficacy of Ginkgo biloba on cognitive function in Alzheimer disease. Arch Neurol. 1998;55:1409-1415. doi:10.1001/archneur.55.11.1409
- Le Bars PL, Katz MM, Berman N, et al. A placebo-controlled, double-blind, randomized trial of an extract of Ginkgo biloba for dementia. North American EGb Study Group. JAMA. 1997;278:1327-1332. doi:10.1001/jama.278.16.1327
- Koltermann A, Hartkorn A, Koch E, et al. Ginkgo biloba extract EGb 761 increases endothelial nitric oxide production in vitro and in vivo. Cell Mol Life Sci. 2007;64:1715-1722. doi:10.1007/s00018-007-7085-z
- Touvay C, Vilain B, Taylor JE, et al. Proof of the involvement of platelet activating factor (paf-acether) in pulmonary complex immune systems using a specific paf-acether receptor antagonist: BN 52021. Prog Lipid Res. 1986;25:277-288. doi:10.1016/0163-7827(86)90057-3
- Parsad D, Pandhi R, Juneja A. Effectiveness of oral Ginkgo biloba in treating limited, slowly spreading vitiligo. Clin Exp Dermatol. 2003;28:285-287. doi:10.1046/j.1365-2230.2003.01207.x
- Jacobsson I, Jönsson AK, Gerdén B, et al. Spontaneously reported adverse reactions in association with complementary and alternative medicine substances in Sweden. Pharmacoepidemiol Drug Saf. 2009;18:1039-1047. doi:10.1002/pds.1818
- Bolus M. Dermatitis venenata due to Ginkgo berries. Arch Derm Syphilol. 1939;39:530.
- Becker LE, Skipworth GB. Ginkgo-tree dermatitis, stomatitis, and proctitis. JAMA. 1975;231:1162-1163.
- Nakamura T. Ginkgo tree dermatitis. Contact Dermatitis. 1985;12:281-282. doi:10.1111/j.1600-0536.1985.tb01138.x
- Jiang J, Ding Y, Qian G. Airborne contact dermatitis caused by the sarcotesta of Ginkgo biloba. Contact Dermatitis. 2016;75:384-385. doi:10.1111/cod.12646
- Hotta E, Tamagawa-Mineoka R, Katoh N. Allergic contact dermatitis due to ginkgo tree fruit and leaf. Eur J Dermatol. 2013;23:548-549. doi:10.1684/ejd.2013.2102
- Chiu AE, Lane AT, Kimball AB. Diffuse morbilliform eruption after consumption of Ginkgo biloba supplement. J Am Acad Dermatol. 2002;46:145-146. doi:10.1067/mjd.2001.118545
- Pennisi RS. Acute generalised exanthematous pustulosis induced by the herbal remedy Ginkgo biloba. Med J Aust. 2006;184:583-584. doi:10.5694/j.1326-5377.2006.tb00386.x
- Yuste M, Sánchez-Estella J, Santos JC, et al. Stevens-Johnson syndrome/toxic epidermal necrolysis treated with intravenous immunoglobulins. Actas Dermosifiliogr. 2005;96:589-592. doi:10.1016/s0001-7310(05)73141-0
- Jeyamani VP, Sabishruthi S, Kavitha S, et al. An illustrative case study on drug induced Steven-Johnson syndrome by Ginkgo biloba. J Clin Res. 2018;2:1-3.
- Davydov L, Stirling AL. Stevens-Johnson syndrome with Ginkgo biloba. J Herbal Pharmacother. 2001;1:65-69. doi:10.1080/J157v01n03_06
- Yin OQP, Tomlinson B, Waye MMY, et al. Pharmacogenetics and herb–drug interactions: experience with Ginkgo biloba and omeprazole. Pharmacogenetics. 2004;14:841-850. doi:10.1097/00008571-200412000-00007
- Kupiec T, Raj V. Fatal seizures due to potential herb–drug interactions with Ginkgo biloba. J Anal Toxicol. 2005;29:755-758. doi:10.1093/jat/29.7.755
- Zadoyan G, Rokitta D, Klement S, et al. Effect of Ginkgo biloba special extract EGb 761® on human cytochrome P450 activity: a cocktail interaction study in healthy volunteers. Eur J Clin Pharmacol. 2012;68:553-560. doi:10.1007/s00228-011-1174-5
- Zhou S-F, Deng Y, Bi H-c, et al. Induction of cytochrome P450 3A by the Ginkgo biloba extract and bilobalides in human and rat primary hepatocytes. Drug Metab Lett. 2008;2:60-66. doi:10.2174/187231208783478489
- Kellermann AJ, Kloft C. Is there a risk of bleeding associated with standardized Ginkgo biloba extract therapy? a systematic review and meta-analysis. Pharmacotherapy. 2011;31:490-502. doi:10.1592/phco.31.5.490
- Gardner CD, Zehnder JL, Rigby AJ, et al. Effect of Ginkgo biloba (EGb 761) and aspirin on platelet aggregation and platelet function analysis among older adults at risk of cardiovascular disease: a randomized clinical trial. Blood Coagul Fibrinolysis. 2007;18:787-79. doi:10.1097/MBC.0b013e3282f102b1
- Jiang X, Williams KM, Liauw WS, et al. Effect of ginkgo and ginger on the pharmacokinetics and pharmacodynamics of warfarin in healthy subjects. Br J Clin Pharmacol. 2005;59:425-432. doi:10.1111/j.1365-2125.2005.02322.x
- Toxicology and carcinogenesis studies of Ginkgo biloba extract (CAS No. 90045-36-6) in F344/N rats and B6C3F1/N mice (gavage studies). Natl Toxicol Program Tech Rep Ser. 2013:1-183.
- Azuma F, Nokura K, Kako T, et al. An adult case of generalized convulsions caused by the ingestion of Ginkgo biloba seeds with alcohol. Intern Med. 2020;59:1555-1558. doi:10.2169/internalmedicine.4196-19
- Cohen PR. Fixed drug eruption to supplement containing Ginkgo biloba and vinpocetine: a case report and review of related cutaneous side effects. J Clin Aesthet Dermatol. 2017;10:44-47.
An ancient tree of the Ginkgoaceae family, Ginkgo biloba is known as a living fossil because its genome has been identified in fossils older than 200 million years.1 An individual tree can live longer than 1000 years. Originating in China, G biloba (here, “ginkgo”) is cultivated worldwide for its attractive foliage (Figure 1). Ginkgo extract has long been used in traditional Chinese medicine; however, contact with the plant proper can provoke allergic contact dermatitis.

Dermatitis-Inducing Components
The allergenic component of the ginkgo tree is ginkgolic acid, which is structurally similar to urushiol and anacardic acid.2,3 This compound can cause a cross-reaction in a person previously sensitized by contact with other plants. Urushiol is found in poison ivy(Toxicodendron radicans); anacardic acid is found in the cashew tree (Anacardium occidentale). Both plants belong to the family Anacardiaceae, commonly known as the cashew family.
Members of Anacardiaceae are the most common causes of plant-induced allergic contact dermatitis and include the cashew tree, mango tree, poison ivy, poison oak, and poison sumac. These plants can cross-react to cause contact dermatitis (Table).3 Patch tests have revealed that some individuals who are sensitive to components of the ginkgo tree also demonstrate sensitivity to poison ivy and poison sumac4,5; countering this finding, Lepoittevin and colleagues6 demonstrated in animal studies that there was no cross-reactivity between ginkgo and urushiol, suggesting that patients with a reported cross-reaction might truly have been previously sensitized to both plants. In general, patients who have a history of a reaction to any Anacardiaceae plant should take precautions when handling them.

Therapeutic Benefit of Ginkgo
Ginkgo extract is sold as the herbal supplement EGB761, which acts as an antioxidant.7 In France, Germany, and China, it is a commonly prescribed herbal medicine.8 It is purported to support memory and attention; studies have shown improvement in cognition and in involvement with activities of daily living for patients with dementia.9,10 Ginkgo extract might lessen peripheral vascular disease and cerebral circulatory disease, having been shown in vitro and in animal models to prevent platelet aggregation induced by platelet-activating factor and to stimulate vasodilation by increasing production of nitric oxide.11,12
Furthermore, purified ginkgo extract might have beneficial effects on skin. A study in rats showed that when intraperitoneal ginkgo extract was given prior to radiation therapy, 100% of rats receiving placebo developed radiation dermatitis vs 13% of those that received ginkgo extract (P<.0001). An excisional skin biopsy showed a decrease in markers of oxidative stress in rats that received ginkgo extract prior to radiation.7
A randomized, double-blind clinical trial showed a significant reduction in disease progression in vitiligo patients assigned to receive ginkgo extract orally compared to placebo (P=.006).13 Research for many possible uses of ginkgo extract is ongoing.
Cutaneous Manifestations
Contact with the fruit of the ginkgo tree can induce allergic contact dermatitis,14 most often as erythematous papules, vesicles, and in some cases edema.5,15
Exposures While Picking Berries—In 1939, Bolus15 reported the case of a patient who presented with edema, erythema, and vesicular lesions involving the hands and face after picking berries from a ginkgo tree. Later, patch testing on this patient, using ginkgo fruit, resulted in burning and stinging that necessitated removal of the patch, suggesting an irritant reaction. This was followed by a vesicular reaction that then developed within 24 hours, which was more consistent with allergy. Similarly, in 1988, a case series of contact dermatitis was reported in 3 patients after gathering ginkgo fruit.5
Incidental Exposure While Walking—In 1965, dermatitis broke out in 35 high school students, mainly affecting exposed portions of the leg, after ginkgo fruit fell and its pulp was exposed on a path at their school.4 Subsequently, patch testing was performed on 29 volunteers—some who had been exposed to ginkgo on that path, others without prior exposure. It was established that testing with ginkgo pulp directly caused an irritant reaction in all students, regardless of prior ginkgo exposure, but all prior ginkgo-exposed students in this study reacted positively to an acetone extract of ginkgo pulp and either poison ivy extract or pentadecylcatechol.4
Systemic Contact After Eating Fruit—An illustrative case of dermatitis, stomatitis, and proctitis was reported in a man with history of poison oak contact dermatitis who had eaten fruit from a ginkgo tree, suggesting systemic contact dermatitis. Weeks after resolution of symptoms, he reacted positively to ginkgo fruit and poison ivy extracts on patch testing.16
Ginkgo dermatitis tends to resolve upon removal of the inciting agent and application of a topical steroid.8,17 Although many reported cases involve the fruit, allergic contact dermatitis can result from exposure to any part of the plant. In a reported case, a woman developed airborne contact dermatitis from working with sarcotesta of the ginkgo plant.18 Despite wearing rubber gloves, she broke out 1 week after exposure with erythema on the face and arms and severe facial edema.
Ginkgo leaves also can cause allergic contact dermatitis.19 Precautions should be taken when handling any component of the ginkgo tree.
Oral ginkgo supplementation has been implicated in a variety of other cutaneous reactions—from benign to life-threatening. When the ginkgo allergen concentration is too high within the supplement, as has been noted in some formulations, patients have presented with a diffuse morbilliform eruption within 1 or 2 weeks after taking ginkgo.20 One patient—who was not taking any other medication—experienced an episode of acute generalized exanthematous pustulosis 48 hours after taking ginkgo.21 Ingestion of ginkgo extract also has been associated with Stevens-Johnson syndrome.22-24
Other Adverse Reactions
The adverse effects of ginkgo supplement vary widely. In addition to dermatitis, ginkgo supplement can cause headaches, palpitations, tachycardia, vasculitis, nausea, and other symptoms.14
Metabolic Disturbance—One patient taking ginkgo who died after a seizure was found to have subtherapeutic levels of valproate and phenytoin,25 which could be due to ginkgo’s effect on cytochrome p450 enzyme CYP2C19.26 Ginkgo interactions with many cytochrome enzymes have been studied for potential drug interactions. Any other direct effects remain variable and controversial.27,28
Hemorrhage—Another serious effect associated with taking ginkgo supplements is hemorrhage, often in conjunction with warfarin14; however, a meta-analysis indicated that ginkgo generally does not increase the risk of bleeding.29 Other studies have shown that taking ginkgo with warfarin showed no difference in clotting status, and ginkgo with aspirin resulted in no clinically significant difference in bruising, bleeding, or platelet function in an analysis over a period of 1 month.30,31 These findings notwithstanding, pregnant women, surgical patients, and those taking a blood thinner are advised as a general precaution not to take ginkgo extract.
Carcinogenesis—Ginkgo extract has antioxidant properties, but there is evidence that it might act as a carcinogen. An animal study reported by the US National Toxicology Program found that ginkgo induced mutagenic activity in the liver, thyroid, and nose of mice and rats. Over time, rodent liver underwent changes consistent with hepatic enzyme induction.32 More research is needed to clarify the role of ginkgo in this process.
Toxicity by Ingestion—Ginkgo seeds can cause food poisoning due to the compound 4’-O-methylpyridoxine (also known as ginkgotoxin).33 Because methylpyridoxine can cause depletion of pyridoxal phosphate (a form of vitamin B6 necessary for the synthesis of γ-aminobutyric acid), overconsumption of ginkgo seeds, even when fully cooked, might result in convulsions and even death.33
Nomenclature and Distribution of Plants
Gingko biloba belongs to the Ginkgoaceae family (class Ginkgophytes). The tree originated in China but might no longer exist in a truly wild form. It is grown worldwide for its beauty and longevity. The female ginkgo tree is a gymnosperm, producing fruit with seeds that are not coated by an ovary wall15; male (nonfruiting) trees are preferentially planted because the fruit is surrounded by a pulp that, when dropped, emits a sour smell described variously as rancid butter, vomit, or excrement.5
Identifying Features and Plant Facts
The deciduous ginkgo tree has unique fan-shaped leaves and is cultivated for its beauty and resistance to disease (Figure 2).4,34 It is nicknamed the maidenhair tree because the leaves are similar to the pinnae of the maidenhair fern.34 Because G biloba is resistant to pollution, it often is planted along city streets.17 The leaf—5- to 8-cm wide and a symbol of the city of Tokyo, Japan34—grows in clusters (Figure 3)5 and is green but turns yellow before it falls in autumn.34 Leaf veins branch out into the blade without anastomosing.34

Male flowers grow in a catkinlike pattern; female flowers grow on long stems.5 The fruit is small, dark, and shriveled, with a hint of silver4; it typically is 2 to 2.5 cm in diameter and contains the ginkgo nut or seed. The kernel of the ginkgo nut is edible when roasted and is used in traditional Chinese and Japanese cuisine as a dish served on special occasions in autumn.33

Final Thoughts
Given that G biloba is a beautiful, commonly planted ornamental tree, gardeners and landscapers should be aware of the risk for allergic contact dermatitis and use proper protection. Dermatologists should be aware of its cross-reactivity with other common plants such as poison ivy and poison oak to help patients identify the cause of their reactions and avoid the inciting agent. Because ginkgo extract also can cause a cutaneous reaction or interact with other medications, providers should remember to take a thorough medication history that includes herbal medicines and supplements.
An ancient tree of the Ginkgoaceae family, Ginkgo biloba is known as a living fossil because its genome has been identified in fossils older than 200 million years.1 An individual tree can live longer than 1000 years. Originating in China, G biloba (here, “ginkgo”) is cultivated worldwide for its attractive foliage (Figure 1). Ginkgo extract has long been used in traditional Chinese medicine; however, contact with the plant proper can provoke allergic contact dermatitis.

Dermatitis-Inducing Components
The allergenic component of the ginkgo tree is ginkgolic acid, which is structurally similar to urushiol and anacardic acid.2,3 This compound can cause a cross-reaction in a person previously sensitized by contact with other plants. Urushiol is found in poison ivy(Toxicodendron radicans); anacardic acid is found in the cashew tree (Anacardium occidentale). Both plants belong to the family Anacardiaceae, commonly known as the cashew family.
Members of Anacardiaceae are the most common causes of plant-induced allergic contact dermatitis and include the cashew tree, mango tree, poison ivy, poison oak, and poison sumac. These plants can cross-react to cause contact dermatitis (Table).3 Patch tests have revealed that some individuals who are sensitive to components of the ginkgo tree also demonstrate sensitivity to poison ivy and poison sumac4,5; countering this finding, Lepoittevin and colleagues6 demonstrated in animal studies that there was no cross-reactivity between ginkgo and urushiol, suggesting that patients with a reported cross-reaction might truly have been previously sensitized to both plants. In general, patients who have a history of a reaction to any Anacardiaceae plant should take precautions when handling them.

Therapeutic Benefit of Ginkgo
Ginkgo extract is sold as the herbal supplement EGB761, which acts as an antioxidant.7 In France, Germany, and China, it is a commonly prescribed herbal medicine.8 It is purported to support memory and attention; studies have shown improvement in cognition and in involvement with activities of daily living for patients with dementia.9,10 Ginkgo extract might lessen peripheral vascular disease and cerebral circulatory disease, having been shown in vitro and in animal models to prevent platelet aggregation induced by platelet-activating factor and to stimulate vasodilation by increasing production of nitric oxide.11,12
Furthermore, purified ginkgo extract might have beneficial effects on skin. A study in rats showed that when intraperitoneal ginkgo extract was given prior to radiation therapy, 100% of rats receiving placebo developed radiation dermatitis vs 13% of those that received ginkgo extract (P<.0001). An excisional skin biopsy showed a decrease in markers of oxidative stress in rats that received ginkgo extract prior to radiation.7
A randomized, double-blind clinical trial showed a significant reduction in disease progression in vitiligo patients assigned to receive ginkgo extract orally compared to placebo (P=.006).13 Research for many possible uses of ginkgo extract is ongoing.
Cutaneous Manifestations
Contact with the fruit of the ginkgo tree can induce allergic contact dermatitis,14 most often as erythematous papules, vesicles, and in some cases edema.5,15
Exposures While Picking Berries—In 1939, Bolus15 reported the case of a patient who presented with edema, erythema, and vesicular lesions involving the hands and face after picking berries from a ginkgo tree. Later, patch testing on this patient, using ginkgo fruit, resulted in burning and stinging that necessitated removal of the patch, suggesting an irritant reaction. This was followed by a vesicular reaction that then developed within 24 hours, which was more consistent with allergy. Similarly, in 1988, a case series of contact dermatitis was reported in 3 patients after gathering ginkgo fruit.5
Incidental Exposure While Walking—In 1965, dermatitis broke out in 35 high school students, mainly affecting exposed portions of the leg, after ginkgo fruit fell and its pulp was exposed on a path at their school.4 Subsequently, patch testing was performed on 29 volunteers—some who had been exposed to ginkgo on that path, others without prior exposure. It was established that testing with ginkgo pulp directly caused an irritant reaction in all students, regardless of prior ginkgo exposure, but all prior ginkgo-exposed students in this study reacted positively to an acetone extract of ginkgo pulp and either poison ivy extract or pentadecylcatechol.4
Systemic Contact After Eating Fruit—An illustrative case of dermatitis, stomatitis, and proctitis was reported in a man with history of poison oak contact dermatitis who had eaten fruit from a ginkgo tree, suggesting systemic contact dermatitis. Weeks after resolution of symptoms, he reacted positively to ginkgo fruit and poison ivy extracts on patch testing.16
Ginkgo dermatitis tends to resolve upon removal of the inciting agent and application of a topical steroid.8,17 Although many reported cases involve the fruit, allergic contact dermatitis can result from exposure to any part of the plant. In a reported case, a woman developed airborne contact dermatitis from working with sarcotesta of the ginkgo plant.18 Despite wearing rubber gloves, she broke out 1 week after exposure with erythema on the face and arms and severe facial edema.
Ginkgo leaves also can cause allergic contact dermatitis.19 Precautions should be taken when handling any component of the ginkgo tree.
Oral ginkgo supplementation has been implicated in a variety of other cutaneous reactions—from benign to life-threatening. When the ginkgo allergen concentration is too high within the supplement, as has been noted in some formulations, patients have presented with a diffuse morbilliform eruption within 1 or 2 weeks after taking ginkgo.20 One patient—who was not taking any other medication—experienced an episode of acute generalized exanthematous pustulosis 48 hours after taking ginkgo.21 Ingestion of ginkgo extract also has been associated with Stevens-Johnson syndrome.22-24
Other Adverse Reactions
The adverse effects of ginkgo supplement vary widely. In addition to dermatitis, ginkgo supplement can cause headaches, palpitations, tachycardia, vasculitis, nausea, and other symptoms.14
Metabolic Disturbance—One patient taking ginkgo who died after a seizure was found to have subtherapeutic levels of valproate and phenytoin,25 which could be due to ginkgo’s effect on cytochrome p450 enzyme CYP2C19.26 Ginkgo interactions with many cytochrome enzymes have been studied for potential drug interactions. Any other direct effects remain variable and controversial.27,28
Hemorrhage—Another serious effect associated with taking ginkgo supplements is hemorrhage, often in conjunction with warfarin14; however, a meta-analysis indicated that ginkgo generally does not increase the risk of bleeding.29 Other studies have shown that taking ginkgo with warfarin showed no difference in clotting status, and ginkgo with aspirin resulted in no clinically significant difference in bruising, bleeding, or platelet function in an analysis over a period of 1 month.30,31 These findings notwithstanding, pregnant women, surgical patients, and those taking a blood thinner are advised as a general precaution not to take ginkgo extract.
Carcinogenesis—Ginkgo extract has antioxidant properties, but there is evidence that it might act as a carcinogen. An animal study reported by the US National Toxicology Program found that ginkgo induced mutagenic activity in the liver, thyroid, and nose of mice and rats. Over time, rodent liver underwent changes consistent with hepatic enzyme induction.32 More research is needed to clarify the role of ginkgo in this process.
Toxicity by Ingestion—Ginkgo seeds can cause food poisoning due to the compound 4’-O-methylpyridoxine (also known as ginkgotoxin).33 Because methylpyridoxine can cause depletion of pyridoxal phosphate (a form of vitamin B6 necessary for the synthesis of γ-aminobutyric acid), overconsumption of ginkgo seeds, even when fully cooked, might result in convulsions and even death.33
Nomenclature and Distribution of Plants
Gingko biloba belongs to the Ginkgoaceae family (class Ginkgophytes). The tree originated in China but might no longer exist in a truly wild form. It is grown worldwide for its beauty and longevity. The female ginkgo tree is a gymnosperm, producing fruit with seeds that are not coated by an ovary wall15; male (nonfruiting) trees are preferentially planted because the fruit is surrounded by a pulp that, when dropped, emits a sour smell described variously as rancid butter, vomit, or excrement.5
Identifying Features and Plant Facts
The deciduous ginkgo tree has unique fan-shaped leaves and is cultivated for its beauty and resistance to disease (Figure 2).4,34 It is nicknamed the maidenhair tree because the leaves are similar to the pinnae of the maidenhair fern.34 Because G biloba is resistant to pollution, it often is planted along city streets.17 The leaf—5- to 8-cm wide and a symbol of the city of Tokyo, Japan34—grows in clusters (Figure 3)5 and is green but turns yellow before it falls in autumn.34 Leaf veins branch out into the blade without anastomosing.34

Male flowers grow in a catkinlike pattern; female flowers grow on long stems.5 The fruit is small, dark, and shriveled, with a hint of silver4; it typically is 2 to 2.5 cm in diameter and contains the ginkgo nut or seed. The kernel of the ginkgo nut is edible when roasted and is used in traditional Chinese and Japanese cuisine as a dish served on special occasions in autumn.33

