User login
When Female Patients with MS Ask About Breastfeeding, Here’s What to Tell Them
Chances are your female patients of childbearing age with multiple sclerosis—particularly if they become pregnant—will ask about breastfeeding. What are they likely to ask, and how should you answer? Here’s a quick rundown.
What kind of impact will breastfeeding have on my child?
We know that MS is not a genetic disease per se-it is neither autosomal recessive nor dominant. But there is an increased risk among family members, particularly first-degree relatives. If a patient asks, you can tell them it appears that infants who are breastfed are less likely to develop pediatric-onset MS.
In 2017, Brenton and colleagues asked individuals who experienced pediatric-onset MS (n=36) and those in a control group (n=72) to complete a questionnaire that covered breastfeeding history and other birth and demographic features. While most demographic and birth features were similar, 36% of those in the pediatric-onset MS group reported being breastfed, compared with 71% of controls. Individuals who were not breastfed were nearly 4.5 times more likely to be diagnosed with pediatric-onset MS.
How will breastfeeding impact my risk of MS relapse after giving birth?
The issue of breastfeeding and MS relapses is somewhat controversial. In 1988, Nelson and colleagues found that among 191 women with MS who became pregnant, 10% relapsed during pregnancy, but relapse rate rose to 34% during the 9 months after birth. Moreover, nearly 4 in 10 of those who breastfed experienced exacerbations, versus 3 in 10 among those who did not.
However, more recent studies demonstrate no association with breastfeeding and relapse. Just this year, Gould and colleagues published a study showing that among 466 pregnancies, annualized relapse rates declined during pregnancy, and there was no increase seen in the postpartum period. Moreover, women who exclusively breastfed saw their risk of an early postpartum relapse lowered by 63%.
In late 2019, Krysko and colleagues published a meta-analysis of 24 studies involving nearly 3,000 women with MS which showed that breastfeeds were 43% less likely to experience postpartum relapse compared with their non-breastfeeding counterparts. The link was stronger in studies where women breastfed exclusively.
The bottom line: There is a plurality of physicians who believe that breastfeeding has a protective effect – and most will tell you that you should recommend exclusive breastfeeding.
What medicines can I take that will not adversely affect me and my baby?
Once a woman knows that breastfeeding could help her offspring avoid developing MS, and minimize her chance of a postpartum relapse, she will likely ask what to do about medications. You answer will depends on what she’s taking.
- Drugs she can take with relative peace of mind. Most experts believe it is safe to take corticosteroids and breastfeed. In fact, women who relapse while breastfeeding will in all likelihood be given intravenous corticosteroids, such as methylprednisolone. These medications are present in the blood at very low levels, peak an hour after infusion, and quickly dissipate. So, it’s important to tell your patients to delay breastfeeding by 2 to 4 hours after they receive the steroid.
- Drugs that are potentially concerning and require close monitoring. For the so-called platform therapies—such as interferon beta/glatiramer acetate, natalizumab, and their generic equivalents—there are no large studies that clearly demonstrate safety. Still, they are generally thought to be safe. Be sure to heed FDA labeling: weigh breastfeeding benefit against the potential risk
- Drug to avoid entirely. Under no circumstances should breastfeeding women receive teriflunomide, cladribine, alemtuzumab, or mitoxantrone. The jury is still out on rituximab—which is not yet approved for MS in the United States—and ocrelizumab. For now, err on the safe side and switch to another therapy.
Chances are your female patients of childbearing age with multiple sclerosis—particularly if they become pregnant—will ask about breastfeeding. What are they likely to ask, and how should you answer? Here’s a quick rundown.
What kind of impact will breastfeeding have on my child?
We know that MS is not a genetic disease per se-it is neither autosomal recessive nor dominant. But there is an increased risk among family members, particularly first-degree relatives. If a patient asks, you can tell them it appears that infants who are breastfed are less likely to develop pediatric-onset MS.
In 2017, Brenton and colleagues asked individuals who experienced pediatric-onset MS (n=36) and those in a control group (n=72) to complete a questionnaire that covered breastfeeding history and other birth and demographic features. While most demographic and birth features were similar, 36% of those in the pediatric-onset MS group reported being breastfed, compared with 71% of controls. Individuals who were not breastfed were nearly 4.5 times more likely to be diagnosed with pediatric-onset MS.
How will breastfeeding impact my risk of MS relapse after giving birth?
The issue of breastfeeding and MS relapses is somewhat controversial. In 1988, Nelson and colleagues found that among 191 women with MS who became pregnant, 10% relapsed during pregnancy, but relapse rate rose to 34% during the 9 months after birth. Moreover, nearly 4 in 10 of those who breastfed experienced exacerbations, versus 3 in 10 among those who did not.
However, more recent studies demonstrate no association with breastfeeding and relapse. Just this year, Gould and colleagues published a study showing that among 466 pregnancies, annualized relapse rates declined during pregnancy, and there was no increase seen in the postpartum period. Moreover, women who exclusively breastfed saw their risk of an early postpartum relapse lowered by 63%.
In late 2019, Krysko and colleagues published a meta-analysis of 24 studies involving nearly 3,000 women with MS which showed that breastfeeds were 43% less likely to experience postpartum relapse compared with their non-breastfeeding counterparts. The link was stronger in studies where women breastfed exclusively.
The bottom line: There is a plurality of physicians who believe that breastfeeding has a protective effect – and most will tell you that you should recommend exclusive breastfeeding.
What medicines can I take that will not adversely affect me and my baby?
Once a woman knows that breastfeeding could help her offspring avoid developing MS, and minimize her chance of a postpartum relapse, she will likely ask what to do about medications. You answer will depends on what she’s taking.
- Drugs she can take with relative peace of mind. Most experts believe it is safe to take corticosteroids and breastfeed. In fact, women who relapse while breastfeeding will in all likelihood be given intravenous corticosteroids, such as methylprednisolone. These medications are present in the blood at very low levels, peak an hour after infusion, and quickly dissipate. So, it’s important to tell your patients to delay breastfeeding by 2 to 4 hours after they receive the steroid.
- Drugs that are potentially concerning and require close monitoring. For the so-called platform therapies—such as interferon beta/glatiramer acetate, natalizumab, and their generic equivalents—there are no large studies that clearly demonstrate safety. Still, they are generally thought to be safe. Be sure to heed FDA labeling: weigh breastfeeding benefit against the potential risk
- Drug to avoid entirely. Under no circumstances should breastfeeding women receive teriflunomide, cladribine, alemtuzumab, or mitoxantrone. The jury is still out on rituximab—which is not yet approved for MS in the United States—and ocrelizumab. For now, err on the safe side and switch to another therapy.
Chances are your female patients of childbearing age with multiple sclerosis—particularly if they become pregnant—will ask about breastfeeding. What are they likely to ask, and how should you answer? Here’s a quick rundown.
What kind of impact will breastfeeding have on my child?
We know that MS is not a genetic disease per se-it is neither autosomal recessive nor dominant. But there is an increased risk among family members, particularly first-degree relatives. If a patient asks, you can tell them it appears that infants who are breastfed are less likely to develop pediatric-onset MS.
In 2017, Brenton and colleagues asked individuals who experienced pediatric-onset MS (n=36) and those in a control group (n=72) to complete a questionnaire that covered breastfeeding history and other birth and demographic features. While most demographic and birth features were similar, 36% of those in the pediatric-onset MS group reported being breastfed, compared with 71% of controls. Individuals who were not breastfed were nearly 4.5 times more likely to be diagnosed with pediatric-onset MS.
How will breastfeeding impact my risk of MS relapse after giving birth?
The issue of breastfeeding and MS relapses is somewhat controversial. In 1988, Nelson and colleagues found that among 191 women with MS who became pregnant, 10% relapsed during pregnancy, but relapse rate rose to 34% during the 9 months after birth. Moreover, nearly 4 in 10 of those who breastfed experienced exacerbations, versus 3 in 10 among those who did not.
However, more recent studies demonstrate no association with breastfeeding and relapse. Just this year, Gould and colleagues published a study showing that among 466 pregnancies, annualized relapse rates declined during pregnancy, and there was no increase seen in the postpartum period. Moreover, women who exclusively breastfed saw their risk of an early postpartum relapse lowered by 63%.
In late 2019, Krysko and colleagues published a meta-analysis of 24 studies involving nearly 3,000 women with MS which showed that breastfeeds were 43% less likely to experience postpartum relapse compared with their non-breastfeeding counterparts. The link was stronger in studies where women breastfed exclusively.
The bottom line: There is a plurality of physicians who believe that breastfeeding has a protective effect – and most will tell you that you should recommend exclusive breastfeeding.
What medicines can I take that will not adversely affect me and my baby?
Once a woman knows that breastfeeding could help her offspring avoid developing MS, and minimize her chance of a postpartum relapse, she will likely ask what to do about medications. You answer will depends on what she’s taking.
- Drugs she can take with relative peace of mind. Most experts believe it is safe to take corticosteroids and breastfeed. In fact, women who relapse while breastfeeding will in all likelihood be given intravenous corticosteroids, such as methylprednisolone. These medications are present in the blood at very low levels, peak an hour after infusion, and quickly dissipate. So, it’s important to tell your patients to delay breastfeeding by 2 to 4 hours after they receive the steroid.
- Drugs that are potentially concerning and require close monitoring. For the so-called platform therapies—such as interferon beta/glatiramer acetate, natalizumab, and their generic equivalents—there are no large studies that clearly demonstrate safety. Still, they are generally thought to be safe. Be sure to heed FDA labeling: weigh breastfeeding benefit against the potential risk
- Drug to avoid entirely. Under no circumstances should breastfeeding women receive teriflunomide, cladribine, alemtuzumab, or mitoxantrone. The jury is still out on rituximab—which is not yet approved for MS in the United States—and ocrelizumab. For now, err on the safe side and switch to another therapy.
ICYMI: MSVirtual2020 Virtual Joint ACTRIMS-ECTRIMS Meeting Summary from MS Resource Center Editor in Chief, Joseph R. Berger, MD
I had the privilege of attending and speaking at the recent MSVirtual2020—the 8th Joint ACTRIMS-ECTRIMS Meeting. I came away with a wealth of knowledge, much of which can be put to immediate use in practice, and some that shows the promise of eventual clinical utility.
Dr. Helen Tremlett, PhD, kicked off the meeting with a keynote address covering her important work on the MS prodrome. The Canada research chair in neuroepidemiology and multiple sclerosis at the University of British Columbia summarized her team’s research to date and offered her thoughts on clinical implications.
Dr. Tremlett’s group has observed that in the five years before an MS symptom onset, individuals who would ultimately be diagnosed tended to experience more hospitalizations, visit their provider more, and fill more prescriptions than did those in the general population. The team dug deeper and found that these individuals experienced a range of issues prior to symptom onset, including pain, headache, migraine, fibromyalgia, irritable bowel syndrome, sleep disturbances, depression/anxiety, and dermatologic issues.
Interestingly, females in this group were less likely to become pregnant and more likely than healthy females to fill an oral contraceptive prescription, suggesting that they were trying to delay pregnancy due to these prodromal symptoms.
Dr. Tremlett noted that the more immediate implications of her group’s work are for clinical researchers, who can now use these findings to understand that there is a prodromal stage as they conduct clinical trials. The ultimate aim is to use this work to develop a diagnostic tool, but that will take more time and study.
COVID-19’s Impact on MS
The impact on COVID-19 on individuals with MS was addressed in a number of sessions. I presented data that clearly shows the risk of infection from COVID-19 is similar to that of the population at large.
- A critical evaluation of MS disease modifying therapies (DMTs) and their potential effects on COVID-19 that I published with my colleagues at the University of Pennsylvania suggested that DMTs might not increase the risk of morbidity and mortality associated with COVID-19 as some had feared. We based this conclusion on an evaluation of pathogenesis of COVID, the importance of the innate immune system in control of exposure to a novel pathogen, and the likely effects, both salutary and pernicious, of DMTs on COVID morbidity and mortality.
- Investigators from Italy looked at 232 patients from 38 centers with MS and confirmed or suspected COVID and found that the vast majority of them (96%) had mild disease consisting of no or mild pneumonia. The remainder had either severe (2%) or critical (3%) disease. These investigators have since expanded their observations and suggested that anti-CD20 monoclonal antibody treatment may be associated with a higher risk of hospitalization, though there did not appear to be an increase in the risk of death with their use. Importantly, the anti-CD20 monoclonal antibody therapies are the DMTs routinely used in patients with progressive MS, generally, the MS population at greatest risk of hospitalization with COVID-19 due to their older age, co-morbidities, and level of debility.
- Recently, French researchers evaluated 347 individuals with MS and COVID by COVID disease severity. They found that there was a higher proportion of patients with severe COVID not receiving DMT compared with individuals receiving treatment (46% and 15%, respectively).
The Increasing Importance of sNfL Concentration
Serum neurofilament light chain (sNfL) concentration continues to be a hot topic. Dr. Jens Kuhle, head of the Multiple Sclerosis Centre at the University of Basel, and colleagues have demonstrated that sNfL levels can play a role in monitoring MS treatment in practice. They evaluated more than 1000 individuals who were taking DMTs, measuring sNfL and deriving a score that reflected how participants fared relative to healthy controls of the same age. Among their findings:
- The resulting score predicted clinical events in the following year, with the effect escalating in magnitude in those whose scores were higher.
- This same predictive effect was seen with respect to future new/enlarging T2 lesions and brain volume loss.
- Score change in patients with NEDA-03 status was linked with a 37% increased risk of clinical events in the following year.
New Radiologic Techniques
Encouraging findings on new radiologic techniques were presented. I found three studies extremely informative. The first two have immediate or near-immediate clinical implications, and the third shows promise.
- In a comparison of patients with MS and healthy individuals who underwent brain 3T MRI to assess lesions and atrophy, R. Bonacchi and colleagues from Milan, Italy found that cardiovascular (CV) risk factors are linked with brain atrophy in patients with MS, even those <50 years of age. Specifically, the presence of at least two CV risk factors was linked with reduced normalized grey matter volume, white matter volume, and brain volume.
- Another comparison of individuals with MS and healthy controls—this one from O. Al-Louzi and colleagues at the National Institute of Neurological Disorders and Stroke—looked at the central vein sign (CVS) biomarker and determined that excluding lesions only if all dimensions of 3T MRI results were less than threshold (versus if any dimension was less than threshold) led to the inclusion of more CVS-positive lesions. Investigators suggested this work could lead to modified clinical guidelines.
- In an evaluation of patients with MS using 3T MRI, F. LaRosa and colleagues from Lausanne, Switzerland reported that RimNet, a prototype built upon two convolutional neural networks, was better than two alternative methods at detecting pragmatic rim lesions, which are linked with higher disease burden. Compared with expert raters, RimNet had higher sensitivity (87% vs 76%) but lower specificity (91% vs 99%).
There were many other valuable presentations at MSVirtual2020, but perhaps the most appreciated experience was the ability to hear more experts deliver their important work. Unlike a live meeting, I was able to easily attend parallel sessions and to do so at my leisure. ECTRIMS has become so big that I often left the live meeting feeling as if I missed out on a lot. Not this year. I heard almost all of it and came away with a greater appreciation of the breadth and depth of the meeting. I hope that in the future, even following the return of in-person meetings, a virtual format coexists to afford attendees and those unable to attend live the opportunity to experience the totality of the meeting.
I had the privilege of attending and speaking at the recent MSVirtual2020—the 8th Joint ACTRIMS-ECTRIMS Meeting. I came away with a wealth of knowledge, much of which can be put to immediate use in practice, and some that shows the promise of eventual clinical utility.
Dr. Helen Tremlett, PhD, kicked off the meeting with a keynote address covering her important work on the MS prodrome. The Canada research chair in neuroepidemiology and multiple sclerosis at the University of British Columbia summarized her team’s research to date and offered her thoughts on clinical implications.
Dr. Tremlett’s group has observed that in the five years before an MS symptom onset, individuals who would ultimately be diagnosed tended to experience more hospitalizations, visit their provider more, and fill more prescriptions than did those in the general population. The team dug deeper and found that these individuals experienced a range of issues prior to symptom onset, including pain, headache, migraine, fibromyalgia, irritable bowel syndrome, sleep disturbances, depression/anxiety, and dermatologic issues.
Interestingly, females in this group were less likely to become pregnant and more likely than healthy females to fill an oral contraceptive prescription, suggesting that they were trying to delay pregnancy due to these prodromal symptoms.
Dr. Tremlett noted that the more immediate implications of her group’s work are for clinical researchers, who can now use these findings to understand that there is a prodromal stage as they conduct clinical trials. The ultimate aim is to use this work to develop a diagnostic tool, but that will take more time and study.
COVID-19’s Impact on MS
The impact on COVID-19 on individuals with MS was addressed in a number of sessions. I presented data that clearly shows the risk of infection from COVID-19 is similar to that of the population at large.
- A critical evaluation of MS disease modifying therapies (DMTs) and their potential effects on COVID-19 that I published with my colleagues at the University of Pennsylvania suggested that DMTs might not increase the risk of morbidity and mortality associated with COVID-19 as some had feared. We based this conclusion on an evaluation of pathogenesis of COVID, the importance of the innate immune system in control of exposure to a novel pathogen, and the likely effects, both salutary and pernicious, of DMTs on COVID morbidity and mortality.
