User login
Child of The New Gastroenterologist
Role of gastroenterologists in the U.S. in the management of gastric cancer
Introduction
Although gastric cancer is one of the most common causes of cancer death in the world, the burden of gastric cancer in the United States tends to be underestimated relative to that of other cancers of the digestive system. In fact, the 5-year survival rate from gastric cancer remains poor (~32%)1 in the United States, and this is largely because gastric cancers are not diagnosed at an early stage when curative therapeutic options are available. Cumulative epidemiologic data consistently demonstrate that the incidence of gastric cancer in the United States varies according to ethnicity, immigrant status, and country of origin. It is important for practicing gastroenterologists in the United States to recognize individual risk profiles and identify people at higher risk for gastric cancer. Hereditary diffuse gastric cancer is an inherited form of diffuse-type gastric cancer and has pathogenic variants in the E-cadherin gene that are inherited in an autosomal dominant pattern. The lifetime risk of gastric cancer in individuals with HDGC is very high, and prophylactic total gastrectomy is usually advised. This article focuses on intestinal type cancer.
Epidemiology
Gastric cancer (proximal and distal gastric cancer combined) is the fifth most frequently diagnosed cancer and the third most common cause of cancer death worldwide, with 1,033,701 new cases and 782,685 deaths in 2018.2 Gastric cancer is subcategorized based on location (proximal [i.e., esophagogastric junctional, gastric cardia] and distal) and histology (intestinal and diffuse type), and each subtype is considered to have a distinct pathogenesis. Distal intestinal type gastric cancer is most commonly encountered in clinical practice. In this article, gastric cancer will signify distal intestinal type gastric cancer unless it is otherwise noted. In general, incidence rates are about twofold higher in men than in women. There is marked geographic variation in incidence rates, and the age-standardized incidence rates in eastern Asia (32.1 and 13.2, per 100,000) are approximately six times higher than those in northern America (5.6 and 2.8, per 100,000) in both men and women, respectively.2 Recent studies evaluating global trends in the incidence and mortality of gastric cancer have demonstrated decreases worldwide.3-5 However, the degree of decrease in the incidence and mortality of gastric cancer varies substantially across geographic regions, reflecting the heterogeneous distribution of risk profiles. A comprehensive analysis of a U.S. population registry demonstrated a linear decrease in the incidence of gastric cancer in the United States (0.94% decrease per year between 2001 and 2015),6 though the annual percent change in the gastric cancer mortality in the United States was lower (around 2% decrease per year between 1980 and 2011) than in other countries.3Several population-based studies conducted in the United States have demonstrated that the incidence of gastric cancer varied by ethnicity, immigrant status, and country of origin, and the highest incidence was observed among Asian immigrants.7,8 A comprehensive meta-analysis examining the risk of gastric cancer in immigrants from high-incidence regions to low-incidence regions found a persistently higher risk of gastric cancer and related mortality among immigrants.9 These results indicate that there are important risk factors such as environmental and dietary factors in addition to the traditionally considered risk factors including male gender, age, family history, and tobacco use. A survey conducted in an ethnically and culturally diverse U.S. city showed that gastroenterology providers demonstrated knowledge deficiencies in identifying and managing patients with increased risk of gastric cancer.10 Recognizing individualized risk profiles in higher-risk groups (e.g., immigrants from higher-incidence/prevalence regions) is important for optimizing management of gastric cancer in the United States.
Assessment and management of modifiable risk factors
Helicobacter pylori, a group 1 carcinogen, is the most well-recognized risk factor for gastric cancer, particularly noncardia gastric cancer.11 Since a landmark longitudinal follow-up study in Japan demonstrated that people with H. pylori infection are more likely to develop gastric cancer than those without H. pylori infection,12 accumulating evidence largely from Asian countries has shown that eradication of H. pylori is associated with a reduced incidence of gastric cancer regardless of baseline risk.13 There are also data on the protective effect for gastric cancer of H. pylori eradication in asymptomatic individuals. Another meta-analysis of six international randomized control trials demonstrated a 34% relative risk reduction of gastric cancer occurrence in asymptomatic people (relative risk of developing gastric cancer was 0.66 in those who received eradication therapy compared with those with placebo or no treatment, 95% CI, 0.46-0.95).14 A U.S. practice guideline published after these meta-analyses recommends that all patients with a positive test indicating active infection with H. pylori should be offered treatment and testing to prove eradication,15 though the recommendation was not purely intended to reduce the gastric cancer risk in U.S. population. Subsequently, a Department of Veterans Affairs cohort study added valuable insights from a U.S. experience to the body of evidence from other countries with higher prevalence. In this study of more than 370,000 patients with a history of H. pylori infection, the detection and successful eradication of H. pylori was associated with a 76% lower incidence of gastric cancer compared with people without H. pylori treatment.16 This study also provided insight into H. pylori treatment practice patterns. Of patients with a positive H. pylori test result (stool antigen, urea breath test, or pathology), approximately 75% were prescribed an eradication regimen and only 21% of those underwent eradication tests. A low rate (24%) of eradication testing was subsequently reported by the same group among U.S. patients regardless of gastric cancer risk profiles.17 The lesson from the aforementioned study is that treatment and eradication of H. pylori even among asymptomatic U.S. patients reduces the risk of subsequent gastric cancer. However, it may be difficult to generalize the results of this study given the nature of the Veterans Affairs cohort, and more data are required to justify the implementation of nationwide preventive H. pylori screening in the general U.S. population.
Smoking has been recognized as the other important risk factor. A study from the European prospective multicenter cohort demonstrated a significant association of cigarette smoking and gastric cancer risk (HR for ever-smokers 1.45 [95% CI, 1.08-1.94], current-smokers in males 1.73 [95% CI, 1.06-2.83], and current smokers in females 1.87 [95% CI, 1.12-3.12], respectively) after adjustment for educational level, dietary consumption profiles, alcohol intake, and body mass index (BMI).18 A subsequent meta-analysis provided solid evidence of smoking as the important behavioral risk factor for gastric cancer.19 Smoking also predisposed to the development of proximal gastric cancer.20 Along with other cancers in the digestive system such as in the esophagus, colon and rectum, liver, gallbladder, and pancreas, a significant association of BMI and the risk of proximal gastric cancer (RR of the highest BMI category compared with normal BMI, 1.8 [95% CI, 1.3-2.5]) was reported, with positive dose-response relationships; however, the association was not sufficient for distal gastric cancer.21 There is also evidence to show a trend of greater alcohol consumption (>45 grams per day [about 3 drinks a day]) associated with the increased risk of gastric cancer.21 It has been thought that salt and salt-preserved food increase the risk of gastric cancer. It should be noted that the observational studies showing the associations were published from Asian countries where such foods were a substantial part of traditional diets (e.g., salted vegetables in Japan) and the incidence of gastric cancer is high. There is also a speculation that preserved foods may have been eaten in more underserved, low socioeconomic regions where refrigeration was not available and prevalence of H. pylori infection was higher. Except for documented inherited form of gastric cancer (e.g., HDGC or hereditary cancer syndromes), most gastric cancers are considered sporadic. A recent randomized study published from South Korea investigated a cohort of higher-risk asymptomatic patients with family history significant for gastric cancer. This study of 1,676 subjects with a median follow-up of 9.2 years showed that successful eradication of H. pylori in the first-degree relatives of those with gastric cancer significantly reduced the risk (HR 0.45 [95% CI, 0.21-0.94]) of developing gastric cancer.22 As previously discussed, in the United States where the prevalence of H. pylori and the incidence of gastric cancer are both lower than in some Asian countries, routine screening of asymptomatic individuals for H. pylori is not justified yet. There may be a role for screening individuals who are first-generation immigrants from areas of high gastric cancer incidence and also have a first-degree relative with gastric cancer.
Who should we consider high risk and offer screening EGD?
With available evidence to date, screening for gastric cancer in a general U.S. population is not recommended. However, it is important to acknowledge the aforementioned varying incidence of gastric cancer in the United States among ethnicity, immigrant status, and country of origin. Immigrants from high-incidence regions maintain a higher risk of gastric cancer and related mortality even after migration to lower-incidence regions. The latter comprehensive study estimated that as many as 12.7 million people (29.4% of total U.S. immigrant population) have emigrated from higher-incidence regions including East Asian and some Central American countries.9 Indeed, an opportunistic nationwide gastric cancer screening program has been implemented in South Korea (beginning at age 40, biannually)23 and Japan (beginning at age 50, biannually).24 Two decision-analytic simulation studies have provided insight into the uncertainty about the cost effectiveness for potential targeted gastric cancer screening in higher-risk populations in the United States. One study demonstrated that esophagogastroduodenoscopy (EGD) screening for otherwise asymptomatic Asian American people (as well as Hispanics and non-Hispanic Blacks) at the time of screening colonoscopy at 50 years of age with continued endoscopic surveillance every 3 years was cost effective, only if gastric intestinal metaplasia (GIM) or more advanced lesions were diagnosed at the index screening EGD.25 Previous studies analyzing the cost effectiveness for gastric cancer screening in the United States had the limitation of not stratifying according to race or ethnicity, or accounting for patients diagnosed with GIM. Subsequently, the same research group extended this model analysis and has published additional findings that this strategy is cost effective for each of the most prevalent Asian American ethnicities (Chinese, Filipino, Southeast Asian, Vietnamese, Korean, and Japanese Americans) in the United States irrespective of sex.26 Although the authors raised a limitation that additional risk factors such as family history, tobacco use, or persistent H. pylori infection were not considered in the model because data regarding differentiated noncardia gastric cancer risk among Asian American ethnicities based on these risk factors are not available.
These two model analytic studies added valuable insights to the body of evidence that subsequent EGDs after the one-time bundled EGD is cost effective for higher-risk asymptomatic people in the United States, if the index screening EGD with gastric mucosal biopsies demonstrates at least GIM. Further population-based research to elucidate risk stratification among higher-risk people will provide a schema that could standardize management and resource allocation as well as increase the cost effectiveness of a gastric cancer screening program in the United States. The degree of risk of developing gastric cancer in autoimmune gastritis varies among the reported studies.27-29 Although the benefit of endoscopic screening in patients with autoimmune gastritis has not been established, a single endoscopic evaluation should be recommended soon after the diagnosis of autoimmune gastritis in order to identify prevalent neoplastic lesions.30
Practical consideration when we perform EGD for early gastric cancer screening
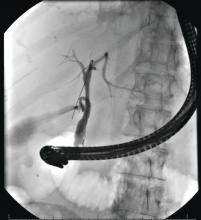
Identification of higher-risk patients should alert an endoscopist to observe mucosa with greater care with a lower threshold to biopsy any suspicious lesions. Preprocedural risk stratification for each individual before performing diagnostic EGD will improve early gastric cancer detection. While we perform EGD, detecting precursor lesions (atrophic gastritis and GIM) is as important as diagnosing an early gastric cancer. Screening and management of patients with precursor lesions (i.e., atrophic gastritis and GIM) is beyond the scope of this article, and this was published in a previous issue of the New Gastroenterologist. It is important to first grossly survey the entire gastric mucosa using high-definition while light (HDWL) endoscopy and screen for any focal irregular (raised or depressed) mucosal lesions. These lesions are often erythematous and should be examined carefully. Use of mucolytic and/or deforming agents (e.g., N-acetylcysteine or simethicone) is recommended for the improvement of visual clarity of gastric mucosa.31 Simethicone is widely used in the United States for colonoscopy and should also be available at the time of EGD for better gastric mucosal visibility. If irregular mucosal lesions are noted, this area should also be examined under narrowband imaging (NBI) in addition to HDWL. According to a simplified classification consisting of mucosal and vascular irregularity, NBI provides better mucosal surface morphology for diagnosis of early gastric cancer compared with HDWL, and a thorough examination of the surface characteristics is a prerequisite.32 This classification was further validated in a randomized control trial, and NBI increased sensitivity for the diagnosis of neoplasia compared with HDWL (92 % vs. 74 %).33 The majority of institutions in the United States have a newer-generation NBI (Olympus America, EVIS EXERA III video system, GIF-HQ190), which provides brighter endoscopic images to better characterize gastric neoplastic lesions. Once we recognize an area suspicious for neoplasia, we should describe the macroscopic features according to a classification system.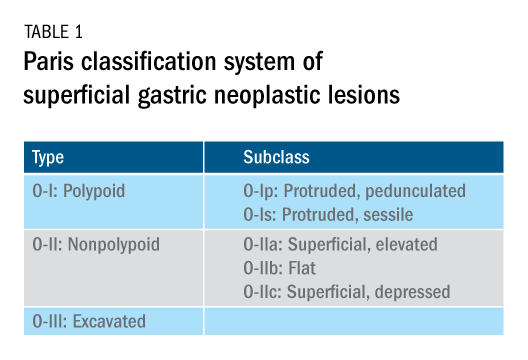
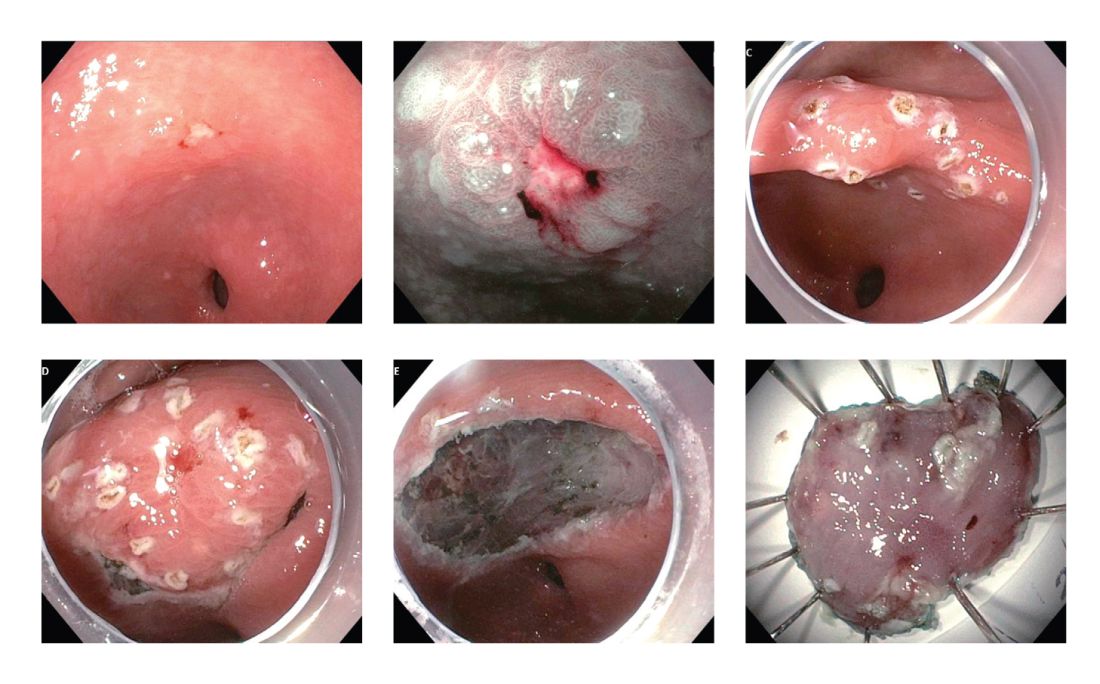
The Paris classification, one of the most widely recognized classification systems among U.S. gastroenterologists, is recommended for gastric neoplastic lesions.34Gastric neoplastic lesions with a “superficial” endoscopic appearance are classified as subtypes of “type 0.” The term “type 0” was chosen to distinguish the classification of “superficial” lesions from the Borrmann classification for “advanced” gastric tumors, which includes types 1 to 4. In the classification, a neoplastic lesion is called “superficial” when its endoscopic appearance suggests that the depth of penetration in the digestive wall is not more than into the submucosa (i.e., there is no infiltration of the muscularis propria). The distinctive characters of polypoid and nonpolypoid lesions are summarized in Table 1. Endoscopic submucosal dissection (ESD) has steadily gained acceptance for the treatment of early gastric cancer in the United States. The American Gastroenterological Association recommended in the 2019 institutional updated clinical practice guideline that ESD should be considered the first-line therapy for visible, endoscopically resectable, superficial gastric neoplasia.35 This recommendation is further supported by the published data on efficacy and safety of ESD for early gastric neoplasia in a large multicenter cohort in the United States.36 For all suspicious lesions, irrespective of pathological neoplastic confirmation, referral to an experienced center for further evaluation and endoscopic management should be considered. Lastly, all patients with early gastric cancer should be evaluated for H. pylori infection and treated if the test is positive. Eradication of H. pylori is associated with a lower rate of metachronous gastric cancer,37 and treatment of H. pylori as secondary prevention is also recommended.
Conclusion
As summarized above, cumulative epidemiologic data consistently demonstrate that the incidence of gastric cancer in the U.S. varies according to ethnicity, immigrant status, and country of origin. New gastroenterologists will need to recognize individual risk profiles and identify people at higher risk for gastric cancer. Risk stratification before performing endoscopic evaluation will improve early gastric cancer detection and make noninvasive, effective therapies an option.
References
1. Surveillance, Epidemiology, and End Results Program cancer statistics. https://seer.cancer.gov/statfacts/html/stomach.html.
2. Bray F et al. Ca Cancer J Clin. 2018;68:394-424.
3. Ferro A et al. Eur J Cancer. 2014;50:1330-44.
4. Luo G et al. Int J Cancer. 2017;141:1333-44.
5. Arnold M et al. Eur J Cancer. 2015;51:1164-87.
6. Thrift AP, El-Serag HB. Clin Gastroenterol Hepatol. 2020;18:534-42.
7. Kim Y et al. Epidemiol Health. 2015;37:e2015066.
8. Kamineni A et al. Cancer Causes Control. 1999;10:77-83.
9. Pabla BS et al. Clin Gastroenterol Hepatol. 2020;18:347-59.
10. Shah SC et al. Knowledge Gaps among Physicians Caring for Multiethnic Populations at Increased Gastric Cancer Risk. Gut Liver. 2018 Jan 15;12(1):38-45.
11. International Agency for Research on Cancer. Monographs on the Identification of Carcinogenic Hazards to Humans. IARC. July 7, 2019. 12. Uemura N et al. N Engl J Med. 2001;345:784-9.
13. Lee YC et al. Gastroenterology. 2016;150:1113-24.
14. Ford AC et al. BMJ. 2014;348:g3174.
15. Chey W et al. Am J Gastroenterol. 2017;112:212-39.
16. Kumar S et al. Gastroenterology. 2020;158:527-36.
17. Kumar S et al. Clin Gastroenterol Hepatol. 2020 Apr 6;S1542-3565(20)30436-5.
18. González CA et al. Int J Cancer. 2003;107:629-34.
19. Ladeiras-Lopes R et al. Cancer Causes Control. 2008;19:689-701.
20. Cavaleiro-Pinto M et al. Cancer Causes Control. 2011;22:375-87.
21. Lauby-Secretan B et al. N Engl J Med. 2016;375:794-8.
22. Choi IJ et al. N Engl J Med. 2020;382:427-36.
23. Kim BJ et al. World J Gastroenterol. 2013;19:736-41.
24. Hamashima C. Jpn J Clin Oncol. 2018;48:278–86.
25. Saumoy M et al. Gastroenterology. 2018;155:648-60.
26. Shah SC et al. Clin Gastroenterol Hepatol. 2020 Jul 21:S1542-3565(20)30993-9. doi: 10.1016/j.cgh.2020.07.031.
27. Brinton LA et al. Br J Cancer. 1989;59:810-3.
28. Hsing AW et al. Cancer. 1993;71:745-50.
29. Schafer LW et al. Mayo Clin Proc. 1985;60:444-8.
30. American Society for Gastrointestinal Endoscopy Standards of Practice Committee. Gastrointest Endosc. 2015;82:1-8.
31. Chiu PWY et al. Gut. 2019;68:186-97.
32. Pimentel-Nunes P et al. Endoscopy. 2012;44:236-46.
33. Pimentel-Nunes P et al. Endoscopy. 2016;48:723-30.
34. Participants in the Paris Workshop. Gastrointest Endosc. 2003;58:S3-43.
35. Draganov PV et al. Clin Gastroenterol Hepatol. 2019;17:16-25.
36. Ngamruengphong S et al. Clin Gastroenterol Hepatol. 2020 Jun 18;S1542-3565(20)30834-X. Online ahead of print.
37. Choi IJ et al. N Engl J Med. 2018;378:1085-95.
Dr. Tomizawa is a clinical assistant professor of medicine in the division of gastroenterology, University of Washington, Seattle.
Introduction
Although gastric cancer is one of the most common causes of cancer death in the world, the burden of gastric cancer in the United States tends to be underestimated relative to that of other cancers of the digestive system. In fact, the 5-year survival rate from gastric cancer remains poor (~32%)1 in the United States, and this is largely because gastric cancers are not diagnosed at an early stage when curative therapeutic options are available. Cumulative epidemiologic data consistently demonstrate that the incidence of gastric cancer in the United States varies according to ethnicity, immigrant status, and country of origin. It is important for practicing gastroenterologists in the United States to recognize individual risk profiles and identify people at higher risk for gastric cancer. Hereditary diffuse gastric cancer is an inherited form of diffuse-type gastric cancer and has pathogenic variants in the E-cadherin gene that are inherited in an autosomal dominant pattern. The lifetime risk of gastric cancer in individuals with HDGC is very high, and prophylactic total gastrectomy is usually advised. This article focuses on intestinal type cancer.
Epidemiology
Gastric cancer (proximal and distal gastric cancer combined) is the fifth most frequently diagnosed cancer and the third most common cause of cancer death worldwide, with 1,033,701 new cases and 782,685 deaths in 2018.2 Gastric cancer is subcategorized based on location (proximal [i.e., esophagogastric junctional, gastric cardia] and distal) and histology (intestinal and diffuse type), and each subtype is considered to have a distinct pathogenesis. Distal intestinal type gastric cancer is most commonly encountered in clinical practice. In this article, gastric cancer will signify distal intestinal type gastric cancer unless it is otherwise noted. In general, incidence rates are about twofold higher in men than in women. There is marked geographic variation in incidence rates, and the age-standardized incidence rates in eastern Asia (32.1 and 13.2, per 100,000) are approximately six times higher than those in northern America (5.6 and 2.8, per 100,000) in both men and women, respectively.2 Recent studies evaluating global trends in the incidence and mortality of gastric cancer have demonstrated decreases worldwide.3-5 However, the degree of decrease in the incidence and mortality of gastric cancer varies substantially across geographic regions, reflecting the heterogeneous distribution of risk profiles. A comprehensive analysis of a U.S. population registry demonstrated a linear decrease in the incidence of gastric cancer in the United States (0.94% decrease per year between 2001 and 2015),6 though the annual percent change in the gastric cancer mortality in the United States was lower (around 2% decrease per year between 1980 and 2011) than in other countries.3Several population-based studies conducted in the United States have demonstrated that the incidence of gastric cancer varied by ethnicity, immigrant status, and country of origin, and the highest incidence was observed among Asian immigrants.7,8 A comprehensive meta-analysis examining the risk of gastric cancer in immigrants from high-incidence regions to low-incidence regions found a persistently higher risk of gastric cancer and related mortality among immigrants.9 These results indicate that there are important risk factors such as environmental and dietary factors in addition to the traditionally considered risk factors including male gender, age, family history, and tobacco use. A survey conducted in an ethnically and culturally diverse U.S. city showed that gastroenterology providers demonstrated knowledge deficiencies in identifying and managing patients with increased risk of gastric cancer.10 Recognizing individualized risk profiles in higher-risk groups (e.g., immigrants from higher-incidence/prevalence regions) is important for optimizing management of gastric cancer in the United States.
Assessment and management of modifiable risk factors
Helicobacter pylori, a group 1 carcinogen, is the most well-recognized risk factor for gastric cancer, particularly noncardia gastric cancer.11 Since a landmark longitudinal follow-up study in Japan demonstrated that people with H. pylori infection are more likely to develop gastric cancer than those without H. pylori infection,12 accumulating evidence largely from Asian countries has shown that eradication of H. pylori is associated with a reduced incidence of gastric cancer regardless of baseline risk.13 There are also data on the protective effect for gastric cancer of H. pylori eradication in asymptomatic individuals. Another meta-analysis of six international randomized control trials demonstrated a 34% relative risk reduction of gastric cancer occurrence in asymptomatic people (relative risk of developing gastric cancer was 0.66 in those who received eradication therapy compared with those with placebo or no treatment, 95% CI, 0.46-0.95).14 A U.S. practice guideline published after these meta-analyses recommends that all patients with a positive test indicating active infection with H. pylori should be offered treatment and testing to prove eradication,15 though the recommendation was not purely intended to reduce the gastric cancer risk in U.S. population. Subsequently, a Department of Veterans Affairs cohort study added valuable insights from a U.S. experience to the body of evidence from other countries with higher prevalence. In this study of more than 370,000 patients with a history of H. pylori infection, the detection and successful eradication of H. pylori was associated with a 76% lower incidence of gastric cancer compared with people without H. pylori treatment.16 This study also provided insight into H. pylori treatment practice patterns. Of patients with a positive H. pylori test result (stool antigen, urea breath test, or pathology), approximately 75% were prescribed an eradication regimen and only 21% of those underwent eradication tests. A low rate (24%) of eradication testing was subsequently reported by the same group among U.S. patients regardless of gastric cancer risk profiles.17 The lesson from the aforementioned study is that treatment and eradication of H. pylori even among asymptomatic U.S. patients reduces the risk of subsequent gastric cancer. However, it may be difficult to generalize the results of this study given the nature of the Veterans Affairs cohort, and more data are required to justify the implementation of nationwide preventive H. pylori screening in the general U.S. population.
Smoking has been recognized as the other important risk factor. A study from the European prospective multicenter cohort demonstrated a significant association of cigarette smoking and gastric cancer risk (HR for ever-smokers 1.45 [95% CI, 1.08-1.94], current-smokers in males 1.73 [95% CI, 1.06-2.83], and current smokers in females 1.87 [95% CI, 1.12-3.12], respectively) after adjustment for educational level, dietary consumption profiles, alcohol intake, and body mass index (BMI).18 A subsequent meta-analysis provided solid evidence of smoking as the important behavioral risk factor for gastric cancer.19 Smoking also predisposed to the development of proximal gastric cancer.20 Along with other cancers in the digestive system such as in the esophagus, colon and rectum, liver, gallbladder, and pancreas, a significant association of BMI and the risk of proximal gastric cancer (RR of the highest BMI category compared with normal BMI, 1.8 [95% CI, 1.3-2.5]) was reported, with positive dose-response relationships; however, the association was not sufficient for distal gastric cancer.21 There is also evidence to show a trend of greater alcohol consumption (>45 grams per day [about 3 drinks a day]) associated with the increased risk of gastric cancer.21 It has been thought that salt and salt-preserved food increase the risk of gastric cancer. It should be noted that the observational studies showing the associations were published from Asian countries where such foods were a substantial part of traditional diets (e.g., salted vegetables in Japan) and the incidence of gastric cancer is high. There is also a speculation that preserved foods may have been eaten in more underserved, low socioeconomic regions where refrigeration was not available and prevalence of H. pylori infection was higher. Except for documented inherited form of gastric cancer (e.g., HDGC or hereditary cancer syndromes), most gastric cancers are considered sporadic. A recent randomized study published from South Korea investigated a cohort of higher-risk asymptomatic patients with family history significant for gastric cancer. This study of 1,676 subjects with a median follow-up of 9.2 years showed that successful eradication of H. pylori in the first-degree relatives of those with gastric cancer significantly reduced the risk (HR 0.45 [95% CI, 0.21-0.94]) of developing gastric cancer.22 As previously discussed, in the United States where the prevalence of H. pylori and the incidence of gastric cancer are both lower than in some Asian countries, routine screening of asymptomatic individuals for H. pylori is not justified yet. There may be a role for screening individuals who are first-generation immigrants from areas of high gastric cancer incidence and also have a first-degree relative with gastric cancer.
Who should we consider high risk and offer screening EGD?
With available evidence to date, screening for gastric cancer in a general U.S. population is not recommended. However, it is important to acknowledge the aforementioned varying incidence of gastric cancer in the United States among ethnicity, immigrant status, and country of origin. Immigrants from high-incidence regions maintain a higher risk of gastric cancer and related mortality even after migration to lower-incidence regions. The latter comprehensive study estimated that as many as 12.7 million people (29.4% of total U.S. immigrant population) have emigrated from higher-incidence regions including East Asian and some Central American countries.9 Indeed, an opportunistic nationwide gastric cancer screening program has been implemented in South Korea (beginning at age 40, biannually)23 and Japan (beginning at age 50, biannually).24 Two decision-analytic simulation studies have provided insight into the uncertainty about the cost effectiveness for potential targeted gastric cancer screening in higher-risk populations in the United States. One study demonstrated that esophagogastroduodenoscopy (EGD) screening for otherwise asymptomatic Asian American people (as well as Hispanics and non-Hispanic Blacks) at the time of screening colonoscopy at 50 years of age with continued endoscopic surveillance every 3 years was cost effective, only if gastric intestinal metaplasia (GIM) or more advanced lesions were diagnosed at the index screening EGD.25 Previous studies analyzing the cost effectiveness for gastric cancer screening in the United States had the limitation of not stratifying according to race or ethnicity, or accounting for patients diagnosed with GIM. Subsequently, the same research group extended this model analysis and has published additional findings that this strategy is cost effective for each of the most prevalent Asian American ethnicities (Chinese, Filipino, Southeast Asian, Vietnamese, Korean, and Japanese Americans) in the United States irrespective of sex.26 Although the authors raised a limitation that additional risk factors such as family history, tobacco use, or persistent H. pylori infection were not considered in the model because data regarding differentiated noncardia gastric cancer risk among Asian American ethnicities based on these risk factors are not available.

These two model analytic studies added valuable insights to the body of evidence that subsequent EGDs after the one-time bundled EGD is cost effective for higher-risk asymptomatic people in the United States, if the index screening EGD with gastric mucosal biopsies demonstrates at least GIM. Further population-based research to elucidate risk stratification among higher-risk people will provide a schema that could standardize management and resource allocation as well as increase the cost effectiveness of a gastric cancer screening program in the United States. The degree of risk of developing gastric cancer in autoimmune gastritis varies among the reported studies.27-29 Although the benefit of endoscopic screening in patients with autoimmune gastritis has not been established, a single endoscopic evaluation should be recommended soon after the diagnosis of autoimmune gastritis in order to identify prevalent neoplastic lesions.30
Practical consideration when we perform EGD for early gastric cancer screening
Identification of higher-risk patients should alert an endoscopist to observe mucosa with greater care with a lower threshold to biopsy any suspicious lesions. Preprocedural risk stratification for each individual before performing diagnostic EGD will improve early gastric cancer detection. While we perform EGD, detecting precursor lesions (atrophic gastritis and GIM) is as important as diagnosing an early gastric cancer. Screening and management of patients with precursor lesions (i.e., atrophic gastritis and GIM) is beyond the scope of this article, and this was published in a previous issue of the New Gastroenterologist. It is important to first grossly survey the entire gastric mucosa using high-definition while light (HDWL) endoscopy and screen for any focal irregular (raised or depressed) mucosal lesions. These lesions are often erythematous and should be examined carefully. Use of mucolytic and/or deforming agents (e.g., N-acetylcysteine or simethicone) is recommended for the improvement of visual clarity of gastric mucosa.31 Simethicone is widely used in the United States for colonoscopy and should also be available at the time of EGD for better gastric mucosal visibility. If irregular mucosal lesions are noted, this area should also be examined under narrowband imaging (NBI) in addition to HDWL. According to a simplified classification consisting of mucosal and vascular irregularity, NBI provides better mucosal surface morphology for diagnosis of early gastric cancer compared with HDWL, and a thorough examination of the surface characteristics is a prerequisite.32 This classification was further validated in a randomized control trial, and NBI increased sensitivity for the diagnosis of neoplasia compared with HDWL (92 % vs. 74 %).33 The majority of institutions in the United States have a newer-generation NBI (Olympus America, EVIS EXERA III video system, GIF-HQ190), which provides brighter endoscopic images to better characterize gastric neoplastic lesions. Once we recognize an area suspicious for neoplasia, we should describe the macroscopic features according to a classification system.
The Paris classification, one of the most widely recognized classification systems among U.S. gastroenterologists, is recommended for gastric neoplastic lesions.34Gastric neoplastic lesions with a “superficial” endoscopic appearance are classified as subtypes of “type 0.” The term “type 0” was chosen to distinguish the classification of “superficial” lesions from the Borrmann classification for “advanced” gastric tumors, which includes types 1 to 4. In the classification, a neoplastic lesion is called “superficial” when its endoscopic appearance suggests that the depth of penetration in the digestive wall is not more than into the submucosa (i.e., there is no infiltration of the muscularis propria). The distinctive characters of polypoid and nonpolypoid lesions are summarized in Table 1. Endoscopic submucosal dissection (ESD) has steadily gained acceptance for the treatment of early gastric cancer in the United States. The American Gastroenterological Association recommended in the 2019 institutional updated clinical practice guideline that ESD should be considered the first-line therapy for visible, endoscopically resectable, superficial gastric neoplasia.35 This recommendation is further supported by the published data on efficacy and safety of ESD for early gastric neoplasia in a large multicenter cohort in the United States.36 For all suspicious lesions, irrespective of pathological neoplastic confirmation, referral to an experienced center for further evaluation and endoscopic management should be considered. Lastly, all patients with early gastric cancer should be evaluated for H. pylori infection and treated if the test is positive. Eradication of H. pylori is associated with a lower rate of metachronous gastric cancer,37 and treatment of H. pylori as secondary prevention is also recommended.
Conclusion
As summarized above, cumulative epidemiologic data consistently demonstrate that the incidence of gastric cancer in the U.S. varies according to ethnicity, immigrant status, and country of origin. New gastroenterologists will need to recognize individual risk profiles and identify people at higher risk for gastric cancer. Risk stratification before performing endoscopic evaluation will improve early gastric cancer detection and make noninvasive, effective therapies an option.
References
1. Surveillance, Epidemiology, and End Results Program cancer statistics. https://seer.cancer.gov/statfacts/html/stomach.html.
2. Bray F et al. Ca Cancer J Clin. 2018;68:394-424.
3. Ferro A et al. Eur J Cancer. 2014;50:1330-44.
4. Luo G et al. Int J Cancer. 2017;141:1333-44.
5. Arnold M et al. Eur J Cancer. 2015;51:1164-87.
6. Thrift AP, El-Serag HB. Clin Gastroenterol Hepatol. 2020;18:534-42.
7. Kim Y et al. Epidemiol Health. 2015;37:e2015066.
8. Kamineni A et al. Cancer Causes Control. 1999;10:77-83.
9. Pabla BS et al. Clin Gastroenterol Hepatol. 2020;18:347-59.
10. Shah SC et al. Knowledge Gaps among Physicians Caring for Multiethnic Populations at Increased Gastric Cancer Risk. Gut Liver. 2018 Jan 15;12(1):38-45.
11. International Agency for Research on Cancer. Monographs on the Identification of Carcinogenic Hazards to Humans. IARC. July 7, 2019. 12. Uemura N et al. N Engl J Med. 2001;345:784-9.
13. Lee YC et al. Gastroenterology. 2016;150:1113-24.
14. Ford AC et al. BMJ. 2014;348:g3174.
15. Chey W et al. Am J Gastroenterol. 2017;112:212-39.
16. Kumar S et al. Gastroenterology. 2020;158:527-36.
17. Kumar S et al. Clin Gastroenterol Hepatol. 2020 Apr 6;S1542-3565(20)30436-5.
18. González CA et al. Int J Cancer. 2003;107:629-34.
19. Ladeiras-Lopes R et al. Cancer Causes Control. 2008;19:689-701.
20. Cavaleiro-Pinto M et al. Cancer Causes Control. 2011;22:375-87.
21. Lauby-Secretan B et al. N Engl J Med. 2016;375:794-8.
22. Choi IJ et al. N Engl J Med. 2020;382:427-36.
23. Kim BJ et al. World J Gastroenterol. 2013;19:736-41.
24. Hamashima C. Jpn J Clin Oncol. 2018;48:278–86.
25. Saumoy M et al. Gastroenterology. 2018;155:648-60.
26. Shah SC et al. Clin Gastroenterol Hepatol. 2020 Jul 21:S1542-3565(20)30993-9. doi: 10.1016/j.cgh.2020.07.031.
27. Brinton LA et al. Br J Cancer. 1989;59:810-3.
28. Hsing AW et al. Cancer. 1993;71:745-50.
29. Schafer LW et al. Mayo Clin Proc. 1985;60:444-8.
30. American Society for Gastrointestinal Endoscopy Standards of Practice Committee. Gastrointest Endosc. 2015;82:1-8.
31. Chiu PWY et al. Gut. 2019;68:186-97.
32. Pimentel-Nunes P et al. Endoscopy. 2012;44:236-46.
33. Pimentel-Nunes P et al. Endoscopy. 2016;48:723-30.
34. Participants in the Paris Workshop. Gastrointest Endosc. 2003;58:S3-43.
35. Draganov PV et al. Clin Gastroenterol Hepatol. 2019;17:16-25.
36. Ngamruengphong S et al. Clin Gastroenterol Hepatol. 2020 Jun 18;S1542-3565(20)30834-X. Online ahead of print.
37. Choi IJ et al. N Engl J Med. 2018;378:1085-95.
Dr. Tomizawa is a clinical assistant professor of medicine in the division of gastroenterology, University of Washington, Seattle.
Introduction
Although gastric cancer is one of the most common causes of cancer death in the world, the burden of gastric cancer in the United States tends to be underestimated relative to that of other cancers of the digestive system. In fact, the 5-year survival rate from gastric cancer remains poor (~32%)1 in the United States, and this is largely because gastric cancers are not diagnosed at an early stage when curative therapeutic options are available. Cumulative epidemiologic data consistently demonstrate that the incidence of gastric cancer in the United States varies according to ethnicity, immigrant status, and country of origin. It is important for practicing gastroenterologists in the United States to recognize individual risk profiles and identify people at higher risk for gastric cancer. Hereditary diffuse gastric cancer is an inherited form of diffuse-type gastric cancer and has pathogenic variants in the E-cadherin gene that are inherited in an autosomal dominant pattern. The lifetime risk of gastric cancer in individuals with HDGC is very high, and prophylactic total gastrectomy is usually advised. This article focuses on intestinal type cancer.
Epidemiology
Gastric cancer (proximal and distal gastric cancer combined) is the fifth most frequently diagnosed cancer and the third most common cause of cancer death worldwide, with 1,033,701 new cases and 782,685 deaths in 2018.2 Gastric cancer is subcategorized based on location (proximal [i.e., esophagogastric junctional, gastric cardia] and distal) and histology (intestinal and diffuse type), and each subtype is considered to have a distinct pathogenesis. Distal intestinal type gastric cancer is most commonly encountered in clinical practice. In this article, gastric cancer will signify distal intestinal type gastric cancer unless it is otherwise noted. In general, incidence rates are about twofold higher in men than in women. There is marked geographic variation in incidence rates, and the age-standardized incidence rates in eastern Asia (32.1 and 13.2, per 100,000) are approximately six times higher than those in northern America (5.6 and 2.8, per 100,000) in both men and women, respectively.2 Recent studies evaluating global trends in the incidence and mortality of gastric cancer have demonstrated decreases worldwide.3-5 However, the degree of decrease in the incidence and mortality of gastric cancer varies substantially across geographic regions, reflecting the heterogeneous distribution of risk profiles. A comprehensive analysis of a U.S. population registry demonstrated a linear decrease in the incidence of gastric cancer in the United States (0.94% decrease per year between 2001 and 2015),6 though the annual percent change in the gastric cancer mortality in the United States was lower (around 2% decrease per year between 1980 and 2011) than in other countries.3Several population-based studies conducted in the United States have demonstrated that the incidence of gastric cancer varied by ethnicity, immigrant status, and country of origin, and the highest incidence was observed among Asian immigrants.7,8 A comprehensive meta-analysis examining the risk of gastric cancer in immigrants from high-incidence regions to low-incidence regions found a persistently higher risk of gastric cancer and related mortality among immigrants.9 These results indicate that there are important risk factors such as environmental and dietary factors in addition to the traditionally considered risk factors including male gender, age, family history, and tobacco use. A survey conducted in an ethnically and culturally diverse U.S. city showed that gastroenterology providers demonstrated knowledge deficiencies in identifying and managing patients with increased risk of gastric cancer.10 Recognizing individualized risk profiles in higher-risk groups (e.g., immigrants from higher-incidence/prevalence regions) is important for optimizing management of gastric cancer in the United States.
Assessment and management of modifiable risk factors
Helicobacter pylori, a group 1 carcinogen, is the most well-recognized risk factor for gastric cancer, particularly noncardia gastric cancer.11 Since a landmark longitudinal follow-up study in Japan demonstrated that people with H. pylori infection are more likely to develop gastric cancer than those without H. pylori infection,12 accumulating evidence largely from Asian countries has shown that eradication of H. pylori is associated with a reduced incidence of gastric cancer regardless of baseline risk.13 There are also data on the protective effect for gastric cancer of H. pylori eradication in asymptomatic individuals. Another meta-analysis of six international randomized control trials demonstrated a 34% relative risk reduction of gastric cancer occurrence in asymptomatic people (relative risk of developing gastric cancer was 0.66 in those who received eradication therapy compared with those with placebo or no treatment, 95% CI, 0.46-0.95).14 A U.S. practice guideline published after these meta-analyses recommends that all patients with a positive test indicating active infection with H. pylori should be offered treatment and testing to prove eradication,15 though the recommendation was not purely intended to reduce the gastric cancer risk in U.S. population. Subsequently, a Department of Veterans Affairs cohort study added valuable insights from a U.S. experience to the body of evidence from other countries with higher prevalence. In this study of more than 370,000 patients with a history of H. pylori infection, the detection and successful eradication of H. pylori was associated with a 76% lower incidence of gastric cancer compared with people without H. pylori treatment.16 This study also provided insight into H. pylori treatment practice patterns. Of patients with a positive H. pylori test result (stool antigen, urea breath test, or pathology), approximately 75% were prescribed an eradication regimen and only 21% of those underwent eradication tests. A low rate (24%) of eradication testing was subsequently reported by the same group among U.S. patients regardless of gastric cancer risk profiles.17 The lesson from the aforementioned study is that treatment and eradication of H. pylori even among asymptomatic U.S. patients reduces the risk of subsequent gastric cancer. However, it may be difficult to generalize the results of this study given the nature of the Veterans Affairs cohort, and more data are required to justify the implementation of nationwide preventive H. pylori screening in the general U.S. population.
Smoking has been recognized as the other important risk factor. A study from the European prospective multicenter cohort demonstrated a significant association of cigarette smoking and gastric cancer risk (HR for ever-smokers 1.45 [95% CI, 1.08-1.94], current-smokers in males 1.73 [95% CI, 1.06-2.83], and current smokers in females 1.87 [95% CI, 1.12-3.12], respectively) after adjustment for educational level, dietary consumption profiles, alcohol intake, and body mass index (BMI).18 A subsequent meta-analysis provided solid evidence of smoking as the important behavioral risk factor for gastric cancer.19 Smoking also predisposed to the development of proximal gastric cancer.20 Along with other cancers in the digestive system such as in the esophagus, colon and rectum, liver, gallbladder, and pancreas, a significant association of BMI and the risk of proximal gastric cancer (RR of the highest BMI category compared with normal BMI, 1.8 [95% CI, 1.3-2.5]) was reported, with positive dose-response relationships; however, the association was not sufficient for distal gastric cancer.21 There is also evidence to show a trend of greater alcohol consumption (>45 grams per day [about 3 drinks a day]) associated with the increased risk of gastric cancer.21 It has been thought that salt and salt-preserved food increase the risk of gastric cancer. It should be noted that the observational studies showing the associations were published from Asian countries where such foods were a substantial part of traditional diets (e.g., salted vegetables in Japan) and the incidence of gastric cancer is high. There is also a speculation that preserved foods may have been eaten in more underserved, low socioeconomic regions where refrigeration was not available and prevalence of H. pylori infection was higher. Except for documented inherited form of gastric cancer (e.g., HDGC or hereditary cancer syndromes), most gastric cancers are considered sporadic. A recent randomized study published from South Korea investigated a cohort of higher-risk asymptomatic patients with family history significant for gastric cancer. This study of 1,676 subjects with a median follow-up of 9.2 years showed that successful eradication of H. pylori in the first-degree relatives of those with gastric cancer significantly reduced the risk (HR 0.45 [95% CI, 0.21-0.94]) of developing gastric cancer.22 As previously discussed, in the United States where the prevalence of H. pylori and the incidence of gastric cancer are both lower than in some Asian countries, routine screening of asymptomatic individuals for H. pylori is not justified yet. There may be a role for screening individuals who are first-generation immigrants from areas of high gastric cancer incidence and also have a first-degree relative with gastric cancer.
Who should we consider high risk and offer screening EGD?
With available evidence to date, screening for gastric cancer in a general U.S. population is not recommended. However, it is important to acknowledge the aforementioned varying incidence of gastric cancer in the United States among ethnicity, immigrant status, and country of origin. Immigrants from high-incidence regions maintain a higher risk of gastric cancer and related mortality even after migration to lower-incidence regions. The latter comprehensive study estimated that as many as 12.7 million people (29.4% of total U.S. immigrant population) have emigrated from higher-incidence regions including East Asian and some Central American countries.9 Indeed, an opportunistic nationwide gastric cancer screening program has been implemented in South Korea (beginning at age 40, biannually)23 and Japan (beginning at age 50, biannually).24 Two decision-analytic simulation studies have provided insight into the uncertainty about the cost effectiveness for potential targeted gastric cancer screening in higher-risk populations in the United States. One study demonstrated that esophagogastroduodenoscopy (EGD) screening for otherwise asymptomatic Asian American people (as well as Hispanics and non-Hispanic Blacks) at the time of screening colonoscopy at 50 years of age with continued endoscopic surveillance every 3 years was cost effective, only if gastric intestinal metaplasia (GIM) or more advanced lesions were diagnosed at the index screening EGD.25 Previous studies analyzing the cost effectiveness for gastric cancer screening in the United States had the limitation of not stratifying according to race or ethnicity, or accounting for patients diagnosed with GIM. Subsequently, the same research group extended this model analysis and has published additional findings that this strategy is cost effective for each of the most prevalent Asian American ethnicities (Chinese, Filipino, Southeast Asian, Vietnamese, Korean, and Japanese Americans) in the United States irrespective of sex.26 Although the authors raised a limitation that additional risk factors such as family history, tobacco use, or persistent H. pylori infection were not considered in the model because data regarding differentiated noncardia gastric cancer risk among Asian American ethnicities based on these risk factors are not available.