Final Thoughts
Given that G biloba is a beautiful, commonly planted ornamental tree, gardeners and landscapers should be aware of the risk for allergic contact dermatitis and use proper protection. Dermatologists should be aware of its cross-reactivity with other common plants such as poison ivy and poison oak to help patients identify the cause of their reactions and avoid the inciting agent. Because ginkgo extract also can cause a cutaneous reaction or interact with other medications, providers should remember to take a thorough medication history that includes herbal medicines and supplements.
- Lyu J. Ginkgo history told by genomes. Nat Plants. 2019;5:1029. doi:10.1038/s41477-019-0529-2
- ElSohly MA, Adawadkar PD, Benigni DA, et al. Analogues of poison ivy urushiol. Synthesis and biological activity of disubstituted n-alkylbenzenes. J Med Chem. 1986;29:606-611. doi:10.1021/jm00155a003
- He X, Bernart MW, Nolan GS, et al. High-performance liquid chromatography–electrospray ionization-mass spectrometry study of ginkgolic acid in the leaves and fruits of the ginkgo tree (Ginkgo biloba). J Chromatogr Sci. 2000;38:169-173. doi:10.1093/chromsci/38.4.169
- Sowers WF, Weary PE, Collins OD, et al. Ginkgo-tree dermatitis. Arch Dermatol. 1965;91:452-456. doi:10.1001/archderm.1965.01600110038009
- Tomb RR, Foussereau J, Sell Y. Mini-epidemic of contact dermatitis from ginkgo tree fruit (Ginkgo biloba L.). Contact Dermatitis. 1988;19:281-283. doi:10.1111/j.1600-0536.1988.tb02928.x
- Lepoittevin J-P, Benezra C, Asakawa Y. Allergic contact dermatitis to Ginkgo biloba L.: relationship with urushiol. Arch Dermatol Res. 1989;281:227-230. doi:10.1007/BF00431055
- Yirmibesoglu E, Karahacioglu E, Kilic D, et al. The protective effects of Ginkgo biloba extract (EGb-761) on radiation-induced dermatitis: an experimental study. Clin Exp Dermatol. 2012;37:387-394. doi:10.1111/j.1365-2230.2011.04253.x
- Jiang L, Su L, Cui H, et al. Ginkgo biloba extract for dementia: a systematic review. Shanghai Arch Psychiatry. 2013;25:10-21. doi:10.3969/j.issn.1002-0829.2013.01.005
- Oken BS, Storzbach DM, Kaye JA. The efficacy of Ginkgo biloba on cognitive function in Alzheimer disease. Arch Neurol. 1998;55:1409-1415. doi:10.1001/archneur.55.11.1409
- Le Bars PL, Katz MM, Berman N, et al. A placebo-controlled, double-blind, randomized trial of an extract of Ginkgo biloba for dementia. North American EGb Study Group. JAMA. 1997;278:1327-1332. doi:10.1001/jama.278.16.1327
- Koltermann A, Hartkorn A, Koch E, et al. Ginkgo biloba extract EGb 761 increases endothelial nitric oxide production in vitro and in vivo. Cell Mol Life Sci. 2007;64:1715-1722. doi:10.1007/s00018-007-7085-z
- Touvay C, Vilain B, Taylor JE, et al. Proof of the involvement of platelet activating factor (paf-acether) in pulmonary complex immune systems using a specific paf-acether receptor antagonist: BN 52021. Prog Lipid Res. 1986;25:277-288. doi:10.1016/0163-7827(86)90057-3
- Parsad D, Pandhi R, Juneja A. Effectiveness of oral Ginkgo biloba in treating limited, slowly spreading vitiligo. Clin Exp Dermatol. 2003;28:285-287. doi:10.1046/j.1365-2230.2003.01207.x
- Jacobsson I, Jönsson AK, Gerdén B, et al. Spontaneously reported adverse reactions in association with complementary and alternative medicine substances in Sweden. Pharmacoepidemiol Drug Saf. 2009;18:1039-1047. doi:10.1002/pds.1818
- Bolus M. Dermatitis venenata due to Ginkgo berries. Arch Derm Syphilol. 1939;39:530.
- Becker LE, Skipworth GB. Ginkgo-tree dermatitis, stomatitis, and proctitis. JAMA. 1975;231:1162-1163.
- Nakamura T. Ginkgo tree dermatitis. Contact Dermatitis. 1985;12:281-282. doi:10.1111/j.1600-0536.1985.tb01138.x
- Jiang J, Ding Y, Qian G. Airborne contact dermatitis caused by the sarcotesta of Ginkgo biloba. Contact Dermatitis. 2016;75:384-385. doi:10.1111/cod.12646
- Hotta E, Tamagawa-Mineoka R, Katoh N. Allergic contact dermatitis due to ginkgo tree fruit and leaf. Eur J Dermatol. 2013;23:548-549. doi:10.1684/ejd.2013.2102
- Chiu AE, Lane AT, Kimball AB. Diffuse morbilliform eruption after consumption of Ginkgo biloba supplement. J Am Acad Dermatol. 2002;46:145-146. doi:10.1067/mjd.2001.118545
- Pennisi RS. Acute generalised exanthematous pustulosis induced by the herbal remedy Ginkgo biloba. Med J Aust. 2006;184:583-584. doi:10.5694/j.1326-5377.2006.tb00386.x
- Yuste M, Sánchez-Estella J, Santos JC, et al. Stevens-Johnson syndrome/toxic epidermal necrolysis treated with intravenous immunoglobulins. Actas Dermosifiliogr. 2005;96:589-592. doi:10.1016/s0001-7310(05)73141-0
- Jeyamani VP, Sabishruthi S, Kavitha S, et al. An illustrative case study on drug induced Steven-Johnson syndrome by Ginkgo biloba. J Clin Res. 2018;2:1-3.
- Davydov L, Stirling AL. Stevens-Johnson syndrome with Ginkgo biloba. J Herbal Pharmacother. 2001;1:65-69. doi:10.1080/J157v01n03_06
- Yin OQP, Tomlinson B, Waye MMY, et al. Pharmacogenetics and herb–drug interactions: experience with Ginkgo biloba and omeprazole. Pharmacogenetics. 2004;14:841-850. doi:10.1097/00008571-200412000-00007
- Kupiec T, Raj V. Fatal seizures due to potential herb–drug interactions with Ginkgo biloba. J Anal Toxicol. 2005;29:755-758. doi:10.1093/jat/29.7.755
- Zadoyan G, Rokitta D, Klement S, et al. Effect of Ginkgo biloba special extract EGb 761® on human cytochrome P450 activity: a cocktail interaction study in healthy volunteers. Eur J Clin Pharmacol. 2012;68:553-560. doi:10.1007/s00228-011-1174-5
- Zhou S-F, Deng Y, Bi H-c, et al. Induction of cytochrome P450 3A by the Ginkgo biloba extract and bilobalides in human and rat primary hepatocytes. Drug Metab Lett. 2008;2:60-66. doi:10.2174/187231208783478489
- Kellermann AJ, Kloft C. Is there a risk of bleeding associated with standardized Ginkgo biloba extract therapy? a systematic review and meta-analysis. Pharmacotherapy. 2011;31:490-502. doi:10.1592/phco.31.5.490
- Gardner CD, Zehnder JL, Rigby AJ, et al. Effect of Ginkgo biloba (EGb 761) and aspirin on platelet aggregation and platelet function analysis among older adults at risk of cardiovascular disease: a randomized clinical trial. Blood Coagul Fibrinolysis. 2007;18:787-79. doi:10.1097/MBC.0b013e3282f102b1
- Jiang X, Williams KM, Liauw WS, et al. Effect of ginkgo and ginger on the pharmacokinetics and pharmacodynamics of warfarin in healthy subjects. Br J Clin Pharmacol. 2005;59:425-432. doi:10.1111/j.1365-2125.2005.02322.x
- Toxicology and carcinogenesis studies of Ginkgo biloba extract (CAS No. 90045-36-6) in F344/N rats and B6C3F1/N mice (gavage studies). Natl Toxicol Program Tech Rep Ser. 2013:1-183.
- Azuma F, Nokura K, Kako T, et al. An adult case of generalized convulsions caused by the ingestion of Ginkgo biloba seeds with alcohol. Intern Med. 2020;59:1555-1558. doi:10.2169/internalmedicine.4196-19
- Cohen PR. Fixed drug eruption to supplement containing Ginkgo biloba and vinpocetine: a case report and review of related cutaneous side effects. J Clin Aesthet Dermatol. 2017;10:44-47.
- Lyu J. Ginkgo history told by genomes. Nat Plants. 2019;5:1029. doi:10.1038/s41477-019-0529-2
- ElSohly MA, Adawadkar PD, Benigni DA, et al. Analogues of poison ivy urushiol. Synthesis and biological activity of disubstituted n-alkylbenzenes. J Med Chem. 1986;29:606-611. doi:10.1021/jm00155a003
- He X, Bernart MW, Nolan GS, et al. High-performance liquid chromatography–electrospray ionization-mass spectrometry study of ginkgolic acid in the leaves and fruits of the ginkgo tree (Ginkgo biloba). J Chromatogr Sci. 2000;38:169-173. doi:10.1093/chromsci/38.4.169
- Sowers WF, Weary PE, Collins OD, et al. Ginkgo-tree dermatitis. Arch Dermatol. 1965;91:452-456. doi:10.1001/archderm.1965.01600110038009
- Tomb RR, Foussereau J, Sell Y. Mini-epidemic of contact dermatitis from ginkgo tree fruit (Ginkgo biloba L.). Contact Dermatitis. 1988;19:281-283. doi:10.1111/j.1600-0536.1988.tb02928.x
- Lepoittevin J-P, Benezra C, Asakawa Y. Allergic contact dermatitis to Ginkgo biloba L.: relationship with urushiol. Arch Dermatol Res. 1989;281:227-230. doi:10.1007/BF00431055
- Yirmibesoglu E, Karahacioglu E, Kilic D, et al. The protective effects of Ginkgo biloba extract (EGb-761) on radiation-induced dermatitis: an experimental study. Clin Exp Dermatol. 2012;37:387-394. doi:10.1111/j.1365-2230.2011.04253.x
- Jiang L, Su L, Cui H, et al. Ginkgo biloba extract for dementia: a systematic review. Shanghai Arch Psychiatry. 2013;25:10-21. doi:10.3969/j.issn.1002-0829.2013.01.005
- Oken BS, Storzbach DM, Kaye JA. The efficacy of Ginkgo biloba on cognitive function in Alzheimer disease. Arch Neurol. 1998;55:1409-1415. doi:10.1001/archneur.55.11.1409
- Le Bars PL, Katz MM, Berman N, et al. A placebo-controlled, double-blind, randomized trial of an extract of Ginkgo biloba for dementia. North American EGb Study Group. JAMA. 1997;278:1327-1332. doi:10.1001/jama.278.16.1327
- Koltermann A, Hartkorn A, Koch E, et al. Ginkgo biloba extract EGb 761 increases endothelial nitric oxide production in vitro and in vivo. Cell Mol Life Sci. 2007;64:1715-1722. doi:10.1007/s00018-007-7085-z
- Touvay C, Vilain B, Taylor JE, et al. Proof of the involvement of platelet activating factor (paf-acether) in pulmonary complex immune systems using a specific paf-acether receptor antagonist: BN 52021. Prog Lipid Res. 1986;25:277-288. doi:10.1016/0163-7827(86)90057-3
- Parsad D, Pandhi R, Juneja A. Effectiveness of oral Ginkgo biloba in treating limited, slowly spreading vitiligo. Clin Exp Dermatol. 2003;28:285-287. doi:10.1046/j.1365-2230.2003.01207.x
- Jacobsson I, Jönsson AK, Gerdén B, et al. Spontaneously reported adverse reactions in association with complementary and alternative medicine substances in Sweden. Pharmacoepidemiol Drug Saf. 2009;18:1039-1047. doi:10.1002/pds.1818
- Bolus M. Dermatitis venenata due to Ginkgo berries. Arch Derm Syphilol. 1939;39:530.
- Becker LE, Skipworth GB. Ginkgo-tree dermatitis, stomatitis, and proctitis. JAMA. 1975;231:1162-1163.
- Nakamura T. Ginkgo tree dermatitis. Contact Dermatitis. 1985;12:281-282. doi:10.1111/j.1600-0536.1985.tb01138.x
- Jiang J, Ding Y, Qian G. Airborne contact dermatitis caused by the sarcotesta of Ginkgo biloba. Contact Dermatitis. 2016;75:384-385. doi:10.1111/cod.12646
- Hotta E, Tamagawa-Mineoka R, Katoh N. Allergic contact dermatitis due to ginkgo tree fruit and leaf. Eur J Dermatol. 2013;23:548-549. doi:10.1684/ejd.2013.2102
- Chiu AE, Lane AT, Kimball AB. Diffuse morbilliform eruption after consumption of Ginkgo biloba supplement. J Am Acad Dermatol. 2002;46:145-146. doi:10.1067/mjd.2001.118545
- Pennisi RS. Acute generalised exanthematous pustulosis induced by the herbal remedy Ginkgo biloba. Med J Aust. 2006;184:583-584. doi:10.5694/j.1326-5377.2006.tb00386.x
- Yuste M, Sánchez-Estella J, Santos JC, et al. Stevens-Johnson syndrome/toxic epidermal necrolysis treated with intravenous immunoglobulins. Actas Dermosifiliogr. 2005;96:589-592. doi:10.1016/s0001-7310(05)73141-0
- Jeyamani VP, Sabishruthi S, Kavitha S, et al. An illustrative case study on drug induced Steven-Johnson syndrome by Ginkgo biloba. J Clin Res. 2018;2:1-3.
- Davydov L, Stirling AL. Stevens-Johnson syndrome with Ginkgo biloba. J Herbal Pharmacother. 2001;1:65-69. doi:10.1080/J157v01n03_06
- Yin OQP, Tomlinson B, Waye MMY, et al. Pharmacogenetics and herb–drug interactions: experience with Ginkgo biloba and omeprazole. Pharmacogenetics. 2004;14:841-850. doi:10.1097/00008571-200412000-00007
- Kupiec T, Raj V. Fatal seizures due to potential herb–drug interactions with Ginkgo biloba. J Anal Toxicol. 2005;29:755-758. doi:10.1093/jat/29.7.755
- Zadoyan G, Rokitta D, Klement S, et al. Effect of Ginkgo biloba special extract EGb 761® on human cytochrome P450 activity: a cocktail interaction study in healthy volunteers. Eur J Clin Pharmacol. 2012;68:553-560. doi:10.1007/s00228-011-1174-5
- Zhou S-F, Deng Y, Bi H-c, et al. Induction of cytochrome P450 3A by the Ginkgo biloba extract and bilobalides in human and rat primary hepatocytes. Drug Metab Lett. 2008;2:60-66. doi:10.2174/187231208783478489
- Kellermann AJ, Kloft C. Is there a risk of bleeding associated with standardized Ginkgo biloba extract therapy? a systematic review and meta-analysis. Pharmacotherapy. 2011;31:490-502. doi:10.1592/phco.31.5.490
- Gardner CD, Zehnder JL, Rigby AJ, et al. Effect of Ginkgo biloba (EGb 761) and aspirin on platelet aggregation and platelet function analysis among older adults at risk of cardiovascular disease: a randomized clinical trial. Blood Coagul Fibrinolysis. 2007;18:787-79. doi:10.1097/MBC.0b013e3282f102b1
- Jiang X, Williams KM, Liauw WS, et al. Effect of ginkgo and ginger on the pharmacokinetics and pharmacodynamics of warfarin in healthy subjects. Br J Clin Pharmacol. 2005;59:425-432. doi:10.1111/j.1365-2125.2005.02322.x
- Toxicology and carcinogenesis studies of Ginkgo biloba extract (CAS No. 90045-36-6) in F344/N rats and B6C3F1/N mice (gavage studies). Natl Toxicol Program Tech Rep Ser. 2013:1-183.
- Azuma F, Nokura K, Kako T, et al. An adult case of generalized convulsions caused by the ingestion of Ginkgo biloba seeds with alcohol. Intern Med. 2020;59:1555-1558. doi:10.2169/internalmedicine.4196-19
- Cohen PR. Fixed drug eruption to supplement containing Ginkgo biloba and vinpocetine: a case report and review of related cutaneous side effects. J Clin Aesthet Dermatol. 2017;10:44-47.
PRACTICE POINTS
- Contact with the Ginkgo biloba tree can cause allergic contact dermatitis; ingestion can cause systemic dermatitis in a previously sensitized patient.
- Ginkgo biloba can cross-react with plants of the family Anacardiaceae, such as poison ivy, poison oak, poison sumac, cashew tree, and mango.
- Ginkgo extract is widely considered safe for use; however, dermatologists should be aware that it can cause systemic dermatitis and serious adverse effects, including internal hemorrhage and convulsions.
Aquatic Antagonists: Marine Rashes (Seabather’s Eruption and Diver’s Dermatitis)
Background and Clinical Presentation
Seabather’s Eruption—Seabather’s eruption is a type I and IV hypersensitivity reaction caused by nematocysts of larval-stage thimble jellyfish (Linuche unguiculata), sea anemones (eg, Edwardsiella lineata), and larval cnidarians.1Linuche unguiculata commonly is found along the southeast coast of the United States and in the Caribbean, the Gulf of Mexico, and the coasts of Florida; less commonly, it has been reported along the coasts of Brazil and Papua New Guinea. Edwardsiella lineata more commonly is seen along the East Coast of the United States.2 Seabather’s eruption presents as numerous scattered, pruritic, red macules and papules (measuring 1 mm to 1.5 cm in size) distributed in areas covered by skin folds, wet clothing, or hair following exposure to marine water (Figure 1). This maculopapular rash generally appears shortly after exiting the water and can last up to several weeks in some cases.3 The cause for this delayed presentation is that the marine organisms become entrapped between the skin of the human contact and another object (eg, swimwear) but do not release their preformed antivenom until they are exposed to air after removal from the water, at which point the organisms die and cell lysis results in injection of the venom.
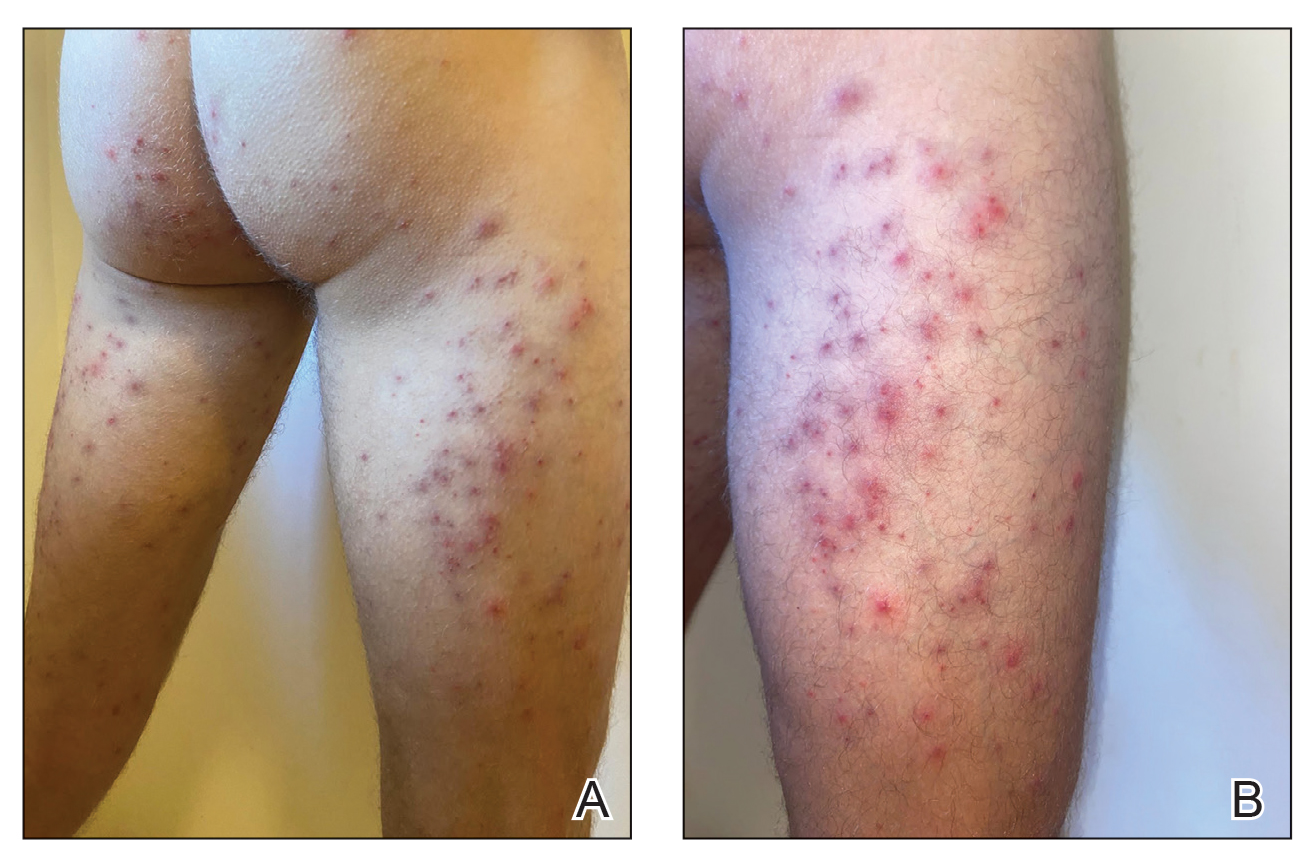
Diver’s Dermatitis—Diver’s dermatitis (also referred to as “swimmer’s itch”) is a type I and IV hypersensitivity reaction caused by schistosome cercariae released by aquatic snails.4 There are several different cercarial species known to be capable of causing diver dermatitis, but the most commonly implicated genera are Trichobilharzia and Gigantobilharzia. These parasites most commonly are found in freshwater lakes but also occur in oceans, particularly in brackish areas adjacent to freshwater access. Factors associated with increased concentrations of these parasites include shallow, slow-moving water and prolonged onshore wind causing accumulation near the shoreline. It also is thought that the snail host will shed greater concentrations of the parasitic worm in the morning hours and after prolonged exposure to sunlight.4 These flatworm trematodes have a 2-host life cycle. The snails function as intermediate hosts for the parasites before they enter their final host, which are birds. Humans only function as incidental and nonviable hosts for these worms. The parasites gain access to the human body by burrowing into exposed skin. Because the parasite is unable to survive on human hosts, it dies shortly after penetrating the skin, which leads to an intense inflammatory response causing symptoms of pruritus within hours of exposure (Figure 2). The initial eruption progresses over a few days into a diffuse, maculopapular, pruritic rash, similar to that seen in seabather’s eruption. This rash then regresses completely in 1 to 3 weeks. Subsequent exposure to the same parasite is associated with increased severity of future rashes, likely due to antibody-mediated sensitization.4
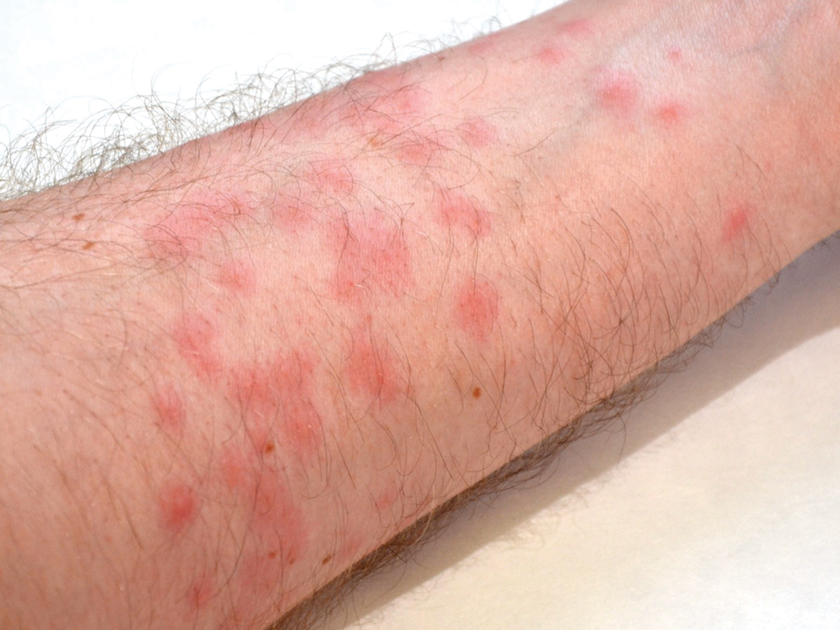
Diagnosis—Marine-derived dermatoses from various sources can present very similarly; thus, it is difficult to discern the specific etiology behind the clinical presentation. No commonly utilized imaging modalities can differentiate between seabather’s eruption and diver’s dermatitis, but eliciting a thorough patient history often can aid in differentiation of the cause of the eruption. For example, lesions located only on nonexposed areas of the skin increases the likelihood of seabather’s eruption due to nematocysts being trapped between clothing and the skin. In contrast, diver’s dermatitis generally appears on areas of the skin that were directly exposed to water and uncovered by clothing.5 Patient reports of a lack of symptoms until shortly after exiting the water further support a diagnosis of seabather’s eruption, as this delayed presentation of symptoms is caused by lysis of the culprit organisms following removal from the marine environment. The cell lysis is responsible for the widespread injection of preformed venom via the numerous nematocysts trapped between clothing and the patient’s body.1
Treatment
For both conditions, the symptoms are treated with hydrocortisone or other topical steroid solutions in conjunction with oral hydroxyzine. Alternative treatments include calamine lotion with 1% menthol and nonsteroidal anti-inflammatory drugs. Taking baths with oatmeal, Epsom salts, or baking soda also may alleviate some of the pruritic symptoms.2
Prevention
The ability to diagnose the precise cause of these similar marine rashes can bring peace of mind to both patients and physicians regardless of their similar management strategies. Severe contact dermatitis of unknown etiology can be disconcerting for patients. Additionally, documenting the causes of marine rashes in particular geographic locations can be beneficial for establishing which organisms are most likely to affect visitors to those areas. This type of data collection can be utilized to develop preventative recommendations, such as deciding when to avoid the water. Education of the public can be done with the use of informational posters located near popular swimming areas and online public service announcements. Informing the general public about the dangers of entering the ocean, especially during certain times of the year when nematocyst-equipped sea creatures are in abundance, could serve to prevent numerous cases of seabather’s eruption. Likewise, advising against immersion in shallow, slow-moving water during the morning hours or after prolonged sun exposure in trematode-endemic areas could prevent numerous cases of diver’s dermatitis. Basic information on what to expect if afflicted by a marine rash also may reduce the number of emergency department visits for these conditions, thus providing economic benefit for patients and for hospitals since patients would better know how to acutely treat these rashes and lessen the patient load at hospital emergency departments. If individuals can assure themselves of the self-limited nature of these types of dermatoses, they may be less inclined to seek medical consultation.
Final Thoughts
As the climate continues to change, the incidence of marine rashes such as seabather’s eruption and diver’s dermatitis is expected to increase due to warmer surface temperatures causing more frequent and earlier blooms of L unguiculata and E lineata. Cases of diver’s dermatitis also could increase due to a longer season of more frequent human exposure from an increase in warmer temperatures. The projected uptick in incidences of these marine rashes makes understanding these pathologies even more pertinent for physicians.6 Increasing our understanding of the different types of marine rashes and their causes will help guide future recommendations for the general public when visiting the ocean.
Future research may wish to investigate unique ways in which to prevent contact between these organisms and humans. Past research on mice indicated that topical application of DEET (N,N-diethyl-meta-toluamide) prior to trematode exposure prevented penetration of the skin by parasitic worms.7 Future studies are needed to examine the effectiveness of this preventative technique on humans. For now, dermatologists may counsel our ocean-going patients on preventative behaviors as well as provide reassurance and symptomatic relief when they present to our clinics with marine rashes.
- Parrish DO. Seabather’s eruption or diver’s dermatitis? JAMA. 1993;270:2300-2301. doi:10.1001/jama.1993.03510190054021
- Tomchik RS, Russell MT, Szmant AM, et al. Clinical perspectives on seabather’s eruption, also known as ‘sea lice’. JAMA. 1993;269:1669-1672. doi:10.1001/jama.1993.03500130083037
- Bonamonte D, Filoni A, Verni P, et al. Dermatitis caused by algae and Bryozoans. In: Bonamonte D, Angelini G, eds. Aquatic Dermatology: Biotic, Chemical, and Physical Agents. Springer; 2016:127-137.
- Tracz ES, Al-Jubury A, Buchmann K, et al. Outbreak of swimmer’s itch in Denmark. Acta Derm Venereol. 2019;99:1116-1120. doi:10.2340/00015555-3309
- Freudenthal AR, Joseph PR. Seabather’s eruption. N Engl J Med. 1993;329:542-544. doi:10.1056/NEJM199308193290805
- Kaffenberger BH, Shetlar D, Norton SA, et al. The effect of climate change on skin disease in North America. JAAD. 2016;76:140-147. doi:10.1016/j.jaad.2016.08.014
- Salafsky B, Ramaswamy K, He YX, et al. Development and evaluation of LIPODEET, a new long-acting formulation of N, N-diethyl-m-toluamide (DEET) for the prevention of schistosomiasis. Am J Trop Med Hyg. 1999;61:743-750. doi:10.4269/ajtmh.1999.61.743
Background and Clinical Presentation
Seabather’s Eruption—Seabather’s eruption is a type I and IV hypersensitivity reaction caused by nematocysts of larval-stage thimble jellyfish (Linuche unguiculata), sea anemones (eg, Edwardsiella lineata), and larval cnidarians.1Linuche unguiculata commonly is found along the southeast coast of the United States and in the Caribbean, the Gulf of Mexico, and the coasts of Florida; less commonly, it has been reported along the coasts of Brazil and Papua New Guinea. Edwardsiella lineata more commonly is seen along the East Coast of the United States.2 Seabather’s eruption presents as numerous scattered, pruritic, red macules and papules (measuring 1 mm to 1.5 cm in size) distributed in areas covered by skin folds, wet clothing, or hair following exposure to marine water (Figure 1). This maculopapular rash generally appears shortly after exiting the water and can last up to several weeks in some cases.3 The cause for this delayed presentation is that the marine organisms become entrapped between the skin of the human contact and another object (eg, swimwear) but do not release their preformed antivenom until they are exposed to air after removal from the water, at which point the organisms die and cell lysis results in injection of the venom.

Diver’s Dermatitis—Diver’s dermatitis (also referred to as “swimmer’s itch”) is a type I and IV hypersensitivity reaction caused by schistosome cercariae released by aquatic snails.4 There are several different cercarial species known to be capable of causing diver dermatitis, but the most commonly implicated genera are Trichobilharzia and Gigantobilharzia. These parasites most commonly are found in freshwater lakes but also occur in oceans, particularly in brackish areas adjacent to freshwater access. Factors associated with increased concentrations of these parasites include shallow, slow-moving water and prolonged onshore wind causing accumulation near the shoreline. It also is thought that the snail host will shed greater concentrations of the parasitic worm in the morning hours and after prolonged exposure to sunlight.4 These flatworm trematodes have a 2-host life cycle. The snails function as intermediate hosts for the parasites before they enter their final host, which are birds. Humans only function as incidental and nonviable hosts for these worms. The parasites gain access to the human body by burrowing into exposed skin. Because the parasite is unable to survive on human hosts, it dies shortly after penetrating the skin, which leads to an intense inflammatory response causing symptoms of pruritus within hours of exposure (Figure 2). The initial eruption progresses over a few days into a diffuse, maculopapular, pruritic rash, similar to that seen in seabather’s eruption. This rash then regresses completely in 1 to 3 weeks. Subsequent exposure to the same parasite is associated with increased severity of future rashes, likely due to antibody-mediated sensitization.4

Diagnosis—Marine-derived dermatoses from various sources can present very similarly; thus, it is difficult to discern the specific etiology behind the clinical presentation. No commonly utilized imaging modalities can differentiate between seabather’s eruption and diver’s dermatitis, but eliciting a thorough patient history often can aid in differentiation of the cause of the eruption. For example, lesions located only on nonexposed areas of the skin increases the likelihood of seabather’s eruption due to nematocysts being trapped between clothing and the skin. In contrast, diver’s dermatitis generally appears on areas of the skin that were directly exposed to water and uncovered by clothing.5 Patient reports of a lack of symptoms until shortly after exiting the water further support a diagnosis of seabather’s eruption, as this delayed presentation of symptoms is caused by lysis of the culprit organisms following removal from the marine environment. The cell lysis is responsible for the widespread injection of preformed venom via the numerous nematocysts trapped between clothing and the patient’s body.1
Treatment
For both conditions, the symptoms are treated with hydrocortisone or other topical steroid solutions in conjunction with oral hydroxyzine. Alternative treatments include calamine lotion with 1% menthol and nonsteroidal anti-inflammatory drugs. Taking baths with oatmeal, Epsom salts, or baking soda also may alleviate some of the pruritic symptoms.2
Prevention
The ability to diagnose the precise cause of these similar marine rashes can bring peace of mind to both patients and physicians regardless of their similar management strategies. Severe contact dermatitis of unknown etiology can be disconcerting for patients. Additionally, documenting the causes of marine rashes in particular geographic locations can be beneficial for establishing which organisms are most likely to affect visitors to those areas. This type of data collection can be utilized to develop preventative recommendations, such as deciding when to avoid the water. Education of the public can be done with the use of informational posters located near popular swimming areas and online public service announcements. Informing the general public about the dangers of entering the ocean, especially during certain times of the year when nematocyst-equipped sea creatures are in abundance, could serve to prevent numerous cases of seabather’s eruption. Likewise, advising against immersion in shallow, slow-moving water during the morning hours or after prolonged sun exposure in trematode-endemic areas could prevent numerous cases of diver’s dermatitis. Basic information on what to expect if afflicted by a marine rash also may reduce the number of emergency department visits for these conditions, thus providing economic benefit for patients and for hospitals since patients would better know how to acutely treat these rashes and lessen the patient load at hospital emergency departments. If individuals can assure themselves of the self-limited nature of these types of dermatoses, they may be less inclined to seek medical consultation.
Final Thoughts
As the climate continues to change, the incidence of marine rashes such as seabather’s eruption and diver’s dermatitis is expected to increase due to warmer surface temperatures causing more frequent and earlier blooms of L unguiculata and E lineata. Cases of diver’s dermatitis also could increase due to a longer season of more frequent human exposure from an increase in warmer temperatures. The projected uptick in incidences of these marine rashes makes understanding these pathologies even more pertinent for physicians.6 Increasing our understanding of the different types of marine rashes and their causes will help guide future recommendations for the general public when visiting the ocean.
Future research may wish to investigate unique ways in which to prevent contact between these organisms and humans. Past research on mice indicated that topical application of DEET (N,N-diethyl-meta-toluamide) prior to trematode exposure prevented penetration of the skin by parasitic worms.7 Future studies are needed to examine the effectiveness of this preventative technique on humans. For now, dermatologists may counsel our ocean-going patients on preventative behaviors as well as provide reassurance and symptomatic relief when they present to our clinics with marine rashes.
Background and Clinical Presentation
Seabather’s Eruption—Seabather’s eruption is a type I and IV hypersensitivity reaction caused by nematocysts of larval-stage thimble jellyfish (Linuche unguiculata), sea anemones (eg, Edwardsiella lineata), and larval cnidarians.1Linuche unguiculata commonly is found along the southeast coast of the United States and in the Caribbean, the Gulf of Mexico, and the coasts of Florida; less commonly, it has been reported along the coasts of Brazil and Papua New Guinea. Edwardsiella lineata more commonly is seen along the East Coast of the United States.2 Seabather’s eruption presents as numerous scattered, pruritic, red macules and papules (measuring 1 mm to 1.5 cm in size) distributed in areas covered by skin folds, wet clothing, or hair following exposure to marine water (Figure 1). This maculopapular rash generally appears shortly after exiting the water and can last up to several weeks in some cases.3 The cause for this delayed presentation is that the marine organisms become entrapped between the skin of the human contact and another object (eg, swimwear) but do not release their preformed antivenom until they are exposed to air after removal from the water, at which point the organisms die and cell lysis results in injection of the venom.