- Investigators from Italy looked at 232 patients from 38 centers with MS and confirmed or suspected COVID and found that the vast majority of them (96%) had mild disease consisting of no or mild pneumonia. The remainder had either severe (2%) or critical (3%) disease. These investigators have since expanded their observations and suggested that anti-CD20 monoclonal antibody treatment may be associated with a higher risk of hospitalization, though there did not appear to be an increase in the risk of death with their use. Importantly, the anti-CD20 monoclonal antibody therapies are the DMTs routinely used in patients with progressive MS, generally, the MS population at greatest risk of hospitalization with COVID-19 due to their older age, co-morbidities, and level of debility.
- Recently, French researchers evaluated 347 individuals with MS and COVID by COVID disease severity. They found that there was a higher proportion of patients with severe COVID not receiving DMT compared with individuals receiving treatment (46% and 15%, respectively).
The Increasing Importance of sNfL Concentration
Serum neurofilament light chain (sNfL) concentration continues to be a hot topic. Dr. Jens Kuhle, head of the Multiple Sclerosis Centre at the University of Basel, and colleagues have demonstrated that sNfL levels can play a role in monitoring MS treatment in practice. They evaluated more than 1000 individuals who were taking DMTs, measuring sNfL and deriving a score that reflected how participants fared relative to healthy controls of the same age. Among their findings:
- The resulting score predicted clinical events in the following year, with the effect escalating in magnitude in those whose scores were higher.
- This same predictive effect was seen with respect to future new/enlarging T2 lesions and brain volume loss.
- Score change in patients with NEDA-03 status was linked with a 37% increased risk of clinical events in the following year.
New Radiologic Techniques
Encouraging findings on new radiologic techniques were presented. I found three studies extremely informative. The first two have immediate or near-immediate clinical implications, and the third shows promise.
- In a comparison of patients with MS and healthy individuals who underwent brain 3T MRI to assess lesions and atrophy, R. Bonacchi and colleagues from Milan, Italy found that cardiovascular (CV) risk factors are linked with brain atrophy in patients with MS, even those <50 years of age. Specifically, the presence of at least two CV risk factors was linked with reduced normalized grey matter volume, white matter volume, and brain volume.
- Another comparison of individuals with MS and healthy controls—this one from O. Al-Louzi and colleagues at the National Institute of Neurological Disorders and Stroke—looked at the central vein sign (CVS) biomarker and determined that excluding lesions only if all dimensions of 3T MRI results were less than threshold (versus if any dimension was less than threshold) led to the inclusion of more CVS-positive lesions. Investigators suggested this work could lead to modified clinical guidelines.
- In an evaluation of patients with MS using 3T MRI, F. LaRosa and colleagues from Lausanne, Switzerland reported that RimNet, a prototype built upon two convolutional neural networks, was better than two alternative methods at detecting pragmatic rim lesions, which are linked with higher disease burden. Compared with expert raters, RimNet had higher sensitivity (87% vs 76%) but lower specificity (91% vs 99%).
There were many other valuable presentations at MSVirtual2020, but perhaps the most appreciated experience was the ability to hear more experts deliver their important work. Unlike a live meeting, I was able to easily attend parallel sessions and to do so at my leisure. ECTRIMS has become so big that I often left the live meeting feeling as if I missed out on a lot. Not this year. I heard almost all of it and came away with a greater appreciation of the breadth and depth of the meeting. I hope that in the future, even following the return of in-person meetings, a virtual format coexists to afford attendees and those unable to attend live the opportunity to experience the totality of the meeting.
I had the privilege of attending and speaking at the recent MSVirtual2020—the 8th Joint ACTRIMS-ECTRIMS Meeting. I came away with a wealth of knowledge, much of which can be put to immediate use in practice, and some that shows the promise of eventual clinical utility.
Dr. Helen Tremlett, PhD, kicked off the meeting with a keynote address covering her important work on the MS prodrome. The Canada research chair in neuroepidemiology and multiple sclerosis at the University of British Columbia summarized her team’s research to date and offered her thoughts on clinical implications.
Dr. Tremlett’s group has observed that in the five years before an MS symptom onset, individuals who would ultimately be diagnosed tended to experience more hospitalizations, visit their provider more, and fill more prescriptions than did those in the general population. The team dug deeper and found that these individuals experienced a range of issues prior to symptom onset, including pain, headache, migraine, fibromyalgia, irritable bowel syndrome, sleep disturbances, depression/anxiety, and dermatologic issues.
Interestingly, females in this group were less likely to become pregnant and more likely than healthy females to fill an oral contraceptive prescription, suggesting that they were trying to delay pregnancy due to these prodromal symptoms.
Dr. Tremlett noted that the more immediate implications of her group’s work are for clinical researchers, who can now use these findings to understand that there is a prodromal stage as they conduct clinical trials. The ultimate aim is to use this work to develop a diagnostic tool, but that will take more time and study.
COVID-19’s Impact on MS
The impact on COVID-19 on individuals with MS was addressed in a number of sessions. I presented data that clearly shows the risk of infection from COVID-19 is similar to that of the population at large.
- A critical evaluation of MS disease modifying therapies (DMTs) and their potential effects on COVID-19 that I published with my colleagues at the University of Pennsylvania suggested that DMTs might not increase the risk of morbidity and mortality associated with COVID-19 as some had feared. We based this conclusion on an evaluation of pathogenesis of COVID, the importance of the innate immune system in control of exposure to a novel pathogen, and the likely effects, both salutary and pernicious, of DMTs on COVID morbidity and mortality.
- Investigators from Italy looked at 232 patients from 38 centers with MS and confirmed or suspected COVID and found that the vast majority of them (96%) had mild disease consisting of no or mild pneumonia. The remainder had either severe (2%) or critical (3%) disease. These investigators have since expanded their observations and suggested that anti-CD20 monoclonal antibody treatment may be associated with a higher risk of hospitalization, though there did not appear to be an increase in the risk of death with their use. Importantly, the anti-CD20 monoclonal antibody therapies are the DMTs routinely used in patients with progressive MS, generally, the MS population at greatest risk of hospitalization with COVID-19 due to their older age, co-morbidities, and level of debility.
- Recently, French researchers evaluated 347 individuals with MS and COVID by COVID disease severity. They found that there was a higher proportion of patients with severe COVID not receiving DMT compared with individuals receiving treatment (46% and 15%, respectively).
The Increasing Importance of sNfL Concentration
Serum neurofilament light chain (sNfL) concentration continues to be a hot topic. Dr. Jens Kuhle, head of the Multiple Sclerosis Centre at the University of Basel, and colleagues have demonstrated that sNfL levels can play a role in monitoring MS treatment in practice. They evaluated more than 1000 individuals who were taking DMTs, measuring sNfL and deriving a score that reflected how participants fared relative to healthy controls of the same age. Among their findings:
- The resulting score predicted clinical events in the following year, with the effect escalating in magnitude in those whose scores were higher.
- This same predictive effect was seen with respect to future new/enlarging T2 lesions and brain volume loss.
- Score change in patients with NEDA-03 status was linked with a 37% increased risk of clinical events in the following year.
New Radiologic Techniques
Encouraging findings on new radiologic techniques were presented. I found three studies extremely informative. The first two have immediate or near-immediate clinical implications, and the third shows promise.
- In a comparison of patients with MS and healthy individuals who underwent brain 3T MRI to assess lesions and atrophy, R. Bonacchi and colleagues from Milan, Italy found that cardiovascular (CV) risk factors are linked with brain atrophy in patients with MS, even those <50 years of age. Specifically, the presence of at least two CV risk factors was linked with reduced normalized grey matter volume, white matter volume, and brain volume.
- Another comparison of individuals with MS and healthy controls—this one from O. Al-Louzi and colleagues at the National Institute of Neurological Disorders and Stroke—looked at the central vein sign (CVS) biomarker and determined that excluding lesions only if all dimensions of 3T MRI results were less than threshold (versus if any dimension was less than threshold) led to the inclusion of more CVS-positive lesions. Investigators suggested this work could lead to modified clinical guidelines.
- In an evaluation of patients with MS using 3T MRI, F. LaRosa and colleagues from Lausanne, Switzerland reported that RimNet, a prototype built upon two convolutional neural networks, was better than two alternative methods at detecting pragmatic rim lesions, which are linked with higher disease burden. Compared with expert raters, RimNet had higher sensitivity (87% vs 76%) but lower specificity (91% vs 99%).
There were many other valuable presentations at MSVirtual2020, but perhaps the most appreciated experience was the ability to hear more experts deliver their important work. Unlike a live meeting, I was able to easily attend parallel sessions and to do so at my leisure. ECTRIMS has become so big that I often left the live meeting feeling as if I missed out on a lot. Not this year. I heard almost all of it and came away with a greater appreciation of the breadth and depth of the meeting. I hope that in the future, even following the return of in-person meetings, a virtual format coexists to afford attendees and those unable to attend live the opportunity to experience the totality of the meeting.
Please stop using the adjective “elective” to describe the important health services ObGyns provide
During the April 2020 peak of patient admissions to our hospital caused by coronavirus disease 2019 (COVID-19), we severely limited the number of surgical procedures performed to conserve health system resources. During this stressful time, some administrators and physicians began categorizing operations for cancer as "elective" procedures that could be postponed for months. Personally, I think the use of elective to describe cancer surgery is not optimal, even during a pandemic. In reality, the surgeries for patients with cancer were being postponed to ensure that services were available for patients with severe and critical COVID-19 disease, not because the surgeries were "elective." The health system leaders were making the rational decision to prioritize the needs of patients with COVID-19 infections over the needs of patients with cancer. However, they were using an inappropriate description of the rationale for postponing the surgery for patients with cancer—an intellectual short-cut.
This experience prompted me to explore all the medical interventions commonly described as elective. Surprisingly, among medical specialists, obstetricians excel in using the adjective elective to describe our important work. For example, in the medical record we commonly use terms such as “elective induction of labor,” “elective cesarean delivery” (CD) and “elective termination of pregnancy.” I believe it would advance our field if obstetricians stopped using the term elective to describe the important health services we provide.
Stop using the term “elective induction of labor”
Ghartey and Macones recently advocated for all obstetricians to stop using the term elective when describing induction of labor.1 The ARRIVE trial (A Randomized Trial of Induction vs Expectant Management)2 demonstrated that, among nulliparous women at 39 weeks’ gestation, induction of labor resulted in a lower CD rate than expectant management (18.6% vs 22.2%, respectively; relative risk, 0.84; 95% confidence interval [CI], 0.76-0.93). These findings indicate that induction of labor is not elective because it provides a clear health benefit over the alternative of expectant management. Given current expert guidance, induction of labor prior to 39 weeks’ gestation must be based on an accepted medical indication and provide a health benefit; hence, these inductions are medically indicated. Similarly, since induction of labor at 39 weeks’ gestation also provides a clear health benefit it is also medically indicated and not “elective.” Ghartey and Macones conclude1:
"The words we choose to
describe medical interventions
matter. They send a message
to patients, physicians, nurses,
and hospital administrators.
When the term 'elective' is applied to a medical intervention,
it implies that it is not really
necessary. That is certainly not
the case when it comes to 39-
week nulliparous induction. The
ARRIVE trial provides grade A
(good and consistent) evidence
that labor induction provided
benefit with no harm to women
and their infants. These inductions are not 'elective'."
An alternative descriptor is “medically indicated” induction.
Continue to: Stop using the term “elective cesarean delivery”...
Stop using the term “elective cesarean delivery”
I recently searched PubMed for publications using the key words, “elective cesarean delivery,” and more than 7,000 publications were identified by the National Library of Medicine. “Elective cesarean delivery” is clearly an important term used by obstetrical authorities. What do we mean by elective CD?
At 39 weeks’ gestation, a low-risk nulliparous pregnant woman has a limited number of options:
- induction of labor
- expectant management awaiting the onset of labor
- scheduled CD before the onset of labor.
For a low-risk pregnant woman at 39 weeks’ gestation, the American College of Obstetricians and Gynecologists recommends vaginal delivery because it best balances the risks and benefits for the woman and newborn.3 When a low-risk nulliparous pregnant woman asks a clinician about a scheduled CD, we are trained to thoroughly explore the reasons for the woman’s request, including her intellectual, fact-based, concerns about labor and vaginal birth and her emotional reaction to the thought of a vaginal or cesarean birth. In this situation the clinician will provide information about the risks and benefits of vaginal versus CD. In the vast majority of situations, the pregnant woman will agree to attempting vaginal delivery. In one study of 458,767 births, only 0.2% of women choose a “maternal request cesarean delivery.”4
After thorough counseling, if a woman and her clinician jointly agree to schedule a primary CD it will be the result of hours of intensive discussion, not an imprudent and hasty decision. In this case, the delivery is best characterized as a “maternal request cesarean delivery,” not an “elective” CD.
Stop using the terms “elective termination of pregnancy” and “elective abortion”
Janiak and Goldberg have advocated for the elimination of the phrase elective abortion.5 They write5:
"Support for abortion varies
depending on the reason for
the abortion—whether it is
'elective' or 'indicated.' In the
case of abortion, these terms
generally differentiate between
women seeking abortion for
reasons of maternal or fetal
health (an 'indicated abortion')
defined in contrast to women
seeking abortion for other
reasons (an 'elective abortion').
We argue that such a distinction is impossible to operationalize in a just manner. The use
of the phrase 'elective abortion'
promotes the institutionalization of a false hierarchy of need
among abortion patients."
My experience is that pregnant women never seek an abortion based on whimsy. Most pregnant women who consider an abortion struggle greatly with the choice, using reason and judgment to arrive at their final decision. The choice to seek an abortion is always a difficult one, influenced by a constellation of hard facts that impact the woman’s life. Using the term elective to describe an abortion implies a moral judgment and stigmatizes the choice to have an abortion. Janiak and Goldberg conclude by recommending the elimination of the phrase 'elective abortion' in favor of the phrase “induced abortion.”5
Continue to: Time for change...
Time for change
Shockingly, in searching the International Statistical Classification of Diseases and Related Health Problems, 10th revision (ICD10), the word elective is most commonly used in the context of health services provided to pregnant women, including: elective induction of labor (Z34.90), elective cesarean delivery (O82), elective termination of pregnancy (Z33.2), and elective fetal reduction (Z031.30X0). In ICD10, other specialties do not describe the scope of their health services with the adjective elective.
There are many definitions and interpretations of elective. The most benign use of the word in the context of surgery is to contrast procedures that can be scheduled in the future with those that need to be performed urgently. In this context elective only refers to the timing, not the medical necessity, of the procedure. By contrast, describing a procedure as elective may signal that it is not medically necessary and is being performed based on the capricious preference of the patient or physician. Given the confusion and misunderstanding that may be caused by describing our important health services as “elective,” I hope that we can permanently sunset use of the term. ●
- Ghartey J, Macones GA. 39-week nulliparous inductions are not elective. Am J Obstet Gynecol. 2020;222:519-520.
- Grobman WA, Rice MM, Reddy UM, et al. Labor induction versus expectant management in low-risk nulliparous women. N Engl J Med. 2018;379:513-523.
- ACOG Committee Opinion No 761: cesarean delivery on maternal request. Obstet Gynecol. 2019;133.e73-e77.
- Gossman GL, Joesch JM, Tanfer K. Trends in maternal request cesarean delivery from 1991 to 2004. Obstet Gynecol. 2006;108:1506-1516.
- Janiak E, Goldberg AB. Eliminating the phrase “elective abortion”: why language matters. Contraception. 2016;93:89-92.
During the April 2020 peak of patient admissions to our hospital caused by coronavirus disease 2019 (COVID-19), we severely limited the number of surgical procedures performed to conserve health system resources. During this stressful time, some administrators and physicians began categorizing operations for cancer as "elective" procedures that could be postponed for months. Personally, I think the use of elective to describe cancer surgery is not optimal, even during a pandemic. In reality, the surgeries for patients with cancer were being postponed to ensure that services were available for patients with severe and critical COVID-19 disease, not because the surgeries were "elective." The health system leaders were making the rational decision to prioritize the needs of patients with COVID-19 infections over the needs of patients with cancer. However, they were using an inappropriate description of the rationale for postponing the surgery for patients with cancer—an intellectual short-cut.
This experience prompted me to explore all the medical interventions commonly described as elective. Surprisingly, among medical specialists, obstetricians excel in using the adjective elective to describe our important work. For example, in the medical record we commonly use terms such as “elective induction of labor,” “elective cesarean delivery” (CD) and “elective termination of pregnancy.” I believe it would advance our field if obstetricians stopped using the term elective to describe the important health services we provide.
Stop using the term “elective induction of labor”
Ghartey and Macones recently advocated for all obstetricians to stop using the term elective when describing induction of labor.1 The ARRIVE trial (A Randomized Trial of Induction vs Expectant Management)2 demonstrated that, among nulliparous women at 39 weeks’ gestation, induction of labor resulted in a lower CD rate than expectant management (18.6% vs 22.2%, respectively; relative risk, 0.84; 95% confidence interval [CI], 0.76-0.93). These findings indicate that induction of labor is not elective because it provides a clear health benefit over the alternative of expectant management. Given current expert guidance, induction of labor prior to 39 weeks’ gestation must be based on an accepted medical indication and provide a health benefit; hence, these inductions are medically indicated. Similarly, since induction of labor at 39 weeks’ gestation also provides a clear health benefit it is also medically indicated and not “elective.” Ghartey and Macones conclude1:
"The words we choose to
describe medical interventions
matter. They send a message
to patients, physicians, nurses,
and hospital administrators.
