User login
Daily calorie intake requirements during pregnancy: Does one size fit all?
Most J, St Amant M, Hsia DS, et al. Evidence-based recommendations for energy intake in pregnant women with obesity. J Clin Invest. 2019;129:4682-4690.
EXPERT COMMENTARY
In 2009, the Institute of Medicine, now known as the National Academy of Medicine, updated its gestational weight gain guideline. This guideline’s major difference, compared with the 1990 guideline, is a specific weight gain range for women with obesity: 5 to 9 kg, or 11 to 20 lb.1 This weight gain range was chosen in part because it allows for a minimum weight gain that supports the growth and development of tissues (fetus, placenta, breast, uterus) and fluids (blood volume, intracellular and extracellular fluid), also known as the “fat-free” mass.
Many studies have since shown not only associations between lower-than-guideline-recommended weight gain and improved pregnancy outcomes (for example, reductions in preeclampsia and cesarean deliveries), but also increases in low birth weight for infants of women with obesity.2,3 Although the weight gain guideline differs based on a woman’s prepregnancy body mass index, the energy requirements, or how many additional calories a woman should consume daily, are the same for all, regardless of weight prior to pregnancy: an increase by 340 to 452 kcal/day in the second and third trimesters.1
Recently, Most and colleagues challenged this recommendation for energy requirements with results from their prospective observational study of 54 women with obesity during pregnancy.4 They aimed to evaluate energy intake with the energy intake-balance method (doubly labeled water and whole-room indirect calorimetry and body composition) according to tests done at 13 to 16 weeks’ gestation and 35 to 37 weeks’ gestation and according to the current National Academy of Medicine gestational weight gain guideline (inadequate, recommended, or excessive weight gain groups).4
Details of the study
Women who participated in this study were recruited from the Pennington Biomedical Research Center in Louisiana and were mostly multiparas (57%); about half had a college degree or higher (52%) and 41% were African American. The investigators found that gestational weight gain in their participants was similar to that found in other large epidemiologic studies in that 67% of women had excessive gestational weight gain.5
Findings. For women who gained the recommended amount of weight (n = 8), mean (SD) daily energy intake was 2,698 (99) kcal/day and energy expenditure was 2,824 (105) kcal/day. Therefore, to meet the recommended amount of weight gain, these women had a negative energy balance (-125 [52] kcal/day). Women with inadequate weight gain
(n = 10) also had a negative energy balance (-262 [32] kcal/day), but the difference was not significantly different compared with that in the recommended gestational weight gain group (P = .08). By contrast, women with excessive gestational weight gain (n = 36) had a mean (SD) positive energy balance of 186 (29) kcal/day.
Fat-free mass and fat mass weight gains. The body weight gains of the fat-free and fat mass compartments also were compared with linear mixed effect models among the 3 weight gain groups. There were no differences in the amount of fat-free mass gained among the 3 weight gain groups (P>.05), but women with excessive gestational weight gain had significantly higher increases in fat mass compared with the other 2 weight gain groups (P<.001).
Pregnancy outcomes. Although there were no differences in cesarean deliveries or birth weight among the 3 weight gain groups, the study was not powered to detect these differences.
Continue to: Study strengths and limitations...
Study strengths and limitations
It is important to note that this study by Most and colleagues was not a health behavior intervention for gestational weight gain. Women who participated in the study did not receive specific directions or advice on diet or physical activity. Furthermore, the study used the current gestational weight gain guideline as a reference to determine energy intake. As such, findings from this study alone cannot be used to adapt the current gestational weight gain guideline for women with obesity.
The study methods were rigorous in terms of the energy intake measurements, but a larger and more diverse sample size is needed to confirm the study findings.
Most and colleagues’ data suggest that maintaining energy balance can support obligatory growth and development of women and their fetuses during pregnancy (fat-free mass). In doing so, women with obesity meet the current gestational weight gain guideline. It is hoped that this important research will be used in future studies, with larger sample sizes, to evaluate energy requirements during pregnancy, especially in women with different classes of obesity. Ultimately, these new recommendations for energy requirements should be combined with studies of health behavior interventions for gestational weight gain.
The study by Most and colleagues supports the concept that energy requirements need to be individualized for women to meet the recommended amount of gestational weight gain. If women meet their gestational weight gain goals, they have the potential to improve their health and the health of their offspring.
MICHELLE A. KOMINIAREK, MD, MS
- Institute of Medicine and National Research Council Committee to Reexamine IOM Pregnacy Weight Guidelines. Rasmussen KM, Yaktine AL, eds. Weight Gain During Pregnancy: Reexamining the Guidelines. Washington, DC: National Academies Press; 2009.
- Kapadia MZ, Park CK, Beyene J, et al. Weight loss instead of weight gain within the guidelines in obese women during pregnancy: a systematic review and metaanalyses of maternal and infant outcomes. PLoS One. 2015;10:e0132650.
- Kapadia MZ, Park CK, Beyene J, et al. Can we safely recommend gestational weight gain below the 2009 guidelines in obese women? A systematic review and metaanalysis. Obes Rev. 2015;16:189-206.
- Most J, St Amant M, Hsia DS, et al. Evidence-based recommendations for energy intake in pregnant women with obesity. J Clin Invest. 2019;129:4682-4690.
- Deputy NP, Sharma AJ, Kim SY, et al. Prevalence and characteristics associated with gestational weight gain adequacy. Obstet Gynecol. 2015;125:773-781.
Most J, St Amant M, Hsia DS, et al. Evidence-based recommendations for energy intake in pregnant women with obesity. J Clin Invest. 2019;129:4682-4690.
EXPERT COMMENTARY
In 2009, the Institute of Medicine, now known as the National Academy of Medicine, updated its gestational weight gain guideline. This guideline’s major difference, compared with the 1990 guideline, is a specific weight gain range for women with obesity: 5 to 9 kg, or 11 to 20 lb.1 This weight gain range was chosen in part because it allows for a minimum weight gain that supports the growth and development of tissues (fetus, placenta, breast, uterus) and fluids (blood volume, intracellular and extracellular fluid), also known as the “fat-free” mass.
Many studies have since shown not only associations between lower-than-guideline-recommended weight gain and improved pregnancy outcomes (for example, reductions in preeclampsia and cesarean deliveries), but also increases in low birth weight for infants of women with obesity.2,3 Although the weight gain guideline differs based on a woman’s prepregnancy body mass index, the energy requirements, or how many additional calories a woman should consume daily, are the same for all, regardless of weight prior to pregnancy: an increase by 340 to 452 kcal/day in the second and third trimesters.1
Recently, Most and colleagues challenged this recommendation for energy requirements with results from their prospective observational study of 54 women with obesity during pregnancy.4 They aimed to evaluate energy intake with the energy intake-balance method (doubly labeled water and whole-room indirect calorimetry and body composition) according to tests done at 13 to 16 weeks’ gestation and 35 to 37 weeks’ gestation and according to the current National Academy of Medicine gestational weight gain guideline (inadequate, recommended, or excessive weight gain groups).4
Details of the study
Women who participated in this study were recruited from the Pennington Biomedical Research Center in Louisiana and were mostly multiparas (57%); about half had a college degree or higher (52%) and 41% were African American. The investigators found that gestational weight gain in their participants was similar to that found in other large epidemiologic studies in that 67% of women had excessive gestational weight gain.5
Findings. For women who gained the recommended amount of weight (n = 8), mean (SD) daily energy intake was 2,698 (99) kcal/day and energy expenditure was 2,824 (105) kcal/day. Therefore, to meet the recommended amount of weight gain, these women had a negative energy balance (-125 [52] kcal/day). Women with inadequate weight gain
(n = 10) also had a negative energy balance (-262 [32] kcal/day), but the difference was not significantly different compared with that in the recommended gestational weight gain group (P = .08). By contrast, women with excessive gestational weight gain (n = 36) had a mean (SD) positive energy balance of 186 (29) kcal/day.
Fat-free mass and fat mass weight gains. The body weight gains of the fat-free and fat mass compartments also were compared with linear mixed effect models among the 3 weight gain groups. There were no differences in the amount of fat-free mass gained among the 3 weight gain groups (P>.05), but women with excessive gestational weight gain had significantly higher increases in fat mass compared with the other 2 weight gain groups (P<.001).
Pregnancy outcomes. Although there were no differences in cesarean deliveries or birth weight among the 3 weight gain groups, the study was not powered to detect these differences.
Continue to: Study strengths and limitations...
Study strengths and limitations
It is important to note that this study by Most and colleagues was not a health behavior intervention for gestational weight gain. Women who participated in the study did not receive specific directions or advice on diet or physical activity. Furthermore, the study used the current gestational weight gain guideline as a reference to determine energy intake. As such, findings from this study alone cannot be used to adapt the current gestational weight gain guideline for women with obesity.
The study methods were rigorous in terms of the energy intake measurements, but a larger and more diverse sample size is needed to confirm the study findings.
Most and colleagues’ data suggest that maintaining energy balance can support obligatory growth and development of women and their fetuses during pregnancy (fat-free mass). In doing so, women with obesity meet the current gestational weight gain guideline. It is hoped that this important research will be used in future studies, with larger sample sizes, to evaluate energy requirements during pregnancy, especially in women with different classes of obesity. Ultimately, these new recommendations for energy requirements should be combined with studies of health behavior interventions for gestational weight gain.
The study by Most and colleagues supports the concept that energy requirements need to be individualized for women to meet the recommended amount of gestational weight gain. If women meet their gestational weight gain goals, they have the potential to improve their health and the health of their offspring.
MICHELLE A. KOMINIAREK, MD, MS
Most J, St Amant M, Hsia DS, et al. Evidence-based recommendations for energy intake in pregnant women with obesity. J Clin Invest. 2019;129:4682-4690.
EXPERT COMMENTARY
In 2009, the Institute of Medicine, now known as the National Academy of Medicine, updated its gestational weight gain guideline. This guideline’s major difference, compared with the 1990 guideline, is a specific weight gain range for women with obesity: 5 to 9 kg, or 11 to 20 lb.1 This weight gain range was chosen in part because it allows for a minimum weight gain that supports the growth and development of tissues (fetus, placenta, breast, uterus) and fluids (blood volume, intracellular and extracellular fluid), also known as the “fat-free” mass.
Many studies have since shown not only associations between lower-than-guideline-recommended weight gain and improved pregnancy outcomes (for example, reductions in preeclampsia and cesarean deliveries), but also increases in low birth weight for infants of women with obesity.2,3 Although the weight gain guideline differs based on a woman’s prepregnancy body mass index, the energy requirements, or how many additional calories a woman should consume daily, are the same for all, regardless of weight prior to pregnancy: an increase by 340 to 452 kcal/day in the second and third trimesters.1
Recently, Most and colleagues challenged this recommendation for energy requirements with results from their prospective observational study of 54 women with obesity during pregnancy.4 They aimed to evaluate energy intake with the energy intake-balance method (doubly labeled water and whole-room indirect calorimetry and body composition) according to tests done at 13 to 16 weeks’ gestation and 35 to 37 weeks’ gestation and according to the current National Academy of Medicine gestational weight gain guideline (inadequate, recommended, or excessive weight gain groups).4
Details of the study
Women who participated in this study were recruited from the Pennington Biomedical Research Center in Louisiana and were mostly multiparas (57%); about half had a college degree or higher (52%) and 41% were African American. The investigators found that gestational weight gain in their participants was similar to that found in other large epidemiologic studies in that 67% of women had excessive gestational weight gain.5
Findings. For women who gained the recommended amount of weight (n = 8), mean (SD) daily energy intake was 2,698 (99) kcal/day and energy expenditure was 2,824 (105) kcal/day. Therefore, to meet the recommended amount of weight gain, these women had a negative energy balance (-125 [52] kcal/day). Women with inadequate weight gain
(n = 10) also had a negative energy balance (-262 [32] kcal/day), but the difference was not significantly different compared with that in the recommended gestational weight gain group (P = .08). By contrast, women with excessive gestational weight gain (n = 36) had a mean (SD) positive energy balance of 186 (29) kcal/day.
Fat-free mass and fat mass weight gains. The body weight gains of the fat-free and fat mass compartments also were compared with linear mixed effect models among the 3 weight gain groups. There were no differences in the amount of fat-free mass gained among the 3 weight gain groups (P>.05), but women with excessive gestational weight gain had significantly higher increases in fat mass compared with the other 2 weight gain groups (P<.001).
Pregnancy outcomes. Although there were no differences in cesarean deliveries or birth weight among the 3 weight gain groups, the study was not powered to detect these differences.
Continue to: Study strengths and limitations...
Study strengths and limitations
It is important to note that this study by Most and colleagues was not a health behavior intervention for gestational weight gain. Women who participated in the study did not receive specific directions or advice on diet or physical activity. Furthermore, the study used the current gestational weight gain guideline as a reference to determine energy intake. As such, findings from this study alone cannot be used to adapt the current gestational weight gain guideline for women with obesity.
The study methods were rigorous in terms of the energy intake measurements, but a larger and more diverse sample size is needed to confirm the study findings.
Most and colleagues’ data suggest that maintaining energy balance can support obligatory growth and development of women and their fetuses during pregnancy (fat-free mass). In doing so, women with obesity meet the current gestational weight gain guideline. It is hoped that this important research will be used in future studies, with larger sample sizes, to evaluate energy requirements during pregnancy, especially in women with different classes of obesity. Ultimately, these new recommendations for energy requirements should be combined with studies of health behavior interventions for gestational weight gain.
The study by Most and colleagues supports the concept that energy requirements need to be individualized for women to meet the recommended amount of gestational weight gain. If women meet their gestational weight gain goals, they have the potential to improve their health and the health of their offspring.
MICHELLE A. KOMINIAREK, MD, MS
- Institute of Medicine and National Research Council Committee to Reexamine IOM Pregnacy Weight Guidelines. Rasmussen KM, Yaktine AL, eds. Weight Gain During Pregnancy: Reexamining the Guidelines. Washington, DC: National Academies Press; 2009.
- Kapadia MZ, Park CK, Beyene J, et al. Weight loss instead of weight gain within the guidelines in obese women during pregnancy: a systematic review and metaanalyses of maternal and infant outcomes. PLoS One. 2015;10:e0132650.
- Kapadia MZ, Park CK, Beyene J, et al. Can we safely recommend gestational weight gain below the 2009 guidelines in obese women? A systematic review and metaanalysis. Obes Rev. 2015;16:189-206.
- Most J, St Amant M, Hsia DS, et al. Evidence-based recommendations for energy intake in pregnant women with obesity. J Clin Invest. 2019;129:4682-4690.
- Deputy NP, Sharma AJ, Kim SY, et al. Prevalence and characteristics associated with gestational weight gain adequacy. Obstet Gynecol. 2015;125:773-781.
- Institute of Medicine and National Research Council Committee to Reexamine IOM Pregnacy Weight Guidelines. Rasmussen KM, Yaktine AL, eds. Weight Gain During Pregnancy: Reexamining the Guidelines. Washington, DC: National Academies Press; 2009.
- Kapadia MZ, Park CK, Beyene J, et al. Weight loss instead of weight gain within the guidelines in obese women during pregnancy: a systematic review and metaanalyses of maternal and infant outcomes. PLoS One. 2015;10:e0132650.
- Kapadia MZ, Park CK, Beyene J, et al. Can we safely recommend gestational weight gain below the 2009 guidelines in obese women? A systematic review and metaanalysis. Obes Rev. 2015;16:189-206.
- Most J, St Amant M, Hsia DS, et al. Evidence-based recommendations for energy intake in pregnant women with obesity. J Clin Invest. 2019;129:4682-4690.
- Deputy NP, Sharma AJ, Kim SY, et al. Prevalence and characteristics associated with gestational weight gain adequacy. Obstet Gynecol. 2015;125:773-781.
The IUD string check: Benefit or burden?
CASE A patient experiences unnessary inconvenience, distress, and cost following IUD placement
Ms. J had a levonorgestrel intrauterine device (IUD) placed at her postpartum visit. Her physician asked her to return for a string check in 4 to 6 weeks. She was dismayed at the prospect of re-presenting for care, as she is losing the Medicaid coverage that paid for her pregnancy care. One month later, she arranged for a babysitter so she could obtain the recommended string check. The physician told her the strings seemed longer than expected and ordered ultrasonography. Ms. J is distressed because of the mounting cost of care but is anxious to ensure that the IUD will prevent future pregnancy.
Should the routine IUD string check be reconsidered?
The string check dissension
Intrauterine devices offer reliable contraception with a high rate of satisfaction and a remarkably low rate of complications.1-3 With the increased uptake of IUDs, the value of “string checks” is being debated, with myriad responses from professional groups, manufacturers, and individual clinicians. For many practicing ObGyns, the question remains: Should patients be counseled about presenting for or doing their own IUD string checks?
Indeed, all IUD manufacturers recommend monthly self-examination to evaluate string presence.4-8 Manufacturers’ websites prominently display this information in material directed toward current or potential users, so many patients may be familiar already with this recommendation before their clinician visit. Yet, the Centers for Disease Control and Prevention state that no routine follow-up or monitoring is needed.9
In our case scenario, follow-up is clearly burdensome and ultimately costly. Instead, clinicians can advise patients to return with rare but important to recognize complications (such as perforation, expulsion, infection), adverse effects, or desire for change. While no data are available to support in-office or at-home string checks, data do show that women reliably present when intervention is needed.
Here, we explore 5 questions relevant to IUD string checks and discuss why it is time to rethink this practice habit.
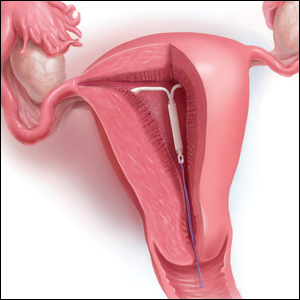
What is the purpose of a string check?
String checks serve as a surrogate for assessing an IUD’s position and function. A string check can be performed by a clinician, who observes the IUD strings on speculum exam or palpates the strings on bimanual exam, or by the patient doing a self-exam. A positive string check purportedly assures both the IUD user and the health care provider that an IUD remains in a fundal, intrauterine position, thus providing an ongoing reliable contraceptive effect.
However, string check reliability in detecting contraceptive effectiveness is uncertain. Strings that subjectively feel or appear longer than anticipated can lead to unnecessary additional evaluation and emotional distress: These are harms. By contrast, when an expulsion occurs, it often is a partial expulsion or displacement, with unclear effect on patient or physician perception of the strings on examination. One retrospective review identified women with a history of IUD placement and a positive pregnancy test; those with an intrauterine pregnancy (74%) frequently also had a malpositioned IUD (55%) and rarely identifiable string issues (16%).10 Before asking patients and clinicians to use resources for performing string evaluations, the association between this action and outcomes of interest must be elucidated.
If not for assessing risk of expulsion, IUD follow-up allows the clinician to evaluate for other complications or adverse effects and to address patient concerns. This practice often is performed when the patient is starting a new medication or medical intervention. However, a systematic review involving 4 studies of IUD follow-up visits or phone calls after contraceptive initiation generated limited data, with no notable impact on contraceptive continuation or indicated use.11
Most important, data show that patients present to their clinician when issues arise with IUD use. One prospective study of 280 women compared multiple follow-up visits with a single 6-week follow-up visit after IUD placement; 10 expulsions were identified, and 8 of these were noted at unscheduled visits when patients presented with symptoms.12 This study suggests that there is little benefit in scheduled follow-up or set self-checks.
Furthermore, in a study in Finland of more than 17,000 IUD users, the rare participants who became pregnant during IUD use promptly presented for care because of a change in menses, pain, or symptoms of pregnancy.13 While IUDs are touted as user independent, this overlooks the reality: Data show that device failure, although rare, is rapidly and appropriately addressed by the user.
Continue to: Does the risk of IUD expulsion warrant string checks?...
Does the risk of IUD expulsion warrant string checks?
The risk of IUD expulsion is estimated to be 1% at 1 month and 4% at 1 year, with a contraceptive failure rate of 0.4% at 1 year. The risk of expulsion does not differ by age group, including adolescents, or parity, but it is higher with use of the copper IUD (2% at 1 month, 6% at 1 year) and with prior expulsion (14%, limited by small numbers).1 Furthermore, risk of expulsion is higher with postplacental placement and second trimester abortion.14,15 Despite this risk, the contraceptive failure rate of all types of IUDs remains consistently lower than all other reversible methods besides the contraceptive implant.16
Furthermore, while IUD expulsion is rare, unnoticed expulsion is even more rare. In one study with more than 58,000 person-years of use, 132 pregnancies were noted, and 7 of these occurred in the setting of an unnoticed expulsion.13 Notably, a higher risk threshold is held for other medications. For example, statins are associated with a 3% risk of irreversible hepatic injury, yet serial liver function tests are not performed in patients without baseline liver dysfunction.17 A less than 0.1% risk of a non–life-threatening complication—unnoticed expulsion—does not warrant routine follow-up. Instead, the patient gauges the tolerability of that risk in making a follow-up plan, particularly given the varied individual preferences in patients’ management of the associated outcome of unintended pregnancy.
Are women interested in and able to perform their own string checks?
Recommendations to perform IUD string self-checks should consider whether women are willing and able to do so. In a study of 126 IUD users, 59% of women had attempted to check their IUD strings at home, and one-third were unable to do so successfully; all participants had visible strings on subsequent speculum exam.18 The women also were given the opportunity to perform a string self-check at the study visit. Overall, only 46% of participants found the exercise acceptable and were able to palpate the IUD strings.18 The authors aptly stated, “A universal recommendation for practice that is meant to identify a rare complication has no clinical utility if at least half of the women are unable to follow it.”
In which scenarios might a string check have clear utility?
The most important reason for follow-up after IUD placement or for patients to perform string self-checks is patient preference. At least anecdotally, some patients take comfort, particularly in the absence of menses, in palpating IUD strings regularly; these individuals should know that there is no necessity for but also no harm in this practice. In addition, patients may desire a string check or follow-up visit to discuss their new contraceptive’s goodness-of-fit.
While limited data show that routinely scheduling such visits does not improve contraceptive continuation, it is difficult to extrapolate these data to the select individuals who independently desire follow-up. (In addition, contraceptive continuance is hardly a metric of success, as clinicians and patients can agree that discontinuation in the setting of patient dissatisfaction is always appropriate.)
Clinicians should share with patients differing risks of IUD expulsion, and this may prompt more nuanced decisions about string checks and/or follow-up. Patients with postplacental or postabortion (second trimester) IUD placement or placement following prior expulsion may opt to perform string checks given the relatively higher risk of expulsion despite the maintained, absolutely low risk that such an event is unnoticed.
If a patient does present for a string check and strings are not visualized on exam, reasonable attempts should be made to identify the strings at that time. A cytobrush can be used to liberate and identify strings within the cervical canal. If the clinician cannot identify the strings or the patient is unable to tolerate such attempts, ultrasonography should be performed to localize the IUD. The ultrasound scan can be done in the office, if available, which is more cost-effective for women than a referral to radiology. If ultrasonography does not identify an intrauterine IUD, an x-ray is the next step to determine if the IUD has expulsed or perforated.
Continue to: Is a string check worth the cost?...
Is a string check worth the cost?
Health care providers may not be aware of the cost of care from the patient perspective. While the Affordable Care Act of 2010 mandates contraceptive coverage for women with insurance, a string check often is coded as a problem-based visit and thus may require a significant copay or out-of-pocket cost for high-deductible plans—without a proven benefit.19 Women who lack insurance coverage may forgo even necessary care due to the cost.20
The bottom line
The medical community and ObGyns specifically are familiar with a practice of patient self-examination falling by the wayside, as has been the case with breast self-examination.21 While counseling on string checks can complement conversations about risks and patients’ personal preferences regarding follow-up, no data support routine string checks in the clinic or at home. One of the great benefits of IUD use is its lack of barriers and resources for ongoing use. Physicians need not reintroduce burdens without benefits to those who desire this contraceptive method.
- Aoun J, Dines VA, Stovall DW, et al. Effects of age, parity, and device type on complications and discontinuation of intrauterine devices. Obstet Gynecol. 2014;123:585-592.
- Peipert JF, Zhao Q, Allsworth JE, et al. Continuation and satisfaction of reversible contraception. Obstet Gynecol. 2011;117:1105-1113.
- American College of Obstetricians and Gynecologists Committee on Gynecology Practice. Committee opinion No. 672. Clinical challenges of long-acting reversible contraceptive methods. Obstet Gynecol. 2016;128:e69-e77.
- Mirena website. Placement of Mirena. 2019. https://www.mirena-us.com/placement-of-mirena/. Accessed December 7, 2019.
- Kyleena website. Let’s get started. 2019. https://www.kyleena-us.com/lets-get-started/what-to-expect/. Accessed December 7, 2019.
- Skyla website. What to expect. 2019. https://www.skyla-us.com/getting-skyla/index.php. Accessed December 7, 2019.
- Liletta website. What should I expect after Liletta insertion? 2020. https://www.liletta.com/about/what-to-expect-afterinsertion. Accessed December 7, 2019.
- Paragard website. What to expect with Paragard. 2019. https://www.paragard.com/what-can-i-expect-with-paragard/. Accessed December 7, 2019.
- Curtis KM, Jatlaoui TC, Tepper NK, et al. US selected practice recommendations for contraceptive use, 2016. MMWR Recomm Rep. 2016;65(4):1-66. https://www.cdc.gov/mmwr/ volumes/65/rr/pdfs/rr6504.pdf. Accessed February 19, 2020.
- Moschos E, Twickler DM. Intrauterine devices in early pregnancy: findings on ultrasound and clinical outcomes. Am J Obstet Gynecol. 2011;204:427.e1-6.
- Steenland MW, Zapata LB, Brahmi D, et al. Appropriate follow up to detect potential adverse events after initiation of select contraceptive methods: a systematic review. Contraception 2013;87:611-624.
- Neuteboom K, de Kroon CD, Dersjant-Roorda M, et al. Follow-up visits after IUD-insertion: sense or nonsense? Contraception. 2003;68:101-104.
- Backman T, Rauramo I, Huhtala S, et al. Pregnancy during the use of levonorgestrel intrauterine system. Am J Obstet Gynecol. 2004;190:50-54.
- Whitaker AK, Chen BA. Society of Family Planning guidelines: postplacental insertion of intrauterine devices. Contraception. 2018;97:2-13.
- Roe AH, Bartz D. Society of Family Planning clinical recommendations: contraception after surgical abortion. Contraception. 2019;99:2-9.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins–Gynecology. Practice bulletin No. 186. Long-acting reversible contraception: implants and intrauterine devices. Obstet Gynecol. 2017;130:e251-e269.
- US Food and Drug Administration. FDA drug safety communication: important safety label changes to cholesterol-lowering statin drugs. 2016. https://www .fda.gov/drugs/drug-safety-and-availability/fda-drugsafety-communication-important-safety-label-changescholesterol-lowering-statin-drugs. Accessed January 9, 2020.
- Melo J, Tschann M, Soon R, et al. Women’s willingness and ability to feel the strings of their intrauterine device. Int J Gynaecol Obstet. 2017;137:309-313.
- Healthcare.gov website. Health benefits & coverage: birth control benefits. 2020. https://www.healthcare.gov/ coverage/birth-control-benefits/. Accessed January 6, 2020.
