User login
Performance Evaluation Program for Individual Physicians Directional at Best
What makes a great doctor? Heck if I know. Maybe it’s like pornography. A great physician, well, “You know one when you see one.” That approach worked from the time of Hippocrates until the recent past, when the Centers for Medicare & Medicaid Services (CMS), the Joint Commission, and others embarked on programs to measure and report physician quality. Of course, bodies like the American Board of Internal Medicine have been certifying physicians for a long time.
Ongoing Professional Practice Evaluation (OPPE) is one such measurement program, now over four years old, with standards put forth by the Joint Commission in an effort to monitor individual physician—and non-physician provider—performance across a number of domains. The program requires accredited hospitals to monitor and report performance to the physician/provider at least every 11 months, and to use such information in the credentialing process.
This year, I received two OPPE reports, causing me to reflect on how helpful these reports are in judging and improving the quality of my practice. Before I discuss some of the “grades” I received, let me start with my conclusion: Physician quality measurement is in its infancy, and the measures are at best “directional” for most physicians, including hospitalists. Some measurement is better than none at all, however, and selected measures, such as surgical site infection and other measures of harm, may be grounds for closer monitoring, or even corrective action, of a physician’s practice. Unfortunately, my stance that OPPE quality measures are “directional” might not help a physician whose privileges are on the line.
Attribution
For hospitalists, the first concern in measuring and reporting quality is, “How can I attribute quality to an individual hospitalist, when several different hospitalists see the patient?” My perspective is that unless a quality measure can be attributed to an individual hospitalist (e.g. discharge medication reconciliation), it should be attributed at the group level.
However, the OPPE program is specifically intended to address the individual physician/provider for purposes of credentialing, and group attribution is a non-starter. In my performance examples below, I believe that attributing outcomes like mortality, readmissions, or resource utilization to individual hospitalists does not make sense—and is probably unfair.
Resource Utilization
The report lists my performance (Practitioner) compared to an Internal Comparison Group for a specified time period (see Figure 1). The comparison group is described as “practitioners in your specialty...from within your health system.” My data were generated based on only 45 cases (I see patients only part time), while the comparison group was based on 4,530 cases. What I take home from this is that, for cost/resource, I look favorable in “supplies” and “pharmacy”; for most of the others, I’m expensive in comparison.
Will this change my practice? Maybe I will think twice about incurring laboratory or pharmacy costs, but I can’t say I am going to fundamentally rethink how or what I order. And I take all these data with a grain of salt, because I share responsibility for patients with several other hospitalists.
Readmissions
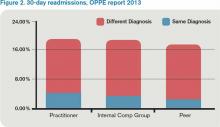
My 30-day readmissions performance (see Figure 2) is weak compared to the Internal Comp Group, which I defined above, and the Peer group, which in my report is defined as derived from practitioners at facilities with 501 beds or more (my facility has 700-plus beds). I accept the “directional” nature of the data, meaning that it provides a general idea but not a precise measurement, and vow to reflect on the processes underlying my approach to hospital discharge (teach back, medication reconciliation, PCP communication, and so on).
Mortality
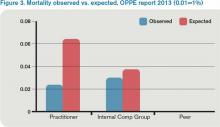
For this category (see Figure 3), I’m looking better. The blue bar is “observed,” while the red bar is “expected.” Although my patients are sicker (higher “expected” mortality), my “observed” mortality is lower than the comparison group. I’m not sure why my observed mortality is lower, but I’m convinced that part of the reason for a higher expected mortality is that my documentation is better than the comparison group.
Will OPPE Change My Practice?
There are other data in my report, including process (core) measures, length of stay, hospital-acquired conditions, and patient flow measures. The OPPE report is but one of a growing number of physician report cards: The Massachusetts Board of Medicine, Physician Compare (CMS), and Health Grades are just a few of the organizations that have public websites reporting my performance. Perhaps at this stage, the primary impact of these reports is through the oft-invoked “Hawthorne Effect,” where subjects modify behavior simply because they are being observed, as opposed to any particular piece of feedback.
My sense is that hospitalists are particularly open to the type of feedback offered in OPPE and similar reports, as long as the data are credible, even if reflecting group level performance. The 2012 SHM State of Hospital Medicine survey shows that the percent of hospitalist compensation based on performance (other than production/billings) increased to 7% from 4% in 2011. It seems that performance measurement with consequences, be it for credentialing or compensation, is here to stay.
Dr. Whitcombis Chief Medical Officer of Remedy Partners. He is co-founder and past president of SHM. Email him at [email protected].
What makes a great doctor? Heck if I know. Maybe it’s like pornography. A great physician, well, “You know one when you see one.” That approach worked from the time of Hippocrates until the recent past, when the Centers for Medicare & Medicaid Services (CMS), the Joint Commission, and others embarked on programs to measure and report physician quality. Of course, bodies like the American Board of Internal Medicine have been certifying physicians for a long time.
Ongoing Professional Practice Evaluation (OPPE) is one such measurement program, now over four years old, with standards put forth by the Joint Commission in an effort to monitor individual physician—and non-physician provider—performance across a number of domains. The program requires accredited hospitals to monitor and report performance to the physician/provider at least every 11 months, and to use such information in the credentialing process.
This year, I received two OPPE reports, causing me to reflect on how helpful these reports are in judging and improving the quality of my practice. Before I discuss some of the “grades” I received, let me start with my conclusion: Physician quality measurement is in its infancy, and the measures are at best “directional” for most physicians, including hospitalists. Some measurement is better than none at all, however, and selected measures, such as surgical site infection and other measures of harm, may be grounds for closer monitoring, or even corrective action, of a physician’s practice. Unfortunately, my stance that OPPE quality measures are “directional” might not help a physician whose privileges are on the line.
Attribution
For hospitalists, the first concern in measuring and reporting quality is, “How can I attribute quality to an individual hospitalist, when several different hospitalists see the patient?” My perspective is that unless a quality measure can be attributed to an individual hospitalist (e.g. discharge medication reconciliation), it should be attributed at the group level.
However, the OPPE program is specifically intended to address the individual physician/provider for purposes of credentialing, and group attribution is a non-starter. In my performance examples below, I believe that attributing outcomes like mortality, readmissions, or resource utilization to individual hospitalists does not make sense—and is probably unfair.
Resource Utilization
The report lists my performance (Practitioner) compared to an Internal Comparison Group for a specified time period (see Figure 1). The comparison group is described as “practitioners in your specialty...from within your health system.” My data were generated based on only 45 cases (I see patients only part time), while the comparison group was based on 4,530 cases. What I take home from this is that, for cost/resource, I look favorable in “supplies” and “pharmacy”; for most of the others, I’m expensive in comparison.
Will this change my practice? Maybe I will think twice about incurring laboratory or pharmacy costs, but I can’t say I am going to fundamentally rethink how or what I order. And I take all these data with a grain of salt, because I share responsibility for patients with several other hospitalists.
Readmissions
My 30-day readmissions performance (see Figure 2) is weak compared to the Internal Comp Group, which I defined above, and the Peer group, which in my report is defined as derived from practitioners at facilities with 501 beds or more (my facility has 700-plus beds). I accept the “directional” nature of the data, meaning that it provides a general idea but not a precise measurement, and vow to reflect on the processes underlying my approach to hospital discharge (teach back, medication reconciliation, PCP communication, and so on).
Mortality
For this category (see Figure 3), I’m looking better. The blue bar is “observed,” while the red bar is “expected.” Although my patients are sicker (higher “expected” mortality), my “observed” mortality is lower than the comparison group. I’m not sure why my observed mortality is lower, but I’m convinced that part of the reason for a higher expected mortality is that my documentation is better than the comparison group.
Will OPPE Change My Practice?
There are other data in my report, including process (core) measures, length of stay, hospital-acquired conditions, and patient flow measures. The OPPE report is but one of a growing number of physician report cards: The Massachusetts Board of Medicine, Physician Compare (CMS), and Health Grades are just a few of the organizations that have public websites reporting my performance. Perhaps at this stage, the primary impact of these reports is through the oft-invoked “Hawthorne Effect,” where subjects modify behavior simply because they are being observed, as opposed to any particular piece of feedback.
My sense is that hospitalists are particularly open to the type of feedback offered in OPPE and similar reports, as long as the data are credible, even if reflecting group level performance. The 2012 SHM State of Hospital Medicine survey shows that the percent of hospitalist compensation based on performance (other than production/billings) increased to 7% from 4% in 2011. It seems that performance measurement with consequences, be it for credentialing or compensation, is here to stay.
Dr. Whitcombis Chief Medical Officer of Remedy Partners. He is co-founder and past president of SHM. Email him at [email protected].
What makes a great doctor? Heck if I know. Maybe it’s like pornography. A great physician, well, “You know one when you see one.” That approach worked from the time of Hippocrates until the recent past, when the Centers for Medicare & Medicaid Services (CMS), the Joint Commission, and others embarked on programs to measure and report physician quality. Of course, bodies like the American Board of Internal Medicine have been certifying physicians for a long time.
Ongoing Professional Practice Evaluation (OPPE) is one such measurement program, now over four years old, with standards put forth by the Joint Commission in an effort to monitor individual physician—and non-physician provider—performance across a number of domains. The program requires accredited hospitals to monitor and report performance to the physician/provider at least every 11 months, and to use such information in the credentialing process.
This year, I received two OPPE reports, causing me to reflect on how helpful these reports are in judging and improving the quality of my practice. Before I discuss some of the “grades” I received, let me start with my conclusion: Physician quality measurement is in its infancy, and the measures are at best “directional” for most physicians, including hospitalists. Some measurement is better than none at all, however, and selected measures, such as surgical site infection and other measures of harm, may be grounds for closer monitoring, or even corrective action, of a physician’s practice. Unfortunately, my stance that OPPE quality measures are “directional” might not help a physician whose privileges are on the line.
Attribution
For hospitalists, the first concern in measuring and reporting quality is, “How can I attribute quality to an individual hospitalist, when several different hospitalists see the patient?” My perspective is that unless a quality measure can be attributed to an individual hospitalist (e.g. discharge medication reconciliation), it should be attributed at the group level.
However, the OPPE program is specifically intended to address the individual physician/provider for purposes of credentialing, and group attribution is a non-starter. In my performance examples below, I believe that attributing outcomes like mortality, readmissions, or resource utilization to individual hospitalists does not make sense—and is probably unfair.
Resource Utilization
The report lists my performance (Practitioner) compared to an Internal Comparison Group for a specified time period (see Figure 1). The comparison group is described as “practitioners in your specialty...from within your health system.” My data were generated based on only 45 cases (I see patients only part time), while the comparison group was based on 4,530 cases. What I take home from this is that, for cost/resource, I look favorable in “supplies” and “pharmacy”; for most of the others, I’m expensive in comparison.
Will this change my practice? Maybe I will think twice about incurring laboratory or pharmacy costs, but I can’t say I am going to fundamentally rethink how or what I order. And I take all these data with a grain of salt, because I share responsibility for patients with several other hospitalists.
Readmissions
My 30-day readmissions performance (see Figure 2) is weak compared to the Internal Comp Group, which I defined above, and the Peer group, which in my report is defined as derived from practitioners at facilities with 501 beds or more (my facility has 700-plus beds). I accept the “directional” nature of the data, meaning that it provides a general idea but not a precise measurement, and vow to reflect on the processes underlying my approach to hospital discharge (teach back, medication reconciliation, PCP communication, and so on).
Mortality
For this category (see Figure 3), I’m looking better. The blue bar is “observed,” while the red bar is “expected.” Although my patients are sicker (higher “expected” mortality), my “observed” mortality is lower than the comparison group. I’m not sure why my observed mortality is lower, but I’m convinced that part of the reason for a higher expected mortality is that my documentation is better than the comparison group.
Will OPPE Change My Practice?
There are other data in my report, including process (core) measures, length of stay, hospital-acquired conditions, and patient flow measures. The OPPE report is but one of a growing number of physician report cards: The Massachusetts Board of Medicine, Physician Compare (CMS), and Health Grades are just a few of the organizations that have public websites reporting my performance. Perhaps at this stage, the primary impact of these reports is through the oft-invoked “Hawthorne Effect,” where subjects modify behavior simply because they are being observed, as opposed to any particular piece of feedback.
My sense is that hospitalists are particularly open to the type of feedback offered in OPPE and similar reports, as long as the data are credible, even if reflecting group level performance. The 2012 SHM State of Hospital Medicine survey shows that the percent of hospitalist compensation based on performance (other than production/billings) increased to 7% from 4% in 2011. It seems that performance measurement with consequences, be it for credentialing or compensation, is here to stay.
Dr. Whitcombis Chief Medical Officer of Remedy Partners. He is co-founder and past president of SHM. Email him at [email protected].
Is one oral estrogen formulation safer than another for menopausal women?
Although numerous investigators, including the Women’s Health Initiative (WHI) team, have compared cardiovascular risks in women using menopausal hormone therapy (HT) versus nonusers, few researchers have addressed the comparative safety of different oral estrogen formulations.
Related Article: Update on Menopause (Andrew M. Kaunitz, MD, June 2013)
In this case-control study, Smith and colleagues compared the safety of oral estradiol and CEE in menopausal members of a large US Health Maintenance Organization who were using these oral estrogens between 2003 and 2009.
Details of the study
Cases were women diagnosed with deep venous thrombosis, including pulmonary embolism; myocardial infarction; or ischemic stroke. Women in the control group had no history of cardiovascular events. The endogenous thrombin potential-based normalized activated protein C sensitivity ratio (nAPCsr), which has been shown to predict venous thromboembolism (VTE) in the setting of estrogen therapy, was measured in the control group.
Between 2003 and 2009, incident VTE, MI, and stroke were diagnosed in 68, 67, and 49 cases, respectively, and 201 controls were identified. Cases were more likely than controls to have cardiovascular risk factors.
More than 90% of participants were white, with a mean age ranging from 63.2 to 67.6 years.
Among women in the control group, those using oral estradiol had slightly more cardiovascular risk factors than those using CEE, although age, body mass index, and the recency of HT initiation were similar among women using the two oral estrogens.
Although the ORs for VTE and MI were elevated among CEE users, the risk for ischemic stroke was similar for estradiol and CEE users. Women using CEE had higher nAPCsrs (P <.001), however, suggesting a greater tendency to clot.
WHAT THIS EVIDENCE MEANS FOR PRACTICE
Although the risk of VTE appears to be higher among users of oral estrogen than among those using a transdermal formulation,1 many menopausal women prefer oral estrogen for its convenience and because patch adherence can sometimes be an issue.
Oral estradiol and oral CEE appear to be equally effective in relieving menopausal symptoms. However, there is a significant cost differential: A 1-month supply of 1-mg estradiol tablets costs $4 at some chain pharmacies, whereas 0.625-mg tablets of CEE cost $84.92 (according to goodrx.com). Therefore, for menopausal women who elect to use an oral estrogen formulation, estradiol appears to be a wise choice for both safety and economy.
Andrew M. Kaunitz, MD
WE WANT TO HEAR FROM YOU. Tell us what you think.
Reference
- Postmenopausal estrogen therapy: route of administration and risk of venous thromboembolism. Committee Opinion #556. American College of Obstetricians and Gynecologists. Obstet Gynecol. 2013;121(4):887–890.
Although numerous investigators, including the Women’s Health Initiative (WHI) team, have compared cardiovascular risks in women using menopausal hormone therapy (HT) versus nonusers, few researchers have addressed the comparative safety of different oral estrogen formulations.
Related Article: Update on Menopause (Andrew M. Kaunitz, MD, June 2013)
In this case-control study, Smith and colleagues compared the safety of oral estradiol and CEE in menopausal members of a large US Health Maintenance Organization who were using these oral estrogens between 2003 and 2009.
Details of the study
Cases were women diagnosed with deep venous thrombosis, including pulmonary embolism; myocardial infarction; or ischemic stroke. Women in the control group had no history of cardiovascular events. The endogenous thrombin potential-based normalized activated protein C sensitivity ratio (nAPCsr), which has been shown to predict venous thromboembolism (VTE) in the setting of estrogen therapy, was measured in the control group.
Between 2003 and 2009, incident VTE, MI, and stroke were diagnosed in 68, 67, and 49 cases, respectively, and 201 controls were identified. Cases were more likely than controls to have cardiovascular risk factors.
More than 90% of participants were white, with a mean age ranging from 63.2 to 67.6 years.
Among women in the control group, those using oral estradiol had slightly more cardiovascular risk factors than those using CEE, although age, body mass index, and the recency of HT initiation were similar among women using the two oral estrogens.
Although the ORs for VTE and MI were elevated among CEE users, the risk for ischemic stroke was similar for estradiol and CEE users. Women using CEE had higher nAPCsrs (P <.001), however, suggesting a greater tendency to clot.
WHAT THIS EVIDENCE MEANS FOR PRACTICE
Although the risk of VTE appears to be higher among users of oral estrogen than among those using a transdermal formulation,1 many menopausal women prefer oral estrogen for its convenience and because patch adherence can sometimes be an issue.