These two model analytic studies added valuable insights to the body of evidence that subsequent EGDs after the one-time bundled EGD is cost effective for higher-risk asymptomatic people in the United States, if the index screening EGD with gastric mucosal biopsies demonstrates at least GIM. Further population-based research to elucidate risk stratification among higher-risk people will provide a schema that could standardize management and resource allocation as well as increase the cost effectiveness of a gastric cancer screening program in the United States. The degree of risk of developing gastric cancer in autoimmune gastritis varies among the reported studies.27-29 Although the benefit of endoscopic screening in patients with autoimmune gastritis has not been established, a single endoscopic evaluation should be recommended soon after the diagnosis of autoimmune gastritis in order to identify prevalent neoplastic lesions.30
Practical consideration when we perform EGD for early gastric cancer screening
Identification of higher-risk patients should alert an endoscopist to observe mucosa with greater care with a lower threshold to biopsy any suspicious lesions. Preprocedural risk stratification for each individual before performing diagnostic EGD will improve early gastric cancer detection. While we perform EGD, detecting precursor lesions (atrophic gastritis and GIM) is as important as diagnosing an early gastric cancer. Screening and management of patients with precursor lesions (i.e., atrophic gastritis and GIM) is beyond the scope of this article, and this was published in a previous issue of the New Gastroenterologist. It is important to first grossly survey the entire gastric mucosa using high-definition while light (HDWL) endoscopy and screen for any focal irregular (raised or depressed) mucosal lesions. These lesions are often erythematous and should be examined carefully. Use of mucolytic and/or deforming agents (e.g., N-acetylcysteine or simethicone) is recommended for the improvement of visual clarity of gastric mucosa.31 Simethicone is widely used in the United States for colonoscopy and should also be available at the time of EGD for better gastric mucosal visibility. If irregular mucosal lesions are noted, this area should also be examined under narrowband imaging (NBI) in addition to HDWL. According to a simplified classification consisting of mucosal and vascular irregularity, NBI provides better mucosal surface morphology for diagnosis of early gastric cancer compared with HDWL, and a thorough examination of the surface characteristics is a prerequisite.32 This classification was further validated in a randomized control trial, and NBI increased sensitivity for the diagnosis of neoplasia compared with HDWL (92 % vs. 74 %).33 The majority of institutions in the United States have a newer-generation NBI (Olympus America, EVIS EXERA III video system, GIF-HQ190), which provides brighter endoscopic images to better characterize gastric neoplastic lesions. Once we recognize an area suspicious for neoplasia, we should describe the macroscopic features according to a classification system.
The Paris classification, one of the most widely recognized classification systems among U.S. gastroenterologists, is recommended for gastric neoplastic lesions.34Gastric neoplastic lesions with a “superficial” endoscopic appearance are classified as subtypes of “type 0.” The term “type 0” was chosen to distinguish the classification of “superficial” lesions from the Borrmann classification for “advanced” gastric tumors, which includes types 1 to 4. In the classification, a neoplastic lesion is called “superficial” when its endoscopic appearance suggests that the depth of penetration in the digestive wall is not more than into the submucosa (i.e., there is no infiltration of the muscularis propria). The distinctive characters of polypoid and nonpolypoid lesions are summarized in Table 1. Endoscopic submucosal dissection (ESD) has steadily gained acceptance for the treatment of early gastric cancer in the United States. The American Gastroenterological Association recommended in the 2019 institutional updated clinical practice guideline that ESD should be considered the first-line therapy for visible, endoscopically resectable, superficial gastric neoplasia.35 This recommendation is further supported by the published data on efficacy and safety of ESD for early gastric neoplasia in a large multicenter cohort in the United States.36 For all suspicious lesions, irrespective of pathological neoplastic confirmation, referral to an experienced center for further evaluation and endoscopic management should be considered. Lastly, all patients with early gastric cancer should be evaluated for H. pylori infection and treated if the test is positive. Eradication of H. pylori is associated with a lower rate of metachronous gastric cancer,37 and treatment of H. pylori as secondary prevention is also recommended.
Conclusion
As summarized above, cumulative epidemiologic data consistently demonstrate that the incidence of gastric cancer in the U.S. varies according to ethnicity, immigrant status, and country of origin. New gastroenterologists will need to recognize individual risk profiles and identify people at higher risk for gastric cancer. Risk stratification before performing endoscopic evaluation will improve early gastric cancer detection and make noninvasive, effective therapies an option.
References
1. Surveillance, Epidemiology, and End Results Program cancer statistics. https://seer.cancer.gov/statfacts/html/stomach.html.
2. Bray F et al. Ca Cancer J Clin. 2018;68:394-424.
3. Ferro A et al. Eur J Cancer. 2014;50:1330-44.
4. Luo G et al. Int J Cancer. 2017;141:1333-44.
5. Arnold M et al. Eur J Cancer. 2015;51:1164-87.
6. Thrift AP, El-Serag HB. Clin Gastroenterol Hepatol. 2020;18:534-42.
7. Kim Y et al. Epidemiol Health. 2015;37:e2015066.
8. Kamineni A et al. Cancer Causes Control. 1999;10:77-83.
9. Pabla BS et al. Clin Gastroenterol Hepatol. 2020;18:347-59.
10. Shah SC et al. Knowledge Gaps among Physicians Caring for Multiethnic Populations at Increased Gastric Cancer Risk. Gut Liver. 2018 Jan 15;12(1):38-45.
11. International Agency for Research on Cancer. Monographs on the Identification of Carcinogenic Hazards to Humans. IARC. July 7, 2019. 12. Uemura N et al. N Engl J Med. 2001;345:784-9.
13. Lee YC et al. Gastroenterology. 2016;150:1113-24.
14. Ford AC et al. BMJ. 2014;348:g3174.
15. Chey W et al. Am J Gastroenterol. 2017;112:212-39.
16. Kumar S et al. Gastroenterology. 2020;158:527-36.
17. Kumar S et al. Clin Gastroenterol Hepatol. 2020 Apr 6;S1542-3565(20)30436-5.
18. González CA et al. Int J Cancer. 2003;107:629-34.
19. Ladeiras-Lopes R et al. Cancer Causes Control. 2008;19:689-701.
20. Cavaleiro-Pinto M et al. Cancer Causes Control. 2011;22:375-87.
21. Lauby-Secretan B et al. N Engl J Med. 2016;375:794-8.
22. Choi IJ et al. N Engl J Med. 2020;382:427-36.
23. Kim BJ et al. World J Gastroenterol. 2013;19:736-41.
24. Hamashima C. Jpn J Clin Oncol. 2018;48:278–86.
25. Saumoy M et al. Gastroenterology. 2018;155:648-60.
26. Shah SC et al. Clin Gastroenterol Hepatol. 2020 Jul 21:S1542-3565(20)30993-9. doi: 10.1016/j.cgh.2020.07.031.
27. Brinton LA et al. Br J Cancer. 1989;59:810-3.
28. Hsing AW et al. Cancer. 1993;71:745-50.
29. Schafer LW et al. Mayo Clin Proc. 1985;60:444-8.
30. American Society for Gastrointestinal Endoscopy Standards of Practice Committee. Gastrointest Endosc. 2015;82:1-8.
31. Chiu PWY et al. Gut. 2019;68:186-97.
32. Pimentel-Nunes P et al. Endoscopy. 2012;44:236-46.
33. Pimentel-Nunes P et al. Endoscopy. 2016;48:723-30.
34. Participants in the Paris Workshop. Gastrointest Endosc. 2003;58:S3-43.
35. Draganov PV et al. Clin Gastroenterol Hepatol. 2019;17:16-25.
36. Ngamruengphong S et al. Clin Gastroenterol Hepatol. 2020 Jun 18;S1542-3565(20)30834-X. Online ahead of print.
37. Choi IJ et al. N Engl J Med. 2018;378:1085-95.
Dr. Tomizawa is a clinical assistant professor of medicine in the division of gastroenterology, University of Washington, Seattle.
Eosinophilic esophagitis: Frequently asked questions (and answers) for the early-career gastroenterologist
Introduction
Eosinophilic esophagitis (EoE) has transformed over the past 3 decades from a rarely encountered entity to one of the most common causes of dysphagia in adults.1 Given the marked rise in prevalence, the early-career gastroenterologist will undoubtedly be involved with managing this disease.2 The typical presentation includes a young, atopic male presenting with dysphagia in the outpatient setting or, more acutely, with a food impaction when on call. As every fellow is keenly aware, the calls often come late at night as patients commonly have meat impactions while consuming dinner. Current management focuses on symptomatic, histologic, and endoscopic improvement with medication, dietary, and mechanical (i.e., dilation) modalities.
EoE is defined by the presence of esophageal dysfunction and esophageal eosinophilic inflammation with ≥15 eosinophils/high-powered field (eos/hpf) required for the diagnosis. With better understanding of the pathogenesis of EoE involving the complex interaction of environmental, host, and genetic factors, advancements have been made as it relates to the diagnostic criteria, endoscopic evaluation, and therapeutic options. In this article, we review the current management of adult patients with EoE and offer practical guidance to key questions for the young gastroenterologist as well as insights into future areas of interest.
What should I consider when diagnosing EoE?
Symptoms are central to the diagnosis and clinical presentation of EoE. In assessing symptoms, clinicians should be aware of adaptive “IMPACT” strategies patients often subconsciously develop in response to their chronic and progressive condition: Imbibing fluids with meals, modifying foods by cutting or pureeing, prolonging meal times, avoiding harder texture foods, chewing excessively, and turning away tablets/pills.3 Failure to query such adaptive behaviors may lead to an underestimation of disease activity and severity.
An important aspect to confirming the diagnosis of EoE is to exclude other causes of esophageal eosinophilia. Gastroesophageal reflux disease (GERD) is known to cause esophageal eosinophilia and historically has been viewed as a distinct disease process. In fact, initial guidelines included lack of response to a proton pump inhibitor (PPI) trial or normal esophageal pH monitoring as diagnostic criteria.4 However, as experience was garnered, it became clear that PPI therapy was effective at improving inflammation in 30%-50% of patients with clinical presentations and histologic features consistent with EoE. As such, the concept of PPI–responsive esophageal eosinophilia (PPI-REE) was introduced in 2011.5 Further investigation then highlighted that PPI-REE and EoE had nearly identical clinical, endoscopic, and histologic features as well as eosinophil biomarker and gene expression profiles. Hence, recent international guidelines no longer necessitate a PPI trial to establish a diagnosis of EoE.6
The young gastroenterologist should also be mindful of other issues related to the initial diagnosis of EoE. EoE may present concomitantly with other disease entities including GERD, “extra-esophageal” eosinophilic gastrointestinal diseases, concomitant IgE-mediated food allergy, hypereosinophilic syndromes, connective tissue disorders, autoimmune diseases, celiac disease, and inflammatory bowel disease.3 It has been speculated that some of these disorders share common aspects of genetic and environmental predisposing factors as well as shared pathogenesis. Careful history taking should include a full review of atopic conditions and GI-related symptoms and endoscopy should carefully inspect not only the esophagus, but also gastric and duodenal mucosa. The endoscopic features almost always reveal edema, rings, exudates, furrows, and strictures and can be assessed using the EoE Endoscopic Reference Scoring system (EREFS).7 EREFS allows for systematic identification of abnormalities that can inform decisions regarding treatment efficacy and decisions on the need for esophageal dilation. When the esophageal mucosa is evaluated for biopsies, furrows and exudates should be targeted, if present, and multiple biopsies (minimum of five to six) should be taken throughout the esophagus given the patchy nature of the disease.
How do I choose an initial therapy?
The choice of initial therapy considers patient preferences, medication availability, disease severity, impact on quality of life, and need for repeated endoscopies. While there are many novel agents currently being investigated in phase 2 and 3 clinical trials, the current mainstays of treatment include PPI therapy, topical steroids, dietary therapy, and dilation. Of note, there have been no head-to-head trials comparing these different modalities. A recent systematic review reported that PPIs can induce histologic remission in 42% of patients.8 The ease of use and availability of PPI therapy make this an attractive first choice for patients. Pooled estimates show that topical steroids can induce remission in 66% of patients.8 It is important to note that there is currently no Food and Drug Administration–approved formulation of steroids for the treatment of EoE. As such, there are several practical aspects to consider when instructing patients to use agents not designed for esophageal delivery (Figure 1).
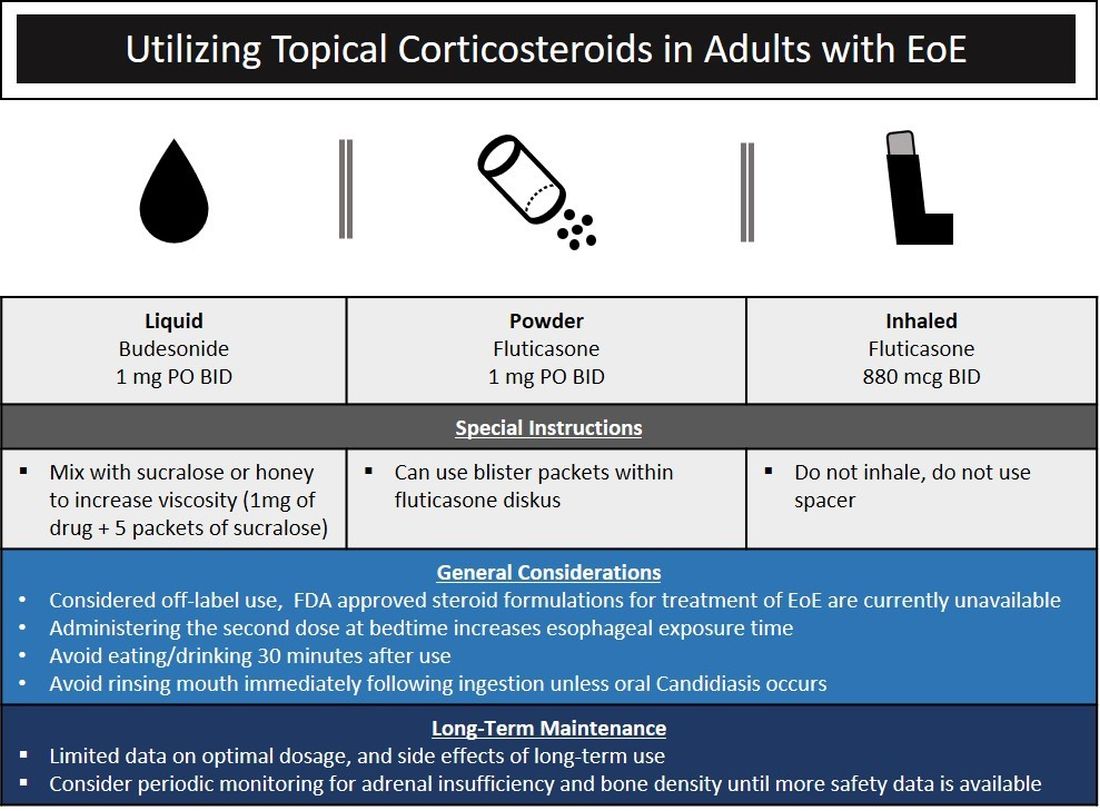
Source: Dr. Patel, Dr. Hirano
Lack of insurance coverage for topical steroids can make cost of a prescription a deterrent to use. While topical steroids are well tolerated, concerns for candidiasis and adrenal insufficiency are being monitored in prospective, long-term clinical trials. Concomitant use of steroids with PPI would be appropriate for EoE patients with coexisting GERD (severe heartburn, erosive esophagitis, Barrett’s esophagus). In addition, we often combine steroids with PPI therapy for EoE patients who demonstrate a convincing but incomplete response to PPI monotherapy (i.e., reduction of baseline inflammation from 75 eos/hpf to 20 eos/hpf).
Diet therapy is a popular choice for management of EoE by patients, given the ability to remove food triggers that initiate the immune dysregulation and to avoid chronic medication use. Three dietary options have been described including an elemental, amino acid–based diet which eliminates all common food allergens, allergy testing–directed elimination diet, and an empiric elimination diet. Though elemental diets have shown the most efficacy, practical aspects of implementing, maintaining, and identifying triggers restrict their adoption by most patients and clinicians.9 Allergy-directed elimination diets, where allergens are eliminated based on office-based allergy testing, initially seemed promising, though studies have shown limited histologic remission, compared with other diet therapies as well as the inability to identify true food triggers. Advancement of office-based testing to identify food triggers is needed to streamline this dietary approach. In the adult patient, the empiric elimination diet remains an attractive choice of the available dietary therapies. In this dietary approach, which has shown efficacy in both children and adults, the most common food allergens (milk, wheat, soy, egg, nuts, and seafood) are eliminated.9
How do I make dietary therapy work in clinical practice?
Before dietary therapy is initiated, it is important that your practice is situated to support this approach and that patients fully understand the process. A multidisciplinary approach optimizes dietary therapy. Dietitians provide expert guidance on eliminating trigger foods, maintaining nutrition, and avoiding inadvertent cross-contamination. Patient questions may include the safety of consumption of non–cow-based cheese/milk, alcoholic beverages, wheat alternatives, and restaurant food. Allergists address concerns for a concomitant IgE food allergy based on a clinical history or previous testing. Patients should be informed that identifying a food trigger often takes several months and multiple endoscopies. Clinicians should be aware of potential food cost and accessibility issues as well as the reported, albeit uncommon, development of de novo IgE-mediated food allergy during reintroduction. Timing of diet therapy is also a factor in success. Patients should avoid starting diets during major holidays, family celebrations, college years, and busy travel months.
Particularly empiric elimination diets, frequently used in adults, several approaches have been described (Figure 2).
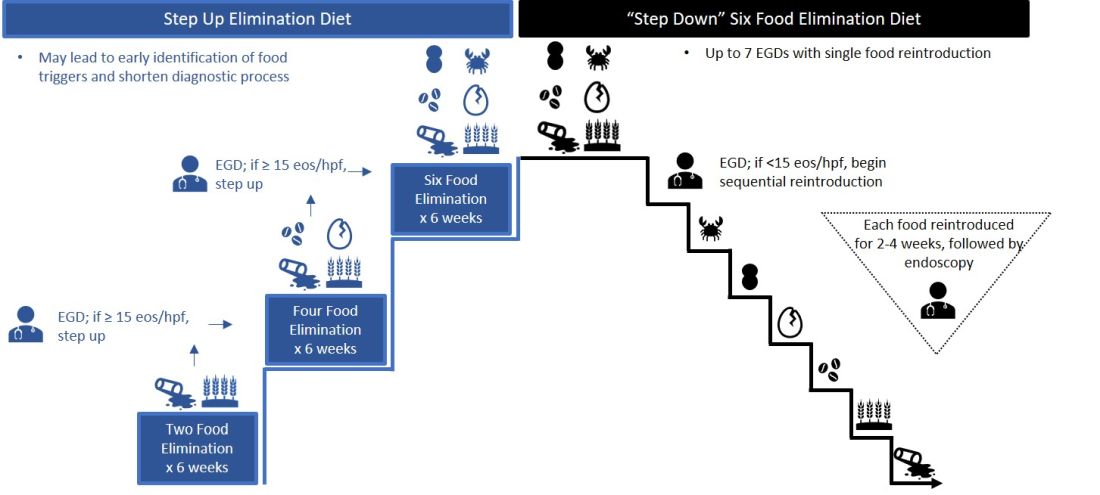
Source: Dr. Patel, Dr. Hirano
Initially, a step-down approach was described, with patients pursuing a six-food elimination diet (SFED), which eliminates the six most common triggers: milk, wheat, soy/legumes, egg, nuts, and seafood. Once in histologic remission, patients then systematically reintroduce foods in order to identify a causative trigger. Given that many patients have only one or two identified food triggers, other approaches were created including a single-food elimination diet eliminating milk, the two-food elimination diet (TFED) eliminating milk and wheat, and the four-food elimination diet (FFED) eliminating milk, wheat, soy/legumes, and eggs. A novel step-up approach has also now been described where patients start with the TFED and progress to the FFED and then potentially SFED based on histologic response.10 This approach has the potential to more readily identify triggers, decrease diagnostic time, and reduce endoscopic interventions. There are pros and cons to each elimination diet approach that should be discussed with patients. Many patients may find a one- or two-food elimination diet more feasible than a full SFED.
What should I consider when performing dilation?
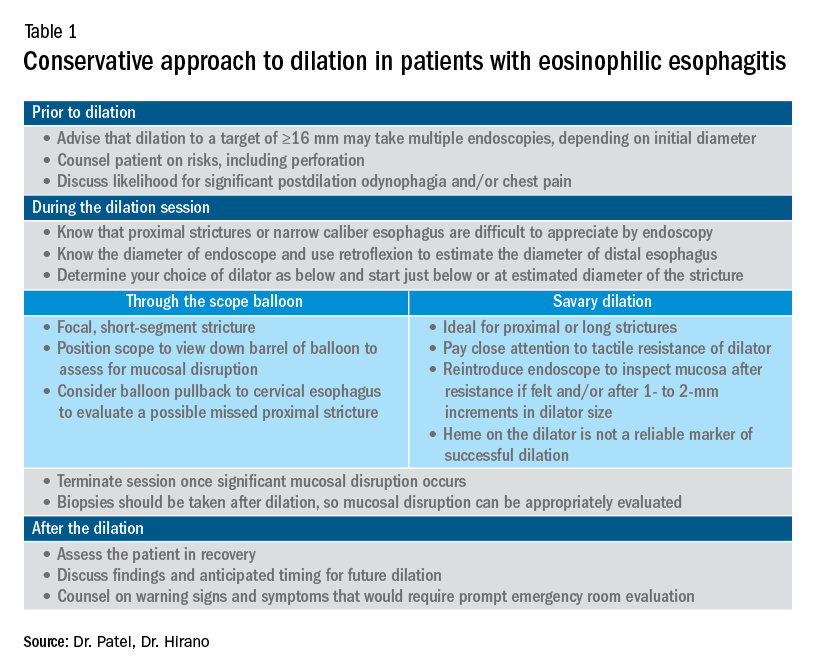
Esophageal dilation is frequently used to address the fibrostenotic complications of EoE that do not as readily respond to PPI, steroid, or diet therapy. The majority of patients note symptomatic improvement following dilation, though dilation alone does not address the inflammatory component of disease.8 With a conservative approach, the complication rates of esophageal dilation in EoE are similar to that of benign, esophageal strictures. Endoscopists should be aware that endoscopy alone can miss strictures and consider both practical and technical aspects when performing dilations (Table 1).11,12
When should an allergist be consulted?
The role of the allergist in the management of patients with EoE varies by patient and practice. IgE serologic or skin testing have limited accuracy in identifying food triggers for EoE. Nevertheless, the majority of patients with EoE have an atopic condition which may include asthma, allergic rhinitis, atopic dermatitis, or IgE-mediated food allergy. Although EoE is thought to primarily occur from an immune response to ingested oral allergens, aeroallergens may exacerbate disease as evidenced by the seasonal variation in EoE symptoms in some patients. The allergist provides treatment for these “extraesophageal” atopic conditions which may, in turn, have synergistic effects on the treatment of EoE. Furthermore, allergists may prescribe biologic therapies that are FDA approved for the treatment of atopic dermatitis, asthma, and allergic rhinitis. While not approved for EoE, several of these agents have shown efficacy in phase 2 clinical trials in EoE. In some practice settings, allergists primarily manage EoE patients with the assistance of gastroenterologists for periodic endoscopic activity assessment.
What are the key aspects of maintenance therapy?
The goals of treatment focus on symptomatic, histologic, and endoscopic improvement, and the prevention of future or ongoing fibrostenotic complications.2 Because of the adaptive eating behaviors discussed above, symptom response may not reliably correlate with histologic and/or endoscopic improvement. Moreover, dysphagia is related to strictures that often do not resolve in spite of resolution of mucosal inflammation. As such, histology and endoscopy are more objective and reliable targets of a successful response to therapy. Though studies have used variable esophageal density levels for response, using a cutoff of <15 eos/hpf as a therapeutic endpoint is reasonable for both initial response to therapy and long-term monitoring.13 We advocate for standardization of reporting endoscopic findings to better track change over time using the EREFS scoring system.7 While inflammatory features improve, the fibrostenotic features may persist despite improvement in histology. Dilation is often performed in these situations, especially for symptomatic individuals.
During clinical follow-up, the frequency of monitoring as it relates to symptom and endoscopic assessment is not well defined. It is reasonable to repeat endoscopic intervention following changes in therapy (i.e., reduction in steroid dosing or reintroduction of putative food triggers) or in symptoms.13 It is unclear if patients benefit from repeated endoscopies at set intervals without symptom change and after histologic response has been confirmed. In our practice, endoscopies are often considered on an annual basis. This interval is increased for patients with demonstrated stability of disease.
For patients who opt for dietary therapy and have one or two food triggers identified, long-term maintenance therapy can be straightforward with ongoing food avoidance. Limited data exist regarding long-term effectiveness of dietary therapy but loss of initial response has been reported that is often attributed to problems with adherence. Use of “diet holidays” or “planned cheats” to allow for intermittent consumption of trigger foods, often under the cover of short-term use of steroids, may improve the long-term feasibility of diet approaches.
In the recent American Gastroenterological Association guidelines, continuation of swallowed, topical steroids is recommended following remission with short-term treatment. The recurrence of both symptoms and inflammation following medication withdrawal supports this practice. Furthermore, natural history studies demonstrate progression of esophageal strictures with untreated disease.
There are no clear guidelines for long-term dosage and use of PPI or topical steroid therapy. Our practice is to down-titrate the dose of PPI or steroid following remission with short-term therapy, often starting with a reduction from twice a day to daily dosing. Although topical steroid therapy has fewer side effects, compared with systemic steroids, patients should be aware of the potential for adrenal suppression especially in an atopic population who may be exposed to multiple forms of topical steroids. Shared decision-making between patients and providers is recommended to determine comfort level with long-term use of prescription medications and dosage.
What’s on the horizon?
Several areas of development are underway to better assess and manage EoE. Novel histologic scoring tools now assess characteristics on pathology beyond eosinophil density, office-based testing modalities have been developed to assess inflammatory activity and thereby obviate the need for endoscopy, new technology can provide measures of esophageal remodeling and provide assessment of disease severity, and several biologic agents are being studied that target specific allergic mediators of the immune response in EoE.3,14-18 These novel tools, technologies, and therapies will undoubtedly change the management approach to EoE. Referral of patients into ongoing clinical trials will help inform advances in the field.
Conclusion
As an increasingly prevalent disease with a high degree of upper GI morbidity, EoE has transitioned from a rare entity to a commonly encountered disease. The new gastroenterologist will confront both straightforward as well as complex patients with EoE, and we offer several practical aspects on management. In the years ahead, the care of patients with EoE will continue to evolve to a more streamlined, effective, and personalized approach.
References
1. Kidambi T et al. World J Gastroenterol. 2012;18:4335-41.
2. Dellon ES et al. Gastroenterology. 2018;154:319-32 e3.
3. Hirano I et al. Gastroenterology. 2020;158:840-51.
4. Furuta GT et al. Gastroenterology. 2007;133:1342-63.
5. Liacouras CA et al. J Allergy Clin Immunol. 2011;128:3-20 e6; quiz 1-2.
6. Dellon ES et al. Gastroenterology. 2018;155:1022-33 e10.
7. Hirano I et al. Gut. 2013;62:489-95.
8. Rank MA et al. Gastroenterology. 2020;158:1789-810 e15.
9. Arias A et al. Gastroenterology. 2014;146:1639-48.
10. Molina-Infante J et al. J Allergy Clin Immunol. 2018;141:1365-72.
11. Gentile N et al. Aliment Pharmacol Ther. 2014;40:1333-40.
12. Hirano I. Gastroenterology. 2018;155:601-6.
13. Hirano I et al. Gastroenterology. 2020;158:1776-86.
14. Collins MH et al. Dis Esophagus. 2017;30:1-8.
15. Furuta GT et al. Gut. 2013;62:1395-405.
16. Katzka DA et al. Clin Gastroenterol Hepatol. 2015;13:77-83 e2.
17. Kwiatek MA et al. Gastroenterology. 2011;140:82-90.
18. Nicodeme F et al. Clin Gastroenterol Hepatol. 2013;11:1101-7 e1.
Introduction
Eosinophilic esophagitis (EoE) has transformed over the past 3 decades from a rarely encountered entity to one of the most common causes of dysphagia in adults.1 Given the marked rise in prevalence, the early-career gastroenterologist will undoubtedly be involved with managing this disease.2 The typical presentation includes a young, atopic male presenting with dysphagia in the outpatient setting or, more acutely, with a food impaction when on call. As every fellow is keenly aware, the calls often come late at night as patients commonly have meat impactions while consuming dinner. Current management focuses on symptomatic, histologic, and endoscopic improvement with medication, dietary, and mechanical (i.e., dilation) modalities.
EoE is defined by the presence of esophageal dysfunction and esophageal eosinophilic inflammation with ≥15 eosinophils/high-powered field (eos/hpf) required for the diagnosis. With better understanding of the pathogenesis of EoE involving the complex interaction of environmental, host, and genetic factors, advancements have been made as it relates to the diagnostic criteria, endoscopic evaluation, and therapeutic options. In this article, we review the current management of adult patients with EoE and offer practical guidance to key questions for the young gastroenterologist as well as insights into future areas of interest.
What should I consider when diagnosing EoE?
Symptoms are central to the diagnosis and clinical presentation of EoE. In assessing symptoms, clinicians should be aware of adaptive “IMPACT” strategies patients often subconsciously develop in response to their chronic and progressive condition: Imbibing fluids with meals, modifying foods by cutting or pureeing, prolonging meal times, avoiding harder texture foods, chewing excessively, and turning away tablets/pills.3 Failure to query such adaptive behaviors may lead to an underestimation of disease activity and severity.
An important aspect to confirming the diagnosis of EoE is to exclude other causes of esophageal eosinophilia. Gastroesophageal reflux disease (GERD) is known to cause esophageal eosinophilia and historically has been viewed as a distinct disease process. In fact, initial guidelines included lack of response to a proton pump inhibitor (PPI) trial or normal esophageal pH monitoring as diagnostic criteria.4 However, as experience was garnered, it became clear that PPI therapy was effective at improving inflammation in 30%-50% of patients with clinical presentations and histologic features consistent with EoE. As such, the concept of PPI–responsive esophageal eosinophilia (PPI-REE) was introduced in 2011.5 Further investigation then highlighted that PPI-REE and EoE had nearly identical clinical, endoscopic, and histologic features as well as eosinophil biomarker and gene expression profiles. Hence, recent international guidelines no longer necessitate a PPI trial to establish a diagnosis of EoE.6
The young gastroenterologist should also be mindful of other issues related to the initial diagnosis of EoE. EoE may present concomitantly with other disease entities including GERD, “extra-esophageal” eosinophilic gastrointestinal diseases, concomitant IgE-mediated food allergy, hypereosinophilic syndromes, connective tissue disorders, autoimmune diseases, celiac disease, and inflammatory bowel disease.3 It has been speculated that some of these disorders share common aspects of genetic and environmental predisposing factors as well as shared pathogenesis. Careful history taking should include a full review of atopic conditions and GI-related symptoms and endoscopy should carefully inspect not only the esophagus, but also gastric and duodenal mucosa. The endoscopic features almost always reveal edema, rings, exudates, furrows, and strictures and can be assessed using the EoE Endoscopic Reference Scoring system (EREFS).7 EREFS allows for systematic identification of abnormalities that can inform decisions regarding treatment efficacy and decisions on the need for esophageal dilation. When the esophageal mucosa is evaluated for biopsies, furrows and exudates should be targeted, if present, and multiple biopsies (minimum of five to six) should be taken throughout the esophagus given the patchy nature of the disease.
How do I choose an initial therapy?
The choice of initial therapy considers patient preferences, medication availability, disease severity, impact on quality of life, and need for repeated endoscopies. While there are many novel agents currently being investigated in phase 2 and 3 clinical trials, the current mainstays of treatment include PPI therapy, topical steroids, dietary therapy, and dilation. Of note, there have been no head-to-head trials comparing these different modalities. A recent systematic review reported that PPIs can induce histologic remission in 42% of patients.8 The ease of use and availability of PPI therapy make this an attractive first choice for patients. Pooled estimates show that topical steroids can induce remission in 66% of patients.8 It is important to note that there is currently no Food and Drug Administration–approved formulation of steroids for the treatment of EoE. As such, there are several practical aspects to consider when instructing patients to use agents not designed for esophageal delivery (Figure 1).