- NORC at the University of Chicago. Americans’ views of healthcare costs, coverage, and policy. 2018;1-15. https:// www.norc.org/PDFs/WHI%20Healthcare%20Costs%20 Coverage%20and%20Policy/WHI%20Healthcare%20 Costs%20Coverage%20and%20Policy%20Issue%20Brief.pdf. Accessed February 19, 2020.
- Kosters JP, Gotzsche PC. Regular self-examination or clinical examination for early detection of breast cancer. Cochrane Database Syst Rev. 2003. CD003373.
CASE A patient experiences unnessary inconvenience, distress, and cost following IUD placement
Ms. J had a levonorgestrel intrauterine device (IUD) placed at her postpartum visit. Her physician asked her to return for a string check in 4 to 6 weeks. She was dismayed at the prospect of re-presenting for care, as she is losing the Medicaid coverage that paid for her pregnancy care. One month later, she arranged for a babysitter so she could obtain the recommended string check. The physician told her the strings seemed longer than expected and ordered ultrasonography. Ms. J is distressed because of the mounting cost of care but is anxious to ensure that the IUD will prevent future pregnancy.
Should the routine IUD string check be reconsidered?
The string check dissension
Intrauterine devices offer reliable contraception with a high rate of satisfaction and a remarkably low rate of complications.1-3 With the increased uptake of IUDs, the value of “string checks” is being debated, with myriad responses from professional groups, manufacturers, and individual clinicians. For many practicing ObGyns, the question remains: Should patients be counseled about presenting for or doing their own IUD string checks?
Indeed, all IUD manufacturers recommend monthly self-examination to evaluate string presence.4-8 Manufacturers’ websites prominently display this information in material directed toward current or potential users, so many patients may be familiar already with this recommendation before their clinician visit. Yet, the Centers for Disease Control and Prevention state that no routine follow-up or monitoring is needed.9
In our case scenario, follow-up is clearly burdensome and ultimately costly. Instead, clinicians can advise patients to return with rare but important to recognize complications (such as perforation, expulsion, infection), adverse effects, or desire for change. While no data are available to support in-office or at-home string checks, data do show that women reliably present when intervention is needed.
Here, we explore 5 questions relevant to IUD string checks and discuss why it is time to rethink this practice habit.

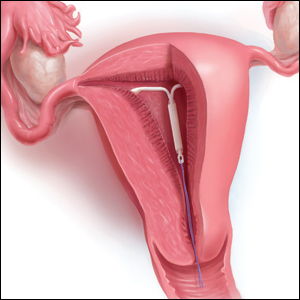
What is the purpose of a string check?
String checks serve as a surrogate for assessing an IUD’s position and function. A string check can be performed by a clinician, who observes the IUD strings on speculum exam or palpates the strings on bimanual exam, or by the patient doing a self-exam. A positive string check purportedly assures both the IUD user and the health care provider that an IUD remains in a fundal, intrauterine position, thus providing an ongoing reliable contraceptive effect.
However, string check reliability in detecting contraceptive effectiveness is uncertain. Strings that subjectively feel or appear longer than anticipated can lead to unnecessary additional evaluation and emotional distress: These are harms. By contrast, when an expulsion occurs, it often is a partial expulsion or displacement, with unclear effect on patient or physician perception of the strings on examination. One retrospective review identified women with a history of IUD placement and a positive pregnancy test; those with an intrauterine pregnancy (74%) frequently also had a malpositioned IUD (55%) and rarely identifiable string issues (16%).10 Before asking patients and clinicians to use resources for performing string evaluations, the association between this action and outcomes of interest must be elucidated.
If not for assessing risk of expulsion, IUD follow-up allows the clinician to evaluate for other complications or adverse effects and to address patient concerns. This practice often is performed when the patient is starting a new medication or medical intervention. However, a systematic review involving 4 studies of IUD follow-up visits or phone calls after contraceptive initiation generated limited data, with no notable impact on contraceptive continuation or indicated use.11
Most important, data show that patients present to their clinician when issues arise with IUD use. One prospective study of 280 women compared multiple follow-up visits with a single 6-week follow-up visit after IUD placement; 10 expulsions were identified, and 8 of these were noted at unscheduled visits when patients presented with symptoms.12 This study suggests that there is little benefit in scheduled follow-up or set self-checks.
Furthermore, in a study in Finland of more than 17,000 IUD users, the rare participants who became pregnant during IUD use promptly presented for care because of a change in menses, pain, or symptoms of pregnancy.13 While IUDs are touted as user independent, this overlooks the reality: Data show that device failure, although rare, is rapidly and appropriately addressed by the user.
Continue to: Does the risk of IUD expulsion warrant string checks?...
Does the risk of IUD expulsion warrant string checks?
The risk of IUD expulsion is estimated to be 1% at 1 month and 4% at 1 year, with a contraceptive failure rate of 0.4% at 1 year. The risk of expulsion does not differ by age group, including adolescents, or parity, but it is higher with use of the copper IUD (2% at 1 month, 6% at 1 year) and with prior expulsion (14%, limited by small numbers).1 Furthermore, risk of expulsion is higher with postplacental placement and second trimester abortion.14,15 Despite this risk, the contraceptive failure rate of all types of IUDs remains consistently lower than all other reversible methods besides the contraceptive implant.16
Furthermore, while IUD expulsion is rare, unnoticed expulsion is even more rare. In one study with more than 58,000 person-years of use, 132 pregnancies were noted, and 7 of these occurred in the setting of an unnoticed expulsion.13 Notably, a higher risk threshold is held for other medications. For example, statins are associated with a 3% risk of irreversible hepatic injury, yet serial liver function tests are not performed in patients without baseline liver dysfunction.17 A less than 0.1% risk of a non–life-threatening complication—unnoticed expulsion—does not warrant routine follow-up. Instead, the patient gauges the tolerability of that risk in making a follow-up plan, particularly given the varied individual preferences in patients’ management of the associated outcome of unintended pregnancy.

Are women interested in and able to perform their own string checks?
Recommendations to perform IUD string self-checks should consider whether women are willing and able to do so. In a study of 126 IUD users, 59% of women had attempted to check their IUD strings at home, and one-third were unable to do so successfully; all participants had visible strings on subsequent speculum exam.18 The women also were given the opportunity to perform a string self-check at the study visit. Overall, only 46% of participants found the exercise acceptable and were able to palpate the IUD strings.18 The authors aptly stated, “A universal recommendation for practice that is meant to identify a rare complication has no clinical utility if at least half of the women are unable to follow it.”
In which scenarios might a string check have clear utility?
The most important reason for follow-up after IUD placement or for patients to perform string self-checks is patient preference. At least anecdotally, some patients take comfort, particularly in the absence of menses, in palpating IUD strings regularly; these individuals should know that there is no necessity for but also no harm in this practice. In addition, patients may desire a string check or follow-up visit to discuss their new contraceptive’s goodness-of-fit.
While limited data show that routinely scheduling such visits does not improve contraceptive continuation, it is difficult to extrapolate these data to the select individuals who independently desire follow-up. (In addition, contraceptive continuance is hardly a metric of success, as clinicians and patients can agree that discontinuation in the setting of patient dissatisfaction is always appropriate.)
Clinicians should share with patients differing risks of IUD expulsion, and this may prompt more nuanced decisions about string checks and/or follow-up. Patients with postplacental or postabortion (second trimester) IUD placement or placement following prior expulsion may opt to perform string checks given the relatively higher risk of expulsion despite the maintained, absolutely low risk that such an event is unnoticed.
If a patient does present for a string check and strings are not visualized on exam, reasonable attempts should be made to identify the strings at that time. A cytobrush can be used to liberate and identify strings within the cervical canal. If the clinician cannot identify the strings or the patient is unable to tolerate such attempts, ultrasonography should be performed to localize the IUD. The ultrasound scan can be done in the office, if available, which is more cost-effective for women than a referral to radiology. If ultrasonography does not identify an intrauterine IUD, an x-ray is the next step to determine if the IUD has expulsed or perforated.
Continue to: Is a string check worth the cost?...
Is a string check worth the cost?
Health care providers may not be aware of the cost of care from the patient perspective. While the Affordable Care Act of 2010 mandates contraceptive coverage for women with insurance, a string check often is coded as a problem-based visit and thus may require a significant copay or out-of-pocket cost for high-deductible plans—without a proven benefit.19 Women who lack insurance coverage may forgo even necessary care due to the cost.20
The bottom line
The medical community and ObGyns specifically are familiar with a practice of patient self-examination falling by the wayside, as has been the case with breast self-examination.21 While counseling on string checks can complement conversations about risks and patients’ personal preferences regarding follow-up, no data support routine string checks in the clinic or at home. One of the great benefits of IUD use is its lack of barriers and resources for ongoing use. Physicians need not reintroduce burdens without benefits to those who desire this contraceptive method.
CASE A patient experiences unnessary inconvenience, distress, and cost following IUD placement
Ms. J had a levonorgestrel intrauterine device (IUD) placed at her postpartum visit. Her physician asked her to return for a string check in 4 to 6 weeks. She was dismayed at the prospect of re-presenting for care, as she is losing the Medicaid coverage that paid for her pregnancy care. One month later, she arranged for a babysitter so she could obtain the recommended string check. The physician told her the strings seemed longer than expected and ordered ultrasonography. Ms. J is distressed because of the mounting cost of care but is anxious to ensure that the IUD will prevent future pregnancy.
Should the routine IUD string check be reconsidered?
The string check dissension
Intrauterine devices offer reliable contraception with a high rate of satisfaction and a remarkably low rate of complications.1-3 With the increased uptake of IUDs, the value of “string checks” is being debated, with myriad responses from professional groups, manufacturers, and individual clinicians. For many practicing ObGyns, the question remains: Should patients be counseled about presenting for or doing their own IUD string checks?
Indeed, all IUD manufacturers recommend monthly self-examination to evaluate string presence.4-8 Manufacturers’ websites prominently display this information in material directed toward current or potential users, so many patients may be familiar already with this recommendation before their clinician visit. Yet, the Centers for Disease Control and Prevention state that no routine follow-up or monitoring is needed.9
In our case scenario, follow-up is clearly burdensome and ultimately costly. Instead, clinicians can advise patients to return with rare but important to recognize complications (such as perforation, expulsion, infection), adverse effects, or desire for change. While no data are available to support in-office or at-home string checks, data do show that women reliably present when intervention is needed.
Here, we explore 5 questions relevant to IUD string checks and discuss why it is time to rethink this practice habit.

What is the purpose of a string check?
String checks serve as a surrogate for assessing an IUD’s position and function. A string check can be performed by a clinician, who observes the IUD strings on speculum exam or palpates the strings on bimanual exam, or by the patient doing a self-exam. A positive string check purportedly assures both the IUD user and the health care provider that an IUD remains in a fundal, intrauterine position, thus providing an ongoing reliable contraceptive effect.
However, string check reliability in detecting contraceptive effectiveness is uncertain. Strings that subjectively feel or appear longer than anticipated can lead to unnecessary additional evaluation and emotional distress: These are harms. By contrast, when an expulsion occurs, it often is a partial expulsion or displacement, with unclear effect on patient or physician perception of the strings on examination. One retrospective review identified women with a history of IUD placement and a positive pregnancy test; those with an intrauterine pregnancy (74%) frequently also had a malpositioned IUD (55%) and rarely identifiable string issues (16%).10 Before asking patients and clinicians to use resources for performing string evaluations, the association between this action and outcomes of interest must be elucidated.
If not for assessing risk of expulsion, IUD follow-up allows the clinician to evaluate for other complications or adverse effects and to address patient concerns. This practice often is performed when the patient is starting a new medication or medical intervention. However, a systematic review involving 4 studies of IUD follow-up visits or phone calls after contraceptive initiation generated limited data, with no notable impact on contraceptive continuation or indicated use.11
Most important, data show that patients present to their clinician when issues arise with IUD use. One prospective study of 280 women compared multiple follow-up visits with a single 6-week follow-up visit after IUD placement; 10 expulsions were identified, and 8 of these were noted at unscheduled visits when patients presented with symptoms.12 This study suggests that there is little benefit in scheduled follow-up or set self-checks.
Furthermore, in a study in Finland of more than 17,000 IUD users, the rare participants who became pregnant during IUD use promptly presented for care because of a change in menses, pain, or symptoms of pregnancy.13 While IUDs are touted as user independent, this overlooks the reality: Data show that device failure, although rare, is rapidly and appropriately addressed by the user.
Continue to: Does the risk of IUD expulsion warrant string checks?...
Does the risk of IUD expulsion warrant string checks?
The risk of IUD expulsion is estimated to be 1% at 1 month and 4% at 1 year, with a contraceptive failure rate of 0.4% at 1 year. The risk of expulsion does not differ by age group, including adolescents, or parity, but it is higher with use of the copper IUD (2% at 1 month, 6% at 1 year) and with prior expulsion (14%, limited by small numbers).1 Furthermore, risk of expulsion is higher with postplacental placement and second trimester abortion.14,15 Despite this risk, the contraceptive failure rate of all types of IUDs remains consistently lower than all other reversible methods besides the contraceptive implant.16
Furthermore, while IUD expulsion is rare, unnoticed expulsion is even more rare. In one study with more than 58,000 person-years of use, 132 pregnancies were noted, and 7 of these occurred in the setting of an unnoticed expulsion.13 Notably, a higher risk threshold is held for other medications. For example, statins are associated with a 3% risk of irreversible hepatic injury, yet serial liver function tests are not performed in patients without baseline liver dysfunction.17 A less than 0.1% risk of a non–life-threatening complication—unnoticed expulsion—does not warrant routine follow-up. Instead, the patient gauges the tolerability of that risk in making a follow-up plan, particularly given the varied individual preferences in patients’ management of the associated outcome of unintended pregnancy.

Are women interested in and able to perform their own string checks?
Recommendations to perform IUD string self-checks should consider whether women are willing and able to do so. In a study of 126 IUD users, 59% of women had attempted to check their IUD strings at home, and one-third were unable to do so successfully; all participants had visible strings on subsequent speculum exam.18 The women also were given the opportunity to perform a string self-check at the study visit. Overall, only 46% of participants found the exercise acceptable and were able to palpate the IUD strings.18 The authors aptly stated, “A universal recommendation for practice that is meant to identify a rare complication has no clinical utility if at least half of the women are unable to follow it.”
In which scenarios might a string check have clear utility?
The most important reason for follow-up after IUD placement or for patients to perform string self-checks is patient preference. At least anecdotally, some patients take comfort, particularly in the absence of menses, in palpating IUD strings regularly; these individuals should know that there is no necessity for but also no harm in this practice. In addition, patients may desire a string check or follow-up visit to discuss their new contraceptive’s goodness-of-fit.
While limited data show that routinely scheduling such visits does not improve contraceptive continuation, it is difficult to extrapolate these data to the select individuals who independently desire follow-up. (In addition, contraceptive continuance is hardly a metric of success, as clinicians and patients can agree that discontinuation in the setting of patient dissatisfaction is always appropriate.)
Clinicians should share with patients differing risks of IUD expulsion, and this may prompt more nuanced decisions about string checks and/or follow-up. Patients with postplacental or postabortion (second trimester) IUD placement or placement following prior expulsion may opt to perform string checks given the relatively higher risk of expulsion despite the maintained, absolutely low risk that such an event is unnoticed.
If a patient does present for a string check and strings are not visualized on exam, reasonable attempts should be made to identify the strings at that time. A cytobrush can be used to liberate and identify strings within the cervical canal. If the clinician cannot identify the strings or the patient is unable to tolerate such attempts, ultrasonography should be performed to localize the IUD. The ultrasound scan can be done in the office, if available, which is more cost-effective for women than a referral to radiology. If ultrasonography does not identify an intrauterine IUD, an x-ray is the next step to determine if the IUD has expulsed or perforated.
Continue to: Is a string check worth the cost?...
Is a string check worth the cost?
Health care providers may not be aware of the cost of care from the patient perspective. While the Affordable Care Act of 2010 mandates contraceptive coverage for women with insurance, a string check often is coded as a problem-based visit and thus may require a significant copay or out-of-pocket cost for high-deductible plans—without a proven benefit.19 Women who lack insurance coverage may forgo even necessary care due to the cost.20
The bottom line
The medical community and ObGyns specifically are familiar with a practice of patient self-examination falling by the wayside, as has been the case with breast self-examination.21 While counseling on string checks can complement conversations about risks and patients’ personal preferences regarding follow-up, no data support routine string checks in the clinic or at home. One of the great benefits of IUD use is its lack of barriers and resources for ongoing use. Physicians need not reintroduce burdens without benefits to those who desire this contraceptive method.
- Aoun J, Dines VA, Stovall DW, et al. Effects of age, parity, and device type on complications and discontinuation of intrauterine devices. Obstet Gynecol. 2014;123:585-592.
- Peipert JF, Zhao Q, Allsworth JE, et al. Continuation and satisfaction of reversible contraception. Obstet Gynecol. 2011;117:1105-1113.
- American College of Obstetricians and Gynecologists Committee on Gynecology Practice. Committee opinion No. 672. Clinical challenges of long-acting reversible contraceptive methods. Obstet Gynecol. 2016;128:e69-e77.
- Mirena website. Placement of Mirena. 2019. https://www.mirena-us.com/placement-of-mirena/. Accessed December 7, 2019.
- Kyleena website. Let’s get started. 2019. https://www.kyleena-us.com/lets-get-started/what-to-expect/. Accessed December 7, 2019.
- Skyla website. What to expect. 2019. https://www.skyla-us.com/getting-skyla/index.php. Accessed December 7, 2019.
- Liletta website. What should I expect after Liletta insertion? 2020. https://www.liletta.com/about/what-to-expect-afterinsertion. Accessed December 7, 2019.
- Paragard website. What to expect with Paragard. 2019. https://www.paragard.com/what-can-i-expect-with-paragard/. Accessed December 7, 2019.
- Curtis KM, Jatlaoui TC, Tepper NK, et al. US selected practice recommendations for contraceptive use, 2016. MMWR Recomm Rep. 2016;65(4):1-66. https://www.cdc.gov/mmwr/ volumes/65/rr/pdfs/rr6504.pdf. Accessed February 19, 2020.
- Moschos E, Twickler DM. Intrauterine devices in early pregnancy: findings on ultrasound and clinical outcomes. Am J Obstet Gynecol. 2011;204:427.e1-6.
- Steenland MW, Zapata LB, Brahmi D, et al. Appropriate follow up to detect potential adverse events after initiation of select contraceptive methods: a systematic review. Contraception 2013;87:611-624.
- Neuteboom K, de Kroon CD, Dersjant-Roorda M, et al. Follow-up visits after IUD-insertion: sense or nonsense? Contraception. 2003;68:101-104.
- Backman T, Rauramo I, Huhtala S, et al. Pregnancy during the use of levonorgestrel intrauterine system. Am J Obstet Gynecol. 2004;190:50-54.
- Whitaker AK, Chen BA. Society of Family Planning guidelines: postplacental insertion of intrauterine devices. Contraception. 2018;97:2-13.
- Roe AH, Bartz D. Society of Family Planning clinical recommendations: contraception after surgical abortion. Contraception. 2019;99:2-9.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins–Gynecology. Practice bulletin No. 186. Long-acting reversible contraception: implants and intrauterine devices. Obstet Gynecol. 2017;130:e251-e269.
- US Food and Drug Administration. FDA drug safety communication: important safety label changes to cholesterol-lowering statin drugs. 2016. https://www .fda.gov/drugs/drug-safety-and-availability/fda-drugsafety-communication-important-safety-label-changescholesterol-lowering-statin-drugs. Accessed January 9, 2020.
- Melo J, Tschann M, Soon R, et al. Women’s willingness and ability to feel the strings of their intrauterine device. Int J Gynaecol Obstet. 2017;137:309-313.
- Healthcare.gov website. Health benefits & coverage: birth control benefits. 2020. https://www.healthcare.gov/ coverage/birth-control-benefits/. Accessed January 6, 2020.
- NORC at the University of Chicago. Americans’ views of healthcare costs, coverage, and policy. 2018;1-15. https:// www.norc.org/PDFs/WHI%20Healthcare%20Costs%20 Coverage%20and%20Policy/WHI%20Healthcare%20 Costs%20Coverage%20and%20Policy%20Issue%20Brief.pdf. Accessed February 19, 2020.
- Kosters JP, Gotzsche PC. Regular self-examination or clinical examination for early detection of breast cancer. Cochrane Database Syst Rev. 2003. CD003373.
- Aoun J, Dines VA, Stovall DW, et al. Effects of age, parity, and device type on complications and discontinuation of intrauterine devices. Obstet Gynecol. 2014;123:585-592.
- Peipert JF, Zhao Q, Allsworth JE, et al. Continuation and satisfaction of reversible contraception. Obstet Gynecol. 2011;117:1105-1113.
- American College of Obstetricians and Gynecologists Committee on Gynecology Practice. Committee opinion No. 672. Clinical challenges of long-acting reversible contraceptive methods. Obstet Gynecol. 2016;128:e69-e77.
- Mirena website. Placement of Mirena. 2019. https://www.mirena-us.com/placement-of-mirena/. Accessed December 7, 2019.
- Kyleena website. Let’s get started. 2019. https://www.kyleena-us.com/lets-get-started/what-to-expect/. Accessed December 7, 2019.
- Skyla website. What to expect. 2019. https://www.skyla-us.com/getting-skyla/index.php. Accessed December 7, 2019.
- Liletta website. What should I expect after Liletta insertion? 2020. https://www.liletta.com/about/what-to-expect-afterinsertion. Accessed December 7, 2019.
- Paragard website. What to expect with Paragard. 2019. https://www.paragard.com/what-can-i-expect-with-paragard/. Accessed December 7, 2019.
- Curtis KM, Jatlaoui TC, Tepper NK, et al. US selected practice recommendations for contraceptive use, 2016. MMWR Recomm Rep. 2016;65(4):1-66. https://www.cdc.gov/mmwr/ volumes/65/rr/pdfs/rr6504.pdf. Accessed February 19, 2020.
- Moschos E, Twickler DM. Intrauterine devices in early pregnancy: findings on ultrasound and clinical outcomes. Am J Obstet Gynecol. 2011;204:427.e1-6.
- Steenland MW, Zapata LB, Brahmi D, et al. Appropriate follow up to detect potential adverse events after initiation of select contraceptive methods: a systematic review. Contraception 2013;87:611-624.
- Neuteboom K, de Kroon CD, Dersjant-Roorda M, et al. Follow-up visits after IUD-insertion: sense or nonsense? Contraception. 2003;68:101-104.
- Backman T, Rauramo I, Huhtala S, et al. Pregnancy during the use of levonorgestrel intrauterine system. Am J Obstet Gynecol. 2004;190:50-54.
- Whitaker AK, Chen BA. Society of Family Planning guidelines: postplacental insertion of intrauterine devices. Contraception. 2018;97:2-13.
- Roe AH, Bartz D. Society of Family Planning clinical recommendations: contraception after surgical abortion. Contraception. 2019;99:2-9.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins–Gynecology. Practice bulletin No. 186. Long-acting reversible contraception: implants and intrauterine devices. Obstet Gynecol. 2017;130:e251-e269.
- US Food and Drug Administration. FDA drug safety communication: important safety label changes to cholesterol-lowering statin drugs. 2016. https://www .fda.gov/drugs/drug-safety-and-availability/fda-drugsafety-communication-important-safety-label-changescholesterol-lowering-statin-drugs. Accessed January 9, 2020.
- Melo J, Tschann M, Soon R, et al. Women’s willingness and ability to feel the strings of their intrauterine device. Int J Gynaecol Obstet. 2017;137:309-313.
- Healthcare.gov website. Health benefits & coverage: birth control benefits. 2020. https://www.healthcare.gov/ coverage/birth-control-benefits/. Accessed January 6, 2020.
- NORC at the University of Chicago. Americans’ views of healthcare costs, coverage, and policy. 2018;1-15. https:// www.norc.org/PDFs/WHI%20Healthcare%20Costs%20 Coverage%20and%20Policy/WHI%20Healthcare%20 Costs%20Coverage%20and%20Policy%20Issue%20Brief.pdf. Accessed February 19, 2020.
- Kosters JP, Gotzsche PC. Regular self-examination or clinical examination for early detection of breast cancer. Cochrane Database Syst Rev. 2003. CD003373.
What is the role of the ObGyn in preventing and treating obesity?
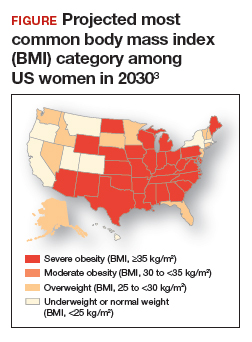
Obesity is a disease causing a public health crisis. In the United States, tobacco use and obesity are the two most important causes of preventable premature death. They result in an estimated 480,0001 and 300,0002 premature deaths per year, respectively. Obesity is a major contributor to diabetes mellitus, hypertension, dyslipidemia, and coronary heart disease. Obesity is also associated with increased rates of colon, breast, and endometrial cancer. Experts predict that in 2030, 50% of adults in the United States will have a body mass index (BMI) ≥ 30 kg/m2, and 25% will have a BMI ≥ 35 kg/m2.3 More women than men are predicted to be severely obese (FIGURE).3
As clinicians we need to increase our efforts to reduce the epidemic of obesity. ObGyns can play an important role in preventing and managing obesity, by recommending primary-care weight management practices, prescribing medications that influence central metabolism, and referring appropriate patients to bariatric surgery centers of excellence.
Primary-care weight management
Measuring BMI and recommending interventions to prevent and treat obesity are important components of a health maintenance encounter. For women who are overweight or obese, dietary changes and exercise are important recommendations. The American Heart Association recommends the following lifestyle interventions4:
- Eat a high-quality diet that includes vegetables, fruit, whole grains, beans, legumes, nuts, plant-based protein, lean animal protein, and fish.
- Limit intake of sugary drinks and foods, fatty or processed meats, full-fat dairy products, eggs, highly processed foods, and tropical oils.
- Exercise at least 150 minutes weekly at a moderate activity level, including muscle-strengthening activity.
- Reduce prolonged intervals of sitting.
- Consider using an activity tracker to monitor activity level.
Clinicians should consider referring overweight and obese patients to a nutritionist for a consultation to plan how to consume a high-quality, low-calorie diet. A nutritionist can spend time with patients explaining options for implementing a calorie-restricted diet. In addition, some health insurers will require patients to participate in a supervised calorie-restricted diet plan for at least 6 months before authorizing coverage of expensive weight loss medications or bariatric surgery. In addition to recommending diet and exercise, ObGyns may consider prescribing metformin for their obese patients.
Continue to: Metformin...
Metformin
Metformin is approved for the treatment of type 2 diabetes mellitus. Unlike insulin therapy, which is associated with weight gain, metformin is associated with modest weight loss. The Diabetes Prevention Program (DPP) randomly assigned 3,234 nondiabetic participants with a fasting glucose level between 95 and 125 mg/dL and impaired glucose tolerance (140 to 199 mg/dL) after a 75-g oral glucose load to intensive lifestyle changes (calorie-restricted diet to achieve 7% weight loss plus 150 minutes of exercise weekly), metformin (850 mg twice daily), or placebo.5,6 The mean age of the participants was 51 years, with a mean BMI of 34 kg/m2. Most (68%) of the participants were women.