Oral estradiol and oral CEE appear to be equally effective in relieving menopausal symptoms. However, there is a significant cost differential: A 1-month supply of 1-mg estradiol tablets costs $4 at some chain pharmacies, whereas 0.625-mg tablets of CEE cost $84.92 (according to goodrx.com). Therefore, for menopausal women who elect to use an oral estrogen formulation, estradiol appears to be a wise choice for both safety and economy.
Andrew M. Kaunitz, MD
WE WANT TO HEAR FROM YOU. Tell us what you think.
Although numerous investigators, including the Women’s Health Initiative (WHI) team, have compared cardiovascular risks in women using menopausal hormone therapy (HT) versus nonusers, few researchers have addressed the comparative safety of different oral estrogen formulations.
Related Article: Update on Menopause (Andrew M. Kaunitz, MD, June 2013)
In this case-control study, Smith and colleagues compared the safety of oral estradiol and CEE in menopausal members of a large US Health Maintenance Organization who were using these oral estrogens between 2003 and 2009.
Details of the study
Cases were women diagnosed with deep venous thrombosis, including pulmonary embolism; myocardial infarction; or ischemic stroke. Women in the control group had no history of cardiovascular events. The endogenous thrombin potential-based normalized activated protein C sensitivity ratio (nAPCsr), which has been shown to predict venous thromboembolism (VTE) in the setting of estrogen therapy, was measured in the control group.
Between 2003 and 2009, incident VTE, MI, and stroke were diagnosed in 68, 67, and 49 cases, respectively, and 201 controls were identified. Cases were more likely than controls to have cardiovascular risk factors.
More than 90% of participants were white, with a mean age ranging from 63.2 to 67.6 years.
Among women in the control group, those using oral estradiol had slightly more cardiovascular risk factors than those using CEE, although age, body mass index, and the recency of HT initiation were similar among women using the two oral estrogens.
Although the ORs for VTE and MI were elevated among CEE users, the risk for ischemic stroke was similar for estradiol and CEE users. Women using CEE had higher nAPCsrs (P <.001), however, suggesting a greater tendency to clot.
WHAT THIS EVIDENCE MEANS FOR PRACTICE
Although the risk of VTE appears to be higher among users of oral estrogen than among those using a transdermal formulation,1 many menopausal women prefer oral estrogen for its convenience and because patch adherence can sometimes be an issue.
Oral estradiol and oral CEE appear to be equally effective in relieving menopausal symptoms. However, there is a significant cost differential: A 1-month supply of 1-mg estradiol tablets costs $4 at some chain pharmacies, whereas 0.625-mg tablets of CEE cost $84.92 (according to goodrx.com). Therefore, for menopausal women who elect to use an oral estrogen formulation, estradiol appears to be a wise choice for both safety and economy.
Andrew M. Kaunitz, MD
WE WANT TO HEAR FROM YOU. Tell us what you think.
Reference
- Postmenopausal estrogen therapy: route of administration and risk of venous thromboembolism. Committee Opinion #556. American College of Obstetricians and Gynecologists. Obstet Gynecol. 2013;121(4):887–890.
Reference
- Postmenopausal estrogen therapy: route of administration and risk of venous thromboembolism. Committee Opinion #556. American College of Obstetricians and Gynecologists. Obstet Gynecol. 2013;121(4):887–890.
Get Ready for Transition to ICD-10 Medical Coding
By now, I’m sure you’re knowledgeable about things like healthcare exchanges and other parts of the Accountable Care Act, the increasing number of metrics within hospital value-based purchasing, the physician value-based payment modifier, the physician quality reporting system (PQRS), how to use your hospital’s new EHR efficiently, the new “two-midnight rule” to determine inpatient vs. observation status, and so on.
You’re to be commended if you’re staying on top of all these things and have effective plans in place to ensure good performance on each. And if you haven’t already, you should add at least one more important issue to this list—the transition to ICD-10 coding on Oct. 1, 2014.
An Overview
ICD stands for International Classification of Diseases, and the U.S. has been using the 9th revision (ICD-9) since 1978. ICD-9 is now significantly out of step with current medical knowledge and has run out of codes in some disease sections (“chapters”). This might mean, for example, that new codes for heart diseases would be assigned to the chapter for eye disease, because the former is full.
ICD-10 provides a way to fix these problems and, through more specific coding of diseases, should be able to yield more useful “big data” to measure things like safety and efficacy of care and more accurately identify diagnosis trends and epidemics. And, in theory, it could reduce the number of rejected billing claims, though I’m waiting to see if that happens. I worry that even after fixing all the initial bugs related to the ICD-10 transition, we will see more claim rejections than we experience today.
ICD codes can be thought of as diagnosis codes. CPT codes (Current Procedural Terminology) are an entirely separate set of codes that we use to report the work we do for the purposes of billing. We need to be familiar with both, but it is the ICD codes that are changing.
ICD-10 Basics and Trivia
The World Health Organization issued the ICD-10 in 1994, and it is already in use in many countries. Like some other countries, the U.S. made modifications to the WHO’s original code set, so we refer to ICD-10-CM (Clinical Modification), which contains diagnosis codes. The National Center for Health Statistics, a department of the CDC, is responsible for these modifications.
The WHO version of ICD-10 doesn’t have any procedure codes, so CMS developed ICD-10-PCS (Procedure Coding System) to report procedures, such as surgeries, done in U.S. hospitals. Most hospitalists won’t use these procedure codes often.
Table 1 (left) compares ICD-10-CM to ICD-9-CM. Most of the additional codes in the new version simply add information regarding whether the diagnosis is on the left or right of the body, acute or chronic, or an initial or subsequent visit for the condition. But the standard structure for each code had to be modified significantly to capture this additional information. Some highlights of the seven-character code structure are:
- Characters 1–3: category; first digit always a letter, second digit always a number, all other digits can be either; not case sensitive;
- Characters 4–6: etiology, anatomic site, severity, or other clinical detail; for example, 1=right, 2=left, 3-bilateral, and 0 or 9=unspecified; and
- Character 7: extension (i.e., A=initial encounter, D=subsequent encounter, S=sequelae).
- A placeholder “x” is used as needed to fill in empty characters to ensure that the seventh character stays in the seventh position. For example, T79.1xxA equates to “fat embolism, initial encounter.” (Note that the “dummy” characters could create problems for some IT systems.)
An example of more information contained in additional characters:
- S52=fracture of forearm.
- S52.5=fracture of lower end of radius.
- S52.52=torus fracture of lower end of radius.
- S52.521=torus fracture of lower end of right radius.
- S52.521A=torus fracture of lower end of right radius, initial encounter for closed fracture.
Compared to its predecessor, ICD-10 expands use of combination codes. These are single codes that can be used to classify either two diagnoses, a diagnosis with an associated secondary process, or a diagnosis with an associated complication. For example, rather than reporting acute cor pulmonale and septic pulmonary embolism separately, ICD-10 allows use of the code I26.01: septic pulmonary embolism with acute cor pulmonale.
Resources
In addition to resources on the SHM website, both the American Medical Association (www.ama-assn.org, search “ICD-10”) and the Centers for Medicare and Medicaid Services (www.cms.gov/icd10) have very informative microsites offering detailed ICD-10 information. Much of the information in this column, including the examples above, comes from those sites.
What to Expect
Your hospital and your employer are probably already working in earnest to prepare for the change. In some cases, hospitalists are actively involved in these preparations, but in most cases they will simply wait for an organization to notify them that they should begin training to understand the new coding system. Experts say that most physicians will need two to four hours of training on ICD-10, but because we use a universe of diagnosis codes that is much larger than many specialties, I wonder if hospitalists may need additional training.
Like nearly all the programs I listed at the beginning, the transition to ICD-10 has me concerned. Managing it poorly could mean significant loss in hospital and physician professional fee revenue, as well as lots of tedious and time-consuming work. So, doing it right is important. But, it is also important to do well on all the programs I listed at the beginning of this column, and many others, and there is a limit to just how much we can do effectively as individuals.
Collectively, these programs risk taking too much time and too many brain cells away from keeping up with clinical medicine. So, I wonder if, for many of us, ICD-10 will serve as a tipping point that results in physicians hiring professional coders to choose our diagnosis codes and CPT codes rather than doing it ourselves.
As with EHRs, ICD-10 is said to have many benefits. But the introduction of EHRs in many hospitals had the unintended effect of significantly reducing the number of doctors who were willing to serve as admitting and attending physicians; instead, many chose to refer to hospitalists. In a similar way, ICD-10 might lead many organizations to relieve physicians of the responsibility of looking up and entering codes for each patient, leaving them with more time and energy to be clinicians. We’ll have to wait and see.
Dr. Nelson has been a practicing hospitalist since 1988. He is co-founder and past president of SHM, and principal in Nelson Flores Hospital Medicine Consultants. He is co-director for SHM’s “Best Practices in Managing a Hospital Medicine Program” course. Write to him at [email protected].
By now, I’m sure you’re knowledgeable about things like healthcare exchanges and other parts of the Accountable Care Act, the increasing number of metrics within hospital value-based purchasing, the physician value-based payment modifier, the physician quality reporting system (PQRS), how to use your hospital’s new EHR efficiently, the new “two-midnight rule” to determine inpatient vs. observation status, and so on.
You’re to be commended if you’re staying on top of all these things and have effective plans in place to ensure good performance on each. And if you haven’t already, you should add at least one more important issue to this list—the transition to ICD-10 coding on Oct. 1, 2014.
An Overview
ICD stands for International Classification of Diseases, and the U.S. has been using the 9th revision (ICD-9) since 1978. ICD-9 is now significantly out of step with current medical knowledge and has run out of codes in some disease sections (“chapters”). This might mean, for example, that new codes for heart diseases would be assigned to the chapter for eye disease, because the former is full.
ICD-10 provides a way to fix these problems and, through more specific coding of diseases, should be able to yield more useful “big data” to measure things like safety and efficacy of care and more accurately identify diagnosis trends and epidemics. And, in theory, it could reduce the number of rejected billing claims, though I’m waiting to see if that happens. I worry that even after fixing all the initial bugs related to the ICD-10 transition, we will see more claim rejections than we experience today.
ICD codes can be thought of as diagnosis codes. CPT codes (Current Procedural Terminology) are an entirely separate set of codes that we use to report the work we do for the purposes of billing. We need to be familiar with both, but it is the ICD codes that are changing.
ICD-10 Basics and Trivia
The World Health Organization issued the ICD-10 in 1994, and it is already in use in many countries. Like some other countries, the U.S. made modifications to the WHO’s original code set, so we refer to ICD-10-CM (Clinical Modification), which contains diagnosis codes. The National Center for Health Statistics, a department of the CDC, is responsible for these modifications.
The WHO version of ICD-10 doesn’t have any procedure codes, so CMS developed ICD-10-PCS (Procedure Coding System) to report procedures, such as surgeries, done in U.S. hospitals. Most hospitalists won’t use these procedure codes often.
Table 1 (left) compares ICD-10-CM to ICD-9-CM. Most of the additional codes in the new version simply add information regarding whether the diagnosis is on the left or right of the body, acute or chronic, or an initial or subsequent visit for the condition. But the standard structure for each code had to be modified significantly to capture this additional information. Some highlights of the seven-character code structure are:
- Characters 1–3: category; first digit always a letter, second digit always a number, all other digits can be either; not case sensitive;
- Characters 4–6: etiology, anatomic site, severity, or other clinical detail; for example, 1=right, 2=left, 3-bilateral, and 0 or 9=unspecified; and
- Character 7: extension (i.e., A=initial encounter, D=subsequent encounter, S=sequelae).
- A placeholder “x” is used as needed to fill in empty characters to ensure that the seventh character stays in the seventh position. For example, T79.1xxA equates to “fat embolism, initial encounter.” (Note that the “dummy” characters could create problems for some IT systems.)
An example of more information contained in additional characters:
- S52=fracture of forearm.
- S52.5=fracture of lower end of radius.
- S52.52=torus fracture of lower end of radius.
- S52.521=torus fracture of lower end of right radius.
- S52.521A=torus fracture of lower end of right radius, initial encounter for closed fracture.
Compared to its predecessor, ICD-10 expands use of combination codes. These are single codes that can be used to classify either two diagnoses, a diagnosis with an associated secondary process, or a diagnosis with an associated complication. For example, rather than reporting acute cor pulmonale and septic pulmonary embolism separately, ICD-10 allows use of the code I26.01: septic pulmonary embolism with acute cor pulmonale.
Resources
In addition to resources on the SHM website, both the American Medical Association (www.ama-assn.org, search “ICD-10”) and the Centers for Medicare and Medicaid Services (www.cms.gov/icd10) have very informative microsites offering detailed ICD-10 information. Much of the information in this column, including the examples above, comes from those sites.
What to Expect
Your hospital and your employer are probably already working in earnest to prepare for the change. In some cases, hospitalists are actively involved in these preparations, but in most cases they will simply wait for an organization to notify them that they should begin training to understand the new coding system. Experts say that most physicians will need two to four hours of training on ICD-10, but because we use a universe of diagnosis codes that is much larger than many specialties, I wonder if hospitalists may need additional training.
Like nearly all the programs I listed at the beginning, the transition to ICD-10 has me concerned. Managing it poorly could mean significant loss in hospital and physician professional fee revenue, as well as lots of tedious and time-consuming work. So, doing it right is important. But, it is also important to do well on all the programs I listed at the beginning of this column, and many others, and there is a limit to just how much we can do effectively as individuals.
Collectively, these programs risk taking too much time and too many brain cells away from keeping up with clinical medicine. So, I wonder if, for many of us, ICD-10 will serve as a tipping point that results in physicians hiring professional coders to choose our diagnosis codes and CPT codes rather than doing it ourselves.
As with EHRs, ICD-10 is said to have many benefits. But the introduction of EHRs in many hospitals had the unintended effect of significantly reducing the number of doctors who were willing to serve as admitting and attending physicians; instead, many chose to refer to hospitalists. In a similar way, ICD-10 might lead many organizations to relieve physicians of the responsibility of looking up and entering codes for each patient, leaving them with more time and energy to be clinicians. We’ll have to wait and see.
Dr. Nelson has been a practicing hospitalist since 1988. He is co-founder and past president of SHM, and principal in Nelson Flores Hospital Medicine Consultants. He is co-director for SHM’s “Best Practices in Managing a Hospital Medicine Program” course. Write to him at [email protected].
By now, I’m sure you’re knowledgeable about things like healthcare exchanges and other parts of the Accountable Care Act, the increasing number of metrics within hospital value-based purchasing, the physician value-based payment modifier, the physician quality reporting system (PQRS), how to use your hospital’s new EHR efficiently, the new “two-midnight rule” to determine inpatient vs. observation status, and so on.
You’re to be commended if you’re staying on top of all these things and have effective plans in place to ensure good performance on each. And if you haven’t already, you should add at least one more important issue to this list—the transition to ICD-10 coding on Oct. 1, 2014.
An Overview
ICD stands for International Classification of Diseases, and the U.S. has been using the 9th revision (ICD-9) since 1978. ICD-9 is now significantly out of step with current medical knowledge and has run out of codes in some disease sections (“chapters”). This might mean, for example, that new codes for heart diseases would be assigned to the chapter for eye disease, because the former is full.
ICD-10 provides a way to fix these problems and, through more specific coding of diseases, should be able to yield more useful “big data” to measure things like safety and efficacy of care and more accurately identify diagnosis trends and epidemics. And, in theory, it could reduce the number of rejected billing claims, though I’m waiting to see if that happens. I worry that even after fixing all the initial bugs related to the ICD-10 transition, we will see more claim rejections than we experience today.
ICD codes can be thought of as diagnosis codes. CPT codes (Current Procedural Terminology) are an entirely separate set of codes that we use to report the work we do for the purposes of billing. We need to be familiar with both, but it is the ICD codes that are changing.
ICD-10 Basics and Trivia
The World Health Organization issued the ICD-10 in 1994, and it is already in use in many countries. Like some other countries, the U.S. made modifications to the WHO’s original code set, so we refer to ICD-10-CM (Clinical Modification), which contains diagnosis codes. The National Center for Health Statistics, a department of the CDC, is responsible for these modifications.
The WHO version of ICD-10 doesn’t have any procedure codes, so CMS developed ICD-10-PCS (Procedure Coding System) to report procedures, such as surgeries, done in U.S. hospitals. Most hospitalists won’t use these procedure codes often.
Table 1 (left) compares ICD-10-CM to ICD-9-CM. Most of the additional codes in the new version simply add information regarding whether the diagnosis is on the left or right of the body, acute or chronic, or an initial or subsequent visit for the condition. But the standard structure for each code had to be modified significantly to capture this additional information. Some highlights of the seven-character code structure are:
- Characters 1–3: category; first digit always a letter, second digit always a number, all other digits can be either; not case sensitive;
- Characters 4–6: etiology, anatomic site, severity, or other clinical detail; for example, 1=right, 2=left, 3-bilateral, and 0 or 9=unspecified; and
- Character 7: extension (i.e., A=initial encounter, D=subsequent encounter, S=sequelae).
