User login
Aquatic Antagonists: Jellyfish Stings
Jellyfish stings are one of the most common marine injuries, with an estimated 150 million stings occurring annually worldwide.1 Most jellyfish stings result in painful localized skin reactions that are self-limited and can be treated with conservative measures including hot water immersion and topical anesthetics. Life-threatening systemic reactions (eg, anaphylaxis, Irukandji syndrome) can occur with some species.2-4 Mainstream media reports do not reflect the true incidence and variability of jellyfish-related injuries that are commonly encountered in the clinic.3
Characteristics of Jellyfish
There are roughly 10,000 known species of jellyfish, with approximately 100 of them posing danger to humans.5 Jellyfish belong to the phylum Cnidaria, which is comprised of 5 classes of both free-floating and sessile animals: Staurozoa (stauromedusae), Hydrozoa (hydroids, fire corals, and Portuguese man-of-war), Scyphozoa (true jellyfish), Anthozoa (corals and sea anemones), and Cubozoa (box jellyfish and Irukandji jellyfish).1,2,6 Jellyfish typically have several tentacles suspended from a free-floating gelatinous body or bell; these tentacles are covered with thousands of cells unique to Cnidaria called nematocytes or cnidocytes containing specialized stinging organelles known as nematocysts. When triggered by physical (eg, human or foreign-body contact) or chemical stimuli, each nematocyst ejects a hollow filament or barb externally, releasing venom into the victim.7,8

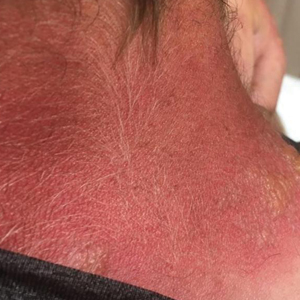
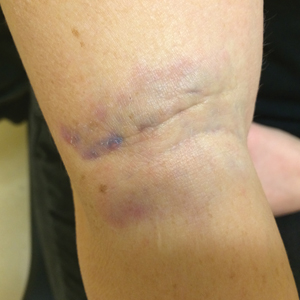
The scyphozoan, hydrozoan, and cubozoan life cycles generally consist of a bottom-dwelling, sessile polyp form that produces multiple free-swimming ephyrae through an asexual reproductive process called strobilation. These ephyrae grow into the fully mature medusae, recognizable as jellyfish (Figure 1).5 Additionally, jellyfish populations experience cycles of temporal and spatial population abundance and crashes known as jellyfish blooms. In 2017, Kaffenberger et al9 reviewed the shifting landscape of skin diseases in North America attributable to major changes in climate and weather patterns, including the rise in jellyfish blooms and envenomation outbreaks worldwide (eg, Physalia physalis [Portuguese man-of-war][Figure 2] along the southeastern US coastline, Porpita pacifica off Japanese beaches). Some research suggests jellyfish surges relate to climate change and human interactions with jellyfish habitats by way of eutrophication and fishing (removing predators of jellyfish).9,10

Clinical Presentation
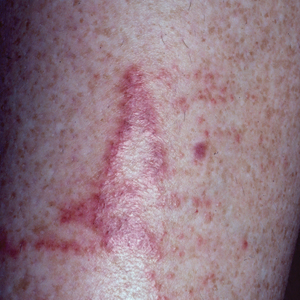
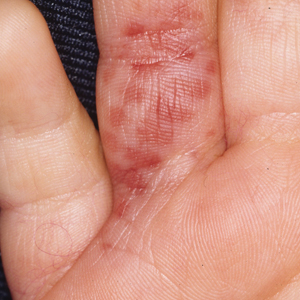
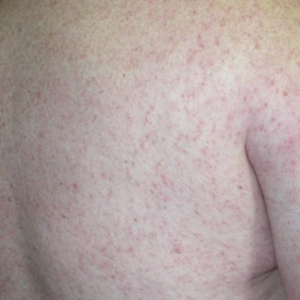
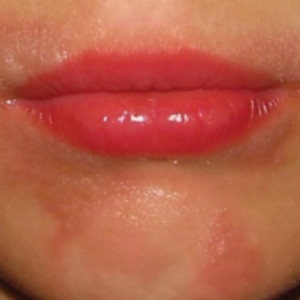
Jellyfish injuries can vary greatly in clinical symptoms, but they do follow some basic patterns. The severity of pain and symptoms is related to the jellyfish species, the number of stinging cells (nematocysts) that are triggered, and the potency of the venom that is absorbed by the victim.11-13 Most stings are minor, and patients experience immediate localized pain with serpiginous raised erythematous or urticarial lesions following the distribution of tentacle contact; these lesions have been described as tentaclelike and resembling a string of beads (Figure 3).12 Pain usually lasts a couple hours, while the skin lesions can last hours to days and can even recur years later. This pattern fits that of the well-known hydrozoans P physalis and Physalia utriculus (bluebottle), which are endemic to the Atlantic and Indo-Pacific Oceans, respectively. The scyphozoan jellyfish causing similar presentations include Pelagia noctiluca (Mauve stinger), Aurelia aurita (Moon jellyfish), and Cyanea species. The cubozoan Chironex fleckeri (Australian box jellyfish or sea wasp) also causes tentaclelike stings but is widely considered the most dangerous jellyfish, as its venom is known to cause cardiac or respiratory arrest.4,11 More than 100 fatalities have been reported following severe envenomations from C fleckeri in Australian and Indo-Pacific waters.6
Stings from another box jellyfish species, Carukia barnesi, cause a unique presentation known as Irukandji syndrome. Carukia barnesi is a small box jellyfish with a bell measuring roughly 2 cm in diameter. It has nematocysts on both its bell and tentacles. It inhabits deeper waters and typically stings divers but also can wash ashore and injure beach tourists. Although Irukandji syndrome usually is associated with C barnesi, which is endemic to Northern Australian beaches, other jellyfish species including P physalis rarely have been linked to this potentially fatal syndrome.6,11 Unlike the immediate cutaneous and systemic findings described in C fleckeri encounters, symptoms of Irukandji-like stings can be delayed by up to 30 minutes. Patients may present with severe generalized pain (lower back, chest, headache), signs of excess catecholamine release (tachycardia, hypertension, anxiety, diaphoresis, agitation), or cardiopulmonary decompensation (arrhythmia, cardiac arrest, pulmonary edema).6,11,14.15 Anaphylactic reactions also have been reported in those sensitized by prior stings.16
Management
Prevention of drowning is key in all marine injuries. Rescuers should remove the individual from the water, establish the ABCs—airway, breathing, and circulation—and seek acute medical attention. If immediate resuscitation is not required, douse the wound as soon as possible with a solution that halts further nematocyst discharge, which may contain alcohol, vinegar, or bicarbonate, depending on the prevalent species. General guidance is available to providers through evidence-based, point-of-care databases including UpToDate and DynaMed, as well as through the American Heart Association (AHA) or a country’s equivalent council on emergency care if residing outside the United States. Pressure immobilization bandages as a means of decreasing venom redistribution is no longer recommended by the AHA because animal studies have shown increased nematocyst discharge after pressure application.17 As such, touching or applying pressure to the affected area is not recommended until after a proper rinse solution has been applied. Tentacles may be removed mechanically with gloved hands or sand and seawater with minimal compression or agitation.
When acetic acid is appropriate, such as for cubozoan stings, commercially available vinegar (5% acetic acid in the United States) is preferred.16,17 Tap water can cause discharge of nematocysts, and seawater is preferred when no other solution is available.18 Most marine venoms are heat labile. Immersion in hot water can produce pain relief, but ice can be just as efficacious and is preferred by some patients. Prior reports of patients stung by Physalia species demonstrated greater pain relief with hot water immersion compared to ice pack application.18,19
In the setting of anaphylaxis, patients should receive epinephrine and be transported to a hospital with appropriate hemodynamic monitoring and supportive care. If the species of jellyfish has been identified, species-specific antivenin also may be available in certain regions (eg, C fleckeri antivenin manufactured in Australia), but it is unclear if it improves outcomes when compared with supportive care alone.6,16
Conclusion
Following jellyfish stings, most skin lesions will spontaneously resolve. Patients likely will present days to weeks following the inciting event with mild cutaneous symptoms that are amenable to topical corticosteroids. Recurrent dermatitis following a jellyfish sting is uncommon and is thought to be due to an immunologic mechanism consistent with type IV hypersensitivity reactions. Patients may require multiple courses of treatment before complete resolution.20
Patient education regarding marine envenomation and mechanical barriers such as wetsuits or stinger suits can reduce the risk for injury from jellyfish stings. Sting-inhibiting lotions also are commercially available, though more research is needed.21 Many beaches that are known to harbor the dangerous box jellyfish provide stinger nets to direct travelers to safer waters. Complete avoidance during jellyfish season is recommended in highly endemic areas.1
- Cegolon L, Heymann WC, Lange JH, et al. Jellyfish stings and their management: a review. Mar Drugs. 2013;11:523-550.
- Hornbeak KB, Auerbach PS. Marine envenomation. Emerg Med Clin North Am. 2017;35:321-337.
- Ward NT, Darracq MA, Tomaszewski C, et al. Evidence-based treatment of jellyfish stings in North America and Hawaii. Ann Emerg Med. 2012;60:399-414.
- Burnett JW, Calton GJ, Burnett HW. Jellyfish envenomation syndromes. J Am Acad Dermatol. 1986;14:100-106.
- Brotz L, Cheung WWL, Kleisner K, et al. Increasing jellyfish populations: trends in large marine ecosystems. Hydrobiologia. 2012;690:3-20.
- Ottuso PT. Aquatic antagonists: Cubozoan jellyfish (Chironex fleckeri and Carukia barnesi). Cutis. 2010;85:133-136.
- Lakkis NA, Maalouf GJ, Mahmassani DM. Jellyfish stings: a practical approach. Wilderness Environ Med. 2015;26:422-429.
- Li L, McGee RG, Isbister G, et al. Interventions for the symptoms and signs resulting from jellyfish stings. Cochrane Database Syst Rev. 2013;12:CD009688.
- Kaffenberger BH, Shetlar D, Norton SA, et al. The effect of climate change on skin disease in North America. J Am Acad Dermatol. 2017;76:140-147.
- Purcell JE, Uye S, Lo W. Anthropogenic causes of jellyfish blooms and their direct consequences for humans: a review. Marine Ecology Progress Series. 2007;350:153-174.
- Berling I, Isbister G. Marine envenomations. Aust Fam Physician. 2015;44:28-32.
- Tibballs J, Yanagihara AA, Turner HC, et al. Immunological and toxinological responses to jellyfish stings. Inflamm Allergy Drug Targets. 2011;10:438-446.
- Tibballs J. Australian venomous jellyfish, envenomation syndromes, toxins and therapy. Toxicon. 2006;48:830-859.
- Stein MR, Marracini JV, Rothschild NE, et al. Fatal Portuguese man-o’-war (Physalia physalis) envenomation. Ann Emerg Med. 1989;18:312-315.
- Burnett JW, Gable WD. A fatal jellyfish envenomation by the Portuguese man-o’war. Toxicon. 1989;27:823-824.
- Warrell DA. Venomous bites, stings, and poisoning: an update. Infect Dis Clin North Am. 2019;33:17-38.
- Neumar RW, Shuster M, Callaway CW, et al. Part 1: executive summary: 2015 American Heart Association guidelines update for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation. 2015;132(18 suppl 2):S315-S367.
- Wilcox CL, Headlam JL, Doyle TK, et al. Assessing the efficacy of first-aid measures in Physalia sp. envenomation, using solution- and blood agarose-based models. Toxins (Basel). 2017;9:149.
- Wilcox CL, Yanagihara AA. Heated debates: hot-water immersion or ice packs as first aid for cnidarian envenomations? Toxins (Basel). 2016;8:97.
- Loredana Asztalos M, Rubin AI, Elenitsas R, et al. Recurrent dermatitis and dermal hypersensitivity following a jellyfish sting: a case report and review of literature. Pediatr Dermatol. 2014;31:217-219.
- Boulware DR. A randomized, controlled field trial for the prevention of jellyfish stings with a topical sting inhibitor. J Travel Med. 2006;13:166-171.
Jellyfish stings are one of the most common marine injuries, with an estimated 150 million stings occurring annually worldwide.1 Most jellyfish stings result in painful localized skin reactions that are self-limited and can be treated with conservative measures including hot water immersion and topical anesthetics. Life-threatening systemic reactions (eg, anaphylaxis, Irukandji syndrome) can occur with some species.2-4 Mainstream media reports do not reflect the true incidence and variability of jellyfish-related injuries that are commonly encountered in the clinic.3
Characteristics of Jellyfish
There are roughly 10,000 known species of jellyfish, with approximately 100 of them posing danger to humans.5 Jellyfish belong to the phylum Cnidaria, which is comprised of 5 classes of both free-floating and sessile animals: Staurozoa (stauromedusae), Hydrozoa (hydroids, fire corals, and Portuguese man-of-war), Scyphozoa (true jellyfish), Anthozoa (corals and sea anemones), and Cubozoa (box jellyfish and Irukandji jellyfish).1,2,6 Jellyfish typically have several tentacles suspended from a free-floating gelatinous body or bell; these tentacles are covered with thousands of cells unique to Cnidaria called nematocytes or cnidocytes containing specialized stinging organelles known as nematocysts. When triggered by physical (eg, human or foreign-body contact) or chemical stimuli, each nematocyst ejects a hollow filament or barb externally, releasing venom into the victim.7,8

The scyphozoan, hydrozoan, and cubozoan life cycles generally consist of a bottom-dwelling, sessile polyp form that produces multiple free-swimming ephyrae through an asexual reproductive process called strobilation. These ephyrae grow into the fully mature medusae, recognizable as jellyfish (Figure 1).5 Additionally, jellyfish populations experience cycles of temporal and spatial population abundance and crashes known as jellyfish blooms. In 2017, Kaffenberger et al9 reviewed the shifting landscape of skin diseases in North America attributable to major changes in climate and weather patterns, including the rise in jellyfish blooms and envenomation outbreaks worldwide (eg, Physalia physalis [Portuguese man-of-war][Figure 2] along the southeastern US coastline, Porpita pacifica off Japanese beaches). Some research suggests jellyfish surges relate to climate change and human interactions with jellyfish habitats by way of eutrophication and fishing (removing predators of jellyfish).9,10

Clinical Presentation
Jellyfish injuries can vary greatly in clinical symptoms, but they do follow some basic patterns. The severity of pain and symptoms is related to the jellyfish species, the number of stinging cells (nematocysts) that are triggered, and the potency of the venom that is absorbed by the victim.11-13 Most stings are minor, and patients experience immediate localized pain with serpiginous raised erythematous or urticarial lesions following the distribution of tentacle contact; these lesions have been described as tentaclelike and resembling a string of beads (Figure 3).12 Pain usually lasts a couple hours, while the skin lesions can last hours to days and can even recur years later. This pattern fits that of the well-known hydrozoans P physalis and Physalia utriculus (bluebottle), which are endemic to the Atlantic and Indo-Pacific Oceans, respectively. The scyphozoan jellyfish causing similar presentations include Pelagia noctiluca (Mauve stinger), Aurelia aurita (Moon jellyfish), and Cyanea species. The cubozoan Chironex fleckeri (Australian box jellyfish or sea wasp) also causes tentaclelike stings but is widely considered the most dangerous jellyfish, as its venom is known to cause cardiac or respiratory arrest.4,11 More than 100 fatalities have been reported following severe envenomations from C fleckeri in Australian and Indo-Pacific waters.6

Stings from another box jellyfish species, Carukia barnesi, cause a unique presentation known as Irukandji syndrome. Carukia barnesi is a small box jellyfish with a bell measuring roughly 2 cm in diameter. It has nematocysts on both its bell and tentacles. It inhabits deeper waters and typically stings divers but also can wash ashore and injure beach tourists. Although Irukandji syndrome usually is associated with C barnesi, which is endemic to Northern Australian beaches, other jellyfish species including P physalis rarely have been linked to this potentially fatal syndrome.6,11 Unlike the immediate cutaneous and systemic findings described in C fleckeri encounters, symptoms of Irukandji-like stings can be delayed by up to 30 minutes. Patients may present with severe generalized pain (lower back, chest, headache), signs of excess catecholamine release (tachycardia, hypertension, anxiety, diaphoresis, agitation), or cardiopulmonary decompensation (arrhythmia, cardiac arrest, pulmonary edema).6,11,14.15 Anaphylactic reactions also have been reported in those sensitized by prior stings.16
Management
Prevention of drowning is key in all marine injuries. Rescuers should remove the individual from the water, establish the ABCs—airway, breathing, and circulation—and seek acute medical attention. If immediate resuscitation is not required, douse the wound as soon as possible with a solution that halts further nematocyst discharge, which may contain alcohol, vinegar, or bicarbonate, depending on the prevalent species. General guidance is available to providers through evidence-based, point-of-care databases including UpToDate and DynaMed, as well as through the American Heart Association (AHA) or a country’s equivalent council on emergency care if residing outside the United States. Pressure immobilization bandages as a means of decreasing venom redistribution is no longer recommended by the AHA because animal studies have shown increased nematocyst discharge after pressure application.17 As such, touching or applying pressure to the affected area is not recommended until after a proper rinse solution has been applied. Tentacles may be removed mechanically with gloved hands or sand and seawater with minimal compression or agitation.
When acetic acid is appropriate, such as for cubozoan stings, commercially available vinegar (5% acetic acid in the United States) is preferred.16,17 Tap water can cause discharge of nematocysts, and seawater is preferred when no other solution is available.18 Most marine venoms are heat labile. Immersion in hot water can produce pain relief, but ice can be just as efficacious and is preferred by some patients. Prior reports of patients stung by Physalia species demonstrated greater pain relief with hot water immersion compared to ice pack application.18,19
In the setting of anaphylaxis, patients should receive epinephrine and be transported to a hospital with appropriate hemodynamic monitoring and supportive care. If the species of jellyfish has been identified, species-specific antivenin also may be available in certain regions (eg, C fleckeri antivenin manufactured in Australia), but it is unclear if it improves outcomes when compared with supportive care alone.6,16
Conclusion
Following jellyfish stings, most skin lesions will spontaneously resolve. Patients likely will present days to weeks following the inciting event with mild cutaneous symptoms that are amenable to topical corticosteroids. Recurrent dermatitis following a jellyfish sting is uncommon and is thought to be due to an immunologic mechanism consistent with type IV hypersensitivity reactions. Patients may require multiple courses of treatment before complete resolution.20
Patient education regarding marine envenomation and mechanical barriers such as wetsuits or stinger suits can reduce the risk for injury from jellyfish stings. Sting-inhibiting lotions also are commercially available, though more research is needed.21 Many beaches that are known to harbor the dangerous box jellyfish provide stinger nets to direct travelers to safer waters. Complete avoidance during jellyfish season is recommended in highly endemic areas.1
Jellyfish stings are one of the most common marine injuries, with an estimated 150 million stings occurring annually worldwide.1 Most jellyfish stings result in painful localized skin reactions that are self-limited and can be treated with conservative measures including hot water immersion and topical anesthetics. Life-threatening systemic reactions (eg, anaphylaxis, Irukandji syndrome) can occur with some species.2-4 Mainstream media reports do not reflect the true incidence and variability of jellyfish-related injuries that are commonly encountered in the clinic.3
Characteristics of Jellyfish
There are roughly 10,000 known species of jellyfish, with approximately 100 of them posing danger to humans.5 Jellyfish belong to the phylum Cnidaria, which is comprised of 5 classes of both free-floating and sessile animals: Staurozoa (stauromedusae), Hydrozoa (hydroids, fire corals, and Portuguese man-of-war), Scyphozoa (true jellyfish), Anthozoa (corals and sea anemones), and Cubozoa (box jellyfish and Irukandji jellyfish).1,2,6 Jellyfish typically have several tentacles suspended from a free-floating gelatinous body or bell; these tentacles are covered with thousands of cells unique to Cnidaria called nematocytes or cnidocytes containing specialized stinging organelles known as nematocysts. When triggered by physical (eg, human or foreign-body contact) or chemical stimuli, each nematocyst ejects a hollow filament or barb externally, releasing venom into the victim.7,8

The scyphozoan, hydrozoan, and cubozoan life cycles generally consist of a bottom-dwelling, sessile polyp form that produces multiple free-swimming ephyrae through an asexual reproductive process called strobilation. These ephyrae grow into the fully mature medusae, recognizable as jellyfish (Figure 1).5 Additionally, jellyfish populations experience cycles of temporal and spatial population abundance and crashes known as jellyfish blooms. In 2017, Kaffenberger et al9 reviewed the shifting landscape of skin diseases in North America attributable to major changes in climate and weather patterns, including the rise in jellyfish blooms and envenomation outbreaks worldwide (eg, Physalia physalis [Portuguese man-of-war][Figure 2] along the southeastern US coastline, Porpita pacifica off Japanese beaches). Some research suggests jellyfish surges relate to climate change and human interactions with jellyfish habitats by way of eutrophication and fishing (removing predators of jellyfish).9,10

Clinical Presentation
Jellyfish injuries can vary greatly in clinical symptoms, but they do follow some basic patterns. The severity of pain and symptoms is related to the jellyfish species, the number of stinging cells (nematocysts) that are triggered, and the potency of the venom that is absorbed by the victim.11-13 Most stings are minor, and patients experience immediate localized pain with serpiginous raised erythematous or urticarial lesions following the distribution of tentacle contact; these lesions have been described as tentaclelike and resembling a string of beads (Figure 3).12 Pain usually lasts a couple hours, while the skin lesions can last hours to days and can even recur years later. This pattern fits that of the well-known hydrozoans P physalis and Physalia utriculus (bluebottle), which are endemic to the Atlantic and Indo-Pacific Oceans, respectively. The scyphozoan jellyfish causing similar presentations include Pelagia noctiluca (Mauve stinger), Aurelia aurita (Moon jellyfish), and Cyanea species. The cubozoan Chironex fleckeri (Australian box jellyfish or sea wasp) also causes tentaclelike stings but is widely considered the most dangerous jellyfish, as its venom is known to cause cardiac or respiratory arrest.4,11 More than 100 fatalities have been reported following severe envenomations from C fleckeri in Australian and Indo-Pacific waters.6

Stings from another box jellyfish species, Carukia barnesi, cause a unique presentation known as Irukandji syndrome. Carukia barnesi is a small box jellyfish with a bell measuring roughly 2 cm in diameter. It has nematocysts on both its bell and tentacles. It inhabits deeper waters and typically stings divers but also can wash ashore and injure beach tourists. Although Irukandji syndrome usually is associated with C barnesi, which is endemic to Northern Australian beaches, other jellyfish species including P physalis rarely have been linked to this potentially fatal syndrome.6,11 Unlike the immediate cutaneous and systemic findings described in C fleckeri encounters, symptoms of Irukandji-like stings can be delayed by up to 30 minutes. Patients may present with severe generalized pain (lower back, chest, headache), signs of excess catecholamine release (tachycardia, hypertension, anxiety, diaphoresis, agitation), or cardiopulmonary decompensation (arrhythmia, cardiac arrest, pulmonary edema).6,11,14.15 Anaphylactic reactions also have been reported in those sensitized by prior stings.16
Management
Prevention of drowning is key in all marine injuries. Rescuers should remove the individual from the water, establish the ABCs—airway, breathing, and circulation—and seek acute medical attention. If immediate resuscitation is not required, douse the wound as soon as possible with a solution that halts further nematocyst discharge, which may contain alcohol, vinegar, or bicarbonate, depending on the prevalent species. General guidance is available to providers through evidence-based, point-of-care databases including UpToDate and DynaMed, as well as through the American Heart Association (AHA) or a country’s equivalent council on emergency care if residing outside the United States. Pressure immobilization bandages as a means of decreasing venom redistribution is no longer recommended by the AHA because animal studies have shown increased nematocyst discharge after pressure application.17 As such, touching or applying pressure to the affected area is not recommended until after a proper rinse solution has been applied. Tentacles may be removed mechanically with gloved hands or sand and seawater with minimal compression or agitation.
When acetic acid is appropriate, such as for cubozoan stings, commercially available vinegar (5% acetic acid in the United States) is preferred.16,17 Tap water can cause discharge of nematocysts, and seawater is preferred when no other solution is available.18 Most marine venoms are heat labile. Immersion in hot water can produce pain relief, but ice can be just as efficacious and is preferred by some patients. Prior reports of patients stung by Physalia species demonstrated greater pain relief with hot water immersion compared to ice pack application.18,19
In the setting of anaphylaxis, patients should receive epinephrine and be transported to a hospital with appropriate hemodynamic monitoring and supportive care. If the species of jellyfish has been identified, species-specific antivenin also may be available in certain regions (eg, C fleckeri antivenin manufactured in Australia), but it is unclear if it improves outcomes when compared with supportive care alone.6,16
Conclusion
Following jellyfish stings, most skin lesions will spontaneously resolve. Patients likely will present days to weeks following the inciting event with mild cutaneous symptoms that are amenable to topical corticosteroids. Recurrent dermatitis following a jellyfish sting is uncommon and is thought to be due to an immunologic mechanism consistent with type IV hypersensitivity reactions. Patients may require multiple courses of treatment before complete resolution.20
Patient education regarding marine envenomation and mechanical barriers such as wetsuits or stinger suits can reduce the risk for injury from jellyfish stings. Sting-inhibiting lotions also are commercially available, though more research is needed.21 Many beaches that are known to harbor the dangerous box jellyfish provide stinger nets to direct travelers to safer waters. Complete avoidance during jellyfish season is recommended in highly endemic areas.1
- Cegolon L, Heymann WC, Lange JH, et al. Jellyfish stings and their management: a review. Mar Drugs. 2013;11:523-550.
- Hornbeak KB, Auerbach PS. Marine envenomation. Emerg Med Clin North Am. 2017;35:321-337.
- Ward NT, Darracq MA, Tomaszewski C, et al. Evidence-based treatment of jellyfish stings in North America and Hawaii. Ann Emerg Med. 2012;60:399-414.
- Burnett JW, Calton GJ, Burnett HW. Jellyfish envenomation syndromes. J Am Acad Dermatol. 1986;14:100-106.
- Brotz L, Cheung WWL, Kleisner K, et al. Increasing jellyfish populations: trends in large marine ecosystems. Hydrobiologia. 2012;690:3-20.
- Ottuso PT. Aquatic antagonists: Cubozoan jellyfish (Chironex fleckeri and Carukia barnesi). Cutis. 2010;85:133-136.
- Lakkis NA, Maalouf GJ, Mahmassani DM. Jellyfish stings: a practical approach. Wilderness Environ Med. 2015;26:422-429.
- Li L, McGee RG, Isbister G, et al. Interventions for the symptoms and signs resulting from jellyfish stings. Cochrane Database Syst Rev. 2013;12:CD009688.
- Kaffenberger BH, Shetlar D, Norton SA, et al. The effect of climate change on skin disease in North America. J Am Acad Dermatol. 2017;76:140-147.
- Purcell JE, Uye S, Lo W. Anthropogenic causes of jellyfish blooms and their direct consequences for humans: a review. Marine Ecology Progress Series. 2007;350:153-174.
- Berling I, Isbister G. Marine envenomations. Aust Fam Physician. 2015;44:28-32.
- Tibballs J, Yanagihara AA, Turner HC, et al. Immunological and toxinological responses to jellyfish stings. Inflamm Allergy Drug Targets. 2011;10:438-446.
- Tibballs J. Australian venomous jellyfish, envenomation syndromes, toxins and therapy. Toxicon. 2006;48:830-859.
- Stein MR, Marracini JV, Rothschild NE, et al. Fatal Portuguese man-o’-war (Physalia physalis) envenomation. Ann Emerg Med. 1989;18:312-315.
- Burnett JW, Gable WD. A fatal jellyfish envenomation by the Portuguese man-o’war. Toxicon. 1989;27:823-824.
- Warrell DA. Venomous bites, stings, and poisoning: an update. Infect Dis Clin North Am. 2019;33:17-38.
- Neumar RW, Shuster M, Callaway CW, et al. Part 1: executive summary: 2015 American Heart Association guidelines update for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation. 2015;132(18 suppl 2):S315-S367.
- Wilcox CL, Headlam JL, Doyle TK, et al. Assessing the efficacy of first-aid measures in Physalia sp. envenomation, using solution- and blood agarose-based models. Toxins (Basel). 2017;9:149.
- Wilcox CL, Yanagihara AA. Heated debates: hot-water immersion or ice packs as first aid for cnidarian envenomations? Toxins (Basel). 2016;8:97.
- Loredana Asztalos M, Rubin AI, Elenitsas R, et al. Recurrent dermatitis and dermal hypersensitivity following a jellyfish sting: a case report and review of literature. Pediatr Dermatol. 2014;31:217-219.
- Boulware DR. A randomized, controlled field trial for the prevention of jellyfish stings with a topical sting inhibitor. J Travel Med. 2006;13:166-171.
- Cegolon L, Heymann WC, Lange JH, et al. Jellyfish stings and their management: a review. Mar Drugs. 2013;11:523-550.
- Hornbeak KB, Auerbach PS. Marine envenomation. Emerg Med Clin North Am. 2017;35:321-337.
- Ward NT, Darracq MA, Tomaszewski C, et al. Evidence-based treatment of jellyfish stings in North America and Hawaii. Ann Emerg Med. 2012;60:399-414.
- Burnett JW, Calton GJ, Burnett HW. Jellyfish envenomation syndromes. J Am Acad Dermatol. 1986;14:100-106.
- Brotz L, Cheung WWL, Kleisner K, et al. Increasing jellyfish populations: trends in large marine ecosystems. Hydrobiologia. 2012;690:3-20.
- Ottuso PT. Aquatic antagonists: Cubozoan jellyfish (Chironex fleckeri and Carukia barnesi). Cutis. 2010;85:133-136.
- Lakkis NA, Maalouf GJ, Mahmassani DM. Jellyfish stings: a practical approach. Wilderness Environ Med. 2015;26:422-429.
- Li L, McGee RG, Isbister G, et al. Interventions for the symptoms and signs resulting from jellyfish stings. Cochrane Database Syst Rev. 2013;12:CD009688.
- Kaffenberger BH, Shetlar D, Norton SA, et al. The effect of climate change on skin disease in North America. J Am Acad Dermatol. 2017;76:140-147.
- Purcell JE, Uye S, Lo W. Anthropogenic causes of jellyfish blooms and their direct consequences for humans: a review. Marine Ecology Progress Series. 2007;350:153-174.
- Berling I, Isbister G. Marine envenomations. Aust Fam Physician. 2015;44:28-32.
- Tibballs J, Yanagihara AA, Turner HC, et al. Immunological and toxinological responses to jellyfish stings. Inflamm Allergy Drug Targets. 2011;10:438-446.
- Tibballs J. Australian venomous jellyfish, envenomation syndromes, toxins and therapy. Toxicon. 2006;48:830-859.
- Stein MR, Marracini JV, Rothschild NE, et al. Fatal Portuguese man-o’-war (Physalia physalis) envenomation. Ann Emerg Med. 1989;18:312-315.
- Burnett JW, Gable WD. A fatal jellyfish envenomation by the Portuguese man-o’war. Toxicon. 1989;27:823-824.
- Warrell DA. Venomous bites, stings, and poisoning: an update. Infect Dis Clin North Am. 2019;33:17-38.
- Neumar RW, Shuster M, Callaway CW, et al. Part 1: executive summary: 2015 American Heart Association guidelines update for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation. 2015;132(18 suppl 2):S315-S367.
- Wilcox CL, Headlam JL, Doyle TK, et al. Assessing the efficacy of first-aid measures in Physalia sp. envenomation, using solution- and blood agarose-based models. Toxins (Basel). 2017;9:149.
- Wilcox CL, Yanagihara AA. Heated debates: hot-water immersion or ice packs as first aid for cnidarian envenomations? Toxins (Basel). 2016;8:97.
- Loredana Asztalos M, Rubin AI, Elenitsas R, et al. Recurrent dermatitis and dermal hypersensitivity following a jellyfish sting: a case report and review of literature. Pediatr Dermatol. 2014;31:217-219.
- Boulware DR. A randomized, controlled field trial for the prevention of jellyfish stings with a topical sting inhibitor. J Travel Med. 2006;13:166-171.
Practice Points
- Jellyfish stings occur an estimated 150 million times annually worldwide, with numbers expected to rise due to climate change.
- Most stings result in painful self-limited cutaneous symptoms that resolve spontaneously. Box jellyfish (Cubozoa) stings carry a greater risk for causing severe systemic reactions.
- Treatment of skin reactions includes removal of tentacles and hot water immersion. Vinegar dousing for at least 30 seconds is recommended for box jellyfish stings. Supportive care and monitoring for cardiovascular collapse are key. The role of antivenin is uncertain.
What’s Eating You? Caterpillars
Causes of Lepidopterism
Caterpillars are wormlike organisms that serve as the larval stage of moths and butterflies, which belong to the order Lepidoptera. There are almost 165,000 discovered species, with 13,000 found in the United States.1,2 Roughly 150 species are known to have the potential to cause an adverse reaction in humans, with 50 of these in the United States.1Lepidopterism describes systemic and cutaneous reactions to moths, butterflies, and caterpillars; erucism describes strictly cutaneous reactions.1
Although the rate of lepidopterism is thought to be underreported because it often is self-limited and of a mild nature, a review found caterpillars to be the cause of roughly 2.2% of reported bites and stings annually.2 Cases increase in number with seasonal increases in caterpillars, which vary by region and species. For example, the Megalopyge opercularis (southern flannel moth) caterpillar was noted to have 2 peaks in a Texas-based study: 12% of reported stings occurred in July; 59% from October through November.3 In general, the likelihood of exposure increases during warmer months, and exposure is more common in people who work outdoors in a rural area or in a suburban area where there are many caterpillar-infested trees.4
Most cases of lepidopterism are caused by caterpillars, not by adult butterflies and moths, because the former have many tubular, or porous, hairlike structures called setae that are embedded in the integument. Setae were once thought to be connected to poison-secreting glandular cells, but current belief is that venomous caterpillars lack specialized gland cells and instead produce venom through secretory epithelial cells located above the integument.1 Venom accumulates in the hemolymph and is stored in the setae or other types of bristles, such as scoli (wartlike bumps that bear setae) or spines.5 With a large amount of chitin, bristles have a tendency to fracture and release venom upon contact.1 It is thought that some species of caterpillars formulate venom by ingesting toxins or toxin precursors from plants; for example, the tiger moth (family Arctiidae) is known to produce venom containing biogenic amines, pyrrolizidine, alkaloids, and cardiac glycosides obtained through food sources.5
Even if a caterpillar does not produce venom, its setae might embed into skin or mucous membranes and cause an adverse irritant reaction.1 Setae also might dislodge and be transported in the air to embed in objects—some remaining stable in the environment for longer than a year.2 In contrast to setae, spines are permanently fixed into the integument; for that reason, only direct contact with the caterpillar can result in an adverse reaction. Although it is mostly caterpillars that contain setae and spines, certain species of moths also might contain these structures or might acquire them as they emerge from the cocoon, which often contains incorporated setae.2
Reactions in Humans
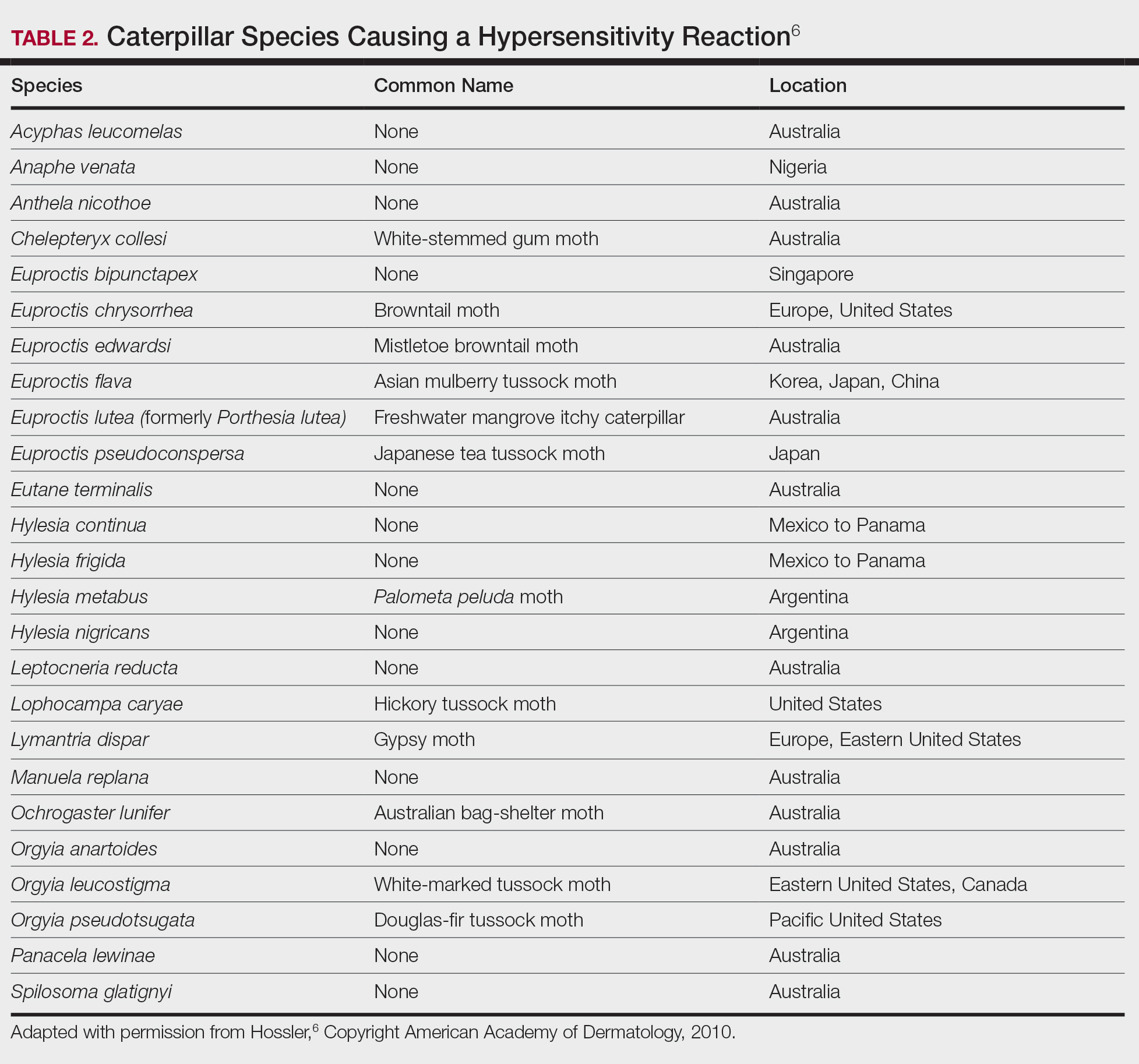
Lepidopterism encompasses 3 principal reactions in humans: sting reaction, hypersensitivity reaction, and lonomism (a hemorrhagic diathesis produced by Lonomia caterpillars). The type and severity of the reaction depends on (1) the species of caterpillar or moth and (2) the individual patient.2 There are approximately 12 families of caterpillars, mainly of the moth variety, that can cause an adverse reaction in humans.1 Tables 1 and 2 list examples of species that cause each type of reaction.6
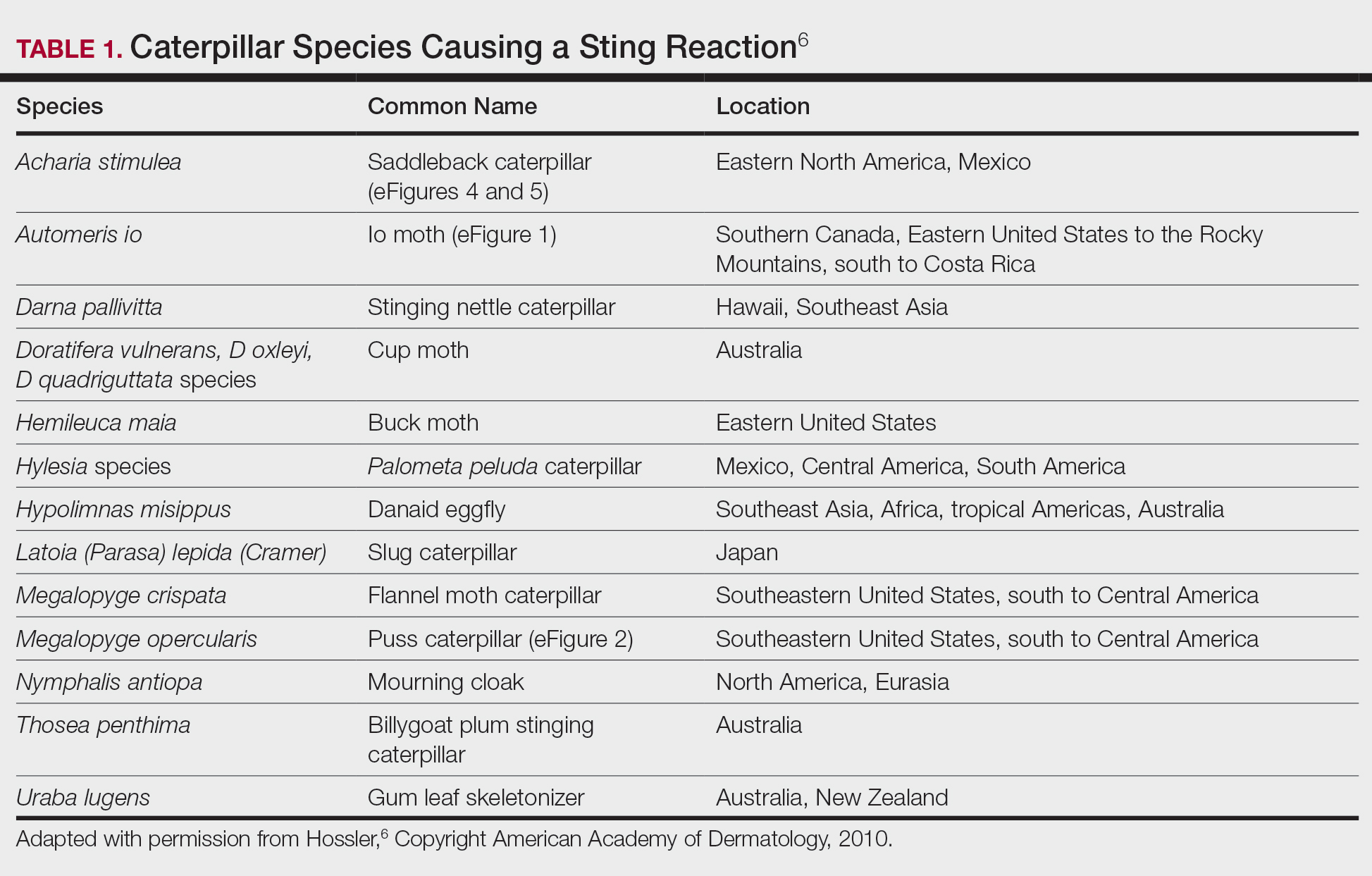
Chemicals and toxins contained in the poison of setae and spines vary by species of caterpillar. Numerous kinds have been isolated from different venoms,1,2 including several peptides, histamine, histamine-releasing substances, acetylcholine, phospholipase A, hyaluronidase, formic acid, proteins with trypsinlike activity, serine proteases such as kallikrein, and other enzymes with vasodegenerative and fibrinolytic properties
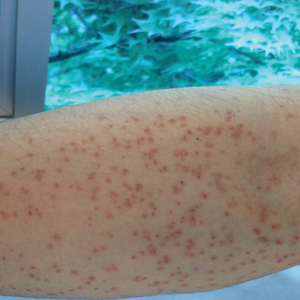
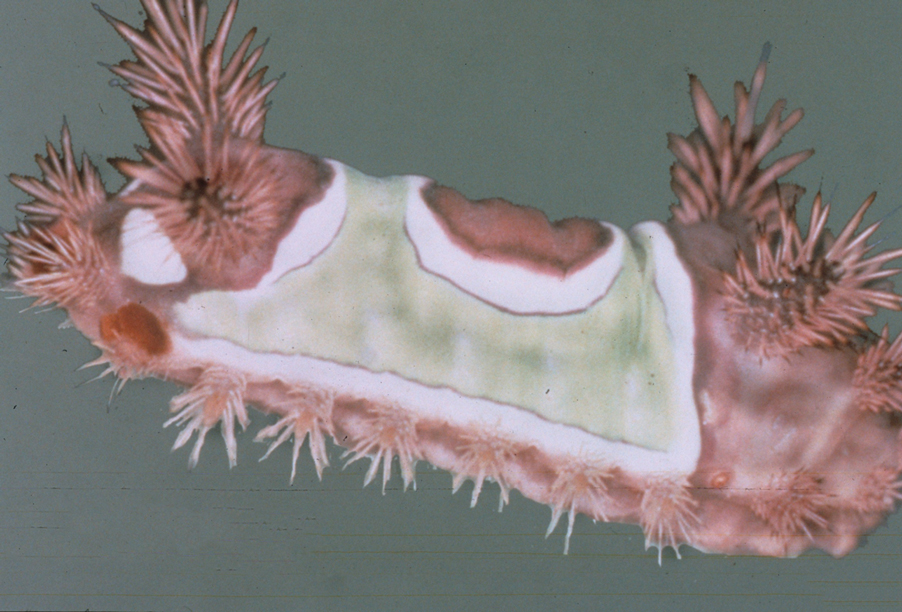
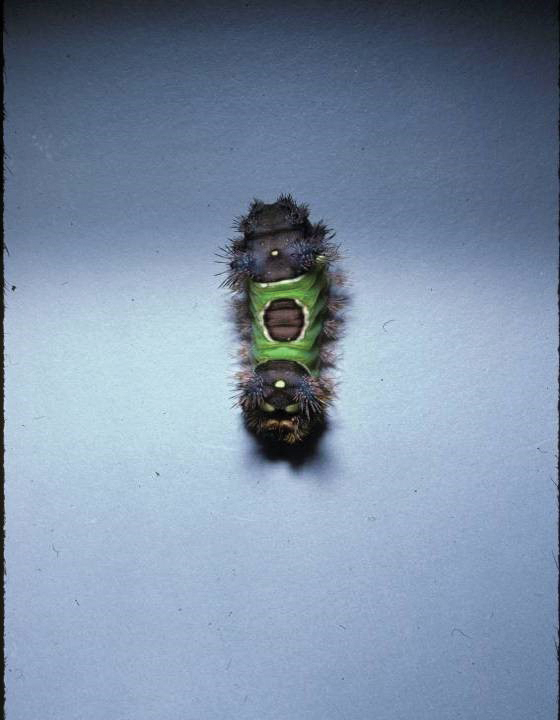
Stings: An Immediate Adverse Reaction—Depending on the venom, a sting might result in mild to severe burning pain, accompanied by welts, vesicles, and red papules or plaques.2 Figure 1 demonstrates a particularly mild sting from a caterpillar of the family Automeris, examples of which are seen in Figures 2 and 3 and eFigure 1. Components of the venom determine the mechanism of the sting and the pain that accompanies it. For example, a recent study demonstrated that the venom of the Latoia consocia caterpillar induces pain through the ion-channel receptor known as transient receptor potential vanilloid 1, which integrates and sends painful stimuli from the peripheral nervous system to the central nervous system.7 It is thought that a variety of ion channels are targets of the venom of caterpillars.
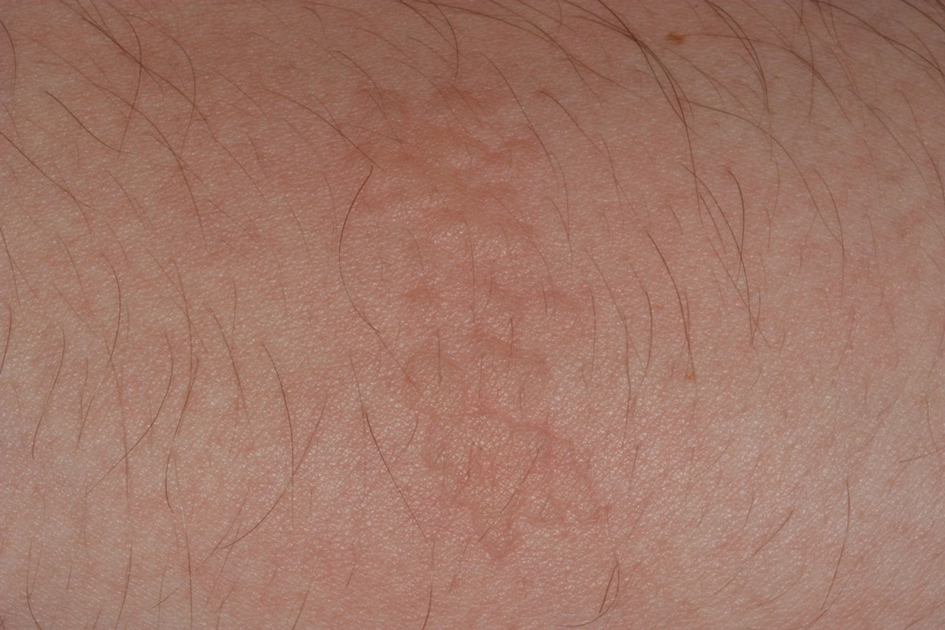



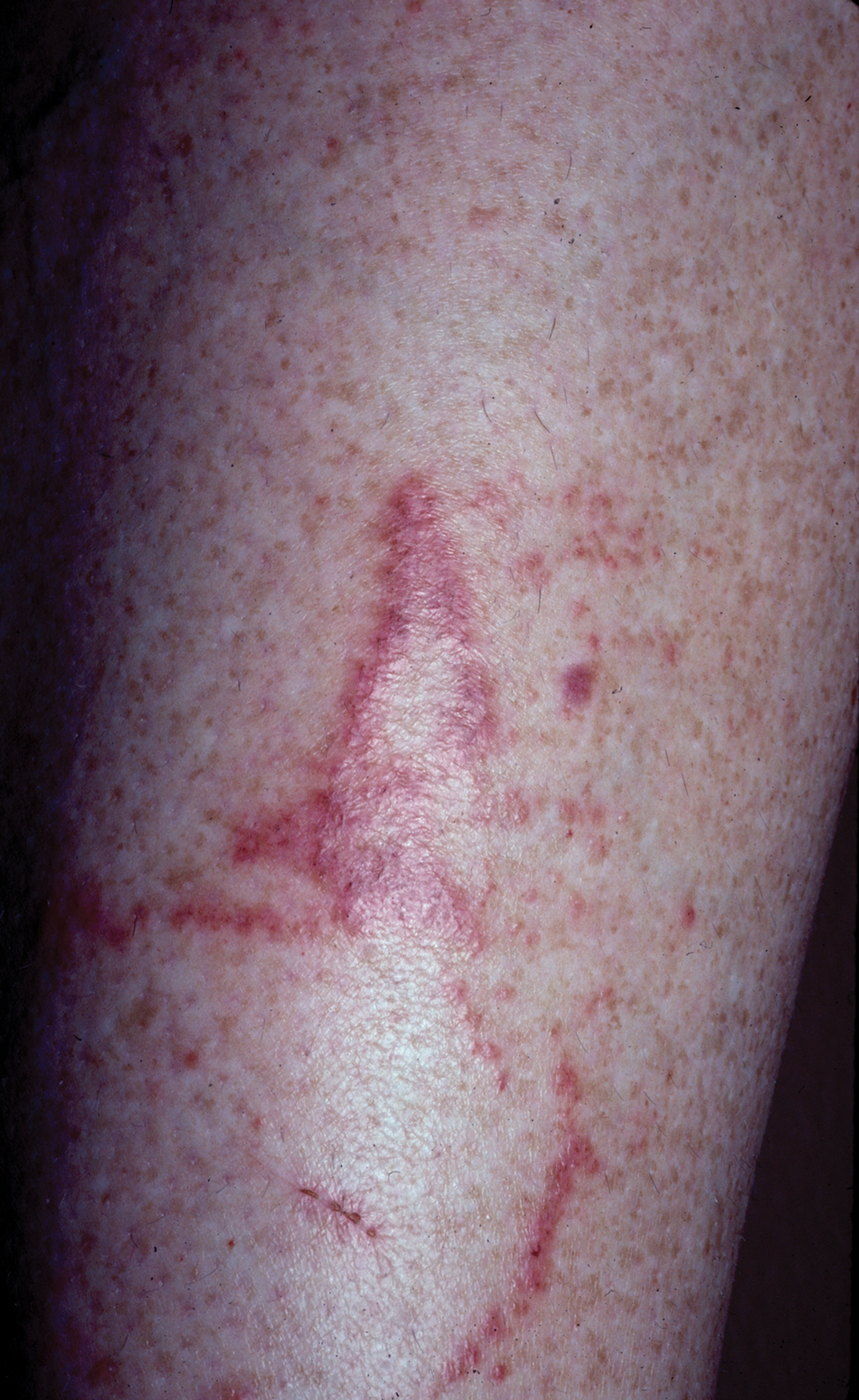
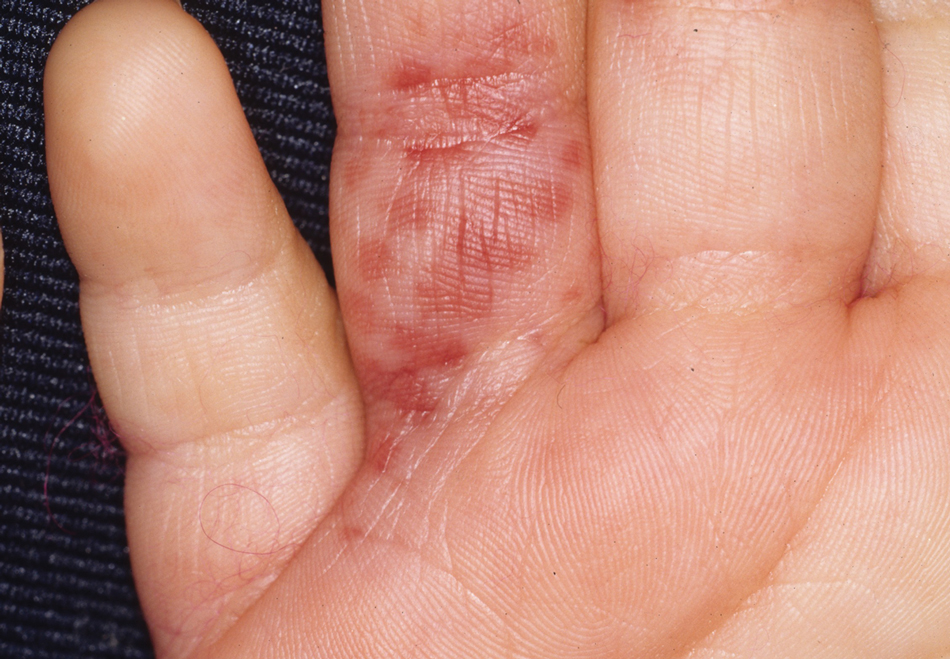
One of the most characteristic sting patterns is that of the caterpillar of family Megalopygidae (flannel moth)(eFigures 2 and 3). The stings of these caterpillars create a unique tram-track pattern of hemorrhagic macules or papules (Figure 4).4 A study found that 90% of reported M opercularis envenomations consist primarily of cutaneous symptoms, with 84% of those symptoms being irritation or pain; 45% a puncture or wound; 29% erythema; and 15% edema.3 Systemic findings can include headache, fever, adenopathy, nausea, vomiting, abdominal pain, and chest pain.4 Symptoms normally are self-limited, though they can last minutes or hours.