After 12 months of follow-up, mean weight loss in the intensive lifestyle change, metformin, and placebo groups was 6.5%, 2.7%, and 0.4%, respectively. After 2 years of treatment, weight loss among those who reliably took their metformin pills was approximately 4%, while participants in the placebo group had a 1% weight gain. Among those who continued to reliably take their metformin pills, the weight loss persisted through 9 years of follow up.
The mechanisms by which metformin causes weight loss are not clear. Metformin stimulates phosphorylation of adenosine monophosphate (AMP)-activated protein kinase, which regulates mitochondrial function, hepatic and muscle fatty acid oxidation, glucose transport, insulin secretion, and lipogenesis.7
Many ObGyns have experience in using metformin for the treatment of polycystic ovary syndrome or gestational diabetes. Hence, the dosing and adverse effects of metformin are familiar to many obstetricians-gynecologists. Metformin is contraindicated in individuals with creatinine clearance less than 30 mL/min. Rarely, metformin can cause lactic acidosis. According to Lexicomp,8 the most common adverse effects of metformin extended release (metformin ER) are diarrhea (17%), nausea and vomiting (7%), and decreased vitamin B12 concentration (7%) due to malabsorption in the terminal ileum. Of note, in the DPP study, hemoglobin concentration was slightly lower over time in the metformin compared with the placebo group (13.6 mg/dL vs 13.8 mg/dL, respectively; P<.001).6 Some experts recommend annual vitamin B12 measurement in individuals taking metformin.
In my practice, I only prescribe metformin ER. I usually start metformin treatment with one 750 mg ER tablet with dinner. If the patient tolerates that dose, I increase the dose to two 750 mg ER tablets with dinner. Metformin-induced adverse effects include diarrhea (17%) and nausea and vomiting (7%). Metformin ER is inexpensive. A one-month supply of metformin (sixty 750 mg tablets) costs between $4 and $21 at major pharmacies.9 Health insurance companies generally do not require preauthorization to cover metformin prescriptions.
Weight loss medications
US Food and Drug Administration (FDA)-approved weight loss medications include: liraglutide (Victoza), orlistat (Xenical, Alli), combination phentermine-extended release topiramate (Qsymia), and combination extended release naltrexone-bupropion (Contrave). All FDA-approved weight loss medications result in mean weight loss in the range of 6% to 10%. Many of these medications are very expensive (more than $200 per month).10 Insurance preauthorization is commonly required for these medications. For ObGyns, it may be best to refer patients who would like to use a weight loss medication to a specialist or specialty center with expertise in using these medications.
Sustainable weight loss is very difficult to achieve through dieting alone. A multitude of dietary interventions have been presented as “revolutionary approaches” to the challenging problem of sustainable weight loss, including the Paleo diet, the Vegan diet, the low-carb diet, the Dukan diet, the ultra-lowfat diet, the Atkins diet, the HCG diet, the Zone diet, the South Beach diet, the plant-based diet, the Mediterranean diet, the Asian diet, and intermittent fasting. Recently, intermittent fasting has been presented as the latest and greatest approach to dieting, with the dual goals of achieving weight loss and improved health.1 In some animal models, intermittent dieting has been shown to increase life-span, a finding that has attracted great interest. A major goal of intermittent fasting is to promote “metabolic switching” with increased reliance on ketones to fuel cellular energy needs.
Two approaches to “prescribing” an intermittent fasting diet are to limit food intake to a period of 6 to 10 hours each day or to markedly reduce caloric intake one or two days per week, for example to 750 calories in a 24-hour period. There are no long-term studies of the health outcomes associated with intermittent fasting. In head-to-head clinical trials of intermittent fasting and daily calorie restriction (classic dieting), both diets result in similar weight loss. For example, in one clinical trial 100 obese participants, with a mean body mass index (BMI) of 34 kg/m2 , including 86 women, were randomly assigned to2:
1. intermittent fasting (25% of energy needs every other day)
2. daily calorie restriction (75% of energy needs every day), or
3. no intervention.
After 12 months of follow up, the participants in the no intervention group had gained 0.5% of their starting weight. The intermittent fasting and the daily calorie restriction groups had similar amounts of weight loss, approximately 5% of their starting weight. More individuals dropped out of the study from the intermittent fasting group than the daily calorie restriction group (38% vs 29%, respectively).
In another clinical trial, 107 overweight or obese premenopausal women, average age 40 years and mean BMI 31 kg/m2 , were randomly assigned to intermittent fasting (25% of energy needs 2 days per week) or daily calorie restriction (75% of energy needs daily) for 6 months. The mean weight of the participants at baseline was 83 kg. Weight loss was similar in the intermittent fasting and daily calorie restriction groups, 6.4 kg (-7.7%) and 5.6 kg (-6.7%), respectively (P=.4).3
The investigators concluded that intermittent fasting and daily calorie restriction could both be offered as effective approaches to weight loss. My conclusion is that intermittent fasting is not a miracle dietary intervention, but it is another important option in the armamentarium of weight loss interventions.
References
1. de Cabo R, Mattson MP. Effects of intermittent fasting on health, aging and disease. N Engl J Med. 2019;381:2541-2551.
2. Trepanowski JF, Kroeger CM, Barnosky A, et al. Effect of alternate-day fasting on weight loss, weight maintenance, and cardioprotection among metabolically healthy obese adults: a randomized clinical trial. JAMA Intern Med. 2017;177:930-938.
3. Harvie MN, Pegington M, Mattson MP, et al. The effects of intermittent or continuous energy restriction on weight loss and metabolic disc disease risk markers: a randomized trial in young overweight women. Int J Obes (Lond). 2011;35:714-727.
Sleeve gastrectomy
Two children are playing in a school yard. One child proudly states, “My mother is an endocrinologist. She treats diabetes.” Not to be outdone, the other child replies, “My mother is a bariatric surgeon. She cures diabetes.”
The dialogue reflects the reality that bariatric surgery results in more reliable and significant weight loss than diet, exercise, or weight loss medications. Diet, exercise, and weight loss medications often result in a 5% to 10% decrease in weight, but bariatric surgery typically results in a 25% decrease in weight. Until recently, 3 bariatric surgical procedures were commonly performed: Roux-en-Y gastric bypass (RYGB), sleeve gastrectomy (SG), and adjustable gastric banding (AGB). AGB is now seldom performed because it is less effective than RYGB and SG. Two recently published randomized trials compared the long-term outcomes associated with RYGB and SG. The studies found that SG and RYGB result in a similar degree of weight loss. RYGB resulted in slightly more weight loss than SG, but SG was associated with a lower rate of major complications, such as internal hernias. SG takes much less time to perform than RYGB. SG has become the most commonly performed bariatric surgery in premenopausal women considering pregnancy because of the low risk of internal hernias.
In the Swiss Multicenter Bypass or Sleeve Study (SM-BOSS), 217 participants with a mean BMI of 44 kg/m2 and mean age of 45.5 years were randomly assigned to RYGB or SG and followed for 5 years.11 The majority (72%) of the participants were women. At 5 years of follow-up, in the RYGB and SG groups, mean weight loss was 37 kg and 33 kg, respectively (P=.19). In both groups, weight loss nadir was reached 12 to 24 months after surgery. Expressed as a percentage of original weight, weight loss in the RYGB and SG groups was -29% and -25%, respectively (P=.02). Gastric reflux worsened in both the RYGB and SG groups (6% vs 32%, respectively). The number of reoperations in the RYGB and SG groups was 22% and 16%. Of note, among individuals with prevalent diabetes, RYGB and SG resulted in remission of the diabetes in 68% and 62% of participants, respectively.
In the Sleeve vs Bypass study (SLEEVEPASS), 240 participants, with mean BMI of 46 kg/m2 and mean age of 48 years, were randomly assigned to RYGB or SG and followed for 5 years.12 Most (70%) of the participants were women. Following bariatric surgery, BMI decreased significantly in both groups. In the RYGB group, BMI decreased from 48 kg/m2 preoperatively to 35.4 kg/m2 at 5 years of follow up. In the SG group, BMI decreased from 47 kg/m2 preoperatively to 36.5 kg/m2 at 5 years of follow up. Late major complications (defined as complications occurring from 30 days to 5 years postoperatively) occurred more frequently in the RYGB group (15%) versus the SG group (8%). All the late major complications required reoperation. In the SG group, 7 of 10 reoperations were for severe gastric reflux disease. In the RYGB group 17 of 18 reoperations were for suspected internal hernia, requiring closure of a mesenteric defect at reoperation. There was no treatment-related mortality during the 5-year follow up.
Guidelines for bariatric surgery are BMI ≥ 40 kg/m2 without a comorbid illness or BMI ≥ 35 kg/m2 with at least one serious comorbid disease, such as diabetes.13 ObGyns can build a synergistic relationship with bariatric surgeons by referring eligible patients for surgical consultation and, in return, accepting referrals. A paradox and challenge is that many health insurers require patients to complete a supervised medical weight loss management program prior to being approved for bariatric surgery. However, the medical weight loss program might result in the patient no longer being eligible for insurance coverage of their surgery. For example, a patient who had a BMI of 42 kg/m2 prior to a medical weight loss management program who then lost enough weight to achieve a BMI of 38 kg/m2 might no longer be eligible for insurance coverage of a bariatric operation.14
Continue to: ObGyns need to prioritize treatment for obesity...
ObGyns need to prioritize treatment for obesity
Between 1959 and 2014, US life expectancy increased from 69.9 years to 79.1 years. However, in 2015 and 2016 life expectancy in the United States decreased slightly to 78.9 years, while continuing to improve in other countries.15 What could cause such an unexpected trend? Some experts believe that excess overweight and obesity in the US population, resulting in increased rates of diabetes, hypertension, and heart disease, accounts for a significant proportion of the life expectancy gap between US citizens and those who reside in Australia, Finland, Japan, and Sweden.16,17 All frontline clinicians play an important role in reversing the decades-long trend of increasing rates of overweight and obesity. Interventions that ObGyns could prioritize in their practices for treating overweight and obese patients include: a calorie-restricted diet, exercise, metformin, and SG.
- U.S. Department of Health and Human Services. The Health Consequences of Smoking—50 Years of Progress. A Report of the Surgeon General. Atlanta: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2014.
- Allison DB, Fontaine KR, Manson JE, et al. Annual deaths attributable to obesity in the United States. JAMA. 1999;282:1530-1538.
- Ward ZJ, Bleich SN, Cradock AL, et al. Projected U.S. state-level prevalence of adult obesity and severe obesity. N Engl J Med. 2019;381:2440-2450.
- American Heart Association. My life check | Life’s simple 7. https://www.heart.org/en/healthyliving/healthy-lifestyle/my-life-check--lifessimple-7. Reviewed May 2, 2018. Accessed February 10, 2020.
- Knowler WC, Barrett-Connor E, Fowler SE, et al; Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393-403.
- Diabetes Prevention Program Research Group. Long-term safety, tolerability and weight loss associated with metformin in the Diabetes Prevention Program Outcomes Study. Diabetes Care. 2012;35:731-737.
- Winder WW, Hardie DG. Inactivation of acetylCoA carboxylase and activation of AMP-activated protein kinase in muscle during exercise. Am J Physiol. 1996;270(2 pt 1):E299-E304.
- Lexicomp. https://online.lexi.com/lco/action/ home. Accessed February 13, 2020.
- Metformin ER (Glucophage XR). GoodRX website. https://www.goodrx.com/metformin-erglucophage-xr?dosage=750mg&form=tablet&la bel_override=metformin+ER+%28Glucophage+X R%29&quantity=60. Accessed February 13, 2020.
- GoodRX website. www.goodrx.com. Accessed February 10, 2020.
- Peterli R, Wolnerhanssen BK, Peters T, et al. Effect of laparoscopic sleeve gastrectomy vs laparoscopic Roux-en-Y gastric bypass on weight loss in patients with morbid obesity: the SM-BOSS randomized clinical trial. JAMA. 2018;319:255-265.
- Salminen P, Helmiö M, Ovaska J, et al. Effect of laparoscopic sleeve gastrectomy versus laparoscopic Roux-en-Y gastric bypass on weight loss at 5 years among patients with morbid obesity: The SLEEVEPASS randomized clinical trial. JAMA. 2018;319:241-254.
- Rubino F, Nathan DM, Eckel RH, et al; Delegates of the 2nd Diabetes Surgery Summit. Metabolic surgery in the treatment algorithm for type 2 diabetes: a joint statement by international diabetes organizations. Obes Surg. 2017;27:2-21.
- Gebran SG, Knighton B, Ngaage LM, et al. Insurance coverage criteria for bariatric surgery: a survey of policies. Obes Surg. 2020;30:707-713.
- Woolf SH, Schoomaker H. Life expectancy and mortality rates in the United States, 1959-2017. JAMA. 2019;322:1996-2016.
- Preston SH, Vierboom YC, Stokes A. The role of obesity in exceptionally slow US mortality improvement. Proc Natl Acad Sci U S A. 2019;115:957-961.
- Xu H, Cupples LA, Stokes A, et al. Association of obesity with mortality over 24 years of weight history: findings from the Framingham Heart Study. JAMA Network Open. 2018;1:e184587.
Obesity is a disease causing a public health crisis. In the United States, tobacco use and obesity are the two most important causes of preventable premature death. They result in an estimated 480,0001 and 300,0002 premature deaths per year, respectively. Obesity is a major contributor to diabetes mellitus, hypertension, dyslipidemia, and coronary heart disease. Obesity is also associated with increased rates of colon, breast, and endometrial cancer. Experts predict that in 2030, 50% of adults in the United States will have a body mass index (BMI) ≥ 30 kg/m2, and 25% will have a BMI ≥ 35 kg/m2.3 More women than men are predicted to be severely obese (FIGURE).3

As clinicians we need to increase our efforts to reduce the epidemic of obesity. ObGyns can play an important role in preventing and managing obesity, by recommending primary-care weight management practices, prescribing medications that influence central metabolism, and referring appropriate patients to bariatric surgery centers of excellence.
Primary-care weight management
Measuring BMI and recommending interventions to prevent and treat obesity are important components of a health maintenance encounter. For women who are overweight or obese, dietary changes and exercise are important recommendations. The American Heart Association recommends the following lifestyle interventions4:
- Eat a high-quality diet that includes vegetables, fruit, whole grains, beans, legumes, nuts, plant-based protein, lean animal protein, and fish.
- Limit intake of sugary drinks and foods, fatty or processed meats, full-fat dairy products, eggs, highly processed foods, and tropical oils.
- Exercise at least 150 minutes weekly at a moderate activity level, including muscle-strengthening activity.
- Reduce prolonged intervals of sitting.
- Consider using an activity tracker to monitor activity level.
Clinicians should consider referring overweight and obese patients to a nutritionist for a consultation to plan how to consume a high-quality, low-calorie diet. A nutritionist can spend time with patients explaining options for implementing a calorie-restricted diet. In addition, some health insurers will require patients to participate in a supervised calorie-restricted diet plan for at least 6 months before authorizing coverage of expensive weight loss medications or bariatric surgery. In addition to recommending diet and exercise, ObGyns may consider prescribing metformin for their obese patients.
Continue to: Metformin...
Metformin
Metformin is approved for the treatment of type 2 diabetes mellitus. Unlike insulin therapy, which is associated with weight gain, metformin is associated with modest weight loss. The Diabetes Prevention Program (DPP) randomly assigned 3,234 nondiabetic participants with a fasting glucose level between 95 and 125 mg/dL and impaired glucose tolerance (140 to 199 mg/dL) after a 75-g oral glucose load to intensive lifestyle changes (calorie-restricted diet to achieve 7% weight loss plus 150 minutes of exercise weekly), metformin (850 mg twice daily), or placebo.5,6 The mean age of the participants was 51 years, with a mean BMI of 34 kg/m2. Most (68%) of the participants were women.
After 12 months of follow-up, mean weight loss in the intensive lifestyle change, metformin, and placebo groups was 6.5%, 2.7%, and 0.4%, respectively. After 2 years of treatment, weight loss among those who reliably took their metformin pills was approximately 4%, while participants in the placebo group had a 1% weight gain. Among those who continued to reliably take their metformin pills, the weight loss persisted through 9 years of follow up.
The mechanisms by which metformin causes weight loss are not clear. Metformin stimulates phosphorylation of adenosine monophosphate (AMP)-activated protein kinase, which regulates mitochondrial function, hepatic and muscle fatty acid oxidation, glucose transport, insulin secretion, and lipogenesis.7
Many ObGyns have experience in using metformin for the treatment of polycystic ovary syndrome or gestational diabetes. Hence, the dosing and adverse effects of metformin are familiar to many obstetricians-gynecologists. Metformin is contraindicated in individuals with creatinine clearance less than 30 mL/min. Rarely, metformin can cause lactic acidosis. According to Lexicomp,8 the most common adverse effects of metformin extended release (metformin ER) are diarrhea (17%), nausea and vomiting (7%), and decreased vitamin B12 concentration (7%) due to malabsorption in the terminal ileum. Of note, in the DPP study, hemoglobin concentration was slightly lower over time in the metformin compared with the placebo group (13.6 mg/dL vs 13.8 mg/dL, respectively; P<.001).6 Some experts recommend annual vitamin B12 measurement in individuals taking metformin.
In my practice, I only prescribe metformin ER. I usually start metformin treatment with one 750 mg ER tablet with dinner. If the patient tolerates that dose, I increase the dose to two 750 mg ER tablets with dinner. Metformin-induced adverse effects include diarrhea (17%) and nausea and vomiting (7%). Metformin ER is inexpensive. A one-month supply of metformin (sixty 750 mg tablets) costs between $4 and $21 at major pharmacies.9 Health insurance companies generally do not require preauthorization to cover metformin prescriptions.
Weight loss medications
US Food and Drug Administration (FDA)-approved weight loss medications include: liraglutide (Victoza), orlistat (Xenical, Alli), combination phentermine-extended release topiramate (Qsymia), and combination extended release naltrexone-bupropion (Contrave). All FDA-approved weight loss medications result in mean weight loss in the range of 6% to 10%. Many of these medications are very expensive (more than $200 per month).10 Insurance preauthorization is commonly required for these medications. For ObGyns, it may be best to refer patients who would like to use a weight loss medication to a specialist or specialty center with expertise in using these medications.
Sustainable weight loss is very difficult to achieve through dieting alone. A multitude of dietary interventions have been presented as “revolutionary approaches” to the challenging problem of sustainable weight loss, including the Paleo diet, the Vegan diet, the low-carb diet, the Dukan diet, the ultra-lowfat diet, the Atkins diet, the HCG diet, the Zone diet, the South Beach diet, the plant-based diet, the Mediterranean diet, the Asian diet, and intermittent fasting. Recently, intermittent fasting has been presented as the latest and greatest approach to dieting, with the dual goals of achieving weight loss and improved health.1 In some animal models, intermittent dieting has been shown to increase life-span, a finding that has attracted great interest. A major goal of intermittent fasting is to promote “metabolic switching” with increased reliance on ketones to fuel cellular energy needs.
Two approaches to “prescribing” an intermittent fasting diet are to limit food intake to a period of 6 to 10 hours each day or to markedly reduce caloric intake one or two days per week, for example to 750 calories in a 24-hour period. There are no long-term studies of the health outcomes associated with intermittent fasting. In head-to-head clinical trials of intermittent fasting and daily calorie restriction (classic dieting), both diets result in similar weight loss. For example, in one clinical trial 100 obese participants, with a mean body mass index (BMI) of 34 kg/m2 , including 86 women, were randomly assigned to2:
1. intermittent fasting (25% of energy needs every other day)
2. daily calorie restriction (75% of energy needs every day), or
3. no intervention.
After 12 months of follow up, the participants in the no intervention group had gained 0.5% of their starting weight. The intermittent fasting and the daily calorie restriction groups had similar amounts of weight loss, approximately 5% of their starting weight. More individuals dropped out of the study from the intermittent fasting group than the daily calorie restriction group (38% vs 29%, respectively).
In another clinical trial, 107 overweight or obese premenopausal women, average age 40 years and mean BMI 31 kg/m2 , were randomly assigned to intermittent fasting (25% of energy needs 2 days per week) or daily calorie restriction (75% of energy needs daily) for 6 months. The mean weight of the participants at baseline was 83 kg. Weight loss was similar in the intermittent fasting and daily calorie restriction groups, 6.4 kg (-7.7%) and 5.6 kg (-6.7%), respectively (P=.4).3
The investigators concluded that intermittent fasting and daily calorie restriction could both be offered as effective approaches to weight loss. My conclusion is that intermittent fasting is not a miracle dietary intervention, but it is another important option in the armamentarium of weight loss interventions.
References
1. de Cabo R, Mattson MP. Effects of intermittent fasting on health, aging and disease. N Engl J Med. 2019;381:2541-2551.
2. Trepanowski JF, Kroeger CM, Barnosky A, et al. Effect of alternate-day fasting on weight loss, weight maintenance, and cardioprotection among metabolically healthy obese adults: a randomized clinical trial. JAMA Intern Med. 2017;177:930-938.
3. Harvie MN, Pegington M, Mattson MP, et al. The effects of intermittent or continuous energy restriction on weight loss and metabolic disc disease risk markers: a randomized trial in young overweight women. Int J Obes (Lond). 2011;35:714-727.
Sleeve gastrectomy
Two children are playing in a school yard. One child proudly states, “My mother is an endocrinologist. She treats diabetes.” Not to be outdone, the other child replies, “My mother is a bariatric surgeon. She cures diabetes.”
The dialogue reflects the reality that bariatric surgery results in more reliable and significant weight loss than diet, exercise, or weight loss medications. Diet, exercise, and weight loss medications often result in a 5% to 10% decrease in weight, but bariatric surgery typically results in a 25% decrease in weight. Until recently, 3 bariatric surgical procedures were commonly performed: Roux-en-Y gastric bypass (RYGB), sleeve gastrectomy (SG), and adjustable gastric banding (AGB). AGB is now seldom performed because it is less effective than RYGB and SG. Two recently published randomized trials compared the long-term outcomes associated with RYGB and SG. The studies found that SG and RYGB result in a similar degree of weight loss. RYGB resulted in slightly more weight loss than SG, but SG was associated with a lower rate of major complications, such as internal hernias. SG takes much less time to perform than RYGB. SG has become the most commonly performed bariatric surgery in premenopausal women considering pregnancy because of the low risk of internal hernias.
In the Swiss Multicenter Bypass or Sleeve Study (SM-BOSS), 217 participants with a mean BMI of 44 kg/m2 and mean age of 45.5 years were randomly assigned to RYGB or SG and followed for 5 years.11 The majority (72%) of the participants were women. At 5 years of follow-up, in the RYGB and SG groups, mean weight loss was 37 kg and 33 kg, respectively (P=.19). In both groups, weight loss nadir was reached 12 to 24 months after surgery. Expressed as a percentage of original weight, weight loss in the RYGB and SG groups was -29% and -25%, respectively (P=.02). Gastric reflux worsened in both the RYGB and SG groups (6% vs 32%, respectively). The number of reoperations in the RYGB and SG groups was 22% and 16%. Of note, among individuals with prevalent diabetes, RYGB and SG resulted in remission of the diabetes in 68% and 62% of participants, respectively.
In the Sleeve vs Bypass study (SLEEVEPASS), 240 participants, with mean BMI of 46 kg/m2 and mean age of 48 years, were randomly assigned to RYGB or SG and followed for 5 years.12 Most (70%) of the participants were women. Following bariatric surgery, BMI decreased significantly in both groups. In the RYGB group, BMI decreased from 48 kg/m2 preoperatively to 35.4 kg/m2 at 5 years of follow up. In the SG group, BMI decreased from 47 kg/m2 preoperatively to 36.5 kg/m2 at 5 years of follow up. Late major complications (defined as complications occurring from 30 days to 5 years postoperatively) occurred more frequently in the RYGB group (15%) versus the SG group (8%). All the late major complications required reoperation. In the SG group, 7 of 10 reoperations were for severe gastric reflux disease. In the RYGB group 17 of 18 reoperations were for suspected internal hernia, requiring closure of a mesenteric defect at reoperation. There was no treatment-related mortality during the 5-year follow up.
Guidelines for bariatric surgery are BMI ≥ 40 kg/m2 without a comorbid illness or BMI ≥ 35 kg/m2 with at least one serious comorbid disease, such as diabetes.13 ObGyns can build a synergistic relationship with bariatric surgeons by referring eligible patients for surgical consultation and, in return, accepting referrals. A paradox and challenge is that many health insurers require patients to complete a supervised medical weight loss management program prior to being approved for bariatric surgery. However, the medical weight loss program might result in the patient no longer being eligible for insurance coverage of their surgery. For example, a patient who had a BMI of 42 kg/m2 prior to a medical weight loss management program who then lost enough weight to achieve a BMI of 38 kg/m2 might no longer be eligible for insurance coverage of a bariatric operation.14
Continue to: ObGyns need to prioritize treatment for obesity...
ObGyns need to prioritize treatment for obesity
Between 1959 and 2014, US life expectancy increased from 69.9 years to 79.1 years. However, in 2015 and 2016 life expectancy in the United States decreased slightly to 78.9 years, while continuing to improve in other countries.15 What could cause such an unexpected trend? Some experts believe that excess overweight and obesity in the US population, resulting in increased rates of diabetes, hypertension, and heart disease, accounts for a significant proportion of the life expectancy gap between US citizens and those who reside in Australia, Finland, Japan, and Sweden.16,17 All frontline clinicians play an important role in reversing the decades-long trend of increasing rates of overweight and obesity. Interventions that ObGyns could prioritize in their practices for treating overweight and obese patients include: a calorie-restricted diet, exercise, metformin, and SG.
Obesity is a disease causing a public health crisis. In the United States, tobacco use and obesity are the two most important causes of preventable premature death. They result in an estimated 480,0001 and 300,0002 premature deaths per year, respectively. Obesity is a major contributor to diabetes mellitus, hypertension, dyslipidemia, and coronary heart disease. Obesity is also associated with increased rates of colon, breast, and endometrial cancer. Experts predict that in 2030, 50% of adults in the United States will have a body mass index (BMI) ≥ 30 kg/m2, and 25% will have a BMI ≥ 35 kg/m2.3 More women than men are predicted to be severely obese (FIGURE).3

As clinicians we need to increase our efforts to reduce the epidemic of obesity. ObGyns can play an important role in preventing and managing obesity, by recommending primary-care weight management practices, prescribing medications that influence central metabolism, and referring appropriate patients to bariatric surgery centers of excellence.
Primary-care weight management
Measuring BMI and recommending interventions to prevent and treat obesity are important components of a health maintenance encounter. For women who are overweight or obese, dietary changes and exercise are important recommendations. The American Heart Association recommends the following lifestyle interventions4:
- Eat a high-quality diet that includes vegetables, fruit, whole grains, beans, legumes, nuts, plant-based protein, lean animal protein, and fish.
- Limit intake of sugary drinks and foods, fatty or processed meats, full-fat dairy products, eggs, highly processed foods, and tropical oils.
- Exercise at least 150 minutes weekly at a moderate activity level, including muscle-strengthening activity.
- Reduce prolonged intervals of sitting.
- Consider using an activity tracker to monitor activity level.