When the term 'elective' is applied to a medical intervention,
it implies that it is not really
necessary. That is certainly not
the case when it comes to 39-
week nulliparous induction. The
ARRIVE trial provides grade A
(good and consistent) evidence
that labor induction provided
benefit with no harm to women
and their infants. These inductions are not 'elective'."
An alternative descriptor is “medically indicated” induction.
Continue to: Stop using the term “elective cesarean delivery”...
Stop using the term “elective cesarean delivery”
I recently searched PubMed for publications using the key words, “elective cesarean delivery,” and more than 7,000 publications were identified by the National Library of Medicine. “Elective cesarean delivery” is clearly an important term used by obstetrical authorities. What do we mean by elective CD?
At 39 weeks’ gestation, a low-risk nulliparous pregnant woman has a limited number of options:
- induction of labor
- expectant management awaiting the onset of labor
- scheduled CD before the onset of labor.
For a low-risk pregnant woman at 39 weeks’ gestation, the American College of Obstetricians and Gynecologists recommends vaginal delivery because it best balances the risks and benefits for the woman and newborn.3 When a low-risk nulliparous pregnant woman asks a clinician about a scheduled CD, we are trained to thoroughly explore the reasons for the woman’s request, including her intellectual, fact-based, concerns about labor and vaginal birth and her emotional reaction to the thought of a vaginal or cesarean birth. In this situation the clinician will provide information about the risks and benefits of vaginal versus CD. In the vast majority of situations, the pregnant woman will agree to attempting vaginal delivery. In one study of 458,767 births, only 0.2% of women choose a “maternal request cesarean delivery.”4
After thorough counseling, if a woman and her clinician jointly agree to schedule a primary CD it will be the result of hours of intensive discussion, not an imprudent and hasty decision. In this case, the delivery is best characterized as a “maternal request cesarean delivery,” not an “elective” CD.
Stop using the terms “elective termination of pregnancy” and “elective abortion”
Janiak and Goldberg have advocated for the elimination of the phrase elective abortion.5 They write5:
"Support for abortion varies
depending on the reason for
the abortion—whether it is
'elective' or 'indicated.' In the
case of abortion, these terms
generally differentiate between
women seeking abortion for
reasons of maternal or fetal
health (an 'indicated abortion')
defined in contrast to women
seeking abortion for other
reasons (an 'elective abortion').
We argue that such a distinction is impossible to operationalize in a just manner. The use
of the phrase 'elective abortion'
promotes the institutionalization of a false hierarchy of need
among abortion patients."
My experience is that pregnant women never seek an abortion based on whimsy. Most pregnant women who consider an abortion struggle greatly with the choice, using reason and judgment to arrive at their final decision. The choice to seek an abortion is always a difficult one, influenced by a constellation of hard facts that impact the woman’s life. Using the term elective to describe an abortion implies a moral judgment and stigmatizes the choice to have an abortion. Janiak and Goldberg conclude by recommending the elimination of the phrase 'elective abortion' in favor of the phrase “induced abortion.”5
Continue to: Time for change...
Time for change
Shockingly, in searching the International Statistical Classification of Diseases and Related Health Problems, 10th revision (ICD10), the word elective is most commonly used in the context of health services provided to pregnant women, including: elective induction of labor (Z34.90), elective cesarean delivery (O82), elective termination of pregnancy (Z33.2), and elective fetal reduction (Z031.30X0). In ICD10, other specialties do not describe the scope of their health services with the adjective elective.
There are many definitions and interpretations of elective. The most benign use of the word in the context of surgery is to contrast procedures that can be scheduled in the future with those that need to be performed urgently. In this context elective only refers to the timing, not the medical necessity, of the procedure. By contrast, describing a procedure as elective may signal that it is not medically necessary and is being performed based on the capricious preference of the patient or physician. Given the confusion and misunderstanding that may be caused by describing our important health services as “elective,” I hope that we can permanently sunset use of the term. ●
During the April 2020 peak of patient admissions to our hospital caused by coronavirus disease 2019 (COVID-19), we severely limited the number of surgical procedures performed to conserve health system resources. During this stressful time, some administrators and physicians began categorizing operations for cancer as "elective" procedures that could be postponed for months. Personally, I think the use of elective to describe cancer surgery is not optimal, even during a pandemic. In reality, the surgeries for patients with cancer were being postponed to ensure that services were available for patients with severe and critical COVID-19 disease, not because the surgeries were "elective." The health system leaders were making the rational decision to prioritize the needs of patients with COVID-19 infections over the needs of patients with cancer. However, they were using an inappropriate description of the rationale for postponing the surgery for patients with cancer—an intellectual short-cut.
This experience prompted me to explore all the medical interventions commonly described as elective. Surprisingly, among medical specialists, obstetricians excel in using the adjective elective to describe our important work. For example, in the medical record we commonly use terms such as “elective induction of labor,” “elective cesarean delivery” (CD) and “elective termination of pregnancy.” I believe it would advance our field if obstetricians stopped using the term elective to describe the important health services we provide.
Stop using the term “elective induction of labor”
Ghartey and Macones recently advocated for all obstetricians to stop using the term elective when describing induction of labor.1 The ARRIVE trial (A Randomized Trial of Induction vs Expectant Management)2 demonstrated that, among nulliparous women at 39 weeks’ gestation, induction of labor resulted in a lower CD rate than expectant management (18.6% vs 22.2%, respectively; relative risk, 0.84; 95% confidence interval [CI], 0.76-0.93). These findings indicate that induction of labor is not elective because it provides a clear health benefit over the alternative of expectant management. Given current expert guidance, induction of labor prior to 39 weeks’ gestation must be based on an accepted medical indication and provide a health benefit; hence, these inductions are medically indicated. Similarly, since induction of labor at 39 weeks’ gestation also provides a clear health benefit it is also medically indicated and not “elective.” Ghartey and Macones conclude1:
"The words we choose to
describe medical interventions
matter. They send a message
to patients, physicians, nurses,
and hospital administrators.
When the term 'elective' is applied to a medical intervention,
it implies that it is not really
necessary. That is certainly not
the case when it comes to 39-
week nulliparous induction. The
ARRIVE trial provides grade A
(good and consistent) evidence
that labor induction provided
benefit with no harm to women
and their infants. These inductions are not 'elective'."
An alternative descriptor is “medically indicated” induction.
Continue to: Stop using the term “elective cesarean delivery”...
Stop using the term “elective cesarean delivery”
I recently searched PubMed for publications using the key words, “elective cesarean delivery,” and more than 7,000 publications were identified by the National Library of Medicine. “Elective cesarean delivery” is clearly an important term used by obstetrical authorities. What do we mean by elective CD?
At 39 weeks’ gestation, a low-risk nulliparous pregnant woman has a limited number of options:
- induction of labor
- expectant management awaiting the onset of labor
- scheduled CD before the onset of labor.
For a low-risk pregnant woman at 39 weeks’ gestation, the American College of Obstetricians and Gynecologists recommends vaginal delivery because it best balances the risks and benefits for the woman and newborn.3 When a low-risk nulliparous pregnant woman asks a clinician about a scheduled CD, we are trained to thoroughly explore the reasons for the woman’s request, including her intellectual, fact-based, concerns about labor and vaginal birth and her emotional reaction to the thought of a vaginal or cesarean birth. In this situation the clinician will provide information about the risks and benefits of vaginal versus CD. In the vast majority of situations, the pregnant woman will agree to attempting vaginal delivery. In one study of 458,767 births, only 0.2% of women choose a “maternal request cesarean delivery.”4
After thorough counseling, if a woman and her clinician jointly agree to schedule a primary CD it will be the result of hours of intensive discussion, not an imprudent and hasty decision. In this case, the delivery is best characterized as a “maternal request cesarean delivery,” not an “elective” CD.
Stop using the terms “elective termination of pregnancy” and “elective abortion”
Janiak and Goldberg have advocated for the elimination of the phrase elective abortion.5 They write5:
"Support for abortion varies
depending on the reason for
the abortion—whether it is
'elective' or 'indicated.' In the
case of abortion, these terms
generally differentiate between
women seeking abortion for
reasons of maternal or fetal
health (an 'indicated abortion')
defined in contrast to women
seeking abortion for other
reasons (an 'elective abortion').
We argue that such a distinction is impossible to operationalize in a just manner. The use
of the phrase 'elective abortion'
promotes the institutionalization of a false hierarchy of need
among abortion patients."
My experience is that pregnant women never seek an abortion based on whimsy. Most pregnant women who consider an abortion struggle greatly with the choice, using reason and judgment to arrive at their final decision. The choice to seek an abortion is always a difficult one, influenced by a constellation of hard facts that impact the woman’s life. Using the term elective to describe an abortion implies a moral judgment and stigmatizes the choice to have an abortion. Janiak and Goldberg conclude by recommending the elimination of the phrase 'elective abortion' in favor of the phrase “induced abortion.”5
Continue to: Time for change...
Time for change
Shockingly, in searching the International Statistical Classification of Diseases and Related Health Problems, 10th revision (ICD10), the word elective is most commonly used in the context of health services provided to pregnant women, including: elective induction of labor (Z34.90), elective cesarean delivery (O82), elective termination of pregnancy (Z33.2), and elective fetal reduction (Z031.30X0). In ICD10, other specialties do not describe the scope of their health services with the adjective elective.
There are many definitions and interpretations of elective. The most benign use of the word in the context of surgery is to contrast procedures that can be scheduled in the future with those that need to be performed urgently. In this context elective only refers to the timing, not the medical necessity, of the procedure. By contrast, describing a procedure as elective may signal that it is not medically necessary and is being performed based on the capricious preference of the patient or physician. Given the confusion and misunderstanding that may be caused by describing our important health services as “elective,” I hope that we can permanently sunset use of the term. ●
- Ghartey J, Macones GA. 39-week nulliparous inductions are not elective. Am J Obstet Gynecol. 2020;222:519-520.
- Grobman WA, Rice MM, Reddy UM, et al. Labor induction versus expectant management in low-risk nulliparous women. N Engl J Med. 2018;379:513-523.
- ACOG Committee Opinion No 761: cesarean delivery on maternal request. Obstet Gynecol. 2019;133.e73-e77.
- Gossman GL, Joesch JM, Tanfer K. Trends in maternal request cesarean delivery from 1991 to 2004. Obstet Gynecol. 2006;108:1506-1516.
- Janiak E, Goldberg AB. Eliminating the phrase “elective abortion”: why language matters. Contraception. 2016;93:89-92.
- Ghartey J, Macones GA. 39-week nulliparous inductions are not elective. Am J Obstet Gynecol. 2020;222:519-520.
- Grobman WA, Rice MM, Reddy UM, et al. Labor induction versus expectant management in low-risk nulliparous women. N Engl J Med. 2018;379:513-523.
- ACOG Committee Opinion No 761: cesarean delivery on maternal request. Obstet Gynecol. 2019;133.e73-e77.
- Gossman GL, Joesch JM, Tanfer K. Trends in maternal request cesarean delivery from 1991 to 2004. Obstet Gynecol. 2006;108:1506-1516.
- Janiak E, Goldberg AB. Eliminating the phrase “elective abortion”: why language matters. Contraception. 2016;93:89-92.
Even in a virtual environment, the Society of Gynecologic Surgeons delivers without a “glitch”
Earlier this year, I was honored to serve as the Scientific Program Chair for the 46th Annual Scientific Meeting of the Society of Gynecologic Surgeons (SGS). This year’s meeting was the first ever (and hopefully last) “virtual” scientific meeting, which consisted of a hybrid of prerecorded and live presentations. Although faculty and attendees were not able to be together physically, the essence of the lively SGS meetings came through loud and clear. We still had “discussants” comment on the oral presentations and ask questions of the presenters. These questions and answers were all done live—without a glitch! Many thanks to all who made this meeting possible.
In addition to the outstanding abstract and video presentations, there were 4 superb postgraduate courses:
- Mikio Nihira, MD, chaired “Enhanced recovery after surgery: Overcoming barriers to implementation.”
- Charles Hanes, MD, headed up “It’s all about the apex: The key to successful POP surgery.”
- Cara King, DO, MS, led “Total laparoscopic hysterectomy: Pushing the envelope.”
- Vincent Lucente, MD, chaired “Transvaginal reconstructive pelvic surgery using graft augmentation post-FDA.”
Many special thanks to Dr. Lucente who transformed his course into a wonderful article for this special section of
One of our exceptional keynote speakers was Marc Beer (a serial entrepreneur and cofounder, chairman, and CEO of Renovia, Inc.), whose talk was entitled “A primer on medical device innovation—How to avoid common pitfalls while realizing your vision.” Mr. Beer has turned this topic into a unique article for this special section (see next month’s issue for Part 2).
Our TeLinde Lecture, entitled “Artificial intelligence in surgery,” was delivered by the dynamic Vicente Gracias, MD, professor of surgery at Robert Wood Johnson University Hospital, New Brunswick, New Jersey. We also held 2 live panel discussions that were very popular. The first, “Work-life balance and gynecologic surgery,” featured various perspectives from Drs. Kristie Green, Sally Huber, Catherine Matthews, and Charles Rardin. The second panel discussion, entitled “Understanding, managing, and benefiting from your e-presence,” by experts Heather Schueppert; Chief Marketing Officer at Unified Physician Management, Brad Bowman, MD; and Peter Lotze, MD. Both of these panel discussions are included in this special section as well.
I hope you enjoy the content of this special section of
Earlier this year, I was honored to serve as the Scientific Program Chair for the 46th Annual Scientific Meeting of the Society of Gynecologic Surgeons (SGS). This year’s meeting was the first ever (and hopefully last) “virtual” scientific meeting, which consisted of a hybrid of prerecorded and live presentations. Although faculty and attendees were not able to be together physically, the essence of the lively SGS meetings came through loud and clear. We still had “discussants” comment on the oral presentations and ask questions of the presenters. These questions and answers were all done live—without a glitch! Many thanks to all who made this meeting possible.
In addition to the outstanding abstract and video presentations, there were 4 superb postgraduate courses:
- Mikio Nihira, MD, chaired “Enhanced recovery after surgery: Overcoming barriers to implementation.”
- Charles Hanes, MD, headed up “It’s all about the apex: The key to successful POP surgery.”
- Cara King, DO, MS, led “Total laparoscopic hysterectomy: Pushing the envelope.”
- Vincent Lucente, MD, chaired “Transvaginal reconstructive pelvic surgery using graft augmentation post-FDA.”
Many special thanks to Dr. Lucente who transformed his course into a wonderful article for this special section of
One of our exceptional keynote speakers was Marc Beer (a serial entrepreneur and cofounder, chairman, and CEO of Renovia, Inc.), whose talk was entitled “A primer on medical device innovation—How to avoid common pitfalls while realizing your vision.” Mr. Beer has turned this topic into a unique article for this special section (see next month’s issue for Part 2).
Our TeLinde Lecture, entitled “Artificial intelligence in surgery,” was delivered by the dynamic Vicente Gracias, MD, professor of surgery at Robert Wood Johnson University Hospital, New Brunswick, New Jersey. We also held 2 live panel discussions that were very popular. The first, “Work-life balance and gynecologic surgery,” featured various perspectives from Drs. Kristie Green, Sally Huber, Catherine Matthews, and Charles Rardin. The second panel discussion, entitled “Understanding, managing, and benefiting from your e-presence,” by experts Heather Schueppert; Chief Marketing Officer at Unified Physician Management, Brad Bowman, MD; and Peter Lotze, MD. Both of these panel discussions are included in this special section as well.
I hope you enjoy the content of this special section of
Earlier this year, I was honored to serve as the Scientific Program Chair for the 46th Annual Scientific Meeting of the Society of Gynecologic Surgeons (SGS). This year’s meeting was the first ever (and hopefully last) “virtual” scientific meeting, which consisted of a hybrid of prerecorded and live presentations. Although faculty and attendees were not able to be together physically, the essence of the lively SGS meetings came through loud and clear. We still had “discussants” comment on the oral presentations and ask questions of the presenters. These questions and answers were all done live—without a glitch! Many thanks to all who made this meeting possible.
In addition to the outstanding abstract and video presentations, there were 4 superb postgraduate courses:
- Mikio Nihira, MD, chaired “Enhanced recovery after surgery: Overcoming barriers to implementation.”
- Charles Hanes, MD, headed up “It’s all about the apex: The key to successful POP surgery.”
- Cara King, DO, MS, led “Total laparoscopic hysterectomy: Pushing the envelope.”
- Vincent Lucente, MD, chaired “Transvaginal reconstructive pelvic surgery using graft augmentation post-FDA.”
Many special thanks to Dr. Lucente who transformed his course into a wonderful article for this special section of
One of our exceptional keynote speakers was Marc Beer (a serial entrepreneur and cofounder, chairman, and CEO of Renovia, Inc.), whose talk was entitled “A primer on medical device innovation—How to avoid common pitfalls while realizing your vision.” Mr. Beer has turned this topic into a unique article for this special section (see next month’s issue for Part 2).