Hypersensitivity Reaction—Studies demonstrate that the symptoms of this reaction are a mixture of type I hypersensitivity, type IV hypersensitivity, and a foreign-body response.2 The specific hypersensitivity reaction depends on the venom and the exposed individual—most commonly including a combination of pruritic papules, urticarial wheals, flares, and dermatitis.2 A reaction that is a result of direct contact with the caterpillar or moth will appear on exposed areas; however, because setae embed in linens and clothing, they might cause a reaction anywhere on the body. Although usually self-limited, a hypersensitivity reaction might develop within minutes and can last for days or weeks.
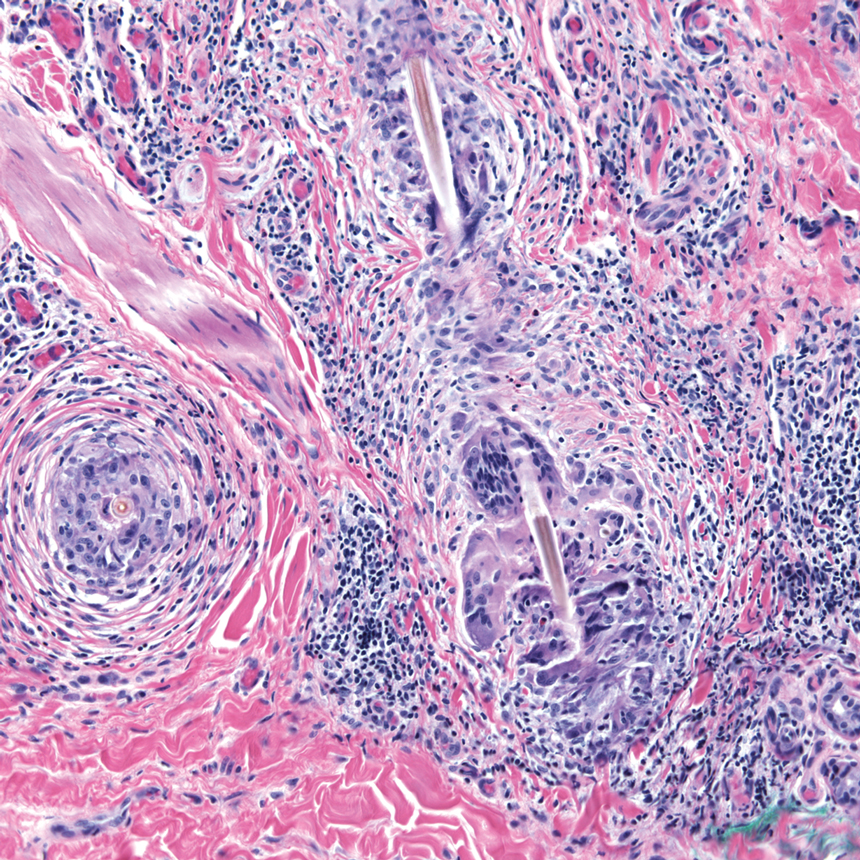
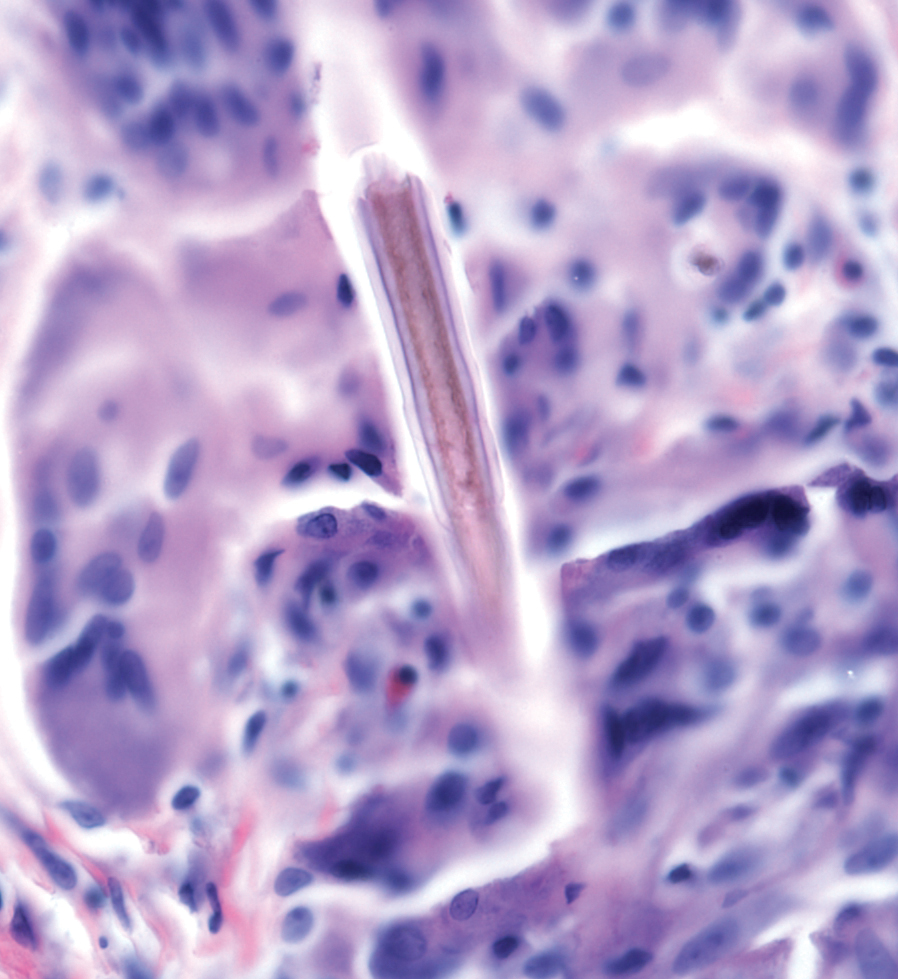
Stings and hypersensitivity reactions to caterpillars and moths tend to lead to a nonspecific histologic presentation characterized by epidermal edema and a superficial perivascular lymphocytic infiltrate, often with eosinophils.6 After approximately 1 week, a foreign-body response to setae can lead to tuberculoid granulomas accompanied by neutrophils in the dermis and occasionally in subcutaneous tissues (Figures 5 and 6).8 If setae have not yet been removed, they also might be visible in skin scrapings.
Additional complications can accompany the hypersensitivity reaction to setae or spines. Type I hypersensitivity reactions can lead to severe reactions on second contact due to previously sensitized IgE antibodies. Although the first reaction appears mild, second contact might result in angioedema, wheezing, dyspnea, or anaphylaxis, or a combination of these findings.9 In addition, some patients who come in contact with Dendrolimus caterpillars might develop a condition known as dendrolimiasis, characterized by dermatitis in addition to arthritis or chondritis.6 The arthritis is normally monoarticular and can result in complete destruction of the joint. Pararamose, a condition with a similar presentation, is caused by the Brazilian moth Premolis semirufa.6
Contact of setae or spines with mucous membranes or inhalation of setae also might result in edema, dysphagia, dyspnea, drooling, rhinitis, or conjunctivitis, or a combination of these findings.6 In addition, setae can embed in the eye and cause an inflammatory reaction—ophthalmia nodosa—most commonly caused by caterpillars of the pine processionary moth (Thaumetopoea pityocampa) and characterized by immediate chemosis, which can progress to liquefactive necrosis and hypopyon, later developing into a granulomatous foreign-body response.2,10 The process is thought to be the result of a combination of the thaumetopoein toxin in the setae and an IgE-mediated response to other proteins.10
Due to their harpoon shape and forward-only motion, setae might migrate deeper, potentially even to the optic nerve.11 Because migration might take years and the barbed shape of setae does not always allow removal, some patients require lifetime monitoring with slit-lamp examination.Chronic problems, such as cataracts and persistent posterior uveitis, have been reported.10,11
Lonomism—One of the most serious (though rarest) reactions to caterpillars is lonomism, a condition caused by the caterpillars of Lonomia achelous and Lonomia obliqua moths. These caterpillars have a unique combination of toxins filling their branched spines, which ultimately leads to the same outcome: a hemorrhagic diathesis.
The toxin of L achelous comprises several proteases that degrade fibrin, fibrinogen, and factor XIII while activating prothrombin. In contrast, L obliqua poison causes a hemorrhagic diathesis by promoting a consumptive coagulopathy through enzymes that activate factor X and prothrombin.
With initial contact with either of these Lonomia caterpillars, the patient experiences severe pain accompanied by systemic symptoms, including headache, nausea, and vomiting. Shortly afterward, symptoms of a hemorrhagic diathesis manifest, including bleeding gums, hematuria, bleeding from prior wounds, and epistaxis.5 Serious complications of the hemorrhagic diathesis, such as hemorrhage of major organs, leads to death in 4% of patients.5 A reported case of a patient whose Lonomia caterpillar sting went unrecognized until a week after the accident ended with progression to stage V chronic renal disease.12
Recent research has focused on the specific mechanism of injury caused by Lonomia species. A study found that the venom of L obliqua causes cytoskeleton rearrangement and migration in vascular smooth muscle cells (VSMCs) by inducing formation of reactive oxygen species through activation of nicotinamide adenine dinucleotide phosphate oxidase.13 Thus, the venom directly contributes to the proinflammatory phenotype of endothelial cells seen following envenomation. The same study also demonstrated that elevated reactive oxygen species trigger extracellular signal-regulated kinase pathway activation in VSMCs, leading to cell proliferation, re-stenosis, and ischemia.13 This finding was confirmed by another study,14 which demonstrated an increase in Rac1, a signaling protein involved in the extracellular signal-regulated kinase pathway, in VSMCs upon exposure to L obliqua venom. These studies propose potential new targets for treatment to prevent vascular damage.
Reactions to Adult Organisms—Although it is more common for the caterpillar form of these organisms to cause an adverse reaction, the adult moth also might be capable of causing a similar reaction by retaining setae from the cocoon or by their own spines. The most notable example of this is female moths of the genus Hylesia, which possess spines attached to glands on the abdomen. The poison in these spines—a mixture of proteases and chitinase—causes a dermatitis known as Caripito itch—the name derived from a river port in Venezuela where this moth caused a memorable epidemic of moth-induced dermatitis.7,15 Caripito itch is known for intense pruritus that most commonly lasts days or weeks, possibly longer than 1 year.
Diagnostic Difficulties
The challenge of diagnosing a caterpillar- or moth-induced reaction in humans arises from (1) the lack of clinical history (the caterpillar might not be seen at all by the patient or the examiner) and (2) the similarity of these reactions to those with more common triggers.
When setae remain embedded in the skin or mucous membranes, skin scrapings allow accelerated diagnosis. On a skin scraping prepared with 20% potassium hydroxide, setae appear as tapered and barbed hairlike structures, which allows them to be distinguished from other similar-appearing but differently shaped structures, such as glass fibers.
When setae do not remain embedded in the skin or when the cause of the reaction is due to spines, the physician is left with a nonspecific histologic picture and a large differential diagnosis to be narrowed down based on the history and occasionally the pattern of the skin lesion.
A challenge in sting diagnosis is differentiating a caterpillar or moth sting from that of another organism. In certain cases, such as those of the family Megalopygidae, specific patterns of stings might assist in making the diagnosis. Hypersensitivity reactions are associated with a wider differential diagnosis, including irritant or allergic dermatitis from other causes, scabies, eczema, lichen planus, lichen simplex chronicus, seborrheic dermatitis, and tinea corporis, to name a few.6 Skin scrapings can be examined for other features, such as burrows in the case of scabies, to further narrow the differential.
Stings and hypersensitivity reactions lacking a proper history and associated with more severe systemic symptoms have caused misdiagnosis or led to a workup for the wrong condition; for example, the picture of abdominal pain, nausea, vomiting, tachycardia, leukocytosis, hypokalemia, and metabolic acidosis can simulate appendicitis.16 Upon discovery of a puss caterpillar sting in a patient, her symptoms resolved after treatment with ondansetron, morphine, and intravenous fluids.16
In lonomism, the diagnosis must be established by laboratory measurement of the fibrinogen level, clotting factors, prothrombin time, and activated partial thromboplastin time.4 The differential diagnosis associated with lonomism includes disseminated intravascular coagulation (DIC), snakebite, and a hereditary bleeding disorder.4 The combination of laboratory tests and an extensive medical history allows a diagnosis. Absence of a personal or family history of bleeding excludes a diagnosis of hereditary bleeding disorder, whereas the absence of known causes of DIC or thrombocytopenia allows DIC to be excluded from the differential.
Treatment Options and Prevention
Treatment—The first step is to remove any embedded setae from the skin or mucous membranes. The stepwise recommendation is to remove any constricted clothing, detach setae with adhesive tape, wash with soap and water, and dry without touching the skin.1 Any remaining setae can be removed with additional tape or forceps; setae tend to be fragile and are difficult to remove in their entirety.
Other than removal of the setae, skin reactions are treated symptomatically. Ice packs and isopropyl alcohol have been utilized to cool burning or stinging areas. Pain, pruritus, and inflammation have been alleviated with antihistamines and topical corticosteroids.1 When pain is severe, oral codeine or local injection of anesthetic can be used. For severe and persistent skin lesions, a course of an oral glucocorticoid can be administered. Intramuscular triamcinolone acetonide has been shown to treat pain, dermatitis, and subcutaneous nodules otherwise refractory to treatment.8
Antivenin specific for L obliqua exists to treat lonomism and is therefore effective only when lonomism is caused by that species. Lonomism caused by L achelous is treated with cryoprecipitate, purified fibrinogen, and antifibrinolytic drugs, such as aprotinin.6 Whole blood and fresh-frozen plasma have been noted to make hemorrhage worse when utilized to treat lonomism. Because the mechanism of action of the venom of Lonomia species is based, in part, on inducing a proinflammatory profile in endothelial cells, studies have demonstrated that inhibition of kallikrein might prevent vascular injury and thus prevent serious adverse effects, such as renal failure.17
Prevention—People should wear proper protective clothing when outdoors in potentially infested areas. Measures should be taken to ensure that linens and clothing are not left outside in areas where setae might be carried on the wind. Infestation control is necessary if the population of caterpillars reaches a high enough level.
Conclusion
Several species of caterpillars and moths cause adverse reactions in humans: stings, hypersensitivity reactions, and lonomism. Although most reactions are self-limited, some might have more serious effects, including organ failure and death. Mechanisms of injury vary by species of caterpillar, moth, and butterfly; current research is focused on further defining venom components and signaling pathways to isolate potential targets to aid in the diagnosis and treatment of lepidopterism.
- Goldman BS, Bragg BN. Caterpillar and moth bites. Stat Pearls [Internet]. StatPearls Publishing. Updated August 3, 2021. Accessed November 4, 2021. https://www.ncbi.nlm.nih.gov/books/NBK539851/
- Hossler EW. Caterpillars and moths: part I. Dermatologic manifestations of encounters with Lepidoptera. J Am Acad Dermatol. 2010;62:1-10. doi:10.1016/j.jaad.2009.08.060
- Forrester MB. Megalopyge opercularis caterpillar stings reported to Texas poison centers. Wilderness Environ Med. 2018;29:215-220. doi:10.1016/j.wem.2018.02.002
- Hossler EW. Lepidopterism: skin disorders secondary to caterpillars and moths. UpToDate website. Published October 20, 2021. Accessed November 18, 2021. https://www.uptodate.com/contents/lepidopterism-skin-disorders-secondary-to-caterpillars-and-moths
- Villas-Boas IM, Bonfá G, Tambourgi DV. Venomous caterpillars: from inoculation apparatus to venom composition and envenomation. Toxicon. 2018;153:39-52. doi:10.1016/j.toxicon.2018.08.007
- Hossler EW. Caterpillars and moths: part II. dermatologic manifestations of encounters with Lepidoptera. J Am Acad Dermatol. 2010;62:13-28. doi:10.1016/j.jaad.2009.08.061
- Yao Z, Kamau PM, Han Y, et al. The Latoia consocia caterpillar induces pain by targeting nociceptive ion channel TRPV1. Toxins (Basel). 2019;11:695. doi:10.3390/toxins11120695
- Paniz-Mondolfi AE, Pérez-Alvarez AM, Lundberg U, et al. Cutaneous lepidopterism: dermatitis from contact with moths of Hylesia metabus (Cramer 1775) (Lepidoptera: Saturniidae), the causative agent of caripito itch. Int J Dermatol. 2011;50:535-541. doi:10.1111/j.1365-4632.2010.04683.x
- Santos-Magadán S, González de Olano D, Bartolomé-Zavala B, et al. Adverse reactions to the processionary caterpillar: irritant or allergic mechanism? Contact Dermatitis. 2009;60:109-110. doi:10.1111/j.1600-0536.2008.01464.x
- González-Martín-Moro J, Contreras-Martín I, Castro-Rebollo M, et al. Focal cortical cataract due to caterpillar hair migration. Clin Exp Optom. 2019;102:89-90. doi:10.1111/cxo.12809
- Singh A, Behera UC, Agrawal H. Intra-lenticular caterpillar seta in ophthalmia nodosa. Eur J Ophthalmol. 2021;31:NP109-NP111. doi:10.1177/1120672119858899
- Schmitberger PA, Fernandes TC, Santos RC, et al. Probable chronic renal failure caused by Lonomia caterpillar envenomation. J Venom Anim Toxins Incl Trop Dis. 2013;19:14. doi:10.1186/1678-9199-19-14
- Moraes JA, Rodrigues G, Nascimento-Silva V, et al. Effects of Lonomia obliqua venom on vascular smooth muscle cells: contribution of NADPH oxidase-derived reactive oxygen species. Toxins (Basel). 2017;9:360. doi:10.3390/toxins9110360
- Bernardi L, Pinto AFM, Mendes E, et al. Lonomia obliqua bristle extract modulates Rac1 activation, membrane dynamics and cell adhesion properties. Toxicon. 2019;162:32-39. doi:10.1016/j.toxicon.2019.02.019
- Cabrera G, Lundberg U, Rodríguez-Ulloa A, et al. Protein content of the Hylesia metabus egg nest setae (Cramer [1775]) (Lepidoptera: Saturniidae) and its association with the parental investment for the reproductive success and lepidopterism. J Proteomics. 2017;150:183-200. doi:10.1016/j.jprot.2016.08.010
- Greene SC, Carey JM. Puss caterpillar envenomation: erucism mimicking appendicitis in a young child. Pediatr Emerg Care. 2020;36:E732-E734. doi:10.1097/PEC.0000000000001514
- Berger M, de Moraes JA, Beys-da-Silva WO, et al. Renal and vascular effects of kallikrein inhibition in a model of Lonomia obliqua venom-induced acute kidney injury. PLoS Negl Trop Dis. 2019;13:e0007197. doi:10.1371/journal.pntd.0007197
Causes of Lepidopterism
Caterpillars are wormlike organisms that serve as the larval stage of moths and butterflies, which belong to the order Lepidoptera. There are almost 165,000 discovered species, with 13,000 found in the United States.1,2 Roughly 150 species are known to have the potential to cause an adverse reaction in humans, with 50 of these in the United States.1Lepidopterism describes systemic and cutaneous reactions to moths, butterflies, and caterpillars; erucism describes strictly cutaneous reactions.1
Although the rate of lepidopterism is thought to be underreported because it often is self-limited and of a mild nature, a review found caterpillars to be the cause of roughly 2.2% of reported bites and stings annually.2 Cases increase in number with seasonal increases in caterpillars, which vary by region and species. For example, the Megalopyge opercularis (southern flannel moth) caterpillar was noted to have 2 peaks in a Texas-based study: 12% of reported stings occurred in July; 59% from October through November.3 In general, the likelihood of exposure increases during warmer months, and exposure is more common in people who work outdoors in a rural area or in a suburban area where there are many caterpillar-infested trees.4
Most cases of lepidopterism are caused by caterpillars, not by adult butterflies and moths, because the former have many tubular, or porous, hairlike structures called setae that are embedded in the integument. Setae were once thought to be connected to poison-secreting glandular cells, but current belief is that venomous caterpillars lack specialized gland cells and instead produce venom through secretory epithelial cells located above the integument.1 Venom accumulates in the hemolymph and is stored in the setae or other types of bristles, such as scoli (wartlike bumps that bear setae) or spines.5 With a large amount of chitin, bristles have a tendency to fracture and release venom upon contact.1 It is thought that some species of caterpillars formulate venom by ingesting toxins or toxin precursors from plants; for example, the tiger moth (family Arctiidae) is known to produce venom containing biogenic amines, pyrrolizidine, alkaloids, and cardiac glycosides obtained through food sources.5
Even if a caterpillar does not produce venom, its setae might embed into skin or mucous membranes and cause an adverse irritant reaction.1 Setae also might dislodge and be transported in the air to embed in objects—some remaining stable in the environment for longer than a year.2 In contrast to setae, spines are permanently fixed into the integument; for that reason, only direct contact with the caterpillar can result in an adverse reaction. Although it is mostly caterpillars that contain setae and spines, certain species of moths also might contain these structures or might acquire them as they emerge from the cocoon, which often contains incorporated setae.2
Reactions in Humans
Lepidopterism encompasses 3 principal reactions in humans: sting reaction, hypersensitivity reaction, and lonomism (a hemorrhagic diathesis produced by Lonomia caterpillars). The type and severity of the reaction depends on (1) the species of caterpillar or moth and (2) the individual patient.2 There are approximately 12 families of caterpillars, mainly of the moth variety, that can cause an adverse reaction in humans.1 Tables 1 and 2 list examples of species that cause each type of reaction.6



Chemicals and toxins contained in the poison of setae and spines vary by species of caterpillar. Numerous kinds have been isolated from different venoms,1,2 including several peptides, histamine, histamine-releasing substances, acetylcholine, phospholipase A, hyaluronidase, formic acid, proteins with trypsinlike activity, serine proteases such as kallikrein, and other enzymes with vasodegenerative and fibrinolytic properties

Stings: An Immediate Adverse Reaction—Depending on the venom, a sting might result in mild to severe burning pain, accompanied by welts, vesicles, and red papules or plaques.2 Figure 1 demonstrates a particularly mild sting from a caterpillar of the family Automeris, examples of which are seen in Figures 2 and 3 and eFigure 1. Components of the venom determine the mechanism of the sting and the pain that accompanies it. For example, a recent study demonstrated that the venom of the Latoia consocia caterpillar induces pain through the ion-channel receptor known as transient receptor potential vanilloid 1, which integrates and sends painful stimuli from the peripheral nervous system to the central nervous system.7 It is thought that a variety of ion channels are targets of the venom of caterpillars.




One of the most characteristic sting patterns is that of the caterpillar of family Megalopygidae (flannel moth)(eFigures 2 and 3). The stings of these caterpillars create a unique tram-track pattern of hemorrhagic macules or papules (Figure 4).4 A study found that 90% of reported M opercularis envenomations consist primarily of cutaneous symptoms, with 84% of those symptoms being irritation or pain; 45% a puncture or wound; 29% erythema; and 15% edema.3 Systemic findings can include headache, fever, adenopathy, nausea, vomiting, abdominal pain, and chest pain.4 Symptoms normally are self-limited, though they can last minutes or hours.



Hypersensitivity Reaction—Studies demonstrate that the symptoms of this reaction are a mixture of type I hypersensitivity, type IV hypersensitivity, and a foreign-body response.2 The specific hypersensitivity reaction depends on the venom and the exposed individual—most commonly including a combination of pruritic papules, urticarial wheals, flares, and dermatitis.2 A reaction that is a result of direct contact with the caterpillar or moth will appear on exposed areas; however, because setae embed in linens and clothing, they might cause a reaction anywhere on the body. Although usually self-limited, a hypersensitivity reaction might develop within minutes and can last for days or weeks.
Stings and hypersensitivity reactions to caterpillars and moths tend to lead to a nonspecific histologic presentation characterized by epidermal edema and a superficial perivascular lymphocytic infiltrate, often with eosinophils.6 After approximately 1 week, a foreign-body response to setae can lead to tuberculoid granulomas accompanied by neutrophils in the dermis and occasionally in subcutaneous tissues (Figures 5 and 6).8 If setae have not yet been removed, they also might be visible in skin scrapings.


Additional complications can accompany the hypersensitivity reaction to setae or spines. Type I hypersensitivity reactions can lead to severe reactions on second contact due to previously sensitized IgE antibodies. Although the first reaction appears mild, second contact might result in angioedema, wheezing, dyspnea, or anaphylaxis, or a combination of these findings.9 In addition, some patients who come in contact with Dendrolimus caterpillars might develop a condition known as dendrolimiasis, characterized by dermatitis in addition to arthritis or chondritis.6 The arthritis is normally monoarticular and can result in complete destruction of the joint. Pararamose, a condition with a similar presentation, is caused by the Brazilian moth Premolis semirufa.6
Contact of setae or spines with mucous membranes or inhalation of setae also might result in edema, dysphagia, dyspnea, drooling, rhinitis, or conjunctivitis, or a combination of these findings.6 In addition, setae can embed in the eye and cause an inflammatory reaction—ophthalmia nodosa—most commonly caused by caterpillars of the pine processionary moth (Thaumetopoea pityocampa) and characterized by immediate chemosis, which can progress to liquefactive necrosis and hypopyon, later developing into a granulomatous foreign-body response.2,10 The process is thought to be the result of a combination of the thaumetopoein toxin in the setae and an IgE-mediated response to other proteins.10
Due to their harpoon shape and forward-only motion, setae might migrate deeper, potentially even to the optic nerve.11 Because migration might take years and the barbed shape of setae does not always allow removal, some patients require lifetime monitoring with slit-lamp examination.Chronic problems, such as cataracts and persistent posterior uveitis, have been reported.10,11
Lonomism—One of the most serious (though rarest) reactions to caterpillars is lonomism, a condition caused by the caterpillars of Lonomia achelous and Lonomia obliqua moths. These caterpillars have a unique combination of toxins filling their branched spines, which ultimately leads to the same outcome: a hemorrhagic diathesis.
The toxin of L achelous comprises several proteases that degrade fibrin, fibrinogen, and factor XIII while activating prothrombin. In contrast, L obliqua poison causes a hemorrhagic diathesis by promoting a consumptive coagulopathy through enzymes that activate factor X and prothrombin.
With initial contact with either of these Lonomia caterpillars, the patient experiences severe pain accompanied by systemic symptoms, including headache, nausea, and vomiting. Shortly afterward, symptoms of a hemorrhagic diathesis manifest, including bleeding gums, hematuria, bleeding from prior wounds, and epistaxis.5 Serious complications of the hemorrhagic diathesis, such as hemorrhage of major organs, leads to death in 4% of patients.5 A reported case of a patient whose Lonomia caterpillar sting went unrecognized until a week after the accident ended with progression to stage V chronic renal disease.12
Recent research has focused on the specific mechanism of injury caused by Lonomia species. A study found that the venom of L obliqua causes cytoskeleton rearrangement and migration in vascular smooth muscle cells (VSMCs) by inducing formation of reactive oxygen species through activation of nicotinamide adenine dinucleotide phosphate oxidase.13 Thus, the venom directly contributes to the proinflammatory phenotype of endothelial cells seen following envenomation. The same study also demonstrated that elevated reactive oxygen species trigger extracellular signal-regulated kinase pathway activation in VSMCs, leading to cell proliferation, re-stenosis, and ischemia.13 This finding was confirmed by another study,14 which demonstrated an increase in Rac1, a signaling protein involved in the extracellular signal-regulated kinase pathway, in VSMCs upon exposure to L obliqua venom. These studies propose potential new targets for treatment to prevent vascular damage.
Reactions to Adult Organisms—Although it is more common for the caterpillar form of these organisms to cause an adverse reaction, the adult moth also might be capable of causing a similar reaction by retaining setae from the cocoon or by their own spines. The most notable example of this is female moths of the genus Hylesia, which possess spines attached to glands on the abdomen. The poison in these spines—a mixture of proteases and chitinase—causes a dermatitis known as Caripito itch—the name derived from a river port in Venezuela where this moth caused a memorable epidemic of moth-induced dermatitis.7,15 Caripito itch is known for intense pruritus that most commonly lasts days or weeks, possibly longer than 1 year.
Diagnostic Difficulties
The challenge of diagnosing a caterpillar- or moth-induced reaction in humans arises from (1) the lack of clinical history (the caterpillar might not be seen at all by the patient or the examiner) and (2) the similarity of these reactions to those with more common triggers.
When setae remain embedded in the skin or mucous membranes, skin scrapings allow accelerated diagnosis. On a skin scraping prepared with 20% potassium hydroxide, setae appear as tapered and barbed hairlike structures, which allows them to be distinguished from other similar-appearing but differently shaped structures, such as glass fibers.
When setae do not remain embedded in the skin or when the cause of the reaction is due to spines, the physician is left with a nonspecific histologic picture and a large differential diagnosis to be narrowed down based on the history and occasionally the pattern of the skin lesion.
A challenge in sting diagnosis is differentiating a caterpillar or moth sting from that of another organism. In certain cases, such as those of the family Megalopygidae, specific patterns of stings might assist in making the diagnosis. Hypersensitivity reactions are associated with a wider differential diagnosis, including irritant or allergic dermatitis from other causes, scabies, eczema, lichen planus, lichen simplex chronicus, seborrheic dermatitis, and tinea corporis, to name a few.6 Skin scrapings can be examined for other features, such as burrows in the case of scabies, to further narrow the differential.
Stings and hypersensitivity reactions lacking a proper history and associated with more severe systemic symptoms have caused misdiagnosis or led to a workup for the wrong condition; for example, the picture of abdominal pain, nausea, vomiting, tachycardia, leukocytosis, hypokalemia, and metabolic acidosis can simulate appendicitis.16 Upon discovery of a puss caterpillar sting in a patient, her symptoms resolved after treatment with ondansetron, morphine, and intravenous fluids.16
In lonomism, the diagnosis must be established by laboratory measurement of the fibrinogen level, clotting factors, prothrombin time, and activated partial thromboplastin time.4 The differential diagnosis associated with lonomism includes disseminated intravascular coagulation (DIC), snakebite, and a hereditary bleeding disorder.4 The combination of laboratory tests and an extensive medical history allows a diagnosis. Absence of a personal or family history of bleeding excludes a diagnosis of hereditary bleeding disorder, whereas the absence of known causes of DIC or thrombocytopenia allows DIC to be excluded from the differential.
Treatment Options and Prevention
Treatment—The first step is to remove any embedded setae from the skin or mucous membranes. The stepwise recommendation is to remove any constricted clothing, detach setae with adhesive tape, wash with soap and water, and dry without touching the skin.1 Any remaining setae can be removed with additional tape or forceps; setae tend to be fragile and are difficult to remove in their entirety.
Other than removal of the setae, skin reactions are treated symptomatically. Ice packs and isopropyl alcohol have been utilized to cool burning or stinging areas. Pain, pruritus, and inflammation have been alleviated with antihistamines and topical corticosteroids.1 When pain is severe, oral codeine or local injection of anesthetic can be used. For severe and persistent skin lesions, a course of an oral glucocorticoid can be administered. Intramuscular triamcinolone acetonide has been shown to treat pain, dermatitis, and subcutaneous nodules otherwise refractory to treatment.8
Antivenin specific for L obliqua exists to treat lonomism and is therefore effective only when lonomism is caused by that species. Lonomism caused by L achelous is treated with cryoprecipitate, purified fibrinogen, and antifibrinolytic drugs, such as aprotinin.6 Whole blood and fresh-frozen plasma have been noted to make hemorrhage worse when utilized to treat lonomism. Because the mechanism of action of the venom of Lonomia species is based, in part, on inducing a proinflammatory profile in endothelial cells, studies have demonstrated that inhibition of kallikrein might prevent vascular injury and thus prevent serious adverse effects, such as renal failure.17
Prevention—People should wear proper protective clothing when outdoors in potentially infested areas. Measures should be taken to ensure that linens and clothing are not left outside in areas where setae might be carried on the wind. Infestation control is necessary if the population of caterpillars reaches a high enough level.
Conclusion
Several species of caterpillars and moths cause adverse reactions in humans: stings, hypersensitivity reactions, and lonomism. Although most reactions are self-limited, some might have more serious effects, including organ failure and death. Mechanisms of injury vary by species of caterpillar, moth, and butterfly; current research is focused on further defining venom components and signaling pathways to isolate potential targets to aid in the diagnosis and treatment of lepidopterism.
Causes of Lepidopterism
Caterpillars are wormlike organisms that serve as the larval stage of moths and butterflies, which belong to the order Lepidoptera. There are almost 165,000 discovered species, with 13,000 found in the United States.1,2 Roughly 150 species are known to have the potential to cause an adverse reaction in humans, with 50 of these in the United States.1Lepidopterism describes systemic and cutaneous reactions to moths, butterflies, and caterpillars; erucism describes strictly cutaneous reactions.1
Although the rate of lepidopterism is thought to be underreported because it often is self-limited and of a mild nature, a review found caterpillars to be the cause of roughly 2.2% of reported bites and stings annually.2 Cases increase in number with seasonal increases in caterpillars, which vary by region and species. For example, the Megalopyge opercularis (southern flannel moth) caterpillar was noted to have 2 peaks in a Texas-based study: 12% of reported stings occurred in July; 59% from October through November.3 In general, the likelihood of exposure increases during warmer months, and exposure is more common in people who work outdoors in a rural area or in a suburban area where there are many caterpillar-infested trees.4
Most cases of lepidopterism are caused by caterpillars, not by adult butterflies and moths, because the former have many tubular, or porous, hairlike structures called setae that are embedded in the integument. Setae were once thought to be connected to poison-secreting glandular cells, but current belief is that venomous caterpillars lack specialized gland cells and instead produce venom through secretory epithelial cells located above the integument.1 Venom accumulates in the hemolymph and is stored in the setae or other types of bristles, such as scoli (wartlike bumps that bear setae) or spines.5 With a large amount of chitin, bristles have a tendency to fracture and release venom upon contact.1 It is thought that some species of caterpillars formulate venom by ingesting toxins or toxin precursors from plants; for example, the tiger moth (family Arctiidae) is known to produce venom containing biogenic amines, pyrrolizidine, alkaloids, and cardiac glycosides obtained through food sources.5
Even if a caterpillar does not produce venom, its setae might embed into skin or mucous membranes and cause an adverse irritant reaction.1 Setae also might dislodge and be transported in the air to embed in objects—some remaining stable in the environment for longer than a year.2 In contrast to setae, spines are permanently fixed into the integument; for that reason, only direct contact with the caterpillar can result in an adverse reaction. Although it is mostly caterpillars that contain setae and spines, certain species of moths also might contain these structures or might acquire them as they emerge from the cocoon, which often contains incorporated setae.2
Reactions in Humans
Lepidopterism encompasses 3 principal reactions in humans: sting reaction, hypersensitivity reaction, and lonomism (a hemorrhagic diathesis produced by Lonomia caterpillars). The type and severity of the reaction depends on (1) the species of caterpillar or moth and (2) the individual patient.2 There are approximately 12 families of caterpillars, mainly of the moth variety, that can cause an adverse reaction in humans.1 Tables 1 and 2 list examples of species that cause each type of reaction.6



Chemicals and toxins contained in the poison of setae and spines vary by species of caterpillar. Numerous kinds have been isolated from different venoms,1,2 including several peptides, histamine, histamine-releasing substances, acetylcholine, phospholipase A, hyaluronidase, formic acid, proteins with trypsinlike activity, serine proteases such as kallikrein, and other enzymes with vasodegenerative and fibrinolytic properties

Stings: An Immediate Adverse Reaction—Depending on the venom, a sting might result in mild to severe burning pain, accompanied by welts, vesicles, and red papules or plaques.2 Figure 1 demonstrates a particularly mild sting from a caterpillar of the family Automeris, examples of which are seen in Figures 2 and 3 and eFigure 1. Components of the venom determine the mechanism of the sting and the pain that accompanies it. For example, a recent study demonstrated that the venom of the Latoia consocia caterpillar induces pain through the ion-channel receptor known as transient receptor potential vanilloid 1, which integrates and sends painful stimuli from the peripheral nervous system to the central nervous system.7 It is thought that a variety of ion channels are targets of the venom of caterpillars.




One of the most characteristic sting patterns is that of the caterpillar of family Megalopygidae (flannel moth)(eFigures 2 and 3). The stings of these caterpillars create a unique tram-track pattern of hemorrhagic macules or papules (Figure 4).4 A study found that 90% of reported M opercularis envenomations consist primarily of cutaneous symptoms, with 84% of those symptoms being irritation or pain; 45% a puncture or wound; 29% erythema; and 15% edema.3 Systemic findings can include headache, fever, adenopathy, nausea, vomiting, abdominal pain, and chest pain.4 Symptoms normally are self-limited, though they can last minutes or hours.



Hypersensitivity Reaction—Studies demonstrate that the symptoms of this reaction are a mixture of type I hypersensitivity, type IV hypersensitivity, and a foreign-body response.2 The specific hypersensitivity reaction depends on the venom and the exposed individual—most commonly including a combination of pruritic papules, urticarial wheals, flares, and dermatitis.2 A reaction that is a result of direct contact with the caterpillar or moth will appear on exposed areas; however, because setae embed in linens and clothing, they might cause a reaction anywhere on the body. Although usually self-limited, a hypersensitivity reaction might develop within minutes and can last for days or weeks.
Stings and hypersensitivity reactions to caterpillars and moths tend to lead to a nonspecific histologic presentation characterized by epidermal edema and a superficial perivascular lymphocytic infiltrate, often with eosinophils.6 After approximately 1 week, a foreign-body response to setae can lead to tuberculoid granulomas accompanied by neutrophils in the dermis and occasionally in subcutaneous tissues (Figures 5 and 6).8 If setae have not yet been removed, they also might be visible in skin scrapings.