Clinicians should consider referring overweight and obese patients to a nutritionist for a consultation to plan how to consume a high-quality, low-calorie diet. A nutritionist can spend time with patients explaining options for implementing a calorie-restricted diet. In addition, some health insurers will require patients to participate in a supervised calorie-restricted diet plan for at least 6 months before authorizing coverage of expensive weight loss medications or bariatric surgery. In addition to recommending diet and exercise, ObGyns may consider prescribing metformin for their obese patients.
Continue to: Metformin...
Metformin
Metformin is approved for the treatment of type 2 diabetes mellitus. Unlike insulin therapy, which is associated with weight gain, metformin is associated with modest weight loss. The Diabetes Prevention Program (DPP) randomly assigned 3,234 nondiabetic participants with a fasting glucose level between 95 and 125 mg/dL and impaired glucose tolerance (140 to 199 mg/dL) after a 75-g oral glucose load to intensive lifestyle changes (calorie-restricted diet to achieve 7% weight loss plus 150 minutes of exercise weekly), metformin (850 mg twice daily), or placebo.5,6 The mean age of the participants was 51 years, with a mean BMI of 34 kg/m2. Most (68%) of the participants were women.
After 12 months of follow-up, mean weight loss in the intensive lifestyle change, metformin, and placebo groups was 6.5%, 2.7%, and 0.4%, respectively. After 2 years of treatment, weight loss among those who reliably took their metformin pills was approximately 4%, while participants in the placebo group had a 1% weight gain. Among those who continued to reliably take their metformin pills, the weight loss persisted through 9 years of follow up.
The mechanisms by which metformin causes weight loss are not clear. Metformin stimulates phosphorylation of adenosine monophosphate (AMP)-activated protein kinase, which regulates mitochondrial function, hepatic and muscle fatty acid oxidation, glucose transport, insulin secretion, and lipogenesis.7
Many ObGyns have experience in using metformin for the treatment of polycystic ovary syndrome or gestational diabetes. Hence, the dosing and adverse effects of metformin are familiar to many obstetricians-gynecologists. Metformin is contraindicated in individuals with creatinine clearance less than 30 mL/min. Rarely, metformin can cause lactic acidosis. According to Lexicomp,8 the most common adverse effects of metformin extended release (metformin ER) are diarrhea (17%), nausea and vomiting (7%), and decreased vitamin B12 concentration (7%) due to malabsorption in the terminal ileum. Of note, in the DPP study, hemoglobin concentration was slightly lower over time in the metformin compared with the placebo group (13.6 mg/dL vs 13.8 mg/dL, respectively; P<.001).6 Some experts recommend annual vitamin B12 measurement in individuals taking metformin.
In my practice, I only prescribe metformin ER. I usually start metformin treatment with one 750 mg ER tablet with dinner. If the patient tolerates that dose, I increase the dose to two 750 mg ER tablets with dinner. Metformin-induced adverse effects include diarrhea (17%) and nausea and vomiting (7%). Metformin ER is inexpensive. A one-month supply of metformin (sixty 750 mg tablets) costs between $4 and $21 at major pharmacies.9 Health insurance companies generally do not require preauthorization to cover metformin prescriptions.
Weight loss medications
US Food and Drug Administration (FDA)-approved weight loss medications include: liraglutide (Victoza), orlistat (Xenical, Alli), combination phentermine-extended release topiramate (Qsymia), and combination extended release naltrexone-bupropion (Contrave). All FDA-approved weight loss medications result in mean weight loss in the range of 6% to 10%. Many of these medications are very expensive (more than $200 per month).10 Insurance preauthorization is commonly required for these medications. For ObGyns, it may be best to refer patients who would like to use a weight loss medication to a specialist or specialty center with expertise in using these medications.
Sustainable weight loss is very difficult to achieve through dieting alone. A multitude of dietary interventions have been presented as “revolutionary approaches” to the challenging problem of sustainable weight loss, including the Paleo diet, the Vegan diet, the low-carb diet, the Dukan diet, the ultra-lowfat diet, the Atkins diet, the HCG diet, the Zone diet, the South Beach diet, the plant-based diet, the Mediterranean diet, the Asian diet, and intermittent fasting. Recently, intermittent fasting has been presented as the latest and greatest approach to dieting, with the dual goals of achieving weight loss and improved health.1 In some animal models, intermittent dieting has been shown to increase life-span, a finding that has attracted great interest. A major goal of intermittent fasting is to promote “metabolic switching” with increased reliance on ketones to fuel cellular energy needs.
Two approaches to “prescribing” an intermittent fasting diet are to limit food intake to a period of 6 to 10 hours each day or to markedly reduce caloric intake one or two days per week, for example to 750 calories in a 24-hour period. There are no long-term studies of the health outcomes associated with intermittent fasting. In head-to-head clinical trials of intermittent fasting and daily calorie restriction (classic dieting), both diets result in similar weight loss. For example, in one clinical trial 100 obese participants, with a mean body mass index (BMI) of 34 kg/m2 , including 86 women, were randomly assigned to2:
1. intermittent fasting (25% of energy needs every other day)
2. daily calorie restriction (75% of energy needs every day), or
3. no intervention.
After 12 months of follow up, the participants in the no intervention group had gained 0.5% of their starting weight. The intermittent fasting and the daily calorie restriction groups had similar amounts of weight loss, approximately 5% of their starting weight. More individuals dropped out of the study from the intermittent fasting group than the daily calorie restriction group (38% vs 29%, respectively).
In another clinical trial, 107 overweight or obese premenopausal women, average age 40 years and mean BMI 31 kg/m2 , were randomly assigned to intermittent fasting (25% of energy needs 2 days per week) or daily calorie restriction (75% of energy needs daily) for 6 months. The mean weight of the participants at baseline was 83 kg. Weight loss was similar in the intermittent fasting and daily calorie restriction groups, 6.4 kg (-7.7%) and 5.6 kg (-6.7%), respectively (P=.4).3
The investigators concluded that intermittent fasting and daily calorie restriction could both be offered as effective approaches to weight loss. My conclusion is that intermittent fasting is not a miracle dietary intervention, but it is another important option in the armamentarium of weight loss interventions.
References
1. de Cabo R, Mattson MP. Effects of intermittent fasting on health, aging and disease. N Engl J Med. 2019;381:2541-2551.
2. Trepanowski JF, Kroeger CM, Barnosky A, et al. Effect of alternate-day fasting on weight loss, weight maintenance, and cardioprotection among metabolically healthy obese adults: a randomized clinical trial. JAMA Intern Med. 2017;177:930-938.
3. Harvie MN, Pegington M, Mattson MP, et al. The effects of intermittent or continuous energy restriction on weight loss and metabolic disc disease risk markers: a randomized trial in young overweight women. Int J Obes (Lond). 2011;35:714-727.
Sleeve gastrectomy
Two children are playing in a school yard. One child proudly states, “My mother is an endocrinologist. She treats diabetes.” Not to be outdone, the other child replies, “My mother is a bariatric surgeon. She cures diabetes.”
The dialogue reflects the reality that bariatric surgery results in more reliable and significant weight loss than diet, exercise, or weight loss medications. Diet, exercise, and weight loss medications often result in a 5% to 10% decrease in weight, but bariatric surgery typically results in a 25% decrease in weight. Until recently, 3 bariatric surgical procedures were commonly performed: Roux-en-Y gastric bypass (RYGB), sleeve gastrectomy (SG), and adjustable gastric banding (AGB). AGB is now seldom performed because it is less effective than RYGB and SG. Two recently published randomized trials compared the long-term outcomes associated with RYGB and SG. The studies found that SG and RYGB result in a similar degree of weight loss. RYGB resulted in slightly more weight loss than SG, but SG was associated with a lower rate of major complications, such as internal hernias. SG takes much less time to perform than RYGB. SG has become the most commonly performed bariatric surgery in premenopausal women considering pregnancy because of the low risk of internal hernias.
In the Swiss Multicenter Bypass or Sleeve Study (SM-BOSS), 217 participants with a mean BMI of 44 kg/m2 and mean age of 45.5 years were randomly assigned to RYGB or SG and followed for 5 years.11 The majority (72%) of the participants were women. At 5 years of follow-up, in the RYGB and SG groups, mean weight loss was 37 kg and 33 kg, respectively (P=.19). In both groups, weight loss nadir was reached 12 to 24 months after surgery. Expressed as a percentage of original weight, weight loss in the RYGB and SG groups was -29% and -25%, respectively (P=.02). Gastric reflux worsened in both the RYGB and SG groups (6% vs 32%, respectively). The number of reoperations in the RYGB and SG groups was 22% and 16%. Of note, among individuals with prevalent diabetes, RYGB and SG resulted in remission of the diabetes in 68% and 62% of participants, respectively.
In the Sleeve vs Bypass study (SLEEVEPASS), 240 participants, with mean BMI of 46 kg/m2 and mean age of 48 years, were randomly assigned to RYGB or SG and followed for 5 years.12 Most (70%) of the participants were women. Following bariatric surgery, BMI decreased significantly in both groups. In the RYGB group, BMI decreased from 48 kg/m2 preoperatively to 35.4 kg/m2 at 5 years of follow up. In the SG group, BMI decreased from 47 kg/m2 preoperatively to 36.5 kg/m2 at 5 years of follow up. Late major complications (defined as complications occurring from 30 days to 5 years postoperatively) occurred more frequently in the RYGB group (15%) versus the SG group (8%). All the late major complications required reoperation. In the SG group, 7 of 10 reoperations were for severe gastric reflux disease. In the RYGB group 17 of 18 reoperations were for suspected internal hernia, requiring closure of a mesenteric defect at reoperation. There was no treatment-related mortality during the 5-year follow up.
Guidelines for bariatric surgery are BMI ≥ 40 kg/m2 without a comorbid illness or BMI ≥ 35 kg/m2 with at least one serious comorbid disease, such as diabetes.13 ObGyns can build a synergistic relationship with bariatric surgeons by referring eligible patients for surgical consultation and, in return, accepting referrals. A paradox and challenge is that many health insurers require patients to complete a supervised medical weight loss management program prior to being approved for bariatric surgery. However, the medical weight loss program might result in the patient no longer being eligible for insurance coverage of their surgery. For example, a patient who had a BMI of 42 kg/m2 prior to a medical weight loss management program who then lost enough weight to achieve a BMI of 38 kg/m2 might no longer be eligible for insurance coverage of a bariatric operation.14
Continue to: ObGyns need to prioritize treatment for obesity...
ObGyns need to prioritize treatment for obesity
Between 1959 and 2014, US life expectancy increased from 69.9 years to 79.1 years. However, in 2015 and 2016 life expectancy in the United States decreased slightly to 78.9 years, while continuing to improve in other countries.15 What could cause such an unexpected trend? Some experts believe that excess overweight and obesity in the US population, resulting in increased rates of diabetes, hypertension, and heart disease, accounts for a significant proportion of the life expectancy gap between US citizens and those who reside in Australia, Finland, Japan, and Sweden.16,17 All frontline clinicians play an important role in reversing the decades-long trend of increasing rates of overweight and obesity. Interventions that ObGyns could prioritize in their practices for treating overweight and obese patients include: a calorie-restricted diet, exercise, metformin, and SG.
- U.S. Department of Health and Human Services. The Health Consequences of Smoking—50 Years of Progress. A Report of the Surgeon General. Atlanta: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2014.
- Allison DB, Fontaine KR, Manson JE, et al. Annual deaths attributable to obesity in the United States. JAMA. 1999;282:1530-1538.
- Ward ZJ, Bleich SN, Cradock AL, et al. Projected U.S. state-level prevalence of adult obesity and severe obesity. N Engl J Med. 2019;381:2440-2450.
- American Heart Association. My life check | Life’s simple 7. https://www.heart.org/en/healthyliving/healthy-lifestyle/my-life-check--lifessimple-7. Reviewed May 2, 2018. Accessed February 10, 2020.
- Knowler WC, Barrett-Connor E, Fowler SE, et al; Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393-403.
- Diabetes Prevention Program Research Group. Long-term safety, tolerability and weight loss associated with metformin in the Diabetes Prevention Program Outcomes Study. Diabetes Care. 2012;35:731-737.
- Winder WW, Hardie DG. Inactivation of acetylCoA carboxylase and activation of AMP-activated protein kinase in muscle during exercise. Am J Physiol. 1996;270(2 pt 1):E299-E304.
- Lexicomp. https://online.lexi.com/lco/action/ home. Accessed February 13, 2020.
- Metformin ER (Glucophage XR). GoodRX website. https://www.goodrx.com/metformin-erglucophage-xr?dosage=750mg&form=tablet&la bel_override=metformin+ER+%28Glucophage+X R%29&quantity=60. Accessed February 13, 2020.
- GoodRX website. www.goodrx.com. Accessed February 10, 2020.
- Peterli R, Wolnerhanssen BK, Peters T, et al. Effect of laparoscopic sleeve gastrectomy vs laparoscopic Roux-en-Y gastric bypass on weight loss in patients with morbid obesity: the SM-BOSS randomized clinical trial. JAMA. 2018;319:255-265.
- Salminen P, Helmiö M, Ovaska J, et al. Effect of laparoscopic sleeve gastrectomy versus laparoscopic Roux-en-Y gastric bypass on weight loss at 5 years among patients with morbid obesity: The SLEEVEPASS randomized clinical trial. JAMA. 2018;319:241-254.
- Rubino F, Nathan DM, Eckel RH, et al; Delegates of the 2nd Diabetes Surgery Summit. Metabolic surgery in the treatment algorithm for type 2 diabetes: a joint statement by international diabetes organizations. Obes Surg. 2017;27:2-21.
- Gebran SG, Knighton B, Ngaage LM, et al. Insurance coverage criteria for bariatric surgery: a survey of policies. Obes Surg. 2020;30:707-713.
- Woolf SH, Schoomaker H. Life expectancy and mortality rates in the United States, 1959-2017. JAMA. 2019;322:1996-2016.
- Preston SH, Vierboom YC, Stokes A. The role of obesity in exceptionally slow US mortality improvement. Proc Natl Acad Sci U S A. 2019;115:957-961.
- Xu H, Cupples LA, Stokes A, et al. Association of obesity with mortality over 24 years of weight history: findings from the Framingham Heart Study. JAMA Network Open. 2018;1:e184587.
- U.S. Department of Health and Human Services. The Health Consequences of Smoking—50 Years of Progress. A Report of the Surgeon General. Atlanta: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2014.
- Allison DB, Fontaine KR, Manson JE, et al. Annual deaths attributable to obesity in the United States. JAMA. 1999;282:1530-1538.
- Ward ZJ, Bleich SN, Cradock AL, et al. Projected U.S. state-level prevalence of adult obesity and severe obesity. N Engl J Med. 2019;381:2440-2450.
- American Heart Association. My life check | Life’s simple 7. https://www.heart.org/en/healthyliving/healthy-lifestyle/my-life-check--lifessimple-7. Reviewed May 2, 2018. Accessed February 10, 2020.
- Knowler WC, Barrett-Connor E, Fowler SE, et al; Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393-403.
- Diabetes Prevention Program Research Group. Long-term safety, tolerability and weight loss associated with metformin in the Diabetes Prevention Program Outcomes Study. Diabetes Care. 2012;35:731-737.
- Winder WW, Hardie DG. Inactivation of acetylCoA carboxylase and activation of AMP-activated protein kinase in muscle during exercise. Am J Physiol. 1996;270(2 pt 1):E299-E304.
- Lexicomp. https://online.lexi.com/lco/action/ home. Accessed February 13, 2020.
- Metformin ER (Glucophage XR). GoodRX website. https://www.goodrx.com/metformin-erglucophage-xr?dosage=750mg&form=tablet&la bel_override=metformin+ER+%28Glucophage+X R%29&quantity=60. Accessed February 13, 2020.
- GoodRX website. www.goodrx.com. Accessed February 10, 2020.
- Peterli R, Wolnerhanssen BK, Peters T, et al. Effect of laparoscopic sleeve gastrectomy vs laparoscopic Roux-en-Y gastric bypass on weight loss in patients with morbid obesity: the SM-BOSS randomized clinical trial. JAMA. 2018;319:255-265.
- Salminen P, Helmiö M, Ovaska J, et al. Effect of laparoscopic sleeve gastrectomy versus laparoscopic Roux-en-Y gastric bypass on weight loss at 5 years among patients with morbid obesity: The SLEEVEPASS randomized clinical trial. JAMA. 2018;319:241-254.
- Rubino F, Nathan DM, Eckel RH, et al; Delegates of the 2nd Diabetes Surgery Summit. Metabolic surgery in the treatment algorithm for type 2 diabetes: a joint statement by international diabetes organizations. Obes Surg. 2017;27:2-21.
- Gebran SG, Knighton B, Ngaage LM, et al. Insurance coverage criteria for bariatric surgery: a survey of policies. Obes Surg. 2020;30:707-713.
- Woolf SH, Schoomaker H. Life expectancy and mortality rates in the United States, 1959-2017. JAMA. 2019;322:1996-2016.
- Preston SH, Vierboom YC, Stokes A. The role of obesity in exceptionally slow US mortality improvement. Proc Natl Acad Sci U S A. 2019;115:957-961.
- Xu H, Cupples LA, Stokes A, et al. Association of obesity with mortality over 24 years of weight history: findings from the Framingham Heart Study. JAMA Network Open. 2018;1:e184587.
June Medical Services v. Russo: Understanding this high-stakes abortion case
On March 4, 2020, the Supreme Court of the United States (SCOTUS) will hear opening arguments for June Medical Services v. Russo. (Please note that this case was originally referred to as June Medical Services v. Gee. However, Secretary Rebekah Gee resigned from her position on January 31, 2020, and was replaced by Interim Secretary Stephen Russo.) The case will examine a Louisiana law (Louisiana Act 620, or LA 620), originally passed in 2014, that requires physicians to have hospital admitting privileges within 30 miles of where they provide abortion services.1 When LA 620 was signed into law in 2014, 5 of Louisiana’s 6 abortion clinics would not have met the standards created by this legislation and would have been forced to close, potentially leaving the vast majority of women in Louisiana without access to an abortion provider, and disproportionately impacting poor and rural women. Prior to enactment of this law, physicians at these 5 clinics attempted to obtain admitting privileges, and all were denied. The denials occurred due to two main reasons—because the providers admitted too few patients each year to qualify for hospital privileges or simply because they provided abortion care.2 Shortly after this legislation was signed into law, the Center for Reproductive Rights (CRR) challenged the law, citing the undue burden it created for patients attempting to access abortion care.
Prior case also considered question of hospital privileges for abortion providers
Interestingly, SCOTUS already has ruled on this very question. In 1992, the Court ruled in Planned Parenthood of Southeastern Pennsylvania v. Casey that it is unconstitutional for a state to create an “undue burden” on a woman’s right to abortion prior to fetal viability.3 And in 2016, when considering whether or not requiring abortion providers to obtain hospital privileges creates an undue burden in Whole Women’s Health (WWH) v. Hellerstedt, the Supreme Court’s answer was yes, it does. WWH, with legal aid from CRR, challenged Texas House Bill 2 (H.B. 2), which similar to LA 620, required abortion providers to have local admitting privileges. Based largely on the precedent set in Casey, SCOTUS ruled 5-3 in favor of WWH.
The Louisiana law currently in question was written and challenged in district court simultaneous to the Supreme Court’s review of WWH. The district court declared LA 620 invalid and permanently enjoined its enforcement, finding the law would “drastically burden women’s right to choose abortions.”4 However, the US Court of Appeals for the Fifth Circuit reviewed the case and overturned the district court decision, finding the lower court’s analysis erroneous and stating, “no clinics will likely be forced to close on account of [LA 620].” The Fifth Circuit panel ruled that, despite the precedent of WWH, LA 620 did not create an undue burden because of state-level differences in admitting privileges, demographics, and geography. They also found that only 30% of the 2 million women living in Louisiana would be impacted by the law, predominantly via longer wait times, and argued that this does not represent significant burden. The plaintiffs filed for an emergency stay with SCOTUS, who granted the stay pending a full hearing. On March 4, the Supreme Court will hear arguments to determine if the Fifth Circuit was correct in drawing a distinction between LA 620 and the SCOTUS verdict in WWH.
Targeted restrictions on abortion providers
LA 620 joins a long series of laws meant to enact targeted restrictions on abortion providers, or “TRAP” laws. TRAP laws are written to limit access to abortion under the guise of improving patient safety, despite ample evidence to the contrary, and include such various regulations as admitting privileges, facilities requirements, waiting periods, and parental or partner notification. Many such laws have been enacted in the last decade, and many struck down based on judicial precedent.
How the Supreme Court has ruled in the past
When a case is appealed to the Supreme Court, the court can either decline to hear the case, thereby leaving the lower courts’ ruling in place, or choose to hear the case in full and either affirm or overturn the lower court’s decision. After issuing a ruling in WWH, the 2016-2017 Roberts Court declined to hear challenges from other states with similarly overturned laws, leaving the laws struck down. In electing to hear June Medical Services v. Russo, the court has the opportunity to uphold or overturn the Fifth Circuit Court’s decision. However, today’s Supreme Court differs greatly from the Supreme Court in 2016.
In 2016, the court ruled 5-3 to overturn H.B. 2 in WWH shortly after the death of Justice Antonin Scalia. Scalia was replaced by Justice Neil Gorsuch, a Constitutional originalist who has never directly ruled on an abortion case.5 In 2018, Justice Anthony Kennedy, who authored the court’s majority opinion on Casey and was among the majority on WWH, retired, and was replaced by Justice Brett Kavanaugh. Kavanaugh has ruled once on the right to abortion in Garza v. Hargan in 2017, where he argued that precedent states that the government has “permissible interests in favoring fetal life…and refraining from facilitating abortion,” and that significant delay in care did not constitute undue burden.6 In regard to the 5-4 stay issued by the court in June Medical Services, Kavanaugh joined Gorsuch in voting to deny the application for stay, and was the only justice to issue an opinion alongside the ruling, arguing that because the doctors in question had not applied for and been denied admitting privileges since the WWH ruling, the case hinges on theoretical rather than demonstrable undue burden.7 Appointed by President Donald Trump, both Gorsuch and Kavanaugh are widely considered to be conservative judges, and while neither has a strong judicial record on abortion rights, both are anticipated to side with the conservative majority on the court.
The Supreme Court rarely overturns its own precedent, but concerns are high
The question of precedent will be central in SCOTUS hearing June Medical Services v. Russo so quickly after the WWH decision. Additionally, in hearing this case, the court will have the opportunity to reexamine all relevant precedent, including the Planned Parenthood of Southeastern Pennsylvania v. Casey decision and even Roe v. Wade. With a conservative court and an increasingly charged political environment, reproductive rights advocates fear that the June Medical Services v. Russo ruling may be the first step toward dismantling judicial protection of abortion rights in the United States.
If SCOTUS rules against June Medical Services, stating that admitting privileges do not cause an undue burden for women seeking to access abortion care, other states likely will introduce and enact similar legislation. These TRAP laws have the potential to limit or eliminate access to abortion for 25 million people of reproductive age. Numerous studies have demonstrated that limiting access to abortion care does not decrease the number of abortions but can result in patients using unsafe means to obtain an abortion.8
The medical community recognizes the danger of enacting restrictive legislation. The American College of Obstetricians and Gynecologists (ACOG), along with the American Medical Association, the Society of Maternal-Fetal Medicine, the Association for Sexual and Reproductive Medicine, the American Association of Family Practitioners, and many others, filed an amicus curiae in support of the June Medical Services plaintiffs.9 These brief filings are critical to ensuring the courts hear physician voices in this important legal decision, and ACOG’s briefs have been quoted in several previous Supreme Court opinions, concurrences, and dissents.
Action items
- Although June Medical Services v. Russo’s decision will not be made until early summer 2020, we can continue to use our voices and expertise to speak out against laws designed to limit access to abortion—at the state and federal levels. As women’s health clinicians, we see the impact abortion restrictions have on our patients, especially our low income and rural patients. Sharing these stories with our legislators, testifying for or against legislation, and speaking out in our communities can have a powerful impact. Check with your local ACOG chapter or with ACOG’s state and government affairs office for more information.
- Follow along with this case at SCOTUS Blog.
- Lastly, make sure you are registered to vote. We are in an election year, and using our voices in and out of the ballot box is critical. You can register here.
- HB338. Louisiana State Legislature. 2014. http://www.legis.la.gov/legis/BillInfo.aspx?s=14RS&b=ACT620&sbi=y. Accessed February 19, 2020.
- Nash E, Donovan MK. Admitting priveleges are back at the U.S. Supreme Court with serious implications for abortion access. Guttmacher Institute. Updated December 3, 2019.
- Planned Parenthood of Southeastern Pennsylvania v. Casey. Cornell Law School Legal Information Institute. https://www.law.cornell.edu/supremecourt/text/505/833. Accessed February 20, 2020.
- June Medical Services LLC v Gee. Oyez. www.oyez.org/cases/2019/18-1323. Accessed February 20, 2020.
- Neil Gorsuch. Oyez. https://www.oyez.org/justices/neil_gorsuch. Accessed February 20, 2020.
- Judge Kavanaugh’s Judicial Record on the Right to Abortion. Center for Reproductive Rights. https://www.reproductiverights.org/sites/crr.civicactions.net/files/documents/factsheets/Judge-Kavanaugh-Judicial-Record-on-the-Right-to-Abortion2.pdf. Accessed February 20, 2020.
- Kavanaugh B. (2019, February 7). June Medical Services, L.L.C, v. Gee, 586 U.S. ____ (2019). Supreme Court of the United States. https://www.supremecourt.gov/opinions/18pdf/18a774_3ebh.pdf. Accessed February 20, 2020.
- Cohen SA. Facts and consequences: Legality, incidence and safety of abortion worldwide. November 20, 2009.
- June Medical Services, LLC v. Russo. SCOTUSblog. February 6, 2020. https://www.scotusblog.com/case-files/cases/june-medical-services-llc-v-russo/. Accessed February 20, 2020.
On March 4, 2020, the Supreme Court of the United States (SCOTUS) will hear opening arguments for June Medical Services v. Russo. (Please note that this case was originally referred to as June Medical Services v. Gee. However, Secretary Rebekah Gee resigned from her position on January 31, 2020, and was replaced by Interim Secretary Stephen Russo.) The case will examine a Louisiana law (Louisiana Act 620, or LA 620), originally passed in 2014, that requires physicians to have hospital admitting privileges within 30 miles of where they provide abortion services.1 When LA 620 was signed into law in 2014, 5 of Louisiana’s 6 abortion clinics would not have met the standards created by this legislation and would have been forced to close, potentially leaving the vast majority of women in Louisiana without access to an abortion provider, and disproportionately impacting poor and rural women. Prior to enactment of this law, physicians at these 5 clinics attempted to obtain admitting privileges, and all were denied. The denials occurred due to two main reasons—because the providers admitted too few patients each year to qualify for hospital privileges or simply because they provided abortion care.2 Shortly after this legislation was signed into law, the Center for Reproductive Rights (CRR) challenged the law, citing the undue burden it created for patients attempting to access abortion care.
Prior case also considered question of hospital privileges for abortion providers
Interestingly, SCOTUS already has ruled on this very question. In 1992, the Court ruled in Planned Parenthood of Southeastern Pennsylvania v. Casey that it is unconstitutional for a state to create an “undue burden” on a woman’s right to abortion prior to fetal viability.3 And in 2016, when considering whether or not requiring abortion providers to obtain hospital privileges creates an undue burden in Whole Women’s Health (WWH) v. Hellerstedt, the Supreme Court’s answer was yes, it does. WWH, with legal aid from CRR, challenged Texas House Bill 2 (H.B. 2), which similar to LA 620, required abortion providers to have local admitting privileges. Based largely on the precedent set in Casey, SCOTUS ruled 5-3 in favor of WWH.