- A placeholder “x” is used as needed to fill in empty characters to ensure that the seventh character stays in the seventh position. For example, T79.1xxA equates to “fat embolism, initial encounter.” (Note that the “dummy” characters could create problems for some IT systems.)
An example of more information contained in additional characters:
- S52=fracture of forearm.
- S52.5=fracture of lower end of radius.
- S52.52=torus fracture of lower end of radius.
- S52.521=torus fracture of lower end of right radius.
- S52.521A=torus fracture of lower end of right radius, initial encounter for closed fracture.
Compared to its predecessor, ICD-10 expands use of combination codes. These are single codes that can be used to classify either two diagnoses, a diagnosis with an associated secondary process, or a diagnosis with an associated complication. For example, rather than reporting acute cor pulmonale and septic pulmonary embolism separately, ICD-10 allows use of the code I26.01: septic pulmonary embolism with acute cor pulmonale.
Resources
In addition to resources on the SHM website, both the American Medical Association (www.ama-assn.org, search “ICD-10”) and the Centers for Medicare and Medicaid Services (www.cms.gov/icd10) have very informative microsites offering detailed ICD-10 information. Much of the information in this column, including the examples above, comes from those sites.
What to Expect
Your hospital and your employer are probably already working in earnest to prepare for the change. In some cases, hospitalists are actively involved in these preparations, but in most cases they will simply wait for an organization to notify them that they should begin training to understand the new coding system. Experts say that most physicians will need two to four hours of training on ICD-10, but because we use a universe of diagnosis codes that is much larger than many specialties, I wonder if hospitalists may need additional training.
Like nearly all the programs I listed at the beginning, the transition to ICD-10 has me concerned. Managing it poorly could mean significant loss in hospital and physician professional fee revenue, as well as lots of tedious and time-consuming work. So, doing it right is important. But, it is also important to do well on all the programs I listed at the beginning of this column, and many others, and there is a limit to just how much we can do effectively as individuals.
Collectively, these programs risk taking too much time and too many brain cells away from keeping up with clinical medicine. So, I wonder if, for many of us, ICD-10 will serve as a tipping point that results in physicians hiring professional coders to choose our diagnosis codes and CPT codes rather than doing it ourselves.
As with EHRs, ICD-10 is said to have many benefits. But the introduction of EHRs in many hospitals had the unintended effect of significantly reducing the number of doctors who were willing to serve as admitting and attending physicians; instead, many chose to refer to hospitalists. In a similar way, ICD-10 might lead many organizations to relieve physicians of the responsibility of looking up and entering codes for each patient, leaving them with more time and energy to be clinicians. We’ll have to wait and see.
Dr. Nelson has been a practicing hospitalist since 1988. He is co-founder and past president of SHM, and principal in Nelson Flores Hospital Medicine Consultants. He is co-director for SHM’s “Best Practices in Managing a Hospital Medicine Program” course. Write to him at [email protected].
Workflow Interruptions Threaten Patient Safety, Hospitalists' Job Satisfaction
When I visit hospitalist programs, one of the things I am most interested in learning about is the degree to which the hospitalists enjoy their work and why. On a recent visit, in my usual meeting with the hospitalist group, we talked a lot about what it is like to be a hospitalist. When I asked them what the greatest threat to their job satisfaction was, there was a chorus of consistency in their answers: interruptions. The hospitalists were deeply frustrated by minute-to-minute intrusions into their workflow. The emergency department, nurses, pharmacy, the admitting department, the lab, radiology—you name it, everyone wants a piece of them.
Constant interruptions are a career satisfaction issue for hospitalists. But for patients, the interruptions represent a safety and quality of care issue. Why?
The Myth of Multi-tasking
Some of us take pride in our ability to multi-task. Others freely admit they aren’t very good at it. In any case, we know through cognitive psychology that the brain cannot multi-task, at least in the realm of conscious work. (The brain, of course, carries out basic, life-sustaining functions while we are doing other work cognitively.) The brain is actually a “sequential processor,” and multi-tasking actually is “task-switching.” Those of us who “multi-task” well are able to switch tasks easily and effectively.
But, task switching comes at a cost. When we switch tasks, we are prone to errors in the performance of those tasks. Two psychologists, Rogers and Monsell, demonstrated this in a study that looked at error rates when subjects performed tasks involving numerical or letter manipulations.1 The tasks involved classifying either the digit member of a pair of characters as even/odd or the letter member as consonant/vowel. When subjects performed the tasks while switching among multiple tasks, the error rate was fourfold the rate with no task switching (see Figure 1).1 These findings have been replicated since the original study. Further, there is now well-developed literature devoted to interruptions and patient safety.
It Takes Time
We also know that switching between tasks takes time. Why? Because changing one’s attention from one subject to another involves neurologic processes that are not instantaneous. In a simulated driving study comparing mean reaction times between intoxicated subjects (blood alcohol 0.08%) and those talking on a cell phone, Strayer and Drews found the mean time to brake onset was significantly slower in the cell phone group than in the drunk driving group, presumably because cell phone users had to switch tasks.2
How Can We Tame Interruptions?
I submit that we need to be realistic about our ability to control the number of interruptions hospitalists experience in a given workday. One approach is to identify “high stakes moments” that are protected from excessive interruptions. Taking an example from aviation, airplane takeoffs and landings are “no interruption” zones, meaning that no needless talking or tasking is allowed in the cockpit during these tasks. Potential “no interruption” zones in hospital medicine might include times when hospitalists are developing an assessment and plan, engaged in complex decision-making, or performing medication reconciliation.
But is it realistic to think that we can cordon off hospitalists during these tasks?
Another approach is to establish practices that may decrease interruptions. Interruptions likely are reduced by:
- Having unit-based hospitalist staffing;
- Holding multidisciplinary rounds;
- Training nurses to batch pages;
- Conducting structured evening and night rounds on all nursing units for non-urgent matters; and
- Developing paging “levels” so that a receiving physician knows if a call back is needed and, if so, if it is urgent or not.
In talking to hospitalists who cite interruptions as job dissatisfiers, it occurs to me that anything that erodes career engagement also threatens patient safety. If we could figure out how to control interruptions, we would kill two birds with one stone.
Dr. Whitcomb is Chief Medical Officer of Remedy Partners. He is co-founder and past president of SHM. Email him at [email protected].
References
When I visit hospitalist programs, one of the things I am most interested in learning about is the degree to which the hospitalists enjoy their work and why. On a recent visit, in my usual meeting with the hospitalist group, we talked a lot about what it is like to be a hospitalist. When I asked them what the greatest threat to their job satisfaction was, there was a chorus of consistency in their answers: interruptions. The hospitalists were deeply frustrated by minute-to-minute intrusions into their workflow. The emergency department, nurses, pharmacy, the admitting department, the lab, radiology—you name it, everyone wants a piece of them.
Constant interruptions are a career satisfaction issue for hospitalists. But for patients, the interruptions represent a safety and quality of care issue. Why?
The Myth of Multi-tasking
Some of us take pride in our ability to multi-task. Others freely admit they aren’t very good at it. In any case, we know through cognitive psychology that the brain cannot multi-task, at least in the realm of conscious work. (The brain, of course, carries out basic, life-sustaining functions while we are doing other work cognitively.) The brain is actually a “sequential processor,” and multi-tasking actually is “task-switching.” Those of us who “multi-task” well are able to switch tasks easily and effectively.
But, task switching comes at a cost. When we switch tasks, we are prone to errors in the performance of those tasks. Two psychologists, Rogers and Monsell, demonstrated this in a study that looked at error rates when subjects performed tasks involving numerical or letter manipulations.1 The tasks involved classifying either the digit member of a pair of characters as even/odd or the letter member as consonant/vowel. When subjects performed the tasks while switching among multiple tasks, the error rate was fourfold the rate with no task switching (see Figure 1).1 These findings have been replicated since the original study. Further, there is now well-developed literature devoted to interruptions and patient safety.
It Takes Time
We also know that switching between tasks takes time. Why? Because changing one’s attention from one subject to another involves neurologic processes that are not instantaneous. In a simulated driving study comparing mean reaction times between intoxicated subjects (blood alcohol 0.08%) and those talking on a cell phone, Strayer and Drews found the mean time to brake onset was significantly slower in the cell phone group than in the drunk driving group, presumably because cell phone users had to switch tasks.2
How Can We Tame Interruptions?
I submit that we need to be realistic about our ability to control the number of interruptions hospitalists experience in a given workday. One approach is to identify “high stakes moments” that are protected from excessive interruptions. Taking an example from aviation, airplane takeoffs and landings are “no interruption” zones, meaning that no needless talking or tasking is allowed in the cockpit during these tasks. Potential “no interruption” zones in hospital medicine might include times when hospitalists are developing an assessment and plan, engaged in complex decision-making, or performing medication reconciliation.
But is it realistic to think that we can cordon off hospitalists during these tasks?
Another approach is to establish practices that may decrease interruptions. Interruptions likely are reduced by:
- Having unit-based hospitalist staffing;
- Holding multidisciplinary rounds;
- Training nurses to batch pages;
- Conducting structured evening and night rounds on all nursing units for non-urgent matters; and
- Developing paging “levels” so that a receiving physician knows if a call back is needed and, if so, if it is urgent or not.
In talking to hospitalists who cite interruptions as job dissatisfiers, it occurs to me that anything that erodes career engagement also threatens patient safety. If we could figure out how to control interruptions, we would kill two birds with one stone.
Dr. Whitcomb is Chief Medical Officer of Remedy Partners. He is co-founder and past president of SHM. Email him at [email protected].
References
When I visit hospitalist programs, one of the things I am most interested in learning about is the degree to which the hospitalists enjoy their work and why. On a recent visit, in my usual meeting with the hospitalist group, we talked a lot about what it is like to be a hospitalist. When I asked them what the greatest threat to their job satisfaction was, there was a chorus of consistency in their answers: interruptions. The hospitalists were deeply frustrated by minute-to-minute intrusions into their workflow. The emergency department, nurses, pharmacy, the admitting department, the lab, radiology—you name it, everyone wants a piece of them.
Constant interruptions are a career satisfaction issue for hospitalists. But for patients, the interruptions represent a safety and quality of care issue. Why?
The Myth of Multi-tasking
Some of us take pride in our ability to multi-task. Others freely admit they aren’t very good at it. In any case, we know through cognitive psychology that the brain cannot multi-task, at least in the realm of conscious work. (The brain, of course, carries out basic, life-sustaining functions while we are doing other work cognitively.) The brain is actually a “sequential processor,” and multi-tasking actually is “task-switching.” Those of us who “multi-task” well are able to switch tasks easily and effectively.
But, task switching comes at a cost. When we switch tasks, we are prone to errors in the performance of those tasks. Two psychologists, Rogers and Monsell, demonstrated this in a study that looked at error rates when subjects performed tasks involving numerical or letter manipulations.1 The tasks involved classifying either the digit member of a pair of characters as even/odd or the letter member as consonant/vowel. When subjects performed the tasks while switching among multiple tasks, the error rate was fourfold the rate with no task switching (see Figure 1).1 These findings have been replicated since the original study. Further, there is now well-developed literature devoted to interruptions and patient safety.
It Takes Time
We also know that switching between tasks takes time. Why? Because changing one’s attention from one subject to another involves neurologic processes that are not instantaneous. In a simulated driving study comparing mean reaction times between intoxicated subjects (blood alcohol 0.08%) and those talking on a cell phone, Strayer and Drews found the mean time to brake onset was significantly slower in the cell phone group than in the drunk driving group, presumably because cell phone users had to switch tasks.2
How Can We Tame Interruptions?
I submit that we need to be realistic about our ability to control the number of interruptions hospitalists experience in a given workday. One approach is to identify “high stakes moments” that are protected from excessive interruptions. Taking an example from aviation, airplane takeoffs and landings are “no interruption” zones, meaning that no needless talking or tasking is allowed in the cockpit during these tasks. Potential “no interruption” zones in hospital medicine might include times when hospitalists are developing an assessment and plan, engaged in complex decision-making, or performing medication reconciliation.
But is it realistic to think that we can cordon off hospitalists during these tasks?
Another approach is to establish practices that may decrease interruptions. Interruptions likely are reduced by:
- Having unit-based hospitalist staffing;
- Holding multidisciplinary rounds;
- Training nurses to batch pages;
- Conducting structured evening and night rounds on all nursing units for non-urgent matters; and
- Developing paging “levels” so that a receiving physician knows if a call back is needed and, if so, if it is urgent or not.
In talking to hospitalists who cite interruptions as job dissatisfiers, it occurs to me that anything that erodes career engagement also threatens patient safety. If we could figure out how to control interruptions, we would kill two birds with one stone.
Dr. Whitcomb is Chief Medical Officer of Remedy Partners. He is co-founder and past president of SHM. Email him at [email protected].
References
Is the risk of placenta accreta in a subsequent pregnancy higher after emergent primary cesarean or after elective primary cesarean?
Invasive disorders of the placenta (placenta accreta, increta, and percreta) are increasingly common. These conditions are associated with a high risk of massive obstetric hemorrhage, are the leading cause of peripartum hysterectomy, and are an important cause of pregnancy-related death in the United States and Western world.1 It is clear that strategies must be developed to reduce the incidence of these disorders, and that most of these strategies must focus on prevention.
Earlier studies have consistently found that cesarean delivery is the most important risk factor for placenta accreta in a subsequent pregnancy, with the risk rising with the number of prior cesarean deliveries.1 In recent years, the cesarean delivery rate has skyrocketed in most Western nations and is the major contributor to the increased incidence of placenta accreta.2 A major effort is in place to prevent the first cesarean delivery.2
In this study, Kamara and colleagues focused on the timing of cesarean delivery to determine the impact on the likelihood of placenta accreta in subsequent pregnancies.
Details of the study
Kamara and colleagues found that elective cesarean delivery carries a threefold increased risk of placenta accreta in a subsequent pregnancy, compared with cesarean delivery during labor. To my knowledge, until now, no one has attempted to determine whether the timing of cesarean delivery affects the risk of subsequent placenta accreta.
The investigators hypothesize that the increased risk arises when a thick, nonlaboring myometrium is incised, as opposed to the thinned-out myometrium that occurs in labor. This theory is in keeping with the theory that placenta accreta develops when a gestation implants into a cesarean scar.
Limitations of the study
The cases analyzed in this investigation came from a 15-year period, a time when practice patterns changed considerably.
The timing of cesareans performed during labor was not examined.
Primary cesarean was defined as the woman’s first cesarean delivery, regardless of whether she had vaginal deliveries before or after the cesarean.
Retrospective case-control study methodology cannot address causality and may not be ideally suited to provide definitive findings. However, the findings are novel and deserve further investigation.
Related Article: Evolving applications of first-trimester ultrasound Ilan E. Timor-Tritsch, MD, and Simi K. Gupta, MD (December 2012)
What this evidence means for practice
The rate of cesarean delivery continues to rise. It is increasingly performed on an elective basis for such reasons as maternal request, suspected macrosomia, and breech presentation (often without giving the patient the option of version). Although additional investigations are necessary to validate the findings of this study, patients should be counseled that elective primary cesarean is not without risk, and that placenta accreta in a subsequent pregnancy is a potential consequence.
This study provides one more reason to attempt to find methods to reduce the cesarean delivery rate, particularly the rate of elective cesarean.
--Yinka Oyelese, MD
- Oyelese Y, Smulian J. Placenta previa, placenta accreta, and vasa previa. Obstet Gynecol. 2006;107(4):927–941.
- Spong CY, Berghella V, Wenstrom KD, Mercer BM, Saade GR. Preventing the first cesarean delivery: Summary of a joint Eunice Kennedy Shriver National Institute of Child Health and Human Development, Society for Maternal-Fetal Medicine, and American College of Obstetricians and Gynecologists Workshop. Obstet Gynecol. 2012;120(5):1181–1193.
Invasive disorders of the placenta (placenta accreta, increta, and percreta) are increasingly common. These conditions are associated with a high risk of massive obstetric hemorrhage, are the leading cause of peripartum hysterectomy, and are an important cause of pregnancy-related death in the United States and Western world.1 It is clear that strategies must be developed to reduce the incidence of these disorders, and that most of these strategies must focus on prevention.
Earlier studies have consistently found that cesarean delivery is the most important risk factor for placenta accreta in a subsequent pregnancy, with the risk rising with the number of prior cesarean deliveries.1 In recent years, the cesarean delivery rate has skyrocketed in most Western nations and is the major contributor to the increased incidence of placenta accreta.2 A major effort is in place to prevent the first cesarean delivery.2
In this study, Kamara and colleagues focused on the timing of cesarean delivery to determine the impact on the likelihood of placenta accreta in subsequent pregnancies.
Details of the study
Kamara and colleagues found that elective cesarean delivery carries a threefold increased risk of placenta accreta in a subsequent pregnancy, compared with cesarean delivery during labor. To my knowledge, until now, no one has attempted to determine whether the timing of cesarean delivery affects the risk of subsequent placenta accreta.
The investigators hypothesize that the increased risk arises when a thick, nonlaboring myometrium is incised, as opposed to the thinned-out myometrium that occurs in labor. This theory is in keeping with the theory that placenta accreta develops when a gestation implants into a cesarean scar.
