User login
Noninvasive prenatal testing: Where we are and where we’re going
The introduction of amniocentesis in the 1960s brought to prenatal diagnosticians the ability to detect fetal chromosome abnormalities and certain structural defects (including neural tube defects). Since that time, a goal for these practitioners has been the development of effective screening algorithms to better identify women at high risk for detectable fetal abnormalities in concert with the advent of safer and more accessible diagnostic tests, with the eventual aim being the development of a noninvasive prenatal diagnostic test.
Postamniocentesis advancements have included the identification of maternal serum analytes as well as the incorporation of first-trimester ultrasonographic measurements of the fetal nuchal translucency (NT) and nasal bone, all associated with an improved ability to identify women at increased risk for fetal trisomies 21 and 18 as well as some other fetal abnormalities. In addition, targeted ultrasound has greatly improved the ability to detect fetal structural and growth abnormalities in women of all risk levels, although it remains a highly subjective process with considerable inter/intraoperator and equipment variability.
Related article: NIPT is expanding rapidly--but don't throw out that CVS kit just yet! (Update on Obstetrics; Jaimey M. Pauli, MD, and John T. Repke, MD; January 2014)
Noninvasive prenatal screening has the advantages of being noninvasive and carrying no increased risk for fetal loss compared with chorionic villus sampling (CVS) and amniocentesis, which are associated with a small increased risk for pregnancy loss (1/500 to 1/1,500 over baseline risk for loss). However, noninvasive screening is limited compared with diagnostic procedures because it provides only a risk adjustment rather than a definitive diagnostic outcome and is mostly limited to assessment for fetal trisomies 18 and 21.
Targeted ultrasound can identify structural abnormalities associated with other chromosomal, genetic, and genomic abnormalities, but again depends on operator experience, equipment used, maternal habitus, and fetal position. Accordingly, considerable interest has remained in developing a more effective approach for detecting fetal aneuploidy and other fetal abnormalities, including assays that eventually could serve to provide noninvasive prenatal diagnosis.
RECENT ADVANCES BRING US CLOSER TO OUR ULTIMATE GOAL
The recent introduction of circulating cell-free nucleic acids (ccfna) technologies for prenatal screening for common fetal aneuploidies, better known as noninvasive prenatal testing, or NIPT, has presented a far more effective prenatal screening protocol for certain groups of women compared with the aforementioned screening algorithms that rely on measurements of the fetal NT in the late first trimester and maternal serum measurements of analytes in the first and second trimesters.
Currently, four NIPT screening products are available commercially in the United States: MaterniT21 Plus (Sequenom, San Diego, California); Verifi (Illumina, San Diego, California); Harmony Prenatal Test (Ariosa Diagnostics, San Jose, California); and Panorama Prenatal Test (Natera, San Carlos, California). While the technologies and algorithms used by each of the companies differ, they all rely on the premise that 5% to 10% of ccfna in maternal blood are fetal in nature.1 Calculating the ratios of the expected amount of each chromosome-specific nucleic acid to that actually measured in the sample, a prediction of a normal or abnormal complement for that specific chromosome is then made. None of the commercially available tests specifically identify fetal DNA or differentiate fetal from maternal DNA.
Current validation studies have thus far limited the offering of NIPT to women at increased risk for fetal aneuploidy, including those:2–6
- of advanced maternal age
- with a positive conventional screening test
- with abnormal ultrasound results suggestive of aneuploidy, or
- who have had a prior pregnancy with a chromosome aneuploidy found in the NIPT panel.
Studies of all available technologies tested on women at increased risk for fetal aneuploidy have thus far shown considerably higher sensitivities and specificities and detection rates for fetal trisomies 21, 18, and 13 than conventional screening algorithms, although detection rates for trisomy 13 are slightly lower than those observed for trisomies 21 and 18.
WE STILL HAVE MANY HURDLES TO LEAP
However, the groups of women at high risk for fetal aneuploidy just outlined represent only a small segment of the community of pregnant women. A multicenter study involving 1,914 women published February 2014 in the New England Journal of Medicine7 showed considerably and significantly lower false-positive rates and higher positive predictive values for the detection of trisomies 21 and 18 by NIPT compared with conventional fetal aneuploidy screening. This study incorporated women at low risk for fetal aneuploidies in the study cohort, although women at high risk (based on the stated range of maternal age) also were included in the cohort. Unfortunately, no information was provided in the report about the percentage of low-risk women among the study participants.
Related articles:
Noninvasive prenatal DNA testing: Who is using it, and how? Audiocast, June 2013
Noninvasive prenatal DNA tests are unproven and costly David A. Carpenter, MD (Comment & Controversy; September 2013)
Another concern about the published accuracy of NIPT clinical assays was recently sounded by Menutti and colleagues.8 The authors cited recent cases of positive NIPT outcomes for fetal trisomies 18 and 13 that were not confirmed by diagnostic testing of the pregnancies in question. The authors pondered whether such cases may reflect a limitation of the positive predictive values attributed to NIPT assays and that such limitations may carry profound inaccuracies in determining the accuracy of such protocols for rare aneuploidies.
While the improved detection rates for NIPT compared with conventional screening are not surprising, guidelines published by the American College of Obstetricians and Gynecologists still do not recommend the use of NIPT for the screening of low-risk women because of insufficient evaluation of ccfna technologies in the screening of such pregnancies.3 This also applies to twin pregnancies, despite preliminary studies showing comparable detection of trisomies 18 and 21 in such pregnancies compared with singleton pregnancies.3,9
There are no direct comparative studies of the four commercially available screening products, thus precluding a robust comparison and determination of the best existing method to use.
SO, WHERE ARE WE WITH NIPT EXACTLY?
The recent introduction of NIPT into routine obstetric care has left many clinicians with a wide range of questions, many of which cannot be answered because of little or no information, robust or otherwise, to formulate an accurate and cogent response. So let’s state what we know based on the available evidence, recognizing that this will likely change, perhaps considerably, in the weeks and months ahead.
NIPT is a far superior approach, compared with conventional screening approaches, to screening for fetal trisomies 21, 18, and 13 in women carrying singleton pregnancies who are at an increased risk for fetal chromosome abnormalities.
In our current understanding of prenatal screening and diagnosis, NIPT does not provide either the comprehensive approach or the diagnostic accuracy associated with CVS and amniocentesis. As such, NIPT is not a suitable replacement for prenatal diagnostic procedures.
However, its application to screening a low-risk population for the common fetal aneuploidies, as well as in twin pregnancies, has been supported by initial studies, and the inclusion of other clinical outcomes—including other chromosome abnormalities, such as X and Y aneuploidies, trisomy 16, and triploidy10,11 and certain genomic abnormalities (eg, 22q deletions)—in the screening algorithm will expand the future clinical applications of NIPT screening.
DOES NIPT CHANGE OUR CONCEPTS OF SCREENING AND DIAGNOSIS?
This question is simple but profound and is perhaps the most important to be asked and addressed. Is a screening algorithm that has a similar sensitivity and specificity to that of CVS and amniocentesis for the most common fetal trisomies in the first and second trimesters sufficient to replace invasive testing for most women? Does the ability to detect fetal genomic abnormalities with microarray analyses of fetal cells obtained by CVS or amniocentesis provide a far greater benefit than that possible with any screening algorithm?
With renewed interest in the cost of health-care screening and diagnosis, we need to consider how comprehensive and accurate our prenatal screening and diagnostic tests should be and whether such improvements are desired or even possible from a clinical or economic viewpoint. In addition, the development of new technologies, such as the capture and analysis of fetal cells in maternal blood, presents the potential for a direct diagnostic fetal assay without the risks of an invasive procedure.
BIAS-FREE COUNSELING CANNOT BE OVERLOOKED
That being said, the current role of NIPT and other screening protocols in obstetric care needs to be clearly communicated to women who are considering their fetal assessment options, with emphasis placed on the capabilities and limitations of prenatal screening (even the newer ccfna-based options), the actual risks associated with invasive testing, and the ability of invasive testing to provide expanded fetal information with the use of microarray analyses.
As it has been from the beginning of prenatal testing in the 1960s, counseling continues to be the most important part of the prenatal screening and diagnostic process and it is needed to facilitate clinical decisions made by women and couples. Counseling must include an accurate communication of the risks, benefits, and limitations of the aforementioned options and issues, and should be provided in a manner that strives to be free of bias, direction, and the personal opinions of the counselor.
In order to provide such counseling, we must remain informed of the ongoing work in the field of prenatal testing, a task that has become more challenging with the rapid release of a considerable amount of new information on prenatal screening technologies over the past 2 years. This will likely continue, and perhaps become even more frenetic, with the expected release of additional information on the clinical applications of ccfna technologies in the near future as well as the development of new technologies applicable for the screening and diagnosis of fetal abnormalities.
WE WANT TO HEAR FROM YOU!
Share your thoughts on this article. Send your letter to: [email protected] Please include the city and state in which you practice.
- Lo YM, Corbetta N, Chamberlain PF, et al. Presence of fetal DNA in maternal plasma and serum. Lancet. 1997;350(9076):485–487.
- Ashoor G, Syngelaki A, Wagner M, Birdir C, Nicolaides KH. Chromosome-selective sequencing of maternal plasma cell–free DNA for first-trimester detection of trisomy 21 and trisomy 18. Am J Obstet Gynecol. 2012;206(4):322.e1–e5.
- American College of Obstetricians and Gynecologists Committee on Genetics. Committee Opinion No. 545: Noninvasive prenatal testing for fetal aneuploidy. Obstet Gynecol. 2012;120(6):1532–1534.
- Bianchi DW, Platt LD, Goldberg JD, et al; MatErnal Blood IS Source to Accurately diagnose fetal aneuploidy (MELISSA) Study Group. Genome-wide fetal aneuploidy detection by maternal plasma DNA-sequencing. Obstet Gynecol. 2012;119(5):890–901.
- Palomaki GE, Kloza EM, Lambert-Messerlian GM, et al. DNA sequencing of maternal plasma to detect Down syndrome: An international clinical validation study. Genet Med. 2011;13(11):913–920.
- Palomaki GE, Deciu C, Kloza EM, et al. DNA sequencing of maternal plasma reliably identifies trisomy 18 and trisomy 13 as well as Down syndrome: An international collaborative study. Genet Med. 2012;14(3):296–305.
- Bianchi DW, Parker RL, Wentworth J, et al; CARE Study Group. DNA sequencing versus standard prenatal aneuploidy screening. N Engl J Med. 2014;370(9):799–808.
- Menutti MT, Cherry AM, Morrissette JJ, Dugoff L. Is it time to sound an alarm about false-positive cell-free DNA testing for fetal aneuploidy. Am J Obstet Gynecol. 2013;209(5):415−419.
- Canick JA, Kloza EM, Lambert-Messerlian GM, et al. DNA sequencing of maternal plasma to identify Down syndrome and other trisomies in multiple gestations. Prenat Diagn. 2012;32(8):730–734.
- Nicolaides KH, Syngelaki A, Gil MM, Quezada MS, Zinevich Y. Prenatal detection of fetal triploidy from cell-free DNA testing in maternal blood [published online ahead of print October 10, 2013]. Fetal Diagn Ther.
- Semango-Sprouse C, Banjevic M, Ryan A, et al. SNP-based non-invasive prenatal testing detects sex chromosome aneuploidies with high accuracy. Prenat Diagn. 2013;33(7):643–649.
The introduction of amniocentesis in the 1960s brought to prenatal diagnosticians the ability to detect fetal chromosome abnormalities and certain structural defects (including neural tube defects). Since that time, a goal for these practitioners has been the development of effective screening algorithms to better identify women at high risk for detectable fetal abnormalities in concert with the advent of safer and more accessible diagnostic tests, with the eventual aim being the development of a noninvasive prenatal diagnostic test.
Postamniocentesis advancements have included the identification of maternal serum analytes as well as the incorporation of first-trimester ultrasonographic measurements of the fetal nuchal translucency (NT) and nasal bone, all associated with an improved ability to identify women at increased risk for fetal trisomies 21 and 18 as well as some other fetal abnormalities. In addition, targeted ultrasound has greatly improved the ability to detect fetal structural and growth abnormalities in women of all risk levels, although it remains a highly subjective process with considerable inter/intraoperator and equipment variability.
Related article: NIPT is expanding rapidly--but don't throw out that CVS kit just yet! (Update on Obstetrics; Jaimey M. Pauli, MD, and John T. Repke, MD; January 2014)
Noninvasive prenatal screening has the advantages of being noninvasive and carrying no increased risk for fetal loss compared with chorionic villus sampling (CVS) and amniocentesis, which are associated with a small increased risk for pregnancy loss (1/500 to 1/1,500 over baseline risk for loss). However, noninvasive screening is limited compared with diagnostic procedures because it provides only a risk adjustment rather than a definitive diagnostic outcome and is mostly limited to assessment for fetal trisomies 18 and 21.
Targeted ultrasound can identify structural abnormalities associated with other chromosomal, genetic, and genomic abnormalities, but again depends on operator experience, equipment used, maternal habitus, and fetal position. Accordingly, considerable interest has remained in developing a more effective approach for detecting fetal aneuploidy and other fetal abnormalities, including assays that eventually could serve to provide noninvasive prenatal diagnosis.
RECENT ADVANCES BRING US CLOSER TO OUR ULTIMATE GOAL
The recent introduction of circulating cell-free nucleic acids (ccfna) technologies for prenatal screening for common fetal aneuploidies, better known as noninvasive prenatal testing, or NIPT, has presented a far more effective prenatal screening protocol for certain groups of women compared with the aforementioned screening algorithms that rely on measurements of the fetal NT in the late first trimester and maternal serum measurements of analytes in the first and second trimesters.
Currently, four NIPT screening products are available commercially in the United States: MaterniT21 Plus (Sequenom, San Diego, California); Verifi (Illumina, San Diego, California); Harmony Prenatal Test (Ariosa Diagnostics, San Jose, California); and Panorama Prenatal Test (Natera, San Carlos, California). While the technologies and algorithms used by each of the companies differ, they all rely on the premise that 5% to 10% of ccfna in maternal blood are fetal in nature.1 Calculating the ratios of the expected amount of each chromosome-specific nucleic acid to that actually measured in the sample, a prediction of a normal or abnormal complement for that specific chromosome is then made. None of the commercially available tests specifically identify fetal DNA or differentiate fetal from maternal DNA.
Current validation studies have thus far limited the offering of NIPT to women at increased risk for fetal aneuploidy, including those:2–6
- of advanced maternal age
- with a positive conventional screening test
- with abnormal ultrasound results suggestive of aneuploidy, or
- who have had a prior pregnancy with a chromosome aneuploidy found in the NIPT panel.
Studies of all available technologies tested on women at increased risk for fetal aneuploidy have thus far shown considerably higher sensitivities and specificities and detection rates for fetal trisomies 21, 18, and 13 than conventional screening algorithms, although detection rates for trisomy 13 are slightly lower than those observed for trisomies 21 and 18.