The Louisiana law currently in question was written and challenged in district court simultaneous to the Supreme Court’s review of WWH. The district court declared LA 620 invalid and permanently enjoined its enforcement, finding the law would “drastically burden women’s right to choose abortions.”4 However, the US Court of Appeals for the Fifth Circuit reviewed the case and overturned the district court decision, finding the lower court’s analysis erroneous and stating, “no clinics will likely be forced to close on account of [LA 620].” The Fifth Circuit panel ruled that, despite the precedent of WWH, LA 620 did not create an undue burden because of state-level differences in admitting privileges, demographics, and geography. They also found that only 30% of the 2 million women living in Louisiana would be impacted by the law, predominantly via longer wait times, and argued that this does not represent significant burden. The plaintiffs filed for an emergency stay with SCOTUS, who granted the stay pending a full hearing. On March 4, the Supreme Court will hear arguments to determine if the Fifth Circuit was correct in drawing a distinction between LA 620 and the SCOTUS verdict in WWH.
Targeted restrictions on abortion providers
LA 620 joins a long series of laws meant to enact targeted restrictions on abortion providers, or “TRAP” laws. TRAP laws are written to limit access to abortion under the guise of improving patient safety, despite ample evidence to the contrary, and include such various regulations as admitting privileges, facilities requirements, waiting periods, and parental or partner notification. Many such laws have been enacted in the last decade, and many struck down based on judicial precedent.
How the Supreme Court has ruled in the past
When a case is appealed to the Supreme Court, the court can either decline to hear the case, thereby leaving the lower courts’ ruling in place, or choose to hear the case in full and either affirm or overturn the lower court’s decision. After issuing a ruling in WWH, the 2016-2017 Roberts Court declined to hear challenges from other states with similarly overturned laws, leaving the laws struck down. In electing to hear June Medical Services v. Russo, the court has the opportunity to uphold or overturn the Fifth Circuit Court’s decision. However, today’s Supreme Court differs greatly from the Supreme Court in 2016.
In 2016, the court ruled 5-3 to overturn H.B. 2 in WWH shortly after the death of Justice Antonin Scalia. Scalia was replaced by Justice Neil Gorsuch, a Constitutional originalist who has never directly ruled on an abortion case.5 In 2018, Justice Anthony Kennedy, who authored the court’s majority opinion on Casey and was among the majority on WWH, retired, and was replaced by Justice Brett Kavanaugh. Kavanaugh has ruled once on the right to abortion in Garza v. Hargan in 2017, where he argued that precedent states that the government has “permissible interests in favoring fetal life…and refraining from facilitating abortion,” and that significant delay in care did not constitute undue burden.6 In regard to the 5-4 stay issued by the court in June Medical Services, Kavanaugh joined Gorsuch in voting to deny the application for stay, and was the only justice to issue an opinion alongside the ruling, arguing that because the doctors in question had not applied for and been denied admitting privileges since the WWH ruling, the case hinges on theoretical rather than demonstrable undue burden.7 Appointed by President Donald Trump, both Gorsuch and Kavanaugh are widely considered to be conservative judges, and while neither has a strong judicial record on abortion rights, both are anticipated to side with the conservative majority on the court.
The Supreme Court rarely overturns its own precedent, but concerns are high
The question of precedent will be central in SCOTUS hearing June Medical Services v. Russo so quickly after the WWH decision. Additionally, in hearing this case, the court will have the opportunity to reexamine all relevant precedent, including the Planned Parenthood of Southeastern Pennsylvania v. Casey decision and even Roe v. Wade. With a conservative court and an increasingly charged political environment, reproductive rights advocates fear that the June Medical Services v. Russo ruling may be the first step toward dismantling judicial protection of abortion rights in the United States.
If SCOTUS rules against June Medical Services, stating that admitting privileges do not cause an undue burden for women seeking to access abortion care, other states likely will introduce and enact similar legislation. These TRAP laws have the potential to limit or eliminate access to abortion for 25 million people of reproductive age. Numerous studies have demonstrated that limiting access to abortion care does not decrease the number of abortions but can result in patients using unsafe means to obtain an abortion.8
The medical community recognizes the danger of enacting restrictive legislation. The American College of Obstetricians and Gynecologists (ACOG), along with the American Medical Association, the Society of Maternal-Fetal Medicine, the Association for Sexual and Reproductive Medicine, the American Association of Family Practitioners, and many others, filed an amicus curiae in support of the June Medical Services plaintiffs.9 These brief filings are critical to ensuring the courts hear physician voices in this important legal decision, and ACOG’s briefs have been quoted in several previous Supreme Court opinions, concurrences, and dissents.
Action items
- Although June Medical Services v. Russo’s decision will not be made until early summer 2020, we can continue to use our voices and expertise to speak out against laws designed to limit access to abortion—at the state and federal levels. As women’s health clinicians, we see the impact abortion restrictions have on our patients, especially our low income and rural patients. Sharing these stories with our legislators, testifying for or against legislation, and speaking out in our communities can have a powerful impact. Check with your local ACOG chapter or with ACOG’s state and government affairs office for more information.
- Follow along with this case at SCOTUS Blog.
- Lastly, make sure you are registered to vote. We are in an election year, and using our voices in and out of the ballot box is critical. You can register here.
On March 4, 2020, the Supreme Court of the United States (SCOTUS) will hear opening arguments for June Medical Services v. Russo. (Please note that this case was originally referred to as June Medical Services v. Gee. However, Secretary Rebekah Gee resigned from her position on January 31, 2020, and was replaced by Interim Secretary Stephen Russo.) The case will examine a Louisiana law (Louisiana Act 620, or LA 620), originally passed in 2014, that requires physicians to have hospital admitting privileges within 30 miles of where they provide abortion services.1 When LA 620 was signed into law in 2014, 5 of Louisiana’s 6 abortion clinics would not have met the standards created by this legislation and would have been forced to close, potentially leaving the vast majority of women in Louisiana without access to an abortion provider, and disproportionately impacting poor and rural women. Prior to enactment of this law, physicians at these 5 clinics attempted to obtain admitting privileges, and all were denied. The denials occurred due to two main reasons—because the providers admitted too few patients each year to qualify for hospital privileges or simply because they provided abortion care.2 Shortly after this legislation was signed into law, the Center for Reproductive Rights (CRR) challenged the law, citing the undue burden it created for patients attempting to access abortion care.
Prior case also considered question of hospital privileges for abortion providers
Interestingly, SCOTUS already has ruled on this very question. In 1992, the Court ruled in Planned Parenthood of Southeastern Pennsylvania v. Casey that it is unconstitutional for a state to create an “undue burden” on a woman’s right to abortion prior to fetal viability.3 And in 2016, when considering whether or not requiring abortion providers to obtain hospital privileges creates an undue burden in Whole Women’s Health (WWH) v. Hellerstedt, the Supreme Court’s answer was yes, it does. WWH, with legal aid from CRR, challenged Texas House Bill 2 (H.B. 2), which similar to LA 620, required abortion providers to have local admitting privileges. Based largely on the precedent set in Casey, SCOTUS ruled 5-3 in favor of WWH.
The Louisiana law currently in question was written and challenged in district court simultaneous to the Supreme Court’s review of WWH. The district court declared LA 620 invalid and permanently enjoined its enforcement, finding the law would “drastically burden women’s right to choose abortions.”4 However, the US Court of Appeals for the Fifth Circuit reviewed the case and overturned the district court decision, finding the lower court’s analysis erroneous and stating, “no clinics will likely be forced to close on account of [LA 620].” The Fifth Circuit panel ruled that, despite the precedent of WWH, LA 620 did not create an undue burden because of state-level differences in admitting privileges, demographics, and geography. They also found that only 30% of the 2 million women living in Louisiana would be impacted by the law, predominantly via longer wait times, and argued that this does not represent significant burden. The plaintiffs filed for an emergency stay with SCOTUS, who granted the stay pending a full hearing. On March 4, the Supreme Court will hear arguments to determine if the Fifth Circuit was correct in drawing a distinction between LA 620 and the SCOTUS verdict in WWH.
Targeted restrictions on abortion providers
LA 620 joins a long series of laws meant to enact targeted restrictions on abortion providers, or “TRAP” laws. TRAP laws are written to limit access to abortion under the guise of improving patient safety, despite ample evidence to the contrary, and include such various regulations as admitting privileges, facilities requirements, waiting periods, and parental or partner notification. Many such laws have been enacted in the last decade, and many struck down based on judicial precedent.
How the Supreme Court has ruled in the past
When a case is appealed to the Supreme Court, the court can either decline to hear the case, thereby leaving the lower courts’ ruling in place, or choose to hear the case in full and either affirm or overturn the lower court’s decision. After issuing a ruling in WWH, the 2016-2017 Roberts Court declined to hear challenges from other states with similarly overturned laws, leaving the laws struck down. In electing to hear June Medical Services v. Russo, the court has the opportunity to uphold or overturn the Fifth Circuit Court’s decision. However, today’s Supreme Court differs greatly from the Supreme Court in 2016.
In 2016, the court ruled 5-3 to overturn H.B. 2 in WWH shortly after the death of Justice Antonin Scalia. Scalia was replaced by Justice Neil Gorsuch, a Constitutional originalist who has never directly ruled on an abortion case.5 In 2018, Justice Anthony Kennedy, who authored the court’s majority opinion on Casey and was among the majority on WWH, retired, and was replaced by Justice Brett Kavanaugh. Kavanaugh has ruled once on the right to abortion in Garza v. Hargan in 2017, where he argued that precedent states that the government has “permissible interests in favoring fetal life…and refraining from facilitating abortion,” and that significant delay in care did not constitute undue burden.6 In regard to the 5-4 stay issued by the court in June Medical Services, Kavanaugh joined Gorsuch in voting to deny the application for stay, and was the only justice to issue an opinion alongside the ruling, arguing that because the doctors in question had not applied for and been denied admitting privileges since the WWH ruling, the case hinges on theoretical rather than demonstrable undue burden.7 Appointed by President Donald Trump, both Gorsuch and Kavanaugh are widely considered to be conservative judges, and while neither has a strong judicial record on abortion rights, both are anticipated to side with the conservative majority on the court.
The Supreme Court rarely overturns its own precedent, but concerns are high
The question of precedent will be central in SCOTUS hearing June Medical Services v. Russo so quickly after the WWH decision. Additionally, in hearing this case, the court will have the opportunity to reexamine all relevant precedent, including the Planned Parenthood of Southeastern Pennsylvania v. Casey decision and even Roe v. Wade. With a conservative court and an increasingly charged political environment, reproductive rights advocates fear that the June Medical Services v. Russo ruling may be the first step toward dismantling judicial protection of abortion rights in the United States.
If SCOTUS rules against June Medical Services, stating that admitting privileges do not cause an undue burden for women seeking to access abortion care, other states likely will introduce and enact similar legislation. These TRAP laws have the potential to limit or eliminate access to abortion for 25 million people of reproductive age. Numerous studies have demonstrated that limiting access to abortion care does not decrease the number of abortions but can result in patients using unsafe means to obtain an abortion.8
The medical community recognizes the danger of enacting restrictive legislation. The American College of Obstetricians and Gynecologists (ACOG), along with the American Medical Association, the Society of Maternal-Fetal Medicine, the Association for Sexual and Reproductive Medicine, the American Association of Family Practitioners, and many others, filed an amicus curiae in support of the June Medical Services plaintiffs.9 These brief filings are critical to ensuring the courts hear physician voices in this important legal decision, and ACOG’s briefs have been quoted in several previous Supreme Court opinions, concurrences, and dissents.
Action items
- Although June Medical Services v. Russo’s decision will not be made until early summer 2020, we can continue to use our voices and expertise to speak out against laws designed to limit access to abortion—at the state and federal levels. As women’s health clinicians, we see the impact abortion restrictions have on our patients, especially our low income and rural patients. Sharing these stories with our legislators, testifying for or against legislation, and speaking out in our communities can have a powerful impact. Check with your local ACOG chapter or with ACOG’s state and government affairs office for more information.
- Follow along with this case at SCOTUS Blog.
- Lastly, make sure you are registered to vote. We are in an election year, and using our voices in and out of the ballot box is critical. You can register here.
- HB338. Louisiana State Legislature. 2014. http://www.legis.la.gov/legis/BillInfo.aspx?s=14RS&b=ACT620&sbi=y. Accessed February 19, 2020.
- Nash E, Donovan MK. Admitting priveleges are back at the U.S. Supreme Court with serious implications for abortion access. Guttmacher Institute. Updated December 3, 2019.
- Planned Parenthood of Southeastern Pennsylvania v. Casey. Cornell Law School Legal Information Institute. https://www.law.cornell.edu/supremecourt/text/505/833. Accessed February 20, 2020.
- June Medical Services LLC v Gee. Oyez. www.oyez.org/cases/2019/18-1323. Accessed February 20, 2020.
- Neil Gorsuch. Oyez. https://www.oyez.org/justices/neil_gorsuch. Accessed February 20, 2020.
- Judge Kavanaugh’s Judicial Record on the Right to Abortion. Center for Reproductive Rights. https://www.reproductiverights.org/sites/crr.civicactions.net/files/documents/factsheets/Judge-Kavanaugh-Judicial-Record-on-the-Right-to-Abortion2.pdf. Accessed February 20, 2020.
- Kavanaugh B. (2019, February 7). June Medical Services, L.L.C, v. Gee, 586 U.S. ____ (2019). Supreme Court of the United States. https://www.supremecourt.gov/opinions/18pdf/18a774_3ebh.pdf. Accessed February 20, 2020.
- Cohen SA. Facts and consequences: Legality, incidence and safety of abortion worldwide. November 20, 2009.
- June Medical Services, LLC v. Russo. SCOTUSblog. February 6, 2020. https://www.scotusblog.com/case-files/cases/june-medical-services-llc-v-russo/. Accessed February 20, 2020.
- HB338. Louisiana State Legislature. 2014. http://www.legis.la.gov/legis/BillInfo.aspx?s=14RS&b=ACT620&sbi=y. Accessed February 19, 2020.
- Nash E, Donovan MK. Admitting priveleges are back at the U.S. Supreme Court with serious implications for abortion access. Guttmacher Institute. Updated December 3, 2019.
- Planned Parenthood of Southeastern Pennsylvania v. Casey. Cornell Law School Legal Information Institute. https://www.law.cornell.edu/supremecourt/text/505/833. Accessed February 20, 2020.
- June Medical Services LLC v Gee. Oyez. www.oyez.org/cases/2019/18-1323. Accessed February 20, 2020.
- Neil Gorsuch. Oyez. https://www.oyez.org/justices/neil_gorsuch. Accessed February 20, 2020.
- Judge Kavanaugh’s Judicial Record on the Right to Abortion. Center for Reproductive Rights. https://www.reproductiverights.org/sites/crr.civicactions.net/files/documents/factsheets/Judge-Kavanaugh-Judicial-Record-on-the-Right-to-Abortion2.pdf. Accessed February 20, 2020.
- Kavanaugh B. (2019, February 7). June Medical Services, L.L.C, v. Gee, 586 U.S. ____ (2019). Supreme Court of the United States. https://www.supremecourt.gov/opinions/18pdf/18a774_3ebh.pdf. Accessed February 20, 2020.
- Cohen SA. Facts and consequences: Legality, incidence and safety of abortion worldwide. November 20, 2009.
- June Medical Services, LLC v. Russo. SCOTUSblog. February 6, 2020. https://www.scotusblog.com/case-files/cases/june-medical-services-llc-v-russo/. Accessed February 20, 2020.
Progestin-only systemic hormone therapy for menopausal hot flashes
The field of menopause medicine is dominated by studies documenting the effectiveness of systemic estrogen or estrogen-progestin hormone therapy for the treatment of hot flashes caused by hypoestrogenism. The effectiveness of progestin-only systemic hormone therapy for the treatment of hot flashes is much less studied and seldom is utilized in clinical practice. A small number of studies have reported that progestins, including micronized progesterone, medroxyprogesterone acetate, and norethindrone acetate, are effective treatment for hot flashes. Progestin-only systemic hormone therapy might be especially helpful for postmenopausal women with moderate to severe hot flashes who have a contraindication to estrogen treatment.
Micronized progesterone
Micronized progesterone (Prometrium) 300 mg daily taken at bedtime has been reported to effectively treat hot flashes in postmenopausal women. In one study, 133 postmenopausal women with an average age of 55 years and approximately 3 years from their last menstrual period were randomly assigned to 12 weeks of treatment with placebo or micronized progesterone 300 mg daily taken at bedtime.1 Mean serum progesterone levels were 0.28 ng/mL (0.89 nM) and 27 ng/mL (86 nM) in the women taking placebo and micronized progesterone, respectively. Compared with placebo, micronized progesterone reduced daytime and nighttime hot flash frequency and severity. In addition, compared with placebo, micronized progesterone improved the quality of sleep.1
Most reviews conclude that micronized progesterone has minimal cardiovascular risk.2 Micronized progesterone therapy might be especially helpful for postmenopausal women with moderate to severe hot flashes who have a contraindication to estrogen treatment such as those at increased risk for cardiovascular disease or women with a thrombophilia. Many experts believe that systemic estrogen therapy is contraindicated in postmenopausal women with an American Heart Association risk score greater than 10% over 10 years.3 Additional contraindications to systemic estrogen include women with cardiac disease who have a thrombophilia, such as the Factor V Leiden mutation.4
For women who are at high risk for estrogen-induced cardiovascular events, micronized progesterone may be a better option than systemic estrogen for treating hot flashes. Alternatively, in these women at risk of cardiovascular disease a selective serotonin reuptake inhibitor, such as escitalopram, 10 mg to 20 mg daily, may be a good option for treating postmenopausal hot flashes.5
Medroxyprogesterone acetate
Medroxyprogesterone acetate, at a dosage of 20 mg daily, is an effective treatment for hot flashes. In a randomized clinical trial 27 postmenopausal women with hot flashes were randomly assigned to treatment with placebo or medroxyprogesterone acetate 20 mg daily for 4 weeks. Vasomotor flushes were decreased by 26% and 74% in the placebo and medroxyprogesterone groups, respectively.6
Depot medroxyprogesterone acetate injections at dosages from 150 mg to 400 mg also have been reported to effectively treat hot flashes.7,8 In a trial comparing the effectiveness of estrogen monotherapy (conjugated equine estrogen 0.6 mg daily) with progestin monotherapy (medroxyprogesterone acetate 10 mg daily), both treatments were equally effective in reducing hot flashes.9
Continue to: Micronized progesterone vs medroxyprogesterone acetate...
Micronized progesterone vs medroxyprogesterone acetate
Experts in menopause medicine have suggested that in postmenopausal women micronized progesterone has a better pattern of benefits and fewer risks than medroxyprogesterone acetate.10,11 For example, in the E3N observational study of hormones and breast cancer risk, among 80,377 French postmenopausal women followed for a mean of 8 years, the combination of transdermal estradiol plus oral micronized progesterone was associated with no significantly increased risk of breast cancer (relative risk [RR], 1.08, 95% confidence interval [CI], 0.89–1.31) compared with never users of postmenopausal hormone therapy.12 By contrast, the combination of oral estrogen plus medroxyprogesterone acetate was associated with an increased risk of breast cancer (RR, 1.48; 95% CI, 1.02–2.16) compared with never users of postmenopausal hormone therapy. The E3N study indicates that micronized progesterone may have a more favorable breast health profile than medroxyprogesterone acetate.12

Norethindrone acetate
Norethindrone acetate monotherapy is not commonly prescribed for the treatment of menopausal hot flashes. However, a large clinical trial has demonstrated that norethindrone acetate effectively suppresses hot flashes in women with endometriosis treated with depot leuprolide acetate (LA). In one trial 201 women with endometriosis were randomly assigned to 12 months of treatment with13:
- LA plus placebo pills
- LA plus norethindrone acetate (NEA) 5 mg daily
- LA plus NEA 5 mg daily plus conjugated equine estrogen (CEE) 0.625 mg daily, or
- LA plus NEA 5 mg daily plus CEE 1.25 mg daily.
The median number of hot flashes in 24 hours was 6 in the LA plus placebo group and 0 in both the LA plus NEA 5 mg daily group and the LA plus NEA 5 mg plus CEE 1.25 mg daily group. This study demonstrates that NEA 5 mg daily is an effective treatment for hot flashes.
In the same study, LA plus placebo was associated with a significant decrease in lumbar spine bone mineral density. No significant decrease in bone mineral density was observed in the women who received LA plus NEA 5 mg daily. This finding indicates that NEA 5 mg reduces bone absorption caused by hypoestrogenism. In humans, norethindrone is a substrate for the aromatase enzyme system.14 Small quantities of ethinyl estradiol may be formed by aromatization of norethindrone in vivo,15,16 contributing to the effectiveness of NEA in suppressing hot flashes and preserving bone density.
Progestin: The estrogen alternative to hot flashes
For postmenopausal women with moderate to severe hot flashes, estrogen treatment reliably suppresses hot flashes and often improves sleep quality and mood. For postmenopausal women with a contraindication to estrogen treatment, progestin-only treatment with micronized progesterone or norethindrone acetate may be an effective option.
- Hitchcock CL, Prior JC. Oral micronized progesterone for vasomotor symptoms—a placebo-controlled randomized trial in healthy postmenopausal women. Menopause. 2012;19:886-893.
- Spark MJ, Willis J. Systematic review of progesterone use by midlife menopausal women. Maturitas 2012; 72: 192-202.
- Manson JE, Ames JM, Shapiro M, et al. Algorithm and mobile app for menopausal symptom management and hormonal/nonhormonal therapy decision making: a clinical decision-suport tool from The North American Menopause Society. Menopause. 2015;22:247-253.
- Herrington DM, Vittinghoff E, Howard TD, et al. Factor V Leiden, hormone replacement therapy, and risk of venous thromboembolic events in women with coronary disease. Arterioscler Thromb Vasc Biol. 2002;22:1012-1017.
- Ensrud KE, Joffe H, Guthrie KA, et al. Effect of escitalopram on insomnia symptoms and subjective sleep quality in healthy perimenopausal and postmenopausal women with hot flashes: a randomized controlled trial. Menopause. 2012;19:848-855.
- Schiff I, Tulchinsky D, Cramer D, et al. Oral medroxyprogesterone in the treatment of postmenopausal symptoms. JAMA. 1980;244:1443-1445.
- Bullock JL, Massey FM, Gambrell RD Jr. Use of medroxyprogesterone acetate to prevent menopausal symptoms. Obstet Gynecol. 1975;46:165-168.
- Loprinzi CL, Levitt R, Barton D, et al. Phase III comparison of depot medroxyprogesterone acetate to venlafaxine for managing hot flashes: North Central Cancer Treatment Group Trial N99C7. J Clin Oncol. 2006;24:1409-1414.
- Prior JC, Nielsen JD, Hitchcock CL, et al. Medroxyprogesterone and conjugated oestrogen are equivalent for hot flushes: 1-year randomized double-blind trial following premenopausal ovariectomy. Clin Sci (Lond). 2007;112:517-525.
- L’hermite M, Simoncini T, Fuller S, et al. Could transdermal estradiol + progesterone be a safer postmenopausal HRT? A review. Maturitas. 2008;60:185-201.
- Simon JA. What if the Women’s Health Initiative had used transdermal estradiol and oral progesterone instead? Menopause. 2014;21:769-783.
- Fournier A, Berrino F, Clavel-Chapelon F. Unequal risks for breast cancer associated with different hormone replacement therapies: results from the E3N cohort study. Breast Cancer Res Treat. 2008;107:103-111.
- Hornstein MD, Surrey ES, Weisberg GW, et al. Leuprolide acetate depot and hormonal add-back in endometriosis: a 12-month study. Lupron Add-Back Study Group. Obstet Gynecol. 1998;91:16-24.
- Barbieri RL, Canick JA, Ryan KJ. High-affinity steroid binding to rat testis 17 alpha-hydroxylase and human placental aromatase. J Steroid Biochem. 1981;14:387-393.
- Chu MC, Zhang X, Gentzschein E, et al. Formation of ethinyl estradiol in women during treatment with norethindrone acetate. J Clin Endocrinol Metab. 2007;92:2205-2207.
- Chwalisz K, Surrey E, Stanczyk FZ. The hormonal profile of norethindrone acetate: rationale for add-back therapy with gonadotropin-releasing hormone agonists in women with endometriosis. Reprod Sci. 2012;19:563-571.
The field of menopause medicine is dominated by studies documenting the effectiveness of systemic estrogen or estrogen-progestin hormone therapy for the treatment of hot flashes caused by hypoestrogenism. The effectiveness of progestin-only systemic hormone therapy for the treatment of hot flashes is much less studied and seldom is utilized in clinical practice. A small number of studies have reported that progestins, including micronized progesterone, medroxyprogesterone acetate, and norethindrone acetate, are effective treatment for hot flashes. Progestin-only systemic hormone therapy might be especially helpful for postmenopausal women with moderate to severe hot flashes who have a contraindication to estrogen treatment.
Micronized progesterone
Micronized progesterone (Prometrium) 300 mg daily taken at bedtime has been reported to effectively treat hot flashes in postmenopausal women. In one study, 133 postmenopausal women with an average age of 55 years and approximately 3 years from their last menstrual period were randomly assigned to 12 weeks of treatment with placebo or micronized progesterone 300 mg daily taken at bedtime.1 Mean serum progesterone levels were 0.28 ng/mL (0.89 nM) and 27 ng/mL (86 nM) in the women taking placebo and micronized progesterone, respectively. Compared with placebo, micronized progesterone reduced daytime and nighttime hot flash frequency and severity. In addition, compared with placebo, micronized progesterone improved the quality of sleep.1
Most reviews conclude that micronized progesterone has minimal cardiovascular risk.2 Micronized progesterone therapy might be especially helpful for postmenopausal women with moderate to severe hot flashes who have a contraindication to estrogen treatment such as those at increased risk for cardiovascular disease or women with a thrombophilia. Many experts believe that systemic estrogen therapy is contraindicated in postmenopausal women with an American Heart Association risk score greater than 10% over 10 years.3 Additional contraindications to systemic estrogen include women with cardiac disease who have a thrombophilia, such as the Factor V Leiden mutation.4
For women who are at high risk for estrogen-induced cardiovascular events, micronized progesterone may be a better option than systemic estrogen for treating hot flashes. Alternatively, in these women at risk of cardiovascular disease a selective serotonin reuptake inhibitor, such as escitalopram, 10 mg to 20 mg daily, may be a good option for treating postmenopausal hot flashes.5
Medroxyprogesterone acetate
Medroxyprogesterone acetate, at a dosage of 20 mg daily, is an effective treatment for hot flashes. In a randomized clinical trial 27 postmenopausal women with hot flashes were randomly assigned to treatment with placebo or medroxyprogesterone acetate 20 mg daily for 4 weeks. Vasomotor flushes were decreased by 26% and 74% in the placebo and medroxyprogesterone groups, respectively.6
Depot medroxyprogesterone acetate injections at dosages from 150 mg to 400 mg also have been reported to effectively treat hot flashes.7,8 In a trial comparing the effectiveness of estrogen monotherapy (conjugated equine estrogen 0.6 mg daily) with progestin monotherapy (medroxyprogesterone acetate 10 mg daily), both treatments were equally effective in reducing hot flashes.9
Continue to: Micronized progesterone vs medroxyprogesterone acetate...