Limitations of the study
The cases analyzed in this investigation came from a 15-year period, a time when practice patterns changed considerably.
The timing of cesareans performed during labor was not examined.
Primary cesarean was defined as the woman’s first cesarean delivery, regardless of whether she had vaginal deliveries before or after the cesarean.
Retrospective case-control study methodology cannot address causality and may not be ideally suited to provide definitive findings. However, the findings are novel and deserve further investigation.
Related Article: Evolving applications of first-trimester ultrasound Ilan E. Timor-Tritsch, MD, and Simi K. Gupta, MD (December 2012)
What this evidence means for practice
The rate of cesarean delivery continues to rise. It is increasingly performed on an elective basis for such reasons as maternal request, suspected macrosomia, and breech presentation (often without giving the patient the option of version). Although additional investigations are necessary to validate the findings of this study, patients should be counseled that elective primary cesarean is not without risk, and that placenta accreta in a subsequent pregnancy is a potential consequence.
This study provides one more reason to attempt to find methods to reduce the cesarean delivery rate, particularly the rate of elective cesarean.
--Yinka Oyelese, MD
Invasive disorders of the placenta (placenta accreta, increta, and percreta) are increasingly common. These conditions are associated with a high risk of massive obstetric hemorrhage, are the leading cause of peripartum hysterectomy, and are an important cause of pregnancy-related death in the United States and Western world.1 It is clear that strategies must be developed to reduce the incidence of these disorders, and that most of these strategies must focus on prevention.
Earlier studies have consistently found that cesarean delivery is the most important risk factor for placenta accreta in a subsequent pregnancy, with the risk rising with the number of prior cesarean deliveries.1 In recent years, the cesarean delivery rate has skyrocketed in most Western nations and is the major contributor to the increased incidence of placenta accreta.2 A major effort is in place to prevent the first cesarean delivery.2
In this study, Kamara and colleagues focused on the timing of cesarean delivery to determine the impact on the likelihood of placenta accreta in subsequent pregnancies.
Details of the study
Kamara and colleagues found that elective cesarean delivery carries a threefold increased risk of placenta accreta in a subsequent pregnancy, compared with cesarean delivery during labor. To my knowledge, until now, no one has attempted to determine whether the timing of cesarean delivery affects the risk of subsequent placenta accreta.
The investigators hypothesize that the increased risk arises when a thick, nonlaboring myometrium is incised, as opposed to the thinned-out myometrium that occurs in labor. This theory is in keeping with the theory that placenta accreta develops when a gestation implants into a cesarean scar.
Limitations of the study
The cases analyzed in this investigation came from a 15-year period, a time when practice patterns changed considerably.
The timing of cesareans performed during labor was not examined.
Primary cesarean was defined as the woman’s first cesarean delivery, regardless of whether she had vaginal deliveries before or after the cesarean.
Retrospective case-control study methodology cannot address causality and may not be ideally suited to provide definitive findings. However, the findings are novel and deserve further investigation.
Related Article: Evolving applications of first-trimester ultrasound Ilan E. Timor-Tritsch, MD, and Simi K. Gupta, MD (December 2012)
What this evidence means for practice
The rate of cesarean delivery continues to rise. It is increasingly performed on an elective basis for such reasons as maternal request, suspected macrosomia, and breech presentation (often without giving the patient the option of version). Although additional investigations are necessary to validate the findings of this study, patients should be counseled that elective primary cesarean is not without risk, and that placenta accreta in a subsequent pregnancy is a potential consequence.
This study provides one more reason to attempt to find methods to reduce the cesarean delivery rate, particularly the rate of elective cesarean.
--Yinka Oyelese, MD
- Oyelese Y, Smulian J. Placenta previa, placenta accreta, and vasa previa. Obstet Gynecol. 2006;107(4):927–941.
- Spong CY, Berghella V, Wenstrom KD, Mercer BM, Saade GR. Preventing the first cesarean delivery: Summary of a joint Eunice Kennedy Shriver National Institute of Child Health and Human Development, Society for Maternal-Fetal Medicine, and American College of Obstetricians and Gynecologists Workshop. Obstet Gynecol. 2012;120(5):1181–1193.
- Oyelese Y, Smulian J. Placenta previa, placenta accreta, and vasa previa. Obstet Gynecol. 2006;107(4):927–941.
- Spong CY, Berghella V, Wenstrom KD, Mercer BM, Saade GR. Preventing the first cesarean delivery: Summary of a joint Eunice Kennedy Shriver National Institute of Child Health and Human Development, Society for Maternal-Fetal Medicine, and American College of Obstetricians and Gynecologists Workshop. Obstet Gynecol. 2012;120(5):1181–1193.
STOP enforcing a 5-year rule for menopausal hormone therapy
Immediately after the worrisome initial findings of the Women’s Health Initiative (WHI) were published in July 2002,1 leading organizations and experts in menopausal medicine began advising practitioners to prescribe the “lowest dose of hormones for the shortest period of time.” News headlines that cited menopausal hormone therapy (HT) as a risk factor for myocardial infarction, venous thromboembolism (VTE), gall bladder disease, stroke, urinary incontinence, dementia, and cancers of the breast and lung fueled fear among the lay public and led to a burgeoning market for alternative therapies to address menopausal symptoms.2 Companies that marketed alternative therapies, including bioidentical hormones, often exaggerated the reported risks of menopausal HT and implied that their products were safe and effective, although supporting evidence was lacking.3
More than a decade later, despite a growing body of data reinforcing the safety and efficacy of HT for recently menopausal women,4 many medical professionals remain reluctant to prescribe HT—and when they do prescribe it, they push for a 5-year limit.4,5 This has led to needless suffering and reduced quality of life among thousands of women entering the menopausal transition.6,7
THE IMPORTANCE OF TARGETING HT TO THE APPROPRIATE POPULATION
Over the past decade, experts have conducted in-depth analyses of WHI findings and other contemporary data on the benefits and risks of HT. One fact is clear: The original reports and the way the data were portrayed in the media overstated the risks of HT in newly menopausal women.2,8 Reanalysis has shown that when HT is initiated within 10 years of menopause, the risks are few and generally are outweighed by benefits.9–11 When HT is initiated by women in their 60s and 70s, however, the reverse may be true.
HT is the best therapy for menopausal vasomotor symptoms and has a secondary benefit of preventing osteoporosis.12 HT also may offer cardiovascular benefits in younger menopausal women, although no appropriately powered randomized, clinical trial has yet confirmed this presumption.9,13
Related Article: In young hysterectomized women, does unopposed estrogen therapy increase overall survival? Andrew M. Kaunitz, MD (Examining the Evidence, October 2013)
HT AND BREAST CANCER: CONTEXT IS CRITICAL
The original WHI publication and the news reports that followed emphasized that women using combination estrogen-progestin HT experienced a 24% increase in the incidence of breast cancer, which became apparent in the fifth year of therapy.1 A closer look at the data reveals that the increased incidence of breast cancer reported in this arm of the WHI involved just 38 breast cancers per 10,000 women using HT per year, compared with 30 breast cancers per 10,000 women using placebo. The absolute risk increased by eight breast cancers per 10,000 women, or 0.08%, for each year of use. In the WHI, the 75% of women who were new users of HT actually had no increased risk of breast cancer (hazard ratio [HR], 1.06; 95% confidence interval [CI], 0.81–1.38).
Related Article: Osteoporosis treatment and breast cancer prevention: Two goals, one treatment? Robert L. Barbieri, MD (Editorial, November 2013)
It is important to put this degree of increased risk into perspective. An increase of 0.08% per year is less than one-tenth of a percentage point and is comparable to the risk of breast cancer that a woman accepts if she drinks alcohol regularly, allows herself to become overweight during perimenopause, or fails to exercise at least three times a week.14 Cumulative data from a number of observational studies suggest that the effect of estrogen alone (without a progestin) on breast cancer is even lower, and that estrogen can be taken for many years before any effect is seen. Indeed, among women receiving estrogen alone in the WHI, the risk of breast cancer did not increase. In fact, there was a statistically significant decrease in breast cancer in this population.
Related Article: USPSTF recommends tamoxifen or raloxifene to reduce breast cancer risk in high-risk patients (October 2013)
WHY A 5-YEAR LIMIT IS INAPPROPRIATE
As I explained above, the increase in the incidence of breast cancer observed in the estrogen-progestin arm of the WHI after 5 years represents an increase in the absolute risk of breast cancer of only 0.08% per year. Although HT carries other small potential risks, most experts agree that they are outweighed by the potential benefits among most perimenopausal women. Because an individual’s risks and benefits probably vary according to her personal and family history, clinicians can mitigate the risks, in part, by tailoring the dose, regimen, and route of delivery to the individual’s situation. The risk of VTE is greatest during the first year of HT and approaches background rates thereafter. The risk of stroke in newly menopausal women who initiated HT in the WHI was approximately 1/1,000.13
Health-care practitioners also can minimize the risks of HT by monitoring outcomes, such as blood pressure, unscheduled bleeding, and so on.15 It also may be helpful to counsel patients about interventions for other conditions that contribute to risk, including obesity, smoking, inactivity, hypertension, and hyperlipidemia.
Quality of life was largely ignored in the decade after publication of the initial WHI findings because it was thought that the lives saved by avoiding HT would justify some level of distress.6,7 There also was a presumption—promoted by advocates of natural products and alternative therapies—that interventions such as acupuncture, paced respiration, and herbal remedies were safe and effective at alleviating hot flashes, night sweats, mood swings, and sleep disruption. Complaints of vaginal dryness and dyspareunia from urogenital atrophy often were inadequately addressed because local estrogen was incorrectly thought to increase the risk of hormone-induced breast cancer. Rates of osteoporosis and hip fracture also have risen over the past decade as the protective effect of systemic HT for many women was lost.16
Although most postmenopausal women (60%) experience hot flashes for less than 7 years, as many as 15% report that hot flashes persist for 15 years or longer. The symptoms that can accompany hot flashes (including sweating, palpitations, apprehension, and anxiety) contribute to a woman’s discomfort, inconvenience, and distress, particularly when the hot flashes are frequent, and can be a significant contributor to sleep disturbance. Vasomotor symptoms adversely affect quality of life for 20% to 25% of women, primarily due to the physical discomfort and social embarrassment that they evoke—although night sweats and sleep disturbance also are reported to exert a negative impact.17–19
THE BOTTOM LINE
Nothing magical happens after 5 years of HT to increase a woman’s risk of breast cancer. Any cumulative effect of combination HT on the risk of breast cancer is gradual and small. It is not appropriate to demand that a patient stop HT after 5 years if it affords dramatic improvement in her quality of life, provided she has been correctly informed about potential risks and chooses to continue with therapy.
- Writing Group for the Women`s Health Initiative Investigators. Risks and benefits of estrogen and progestin in healthy postmenopausal women: Principal results of the Women`s Health Initiative randomized controlled trial. JAMA. 2002;288(3):321–333.
- Brown S. Shock, terror and controversy: how the media reacted to the Women’s Health Initiative. Climacteric. 2012;15(3):275–280.
- Bioidentical hormones. Med Lett Drugs Ther. 2010;52(1339):43–44.
- North American Menopause Society. The 2012 Hormone Therapy Position Statement of The North American Menopause Society. Menopause. 2012;19(3):257–271.
- Rossouw JE, Manson JE, Kaunitz AM, Anderson GL. Lessons learned from the Women’s Health Initiative trials of menopausal hormone therapy. Obstet Gynecol. 2013;121(1):172–176.
- Pines A, Sturdee DW, MacLennan AH. Quality of life and the role of menopausal hormone therapy. Climacteric. 2012;15(3):213–216.
- Burger HG, MacLennan AH, Huang K-E, Castelo-Branco C. Evidence-based assessment of the impact of the WHI on women’s health. Climacteric. 2012;15(3):281–287.
- Utian WH. NIH and WHI: Time for a mea culpa and steps beyond. Menopause. 2007;14(6):1056–1059.
- LaCroix AZ, Chlebowski RT, Manson JE, et al. Health outcomes after stopping conjugated equine estrogens among postmenopausal women with prior hysterectomy: A randomized controlled trial. JAMA. 2011;305(13):1305–1314.
- Stuenkel CA, Gass MLS, Manson JE, et al. A decade after the Women`s Health Initiative—The experts do agree. Menopause. 2012;19(8):846-847.
- Langer RD, Manson JE, Allison MA. Have we come full circle—or moved forward? The Women’s Health Initiative 10 years on. Climacteric. 2012;15(3):206–212.
- Gallagher JC, Levine JP. Preventing osteoporosis in symptomatic postmenopausal women. Menopause. 2011;18(1):109–118.
- Hodis HN, Mack WJ. Postmenopausal hormone therapy in clinical perspective. Menopause. 2007;14(5):944–957.
- Singletary SE. Rating the risk factors for breast cancer. Ann Surg. 2003;237(4):474–482.
- Archer DF, Oger E. Estrogen and progestogen effect on venous thromboembolism in menopausal women. Climacteric. 2012;15(3):235–240.
- Islam S, Liu Q, Chines A, Helzner E. Trend in incidence of osteoporosis-related fractures among 40- to 69-year-old women: Analysis of a large insurance claims database, 2000-2005. Menopause. 2009;16(1):77–83.
- Whiteman MK, Staropoli CA, Langenberg PW, McCarter RJ, Kjerulff KH, Flaws JA. Smoking, body mass and hot flashes in midlife women. Obstet Gynecol. 2003;101(2):264–272.
- Utian WH. Psychosocial and socioeconomic burden of vasomotor symptoms in menopause: A comprehensive review. Health Qual Life Outcomes. 2005;3:47.
- Hunter M, Rendall M. Bio-psycho-socio-cultural perspectives on menopause. Best Pract Res Clin Obstet Gynaecol. 2007;21(2):261–274.
Immediately after the worrisome initial findings of the Women’s Health Initiative (WHI) were published in July 2002,1 leading organizations and experts in menopausal medicine began advising practitioners to prescribe the “lowest dose of hormones for the shortest period of time.” News headlines that cited menopausal hormone therapy (HT) as a risk factor for myocardial infarction, venous thromboembolism (VTE), gall bladder disease, stroke, urinary incontinence, dementia, and cancers of the breast and lung fueled fear among the lay public and led to a burgeoning market for alternative therapies to address menopausal symptoms.2 Companies that marketed alternative therapies, including bioidentical hormones, often exaggerated the reported risks of menopausal HT and implied that their products were safe and effective, although supporting evidence was lacking.3
More than a decade later, despite a growing body of data reinforcing the safety and efficacy of HT for recently menopausal women,4 many medical professionals remain reluctant to prescribe HT—and when they do prescribe it, they push for a 5-year limit.4,5 This has led to needless suffering and reduced quality of life among thousands of women entering the menopausal transition.6,7
THE IMPORTANCE OF TARGETING HT TO THE APPROPRIATE POPULATION
Over the past decade, experts have conducted in-depth analyses of WHI findings and other contemporary data on the benefits and risks of HT. One fact is clear: The original reports and the way the data were portrayed in the media overstated the risks of HT in newly menopausal women.2,8 Reanalysis has shown that when HT is initiated within 10 years of menopause, the risks are few and generally are outweighed by benefits.9–11 When HT is initiated by women in their 60s and 70s, however, the reverse may be true.
HT is the best therapy for menopausal vasomotor symptoms and has a secondary benefit of preventing osteoporosis.12 HT also may offer cardiovascular benefits in younger menopausal women, although no appropriately powered randomized, clinical trial has yet confirmed this presumption.9,13
Related Article: In young hysterectomized women, does unopposed estrogen therapy increase overall survival? Andrew M. Kaunitz, MD (Examining the Evidence, October 2013)
HT AND BREAST CANCER: CONTEXT IS CRITICAL
The original WHI publication and the news reports that followed emphasized that women using combination estrogen-progestin HT experienced a 24% increase in the incidence of breast cancer, which became apparent in the fifth year of therapy.1 A closer look at the data reveals that the increased incidence of breast cancer reported in this arm of the WHI involved just 38 breast cancers per 10,000 women using HT per year, compared with 30 breast cancers per 10,000 women using placebo. The absolute risk increased by eight breast cancers per 10,000 women, or 0.08%, for each year of use. In the WHI, the 75% of women who were new users of HT actually had no increased risk of breast cancer (hazard ratio [HR], 1.06; 95% confidence interval [CI], 0.81–1.38).
Related Article: Osteoporosis treatment and breast cancer prevention: Two goals, one treatment? Robert L. Barbieri, MD (Editorial, November 2013)
It is important to put this degree of increased risk into perspective. An increase of 0.08% per year is less than one-tenth of a percentage point and is comparable to the risk of breast cancer that a woman accepts if she drinks alcohol regularly, allows herself to become overweight during perimenopause, or fails to exercise at least three times a week.14 Cumulative data from a number of observational studies suggest that the effect of estrogen alone (without a progestin) on breast cancer is even lower, and that estrogen can be taken for many years before any effect is seen. Indeed, among women receiving estrogen alone in the WHI, the risk of breast cancer did not increase. In fact, there was a statistically significant decrease in breast cancer in this population.