Our TeLinde Lecture, entitled “Artificial intelligence in surgery,” was delivered by the dynamic Vicente Gracias, MD, professor of surgery at Robert Wood Johnson University Hospital, New Brunswick, New Jersey. We also held 2 live panel discussions that were very popular. The first, “Work-life balance and gynecologic surgery,” featured various perspectives from Drs. Kristie Green, Sally Huber, Catherine Matthews, and Charles Rardin. The second panel discussion, entitled “Understanding, managing, and benefiting from your e-presence,” by experts Heather Schueppert; Chief Marketing Officer at Unified Physician Management, Brad Bowman, MD; and Peter Lotze, MD. Both of these panel discussions are included in this special section as well.
I hope you enjoy the content of this special section of
Is vertical transmission of SARS-CoV-2 possible? Is that the right question?
Pique-Regi R, Romero R, Tarca AL, et al. Does the human placenta express the canonical cell entry mediators for SARS CoV-2? eLife. 2020;9:e58716.
EXPERT COMMENTARY
Maternal infection with the novel SARS-CoV-2 virus has been associated with severe maternal morbidity and mortality causing adverse pregnancy outcomes, such as preterm birth and, potentially, stillbirth, with vertical transmission of the virus to the fetus possible.1,2
Uniquely, maternal physiology supports both pro- and anti-inflammatory states within pregnancy—a system that not only must protect the mother but also must tolerate a semi-allogenic fetus. Studies demonstrate that the first and third trimesters are pro-inflammatory, while the second trimester is thought to be anti-inflammatory.3 Since the discovery of the SARS-CoV-2 virus, the question surrounding vertical transmission (infectivity from mother to fetus via the placenta) has occupied the imagination of physicians, scientists, and pregnant women. Importantly, the virus is transmitted to human cells via the ACE2 (angiotensin-converting enzyme 2) receptor, which aids in viral cell attachment. ACE2 receptors are expressed in placental stromal cells, perivascular cells of decidua, cytotrophoblast and syncytiotrophoblast,4 as well as blood vessel endothelium and vascular smooth muscle from both primary and secondary villi.
Details of the study
In their recent study, Pique-Regi and colleagues used single-cell RNA sequencing data to investigate whether the receptors responsible for SARS-CoV-2 infection are expressed in the human placenta.5 Their findings suggest that TMPRSS2 is present in insufficient quantity in the placenta to make vertical transmission possible and/or clinically relevant. Thus, despite the presence of ACE2 receptors in placental tissue, without the enzymatic assistance of a helper protein like TMPRSS2 (transmembrane protease, serine 2), vertical transmission is highly unlikely. The researchers found that there was negligible co-transcription for ACE2 and TMPRSS2 in the placenta and that placental tissue lacks the mRNA necessary to produce the enzyme; they concluded that the likelihood of vertical transmission to the fetus was therefore unlikely.
As a caveat to their research, the authors noted that:
- transcription levels do not always correlate with protein expression
- it is possible that a noncanonical cell-entry mediator facilitates entry
- individuals with complications related to the renin-angiotensin-aldosterone system (such as hypertensive disease) may have alterations to the expression of ACE2.
Study strengths and limitations
Methods for this study reveal that the researchers examined 32 placentas, all taken in the third trimester (32.9-39.1 weeks), with a median gestational age of 36.9 weeks. Notably, 81.3% of placentas were from Black women, 6.2% from White women, and 12.5% from Other women. The median maternal age was 25 years, median body mass index was 27.8 kg/m2, and 84.4% of women were multiparous. While this sample was not representative of race, gestational age, or parity, it is difficult to know whether those selection biases would have changed the researchers' findings.
The question regarding vertical transmission is one not answered solely on the basis of RNA sequencing data. Clinically, we know that neonates of mothers infected with SARS-CoV-2 have been born with immunoglobulin M antibodies, indicating antenatal exposure to the virus.6,7 In addition, infants have tested positive immediately after birth for coronavirus disease 2019 (COVID-19) via nasopharyngeal swab and amniotic fluid, and there are ample cases of histologic and polymerase chain reaction evidence of placental infection.8,9 We also know that inflammatory damage to the placenta could possibly break down the placental barrier.10
The destruction that SARS-CoV-2 often leaves in its wake is devastating for the maternal-fetal dyad. The effects of maternal infection on the placenta—where additional research is needed—can be profound, causing profuse endothelial damage, vascular malperfusion, thrombi, and infarcts, all of which can be lethal to some developing fetuses.
While the study by Pique-Regi and colleagues is an important contribution to the literature, it does not satisfactorily answer the question regarding vertical transmission. More research is needed, especially regarding maternal infection in the first and second trimesters, on the effects on placental vasculature (and timing of infection in each trimester), the potential breakdown of the maternal-fetal barrier, and, most important, the clinical courses and outcomes in both mother and infant.
JANE VAN DIS, MD
- Khalil A, Kalafat E, Benlioglu C, et al. SARS-CoV-2 infection in pregnancy: a systematic review and meta-analysis of clinical features and pregnancy outcomes. EClinical Medicine. 2020;25:100446.
- Khalil A, von Dadelszen P, Draycott T, et al. Change in the incidence of stillbirth and preterm delivery during the COVID-19 pandemic. JAMA. 2020;324:705-706.
- Liu H, Wang LL, Zhao SJ, et al. Why are pregnant women susceptible to COVID-19? An immunological viewpoint. J Reprod Immunol. 2020;139;103122.
- Li M, Chen L, Zhang J, et al. The SARS-CoV-2 receptor ACE2 expression of maternal-fetal interface and fetal organs by single-cell transcriptome study. PLoS One. 2020;15:e0230295.
- Pique-Regi R, Romero R, Tarca AL, et al. Does the human placenta express the canonical cell entry mediators for SARS-CoV-2? eLife. 2020;9:e58716.
- Zeng H, Xu C, Fan J, et al. Antibodies in infants born to mothers with COVID-19 pneumonia. JAMA. 2020;323:1848-1849.
- Dong L, Tian J, He S, et al. Possible vertical transmission of SARS-CoV-2 from an infected mother to her newborn. JAMA. 2020;323:1846-1848.
- Kotlyar A, Grechukhina O, Chen A, et al. Vertical transmission of COVID-19: a systematic review and meta-analysis. Am J Obstet Gynecol. 2020;S0002-9378(20)30823-1.
- Richtmann R, Torloni MR, Oyamada Otani AR, et al. Fetal deaths in pregnancies with SARS-CoV-2 infection in Brazil: a case series. Case Rep Womens Health. 2020;e00243.
- Wang C, Zhou YH, Yang HX, et al. Intrauterine vertical transmission of SARS-CoV-2: what we know so far. Ultrasound Obstet Gynecol. 2020;55:724-725.
Pique-Regi R, Romero R, Tarca AL, et al. Does the human placenta express the canonical cell entry mediators for SARS CoV-2? eLife. 2020;9:e58716.
EXPERT COMMENTARY
Maternal infection with the novel SARS-CoV-2 virus has been associated with severe maternal morbidity and mortality causing adverse pregnancy outcomes, such as preterm birth and, potentially, stillbirth, with vertical transmission of the virus to the fetus possible.1,2
Uniquely, maternal physiology supports both pro- and anti-inflammatory states within pregnancy—a system that not only must protect the mother but also must tolerate a semi-allogenic fetus. Studies demonstrate that the first and third trimesters are pro-inflammatory, while the second trimester is thought to be anti-inflammatory.3 Since the discovery of the SARS-CoV-2 virus, the question surrounding vertical transmission (infectivity from mother to fetus via the placenta) has occupied the imagination of physicians, scientists, and pregnant women. Importantly, the virus is transmitted to human cells via the ACE2 (angiotensin-converting enzyme 2) receptor, which aids in viral cell attachment. ACE2 receptors are expressed in placental stromal cells, perivascular cells of decidua, cytotrophoblast and syncytiotrophoblast,4 as well as blood vessel endothelium and vascular smooth muscle from both primary and secondary villi.
Details of the study
In their recent study, Pique-Regi and colleagues used single-cell RNA sequencing data to investigate whether the receptors responsible for SARS-CoV-2 infection are expressed in the human placenta.5 Their findings suggest that TMPRSS2 is present in insufficient quantity in the placenta to make vertical transmission possible and/or clinically relevant. Thus, despite the presence of ACE2 receptors in placental tissue, without the enzymatic assistance of a helper protein like TMPRSS2 (transmembrane protease, serine 2), vertical transmission is highly unlikely. The researchers found that there was negligible co-transcription for ACE2 and TMPRSS2 in the placenta and that placental tissue lacks the mRNA necessary to produce the enzyme; they concluded that the likelihood of vertical transmission to the fetus was therefore unlikely.
As a caveat to their research, the authors noted that:
- transcription levels do not always correlate with protein expression
- it is possible that a noncanonical cell-entry mediator facilitates entry
- individuals with complications related to the renin-angiotensin-aldosterone system (such as hypertensive disease) may have alterations to the expression of ACE2.
Study strengths and limitations
Methods for this study reveal that the researchers examined 32 placentas, all taken in the third trimester (32.9-39.1 weeks), with a median gestational age of 36.9 weeks. Notably, 81.3% of placentas were from Black women, 6.2% from White women, and 12.5% from Other women. The median maternal age was 25 years, median body mass index was 27.8 kg/m2, and 84.4% of women were multiparous. While this sample was not representative of race, gestational age, or parity, it is difficult to know whether those selection biases would have changed the researchers' findings.
The question regarding vertical transmission is one not answered solely on the basis of RNA sequencing data. Clinically, we know that neonates of mothers infected with SARS-CoV-2 have been born with immunoglobulin M antibodies, indicating antenatal exposure to the virus.6,7 In addition, infants have tested positive immediately after birth for coronavirus disease 2019 (COVID-19) via nasopharyngeal swab and amniotic fluid, and there are ample cases of histologic and polymerase chain reaction evidence of placental infection.8,9 We also know that inflammatory damage to the placenta could possibly break down the placental barrier.10
The destruction that SARS-CoV-2 often leaves in its wake is devastating for the maternal-fetal dyad. The effects of maternal infection on the placenta—where additional research is needed—can be profound, causing profuse endothelial damage, vascular malperfusion, thrombi, and infarcts, all of which can be lethal to some developing fetuses.
While the study by Pique-Regi and colleagues is an important contribution to the literature, it does not satisfactorily answer the question regarding vertical transmission. More research is needed, especially regarding maternal infection in the first and second trimesters, on the effects on placental vasculature (and timing of infection in each trimester), the potential breakdown of the maternal-fetal barrier, and, most important, the clinical courses and outcomes in both mother and infant.
JANE VAN DIS, MD
Pique-Regi R, Romero R, Tarca AL, et al. Does the human placenta express the canonical cell entry mediators for SARS CoV-2? eLife. 2020;9:e58716.
EXPERT COMMENTARY
Maternal infection with the novel SARS-CoV-2 virus has been associated with severe maternal morbidity and mortality causing adverse pregnancy outcomes, such as preterm birth and, potentially, stillbirth, with vertical transmission of the virus to the fetus possible.1,2
Uniquely, maternal physiology supports both pro- and anti-inflammatory states within pregnancy—a system that not only must protect the mother but also must tolerate a semi-allogenic fetus. Studies demonstrate that the first and third trimesters are pro-inflammatory, while the second trimester is thought to be anti-inflammatory.3 Since the discovery of the SARS-CoV-2 virus, the question surrounding vertical transmission (infectivity from mother to fetus via the placenta) has occupied the imagination of physicians, scientists, and pregnant women. Importantly, the virus is transmitted to human cells via the ACE2 (angiotensin-converting enzyme 2) receptor, which aids in viral cell attachment. ACE2 receptors are expressed in placental stromal cells, perivascular cells of decidua, cytotrophoblast and syncytiotrophoblast,4 as well as blood vessel endothelium and vascular smooth muscle from both primary and secondary villi.
Details of the study
In their recent study, Pique-Regi and colleagues used single-cell RNA sequencing data to investigate whether the receptors responsible for SARS-CoV-2 infection are expressed in the human placenta.5 Their findings suggest that TMPRSS2 is present in insufficient quantity in the placenta to make vertical transmission possible and/or clinically relevant. Thus, despite the presence of ACE2 receptors in placental tissue, without the enzymatic assistance of a helper protein like TMPRSS2 (transmembrane protease, serine 2), vertical transmission is highly unlikely. The researchers found that there was negligible co-transcription for ACE2 and TMPRSS2 in the placenta and that placental tissue lacks the mRNA necessary to produce the enzyme; they concluded that the likelihood of vertical transmission to the fetus was therefore unlikely.
As a caveat to their research, the authors noted that:
- transcription levels do not always correlate with protein expression
- it is possible that a noncanonical cell-entry mediator facilitates entry
- individuals with complications related to the renin-angiotensin-aldosterone system (such as hypertensive disease) may have alterations to the expression of ACE2.
Study strengths and limitations
Methods for this study reveal that the researchers examined 32 placentas, all taken in the third trimester (32.9-39.1 weeks), with a median gestational age of 36.9 weeks. Notably, 81.3% of placentas were from Black women, 6.2% from White women, and 12.5% from Other women. The median maternal age was 25 years, median body mass index was 27.8 kg/m2, and 84.4% of women were multiparous. While this sample was not representative of race, gestational age, or parity, it is difficult to know whether those selection biases would have changed the researchers' findings.
The question regarding vertical transmission is one not answered solely on the basis of RNA sequencing data. Clinically, we know that neonates of mothers infected with SARS-CoV-2 have been born with immunoglobulin M antibodies, indicating antenatal exposure to the virus.6,7 In addition, infants have tested positive immediately after birth for coronavirus disease 2019 (COVID-19) via nasopharyngeal swab and amniotic fluid, and there are ample cases of histologic and polymerase chain reaction evidence of placental infection.8,9 We also know that inflammatory damage to the placenta could possibly break down the placental barrier.10
The destruction that SARS-CoV-2 often leaves in its wake is devastating for the maternal-fetal dyad. The effects of maternal infection on the placenta—where additional research is needed—can be profound, causing profuse endothelial damage, vascular malperfusion, thrombi, and infarcts, all of which can be lethal to some developing fetuses.
While the study by Pique-Regi and colleagues is an important contribution to the literature, it does not satisfactorily answer the question regarding vertical transmission. More research is needed, especially regarding maternal infection in the first and second trimesters, on the effects on placental vasculature (and timing of infection in each trimester), the potential breakdown of the maternal-fetal barrier, and, most important, the clinical courses and outcomes in both mother and infant.
JANE VAN DIS, MD
- Khalil A, Kalafat E, Benlioglu C, et al. SARS-CoV-2 infection in pregnancy: a systematic review and meta-analysis of clinical features and pregnancy outcomes. EClinical Medicine. 2020;25:100446.
- Khalil A, von Dadelszen P, Draycott T, et al. Change in the incidence of stillbirth and preterm delivery during the COVID-19 pandemic. JAMA. 2020;324:705-706.
- Liu H, Wang LL, Zhao SJ, et al. Why are pregnant women susceptible to COVID-19? An immunological viewpoint. J Reprod Immunol. 2020;139;103122.
- Li M, Chen L, Zhang J, et al. The SARS-CoV-2 receptor ACE2 expression of maternal-fetal interface and fetal organs by single-cell transcriptome study. PLoS One. 2020;15:e0230295.
- Pique-Regi R, Romero R, Tarca AL, et al. Does the human placenta express the canonical cell entry mediators for SARS-CoV-2? eLife. 2020;9:e58716.
- Zeng H, Xu C, Fan J, et al. Antibodies in infants born to mothers with COVID-19 pneumonia. JAMA. 2020;323:1848-1849.
- Dong L, Tian J, He S, et al. Possible vertical transmission of SARS-CoV-2 from an infected mother to her newborn. JAMA. 2020;323:1846-1848.
- Kotlyar A, Grechukhina O, Chen A, et al. Vertical transmission of COVID-19: a systematic review and meta-analysis. Am J Obstet Gynecol. 2020;S0002-9378(20)30823-1.
- Richtmann R, Torloni MR, Oyamada Otani AR, et al. Fetal deaths in pregnancies with SARS-CoV-2 infection in Brazil: a case series. Case Rep Womens Health. 2020;e00243.
- Wang C, Zhou YH, Yang HX, et al. Intrauterine vertical transmission of SARS-CoV-2: what we know so far. Ultrasound Obstet Gynecol. 2020;55:724-725.
- Khalil A, Kalafat E, Benlioglu C, et al. SARS-CoV-2 infection in pregnancy: a systematic review and meta-analysis of clinical features and pregnancy outcomes. EClinical Medicine. 2020;25:100446.
- Khalil A, von Dadelszen P, Draycott T, et al. Change in the incidence of stillbirth and preterm delivery during the COVID-19 pandemic. JAMA. 2020;324:705-706.
- Liu H, Wang LL, Zhao SJ, et al. Why are pregnant women susceptible to COVID-19? An immunological viewpoint. J Reprod Immunol. 2020;139;103122.
- Li M, Chen L, Zhang J, et al. The SARS-CoV-2 receptor ACE2 expression of maternal-fetal interface and fetal organs by single-cell transcriptome study. PLoS One. 2020;15:e0230295.