WE STILL HAVE MANY HURDLES TO LEAP
However, the groups of women at high risk for fetal aneuploidy just outlined represent only a small segment of the community of pregnant women. A multicenter study involving 1,914 women published February 2014 in the New England Journal of Medicine7 showed considerably and significantly lower false-positive rates and higher positive predictive values for the detection of trisomies 21 and 18 by NIPT compared with conventional fetal aneuploidy screening. This study incorporated women at low risk for fetal aneuploidies in the study cohort, although women at high risk (based on the stated range of maternal age) also were included in the cohort. Unfortunately, no information was provided in the report about the percentage of low-risk women among the study participants.
Related articles:
Noninvasive prenatal DNA testing: Who is using it, and how? Audiocast, June 2013
Noninvasive prenatal DNA tests are unproven and costly David A. Carpenter, MD (Comment & Controversy; September 2013)
Another concern about the published accuracy of NIPT clinical assays was recently sounded by Menutti and colleagues.8 The authors cited recent cases of positive NIPT outcomes for fetal trisomies 18 and 13 that were not confirmed by diagnostic testing of the pregnancies in question. The authors pondered whether such cases may reflect a limitation of the positive predictive values attributed to NIPT assays and that such limitations may carry profound inaccuracies in determining the accuracy of such protocols for rare aneuploidies.
While the improved detection rates for NIPT compared with conventional screening are not surprising, guidelines published by the American College of Obstetricians and Gynecologists still do not recommend the use of NIPT for the screening of low-risk women because of insufficient evaluation of ccfna technologies in the screening of such pregnancies.3 This also applies to twin pregnancies, despite preliminary studies showing comparable detection of trisomies 18 and 21 in such pregnancies compared with singleton pregnancies.3,9
There are no direct comparative studies of the four commercially available screening products, thus precluding a robust comparison and determination of the best existing method to use.
SO, WHERE ARE WE WITH NIPT EXACTLY?
The recent introduction of NIPT into routine obstetric care has left many clinicians with a wide range of questions, many of which cannot be answered because of little or no information, robust or otherwise, to formulate an accurate and cogent response. So let’s state what we know based on the available evidence, recognizing that this will likely change, perhaps considerably, in the weeks and months ahead.
NIPT is a far superior approach, compared with conventional screening approaches, to screening for fetal trisomies 21, 18, and 13 in women carrying singleton pregnancies who are at an increased risk for fetal chromosome abnormalities.
In our current understanding of prenatal screening and diagnosis, NIPT does not provide either the comprehensive approach or the diagnostic accuracy associated with CVS and amniocentesis. As such, NIPT is not a suitable replacement for prenatal diagnostic procedures.
However, its application to screening a low-risk population for the common fetal aneuploidies, as well as in twin pregnancies, has been supported by initial studies, and the inclusion of other clinical outcomes—including other chromosome abnormalities, such as X and Y aneuploidies, trisomy 16, and triploidy10,11 and certain genomic abnormalities (eg, 22q deletions)—in the screening algorithm will expand the future clinical applications of NIPT screening.
DOES NIPT CHANGE OUR CONCEPTS OF SCREENING AND DIAGNOSIS?
This question is simple but profound and is perhaps the most important to be asked and addressed. Is a screening algorithm that has a similar sensitivity and specificity to that of CVS and amniocentesis for the most common fetal trisomies in the first and second trimesters sufficient to replace invasive testing for most women? Does the ability to detect fetal genomic abnormalities with microarray analyses of fetal cells obtained by CVS or amniocentesis provide a far greater benefit than that possible with any screening algorithm?
With renewed interest in the cost of health-care screening and diagnosis, we need to consider how comprehensive and accurate our prenatal screening and diagnostic tests should be and whether such improvements are desired or even possible from a clinical or economic viewpoint. In addition, the development of new technologies, such as the capture and analysis of fetal cells in maternal blood, presents the potential for a direct diagnostic fetal assay without the risks of an invasive procedure.
BIAS-FREE COUNSELING CANNOT BE OVERLOOKED
That being said, the current role of NIPT and other screening protocols in obstetric care needs to be clearly communicated to women who are considering their fetal assessment options, with emphasis placed on the capabilities and limitations of prenatal screening (even the newer ccfna-based options), the actual risks associated with invasive testing, and the ability of invasive testing to provide expanded fetal information with the use of microarray analyses.
As it has been from the beginning of prenatal testing in the 1960s, counseling continues to be the most important part of the prenatal screening and diagnostic process and it is needed to facilitate clinical decisions made by women and couples. Counseling must include an accurate communication of the risks, benefits, and limitations of the aforementioned options and issues, and should be provided in a manner that strives to be free of bias, direction, and the personal opinions of the counselor.
In order to provide such counseling, we must remain informed of the ongoing work in the field of prenatal testing, a task that has become more challenging with the rapid release of a considerable amount of new information on prenatal screening technologies over the past 2 years. This will likely continue, and perhaps become even more frenetic, with the expected release of additional information on the clinical applications of ccfna technologies in the near future as well as the development of new technologies applicable for the screening and diagnosis of fetal abnormalities.
WE WANT TO HEAR FROM YOU!
Share your thoughts on this article. Send your letter to: [email protected] Please include the city and state in which you practice.
The introduction of amniocentesis in the 1960s brought to prenatal diagnosticians the ability to detect fetal chromosome abnormalities and certain structural defects (including neural tube defects). Since that time, a goal for these practitioners has been the development of effective screening algorithms to better identify women at high risk for detectable fetal abnormalities in concert with the advent of safer and more accessible diagnostic tests, with the eventual aim being the development of a noninvasive prenatal diagnostic test.
Postamniocentesis advancements have included the identification of maternal serum analytes as well as the incorporation of first-trimester ultrasonographic measurements of the fetal nuchal translucency (NT) and nasal bone, all associated with an improved ability to identify women at increased risk for fetal trisomies 21 and 18 as well as some other fetal abnormalities. In addition, targeted ultrasound has greatly improved the ability to detect fetal structural and growth abnormalities in women of all risk levels, although it remains a highly subjective process with considerable inter/intraoperator and equipment variability.
Related article: NIPT is expanding rapidly--but don't throw out that CVS kit just yet! (Update on Obstetrics; Jaimey M. Pauli, MD, and John T. Repke, MD; January 2014)
Noninvasive prenatal screening has the advantages of being noninvasive and carrying no increased risk for fetal loss compared with chorionic villus sampling (CVS) and amniocentesis, which are associated with a small increased risk for pregnancy loss (1/500 to 1/1,500 over baseline risk for loss). However, noninvasive screening is limited compared with diagnostic procedures because it provides only a risk adjustment rather than a definitive diagnostic outcome and is mostly limited to assessment for fetal trisomies 18 and 21.
Targeted ultrasound can identify structural abnormalities associated with other chromosomal, genetic, and genomic abnormalities, but again depends on operator experience, equipment used, maternal habitus, and fetal position. Accordingly, considerable interest has remained in developing a more effective approach for detecting fetal aneuploidy and other fetal abnormalities, including assays that eventually could serve to provide noninvasive prenatal diagnosis.
RECENT ADVANCES BRING US CLOSER TO OUR ULTIMATE GOAL
The recent introduction of circulating cell-free nucleic acids (ccfna) technologies for prenatal screening for common fetal aneuploidies, better known as noninvasive prenatal testing, or NIPT, has presented a far more effective prenatal screening protocol for certain groups of women compared with the aforementioned screening algorithms that rely on measurements of the fetal NT in the late first trimester and maternal serum measurements of analytes in the first and second trimesters.
Currently, four NIPT screening products are available commercially in the United States: MaterniT21 Plus (Sequenom, San Diego, California); Verifi (Illumina, San Diego, California); Harmony Prenatal Test (Ariosa Diagnostics, San Jose, California); and Panorama Prenatal Test (Natera, San Carlos, California). While the technologies and algorithms used by each of the companies differ, they all rely on the premise that 5% to 10% of ccfna in maternal blood are fetal in nature.1 Calculating the ratios of the expected amount of each chromosome-specific nucleic acid to that actually measured in the sample, a prediction of a normal or abnormal complement for that specific chromosome is then made. None of the commercially available tests specifically identify fetal DNA or differentiate fetal from maternal DNA.
Current validation studies have thus far limited the offering of NIPT to women at increased risk for fetal aneuploidy, including those:2–6
- of advanced maternal age
- with a positive conventional screening test
- with abnormal ultrasound results suggestive of aneuploidy, or
- who have had a prior pregnancy with a chromosome aneuploidy found in the NIPT panel.
Studies of all available technologies tested on women at increased risk for fetal aneuploidy have thus far shown considerably higher sensitivities and specificities and detection rates for fetal trisomies 21, 18, and 13 than conventional screening algorithms, although detection rates for trisomy 13 are slightly lower than those observed for trisomies 21 and 18.
WE STILL HAVE MANY HURDLES TO LEAP
However, the groups of women at high risk for fetal aneuploidy just outlined represent only a small segment of the community of pregnant women. A multicenter study involving 1,914 women published February 2014 in the New England Journal of Medicine7 showed considerably and significantly lower false-positive rates and higher positive predictive values for the detection of trisomies 21 and 18 by NIPT compared with conventional fetal aneuploidy screening. This study incorporated women at low risk for fetal aneuploidies in the study cohort, although women at high risk (based on the stated range of maternal age) also were included in the cohort. Unfortunately, no information was provided in the report about the percentage of low-risk women among the study participants.
Related articles:
Noninvasive prenatal DNA testing: Who is using it, and how? Audiocast, June 2013
Noninvasive prenatal DNA tests are unproven and costly David A. Carpenter, MD (Comment & Controversy; September 2013)
Another concern about the published accuracy of NIPT clinical assays was recently sounded by Menutti and colleagues.8 The authors cited recent cases of positive NIPT outcomes for fetal trisomies 18 and 13 that were not confirmed by diagnostic testing of the pregnancies in question. The authors pondered whether such cases may reflect a limitation of the positive predictive values attributed to NIPT assays and that such limitations may carry profound inaccuracies in determining the accuracy of such protocols for rare aneuploidies.
While the improved detection rates for NIPT compared with conventional screening are not surprising, guidelines published by the American College of Obstetricians and Gynecologists still do not recommend the use of NIPT for the screening of low-risk women because of insufficient evaluation of ccfna technologies in the screening of such pregnancies.3 This also applies to twin pregnancies, despite preliminary studies showing comparable detection of trisomies 18 and 21 in such pregnancies compared with singleton pregnancies.3,9
There are no direct comparative studies of the four commercially available screening products, thus precluding a robust comparison and determination of the best existing method to use.
SO, WHERE ARE WE WITH NIPT EXACTLY?
The recent introduction of NIPT into routine obstetric care has left many clinicians with a wide range of questions, many of which cannot be answered because of little or no information, robust or otherwise, to formulate an accurate and cogent response. So let’s state what we know based on the available evidence, recognizing that this will likely change, perhaps considerably, in the weeks and months ahead.
NIPT is a far superior approach, compared with conventional screening approaches, to screening for fetal trisomies 21, 18, and 13 in women carrying singleton pregnancies who are at an increased risk for fetal chromosome abnormalities.
In our current understanding of prenatal screening and diagnosis, NIPT does not provide either the comprehensive approach or the diagnostic accuracy associated with CVS and amniocentesis. As such, NIPT is not a suitable replacement for prenatal diagnostic procedures.
However, its application to screening a low-risk population for the common fetal aneuploidies, as well as in twin pregnancies, has been supported by initial studies, and the inclusion of other clinical outcomes—including other chromosome abnormalities, such as X and Y aneuploidies, trisomy 16, and triploidy10,11 and certain genomic abnormalities (eg, 22q deletions)—in the screening algorithm will expand the future clinical applications of NIPT screening.
DOES NIPT CHANGE OUR CONCEPTS OF SCREENING AND DIAGNOSIS?
This question is simple but profound and is perhaps the most important to be asked and addressed. Is a screening algorithm that has a similar sensitivity and specificity to that of CVS and amniocentesis for the most common fetal trisomies in the first and second trimesters sufficient to replace invasive testing for most women? Does the ability to detect fetal genomic abnormalities with microarray analyses of fetal cells obtained by CVS or amniocentesis provide a far greater benefit than that possible with any screening algorithm?
With renewed interest in the cost of health-care screening and diagnosis, we need to consider how comprehensive and accurate our prenatal screening and diagnostic tests should be and whether such improvements are desired or even possible from a clinical or economic viewpoint. In addition, the development of new technologies, such as the capture and analysis of fetal cells in maternal blood, presents the potential for a direct diagnostic fetal assay without the risks of an invasive procedure.
BIAS-FREE COUNSELING CANNOT BE OVERLOOKED
That being said, the current role of NIPT and other screening protocols in obstetric care needs to be clearly communicated to women who are considering their fetal assessment options, with emphasis placed on the capabilities and limitations of prenatal screening (even the newer ccfna-based options), the actual risks associated with invasive testing, and the ability of invasive testing to provide expanded fetal information with the use of microarray analyses.
As it has been from the beginning of prenatal testing in the 1960s, counseling continues to be the most important part of the prenatal screening and diagnostic process and it is needed to facilitate clinical decisions made by women and couples. Counseling must include an accurate communication of the risks, benefits, and limitations of the aforementioned options and issues, and should be provided in a manner that strives to be free of bias, direction, and the personal opinions of the counselor.
In order to provide such counseling, we must remain informed of the ongoing work in the field of prenatal testing, a task that has become more challenging with the rapid release of a considerable amount of new information on prenatal screening technologies over the past 2 years. This will likely continue, and perhaps become even more frenetic, with the expected release of additional information on the clinical applications of ccfna technologies in the near future as well as the development of new technologies applicable for the screening and diagnosis of fetal abnormalities.
WE WANT TO HEAR FROM YOU!
Share your thoughts on this article. Send your letter to: [email protected] Please include the city and state in which you practice.
- Lo YM, Corbetta N, Chamberlain PF, et al. Presence of fetal DNA in maternal plasma and serum. Lancet. 1997;350(9076):485–487.
- Ashoor G, Syngelaki A, Wagner M, Birdir C, Nicolaides KH. Chromosome-selective sequencing of maternal plasma cell–free DNA for first-trimester detection of trisomy 21 and trisomy 18. Am J Obstet Gynecol. 2012;206(4):322.e1–e5.
- American College of Obstetricians and Gynecologists Committee on Genetics. Committee Opinion No. 545: Noninvasive prenatal testing for fetal aneuploidy. Obstet Gynecol. 2012;120(6):1532–1534.
- Bianchi DW, Platt LD, Goldberg JD, et al; MatErnal Blood IS Source to Accurately diagnose fetal aneuploidy (MELISSA) Study Group. Genome-wide fetal aneuploidy detection by maternal plasma DNA-sequencing. Obstet Gynecol. 2012;119(5):890–901.
- Palomaki GE, Kloza EM, Lambert-Messerlian GM, et al. DNA sequencing of maternal plasma to detect Down syndrome: An international clinical validation study. Genet Med. 2011;13(11):913–920.