Related Article: USPSTF recommends tamoxifen or raloxifene to reduce breast cancer risk in high-risk patients (October 2013)
WHY A 5-YEAR LIMIT IS INAPPROPRIATE
As I explained above, the increase in the incidence of breast cancer observed in the estrogen-progestin arm of the WHI after 5 years represents an increase in the absolute risk of breast cancer of only 0.08% per year. Although HT carries other small potential risks, most experts agree that they are outweighed by the potential benefits among most perimenopausal women. Because an individual’s risks and benefits probably vary according to her personal and family history, clinicians can mitigate the risks, in part, by tailoring the dose, regimen, and route of delivery to the individual’s situation. The risk of VTE is greatest during the first year of HT and approaches background rates thereafter. The risk of stroke in newly menopausal women who initiated HT in the WHI was approximately 1/1,000.13
Health-care practitioners also can minimize the risks of HT by monitoring outcomes, such as blood pressure, unscheduled bleeding, and so on.15 It also may be helpful to counsel patients about interventions for other conditions that contribute to risk, including obesity, smoking, inactivity, hypertension, and hyperlipidemia.
Quality of life was largely ignored in the decade after publication of the initial WHI findings because it was thought that the lives saved by avoiding HT would justify some level of distress.6,7 There also was a presumption—promoted by advocates of natural products and alternative therapies—that interventions such as acupuncture, paced respiration, and herbal remedies were safe and effective at alleviating hot flashes, night sweats, mood swings, and sleep disruption. Complaints of vaginal dryness and dyspareunia from urogenital atrophy often were inadequately addressed because local estrogen was incorrectly thought to increase the risk of hormone-induced breast cancer. Rates of osteoporosis and hip fracture also have risen over the past decade as the protective effect of systemic HT for many women was lost.16
Although most postmenopausal women (60%) experience hot flashes for less than 7 years, as many as 15% report that hot flashes persist for 15 years or longer. The symptoms that can accompany hot flashes (including sweating, palpitations, apprehension, and anxiety) contribute to a woman’s discomfort, inconvenience, and distress, particularly when the hot flashes are frequent, and can be a significant contributor to sleep disturbance. Vasomotor symptoms adversely affect quality of life for 20% to 25% of women, primarily due to the physical discomfort and social embarrassment that they evoke—although night sweats and sleep disturbance also are reported to exert a negative impact.17–19
THE BOTTOM LINE
Nothing magical happens after 5 years of HT to increase a woman’s risk of breast cancer. Any cumulative effect of combination HT on the risk of breast cancer is gradual and small. It is not appropriate to demand that a patient stop HT after 5 years if it affords dramatic improvement in her quality of life, provided she has been correctly informed about potential risks and chooses to continue with therapy.
Immediately after the worrisome initial findings of the Women’s Health Initiative (WHI) were published in July 2002,1 leading organizations and experts in menopausal medicine began advising practitioners to prescribe the “lowest dose of hormones for the shortest period of time.” News headlines that cited menopausal hormone therapy (HT) as a risk factor for myocardial infarction, venous thromboembolism (VTE), gall bladder disease, stroke, urinary incontinence, dementia, and cancers of the breast and lung fueled fear among the lay public and led to a burgeoning market for alternative therapies to address menopausal symptoms.2 Companies that marketed alternative therapies, including bioidentical hormones, often exaggerated the reported risks of menopausal HT and implied that their products were safe and effective, although supporting evidence was lacking.3
More than a decade later, despite a growing body of data reinforcing the safety and efficacy of HT for recently menopausal women,4 many medical professionals remain reluctant to prescribe HT—and when they do prescribe it, they push for a 5-year limit.4,5 This has led to needless suffering and reduced quality of life among thousands of women entering the menopausal transition.6,7
THE IMPORTANCE OF TARGETING HT TO THE APPROPRIATE POPULATION
Over the past decade, experts have conducted in-depth analyses of WHI findings and other contemporary data on the benefits and risks of HT. One fact is clear: The original reports and the way the data were portrayed in the media overstated the risks of HT in newly menopausal women.2,8 Reanalysis has shown that when HT is initiated within 10 years of menopause, the risks are few and generally are outweighed by benefits.9–11 When HT is initiated by women in their 60s and 70s, however, the reverse may be true.
HT is the best therapy for menopausal vasomotor symptoms and has a secondary benefit of preventing osteoporosis.12 HT also may offer cardiovascular benefits in younger menopausal women, although no appropriately powered randomized, clinical trial has yet confirmed this presumption.9,13
Related Article: In young hysterectomized women, does unopposed estrogen therapy increase overall survival? Andrew M. Kaunitz, MD (Examining the Evidence, October 2013)
HT AND BREAST CANCER: CONTEXT IS CRITICAL
The original WHI publication and the news reports that followed emphasized that women using combination estrogen-progestin HT experienced a 24% increase in the incidence of breast cancer, which became apparent in the fifth year of therapy.1 A closer look at the data reveals that the increased incidence of breast cancer reported in this arm of the WHI involved just 38 breast cancers per 10,000 women using HT per year, compared with 30 breast cancers per 10,000 women using placebo. The absolute risk increased by eight breast cancers per 10,000 women, or 0.08%, for each year of use. In the WHI, the 75% of women who were new users of HT actually had no increased risk of breast cancer (hazard ratio [HR], 1.06; 95% confidence interval [CI], 0.81–1.38).
Related Article: Osteoporosis treatment and breast cancer prevention: Two goals, one treatment? Robert L. Barbieri, MD (Editorial, November 2013)
It is important to put this degree of increased risk into perspective. An increase of 0.08% per year is less than one-tenth of a percentage point and is comparable to the risk of breast cancer that a woman accepts if she drinks alcohol regularly, allows herself to become overweight during perimenopause, or fails to exercise at least three times a week.14 Cumulative data from a number of observational studies suggest that the effect of estrogen alone (without a progestin) on breast cancer is even lower, and that estrogen can be taken for many years before any effect is seen. Indeed, among women receiving estrogen alone in the WHI, the risk of breast cancer did not increase. In fact, there was a statistically significant decrease in breast cancer in this population.
Related Article: USPSTF recommends tamoxifen or raloxifene to reduce breast cancer risk in high-risk patients (October 2013)
WHY A 5-YEAR LIMIT IS INAPPROPRIATE
As I explained above, the increase in the incidence of breast cancer observed in the estrogen-progestin arm of the WHI after 5 years represents an increase in the absolute risk of breast cancer of only 0.08% per year. Although HT carries other small potential risks, most experts agree that they are outweighed by the potential benefits among most perimenopausal women. Because an individual’s risks and benefits probably vary according to her personal and family history, clinicians can mitigate the risks, in part, by tailoring the dose, regimen, and route of delivery to the individual’s situation. The risk of VTE is greatest during the first year of HT and approaches background rates thereafter. The risk of stroke in newly menopausal women who initiated HT in the WHI was approximately 1/1,000.13
Health-care practitioners also can minimize the risks of HT by monitoring outcomes, such as blood pressure, unscheduled bleeding, and so on.15 It also may be helpful to counsel patients about interventions for other conditions that contribute to risk, including obesity, smoking, inactivity, hypertension, and hyperlipidemia.
Quality of life was largely ignored in the decade after publication of the initial WHI findings because it was thought that the lives saved by avoiding HT would justify some level of distress.6,7 There also was a presumption—promoted by advocates of natural products and alternative therapies—that interventions such as acupuncture, paced respiration, and herbal remedies were safe and effective at alleviating hot flashes, night sweats, mood swings, and sleep disruption. Complaints of vaginal dryness and dyspareunia from urogenital atrophy often were inadequately addressed because local estrogen was incorrectly thought to increase the risk of hormone-induced breast cancer. Rates of osteoporosis and hip fracture also have risen over the past decade as the protective effect of systemic HT for many women was lost.16
Although most postmenopausal women (60%) experience hot flashes for less than 7 years, as many as 15% report that hot flashes persist for 15 years or longer. The symptoms that can accompany hot flashes (including sweating, palpitations, apprehension, and anxiety) contribute to a woman’s discomfort, inconvenience, and distress, particularly when the hot flashes are frequent, and can be a significant contributor to sleep disturbance. Vasomotor symptoms adversely affect quality of life for 20% to 25% of women, primarily due to the physical discomfort and social embarrassment that they evoke—although night sweats and sleep disturbance also are reported to exert a negative impact.17–19
THE BOTTOM LINE
Nothing magical happens after 5 years of HT to increase a woman’s risk of breast cancer. Any cumulative effect of combination HT on the risk of breast cancer is gradual and small. It is not appropriate to demand that a patient stop HT after 5 years if it affords dramatic improvement in her quality of life, provided she has been correctly informed about potential risks and chooses to continue with therapy.
- Writing Group for the Women`s Health Initiative Investigators. Risks and benefits of estrogen and progestin in healthy postmenopausal women: Principal results of the Women`s Health Initiative randomized controlled trial. JAMA. 2002;288(3):321–333.
- Brown S. Shock, terror and controversy: how the media reacted to the Women’s Health Initiative. Climacteric. 2012;15(3):275–280.
- Bioidentical hormones. Med Lett Drugs Ther. 2010;52(1339):43–44.
- North American Menopause Society. The 2012 Hormone Therapy Position Statement of The North American Menopause Society. Menopause. 2012;19(3):257–271.
- Rossouw JE, Manson JE, Kaunitz AM, Anderson GL. Lessons learned from the Women’s Health Initiative trials of menopausal hormone therapy. Obstet Gynecol. 2013;121(1):172–176.
- Pines A, Sturdee DW, MacLennan AH. Quality of life and the role of menopausal hormone therapy. Climacteric. 2012;15(3):213–216.
- Burger HG, MacLennan AH, Huang K-E, Castelo-Branco C. Evidence-based assessment of the impact of the WHI on women’s health. Climacteric. 2012;15(3):281–287.
- Utian WH. NIH and WHI: Time for a mea culpa and steps beyond. Menopause. 2007;14(6):1056–1059.
- LaCroix AZ, Chlebowski RT, Manson JE, et al. Health outcomes after stopping conjugated equine estrogens among postmenopausal women with prior hysterectomy: A randomized controlled trial. JAMA. 2011;305(13):1305–1314.
- Stuenkel CA, Gass MLS, Manson JE, et al. A decade after the Women`s Health Initiative—The experts do agree. Menopause. 2012;19(8):846-847.
- Langer RD, Manson JE, Allison MA. Have we come full circle—or moved forward? The Women’s Health Initiative 10 years on. Climacteric. 2012;15(3):206–212.
- Gallagher JC, Levine JP. Preventing osteoporosis in symptomatic postmenopausal women. Menopause. 2011;18(1):109–118.
- Hodis HN, Mack WJ. Postmenopausal hormone therapy in clinical perspective. Menopause. 2007;14(5):944–957.
- Singletary SE. Rating the risk factors for breast cancer. Ann Surg. 2003;237(4):474–482.
- Archer DF, Oger E. Estrogen and progestogen effect on venous thromboembolism in menopausal women. Climacteric. 2012;15(3):235–240.
- Islam S, Liu Q, Chines A, Helzner E. Trend in incidence of osteoporosis-related fractures among 40- to 69-year-old women: Analysis of a large insurance claims database, 2000-2005. Menopause. 2009;16(1):77–83.
- Whiteman MK, Staropoli CA, Langenberg PW, McCarter RJ, Kjerulff KH, Flaws JA. Smoking, body mass and hot flashes in midlife women. Obstet Gynecol. 2003;101(2):264–272.
- Utian WH. Psychosocial and socioeconomic burden of vasomotor symptoms in menopause: A comprehensive review. Health Qual Life Outcomes. 2005;3:47.
- Hunter M, Rendall M. Bio-psycho-socio-cultural perspectives on menopause. Best Pract Res Clin Obstet Gynaecol. 2007;21(2):261–274.
- Writing Group for the Women`s Health Initiative Investigators. Risks and benefits of estrogen and progestin in healthy postmenopausal women: Principal results of the Women`s Health Initiative randomized controlled trial. JAMA. 2002;288(3):321–333.
- Brown S. Shock, terror and controversy: how the media reacted to the Women’s Health Initiative. Climacteric. 2012;15(3):275–280.
- Bioidentical hormones. Med Lett Drugs Ther. 2010;52(1339):43–44.
- North American Menopause Society. The 2012 Hormone Therapy Position Statement of The North American Menopause Society. Menopause. 2012;19(3):257–271.
- Rossouw JE, Manson JE, Kaunitz AM, Anderson GL. Lessons learned from the Women’s Health Initiative trials of menopausal hormone therapy. Obstet Gynecol. 2013;121(1):172–176.
- Pines A, Sturdee DW, MacLennan AH. Quality of life and the role of menopausal hormone therapy. Climacteric. 2012;15(3):213–216.
- Burger HG, MacLennan AH, Huang K-E, Castelo-Branco C. Evidence-based assessment of the impact of the WHI on women’s health. Climacteric. 2012;15(3):281–287.
- Utian WH. NIH and WHI: Time for a mea culpa and steps beyond. Menopause. 2007;14(6):1056–1059.
- LaCroix AZ, Chlebowski RT, Manson JE, et al. Health outcomes after stopping conjugated equine estrogens among postmenopausal women with prior hysterectomy: A randomized controlled trial. JAMA. 2011;305(13):1305–1314.
- Stuenkel CA, Gass MLS, Manson JE, et al. A decade after the Women`s Health Initiative—The experts do agree. Menopause. 2012;19(8):846-847.
- Langer RD, Manson JE, Allison MA. Have we come full circle—or moved forward? The Women’s Health Initiative 10 years on. Climacteric. 2012;15(3):206–212.
- Gallagher JC, Levine JP. Preventing osteoporosis in symptomatic postmenopausal women. Menopause. 2011;18(1):109–118.
- Hodis HN, Mack WJ. Postmenopausal hormone therapy in clinical perspective. Menopause. 2007;14(5):944–957.
- Singletary SE. Rating the risk factors for breast cancer. Ann Surg. 2003;237(4):474–482.
- Archer DF, Oger E. Estrogen and progestogen effect on venous thromboembolism in menopausal women. Climacteric. 2012;15(3):235–240.
- Islam S, Liu Q, Chines A, Helzner E. Trend in incidence of osteoporosis-related fractures among 40- to 69-year-old women: Analysis of a large insurance claims database, 2000-2005. Menopause. 2009;16(1):77–83.
- Whiteman MK, Staropoli CA, Langenberg PW, McCarter RJ, Kjerulff KH, Flaws JA. Smoking, body mass and hot flashes in midlife women. Obstet Gynecol. 2003;101(2):264–272.
- Utian WH. Psychosocial and socioeconomic burden of vasomotor symptoms in menopause: A comprehensive review. Health Qual Life Outcomes. 2005;3:47.
- Hunter M, Rendall M. Bio-psycho-socio-cultural perspectives on menopause. Best Pract Res Clin Obstet Gynaecol. 2007;21(2):261–274.
Best age to begin screening mammograms: How I manage my patients
Controversy has surrounded the utility of screening mammograms, particularly in women in their 40s. In 2009, the US Preventive Services Task Force recommended that screening mammography begin at age 50 and that women aged 50 to 74 receive a mammogram every 2 years.1 However, the American Cancer Society2 and other professional groups continue to recommend that annual screening begin at age 40, leading to controversy and confusion among women’s health clinicians and our patients.
In a recent study, Webb and colleagues3 used registry data based on a health plan in a single US city to assess the cause of death and mammogram history of 1,705 women who died following a diagnosis of invasive breast cancer from 1990 to 1999. They confirmed that 609 of these deaths were from breast cancer. How many of these patients were screened?
What did they find?
The investigators found that 29% of the 609 women who died from breast cancer had been screened for it—19% of the cancers that caused death were screen-detected and 10% were interval cancers. (Interval cancers were defined as symptomatic or palpable tumors that presented less than 2 years after the prior screening mammogram.) That means that 71% of 609 deaths from breast cancer were among unscreened women, with 6% of the fatal cancers diagnosed more than 2 years after the last mammogram and 65% never found upon screening because screening did not occur.
Among deaths caused (n = 609) and not caused (n = 905) by breast cancer, the median age at diagnosis was 49 and 72 years, respectively. Investigators concluded that regular screening of women younger than age 50 years would lower the death rate from breast cancer.
Related Article: Biennial vs annual mammography: How I manage my patients Andrew M. Kaunitz, MD (June 2013)
Let’s not jump to any conclusions
Although some may find the report by Webb and colleagues persuasive, I am concerned about this study’s limitations, of which there are a few. First, analyses that focus on women diagnosed with breast cancers do not allow comparison of outcomes among screened and unscreened populations.
Moreover, this report provides no information on treatment received by screened and unscreened women. It is likely that women who have never been screened, or who have been screened only infrequently, are considerably less affluent and less educated than women who are regularly screened. Accordingly, upon noting a palpable breast mass, unscreened women may be less likely to seek timely medical attention than regularly screened women, leading to differences in breast cancer outcomes, which are independent of screening history.