Additional complications can accompany the hypersensitivity reaction to setae or spines. Type I hypersensitivity reactions can lead to severe reactions on second contact due to previously sensitized IgE antibodies. Although the first reaction appears mild, second contact might result in angioedema, wheezing, dyspnea, or anaphylaxis, or a combination of these findings.9 In addition, some patients who come in contact with Dendrolimus caterpillars might develop a condition known as dendrolimiasis, characterized by dermatitis in addition to arthritis or chondritis.6 The arthritis is normally monoarticular and can result in complete destruction of the joint. Pararamose, a condition with a similar presentation, is caused by the Brazilian moth Premolis semirufa.6
Contact of setae or spines with mucous membranes or inhalation of setae also might result in edema, dysphagia, dyspnea, drooling, rhinitis, or conjunctivitis, or a combination of these findings.6 In addition, setae can embed in the eye and cause an inflammatory reaction—ophthalmia nodosa—most commonly caused by caterpillars of the pine processionary moth (Thaumetopoea pityocampa) and characterized by immediate chemosis, which can progress to liquefactive necrosis and hypopyon, later developing into a granulomatous foreign-body response.2,10 The process is thought to be the result of a combination of the thaumetopoein toxin in the setae and an IgE-mediated response to other proteins.10
Due to their harpoon shape and forward-only motion, setae might migrate deeper, potentially even to the optic nerve.11 Because migration might take years and the barbed shape of setae does not always allow removal, some patients require lifetime monitoring with slit-lamp examination.Chronic problems, such as cataracts and persistent posterior uveitis, have been reported.10,11
Lonomism—One of the most serious (though rarest) reactions to caterpillars is lonomism, a condition caused by the caterpillars of Lonomia achelous and Lonomia obliqua moths. These caterpillars have a unique combination of toxins filling their branched spines, which ultimately leads to the same outcome: a hemorrhagic diathesis.
The toxin of L achelous comprises several proteases that degrade fibrin, fibrinogen, and factor XIII while activating prothrombin. In contrast, L obliqua poison causes a hemorrhagic diathesis by promoting a consumptive coagulopathy through enzymes that activate factor X and prothrombin.
With initial contact with either of these Lonomia caterpillars, the patient experiences severe pain accompanied by systemic symptoms, including headache, nausea, and vomiting. Shortly afterward, symptoms of a hemorrhagic diathesis manifest, including bleeding gums, hematuria, bleeding from prior wounds, and epistaxis.5 Serious complications of the hemorrhagic diathesis, such as hemorrhage of major organs, leads to death in 4% of patients.5 A reported case of a patient whose Lonomia caterpillar sting went unrecognized until a week after the accident ended with progression to stage V chronic renal disease.12
Recent research has focused on the specific mechanism of injury caused by Lonomia species. A study found that the venom of L obliqua causes cytoskeleton rearrangement and migration in vascular smooth muscle cells (VSMCs) by inducing formation of reactive oxygen species through activation of nicotinamide adenine dinucleotide phosphate oxidase.13 Thus, the venom directly contributes to the proinflammatory phenotype of endothelial cells seen following envenomation. The same study also demonstrated that elevated reactive oxygen species trigger extracellular signal-regulated kinase pathway activation in VSMCs, leading to cell proliferation, re-stenosis, and ischemia.13 This finding was confirmed by another study,14 which demonstrated an increase in Rac1, a signaling protein involved in the extracellular signal-regulated kinase pathway, in VSMCs upon exposure to L obliqua venom. These studies propose potential new targets for treatment to prevent vascular damage.
Reactions to Adult Organisms—Although it is more common for the caterpillar form of these organisms to cause an adverse reaction, the adult moth also might be capable of causing a similar reaction by retaining setae from the cocoon or by their own spines. The most notable example of this is female moths of the genus Hylesia, which possess spines attached to glands on the abdomen. The poison in these spines—a mixture of proteases and chitinase—causes a dermatitis known as Caripito itch—the name derived from a river port in Venezuela where this moth caused a memorable epidemic of moth-induced dermatitis.7,15 Caripito itch is known for intense pruritus that most commonly lasts days or weeks, possibly longer than 1 year.
Diagnostic Difficulties
The challenge of diagnosing a caterpillar- or moth-induced reaction in humans arises from (1) the lack of clinical history (the caterpillar might not be seen at all by the patient or the examiner) and (2) the similarity of these reactions to those with more common triggers.
When setae remain embedded in the skin or mucous membranes, skin scrapings allow accelerated diagnosis. On a skin scraping prepared with 20% potassium hydroxide, setae appear as tapered and barbed hairlike structures, which allows them to be distinguished from other similar-appearing but differently shaped structures, such as glass fibers.
When setae do not remain embedded in the skin or when the cause of the reaction is due to spines, the physician is left with a nonspecific histologic picture and a large differential diagnosis to be narrowed down based on the history and occasionally the pattern of the skin lesion.
A challenge in sting diagnosis is differentiating a caterpillar or moth sting from that of another organism. In certain cases, such as those of the family Megalopygidae, specific patterns of stings might assist in making the diagnosis. Hypersensitivity reactions are associated with a wider differential diagnosis, including irritant or allergic dermatitis from other causes, scabies, eczema, lichen planus, lichen simplex chronicus, seborrheic dermatitis, and tinea corporis, to name a few.6 Skin scrapings can be examined for other features, such as burrows in the case of scabies, to further narrow the differential.
Stings and hypersensitivity reactions lacking a proper history and associated with more severe systemic symptoms have caused misdiagnosis or led to a workup for the wrong condition; for example, the picture of abdominal pain, nausea, vomiting, tachycardia, leukocytosis, hypokalemia, and metabolic acidosis can simulate appendicitis.16 Upon discovery of a puss caterpillar sting in a patient, her symptoms resolved after treatment with ondansetron, morphine, and intravenous fluids.16
In lonomism, the diagnosis must be established by laboratory measurement of the fibrinogen level, clotting factors, prothrombin time, and activated partial thromboplastin time.4 The differential diagnosis associated with lonomism includes disseminated intravascular coagulation (DIC), snakebite, and a hereditary bleeding disorder.4 The combination of laboratory tests and an extensive medical history allows a diagnosis. Absence of a personal or family history of bleeding excludes a diagnosis of hereditary bleeding disorder, whereas the absence of known causes of DIC or thrombocytopenia allows DIC to be excluded from the differential.
Treatment Options and Prevention
Treatment—The first step is to remove any embedded setae from the skin or mucous membranes. The stepwise recommendation is to remove any constricted clothing, detach setae with adhesive tape, wash with soap and water, and dry without touching the skin.1 Any remaining setae can be removed with additional tape or forceps; setae tend to be fragile and are difficult to remove in their entirety.
Other than removal of the setae, skin reactions are treated symptomatically. Ice packs and isopropyl alcohol have been utilized to cool burning or stinging areas. Pain, pruritus, and inflammation have been alleviated with antihistamines and topical corticosteroids.1 When pain is severe, oral codeine or local injection of anesthetic can be used. For severe and persistent skin lesions, a course of an oral glucocorticoid can be administered. Intramuscular triamcinolone acetonide has been shown to treat pain, dermatitis, and subcutaneous nodules otherwise refractory to treatment.8
Antivenin specific for L obliqua exists to treat lonomism and is therefore effective only when lonomism is caused by that species. Lonomism caused by L achelous is treated with cryoprecipitate, purified fibrinogen, and antifibrinolytic drugs, such as aprotinin.6 Whole blood and fresh-frozen plasma have been noted to make hemorrhage worse when utilized to treat lonomism. Because the mechanism of action of the venom of Lonomia species is based, in part, on inducing a proinflammatory profile in endothelial cells, studies have demonstrated that inhibition of kallikrein might prevent vascular injury and thus prevent serious adverse effects, such as renal failure.17
Prevention—People should wear proper protective clothing when outdoors in potentially infested areas. Measures should be taken to ensure that linens and clothing are not left outside in areas where setae might be carried on the wind. Infestation control is necessary if the population of caterpillars reaches a high enough level.
Conclusion
Several species of caterpillars and moths cause adverse reactions in humans: stings, hypersensitivity reactions, and lonomism. Although most reactions are self-limited, some might have more serious effects, including organ failure and death. Mechanisms of injury vary by species of caterpillar, moth, and butterfly; current research is focused on further defining venom components and signaling pathways to isolate potential targets to aid in the diagnosis and treatment of lepidopterism.
- Goldman BS, Bragg BN. Caterpillar and moth bites. Stat Pearls [Internet]. StatPearls Publishing. Updated August 3, 2021. Accessed November 4, 2021. https://www.ncbi.nlm.nih.gov/books/NBK539851/
- Hossler EW. Caterpillars and moths: part I. Dermatologic manifestations of encounters with Lepidoptera. J Am Acad Dermatol. 2010;62:1-10. doi:10.1016/j.jaad.2009.08.060
- Forrester MB. Megalopyge opercularis caterpillar stings reported to Texas poison centers. Wilderness Environ Med. 2018;29:215-220. doi:10.1016/j.wem.2018.02.002
- Hossler EW. Lepidopterism: skin disorders secondary to caterpillars and moths. UpToDate website. Published October 20, 2021. Accessed November 18, 2021. https://www.uptodate.com/contents/lepidopterism-skin-disorders-secondary-to-caterpillars-and-moths
- Villas-Boas IM, Bonfá G, Tambourgi DV. Venomous caterpillars: from inoculation apparatus to venom composition and envenomation. Toxicon. 2018;153:39-52. doi:10.1016/j.toxicon.2018.08.007
- Hossler EW. Caterpillars and moths: part II. dermatologic manifestations of encounters with Lepidoptera. J Am Acad Dermatol. 2010;62:13-28. doi:10.1016/j.jaad.2009.08.061
- Yao Z, Kamau PM, Han Y, et al. The Latoia consocia caterpillar induces pain by targeting nociceptive ion channel TRPV1. Toxins (Basel). 2019;11:695. doi:10.3390/toxins11120695
- Paniz-Mondolfi AE, Pérez-Alvarez AM, Lundberg U, et al. Cutaneous lepidopterism: dermatitis from contact with moths of Hylesia metabus (Cramer 1775) (Lepidoptera: Saturniidae), the causative agent of caripito itch. Int J Dermatol. 2011;50:535-541. doi:10.1111/j.1365-4632.2010.04683.x
- Santos-Magadán S, González de Olano D, Bartolomé-Zavala B, et al. Adverse reactions to the processionary caterpillar: irritant or allergic mechanism? Contact Dermatitis. 2009;60:109-110. doi:10.1111/j.1600-0536.2008.01464.x
- González-Martín-Moro J, Contreras-Martín I, Castro-Rebollo M, et al. Focal cortical cataract due to caterpillar hair migration. Clin Exp Optom. 2019;102:89-90. doi:10.1111/cxo.12809
- Singh A, Behera UC, Agrawal H. Intra-lenticular caterpillar seta in ophthalmia nodosa. Eur J Ophthalmol. 2021;31:NP109-NP111. doi:10.1177/1120672119858899
- Schmitberger PA, Fernandes TC, Santos RC, et al. Probable chronic renal failure caused by Lonomia caterpillar envenomation. J Venom Anim Toxins Incl Trop Dis. 2013;19:14. doi:10.1186/1678-9199-19-14
- Moraes JA, Rodrigues G, Nascimento-Silva V, et al. Effects of Lonomia obliqua venom on vascular smooth muscle cells: contribution of NADPH oxidase-derived reactive oxygen species. Toxins (Basel). 2017;9:360. doi:10.3390/toxins9110360
- Bernardi L, Pinto AFM, Mendes E, et al. Lonomia obliqua bristle extract modulates Rac1 activation, membrane dynamics and cell adhesion properties. Toxicon. 2019;162:32-39. doi:10.1016/j.toxicon.2019.02.019
- Cabrera G, Lundberg U, Rodríguez-Ulloa A, et al. Protein content of the Hylesia metabus egg nest setae (Cramer [1775]) (Lepidoptera: Saturniidae) and its association with the parental investment for the reproductive success and lepidopterism. J Proteomics. 2017;150:183-200. doi:10.1016/j.jprot.2016.08.010
- Greene SC, Carey JM. Puss caterpillar envenomation: erucism mimicking appendicitis in a young child. Pediatr Emerg Care. 2020;36:E732-E734. doi:10.1097/PEC.0000000000001514
- Berger M, de Moraes JA, Beys-da-Silva WO, et al. Renal and vascular effects of kallikrein inhibition in a model of Lonomia obliqua venom-induced acute kidney injury. PLoS Negl Trop Dis. 2019;13:e0007197. doi:10.1371/journal.pntd.0007197
- Goldman BS, Bragg BN. Caterpillar and moth bites. Stat Pearls [Internet]. StatPearls Publishing. Updated August 3, 2021. Accessed November 4, 2021. https://www.ncbi.nlm.nih.gov/books/NBK539851/
- Hossler EW. Caterpillars and moths: part I. Dermatologic manifestations of encounters with Lepidoptera. J Am Acad Dermatol. 2010;62:1-10. doi:10.1016/j.jaad.2009.08.060
- Forrester MB. Megalopyge opercularis caterpillar stings reported to Texas poison centers. Wilderness Environ Med. 2018;29:215-220. doi:10.1016/j.wem.2018.02.002
- Hossler EW. Lepidopterism: skin disorders secondary to caterpillars and moths. UpToDate website. Published October 20, 2021. Accessed November 18, 2021. https://www.uptodate.com/contents/lepidopterism-skin-disorders-secondary-to-caterpillars-and-moths
- Villas-Boas IM, Bonfá G, Tambourgi DV. Venomous caterpillars: from inoculation apparatus to venom composition and envenomation. Toxicon. 2018;153:39-52. doi:10.1016/j.toxicon.2018.08.007
- Hossler EW. Caterpillars and moths: part II. dermatologic manifestations of encounters with Lepidoptera. J Am Acad Dermatol. 2010;62:13-28. doi:10.1016/j.jaad.2009.08.061
- Yao Z, Kamau PM, Han Y, et al. The Latoia consocia caterpillar induces pain by targeting nociceptive ion channel TRPV1. Toxins (Basel). 2019;11:695. doi:10.3390/toxins11120695
- Paniz-Mondolfi AE, Pérez-Alvarez AM, Lundberg U, et al. Cutaneous lepidopterism: dermatitis from contact with moths of Hylesia metabus (Cramer 1775) (Lepidoptera: Saturniidae), the causative agent of caripito itch. Int J Dermatol. 2011;50:535-541. doi:10.1111/j.1365-4632.2010.04683.x
- Santos-Magadán S, González de Olano D, Bartolomé-Zavala B, et al. Adverse reactions to the processionary caterpillar: irritant or allergic mechanism? Contact Dermatitis. 2009;60:109-110. doi:10.1111/j.1600-0536.2008.01464.x
- González-Martín-Moro J, Contreras-Martín I, Castro-Rebollo M, et al. Focal cortical cataract due to caterpillar hair migration. Clin Exp Optom. 2019;102:89-90. doi:10.1111/cxo.12809
- Singh A, Behera UC, Agrawal H. Intra-lenticular caterpillar seta in ophthalmia nodosa. Eur J Ophthalmol. 2021;31:NP109-NP111. doi:10.1177/1120672119858899
- Schmitberger PA, Fernandes TC, Santos RC, et al. Probable chronic renal failure caused by Lonomia caterpillar envenomation. J Venom Anim Toxins Incl Trop Dis. 2013;19:14. doi:10.1186/1678-9199-19-14
- Moraes JA, Rodrigues G, Nascimento-Silva V, et al. Effects of Lonomia obliqua venom on vascular smooth muscle cells: contribution of NADPH oxidase-derived reactive oxygen species. Toxins (Basel). 2017;9:360. doi:10.3390/toxins9110360
- Bernardi L, Pinto AFM, Mendes E, et al. Lonomia obliqua bristle extract modulates Rac1 activation, membrane dynamics and cell adhesion properties. Toxicon. 2019;162:32-39. doi:10.1016/j.toxicon.2019.02.019
- Cabrera G, Lundberg U, Rodríguez-Ulloa A, et al. Protein content of the Hylesia metabus egg nest setae (Cramer [1775]) (Lepidoptera: Saturniidae) and its association with the parental investment for the reproductive success and lepidopterism. J Proteomics. 2017;150:183-200. doi:10.1016/j.jprot.2016.08.010
- Greene SC, Carey JM. Puss caterpillar envenomation: erucism mimicking appendicitis in a young child. Pediatr Emerg Care. 2020;36:E732-E734. doi:10.1097/PEC.0000000000001514
- Berger M, de Moraes JA, Beys-da-Silva WO, et al. Renal and vascular effects of kallikrein inhibition in a model of Lonomia obliqua venom-induced acute kidney injury. PLoS Negl Trop Dis. 2019;13:e0007197. doi:10.1371/journal.pntd.0007197
Practice Points
- Lepidopterism describes adverse reactions caused by the stings, hypersensitivity reactions, and lonomism (a hemorrhagic diathesis) of caterpillars, moths, and butterflies.
- Caterpillars can induce an adverse reaction by injecting venom stored in their bristles, inducing a foreign-body reaction to embedded bristles, or a combination of these mechanisms.
- A thorough history, skin scrapings, relevant examination of affected body parts (such as slit-lamp examination, in the case of eyes), and laboratory testing should be conducted to narrow the wide differential diagnosis associated with lepidopterism.
Botanical Briefs: Phytophotodermatitis Caused by Giant Hogweed (Heracleum mantegazzianum)
Giant hogweed (Heracleum mantegazzianum) is an invasive flowering weed of the family Apiaceae that typically reaches a height of 13 feet, with thick stems; large green leaves; and umbrella-shaped, flat-topped, radial clusters (umbels) of small individual white flowers1 (Figure 1). Because of the size and beauty of giant hogweed, it was widely planted in 19th century ornamental gardens in the United Kingdom and has since naturalized and spread throughout central Europe, Canada, and the United States.1,2 The plant most commonly is found in shady areas near rivers and woodlands.1

Due to the invasive nature of the giant hogweed, its prevalence continues to grow, its eradication remains difficult, and reports of phytophotodermatitis are increasing in number and distribution. In fact, there has been widespread media coverage of the dangers of giant hogweed in the United Kingdom since 20161 and in the United States in 2018 and 2019.3-6
Transmission
Phytophotodermatitis is a type of nonimmunologic dermatitis caused by UV light reacting with a plant-based photosensitizing agent. In the case of giant hogweed, sap from the plant’s fruits, leaves, and stem contain furocoumarins or psoralens.7 Upon activation by UVA radiation, furan rings of these compounds create reactive oxygen species and intercalate with DNA pyrimidine bases, which results in cellular death, damage to successive skin layers, and reduced wound healing at the cellular level.8 This effect is intensified with increased percutaneous absorption of furocoumarin, which can result from high temperature, humidity, skin infection, lack of protective clothing, and moist conditions.9
The highest concentration of phototoxic compounds is found in giant hogweed from June through August,7 which, in combination with people increasing their outdoor activity in the summer, results in a greater prevalence and severity of H mantegazzianum phytophotodermatitis during summer months.
Presentation
Phytophotodermatitis caused by giant hogweed can range from burning and erythema to full-thickness chemical burns that require surgical debridement and skin grafting.8 After exposure to the offending agent, a harmful skin reaction can start within 15 minutes. After a latent period of approximately 24 hours, erythema, edema, and bullae can appear and generally peak by 72 hours.10 In addition to cutaneous injury, inhalation of giant hogweed traces can result in obstructive pulmonary symptoms. Eye contact can result in blindness.9
In addition to the rash caused by giant hogweed, a “weed-wacker dermatitis” or “strimmer rash” can be caused by the similar-appearing but smaller common hogweed (Heracleum sphondylium). Common hogweed is highly prevalent in the United States and often is confused with the larger giant hogweed because of tall stems and white, flat-topped flowers.
Management
Following contact with giant hogweed, a person should immediately avoid UV exposure and rinse the area with soap and water. UV radiation must be avoided for at least 48 hours. If erythema occurs, a topical steroid can be applied to the affected area; pain can be alleviated by a nonsteroidal anti-inflammatory drug.9
Further treatment might be required if bullous lesions are present. Small blisters can be punctured and drained; however, large blisters, extensive epidermal-dermal separation, and large areas of detached epidermis should simply be cleansed and dressed. An oral steroid also can be used to reduce inflammation in moderate and severe cases. Full-thickness injury might require surgical intervention.8
Clinical Case
A 27-year-old male landscaper presented to the emergency department with an increasingly painful blistering rash on the arms and neck of 1 day’s duration. He noticed bright red skin and blisters 18 to 24 hours after trimming what he identified as shoulder-high giant hogweed plants. Neither he nor his coworkers were wearing sunscreen or protective clothing as they cleared the plants for several hours. His coworkers developed similar rashes, but his rash was the most severe, requiring treatment in the emergency department.
Physical examination showed innumerable 2- to 10-mm, tense vesicles and bullae on a background of blanching erythema in a striking photodistribution along the neck (Figure 2) and arms (Figure 3). He had notable edema of both arms and several large 3- to 4-cm bullae on the ventral aspects of the forearms.
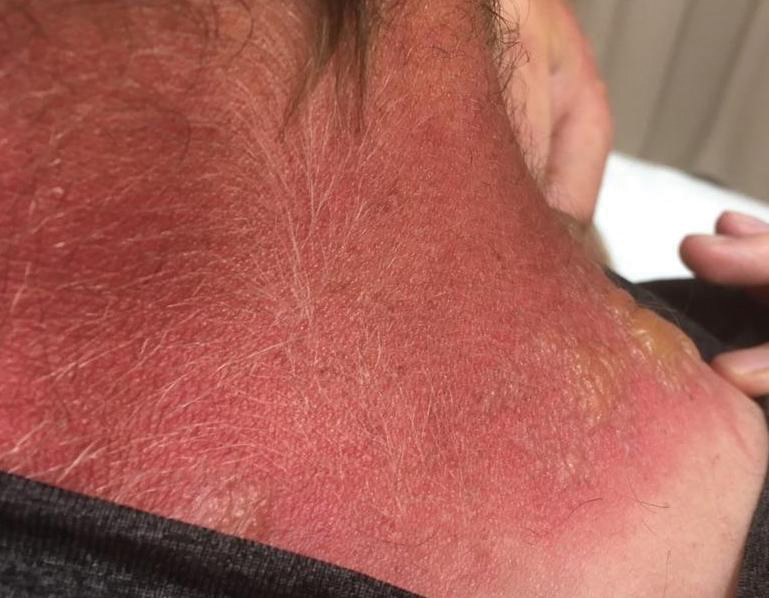
The patient was diagnosed with severe phytophotodermatitis secondary to contact with H mantegazzianum and was started on oral prednisone 70 mg daily (1 mg/kg/d), which was decreased by 10 mg every 3 days until the course of treatment was complete. He also was instructed to apply mupirocin ointment to open areas and petroleum jelly to intact skin. Additionally, he was advised to practice strict photoprotection for the near and distant future.
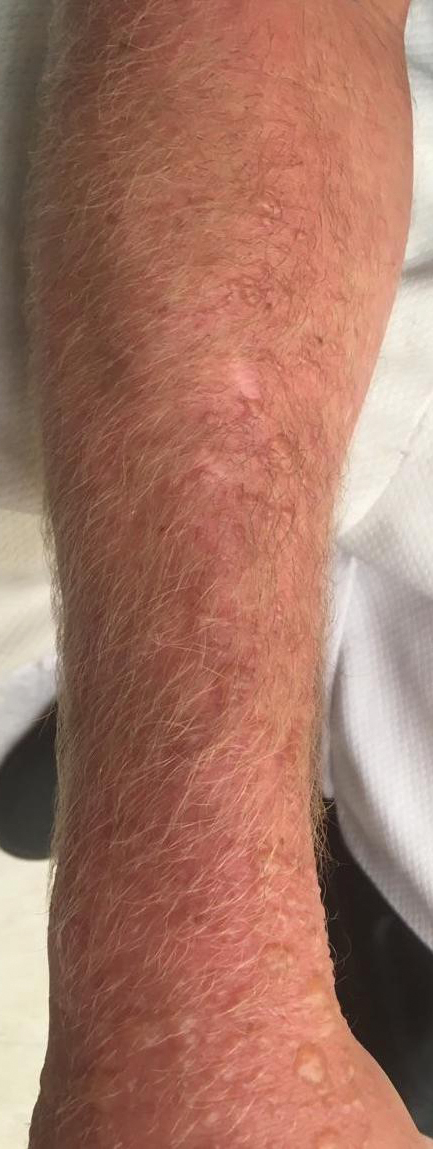
Within several days after treatment began, the phytophotodermatitis dramatically improved, with complete resolution in 1 week. Postinflammatory hyperpigmentation resolved after several weeks.
- Baker B, Bedford J, Kanitkar S. Keeping pace with the media; giant hogweed burns—a case series and comprehensive review [published online December 26, 2016]. Burns. 2017;13:933-938. doi:10.1016/j.burns.2016.10.018
- Klimaszyk P, Klimaszyk D, Piotrowiak M, et al. Unusual complications after occupational exposure to giant hogweed (Heracleum mantegazzianum): a case report. Int J Occup Med Environ Health. 2014;27:141-144. doi:10.2478/s13382-014-0238-z
- Zaveria M, Hauser C. Giant hogweed: a plant that can burn and blind you. but don’t panic. New York Times. July 2, 2018. Accessed October 18, 2021. https://www.nytimes.com/2018/07/02/us/giant-hogweed-nyt.html
- Hignett K. Giant hogweed: New York officials warn residents about dangerous plant that causes serious burns, blisters and scars. Newsweek. June 25, 2019. Accessed October 18, 2021. https://www.newsweek.com/giant-hogweed-new-york-dangerous-plant-burns-skin-sunlight-1445785
- Eastman J. Toxic giant hogweed sap that burns, blisters skin found in Clark County. The Oregonian. Updated July 16, 2019. Accessed October 18, 2021. https://www.oregonlive.com/news/2019/07/toxic-giant-hogweed-plant-that-burns-blisters-skin-found-in-clark-county.html
- O’Kane C. Giant hogweed, plant that causes blindness and third-degree burns, discovered in Virginia. CBS News. June 18, 2018. Accessed October 18, 2021. https://www.cbsnews.com/news/giant-hogweed-plant-causes-blindness-third-degree-burns-discovered-in-virginia-otherstates/
- Pira E, Romano C, Sulotto F, et al. Heracleum mantegazzianum growth phases and furocoumarin content. Contact Dermatitis. 1989;21:300-303. doi:10.1111/j.1600-0536.1989.tb04747.x
- Chan JCY, Sullivan PJ, O’Sullivan MJ, et al. Full thickness burn caused by exposure to giant hogweed: delayed presentation, histological features and surgical management. J Plast Reconstr Aesthet Surg. 2011;64:128-130. doi:10.1016/j.bjps.2010.03.030
- Pfurtscheller K, Trop M. Phototoxic plant burns: report of a case and review of topical wound treatment in children. Pediatr Dermatol. 2014;31:E156-E159. doi:10.1111/pde.12396
- Kavli G, Volden G: Phytophotodermatitis. Photodermatol. 1984;1:65-75.
Giant hogweed (Heracleum mantegazzianum) is an invasive flowering weed of the family Apiaceae that typically reaches a height of 13 feet, with thick stems; large green leaves; and umbrella-shaped, flat-topped, radial clusters (umbels) of small individual white flowers1 (Figure 1). Because of the size and beauty of giant hogweed, it was widely planted in 19th century ornamental gardens in the United Kingdom and has since naturalized and spread throughout central Europe, Canada, and the United States.1,2 The plant most commonly is found in shady areas near rivers and woodlands.1

Due to the invasive nature of the giant hogweed, its prevalence continues to grow, its eradication remains difficult, and reports of phytophotodermatitis are increasing in number and distribution. In fact, there has been widespread media coverage of the dangers of giant hogweed in the United Kingdom since 20161 and in the United States in 2018 and 2019.3-6
Transmission
Phytophotodermatitis is a type of nonimmunologic dermatitis caused by UV light reacting with a plant-based photosensitizing agent. In the case of giant hogweed, sap from the plant’s fruits, leaves, and stem contain furocoumarins or psoralens.7 Upon activation by UVA radiation, furan rings of these compounds create reactive oxygen species and intercalate with DNA pyrimidine bases, which results in cellular death, damage to successive skin layers, and reduced wound healing at the cellular level.8 This effect is intensified with increased percutaneous absorption of furocoumarin, which can result from high temperature, humidity, skin infection, lack of protective clothing, and moist conditions.9
The highest concentration of phototoxic compounds is found in giant hogweed from June through August,7 which, in combination with people increasing their outdoor activity in the summer, results in a greater prevalence and severity of H mantegazzianum phytophotodermatitis during summer months.
Presentation
Phytophotodermatitis caused by giant hogweed can range from burning and erythema to full-thickness chemical burns that require surgical debridement and skin grafting.8 After exposure to the offending agent, a harmful skin reaction can start within 15 minutes. After a latent period of approximately 24 hours, erythema, edema, and bullae can appear and generally peak by 72 hours.10 In addition to cutaneous injury, inhalation of giant hogweed traces can result in obstructive pulmonary symptoms. Eye contact can result in blindness.9
In addition to the rash caused by giant hogweed, a “weed-wacker dermatitis” or “strimmer rash” can be caused by the similar-appearing but smaller common hogweed (Heracleum sphondylium). Common hogweed is highly prevalent in the United States and often is confused with the larger giant hogweed because of tall stems and white, flat-topped flowers.
Management
Following contact with giant hogweed, a person should immediately avoid UV exposure and rinse the area with soap and water. UV radiation must be avoided for at least 48 hours. If erythema occurs, a topical steroid can be applied to the affected area; pain can be alleviated by a nonsteroidal anti-inflammatory drug.9
Further treatment might be required if bullous lesions are present. Small blisters can be punctured and drained; however, large blisters, extensive epidermal-dermal separation, and large areas of detached epidermis should simply be cleansed and dressed. An oral steroid also can be used to reduce inflammation in moderate and severe cases. Full-thickness injury might require surgical intervention.8
Clinical Case
A 27-year-old male landscaper presented to the emergency department with an increasingly painful blistering rash on the arms and neck of 1 day’s duration. He noticed bright red skin and blisters 18 to 24 hours after trimming what he identified as shoulder-high giant hogweed plants. Neither he nor his coworkers were wearing sunscreen or protective clothing as they cleared the plants for several hours. His coworkers developed similar rashes, but his rash was the most severe, requiring treatment in the emergency department.
Physical examination showed innumerable 2- to 10-mm, tense vesicles and bullae on a background of blanching erythema in a striking photodistribution along the neck (Figure 2) and arms (Figure 3). He had notable edema of both arms and several large 3- to 4-cm bullae on the ventral aspects of the forearms.

The patient was diagnosed with severe phytophotodermatitis secondary to contact with H mantegazzianum and was started on oral prednisone 70 mg daily (1 mg/kg/d), which was decreased by 10 mg every 3 days until the course of treatment was complete. He also was instructed to apply mupirocin ointment to open areas and petroleum jelly to intact skin. Additionally, he was advised to practice strict photoprotection for the near and distant future.

Within several days after treatment began, the phytophotodermatitis dramatically improved, with complete resolution in 1 week. Postinflammatory hyperpigmentation resolved after several weeks.
Giant hogweed (Heracleum mantegazzianum) is an invasive flowering weed of the family Apiaceae that typically reaches a height of 13 feet, with thick stems; large green leaves; and umbrella-shaped, flat-topped, radial clusters (umbels) of small individual white flowers1 (Figure 1). Because of the size and beauty of giant hogweed, it was widely planted in 19th century ornamental gardens in the United Kingdom and has since naturalized and spread throughout central Europe, Canada, and the United States.1,2 The plant most commonly is found in shady areas near rivers and woodlands.1

Due to the invasive nature of the giant hogweed, its prevalence continues to grow, its eradication remains difficult, and reports of phytophotodermatitis are increasing in number and distribution. In fact, there has been widespread media coverage of the dangers of giant hogweed in the United Kingdom since 20161 and in the United States in 2018 and 2019.3-6
Transmission
Phytophotodermatitis is a type of nonimmunologic dermatitis caused by UV light reacting with a plant-based photosensitizing agent. In the case of giant hogweed, sap from the plant’s fruits, leaves, and stem contain furocoumarins or psoralens.7 Upon activation by UVA radiation, furan rings of these compounds create reactive oxygen species and intercalate with DNA pyrimidine bases, which results in cellular death, damage to successive skin layers, and reduced wound healing at the cellular level.8 This effect is intensified with increased percutaneous absorption of furocoumarin, which can result from high temperature, humidity, skin infection, lack of protective clothing, and moist conditions.9
The highest concentration of phototoxic compounds is found in giant hogweed from June through August,7 which, in combination with people increasing their outdoor activity in the summer, results in a greater prevalence and severity of H mantegazzianum phytophotodermatitis during summer months.
Presentation
Phytophotodermatitis caused by giant hogweed can range from burning and erythema to full-thickness chemical burns that require surgical debridement and skin grafting.8 After exposure to the offending agent, a harmful skin reaction can start within 15 minutes. After a latent period of approximately 24 hours, erythema, edema, and bullae can appear and generally peak by 72 hours.10 In addition to cutaneous injury, inhalation of giant hogweed traces can result in obstructive pulmonary symptoms. Eye contact can result in blindness.9
In addition to the rash caused by giant hogweed, a “weed-wacker dermatitis” or “strimmer rash” can be caused by the similar-appearing but smaller common hogweed (Heracleum sphondylium). Common hogweed is highly prevalent in the United States and often is confused with the larger giant hogweed because of tall stems and white, flat-topped flowers.
Management
Following contact with giant hogweed, a person should immediately avoid UV exposure and rinse the area with soap and water. UV radiation must be avoided for at least 48 hours. If erythema occurs, a topical steroid can be applied to the affected area; pain can be alleviated by a nonsteroidal anti-inflammatory drug.9
Further treatment might be required if bullous lesions are present. Small blisters can be punctured and drained; however, large blisters, extensive epidermal-dermal separation, and large areas of detached epidermis should simply be cleansed and dressed. An oral steroid also can be used to reduce inflammation in moderate and severe cases. Full-thickness injury might require surgical intervention.8
Clinical Case
A 27-year-old male landscaper presented to the emergency department with an increasingly painful blistering rash on the arms and neck of 1 day’s duration. He noticed bright red skin and blisters 18 to 24 hours after trimming what he identified as shoulder-high giant hogweed plants. Neither he nor his coworkers were wearing sunscreen or protective clothing as they cleared the plants for several hours. His coworkers developed similar rashes, but his rash was the most severe, requiring treatment in the emergency department.
Physical examination showed innumerable 2- to 10-mm, tense vesicles and bullae on a background of blanching erythema in a striking photodistribution along the neck (Figure 2) and arms (Figure 3). He had notable edema of both arms and several large 3- to 4-cm bullae on the ventral aspects of the forearms.

The patient was diagnosed with severe phytophotodermatitis secondary to contact with H mantegazzianum and was started on oral prednisone 70 mg daily (1 mg/kg/d), which was decreased by 10 mg every 3 days until the course of treatment was complete. He also was instructed to apply mupirocin ointment to open areas and petroleum jelly to intact skin. Additionally, he was advised to practice strict photoprotection for the near and distant future.

Within several days after treatment began, the phytophotodermatitis dramatically improved, with complete resolution in 1 week. Postinflammatory hyperpigmentation resolved after several weeks.
- Baker B, Bedford J, Kanitkar S. Keeping pace with the media; giant hogweed burns—a case series and comprehensive review [published online December 26, 2016]. Burns. 2017;13:933-938. doi:10.1016/j.burns.2016.10.018
- Klimaszyk P, Klimaszyk D, Piotrowiak M, et al. Unusual complications after occupational exposure to giant hogweed (Heracleum mantegazzianum): a case report. Int J Occup Med Environ Health. 2014;27:141-144. doi:10.2478/s13382-014-0238-z
- Zaveria M, Hauser C. Giant hogweed: a plant that can burn and blind you. but don’t panic. New York Times. July 2, 2018. Accessed October 18, 2021. https://www.nytimes.com/2018/07/02/us/giant-hogweed-nyt.html
- Hignett K. Giant hogweed: New York officials warn residents about dangerous plant that causes serious burns, blisters and scars. Newsweek. June 25, 2019. Accessed October 18, 2021. https://www.newsweek.com/giant-hogweed-new-york-dangerous-plant-burns-skin-sunlight-1445785
- Eastman J. Toxic giant hogweed sap that burns, blisters skin found in Clark County. The Oregonian. Updated July 16, 2019. Accessed October 18, 2021. https://www.oregonlive.com/news/2019/07/toxic-giant-hogweed-plant-that-burns-blisters-skin-found-in-clark-county.html
- O’Kane C. Giant hogweed, plant that causes blindness and third-degree burns, discovered in Virginia. CBS News. June 18, 2018. Accessed October 18, 2021. https://www.cbsnews.com/news/giant-hogweed-plant-causes-blindness-third-degree-burns-discovered-in-virginia-otherstates/
- Pira E, Romano C, Sulotto F, et al. Heracleum mantegazzianum growth phases and furocoumarin content. Contact Dermatitis. 1989;21:300-303. doi:10.1111/j.1600-0536.1989.tb04747.x
- Chan JCY, Sullivan PJ, O’Sullivan MJ, et al. Full thickness burn caused by exposure to giant hogweed: delayed presentation, histological features and surgical management. J Plast Reconstr Aesthet Surg. 2011;64:128-130. doi:10.1016/j.bjps.2010.03.030
- Pfurtscheller K, Trop M. Phototoxic plant burns: report of a case and review of topical wound treatment in children. Pediatr Dermatol. 2014;31:E156-E159. doi:10.1111/pde.12396
- Kavli G, Volden G: Phytophotodermatitis. Photodermatol. 1984;1:65-75.
- Baker B, Bedford J, Kanitkar S. Keeping pace with the media; giant hogweed burns—a case series and comprehensive review [published online December 26, 2016]. Burns. 2017;13:933-938. doi:10.1016/j.burns.2016.10.018
- Klimaszyk P, Klimaszyk D, Piotrowiak M, et al. Unusual complications after occupational exposure to giant hogweed (Heracleum mantegazzianum): a case report. Int J Occup Med Environ Health. 2014;27:141-144. doi:10.2478/s13382-014-0238-z
- Zaveria M, Hauser C. Giant hogweed: a plant that can burn and blind you. but don’t panic. New York Times. July 2, 2018. Accessed October 18, 2021. https://www.nytimes.com/2018/07/02/us/giant-hogweed-nyt.html
- Hignett K. Giant hogweed: New York officials warn residents about dangerous plant that causes serious burns, blisters and scars. Newsweek. June 25, 2019. Accessed October 18, 2021. https://www.newsweek.com/giant-hogweed-new-york-dangerous-plant-burns-skin-sunlight-1445785
- Eastman J. Toxic giant hogweed sap that burns, blisters skin found in Clark County. The Oregonian. Updated July 16, 2019. Accessed October 18, 2021. https://www.oregonlive.com/news/2019/07/toxic-giant-hogweed-plant-that-burns-blisters-skin-found-in-clark-county.html
- O’Kane C. Giant hogweed, plant that causes blindness and third-degree burns, discovered in Virginia. CBS News. June 18, 2018. Accessed October 18, 2021. https://www.cbsnews.com/news/giant-hogweed-plant-causes-blindness-third-degree-burns-discovered-in-virginia-otherstates/
- Pira E, Romano C, Sulotto F, et al. Heracleum mantegazzianum growth phases and furocoumarin content. Contact Dermatitis. 1989;21:300-303. doi:10.1111/j.1600-0536.1989.tb04747.x
- Chan JCY, Sullivan PJ, O’Sullivan MJ, et al. Full thickness burn caused by exposure to giant hogweed: delayed presentation, histological features and surgical management. J Plast Reconstr Aesthet Surg. 2011;64:128-130. doi:10.1016/j.bjps.2010.03.030
- Pfurtscheller K, Trop M. Phototoxic plant burns: report of a case and review of topical wound treatment in children. Pediatr Dermatol. 2014;31:E156-E159. doi:10.1111/pde.12396
- Kavli G, Volden G: Phytophotodermatitis. Photodermatol. 1984;1:65-75.
PRACTICE POINTS
- The public should be educated, especially during summer months, about how to identify giant hogweed, reduce exposure to the plant’s phototoxin, and thus reduce the risk for severe phytophotodermatitis.
- Phytophotodermatitis should be included in the differential diagnosis when a patient presents with acute erythema and bullae in sun-exposed areas.
- Phytophotodermatitis can be treated by promptly washing the skin with soap and water, protecting the skin from exposure to UV light, and utilizing topical and oral steroids.
Botanical Briefs: Bloodroot (Sanguinaria canadensis)
Bloodroot (Sanguinaria canadensis) is a member of the family Papaveraceae.1 This North American plant commonly is found in widespread distribution from Nova Scotia, Canada, to Florida and from the Great Lakes to Mississippi.2 Historically, Native Americans used bloodroot as a skin dye and as a medicine for many ailments.3
Bloodroot blooms for only a few days, starting in March, and fruits in June. The flowers comprise 8 to 10 white petals, surrounding a bed of yellow stamens (Figure). The plant thrives in wooded areas and grows to 12 inches tall. In its off-season, the plant remains dormant and can survive below-freezing temperatures.4

Chemical Constituents
Bloodroot gets its colloquial name from its red sap, which is released when the plant’s rhizome is cut. This sap contains a high concentration of alkaloids that are used for protection against predators. The rhizome itself has a rusty, red-brown color; the roots are a brighter red-orange.4
The rhizome of S canadensis contains the highest concentration of active alkaloids; the roots also contain these chemicals, though to a lesser degree; and the leaves, flowers, and fruits harvest approximately 1% of the alkaloids found in the roots.4 The concentration of alkaloids can vary from one plant to the next, depending on environmental conditions.5,6
The major alkaloids in S canadensis include both quaternary benzophenanthridine alkaloids (eg, sanguinarine, chelerythrine, sanguilutine, chelilutine, sanguirubine, chelirubine) and protopin alkaloids (eg, protopine, allocryptopine).3,7 Of these, sanguinarine and chelerythrine typically are the most potent.1 Oral ingestion or topical application of these molecules can have therapeutic and toxic effects.8
Biophysiological Effects
Bloodroot has been shown to have remarkable antimicrobial effects.9 The plant produces hydrogen peroxide and superoxide anion.10 These mediators cause oxidative stress, thus inducing destruction of cellular DNA and the cell membrane.11 Although these effects can be helpful when fighting infection, they are not necessarily selective against healthy cells.12
Alkaloids of bloodroot also have cardiovascular therapeutic effects. Sanguinarine blocks angiotensin II and causes vasodilation, thus helping treat hypertension.13 It also acts as an inotrope by blocking the Na+/K+ ATPase pump. These effects in a patient who is already taking digoxin can cause notable cardiotoxicity because the 2 drugs share a mechanism of action.14
Chelerythrine blocks production of cyclooxygenase 2 and prostaglandin E2.15 This pathway modification results in anti-inflammatory effects that can help treat arthritis, edema, and other inflammatory conditions.16 Moreover, sanguinarine has demonstrated efficacy in numerous anticancer pathways,17 including downregulation of intercellular adhesion molecules, vascular cell adhesion molecules, and vascular endothelial growth factor (VEGF).18-20 Blocking VEGF is one way to inhibit angiogenesis,21 which is upregulated in tumor formation, thus sanguinarine can have an antiproliferative anticancer effect.22 Sanguinarine also upregulates molecules such as nuclear factor–κB and the protease enzymes known as caspases to cause proapoptotic effects, furthering its antitumor potential.23,24
Treatment of Dermatologic Conditions
The initial technique of Mohs micrographic surgery employed a chemopaste that utilized an extract of S canadensis to preserve tissue.25 Outside the dermatologist’s office, bloodroot is used as a topical home remedy for a variety of cutaneous conditions, including cancer, skin tags, and warts.26 Bloodroot is advertised as black salve, an alternative anticancer treatment.27,28
As useful as this natural agent sounds, it has a pitfall: The alkaloids of S canadensis are nonspecific in their cytotoxicity, damaging neoplastic and healthy tissue.29 This cytotoxic effect can cause escharification through diffuse tissue destruction and has been observed to result in formation of a keloid scar.30 The alkaloids in black salve also have been shown to cause skin erosions and cellular atypia.28,31 Therefore, the utility of this escharotic in medical treatment is limited.32 Fortuitously, oral antibiotics and wound care can help address this adverse effect.28
Bloodroot was once used as a mouth rinse and toothpaste to treat gingivitis, but this application was later associated with oral leukoplakia, a premalignant condition.33 Leukoplakia associated with S canadensis extract often is unremitting. Immediate discontinuation of the offending agent produces little regression, suggesting that cellular damage is irreversible.34
Final Thoughts
Although bloodroot demonstrates efficacy as a phytotherapeutic, it does come with notable toxicity. Physicians should warn patients of the unwanted cosmetic effects of black salve, especially oral products that incorporate sanguinarine. Adverse effects on the oropharynx can be irreversible, though the eschar associated with black salve can be treated with a topical or oral corticosteroid.29
- Vogel M, Lawson M, Sippl W, et al. Structure and mechanism of sanguinarine reductase, an enzyme of alkaloid detoxification. J Biol Chem. 2010;285:18397-18406. doi:10.1074/jbc.M109.088989
- Maranda EL, Wang MX, Cortizo J, et al. Flower power—the versatility of bloodroot. JAMA Dermatol. 2016;152:824. doi:10.1001/jamadermatol.2015.5522
- Setzer WN. The phytochemistry of Cherokee aromatic medicinal plants. Medicines (Basel). 2018;5:121. doi:10.3390/medicines5040121
- Croaker A, King GJ, Pyne JH, et al. Sanguinaria canadensis: traditional medicine, phytochemical composition, biological activities and current uses. Int J Mol Sci. 2016;17:1414. doi:10.3390/ijms17091414
- Graf TN, Levine KE, Andrews ME, et al. Variability in the yield of benzophenanthridine alkaloids in wildcrafted vs cultivated bloodroot (Sanguinaria canadensis L.) J Agric Food Chem. 2007; 55:1205-1211. doi:10.1021/jf062498f
- Bennett BC, Bell CR, Boulware RT. Geographic variation in alkaloid content of Sanguinaria canadensis (Papaveraceae). Rhodora. 1990;92:57-69.
- Leaver CA, Yuan H, Wallen GR. Apoptotic activities of Sanguinaria canadensis: primary human keratinocytes, C-33A, and human papillomavirus HeLa cervical cancer lines. Integr Med (Encinitas). 2018;17:32-37.
- Kutchan TM. Molecular genetics of plant alkaloid biosynthesis. In: Cordell GA, ed. The Alkaloids. Vol 50. Elsevier Science Publishing Co, Inc; 1997:257-316.
- Obiang-Obounou BW, Kang O-H, Choi J-G, et al. The mechanism of action of sanguinarine against methicillin-resistant Staphylococcus aureus. J Toxicol Sci. 2011;36:277-283. doi:10.2131/jts.36.277
- Z˙abka A, Winnicki K, Polit JT, et al. Sanguinarine-induced oxidative stress and apoptosis-like programmed cell death (AL-PCD) in root meristem cells of Allium cepa. Plant Physiol Biochem. 2017;112:193-206. doi:10.1016/j.plaphy.2017.01.004
- Kumar GS, Hazra S. Sanguinarine, a promising anticancer therapeutic: photochemical and nucleic acid binding properties. RSC Advances. 2014;4:56518-56531.
- Ping G, Wang Y, Shen L, et al. Highly efficient complexation of sanguinarine alkaloid by carboxylatopillar[6]arene: pKa shift, increased solubility and enhanced antibacterial activity. Chemical Commun (Camb). 2017;53:7381-7384. doi:10.1039/c7cc02799k
- Caballero-George C, Vanderheyden PM, Solis PN, et al. Biological screening of selected medicinal Panamanian plants by radioligand-binding techniques. Phytomedicine. 2001;8:59-70. doi:10.1078/0944-7113-00011
- Seifen E, Adams RJ, Riemer RK. Sanguinarine: a positive inotropic alkaloid which inhibits cardiac Na+, K+-ATPase. Eur J Pharmacol. 1979;60:373-377. doi:10.1016/0014-2999(79)90245-0
- Debprasad C, Hemanta M, Paromita B, et al. Inhibition of NO2, PGE2, TNF-α, and iNOS EXpression by Shorea robusta L.: an ethnomedicine used for anti-inflammatory and analgesic activity. Evid Based Complement Alternat Med. 2012; 2012:254849. doi:10.1155/2012/254849
- Melov S, Ravenscroft J, Malik S, et al. Extension of life-span with superoxide dismutase/catalase mimetics. Science. 2000;289:1567-1569. doi:10.1126/science.289.5484.1567
- Basu P, Kumar GS. Sanguinarine and its role in chronic diseases. In: Gupta SC, Prasad S, Aggarwal BB, eds. Advances in Experimental Medicine and Biology: Anti-inflammatory Nutraceuticals and Chronic Diseases. Vol 928. Springer International Publishing; 2016:155-172.
- Alasvand M, Assadollahi V, Ambra R, et al. Antiangiogenic effect of alkaloids. Oxid Med Cell Longev. 2019;2019:9475908. doi:10.1155/2019/9475908
- Basini G, Santini SE, Bussolati S, et al. The plant alkaloid sanguinarine is a potential inhibitor of follicular angiogenesis. J Reprod Dev. 2007;53:573-579. doi:10.1262/jrd.18126
- Xu J-Y, Meng Q-H, Chong Y, et al. Sanguinarine is a novel VEGF inhibitor involved in the suppression of angiogenesis and cell migration. Mol Clin Oncol. 2013;1:331-336. doi:10.3892/mco.2012.41
- Lu K, Bhat M, Basu S. Plants and their active compounds: natural molecules to target angiogenesis. Angiogenesis. 2016;19:287-295. doi:10.1007/s10456-016-9512-y
- Achkar IW, Mraiche F, Mohammad RM, et al. Anticancer potential of sanguinarine for various human malignancies. Future Med Chem. 2017;9:933-950. doi:10.4155/fmc-2017-0041
- Lee TK, Park C, Jeong S-J, et al. Sanguinarine induces apoptosis of human oral squamous cell carcinoma KB cells via inactivation of the PI3K/Akt signaling pathway. Drug Dev Res. 2016;77:227-240. doi:10.1002/ddr.21315
- Gaziano R, Moroni G, Buè C, et al. Antitumor effects of the benzophenanthridine alkaloid sanguinarine: evidence and perspectives. World J Gastrointest Oncol. 2016;8:30-39. doi:10.4251/wjgo.v8.i1.30
- Mohs FE. Chemosurgery for skin cancer: fixed tissue and fresh tissue techniques. Arch Dermatol. 1976;112:211-215.
- Affleck AG, Varma S. A case of do-it-yourself Mohs’ surgery using bloodroot obtained from the internet. Br J Dermatol. 2007;157:1078-1079. doi:10.1111/j.1365-2133.2007.08180.x
- Eastman KL, McFarland LV, Raugi GJ. Buyer beware: a black salve caution. J Am Acad Dermatol. 2011;65:E154-E155. doi:10.1016/j.jaad.2011.07.031
- Osswald SS, Elston DM, Farley MF, et al. Self-treatment of a basal cell carcinoma with “black and yellow salve.” J Am Acad Dermatol. 2005;53:508-510. doi:10.1016/j.jaad.2005.04.007
- Schlichte MJ, Downing CP, Ramirez-Fort M, et al. Bloodroot associated eschar. Dermatol Online J. 2015;20:13030/qt05r0r2wr.
- Wang MZ, Warshaw EM. Bloodroot. Dermatitis. 2012;23:281-283. doi:10.1097/DER.0b013e318273a4dd
- Tan JM, Peters P, Ong N, et al. Histopathological features after topical black salve application. Australas J Dermatol. 2015;56:75-76.
- Hou JL, Brewer JD. Black salve and bloodroot extract in dermatologic conditions. Cutis. 2015;95:309-311.
- Eversole LR, Eversole GM, Kopcik J. Sanguinaria-associated oral leukoplakia: comparison with other benign and dysplastic leukoplakic lesions. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000;89:455-464. doi:10.1016/s1079-2104(00)70125-9
- Mascarenhas AK, Allen CM, Moeschberger ML. The association between Viadent® use and oral leukoplakia—results of a matched case-control study. J Public Health Dent. 2002;62:158-162. doi:10.1111/j.1752-7325.2002.tb03437.x
Bloodroot (Sanguinaria canadensis) is a member of the family Papaveraceae.1 This North American plant commonly is found in widespread distribution from Nova Scotia, Canada, to Florida and from the Great Lakes to Mississippi.2 Historically, Native Americans used bloodroot as a skin dye and as a medicine for many ailments.3
Bloodroot blooms for only a few days, starting in March, and fruits in June. The flowers comprise 8 to 10 white petals, surrounding a bed of yellow stamens (Figure). The plant thrives in wooded areas and grows to 12 inches tall. In its off-season, the plant remains dormant and can survive below-freezing temperatures.4