- Palomaki GE, Deciu C, Kloza EM, et al. DNA sequencing of maternal plasma reliably identifies trisomy 18 and trisomy 13 as well as Down syndrome: An international collaborative study. Genet Med. 2012;14(3):296–305.
- Bianchi DW, Parker RL, Wentworth J, et al; CARE Study Group. DNA sequencing versus standard prenatal aneuploidy screening. N Engl J Med. 2014;370(9):799–808.
- Menutti MT, Cherry AM, Morrissette JJ, Dugoff L. Is it time to sound an alarm about false-positive cell-free DNA testing for fetal aneuploidy. Am J Obstet Gynecol. 2013;209(5):415−419.
- Canick JA, Kloza EM, Lambert-Messerlian GM, et al. DNA sequencing of maternal plasma to identify Down syndrome and other trisomies in multiple gestations. Prenat Diagn. 2012;32(8):730–734.
- Nicolaides KH, Syngelaki A, Gil MM, Quezada MS, Zinevich Y. Prenatal detection of fetal triploidy from cell-free DNA testing in maternal blood [published online ahead of print October 10, 2013]. Fetal Diagn Ther.
- Semango-Sprouse C, Banjevic M, Ryan A, et al. SNP-based non-invasive prenatal testing detects sex chromosome aneuploidies with high accuracy. Prenat Diagn. 2013;33(7):643–649.
- Lo YM, Corbetta N, Chamberlain PF, et al. Presence of fetal DNA in maternal plasma and serum. Lancet. 1997;350(9076):485–487.
- Ashoor G, Syngelaki A, Wagner M, Birdir C, Nicolaides KH. Chromosome-selective sequencing of maternal plasma cell–free DNA for first-trimester detection of trisomy 21 and trisomy 18. Am J Obstet Gynecol. 2012;206(4):322.e1–e5.
- American College of Obstetricians and Gynecologists Committee on Genetics. Committee Opinion No. 545: Noninvasive prenatal testing for fetal aneuploidy. Obstet Gynecol. 2012;120(6):1532–1534.
- Bianchi DW, Platt LD, Goldberg JD, et al; MatErnal Blood IS Source to Accurately diagnose fetal aneuploidy (MELISSA) Study Group. Genome-wide fetal aneuploidy detection by maternal plasma DNA-sequencing. Obstet Gynecol. 2012;119(5):890–901.
- Palomaki GE, Kloza EM, Lambert-Messerlian GM, et al. DNA sequencing of maternal plasma to detect Down syndrome: An international clinical validation study. Genet Med. 2011;13(11):913–920.
- Palomaki GE, Deciu C, Kloza EM, et al. DNA sequencing of maternal plasma reliably identifies trisomy 18 and trisomy 13 as well as Down syndrome: An international collaborative study. Genet Med. 2012;14(3):296–305.
- Bianchi DW, Parker RL, Wentworth J, et al; CARE Study Group. DNA sequencing versus standard prenatal aneuploidy screening. N Engl J Med. 2014;370(9):799–808.
- Menutti MT, Cherry AM, Morrissette JJ, Dugoff L. Is it time to sound an alarm about false-positive cell-free DNA testing for fetal aneuploidy. Am J Obstet Gynecol. 2013;209(5):415−419.
- Canick JA, Kloza EM, Lambert-Messerlian GM, et al. DNA sequencing of maternal plasma to identify Down syndrome and other trisomies in multiple gestations. Prenat Diagn. 2012;32(8):730–734.
- Nicolaides KH, Syngelaki A, Gil MM, Quezada MS, Zinevich Y. Prenatal detection of fetal triploidy from cell-free DNA testing in maternal blood [published online ahead of print October 10, 2013]. Fetal Diagn Ther.
- Semango-Sprouse C, Banjevic M, Ryan A, et al. SNP-based non-invasive prenatal testing detects sex chromosome aneuploidies with high accuracy. Prenat Diagn. 2013;33(7):643–649.
Adding infertility assessment and treatment to your practice
At the 62nd Annual Clinical Meeting of the American College of Obstetricians and Gynecologists, Dr. Jensen offered efficient strategies to generalist ObGyns to incorporate infertility assessment and treatment into their already busy practices. Here, a 7-minute audiocast with Dr. Jensen on the key takeaways from her seminar, "Managing infertility without IVF: The old-fashioned way."
At the 62nd Annual Clinical Meeting of the American College of Obstetricians and Gynecologists, Dr. Jensen offered efficient strategies to generalist ObGyns to incorporate infertility assessment and treatment into their already busy practices. Here, a 7-minute audiocast with Dr. Jensen on the key takeaways from her seminar, "Managing infertility without IVF: The old-fashioned way."
At the 62nd Annual Clinical Meeting of the American College of Obstetricians and Gynecologists, Dr. Jensen offered efficient strategies to generalist ObGyns to incorporate infertility assessment and treatment into their already busy practices. Here, a 7-minute audiocast with Dr. Jensen on the key takeaways from her seminar, "Managing infertility without IVF: The old-fashioned way."
Why it's important to open the sexual health dialogue
In this 5-minute audiocast, Dr. Krychman offers key takeaways from his seminar, "Sexuality in the Elder Woman," at the 62nd Annual Clinical Meeting of the American College of Obstetricians and Gynecologists.
In this 5-minute audiocast, Dr. Krychman offers key takeaways from his seminar, "Sexuality in the Elder Woman," at the 62nd Annual Clinical Meeting of the American College of Obstetricians and Gynecologists.
In this 5-minute audiocast, Dr. Krychman offers key takeaways from his seminar, "Sexuality in the Elder Woman," at the 62nd Annual Clinical Meeting of the American College of Obstetricians and Gynecologists.
Should the adnexae be removed during hysterectomy for benign disease to reduce the risk of ovarian cancer?
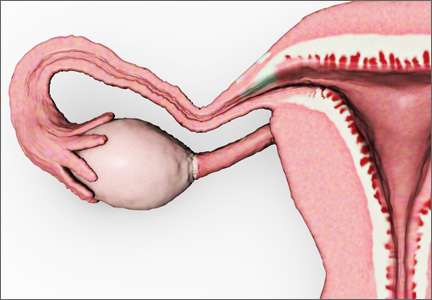
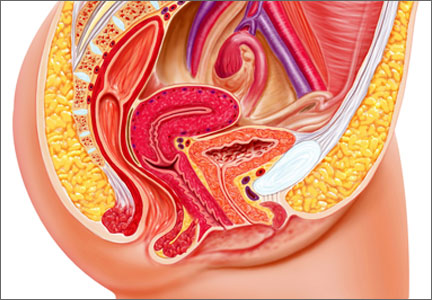
The decision-making surrounding gynecologic surgery for benign disease is increasingly complex. Patients and their physicians must balance the potential benefits of salpingo-oophorectomy against possible adverse consequence as they consider various health goals, including longevity, cancer risk, and quality of life.
Chan and colleagues add important data to our understanding of this equation. Analyzing a large cohort of patients from Kaiser Permanente Northern California who underwent hysterectomy for benign disease, they found that removal of the fallopian tubes and ovaries significantly reduced the risk of developing ovarian cancer. The incidence of ovarian cancer per 100,000 person-years was 26.2 for women undergoing hysterectomy alone (95% confidence interval [CI], 15.5–37.0), 17.5 for hysterectomy with unilateral salpingo-oophorectomy (95% CI, 0–39.1), and 1.7 for hysterectomy with bilateral salpingo-oophorectomy (95% CI, 0.4–3.0).
The hazard ratio (HR) for ovarian cancer was 0.58 for women undergoing unilateral salpingo-oophorectomy (95% CI, 0.18–1.90) and 0.12 for women undergoing bilateral salpingo-oophorectomy (95% CI, 0.05–0.28), compared with women undergoing hysterectomy alone.
Notable strengths of the analysis include the large size of the study population and the duration of patient follow-up (18 years). The authors acknowledge several limitations of the study, including the lack of data on BRCA mutation status and family history of cancer, as well as several other demographic data points possibly relevant to a risk of developing adnexal or peritoneal malignancy.
Related article: What is the gynecologist’s role in the care of BRCA previvors? Robert L. Barbieri, MD (Editorial, September 2013)
Keep these findings in context
As the authors discuss, this report should be considered in the context of other work suggesting that the lower mortality rate associated with ovarian conservation at the time of hysterectomy for benign disease arises mostly from a protective effect against cardiovascular disease (CVD)—perhaps from subclinical hormone production following menopause. Given that CVD remains the leading cause of death among American women, an individualized assessment of risk is necessary when planning the extent of surgery in this circumstance.
Related article: Oophorectomy or salpingectomy—which makes more sense? William H. Parker, MD (March 2014)
It also is interesting to consider the authors’ finding of a notable but statistically insignificant decrease in the risk of ovarian cancer associated with removal of only one tube and ovary. However, recognizing the possible limitations of their demographic information on this point, they suggest that this may be an area for further investigation, which would necessarily include characterization of the trends in the laterality of adnexal cancers. The preservation of hormonal function makes this an interesting option to consider.
We also need to further investigate the role of bilateral salpingectomy at the time of hysterectomy, with ovarian conservation, as an alternate therapeutic option, based upon evidence that extrauterine serous carcinoma may to a significant degree arise from the tubal epithelium rather than the ovarian cortex.
WHAT THIS EVIDENCE MEANS FOR PRACTICE
Removal of the adnexae significantly reduces the risk of ovarian cancer among the cohort of women undergoing hysterectomy for benign disease. However, the decision of whether or not to remove the adnexae when planning surgery should take into account other factors that may affect the risk of adnexal malignancy, including family history and BRCA mutation status, as well as other patient comorbidities.
Andrew W. Menzin, MD
Tell us what you think!
Drop us a line and let us know what you think about this or other current articles, which topics you'd like to see covered in future issues, and what challenges you face in daily practice. Tell us what you think by emailing us at: [email protected] Please include your name, city and state.
Stay in touch! Your feedback is important to us!
The decision-making surrounding gynecologic surgery for benign disease is increasingly complex. Patients and their physicians must balance the potential benefits of salpingo-oophorectomy against possible adverse consequence as they consider various health goals, including longevity, cancer risk, and quality of life.
Chan and colleagues add important data to our understanding of this equation. Analyzing a large cohort of patients from Kaiser Permanente Northern California who underwent hysterectomy for benign disease, they found that removal of the fallopian tubes and ovaries significantly reduced the risk of developing ovarian cancer. The incidence of ovarian cancer per 100,000 person-years was 26.2 for women undergoing hysterectomy alone (95% confidence interval [CI], 15.5–37.0), 17.5 for hysterectomy with unilateral salpingo-oophorectomy (95% CI, 0–39.1), and 1.7 for hysterectomy with bilateral salpingo-oophorectomy (95% CI, 0.4–3.0).
The hazard ratio (HR) for ovarian cancer was 0.58 for women undergoing unilateral salpingo-oophorectomy (95% CI, 0.18–1.90) and 0.12 for women undergoing bilateral salpingo-oophorectomy (95% CI, 0.05–0.28), compared with women undergoing hysterectomy alone.
Notable strengths of the analysis include the large size of the study population and the duration of patient follow-up (18 years). The authors acknowledge several limitations of the study, including the lack of data on BRCA mutation status and family history of cancer, as well as several other demographic data points possibly relevant to a risk of developing adnexal or peritoneal malignancy.
Related article: What is the gynecologist’s role in the care of BRCA previvors? Robert L. Barbieri, MD (Editorial, September 2013)
Keep these findings in context
As the authors discuss, this report should be considered in the context of other work suggesting that the lower mortality rate associated with ovarian conservation at the time of hysterectomy for benign disease arises mostly from a protective effect against cardiovascular disease (CVD)—perhaps from subclinical hormone production following menopause. Given that CVD remains the leading cause of death among American women, an individualized assessment of risk is necessary when planning the extent of surgery in this circumstance.
Related article: Oophorectomy or salpingectomy—which makes more sense? William H. Parker, MD (March 2014)
It also is interesting to consider the authors’ finding of a notable but statistically insignificant decrease in the risk of ovarian cancer associated with removal of only one tube and ovary. However, recognizing the possible limitations of their demographic information on this point, they suggest that this may be an area for further investigation, which would necessarily include characterization of the trends in the laterality of adnexal cancers. The preservation of hormonal function makes this an interesting option to consider.
We also need to further investigate the role of bilateral salpingectomy at the time of hysterectomy, with ovarian conservation, as an alternate therapeutic option, based upon evidence that extrauterine serous carcinoma may to a significant degree arise from the tubal epithelium rather than the ovarian cortex.
WHAT THIS EVIDENCE MEANS FOR PRACTICE
Removal of the adnexae significantly reduces the risk of ovarian cancer among the cohort of women undergoing hysterectomy for benign disease. However, the decision of whether or not to remove the adnexae when planning surgery should take into account other factors that may affect the risk of adnexal malignancy, including family history and BRCA mutation status, as well as other patient comorbidities.
Andrew W. Menzin, MD
Tell us what you think!
Drop us a line and let us know what you think about this or other current articles, which topics you'd like to see covered in future issues, and what challenges you face in daily practice. Tell us what you think by emailing us at: [email protected] Please include your name, city and state.
Stay in touch! Your feedback is important to us!
The decision-making surrounding gynecologic surgery for benign disease is increasingly complex. Patients and their physicians must balance the potential benefits of salpingo-oophorectomy against possible adverse consequence as they consider various health goals, including longevity, cancer risk, and quality of life.
Chan and colleagues add important data to our understanding of this equation. Analyzing a large cohort of patients from Kaiser Permanente Northern California who underwent hysterectomy for benign disease, they found that removal of the fallopian tubes and ovaries significantly reduced the risk of developing ovarian cancer. The incidence of ovarian cancer per 100,000 person-years was 26.2 for women undergoing hysterectomy alone (95% confidence interval [CI], 15.5–37.0), 17.5 for hysterectomy with unilateral salpingo-oophorectomy (95% CI, 0–39.1), and 1.7 for hysterectomy with bilateral salpingo-oophorectomy (95% CI, 0.4–3.0).
The hazard ratio (HR) for ovarian cancer was 0.58 for women undergoing unilateral salpingo-oophorectomy (95% CI, 0.18–1.90) and 0.12 for women undergoing bilateral salpingo-oophorectomy (95% CI, 0.05–0.28), compared with women undergoing hysterectomy alone.
Notable strengths of the analysis include the large size of the study population and the duration of patient follow-up (18 years). The authors acknowledge several limitations of the study, including the lack of data on BRCA mutation status and family history of cancer, as well as several other demographic data points possibly relevant to a risk of developing adnexal or peritoneal malignancy.
Related article: What is the gynecologist’s role in the care of BRCA previvors? Robert L. Barbieri, MD (Editorial, September 2013)
Keep these findings in context
As the authors discuss, this report should be considered in the context of other work suggesting that the lower mortality rate associated with ovarian conservation at the time of hysterectomy for benign disease arises mostly from a protective effect against cardiovascular disease (CVD)—perhaps from subclinical hormone production following menopause. Given that CVD remains the leading cause of death among American women, an individualized assessment of risk is necessary when planning the extent of surgery in this circumstance.