Micronized progesterone vs medroxyprogesterone acetate
Experts in menopause medicine have suggested that in postmenopausal women micronized progesterone has a better pattern of benefits and fewer risks than medroxyprogesterone acetate.10,11 For example, in the E3N observational study of hormones and breast cancer risk, among 80,377 French postmenopausal women followed for a mean of 8 years, the combination of transdermal estradiol plus oral micronized progesterone was associated with no significantly increased risk of breast cancer (relative risk [RR], 1.08, 95% confidence interval [CI], 0.89–1.31) compared with never users of postmenopausal hormone therapy.12 By contrast, the combination of oral estrogen plus medroxyprogesterone acetate was associated with an increased risk of breast cancer (RR, 1.48; 95% CI, 1.02–2.16) compared with never users of postmenopausal hormone therapy. The E3N study indicates that micronized progesterone may have a more favorable breast health profile than medroxyprogesterone acetate.12

Norethindrone acetate
Norethindrone acetate monotherapy is not commonly prescribed for the treatment of menopausal hot flashes. However, a large clinical trial has demonstrated that norethindrone acetate effectively suppresses hot flashes in women with endometriosis treated with depot leuprolide acetate (LA). In one trial 201 women with endometriosis were randomly assigned to 12 months of treatment with13:
- LA plus placebo pills
- LA plus norethindrone acetate (NEA) 5 mg daily
- LA plus NEA 5 mg daily plus conjugated equine estrogen (CEE) 0.625 mg daily, or
- LA plus NEA 5 mg daily plus CEE 1.25 mg daily.
The median number of hot flashes in 24 hours was 6 in the LA plus placebo group and 0 in both the LA plus NEA 5 mg daily group and the LA plus NEA 5 mg plus CEE 1.25 mg daily group. This study demonstrates that NEA 5 mg daily is an effective treatment for hot flashes.
In the same study, LA plus placebo was associated with a significant decrease in lumbar spine bone mineral density. No significant decrease in bone mineral density was observed in the women who received LA plus NEA 5 mg daily. This finding indicates that NEA 5 mg reduces bone absorption caused by hypoestrogenism. In humans, norethindrone is a substrate for the aromatase enzyme system.14 Small quantities of ethinyl estradiol may be formed by aromatization of norethindrone in vivo,15,16 contributing to the effectiveness of NEA in suppressing hot flashes and preserving bone density.
Progestin: The estrogen alternative to hot flashes
For postmenopausal women with moderate to severe hot flashes, estrogen treatment reliably suppresses hot flashes and often improves sleep quality and mood. For postmenopausal women with a contraindication to estrogen treatment, progestin-only treatment with micronized progesterone or norethindrone acetate may be an effective option.
The field of menopause medicine is dominated by studies documenting the effectiveness of systemic estrogen or estrogen-progestin hormone therapy for the treatment of hot flashes caused by hypoestrogenism. The effectiveness of progestin-only systemic hormone therapy for the treatment of hot flashes is much less studied and seldom is utilized in clinical practice. A small number of studies have reported that progestins, including micronized progesterone, medroxyprogesterone acetate, and norethindrone acetate, are effective treatment for hot flashes. Progestin-only systemic hormone therapy might be especially helpful for postmenopausal women with moderate to severe hot flashes who have a contraindication to estrogen treatment.
Micronized progesterone
Micronized progesterone (Prometrium) 300 mg daily taken at bedtime has been reported to effectively treat hot flashes in postmenopausal women. In one study, 133 postmenopausal women with an average age of 55 years and approximately 3 years from their last menstrual period were randomly assigned to 12 weeks of treatment with placebo or micronized progesterone 300 mg daily taken at bedtime.1 Mean serum progesterone levels were 0.28 ng/mL (0.89 nM) and 27 ng/mL (86 nM) in the women taking placebo and micronized progesterone, respectively. Compared with placebo, micronized progesterone reduced daytime and nighttime hot flash frequency and severity. In addition, compared with placebo, micronized progesterone improved the quality of sleep.1
Most reviews conclude that micronized progesterone has minimal cardiovascular risk.2 Micronized progesterone therapy might be especially helpful for postmenopausal women with moderate to severe hot flashes who have a contraindication to estrogen treatment such as those at increased risk for cardiovascular disease or women with a thrombophilia. Many experts believe that systemic estrogen therapy is contraindicated in postmenopausal women with an American Heart Association risk score greater than 10% over 10 years.3 Additional contraindications to systemic estrogen include women with cardiac disease who have a thrombophilia, such as the Factor V Leiden mutation.4
For women who are at high risk for estrogen-induced cardiovascular events, micronized progesterone may be a better option than systemic estrogen for treating hot flashes. Alternatively, in these women at risk of cardiovascular disease a selective serotonin reuptake inhibitor, such as escitalopram, 10 mg to 20 mg daily, may be a good option for treating postmenopausal hot flashes.5
Medroxyprogesterone acetate
Medroxyprogesterone acetate, at a dosage of 20 mg daily, is an effective treatment for hot flashes. In a randomized clinical trial 27 postmenopausal women with hot flashes were randomly assigned to treatment with placebo or medroxyprogesterone acetate 20 mg daily for 4 weeks. Vasomotor flushes were decreased by 26% and 74% in the placebo and medroxyprogesterone groups, respectively.6
Depot medroxyprogesterone acetate injections at dosages from 150 mg to 400 mg also have been reported to effectively treat hot flashes.7,8 In a trial comparing the effectiveness of estrogen monotherapy (conjugated equine estrogen 0.6 mg daily) with progestin monotherapy (medroxyprogesterone acetate 10 mg daily), both treatments were equally effective in reducing hot flashes.9
Continue to: Micronized progesterone vs medroxyprogesterone acetate...
Micronized progesterone vs medroxyprogesterone acetate
Experts in menopause medicine have suggested that in postmenopausal women micronized progesterone has a better pattern of benefits and fewer risks than medroxyprogesterone acetate.10,11 For example, in the E3N observational study of hormones and breast cancer risk, among 80,377 French postmenopausal women followed for a mean of 8 years, the combination of transdermal estradiol plus oral micronized progesterone was associated with no significantly increased risk of breast cancer (relative risk [RR], 1.08, 95% confidence interval [CI], 0.89–1.31) compared with never users of postmenopausal hormone therapy.12 By contrast, the combination of oral estrogen plus medroxyprogesterone acetate was associated with an increased risk of breast cancer (RR, 1.48; 95% CI, 1.02–2.16) compared with never users of postmenopausal hormone therapy. The E3N study indicates that micronized progesterone may have a more favorable breast health profile than medroxyprogesterone acetate.12

Norethindrone acetate
Norethindrone acetate monotherapy is not commonly prescribed for the treatment of menopausal hot flashes. However, a large clinical trial has demonstrated that norethindrone acetate effectively suppresses hot flashes in women with endometriosis treated with depot leuprolide acetate (LA). In one trial 201 women with endometriosis were randomly assigned to 12 months of treatment with13:
- LA plus placebo pills
- LA plus norethindrone acetate (NEA) 5 mg daily
- LA plus NEA 5 mg daily plus conjugated equine estrogen (CEE) 0.625 mg daily, or
- LA plus NEA 5 mg daily plus CEE 1.25 mg daily.
The median number of hot flashes in 24 hours was 6 in the LA plus placebo group and 0 in both the LA plus NEA 5 mg daily group and the LA plus NEA 5 mg plus CEE 1.25 mg daily group. This study demonstrates that NEA 5 mg daily is an effective treatment for hot flashes.
In the same study, LA plus placebo was associated with a significant decrease in lumbar spine bone mineral density. No significant decrease in bone mineral density was observed in the women who received LA plus NEA 5 mg daily. This finding indicates that NEA 5 mg reduces bone absorption caused by hypoestrogenism. In humans, norethindrone is a substrate for the aromatase enzyme system.14 Small quantities of ethinyl estradiol may be formed by aromatization of norethindrone in vivo,15,16 contributing to the effectiveness of NEA in suppressing hot flashes and preserving bone density.
Progestin: The estrogen alternative to hot flashes
For postmenopausal women with moderate to severe hot flashes, estrogen treatment reliably suppresses hot flashes and often improves sleep quality and mood. For postmenopausal women with a contraindication to estrogen treatment, progestin-only treatment with micronized progesterone or norethindrone acetate may be an effective option.
- Hitchcock CL, Prior JC. Oral micronized progesterone for vasomotor symptoms—a placebo-controlled randomized trial in healthy postmenopausal women. Menopause. 2012;19:886-893.
- Spark MJ, Willis J. Systematic review of progesterone use by midlife menopausal women. Maturitas 2012; 72: 192-202.
- Manson JE, Ames JM, Shapiro M, et al. Algorithm and mobile app for menopausal symptom management and hormonal/nonhormonal therapy decision making: a clinical decision-suport tool from The North American Menopause Society. Menopause. 2015;22:247-253.
- Herrington DM, Vittinghoff E, Howard TD, et al. Factor V Leiden, hormone replacement therapy, and risk of venous thromboembolic events in women with coronary disease. Arterioscler Thromb Vasc Biol. 2002;22:1012-1017.
- Ensrud KE, Joffe H, Guthrie KA, et al. Effect of escitalopram on insomnia symptoms and subjective sleep quality in healthy perimenopausal and postmenopausal women with hot flashes: a randomized controlled trial. Menopause. 2012;19:848-855.
- Schiff I, Tulchinsky D, Cramer D, et al. Oral medroxyprogesterone in the treatment of postmenopausal symptoms. JAMA. 1980;244:1443-1445.
- Bullock JL, Massey FM, Gambrell RD Jr. Use of medroxyprogesterone acetate to prevent menopausal symptoms. Obstet Gynecol. 1975;46:165-168.
- Loprinzi CL, Levitt R, Barton D, et al. Phase III comparison of depot medroxyprogesterone acetate to venlafaxine for managing hot flashes: North Central Cancer Treatment Group Trial N99C7. J Clin Oncol. 2006;24:1409-1414.
- Prior JC, Nielsen JD, Hitchcock CL, et al. Medroxyprogesterone and conjugated oestrogen are equivalent for hot flushes: 1-year randomized double-blind trial following premenopausal ovariectomy. Clin Sci (Lond). 2007;112:517-525.
- L’hermite M, Simoncini T, Fuller S, et al. Could transdermal estradiol + progesterone be a safer postmenopausal HRT? A review. Maturitas. 2008;60:185-201.
- Simon JA. What if the Women’s Health Initiative had used transdermal estradiol and oral progesterone instead? Menopause. 2014;21:769-783.
- Fournier A, Berrino F, Clavel-Chapelon F. Unequal risks for breast cancer associated with different hormone replacement therapies: results from the E3N cohort study. Breast Cancer Res Treat. 2008;107:103-111.
- Hornstein MD, Surrey ES, Weisberg GW, et al. Leuprolide acetate depot and hormonal add-back in endometriosis: a 12-month study. Lupron Add-Back Study Group. Obstet Gynecol. 1998;91:16-24.
- Barbieri RL, Canick JA, Ryan KJ. High-affinity steroid binding to rat testis 17 alpha-hydroxylase and human placental aromatase. J Steroid Biochem. 1981;14:387-393.
- Chu MC, Zhang X, Gentzschein E, et al. Formation of ethinyl estradiol in women during treatment with norethindrone acetate. J Clin Endocrinol Metab. 2007;92:2205-2207.
- Chwalisz K, Surrey E, Stanczyk FZ. The hormonal profile of norethindrone acetate: rationale for add-back therapy with gonadotropin-releasing hormone agonists in women with endometriosis. Reprod Sci. 2012;19:563-571.
- Hitchcock CL, Prior JC. Oral micronized progesterone for vasomotor symptoms—a placebo-controlled randomized trial in healthy postmenopausal women. Menopause. 2012;19:886-893.
- Spark MJ, Willis J. Systematic review of progesterone use by midlife menopausal women. Maturitas 2012; 72: 192-202.
- Manson JE, Ames JM, Shapiro M, et al. Algorithm and mobile app for menopausal symptom management and hormonal/nonhormonal therapy decision making: a clinical decision-suport tool from The North American Menopause Society. Menopause. 2015;22:247-253.
- Herrington DM, Vittinghoff E, Howard TD, et al. Factor V Leiden, hormone replacement therapy, and risk of venous thromboembolic events in women with coronary disease. Arterioscler Thromb Vasc Biol. 2002;22:1012-1017.
- Ensrud KE, Joffe H, Guthrie KA, et al. Effect of escitalopram on insomnia symptoms and subjective sleep quality in healthy perimenopausal and postmenopausal women with hot flashes: a randomized controlled trial. Menopause. 2012;19:848-855.
- Schiff I, Tulchinsky D, Cramer D, et al. Oral medroxyprogesterone in the treatment of postmenopausal symptoms. JAMA. 1980;244:1443-1445.
- Bullock JL, Massey FM, Gambrell RD Jr. Use of medroxyprogesterone acetate to prevent menopausal symptoms. Obstet Gynecol. 1975;46:165-168.
- Loprinzi CL, Levitt R, Barton D, et al. Phase III comparison of depot medroxyprogesterone acetate to venlafaxine for managing hot flashes: North Central Cancer Treatment Group Trial N99C7. J Clin Oncol. 2006;24:1409-1414.
- Prior JC, Nielsen JD, Hitchcock CL, et al. Medroxyprogesterone and conjugated oestrogen are equivalent for hot flushes: 1-year randomized double-blind trial following premenopausal ovariectomy. Clin Sci (Lond). 2007;112:517-525.
- L’hermite M, Simoncini T, Fuller S, et al. Could transdermal estradiol + progesterone be a safer postmenopausal HRT? A review. Maturitas. 2008;60:185-201.
- Simon JA. What if the Women’s Health Initiative had used transdermal estradiol and oral progesterone instead? Menopause. 2014;21:769-783.
- Fournier A, Berrino F, Clavel-Chapelon F. Unequal risks for breast cancer associated with different hormone replacement therapies: results from the E3N cohort study. Breast Cancer Res Treat. 2008;107:103-111.
- Hornstein MD, Surrey ES, Weisberg GW, et al. Leuprolide acetate depot and hormonal add-back in endometriosis: a 12-month study. Lupron Add-Back Study Group. Obstet Gynecol. 1998;91:16-24.
- Barbieri RL, Canick JA, Ryan KJ. High-affinity steroid binding to rat testis 17 alpha-hydroxylase and human placental aromatase. J Steroid Biochem. 1981;14:387-393.
- Chu MC, Zhang X, Gentzschein E, et al. Formation of ethinyl estradiol in women during treatment with norethindrone acetate. J Clin Endocrinol Metab. 2007;92:2205-2207.
- Chwalisz K, Surrey E, Stanczyk FZ. The hormonal profile of norethindrone acetate: rationale for add-back therapy with gonadotropin-releasing hormone agonists in women with endometriosis. Reprod Sci. 2012;19:563-571.
Breast cancer chemoprophylaxis in high-risk women: How persistent is the impact of an aromatase inhibitor after 5 years of use?
Cuzick J, Sestak I, Forbes JF, et al; IBIS-II Investigators. Use of anastrozole for breast cancer prevention (IBIS-II): long-term results of a randomised controlled trial. Lancet. 2020;395;117-122.
EXPERT COMMENTARY
A manufacturer-sponsored trial initiated in 2003, IBIS-II (International Breast Cancer Intervention Study II) included 3,864 menopausal women (mean age at baseline, 59.4 years) at elevated risk for breast cancer. The women were randomly assigned to 5-year treatment with either placebo (N = 1,944) or the aromatase inhibitor anastrozole 1 mg daily (N = 1,920).1
Reporting on the long-term follow-up results of the trial, Cuzick and colleagues found that anastrozole use substantially reduced the incidence of all breast cancer, including invasive breast cancer and ductal carcinoma in situ. Key adverse events associated with anastrozole were fractures, arthralgias, and menopausal symptoms (vasomotor symptoms and vaginal dryness).
To determine whether anastrozole had any persistent impact, the investigators continued to follow participants for all breast cancers and other outcomes.2
Details of the study
This randomized controlled trial that included 3,864 postmenopausal women had a median overall follow-up of 131 months; the primary outcome was all breast cancer. Random assignment to anastrozole use (1,920 women) was associated with a 49% reduction in all breast cancer (85 cases vs 165 cases in the placebo group [N = 1,944]; HR, 0.51; 95% CI, 0.39–0.66; P<.0001).
In the first 5 years, risk reduction was 61% with anastrozole (P<.0001 for overall and the first 5 years of follow-up). Subsequently, the magnitude of the risk reduction attenuated to 37% (P = .014). With 12 years of follow-up, the estimated risk of being diagnosed with breast cancer was 8.8% and 5.3% in the placebo and anastrozole groups, respectively. The number needed to treat for 5 years to prevent 1 breast cancer was 29.
With anastrozole, prevention of estrogen–receptor positive tumors was substantially more robust at 54% (HR, 0.46; 95% CI, 0.33–0.65; P<.0001) than for estrogen–receptor negative tumors at 27% (HR, 0.77; 95% CI, 0.41–1.44; P = .41).
Over the course of the long-term study, the incidence of fractures and cardiovascular events was similar in the placebo and anastrozole groups. Arthralgias and menopausal symptoms were not assessed after the trial’s initial 5 years. Overall, the number of deaths (all cause as well as breast cancer related) were similar in the placebo and anastrozole groups.
Continue to: Study strengths and limitations...
Study strengths and limitations
The authors noted that this updated analysis of the IBIS-II trial data offers further support for the use of anastrozole in breast cancer prevention for high-risk postmenopausal women. The extended posttreatment follow-up showed a significant continuing reduction in breast cancer, and there was no evidence of new late adverse effects. A limitation of the analysis, however, is that very few deaths from breast cancer occurred during the study timeframe. Thus, additional follow-up would be needed to assess anastrozole’s effect on breast cancer mortality.
The breast cancer chemoprophylactic efficacy of anastrozole compares favorably with that of tamoxifen. Furthermore, in women with an intact uterus, the increased risks of gynecologic problems, including endometrial cancer, associated with tamoxifen do not occur with aromatase inhibitors. However, the lack of any obvious mortality benefit means the ultimate value of estrogen deprivation breast cancer chemoprophylaxis continues to be uncertain, especially given other risks, including bone loss. In view of these new data, it will be important for high-risk women considering aromatase inhibitor prophylaxis to understand that these medications have not been associated with a mortality benefit.
ANDREW M. KAUNITZ, MD, NCMP
- Cuzick J, Sestak I, Forbes JF, et al; IBIS-II Investigators. Anastrozole for prevention of breast cancer in high-risk postmenopausal women (IBIS-II): an international, double-blind, randomised placebo-controlled trial. Lancet. 2014;383:1041-1048.
- Cuzick J, Sestak I, Forbes JF, et al; IBIS-II Investigators. Use of anastrozole for breast cancer prevention (IBIS-II): long-term results of a randomised controlled trial. Lancet. 2020;395;117-122.
Cuzick J, Sestak I, Forbes JF, et al; IBIS-II Investigators. Use of anastrozole for breast cancer prevention (IBIS-II): long-term results of a randomised controlled trial. Lancet. 2020;395;117-122.
EXPERT COMMENTARY
A manufacturer-sponsored trial initiated in 2003, IBIS-II (International Breast Cancer Intervention Study II) included 3,864 menopausal women (mean age at baseline, 59.4 years) at elevated risk for breast cancer. The women were randomly assigned to 5-year treatment with either placebo (N = 1,944) or the aromatase inhibitor anastrozole 1 mg daily (N = 1,920).1
Reporting on the long-term follow-up results of the trial, Cuzick and colleagues found that anastrozole use substantially reduced the incidence of all breast cancer, including invasive breast cancer and ductal carcinoma in situ. Key adverse events associated with anastrozole were fractures, arthralgias, and menopausal symptoms (vasomotor symptoms and vaginal dryness).
To determine whether anastrozole had any persistent impact, the investigators continued to follow participants for all breast cancers and other outcomes.2
Details of the study
This randomized controlled trial that included 3,864 postmenopausal women had a median overall follow-up of 131 months; the primary outcome was all breast cancer. Random assignment to anastrozole use (1,920 women) was associated with a 49% reduction in all breast cancer (85 cases vs 165 cases in the placebo group [N = 1,944]; HR, 0.51; 95% CI, 0.39–0.66; P<.0001).
In the first 5 years, risk reduction was 61% with anastrozole (P<.0001 for overall and the first 5 years of follow-up). Subsequently, the magnitude of the risk reduction attenuated to 37% (P = .014). With 12 years of follow-up, the estimated risk of being diagnosed with breast cancer was 8.8% and 5.3% in the placebo and anastrozole groups, respectively. The number needed to treat for 5 years to prevent 1 breast cancer was 29.
With anastrozole, prevention of estrogen–receptor positive tumors was substantially more robust at 54% (HR, 0.46; 95% CI, 0.33–0.65; P<.0001) than for estrogen–receptor negative tumors at 27% (HR, 0.77; 95% CI, 0.41–1.44; P = .41).
Over the course of the long-term study, the incidence of fractures and cardiovascular events was similar in the placebo and anastrozole groups. Arthralgias and menopausal symptoms were not assessed after the trial’s initial 5 years. Overall, the number of deaths (all cause as well as breast cancer related) were similar in the placebo and anastrozole groups.
Continue to: Study strengths and limitations...
Study strengths and limitations
The authors noted that this updated analysis of the IBIS-II trial data offers further support for the use of anastrozole in breast cancer prevention for high-risk postmenopausal women. The extended posttreatment follow-up showed a significant continuing reduction in breast cancer, and there was no evidence of new late adverse effects. A limitation of the analysis, however, is that very few deaths from breast cancer occurred during the study timeframe. Thus, additional follow-up would be needed to assess anastrozole’s effect on breast cancer mortality.
The breast cancer chemoprophylactic efficacy of anastrozole compares favorably with that of tamoxifen. Furthermore, in women with an intact uterus, the increased risks of gynecologic problems, including endometrial cancer, associated with tamoxifen do not occur with aromatase inhibitors. However, the lack of any obvious mortality benefit means the ultimate value of estrogen deprivation breast cancer chemoprophylaxis continues to be uncertain, especially given other risks, including bone loss. In view of these new data, it will be important for high-risk women considering aromatase inhibitor prophylaxis to understand that these medications have not been associated with a mortality benefit.
ANDREW M. KAUNITZ, MD, NCMP
Cuzick J, Sestak I, Forbes JF, et al; IBIS-II Investigators. Use of anastrozole for breast cancer prevention (IBIS-II): long-term results of a randomised controlled trial. Lancet. 2020;395;117-122.
EXPERT COMMENTARY
A manufacturer-sponsored trial initiated in 2003, IBIS-II (International Breast Cancer Intervention Study II) included 3,864 menopausal women (mean age at baseline, 59.4 years) at elevated risk for breast cancer. The women were randomly assigned to 5-year treatment with either placebo (N = 1,944) or the aromatase inhibitor anastrozole 1 mg daily (N = 1,920).1
Reporting on the long-term follow-up results of the trial, Cuzick and colleagues found that anastrozole use substantially reduced the incidence of all breast cancer, including invasive breast cancer and ductal carcinoma in situ. Key adverse events associated with anastrozole were fractures, arthralgias, and menopausal symptoms (vasomotor symptoms and vaginal dryness).
To determine whether anastrozole had any persistent impact, the investigators continued to follow participants for all breast cancers and other outcomes.2
Details of the study
This randomized controlled trial that included 3,864 postmenopausal women had a median overall follow-up of 131 months; the primary outcome was all breast cancer. Random assignment to anastrozole use (1,920 women) was associated with a 49% reduction in all breast cancer (85 cases vs 165 cases in the placebo group [N = 1,944]; HR, 0.51; 95% CI, 0.39–0.66; P<.0001).
In the first 5 years, risk reduction was 61% with anastrozole (P<.0001 for overall and the first 5 years of follow-up). Subsequently, the magnitude of the risk reduction attenuated to 37% (P = .014). With 12 years of follow-up, the estimated risk of being diagnosed with breast cancer was 8.8% and 5.3% in the placebo and anastrozole groups, respectively. The number needed to treat for 5 years to prevent 1 breast cancer was 29.
With anastrozole, prevention of estrogen–receptor positive tumors was substantially more robust at 54% (HR, 0.46; 95% CI, 0.33–0.65; P<.0001) than for estrogen–receptor negative tumors at 27% (HR, 0.77; 95% CI, 0.41–1.44; P = .41).
Over the course of the long-term study, the incidence of fractures and cardiovascular events was similar in the placebo and anastrozole groups. Arthralgias and menopausal symptoms were not assessed after the trial’s initial 5 years. Overall, the number of deaths (all cause as well as breast cancer related) were similar in the placebo and anastrozole groups.
Continue to: Study strengths and limitations...
Study strengths and limitations
The authors noted that this updated analysis of the IBIS-II trial data offers further support for the use of anastrozole in breast cancer prevention for high-risk postmenopausal women. The extended posttreatment follow-up showed a significant continuing reduction in breast cancer, and there was no evidence of new late adverse effects. A limitation of the analysis, however, is that very few deaths from breast cancer occurred during the study timeframe. Thus, additional follow-up would be needed to assess anastrozole’s effect on breast cancer mortality.
The breast cancer chemoprophylactic efficacy of anastrozole compares favorably with that of tamoxifen. Furthermore, in women with an intact uterus, the increased risks of gynecologic problems, including endometrial cancer, associated with tamoxifen do not occur with aromatase inhibitors. However, the lack of any obvious mortality benefit means the ultimate value of estrogen deprivation breast cancer chemoprophylaxis continues to be uncertain, especially given other risks, including bone loss. In view of these new data, it will be important for high-risk women considering aromatase inhibitor prophylaxis to understand that these medications have not been associated with a mortality benefit.
ANDREW M. KAUNITZ, MD, NCMP
- Cuzick J, Sestak I, Forbes JF, et al; IBIS-II Investigators. Anastrozole for prevention of breast cancer in high-risk postmenopausal women (IBIS-II): an international, double-blind, randomised placebo-controlled trial. Lancet. 2014;383:1041-1048.
- Cuzick J, Sestak I, Forbes JF, et al; IBIS-II Investigators. Use of anastrozole for breast cancer prevention (IBIS-II): long-term results of a randomised controlled trial. Lancet. 2020;395;117-122.
- Cuzick J, Sestak I, Forbes JF, et al; IBIS-II Investigators. Anastrozole for prevention of breast cancer in high-risk postmenopausal women (IBIS-II): an international, double-blind, randomised placebo-controlled trial. Lancet. 2014;383:1041-1048.
- Cuzick J, Sestak I, Forbes JF, et al; IBIS-II Investigators. Use of anastrozole for breast cancer prevention (IBIS-II): long-term results of a randomised controlled trial. Lancet. 2020;395;117-122.
Should supplemental MRI be used in otherwise average-risk women with extremely dense breasts?