Diver’s Dermatitis—Diver’s dermatitis (also referred to as “swimmer’s itch”) is a type I and IV hypersensitivity reaction caused by schistosome cercariae released by aquatic snails.4 There are several different cercarial species known to be capable of causing diver dermatitis, but the most commonly implicated genera are Trichobilharzia and Gigantobilharzia. These parasites most commonly are found in freshwater lakes but also occur in oceans, particularly in brackish areas adjacent to freshwater access. Factors associated with increased concentrations of these parasites include shallow, slow-moving water and prolonged onshore wind causing accumulation near the shoreline. It also is thought that the snail host will shed greater concentrations of the parasitic worm in the morning hours and after prolonged exposure to sunlight.4 These flatworm trematodes have a 2-host life cycle. The snails function as intermediate hosts for the parasites before they enter their final host, which are birds. Humans only function as incidental and nonviable hosts for these worms. The parasites gain access to the human body by burrowing into exposed skin. Because the parasite is unable to survive on human hosts, it dies shortly after penetrating the skin, which leads to an intense inflammatory response causing symptoms of pruritus within hours of exposure (Figure 2). The initial eruption progresses over a few days into a diffuse, maculopapular, pruritic rash, similar to that seen in seabather’s eruption. This rash then regresses completely in 1 to 3 weeks. Subsequent exposure to the same parasite is associated with increased severity of future rashes, likely due to antibody-mediated sensitization.4

Diagnosis—Marine-derived dermatoses from various sources can present very similarly; thus, it is difficult to discern the specific etiology behind the clinical presentation. No commonly utilized imaging modalities can differentiate between seabather’s eruption and diver’s dermatitis, but eliciting a thorough patient history often can aid in differentiation of the cause of the eruption. For example, lesions located only on nonexposed areas of the skin increases the likelihood of seabather’s eruption due to nematocysts being trapped between clothing and the skin. In contrast, diver’s dermatitis generally appears on areas of the skin that were directly exposed to water and uncovered by clothing.5 Patient reports of a lack of symptoms until shortly after exiting the water further support a diagnosis of seabather’s eruption, as this delayed presentation of symptoms is caused by lysis of the culprit organisms following removal from the marine environment. The cell lysis is responsible for the widespread injection of preformed venom via the numerous nematocysts trapped between clothing and the patient’s body.1
Treatment
For both conditions, the symptoms are treated with hydrocortisone or other topical steroid solutions in conjunction with oral hydroxyzine. Alternative treatments include calamine lotion with 1% menthol and nonsteroidal anti-inflammatory drugs. Taking baths with oatmeal, Epsom salts, or baking soda also may alleviate some of the pruritic symptoms.2
Prevention
The ability to diagnose the precise cause of these similar marine rashes can bring peace of mind to both patients and physicians regardless of their similar management strategies. Severe contact dermatitis of unknown etiology can be disconcerting for patients. Additionally, documenting the causes of marine rashes in particular geographic locations can be beneficial for establishing which organisms are most likely to affect visitors to those areas. This type of data collection can be utilized to develop preventative recommendations, such as deciding when to avoid the water. Education of the public can be done with the use of informational posters located near popular swimming areas and online public service announcements. Informing the general public about the dangers of entering the ocean, especially during certain times of the year when nematocyst-equipped sea creatures are in abundance, could serve to prevent numerous cases of seabather’s eruption. Likewise, advising against immersion in shallow, slow-moving water during the morning hours or after prolonged sun exposure in trematode-endemic areas could prevent numerous cases of diver’s dermatitis. Basic information on what to expect if afflicted by a marine rash also may reduce the number of emergency department visits for these conditions, thus providing economic benefit for patients and for hospitals since patients would better know how to acutely treat these rashes and lessen the patient load at hospital emergency departments. If individuals can assure themselves of the self-limited nature of these types of dermatoses, they may be less inclined to seek medical consultation.
Final Thoughts
As the climate continues to change, the incidence of marine rashes such as seabather’s eruption and diver’s dermatitis is expected to increase due to warmer surface temperatures causing more frequent and earlier blooms of L unguiculata and E lineata. Cases of diver’s dermatitis also could increase due to a longer season of more frequent human exposure from an increase in warmer temperatures. The projected uptick in incidences of these marine rashes makes understanding these pathologies even more pertinent for physicians.6 Increasing our understanding of the different types of marine rashes and their causes will help guide future recommendations for the general public when visiting the ocean.
Future research may wish to investigate unique ways in which to prevent contact between these organisms and humans. Past research on mice indicated that topical application of DEET (N,N-diethyl-meta-toluamide) prior to trematode exposure prevented penetration of the skin by parasitic worms.7 Future studies are needed to examine the effectiveness of this preventative technique on humans. For now, dermatologists may counsel our ocean-going patients on preventative behaviors as well as provide reassurance and symptomatic relief when they present to our clinics with marine rashes.
- Parrish DO. Seabather’s eruption or diver’s dermatitis? JAMA. 1993;270:2300-2301. doi:10.1001/jama.1993.03510190054021
- Tomchik RS, Russell MT, Szmant AM, et al. Clinical perspectives on seabather’s eruption, also known as ‘sea lice’. JAMA. 1993;269:1669-1672. doi:10.1001/jama.1993.03500130083037
- Bonamonte D, Filoni A, Verni P, et al. Dermatitis caused by algae and Bryozoans. In: Bonamonte D, Angelini G, eds. Aquatic Dermatology: Biotic, Chemical, and Physical Agents. Springer; 2016:127-137.
- Tracz ES, Al-Jubury A, Buchmann K, et al. Outbreak of swimmer’s itch in Denmark. Acta Derm Venereol. 2019;99:1116-1120. doi:10.2340/00015555-3309
- Freudenthal AR, Joseph PR. Seabather’s eruption. N Engl J Med. 1993;329:542-544. doi:10.1056/NEJM199308193290805
- Kaffenberger BH, Shetlar D, Norton SA, et al. The effect of climate change on skin disease in North America. JAAD. 2016;76:140-147. doi:10.1016/j.jaad.2016.08.014
- Salafsky B, Ramaswamy K, He YX, et al. Development and evaluation of LIPODEET, a new long-acting formulation of N, N-diethyl-m-toluamide (DEET) for the prevention of schistosomiasis. Am J Trop Med Hyg. 1999;61:743-750. doi:10.4269/ajtmh.1999.61.743
- Parrish DO. Seabather’s eruption or diver’s dermatitis? JAMA. 1993;270:2300-2301. doi:10.1001/jama.1993.03510190054021
- Tomchik RS, Russell MT, Szmant AM, et al. Clinical perspectives on seabather’s eruption, also known as ‘sea lice’. JAMA. 1993;269:1669-1672. doi:10.1001/jama.1993.03500130083037
- Bonamonte D, Filoni A, Verni P, et al. Dermatitis caused by algae and Bryozoans. In: Bonamonte D, Angelini G, eds. Aquatic Dermatology: Biotic, Chemical, and Physical Agents. Springer; 2016:127-137.
- Tracz ES, Al-Jubury A, Buchmann K, et al. Outbreak of swimmer’s itch in Denmark. Acta Derm Venereol. 2019;99:1116-1120. doi:10.2340/00015555-3309
- Freudenthal AR, Joseph PR. Seabather’s eruption. N Engl J Med. 1993;329:542-544. doi:10.1056/NEJM199308193290805
- Kaffenberger BH, Shetlar D, Norton SA, et al. The effect of climate change on skin disease in North America. JAAD. 2016;76:140-147. doi:10.1016/j.jaad.2016.08.014
- Salafsky B, Ramaswamy K, He YX, et al. Development and evaluation of LIPODEET, a new long-acting formulation of N, N-diethyl-m-toluamide (DEET) for the prevention of schistosomiasis. Am J Trop Med Hyg. 1999;61:743-750. doi:10.4269/ajtmh.1999.61.743
Practice Points
- Seabather’s eruption and diver’s dermatitis have similar clinical presentations but differ in the ways that organisms come in contact with the skin.
- No commonly utilized imaging modality can differentiate between seabather’s eruption and diver’s dermatitis, but eliciting a thorough history often can aid in differentiating these marine rashes.
- Physicians should understand the pathologies of common marine rashes due to a projected uptick in the number of cases related to climate change.
What’s Eating You? Mosquitoes (Culicidae)
Incidence and Characteristics
Mosquitoes are insects categorized into the order of Diptera and family of Culicidae, and more than 3500 different species have been identified.1 In the United States, the most common genus of mosquitoes is Aedes, with other common genera including Culex, Anopheles, Culiseta, and Coquillettidia. Most bites are performed by female rather than male mosquitoes, as it serves to complete their life cycle (Figure 1).1

There are a variety of possible reactions to mosquito bites. Severe local reactions that are large (papules >30 mm in diameter) or are accompanied by systemic manifestations are referred to as hypersensitivity to mosquito bites (HMB).2 These hypersensitivity reactions vary according to multiple factors, including comorbid conditions, genetic predisposition, and geographic location. The majority of the world’s population will exhibit local reactions to mosquito bites at some point during life, with the median age of onset of the first bite at 2 years of age.3 In a study by Arias-Cruz et al,4 the incidence of patient-reported large local reactions was 2.5%. Hypersensitivity to mosquito bites, perhaps the most rare reaction, is more common among Asian and Central American children.5 The median age of diagnosis for HMB is 7 years, and most reactions occur during the first 2 decades of life.6,7
Clinical Presentation
Mosquitoes bite vertebrates in an attempt to feed and thus must locate the host’s blood vessels through a process known as probing, which often necessitates changing the bite site several times. Once the vessel is located and lacerated, the mosquito feeds either from the vessel directly or the hematoma around it. Not only does the bite cause trauma to the skin, but a cutaneous reaction also may occur in response to salivary gland secretions that concurrently are deposited in the host tissue.8 Mosquitoes’ salivary gland components are the primary cause of cutaneous reactions, as one study showed that bites from mosquitoes lacking salivary gland ducts were not associated with these reactions.9 Mosquito saliva contains a large number of compounds with biologic activities, including lysozymes, antibacterial glucosidases, anticoagulants, antiplatelet aggregating factors, and vasodilators, as well as a potentially large number of unknown allergenic proteins. As of 2016, 70 mosquito-derived allergens have been identified, but this number continues to grow.2 After a bite from a mosquito, these compounds may result in host sensitization over time, though interestingly, sensitization to mosquito bites from a species different from the original offender does not occur due to lack of cross-reactivity between species.1
Because mosquitoes reproduce by laying their eggs directly on or near water, people who live near bodies of water or wetlands are at the highest risk for mosquito bites. Patient factors that have been found to lead to increased rates of mosquito bites include lower microbial diversity on the skin, the presence of sweat or body odor, pregnancy, increased body temperature, type O blood, dark clothing, and perfumes.2 Exaggerated bite reactions are associated with Epstein-Barr virus (EBV) infection and hematologic malignancies.10
Immediate hypersensitivity is mediated by a specific IgE antibody and is characterized by erythema and a wheal at the bite site that peaks within minutes of the bite. In contrast, delayed hypersensitivity is lymphocyte mediated; occurs 24 hours after the bite; and causes an indurated, pruritic, and erythematous 2- to 10-mm papule that may blister.11 Although the evidence of immediate hypersensitivity disappears within hours, symptoms of delayed hypersensitivity may last days to weeks. Accompanying symptoms may include local swelling, pain, and warmth. The itch that often is experienced in conjunction with erythema and papule formation is elicited in 3 main ways: direct induction utilizing classic pruritic pathways, IgE-mediated hypersensitivity reaction to salivary components, and IgE-independent host immune response to salivary antigens. Papular urticaria is a common additional finding in children with mosquito bites.1 As an individual is repeatedly bitten, they may undergo 5 stages of sensitization: stage I (neither immediate nor delayed reaction), stage II (delayed reaction), stage III (immediate and delayed reaction), stage IV (immediate reaction), and stage V (neither immediate or delayed reaction).11
Although most mosquito bites cause common local reactions, patients rarely demonstrate systemic reactions that can be much more severe. Skeeter syndrome is a milder systemic response characterized by large local reactions (papules >30 mm in diameter) developing hours after a bite with accompanying fever.12 The reaction typically peaks over days to weeks.2 Although the reaction may resemble cellulitis clinically, a history of a preceding mosquito bite can help make the distinction.13
A more severe systemic reaction is HMB, which is characterized by intense local skin findings as well as generalized systemic symptoms. Initially, indurated, clear, or hemorrhagic bullae appear at the bite site (Figure 2). Later, there is progression to swelling, necrosis, and ulceration.10 Biopsies from the skin lesions associated with HMB reveal necrosis, interstitial and perivascular eosinophilic and lymphocytic infiltrates, and small vessels with fibrinoid necrosis.7 Systemically, high fever, general malaise, liver dysfunction, proteinuria, hematuria, hepatosplenomegaly, and lymph node enlargement may occur. Patients typically experience these severe symptoms each time they are bitten.10
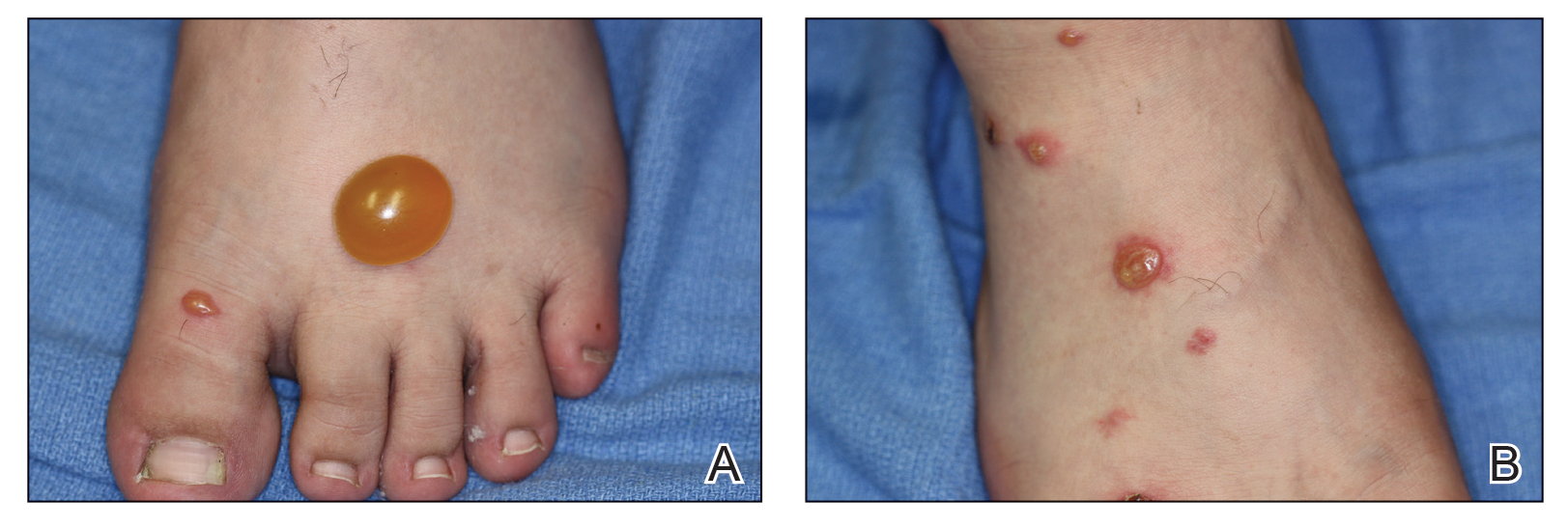
The mechanism of the HMB reaction is complex but has a close association with natural killer (NK) cell lymphoproliferative disorder and EBV infection (Figure 3). In fact, it is not uncommon for HMB patients to develop malignant lymphomas during their clinical course, even those unrelated to EBV.14 Epstein-Barr virus, one of the human herpesviruses, produces latent infection in NK cells. It is hypothesized that after a mosquito bite, EBV may be reactivated within these cells by induced expression of the viral lytic-cycle transactivator gene BamHI Z fragment leftward open reading frame 1, BZLF1.6 In response to mosquito salivary gland components, CD4+ T cells proliferate and induce expression of the EBV oncogene latent membrane protein 1, LMP1, on NK cells, which then infiltrate the bite site.15 These EBV-infected NK cells also overexpress the Fas ligand, thus contributing to organ and tissue damage.6 In addition to activating oncogene expression on NK cells, T cells also activate the basophils and mast cells carrying mosquito-specific IgE, both of which also add to the severe skin reaction of HMB.15 The particular triad of HMB, chronic active EBV infection, and NK cell lymphoproliferative disorder commonly is known as HMB-EBV-NK or HEN disease.1 Patients with HMB should be monitored for malignancy. The mortality of HMB is increased in patients in whom onset occurs when they are older than 9 years and with BZLF1 messenger RNA in skin lesions.6

Other rare reactions to mosquito bites include Wells syndrome, anaphylaxis, and superficial lymphangitis. Wells syndrome (also known as eosinophilic cellulitis) is characterized by erythematous or violaceous plaques and pruritic blisters. Although its etiology has not been defined, it is thought to be evoked or exacerbated by insect bites, with CD4+ T cells playing a primary role.1 Anaphylaxis (angioedema, urticaria, and wheezing) rarely may occur due to mosquito salivary gland components but typically is caused by other stinging insects. Superficial lymphangitis, often misdiagnosed as an infection of the lymphatic system, presents within minutes as nontender pink streaks originating from the bite site. A biopsy with eosinophil and mast cell infiltrates consistent with an allergic-type reaction confirms the absence of infection. Patients respond well to glucocorticoid treatment.
Mosquitoes are vectors for many blood-borne diseases, including dengue hemorrhagic fever, malaria, Chikungunya virus, La Crosse encephalitis, St. Louis encephalitis, West Nile virus, and yellow fever.16 Additionally, scratching the bites may lead to superinfection and scarring.1
Prevention and Treatment
Patients with known mosquito sensitivity should avoid areas of stagnant water and utilize preventative measures such as wearing protective clothing and using mosquito repellent containing DEET (N,N-diethyl-meta-toluamide), IR3535 (ethyl butylacetylaminopropionate), picaridin, or 2-undecanone (methyl nonyl ketone or IBI-246) when outdoors. Essential oils such as lemon, eucalyptus, citronella, and garlic are somewhat effective.1 Additionally, prophylactic dosing of antihistamines may prevent milder reactions.
Although often supportive, treatment and management of mosquito bites depends on the extent of the reaction. For common local reactions, symptomatic management with topical anesthetics, calamine lotion, or corticosteroid creams is appropriate. If superinfection from scratching is a concern, antibiotics may be appropriate.
Management of more severe and systemic reactions such as HMB also is supportive, and the addition of oral corticosteroids to decrease inflammation is required.7 Severe HMB also has been treated with immunosuppressive and anticancer drugs, though the efficacy is limited. Venom immunotherapy is a preventative option for patients with mosquito-specific IgE antibodies, and hematopoietic stem cell transplant may be required in patients with HMB.14,16
Conclusion
Mosquito allergens can cause a variety of reactions, ranging from those limited to the skin to those characterized by severe systemic effects. Although common local reactions can be symptomatically treated with topical medication, more severe reactions such as HMB require more involved clinical management. Hypersensitivity to mosquito bites is an important condition to recognize, as it is related to multiple organ impairment as well as later development of malignancy. Patients should be closely monitored during the entire clinical course and in the years following.
- Fostini AC, Golpanian RS, Rosen JD, et al. Beat the bite: pathophysiology and management of itch in mosquito bites. Itch. 2019;4:1.
- Engler RJ, Crisp HC, Freeman T, et al. Mosquito hypersensitivity: clinical updates. In: Freeman TM, Tracy JM, eds. Stinging Insect Allergy: A Clinician’s Guide. Springer; 2017:203-230.
- Manuyakorn W, Itsaradisaikul S, Benjaponpitak S, et al. Mosquito allergy in children: clinical features and limitation of commercially-available diagnostic tests. Asian Pac J Allergy Immunol. 2017;35:186-190.
- Arias-Cruz A, Avitia-Valenzuela E, González-Díaz SN, et al. Epidemiology of mosquito bite allergy in the Centre of Allergy and Clinical Immunology of Monterrey, Mexico. J Allergy Clin Immunol. 2006;117:S128.
- Jiang S, Manandhar U, Zheng KP, et al. A case of nodal marginal zone lymphoma with hypersensitivity to mosquito bites as initial symptom. J Cutan Pathol. 2019;46:769-774.
- Kyriakidis I, Vasileiou E, Karastrati S, et al. Primary EBV infection and hypersensitivity to mosquito bites: a case report. Virol Sin. 2016;31:517-520.
- Chiu TM, Lin YM, Wang SC, et al. Hypersensitivity to mosquito bites as the primary clinical manifestation of an Epstein-Barr virus infection. J Microbiol Immunol Infect. 2016;49:613-616.
- Henrique MO, Neto LS, Assis JB, et al. Evaluation of inflammatory skin infiltrate following Aedes aegypti bites in sensitized and non-sensitized mice reveals saliva-dependent and immune-dependent phenotypes. Immunology. 2019;158:47-59.
- Hudson A, Bowman L, Orr CWM. Effects of absence of saliva on blood feeding by mosquitoes. Science. 1960;131:1730-1731.
- Tatsuno K, Fujiyama T, Matsuoka H, et al. Clinical categories of exaggerated skin reactions to mosquito bites and their pathophysiology. J Dermatol Sci. 2016;82:145-152.
- Oka K, Ohtaki N, Igawa K, et al. Study on the correlation between age and changes in mosquito bite response. J Dermatol. 2018;45:1471-1474.
- Ferdman RM. Superficial allergic lymphangitis with a cutaneous recall reaction to a mosquito bite. Ann Allergy Asthma Immunol. 2019;123:521-522.
- Crisp HS, Johnson KS. Mosquito allergy. Ann Allergy Asthma Immunol. 2013;110:65-69.
- Washio K, Oka T, Abdalkader L, et al. Gene expression analysis of hypersensitivity to mosquito bite, chronic active EBV infection and NK/T-lymphoma/leukemia. Leuk Lymphoma. 2017;58:2683-2694.
- Sakakibara Y, Wada T, Muraoka M, et al. Basophil activation by mosquito extracts in patients with hypersensitivity to mosquito bites. Cancer Sci. 2015;106:965-971.
- Lee H, Halvorsen S, Mackey R, et al. Insect allergy. Prim Care. 2016;43:417-431.
Incidence and Characteristics
Mosquitoes are insects categorized into the order of Diptera and family of Culicidae, and more than 3500 different species have been identified.1 In the United States, the most common genus of mosquitoes is Aedes, with other common genera including Culex, Anopheles, Culiseta, and Coquillettidia. Most bites are performed by female rather than male mosquitoes, as it serves to complete their life cycle (Figure 1).1

There are a variety of possible reactions to mosquito bites. Severe local reactions that are large (papules >30 mm in diameter) or are accompanied by systemic manifestations are referred to as hypersensitivity to mosquito bites (HMB).2 These hypersensitivity reactions vary according to multiple factors, including comorbid conditions, genetic predisposition, and geographic location. The majority of the world’s population will exhibit local reactions to mosquito bites at some point during life, with the median age of onset of the first bite at 2 years of age.3 In a study by Arias-Cruz et al,4 the incidence of patient-reported large local reactions was 2.5%. Hypersensitivity to mosquito bites, perhaps the most rare reaction, is more common among Asian and Central American children.5 The median age of diagnosis for HMB is 7 years, and most reactions occur during the first 2 decades of life.6,7
Clinical Presentation
Mosquitoes bite vertebrates in an attempt to feed and thus must locate the host’s blood vessels through a process known as probing, which often necessitates changing the bite site several times. Once the vessel is located and lacerated, the mosquito feeds either from the vessel directly or the hematoma around it. Not only does the bite cause trauma to the skin, but a cutaneous reaction also may occur in response to salivary gland secretions that concurrently are deposited in the host tissue.8 Mosquitoes’ salivary gland components are the primary cause of cutaneous reactions, as one study showed that bites from mosquitoes lacking salivary gland ducts were not associated with these reactions.9 Mosquito saliva contains a large number of compounds with biologic activities, including lysozymes, antibacterial glucosidases, anticoagulants, antiplatelet aggregating factors, and vasodilators, as well as a potentially large number of unknown allergenic proteins. As of 2016, 70 mosquito-derived allergens have been identified, but this number continues to grow.2 After a bite from a mosquito, these compounds may result in host sensitization over time, though interestingly, sensitization to mosquito bites from a species different from the original offender does not occur due to lack of cross-reactivity between species.1
Because mosquitoes reproduce by laying their eggs directly on or near water, people who live near bodies of water or wetlands are at the highest risk for mosquito bites. Patient factors that have been found to lead to increased rates of mosquito bites include lower microbial diversity on the skin, the presence of sweat or body odor, pregnancy, increased body temperature, type O blood, dark clothing, and perfumes.2 Exaggerated bite reactions are associated with Epstein-Barr virus (EBV) infection and hematologic malignancies.10
Immediate hypersensitivity is mediated by a specific IgE antibody and is characterized by erythema and a wheal at the bite site that peaks within minutes of the bite. In contrast, delayed hypersensitivity is lymphocyte mediated; occurs 24 hours after the bite; and causes an indurated, pruritic, and erythematous 2- to 10-mm papule that may blister.11 Although the evidence of immediate hypersensitivity disappears within hours, symptoms of delayed hypersensitivity may last days to weeks. Accompanying symptoms may include local swelling, pain, and warmth. The itch that often is experienced in conjunction with erythema and papule formation is elicited in 3 main ways: direct induction utilizing classic pruritic pathways, IgE-mediated hypersensitivity reaction to salivary components, and IgE-independent host immune response to salivary antigens. Papular urticaria is a common additional finding in children with mosquito bites.1 As an individual is repeatedly bitten, they may undergo 5 stages of sensitization: stage I (neither immediate nor delayed reaction), stage II (delayed reaction), stage III (immediate and delayed reaction), stage IV (immediate reaction), and stage V (neither immediate or delayed reaction).11
Although most mosquito bites cause common local reactions, patients rarely demonstrate systemic reactions that can be much more severe. Skeeter syndrome is a milder systemic response characterized by large local reactions (papules >30 mm in diameter) developing hours after a bite with accompanying fever.12 The reaction typically peaks over days to weeks.2 Although the reaction may resemble cellulitis clinically, a history of a preceding mosquito bite can help make the distinction.13
A more severe systemic reaction is HMB, which is characterized by intense local skin findings as well as generalized systemic symptoms. Initially, indurated, clear, or hemorrhagic bullae appear at the bite site (Figure 2). Later, there is progression to swelling, necrosis, and ulceration.10 Biopsies from the skin lesions associated with HMB reveal necrosis, interstitial and perivascular eosinophilic and lymphocytic infiltrates, and small vessels with fibrinoid necrosis.7 Systemically, high fever, general malaise, liver dysfunction, proteinuria, hematuria, hepatosplenomegaly, and lymph node enlargement may occur. Patients typically experience these severe symptoms each time they are bitten.10

The mechanism of the HMB reaction is complex but has a close association with natural killer (NK) cell lymphoproliferative disorder and EBV infection (Figure 3). In fact, it is not uncommon for HMB patients to develop malignant lymphomas during their clinical course, even those unrelated to EBV.14 Epstein-Barr virus, one of the human herpesviruses, produces latent infection in NK cells. It is hypothesized that after a mosquito bite, EBV may be reactivated within these cells by induced expression of the viral lytic-cycle transactivator gene BamHI Z fragment leftward open reading frame 1, BZLF1.6 In response to mosquito salivary gland components, CD4+ T cells proliferate and induce expression of the EBV oncogene latent membrane protein 1, LMP1, on NK cells, which then infiltrate the bite site.15 These EBV-infected NK cells also overexpress the Fas ligand, thus contributing to organ and tissue damage.6 In addition to activating oncogene expression on NK cells, T cells also activate the basophils and mast cells carrying mosquito-specific IgE, both of which also add to the severe skin reaction of HMB.15 The particular triad of HMB, chronic active EBV infection, and NK cell lymphoproliferative disorder commonly is known as HMB-EBV-NK or HEN disease.1 Patients with HMB should be monitored for malignancy. The mortality of HMB is increased in patients in whom onset occurs when they are older than 9 years and with BZLF1 messenger RNA in skin lesions.6