Related article: Oophorectomy or salpingectomy—which makes more sense? William H. Parker, MD (March 2014)
It also is interesting to consider the authors’ finding of a notable but statistically insignificant decrease in the risk of ovarian cancer associated with removal of only one tube and ovary. However, recognizing the possible limitations of their demographic information on this point, they suggest that this may be an area for further investigation, which would necessarily include characterization of the trends in the laterality of adnexal cancers. The preservation of hormonal function makes this an interesting option to consider.
We also need to further investigate the role of bilateral salpingectomy at the time of hysterectomy, with ovarian conservation, as an alternate therapeutic option, based upon evidence that extrauterine serous carcinoma may to a significant degree arise from the tubal epithelium rather than the ovarian cortex.
WHAT THIS EVIDENCE MEANS FOR PRACTICE
Removal of the adnexae significantly reduces the risk of ovarian cancer among the cohort of women undergoing hysterectomy for benign disease. However, the decision of whether or not to remove the adnexae when planning surgery should take into account other factors that may affect the risk of adnexal malignancy, including family history and BRCA mutation status, as well as other patient comorbidities.
Andrew W. Menzin, MD
Tell us what you think!
Drop us a line and let us know what you think about this or other current articles, which topics you'd like to see covered in future issues, and what challenges you face in daily practice. Tell us what you think by emailing us at: [email protected] Please include your name, city and state.
Stay in touch! Your feedback is important to us!
Problem Solving In Multi-Site Hospital Medicine Groups
Serving as the lead physician for a hospital medicine group (HMG) makes for challenging work. And the challenges and complexity only increase for anyone who serves as the physician leader for multiple practice sites in the same hospital system. In my November 2013 column on multi-site HMG leaders, I listed a few of the tricky issues they face and will mention a few more here.
Large-Small Friction
Unfortunately, tension between hospitalists at the big hospital and doctors at the small, “feeder” hospitals seems pretty common, and I think it’s due largely to high stress and a wide variation in workload, neither of which are in our direct control. At facilities where there is significant tension, I’m impressed by how vigorously the hospitalists at both the small and large hospitals argue that their own site faces the most stress and challenges. (This is a little like the endless debate about who works harder, those who work with residents and those who don’t.)
The hospitalists at the small site point out that they work with little or no subspecialty help and might even have to take night call from home while working during the day. Those at the big hospital say they are the ones with the very large scope of clinical practice and that, rather than making their life easier, the presence of lots of subspecialists makes for additional work coordinating care and communicating with all parties.
Where it exists, this tension is most evident during a transfer from one of the small hospitals to the large one. After all, one of the reasons to form a system of hospitals is so that nearly all patient needs can be met at one of the facilities in the system. Yet, for many reasons, the hospitalists at the large hospital are—sometimes—not as receptive to transfers as might be ideal. They might be short staffed or facing a high census or an unusually high number of admissions from their own ED. Or, perhaps, they’re concerned that the subspecialty services for which the patient is being transferred (e.g. to be scoped by a GI doctor) won’t be as helpful or prompt as needed. Or maybe they’ve felt “burned” by their colleagues at the small hospital for past transfers that didn’t seem necessary.
The result can be that the doctors at the smaller hospital complain that the “mother ship” hospitalists often are unfriendly and unreceptive to transfer requests. Although there may not be a definitive “cure” for this issue, there are several ways to help address the problem.
- In my last column, I mentioned the value of one or more in-person meetings between those who tend to be on the sending and receiving end of transfers, to establish some criteria regarding transfers that are appropriate and review the process of requesting a transfer and making the associated arrangements. In most cases there will be value in the parties meeting routinely—perhaps two to four times annually—to review how the system is working and address any difficulties.
- Periodic social meetings among the hospitalists at each site will help to form relationships that can make it less likely that any conversation about transfers will go in an unhelpful direction. Things can be very different when the people on each end of the phone call know each other personally.
- Record the phone calls between those seeking and accepting/declining each transfer. Scott Rissmiller, MD, the lead hospitalist for the 17 practice sites in Carolinas Healthcare, has said that having underperforming doctors listen to recordings of their phone calls about transfers has, in most cases he’s been involved with, proven to be a very effective way to encourage improvement.
Shared Staffing
The small hospitals in many systems sometimes struggle to find a way to provide economical night coverage. Hospitals below a certain size find it very difficult to justify a separate, in-house night provider. Some hospital systems have had success sharing night staffing, with the large hospital’s night hospitalist, nurse practitioner, or physician assistant providing telephone coverage for “cross cover” issues that arise after hours.
For example, when a nurse at the small hospital needs to contact a night hospitalist, staff will page the provider at the big hospital, and, in many cases, the issue can be managed effectively by phone. This works best when both hospitals are on the same electronic medical record, so that the responding provider can look through the record as needed.
The hospitalist at the small hospital typically stays on back-up call and is contacted if bedside attention is required.
Or, if the large and small hospitals are a short drive apart, the night hospitalist at the large facility might make the short drive to the small hospital when needed. In the case of emergencies (i.e., a code blue), the in-house night ED physician is relied on as the first responder.
Dr. Nelson has been a practicing hospitalist since 1988. He is co-founder and past president of SHM, and principal in Nelson Flores Hospital Medicine Consultants. He is co-director for SHM’s “Best Practices in Managing a Hospital Medicine Program” course. Write to him at [email protected].
Serving as the lead physician for a hospital medicine group (HMG) makes for challenging work. And the challenges and complexity only increase for anyone who serves as the physician leader for multiple practice sites in the same hospital system. In my November 2013 column on multi-site HMG leaders, I listed a few of the tricky issues they face and will mention a few more here.
Large-Small Friction
Unfortunately, tension between hospitalists at the big hospital and doctors at the small, “feeder” hospitals seems pretty common, and I think it’s due largely to high stress and a wide variation in workload, neither of which are in our direct control. At facilities where there is significant tension, I’m impressed by how vigorously the hospitalists at both the small and large hospitals argue that their own site faces the most stress and challenges. (This is a little like the endless debate about who works harder, those who work with residents and those who don’t.)
The hospitalists at the small site point out that they work with little or no subspecialty help and might even have to take night call from home while working during the day. Those at the big hospital say they are the ones with the very large scope of clinical practice and that, rather than making their life easier, the presence of lots of subspecialists makes for additional work coordinating care and communicating with all parties.
Where it exists, this tension is most evident during a transfer from one of the small hospitals to the large one. After all, one of the reasons to form a system of hospitals is so that nearly all patient needs can be met at one of the facilities in the system. Yet, for many reasons, the hospitalists at the large hospital are—sometimes—not as receptive to transfers as might be ideal. They might be short staffed or facing a high census or an unusually high number of admissions from their own ED. Or, perhaps, they’re concerned that the subspecialty services for which the patient is being transferred (e.g. to be scoped by a GI doctor) won’t be as helpful or prompt as needed. Or maybe they’ve felt “burned” by their colleagues at the small hospital for past transfers that didn’t seem necessary.
The result can be that the doctors at the smaller hospital complain that the “mother ship” hospitalists often are unfriendly and unreceptive to transfer requests. Although there may not be a definitive “cure” for this issue, there are several ways to help address the problem.
- In my last column, I mentioned the value of one or more in-person meetings between those who tend to be on the sending and receiving end of transfers, to establish some criteria regarding transfers that are appropriate and review the process of requesting a transfer and making the associated arrangements. In most cases there will be value in the parties meeting routinely—perhaps two to four times annually—to review how the system is working and address any difficulties.
- Periodic social meetings among the hospitalists at each site will help to form relationships that can make it less likely that any conversation about transfers will go in an unhelpful direction. Things can be very different when the people on each end of the phone call know each other personally.
- Record the phone calls between those seeking and accepting/declining each transfer. Scott Rissmiller, MD, the lead hospitalist for the 17 practice sites in Carolinas Healthcare, has said that having underperforming doctors listen to recordings of their phone calls about transfers has, in most cases he’s been involved with, proven to be a very effective way to encourage improvement.
Shared Staffing
The small hospitals in many systems sometimes struggle to find a way to provide economical night coverage. Hospitals below a certain size find it very difficult to justify a separate, in-house night provider. Some hospital systems have had success sharing night staffing, with the large hospital’s night hospitalist, nurse practitioner, or physician assistant providing telephone coverage for “cross cover” issues that arise after hours.
For example, when a nurse at the small hospital needs to contact a night hospitalist, staff will page the provider at the big hospital, and, in many cases, the issue can be managed effectively by phone. This works best when both hospitals are on the same electronic medical record, so that the responding provider can look through the record as needed.
The hospitalist at the small hospital typically stays on back-up call and is contacted if bedside attention is required.
Or, if the large and small hospitals are a short drive apart, the night hospitalist at the large facility might make the short drive to the small hospital when needed. In the case of emergencies (i.e., a code blue), the in-house night ED physician is relied on as the first responder.
Dr. Nelson has been a practicing hospitalist since 1988. He is co-founder and past president of SHM, and principal in Nelson Flores Hospital Medicine Consultants. He is co-director for SHM’s “Best Practices in Managing a Hospital Medicine Program” course. Write to him at [email protected].
Serving as the lead physician for a hospital medicine group (HMG) makes for challenging work. And the challenges and complexity only increase for anyone who serves as the physician leader for multiple practice sites in the same hospital system. In my November 2013 column on multi-site HMG leaders, I listed a few of the tricky issues they face and will mention a few more here.
Large-Small Friction
Unfortunately, tension between hospitalists at the big hospital and doctors at the small, “feeder” hospitals seems pretty common, and I think it’s due largely to high stress and a wide variation in workload, neither of which are in our direct control. At facilities where there is significant tension, I’m impressed by how vigorously the hospitalists at both the small and large hospitals argue that their own site faces the most stress and challenges. (This is a little like the endless debate about who works harder, those who work with residents and those who don’t.)
The hospitalists at the small site point out that they work with little or no subspecialty help and might even have to take night call from home while working during the day. Those at the big hospital say they are the ones with the very large scope of clinical practice and that, rather than making their life easier, the presence of lots of subspecialists makes for additional work coordinating care and communicating with all parties.
Where it exists, this tension is most evident during a transfer from one of the small hospitals to the large one. After all, one of the reasons to form a system of hospitals is so that nearly all patient needs can be met at one of the facilities in the system. Yet, for many reasons, the hospitalists at the large hospital are—sometimes—not as receptive to transfers as might be ideal. They might be short staffed or facing a high census or an unusually high number of admissions from their own ED. Or, perhaps, they’re concerned that the subspecialty services for which the patient is being transferred (e.g. to be scoped by a GI doctor) won’t be as helpful or prompt as needed. Or maybe they’ve felt “burned” by their colleagues at the small hospital for past transfers that didn’t seem necessary.
The result can be that the doctors at the smaller hospital complain that the “mother ship” hospitalists often are unfriendly and unreceptive to transfer requests. Although there may not be a definitive “cure” for this issue, there are several ways to help address the problem.
- In my last column, I mentioned the value of one or more in-person meetings between those who tend to be on the sending and receiving end of transfers, to establish some criteria regarding transfers that are appropriate and review the process of requesting a transfer and making the associated arrangements. In most cases there will be value in the parties meeting routinely—perhaps two to four times annually—to review how the system is working and address any difficulties.
- Periodic social meetings among the hospitalists at each site will help to form relationships that can make it less likely that any conversation about transfers will go in an unhelpful direction. Things can be very different when the people on each end of the phone call know each other personally.
- Record the phone calls between those seeking and accepting/declining each transfer. Scott Rissmiller, MD, the lead hospitalist for the 17 practice sites in Carolinas Healthcare, has said that having underperforming doctors listen to recordings of their phone calls about transfers has, in most cases he’s been involved with, proven to be a very effective way to encourage improvement.
Shared Staffing
The small hospitals in many systems sometimes struggle to find a way to provide economical night coverage. Hospitals below a certain size find it very difficult to justify a separate, in-house night provider. Some hospital systems have had success sharing night staffing, with the large hospital’s night hospitalist, nurse practitioner, or physician assistant providing telephone coverage for “cross cover” issues that arise after hours.
For example, when a nurse at the small hospital needs to contact a night hospitalist, staff will page the provider at the big hospital, and, in many cases, the issue can be managed effectively by phone. This works best when both hospitals are on the same electronic medical record, so that the responding provider can look through the record as needed.
The hospitalist at the small hospital typically stays on back-up call and is contacted if bedside attention is required.
Or, if the large and small hospitals are a short drive apart, the night hospitalist at the large facility might make the short drive to the small hospital when needed. In the case of emergencies (i.e., a code blue), the in-house night ED physician is relied on as the first responder.
Dr. Nelson has been a practicing hospitalist since 1988. He is co-founder and past president of SHM, and principal in Nelson Flores Hospital Medicine Consultants. He is co-director for SHM’s “Best Practices in Managing a Hospital Medicine Program” course. Write to him at [email protected].
Does maternal obesity increase the risk of preterm delivery?
The developed world is in the midst of an unprecedented increase in human body mass. This increase can be attributed to widespread access to large amounts of inexpensive calories—particularly carbohydrates—and diminishing physical exertion. We have “evolved” from creatures struggling to get enough food to survive to vertebrates drowning in an ocean of calories and ease.
Although obesity clearly lies on the causal pathway for diseases such as diabetes and endometrial cancer, it also is associated with many other unhealthy behaviors and exposures. For example, obese women tend to earn less money, achieve less in school, smoke, and live farther away from markets and playgrounds—and the list of confounders goes on and on. We can measure height and weight with ease and precision, but we can’t assess and quantify most of these other confounders.
Related Article: Should you start prescribing lorcaserin or orlistat to your overweight or obese patients? Robert L. Barbieri, MD (Editorial, October 2013)
Cnattingius and colleagues give us another “obesity is bad” paper, this time with the outcome of preterm delivery. They found not only an association between obesity and preterm delivery but also a “mass response effect”—that is, the association increased along with maternal BMI.
The magnitude of the associations was small (odds ratios <3.0 for preterm delivery among overweight and obese women, compared with women of normal weight), and despite valiant efforts by the investigators to control for confounding, the imprecision I mentioned above limits their findings.
I declared a personal moratorium on reading “obesity is bad” papers a few years back. Even if obesity is a real risk factor for poor perinatal outcomes and not a proxy for residual confounding, we still have no idea, short of invasive surgery, how to modify that risk. Real progress will require effective lifestyle intervention—and we know so little about how to get people to lead healthy lives. It is difficult enough to modify our own behavior (recall your New Year’s resolutions), even harder to motivate our patients to lose weight and exercise.
What this evidence means for practice
Obesity is at best a weak risk factor for preterm delivery. Unless you are more successful than I have been at getting women to modify their diet and exercise, I would not make heavy mothers feel any worse.
