User login
What is optimal hormonal treatment for women with polycystic ovary syndrome?
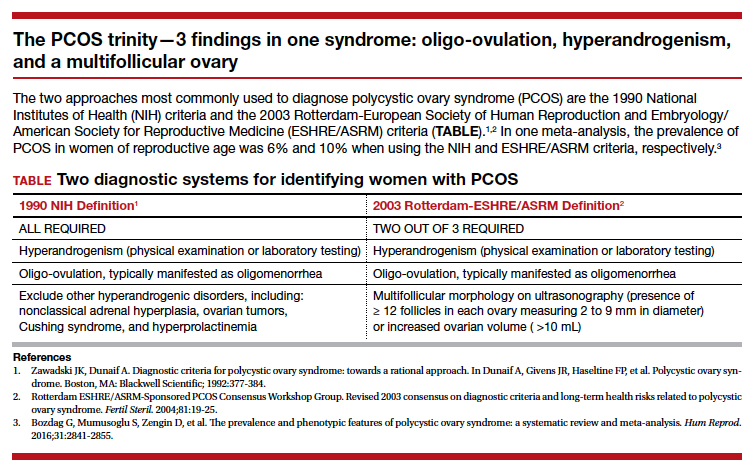
Polycystic ovary syndrome (PCOS) is the triad of oligo-ovulation resulting in oligomenorrhea, hyperandrogenism and, often, an excess number of small antral follicles on high-resolution pelvic ultrasound. One meta-analysis reported that, in women of reproductive age, the prevalence of PCOS was 10% using the Rotterdam-European Society of Human Reproduction and Embryology/American Society for Reproductive Medicine (ESHRE/ASRM) criteria1 and 6% using the National Institutes of Health 1990 diagnostic criteria.2 (See “The PCOS trinity—3 findings in one syndrome: oligo-ovulation, hyperandrogenism, and a multifollicular ovary.”3)
PCOS is caused by abnormalities in 3 systems: reproductive, metabolic, and dermatologic. Reproductive abnormalities commonly observed in women with PCOS include4:
- an increase in pituitary secretion of luteinizing hormone (LH), resulting from both an increase in LH pulse amplitude and LH pulse frequency, suggesting a primary hypothalamic disorder
- an increase in ovarian secretion of androstenedione and testosterone due to stimulation by LH and possibly insulin
- oligo-ovulation with chronically low levels of progesterone that can result in endometrial hyperplasia
- ovulatory infertility.
Metabolic abnormalities commonly observed in women with PCOS include5,6:
- insulin resistance and hyperinsulinemia
- excess adipose tissue in the liver
- excess visceral fat
- elevated adipokines
- obesity
- an increased prevalence of glucose intolerance and frank diabetes.
Dermatologic abnormalities commonly observed in women with PCOS include7:
- facial hirsutism
- acne
- androgenetic alopecia.
Given that PCOS is caused by abnormalities in the reproductive, metabolic, and dermatologic systems, it is appropriate to consider multimodal hormonal therapy that addresses all 3 problems. In my practice, I believe that the best approach to the long-term hormonal treatment of PCOS for many women is to prescribe a combination of 3 medicines: a combination estrogen-progestin oral contraceptive (COC), an insulin sensitizer, and an antiandrogen.
The COC reduces pituitary secretion of LH, decreases ovarian androgen production, and prevents the development of endometrial hyperplasia. When taken cyclically, the COC treatment also restores regular withdrawal uterine bleeding.
An insulin sensitizer, such as metformin or pioglitazone, helps to reduce insulin resistance, glucose intolerance, and hepatic adipose content, rebalancing central metabolism. It is important to include diet and exercise in the long-term treatment of PCOS, and I always encourage these lifestyle changes. However, my patients usually report that they have tried multiple times to restrict dietary caloric intake and increase exercise and have been unable to rebalance their metabolism with these interventions alone. Of note, in the women with PCOS and a body mass index >35 kg/m2, bariatric surgery, such as a sleeve gastrectomy, often results in marked improvement of their PCOS.8
The antiandrogen spironolactone provides effective treatment for the dermatologic problems of facial hirsutism and acne. Some COCs containing the progestins drospirenone, norgestimate, and norethindrone acetate are approved by the US Food and Drug Administration for the treatment of acne. A common approach I use in practice is to prescribe a COC, plus spironolactone 100 mg daily plus metformin extended-release 750 mg to 1,500 mg daily.
Continue to: Which COCs have low androgenicity?...
Which COCs have low androgenicity?
I believe that every COC is an effective treatment for PCOS, regardless of the androgenicity of the progestin in the contraceptive. However, some dermatologists believe that combination contraceptives containing progestins with low androgenicity, such as drospirenone, norgestimate, and desogestrel, are more likely to improve acne than contraceptives with an androgenic progestin such as levonorgestrel. In one study in which 2,147 women with acne were treated by one dermatologic practice, the percentage of women reporting that a birth control pill helped to improve their acne was 66% for pills containing drospirenone, 53% for pills containing norgestimate, 44% for pills containing desogestrel, 30% for pills containing norethindrone, and 25% for pills containing levonorgestrel. In the same study, the percent of women reporting that a birth control pill made their acne worse was 3% for pills containing drospirenone, 6% for pills containing norgestimate, 2% for pills containing desogestrel, 8% for pills containing norethindrone, and 10% for pills containing levonorgestrel.9 Given these findings, when treating a woman with PCOS, I generally prescribe a contraceptive that does not contain levonorgestrel.
Why is a spironolactone dose of 100 mg a good choice for PCOS treatment?
Spironolactone, an antiandrogen and inhibitor of 5-alpha-reductase, is commonly prescribed for the treatment of hirsutism and acne at doses ranging from 50 mg to 200 mg daily.10,11 In my clinical experience, spironolactone at a dose of 200 mg daily commonly causes irregular and bothersome uterine bleeding while spironolactone at a dose of 100 mg daily is seldom associated with irregular bleeding. I believe that spironolactone at a dose of 100 mg daily results in superior clinical efficacy than a 50-mg daily dose, although studies report that both doses are effective in the treatment of acne and hirsutism. Spironolactone should not be prescribed to women with renal failure because it can result in severe hyperkalemia. In a study of spironolactone safety in the treatment of acne, no adverse effects on the kidney, liver, or adrenal glands were reported over 8 years of use.12
What insulin sensitizers are useful in rebalancing the metabolic abnormalities observed with PCOS?
Diet and exercise are superb approaches to rebalancing metabolic abnormalities, but for many of my patients they are insufficient and treatment with an insulin sensitizer is warranted. The most commonly utilized insulin sensitizer for the treatment of PCOS is metformin because it is very inexpensive and has a low risk of serious adverse effects such as lactic acidosis. Metformin increases peripheral glucose uptake and reduces gastrointestinal glucose absorption. Insulin sensitizers also decrease visceral fat, a major source of adipokines. One major disadvantage of metformin is that at doses in the range of 1,500 mg to 2,250 mg it often causes gastrointestinal adverse effects such as borborygmi, nausea, abdominal discomfort, and loose stools.
Thiazolidinediones, including pioglitazone, have been reported to be effective in rebalancing central metabolism in women with PCOS. Pioglitazone carries a black box warning of an increased risk of congestive heart failure and nonfatal myocardial infarction. Pioglitazone is also associated with a risk of hepatotoxicity. However, at the pioglitazone dose commonly used in the treatment of PCOS (7.5 mg daily), these serious adverse effects are rare. In practice, I initiate metformin at a dose of 750 mg daily using the extended-release formulation. I increase the metformin dose to 1,500 mg daily if the patient has no bothersome gastrointestinal symptoms on the lower dose. If the patient cannot tolerate metformin treatment because of adverse effects, I will use pioglitazone 7.5 mg daily.
Continue to: Treatment of PCOS in women who are carriers of the Factor V Leiden mutation...
Treatment of PCOS in women who are carriers of the Factor V Leiden mutation
The Factor V Leiden allele is associated with an increased risk of venous thromboembolism. Estrogen-progestin contraception is contraindicated in women with the Factor V Leiden mutation. The prevalence of this mutation varies by race and ethnicity. It is present in about 5% of white, 2% of Hispanic, 1% of black, 1% of Native American, and 0.5% of Asian women. In women with PCOS who are known to be carriers of the mutation, dual therapy with metformin and spironolactone is highly effective.13-15 For these women I also offer a levonorgestrel IUD to provide contraception and reduce the risk of endometrial hyperplasia.
Combination triple medication treatment of PCOS
Optimal treatment of the reproductive, metabolic, and dermatologic problems associated with PCOS requires multimodal medications including an estrogen-progestin contraceptive, an antiandrogen, and an insulin sensitizer. In my practice, I initiate treatment of PCOS by offering patients 3 medications: a COC, spironolactone 100 mg daily, and metformin extended-release formulation 750 mg daily. Some patients elect dual medication therapy (COC plus spironolactone or COC plus metformin), but many patients select treatment with all 3 medications. Although triple medication treatment of PCOS has not been tested in large randomized clinical trials, small trials report that triple medication treatment produces optimal improvement in the reproductive, metabolic, and dermatologic problems associated with PCOS.16-18
- Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril. 2004;81:19-25.
- Zawadski JK, Dunaif A. Diagnostic criteria for polycystic ovary syndrome: towards a rational approach. In Dunaif A, Givens JR, Haseltine FP, et al. Polycystic ovary syndrome. Boston, MA: Blackwell Scientific; 1992:377-384.
- Bozdag G, Mumusoglu S, Zengin D, et al. The prevalence and phenotypic features of polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod. 2016;31:2841-2855.
- Baskind NE, Balen AH. Hypothalamic-pituitary, ovarian and adrenal contributions to polycystic ovary syndrome. Best Pract Res Clin Obstet Gynaecol. 2016;37:80-97.
- Gilbert EW, Tay CT, Hiam DS, et al. Comorbidities and complications of polycystic ovary syndrome: an overview of systematic reviews. Clin Endocrinol (Oxf). 2018;89:683-699.
- Harsha Varma S, Tirupati S, Pradeep TV, et al. Insulin resistance and hyperandrogenemia independently predict nonalcoholic fatty liver disease in women with polycystic ovary syndrome. Diabetes Metab Syndr. 2019;13:1065-1069.
- Housman E, Reynolds RV. Polycystic ovary syndrome: a review for dermatologists: Part I. Diagnosis and manifestations. J Am Acad Dermatol. 2014;71:847.e1-e10.
- Dilday J, Derickson M, Kuckelman J, et al. Sleeve gastrectomy for obesity in polycystic ovarian syndrome: a pilot study evaluating weight loss and fertility outcomes. Obes Surg. 2019;29:93-98.
- Lortscher D, Admani S, Satur N, et al. Hormonal contraceptives and acne: a retrospective analysis of 2147 patients. J Drugs Dermatol. 2016;15:670-674.
- Brown J, Farquhar C, Lee O, et al. Spironolactone versus placebo or in combination with steroids for hirsutism and/or acne. Cochrane Database Syst Rev. 2009;CD000194.
- Shaw JC. Low-dose adjunctive spironolactone in the treatment of acne in women: a retrospective analysis of 85 consecutively treated patients. J Am Acad Dermatol. 2000;43:498-502.
- Shaw JC, White LE. Long-term safety of spironolactone in acne: results of an 8-year follow-up study. J Cutan Med Surg. 2002;6:541-545.
- Ganie MA, Khurana ML, Nisar S, et al. Improved efficacy of low-dose spironolactone and metformin combination than either drug alone in the management of women with polycystic ovary syndrome (PCOS): a six-month, open-label randomized study. J Clin Endocrinol Metab. 2013;98:3599-3607.
- Mazza A, Fruci B, Guzzi P, et al. In PCOS patients the addition of low-dose spironolactone induces a more marked reduction of clinical and biochemical hyperandrogenism than metformin alone. Nutr Metab Cardiovascular Dis. 2014;24:132-139.
- Ganie MA, Khurana ML, Eunice M, et al. Comparison of efficacy of spironolactone with metformin in the management of polycystic ovary syndrome: an open-labeled study. J Clin Endocrinol Metab. 2004;89:2756-2762.
- Ibanez L, de Zegher F. Low-dose combination flutamide, metformin and an oral contraceptive for non-obese, young women with polycystic ovary syndrome. Hum Reprod. 2003;18:57-60.
- Ibanez L, de Zegher F. Flutamide-metformin plus an oral contraceptive (OC) for young women with polycystic ovary syndrome: switch from third- to fourth-generation OC reduces body adiposity. Hum Reprod. 2004;19:1725-1727.
Polycystic ovary syndrome (PCOS) is the triad of oligo-ovulation resulting in oligomenorrhea, hyperandrogenism and, often, an excess number of small antral follicles on high-resolution pelvic ultrasound. One meta-analysis reported that, in women of reproductive age, the prevalence of PCOS was 10% using the Rotterdam-European Society of Human Reproduction and Embryology/American Society for Reproductive Medicine (ESHRE/ASRM) criteria1 and 6% using the National Institutes of Health 1990 diagnostic criteria.2 (See “The PCOS trinity—3 findings in one syndrome: oligo-ovulation, hyperandrogenism, and a multifollicular ovary.”3)
PCOS is caused by abnormalities in 3 systems: reproductive, metabolic, and dermatologic. Reproductive abnormalities commonly observed in women with PCOS include4:
- an increase in pituitary secretion of luteinizing hormone (LH), resulting from both an increase in LH pulse amplitude and LH pulse frequency, suggesting a primary hypothalamic disorder
- an increase in ovarian secretion of androstenedione and testosterone due to stimulation by LH and possibly insulin
- oligo-ovulation with chronically low levels of progesterone that can result in endometrial hyperplasia
- ovulatory infertility.
Metabolic abnormalities commonly observed in women with PCOS include5,6:
- insulin resistance and hyperinsulinemia
- excess adipose tissue in the liver
- excess visceral fat
- elevated adipokines
- obesity
- an increased prevalence of glucose intolerance and frank diabetes.
Dermatologic abnormalities commonly observed in women with PCOS include7:
- facial hirsutism
- acne
- androgenetic alopecia.
Given that PCOS is caused by abnormalities in the reproductive, metabolic, and dermatologic systems, it is appropriate to consider multimodal hormonal therapy that addresses all 3 problems. In my practice, I believe that the best approach to the long-term hormonal treatment of PCOS for many women is to prescribe a combination of 3 medicines: a combination estrogen-progestin oral contraceptive (COC), an insulin sensitizer, and an antiandrogen.

The COC reduces pituitary secretion of LH, decreases ovarian androgen production, and prevents the development of endometrial hyperplasia. When taken cyclically, the COC treatment also restores regular withdrawal uterine bleeding.
An insulin sensitizer, such as metformin or pioglitazone, helps to reduce insulin resistance, glucose intolerance, and hepatic adipose content, rebalancing central metabolism. It is important to include diet and exercise in the long-term treatment of PCOS, and I always encourage these lifestyle changes. However, my patients usually report that they have tried multiple times to restrict dietary caloric intake and increase exercise and have been unable to rebalance their metabolism with these interventions alone. Of note, in the women with PCOS and a body mass index >35 kg/m2, bariatric surgery, such as a sleeve gastrectomy, often results in marked improvement of their PCOS.8
The antiandrogen spironolactone provides effective treatment for the dermatologic problems of facial hirsutism and acne. Some COCs containing the progestins drospirenone, norgestimate, and norethindrone acetate are approved by the US Food and Drug Administration for the treatment of acne. A common approach I use in practice is to prescribe a COC, plus spironolactone 100 mg daily plus metformin extended-release 750 mg to 1,500 mg daily.
Continue to: Which COCs have low androgenicity?...
Which COCs have low androgenicity?
I believe that every COC is an effective treatment for PCOS, regardless of the androgenicity of the progestin in the contraceptive. However, some dermatologists believe that combination contraceptives containing progestins with low androgenicity, such as drospirenone, norgestimate, and desogestrel, are more likely to improve acne than contraceptives with an androgenic progestin such as levonorgestrel. In one study in which 2,147 women with acne were treated by one dermatologic practice, the percentage of women reporting that a birth control pill helped to improve their acne was 66% for pills containing drospirenone, 53% for pills containing norgestimate, 44% for pills containing desogestrel, 30% for pills containing norethindrone, and 25% for pills containing levonorgestrel. In the same study, the percent of women reporting that a birth control pill made their acne worse was 3% for pills containing drospirenone, 6% for pills containing norgestimate, 2% for pills containing desogestrel, 8% for pills containing norethindrone, and 10% for pills containing levonorgestrel.9 Given these findings, when treating a woman with PCOS, I generally prescribe a contraceptive that does not contain levonorgestrel.

Why is a spironolactone dose of 100 mg a good choice for PCOS treatment?
Spironolactone, an antiandrogen and inhibitor of 5-alpha-reductase, is commonly prescribed for the treatment of hirsutism and acne at doses ranging from 50 mg to 200 mg daily.10,11 In my clinical experience, spironolactone at a dose of 200 mg daily commonly causes irregular and bothersome uterine bleeding while spironolactone at a dose of 100 mg daily is seldom associated with irregular bleeding. I believe that spironolactone at a dose of 100 mg daily results in superior clinical efficacy than a 50-mg daily dose, although studies report that both doses are effective in the treatment of acne and hirsutism. Spironolactone should not be prescribed to women with renal failure because it can result in severe hyperkalemia. In a study of spironolactone safety in the treatment of acne, no adverse effects on the kidney, liver, or adrenal glands were reported over 8 years of use.12
What insulin sensitizers are useful in rebalancing the metabolic abnormalities observed with PCOS?
Diet and exercise are superb approaches to rebalancing metabolic abnormalities, but for many of my patients they are insufficient and treatment with an insulin sensitizer is warranted. The most commonly utilized insulin sensitizer for the treatment of PCOS is metformin because it is very inexpensive and has a low risk of serious adverse effects such as lactic acidosis. Metformin increases peripheral glucose uptake and reduces gastrointestinal glucose absorption. Insulin sensitizers also decrease visceral fat, a major source of adipokines. One major disadvantage of metformin is that at doses in the range of 1,500 mg to 2,250 mg it often causes gastrointestinal adverse effects such as borborygmi, nausea, abdominal discomfort, and loose stools.
Thiazolidinediones, including pioglitazone, have been reported to be effective in rebalancing central metabolism in women with PCOS. Pioglitazone carries a black box warning of an increased risk of congestive heart failure and nonfatal myocardial infarction. Pioglitazone is also associated with a risk of hepatotoxicity. However, at the pioglitazone dose commonly used in the treatment of PCOS (7.5 mg daily), these serious adverse effects are rare. In practice, I initiate metformin at a dose of 750 mg daily using the extended-release formulation. I increase the metformin dose to 1,500 mg daily if the patient has no bothersome gastrointestinal symptoms on the lower dose. If the patient cannot tolerate metformin treatment because of adverse effects, I will use pioglitazone 7.5 mg daily.
Continue to: Treatment of PCOS in women who are carriers of the Factor V Leiden mutation...
Treatment of PCOS in women who are carriers of the Factor V Leiden mutation
The Factor V Leiden allele is associated with an increased risk of venous thromboembolism. Estrogen-progestin contraception is contraindicated in women with the Factor V Leiden mutation. The prevalence of this mutation varies by race and ethnicity. It is present in about 5% of white, 2% of Hispanic, 1% of black, 1% of Native American, and 0.5% of Asian women. In women with PCOS who are known to be carriers of the mutation, dual therapy with metformin and spironolactone is highly effective.13-15 For these women I also offer a levonorgestrel IUD to provide contraception and reduce the risk of endometrial hyperplasia.
Combination triple medication treatment of PCOS
Optimal treatment of the reproductive, metabolic, and dermatologic problems associated with PCOS requires multimodal medications including an estrogen-progestin contraceptive, an antiandrogen, and an insulin sensitizer. In my practice, I initiate treatment of PCOS by offering patients 3 medications: a COC, spironolactone 100 mg daily, and metformin extended-release formulation 750 mg daily. Some patients elect dual medication therapy (COC plus spironolactone or COC plus metformin), but many patients select treatment with all 3 medications. Although triple medication treatment of PCOS has not been tested in large randomized clinical trials, small trials report that triple medication treatment produces optimal improvement in the reproductive, metabolic, and dermatologic problems associated with PCOS.16-18
Polycystic ovary syndrome (PCOS) is the triad of oligo-ovulation resulting in oligomenorrhea, hyperandrogenism and, often, an excess number of small antral follicles on high-resolution pelvic ultrasound. One meta-analysis reported that, in women of reproductive age, the prevalence of PCOS was 10% using the Rotterdam-European Society of Human Reproduction and Embryology/American Society for Reproductive Medicine (ESHRE/ASRM) criteria1 and 6% using the National Institutes of Health 1990 diagnostic criteria.2 (See “The PCOS trinity—3 findings in one syndrome: oligo-ovulation, hyperandrogenism, and a multifollicular ovary.”3)
PCOS is caused by abnormalities in 3 systems: reproductive, metabolic, and dermatologic. Reproductive abnormalities commonly observed in women with PCOS include4:
- an increase in pituitary secretion of luteinizing hormone (LH), resulting from both an increase in LH pulse amplitude and LH pulse frequency, suggesting a primary hypothalamic disorder
- an increase in ovarian secretion of androstenedione and testosterone due to stimulation by LH and possibly insulin
- oligo-ovulation with chronically low levels of progesterone that can result in endometrial hyperplasia
- ovulatory infertility.
Metabolic abnormalities commonly observed in women with PCOS include5,6:
- insulin resistance and hyperinsulinemia
- excess adipose tissue in the liver
- excess visceral fat
- elevated adipokines
- obesity
- an increased prevalence of glucose intolerance and frank diabetes.
Dermatologic abnormalities commonly observed in women with PCOS include7:
- facial hirsutism
- acne
- androgenetic alopecia.
Given that PCOS is caused by abnormalities in the reproductive, metabolic, and dermatologic systems, it is appropriate to consider multimodal hormonal therapy that addresses all 3 problems. In my practice, I believe that the best approach to the long-term hormonal treatment of PCOS for many women is to prescribe a combination of 3 medicines: a combination estrogen-progestin oral contraceptive (COC), an insulin sensitizer, and an antiandrogen.

The COC reduces pituitary secretion of LH, decreases ovarian androgen production, and prevents the development of endometrial hyperplasia. When taken cyclically, the COC treatment also restores regular withdrawal uterine bleeding.
An insulin sensitizer, such as metformin or pioglitazone, helps to reduce insulin resistance, glucose intolerance, and hepatic adipose content, rebalancing central metabolism. It is important to include diet and exercise in the long-term treatment of PCOS, and I always encourage these lifestyle changes. However, my patients usually report that they have tried multiple times to restrict dietary caloric intake and increase exercise and have been unable to rebalance their metabolism with these interventions alone. Of note, in the women with PCOS and a body mass index >35 kg/m2, bariatric surgery, such as a sleeve gastrectomy, often results in marked improvement of their PCOS.8
The antiandrogen spironolactone provides effective treatment for the dermatologic problems of facial hirsutism and acne. Some COCs containing the progestins drospirenone, norgestimate, and norethindrone acetate are approved by the US Food and Drug Administration for the treatment of acne. A common approach I use in practice is to prescribe a COC, plus spironolactone 100 mg daily plus metformin extended-release 750 mg to 1,500 mg daily.
Continue to: Which COCs have low androgenicity?...
Which COCs have low androgenicity?