Other rare reactions to mosquito bites include Wells syndrome, anaphylaxis, and superficial lymphangitis. Wells syndrome (also known as eosinophilic cellulitis) is characterized by erythematous or violaceous plaques and pruritic blisters. Although its etiology has not been defined, it is thought to be evoked or exacerbated by insect bites, with CD4+ T cells playing a primary role.1 Anaphylaxis (angioedema, urticaria, and wheezing) rarely may occur due to mosquito salivary gland components but typically is caused by other stinging insects. Superficial lymphangitis, often misdiagnosed as an infection of the lymphatic system, presents within minutes as nontender pink streaks originating from the bite site. A biopsy with eosinophil and mast cell infiltrates consistent with an allergic-type reaction confirms the absence of infection. Patients respond well to glucocorticoid treatment.
Mosquitoes are vectors for many blood-borne diseases, including dengue hemorrhagic fever, malaria, Chikungunya virus, La Crosse encephalitis, St. Louis encephalitis, West Nile virus, and yellow fever.16 Additionally, scratching the bites may lead to superinfection and scarring.1
Prevention and Treatment
Patients with known mosquito sensitivity should avoid areas of stagnant water and utilize preventative measures such as wearing protective clothing and using mosquito repellent containing DEET (N,N-diethyl-meta-toluamide), IR3535 (ethyl butylacetylaminopropionate), picaridin, or 2-undecanone (methyl nonyl ketone or IBI-246) when outdoors. Essential oils such as lemon, eucalyptus, citronella, and garlic are somewhat effective.1 Additionally, prophylactic dosing of antihistamines may prevent milder reactions.
Although often supportive, treatment and management of mosquito bites depends on the extent of the reaction. For common local reactions, symptomatic management with topical anesthetics, calamine lotion, or corticosteroid creams is appropriate. If superinfection from scratching is a concern, antibiotics may be appropriate.
Management of more severe and systemic reactions such as HMB also is supportive, and the addition of oral corticosteroids to decrease inflammation is required.7 Severe HMB also has been treated with immunosuppressive and anticancer drugs, though the efficacy is limited. Venom immunotherapy is a preventative option for patients with mosquito-specific IgE antibodies, and hematopoietic stem cell transplant may be required in patients with HMB.14,16
Conclusion
Mosquito allergens can cause a variety of reactions, ranging from those limited to the skin to those characterized by severe systemic effects. Although common local reactions can be symptomatically treated with topical medication, more severe reactions such as HMB require more involved clinical management. Hypersensitivity to mosquito bites is an important condition to recognize, as it is related to multiple organ impairment as well as later development of malignancy. Patients should be closely monitored during the entire clinical course and in the years following.
Incidence and Characteristics
Mosquitoes are insects categorized into the order of Diptera and family of Culicidae, and more than 3500 different species have been identified.1 In the United States, the most common genus of mosquitoes is Aedes, with other common genera including Culex, Anopheles, Culiseta, and Coquillettidia. Most bites are performed by female rather than male mosquitoes, as it serves to complete their life cycle (Figure 1).1

There are a variety of possible reactions to mosquito bites. Severe local reactions that are large (papules >30 mm in diameter) or are accompanied by systemic manifestations are referred to as hypersensitivity to mosquito bites (HMB).2 These hypersensitivity reactions vary according to multiple factors, including comorbid conditions, genetic predisposition, and geographic location. The majority of the world’s population will exhibit local reactions to mosquito bites at some point during life, with the median age of onset of the first bite at 2 years of age.3 In a study by Arias-Cruz et al,4 the incidence of patient-reported large local reactions was 2.5%. Hypersensitivity to mosquito bites, perhaps the most rare reaction, is more common among Asian and Central American children.5 The median age of diagnosis for HMB is 7 years, and most reactions occur during the first 2 decades of life.6,7
Clinical Presentation
Mosquitoes bite vertebrates in an attempt to feed and thus must locate the host’s blood vessels through a process known as probing, which often necessitates changing the bite site several times. Once the vessel is located and lacerated, the mosquito feeds either from the vessel directly or the hematoma around it. Not only does the bite cause trauma to the skin, but a cutaneous reaction also may occur in response to salivary gland secretions that concurrently are deposited in the host tissue.8 Mosquitoes’ salivary gland components are the primary cause of cutaneous reactions, as one study showed that bites from mosquitoes lacking salivary gland ducts were not associated with these reactions.9 Mosquito saliva contains a large number of compounds with biologic activities, including lysozymes, antibacterial glucosidases, anticoagulants, antiplatelet aggregating factors, and vasodilators, as well as a potentially large number of unknown allergenic proteins. As of 2016, 70 mosquito-derived allergens have been identified, but this number continues to grow.2 After a bite from a mosquito, these compounds may result in host sensitization over time, though interestingly, sensitization to mosquito bites from a species different from the original offender does not occur due to lack of cross-reactivity between species.1
Because mosquitoes reproduce by laying their eggs directly on or near water, people who live near bodies of water or wetlands are at the highest risk for mosquito bites. Patient factors that have been found to lead to increased rates of mosquito bites include lower microbial diversity on the skin, the presence of sweat or body odor, pregnancy, increased body temperature, type O blood, dark clothing, and perfumes.2 Exaggerated bite reactions are associated with Epstein-Barr virus (EBV) infection and hematologic malignancies.10
Immediate hypersensitivity is mediated by a specific IgE antibody and is characterized by erythema and a wheal at the bite site that peaks within minutes of the bite. In contrast, delayed hypersensitivity is lymphocyte mediated; occurs 24 hours after the bite; and causes an indurated, pruritic, and erythematous 2- to 10-mm papule that may blister.11 Although the evidence of immediate hypersensitivity disappears within hours, symptoms of delayed hypersensitivity may last days to weeks. Accompanying symptoms may include local swelling, pain, and warmth. The itch that often is experienced in conjunction with erythema and papule formation is elicited in 3 main ways: direct induction utilizing classic pruritic pathways, IgE-mediated hypersensitivity reaction to salivary components, and IgE-independent host immune response to salivary antigens. Papular urticaria is a common additional finding in children with mosquito bites.1 As an individual is repeatedly bitten, they may undergo 5 stages of sensitization: stage I (neither immediate nor delayed reaction), stage II (delayed reaction), stage III (immediate and delayed reaction), stage IV (immediate reaction), and stage V (neither immediate or delayed reaction).11
Although most mosquito bites cause common local reactions, patients rarely demonstrate systemic reactions that can be much more severe. Skeeter syndrome is a milder systemic response characterized by large local reactions (papules >30 mm in diameter) developing hours after a bite with accompanying fever.12 The reaction typically peaks over days to weeks.2 Although the reaction may resemble cellulitis clinically, a history of a preceding mosquito bite can help make the distinction.13
A more severe systemic reaction is HMB, which is characterized by intense local skin findings as well as generalized systemic symptoms. Initially, indurated, clear, or hemorrhagic bullae appear at the bite site (Figure 2). Later, there is progression to swelling, necrosis, and ulceration.10 Biopsies from the skin lesions associated with HMB reveal necrosis, interstitial and perivascular eosinophilic and lymphocytic infiltrates, and small vessels with fibrinoid necrosis.7 Systemically, high fever, general malaise, liver dysfunction, proteinuria, hematuria, hepatosplenomegaly, and lymph node enlargement may occur. Patients typically experience these severe symptoms each time they are bitten.10

The mechanism of the HMB reaction is complex but has a close association with natural killer (NK) cell lymphoproliferative disorder and EBV infection (Figure 3). In fact, it is not uncommon for HMB patients to develop malignant lymphomas during their clinical course, even those unrelated to EBV.14 Epstein-Barr virus, one of the human herpesviruses, produces latent infection in NK cells. It is hypothesized that after a mosquito bite, EBV may be reactivated within these cells by induced expression of the viral lytic-cycle transactivator gene BamHI Z fragment leftward open reading frame 1, BZLF1.6 In response to mosquito salivary gland components, CD4+ T cells proliferate and induce expression of the EBV oncogene latent membrane protein 1, LMP1, on NK cells, which then infiltrate the bite site.15 These EBV-infected NK cells also overexpress the Fas ligand, thus contributing to organ and tissue damage.6 In addition to activating oncogene expression on NK cells, T cells also activate the basophils and mast cells carrying mosquito-specific IgE, both of which also add to the severe skin reaction of HMB.15 The particular triad of HMB, chronic active EBV infection, and NK cell lymphoproliferative disorder commonly is known as HMB-EBV-NK or HEN disease.1 Patients with HMB should be monitored for malignancy. The mortality of HMB is increased in patients in whom onset occurs when they are older than 9 years and with BZLF1 messenger RNA in skin lesions.6

Other rare reactions to mosquito bites include Wells syndrome, anaphylaxis, and superficial lymphangitis. Wells syndrome (also known as eosinophilic cellulitis) is characterized by erythematous or violaceous plaques and pruritic blisters. Although its etiology has not been defined, it is thought to be evoked or exacerbated by insect bites, with CD4+ T cells playing a primary role.1 Anaphylaxis (angioedema, urticaria, and wheezing) rarely may occur due to mosquito salivary gland components but typically is caused by other stinging insects. Superficial lymphangitis, often misdiagnosed as an infection of the lymphatic system, presents within minutes as nontender pink streaks originating from the bite site. A biopsy with eosinophil and mast cell infiltrates consistent with an allergic-type reaction confirms the absence of infection. Patients respond well to glucocorticoid treatment.
Mosquitoes are vectors for many blood-borne diseases, including dengue hemorrhagic fever, malaria, Chikungunya virus, La Crosse encephalitis, St. Louis encephalitis, West Nile virus, and yellow fever.16 Additionally, scratching the bites may lead to superinfection and scarring.1
Prevention and Treatment
Patients with known mosquito sensitivity should avoid areas of stagnant water and utilize preventative measures such as wearing protective clothing and using mosquito repellent containing DEET (N,N-diethyl-meta-toluamide), IR3535 (ethyl butylacetylaminopropionate), picaridin, or 2-undecanone (methyl nonyl ketone or IBI-246) when outdoors. Essential oils such as lemon, eucalyptus, citronella, and garlic are somewhat effective.1 Additionally, prophylactic dosing of antihistamines may prevent milder reactions.
Although often supportive, treatment and management of mosquito bites depends on the extent of the reaction. For common local reactions, symptomatic management with topical anesthetics, calamine lotion, or corticosteroid creams is appropriate. If superinfection from scratching is a concern, antibiotics may be appropriate.
Management of more severe and systemic reactions such as HMB also is supportive, and the addition of oral corticosteroids to decrease inflammation is required.7 Severe HMB also has been treated with immunosuppressive and anticancer drugs, though the efficacy is limited. Venom immunotherapy is a preventative option for patients with mosquito-specific IgE antibodies, and hematopoietic stem cell transplant may be required in patients with HMB.14,16
Conclusion
Mosquito allergens can cause a variety of reactions, ranging from those limited to the skin to those characterized by severe systemic effects. Although common local reactions can be symptomatically treated with topical medication, more severe reactions such as HMB require more involved clinical management. Hypersensitivity to mosquito bites is an important condition to recognize, as it is related to multiple organ impairment as well as later development of malignancy. Patients should be closely monitored during the entire clinical course and in the years following.
- Fostini AC, Golpanian RS, Rosen JD, et al. Beat the bite: pathophysiology and management of itch in mosquito bites. Itch. 2019;4:1.
- Engler RJ, Crisp HC, Freeman T, et al. Mosquito hypersensitivity: clinical updates. In: Freeman TM, Tracy JM, eds. Stinging Insect Allergy: A Clinician’s Guide. Springer; 2017:203-230.
- Manuyakorn W, Itsaradisaikul S, Benjaponpitak S, et al. Mosquito allergy in children: clinical features and limitation of commercially-available diagnostic tests. Asian Pac J Allergy Immunol. 2017;35:186-190.
- Arias-Cruz A, Avitia-Valenzuela E, González-Díaz SN, et al. Epidemiology of mosquito bite allergy in the Centre of Allergy and Clinical Immunology of Monterrey, Mexico. J Allergy Clin Immunol. 2006;117:S128.
- Jiang S, Manandhar U, Zheng KP, et al. A case of nodal marginal zone lymphoma with hypersensitivity to mosquito bites as initial symptom. J Cutan Pathol. 2019;46:769-774.
- Kyriakidis I, Vasileiou E, Karastrati S, et al. Primary EBV infection and hypersensitivity to mosquito bites: a case report. Virol Sin. 2016;31:517-520.
- Chiu TM, Lin YM, Wang SC, et al. Hypersensitivity to mosquito bites as the primary clinical manifestation of an Epstein-Barr virus infection. J Microbiol Immunol Infect. 2016;49:613-616.
- Henrique MO, Neto LS, Assis JB, et al. Evaluation of inflammatory skin infiltrate following Aedes aegypti bites in sensitized and non-sensitized mice reveals saliva-dependent and immune-dependent phenotypes. Immunology. 2019;158:47-59.
- Hudson A, Bowman L, Orr CWM. Effects of absence of saliva on blood feeding by mosquitoes. Science. 1960;131:1730-1731.
- Tatsuno K, Fujiyama T, Matsuoka H, et al. Clinical categories of exaggerated skin reactions to mosquito bites and their pathophysiology. J Dermatol Sci. 2016;82:145-152.
- Oka K, Ohtaki N, Igawa K, et al. Study on the correlation between age and changes in mosquito bite response. J Dermatol. 2018;45:1471-1474.
- Ferdman RM. Superficial allergic lymphangitis with a cutaneous recall reaction to a mosquito bite. Ann Allergy Asthma Immunol. 2019;123:521-522.
- Crisp HS, Johnson KS. Mosquito allergy. Ann Allergy Asthma Immunol. 2013;110:65-69.
- Washio K, Oka T, Abdalkader L, et al. Gene expression analysis of hypersensitivity to mosquito bite, chronic active EBV infection and NK/T-lymphoma/leukemia. Leuk Lymphoma. 2017;58:2683-2694.
- Sakakibara Y, Wada T, Muraoka M, et al. Basophil activation by mosquito extracts in patients with hypersensitivity to mosquito bites. Cancer Sci. 2015;106:965-971.
- Lee H, Halvorsen S, Mackey R, et al. Insect allergy. Prim Care. 2016;43:417-431.
- Fostini AC, Golpanian RS, Rosen JD, et al. Beat the bite: pathophysiology and management of itch in mosquito bites. Itch. 2019;4:1.
- Engler RJ, Crisp HC, Freeman T, et al. Mosquito hypersensitivity: clinical updates. In: Freeman TM, Tracy JM, eds. Stinging Insect Allergy: A Clinician’s Guide. Springer; 2017:203-230.
- Manuyakorn W, Itsaradisaikul S, Benjaponpitak S, et al. Mosquito allergy in children: clinical features and limitation of commercially-available diagnostic tests. Asian Pac J Allergy Immunol. 2017;35:186-190.
- Arias-Cruz A, Avitia-Valenzuela E, González-Díaz SN, et al. Epidemiology of mosquito bite allergy in the Centre of Allergy and Clinical Immunology of Monterrey, Mexico. J Allergy Clin Immunol. 2006;117:S128.
- Jiang S, Manandhar U, Zheng KP, et al. A case of nodal marginal zone lymphoma with hypersensitivity to mosquito bites as initial symptom. J Cutan Pathol. 2019;46:769-774.
- Kyriakidis I, Vasileiou E, Karastrati S, et al. Primary EBV infection and hypersensitivity to mosquito bites: a case report. Virol Sin. 2016;31:517-520.
- Chiu TM, Lin YM, Wang SC, et al. Hypersensitivity to mosquito bites as the primary clinical manifestation of an Epstein-Barr virus infection. J Microbiol Immunol Infect. 2016;49:613-616.
- Henrique MO, Neto LS, Assis JB, et al. Evaluation of inflammatory skin infiltrate following Aedes aegypti bites in sensitized and non-sensitized mice reveals saliva-dependent and immune-dependent phenotypes. Immunology. 2019;158:47-59.
- Hudson A, Bowman L, Orr CWM. Effects of absence of saliva on blood feeding by mosquitoes. Science. 1960;131:1730-1731.
- Tatsuno K, Fujiyama T, Matsuoka H, et al. Clinical categories of exaggerated skin reactions to mosquito bites and their pathophysiology. J Dermatol Sci. 2016;82:145-152.
- Oka K, Ohtaki N, Igawa K, et al. Study on the correlation between age and changes in mosquito bite response. J Dermatol. 2018;45:1471-1474.
- Ferdman RM. Superficial allergic lymphangitis with a cutaneous recall reaction to a mosquito bite. Ann Allergy Asthma Immunol. 2019;123:521-522.
- Crisp HS, Johnson KS. Mosquito allergy. Ann Allergy Asthma Immunol. 2013;110:65-69.
- Washio K, Oka T, Abdalkader L, et al. Gene expression analysis of hypersensitivity to mosquito bite, chronic active EBV infection and NK/T-lymphoma/leukemia. Leuk Lymphoma. 2017;58:2683-2694.
- Sakakibara Y, Wada T, Muraoka M, et al. Basophil activation by mosquito extracts in patients with hypersensitivity to mosquito bites. Cancer Sci. 2015;106:965-971.
- Lee H, Halvorsen S, Mackey R, et al. Insect allergy. Prim Care. 2016;43:417-431.
Practice Points
- Common local reactions to mosquito bites include immediate and delayed hypersensitivity reactions. With repeated exposure, reactions can increase in severity.
- Hypersensitivity to mosquito bites is a severe systemic reaction to mosquito salivary gland components characterized by bullous necrotic skin lesions associated with systemic manifestations such as high fever, malaise, liver dysfunction, proteinuria, hematuria, hepatosplenomegaly, and lymph node enlargement.
- Hypersensitivity to mosquito bites is closely associated with chronic Epstein-Barr virus infection and lymphoproliferative disorders.
Aquatic Antagonists: Jellyfish Stings
Jellyfish stings are one of the most common marine injuries, with an estimated 150 million stings occurring annually worldwide.1 Most jellyfish stings result in painful localized skin reactions that are self-limited and can be treated with conservative measures including hot water immersion and topical anesthetics. Life-threatening systemic reactions (eg, anaphylaxis, Irukandji syndrome) can occur with some species.2-4 Mainstream media reports do not reflect the true incidence and variability of jellyfish-related injuries that are commonly encountered in the clinic.3
Characteristics of Jellyfish
There are roughly 10,000 known species of jellyfish, with approximately 100 of them posing danger to humans.5 Jellyfish belong to the phylum Cnidaria, which is comprised of 5 classes of both free-floating and sessile animals: Staurozoa (stauromedusae), Hydrozoa (hydroids, fire corals, and Portuguese man-of-war), Scyphozoa (true jellyfish), Anthozoa (corals and sea anemones), and Cubozoa (box jellyfish and Irukandji jellyfish).1,2,6 Jellyfish typically have several tentacles suspended from a free-floating gelatinous body or bell; these tentacles are covered with thousands of cells unique to Cnidaria called nematocytes or cnidocytes containing specialized stinging organelles known as nematocysts. When triggered by physical (eg, human or foreign-body contact) or chemical stimuli, each nematocyst ejects a hollow filament or barb externally, releasing venom into the victim.7,8

The scyphozoan, hydrozoan, and cubozoan life cycles generally consist of a bottom-dwelling, sessile polyp form that produces multiple free-swimming ephyrae through an asexual reproductive process called strobilation. These ephyrae grow into the fully mature medusae, recognizable as jellyfish (Figure 1).5 Additionally, jellyfish populations experience cycles of temporal and spatial population abundance and crashes known as jellyfish blooms. In 2017, Kaffenberger et al9 reviewed the shifting landscape of skin diseases in North America attributable to major changes in climate and weather patterns, including the rise in jellyfish blooms and envenomation outbreaks worldwide (eg, Physalia physalis [Portuguese man-of-war][Figure 2] along the southeastern US coastline, Porpita pacifica off Japanese beaches). Some research suggests jellyfish surges relate to climate change and human interactions with jellyfish habitats by way of eutrophication and fishing (removing predators of jellyfish).9,10

Clinical Presentation
Jellyfish injuries can vary greatly in clinical symptoms, but they do follow some basic patterns. The severity of pain and symptoms is related to the jellyfish species, the number of stinging cells (nematocysts) that are triggered, and the potency of the venom that is absorbed by the victim.11-13 Most stings are minor, and patients experience immediate localized pain with serpiginous raised erythematous or urticarial lesions following the distribution of tentacle contact; these lesions have been described as tentaclelike and resembling a string of beads (Figure 3).12 Pain usually lasts a couple hours, while the skin lesions can last hours to days and can even recur years later. This pattern fits that of the well-known hydrozoans P physalis and Physalia utriculus (bluebottle), which are endemic to the Atlantic and Indo-Pacific Oceans, respectively. The scyphozoan jellyfish causing similar presentations include Pelagia noctiluca (Mauve stinger), Aurelia aurita (Moon jellyfish), and Cyanea species. The cubozoan Chironex fleckeri (Australian box jellyfish or sea wasp) also causes tentaclelike stings but is widely considered the most dangerous jellyfish, as its venom is known to cause cardiac or respiratory arrest.4,11 More than 100 fatalities have been reported following severe envenomations from C fleckeri in Australian and Indo-Pacific waters.6
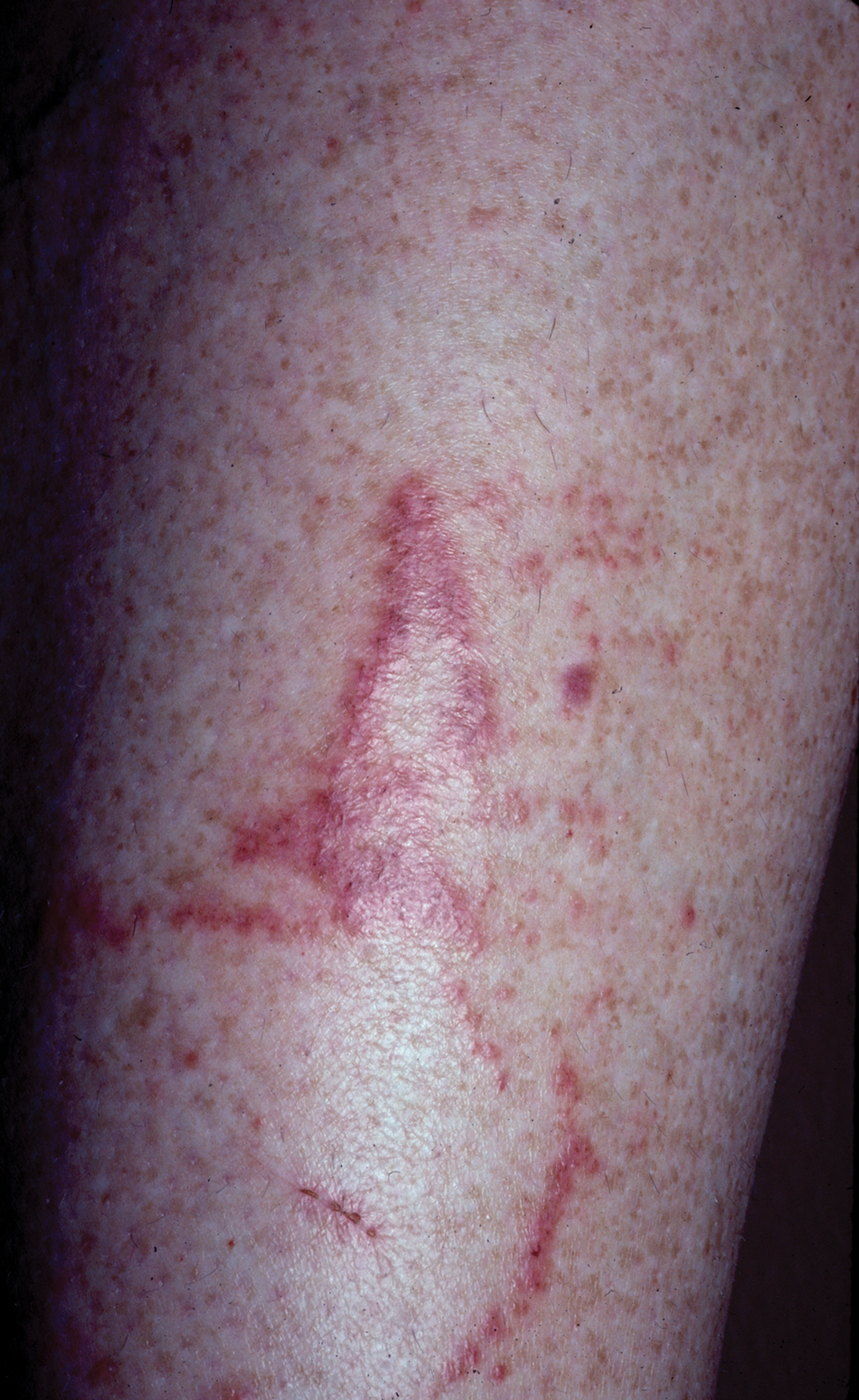
Stings from another box jellyfish species, Carukia barnesi, cause a unique presentation known as Irukandji syndrome. Carukia barnesi is a small box jellyfish with a bell measuring roughly 2 cm in diameter. It has nematocysts on both its bell and tentacles. It inhabits deeper waters and typically stings divers but also can wash ashore and injure beach tourists. Although Irukandji syndrome usually is associated with C barnesi, which is endemic to Northern Australian beaches, other jellyfish species including P physalis rarely have been linked to this potentially fatal syndrome.6,11 Unlike the immediate cutaneous and systemic findings described in C fleckeri encounters, symptoms of Irukandji-like stings can be delayed by up to 30 minutes. Patients may present with severe generalized pain (lower back, chest, headache), signs of excess catecholamine release (tachycardia, hypertension, anxiety, diaphoresis, agitation), or cardiopulmonary decompensation (arrhythmia, cardiac arrest, pulmonary edema).6,11,14.15 Anaphylactic reactions also have been reported in those sensitized by prior stings.16
Management
Prevention of drowning is key in all marine injuries. Rescuers should remove the individual from the water, establish the ABCs—airway, breathing, and circulation—and seek acute medical attention. If immediate resuscitation is not required, douse the wound as soon as possible with a solution that halts further nematocyst discharge, which may contain alcohol, vinegar, or bicarbonate, depending on the prevalent species. General guidance is available to providers through evidence-based, point-of-care databases including UpToDate and DynaMed, as well as through the American Heart Association (AHA) or a country’s equivalent council on emergency care if residing outside the United States. Pressure immobilization bandages as a means of decreasing venom redistribution is no longer recommended by the AHA because animal studies have shown increased nematocyst discharge after pressure application.17 As such, touching or applying pressure to the affected area is not recommended until after a proper rinse solution has been applied. Tentacles may be removed mechanically with gloved hands or sand and seawater with minimal compression or agitation.
When acetic acid is appropriate, such as for cubozoan stings, commercially available vinegar (5% acetic acid in the United States) is preferred.16,17 Tap water can cause discharge of nematocysts, and seawater is preferred when no other solution is available.18 Most marine venoms are heat labile. Immersion in hot water can produce pain relief, but ice can be just as efficacious and is preferred by some patients. Prior reports of patients stung by Physalia species demonstrated greater pain relief with hot water immersion compared to ice pack application.18,19
In the setting of anaphylaxis, patients should receive epinephrine and be transported to a hospital with appropriate hemodynamic monitoring and supportive care. If the species of jellyfish has been identified, species-specific antivenin also may be available in certain regions (eg, C fleckeri antivenin manufactured in Australia), but it is unclear if it improves outcomes when compared with supportive care alone.6,16
Conclusion
Following jellyfish stings, most skin lesions will spontaneously resolve. Patients likely will present days to weeks following the inciting event with mild cutaneous symptoms that are amenable to topical corticosteroids. Recurrent dermatitis following a jellyfish sting is uncommon and is thought to be due to an immunologic mechanism consistent with type IV hypersensitivity reactions. Patients may require multiple courses of treatment before complete resolution.20
Patient education regarding marine envenomation and mechanical barriers such as wetsuits or stinger suits can reduce the risk for injury from jellyfish stings. Sting-inhibiting lotions also are commercially available, though more research is needed.21 Many beaches that are known to harbor the dangerous box jellyfish provide stinger nets to direct travelers to safer waters. Complete avoidance during jellyfish season is recommended in highly endemic areas.1
- Cegolon L, Heymann WC, Lange JH, et al. Jellyfish stings and their management: a review. Mar Drugs. 2013;11:523-550.
- Hornbeak KB, Auerbach PS. Marine envenomation. Emerg Med Clin North Am. 2017;35:321-337.
- Ward NT, Darracq MA, Tomaszewski C, et al. Evidence-based treatment of jellyfish stings in North America and Hawaii. Ann Emerg Med. 2012;60:399-414.
- Burnett JW, Calton GJ, Burnett HW. Jellyfish envenomation syndromes. J Am Acad Dermatol. 1986;14:100-106.
- Brotz L, Cheung WWL, Kleisner K, et al. Increasing jellyfish populations: trends in large marine ecosystems. Hydrobiologia. 2012;690:3-20.
- Ottuso PT. Aquatic antagonists: Cubozoan jellyfish (Chironex fleckeri and Carukia barnesi). Cutis. 2010;85:133-136.
- Lakkis NA, Maalouf GJ, Mahmassani DM. Jellyfish stings: a practical approach. Wilderness Environ Med. 2015;26:422-429.
- Li L, McGee RG, Isbister G, et al. Interventions for the symptoms and signs resulting from jellyfish stings. Cochrane Database Syst Rev. 2013;12:CD009688.
- Kaffenberger BH, Shetlar D, Norton SA, et al. The effect of climate change on skin disease in North America. J Am Acad Dermatol. 2017;76:140-147.
- Purcell JE, Uye S, Lo W. Anthropogenic causes of jellyfish blooms and their direct consequences for humans: a review. Marine Ecology Progress Series. 2007;350:153-174.
- Berling I, Isbister G. Marine envenomations. Aust Fam Physician. 2015;44:28-32.
- Tibballs J, Yanagihara AA, Turner HC, et al. Immunological and toxinological responses to jellyfish stings. Inflamm Allergy Drug Targets. 2011;10:438-446.
- Tibballs J. Australian venomous jellyfish, envenomation syndromes, toxins and therapy. Toxicon. 2006;48:830-859.
- Stein MR, Marracini JV, Rothschild NE, et al. Fatal Portuguese man-o’-war (Physalia physalis) envenomation. Ann Emerg Med. 1989;18:312-315.
- Burnett JW, Gable WD. A fatal jellyfish envenomation by the Portuguese man-o’war. Toxicon. 1989;27:823-824.
- Warrell DA. Venomous bites, stings, and poisoning: an update. Infect Dis Clin North Am. 2019;33:17-38.
- Neumar RW, Shuster M, Callaway CW, et al. Part 1: executive summary: 2015 American Heart Association guidelines update for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation. 2015;132(18 suppl 2):S315-S367.
- Wilcox CL, Headlam JL, Doyle TK, et al. Assessing the efficacy of first-aid measures in Physalia sp. envenomation, using solution- and blood agarose-based models. Toxins (Basel). 2017;9:149.
- Wilcox CL, Yanagihara AA. Heated debates: hot-water immersion or ice packs as first aid for cnidarian envenomations? Toxins (Basel). 2016;8:97.
- Loredana Asztalos M, Rubin AI, Elenitsas R, et al. Recurrent dermatitis and dermal hypersensitivity following a jellyfish sting: a case report and review of literature. Pediatr Dermatol. 2014;31:217-219.
- Boulware DR. A randomized, controlled field trial for the prevention of jellyfish stings with a topical sting inhibitor. J Travel Med. 2006;13:166-171.
Jellyfish stings are one of the most common marine injuries, with an estimated 150 million stings occurring annually worldwide.1 Most jellyfish stings result in painful localized skin reactions that are self-limited and can be treated with conservative measures including hot water immersion and topical anesthetics. Life-threatening systemic reactions (eg, anaphylaxis, Irukandji syndrome) can occur with some species.2-4 Mainstream media reports do not reflect the true incidence and variability of jellyfish-related injuries that are commonly encountered in the clinic.3
Characteristics of Jellyfish
There are roughly 10,000 known species of jellyfish, with approximately 100 of them posing danger to humans.5 Jellyfish belong to the phylum Cnidaria, which is comprised of 5 classes of both free-floating and sessile animals: Staurozoa (stauromedusae), Hydrozoa (hydroids, fire corals, and Portuguese man-of-war), Scyphozoa (true jellyfish), Anthozoa (corals and sea anemones), and Cubozoa (box jellyfish and Irukandji jellyfish).1,2,6 Jellyfish typically have several tentacles suspended from a free-floating gelatinous body or bell; these tentacles are covered with thousands of cells unique to Cnidaria called nematocytes or cnidocytes containing specialized stinging organelles known as nematocysts. When triggered by physical (eg, human or foreign-body contact) or chemical stimuli, each nematocyst ejects a hollow filament or barb externally, releasing venom into the victim.7,8