John M. Thorp Jr., MD
TELL US WHAT YOU THINK!
Share your thoughts on this article or on any topic relevant to ObGyns and women’s health practitioners. Tell us which topics you’d like to see covered in future issues, and what challenges you face in daily practice. We will consider publishing your letter and in a future issue.
Send your letter to: [email protected] Please include the city and state in which you practice.
Stay in touch! Your feedback is important to us!
The developed world is in the midst of an unprecedented increase in human body mass. This increase can be attributed to widespread access to large amounts of inexpensive calories—particularly carbohydrates—and diminishing physical exertion. We have “evolved” from creatures struggling to get enough food to survive to vertebrates drowning in an ocean of calories and ease.
Although obesity clearly lies on the causal pathway for diseases such as diabetes and endometrial cancer, it also is associated with many other unhealthy behaviors and exposures. For example, obese women tend to earn less money, achieve less in school, smoke, and live farther away from markets and playgrounds—and the list of confounders goes on and on. We can measure height and weight with ease and precision, but we can’t assess and quantify most of these other confounders.
Related Article: Should you start prescribing lorcaserin or orlistat to your overweight or obese patients? Robert L. Barbieri, MD (Editorial, October 2013)
Cnattingius and colleagues give us another “obesity is bad” paper, this time with the outcome of preterm delivery. They found not only an association between obesity and preterm delivery but also a “mass response effect”—that is, the association increased along with maternal BMI.
The magnitude of the associations was small (odds ratios <3.0 for preterm delivery among overweight and obese women, compared with women of normal weight), and despite valiant efforts by the investigators to control for confounding, the imprecision I mentioned above limits their findings.
I declared a personal moratorium on reading “obesity is bad” papers a few years back. Even if obesity is a real risk factor for poor perinatal outcomes and not a proxy for residual confounding, we still have no idea, short of invasive surgery, how to modify that risk. Real progress will require effective lifestyle intervention—and we know so little about how to get people to lead healthy lives. It is difficult enough to modify our own behavior (recall your New Year’s resolutions), even harder to motivate our patients to lose weight and exercise.
What this evidence means for practice
Obesity is at best a weak risk factor for preterm delivery. Unless you are more successful than I have been at getting women to modify their diet and exercise, I would not make heavy mothers feel any worse.
John M. Thorp Jr., MD
TELL US WHAT YOU THINK!
Share your thoughts on this article or on any topic relevant to ObGyns and women’s health practitioners. Tell us which topics you’d like to see covered in future issues, and what challenges you face in daily practice. We will consider publishing your letter and in a future issue.
Send your letter to: [email protected] Please include the city and state in which you practice.
Stay in touch! Your feedback is important to us!
The developed world is in the midst of an unprecedented increase in human body mass. This increase can be attributed to widespread access to large amounts of inexpensive calories—particularly carbohydrates—and diminishing physical exertion. We have “evolved” from creatures struggling to get enough food to survive to vertebrates drowning in an ocean of calories and ease.
Although obesity clearly lies on the causal pathway for diseases such as diabetes and endometrial cancer, it also is associated with many other unhealthy behaviors and exposures. For example, obese women tend to earn less money, achieve less in school, smoke, and live farther away from markets and playgrounds—and the list of confounders goes on and on. We can measure height and weight with ease and precision, but we can’t assess and quantify most of these other confounders.
Related Article: Should you start prescribing lorcaserin or orlistat to your overweight or obese patients? Robert L. Barbieri, MD (Editorial, October 2013)
Cnattingius and colleagues give us another “obesity is bad” paper, this time with the outcome of preterm delivery. They found not only an association between obesity and preterm delivery but also a “mass response effect”—that is, the association increased along with maternal BMI.
The magnitude of the associations was small (odds ratios <3.0 for preterm delivery among overweight and obese women, compared with women of normal weight), and despite valiant efforts by the investigators to control for confounding, the imprecision I mentioned above limits their findings.
I declared a personal moratorium on reading “obesity is bad” papers a few years back. Even if obesity is a real risk factor for poor perinatal outcomes and not a proxy for residual confounding, we still have no idea, short of invasive surgery, how to modify that risk. Real progress will require effective lifestyle intervention—and we know so little about how to get people to lead healthy lives. It is difficult enough to modify our own behavior (recall your New Year’s resolutions), even harder to motivate our patients to lose weight and exercise.
What this evidence means for practice
Obesity is at best a weak risk factor for preterm delivery. Unless you are more successful than I have been at getting women to modify their diet and exercise, I would not make heavy mothers feel any worse.
John M. Thorp Jr., MD
TELL US WHAT YOU THINK!
Share your thoughts on this article or on any topic relevant to ObGyns and women’s health practitioners. Tell us which topics you’d like to see covered in future issues, and what challenges you face in daily practice. We will consider publishing your letter and in a future issue.
Send your letter to: [email protected] Please include the city and state in which you practice.
Stay in touch! Your feedback is important to us!
In the latest report from the WHI, the data contradict the conclusions
In October 2013, the Women’s Health Initiative (WHI) investigators published a comprehensive overview of findings from their two hormone therapy (HT) trials, including extended follow-up representing 13 years of cumulative data.1 When I analyzed this latest WHI report, I initially focused almost exclusively on the data presented in figures and tables within the article itself, as well as on supplemental data presented on the Internet.2 Only then did I read the discussion comments by its authors. I would recommend this approach to anyone who has not yet reviewed this publication.
Overall, the WHI investigators maintain a negative stance toward the preventive and therapeutic benefits of menopausal HT. In my opinion, they also under-emphasize the importance of time since menopause in patient selection. These are the same WHI investigators who initially published un-adjudicated data3 and who delayed reporting age-stratified data.4 They also erroneously concluded that HT might increase the risk of ovarian cancer, even though their own data showed otherwise.5,6
The tables and figures contain the most important data point from this extended WHI follow-up: a reduction in all-cause mortality among women who initiated HT within 10 years of menopause, whether they used estrogen-alone (hysterectomized women) or estrogen-progestin therapy (women with an intact uterus), compared with women in the placebo group.1
Related Article: When should a menopausal woman discontinue hormone therapy? Andrew M. Kaunitz, MD (Cases in Menopause, February 2014)
DO THE RISKS OF HT REALLY OUTWEIGH THE BENEFITS?
The dramatic benefits of estrogen-alone HT, in particular, recently were highlighted by Sarrel and colleagues in an analysis that suggests that as many as 90,000 deaths may have occurred after publication of the initial WHI findings, when estrogen therapy was widely withheld.7 The study by Sarrel and colleagues also was highlighted in a recent issue of this journal.8
However, based on a “global index,” which has not been validated, the WHI investigators concluded that the risks of estrogen-progestin therapy outweigh the benefits regardless of age. Yet, the global index does not include all key concerns, omitting several quality-of-life concerns, including sleep disturbance, work productivity, and sexual function, as well as type 2 diabetes mellitus, osteoarthritis, and nonosteoporotic musculoskeletal problems. Nor does the global index provide individual weights.
Although the WHI data show reductions in the incidence of some serious chronic diseases, such as osteoporotic fracture and cardiovascular disease (in women within 10 years of menopause), Manson and colleagues make the blanket statement that HT should not be used for disease prevention, although they admit that it may be a “reasonable option for the management of moderate to severe menopausal symptoms among generally healthy women during early menopause.”1
Related Article: Update on Osteoporosis Steven R. Goldstein, MD (December 2013)
For some time, Wulf H. Utian, MD, PhD, a founder of both the International Menopause Society and the North American Menopause Society, has been calling for an independent commission to reevaluate all of the major WHI reports “to determine whether the data justified the conclusions drawn.”9 I support his call and suggest that this latest WHI publication be included in that reevaluation. The fact that total mortality is reduced among women using HT—according to the WHI’s own data—is not only impressive, it argues for, not against, the use of HT for chronic disease reduction.
KEEP YOUR EYE ON THE DATA
I have no doubt that medical history books will note the destructive effects of misinterpretation of WHI data. Until then, it is up to all practitioners and educators to counsel our patients and our trainees about menopause and menopausal HT and to look beyond the textual conclusions of WHI reports to assess the data themselves.
Other important research, such as the Study of Women’s Health Across the Nation (SWAN), shows that untreated menopausal women fall off the work productivity ladder.10 This is important because economic stability is a critical component of health and wellness. I have heard many women remark that someone will have to “pry” their hormones out of their “cold, dead hands”—meaning that they intend to take HT even if it shortens their lifespan—which is ironic, given that HT is likely to extend their lifespan!
Coronary heart disease (CHD) is the major killer of American women, and the long-term WHI data actually suggest that HT can prevent it, provided it is initiated within 10 years of menopause. In a two-part article, Hodis and Mack shrewdly compare the risks associated with HT with those associated with other commonly used medications in women’s health.11,12 They note that evidence-based data from randomized, clinical trials are very reassuring, as HT-associated risks are rare (less than 1 event per 1,000 women treated)—and even rarer when HT is initiated within 10 years of menopause. HT reduces CHD and total mortality, whereas aspirin and statins (as primary preventives) do not.11,12
Related Article: Update: Menopause Andrew M. Kaunitz, MD (May 2010)
THE BOTTOM LINE
We need to look at the totality of the data on menopausal HT, evaluate our patients individually, treat those who are truly hormonally deficient and suffering, and counsel them that many of the harms linked to HT have been exaggerated.
The pendulum is finally swinging back toward a more balanced assessment of the benefits and risks of HT, indicating that it may be appropriate for primary prevention of cardiovascular disease, osteoporosis, and type 2 diabetes—and thus can potentially expand the lifespan. It’s up to us to communicate this fact to our patients.
TELL US WHAT YOU THINK!
Share your thoughts on this article or on any topic relevant to ObGyns and women’s health practitioners. Tell us which topics you’d like to see covered in future issues, and what challenges you face in daily practice.
We will consider publishing your letter and in a future issue.
Send your letter to: [email protected]
Please include the city and state in which you practice.
Stay in touch! Your feedback is important to us!
- Manson JE, Chlebowski RT, Stefanick ML, et al. Menopausal hormone therapy and health outcomes during the intervention and extended poststopping phases of the Women’s Health Initiative randomized trials. JAMA. 2013;310(13):1353–1368.
- Supplemental content published online by the Journal of the American Medical Association. http://jama.jamanetwork.com/article
.aspx?articleid=1745676. Accessed January 27, 2014 [subscription required]. - Rossouw JE, Anderson GL, Prentice RL, et al; Writing Group for the Women’s Health Initiative Investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women. JAMA. 2002;288(3):321–333.
- Rossouw JE, Prentice RL, Manson JE, et al. Postmenopausal hormone therapy and risk of cardiovascular disease by age and years since menopause. JAMA. 2007;297(13):1465–1477.
- Utian WH. Hormone therapy and risk of gynecologic cancers [letter]. JAMA. 2004;291(1):42.
- Anderson GL, Judd HL, Kaunitz AM, et al. Hormone therapy and risk of gynecologic cancers—reply [letter]. JAMA. 2004;291(1):42.
- Sarrel PM, Njike VY, Vinante V, Katz DL. The mortality toll of estrogen avoidance: an analysis of excess deaths among hysterectomized women aged 50 to 59. Am J Pub Health. 2013;103(9):1583–1588.
- Kaunitz AM. In young hysterectomized women, does unopposed estrogen therapy increase overall survival? OBG Manag. 2013;25(10):55–56.
- Utian WH. A decade post WHI, menopausal hormone therapy comes full circule—need for independent commission. Climacteric. 2012;15(4):320–325.
- Tseng LA, El Khoudary SR, Young EA, et al. The association of menopausal status with physical function: The Study of Women’s Health Across the Nation. Menopause. 2012;19(11):1186–1192.
- Hodis HN, Mack WJ. The timing hypothesis and hormone replacement therapy: a paradigm shift in the primary prevention of coronary heart disease in women. Part 1: Comparison of therapeutic efficacy. J Am Geriatric Soc. 2013;61(6):1005–1010.
- Hodis HN, Mack WJ. The timing hypothesis and hormone replacement therapy: a paradigm shift in the primary prevention of coronary heart disease in women. Part 2: Comparative risks. J Am Geriatric Soc. 2013;61(6):1011–1018.
In October 2013, the Women’s Health Initiative (WHI) investigators published a comprehensive overview of findings from their two hormone therapy (HT) trials, including extended follow-up representing 13 years of cumulative data.1 When I analyzed this latest WHI report, I initially focused almost exclusively on the data presented in figures and tables within the article itself, as well as on supplemental data presented on the Internet.2 Only then did I read the discussion comments by its authors. I would recommend this approach to anyone who has not yet reviewed this publication.
Overall, the WHI investigators maintain a negative stance toward the preventive and therapeutic benefits of menopausal HT. In my opinion, they also under-emphasize the importance of time since menopause in patient selection. These are the same WHI investigators who initially published un-adjudicated data3 and who delayed reporting age-stratified data.4 They also erroneously concluded that HT might increase the risk of ovarian cancer, even though their own data showed otherwise.5,6
The tables and figures contain the most important data point from this extended WHI follow-up: a reduction in all-cause mortality among women who initiated HT within 10 years of menopause, whether they used estrogen-alone (hysterectomized women) or estrogen-progestin therapy (women with an intact uterus), compared with women in the placebo group.1
Related Article: When should a menopausal woman discontinue hormone therapy? Andrew M. Kaunitz, MD (Cases in Menopause, February 2014)
DO THE RISKS OF HT REALLY OUTWEIGH THE BENEFITS?
The dramatic benefits of estrogen-alone HT, in particular, recently were highlighted by Sarrel and colleagues in an analysis that suggests that as many as 90,000 deaths may have occurred after publication of the initial WHI findings, when estrogen therapy was widely withheld.7 The study by Sarrel and colleagues also was highlighted in a recent issue of this journal.8
However, based on a “global index,” which has not been validated, the WHI investigators concluded that the risks of estrogen-progestin therapy outweigh the benefits regardless of age. Yet, the global index does not include all key concerns, omitting several quality-of-life concerns, including sleep disturbance, work productivity, and sexual function, as well as type 2 diabetes mellitus, osteoarthritis, and nonosteoporotic musculoskeletal problems. Nor does the global index provide individual weights.
Although the WHI data show reductions in the incidence of some serious chronic diseases, such as osteoporotic fracture and cardiovascular disease (in women within 10 years of menopause), Manson and colleagues make the blanket statement that HT should not be used for disease prevention, although they admit that it may be a “reasonable option for the management of moderate to severe menopausal symptoms among generally healthy women during early menopause.”1
Related Article: Update on Osteoporosis Steven R. Goldstein, MD (December 2013)
For some time, Wulf H. Utian, MD, PhD, a founder of both the International Menopause Society and the North American Menopause Society, has been calling for an independent commission to reevaluate all of the major WHI reports “to determine whether the data justified the conclusions drawn.”9 I support his call and suggest that this latest WHI publication be included in that reevaluation. The fact that total mortality is reduced among women using HT—according to the WHI’s own data—is not only impressive, it argues for, not against, the use of HT for chronic disease reduction.