I believe that every COC is an effective treatment for PCOS, regardless of the androgenicity of the progestin in the contraceptive. However, some dermatologists believe that combination contraceptives containing progestins with low androgenicity, such as drospirenone, norgestimate, and desogestrel, are more likely to improve acne than contraceptives with an androgenic progestin such as levonorgestrel. In one study in which 2,147 women with acne were treated by one dermatologic practice, the percentage of women reporting that a birth control pill helped to improve their acne was 66% for pills containing drospirenone, 53% for pills containing norgestimate, 44% for pills containing desogestrel, 30% for pills containing norethindrone, and 25% for pills containing levonorgestrel. In the same study, the percent of women reporting that a birth control pill made their acne worse was 3% for pills containing drospirenone, 6% for pills containing norgestimate, 2% for pills containing desogestrel, 8% for pills containing norethindrone, and 10% for pills containing levonorgestrel.9 Given these findings, when treating a woman with PCOS, I generally prescribe a contraceptive that does not contain levonorgestrel.

Why is a spironolactone dose of 100 mg a good choice for PCOS treatment?
Spironolactone, an antiandrogen and inhibitor of 5-alpha-reductase, is commonly prescribed for the treatment of hirsutism and acne at doses ranging from 50 mg to 200 mg daily.10,11 In my clinical experience, spironolactone at a dose of 200 mg daily commonly causes irregular and bothersome uterine bleeding while spironolactone at a dose of 100 mg daily is seldom associated with irregular bleeding. I believe that spironolactone at a dose of 100 mg daily results in superior clinical efficacy than a 50-mg daily dose, although studies report that both doses are effective in the treatment of acne and hirsutism. Spironolactone should not be prescribed to women with renal failure because it can result in severe hyperkalemia. In a study of spironolactone safety in the treatment of acne, no adverse effects on the kidney, liver, or adrenal glands were reported over 8 years of use.12
What insulin sensitizers are useful in rebalancing the metabolic abnormalities observed with PCOS?
Diet and exercise are superb approaches to rebalancing metabolic abnormalities, but for many of my patients they are insufficient and treatment with an insulin sensitizer is warranted. The most commonly utilized insulin sensitizer for the treatment of PCOS is metformin because it is very inexpensive and has a low risk of serious adverse effects such as lactic acidosis. Metformin increases peripheral glucose uptake and reduces gastrointestinal glucose absorption. Insulin sensitizers also decrease visceral fat, a major source of adipokines. One major disadvantage of metformin is that at doses in the range of 1,500 mg to 2,250 mg it often causes gastrointestinal adverse effects such as borborygmi, nausea, abdominal discomfort, and loose stools.
Thiazolidinediones, including pioglitazone, have been reported to be effective in rebalancing central metabolism in women with PCOS. Pioglitazone carries a black box warning of an increased risk of congestive heart failure and nonfatal myocardial infarction. Pioglitazone is also associated with a risk of hepatotoxicity. However, at the pioglitazone dose commonly used in the treatment of PCOS (7.5 mg daily), these serious adverse effects are rare. In practice, I initiate metformin at a dose of 750 mg daily using the extended-release formulation. I increase the metformin dose to 1,500 mg daily if the patient has no bothersome gastrointestinal symptoms on the lower dose. If the patient cannot tolerate metformin treatment because of adverse effects, I will use pioglitazone 7.5 mg daily.
Continue to: Treatment of PCOS in women who are carriers of the Factor V Leiden mutation...
Treatment of PCOS in women who are carriers of the Factor V Leiden mutation
The Factor V Leiden allele is associated with an increased risk of venous thromboembolism. Estrogen-progestin contraception is contraindicated in women with the Factor V Leiden mutation. The prevalence of this mutation varies by race and ethnicity. It is present in about 5% of white, 2% of Hispanic, 1% of black, 1% of Native American, and 0.5% of Asian women. In women with PCOS who are known to be carriers of the mutation, dual therapy with metformin and spironolactone is highly effective.13-15 For these women I also offer a levonorgestrel IUD to provide contraception and reduce the risk of endometrial hyperplasia.
Combination triple medication treatment of PCOS
Optimal treatment of the reproductive, metabolic, and dermatologic problems associated with PCOS requires multimodal medications including an estrogen-progestin contraceptive, an antiandrogen, and an insulin sensitizer. In my practice, I initiate treatment of PCOS by offering patients 3 medications: a COC, spironolactone 100 mg daily, and metformin extended-release formulation 750 mg daily. Some patients elect dual medication therapy (COC plus spironolactone or COC plus metformin), but many patients select treatment with all 3 medications. Although triple medication treatment of PCOS has not been tested in large randomized clinical trials, small trials report that triple medication treatment produces optimal improvement in the reproductive, metabolic, and dermatologic problems associated with PCOS.16-18
- Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril. 2004;81:19-25.
- Zawadski JK, Dunaif A. Diagnostic criteria for polycystic ovary syndrome: towards a rational approach. In Dunaif A, Givens JR, Haseltine FP, et al. Polycystic ovary syndrome. Boston, MA: Blackwell Scientific; 1992:377-384.
- Bozdag G, Mumusoglu S, Zengin D, et al. The prevalence and phenotypic features of polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod. 2016;31:2841-2855.
- Baskind NE, Balen AH. Hypothalamic-pituitary, ovarian and adrenal contributions to polycystic ovary syndrome. Best Pract Res Clin Obstet Gynaecol. 2016;37:80-97.
- Gilbert EW, Tay CT, Hiam DS, et al. Comorbidities and complications of polycystic ovary syndrome: an overview of systematic reviews. Clin Endocrinol (Oxf). 2018;89:683-699.
- Harsha Varma S, Tirupati S, Pradeep TV, et al. Insulin resistance and hyperandrogenemia independently predict nonalcoholic fatty liver disease in women with polycystic ovary syndrome. Diabetes Metab Syndr. 2019;13:1065-1069.
- Housman E, Reynolds RV. Polycystic ovary syndrome: a review for dermatologists: Part I. Diagnosis and manifestations. J Am Acad Dermatol. 2014;71:847.e1-e10.
- Dilday J, Derickson M, Kuckelman J, et al. Sleeve gastrectomy for obesity in polycystic ovarian syndrome: a pilot study evaluating weight loss and fertility outcomes. Obes Surg. 2019;29:93-98.
- Lortscher D, Admani S, Satur N, et al. Hormonal contraceptives and acne: a retrospective analysis of 2147 patients. J Drugs Dermatol. 2016;15:670-674.
- Brown J, Farquhar C, Lee O, et al. Spironolactone versus placebo or in combination with steroids for hirsutism and/or acne. Cochrane Database Syst Rev. 2009;CD000194.
- Shaw JC. Low-dose adjunctive spironolactone in the treatment of acne in women: a retrospective analysis of 85 consecutively treated patients. J Am Acad Dermatol. 2000;43:498-502.
- Shaw JC, White LE. Long-term safety of spironolactone in acne: results of an 8-year follow-up study. J Cutan Med Surg. 2002;6:541-545.
- Ganie MA, Khurana ML, Nisar S, et al. Improved efficacy of low-dose spironolactone and metformin combination than either drug alone in the management of women with polycystic ovary syndrome (PCOS): a six-month, open-label randomized study. J Clin Endocrinol Metab. 2013;98:3599-3607.
- Mazza A, Fruci B, Guzzi P, et al. In PCOS patients the addition of low-dose spironolactone induces a more marked reduction of clinical and biochemical hyperandrogenism than metformin alone. Nutr Metab Cardiovascular Dis. 2014;24:132-139.
- Ganie MA, Khurana ML, Eunice M, et al. Comparison of efficacy of spironolactone with metformin in the management of polycystic ovary syndrome: an open-labeled study. J Clin Endocrinol Metab. 2004;89:2756-2762.
- Ibanez L, de Zegher F. Low-dose combination flutamide, metformin and an oral contraceptive for non-obese, young women with polycystic ovary syndrome. Hum Reprod. 2003;18:57-60.
- Ibanez L, de Zegher F. Flutamide-metformin plus an oral contraceptive (OC) for young women with polycystic ovary syndrome: switch from third- to fourth-generation OC reduces body adiposity. Hum Reprod. 2004;19:1725-1727.
- Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril. 2004;81:19-25.
- Zawadski JK, Dunaif A. Diagnostic criteria for polycystic ovary syndrome: towards a rational approach. In Dunaif A, Givens JR, Haseltine FP, et al. Polycystic ovary syndrome. Boston, MA: Blackwell Scientific; 1992:377-384.
- Bozdag G, Mumusoglu S, Zengin D, et al. The prevalence and phenotypic features of polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod. 2016;31:2841-2855.
- Baskind NE, Balen AH. Hypothalamic-pituitary, ovarian and adrenal contributions to polycystic ovary syndrome. Best Pract Res Clin Obstet Gynaecol. 2016;37:80-97.
- Gilbert EW, Tay CT, Hiam DS, et al. Comorbidities and complications of polycystic ovary syndrome: an overview of systematic reviews. Clin Endocrinol (Oxf). 2018;89:683-699.
- Harsha Varma S, Tirupati S, Pradeep TV, et al. Insulin resistance and hyperandrogenemia independently predict nonalcoholic fatty liver disease in women with polycystic ovary syndrome. Diabetes Metab Syndr. 2019;13:1065-1069.
- Housman E, Reynolds RV. Polycystic ovary syndrome: a review for dermatologists: Part I. Diagnosis and manifestations. J Am Acad Dermatol. 2014;71:847.e1-e10.
- Dilday J, Derickson M, Kuckelman J, et al. Sleeve gastrectomy for obesity in polycystic ovarian syndrome: a pilot study evaluating weight loss and fertility outcomes. Obes Surg. 2019;29:93-98.
- Lortscher D, Admani S, Satur N, et al. Hormonal contraceptives and acne: a retrospective analysis of 2147 patients. J Drugs Dermatol. 2016;15:670-674.
- Brown J, Farquhar C, Lee O, et al. Spironolactone versus placebo or in combination with steroids for hirsutism and/or acne. Cochrane Database Syst Rev. 2009;CD000194.
- Shaw JC. Low-dose adjunctive spironolactone in the treatment of acne in women: a retrospective analysis of 85 consecutively treated patients. J Am Acad Dermatol. 2000;43:498-502.
- Shaw JC, White LE. Long-term safety of spironolactone in acne: results of an 8-year follow-up study. J Cutan Med Surg. 2002;6:541-545.
- Ganie MA, Khurana ML, Nisar S, et al. Improved efficacy of low-dose spironolactone and metformin combination than either drug alone in the management of women with polycystic ovary syndrome (PCOS): a six-month, open-label randomized study. J Clin Endocrinol Metab. 2013;98:3599-3607.
- Mazza A, Fruci B, Guzzi P, et al. In PCOS patients the addition of low-dose spironolactone induces a more marked reduction of clinical and biochemical hyperandrogenism than metformin alone. Nutr Metab Cardiovascular Dis. 2014;24:132-139.
- Ganie MA, Khurana ML, Eunice M, et al. Comparison of efficacy of spironolactone with metformin in the management of polycystic ovary syndrome: an open-labeled study. J Clin Endocrinol Metab. 2004;89:2756-2762.
- Ibanez L, de Zegher F. Low-dose combination flutamide, metformin and an oral contraceptive for non-obese, young women with polycystic ovary syndrome. Hum Reprod. 2003;18:57-60.
- Ibanez L, de Zegher F. Flutamide-metformin plus an oral contraceptive (OC) for young women with polycystic ovary syndrome: switch from third- to fourth-generation OC reduces body adiposity. Hum Reprod. 2004;19:1725-1727.
Can the office visit interval for routine pessary care be extended safely?
Propst K, Mellen C, O’Sullivan DM, et al. Timing of office-based pessary care: a randomized controlled trial. Obstet Gynecol. 2019 Dec 5. Doi: 10.1097/AOG.0000000000003580.
EXPERT COMMENTARY
Vaginal pessaries are a common and effective approach for managing pelvic organ prolapse (POP) as well as stress urinary incontinence (SUI). Vaginal mucosal erosions, however, may complicate pessary use. The risk for erosions may be associated with the frequency of pessary change, which involves removing the pessary, washing it, and replacing it in the vagina. Existing data do not address the frequency of pessary change. Recently, however, investigators conducted a randomized noninferiority trial to evaluate the effect of pessary visit intervals on the development of vaginal epithelial abnormalities.
Details of the study
At a single US hospital, Propst and colleagues randomly assigned women who used pessaries for POP, SUI, or both to routine pessary care (offices visits every 12 weeks) or to extended interval pessary care (office visits every 24 weeks). The women used ring, incontinence dish, or Gelhorn pessaries, did not change their pessaries on their own, and had no vaginal mucosal abnormalities.
A total of 130 women were randomly assigned, 64 to the routine care group and 66 to the extended interval care group. The mean age was 79 years and 90% were white, 4.6% were black, and 4% were Hispanic. Approximately 74% of the women used vaginal estrogen.
The primary outcome was the rate of vaginal epithelial abnormalities, including epithelial breaks or erosions. The predetermined noninferiority margin was set at 7.5%.
Results. At the 48-week follow-up, the rate of epithelial erosion was 7.4% in the routine care group and 1.7% in the extended interval care group, thus meeting the prespecified criteria for noninferiority of extended interval pessary care.
Women in each care group reported a similar amount of bothersome vaginal discharge. This was reported on a 5-point scale, with higher numbers indicating greater degree of bother. The mean scores were 1.39 in the routine care group and 1.34 in the extended interval care group. No other pessary-related adverse events occurred in either care group.
Study strengths and limitations
This trial provides good evidence that the timing of office pessary care can be extended to 24 weeks without compromising outcomes. However, since nearly three-quarters of the study participants used vaginal estrogen, the results may not be applicable to pessary users who do not use vaginal estrogen.
Many women change their pessary at home as often as weekly or daily. For women who rely on office visits for pessary care, however, the trial by Propst and colleagues provides good quality evidence that pessaries can be changed as infrequently as every 24 weeks without compromising outcomes. An important limitation of these data is that since most study participants used vaginal estrogen, the findings may not apply to pessary use among women who do not use vaginal estrogen.
ANDREW M. KAUNITZ, MD, NCMP
Propst K, Mellen C, O’Sullivan DM, et al. Timing of office-based pessary care: a randomized controlled trial. Obstet Gynecol. 2019 Dec 5. Doi: 10.1097/AOG.0000000000003580.
EXPERT COMMENTARY
Vaginal pessaries are a common and effective approach for managing pelvic organ prolapse (POP) as well as stress urinary incontinence (SUI). Vaginal mucosal erosions, however, may complicate pessary use. The risk for erosions may be associated with the frequency of pessary change, which involves removing the pessary, washing it, and replacing it in the vagina. Existing data do not address the frequency of pessary change. Recently, however, investigators conducted a randomized noninferiority trial to evaluate the effect of pessary visit intervals on the development of vaginal epithelial abnormalities.
Details of the study
At a single US hospital, Propst and colleagues randomly assigned women who used pessaries for POP, SUI, or both to routine pessary care (offices visits every 12 weeks) or to extended interval pessary care (office visits every 24 weeks). The women used ring, incontinence dish, or Gelhorn pessaries, did not change their pessaries on their own, and had no vaginal mucosal abnormalities.
A total of 130 women were randomly assigned, 64 to the routine care group and 66 to the extended interval care group. The mean age was 79 years and 90% were white, 4.6% were black, and 4% were Hispanic. Approximately 74% of the women used vaginal estrogen.
The primary outcome was the rate of vaginal epithelial abnormalities, including epithelial breaks or erosions. The predetermined noninferiority margin was set at 7.5%.
Results. At the 48-week follow-up, the rate of epithelial erosion was 7.4% in the routine care group and 1.7% in the extended interval care group, thus meeting the prespecified criteria for noninferiority of extended interval pessary care.
Women in each care group reported a similar amount of bothersome vaginal discharge. This was reported on a 5-point scale, with higher numbers indicating greater degree of bother. The mean scores were 1.39 in the routine care group and 1.34 in the extended interval care group. No other pessary-related adverse events occurred in either care group.
Study strengths and limitations
This trial provides good evidence that the timing of office pessary care can be extended to 24 weeks without compromising outcomes. However, since nearly three-quarters of the study participants used vaginal estrogen, the results may not be applicable to pessary users who do not use vaginal estrogen.
Many women change their pessary at home as often as weekly or daily. For women who rely on office visits for pessary care, however, the trial by Propst and colleagues provides good quality evidence that pessaries can be changed as infrequently as every 24 weeks without compromising outcomes. An important limitation of these data is that since most study participants used vaginal estrogen, the findings may not apply to pessary use among women who do not use vaginal estrogen.
ANDREW M. KAUNITZ, MD, NCMP
Propst K, Mellen C, O’Sullivan DM, et al. Timing of office-based pessary care: a randomized controlled trial. Obstet Gynecol. 2019 Dec 5. Doi: 10.1097/AOG.0000000000003580.
EXPERT COMMENTARY
Vaginal pessaries are a common and effective approach for managing pelvic organ prolapse (POP) as well as stress urinary incontinence (SUI). Vaginal mucosal erosions, however, may complicate pessary use. The risk for erosions may be associated with the frequency of pessary change, which involves removing the pessary, washing it, and replacing it in the vagina. Existing data do not address the frequency of pessary change. Recently, however, investigators conducted a randomized noninferiority trial to evaluate the effect of pessary visit intervals on the development of vaginal epithelial abnormalities.
Details of the study
At a single US hospital, Propst and colleagues randomly assigned women who used pessaries for POP, SUI, or both to routine pessary care (offices visits every 12 weeks) or to extended interval pessary care (office visits every 24 weeks). The women used ring, incontinence dish, or Gelhorn pessaries, did not change their pessaries on their own, and had no vaginal mucosal abnormalities.
A total of 130 women were randomly assigned, 64 to the routine care group and 66 to the extended interval care group. The mean age was 79 years and 90% were white, 4.6% were black, and 4% were Hispanic. Approximately 74% of the women used vaginal estrogen.
The primary outcome was the rate of vaginal epithelial abnormalities, including epithelial breaks or erosions. The predetermined noninferiority margin was set at 7.5%.
Results. At the 48-week follow-up, the rate of epithelial erosion was 7.4% in the routine care group and 1.7% in the extended interval care group, thus meeting the prespecified criteria for noninferiority of extended interval pessary care.
Women in each care group reported a similar amount of bothersome vaginal discharge. This was reported on a 5-point scale, with higher numbers indicating greater degree of bother. The mean scores were 1.39 in the routine care group and 1.34 in the extended interval care group. No other pessary-related adverse events occurred in either care group.
Study strengths and limitations
This trial provides good evidence that the timing of office pessary care can be extended to 24 weeks without compromising outcomes. However, since nearly three-quarters of the study participants used vaginal estrogen, the results may not be applicable to pessary users who do not use vaginal estrogen.
Many women change their pessary at home as often as weekly or daily. For women who rely on office visits for pessary care, however, the trial by Propst and colleagues provides good quality evidence that pessaries can be changed as infrequently as every 24 weeks without compromising outcomes. An important limitation of these data is that since most study participants used vaginal estrogen, the findings may not apply to pessary use among women who do not use vaginal estrogen.
ANDREW M. KAUNITZ, MD, NCMP
Progesterone supplementation does not PROLONG pregnancy in women at risk for preterm birth: What do we do now?
Preterm birth (PTB) remains a significant public health concern and a major cause of newborn morbidity and mortality. In the United States, 1 in 10 babies are born preterm (< 37 weeks), and this rate has changed little in 30 years.1
In 2011, the US Food and Drug Administration (FDA) approved progesterone supplementation—specifically, α-hydroxyprogesterone caproate (17P) injection (Makena)—to prevent recurrent PTB in women with a singleton pregnancy at high risk by virtue of a prior spontaneous PTB.2 This was the first-ever FDA-approved drug for PTB prevention, and it was the first drug approved by the FDA for use in pregnancy in more than 15 years. The approval of 17P utilized the FDA's Subpart H Accelerated Approval Pathway, which applies to therapies that: 1) treat serious conditions with unmet need, and 2) demonstrate safety and efficacy on surrogate end points reasonably likely to predict clinical benefit.3
By voting their approval of 17P in 2011, the FDA affirmed that PTB was a serious condition with unmet need, that birth < 37 weeks was an accepted surrogate end point, and that there was compelling evidence of safety and benefit. The compelling evidence presented was a single, randomized, vehicle-controlled clinical trial conducted by the Maternal-Fetal Medicine Units (MFMU) Network, which showed significant reduction in recurrent PTB < 37 weeks (from 54.9% in the placebo group to 36.3% in the 17P group; P<.001; relative risk [RR], 0.66; 95% confidence interval [CI], 0.54-0.81).4
In 2017, the Society for Maternal-Fetal Medicine (SMFM) reaffirmed the use of 17P to prevent recurrent PTB and, that same year, it was estimated that 75% of eligible patients received 17P.5,6 Importantly, Subpart H approval requires one or more follow-up clinical trials confirming safety and efficacy. And the FDA has the right—the responsibility—to revisit approval if such trials are either not performed or are unfavorable.
The recently published PROLONG study by Blackwell and colleagues is this required postapproval confirmatory trial conducted to verify the clinical benefit of 17P supplementation.7
Continue to: Study design, and stunning results...
Study design, and stunning results
PROLONG (Progestin's Role in Optimizing Neonatal Gestation) was a randomized (2:1), double-blind, vehicle-controlled, multicenter international trial (2009-2018) conducted to assess the safety and efficacy of 17P injection in 1,708 women with a singleton pregnancy and one or more prior spontaneous PTBs.7 Women in the active treatment group (n = 1,130) received weekly intramuscular injections of 17P, while those in the control group (n = 578) received weekly injections of inert oil vehicle.
Results of the trial showed no significant reduction in the co-primary end points, which were PTB < 35 weeks (11.0% in the 17P group vs 11.5% in the placebo group; RR, 0.95; 95% CI, 0.71-1.26) and neonatal morbidity index (5.6% in the 17P group vs 5.0% in the placebo group; RR, 1.12; 95% CI, 0.68-1.61). There was no evidence of benefit for any subpopulation (geographic region, race, or other PTB risk factor). Maternal outcomes also were similar between the groups. No significant safety concerns were identified.
Important differences between MFMU and PROLONG trials
Strengths of the PROLONG trial include its randomized, placebo-controlled design, excellent follow-up rate, and use of a protocol that mirrored that of the MFMU trial. The primary limitation of PROLONG is that participants experienced a lower rate of PTB compared with those in the MFMU trial. The rate of PTB < 37 weeks was 54.9% in the control group of the MFMU trial compared with 21.9% in PROLONG.
Given the low rate of PTB in PROLONG, the study was underpowered for the co-primary outcomes. In addition, lower rates of PTB in PROLONG compared with in the MFMU trial likely reflected different patient populations.8 Moreover, PROLONG was an international trial. Of the 1,708 participants, most were recruited in Russia (36%) and Ukraine (25%); only 23% were from the United States. By contrast, participants in the MFMU trial were recruited from US academic medical centers. Also, participants in the MFMU trial were significantly more likely to have a short cervix, to have a history of more than one PTB, and to be African American.
Discrepant trial results create clinical quandary
In October 2019, the FDA's Bone, Reproductive and Urologic Drugs Advisory Committee voted 9-7 to withdraw approval for 17P. Committee members struggled with the conflicting data between the 2 trials and hesitated to remove a medication whose use has become standard practice. Ultimately, however, it was lack of substantial evidence of effectiveness of 17P that swayed the committee's vote. While the FDA generally follows the recommendation of an advisory committee, it is not bound to do so.