Source: Dr. Patel, Dr. Hirano
Lack of insurance coverage for topical steroids can make cost of a prescription a deterrent to use. While topical steroids are well tolerated, concerns for candidiasis and adrenal insufficiency are being monitored in prospective, long-term clinical trials. Concomitant use of steroids with PPI would be appropriate for EoE patients with coexisting GERD (severe heartburn, erosive esophagitis, Barrett’s esophagus). In addition, we often combine steroids with PPI therapy for EoE patients who demonstrate a convincing but incomplete response to PPI monotherapy (i.e., reduction of baseline inflammation from 75 eos/hpf to 20 eos/hpf).
Diet therapy is a popular choice for management of EoE by patients, given the ability to remove food triggers that initiate the immune dysregulation and to avoid chronic medication use. Three dietary options have been described including an elemental, amino acid–based diet which eliminates all common food allergens, allergy testing–directed elimination diet, and an empiric elimination diet. Though elemental diets have shown the most efficacy, practical aspects of implementing, maintaining, and identifying triggers restrict their adoption by most patients and clinicians.9 Allergy-directed elimination diets, where allergens are eliminated based on office-based allergy testing, initially seemed promising, though studies have shown limited histologic remission, compared with other diet therapies as well as the inability to identify true food triggers. Advancement of office-based testing to identify food triggers is needed to streamline this dietary approach. In the adult patient, the empiric elimination diet remains an attractive choice of the available dietary therapies. In this dietary approach, which has shown efficacy in both children and adults, the most common food allergens (milk, wheat, soy, egg, nuts, and seafood) are eliminated.9
How do I make dietary therapy work in clinical practice?
Before dietary therapy is initiated, it is important that your practice is situated to support this approach and that patients fully understand the process. A multidisciplinary approach optimizes dietary therapy. Dietitians provide expert guidance on eliminating trigger foods, maintaining nutrition, and avoiding inadvertent cross-contamination. Patient questions may include the safety of consumption of non–cow-based cheese/milk, alcoholic beverages, wheat alternatives, and restaurant food. Allergists address concerns for a concomitant IgE food allergy based on a clinical history or previous testing. Patients should be informed that identifying a food trigger often takes several months and multiple endoscopies. Clinicians should be aware of potential food cost and accessibility issues as well as the reported, albeit uncommon, development of de novo IgE-mediated food allergy during reintroduction. Timing of diet therapy is also a factor in success. Patients should avoid starting diets during major holidays, family celebrations, college years, and busy travel months.
Particularly empiric elimination diets, frequently used in adults, several approaches have been described (Figure 2).

Source: Dr. Patel, Dr. Hirano
Initially, a step-down approach was described, with patients pursuing a six-food elimination diet (SFED), which eliminates the six most common triggers: milk, wheat, soy/legumes, egg, nuts, and seafood. Once in histologic remission, patients then systematically reintroduce foods in order to identify a causative trigger. Given that many patients have only one or two identified food triggers, other approaches were created including a single-food elimination diet eliminating milk, the two-food elimination diet (TFED) eliminating milk and wheat, and the four-food elimination diet (FFED) eliminating milk, wheat, soy/legumes, and eggs. A novel step-up approach has also now been described where patients start with the TFED and progress to the FFED and then potentially SFED based on histologic response.10 This approach has the potential to more readily identify triggers, decrease diagnostic time, and reduce endoscopic interventions. There are pros and cons to each elimination diet approach that should be discussed with patients. Many patients may find a one- or two-food elimination diet more feasible than a full SFED.
What should I consider when performing dilation?
Esophageal dilation is frequently used to address the fibrostenotic complications of EoE that do not as readily respond to PPI, steroid, or diet therapy. The majority of patients note symptomatic improvement following dilation, though dilation alone does not address the inflammatory component of disease.8 With a conservative approach, the complication rates of esophageal dilation in EoE are similar to that of benign, esophageal strictures. Endoscopists should be aware that endoscopy alone can miss strictures and consider both practical and technical aspects when performing dilations (Table 1).11,12

When should an allergist be consulted?
The role of the allergist in the management of patients with EoE varies by patient and practice. IgE serologic or skin testing have limited accuracy in identifying food triggers for EoE. Nevertheless, the majority of patients with EoE have an atopic condition which may include asthma, allergic rhinitis, atopic dermatitis, or IgE-mediated food allergy. Although EoE is thought to primarily occur from an immune response to ingested oral allergens, aeroallergens may exacerbate disease as evidenced by the seasonal variation in EoE symptoms in some patients. The allergist provides treatment for these “extraesophageal” atopic conditions which may, in turn, have synergistic effects on the treatment of EoE. Furthermore, allergists may prescribe biologic therapies that are FDA approved for the treatment of atopic dermatitis, asthma, and allergic rhinitis. While not approved for EoE, several of these agents have shown efficacy in phase 2 clinical trials in EoE. In some practice settings, allergists primarily manage EoE patients with the assistance of gastroenterologists for periodic endoscopic activity assessment.
What are the key aspects of maintenance therapy?
The goals of treatment focus on symptomatic, histologic, and endoscopic improvement, and the prevention of future or ongoing fibrostenotic complications.2 Because of the adaptive eating behaviors discussed above, symptom response may not reliably correlate with histologic and/or endoscopic improvement. Moreover, dysphagia is related to strictures that often do not resolve in spite of resolution of mucosal inflammation. As such, histology and endoscopy are more objective and reliable targets of a successful response to therapy. Though studies have used variable esophageal density levels for response, using a cutoff of <15 eos/hpf as a therapeutic endpoint is reasonable for both initial response to therapy and long-term monitoring.13 We advocate for standardization of reporting endoscopic findings to better track change over time using the EREFS scoring system.7 While inflammatory features improve, the fibrostenotic features may persist despite improvement in histology. Dilation is often performed in these situations, especially for symptomatic individuals.
During clinical follow-up, the frequency of monitoring as it relates to symptom and endoscopic assessment is not well defined. It is reasonable to repeat endoscopic intervention following changes in therapy (i.e., reduction in steroid dosing or reintroduction of putative food triggers) or in symptoms.13 It is unclear if patients benefit from repeated endoscopies at set intervals without symptom change and after histologic response has been confirmed. In our practice, endoscopies are often considered on an annual basis. This interval is increased for patients with demonstrated stability of disease.
For patients who opt for dietary therapy and have one or two food triggers identified, long-term maintenance therapy can be straightforward with ongoing food avoidance. Limited data exist regarding long-term effectiveness of dietary therapy but loss of initial response has been reported that is often attributed to problems with adherence. Use of “diet holidays” or “planned cheats” to allow for intermittent consumption of trigger foods, often under the cover of short-term use of steroids, may improve the long-term feasibility of diet approaches.
In the recent American Gastroenterological Association guidelines, continuation of swallowed, topical steroids is recommended following remission with short-term treatment. The recurrence of both symptoms and inflammation following medication withdrawal supports this practice. Furthermore, natural history studies demonstrate progression of esophageal strictures with untreated disease.
There are no clear guidelines for long-term dosage and use of PPI or topical steroid therapy. Our practice is to down-titrate the dose of PPI or steroid following remission with short-term therapy, often starting with a reduction from twice a day to daily dosing. Although topical steroid therapy has fewer side effects, compared with systemic steroids, patients should be aware of the potential for adrenal suppression especially in an atopic population who may be exposed to multiple forms of topical steroids. Shared decision-making between patients and providers is recommended to determine comfort level with long-term use of prescription medications and dosage.
What’s on the horizon?
Several areas of development are underway to better assess and manage EoE. Novel histologic scoring tools now assess characteristics on pathology beyond eosinophil density, office-based testing modalities have been developed to assess inflammatory activity and thereby obviate the need for endoscopy, new technology can provide measures of esophageal remodeling and provide assessment of disease severity, and several biologic agents are being studied that target specific allergic mediators of the immune response in EoE.3,14-18 These novel tools, technologies, and therapies will undoubtedly change the management approach to EoE. Referral of patients into ongoing clinical trials will help inform advances in the field.
Conclusion
As an increasingly prevalent disease with a high degree of upper GI morbidity, EoE has transitioned from a rare entity to a commonly encountered disease. The new gastroenterologist will confront both straightforward as well as complex patients with EoE, and we offer several practical aspects on management. In the years ahead, the care of patients with EoE will continue to evolve to a more streamlined, effective, and personalized approach.
References
1. Kidambi T et al. World J Gastroenterol. 2012;18:4335-41.
2. Dellon ES et al. Gastroenterology. 2018;154:319-32 e3.
3. Hirano I et al. Gastroenterology. 2020;158:840-51.
4. Furuta GT et al. Gastroenterology. 2007;133:1342-63.
5. Liacouras CA et al. J Allergy Clin Immunol. 2011;128:3-20 e6; quiz 1-2.
6. Dellon ES et al. Gastroenterology. 2018;155:1022-33 e10.
7. Hirano I et al. Gut. 2013;62:489-95.
8. Rank MA et al. Gastroenterology. 2020;158:1789-810 e15.
9. Arias A et al. Gastroenterology. 2014;146:1639-48.
10. Molina-Infante J et al. J Allergy Clin Immunol. 2018;141:1365-72.
11. Gentile N et al. Aliment Pharmacol Ther. 2014;40:1333-40.
12. Hirano I. Gastroenterology. 2018;155:601-6.
13. Hirano I et al. Gastroenterology. 2020;158:1776-86.
14. Collins MH et al. Dis Esophagus. 2017;30:1-8.
15. Furuta GT et al. Gut. 2013;62:1395-405.
16. Katzka DA et al. Clin Gastroenterol Hepatol. 2015;13:77-83 e2.
17. Kwiatek MA et al. Gastroenterology. 2011;140:82-90.
18. Nicodeme F et al. Clin Gastroenterol Hepatol. 2013;11:1101-7 e1.
Introduction
Eosinophilic esophagitis (EoE) has transformed over the past 3 decades from a rarely encountered entity to one of the most common causes of dysphagia in adults.1 Given the marked rise in prevalence, the early-career gastroenterologist will undoubtedly be involved with managing this disease.2 The typical presentation includes a young, atopic male presenting with dysphagia in the outpatient setting or, more acutely, with a food impaction when on call. As every fellow is keenly aware, the calls often come late at night as patients commonly have meat impactions while consuming dinner. Current management focuses on symptomatic, histologic, and endoscopic improvement with medication, dietary, and mechanical (i.e., dilation) modalities.
EoE is defined by the presence of esophageal dysfunction and esophageal eosinophilic inflammation with ≥15 eosinophils/high-powered field (eos/hpf) required for the diagnosis. With better understanding of the pathogenesis of EoE involving the complex interaction of environmental, host, and genetic factors, advancements have been made as it relates to the diagnostic criteria, endoscopic evaluation, and therapeutic options. In this article, we review the current management of adult patients with EoE and offer practical guidance to key questions for the young gastroenterologist as well as insights into future areas of interest.
What should I consider when diagnosing EoE?
Symptoms are central to the diagnosis and clinical presentation of EoE. In assessing symptoms, clinicians should be aware of adaptive “IMPACT” strategies patients often subconsciously develop in response to their chronic and progressive condition: Imbibing fluids with meals, modifying foods by cutting or pureeing, prolonging meal times, avoiding harder texture foods, chewing excessively, and turning away tablets/pills.3 Failure to query such adaptive behaviors may lead to an underestimation of disease activity and severity.
An important aspect to confirming the diagnosis of EoE is to exclude other causes of esophageal eosinophilia. Gastroesophageal reflux disease (GERD) is known to cause esophageal eosinophilia and historically has been viewed as a distinct disease process. In fact, initial guidelines included lack of response to a proton pump inhibitor (PPI) trial or normal esophageal pH monitoring as diagnostic criteria.4 However, as experience was garnered, it became clear that PPI therapy was effective at improving inflammation in 30%-50% of patients with clinical presentations and histologic features consistent with EoE. As such, the concept of PPI–responsive esophageal eosinophilia (PPI-REE) was introduced in 2011.5 Further investigation then highlighted that PPI-REE and EoE had nearly identical clinical, endoscopic, and histologic features as well as eosinophil biomarker and gene expression profiles. Hence, recent international guidelines no longer necessitate a PPI trial to establish a diagnosis of EoE.6
The young gastroenterologist should also be mindful of other issues related to the initial diagnosis of EoE. EoE may present concomitantly with other disease entities including GERD, “extra-esophageal” eosinophilic gastrointestinal diseases, concomitant IgE-mediated food allergy, hypereosinophilic syndromes, connective tissue disorders, autoimmune diseases, celiac disease, and inflammatory bowel disease.3 It has been speculated that some of these disorders share common aspects of genetic and environmental predisposing factors as well as shared pathogenesis. Careful history taking should include a full review of atopic conditions and GI-related symptoms and endoscopy should carefully inspect not only the esophagus, but also gastric and duodenal mucosa. The endoscopic features almost always reveal edema, rings, exudates, furrows, and strictures and can be assessed using the EoE Endoscopic Reference Scoring system (EREFS).7 EREFS allows for systematic identification of abnormalities that can inform decisions regarding treatment efficacy and decisions on the need for esophageal dilation. When the esophageal mucosa is evaluated for biopsies, furrows and exudates should be targeted, if present, and multiple biopsies (minimum of five to six) should be taken throughout the esophagus given the patchy nature of the disease.
How do I choose an initial therapy?
The choice of initial therapy considers patient preferences, medication availability, disease severity, impact on quality of life, and need for repeated endoscopies. While there are many novel agents currently being investigated in phase 2 and 3 clinical trials, the current mainstays of treatment include PPI therapy, topical steroids, dietary therapy, and dilation. Of note, there have been no head-to-head trials comparing these different modalities. A recent systematic review reported that PPIs can induce histologic remission in 42% of patients.8 The ease of use and availability of PPI therapy make this an attractive first choice for patients. Pooled estimates show that topical steroids can induce remission in 66% of patients.8 It is important to note that there is currently no Food and Drug Administration–approved formulation of steroids for the treatment of EoE. As such, there are several practical aspects to consider when instructing patients to use agents not designed for esophageal delivery (Figure 1).

Source: Dr. Patel, Dr. Hirano
Lack of insurance coverage for topical steroids can make cost of a prescription a deterrent to use. While topical steroids are well tolerated, concerns for candidiasis and adrenal insufficiency are being monitored in prospective, long-term clinical trials. Concomitant use of steroids with PPI would be appropriate for EoE patients with coexisting GERD (severe heartburn, erosive esophagitis, Barrett’s esophagus). In addition, we often combine steroids with PPI therapy for EoE patients who demonstrate a convincing but incomplete response to PPI monotherapy (i.e., reduction of baseline inflammation from 75 eos/hpf to 20 eos/hpf).
Diet therapy is a popular choice for management of EoE by patients, given the ability to remove food triggers that initiate the immune dysregulation and to avoid chronic medication use. Three dietary options have been described including an elemental, amino acid–based diet which eliminates all common food allergens, allergy testing–directed elimination diet, and an empiric elimination diet. Though elemental diets have shown the most efficacy, practical aspects of implementing, maintaining, and identifying triggers restrict their adoption by most patients and clinicians.9 Allergy-directed elimination diets, where allergens are eliminated based on office-based allergy testing, initially seemed promising, though studies have shown limited histologic remission, compared with other diet therapies as well as the inability to identify true food triggers. Advancement of office-based testing to identify food triggers is needed to streamline this dietary approach. In the adult patient, the empiric elimination diet remains an attractive choice of the available dietary therapies. In this dietary approach, which has shown efficacy in both children and adults, the most common food allergens (milk, wheat, soy, egg, nuts, and seafood) are eliminated.9
How do I make dietary therapy work in clinical practice?
Before dietary therapy is initiated, it is important that your practice is situated to support this approach and that patients fully understand the process. A multidisciplinary approach optimizes dietary therapy. Dietitians provide expert guidance on eliminating trigger foods, maintaining nutrition, and avoiding inadvertent cross-contamination. Patient questions may include the safety of consumption of non–cow-based cheese/milk, alcoholic beverages, wheat alternatives, and restaurant food. Allergists address concerns for a concomitant IgE food allergy based on a clinical history or previous testing. Patients should be informed that identifying a food trigger often takes several months and multiple endoscopies. Clinicians should be aware of potential food cost and accessibility issues as well as the reported, albeit uncommon, development of de novo IgE-mediated food allergy during reintroduction. Timing of diet therapy is also a factor in success. Patients should avoid starting diets during major holidays, family celebrations, college years, and busy travel months.
Particularly empiric elimination diets, frequently used in adults, several approaches have been described (Figure 2).

Source: Dr. Patel, Dr. Hirano
Initially, a step-down approach was described, with patients pursuing a six-food elimination diet (SFED), which eliminates the six most common triggers: milk, wheat, soy/legumes, egg, nuts, and seafood. Once in histologic remission, patients then systematically reintroduce foods in order to identify a causative trigger. Given that many patients have only one or two identified food triggers, other approaches were created including a single-food elimination diet eliminating milk, the two-food elimination diet (TFED) eliminating milk and wheat, and the four-food elimination diet (FFED) eliminating milk, wheat, soy/legumes, and eggs. A novel step-up approach has also now been described where patients start with the TFED and progress to the FFED and then potentially SFED based on histologic response.10 This approach has the potential to more readily identify triggers, decrease diagnostic time, and reduce endoscopic interventions. There are pros and cons to each elimination diet approach that should be discussed with patients. Many patients may find a one- or two-food elimination diet more feasible than a full SFED.
What should I consider when performing dilation?
Esophageal dilation is frequently used to address the fibrostenotic complications of EoE that do not as readily respond to PPI, steroid, or diet therapy. The majority of patients note symptomatic improvement following dilation, though dilation alone does not address the inflammatory component of disease.8 With a conservative approach, the complication rates of esophageal dilation in EoE are similar to that of benign, esophageal strictures. Endoscopists should be aware that endoscopy alone can miss strictures and consider both practical and technical aspects when performing dilations (Table 1).11,12

When should an allergist be consulted?
The role of the allergist in the management of patients with EoE varies by patient and practice. IgE serologic or skin testing have limited accuracy in identifying food triggers for EoE. Nevertheless, the majority of patients with EoE have an atopic condition which may include asthma, allergic rhinitis, atopic dermatitis, or IgE-mediated food allergy. Although EoE is thought to primarily occur from an immune response to ingested oral allergens, aeroallergens may exacerbate disease as evidenced by the seasonal variation in EoE symptoms in some patients. The allergist provides treatment for these “extraesophageal” atopic conditions which may, in turn, have synergistic effects on the treatment of EoE. Furthermore, allergists may prescribe biologic therapies that are FDA approved for the treatment of atopic dermatitis, asthma, and allergic rhinitis. While not approved for EoE, several of these agents have shown efficacy in phase 2 clinical trials in EoE. In some practice settings, allergists primarily manage EoE patients with the assistance of gastroenterologists for periodic endoscopic activity assessment.
What are the key aspects of maintenance therapy?
The goals of treatment focus on symptomatic, histologic, and endoscopic improvement, and the prevention of future or ongoing fibrostenotic complications.2 Because of the adaptive eating behaviors discussed above, symptom response may not reliably correlate with histologic and/or endoscopic improvement. Moreover, dysphagia is related to strictures that often do not resolve in spite of resolution of mucosal inflammation. As such, histology and endoscopy are more objective and reliable targets of a successful response to therapy. Though studies have used variable esophageal density levels for response, using a cutoff of <15 eos/hpf as a therapeutic endpoint is reasonable for both initial response to therapy and long-term monitoring.13 We advocate for standardization of reporting endoscopic findings to better track change over time using the EREFS scoring system.7 While inflammatory features improve, the fibrostenotic features may persist despite improvement in histology. Dilation is often performed in these situations, especially for symptomatic individuals.
During clinical follow-up, the frequency of monitoring as it relates to symptom and endoscopic assessment is not well defined. It is reasonable to repeat endoscopic intervention following changes in therapy (i.e., reduction in steroid dosing or reintroduction of putative food triggers) or in symptoms.13 It is unclear if patients benefit from repeated endoscopies at set intervals without symptom change and after histologic response has been confirmed. In our practice, endoscopies are often considered on an annual basis. This interval is increased for patients with demonstrated stability of disease.
For patients who opt for dietary therapy and have one or two food triggers identified, long-term maintenance therapy can be straightforward with ongoing food avoidance. Limited data exist regarding long-term effectiveness of dietary therapy but loss of initial response has been reported that is often attributed to problems with adherence. Use of “diet holidays” or “planned cheats” to allow for intermittent consumption of trigger foods, often under the cover of short-term use of steroids, may improve the long-term feasibility of diet approaches.
In the recent American Gastroenterological Association guidelines, continuation of swallowed, topical steroids is recommended following remission with short-term treatment. The recurrence of both symptoms and inflammation following medication withdrawal supports this practice. Furthermore, natural history studies demonstrate progression of esophageal strictures with untreated disease.
There are no clear guidelines for long-term dosage and use of PPI or topical steroid therapy. Our practice is to down-titrate the dose of PPI or steroid following remission with short-term therapy, often starting with a reduction from twice a day to daily dosing. Although topical steroid therapy has fewer side effects, compared with systemic steroids, patients should be aware of the potential for adrenal suppression especially in an atopic population who may be exposed to multiple forms of topical steroids. Shared decision-making between patients and providers is recommended to determine comfort level with long-term use of prescription medications and dosage.
What’s on the horizon?
Several areas of development are underway to better assess and manage EoE. Novel histologic scoring tools now assess characteristics on pathology beyond eosinophil density, office-based testing modalities have been developed to assess inflammatory activity and thereby obviate the need for endoscopy, new technology can provide measures of esophageal remodeling and provide assessment of disease severity, and several biologic agents are being studied that target specific allergic mediators of the immune response in EoE.3,14-18 These novel tools, technologies, and therapies will undoubtedly change the management approach to EoE. Referral of patients into ongoing clinical trials will help inform advances in the field.
Conclusion
As an increasingly prevalent disease with a high degree of upper GI morbidity, EoE has transitioned from a rare entity to a commonly encountered disease. The new gastroenterologist will confront both straightforward as well as complex patients with EoE, and we offer several practical aspects on management. In the years ahead, the care of patients with EoE will continue to evolve to a more streamlined, effective, and personalized approach.
References
1. Kidambi T et al. World J Gastroenterol. 2012;18:4335-41.
2. Dellon ES et al. Gastroenterology. 2018;154:319-32 e3.
3. Hirano I et al. Gastroenterology. 2020;158:840-51.
4. Furuta GT et al. Gastroenterology. 2007;133:1342-63.
5. Liacouras CA et al. J Allergy Clin Immunol. 2011;128:3-20 e6; quiz 1-2.
6. Dellon ES et al. Gastroenterology. 2018;155:1022-33 e10.
7. Hirano I et al. Gut. 2013;62:489-95.
8. Rank MA et al. Gastroenterology. 2020;158:1789-810 e15.
9. Arias A et al. Gastroenterology. 2014;146:1639-48.
10. Molina-Infante J et al. J Allergy Clin Immunol. 2018;141:1365-72.
11. Gentile N et al. Aliment Pharmacol Ther. 2014;40:1333-40.
12. Hirano I. Gastroenterology. 2018;155:601-6.
13. Hirano I et al. Gastroenterology. 2020;158:1776-86.
14. Collins MH et al. Dis Esophagus. 2017;30:1-8.
15. Furuta GT et al. Gut. 2013;62:1395-405.
16. Katzka DA et al. Clin Gastroenterol Hepatol. 2015;13:77-83 e2.
17. Kwiatek MA et al. Gastroenterology. 2011;140:82-90.
18. Nicodeme F et al. Clin Gastroenterol Hepatol. 2013;11:1101-7 e1.
Cyclic vomiting syndrome: A GI primer
Introduction
Cyclic vomiting syndrome (CVS) is a chronic disorder of gut-brain interaction (DGBI) and is characterized by recurrent episodes of severe nausea, vomiting, and often, abdominal pain. Patients are usually asymptomatic in between episodes.1 CVS was considered a pediatric disease but is now known to be as common in adults. The prevalence of CVS in adults was 2% in a recent population-based study.2 Patients are predominantly white. Both males and females are affected with some studies showing a female preponderance. The mean age of onset is 5 years in children and 35 years in adults.3
The etiology of CVS is not known, but various hypotheses have been proposed. Zaki et al. showed that two mitochondrial DNA polymorphisms 16519T and 3010A were associated with a 17-fold increased odds of having CVS in children.4 These polymorphisms were not associated with CVS in adults.5 Alterations in the brain-gut axis also have been shown in CVS. Functional neuroimaging studies demonstrate that patients with CVS displayed increased connectivity between insula and salience networks with concomitant decrease in connectivity to somatosensory networks.6 Recent data also indicate that the endocannabinoid system (ECS) and the hypothalamic-pituitary-adrenal axis are implicated in CVS with an increase in serum endocannabinoid concentration during an episode.7 The same study also showed a significant increase in salivary cortisol in CVS patients who used cannabis. Further, single nucleotide polymorphisms (SNPs) in the gene that encodes for the cannabinoid receptor type 1 (CB1R) are implicated in CVS.8 The CB1R is part of the ECS and is densely expressed in brain areas involved in emesis, such as the dorsal vagal complex consisting of the area postrema (AP), nucleus of the solitary tract (NTS), and also the dorsal motor nucleus of the vagus.9 Wasilewski et al. showed an increased risk of CVS among individuals with AG and GG genotypes of CNR1 rs806380 (P less than .01), whereas the CC genotype of CNR1 rs806368 was associated with a decreased risk of CVS (P less than .05).8 CB1R agonists – endocannabinoids and tetrahydrocannabinol (THC) – have acute antiemetic and anxiolytic effects.9-11 The apparent paradoxical effects of cannabis in this patient population are yet to be explained and need further study.
Diagnosis and clinical features of CVS
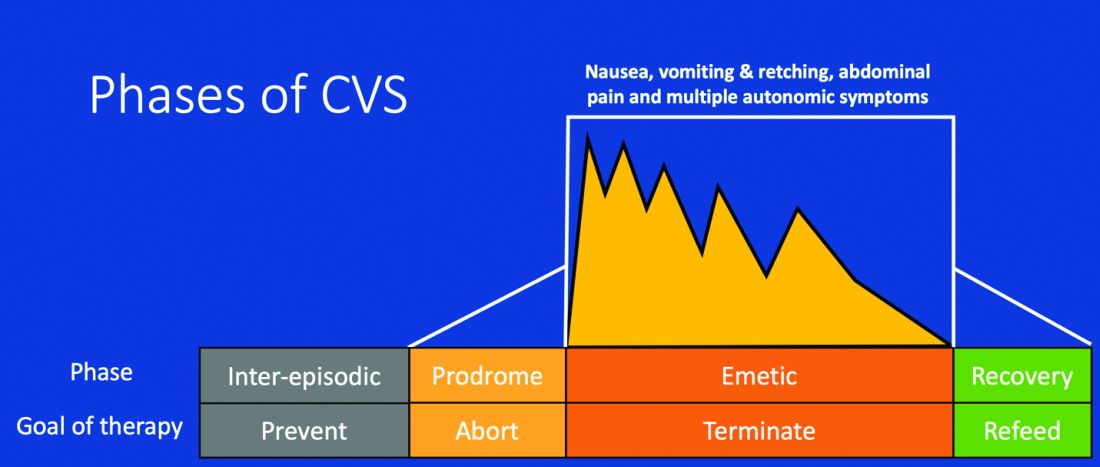
Figure 1: Phases of Cyclic Vomiting Syndrome12
Adapted from Fleisher DR, Gornowicz B, Adams K, Burch R, Feldman EJ. Cyclic Vomiting Syndrome in 41 adults: The illness, the patients, and problems of management. BMC Med 2005;3:20. This work is licensed under the Creative Commons Attribution 4.0 International License https://creativecommons.org/licenses/by/4.0/, which permits unrestricted use, distribution, modification, and reproduction in any medium.
CVS consists of four phases which include the a) prodromal phase, b) the episodic phase, c) recovery phase, and d) the interepisodic phase; and was first described by David Fleisher.12 The phases of CVS are important for clinicians and patients alike as they have therapeutic implications. The administration of abortive medications during a prodrome can terminate an episode. The phases of CVS are shown above.
Most patients (~ 93%) have a prodromal phase. Symptoms during this phase can include nausea, abdominal pain, diaphoresis, fatigue, weakness, hot flashes, chills, shivering, increased thirst, loss of appetite, burping, lightheadedness, and paresthesia.13 Some patients report a sense of impending doom and many have symptoms consistent with panic. If untreated, this progresses to the emetic phase and patients have unrelenting nausea, retching, vomiting, and other symptoms. During an episode, patients may vomit up to 20 times per hour and the episode may last several hours to days. During this phase, patients are sometimes described as being in a “conscious coma” and exhibit lethargy, listlessness, withdrawal, and sometimes disorientation.14,15 The emetic phase is followed by the recovery phase, during which symptoms subside and patients are able to resume oral intake. Patients are usually asymptomatic between episodes but ~ 30% can have interepisodic nausea and dyspepsia. In some patients, episodes become progressively longer and the interepisodic phase is considerably shortened and patients have a “coalescence of symptoms.”12 It is important to elicit a thorough history in all patients with vomiting in order to make an accurate diagnosis of CVS since coalescence of symptoms only occurs over a period of time. Episodes often are triggered by psychological stress, both positive and negative. Common triggers can include positive events such as birthdays, holidays, and negative ones like examinations, the death of a loved one, etc. Sleep deprivation and physical exhaustion also can trigger an episode.12
CVS remains a clinical diagnosis since there are no biomarkers. While there is a lack of data on the optimal work-up in these patients, experts recommend an upper endoscopy or upper GI series in order to rule out alternative gastric and intestinal pathology (e.g., malrotation with volvulus).16 Of note, a gastric-emptying study is not recommended as part of the routine work-up as per recent guidelines because of the poor specificity of this test in establishing a diagnosis of CVS.16 Biochemical testing including a complete blood count, serum electrolytes, serum glucose, liver panel, and urinalysis is also warranted. Any additional testing is indicated when clinical features suggest an alternative diagnosis. For instance, neurologic symptoms might warrant a cranial MRI to exclude an intracerebral tumor or other lesions of the brain.