KEEP YOUR EYE ON THE DATA
I have no doubt that medical history books will note the destructive effects of misinterpretation of WHI data. Until then, it is up to all practitioners and educators to counsel our patients and our trainees about menopause and menopausal HT and to look beyond the textual conclusions of WHI reports to assess the data themselves.
Other important research, such as the Study of Women’s Health Across the Nation (SWAN), shows that untreated menopausal women fall off the work productivity ladder.10 This is important because economic stability is a critical component of health and wellness. I have heard many women remark that someone will have to “pry” their hormones out of their “cold, dead hands”—meaning that they intend to take HT even if it shortens their lifespan—which is ironic, given that HT is likely to extend their lifespan!
Coronary heart disease (CHD) is the major killer of American women, and the long-term WHI data actually suggest that HT can prevent it, provided it is initiated within 10 years of menopause. In a two-part article, Hodis and Mack shrewdly compare the risks associated with HT with those associated with other commonly used medications in women’s health.11,12 They note that evidence-based data from randomized, clinical trials are very reassuring, as HT-associated risks are rare (less than 1 event per 1,000 women treated)—and even rarer when HT is initiated within 10 years of menopause. HT reduces CHD and total mortality, whereas aspirin and statins (as primary preventives) do not.11,12
Related Article: Update: Menopause Andrew M. Kaunitz, MD (May 2010)
THE BOTTOM LINE
We need to look at the totality of the data on menopausal HT, evaluate our patients individually, treat those who are truly hormonally deficient and suffering, and counsel them that many of the harms linked to HT have been exaggerated.
The pendulum is finally swinging back toward a more balanced assessment of the benefits and risks of HT, indicating that it may be appropriate for primary prevention of cardiovascular disease, osteoporosis, and type 2 diabetes—and thus can potentially expand the lifespan. It’s up to us to communicate this fact to our patients.
TELL US WHAT YOU THINK!
Share your thoughts on this article or on any topic relevant to ObGyns and women’s health practitioners. Tell us which topics you’d like to see covered in future issues, and what challenges you face in daily practice.
We will consider publishing your letter and in a future issue.
Send your letter to: [email protected]
Please include the city and state in which you practice.
Stay in touch! Your feedback is important to us!
In October 2013, the Women’s Health Initiative (WHI) investigators published a comprehensive overview of findings from their two hormone therapy (HT) trials, including extended follow-up representing 13 years of cumulative data.1 When I analyzed this latest WHI report, I initially focused almost exclusively on the data presented in figures and tables within the article itself, as well as on supplemental data presented on the Internet.2 Only then did I read the discussion comments by its authors. I would recommend this approach to anyone who has not yet reviewed this publication.
Overall, the WHI investigators maintain a negative stance toward the preventive and therapeutic benefits of menopausal HT. In my opinion, they also under-emphasize the importance of time since menopause in patient selection. These are the same WHI investigators who initially published un-adjudicated data3 and who delayed reporting age-stratified data.4 They also erroneously concluded that HT might increase the risk of ovarian cancer, even though their own data showed otherwise.5,6
The tables and figures contain the most important data point from this extended WHI follow-up: a reduction in all-cause mortality among women who initiated HT within 10 years of menopause, whether they used estrogen-alone (hysterectomized women) or estrogen-progestin therapy (women with an intact uterus), compared with women in the placebo group.1
Related Article: When should a menopausal woman discontinue hormone therapy? Andrew M. Kaunitz, MD (Cases in Menopause, February 2014)
DO THE RISKS OF HT REALLY OUTWEIGH THE BENEFITS?
The dramatic benefits of estrogen-alone HT, in particular, recently were highlighted by Sarrel and colleagues in an analysis that suggests that as many as 90,000 deaths may have occurred after publication of the initial WHI findings, when estrogen therapy was widely withheld.7 The study by Sarrel and colleagues also was highlighted in a recent issue of this journal.8
However, based on a “global index,” which has not been validated, the WHI investigators concluded that the risks of estrogen-progestin therapy outweigh the benefits regardless of age. Yet, the global index does not include all key concerns, omitting several quality-of-life concerns, including sleep disturbance, work productivity, and sexual function, as well as type 2 diabetes mellitus, osteoarthritis, and nonosteoporotic musculoskeletal problems. Nor does the global index provide individual weights.
Although the WHI data show reductions in the incidence of some serious chronic diseases, such as osteoporotic fracture and cardiovascular disease (in women within 10 years of menopause), Manson and colleagues make the blanket statement that HT should not be used for disease prevention, although they admit that it may be a “reasonable option for the management of moderate to severe menopausal symptoms among generally healthy women during early menopause.”1
Related Article: Update on Osteoporosis Steven R. Goldstein, MD (December 2013)
For some time, Wulf H. Utian, MD, PhD, a founder of both the International Menopause Society and the North American Menopause Society, has been calling for an independent commission to reevaluate all of the major WHI reports “to determine whether the data justified the conclusions drawn.”9 I support his call and suggest that this latest WHI publication be included in that reevaluation. The fact that total mortality is reduced among women using HT—according to the WHI’s own data—is not only impressive, it argues for, not against, the use of HT for chronic disease reduction.
KEEP YOUR EYE ON THE DATA
I have no doubt that medical history books will note the destructive effects of misinterpretation of WHI data. Until then, it is up to all practitioners and educators to counsel our patients and our trainees about menopause and menopausal HT and to look beyond the textual conclusions of WHI reports to assess the data themselves.
Other important research, such as the Study of Women’s Health Across the Nation (SWAN), shows that untreated menopausal women fall off the work productivity ladder.10 This is important because economic stability is a critical component of health and wellness. I have heard many women remark that someone will have to “pry” their hormones out of their “cold, dead hands”—meaning that they intend to take HT even if it shortens their lifespan—which is ironic, given that HT is likely to extend their lifespan!
Coronary heart disease (CHD) is the major killer of American women, and the long-term WHI data actually suggest that HT can prevent it, provided it is initiated within 10 years of menopause. In a two-part article, Hodis and Mack shrewdly compare the risks associated with HT with those associated with other commonly used medications in women’s health.11,12 They note that evidence-based data from randomized, clinical trials are very reassuring, as HT-associated risks are rare (less than 1 event per 1,000 women treated)—and even rarer when HT is initiated within 10 years of menopause. HT reduces CHD and total mortality, whereas aspirin and statins (as primary preventives) do not.11,12
Related Article: Update: Menopause Andrew M. Kaunitz, MD (May 2010)
THE BOTTOM LINE
We need to look at the totality of the data on menopausal HT, evaluate our patients individually, treat those who are truly hormonally deficient and suffering, and counsel them that many of the harms linked to HT have been exaggerated.
The pendulum is finally swinging back toward a more balanced assessment of the benefits and risks of HT, indicating that it may be appropriate for primary prevention of cardiovascular disease, osteoporosis, and type 2 diabetes—and thus can potentially expand the lifespan. It’s up to us to communicate this fact to our patients.
TELL US WHAT YOU THINK!
Share your thoughts on this article or on any topic relevant to ObGyns and women’s health practitioners. Tell us which topics you’d like to see covered in future issues, and what challenges you face in daily practice.
We will consider publishing your letter and in a future issue.
Send your letter to: [email protected]
Please include the city and state in which you practice.
Stay in touch! Your feedback is important to us!
- Manson JE, Chlebowski RT, Stefanick ML, et al. Menopausal hormone therapy and health outcomes during the intervention and extended poststopping phases of the Women’s Health Initiative randomized trials. JAMA. 2013;310(13):1353–1368.
- Supplemental content published online by the Journal of the American Medical Association. http://jama.jamanetwork.com/article
.aspx?articleid=1745676. Accessed January 27, 2014 [subscription required]. - Rossouw JE, Anderson GL, Prentice RL, et al; Writing Group for the Women’s Health Initiative Investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women. JAMA. 2002;288(3):321–333.
- Rossouw JE, Prentice RL, Manson JE, et al. Postmenopausal hormone therapy and risk of cardiovascular disease by age and years since menopause. JAMA. 2007;297(13):1465–1477.
- Utian WH. Hormone therapy and risk of gynecologic cancers [letter]. JAMA. 2004;291(1):42.
- Anderson GL, Judd HL, Kaunitz AM, et al. Hormone therapy and risk of gynecologic cancers—reply [letter]. JAMA. 2004;291(1):42.
- Sarrel PM, Njike VY, Vinante V, Katz DL. The mortality toll of estrogen avoidance: an analysis of excess deaths among hysterectomized women aged 50 to 59. Am J Pub Health. 2013;103(9):1583–1588.
- Kaunitz AM. In young hysterectomized women, does unopposed estrogen therapy increase overall survival? OBG Manag. 2013;25(10):55–56.
- Utian WH. A decade post WHI, menopausal hormone therapy comes full circule—need for independent commission. Climacteric. 2012;15(4):320–325.
- Tseng LA, El Khoudary SR, Young EA, et al. The association of menopausal status with physical function: The Study of Women’s Health Across the Nation. Menopause. 2012;19(11):1186–1192.
- Hodis HN, Mack WJ. The timing hypothesis and hormone replacement therapy: a paradigm shift in the primary prevention of coronary heart disease in women. Part 1: Comparison of therapeutic efficacy. J Am Geriatric Soc. 2013;61(6):1005–1010.
- Hodis HN, Mack WJ. The timing hypothesis and hormone replacement therapy: a paradigm shift in the primary prevention of coronary heart disease in women. Part 2: Comparative risks. J Am Geriatric Soc. 2013;61(6):1011–1018.
- Manson JE, Chlebowski RT, Stefanick ML, et al. Menopausal hormone therapy and health outcomes during the intervention and extended poststopping phases of the Women’s Health Initiative randomized trials. JAMA. 2013;310(13):1353–1368.
- Supplemental content published online by the Journal of the American Medical Association. http://jama.jamanetwork.com/article
.aspx?articleid=1745676. Accessed January 27, 2014 [subscription required]. - Rossouw JE, Anderson GL, Prentice RL, et al; Writing Group for the Women’s Health Initiative Investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women. JAMA. 2002;288(3):321–333.
- Rossouw JE, Prentice RL, Manson JE, et al. Postmenopausal hormone therapy and risk of cardiovascular disease by age and years since menopause. JAMA. 2007;297(13):1465–1477.
- Utian WH. Hormone therapy and risk of gynecologic cancers [letter]. JAMA. 2004;291(1):42.
- Anderson GL, Judd HL, Kaunitz AM, et al. Hormone therapy and risk of gynecologic cancers—reply [letter]. JAMA. 2004;291(1):42.
- Sarrel PM, Njike VY, Vinante V, Katz DL. The mortality toll of estrogen avoidance: an analysis of excess deaths among hysterectomized women aged 50 to 59. Am J Pub Health. 2013;103(9):1583–1588.
- Kaunitz AM. In young hysterectomized women, does unopposed estrogen therapy increase overall survival? OBG Manag. 2013;25(10):55–56.
- Utian WH. A decade post WHI, menopausal hormone therapy comes full circule—need for independent commission. Climacteric. 2012;15(4):320–325.
- Tseng LA, El Khoudary SR, Young EA, et al. The association of menopausal status with physical function: The Study of Women’s Health Across the Nation. Menopause. 2012;19(11):1186–1192.
- Hodis HN, Mack WJ. The timing hypothesis and hormone replacement therapy: a paradigm shift in the primary prevention of coronary heart disease in women. Part 1: Comparison of therapeutic efficacy. J Am Geriatric Soc. 2013;61(6):1005–1010.
- Hodis HN, Mack WJ. The timing hypothesis and hormone replacement therapy: a paradigm shift in the primary prevention of coronary heart disease in women. Part 2: Comparative risks. J Am Geriatric Soc. 2013;61(6):1011–1018.
Does vaginal prolapse repair using synthetic mesh confer long-term benefit over native-tissue colpopexy?
This is the third report from Gutman and colleagues on the outcomes of a double-blind, multicenter, randomized, controlled trial of vaginal prolapse repair using synthetic mesh versus native-tissue colpopexy in women with significant vaginal prolapse.
The trial involved 33 women who underwent mesh repair and 32 who underwent repair without mesh. (The mesh-free repair consisted primarily of uterosacral suspension and concurrent colporrhaphy.) It was halted when it reached a predetermined threshold for discontinuation, which was a mesh erosion rate of 15% or more.
Investigators found no difference in long-term cure rates between the mesh and no-mesh groups, regardless of the definition of cure (ie, anatomic, symptomatic, or combined). Nor was there a difference in the overall recurrence rate.
Summary of earlier reports
Three-month outcomes. The first report from this trial described 3-month objective treatment outcomes, with success described as prolapse no greater than stage 1.1 It found a high erosion rate (15.6%) for vaginal mesh, with no differences between groups in overall subjective or objective cure rates, with an overall recurrence rate of 59.4% (19 cases) in the mesh group versus 70.4% (24 cases) in the no-mesh group (P = .28), with recurrence defined as prolapse beyond stage 1 in any compartment. Investigators also observed potential benefit in the mesh group in the anterior vaginal wall at point Ba at a median of 9.7 months after surgery.
Related Article: Stop using synthetic mesh for routine repair of pelvic organ prolapse Cheryl B. Iglesia, MD (Stop/Start, April 2013)
One-year outcomes. The second report described 1-year objective and functional outcomes in all participants of the trial.2 It found comparable objective and subjective cure rates between groups but a higher reoperation rate for mesh repairs. Prolapse recurred in the anterior department in 46.9% of women in the mesh group versus 60.6% in the no-mesh group (P = .40).
Subjective quality-of-life assessments continued to reflect significant improvement in symptoms from baseline. Vaginal bulging was relieved in 96.2% of women in the mesh group, compared with 90.9% in the no-mesh group (P = .62).
More women in the mesh group required reoperation for recurrent prolapse or mesh exposure (5 in the mesh group vs 0 in the no-mesh group; P = .017).
Strengths and limitations of the trial
Gutman and colleagues are to be congratulated for continuing to monitor longer-term outcomes of vaginal prolapse repairs augmented with synthetic mesh, as data are sorely needed on both early complications and those more remote from surgery. However, it is regrettable that continued attrition in this trial led to minimal power to compare outcomes between groups.
Cure rates were assessed three ways: anatomically, by virtue of symptoms, and by a combination of the two measures. Participants had documentation of at least 2-year anatomic outcomes and 3-year subjective outcomes using validated measures.
Forty-one (63%) of the original 65 women in the trial had anatomic outcomes (20 in the mesh group vs 21 in the no-mesh group), and 51 (78%) of the original 65 women had evaluable subjective outcomes (25 in the mesh group vs 26 in the no-mesh group).
Women who underwent reoperation for recurrent prolapse were removed from any outcomes analysis and considered to have failed composite outcomes measures (anatomic and subjective assessment and whether reoperation or a pessary was required for recurrent prolapse).