Societies' perspectives
So what are physicians and patients to do? It is possible that a small subgroup of women at extremely high risk for early PTB may benefit from 17P administration. SMFM stated: "...it is reasonable for providers to use 17-OHPC [17P] in women with a profile more representative of the very high-risk population reported in the Meis [MFMU] trial."8 Further, the American College of Obstetricians and Gynecologists (ACOG) stated in a Practice Advisory dated October 25, 2019, that "ACOG is not changing our clinical recommendations at this time... [We] will be reviewing subsequent forthcoming analyses and will issue updated clinical guidance as appropriate."9
Where we stand on 17P use going forward
17P should be available to women who previously may have benefited from its use. However, 17P should not be recommended routinely to prevent recurrent spontaneous PTB in women with one prior PTB and no other risk factors. Of note, the PROLONG trial does not change recommendations for cervical length screening. Women with a history of a prior spontaneous PTB should undergo cervical length screening to identify those individuals who may benefit from an ultrasound-indicated cerclage.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins--Obstetrics. ACOG practice bulletin no. 127: Management of preterm labor. Obstet Gynecol. 2012;119:1308-1317.
- Makena [package insert]. Waltham, MA: AMAG Pharmaceuticals, Inc; 2018. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/021945s012lbl.pdf. Accessed November 10, 2019.
- US Food and Drug Administration. Code of Federal Regulations Title 21. Subpart H--Acceleratedapproval of new drugs for serious or life-threatening illnesses. April 1, 2019. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?CFRPart=314&showFR=1&subpartNode=21:5.0.1.1.4.8. Accessed November 10, 2019.
- Meis PJ, Klebanoff M, Thom E, et al; for the National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Prevention of recurrent preterm delivery by 17 alpha-hydroxyprogesterone caproate. N Engl J Med. 2003;348:2379-2385.
- Society for Maternal-Fetal Medicine Publications Committee. The choice of progestogen for the prevention of preterm birth in women with singleton pregnancy and prior preterm birth. Am J Obstet Gynecol. 2017;216:B11-B13.
- Gallagher JR, Gudeman J, Heap K, et al. Understanding if, how, and why women with prior spontaneous preterm births are treated with progestogens: a national survey of obstetrician practice patterns. AJP Rep. 2018;8:e315-e324.
- Blackwell SC, Gyamfi-Bannerman C, Biggio JR Jr, et al. 17-OHPC to prevent recurrent preterm birth in singleton gestations (PROLONG study): a multicenter, international, randomized double-blind trial. Am J Perinatol. 2019. doi:10.1055/s-0039-3400227.
- Society for Maternal-Fetal Medicine Publications Committee. SMFM statement: Use of 17-alpha hydroxyprogesterone caproate for prevention of recurrent preterm birth. https://els-jbs-prod-cdn.literatumonline.com/pb/assets/raw/Health%20Advance/journals/ymob/SMFM_Statement_PRO LONG-1572023839767.pdf. Accessed November 10, 2019.
- American College of Obstetricians and Gynecologists. Practice advisory: Clinical guidance for integration of the findings of the PROLONG study: progestin's role in optimizing neonatal gestation. https://www.acog.org/Clinical-Guidance-and-Publications/Practice-Advisories/Clinical-guidance-for-integration-of-the-findings-of-The-PROLONG-study-Progestins-Role-in-Optimizing?IsMobileSet=false. Accessed November 10, 2019.
Preterm birth (PTB) remains a significant public health concern and a major cause of newborn morbidity and mortality. In the United States, 1 in 10 babies are born preterm (< 37 weeks), and this rate has changed little in 30 years.1
In 2011, the US Food and Drug Administration (FDA) approved progesterone supplementation—specifically, α-hydroxyprogesterone caproate (17P) injection (Makena)—to prevent recurrent PTB in women with a singleton pregnancy at high risk by virtue of a prior spontaneous PTB.2 This was the first-ever FDA-approved drug for PTB prevention, and it was the first drug approved by the FDA for use in pregnancy in more than 15 years. The approval of 17P utilized the FDA's Subpart H Accelerated Approval Pathway, which applies to therapies that: 1) treat serious conditions with unmet need, and 2) demonstrate safety and efficacy on surrogate end points reasonably likely to predict clinical benefit.3
By voting their approval of 17P in 2011, the FDA affirmed that PTB was a serious condition with unmet need, that birth < 37 weeks was an accepted surrogate end point, and that there was compelling evidence of safety and benefit. The compelling evidence presented was a single, randomized, vehicle-controlled clinical trial conducted by the Maternal-Fetal Medicine Units (MFMU) Network, which showed significant reduction in recurrent PTB < 37 weeks (from 54.9% in the placebo group to 36.3% in the 17P group; P<.001; relative risk [RR], 0.66; 95% confidence interval [CI], 0.54-0.81).4
In 2017, the Society for Maternal-Fetal Medicine (SMFM) reaffirmed the use of 17P to prevent recurrent PTB and, that same year, it was estimated that 75% of eligible patients received 17P.5,6 Importantly, Subpart H approval requires one or more follow-up clinical trials confirming safety and efficacy. And the FDA has the right—the responsibility—to revisit approval if such trials are either not performed or are unfavorable.
The recently published PROLONG study by Blackwell and colleagues is this required postapproval confirmatory trial conducted to verify the clinical benefit of 17P supplementation.7
Continue to: Study design, and stunning results...
Study design, and stunning results
PROLONG (Progestin's Role in Optimizing Neonatal Gestation) was a randomized (2:1), double-blind, vehicle-controlled, multicenter international trial (2009-2018) conducted to assess the safety and efficacy of 17P injection in 1,708 women with a singleton pregnancy and one or more prior spontaneous PTBs.7 Women in the active treatment group (n = 1,130) received weekly intramuscular injections of 17P, while those in the control group (n = 578) received weekly injections of inert oil vehicle.
Results of the trial showed no significant reduction in the co-primary end points, which were PTB < 35 weeks (11.0% in the 17P group vs 11.5% in the placebo group; RR, 0.95; 95% CI, 0.71-1.26) and neonatal morbidity index (5.6% in the 17P group vs 5.0% in the placebo group; RR, 1.12; 95% CI, 0.68-1.61). There was no evidence of benefit for any subpopulation (geographic region, race, or other PTB risk factor). Maternal outcomes also were similar between the groups. No significant safety concerns were identified.
Important differences between MFMU and PROLONG trials
Strengths of the PROLONG trial include its randomized, placebo-controlled design, excellent follow-up rate, and use of a protocol that mirrored that of the MFMU trial. The primary limitation of PROLONG is that participants experienced a lower rate of PTB compared with those in the MFMU trial. The rate of PTB < 37 weeks was 54.9% in the control group of the MFMU trial compared with 21.9% in PROLONG.
Given the low rate of PTB in PROLONG, the study was underpowered for the co-primary outcomes. In addition, lower rates of PTB in PROLONG compared with in the MFMU trial likely reflected different patient populations.8 Moreover, PROLONG was an international trial. Of the 1,708 participants, most were recruited in Russia (36%) and Ukraine (25%); only 23% were from the United States. By contrast, participants in the MFMU trial were recruited from US academic medical centers. Also, participants in the MFMU trial were significantly more likely to have a short cervix, to have a history of more than one PTB, and to be African American.
Discrepant trial results create clinical quandary
In October 2019, the FDA's Bone, Reproductive and Urologic Drugs Advisory Committee voted 9-7 to withdraw approval for 17P. Committee members struggled with the conflicting data between the 2 trials and hesitated to remove a medication whose use has become standard practice. Ultimately, however, it was lack of substantial evidence of effectiveness of 17P that swayed the committee's vote. While the FDA generally follows the recommendation of an advisory committee, it is not bound to do so.
Societies' perspectives
So what are physicians and patients to do? It is possible that a small subgroup of women at extremely high risk for early PTB may benefit from 17P administration. SMFM stated: "...it is reasonable for providers to use 17-OHPC [17P] in women with a profile more representative of the very high-risk population reported in the Meis [MFMU] trial."8 Further, the American College of Obstetricians and Gynecologists (ACOG) stated in a Practice Advisory dated October 25, 2019, that "ACOG is not changing our clinical recommendations at this time... [We] will be reviewing subsequent forthcoming analyses and will issue updated clinical guidance as appropriate."9
Where we stand on 17P use going forward
17P should be available to women who previously may have benefited from its use. However, 17P should not be recommended routinely to prevent recurrent spontaneous PTB in women with one prior PTB and no other risk factors. Of note, the PROLONG trial does not change recommendations for cervical length screening. Women with a history of a prior spontaneous PTB should undergo cervical length screening to identify those individuals who may benefit from an ultrasound-indicated cerclage.
Preterm birth (PTB) remains a significant public health concern and a major cause of newborn morbidity and mortality. In the United States, 1 in 10 babies are born preterm (< 37 weeks), and this rate has changed little in 30 years.1
In 2011, the US Food and Drug Administration (FDA) approved progesterone supplementation—specifically, α-hydroxyprogesterone caproate (17P) injection (Makena)—to prevent recurrent PTB in women with a singleton pregnancy at high risk by virtue of a prior spontaneous PTB.2 This was the first-ever FDA-approved drug for PTB prevention, and it was the first drug approved by the FDA for use in pregnancy in more than 15 years. The approval of 17P utilized the FDA's Subpart H Accelerated Approval Pathway, which applies to therapies that: 1) treat serious conditions with unmet need, and 2) demonstrate safety and efficacy on surrogate end points reasonably likely to predict clinical benefit.3
By voting their approval of 17P in 2011, the FDA affirmed that PTB was a serious condition with unmet need, that birth < 37 weeks was an accepted surrogate end point, and that there was compelling evidence of safety and benefit. The compelling evidence presented was a single, randomized, vehicle-controlled clinical trial conducted by the Maternal-Fetal Medicine Units (MFMU) Network, which showed significant reduction in recurrent PTB < 37 weeks (from 54.9% in the placebo group to 36.3% in the 17P group; P<.001; relative risk [RR], 0.66; 95% confidence interval [CI], 0.54-0.81).4
In 2017, the Society for Maternal-Fetal Medicine (SMFM) reaffirmed the use of 17P to prevent recurrent PTB and, that same year, it was estimated that 75% of eligible patients received 17P.5,6 Importantly, Subpart H approval requires one or more follow-up clinical trials confirming safety and efficacy. And the FDA has the right—the responsibility—to revisit approval if such trials are either not performed or are unfavorable.
The recently published PROLONG study by Blackwell and colleagues is this required postapproval confirmatory trial conducted to verify the clinical benefit of 17P supplementation.7
Continue to: Study design, and stunning results...
Study design, and stunning results
PROLONG (Progestin's Role in Optimizing Neonatal Gestation) was a randomized (2:1), double-blind, vehicle-controlled, multicenter international trial (2009-2018) conducted to assess the safety and efficacy of 17P injection in 1,708 women with a singleton pregnancy and one or more prior spontaneous PTBs.7 Women in the active treatment group (n = 1,130) received weekly intramuscular injections of 17P, while those in the control group (n = 578) received weekly injections of inert oil vehicle.
Results of the trial showed no significant reduction in the co-primary end points, which were PTB < 35 weeks (11.0% in the 17P group vs 11.5% in the placebo group; RR, 0.95; 95% CI, 0.71-1.26) and neonatal morbidity index (5.6% in the 17P group vs 5.0% in the placebo group; RR, 1.12; 95% CI, 0.68-1.61). There was no evidence of benefit for any subpopulation (geographic region, race, or other PTB risk factor). Maternal outcomes also were similar between the groups. No significant safety concerns were identified.
Important differences between MFMU and PROLONG trials
Strengths of the PROLONG trial include its randomized, placebo-controlled design, excellent follow-up rate, and use of a protocol that mirrored that of the MFMU trial. The primary limitation of PROLONG is that participants experienced a lower rate of PTB compared with those in the MFMU trial. The rate of PTB < 37 weeks was 54.9% in the control group of the MFMU trial compared with 21.9% in PROLONG.
Given the low rate of PTB in PROLONG, the study was underpowered for the co-primary outcomes. In addition, lower rates of PTB in PROLONG compared with in the MFMU trial likely reflected different patient populations.8 Moreover, PROLONG was an international trial. Of the 1,708 participants, most were recruited in Russia (36%) and Ukraine (25%); only 23% were from the United States. By contrast, participants in the MFMU trial were recruited from US academic medical centers. Also, participants in the MFMU trial were significantly more likely to have a short cervix, to have a history of more than one PTB, and to be African American.
Discrepant trial results create clinical quandary
In October 2019, the FDA's Bone, Reproductive and Urologic Drugs Advisory Committee voted 9-7 to withdraw approval for 17P. Committee members struggled with the conflicting data between the 2 trials and hesitated to remove a medication whose use has become standard practice. Ultimately, however, it was lack of substantial evidence of effectiveness of 17P that swayed the committee's vote. While the FDA generally follows the recommendation of an advisory committee, it is not bound to do so.
Societies' perspectives
So what are physicians and patients to do? It is possible that a small subgroup of women at extremely high risk for early PTB may benefit from 17P administration. SMFM stated: "...it is reasonable for providers to use 17-OHPC [17P] in women with a profile more representative of the very high-risk population reported in the Meis [MFMU] trial."8 Further, the American College of Obstetricians and Gynecologists (ACOG) stated in a Practice Advisory dated October 25, 2019, that "ACOG is not changing our clinical recommendations at this time... [We] will be reviewing subsequent forthcoming analyses and will issue updated clinical guidance as appropriate."9
Where we stand on 17P use going forward
17P should be available to women who previously may have benefited from its use. However, 17P should not be recommended routinely to prevent recurrent spontaneous PTB in women with one prior PTB and no other risk factors. Of note, the PROLONG trial does not change recommendations for cervical length screening. Women with a history of a prior spontaneous PTB should undergo cervical length screening to identify those individuals who may benefit from an ultrasound-indicated cerclage.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins--Obstetrics. ACOG practice bulletin no. 127: Management of preterm labor. Obstet Gynecol. 2012;119:1308-1317.
- Makena [package insert]. Waltham, MA: AMAG Pharmaceuticals, Inc; 2018. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/021945s012lbl.pdf. Accessed November 10, 2019.
- US Food and Drug Administration. Code of Federal Regulations Title 21. Subpart H--Acceleratedapproval of new drugs for serious or life-threatening illnesses. April 1, 2019. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?CFRPart=314&showFR=1&subpartNode=21:5.0.1.1.4.8. Accessed November 10, 2019.
- Meis PJ, Klebanoff M, Thom E, et al; for the National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Prevention of recurrent preterm delivery by 17 alpha-hydroxyprogesterone caproate. N Engl J Med. 2003;348:2379-2385.
- Society for Maternal-Fetal Medicine Publications Committee. The choice of progestogen for the prevention of preterm birth in women with singleton pregnancy and prior preterm birth. Am J Obstet Gynecol. 2017;216:B11-B13.
- Gallagher JR, Gudeman J, Heap K, et al. Understanding if, how, and why women with prior spontaneous preterm births are treated with progestogens: a national survey of obstetrician practice patterns. AJP Rep. 2018;8:e315-e324.
- Blackwell SC, Gyamfi-Bannerman C, Biggio JR Jr, et al. 17-OHPC to prevent recurrent preterm birth in singleton gestations (PROLONG study): a multicenter, international, randomized double-blind trial. Am J Perinatol. 2019. doi:10.1055/s-0039-3400227.
- Society for Maternal-Fetal Medicine Publications Committee. SMFM statement: Use of 17-alpha hydroxyprogesterone caproate for prevention of recurrent preterm birth. https://els-jbs-prod-cdn.literatumonline.com/pb/assets/raw/Health%20Advance/journals/ymob/SMFM_Statement_PRO LONG-1572023839767.pdf. Accessed November 10, 2019.
- American College of Obstetricians and Gynecologists. Practice advisory: Clinical guidance for integration of the findings of the PROLONG study: progestin's role in optimizing neonatal gestation. https://www.acog.org/Clinical-Guidance-and-Publications/Practice-Advisories/Clinical-guidance-for-integration-of-the-findings-of-The-PROLONG-study-Progestins-Role-in-Optimizing?IsMobileSet=false. Accessed November 10, 2019.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins--Obstetrics. ACOG practice bulletin no. 127: Management of preterm labor. Obstet Gynecol. 2012;119:1308-1317.
- Makena [package insert]. Waltham, MA: AMAG Pharmaceuticals, Inc; 2018. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/021945s012lbl.pdf. Accessed November 10, 2019.
- US Food and Drug Administration. Code of Federal Regulations Title 21. Subpart H--Acceleratedapproval of new drugs for serious or life-threatening illnesses. April 1, 2019. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?CFRPart=314&showFR=1&subpartNode=21:5.0.1.1.4.8. Accessed November 10, 2019.
- Meis PJ, Klebanoff M, Thom E, et al; for the National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Prevention of recurrent preterm delivery by 17 alpha-hydroxyprogesterone caproate. N Engl J Med. 2003;348:2379-2385.
- Society for Maternal-Fetal Medicine Publications Committee. The choice of progestogen for the prevention of preterm birth in women with singleton pregnancy and prior preterm birth. Am J Obstet Gynecol. 2017;216:B11-B13.
- Gallagher JR, Gudeman J, Heap K, et al. Understanding if, how, and why women with prior spontaneous preterm births are treated with progestogens: a national survey of obstetrician practice patterns. AJP Rep. 2018;8:e315-e324.
- Blackwell SC, Gyamfi-Bannerman C, Biggio JR Jr, et al. 17-OHPC to prevent recurrent preterm birth in singleton gestations (PROLONG study): a multicenter, international, randomized double-blind trial. Am J Perinatol. 2019. doi:10.1055/s-0039-3400227.
- Society for Maternal-Fetal Medicine Publications Committee. SMFM statement: Use of 17-alpha hydroxyprogesterone caproate for prevention of recurrent preterm birth. https://els-jbs-prod-cdn.literatumonline.com/pb/assets/raw/Health%20Advance/journals/ymob/SMFM_Statement_PRO LONG-1572023839767.pdf. Accessed November 10, 2019.
- American College of Obstetricians and Gynecologists. Practice advisory: Clinical guidance for integration of the findings of the PROLONG study: progestin's role in optimizing neonatal gestation. https://www.acog.org/Clinical-Guidance-and-Publications/Practice-Advisories/Clinical-guidance-for-integration-of-the-findings-of-The-PROLONG-study-Progestins-Role-in-Optimizing?IsMobileSet=false. Accessed November 10, 2019.
Retained placenta after vaginal birth: How long should you wait to manually remove the placenta?
You have just safely delivered the baby who is quietly resting on her mother’s chest. You begin active management of the third stage of labor, administering oxytocin, performing uterine massage and applying controlled tension on the umbilical cord. There is no evidence of excess postpartum bleeding.
How long will you wait to deliver the placenta?
Active management of the third stage of labor
Most authorities recommend active management of the third stage of labor because active management reduces the risk of maternal hemorrhage >1,000 mL (relative risk [RR], 0.34), postpartum hemoglobin levels < 9 g/dL (RR, 0.50), and maternal blood transfusion (RR, 0.35) compared with expectant management.1
The most important component of active management of the third stage of labor is the administration of a uterotonic after delivery of the newborn. In the United States, oxytocin is the uterotonic most often utilized for the active management of the third stage of labor. Authors of a recent randomized clinical trial reported that intravenous oxytocin is superior to intramuscular oxytocin for reducing postpartum blood loss (385 vs 445 mL), the frequency of blood loss greater than 1,000 mL (4.6% vs 8.1%), and the rate of maternal blood transfusion (1.5% vs 4.4%).2
In addition to administering oxytocin, the active management of the third stage often involves maneuvers to accelerate placental delivery, including the Crede and Brandt-Andrews maneuvers and controlled tension on the umbilical cord. The Crede maneuver, described in 1853, involves placing a hand on the abdominal wall near the uterine fundus and squeezing the uterine fundus between the thumb and fingers.3,4
The Brandt-Andrews maneuver, described in 1933, involves placing a clamp on the umbilical cord close to the vulva.5 The clamp is used to apply judicious tension on the cord with one hand, while the other hand is placed on the mother’s abdomen with the palm and fingers overlying the junction between the uterine corpus and the lower segment. With judicious tension on the cord, the abdominal hand pushes the uterus upward toward the umbilicus. Placental separation is indicated when lengthening of the umbilical cord occurs. The Brandt-Andrews maneuver may be associated with fewer cases of uterine inversion than the Crede maneuver.5-7
Of note, umbilical cord traction has not been demonstrated to reduce the need for blood transfusion or the incidence of postpartum hemorrhage (PPH) >1,000 mL, and it is commonly utilized by obstetricians and midwives.8,9 Hence, in the third stage, the delivering clinician should routinely administer a uterotonic, but use of judicious tension on the cord can be deferred if the woman prefers a noninterventional approach to delivery.
Following a vaginal birth, when should the diagnosis of retained placenta be made?
The historic definition of retained placenta is nonexpulsion of the placenta 30 minutes after delivery of the newborn. However, many observational studies report that, when active management of the third stage is utilized, 90%, 95%, and 99% of placentas deliver by 9 minutes, 13 minutes, and 28 minutes, respectively.10 In addition, many observational studies report that the incidence of PPH increases significantly with longer intervals between birth of the newborn and delivery of the placenta. In one study the rate of blood loss >500 mL was 8.5% when the placenta delivered between 5 and 9 minutes and 35.1% when the placenta delivered ≥30 minutes following birth of the baby.10 In another observational study, compared with women delivering the placenta < 10 minutes after birth, women delivering the placenta ≥30 minutes after birth had a 3-fold increased risk of PPH.11 Similar findings have been reported in other studies.12-14
Continue to: Based on the association between a delay in delivery...
Based on the association between a delay in delivery of the placenta and an increased risk of PPH, some authorities recommend that, in term pregnancy, the diagnosis of retained placenta should be made at 20 minutes following birth and consideration should be given to removing the placenta at this time. For women with effective neuraxial anesthesia, manual removal of the placenta 20 minutes following birth may be the best decision for balancing the benefit of preventing PPH with the risk of unnecessary intervention. For women with no anesthesia, delaying manual removal of the placenta to 30 minutes or more following birth may permit more time for the placenta to deliver prior to performing an intervention that might cause pain, but the delay increases the risk of PPH.
The retained placenta may prevent the uterine muscle from effectively contracting around penetrating veins and arteries, thereby increasing the risk of postpartum hemorrhage. The placenta that has separated from the uterine wall but is trapped inside the uterine cavity can be removed easily with manual extraction. If the placenta is physiologically adherent to the uterine wall, a gentle sweeping motion with an intrauterine hand usually can separate the placenta from the uterus in preparation for manual extraction. However, if a placenta accreta spectrum disorder is contributing to a retained placenta, it may be difficult to separate the densely adherent portion of the uterus from the uterine wall. In the presence of placenta accreta spectrum disorder, vigorous attempts to remove the placenta may precipitate massive bleeding. In some cases, the acchoucheur/midwife may recognize the presence of a focal accreta and cease attempts to remove the placenta in order to organize the personnel and equipment needed to effectively treat a potential case of placenta accreta. In one study, when a placenta accreta was recognized or suspected, immediately ceasing attempts at manually removing the placenta resulted in better case outcomes than continued attempts to remove the placenta.1
Uterine inversion may occur during an attempt to manually remove the placenta. There is universal agreement that once a uterine inversion is recognized it is critically important to immediately restore normal uterine anatomy to avoid massive hemorrhage and maternal shock. The initial management of uterine inversion includes:
- stopping oxytocin infusion
- initiating high volume fluid resuscitation
- considering a dose of a uterine relaxant, such as nitroglycerin or terbutaline
- preparing for blood product replacement.