The scyphozoan, hydrozoan, and cubozoan life cycles generally consist of a bottom-dwelling, sessile polyp form that produces multiple free-swimming ephyrae through an asexual reproductive process called strobilation. These ephyrae grow into the fully mature medusae, recognizable as jellyfish (Figure 1).5 Additionally, jellyfish populations experience cycles of temporal and spatial population abundance and crashes known as jellyfish blooms. In 2017, Kaffenberger et al9 reviewed the shifting landscape of skin diseases in North America attributable to major changes in climate and weather patterns, including the rise in jellyfish blooms and envenomation outbreaks worldwide (eg, Physalia physalis [Portuguese man-of-war][Figure 2] along the southeastern US coastline, Porpita pacifica off Japanese beaches). Some research suggests jellyfish surges relate to climate change and human interactions with jellyfish habitats by way of eutrophication and fishing (removing predators of jellyfish).9,10

Clinical Presentation
Jellyfish injuries can vary greatly in clinical symptoms, but they do follow some basic patterns. The severity of pain and symptoms is related to the jellyfish species, the number of stinging cells (nematocysts) that are triggered, and the potency of the venom that is absorbed by the victim.11-13 Most stings are minor, and patients experience immediate localized pain with serpiginous raised erythematous or urticarial lesions following the distribution of tentacle contact; these lesions have been described as tentaclelike and resembling a string of beads (Figure 3).12 Pain usually lasts a couple hours, while the skin lesions can last hours to days and can even recur years later. This pattern fits that of the well-known hydrozoans P physalis and Physalia utriculus (bluebottle), which are endemic to the Atlantic and Indo-Pacific Oceans, respectively. The scyphozoan jellyfish causing similar presentations include Pelagia noctiluca (Mauve stinger), Aurelia aurita (Moon jellyfish), and Cyanea species. The cubozoan Chironex fleckeri (Australian box jellyfish or sea wasp) also causes tentaclelike stings but is widely considered the most dangerous jellyfish, as its venom is known to cause cardiac or respiratory arrest.4,11 More than 100 fatalities have been reported following severe envenomations from C fleckeri in Australian and Indo-Pacific waters.6

Stings from another box jellyfish species, Carukia barnesi, cause a unique presentation known as Irukandji syndrome. Carukia barnesi is a small box jellyfish with a bell measuring roughly 2 cm in diameter. It has nematocysts on both its bell and tentacles. It inhabits deeper waters and typically stings divers but also can wash ashore and injure beach tourists. Although Irukandji syndrome usually is associated with C barnesi, which is endemic to Northern Australian beaches, other jellyfish species including P physalis rarely have been linked to this potentially fatal syndrome.6,11 Unlike the immediate cutaneous and systemic findings described in C fleckeri encounters, symptoms of Irukandji-like stings can be delayed by up to 30 minutes. Patients may present with severe generalized pain (lower back, chest, headache), signs of excess catecholamine release (tachycardia, hypertension, anxiety, diaphoresis, agitation), or cardiopulmonary decompensation (arrhythmia, cardiac arrest, pulmonary edema).6,11,14.15 Anaphylactic reactions also have been reported in those sensitized by prior stings.16
Management
Prevention of drowning is key in all marine injuries. Rescuers should remove the individual from the water, establish the ABCs—airway, breathing, and circulation—and seek acute medical attention. If immediate resuscitation is not required, douse the wound as soon as possible with a solution that halts further nematocyst discharge, which may contain alcohol, vinegar, or bicarbonate, depending on the prevalent species. General guidance is available to providers through evidence-based, point-of-care databases including UpToDate and DynaMed, as well as through the American Heart Association (AHA) or a country’s equivalent council on emergency care if residing outside the United States. Pressure immobilization bandages as a means of decreasing venom redistribution is no longer recommended by the AHA because animal studies have shown increased nematocyst discharge after pressure application.17 As such, touching or applying pressure to the affected area is not recommended until after a proper rinse solution has been applied. Tentacles may be removed mechanically with gloved hands or sand and seawater with minimal compression or agitation.
When acetic acid is appropriate, such as for cubozoan stings, commercially available vinegar (5% acetic acid in the United States) is preferred.16,17 Tap water can cause discharge of nematocysts, and seawater is preferred when no other solution is available.18 Most marine venoms are heat labile. Immersion in hot water can produce pain relief, but ice can be just as efficacious and is preferred by some patients. Prior reports of patients stung by Physalia species demonstrated greater pain relief with hot water immersion compared to ice pack application.18,19
In the setting of anaphylaxis, patients should receive epinephrine and be transported to a hospital with appropriate hemodynamic monitoring and supportive care. If the species of jellyfish has been identified, species-specific antivenin also may be available in certain regions (eg, C fleckeri antivenin manufactured in Australia), but it is unclear if it improves outcomes when compared with supportive care alone.6,16
Conclusion
Following jellyfish stings, most skin lesions will spontaneously resolve. Patients likely will present days to weeks following the inciting event with mild cutaneous symptoms that are amenable to topical corticosteroids. Recurrent dermatitis following a jellyfish sting is uncommon and is thought to be due to an immunologic mechanism consistent with type IV hypersensitivity reactions. Patients may require multiple courses of treatment before complete resolution.20
Patient education regarding marine envenomation and mechanical barriers such as wetsuits or stinger suits can reduce the risk for injury from jellyfish stings. Sting-inhibiting lotions also are commercially available, though more research is needed.21 Many beaches that are known to harbor the dangerous box jellyfish provide stinger nets to direct travelers to safer waters. Complete avoidance during jellyfish season is recommended in highly endemic areas.1
Jellyfish stings are one of the most common marine injuries, with an estimated 150 million stings occurring annually worldwide.1 Most jellyfish stings result in painful localized skin reactions that are self-limited and can be treated with conservative measures including hot water immersion and topical anesthetics. Life-threatening systemic reactions (eg, anaphylaxis, Irukandji syndrome) can occur with some species.2-4 Mainstream media reports do not reflect the true incidence and variability of jellyfish-related injuries that are commonly encountered in the clinic.3
Characteristics of Jellyfish
There are roughly 10,000 known species of jellyfish, with approximately 100 of them posing danger to humans.5 Jellyfish belong to the phylum Cnidaria, which is comprised of 5 classes of both free-floating and sessile animals: Staurozoa (stauromedusae), Hydrozoa (hydroids, fire corals, and Portuguese man-of-war), Scyphozoa (true jellyfish), Anthozoa (corals and sea anemones), and Cubozoa (box jellyfish and Irukandji jellyfish).1,2,6 Jellyfish typically have several tentacles suspended from a free-floating gelatinous body or bell; these tentacles are covered with thousands of cells unique to Cnidaria called nematocytes or cnidocytes containing specialized stinging organelles known as nematocysts. When triggered by physical (eg, human or foreign-body contact) or chemical stimuli, each nematocyst ejects a hollow filament or barb externally, releasing venom into the victim.7,8

The scyphozoan, hydrozoan, and cubozoan life cycles generally consist of a bottom-dwelling, sessile polyp form that produces multiple free-swimming ephyrae through an asexual reproductive process called strobilation. These ephyrae grow into the fully mature medusae, recognizable as jellyfish (Figure 1).5 Additionally, jellyfish populations experience cycles of temporal and spatial population abundance and crashes known as jellyfish blooms. In 2017, Kaffenberger et al9 reviewed the shifting landscape of skin diseases in North America attributable to major changes in climate and weather patterns, including the rise in jellyfish blooms and envenomation outbreaks worldwide (eg, Physalia physalis [Portuguese man-of-war][Figure 2] along the southeastern US coastline, Porpita pacifica off Japanese beaches). Some research suggests jellyfish surges relate to climate change and human interactions with jellyfish habitats by way of eutrophication and fishing (removing predators of jellyfish).9,10

Clinical Presentation
Jellyfish injuries can vary greatly in clinical symptoms, but they do follow some basic patterns. The severity of pain and symptoms is related to the jellyfish species, the number of stinging cells (nematocysts) that are triggered, and the potency of the venom that is absorbed by the victim.11-13 Most stings are minor, and patients experience immediate localized pain with serpiginous raised erythematous or urticarial lesions following the distribution of tentacle contact; these lesions have been described as tentaclelike and resembling a string of beads (Figure 3).12 Pain usually lasts a couple hours, while the skin lesions can last hours to days and can even recur years later. This pattern fits that of the well-known hydrozoans P physalis and Physalia utriculus (bluebottle), which are endemic to the Atlantic and Indo-Pacific Oceans, respectively. The scyphozoan jellyfish causing similar presentations include Pelagia noctiluca (Mauve stinger), Aurelia aurita (Moon jellyfish), and Cyanea species. The cubozoan Chironex fleckeri (Australian box jellyfish or sea wasp) also causes tentaclelike stings but is widely considered the most dangerous jellyfish, as its venom is known to cause cardiac or respiratory arrest.4,11 More than 100 fatalities have been reported following severe envenomations from C fleckeri in Australian and Indo-Pacific waters.6

Stings from another box jellyfish species, Carukia barnesi, cause a unique presentation known as Irukandji syndrome. Carukia barnesi is a small box jellyfish with a bell measuring roughly 2 cm in diameter. It has nematocysts on both its bell and tentacles. It inhabits deeper waters and typically stings divers but also can wash ashore and injure beach tourists. Although Irukandji syndrome usually is associated with C barnesi, which is endemic to Northern Australian beaches, other jellyfish species including P physalis rarely have been linked to this potentially fatal syndrome.6,11 Unlike the immediate cutaneous and systemic findings described in C fleckeri encounters, symptoms of Irukandji-like stings can be delayed by up to 30 minutes. Patients may present with severe generalized pain (lower back, chest, headache), signs of excess catecholamine release (tachycardia, hypertension, anxiety, diaphoresis, agitation), or cardiopulmonary decompensation (arrhythmia, cardiac arrest, pulmonary edema).6,11,14.15 Anaphylactic reactions also have been reported in those sensitized by prior stings.16
Management
Prevention of drowning is key in all marine injuries. Rescuers should remove the individual from the water, establish the ABCs—airway, breathing, and circulation—and seek acute medical attention. If immediate resuscitation is not required, douse the wound as soon as possible with a solution that halts further nematocyst discharge, which may contain alcohol, vinegar, or bicarbonate, depending on the prevalent species. General guidance is available to providers through evidence-based, point-of-care databases including UpToDate and DynaMed, as well as through the American Heart Association (AHA) or a country’s equivalent council on emergency care if residing outside the United States. Pressure immobilization bandages as a means of decreasing venom redistribution is no longer recommended by the AHA because animal studies have shown increased nematocyst discharge after pressure application.17 As such, touching or applying pressure to the affected area is not recommended until after a proper rinse solution has been applied. Tentacles may be removed mechanically with gloved hands or sand and seawater with minimal compression or agitation.
When acetic acid is appropriate, such as for cubozoan stings, commercially available vinegar (5% acetic acid in the United States) is preferred.16,17 Tap water can cause discharge of nematocysts, and seawater is preferred when no other solution is available.18 Most marine venoms are heat labile. Immersion in hot water can produce pain relief, but ice can be just as efficacious and is preferred by some patients. Prior reports of patients stung by Physalia species demonstrated greater pain relief with hot water immersion compared to ice pack application.18,19
In the setting of anaphylaxis, patients should receive epinephrine and be transported to a hospital with appropriate hemodynamic monitoring and supportive care. If the species of jellyfish has been identified, species-specific antivenin also may be available in certain regions (eg, C fleckeri antivenin manufactured in Australia), but it is unclear if it improves outcomes when compared with supportive care alone.6,16
Conclusion
Following jellyfish stings, most skin lesions will spontaneously resolve. Patients likely will present days to weeks following the inciting event with mild cutaneous symptoms that are amenable to topical corticosteroids. Recurrent dermatitis following a jellyfish sting is uncommon and is thought to be due to an immunologic mechanism consistent with type IV hypersensitivity reactions. Patients may require multiple courses of treatment before complete resolution.20
Patient education regarding marine envenomation and mechanical barriers such as wetsuits or stinger suits can reduce the risk for injury from jellyfish stings. Sting-inhibiting lotions also are commercially available, though more research is needed.21 Many beaches that are known to harbor the dangerous box jellyfish provide stinger nets to direct travelers to safer waters. Complete avoidance during jellyfish season is recommended in highly endemic areas.1
- Cegolon L, Heymann WC, Lange JH, et al. Jellyfish stings and their management: a review. Mar Drugs. 2013;11:523-550.
- Hornbeak KB, Auerbach PS. Marine envenomation. Emerg Med Clin North Am. 2017;35:321-337.
- Ward NT, Darracq MA, Tomaszewski C, et al. Evidence-based treatment of jellyfish stings in North America and Hawaii. Ann Emerg Med. 2012;60:399-414.
- Burnett JW, Calton GJ, Burnett HW. Jellyfish envenomation syndromes. J Am Acad Dermatol. 1986;14:100-106.
- Brotz L, Cheung WWL, Kleisner K, et al. Increasing jellyfish populations: trends in large marine ecosystems. Hydrobiologia. 2012;690:3-20.
- Ottuso PT. Aquatic antagonists: Cubozoan jellyfish (Chironex fleckeri and Carukia barnesi). Cutis. 2010;85:133-136.
- Lakkis NA, Maalouf GJ, Mahmassani DM. Jellyfish stings: a practical approach. Wilderness Environ Med. 2015;26:422-429.
- Li L, McGee RG, Isbister G, et al. Interventions for the symptoms and signs resulting from jellyfish stings. Cochrane Database Syst Rev. 2013;12:CD009688.
- Kaffenberger BH, Shetlar D, Norton SA, et al. The effect of climate change on skin disease in North America. J Am Acad Dermatol. 2017;76:140-147.
- Purcell JE, Uye S, Lo W. Anthropogenic causes of jellyfish blooms and their direct consequences for humans: a review. Marine Ecology Progress Series. 2007;350:153-174.
- Berling I, Isbister G. Marine envenomations. Aust Fam Physician. 2015;44:28-32.
- Tibballs J, Yanagihara AA, Turner HC, et al. Immunological and toxinological responses to jellyfish stings. Inflamm Allergy Drug Targets. 2011;10:438-446.
- Tibballs J. Australian venomous jellyfish, envenomation syndromes, toxins and therapy. Toxicon. 2006;48:830-859.
- Stein MR, Marracini JV, Rothschild NE, et al. Fatal Portuguese man-o’-war (Physalia physalis) envenomation. Ann Emerg Med. 1989;18:312-315.
- Burnett JW, Gable WD. A fatal jellyfish envenomation by the Portuguese man-o’war. Toxicon. 1989;27:823-824.
- Warrell DA. Venomous bites, stings, and poisoning: an update. Infect Dis Clin North Am. 2019;33:17-38.
- Neumar RW, Shuster M, Callaway CW, et al. Part 1: executive summary: 2015 American Heart Association guidelines update for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation. 2015;132(18 suppl 2):S315-S367.
- Wilcox CL, Headlam JL, Doyle TK, et al. Assessing the efficacy of first-aid measures in Physalia sp. envenomation, using solution- and blood agarose-based models. Toxins (Basel). 2017;9:149.
- Wilcox CL, Yanagihara AA. Heated debates: hot-water immersion or ice packs as first aid for cnidarian envenomations? Toxins (Basel). 2016;8:97.
- Loredana Asztalos M, Rubin AI, Elenitsas R, et al. Recurrent dermatitis and dermal hypersensitivity following a jellyfish sting: a case report and review of literature. Pediatr Dermatol. 2014;31:217-219.
- Boulware DR. A randomized, controlled field trial for the prevention of jellyfish stings with a topical sting inhibitor. J Travel Med. 2006;13:166-171.
- Cegolon L, Heymann WC, Lange JH, et al. Jellyfish stings and their management: a review. Mar Drugs. 2013;11:523-550.
- Hornbeak KB, Auerbach PS. Marine envenomation. Emerg Med Clin North Am. 2017;35:321-337.
- Ward NT, Darracq MA, Tomaszewski C, et al. Evidence-based treatment of jellyfish stings in North America and Hawaii. Ann Emerg Med. 2012;60:399-414.
- Burnett JW, Calton GJ, Burnett HW. Jellyfish envenomation syndromes. J Am Acad Dermatol. 1986;14:100-106.
- Brotz L, Cheung WWL, Kleisner K, et al. Increasing jellyfish populations: trends in large marine ecosystems. Hydrobiologia. 2012;690:3-20.
- Ottuso PT. Aquatic antagonists: Cubozoan jellyfish (Chironex fleckeri and Carukia barnesi). Cutis. 2010;85:133-136.
- Lakkis NA, Maalouf GJ, Mahmassani DM. Jellyfish stings: a practical approach. Wilderness Environ Med. 2015;26:422-429.
- Li L, McGee RG, Isbister G, et al. Interventions for the symptoms and signs resulting from jellyfish stings. Cochrane Database Syst Rev. 2013;12:CD009688.
- Kaffenberger BH, Shetlar D, Norton SA, et al. The effect of climate change on skin disease in North America. J Am Acad Dermatol. 2017;76:140-147.
- Purcell JE, Uye S, Lo W. Anthropogenic causes of jellyfish blooms and their direct consequences for humans: a review. Marine Ecology Progress Series. 2007;350:153-174.
- Berling I, Isbister G. Marine envenomations. Aust Fam Physician. 2015;44:28-32.
- Tibballs J, Yanagihara AA, Turner HC, et al. Immunological and toxinological responses to jellyfish stings. Inflamm Allergy Drug Targets. 2011;10:438-446.
- Tibballs J. Australian venomous jellyfish, envenomation syndromes, toxins and therapy. Toxicon. 2006;48:830-859.
- Stein MR, Marracini JV, Rothschild NE, et al. Fatal Portuguese man-o’-war (Physalia physalis) envenomation. Ann Emerg Med. 1989;18:312-315.
- Burnett JW, Gable WD. A fatal jellyfish envenomation by the Portuguese man-o’war. Toxicon. 1989;27:823-824.
- Warrell DA. Venomous bites, stings, and poisoning: an update. Infect Dis Clin North Am. 2019;33:17-38.
- Neumar RW, Shuster M, Callaway CW, et al. Part 1: executive summary: 2015 American Heart Association guidelines update for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation. 2015;132(18 suppl 2):S315-S367.
- Wilcox CL, Headlam JL, Doyle TK, et al. Assessing the efficacy of first-aid measures in Physalia sp. envenomation, using solution- and blood agarose-based models. Toxins (Basel). 2017;9:149.
- Wilcox CL, Yanagihara AA. Heated debates: hot-water immersion or ice packs as first aid for cnidarian envenomations? Toxins (Basel). 2016;8:97.
- Loredana Asztalos M, Rubin AI, Elenitsas R, et al. Recurrent dermatitis and dermal hypersensitivity following a jellyfish sting: a case report and review of literature. Pediatr Dermatol. 2014;31:217-219.
- Boulware DR. A randomized, controlled field trial for the prevention of jellyfish stings with a topical sting inhibitor. J Travel Med. 2006;13:166-171.
Practice Points
- Jellyfish stings occur an estimated 150 million times annually worldwide, with numbers expected to rise due to climate change.
- Most stings result in painful self-limited cutaneous symptoms that resolve spontaneously. Box jellyfish (Cubozoa) stings carry a greater risk for causing severe systemic reactions.
- Treatment of skin reactions includes removal of tentacles and hot water immersion. Vinegar dousing for at least 30 seconds is recommended for box jellyfish stings. Supportive care and monitoring for cardiovascular collapse are key. The role of antivenin is uncertain.
What’s Eating You? Caterpillars
Causes of Lepidopterism
Caterpillars are wormlike organisms that serve as the larval stage of moths and butterflies, which belong to the order Lepidoptera. There are almost 165,000 discovered species, with 13,000 found in the United States.1,2 Roughly 150 species are known to have the potential to cause an adverse reaction in humans, with 50 of these in the United States.1Lepidopterism describes systemic and cutaneous reactions to moths, butterflies, and caterpillars; erucism describes strictly cutaneous reactions.1
Although the rate of lepidopterism is thought to be underreported because it often is self-limited and of a mild nature, a review found caterpillars to be the cause of roughly 2.2% of reported bites and stings annually.2 Cases increase in number with seasonal increases in caterpillars, which vary by region and species. For example, the Megalopyge opercularis (southern flannel moth) caterpillar was noted to have 2 peaks in a Texas-based study: 12% of reported stings occurred in July; 59% from October through November.3 In general, the likelihood of exposure increases during warmer months, and exposure is more common in people who work outdoors in a rural area or in a suburban area where there are many caterpillar-infested trees.4
Most cases of lepidopterism are caused by caterpillars, not by adult butterflies and moths, because the former have many tubular, or porous, hairlike structures called setae that are embedded in the integument. Setae were once thought to be connected to poison-secreting glandular cells, but current belief is that venomous caterpillars lack specialized gland cells and instead produce venom through secretory epithelial cells located above the integument.1 Venom accumulates in the hemolymph and is stored in the setae or other types of bristles, such as scoli (wartlike bumps that bear setae) or spines.5 With a large amount of chitin, bristles have a tendency to fracture and release venom upon contact.1 It is thought that some species of caterpillars formulate venom by ingesting toxins or toxin precursors from plants; for example, the tiger moth (family Arctiidae) is known to produce venom containing biogenic amines, pyrrolizidine, alkaloids, and cardiac glycosides obtained through food sources.5
Even if a caterpillar does not produce venom, its setae might embed into skin or mucous membranes and cause an adverse irritant reaction.1 Setae also might dislodge and be transported in the air to embed in objects—some remaining stable in the environment for longer than a year.2 In contrast to setae, spines are permanently fixed into the integument; for that reason, only direct contact with the caterpillar can result in an adverse reaction. Although it is mostly caterpillars that contain setae and spines, certain species of moths also might contain these structures or might acquire them as they emerge from the cocoon, which often contains incorporated setae.2
Reactions in Humans
Lepidopterism encompasses 3 principal reactions in humans: sting reaction, hypersensitivity reaction, and lonomism (a hemorrhagic diathesis produced by Lonomia caterpillars). The type and severity of the reaction depends on (1) the species of caterpillar or moth and (2) the individual patient.2 There are approximately 12 families of caterpillars, mainly of the moth variety, that can cause an adverse reaction in humans.1 Tables 1 and 2 list examples of species that cause each type of reaction.6
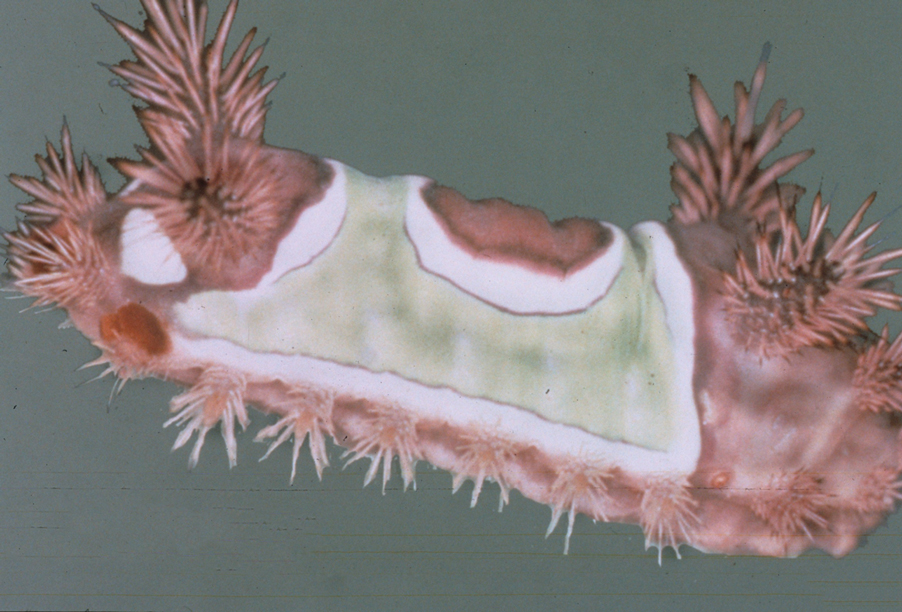
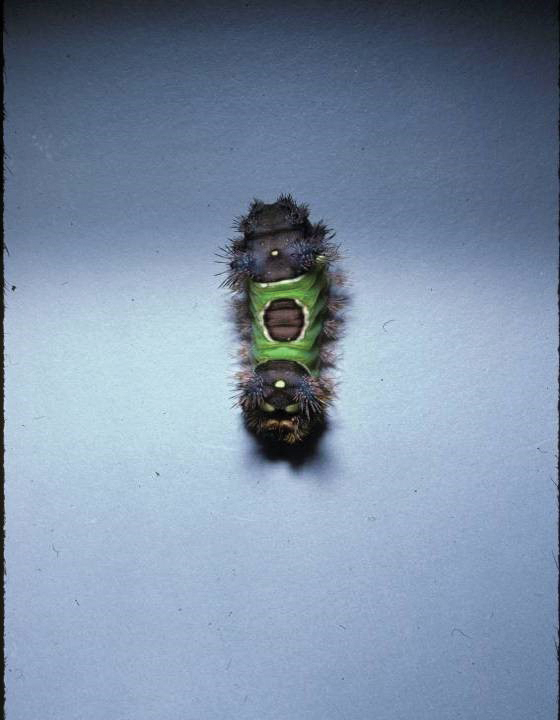
Chemicals and toxins contained in the poison of setae and spines vary by species of caterpillar. Numerous kinds have been isolated from different venoms,1,2 including several peptides, histamine, histamine-releasing substances, acetylcholine, phospholipase A, hyaluronidase, formic acid, proteins with trypsinlike activity, serine proteases such as kallikrein, and other enzymes with vasodegenerative and fibrinolytic properties
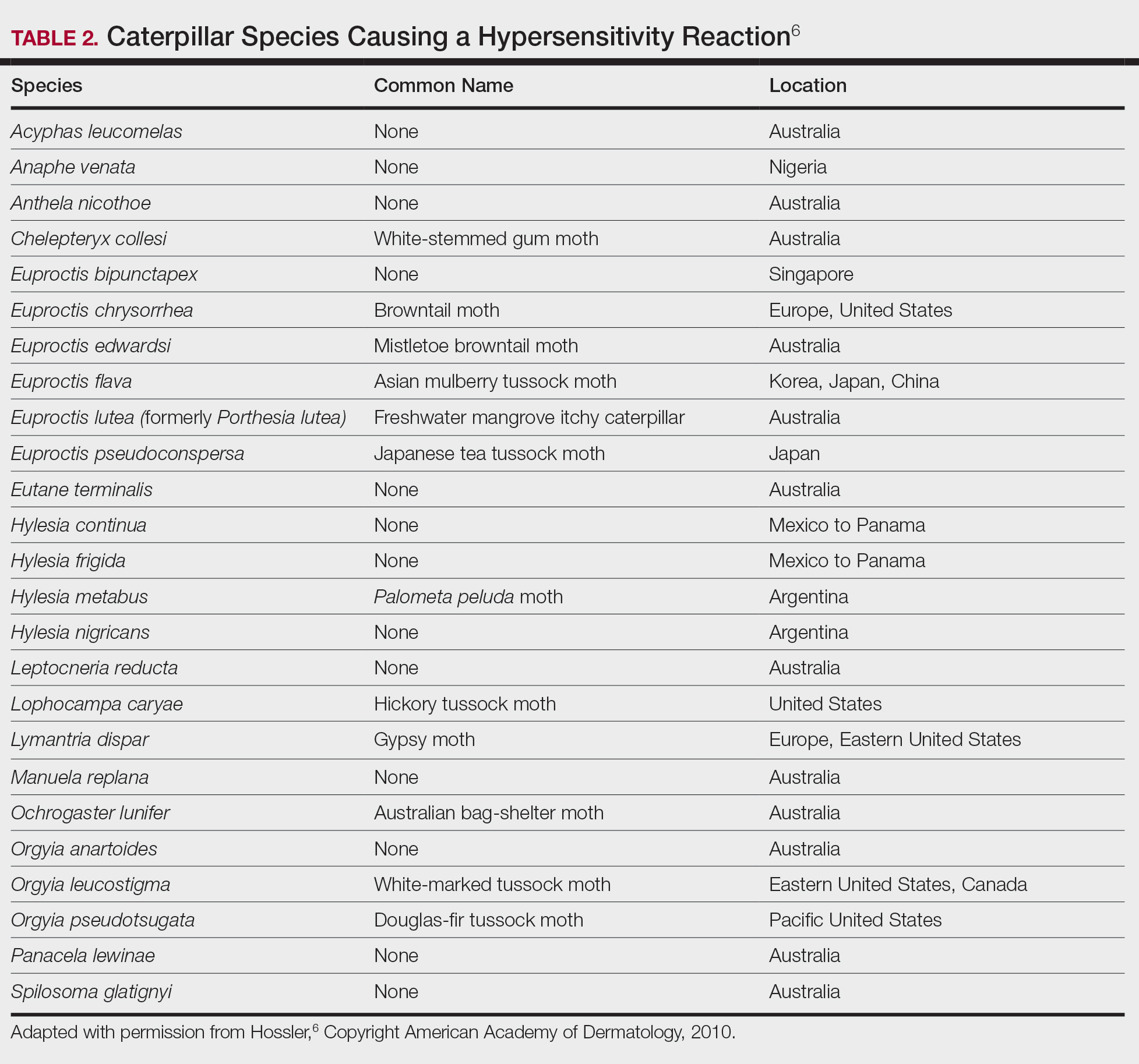
Stings: An Immediate Adverse Reaction—Depending on the venom, a sting might result in mild to severe burning pain, accompanied by welts, vesicles, and red papules or plaques.2 Figure 1 demonstrates a particularly mild sting from a caterpillar of the family Automeris, examples of which are seen in Figures 2 and 3 and eFigure 1. Components of the venom determine the mechanism of the sting and the pain that accompanies it. For example, a recent study demonstrated that the venom of the Latoia consocia caterpillar induces pain through the ion-channel receptor known as transient receptor potential vanilloid 1, which integrates and sends painful stimuli from the peripheral nervous system to the central nervous system.7 It is thought that a variety of ion channels are targets of the venom of caterpillars.
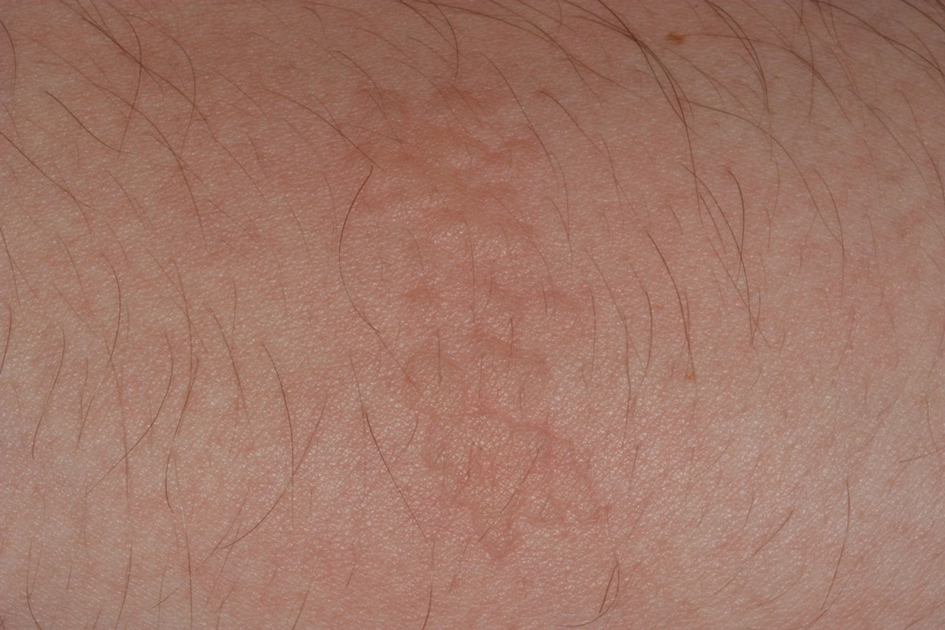