The severity and unpredictable nature of symptoms makes it difficult for some patients to attend school or work; one study found that 32% of patients with CVS were completely disabled.12 Despite increasing awareness of this disorder, patients often are misdiagnosed. The prevalence of CVS in an outpatient gastroenterology clinic in the United Kingdom was 11% and was markedly underdiagnosed in the community.17 Only 5% of patients who were subsequently diagnosed with CVS were initially diagnosed accurately by their referring physician despite meeting criteria for the disorder.17 A subset of patients with CVS even undergo futile surgeries.13 Fleisher et al. noted that 30% of a 41-patient cohort underwent cholecystectomy for CVS symptoms without any improvement in disease.12 Prompt diagnosis and appropriate therapy is essential to improve patient outcomes and improve quality of life.
CVS is associated with various comorbidities such as migraine, anxiety, depression and dysautonomia, which can further impair quality of life.18,19 Approximately 70% of CVS patients report a personal or family history of migraine. Anxiety and depression affects nearly half of patients with CVS.13 Cannabis use is significantly more prevalent among patients with CVS than patients without CVS.20
Role of cannabis in CVS
The role of cannabis in the pathogenesis of symptoms in CVS is controversial. While cannabis has antiemetic properties, there is a strong link between its use and CVS. The use of cannabis has increased over the past decade with increasing legalization.21 Several studies have shown that 40%-80% of patients with CVS use cannabis.22,23 Following this, cannabinoid hyperemesis syndrome (CHS) was coined as a separate entity based on this statistical association, though there are no data to support the notion that cannabis causes vomiting.24,25 CHS has clinical features that are indistinguishable from CVS except for the chronic heavy cannabis use. A peculiar bathing behavior called “compulsive hot-water bathing” has been described and was thought to be pathognomonic of cannabis use.26 During an episode, patients will take multiple hot showers/baths, which temporarily alleviate their symptoms. Many patients even report running out of hot water and sometimes check into a hotel for a continuous supply of hot water. A small number of patients may sustain burns from the hot-water bathing. However, studies show that this hot-water bathing behavior also is seen in about 50% of patents with CVS who do not use cannabis.22
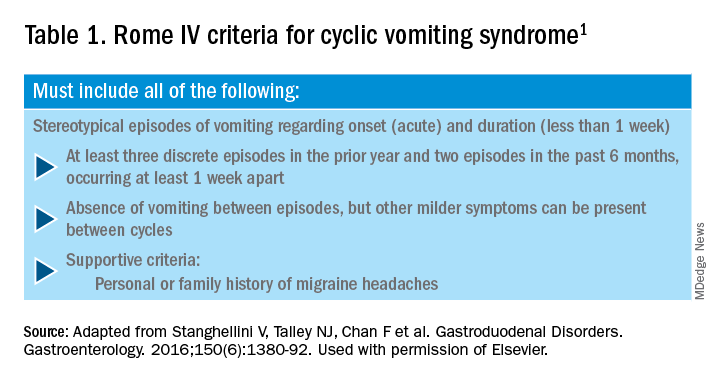
CHS is now defined by Rome IV criteria, which include episodes of nausea and vomiting similar to CVS preceded by chronic, heavy cannabis use. Patients must have complete resolution of symptoms following cessation.1 A recent systematic review of 376 cases of purported CHS showed that only 59 (15.7%) met Rome IV criteria for this disorder.27 This is because of considerable heterogeneity in how the diagnosis of CHS was made and the lack of standard diagnostic criteria at the time. Some cases of CHS were diagnosed merely based on an association of vomiting, hot-water bathing, and cannabis use.28 Only a minority of patients (71,19%) had a duration of follow-up more than 4 weeks, which would make it impossible to establish a diagnosis of CHS. A period of at least a year or a duration of time that spans at least three episodes is generally recommended to determine if abstinence from cannabis causes a true resolution of symptoms.27 Whether CHS is a separate entity or a subtype of CVS remains to be determined. The paradoxical effects of cannabis may happen because of the use of highly potent cannabis products that are currently in use. A complete discussion of the role of cannabis in CVS is beyond the scope of this article, and the reader is referred to a recent systematic review and discussion.27
Treatment
CVS should be treated based on a biopsychosocial model with a multidisciplinary team that includes a gastroenterologist with knowledge of CVS, primary care physician, psychologist, psychiatrist, and sleep specialist if needed.16 Initiating prophylactic treatment is based on the severity of disease. An algorithm for the treatment of CVS based on severity of symptoms is shown below.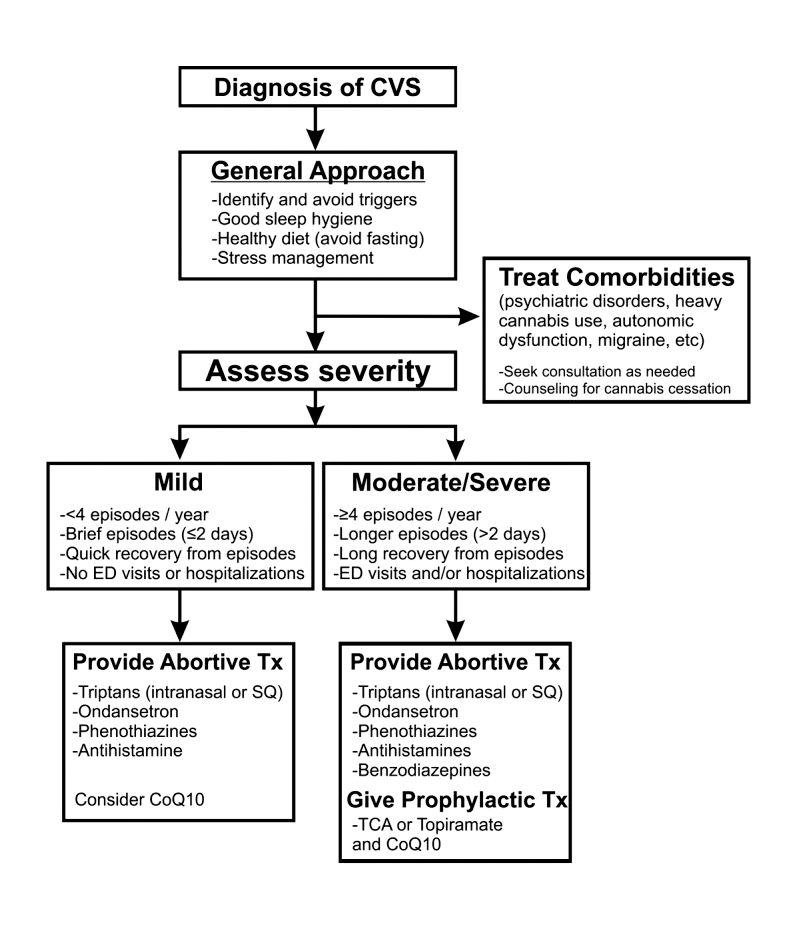
Figure 2. Adapted and reprinted by permission from the Licensor: Springer Nature, Current Treatment Options in Gastroenterology, Bhandari S, Venkatesan T. Novel Treatments for Cyclic Vomiting Syndrome: Beyond Ondansetron and Amitriptyline, 14:495-506, Copyright 2016.
Patients who have mild disease (defined as fewer than four episodes/year, episodes lasting up to 2 days, quick recovery from episodes, or episodes not requiring ED care or hospitalization) are usually prescribed abortive medications.16 These medications are best administered during the prodromal phase and can prevent progression to the emetic phase. Medications used for aborting episodes include sumatriptan (20 mg intranasal or 6 mg subcutaneous), ondansetron (8 mg sublingual), and diphenhydramine (25-50 mg).30,31 This combination can help abort symptoms and potentially avoid ED visits or hospitalizations. Patients with moderate-to-severe CVS are offered prophylactic therapy in addition to abortive therapy.16
Recent guidelines recommend tricyclic antidepressants (TCAs) as the first-line agent in the prophylaxis of CVS episodes. Data from 14 studies determined that 70% (413/600) of patients responded partially or completely to TCAs.16 An open-label study of 46 patients by Hejazi et al. noted a decline in the number of CVS episodes from 17 to 3, in the duration of a CVS episode from 6 to 2 days, and in the number of ED visits/ hospitalizations from 15 to 3.3.32Amitriptyline should be started at 25 mg at night and titrated up by 10-25 mg each week to minimize emergence of side effects. The mean effective dose is 75-100 mg or 1.0-1.5 mg/kg. An EKG should be checked at baseline and during titration to monitor the QT interval. Unfortunately, side effects from TCAs are quite common and include cognitive impairment, drowsiness, dryness of mouth, weight gain, constipation, and mood changes, which may warrant dose reduction or discontinuation. Antiepileptics such as topiramate, mitochondrial supplements such as Coenzyme Q10 and riboflavin are alternative prophylactic agents in CVS.33 Aprepitant, a newer NK1 receptor antagonist has been found to be effective in refractory CVS.34 In addition to pharmacotherapy, addressing comorbid conditions such as anxiety and depression and counseling patients to abstain from heavy cannabis use is also important to achieve good health care outcomes.
In summary, CVS is a common, chronic functional GI disorder with episodic nausea, vomiting, and often, abdominal pain. Symptoms can be disabling, and prompt diagnosis and therapy is important. CVS is associated with multiple comorbid conditions such as migraine, anxiety and depression, and a biopsychosocial model of care is essential. Medications such as amitriptyline are effective in the prophylaxis of CVS, but side effects hamper their use. Recent recommendations for management of CVS have been published.16 Cannabis is frequently used by patients for symptom relief but use of high potency products may cause worsening of symptoms or unmask symptoms in genetically predisposed individuals.23 Studies to elucidate the pathophysiology of CVS should help in the development of better therapies.
Dr. Mooers is PGY-2, an internal medicine resident in the department of medicine, Medical College of Wisconsin, Milwaukee; Dr. Venkatesan is professor of medicine, division of gastroenterology and hepatology, department of medicine, Medical College of Wisconsin, Milwaukee. The authors have no conflicts to disclose.
References
1. Stanghellini V et al. Gastroenterology. 2016;150:1380-92.
2. Aziz I et al. Clin Gastroenterol Hepatol. 2019 Apr;17(5):878-86.
3. Kovacic K et al. Curr Gastroenterol Rep. 2018;20(10):46.
4. Zaki EA et al. Cephalalgia. 2009;29:719-28.
5. Venkatesan T et al. BMC Gastroenterol. 2014;14:181.
6. Ellingsen DM et al. Neurogastroenterol Motil. 2017;29 (6)e13004 9.
7. Venkatesan T et al. Neurogastroenterol Motil. 2016;28:1409-18.
8. Wasilewski A et al. Am J Gastroenterol. 2017;112:933-9.
9. van Sickle MD et al. Am J Physiol Gastrointest Liver Physiol 2003;285:G566-76.
10. Parker LA et al. Br J Pharmacol. 2011;163:1411-22.
11. van Sickle MD et al. Gastroenterology. 2001;121:767-74.
12. Fleisher DR et al. BMC Med. 2005;3:20.
13. Kumar N et al. BMC Gastroenterol. 2012;12:52.
14. Li BU et al. J Pediatr Gastroenterol Nutr. 2008;47:379-93.
15. Bhandari S et al. Clin Auton Res. 2018 Apr;28(2):203-9.
16. Venkatesan T et al. Neurogastroenterol Motil. 2019;31 Suppl 2:e13604. doi: 10.1111/nmo.13604.
17. Sagar RC et al. Neurogastroenterol Motil. 2018;30. doi: 10.1111/nmo.13174.
18. Taranukha T et al. Neurogastroenterol Motil. 2018 Apr;30(4):e13245. doi: 10.1111/nmo.13245.
19. Bhandari S and Venkatesan T. Dig Dis Sci. 2017;62:2035-44.
20. Choung RS et al. Neurogastroenterol Motil. 2012;24:20-6, e21. doi: 10.1111/j.1365-2982.2011.01791.x.
21. Bhandari S et al. Intern Med J. 2019 May;49(5):649-55.
22. Venkatesan T et al. Exp Brain Res. 2014; 232:2563-70.
23. Venkatesan T et al. Clin Gastroenterol Hepatol. 2019 Jul 25. doi: 10.1016/j.cgh.2019.07.039.
24. Simonetto DA et al. Mayo Clin Proc. 2012;87:114-9.
25. Wallace EA et al. South Med J. 2011;104:659-64.
26. Allen JH et al. Gut. 2004;53:1566-70.
27. Venkatesan T et al. Neurogastroenterol Motil. 2019;31 Suppl 2:e13606. doi: 10.1111/nmo.13606.
28. Habboushe J et al. Basic Clin Pharmacol Toxicol. 2018;122:660-2.
29. Bhandari S and Venkatesan T. Curr Treat Options Gastroenterol. 2016;14:495-506.
30. Hikita T et al. Cephalalgia. 2011;31:504-7.
31. Fuseau E et al. Clin Pharmacokinet 2002;41:801-11.
32. Hejazi RA et al. J Clin Gastroenterol. 2010;44:18-21.
33. Sezer OB and Sezer T. J Neurogastroenterol Motil. 2016;22:656-60.
34. Cristofori F et al. Aliment Pharmacol Ther. 2014;40:309-17.
Introduction
Cyclic vomiting syndrome (CVS) is a chronic disorder of gut-brain interaction (DGBI) and is characterized by recurrent episodes of severe nausea, vomiting, and often, abdominal pain. Patients are usually asymptomatic in between episodes.1 CVS was considered a pediatric disease but is now known to be as common in adults. The prevalence of CVS in adults was 2% in a recent population-based study.2 Patients are predominantly white. Both males and females are affected with some studies showing a female preponderance. The mean age of onset is 5 years in children and 35 years in adults.3
The etiology of CVS is not known, but various hypotheses have been proposed. Zaki et al. showed that two mitochondrial DNA polymorphisms 16519T and 3010A were associated with a 17-fold increased odds of having CVS in children.4 These polymorphisms were not associated with CVS in adults.5 Alterations in the brain-gut axis also have been shown in CVS. Functional neuroimaging studies demonstrate that patients with CVS displayed increased connectivity between insula and salience networks with concomitant decrease in connectivity to somatosensory networks.6 Recent data also indicate that the endocannabinoid system (ECS) and the hypothalamic-pituitary-adrenal axis are implicated in CVS with an increase in serum endocannabinoid concentration during an episode.7 The same study also showed a significant increase in salivary cortisol in CVS patients who used cannabis. Further, single nucleotide polymorphisms (SNPs) in the gene that encodes for the cannabinoid receptor type 1 (CB1R) are implicated in CVS.8 The CB1R is part of the ECS and is densely expressed in brain areas involved in emesis, such as the dorsal vagal complex consisting of the area postrema (AP), nucleus of the solitary tract (NTS), and also the dorsal motor nucleus of the vagus.9 Wasilewski et al. showed an increased risk of CVS among individuals with AG and GG genotypes of CNR1 rs806380 (P less than .01), whereas the CC genotype of CNR1 rs806368 was associated with a decreased risk of CVS (P less than .05).8 CB1R agonists – endocannabinoids and tetrahydrocannabinol (THC) – have acute antiemetic and anxiolytic effects.9-11 The apparent paradoxical effects of cannabis in this patient population are yet to be explained and need further study.
Diagnosis and clinical features of CVS

Figure 1: Phases of Cyclic Vomiting Syndrome12
Adapted from Fleisher DR, Gornowicz B, Adams K, Burch R, Feldman EJ. Cyclic Vomiting Syndrome in 41 adults: The illness, the patients, and problems of management. BMC Med 2005;3:20. This work is licensed under the Creative Commons Attribution 4.0 International License https://creativecommons.org/licenses/by/4.0/, which permits unrestricted use, distribution, modification, and reproduction in any medium.
CVS consists of four phases which include the a) prodromal phase, b) the episodic phase, c) recovery phase, and d) the interepisodic phase; and was first described by David Fleisher.12 The phases of CVS are important for clinicians and patients alike as they have therapeutic implications. The administration of abortive medications during a prodrome can terminate an episode. The phases of CVS are shown above.
Most patients (~ 93%) have a prodromal phase. Symptoms during this phase can include nausea, abdominal pain, diaphoresis, fatigue, weakness, hot flashes, chills, shivering, increased thirst, loss of appetite, burping, lightheadedness, and paresthesia.13 Some patients report a sense of impending doom and many have symptoms consistent with panic. If untreated, this progresses to the emetic phase and patients have unrelenting nausea, retching, vomiting, and other symptoms. During an episode, patients may vomit up to 20 times per hour and the episode may last several hours to days. During this phase, patients are sometimes described as being in a “conscious coma” and exhibit lethargy, listlessness, withdrawal, and sometimes disorientation.14,15 The emetic phase is followed by the recovery phase, during which symptoms subside and patients are able to resume oral intake. Patients are usually asymptomatic between episodes but ~ 30% can have interepisodic nausea and dyspepsia. In some patients, episodes become progressively longer and the interepisodic phase is considerably shortened and patients have a “coalescence of symptoms.”12 It is important to elicit a thorough history in all patients with vomiting in order to make an accurate diagnosis of CVS since coalescence of symptoms only occurs over a period of time. Episodes often are triggered by psychological stress, both positive and negative. Common triggers can include positive events such as birthdays, holidays, and negative ones like examinations, the death of a loved one, etc. Sleep deprivation and physical exhaustion also can trigger an episode.12
CVS remains a clinical diagnosis since there are no biomarkers. While there is a lack of data on the optimal work-up in these patients, experts recommend an upper endoscopy or upper GI series in order to rule out alternative gastric and intestinal pathology (e.g., malrotation with volvulus).16 Of note, a gastric-emptying study is not recommended as part of the routine work-up as per recent guidelines because of the poor specificity of this test in establishing a diagnosis of CVS.16 Biochemical testing including a complete blood count, serum electrolytes, serum glucose, liver panel, and urinalysis is also warranted. Any additional testing is indicated when clinical features suggest an alternative diagnosis. For instance, neurologic symptoms might warrant a cranial MRI to exclude an intracerebral tumor or other lesions of the brain.

The severity and unpredictable nature of symptoms makes it difficult for some patients to attend school or work; one study found that 32% of patients with CVS were completely disabled.12 Despite increasing awareness of this disorder, patients often are misdiagnosed. The prevalence of CVS in an outpatient gastroenterology clinic in the United Kingdom was 11% and was markedly underdiagnosed in the community.17 Only 5% of patients who were subsequently diagnosed with CVS were initially diagnosed accurately by their referring physician despite meeting criteria for the disorder.17 A subset of patients with CVS even undergo futile surgeries.13 Fleisher et al. noted that 30% of a 41-patient cohort underwent cholecystectomy for CVS symptoms without any improvement in disease.12 Prompt diagnosis and appropriate therapy is essential to improve patient outcomes and improve quality of life.
CVS is associated with various comorbidities such as migraine, anxiety, depression and dysautonomia, which can further impair quality of life.18,19 Approximately 70% of CVS patients report a personal or family history of migraine. Anxiety and depression affects nearly half of patients with CVS.13 Cannabis use is significantly more prevalent among patients with CVS than patients without CVS.20
Role of cannabis in CVS
The role of cannabis in the pathogenesis of symptoms in CVS is controversial. While cannabis has antiemetic properties, there is a strong link between its use and CVS. The use of cannabis has increased over the past decade with increasing legalization.21 Several studies have shown that 40%-80% of patients with CVS use cannabis.22,23 Following this, cannabinoid hyperemesis syndrome (CHS) was coined as a separate entity based on this statistical association, though there are no data to support the notion that cannabis causes vomiting.24,25 CHS has clinical features that are indistinguishable from CVS except for the chronic heavy cannabis use. A peculiar bathing behavior called “compulsive hot-water bathing” has been described and was thought to be pathognomonic of cannabis use.26 During an episode, patients will take multiple hot showers/baths, which temporarily alleviate their symptoms. Many patients even report running out of hot water and sometimes check into a hotel for a continuous supply of hot water. A small number of patients may sustain burns from the hot-water bathing. However, studies show that this hot-water bathing behavior also is seen in about 50% of patents with CVS who do not use cannabis.22

CHS is now defined by Rome IV criteria, which include episodes of nausea and vomiting similar to CVS preceded by chronic, heavy cannabis use. Patients must have complete resolution of symptoms following cessation.1 A recent systematic review of 376 cases of purported CHS showed that only 59 (15.7%) met Rome IV criteria for this disorder.27 This is because of considerable heterogeneity in how the diagnosis of CHS was made and the lack of standard diagnostic criteria at the time. Some cases of CHS were diagnosed merely based on an association of vomiting, hot-water bathing, and cannabis use.28 Only a minority of patients (71,19%) had a duration of follow-up more than 4 weeks, which would make it impossible to establish a diagnosis of CHS. A period of at least a year or a duration of time that spans at least three episodes is generally recommended to determine if abstinence from cannabis causes a true resolution of symptoms.27 Whether CHS is a separate entity or a subtype of CVS remains to be determined. The paradoxical effects of cannabis may happen because of the use of highly potent cannabis products that are currently in use. A complete discussion of the role of cannabis in CVS is beyond the scope of this article, and the reader is referred to a recent systematic review and discussion.27
Treatment
CVS should be treated based on a biopsychosocial model with a multidisciplinary team that includes a gastroenterologist with knowledge of CVS, primary care physician, psychologist, psychiatrist, and sleep specialist if needed.16 Initiating prophylactic treatment is based on the severity of disease. An algorithm for the treatment of CVS based on severity of symptoms is shown below.
Figure 2. Adapted and reprinted by permission from the Licensor: Springer Nature, Current Treatment Options in Gastroenterology, Bhandari S, Venkatesan T. Novel Treatments for Cyclic Vomiting Syndrome: Beyond Ondansetron and Amitriptyline, 14:495-506, Copyright 2016.
Patients who have mild disease (defined as fewer than four episodes/year, episodes lasting up to 2 days, quick recovery from episodes, or episodes not requiring ED care or hospitalization) are usually prescribed abortive medications.16 These medications are best administered during the prodromal phase and can prevent progression to the emetic phase. Medications used for aborting episodes include sumatriptan (20 mg intranasal or 6 mg subcutaneous), ondansetron (8 mg sublingual), and diphenhydramine (25-50 mg).30,31 This combination can help abort symptoms and potentially avoid ED visits or hospitalizations. Patients with moderate-to-severe CVS are offered prophylactic therapy in addition to abortive therapy.16
Recent guidelines recommend tricyclic antidepressants (TCAs) as the first-line agent in the prophylaxis of CVS episodes. Data from 14 studies determined that 70% (413/600) of patients responded partially or completely to TCAs.16 An open-label study of 46 patients by Hejazi et al. noted a decline in the number of CVS episodes from 17 to 3, in the duration of a CVS episode from 6 to 2 days, and in the number of ED visits/ hospitalizations from 15 to 3.3.32Amitriptyline should be started at 25 mg at night and titrated up by 10-25 mg each week to minimize emergence of side effects. The mean effective dose is 75-100 mg or 1.0-1.5 mg/kg. An EKG should be checked at baseline and during titration to monitor the QT interval. Unfortunately, side effects from TCAs are quite common and include cognitive impairment, drowsiness, dryness of mouth, weight gain, constipation, and mood changes, which may warrant dose reduction or discontinuation. Antiepileptics such as topiramate, mitochondrial supplements such as Coenzyme Q10 and riboflavin are alternative prophylactic agents in CVS.33 Aprepitant, a newer NK1 receptor antagonist has been found to be effective in refractory CVS.34 In addition to pharmacotherapy, addressing comorbid conditions such as anxiety and depression and counseling patients to abstain from heavy cannabis use is also important to achieve good health care outcomes.
In summary, CVS is a common, chronic functional GI disorder with episodic nausea, vomiting, and often, abdominal pain. Symptoms can be disabling, and prompt diagnosis and therapy is important. CVS is associated with multiple comorbid conditions such as migraine, anxiety and depression, and a biopsychosocial model of care is essential. Medications such as amitriptyline are effective in the prophylaxis of CVS, but side effects hamper their use. Recent recommendations for management of CVS have been published.16 Cannabis is frequently used by patients for symptom relief but use of high potency products may cause worsening of symptoms or unmask symptoms in genetically predisposed individuals.23 Studies to elucidate the pathophysiology of CVS should help in the development of better therapies.
Dr. Mooers is PGY-2, an internal medicine resident in the department of medicine, Medical College of Wisconsin, Milwaukee; Dr. Venkatesan is professor of medicine, division of gastroenterology and hepatology, department of medicine, Medical College of Wisconsin, Milwaukee. The authors have no conflicts to disclose.
References
1. Stanghellini V et al. Gastroenterology. 2016;150:1380-92.
2. Aziz I et al. Clin Gastroenterol Hepatol. 2019 Apr;17(5):878-86.
3. Kovacic K et al. Curr Gastroenterol Rep. 2018;20(10):46.
4. Zaki EA et al. Cephalalgia. 2009;29:719-28.
5. Venkatesan T et al. BMC Gastroenterol. 2014;14:181.
6. Ellingsen DM et al. Neurogastroenterol Motil. 2017;29 (6)e13004 9.
7. Venkatesan T et al. Neurogastroenterol Motil. 2016;28:1409-18.
8. Wasilewski A et al. Am J Gastroenterol. 2017;112:933-9.
9. van Sickle MD et al. Am J Physiol Gastrointest Liver Physiol 2003;285:G566-76.
10. Parker LA et al. Br J Pharmacol. 2011;163:1411-22.
11. van Sickle MD et al. Gastroenterology. 2001;121:767-74.
12. Fleisher DR et al. BMC Med. 2005;3:20.
13. Kumar N et al. BMC Gastroenterol. 2012;12:52.
14. Li BU et al. J Pediatr Gastroenterol Nutr. 2008;47:379-93.
15. Bhandari S et al. Clin Auton Res. 2018 Apr;28(2):203-9.
16. Venkatesan T et al. Neurogastroenterol Motil. 2019;31 Suppl 2:e13604. doi: 10.1111/nmo.13604.
17. Sagar RC et al. Neurogastroenterol Motil. 2018;30. doi: 10.1111/nmo.13174.
18. Taranukha T et al. Neurogastroenterol Motil. 2018 Apr;30(4):e13245. doi: 10.1111/nmo.13245.
19. Bhandari S and Venkatesan T. Dig Dis Sci. 2017;62:2035-44.
20. Choung RS et al. Neurogastroenterol Motil. 2012;24:20-6, e21. doi: 10.1111/j.1365-2982.2011.01791.x.
21. Bhandari S et al. Intern Med J. 2019 May;49(5):649-55.
22. Venkatesan T et al. Exp Brain Res. 2014; 232:2563-70.
23. Venkatesan T et al. Clin Gastroenterol Hepatol. 2019 Jul 25. doi: 10.1016/j.cgh.2019.07.039.
24. Simonetto DA et al. Mayo Clin Proc. 2012;87:114-9.
25. Wallace EA et al. South Med J. 2011;104:659-64.
26. Allen JH et al. Gut. 2004;53:1566-70.
27. Venkatesan T et al. Neurogastroenterol Motil. 2019;31 Suppl 2:e13606. doi: 10.1111/nmo.13606.
28. Habboushe J et al. Basic Clin Pharmacol Toxicol. 2018;122:660-2.
29. Bhandari S and Venkatesan T. Curr Treat Options Gastroenterol. 2016;14:495-506.
30. Hikita T et al. Cephalalgia. 2011;31:504-7.
31. Fuseau E et al. Clin Pharmacokinet 2002;41:801-11.
32. Hejazi RA et al. J Clin Gastroenterol. 2010;44:18-21.
33. Sezer OB and Sezer T. J Neurogastroenterol Motil. 2016;22:656-60.
34. Cristofori F et al. Aliment Pharmacol Ther. 2014;40:309-17.
Introduction
Cyclic vomiting syndrome (CVS) is a chronic disorder of gut-brain interaction (DGBI) and is characterized by recurrent episodes of severe nausea, vomiting, and often, abdominal pain. Patients are usually asymptomatic in between episodes.1 CVS was considered a pediatric disease but is now known to be as common in adults. The prevalence of CVS in adults was 2% in a recent population-based study.2 Patients are predominantly white. Both males and females are affected with some studies showing a female preponderance. The mean age of onset is 5 years in children and 35 years in adults.3
The etiology of CVS is not known, but various hypotheses have been proposed. Zaki et al. showed that two mitochondrial DNA polymorphisms 16519T and 3010A were associated with a 17-fold increased odds of having CVS in children.4 These polymorphisms were not associated with CVS in adults.5 Alterations in the brain-gut axis also have been shown in CVS. Functional neuroimaging studies demonstrate that patients with CVS displayed increased connectivity between insula and salience networks with concomitant decrease in connectivity to somatosensory networks.6 Recent data also indicate that the endocannabinoid system (ECS) and the hypothalamic-pituitary-adrenal axis are implicated in CVS with an increase in serum endocannabinoid concentration during an episode.7 The same study also showed a significant increase in salivary cortisol in CVS patients who used cannabis. Further, single nucleotide polymorphisms (SNPs) in the gene that encodes for the cannabinoid receptor type 1 (CB1R) are implicated in CVS.8 The CB1R is part of the ECS and is densely expressed in brain areas involved in emesis, such as the dorsal vagal complex consisting of the area postrema (AP), nucleus of the solitary tract (NTS), and also the dorsal motor nucleus of the vagus.9 Wasilewski et al. showed an increased risk of CVS among individuals with AG and GG genotypes of CNR1 rs806380 (P less than .01), whereas the CC genotype of CNR1 rs806368 was associated with a decreased risk of CVS (P less than .05).8 CB1R agonists – endocannabinoids and tetrahydrocannabinol (THC) – have acute antiemetic and anxiolytic effects.9-11 The apparent paradoxical effects of cannabis in this patient population are yet to be explained and need further study.
Diagnosis and clinical features of CVS

Figure 1: Phases of Cyclic Vomiting Syndrome12
Adapted from Fleisher DR, Gornowicz B, Adams K, Burch R, Feldman EJ. Cyclic Vomiting Syndrome in 41 adults: The illness, the patients, and problems of management. BMC Med 2005;3:20. This work is licensed under the Creative Commons Attribution 4.0 International License https://creativecommons.org/licenses/by/4.0/, which permits unrestricted use, distribution, modification, and reproduction in any medium.
CVS consists of four phases which include the a) prodromal phase, b) the episodic phase, c) recovery phase, and d) the interepisodic phase; and was first described by David Fleisher.12 The phases of CVS are important for clinicians and patients alike as they have therapeutic implications. The administration of abortive medications during a prodrome can terminate an episode. The phases of CVS are shown above.
Most patients (~ 93%) have a prodromal phase. Symptoms during this phase can include nausea, abdominal pain, diaphoresis, fatigue, weakness, hot flashes, chills, shivering, increased thirst, loss of appetite, burping, lightheadedness, and paresthesia.13 Some patients report a sense of impending doom and many have symptoms consistent with panic. If untreated, this progresses to the emetic phase and patients have unrelenting nausea, retching, vomiting, and other symptoms. During an episode, patients may vomit up to 20 times per hour and the episode may last several hours to days. During this phase, patients are sometimes described as being in a “conscious coma” and exhibit lethargy, listlessness, withdrawal, and sometimes disorientation.14,15 The emetic phase is followed by the recovery phase, during which symptoms subside and patients are able to resume oral intake. Patients are usually asymptomatic between episodes but ~ 30% can have interepisodic nausea and dyspepsia. In some patients, episodes become progressively longer and the interepisodic phase is considerably shortened and patients have a “coalescence of symptoms.”12 It is important to elicit a thorough history in all patients with vomiting in order to make an accurate diagnosis of CVS since coalescence of symptoms only occurs over a period of time. Episodes often are triggered by psychological stress, both positive and negative. Common triggers can include positive events such as birthdays, holidays, and negative ones like examinations, the death of a loved one, etc. Sleep deprivation and physical exhaustion also can trigger an episode.12
CVS remains a clinical diagnosis since there are no biomarkers. While there is a lack of data on the optimal work-up in these patients, experts recommend an upper endoscopy or upper GI series in order to rule out alternative gastric and intestinal pathology (e.g., malrotation with volvulus).16 Of note, a gastric-emptying study is not recommended as part of the routine work-up as per recent guidelines because of the poor specificity of this test in establishing a diagnosis of CVS.16 Biochemical testing including a complete blood count, serum electrolytes, serum glucose, liver panel, and urinalysis is also warranted. Any additional testing is indicated when clinical features suggest an alternative diagnosis. For instance, neurologic symptoms might warrant a cranial MRI to exclude an intracerebral tumor or other lesions of the brain.

The severity and unpredictable nature of symptoms makes it difficult for some patients to attend school or work; one study found that 32% of patients with CVS were completely disabled.12 Despite increasing awareness of this disorder, patients often are misdiagnosed. The prevalence of CVS in an outpatient gastroenterology clinic in the United Kingdom was 11% and was markedly underdiagnosed in the community.17 Only 5% of patients who were subsequently diagnosed with CVS were initially diagnosed accurately by their referring physician despite meeting criteria for the disorder.17 A subset of patients with CVS even undergo futile surgeries.13 Fleisher et al. noted that 30% of a 41-patient cohort underwent cholecystectomy for CVS symptoms without any improvement in disease.12 Prompt diagnosis and appropriate therapy is essential to improve patient outcomes and improve quality of life.
CVS is associated with various comorbidities such as migraine, anxiety, depression and dysautonomia, which can further impair quality of life.18,19 Approximately 70% of CVS patients report a personal or family history of migraine. Anxiety and depression affects nearly half of patients with CVS.13 Cannabis use is significantly more prevalent among patients with CVS than patients without CVS.20
Role of cannabis in CVS
The role of cannabis in the pathogenesis of symptoms in CVS is controversial. While cannabis has antiemetic properties, there is a strong link between its use and CVS. The use of cannabis has increased over the past decade with increasing legalization.21 Several studies have shown that 40%-80% of patients with CVS use cannabis.22,23 Following this, cannabinoid hyperemesis syndrome (CHS) was coined as a separate entity based on this statistical association, though there are no data to support the notion that cannabis causes vomiting.24,25 CHS has clinical features that are indistinguishable from CVS except for the chronic heavy cannabis use. A peculiar bathing behavior called “compulsive hot-water bathing” has been described and was thought to be pathognomonic of cannabis use.26 During an episode, patients will take multiple hot showers/baths, which temporarily alleviate their symptoms. Many patients even report running out of hot water and sometimes check into a hotel for a continuous supply of hot water. A small number of patients may sustain burns from the hot-water bathing. However, studies show that this hot-water bathing behavior also is seen in about 50% of patents with CVS who do not use cannabis.22

CHS is now defined by Rome IV criteria, which include episodes of nausea and vomiting similar to CVS preceded by chronic, heavy cannabis use. Patients must have complete resolution of symptoms following cessation.1 A recent systematic review of 376 cases of purported CHS showed that only 59 (15.7%) met Rome IV criteria for this disorder.27 This is because of considerable heterogeneity in how the diagnosis of CHS was made and the lack of standard diagnostic criteria at the time. Some cases of CHS were diagnosed merely based on an association of vomiting, hot-water bathing, and cannabis use.28 Only a minority of patients (71,19%) had a duration of follow-up more than 4 weeks, which would make it impossible to establish a diagnosis of CHS. A period of at least a year or a duration of time that spans at least three episodes is generally recommended to determine if abstinence from cannabis causes a true resolution of symptoms.27 Whether CHS is a separate entity or a subtype of CVS remains to be determined. The paradoxical effects of cannabis may happen because of the use of highly potent cannabis products that are currently in use. A complete discussion of the role of cannabis in CVS is beyond the scope of this article, and the reader is referred to a recent systematic review and discussion.27
Treatment
CVS should be treated based on a biopsychosocial model with a multidisciplinary team that includes a gastroenterologist with knowledge of CVS, primary care physician, psychologist, psychiatrist, and sleep specialist if needed.16 Initiating prophylactic treatment is based on the severity of disease. An algorithm for the treatment of CVS based on severity of symptoms is shown below.
Figure 2. Adapted and reprinted by permission from the Licensor: Springer Nature, Current Treatment Options in Gastroenterology, Bhandari S, Venkatesan T. Novel Treatments for Cyclic Vomiting Syndrome: Beyond Ondansetron and Amitriptyline, 14:495-506, Copyright 2016.
Patients who have mild disease (defined as fewer than four episodes/year, episodes lasting up to 2 days, quick recovery from episodes, or episodes not requiring ED care or hospitalization) are usually prescribed abortive medications.16 These medications are best administered during the prodromal phase and can prevent progression to the emetic phase. Medications used for aborting episodes include sumatriptan (20 mg intranasal or 6 mg subcutaneous), ondansetron (8 mg sublingual), and diphenhydramine (25-50 mg).30,31 This combination can help abort symptoms and potentially avoid ED visits or hospitalizations. Patients with moderate-to-severe CVS are offered prophylactic therapy in addition to abortive therapy.16
Recent guidelines recommend tricyclic antidepressants (TCAs) as the first-line agent in the prophylaxis of CVS episodes. Data from 14 studies determined that 70% (413/600) of patients responded partially or completely to TCAs.16 An open-label study of 46 patients by Hejazi et al. noted a decline in the number of CVS episodes from 17 to 3, in the duration of a CVS episode from 6 to 2 days, and in the number of ED visits/ hospitalizations from 15 to 3.3.32Amitriptyline should be started at 25 mg at night and titrated up by 10-25 mg each week to minimize emergence of side effects. The mean effective dose is 75-100 mg or 1.0-1.5 mg/kg. An EKG should be checked at baseline and during titration to monitor the QT interval. Unfortunately, side effects from TCAs are quite common and include cognitive impairment, drowsiness, dryness of mouth, weight gain, constipation, and mood changes, which may warrant dose reduction or discontinuation. Antiepileptics such as topiramate, mitochondrial supplements such as Coenzyme Q10 and riboflavin are alternative prophylactic agents in CVS.33 Aprepitant, a newer NK1 receptor antagonist has been found to be effective in refractory CVS.34 In addition to pharmacotherapy, addressing comorbid conditions such as anxiety and depression and counseling patients to abstain from heavy cannabis use is also important to achieve good health care outcomes.
In summary, CVS is a common, chronic functional GI disorder with episodic nausea, vomiting, and often, abdominal pain. Symptoms can be disabling, and prompt diagnosis and therapy is important. CVS is associated with multiple comorbid conditions such as migraine, anxiety and depression, and a biopsychosocial model of care is essential. Medications such as amitriptyline are effective in the prophylaxis of CVS, but side effects hamper their use. Recent recommendations for management of CVS have been published.16 Cannabis is frequently used by patients for symptom relief but use of high potency products may cause worsening of symptoms or unmask symptoms in genetically predisposed individuals.23 Studies to elucidate the pathophysiology of CVS should help in the development of better therapies.
Dr. Mooers is PGY-2, an internal medicine resident in the department of medicine, Medical College of Wisconsin, Milwaukee; Dr. Venkatesan is professor of medicine, division of gastroenterology and hepatology, department of medicine, Medical College of Wisconsin, Milwaukee. The authors have no conflicts to disclose.
References
1. Stanghellini V et al. Gastroenterology. 2016;150:1380-92.
2. Aziz I et al. Clin Gastroenterol Hepatol. 2019 Apr;17(5):878-86.
3. Kovacic K et al. Curr Gastroenterol Rep. 2018;20(10):46.
4. Zaki EA et al. Cephalalgia. 2009;29:719-28.
5. Venkatesan T et al. BMC Gastroenterol. 2014;14:181.
6. Ellingsen DM et al. Neurogastroenterol Motil. 2017;29 (6)e13004 9.
7. Venkatesan T et al. Neurogastroenterol Motil. 2016;28:1409-18.
8. Wasilewski A et al. Am J Gastroenterol. 2017;112:933-9.
9. van Sickle MD et al. Am J Physiol Gastrointest Liver Physiol 2003;285:G566-76.
10. Parker LA et al. Br J Pharmacol. 2011;163:1411-22.
11. van Sickle MD et al. Gastroenterology. 2001;121:767-74.
12. Fleisher DR et al. BMC Med. 2005;3:20.
13. Kumar N et al. BMC Gastroenterol. 2012;12:52.
14. Li BU et al. J Pediatr Gastroenterol Nutr. 2008;47:379-93.
15. Bhandari S et al. Clin Auton Res. 2018 Apr;28(2):203-9.
16. Venkatesan T et al. Neurogastroenterol Motil. 2019;31 Suppl 2:e13604. doi: 10.1111/nmo.13604.
17. Sagar RC et al. Neurogastroenterol Motil. 2018;30. doi: 10.1111/nmo.13174.
18. Taranukha T et al. Neurogastroenterol Motil. 2018 Apr;30(4):e13245. doi: 10.1111/nmo.13245.
19. Bhandari S and Venkatesan T. Dig Dis Sci. 2017;62:2035-44.
20. Choung RS et al. Neurogastroenterol Motil. 2012;24:20-6, e21. doi: 10.1111/j.1365-2982.2011.01791.x.
21. Bhandari S et al. Intern Med J. 2019 May;49(5):649-55.
22. Venkatesan T et al. Exp Brain Res. 2014; 232:2563-70.
23. Venkatesan T et al. Clin Gastroenterol Hepatol. 2019 Jul 25. doi: 10.1016/j.cgh.2019.07.039.
24. Simonetto DA et al. Mayo Clin Proc. 2012;87:114-9.
25. Wallace EA et al. South Med J. 2011;104:659-64.
26. Allen JH et al. Gut. 2004;53:1566-70.
27. Venkatesan T et al. Neurogastroenterol Motil. 2019;31 Suppl 2:e13606. doi: 10.1111/nmo.13606.
28. Habboushe J et al. Basic Clin Pharmacol Toxicol. 2018;122:660-2.
29. Bhandari S and Venkatesan T. Curr Treat Options Gastroenterol. 2016;14:495-506.
30. Hikita T et al. Cephalalgia. 2011;31:504-7.
31. Fuseau E et al. Clin Pharmacokinet 2002;41:801-11.
32. Hejazi RA et al. J Clin Gastroenterol. 2010;44:18-21.
33. Sezer OB and Sezer T. J Neurogastroenterol Motil. 2016;22:656-60.
34. Cristofori F et al. Aliment Pharmacol Ther. 2014;40:309-17.
Colorectal polyps and cancer – when to refer to genetics
Introduction
Genetic predisposition to colorectal polyps and colorectal cancer (CRC) is more common than previously recognized. Approximately 5%-10% of all individuals diagnosed with CRC have a known genetic association. However, among those with early-onset CRC (diagnosed at age less than 50 years), recent studies show that up to 20% have an associated genetic mutation.1,2 In addition, the risk of CRC in patients with certain hereditary syndromes, such as familial adenomatous polyposis (FAP), approaches 80%-90% without timely management.3 This overall high risk of CRC and extracolonic malignancies in patients with a hereditary syndrome, along with the rising rates of early-onset CRC, underscores the importance of early diagnosis and management of a hereditary condition.
Despite increasing awareness of hereditary polyposis and nonpolyposis syndromes, referral rates for genetic counseling and testing remain low.4 As gastroenterologists we have several unique opportunities, in clinic and in endoscopy, to identify patients at risk for hereditary syndromes.
Risk stratification
Personal and family history
Reviewing personal medical history and family history in detail should be a routine part of our practice. This is often when initial signs of a potential hereditary syndrome can be detected. For example, if a patient reports a personal or family history of colorectal polyps or CRC, additional information that becomes important includes age at time of diagnosis, polyp burden (number and histologic subtype), presence of inflammatory bowel disease, and history of any extracolonic malignancies. Patients with multiple colorectal polyps (e.g. more than 10-20 adenomas or more than 2 hamartomas) and those with CRC diagnosed at a young age (younger than 50 years) should be considered candidates for genetic evaluation.5
Lynch syndrome (LS), an autosomal dominant condition caused by loss of DNA mismatch repair (MMR) genes, is the most common hereditary CRC syndrome, accounting for 2%-4% of all CRCs.3,6 Extracolonic LS-associated cancers to keep in mind while reviewing personal and family histories include those involving the gastrointestinal (GI) tract such as gastric, pancreatic, biliary tract, and small intestine cancers, and also non-GI tract cancers including endometrial, ovarian, urinary tract, and renal cancers along with brain tumors, and skin lesions including sebaceous adenomas, sebaceous carcinomas, and keratoacanthomas. Notably, after CRC, endometrial cancer is the second most common cancer among women with LS. Prior diagnosis of endometrial cancer should also prompt additional history-taking and evaluation for LS.
As the National Comprehensive Cancer Network (NCCN) highlights in its recent guidelines, several key findings in family history that should prompt referral to genetics for evaluation and testing for LS include: one or more first-degree relatives (FDR) with CRC or endometrial cancer diagnosed at less than 50 years of age, one or more FDR with CRC or endometrial cancer and another synchronous or metachronous LS-related cancer, two or more FDR or second-degree relatives (SDR) with LS-related cancer (including at least one diagnosed at age less than 50 years), and three or more FDR or SDR with LS-related cancers (regardless of age).5
Comprehensive assessment of family history should include all cancer diagnoses in first- and second-degree relatives, including age at diagnosis and cancer type, as well as ethnicity, as these inform the likelihood that the patient harbors a germline pathogenic variant associated with cancer predisposition.5 Given the difficulty of eliciting this level of detail, the family histories elicited in clinical settings are often limited or incomplete. Unknown family history should not be mistaken for unremarkable family history. Alternatively, if family history is unimpressive, this is not necessarily reassuring, as there can be variability in disease penetrance, including autosomal recessive syndromes that may skip generations, and de novo mutations do occur. In fact, among individuals with early-onset CRC diagnosed at age less than 50, only half of mutation carriers reported a family history of CRC in an FDR.2 Thus, individuals with concerning personal histories should undergo a genetic evaluation even if family history is not concerning.
Polyp phenotype
In addition to personal and family history, colon polyp history (including number, size, and histology) can provide important clues to identifying individuals with genetic predisposition to CRC. Table 1 highlights hereditary syndromes and polyp phenotypes associated with increased CRC risk. Based on consensus guidelines, individuals with a history of greater than 10-20 adenomas, 2 or more hamartomas, or 5 or more sessile serrated polyps should be referred for genetic testing.5,7 Serrated polyposis syndrome (SPS) is diagnosed based on at least one of the following criteria: 1) 5 or more serrated polyps, all at least 5 mm in size, proximal to the rectum including at least 2 that are 10 mm or larger in size, or 2) more than 20 serrated polyps distributed throughout the colon with at least 5 proximal to the rectum.8 Pathogenic germline variants in RNF43, a tumor suppressor gene, have been associated with SPS in rare families; however, in most cases genetic testing is uninformative and further genetic and environmental discovery studies are needed to determine the underlying cause.8,9

Risk prediction models
Models have been developed that integrate family history and phenotype data to help identify patients who may be at risk for LS. The Amsterdam criteria (more than 3 relatives with LS-associated cancers, more than 2 generations involving LS-associated cancers, and more than 1 cancer diagnosed before the age of 50; “3:2:1” criteria) were initially developed for research purposes to identify individuals who were likely to be carriers of mutations of LS based on CRC and later revised to include extracolonic malignancies (Amsterdam II).11 However, they have limited sensitivity for identifying high-risk patients. Similarly, the Bethesda guidelines have also been modified and revised to identify patients at risk for LS whose tumors should be tested with microsatellite instability (MSI), but also with limited sensitivity.12
Several risk prediction models have been developed that perform better than the Amsterdam criteria or Bethesda guidelines for determining which patients should be referred for genetic testing for LS. These include MMRPredict, MMRpro, and PREMM5.13-16 These models use clinical data (personal and family history of cancer and tumor phenotypes) to calculate the probability of a germline mutation in one of the mismatch repair (MMR) genes associated with LS. The current threshold at which to refer a patient for genetic counseling and testing is a predicted probability of 5% or greater using any one of these models, though some have proposed lowering the threshold to 2.5%.16,17
Universal tumor testing
Because of the limitations of relying on clinical family history, such as with the Amsterdam criteria and the Bethesda guidelines,18,19 as of 2014 the NCCN recommended universal tumor screening for DNA MMR deficiency associated with LS. This approach, also known as “universal testing,” has been shown to be cost effective and more sensitive in identifying at-risk patients than clinical criteria alone.20,21 Specifically, the NCCN recommends that tumor specimens of all patients diagnosed with CRC undergo testing for microsatellite instability (MSI) or loss of MMR proteins (MLH1, MSH2, MSH6, PMS2) expression by immunohistochemistry (IHC).5 Loss of MMR proteins or MSI-high findings should prompt a referral to genetics for counseling and consideration of testing for germline mutations. Universal testing of CRC and endometrial cancers is considered the most reliable way to screen patients for LS.