In my experience, when uterine inversion is immediately recognized and successfully treated, blood product replacement is not usually necessary. However, if uterine inversion has not been immediately recognized or treated, massive hemorrhage and shock may occur.
Two approaches to the vaginal restoration of uterine anatomy involve using the tips of the fingers and palm of the hand to guide the wall of the uterus back to its normal position (FIGURE 1) or to forcefully use a fist to force the uterine wall back to its normal position (FIGURE 2). If these maneuvers are unsuccessful, a laparotomy may be necessary.
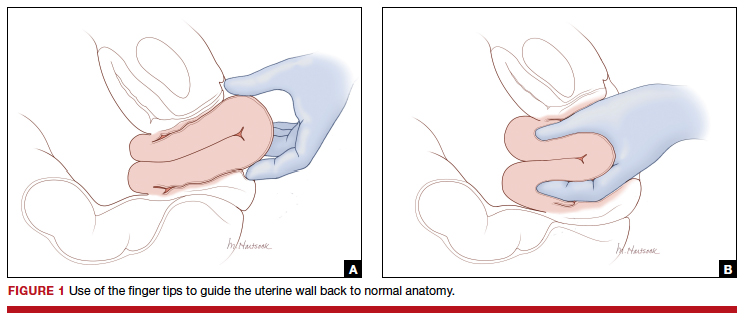
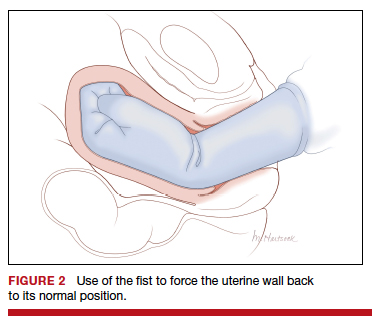
At laparotomy, the Huntington or Haultain procedures may help restore normal uterine anatomy. The Huntington procedure involves using clamps to apply symmetrical tension to the left and right round ligaments and/or uterine serosa to sequentially tease the uterus back to normal anatomy.2,3 The Haultain procedure involves a vertical incision on the posterior wall of the uterus to release the uterine constriction ring that is preventing the return of the uterine fundus to its normal position (FIGURE 3).4,5
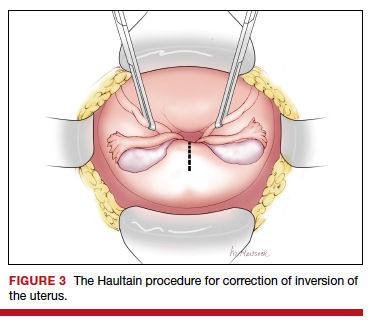
References
- Kayem G, Anselem O, Schmitz T, et al. Conservative versus radical management in cases of placenta accreta: a historical study. J Gynecol Obstet Biol Reprod (Paris). 2007;36:680-687.
- Huntington JL. Acute inversion of the uterus. Boston Med Surg J. 1921;184:376-378.
- Huntington JL, Irving FC, Kellogg FS. Abdominal reposition in acute inversion of the puerperal uterus. Am J Obstet Gynecol. 1928;15:34-40.
- Haultain FW. Abdominal hysterotomy for chronic uterine inversion: a record of 3 cases. Proc Roy Soc Med. 1908;1:528-535.
- Easterday CL, Reid DE. Inversion of the puerperal uterus managed by the Haultain technique; A case report. Am J Obstet Gynecol. 1959;78:1224-1226.
Manual extraction of the placenta
Prior to performing manual extraction of the placenta, a decision should be made regarding the approach to anesthesia and perioperative antibiotics. Manual extraction of the placenta is performed by placing one hand on the uterine fundus to stabilize the uterus and using the other hand to follow the umbilical cord into the uterine cavity. The intrauterine hand is used to separate the uterine-placental interface with a gentle sweeping motion. The placental mass is grasped and gently teased through the cervix and vagina. Inspection of the placenta to ensure complete removal is necessary.
An alternative to manual extraction of the placenta is the use of Bierer forceps and ultrasound guidance to tease the placenta through the cervical os. This technique involves the following steps15:
1. use ultrasound to locate the placenta
2. place a ring forceps on the anterior lip of the cervix
3. introduce the Bierer forcep into the uterus
4. use the forceps to grasp the placenta and pull it toward the vagina
5. stop frequently to re-grasp placental tissue that is deeper in the uterine cavity
6. once the placenta is extracted, examine the placenta to ensure complete removal.
Of note when manual extraction is used to deliver a retained placenta, randomized clinical trials report no benefit for the following interventions:
- perioperative antibiotics16
- nitroglycerin to relax the uterus17
- ultrasound to detect retained placental tissue.18
Best timing for manual extraction of the placenta
The timing for the diagnosis of retained placenta, and the risks and benefits of manual extraction would be best evaluated in a large, randomized clinical trial. However, based on observational studies, in a term pregnancy, the diagnosis of retained placenta is best made using a 20-minute interval. In women with effective neuraxial anesthesia, consideration should be given to manual removal of the placenta at that time.
- Begley CM, Gyte GM, Devane D, et al. Active versus expectant management for women in the third stage of labor. Cochrane Database Syst Rev. 2019;2:CD007412.
- Adnan N, Conlan-Trant R, McCormick C, et al. Intramuscular versus intravenous oxytocin to prevent postpartum haemorrhage at vaginal delivery: randomized controlled trial. BMJ. 2018;362:k3546.
- Gülmezoglu AM, Souza JP. The evolving management of the third stage of labour. BJOG. 2009;116(suppl 1):26-28.
- Ebert AD, David M. Meilensteine der Praventionsmedizin. Carl Siegmund Franz Credé (1819-1882), der Credesche Handgriff und die Credesche Augenprophylaxe. Geburtshilfe Frauenheilkd. 2016;76:675-678.
- Brandt ML. The mechanism and management of the third stage of labor. Am J Obstet Gynecol. 1933;25:662-667.
- Kimbell N. Brandt-Andrews technique of delivery of the placenta. Br Med J. 1958;1:203-204.
- De Lee JB, Greenhill JP. Principles and Practice of Obstetrics. 9th ed. Philadelphia, PA: Saunders; 1947:275.
- Du Y, Ye M, Zheng F. Active management of the third stage of labor with and without controlled cord traction: a systematic review and meta-analysis of randomized controlled trials. Acta Obstet Gynecol Scand. 2014;93:626-633.
- Hofmeyr GJ, Mshweshwe NT, Gülmezoglu AM. Controlled cord traction for the third stage of labor. Cochrane Database Syst Rev. 2015;1:CD008020.
- Frolova AI, Stout MJ, Tuuli MG, et al. Duration of the third stage of labor and risk of postpartum hemorrhage. Obstet Gynecol. 2016;127:951-956.
- Shinar S, Schwartz A, Maslovitz S, et al. How long is safe? Setting the cutoff for uncomplicated third stage length: a retrospective case-control study. Birth. 2016;43:36-41.
- Magann EF, Evans S, Chauhan SP, et al. The length of the third stage of labor and the risk of postpartum hemorrhage. Obstet Gynecol. 2005;105:290-293.
- Cummings K, Doherty DA, Magann EF, et al. Timing of manual placenta removal to prevent postpartum hemorrhage: is it time to act? J Matern Fetal Neonatal Med. 2016;29:3930-3933.
- Rabie NZ, Ounpraseuth S, Hughes D, et al. Association of the length of the third stage of labor and blood loss following vaginal delivery. South Med J. 2018;111:178-182.
- Rosenstein MG, Vargas JE, Drey EA. Ultrasound-guided instrumental removal of the retained placenta after vaginal delivery. Am J Obstet Gynecol. 2014;211:180.e1-e3.
- Chibueze EC, Parsons AJ, Ota E, et al. Prophylactic antibiotics for manual removal of retained placenta during vaginal birth: a systematic review of observational studies and meta-analysis. BMC Pregnancy Childbirth. 2015;15:313.
- Abdel-Aleem H, Abdel-Aleem MA, Shaaban OM. Nitroglycerin for management of retained placenta. Cochrane Database Syst Rev. 2015;(11):CD007708.
- Weissback T, Haikin-Herzberger E, Bacci-Hugger K, et al. Immediate postpartum ultrasound evaluation for suspected retained placental tissue in patients undergoing manual removal of placenta. Eur J Obstet Gynecol Reprod Biol. 2015;192:37-40.
You have just safely delivered the baby who is quietly resting on her mother’s chest. You begin active management of the third stage of labor, administering oxytocin, performing uterine massage and applying controlled tension on the umbilical cord. There is no evidence of excess postpartum bleeding.
How long will you wait to deliver the placenta?
Active management of the third stage of labor
Most authorities recommend active management of the third stage of labor because active management reduces the risk of maternal hemorrhage >1,000 mL (relative risk [RR], 0.34), postpartum hemoglobin levels < 9 g/dL (RR, 0.50), and maternal blood transfusion (RR, 0.35) compared with expectant management.1
The most important component of active management of the third stage of labor is the administration of a uterotonic after delivery of the newborn. In the United States, oxytocin is the uterotonic most often utilized for the active management of the third stage of labor. Authors of a recent randomized clinical trial reported that intravenous oxytocin is superior to intramuscular oxytocin for reducing postpartum blood loss (385 vs 445 mL), the frequency of blood loss greater than 1,000 mL (4.6% vs 8.1%), and the rate of maternal blood transfusion (1.5% vs 4.4%).2
In addition to administering oxytocin, the active management of the third stage often involves maneuvers to accelerate placental delivery, including the Crede and Brandt-Andrews maneuvers and controlled tension on the umbilical cord. The Crede maneuver, described in 1853, involves placing a hand on the abdominal wall near the uterine fundus and squeezing the uterine fundus between the thumb and fingers.3,4
The Brandt-Andrews maneuver, described in 1933, involves placing a clamp on the umbilical cord close to the vulva.5 The clamp is used to apply judicious tension on the cord with one hand, while the other hand is placed on the mother’s abdomen with the palm and fingers overlying the junction between the uterine corpus and the lower segment. With judicious tension on the cord, the abdominal hand pushes the uterus upward toward the umbilicus. Placental separation is indicated when lengthening of the umbilical cord occurs. The Brandt-Andrews maneuver may be associated with fewer cases of uterine inversion than the Crede maneuver.5-7
Of note, umbilical cord traction has not been demonstrated to reduce the need for blood transfusion or the incidence of postpartum hemorrhage (PPH) >1,000 mL, and it is commonly utilized by obstetricians and midwives.8,9 Hence, in the third stage, the delivering clinician should routinely administer a uterotonic, but use of judicious tension on the cord can be deferred if the woman prefers a noninterventional approach to delivery.
Following a vaginal birth, when should the diagnosis of retained placenta be made?
The historic definition of retained placenta is nonexpulsion of the placenta 30 minutes after delivery of the newborn. However, many observational studies report that, when active management of the third stage is utilized, 90%, 95%, and 99% of placentas deliver by 9 minutes, 13 minutes, and 28 minutes, respectively.10 In addition, many observational studies report that the incidence of PPH increases significantly with longer intervals between birth of the newborn and delivery of the placenta. In one study the rate of blood loss >500 mL was 8.5% when the placenta delivered between 5 and 9 minutes and 35.1% when the placenta delivered ≥30 minutes following birth of the baby.10 In another observational study, compared with women delivering the placenta < 10 minutes after birth, women delivering the placenta ≥30 minutes after birth had a 3-fold increased risk of PPH.11 Similar findings have been reported in other studies.12-14
Continue to: Based on the association between a delay in delivery...
Based on the association between a delay in delivery of the placenta and an increased risk of PPH, some authorities recommend that, in term pregnancy, the diagnosis of retained placenta should be made at 20 minutes following birth and consideration should be given to removing the placenta at this time. For women with effective neuraxial anesthesia, manual removal of the placenta 20 minutes following birth may be the best decision for balancing the benefit of preventing PPH with the risk of unnecessary intervention. For women with no anesthesia, delaying manual removal of the placenta to 30 minutes or more following birth may permit more time for the placenta to deliver prior to performing an intervention that might cause pain, but the delay increases the risk of PPH.
The retained placenta may prevent the uterine muscle from effectively contracting around penetrating veins and arteries, thereby increasing the risk of postpartum hemorrhage. The placenta that has separated from the uterine wall but is trapped inside the uterine cavity can be removed easily with manual extraction. If the placenta is physiologically adherent to the uterine wall, a gentle sweeping motion with an intrauterine hand usually can separate the placenta from the uterus in preparation for manual extraction. However, if a placenta accreta spectrum disorder is contributing to a retained placenta, it may be difficult to separate the densely adherent portion of the uterus from the uterine wall. In the presence of placenta accreta spectrum disorder, vigorous attempts to remove the placenta may precipitate massive bleeding. In some cases, the acchoucheur/midwife may recognize the presence of a focal accreta and cease attempts to remove the placenta in order to organize the personnel and equipment needed to effectively treat a potential case of placenta accreta. In one study, when a placenta accreta was recognized or suspected, immediately ceasing attempts at manually removing the placenta resulted in better case outcomes than continued attempts to remove the placenta.1
Uterine inversion may occur during an attempt to manually remove the placenta. There is universal agreement that once a uterine inversion is recognized it is critically important to immediately restore normal uterine anatomy to avoid massive hemorrhage and maternal shock. The initial management of uterine inversion includes:
- stopping oxytocin infusion
- initiating high volume fluid resuscitation
- considering a dose of a uterine relaxant, such as nitroglycerin or terbutaline
- preparing for blood product replacement.
In my experience, when uterine inversion is immediately recognized and successfully treated, blood product replacement is not usually necessary. However, if uterine inversion has not been immediately recognized or treated, massive hemorrhage and shock may occur.
Two approaches to the vaginal restoration of uterine anatomy involve using the tips of the fingers and palm of the hand to guide the wall of the uterus back to its normal position (FIGURE 1) or to forcefully use a fist to force the uterine wall back to its normal position (FIGURE 2). If these maneuvers are unsuccessful, a laparotomy may be necessary.


At laparotomy, the Huntington or Haultain procedures may help restore normal uterine anatomy. The Huntington procedure involves using clamps to apply symmetrical tension to the left and right round ligaments and/or uterine serosa to sequentially tease the uterus back to normal anatomy.2,3 The Haultain procedure involves a vertical incision on the posterior wall of the uterus to release the uterine constriction ring that is preventing the return of the uterine fundus to its normal position (FIGURE 3).4,5

References
- Kayem G, Anselem O, Schmitz T, et al. Conservative versus radical management in cases of placenta accreta: a historical study. J Gynecol Obstet Biol Reprod (Paris). 2007;36:680-687.
- Huntington JL. Acute inversion of the uterus. Boston Med Surg J. 1921;184:376-378.
- Huntington JL, Irving FC, Kellogg FS. Abdominal reposition in acute inversion of the puerperal uterus. Am J Obstet Gynecol. 1928;15:34-40.
- Haultain FW. Abdominal hysterotomy for chronic uterine inversion: a record of 3 cases. Proc Roy Soc Med. 1908;1:528-535.
- Easterday CL, Reid DE. Inversion of the puerperal uterus managed by the Haultain technique; A case report. Am J Obstet Gynecol. 1959;78:1224-1226.
Manual extraction of the placenta
Prior to performing manual extraction of the placenta, a decision should be made regarding the approach to anesthesia and perioperative antibiotics. Manual extraction of the placenta is performed by placing one hand on the uterine fundus to stabilize the uterus and using the other hand to follow the umbilical cord into the uterine cavity. The intrauterine hand is used to separate the uterine-placental interface with a gentle sweeping motion. The placental mass is grasped and gently teased through the cervix and vagina. Inspection of the placenta to ensure complete removal is necessary.
An alternative to manual extraction of the placenta is the use of Bierer forceps and ultrasound guidance to tease the placenta through the cervical os. This technique involves the following steps15:
1. use ultrasound to locate the placenta
2. place a ring forceps on the anterior lip of the cervix
3. introduce the Bierer forcep into the uterus
4. use the forceps to grasp the placenta and pull it toward the vagina
5. stop frequently to re-grasp placental tissue that is deeper in the uterine cavity
6. once the placenta is extracted, examine the placenta to ensure complete removal.
Of note when manual extraction is used to deliver a retained placenta, randomized clinical trials report no benefit for the following interventions:
- perioperative antibiotics16
- nitroglycerin to relax the uterus17
- ultrasound to detect retained placental tissue.18
Best timing for manual extraction of the placenta
The timing for the diagnosis of retained placenta, and the risks and benefits of manual extraction would be best evaluated in a large, randomized clinical trial. However, based on observational studies, in a term pregnancy, the diagnosis of retained placenta is best made using a 20-minute interval. In women with effective neuraxial anesthesia, consideration should be given to manual removal of the placenta at that time.
You have just safely delivered the baby who is quietly resting on her mother’s chest. You begin active management of the third stage of labor, administering oxytocin, performing uterine massage and applying controlled tension on the umbilical cord. There is no evidence of excess postpartum bleeding.
How long will you wait to deliver the placenta?
Active management of the third stage of labor
Most authorities recommend active management of the third stage of labor because active management reduces the risk of maternal hemorrhage >1,000 mL (relative risk [RR], 0.34), postpartum hemoglobin levels < 9 g/dL (RR, 0.50), and maternal blood transfusion (RR, 0.35) compared with expectant management.1
The most important component of active management of the third stage of labor is the administration of a uterotonic after delivery of the newborn. In the United States, oxytocin is the uterotonic most often utilized for the active management of the third stage of labor. Authors of a recent randomized clinical trial reported that intravenous oxytocin is superior to intramuscular oxytocin for reducing postpartum blood loss (385 vs 445 mL), the frequency of blood loss greater than 1,000 mL (4.6% vs 8.1%), and the rate of maternal blood transfusion (1.5% vs 4.4%).2
In addition to administering oxytocin, the active management of the third stage often involves maneuvers to accelerate placental delivery, including the Crede and Brandt-Andrews maneuvers and controlled tension on the umbilical cord. The Crede maneuver, described in 1853, involves placing a hand on the abdominal wall near the uterine fundus and squeezing the uterine fundus between the thumb and fingers.3,4
The Brandt-Andrews maneuver, described in 1933, involves placing a clamp on the umbilical cord close to the vulva.5 The clamp is used to apply judicious tension on the cord with one hand, while the other hand is placed on the mother’s abdomen with the palm and fingers overlying the junction between the uterine corpus and the lower segment. With judicious tension on the cord, the abdominal hand pushes the uterus upward toward the umbilicus. Placental separation is indicated when lengthening of the umbilical cord occurs. The Brandt-Andrews maneuver may be associated with fewer cases of uterine inversion than the Crede maneuver.5-7
Of note, umbilical cord traction has not been demonstrated to reduce the need for blood transfusion or the incidence of postpartum hemorrhage (PPH) >1,000 mL, and it is commonly utilized by obstetricians and midwives.8,9 Hence, in the third stage, the delivering clinician should routinely administer a uterotonic, but use of judicious tension on the cord can be deferred if the woman prefers a noninterventional approach to delivery.
Following a vaginal birth, when should the diagnosis of retained placenta be made?
The historic definition of retained placenta is nonexpulsion of the placenta 30 minutes after delivery of the newborn. However, many observational studies report that, when active management of the third stage is utilized, 90%, 95%, and 99% of placentas deliver by 9 minutes, 13 minutes, and 28 minutes, respectively.10 In addition, many observational studies report that the incidence of PPH increases significantly with longer intervals between birth of the newborn and delivery of the placenta. In one study the rate of blood loss >500 mL was 8.5% when the placenta delivered between 5 and 9 minutes and 35.1% when the placenta delivered ≥30 minutes following birth of the baby.10 In another observational study, compared with women delivering the placenta < 10 minutes after birth, women delivering the placenta ≥30 minutes after birth had a 3-fold increased risk of PPH.11 Similar findings have been reported in other studies.12-14
Continue to: Based on the association between a delay in delivery...
Based on the association between a delay in delivery of the placenta and an increased risk of PPH, some authorities recommend that, in term pregnancy, the diagnosis of retained placenta should be made at 20 minutes following birth and consideration should be given to removing the placenta at this time. For women with effective neuraxial anesthesia, manual removal of the placenta 20 minutes following birth may be the best decision for balancing the benefit of preventing PPH with the risk of unnecessary intervention. For women with no anesthesia, delaying manual removal of the placenta to 30 minutes or more following birth may permit more time for the placenta to deliver prior to performing an intervention that might cause pain, but the delay increases the risk of PPH.
The retained placenta may prevent the uterine muscle from effectively contracting around penetrating veins and arteries, thereby increasing the risk of postpartum hemorrhage. The placenta that has separated from the uterine wall but is trapped inside the uterine cavity can be removed easily with manual extraction. If the placenta is physiologically adherent to the uterine wall, a gentle sweeping motion with an intrauterine hand usually can separate the placenta from the uterus in preparation for manual extraction. However, if a placenta accreta spectrum disorder is contributing to a retained placenta, it may be difficult to separate the densely adherent portion of the uterus from the uterine wall. In the presence of placenta accreta spectrum disorder, vigorous attempts to remove the placenta may precipitate massive bleeding. In some cases, the acchoucheur/midwife may recognize the presence of a focal accreta and cease attempts to remove the placenta in order to organize the personnel and equipment needed to effectively treat a potential case of placenta accreta. In one study, when a placenta accreta was recognized or suspected, immediately ceasing attempts at manually removing the placenta resulted in better case outcomes than continued attempts to remove the placenta.1
Uterine inversion may occur during an attempt to manually remove the placenta. There is universal agreement that once a uterine inversion is recognized it is critically important to immediately restore normal uterine anatomy to avoid massive hemorrhage and maternal shock. The initial management of uterine inversion includes:
- stopping oxytocin infusion
- initiating high volume fluid resuscitation
- considering a dose of a uterine relaxant, such as nitroglycerin or terbutaline
- preparing for blood product replacement.
In my experience, when uterine inversion is immediately recognized and successfully treated, blood product replacement is not usually necessary. However, if uterine inversion has not been immediately recognized or treated, massive hemorrhage and shock may occur.
Two approaches to the vaginal restoration of uterine anatomy involve using the tips of the fingers and palm of the hand to guide the wall of the uterus back to its normal position (FIGURE 1) or to forcefully use a fist to force the uterine wall back to its normal position (FIGURE 2). If these maneuvers are unsuccessful, a laparotomy may be necessary.


At laparotomy, the Huntington or Haultain procedures may help restore normal uterine anatomy. The Huntington procedure involves using clamps to apply symmetrical tension to the left and right round ligaments and/or uterine serosa to sequentially tease the uterus back to normal anatomy.2,3 The Haultain procedure involves a vertical incision on the posterior wall of the uterus to release the uterine constriction ring that is preventing the return of the uterine fundus to its normal position (FIGURE 3).4,5

References
- Kayem G, Anselem O, Schmitz T, et al. Conservative versus radical management in cases of placenta accreta: a historical study. J Gynecol Obstet Biol Reprod (Paris). 2007;36:680-687.