Chemical Constituents
Bloodroot gets its colloquial name from its red sap, which is released when the plant’s rhizome is cut. This sap contains a high concentration of alkaloids that are used for protection against predators. The rhizome itself has a rusty, red-brown color; the roots are a brighter red-orange.4
The rhizome of S canadensis contains the highest concentration of active alkaloids; the roots also contain these chemicals, though to a lesser degree; and the leaves, flowers, and fruits harvest approximately 1% of the alkaloids found in the roots.4 The concentration of alkaloids can vary from one plant to the next, depending on environmental conditions.5,6
The major alkaloids in S canadensis include both quaternary benzophenanthridine alkaloids (eg, sanguinarine, chelerythrine, sanguilutine, chelilutine, sanguirubine, chelirubine) and protopin alkaloids (eg, protopine, allocryptopine).3,7 Of these, sanguinarine and chelerythrine typically are the most potent.1 Oral ingestion or topical application of these molecules can have therapeutic and toxic effects.8
Biophysiological Effects
Bloodroot has been shown to have remarkable antimicrobial effects.9 The plant produces hydrogen peroxide and superoxide anion.10 These mediators cause oxidative stress, thus inducing destruction of cellular DNA and the cell membrane.11 Although these effects can be helpful when fighting infection, they are not necessarily selective against healthy cells.12
Alkaloids of bloodroot also have cardiovascular therapeutic effects. Sanguinarine blocks angiotensin II and causes vasodilation, thus helping treat hypertension.13 It also acts as an inotrope by blocking the Na+/K+ ATPase pump. These effects in a patient who is already taking digoxin can cause notable cardiotoxicity because the 2 drugs share a mechanism of action.14
Chelerythrine blocks production of cyclooxygenase 2 and prostaglandin E2.15 This pathway modification results in anti-inflammatory effects that can help treat arthritis, edema, and other inflammatory conditions.16 Moreover, sanguinarine has demonstrated efficacy in numerous anticancer pathways,17 including downregulation of intercellular adhesion molecules, vascular cell adhesion molecules, and vascular endothelial growth factor (VEGF).18-20 Blocking VEGF is one way to inhibit angiogenesis,21 which is upregulated in tumor formation, thus sanguinarine can have an antiproliferative anticancer effect.22 Sanguinarine also upregulates molecules such as nuclear factor–κB and the protease enzymes known as caspases to cause proapoptotic effects, furthering its antitumor potential.23,24
Treatment of Dermatologic Conditions
The initial technique of Mohs micrographic surgery employed a chemopaste that utilized an extract of S canadensis to preserve tissue.25 Outside the dermatologist’s office, bloodroot is used as a topical home remedy for a variety of cutaneous conditions, including cancer, skin tags, and warts.26 Bloodroot is advertised as black salve, an alternative anticancer treatment.27,28
As useful as this natural agent sounds, it has a pitfall: The alkaloids of S canadensis are nonspecific in their cytotoxicity, damaging neoplastic and healthy tissue.29 This cytotoxic effect can cause escharification through diffuse tissue destruction and has been observed to result in formation of a keloid scar.30 The alkaloids in black salve also have been shown to cause skin erosions and cellular atypia.28,31 Therefore, the utility of this escharotic in medical treatment is limited.32 Fortuitously, oral antibiotics and wound care can help address this adverse effect.28
Bloodroot was once used as a mouth rinse and toothpaste to treat gingivitis, but this application was later associated with oral leukoplakia, a premalignant condition.33 Leukoplakia associated with S canadensis extract often is unremitting. Immediate discontinuation of the offending agent produces little regression, suggesting that cellular damage is irreversible.34
Final Thoughts
Although bloodroot demonstrates efficacy as a phytotherapeutic, it does come with notable toxicity. Physicians should warn patients of the unwanted cosmetic effects of black salve, especially oral products that incorporate sanguinarine. Adverse effects on the oropharynx can be irreversible, though the eschar associated with black salve can be treated with a topical or oral corticosteroid.29
Bloodroot (Sanguinaria canadensis) is a member of the family Papaveraceae.1 This North American plant commonly is found in widespread distribution from Nova Scotia, Canada, to Florida and from the Great Lakes to Mississippi.2 Historically, Native Americans used bloodroot as a skin dye and as a medicine for many ailments.3
Bloodroot blooms for only a few days, starting in March, and fruits in June. The flowers comprise 8 to 10 white petals, surrounding a bed of yellow stamens (Figure). The plant thrives in wooded areas and grows to 12 inches tall. In its off-season, the plant remains dormant and can survive below-freezing temperatures.4

Chemical Constituents
Bloodroot gets its colloquial name from its red sap, which is released when the plant’s rhizome is cut. This sap contains a high concentration of alkaloids that are used for protection against predators. The rhizome itself has a rusty, red-brown color; the roots are a brighter red-orange.4
The rhizome of S canadensis contains the highest concentration of active alkaloids; the roots also contain these chemicals, though to a lesser degree; and the leaves, flowers, and fruits harvest approximately 1% of the alkaloids found in the roots.4 The concentration of alkaloids can vary from one plant to the next, depending on environmental conditions.5,6
The major alkaloids in S canadensis include both quaternary benzophenanthridine alkaloids (eg, sanguinarine, chelerythrine, sanguilutine, chelilutine, sanguirubine, chelirubine) and protopin alkaloids (eg, protopine, allocryptopine).3,7 Of these, sanguinarine and chelerythrine typically are the most potent.1 Oral ingestion or topical application of these molecules can have therapeutic and toxic effects.8
Biophysiological Effects
Bloodroot has been shown to have remarkable antimicrobial effects.9 The plant produces hydrogen peroxide and superoxide anion.10 These mediators cause oxidative stress, thus inducing destruction of cellular DNA and the cell membrane.11 Although these effects can be helpful when fighting infection, they are not necessarily selective against healthy cells.12
Alkaloids of bloodroot also have cardiovascular therapeutic effects. Sanguinarine blocks angiotensin II and causes vasodilation, thus helping treat hypertension.13 It also acts as an inotrope by blocking the Na+/K+ ATPase pump. These effects in a patient who is already taking digoxin can cause notable cardiotoxicity because the 2 drugs share a mechanism of action.14
Chelerythrine blocks production of cyclooxygenase 2 and prostaglandin E2.15 This pathway modification results in anti-inflammatory effects that can help treat arthritis, edema, and other inflammatory conditions.16 Moreover, sanguinarine has demonstrated efficacy in numerous anticancer pathways,17 including downregulation of intercellular adhesion molecules, vascular cell adhesion molecules, and vascular endothelial growth factor (VEGF).18-20 Blocking VEGF is one way to inhibit angiogenesis,21 which is upregulated in tumor formation, thus sanguinarine can have an antiproliferative anticancer effect.22 Sanguinarine also upregulates molecules such as nuclear factor–κB and the protease enzymes known as caspases to cause proapoptotic effects, furthering its antitumor potential.23,24
Treatment of Dermatologic Conditions
The initial technique of Mohs micrographic surgery employed a chemopaste that utilized an extract of S canadensis to preserve tissue.25 Outside the dermatologist’s office, bloodroot is used as a topical home remedy for a variety of cutaneous conditions, including cancer, skin tags, and warts.26 Bloodroot is advertised as black salve, an alternative anticancer treatment.27,28
As useful as this natural agent sounds, it has a pitfall: The alkaloids of S canadensis are nonspecific in their cytotoxicity, damaging neoplastic and healthy tissue.29 This cytotoxic effect can cause escharification through diffuse tissue destruction and has been observed to result in formation of a keloid scar.30 The alkaloids in black salve also have been shown to cause skin erosions and cellular atypia.28,31 Therefore, the utility of this escharotic in medical treatment is limited.32 Fortuitously, oral antibiotics and wound care can help address this adverse effect.28
Bloodroot was once used as a mouth rinse and toothpaste to treat gingivitis, but this application was later associated with oral leukoplakia, a premalignant condition.33 Leukoplakia associated with S canadensis extract often is unremitting. Immediate discontinuation of the offending agent produces little regression, suggesting that cellular damage is irreversible.34
Final Thoughts
Although bloodroot demonstrates efficacy as a phytotherapeutic, it does come with notable toxicity. Physicians should warn patients of the unwanted cosmetic effects of black salve, especially oral products that incorporate sanguinarine. Adverse effects on the oropharynx can be irreversible, though the eschar associated with black salve can be treated with a topical or oral corticosteroid.29
- Vogel M, Lawson M, Sippl W, et al. Structure and mechanism of sanguinarine reductase, an enzyme of alkaloid detoxification. J Biol Chem. 2010;285:18397-18406. doi:10.1074/jbc.M109.088989
- Maranda EL, Wang MX, Cortizo J, et al. Flower power—the versatility of bloodroot. JAMA Dermatol. 2016;152:824. doi:10.1001/jamadermatol.2015.5522
- Setzer WN. The phytochemistry of Cherokee aromatic medicinal plants. Medicines (Basel). 2018;5:121. doi:10.3390/medicines5040121
- Croaker A, King GJ, Pyne JH, et al. Sanguinaria canadensis: traditional medicine, phytochemical composition, biological activities and current uses. Int J Mol Sci. 2016;17:1414. doi:10.3390/ijms17091414
- Graf TN, Levine KE, Andrews ME, et al. Variability in the yield of benzophenanthridine alkaloids in wildcrafted vs cultivated bloodroot (Sanguinaria canadensis L.) J Agric Food Chem. 2007; 55:1205-1211. doi:10.1021/jf062498f
- Bennett BC, Bell CR, Boulware RT. Geographic variation in alkaloid content of Sanguinaria canadensis (Papaveraceae). Rhodora. 1990;92:57-69.
- Leaver CA, Yuan H, Wallen GR. Apoptotic activities of Sanguinaria canadensis: primary human keratinocytes, C-33A, and human papillomavirus HeLa cervical cancer lines. Integr Med (Encinitas). 2018;17:32-37.
- Kutchan TM. Molecular genetics of plant alkaloid biosynthesis. In: Cordell GA, ed. The Alkaloids. Vol 50. Elsevier Science Publishing Co, Inc; 1997:257-316.
- Obiang-Obounou BW, Kang O-H, Choi J-G, et al. The mechanism of action of sanguinarine against methicillin-resistant Staphylococcus aureus. J Toxicol Sci. 2011;36:277-283. doi:10.2131/jts.36.277
- Z˙abka A, Winnicki K, Polit JT, et al. Sanguinarine-induced oxidative stress and apoptosis-like programmed cell death (AL-PCD) in root meristem cells of Allium cepa. Plant Physiol Biochem. 2017;112:193-206. doi:10.1016/j.plaphy.2017.01.004
- Kumar GS, Hazra S. Sanguinarine, a promising anticancer therapeutic: photochemical and nucleic acid binding properties. RSC Advances. 2014;4:56518-56531.
- Ping G, Wang Y, Shen L, et al. Highly efficient complexation of sanguinarine alkaloid by carboxylatopillar[6]arene: pKa shift, increased solubility and enhanced antibacterial activity. Chemical Commun (Camb). 2017;53:7381-7384. doi:10.1039/c7cc02799k
- Caballero-George C, Vanderheyden PM, Solis PN, et al. Biological screening of selected medicinal Panamanian plants by radioligand-binding techniques. Phytomedicine. 2001;8:59-70. doi:10.1078/0944-7113-00011
- Seifen E, Adams RJ, Riemer RK. Sanguinarine: a positive inotropic alkaloid which inhibits cardiac Na+, K+-ATPase. Eur J Pharmacol. 1979;60:373-377. doi:10.1016/0014-2999(79)90245-0
- Debprasad C, Hemanta M, Paromita B, et al. Inhibition of NO2, PGE2, TNF-α, and iNOS EXpression by Shorea robusta L.: an ethnomedicine used for anti-inflammatory and analgesic activity. Evid Based Complement Alternat Med. 2012; 2012:254849. doi:10.1155/2012/254849
- Melov S, Ravenscroft J, Malik S, et al. Extension of life-span with superoxide dismutase/catalase mimetics. Science. 2000;289:1567-1569. doi:10.1126/science.289.5484.1567
- Basu P, Kumar GS. Sanguinarine and its role in chronic diseases. In: Gupta SC, Prasad S, Aggarwal BB, eds. Advances in Experimental Medicine and Biology: Anti-inflammatory Nutraceuticals and Chronic Diseases. Vol 928. Springer International Publishing; 2016:155-172.
- Alasvand M, Assadollahi V, Ambra R, et al. Antiangiogenic effect of alkaloids. Oxid Med Cell Longev. 2019;2019:9475908. doi:10.1155/2019/9475908
- Basini G, Santini SE, Bussolati S, et al. The plant alkaloid sanguinarine is a potential inhibitor of follicular angiogenesis. J Reprod Dev. 2007;53:573-579. doi:10.1262/jrd.18126
- Xu J-Y, Meng Q-H, Chong Y, et al. Sanguinarine is a novel VEGF inhibitor involved in the suppression of angiogenesis and cell migration. Mol Clin Oncol. 2013;1:331-336. doi:10.3892/mco.2012.41
- Lu K, Bhat M, Basu S. Plants and their active compounds: natural molecules to target angiogenesis. Angiogenesis. 2016;19:287-295. doi:10.1007/s10456-016-9512-y
- Achkar IW, Mraiche F, Mohammad RM, et al. Anticancer potential of sanguinarine for various human malignancies. Future Med Chem. 2017;9:933-950. doi:10.4155/fmc-2017-0041
- Lee TK, Park C, Jeong S-J, et al. Sanguinarine induces apoptosis of human oral squamous cell carcinoma KB cells via inactivation of the PI3K/Akt signaling pathway. Drug Dev Res. 2016;77:227-240. doi:10.1002/ddr.21315
- Gaziano R, Moroni G, Buè C, et al. Antitumor effects of the benzophenanthridine alkaloid sanguinarine: evidence and perspectives. World J Gastrointest Oncol. 2016;8:30-39. doi:10.4251/wjgo.v8.i1.30
- Mohs FE. Chemosurgery for skin cancer: fixed tissue and fresh tissue techniques. Arch Dermatol. 1976;112:211-215.
- Affleck AG, Varma S. A case of do-it-yourself Mohs’ surgery using bloodroot obtained from the internet. Br J Dermatol. 2007;157:1078-1079. doi:10.1111/j.1365-2133.2007.08180.x
- Eastman KL, McFarland LV, Raugi GJ. Buyer beware: a black salve caution. J Am Acad Dermatol. 2011;65:E154-E155. doi:10.1016/j.jaad.2011.07.031
- Osswald SS, Elston DM, Farley MF, et al. Self-treatment of a basal cell carcinoma with “black and yellow salve.” J Am Acad Dermatol. 2005;53:508-510. doi:10.1016/j.jaad.2005.04.007
- Schlichte MJ, Downing CP, Ramirez-Fort M, et al. Bloodroot associated eschar. Dermatol Online J. 2015;20:13030/qt05r0r2wr.
- Wang MZ, Warshaw EM. Bloodroot. Dermatitis. 2012;23:281-283. doi:10.1097/DER.0b013e318273a4dd
- Tan JM, Peters P, Ong N, et al. Histopathological features after topical black salve application. Australas J Dermatol. 2015;56:75-76.
- Hou JL, Brewer JD. Black salve and bloodroot extract in dermatologic conditions. Cutis. 2015;95:309-311.
- Eversole LR, Eversole GM, Kopcik J. Sanguinaria-associated oral leukoplakia: comparison with other benign and dysplastic leukoplakic lesions. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000;89:455-464. doi:10.1016/s1079-2104(00)70125-9
- Mascarenhas AK, Allen CM, Moeschberger ML. The association between Viadent® use and oral leukoplakia—results of a matched case-control study. J Public Health Dent. 2002;62:158-162. doi:10.1111/j.1752-7325.2002.tb03437.x
- Vogel M, Lawson M, Sippl W, et al. Structure and mechanism of sanguinarine reductase, an enzyme of alkaloid detoxification. J Biol Chem. 2010;285:18397-18406. doi:10.1074/jbc.M109.088989
- Maranda EL, Wang MX, Cortizo J, et al. Flower power—the versatility of bloodroot. JAMA Dermatol. 2016;152:824. doi:10.1001/jamadermatol.2015.5522
- Setzer WN. The phytochemistry of Cherokee aromatic medicinal plants. Medicines (Basel). 2018;5:121. doi:10.3390/medicines5040121
- Croaker A, King GJ, Pyne JH, et al. Sanguinaria canadensis: traditional medicine, phytochemical composition, biological activities and current uses. Int J Mol Sci. 2016;17:1414. doi:10.3390/ijms17091414
- Graf TN, Levine KE, Andrews ME, et al. Variability in the yield of benzophenanthridine alkaloids in wildcrafted vs cultivated bloodroot (Sanguinaria canadensis L.) J Agric Food Chem. 2007; 55:1205-1211. doi:10.1021/jf062498f
- Bennett BC, Bell CR, Boulware RT. Geographic variation in alkaloid content of Sanguinaria canadensis (Papaveraceae). Rhodora. 1990;92:57-69.
- Leaver CA, Yuan H, Wallen GR. Apoptotic activities of Sanguinaria canadensis: primary human keratinocytes, C-33A, and human papillomavirus HeLa cervical cancer lines. Integr Med (Encinitas). 2018;17:32-37.
- Kutchan TM. Molecular genetics of plant alkaloid biosynthesis. In: Cordell GA, ed. The Alkaloids. Vol 50. Elsevier Science Publishing Co, Inc; 1997:257-316.
- Obiang-Obounou BW, Kang O-H, Choi J-G, et al. The mechanism of action of sanguinarine against methicillin-resistant Staphylococcus aureus. J Toxicol Sci. 2011;36:277-283. doi:10.2131/jts.36.277
- Z˙abka A, Winnicki K, Polit JT, et al. Sanguinarine-induced oxidative stress and apoptosis-like programmed cell death (AL-PCD) in root meristem cells of Allium cepa. Plant Physiol Biochem. 2017;112:193-206. doi:10.1016/j.plaphy.2017.01.004
- Kumar GS, Hazra S. Sanguinarine, a promising anticancer therapeutic: photochemical and nucleic acid binding properties. RSC Advances. 2014;4:56518-56531.
- Ping G, Wang Y, Shen L, et al. Highly efficient complexation of sanguinarine alkaloid by carboxylatopillar[6]arene: pKa shift, increased solubility and enhanced antibacterial activity. Chemical Commun (Camb). 2017;53:7381-7384. doi:10.1039/c7cc02799k
- Caballero-George C, Vanderheyden PM, Solis PN, et al. Biological screening of selected medicinal Panamanian plants by radioligand-binding techniques. Phytomedicine. 2001;8:59-70. doi:10.1078/0944-7113-00011
- Seifen E, Adams RJ, Riemer RK. Sanguinarine: a positive inotropic alkaloid which inhibits cardiac Na+, K+-ATPase. Eur J Pharmacol. 1979;60:373-377. doi:10.1016/0014-2999(79)90245-0
- Debprasad C, Hemanta M, Paromita B, et al. Inhibition of NO2, PGE2, TNF-α, and iNOS EXpression by Shorea robusta L.: an ethnomedicine used for anti-inflammatory and analgesic activity. Evid Based Complement Alternat Med. 2012; 2012:254849. doi:10.1155/2012/254849
- Melov S, Ravenscroft J, Malik S, et al. Extension of life-span with superoxide dismutase/catalase mimetics. Science. 2000;289:1567-1569. doi:10.1126/science.289.5484.1567
- Basu P, Kumar GS. Sanguinarine and its role in chronic diseases. In: Gupta SC, Prasad S, Aggarwal BB, eds. Advances in Experimental Medicine and Biology: Anti-inflammatory Nutraceuticals and Chronic Diseases. Vol 928. Springer International Publishing; 2016:155-172.
- Alasvand M, Assadollahi V, Ambra R, et al. Antiangiogenic effect of alkaloids. Oxid Med Cell Longev. 2019;2019:9475908. doi:10.1155/2019/9475908
- Basini G, Santini SE, Bussolati S, et al. The plant alkaloid sanguinarine is a potential inhibitor of follicular angiogenesis. J Reprod Dev. 2007;53:573-579. doi:10.1262/jrd.18126
- Xu J-Y, Meng Q-H, Chong Y, et al. Sanguinarine is a novel VEGF inhibitor involved in the suppression of angiogenesis and cell migration. Mol Clin Oncol. 2013;1:331-336. doi:10.3892/mco.2012.41
- Lu K, Bhat M, Basu S. Plants and their active compounds: natural molecules to target angiogenesis. Angiogenesis. 2016;19:287-295. doi:10.1007/s10456-016-9512-y
- Achkar IW, Mraiche F, Mohammad RM, et al. Anticancer potential of sanguinarine for various human malignancies. Future Med Chem. 2017;9:933-950. doi:10.4155/fmc-2017-0041
- Lee TK, Park C, Jeong S-J, et al. Sanguinarine induces apoptosis of human oral squamous cell carcinoma KB cells via inactivation of the PI3K/Akt signaling pathway. Drug Dev Res. 2016;77:227-240. doi:10.1002/ddr.21315
- Gaziano R, Moroni G, Buè C, et al. Antitumor effects of the benzophenanthridine alkaloid sanguinarine: evidence and perspectives. World J Gastrointest Oncol. 2016;8:30-39. doi:10.4251/wjgo.v8.i1.30
- Mohs FE. Chemosurgery for skin cancer: fixed tissue and fresh tissue techniques. Arch Dermatol. 1976;112:211-215.
- Affleck AG, Varma S. A case of do-it-yourself Mohs’ surgery using bloodroot obtained from the internet. Br J Dermatol. 2007;157:1078-1079. doi:10.1111/j.1365-2133.2007.08180.x
- Eastman KL, McFarland LV, Raugi GJ. Buyer beware: a black salve caution. J Am Acad Dermatol. 2011;65:E154-E155. doi:10.1016/j.jaad.2011.07.031
- Osswald SS, Elston DM, Farley MF, et al. Self-treatment of a basal cell carcinoma with “black and yellow salve.” J Am Acad Dermatol. 2005;53:508-510. doi:10.1016/j.jaad.2005.04.007
- Schlichte MJ, Downing CP, Ramirez-Fort M, et al. Bloodroot associated eschar. Dermatol Online J. 2015;20:13030/qt05r0r2wr.
- Wang MZ, Warshaw EM. Bloodroot. Dermatitis. 2012;23:281-283. doi:10.1097/DER.0b013e318273a4dd
- Tan JM, Peters P, Ong N, et al. Histopathological features after topical black salve application. Australas J Dermatol. 2015;56:75-76.
- Hou JL, Brewer JD. Black salve and bloodroot extract in dermatologic conditions. Cutis. 2015;95:309-311.
- Eversole LR, Eversole GM, Kopcik J. Sanguinaria-associated oral leukoplakia: comparison with other benign and dysplastic leukoplakic lesions. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000;89:455-464. doi:10.1016/s1079-2104(00)70125-9
- Mascarenhas AK, Allen CM, Moeschberger ML. The association between Viadent® use and oral leukoplakia—results of a matched case-control study. J Public Health Dent. 2002;62:158-162. doi:10.1111/j.1752-7325.2002.tb03437.x
Practice Points
- Bloodroot (Sanguinaria canadensis) is a plant historically used in Mohs micrographic surgery as chemopaste.
- Bloodroot has been shown to have remarkable antimicrobial effects.
- The alkaloids of S canadensis are nonspecific in their cytotoxicity, damaging both neoplastic and healthy tissue. They have been shown to cause skin erosions and cellular atypia.
Aquatic Antagonists: Sea Cucumbers (Holothuroidea)
Sea cucumbers—commonly known as trepang in Indonesia, namako in Japan, and hai shen in China, where they are treasured as a food delicacy—are sea creatures belonging to the phylum Echinodermata, class Holothuridea, and family Cucumariidae . 1,2 They are an integral part of a variety of marine habitats, serving as cleaners as they filter through sediment for nutrients. They can be found on the ocean floor under hundreds of feet of water or in shallow sandy waters along the coast, but they most commonly are found living among coral reefs. Sea cucumbers look just as they sound—shaped like cucumbers or sausages, ranging from under 1 inch to upwards of 6 feet in length depending on the specific species (Figure 1). They have a group of tentacles around the mouth used for filtering sediment, and they move about the ocean floor on tubular feet protruding through the body wall, similar to a sea star.

Beneficial Properties and Cultural Relevance
Although more than 1200 species of sea cucumbers have been identified thus far, only about 20 of these are edible.2 The most common of the edible species is Stichopus japonicus, which can be found off the coasts of Korea, China, Japan, and Russia. This particular species most commonly is used in traditional dishes and is divided into 3 groups based on the color: red, green, or black. The price and taste of sea cucumbers varies based on the color, with red being the most expensive.2 The body wall of the sea cucumber is cleaned, repeatedly boiled, and dried until edible. It is considered a delicacy, not only in food but also in pharmaceutical forms, as it is comprised of a variety of vitamins, minerals, and other nutrients that are thought to provide anticancer, anticoagulant, antioxidant, antifungal, and anti-inflammatory properties. Components of the body wall include collagen, mucopolysaccharides, peptides, gelatin, glycosaminoglycans, glycosides (including various holotoxins), hydroxylates, saponins, and fatty acids.2 The regenerative properties of the sea cucumber also are important in future biomedical developments.
Toxic Properties
Although sea cucumbers have proven to have many beneficial properties, at least 30 species also produce potent toxins that pose a danger to both humans and other wildlife.3 The toxins are collectively referred to as holothurin; however, specific species actually produce a variety of holothurin toxins with unique chemical structures. Each toxin is a variation of a specific triterpene glycoside called saponins, which are common glycosides in the plant world. Holothurin was the first saponin to be found in animals. The only animals known to contain holothurin are the echinoderms, including sea cucumbers and sea stars.1 Holothurins A and B are the 2 groups of holothurin toxins produced specifically by sea cucumbers. The toxins are composed of roughly 60% glycosides and pigment; 30% free amino acids (alanine, arginine, cysteine, glycine, glutamic acid, histidine, serine, and valine); 5% to 10% insoluble proteins; and 1% cholesterol, salts, and polypeptides.3
Holothurins are concentrated in granules within specialized structures of the sea cucumber called Cuvierian tubules, which freely float in the posterior coelomic cavity of the sea cucumber and are attached at the base of the respiratory tree. It is with these tubules that sea cucumbers utilize a unique defensive mechanism. Upon disturbance, the sea cucumber will turn its posterior end to the threat and squeeze its body in a series of violent contractions, inducing a tear in the cloacal wall.4 The tubules pass through this tear, are autotomized from the attachment point at the respiratory tree, and are finally expelled through the anus onto the predator and into the surrounding waters. The tubules are both sticky on contact and poisonous due to the holothurin, allowing the sea cucumber to crawl away from the threat unscathed. Over time, the tubules will regenerate, allowing the sea cucumber to protect itself again in the face of future danger.
Aside from direct disturbance by a threat, sea cucumbers also are known to undergo evisceration due to high temperatures and oxygen deficiency.3 Species that lack Cuvierian tubules can still produce holothurin toxins, though the toxins are secreted onto the outer surface of the body wall and mainly pose a risk with direct contact undiluted by seawater.5 The toxin induces a neural blockade in other sea creatures through its interaction with ion channels. On Asian islands, sea cucumbers have been exploited for this ability and commonly are thrown into tidal pools by fishermen to paralyze fish for easier capture.1
Effects on Human Skin
In humans, the holothurin toxins of sea cucumbers cause an acute irritant dermatitis upon contact with the skin.6 Fishermen or divers handling sea cucumbers without gloves may present with an irritant contact dermatitis characterized by marked erythema and swelling (Figure 2).6-8 Additionally, holothurin toxins can cause irritation of the mucous membranes of the eyes and mouth. Contact with the mucous membranes of the eyes can induce a painful conjunctivitis that may result in blindness.6,8 Ingestion of large quantities of sea cucumber can produce an anticoagulant effect, and toxins in some species act similar to cardiac glycosides.3,9
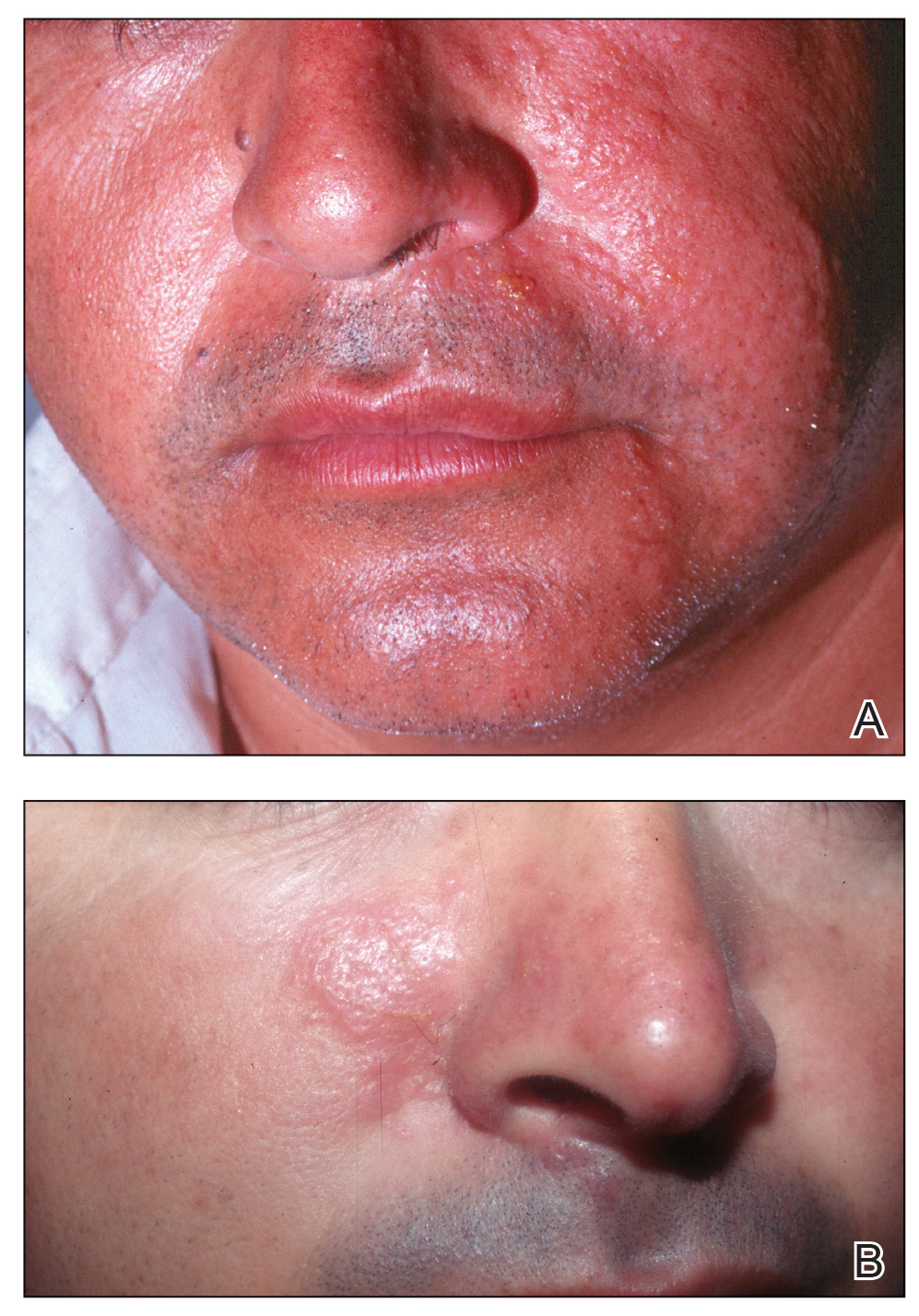
In addition to their own toxins, sea cucumbers also can secrete undigested nematocysts of previously consumed cnidarians through the integument.7,10 In this case, the result of direct contact with the body wall is similar to a jellyfish sting in addition to the irritant contact dermatitis caused by the holothurin toxin.
Treatment and Prevention
Irritant dermatitis resulting from contact with a holothurin toxin is first treated with cleansing of the affected area at the time of exposure with generous amounts of seawater or preferably hot seawater and soap. Most marine toxins are inactivated by heat, but holothurin is partially heat stable. Vinegar or isopropyl alcohol also have been used.9 The result is removal of the slime containing the holothurin toxin rather than deactivation of the toxin. Although this alone may relieve symptoms, dermatitis also may be addressed with topical anesthetics, corticosteroids, or, if a severe reaction has occurred, systemic steroids.9
Conjunctivitis should be addressed with copious irrigation with tap water and topical anesthesia. Following proper irrigation, providers may choose to follow up with fluorescein staining to rule out corneal injury.10
The dermatologic effects of holothurin toxins can be prevented with the use of gloves and diving masks or goggles. Proper protective wear should be utilized not only when directly handling sea cucumbers but also when swimming in water where sea cucumbers may be present. Systemic toxicity can be prevented by proper cooking, as holothurin toxins are only partially heat resistant and also are hydrolyzed into nontoxic products by gastric acid. Additionally, the species of the sea cucumber should be confirmed prior to consumption, as edible species are known to contain less toxin.1
Conclusion
Although sea cucumbers have ecologic, culinary, and pharmaceutical value, they also can pose a threat to both humans and wildlife. The holothurin toxins produced by sea cucumbers cause a painful contact dermatitis and can lead to conjunctivitis and even blindness following eye exposure. Although the toxin is broken down into nontoxic metabolites by gastric acid, large amounts of potent variants can induce systemic effects. Individuals who come in contact with sea cucumbers, such as fishermen and divers, should utilize proper protection including gloves and protective eyewear.
- Burnett K, Fenner P, Williamson J. Venomous and Poisonous Marine Animals: A Medical and Biological Handbook. University of New South Wales Press; 1996.
- Oh GW, Ko SC, Lee DH, et al. Biological activities and biomedical potential of sea cucumber (Stichopus japonicus): a review. Fisheries Aquatic Sci. 2017;20:28.
- Nigrelli RF, Jakowska S. Effects of holothurian, a steroid saponin from the Bahamian sea cucumber (Actinopyga agassizi), on various biological systems. Ann NY Acad Sci. 1960;90:884-892.
- Demeuldre M, Hennebert E, Bonneel M, et al. Mechanical adaptability of sea cucumber Cuvierian tubules involves a mutable collagenous tissue. J Exp Biol. 2017;220:2108-2119.
- Matranga V, ed. Echinodermata: Progress in Molecular and Subcellular Biology. Springer; 2005.
- Tlougan, BE, Podjasek, JO, Adams BB. Aquatic sports dermatoses. part 2—in the water: saltwater dermatoses. Int J Dermatol. 2010;49:994-1002.
- Bonamonte D, Verni P, Filoni A, et al. Dermatitis caused by echinoderms. In: Bonamonte D, Angelini G, eds. Springer; 2016:59-72.
- Haddad V Jr. Medical Emergencies Caused by Aquatic Animals: A Zoological and Clinical Guide. Springer International Publishing; 2016.
- French LK, Horowitz BZ. Marine vertebrates, cnidarians, and mollusks. In: Brent J, Burkhart K, Dargan P, et al, eds. Critical Care Toxicology. Springer; 2017:1-30.
- Smith ML. Skin problems from marine echinoderms. Dermatol Ther. 2002;15:30-33.
Sea cucumbers—commonly known as trepang in Indonesia, namako in Japan, and hai shen in China, where they are treasured as a food delicacy—are sea creatures belonging to the phylum Echinodermata, class Holothuridea, and family Cucumariidae . 1,2 They are an integral part of a variety of marine habitats, serving as cleaners as they filter through sediment for nutrients. They can be found on the ocean floor under hundreds of feet of water or in shallow sandy waters along the coast, but they most commonly are found living among coral reefs. Sea cucumbers look just as they sound—shaped like cucumbers or sausages, ranging from under 1 inch to upwards of 6 feet in length depending on the specific species (Figure 1). They have a group of tentacles around the mouth used for filtering sediment, and they move about the ocean floor on tubular feet protruding through the body wall, similar to a sea star.

Beneficial Properties and Cultural Relevance
Although more than 1200 species of sea cucumbers have been identified thus far, only about 20 of these are edible.2 The most common of the edible species is Stichopus japonicus, which can be found off the coasts of Korea, China, Japan, and Russia. This particular species most commonly is used in traditional dishes and is divided into 3 groups based on the color: red, green, or black. The price and taste of sea cucumbers varies based on the color, with red being the most expensive.2 The body wall of the sea cucumber is cleaned, repeatedly boiled, and dried until edible. It is considered a delicacy, not only in food but also in pharmaceutical forms, as it is comprised of a variety of vitamins, minerals, and other nutrients that are thought to provide anticancer, anticoagulant, antioxidant, antifungal, and anti-inflammatory properties. Components of the body wall include collagen, mucopolysaccharides, peptides, gelatin, glycosaminoglycans, glycosides (including various holotoxins), hydroxylates, saponins, and fatty acids.2 The regenerative properties of the sea cucumber also are important in future biomedical developments.
Toxic Properties
Although sea cucumbers have proven to have many beneficial properties, at least 30 species also produce potent toxins that pose a danger to both humans and other wildlife.3 The toxins are collectively referred to as holothurin; however, specific species actually produce a variety of holothurin toxins with unique chemical structures. Each toxin is a variation of a specific triterpene glycoside called saponins, which are common glycosides in the plant world. Holothurin was the first saponin to be found in animals. The only animals known to contain holothurin are the echinoderms, including sea cucumbers and sea stars.1 Holothurins A and B are the 2 groups of holothurin toxins produced specifically by sea cucumbers. The toxins are composed of roughly 60% glycosides and pigment; 30% free amino acids (alanine, arginine, cysteine, glycine, glutamic acid, histidine, serine, and valine); 5% to 10% insoluble proteins; and 1% cholesterol, salts, and polypeptides.3
Holothurins are concentrated in granules within specialized structures of the sea cucumber called Cuvierian tubules, which freely float in the posterior coelomic cavity of the sea cucumber and are attached at the base of the respiratory tree. It is with these tubules that sea cucumbers utilize a unique defensive mechanism. Upon disturbance, the sea cucumber will turn its posterior end to the threat and squeeze its body in a series of violent contractions, inducing a tear in the cloacal wall.4 The tubules pass through this tear, are autotomized from the attachment point at the respiratory tree, and are finally expelled through the anus onto the predator and into the surrounding waters. The tubules are both sticky on contact and poisonous due to the holothurin, allowing the sea cucumber to crawl away from the threat unscathed. Over time, the tubules will regenerate, allowing the sea cucumber to protect itself again in the face of future danger.
Aside from direct disturbance by a threat, sea cucumbers also are known to undergo evisceration due to high temperatures and oxygen deficiency.3 Species that lack Cuvierian tubules can still produce holothurin toxins, though the toxins are secreted onto the outer surface of the body wall and mainly pose a risk with direct contact undiluted by seawater.5 The toxin induces a neural blockade in other sea creatures through its interaction with ion channels. On Asian islands, sea cucumbers have been exploited for this ability and commonly are thrown into tidal pools by fishermen to paralyze fish for easier capture.1
Effects on Human Skin
In humans, the holothurin toxins of sea cucumbers cause an acute irritant dermatitis upon contact with the skin.6 Fishermen or divers handling sea cucumbers without gloves may present with an irritant contact dermatitis characterized by marked erythema and swelling (Figure 2).6-8 Additionally, holothurin toxins can cause irritation of the mucous membranes of the eyes and mouth. Contact with the mucous membranes of the eyes can induce a painful conjunctivitis that may result in blindness.6,8 Ingestion of large quantities of sea cucumber can produce an anticoagulant effect, and toxins in some species act similar to cardiac glycosides.3,9

In addition to their own toxins, sea cucumbers also can secrete undigested nematocysts of previously consumed cnidarians through the integument.7,10 In this case, the result of direct contact with the body wall is similar to a jellyfish sting in addition to the irritant contact dermatitis caused by the holothurin toxin.
Treatment and Prevention
Irritant dermatitis resulting from contact with a holothurin toxin is first treated with cleansing of the affected area at the time of exposure with generous amounts of seawater or preferably hot seawater and soap. Most marine toxins are inactivated by heat, but holothurin is partially heat stable. Vinegar or isopropyl alcohol also have been used.9 The result is removal of the slime containing the holothurin toxin rather than deactivation of the toxin. Although this alone may relieve symptoms, dermatitis also may be addressed with topical anesthetics, corticosteroids, or, if a severe reaction has occurred, systemic steroids.9
Conjunctivitis should be addressed with copious irrigation with tap water and topical anesthesia. Following proper irrigation, providers may choose to follow up with fluorescein staining to rule out corneal injury.10
The dermatologic effects of holothurin toxins can be prevented with the use of gloves and diving masks or goggles. Proper protective wear should be utilized not only when directly handling sea cucumbers but also when swimming in water where sea cucumbers may be present. Systemic toxicity can be prevented by proper cooking, as holothurin toxins are only partially heat resistant and also are hydrolyzed into nontoxic products by gastric acid. Additionally, the species of the sea cucumber should be confirmed prior to consumption, as edible species are known to contain less toxin.1
Conclusion
Although sea cucumbers have ecologic, culinary, and pharmaceutical value, they also can pose a threat to both humans and wildlife. The holothurin toxins produced by sea cucumbers cause a painful contact dermatitis and can lead to conjunctivitis and even blindness following eye exposure. Although the toxin is broken down into nontoxic metabolites by gastric acid, large amounts of potent variants can induce systemic effects. Individuals who come in contact with sea cucumbers, such as fishermen and divers, should utilize proper protection including gloves and protective eyewear.
Sea cucumbers—commonly known as trepang in Indonesia, namako in Japan, and hai shen in China, where they are treasured as a food delicacy—are sea creatures belonging to the phylum Echinodermata, class Holothuridea, and family Cucumariidae . 1,2 They are an integral part of a variety of marine habitats, serving as cleaners as they filter through sediment for nutrients. They can be found on the ocean floor under hundreds of feet of water or in shallow sandy waters along the coast, but they most commonly are found living among coral reefs. Sea cucumbers look just as they sound—shaped like cucumbers or sausages, ranging from under 1 inch to upwards of 6 feet in length depending on the specific species (Figure 1). They have a group of tentacles around the mouth used for filtering sediment, and they move about the ocean floor on tubular feet protruding through the body wall, similar to a sea star.

Beneficial Properties and Cultural Relevance
Although more than 1200 species of sea cucumbers have been identified thus far, only about 20 of these are edible.2 The most common of the edible species is Stichopus japonicus, which can be found off the coasts of Korea, China, Japan, and Russia. This particular species most commonly is used in traditional dishes and is divided into 3 groups based on the color: red, green, or black. The price and taste of sea cucumbers varies based on the color, with red being the most expensive.2 The body wall of the sea cucumber is cleaned, repeatedly boiled, and dried until edible. It is considered a delicacy, not only in food but also in pharmaceutical forms, as it is comprised of a variety of vitamins, minerals, and other nutrients that are thought to provide anticancer, anticoagulant, antioxidant, antifungal, and anti-inflammatory properties. Components of the body wall include collagen, mucopolysaccharides, peptides, gelatin, glycosaminoglycans, glycosides (including various holotoxins), hydroxylates, saponins, and fatty acids.2 The regenerative properties of the sea cucumber also are important in future biomedical developments.
Toxic Properties
Although sea cucumbers have proven to have many beneficial properties, at least 30 species also produce potent toxins that pose a danger to both humans and other wildlife.3 The toxins are collectively referred to as holothurin; however, specific species actually produce a variety of holothurin toxins with unique chemical structures. Each toxin is a variation of a specific triterpene glycoside called saponins, which are common glycosides in the plant world. Holothurin was the first saponin to be found in animals. The only animals known to contain holothurin are the echinoderms, including sea cucumbers and sea stars.1 Holothurins A and B are the 2 groups of holothurin toxins produced specifically by sea cucumbers. The toxins are composed of roughly 60% glycosides and pigment; 30% free amino acids (alanine, arginine, cysteine, glycine, glutamic acid, histidine, serine, and valine); 5% to 10% insoluble proteins; and 1% cholesterol, salts, and polypeptides.3
Holothurins are concentrated in granules within specialized structures of the sea cucumber called Cuvierian tubules, which freely float in the posterior coelomic cavity of the sea cucumber and are attached at the base of the respiratory tree. It is with these tubules that sea cucumbers utilize a unique defensive mechanism. Upon disturbance, the sea cucumber will turn its posterior end to the threat and squeeze its body in a series of violent contractions, inducing a tear in the cloacal wall.4 The tubules pass through this tear, are autotomized from the attachment point at the respiratory tree, and are finally expelled through the anus onto the predator and into the surrounding waters. The tubules are both sticky on contact and poisonous due to the holothurin, allowing the sea cucumber to crawl away from the threat unscathed. Over time, the tubules will regenerate, allowing the sea cucumber to protect itself again in the face of future danger.
Aside from direct disturbance by a threat, sea cucumbers also are known to undergo evisceration due to high temperatures and oxygen deficiency.3 Species that lack Cuvierian tubules can still produce holothurin toxins, though the toxins are secreted onto the outer surface of the body wall and mainly pose a risk with direct contact undiluted by seawater.5 The toxin induces a neural blockade in other sea creatures through its interaction with ion channels. On Asian islands, sea cucumbers have been exploited for this ability and commonly are thrown into tidal pools by fishermen to paralyze fish for easier capture.1
Effects on Human Skin
In humans, the holothurin toxins of sea cucumbers cause an acute irritant dermatitis upon contact with the skin.6 Fishermen or divers handling sea cucumbers without gloves may present with an irritant contact dermatitis characterized by marked erythema and swelling (Figure 2).6-8 Additionally, holothurin toxins can cause irritation of the mucous membranes of the eyes and mouth. Contact with the mucous membranes of the eyes can induce a painful conjunctivitis that may result in blindness.6,8 Ingestion of large quantities of sea cucumber can produce an anticoagulant effect, and toxins in some species act similar to cardiac glycosides.3,9