How I counsel my patients
For now, I will continue to be laissez-fare in my recommendations about screening mammograms for average-risk women in their 40s by supporting their individual preferences about when to initiate such screening.
- Screening for breast cancer, Topic Page. US Preventive Services Task Force. http://www.uspreventiveservicestaskforce.org/uspstf/uspsbrca.htm. Updated July 2010. Accessed October 28, 2013.
- American Cancer Society Guidelines for the Early Detection of Cancer: Breast cancer. American Cancer Society Web site. http://www.cancer.org/healthy/findcancerearly/cancerscreeningguidelines/american-cancer-society-guidelines-for-the-early-detection-of-cancer. Updated May 3, 2013. Accessed October 28, 2013.
- Webb ML, Cady B, Michaelson JS, et al. A failure analysis of invasive breast cancer: Most deaths from disease occur in women not regularly screened [published online ahead of print September 9, 2013]. Cancer. doi:10.1002/cncr.28199.
Controversy has surrounded the utility of screening mammograms, particularly in women in their 40s. In 2009, the US Preventive Services Task Force recommended that screening mammography begin at age 50 and that women aged 50 to 74 receive a mammogram every 2 years.1 However, the American Cancer Society2 and other professional groups continue to recommend that annual screening begin at age 40, leading to controversy and confusion among women’s health clinicians and our patients.
In a recent study, Webb and colleagues3 used registry data based on a health plan in a single US city to assess the cause of death and mammogram history of 1,705 women who died following a diagnosis of invasive breast cancer from 1990 to 1999. They confirmed that 609 of these deaths were from breast cancer. How many of these patients were screened?
What did they find?
The investigators found that 29% of the 609 women who died from breast cancer had been screened for it—19% of the cancers that caused death were screen-detected and 10% were interval cancers. (Interval cancers were defined as symptomatic or palpable tumors that presented less than 2 years after the prior screening mammogram.) That means that 71% of 609 deaths from breast cancer were among unscreened women, with 6% of the fatal cancers diagnosed more than 2 years after the last mammogram and 65% never found upon screening because screening did not occur.
Among deaths caused (n = 609) and not caused (n = 905) by breast cancer, the median age at diagnosis was 49 and 72 years, respectively. Investigators concluded that regular screening of women younger than age 50 years would lower the death rate from breast cancer.
Related Article: Biennial vs annual mammography: How I manage my patients Andrew M. Kaunitz, MD (June 2013)
Let’s not jump to any conclusions
Although some may find the report by Webb and colleagues persuasive, I am concerned about this study’s limitations, of which there are a few. First, analyses that focus on women diagnosed with breast cancers do not allow comparison of outcomes among screened and unscreened populations.
Moreover, this report provides no information on treatment received by screened and unscreened women. It is likely that women who have never been screened, or who have been screened only infrequently, are considerably less affluent and less educated than women who are regularly screened. Accordingly, upon noting a palpable breast mass, unscreened women may be less likely to seek timely medical attention than regularly screened women, leading to differences in breast cancer outcomes, which are independent of screening history.
How I counsel my patients
For now, I will continue to be laissez-fare in my recommendations about screening mammograms for average-risk women in their 40s by supporting their individual preferences about when to initiate such screening.
Controversy has surrounded the utility of screening mammograms, particularly in women in their 40s. In 2009, the US Preventive Services Task Force recommended that screening mammography begin at age 50 and that women aged 50 to 74 receive a mammogram every 2 years.1 However, the American Cancer Society2 and other professional groups continue to recommend that annual screening begin at age 40, leading to controversy and confusion among women’s health clinicians and our patients.
In a recent study, Webb and colleagues3 used registry data based on a health plan in a single US city to assess the cause of death and mammogram history of 1,705 women who died following a diagnosis of invasive breast cancer from 1990 to 1999. They confirmed that 609 of these deaths were from breast cancer. How many of these patients were screened?
What did they find?
The investigators found that 29% of the 609 women who died from breast cancer had been screened for it—19% of the cancers that caused death were screen-detected and 10% were interval cancers. (Interval cancers were defined as symptomatic or palpable tumors that presented less than 2 years after the prior screening mammogram.) That means that 71% of 609 deaths from breast cancer were among unscreened women, with 6% of the fatal cancers diagnosed more than 2 years after the last mammogram and 65% never found upon screening because screening did not occur.
Among deaths caused (n = 609) and not caused (n = 905) by breast cancer, the median age at diagnosis was 49 and 72 years, respectively. Investigators concluded that regular screening of women younger than age 50 years would lower the death rate from breast cancer.
Related Article: Biennial vs annual mammography: How I manage my patients Andrew M. Kaunitz, MD (June 2013)
Let’s not jump to any conclusions
Although some may find the report by Webb and colleagues persuasive, I am concerned about this study’s limitations, of which there are a few. First, analyses that focus on women diagnosed with breast cancers do not allow comparison of outcomes among screened and unscreened populations.
Moreover, this report provides no information on treatment received by screened and unscreened women. It is likely that women who have never been screened, or who have been screened only infrequently, are considerably less affluent and less educated than women who are regularly screened. Accordingly, upon noting a palpable breast mass, unscreened women may be less likely to seek timely medical attention than regularly screened women, leading to differences in breast cancer outcomes, which are independent of screening history.
How I counsel my patients
For now, I will continue to be laissez-fare in my recommendations about screening mammograms for average-risk women in their 40s by supporting their individual preferences about when to initiate such screening.
- Screening for breast cancer, Topic Page. US Preventive Services Task Force. http://www.uspreventiveservicestaskforce.org/uspstf/uspsbrca.htm. Updated July 2010. Accessed October 28, 2013.
- American Cancer Society Guidelines for the Early Detection of Cancer: Breast cancer. American Cancer Society Web site. http://www.cancer.org/healthy/findcancerearly/cancerscreeningguidelines/american-cancer-society-guidelines-for-the-early-detection-of-cancer. Updated May 3, 2013. Accessed October 28, 2013.
- Webb ML, Cady B, Michaelson JS, et al. A failure analysis of invasive breast cancer: Most deaths from disease occur in women not regularly screened [published online ahead of print September 9, 2013]. Cancer. doi:10.1002/cncr.28199.
- Screening for breast cancer, Topic Page. US Preventive Services Task Force. http://www.uspreventiveservicestaskforce.org/uspstf/uspsbrca.htm. Updated July 2010. Accessed October 28, 2013.
- American Cancer Society Guidelines for the Early Detection of Cancer: Breast cancer. American Cancer Society Web site. http://www.cancer.org/healthy/findcancerearly/cancerscreeningguidelines/american-cancer-society-guidelines-for-the-early-detection-of-cancer. Updated May 3, 2013. Accessed October 28, 2013.
- Webb ML, Cady B, Michaelson JS, et al. A failure analysis of invasive breast cancer: Most deaths from disease occur in women not regularly screened [published online ahead of print September 9, 2013]. Cancer. doi:10.1002/cncr.28199.
Multi-Site Hospital Medicine Group Leaders Face Similar Challenges
Let’s call them multi-site, hospital medicine group leaders, or just multi-site HMG leaders. Once rare, they’re now becoming common, and among the many people now holding this job are:
- Dr. Doug Apple at Spectrum Health Medical Group in Grand Rapids, Mich;
- Dr. Tierza Stephan at Allina Health in Minneapolis, Minn.;
- Dr. Darren Thomas at St. John Health System in Tulsa, Okla.;
- Dr. Thomas McIlraith at Dignity Health in Sacremento, Calif.; and
- Dr. Rohit Uppal at Ohio Health in Columbus, Ohio.
The career path that led to their current position usually follows a standard pattern. They are a successful leader of a single-site hospitalist program when, through merger or acquisition, their hospital becomes part of a larger system. The executives responsible for this larger system—typically four to eight hospitals—realize that the HMGs serving each hospital in the system vary significantly in their cost, productivity, and performance on things like patient satisfaction and quality metrics. So they tap the leader of the largest (or best performing) HMG in the system to be system-wide hospitalist medical director. They nearly always choose an internal candidate rather than recruiting from outside, which brings some level of cohesion in operations and performance improvement.
Multi-Site Challenges
This is not an easy job. After all, it isn’t easy to serve as lead hospitalist for a single-site group, so it makes sense that the difficulties and challenges only increase when trying to manage groups at different locations.
The new multi-site HMG leader is busy from the first day on the job. The HMG at one site is short on staffing and needs help right away, patient satisfaction scores are poor at the next site, and so on. Although putting out these fires is important, the new leader also needs to think about how to accomplish a broader mission: ensuring greater cohesion across all groups.
I don’t think there is a secret recipe to ensure success in such a job. Prerequisites include the usual leadership skills, such as patience, good listening, and diplomacy (collectively, one’s EQ, or emotional quotient), along with lots of energy and decisive action. But there are a number of practical matters to address that can influence the level of success.
Cohesion vs. Independence
In most situations, a health system will benefit from some common operating principles across all the HMGs who serve its hospitals. For example, it usually makes sense for any portion of compensation tied to performance (e.g., a bonus) to be based on the same performance domains at all sites. For example, if metrics such as the observed-to-expected mortality ratio (O:E ratio) and patient satisfaction are important to the hospital system, then they should probably influence hospitalist compensation at every site. However, it might be reasonable to target a level of performance for any given domain higher at one site than at another.
Among the many things that should be the same across all sites are operational practices: charge capture, coding audits, performance reviews, dashboard elements and format, and credentialing for new hires. Other things, like individual hospitalist productivity, work schedule, and method and amount of compensation, should vary by site because of the unique attributes of the work at each place.
Fixed Locale vs. Rotations
The travel time between hospitals and the value of extensive experience in the details of how each particular hospital operates usually make it most practical for each individual hospitalist to work nearly all of the time at one hospital. But every doctor should be credentialed at every other hospital in the system so that he can cover a staffing shortage elsewhere.
And, hospitalists hired to work primarily at one of the small hospitals would probably benefit from working at the large referral hospital for the first few weeks of employment. This seems like a great way for them to become familiar with the people and operations at the big hospital, especially since they will be transferring patients there periodically.
Governance
Some mix of central control vs. local autonomy in decision making at each site is important for success. There aren’t any clear guidelines here, but providing the local doctors at each location with the ability to make their own decisions on things like work schedule will contribute to their sense of ownership of the practice. That feeling is valuable and supports good performance.
My bias is that each site in a practice could adopt the same “internal governance” guidelines, or rules by which they make decisions when unable to reach consensus (see “Play by the Rules,” December 2007, for sample guidelines.)
There should also be some form of “umbrella” governance structure in which the local site leaders meet regularly with the multi-site HMG leader.
Patient Transfers
One reason hospitals merge into a single system is the hope that they can more effectively meet the needs of all patients in the system’s hospitals. A typical configuration is several small hospitals, along with a single, large, referral center, to which patients are sent if the small hospital can’t meet their needs. The hope is that if all the hospitals are in the same system, the process of transfer can be smoother and more efficient.
A large portion—maybe even the majority—of all transfers in the system will be between a hospitalist at the small hospital and a partner hospitalist at the large hospital. Things will work best when the transferring and receiving hospitalists know something about the strengths and weaknesses of each other’s hospitals. And, you only know one another reasonably well from working together on committees or being on clinical service together at the same hospital, as well as social functions that include hospitalists from all sites.
Therefore, the multi-site HMG leader should think deliberately about how to ensure that the hospitalists interact with one another often, and not just when a transfer needs to take place.
A written agreement outlining the criteria for an appropriate transfer can be helpful. But such agreements cannot address all the situations that will arise, so good relationships between doctors at the different sites are invaluable and worth taking the time to cultivate.
Communication
Like the five people I mentioned above, anyone holding the position of multi-site HMG leader would benefit from talking with others in the same position. I’m working to arrange some forum for such communication, potentially including an in-person meeting at HM14 in Las Vegas in March (www.hospitalmedicine2014.org). If you are a health system-employed, multi-site HMG leader and want to be part of this conversation, I would love to hear from you.
Dr. Nelson has been a practicing hospitalist since 1988. He is co-founder and past president of SHM, and principal in Nelson Flores Hospital Medicine Consultants. He is co-director for SHM’s “Best Practices in Managing a Hospital Medicine Program” course. Write to him at [email protected].
Let’s call them multi-site, hospital medicine group leaders, or just multi-site HMG leaders. Once rare, they’re now becoming common, and among the many people now holding this job are:
- Dr. Doug Apple at Spectrum Health Medical Group in Grand Rapids, Mich;
- Dr. Tierza Stephan at Allina Health in Minneapolis, Minn.;
- Dr. Darren Thomas at St. John Health System in Tulsa, Okla.;
- Dr. Thomas McIlraith at Dignity Health in Sacremento, Calif.; and
- Dr. Rohit Uppal at Ohio Health in Columbus, Ohio.
The career path that led to their current position usually follows a standard pattern. They are a successful leader of a single-site hospitalist program when, through merger or acquisition, their hospital becomes part of a larger system. The executives responsible for this larger system—typically four to eight hospitals—realize that the HMGs serving each hospital in the system vary significantly in their cost, productivity, and performance on things like patient satisfaction and quality metrics. So they tap the leader of the largest (or best performing) HMG in the system to be system-wide hospitalist medical director. They nearly always choose an internal candidate rather than recruiting from outside, which brings some level of cohesion in operations and performance improvement.
Multi-Site Challenges
This is not an easy job. After all, it isn’t easy to serve as lead hospitalist for a single-site group, so it makes sense that the difficulties and challenges only increase when trying to manage groups at different locations.
The new multi-site HMG leader is busy from the first day on the job. The HMG at one site is short on staffing and needs help right away, patient satisfaction scores are poor at the next site, and so on. Although putting out these fires is important, the new leader also needs to think about how to accomplish a broader mission: ensuring greater cohesion across all groups.
I don’t think there is a secret recipe to ensure success in such a job. Prerequisites include the usual leadership skills, such as patience, good listening, and diplomacy (collectively, one’s EQ, or emotional quotient), along with lots of energy and decisive action. But there are a number of practical matters to address that can influence the level of success.
Cohesion vs. Independence
In most situations, a health system will benefit from some common operating principles across all the HMGs who serve its hospitals. For example, it usually makes sense for any portion of compensation tied to performance (e.g., a bonus) to be based on the same performance domains at all sites. For example, if metrics such as the observed-to-expected mortality ratio (O:E ratio) and patient satisfaction are important to the hospital system, then they should probably influence hospitalist compensation at every site. However, it might be reasonable to target a level of performance for any given domain higher at one site than at another.
Among the many things that should be the same across all sites are operational practices: charge capture, coding audits, performance reviews, dashboard elements and format, and credentialing for new hires. Other things, like individual hospitalist productivity, work schedule, and method and amount of compensation, should vary by site because of the unique attributes of the work at each place.
Fixed Locale vs. Rotations
The travel time between hospitals and the value of extensive experience in the details of how each particular hospital operates usually make it most practical for each individual hospitalist to work nearly all of the time at one hospital. But every doctor should be credentialed at every other hospital in the system so that he can cover a staffing shortage elsewhere.
And, hospitalists hired to work primarily at one of the small hospitals would probably benefit from working at the large referral hospital for the first few weeks of employment. This seems like a great way for them to become familiar with the people and operations at the big hospital, especially since they will be transferring patients there periodically.
Governance
Some mix of central control vs. local autonomy in decision making at each site is important for success. There aren’t any clear guidelines here, but providing the local doctors at each location with the ability to make their own decisions on things like work schedule will contribute to their sense of ownership of the practice. That feeling is valuable and supports good performance.
My bias is that each site in a practice could adopt the same “internal governance” guidelines, or rules by which they make decisions when unable to reach consensus (see “Play by the Rules,” December 2007, for sample guidelines.)
There should also be some form of “umbrella” governance structure in which the local site leaders meet regularly with the multi-site HMG leader.
Patient Transfers
One reason hospitals merge into a single system is the hope that they can more effectively meet the needs of all patients in the system’s hospitals. A typical configuration is several small hospitals, along with a single, large, referral center, to which patients are sent if the small hospital can’t meet their needs. The hope is that if all the hospitals are in the same system, the process of transfer can be smoother and more efficient.
A large portion—maybe even the majority—of all transfers in the system will be between a hospitalist at the small hospital and a partner hospitalist at the large hospital. Things will work best when the transferring and receiving hospitalists know something about the strengths and weaknesses of each other’s hospitals. And, you only know one another reasonably well from working together on committees or being on clinical service together at the same hospital, as well as social functions that include hospitalists from all sites.
Therefore, the multi-site HMG leader should think deliberately about how to ensure that the hospitalists interact with one another often, and not just when a transfer needs to take place.
A written agreement outlining the criteria for an appropriate transfer can be helpful. But such agreements cannot address all the situations that will arise, so good relationships between doctors at the different sites are invaluable and worth taking the time to cultivate.
Communication
Like the five people I mentioned above, anyone holding the position of multi-site HMG leader would benefit from talking with others in the same position. I’m working to arrange some forum for such communication, potentially including an in-person meeting at HM14 in Las Vegas in March (www.hospitalmedicine2014.org). If you are a health system-employed, multi-site HMG leader and want to be part of this conversation, I would love to hear from you.