- Huntington JL. Acute inversion of the uterus. Boston Med Surg J. 1921;184:376-378.
- Huntington JL, Irving FC, Kellogg FS. Abdominal reposition in acute inversion of the puerperal uterus. Am J Obstet Gynecol. 1928;15:34-40.
- Haultain FW. Abdominal hysterotomy for chronic uterine inversion: a record of 3 cases. Proc Roy Soc Med. 1908;1:528-535.
- Easterday CL, Reid DE. Inversion of the puerperal uterus managed by the Haultain technique; A case report. Am J Obstet Gynecol. 1959;78:1224-1226.
Manual extraction of the placenta
Prior to performing manual extraction of the placenta, a decision should be made regarding the approach to anesthesia and perioperative antibiotics. Manual extraction of the placenta is performed by placing one hand on the uterine fundus to stabilize the uterus and using the other hand to follow the umbilical cord into the uterine cavity. The intrauterine hand is used to separate the uterine-placental interface with a gentle sweeping motion. The placental mass is grasped and gently teased through the cervix and vagina. Inspection of the placenta to ensure complete removal is necessary.
An alternative to manual extraction of the placenta is the use of Bierer forceps and ultrasound guidance to tease the placenta through the cervical os. This technique involves the following steps15:
1. use ultrasound to locate the placenta
2. place a ring forceps on the anterior lip of the cervix
3. introduce the Bierer forcep into the uterus
4. use the forceps to grasp the placenta and pull it toward the vagina
5. stop frequently to re-grasp placental tissue that is deeper in the uterine cavity
6. once the placenta is extracted, examine the placenta to ensure complete removal.
Of note when manual extraction is used to deliver a retained placenta, randomized clinical trials report no benefit for the following interventions:
- perioperative antibiotics16
- nitroglycerin to relax the uterus17
- ultrasound to detect retained placental tissue.18
Best timing for manual extraction of the placenta
The timing for the diagnosis of retained placenta, and the risks and benefits of manual extraction would be best evaluated in a large, randomized clinical trial. However, based on observational studies, in a term pregnancy, the diagnosis of retained placenta is best made using a 20-minute interval. In women with effective neuraxial anesthesia, consideration should be given to manual removal of the placenta at that time.
- Begley CM, Gyte GM, Devane D, et al. Active versus expectant management for women in the third stage of labor. Cochrane Database Syst Rev. 2019;2:CD007412.
- Adnan N, Conlan-Trant R, McCormick C, et al. Intramuscular versus intravenous oxytocin to prevent postpartum haemorrhage at vaginal delivery: randomized controlled trial. BMJ. 2018;362:k3546.
- Gülmezoglu AM, Souza JP. The evolving management of the third stage of labour. BJOG. 2009;116(suppl 1):26-28.
- Ebert AD, David M. Meilensteine der Praventionsmedizin. Carl Siegmund Franz Credé (1819-1882), der Credesche Handgriff und die Credesche Augenprophylaxe. Geburtshilfe Frauenheilkd. 2016;76:675-678.
- Brandt ML. The mechanism and management of the third stage of labor. Am J Obstet Gynecol. 1933;25:662-667.
- Kimbell N. Brandt-Andrews technique of delivery of the placenta. Br Med J. 1958;1:203-204.
- De Lee JB, Greenhill JP. Principles and Practice of Obstetrics. 9th ed. Philadelphia, PA: Saunders; 1947:275.
- Du Y, Ye M, Zheng F. Active management of the third stage of labor with and without controlled cord traction: a systematic review and meta-analysis of randomized controlled trials. Acta Obstet Gynecol Scand. 2014;93:626-633.
- Hofmeyr GJ, Mshweshwe NT, Gülmezoglu AM. Controlled cord traction for the third stage of labor. Cochrane Database Syst Rev. 2015;1:CD008020.
- Frolova AI, Stout MJ, Tuuli MG, et al. Duration of the third stage of labor and risk of postpartum hemorrhage. Obstet Gynecol. 2016;127:951-956.
- Shinar S, Schwartz A, Maslovitz S, et al. How long is safe? Setting the cutoff for uncomplicated third stage length: a retrospective case-control study. Birth. 2016;43:36-41.
- Magann EF, Evans S, Chauhan SP, et al. The length of the third stage of labor and the risk of postpartum hemorrhage. Obstet Gynecol. 2005;105:290-293.
- Cummings K, Doherty DA, Magann EF, et al. Timing of manual placenta removal to prevent postpartum hemorrhage: is it time to act? J Matern Fetal Neonatal Med. 2016;29:3930-3933.
- Rabie NZ, Ounpraseuth S, Hughes D, et al. Association of the length of the third stage of labor and blood loss following vaginal delivery. South Med J. 2018;111:178-182.
- Rosenstein MG, Vargas JE, Drey EA. Ultrasound-guided instrumental removal of the retained placenta after vaginal delivery. Am J Obstet Gynecol. 2014;211:180.e1-e3.
- Chibueze EC, Parsons AJ, Ota E, et al. Prophylactic antibiotics for manual removal of retained placenta during vaginal birth: a systematic review of observational studies and meta-analysis. BMC Pregnancy Childbirth. 2015;15:313.
- Abdel-Aleem H, Abdel-Aleem MA, Shaaban OM. Nitroglycerin for management of retained placenta. Cochrane Database Syst Rev. 2015;(11):CD007708.
- Weissback T, Haikin-Herzberger E, Bacci-Hugger K, et al. Immediate postpartum ultrasound evaluation for suspected retained placental tissue in patients undergoing manual removal of placenta. Eur J Obstet Gynecol Reprod Biol. 2015;192:37-40.
- Begley CM, Gyte GM, Devane D, et al. Active versus expectant management for women in the third stage of labor. Cochrane Database Syst Rev. 2019;2:CD007412.
- Adnan N, Conlan-Trant R, McCormick C, et al. Intramuscular versus intravenous oxytocin to prevent postpartum haemorrhage at vaginal delivery: randomized controlled trial. BMJ. 2018;362:k3546.
- Gülmezoglu AM, Souza JP. The evolving management of the third stage of labour. BJOG. 2009;116(suppl 1):26-28.
- Ebert AD, David M. Meilensteine der Praventionsmedizin. Carl Siegmund Franz Credé (1819-1882), der Credesche Handgriff und die Credesche Augenprophylaxe. Geburtshilfe Frauenheilkd. 2016;76:675-678.
- Brandt ML. The mechanism and management of the third stage of labor. Am J Obstet Gynecol. 1933;25:662-667.
- Kimbell N. Brandt-Andrews technique of delivery of the placenta. Br Med J. 1958;1:203-204.
- De Lee JB, Greenhill JP. Principles and Practice of Obstetrics. 9th ed. Philadelphia, PA: Saunders; 1947:275.
- Du Y, Ye M, Zheng F. Active management of the third stage of labor with and without controlled cord traction: a systematic review and meta-analysis of randomized controlled trials. Acta Obstet Gynecol Scand. 2014;93:626-633.
- Hofmeyr GJ, Mshweshwe NT, Gülmezoglu AM. Controlled cord traction for the third stage of labor. Cochrane Database Syst Rev. 2015;1:CD008020.
- Frolova AI, Stout MJ, Tuuli MG, et al. Duration of the third stage of labor and risk of postpartum hemorrhage. Obstet Gynecol. 2016;127:951-956.
- Shinar S, Schwartz A, Maslovitz S, et al. How long is safe? Setting the cutoff for uncomplicated third stage length: a retrospective case-control study. Birth. 2016;43:36-41.
- Magann EF, Evans S, Chauhan SP, et al. The length of the third stage of labor and the risk of postpartum hemorrhage. Obstet Gynecol. 2005;105:290-293.
- Cummings K, Doherty DA, Magann EF, et al. Timing of manual placenta removal to prevent postpartum hemorrhage: is it time to act? J Matern Fetal Neonatal Med. 2016;29:3930-3933.
- Rabie NZ, Ounpraseuth S, Hughes D, et al. Association of the length of the third stage of labor and blood loss following vaginal delivery. South Med J. 2018;111:178-182.
- Rosenstein MG, Vargas JE, Drey EA. Ultrasound-guided instrumental removal of the retained placenta after vaginal delivery. Am J Obstet Gynecol. 2014;211:180.e1-e3.
- Chibueze EC, Parsons AJ, Ota E, et al. Prophylactic antibiotics for manual removal of retained placenta during vaginal birth: a systematic review of observational studies and meta-analysis. BMC Pregnancy Childbirth. 2015;15:313.
- Abdel-Aleem H, Abdel-Aleem MA, Shaaban OM. Nitroglycerin for management of retained placenta. Cochrane Database Syst Rev. 2015;(11):CD007708.
- Weissback T, Haikin-Herzberger E, Bacci-Hugger K, et al. Immediate postpartum ultrasound evaluation for suspected retained placental tissue in patients undergoing manual removal of placenta. Eur J Obstet Gynecol Reprod Biol. 2015;192:37-40.
The One Step test: The better diagnostic approach for gestational diabetes mellitus
Gestational diabetes mellitus (GDM) generally is defined as any degree of glucose intolerance with onset or first recognition during pregnancy.1-14 The best approach and exact criteria to use for GDM screening and diagnosis are under worldwide debate. In TABLE 1 we present just some of the many differing suggestions by varying organizations.2,7-9,11,12,15-17 The American College of Obstetricians and Gynecologists, for instance, suggests a Two Step approach to diagnosis.15 We will make the argument in this article, however, that diagnosis should be defined universally as an abnormal result with the One Step 75-g glucose testing, as adopted by the World Health Organization, International Federation of Gynecology and Obstetrics, and others. Approximately 8% of all pregnancies are complicated by GDM by the One Step test in the United States.18-22 The prevalence may range from 1% to 14% of all pregnancies, depending on the population studied and the diagnostic tests employed.1,19
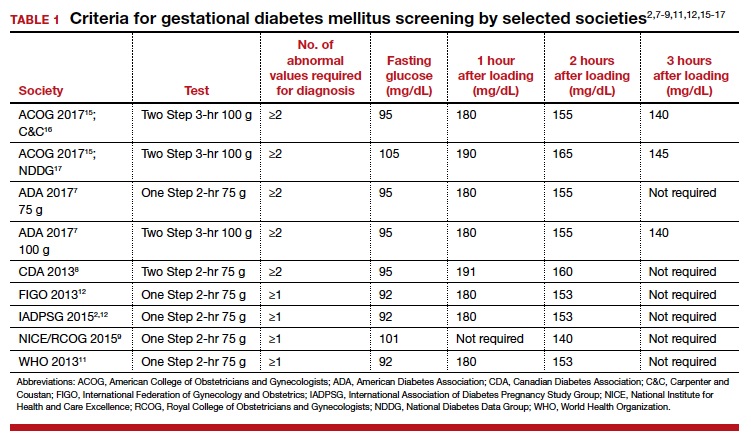
Diagnostic options
Different methods for screening and diagnosis of GDM have been proposed by international societies; there is controversy regarding the diagnosis of GDM by either the One Step or the Two Step approach.6
The One Step approach includes an oral glucose tolerance test with a 75-g glucose load with measurement of plasma glucose concentration at fasting state and 1 hour and 2 hours post–glucose administration. A positive result for the One Step approach is defined as at least 1 measurement higher than 92, 180, or 153 mg/dL at fasting, 1 hour, or 2 hours, respectively.
The Two Step approach includes a nonfasting oral 50-g glucose load, with a glucose blood measurement 1 hour later. A positive screening, defined often as a blood glucose value higher than 135 mg/dL (range, 130 to 140 mg/dL), is followed by a diagnostic test with a 100-g glucose load with measurements at fasting and 1, 2, and 3 hours post–glucose administration. A positive diagnostic test is defined as 2 measurements higher than the target value.
Why we support the One Step test
There are several reasons to prefer the One Step approach for the diagnosis of GDM, compared with the Two Step approach.
Women testing negative for GDM with Two Step still experience complications pregnancy. Women who test positive for GDM with the One Step test, but negative with the Two Step test, despite having therefore a milder degree of glucose intolerance, do have a higher risk of experiencing several complications.23 For the mother, these complications include gestational hypertension, preeclampsia, and cesarean delivery. The baby also can experience problems at birth (TABLE 2).23 Therefore, women who test positive for GDM with the One Step test deserve to be diagnosed with and treated for the condition, as not only are they at risk for these complications but also treatment of the GDM decreases the incidence of these complications.18,19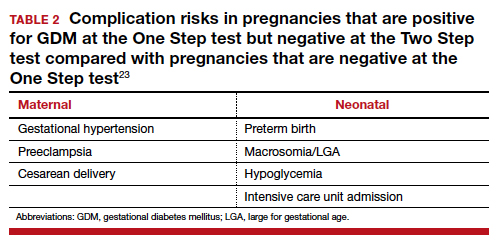
There is indeed an increased GDM diagnosis rate with the One Step (about 8%) compared with the Two Step test (about 4%). Nonetheless, this increase is mild and nonsignificant in the meta-analysis of randomized controlled trials (RCTs),18,19 is less than the 18% difference in diagnosis rate previously hypothesized, is consistent with the increased diabetes/prediabetes rates in the general population, and is linked to the increasing incidence of obesity and insulin resistance.
Overall test adherence is better. Five percent to 15% of patients, depending on the study, are not adherent with taking the second part of the Two Step test. Women indeed prefer the One Step approach; the second step in the Two Step approach may be a burden.
Less costly. The One Step process is cost-effective when postpregnancy diabetes mellitus prevention is considered.
Better maternal and perinatal outcomes. Probably the most important and convincing reason the One Step test should be used is that meta-analysis of the 4 RCTs comparing the approaches (including 2 US trials) shows that diagnosing and treating mild GDM as per the One Step approach, compared with screening and treating using the Two Step approach, is associated with increased incidence of GDM (8% vs 4%) and with better maternal and perinatal outcomes.13,18,19 In fact, the One Step approach is associated with significant reductions in: large for gestational age (56%), admission to neonatal intensive care unit (51%), and neonatal hypoglycemia (48%). Tests of heterogeneity in the meta-analysis and of quality all pointed to better outcomes in the One Step test group.13,19
The need for a second step in the Two Step approach delays diagnosis and treatment. The One Step approach is associated with an increase in GDM test adherence and earlier diagnosis,13 which is another reason for better outcomes with the One Step approach. In the presence of risk factors, such as prior GDM, prior macrosomia, advanced maternal age, multiple gestations, and others, the One Step test should be done at the first prenatal visit.
Continue to: US guidelines should be reconsidered...
US guidelines should be reconsidered
The One Step, 75-g, 2-hour oral glucose tolerance test is universally used to diagnose diabetes mellitus outside of pregnancy. Given our many noted reasons (TABLE 3), we recommend universal screening of GDM by using the One Step approach. It is time, indeed, for the United States to reconsider its guidelines for screening for GDM.
- Kampmann U, Madsen LR, Skajaa GO, et al. Gestational diabetes: a clinical update. World J Diabetes. 2015;6:1065-1072.
- HAPO Study Cooperative Research Group. Hyperglycemia and adverse pregnancy outcomes. N Engl J Med. 2008;358:1991-2002.
- Meltzer SJ, Snyder J, Penrod JR, et al. Gestational diabetes mellitus screening and diagnosis: a prospective randomised controlled trial comparing costs of one-step and two-step methods. BJOG. 2010;117:407-415.
- Sevket O, Ates S, Uysal O, et al. To evaluate the prevalence and clinical outcomes using a one-step method versus a two-step method to screen gestational diabetes mellitus. J Matern Fetal Neonatal Med. 2014;27:36-41.
- Scifres CM, Abebe KZ, Jones KA, et al. Gestational diabetes diagnostic methods (GD2M) pilot randomized trial. Matern Child Health J. 2015;19:1472-1480.
- Farrar D, Duley L, Medley N, et al. Different strategies for diagnosing gestational diabetes to improve maternal and infant health. Cochrane Database Syst Rev. 2015;1:CD007122.
- American Diabetes Association. Classification and diagnosis of diabetes. Diabetes Care. 2017;40(suppl 1):S11-S24.
- Canadian Diabetes Association Clinical Practice Guidelines Expert Committee. Diabetes and Pregnancy. Can J Diabetes. 2013;37(suppl 1):S168-S183.
- NICE guideline. Diabetes in pregnancy: management from preconception to the postnatal period. February 2015. https://www.nice.org.uk/guidance/ng3/. Last updated August 2015. Accessed November 18, 2019.
- WHO 1999. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus. From http://apps.who.int/iris/bitstream/10665/66040/1/WHO
_NCD_NCS_99.2.pdf. Accessed November 18, 2019. - World Health Organization. Diagnostic criteria and classification of hyperglycaemia first detected in pregnancy. 2013. http://apps.who.int/iris/bitstream/10665/85975/1/WHO_
NMH_MND_13.2_eng.pdf. Accessed November 18, 2019. - Hod M, Kapur A, Sacks DA, et al. The International Federation of Gynecology and Obstetrics (FIGO) Initiative on gestational diabetes mellitus: a pragmatic guide for diagnosis, management, and care. Int J Gynaecol Obstet. 2015;131(suppl 3):S173-S211.
- Berghella V, Caissutti C, Saccone G, et al. The One Step approach for diagnosing gestational diabetes is associated with better perinatal outcomes than using the Two Step approach: evidence of randomized clinical trials. Am J Obstet Gynecol. 2019;220:562-564.
- Berghella V, Caissutti C, Saccone G, et al. One-Step approach to identifying gestational diabetes mellitus: association with perinatal outcomes. Obstet Gynecol. 2019;133:383.
- American College of Obstetricians and Gynecologists. Committee on Practice Bulletins—Obstetrics. Practice Bulletin No. 180: gestational diabetes mellitus. Obstet Gynecol. 2017;130:e17-e31.
- Carpenter MW, Coustan DR. Criteria for screening tests for gestational diabetes. Am J Obstet Gynecol. 1982;144:768-773.
- National Diabetes Data Group. Classification and diagnosis of diabetes mellitus and other categories of glucose intolerance. Diabetes. 1979;28:1039-1057.
- Saccone G, Khalifeh A, Al-Kouatly HB, et al. Screening for gestational diabetes mellitus: one step versus two step approach. A meta-analysis of randomized trials. J Matern Fetal Neonatal Med. 2018:1-9.
- Saccone G, Caissutti C, Khalifeh A, et al. One step versus two step approach for gestational diabetes screening: systematic review and meta-analysis of the randomized trials. J Matern Fetal Neonatal Med. 2019;32:1547-1555.
- Khalifeh A, Eckler R, Felder L, et al. One-step versus two-step diagnostic testing for gestational diabetes: a randomized controlled trial. J Matern Fetal Neonatal Med. 2018:1-6.
- Caissutti C, Saccone G, Khalifeh A, et al. Which criteria should be used for starting pharmacologic therapy for management of gestational diabetes in pregnancy? Evidence from randomized controlled trials. J Matern Fetal Neonatal Med. 2019;32:2905-2914.
- Caissutti C, Saccone G, Ciardulli A, et al. Very tight vs. tight control: which should be the criteria for pharmacologic therapy dose adjustment in diabetes in pregnancy? Evidence from randomized controlled trials. Acta Obstet Gynecol Scand. 2018;97:235-247.
- Caissutti C, Khalifeh A, Saccone G, et al. Are women positive for the One Step but negative for the Two Step screening tests for gestational diabetes at higher risk for adverse outcomes? Acta Obstet Gynecol Scand. 2018;97:122-134.
Gestational diabetes mellitus (GDM) generally is defined as any degree of glucose intolerance with onset or first recognition during pregnancy.1-14 The best approach and exact criteria to use for GDM screening and diagnosis are under worldwide debate. In TABLE 1 we present just some of the many differing suggestions by varying organizations.2,7-9,11,12,15-17 The American College of Obstetricians and Gynecologists, for instance, suggests a Two Step approach to diagnosis.15 We will make the argument in this article, however, that diagnosis should be defined universally as an abnormal result with the One Step 75-g glucose testing, as adopted by the World Health Organization, International Federation of Gynecology and Obstetrics, and others. Approximately 8% of all pregnancies are complicated by GDM by the One Step test in the United States.18-22 The prevalence may range from 1% to 14% of all pregnancies, depending on the population studied and the diagnostic tests employed.1,19

Diagnostic options
Different methods for screening and diagnosis of GDM have been proposed by international societies; there is controversy regarding the diagnosis of GDM by either the One Step or the Two Step approach.6
The One Step approach includes an oral glucose tolerance test with a 75-g glucose load with measurement of plasma glucose concentration at fasting state and 1 hour and 2 hours post–glucose administration. A positive result for the One Step approach is defined as at least 1 measurement higher than 92, 180, or 153 mg/dL at fasting, 1 hour, or 2 hours, respectively.
The Two Step approach includes a nonfasting oral 50-g glucose load, with a glucose blood measurement 1 hour later. A positive screening, defined often as a blood glucose value higher than 135 mg/dL (range, 130 to 140 mg/dL), is followed by a diagnostic test with a 100-g glucose load with measurements at fasting and 1, 2, and 3 hours post–glucose administration. A positive diagnostic test is defined as 2 measurements higher than the target value.
Why we support the One Step test
There are several reasons to prefer the One Step approach for the diagnosis of GDM, compared with the Two Step approach.
Women testing negative for GDM with Two Step still experience complications pregnancy. Women who test positive for GDM with the One Step test, but negative with the Two Step test, despite having therefore a milder degree of glucose intolerance, do have a higher risk of experiencing several complications.23 For the mother, these complications include gestational hypertension, preeclampsia, and cesarean delivery. The baby also can experience problems at birth (TABLE 2).23 Therefore, women who test positive for GDM with the One Step test deserve to be diagnosed with and treated for the condition, as not only are they at risk for these complications but also treatment of the GDM decreases the incidence of these complications.18,19
There is indeed an increased GDM diagnosis rate with the One Step (about 8%) compared with the Two Step test (about 4%). Nonetheless, this increase is mild and nonsignificant in the meta-analysis of randomized controlled trials (RCTs),18,19 is less than the 18% difference in diagnosis rate previously hypothesized, is consistent with the increased diabetes/prediabetes rates in the general population, and is linked to the increasing incidence of obesity and insulin resistance.
Overall test adherence is better. Five percent to 15% of patients, depending on the study, are not adherent with taking the second part of the Two Step test. Women indeed prefer the One Step approach; the second step in the Two Step approach may be a burden.
Less costly. The One Step process is cost-effective when postpregnancy diabetes mellitus prevention is considered.
Better maternal and perinatal outcomes. Probably the most important and convincing reason the One Step test should be used is that meta-analysis of the 4 RCTs comparing the approaches (including 2 US trials) shows that diagnosing and treating mild GDM as per the One Step approach, compared with screening and treating using the Two Step approach, is associated with increased incidence of GDM (8% vs 4%) and with better maternal and perinatal outcomes.13,18,19 In fact, the One Step approach is associated with significant reductions in: large for gestational age (56%), admission to neonatal intensive care unit (51%), and neonatal hypoglycemia (48%). Tests of heterogeneity in the meta-analysis and of quality all pointed to better outcomes in the One Step test group.13,19
The need for a second step in the Two Step approach delays diagnosis and treatment. The One Step approach is associated with an increase in GDM test adherence and earlier diagnosis,13 which is another reason for better outcomes with the One Step approach. In the presence of risk factors, such as prior GDM, prior macrosomia, advanced maternal age, multiple gestations, and others, the One Step test should be done at the first prenatal visit.