In addition to their own toxins, sea cucumbers also can secrete undigested nematocysts of previously consumed cnidarians through the integument.7,10 In this case, the result of direct contact with the body wall is similar to a jellyfish sting in addition to the irritant contact dermatitis caused by the holothurin toxin.
Treatment and Prevention
Irritant dermatitis resulting from contact with a holothurin toxin is first treated with cleansing of the affected area at the time of exposure with generous amounts of seawater or preferably hot seawater and soap. Most marine toxins are inactivated by heat, but holothurin is partially heat stable. Vinegar or isopropyl alcohol also have been used.9 The result is removal of the slime containing the holothurin toxin rather than deactivation of the toxin. Although this alone may relieve symptoms, dermatitis also may be addressed with topical anesthetics, corticosteroids, or, if a severe reaction has occurred, systemic steroids.9
Conjunctivitis should be addressed with copious irrigation with tap water and topical anesthesia. Following proper irrigation, providers may choose to follow up with fluorescein staining to rule out corneal injury.10
The dermatologic effects of holothurin toxins can be prevented with the use of gloves and diving masks or goggles. Proper protective wear should be utilized not only when directly handling sea cucumbers but also when swimming in water where sea cucumbers may be present. Systemic toxicity can be prevented by proper cooking, as holothurin toxins are only partially heat resistant and also are hydrolyzed into nontoxic products by gastric acid. Additionally, the species of the sea cucumber should be confirmed prior to consumption, as edible species are known to contain less toxin.1
Conclusion
Although sea cucumbers have ecologic, culinary, and pharmaceutical value, they also can pose a threat to both humans and wildlife. The holothurin toxins produced by sea cucumbers cause a painful contact dermatitis and can lead to conjunctivitis and even blindness following eye exposure. Although the toxin is broken down into nontoxic metabolites by gastric acid, large amounts of potent variants can induce systemic effects. Individuals who come in contact with sea cucumbers, such as fishermen and divers, should utilize proper protection including gloves and protective eyewear.
- Burnett K, Fenner P, Williamson J. Venomous and Poisonous Marine Animals: A Medical and Biological Handbook. University of New South Wales Press; 1996.
- Oh GW, Ko SC, Lee DH, et al. Biological activities and biomedical potential of sea cucumber (Stichopus japonicus): a review. Fisheries Aquatic Sci. 2017;20:28.
- Nigrelli RF, Jakowska S. Effects of holothurian, a steroid saponin from the Bahamian sea cucumber (Actinopyga agassizi), on various biological systems. Ann NY Acad Sci. 1960;90:884-892.
- Demeuldre M, Hennebert E, Bonneel M, et al. Mechanical adaptability of sea cucumber Cuvierian tubules involves a mutable collagenous tissue. J Exp Biol. 2017;220:2108-2119.
- Matranga V, ed. Echinodermata: Progress in Molecular and Subcellular Biology. Springer; 2005.
- Tlougan, BE, Podjasek, JO, Adams BB. Aquatic sports dermatoses. part 2—in the water: saltwater dermatoses. Int J Dermatol. 2010;49:994-1002.
- Bonamonte D, Verni P, Filoni A, et al. Dermatitis caused by echinoderms. In: Bonamonte D, Angelini G, eds. Springer; 2016:59-72.
- Haddad V Jr. Medical Emergencies Caused by Aquatic Animals: A Zoological and Clinical Guide. Springer International Publishing; 2016.
- French LK, Horowitz BZ. Marine vertebrates, cnidarians, and mollusks. In: Brent J, Burkhart K, Dargan P, et al, eds. Critical Care Toxicology. Springer; 2017:1-30.
- Smith ML. Skin problems from marine echinoderms. Dermatol Ther. 2002;15:30-33.
- Burnett K, Fenner P, Williamson J. Venomous and Poisonous Marine Animals: A Medical and Biological Handbook. University of New South Wales Press; 1996.
- Oh GW, Ko SC, Lee DH, et al. Biological activities and biomedical potential of sea cucumber (Stichopus japonicus): a review. Fisheries Aquatic Sci. 2017;20:28.
- Nigrelli RF, Jakowska S. Effects of holothurian, a steroid saponin from the Bahamian sea cucumber (Actinopyga agassizi), on various biological systems. Ann NY Acad Sci. 1960;90:884-892.
- Demeuldre M, Hennebert E, Bonneel M, et al. Mechanical adaptability of sea cucumber Cuvierian tubules involves a mutable collagenous tissue. J Exp Biol. 2017;220:2108-2119.
- Matranga V, ed. Echinodermata: Progress in Molecular and Subcellular Biology. Springer; 2005.
- Tlougan, BE, Podjasek, JO, Adams BB. Aquatic sports dermatoses. part 2—in the water: saltwater dermatoses. Int J Dermatol. 2010;49:994-1002.
- Bonamonte D, Verni P, Filoni A, et al. Dermatitis caused by echinoderms. In: Bonamonte D, Angelini G, eds. Springer; 2016:59-72.
- Haddad V Jr. Medical Emergencies Caused by Aquatic Animals: A Zoological and Clinical Guide. Springer International Publishing; 2016.
- French LK, Horowitz BZ. Marine vertebrates, cnidarians, and mollusks. In: Brent J, Burkhart K, Dargan P, et al, eds. Critical Care Toxicology. Springer; 2017:1-30.
- Smith ML. Skin problems from marine echinoderms. Dermatol Ther. 2002;15:30-33.
Practice Points
- Sea cucumbers produce a toxin known as holothurin, which is contained in specialized structures called Cuvierian tubules and secreted onto the outer surface of the body wall. Some species also eject portions of their toxic inner organs through the anus as a defensive mechanism.
- In humans, the holothurin toxins cause an acute irritant dermatitis upon contact with the skin and a painful chemical conjunctivitis upon contact with the eyes.
- In addition to their own toxin, sea cucumbers also can secrete undigested nematocysts of previously consumed cnidarians through their integument, causing additional effects on human skin.
- The dermatologic effects of sea cucumbers can be prevented with the use of gloves and swim masks or goggles.
What’s Eating You? Culex Mosquitoes and West Nile Virus
What is West Nile virus? How is it contracted, and who can become infected?
West Nile virus (WNV) is a single-stranded RNA virus of the Flaviviridae family and Flavivirus genus, a lineage that also includes the yellow fever, dengue, Zika, Japanese encephalitis, and Saint Louis encephalitis viruses.1 Birds serve as the reservoir hosts of WNV, and mosquitoes acquire the virus during feeding.2 West Nile virus then is transmitted to humans primarily by bites from Culex mosquitoes, which are especially prevalent in wooded areas during peak mosquito season (summer through early fall in North America).1 Mosquitoes also can infect horses; however, humans and horses are dead-end hosts, meaning they do not pass the virus on to other biting mosquitoes.3 There also have been rare reports of transmission of WNV through blood and donation as well as mother-to-baby transmission.2
What is the epidemiology of WNV in the United States?
Since the introduction of WNV to the United States in 1999, it has become an important public health concern, with 48,183 cases and 2163 deaths reported since 1999.2,3 In 2018, Nebraska had the highest number of cases of WNV (n=251), followed by California (n=217), North Dakota (n=204), Illinois (n=176), and South Dakota (n=169).3 West Nile virus is endemic to all 48 contiguous states and Canada, though the Great Plains region is especially affected by WNV due to several factors, such as a greater percentage of rural land, forests, and irrigated areas.4 The Great Plains region also has been thought to be an ecological niche for a more virulent species (Culex tarsalis) compared to other regions in the United States.5
The annual incidence of WNV in the United States peaked in 2003 at 9862 cases (up from 62 cases in 1999), then declined gradually until 2008 to 2011, during which the incidence was stable at 700 to 1100 new cases per year. However, there was a resurgence of cases (n=5674) in 2012 that steadied at around 2200 cases annually in subsequent years.6 Although there likely are several factors affecting WNV incidence trends in the United States, interannual changes in temperature and precipitation have been described. An increased mean annual temperature (from September through October, the end of peak mosquito season) and an increased temperature in winter months (from January through March, prior to peak mosquito season) have both been associated with an increased incidence of WNV.7 An increased temperature is thought to increase population numbers of mosquitoes both by increasing reproductive rates and creating ideal breeding environments via pooled water areas.8 Depending on the region, both above average and below average precipitation levels in the United States can increase WNV incidence the following year.7,9
What are the signs and symptoms of WNV infection?
Up to 80% of those infected with WNV are asymptomatic.3 After an incubation period of roughly 2 to 14 days, the remaining 20% may develop symptoms of West Nile fever (WNF), typically a self-limited illness that consists of 3 to 10 days of nonspecific symptoms such as fever, headache, fatigue, muscle pain and/or weakness, eye pain, gastrointestinal tract upset, and a macular rash that usually presents on the trunk or extremities.1,3 Less than 1% of patients affected by WNV develop neuroinvasive disease, including meningitis, encephalitis, and/or acute flaccid paralysis.10 West Nile virus neuroinvasive disease can cause permanent neurologic sequelae such as muscle weakness, confusion, memory loss, and fatigue; it carries a mortality rate of 10% to 30%, which is mainly dependent on older age and immunosuppression status.1,10
What is the reported spectrum of cutaneous findings in WNV?
Of the roughly 20% of patients infected with WNV that develop WNF, approximately 25% to 50% will develop an associated rash.1 It most commonly is described as a morbilliform or maculopapular rash located on the chest, back, and arms, usually sparing the palms and soles, though 1 case report noted involvement with these areas (Figure).11,12 It typically appears 5 days after symptom onset, can be associated with defervescence, and lasts less than a week.1,13 Pruritus and dysesthesia are sometimes present.13 Other rare presentations that have been reported include an ill-defined pseudovesicular rash with erythematous papules on the palms and pink, scaly, psoriasiform papules on the feet and thighs, as well as neuroinvasive WNV leading to purpura fulminans.14,15 A diffuse, erythematous, petechial rash on the face, neck, trunk, and extremities was reported in a pediatric patient, but there have been no reports of a petechial rash associated with WNV in adult patients.16 These findings suggest some potential variability in the presentation of the WNV rash.
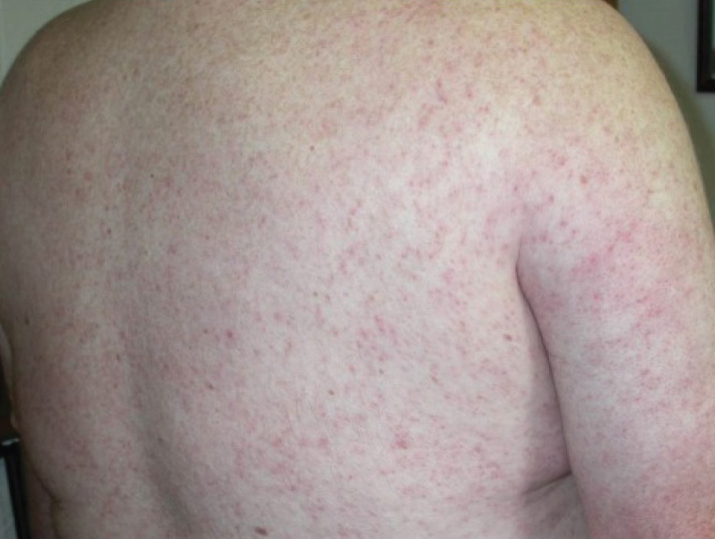
What role does the presence of rash play diagnostically and prognostically?
The rash of WNV has been implicated as a potential prognostic factor in predicting more favorable outcomes.17 Using 2002 data from the Illinois Department of Public Health and 2003 data from the Colorado Department of Public Health, Huhn and Dworkin17 found the age-adjusted risk of encephalitis and death to be decreased in WNV patients with a rash (relative risk, 0.44; 95% CI, 0.21-0.92). The reasons for this are not definitively known, but we hypothesize that the rash may prompt patients to seek earlier medical attention or indicate a more robust immune response. Additionally, a rash in WNV more commonly is seen in younger patients, whereas WNV neuroinvasive disease is more common in older patients, who also tend to have worse outcomes.10 One study found rash to be the only symptom that demonstrated a significant association with seropositivity (overall risk=6.35; P<.05; 95% CI, 3.75-10.80) by multivariate analysis.18
How is WNV diagnosed? What are the downsides to WNV testing?
Given that the presenting symptoms of WNV and WNF are nonspecific, it becomes challenging to arrive at the diagnosis based solely on physical examination. As such, the patient’s clinical and epidemiologic history, such as timing, pattern, and appearance of the rash or recent history of mosquito bites, is key to arriving at the correct diagnosis. With clinical suspicion, possible diagnostic tests include an IgM enzyme-linked immunosorbent assay (ELISA) for WNV, a plaque reduction neutralization test (PNRT), and blood polymerase chain reaction (PCR).
An ELISA is a confirmatory test to detect IgM antibodies to WNV in the serum. Because IgM seroconversion typically occurs between days 4 and 10 of symptom onset, there is a high probability of initial false-negative testing within the first 8 days after symptom onset.19,20 Clinical understanding of this fact is imperative, as an initial negative ELISA does not rule out WNV, and a retest is warranted if clinical suspicion is high. In addition to a high initial false-negative rate with ELISA, there are several other limitations to note. IgM antibodies remain elevated for 1 to 3 months or possibly up to a year in immunocompromised patients.1 Due to this, false positives may be present if there was a recent prior infection. Enzyme-linked immunosorbent assay may not distinguish from different flaviviruses, including the yellow fever, dengue, Zika, Japanese encephalitis, and Saint Louis encephalitis viruses. Seropositivity has been estimated in some states, including 1999 data from New York (2.6%), 2003 data from Nebraska (9.5%), and 2012-2014 data from Connecticut (8.5%).21-23 Regional variance may be expected, as there also were significant differences in WNV seropositivity between different regions in Nebraska (P<.001).23
Because ELISA testing for WNV has readily apparent flaws, other tests have been utilized in its diagnosis. The PNRT is the most specific test, and it works by measuring neutralizing antibody titers for different flaviviruses. It has the ability to determine cross-reactivity with other flaviviruses; however, it does not discriminate between a current infection and a prior infection or prior flavivirus vaccine (ie, yellow fever vaccine). Despite this, a positive PNRT can lend credibility to a positive ELISA test and determine specificity for WNV for those with no prior flavivirus exposure.24 According to the Centers for Disease Control and Prevention (CDC), this test can be performed by the CDC or in reference laboratories designated by the CDC.3 Additionally, some state health laboratories may perform PRNTs.
Viral detection with PCR currently is used to screen blood donations and may be beneficial for immunocompromised patients that lack the ability to form a robust antibody response or if a patient presents early, as PCR works best within the first week of symptom onset.1 Tilley et al25 showed that a combination of PCR and ELISA were able to accurately predict 94.2% of patients (180/191) with documented WNV on a first blood sample compared to 45% and 58.1% for only viral detection or ELISA, respectively. Based on costs from a Midwest academic center, antibody detection tests are around $100 while PCR may range from $500 to $1000 and is only performed in reference laboratories. Although these tests remain in the repertoire for WNV diagnosis, financial stewardship is important.
If there are symptoms of photophobia, phonophobia, nuchal rigidity, loss of consciousness, or marked personality changes, a lumbar puncture for WNV IgM in the cerebrospinal fluid can be performed. As with most viral infections, cerebrospinal fluid findings normally include an elevated protein and lymphocyte count, but neutrophils may be predominantly elevated if the infection is early in its course.26
What are the management options?
To date, there is no curative treatment for WNV, and management is largely supportive. For WNF, over-the-counter pain medications may be helpful to reduce fever and pain. If more severe disease develops, hospitalization for further supportive care may be needed.27 If meningitis or encephalitis is suspected, broad-spectrum antibiotics may need to be started until other common etiologies are ruled out.28
How can you prevent WNV infection?
Disease prevention largely consists of educating the public to avoid heavily wooded areas, especially in areas of high prevalence and during peak months, and to use protective clothing and insect repellant that has been approved by the Environmental Protection Agency.3 Insect repellants approved by the Environmental Protection Agency contain ingredients such as DEET (N, N-diethyl-meta-toluamide), picaridin, IR3535 (ethyl butylacetylaminopropionate), and oil of lemon eucalyptus, which have been proven safe and effective.29 Patients also can protect their homes by using window screens and promptly repairing screens with holes.3
What is the differential diagnosis for WNV?
The differential diagnosis for fever with generalized maculopapular rash broadly ranges from viral etiologies (eg, WNV, Zika, measles), to tick bites (eg, Rocky Mountain spotted fever, ehrlichiosis), to drug-induced rashes. A detailed patient history inquiring on recent sick contacts, travel (WNV in the Midwest, ehrlichiosis in the Southeast), environmental exposures (ticks, mosquitoes), and new medications (typically 7–10 days after starting) is imperative to narrow the differential.30 In addition, the distribution, timing, and clinical characteristics of the rash may aid in diagnosis, along with an appropriately correlated clinical picture. West Nile virus likely will present in the summer in mid central geographic locations and often develops on the trunk and extremities as a blanching, generalized, maculopapular rash around 5 days after symptom onset or with defervescence.1
- Petersen LR. Clinical manifestations and diagnosis of West Nile virus infection. UpToDate website. Updated August 7, 2020. Accessed April 16, 2021. https://www.uptodate.com/contents/clinical-manifestations-and-diagnosis-of-west-nile-virus-infection?search=clinical-manifestations-and-diagnosis-of-west-nile-virusinfection.&source=search_result&selectedTitle=1~78&usage_type=default&display_rank=1
- Sampathkumar P. West Nile virus: epidemiology, clinical presentation, diagnosis, and prevention. Mayo Clin Proc. 2003;78:1137-1144.
- Centers for Disease Control and Prevention. West Nile virus. Updated June 3, 2020. Accessed April 16, 2021. https://www.cdc.gov/westnile/index.html
- Chuang TW, Hockett CW, Kightlinger L, et al. Landscape-level spatial patterns of West Nile virus risk in the northern Great Plains. Am J Trop Med Hyg. 2012;86:724-731.
- Wimberly MC, Hildreth MB, Boyte SP, et al. Ecological niche of the 2003 West Nile virus epidemic in the northern great plains of the United States. PLoS One. 2008;3:E3744. doi:10.1371/journal.pone.0003744
- Centers for Disease Control and Prevention. West Nile virus disease cases reported to CDC by state of residence, 1999-2019. Accessed April 26, 2021. https://www.cdc.gov/westnile/resources/pdfs/data/West-Nile-virus-disease-cases-by-state_1999-2019-P.pdf
- Hahn MB, Monaghan AJ, Hayden MH, et al. Meteorological conditions associated with increased incidence of West Nile virus disease in the United States, 2004–2012. Am J Trop Med Hyg. 2015;92:1013-1022.
- Brown CM, DeMaria A Jr. The resurgence of West Nile virus. Ann Intern Med. 2012;157:823-824.
- Landesman WJ, Allan BF, Langerhans RB, et al. Inter-annual associations between precipitation and human incidence of West Nile virus in the United States. Vector Borne Zoonotic Dis. 2007;7:337-343.
- Hart J Jr, Tillman G, Kraut MA, et al. West Nile virus neuroinvasive disease: neurological manifestations and prospective longitudinal outcomes. BMC Infect Dis. 2014;14:248.
- Wu JJ, Huang DB, Tyring SK. West Nile virus rash on the palms and soles of the feet. J Eur Acad Dermatol Venereol. 2006;20:1393-1394.
- Sejvar J. Clinical manifestations and outcomes of West Nile virus infection. Viruses. 2014;6:606-623.
- Ferguson DD, Gershman K, LeBailly A, et al. Characteristics of the rash associated with West Nile virus fever. Clin Infect Dis. 2005;41:1204-1207.
- Marszalek R, Chen A, Gjede J. Psoriasiform eruption in the setting of West Nile virus. J Am Acad Dermatol. 2014;70:AB4. doi:10.1016/j.jaad.2014.01.017
- Shah S, Fite LP, Lane N, et al. Purpura fulminans associated with acute West Nile virus encephalitis. J Clin Virol. 2016;75:1-4.
- Civen R, Villacorte F, Robles DT, et al. West Nile virus infection in the pediatric population. Pediatr Infect Dis J. 2006;25:75-78.
- Huhn GD, Dworkin MS. Rash as a prognostic factor in West Nile virus disease. Clin Infect Dis. 2006;43:388-389.
- Murphy TD, Grandpre J, Novick SL, et al. West Nile virus infection among health-fair participants, Wyoming 2003: assessment of symptoms and risk factors. Vector Borne Zoonotic Dis. 2005;5:246-251.
- Prince HE, Tobler LH, Lapé-Nixon M, et al. Development and persistence of West Nile virus–specific immunoglobulin M (IgM), IgA, and IgG in viremic blood donors. J Clin Microbiol. 2005;43:4316-4320.
- Busch MP, Kleinman SH, Tobler LH, et al. Virus and antibody dynamics in acute West Nile Virus infection. J Infect Dis. 2008;198:984-993.
- Mostashari F, Bunning ML, Kitsutani PT, et al. Epidemic West Nile encephalitis, New York, 1999: results of a household-based seroepidemiological survey. Lancet. 2001;358:261-264.
- Cahill ME, Yao Y, Nock D, et al. West Nile virus seroprevalence, Connecticut, USA, 2000–2014. Emerg Infect Dis. 2017;23:708-710.
- Schweitzer BK, Kramer WL, Sambol AR, et al. Geographic factors contributing to a high seroprevalence of West Nile virus-specific antibodies in humans following an epidemic. Clin Vaccine Immunol. 2006;13:314-318.
- Maeda A, Maeda J. Review of diagnostic plaque reduction neutralization tests for flavivirus infection. Vet J. 2013;195:33-40.
- Tilley PA, Fox JD, Jayaraman GC, et al. Nucleic acid testing for west nile virus RNA in plasma enhances rapid diagnosis of acute infection in symptomatic patients. J Infect Dis. 2006;193:1361-1364.
- Petersen LR, Brault AC, Nasci RS. West Nile virus: review of the literature. JAMA. 2013;310:308-315.
- Yu A, Ferenczi E, Moussa K, et al. Clinical spectrum of West Nile virus neuroinvasive disease. Neurohospitalist. 2020;10:43-47.
- Michaelis M, Kleinschmidt MC, Doerr HW, et al. Minocycline inhibits West Nile virus replication and apoptosis in human neuronal cells. J Antimicrob Chemother. 2007;60:981-986.
- United State Environmental Protection Agency. Skin-applied repellent ingredients. https://www.epa.gov/insect-repellents/skin-applied-repellent-ingredients. Accessed April 16, 2021.
- Muzumdar S, Rothe MJ, Grant-Kels JM. The rash with maculopapules and fever in adults. Clin Dermatol. 2019;37:109-118.
What is West Nile virus? How is it contracted, and who can become infected?
West Nile virus (WNV) is a single-stranded RNA virus of the Flaviviridae family and Flavivirus genus, a lineage that also includes the yellow fever, dengue, Zika, Japanese encephalitis, and Saint Louis encephalitis viruses.1 Birds serve as the reservoir hosts of WNV, and mosquitoes acquire the virus during feeding.2 West Nile virus then is transmitted to humans primarily by bites from Culex mosquitoes, which are especially prevalent in wooded areas during peak mosquito season (summer through early fall in North America).1 Mosquitoes also can infect horses; however, humans and horses are dead-end hosts, meaning they do not pass the virus on to other biting mosquitoes.3 There also have been rare reports of transmission of WNV through blood and donation as well as mother-to-baby transmission.2
What is the epidemiology of WNV in the United States?
Since the introduction of WNV to the United States in 1999, it has become an important public health concern, with 48,183 cases and 2163 deaths reported since 1999.2,3 In 2018, Nebraska had the highest number of cases of WNV (n=251), followed by California (n=217), North Dakota (n=204), Illinois (n=176), and South Dakota (n=169).3 West Nile virus is endemic to all 48 contiguous states and Canada, though the Great Plains region is especially affected by WNV due to several factors, such as a greater percentage of rural land, forests, and irrigated areas.4 The Great Plains region also has been thought to be an ecological niche for a more virulent species (Culex tarsalis) compared to other regions in the United States.5
The annual incidence of WNV in the United States peaked in 2003 at 9862 cases (up from 62 cases in 1999), then declined gradually until 2008 to 2011, during which the incidence was stable at 700 to 1100 new cases per year. However, there was a resurgence of cases (n=5674) in 2012 that steadied at around 2200 cases annually in subsequent years.6 Although there likely are several factors affecting WNV incidence trends in the United States, interannual changes in temperature and precipitation have been described. An increased mean annual temperature (from September through October, the end of peak mosquito season) and an increased temperature in winter months (from January through March, prior to peak mosquito season) have both been associated with an increased incidence of WNV.7 An increased temperature is thought to increase population numbers of mosquitoes both by increasing reproductive rates and creating ideal breeding environments via pooled water areas.8 Depending on the region, both above average and below average precipitation levels in the United States can increase WNV incidence the following year.7,9
What are the signs and symptoms of WNV infection?
Up to 80% of those infected with WNV are asymptomatic.3 After an incubation period of roughly 2 to 14 days, the remaining 20% may develop symptoms of West Nile fever (WNF), typically a self-limited illness that consists of 3 to 10 days of nonspecific symptoms such as fever, headache, fatigue, muscle pain and/or weakness, eye pain, gastrointestinal tract upset, and a macular rash that usually presents on the trunk or extremities.1,3 Less than 1% of patients affected by WNV develop neuroinvasive disease, including meningitis, encephalitis, and/or acute flaccid paralysis.10 West Nile virus neuroinvasive disease can cause permanent neurologic sequelae such as muscle weakness, confusion, memory loss, and fatigue; it carries a mortality rate of 10% to 30%, which is mainly dependent on older age and immunosuppression status.1,10
What is the reported spectrum of cutaneous findings in WNV?
Of the roughly 20% of patients infected with WNV that develop WNF, approximately 25% to 50% will develop an associated rash.1 It most commonly is described as a morbilliform or maculopapular rash located on the chest, back, and arms, usually sparing the palms and soles, though 1 case report noted involvement with these areas (Figure).11,12 It typically appears 5 days after symptom onset, can be associated with defervescence, and lasts less than a week.1,13 Pruritus and dysesthesia are sometimes present.13 Other rare presentations that have been reported include an ill-defined pseudovesicular rash with erythematous papules on the palms and pink, scaly, psoriasiform papules on the feet and thighs, as well as neuroinvasive WNV leading to purpura fulminans.14,15 A diffuse, erythematous, petechial rash on the face, neck, trunk, and extremities was reported in a pediatric patient, but there have been no reports of a petechial rash associated with WNV in adult patients.16 These findings suggest some potential variability in the presentation of the WNV rash.

What role does the presence of rash play diagnostically and prognostically?
The rash of WNV has been implicated as a potential prognostic factor in predicting more favorable outcomes.17 Using 2002 data from the Illinois Department of Public Health and 2003 data from the Colorado Department of Public Health, Huhn and Dworkin17 found the age-adjusted risk of encephalitis and death to be decreased in WNV patients with a rash (relative risk, 0.44; 95% CI, 0.21-0.92). The reasons for this are not definitively known, but we hypothesize that the rash may prompt patients to seek earlier medical attention or indicate a more robust immune response. Additionally, a rash in WNV more commonly is seen in younger patients, whereas WNV neuroinvasive disease is more common in older patients, who also tend to have worse outcomes.10 One study found rash to be the only symptom that demonstrated a significant association with seropositivity (overall risk=6.35; P<.05; 95% CI, 3.75-10.80) by multivariate analysis.18
How is WNV diagnosed? What are the downsides to WNV testing?
Given that the presenting symptoms of WNV and WNF are nonspecific, it becomes challenging to arrive at the diagnosis based solely on physical examination. As such, the patient’s clinical and epidemiologic history, such as timing, pattern, and appearance of the rash or recent history of mosquito bites, is key to arriving at the correct diagnosis. With clinical suspicion, possible diagnostic tests include an IgM enzyme-linked immunosorbent assay (ELISA) for WNV, a plaque reduction neutralization test (PNRT), and blood polymerase chain reaction (PCR).
An ELISA is a confirmatory test to detect IgM antibodies to WNV in the serum. Because IgM seroconversion typically occurs between days 4 and 10 of symptom onset, there is a high probability of initial false-negative testing within the first 8 days after symptom onset.19,20 Clinical understanding of this fact is imperative, as an initial negative ELISA does not rule out WNV, and a retest is warranted if clinical suspicion is high. In addition to a high initial false-negative rate with ELISA, there are several other limitations to note. IgM antibodies remain elevated for 1 to 3 months or possibly up to a year in immunocompromised patients.1 Due to this, false positives may be present if there was a recent prior infection. Enzyme-linked immunosorbent assay may not distinguish from different flaviviruses, including the yellow fever, dengue, Zika, Japanese encephalitis, and Saint Louis encephalitis viruses. Seropositivity has been estimated in some states, including 1999 data from New York (2.6%), 2003 data from Nebraska (9.5%), and 2012-2014 data from Connecticut (8.5%).21-23 Regional variance may be expected, as there also were significant differences in WNV seropositivity between different regions in Nebraska (P<.001).23
Because ELISA testing for WNV has readily apparent flaws, other tests have been utilized in its diagnosis. The PNRT is the most specific test, and it works by measuring neutralizing antibody titers for different flaviviruses. It has the ability to determine cross-reactivity with other flaviviruses; however, it does not discriminate between a current infection and a prior infection or prior flavivirus vaccine (ie, yellow fever vaccine). Despite this, a positive PNRT can lend credibility to a positive ELISA test and determine specificity for WNV for those with no prior flavivirus exposure.24 According to the Centers for Disease Control and Prevention (CDC), this test can be performed by the CDC or in reference laboratories designated by the CDC.3 Additionally, some state health laboratories may perform PRNTs.
Viral detection with PCR currently is used to screen blood donations and may be beneficial for immunocompromised patients that lack the ability to form a robust antibody response or if a patient presents early, as PCR works best within the first week of symptom onset.1 Tilley et al25 showed that a combination of PCR and ELISA were able to accurately predict 94.2% of patients (180/191) with documented WNV on a first blood sample compared to 45% and 58.1% for only viral detection or ELISA, respectively. Based on costs from a Midwest academic center, antibody detection tests are around $100 while PCR may range from $500 to $1000 and is only performed in reference laboratories. Although these tests remain in the repertoire for WNV diagnosis, financial stewardship is important.
If there are symptoms of photophobia, phonophobia, nuchal rigidity, loss of consciousness, or marked personality changes, a lumbar puncture for WNV IgM in the cerebrospinal fluid can be performed. As with most viral infections, cerebrospinal fluid findings normally include an elevated protein and lymphocyte count, but neutrophils may be predominantly elevated if the infection is early in its course.26
What are the management options?
To date, there is no curative treatment for WNV, and management is largely supportive. For WNF, over-the-counter pain medications may be helpful to reduce fever and pain. If more severe disease develops, hospitalization for further supportive care may be needed.27 If meningitis or encephalitis is suspected, broad-spectrum antibiotics may need to be started until other common etiologies are ruled out.28
How can you prevent WNV infection?
Disease prevention largely consists of educating the public to avoid heavily wooded areas, especially in areas of high prevalence and during peak months, and to use protective clothing and insect repellant that has been approved by the Environmental Protection Agency.3 Insect repellants approved by the Environmental Protection Agency contain ingredients such as DEET (N, N-diethyl-meta-toluamide), picaridin, IR3535 (ethyl butylacetylaminopropionate), and oil of lemon eucalyptus, which have been proven safe and effective.29 Patients also can protect their homes by using window screens and promptly repairing screens with holes.3
What is the differential diagnosis for WNV?
The differential diagnosis for fever with generalized maculopapular rash broadly ranges from viral etiologies (eg, WNV, Zika, measles), to tick bites (eg, Rocky Mountain spotted fever, ehrlichiosis), to drug-induced rashes. A detailed patient history inquiring on recent sick contacts, travel (WNV in the Midwest, ehrlichiosis in the Southeast), environmental exposures (ticks, mosquitoes), and new medications (typically 7–10 days after starting) is imperative to narrow the differential.30 In addition, the distribution, timing, and clinical characteristics of the rash may aid in diagnosis, along with an appropriately correlated clinical picture. West Nile virus likely will present in the summer in mid central geographic locations and often develops on the trunk and extremities as a blanching, generalized, maculopapular rash around 5 days after symptom onset or with defervescence.1
What is West Nile virus? How is it contracted, and who can become infected?
West Nile virus (WNV) is a single-stranded RNA virus of the Flaviviridae family and Flavivirus genus, a lineage that also includes the yellow fever, dengue, Zika, Japanese encephalitis, and Saint Louis encephalitis viruses.1 Birds serve as the reservoir hosts of WNV, and mosquitoes acquire the virus during feeding.2 West Nile virus then is transmitted to humans primarily by bites from Culex mosquitoes, which are especially prevalent in wooded areas during peak mosquito season (summer through early fall in North America).1 Mosquitoes also can infect horses; however, humans and horses are dead-end hosts, meaning they do not pass the virus on to other biting mosquitoes.3 There also have been rare reports of transmission of WNV through blood and donation as well as mother-to-baby transmission.2
What is the epidemiology of WNV in the United States?
Since the introduction of WNV to the United States in 1999, it has become an important public health concern, with 48,183 cases and 2163 deaths reported since 1999.2,3 In 2018, Nebraska had the highest number of cases of WNV (n=251), followed by California (n=217), North Dakota (n=204), Illinois (n=176), and South Dakota (n=169).3 West Nile virus is endemic to all 48 contiguous states and Canada, though the Great Plains region is especially affected by WNV due to several factors, such as a greater percentage of rural land, forests, and irrigated areas.4 The Great Plains region also has been thought to be an ecological niche for a more virulent species (Culex tarsalis) compared to other regions in the United States.5
The annual incidence of WNV in the United States peaked in 2003 at 9862 cases (up from 62 cases in 1999), then declined gradually until 2008 to 2011, during which the incidence was stable at 700 to 1100 new cases per year. However, there was a resurgence of cases (n=5674) in 2012 that steadied at around 2200 cases annually in subsequent years.6 Although there likely are several factors affecting WNV incidence trends in the United States, interannual changes in temperature and precipitation have been described. An increased mean annual temperature (from September through October, the end of peak mosquito season) and an increased temperature in winter months (from January through March, prior to peak mosquito season) have both been associated with an increased incidence of WNV.7 An increased temperature is thought to increase population numbers of mosquitoes both by increasing reproductive rates and creating ideal breeding environments via pooled water areas.8 Depending on the region, both above average and below average precipitation levels in the United States can increase WNV incidence the following year.7,9
What are the signs and symptoms of WNV infection?
Up to 80% of those infected with WNV are asymptomatic.3 After an incubation period of roughly 2 to 14 days, the remaining 20% may develop symptoms of West Nile fever (WNF), typically a self-limited illness that consists of 3 to 10 days of nonspecific symptoms such as fever, headache, fatigue, muscle pain and/or weakness, eye pain, gastrointestinal tract upset, and a macular rash that usually presents on the trunk or extremities.1,3 Less than 1% of patients affected by WNV develop neuroinvasive disease, including meningitis, encephalitis, and/or acute flaccid paralysis.10 West Nile virus neuroinvasive disease can cause permanent neurologic sequelae such as muscle weakness, confusion, memory loss, and fatigue; it carries a mortality rate of 10% to 30%, which is mainly dependent on older age and immunosuppression status.1,10
What is the reported spectrum of cutaneous findings in WNV?
Of the roughly 20% of patients infected with WNV that develop WNF, approximately 25% to 50% will develop an associated rash.1 It most commonly is described as a morbilliform or maculopapular rash located on the chest, back, and arms, usually sparing the palms and soles, though 1 case report noted involvement with these areas (Figure).11,12 It typically appears 5 days after symptom onset, can be associated with defervescence, and lasts less than a week.1,13 Pruritus and dysesthesia are sometimes present.13 Other rare presentations that have been reported include an ill-defined pseudovesicular rash with erythematous papules on the palms and pink, scaly, psoriasiform papules on the feet and thighs, as well as neuroinvasive WNV leading to purpura fulminans.14,15 A diffuse, erythematous, petechial rash on the face, neck, trunk, and extremities was reported in a pediatric patient, but there have been no reports of a petechial rash associated with WNV in adult patients.16 These findings suggest some potential variability in the presentation of the WNV rash.

What role does the presence of rash play diagnostically and prognostically?
The rash of WNV has been implicated as a potential prognostic factor in predicting more favorable outcomes.17 Using 2002 data from the Illinois Department of Public Health and 2003 data from the Colorado Department of Public Health, Huhn and Dworkin17 found the age-adjusted risk of encephalitis and death to be decreased in WNV patients with a rash (relative risk, 0.44; 95% CI, 0.21-0.92). The reasons for this are not definitively known, but we hypothesize that the rash may prompt patients to seek earlier medical attention or indicate a more robust immune response. Additionally, a rash in WNV more commonly is seen in younger patients, whereas WNV neuroinvasive disease is more common in older patients, who also tend to have worse outcomes.10 One study found rash to be the only symptom that demonstrated a significant association with seropositivity (overall risk=6.35; P<.05; 95% CI, 3.75-10.80) by multivariate analysis.18
How is WNV diagnosed? What are the downsides to WNV testing?
Given that the presenting symptoms of WNV and WNF are nonspecific, it becomes challenging to arrive at the diagnosis based solely on physical examination. As such, the patient’s clinical and epidemiologic history, such as timing, pattern, and appearance of the rash or recent history of mosquito bites, is key to arriving at the correct diagnosis. With clinical suspicion, possible diagnostic tests include an IgM enzyme-linked immunosorbent assay (ELISA) for WNV, a plaque reduction neutralization test (PNRT), and blood polymerase chain reaction (PCR).
An ELISA is a confirmatory test to detect IgM antibodies to WNV in the serum. Because IgM seroconversion typically occurs between days 4 and 10 of symptom onset, there is a high probability of initial false-negative testing within the first 8 days after symptom onset.19,20 Clinical understanding of this fact is imperative, as an initial negative ELISA does not rule out WNV, and a retest is warranted if clinical suspicion is high. In addition to a high initial false-negative rate with ELISA, there are several other limitations to note. IgM antibodies remain elevated for 1 to 3 months or possibly up to a year in immunocompromised patients.1 Due to this, false positives may be present if there was a recent prior infection. Enzyme-linked immunosorbent assay may not distinguish from different flaviviruses, including the yellow fever, dengue, Zika, Japanese encephalitis, and Saint Louis encephalitis viruses. Seropositivity has been estimated in some states, including 1999 data from New York (2.6%), 2003 data from Nebraska (9.5%), and 2012-2014 data from Connecticut (8.5%).21-23 Regional variance may be expected, as there also were significant differences in WNV seropositivity between different regions in Nebraska (P<.001).23
Because ELISA testing for WNV has readily apparent flaws, other tests have been utilized in its diagnosis. The PNRT is the most specific test, and it works by measuring neutralizing antibody titers for different flaviviruses. It has the ability to determine cross-reactivity with other flaviviruses; however, it does not discriminate between a current infection and a prior infection or prior flavivirus vaccine (ie, yellow fever vaccine). Despite this, a positive PNRT can lend credibility to a positive ELISA test and determine specificity for WNV for those with no prior flavivirus exposure.24 According to the Centers for Disease Control and Prevention (CDC), this test can be performed by the CDC or in reference laboratories designated by the CDC.3 Additionally, some state health laboratories may perform PRNTs.
Viral detection with PCR currently is used to screen blood donations and may be beneficial for immunocompromised patients that lack the ability to form a robust antibody response or if a patient presents early, as PCR works best within the first week of symptom onset.1 Tilley et al25 showed that a combination of PCR and ELISA were able to accurately predict 94.2% of patients (180/191) with documented WNV on a first blood sample compared to 45% and 58.1% for only viral detection or ELISA, respectively. Based on costs from a Midwest academic center, antibody detection tests are around $100 while PCR may range from $500 to $1000 and is only performed in reference laboratories. Although these tests remain in the repertoire for WNV diagnosis, financial stewardship is important.
If there are symptoms of photophobia, phonophobia, nuchal rigidity, loss of consciousness, or marked personality changes, a lumbar puncture for WNV IgM in the cerebrospinal fluid can be performed. As with most viral infections, cerebrospinal fluid findings normally include an elevated protein and lymphocyte count, but neutrophils may be predominantly elevated if the infection is early in its course.26
What are the management options?
To date, there is no curative treatment for WNV, and management is largely supportive. For WNF, over-the-counter pain medications may be helpful to reduce fever and pain. If more severe disease develops, hospitalization for further supportive care may be needed.27 If meningitis or encephalitis is suspected, broad-spectrum antibiotics may need to be started until other common etiologies are ruled out.28
How can you prevent WNV infection?
Disease prevention largely consists of educating the public to avoid heavily wooded areas, especially in areas of high prevalence and during peak months, and to use protective clothing and insect repellant that has been approved by the Environmental Protection Agency.3 Insect repellants approved by the Environmental Protection Agency contain ingredients such as DEET (N, N-diethyl-meta-toluamide), picaridin, IR3535 (ethyl butylacetylaminopropionate), and oil of lemon eucalyptus, which have been proven safe and effective.29 Patients also can protect their homes by using window screens and promptly repairing screens with holes.3
What is the differential diagnosis for WNV?
The differential diagnosis for fever with generalized maculopapular rash broadly ranges from viral etiologies (eg, WNV, Zika, measles), to tick bites (eg, Rocky Mountain spotted fever, ehrlichiosis), to drug-induced rashes. A detailed patient history inquiring on recent sick contacts, travel (WNV in the Midwest, ehrlichiosis in the Southeast), environmental exposures (ticks, mosquitoes), and new medications (typically 7–10 days after starting) is imperative to narrow the differential.30 In addition, the distribution, timing, and clinical characteristics of the rash may aid in diagnosis, along with an appropriately correlated clinical picture. West Nile virus likely will present in the summer in mid central geographic locations and often develops on the trunk and extremities as a blanching, generalized, maculopapular rash around 5 days after symptom onset or with defervescence.1
- Petersen LR. Clinical manifestations and diagnosis of West Nile virus infection. UpToDate website. Updated August 7, 2020. Accessed April 16, 2021. https://www.uptodate.com/contents/clinical-manifestations-and-diagnosis-of-west-nile-virus-infection?search=clinical-manifestations-and-diagnosis-of-west-nile-virusinfection.&source=search_result&selectedTitle=1~78&usage_type=default&display_rank=1
- Sampathkumar P. West Nile virus: epidemiology, clinical presentation, diagnosis, and prevention. Mayo Clin Proc. 2003;78:1137-1144.
- Centers for Disease Control and Prevention. West Nile virus. Updated June 3, 2020. Accessed April 16, 2021. https://www.cdc.gov/westnile/index.html
- Chuang TW, Hockett CW, Kightlinger L, et al. Landscape-level spatial patterns of West Nile virus risk in the northern Great Plains. Am J Trop Med Hyg. 2012;86:724-731.
- Wimberly MC, Hildreth MB, Boyte SP, et al. Ecological niche of the 2003 West Nile virus epidemic in the northern great plains of the United States. PLoS One. 2008;3:E3744. doi:10.1371/journal.pone.0003744
- Centers for Disease Control and Prevention. West Nile virus disease cases reported to CDC by state of residence, 1999-2019. Accessed April 26, 2021. https://www.cdc.gov/westnile/resources/pdfs/data/West-Nile-virus-disease-cases-by-state_1999-2019-P.pdf
- Hahn MB, Monaghan AJ, Hayden MH, et al. Meteorological conditions associated with increased incidence of West Nile virus disease in the United States, 2004–2012. Am J Trop Med Hyg. 2015;92:1013-1022.
- Brown CM, DeMaria A Jr. The resurgence of West Nile virus. Ann Intern Med. 2012;157:823-824.
- Landesman WJ, Allan BF, Langerhans RB, et al. Inter-annual associations between precipitation and human incidence of West Nile virus in the United States. Vector Borne Zoonotic Dis. 2007;7:337-343.
- Hart J Jr, Tillman G, Kraut MA, et al. West Nile virus neuroinvasive disease: neurological manifestations and prospective longitudinal outcomes. BMC Infect Dis. 2014;14:248.
- Wu JJ, Huang DB, Tyring SK. West Nile virus rash on the palms and soles of the feet. J Eur Acad Dermatol Venereol. 2006;20:1393-1394.
- Sejvar J. Clinical manifestations and outcomes of West Nile virus infection. Viruses. 2014;6:606-623.
- Ferguson DD, Gershman K, LeBailly A, et al. Characteristics of the rash associated with West Nile virus fever. Clin Infect Dis. 2005;41:1204-1207.
- Marszalek R, Chen A, Gjede J. Psoriasiform eruption in the setting of West Nile virus. J Am Acad Dermatol. 2014;70:AB4. doi:10.1016/j.jaad.2014.01.017
- Shah S, Fite LP, Lane N, et al. Purpura fulminans associated with acute West Nile virus encephalitis. J Clin Virol. 2016;75:1-4.
- Civen R, Villacorte F, Robles DT, et al. West Nile virus infection in the pediatric population. Pediatr Infect Dis J. 2006;25:75-78.
- Huhn GD, Dworkin MS. Rash as a prognostic factor in West Nile virus disease. Clin Infect Dis. 2006;43:388-389.
- Murphy TD, Grandpre J, Novick SL, et al. West Nile virus infection among health-fair participants, Wyoming 2003: assessment of symptoms and risk factors. Vector Borne Zoonotic Dis. 2005;5:246-251.
- Prince HE, Tobler LH, Lapé-Nixon M, et al. Development and persistence of West Nile virus–specific immunoglobulin M (IgM), IgA, and IgG in viremic blood donors. J Clin Microbiol. 2005;43:4316-4320.
- Busch MP, Kleinman SH, Tobler LH, et al. Virus and antibody dynamics in acute West Nile Virus infection. J Infect Dis. 2008;198:984-993.
- Mostashari F, Bunning ML, Kitsutani PT, et al. Epidemic West Nile encephalitis, New York, 1999: results of a household-based seroepidemiological survey. Lancet. 2001;358:261-264.
- Cahill ME, Yao Y, Nock D, et al. West Nile virus seroprevalence, Connecticut, USA, 2000–2014. Emerg Infect Dis. 2017;23:708-710.
- Schweitzer BK, Kramer WL, Sambol AR, et al. Geographic factors contributing to a high seroprevalence of West Nile virus-specific antibodies in humans following an epidemic. Clin Vaccine Immunol. 2006;13:314-318.
- Maeda A, Maeda J. Review of diagnostic plaque reduction neutralization tests for flavivirus infection. Vet J. 2013;195:33-40.
- Tilley PA, Fox JD, Jayaraman GC, et al. Nucleic acid testing for west nile virus RNA in plasma enhances rapid diagnosis of acute infection in symptomatic patients. J Infect Dis. 2006;193:1361-1364.
- Petersen LR, Brault AC, Nasci RS. West Nile virus: review of the literature. JAMA. 2013;310:308-315.
- Yu A, Ferenczi E, Moussa K, et al. Clinical spectrum of West Nile virus neuroinvasive disease. Neurohospitalist. 2020;10:43-47.
- Michaelis M, Kleinschmidt MC, Doerr HW, et al. Minocycline inhibits West Nile virus replication and apoptosis in human neuronal cells. J Antimicrob Chemother. 2007;60:981-986.
- United State Environmental Protection Agency. Skin-applied repellent ingredients. https://www.epa.gov/insect-repellents/skin-applied-repellent-ingredients. Accessed April 16, 2021.
- Muzumdar S, Rothe MJ, Grant-Kels JM. The rash with maculopapules and fever in adults. Clin Dermatol. 2019;37:109-118.
- Petersen LR. Clinical manifestations and diagnosis of West Nile virus infection. UpToDate website. Updated August 7, 2020. Accessed April 16, 2021. https://www.uptodate.com/contents/clinical-manifestations-and-diagnosis-of-west-nile-virus-infection?search=clinical-manifestations-and-diagnosis-of-west-nile-virusinfection.&source=search_result&selectedTitle=1~78&usage_type=default&display_rank=1
- Sampathkumar P. West Nile virus: epidemiology, clinical presentation, diagnosis, and prevention. Mayo Clin Proc. 2003;78:1137-1144.
- Centers for Disease Control and Prevention. West Nile virus. Updated June 3, 2020. Accessed April 16, 2021. https://www.cdc.gov/westnile/index.html
- Chuang TW, Hockett CW, Kightlinger L, et al. Landscape-level spatial patterns of West Nile virus risk in the northern Great Plains. Am J Trop Med Hyg. 2012;86:724-731.
- Wimberly MC, Hildreth MB, Boyte SP, et al. Ecological niche of the 2003 West Nile virus epidemic in the northern great plains of the United States. PLoS One. 2008;3:E3744. doi:10.1371/journal.pone.0003744
- Centers for Disease Control and Prevention. West Nile virus disease cases reported to CDC by state of residence, 1999-2019. Accessed April 26, 2021. https://www.cdc.gov/westnile/resources/pdfs/data/West-Nile-virus-disease-cases-by-state_1999-2019-P.pdf
- Hahn MB, Monaghan AJ, Hayden MH, et al. Meteorological conditions associated with increased incidence of West Nile virus disease in the United States, 2004–2012. Am J Trop Med Hyg. 2015;92:1013-1022.
- Brown CM, DeMaria A Jr. The resurgence of West Nile virus. Ann Intern Med. 2012;157:823-824.
- Landesman WJ, Allan BF, Langerhans RB, et al. Inter-annual associations between precipitation and human incidence of West Nile virus in the United States. Vector Borne Zoonotic Dis. 2007;7:337-343.
- Hart J Jr, Tillman G, Kraut MA, et al. West Nile virus neuroinvasive disease: neurological manifestations and prospective longitudinal outcomes. BMC Infect Dis. 2014;14:248.
- Wu JJ, Huang DB, Tyring SK. West Nile virus rash on the palms and soles of the feet. J Eur Acad Dermatol Venereol. 2006;20:1393-1394.
- Sejvar J. Clinical manifestations and outcomes of West Nile virus infection. Viruses. 2014;6:606-623.
- Ferguson DD, Gershman K, LeBailly A, et al. Characteristics of the rash associated with West Nile virus fever. Clin Infect Dis. 2005;41:1204-1207.
- Marszalek R, Chen A, Gjede J. Psoriasiform eruption in the setting of West Nile virus. J Am Acad Dermatol. 2014;70:AB4. doi:10.1016/j.jaad.2014.01.017
- Shah S, Fite LP, Lane N, et al. Purpura fulminans associated with acute West Nile virus encephalitis. J Clin Virol. 2016;75:1-4.
- Civen R, Villacorte F, Robles DT, et al. West Nile virus infection in the pediatric population. Pediatr Infect Dis J. 2006;25:75-78.
- Huhn GD, Dworkin MS. Rash as a prognostic factor in West Nile virus disease. Clin Infect Dis. 2006;43:388-389.
- Murphy TD, Grandpre J, Novick SL, et al. West Nile virus infection among health-fair participants, Wyoming 2003: assessment of symptoms and risk factors. Vector Borne Zoonotic Dis. 2005;5:246-251.
- Prince HE, Tobler LH, Lapé-Nixon M, et al. Development and persistence of West Nile virus–specific immunoglobulin M (IgM), IgA, and IgG in viremic blood donors. J Clin Microbiol. 2005;43:4316-4320.
- Busch MP, Kleinman SH, Tobler LH, et al. Virus and antibody dynamics in acute West Nile Virus infection. J Infect Dis. 2008;198:984-993.
- Mostashari F, Bunning ML, Kitsutani PT, et al. Epidemic West Nile encephalitis, New York, 1999: results of a household-based seroepidemiological survey. Lancet. 2001;358:261-264.
- Cahill ME, Yao Y, Nock D, et al. West Nile virus seroprevalence, Connecticut, USA, 2000–2014. Emerg Infect Dis. 2017;23:708-710.
- Schweitzer BK, Kramer WL, Sambol AR, et al. Geographic factors contributing to a high seroprevalence of West Nile virus-specific antibodies in humans following an epidemic. Clin Vaccine Immunol. 2006;13:314-318.
- Maeda A, Maeda J. Review of diagnostic plaque reduction neutralization tests for flavivirus infection. Vet J. 2013;195:33-40.
- Tilley PA, Fox JD, Jayaraman GC, et al. Nucleic acid testing for west nile virus RNA in plasma enhances rapid diagnosis of acute infection in symptomatic patients. J Infect Dis. 2006;193:1361-1364.
- Petersen LR, Brault AC, Nasci RS. West Nile virus: review of the literature. JAMA. 2013;310:308-315.
- Yu A, Ferenczi E, Moussa K, et al. Clinical spectrum of West Nile virus neuroinvasive disease. Neurohospitalist. 2020;10:43-47.
- Michaelis M, Kleinschmidt MC, Doerr HW, et al. Minocycline inhibits West Nile virus replication and apoptosis in human neuronal cells. J Antimicrob Chemother. 2007;60:981-986.
- United State Environmental Protection Agency. Skin-applied repellent ingredients. https://www.epa.gov/insect-repellents/skin-applied-repellent-ingredients. Accessed April 16, 2021.
- Muzumdar S, Rothe MJ, Grant-Kels JM. The rash with maculopapules and fever in adults. Clin Dermatol. 2019;37:109-118.
Practice Points
- Dermatologists should be aware of the most common rash associated with West Nile virus (WNV), which is a nonspecific maculopapular rash appearing on the trunk and extremities around 5 days after the onset of fever, fatigue, and other nonspecific symptoms.
- Rash may serve as a prognostic indicator for improved outcomes in WNV due to its association with decreased risk of encephalitis and death.
- An IgM enzyme-linked immunosorbent assay for WNV initially may yield false-negative results, as the development of detectable antibodies against the virus may take up to 8 days after symptom onset.
Botanical Briefs: Phytophotodermatitis Is an Occupational and Recreational Dermatosis in the Limelight
Phytophotodermatitis (PPD) is a nonallergic contact dermatitis and thus is independent of the immune system, so prior sensitization is not required.1-3 It sometimes is known by colorful names such as margarita photodermatitis, in which a slice of lime in a refreshing summer drink may be etiologic,4,5 or berloque dermatitis, caused by exposure to perfumes containing bergapten (5-methoxypsoralen).6,7 Phytophotodermatitis may develop when phototoxic agents such as furocoumarins, which protect plants from fungal pathogens, and psoralens are applied to the skin followed by exposure to UV light, more specifically in the UVA range of 320 to 400 nm. Thus, these chemicals produce a phototoxic rather than photoallergic reaction, leading to cellular damage. Furocoumarins and psoralens often are found in plants such as celery and figs as well as in citrus fruits such as limes, lemons, and grapefruits. Exposure may be cryptic, as the patient may not consider or mention the eruption as possibly caused by activities such as soaking one’s feet in a folk remedy containing fig leaves.7,8 Once these phototoxic agents come in contact with the skin, the symptoms of PPD may arise within 24 hours of exposure, beginning as an acute dermatitis with erythema, edema, vesicles, or bullae accompanied by pain and itching.
Etiology
Phytophotodermatitis is caused by exposure to several different types of plants, including Ficus carica (common fig), the genus Citrus (eg, lime, lemon), or Pastina sativa (wild parsnip). Each of these contain furocoumarins and psoralens—phototoxic agents that cause cellular damage with epidermal necrosis and resultant pain when the skin is exposed to UVA light.1-4 There are 2 types of photochemical reactions in PPD: type I reactions occur in the absence of oxygen, whereas oxygen is present in type II reactions. Both damage cell membranes and DNA, which then results in DNA interstrand cross-linking between the psoralen furan ring and the thymine or cytosine of DNA, activating arachidonic acid metabolic pathways to produce cell death.1
Epidemiology
The incidence of PPD is unknown due to the high variability of reactions in individuals spanning from children to the elderly. It can be caused by many different wild and domestic plants in many areas of the world and can affect any individual regardless of age, race, gender, or ethnicity. Some individuals may be affected by hyperpigmentation without prominent inflammation.8 Diagnosis of PPD can be challenging, and an occupation and recreational history of exposure or recent travel with possible contact with plants may be required.
Occupational Dermatitis
Recreational Dermatitis
Phytophotodermatitis may be caused by exposure to phototoxic agents during leisure activities. Recreational exposure can occur almost anywhere, including in the kitchen, backyard, park, or woods, as well as at the beach. One notable culprit in recreational PPD is cooking with limes, parsley, or parsnips—plants that often are employed as garnishes in dishes, allowing early exposure of juices on the hands. Individuals who garden recreationally should be aware of ornamental plants such as hogweed and figs, which are notorious for causing PPD.13 Children’s camp counselors should have knowledge of PPD, as children have considerable curiosity and may touch or play with attractive plants such as hogweed. Children enjoying sports in parks can accidentally fall onto or be exposed to wild parsnip or hogweed growing nearby and wake up the next day with erythema and burning.14 Photoprotection is important, but sunscreens containing carrot extract can produce PPD.15 Widespread PPD over 80% of the body surface area due to sunbathing after applying fig leaf tea as a tanning agent has been described.16 Eating figs does not cause photosensitization unless the juice is smeared onto the skin. Margarita dermatitis and “Mexican beer dermatitis” can occur due to limes and other citrus fruits being used as ingredients in summer drinks.5 Similarly, preparing sangria may produce PPD from lime and lemon juices.17 In one report, hiking in Corsica resulted in PPD following incidental contact with the endemic plant Peucedanum paniculatum.18
Perfume (Berloque) Dermatitis
Perfume dermatitis, or berloque dermatitis, is a type of PPD for which the name is derived from the German word berlock or the French word berloque meaning trinket or charm; it was first described in 1925 by Rosenthal7 with regard to pendantlike streaks of pigmentation on the neck, face, arms, or trunk. The dermatitis develops due to bergapten, a component of bergamot oil, which is derived from the rind of Citrus bergamia. Many perfumes contain bergamot oil, but the incidence of this condition has been diminished due to use of artificial bergamot oil.6
Clinical Manifestation
Phytophotodermatitis is first evident as erythematous patches that appear within 24 hours of initial exposure to a phototoxic agent and UVA light, sometimes with a burning sensation. Solar exposure within 48 hours of sufficient plant exposure is required. Perfuse sweating may enhance the reaction.19 Rarely, it first may be seen with the sudden appearance of
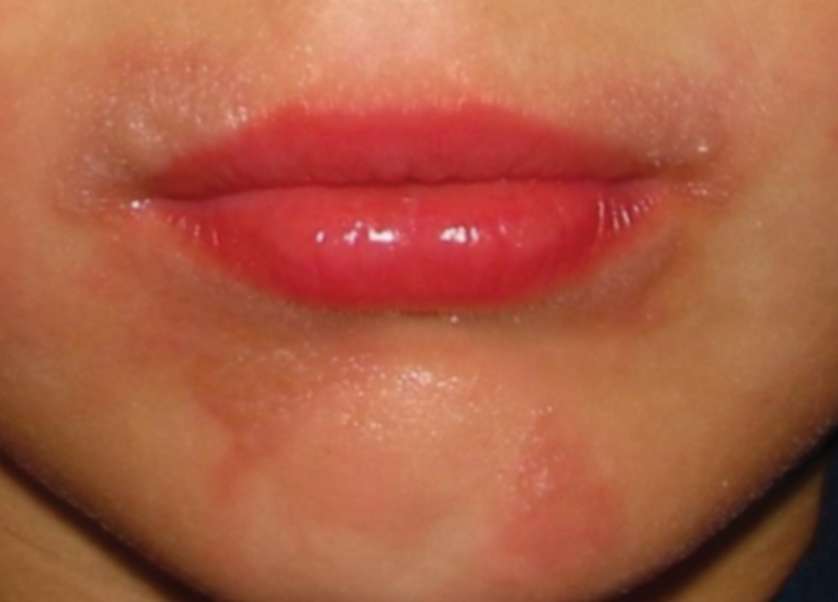
Differential Diagnosis
Phytophotodermatitis may resemble other types of dermatitis, particularly other forms of contact dermatitis such poison ivy, and occasionally other environmental simulants such as jellyfish stings.1-6,20,21 Photosensitizing disorders including porphyria cutanea tarda, pseudoporphyria, and lupus erythematosus must be distinguished from PPD.22-24 Photosensitizing medications such tetracyclines, thiazide diuretics, sulfonamides, griseofulvin, and sulfonylureas should be considered. Airborne contact dermatitis may resemble PPD, as when poison ivy is burned and is exposed to the skin in sites of airborne contact.20 Excessive solar exposure is popular, particularly among adolescents, so sunburn and sunburnlike reactions can be noteworthy.25,26
Treatment
Phytophotodermatitis can be treated with topical steroids, sometimes adding an oral antihistamine, and occasionally oral steroids.2-4 Localized pain or a burning sensation should respond to therapy. Alternatively, a cold compress applied to the skin can relieve the pain and pruritus, and the burn can be debrided and dressed daily with silver sulfadiazine plus an oral nonsteroidal anti-inflammatory drug. This eruption should be self-limited as long as it is recognized early and the cause avoided. Management of acute exposure includes prompt application of soap and water and avoidance of UV light exposure for 48 to 72 hours to prevent psoralen photoactivation.
Because PPD is essentially a chemical burn, a burn protocol and possible referral to a burn center may be needed, whether the reaction is acute or widespread.11,12,14,27,28 Surgical debridement and skin grafting rarely may be mandated.14 Postinflammatory hyperpigmentation may ensue as the dermatitis resolves but is not common.
The best approach for PPD is prevention (Figure 2). Individuals who are at risk should be aware of their surroundings and potential plants of concern and employ personal protective equipment to shield the skin from plant sap, which should be promptly removed if it comes in contact with the skin.