One of the most characteristic sting patterns is that of the caterpillar of family Megalopygidae (flannel moth)(eFigures 2 and 3). The stings of these caterpillars create a unique tram-track pattern of hemorrhagic macules or papules (Figure 4).4 A study found that 90% of reported M opercularis envenomations consist primarily of cutaneous symptoms, with 84% of those symptoms being irritation or pain; 45% a puncture or wound; 29% erythema; and 15% edema.3 Systemic findings can include headache, fever, adenopathy, nausea, vomiting, abdominal pain, and chest pain.4 Symptoms normally are self-limited, though they can last minutes or hours.


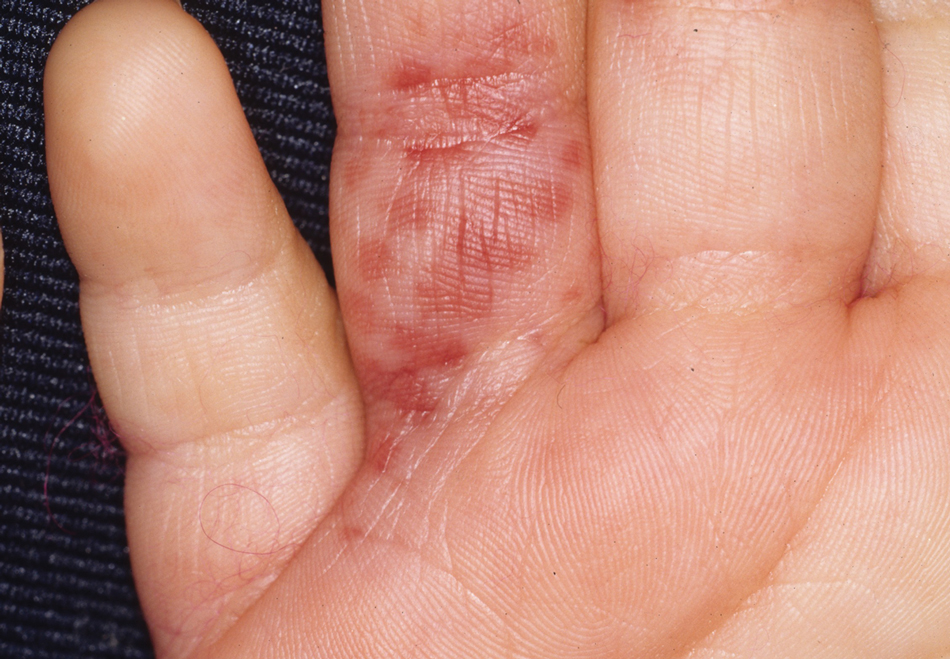
Hypersensitivity Reaction—Studies demonstrate that the symptoms of this reaction are a mixture of type I hypersensitivity, type IV hypersensitivity, and a foreign-body response.2 The specific hypersensitivity reaction depends on the venom and the exposed individual—most commonly including a combination of pruritic papules, urticarial wheals, flares, and dermatitis.2 A reaction that is a result of direct contact with the caterpillar or moth will appear on exposed areas; however, because setae embed in linens and clothing, they might cause a reaction anywhere on the body. Although usually self-limited, a hypersensitivity reaction might develop within minutes and can last for days or weeks.
Stings and hypersensitivity reactions to caterpillars and moths tend to lead to a nonspecific histologic presentation characterized by epidermal edema and a superficial perivascular lymphocytic infiltrate, often with eosinophils.6 After approximately 1 week, a foreign-body response to setae can lead to tuberculoid granulomas accompanied by neutrophils in the dermis and occasionally in subcutaneous tissues (Figures 5 and 6).8 If setae have not yet been removed, they also might be visible in skin scrapings.
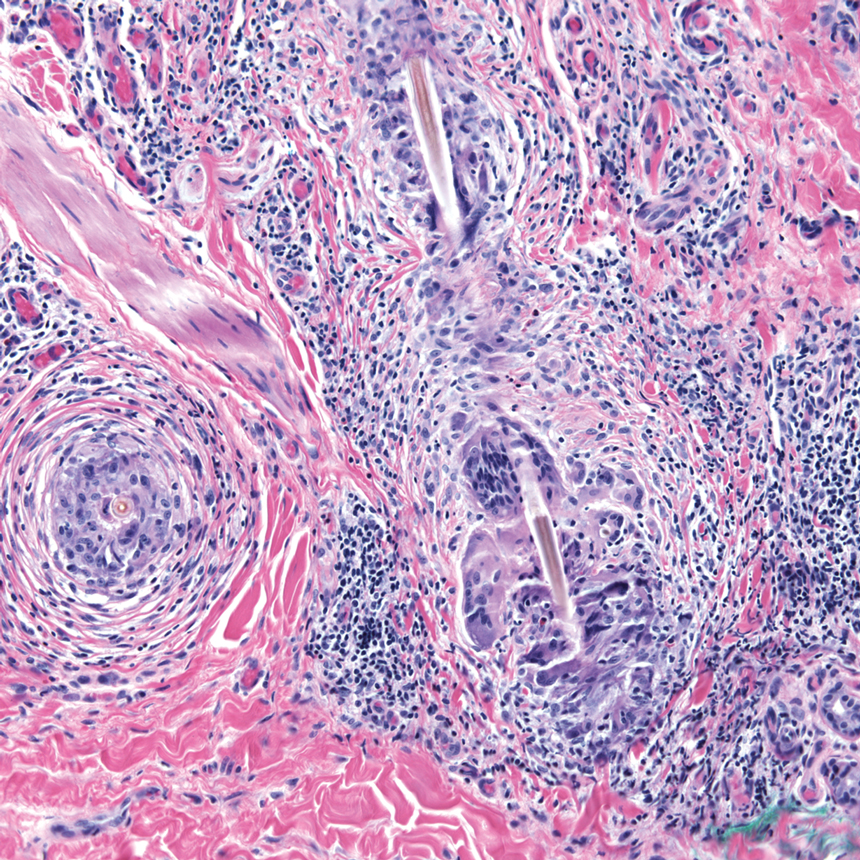
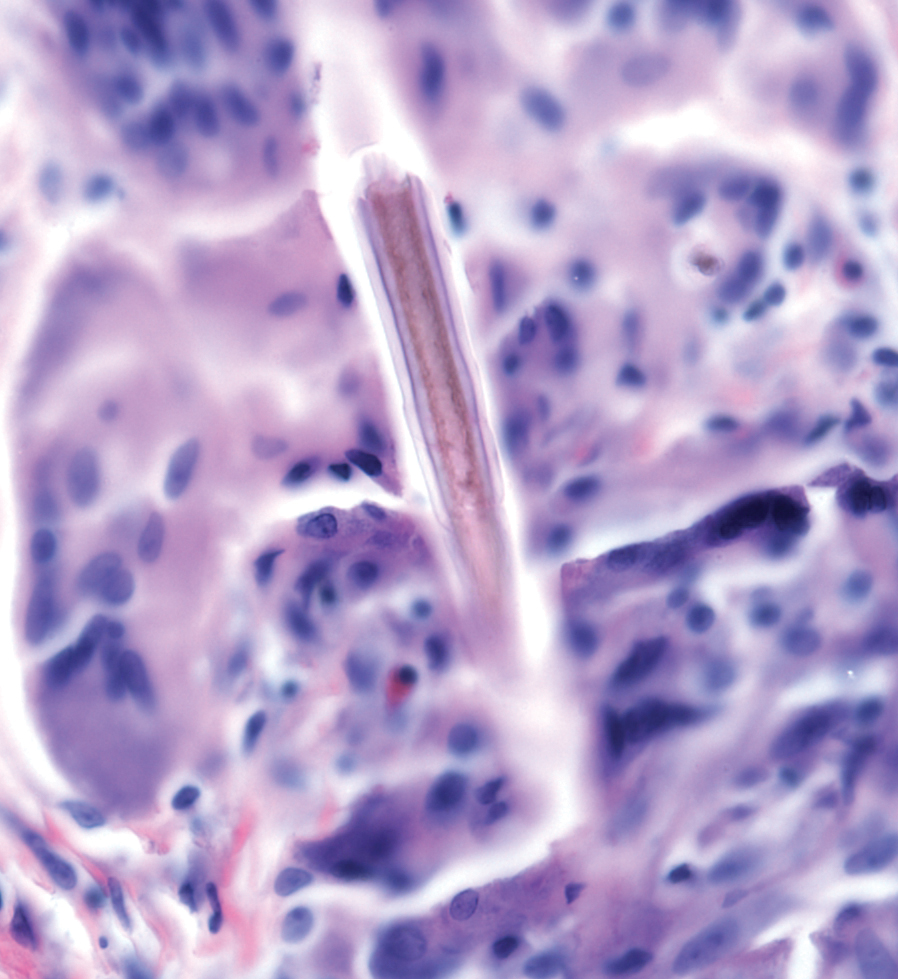
Additional complications can accompany the hypersensitivity reaction to setae or spines. Type I hypersensitivity reactions can lead to severe reactions on second contact due to previously sensitized IgE antibodies. Although the first reaction appears mild, second contact might result in angioedema, wheezing, dyspnea, or anaphylaxis, or a combination of these findings.9 In addition, some patients who come in contact with Dendrolimus caterpillars might develop a condition known as dendrolimiasis, characterized by dermatitis in addition to arthritis or chondritis.6 The arthritis is normally monoarticular and can result in complete destruction of the joint. Pararamose, a condition with a similar presentation, is caused by the Brazilian moth Premolis semirufa.6
Contact of setae or spines with mucous membranes or inhalation of setae also might result in edema, dysphagia, dyspnea, drooling, rhinitis, or conjunctivitis, or a combination of these findings.6 In addition, setae can embed in the eye and cause an inflammatory reaction—ophthalmia nodosa—most commonly caused by caterpillars of the pine processionary moth (Thaumetopoea pityocampa) and characterized by immediate chemosis, which can progress to liquefactive necrosis and hypopyon, later developing into a granulomatous foreign-body response.2,10 The process is thought to be the result of a combination of the thaumetopoein toxin in the setae and an IgE-mediated response to other proteins.10
Due to their harpoon shape and forward-only motion, setae might migrate deeper, potentially even to the optic nerve.11 Because migration might take years and the barbed shape of setae does not always allow removal, some patients require lifetime monitoring with slit-lamp examination.Chronic problems, such as cataracts and persistent posterior uveitis, have been reported.10,11
Lonomism—One of the most serious (though rarest) reactions to caterpillars is lonomism, a condition caused by the caterpillars of Lonomia achelous and Lonomia obliqua moths. These caterpillars have a unique combination of toxins filling their branched spines, which ultimately leads to the same outcome: a hemorrhagic diathesis.
The toxin of L achelous comprises several proteases that degrade fibrin, fibrinogen, and factor XIII while activating prothrombin. In contrast, L obliqua poison causes a hemorrhagic diathesis by promoting a consumptive coagulopathy through enzymes that activate factor X and prothrombin.
With initial contact with either of these Lonomia caterpillars, the patient experiences severe pain accompanied by systemic symptoms, including headache, nausea, and vomiting. Shortly afterward, symptoms of a hemorrhagic diathesis manifest, including bleeding gums, hematuria, bleeding from prior wounds, and epistaxis.5 Serious complications of the hemorrhagic diathesis, such as hemorrhage of major organs, leads to death in 4% of patients.5 A reported case of a patient whose Lonomia caterpillar sting went unrecognized until a week after the accident ended with progression to stage V chronic renal disease.12
Recent research has focused on the specific mechanism of injury caused by Lonomia species. A study found that the venom of L obliqua causes cytoskeleton rearrangement and migration in vascular smooth muscle cells (VSMCs) by inducing formation of reactive oxygen species through activation of nicotinamide adenine dinucleotide phosphate oxidase.13 Thus, the venom directly contributes to the proinflammatory phenotype of endothelial cells seen following envenomation. The same study also demonstrated that elevated reactive oxygen species trigger extracellular signal-regulated kinase pathway activation in VSMCs, leading to cell proliferation, re-stenosis, and ischemia.13 This finding was confirmed by another study,14 which demonstrated an increase in Rac1, a signaling protein involved in the extracellular signal-regulated kinase pathway, in VSMCs upon exposure to L obliqua venom. These studies propose potential new targets for treatment to prevent vascular damage.
Reactions to Adult Organisms—Although it is more common for the caterpillar form of these organisms to cause an adverse reaction, the adult moth also might be capable of causing a similar reaction by retaining setae from the cocoon or by their own spines. The most notable example of this is female moths of the genus Hylesia, which possess spines attached to glands on the abdomen. The poison in these spines—a mixture of proteases and chitinase—causes a dermatitis known as Caripito itch—the name derived from a river port in Venezuela where this moth caused a memorable epidemic of moth-induced dermatitis.7,15 Caripito itch is known for intense pruritus that most commonly lasts days or weeks, possibly longer than 1 year.
Diagnostic Difficulties
The challenge of diagnosing a caterpillar- or moth-induced reaction in humans arises from (1) the lack of clinical history (the caterpillar might not be seen at all by the patient or the examiner) and (2) the similarity of these reactions to those with more common triggers.
When setae remain embedded in the skin or mucous membranes, skin scrapings allow accelerated diagnosis. On a skin scraping prepared with 20% potassium hydroxide, setae appear as tapered and barbed hairlike structures, which allows them to be distinguished from other similar-appearing but differently shaped structures, such as glass fibers.
When setae do not remain embedded in the skin or when the cause of the reaction is due to spines, the physician is left with a nonspecific histologic picture and a large differential diagnosis to be narrowed down based on the history and occasionally the pattern of the skin lesion.
A challenge in sting diagnosis is differentiating a caterpillar or moth sting from that of another organism. In certain cases, such as those of the family Megalopygidae, specific patterns of stings might assist in making the diagnosis. Hypersensitivity reactions are associated with a wider differential diagnosis, including irritant or allergic dermatitis from other causes, scabies, eczema, lichen planus, lichen simplex chronicus, seborrheic dermatitis, and tinea corporis, to name a few.6 Skin scrapings can be examined for other features, such as burrows in the case of scabies, to further narrow the differential.
Stings and hypersensitivity reactions lacking a proper history and associated with more severe systemic symptoms have caused misdiagnosis or led to a workup for the wrong condition; for example, the picture of abdominal pain, nausea, vomiting, tachycardia, leukocytosis, hypokalemia, and metabolic acidosis can simulate appendicitis.16 Upon discovery of a puss caterpillar sting in a patient, her symptoms resolved after treatment with ondansetron, morphine, and intravenous fluids.16
In lonomism, the diagnosis must be established by laboratory measurement of the fibrinogen level, clotting factors, prothrombin time, and activated partial thromboplastin time.4 The differential diagnosis associated with lonomism includes disseminated intravascular coagulation (DIC), snakebite, and a hereditary bleeding disorder.4 The combination of laboratory tests and an extensive medical history allows a diagnosis. Absence of a personal or family history of bleeding excludes a diagnosis of hereditary bleeding disorder, whereas the absence of known causes of DIC or thrombocytopenia allows DIC to be excluded from the differential.
Treatment Options and Prevention
Treatment—The first step is to remove any embedded setae from the skin or mucous membranes. The stepwise recommendation is to remove any constricted clothing, detach setae with adhesive tape, wash with soap and water, and dry without touching the skin.1 Any remaining setae can be removed with additional tape or forceps; setae tend to be fragile and are difficult to remove in their entirety.
Other than removal of the setae, skin reactions are treated symptomatically. Ice packs and isopropyl alcohol have been utilized to cool burning or stinging areas. Pain, pruritus, and inflammation have been alleviated with antihistamines and topical corticosteroids.1 When pain is severe, oral codeine or local injection of anesthetic can be used. For severe and persistent skin lesions, a course of an oral glucocorticoid can be administered. Intramuscular triamcinolone acetonide has been shown to treat pain, dermatitis, and subcutaneous nodules otherwise refractory to treatment.8
Antivenin specific for L obliqua exists to treat lonomism and is therefore effective only when lonomism is caused by that species. Lonomism caused by L achelous is treated with cryoprecipitate, purified fibrinogen, and antifibrinolytic drugs, such as aprotinin.6 Whole blood and fresh-frozen plasma have been noted to make hemorrhage worse when utilized to treat lonomism. Because the mechanism of action of the venom of Lonomia species is based, in part, on inducing a proinflammatory profile in endothelial cells, studies have demonstrated that inhibition of kallikrein might prevent vascular injury and thus prevent serious adverse effects, such as renal failure.17
Prevention—People should wear proper protective clothing when outdoors in potentially infested areas. Measures should be taken to ensure that linens and clothing are not left outside in areas where setae might be carried on the wind. Infestation control is necessary if the population of caterpillars reaches a high enough level.
Conclusion
Several species of caterpillars and moths cause adverse reactions in humans: stings, hypersensitivity reactions, and lonomism. Although most reactions are self-limited, some might have more serious effects, including organ failure and death. Mechanisms of injury vary by species of caterpillar, moth, and butterfly; current research is focused on further defining venom components and signaling pathways to isolate potential targets to aid in the diagnosis and treatment of lepidopterism.
- Goldman BS, Bragg BN. Caterpillar and moth bites. Stat Pearls [Internet]. StatPearls Publishing. Updated August 3, 2021. Accessed November 4, 2021. https://www.ncbi.nlm.nih.gov/books/NBK539851/
- Hossler EW. Caterpillars and moths: part I. Dermatologic manifestations of encounters with Lepidoptera. J Am Acad Dermatol. 2010;62:1-10. doi:10.1016/j.jaad.2009.08.060
- Forrester MB. Megalopyge opercularis caterpillar stings reported to Texas poison centers. Wilderness Environ Med. 2018;29:215-220. doi:10.1016/j.wem.2018.02.002
- Hossler EW. Lepidopterism: skin disorders secondary to caterpillars and moths. UpToDate website. Published October 20, 2021. Accessed November 18, 2021. https://www.uptodate.com/contents/lepidopterism-skin-disorders-secondary-to-caterpillars-and-moths
- Villas-Boas IM, Bonfá G, Tambourgi DV. Venomous caterpillars: from inoculation apparatus to venom composition and envenomation. Toxicon. 2018;153:39-52. doi:10.1016/j.toxicon.2018.08.007
- Hossler EW. Caterpillars and moths: part II. dermatologic manifestations of encounters with Lepidoptera. J Am Acad Dermatol. 2010;62:13-28. doi:10.1016/j.jaad.2009.08.061
- Yao Z, Kamau PM, Han Y, et al. The Latoia consocia caterpillar induces pain by targeting nociceptive ion channel TRPV1. Toxins (Basel). 2019;11:695. doi:10.3390/toxins11120695
- Paniz-Mondolfi AE, Pérez-Alvarez AM, Lundberg U, et al. Cutaneous lepidopterism: dermatitis from contact with moths of Hylesia metabus (Cramer 1775) (Lepidoptera: Saturniidae), the causative agent of caripito itch. Int J Dermatol. 2011;50:535-541. doi:10.1111/j.1365-4632.2010.04683.x
- Santos-Magadán S, González de Olano D, Bartolomé-Zavala B, et al. Adverse reactions to the processionary caterpillar: irritant or allergic mechanism? Contact Dermatitis. 2009;60:109-110. doi:10.1111/j.1600-0536.2008.01464.x
- González-Martín-Moro J, Contreras-Martín I, Castro-Rebollo M, et al. Focal cortical cataract due to caterpillar hair migration. Clin Exp Optom. 2019;102:89-90. doi:10.1111/cxo.12809
- Singh A, Behera UC, Agrawal H. Intra-lenticular caterpillar seta in ophthalmia nodosa. Eur J Ophthalmol. 2021;31:NP109-NP111. doi:10.1177/1120672119858899
- Schmitberger PA, Fernandes TC, Santos RC, et al. Probable chronic renal failure caused by Lonomia caterpillar envenomation. J Venom Anim Toxins Incl Trop Dis. 2013;19:14. doi:10.1186/1678-9199-19-14
- Moraes JA, Rodrigues G, Nascimento-Silva V, et al. Effects of Lonomia obliqua venom on vascular smooth muscle cells: contribution of NADPH oxidase-derived reactive oxygen species. Toxins (Basel). 2017;9:360. doi:10.3390/toxins9110360
- Bernardi L, Pinto AFM, Mendes E, et al. Lonomia obliqua bristle extract modulates Rac1 activation, membrane dynamics and cell adhesion properties. Toxicon. 2019;162:32-39. doi:10.1016/j.toxicon.2019.02.019
- Cabrera G, Lundberg U, Rodríguez-Ulloa A, et al. Protein content of the Hylesia metabus egg nest setae (Cramer [1775]) (Lepidoptera: Saturniidae) and its association with the parental investment for the reproductive success and lepidopterism. J Proteomics. 2017;150:183-200. doi:10.1016/j.jprot.2016.08.010
- Greene SC, Carey JM. Puss caterpillar envenomation: erucism mimicking appendicitis in a young child. Pediatr Emerg Care. 2020;36:E732-E734. doi:10.1097/PEC.0000000000001514
- Berger M, de Moraes JA, Beys-da-Silva WO, et al. Renal and vascular effects of kallikrein inhibition in a model of Lonomia obliqua venom-induced acute kidney injury. PLoS Negl Trop Dis. 2019;13:e0007197. doi:10.1371/journal.pntd.0007197
Causes of Lepidopterism
Caterpillars are wormlike organisms that serve as the larval stage of moths and butterflies, which belong to the order Lepidoptera. There are almost 165,000 discovered species, with 13,000 found in the United States.1,2 Roughly 150 species are known to have the potential to cause an adverse reaction in humans, with 50 of these in the United States.1Lepidopterism describes systemic and cutaneous reactions to moths, butterflies, and caterpillars; erucism describes strictly cutaneous reactions.1
Although the rate of lepidopterism is thought to be underreported because it often is self-limited and of a mild nature, a review found caterpillars to be the cause of roughly 2.2% of reported bites and stings annually.2 Cases increase in number with seasonal increases in caterpillars, which vary by region and species. For example, the Megalopyge opercularis (southern flannel moth) caterpillar was noted to have 2 peaks in a Texas-based study: 12% of reported stings occurred in July; 59% from October through November.3 In general, the likelihood of exposure increases during warmer months, and exposure is more common in people who work outdoors in a rural area or in a suburban area where there are many caterpillar-infested trees.4
Most cases of lepidopterism are caused by caterpillars, not by adult butterflies and moths, because the former have many tubular, or porous, hairlike structures called setae that are embedded in the integument. Setae were once thought to be connected to poison-secreting glandular cells, but current belief is that venomous caterpillars lack specialized gland cells and instead produce venom through secretory epithelial cells located above the integument.1 Venom accumulates in the hemolymph and is stored in the setae or other types of bristles, such as scoli (wartlike bumps that bear setae) or spines.5 With a large amount of chitin, bristles have a tendency to fracture and release venom upon contact.1 It is thought that some species of caterpillars formulate venom by ingesting toxins or toxin precursors from plants; for example, the tiger moth (family Arctiidae) is known to produce venom containing biogenic amines, pyrrolizidine, alkaloids, and cardiac glycosides obtained through food sources.5
Even if a caterpillar does not produce venom, its setae might embed into skin or mucous membranes and cause an adverse irritant reaction.1 Setae also might dislodge and be transported in the air to embed in objects—some remaining stable in the environment for longer than a year.2 In contrast to setae, spines are permanently fixed into the integument; for that reason, only direct contact with the caterpillar can result in an adverse reaction. Although it is mostly caterpillars that contain setae and spines, certain species of moths also might contain these structures or might acquire them as they emerge from the cocoon, which often contains incorporated setae.2
Reactions in Humans
Lepidopterism encompasses 3 principal reactions in humans: sting reaction, hypersensitivity reaction, and lonomism (a hemorrhagic diathesis produced by Lonomia caterpillars). The type and severity of the reaction depends on (1) the species of caterpillar or moth and (2) the individual patient.2 There are approximately 12 families of caterpillars, mainly of the moth variety, that can cause an adverse reaction in humans.1 Tables 1 and 2 list examples of species that cause each type of reaction.6



Chemicals and toxins contained in the poison of setae and spines vary by species of caterpillar. Numerous kinds have been isolated from different venoms,1,2 including several peptides, histamine, histamine-releasing substances, acetylcholine, phospholipase A, hyaluronidase, formic acid, proteins with trypsinlike activity, serine proteases such as kallikrein, and other enzymes with vasodegenerative and fibrinolytic properties

Stings: An Immediate Adverse Reaction—Depending on the venom, a sting might result in mild to severe burning pain, accompanied by welts, vesicles, and red papules or plaques.2 Figure 1 demonstrates a particularly mild sting from a caterpillar of the family Automeris, examples of which are seen in Figures 2 and 3 and eFigure 1. Components of the venom determine the mechanism of the sting and the pain that accompanies it. For example, a recent study demonstrated that the venom of the Latoia consocia caterpillar induces pain through the ion-channel receptor known as transient receptor potential vanilloid 1, which integrates and sends painful stimuli from the peripheral nervous system to the central nervous system.7 It is thought that a variety of ion channels are targets of the venom of caterpillars.