- Pique-Regi R, Romero R, Tarca AL, et al. Does the human placenta express the canonical cell entry mediators for SARS-CoV-2? eLife. 2020;9:e58716.
- Zeng H, Xu C, Fan J, et al. Antibodies in infants born to mothers with COVID-19 pneumonia. JAMA. 2020;323:1848-1849.
- Dong L, Tian J, He S, et al. Possible vertical transmission of SARS-CoV-2 from an infected mother to her newborn. JAMA. 2020;323:1846-1848.
- Kotlyar A, Grechukhina O, Chen A, et al. Vertical transmission of COVID-19: a systematic review and meta-analysis. Am J Obstet Gynecol. 2020;S0002-9378(20)30823-1.
- Richtmann R, Torloni MR, Oyamada Otani AR, et al. Fetal deaths in pregnancies with SARS-CoV-2 infection in Brazil: a case series. Case Rep Womens Health. 2020;e00243.
- Wang C, Zhou YH, Yang HX, et al. Intrauterine vertical transmission of SARS-CoV-2: what we know so far. Ultrasound Obstet Gynecol. 2020;55:724-725.
Major changes in Medicare billing are planned for January 2021: Some specialties fare better than others
The Centers for Medicare and Medicaid Services (CMS) finalized an increase in the relative value of evaluation and management (E/M) service codes effective January 1, 2021, which results in an overall decrease in the payment for procedural services in the Medicare program. (Due to the mandate for budget neutrality, an increase in relative value units [RVUs] for E/M resulted in a large decrease in the conversion factor—the number of dollars per RVU). This has increased payments for endocrinologists, rheumatologists, and family medicine clinicians and decreased payments for radiologists, pathologists, and surgeons.
In a major win for physicians, CMS proposes to simplify documentation requirements for billing and focus on the complexity of the medical decision making (MDM) or the total time needed to care for the patient on the date of the service as the foundation for determining the relative value of the service. Therefore, there is no more counting bullets—ie, we don’t have to perform a comprehensive physical exam or review of systems to achieve a high level code! Prior to this change, time was only available for coding purposes when counseling and coordination of care was the predominant service (>50%), and only face-to-face time with the patient was considered. Effective January 1, for office and other outpatient services, total time on the calendar date of the encounter will be used. This acknowledges the intensity and value of non–face-to-face work.
Acting through CMS, the federal government influences greatly the US health care system. CMS is an agency in the Department of Health and Human Services that administers the Medicare program and partners with state governments to administer the Health Insurance Exchanges, Medicaid, and the Children’s Health Insurance programs (CHIP).1 In addition, CMS is responsible for enforcing quality care standards in long-term care facilities and clinical laboratories and the implementation of the Health Insurance Portability and Accountability Act.1
In January, CMS plans the following major changes to coding and documentation2,3:
- Selection of the level of E/M service will no longer require documentation of bullet points in the history, physical exam, and MDM. The simplified system allows physicians and qualified health care professionals to code either by total time (both face-to-face and non–face-to-face) on the date of the encounter or by level of MDM.
- For established office patients, 5 levels of office-based evaluation and management services will be retained. CMS had initially proposed to reduce the number of office-based E/M codes from 5 to 3, combining code levels 2, 3, and 4 into 1 code.4 However, after receiving feedback from professional societies and the public, CMS abandoned the plan for radical simplification of coding levels.2,3 Implementation of their proposal would have resulted in the same payment for treatment of a hang nail as for a complex gyn patient with multiple medical problems. Both patient advocacy groups and professional societies argued that incentives originally were misaligned.
- For new office patients, since both 99201 and 99202 require straightforward MDM, the level 1 code (99201) has been eliminated, reducing the number of code levels from 5 to 4.
- History and physical exam will no longer be used to determine code level for office E/M codes. These elements will be required only as medically appropriate. This means that documentation review will no longer focus on “bean counting” the elements in the history and physical exam.
- Following a reassessment of the actual time required to provide E/M services in real-life practice, CMS plans to markedly increase the relative value of office visits for established patients and modestly increase the relative value of office visits for new patients. CMS operates under the principle of “neutral budgeting,” meaning that an increase of the relative value of E/M codes will result in a decrease in the payment for procedural codes. The actual RVUs for procedural services do not change; however, budget neutrality requires a decrease in the dollar conversion factor. The proposed changes will increase the payment for E/M services and decrease payments for procedural services.
Continue to: Refocusing practice on MDM complexity...
Refocusing practice on MDM complexity
The practice of medicine is a calling with great rewards. Prominent among those rewards are improving the health of women, children, and the community, developing deep and trusting relationships with patients, families, and clinical colleagues. The practice of medicine is also replete with a host of punishing administrative burdens, including prior authorizations, clunky electronic medical records, poorly designed quality metrics that are applied to clinicians, and billing compliance rules that emphasize the repetitive documentation of clinical information with minimal value.
Some of the most irritating aspects of medical practice are the CMS rules governing medical record documentation required for billing ambulatory office visits. Current coding compliance focuses on counting the number of systems reviewed in the review of systems; the documentation of past history, social history, and family history; the number of organs and organ elements examined during the physical examination; and the complexity of MDM.
In January 2021, CMS plans to adopt new Current Procedural Terminology (CPT) code descriptors for the office and other outpatient E/M services that sunset most of the “bean-counting” metrics and emphasize the importance of the complexity of MDM in guiding selection of a correct code.2 Beginning in January 2021, clinicians will have the option of selecting an E/M code level based on the total amount of time required to provide the office visit service or the complexity of MDM. When selecting a code level based on MDM the new guidance emphasizes the importance of reviewing notes from other clinicians, reviewing test results, ordering of tests, and discussing and coordinating the care of the patient with other treating physicians. These changes reflect a better understanding of what is most important in good medical practice, promoting better patient care. TABLES 1 and 2 provide the initial guidance from CMS concerning selection of E/M code level based on time and MDM, respectively.2 The guidance for using MDM to select an E/M code level is likely to evolve following implementation, so stay tuned. When using MDM to select a code, 2 of the 3 general categories are required to select that level of service.
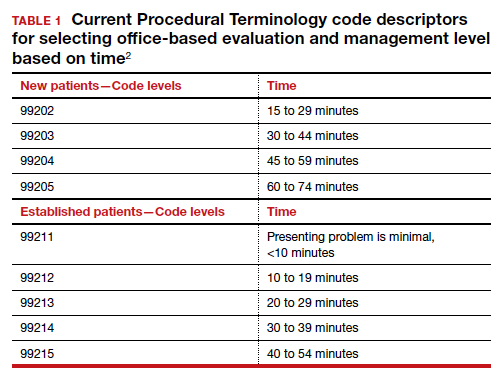
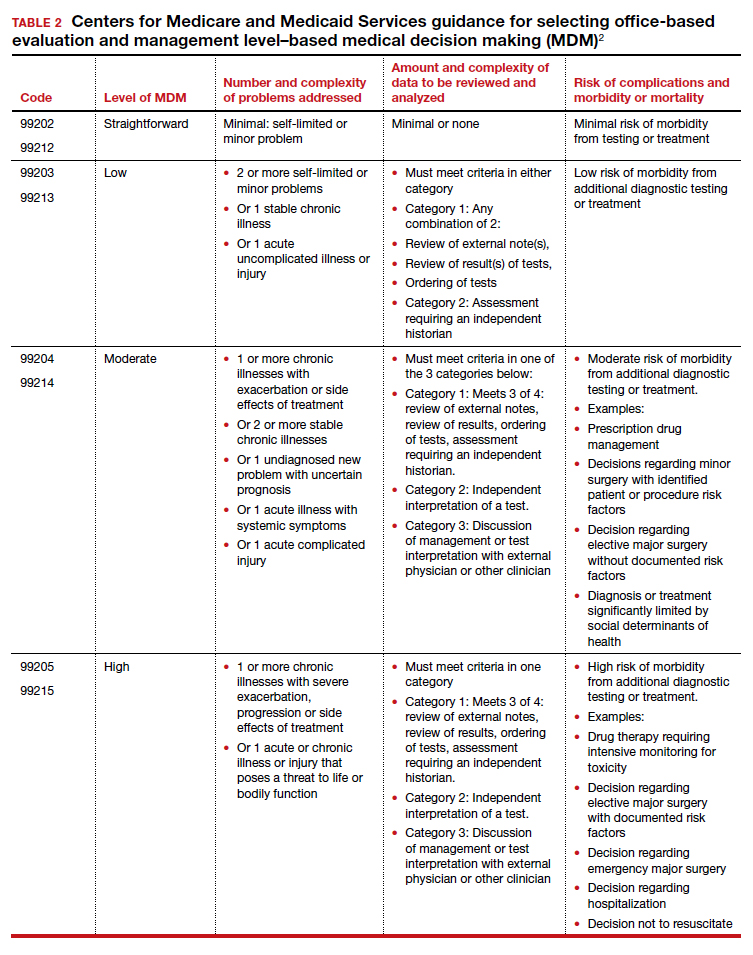
Increase in the valuation of office-based E/M services
The Medicare Physician Fee Schedule uses a resource-based relative value system to determine time and intensity of the work of clinical practice. This system recognizes 3 major factors that influence the resources required to provide a service:
- work of the clinician
- practice expense for technical components
- cost of professional liability insurance.
Many primary care professional associations have long contended that CMS has undervalued office-based E/M services relative to procedures, resulting in the devaluing of primary care practice. After the CPT code descriptors were updated by the CPT editorial panel, 52 specialty societies surveyed their members to provide inputs to CMS on the time and intensity of the office and other outpatient E/M codes as currently practiced. The American Medical Association’s Specialty Society Resource-Based Relative Value Scale Update Committee (RUC) reviewed the surveys and provided new inputs via open comment to CMS. CMS has responded to this feedback with a review of the intensity of clinical work required to provide an ambulatory visit service. In response to the review, CMS proposes to accept the recommendations of the RUC representing the house of medicine and increase the work and practice expense relative value assigned to new and established office visit codes. Overall, the combination of changes in relative values assigned for the work of the clinician and the expense of practice, increases the total value of office-based E/M codes for new patients by 7% to 14% and for established patients from 28% to 46% (see supplemental table in the sidebar at the end of this article).
Continue to: Decreased payments for procedural services...
Decreased payments for procedural services
Medicare is required to offset increased payment in one arena of health care delivery with decreased payment in other arenas of care, thereby achieving “budget-neutrality.” As detailed above, CMS plans to increase Medicare payments for office-based E/M services. Payment for services is calculated by multiplying the total RVUs for a particular service by a “conversion factor” (ie, number of dollars per RVU). To achieve budget-neutrality, CMS has proposed substantially reducing the conversion factor for 2021 (from $36.09 to $32.26), which will effectively decrease Medicare payments for procedural services since their RVUs have not changed. While the AMA RUC and many specialty societies continue to strongly advocate for the E/M work RVU increases to be included in the E/M components of 10- and 90-day global services, CMS has proposed to implement them only for “stand alone” E/M services.
Organizations are lobbying to delay or prevent the planned decrease in conversion factor, which results in substantial declines in payment for procedural services. (See "What do the Medicare billing changes mean for the Obstetrical Bundled services?" at the end of this article.) Due to the economic and clinical practice challenges caused by the coronavirus disease 2019 (COVID-19) pandemic it would be best if CMS did not reduce payments to physicians who are experts in procedural health care, thereby avoiding the risk of reduced access to these vital services.
If the current CMS changes in payment are implemented, endocrinologists, rheumatologists, and family physicians will have an increase in payment, and radiologists, pathologists, and surgeons will have a decrease in payment (TABLE 3).6 Obstetrics and gynecology is projected to have an 8% increase in Medicare payment. However, if an obstetrician-gynecologist derives most of their Medicare payments from surgical procedures, they are likely to have a decrease in payment from Medicare. Other payers will be incorporating the new coding structure for 2021; however, their payment structures and conversion factors are likely to vary. It is important to note that the RVUs for procedures have not changed. The budget neutrality adjustment resulted in a much lower conversion factor and therefore a decrease in payment for those specialties whose RVUs did not increase.
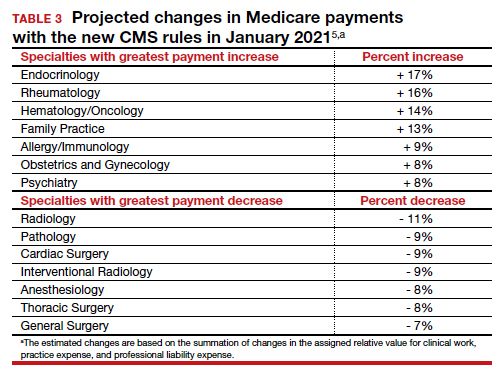
Bottom line
Working through the Medicare, Medicaid, and CHIP programs, CMS can influence greatly the practice of medicine including medical record documentation practices and payment rates for every clinical service. CMS proposes to end the onerous “bean counting” approach to billing compliance and refocus on the complexity of MDM as the foundation for selecting a billing code level. This change is long overdue, valuing the effective management of complex patients in office practice. Hopefully, CMS will reverse the planned reduction in the payment for procedural services, preserving patient access to important health care services. ●
The CY 2020 Medicare Physician Fee Schedule Final Rule was published electronically in the Federal Register on November 1, 2019. This final rule aligns the evaluation and management (E/M) coding and payment with changes recommended by the Current Procedural Terminology (CPT) Editorial Panel and American Medical Association’s (AMA) Specialty Society Resource-Based Relative Value Scale Update Committee (RUC) for office/outpatient E/M visits. Unfortunately, the Centers for Medicare and Medicaid Services (CMS) did not agree with the RUC, AMA, and specialty societies that the E/M payment changes should be applicable across all global services that incorporate E/M visits—despite the fact that the values proposed by the RUC incorporated survey data from 52 specialties, representing most of medicine (including those specialties that predominantly perform procedures). Specifically, CMS expressed the view that the number of E/M visits within the 10- and 90-day global codes, as well as the maternity care bundle, were difficult to validate; therefore, the increased values would not be distributed to those procedural services.
Many professional societies expressed significant concerns about the resulting budget neutrality adjustments that would occur effective January 2021. The great news for ObGyns is that the American College of Obstetricians and Gynecologists (ACOG) was able to respond directly to CMS’s concerns with data to support the number of prenatal visits within the Obstetrical Bundle. Tapping into a de-identified, cloud-based data set of prenatal records—representing more than 1,100 obstetric providers with close to 30,000 recently completed pregnancies—ACOG was able to document both a mean and median number of prenatal visits across a broad geographic, payer, and patient demographic that supported the 13 prenatal visits in the Obstetrical Bundle.
With ACOG’s advocacy and ability to provide data to CMS, the proposed physician fee schedule rule for 2021 has proposed to incorporate the E/M increased reimbursement into the prenatal care codes. Now we urge the CMS to finalize this proposal. Although Medicare pays for a tiny number of pregnancies annually, we hope that all payers, including Medicaid and managed care plans, will agree with this acknowledgement of the increased work of evaluation and management that obstetricians provide during prenatal care. Join ACOG in telling CMS to finalize their proposal to increase the values of the global obstetric codes: https://acog.quorum.us/campaign/28579/.
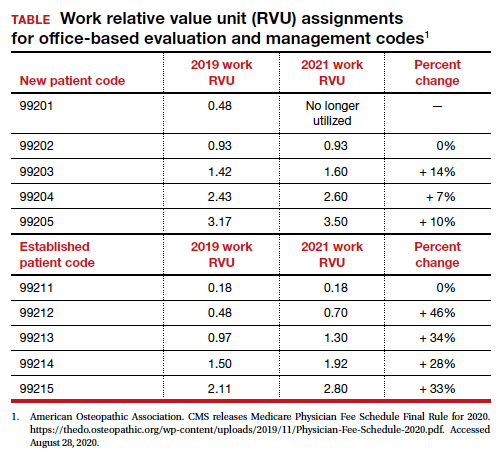
- Centers for Medicare and Medicaid Services. https://www.cms.gov/. Accessed August 28, 2020.
- American Medical Association. CPT Evaluation and Management (E/M) Office or Other Outpatient (99202-99215) and Prolonged Services (99354, 99355, 99356, 99XXX) Code and Guideline Changes. 2019. https://www.ama-assn.org /system/files/2019-06/cpt-office-prolonged-svs -code-changes.pdf. Accessed August 28, 2020.
- The American Academy of Family Physicians. Family medicine updates. Ann Fam Med. 2020;18:84-85. doi: 10.1370/afm.2508.
- Centers for Medicare and Medicaid Services. Final policy, payment and quality provisions changes to the Medicare Physician Fee Schedule for calendar year 2019. November 1, 2018. https://www.cms.gov/newsroom/fact-sheets /final-policy-payment-and-quality-provisionschanges-medicare-physician-fee-schedulecalendar-year. Accessed August 28, 2020.
- Department of Health and Human Services; Centers for Medicare and Medicaid Services. 42 CFR Parts 410, 414, 415, 423, 424, and 425. Federal Register. 2020;85(159). https://www.govinfo.gov /content/pkg/FR-2020-08-17/pdf/2020-17127 .pdf. Accessed August 28, 2020.