Continue to: US guidelines should be reconsidered...
US guidelines should be reconsidered
The One Step, 75-g, 2-hour oral glucose tolerance test is universally used to diagnose diabetes mellitus outside of pregnancy. Given our many noted reasons (TABLE 3), we recommend universal screening of GDM by using the One Step approach. It is time, indeed, for the United States to reconsider its guidelines for screening for GDM.

Gestational diabetes mellitus (GDM) generally is defined as any degree of glucose intolerance with onset or first recognition during pregnancy.1-14 The best approach and exact criteria to use for GDM screening and diagnosis are under worldwide debate. In TABLE 1 we present just some of the many differing suggestions by varying organizations.2,7-9,11,12,15-17 The American College of Obstetricians and Gynecologists, for instance, suggests a Two Step approach to diagnosis.15 We will make the argument in this article, however, that diagnosis should be defined universally as an abnormal result with the One Step 75-g glucose testing, as adopted by the World Health Organization, International Federation of Gynecology and Obstetrics, and others. Approximately 8% of all pregnancies are complicated by GDM by the One Step test in the United States.18-22 The prevalence may range from 1% to 14% of all pregnancies, depending on the population studied and the diagnostic tests employed.1,19

Diagnostic options
Different methods for screening and diagnosis of GDM have been proposed by international societies; there is controversy regarding the diagnosis of GDM by either the One Step or the Two Step approach.6
The One Step approach includes an oral glucose tolerance test with a 75-g glucose load with measurement of plasma glucose concentration at fasting state and 1 hour and 2 hours post–glucose administration. A positive result for the One Step approach is defined as at least 1 measurement higher than 92, 180, or 153 mg/dL at fasting, 1 hour, or 2 hours, respectively.
The Two Step approach includes a nonfasting oral 50-g glucose load, with a glucose blood measurement 1 hour later. A positive screening, defined often as a blood glucose value higher than 135 mg/dL (range, 130 to 140 mg/dL), is followed by a diagnostic test with a 100-g glucose load with measurements at fasting and 1, 2, and 3 hours post–glucose administration. A positive diagnostic test is defined as 2 measurements higher than the target value.
Why we support the One Step test
There are several reasons to prefer the One Step approach for the diagnosis of GDM, compared with the Two Step approach.
Women testing negative for GDM with Two Step still experience complications pregnancy. Women who test positive for GDM with the One Step test, but negative with the Two Step test, despite having therefore a milder degree of glucose intolerance, do have a higher risk of experiencing several complications.23 For the mother, these complications include gestational hypertension, preeclampsia, and cesarean delivery. The baby also can experience problems at birth (TABLE 2).23 Therefore, women who test positive for GDM with the One Step test deserve to be diagnosed with and treated for the condition, as not only are they at risk for these complications but also treatment of the GDM decreases the incidence of these complications.18,19
There is indeed an increased GDM diagnosis rate with the One Step (about 8%) compared with the Two Step test (about 4%). Nonetheless, this increase is mild and nonsignificant in the meta-analysis of randomized controlled trials (RCTs),18,19 is less than the 18% difference in diagnosis rate previously hypothesized, is consistent with the increased diabetes/prediabetes rates in the general population, and is linked to the increasing incidence of obesity and insulin resistance.
Overall test adherence is better. Five percent to 15% of patients, depending on the study, are not adherent with taking the second part of the Two Step test. Women indeed prefer the One Step approach; the second step in the Two Step approach may be a burden.
Less costly. The One Step process is cost-effective when postpregnancy diabetes mellitus prevention is considered.
Better maternal and perinatal outcomes. Probably the most important and convincing reason the One Step test should be used is that meta-analysis of the 4 RCTs comparing the approaches (including 2 US trials) shows that diagnosing and treating mild GDM as per the One Step approach, compared with screening and treating using the Two Step approach, is associated with increased incidence of GDM (8% vs 4%) and with better maternal and perinatal outcomes.13,18,19 In fact, the One Step approach is associated with significant reductions in: large for gestational age (56%), admission to neonatal intensive care unit (51%), and neonatal hypoglycemia (48%). Tests of heterogeneity in the meta-analysis and of quality all pointed to better outcomes in the One Step test group.13,19
The need for a second step in the Two Step approach delays diagnosis and treatment. The One Step approach is associated with an increase in GDM test adherence and earlier diagnosis,13 which is another reason for better outcomes with the One Step approach. In the presence of risk factors, such as prior GDM, prior macrosomia, advanced maternal age, multiple gestations, and others, the One Step test should be done at the first prenatal visit.
Continue to: US guidelines should be reconsidered...
US guidelines should be reconsidered
The One Step, 75-g, 2-hour oral glucose tolerance test is universally used to diagnose diabetes mellitus outside of pregnancy. Given our many noted reasons (TABLE 3), we recommend universal screening of GDM by using the One Step approach. It is time, indeed, for the United States to reconsider its guidelines for screening for GDM.

- Kampmann U, Madsen LR, Skajaa GO, et al. Gestational diabetes: a clinical update. World J Diabetes. 2015;6:1065-1072.
- HAPO Study Cooperative Research Group. Hyperglycemia and adverse pregnancy outcomes. N Engl J Med. 2008;358:1991-2002.
- Meltzer SJ, Snyder J, Penrod JR, et al. Gestational diabetes mellitus screening and diagnosis: a prospective randomised controlled trial comparing costs of one-step and two-step methods. BJOG. 2010;117:407-415.
- Sevket O, Ates S, Uysal O, et al. To evaluate the prevalence and clinical outcomes using a one-step method versus a two-step method to screen gestational diabetes mellitus. J Matern Fetal Neonatal Med. 2014;27:36-41.
- Scifres CM, Abebe KZ, Jones KA, et al. Gestational diabetes diagnostic methods (GD2M) pilot randomized trial. Matern Child Health J. 2015;19:1472-1480.
- Farrar D, Duley L, Medley N, et al. Different strategies for diagnosing gestational diabetes to improve maternal and infant health. Cochrane Database Syst Rev. 2015;1:CD007122.
- American Diabetes Association. Classification and diagnosis of diabetes. Diabetes Care. 2017;40(suppl 1):S11-S24.
- Canadian Diabetes Association Clinical Practice Guidelines Expert Committee. Diabetes and Pregnancy. Can J Diabetes. 2013;37(suppl 1):S168-S183.
- NICE guideline. Diabetes in pregnancy: management from preconception to the postnatal period. February 2015. https://www.nice.org.uk/guidance/ng3/. Last updated August 2015. Accessed November 18, 2019.
- WHO 1999. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus. From http://apps.who.int/iris/bitstream/10665/66040/1/WHO
_NCD_NCS_99.2.pdf. Accessed November 18, 2019. - World Health Organization. Diagnostic criteria and classification of hyperglycaemia first detected in pregnancy. 2013. http://apps.who.int/iris/bitstream/10665/85975/1/WHO_
NMH_MND_13.2_eng.pdf. Accessed November 18, 2019. - Hod M, Kapur A, Sacks DA, et al. The International Federation of Gynecology and Obstetrics (FIGO) Initiative on gestational diabetes mellitus: a pragmatic guide for diagnosis, management, and care. Int J Gynaecol Obstet. 2015;131(suppl 3):S173-S211.
- Berghella V, Caissutti C, Saccone G, et al. The One Step approach for diagnosing gestational diabetes is associated with better perinatal outcomes than using the Two Step approach: evidence of randomized clinical trials. Am J Obstet Gynecol. 2019;220:562-564.
- Berghella V, Caissutti C, Saccone G, et al. One-Step approach to identifying gestational diabetes mellitus: association with perinatal outcomes. Obstet Gynecol. 2019;133:383.
- American College of Obstetricians and Gynecologists. Committee on Practice Bulletins—Obstetrics. Practice Bulletin No. 180: gestational diabetes mellitus. Obstet Gynecol. 2017;130:e17-e31.
- Carpenter MW, Coustan DR. Criteria for screening tests for gestational diabetes. Am J Obstet Gynecol. 1982;144:768-773.
- National Diabetes Data Group. Classification and diagnosis of diabetes mellitus and other categories of glucose intolerance. Diabetes. 1979;28:1039-1057.
- Saccone G, Khalifeh A, Al-Kouatly HB, et al. Screening for gestational diabetes mellitus: one step versus two step approach. A meta-analysis of randomized trials. J Matern Fetal Neonatal Med. 2018:1-9.
- Saccone G, Caissutti C, Khalifeh A, et al. One step versus two step approach for gestational diabetes screening: systematic review and meta-analysis of the randomized trials. J Matern Fetal Neonatal Med. 2019;32:1547-1555.
- Khalifeh A, Eckler R, Felder L, et al. One-step versus two-step diagnostic testing for gestational diabetes: a randomized controlled trial. J Matern Fetal Neonatal Med. 2018:1-6.
- Caissutti C, Saccone G, Khalifeh A, et al. Which criteria should be used for starting pharmacologic therapy for management of gestational diabetes in pregnancy? Evidence from randomized controlled trials. J Matern Fetal Neonatal Med. 2019;32:2905-2914.
- Caissutti C, Saccone G, Ciardulli A, et al. Very tight vs. tight control: which should be the criteria for pharmacologic therapy dose adjustment in diabetes in pregnancy? Evidence from randomized controlled trials. Acta Obstet Gynecol Scand. 2018;97:235-247.
- Caissutti C, Khalifeh A, Saccone G, et al. Are women positive for the One Step but negative for the Two Step screening tests for gestational diabetes at higher risk for adverse outcomes? Acta Obstet Gynecol Scand. 2018;97:122-134.
- Kampmann U, Madsen LR, Skajaa GO, et al. Gestational diabetes: a clinical update. World J Diabetes. 2015;6:1065-1072.
- HAPO Study Cooperative Research Group. Hyperglycemia and adverse pregnancy outcomes. N Engl J Med. 2008;358:1991-2002.
- Meltzer SJ, Snyder J, Penrod JR, et al. Gestational diabetes mellitus screening and diagnosis: a prospective randomised controlled trial comparing costs of one-step and two-step methods. BJOG. 2010;117:407-415.
- Sevket O, Ates S, Uysal O, et al. To evaluate the prevalence and clinical outcomes using a one-step method versus a two-step method to screen gestational diabetes mellitus. J Matern Fetal Neonatal Med. 2014;27:36-41.
- Scifres CM, Abebe KZ, Jones KA, et al. Gestational diabetes diagnostic methods (GD2M) pilot randomized trial. Matern Child Health J. 2015;19:1472-1480.
- Farrar D, Duley L, Medley N, et al. Different strategies for diagnosing gestational diabetes to improve maternal and infant health. Cochrane Database Syst Rev. 2015;1:CD007122.
- American Diabetes Association. Classification and diagnosis of diabetes. Diabetes Care. 2017;40(suppl 1):S11-S24.
- Canadian Diabetes Association Clinical Practice Guidelines Expert Committee. Diabetes and Pregnancy. Can J Diabetes. 2013;37(suppl 1):S168-S183.
- NICE guideline. Diabetes in pregnancy: management from preconception to the postnatal period. February 2015. https://www.nice.org.uk/guidance/ng3/. Last updated August 2015. Accessed November 18, 2019.
- WHO 1999. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus. From http://apps.who.int/iris/bitstream/10665/66040/1/WHO
_NCD_NCS_99.2.pdf. Accessed November 18, 2019. - World Health Organization. Diagnostic criteria and classification of hyperglycaemia first detected in pregnancy. 2013. http://apps.who.int/iris/bitstream/10665/85975/1/WHO_
NMH_MND_13.2_eng.pdf. Accessed November 18, 2019. - Hod M, Kapur A, Sacks DA, et al. The International Federation of Gynecology and Obstetrics (FIGO) Initiative on gestational diabetes mellitus: a pragmatic guide for diagnosis, management, and care. Int J Gynaecol Obstet. 2015;131(suppl 3):S173-S211.
- Berghella V, Caissutti C, Saccone G, et al. The One Step approach for diagnosing gestational diabetes is associated with better perinatal outcomes than using the Two Step approach: evidence of randomized clinical trials. Am J Obstet Gynecol. 2019;220:562-564.
- Berghella V, Caissutti C, Saccone G, et al. One-Step approach to identifying gestational diabetes mellitus: association with perinatal outcomes. Obstet Gynecol. 2019;133:383.
- American College of Obstetricians and Gynecologists. Committee on Practice Bulletins—Obstetrics. Practice Bulletin No. 180: gestational diabetes mellitus. Obstet Gynecol. 2017;130:e17-e31.
- Carpenter MW, Coustan DR. Criteria for screening tests for gestational diabetes. Am J Obstet Gynecol. 1982;144:768-773.
- National Diabetes Data Group. Classification and diagnosis of diabetes mellitus and other categories of glucose intolerance. Diabetes. 1979;28:1039-1057.
- Saccone G, Khalifeh A, Al-Kouatly HB, et al. Screening for gestational diabetes mellitus: one step versus two step approach. A meta-analysis of randomized trials. J Matern Fetal Neonatal Med. 2018:1-9.
- Saccone G, Caissutti C, Khalifeh A, et al. One step versus two step approach for gestational diabetes screening: systematic review and meta-analysis of the randomized trials. J Matern Fetal Neonatal Med. 2019;32:1547-1555.
- Khalifeh A, Eckler R, Felder L, et al. One-step versus two-step diagnostic testing for gestational diabetes: a randomized controlled trial. J Matern Fetal Neonatal Med. 2018:1-6.
- Caissutti C, Saccone G, Khalifeh A, et al. Which criteria should be used for starting pharmacologic therapy for management of gestational diabetes in pregnancy? Evidence from randomized controlled trials. J Matern Fetal Neonatal Med. 2019;32:2905-2914.
- Caissutti C, Saccone G, Ciardulli A, et al. Very tight vs. tight control: which should be the criteria for pharmacologic therapy dose adjustment in diabetes in pregnancy? Evidence from randomized controlled trials. Acta Obstet Gynecol Scand. 2018;97:235-247.
- Caissutti C, Khalifeh A, Saccone G, et al. Are women positive for the One Step but negative for the Two Step screening tests for gestational diabetes at higher risk for adverse outcomes? Acta Obstet Gynecol Scand. 2018;97:122-134.
Does planned early delivery make sense in women with preterm preeclampsia?
Chappell LC, Brocklehurst P, Green ME, et al; PHOENIX Study Group. Planned early delivery or expectant management for late preterm pre-eclampsia (PHOENIX): a randomised controlled trial. Lancet. 2019;394:1181-1190.
EXPERT COMMENTARY
Preeclampsia is a common hypertensive disorder of pregnancy. Among women who develop the disease at late preterm gestation, the question remains, “What is the optimal timing for delivery?” The American College of Obstetricians and Gynecologists (ACOG) categorizes preeclampsia as “with and without severe features.”1 Delivery is recommended for women with preeclampsia with severe features at or beyond 34 weeks’ gestation, and for women with preeclampsia without severe features at or beyond 37 weeks’ gestation.1 For patients with fetal growth restriction and preeclampsia, ACOG also recommends delivery between 34 and 37 weeks’ gestation.
Details of the study
Chappell and colleagues conducted a randomized controlled trial among women with singleton or dichorionic diamniotic twin pregnancy between 34 and 36.6 weeks’ gestation. Women were assigned to either planned delivery within 48 hours of randomization or expectant management until 37 weeks or earlier with clinical deterioration.
Among the 901 women included in the study, 450 were allocated to planned delivery and 451 to expectant management.
Study outcomes. The co-primary short-term maternal outcome was a composite of maternal morbidity with the addition of recorded systolic blood pressure of at least 160 mm Hg postrandomization (on any occasion). The co-primary short-term perinatal outcome was a composite of neonatal deaths within 7 days of delivery and perinatal deaths or neonatal unit admissions.
Participant details. At baseline, the average gestational age at randomization was 35.6 weeks, with equal distribution through the 3 weeks (34 through 36 weeks). About 37% of the women had severe hypertension (≥ 160 mm Hg) in the previous 48 hours prior to randomization, and approximately 22% had fetal growth restriction. The authors did not categorize the women based on severe features of preeclampsia.
Results. The investigators found that the proportion of women with the maternal co-primary outcome was significantly lower in the planned delivery group compared with the expectant management group (65% vs 75%), and the proportion of infants with the perinatal co-primary outcome was significantly higher in the planned delivery group compared with the expectant management group (42% vs 34%). The fact that early delivery led to more neonatal unit admissions for the infant, principally for a listed indication of prematurity and without an excess of respiratory or other morbidity, intensity of care, or length of stay, is very reassuring.
Study strengths and limitations
This is the largest study of women in this group allocated, randomized, and multicenter investigation addressing a very important clinical question. The patient population was mostly white, with only 13% black women, and had an average body mass index of 29 kg/m2 (which is low compared with many practices in the United States). The average difference between the 2 study groups was the additional prolongation of pregnancy from enrollment to delivery of only 3 days, which may not be clinically relevant. More than half of the women in the expectant management group had medically indicated delivery before 37 weeks’ gestation.
Continue to: A limitation of this study...
A limitation of this study is that all women with preeclampsia were considered the same—that is, no distinction was made between severe and nonsevere preeclampsia, and a significant proportion of women had severe hypertension at enrollment, which would make them ineligible for expectant management anyway.
The maternal composite outcome was driven mostly by severe hypertension and progression to severe preeclampsia (likely driven by severe hypertension). All other maternal outcomes were very rare or did not happen; however, the incidence of delivery indications for various preeclampsia-related complications was higher in the expectant management group.
The takeaway
In the absence of biomarkers for risk stratification and treatment of preeclampsia, delivering women who have a diagnosis of preeclampsia at or beyond 34 weeks’ gestation may be a viable option for preventing maternal complications.
In the United States, preeclampsia is categorized as severe or nonsevere, and gestational age at delivery depends on the type of preeclampsia. Clinicians should discuss expectant management after 34 weeks with patients who have preeclampsia without severe features, noting that this may decrease the chances for adverse maternal outcomes (mostly severe hypertension) at the cost of neonatal intensive care unit admission, which may depend on local practices. Attention also should be paid to particular patient populations (such as obese and African American women) who are at higher risk for developing adverse maternal outcomes. This may be particularly relevant in a smaller hospital setting in which patient follow-up may not be universal or access to a maternal-fetal medicine specialist may not be available to discuss management plans.
My personal take: I work in a large tertiary medical center. I worry about added prematurity, especially among women with superimposed preeclampsia where the diagnosis may be unclear. In my practice, we monitor patients with preeclampsia very closely, and with any signs of severe features we deliver them after 34 weeks. We follow ACOG guidelines for managing preeclampsia based on severity of disease and gestational age. I am not planning to immediately change my practice based on this study by Chappell and colleagues, and I will wait for results of long-term effects on neonatal outcomes, studies using biomarkers for risk assessment of women at risk for adverse outcomes, and opinions from ACOG and the Society for Maternal-Fetal Medicine about this management plan.
SAROSH RANA, MD, MPH
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins--Obstetrics. Gestational hypertension and preeclampsia. Obstet Gynecol. 2019;133:e1-e25.
Chappell LC, Brocklehurst P, Green ME, et al; PHOENIX Study Group. Planned early delivery or expectant management for late preterm pre-eclampsia (PHOENIX): a randomised controlled trial. Lancet. 2019;394:1181-1190.
EXPERT COMMENTARY
Preeclampsia is a common hypertensive disorder of pregnancy. Among women who develop the disease at late preterm gestation, the question remains, “What is the optimal timing for delivery?” The American College of Obstetricians and Gynecologists (ACOG) categorizes preeclampsia as “with and without severe features.”1 Delivery is recommended for women with preeclampsia with severe features at or beyond 34 weeks’ gestation, and for women with preeclampsia without severe features at or beyond 37 weeks’ gestation.1 For patients with fetal growth restriction and preeclampsia, ACOG also recommends delivery between 34 and 37 weeks’ gestation.
Details of the study
Chappell and colleagues conducted a randomized controlled trial among women with singleton or dichorionic diamniotic twin pregnancy between 34 and 36.6 weeks’ gestation. Women were assigned to either planned delivery within 48 hours of randomization or expectant management until 37 weeks or earlier with clinical deterioration.
Among the 901 women included in the study, 450 were allocated to planned delivery and 451 to expectant management.
Study outcomes. The co-primary short-term maternal outcome was a composite of maternal morbidity with the addition of recorded systolic blood pressure of at least 160 mm Hg postrandomization (on any occasion). The co-primary short-term perinatal outcome was a composite of neonatal deaths within 7 days of delivery and perinatal deaths or neonatal unit admissions.
Participant details. At baseline, the average gestational age at randomization was 35.6 weeks, with equal distribution through the 3 weeks (34 through 36 weeks). About 37% of the women had severe hypertension (≥ 160 mm Hg) in the previous 48 hours prior to randomization, and approximately 22% had fetal growth restriction. The authors did not categorize the women based on severe features of preeclampsia.
Results. The investigators found that the proportion of women with the maternal co-primary outcome was significantly lower in the planned delivery group compared with the expectant management group (65% vs 75%), and the proportion of infants with the perinatal co-primary outcome was significantly higher in the planned delivery group compared with the expectant management group (42% vs 34%). The fact that early delivery led to more neonatal unit admissions for the infant, principally for a listed indication of prematurity and without an excess of respiratory or other morbidity, intensity of care, or length of stay, is very reassuring.
Study strengths and limitations
This is the largest study of women in this group allocated, randomized, and multicenter investigation addressing a very important clinical question. The patient population was mostly white, with only 13% black women, and had an average body mass index of 29 kg/m2 (which is low compared with many practices in the United States). The average difference between the 2 study groups was the additional prolongation of pregnancy from enrollment to delivery of only 3 days, which may not be clinically relevant. More than half of the women in the expectant management group had medically indicated delivery before 37 weeks’ gestation.
Continue to: A limitation of this study...
A limitation of this study is that all women with preeclampsia were considered the same—that is, no distinction was made between severe and nonsevere preeclampsia, and a significant proportion of women had severe hypertension at enrollment, which would make them ineligible for expectant management anyway.
The maternal composite outcome was driven mostly by severe hypertension and progression to severe preeclampsia (likely driven by severe hypertension). All other maternal outcomes were very rare or did not happen; however, the incidence of delivery indications for various preeclampsia-related complications was higher in the expectant management group.
The takeaway
In the absence of biomarkers for risk stratification and treatment of preeclampsia, delivering women who have a diagnosis of preeclampsia at or beyond 34 weeks’ gestation may be a viable option for preventing maternal complications.
In the United States, preeclampsia is categorized as severe or nonsevere, and gestational age at delivery depends on the type of preeclampsia. Clinicians should discuss expectant management after 34 weeks with patients who have preeclampsia without severe features, noting that this may decrease the chances for adverse maternal outcomes (mostly severe hypertension) at the cost of neonatal intensive care unit admission, which may depend on local practices. Attention also should be paid to particular patient populations (such as obese and African American women) who are at higher risk for developing adverse maternal outcomes. This may be particularly relevant in a smaller hospital setting in which patient follow-up may not be universal or access to a maternal-fetal medicine specialist may not be available to discuss management plans.