One of the most characteristic sting patterns is that of the caterpillar of family Megalopygidae (flannel moth)(eFigures 2 and 3). The stings of these caterpillars create a unique tram-track pattern of hemorrhagic macules or papules (Figure 4).4 A study found that 90% of reported M opercularis envenomations consist primarily of cutaneous symptoms, with 84% of those symptoms being irritation or pain; 45% a puncture or wound; 29% erythema; and 15% edema.3 Systemic findings can include headache, fever, adenopathy, nausea, vomiting, abdominal pain, and chest pain.4 Symptoms normally are self-limited, though they can last minutes or hours.



Hypersensitivity Reaction—Studies demonstrate that the symptoms of this reaction are a mixture of type I hypersensitivity, type IV hypersensitivity, and a foreign-body response.2 The specific hypersensitivity reaction depends on the venom and the exposed individual—most commonly including a combination of pruritic papules, urticarial wheals, flares, and dermatitis.2 A reaction that is a result of direct contact with the caterpillar or moth will appear on exposed areas; however, because setae embed in linens and clothing, they might cause a reaction anywhere on the body. Although usually self-limited, a hypersensitivity reaction might develop within minutes and can last for days or weeks.
Stings and hypersensitivity reactions to caterpillars and moths tend to lead to a nonspecific histologic presentation characterized by epidermal edema and a superficial perivascular lymphocytic infiltrate, often with eosinophils.6 After approximately 1 week, a foreign-body response to setae can lead to tuberculoid granulomas accompanied by neutrophils in the dermis and occasionally in subcutaneous tissues (Figures 5 and 6).8 If setae have not yet been removed, they also might be visible in skin scrapings.


Additional complications can accompany the hypersensitivity reaction to setae or spines. Type I hypersensitivity reactions can lead to severe reactions on second contact due to previously sensitized IgE antibodies. Although the first reaction appears mild, second contact might result in angioedema, wheezing, dyspnea, or anaphylaxis, or a combination of these findings.9 In addition, some patients who come in contact with Dendrolimus caterpillars might develop a condition known as dendrolimiasis, characterized by dermatitis in addition to arthritis or chondritis.6 The arthritis is normally monoarticular and can result in complete destruction of the joint. Pararamose, a condition with a similar presentation, is caused by the Brazilian moth Premolis semirufa.6
Contact of setae or spines with mucous membranes or inhalation of setae also might result in edema, dysphagia, dyspnea, drooling, rhinitis, or conjunctivitis, or a combination of these findings.6 In addition, setae can embed in the eye and cause an inflammatory reaction—ophthalmia nodosa—most commonly caused by caterpillars of the pine processionary moth (Thaumetopoea pityocampa) and characterized by immediate chemosis, which can progress to liquefactive necrosis and hypopyon, later developing into a granulomatous foreign-body response.2,10 The process is thought to be the result of a combination of the thaumetopoein toxin in the setae and an IgE-mediated response to other proteins.10
Due to their harpoon shape and forward-only motion, setae might migrate deeper, potentially even to the optic nerve.11 Because migration might take years and the barbed shape of setae does not always allow removal, some patients require lifetime monitoring with slit-lamp examination.Chronic problems, such as cataracts and persistent posterior uveitis, have been reported.10,11
Lonomism—One of the most serious (though rarest) reactions to caterpillars is lonomism, a condition caused by the caterpillars of Lonomia achelous and Lonomia obliqua moths. These caterpillars have a unique combination of toxins filling their branched spines, which ultimately leads to the same outcome: a hemorrhagic diathesis.
The toxin of L achelous comprises several proteases that degrade fibrin, fibrinogen, and factor XIII while activating prothrombin. In contrast, L obliqua poison causes a hemorrhagic diathesis by promoting a consumptive coagulopathy through enzymes that activate factor X and prothrombin.
With initial contact with either of these Lonomia caterpillars, the patient experiences severe pain accompanied by systemic symptoms, including headache, nausea, and vomiting. Shortly afterward, symptoms of a hemorrhagic diathesis manifest, including bleeding gums, hematuria, bleeding from prior wounds, and epistaxis.5 Serious complications of the hemorrhagic diathesis, such as hemorrhage of major organs, leads to death in 4% of patients.5 A reported case of a patient whose Lonomia caterpillar sting went unrecognized until a week after the accident ended with progression to stage V chronic renal disease.12
Recent research has focused on the specific mechanism of injury caused by Lonomia species. A study found that the venom of L obliqua causes cytoskeleton rearrangement and migration in vascular smooth muscle cells (VSMCs) by inducing formation of reactive oxygen species through activation of nicotinamide adenine dinucleotide phosphate oxidase.13 Thus, the venom directly contributes to the proinflammatory phenotype of endothelial cells seen following envenomation. The same study also demonstrated that elevated reactive oxygen species trigger extracellular signal-regulated kinase pathway activation in VSMCs, leading to cell proliferation, re-stenosis, and ischemia.13 This finding was confirmed by another study,14 which demonstrated an increase in Rac1, a signaling protein involved in the extracellular signal-regulated kinase pathway, in VSMCs upon exposure to L obliqua venom. These studies propose potential new targets for treatment to prevent vascular damage.
Reactions to Adult Organisms—Although it is more common for the caterpillar form of these organisms to cause an adverse reaction, the adult moth also might be capable of causing a similar reaction by retaining setae from the cocoon or by their own spines. The most notable example of this is female moths of the genus Hylesia, which possess spines attached to glands on the abdomen. The poison in these spines—a mixture of proteases and chitinase—causes a dermatitis known as Caripito itch—the name derived from a river port in Venezuela where this moth caused a memorable epidemic of moth-induced dermatitis.7,15 Caripito itch is known for intense pruritus that most commonly lasts days or weeks, possibly longer than 1 year.
Diagnostic Difficulties
The challenge of diagnosing a caterpillar- or moth-induced reaction in humans arises from (1) the lack of clinical history (the caterpillar might not be seen at all by the patient or the examiner) and (2) the similarity of these reactions to those with more common triggers.
When setae remain embedded in the skin or mucous membranes, skin scrapings allow accelerated diagnosis. On a skin scraping prepared with 20% potassium hydroxide, setae appear as tapered and barbed hairlike structures, which allows them to be distinguished from other similar-appearing but differently shaped structures, such as glass fibers.
When setae do not remain embedded in the skin or when the cause of the reaction is due to spines, the physician is left with a nonspecific histologic picture and a large differential diagnosis to be narrowed down based on the history and occasionally the pattern of the skin lesion.
A challenge in sting diagnosis is differentiating a caterpillar or moth sting from that of another organism. In certain cases, such as those of the family Megalopygidae, specific patterns of stings might assist in making the diagnosis. Hypersensitivity reactions are associated with a wider differential diagnosis, including irritant or allergic dermatitis from other causes, scabies, eczema, lichen planus, lichen simplex chronicus, seborrheic dermatitis, and tinea corporis, to name a few.6 Skin scrapings can be examined for other features, such as burrows in the case of scabies, to further narrow the differential.
Stings and hypersensitivity reactions lacking a proper history and associated with more severe systemic symptoms have caused misdiagnosis or led to a workup for the wrong condition; for example, the picture of abdominal pain, nausea, vomiting, tachycardia, leukocytosis, hypokalemia, and metabolic acidosis can simulate appendicitis.16 Upon discovery of a puss caterpillar sting in a patient, her symptoms resolved after treatment with ondansetron, morphine, and intravenous fluids.16
In lonomism, the diagnosis must be established by laboratory measurement of the fibrinogen level, clotting factors, prothrombin time, and activated partial thromboplastin time.4 The differential diagnosis associated with lonomism includes disseminated intravascular coagulation (DIC), snakebite, and a hereditary bleeding disorder.4 The combination of laboratory tests and an extensive medical history allows a diagnosis. Absence of a personal or family history of bleeding excludes a diagnosis of hereditary bleeding disorder, whereas the absence of known causes of DIC or thrombocytopenia allows DIC to be excluded from the differential.
Treatment Options and Prevention
Treatment—The first step is to remove any embedded setae from the skin or mucous membranes. The stepwise recommendation is to remove any constricted clothing, detach setae with adhesive tape, wash with soap and water, and dry without touching the skin.1 Any remaining setae can be removed with additional tape or forceps; setae tend to be fragile and are difficult to remove in their entirety.
Other than removal of the setae, skin reactions are treated symptomatically. Ice packs and isopropyl alcohol have been utilized to cool burning or stinging areas. Pain, pruritus, and inflammation have been alleviated with antihistamines and topical corticosteroids.1 When pain is severe, oral codeine or local injection of anesthetic can be used. For severe and persistent skin lesions, a course of an oral glucocorticoid can be administered. Intramuscular triamcinolone acetonide has been shown to treat pain, dermatitis, and subcutaneous nodules otherwise refractory to treatment.8
Antivenin specific for L obliqua exists to treat lonomism and is therefore effective only when lonomism is caused by that species. Lonomism caused by L achelous is treated with cryoprecipitate, purified fibrinogen, and antifibrinolytic drugs, such as aprotinin.6 Whole blood and fresh-frozen plasma have been noted to make hemorrhage worse when utilized to treat lonomism. Because the mechanism of action of the venom of Lonomia species is based, in part, on inducing a proinflammatory profile in endothelial cells, studies have demonstrated that inhibition of kallikrein might prevent vascular injury and thus prevent serious adverse effects, such as renal failure.17
Prevention—People should wear proper protective clothing when outdoors in potentially infested areas. Measures should be taken to ensure that linens and clothing are not left outside in areas where setae might be carried on the wind. Infestation control is necessary if the population of caterpillars reaches a high enough level.
Conclusion
Several species of caterpillars and moths cause adverse reactions in humans: stings, hypersensitivity reactions, and lonomism. Although most reactions are self-limited, some might have more serious effects, including organ failure and death. Mechanisms of injury vary by species of caterpillar, moth, and butterfly; current research is focused on further defining venom components and signaling pathways to isolate potential targets to aid in the diagnosis and treatment of lepidopterism.
Causes of Lepidopterism
Caterpillars are wormlike organisms that serve as the larval stage of moths and butterflies, which belong to the order Lepidoptera. There are almost 165,000 discovered species, with 13,000 found in the United States.1,2 Roughly 150 species are known to have the potential to cause an adverse reaction in humans, with 50 of these in the United States.1Lepidopterism describes systemic and cutaneous reactions to moths, butterflies, and caterpillars; erucism describes strictly cutaneous reactions.1
Although the rate of lepidopterism is thought to be underreported because it often is self-limited and of a mild nature, a review found caterpillars to be the cause of roughly 2.2% of reported bites and stings annually.2 Cases increase in number with seasonal increases in caterpillars, which vary by region and species. For example, the Megalopyge opercularis (southern flannel moth) caterpillar was noted to have 2 peaks in a Texas-based study: 12% of reported stings occurred in July; 59% from October through November.3 In general, the likelihood of exposure increases during warmer months, and exposure is more common in people who work outdoors in a rural area or in a suburban area where there are many caterpillar-infested trees.4
Most cases of lepidopterism are caused by caterpillars, not by adult butterflies and moths, because the former have many tubular, or porous, hairlike structures called setae that are embedded in the integument. Setae were once thought to be connected to poison-secreting glandular cells, but current belief is that venomous caterpillars lack specialized gland cells and instead produce venom through secretory epithelial cells located above the integument.1 Venom accumulates in the hemolymph and is stored in the setae or other types of bristles, such as scoli (wartlike bumps that bear setae) or spines.5 With a large amount of chitin, bristles have a tendency to fracture and release venom upon contact.1 It is thought that some species of caterpillars formulate venom by ingesting toxins or toxin precursors from plants; for example, the tiger moth (family Arctiidae) is known to produce venom containing biogenic amines, pyrrolizidine, alkaloids, and cardiac glycosides obtained through food sources.5
Even if a caterpillar does not produce venom, its setae might embed into skin or mucous membranes and cause an adverse irritant reaction.1 Setae also might dislodge and be transported in the air to embed in objects—some remaining stable in the environment for longer than a year.2 In contrast to setae, spines are permanently fixed into the integument; for that reason, only direct contact with the caterpillar can result in an adverse reaction. Although it is mostly caterpillars that contain setae and spines, certain species of moths also might contain these structures or might acquire them as they emerge from the cocoon, which often contains incorporated setae.2
Reactions in Humans
Lepidopterism encompasses 3 principal reactions in humans: sting reaction, hypersensitivity reaction, and lonomism (a hemorrhagic diathesis produced by Lonomia caterpillars). The type and severity of the reaction depends on (1) the species of caterpillar or moth and (2) the individual patient.2 There are approximately 12 families of caterpillars, mainly of the moth variety, that can cause an adverse reaction in humans.1 Tables 1 and 2 list examples of species that cause each type of reaction.6



Chemicals and toxins contained in the poison of setae and spines vary by species of caterpillar. Numerous kinds have been isolated from different venoms,1,2 including several peptides, histamine, histamine-releasing substances, acetylcholine, phospholipase A, hyaluronidase, formic acid, proteins with trypsinlike activity, serine proteases such as kallikrein, and other enzymes with vasodegenerative and fibrinolytic properties

Stings: An Immediate Adverse Reaction—Depending on the venom, a sting might result in mild to severe burning pain, accompanied by welts, vesicles, and red papules or plaques.2 Figure 1 demonstrates a particularly mild sting from a caterpillar of the family Automeris, examples of which are seen in Figures 2 and 3 and eFigure 1. Components of the venom determine the mechanism of the sting and the pain that accompanies it. For example, a recent study demonstrated that the venom of the Latoia consocia caterpillar induces pain through the ion-channel receptor known as transient receptor potential vanilloid 1, which integrates and sends painful stimuli from the peripheral nervous system to the central nervous system.7 It is thought that a variety of ion channels are targets of the venom of caterpillars.




One of the most characteristic sting patterns is that of the caterpillar of family Megalopygidae (flannel moth)(eFigures 2 and 3). The stings of these caterpillars create a unique tram-track pattern of hemorrhagic macules or papules (Figure 4).4 A study found that 90% of reported M opercularis envenomations consist primarily of cutaneous symptoms, with 84% of those symptoms being irritation or pain; 45% a puncture or wound; 29% erythema; and 15% edema.3 Systemic findings can include headache, fever, adenopathy, nausea, vomiting, abdominal pain, and chest pain.4 Symptoms normally are self-limited, though they can last minutes or hours.



Hypersensitivity Reaction—Studies demonstrate that the symptoms of this reaction are a mixture of type I hypersensitivity, type IV hypersensitivity, and a foreign-body response.2 The specific hypersensitivity reaction depends on the venom and the exposed individual—most commonly including a combination of pruritic papules, urticarial wheals, flares, and dermatitis.2 A reaction that is a result of direct contact with the caterpillar or moth will appear on exposed areas; however, because setae embed in linens and clothing, they might cause a reaction anywhere on the body. Although usually self-limited, a hypersensitivity reaction might develop within minutes and can last for days or weeks.
Stings and hypersensitivity reactions to caterpillars and moths tend to lead to a nonspecific histologic presentation characterized by epidermal edema and a superficial perivascular lymphocytic infiltrate, often with eosinophils.6 After approximately 1 week, a foreign-body response to setae can lead to tuberculoid granulomas accompanied by neutrophils in the dermis and occasionally in subcutaneous tissues (Figures 5 and 6).8 If setae have not yet been removed, they also might be visible in skin scrapings.


Additional complications can accompany the hypersensitivity reaction to setae or spines. Type I hypersensitivity reactions can lead to severe reactions on second contact due to previously sensitized IgE antibodies. Although the first reaction appears mild, second contact might result in angioedema, wheezing, dyspnea, or anaphylaxis, or a combination of these findings.9 In addition, some patients who come in contact with Dendrolimus caterpillars might develop a condition known as dendrolimiasis, characterized by dermatitis in addition to arthritis or chondritis.6 The arthritis is normally monoarticular and can result in complete destruction of the joint. Pararamose, a condition with a similar presentation, is caused by the Brazilian moth Premolis semirufa.6
Contact of setae or spines with mucous membranes or inhalation of setae also might result in edema, dysphagia, dyspnea, drooling, rhinitis, or conjunctivitis, or a combination of these findings.6 In addition, setae can embed in the eye and cause an inflammatory reaction—ophthalmia nodosa—most commonly caused by caterpillars of the pine processionary moth (Thaumetopoea pityocampa) and characterized by immediate chemosis, which can progress to liquefactive necrosis and hypopyon, later developing into a granulomatous foreign-body response.2,10 The process is thought to be the result of a combination of the thaumetopoein toxin in the setae and an IgE-mediated response to other proteins.10
Due to their harpoon shape and forward-only motion, setae might migrate deeper, potentially even to the optic nerve.11 Because migration might take years and the barbed shape of setae does not always allow removal, some patients require lifetime monitoring with slit-lamp examination.Chronic problems, such as cataracts and persistent posterior uveitis, have been reported.10,11
Lonomism—One of the most serious (though rarest) reactions to caterpillars is lonomism, a condition caused by the caterpillars of Lonomia achelous and Lonomia obliqua moths. These caterpillars have a unique combination of toxins filling their branched spines, which ultimately leads to the same outcome: a hemorrhagic diathesis.
The toxin of L achelous comprises several proteases that degrade fibrin, fibrinogen, and factor XIII while activating prothrombin. In contrast, L obliqua poison causes a hemorrhagic diathesis by promoting a consumptive coagulopathy through enzymes that activate factor X and prothrombin.
With initial contact with either of these Lonomia caterpillars, the patient experiences severe pain accompanied by systemic symptoms, including headache, nausea, and vomiting. Shortly afterward, symptoms of a hemorrhagic diathesis manifest, including bleeding gums, hematuria, bleeding from prior wounds, and epistaxis.5 Serious complications of the hemorrhagic diathesis, such as hemorrhage of major organs, leads to death in 4% of patients.5 A reported case of a patient whose Lonomia caterpillar sting went unrecognized until a week after the accident ended with progression to stage V chronic renal disease.12
Recent research has focused on the specific mechanism of injury caused by Lonomia species. A study found that the venom of L obliqua causes cytoskeleton rearrangement and migration in vascular smooth muscle cells (VSMCs) by inducing formation of reactive oxygen species through activation of nicotinamide adenine dinucleotide phosphate oxidase.13 Thus, the venom directly contributes to the proinflammatory phenotype of endothelial cells seen following envenomation. The same study also demonstrated that elevated reactive oxygen species trigger extracellular signal-regulated kinase pathway activation in VSMCs, leading to cell proliferation, re-stenosis, and ischemia.13 This finding was confirmed by another study,14 which demonstrated an increase in Rac1, a signaling protein involved in the extracellular signal-regulated kinase pathway, in VSMCs upon exposure to L obliqua venom. These studies propose potential new targets for treatment to prevent vascular damage.
Reactions to Adult Organisms—Although it is more common for the caterpillar form of these organisms to cause an adverse reaction, the adult moth also might be capable of causing a similar reaction by retaining setae from the cocoon or by their own spines. The most notable example of this is female moths of the genus Hylesia, which possess spines attached to glands on the abdomen. The poison in these spines—a mixture of proteases and chitinase—causes a dermatitis known as Caripito itch—the name derived from a river port in Venezuela where this moth caused a memorable epidemic of moth-induced dermatitis.7,15 Caripito itch is known for intense pruritus that most commonly lasts days or weeks, possibly longer than 1 year.
Diagnostic Difficulties
The challenge of diagnosing a caterpillar- or moth-induced reaction in humans arises from (1) the lack of clinical history (the caterpillar might not be seen at all by the patient or the examiner) and (2) the similarity of these reactions to those with more common triggers.
When setae remain embedded in the skin or mucous membranes, skin scrapings allow accelerated diagnosis. On a skin scraping prepared with 20% potassium hydroxide, setae appear as tapered and barbed hairlike structures, which allows them to be distinguished from other similar-appearing but differently shaped structures, such as glass fibers.
When setae do not remain embedded in the skin or when the cause of the reaction is due to spines, the physician is left with a nonspecific histologic picture and a large differential diagnosis to be narrowed down based on the history and occasionally the pattern of the skin lesion.
A challenge in sting diagnosis is differentiating a caterpillar or moth sting from that of another organism. In certain cases, such as those of the family Megalopygidae, specific patterns of stings might assist in making the diagnosis. Hypersensitivity reactions are associated with a wider differential diagnosis, including irritant or allergic dermatitis from other causes, scabies, eczema, lichen planus, lichen simplex chronicus, seborrheic dermatitis, and tinea corporis, to name a few.6 Skin scrapings can be examined for other features, such as burrows in the case of scabies, to further narrow the differential.
Stings and hypersensitivity reactions lacking a proper history and associated with more severe systemic symptoms have caused misdiagnosis or led to a workup for the wrong condition; for example, the picture of abdominal pain, nausea, vomiting, tachycardia, leukocytosis, hypokalemia, and metabolic acidosis can simulate appendicitis.16 Upon discovery of a puss caterpillar sting in a patient, her symptoms resolved after treatment with ondansetron, morphine, and intravenous fluids.16
In lonomism, the diagnosis must be established by laboratory measurement of the fibrinogen level, clotting factors, prothrombin time, and activated partial thromboplastin time.4 The differential diagnosis associated with lonomism includes disseminated intravascular coagulation (DIC), snakebite, and a hereditary bleeding disorder.4 The combination of laboratory tests and an extensive medical history allows a diagnosis. Absence of a personal or family history of bleeding excludes a diagnosis of hereditary bleeding disorder, whereas the absence of known causes of DIC or thrombocytopenia allows DIC to be excluded from the differential.
Treatment Options and Prevention
Treatment—The first step is to remove any embedded setae from the skin or mucous membranes. The stepwise recommendation is to remove any constricted clothing, detach setae with adhesive tape, wash with soap and water, and dry without touching the skin.1 Any remaining setae can be removed with additional tape or forceps; setae tend to be fragile and are difficult to remove in their entirety.
Other than removal of the setae, skin reactions are treated symptomatically. Ice packs and isopropyl alcohol have been utilized to cool burning or stinging areas. Pain, pruritus, and inflammation have been alleviated with antihistamines and topical corticosteroids.1 When pain is severe, oral codeine or local injection of anesthetic can be used. For severe and persistent skin lesions, a course of an oral glucocorticoid can be administered. Intramuscular triamcinolone acetonide has been shown to treat pain, dermatitis, and subcutaneous nodules otherwise refractory to treatment.8
Antivenin specific for L obliqua exists to treat lonomism and is therefore effective only when lonomism is caused by that species. Lonomism caused by L achelous is treated with cryoprecipitate, purified fibrinogen, and antifibrinolytic drugs, such as aprotinin.6 Whole blood and fresh-frozen plasma have been noted to make hemorrhage worse when utilized to treat lonomism. Because the mechanism of action of the venom of Lonomia species is based, in part, on inducing a proinflammatory profile in endothelial cells, studies have demonstrated that inhibition of kallikrein might prevent vascular injury and thus prevent serious adverse effects, such as renal failure.17
Prevention—People should wear proper protective clothing when outdoors in potentially infested areas. Measures should be taken to ensure that linens and clothing are not left outside in areas where setae might be carried on the wind. Infestation control is necessary if the population of caterpillars reaches a high enough level.
Conclusion
Several species of caterpillars and moths cause adverse reactions in humans: stings, hypersensitivity reactions, and lonomism. Although most reactions are self-limited, some might have more serious effects, including organ failure and death. Mechanisms of injury vary by species of caterpillar, moth, and butterfly; current research is focused on further defining venom components and signaling pathways to isolate potential targets to aid in the diagnosis and treatment of lepidopterism.
- Goldman BS, Bragg BN. Caterpillar and moth bites. Stat Pearls [Internet]. StatPearls Publishing. Updated August 3, 2021. Accessed November 4, 2021. https://www.ncbi.nlm.nih.gov/books/NBK539851/
- Hossler EW. Caterpillars and moths: part I. Dermatologic manifestations of encounters with Lepidoptera. J Am Acad Dermatol. 2010;62:1-10. doi:10.1016/j.jaad.2009.08.060
- Forrester MB. Megalopyge opercularis caterpillar stings reported to Texas poison centers. Wilderness Environ Med. 2018;29:215-220. doi:10.1016/j.wem.2018.02.002
- Hossler EW. Lepidopterism: skin disorders secondary to caterpillars and moths. UpToDate website. Published October 20, 2021. Accessed November 18, 2021. https://www.uptodate.com/contents/lepidopterism-skin-disorders-secondary-to-caterpillars-and-moths
- Villas-Boas IM, Bonfá G, Tambourgi DV. Venomous caterpillars: from inoculation apparatus to venom composition and envenomation. Toxicon. 2018;153:39-52. doi:10.1016/j.toxicon.2018.08.007
- Hossler EW. Caterpillars and moths: part II. dermatologic manifestations of encounters with Lepidoptera. J Am Acad Dermatol. 2010;62:13-28. doi:10.1016/j.jaad.2009.08.061
- Yao Z, Kamau PM, Han Y, et al. The Latoia consocia caterpillar induces pain by targeting nociceptive ion channel TRPV1. Toxins (Basel). 2019;11:695. doi:10.3390/toxins11120695
- Paniz-Mondolfi AE, Pérez-Alvarez AM, Lundberg U, et al. Cutaneous lepidopterism: dermatitis from contact with moths of Hylesia metabus (Cramer 1775) (Lepidoptera: Saturniidae), the causative agent of caripito itch. Int J Dermatol. 2011;50:535-541. doi:10.1111/j.1365-4632.2010.04683.x
- Santos-Magadán S, González de Olano D, Bartolomé-Zavala B, et al. Adverse reactions to the processionary caterpillar: irritant or allergic mechanism? Contact Dermatitis. 2009;60:109-110. doi:10.1111/j.1600-0536.2008.01464.x
- González-Martín-Moro J, Contreras-Martín I, Castro-Rebollo M, et al. Focal cortical cataract due to caterpillar hair migration. Clin Exp Optom. 2019;102:89-90. doi:10.1111/cxo.12809
- Singh A, Behera UC, Agrawal H. Intra-lenticular caterpillar seta in ophthalmia nodosa. Eur J Ophthalmol. 2021;31:NP109-NP111. doi:10.1177/1120672119858899
- Schmitberger PA, Fernandes TC, Santos RC, et al. Probable chronic renal failure caused by Lonomia caterpillar envenomation. J Venom Anim Toxins Incl Trop Dis. 2013;19:14. doi:10.1186/1678-9199-19-14
- Moraes JA, Rodrigues G, Nascimento-Silva V, et al. Effects of Lonomia obliqua venom on vascular smooth muscle cells: contribution of NADPH oxidase-derived reactive oxygen species. Toxins (Basel). 2017;9:360. doi:10.3390/toxins9110360
- Bernardi L, Pinto AFM, Mendes E, et al. Lonomia obliqua bristle extract modulates Rac1 activation, membrane dynamics and cell adhesion properties. Toxicon. 2019;162:32-39. doi:10.1016/j.toxicon.2019.02.019
- Cabrera G, Lundberg U, Rodríguez-Ulloa A, et al. Protein content of the Hylesia metabus egg nest setae (Cramer [1775]) (Lepidoptera: Saturniidae) and its association with the parental investment for the reproductive success and lepidopterism. J Proteomics. 2017;150:183-200. doi:10.1016/j.jprot.2016.08.010
- Greene SC, Carey JM. Puss caterpillar envenomation: erucism mimicking appendicitis in a young child. Pediatr Emerg Care. 2020;36:E732-E734. doi:10.1097/PEC.0000000000001514
- Berger M, de Moraes JA, Beys-da-Silva WO, et al. Renal and vascular effects of kallikrein inhibition in a model of Lonomia obliqua venom-induced acute kidney injury. PLoS Negl Trop Dis. 2019;13:e0007197. doi:10.1371/journal.pntd.0007197
- Goldman BS, Bragg BN. Caterpillar and moth bites. Stat Pearls [Internet]. StatPearls Publishing. Updated August 3, 2021. Accessed November 4, 2021. https://www.ncbi.nlm.nih.gov/books/NBK539851/
- Hossler EW. Caterpillars and moths: part I. Dermatologic manifestations of encounters with Lepidoptera. J Am Acad Dermatol. 2010;62:1-10. doi:10.1016/j.jaad.2009.08.060
- Forrester MB. Megalopyge opercularis caterpillar stings reported to Texas poison centers. Wilderness Environ Med. 2018;29:215-220. doi:10.1016/j.wem.2018.02.002
- Hossler EW. Lepidopterism: skin disorders secondary to caterpillars and moths. UpToDate website. Published October 20, 2021. Accessed November 18, 2021. https://www.uptodate.com/contents/lepidopterism-skin-disorders-secondary-to-caterpillars-and-moths
- Villas-Boas IM, Bonfá G, Tambourgi DV. Venomous caterpillars: from inoculation apparatus to venom composition and envenomation. Toxicon. 2018;153:39-52. doi:10.1016/j.toxicon.2018.08.007
- Hossler EW. Caterpillars and moths: part II. dermatologic manifestations of encounters with Lepidoptera. J Am Acad Dermatol. 2010;62:13-28. doi:10.1016/j.jaad.2009.08.061
- Yao Z, Kamau PM, Han Y, et al. The Latoia consocia caterpillar induces pain by targeting nociceptive ion channel TRPV1. Toxins (Basel). 2019;11:695. doi:10.3390/toxins11120695
- Paniz-Mondolfi AE, Pérez-Alvarez AM, Lundberg U, et al. Cutaneous lepidopterism: dermatitis from contact with moths of Hylesia metabus (Cramer 1775) (Lepidoptera: Saturniidae), the causative agent of caripito itch. Int J Dermatol. 2011;50:535-541. doi:10.1111/j.1365-4632.2010.04683.x
- Santos-Magadán S, González de Olano D, Bartolomé-Zavala B, et al. Adverse reactions to the processionary caterpillar: irritant or allergic mechanism? Contact Dermatitis. 2009;60:109-110. doi:10.1111/j.1600-0536.2008.01464.x
- González-Martín-Moro J, Contreras-Martín I, Castro-Rebollo M, et al. Focal cortical cataract due to caterpillar hair migration. Clin Exp Optom. 2019;102:89-90. doi:10.1111/cxo.12809
- Singh A, Behera UC, Agrawal H. Intra-lenticular caterpillar seta in ophthalmia nodosa. Eur J Ophthalmol. 2021;31:NP109-NP111. doi:10.1177/1120672119858899
- Schmitberger PA, Fernandes TC, Santos RC, et al. Probable chronic renal failure caused by Lonomia caterpillar envenomation. J Venom Anim Toxins Incl Trop Dis. 2013;19:14. doi:10.1186/1678-9199-19-14
- Moraes JA, Rodrigues G, Nascimento-Silva V, et al. Effects of Lonomia obliqua venom on vascular smooth muscle cells: contribution of NADPH oxidase-derived reactive oxygen species. Toxins (Basel). 2017;9:360. doi:10.3390/toxins9110360
- Bernardi L, Pinto AFM, Mendes E, et al. Lonomia obliqua bristle extract modulates Rac1 activation, membrane dynamics and cell adhesion properties. Toxicon. 2019;162:32-39. doi:10.1016/j.toxicon.2019.02.019
- Cabrera G, Lundberg U, Rodríguez-Ulloa A, et al. Protein content of the Hylesia metabus egg nest setae (Cramer [1775]) (Lepidoptera: Saturniidae) and its association with the parental investment for the reproductive success and lepidopterism. J Proteomics. 2017;150:183-200. doi:10.1016/j.jprot.2016.08.010
- Greene SC, Carey JM. Puss caterpillar envenomation: erucism mimicking appendicitis in a young child. Pediatr Emerg Care. 2020;36:E732-E734. doi:10.1097/PEC.0000000000001514
- Berger M, de Moraes JA, Beys-da-Silva WO, et al. Renal and vascular effects of kallikrein inhibition in a model of Lonomia obliqua venom-induced acute kidney injury. PLoS Negl Trop Dis. 2019;13:e0007197. doi:10.1371/journal.pntd.0007197
Practice Points
- Lepidopterism describes adverse reactions caused by the stings, hypersensitivity reactions, and lonomism (a hemorrhagic diathesis) of caterpillars, moths, and butterflies.
- Caterpillars can induce an adverse reaction by injecting venom stored in their bristles, inducing a foreign-body reaction to embedded bristles, or a combination of these mechanisms.
- A thorough history, skin scrapings, relevant examination of affected body parts (such as slit-lamp examination, in the case of eyes), and laboratory testing should be conducted to narrow the wide differential diagnosis associated with lepidopterism.
Botanical Briefs: Phytophotodermatitis Caused by Giant Hogweed (Heracleum mantegazzianum)
Giant hogweed (Heracleum mantegazzianum) is an invasive flowering weed of the family Apiaceae that typically reaches a height of 13 feet, with thick stems; large green leaves; and umbrella-shaped, flat-topped, radial clusters (umbels) of small individual white flowers1 (Figure 1). Because of the size and beauty of giant hogweed, it was widely planted in 19th century ornamental gardens in the United Kingdom and has since naturalized and spread throughout central Europe, Canada, and the United States.1,2 The plant most commonly is found in shady areas near rivers and woodlands.1