The Centers for Medicare and Medicaid Services (CMS) finalized an increase in the relative value of evaluation and management (E/M) service codes effective January 1, 2021, which results in an overall decrease in the payment for procedural services in the Medicare program. (Due to the mandate for budget neutrality, an increase in relative value units [RVUs] for E/M resulted in a large decrease in the conversion factor—the number of dollars per RVU). This has increased payments for endocrinologists, rheumatologists, and family medicine clinicians and decreased payments for radiologists, pathologists, and surgeons.
In a major win for physicians, CMS proposes to simplify documentation requirements for billing and focus on the complexity of the medical decision making (MDM) or the total time needed to care for the patient on the date of the service as the foundation for determining the relative value of the service. Therefore, there is no more counting bullets—ie, we don’t have to perform a comprehensive physical exam or review of systems to achieve a high level code! Prior to this change, time was only available for coding purposes when counseling and coordination of care was the predominant service (>50%), and only face-to-face time with the patient was considered. Effective January 1, for office and other outpatient services, total time on the calendar date of the encounter will be used. This acknowledges the intensity and value of non–face-to-face work.
Acting through CMS, the federal government influences greatly the US health care system. CMS is an agency in the Department of Health and Human Services that administers the Medicare program and partners with state governments to administer the Health Insurance Exchanges, Medicaid, and the Children’s Health Insurance programs (CHIP).1 In addition, CMS is responsible for enforcing quality care standards in long-term care facilities and clinical laboratories and the implementation of the Health Insurance Portability and Accountability Act.1
In January, CMS plans the following major changes to coding and documentation2,3:
- Selection of the level of E/M service will no longer require documentation of bullet points in the history, physical exam, and MDM. The simplified system allows physicians and qualified health care professionals to code either by total time (both face-to-face and non–face-to-face) on the date of the encounter or by level of MDM.
- For established office patients, 5 levels of office-based evaluation and management services will be retained. CMS had initially proposed to reduce the number of office-based E/M codes from 5 to 3, combining code levels 2, 3, and 4 into 1 code.4 However, after receiving feedback from professional societies and the public, CMS abandoned the plan for radical simplification of coding levels.2,3 Implementation of their proposal would have resulted in the same payment for treatment of a hang nail as for a complex gyn patient with multiple medical problems. Both patient advocacy groups and professional societies argued that incentives originally were misaligned.
- For new office patients, since both 99201 and 99202 require straightforward MDM, the level 1 code (99201) has been eliminated, reducing the number of code levels from 5 to 4.
- History and physical exam will no longer be used to determine code level for office E/M codes. These elements will be required only as medically appropriate. This means that documentation review will no longer focus on “bean counting” the elements in the history and physical exam.
- Following a reassessment of the actual time required to provide E/M services in real-life practice, CMS plans to markedly increase the relative value of office visits for established patients and modestly increase the relative value of office visits for new patients. CMS operates under the principle of “neutral budgeting,” meaning that an increase of the relative value of E/M codes will result in a decrease in the payment for procedural codes. The actual RVUs for procedural services do not change; however, budget neutrality requires a decrease in the dollar conversion factor. The proposed changes will increase the payment for E/M services and decrease payments for procedural services.
Continue to: Refocusing practice on MDM complexity...
Refocusing practice on MDM complexity
The practice of medicine is a calling with great rewards. Prominent among those rewards are improving the health of women, children, and the community, developing deep and trusting relationships with patients, families, and clinical colleagues. The practice of medicine is also replete with a host of punishing administrative burdens, including prior authorizations, clunky electronic medical records, poorly designed quality metrics that are applied to clinicians, and billing compliance rules that emphasize the repetitive documentation of clinical information with minimal value.
Some of the most irritating aspects of medical practice are the CMS rules governing medical record documentation required for billing ambulatory office visits. Current coding compliance focuses on counting the number of systems reviewed in the review of systems; the documentation of past history, social history, and family history; the number of organs and organ elements examined during the physical examination; and the complexity of MDM.
In January 2021, CMS plans to adopt new Current Procedural Terminology (CPT) code descriptors for the office and other outpatient E/M services that sunset most of the “bean-counting” metrics and emphasize the importance of the complexity of MDM in guiding selection of a correct code.2 Beginning in January 2021, clinicians will have the option of selecting an E/M code level based on the total amount of time required to provide the office visit service or the complexity of MDM. When selecting a code level based on MDM the new guidance emphasizes the importance of reviewing notes from other clinicians, reviewing test results, ordering of tests, and discussing and coordinating the care of the patient with other treating physicians. These changes reflect a better understanding of what is most important in good medical practice, promoting better patient care. TABLES 1 and 2 provide the initial guidance from CMS concerning selection of E/M code level based on time and MDM, respectively.2 The guidance for using MDM to select an E/M code level is likely to evolve following implementation, so stay tuned. When using MDM to select a code, 2 of the 3 general categories are required to select that level of service.


Increase in the valuation of office-based E/M services
The Medicare Physician Fee Schedule uses a resource-based relative value system to determine time and intensity of the work of clinical practice. This system recognizes 3 major factors that influence the resources required to provide a service:
- work of the clinician
- practice expense for technical components
- cost of professional liability insurance.
Many primary care professional associations have long contended that CMS has undervalued office-based E/M services relative to procedures, resulting in the devaluing of primary care practice. After the CPT code descriptors were updated by the CPT editorial panel, 52 specialty societies surveyed their members to provide inputs to CMS on the time and intensity of the office and other outpatient E/M codes as currently practiced. The American Medical Association’s Specialty Society Resource-Based Relative Value Scale Update Committee (RUC) reviewed the surveys and provided new inputs via open comment to CMS. CMS has responded to this feedback with a review of the intensity of clinical work required to provide an ambulatory visit service. In response to the review, CMS proposes to accept the recommendations of the RUC representing the house of medicine and increase the work and practice expense relative value assigned to new and established office visit codes. Overall, the combination of changes in relative values assigned for the work of the clinician and the expense of practice, increases the total value of office-based E/M codes for new patients by 7% to 14% and for established patients from 28% to 46% (see supplemental table in the sidebar at the end of this article).
Continue to: Decreased payments for procedural services...
Decreased payments for procedural services
Medicare is required to offset increased payment in one arena of health care delivery with decreased payment in other arenas of care, thereby achieving “budget-neutrality.” As detailed above, CMS plans to increase Medicare payments for office-based E/M services. Payment for services is calculated by multiplying the total RVUs for a particular service by a “conversion factor” (ie, number of dollars per RVU). To achieve budget-neutrality, CMS has proposed substantially reducing the conversion factor for 2021 (from $36.09 to $32.26), which will effectively decrease Medicare payments for procedural services since their RVUs have not changed. While the AMA RUC and many specialty societies continue to strongly advocate for the E/M work RVU increases to be included in the E/M components of 10- and 90-day global services, CMS has proposed to implement them only for “stand alone” E/M services.
Organizations are lobbying to delay or prevent the planned decrease in conversion factor, which results in substantial declines in payment for procedural services. (See "What do the Medicare billing changes mean for the Obstetrical Bundled services?" at the end of this article.) Due to the economic and clinical practice challenges caused by the coronavirus disease 2019 (COVID-19) pandemic it would be best if CMS did not reduce payments to physicians who are experts in procedural health care, thereby avoiding the risk of reduced access to these vital services.
If the current CMS changes in payment are implemented, endocrinologists, rheumatologists, and family physicians will have an increase in payment, and radiologists, pathologists, and surgeons will have a decrease in payment (TABLE 3).6 Obstetrics and gynecology is projected to have an 8% increase in Medicare payment. However, if an obstetrician-gynecologist derives most of their Medicare payments from surgical procedures, they are likely to have a decrease in payment from Medicare. Other payers will be incorporating the new coding structure for 2021; however, their payment structures and conversion factors are likely to vary. It is important to note that the RVUs for procedures have not changed. The budget neutrality adjustment resulted in a much lower conversion factor and therefore a decrease in payment for those specialties whose RVUs did not increase.

Bottom line
Working through the Medicare, Medicaid, and CHIP programs, CMS can influence greatly the practice of medicine including medical record documentation practices and payment rates for every clinical service. CMS proposes to end the onerous “bean counting” approach to billing compliance and refocus on the complexity of MDM as the foundation for selecting a billing code level. This change is long overdue, valuing the effective management of complex patients in office practice. Hopefully, CMS will reverse the planned reduction in the payment for procedural services, preserving patient access to important health care services. ●
The CY 2020 Medicare Physician Fee Schedule Final Rule was published electronically in the Federal Register on November 1, 2019. This final rule aligns the evaluation and management (E/M) coding and payment with changes recommended by the Current Procedural Terminology (CPT) Editorial Panel and American Medical Association’s (AMA) Specialty Society Resource-Based Relative Value Scale Update Committee (RUC) for office/outpatient E/M visits. Unfortunately, the Centers for Medicare and Medicaid Services (CMS) did not agree with the RUC, AMA, and specialty societies that the E/M payment changes should be applicable across all global services that incorporate E/M visits—despite the fact that the values proposed by the RUC incorporated survey data from 52 specialties, representing most of medicine (including those specialties that predominantly perform procedures). Specifically, CMS expressed the view that the number of E/M visits within the 10- and 90-day global codes, as well as the maternity care bundle, were difficult to validate; therefore, the increased values would not be distributed to those procedural services.
Many professional societies expressed significant concerns about the resulting budget neutrality adjustments that would occur effective January 2021. The great news for ObGyns is that the American College of Obstetricians and Gynecologists (ACOG) was able to respond directly to CMS’s concerns with data to support the number of prenatal visits within the Obstetrical Bundle. Tapping into a de-identified, cloud-based data set of prenatal records—representing more than 1,100 obstetric providers with close to 30,000 recently completed pregnancies—ACOG was able to document both a mean and median number of prenatal visits across a broad geographic, payer, and patient demographic that supported the 13 prenatal visits in the Obstetrical Bundle.
With ACOG’s advocacy and ability to provide data to CMS, the proposed physician fee schedule rule for 2021 has proposed to incorporate the E/M increased reimbursement into the prenatal care codes. Now we urge the CMS to finalize this proposal. Although Medicare pays for a tiny number of pregnancies annually, we hope that all payers, including Medicaid and managed care plans, will agree with this acknowledgement of the increased work of evaluation and management that obstetricians provide during prenatal care. Join ACOG in telling CMS to finalize their proposal to increase the values of the global obstetric codes: https://acog.quorum.us/campaign/28579/.

The Centers for Medicare and Medicaid Services (CMS) finalized an increase in the relative value of evaluation and management (E/M) service codes effective January 1, 2021, which results in an overall decrease in the payment for procedural services in the Medicare program. (Due to the mandate for budget neutrality, an increase in relative value units [RVUs] for E/M resulted in a large decrease in the conversion factor—the number of dollars per RVU). This has increased payments for endocrinologists, rheumatologists, and family medicine clinicians and decreased payments for radiologists, pathologists, and surgeons.
In a major win for physicians, CMS proposes to simplify documentation requirements for billing and focus on the complexity of the medical decision making (MDM) or the total time needed to care for the patient on the date of the service as the foundation for determining the relative value of the service. Therefore, there is no more counting bullets—ie, we don’t have to perform a comprehensive physical exam or review of systems to achieve a high level code! Prior to this change, time was only available for coding purposes when counseling and coordination of care was the predominant service (>50%), and only face-to-face time with the patient was considered. Effective January 1, for office and other outpatient services, total time on the calendar date of the encounter will be used. This acknowledges the intensity and value of non–face-to-face work.
Acting through CMS, the federal government influences greatly the US health care system. CMS is an agency in the Department of Health and Human Services that administers the Medicare program and partners with state governments to administer the Health Insurance Exchanges, Medicaid, and the Children’s Health Insurance programs (CHIP).1 In addition, CMS is responsible for enforcing quality care standards in long-term care facilities and clinical laboratories and the implementation of the Health Insurance Portability and Accountability Act.1
In January, CMS plans the following major changes to coding and documentation2,3:
- Selection of the level of E/M service will no longer require documentation of bullet points in the history, physical exam, and MDM. The simplified system allows physicians and qualified health care professionals to code either by total time (both face-to-face and non–face-to-face) on the date of the encounter or by level of MDM.
- For established office patients, 5 levels of office-based evaluation and management services will be retained. CMS had initially proposed to reduce the number of office-based E/M codes from 5 to 3, combining code levels 2, 3, and 4 into 1 code.4 However, after receiving feedback from professional societies and the public, CMS abandoned the plan for radical simplification of coding levels.2,3 Implementation of their proposal would have resulted in the same payment for treatment of a hang nail as for a complex gyn patient with multiple medical problems. Both patient advocacy groups and professional societies argued that incentives originally were misaligned.
- For new office patients, since both 99201 and 99202 require straightforward MDM, the level 1 code (99201) has been eliminated, reducing the number of code levels from 5 to 4.
- History and physical exam will no longer be used to determine code level for office E/M codes. These elements will be required only as medically appropriate. This means that documentation review will no longer focus on “bean counting” the elements in the history and physical exam.
- Following a reassessment of the actual time required to provide E/M services in real-life practice, CMS plans to markedly increase the relative value of office visits for established patients and modestly increase the relative value of office visits for new patients. CMS operates under the principle of “neutral budgeting,” meaning that an increase of the relative value of E/M codes will result in a decrease in the payment for procedural codes. The actual RVUs for procedural services do not change; however, budget neutrality requires a decrease in the dollar conversion factor. The proposed changes will increase the payment for E/M services and decrease payments for procedural services.
Continue to: Refocusing practice on MDM complexity...
Refocusing practice on MDM complexity
The practice of medicine is a calling with great rewards. Prominent among those rewards are improving the health of women, children, and the community, developing deep and trusting relationships with patients, families, and clinical colleagues. The practice of medicine is also replete with a host of punishing administrative burdens, including prior authorizations, clunky electronic medical records, poorly designed quality metrics that are applied to clinicians, and billing compliance rules that emphasize the repetitive documentation of clinical information with minimal value.
Some of the most irritating aspects of medical practice are the CMS rules governing medical record documentation required for billing ambulatory office visits. Current coding compliance focuses on counting the number of systems reviewed in the review of systems; the documentation of past history, social history, and family history; the number of organs and organ elements examined during the physical examination; and the complexity of MDM.
In January 2021, CMS plans to adopt new Current Procedural Terminology (CPT) code descriptors for the office and other outpatient E/M services that sunset most of the “bean-counting” metrics and emphasize the importance of the complexity of MDM in guiding selection of a correct code.2 Beginning in January 2021, clinicians will have the option of selecting an E/M code level based on the total amount of time required to provide the office visit service or the complexity of MDM. When selecting a code level based on MDM the new guidance emphasizes the importance of reviewing notes from other clinicians, reviewing test results, ordering of tests, and discussing and coordinating the care of the patient with other treating physicians. These changes reflect a better understanding of what is most important in good medical practice, promoting better patient care. TABLES 1 and 2 provide the initial guidance from CMS concerning selection of E/M code level based on time and MDM, respectively.2 The guidance for using MDM to select an E/M code level is likely to evolve following implementation, so stay tuned. When using MDM to select a code, 2 of the 3 general categories are required to select that level of service.


Increase in the valuation of office-based E/M services
The Medicare Physician Fee Schedule uses a resource-based relative value system to determine time and intensity of the work of clinical practice. This system recognizes 3 major factors that influence the resources required to provide a service:
- work of the clinician
- practice expense for technical components
- cost of professional liability insurance.
Many primary care professional associations have long contended that CMS has undervalued office-based E/M services relative to procedures, resulting in the devaluing of primary care practice. After the CPT code descriptors were updated by the CPT editorial panel, 52 specialty societies surveyed their members to provide inputs to CMS on the time and intensity of the office and other outpatient E/M codes as currently practiced. The American Medical Association’s Specialty Society Resource-Based Relative Value Scale Update Committee (RUC) reviewed the surveys and provided new inputs via open comment to CMS. CMS has responded to this feedback with a review of the intensity of clinical work required to provide an ambulatory visit service. In response to the review, CMS proposes to accept the recommendations of the RUC representing the house of medicine and increase the work and practice expense relative value assigned to new and established office visit codes. Overall, the combination of changes in relative values assigned for the work of the clinician and the expense of practice, increases the total value of office-based E/M codes for new patients by 7% to 14% and for established patients from 28% to 46% (see supplemental table in the sidebar at the end of this article).
Continue to: Decreased payments for procedural services...
Decreased payments for procedural services
Medicare is required to offset increased payment in one arena of health care delivery with decreased payment in other arenas of care, thereby achieving “budget-neutrality.” As detailed above, CMS plans to increase Medicare payments for office-based E/M services. Payment for services is calculated by multiplying the total RVUs for a particular service by a “conversion factor” (ie, number of dollars per RVU). To achieve budget-neutrality, CMS has proposed substantially reducing the conversion factor for 2021 (from $36.09 to $32.26), which will effectively decrease Medicare payments for procedural services since their RVUs have not changed. While the AMA RUC and many specialty societies continue to strongly advocate for the E/M work RVU increases to be included in the E/M components of 10- and 90-day global services, CMS has proposed to implement them only for “stand alone” E/M services.