Universal testing by MSI or IHC may be performed on premalignant or malignant lesions. However, it is important to recognize that DNA MMR deficiency testing may not be as reliable when applied to colorectal polyps. Using data from three cancer registries (Dana-Farber Cancer Institute, University of Michigan, MD Anderson Cancer Center), Yurgelun and colleagues investigated the yield of MSI and IHC in colorectal polyps removed from patients with known LS.22 Overall, high-level MSI was found in only 41% of Lynch-associated adenomas and loss of MMR protein expression was evident in only 50%. While adenomas 8 mm in size or greater were more likely to have MSI-high or loss of MMR protein expression compared with those less than 8 mm in size, MMR-deficiency phenotype was less reliable in smaller adenomas. Consequently, results of MSI and/or IHC should therefore be interpreted with caution and in the context of the specimen upon which they are performed.
Considerations for clinical genetic testing
Genetic testing for cancer susceptibility should include informed consent and counseling for patients regarding potential risks and benefits. Clinicians ordering genetic testing should have the expertise necessary to interpret test results, which may be positive (pathogenic or likely pathogenic germline variant identified), or negative (no variants identified), or may yield one or more variants of uncertain clinical significance. Individuals found to carry a pathogenic or likely pathogenic germline variant associated with cancer susceptibility should be referred for additional genetic counseling and may require additional expert consultation for management of extracolonic cancer risks. It is important that individuals diagnosed with a hereditary cancer syndrome be informed that this diagnosis has implications for family members, who may also be at risk for the condition and may benefit from genetic testing.
Practical considerations
Given the difficulty in obtaining a detailed family history while in clinic or in endoscopy, several studies have investigated strategies that may be integrated into practice to identify high-risk patients without substantial burden on providers or patients. Kastrinos and colleagues identified the following three high-yield questions as part of a CRC Risk Assessment Tool that can be used while performing a precolonoscopy assessment: 1) Do you have a first-degree relative with CRC or LS-related cancer diagnosed before age 50?; 2) Have you had CRC or polyps diagnosed prior to age 50?; and 3) Do you have three or more relatives with CRC? The authors found that these three questions alone identified 77% of high-risk individuals.23 In addition, implementation of family history screening instruments using standardized surveys or self-administered risk prediction models at the time of colonoscopy have been shown to improve ascertainment of high-risk patients.24,25 Such strategies may become increasingly easier to implement with integration into patients’ electronic medical records.
Conclusions
Hereditary CRC syndromes are becoming increasingly important to identify, especially in an era where we are seeing rising rates of early-onset CRC. Early identification of high-risk features (Table 2) can lead to timely diagnosis with the goal to implement preventive strategies for screening and/or surveillance, ideally prior to development of cancers.
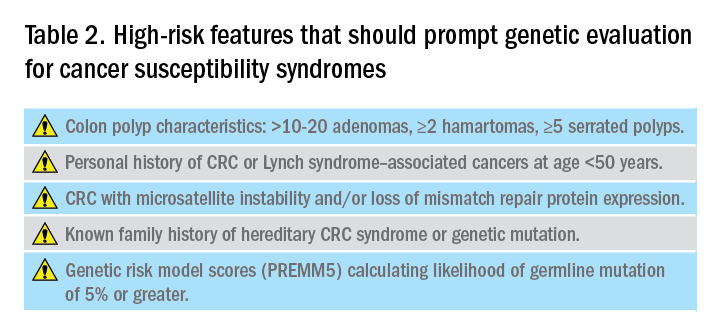
As gastroenterologists, we have several unique opportunities to identify these individuals and must maintain a high level of suspicion with careful attention when obtaining personal and family history details in clinic and in endoscopy.
Dr. Maratt is assistant professor, Indiana University, Richard L. Roudebush VA Medical Center, Indianapolis. Dr. Stoffel is assistant professor, University of Michigan; director of Cancer Genetic Clinic, Rogel Cancer Center, Ann Arbor. They have no conflicts of interest.
References
1. Pearlman R et al. JAMA Oncol. 2017;3(4):464-71.
2. Stoffel EM et al. Gastroenterology. 2018;154(4):897-905.
3. Kanth P et al. Am J Gastroenterol. 2017;112:1509-25.
4. Brennan B et al. Ther Adv Gastroenterol. 2017;10:361-71.
5. National Comprehensive Cancer Network. Available at: nccn.org.
6. Lynch HT et al. Nat Rev Cancer. 2015;15:181-94.
7. Syngal S et al. Am J Gastroenterol. 2015;110:223-62.
8. Mankaney G et al. Clin Gastroenterol Hepatol. 2020:(in press)
9. Yan HHN et al. Gut 2017;66:1645-56.
10. Ma H et al. Pathology. 2018;50:49-59.
11. Vasen H et al. Gastroenterology 1999;116:1453-6.
12. Umar A et al. J Natl Cancer Inst. 2004;96:261-8.
13. Kastrinos F et al. J Natl Cancer Inst. 2015;108(2):1-9.
14. Chen S et al. JAMA. 2006;296(12):1479-87.
15. Barnetson RA et al. N Engl J Med. 2006;354(26):2751-63.
16. Kastrinos F et al. J Clin Oncol. 2017;35:2165-72.
17. Kastrinos F et al. Fam Cancer. 2018;17:567-67.
18. Cohen SA et al. Annu Rev Genomics Hum Genet. 2019;20:293-307.
19. Matloff J et al. J Natl Compr Canc Netw. 2013;11:1380-5.
20. Ladabaum U et al. Ann Intern Med. 2011;155(2):69-79.
21. Hampel H et al. N Engl J Med. 2005;352(18):1851-60.
22. Yurgelun MB et al. Cancer Prev Res. 2012;5:574-82.
23. Kastrinos F et al. Am J Gastroenterol. 2009;104:1508-18.
24. Luba DG et al. Clin Gastroenterol Hepatol. 2018;16:49-58.
25. Guivatchian T et al. Gastrointest Endosc. 2017;86:684-91.
Introduction
Genetic predisposition to colorectal polyps and colorectal cancer (CRC) is more common than previously recognized. Approximately 5%-10% of all individuals diagnosed with CRC have a known genetic association. However, among those with early-onset CRC (diagnosed at age less than 50 years), recent studies show that up to 20% have an associated genetic mutation.1,2 In addition, the risk of CRC in patients with certain hereditary syndromes, such as familial adenomatous polyposis (FAP), approaches 80%-90% without timely management.3 This overall high risk of CRC and extracolonic malignancies in patients with a hereditary syndrome, along with the rising rates of early-onset CRC, underscores the importance of early diagnosis and management of a hereditary condition.
Despite increasing awareness of hereditary polyposis and nonpolyposis syndromes, referral rates for genetic counseling and testing remain low.4 As gastroenterologists we have several unique opportunities, in clinic and in endoscopy, to identify patients at risk for hereditary syndromes.
Risk stratification
Personal and family history
Reviewing personal medical history and family history in detail should be a routine part of our practice. This is often when initial signs of a potential hereditary syndrome can be detected. For example, if a patient reports a personal or family history of colorectal polyps or CRC, additional information that becomes important includes age at time of diagnosis, polyp burden (number and histologic subtype), presence of inflammatory bowel disease, and history of any extracolonic malignancies. Patients with multiple colorectal polyps (e.g. more than 10-20 adenomas or more than 2 hamartomas) and those with CRC diagnosed at a young age (younger than 50 years) should be considered candidates for genetic evaluation.5
Lynch syndrome (LS), an autosomal dominant condition caused by loss of DNA mismatch repair (MMR) genes, is the most common hereditary CRC syndrome, accounting for 2%-4% of all CRCs.3,6 Extracolonic LS-associated cancers to keep in mind while reviewing personal and family histories include those involving the gastrointestinal (GI) tract such as gastric, pancreatic, biliary tract, and small intestine cancers, and also non-GI tract cancers including endometrial, ovarian, urinary tract, and renal cancers along with brain tumors, and skin lesions including sebaceous adenomas, sebaceous carcinomas, and keratoacanthomas. Notably, after CRC, endometrial cancer is the second most common cancer among women with LS. Prior diagnosis of endometrial cancer should also prompt additional history-taking and evaluation for LS.
As the National Comprehensive Cancer Network (NCCN) highlights in its recent guidelines, several key findings in family history that should prompt referral to genetics for evaluation and testing for LS include: one or more first-degree relatives (FDR) with CRC or endometrial cancer diagnosed at less than 50 years of age, one or more FDR with CRC or endometrial cancer and another synchronous or metachronous LS-related cancer, two or more FDR or second-degree relatives (SDR) with LS-related cancer (including at least one diagnosed at age less than 50 years), and three or more FDR or SDR with LS-related cancers (regardless of age).5
Comprehensive assessment of family history should include all cancer diagnoses in first- and second-degree relatives, including age at diagnosis and cancer type, as well as ethnicity, as these inform the likelihood that the patient harbors a germline pathogenic variant associated with cancer predisposition.5 Given the difficulty of eliciting this level of detail, the family histories elicited in clinical settings are often limited or incomplete. Unknown family history should not be mistaken for unremarkable family history. Alternatively, if family history is unimpressive, this is not necessarily reassuring, as there can be variability in disease penetrance, including autosomal recessive syndromes that may skip generations, and de novo mutations do occur. In fact, among individuals with early-onset CRC diagnosed at age less than 50, only half of mutation carriers reported a family history of CRC in an FDR.2 Thus, individuals with concerning personal histories should undergo a genetic evaluation even if family history is not concerning.
Polyp phenotype
In addition to personal and family history, colon polyp history (including number, size, and histology) can provide important clues to identifying individuals with genetic predisposition to CRC. Table 1 highlights hereditary syndromes and polyp phenotypes associated with increased CRC risk. Based on consensus guidelines, individuals with a history of greater than 10-20 adenomas, 2 or more hamartomas, or 5 or more sessile serrated polyps should be referred for genetic testing.5,7 Serrated polyposis syndrome (SPS) is diagnosed based on at least one of the following criteria: 1) 5 or more serrated polyps, all at least 5 mm in size, proximal to the rectum including at least 2 that are 10 mm or larger in size, or 2) more than 20 serrated polyps distributed throughout the colon with at least 5 proximal to the rectum.8 Pathogenic germline variants in RNF43, a tumor suppressor gene, have been associated with SPS in rare families; however, in most cases genetic testing is uninformative and further genetic and environmental discovery studies are needed to determine the underlying cause.8,9

Risk prediction models
Models have been developed that integrate family history and phenotype data to help identify patients who may be at risk for LS. The Amsterdam criteria (more than 3 relatives with LS-associated cancers, more than 2 generations involving LS-associated cancers, and more than 1 cancer diagnosed before the age of 50; “3:2:1” criteria) were initially developed for research purposes to identify individuals who were likely to be carriers of mutations of LS based on CRC and later revised to include extracolonic malignancies (Amsterdam II).11 However, they have limited sensitivity for identifying high-risk patients. Similarly, the Bethesda guidelines have also been modified and revised to identify patients at risk for LS whose tumors should be tested with microsatellite instability (MSI), but also with limited sensitivity.12
Several risk prediction models have been developed that perform better than the Amsterdam criteria or Bethesda guidelines for determining which patients should be referred for genetic testing for LS. These include MMRPredict, MMRpro, and PREMM5.13-16 These models use clinical data (personal and family history of cancer and tumor phenotypes) to calculate the probability of a germline mutation in one of the mismatch repair (MMR) genes associated with LS. The current threshold at which to refer a patient for genetic counseling and testing is a predicted probability of 5% or greater using any one of these models, though some have proposed lowering the threshold to 2.5%.16,17
Universal tumor testing
Because of the limitations of relying on clinical family history, such as with the Amsterdam criteria and the Bethesda guidelines,18,19 as of 2014 the NCCN recommended universal tumor screening for DNA MMR deficiency associated with LS. This approach, also known as “universal testing,” has been shown to be cost effective and more sensitive in identifying at-risk patients than clinical criteria alone.20,21 Specifically, the NCCN recommends that tumor specimens of all patients diagnosed with CRC undergo testing for microsatellite instability (MSI) or loss of MMR proteins (MLH1, MSH2, MSH6, PMS2) expression by immunohistochemistry (IHC).5 Loss of MMR proteins or MSI-high findings should prompt a referral to genetics for counseling and consideration of testing for germline mutations. Universal testing of CRC and endometrial cancers is considered the most reliable way to screen patients for LS.

Universal testing by MSI or IHC may be performed on premalignant or malignant lesions. However, it is important to recognize that DNA MMR deficiency testing may not be as reliable when applied to colorectal polyps. Using data from three cancer registries (Dana-Farber Cancer Institute, University of Michigan, MD Anderson Cancer Center), Yurgelun and colleagues investigated the yield of MSI and IHC in colorectal polyps removed from patients with known LS.22 Overall, high-level MSI was found in only 41% of Lynch-associated adenomas and loss of MMR protein expression was evident in only 50%. While adenomas 8 mm in size or greater were more likely to have MSI-high or loss of MMR protein expression compared with those less than 8 mm in size, MMR-deficiency phenotype was less reliable in smaller adenomas. Consequently, results of MSI and/or IHC should therefore be interpreted with caution and in the context of the specimen upon which they are performed.
Considerations for clinical genetic testing
Genetic testing for cancer susceptibility should include informed consent and counseling for patients regarding potential risks and benefits. Clinicians ordering genetic testing should have the expertise necessary to interpret test results, which may be positive (pathogenic or likely pathogenic germline variant identified), or negative (no variants identified), or may yield one or more variants of uncertain clinical significance. Individuals found to carry a pathogenic or likely pathogenic germline variant associated with cancer susceptibility should be referred for additional genetic counseling and may require additional expert consultation for management of extracolonic cancer risks. It is important that individuals diagnosed with a hereditary cancer syndrome be informed that this diagnosis has implications for family members, who may also be at risk for the condition and may benefit from genetic testing.
Practical considerations
Given the difficulty in obtaining a detailed family history while in clinic or in endoscopy, several studies have investigated strategies that may be integrated into practice to identify high-risk patients without substantial burden on providers or patients. Kastrinos and colleagues identified the following three high-yield questions as part of a CRC Risk Assessment Tool that can be used while performing a precolonoscopy assessment: 1) Do you have a first-degree relative with CRC or LS-related cancer diagnosed before age 50?; 2) Have you had CRC or polyps diagnosed prior to age 50?; and 3) Do you have three or more relatives with CRC? The authors found that these three questions alone identified 77% of high-risk individuals.23 In addition, implementation of family history screening instruments using standardized surveys or self-administered risk prediction models at the time of colonoscopy have been shown to improve ascertainment of high-risk patients.24,25 Such strategies may become increasingly easier to implement with integration into patients’ electronic medical records.
Conclusions
Hereditary CRC syndromes are becoming increasingly important to identify, especially in an era where we are seeing rising rates of early-onset CRC. Early identification of high-risk features (Table 2) can lead to timely diagnosis with the goal to implement preventive strategies for screening and/or surveillance, ideally prior to development of cancers.

As gastroenterologists, we have several unique opportunities to identify these individuals and must maintain a high level of suspicion with careful attention when obtaining personal and family history details in clinic and in endoscopy.
Dr. Maratt is assistant professor, Indiana University, Richard L. Roudebush VA Medical Center, Indianapolis. Dr. Stoffel is assistant professor, University of Michigan; director of Cancer Genetic Clinic, Rogel Cancer Center, Ann Arbor. They have no conflicts of interest.
References
1. Pearlman R et al. JAMA Oncol. 2017;3(4):464-71.
2. Stoffel EM et al. Gastroenterology. 2018;154(4):897-905.
3. Kanth P et al. Am J Gastroenterol. 2017;112:1509-25.
4. Brennan B et al. Ther Adv Gastroenterol. 2017;10:361-71.
5. National Comprehensive Cancer Network. Available at: nccn.org.
6. Lynch HT et al. Nat Rev Cancer. 2015;15:181-94.
7. Syngal S et al. Am J Gastroenterol. 2015;110:223-62.
8. Mankaney G et al. Clin Gastroenterol Hepatol. 2020:(in press)
9. Yan HHN et al. Gut 2017;66:1645-56.
10. Ma H et al. Pathology. 2018;50:49-59.
11. Vasen H et al. Gastroenterology 1999;116:1453-6.
12. Umar A et al. J Natl Cancer Inst. 2004;96:261-8.
13. Kastrinos F et al. J Natl Cancer Inst. 2015;108(2):1-9.
14. Chen S et al. JAMA. 2006;296(12):1479-87.
15. Barnetson RA et al. N Engl J Med. 2006;354(26):2751-63.
16. Kastrinos F et al. J Clin Oncol. 2017;35:2165-72.
17. Kastrinos F et al. Fam Cancer. 2018;17:567-67.
18. Cohen SA et al. Annu Rev Genomics Hum Genet. 2019;20:293-307.
19. Matloff J et al. J Natl Compr Canc Netw. 2013;11:1380-5.
20. Ladabaum U et al. Ann Intern Med. 2011;155(2):69-79.
21. Hampel H et al. N Engl J Med. 2005;352(18):1851-60.
22. Yurgelun MB et al. Cancer Prev Res. 2012;5:574-82.
23. Kastrinos F et al. Am J Gastroenterol. 2009;104:1508-18.
24. Luba DG et al. Clin Gastroenterol Hepatol. 2018;16:49-58.
25. Guivatchian T et al. Gastrointest Endosc. 2017;86:684-91.
Introduction
Genetic predisposition to colorectal polyps and colorectal cancer (CRC) is more common than previously recognized. Approximately 5%-10% of all individuals diagnosed with CRC have a known genetic association. However, among those with early-onset CRC (diagnosed at age less than 50 years), recent studies show that up to 20% have an associated genetic mutation.1,2 In addition, the risk of CRC in patients with certain hereditary syndromes, such as familial adenomatous polyposis (FAP), approaches 80%-90% without timely management.3 This overall high risk of CRC and extracolonic malignancies in patients with a hereditary syndrome, along with the rising rates of early-onset CRC, underscores the importance of early diagnosis and management of a hereditary condition.
Despite increasing awareness of hereditary polyposis and nonpolyposis syndromes, referral rates for genetic counseling and testing remain low.4 As gastroenterologists we have several unique opportunities, in clinic and in endoscopy, to identify patients at risk for hereditary syndromes.
Risk stratification
Personal and family history
Reviewing personal medical history and family history in detail should be a routine part of our practice. This is often when initial signs of a potential hereditary syndrome can be detected. For example, if a patient reports a personal or family history of colorectal polyps or CRC, additional information that becomes important includes age at time of diagnosis, polyp burden (number and histologic subtype), presence of inflammatory bowel disease, and history of any extracolonic malignancies. Patients with multiple colorectal polyps (e.g. more than 10-20 adenomas or more than 2 hamartomas) and those with CRC diagnosed at a young age (younger than 50 years) should be considered candidates for genetic evaluation.5
Lynch syndrome (LS), an autosomal dominant condition caused by loss of DNA mismatch repair (MMR) genes, is the most common hereditary CRC syndrome, accounting for 2%-4% of all CRCs.3,6 Extracolonic LS-associated cancers to keep in mind while reviewing personal and family histories include those involving the gastrointestinal (GI) tract such as gastric, pancreatic, biliary tract, and small intestine cancers, and also non-GI tract cancers including endometrial, ovarian, urinary tract, and renal cancers along with brain tumors, and skin lesions including sebaceous adenomas, sebaceous carcinomas, and keratoacanthomas. Notably, after CRC, endometrial cancer is the second most common cancer among women with LS. Prior diagnosis of endometrial cancer should also prompt additional history-taking and evaluation for LS.
As the National Comprehensive Cancer Network (NCCN) highlights in its recent guidelines, several key findings in family history that should prompt referral to genetics for evaluation and testing for LS include: one or more first-degree relatives (FDR) with CRC or endometrial cancer diagnosed at less than 50 years of age, one or more FDR with CRC or endometrial cancer and another synchronous or metachronous LS-related cancer, two or more FDR or second-degree relatives (SDR) with LS-related cancer (including at least one diagnosed at age less than 50 years), and three or more FDR or SDR with LS-related cancers (regardless of age).5
Comprehensive assessment of family history should include all cancer diagnoses in first- and second-degree relatives, including age at diagnosis and cancer type, as well as ethnicity, as these inform the likelihood that the patient harbors a germline pathogenic variant associated with cancer predisposition.5 Given the difficulty of eliciting this level of detail, the family histories elicited in clinical settings are often limited or incomplete. Unknown family history should not be mistaken for unremarkable family history. Alternatively, if family history is unimpressive, this is not necessarily reassuring, as there can be variability in disease penetrance, including autosomal recessive syndromes that may skip generations, and de novo mutations do occur. In fact, among individuals with early-onset CRC diagnosed at age less than 50, only half of mutation carriers reported a family history of CRC in an FDR.2 Thus, individuals with concerning personal histories should undergo a genetic evaluation even if family history is not concerning.
Polyp phenotype
In addition to personal and family history, colon polyp history (including number, size, and histology) can provide important clues to identifying individuals with genetic predisposition to CRC. Table 1 highlights hereditary syndromes and polyp phenotypes associated with increased CRC risk. Based on consensus guidelines, individuals with a history of greater than 10-20 adenomas, 2 or more hamartomas, or 5 or more sessile serrated polyps should be referred for genetic testing.5,7 Serrated polyposis syndrome (SPS) is diagnosed based on at least one of the following criteria: 1) 5 or more serrated polyps, all at least 5 mm in size, proximal to the rectum including at least 2 that are 10 mm or larger in size, or 2) more than 20 serrated polyps distributed throughout the colon with at least 5 proximal to the rectum.8 Pathogenic germline variants in RNF43, a tumor suppressor gene, have been associated with SPS in rare families; however, in most cases genetic testing is uninformative and further genetic and environmental discovery studies are needed to determine the underlying cause.8,9

Risk prediction models
Models have been developed that integrate family history and phenotype data to help identify patients who may be at risk for LS. The Amsterdam criteria (more than 3 relatives with LS-associated cancers, more than 2 generations involving LS-associated cancers, and more than 1 cancer diagnosed before the age of 50; “3:2:1” criteria) were initially developed for research purposes to identify individuals who were likely to be carriers of mutations of LS based on CRC and later revised to include extracolonic malignancies (Amsterdam II).11 However, they have limited sensitivity for identifying high-risk patients. Similarly, the Bethesda guidelines have also been modified and revised to identify patients at risk for LS whose tumors should be tested with microsatellite instability (MSI), but also with limited sensitivity.12
Several risk prediction models have been developed that perform better than the Amsterdam criteria or Bethesda guidelines for determining which patients should be referred for genetic testing for LS. These include MMRPredict, MMRpro, and PREMM5.13-16 These models use clinical data (personal and family history of cancer and tumor phenotypes) to calculate the probability of a germline mutation in one of the mismatch repair (MMR) genes associated with LS. The current threshold at which to refer a patient for genetic counseling and testing is a predicted probability of 5% or greater using any one of these models, though some have proposed lowering the threshold to 2.5%.16,17
Universal tumor testing
Because of the limitations of relying on clinical family history, such as with the Amsterdam criteria and the Bethesda guidelines,18,19 as of 2014 the NCCN recommended universal tumor screening for DNA MMR deficiency associated with LS. This approach, also known as “universal testing,” has been shown to be cost effective and more sensitive in identifying at-risk patients than clinical criteria alone.20,21 Specifically, the NCCN recommends that tumor specimens of all patients diagnosed with CRC undergo testing for microsatellite instability (MSI) or loss of MMR proteins (MLH1, MSH2, MSH6, PMS2) expression by immunohistochemistry (IHC).5 Loss of MMR proteins or MSI-high findings should prompt a referral to genetics for counseling and consideration of testing for germline mutations. Universal testing of CRC and endometrial cancers is considered the most reliable way to screen patients for LS.

Universal testing by MSI or IHC may be performed on premalignant or malignant lesions. However, it is important to recognize that DNA MMR deficiency testing may not be as reliable when applied to colorectal polyps. Using data from three cancer registries (Dana-Farber Cancer Institute, University of Michigan, MD Anderson Cancer Center), Yurgelun and colleagues investigated the yield of MSI and IHC in colorectal polyps removed from patients with known LS.22 Overall, high-level MSI was found in only 41% of Lynch-associated adenomas and loss of MMR protein expression was evident in only 50%. While adenomas 8 mm in size or greater were more likely to have MSI-high or loss of MMR protein expression compared with those less than 8 mm in size, MMR-deficiency phenotype was less reliable in smaller adenomas. Consequently, results of MSI and/or IHC should therefore be interpreted with caution and in the context of the specimen upon which they are performed.
Considerations for clinical genetic testing
Genetic testing for cancer susceptibility should include informed consent and counseling for patients regarding potential risks and benefits. Clinicians ordering genetic testing should have the expertise necessary to interpret test results, which may be positive (pathogenic or likely pathogenic germline variant identified), or negative (no variants identified), or may yield one or more variants of uncertain clinical significance. Individuals found to carry a pathogenic or likely pathogenic germline variant associated with cancer susceptibility should be referred for additional genetic counseling and may require additional expert consultation for management of extracolonic cancer risks. It is important that individuals diagnosed with a hereditary cancer syndrome be informed that this diagnosis has implications for family members, who may also be at risk for the condition and may benefit from genetic testing.
Practical considerations
Given the difficulty in obtaining a detailed family history while in clinic or in endoscopy, several studies have investigated strategies that may be integrated into practice to identify high-risk patients without substantial burden on providers or patients. Kastrinos and colleagues identified the following three high-yield questions as part of a CRC Risk Assessment Tool that can be used while performing a precolonoscopy assessment: 1) Do you have a first-degree relative with CRC or LS-related cancer diagnosed before age 50?; 2) Have you had CRC or polyps diagnosed prior to age 50?; and 3) Do you have three or more relatives with CRC? The authors found that these three questions alone identified 77% of high-risk individuals.23 In addition, implementation of family history screening instruments using standardized surveys or self-administered risk prediction models at the time of colonoscopy have been shown to improve ascertainment of high-risk patients.24,25 Such strategies may become increasingly easier to implement with integration into patients’ electronic medical records.
Conclusions
Hereditary CRC syndromes are becoming increasingly important to identify, especially in an era where we are seeing rising rates of early-onset CRC. Early identification of high-risk features (Table 2) can lead to timely diagnosis with the goal to implement preventive strategies for screening and/or surveillance, ideally prior to development of cancers.