While the frequency of dense breasts decreases with age, approximately 10% of women in the United States have extremely dense breasts (Breast Imaging, Reporting, and Data System [BI-RADS] category D), and another 40% have heterogeneously dense breasts (BI-RADS category C).1 Women with dense breasts have both an increased risk for developing breast cancer and reduced mammographic sensitivity for breast cancer detection compared with women who have nondense breasts.2
These 2 observations have led the majority of states to pass legislation requiring that women with dense breasts be informed of their breast density, and most require that providers discuss these results with their patients. Thoughtful clinicians who review the available literature, however, will find sparse evidence on which to counsel patients as to next steps.
Now, a recent trial adds to our knowledge about supplemental magnetic resonance imaging (MRI) breast screening in women with extremely dense breasts.
DENSE trial offers high-quality data
Bakker and colleagues studied women aged 50 to 74 who were participating in a Netherlands population-based biennial mammography screening program.3 They enrolled average-risk women with extremely dense breasts who had a negative screening digital mammogram into the Dense Tissue and Early Breast Neoplasm Screening (DENSE) multicenter trial. The women were randomly assigned to receive either continued biennial digital mammography or supplemental breast MRI.
The primary outcome was the between-group difference in the development of interval breast cancers—that is, breast cancers detected by women or their providers between rounds of screening mammography. Interval breast cancers were chosen as the primary outcome for 2 reasons:
- interval cancers appear to be more aggressive tumors than those cancers detected by screening mammography
- interval cancers can be identified over a shorter time interval, making them easier to study than outcomes such as breast cancer mortality, which typically require more than a decade to identify.
The DENSE trial’s secondary outcomes included recall rates from MRI, cancer detection rates on MRI, positive predictive value of MRIs requiring biopsy, and breast cancer characteristics (size, stage) diagnosed in the different groups.
Between-group difference in incidence of interval cancers
A total of 40,373 women with extremely dense breasts were screened; 8,061 of these were randomly assigned to receive breast MRI and 32,312 to continued mammography only (1:4 cluster randomization) across 12 mammography centers in the Netherlands. Among the women assigned to the MRI group, 59% actually underwent MRI (4,783 of the 8,061).
The interval cancer rate in the mammography-only group was 5.0 per 1,000 screenings (95% confidence interval [CI], 4.3–5.8), while the interval cancer rate in the MRI-assigned group was 2.5 per 1,000 screenings (95% CI, 1.6–3.8) (TABLE 1).3
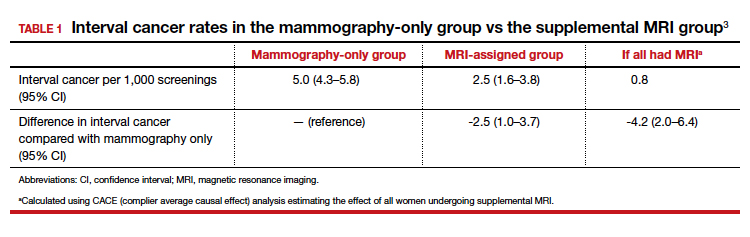
Key secondary outcomes
Of the women who underwent supplemental MRI, 9.49% were recalled for additional imaging, follow-up, or biopsy. Of the 4,783 women who had an MRI, 300 (6.3%) underwent a breast biopsy, and 79 breast cancers (1.65%) were detected. Sixty-four of these cancers were invasive, and 15 were ductal carcinoma in situ (DCIS). Among women who underwent a biopsy for an MRI-detected abnormality, the positive predictive value was 26.3%.
Tumor characteristics. For women who developed breast cancer during the study, both tumor size at diagnosis and tumor stage (early vs late) were described. TABLE 2 shows these results in the women who had their breast cancer detected on MRI, those in the MRI-assigned group who developed interval cancer, and those in the mammography-only group who had interval cancers.3 Overall, tumor size was smaller in the interval group who underwent MRI compared with those who underwent mammography only.
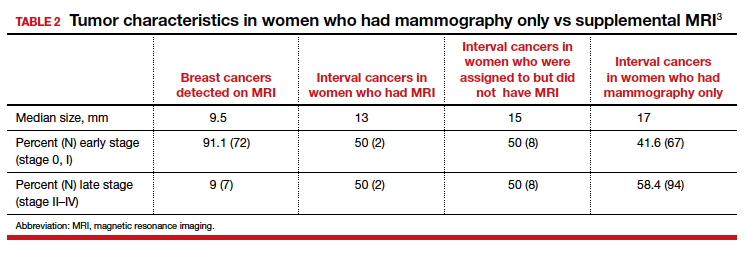
Continue to: Study contributes valuable data, but we need more on long-term outcomes...
Study contributes valuable data, but we need more on long-term outcomes
The trial by Bakker and colleagues employed a solid study design as women were randomly assigned to supplemental MRI screening or ongoing biennial mammography, and nearly all cancers were identified in the short-term of follow-up. In addition, very few women were lost to follow-up, and secondary outcomes, including false-positive rates, were collected to help providers and patients better understand some of the potential downsides of supplemental screening.
The substantial reduction in interval cancers (50% in the intent-to-screen analysis and 84% in the women who actually underwent supplemental MRI) was highly statistically significant (P<.001). While there were substantially fewer interval cancers in the MRI-assigned group, the interval cancers that did occur were of similar stage as those in the women assigned to the mammography-only group (TABLE 2).
Data demonstrate that interval cancers appear to be more aggressive than screen-detected cancers.4 While reducing interval cancers should be a good thing overall, it remains unproven that using supplemental MRI in all women with dense breasts would reduce breast cancer specific mortality, all-cause mortality, or the risk of more invasive treatments (for example, the need for chemotherapy or requirement for mastectomy).
On the other hand, using routine supplemental breast MRI in women with extremely dense breasts would result in very substantial use of resources, including cost, radiologist time, provider time, and machine time. In the United States, approximately 49 million women are aged 50 to 74.5 Breast MRI charges commonly range from $1,000 to $4,000. If the 4.9 million women with extremely dense breasts underwent supplemental MRI this year, the approximate cost would be somewhere between $4.9 and $19.5 billion for imaging alone. This does not include callbacks, biopsies, or provider time for ordering, interpreting, and arranging for follow-up.
While the reduction in interval cancers seen in this study is promising, more assurance of improvement in important outcomes—such as reduced mortality or reduced need for more invasive breast cancer treatments—should precede any routine change in practice.
Unanswered questions
This study did not address a number of other important questions, including:
Should MRI be done with every round of breast cancer screening given the possibility of prevalence bias? Prevalence bias can be defined as more cancers detected in the first round of MRI screening with possible reduced benefit in future rounds of screening. The study authors indicated that they will continue to analyze the study results to see what occurs in the next round of screening.
Is there a similar impact on decreased interval cancers in women undergoing annual mammography or in women screened between ages 40 and 49? This study was conducted in women aged 50 to 74 undergoing mammography every 2 years. In the United States, annual mammography in women aged 40 to 49 is frequently recommended.
What effect does supplemental MRI screening have in women with heterogeneously dense breasts, which represents 40% of the population? The US Food and Drug Administration recommends that all women with dense breasts be counseled regarding options for management.6
Do these results translate to the more racially and ethnically diverse populations of the United States? In the Netherlands, where this study was conducted, 85% to 90% of women are either Dutch or of western European origin. Women of different racial and ancestral backgrounds have biologically different breast cancers and cancer risk (for example, higher rates of triple-negative breast cancers in African American women; 10-fold higher rates of BRCA pathogenic variants in Ashkenazi Jewish women).
Continue to: Use validated tools to assess risk comprehensively...
Use validated tools to assess risk comprehensively
Women aged 50 to 74 with extremely dense breasts have reduced interval cancers following a normal biennial mammogram if supplemental MRI is offered, but the long-term benefit of identifying these cancers earlier is unclear. Until more data are available on important long-term outcomes (such as breast cancer mortality and need for more invasive treatments), providers should consider breast density in the context of a more comprehensive assessment of breast cancer risk using a validated breast cancer risk assessment tool.
I prefer the modified version of the International Breast Cancer Intervention Study (IBIS) tool, which is readily available online (https://ibis.ikonopedia.com/).7 This tool incorporates several breast cancer risk factors, including reproductive risk factors, body mass index, BRCA gene status, breast density, and family history. The tool takes 1 to 2 minutes to complete and provides an estimate of a woman’s 10-year risk and lifetime risk of breast cancer.
If the lifetime risk exceeds 20%, I offer the patient supplemental MRI screening, consistent with current recommendations of the National Comprehensive Cancer Network and the American Cancer Society.8,9 I generally recommend starting breast imaging screening 7 to 10 years prior to the youngest breast cancer occurrence in the family, with mammography starting no earlier than age 30 and MRI no earlier than age 25. Other validated tools also can be used.10-13
Incorporating breast density and other important risk factors allows a more comprehensive analysis upon which to counsel women about the value (benefits and harms) of breast imaging.8
- Sprague BL, Gagnon RE, Burt V, et al. Prevalence of mammographically dense breasts in the United States. J Natl Cancer Inst. 2014;106:dju255. doi: 10.1093/jcni/dju255.
- Boyd NF, Guo H, Martin LJ, et al. Mammographic density and the risk and detection of breast cancer. N Engl J Med. 2007;356:227-236.
- Bakker MF, de Lange SV, Pijnappel RM, et al; for the DENSE Trial Study Group. Supplemental MRI screening for women with extremely dense breast tissue. N Engl J Med. 2019;381:2091-2102.
- Drukker CA, Schmidt MK, Rutgers EJT, et al. Mammographic screening detects low-risk tumor biology breast cancers. Breast Cancer Res Treat. 2014;144:103-111.
- Statista website. Resident population of the United States by sex and age as of July 1, 2018. https://www.statista.com/statistics/241488/population-of-the-us-by-sex-and-age. Accessed January 6, 2020.
- US Food and Drug Administration website. Mammography: what you need to know. https://www.fda.gov/consumers/consumer-updates/mammography-what-you-need-know. Accessed January 13, 2020.
- IBIS (International Breast Cancer Intervention Study) website. Online Tyrer-Cuzick Model Breast Cancer Risk Evaluation Tool. ibis.ikonopedia.com. Accessed January 13, 2020.
- Bevers TB, Anderson BO, Bonaccio E, et al; National Comprehensive Cancer Network. Breast cancer screening and diagnosis: NCCN practice guidelines in oncology. JNCCN. 2009;7:1060-1096.
- Saslow D, Boetes C, Burke W, et al. American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography. CA Cancer J Clin. 2007;57:75-89.
- Antoniou AC, Cunningham AP, Peto J, et al. The BOADICEA model of genetic susceptibility to breast and ovarian cancers: updates and extensions. Br J Cancer. 2008;98:1457-1466.
- Claus EB, Risch N, Thompson WD. Autosomal dominant inheritance of early-onset breast cancer: implications for risk prediction. Cancer. 1994;73:643-651.
- Parmigiani G, Berry D, Aguilar O. Determining carrier probabilities for breast cancer-susceptibility genes BRCA1 and BRCA2. Am J Hum Genet. 1998;62:145-158.
- Tyrer J, Duffy SW, Cuzick J. A breast cancer prediction model incorporating familial and personal risk factors. Stat Med. 2004;23:1111-1130.
While the frequency of dense breasts decreases with age, approximately 10% of women in the United States have extremely dense breasts (Breast Imaging, Reporting, and Data System [BI-RADS] category D), and another 40% have heterogeneously dense breasts (BI-RADS category C).1 Women with dense breasts have both an increased risk for developing breast cancer and reduced mammographic sensitivity for breast cancer detection compared with women who have nondense breasts.2
These 2 observations have led the majority of states to pass legislation requiring that women with dense breasts be informed of their breast density, and most require that providers discuss these results with their patients. Thoughtful clinicians who review the available literature, however, will find sparse evidence on which to counsel patients as to next steps.
Now, a recent trial adds to our knowledge about supplemental magnetic resonance imaging (MRI) breast screening in women with extremely dense breasts.
DENSE trial offers high-quality data
Bakker and colleagues studied women aged 50 to 74 who were participating in a Netherlands population-based biennial mammography screening program.3 They enrolled average-risk women with extremely dense breasts who had a negative screening digital mammogram into the Dense Tissue and Early Breast Neoplasm Screening (DENSE) multicenter trial. The women were randomly assigned to receive either continued biennial digital mammography or supplemental breast MRI.
The primary outcome was the between-group difference in the development of interval breast cancers—that is, breast cancers detected by women or their providers between rounds of screening mammography. Interval breast cancers were chosen as the primary outcome for 2 reasons:
- interval cancers appear to be more aggressive tumors than those cancers detected by screening mammography
- interval cancers can be identified over a shorter time interval, making them easier to study than outcomes such as breast cancer mortality, which typically require more than a decade to identify.
The DENSE trial’s secondary outcomes included recall rates from MRI, cancer detection rates on MRI, positive predictive value of MRIs requiring biopsy, and breast cancer characteristics (size, stage) diagnosed in the different groups.
Between-group difference in incidence of interval cancers
A total of 40,373 women with extremely dense breasts were screened; 8,061 of these were randomly assigned to receive breast MRI and 32,312 to continued mammography only (1:4 cluster randomization) across 12 mammography centers in the Netherlands. Among the women assigned to the MRI group, 59% actually underwent MRI (4,783 of the 8,061).
The interval cancer rate in the mammography-only group was 5.0 per 1,000 screenings (95% confidence interval [CI], 4.3–5.8), while the interval cancer rate in the MRI-assigned group was 2.5 per 1,000 screenings (95% CI, 1.6–3.8) (TABLE 1).3

Key secondary outcomes
Of the women who underwent supplemental MRI, 9.49% were recalled for additional imaging, follow-up, or biopsy. Of the 4,783 women who had an MRI, 300 (6.3%) underwent a breast biopsy, and 79 breast cancers (1.65%) were detected. Sixty-four of these cancers were invasive, and 15 were ductal carcinoma in situ (DCIS). Among women who underwent a biopsy for an MRI-detected abnormality, the positive predictive value was 26.3%.
Tumor characteristics. For women who developed breast cancer during the study, both tumor size at diagnosis and tumor stage (early vs late) were described. TABLE 2 shows these results in the women who had their breast cancer detected on MRI, those in the MRI-assigned group who developed interval cancer, and those in the mammography-only group who had interval cancers.3 Overall, tumor size was smaller in the interval group who underwent MRI compared with those who underwent mammography only.

Continue to: Study contributes valuable data, but we need more on long-term outcomes...
Study contributes valuable data, but we need more on long-term outcomes
The trial by Bakker and colleagues employed a solid study design as women were randomly assigned to supplemental MRI screening or ongoing biennial mammography, and nearly all cancers were identified in the short-term of follow-up. In addition, very few women were lost to follow-up, and secondary outcomes, including false-positive rates, were collected to help providers and patients better understand some of the potential downsides of supplemental screening.
The substantial reduction in interval cancers (50% in the intent-to-screen analysis and 84% in the women who actually underwent supplemental MRI) was highly statistically significant (P<.001). While there were substantially fewer interval cancers in the MRI-assigned group, the interval cancers that did occur were of similar stage as those in the women assigned to the mammography-only group (TABLE 2).
Data demonstrate that interval cancers appear to be more aggressive than screen-detected cancers.4 While reducing interval cancers should be a good thing overall, it remains unproven that using supplemental MRI in all women with dense breasts would reduce breast cancer specific mortality, all-cause mortality, or the risk of more invasive treatments (for example, the need for chemotherapy or requirement for mastectomy).
On the other hand, using routine supplemental breast MRI in women with extremely dense breasts would result in very substantial use of resources, including cost, radiologist time, provider time, and machine time. In the United States, approximately 49 million women are aged 50 to 74.5 Breast MRI charges commonly range from $1,000 to $4,000. If the 4.9 million women with extremely dense breasts underwent supplemental MRI this year, the approximate cost would be somewhere between $4.9 and $19.5 billion for imaging alone. This does not include callbacks, biopsies, or provider time for ordering, interpreting, and arranging for follow-up.
While the reduction in interval cancers seen in this study is promising, more assurance of improvement in important outcomes—such as reduced mortality or reduced need for more invasive breast cancer treatments—should precede any routine change in practice.
Unanswered questions
This study did not address a number of other important questions, including:
Should MRI be done with every round of breast cancer screening given the possibility of prevalence bias? Prevalence bias can be defined as more cancers detected in the first round of MRI screening with possible reduced benefit in future rounds of screening. The study authors indicated that they will continue to analyze the study results to see what occurs in the next round of screening.
Is there a similar impact on decreased interval cancers in women undergoing annual mammography or in women screened between ages 40 and 49? This study was conducted in women aged 50 to 74 undergoing mammography every 2 years. In the United States, annual mammography in women aged 40 to 49 is frequently recommended.
What effect does supplemental MRI screening have in women with heterogeneously dense breasts, which represents 40% of the population? The US Food and Drug Administration recommends that all women with dense breasts be counseled regarding options for management.6
Do these results translate to the more racially and ethnically diverse populations of the United States? In the Netherlands, where this study was conducted, 85% to 90% of women are either Dutch or of western European origin. Women of different racial and ancestral backgrounds have biologically different breast cancers and cancer risk (for example, higher rates of triple-negative breast cancers in African American women; 10-fold higher rates of BRCA pathogenic variants in Ashkenazi Jewish women).
Continue to: Use validated tools to assess risk comprehensively...
Use validated tools to assess risk comprehensively
Women aged 50 to 74 with extremely dense breasts have reduced interval cancers following a normal biennial mammogram if supplemental MRI is offered, but the long-term benefit of identifying these cancers earlier is unclear. Until more data are available on important long-term outcomes (such as breast cancer mortality and need for more invasive treatments), providers should consider breast density in the context of a more comprehensive assessment of breast cancer risk using a validated breast cancer risk assessment tool.
I prefer the modified version of the International Breast Cancer Intervention Study (IBIS) tool, which is readily available online (https://ibis.ikonopedia.com/).7 This tool incorporates several breast cancer risk factors, including reproductive risk factors, body mass index, BRCA gene status, breast density, and family history. The tool takes 1 to 2 minutes to complete and provides an estimate of a woman’s 10-year risk and lifetime risk of breast cancer.
If the lifetime risk exceeds 20%, I offer the patient supplemental MRI screening, consistent with current recommendations of the National Comprehensive Cancer Network and the American Cancer Society.8,9 I generally recommend starting breast imaging screening 7 to 10 years prior to the youngest breast cancer occurrence in the family, with mammography starting no earlier than age 30 and MRI no earlier than age 25. Other validated tools also can be used.10-13
Incorporating breast density and other important risk factors allows a more comprehensive analysis upon which to counsel women about the value (benefits and harms) of breast imaging.8
While the frequency of dense breasts decreases with age, approximately 10% of women in the United States have extremely dense breasts (Breast Imaging, Reporting, and Data System [BI-RADS] category D), and another 40% have heterogeneously dense breasts (BI-RADS category C).1 Women with dense breasts have both an increased risk for developing breast cancer and reduced mammographic sensitivity for breast cancer detection compared with women who have nondense breasts.2
These 2 observations have led the majority of states to pass legislation requiring that women with dense breasts be informed of their breast density, and most require that providers discuss these results with their patients. Thoughtful clinicians who review the available literature, however, will find sparse evidence on which to counsel patients as to next steps.
Now, a recent trial adds to our knowledge about supplemental magnetic resonance imaging (MRI) breast screening in women with extremely dense breasts.
DENSE trial offers high-quality data
Bakker and colleagues studied women aged 50 to 74 who were participating in a Netherlands population-based biennial mammography screening program.3 They enrolled average-risk women with extremely dense breasts who had a negative screening digital mammogram into the Dense Tissue and Early Breast Neoplasm Screening (DENSE) multicenter trial. The women were randomly assigned to receive either continued biennial digital mammography or supplemental breast MRI.
The primary outcome was the between-group difference in the development of interval breast cancers—that is, breast cancers detected by women or their providers between rounds of screening mammography. Interval breast cancers were chosen as the primary outcome for 2 reasons:
- interval cancers appear to be more aggressive tumors than those cancers detected by screening mammography
- interval cancers can be identified over a shorter time interval, making them easier to study than outcomes such as breast cancer mortality, which typically require more than a decade to identify.
The DENSE trial’s secondary outcomes included recall rates from MRI, cancer detection rates on MRI, positive predictive value of MRIs requiring biopsy, and breast cancer characteristics (size, stage) diagnosed in the different groups.
Between-group difference in incidence of interval cancers
A total of 40,373 women with extremely dense breasts were screened; 8,061 of these were randomly assigned to receive breast MRI and 32,312 to continued mammography only (1:4 cluster randomization) across 12 mammography centers in the Netherlands. Among the women assigned to the MRI group, 59% actually underwent MRI (4,783 of the 8,061).
The interval cancer rate in the mammography-only group was 5.0 per 1,000 screenings (95% confidence interval [CI], 4.3–5.8), while the interval cancer rate in the MRI-assigned group was 2.5 per 1,000 screenings (95% CI, 1.6–3.8) (TABLE 1).3

Key secondary outcomes
Of the women who underwent supplemental MRI, 9.49% were recalled for additional imaging, follow-up, or biopsy. Of the 4,783 women who had an MRI, 300 (6.3%) underwent a breast biopsy, and 79 breast cancers (1.65%) were detected. Sixty-four of these cancers were invasive, and 15 were ductal carcinoma in situ (DCIS). Among women who underwent a biopsy for an MRI-detected abnormality, the positive predictive value was 26.3%.
Tumor characteristics. For women who developed breast cancer during the study, both tumor size at diagnosis and tumor stage (early vs late) were described. TABLE 2 shows these results in the women who had their breast cancer detected on MRI, those in the MRI-assigned group who developed interval cancer, and those in the mammography-only group who had interval cancers.3 Overall, tumor size was smaller in the interval group who underwent MRI compared with those who underwent mammography only.

Continue to: Study contributes valuable data, but we need more on long-term outcomes...
Study contributes valuable data, but we need more on long-term outcomes
The trial by Bakker and colleagues employed a solid study design as women were randomly assigned to supplemental MRI screening or ongoing biennial mammography, and nearly all cancers were identified in the short-term of follow-up. In addition, very few women were lost to follow-up, and secondary outcomes, including false-positive rates, were collected to help providers and patients better understand some of the potential downsides of supplemental screening.
The substantial reduction in interval cancers (50% in the intent-to-screen analysis and 84% in the women who actually underwent supplemental MRI) was highly statistically significant (P<.001). While there were substantially fewer interval cancers in the MRI-assigned group, the interval cancers that did occur were of similar stage as those in the women assigned to the mammography-only group (TABLE 2).
Data demonstrate that interval cancers appear to be more aggressive than screen-detected cancers.4 While reducing interval cancers should be a good thing overall, it remains unproven that using supplemental MRI in all women with dense breasts would reduce breast cancer specific mortality, all-cause mortality, or the risk of more invasive treatments (for example, the need for chemotherapy or requirement for mastectomy).
On the other hand, using routine supplemental breast MRI in women with extremely dense breasts would result in very substantial use of resources, including cost, radiologist time, provider time, and machine time. In the United States, approximately 49 million women are aged 50 to 74.5 Breast MRI charges commonly range from $1,000 to $4,000. If the 4.9 million women with extremely dense breasts underwent supplemental MRI this year, the approximate cost would be somewhere between $4.9 and $19.5 billion for imaging alone. This does not include callbacks, biopsies, or provider time for ordering, interpreting, and arranging for follow-up.
While the reduction in interval cancers seen in this study is promising, more assurance of improvement in important outcomes—such as reduced mortality or reduced need for more invasive breast cancer treatments—should precede any routine change in practice.
Unanswered questions
This study did not address a number of other important questions, including:
Should MRI be done with every round of breast cancer screening given the possibility of prevalence bias? Prevalence bias can be defined as more cancers detected in the first round of MRI screening with possible reduced benefit in future rounds of screening. The study authors indicated that they will continue to analyze the study results to see what occurs in the next round of screening.
Is there a similar impact on decreased interval cancers in women undergoing annual mammography or in women screened between ages 40 and 49? This study was conducted in women aged 50 to 74 undergoing mammography every 2 years. In the United States, annual mammography in women aged 40 to 49 is frequently recommended.
What effect does supplemental MRI screening have in women with heterogeneously dense breasts, which represents 40% of the population? The US Food and Drug Administration recommends that all women with dense breasts be counseled regarding options for management.6
Do these results translate to the more racially and ethnically diverse populations of the United States? In the Netherlands, where this study was conducted, 85% to 90% of women are either Dutch or of western European origin. Women of different racial and ancestral backgrounds have biologically different breast cancers and cancer risk (for example, higher rates of triple-negative breast cancers in African American women; 10-fold higher rates of BRCA pathogenic variants in Ashkenazi Jewish women).
Continue to: Use validated tools to assess risk comprehensively...
Use validated tools to assess risk comprehensively
Women aged 50 to 74 with extremely dense breasts have reduced interval cancers following a normal biennial mammogram if supplemental MRI is offered, but the long-term benefit of identifying these cancers earlier is unclear. Until more data are available on important long-term outcomes (such as breast cancer mortality and need for more invasive treatments), providers should consider breast density in the context of a more comprehensive assessment of breast cancer risk using a validated breast cancer risk assessment tool.
I prefer the modified version of the International Breast Cancer Intervention Study (IBIS) tool, which is readily available online (https://ibis.ikonopedia.com/).7 This tool incorporates several breast cancer risk factors, including reproductive risk factors, body mass index, BRCA gene status, breast density, and family history. The tool takes 1 to 2 minutes to complete and provides an estimate of a woman’s 10-year risk and lifetime risk of breast cancer.
If the lifetime risk exceeds 20%, I offer the patient supplemental MRI screening, consistent with current recommendations of the National Comprehensive Cancer Network and the American Cancer Society.8,9 I generally recommend starting breast imaging screening 7 to 10 years prior to the youngest breast cancer occurrence in the family, with mammography starting no earlier than age 30 and MRI no earlier than age 25. Other validated tools also can be used.10-13
Incorporating breast density and other important risk factors allows a more comprehensive analysis upon which to counsel women about the value (benefits and harms) of breast imaging.8
- Sprague BL, Gagnon RE, Burt V, et al. Prevalence of mammographically dense breasts in the United States. J Natl Cancer Inst. 2014;106:dju255. doi: 10.1093/jcni/dju255.
- Boyd NF, Guo H, Martin LJ, et al. Mammographic density and the risk and detection of breast cancer. N Engl J Med. 2007;356:227-236.
- Bakker MF, de Lange SV, Pijnappel RM, et al; for the DENSE Trial Study Group. Supplemental MRI screening for women with extremely dense breast tissue. N Engl J Med. 2019;381:2091-2102.
- Drukker CA, Schmidt MK, Rutgers EJT, et al. Mammographic screening detects low-risk tumor biology breast cancers. Breast Cancer Res Treat. 2014;144:103-111.
- Statista website. Resident population of the United States by sex and age as of July 1, 2018. https://www.statista.com/statistics/241488/population-of-the-us-by-sex-and-age. Accessed January 6, 2020.
- US Food and Drug Administration website. Mammography: what you need to know. https://www.fda.gov/consumers/consumer-updates/mammography-what-you-need-know. Accessed January 13, 2020.
- IBIS (International Breast Cancer Intervention Study) website. Online Tyrer-Cuzick Model Breast Cancer Risk Evaluation Tool. ibis.ikonopedia.com. Accessed January 13, 2020.