Organizations are lobbying to delay or prevent the planned decrease in conversion factor, which results in substantial declines in payment for procedural services. (See "What do the Medicare billing changes mean for the Obstetrical Bundled services?" at the end of this article.) Due to the economic and clinical practice challenges caused by the coronavirus disease 2019 (COVID-19) pandemic it would be best if CMS did not reduce payments to physicians who are experts in procedural health care, thereby avoiding the risk of reduced access to these vital services.
If the current CMS changes in payment are implemented, endocrinologists, rheumatologists, and family physicians will have an increase in payment, and radiologists, pathologists, and surgeons will have a decrease in payment (TABLE 3).6 Obstetrics and gynecology is projected to have an 8% increase in Medicare payment. However, if an obstetrician-gynecologist derives most of their Medicare payments from surgical procedures, they are likely to have a decrease in payment from Medicare. Other payers will be incorporating the new coding structure for 2021; however, their payment structures and conversion factors are likely to vary. It is important to note that the RVUs for procedures have not changed. The budget neutrality adjustment resulted in a much lower conversion factor and therefore a decrease in payment for those specialties whose RVUs did not increase.

Bottom line
Working through the Medicare, Medicaid, and CHIP programs, CMS can influence greatly the practice of medicine including medical record documentation practices and payment rates for every clinical service. CMS proposes to end the onerous “bean counting” approach to billing compliance and refocus on the complexity of MDM as the foundation for selecting a billing code level. This change is long overdue, valuing the effective management of complex patients in office practice. Hopefully, CMS will reverse the planned reduction in the payment for procedural services, preserving patient access to important health care services. ●
The CY 2020 Medicare Physician Fee Schedule Final Rule was published electronically in the Federal Register on November 1, 2019. This final rule aligns the evaluation and management (E/M) coding and payment with changes recommended by the Current Procedural Terminology (CPT) Editorial Panel and American Medical Association’s (AMA) Specialty Society Resource-Based Relative Value Scale Update Committee (RUC) for office/outpatient E/M visits. Unfortunately, the Centers for Medicare and Medicaid Services (CMS) did not agree with the RUC, AMA, and specialty societies that the E/M payment changes should be applicable across all global services that incorporate E/M visits—despite the fact that the values proposed by the RUC incorporated survey data from 52 specialties, representing most of medicine (including those specialties that predominantly perform procedures). Specifically, CMS expressed the view that the number of E/M visits within the 10- and 90-day global codes, as well as the maternity care bundle, were difficult to validate; therefore, the increased values would not be distributed to those procedural services.
Many professional societies expressed significant concerns about the resulting budget neutrality adjustments that would occur effective January 2021. The great news for ObGyns is that the American College of Obstetricians and Gynecologists (ACOG) was able to respond directly to CMS’s concerns with data to support the number of prenatal visits within the Obstetrical Bundle. Tapping into a de-identified, cloud-based data set of prenatal records—representing more than 1,100 obstetric providers with close to 30,000 recently completed pregnancies—ACOG was able to document both a mean and median number of prenatal visits across a broad geographic, payer, and patient demographic that supported the 13 prenatal visits in the Obstetrical Bundle.
With ACOG’s advocacy and ability to provide data to CMS, the proposed physician fee schedule rule for 2021 has proposed to incorporate the E/M increased reimbursement into the prenatal care codes. Now we urge the CMS to finalize this proposal. Although Medicare pays for a tiny number of pregnancies annually, we hope that all payers, including Medicaid and managed care plans, will agree with this acknowledgement of the increased work of evaluation and management that obstetricians provide during prenatal care. Join ACOG in telling CMS to finalize their proposal to increase the values of the global obstetric codes: https://acog.quorum.us/campaign/28579/.

- Centers for Medicare and Medicaid Services. https://www.cms.gov/. Accessed August 28, 2020.
- American Medical Association. CPT Evaluation and Management (E/M) Office or Other Outpatient (99202-99215) and Prolonged Services (99354, 99355, 99356, 99XXX) Code and Guideline Changes. 2019. https://www.ama-assn.org /system/files/2019-06/cpt-office-prolonged-svs -code-changes.pdf. Accessed August 28, 2020.
- The American Academy of Family Physicians. Family medicine updates. Ann Fam Med. 2020;18:84-85. doi: 10.1370/afm.2508.
- Centers for Medicare and Medicaid Services. Final policy, payment and quality provisions changes to the Medicare Physician Fee Schedule for calendar year 2019. November 1, 2018. https://www.cms.gov/newsroom/fact-sheets /final-policy-payment-and-quality-provisionschanges-medicare-physician-fee-schedulecalendar-year. Accessed August 28, 2020.
- Department of Health and Human Services; Centers for Medicare and Medicaid Services. 42 CFR Parts 410, 414, 415, 423, 424, and 425. Federal Register. 2020;85(159). https://www.govinfo.gov /content/pkg/FR-2020-08-17/pdf/2020-17127 .pdf. Accessed August 28, 2020.
- Centers for Medicare and Medicaid Services. https://www.cms.gov/. Accessed August 28, 2020.
- American Medical Association. CPT Evaluation and Management (E/M) Office or Other Outpatient (99202-99215) and Prolonged Services (99354, 99355, 99356, 99XXX) Code and Guideline Changes. 2019. https://www.ama-assn.org /system/files/2019-06/cpt-office-prolonged-svs -code-changes.pdf. Accessed August 28, 2020.
- The American Academy of Family Physicians. Family medicine updates. Ann Fam Med. 2020;18:84-85. doi: 10.1370/afm.2508.
- Centers for Medicare and Medicaid Services. Final policy, payment and quality provisions changes to the Medicare Physician Fee Schedule for calendar year 2019. November 1, 2018. https://www.cms.gov/newsroom/fact-sheets /final-policy-payment-and-quality-provisionschanges-medicare-physician-fee-schedulecalendar-year. Accessed August 28, 2020.
- Department of Health and Human Services; Centers for Medicare and Medicaid Services. 42 CFR Parts 410, 414, 415, 423, 424, and 425. Federal Register. 2020;85(159). https://www.govinfo.gov /content/pkg/FR-2020-08-17/pdf/2020-17127 .pdf. Accessed August 28, 2020.
Lifting the restrictions on mifepristone during COVID-19: A step in the right direction
Mifepristone is a safe, effective, and well-tolerated medication for managing miscarriage and for medical abortion when combined with misoprostol.1,2 Since the US Food and Drug Administration (FDA) approved its use in 2000, more than 4 million women have used this medication.3 The combination of mifepristone with misoprostol was used for 39% of all US abortions in 2017.4 Approximately 10% of all clinically recognized pregnancies end in miscarriages, and many are safely managed with either misoprostol alone or with the combination of mifepristone and misoprostol.5
The issue
The prescription and distribution of mifepristone is highly regulated by the FDA via requirements outlined in the Risk Evaluation and Mitigation Strategies (REMS) drug safety program. The FDA may determine a REMS is necessary for a specific drug to ensure the benefits of a drug outweigh the potential risks. A REMS may include an informative package insert for patients, follow-up communication to prescribers—including letters, safety protocols or recommended laboratory tests, or Elements to Assure Safe Use (ETASU). ETASU are types of REMS that are placed on medications that have significant potential for serious adverse effects, and without such restrictions FDA approval would be rescinded.
Are mifepristone requirements fairly applied?
The 3 ETASU restrictions on the distribution of mifepristone are in-person dispensation, prescriber certification, and patient signatures on special forms.6 The in-person dispensing requirement is applied to only 16 other medications (one of which is Mifeprex, the brand version of mifepristone), and Mifeprex/mifepristone are the only ones deemed safe for self-administration—meaning that patients receive the drug from a clinic but then may take it at a site of their choosing. The prescriber certification requirement places expectations on providers to account for distribution of doses and keep records of serial numbers (in effect, having clinicians act as both physician and pharmacist, as most medications are distributed and recorded in pharmacies). The patient form was recommended for elimination in 2016 due to its duplicative information and burden on patients—a recommendation that was then overruled by the FDA commissioner.7
These 3 requirements placed on mifepristone specifically target dosages for use related to abortions and miscarriages. Mifepristone is used to treat other medical conditions, with much higher doses, without the same restrictions—in fact, the FDA has allowed much higher doses of mifepristone to be mailed directly to a patient when prescribed for different disorders. The American College of Obstetricians and Gynecologists (ACOG) has long opposed the burdensome REMS requirements on mifepristone for reproductive health indications.8
Arguments regarding the safety of mifepristone must be understood in the context of how the medication is taken, and the unique difference with other medications that must be administered by physicians or in health care facilities. Mifepristone is self-administered, and the desired effect—evacuation of uterine contents—typically occurs after a patient takes the accompanying medication misoprostol, which is some 24 to 72 hours later. This timeframe makes it highly unlikely that any patient would be in the presence of their provider at the time of medication effect, thus an in-person dispensing requirement has no medical bearing on the outcome of the health of the patient.
REMS changes during the COVID-19 pandemic
The coronavirus disease 2019 (COVID-19) pandemic has necessarily changed the structure of REMS and ETASU requirements for many medications, with changes made in order to mitigate viral transmission through the limitation of unnecessary visits to clinics or hospitals. The FDA announced in March of 2020 that it would not enforce pre-prescription requirements, such as laboratory or magnetic resonance imaging results, for many medications (including those more toxic than mifepristone), and that it would lift the requirement for in-person dispensation of several medications.9 Also in March 2020 the Department of Health and Human Services Secretary (HHS) and the Drug Enforcement Agency (DEA) activated a “telemedicine exception” to allow physicians to use telemedicine to satisfy mandatory requirements for prescribing controlled substances, including opioids.10
Despite repeated pleas from organizations, individuals, and physician groups, the FDA continued to enforce the REMS/ETASU for mifepristone as the pandemic decimated communities. Importantly, the pandemic has not had an equal effect on all communities, and the disparities highlighted in outcomes as related to COVID-19 are also reflected in disparities to access to reproductive choices.11 By enforcing REMS/ETASU for mifepristone during a global pandemic, the FDA has placed additional burden on women and people who menstruate. As offices and clinics have closed, and as many jobs have evaporated, additional barriers have emerged, such as lack of childcare, fewer transportation options, and decreased clinic appointments.
As the pandemic continues to affect communities in the United States, ACOG has issued guidance recommending assessment for eligibility for medical abortion remotely, and has encouraged the use of telemedicine and other remote interactions for its members and patients to limit transmission of the virus.
The lawsuit
On May 27, 2020, the American Civil Liberties Union (ACLU) (on behalf of ACOG, the Council of University Chairs of Obstetrics and Gynecology, New York State Academy of Family Physicians, SisterSong, and Honor MacNaughton, MD) filed a civil action against the FDA and HHS challenging the requirement for in-person dispensing of mifepristone and associated ETASU requirements during the COVID-19 pandemic. The plaintiffs sought this injunction based on the claim that these restrictions during the pandemic infringe on the constitutional rights to patients’ privacy and liberty and to equal protection of the law as protected by the Due Process Clause of the Fifth Amendment. Additionally, the ACLU and other organizations said these unnecessary restrictions place patients, providers, and staff at unnecessary risk of viral exposure amidst a global pandemic.
The verdict
On July 13, 2020, a federal court granted the preliminary injunction to suspend FDA’s enforcement of the in-person requirements of mifepristone for abortion during the COVID-19 pandemic. The court denied the motion for suspension of in-person restrictions as applied to miscarriage management. The preliminary injunction applies nationwide without geographic limitation. It will remain in effect until the end of the litigation or for 30 days following the expiration of the public health emergency.
What the outcome means
This injunction is a step in the right direction for patients and providers to allow for autonomy and clinical practice guided by clinician expertise. However, this ruling remains narrow. Patients must be counseled about mifepristone via telemedicine and sign a Patient Agreement Form, which must be returned electronically or by mail. Patients must receive a copy of the mifepristone medication guide, and dispensing of mifepristone must still be conducted by or under the supervision of a certified provider. The medication may not be dispensed by retail pharmacies, thus requiring providers to arrange for mailing of prescriptions to patients. Given state-based legal statutes regarding mailing of medications, this injunction may not lead to an immediate increase in access to care. In addition, patients seeking management for miscarriage must go to clinic to have mifepristone dispensed and thus risk exposure to viral transmission.
What now?
The regulation of mifepristone—in spite of excellent safety and specifically for the narrow purpose of administration in the setting of abortion and miscarriage care—is by definition a discriminatory practice against patients and providers. As clinicians, we are duty-bound to speak out against injustices to our practices and our patients. At a local level, we can work to implement safe practices in the setting of this injunction and continue to work on a national level to ensure this injunction becomes permanent and with more broad scope to eliminate all of the REMS requirements for mifepristone.
ACTION ITEMS
- Act locally! Are you an abortion provider? Contact your local ACLU (find them here) or lawyer in your area for assistance navigating the legal landscape to prescribe after this injunction.
- Act statewide! Press candidates in your state to stand up for science and data. Support legislative acts and bills that address combating discriminatory regulations.
- Act nationally! The President is responsible for appointing the Commissioner of the FDA and the Secretary of Health and Human Services (with Senate advice and consent). Who we elect matters. Seek out opportunities to become involved in increasing access to and awareness of voter registration and Election Day, and speak out against voter suppression. Make sure you are registered to vote here and check your area to review new recommendations amidst the pandemic.
- American College of Obstetricians and Gynecologists. Practice bulletin number 143: medical management of first trimester abortion. Obstet Gynecol. 2014;123:676-692.
- Schreiber CA, Crenin MD, Atrio J, et al. Mifepristone pretreatment for the medical management of early pregnancy loss. N Engl J Med. 2018;378:2161-2170.
- Danco Laboratories. Mifeprex effectiveness and advantages. https://www.earlyoptionpill.com/is-mifeprex-right-for-me/effectiveness-advantages/ Accessed August 2, 2020.
- Jones RK, Witwer E, Jerman J. Abortion incidence and service availability in the United States, 2017. September 2019. https://www.guttmacher.org/report/abortion-incidence-service-availability-us-2017. Accessed September 10, 2020.
- American College of Obstetricians and Gynecologists. Practice bulletin no. 150: early pregnancy loss. Obstet Gynecol. 2015;125:1258-1267.
- US Food and Drug Administration. Risk evaluation and mitigation strategy (REMS) single shared system for mifepristone 200 mg. April 2019. https://www.accessdata.fda.gov/drugsatfda_docs/rems/Mifepristone_2019_04_11_REMS_Full.pdf. Accessed September 10, 2020.
- US Food and Drug Administration; Center for Drug Evaluation and Research. 2016 REMS Review, Summary Review 25. March 29, 2016. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2016/020687Orig1s020SumR.pdf. Accessed September 10, 2020.
- American College of Obstetricians and Gynecologists. Improving access to mifepristone for reproductive health indications. June 2018. https://www.acog.org/clinical-information/policy-and-position-statements/position-statements/2018/improving-access-to-mifepristone-for-reproductive-health-indications. Accessed August 2, 2020.
- US Food and Drug Administration. Policy for certain REMS requirements during the COVID-19 public health emergency: guidance for industry and health care professionals. March 2020. https://www.fda.gov/media/136317/download. Accessed September 10, 2020.
- US Department of Justice. US Drug Enforcement Administration. COVID-19 Information Page, Telemedicine. https://www.deadiversion.usdoj.gov/coronavirus.html#TELE. Accessed May 25, 2020.
- Centers for Disease Control and Prevention. Coronavirus disease 2019: health equity considerations and racial and ethnic minority groups. https://www.cdc.gov/coronavirus/2019-ncov/community/health-equity/race-ethnicity.html. Updated July 24, 2020. Accessed September 10, 2020.
Mifepristone is a safe, effective, and well-tolerated medication for managing miscarriage and for medical abortion when combined with misoprostol.1,2 Since the US Food and Drug Administration (FDA) approved its use in 2000, more than 4 million women have used this medication.3 The combination of mifepristone with misoprostol was used for 39% of all US abortions in 2017.4 Approximately 10% of all clinically recognized pregnancies end in miscarriages, and many are safely managed with either misoprostol alone or with the combination of mifepristone and misoprostol.5
The issue
The prescription and distribution of mifepristone is highly regulated by the FDA via requirements outlined in the Risk Evaluation and Mitigation Strategies (REMS) drug safety program. The FDA may determine a REMS is necessary for a specific drug to ensure the benefits of a drug outweigh the potential risks. A REMS may include an informative package insert for patients, follow-up communication to prescribers—including letters, safety protocols or recommended laboratory tests, or Elements to Assure Safe Use (ETASU). ETASU are types of REMS that are placed on medications that have significant potential for serious adverse effects, and without such restrictions FDA approval would be rescinded.
Are mifepristone requirements fairly applied?