As gastroenterologists, we have several unique opportunities to identify these individuals and must maintain a high level of suspicion with careful attention when obtaining personal and family history details in clinic and in endoscopy.
Dr. Maratt is assistant professor, Indiana University, Richard L. Roudebush VA Medical Center, Indianapolis. Dr. Stoffel is assistant professor, University of Michigan; director of Cancer Genetic Clinic, Rogel Cancer Center, Ann Arbor. They have no conflicts of interest.
References
1. Pearlman R et al. JAMA Oncol. 2017;3(4):464-71.
2. Stoffel EM et al. Gastroenterology. 2018;154(4):897-905.
3. Kanth P et al. Am J Gastroenterol. 2017;112:1509-25.
4. Brennan B et al. Ther Adv Gastroenterol. 2017;10:361-71.
5. National Comprehensive Cancer Network. Available at: nccn.org.
6. Lynch HT et al. Nat Rev Cancer. 2015;15:181-94.
7. Syngal S et al. Am J Gastroenterol. 2015;110:223-62.
8. Mankaney G et al. Clin Gastroenterol Hepatol. 2020:(in press)
9. Yan HHN et al. Gut 2017;66:1645-56.
10. Ma H et al. Pathology. 2018;50:49-59.
11. Vasen H et al. Gastroenterology 1999;116:1453-6.
12. Umar A et al. J Natl Cancer Inst. 2004;96:261-8.
13. Kastrinos F et al. J Natl Cancer Inst. 2015;108(2):1-9.
14. Chen S et al. JAMA. 2006;296(12):1479-87.
15. Barnetson RA et al. N Engl J Med. 2006;354(26):2751-63.
16. Kastrinos F et al. J Clin Oncol. 2017;35:2165-72.
17. Kastrinos F et al. Fam Cancer. 2018;17:567-67.
18. Cohen SA et al. Annu Rev Genomics Hum Genet. 2019;20:293-307.
19. Matloff J et al. J Natl Compr Canc Netw. 2013;11:1380-5.
20. Ladabaum U et al. Ann Intern Med. 2011;155(2):69-79.
21. Hampel H et al. N Engl J Med. 2005;352(18):1851-60.
22. Yurgelun MB et al. Cancer Prev Res. 2012;5:574-82.
23. Kastrinos F et al. Am J Gastroenterol. 2009;104:1508-18.
24. Luba DG et al. Clin Gastroenterol Hepatol. 2018;16:49-58.
25. Guivatchian T et al. Gastrointest Endosc. 2017;86:684-91.
Management of the hospitalized ulcerative colitis patient: A primer for the initial approach to care for the practicing gastroenterologist
Introduction
Inpatient management of acute ulcerative colitis (UC) flares can be challenging because of the multiple patient and disease-related factors influencing therapeutic decision making. The clinical course during the first 24-72 hours of the hospitalization will likely guide the decision between rescue medical and surgical therapy. Using available evidence from clinical practice guidelines, we present a day-by-day guide to managing most hospitalized UC patients.
Day 0 – The emergency department (ED)
When an UC patient presents to the ED for evaluation, the initial assessments should focus on the acuity and severity of the flare. Key clinical features of disease severity include the presence of fever, tachycardia, hypotension, or weight loss in addition to worsened gastrointestinal symptoms of stool frequency relative to baseline, rectal bleeding, and abdominal pain. Acute severe ulcerative colitis (ASUC) is often defined using the modified Truelove and Witts criteria.1 A patient meets criteria for ASUC if they have at least six bloody stools per day and at least one sign of systemic toxicity, such as heart rate greater than 90 bpm, temperature at or above 37.8° C, hemoglobin level below 10.5 g/dL, or elevated inflammatory markers.
Initial laboratory assessments should include complete blood counts to identify anemia, potential superimposed infection, or toxicity and a comprehensive metabolic profile to evaluate for dehydration, electrolyte abnormalities, hepatic injury or hypoalbuminemia (an important predictor of surgery), as well as assessment of response to treatment and readmission.2,3 An evaluation at admission of C-reactive protein (CRP) is crucial because changes from the initial value will determine steroid response and predict need for surgical intervention or rescue therapy. A baseline fecal calprotectin can serve as a noninvasive marker that can be followed after discharge to monitor response to therapy.
Clostridioides difficile infection (CDI) must be ruled out in all patients presenting with ASUC regardless of history of antibiotic use or prior negative testing. Concomitant UC and CDI are associated with a four- to sixfold increased risk of in-hospital mortality and a two- to sixfold increased risk of bowel surgery.4-6 Immunoassay testing is inexpensive and fast with a high specificity but has low sensitivity; nucleic acid amplification testing with polymerase chain reaction has a high sensitivity and specificity.7 Knowing which testing algorithm the hospital lab uses helps guide interpretation of results.
For patients meeting criteria for ASUC, obtaining at least an abdominal x-ray is important to assess for colonic dilation to further stratify the patient by risk. Colonic dilation, defined as a transverse colon diameter greater than 5.5 cm, places the patient in the category of fulminant colitis and colorectal surgical consultation should be obtained.8 A CT scan is often ordered first because it can provide a rapid assessment of intra-abdominal processes but is not routinely needed unless hemodynamic instability, an acute abdomen, or markedly abnormal laboratory testing (specifically white blood cell count with bandemia) is present as these can be indicators of toxic megacolon or perforation.8-10
Day 1 – Assess disease severity and assemble the team
Obtaining a thorough clinical history is essential to classify disease severity and identify potential triggers for the acute exacerbation. Potential triggers may include infections, new medications, recent antibiotic use, recent travel, sick contacts, or cessation of treatments. Standard questions include asking about the timing of onset of symptoms, bowel movements during a 24-hour period, and particularly the presence of nocturnal bowel movements. If patients report bloody stools, inquire how often they see blood relative to the total number of bowel movements. The presence and nature of abdominal pain should be elicited, particularly changes in abdominal pain and comparison with previous disease flares. These clinical parameters are used to assess response to treatment; therefore, ask patients to keep a log of their stool frequency, consistency, rectal urgency, and bleeding each day to report to the team during daily rounds.
For patients with ASUC, a full colonoscopy is rarely indicated in the inpatient setting because it is unlikely to change management and poses a risk of perforation.11 However, a sigmoidoscopy within the first 24 hours of admission will provide useful information about the endoscopic disease activity, particularly if features such as deep or well-like ulcers, large mucosal abrasions, or extensive loss of the mucosal layer are present because these are predictors of colectomy.8 Tissue biopsies can exclude cytomegalovirus (CMV) infection, an important consideration for patients on immunosuppression including corticosteroids.12-16
Venous thromboembolism (VTE) prophylaxis is extremely important for hospitalized inflammatory bowel disease (IBD) patients. At baseline, IBD patients have a threefold higher risk of VTE than do non-IBD patients, which increases to approximately sixfold during flares.17 Pharmacologic VTE prophylaxis is recommended for all hospitalized IBD patients, even those with rectal bleeding. This may seem counterintuitive in the setting of “GI bleeding,” so it is important to counsel both patients and team members regarding VTE risks and the role of the prophylactic regimen to ensure adherence. Mechanical VTE prophylaxis can be used in patients with severe bleeding and hemodynamic instability until pharmacologic VTE prophylaxis can be safely initiated.17
Narcotics should be used sparingly for hospitalized IBD patients. Narcotic use is associated with greater likelihood of subsequent IBD hospitalizations, ED visits, and higher costs of health care for patients with IBD.18 Heavy use of opiates, defined as continuous use for more than 30 days at a dose exceeding 50 mg morphine per day or equivalent, was strongly associated with an increased overall mortality in IBD patients.19 Opiates also slow bowel motility and precipitate toxic megacolon, along with any other agent that slows bowel motility, such as anticholinergic medications.8 These agents may also mask bowel frequency symptoms that would otherwise indicate a failure of medical therapy. Similarly, use of NSAIDS should also be avoided because these have been associated with disease relapse and escalating intestinal inflammation.20
Once disease severity has been determined, intravenous corticosteroid therapy may be initiated, ideally once CDI and CMV have been excluded. The recommended dosing of intravenous corticosteroids is methylprednisolone 20 mg IV every 8 hours or equivalent. There is no evidence to support additional benefit for doses exceeding these amounts.8 Prior to starting parenteral corticosteroids, it is important to keep in mind the possible need for rescue therapy during the admission. Recommended testing includes hepatitis B surface antigen and antibody, hepatitis B core antibody and tuberculosis testing if there is no documented negative testing within the past 6-12 months. These labs should be drawn prior to steroid treatment to avoid delays in care and indeterminate results. Finally, a lipid profile is recommended for patients who may be cyclosporine candidates pending response to intravenous corticosteroids. Unless the patient has been admitted with a bowel obstruction, which should raise the suspicion that the diagnosis is actually Crohn’s disease, enteral feeding is preferred for UC patients even if they may have significant food aversion. The early involvement of a registered dietitian is valuable to guide dietary choices and recommend appropriate enteral nutrition supplements. During acute flares, patients may find a low-residue diet to be less stimulating to their gut while their acute flare is being treated. Electrolyte abnormalities should be repleted and consistently monitored during the hospitalization. Providing parenteral intravenous iron for anemic patients will expedite correction of the anemia alongside treatment of the underlying UC.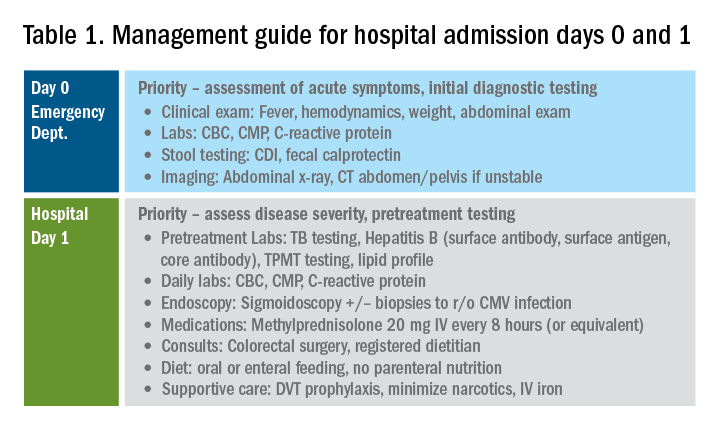
Most UC patients admitted to the hospital will require a multidisciplinary approach with gastroenterologists, surgeons, radiologists, dietitians, and case coordinators/social workers, among others. It is essential to assemble the team, especially the surgeons, earlier during the hospitalization rather than later. It is especially important to discuss the role of the surgeon in the management of UC and explain why the surgeon is being consulted in the context of the patient’s acute presentation. Being transparent about the parameters the GI team are monitoring to determine if and when surgery is the most appropriate and safe approach will improve patients’ acceptance of the surgical team’s role in their care. Specific indications for surgery in ASUC include toxic megacolon, colonic perforation, severe refractory hemorrhage, and failure to respond to medical therapy (Table 1).8
Day 3 – Assessing response to corticosteroids
In addition to daily symptom assessments, a careful abdominal exam should be performed every day with the understanding that steroids (and also narcotics) may mask perforation or pain. Any abrupt decrease or cessation of bowel movements, increasing abdominal distention, or a sudden increase in abdominal pain or tenderness may require abdominal imaging to ensure no interim perforation or severe colonic dilation has occurred while receiving steroid therapy. In these circumstances, the addition of broad spectrum intravenous antibiotics should be considered, particularly if hemodynamic instability (such as tachycardia) is present.
Patients should be assessed for response to intravenous steroid therapy after 3 days of treatment. A meaningful response to corticosteroids is present if the patient has had more than 50% improvement in symptoms, particularly rectal bleeding and stool frequency. A more than 75% improvement in CRP should also be noted from admission to day 3 with an overall trend of improvement.2,21 Additionally, patients should be afebrile, require minimal to no narcotic usage, tolerating oral intake, and be ambulatory. If the patient has met all these parameters, it is reasonable to transition to oral corticosteroids, such as prednisone 40-60 mg daily after a course of 3-5 days of intravenous corticosteroids. Ideally, patients should be observed for 24-48 hours in the hospital after transitioning to oral corticosteroids to make sure that symptoms do not worsen with the switch.
Patients with more than eight bowel movements per day, CRP greater than 4.5 g/dL, deep ulcers on endoscopy, or albumin less than 3.0 g/dL have a higher likelihood of failing intravenous corticosteroid therapy, and these patients should be prepared for rescue therapy.2,21 A patient has failed intravenous corticosteroids by day 3 if they have sustained fever in the absence of an infection, continued CRP elevation or lack of CRP decrease, or ongoing high stool frequency, bleeding, and pain with less than 50% improvement from baseline on admission.8 In the setting of nonresponse to intravenous corticosteroids, it is prudent to involve colorectal surgery to discuss colectomy as an option of equal merit to medical salvage therapies such as infliximab or cyclosporine.
Infliximab is the most readily available rescue therapy for steroid-refractory patients and has been shown to increase colectomy-free survival in patients with ASUC.8 However, patients with the same predictors for intravenous steroid failures (low albumin, high CRP, and/or deep ulcers on endoscopy) are also at the highest risk for infliximab nonresponse. These factors are important to discuss with the patients and colorectal surgery teams when providing the options of treatment strategy, particularly with medication dosing. ASUC with more severe disease biochemically (low albumin, elevated CRP, possibly bandemia) benefit from a higher dose of infliximab at 10 mg/kg, given the likelihood of increased drug clearance in this situation.22,23
From a practical standpoint, it is important to confirm the patient’s insurance status prior to medication administration to make sure therapy can be continued after hospital discharge. Early involvement of the social workers and case coordinators is key to ensuring timely administration of the next dose of treatment. Patients who receive infliximab rescue therapy should be monitored for an additional 1-2 days after administration to ensure they are responding to this therapy with continued monitoring of CRP and symptoms during this period. If there is no response at this point, an additional dose of infliximab may be considered but surgery should not be delayed if there is no meaningful response after the first dose.
Another option for intravenous corticosteroid nonresponders is intravenous cyclosporine because treatment failure rates for cyclosporine and infliximab were similar in head-to-head studies.24 However, patient selection is key to successful utilization of this agent. Unlike infliximab, cyclosporine is primarily an induction agent for steroid nonresponders rather than a maintenance strategy. Therefore, in patients in whom cyclosporine is being considered, thiopurines or vedolizumab are potential options for maintenance therapy. If the patient has poor renal function, low cholesterol, advanced age, significant comorbidities, or a history of nonadherence to therapy, cyclosporine should not be given. Additionally, clinical experience with intravenous cyclosporine administration and monitoring both during inpatient and outpatient care settings should be factored into the decision making for infliximab versus cyclosporine.8
Day 5 and beyond – Discharge planning
Patients who have responded to the initial intravenous steroid course by hospital day 5 should have successfully transitioned to oral steroids with plans to start an appropriate steroid-sparing therapy shortly after discharge. Treatment planning should commence prior to discharge and should be communicated with the outpatient GI team to ensure a smooth transition to the ambulatory care setting, primarily to begin insurance authorizations as soon as possible. If the patient has had a meaningful response to infliximab rescue therapy (improvement by more than 50% in bowel frequency, amount of blood, abdominal pain), discharge planning needs to prioritize obtaining authorization for the second dose within 2 weeks of the initial infusion. These patients are high risk for readmission, and close outpatient follow-up by the ambulatory GI care team is necessary to help direct the tapering of steroids and monitor response to treatment.
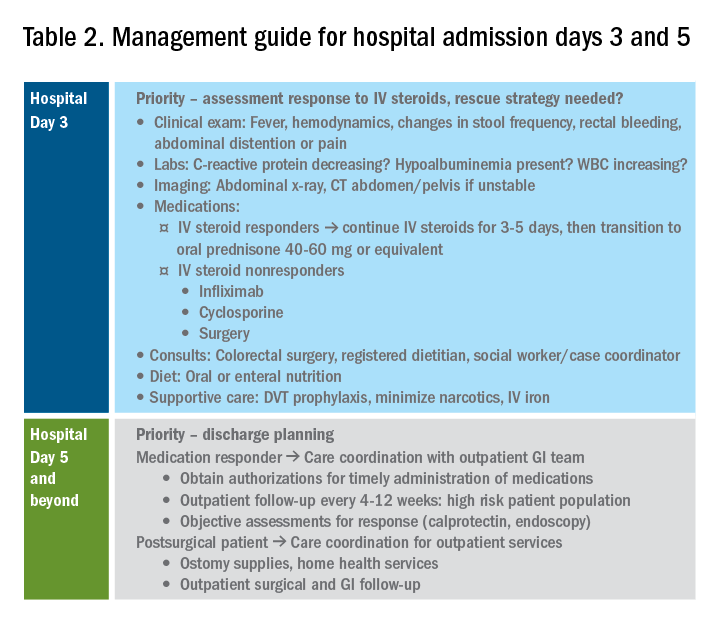
If the patient has not responded to intravenous steroid therapy, infliximab, or cyclosporine by day 5-7, then surgery should be strongly considered. Delaying surgery may worsen outcomes as patients become more malnourished, anemic, and continue to receive intravenous steroids. Additional preoperative optimization may be required depending on the patient’s course up to this point (Table 2).
Summary
The cornerstones of inpatient UC management center on a thorough initial evaluation including imaging and endoscopy as appropriate, establishment of baseline parameters, and daily assessment of response to therapy through a combination of patient-reported outcomes and biomarkers of inflammation. With this strategy in mind, practitioners and care teams can manage these complex patients using a consistent strategy focusing on multidisciplinary, evidence-based care.
References
1. Truelove SC et al. Br Med J. 1955 Oct 23;2(4947):1041-8.
2. Ho GT et al. Aliment Pharmacol Ther. 2004 May 15;19(10):1079-87.
3. Tinsley A et al. Scand J Gastroenterol. 2015;50(9):1103-9.
4. Issa M et al. Clin Gastroenterol Hepatol. 2007 Mar;5(3):345-51.
5. Ananthakrishnan AN et al. Gut. 2008 Feb;57(2):205-10.
6. Negron ME et al. Am J Gastroenterol. 2016 May;111(5):691-704.
7. Taylor KN et al. Gynecol Oncol. 2017 Feb;144(2):428-37.
8. Rubin DT et al. Am J Gastroenterol. 2019 Mar;114(3):384-413.
9. Jalan KN et al. Gastroenterology. 1969 Jul;57(1):68-82.
10. Gan SI et al. Am J Gastroenterol. 2003 Nov;98(11):2363-71.
11. Makkar R et al. Gastroenterol Hepatol (N Y). 2013 Sep;9(9):573-83.
12. Hindryckx P et al. Nat Rev Gastroenterol Hepatol. 2016 Nov;13(11):654-64.
13. Yerushalmy-Feler A et al. Curr Infect Dis Rep. 2019 Feb 15;21(2):5.
14. Shukla T et al. J Clin Gastroenterol. 2017 May/Jun;51(5):394-401.
15. McCurdy JD et al. Clin Gastroenterol Hepatol. 2015 Jan;13(1):131-7; quiz e7.
16. Cottone M et al. Am J Gastroenterol. 2001 Mar;96(3):773-5.
17. Nguyen GC et al. Gastroenterology. 2014 Mar;146(3):835-48 e6.
18. Limsrivilai J et al. Clin Gastroenterol Hepatol. 2017 Mar;15(3):385-92 e2.
19. Targownik LE et al. Am J Gastroenterol. 2014 Oct;109(10):1613-20.
20. Takeuchi K et al. Clin Gastroenterol Hepatol. 2006 Feb;4(2):196-202.
21. Travis SP et al. Gut. 1996 Jun;38(6):905-10.
22. Syal G et al. Mo1891 - Gastroenterology. 2018;154:S841.
23. Ungar B et al. Aliment Pharmacol Ther. 2016 Jun;43(12):1293-9.
24. Laharie D et al. Lancet 2012 Dec 1;380(9857):1909-15.
Dr. Chiplunker is an advanced inflammatory bowel disease fellow; Dr. Ha is associate professor of medicine at the Inflammatory Bowel Disease Center at Cedars-Sinai Medical Center, Los Angeles.
Introduction
Inpatient management of acute ulcerative colitis (UC) flares can be challenging because of the multiple patient and disease-related factors influencing therapeutic decision making. The clinical course during the first 24-72 hours of the hospitalization will likely guide the decision between rescue medical and surgical therapy. Using available evidence from clinical practice guidelines, we present a day-by-day guide to managing most hospitalized UC patients.
Day 0 – The emergency department (ED)
When an UC patient presents to the ED for evaluation, the initial assessments should focus on the acuity and severity of the flare. Key clinical features of disease severity include the presence of fever, tachycardia, hypotension, or weight loss in addition to worsened gastrointestinal symptoms of stool frequency relative to baseline, rectal bleeding, and abdominal pain. Acute severe ulcerative colitis (ASUC) is often defined using the modified Truelove and Witts criteria.1 A patient meets criteria for ASUC if they have at least six bloody stools per day and at least one sign of systemic toxicity, such as heart rate greater than 90 bpm, temperature at or above 37.8° C, hemoglobin level below 10.5 g/dL, or elevated inflammatory markers.
Initial laboratory assessments should include complete blood counts to identify anemia, potential superimposed infection, or toxicity and a comprehensive metabolic profile to evaluate for dehydration, electrolyte abnormalities, hepatic injury or hypoalbuminemia (an important predictor of surgery), as well as assessment of response to treatment and readmission.2,3 An evaluation at admission of C-reactive protein (CRP) is crucial because changes from the initial value will determine steroid response and predict need for surgical intervention or rescue therapy. A baseline fecal calprotectin can serve as a noninvasive marker that can be followed after discharge to monitor response to therapy.
Clostridioides difficile infection (CDI) must be ruled out in all patients presenting with ASUC regardless of history of antibiotic use or prior negative testing. Concomitant UC and CDI are associated with a four- to sixfold increased risk of in-hospital mortality and a two- to sixfold increased risk of bowel surgery.4-6 Immunoassay testing is inexpensive and fast with a high specificity but has low sensitivity; nucleic acid amplification testing with polymerase chain reaction has a high sensitivity and specificity.7 Knowing which testing algorithm the hospital lab uses helps guide interpretation of results.
For patients meeting criteria for ASUC, obtaining at least an abdominal x-ray is important to assess for colonic dilation to further stratify the patient by risk. Colonic dilation, defined as a transverse colon diameter greater than 5.5 cm, places the patient in the category of fulminant colitis and colorectal surgical consultation should be obtained.8 A CT scan is often ordered first because it can provide a rapid assessment of intra-abdominal processes but is not routinely needed unless hemodynamic instability, an acute abdomen, or markedly abnormal laboratory testing (specifically white blood cell count with bandemia) is present as these can be indicators of toxic megacolon or perforation.8-10
Day 1 – Assess disease severity and assemble the team
Obtaining a thorough clinical history is essential to classify disease severity and identify potential triggers for the acute exacerbation. Potential triggers may include infections, new medications, recent antibiotic use, recent travel, sick contacts, or cessation of treatments. Standard questions include asking about the timing of onset of symptoms, bowel movements during a 24-hour period, and particularly the presence of nocturnal bowel movements. If patients report bloody stools, inquire how often they see blood relative to the total number of bowel movements. The presence and nature of abdominal pain should be elicited, particularly changes in abdominal pain and comparison with previous disease flares. These clinical parameters are used to assess response to treatment; therefore, ask patients to keep a log of their stool frequency, consistency, rectal urgency, and bleeding each day to report to the team during daily rounds.
For patients with ASUC, a full colonoscopy is rarely indicated in the inpatient setting because it is unlikely to change management and poses a risk of perforation.11 However, a sigmoidoscopy within the first 24 hours of admission will provide useful information about the endoscopic disease activity, particularly if features such as deep or well-like ulcers, large mucosal abrasions, or extensive loss of the mucosal layer are present because these are predictors of colectomy.8 Tissue biopsies can exclude cytomegalovirus (CMV) infection, an important consideration for patients on immunosuppression including corticosteroids.12-16
Venous thromboembolism (VTE) prophylaxis is extremely important for hospitalized inflammatory bowel disease (IBD) patients. At baseline, IBD patients have a threefold higher risk of VTE than do non-IBD patients, which increases to approximately sixfold during flares.17 Pharmacologic VTE prophylaxis is recommended for all hospitalized IBD patients, even those with rectal bleeding. This may seem counterintuitive in the setting of “GI bleeding,” so it is important to counsel both patients and team members regarding VTE risks and the role of the prophylactic regimen to ensure adherence. Mechanical VTE prophylaxis can be used in patients with severe bleeding and hemodynamic instability until pharmacologic VTE prophylaxis can be safely initiated.17
Narcotics should be used sparingly for hospitalized IBD patients. Narcotic use is associated with greater likelihood of subsequent IBD hospitalizations, ED visits, and higher costs of health care for patients with IBD.18 Heavy use of opiates, defined as continuous use for more than 30 days at a dose exceeding 50 mg morphine per day or equivalent, was strongly associated with an increased overall mortality in IBD patients.19 Opiates also slow bowel motility and precipitate toxic megacolon, along with any other agent that slows bowel motility, such as anticholinergic medications.8 These agents may also mask bowel frequency symptoms that would otherwise indicate a failure of medical therapy. Similarly, use of NSAIDS should also be avoided because these have been associated with disease relapse and escalating intestinal inflammation.20
Once disease severity has been determined, intravenous corticosteroid therapy may be initiated, ideally once CDI and CMV have been excluded. The recommended dosing of intravenous corticosteroids is methylprednisolone 20 mg IV every 8 hours or equivalent. There is no evidence to support additional benefit for doses exceeding these amounts.8 Prior to starting parenteral corticosteroids, it is important to keep in mind the possible need for rescue therapy during the admission. Recommended testing includes hepatitis B surface antigen and antibody, hepatitis B core antibody and tuberculosis testing if there is no documented negative testing within the past 6-12 months. These labs should be drawn prior to steroid treatment to avoid delays in care and indeterminate results. Finally, a lipid profile is recommended for patients who may be cyclosporine candidates pending response to intravenous corticosteroids. Unless the patient has been admitted with a bowel obstruction, which should raise the suspicion that the diagnosis is actually Crohn’s disease, enteral feeding is preferred for UC patients even if they may have significant food aversion. The early involvement of a registered dietitian is valuable to guide dietary choices and recommend appropriate enteral nutrition supplements. During acute flares, patients may find a low-residue diet to be less stimulating to their gut while their acute flare is being treated. Electrolyte abnormalities should be repleted and consistently monitored during the hospitalization. Providing parenteral intravenous iron for anemic patients will expedite correction of the anemia alongside treatment of the underlying UC.
Most UC patients admitted to the hospital will require a multidisciplinary approach with gastroenterologists, surgeons, radiologists, dietitians, and case coordinators/social workers, among others. It is essential to assemble the team, especially the surgeons, earlier during the hospitalization rather than later. It is especially important to discuss the role of the surgeon in the management of UC and explain why the surgeon is being consulted in the context of the patient’s acute presentation. Being transparent about the parameters the GI team are monitoring to determine if and when surgery is the most appropriate and safe approach will improve patients’ acceptance of the surgical team’s role in their care. Specific indications for surgery in ASUC include toxic megacolon, colonic perforation, severe refractory hemorrhage, and failure to respond to medical therapy (Table 1).8
Day 3 – Assessing response to corticosteroids
In addition to daily symptom assessments, a careful abdominal exam should be performed every day with the understanding that steroids (and also narcotics) may mask perforation or pain. Any abrupt decrease or cessation of bowel movements, increasing abdominal distention, or a sudden increase in abdominal pain or tenderness may require abdominal imaging to ensure no interim perforation or severe colonic dilation has occurred while receiving steroid therapy. In these circumstances, the addition of broad spectrum intravenous antibiotics should be considered, particularly if hemodynamic instability (such as tachycardia) is present.
Patients should be assessed for response to intravenous steroid therapy after 3 days of treatment. A meaningful response to corticosteroids is present if the patient has had more than 50% improvement in symptoms, particularly rectal bleeding and stool frequency. A more than 75% improvement in CRP should also be noted from admission to day 3 with an overall trend of improvement.2,21 Additionally, patients should be afebrile, require minimal to no narcotic usage, tolerating oral intake, and be ambulatory. If the patient has met all these parameters, it is reasonable to transition to oral corticosteroids, such as prednisone 40-60 mg daily after a course of 3-5 days of intravenous corticosteroids. Ideally, patients should be observed for 24-48 hours in the hospital after transitioning to oral corticosteroids to make sure that symptoms do not worsen with the switch.
Patients with more than eight bowel movements per day, CRP greater than 4.5 g/dL, deep ulcers on endoscopy, or albumin less than 3.0 g/dL have a higher likelihood of failing intravenous corticosteroid therapy, and these patients should be prepared for rescue therapy.2,21 A patient has failed intravenous corticosteroids by day 3 if they have sustained fever in the absence of an infection, continued CRP elevation or lack of CRP decrease, or ongoing high stool frequency, bleeding, and pain with less than 50% improvement from baseline on admission.8 In the setting of nonresponse to intravenous corticosteroids, it is prudent to involve colorectal surgery to discuss colectomy as an option of equal merit to medical salvage therapies such as infliximab or cyclosporine.
Infliximab is the most readily available rescue therapy for steroid-refractory patients and has been shown to increase colectomy-free survival in patients with ASUC.8 However, patients with the same predictors for intravenous steroid failures (low albumin, high CRP, and/or deep ulcers on endoscopy) are also at the highest risk for infliximab nonresponse. These factors are important to discuss with the patients and colorectal surgery teams when providing the options of treatment strategy, particularly with medication dosing. ASUC with more severe disease biochemically (low albumin, elevated CRP, possibly bandemia) benefit from a higher dose of infliximab at 10 mg/kg, given the likelihood of increased drug clearance in this situation.22,23
From a practical standpoint, it is important to confirm the patient’s insurance status prior to medication administration to make sure therapy can be continued after hospital discharge. Early involvement of the social workers and case coordinators is key to ensuring timely administration of the next dose of treatment. Patients who receive infliximab rescue therapy should be monitored for an additional 1-2 days after administration to ensure they are responding to this therapy with continued monitoring of CRP and symptoms during this period. If there is no response at this point, an additional dose of infliximab may be considered but surgery should not be delayed if there is no meaningful response after the first dose.
Another option for intravenous corticosteroid nonresponders is intravenous cyclosporine because treatment failure rates for cyclosporine and infliximab were similar in head-to-head studies.24 However, patient selection is key to successful utilization of this agent. Unlike infliximab, cyclosporine is primarily an induction agent for steroid nonresponders rather than a maintenance strategy. Therefore, in patients in whom cyclosporine is being considered, thiopurines or vedolizumab are potential options for maintenance therapy. If the patient has poor renal function, low cholesterol, advanced age, significant comorbidities, or a history of nonadherence to therapy, cyclosporine should not be given. Additionally, clinical experience with intravenous cyclosporine administration and monitoring both during inpatient and outpatient care settings should be factored into the decision making for infliximab versus cyclosporine.8
Day 5 and beyond – Discharge planning
Patients who have responded to the initial intravenous steroid course by hospital day 5 should have successfully transitioned to oral steroids with plans to start an appropriate steroid-sparing therapy shortly after discharge. Treatment planning should commence prior to discharge and should be communicated with the outpatient GI team to ensure a smooth transition to the ambulatory care setting, primarily to begin insurance authorizations as soon as possible. If the patient has had a meaningful response to infliximab rescue therapy (improvement by more than 50% in bowel frequency, amount of blood, abdominal pain), discharge planning needs to prioritize obtaining authorization for the second dose within 2 weeks of the initial infusion. These patients are high risk for readmission, and close outpatient follow-up by the ambulatory GI care team is necessary to help direct the tapering of steroids and monitor response to treatment.

If the patient has not responded to intravenous steroid therapy, infliximab, or cyclosporine by day 5-7, then surgery should be strongly considered. Delaying surgery may worsen outcomes as patients become more malnourished, anemic, and continue to receive intravenous steroids. Additional preoperative optimization may be required depending on the patient’s course up to this point (Table 2).
Summary
The cornerstones of inpatient UC management center on a thorough initial evaluation including imaging and endoscopy as appropriate, establishment of baseline parameters, and daily assessment of response to therapy through a combination of patient-reported outcomes and biomarkers of inflammation. With this strategy in mind, practitioners and care teams can manage these complex patients using a consistent strategy focusing on multidisciplinary, evidence-based care.
References
1. Truelove SC et al. Br Med J. 1955 Oct 23;2(4947):1041-8.
2. Ho GT et al. Aliment Pharmacol Ther. 2004 May 15;19(10):1079-87.
3. Tinsley A et al. Scand J Gastroenterol. 2015;50(9):1103-9.
4. Issa M et al. Clin Gastroenterol Hepatol. 2007 Mar;5(3):345-51.
5. Ananthakrishnan AN et al. Gut. 2008 Feb;57(2):205-10.
6. Negron ME et al. Am J Gastroenterol. 2016 May;111(5):691-704.
7. Taylor KN et al. Gynecol Oncol. 2017 Feb;144(2):428-37.
8. Rubin DT et al. Am J Gastroenterol. 2019 Mar;114(3):384-413.
9. Jalan KN et al. Gastroenterology. 1969 Jul;57(1):68-82.
10. Gan SI et al. Am J Gastroenterol. 2003 Nov;98(11):2363-71.
11. Makkar R et al. Gastroenterol Hepatol (N Y). 2013 Sep;9(9):573-83.
12. Hindryckx P et al. Nat Rev Gastroenterol Hepatol. 2016 Nov;13(11):654-64.
13. Yerushalmy-Feler A et al. Curr Infect Dis Rep. 2019 Feb 15;21(2):5.
14. Shukla T et al. J Clin Gastroenterol. 2017 May/Jun;51(5):394-401.
15. McCurdy JD et al. Clin Gastroenterol Hepatol. 2015 Jan;13(1):131-7; quiz e7.
16. Cottone M et al. Am J Gastroenterol. 2001 Mar;96(3):773-5.
17. Nguyen GC et al. Gastroenterology. 2014 Mar;146(3):835-48 e6.
18. Limsrivilai J et al. Clin Gastroenterol Hepatol. 2017 Mar;15(3):385-92 e2.
19. Targownik LE et al. Am J Gastroenterol. 2014 Oct;109(10):1613-20.
20. Takeuchi K et al. Clin Gastroenterol Hepatol. 2006 Feb;4(2):196-202.
21. Travis SP et al. Gut. 1996 Jun;38(6):905-10.
22. Syal G et al. Mo1891 - Gastroenterology. 2018;154:S841.
23. Ungar B et al. Aliment Pharmacol Ther. 2016 Jun;43(12):1293-9.
24. Laharie D et al. Lancet 2012 Dec 1;380(9857):1909-15.
Dr. Chiplunker is an advanced inflammatory bowel disease fellow; Dr. Ha is associate professor of medicine at the Inflammatory Bowel Disease Center at Cedars-Sinai Medical Center, Los Angeles.
Introduction
Inpatient management of acute ulcerative colitis (UC) flares can be challenging because of the multiple patient and disease-related factors influencing therapeutic decision making. The clinical course during the first 24-72 hours of the hospitalization will likely guide the decision between rescue medical and surgical therapy. Using available evidence from clinical practice guidelines, we present a day-by-day guide to managing most hospitalized UC patients.
Day 0 – The emergency department (ED)
When an UC patient presents to the ED for evaluation, the initial assessments should focus on the acuity and severity of the flare. Key clinical features of disease severity include the presence of fever, tachycardia, hypotension, or weight loss in addition to worsened gastrointestinal symptoms of stool frequency relative to baseline, rectal bleeding, and abdominal pain. Acute severe ulcerative colitis (ASUC) is often defined using the modified Truelove and Witts criteria.1 A patient meets criteria for ASUC if they have at least six bloody stools per day and at least one sign of systemic toxicity, such as heart rate greater than 90 bpm, temperature at or above 37.8° C, hemoglobin level below 10.5 g/dL, or elevated inflammatory markers.
Initial laboratory assessments should include complete blood counts to identify anemia, potential superimposed infection, or toxicity and a comprehensive metabolic profile to evaluate for dehydration, electrolyte abnormalities, hepatic injury or hypoalbuminemia (an important predictor of surgery), as well as assessment of response to treatment and readmission.2,3 An evaluation at admission of C-reactive protein (CRP) is crucial because changes from the initial value will determine steroid response and predict need for surgical intervention or rescue therapy. A baseline fecal calprotectin can serve as a noninvasive marker that can be followed after discharge to monitor response to therapy.
Clostridioides difficile infection (CDI) must be ruled out in all patients presenting with ASUC regardless of history of antibiotic use or prior negative testing. Concomitant UC and CDI are associated with a four- to sixfold increased risk of in-hospital mortality and a two- to sixfold increased risk of bowel surgery.4-6 Immunoassay testing is inexpensive and fast with a high specificity but has low sensitivity; nucleic acid amplification testing with polymerase chain reaction has a high sensitivity and specificity.7 Knowing which testing algorithm the hospital lab uses helps guide interpretation of results.
For patients meeting criteria for ASUC, obtaining at least an abdominal x-ray is important to assess for colonic dilation to further stratify the patient by risk. Colonic dilation, defined as a transverse colon diameter greater than 5.5 cm, places the patient in the category of fulminant colitis and colorectal surgical consultation should be obtained.8 A CT scan is often ordered first because it can provide a rapid assessment of intra-abdominal processes but is not routinely needed unless hemodynamic instability, an acute abdomen, or markedly abnormal laboratory testing (specifically white blood cell count with bandemia) is present as these can be indicators of toxic megacolon or perforation.8-10
Day 1 – Assess disease severity and assemble the team
Obtaining a thorough clinical history is essential to classify disease severity and identify potential triggers for the acute exacerbation. Potential triggers may include infections, new medications, recent antibiotic use, recent travel, sick contacts, or cessation of treatments. Standard questions include asking about the timing of onset of symptoms, bowel movements during a 24-hour period, and particularly the presence of nocturnal bowel movements. If patients report bloody stools, inquire how often they see blood relative to the total number of bowel movements. The presence and nature of abdominal pain should be elicited, particularly changes in abdominal pain and comparison with previous disease flares. These clinical parameters are used to assess response to treatment; therefore, ask patients to keep a log of their stool frequency, consistency, rectal urgency, and bleeding each day to report to the team during daily rounds.
For patients with ASUC, a full colonoscopy is rarely indicated in the inpatient setting because it is unlikely to change management and poses a risk of perforation.11 However, a sigmoidoscopy within the first 24 hours of admission will provide useful information about the endoscopic disease activity, particularly if features such as deep or well-like ulcers, large mucosal abrasions, or extensive loss of the mucosal layer are present because these are predictors of colectomy.8 Tissue biopsies can exclude cytomegalovirus (CMV) infection, an important consideration for patients on immunosuppression including corticosteroids.12-16
Venous thromboembolism (VTE) prophylaxis is extremely important for hospitalized inflammatory bowel disease (IBD) patients. At baseline, IBD patients have a threefold higher risk of VTE than do non-IBD patients, which increases to approximately sixfold during flares.17 Pharmacologic VTE prophylaxis is recommended for all hospitalized IBD patients, even those with rectal bleeding. This may seem counterintuitive in the setting of “GI bleeding,” so it is important to counsel both patients and team members regarding VTE risks and the role of the prophylactic regimen to ensure adherence. Mechanical VTE prophylaxis can be used in patients with severe bleeding and hemodynamic instability until pharmacologic VTE prophylaxis can be safely initiated.17
Narcotics should be used sparingly for hospitalized IBD patients. Narcotic use is associated with greater likelihood of subsequent IBD hospitalizations, ED visits, and higher costs of health care for patients with IBD.18 Heavy use of opiates, defined as continuous use for more than 30 days at a dose exceeding 50 mg morphine per day or equivalent, was strongly associated with an increased overall mortality in IBD patients.19 Opiates also slow bowel motility and precipitate toxic megacolon, along with any other agent that slows bowel motility, such as anticholinergic medications.8 These agents may also mask bowel frequency symptoms that would otherwise indicate a failure of medical therapy. Similarly, use of NSAIDS should also be avoided because these have been associated with disease relapse and escalating intestinal inflammation.20
Once disease severity has been determined, intravenous corticosteroid therapy may be initiated, ideally once CDI and CMV have been excluded. The recommended dosing of intravenous corticosteroids is methylprednisolone 20 mg IV every 8 hours or equivalent. There is no evidence to support additional benefit for doses exceeding these amounts.8 Prior to starting parenteral corticosteroids, it is important to keep in mind the possible need for rescue therapy during the admission. Recommended testing includes hepatitis B surface antigen and antibody, hepatitis B core antibody and tuberculosis testing if there is no documented negative testing within the past 6-12 months. These labs should be drawn prior to steroid treatment to avoid delays in care and indeterminate results. Finally, a lipid profile is recommended for patients who may be cyclosporine candidates pending response to intravenous corticosteroids. Unless the patient has been admitted with a bowel obstruction, which should raise the suspicion that the diagnosis is actually Crohn’s disease, enteral feeding is preferred for UC patients even if they may have significant food aversion. The early involvement of a registered dietitian is valuable to guide dietary choices and recommend appropriate enteral nutrition supplements. During acute flares, patients may find a low-residue diet to be less stimulating to their gut while their acute flare is being treated. Electrolyte abnormalities should be repleted and consistently monitored during the hospitalization. Providing parenteral intravenous iron for anemic patients will expedite correction of the anemia alongside treatment of the underlying UC.
Most UC patients admitted to the hospital will require a multidisciplinary approach with gastroenterologists, surgeons, radiologists, dietitians, and case coordinators/social workers, among others. It is essential to assemble the team, especially the surgeons, earlier during the hospitalization rather than later. It is especially important to discuss the role of the surgeon in the management of UC and explain why the surgeon is being consulted in the context of the patient’s acute presentation. Being transparent about the parameters the GI team are monitoring to determine if and when surgery is the most appropriate and safe approach will improve patients’ acceptance of the surgical team’s role in their care. Specific indications for surgery in ASUC include toxic megacolon, colonic perforation, severe refractory hemorrhage, and failure to respond to medical therapy (Table 1).8
Day 3 – Assessing response to corticosteroids
In addition to daily symptom assessments, a careful abdominal exam should be performed every day with the understanding that steroids (and also narcotics) may mask perforation or pain. Any abrupt decrease or cessation of bowel movements, increasing abdominal distention, or a sudden increase in abdominal pain or tenderness may require abdominal imaging to ensure no interim perforation or severe colonic dilation has occurred while receiving steroid therapy. In these circumstances, the addition of broad spectrum intravenous antibiotics should be considered, particularly if hemodynamic instability (such as tachycardia) is present.
Patients should be assessed for response to intravenous steroid therapy after 3 days of treatment. A meaningful response to corticosteroids is present if the patient has had more than 50% improvement in symptoms, particularly rectal bleeding and stool frequency. A more than 75% improvement in CRP should also be noted from admission to day 3 with an overall trend of improvement.2,21 Additionally, patients should be afebrile, require minimal to no narcotic usage, tolerating oral intake, and be ambulatory. If the patient has met all these parameters, it is reasonable to transition to oral corticosteroids, such as prednisone 40-60 mg daily after a course of 3-5 days of intravenous corticosteroids. Ideally, patients should be observed for 24-48 hours in the hospital after transitioning to oral corticosteroids to make sure that symptoms do not worsen with the switch.
Patients with more than eight bowel movements per day, CRP greater than 4.5 g/dL, deep ulcers on endoscopy, or albumin less than 3.0 g/dL have a higher likelihood of failing intravenous corticosteroid therapy, and these patients should be prepared for rescue therapy.2,21 A patient has failed intravenous corticosteroids by day 3 if they have sustained fever in the absence of an infection, continued CRP elevation or lack of CRP decrease, or ongoing high stool frequency, bleeding, and pain with less than 50% improvement from baseline on admission.8 In the setting of nonresponse to intravenous corticosteroids, it is prudent to involve colorectal surgery to discuss colectomy as an option of equal merit to medical salvage therapies such as infliximab or cyclosporine.
Infliximab is the most readily available rescue therapy for steroid-refractory patients and has been shown to increase colectomy-free survival in patients with ASUC.8 However, patients with the same predictors for intravenous steroid failures (low albumin, high CRP, and/or deep ulcers on endoscopy) are also at the highest risk for infliximab nonresponse. These factors are important to discuss with the patients and colorectal surgery teams when providing the options of treatment strategy, particularly with medication dosing. ASUC with more severe disease biochemically (low albumin, elevated CRP, possibly bandemia) benefit from a higher dose of infliximab at 10 mg/kg, given the likelihood of increased drug clearance in this situation.22,23
From a practical standpoint, it is important to confirm the patient’s insurance status prior to medication administration to make sure therapy can be continued after hospital discharge. Early involvement of the social workers and case coordinators is key to ensuring timely administration of the next dose of treatment. Patients who receive infliximab rescue therapy should be monitored for an additional 1-2 days after administration to ensure they are responding to this therapy with continued monitoring of CRP and symptoms during this period. If there is no response at this point, an additional dose of infliximab may be considered but surgery should not be delayed if there is no meaningful response after the first dose.
Another option for intravenous corticosteroid nonresponders is intravenous cyclosporine because treatment failure rates for cyclosporine and infliximab were similar in head-to-head studies.24 However, patient selection is key to successful utilization of this agent. Unlike infliximab, cyclosporine is primarily an induction agent for steroid nonresponders rather than a maintenance strategy. Therefore, in patients in whom cyclosporine is being considered, thiopurines or vedolizumab are potential options for maintenance therapy. If the patient has poor renal function, low cholesterol, advanced age, significant comorbidities, or a history of nonadherence to therapy, cyclosporine should not be given. Additionally, clinical experience with intravenous cyclosporine administration and monitoring both during inpatient and outpatient care settings should be factored into the decision making for infliximab versus cyclosporine.8
Day 5 and beyond – Discharge planning
Patients who have responded to the initial intravenous steroid course by hospital day 5 should have successfully transitioned to oral steroids with plans to start an appropriate steroid-sparing therapy shortly after discharge. Treatment planning should commence prior to discharge and should be communicated with the outpatient GI team to ensure a smooth transition to the ambulatory care setting, primarily to begin insurance authorizations as soon as possible. If the patient has had a meaningful response to infliximab rescue therapy (improvement by more than 50% in bowel frequency, amount of blood, abdominal pain), discharge planning needs to prioritize obtaining authorization for the second dose within 2 weeks of the initial infusion. These patients are high risk for readmission, and close outpatient follow-up by the ambulatory GI care team is necessary to help direct the tapering of steroids and monitor response to treatment.