Dr. Nelson has been a practicing hospitalist since 1988. He is co-founder and past president of SHM, and principal in Nelson Flores Hospital Medicine Consultants. He is co-director for SHM’s “Best Practices in Managing a Hospital Medicine Program” course. Write to him at [email protected].
Let’s call them multi-site, hospital medicine group leaders, or just multi-site HMG leaders. Once rare, they’re now becoming common, and among the many people now holding this job are:
- Dr. Doug Apple at Spectrum Health Medical Group in Grand Rapids, Mich;
- Dr. Tierza Stephan at Allina Health in Minneapolis, Minn.;
- Dr. Darren Thomas at St. John Health System in Tulsa, Okla.;
- Dr. Thomas McIlraith at Dignity Health in Sacremento, Calif.; and
- Dr. Rohit Uppal at Ohio Health in Columbus, Ohio.
The career path that led to their current position usually follows a standard pattern. They are a successful leader of a single-site hospitalist program when, through merger or acquisition, their hospital becomes part of a larger system. The executives responsible for this larger system—typically four to eight hospitals—realize that the HMGs serving each hospital in the system vary significantly in their cost, productivity, and performance on things like patient satisfaction and quality metrics. So they tap the leader of the largest (or best performing) HMG in the system to be system-wide hospitalist medical director. They nearly always choose an internal candidate rather than recruiting from outside, which brings some level of cohesion in operations and performance improvement.
Multi-Site Challenges
This is not an easy job. After all, it isn’t easy to serve as lead hospitalist for a single-site group, so it makes sense that the difficulties and challenges only increase when trying to manage groups at different locations.
The new multi-site HMG leader is busy from the first day on the job. The HMG at one site is short on staffing and needs help right away, patient satisfaction scores are poor at the next site, and so on. Although putting out these fires is important, the new leader also needs to think about how to accomplish a broader mission: ensuring greater cohesion across all groups.
I don’t think there is a secret recipe to ensure success in such a job. Prerequisites include the usual leadership skills, such as patience, good listening, and diplomacy (collectively, one’s EQ, or emotional quotient), along with lots of energy and decisive action. But there are a number of practical matters to address that can influence the level of success.
Cohesion vs. Independence
In most situations, a health system will benefit from some common operating principles across all the HMGs who serve its hospitals. For example, it usually makes sense for any portion of compensation tied to performance (e.g., a bonus) to be based on the same performance domains at all sites. For example, if metrics such as the observed-to-expected mortality ratio (O:E ratio) and patient satisfaction are important to the hospital system, then they should probably influence hospitalist compensation at every site. However, it might be reasonable to target a level of performance for any given domain higher at one site than at another.
Among the many things that should be the same across all sites are operational practices: charge capture, coding audits, performance reviews, dashboard elements and format, and credentialing for new hires. Other things, like individual hospitalist productivity, work schedule, and method and amount of compensation, should vary by site because of the unique attributes of the work at each place.
Fixed Locale vs. Rotations
The travel time between hospitals and the value of extensive experience in the details of how each particular hospital operates usually make it most practical for each individual hospitalist to work nearly all of the time at one hospital. But every doctor should be credentialed at every other hospital in the system so that he can cover a staffing shortage elsewhere.
And, hospitalists hired to work primarily at one of the small hospitals would probably benefit from working at the large referral hospital for the first few weeks of employment. This seems like a great way for them to become familiar with the people and operations at the big hospital, especially since they will be transferring patients there periodically.
Governance
Some mix of central control vs. local autonomy in decision making at each site is important for success. There aren’t any clear guidelines here, but providing the local doctors at each location with the ability to make their own decisions on things like work schedule will contribute to their sense of ownership of the practice. That feeling is valuable and supports good performance.
My bias is that each site in a practice could adopt the same “internal governance” guidelines, or rules by which they make decisions when unable to reach consensus (see “Play by the Rules,” December 2007, for sample guidelines.)
There should also be some form of “umbrella” governance structure in which the local site leaders meet regularly with the multi-site HMG leader.
Patient Transfers
One reason hospitals merge into a single system is the hope that they can more effectively meet the needs of all patients in the system’s hospitals. A typical configuration is several small hospitals, along with a single, large, referral center, to which patients are sent if the small hospital can’t meet their needs. The hope is that if all the hospitals are in the same system, the process of transfer can be smoother and more efficient.
A large portion—maybe even the majority—of all transfers in the system will be between a hospitalist at the small hospital and a partner hospitalist at the large hospital. Things will work best when the transferring and receiving hospitalists know something about the strengths and weaknesses of each other’s hospitals. And, you only know one another reasonably well from working together on committees or being on clinical service together at the same hospital, as well as social functions that include hospitalists from all sites.
Therefore, the multi-site HMG leader should think deliberately about how to ensure that the hospitalists interact with one another often, and not just when a transfer needs to take place.
A written agreement outlining the criteria for an appropriate transfer can be helpful. But such agreements cannot address all the situations that will arise, so good relationships between doctors at the different sites are invaluable and worth taking the time to cultivate.
Communication
Like the five people I mentioned above, anyone holding the position of multi-site HMG leader would benefit from talking with others in the same position. I’m working to arrange some forum for such communication, potentially including an in-person meeting at HM14 in Las Vegas in March (www.hospitalmedicine2014.org). If you are a health system-employed, multi-site HMG leader and want to be part of this conversation, I would love to hear from you.
Dr. Nelson has been a practicing hospitalist since 1988. He is co-founder and past president of SHM, and principal in Nelson Flores Hospital Medicine Consultants. He is co-director for SHM’s “Best Practices in Managing a Hospital Medicine Program” course. Write to him at [email protected].
Massachusetts Hospitalists Experiment with Unit-Based Rounding
Today marks the end of the second week of a three-month experiment we are embarking on to improve team-based care. The main elements of our experiment are two early career hospitalists dedicated to a single nursing unit who are present on the unit throughout the day, structured multidisciplinary rounds, pharmacists doing medication histories to help with medical reconciliation, and a veteran hospitalist serving as a coach, broadly overseeing care coordination and throughput on the unit. (I’m going to focus on multidisciplinary care and leave the coaching part for another day.)
Many have written about and many more have tried to establish unit-based hospitalist models, where a hospitalist is assigned to a single nursing unit. These models often incorporate multidisciplinary rounds, where the hospitalist, case management, social services, physical therapy, and perhaps pharmacy meet each day and review each patient’s progress through the hospitalization. The underlying premise for establishing a unit-based model is that all, or nearly all, of the hospitalist’s patients are located on the nursing unit.
It Can’t Be That Hard
Dedicated units and multidisciplinary rounds are designed to achieve better coordination between the hospitalists and the other members of the hospital team. Most healthcare professionals intuitively support this model; however, many hospitalists have concerns.
To provide the best care for their patients while maintaining career satisfaction, these hospitalists may feel the need for flexibility—the ability to be independent and roam unrestricted through the hallways and departments of the hospital. This goal can be at odds with being limited to a single nursing unit.
For these hospitalists to support the unit-based model, there had better be good reasons for doing so.
Measuring the Effects of Teamwork
Jody Hoffer Gittell, PhD, a professor of management at Brandeis University in Waltham, Mass., has studied relational coordination extensively in healthcare and other service industries. Relational coordination can be defined as “coordinating work through relationships of shared goals, shared knowledge and mutual respect, supported by frequent, timely, accurate, problem-solving communication.”1
Dr. Gittell has developed a validated questionnaire to be completed by each member of the healthcare team, quantifying their perspective on these dimensions for others on the team. I think of relational coordination as a rigorous way of quantifying teamwork.
In 2008, Dr. Gittell published an observational study with SHM senior vice president Joe Miller and hospitalist leader Adrienne L. Bennett, MD, PhD, conducted at a suburban Boston hospital.2 The study looked at relational coordination between members of the hospital team under hospitalist care compared to traditional, PCP-based hospital care. They measured relational coordination by asking the attending physician (hospitalist or PCP providing hospital care), medical resident, floor nurse, case manager, social worker, and therapist (occupational, physical, respiratory, speech) to complete questionnaires about the other team members for a cohort of patients.
The study concluded that relational coordination between other members of the team and the physician was significantly higher for patients treated by hospitalists than for patients treated by traditional PCPs. Further, they found that as relational coordination increased, for patients treated either by hospitalists or PCPs, length of stay, cost, and 30-day readmission rates decreased. I will add that the hospitalists were not unit-based in this study, but were assumed to be more available to the care team than traditional PCPs.
Subsequent studies of multidisciplinary rounds on a “hospitalist unit” conducted by Kevin O’Leary, MD, and colleagues at Northwestern University in Chicago have demonstrated a favorable effect on nurses’ ratings of teamwork and collaboration, as well as the rate of adverse events.3,4 The former study did not, however, find decreased costs or length of stay.
Keys to Success
Before our current experiment, I’ve had the privilege to witness, both at my home institution and at a number of outside ones, many permutations of multidisciplinary rounds and unit-based hospitalists. I’ve seen failures, some mixed results, and occasional success stories. In all cases, participants seem to agree that it takes extra effort to execute on this model, especially once the initial enthusiasm wanes. So, for these arrangements to succeed over time, including our current experiment, I see the following four factors as critical:
- Multidisciplinary rounds must be tightly organized, with case manager, nurse, and hospitalist providing input concisely. Average time per patient should not exceed about three minutes. The total time for rounds, no matter how many patients are under discussion, should not exceed one hour.
- Each team member must be prepared to provide critical information for rounds. For example, hospitalists and nurses should have seen/reviewed their patients, case managers should know expected length of stay and key disposition information, and pharmacists should know medical histories and other pertinent information.
- The fundamental concern of multidisciplinary rounds—that someone’s time is being wasted (when not talking about that team member’s patient at that moment)—must be mitigated one way or another. Solutions include rotating nurses or hospitalists in and out of rounds, and allowing hospitalists to enter orders and do other discreet multitasking during rounds. Careful attention to showing up for the rounds on time and on cue is crucial.
- Hospitalist autonomy and need to roam has to be programmed in by allowing them time to get off the unit, see the broader world, and interact with colleagues.
At the conclusion of three months, as a QI project (as opposed to rigorous research), we will measure a number of things, including cost, throughput, patient satisfaction, and team member satisfaction with the model. If you have predictions, please e-mail me. I’ll report our results in a subsequent column.
Dr. Whitcomb is medical director of healthcare quality at Baystate Medical Center in Springfield, Mass. He is co-founder and past president of SHM. E-mail him at [email protected].
References
- Relational Coordination Research Collaborative. Brandeis University website. Available at: http://rcrc.brandeis.edu/about-rc/What%20is%20Relational%20Coordination.html. Accessed September 23, 2013.
- Gittell JH, Weinberg DB, Bennett AL, Miller JA. Is the doctor in? A relational approach to job design and the coordination of work. Hum Resource Manag J. 2008;47(4):729-755.
- O’Leary KJ, Haviley C, Slade ME, Shah HM, Lee J, Williams MV. Improving teamwork: impact of structured interdisciplinary rounds on a hospitalist unit. J Hosp Med. 2011;6(2):88-93.
- O’Leary KJ, Buck R, Fligiel HM, et al. Structured interdisciplinary rounds in a medical teaching unit: improving patient safety. Arch Intern Med. 2011;171(7):678-684.
Today marks the end of the second week of a three-month experiment we are embarking on to improve team-based care. The main elements of our experiment are two early career hospitalists dedicated to a single nursing unit who are present on the unit throughout the day, structured multidisciplinary rounds, pharmacists doing medication histories to help with medical reconciliation, and a veteran hospitalist serving as a coach, broadly overseeing care coordination and throughput on the unit. (I’m going to focus on multidisciplinary care and leave the coaching part for another day.)
Many have written about and many more have tried to establish unit-based hospitalist models, where a hospitalist is assigned to a single nursing unit. These models often incorporate multidisciplinary rounds, where the hospitalist, case management, social services, physical therapy, and perhaps pharmacy meet each day and review each patient’s progress through the hospitalization. The underlying premise for establishing a unit-based model is that all, or nearly all, of the hospitalist’s patients are located on the nursing unit.
It Can’t Be That Hard
Dedicated units and multidisciplinary rounds are designed to achieve better coordination between the hospitalists and the other members of the hospital team. Most healthcare professionals intuitively support this model; however, many hospitalists have concerns.
To provide the best care for their patients while maintaining career satisfaction, these hospitalists may feel the need for flexibility—the ability to be independent and roam unrestricted through the hallways and departments of the hospital. This goal can be at odds with being limited to a single nursing unit.
For these hospitalists to support the unit-based model, there had better be good reasons for doing so.
Measuring the Effects of Teamwork
Jody Hoffer Gittell, PhD, a professor of management at Brandeis University in Waltham, Mass., has studied relational coordination extensively in healthcare and other service industries. Relational coordination can be defined as “coordinating work through relationships of shared goals, shared knowledge and mutual respect, supported by frequent, timely, accurate, problem-solving communication.”1
Dr. Gittell has developed a validated questionnaire to be completed by each member of the healthcare team, quantifying their perspective on these dimensions for others on the team. I think of relational coordination as a rigorous way of quantifying teamwork.
In 2008, Dr. Gittell published an observational study with SHM senior vice president Joe Miller and hospitalist leader Adrienne L. Bennett, MD, PhD, conducted at a suburban Boston hospital.2 The study looked at relational coordination between members of the hospital team under hospitalist care compared to traditional, PCP-based hospital care. They measured relational coordination by asking the attending physician (hospitalist or PCP providing hospital care), medical resident, floor nurse, case manager, social worker, and therapist (occupational, physical, respiratory, speech) to complete questionnaires about the other team members for a cohort of patients.
The study concluded that relational coordination between other members of the team and the physician was significantly higher for patients treated by hospitalists than for patients treated by traditional PCPs. Further, they found that as relational coordination increased, for patients treated either by hospitalists or PCPs, length of stay, cost, and 30-day readmission rates decreased. I will add that the hospitalists were not unit-based in this study, but were assumed to be more available to the care team than traditional PCPs.
Subsequent studies of multidisciplinary rounds on a “hospitalist unit” conducted by Kevin O’Leary, MD, and colleagues at Northwestern University in Chicago have demonstrated a favorable effect on nurses’ ratings of teamwork and collaboration, as well as the rate of adverse events.3,4 The former study did not, however, find decreased costs or length of stay.
Keys to Success
Before our current experiment, I’ve had the privilege to witness, both at my home institution and at a number of outside ones, many permutations of multidisciplinary rounds and unit-based hospitalists. I’ve seen failures, some mixed results, and occasional success stories. In all cases, participants seem to agree that it takes extra effort to execute on this model, especially once the initial enthusiasm wanes. So, for these arrangements to succeed over time, including our current experiment, I see the following four factors as critical:
- Multidisciplinary rounds must be tightly organized, with case manager, nurse, and hospitalist providing input concisely. Average time per patient should not exceed about three minutes. The total time for rounds, no matter how many patients are under discussion, should not exceed one hour.
- Each team member must be prepared to provide critical information for rounds. For example, hospitalists and nurses should have seen/reviewed their patients, case managers should know expected length of stay and key disposition information, and pharmacists should know medical histories and other pertinent information.
- The fundamental concern of multidisciplinary rounds—that someone’s time is being wasted (when not talking about that team member’s patient at that moment)—must be mitigated one way or another. Solutions include rotating nurses or hospitalists in and out of rounds, and allowing hospitalists to enter orders and do other discreet multitasking during rounds. Careful attention to showing up for the rounds on time and on cue is crucial.
- Hospitalist autonomy and need to roam has to be programmed in by allowing them time to get off the unit, see the broader world, and interact with colleagues.
At the conclusion of three months, as a QI project (as opposed to rigorous research), we will measure a number of things, including cost, throughput, patient satisfaction, and team member satisfaction with the model. If you have predictions, please e-mail me. I’ll report our results in a subsequent column.
Dr. Whitcomb is medical director of healthcare quality at Baystate Medical Center in Springfield, Mass. He is co-founder and past president of SHM. E-mail him at [email protected].
References
- Relational Coordination Research Collaborative. Brandeis University website. Available at: http://rcrc.brandeis.edu/about-rc/What%20is%20Relational%20Coordination.html. Accessed September 23, 2013.
- Gittell JH, Weinberg DB, Bennett AL, Miller JA. Is the doctor in? A relational approach to job design and the coordination of work. Hum Resource Manag J. 2008;47(4):729-755.
- O’Leary KJ, Haviley C, Slade ME, Shah HM, Lee J, Williams MV. Improving teamwork: impact of structured interdisciplinary rounds on a hospitalist unit. J Hosp Med. 2011;6(2):88-93.
- O’Leary KJ, Buck R, Fligiel HM, et al. Structured interdisciplinary rounds in a medical teaching unit: improving patient safety. Arch Intern Med. 2011;171(7):678-684.