The 3 ETASU restrictions on the distribution of mifepristone are in-person dispensation, prescriber certification, and patient signatures on special forms.6 The in-person dispensing requirement is applied to only 16 other medications (one of which is Mifeprex, the brand version of mifepristone), and Mifeprex/mifepristone are the only ones deemed safe for self-administration—meaning that patients receive the drug from a clinic but then may take it at a site of their choosing. The prescriber certification requirement places expectations on providers to account for distribution of doses and keep records of serial numbers (in effect, having clinicians act as both physician and pharmacist, as most medications are distributed and recorded in pharmacies). The patient form was recommended for elimination in 2016 due to its duplicative information and burden on patients—a recommendation that was then overruled by the FDA commissioner.7
These 3 requirements placed on mifepristone specifically target dosages for use related to abortions and miscarriages. Mifepristone is used to treat other medical conditions, with much higher doses, without the same restrictions—in fact, the FDA has allowed much higher doses of mifepristone to be mailed directly to a patient when prescribed for different disorders. The American College of Obstetricians and Gynecologists (ACOG) has long opposed the burdensome REMS requirements on mifepristone for reproductive health indications.8
Arguments regarding the safety of mifepristone must be understood in the context of how the medication is taken, and the unique difference with other medications that must be administered by physicians or in health care facilities. Mifepristone is self-administered, and the desired effect—evacuation of uterine contents—typically occurs after a patient takes the accompanying medication misoprostol, which is some 24 to 72 hours later. This timeframe makes it highly unlikely that any patient would be in the presence of their provider at the time of medication effect, thus an in-person dispensing requirement has no medical bearing on the outcome of the health of the patient.
REMS changes during the COVID-19 pandemic
The coronavirus disease 2019 (COVID-19) pandemic has necessarily changed the structure of REMS and ETASU requirements for many medications, with changes made in order to mitigate viral transmission through the limitation of unnecessary visits to clinics or hospitals. The FDA announced in March of 2020 that it would not enforce pre-prescription requirements, such as laboratory or magnetic resonance imaging results, for many medications (including those more toxic than mifepristone), and that it would lift the requirement for in-person dispensation of several medications.9 Also in March 2020 the Department of Health and Human Services Secretary (HHS) and the Drug Enforcement Agency (DEA) activated a “telemedicine exception” to allow physicians to use telemedicine to satisfy mandatory requirements for prescribing controlled substances, including opioids.10
Despite repeated pleas from organizations, individuals, and physician groups, the FDA continued to enforce the REMS/ETASU for mifepristone as the pandemic decimated communities. Importantly, the pandemic has not had an equal effect on all communities, and the disparities highlighted in outcomes as related to COVID-19 are also reflected in disparities to access to reproductive choices.11 By enforcing REMS/ETASU for mifepristone during a global pandemic, the FDA has placed additional burden on women and people who menstruate. As offices and clinics have closed, and as many jobs have evaporated, additional barriers have emerged, such as lack of childcare, fewer transportation options, and decreased clinic appointments.
As the pandemic continues to affect communities in the United States, ACOG has issued guidance recommending assessment for eligibility for medical abortion remotely, and has encouraged the use of telemedicine and other remote interactions for its members and patients to limit transmission of the virus.
The lawsuit
On May 27, 2020, the American Civil Liberties Union (ACLU) (on behalf of ACOG, the Council of University Chairs of Obstetrics and Gynecology, New York State Academy of Family Physicians, SisterSong, and Honor MacNaughton, MD) filed a civil action against the FDA and HHS challenging the requirement for in-person dispensing of mifepristone and associated ETASU requirements during the COVID-19 pandemic. The plaintiffs sought this injunction based on the claim that these restrictions during the pandemic infringe on the constitutional rights to patients’ privacy and liberty and to equal protection of the law as protected by the Due Process Clause of the Fifth Amendment. Additionally, the ACLU and other organizations said these unnecessary restrictions place patients, providers, and staff at unnecessary risk of viral exposure amidst a global pandemic.
The verdict
On July 13, 2020, a federal court granted the preliminary injunction to suspend FDA’s enforcement of the in-person requirements of mifepristone for abortion during the COVID-19 pandemic. The court denied the motion for suspension of in-person restrictions as applied to miscarriage management. The preliminary injunction applies nationwide without geographic limitation. It will remain in effect until the end of the litigation or for 30 days following the expiration of the public health emergency.
What the outcome means
This injunction is a step in the right direction for patients and providers to allow for autonomy and clinical practice guided by clinician expertise. However, this ruling remains narrow. Patients must be counseled about mifepristone via telemedicine and sign a Patient Agreement Form, which must be returned electronically or by mail. Patients must receive a copy of the mifepristone medication guide, and dispensing of mifepristone must still be conducted by or under the supervision of a certified provider. The medication may not be dispensed by retail pharmacies, thus requiring providers to arrange for mailing of prescriptions to patients. Given state-based legal statutes regarding mailing of medications, this injunction may not lead to an immediate increase in access to care. In addition, patients seeking management for miscarriage must go to clinic to have mifepristone dispensed and thus risk exposure to viral transmission.
What now?
The regulation of mifepristone—in spite of excellent safety and specifically for the narrow purpose of administration in the setting of abortion and miscarriage care—is by definition a discriminatory practice against patients and providers. As clinicians, we are duty-bound to speak out against injustices to our practices and our patients. At a local level, we can work to implement safe practices in the setting of this injunction and continue to work on a national level to ensure this injunction becomes permanent and with more broad scope to eliminate all of the REMS requirements for mifepristone.
ACTION ITEMS
- Act locally! Are you an abortion provider? Contact your local ACLU (find them here) or lawyer in your area for assistance navigating the legal landscape to prescribe after this injunction.
- Act statewide! Press candidates in your state to stand up for science and data. Support legislative acts and bills that address combating discriminatory regulations.
- Act nationally! The President is responsible for appointing the Commissioner of the FDA and the Secretary of Health and Human Services (with Senate advice and consent). Who we elect matters. Seek out opportunities to become involved in increasing access to and awareness of voter registration and Election Day, and speak out against voter suppression. Make sure you are registered to vote here and check your area to review new recommendations amidst the pandemic.
Mifepristone is a safe, effective, and well-tolerated medication for managing miscarriage and for medical abortion when combined with misoprostol.1,2 Since the US Food and Drug Administration (FDA) approved its use in 2000, more than 4 million women have used this medication.3 The combination of mifepristone with misoprostol was used for 39% of all US abortions in 2017.4 Approximately 10% of all clinically recognized pregnancies end in miscarriages, and many are safely managed with either misoprostol alone or with the combination of mifepristone and misoprostol.5
The issue
The prescription and distribution of mifepristone is highly regulated by the FDA via requirements outlined in the Risk Evaluation and Mitigation Strategies (REMS) drug safety program. The FDA may determine a REMS is necessary for a specific drug to ensure the benefits of a drug outweigh the potential risks. A REMS may include an informative package insert for patients, follow-up communication to prescribers—including letters, safety protocols or recommended laboratory tests, or Elements to Assure Safe Use (ETASU). ETASU are types of REMS that are placed on medications that have significant potential for serious adverse effects, and without such restrictions FDA approval would be rescinded.
Are mifepristone requirements fairly applied?
The 3 ETASU restrictions on the distribution of mifepristone are in-person dispensation, prescriber certification, and patient signatures on special forms.6 The in-person dispensing requirement is applied to only 16 other medications (one of which is Mifeprex, the brand version of mifepristone), and Mifeprex/mifepristone are the only ones deemed safe for self-administration—meaning that patients receive the drug from a clinic but then may take it at a site of their choosing. The prescriber certification requirement places expectations on providers to account for distribution of doses and keep records of serial numbers (in effect, having clinicians act as both physician and pharmacist, as most medications are distributed and recorded in pharmacies). The patient form was recommended for elimination in 2016 due to its duplicative information and burden on patients—a recommendation that was then overruled by the FDA commissioner.7
These 3 requirements placed on mifepristone specifically target dosages for use related to abortions and miscarriages. Mifepristone is used to treat other medical conditions, with much higher doses, without the same restrictions—in fact, the FDA has allowed much higher doses of mifepristone to be mailed directly to a patient when prescribed for different disorders. The American College of Obstetricians and Gynecologists (ACOG) has long opposed the burdensome REMS requirements on mifepristone for reproductive health indications.8
Arguments regarding the safety of mifepristone must be understood in the context of how the medication is taken, and the unique difference with other medications that must be administered by physicians or in health care facilities. Mifepristone is self-administered, and the desired effect—evacuation of uterine contents—typically occurs after a patient takes the accompanying medication misoprostol, which is some 24 to 72 hours later. This timeframe makes it highly unlikely that any patient would be in the presence of their provider at the time of medication effect, thus an in-person dispensing requirement has no medical bearing on the outcome of the health of the patient.
REMS changes during the COVID-19 pandemic
The coronavirus disease 2019 (COVID-19) pandemic has necessarily changed the structure of REMS and ETASU requirements for many medications, with changes made in order to mitigate viral transmission through the limitation of unnecessary visits to clinics or hospitals. The FDA announced in March of 2020 that it would not enforce pre-prescription requirements, such as laboratory or magnetic resonance imaging results, for many medications (including those more toxic than mifepristone), and that it would lift the requirement for in-person dispensation of several medications.9 Also in March 2020 the Department of Health and Human Services Secretary (HHS) and the Drug Enforcement Agency (DEA) activated a “telemedicine exception” to allow physicians to use telemedicine to satisfy mandatory requirements for prescribing controlled substances, including opioids.10
Despite repeated pleas from organizations, individuals, and physician groups, the FDA continued to enforce the REMS/ETASU for mifepristone as the pandemic decimated communities. Importantly, the pandemic has not had an equal effect on all communities, and the disparities highlighted in outcomes as related to COVID-19 are also reflected in disparities to access to reproductive choices.11 By enforcing REMS/ETASU for mifepristone during a global pandemic, the FDA has placed additional burden on women and people who menstruate. As offices and clinics have closed, and as many jobs have evaporated, additional barriers have emerged, such as lack of childcare, fewer transportation options, and decreased clinic appointments.
As the pandemic continues to affect communities in the United States, ACOG has issued guidance recommending assessment for eligibility for medical abortion remotely, and has encouraged the use of telemedicine and other remote interactions for its members and patients to limit transmission of the virus.
The lawsuit
On May 27, 2020, the American Civil Liberties Union (ACLU) (on behalf of ACOG, the Council of University Chairs of Obstetrics and Gynecology, New York State Academy of Family Physicians, SisterSong, and Honor MacNaughton, MD) filed a civil action against the FDA and HHS challenging the requirement for in-person dispensing of mifepristone and associated ETASU requirements during the COVID-19 pandemic. The plaintiffs sought this injunction based on the claim that these restrictions during the pandemic infringe on the constitutional rights to patients’ privacy and liberty and to equal protection of the law as protected by the Due Process Clause of the Fifth Amendment. Additionally, the ACLU and other organizations said these unnecessary restrictions place patients, providers, and staff at unnecessary risk of viral exposure amidst a global pandemic.
The verdict
On July 13, 2020, a federal court granted the preliminary injunction to suspend FDA’s enforcement of the in-person requirements of mifepristone for abortion during the COVID-19 pandemic. The court denied the motion for suspension of in-person restrictions as applied to miscarriage management. The preliminary injunction applies nationwide without geographic limitation. It will remain in effect until the end of the litigation or for 30 days following the expiration of the public health emergency.
What the outcome means
This injunction is a step in the right direction for patients and providers to allow for autonomy and clinical practice guided by clinician expertise. However, this ruling remains narrow. Patients must be counseled about mifepristone via telemedicine and sign a Patient Agreement Form, which must be returned electronically or by mail. Patients must receive a copy of the mifepristone medication guide, and dispensing of mifepristone must still be conducted by or under the supervision of a certified provider. The medication may not be dispensed by retail pharmacies, thus requiring providers to arrange for mailing of prescriptions to patients. Given state-based legal statutes regarding mailing of medications, this injunction may not lead to an immediate increase in access to care. In addition, patients seeking management for miscarriage must go to clinic to have mifepristone dispensed and thus risk exposure to viral transmission.
What now?
The regulation of mifepristone—in spite of excellent safety and specifically for the narrow purpose of administration in the setting of abortion and miscarriage care—is by definition a discriminatory practice against patients and providers. As clinicians, we are duty-bound to speak out against injustices to our practices and our patients. At a local level, we can work to implement safe practices in the setting of this injunction and continue to work on a national level to ensure this injunction becomes permanent and with more broad scope to eliminate all of the REMS requirements for mifepristone.
ACTION ITEMS
- Act locally! Are you an abortion provider? Contact your local ACLU (find them here) or lawyer in your area for assistance navigating the legal landscape to prescribe after this injunction.
- Act statewide! Press candidates in your state to stand up for science and data. Support legislative acts and bills that address combating discriminatory regulations.
- Act nationally! The President is responsible for appointing the Commissioner of the FDA and the Secretary of Health and Human Services (with Senate advice and consent). Who we elect matters. Seek out opportunities to become involved in increasing access to and awareness of voter registration and Election Day, and speak out against voter suppression. Make sure you are registered to vote here and check your area to review new recommendations amidst the pandemic.
- American College of Obstetricians and Gynecologists. Practice bulletin number 143: medical management of first trimester abortion. Obstet Gynecol. 2014;123:676-692.
- Schreiber CA, Crenin MD, Atrio J, et al. Mifepristone pretreatment for the medical management of early pregnancy loss. N Engl J Med. 2018;378:2161-2170.
- Danco Laboratories. Mifeprex effectiveness and advantages. https://www.earlyoptionpill.com/is-mifeprex-right-for-me/effectiveness-advantages/ Accessed August 2, 2020.
- Jones RK, Witwer E, Jerman J. Abortion incidence and service availability in the United States, 2017. September 2019. https://www.guttmacher.org/report/abortion-incidence-service-availability-us-2017. Accessed September 10, 2020.
- American College of Obstetricians and Gynecologists. Practice bulletin no. 150: early pregnancy loss. Obstet Gynecol. 2015;125:1258-1267.
- US Food and Drug Administration. Risk evaluation and mitigation strategy (REMS) single shared system for mifepristone 200 mg. April 2019. https://www.accessdata.fda.gov/drugsatfda_docs/rems/Mifepristone_2019_04_11_REMS_Full.pdf. Accessed September 10, 2020.
- US Food and Drug Administration; Center for Drug Evaluation and Research. 2016 REMS Review, Summary Review 25. March 29, 2016. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2016/020687Orig1s020SumR.pdf. Accessed September 10, 2020.
- American College of Obstetricians and Gynecologists. Improving access to mifepristone for reproductive health indications. June 2018. https://www.acog.org/clinical-information/policy-and-position-statements/position-statements/2018/improving-access-to-mifepristone-for-reproductive-health-indications. Accessed August 2, 2020.
- US Food and Drug Administration. Policy for certain REMS requirements during the COVID-19 public health emergency: guidance for industry and health care professionals. March 2020. https://www.fda.gov/media/136317/download. Accessed September 10, 2020.
- US Department of Justice. US Drug Enforcement Administration. COVID-19 Information Page, Telemedicine. https://www.deadiversion.usdoj.gov/coronavirus.html#TELE. Accessed May 25, 2020.
- Centers for Disease Control and Prevention. Coronavirus disease 2019: health equity considerations and racial and ethnic minority groups. https://www.cdc.gov/coronavirus/2019-ncov/community/health-equity/race-ethnicity.html. Updated July 24, 2020. Accessed September 10, 2020.
- American College of Obstetricians and Gynecologists. Practice bulletin number 143: medical management of first trimester abortion. Obstet Gynecol. 2014;123:676-692.
- Schreiber CA, Crenin MD, Atrio J, et al. Mifepristone pretreatment for the medical management of early pregnancy loss. N Engl J Med. 2018;378:2161-2170.
- Danco Laboratories. Mifeprex effectiveness and advantages. https://www.earlyoptionpill.com/is-mifeprex-right-for-me/effectiveness-advantages/ Accessed August 2, 2020.
- Jones RK, Witwer E, Jerman J. Abortion incidence and service availability in the United States, 2017. September 2019. https://www.guttmacher.org/report/abortion-incidence-service-availability-us-2017. Accessed September 10, 2020.
- American College of Obstetricians and Gynecologists. Practice bulletin no. 150: early pregnancy loss. Obstet Gynecol. 2015;125:1258-1267.
- US Food and Drug Administration. Risk evaluation and mitigation strategy (REMS) single shared system for mifepristone 200 mg. April 2019. https://www.accessdata.fda.gov/drugsatfda_docs/rems/Mifepristone_2019_04_11_REMS_Full.pdf. Accessed September 10, 2020.
- US Food and Drug Administration; Center for Drug Evaluation and Research. 2016 REMS Review, Summary Review 25. March 29, 2016. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2016/020687Orig1s020SumR.pdf. Accessed September 10, 2020.
- American College of Obstetricians and Gynecologists. Improving access to mifepristone for reproductive health indications. June 2018. https://www.acog.org/clinical-information/policy-and-position-statements/position-statements/2018/improving-access-to-mifepristone-for-reproductive-health-indications. Accessed August 2, 2020.
- US Food and Drug Administration. Policy for certain REMS requirements during the COVID-19 public health emergency: guidance for industry and health care professionals. March 2020. https://www.fda.gov/media/136317/download. Accessed September 10, 2020.
- US Department of Justice. US Drug Enforcement Administration. COVID-19 Information Page, Telemedicine. https://www.deadiversion.usdoj.gov/coronavirus.html#TELE. Accessed May 25, 2020.
- Centers for Disease Control and Prevention. Coronavirus disease 2019: health equity considerations and racial and ethnic minority groups. https://www.cdc.gov/coronavirus/2019-ncov/community/health-equity/race-ethnicity.html. Updated July 24, 2020. Accessed September 10, 2020.