If the patient has not responded to intravenous steroid therapy, infliximab, or cyclosporine by day 5-7, then surgery should be strongly considered. Delaying surgery may worsen outcomes as patients become more malnourished, anemic, and continue to receive intravenous steroids. Additional preoperative optimization may be required depending on the patient’s course up to this point (Table 2).
Summary
The cornerstones of inpatient UC management center on a thorough initial evaluation including imaging and endoscopy as appropriate, establishment of baseline parameters, and daily assessment of response to therapy through a combination of patient-reported outcomes and biomarkers of inflammation. With this strategy in mind, practitioners and care teams can manage these complex patients using a consistent strategy focusing on multidisciplinary, evidence-based care.
References
1. Truelove SC et al. Br Med J. 1955 Oct 23;2(4947):1041-8.
2. Ho GT et al. Aliment Pharmacol Ther. 2004 May 15;19(10):1079-87.
3. Tinsley A et al. Scand J Gastroenterol. 2015;50(9):1103-9.
4. Issa M et al. Clin Gastroenterol Hepatol. 2007 Mar;5(3):345-51.
5. Ananthakrishnan AN et al. Gut. 2008 Feb;57(2):205-10.
6. Negron ME et al. Am J Gastroenterol. 2016 May;111(5):691-704.
7. Taylor KN et al. Gynecol Oncol. 2017 Feb;144(2):428-37.
8. Rubin DT et al. Am J Gastroenterol. 2019 Mar;114(3):384-413.
9. Jalan KN et al. Gastroenterology. 1969 Jul;57(1):68-82.
10. Gan SI et al. Am J Gastroenterol. 2003 Nov;98(11):2363-71.
11. Makkar R et al. Gastroenterol Hepatol (N Y). 2013 Sep;9(9):573-83.
12. Hindryckx P et al. Nat Rev Gastroenterol Hepatol. 2016 Nov;13(11):654-64.
13. Yerushalmy-Feler A et al. Curr Infect Dis Rep. 2019 Feb 15;21(2):5.
14. Shukla T et al. J Clin Gastroenterol. 2017 May/Jun;51(5):394-401.
15. McCurdy JD et al. Clin Gastroenterol Hepatol. 2015 Jan;13(1):131-7; quiz e7.
16. Cottone M et al. Am J Gastroenterol. 2001 Mar;96(3):773-5.
17. Nguyen GC et al. Gastroenterology. 2014 Mar;146(3):835-48 e6.
18. Limsrivilai J et al. Clin Gastroenterol Hepatol. 2017 Mar;15(3):385-92 e2.
19. Targownik LE et al. Am J Gastroenterol. 2014 Oct;109(10):1613-20.
20. Takeuchi K et al. Clin Gastroenterol Hepatol. 2006 Feb;4(2):196-202.
21. Travis SP et al. Gut. 1996 Jun;38(6):905-10.
22. Syal G et al. Mo1891 - Gastroenterology. 2018;154:S841.
23. Ungar B et al. Aliment Pharmacol Ther. 2016 Jun;43(12):1293-9.
24. Laharie D et al. Lancet 2012 Dec 1;380(9857):1909-15.
Dr. Chiplunker is an advanced inflammatory bowel disease fellow; Dr. Ha is associate professor of medicine at the Inflammatory Bowel Disease Center at Cedars-Sinai Medical Center, Los Angeles.
Diagnosis and management of gastric intestinal metaplasia in the United States
Introduction
Despite a global decline in the incidence of gastric cancer over the past 3 decades, it remains the fifth most commonly diagnosed cancer and the third most common cause of cancer deaths worldwide.1 In the United States it is the fourth most commonly diagnosed GI malignancy, after colorectal, pancreas, and liver cancer. The prevalence remains high in Latin America and Asia, which has implications in the United States because of growing Hispanic and Asian populations.2,3 In recent years, a change in the trend of gastric cancer among non-Hispanic whites has been observed, particularly in women younger than 50 years old.4 5
Etiology
Gastric adenocarcinomas are classified into two subcategories based on location (cardia and noncardia) and histology (intestinal and diffuse types).6,7 Atrophic gastritis and gastric intestinal metaplasia (GIM) are considered precursors of intestinal-type noncardia gastric adenocarcinoma. The Correa cascade is a commonly accepted precancer sequence for noncardia gastric adenocarcinoma that describes mucosal changes from inflammation to atrophy to metaplasia to intraepithelial neoplasia and culminating in carcinoma.8,9 It has been observed that GIM may be the histologic change prior to the development of dysplasia and over 50% of patients with high-grade dysplasia will progress to adenocarcinoma.10-12 In the United States, GIM has the highest prevalence in African Americans, Hispanics, and East Asians, with the overall GIM prevalence regardless of ethnicity reported from 3.05% to 19.2%.5,13
Risk factors and subclassification
Replacement of the foveolar and/or glandular epithelium in the oxyntic and antral mucosa by intestinal epithelium results in GIM. It can be focal when limited to one region of the stomach or extensive when two or more regions are involved.14 The main risk factors for GIM development are Helicobacter pylori infection, tobacco, alcohol consumption, high salt intake, and chronic bile reflux.15,16 Additional risks for developing gastric cancer include older age, certain ethnicities, and male sex.17
CagA strains of H. pylori can promote carcinogenesis by inducing a mitogenic cellular response and downregulating cell adhesion.18,19 Less carcinogenic risk is associated with H. pylori Cag-A negative strains; however, they also have oncogenic potential mediated by expression of babA2 and vacA genes.20 Hence, the combination of multiple virulent factors encoded in babA2, CagA, and vacA genes has been associated with increased risk of GIM, inflammation, and development of gastric cancer.15 The clinical usefulness of genotyping H. pylori strains specifically to survey precancerous gastric lesions remains to be seen because of a lack of sufficient clinical studies. In addition, genotyping H. pylori is not commonly performed as part of clinical practice.
The loss of parietal cells seen in atrophic gastritis due to chronic H. pylori infection has been linked to the development of metaplasia due to possible loss of differentiation-promoting factors. As a result, metaplastic cells emerge that express spasmolytic polypeptide (SP or TFF2); hence, this type of metaplasia is referred to as spasmolytic polypeptide–expressing metaplasia (SPEM). The cellular mechanism that may explain a precursor role of SPEM in the development of GIM remains unknown.14 A second competing theory for the development of GIM is the clonal expansion of stem cells in the gastric isthmus that can lead to dysplasia and cancer development.14
On the basis of histological similarities with small intestinal or colonic epithelium, GIM can be further classified into complete or incomplete intestinal metaplasia.21 Complete intestinal metaplasia most closely resembles small intestinal epithelium with a brush border and goblet cells. Incomplete intestinal metaplasia resembles the colonic epithelium and lacks a brush border. A second classification further classifies GIM into three subtypes: Type I contains nonsecretory absorptive cells and sialomucin secreting goblet cells; type II has few absorptive cells, columnar cells secreting sialomucin, goblet cells secreting mainly sialomucin but some sulphomucin, and presence of Paneth cells; and type III consists of columnar cells secreting predominantly sulphomucin, goblet cells secreting sialomucin or sulphomucin, and absence of Paneth cells.15,22 In this subclassification, type I GIM is known as complete GIM and types II and III as incomplete GIM.23-25
Multiple studies performed outside of the United States have shown a higher progression risk to gastric adenocarcinoma in incomplete intestinal metaplasia, or type III intestinal metaplasia.26-32 Also, the risk of gastric cancer has been demonstrated to be higher among patients with a greater area of metaplasia and extensive intestinal metaplasia, defined as GIM in both the antrum and corpus.33,34 Hence, the extent of the metaplasia determined with mapping biopsies, regardless of the subtype, should also be incorporated into the risk assessment of the patient. Currently, a major limitation in the United States is a standardized method of pathologic reporting including subclassification of incomplete versus complete intestinal metaplasia.
Which patients to screen
Understanding this sequence of carcinogenesis offers a potential window for screening and surveillance. Subsequently, early detection of precancerous mucosal changes would be more amenable for endoscopic submucosal dissection (ESD).35,36 Currently, U.S. society guidelines do not specifically address the management of GIM. The American Society for Gastrointestinal Endoscopy (ASGE) guidelines for management of premalignant and malignant conditions of the stomach recommend surveillance in individuals with a family history of gastric cancer or of high-risk ethnic background but with no specific optimal surveillance interval.37 Also, H. pylori treatment is recommended if identified, but empiric treatment in GIM was felt to be controversial. The AGA recently sought comments on a proposed new guideline for the management of GIM. This guideline should be released after the comment period and help address management of GIM in the United States. In April of 2019, the European Society of Gastrointestinal Endoscopy (ESGE) updated the management of epithelial precancerous conditions and lesions in the stomach (MAPS II) guideline.38 The MAPS II guideline identifies atrophic gastritis and intestinal metaplasia as precancerous lesions. In patients with moderate to marked atrophy or GIM affecting both antral and body mucosa, ESGE recommends endoscopic surveillance with high-definition chromoendoscopy, mapping, and guided biopsies or at least two biopsies taken separately at the lesser and greater curvature of the antrum and body. H. pylori eradication was recommended if the patient tested positive.
Furthermore, MAPS II proposed replacing atrophic gastritis (AG) in the Operative Link on Gastritis Assessment (OLGA) staging by GIM (OLGIM) as it is considered a more reliable predictor of an individual’s gastric neoplasia risk, based on the interobserver agreement kappa value 0.6 for AG versus 0.9 for GIM.39 Five biopsies (two from the antrum, two from the corpus, and one from the incisura angularis) are needed for the OLGA/OLGIM score system to be considered an accurate predictor of this risk.39 This is supported by the early findings of gastric atrophy and GIM in the incisura angularis.23 In addition, for patients with GIM only in either the antrum or the body, a family history of gastric cancer, incomplete GIM, autoimmune gastritis, or persistent H. pylori infection was felt to increase the risk to warrant surveillance every 3 years. In those patients with atrophy or GIM in both the antrum and body with a first-degree relative with gastric cancer, surveillance was recommended every 1-2 years. Patients with any dysplasia and a visible lesion should have staging and resection. With no visible lesion, a follow-up endoscopy should be performed in 6 months with high-grade dysplasia and with low-grade dysplasia a repeat in 12 months. Patients with mild to moderate atrophy in the antrum and no intestinal metaplasia were not felt to warrant any further surveillance. (See Figure 1.)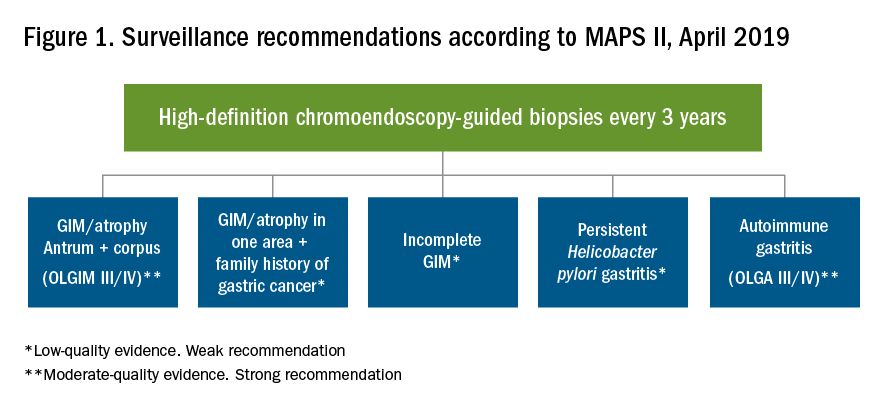
How to screen
Previous studies have found a poor correlation between the endoscopic determination of gastric atrophy and the histologic diagnosis.42 Several studies also found that gastric cancer was missed on initial endoscopic examinations. Sensitivity of endoscopy to detect gastric cancer has ranged from 77% to 93%.43,44 In the United States, there is a lack of standardized quality indicators for upper endoscopy exams. The ESGE has suggested several performance measures to ensure a quality endoscopy exam, including accurate photo documentation, sufficient procedure time of at least 7 minutes, adherence to biopsy protocols, and low complication rates.45 In Asia, a systematic screening protocol is used for photo documentation, and simple techniques such as adequate air insufflation and irrigation to remove mucus are routinely used to improve the endoscopy exam.46,47 The mean time of an endoscopy exam has also been found to increase the detection of neoplastic lesions, as slow endoscopists – with a mean exam duration of 8.6 ± 4.2 min during upper endoscopy – detected threefold more neoplastic lesions than did fast endoscopists.48
A standardized biopsy approach is also important when screening patients. The updated Sydney protocol has been suggested for mapping the stomach to screen for atrophy and GIM. This protocol recommends two biopsies from the antrum (at the lesser and greater curvature), two from the body (at the lesser and greater curvature), and one from the incisura.23 This biopsy protocol was also suggested in the recent MAPS II update, with the biopsy of the incisura felt to be an additional biopsy left to the discretion of the endoscopist. Notably, abnormal appearing mucosal areas should be biopsied separately from the mapping biopsies.
High-definition endoscopy with virtual chromoendoscopy is felt to be better than white-light endoscopy alone at detecting precancerous gastric lesions.38 (See Figure 2.)
In particular, narrow-band imaging (NBI) has been studied and found to increase the diagnostic yield of GIM and dysplasia compared with white light alone.49 Several studies have shown an increased accuracy for the detection of GIM with magnification NBI.50-52 An unfortunate limitation is the geographic availability of magnification NBI: It is not available in the United States. A multicenter study in Portugal developed a new classification system for the appearance of precancerous lesions with NBI and tested its accuracy in endoscopists with a wide range of NBI experience. An abnormal mucosal pattern that showed light blue crests/regular ridge or a tubulovillous appearance and a regular mucosal pattern was found with GIM. An irregular vascular pattern with a white opaque substance and an absent or irregular mucosal pattern was most often found with dysplasia. Furthermore, the reproducibility of these patterns was high between endoscopists.53 Multiple studies have been performed on additional imaging technologies to enhance the detection of gastric neoplasia; however, these technologies are still investigational and currently not recommended for screening.54-57
Serum pepsinogens have been studied in Europe and Asia as noninvasive indicators of gastric atrophy to determine who should be screened with endoscopy.58 A low serum pepsinogen I level below 70 ng/mL and pepsinogen I/II ratio below 3 has generally been used to detect atrophic gastritis and at-risk populations. However, the studies performed in Europe and Asia used different methods for quantifying pepsinogen levels. Therefore, cutoff values cannot be generalized for all assays and should be validated for the specific tests used.38
Summary
Gastric atrophy and gastric intestinal metaplasia are considered precancerous lesions with an increased risk of development of gastric cancer. H. pylori is a major risk factor for the development of GIM. The extent of GIM as well as the presence of incomplete intestinal metaplasia, or type III intestinal metaplasia has been found to have the highest gastric cancer risk. Currently, in the United States, specific guidelines on endoscopic screening and surveillance for noncardia gastric adenocarcinoma based on histological subtype of GIM, location, and extension are lacking. The ESGE recently updated guidelines that recommend surveillance of patients with extensive atrophy and intestinal metaplasia or with a significant family history. Location and extension of intestinal metaplasia plays a role in increased risk. Screening should include a standardized upper endoscopy approach with high-definition white- light endoscopy and NBI, at least a 7-minute examination, adequate insufflation and cleaning, adequate photo documentation, and a standardized biopsy protocol. Further studies are needed to determine an appropriate surveillance interval and standardized pathology reporting approach as well.
Diana Curras-Martin MD, is an internal medicine resident at Hackensack Meridian Jersey Shore University Medical Center. Susana Gonzalez, MD, is assistant professor of medicine in the division of gastroenterology and hepatology (@WCM_GI), Weill Cornell Medicine, New York Presbyterian Hospital–Cornell.
References
1. Bray F et al. CA Cancer J Clin. 2018;68(6):394-424.
2. Global Burden of Disease Cancer Collaboration et al. JAMA Oncol. 2018;4(11):1553-68.
3. Balakrishnan M et al. Curr Gastroenterol Rep. 2017;19(8):36.
4. Anderson WF et al. J Natl Cancer Inst. 2018;110(6):608-15.
5. Trieu JA et al. Dig Dis Sci. 2019;64(5):1079-88.
6. Lauren P. Acta Pathol Microbiol Scand. 1965;64:31-49.
7. Correa P, Schneider BG. Cancer Epidemiol Biomarkers Prev. 2005;14(8):1865-8.
8. Correa P. Cancer Res. 1992;52(24):6735-40.
9. Correa P, Piazuelo MB. J Dig Dis. 2012;13(1):2-9.
10. Correa P et al. J Natl Cancer Inst. 1970;44(2):297-306.
11. Correa P. Semin Oncol. 1985;12(1):2-10.
12. Rugge M et al. Hum Pathol. 1991;22(10):1002-8.
13. Simko V et al. Bratisl Lek Listy. 2015;116(1):3-8.
14. Giroux V, Rustgi AK. Nat Rev Cancer. 2017;17(10):594-604.
15. Jencks DS et al. Gastroenterol Hepatol (N Y). 2018;14(2):92-101.
16. Amieva M, Peek RM Jr. Gastroenterology. 2016;150(1):64-78.
17. Karimi P et al. Cancer Epidemiol Biomarkers Prev. 2014;23(5):700-13.
18. Hatakeyama M. Proc Jpn Acad Ser B Phys Biol Sci. 2017;93(4):196-219.
19. Tsutsumi R et al. Mol Cell Biol. 2006;26(1):261-76.
20. Kikuchi S et al. Am J Gastroenterol. 1999;94(12):3455-9.
21. Jass JR, Filipe MI. Histopathology. 1980;4(3):271-9.
22. Jass JR, Filipe MI. Histochem J. 1981;13(6):931-9.
23. Dixon MF et al. Am J Surg Pathol. 1996;20(10):1161-81.
24. Kang KP et al. J Gastroenterol Hepatol. 2009;24(1):140-8.
25. Gonzalez CA et al. Int J Cancer. 2010;127(11):2654-60.
26. Filipe MI et al. Gut. 1985;26(12):1319-26.
27. Filipe MI et al. Int J Cancer. 1994;57(3):324-9.
28. Gonzalez CA et al. J Gastroenterol Hepatol. 2016;31(5):953-8.
29. Cassaro M et al. Am J Gastroenterol. 2000;95(6):1431-8.
30. Shao L et al. Int J Cancer. Apr 29. 2018.
31. Stemmermann GN. Cancer. 1994;74(2):556-64.
32. Gonzalez CA et al. Int J Cancer. 2013;133(5):1023-32.
33. Reddy KM et al. Clin Gastroenterol Hepatol. 2016;14(10):1420-5.
34. Tava F et al. Hum Pathol. 2006;37(11):1489-97.
35. Fernandez-Esparrach G et al. Rev Esp Enferm Dig. 2014;106(2):120-32.
36. Ono H et al. Dig Endosc. 2016;28(1):3-15.
37. Evans JA, DeWitt JM. Gastrointest Endosc. 2016;83(1):274.
38. Pimentel-Nunes P et al. Endoscopy. 2019;51(4):365-88.
39. Capelle LG et al. Gastrointest Endosc. 2010;71(7):1150-8.
40. Saumoy M et al. Gastroenterology. 2018;155(3):648-60.
41. Gupta N et al. Gastrointest Endosc. 2011;74(3):610-24 e612.
42. Eshmuratov A et al. Dig Dis Sci. 2010;55(5):1364-75.
43. Nam JH et al. Cancer. 2012;118(20):4953-60.
44. Amin A et al. J R Coll Surg Edinb. 2002;47(5):681-4.
45. Bisschops R et al. United European Gastroenterol J. 2016;4(5):629-56.
46. Uedo N et al. Gastroenterol Clin North Am. 2013;42(2):317-35.
47. Yao K. Ann Gastroenterol. 2013;26(1):11-22.
48. Teh JL et al. Clin Gastroenterol Hepatol. 2015;13(3):480-7 e482.
49. Capelle LG et al. Dig Dis Sci. 2010;55(12):3442-8.
50. Bansal A et al. Gastrointest Endosc. 2008;67(2):210-6.
51. Tahara T et al. Gastrointest Endosc. 2009;70(2):246-53.
52. Uedo N et al. Endoscopy. 2006;38(8):819-24.
53. Pimentel-Nunes P et al. Endoscopy. 2012;44(3):236-46.
54. Kato M et al. Gastrointest Endosc. 2009;70(5):899-906.
55. Nishimura J et al. Gastroenterol Res Pract. 2014;2014:819395.
56. Dohi O et al. Gastrointest Endosc. 2019;89(1):47-57.
57. Osawa H et al. World J Gastrointest Endosc. 2012;4(8):356-61.
58. Pasechnikov V et al. World J Gastroenterol. 2014;20(38):13842-62.
Introduction
Despite a global decline in the incidence of gastric cancer over the past 3 decades, it remains the fifth most commonly diagnosed cancer and the third most common cause of cancer deaths worldwide.1 In the United States it is the fourth most commonly diagnosed GI malignancy, after colorectal, pancreas, and liver cancer. The prevalence remains high in Latin America and Asia, which has implications in the United States because of growing Hispanic and Asian populations.2,3 In recent years, a change in the trend of gastric cancer among non-Hispanic whites has been observed, particularly in women younger than 50 years old.4 5
Etiology
Gastric adenocarcinomas are classified into two subcategories based on location (cardia and noncardia) and histology (intestinal and diffuse types).6,7 Atrophic gastritis and gastric intestinal metaplasia (GIM) are considered precursors of intestinal-type noncardia gastric adenocarcinoma. The Correa cascade is a commonly accepted precancer sequence for noncardia gastric adenocarcinoma that describes mucosal changes from inflammation to atrophy to metaplasia to intraepithelial neoplasia and culminating in carcinoma.8,9 It has been observed that GIM may be the histologic change prior to the development of dysplasia and over 50% of patients with high-grade dysplasia will progress to adenocarcinoma.10-12 In the United States, GIM has the highest prevalence in African Americans, Hispanics, and East Asians, with the overall GIM prevalence regardless of ethnicity reported from 3.05% to 19.2%.5,13
Risk factors and subclassification
Replacement of the foveolar and/or glandular epithelium in the oxyntic and antral mucosa by intestinal epithelium results in GIM. It can be focal when limited to one region of the stomach or extensive when two or more regions are involved.14 The main risk factors for GIM development are Helicobacter pylori infection, tobacco, alcohol consumption, high salt intake, and chronic bile reflux.15,16 Additional risks for developing gastric cancer include older age, certain ethnicities, and male sex.17
CagA strains of H. pylori can promote carcinogenesis by inducing a mitogenic cellular response and downregulating cell adhesion.18,19 Less carcinogenic risk is associated with H. pylori Cag-A negative strains; however, they also have oncogenic potential mediated by expression of babA2 and vacA genes.20 Hence, the combination of multiple virulent factors encoded in babA2, CagA, and vacA genes has been associated with increased risk of GIM, inflammation, and development of gastric cancer.15 The clinical usefulness of genotyping H. pylori strains specifically to survey precancerous gastric lesions remains to be seen because of a lack of sufficient clinical studies. In addition, genotyping H. pylori is not commonly performed as part of clinical practice.
The loss of parietal cells seen in atrophic gastritis due to chronic H. pylori infection has been linked to the development of metaplasia due to possible loss of differentiation-promoting factors. As a result, metaplastic cells emerge that express spasmolytic polypeptide (SP or TFF2); hence, this type of metaplasia is referred to as spasmolytic polypeptide–expressing metaplasia (SPEM). The cellular mechanism that may explain a precursor role of SPEM in the development of GIM remains unknown.14 A second competing theory for the development of GIM is the clonal expansion of stem cells in the gastric isthmus that can lead to dysplasia and cancer development.14
On the basis of histological similarities with small intestinal or colonic epithelium, GIM can be further classified into complete or incomplete intestinal metaplasia.21 Complete intestinal metaplasia most closely resembles small intestinal epithelium with a brush border and goblet cells. Incomplete intestinal metaplasia resembles the colonic epithelium and lacks a brush border. A second classification further classifies GIM into three subtypes: Type I contains nonsecretory absorptive cells and sialomucin secreting goblet cells; type II has few absorptive cells, columnar cells secreting sialomucin, goblet cells secreting mainly sialomucin but some sulphomucin, and presence of Paneth cells; and type III consists of columnar cells secreting predominantly sulphomucin, goblet cells secreting sialomucin or sulphomucin, and absence of Paneth cells.15,22 In this subclassification, type I GIM is known as complete GIM and types II and III as incomplete GIM.23-25
Multiple studies performed outside of the United States have shown a higher progression risk to gastric adenocarcinoma in incomplete intestinal metaplasia, or type III intestinal metaplasia.26-32 Also, the risk of gastric cancer has been demonstrated to be higher among patients with a greater area of metaplasia and extensive intestinal metaplasia, defined as GIM in both the antrum and corpus.33,34 Hence, the extent of the metaplasia determined with mapping biopsies, regardless of the subtype, should also be incorporated into the risk assessment of the patient. Currently, a major limitation in the United States is a standardized method of pathologic reporting including subclassification of incomplete versus complete intestinal metaplasia.
Which patients to screen
Understanding this sequence of carcinogenesis offers a potential window for screening and surveillance. Subsequently, early detection of precancerous mucosal changes would be more amenable for endoscopic submucosal dissection (ESD).35,36 Currently, U.S. society guidelines do not specifically address the management of GIM. The American Society for Gastrointestinal Endoscopy (ASGE) guidelines for management of premalignant and malignant conditions of the stomach recommend surveillance in individuals with a family history of gastric cancer or of high-risk ethnic background but with no specific optimal surveillance interval.37 Also, H. pylori treatment is recommended if identified, but empiric treatment in GIM was felt to be controversial. The AGA recently sought comments on a proposed new guideline for the management of GIM. This guideline should be released after the comment period and help address management of GIM in the United States. In April of 2019, the European Society of Gastrointestinal Endoscopy (ESGE) updated the management of epithelial precancerous conditions and lesions in the stomach (MAPS II) guideline.38 The MAPS II guideline identifies atrophic gastritis and intestinal metaplasia as precancerous lesions. In patients with moderate to marked atrophy or GIM affecting both antral and body mucosa, ESGE recommends endoscopic surveillance with high-definition chromoendoscopy, mapping, and guided biopsies or at least two biopsies taken separately at the lesser and greater curvature of the antrum and body. H. pylori eradication was recommended if the patient tested positive.
Furthermore, MAPS II proposed replacing atrophic gastritis (AG) in the Operative Link on Gastritis Assessment (OLGA) staging by GIM (OLGIM) as it is considered a more reliable predictor of an individual’s gastric neoplasia risk, based on the interobserver agreement kappa value 0.6 for AG versus 0.9 for GIM.39 Five biopsies (two from the antrum, two from the corpus, and one from the incisura angularis) are needed for the OLGA/OLGIM score system to be considered an accurate predictor of this risk.39 This is supported by the early findings of gastric atrophy and GIM in the incisura angularis.23 In addition, for patients with GIM only in either the antrum or the body, a family history of gastric cancer, incomplete GIM, autoimmune gastritis, or persistent H. pylori infection was felt to increase the risk to warrant surveillance every 3 years. In those patients with atrophy or GIM in both the antrum and body with a first-degree relative with gastric cancer, surveillance was recommended every 1-2 years. Patients with any dysplasia and a visible lesion should have staging and resection. With no visible lesion, a follow-up endoscopy should be performed in 6 months with high-grade dysplasia and with low-grade dysplasia a repeat in 12 months. Patients with mild to moderate atrophy in the antrum and no intestinal metaplasia were not felt to warrant any further surveillance. (See Figure 1.)
How to screen
Previous studies have found a poor correlation between the endoscopic determination of gastric atrophy and the histologic diagnosis.42 Several studies also found that gastric cancer was missed on initial endoscopic examinations. Sensitivity of endoscopy to detect gastric cancer has ranged from 77% to 93%.43,44 In the United States, there is a lack of standardized quality indicators for upper endoscopy exams. The ESGE has suggested several performance measures to ensure a quality endoscopy exam, including accurate photo documentation, sufficient procedure time of at least 7 minutes, adherence to biopsy protocols, and low complication rates.45 In Asia, a systematic screening protocol is used for photo documentation, and simple techniques such as adequate air insufflation and irrigation to remove mucus are routinely used to improve the endoscopy exam.46,47 The mean time of an endoscopy exam has also been found to increase the detection of neoplastic lesions, as slow endoscopists – with a mean exam duration of 8.6 ± 4.2 min during upper endoscopy – detected threefold more neoplastic lesions than did fast endoscopists.48
A standardized biopsy approach is also important when screening patients. The updated Sydney protocol has been suggested for mapping the stomach to screen for atrophy and GIM. This protocol recommends two biopsies from the antrum (at the lesser and greater curvature), two from the body (at the lesser and greater curvature), and one from the incisura.23 This biopsy protocol was also suggested in the recent MAPS II update, with the biopsy of the incisura felt to be an additional biopsy left to the discretion of the endoscopist. Notably, abnormal appearing mucosal areas should be biopsied separately from the mapping biopsies.
High-definition endoscopy with virtual chromoendoscopy is felt to be better than white-light endoscopy alone at detecting precancerous gastric lesions.38 (See Figure 2.)

In particular, narrow-band imaging (NBI) has been studied and found to increase the diagnostic yield of GIM and dysplasia compared with white light alone.49 Several studies have shown an increased accuracy for the detection of GIM with magnification NBI.50-52 An unfortunate limitation is the geographic availability of magnification NBI: It is not available in the United States. A multicenter study in Portugal developed a new classification system for the appearance of precancerous lesions with NBI and tested its accuracy in endoscopists with a wide range of NBI experience. An abnormal mucosal pattern that showed light blue crests/regular ridge or a tubulovillous appearance and a regular mucosal pattern was found with GIM. An irregular vascular pattern with a white opaque substance and an absent or irregular mucosal pattern was most often found with dysplasia. Furthermore, the reproducibility of these patterns was high between endoscopists.53 Multiple studies have been performed on additional imaging technologies to enhance the detection of gastric neoplasia; however, these technologies are still investigational and currently not recommended for screening.54-57
Serum pepsinogens have been studied in Europe and Asia as noninvasive indicators of gastric atrophy to determine who should be screened with endoscopy.58 A low serum pepsinogen I level below 70 ng/mL and pepsinogen I/II ratio below 3 has generally been used to detect atrophic gastritis and at-risk populations. However, the studies performed in Europe and Asia used different methods for quantifying pepsinogen levels. Therefore, cutoff values cannot be generalized for all assays and should be validated for the specific tests used.38
Summary
Gastric atrophy and gastric intestinal metaplasia are considered precancerous lesions with an increased risk of development of gastric cancer. H. pylori is a major risk factor for the development of GIM. The extent of GIM as well as the presence of incomplete intestinal metaplasia, or type III intestinal metaplasia has been found to have the highest gastric cancer risk. Currently, in the United States, specific guidelines on endoscopic screening and surveillance for noncardia gastric adenocarcinoma based on histological subtype of GIM, location, and extension are lacking. The ESGE recently updated guidelines that recommend surveillance of patients with extensive atrophy and intestinal metaplasia or with a significant family history. Location and extension of intestinal metaplasia plays a role in increased risk. Screening should include a standardized upper endoscopy approach with high-definition white- light endoscopy and NBI, at least a 7-minute examination, adequate insufflation and cleaning, adequate photo documentation, and a standardized biopsy protocol. Further studies are needed to determine an appropriate surveillance interval and standardized pathology reporting approach as well.
Diana Curras-Martin MD, is an internal medicine resident at Hackensack Meridian Jersey Shore University Medical Center. Susana Gonzalez, MD, is assistant professor of medicine in the division of gastroenterology and hepatology (@WCM_GI), Weill Cornell Medicine, New York Presbyterian Hospital–Cornell.
References
1. Bray F et al. CA Cancer J Clin. 2018;68(6):394-424.
2. Global Burden of Disease Cancer Collaboration et al. JAMA Oncol. 2018;4(11):1553-68.
3. Balakrishnan M et al. Curr Gastroenterol Rep. 2017;19(8):36.
4. Anderson WF et al. J Natl Cancer Inst. 2018;110(6):608-15.
5. Trieu JA et al. Dig Dis Sci. 2019;64(5):1079-88.
6. Lauren P. Acta Pathol Microbiol Scand. 1965;64:31-49.
7. Correa P, Schneider BG. Cancer Epidemiol Biomarkers Prev. 2005;14(8):1865-8.
8. Correa P. Cancer Res. 1992;52(24):6735-40.
9. Correa P, Piazuelo MB. J Dig Dis. 2012;13(1):2-9.
10. Correa P et al. J Natl Cancer Inst. 1970;44(2):297-306.
11. Correa P. Semin Oncol. 1985;12(1):2-10.
12. Rugge M et al. Hum Pathol. 1991;22(10):1002-8.
13. Simko V et al. Bratisl Lek Listy. 2015;116(1):3-8.
14. Giroux V, Rustgi AK. Nat Rev Cancer. 2017;17(10):594-604.
15. Jencks DS et al. Gastroenterol Hepatol (N Y). 2018;14(2):92-101.
16. Amieva M, Peek RM Jr. Gastroenterology. 2016;150(1):64-78.
17. Karimi P et al. Cancer Epidemiol Biomarkers Prev. 2014;23(5):700-13.
18. Hatakeyama M. Proc Jpn Acad Ser B Phys Biol Sci. 2017;93(4):196-219.
19. Tsutsumi R et al. Mol Cell Biol. 2006;26(1):261-76.
20. Kikuchi S et al. Am J Gastroenterol. 1999;94(12):3455-9.
21. Jass JR, Filipe MI. Histopathology. 1980;4(3):271-9.
22. Jass JR, Filipe MI. Histochem J. 1981;13(6):931-9.
23. Dixon MF et al. Am J Surg Pathol. 1996;20(10):1161-81.
24. Kang KP et al. J Gastroenterol Hepatol. 2009;24(1):140-8.
25. Gonzalez CA et al. Int J Cancer. 2010;127(11):2654-60.
26. Filipe MI et al. Gut. 1985;26(12):1319-26.
27. Filipe MI et al. Int J Cancer. 1994;57(3):324-9.
28. Gonzalez CA et al. J Gastroenterol Hepatol. 2016;31(5):953-8.
29. Cassaro M et al. Am J Gastroenterol. 2000;95(6):1431-8.
30. Shao L et al. Int J Cancer. Apr 29. 2018.
31. Stemmermann GN. Cancer. 1994;74(2):556-64.
32. Gonzalez CA et al. Int J Cancer. 2013;133(5):1023-32.
33. Reddy KM et al. Clin Gastroenterol Hepatol. 2016;14(10):1420-5.
34. Tava F et al. Hum Pathol. 2006;37(11):1489-97.
35. Fernandez-Esparrach G et al. Rev Esp Enferm Dig. 2014;106(2):120-32.
36. Ono H et al. Dig Endosc. 2016;28(1):3-15.
37. Evans JA, DeWitt JM. Gastrointest Endosc. 2016;83(1):274.
38. Pimentel-Nunes P et al. Endoscopy. 2019;51(4):365-88.
39. Capelle LG et al. Gastrointest Endosc. 2010;71(7):1150-8.
40. Saumoy M et al. Gastroenterology. 2018;155(3):648-60.
41. Gupta N et al. Gastrointest Endosc. 2011;74(3):610-24 e612.
42. Eshmuratov A et al. Dig Dis Sci. 2010;55(5):1364-75.
43. Nam JH et al. Cancer. 2012;118(20):4953-60.
44. Amin A et al. J R Coll Surg Edinb. 2002;47(5):681-4.
45. Bisschops R et al. United European Gastroenterol J. 2016;4(5):629-56.
46. Uedo N et al. Gastroenterol Clin North Am. 2013;42(2):317-35.
47. Yao K. Ann Gastroenterol. 2013;26(1):11-22.
48. Teh JL et al. Clin Gastroenterol Hepatol. 2015;13(3):480-7 e482.
49. Capelle LG et al. Dig Dis Sci. 2010;55(12):3442-8.
50. Bansal A et al. Gastrointest Endosc. 2008;67(2):210-6.
51. Tahara T et al. Gastrointest Endosc. 2009;70(2):246-53.
52. Uedo N et al. Endoscopy. 2006;38(8):819-24.
53. Pimentel-Nunes P et al. Endoscopy. 2012;44(3):236-46.
54. Kato M et al. Gastrointest Endosc. 2009;70(5):899-906.
55. Nishimura J et al. Gastroenterol Res Pract. 2014;2014:819395.
56. Dohi O et al. Gastrointest Endosc. 2019;89(1):47-57.
57. Osawa H et al. World J Gastrointest Endosc. 2012;4(8):356-61.
58. Pasechnikov V et al. World J Gastroenterol. 2014;20(38):13842-62.
Introduction
Despite a global decline in the incidence of gastric cancer over the past 3 decades, it remains the fifth most commonly diagnosed cancer and the third most common cause of cancer deaths worldwide.1 In the United States it is the fourth most commonly diagnosed GI malignancy, after colorectal, pancreas, and liver cancer. The prevalence remains high in Latin America and Asia, which has implications in the United States because of growing Hispanic and Asian populations.2,3 In recent years, a change in the trend of gastric cancer among non-Hispanic whites has been observed, particularly in women younger than 50 years old.4 5
Etiology
Gastric adenocarcinomas are classified into two subcategories based on location (cardia and noncardia) and histology (intestinal and diffuse types).6,7 Atrophic gastritis and gastric intestinal metaplasia (GIM) are considered precursors of intestinal-type noncardia gastric adenocarcinoma. The Correa cascade is a commonly accepted precancer sequence for noncardia gastric adenocarcinoma that describes mucosal changes from inflammation to atrophy to metaplasia to intraepithelial neoplasia and culminating in carcinoma.8,9 It has been observed that GIM may be the histologic change prior to the development of dysplasia and over 50% of patients with high-grade dysplasia will progress to adenocarcinoma.10-12 In the United States, GIM has the highest prevalence in African Americans, Hispanics, and East Asians, with the overall GIM prevalence regardless of ethnicity reported from 3.05% to 19.2%.5,13
Risk factors and subclassification
Replacement of the foveolar and/or glandular epithelium in the oxyntic and antral mucosa by intestinal epithelium results in GIM. It can be focal when limited to one region of the stomach or extensive when two or more regions are involved.14 The main risk factors for GIM development are Helicobacter pylori infection, tobacco, alcohol consumption, high salt intake, and chronic bile reflux.15,16 Additional risks for developing gastric cancer include older age, certain ethnicities, and male sex.17
CagA strains of H. pylori can promote carcinogenesis by inducing a mitogenic cellular response and downregulating cell adhesion.18,19 Less carcinogenic risk is associated with H. pylori Cag-A negative strains; however, they also have oncogenic potential mediated by expression of babA2 and vacA genes.20 Hence, the combination of multiple virulent factors encoded in babA2, CagA, and vacA genes has been associated with increased risk of GIM, inflammation, and development of gastric cancer.15 The clinical usefulness of genotyping H. pylori strains specifically to survey precancerous gastric lesions remains to be seen because of a lack of sufficient clinical studies. In addition, genotyping H. pylori is not commonly performed as part of clinical practice.
The loss of parietal cells seen in atrophic gastritis due to chronic H. pylori infection has been linked to the development of metaplasia due to possible loss of differentiation-promoting factors. As a result, metaplastic cells emerge that express spasmolytic polypeptide (SP or TFF2); hence, this type of metaplasia is referred to as spasmolytic polypeptide–expressing metaplasia (SPEM). The cellular mechanism that may explain a precursor role of SPEM in the development of GIM remains unknown.14 A second competing theory for the development of GIM is the clonal expansion of stem cells in the gastric isthmus that can lead to dysplasia and cancer development.14
On the basis of histological similarities with small intestinal or colonic epithelium, GIM can be further classified into complete or incomplete intestinal metaplasia.21 Complete intestinal metaplasia most closely resembles small intestinal epithelium with a brush border and goblet cells. Incomplete intestinal metaplasia resembles the colonic epithelium and lacks a brush border. A second classification further classifies GIM into three subtypes: Type I contains nonsecretory absorptive cells and sialomucin secreting goblet cells; type II has few absorptive cells, columnar cells secreting sialomucin, goblet cells secreting mainly sialomucin but some sulphomucin, and presence of Paneth cells; and type III consists of columnar cells secreting predominantly sulphomucin, goblet cells secreting sialomucin or sulphomucin, and absence of Paneth cells.15,22 In this subclassification, type I GIM is known as complete GIM and types II and III as incomplete GIM.23-25
Multiple studies performed outside of the United States have shown a higher progression risk to gastric adenocarcinoma in incomplete intestinal metaplasia, or type III intestinal metaplasia.26-32 Also, the risk of gastric cancer has been demonstrated to be higher among patients with a greater area of metaplasia and extensive intestinal metaplasia, defined as GIM in both the antrum and corpus.33,34 Hence, the extent of the metaplasia determined with mapping biopsies, regardless of the subtype, should also be incorporated into the risk assessment of the patient. Currently, a major limitation in the United States is a standardized method of pathologic reporting including subclassification of incomplete versus complete intestinal metaplasia.
Which patients to screen
Understanding this sequence of carcinogenesis offers a potential window for screening and surveillance. Subsequently, early detection of precancerous mucosal changes would be more amenable for endoscopic submucosal dissection (ESD).35,36 Currently, U.S. society guidelines do not specifically address the management of GIM. The American Society for Gastrointestinal Endoscopy (ASGE) guidelines for management of premalignant and malignant conditions of the stomach recommend surveillance in individuals with a family history of gastric cancer or of high-risk ethnic background but with no specific optimal surveillance interval.37 Also, H. pylori treatment is recommended if identified, but empiric treatment in GIM was felt to be controversial. The AGA recently sought comments on a proposed new guideline for the management of GIM. This guideline should be released after the comment period and help address management of GIM in the United States. In April of 2019, the European Society of Gastrointestinal Endoscopy (ESGE) updated the management of epithelial precancerous conditions and lesions in the stomach (MAPS II) guideline.38 The MAPS II guideline identifies atrophic gastritis and intestinal metaplasia as precancerous lesions. In patients with moderate to marked atrophy or GIM affecting both antral and body mucosa, ESGE recommends endoscopic surveillance with high-definition chromoendoscopy, mapping, and guided biopsies or at least two biopsies taken separately at the lesser and greater curvature of the antrum and body. H. pylori eradication was recommended if the patient tested positive.
Furthermore, MAPS II proposed replacing atrophic gastritis (AG) in the Operative Link on Gastritis Assessment (OLGA) staging by GIM (OLGIM) as it is considered a more reliable predictor of an individual’s gastric neoplasia risk, based on the interobserver agreement kappa value 0.6 for AG versus 0.9 for GIM.39 Five biopsies (two from the antrum, two from the corpus, and one from the incisura angularis) are needed for the OLGA/OLGIM score system to be considered an accurate predictor of this risk.39 This is supported by the early findings of gastric atrophy and GIM in the incisura angularis.23 In addition, for patients with GIM only in either the antrum or the body, a family history of gastric cancer, incomplete GIM, autoimmune gastritis, or persistent H. pylori infection was felt to increase the risk to warrant surveillance every 3 years. In those patients with atrophy or GIM in both the antrum and body with a first-degree relative with gastric cancer, surveillance was recommended every 1-2 years. Patients with any dysplasia and a visible lesion should have staging and resection. With no visible lesion, a follow-up endoscopy should be performed in 6 months with high-grade dysplasia and with low-grade dysplasia a repeat in 12 months. Patients with mild to moderate atrophy in the antrum and no intestinal metaplasia were not felt to warrant any further surveillance. (See Figure 1.)
How to screen
Previous studies have found a poor correlation between the endoscopic determination of gastric atrophy and the histologic diagnosis.42 Several studies also found that gastric cancer was missed on initial endoscopic examinations. Sensitivity of endoscopy to detect gastric cancer has ranged from 77% to 93%.43,44 In the United States, there is a lack of standardized quality indicators for upper endoscopy exams. The ESGE has suggested several performance measures to ensure a quality endoscopy exam, including accurate photo documentation, sufficient procedure time of at least 7 minutes, adherence to biopsy protocols, and low complication rates.45 In Asia, a systematic screening protocol is used for photo documentation, and simple techniques such as adequate air insufflation and irrigation to remove mucus are routinely used to improve the endoscopy exam.46,47 The mean time of an endoscopy exam has also been found to increase the detection of neoplastic lesions, as slow endoscopists – with a mean exam duration of 8.6 ± 4.2 min during upper endoscopy – detected threefold more neoplastic lesions than did fast endoscopists.48
A standardized biopsy approach is also important when screening patients. The updated Sydney protocol has been suggested for mapping the stomach to screen for atrophy and GIM. This protocol recommends two biopsies from the antrum (at the lesser and greater curvature), two from the body (at the lesser and greater curvature), and one from the incisura.23 This biopsy protocol was also suggested in the recent MAPS II update, with the biopsy of the incisura felt to be an additional biopsy left to the discretion of the endoscopist. Notably, abnormal appearing mucosal areas should be biopsied separately from the mapping biopsies.
High-definition endoscopy with virtual chromoendoscopy is felt to be better than white-light endoscopy alone at detecting precancerous gastric lesions.38 (See Figure 2.)