- Zhang R, Zhu W. Phytophotodermatitis due to Chinese herbal medicine decoction. Indian J Dermatol. 2011;56:329-331.
- Harshman J, Quan Y, Hsiang D. Phytophotodermatitis: rash with many faces. Can Fam Physician. 2017;63:938-940.
- Imen MS, Ahmadabadi A, Tavousi SH, et al. The curious cases of burn by fig tree leaves. Indian J Dermatol. 2019;64:71-73.
- Hankinson A, Lloyd B, Alweis R. Lime-induced phytophotodermatitis [published online September 29, 2014]. J Community Hosp Intern Med Perspect. doi:10.3402/jchimp.v4.25090
- Abramowitz AI, Resnik KS, Cohen KR. Margarita photodermatitis. N Engl J Med. 2013;328:891.
- Quaak MS, Martens H, Hassing RJ, et al. The sunny side of lime. J Travel Med. 2012;19:327-328.
- Rosenthal O. Berloque dermatitis: Berliner Dermatologische Gesellschaft. Dermatol Zeitschrift. 1925;42:295.
- Choi JY, Hwang S, Lee SH, et al. Asymptomatic hyperpigmentation without preceding inflammation as a clinical feature of citrus fruits–induced phytophotodermatitis. Ann Dermatol. 2018;30:75-78.
- Wynn P, Bell S. Phytophotodermatitis in grounds operatives. Occup Med (Lond). 2005;55:393-395.
- Klimaszyk P, Klimaszyk D, Piotrowiak M, et al. Unusual complications after occupational exposure to giant hogweed (Heracleum mantegazzianum): a case report. Int J Occup Med Environ Health. 2014;27:141-144.
- Downs JW, Cumpston KL, Feldman MJ. Giant hogweed phytophotodermatitis. Clin Toxicol (Phila). 2019;57:822-823.
- Maso MJ, Ruszkowski AM, Bauerle J, et al. Celery phytophotodermatitis in a chef. Arch Dermatol. 1991;127:912-913.
- Derraik JG, Rademaker M. Phytophotodermatitis caused by contact with a fig tree (Ficus carica). New Zealand Med J. 2007;120:U2720.
- Chan JC, Sullivan PJ, O’Sullivan MJ, et al. Full thickness burn caused by exposure to giant hogweed: delayed presentation, histological features and surgical management. J Plast Reconstr Aesthet Surg. 2011;64:128-130.
- Bosanac SS, Clark AK, Sivamani RK. Phytophotodermatitis related to carrot extract–containing sunscreen. Dermatol Online J. 2018;24:1-3.
- Sforza M, Andjelkov K, Zaccheddu R. Severe burn on 81% of body surface after sun tanning. Ulus Travma Acil Cerrahi Derg. 2013;19:383-384.
- Mioduszewski M, Beecker J. Phytophotodermatitis from making sangria: a phototoxic reaction to lime and lemon juice. CMAJ. 2015;187:756.
- Torrents R, Schmitt C, Domangé B, et al. Phytophotodermatitis with Peucedanum paniculatum: an endemic species to Corsica. Clin Toxicol (Phila). 2019;57:68-69.
- Sarhane KA, Ibrahim A, Fagan SP, et al. Phytophotodermatitis. Eplasty. 2013;13:ic57.
- DeLeo VA, Suarez SM, Maso MJ. Photoallergic contact dermatitis. results of photopatch testing in New York, 1985 to 1990. Arch Dermatol. 1992;128:1513-1518.
- Kimyon RS, Warshaw EM. Airborne allergic contact dermatitis: management and responsible allergens on the American Contact Dermatitis Society Core Series. Dermatitis. 2019;30:106-115.
- Miteva L, Broshtilova V, Schwartz RA. Unusual clinical manifestations of chronic discoid lupus erythematosus. Serbian J Dermatol Venereol. 2014;6:69-72.
- Handler NS, Handler MZ, Stephany MP, et al. Porphyria cutanea tarda: an intriguing genetic disease and marker. Int J Dermatol. 2017;56:E106-E117.
- Papadopoulos AJ, Schwartz RA, Fekete Z, et al. Pseudoporphyria: an atypical variant resembling toxic epidermal necrolysis. J Cutan Med Surg. 2001;5:479-485.
- Jasterzbski TJ, Janniger EJ, Schwartz RA. Adolescent tanning practices: understanding the popularity of excessive ultraviolet light exposure. In: Or
anje A, Al-Mutairi N, Shwayder T, eds. Practical Pediatric Dermatology. Controversies in Diagnosis and Treatment. Springer Verlag; 2016:177-185. - Lai YC, Janniger EJ, Schwartz RA. Solar protection policy in school children: proposals for progress. In: Oranje A, Al-Mutairi N, Shwayder T, eds. Practical Pediatric Dermatology. Controversies in Diagnosis and Treatment. Springer Verlag; 2016:165-176.
- Lagey K, Duinslaeger L, Vanderkelen A. Burns induced by plants. Burns. 1995;21:542-543.
- Redgrave N, Solomon J. Severe phytophotodermatitis from fig sap: a little known phenomenon. BMJ Case Rep. 2021;14:e238745.
Phytophotodermatitis (PPD) is a nonallergic contact dermatitis and thus is independent of the immune system, so prior sensitization is not required.1-3 It sometimes is known by colorful names such as margarita photodermatitis, in which a slice of lime in a refreshing summer drink may be etiologic,4,5 or berloque dermatitis, caused by exposure to perfumes containing bergapten (5-methoxypsoralen).6,7 Phytophotodermatitis may develop when phototoxic agents such as furocoumarins, which protect plants from fungal pathogens, and psoralens are applied to the skin followed by exposure to UV light, more specifically in the UVA range of 320 to 400 nm. Thus, these chemicals produce a phototoxic rather than photoallergic reaction, leading to cellular damage. Furocoumarins and psoralens often are found in plants such as celery and figs as well as in citrus fruits such as limes, lemons, and grapefruits. Exposure may be cryptic, as the patient may not consider or mention the eruption as possibly caused by activities such as soaking one’s feet in a folk remedy containing fig leaves.7,8 Once these phototoxic agents come in contact with the skin, the symptoms of PPD may arise within 24 hours of exposure, beginning as an acute dermatitis with erythema, edema, vesicles, or bullae accompanied by pain and itching.
Etiology
Phytophotodermatitis is caused by exposure to several different types of plants, including Ficus carica (common fig), the genus Citrus (eg, lime, lemon), or Pastina sativa (wild parsnip). Each of these contain furocoumarins and psoralens—phototoxic agents that cause cellular damage with epidermal necrosis and resultant pain when the skin is exposed to UVA light.1-4 There are 2 types of photochemical reactions in PPD: type I reactions occur in the absence of oxygen, whereas oxygen is present in type II reactions. Both damage cell membranes and DNA, which then results in DNA interstrand cross-linking between the psoralen furan ring and the thymine or cytosine of DNA, activating arachidonic acid metabolic pathways to produce cell death.1
Epidemiology
The incidence of PPD is unknown due to the high variability of reactions in individuals spanning from children to the elderly. It can be caused by many different wild and domestic plants in many areas of the world and can affect any individual regardless of age, race, gender, or ethnicity. Some individuals may be affected by hyperpigmentation without prominent inflammation.8 Diagnosis of PPD can be challenging, and an occupation and recreational history of exposure or recent travel with possible contact with plants may be required.
Occupational Dermatitis
Recreational Dermatitis
Phytophotodermatitis may be caused by exposure to phototoxic agents during leisure activities. Recreational exposure can occur almost anywhere, including in the kitchen, backyard, park, or woods, as well as at the beach. One notable culprit in recreational PPD is cooking with limes, parsley, or parsnips—plants that often are employed as garnishes in dishes, allowing early exposure of juices on the hands. Individuals who garden recreationally should be aware of ornamental plants such as hogweed and figs, which are notorious for causing PPD.13 Children’s camp counselors should have knowledge of PPD, as children have considerable curiosity and may touch or play with attractive plants such as hogweed. Children enjoying sports in parks can accidentally fall onto or be exposed to wild parsnip or hogweed growing nearby and wake up the next day with erythema and burning.14 Photoprotection is important, but sunscreens containing carrot extract can produce PPD.15 Widespread PPD over 80% of the body surface area due to sunbathing after applying fig leaf tea as a tanning agent has been described.16 Eating figs does not cause photosensitization unless the juice is smeared onto the skin. Margarita dermatitis and “Mexican beer dermatitis” can occur due to limes and other citrus fruits being used as ingredients in summer drinks.5 Similarly, preparing sangria may produce PPD from lime and lemon juices.17 In one report, hiking in Corsica resulted in PPD following incidental contact with the endemic plant Peucedanum paniculatum.18
Perfume (Berloque) Dermatitis
Perfume dermatitis, or berloque dermatitis, is a type of PPD for which the name is derived from the German word berlock or the French word berloque meaning trinket or charm; it was first described in 1925 by Rosenthal7 with regard to pendantlike streaks of pigmentation on the neck, face, arms, or trunk. The dermatitis develops due to bergapten, a component of bergamot oil, which is derived from the rind of Citrus bergamia. Many perfumes contain bergamot oil, but the incidence of this condition has been diminished due to use of artificial bergamot oil.6
Clinical Manifestation
Phytophotodermatitis is first evident as erythematous patches that appear within 24 hours of initial exposure to a phototoxic agent and UVA light, sometimes with a burning sensation. Solar exposure within 48 hours of sufficient plant exposure is required. Perfuse sweating may enhance the reaction.19 Rarely, it first may be seen with the sudden appearance of

Differential Diagnosis
Phytophotodermatitis may resemble other types of dermatitis, particularly other forms of contact dermatitis such poison ivy, and occasionally other environmental simulants such as jellyfish stings.1-6,20,21 Photosensitizing disorders including porphyria cutanea tarda, pseudoporphyria, and lupus erythematosus must be distinguished from PPD.22-24 Photosensitizing medications such tetracyclines, thiazide diuretics, sulfonamides, griseofulvin, and sulfonylureas should be considered. Airborne contact dermatitis may resemble PPD, as when poison ivy is burned and is exposed to the skin in sites of airborne contact.20 Excessive solar exposure is popular, particularly among adolescents, so sunburn and sunburnlike reactions can be noteworthy.25,26
Treatment
Phytophotodermatitis can be treated with topical steroids, sometimes adding an oral antihistamine, and occasionally oral steroids.2-4 Localized pain or a burning sensation should respond to therapy. Alternatively, a cold compress applied to the skin can relieve the pain and pruritus, and the burn can be debrided and dressed daily with silver sulfadiazine plus an oral nonsteroidal anti-inflammatory drug. This eruption should be self-limited as long as it is recognized early and the cause avoided. Management of acute exposure includes prompt application of soap and water and avoidance of UV light exposure for 48 to 72 hours to prevent psoralen photoactivation.
Because PPD is essentially a chemical burn, a burn protocol and possible referral to a burn center may be needed, whether the reaction is acute or widespread.11,12,14,27,28 Surgical debridement and skin grafting rarely may be mandated.14 Postinflammatory hyperpigmentation may ensue as the dermatitis resolves but is not common.
The best approach for PPD is prevention (Figure 2). Individuals who are at risk should be aware of their surroundings and potential plants of concern and employ personal protective equipment to shield the skin from plant sap, which should be promptly removed if it comes in contact with the skin.

Phytophotodermatitis (PPD) is a nonallergic contact dermatitis and thus is independent of the immune system, so prior sensitization is not required.1-3 It sometimes is known by colorful names such as margarita photodermatitis, in which a slice of lime in a refreshing summer drink may be etiologic,4,5 or berloque dermatitis, caused by exposure to perfumes containing bergapten (5-methoxypsoralen).6,7 Phytophotodermatitis may develop when phototoxic agents such as furocoumarins, which protect plants from fungal pathogens, and psoralens are applied to the skin followed by exposure to UV light, more specifically in the UVA range of 320 to 400 nm. Thus, these chemicals produce a phototoxic rather than photoallergic reaction, leading to cellular damage. Furocoumarins and psoralens often are found in plants such as celery and figs as well as in citrus fruits such as limes, lemons, and grapefruits. Exposure may be cryptic, as the patient may not consider or mention the eruption as possibly caused by activities such as soaking one’s feet in a folk remedy containing fig leaves.7,8 Once these phototoxic agents come in contact with the skin, the symptoms of PPD may arise within 24 hours of exposure, beginning as an acute dermatitis with erythema, edema, vesicles, or bullae accompanied by pain and itching.
Etiology
Phytophotodermatitis is caused by exposure to several different types of plants, including Ficus carica (common fig), the genus Citrus (eg, lime, lemon), or Pastina sativa (wild parsnip). Each of these contain furocoumarins and psoralens—phototoxic agents that cause cellular damage with epidermal necrosis and resultant pain when the skin is exposed to UVA light.1-4 There are 2 types of photochemical reactions in PPD: type I reactions occur in the absence of oxygen, whereas oxygen is present in type II reactions. Both damage cell membranes and DNA, which then results in DNA interstrand cross-linking between the psoralen furan ring and the thymine or cytosine of DNA, activating arachidonic acid metabolic pathways to produce cell death.1
Epidemiology
The incidence of PPD is unknown due to the high variability of reactions in individuals spanning from children to the elderly. It can be caused by many different wild and domestic plants in many areas of the world and can affect any individual regardless of age, race, gender, or ethnicity. Some individuals may be affected by hyperpigmentation without prominent inflammation.8 Diagnosis of PPD can be challenging, and an occupation and recreational history of exposure or recent travel with possible contact with plants may be required.
Occupational Dermatitis
Recreational Dermatitis
Phytophotodermatitis may be caused by exposure to phototoxic agents during leisure activities. Recreational exposure can occur almost anywhere, including in the kitchen, backyard, park, or woods, as well as at the beach. One notable culprit in recreational PPD is cooking with limes, parsley, or parsnips—plants that often are employed as garnishes in dishes, allowing early exposure of juices on the hands. Individuals who garden recreationally should be aware of ornamental plants such as hogweed and figs, which are notorious for causing PPD.13 Children’s camp counselors should have knowledge of PPD, as children have considerable curiosity and may touch or play with attractive plants such as hogweed. Children enjoying sports in parks can accidentally fall onto or be exposed to wild parsnip or hogweed growing nearby and wake up the next day with erythema and burning.14 Photoprotection is important, but sunscreens containing carrot extract can produce PPD.15 Widespread PPD over 80% of the body surface area due to sunbathing after applying fig leaf tea as a tanning agent has been described.16 Eating figs does not cause photosensitization unless the juice is smeared onto the skin. Margarita dermatitis and “Mexican beer dermatitis” can occur due to limes and other citrus fruits being used as ingredients in summer drinks.5 Similarly, preparing sangria may produce PPD from lime and lemon juices.17 In one report, hiking in Corsica resulted in PPD following incidental contact with the endemic plant Peucedanum paniculatum.18
Perfume (Berloque) Dermatitis
Perfume dermatitis, or berloque dermatitis, is a type of PPD for which the name is derived from the German word berlock or the French word berloque meaning trinket or charm; it was first described in 1925 by Rosenthal7 with regard to pendantlike streaks of pigmentation on the neck, face, arms, or trunk. The dermatitis develops due to bergapten, a component of bergamot oil, which is derived from the rind of Citrus bergamia. Many perfumes contain bergamot oil, but the incidence of this condition has been diminished due to use of artificial bergamot oil.6
Clinical Manifestation
Phytophotodermatitis is first evident as erythematous patches that appear within 24 hours of initial exposure to a phototoxic agent and UVA light, sometimes with a burning sensation. Solar exposure within 48 hours of sufficient plant exposure is required. Perfuse sweating may enhance the reaction.19 Rarely, it first may be seen with the sudden appearance of

Differential Diagnosis
Phytophotodermatitis may resemble other types of dermatitis, particularly other forms of contact dermatitis such poison ivy, and occasionally other environmental simulants such as jellyfish stings.1-6,20,21 Photosensitizing disorders including porphyria cutanea tarda, pseudoporphyria, and lupus erythematosus must be distinguished from PPD.22-24 Photosensitizing medications such tetracyclines, thiazide diuretics, sulfonamides, griseofulvin, and sulfonylureas should be considered. Airborne contact dermatitis may resemble PPD, as when poison ivy is burned and is exposed to the skin in sites of airborne contact.20 Excessive solar exposure is popular, particularly among adolescents, so sunburn and sunburnlike reactions can be noteworthy.25,26
Treatment
Phytophotodermatitis can be treated with topical steroids, sometimes adding an oral antihistamine, and occasionally oral steroids.2-4 Localized pain or a burning sensation should respond to therapy. Alternatively, a cold compress applied to the skin can relieve the pain and pruritus, and the burn can be debrided and dressed daily with silver sulfadiazine plus an oral nonsteroidal anti-inflammatory drug. This eruption should be self-limited as long as it is recognized early and the cause avoided. Management of acute exposure includes prompt application of soap and water and avoidance of UV light exposure for 48 to 72 hours to prevent psoralen photoactivation.
Because PPD is essentially a chemical burn, a burn protocol and possible referral to a burn center may be needed, whether the reaction is acute or widespread.11,12,14,27,28 Surgical debridement and skin grafting rarely may be mandated.14 Postinflammatory hyperpigmentation may ensue as the dermatitis resolves but is not common.
The best approach for PPD is prevention (Figure 2). Individuals who are at risk should be aware of their surroundings and potential plants of concern and employ personal protective equipment to shield the skin from plant sap, which should be promptly removed if it comes in contact with the skin.

- Zhang R, Zhu W. Phytophotodermatitis due to Chinese herbal medicine decoction. Indian J Dermatol. 2011;56:329-331.
- Harshman J, Quan Y, Hsiang D. Phytophotodermatitis: rash with many faces. Can Fam Physician. 2017;63:938-940.
- Imen MS, Ahmadabadi A, Tavousi SH, et al. The curious cases of burn by fig tree leaves. Indian J Dermatol. 2019;64:71-73.
- Hankinson A, Lloyd B, Alweis R. Lime-induced phytophotodermatitis [published online September 29, 2014]. J Community Hosp Intern Med Perspect. doi:10.3402/jchimp.v4.25090
- Abramowitz AI, Resnik KS, Cohen KR. Margarita photodermatitis. N Engl J Med. 2013;328:891.
- Quaak MS, Martens H, Hassing RJ, et al. The sunny side of lime. J Travel Med. 2012;19:327-328.
- Rosenthal O. Berloque dermatitis: Berliner Dermatologische Gesellschaft. Dermatol Zeitschrift. 1925;42:295.
- Choi JY, Hwang S, Lee SH, et al. Asymptomatic hyperpigmentation without preceding inflammation as a clinical feature of citrus fruits–induced phytophotodermatitis. Ann Dermatol. 2018;30:75-78.
- Wynn P, Bell S. Phytophotodermatitis in grounds operatives. Occup Med (Lond). 2005;55:393-395.
- Klimaszyk P, Klimaszyk D, Piotrowiak M, et al. Unusual complications after occupational exposure to giant hogweed (Heracleum mantegazzianum): a case report. Int J Occup Med Environ Health. 2014;27:141-144.
- Downs JW, Cumpston KL, Feldman MJ. Giant hogweed phytophotodermatitis. Clin Toxicol (Phila). 2019;57:822-823.
- Maso MJ, Ruszkowski AM, Bauerle J, et al. Celery phytophotodermatitis in a chef. Arch Dermatol. 1991;127:912-913.
- Derraik JG, Rademaker M. Phytophotodermatitis caused by contact with a fig tree (Ficus carica). New Zealand Med J. 2007;120:U2720.
- Chan JC, Sullivan PJ, O’Sullivan MJ, et al. Full thickness burn caused by exposure to giant hogweed: delayed presentation, histological features and surgical management. J Plast Reconstr Aesthet Surg. 2011;64:128-130.
- Bosanac SS, Clark AK, Sivamani RK. Phytophotodermatitis related to carrot extract–containing sunscreen. Dermatol Online J. 2018;24:1-3.
- Sforza M, Andjelkov K, Zaccheddu R. Severe burn on 81% of body surface after sun tanning. Ulus Travma Acil Cerrahi Derg. 2013;19:383-384.
- Mioduszewski M, Beecker J. Phytophotodermatitis from making sangria: a phototoxic reaction to lime and lemon juice. CMAJ. 2015;187:756.
- Torrents R, Schmitt C, Domangé B, et al. Phytophotodermatitis with Peucedanum paniculatum: an endemic species to Corsica. Clin Toxicol (Phila). 2019;57:68-69.
- Sarhane KA, Ibrahim A, Fagan SP, et al. Phytophotodermatitis. Eplasty. 2013;13:ic57.
- DeLeo VA, Suarez SM, Maso MJ. Photoallergic contact dermatitis. results of photopatch testing in New York, 1985 to 1990. Arch Dermatol. 1992;128:1513-1518.
- Kimyon RS, Warshaw EM. Airborne allergic contact dermatitis: management and responsible allergens on the American Contact Dermatitis Society Core Series. Dermatitis. 2019;30:106-115.
- Miteva L, Broshtilova V, Schwartz RA. Unusual clinical manifestations of chronic discoid lupus erythematosus. Serbian J Dermatol Venereol. 2014;6:69-72.
- Handler NS, Handler MZ, Stephany MP, et al. Porphyria cutanea tarda: an intriguing genetic disease and marker. Int J Dermatol. 2017;56:E106-E117.
- Papadopoulos AJ, Schwartz RA, Fekete Z, et al. Pseudoporphyria: an atypical variant resembling toxic epidermal necrolysis. J Cutan Med Surg. 2001;5:479-485.
- Jasterzbski TJ, Janniger EJ, Schwartz RA. Adolescent tanning practices: understanding the popularity of excessive ultraviolet light exposure. In: Or
anje A, Al-Mutairi N, Shwayder T, eds. Practical Pediatric Dermatology. Controversies in Diagnosis and Treatment. Springer Verlag; 2016:177-185. - Lai YC, Janniger EJ, Schwartz RA. Solar protection policy in school children: proposals for progress. In: Oranje A, Al-Mutairi N, Shwayder T, eds. Practical Pediatric Dermatology. Controversies in Diagnosis and Treatment. Springer Verlag; 2016:165-176.
- Lagey K, Duinslaeger L, Vanderkelen A. Burns induced by plants. Burns. 1995;21:542-543.
- Redgrave N, Solomon J. Severe phytophotodermatitis from fig sap: a little known phenomenon. BMJ Case Rep. 2021;14:e238745.
- Zhang R, Zhu W. Phytophotodermatitis due to Chinese herbal medicine decoction. Indian J Dermatol. 2011;56:329-331.
- Harshman J, Quan Y, Hsiang D. Phytophotodermatitis: rash with many faces. Can Fam Physician. 2017;63:938-940.
- Imen MS, Ahmadabadi A, Tavousi SH, et al. The curious cases of burn by fig tree leaves. Indian J Dermatol. 2019;64:71-73.
- Hankinson A, Lloyd B, Alweis R. Lime-induced phytophotodermatitis [published online September 29, 2014]. J Community Hosp Intern Med Perspect. doi:10.3402/jchimp.v4.25090
- Abramowitz AI, Resnik KS, Cohen KR. Margarita photodermatitis. N Engl J Med. 2013;328:891.
- Quaak MS, Martens H, Hassing RJ, et al. The sunny side of lime. J Travel Med. 2012;19:327-328.
- Rosenthal O. Berloque dermatitis: Berliner Dermatologische Gesellschaft. Dermatol Zeitschrift. 1925;42:295.
- Choi JY, Hwang S, Lee SH, et al. Asymptomatic hyperpigmentation without preceding inflammation as a clinical feature of citrus fruits–induced phytophotodermatitis. Ann Dermatol. 2018;30:75-78.
- Wynn P, Bell S. Phytophotodermatitis in grounds operatives. Occup Med (Lond). 2005;55:393-395.
- Klimaszyk P, Klimaszyk D, Piotrowiak M, et al. Unusual complications after occupational exposure to giant hogweed (Heracleum mantegazzianum): a case report. Int J Occup Med Environ Health. 2014;27:141-144.
- Downs JW, Cumpston KL, Feldman MJ. Giant hogweed phytophotodermatitis. Clin Toxicol (Phila). 2019;57:822-823.
- Maso MJ, Ruszkowski AM, Bauerle J, et al. Celery phytophotodermatitis in a chef. Arch Dermatol. 1991;127:912-913.
- Derraik JG, Rademaker M. Phytophotodermatitis caused by contact with a fig tree (Ficus carica). New Zealand Med J. 2007;120:U2720.
- Chan JC, Sullivan PJ, O’Sullivan MJ, et al. Full thickness burn caused by exposure to giant hogweed: delayed presentation, histological features and surgical management. J Plast Reconstr Aesthet Surg. 2011;64:128-130.
- Bosanac SS, Clark AK, Sivamani RK. Phytophotodermatitis related to carrot extract–containing sunscreen. Dermatol Online J. 2018;24:1-3.
- Sforza M, Andjelkov K, Zaccheddu R. Severe burn on 81% of body surface after sun tanning. Ulus Travma Acil Cerrahi Derg. 2013;19:383-384.
- Mioduszewski M, Beecker J. Phytophotodermatitis from making sangria: a phototoxic reaction to lime and lemon juice. CMAJ. 2015;187:756.
- Torrents R, Schmitt C, Domangé B, et al. Phytophotodermatitis with Peucedanum paniculatum: an endemic species to Corsica. Clin Toxicol (Phila). 2019;57:68-69.
- Sarhane KA, Ibrahim A, Fagan SP, et al. Phytophotodermatitis. Eplasty. 2013;13:ic57.
- DeLeo VA, Suarez SM, Maso MJ. Photoallergic contact dermatitis. results of photopatch testing in New York, 1985 to 1990. Arch Dermatol. 1992;128:1513-1518.
- Kimyon RS, Warshaw EM. Airborne allergic contact dermatitis: management and responsible allergens on the American Contact Dermatitis Society Core Series. Dermatitis. 2019;30:106-115.
- Miteva L, Broshtilova V, Schwartz RA. Unusual clinical manifestations of chronic discoid lupus erythematosus. Serbian J Dermatol Venereol. 2014;6:69-72.
- Handler NS, Handler MZ, Stephany MP, et al. Porphyria cutanea tarda: an intriguing genetic disease and marker. Int J Dermatol. 2017;56:E106-E117.
- Papadopoulos AJ, Schwartz RA, Fekete Z, et al. Pseudoporphyria: an atypical variant resembling toxic epidermal necrolysis. J Cutan Med Surg. 2001;5:479-485.
- Jasterzbski TJ, Janniger EJ, Schwartz RA. Adolescent tanning practices: understanding the popularity of excessive ultraviolet light exposure. In: Or
anje A, Al-Mutairi N, Shwayder T, eds. Practical Pediatric Dermatology. Controversies in Diagnosis and Treatment. Springer Verlag; 2016:177-185. - Lai YC, Janniger EJ, Schwartz RA. Solar protection policy in school children: proposals for progress. In: Oranje A, Al-Mutairi N, Shwayder T, eds. Practical Pediatric Dermatology. Controversies in Diagnosis and Treatment. Springer Verlag; 2016:165-176.
- Lagey K, Duinslaeger L, Vanderkelen A. Burns induced by plants. Burns. 1995;21:542-543.
- Redgrave N, Solomon J. Severe phytophotodermatitis from fig sap: a little known phenomenon. BMJ Case Rep. 2021;14:e238745.
Practice Points
- Phytophotodermatitis (PPD) can be both an occupational and recreational dermatosis.
- Phytophotodermatitis is a nonallergic contact dermatitis and thus is independent of the immune system, so prior sensitization is not required.
- Individuals who work with plants should be aware of PPD and methods of prevention.
- Phytophotodermatitis may be evident only as asymptomatic hyperpigmentation.
Erethism Mercurialis and Reactions to Elemental Mercury
Evidence of human exposure to mercury dates as far back as the Egyptians in 1500
Mercury release in the environment primarily is a function of human activity, including coal-fired power plants, residential heating, and mining.9,10 Mercury from these sources is commonly found in the sediment of lakes and bays, where it is enzymatically converted to methylmercury by aquatic microorganisms; subsequent food chain biomagnification results in elevated mercury levels in apex predators. Substantial release of mercury into the environment also can be attributed to health care facilities from their use of thermometers containing 0.5 to 3 g of elemental mercury,11 blood pressure monitors, and medical waste incinerators.5
Mercury has been reported as the second most common cause of heavy metal poisoning after lead.12 Standards from the US Food and Drug Administration dictate that methylmercury levels in fish and wheat products must not exceed 1 ppm.13 Most plant and animal food sources contain methylmercury at levels between 0.0001 and 0.01 ppm; mercury concentrations are especially high in tuna, averaging 0.4 ppm, while larger predatory fish contain levels in excess of 1 ppm.14 The use of mercury-containing cosmetic products also presents a substantial exposure risk to consumers.5,10 In one study, 3.3% of skin-lightening creams and soaps purchased within the United States contained concentrations of mercury exceeding 1000 ppm.15
We describe a case of mercury toxicity resulting from intentional injection of liquid mercury into the right antecubital fossa in a suicide attempt.
Case Report
A 31-year-old woman presented to the family practice center for evaluation of a firm stained area on the skin of the right arm. She reported increasing anxiety, depression, tremors, irritability, and difficulty concentrating over the last 6 months. She denied headache and joint or muscle pain. Four years earlier, she had broken apart a thermometer and injected approximately 0.7 mL of its contents into the right arm in a suicide attempt. She intended to inject the thermometer’s contents directly into a vein, but the material instead entered the surrounding tissue. She denied notable pain or itching overlying the injection site. Her medications included aripiprazole and buspirone. She noted that she smoked half a pack of cigarettes per day and had a history of methamphetamine abuse. She was homeless and unemployed. Physical examination revealed an anxious tremulous woman with an erythematous to bluish gray, firm plaque on the right antecubital fossa (Figure 1). There were no notable tremors and no gait disturbance.
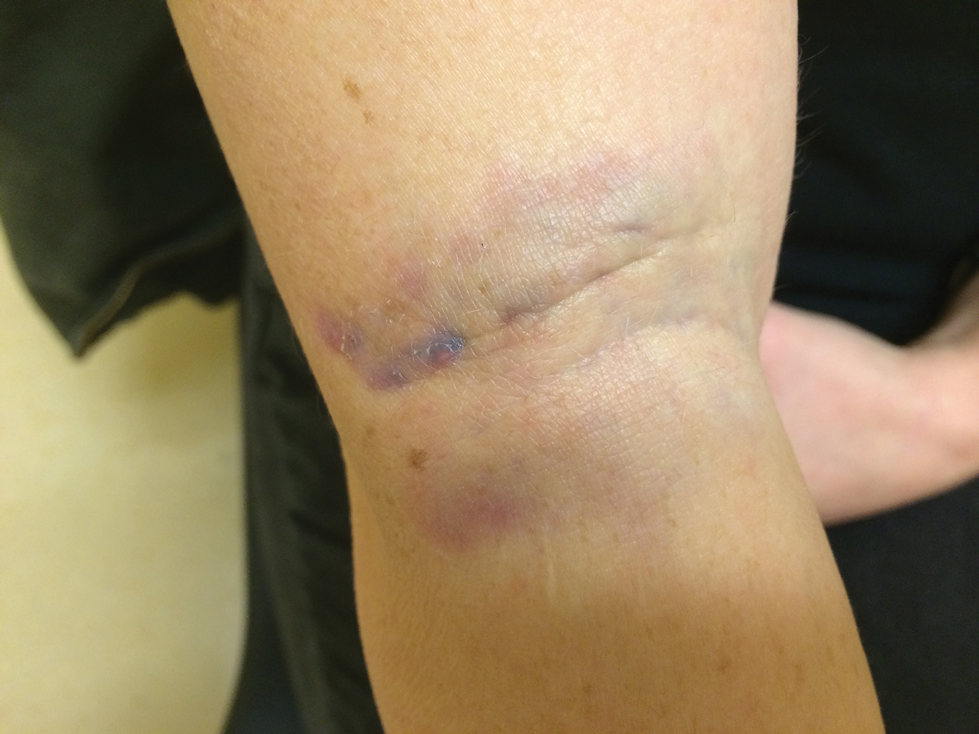
Her blood mercury level was greater than 100 µg/L and urine mercury was 477 µg/g (reference ranges, 1–8 μg/L and 4–5 μg/L, respectively). A radiograph of the right elbow area revealed scattered punctate foci of increased density within or overlying the anterolateral elbow soft tissues. She was diagnosed with mercury granuloma causing chronic mercury elevation. She underwent excision of the granuloma (Figure 2) with endovascular surgery via an elliptical incision. The patient was subsequently lost to follow-up.
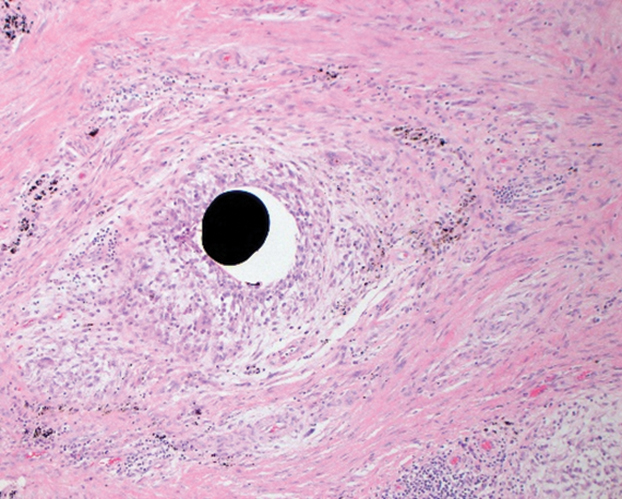
Comment
Elemental mercury is a silver liquid at room temperature that spontaneously evaporates to form mercury vapor, an invisible, odorless, toxic gas. Accidental cutaneous exposure typically is safely managed by washing exposed skin with soap and water,16 though there is a potential risk for systemic absorption, especially when the skin is inflamed. When metallic mercury is subcutaneously injected, it is advised to promptly excise all subcutaneous areas containing mercury, regardless of any symptoms of systemic toxicity. Patients should subsequently be monitored for signs of both central nervous system (CNS) and renal deficits, undergo chelation therapy when systemic effects are apparent, and finally receive psychiatric consultation and treatment when necessary.17
Inorganic mercury compounds are formed when elemental mercury combines with sulfur or oxygen and often take the form of mercury salts, which appear as white crystals.16 These salts occur naturally in the environment and are used in pesticides, antiseptics, and skin-lightening creams and soaps.18
Methylmercury is a highly toxic, organic compound that is capable of crossing the placental and blood-brain barriers. It is the most common organic mercury compound found in the environment.16 Most humans have trace amounts of methylmercury in their bodies, typically as a result of consuming seafood.5
Exposure to mercury most commonly occurs through chronic consumption of methylmercury in seafood or acute inhalation of elemental mercury vapors.9 Iatrogenic cases of mercury exposure via injection also have been reported in the literature, including a case resulting in acute poisoning due to peritoneal lavage with mercury bichloride.19 Acute mercury-induced pulmonary damage typically resolves completely. However, there have been reported cases of exposure progressing to interstitial emphysema, pneumatocele, pneumothorax, pneumomediastinum, interstitial fibrosis, and chronic respiratory insufficiency, with examples of fatal acute respiratory distress syndrome being reported.5,16,20 Although individuals who inhale mercury vapors initially may be unaware of exposure due to little upper airway irritation, symptoms following an initial acute exposure may include ptyalism, a metallic taste, dysphagia, enteritis, diarrhea, nausea, renal damage, and CNS effects.16 Additionally, exposure may lead to confusion with signs and symptoms of metal fume fever, including shortness of breath, pleuritic chest pain, stomatitis, lethargy, and vomiting.20
Chronic exposure to mercury vapor can result in accumulation of mercury in the body, leading to neuropsychiatric, dermatologic, oropharyngeal, and renal manifestations. Sore throat, fever, headache, fatigue, dyspnea, chest pain, and pneumonitis are common.16 Typically, low-level exposure to elemental mercury does not lead to long-lasting health effects. However, individuals exposed to high-level elemental mercury vapors may require hospitalization. Treatment of acute mercury poisoning consists of removing the source of exposure, followed by cardiopulmonary support.16
Specific assays for mercury levels in blood and urine are useful to assess the level of exposure and risk to the patient. Blood mercury concentrations of 20 µg/L or below are considered within reference range; however, once blood and urine concentrations of mercury exceed 100 µg/L, clinical signs of acute mercury poisoning typically manifest.21 Chest radiographs can reveal pulmonary damage, while complete blood cell count, metabolic panel, and urinalysis can assess damage to other organs. Neuropsychiatric testing and nerve conduction studies may provide objective evidence of CNS toxicity. Assays for N-acetyl-β-D-glucosaminidase can provide an indication of early renal tubular dysfunction.16
Elemental mercury is not absorbed from the gastrointestinal tract, posing minimal risk for acute toxicity from ingestion. Generally, less than 10% of ingested inorganic mercury is absorbed from the gut, while elemental mercury is nonabsorbable.10 If an individual ingests a large amount of mercury, it may persist in the gastrointestinal tract for an extended period. Mercury is radiopaque, and abdominal radiographs should be obtained in all cases of ingestion.16
Mercury is toxic to the CNS and peripheral nervous system, resulting in erethism mercurialis, a constellation of neuropsychologic signs and symptoms including restlessness, irritability, insomnia, emotional lability, difficulty concentrating, and impaired memory. In severe cases, delirium and psychosis may develop. Other CNS effects include tremors, paresthesia, dysarthria, neuromuscular changes, headaches, polyneuropathy, and cerebellar ataxia, as well as ophthalmologic and audiologic impairment.5,16
Upon inhalation exposure, patients with respiratory concerns should be given oxygen. Bronchospasms are treated with bronchodilators; however, if multiple chemical exposures are suspected, bronchial-sensitizing agents may pose additional risks. Corticosteroids and antibiotics have been recommended for treatment of chemical pneumonitis, but their efficacy has not been substantiated.16
Skin reactions associated with skin contact to elemental mercury are rare. However, hives and dermatitis have been observed following accidental contact with inorganic mercury compounds.5 Manifestation in children chronically exposed to mercury includes a nonallergic hypersensitivity (acrodynia),5,17 which is characterized by pain and dusky pink discoloration in the hands and feet, most often seen in children chronically exposed to mercury absorbed from vapor inhalation or cutaneous exposure.16
Renal conditions associated with acute inhalation of elemental mercury vapor include proteinuria, nephrotic syndrome, temporary tubular dysfunction, acute tubular necrosis, and oliguric renal failure.16 Chronic exposure to inorganic mercury compounds also has been reported to cause renal damage.5 Chelation therapy should be performed for any symptomatic patient with a clear history of acute elemental mercury exposure.16 The most frequently used chelation agent in cases of acute inorganic mercury exposures is dimercaprol. In rare cases of mercury intoxication, hemodialysis is required in the treatment of renal failure and to expedite removal of dimercaprol-mercury complexes.16
Cardiovascular symptoms associated with acute inhalation of high levels of elemental mercury include tachycardia and hypertension.16 Increases in blood pressure, palpitations, and heart rate also have been observed in instances of acute elemental mercury exposure. Studies show that exposure to mercury increases both the risk for acute myocardial infarction as well as death from coronary heart and cardiovascular diseases.5
Conclusion
Mercury poisoning presents with varied neuropsychologic signs and symptoms. Our case provides insight into a unique route of exposure for mercury toxicity. In addition to the unusual presentation of a mercury granuloma, our case illustrates how surgical techniques can aid in removal of cutaneous reservoirs in the setting of percutaneous exposure.
- History of mercury. Government of Canada website. Modified April 26, 2010. Accessed March 11, 2021. https://www.canada.ca/en/environment-climate-change/services/pollutants/mercury-environment/about/history.html
- Dartmouth Toxic Metals Superfund Research Program website. Accessed March 11, 2021. https://sites.dartmouth.edu/toxmetal/
- Norn S, Permin H, Kruse E, et al. Mercury—a major agent in the history of medicine and alchemy [in Danish]. Dan Medicinhist Arbog. 2008;36:21-40.
- Waldron HA. Did the Mad Hatter have mercury poisoning? Br Med J (Clin Res Ed). 1983;287:1961.
- Poulin J, Gibb H. Mercury: assessing the environmental burden of disease at national and local levels. WHO Environmental Burden of Disease Series No. 16. World Health Organization; 2008.
- Charcot JM. Clinical lectures of the diseases of the nervous system. In: Kinnier Wilson SA. The Landmark Library of Neurology and Neurosurgery. Gryphon Editions; 1994:186.
- Kinnier Wilson SA. Neurology. In: Kinnier Wilson SA. The Landmark Library of Neurology and Neurosurgery. Gryphon Editions; 1994:739-740.
- Harada M. Minamata disease: methylmercury poisoning in Japan caused by environmental pollution. Crit Rev Toxicol. 1995;25:1-24.
- Mercury and health. World Health Organization website. Updated March 31, 2017. Accessed March 12, 2021. http://www.whoint/mediacentre/factsheets/fs361/en/
- Olson DA. Mercury toxicity. Updated November 5, 2018. Accessed March 12, 2021.http://emedicine.medscape.com/article/1175560-overview
- Mercury thermometers. Environmental Protection Agency website. Updated June 26, 2018. https://www.epa.gov/mercury/mercury-thermometers
- Jao-Tan C, Pope E. Cutaneous poisoning syndromes in children: a review. Curr Opin Pediatr. 2006;18:410-416.
- US Department of Health and Human Services: Public Health Service Agency for Toxic Substances and Disease Registry. Toxicological profile for mercury: regulations and advisories. Published March 1999. Accessed March 23, 2021. https://www.atsdr.cdc.gov/toxprofiles/tp46.pdf
- US Food and Drug Administration. Mercury levels in commercial fish and shellfish (1990-2012). Updated October 25, 2017. Accessed March 16, 2021. https://www.fda.gov/food/metals-and-your-food/mercury-levels-commercial-fish-and-shellfish-1990-2012
- Hamann CR, Boonchai W, Wen L, et al. Spectrometric analysis of mercury content in 549 skin-lightening products: is mercury toxicity a hidden global health hazard? J Am Acad Dermatol. 2014;70:281-287.e3.
- Mercury. Managing Hazardous Materials Incidents. Agency for Toxic Substances and Disease Registry website. Accessed March 16, 2021. https://www.atsdr.cdc.gov/MHMI/mmg46.pdf
- Krohn IT, Solof A, Mobini J, et al. Subcutaneous injection of metallic mercury. JAMA. 1980;243:548-549.
- Lai O, Parsi KK, Wu D, et al. Mercury toxicity presenting acrodynia and a papulovesicular eruption in a 5-year-old girl. Dermatol Online J. 2016;16;22:13030/qt6444r7nc.
- Dolianiti M, Tasiopoulou K, Kalostou A, et al. Mercury bichloride iatrogenic poisoning: a case report. J Clin Toxicol. 2016;6:2. doi:10.4172/2161-0495.1000290
- Broussard LA, Hammett-Stabler CA, Winecker RE, et al. The toxicology of mercury. Lab Med. 2002;33:614-625. doi:10.1309/5HY1-V3NE-2LFL-P9MT
- Byeong-Jin Y, Byoung-Gwon K, Man-Joong J, et al. Evaluation of mercury exposure levels, clinical diagnosis and treatment for mercury intoxication. Ann Occup Environ Med. 2016;28:5.
Evidence of human exposure to mercury dates as far back as the Egyptians in 1500
Mercury release in the environment primarily is a function of human activity, including coal-fired power plants, residential heating, and mining.9,10 Mercury from these sources is commonly found in the sediment of lakes and bays, where it is enzymatically converted to methylmercury by aquatic microorganisms; subsequent food chain biomagnification results in elevated mercury levels in apex predators. Substantial release of mercury into the environment also can be attributed to health care facilities from their use of thermometers containing 0.5 to 3 g of elemental mercury,11 blood pressure monitors, and medical waste incinerators.5
Mercury has been reported as the second most common cause of heavy metal poisoning after lead.12 Standards from the US Food and Drug Administration dictate that methylmercury levels in fish and wheat products must not exceed 1 ppm.13 Most plant and animal food sources contain methylmercury at levels between 0.0001 and 0.01 ppm; mercury concentrations are especially high in tuna, averaging 0.4 ppm, while larger predatory fish contain levels in excess of 1 ppm.14 The use of mercury-containing cosmetic products also presents a substantial exposure risk to consumers.5,10 In one study, 3.3% of skin-lightening creams and soaps purchased within the United States contained concentrations of mercury exceeding 1000 ppm.15
We describe a case of mercury toxicity resulting from intentional injection of liquid mercury into the right antecubital fossa in a suicide attempt.
Case Report
A 31-year-old woman presented to the family practice center for evaluation of a firm stained area on the skin of the right arm. She reported increasing anxiety, depression, tremors, irritability, and difficulty concentrating over the last 6 months. She denied headache and joint or muscle pain. Four years earlier, she had broken apart a thermometer and injected approximately 0.7 mL of its contents into the right arm in a suicide attempt. She intended to inject the thermometer’s contents directly into a vein, but the material instead entered the surrounding tissue. She denied notable pain or itching overlying the injection site. Her medications included aripiprazole and buspirone. She noted that she smoked half a pack of cigarettes per day and had a history of methamphetamine abuse. She was homeless and unemployed. Physical examination revealed an anxious tremulous woman with an erythematous to bluish gray, firm plaque on the right antecubital fossa (Figure 1). There were no notable tremors and no gait disturbance.