My personal take: I work in a large tertiary medical center. I worry about added prematurity, especially among women with superimposed preeclampsia where the diagnosis may be unclear. In my practice, we monitor patients with preeclampsia very closely, and with any signs of severe features we deliver them after 34 weeks. We follow ACOG guidelines for managing preeclampsia based on severity of disease and gestational age. I am not planning to immediately change my practice based on this study by Chappell and colleagues, and I will wait for results of long-term effects on neonatal outcomes, studies using biomarkers for risk assessment of women at risk for adverse outcomes, and opinions from ACOG and the Society for Maternal-Fetal Medicine about this management plan.
SAROSH RANA, MD, MPH
Chappell LC, Brocklehurst P, Green ME, et al; PHOENIX Study Group. Planned early delivery or expectant management for late preterm pre-eclampsia (PHOENIX): a randomised controlled trial. Lancet. 2019;394:1181-1190.
EXPERT COMMENTARY
Preeclampsia is a common hypertensive disorder of pregnancy. Among women who develop the disease at late preterm gestation, the question remains, “What is the optimal timing for delivery?” The American College of Obstetricians and Gynecologists (ACOG) categorizes preeclampsia as “with and without severe features.”1 Delivery is recommended for women with preeclampsia with severe features at or beyond 34 weeks’ gestation, and for women with preeclampsia without severe features at or beyond 37 weeks’ gestation.1 For patients with fetal growth restriction and preeclampsia, ACOG also recommends delivery between 34 and 37 weeks’ gestation.
Details of the study
Chappell and colleagues conducted a randomized controlled trial among women with singleton or dichorionic diamniotic twin pregnancy between 34 and 36.6 weeks’ gestation. Women were assigned to either planned delivery within 48 hours of randomization or expectant management until 37 weeks or earlier with clinical deterioration.
Among the 901 women included in the study, 450 were allocated to planned delivery and 451 to expectant management.
Study outcomes. The co-primary short-term maternal outcome was a composite of maternal morbidity with the addition of recorded systolic blood pressure of at least 160 mm Hg postrandomization (on any occasion). The co-primary short-term perinatal outcome was a composite of neonatal deaths within 7 days of delivery and perinatal deaths or neonatal unit admissions.
Participant details. At baseline, the average gestational age at randomization was 35.6 weeks, with equal distribution through the 3 weeks (34 through 36 weeks). About 37% of the women had severe hypertension (≥ 160 mm Hg) in the previous 48 hours prior to randomization, and approximately 22% had fetal growth restriction. The authors did not categorize the women based on severe features of preeclampsia.
Results. The investigators found that the proportion of women with the maternal co-primary outcome was significantly lower in the planned delivery group compared with the expectant management group (65% vs 75%), and the proportion of infants with the perinatal co-primary outcome was significantly higher in the planned delivery group compared with the expectant management group (42% vs 34%). The fact that early delivery led to more neonatal unit admissions for the infant, principally for a listed indication of prematurity and without an excess of respiratory or other morbidity, intensity of care, or length of stay, is very reassuring.
Study strengths and limitations
This is the largest study of women in this group allocated, randomized, and multicenter investigation addressing a very important clinical question. The patient population was mostly white, with only 13% black women, and had an average body mass index of 29 kg/m2 (which is low compared with many practices in the United States). The average difference between the 2 study groups was the additional prolongation of pregnancy from enrollment to delivery of only 3 days, which may not be clinically relevant. More than half of the women in the expectant management group had medically indicated delivery before 37 weeks’ gestation.
Continue to: A limitation of this study...
A limitation of this study is that all women with preeclampsia were considered the same—that is, no distinction was made between severe and nonsevere preeclampsia, and a significant proportion of women had severe hypertension at enrollment, which would make them ineligible for expectant management anyway.
The maternal composite outcome was driven mostly by severe hypertension and progression to severe preeclampsia (likely driven by severe hypertension). All other maternal outcomes were very rare or did not happen; however, the incidence of delivery indications for various preeclampsia-related complications was higher in the expectant management group.
The takeaway
In the absence of biomarkers for risk stratification and treatment of preeclampsia, delivering women who have a diagnosis of preeclampsia at or beyond 34 weeks’ gestation may be a viable option for preventing maternal complications.
In the United States, preeclampsia is categorized as severe or nonsevere, and gestational age at delivery depends on the type of preeclampsia. Clinicians should discuss expectant management after 34 weeks with patients who have preeclampsia without severe features, noting that this may decrease the chances for adverse maternal outcomes (mostly severe hypertension) at the cost of neonatal intensive care unit admission, which may depend on local practices. Attention also should be paid to particular patient populations (such as obese and African American women) who are at higher risk for developing adverse maternal outcomes. This may be particularly relevant in a smaller hospital setting in which patient follow-up may not be universal or access to a maternal-fetal medicine specialist may not be available to discuss management plans.
My personal take: I work in a large tertiary medical center. I worry about added prematurity, especially among women with superimposed preeclampsia where the diagnosis may be unclear. In my practice, we monitor patients with preeclampsia very closely, and with any signs of severe features we deliver them after 34 weeks. We follow ACOG guidelines for managing preeclampsia based on severity of disease and gestational age. I am not planning to immediately change my practice based on this study by Chappell and colleagues, and I will wait for results of long-term effects on neonatal outcomes, studies using biomarkers for risk assessment of women at risk for adverse outcomes, and opinions from ACOG and the Society for Maternal-Fetal Medicine about this management plan.
SAROSH RANA, MD, MPH
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins--Obstetrics. Gestational hypertension and preeclampsia. Obstet Gynecol. 2019;133:e1-e25.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins--Obstetrics. Gestational hypertension and preeclampsia. Obstet Gynecol. 2019;133:e1-e25.
OTC hormonal contraception: An important goal in the fight for reproductive justice
A new American College of Obstetricians and Gynecologists (ACOG) committee opinion addresses how contraception access can be improved through over-the-counter (OTC) hormonal contraception for people of all ages—including oral contraceptive pills (OCPs), progesterone-only pills, the patch, vaginal rings, and depot medroxyprogesterone acetate (DMPA). Although ACOG endorses OTC contraception, some health care providers may be hesitant to support the increase in accessibility for a variety of reasons. We are hopeful that we address these concerns and that all clinicians can move to support ACOG’s position.
Easing access to hormonal contraception is a first step
OCPs are the most widely used contraception among teens and women of reproductive age in the United States.1 Although the Affordable Care Act (ACA) mandated health insurance coverage for contraception, many barriers continue to exist, including obtaining a prescription. Only 13 states have made it legal to obtain hormonal contraception through a pharmacist.2 There also has been an increase in the number of telemedicine and online services that deliver contraceptives to individuals’ homes. While these efforts have helped to decrease barriers to hormonal contraception access for some patients, they only reach a small segment of the population. As clinicians, we should strive to make contraception universally accessible and affordable to everyone who desires to use it. OTC provision can bring us closer to this goal.
Addressing the misconceptions about contraception
Adverse events with hormonal contraception are rarer than one may think. There are few risks associated with hormonal contraception. Venous thromboembolus (VTE) is a serious, although rare, adverse effect (AE) of hormonal contraception. The rate of VTE with combined oral contraception is estimated at 3 to 8 events per 10,000 patient-years, and VTE is even less common with progestin-only contraception (1 to 5 per 10,000 patient-years). For both types of hormonal contraception, the risk of VTE is smaller than with pregnancy, which is 5 to 20 per 10,000 patient-years.3 There are comorbidities that increase the risk of VTE and other AEs of hormonal contraception. In the setting of OTC hormonal contraception, individuals would self-screen for contraindications in order to reduce these complications.
Patients have the aptitude to self-screen for contraindications. Studies looking at the ability of patients over the age of 18 to self-screen for contraindications to hormonal contraception have found that patients do appropriately screen themselves. In fact, they are often more conservative than a physician in avoiding hormonal contraceptive methods.4 Patients younger than age 18 rarely have contraindications to hormonal contraception, but limited studies have shown that they too are able to successfully self-screen.5 ACOG recommends self-screening tools be provided with all OTC combined hormonal contraceptive methods to aid an individual’s contraceptive choice.
Most patients continue their well person care. Some opponents to ACOG’s position also have expressed concern that people who access their contraception OTC will forego their annual exam with their provider. However, studies have shown that the majority of people will continue to make their preventative health care visits.6,7
We need to invest in preventing unplanned pregnancy
Currently, hormonal contraception is covered by health insurance under the ACA, with some caveats. Without a prescription, patients may have to pay full price for their contraception. However, one can find generic OCPs for less than $10 per pack out of pocket. Any cost can be prohibitive to many patients; thus, transition to OTC access to contraception also should ensure limiting the cost to the patient. One possible solution to mitigate costs is to require insurance companies to cover the cost of OTC hormonal contraceptives. (See action item below.)
Reduction in unplanned pregnancies improves public health and public expense, and broadening access to effective forms of contraception is imperative in reducing unplanned pregnancies. Every $1 invested in contraception access realizes $7.09 in savings.8 By making hormonal contraception widely available OTC, access could be improved dramatically—although pharmacist provision of hormonal contraception may be a necessary intermediate step. ACOG’s most recent committee opinion encourages all reproductive health care providers to be strong advocates for this improvement in access. As women’s health providers, we should work to decrease access barriers for our patients; working toward OTC contraception is a critical step in equal access to birth control methods for all of our patients.
Action items
Remember, before a pill can move to OTC access, the manufacturing (pharmaceutical) company must submit an application to the US Food and Drug Administration to obtain this status. Once submitted, the process may take 3 to 4 years to be completed. Currently, no company has submitted an OTC application and no hormonal birth control is available OTC. Find resources for OTC birth control access here: http://ocsotc.org/ and www.freethepill.org.
- Talk to your state representatives about why both OTC birth control access and direct pharmacy availability are important to increasing access and decreasing disparities in reproductive health care. Find your local and federal representatives here and check the status of OCP access in your state here.
- Representative Ayanna Pressley (D-MA) and Senator Patty Murray (D-WA) both have introduced legislation—the Affordability is Access Act (HR 3296/S1847)—to ensure insurance coverage for OTC contraception. Call your representative and ask them to cosponsor this legislation.
- Be mindful of legislation that promotes OTC OCPs but limits access to some populations (minors) and increases cost sharing to the patient. This type of legislation can create harmful barriers to access for some of our patients
- Jones J, Mosher W, Daniels K. Current contraceptive use in the United States, 2006-2010, and changes in patterns of use since 1995. Natl Health Stat Rep. 2012;(60):1-25.
- Free the pill. What’s the law in your state? Ibis Reproductive Health website. http://freethepill.org/statepolicies. Accessed November 15, 2019.
- U.S. Food and Drug Administration. FDA Drug Safety Communication: updated information about the risk of blood clots in women taking birth control pills containing drospirenone. https://www.fda.gov/Drugs/DrugSafety/ucm299305.htm. Accessed November 15, 2019.
- Grossman D, Fernandez L, Hopkins K, et al. Accuracy of self-screening for contraindications to combined oral contraceptive use. Obstet Gynecol. 2008;112:572e8.
- Williams R, Hensel D, Lehmann A, et al. Adolescent self-screening for contraindications to combined oral contraceptive pills [abstract]. Contraception. 2015;92:380.
- Hopkins K, Grossman D, White K, et al. Reproductive health preventive screening among clinic vs. over-the-counter oral contraceptive users. Contraception. 2012;86:376-382.
- Grindlay K, Grossman D. Interest in over-the-counter access to a progestin-only pill among women in the United States. Womens Health Issues. 2018;28:144-151.
- Frost JJ, Sonfield A, Zolna MR, et al. Return on investment: a fuller assessment of the benefits and cost savings of the US publicly funded family planning program. Milbank Q. 2014;92:696-749.
A new American College of Obstetricians and Gynecologists (ACOG) committee opinion addresses how contraception access can be improved through over-the-counter (OTC) hormonal contraception for people of all ages—including oral contraceptive pills (OCPs), progesterone-only pills, the patch, vaginal rings, and depot medroxyprogesterone acetate (DMPA). Although ACOG endorses OTC contraception, some health care providers may be hesitant to support the increase in accessibility for a variety of reasons. We are hopeful that we address these concerns and that all clinicians can move to support ACOG’s position.
Easing access to hormonal contraception is a first step
OCPs are the most widely used contraception among teens and women of reproductive age in the United States.1 Although the Affordable Care Act (ACA) mandated health insurance coverage for contraception, many barriers continue to exist, including obtaining a prescription. Only 13 states have made it legal to obtain hormonal contraception through a pharmacist.2 There also has been an increase in the number of telemedicine and online services that deliver contraceptives to individuals’ homes. While these efforts have helped to decrease barriers to hormonal contraception access for some patients, they only reach a small segment of the population. As clinicians, we should strive to make contraception universally accessible and affordable to everyone who desires to use it. OTC provision can bring us closer to this goal.
Addressing the misconceptions about contraception
Adverse events with hormonal contraception are rarer than one may think. There are few risks associated with hormonal contraception. Venous thromboembolus (VTE) is a serious, although rare, adverse effect (AE) of hormonal contraception. The rate of VTE with combined oral contraception is estimated at 3 to 8 events per 10,000 patient-years, and VTE is even less common with progestin-only contraception (1 to 5 per 10,000 patient-years). For both types of hormonal contraception, the risk of VTE is smaller than with pregnancy, which is 5 to 20 per 10,000 patient-years.3 There are comorbidities that increase the risk of VTE and other AEs of hormonal contraception. In the setting of OTC hormonal contraception, individuals would self-screen for contraindications in order to reduce these complications.
Patients have the aptitude to self-screen for contraindications. Studies looking at the ability of patients over the age of 18 to self-screen for contraindications to hormonal contraception have found that patients do appropriately screen themselves. In fact, they are often more conservative than a physician in avoiding hormonal contraceptive methods.4 Patients younger than age 18 rarely have contraindications to hormonal contraception, but limited studies have shown that they too are able to successfully self-screen.5 ACOG recommends self-screening tools be provided with all OTC combined hormonal contraceptive methods to aid an individual’s contraceptive choice.
Most patients continue their well person care. Some opponents to ACOG’s position also have expressed concern that people who access their contraception OTC will forego their annual exam with their provider. However, studies have shown that the majority of people will continue to make their preventative health care visits.6,7
We need to invest in preventing unplanned pregnancy
Currently, hormonal contraception is covered by health insurance under the ACA, with some caveats. Without a prescription, patients may have to pay full price for their contraception. However, one can find generic OCPs for less than $10 per pack out of pocket. Any cost can be prohibitive to many patients; thus, transition to OTC access to contraception also should ensure limiting the cost to the patient. One possible solution to mitigate costs is to require insurance companies to cover the cost of OTC hormonal contraceptives. (See action item below.)
Reduction in unplanned pregnancies improves public health and public expense, and broadening access to effective forms of contraception is imperative in reducing unplanned pregnancies. Every $1 invested in contraception access realizes $7.09 in savings.8 By making hormonal contraception widely available OTC, access could be improved dramatically—although pharmacist provision of hormonal contraception may be a necessary intermediate step. ACOG’s most recent committee opinion encourages all reproductive health care providers to be strong advocates for this improvement in access. As women’s health providers, we should work to decrease access barriers for our patients; working toward OTC contraception is a critical step in equal access to birth control methods for all of our patients.
Action items
Remember, before a pill can move to OTC access, the manufacturing (pharmaceutical) company must submit an application to the US Food and Drug Administration to obtain this status. Once submitted, the process may take 3 to 4 years to be completed. Currently, no company has submitted an OTC application and no hormonal birth control is available OTC. Find resources for OTC birth control access here: http://ocsotc.org/ and www.freethepill.org.
- Talk to your state representatives about why both OTC birth control access and direct pharmacy availability are important to increasing access and decreasing disparities in reproductive health care. Find your local and federal representatives here and check the status of OCP access in your state here.
- Representative Ayanna Pressley (D-MA) and Senator Patty Murray (D-WA) both have introduced legislation—the Affordability is Access Act (HR 3296/S1847)—to ensure insurance coverage for OTC contraception. Call your representative and ask them to cosponsor this legislation.
- Be mindful of legislation that promotes OTC OCPs but limits access to some populations (minors) and increases cost sharing to the patient. This type of legislation can create harmful barriers to access for some of our patients
A new American College of Obstetricians and Gynecologists (ACOG) committee opinion addresses how contraception access can be improved through over-the-counter (OTC) hormonal contraception for people of all ages—including oral contraceptive pills (OCPs), progesterone-only pills, the patch, vaginal rings, and depot medroxyprogesterone acetate (DMPA). Although ACOG endorses OTC contraception, some health care providers may be hesitant to support the increase in accessibility for a variety of reasons. We are hopeful that we address these concerns and that all clinicians can move to support ACOG’s position.
Easing access to hormonal contraception is a first step
OCPs are the most widely used contraception among teens and women of reproductive age in the United States.1 Although the Affordable Care Act (ACA) mandated health insurance coverage for contraception, many barriers continue to exist, including obtaining a prescription. Only 13 states have made it legal to obtain hormonal contraception through a pharmacist.2 There also has been an increase in the number of telemedicine and online services that deliver contraceptives to individuals’ homes. While these efforts have helped to decrease barriers to hormonal contraception access for some patients, they only reach a small segment of the population. As clinicians, we should strive to make contraception universally accessible and affordable to everyone who desires to use it. OTC provision can bring us closer to this goal.
Addressing the misconceptions about contraception
Adverse events with hormonal contraception are rarer than one may think. There are few risks associated with hormonal contraception. Venous thromboembolus (VTE) is a serious, although rare, adverse effect (AE) of hormonal contraception. The rate of VTE with combined oral contraception is estimated at 3 to 8 events per 10,000 patient-years, and VTE is even less common with progestin-only contraception (1 to 5 per 10,000 patient-years). For both types of hormonal contraception, the risk of VTE is smaller than with pregnancy, which is 5 to 20 per 10,000 patient-years.3 There are comorbidities that increase the risk of VTE and other AEs of hormonal contraception. In the setting of OTC hormonal contraception, individuals would self-screen for contraindications in order to reduce these complications.
Patients have the aptitude to self-screen for contraindications. Studies looking at the ability of patients over the age of 18 to self-screen for contraindications to hormonal contraception have found that patients do appropriately screen themselves. In fact, they are often more conservative than a physician in avoiding hormonal contraceptive methods.4 Patients younger than age 18 rarely have contraindications to hormonal contraception, but limited studies have shown that they too are able to successfully self-screen.5 ACOG recommends self-screening tools be provided with all OTC combined hormonal contraceptive methods to aid an individual’s contraceptive choice.
Most patients continue their well person care. Some opponents to ACOG’s position also have expressed concern that people who access their contraception OTC will forego their annual exam with their provider. However, studies have shown that the majority of people will continue to make their preventative health care visits.6,7
We need to invest in preventing unplanned pregnancy
Currently, hormonal contraception is covered by health insurance under the ACA, with some caveats. Without a prescription, patients may have to pay full price for their contraception. However, one can find generic OCPs for less than $10 per pack out of pocket. Any cost can be prohibitive to many patients; thus, transition to OTC access to contraception also should ensure limiting the cost to the patient. One possible solution to mitigate costs is to require insurance companies to cover the cost of OTC hormonal contraceptives. (See action item below.)
Reduction in unplanned pregnancies improves public health and public expense, and broadening access to effective forms of contraception is imperative in reducing unplanned pregnancies. Every $1 invested in contraception access realizes $7.09 in savings.8 By making hormonal contraception widely available OTC, access could be improved dramatically—although pharmacist provision of hormonal contraception may be a necessary intermediate step. ACOG’s most recent committee opinion encourages all reproductive health care providers to be strong advocates for this improvement in access. As women’s health providers, we should work to decrease access barriers for our patients; working toward OTC contraception is a critical step in equal access to birth control methods for all of our patients.
Action items
Remember, before a pill can move to OTC access, the manufacturing (pharmaceutical) company must submit an application to the US Food and Drug Administration to obtain this status. Once submitted, the process may take 3 to 4 years to be completed. Currently, no company has submitted an OTC application and no hormonal birth control is available OTC. Find resources for OTC birth control access here: http://ocsotc.org/ and www.freethepill.org.
- Talk to your state representatives about why both OTC birth control access and direct pharmacy availability are important to increasing access and decreasing disparities in reproductive health care. Find your local and federal representatives here and check the status of OCP access in your state here.
- Representative Ayanna Pressley (D-MA) and Senator Patty Murray (D-WA) both have introduced legislation—the Affordability is Access Act (HR 3296/S1847)—to ensure insurance coverage for OTC contraception. Call your representative and ask them to cosponsor this legislation.
- Be mindful of legislation that promotes OTC OCPs but limits access to some populations (minors) and increases cost sharing to the patient. This type of legislation can create harmful barriers to access for some of our patients
- Jones J, Mosher W, Daniels K. Current contraceptive use in the United States, 2006-2010, and changes in patterns of use since 1995. Natl Health Stat Rep. 2012;(60):1-25.
- Free the pill. What’s the law in your state? Ibis Reproductive Health website. http://freethepill.org/statepolicies. Accessed November 15, 2019.
- U.S. Food and Drug Administration. FDA Drug Safety Communication: updated information about the risk of blood clots in women taking birth control pills containing drospirenone. https://www.fda.gov/Drugs/DrugSafety/ucm299305.htm. Accessed November 15, 2019.
- Grossman D, Fernandez L, Hopkins K, et al. Accuracy of self-screening for contraindications to combined oral contraceptive use. Obstet Gynecol. 2008;112:572e8.
- Williams R, Hensel D, Lehmann A, et al. Adolescent self-screening for contraindications to combined oral contraceptive pills [abstract]. Contraception. 2015;92:380.
- Hopkins K, Grossman D, White K, et al. Reproductive health preventive screening among clinic vs. over-the-counter oral contraceptive users. Contraception. 2012;86:376-382.
- Grindlay K, Grossman D. Interest in over-the-counter access to a progestin-only pill among women in the United States. Womens Health Issues. 2018;28:144-151.
- Frost JJ, Sonfield A, Zolna MR, et al. Return on investment: a fuller assessment of the benefits and cost savings of the US publicly funded family planning program. Milbank Q. 2014;92:696-749.
- Jones J, Mosher W, Daniels K. Current contraceptive use in the United States, 2006-2010, and changes in patterns of use since 1995. Natl Health Stat Rep. 2012;(60):1-25.
- Free the pill. What’s the law in your state? Ibis Reproductive Health website. http://freethepill.org/statepolicies. Accessed November 15, 2019.
- U.S. Food and Drug Administration. FDA Drug Safety Communication: updated information about the risk of blood clots in women taking birth control pills containing drospirenone. https://www.fda.gov/Drugs/DrugSafety/ucm299305.htm. Accessed November 15, 2019.
- Grossman D, Fernandez L, Hopkins K, et al. Accuracy of self-screening for contraindications to combined oral contraceptive use. Obstet Gynecol. 2008;112:572e8.
- Williams R, Hensel D, Lehmann A, et al. Adolescent self-screening for contraindications to combined oral contraceptive pills [abstract]. Contraception. 2015;92:380.
- Hopkins K, Grossman D, White K, et al. Reproductive health preventive screening among clinic vs. over-the-counter oral contraceptive users. Contraception. 2012;86:376-382.
- Grindlay K, Grossman D. Interest in over-the-counter access to a progestin-only pill among women in the United States. Womens Health Issues. 2018;28:144-151.