- Bevers TB, Anderson BO, Bonaccio E, et al; National Comprehensive Cancer Network. Breast cancer screening and diagnosis: NCCN practice guidelines in oncology. JNCCN. 2009;7:1060-1096.
- Saslow D, Boetes C, Burke W, et al. American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography. CA Cancer J Clin. 2007;57:75-89.
- Antoniou AC, Cunningham AP, Peto J, et al. The BOADICEA model of genetic susceptibility to breast and ovarian cancers: updates and extensions. Br J Cancer. 2008;98:1457-1466.
- Claus EB, Risch N, Thompson WD. Autosomal dominant inheritance of early-onset breast cancer: implications for risk prediction. Cancer. 1994;73:643-651.
- Parmigiani G, Berry D, Aguilar O. Determining carrier probabilities for breast cancer-susceptibility genes BRCA1 and BRCA2. Am J Hum Genet. 1998;62:145-158.
- Tyrer J, Duffy SW, Cuzick J. A breast cancer prediction model incorporating familial and personal risk factors. Stat Med. 2004;23:1111-1130.
- Sprague BL, Gagnon RE, Burt V, et al. Prevalence of mammographically dense breasts in the United States. J Natl Cancer Inst. 2014;106:dju255. doi: 10.1093/jcni/dju255.
- Boyd NF, Guo H, Martin LJ, et al. Mammographic density and the risk and detection of breast cancer. N Engl J Med. 2007;356:227-236.
- Bakker MF, de Lange SV, Pijnappel RM, et al; for the DENSE Trial Study Group. Supplemental MRI screening for women with extremely dense breast tissue. N Engl J Med. 2019;381:2091-2102.
- Drukker CA, Schmidt MK, Rutgers EJT, et al. Mammographic screening detects low-risk tumor biology breast cancers. Breast Cancer Res Treat. 2014;144:103-111.
- Statista website. Resident population of the United States by sex and age as of July 1, 2018. https://www.statista.com/statistics/241488/population-of-the-us-by-sex-and-age. Accessed January 6, 2020.
- US Food and Drug Administration website. Mammography: what you need to know. https://www.fda.gov/consumers/consumer-updates/mammography-what-you-need-know. Accessed January 13, 2020.
- IBIS (International Breast Cancer Intervention Study) website. Online Tyrer-Cuzick Model Breast Cancer Risk Evaluation Tool. ibis.ikonopedia.com. Accessed January 13, 2020.
- Bevers TB, Anderson BO, Bonaccio E, et al; National Comprehensive Cancer Network. Breast cancer screening and diagnosis: NCCN practice guidelines in oncology. JNCCN. 2009;7:1060-1096.
- Saslow D, Boetes C, Burke W, et al. American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography. CA Cancer J Clin. 2007;57:75-89.
- Antoniou AC, Cunningham AP, Peto J, et al. The BOADICEA model of genetic susceptibility to breast and ovarian cancers: updates and extensions. Br J Cancer. 2008;98:1457-1466.
- Claus EB, Risch N, Thompson WD. Autosomal dominant inheritance of early-onset breast cancer: implications for risk prediction. Cancer. 1994;73:643-651.
- Parmigiani G, Berry D, Aguilar O. Determining carrier probabilities for breast cancer-susceptibility genes BRCA1 and BRCA2. Am J Hum Genet. 1998;62:145-158.
- Tyrer J, Duffy SW, Cuzick J. A breast cancer prediction model incorporating familial and personal risk factors. Stat Med. 2004;23:1111-1130.
Should secondary cytoreduction be performed for platinum-sensitive recurrent ovarian cancer?
Coleman RL, Spirtos NM, Enserro D, et al. Secondary surgical cytoreduction for recurrent ovarian cancer. N Engl J Med. 2019;381:1929-1939.
EXPERT COMMENTARY
Ovarian cancer represents the most lethal gynecologic cancer, with an estimated 14,000 deaths in 2019.1 While the incidence of this disease is low in comparison to uterine cancer, the advanced stage at diagnosis portends poor prognosis. While stage is an independent risk factor for death, it is also a risk for recurrence, with more than 80% of women developing recurrent disease.2-4 Secondary cytoreduction remains an option for patients in which disease recurs; up until now this management option was driven by retrospective data.5
Details of the study
Coleman and colleagues conducted the Gynecologic Oncology Group (GOG) 0213 trial—a phase 3, multicenter, randomized clinical trial that included 485 women with recurrent ovarian cancer. The surgical objective of the trial was to determine whether secondary cytoreduction in operable, platinum-sensitive (PS) patients improved overall survival (OS).
Patients were eligible to participate in the surgical portion of the trial if they had PS measurable disease and had the intention to achieve complete gross resection. Women with ascites, evidence of extraabdominal disease, and “diffuse carcinomatosis” were excluded. The primary and secondary end points were OS and progression-free survival (PFS), respectively.
Results. There were no statistical differences between the surgery and no surgery groups with regard to median OS (50.6 months vs 64.7 months, respectively; hazard ratio [HR], 1.29; 95% confidence interval [CI], 0.97–1.72) or median PFS (18.9 months vs 16.2 months; HR, 0.82; 95% CI, 0.66 to 1.01). When comparing patients in which complete gross resection was achieved (150 patients vs 245 who did not receive surgery), there was only a statistical difference in PFS in favor of the surgical group (22.4 months vs 16.2 months; HR, 0.62; 95% CI, 0.48–0.80).
Of note, 67% of the patients who received surgery (63% intention-to-treat) were debulked to complete gross resection. There were 33% more patients with extraabdominal disease (10% vs 7% of total patients in each group) and 15% more patients with upper abdominal disease (40% vs 33% of total patients in each group) included in the surgical group. Finally, the median time to chemotherapy was 40 days in the surgery group versus 7 days in the no surgery group.
Continue to: Study strengths and weaknesses...
Study strengths and weaknesses
The authors deserve to be commended for this well-designed and laborious trial, which is the first of its kind. The strength of the study is its randomized design producing level I data.
Study weaknesses include lack of reporting of BRCA status and the impact of receiving targeted therapies after the trial was over. It is well established that BRCA-mutated patients have an independent survival advantage, even when taking into account platinum sensitivity.6-8BRCA status of the study population is not specifically addressed in this paper. The authors noted in the first GOG 0213 trial publication, which assessed bevacizumab in the recurrent setting, that BRCA status has an impact on patient outcomes. Subsequently, they state that they do not report BRCA status because “…its independent effect on response to an anti-angiogenesis agent was unknown,” but it clearly would affect survival analysis if unbalanced between groups.9
Similarly, in the introduction to their study, Coleman and colleagues list availability of maintenance therapy, for instance poly ADP (adenosine diphosphate–ribose) polymerase (PARP) inhibitors, as rationale for conducting their trial. They subsequently cite this as a possible reason that the median overall survival was 3 times longer than expected. However, they provide no data on which patients received maintenance therapy, which again could have drastically affected survival outcomes.10 They do report in the supplementary information that, when stratifying those receiving bevacizumab adjuvantly during the trial, the median OS was comparable between the surgical and nonsurgical groups (58.5 months vs 61.7 months).
The authors discuss the presence of patient selection bias as a weakness in the study. Selection bias is evident in this trial (as in many surgical trials) because patients with a limited volume of disease were selected to participate over those with large-volume disease. It is reasonable to conclude that this study is likely selecting patients with less aggressive tumor biology, not only evident by low-volume disease at recurrence but also by the 20.4-month median platinum-free interval in the surgical group, which certainly affects the trial’s validity. Despite being considered PS, the disease biology in a patient with a platinum-free interval of 20.4 months is surely different from the disease biology in a patient with a 6.4-month platinum-free interval; therefore, it is difficult to generalize these data to all PS recurrent ovarian cancer patients. Similarly, other research has suggested strict selection criteria, which was not apparent in this study’s methodology.11 While the number of metastatic sites were relatively equal between the surgery and no surgery groups, there were more patients in the surgical group with extraabdominal disease, which the authors used as an exclusion criterion.
Lastly, the time to treatment commencement in each arm, which was 40 days for the surgical arm and 7 days in the nonsurgical arm, could represent a flaw in this trial. While we expect a difference in duration to account for recovery time, many centers start chemotherapy as soon as 21 days after surgery, which is almost half of the median interval in the surgical group in this trial. While the authors address this by stating that they completed a landmark analysis, no data or information about what time points they used for the analysis are provided. They simply report an interquartile range of 28 to 51 days. It is hard to know what effect this may have had on the outcome.
This is the first randomized clinical trial conducted to assess whether secondary surgical cytoreduction is beneficial in PS recurrent ovarian cancer patients. It provides compelling evidence to critically evaluate whether surgical cytoreduction is appropriate in a similar patient population. However, we would recommend using caution applying these data to patients who have different platinum-free intervals or low-volume disease limited to the pelvis.
The trial is not without flaws, as the authors point out in their discussion, but currently, it is the best evidence afforded to gynecologic oncologists. There are multiple trials currently ongoing, including DESTOP-III, which had similar PFS results as GOG 0213. If consensus is reached with these 2 trials, we believe that secondary cytoreduction will be utilized far less often in patients with recurrent ovarian cancer and a long platinum-free interval, thereby changing the current treatment paradigm for these patients.
MICHAEL D. TOBONI, MD, MPH, AND DAVID G. MUTCH, MD
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7-34.
- Parmar MK, Ledermann JA, Colombo N, et al. Paclitaxel plus platinum-based chemotherapy versus conventional platinum-based chemotherapy in women with relapsed ovarian cancer: the ICON4/AGO-OVAR-2.2 trial. Lancet. 2003;361:2099-2106.
- International Collaborative Ovarian Neoplasm Group. Paclitaxel plus carboplatin versus standard chemotherapy with either single-agent carboplatin or cyclophosphamide, doxorubicin, and cisplatin in women with ovarian cancer: the ICON3 randomised trial. Lancet. 2002;360:505-515.
- Mullen MM, Kuroki LM, Thaker PH. Novel treatment options in platinum-sensitive recurrent ovarian cancer: a review. Gynecol Oncol. 2019;152:416-425.
- National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: ovarian cancer. November 26, 2019. https://www.nccn.org/professionals/physician_gls/pdf/ovarian.pdf. Accessed December 18, 2019.
- Cass I, Baldwin RL, Varkey T, et al. Improved survival in women with BRCA-associated ovarian carcinoma. Cancer. 2003;97:2187-2195.
- Gallagher DJ, Konner JA, Bell-McGuinn KM, et al. Survival in epithelial ovarian cancer: a multivariate analysis incorporating BRCA mutation status and platinum sensitivity. Ann Oncol. 2011;22:1127-1132.
- Sun C, Li N, Ding D, et al. The role of BRCA status on the prognosis of patients with epithelial ovarian cancer: a systematic review of the literature with a meta-analysis. PLoS One. 2014;9:e95285.
- Coleman RL, Brady MF, Herzog TJ, et al. Bevacizumab and paclitaxel-carboplatin chemotherapy and secondary cytoreduction in recurrent, platinum-sensitive ovarian cancer (NRG Oncology/Gynecologic Oncology Group study GOG-0213): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2017;18:779-791.
- Coleman RL, Spirtos NM, Enserro D, et al. Secondary surgical cytoreduction for recurrent ovarian cancer. N Engl J Med. 2019;381:1929-1939.
- Chi DS, McCaughty K, Diaz JP, et al. Guidelines and selection criteria for secondary cytoreductive surgery in patients with recurrent, platinum-sensitive epithelial ovarian carcinoma. Cancer. 2006;106:1933-1939.
Coleman RL, Spirtos NM, Enserro D, et al. Secondary surgical cytoreduction for recurrent ovarian cancer. N Engl J Med. 2019;381:1929-1939.
EXPERT COMMENTARY
Ovarian cancer represents the most lethal gynecologic cancer, with an estimated 14,000 deaths in 2019.1 While the incidence of this disease is low in comparison to uterine cancer, the advanced stage at diagnosis portends poor prognosis. While stage is an independent risk factor for death, it is also a risk for recurrence, with more than 80% of women developing recurrent disease.2-4 Secondary cytoreduction remains an option for patients in which disease recurs; up until now this management option was driven by retrospective data.5
Details of the study
Coleman and colleagues conducted the Gynecologic Oncology Group (GOG) 0213 trial—a phase 3, multicenter, randomized clinical trial that included 485 women with recurrent ovarian cancer. The surgical objective of the trial was to determine whether secondary cytoreduction in operable, platinum-sensitive (PS) patients improved overall survival (OS).
Patients were eligible to participate in the surgical portion of the trial if they had PS measurable disease and had the intention to achieve complete gross resection. Women with ascites, evidence of extraabdominal disease, and “diffuse carcinomatosis” were excluded. The primary and secondary end points were OS and progression-free survival (PFS), respectively.
Results. There were no statistical differences between the surgery and no surgery groups with regard to median OS (50.6 months vs 64.7 months, respectively; hazard ratio [HR], 1.29; 95% confidence interval [CI], 0.97–1.72) or median PFS (18.9 months vs 16.2 months; HR, 0.82; 95% CI, 0.66 to 1.01). When comparing patients in which complete gross resection was achieved (150 patients vs 245 who did not receive surgery), there was only a statistical difference in PFS in favor of the surgical group (22.4 months vs 16.2 months; HR, 0.62; 95% CI, 0.48–0.80).
Of note, 67% of the patients who received surgery (63% intention-to-treat) were debulked to complete gross resection. There were 33% more patients with extraabdominal disease (10% vs 7% of total patients in each group) and 15% more patients with upper abdominal disease (40% vs 33% of total patients in each group) included in the surgical group. Finally, the median time to chemotherapy was 40 days in the surgery group versus 7 days in the no surgery group.
Continue to: Study strengths and weaknesses...
Study strengths and weaknesses
The authors deserve to be commended for this well-designed and laborious trial, which is the first of its kind. The strength of the study is its randomized design producing level I data.
Study weaknesses include lack of reporting of BRCA status and the impact of receiving targeted therapies after the trial was over. It is well established that BRCA-mutated patients have an independent survival advantage, even when taking into account platinum sensitivity.6-8BRCA status of the study population is not specifically addressed in this paper. The authors noted in the first GOG 0213 trial publication, which assessed bevacizumab in the recurrent setting, that BRCA status has an impact on patient outcomes. Subsequently, they state that they do not report BRCA status because “…its independent effect on response to an anti-angiogenesis agent was unknown,” but it clearly would affect survival analysis if unbalanced between groups.9
Similarly, in the introduction to their study, Coleman and colleagues list availability of maintenance therapy, for instance poly ADP (adenosine diphosphate–ribose) polymerase (PARP) inhibitors, as rationale for conducting their trial. They subsequently cite this as a possible reason that the median overall survival was 3 times longer than expected. However, they provide no data on which patients received maintenance therapy, which again could have drastically affected survival outcomes.10 They do report in the supplementary information that, when stratifying those receiving bevacizumab adjuvantly during the trial, the median OS was comparable between the surgical and nonsurgical groups (58.5 months vs 61.7 months).
The authors discuss the presence of patient selection bias as a weakness in the study. Selection bias is evident in this trial (as in many surgical trials) because patients with a limited volume of disease were selected to participate over those with large-volume disease. It is reasonable to conclude that this study is likely selecting patients with less aggressive tumor biology, not only evident by low-volume disease at recurrence but also by the 20.4-month median platinum-free interval in the surgical group, which certainly affects the trial’s validity. Despite being considered PS, the disease biology in a patient with a platinum-free interval of 20.4 months is surely different from the disease biology in a patient with a 6.4-month platinum-free interval; therefore, it is difficult to generalize these data to all PS recurrent ovarian cancer patients. Similarly, other research has suggested strict selection criteria, which was not apparent in this study’s methodology.11 While the number of metastatic sites were relatively equal between the surgery and no surgery groups, there were more patients in the surgical group with extraabdominal disease, which the authors used as an exclusion criterion.
Lastly, the time to treatment commencement in each arm, which was 40 days for the surgical arm and 7 days in the nonsurgical arm, could represent a flaw in this trial. While we expect a difference in duration to account for recovery time, many centers start chemotherapy as soon as 21 days after surgery, which is almost half of the median interval in the surgical group in this trial. While the authors address this by stating that they completed a landmark analysis, no data or information about what time points they used for the analysis are provided. They simply report an interquartile range of 28 to 51 days. It is hard to know what effect this may have had on the outcome.
This is the first randomized clinical trial conducted to assess whether secondary surgical cytoreduction is beneficial in PS recurrent ovarian cancer patients. It provides compelling evidence to critically evaluate whether surgical cytoreduction is appropriate in a similar patient population. However, we would recommend using caution applying these data to patients who have different platinum-free intervals or low-volume disease limited to the pelvis.
The trial is not without flaws, as the authors point out in their discussion, but currently, it is the best evidence afforded to gynecologic oncologists. There are multiple trials currently ongoing, including DESTOP-III, which had similar PFS results as GOG 0213. If consensus is reached with these 2 trials, we believe that secondary cytoreduction will be utilized far less often in patients with recurrent ovarian cancer and a long platinum-free interval, thereby changing the current treatment paradigm for these patients.
MICHAEL D. TOBONI, MD, MPH, AND DAVID G. MUTCH, MD
Coleman RL, Spirtos NM, Enserro D, et al. Secondary surgical cytoreduction for recurrent ovarian cancer. N Engl J Med. 2019;381:1929-1939.
EXPERT COMMENTARY
Ovarian cancer represents the most lethal gynecologic cancer, with an estimated 14,000 deaths in 2019.1 While the incidence of this disease is low in comparison to uterine cancer, the advanced stage at diagnosis portends poor prognosis. While stage is an independent risk factor for death, it is also a risk for recurrence, with more than 80% of women developing recurrent disease.2-4 Secondary cytoreduction remains an option for patients in which disease recurs; up until now this management option was driven by retrospective data.5
Details of the study
Coleman and colleagues conducted the Gynecologic Oncology Group (GOG) 0213 trial—a phase 3, multicenter, randomized clinical trial that included 485 women with recurrent ovarian cancer. The surgical objective of the trial was to determine whether secondary cytoreduction in operable, platinum-sensitive (PS) patients improved overall survival (OS).
Patients were eligible to participate in the surgical portion of the trial if they had PS measurable disease and had the intention to achieve complete gross resection. Women with ascites, evidence of extraabdominal disease, and “diffuse carcinomatosis” were excluded. The primary and secondary end points were OS and progression-free survival (PFS), respectively.
Results. There were no statistical differences between the surgery and no surgery groups with regard to median OS (50.6 months vs 64.7 months, respectively; hazard ratio [HR], 1.29; 95% confidence interval [CI], 0.97–1.72) or median PFS (18.9 months vs 16.2 months; HR, 0.82; 95% CI, 0.66 to 1.01). When comparing patients in which complete gross resection was achieved (150 patients vs 245 who did not receive surgery), there was only a statistical difference in PFS in favor of the surgical group (22.4 months vs 16.2 months; HR, 0.62; 95% CI, 0.48–0.80).
Of note, 67% of the patients who received surgery (63% intention-to-treat) were debulked to complete gross resection. There were 33% more patients with extraabdominal disease (10% vs 7% of total patients in each group) and 15% more patients with upper abdominal disease (40% vs 33% of total patients in each group) included in the surgical group. Finally, the median time to chemotherapy was 40 days in the surgery group versus 7 days in the no surgery group.
Continue to: Study strengths and weaknesses...
Study strengths and weaknesses
The authors deserve to be commended for this well-designed and laborious trial, which is the first of its kind. The strength of the study is its randomized design producing level I data.
Study weaknesses include lack of reporting of BRCA status and the impact of receiving targeted therapies after the trial was over. It is well established that BRCA-mutated patients have an independent survival advantage, even when taking into account platinum sensitivity.6-8BRCA status of the study population is not specifically addressed in this paper. The authors noted in the first GOG 0213 trial publication, which assessed bevacizumab in the recurrent setting, that BRCA status has an impact on patient outcomes. Subsequently, they state that they do not report BRCA status because “…its independent effect on response to an anti-angiogenesis agent was unknown,” but it clearly would affect survival analysis if unbalanced between groups.9
Similarly, in the introduction to their study, Coleman and colleagues list availability of maintenance therapy, for instance poly ADP (adenosine diphosphate–ribose) polymerase (PARP) inhibitors, as rationale for conducting their trial. They subsequently cite this as a possible reason that the median overall survival was 3 times longer than expected. However, they provide no data on which patients received maintenance therapy, which again could have drastically affected survival outcomes.10 They do report in the supplementary information that, when stratifying those receiving bevacizumab adjuvantly during the trial, the median OS was comparable between the surgical and nonsurgical groups (58.5 months vs 61.7 months).
The authors discuss the presence of patient selection bias as a weakness in the study. Selection bias is evident in this trial (as in many surgical trials) because patients with a limited volume of disease were selected to participate over those with large-volume disease. It is reasonable to conclude that this study is likely selecting patients with less aggressive tumor biology, not only evident by low-volume disease at recurrence but also by the 20.4-month median platinum-free interval in the surgical group, which certainly affects the trial’s validity. Despite being considered PS, the disease biology in a patient with a platinum-free interval of 20.4 months is surely different from the disease biology in a patient with a 6.4-month platinum-free interval; therefore, it is difficult to generalize these data to all PS recurrent ovarian cancer patients. Similarly, other research has suggested strict selection criteria, which was not apparent in this study’s methodology.11 While the number of metastatic sites were relatively equal between the surgery and no surgery groups, there were more patients in the surgical group with extraabdominal disease, which the authors used as an exclusion criterion.
Lastly, the time to treatment commencement in each arm, which was 40 days for the surgical arm and 7 days in the nonsurgical arm, could represent a flaw in this trial. While we expect a difference in duration to account for recovery time, many centers start chemotherapy as soon as 21 days after surgery, which is almost half of the median interval in the surgical group in this trial. While the authors address this by stating that they completed a landmark analysis, no data or information about what time points they used for the analysis are provided. They simply report an interquartile range of 28 to 51 days. It is hard to know what effect this may have had on the outcome.
This is the first randomized clinical trial conducted to assess whether secondary surgical cytoreduction is beneficial in PS recurrent ovarian cancer patients. It provides compelling evidence to critically evaluate whether surgical cytoreduction is appropriate in a similar patient population. However, we would recommend using caution applying these data to patients who have different platinum-free intervals or low-volume disease limited to the pelvis.
The trial is not without flaws, as the authors point out in their discussion, but currently, it is the best evidence afforded to gynecologic oncologists. There are multiple trials currently ongoing, including DESTOP-III, which had similar PFS results as GOG 0213. If consensus is reached with these 2 trials, we believe that secondary cytoreduction will be utilized far less often in patients with recurrent ovarian cancer and a long platinum-free interval, thereby changing the current treatment paradigm for these patients.
MICHAEL D. TOBONI, MD, MPH, AND DAVID G. MUTCH, MD
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7-34.
- Parmar MK, Ledermann JA, Colombo N, et al. Paclitaxel plus platinum-based chemotherapy versus conventional platinum-based chemotherapy in women with relapsed ovarian cancer: the ICON4/AGO-OVAR-2.2 trial. Lancet. 2003;361:2099-2106.
- International Collaborative Ovarian Neoplasm Group. Paclitaxel plus carboplatin versus standard chemotherapy with either single-agent carboplatin or cyclophosphamide, doxorubicin, and cisplatin in women with ovarian cancer: the ICON3 randomised trial. Lancet. 2002;360:505-515.
- Mullen MM, Kuroki LM, Thaker PH. Novel treatment options in platinum-sensitive recurrent ovarian cancer: a review. Gynecol Oncol. 2019;152:416-425.
- National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: ovarian cancer. November 26, 2019. https://www.nccn.org/professionals/physician_gls/pdf/ovarian.pdf. Accessed December 18, 2019.
- Cass I, Baldwin RL, Varkey T, et al. Improved survival in women with BRCA-associated ovarian carcinoma. Cancer. 2003;97:2187-2195.
- Gallagher DJ, Konner JA, Bell-McGuinn KM, et al. Survival in epithelial ovarian cancer: a multivariate analysis incorporating BRCA mutation status and platinum sensitivity. Ann Oncol. 2011;22:1127-1132.
- Sun C, Li N, Ding D, et al. The role of BRCA status on the prognosis of patients with epithelial ovarian cancer: a systematic review of the literature with a meta-analysis. PLoS One. 2014;9:e95285.
- Coleman RL, Brady MF, Herzog TJ, et al. Bevacizumab and paclitaxel-carboplatin chemotherapy and secondary cytoreduction in recurrent, platinum-sensitive ovarian cancer (NRG Oncology/Gynecologic Oncology Group study GOG-0213): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2017;18:779-791.
- Coleman RL, Spirtos NM, Enserro D, et al. Secondary surgical cytoreduction for recurrent ovarian cancer. N Engl J Med. 2019;381:1929-1939.
- Chi DS, McCaughty K, Diaz JP, et al. Guidelines and selection criteria for secondary cytoreductive surgery in patients with recurrent, platinum-sensitive epithelial ovarian carcinoma. Cancer. 2006;106:1933-1939.
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7-34.
- Parmar MK, Ledermann JA, Colombo N, et al. Paclitaxel plus platinum-based chemotherapy versus conventional platinum-based chemotherapy in women with relapsed ovarian cancer: the ICON4/AGO-OVAR-2.2 trial. Lancet. 2003;361:2099-2106.
- International Collaborative Ovarian Neoplasm Group. Paclitaxel plus carboplatin versus standard chemotherapy with either single-agent carboplatin or cyclophosphamide, doxorubicin, and cisplatin in women with ovarian cancer: the ICON3 randomised trial. Lancet. 2002;360:505-515.
- Mullen MM, Kuroki LM, Thaker PH. Novel treatment options in platinum-sensitive recurrent ovarian cancer: a review. Gynecol Oncol. 2019;152:416-425.
- National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: ovarian cancer. November 26, 2019. https://www.nccn.org/professionals/physician_gls/pdf/ovarian.pdf. Accessed December 18, 2019.
- Cass I, Baldwin RL, Varkey T, et al. Improved survival in women with BRCA-associated ovarian carcinoma. Cancer. 2003;97:2187-2195.
- Gallagher DJ, Konner JA, Bell-McGuinn KM, et al. Survival in epithelial ovarian cancer: a multivariate analysis incorporating BRCA mutation status and platinum sensitivity. Ann Oncol. 2011;22:1127-1132.
- Sun C, Li N, Ding D, et al. The role of BRCA status on the prognosis of patients with epithelial ovarian cancer: a systematic review of the literature with a meta-analysis. PLoS One. 2014;9:e95285.
- Coleman RL, Brady MF, Herzog TJ, et al. Bevacizumab and paclitaxel-carboplatin chemotherapy and secondary cytoreduction in recurrent, platinum-sensitive ovarian cancer (NRG Oncology/Gynecologic Oncology Group study GOG-0213): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2017;18:779-791.
- Coleman RL, Spirtos NM, Enserro D, et al. Secondary surgical cytoreduction for recurrent ovarian cancer. N Engl J Med. 2019;381:1929-1939.
- Chi DS, McCaughty K, Diaz JP, et al. Guidelines and selection criteria for secondary cytoreductive surgery in patients with recurrent, platinum-sensitive epithelial ovarian carcinoma. Cancer. 2006;106:1933-1939.