Her blood mercury level was greater than 100 µg/L and urine mercury was 477 µg/g (reference ranges, 1–8 μg/L and 4–5 μg/L, respectively). A radiograph of the right elbow area revealed scattered punctate foci of increased density within or overlying the anterolateral elbow soft tissues. She was diagnosed with mercury granuloma causing chronic mercury elevation. She underwent excision of the granuloma (Figure 2) with endovascular surgery via an elliptical incision. The patient was subsequently lost to follow-up.

Comment
Elemental mercury is a silver liquid at room temperature that spontaneously evaporates to form mercury vapor, an invisible, odorless, toxic gas. Accidental cutaneous exposure typically is safely managed by washing exposed skin with soap and water,16 though there is a potential risk for systemic absorption, especially when the skin is inflamed. When metallic mercury is subcutaneously injected, it is advised to promptly excise all subcutaneous areas containing mercury, regardless of any symptoms of systemic toxicity. Patients should subsequently be monitored for signs of both central nervous system (CNS) and renal deficits, undergo chelation therapy when systemic effects are apparent, and finally receive psychiatric consultation and treatment when necessary.17
Inorganic mercury compounds are formed when elemental mercury combines with sulfur or oxygen and often take the form of mercury salts, which appear as white crystals.16 These salts occur naturally in the environment and are used in pesticides, antiseptics, and skin-lightening creams and soaps.18
Methylmercury is a highly toxic, organic compound that is capable of crossing the placental and blood-brain barriers. It is the most common organic mercury compound found in the environment.16 Most humans have trace amounts of methylmercury in their bodies, typically as a result of consuming seafood.5
Exposure to mercury most commonly occurs through chronic consumption of methylmercury in seafood or acute inhalation of elemental mercury vapors.9 Iatrogenic cases of mercury exposure via injection also have been reported in the literature, including a case resulting in acute poisoning due to peritoneal lavage with mercury bichloride.19 Acute mercury-induced pulmonary damage typically resolves completely. However, there have been reported cases of exposure progressing to interstitial emphysema, pneumatocele, pneumothorax, pneumomediastinum, interstitial fibrosis, and chronic respiratory insufficiency, with examples of fatal acute respiratory distress syndrome being reported.5,16,20 Although individuals who inhale mercury vapors initially may be unaware of exposure due to little upper airway irritation, symptoms following an initial acute exposure may include ptyalism, a metallic taste, dysphagia, enteritis, diarrhea, nausea, renal damage, and CNS effects.16 Additionally, exposure may lead to confusion with signs and symptoms of metal fume fever, including shortness of breath, pleuritic chest pain, stomatitis, lethargy, and vomiting.20
Chronic exposure to mercury vapor can result in accumulation of mercury in the body, leading to neuropsychiatric, dermatologic, oropharyngeal, and renal manifestations. Sore throat, fever, headache, fatigue, dyspnea, chest pain, and pneumonitis are common.16 Typically, low-level exposure to elemental mercury does not lead to long-lasting health effects. However, individuals exposed to high-level elemental mercury vapors may require hospitalization. Treatment of acute mercury poisoning consists of removing the source of exposure, followed by cardiopulmonary support.16
Specific assays for mercury levels in blood and urine are useful to assess the level of exposure and risk to the patient. Blood mercury concentrations of 20 µg/L or below are considered within reference range; however, once blood and urine concentrations of mercury exceed 100 µg/L, clinical signs of acute mercury poisoning typically manifest.21 Chest radiographs can reveal pulmonary damage, while complete blood cell count, metabolic panel, and urinalysis can assess damage to other organs. Neuropsychiatric testing and nerve conduction studies may provide objective evidence of CNS toxicity. Assays for N-acetyl-β-D-glucosaminidase can provide an indication of early renal tubular dysfunction.16
Elemental mercury is not absorbed from the gastrointestinal tract, posing minimal risk for acute toxicity from ingestion. Generally, less than 10% of ingested inorganic mercury is absorbed from the gut, while elemental mercury is nonabsorbable.10 If an individual ingests a large amount of mercury, it may persist in the gastrointestinal tract for an extended period. Mercury is radiopaque, and abdominal radiographs should be obtained in all cases of ingestion.16
Mercury is toxic to the CNS and peripheral nervous system, resulting in erethism mercurialis, a constellation of neuropsychologic signs and symptoms including restlessness, irritability, insomnia, emotional lability, difficulty concentrating, and impaired memory. In severe cases, delirium and psychosis may develop. Other CNS effects include tremors, paresthesia, dysarthria, neuromuscular changes, headaches, polyneuropathy, and cerebellar ataxia, as well as ophthalmologic and audiologic impairment.5,16
Upon inhalation exposure, patients with respiratory concerns should be given oxygen. Bronchospasms are treated with bronchodilators; however, if multiple chemical exposures are suspected, bronchial-sensitizing agents may pose additional risks. Corticosteroids and antibiotics have been recommended for treatment of chemical pneumonitis, but their efficacy has not been substantiated.16
Skin reactions associated with skin contact to elemental mercury are rare. However, hives and dermatitis have been observed following accidental contact with inorganic mercury compounds.5 Manifestation in children chronically exposed to mercury includes a nonallergic hypersensitivity (acrodynia),5,17 which is characterized by pain and dusky pink discoloration in the hands and feet, most often seen in children chronically exposed to mercury absorbed from vapor inhalation or cutaneous exposure.16
Renal conditions associated with acute inhalation of elemental mercury vapor include proteinuria, nephrotic syndrome, temporary tubular dysfunction, acute tubular necrosis, and oliguric renal failure.16 Chronic exposure to inorganic mercury compounds also has been reported to cause renal damage.5 Chelation therapy should be performed for any symptomatic patient with a clear history of acute elemental mercury exposure.16 The most frequently used chelation agent in cases of acute inorganic mercury exposures is dimercaprol. In rare cases of mercury intoxication, hemodialysis is required in the treatment of renal failure and to expedite removal of dimercaprol-mercury complexes.16
Cardiovascular symptoms associated with acute inhalation of high levels of elemental mercury include tachycardia and hypertension.16 Increases in blood pressure, palpitations, and heart rate also have been observed in instances of acute elemental mercury exposure. Studies show that exposure to mercury increases both the risk for acute myocardial infarction as well as death from coronary heart and cardiovascular diseases.5
Conclusion
Mercury poisoning presents with varied neuropsychologic signs and symptoms. Our case provides insight into a unique route of exposure for mercury toxicity. In addition to the unusual presentation of a mercury granuloma, our case illustrates how surgical techniques can aid in removal of cutaneous reservoirs in the setting of percutaneous exposure.
Evidence of human exposure to mercury dates as far back as the Egyptians in 1500
Mercury release in the environment primarily is a function of human activity, including coal-fired power plants, residential heating, and mining.9,10 Mercury from these sources is commonly found in the sediment of lakes and bays, where it is enzymatically converted to methylmercury by aquatic microorganisms; subsequent food chain biomagnification results in elevated mercury levels in apex predators. Substantial release of mercury into the environment also can be attributed to health care facilities from their use of thermometers containing 0.5 to 3 g of elemental mercury,11 blood pressure monitors, and medical waste incinerators.5
Mercury has been reported as the second most common cause of heavy metal poisoning after lead.12 Standards from the US Food and Drug Administration dictate that methylmercury levels in fish and wheat products must not exceed 1 ppm.13 Most plant and animal food sources contain methylmercury at levels between 0.0001 and 0.01 ppm; mercury concentrations are especially high in tuna, averaging 0.4 ppm, while larger predatory fish contain levels in excess of 1 ppm.14 The use of mercury-containing cosmetic products also presents a substantial exposure risk to consumers.5,10 In one study, 3.3% of skin-lightening creams and soaps purchased within the United States contained concentrations of mercury exceeding 1000 ppm.15
We describe a case of mercury toxicity resulting from intentional injection of liquid mercury into the right antecubital fossa in a suicide attempt.
Case Report
A 31-year-old woman presented to the family practice center for evaluation of a firm stained area on the skin of the right arm. She reported increasing anxiety, depression, tremors, irritability, and difficulty concentrating over the last 6 months. She denied headache and joint or muscle pain. Four years earlier, she had broken apart a thermometer and injected approximately 0.7 mL of its contents into the right arm in a suicide attempt. She intended to inject the thermometer’s contents directly into a vein, but the material instead entered the surrounding tissue. She denied notable pain or itching overlying the injection site. Her medications included aripiprazole and buspirone. She noted that she smoked half a pack of cigarettes per day and had a history of methamphetamine abuse. She was homeless and unemployed. Physical examination revealed an anxious tremulous woman with an erythematous to bluish gray, firm plaque on the right antecubital fossa (Figure 1). There were no notable tremors and no gait disturbance.

Her blood mercury level was greater than 100 µg/L and urine mercury was 477 µg/g (reference ranges, 1–8 μg/L and 4–5 μg/L, respectively). A radiograph of the right elbow area revealed scattered punctate foci of increased density within or overlying the anterolateral elbow soft tissues. She was diagnosed with mercury granuloma causing chronic mercury elevation. She underwent excision of the granuloma (Figure 2) with endovascular surgery via an elliptical incision. The patient was subsequently lost to follow-up.

Comment
Elemental mercury is a silver liquid at room temperature that spontaneously evaporates to form mercury vapor, an invisible, odorless, toxic gas. Accidental cutaneous exposure typically is safely managed by washing exposed skin with soap and water,16 though there is a potential risk for systemic absorption, especially when the skin is inflamed. When metallic mercury is subcutaneously injected, it is advised to promptly excise all subcutaneous areas containing mercury, regardless of any symptoms of systemic toxicity. Patients should subsequently be monitored for signs of both central nervous system (CNS) and renal deficits, undergo chelation therapy when systemic effects are apparent, and finally receive psychiatric consultation and treatment when necessary.17
Inorganic mercury compounds are formed when elemental mercury combines with sulfur or oxygen and often take the form of mercury salts, which appear as white crystals.16 These salts occur naturally in the environment and are used in pesticides, antiseptics, and skin-lightening creams and soaps.18
Methylmercury is a highly toxic, organic compound that is capable of crossing the placental and blood-brain barriers. It is the most common organic mercury compound found in the environment.16 Most humans have trace amounts of methylmercury in their bodies, typically as a result of consuming seafood.5
Exposure to mercury most commonly occurs through chronic consumption of methylmercury in seafood or acute inhalation of elemental mercury vapors.9 Iatrogenic cases of mercury exposure via injection also have been reported in the literature, including a case resulting in acute poisoning due to peritoneal lavage with mercury bichloride.19 Acute mercury-induced pulmonary damage typically resolves completely. However, there have been reported cases of exposure progressing to interstitial emphysema, pneumatocele, pneumothorax, pneumomediastinum, interstitial fibrosis, and chronic respiratory insufficiency, with examples of fatal acute respiratory distress syndrome being reported.5,16,20 Although individuals who inhale mercury vapors initially may be unaware of exposure due to little upper airway irritation, symptoms following an initial acute exposure may include ptyalism, a metallic taste, dysphagia, enteritis, diarrhea, nausea, renal damage, and CNS effects.16 Additionally, exposure may lead to confusion with signs and symptoms of metal fume fever, including shortness of breath, pleuritic chest pain, stomatitis, lethargy, and vomiting.20
Chronic exposure to mercury vapor can result in accumulation of mercury in the body, leading to neuropsychiatric, dermatologic, oropharyngeal, and renal manifestations. Sore throat, fever, headache, fatigue, dyspnea, chest pain, and pneumonitis are common.16 Typically, low-level exposure to elemental mercury does not lead to long-lasting health effects. However, individuals exposed to high-level elemental mercury vapors may require hospitalization. Treatment of acute mercury poisoning consists of removing the source of exposure, followed by cardiopulmonary support.16
Specific assays for mercury levels in blood and urine are useful to assess the level of exposure and risk to the patient. Blood mercury concentrations of 20 µg/L or below are considered within reference range; however, once blood and urine concentrations of mercury exceed 100 µg/L, clinical signs of acute mercury poisoning typically manifest.21 Chest radiographs can reveal pulmonary damage, while complete blood cell count, metabolic panel, and urinalysis can assess damage to other organs. Neuropsychiatric testing and nerve conduction studies may provide objective evidence of CNS toxicity. Assays for N-acetyl-β-D-glucosaminidase can provide an indication of early renal tubular dysfunction.16
Elemental mercury is not absorbed from the gastrointestinal tract, posing minimal risk for acute toxicity from ingestion. Generally, less than 10% of ingested inorganic mercury is absorbed from the gut, while elemental mercury is nonabsorbable.10 If an individual ingests a large amount of mercury, it may persist in the gastrointestinal tract for an extended period. Mercury is radiopaque, and abdominal radiographs should be obtained in all cases of ingestion.16
Mercury is toxic to the CNS and peripheral nervous system, resulting in erethism mercurialis, a constellation of neuropsychologic signs and symptoms including restlessness, irritability, insomnia, emotional lability, difficulty concentrating, and impaired memory. In severe cases, delirium and psychosis may develop. Other CNS effects include tremors, paresthesia, dysarthria, neuromuscular changes, headaches, polyneuropathy, and cerebellar ataxia, as well as ophthalmologic and audiologic impairment.5,16
Upon inhalation exposure, patients with respiratory concerns should be given oxygen. Bronchospasms are treated with bronchodilators; however, if multiple chemical exposures are suspected, bronchial-sensitizing agents may pose additional risks. Corticosteroids and antibiotics have been recommended for treatment of chemical pneumonitis, but their efficacy has not been substantiated.16
Skin reactions associated with skin contact to elemental mercury are rare. However, hives and dermatitis have been observed following accidental contact with inorganic mercury compounds.5 Manifestation in children chronically exposed to mercury includes a nonallergic hypersensitivity (acrodynia),5,17 which is characterized by pain and dusky pink discoloration in the hands and feet, most often seen in children chronically exposed to mercury absorbed from vapor inhalation or cutaneous exposure.16
Renal conditions associated with acute inhalation of elemental mercury vapor include proteinuria, nephrotic syndrome, temporary tubular dysfunction, acute tubular necrosis, and oliguric renal failure.16 Chronic exposure to inorganic mercury compounds also has been reported to cause renal damage.5 Chelation therapy should be performed for any symptomatic patient with a clear history of acute elemental mercury exposure.16 The most frequently used chelation agent in cases of acute inorganic mercury exposures is dimercaprol. In rare cases of mercury intoxication, hemodialysis is required in the treatment of renal failure and to expedite removal of dimercaprol-mercury complexes.16
Cardiovascular symptoms associated with acute inhalation of high levels of elemental mercury include tachycardia and hypertension.16 Increases in blood pressure, palpitations, and heart rate also have been observed in instances of acute elemental mercury exposure. Studies show that exposure to mercury increases both the risk for acute myocardial infarction as well as death from coronary heart and cardiovascular diseases.5
Conclusion
Mercury poisoning presents with varied neuropsychologic signs and symptoms. Our case provides insight into a unique route of exposure for mercury toxicity. In addition to the unusual presentation of a mercury granuloma, our case illustrates how surgical techniques can aid in removal of cutaneous reservoirs in the setting of percutaneous exposure.
- History of mercury. Government of Canada website. Modified April 26, 2010. Accessed March 11, 2021. https://www.canada.ca/en/environment-climate-change/services/pollutants/mercury-environment/about/history.html
- Dartmouth Toxic Metals Superfund Research Program website. Accessed March 11, 2021. https://sites.dartmouth.edu/toxmetal/
- Norn S, Permin H, Kruse E, et al. Mercury—a major agent in the history of medicine and alchemy [in Danish]. Dan Medicinhist Arbog. 2008;36:21-40.
- Waldron HA. Did the Mad Hatter have mercury poisoning? Br Med J (Clin Res Ed). 1983;287:1961.
- Poulin J, Gibb H. Mercury: assessing the environmental burden of disease at national and local levels. WHO Environmental Burden of Disease Series No. 16. World Health Organization; 2008.
- Charcot JM. Clinical lectures of the diseases of the nervous system. In: Kinnier Wilson SA. The Landmark Library of Neurology and Neurosurgery. Gryphon Editions; 1994:186.
- Kinnier Wilson SA. Neurology. In: Kinnier Wilson SA. The Landmark Library of Neurology and Neurosurgery. Gryphon Editions; 1994:739-740.
- Harada M. Minamata disease: methylmercury poisoning in Japan caused by environmental pollution. Crit Rev Toxicol. 1995;25:1-24.
- Mercury and health. World Health Organization website. Updated March 31, 2017. Accessed March 12, 2021. http://www.whoint/mediacentre/factsheets/fs361/en/
- Olson DA. Mercury toxicity. Updated November 5, 2018. Accessed March 12, 2021.http://emedicine.medscape.com/article/1175560-overview
- Mercury thermometers. Environmental Protection Agency website. Updated June 26, 2018. https://www.epa.gov/mercury/mercury-thermometers
- Jao-Tan C, Pope E. Cutaneous poisoning syndromes in children: a review. Curr Opin Pediatr. 2006;18:410-416.
- US Department of Health and Human Services: Public Health Service Agency for Toxic Substances and Disease Registry. Toxicological profile for mercury: regulations and advisories. Published March 1999. Accessed March 23, 2021. https://www.atsdr.cdc.gov/toxprofiles/tp46.pdf
- US Food and Drug Administration. Mercury levels in commercial fish and shellfish (1990-2012). Updated October 25, 2017. Accessed March 16, 2021. https://www.fda.gov/food/metals-and-your-food/mercury-levels-commercial-fish-and-shellfish-1990-2012
- Hamann CR, Boonchai W, Wen L, et al. Spectrometric analysis of mercury content in 549 skin-lightening products: is mercury toxicity a hidden global health hazard? J Am Acad Dermatol. 2014;70:281-287.e3.
- Mercury. Managing Hazardous Materials Incidents. Agency for Toxic Substances and Disease Registry website. Accessed March 16, 2021. https://www.atsdr.cdc.gov/MHMI/mmg46.pdf
- Krohn IT, Solof A, Mobini J, et al. Subcutaneous injection of metallic mercury. JAMA. 1980;243:548-549.
- Lai O, Parsi KK, Wu D, et al. Mercury toxicity presenting acrodynia and a papulovesicular eruption in a 5-year-old girl. Dermatol Online J. 2016;16;22:13030/qt6444r7nc.
- Dolianiti M, Tasiopoulou K, Kalostou A, et al. Mercury bichloride iatrogenic poisoning: a case report. J Clin Toxicol. 2016;6:2. doi:10.4172/2161-0495.1000290
- Broussard LA, Hammett-Stabler CA, Winecker RE, et al. The toxicology of mercury. Lab Med. 2002;33:614-625. doi:10.1309/5HY1-V3NE-2LFL-P9MT
- Byeong-Jin Y, Byoung-Gwon K, Man-Joong J, et al. Evaluation of mercury exposure levels, clinical diagnosis and treatment for mercury intoxication. Ann Occup Environ Med. 2016;28:5.
- History of mercury. Government of Canada website. Modified April 26, 2010. Accessed March 11, 2021. https://www.canada.ca/en/environment-climate-change/services/pollutants/mercury-environment/about/history.html
- Dartmouth Toxic Metals Superfund Research Program website. Accessed March 11, 2021. https://sites.dartmouth.edu/toxmetal/
- Norn S, Permin H, Kruse E, et al. Mercury—a major agent in the history of medicine and alchemy [in Danish]. Dan Medicinhist Arbog. 2008;36:21-40.
- Waldron HA. Did the Mad Hatter have mercury poisoning? Br Med J (Clin Res Ed). 1983;287:1961.
- Poulin J, Gibb H. Mercury: assessing the environmental burden of disease at national and local levels. WHO Environmental Burden of Disease Series No. 16. World Health Organization; 2008.
- Charcot JM. Clinical lectures of the diseases of the nervous system. In: Kinnier Wilson SA. The Landmark Library of Neurology and Neurosurgery. Gryphon Editions; 1994:186.
- Kinnier Wilson SA. Neurology. In: Kinnier Wilson SA. The Landmark Library of Neurology and Neurosurgery. Gryphon Editions; 1994:739-740.
- Harada M. Minamata disease: methylmercury poisoning in Japan caused by environmental pollution. Crit Rev Toxicol. 1995;25:1-24.
- Mercury and health. World Health Organization website. Updated March 31, 2017. Accessed March 12, 2021. http://www.whoint/mediacentre/factsheets/fs361/en/
- Olson DA. Mercury toxicity. Updated November 5, 2018. Accessed March 12, 2021.http://emedicine.medscape.com/article/1175560-overview
- Mercury thermometers. Environmental Protection Agency website. Updated June 26, 2018. https://www.epa.gov/mercury/mercury-thermometers
- Jao-Tan C, Pope E. Cutaneous poisoning syndromes in children: a review. Curr Opin Pediatr. 2006;18:410-416.
- US Department of Health and Human Services: Public Health Service Agency for Toxic Substances and Disease Registry. Toxicological profile for mercury: regulations and advisories. Published March 1999. Accessed March 23, 2021. https://www.atsdr.cdc.gov/toxprofiles/tp46.pdf
- US Food and Drug Administration. Mercury levels in commercial fish and shellfish (1990-2012). Updated October 25, 2017. Accessed March 16, 2021. https://www.fda.gov/food/metals-and-your-food/mercury-levels-commercial-fish-and-shellfish-1990-2012
- Hamann CR, Boonchai W, Wen L, et al. Spectrometric analysis of mercury content in 549 skin-lightening products: is mercury toxicity a hidden global health hazard? J Am Acad Dermatol. 2014;70:281-287.e3.
- Mercury. Managing Hazardous Materials Incidents. Agency for Toxic Substances and Disease Registry website. Accessed March 16, 2021. https://www.atsdr.cdc.gov/MHMI/mmg46.pdf
- Krohn IT, Solof A, Mobini J, et al. Subcutaneous injection of metallic mercury. JAMA. 1980;243:548-549.
- Lai O, Parsi KK, Wu D, et al. Mercury toxicity presenting acrodynia and a papulovesicular eruption in a 5-year-old girl. Dermatol Online J. 2016;16;22:13030/qt6444r7nc.
- Dolianiti M, Tasiopoulou K, Kalostou A, et al. Mercury bichloride iatrogenic poisoning: a case report. J Clin Toxicol. 2016;6:2. doi:10.4172/2161-0495.1000290
- Broussard LA, Hammett-Stabler CA, Winecker RE, et al. The toxicology of mercury. Lab Med. 2002;33:614-625. doi:10.1309/5HY1-V3NE-2LFL-P9MT
- Byeong-Jin Y, Byoung-Gwon K, Man-Joong J, et al. Evaluation of mercury exposure levels, clinical diagnosis and treatment for mercury intoxication. Ann Occup Environ Med. 2016;28:5.
Practice Points
- Chronic mercury granulomas can present as firm, erythematous to bluish gray plaques.
- Accidental skin contact to elemental mercury may cause urticaria and dermatitis.
- Blood mercury concentrations below 20 11µg/L are considered within reference range; once blood and urine concentrations exceed 100 11µg/L, clinical signs of acute mercury poisoning typically manifest.
- Mercury is toxic to the central and peripheral nervous systems, resulting in erethism mercurialis, a constellation of neuropsychologic signs and symptoms including restlessness, irritability, insomnia, emotional lability, difficulty concentrating, and impaired memory.
What’s Eating You? Black Butterfly (Hylesia nigricans)
The order Lepidoptera (phylum Arthropoda, class Hexapoda) is comprised of moths and butterflies.1 Lepidopterism refers to a range of adverse medical conditions resulting from contact with these insects, typically during the caterpillar (larval) stage. It involves multiple pathologic mechanisms, including direct toxicity of venom and mechanical irritant effects.2 Erucism has been defined as any reaction caused by contact with caterpillars or any reaction limited to the skin caused by contact with caterpillars, butterflies, or moths. Lepidopterism can mean any reaction to caterpillars or moths, referring only to reactions from contact with scales or hairs from adult moths or butterflies, or referring only to cases with systemic signs and symptoms (eg, rhinoconjunctival or asthmatic symptoms, angioedema and anaphylaxis, hemorrhagic diathesis) with or without cutaneous findings, resulting from contact with any lepidopteran source.1 Strictly speaking, erucism should refer to any reaction from caterpillars and lepidopterism to reactions from moths or butterflies. Because reactions to both larval and adult lepidoptera can cause a variety of either cutaneous and/or systemic symptoms, classifying reactions into erucism or lepidopterism is only of academic interest.1
We report a documented case of lepidopterism in a patient with acute cutaneous lesions following exposure to an adult-stage black butterfly (Hylesia nigricans).
Case Report
A 21-year-old oil well worker presented with pruritic skin lesions on the right arm and flank of 3 hours’ duration. He reported that a black butterfly had perched on his arm while he was working and left a considerable number of small yellowish hairs on the skin, after which an intense pruritus and skin lesions began to develop. He denied other associated symptoms. Physical examination revealed numerous 1- to 2-mm, nonconfluent, erythematous and edematous papules on the right forearm, arm (Figure 1A), and flank. Some abrasions of the skin due to scratching and crusting were noted (Figure 1B). A skin biopsy from the right arm showed a superficial perivascular dermatitis with a mixed infiltrate of polymorphonuclear predominance with eosinophils (Figure 2A). Importantly, a structure resembling an urticating spicule was identified in the stratum corneum (Figure 2B); spicules are located on the abdomen of arthropods and are associated with an inflammatory response in human skin.
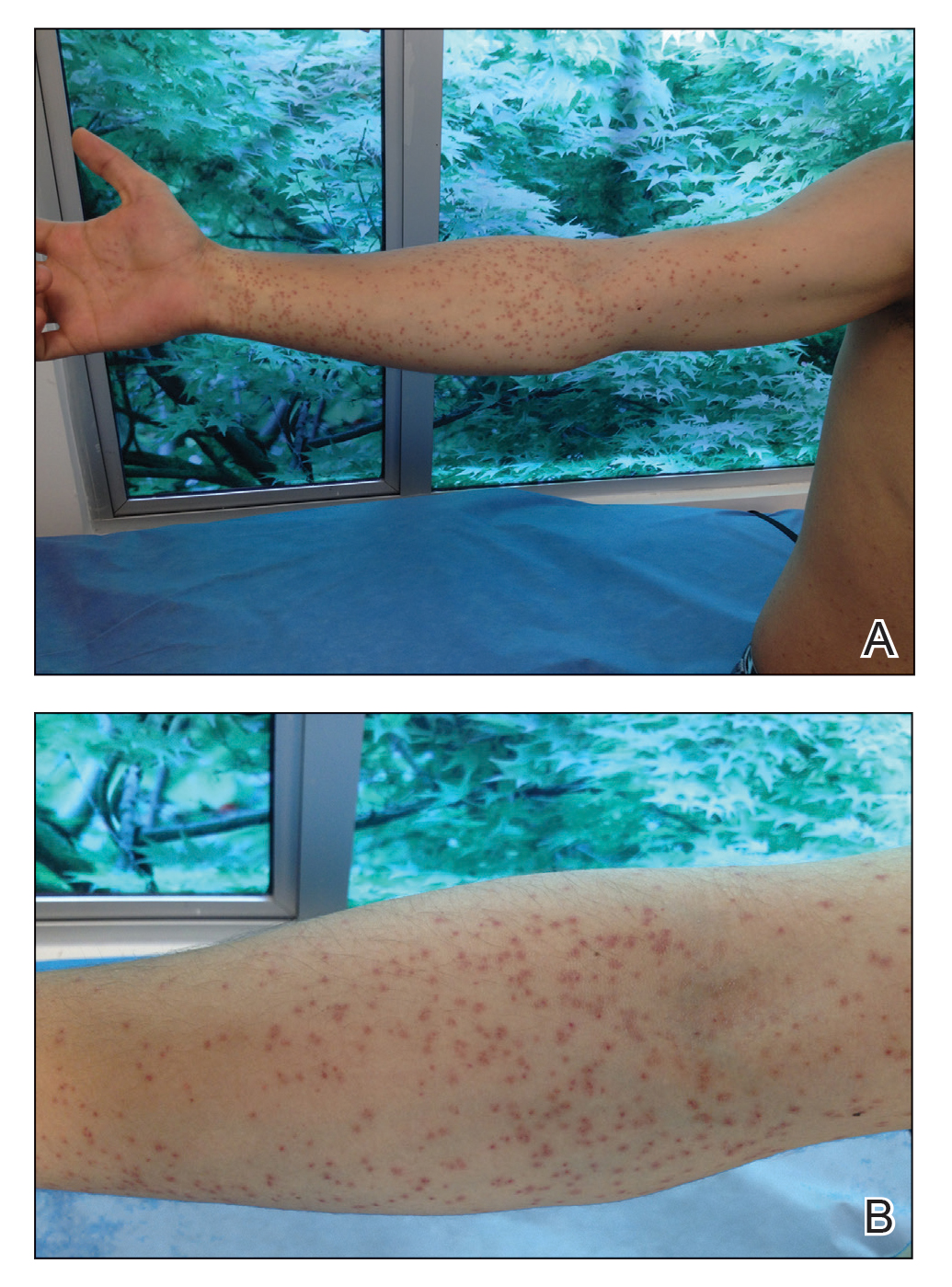
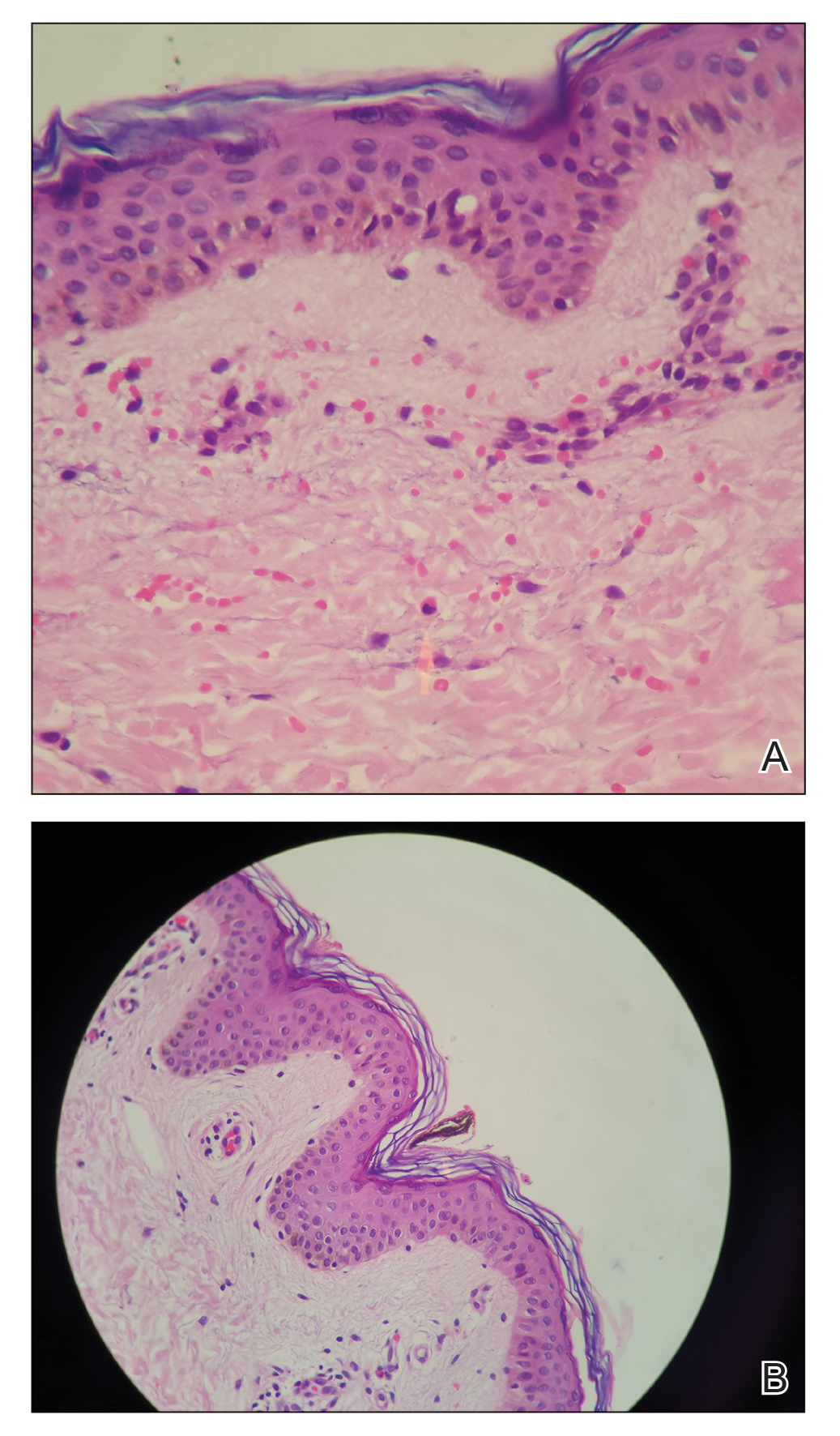
Based on the patient’s history of butterfly exposure, clinical presentation of the lesions, and histopathologic findings demonstrating the presence of the spicules, the diagnosis of lepidopterism was confirmed. The patient was treated with oral antihistamines and topical steroids for 1 week with complete resolution of the lesions.
Comment
Epidemiology of Envenomation
Although many tropical insects carry infectious diseases, cutaneous injury can occur by other mechanisms, such as dermatitis caused by contact with the skin (erucism or lepidopterism). Caterpillar envenomation is common, but this phenomenon rarely has been studied due to few reported cases, which hinders a complete understanding of the problem.3
The order Lepidoptera comprises 2 suborders: Rhopalocera, with adult specimens that fly during the daytime (butterflies), and Heterocera, which are largely nocturnal (moths). The stages of development include egg, larva (caterpillar), pupa (chrysalis), and adult (imago), constituting a holometabolic life cycle.4 Adult butterflies and moths represent the reproductive stage of lepidoptera.
The pathology of lepidopterism is attributed to contact with fluids such as hemolymph and secretions from the spicules, with histamine being identified as the main causative component.3 During the reproductive stage, female insects approach light sources and release clouds of bristles from their abdomens that can penetrate human skin and cause an irritating dermatitis.5 Lepidopterism can occur following contact with bristles from insects of the Hylesia genus (Saturniidae family), such as in our patient.3,6 Epidemic outbreaks have been reported in several countries, mainly Argentina, Brazil, and Venezuela.5 The patient was located in Colombia, a country without any reported cases of lepidopterism from the black butterfly (H nigricans). Cutaneous reactions to lepidoptera insects come in many forms, most commonly presenting as a mild stinging reaction with a papular eruption, pruritic urticarial papules and plaques, or scaly erythematous papules and plaques in exposed areas.7
Histopathologic Findings
The histology of lepidoptera exposure is nonspecific, typically demonstrating epidermal edema, superficial perivascular lymphocytic infiltrate, and eosinophils. Epidermal necrosis and vasculitis rarely are seen. Embedded spines from Hylesia insects have been described.7 The histopathologic examination generally reveals a foreign body reaction in addition to granulomas.3
Therapy
The use of oral antihistamines is indicated for the control of pruritus, and topical treatment with cold compresses, baths, and corticosteroid creams is recommended.3,8,9
Conclusion
We report the case of a patient with lepidopterism, a rare entity confirmed histologically with documentation of a spicule in the stratum corneum in the patient’s biopsy. Changes due to urbanization and industrialization have a closer relationship with various animal species that are pathogenic to humans; therefore, we encourage dermatologists to be aware of these diseases.
- Hossler EW. Caterpillars and moths: part I. dermatologic manifestations of encounters with Lepidoptera. J Am Acad Dermatol. 2010;62:666.
- Redd JT, Voorhees RE, Török TJ. Outbreak of lepidopterism at a Boy Scout camp. J Am Acad Dermatol. 2007;56:952-955.
- Haddad V Jr, Cardoso JL, Lupi O, et al. Tropical dermatology: venomous arthropods and human skin: part I. Insecta. J Am Acad Dermatol. 2012;67:331.
- Cardoso AEC, Haddad V Jr. Accidents caused by lepidopterans (moth larvae and adult): study on the epidemiological, clinical and therapeutic aspects. An Bras Dermatol. 2005;80:571-578.
- Salomón AD, Simón D, Rimoldi JC, et al. Lepidopterism due to the butterfly Hylesia nigricans. preventive research-intervention in Buenos Aires. Medicina (B Aires). 2005;65:241-246.
- Moreira SC, Lima JC, Silva L, et al. Description of an outbreak of lepidopterism (dermatitis associated with contact with moths) among sailors in Salvador, State of Bahia. Rev Soc Bras Med Trop. 2007;40:591-593.
- Hossler EW. Caterpillars and moths: part II. dermatologic manifestations of encounters with Lepidoptera. J Am Acad Dermatol. 2010;62:666.
- Maier H, Spiegel W, Kinaciyan T, et al. The oak processionary caterpillar as the cause of an epidemic airborne disease: survey and analysis. Br J Dermatol. 2003;149:990-997.
- Herrera-Chaumont C, Sojo-Milano M, Pérez-Ybarra LM. Knowledge and practices on lepidopterism by Hylesia metabus (Cramer, 1775)(Lepidoptera: Saturniidae) in Yaguaraparo parish, Sucre state, northeastern Venezuela. Revista Biomédica. 2016;27:11-23.
The order Lepidoptera (phylum Arthropoda, class Hexapoda) is comprised of moths and butterflies.1 Lepidopterism refers to a range of adverse medical conditions resulting from contact with these insects, typically during the caterpillar (larval) stage. It involves multiple pathologic mechanisms, including direct toxicity of venom and mechanical irritant effects.2 Erucism has been defined as any reaction caused by contact with caterpillars or any reaction limited to the skin caused by contact with caterpillars, butterflies, or moths. Lepidopterism can mean any reaction to caterpillars or moths, referring only to reactions from contact with scales or hairs from adult moths or butterflies, or referring only to cases with systemic signs and symptoms (eg, rhinoconjunctival or asthmatic symptoms, angioedema and anaphylaxis, hemorrhagic diathesis) with or without cutaneous findings, resulting from contact with any lepidopteran source.1 Strictly speaking, erucism should refer to any reaction from caterpillars and lepidopterism to reactions from moths or butterflies. Because reactions to both larval and adult lepidoptera can cause a variety of either cutaneous and/or systemic symptoms, classifying reactions into erucism or lepidopterism is only of academic interest.1
We report a documented case of lepidopterism in a patient with acute cutaneous lesions following exposure to an adult-stage black butterfly (Hylesia nigricans).
Case Report
A 21-year-old oil well worker presented with pruritic skin lesions on the right arm and flank of 3 hours’ duration. He reported that a black butterfly had perched on his arm while he was working and left a considerable number of small yellowish hairs on the skin, after which an intense pruritus and skin lesions began to develop. He denied other associated symptoms. Physical examination revealed numerous 1- to 2-mm, nonconfluent, erythematous and edematous papules on the right forearm, arm (Figure 1A), and flank. Some abrasions of the skin due to scratching and crusting were noted (Figure 1B). A skin biopsy from the right arm showed a superficial perivascular dermatitis with a mixed infiltrate of polymorphonuclear predominance with eosinophils (Figure 2A). Importantly, a structure resembling an urticating spicule was identified in the stratum corneum (Figure 2B); spicules are located on the abdomen of arthropods and are associated with an inflammatory response in human skin.


Based on the patient’s history of butterfly exposure, clinical presentation of the lesions, and histopathologic findings demonstrating the presence of the spicules, the diagnosis of lepidopterism was confirmed. The patient was treated with oral antihistamines and topical steroids for 1 week with complete resolution of the lesions.
Comment
Epidemiology of Envenomation
Although many tropical insects carry infectious diseases, cutaneous injury can occur by other mechanisms, such as dermatitis caused by contact with the skin (erucism or lepidopterism). Caterpillar envenomation is common, but this phenomenon rarely has been studied due to few reported cases, which hinders a complete understanding of the problem.3
The order Lepidoptera comprises 2 suborders: Rhopalocera, with adult specimens that fly during the daytime (butterflies), and Heterocera, which are largely nocturnal (moths). The stages of development include egg, larva (caterpillar), pupa (chrysalis), and adult (imago), constituting a holometabolic life cycle.4 Adult butterflies and moths represent the reproductive stage of lepidoptera.
The pathology of lepidopterism is attributed to contact with fluids such as hemolymph and secretions from the spicules, with histamine being identified as the main causative component.3 During the reproductive stage, female insects approach light sources and release clouds of bristles from their abdomens that can penetrate human skin and cause an irritating dermatitis.5 Lepidopterism can occur following contact with bristles from insects of the Hylesia genus (Saturniidae family), such as in our patient.3,6 Epidemic outbreaks have been reported in several countries, mainly Argentina, Brazil, and Venezuela.5 The patient was located in Colombia, a country without any reported cases of lepidopterism from the black butterfly (H nigricans). Cutaneous reactions to lepidoptera insects come in many forms, most commonly presenting as a mild stinging reaction with a papular eruption, pruritic urticarial papules and plaques, or scaly erythematous papules and plaques in exposed areas.7
Histopathologic Findings
The histology of lepidoptera exposure is nonspecific, typically demonstrating epidermal edema, superficial perivascular lymphocytic infiltrate, and eosinophils. Epidermal necrosis and vasculitis rarely are seen. Embedded spines from Hylesia insects have been described.7 The histopathologic examination generally reveals a foreign body reaction in addition to granulomas.3
Therapy
The use of oral antihistamines is indicated for the control of pruritus, and topical treatment with cold compresses, baths, and corticosteroid creams is recommended.3,8,9
Conclusion
We report the case of a patient with lepidopterism, a rare entity confirmed histologically with documentation of a spicule in the stratum corneum in the patient’s biopsy. Changes due to urbanization and industrialization have a closer relationship with various animal species that are pathogenic to humans; therefore, we encourage dermatologists to be aware of these diseases.
The order Lepidoptera (phylum Arthropoda, class Hexapoda) is comprised of moths and butterflies.1 Lepidopterism refers to a range of adverse medical conditions resulting from contact with these insects, typically during the caterpillar (larval) stage. It involves multiple pathologic mechanisms, including direct toxicity of venom and mechanical irritant effects.2 Erucism has been defined as any reaction caused by contact with caterpillars or any reaction limited to the skin caused by contact with caterpillars, butterflies, or moths. Lepidopterism can mean any reaction to caterpillars or moths, referring only to reactions from contact with scales or hairs from adult moths or butterflies, or referring only to cases with systemic signs and symptoms (eg, rhinoconjunctival or asthmatic symptoms, angioedema and anaphylaxis, hemorrhagic diathesis) with or without cutaneous findings, resulting from contact with any lepidopteran source.1 Strictly speaking, erucism should refer to any reaction from caterpillars and lepidopterism to reactions from moths or butterflies. Because reactions to both larval and adult lepidoptera can cause a variety of either cutaneous and/or systemic symptoms, classifying reactions into erucism or lepidopterism is only of academic interest.1
We report a documented case of lepidopterism in a patient with acute cutaneous lesions following exposure to an adult-stage black butterfly (Hylesia nigricans).
Case Report
A 21-year-old oil well worker presented with pruritic skin lesions on the right arm and flank of 3 hours’ duration. He reported that a black butterfly had perched on his arm while he was working and left a considerable number of small yellowish hairs on the skin, after which an intense pruritus and skin lesions began to develop. He denied other associated symptoms. Physical examination revealed numerous 1- to 2-mm, nonconfluent, erythematous and edematous papules on the right forearm, arm (Figure 1A), and flank. Some abrasions of the skin due to scratching and crusting were noted (Figure 1B). A skin biopsy from the right arm showed a superficial perivascular dermatitis with a mixed infiltrate of polymorphonuclear predominance with eosinophils (Figure 2A). Importantly, a structure resembling an urticating spicule was identified in the stratum corneum (Figure 2B); spicules are located on the abdomen of arthropods and are associated with an inflammatory response in human skin.