Today marks the end of the second week of a three-month experiment we are embarking on to improve team-based care. The main elements of our experiment are two early career hospitalists dedicated to a single nursing unit who are present on the unit throughout the day, structured multidisciplinary rounds, pharmacists doing medication histories to help with medical reconciliation, and a veteran hospitalist serving as a coach, broadly overseeing care coordination and throughput on the unit. (I’m going to focus on multidisciplinary care and leave the coaching part for another day.)
Many have written about and many more have tried to establish unit-based hospitalist models, where a hospitalist is assigned to a single nursing unit. These models often incorporate multidisciplinary rounds, where the hospitalist, case management, social services, physical therapy, and perhaps pharmacy meet each day and review each patient’s progress through the hospitalization. The underlying premise for establishing a unit-based model is that all, or nearly all, of the hospitalist’s patients are located on the nursing unit.
It Can’t Be That Hard
Dedicated units and multidisciplinary rounds are designed to achieve better coordination between the hospitalists and the other members of the hospital team. Most healthcare professionals intuitively support this model; however, many hospitalists have concerns.
To provide the best care for their patients while maintaining career satisfaction, these hospitalists may feel the need for flexibility—the ability to be independent and roam unrestricted through the hallways and departments of the hospital. This goal can be at odds with being limited to a single nursing unit.
For these hospitalists to support the unit-based model, there had better be good reasons for doing so.
Measuring the Effects of Teamwork
Jody Hoffer Gittell, PhD, a professor of management at Brandeis University in Waltham, Mass., has studied relational coordination extensively in healthcare and other service industries. Relational coordination can be defined as “coordinating work through relationships of shared goals, shared knowledge and mutual respect, supported by frequent, timely, accurate, problem-solving communication.”1
Dr. Gittell has developed a validated questionnaire to be completed by each member of the healthcare team, quantifying their perspective on these dimensions for others on the team. I think of relational coordination as a rigorous way of quantifying teamwork.
In 2008, Dr. Gittell published an observational study with SHM senior vice president Joe Miller and hospitalist leader Adrienne L. Bennett, MD, PhD, conducted at a suburban Boston hospital.2 The study looked at relational coordination between members of the hospital team under hospitalist care compared to traditional, PCP-based hospital care. They measured relational coordination by asking the attending physician (hospitalist or PCP providing hospital care), medical resident, floor nurse, case manager, social worker, and therapist (occupational, physical, respiratory, speech) to complete questionnaires about the other team members for a cohort of patients.
The study concluded that relational coordination between other members of the team and the physician was significantly higher for patients treated by hospitalists than for patients treated by traditional PCPs. Further, they found that as relational coordination increased, for patients treated either by hospitalists or PCPs, length of stay, cost, and 30-day readmission rates decreased. I will add that the hospitalists were not unit-based in this study, but were assumed to be more available to the care team than traditional PCPs.
Subsequent studies of multidisciplinary rounds on a “hospitalist unit” conducted by Kevin O’Leary, MD, and colleagues at Northwestern University in Chicago have demonstrated a favorable effect on nurses’ ratings of teamwork and collaboration, as well as the rate of adverse events.3,4 The former study did not, however, find decreased costs or length of stay.
Keys to Success
Before our current experiment, I’ve had the privilege to witness, both at my home institution and at a number of outside ones, many permutations of multidisciplinary rounds and unit-based hospitalists. I’ve seen failures, some mixed results, and occasional success stories. In all cases, participants seem to agree that it takes extra effort to execute on this model, especially once the initial enthusiasm wanes. So, for these arrangements to succeed over time, including our current experiment, I see the following four factors as critical:
- Multidisciplinary rounds must be tightly organized, with case manager, nurse, and hospitalist providing input concisely. Average time per patient should not exceed about three minutes. The total time for rounds, no matter how many patients are under discussion, should not exceed one hour.
- Each team member must be prepared to provide critical information for rounds. For example, hospitalists and nurses should have seen/reviewed their patients, case managers should know expected length of stay and key disposition information, and pharmacists should know medical histories and other pertinent information.
- The fundamental concern of multidisciplinary rounds—that someone’s time is being wasted (when not talking about that team member’s patient at that moment)—must be mitigated one way or another. Solutions include rotating nurses or hospitalists in and out of rounds, and allowing hospitalists to enter orders and do other discreet multitasking during rounds. Careful attention to showing up for the rounds on time and on cue is crucial.
- Hospitalist autonomy and need to roam has to be programmed in by allowing them time to get off the unit, see the broader world, and interact with colleagues.
At the conclusion of three months, as a QI project (as opposed to rigorous research), we will measure a number of things, including cost, throughput, patient satisfaction, and team member satisfaction with the model. If you have predictions, please e-mail me. I’ll report our results in a subsequent column.
Dr. Whitcomb is medical director of healthcare quality at Baystate Medical Center in Springfield, Mass. He is co-founder and past president of SHM. E-mail him at [email protected].
References
- Relational Coordination Research Collaborative. Brandeis University website. Available at: http://rcrc.brandeis.edu/about-rc/What%20is%20Relational%20Coordination.html. Accessed September 23, 2013.
- Gittell JH, Weinberg DB, Bennett AL, Miller JA. Is the doctor in? A relational approach to job design and the coordination of work. Hum Resource Manag J. 2008;47(4):729-755.
- O’Leary KJ, Haviley C, Slade ME, Shah HM, Lee J, Williams MV. Improving teamwork: impact of structured interdisciplinary rounds on a hospitalist unit. J Hosp Med. 2011;6(2):88-93.
- O’Leary KJ, Buck R, Fligiel HM, et al. Structured interdisciplinary rounds in a medical teaching unit: improving patient safety. Arch Intern Med. 2011;171(7):678-684.
Which women are most likely to die from breast cancer—those screened annually starting at age 40, biennially starting at age 50, or not at all?
In 2009, the United States Preventive Services Task Force (USPSTF) released a controversial recommendation that breast cancer screening should routinely start at age 50 and be performed biennially.1 The Task Force further stated that the determination to screen women before the age of 50 “should take patient context into account, including the patient’s values regarding specific benefits and harms.”1
Although some professional societies have adopted this recommendation, others, including the American College of Obstetricians and Gynecologists (ACOG), have recommended that annual screening begin at age 40 in average-risk women.2
Related article: Is "overdiagnosis" of breast cancer common among women screened by mammography? Andrew M. Kaunitz, MD (January 2013)
With different professional societies lined up on both sides of this debate, practitioners and women remain confused about why different breast cancer screening guidelines exist in the United States.
Putting the study in context
A review of the data that informed the USPSTF recommendation demonstrate that routine screening mammography has similar efficacy in women in their fifth and sixth decade of life—a reduction in mortality of 16% and 15%, respectively.3 However, more mammograms need to be performed in women in their 40s than in women in their 50s to achieve that benefit. Moreover, because the specificity of screening mammography is less than ideal, about 10% of women are called back for additional imaging, and 10% of that population will undergo a biopsy. Only 30% of these biopsies will result in a diagnosis of cancer.
There has been considerable debate about the mortality improvement with routine screening mammography, including a nationwide administrative data review published in late 2012.4 This data-mining study suggested that routine screening mammography was only a minor contributor to the significant improvement in breast cancer mortality over the past 25 years.
Related article: Update on breast health Mark D. Pearlman, MD, and Jennifer L. Griffin Miller, MD, MPH (March 2013)
Details of the study
In this failure analysis from Massachusetts General Hospital and Brigham and Women’s Hospital, Webb and colleagues reviewed breast cancer deaths over 17 years (new breast cancer cases from 1990 through 1997, with cases followed through 2007). They compared the likelihood of death according to whether screening mammography had been performed in the preceding 2 years.
Of the 609 confirmed deaths from breast cancer, 71% occurred among unscreened women (ie, never screened or screening not done in the preceding 2 years). The median age at diagnosis of fatal cancers was 49 years, whereas women with breast cancer who ultimately died of other causes had a median age at diagnosis of 72 years.
Related article: Biennial vs annual mammography: How I manage my patients Andrew M. Kaunitz, MD (June 2013)
Webb and colleagues concluded: “Most deaths from breast cancer occur in unscreened women. To maximize mortality reduction and life-years gained, initiation of regular screening before age 50 years should be encouraged.”
WHAT THIS EVIDENCE MEANS FOR PRACTICE
The findings of Webb and colleagues are not surprising, given the already demonstrated reduction in breast cancer mortality with the use of routine screening mammography in women in their 40s. What is particularly interesting in this study is that the preponderance of breast cancer deaths occurred in younger women. Biologically, this may be explained by the more aggressive nature of breast cancer in younger women.5
This study provides additional support for the ACOG recommendation to routinely offer breast cancer screening with mammography for women aged 40 to 49 years.
--Mark D. Pearlman, MD
We want to hear from you. Tell us what you think.
- US Preventive Services Task Force. Screening for Breast Cancer. http://www.uspreventiveservicestaskforce.org
/uspstf/uspsbrca.htm. Accessed October 22, 2013. - American College of Obstetricians and Gynecologists. Practice Bulletin No. 122: Breast cancer screening. Obstet Gynecol. 2011;118(2 Pt 1):372–382.
- Nelson HD, Tyne K, Naik A, Bougatsos C, Chan BK, Humphrey L. Screening for breast cancer: An update for the US Preventive Services Task Force. Ann Intern Med. 2009;151(10):727–737.
- Bleyer A, Welch HG. Effect of three decades of screening mammography on breast cancer incidence. N Engl J Med. 2012;367(21):1998–2005.
- Klauber-DeMore N. Tumor biology of breast cancer in young women. Breast Dis. 2006;23:9–15.
In 2009, the United States Preventive Services Task Force (USPSTF) released a controversial recommendation that breast cancer screening should routinely start at age 50 and be performed biennially.1 The Task Force further stated that the determination to screen women before the age of 50 “should take patient context into account, including the patient’s values regarding specific benefits and harms.”1
Although some professional societies have adopted this recommendation, others, including the American College of Obstetricians and Gynecologists (ACOG), have recommended that annual screening begin at age 40 in average-risk women.2
Related article: Is "overdiagnosis" of breast cancer common among women screened by mammography? Andrew M. Kaunitz, MD (January 2013)
With different professional societies lined up on both sides of this debate, practitioners and women remain confused about why different breast cancer screening guidelines exist in the United States.
Putting the study in context
A review of the data that informed the USPSTF recommendation demonstrate that routine screening mammography has similar efficacy in women in their fifth and sixth decade of life—a reduction in mortality of 16% and 15%, respectively.3 However, more mammograms need to be performed in women in their 40s than in women in their 50s to achieve that benefit. Moreover, because the specificity of screening mammography is less than ideal, about 10% of women are called back for additional imaging, and 10% of that population will undergo a biopsy. Only 30% of these biopsies will result in a diagnosis of cancer.
There has been considerable debate about the mortality improvement with routine screening mammography, including a nationwide administrative data review published in late 2012.4 This data-mining study suggested that routine screening mammography was only a minor contributor to the significant improvement in breast cancer mortality over the past 25 years.
Related article: Update on breast health Mark D. Pearlman, MD, and Jennifer L. Griffin Miller, MD, MPH (March 2013)
Details of the study
In this failure analysis from Massachusetts General Hospital and Brigham and Women’s Hospital, Webb and colleagues reviewed breast cancer deaths over 17 years (new breast cancer cases from 1990 through 1997, with cases followed through 2007). They compared the likelihood of death according to whether screening mammography had been performed in the preceding 2 years.
Of the 609 confirmed deaths from breast cancer, 71% occurred among unscreened women (ie, never screened or screening not done in the preceding 2 years). The median age at diagnosis of fatal cancers was 49 years, whereas women with breast cancer who ultimately died of other causes had a median age at diagnosis of 72 years.
Related article: Biennial vs annual mammography: How I manage my patients Andrew M. Kaunitz, MD (June 2013)
Webb and colleagues concluded: “Most deaths from breast cancer occur in unscreened women. To maximize mortality reduction and life-years gained, initiation of regular screening before age 50 years should be encouraged.”
WHAT THIS EVIDENCE MEANS FOR PRACTICE
The findings of Webb and colleagues are not surprising, given the already demonstrated reduction in breast cancer mortality with the use of routine screening mammography in women in their 40s. What is particularly interesting in this study is that the preponderance of breast cancer deaths occurred in younger women. Biologically, this may be explained by the more aggressive nature of breast cancer in younger women.5
This study provides additional support for the ACOG recommendation to routinely offer breast cancer screening with mammography for women aged 40 to 49 years.
--Mark D. Pearlman, MD
We want to hear from you. Tell us what you think.
In 2009, the United States Preventive Services Task Force (USPSTF) released a controversial recommendation that breast cancer screening should routinely start at age 50 and be performed biennially.1 The Task Force further stated that the determination to screen women before the age of 50 “should take patient context into account, including the patient’s values regarding specific benefits and harms.”1
Although some professional societies have adopted this recommendation, others, including the American College of Obstetricians and Gynecologists (ACOG), have recommended that annual screening begin at age 40 in average-risk women.2
Related article: Is "overdiagnosis" of breast cancer common among women screened by mammography? Andrew M. Kaunitz, MD (January 2013)
With different professional societies lined up on both sides of this debate, practitioners and women remain confused about why different breast cancer screening guidelines exist in the United States.
Putting the study in context
A review of the data that informed the USPSTF recommendation demonstrate that routine screening mammography has similar efficacy in women in their fifth and sixth decade of life—a reduction in mortality of 16% and 15%, respectively.3 However, more mammograms need to be performed in women in their 40s than in women in their 50s to achieve that benefit. Moreover, because the specificity of screening mammography is less than ideal, about 10% of women are called back for additional imaging, and 10% of that population will undergo a biopsy. Only 30% of these biopsies will result in a diagnosis of cancer.
There has been considerable debate about the mortality improvement with routine screening mammography, including a nationwide administrative data review published in late 2012.4 This data-mining study suggested that routine screening mammography was only a minor contributor to the significant improvement in breast cancer mortality over the past 25 years.
Related article: Update on breast health Mark D. Pearlman, MD, and Jennifer L. Griffin Miller, MD, MPH (March 2013)
Details of the study
In this failure analysis from Massachusetts General Hospital and Brigham and Women’s Hospital, Webb and colleagues reviewed breast cancer deaths over 17 years (new breast cancer cases from 1990 through 1997, with cases followed through 2007). They compared the likelihood of death according to whether screening mammography had been performed in the preceding 2 years.
Of the 609 confirmed deaths from breast cancer, 71% occurred among unscreened women (ie, never screened or screening not done in the preceding 2 years). The median age at diagnosis of fatal cancers was 49 years, whereas women with breast cancer who ultimately died of other causes had a median age at diagnosis of 72 years.
Related article: Biennial vs annual mammography: How I manage my patients Andrew M. Kaunitz, MD (June 2013)
Webb and colleagues concluded: “Most deaths from breast cancer occur in unscreened women. To maximize mortality reduction and life-years gained, initiation of regular screening before age 50 years should be encouraged.”
WHAT THIS EVIDENCE MEANS FOR PRACTICE
The findings of Webb and colleagues are not surprising, given the already demonstrated reduction in breast cancer mortality with the use of routine screening mammography in women in their 40s. What is particularly interesting in this study is that the preponderance of breast cancer deaths occurred in younger women. Biologically, this may be explained by the more aggressive nature of breast cancer in younger women.5
This study provides additional support for the ACOG recommendation to routinely offer breast cancer screening with mammography for women aged 40 to 49 years.
--Mark D. Pearlman, MD
We want to hear from you. Tell us what you think.
- US Preventive Services Task Force. Screening for Breast Cancer. http://www.uspreventiveservicestaskforce.org
/uspstf/uspsbrca.htm. Accessed October 22, 2013. - American College of Obstetricians and Gynecologists. Practice Bulletin No. 122: Breast cancer screening. Obstet Gynecol. 2011;118(2 Pt 1):372–382.
- Nelson HD, Tyne K, Naik A, Bougatsos C, Chan BK, Humphrey L. Screening for breast cancer: An update for the US Preventive Services Task Force. Ann Intern Med. 2009;151(10):727–737.
- Bleyer A, Welch HG. Effect of three decades of screening mammography on breast cancer incidence. N Engl J Med. 2012;367(21):1998–2005.
- Klauber-DeMore N. Tumor biology of breast cancer in young women. Breast Dis. 2006;23:9–15.
- US Preventive Services Task Force. Screening for Breast Cancer. http://www.uspreventiveservicestaskforce.org
/uspstf/uspsbrca.htm. Accessed October 22, 2013. - American College of Obstetricians and Gynecologists. Practice Bulletin No. 122: Breast cancer screening. Obstet Gynecol. 2011;118(2 Pt 1):372–382.
- Nelson HD, Tyne K, Naik A, Bougatsos C, Chan BK, Humphrey L. Screening for breast cancer: An update for the US Preventive Services Task Force. Ann Intern Med. 2009;151(10):727–737.
- Bleyer A, Welch HG. Effect of three decades of screening mammography on breast cancer incidence. N Engl J Med. 2012;367(21):1998–2005.
- Klauber-DeMore N. Tumor biology of breast cancer in young women. Breast Dis. 2006;23:9–15.