Due to the invasive nature of the giant hogweed, its prevalence continues to grow, its eradication remains difficult, and reports of phytophotodermatitis are increasing in number and distribution. In fact, there has been widespread media coverage of the dangers of giant hogweed in the United Kingdom since 20161 and in the United States in 2018 and 2019.3-6
Transmission
Phytophotodermatitis is a type of nonimmunologic dermatitis caused by UV light reacting with a plant-based photosensitizing agent. In the case of giant hogweed, sap from the plant’s fruits, leaves, and stem contain furocoumarins or psoralens.7 Upon activation by UVA radiation, furan rings of these compounds create reactive oxygen species and intercalate with DNA pyrimidine bases, which results in cellular death, damage to successive skin layers, and reduced wound healing at the cellular level.8 This effect is intensified with increased percutaneous absorption of furocoumarin, which can result from high temperature, humidity, skin infection, lack of protective clothing, and moist conditions.9
The highest concentration of phototoxic compounds is found in giant hogweed from June through August,7 which, in combination with people increasing their outdoor activity in the summer, results in a greater prevalence and severity of H mantegazzianum phytophotodermatitis during summer months.
Presentation
Phytophotodermatitis caused by giant hogweed can range from burning and erythema to full-thickness chemical burns that require surgical debridement and skin grafting.8 After exposure to the offending agent, a harmful skin reaction can start within 15 minutes. After a latent period of approximately 24 hours, erythema, edema, and bullae can appear and generally peak by 72 hours.10 In addition to cutaneous injury, inhalation of giant hogweed traces can result in obstructive pulmonary symptoms. Eye contact can result in blindness.9
In addition to the rash caused by giant hogweed, a “weed-wacker dermatitis” or “strimmer rash” can be caused by the similar-appearing but smaller common hogweed (Heracleum sphondylium). Common hogweed is highly prevalent in the United States and often is confused with the larger giant hogweed because of tall stems and white, flat-topped flowers.
Management
Following contact with giant hogweed, a person should immediately avoid UV exposure and rinse the area with soap and water. UV radiation must be avoided for at least 48 hours. If erythema occurs, a topical steroid can be applied to the affected area; pain can be alleviated by a nonsteroidal anti-inflammatory drug.9
Further treatment might be required if bullous lesions are present. Small blisters can be punctured and drained; however, large blisters, extensive epidermal-dermal separation, and large areas of detached epidermis should simply be cleansed and dressed. An oral steroid also can be used to reduce inflammation in moderate and severe cases. Full-thickness injury might require surgical intervention.8
Clinical Case
A 27-year-old male landscaper presented to the emergency department with an increasingly painful blistering rash on the arms and neck of 1 day’s duration. He noticed bright red skin and blisters 18 to 24 hours after trimming what he identified as shoulder-high giant hogweed plants. Neither he nor his coworkers were wearing sunscreen or protective clothing as they cleared the plants for several hours. His coworkers developed similar rashes, but his rash was the most severe, requiring treatment in the emergency department.
Physical examination showed innumerable 2- to 10-mm, tense vesicles and bullae on a background of blanching erythema in a striking photodistribution along the neck (Figure 2) and arms (Figure 3). He had notable edema of both arms and several large 3- to 4-cm bullae on the ventral aspects of the forearms.
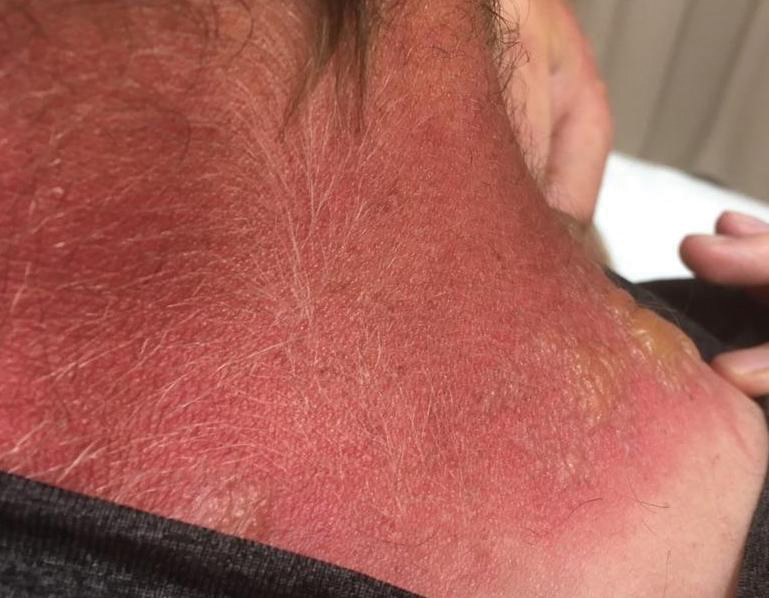
The patient was diagnosed with severe phytophotodermatitis secondary to contact with H mantegazzianum and was started on oral prednisone 70 mg daily (1 mg/kg/d), which was decreased by 10 mg every 3 days until the course of treatment was complete. He also was instructed to apply mupirocin ointment to open areas and petroleum jelly to intact skin. Additionally, he was advised to practice strict photoprotection for the near and distant future.
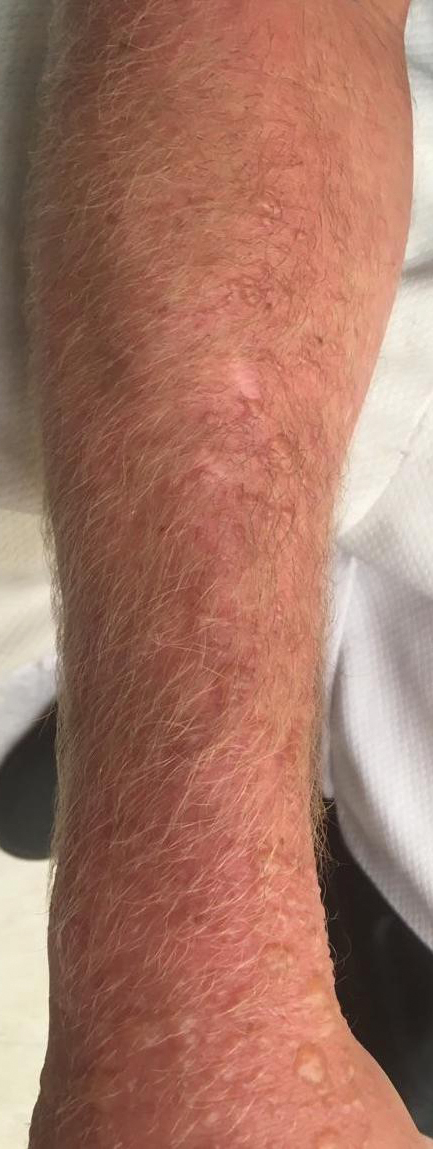
Within several days after treatment began, the phytophotodermatitis dramatically improved, with complete resolution in 1 week. Postinflammatory hyperpigmentation resolved after several weeks.
- Baker B, Bedford J, Kanitkar S. Keeping pace with the media; giant hogweed burns—a case series and comprehensive review [published online December 26, 2016]. Burns. 2017;13:933-938. doi:10.1016/j.burns.2016.10.018
- Klimaszyk P, Klimaszyk D, Piotrowiak M, et al. Unusual complications after occupational exposure to giant hogweed (Heracleum mantegazzianum): a case report. Int J Occup Med Environ Health. 2014;27:141-144. doi:10.2478/s13382-014-0238-z
- Zaveria M, Hauser C. Giant hogweed: a plant that can burn and blind you. but don’t panic. New York Times. July 2, 2018. Accessed October 18, 2021. https://www.nytimes.com/2018/07/02/us/giant-hogweed-nyt.html
- Hignett K. Giant hogweed: New York officials warn residents about dangerous plant that causes serious burns, blisters and scars. Newsweek. June 25, 2019. Accessed October 18, 2021. https://www.newsweek.com/giant-hogweed-new-york-dangerous-plant-burns-skin-sunlight-1445785
- Eastman J. Toxic giant hogweed sap that burns, blisters skin found in Clark County. The Oregonian. Updated July 16, 2019. Accessed October 18, 2021. https://www.oregonlive.com/news/2019/07/toxic-giant-hogweed-plant-that-burns-blisters-skin-found-in-clark-county.html
- O’Kane C. Giant hogweed, plant that causes blindness and third-degree burns, discovered in Virginia. CBS News. June 18, 2018. Accessed October 18, 2021. https://www.cbsnews.com/news/giant-hogweed-plant-causes-blindness-third-degree-burns-discovered-in-virginia-otherstates/
- Pira E, Romano C, Sulotto F, et al. Heracleum mantegazzianum growth phases and furocoumarin content. Contact Dermatitis. 1989;21:300-303. doi:10.1111/j.1600-0536.1989.tb04747.x
- Chan JCY, Sullivan PJ, O’Sullivan MJ, et al. Full thickness burn caused by exposure to giant hogweed: delayed presentation, histological features and surgical management. J Plast Reconstr Aesthet Surg. 2011;64:128-130. doi:10.1016/j.bjps.2010.03.030
- Pfurtscheller K, Trop M. Phototoxic plant burns: report of a case and review of topical wound treatment in children. Pediatr Dermatol. 2014;31:E156-E159. doi:10.1111/pde.12396
- Kavli G, Volden G: Phytophotodermatitis. Photodermatol. 1984;1:65-75.
Giant hogweed (Heracleum mantegazzianum) is an invasive flowering weed of the family Apiaceae that typically reaches a height of 13 feet, with thick stems; large green leaves; and umbrella-shaped, flat-topped, radial clusters (umbels) of small individual white flowers1 (Figure 1). Because of the size and beauty of giant hogweed, it was widely planted in 19th century ornamental gardens in the United Kingdom and has since naturalized and spread throughout central Europe, Canada, and the United States.1,2 The plant most commonly is found in shady areas near rivers and woodlands.1

Due to the invasive nature of the giant hogweed, its prevalence continues to grow, its eradication remains difficult, and reports of phytophotodermatitis are increasing in number and distribution. In fact, there has been widespread media coverage of the dangers of giant hogweed in the United Kingdom since 20161 and in the United States in 2018 and 2019.3-6
Transmission
Phytophotodermatitis is a type of nonimmunologic dermatitis caused by UV light reacting with a plant-based photosensitizing agent. In the case of giant hogweed, sap from the plant’s fruits, leaves, and stem contain furocoumarins or psoralens.7 Upon activation by UVA radiation, furan rings of these compounds create reactive oxygen species and intercalate with DNA pyrimidine bases, which results in cellular death, damage to successive skin layers, and reduced wound healing at the cellular level.8 This effect is intensified with increased percutaneous absorption of furocoumarin, which can result from high temperature, humidity, skin infection, lack of protective clothing, and moist conditions.9
The highest concentration of phototoxic compounds is found in giant hogweed from June through August,7 which, in combination with people increasing their outdoor activity in the summer, results in a greater prevalence and severity of H mantegazzianum phytophotodermatitis during summer months.
Presentation
Phytophotodermatitis caused by giant hogweed can range from burning and erythema to full-thickness chemical burns that require surgical debridement and skin grafting.8 After exposure to the offending agent, a harmful skin reaction can start within 15 minutes. After a latent period of approximately 24 hours, erythema, edema, and bullae can appear and generally peak by 72 hours.10 In addition to cutaneous injury, inhalation of giant hogweed traces can result in obstructive pulmonary symptoms. Eye contact can result in blindness.9
In addition to the rash caused by giant hogweed, a “weed-wacker dermatitis” or “strimmer rash” can be caused by the similar-appearing but smaller common hogweed (Heracleum sphondylium). Common hogweed is highly prevalent in the United States and often is confused with the larger giant hogweed because of tall stems and white, flat-topped flowers.
Management
Following contact with giant hogweed, a person should immediately avoid UV exposure and rinse the area with soap and water. UV radiation must be avoided for at least 48 hours. If erythema occurs, a topical steroid can be applied to the affected area; pain can be alleviated by a nonsteroidal anti-inflammatory drug.9
Further treatment might be required if bullous lesions are present. Small blisters can be punctured and drained; however, large blisters, extensive epidermal-dermal separation, and large areas of detached epidermis should simply be cleansed and dressed. An oral steroid also can be used to reduce inflammation in moderate and severe cases. Full-thickness injury might require surgical intervention.8
Clinical Case
A 27-year-old male landscaper presented to the emergency department with an increasingly painful blistering rash on the arms and neck of 1 day’s duration. He noticed bright red skin and blisters 18 to 24 hours after trimming what he identified as shoulder-high giant hogweed plants. Neither he nor his coworkers were wearing sunscreen or protective clothing as they cleared the plants for several hours. His coworkers developed similar rashes, but his rash was the most severe, requiring treatment in the emergency department.
Physical examination showed innumerable 2- to 10-mm, tense vesicles and bullae on a background of blanching erythema in a striking photodistribution along the neck (Figure 2) and arms (Figure 3). He had notable edema of both arms and several large 3- to 4-cm bullae on the ventral aspects of the forearms.

The patient was diagnosed with severe phytophotodermatitis secondary to contact with H mantegazzianum and was started on oral prednisone 70 mg daily (1 mg/kg/d), which was decreased by 10 mg every 3 days until the course of treatment was complete. He also was instructed to apply mupirocin ointment to open areas and petroleum jelly to intact skin. Additionally, he was advised to practice strict photoprotection for the near and distant future.

Within several days after treatment began, the phytophotodermatitis dramatically improved, with complete resolution in 1 week. Postinflammatory hyperpigmentation resolved after several weeks.
Giant hogweed (Heracleum mantegazzianum) is an invasive flowering weed of the family Apiaceae that typically reaches a height of 13 feet, with thick stems; large green leaves; and umbrella-shaped, flat-topped, radial clusters (umbels) of small individual white flowers1 (Figure 1). Because of the size and beauty of giant hogweed, it was widely planted in 19th century ornamental gardens in the United Kingdom and has since naturalized and spread throughout central Europe, Canada, and the United States.1,2 The plant most commonly is found in shady areas near rivers and woodlands.1

Due to the invasive nature of the giant hogweed, its prevalence continues to grow, its eradication remains difficult, and reports of phytophotodermatitis are increasing in number and distribution. In fact, there has been widespread media coverage of the dangers of giant hogweed in the United Kingdom since 20161 and in the United States in 2018 and 2019.3-6
Transmission
Phytophotodermatitis is a type of nonimmunologic dermatitis caused by UV light reacting with a plant-based photosensitizing agent. In the case of giant hogweed, sap from the plant’s fruits, leaves, and stem contain furocoumarins or psoralens.7 Upon activation by UVA radiation, furan rings of these compounds create reactive oxygen species and intercalate with DNA pyrimidine bases, which results in cellular death, damage to successive skin layers, and reduced wound healing at the cellular level.8 This effect is intensified with increased percutaneous absorption of furocoumarin, which can result from high temperature, humidity, skin infection, lack of protective clothing, and moist conditions.9
The highest concentration of phototoxic compounds is found in giant hogweed from June through August,7 which, in combination with people increasing their outdoor activity in the summer, results in a greater prevalence and severity of H mantegazzianum phytophotodermatitis during summer months.
Presentation
Phytophotodermatitis caused by giant hogweed can range from burning and erythema to full-thickness chemical burns that require surgical debridement and skin grafting.8 After exposure to the offending agent, a harmful skin reaction can start within 15 minutes. After a latent period of approximately 24 hours, erythema, edema, and bullae can appear and generally peak by 72 hours.10 In addition to cutaneous injury, inhalation of giant hogweed traces can result in obstructive pulmonary symptoms. Eye contact can result in blindness.9
In addition to the rash caused by giant hogweed, a “weed-wacker dermatitis” or “strimmer rash” can be caused by the similar-appearing but smaller common hogweed (Heracleum sphondylium). Common hogweed is highly prevalent in the United States and often is confused with the larger giant hogweed because of tall stems and white, flat-topped flowers.
Management
Following contact with giant hogweed, a person should immediately avoid UV exposure and rinse the area with soap and water. UV radiation must be avoided for at least 48 hours. If erythema occurs, a topical steroid can be applied to the affected area; pain can be alleviated by a nonsteroidal anti-inflammatory drug.9
Further treatment might be required if bullous lesions are present. Small blisters can be punctured and drained; however, large blisters, extensive epidermal-dermal separation, and large areas of detached epidermis should simply be cleansed and dressed. An oral steroid also can be used to reduce inflammation in moderate and severe cases. Full-thickness injury might require surgical intervention.8
Clinical Case
A 27-year-old male landscaper presented to the emergency department with an increasingly painful blistering rash on the arms and neck of 1 day’s duration. He noticed bright red skin and blisters 18 to 24 hours after trimming what he identified as shoulder-high giant hogweed plants. Neither he nor his coworkers were wearing sunscreen or protective clothing as they cleared the plants for several hours. His coworkers developed similar rashes, but his rash was the most severe, requiring treatment in the emergency department.
Physical examination showed innumerable 2- to 10-mm, tense vesicles and bullae on a background of blanching erythema in a striking photodistribution along the neck (Figure 2) and arms (Figure 3). He had notable edema of both arms and several large 3- to 4-cm bullae on the ventral aspects of the forearms.

The patient was diagnosed with severe phytophotodermatitis secondary to contact with H mantegazzianum and was started on oral prednisone 70 mg daily (1 mg/kg/d), which was decreased by 10 mg every 3 days until the course of treatment was complete. He also was instructed to apply mupirocin ointment to open areas and petroleum jelly to intact skin. Additionally, he was advised to practice strict photoprotection for the near and distant future.

Within several days after treatment began, the phytophotodermatitis dramatically improved, with complete resolution in 1 week. Postinflammatory hyperpigmentation resolved after several weeks.
- Baker B, Bedford J, Kanitkar S. Keeping pace with the media; giant hogweed burns—a case series and comprehensive review [published online December 26, 2016]. Burns. 2017;13:933-938. doi:10.1016/j.burns.2016.10.018
- Klimaszyk P, Klimaszyk D, Piotrowiak M, et al. Unusual complications after occupational exposure to giant hogweed (Heracleum mantegazzianum): a case report. Int J Occup Med Environ Health. 2014;27:141-144. doi:10.2478/s13382-014-0238-z
- Zaveria M, Hauser C. Giant hogweed: a plant that can burn and blind you. but don’t panic. New York Times. July 2, 2018. Accessed October 18, 2021. https://www.nytimes.com/2018/07/02/us/giant-hogweed-nyt.html
- Hignett K. Giant hogweed: New York officials warn residents about dangerous plant that causes serious burns, blisters and scars. Newsweek. June 25, 2019. Accessed October 18, 2021. https://www.newsweek.com/giant-hogweed-new-york-dangerous-plant-burns-skin-sunlight-1445785
- Eastman J. Toxic giant hogweed sap that burns, blisters skin found in Clark County. The Oregonian. Updated July 16, 2019. Accessed October 18, 2021. https://www.oregonlive.com/news/2019/07/toxic-giant-hogweed-plant-that-burns-blisters-skin-found-in-clark-county.html
- O’Kane C. Giant hogweed, plant that causes blindness and third-degree burns, discovered in Virginia. CBS News. June 18, 2018. Accessed October 18, 2021. https://www.cbsnews.com/news/giant-hogweed-plant-causes-blindness-third-degree-burns-discovered-in-virginia-otherstates/
- Pira E, Romano C, Sulotto F, et al. Heracleum mantegazzianum growth phases and furocoumarin content. Contact Dermatitis. 1989;21:300-303. doi:10.1111/j.1600-0536.1989.tb04747.x
- Chan JCY, Sullivan PJ, O’Sullivan MJ, et al. Full thickness burn caused by exposure to giant hogweed: delayed presentation, histological features and surgical management. J Plast Reconstr Aesthet Surg. 2011;64:128-130. doi:10.1016/j.bjps.2010.03.030
- Pfurtscheller K, Trop M. Phototoxic plant burns: report of a case and review of topical wound treatment in children. Pediatr Dermatol. 2014;31:E156-E159. doi:10.1111/pde.12396
- Kavli G, Volden G: Phytophotodermatitis. Photodermatol. 1984;1:65-75.
- Baker B, Bedford J, Kanitkar S. Keeping pace with the media; giant hogweed burns—a case series and comprehensive review [published online December 26, 2016]. Burns. 2017;13:933-938. doi:10.1016/j.burns.2016.10.018
- Klimaszyk P, Klimaszyk D, Piotrowiak M, et al. Unusual complications after occupational exposure to giant hogweed (Heracleum mantegazzianum): a case report. Int J Occup Med Environ Health. 2014;27:141-144. doi:10.2478/s13382-014-0238-z
- Zaveria M, Hauser C. Giant hogweed: a plant that can burn and blind you. but don’t panic. New York Times. July 2, 2018. Accessed October 18, 2021. https://www.nytimes.com/2018/07/02/us/giant-hogweed-nyt.html
- Hignett K. Giant hogweed: New York officials warn residents about dangerous plant that causes serious burns, blisters and scars. Newsweek. June 25, 2019. Accessed October 18, 2021. https://www.newsweek.com/giant-hogweed-new-york-dangerous-plant-burns-skin-sunlight-1445785
- Eastman J. Toxic giant hogweed sap that burns, blisters skin found in Clark County. The Oregonian. Updated July 16, 2019. Accessed October 18, 2021. https://www.oregonlive.com/news/2019/07/toxic-giant-hogweed-plant-that-burns-blisters-skin-found-in-clark-county.html
- O’Kane C. Giant hogweed, plant that causes blindness and third-degree burns, discovered in Virginia. CBS News. June 18, 2018. Accessed October 18, 2021. https://www.cbsnews.com/news/giant-hogweed-plant-causes-blindness-third-degree-burns-discovered-in-virginia-otherstates/
- Pira E, Romano C, Sulotto F, et al. Heracleum mantegazzianum growth phases and furocoumarin content. Contact Dermatitis. 1989;21:300-303. doi:10.1111/j.1600-0536.1989.tb04747.x
- Chan JCY, Sullivan PJ, O’Sullivan MJ, et al. Full thickness burn caused by exposure to giant hogweed: delayed presentation, histological features and surgical management. J Plast Reconstr Aesthet Surg. 2011;64:128-130. doi:10.1016/j.bjps.2010.03.030
- Pfurtscheller K, Trop M. Phototoxic plant burns: report of a case and review of topical wound treatment in children. Pediatr Dermatol. 2014;31:E156-E159. doi:10.1111/pde.12396
- Kavli G, Volden G: Phytophotodermatitis. Photodermatol. 1984;1:65-75.
PRACTICE POINTS
- The public should be educated, especially during summer months, about how to identify giant hogweed, reduce exposure to the plant’s phototoxin, and thus reduce the risk for severe phytophotodermatitis.
- Phytophotodermatitis should be included in the differential diagnosis when a patient presents with acute erythema and bullae in sun-exposed areas.
- Phytophotodermatitis can be treated by promptly washing the skin with soap and water, protecting the skin from exposure to UV light, and utilizing topical and oral steroids.