Based on the patient’s history of butterfly exposure, clinical presentation of the lesions, and histopathologic findings demonstrating the presence of the spicules, the diagnosis of lepidopterism was confirmed. The patient was treated with oral antihistamines and topical steroids for 1 week with complete resolution of the lesions.
Comment
Epidemiology of Envenomation
Although many tropical insects carry infectious diseases, cutaneous injury can occur by other mechanisms, such as dermatitis caused by contact with the skin (erucism or lepidopterism). Caterpillar envenomation is common, but this phenomenon rarely has been studied due to few reported cases, which hinders a complete understanding of the problem.3
The order Lepidoptera comprises 2 suborders: Rhopalocera, with adult specimens that fly during the daytime (butterflies), and Heterocera, which are largely nocturnal (moths). The stages of development include egg, larva (caterpillar), pupa (chrysalis), and adult (imago), constituting a holometabolic life cycle.4 Adult butterflies and moths represent the reproductive stage of lepidoptera.
The pathology of lepidopterism is attributed to contact with fluids such as hemolymph and secretions from the spicules, with histamine being identified as the main causative component.3 During the reproductive stage, female insects approach light sources and release clouds of bristles from their abdomens that can penetrate human skin and cause an irritating dermatitis.5 Lepidopterism can occur following contact with bristles from insects of the Hylesia genus (Saturniidae family), such as in our patient.3,6 Epidemic outbreaks have been reported in several countries, mainly Argentina, Brazil, and Venezuela.5 The patient was located in Colombia, a country without any reported cases of lepidopterism from the black butterfly (H nigricans). Cutaneous reactions to lepidoptera insects come in many forms, most commonly presenting as a mild stinging reaction with a papular eruption, pruritic urticarial papules and plaques, or scaly erythematous papules and plaques in exposed areas.7
Histopathologic Findings
The histology of lepidoptera exposure is nonspecific, typically demonstrating epidermal edema, superficial perivascular lymphocytic infiltrate, and eosinophils. Epidermal necrosis and vasculitis rarely are seen. Embedded spines from Hylesia insects have been described.7 The histopathologic examination generally reveals a foreign body reaction in addition to granulomas.3
Therapy
The use of oral antihistamines is indicated for the control of pruritus, and topical treatment with cold compresses, baths, and corticosteroid creams is recommended.3,8,9
Conclusion
We report the case of a patient with lepidopterism, a rare entity confirmed histologically with documentation of a spicule in the stratum corneum in the patient’s biopsy. Changes due to urbanization and industrialization have a closer relationship with various animal species that are pathogenic to humans; therefore, we encourage dermatologists to be aware of these diseases.
- Hossler EW. Caterpillars and moths: part I. dermatologic manifestations of encounters with Lepidoptera. J Am Acad Dermatol. 2010;62:666.
- Redd JT, Voorhees RE, Török TJ. Outbreak of lepidopterism at a Boy Scout camp. J Am Acad Dermatol. 2007;56:952-955.
- Haddad V Jr, Cardoso JL, Lupi O, et al. Tropical dermatology: venomous arthropods and human skin: part I. Insecta. J Am Acad Dermatol. 2012;67:331.
- Cardoso AEC, Haddad V Jr. Accidents caused by lepidopterans (moth larvae and adult): study on the epidemiological, clinical and therapeutic aspects. An Bras Dermatol. 2005;80:571-578.
- Salomón AD, Simón D, Rimoldi JC, et al. Lepidopterism due to the butterfly Hylesia nigricans. preventive research-intervention in Buenos Aires. Medicina (B Aires). 2005;65:241-246.
- Moreira SC, Lima JC, Silva L, et al. Description of an outbreak of lepidopterism (dermatitis associated with contact with moths) among sailors in Salvador, State of Bahia. Rev Soc Bras Med Trop. 2007;40:591-593.
- Hossler EW. Caterpillars and moths: part II. dermatologic manifestations of encounters with Lepidoptera. J Am Acad Dermatol. 2010;62:666.
- Maier H, Spiegel W, Kinaciyan T, et al. The oak processionary caterpillar as the cause of an epidemic airborne disease: survey and analysis. Br J Dermatol. 2003;149:990-997.
- Herrera-Chaumont C, Sojo-Milano M, Pérez-Ybarra LM. Knowledge and practices on lepidopterism by Hylesia metabus (Cramer, 1775)(Lepidoptera: Saturniidae) in Yaguaraparo parish, Sucre state, northeastern Venezuela. Revista Biomédica. 2016;27:11-23.
- Hossler EW. Caterpillars and moths: part I. dermatologic manifestations of encounters with Lepidoptera. J Am Acad Dermatol. 2010;62:666.
- Redd JT, Voorhees RE, Török TJ. Outbreak of lepidopterism at a Boy Scout camp. J Am Acad Dermatol. 2007;56:952-955.
- Haddad V Jr, Cardoso JL, Lupi O, et al. Tropical dermatology: venomous arthropods and human skin: part I. Insecta. J Am Acad Dermatol. 2012;67:331.
- Cardoso AEC, Haddad V Jr. Accidents caused by lepidopterans (moth larvae and adult): study on the epidemiological, clinical and therapeutic aspects. An Bras Dermatol. 2005;80:571-578.
- Salomón AD, Simón D, Rimoldi JC, et al. Lepidopterism due to the butterfly Hylesia nigricans. preventive research-intervention in Buenos Aires. Medicina (B Aires). 2005;65:241-246.
- Moreira SC, Lima JC, Silva L, et al. Description of an outbreak of lepidopterism (dermatitis associated with contact with moths) among sailors in Salvador, State of Bahia. Rev Soc Bras Med Trop. 2007;40:591-593.
- Hossler EW. Caterpillars and moths: part II. dermatologic manifestations of encounters with Lepidoptera. J Am Acad Dermatol. 2010;62:666.
- Maier H, Spiegel W, Kinaciyan T, et al. The oak processionary caterpillar as the cause of an epidemic airborne disease: survey and analysis. Br J Dermatol. 2003;149:990-997.
- Herrera-Chaumont C, Sojo-Milano M, Pérez-Ybarra LM. Knowledge and practices on lepidopterism by Hylesia metabus (Cramer, 1775)(Lepidoptera: Saturniidae) in Yaguaraparo parish, Sucre state, northeastern Venezuela. Revista Biomédica. 2016;27:11-23.
Practice Points
- When contact with caterpillars, butterflies, or moths occurs, patients should be advised not to rub or scratch the area or attempt to remove or crush the insect with a bare hand, as this may further spread irritating setae or spines.
- Careful removal of the larva with forceps or a similar instrument, combined with tape stripping of the area and immediate washing with soap and water, can be effective in minimizing exposure.
- Contaminated clothing should be removed and laundered thoroughly.
Aquatic Antagonists: Sponge Dermatitis
Sponges are among the oldest animals on earth, appearing more than 640 million years ago before the Cambrian explosion, a period when most major animal phyla appeared in the fossil records.1 More than 10,000 species of sponges have been identified worldwide and are distributed from polar to tropical regions in both marine (Figure 1) and freshwater (Figure 2) environments. They inhabit both shallow waters as well as depths of more than 2800 m, with shallower sponges tending to be more vibrantly colored than their deeper counterparts. The wide-ranging habitats of sponges have led to size variations from as small as 0.05 mm to more than 3 m in height.2 Their taxonomic phylum, Porifera (meaning pore bearers), is derived from the millions of pores lining the surface of the sponge that are used to filter planktonic organisms.3 Flagellated epithelioid cells called choanocytes line the internal chambers of sponges, creating a water current that promotes filter feeding as well as nutrient absorption across their microvilli.4 The body walls of many sponges consist of a collagenous skeleton made up of spongin and spicules of silicon dioxide (silica) or calcium carbonate embedded in the spongin connective tissue matrix.5 Bath sponges lack silica spicules.
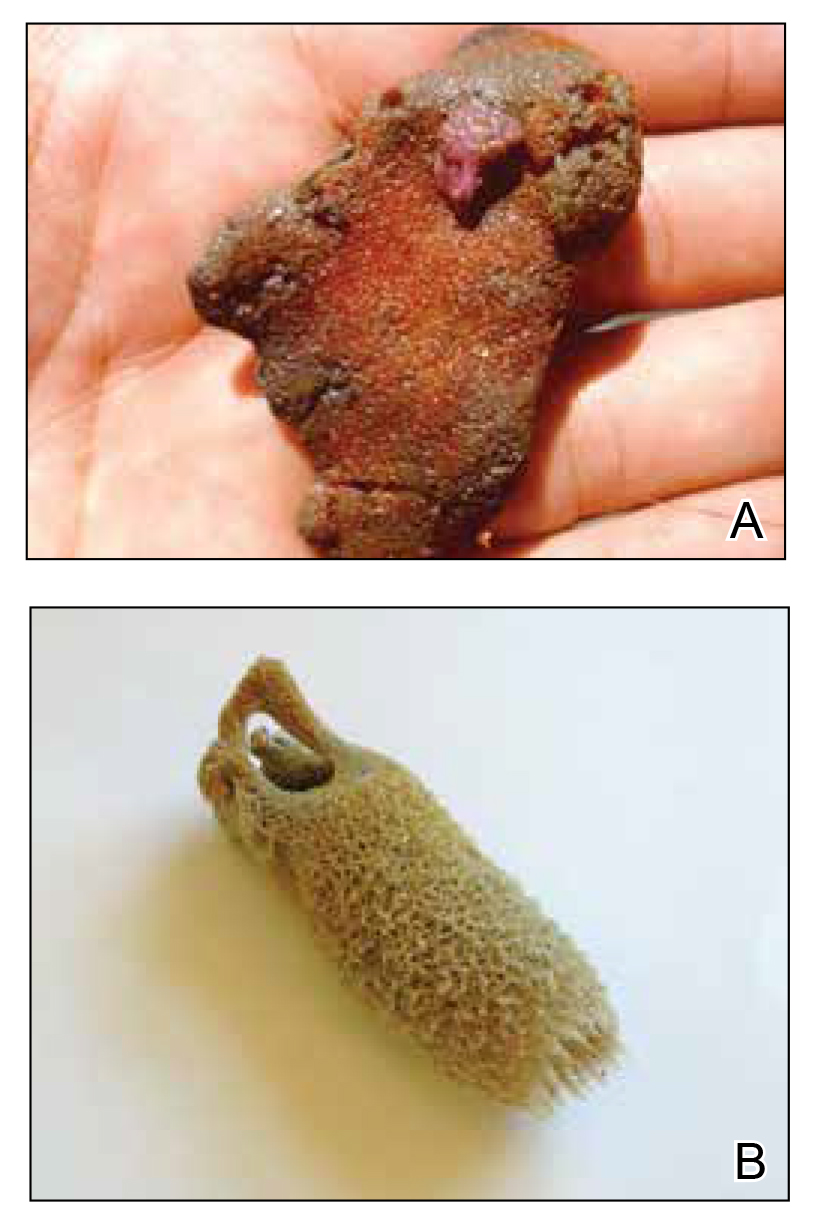

Sponges have been used in medicine for centuries. The first use in Western culture was recorded in 405
Mechanisms and Symptoms of Injury
Bathing sponges (silk sponges) derived from Spongia officinalis are harmless. Other sponges can exert their damaging effects through a variety of mechanisms that lead to dermatologic manifestations (eTable). Some species of sponges produce and secrete toxic metabolites (eg, crinotoxins) onto the body surface or into the surrounding water. They also are capable of synthesizing a mucous slime that can be irritating to human skin. Direct trauma also can be caused by fragments of the silica or calcium carbonate sponge skeleton penetrating the skin. Stinging members of the phylum Cnidaria can colonize the sponge, leading to injury when a human handles the sponge.25-27
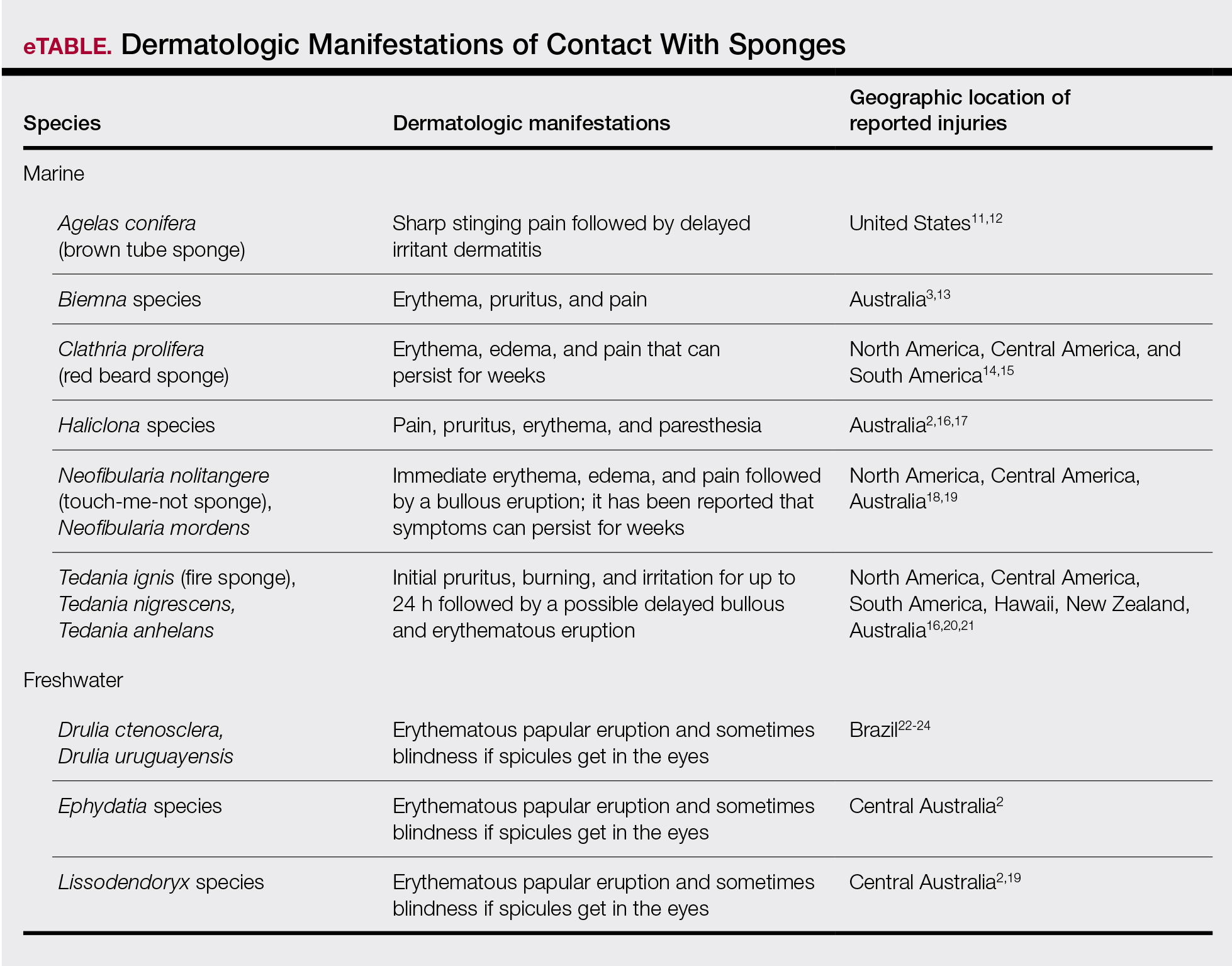
Sponge dermatitis can be divided into 2 major categories: an initial pruritic dermatitis (Figure 3) that occurs within 20 minutes to a few hours after contact and a delayed irritant dermatitis caused by penetration of the spicules and chemical agents into skin.28 Importantly, different species can lead to varying manifestations.
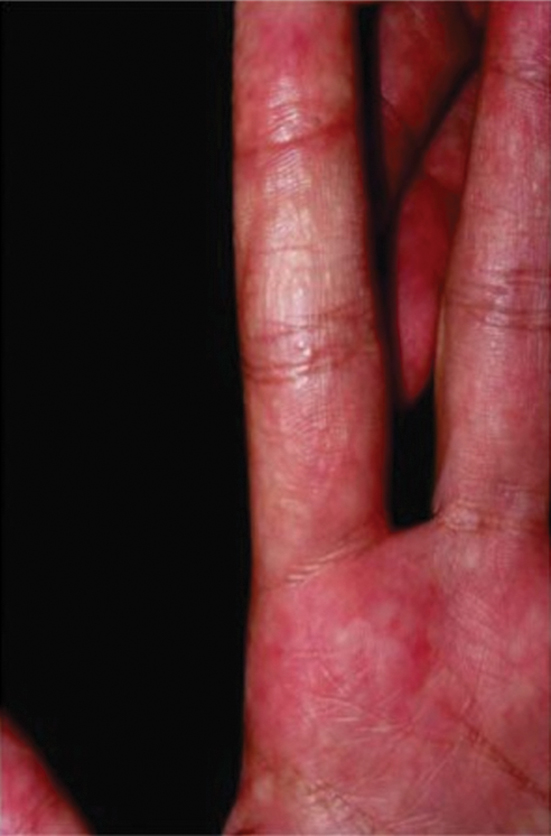
The initial pruritic dermatitis is characterized by itching and burning that progresses to local edema, vesiculation, joint swelling, and stiffness. Because most contact with sponges occurs with handling, joint immobility may ensue within 24 hours of the encounter. Rarely, larger areas of the skin are affected, and fever, chills, malaise, dizziness, nausea, purulent bullae, muscle cramps, and formication may occur.28 Anaphylactic reactions have been described in a small subset of patients. There have even been reports of delayed (ie, 1–2 weeks following exposure) erythema multiforme, livedo reticularis, purpura, and dyshidrotic eczema.16,20,29 The irritant dermatitis caused by spicule trauma is due to a foreign body reaction that can be exacerbated by toxins entering the skin. In severe cases, desquamation, recurrent eczema, and arthralgia can occur.30 In general, more mild cases should self-resolve within 3 to 7 days. Dermatologic conditions also can be caused by organisms that inhabit sponges and as a result produce a dermatitis when the sponge is handled, including sponge divers disease (maladie des plongeurs), a necrotic dermatitis caused by stinging Cnidaria species.31 Dogger Bank itch, first described as a dermatitis caused by sensitization to (2-hydroxyethyl) dimethylsulfoxonium chloride, initially was isolated from the sea chervil (a type of Bryozoan); however, that same chemical also was later found in sponges, producing the same dermatitis after handling the sponge.32 Freshwater sponges also have been reported to be injurious and exist worldwide. In contrast to marine sponges, lesions from freshwater sponges are disseminated pruritic erythematous papules with ulcerations, crusts, and secondary infections.22 The disseminated nature of the dermatitis caused by freshwater sponges is due to contact with the spicules of dead sponges that are dispersed throughout the water rather than from direct handling. Sponge dermatitis occurs mostly in sponge collectors, divers, trawlers, and biology students and has been reported extensively in the United States, Caribbean Islands, Australia, New Zealand, and Brazil.18,27,33,34
Management
Treatment should consist of an initial decontamination; the skin should be dried, and adhesive tape or rubber cement should be utilized to remove any spicules embedded in the skin. Diluted vinegar soaks should be initiated for 10 to 30 minutes on the affected area(s) 3 or 4 times daily.19 The initial decontamination should occur immediately, as delay may lead to persistent purulent bullae that may take months to heal. Topical steroids may be used following the initial decontamination to help relieve inflammation. Antihistamines and nonsteroidal anti-inflammatory drugs may be used to alleviate pruritus and pain, respectively. Severe cases may require systemic glucocorticoids. Additionally, immunization status against tetanus toxoid should be assessed.35 In the event of an anaphylactic reaction, it is important to maintain a patent airway and normalized blood pressure through the use of intramuscular epinephrine.36 Frequent follow-up is warranted, as serious secondary infections can develop.37 Patients also should be counseled on the potential for delayed dermatologic reactions, including erythema multiforme. Contact between humans and coastal environments has been increasing in the last few decades; therefore, an increase in contact with sponges is to be expected.22
- Gold DA, Grabenstatter J, de Mendoza A, et al. Sterol and genomic analyses validate the sponge biomarker hypothesis. Proc Natl Acad Sci U S A. 2016;113:2684-2689.
- Bonamonte D, Filoni A, Verni P, et al. Dermatitis caused by sponges. In: Bonamonte D, Angelini G, eds. Aquatic Dermatology. 2nd ed. Springer; 2016:121-126.
- Marsh LM, Slack-Smith S, Gurry DL. Field Guide to Sea Stingers and Other Venomous and Poisonous Marine Invertebrates. 2nd ed. Western Australian Museum; 2010.
- Eid E, Al-Tawaha M. A Guide to Harmful and Toxic Creatures in the Gulf of Aqaba Jordan. The Royal Marine Conservation Society of Jordan; 2016.
- Reese E, Depenbrock P. Water envenomations and stings. Curr Sports Med Rep. 2014;13:126-131.
- Dormandy TL. Trace element analysis of hair. Br Med J (Clin Res Ed). 1986;293:975-976.
- Voultsiadou E. Sponges: an historical survey of their knowledge in Greek antiquity. J Mar Biol Assoc UK. 2007;87:1757-1763.
- Senthilkumar K, Kim SK. Marine invertebrate natural products for anti-inflammatory and chronic diseases [published online December 31, 2013]. Evid Based Complement Alternat Med. doi:10.1155/2013/572859
- Sagar S, Kaur M, Minneman KP. Antiviral lead compounds from marine sponges. Mar Drugs. 2010;8:2619-2638.
- Usagawa T, Nishimura M, Itoh Y, et al. Preparation of monoclonal antibodies against okadaic acid prepared from the sponge Halichondria okadai. Toxicon. 1989;27:1323-1330.
- Elston DM. Aquatic antagonists: sponge dermatitis. Cutis. 2007;80:279-280.
- Parra-Velandia FJ, Zea S, Van Soest RW. Reef sponges of the genus Agelas (Porifera: Demospongiae) from the Greater Caribbean. Zootaxa. 2014;3794:301-343.
- Hooper JN, Capon RJ, Hodder RA. A new species of toxic marine sponge (Porifera: Demospongiae: Poecilosclerida) from northwest Australia. The Beagle, Records of the Northern Territory Museum of Arts and sciences. 1991;8:27-36.
- Burnett JW, Calton GJ, Morgan RJ. Dermatitis due to stinging sponges. Cutis. 1987;39:476.
- Kizer KW. Marine envenomations. J Toxicol Clin Toxicol. 1983;21:527-555.
- Isbister GK, Hooper JN. Clinical effects of stings by sponges of the genus Tedania and a review of sponge stings worldwide. Toxicon. 2005;46:782-785.
- Fromont J, Abdo DA. New species of Haliclona (Demospongiae: Haplosclerida: Chalinidae) from Western Australia. Zootaxa. 2014;3835:97-109.
- Flachsenberger W, Holmes NJ, Leigh C, et al. Properties of the extract and spicules of the dermatitis inducing sponge Neofibularia mordens Hartman. J Toxicol Clin Toxicol. 1987;25:255-272.
- Southcott RV, Coulter JR. The effects of the southern Australian marine stinging sponges, Neofibularia mordens and Lissodendoryx sp. Med J Aust. 1971;2:895-901.
- Yaffee HS, Stargardter F. Erythema multiforme from Tedania ignis. report of a case and an experimental study of the mechanism of cutaneous irritation from the fire sponge. Arch Dermatol. 1963;87:601-604.
- Yaffee HS. Irritation from red sponge. N Engl J Med. 1970;282:51.
- Haddad V Jr. Environmental dermatology: skin manifestations of injuries caused by invertebrate aquatic animals. An Bras Dermatol. 2013;88:496-506.
- Volkmer-Ribeiro C, Lenzi HL, Orefice F, et al. Freshwater sponge spicules: a new agent of ocular pathology. Mem Inst Oswaldo Cruz. 2006;101:899-903.
- Cruz AA, Alencar VM, Medina NH, et al. Dangerous waters: outbreak of eye lesions caused by fresh water sponge spicules. Eye (Lond). 2013;27:398-402.
- Haddad V Jr. Clinical and therapeutic aspects of envenomations caused by sponges and jellyfish. In: Gopalakrishnakone P, Haddad V Jr, Kem WR, et al, eds. Marine and Freshwater Toxins. Springer; 2016:317-325.
- Haddad V Jr, Lupi O, Lonza JP, et al. Tropical dermatology: marine and aquatic dermatology. J Am Acad Dermatol. 2009;61:733-750.
- Gaastra MT. Aquatic skin disorders. In: Faber WR, Hay RJ, Naafs B, eds. Imported Skin Diseases. 2nd ed. Wiley; 2012:283-292.
- Auerbach P. Envenomation by aquatic invertebrates. In: Auerbach P, ed. Wilderness Medicine. 6th ed. Elsevier Mosby; 2011;1596-1627.
- Sims JK, Irei MY. Human Hawaiian marine sponge poisoning. Hawaii Med J. 1979;38:263-270.
- Haddad V Jr. Aquatic animals of medical importance in Brazil. Rev Soc Bras Med Trop. 2003;36:591-597.
- Tlougan BE, Podjasek JO, Adams BB. Aquatic sports dermatoses. part 2—in the water: saltwater dermatoses. Int J Dermatol. 2010;49:994-1002.
- Warabi K, Nakao Y, Matsunaga S, et al. Dogger Bank itch revisited: isolation of (2-hydroxyethyl) dimethylsulfoxonium chloride as a cytotoxic constituent from the marine sponge Theonella aff. mirabilis. Comp Biochem Physiol B Biochem Mol Biol. 2001;128:27-30.
- Southcott R. Human injuries from invertebrate animals in the Australian seas. Clin Toxicol. 1970;3:617-636.
- Russell FE. Sponge injury—traumatic, toxic or allergic? N Engl J Med. 1970;282:753-754.
- Hornbeak KB, Auerbach PS. Marine envenomation. Emerg Med Clin North Am. 2017;35:321-337.
- Muraro A, Roberts G, Worm M, et al. Anaphylaxis: guidelines from the European Academy of Allergy and Clinical Immunology. Allergy. 2014;69:1026-1045.
- Kizer K, Auerbach P, Dwyer B. Marine envenomations: not just a problem of the tropics. Emerg Med Rep. 1985;6:129-135.
Sponges are among the oldest animals on earth, appearing more than 640 million years ago before the Cambrian explosion, a period when most major animal phyla appeared in the fossil records.1 More than 10,000 species of sponges have been identified worldwide and are distributed from polar to tropical regions in both marine (Figure 1) and freshwater (Figure 2) environments. They inhabit both shallow waters as well as depths of more than 2800 m, with shallower sponges tending to be more vibrantly colored than their deeper counterparts. The wide-ranging habitats of sponges have led to size variations from as small as 0.05 mm to more than 3 m in height.2 Their taxonomic phylum, Porifera (meaning pore bearers), is derived from the millions of pores lining the surface of the sponge that are used to filter planktonic organisms.3 Flagellated epithelioid cells called choanocytes line the internal chambers of sponges, creating a water current that promotes filter feeding as well as nutrient absorption across their microvilli.4 The body walls of many sponges consist of a collagenous skeleton made up of spongin and spicules of silicon dioxide (silica) or calcium carbonate embedded in the spongin connective tissue matrix.5 Bath sponges lack silica spicules.


Sponges have been used in medicine for centuries. The first use in Western culture was recorded in 405
Mechanisms and Symptoms of Injury
Bathing sponges (silk sponges) derived from Spongia officinalis are harmless. Other sponges can exert their damaging effects through a variety of mechanisms that lead to dermatologic manifestations (eTable). Some species of sponges produce and secrete toxic metabolites (eg, crinotoxins) onto the body surface or into the surrounding water. They also are capable of synthesizing a mucous slime that can be irritating to human skin. Direct trauma also can be caused by fragments of the silica or calcium carbonate sponge skeleton penetrating the skin. Stinging members of the phylum Cnidaria can colonize the sponge, leading to injury when a human handles the sponge.25-27

Sponge dermatitis can be divided into 2 major categories: an initial pruritic dermatitis (Figure 3) that occurs within 20 minutes to a few hours after contact and a delayed irritant dermatitis caused by penetration of the spicules and chemical agents into skin.28 Importantly, different species can lead to varying manifestations.

The initial pruritic dermatitis is characterized by itching and burning that progresses to local edema, vesiculation, joint swelling, and stiffness. Because most contact with sponges occurs with handling, joint immobility may ensue within 24 hours of the encounter. Rarely, larger areas of the skin are affected, and fever, chills, malaise, dizziness, nausea, purulent bullae, muscle cramps, and formication may occur.28 Anaphylactic reactions have been described in a small subset of patients. There have even been reports of delayed (ie, 1–2 weeks following exposure) erythema multiforme, livedo reticularis, purpura, and dyshidrotic eczema.16,20,29 The irritant dermatitis caused by spicule trauma is due to a foreign body reaction that can be exacerbated by toxins entering the skin. In severe cases, desquamation, recurrent eczema, and arthralgia can occur.30 In general, more mild cases should self-resolve within 3 to 7 days. Dermatologic conditions also can be caused by organisms that inhabit sponges and as a result produce a dermatitis when the sponge is handled, including sponge divers disease (maladie des plongeurs), a necrotic dermatitis caused by stinging Cnidaria species.31 Dogger Bank itch, first described as a dermatitis caused by sensitization to (2-hydroxyethyl) dimethylsulfoxonium chloride, initially was isolated from the sea chervil (a type of Bryozoan); however, that same chemical also was later found in sponges, producing the same dermatitis after handling the sponge.32 Freshwater sponges also have been reported to be injurious and exist worldwide. In contrast to marine sponges, lesions from freshwater sponges are disseminated pruritic erythematous papules with ulcerations, crusts, and secondary infections.22 The disseminated nature of the dermatitis caused by freshwater sponges is due to contact with the spicules of dead sponges that are dispersed throughout the water rather than from direct handling. Sponge dermatitis occurs mostly in sponge collectors, divers, trawlers, and biology students and has been reported extensively in the United States, Caribbean Islands, Australia, New Zealand, and Brazil.18,27,33,34
Management
Treatment should consist of an initial decontamination; the skin should be dried, and adhesive tape or rubber cement should be utilized to remove any spicules embedded in the skin. Diluted vinegar soaks should be initiated for 10 to 30 minutes on the affected area(s) 3 or 4 times daily.19 The initial decontamination should occur immediately, as delay may lead to persistent purulent bullae that may take months to heal. Topical steroids may be used following the initial decontamination to help relieve inflammation. Antihistamines and nonsteroidal anti-inflammatory drugs may be used to alleviate pruritus and pain, respectively. Severe cases may require systemic glucocorticoids. Additionally, immunization status against tetanus toxoid should be assessed.35 In the event of an anaphylactic reaction, it is important to maintain a patent airway and normalized blood pressure through the use of intramuscular epinephrine.36 Frequent follow-up is warranted, as serious secondary infections can develop.37 Patients also should be counseled on the potential for delayed dermatologic reactions, including erythema multiforme. Contact between humans and coastal environments has been increasing in the last few decades; therefore, an increase in contact with sponges is to be expected.22
Sponges are among the oldest animals on earth, appearing more than 640 million years ago before the Cambrian explosion, a period when most major animal phyla appeared in the fossil records.1 More than 10,000 species of sponges have been identified worldwide and are distributed from polar to tropical regions in both marine (Figure 1) and freshwater (Figure 2) environments. They inhabit both shallow waters as well as depths of more than 2800 m, with shallower sponges tending to be more vibrantly colored than their deeper counterparts. The wide-ranging habitats of sponges have led to size variations from as small as 0.05 mm to more than 3 m in height.2 Their taxonomic phylum, Porifera (meaning pore bearers), is derived from the millions of pores lining the surface of the sponge that are used to filter planktonic organisms.3 Flagellated epithelioid cells called choanocytes line the internal chambers of sponges, creating a water current that promotes filter feeding as well as nutrient absorption across their microvilli.4 The body walls of many sponges consist of a collagenous skeleton made up of spongin and spicules of silicon dioxide (silica) or calcium carbonate embedded in the spongin connective tissue matrix.5 Bath sponges lack silica spicules.


Sponges have been used in medicine for centuries. The first use in Western culture was recorded in 405
Mechanisms and Symptoms of Injury
Bathing sponges (silk sponges) derived from Spongia officinalis are harmless. Other sponges can exert their damaging effects through a variety of mechanisms that lead to dermatologic manifestations (eTable). Some species of sponges produce and secrete toxic metabolites (eg, crinotoxins) onto the body surface or into the surrounding water. They also are capable of synthesizing a mucous slime that can be irritating to human skin. Direct trauma also can be caused by fragments of the silica or calcium carbonate sponge skeleton penetrating the skin. Stinging members of the phylum Cnidaria can colonize the sponge, leading to injury when a human handles the sponge.25-27

Sponge dermatitis can be divided into 2 major categories: an initial pruritic dermatitis (Figure 3) that occurs within 20 minutes to a few hours after contact and a delayed irritant dermatitis caused by penetration of the spicules and chemical agents into skin.28 Importantly, different species can lead to varying manifestations.

The initial pruritic dermatitis is characterized by itching and burning that progresses to local edema, vesiculation, joint swelling, and stiffness. Because most contact with sponges occurs with handling, joint immobility may ensue within 24 hours of the encounter. Rarely, larger areas of the skin are affected, and fever, chills, malaise, dizziness, nausea, purulent bullae, muscle cramps, and formication may occur.28 Anaphylactic reactions have been described in a small subset of patients. There have even been reports of delayed (ie, 1–2 weeks following exposure) erythema multiforme, livedo reticularis, purpura, and dyshidrotic eczema.16,20,29 The irritant dermatitis caused by spicule trauma is due to a foreign body reaction that can be exacerbated by toxins entering the skin. In severe cases, desquamation, recurrent eczema, and arthralgia can occur.30 In general, more mild cases should self-resolve within 3 to 7 days. Dermatologic conditions also can be caused by organisms that inhabit sponges and as a result produce a dermatitis when the sponge is handled, including sponge divers disease (maladie des plongeurs), a necrotic dermatitis caused by stinging Cnidaria species.31 Dogger Bank itch, first described as a dermatitis caused by sensitization to (2-hydroxyethyl) dimethylsulfoxonium chloride, initially was isolated from the sea chervil (a type of Bryozoan); however, that same chemical also was later found in sponges, producing the same dermatitis after handling the sponge.32 Freshwater sponges also have been reported to be injurious and exist worldwide. In contrast to marine sponges, lesions from freshwater sponges are disseminated pruritic erythematous papules with ulcerations, crusts, and secondary infections.22 The disseminated nature of the dermatitis caused by freshwater sponges is due to contact with the spicules of dead sponges that are dispersed throughout the water rather than from direct handling. Sponge dermatitis occurs mostly in sponge collectors, divers, trawlers, and biology students and has been reported extensively in the United States, Caribbean Islands, Australia, New Zealand, and Brazil.18,27,33,34
Management
Treatment should consist of an initial decontamination; the skin should be dried, and adhesive tape or rubber cement should be utilized to remove any spicules embedded in the skin. Diluted vinegar soaks should be initiated for 10 to 30 minutes on the affected area(s) 3 or 4 times daily.19 The initial decontamination should occur immediately, as delay may lead to persistent purulent bullae that may take months to heal. Topical steroids may be used following the initial decontamination to help relieve inflammation. Antihistamines and nonsteroidal anti-inflammatory drugs may be used to alleviate pruritus and pain, respectively. Severe cases may require systemic glucocorticoids. Additionally, immunization status against tetanus toxoid should be assessed.35 In the event of an anaphylactic reaction, it is important to maintain a patent airway and normalized blood pressure through the use of intramuscular epinephrine.36 Frequent follow-up is warranted, as serious secondary infections can develop.37 Patients also should be counseled on the potential for delayed dermatologic reactions, including erythema multiforme. Contact between humans and coastal environments has been increasing in the last few decades; therefore, an increase in contact with sponges is to be expected.22
- Gold DA, Grabenstatter J, de Mendoza A, et al. Sterol and genomic analyses validate the sponge biomarker hypothesis. Proc Natl Acad Sci U S A. 2016;113:2684-2689.
- Bonamonte D, Filoni A, Verni P, et al. Dermatitis caused by sponges. In: Bonamonte D, Angelini G, eds. Aquatic Dermatology. 2nd ed. Springer; 2016:121-126.
- Marsh LM, Slack-Smith S, Gurry DL. Field Guide to Sea Stingers and Other Venomous and Poisonous Marine Invertebrates. 2nd ed. Western Australian Museum; 2010.
- Eid E, Al-Tawaha M. A Guide to Harmful and Toxic Creatures in the Gulf of Aqaba Jordan. The Royal Marine Conservation Society of Jordan; 2016.
- Reese E, Depenbrock P. Water envenomations and stings. Curr Sports Med Rep. 2014;13:126-131.
- Dormandy TL. Trace element analysis of hair. Br Med J (Clin Res Ed). 1986;293:975-976.
- Voultsiadou E. Sponges: an historical survey of their knowledge in Greek antiquity. J Mar Biol Assoc UK. 2007;87:1757-1763.
- Senthilkumar K, Kim SK. Marine invertebrate natural products for anti-inflammatory and chronic diseases [published online December 31, 2013]. Evid Based Complement Alternat Med. doi:10.1155/2013/572859
- Sagar S, Kaur M, Minneman KP. Antiviral lead compounds from marine sponges. Mar Drugs. 2010;8:2619-2638.
- Usagawa T, Nishimura M, Itoh Y, et al. Preparation of monoclonal antibodies against okadaic acid prepared from the sponge Halichondria okadai. Toxicon. 1989;27:1323-1330.
- Elston DM. Aquatic antagonists: sponge dermatitis. Cutis. 2007;80:279-280.
- Parra-Velandia FJ, Zea S, Van Soest RW. Reef sponges of the genus Agelas (Porifera: Demospongiae) from the Greater Caribbean. Zootaxa. 2014;3794:301-343.
- Hooper JN, Capon RJ, Hodder RA. A new species of toxic marine sponge (Porifera: Demospongiae: Poecilosclerida) from northwest Australia. The Beagle, Records of the Northern Territory Museum of Arts and sciences. 1991;8:27-36.
- Burnett JW, Calton GJ, Morgan RJ. Dermatitis due to stinging sponges. Cutis. 1987;39:476.
- Kizer KW. Marine envenomations. J Toxicol Clin Toxicol. 1983;21:527-555.
- Isbister GK, Hooper JN. Clinical effects of stings by sponges of the genus Tedania and a review of sponge stings worldwide. Toxicon. 2005;46:782-785.
- Fromont J, Abdo DA. New species of Haliclona (Demospongiae: Haplosclerida: Chalinidae) from Western Australia. Zootaxa. 2014;3835:97-109.
- Flachsenberger W, Holmes NJ, Leigh C, et al. Properties of the extract and spicules of the dermatitis inducing sponge Neofibularia mordens Hartman. J Toxicol Clin Toxicol. 1987;25:255-272.
- Southcott RV, Coulter JR. The effects of the southern Australian marine stinging sponges, Neofibularia mordens and Lissodendoryx sp. Med J Aust. 1971;2:895-901.
- Yaffee HS, Stargardter F. Erythema multiforme from Tedania ignis. report of a case and an experimental study of the mechanism of cutaneous irritation from the fire sponge. Arch Dermatol. 1963;87:601-604.
- Yaffee HS. Irritation from red sponge. N Engl J Med. 1970;282:51.
- Haddad V Jr. Environmental dermatology: skin manifestations of injuries caused by invertebrate aquatic animals. An Bras Dermatol. 2013;88:496-506.
- Volkmer-Ribeiro C, Lenzi HL, Orefice F, et al. Freshwater sponge spicules: a new agent of ocular pathology. Mem Inst Oswaldo Cruz. 2006;101:899-903.
- Cruz AA, Alencar VM, Medina NH, et al. Dangerous waters: outbreak of eye lesions caused by fresh water sponge spicules. Eye (Lond). 2013;27:398-402.
- Haddad V Jr. Clinical and therapeutic aspects of envenomations caused by sponges and jellyfish. In: Gopalakrishnakone P, Haddad V Jr, Kem WR, et al, eds. Marine and Freshwater Toxins. Springer; 2016:317-325.
- Haddad V Jr, Lupi O, Lonza JP, et al. Tropical dermatology: marine and aquatic dermatology. J Am Acad Dermatol. 2009;61:733-750.
- Gaastra MT. Aquatic skin disorders. In: Faber WR, Hay RJ, Naafs B, eds. Imported Skin Diseases. 2nd ed. Wiley; 2012:283-292.
- Auerbach P. Envenomation by aquatic invertebrates. In: Auerbach P, ed. Wilderness Medicine. 6th ed. Elsevier Mosby; 2011;1596-1627.
- Sims JK, Irei MY. Human Hawaiian marine sponge poisoning. Hawaii Med J. 1979;38:263-270.
- Haddad V Jr. Aquatic animals of medical importance in Brazil. Rev Soc Bras Med Trop. 2003;36:591-597.
- Tlougan BE, Podjasek JO, Adams BB. Aquatic sports dermatoses. part 2—in the water: saltwater dermatoses. Int J Dermatol. 2010;49:994-1002.
- Warabi K, Nakao Y, Matsunaga S, et al. Dogger Bank itch revisited: isolation of (2-hydroxyethyl) dimethylsulfoxonium chloride as a cytotoxic constituent from the marine sponge Theonella aff. mirabilis. Comp Biochem Physiol B Biochem Mol Biol. 2001;128:27-30.
- Southcott R. Human injuries from invertebrate animals in the Australian seas. Clin Toxicol. 1970;3:617-636.
- Russell FE. Sponge injury—traumatic, toxic or allergic? N Engl J Med. 1970;282:753-754.
- Hornbeak KB, Auerbach PS. Marine envenomation. Emerg Med Clin North Am. 2017;35:321-337.
- Muraro A, Roberts G, Worm M, et al. Anaphylaxis: guidelines from the European Academy of Allergy and Clinical Immunology. Allergy. 2014;69:1026-1045.
- Kizer K, Auerbach P, Dwyer B. Marine envenomations: not just a problem of the tropics. Emerg Med Rep. 1985;6:129-135.
- Gold DA, Grabenstatter J, de Mendoza A, et al. Sterol and genomic analyses validate the sponge biomarker hypothesis. Proc Natl Acad Sci U S A. 2016;113:2684-2689.
- Bonamonte D, Filoni A, Verni P, et al. Dermatitis caused by sponges. In: Bonamonte D, Angelini G, eds. Aquatic Dermatology. 2nd ed. Springer; 2016:121-126.
- Marsh LM, Slack-Smith S, Gurry DL. Field Guide to Sea Stingers and Other Venomous and Poisonous Marine Invertebrates. 2nd ed. Western Australian Museum; 2010.
- Eid E, Al-Tawaha M. A Guide to Harmful and Toxic Creatures in the Gulf of Aqaba Jordan. The Royal Marine Conservation Society of Jordan; 2016.
- Reese E, Depenbrock P. Water envenomations and stings. Curr Sports Med Rep. 2014;13:126-131.
- Dormandy TL. Trace element analysis of hair. Br Med J (Clin Res Ed). 1986;293:975-976.
- Voultsiadou E. Sponges: an historical survey of their knowledge in Greek antiquity. J Mar Biol Assoc UK. 2007;87:1757-1763.
- Senthilkumar K, Kim SK. Marine invertebrate natural products for anti-inflammatory and chronic diseases [published online December 31, 2013]. Evid Based Complement Alternat Med. doi:10.1155/2013/572859
- Sagar S, Kaur M, Minneman KP. Antiviral lead compounds from marine sponges. Mar Drugs. 2010;8:2619-2638.
- Usagawa T, Nishimura M, Itoh Y, et al. Preparation of monoclonal antibodies against okadaic acid prepared from the sponge Halichondria okadai. Toxicon. 1989;27:1323-1330.
- Elston DM. Aquatic antagonists: sponge dermatitis. Cutis. 2007;80:279-280.
- Parra-Velandia FJ, Zea S, Van Soest RW. Reef sponges of the genus Agelas (Porifera: Demospongiae) from the Greater Caribbean. Zootaxa. 2014;3794:301-343.
- Hooper JN, Capon RJ, Hodder RA. A new species of toxic marine sponge (Porifera: Demospongiae: Poecilosclerida) from northwest Australia. The Beagle, Records of the Northern Territory Museum of Arts and sciences. 1991;8:27-36.
- Burnett JW, Calton GJ, Morgan RJ. Dermatitis due to stinging sponges. Cutis. 1987;39:476.
- Kizer KW. Marine envenomations. J Toxicol Clin Toxicol. 1983;21:527-555.
- Isbister GK, Hooper JN. Clinical effects of stings by sponges of the genus Tedania and a review of sponge stings worldwide. Toxicon. 2005;46:782-785.
- Fromont J, Abdo DA. New species of Haliclona (Demospongiae: Haplosclerida: Chalinidae) from Western Australia. Zootaxa. 2014;3835:97-109.
- Flachsenberger W, Holmes NJ, Leigh C, et al. Properties of the extract and spicules of the dermatitis inducing sponge Neofibularia mordens Hartman. J Toxicol Clin Toxicol. 1987;25:255-272.
- Southcott RV, Coulter JR. The effects of the southern Australian marine stinging sponges, Neofibularia mordens and Lissodendoryx sp. Med J Aust. 1971;2:895-901.
- Yaffee HS, Stargardter F. Erythema multiforme from Tedania ignis. report of a case and an experimental study of the mechanism of cutaneous irritation from the fire sponge. Arch Dermatol. 1963;87:601-604.
- Yaffee HS. Irritation from red sponge. N Engl J Med. 1970;282:51.
- Haddad V Jr. Environmental dermatology: skin manifestations of injuries caused by invertebrate aquatic animals. An Bras Dermatol. 2013;88:496-506.
- Volkmer-Ribeiro C, Lenzi HL, Orefice F, et al. Freshwater sponge spicules: a new agent of ocular pathology. Mem Inst Oswaldo Cruz. 2006;101:899-903.
- Cruz AA, Alencar VM, Medina NH, et al. Dangerous waters: outbreak of eye lesions caused by fresh water sponge spicules. Eye (Lond). 2013;27:398-402.
- Haddad V Jr. Clinical and therapeutic aspects of envenomations caused by sponges and jellyfish. In: Gopalakrishnakone P, Haddad V Jr, Kem WR, et al, eds. Marine and Freshwater Toxins. Springer; 2016:317-325.
- Haddad V Jr, Lupi O, Lonza JP, et al. Tropical dermatology: marine and aquatic dermatology. J Am Acad Dermatol. 2009;61:733-750.
- Gaastra MT. Aquatic skin disorders. In: Faber WR, Hay RJ, Naafs B, eds. Imported Skin Diseases. 2nd ed. Wiley; 2012:283-292.
- Auerbach P. Envenomation by aquatic invertebrates. In: Auerbach P, ed. Wilderness Medicine. 6th ed. Elsevier Mosby; 2011;1596-1627.
- Sims JK, Irei MY. Human Hawaiian marine sponge poisoning. Hawaii Med J. 1979;38:263-270.
- Haddad V Jr. Aquatic animals of medical importance in Brazil. Rev Soc Bras Med Trop. 2003;36:591-597.
- Tlougan BE, Podjasek JO, Adams BB. Aquatic sports dermatoses. part 2—in the water: saltwater dermatoses. Int J Dermatol. 2010;49:994-1002.
- Warabi K, Nakao Y, Matsunaga S, et al. Dogger Bank itch revisited: isolation of (2-hydroxyethyl) dimethylsulfoxonium chloride as a cytotoxic constituent from the marine sponge Theonella aff. mirabilis. Comp Biochem Physiol B Biochem Mol Biol. 2001;128:27-30.
- Southcott R. Human injuries from invertebrate animals in the Australian seas. Clin Toxicol. 1970;3:617-636.
- Russell FE. Sponge injury—traumatic, toxic or allergic? N Engl J Med. 1970;282:753-754.
- Hornbeak KB, Auerbach PS. Marine envenomation. Emerg Med Clin North Am. 2017;35:321-337.
- Muraro A, Roberts G, Worm M, et al. Anaphylaxis: guidelines from the European Academy of Allergy and Clinical Immunology. Allergy. 2014;69:1026-1045.
- Kizer K, Auerbach P, Dwyer B. Marine envenomations: not just a problem of the tropics. Emerg Med Rep. 1985;6:129-135.
Practice Points
- Sponges exist in both marine and freshwater environments throughout the world.
- Immediate management of sponge dermatitis should include decontamination by removing the sponge spicules with tape or rubber cement followed by dilute vinegar soaks.
- Topical steroids may be used only after initial decontamination. Use of oral steroids may be needed for more severe reactions.