The length of follow-up was similar between groups (median, 3 years; interquartile range, 2.97–3.15), and both groups demonstrated significant anatomic and subjective improvement from baseline.
No difference was observed between groups in the original primary anatomic outcome, which was a POP-Q stage no greater than 1 (45% in the mesh group vs 43% in the no-mesh group; P >.99). Nor was there a difference between groups in any other anatomic outcome, including POP-Q point Ba (median, –1.5 for mesh [range, –2.5, 1.0] vs –0.5 [range, –3.0, 4.0] for the no-mesh group; P = .21) and bulge symptoms (92% for the mesh group vs 81% for the no-mesh group; relative risk, 1.4; 95% confidence interval, 0.91–1.42).
Despite small numbers and markedly reduced comparative validity (readily acknowledged by the investigators), these longer-term outcomes were assessed by examiners blinded to treatment and using validated objective and subjective outcome measures.
The only other randomized trial of mesh versus native-tissue repair with 3-year outcomes had a much larger sample size and follow-up but addressed only anterior-compartment prolapse.3
What this evidence means for practice
The 3-year data presented by Gutman and colleagues should be viewed with caution, owing to the trial’s reduced sample size and power. However, they may be useful in designing future trials.
In the meantime, given the limited longer-term outcomes data available at present, I would recommend continued individualized use of mesh versus native-tissue repair in women presenting with prolapse, including educating patients about the risks and benefits of both approaches. It also is important that outcomes be followed in all of our patients in a robust, unbiased fashion. The new American Urogynecologic Society Pelvic Floor Disorders Registry provides the opportunity for this.
Holly E. Richter, PhD, MD
WE WANT TO HEAR FROM YOU!
Drop us a line and let us know what you think about current articles, which topics you'd like to see covered in future issues, and what challenges you face in daily practice. Tell us what you think by emailing us at: [email protected]
- Iglesia CB, Sokol AI, Sokol ER, et al. Vaginal mesh for prolapse: A randomized controlled trial. Obstet Gynecol. 2010;116(2 Pt 1):293–303.
- Sokol AI, Iglesia CB, Kudish BI, et al. One-year objective and functional outcomes of a randomized clinical trial of vaginal mesh for prolapse. Am J Obstet Gynecol. 2012;206(1):86.e1–e9.
- Nieminen K, Hiltunen R, Takala T, et al. Outcomes after anterior vaginal wall repair with mesh: A randomized, controlled trial with a 3-year follow-up. Am J Obstet Gynecol. 2010;203(3):235.e1–e8.
This is the third report from Gutman and colleagues on the outcomes of a double-blind, multicenter, randomized, controlled trial of vaginal prolapse repair using synthetic mesh versus native-tissue colpopexy in women with significant vaginal prolapse.
The trial involved 33 women who underwent mesh repair and 32 who underwent repair without mesh. (The mesh-free repair consisted primarily of uterosacral suspension and concurrent colporrhaphy.) It was halted when it reached a predetermined threshold for discontinuation, which was a mesh erosion rate of 15% or more.
Investigators found no difference in long-term cure rates between the mesh and no-mesh groups, regardless of the definition of cure (ie, anatomic, symptomatic, or combined). Nor was there a difference in the overall recurrence rate.
Summary of earlier reports
Three-month outcomes. The first report from this trial described 3-month objective treatment outcomes, with success described as prolapse no greater than stage 1.1 It found a high erosion rate (15.6%) for vaginal mesh, with no differences between groups in overall subjective or objective cure rates, with an overall recurrence rate of 59.4% (19 cases) in the mesh group versus 70.4% (24 cases) in the no-mesh group (P = .28), with recurrence defined as prolapse beyond stage 1 in any compartment. Investigators also observed potential benefit in the mesh group in the anterior vaginal wall at point Ba at a median of 9.7 months after surgery.
Related Article: Stop using synthetic mesh for routine repair of pelvic organ prolapse Cheryl B. Iglesia, MD (Stop/Start, April 2013)
One-year outcomes. The second report described 1-year objective and functional outcomes in all participants of the trial.2 It found comparable objective and subjective cure rates between groups but a higher reoperation rate for mesh repairs. Prolapse recurred in the anterior department in 46.9% of women in the mesh group versus 60.6% in the no-mesh group (P = .40).
Subjective quality-of-life assessments continued to reflect significant improvement in symptoms from baseline. Vaginal bulging was relieved in 96.2% of women in the mesh group, compared with 90.9% in the no-mesh group (P = .62).
More women in the mesh group required reoperation for recurrent prolapse or mesh exposure (5 in the mesh group vs 0 in the no-mesh group; P = .017).
Strengths and limitations of the trial
Gutman and colleagues are to be congratulated for continuing to monitor longer-term outcomes of vaginal prolapse repairs augmented with synthetic mesh, as data are sorely needed on both early complications and those more remote from surgery. However, it is regrettable that continued attrition in this trial led to minimal power to compare outcomes between groups.
Cure rates were assessed three ways: anatomically, by virtue of symptoms, and by a combination of the two measures. Participants had documentation of at least 2-year anatomic outcomes and 3-year subjective outcomes using validated measures.
Forty-one (63%) of the original 65 women in the trial had anatomic outcomes (20 in the mesh group vs 21 in the no-mesh group), and 51 (78%) of the original 65 women had evaluable subjective outcomes (25 in the mesh group vs 26 in the no-mesh group).
Women who underwent reoperation for recurrent prolapse were removed from any outcomes analysis and considered to have failed composite outcomes measures (anatomic and subjective assessment and whether reoperation or a pessary was required for recurrent prolapse).
The length of follow-up was similar between groups (median, 3 years; interquartile range, 2.97–3.15), and both groups demonstrated significant anatomic and subjective improvement from baseline.
No difference was observed between groups in the original primary anatomic outcome, which was a POP-Q stage no greater than 1 (45% in the mesh group vs 43% in the no-mesh group; P >.99). Nor was there a difference between groups in any other anatomic outcome, including POP-Q point Ba (median, –1.5 for mesh [range, –2.5, 1.0] vs –0.5 [range, –3.0, 4.0] for the no-mesh group; P = .21) and bulge symptoms (92% for the mesh group vs 81% for the no-mesh group; relative risk, 1.4; 95% confidence interval, 0.91–1.42).
Despite small numbers and markedly reduced comparative validity (readily acknowledged by the investigators), these longer-term outcomes were assessed by examiners blinded to treatment and using validated objective and subjective outcome measures.
The only other randomized trial of mesh versus native-tissue repair with 3-year outcomes had a much larger sample size and follow-up but addressed only anterior-compartment prolapse.3
What this evidence means for practice
The 3-year data presented by Gutman and colleagues should be viewed with caution, owing to the trial’s reduced sample size and power. However, they may be useful in designing future trials.
In the meantime, given the limited longer-term outcomes data available at present, I would recommend continued individualized use of mesh versus native-tissue repair in women presenting with prolapse, including educating patients about the risks and benefits of both approaches. It also is important that outcomes be followed in all of our patients in a robust, unbiased fashion. The new American Urogynecologic Society Pelvic Floor Disorders Registry provides the opportunity for this.
Holly E. Richter, PhD, MD
WE WANT TO HEAR FROM YOU!
Drop us a line and let us know what you think about current articles, which topics you'd like to see covered in future issues, and what challenges you face in daily practice. Tell us what you think by emailing us at: [email protected]
This is the third report from Gutman and colleagues on the outcomes of a double-blind, multicenter, randomized, controlled trial of vaginal prolapse repair using synthetic mesh versus native-tissue colpopexy in women with significant vaginal prolapse.
The trial involved 33 women who underwent mesh repair and 32 who underwent repair without mesh. (The mesh-free repair consisted primarily of uterosacral suspension and concurrent colporrhaphy.) It was halted when it reached a predetermined threshold for discontinuation, which was a mesh erosion rate of 15% or more.
Investigators found no difference in long-term cure rates between the mesh and no-mesh groups, regardless of the definition of cure (ie, anatomic, symptomatic, or combined). Nor was there a difference in the overall recurrence rate.
Summary of earlier reports
Three-month outcomes. The first report from this trial described 3-month objective treatment outcomes, with success described as prolapse no greater than stage 1.1 It found a high erosion rate (15.6%) for vaginal mesh, with no differences between groups in overall subjective or objective cure rates, with an overall recurrence rate of 59.4% (19 cases) in the mesh group versus 70.4% (24 cases) in the no-mesh group (P = .28), with recurrence defined as prolapse beyond stage 1 in any compartment. Investigators also observed potential benefit in the mesh group in the anterior vaginal wall at point Ba at a median of 9.7 months after surgery.
Related Article: Stop using synthetic mesh for routine repair of pelvic organ prolapse Cheryl B. Iglesia, MD (Stop/Start, April 2013)
One-year outcomes. The second report described 1-year objective and functional outcomes in all participants of the trial.2 It found comparable objective and subjective cure rates between groups but a higher reoperation rate for mesh repairs. Prolapse recurred in the anterior department in 46.9% of women in the mesh group versus 60.6% in the no-mesh group (P = .40).
Subjective quality-of-life assessments continued to reflect significant improvement in symptoms from baseline. Vaginal bulging was relieved in 96.2% of women in the mesh group, compared with 90.9% in the no-mesh group (P = .62).
More women in the mesh group required reoperation for recurrent prolapse or mesh exposure (5 in the mesh group vs 0 in the no-mesh group; P = .017).
Strengths and limitations of the trial
Gutman and colleagues are to be congratulated for continuing to monitor longer-term outcomes of vaginal prolapse repairs augmented with synthetic mesh, as data are sorely needed on both early complications and those more remote from surgery. However, it is regrettable that continued attrition in this trial led to minimal power to compare outcomes between groups.
Cure rates were assessed three ways: anatomically, by virtue of symptoms, and by a combination of the two measures. Participants had documentation of at least 2-year anatomic outcomes and 3-year subjective outcomes using validated measures.
Forty-one (63%) of the original 65 women in the trial had anatomic outcomes (20 in the mesh group vs 21 in the no-mesh group), and 51 (78%) of the original 65 women had evaluable subjective outcomes (25 in the mesh group vs 26 in the no-mesh group).
Women who underwent reoperation for recurrent prolapse were removed from any outcomes analysis and considered to have failed composite outcomes measures (anatomic and subjective assessment and whether reoperation or a pessary was required for recurrent prolapse).
The length of follow-up was similar between groups (median, 3 years; interquartile range, 2.97–3.15), and both groups demonstrated significant anatomic and subjective improvement from baseline.
No difference was observed between groups in the original primary anatomic outcome, which was a POP-Q stage no greater than 1 (45% in the mesh group vs 43% in the no-mesh group; P >.99). Nor was there a difference between groups in any other anatomic outcome, including POP-Q point Ba (median, –1.5 for mesh [range, –2.5, 1.0] vs –0.5 [range, –3.0, 4.0] for the no-mesh group; P = .21) and bulge symptoms (92% for the mesh group vs 81% for the no-mesh group; relative risk, 1.4; 95% confidence interval, 0.91–1.42).
Despite small numbers and markedly reduced comparative validity (readily acknowledged by the investigators), these longer-term outcomes were assessed by examiners blinded to treatment and using validated objective and subjective outcome measures.
The only other randomized trial of mesh versus native-tissue repair with 3-year outcomes had a much larger sample size and follow-up but addressed only anterior-compartment prolapse.3
What this evidence means for practice
The 3-year data presented by Gutman and colleagues should be viewed with caution, owing to the trial’s reduced sample size and power. However, they may be useful in designing future trials.
In the meantime, given the limited longer-term outcomes data available at present, I would recommend continued individualized use of mesh versus native-tissue repair in women presenting with prolapse, including educating patients about the risks and benefits of both approaches. It also is important that outcomes be followed in all of our patients in a robust, unbiased fashion. The new American Urogynecologic Society Pelvic Floor Disorders Registry provides the opportunity for this.
Holly E. Richter, PhD, MD
WE WANT TO HEAR FROM YOU!
Drop us a line and let us know what you think about current articles, which topics you'd like to see covered in future issues, and what challenges you face in daily practice. Tell us what you think by emailing us at: [email protected]
- Iglesia CB, Sokol AI, Sokol ER, et al. Vaginal mesh for prolapse: A randomized controlled trial. Obstet Gynecol. 2010;116(2 Pt 1):293–303.
- Sokol AI, Iglesia CB, Kudish BI, et al. One-year objective and functional outcomes of a randomized clinical trial of vaginal mesh for prolapse. Am J Obstet Gynecol. 2012;206(1):86.e1–e9.
- Nieminen K, Hiltunen R, Takala T, et al. Outcomes after anterior vaginal wall repair with mesh: A randomized, controlled trial with a 3-year follow-up. Am J Obstet Gynecol. 2010;203(3):235.e1–e8.
- Iglesia CB, Sokol AI, Sokol ER, et al. Vaginal mesh for prolapse: A randomized controlled trial. Obstet Gynecol. 2010;116(2 Pt 1):293–303.
- Sokol AI, Iglesia CB, Kudish BI, et al. One-year objective and functional outcomes of a randomized clinical trial of vaginal mesh for prolapse. Am J Obstet Gynecol. 2012;206(1):86.e1–e9.
- Nieminen K, Hiltunen R, Takala T, et al. Outcomes after anterior vaginal wall repair with mesh: A randomized, controlled trial with a 3-year follow-up. Am J Obstet Gynecol. 2010;203(3):235.e1–e8.
What's the appropriate lens to use in rigid cystoscopy to evaluate the bladder?
Dr. Gebhart says an angled lens is critical to viewing the bladder, but which angle is ideal?
When Dr. Gebhart surveyed attendees of the Pelvic Anatomy and Gynecology Symposium in Las Vegas, Nevada, in December 2013, as to which lens angle was the best option, the majority chose the 30-degree lens. Listen to why Dr. Gebhart recommends the 70-degree lens.
Dr. Gebhart says an angled lens is critical to viewing the bladder, but which angle is ideal?
When Dr. Gebhart surveyed attendees of the Pelvic Anatomy and Gynecology Symposium in Las Vegas, Nevada, in December 2013, as to which lens angle was the best option, the majority chose the 30-degree lens. Listen to why Dr. Gebhart recommends the 70-degree lens.
Dr. Gebhart says an angled lens is critical to viewing the bladder, but which angle is ideal?
When Dr. Gebhart surveyed attendees of the Pelvic Anatomy and Gynecology Symposium in Las Vegas, Nevada, in December 2013, as to which lens angle was the best option, the majority chose the 30-degree lens. Listen to why Dr. Gebhart recommends the 70-degree lens.
Electronic Health Records Can Complicate Who Does What in a Hospital
The accumulated wisdom, research data, and opinions regarding the use of electronic health records (EHRs) are vast. A quick Internet search turns up many informative articles on their positive and negative effects. But I haven’t found many that explicitly review the unanticipated effects EHRs have on who does what in the hospital.