- Frost JJ, Sonfield A, Zolna MR, et al. Return on investment: a fuller assessment of the benefits and cost savings of the US publicly funded family planning program. Milbank Q. 2014;92:696-749.
Does BSO status affect health outcomes for women taking estrogen for menopause?
Do health effects of menopausal estrogen therapy differ between women with bilateral oophorectomy versus those with conserved ovaries? To answer this question a group of investigators performed a subanalysis of the Women’s Health Initiative (WHI) Estrogen-Alone Trial,1 which included 40 clinical centers across the United States. They examined estrogen therapy outcomes by bilateral salpingo-oophorectomy (BSO) status, with additional stratification by 10-year age groups in 9,939 women aged 50 to 79 years with prior hysterectomy and known oophorectomy status. In the WHI trial, women were randomly assigned to conjugated equine estrogens (CEE) 0.625 mg/d or placebo for a median of 7.2 years. Investigators assessed the incidence of coronary heart disease and invasive breast cancer (the trial’s 2 primary end points), all-cause mortality, and a “global index”—these end points plus stroke, pulmonary embolism, colorectal cancer, and hip fracture—during the intervention phase and 18-year cumulative follow-up.
OBG Management caught up with lead author JoAnn E. Manson, MD, DrPH, NCMP, to discuss the study’s results.
OBG Management : How many women undergo BSO with their hysterectomy?
Dr. JoAnn E. Manson, MD, DrPH, NCMP: Of the 425,000 women who undergo hysterectomy in the United States for benign reasons each year,2,3 about 40% of them undergo BSO—so between 150,000 and 200,000 women per year undergo BSO with their hysterectomy.4,5
OBG Management : Although BSO is performed with hysterectomy to minimize patients’ future ovarian cancer risk, does BSO have health risks of its own, and how has estrogen been shown to affect these risks?
Dr. Manson: First, yes, BSO has been associated with health risks, especially when it is performed at a young age, such as before age 45. It has been linked to an increased risk of heart disease, osteoporosis, cognitive decline, and all-cause mortality. According to observational studies, estrogen therapy appears to offset many of these risks, particularly those related to heart disease and osteoporosis (the evidence is less clear on cognitive deficits).5
OBG Management : What did you find in your trial when you randomly assigned women in the age groups of 50 to 79 who underwent hysterectomy with and without BSO to estrogen therapy or placebo?
Dr. Manson: The WHI is the first study to be conducted in a randomized trial setting to analyze the health risks and benefits of estrogen therapy according to whether or not women had their ovaries removed. What we found was that the woman’s age had a strong influence on the effects of estrogen therapy among women who had BSO but only a negligible effect among women who had conserved ovaries. Overall, across the full age range, the effects of estrogen therapy did not differ substantially between women who had a BSO and those who had their ovaries conserved.
However, there were major differences by age group among the women who had BSO. A significant 32% reduction in all-cause mortality emerged during the 18-year follow-up period among the younger women (below age 60) who had BSO when they received estrogen therapy as compared with placebo. By contrast, the women who had conserved ovaries did not have this significant reduction in all-cause mortality, or in most of the other outcomes on estrogen compared with placebo. Overall, the effects of estrogen therapy tended to be relatively neutral in the women with conserved ovaries.
Now, the reduction in all-cause mortality with estrogen therapy was particularly pronounced among women who had BSO before age 45. They had a 40% statistically significant reduction in all-cause mortality with estrogen therapy compared with placebo. Also, among the women with BSO, there was a strong association between the timing of estrogen initiation and the magnitude of reduction in mortality. Women who started the estrogen therapy within 10 years of having the BSO had a 34% significant reduction in all-cause mortality, and those who started estrogen more than 20 years after having their ovaries removed had no reduction in mortality.
Continue to:
OBG Management : Do your data give support to the timing hypothesis?
Dr. Manson: Yes, our findings do support a timing hypothesis that was particularly pronounced for women who underwent BSO. It was the women who had early surgical menopause (before age 45) and those who started the estrogen therapy within 10 years of having their ovaries removed who had the greatest reduction in all-cause mortality and the most favorable benefit-risk profile from hormone therapy. So, the results do lend support to the timing hypothesis.
By contrast, women who had BSO at hysterectomy and began hormone therapy at age 70 or older had net adverse effects from hormone therapy. They posted a 40% increase in the global index—which is a summary measure of adverse effects on cardiovascular disease, cancer, and other major health outcomes. So, the women with BSO who were randomized in the trial at age 70 and older, had unfavorable results from estrogen therapy and an increase in the global index, in contrast to the women who were below age 60 or within 10 years of menopause.
OBG Management : Given your study findings, in which women would you recommend estrogen therapy? And are there groups of women in which you would advise avoiding estrogen therapy?
Dr. Manson: Current guidelines6,7 recommend estrogen therapy for women who have early menopause, particularly an early surgical menopause and BSO prior to the average age at natural menopause. Unless the woman has contraindications to estrogen therapy, the recommendations are to treat with estrogen until the average age of menopause—until about age 50 to 51.
Our study findings provide reassurance that, if a woman continues to have indications for estrogen (vasomotor symptoms, or other indications for estrogen therapy), there is relative safety of continuing estrogen-alone therapy through her 50s, until age 60. For example, a woman who, after the average age of menopause continues to have vasomotor symptoms, or if she has bone health problems, our study would suggest that estrogen therapy would continue to have a favorable benefit-risk profile until at least the age of 60. Decisions would have to be individualized, especially after age 60, with shared decision-making particularly important for those decisions. (Some women, depending on their risk profile, may continue to be candidates for estrogen therapy past age 60.)
So, this study provides reassurance regarding use of estrogen therapy for women in their 50s if they have had BSO. Actually, the women who had conserved ovaries also had relative safety with estrogen therapy until age 60. They just didn’t show the significant benefits for all-cause mortality. Overall, their pattern of health-related benefits and risks was neutral. Thus, if vasomotor symptom management, quality of life benefits, or bone health effects are sought, taking hormone therapy is a quite reasonable choice for these women.
By contrast, women who have had a BSO and are age 70 or older should really avoid initiating estrogen therapy because it would follow a prolonged period of estrogen deficiency, or very low estrogen levels, and these women appeared to have a net adverse effect from initiating hormone therapy (with increases in the global index found).
Continue to:
OBG Management : Did taking estrogen therapy prior to trial enrollment make a difference when it came to study outcomes?
Dr. Manson: We found minimal if any effect in our analyses. In fact, even the women who did not have prior (pre-randomization) use of estrogen therapy tended to do well on estrogen-alone therapy if they were younger than age 60. This was particularly true for the women who had BSO. Even if they had not used estrogen previously, and they were many years past the BSO, they still did well on estrogen therapy if they were below age 60.
1. Manson JE, Aragaki AK, Bassuk SS. Menopausal estrogen-alone therapy and health outcomes in women with and without bilateral oophorectomy: a randomized trial. Ann Intern Med. 2019 September 10. doi:10.7326/M19-0274.
2. Einarsson J. Are hysterectomy volumes in the US really falling? Contemporary OB/GYN. 1 September 2017. www.contemporaryobgyn.net/gynecology/are-hysterectomy-volumes-us-really-falling. November 4, 2019.
3. Temkin SM, Minasian L, Noone AM. The end of the hysterectomy epidemic and endometrial cancer incidence: what are the unintended consequences of declining hysterectomy rates? Front Oncol. 2016;6:89.
4. Doll KM, Dusetzina SB, Robinson W. Trends in inpatient and outpatient hysterectomy and oophorectomy rates among commercially insured women in the United States, 2000-2014. JAMA Surg. 2016;151:876-877.
5. Adelman MR, Sharp HT. Ovarian conservation vs removal at the time of benign hysterectomy. Am J Obstet Gynecol. 2018;218:269-279.
6. ACOG Practice Bulletin No. 141: management of menopausal symptoms [published corrections appear in: Obstet Gynecol. 2016;127(1):166. and Obstet Gynecol. 2018;131(3):604]. Obstet Gynecol. 2014;123:202-216.
7. The 2017 hormone therapy position statement of The North American Menopause Society. Menopause. 2017;24:728-753.
Do health effects of menopausal estrogen therapy differ between women with bilateral oophorectomy versus those with conserved ovaries? To answer this question a group of investigators performed a subanalysis of the Women’s Health Initiative (WHI) Estrogen-Alone Trial,1 which included 40 clinical centers across the United States. They examined estrogen therapy outcomes by bilateral salpingo-oophorectomy (BSO) status, with additional stratification by 10-year age groups in 9,939 women aged 50 to 79 years with prior hysterectomy and known oophorectomy status. In the WHI trial, women were randomly assigned to conjugated equine estrogens (CEE) 0.625 mg/d or placebo for a median of 7.2 years. Investigators assessed the incidence of coronary heart disease and invasive breast cancer (the trial’s 2 primary end points), all-cause mortality, and a “global index”—these end points plus stroke, pulmonary embolism, colorectal cancer, and hip fracture—during the intervention phase and 18-year cumulative follow-up.
OBG Management caught up with lead author JoAnn E. Manson, MD, DrPH, NCMP, to discuss the study’s results.
OBG Management : How many women undergo BSO with their hysterectomy?
Dr. JoAnn E. Manson, MD, DrPH, NCMP: Of the 425,000 women who undergo hysterectomy in the United States for benign reasons each year,2,3 about 40% of them undergo BSO—so between 150,000 and 200,000 women per year undergo BSO with their hysterectomy.4,5
OBG Management : Although BSO is performed with hysterectomy to minimize patients’ future ovarian cancer risk, does BSO have health risks of its own, and how has estrogen been shown to affect these risks?
Dr. Manson: First, yes, BSO has been associated with health risks, especially when it is performed at a young age, such as before age 45. It has been linked to an increased risk of heart disease, osteoporosis, cognitive decline, and all-cause mortality. According to observational studies, estrogen therapy appears to offset many of these risks, particularly those related to heart disease and osteoporosis (the evidence is less clear on cognitive deficits).5
OBG Management : What did you find in your trial when you randomly assigned women in the age groups of 50 to 79 who underwent hysterectomy with and without BSO to estrogen therapy or placebo?
Dr. Manson: The WHI is the first study to be conducted in a randomized trial setting to analyze the health risks and benefits of estrogen therapy according to whether or not women had their ovaries removed. What we found was that the woman’s age had a strong influence on the effects of estrogen therapy among women who had BSO but only a negligible effect among women who had conserved ovaries. Overall, across the full age range, the effects of estrogen therapy did not differ substantially between women who had a BSO and those who had their ovaries conserved.
However, there were major differences by age group among the women who had BSO. A significant 32% reduction in all-cause mortality emerged during the 18-year follow-up period among the younger women (below age 60) who had BSO when they received estrogen therapy as compared with placebo. By contrast, the women who had conserved ovaries did not have this significant reduction in all-cause mortality, or in most of the other outcomes on estrogen compared with placebo. Overall, the effects of estrogen therapy tended to be relatively neutral in the women with conserved ovaries.
Now, the reduction in all-cause mortality with estrogen therapy was particularly pronounced among women who had BSO before age 45. They had a 40% statistically significant reduction in all-cause mortality with estrogen therapy compared with placebo. Also, among the women with BSO, there was a strong association between the timing of estrogen initiation and the magnitude of reduction in mortality. Women who started the estrogen therapy within 10 years of having the BSO had a 34% significant reduction in all-cause mortality, and those who started estrogen more than 20 years after having their ovaries removed had no reduction in mortality.
Continue to:
OBG Management : Do your data give support to the timing hypothesis?
Dr. Manson: Yes, our findings do support a timing hypothesis that was particularly pronounced for women who underwent BSO. It was the women who had early surgical menopause (before age 45) and those who started the estrogen therapy within 10 years of having their ovaries removed who had the greatest reduction in all-cause mortality and the most favorable benefit-risk profile from hormone therapy. So, the results do lend support to the timing hypothesis.
By contrast, women who had BSO at hysterectomy and began hormone therapy at age 70 or older had net adverse effects from hormone therapy. They posted a 40% increase in the global index—which is a summary measure of adverse effects on cardiovascular disease, cancer, and other major health outcomes. So, the women with BSO who were randomized in the trial at age 70 and older, had unfavorable results from estrogen therapy and an increase in the global index, in contrast to the women who were below age 60 or within 10 years of menopause.
OBG Management : Given your study findings, in which women would you recommend estrogen therapy? And are there groups of women in which you would advise avoiding estrogen therapy?
Dr. Manson: Current guidelines6,7 recommend estrogen therapy for women who have early menopause, particularly an early surgical menopause and BSO prior to the average age at natural menopause. Unless the woman has contraindications to estrogen therapy, the recommendations are to treat with estrogen until the average age of menopause—until about age 50 to 51.
Our study findings provide reassurance that, if a woman continues to have indications for estrogen (vasomotor symptoms, or other indications for estrogen therapy), there is relative safety of continuing estrogen-alone therapy through her 50s, until age 60. For example, a woman who, after the average age of menopause continues to have vasomotor symptoms, or if she has bone health problems, our study would suggest that estrogen therapy would continue to have a favorable benefit-risk profile until at least the age of 60. Decisions would have to be individualized, especially after age 60, with shared decision-making particularly important for those decisions. (Some women, depending on their risk profile, may continue to be candidates for estrogen therapy past age 60.)
So, this study provides reassurance regarding use of estrogen therapy for women in their 50s if they have had BSO. Actually, the women who had conserved ovaries also had relative safety with estrogen therapy until age 60. They just didn’t show the significant benefits for all-cause mortality. Overall, their pattern of health-related benefits and risks was neutral. Thus, if vasomotor symptom management, quality of life benefits, or bone health effects are sought, taking hormone therapy is a quite reasonable choice for these women.
By contrast, women who have had a BSO and are age 70 or older should really avoid initiating estrogen therapy because it would follow a prolonged period of estrogen deficiency, or very low estrogen levels, and these women appeared to have a net adverse effect from initiating hormone therapy (with increases in the global index found).
Continue to:
OBG Management : Did taking estrogen therapy prior to trial enrollment make a difference when it came to study outcomes?
Dr. Manson: We found minimal if any effect in our analyses. In fact, even the women who did not have prior (pre-randomization) use of estrogen therapy tended to do well on estrogen-alone therapy if they were younger than age 60. This was particularly true for the women who had BSO. Even if they had not used estrogen previously, and they were many years past the BSO, they still did well on estrogen therapy if they were below age 60.
Do health effects of menopausal estrogen therapy differ between women with bilateral oophorectomy versus those with conserved ovaries? To answer this question a group of investigators performed a subanalysis of the Women’s Health Initiative (WHI) Estrogen-Alone Trial,1 which included 40 clinical centers across the United States. They examined estrogen therapy outcomes by bilateral salpingo-oophorectomy (BSO) status, with additional stratification by 10-year age groups in 9,939 women aged 50 to 79 years with prior hysterectomy and known oophorectomy status. In the WHI trial, women were randomly assigned to conjugated equine estrogens (CEE) 0.625 mg/d or placebo for a median of 7.2 years. Investigators assessed the incidence of coronary heart disease and invasive breast cancer (the trial’s 2 primary end points), all-cause mortality, and a “global index”—these end points plus stroke, pulmonary embolism, colorectal cancer, and hip fracture—during the intervention phase and 18-year cumulative follow-up.
OBG Management caught up with lead author JoAnn E. Manson, MD, DrPH, NCMP, to discuss the study’s results.
OBG Management : How many women undergo BSO with their hysterectomy?
Dr. JoAnn E. Manson, MD, DrPH, NCMP: Of the 425,000 women who undergo hysterectomy in the United States for benign reasons each year,2,3 about 40% of them undergo BSO—so between 150,000 and 200,000 women per year undergo BSO with their hysterectomy.4,5
OBG Management : Although BSO is performed with hysterectomy to minimize patients’ future ovarian cancer risk, does BSO have health risks of its own, and how has estrogen been shown to affect these risks?
Dr. Manson: First, yes, BSO has been associated with health risks, especially when it is performed at a young age, such as before age 45. It has been linked to an increased risk of heart disease, osteoporosis, cognitive decline, and all-cause mortality. According to observational studies, estrogen therapy appears to offset many of these risks, particularly those related to heart disease and osteoporosis (the evidence is less clear on cognitive deficits).5
OBG Management : What did you find in your trial when you randomly assigned women in the age groups of 50 to 79 who underwent hysterectomy with and without BSO to estrogen therapy or placebo?
Dr. Manson: The WHI is the first study to be conducted in a randomized trial setting to analyze the health risks and benefits of estrogen therapy according to whether or not women had their ovaries removed. What we found was that the woman’s age had a strong influence on the effects of estrogen therapy among women who had BSO but only a negligible effect among women who had conserved ovaries. Overall, across the full age range, the effects of estrogen therapy did not differ substantially between women who had a BSO and those who had their ovaries conserved.
However, there were major differences by age group among the women who had BSO. A significant 32% reduction in all-cause mortality emerged during the 18-year follow-up period among the younger women (below age 60) who had BSO when they received estrogen therapy as compared with placebo. By contrast, the women who had conserved ovaries did not have this significant reduction in all-cause mortality, or in most of the other outcomes on estrogen compared with placebo. Overall, the effects of estrogen therapy tended to be relatively neutral in the women with conserved ovaries.
Now, the reduction in all-cause mortality with estrogen therapy was particularly pronounced among women who had BSO before age 45. They had a 40% statistically significant reduction in all-cause mortality with estrogen therapy compared with placebo. Also, among the women with BSO, there was a strong association between the timing of estrogen initiation and the magnitude of reduction in mortality. Women who started the estrogen therapy within 10 years of having the BSO had a 34% significant reduction in all-cause mortality, and those who started estrogen more than 20 years after having their ovaries removed had no reduction in mortality.
Continue to:
OBG Management : Do your data give support to the timing hypothesis?
Dr. Manson: Yes, our findings do support a timing hypothesis that was particularly pronounced for women who underwent BSO. It was the women who had early surgical menopause (before age 45) and those who started the estrogen therapy within 10 years of having their ovaries removed who had the greatest reduction in all-cause mortality and the most favorable benefit-risk profile from hormone therapy. So, the results do lend support to the timing hypothesis.
By contrast, women who had BSO at hysterectomy and began hormone therapy at age 70 or older had net adverse effects from hormone therapy. They posted a 40% increase in the global index—which is a summary measure of adverse effects on cardiovascular disease, cancer, and other major health outcomes. So, the women with BSO who were randomized in the trial at age 70 and older, had unfavorable results from estrogen therapy and an increase in the global index, in contrast to the women who were below age 60 or within 10 years of menopause.
OBG Management : Given your study findings, in which women would you recommend estrogen therapy? And are there groups of women in which you would advise avoiding estrogen therapy?
Dr. Manson: Current guidelines6,7 recommend estrogen therapy for women who have early menopause, particularly an early surgical menopause and BSO prior to the average age at natural menopause. Unless the woman has contraindications to estrogen therapy, the recommendations are to treat with estrogen until the average age of menopause—until about age 50 to 51.
Our study findings provide reassurance that, if a woman continues to have indications for estrogen (vasomotor symptoms, or other indications for estrogen therapy), there is relative safety of continuing estrogen-alone therapy through her 50s, until age 60. For example, a woman who, after the average age of menopause continues to have vasomotor symptoms, or if she has bone health problems, our study would suggest that estrogen therapy would continue to have a favorable benefit-risk profile until at least the age of 60. Decisions would have to be individualized, especially after age 60, with shared decision-making particularly important for those decisions. (Some women, depending on their risk profile, may continue to be candidates for estrogen therapy past age 60.)
So, this study provides reassurance regarding use of estrogen therapy for women in their 50s if they have had BSO. Actually, the women who had conserved ovaries also had relative safety with estrogen therapy until age 60. They just didn’t show the significant benefits for all-cause mortality. Overall, their pattern of health-related benefits and risks was neutral. Thus, if vasomotor symptom management, quality of life benefits, or bone health effects are sought, taking hormone therapy is a quite reasonable choice for these women.
By contrast, women who have had a BSO and are age 70 or older should really avoid initiating estrogen therapy because it would follow a prolonged period of estrogen deficiency, or very low estrogen levels, and these women appeared to have a net adverse effect from initiating hormone therapy (with increases in the global index found).
Continue to:
OBG Management : Did taking estrogen therapy prior to trial enrollment make a difference when it came to study outcomes?
Dr. Manson: We found minimal if any effect in our analyses. In fact, even the women who did not have prior (pre-randomization) use of estrogen therapy tended to do well on estrogen-alone therapy if they were younger than age 60. This was particularly true for the women who had BSO. Even if they had not used estrogen previously, and they were many years past the BSO, they still did well on estrogen therapy if they were below age 60.
1. Manson JE, Aragaki AK, Bassuk SS. Menopausal estrogen-alone therapy and health outcomes in women with and without bilateral oophorectomy: a randomized trial. Ann Intern Med. 2019 September 10. doi:10.7326/M19-0274.
2. Einarsson J. Are hysterectomy volumes in the US really falling? Contemporary OB/GYN. 1 September 2017. www.contemporaryobgyn.net/gynecology/are-hysterectomy-volumes-us-really-falling. November 4, 2019.
3. Temkin SM, Minasian L, Noone AM. The end of the hysterectomy epidemic and endometrial cancer incidence: what are the unintended consequences of declining hysterectomy rates? Front Oncol. 2016;6:89.
4. Doll KM, Dusetzina SB, Robinson W. Trends in inpatient and outpatient hysterectomy and oophorectomy rates among commercially insured women in the United States, 2000-2014. JAMA Surg. 2016;151:876-877.
5. Adelman MR, Sharp HT. Ovarian conservation vs removal at the time of benign hysterectomy. Am J Obstet Gynecol. 2018;218:269-279.
6. ACOG Practice Bulletin No. 141: management of menopausal symptoms [published corrections appear in: Obstet Gynecol. 2016;127(1):166. and Obstet Gynecol. 2018;131(3):604]. Obstet Gynecol. 2014;123:202-216.
7. The 2017 hormone therapy position statement of The North American Menopause Society. Menopause. 2017;24:728-753.
1. Manson JE, Aragaki AK, Bassuk SS. Menopausal estrogen-alone therapy and health outcomes in women with and without bilateral oophorectomy: a randomized trial. Ann Intern Med. 2019 September 10. doi:10.7326/M19-0274.
2. Einarsson J. Are hysterectomy volumes in the US really falling? Contemporary OB/GYN. 1 September 2017. www.contemporaryobgyn.net/gynecology/are-hysterectomy-volumes-us-really-falling. November 4, 2019.
3. Temkin SM, Minasian L, Noone AM. The end of the hysterectomy epidemic and endometrial cancer incidence: what are the unintended consequences of declining hysterectomy rates? Front Oncol. 2016;6:89.
4. Doll KM, Dusetzina SB, Robinson W. Trends in inpatient and outpatient hysterectomy and oophorectomy rates among commercially insured women in the United States, 2000-2014. JAMA Surg. 2016;151:876-877.
5. Adelman MR, Sharp HT. Ovarian conservation vs removal at the time of benign hysterectomy. Am J Obstet Gynecol. 2018;218:269-279.
6. ACOG Practice Bulletin No. 141: management of menopausal symptoms [published corrections appear in: Obstet Gynecol. 2016;127(1):166. and Obstet Gynecol. 2018;131(3):604]. Obstet Gynecol. 2014;123:202-216.
7. The 2017 hormone therapy position statement of The North American Menopause Society. Menopause. 2017;24:728-753.