For example, when reports such as admission and discharge notes are done via recorded dictation and transcription, the author would typically dictate where copies of the report should be sent (“copy to Dr. Matheny”) and rely on others to ensure it reached its intended destination. In many hospitals, such reports are now typed directly into the EHR, often using speech recognition software, and it is up to the author to click several buttons to ensure that it is routed to the intended recipients. So now a clerical function, sending reports, is handled by providers. This can be a good thing—reduced clerical staffing costs, faster transmission of reports—but often means that there is no documentation within the report itself of whom it was sent to (i.e., no list of “cc’s”). It also means that when the recipient isn’t easy to find, the report author is likely to give up, and the report may never be sent.
Any hospitalist using an EHR could easily list dozens of similar unanticipated effects, both good and bad. The magnitude and risk of these are difficult to quantify.
Altered Referral Patterns, Division of Labor
A hospitalist-specific side effect of EHR adoption is that they tend to cause many other doctors to resist serving as attending physician, instead asking hospitalists to replace them in that role. Even without EHRs, shifting attending responsibility to hospitalists has been a trend at nearly every hospital for years, but it can be accelerated dramatically at the time of a “go live.” So, in addition to the stress of adapting to the new EHR, hospitalists typically face higher than usual patient volumes resulting from increased referrals from other doctors.
If you’re a hospitalist facing an upcoming “go live,” it would be worth talking to other doctors in multiple specialties regarding your capacity to handle additional work. Keep in mind the possibility of higher than typical winter 2014 patient volumes that could result from patients who are newly insured through health exchanges.
Many factors, in addition to EHRs, are moving physicians away from a willingness to serve as attending, including the complexity of managing inpatient vs. observation status, keeping up with ever-changing documentation, pay-for-performance initiatives, the stress of ED call, and so on. As I’ve written before (see my January 2011 column, “Health IT Hurdles,”), I think effective management of hospital systems is becoming as complicated as safely piloting a jumbo jet. It will be increasingly difficult for doctors in any specialty to stay proficient at “piloting” a hospital unless they do it all or most of the time. And, staying proficient at multiple hospitals simultaneously may not be feasible at some point. We’ll see.
When Do Things Get Done?
A friend of mine, Dr. John Maa, is a general surgeon who was instrumental in establishing one of the first general surgery hospitalist practices. He tells a very personal and tragic story of his mother’s death, which, he has come to believe, might have been made more likely because of the unintended effect of an EHR.
She was a healthy 69-year-old who developed new onset atrial fibrillation and went to “one of the most highly regarded academic medical centers on the West Coast,” albeit not a facility where John was practicing. She was admitted with orders for anticoagulation but spent her first night on a stretcher in the ED because no inpatient bed was available. She went to a hospital room the next day, but her late arrival there delayed the planned transesophageal echo and cardioversion by another day.
Tragically, before the cardioversion could be done, she had a very large embolic stroke that led to brain herniation. A short time later, John and his father made the wrenching decision to discontinue mechanical ventilation. She died 112 hours after walking into the hospital.
What John later learned is that the admission orders written while she was in the ED were put into “sign and hold” status in the hospital’s EHR. Her caregivers had not anticipated a significant delay in moving her to an inpatient bed, and for the 18 or so hours she spent boarding in the ED, her admission orders were not acted on, and anticoagulation was delayed many hours. She might have had the same outcome even if anticoagulation had been started promptly, but it would have been much less likely.
John believes that the “sign and hold” status of the admission orders was a major contributor to the treatment delay. It increased the risk that the ED caregivers never acted on those orders, and may not have even seen them, since the EHR essentially holds them for presentation to the receiving inpatient unit.
John only recognized this vulnerability three years after his mother’s passing, when he underwent the physician training for the same EHR system. The course teachers agreed that this problem could arise if a patient was boarded in the ED for a prolonged period but felt that the responsibility rested with hospital administrators to minimize overcrowding in the ED. John also raised the issue with hospital leadership, who shared his concern but believed that a software remedy should be the solution. Ultimately, the answer may come from medical hospitalists, who recognize that every day and night, patients across America are at risk for a repeat of the incident John’s family suffered nearly five years ago.
In a very well written and moving essay, John describes his mother’s care.1 Though he doesn’t specifically mention the likely contribution of the “sign and hold” orders, it is one more example of EHR-related confusion that can arise around who does what and when they should do it. Clearly, the same sort of confusion exists in a non-EHR hospital, but it is the EHR-related change in the previous way of doing things that likely increases risk.
It can be very difficult—even impossible—to see all of these issues in advance. Even when acknowledged, the challenges can be difficult to address. But the first step is to recognize a problem, or potential problem, and think carefully about how it should be addressed.
Dr. Nelson has been a practicing hospitalist since 1988. He is co-founder and past president of SHM, and principal in Nelson Flores Hospital Medicine Consultants. He is co-director for SHM’s “Best Practices in Managing a Hospital Medicine Program” course. Write to him at [email protected].
Reference
The accumulated wisdom, research data, and opinions regarding the use of electronic health records (EHRs) are vast. A quick Internet search turns up many informative articles on their positive and negative effects. But I haven’t found many that explicitly review the unanticipated effects EHRs have on who does what in the hospital.
For example, when reports such as admission and discharge notes are done via recorded dictation and transcription, the author would typically dictate where copies of the report should be sent (“copy to Dr. Matheny”) and rely on others to ensure it reached its intended destination. In many hospitals, such reports are now typed directly into the EHR, often using speech recognition software, and it is up to the author to click several buttons to ensure that it is routed to the intended recipients. So now a clerical function, sending reports, is handled by providers. This can be a good thing—reduced clerical staffing costs, faster transmission of reports—but often means that there is no documentation within the report itself of whom it was sent to (i.e., no list of “cc’s”). It also means that when the recipient isn’t easy to find, the report author is likely to give up, and the report may never be sent.
Any hospitalist using an EHR could easily list dozens of similar unanticipated effects, both good and bad. The magnitude and risk of these are difficult to quantify.
Altered Referral Patterns, Division of Labor
A hospitalist-specific side effect of EHR adoption is that they tend to cause many other doctors to resist serving as attending physician, instead asking hospitalists to replace them in that role. Even without EHRs, shifting attending responsibility to hospitalists has been a trend at nearly every hospital for years, but it can be accelerated dramatically at the time of a “go live.” So, in addition to the stress of adapting to the new EHR, hospitalists typically face higher than usual patient volumes resulting from increased referrals from other doctors.
If you’re a hospitalist facing an upcoming “go live,” it would be worth talking to other doctors in multiple specialties regarding your capacity to handle additional work. Keep in mind the possibility of higher than typical winter 2014 patient volumes that could result from patients who are newly insured through health exchanges.
Many factors, in addition to EHRs, are moving physicians away from a willingness to serve as attending, including the complexity of managing inpatient vs. observation status, keeping up with ever-changing documentation, pay-for-performance initiatives, the stress of ED call, and so on. As I’ve written before (see my January 2011 column, “Health IT Hurdles,”), I think effective management of hospital systems is becoming as complicated as safely piloting a jumbo jet. It will be increasingly difficult for doctors in any specialty to stay proficient at “piloting” a hospital unless they do it all or most of the time. And, staying proficient at multiple hospitals simultaneously may not be feasible at some point. We’ll see.
When Do Things Get Done?
A friend of mine, Dr. John Maa, is a general surgeon who was instrumental in establishing one of the first general surgery hospitalist practices. He tells a very personal and tragic story of his mother’s death, which, he has come to believe, might have been made more likely because of the unintended effect of an EHR.
She was a healthy 69-year-old who developed new onset atrial fibrillation and went to “one of the most highly regarded academic medical centers on the West Coast,” albeit not a facility where John was practicing. She was admitted with orders for anticoagulation but spent her first night on a stretcher in the ED because no inpatient bed was available. She went to a hospital room the next day, but her late arrival there delayed the planned transesophageal echo and cardioversion by another day.
Tragically, before the cardioversion could be done, she had a very large embolic stroke that led to brain herniation. A short time later, John and his father made the wrenching decision to discontinue mechanical ventilation. She died 112 hours after walking into the hospital.
What John later learned is that the admission orders written while she was in the ED were put into “sign and hold” status in the hospital’s EHR. Her caregivers had not anticipated a significant delay in moving her to an inpatient bed, and for the 18 or so hours she spent boarding in the ED, her admission orders were not acted on, and anticoagulation was delayed many hours. She might have had the same outcome even if anticoagulation had been started promptly, but it would have been much less likely.
John believes that the “sign and hold” status of the admission orders was a major contributor to the treatment delay. It increased the risk that the ED caregivers never acted on those orders, and may not have even seen them, since the EHR essentially holds them for presentation to the receiving inpatient unit.
John only recognized this vulnerability three years after his mother’s passing, when he underwent the physician training for the same EHR system. The course teachers agreed that this problem could arise if a patient was boarded in the ED for a prolonged period but felt that the responsibility rested with hospital administrators to minimize overcrowding in the ED. John also raised the issue with hospital leadership, who shared his concern but believed that a software remedy should be the solution. Ultimately, the answer may come from medical hospitalists, who recognize that every day and night, patients across America are at risk for a repeat of the incident John’s family suffered nearly five years ago.
In a very well written and moving essay, John describes his mother’s care.1 Though he doesn’t specifically mention the likely contribution of the “sign and hold” orders, it is one more example of EHR-related confusion that can arise around who does what and when they should do it. Clearly, the same sort of confusion exists in a non-EHR hospital, but it is the EHR-related change in the previous way of doing things that likely increases risk.
It can be very difficult—even impossible—to see all of these issues in advance. Even when acknowledged, the challenges can be difficult to address. But the first step is to recognize a problem, or potential problem, and think carefully about how it should be addressed.
Dr. Nelson has been a practicing hospitalist since 1988. He is co-founder and past president of SHM, and principal in Nelson Flores Hospital Medicine Consultants. He is co-director for SHM’s “Best Practices in Managing a Hospital Medicine Program” course. Write to him at [email protected].
Reference
The accumulated wisdom, research data, and opinions regarding the use of electronic health records (EHRs) are vast. A quick Internet search turns up many informative articles on their positive and negative effects. But I haven’t found many that explicitly review the unanticipated effects EHRs have on who does what in the hospital.
For example, when reports such as admission and discharge notes are done via recorded dictation and transcription, the author would typically dictate where copies of the report should be sent (“copy to Dr. Matheny”) and rely on others to ensure it reached its intended destination. In many hospitals, such reports are now typed directly into the EHR, often using speech recognition software, and it is up to the author to click several buttons to ensure that it is routed to the intended recipients. So now a clerical function, sending reports, is handled by providers. This can be a good thing—reduced clerical staffing costs, faster transmission of reports—but often means that there is no documentation within the report itself of whom it was sent to (i.e., no list of “cc’s”). It also means that when the recipient isn’t easy to find, the report author is likely to give up, and the report may never be sent.
Any hospitalist using an EHR could easily list dozens of similar unanticipated effects, both good and bad. The magnitude and risk of these are difficult to quantify.
Altered Referral Patterns, Division of Labor
A hospitalist-specific side effect of EHR adoption is that they tend to cause many other doctors to resist serving as attending physician, instead asking hospitalists to replace them in that role. Even without EHRs, shifting attending responsibility to hospitalists has been a trend at nearly every hospital for years, but it can be accelerated dramatically at the time of a “go live.” So, in addition to the stress of adapting to the new EHR, hospitalists typically face higher than usual patient volumes resulting from increased referrals from other doctors.
If you’re a hospitalist facing an upcoming “go live,” it would be worth talking to other doctors in multiple specialties regarding your capacity to handle additional work. Keep in mind the possibility of higher than typical winter 2014 patient volumes that could result from patients who are newly insured through health exchanges.
Many factors, in addition to EHRs, are moving physicians away from a willingness to serve as attending, including the complexity of managing inpatient vs. observation status, keeping up with ever-changing documentation, pay-for-performance initiatives, the stress of ED call, and so on. As I’ve written before (see my January 2011 column, “Health IT Hurdles,”), I think effective management of hospital systems is becoming as complicated as safely piloting a jumbo jet. It will be increasingly difficult for doctors in any specialty to stay proficient at “piloting” a hospital unless they do it all or most of the time. And, staying proficient at multiple hospitals simultaneously may not be feasible at some point. We’ll see.
When Do Things Get Done?
A friend of mine, Dr. John Maa, is a general surgeon who was instrumental in establishing one of the first general surgery hospitalist practices. He tells a very personal and tragic story of his mother’s death, which, he has come to believe, might have been made more likely because of the unintended effect of an EHR.
She was a healthy 69-year-old who developed new onset atrial fibrillation and went to “one of the most highly regarded academic medical centers on the West Coast,” albeit not a facility where John was practicing. She was admitted with orders for anticoagulation but spent her first night on a stretcher in the ED because no inpatient bed was available. She went to a hospital room the next day, but her late arrival there delayed the planned transesophageal echo and cardioversion by another day.
Tragically, before the cardioversion could be done, she had a very large embolic stroke that led to brain herniation. A short time later, John and his father made the wrenching decision to discontinue mechanical ventilation. She died 112 hours after walking into the hospital.
What John later learned is that the admission orders written while she was in the ED were put into “sign and hold” status in the hospital’s EHR. Her caregivers had not anticipated a significant delay in moving her to an inpatient bed, and for the 18 or so hours she spent boarding in the ED, her admission orders were not acted on, and anticoagulation was delayed many hours. She might have had the same outcome even if anticoagulation had been started promptly, but it would have been much less likely.
John believes that the “sign and hold” status of the admission orders was a major contributor to the treatment delay. It increased the risk that the ED caregivers never acted on those orders, and may not have even seen them, since the EHR essentially holds them for presentation to the receiving inpatient unit.
John only recognized this vulnerability three years after his mother’s passing, when he underwent the physician training for the same EHR system. The course teachers agreed that this problem could arise if a patient was boarded in the ED for a prolonged period but felt that the responsibility rested with hospital administrators to minimize overcrowding in the ED. John also raised the issue with hospital leadership, who shared his concern but believed that a software remedy should be the solution. Ultimately, the answer may come from medical hospitalists, who recognize that every day and night, patients across America are at risk for a repeat of the incident John’s family suffered nearly five years ago.
In a very well written and moving essay, John describes his mother’s care.1 Though he doesn’t specifically mention the likely contribution of the “sign and hold” orders, it is one more example of EHR-related confusion that can arise around who does what and when they should do it. Clearly, the same sort of confusion exists in a non-EHR hospital, but it is the EHR-related change in the previous way of doing things that likely increases risk.
It can be very difficult—even impossible—to see all of these issues in advance. Even when acknowledged, the challenges can be difficult to address. But the first step is to recognize a problem, or potential problem, and think carefully about how it should be addressed.
Dr. Nelson has been a practicing hospitalist since 1988. He is co-founder and past president of SHM, and principal in Nelson Flores Hospital Medicine Consultants. He is co-director for SHM’s “Best Practices in Managing a Hospital Medicine Program” course. Write to him at [email protected].