In particular, narrow-band imaging (NBI) has been studied and found to increase the diagnostic yield of GIM and dysplasia compared with white light alone.49 Several studies have shown an increased accuracy for the detection of GIM with magnification NBI.50-52 An unfortunate limitation is the geographic availability of magnification NBI: It is not available in the United States. A multicenter study in Portugal developed a new classification system for the appearance of precancerous lesions with NBI and tested its accuracy in endoscopists with a wide range of NBI experience. An abnormal mucosal pattern that showed light blue crests/regular ridge or a tubulovillous appearance and a regular mucosal pattern was found with GIM. An irregular vascular pattern with a white opaque substance and an absent or irregular mucosal pattern was most often found with dysplasia. Furthermore, the reproducibility of these patterns was high between endoscopists.53 Multiple studies have been performed on additional imaging technologies to enhance the detection of gastric neoplasia; however, these technologies are still investigational and currently not recommended for screening.54-57
Serum pepsinogens have been studied in Europe and Asia as noninvasive indicators of gastric atrophy to determine who should be screened with endoscopy.58 A low serum pepsinogen I level below 70 ng/mL and pepsinogen I/II ratio below 3 has generally been used to detect atrophic gastritis and at-risk populations. However, the studies performed in Europe and Asia used different methods for quantifying pepsinogen levels. Therefore, cutoff values cannot be generalized for all assays and should be validated for the specific tests used.38
Summary
Gastric atrophy and gastric intestinal metaplasia are considered precancerous lesions with an increased risk of development of gastric cancer. H. pylori is a major risk factor for the development of GIM. The extent of GIM as well as the presence of incomplete intestinal metaplasia, or type III intestinal metaplasia has been found to have the highest gastric cancer risk. Currently, in the United States, specific guidelines on endoscopic screening and surveillance for noncardia gastric adenocarcinoma based on histological subtype of GIM, location, and extension are lacking. The ESGE recently updated guidelines that recommend surveillance of patients with extensive atrophy and intestinal metaplasia or with a significant family history. Location and extension of intestinal metaplasia plays a role in increased risk. Screening should include a standardized upper endoscopy approach with high-definition white- light endoscopy and NBI, at least a 7-minute examination, adequate insufflation and cleaning, adequate photo documentation, and a standardized biopsy protocol. Further studies are needed to determine an appropriate surveillance interval and standardized pathology reporting approach as well.
Diana Curras-Martin MD, is an internal medicine resident at Hackensack Meridian Jersey Shore University Medical Center. Susana Gonzalez, MD, is assistant professor of medicine in the division of gastroenterology and hepatology (@WCM_GI), Weill Cornell Medicine, New York Presbyterian Hospital–Cornell.
References
1. Bray F et al. CA Cancer J Clin. 2018;68(6):394-424.
2. Global Burden of Disease Cancer Collaboration et al. JAMA Oncol. 2018;4(11):1553-68.
3. Balakrishnan M et al. Curr Gastroenterol Rep. 2017;19(8):36.
4. Anderson WF et al. J Natl Cancer Inst. 2018;110(6):608-15.
5. Trieu JA et al. Dig Dis Sci. 2019;64(5):1079-88.
6. Lauren P. Acta Pathol Microbiol Scand. 1965;64:31-49.
7. Correa P, Schneider BG. Cancer Epidemiol Biomarkers Prev. 2005;14(8):1865-8.
8. Correa P. Cancer Res. 1992;52(24):6735-40.
9. Correa P, Piazuelo MB. J Dig Dis. 2012;13(1):2-9.
10. Correa P et al. J Natl Cancer Inst. 1970;44(2):297-306.
11. Correa P. Semin Oncol. 1985;12(1):2-10.
12. Rugge M et al. Hum Pathol. 1991;22(10):1002-8.
13. Simko V et al. Bratisl Lek Listy. 2015;116(1):3-8.
14. Giroux V, Rustgi AK. Nat Rev Cancer. 2017;17(10):594-604.
15. Jencks DS et al. Gastroenterol Hepatol (N Y). 2018;14(2):92-101.
16. Amieva M, Peek RM Jr. Gastroenterology. 2016;150(1):64-78.
17. Karimi P et al. Cancer Epidemiol Biomarkers Prev. 2014;23(5):700-13.
18. Hatakeyama M. Proc Jpn Acad Ser B Phys Biol Sci. 2017;93(4):196-219.
19. Tsutsumi R et al. Mol Cell Biol. 2006;26(1):261-76.
20. Kikuchi S et al. Am J Gastroenterol. 1999;94(12):3455-9.
21. Jass JR, Filipe MI. Histopathology. 1980;4(3):271-9.
22. Jass JR, Filipe MI. Histochem J. 1981;13(6):931-9.
23. Dixon MF et al. Am J Surg Pathol. 1996;20(10):1161-81.
24. Kang KP et al. J Gastroenterol Hepatol. 2009;24(1):140-8.
25. Gonzalez CA et al. Int J Cancer. 2010;127(11):2654-60.
26. Filipe MI et al. Gut. 1985;26(12):1319-26.
27. Filipe MI et al. Int J Cancer. 1994;57(3):324-9.
28. Gonzalez CA et al. J Gastroenterol Hepatol. 2016;31(5):953-8.
29. Cassaro M et al. Am J Gastroenterol. 2000;95(6):1431-8.
30. Shao L et al. Int J Cancer. Apr 29. 2018.
31. Stemmermann GN. Cancer. 1994;74(2):556-64.
32. Gonzalez CA et al. Int J Cancer. 2013;133(5):1023-32.
33. Reddy KM et al. Clin Gastroenterol Hepatol. 2016;14(10):1420-5.
34. Tava F et al. Hum Pathol. 2006;37(11):1489-97.
35. Fernandez-Esparrach G et al. Rev Esp Enferm Dig. 2014;106(2):120-32.
36. Ono H et al. Dig Endosc. 2016;28(1):3-15.
37. Evans JA, DeWitt JM. Gastrointest Endosc. 2016;83(1):274.
38. Pimentel-Nunes P et al. Endoscopy. 2019;51(4):365-88.
39. Capelle LG et al. Gastrointest Endosc. 2010;71(7):1150-8.
40. Saumoy M et al. Gastroenterology. 2018;155(3):648-60.
41. Gupta N et al. Gastrointest Endosc. 2011;74(3):610-24 e612.
42. Eshmuratov A et al. Dig Dis Sci. 2010;55(5):1364-75.
43. Nam JH et al. Cancer. 2012;118(20):4953-60.
44. Amin A et al. J R Coll Surg Edinb. 2002;47(5):681-4.
45. Bisschops R et al. United European Gastroenterol J. 2016;4(5):629-56.
46. Uedo N et al. Gastroenterol Clin North Am. 2013;42(2):317-35.
47. Yao K. Ann Gastroenterol. 2013;26(1):11-22.
48. Teh JL et al. Clin Gastroenterol Hepatol. 2015;13(3):480-7 e482.
49. Capelle LG et al. Dig Dis Sci. 2010;55(12):3442-8.
50. Bansal A et al. Gastrointest Endosc. 2008;67(2):210-6.
51. Tahara T et al. Gastrointest Endosc. 2009;70(2):246-53.
52. Uedo N et al. Endoscopy. 2006;38(8):819-24.
53. Pimentel-Nunes P et al. Endoscopy. 2012;44(3):236-46.
54. Kato M et al. Gastrointest Endosc. 2009;70(5):899-906.
55. Nishimura J et al. Gastroenterol Res Pract. 2014;2014:819395.
56. Dohi O et al. Gastrointest Endosc. 2019;89(1):47-57.
57. Osawa H et al. World J Gastrointest Endosc. 2012;4(8):356-61.
58. Pasechnikov V et al. World J Gastroenterol. 2014;20(38):13842-62.
Exploring multidisciplinary treatments in the traumatizing aspects of chronic abdominal pain
Introduction
Abdominal pain is a complex phenomenon that involves unpleasant sensory and emotional experiences caused by actual or potential visceral tissue damage. As pain becomes chronic, there is a functional reorganization of the brain involved in emotional and cognitive processing leading to amplification of pain perception and associated pain suffering.1,2 With the rising recognition of the complexity of pain management in the 1960s, the treatment of pain became a recognized field of study, leading to the formation of interdisciplinary teams to treat pain. However, although efficacious, this model lacked adequate reimbursement structures and eventually subsided as opioids (which at the time were widely believed to be nonaddictive) become more prevalent.3 Not only is there a lack of empirical evidence for opioids in the management of chronic abdominal pain, there is a growing list of adverse consequences of prolonged opioid use for both the brain and gastrointestinal tract.4
Recently, there has been more clinical focus on behavioral interventions that can modulate gut pain signals and associated behaviors by reversing maladaptive emotional and cognitive brain processes.5 One such psychological process that has received little attention is the traumatizing nature of chronic abdominal pain. Chronic pain, particularly when it feels uncontrollable to patients, activates the brain’s fear circuitry and drives hyperarousal, emotional numbing, and consolidation of painful somatic memories, which become habitual and further amplify negative visceral signals.6,7 These processes are identical to the symptom manifestations of posttraumatic stress disorder (PTSD) such as intrusiveness, avoidance, negative mood and cognitions, and hyperarousal from life events. In fact, individuals with a history of other traumatizing exposures have an even higher risk of developing chronic pain disorders.8 This review has two objectives: to provide a theoretical framework for understanding chronic pain as a traumatizing experience with posttraumatic manifestations and to discuss behavioral interventions and adjunctive nonopioid pharmacotherapy embedded in multidisciplinary care models essential to reversing this negative brain-gut cycle and reducing pain-related suffering.
Trauma and chronic abdominal pain
Trauma is defined as an individual’s response to a threat to safety. Traumatized patients or those with PTSD are at higher risk for chronic abdominal pain.9 Given the strong neurobiological connection between the brain and gut that has been phylogenetically preserved, emotional (e.g., fear, terror) or physical (e.g., pain) signals represent danger, and with chronicity, there can be a kindling-related consolidation of these maladaptive neurobiological pathways leading to suffering (e.g., hopelessness, sense of failure) and disability (Figure 1).
The interrelationship between chronic pain and trauma is multifaceted and is further complicated by the traumatizing nature of chronic pain itself, when pain is interpreted as a signal that the body is sick or even dangerously ill. Patients with chronic abdominal pain may seek multiple medical opinions and often undergo extensive, unnecessary, and sometimes harmful interventions to find the cause of their pain, with fear of disability and even death driving this search for answers.
The degree to which an individual with long-lasting pain interprets their discomfort as a risk to their well-being is related to the degree of trauma they experience because of their pain.10 Indeed, many of the negative symptoms associated with posttraumatic stress are also found in those with chronic abdominal pain. Trauma impacts the fear circuitry centers of the brain, leading to altered activation of the hypothalamic-pituitary-adrenal axis and the amygdala, as well as chronic activation of the sympathetic nervous system and stress-released hormones, all of which are potential pathways that dysregulate the brain-gut relationship.11-13 Worries for safety, which are reactivated by physiological cues (e.g., GI symptoms, pain), as well as avoidance of potential triggers of GI symptoms (e.g., food, exercise, medications, and situations such as travel or scheduled events, and fear of being trapped without bathroom access), are common. Traumatized individuals can experience a foreshortened sense of the future, which may lead to decreased investment in long-term determinants of health (e.g., balanced diet, exercise, social support) and have higher rates of functional impairment and higher health care utilization.14 Negative mood, including irritability, anxiety, depression, insomnia, and impaired concentration are common in those with trauma and chronic pain and can be accompanied by internalized blame (e.g., depression, substance abuse, suicidality) or externalized blame (e.g., negative relationships with health care providers, rejection from their support or faith system). These can be worsened by an impaired sense of trust, which impacts the patient-provider relationship and other sources of social support leading to lack of behavioral activation, anhedonia, and isolation.
Another commonality is hypervigilance, as those with chronic abdominal pain are often hyperaware of physical symptoms and always “on alert” for a signal indicative of a pain flare. Anxiety and depression frequently co-occur in populations with trauma and chronic pain; these diagnoses are associated with higher rates of catastrophizing and learned helplessness, which may be exacerbated by lack of a “cure” for functional gastrointestinal disorders (FGIDs) and chronic pain.15 These factors could potentially lead to lack of engagement with treatment or, alternatively, risky or destructive attempts to cure pain including dangerous complementary alternative treatments or substance abuse to numb sensations. Another feature of trauma in chronic pain is the sense of dissociation from and lack of control over the body, sometimes induced by negative medical experiences (e.g., unwanted physical examinations, medication side effects, traumatic procedures, or hospitalizations).16,17
The importance of treating trauma in the management of chronic pain
Behavioral treatment is increasingly being recognized as an essential component in the management of any chronic pain syndrome.18 The most studied psychosocial interventions for chronic abdominal pain are cognitive-behavioral therapy (CBT) and gut-focused hypnosis. CBT is usually a problem-focused, short-term intervention that can be delivered individually in the office, via group therapy, or through virtual platforms. CBT is most effective when cognitive distortions and ineffective behaviors create emotional distress, and the therapy targets patient’s stress reactivity, visceral anxiety, catastrophizing, and inflexible coping styles.5 Gut-focused hypnosis is the second most–studied behavioral treatment for chronic abdominal pain and utilizes the trance state to make positive suggestions leading to broad and lasting physiological and psychological improvement.19 In addition to pain management, both CBT and hypnosis are efficacious treatments for PTSD.20,21
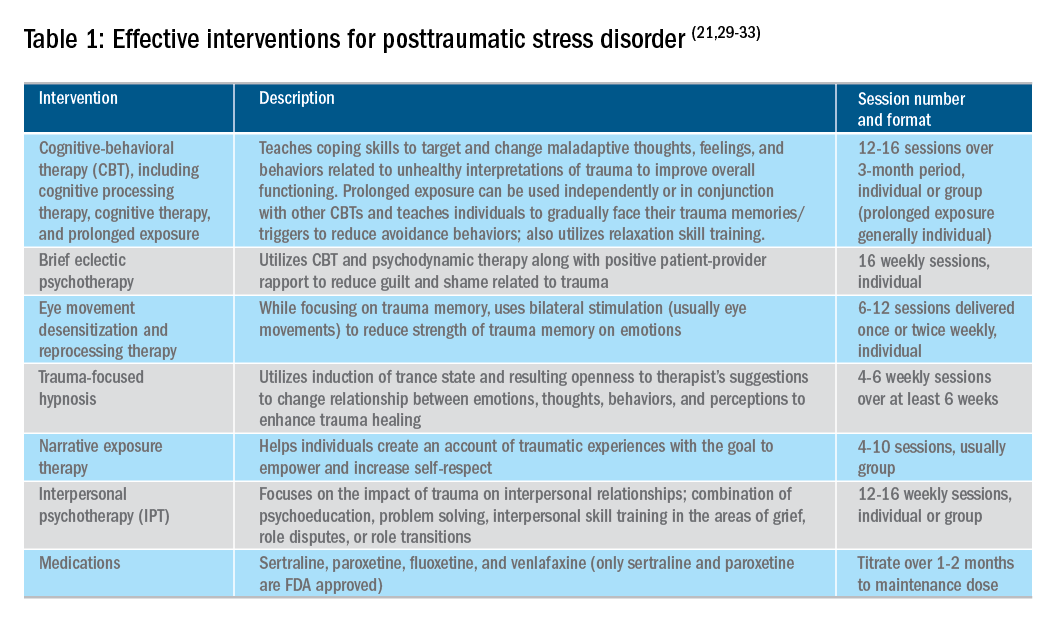
Utilizing a multidisciplinary medical team including integrated behavioral experts, such as in a patient-centered medical home, is considered the standard of care for treatment of chronic pain. The patient-provider relationship is essential, as is consistent follow-up to ensure effective symptom management and improvements in quality of life. Additionally, patient education, including a positive (i.e., clear) diagnosis and information on the brain-gut relationship, is associated with symptom improvement. In our subspecialty medical home for inflammatory bowel disease (IBD), we found that, in our patients who were on opioids for their chronic pain, engagement with our embedded behavioral and pain specialists resulted in significant reduction in opioid use and depression as well as improved self-reported quality of life.22 Gastroenterologists and advanced-practice providers operating without embedded behavioral therapists can consider referring patients to behavioral treatment (e.g., licensed clinical social workers, licensed professional counselors, marriage and family therapists, psychologists, and psychiatrists; the latter often specialize in medication management and may not offer behavioral therapy) for trauma if patients have undergone a traumatic event (e.g., exposure to any potentially life-threatening event, serious injury, or violence) at any point in their lifetime and are experiencing intrusive symptoms (e.g., memories, dreams, or flashbacks to trauma), avoidance of trauma reminders, and negative mood or hyperarousal related to traumatic events (Table 1).23
With the traumatization component of chronic abdominal pain, which can further drive maladaptive coping cycles, incorporation of trauma-informed treatment into gastroenterology clinics is an avenue toward more effective treatment. The core principles of trauma-informed care include safety, choice, collaboration, trustworthiness, and empowerment,24 and are easily aligned with patient-centered models of care such as the interdisciplinary medical home model. Incorporation of screening techniques, interdisciplinary training of clinicians, and use of behavioral providers with experience in evidenced-based treatments of trauma enhance a clinic’s ability to effectively identify and treat individuals who have trauma because of their abdominal pain.25 Additionally, the most common behavioral interventions for functional gastrointestinal disorders (FGIDs) are also efficacious in the treatment of trauma. CBT is a well-validated treatment for PTSD that utilizes exposure therapy to help individuals restructure negative beliefs shaped by their negative experience and develop relaxation skills. Hypnosis is also validated in the treatment of trauma, with the possible mechanism of action being the replacement of the negative or dissociated traumatic trance with a healthy, adaptive trance facilitated by the hypnotherapist.21
Adjunctive nonopioid medications for chronic abdominal pain
While there are few randomized controlled trials establishing efficacy of pharmacotherapy for sustained improvement of abdominal pain or related suffering, several small trials and consensus clinical expert opinion suggest partial improvement in these domains.26,27 Central neuromodulators that can attenuate chronic visceral pain include antidepressants, antipsychotics, and other central nervous system–targeted medications.26 Tricyclic antidepressants (e.g., amitriptyline, nortriptyline, imipramine, desipramine) are often first-line treatment for FGIDs.28 Serotonin noradrenergic reuptake inhibitors (e.g., duloxetine, venlafaxine, desvenlafaxine, milnacipran) are also effective in pain management. Selective serotonin reuptake inhibitors (e.g., paroxetine, fluoxetine, sertraline, citalopram, escitalopram) can be used, especially when comorbid depression, anxiety, and phobic disorders are present. Tetracyclic antidepressants (e.g., mirtazapine, mianserin, trazodone) are effective treatments for early satiety, nausea/vomiting, insomnia, and low weight. Augmenting agents are utilized when single agents do not provide maximum benefit, including quetiapine (disturbed sleep), bupropion (fatigue), aripiprazole, buspirone, and tandospirone (dyspeptic features and anxiety). Delta ligands including gabapentin and pregabalin are helpful for abdominal wall pain or fibromyalgia. Ketamine is a newer but promising pathway for treatment of pain and depression and is increasingly being utilized in outpatient settings. Additionally, partial opioid-receptor agonists including methadone and suboxone have been reported to decrease pain in addition to their efficacy in addiction recovery. Medical marijuana is another area of growing interest, and while research has yet to show a clear effect in pain management, it does appear helpful in nausea and appetite stimulation. Obtaining a therapeutic response is the first treatment goal, after which a patient should be monitored in at least 6-month intervals to ensure sustained benefits and tolerability, and if these are not met, enhancement of treatment or a slow taper is indicated. As in all treatments, a positive patient-provider relationship predicts improved treatment adherence and outcomes.26 However, while these pharmacological interventions can reduce symptom severity, there is little evidence that they reduce traumatization without adjunctive psychotherapy.29
Summary
Both behavioral and pharmacological treatment options are available for chronic abdominal pain and most useful if traumatic manifestations are assessed and included as treatment targets. A multidisciplinary approach to the treatment of chronic abdominal pain with increased screening and treatment of trauma is a promising pathway to improved care and management for patients with chronic pain. If trauma is left untreated, the benefits of otherwise effective treatments are likely to be significantly limited.
References
1. Apkarian AV et al. Prog Neurobiol. 2009 Feb;87(2):81-97.
2. Gallagher RM et al. Pain Med. 2011 Jan;12(1):1-2.
3. Collier R et al. CMAJ. 2018 Jan 8;190(1):E26-7. doi: 10.1503/cmaj.109-5523.
4. Szigethy E et al. Nature Reviews Gastroenterology & Hepatology, 2018;15:168-80.
5. Ballou S et al. Clin Transl Gastroenterol. 2017 Jan;8(1):e214.
6. Egloff N et al. J Pain Res. 2013 Nov 5;6:765-70.
7. Fashler S et al. J Pain Res. 2016 Aug 10;9:551-61.
8. McKernan LC et al. Clin J Pain. 2019 May;35(5):385-93.
9. Ju T et al. J Clin Gastroenterol. 2018 Dec 19. doi: 10.1097/MCG.0000000000001153.
10. Fishbain DA et al. Pain Med. 2017 Apr 1;18(4):711-35.
11. Martin CR et al. Cell Mol Gastroenterol Hepatol. 2018;6(2):133-48.
12. Osadchiy V et al. Clin Gastroenterol Hepatol. 2019 Jan;17(2):322-32.
13. Brzozowski B et al. Curr Neuropharmacol. 2016 Nov;14(8):892-900.
14. Outclat SD et al. Pain Med. 2014;15(11):1872-9.
15. Asmundson GJ et al. Can J Psychiatry. 2002;Dec;47(10):930-7.
16. Taft TH et al. Inflamm Bowel Dis. 2019 Mar 7. doi: 10.1093/ibd/izz032.
17. Duckworth MP et al. International Journal of Rehabilitation and Health, 2000 Apr;5(2):129-39.
18. Scascighini L et al. Rheumatology (Oxford). 2008 May;47(5):670-8.
19. Palsson O et al. European Gastroenterology & Hepatology Review. 2010;6(1):42-6.
20. Watkins LE et al. Frontiers in Behavioral Neuroscience. 2018;12:1-9.
21. O’Toole SK et al. J Trauma Stress. 2016 Feb;29(1):97-100.
22. Goldblum Y et al. Digestive Disease Week. San Diego. 2019. Abstract in press.
23. American Psychiatric Association. Diagnostic and Statistical Manual (of Mental Disorders), Fifth Edition. Arlington, Va: American Psychiatric Publishing, 2013.
24. United States Department of Health and Human Services, Substance Abuse and Mental Health Services Administration. 2018. Trauma-informed approach and trauma-specific interventions. Retrieved from samhsa.gov/nctic/trauma-interventions.
25. Click BH et al. Inflamm Bowel Dis. 2017;23(5):681-94.
26. Drossman DA et al. Gastroenterology. 2018 Mar;154(4):1140-71.
27. Thorkelson G et al. Inflamm Bowel Dis. 2016 Jun 1;22(6):1509-22.
28. Törnblom H et al. Current Gastroenterology Reports. 2018;20(12):58.
29. Watkins LE et al. Front Behav Neurosci. 2018;12:258.
30. American Psychiatric Association. Clinical Practice Guideline for the Treatment of Posttraumatic Stress Disorder (PTSD) in Adults. 2017.
31. Bisson JI et al. Cochrane Database Syst Rev. 2013 Dec 13;(12):CD003388.
32. Department of Veterans Affairs and Department of Defense. VA/DOD clinical practice guideline for the management of posttraumatic stress disorder and acute stress disorder. 2017.
33. Karatzias T et al. Psychol Med. 2019 Mar 12:1-15. doi: 10.1017/S0033291719000436. Advance online publication.
Emily Weaver, LCSW, is a UPMC Total Care–IBD program senior social worker, Eva Szigethy, MD, PhD, is professor of psychiatry and medicine, codirector, IBD Total Care Medical Home, University of Pittsburgh Medical Center, departments of medicine and psychiatry.
Introduction
Abdominal pain is a complex phenomenon that involves unpleasant sensory and emotional experiences caused by actual or potential visceral tissue damage. As pain becomes chronic, there is a functional reorganization of the brain involved in emotional and cognitive processing leading to amplification of pain perception and associated pain suffering.1,2 With the rising recognition of the complexity of pain management in the 1960s, the treatment of pain became a recognized field of study, leading to the formation of interdisciplinary teams to treat pain. However, although efficacious, this model lacked adequate reimbursement structures and eventually subsided as opioids (which at the time were widely believed to be nonaddictive) become more prevalent.3 Not only is there a lack of empirical evidence for opioids in the management of chronic abdominal pain, there is a growing list of adverse consequences of prolonged opioid use for both the brain and gastrointestinal tract.4
Recently, there has been more clinical focus on behavioral interventions that can modulate gut pain signals and associated behaviors by reversing maladaptive emotional and cognitive brain processes.5 One such psychological process that has received little attention is the traumatizing nature of chronic abdominal pain. Chronic pain, particularly when it feels uncontrollable to patients, activates the brain’s fear circuitry and drives hyperarousal, emotional numbing, and consolidation of painful somatic memories, which become habitual and further amplify negative visceral signals.6,7 These processes are identical to the symptom manifestations of posttraumatic stress disorder (PTSD) such as intrusiveness, avoidance, negative mood and cognitions, and hyperarousal from life events. In fact, individuals with a history of other traumatizing exposures have an even higher risk of developing chronic pain disorders.8 This review has two objectives: to provide a theoretical framework for understanding chronic pain as a traumatizing experience with posttraumatic manifestations and to discuss behavioral interventions and adjunctive nonopioid pharmacotherapy embedded in multidisciplinary care models essential to reversing this negative brain-gut cycle and reducing pain-related suffering.
Trauma and chronic abdominal pain
Trauma is defined as an individual’s response to a threat to safety. Traumatized patients or those with PTSD are at higher risk for chronic abdominal pain.9 Given the strong neurobiological connection between the brain and gut that has been phylogenetically preserved, emotional (e.g., fear, terror) or physical (e.g., pain) signals represent danger, and with chronicity, there can be a kindling-related consolidation of these maladaptive neurobiological pathways leading to suffering (e.g., hopelessness, sense of failure) and disability (Figure 1).

The interrelationship between chronic pain and trauma is multifaceted and is further complicated by the traumatizing nature of chronic pain itself, when pain is interpreted as a signal that the body is sick or even dangerously ill. Patients with chronic abdominal pain may seek multiple medical opinions and often undergo extensive, unnecessary, and sometimes harmful interventions to find the cause of their pain, with fear of disability and even death driving this search for answers.
The degree to which an individual with long-lasting pain interprets their discomfort as a risk to their well-being is related to the degree of trauma they experience because of their pain.10 Indeed, many of the negative symptoms associated with posttraumatic stress are also found in those with chronic abdominal pain. Trauma impacts the fear circuitry centers of the brain, leading to altered activation of the hypothalamic-pituitary-adrenal axis and the amygdala, as well as chronic activation of the sympathetic nervous system and stress-released hormones, all of which are potential pathways that dysregulate the brain-gut relationship.11-13 Worries for safety, which are reactivated by physiological cues (e.g., GI symptoms, pain), as well as avoidance of potential triggers of GI symptoms (e.g., food, exercise, medications, and situations such as travel or scheduled events, and fear of being trapped without bathroom access), are common. Traumatized individuals can experience a foreshortened sense of the future, which may lead to decreased investment in long-term determinants of health (e.g., balanced diet, exercise, social support) and have higher rates of functional impairment and higher health care utilization.14 Negative mood, including irritability, anxiety, depression, insomnia, and impaired concentration are common in those with trauma and chronic pain and can be accompanied by internalized blame (e.g., depression, substance abuse, suicidality) or externalized blame (e.g., negative relationships with health care providers, rejection from their support or faith system). These can be worsened by an impaired sense of trust, which impacts the patient-provider relationship and other sources of social support leading to lack of behavioral activation, anhedonia, and isolation.
Another commonality is hypervigilance, as those with chronic abdominal pain are often hyperaware of physical symptoms and always “on alert” for a signal indicative of a pain flare. Anxiety and depression frequently co-occur in populations with trauma and chronic pain; these diagnoses are associated with higher rates of catastrophizing and learned helplessness, which may be exacerbated by lack of a “cure” for functional gastrointestinal disorders (FGIDs) and chronic pain.15 These factors could potentially lead to lack of engagement with treatment or, alternatively, risky or destructive attempts to cure pain including dangerous complementary alternative treatments or substance abuse to numb sensations. Another feature of trauma in chronic pain is the sense of dissociation from and lack of control over the body, sometimes induced by negative medical experiences (e.g., unwanted physical examinations, medication side effects, traumatic procedures, or hospitalizations).16,17
The importance of treating trauma in the management of chronic pain
Behavioral treatment is increasingly being recognized as an essential component in the management of any chronic pain syndrome.18 The most studied psychosocial interventions for chronic abdominal pain are cognitive-behavioral therapy (CBT) and gut-focused hypnosis. CBT is usually a problem-focused, short-term intervention that can be delivered individually in the office, via group therapy, or through virtual platforms. CBT is most effective when cognitive distortions and ineffective behaviors create emotional distress, and the therapy targets patient’s stress reactivity, visceral anxiety, catastrophizing, and inflexible coping styles.5 Gut-focused hypnosis is the second most–studied behavioral treatment for chronic abdominal pain and utilizes the trance state to make positive suggestions leading to broad and lasting physiological and psychological improvement.19 In addition to pain management, both CBT and hypnosis are efficacious treatments for PTSD.20,21

Utilizing a multidisciplinary medical team including integrated behavioral experts, such as in a patient-centered medical home, is considered the standard of care for treatment of chronic pain. The patient-provider relationship is essential, as is consistent follow-up to ensure effective symptom management and improvements in quality of life. Additionally, patient education, including a positive (i.e., clear) diagnosis and information on the brain-gut relationship, is associated with symptom improvement. In our subspecialty medical home for inflammatory bowel disease (IBD), we found that, in our patients who were on opioids for their chronic pain, engagement with our embedded behavioral and pain specialists resulted in significant reduction in opioid use and depression as well as improved self-reported quality of life.22 Gastroenterologists and advanced-practice providers operating without embedded behavioral therapists can consider referring patients to behavioral treatment (e.g., licensed clinical social workers, licensed professional counselors, marriage and family therapists, psychologists, and psychiatrists; the latter often specialize in medication management and may not offer behavioral therapy) for trauma if patients have undergone a traumatic event (e.g., exposure to any potentially life-threatening event, serious injury, or violence) at any point in their lifetime and are experiencing intrusive symptoms (e.g., memories, dreams, or flashbacks to trauma), avoidance of trauma reminders, and negative mood or hyperarousal related to traumatic events (Table 1).23
With the traumatization component of chronic abdominal pain, which can further drive maladaptive coping cycles, incorporation of trauma-informed treatment into gastroenterology clinics is an avenue toward more effective treatment. The core principles of trauma-informed care include safety, choice, collaboration, trustworthiness, and empowerment,24 and are easily aligned with patient-centered models of care such as the interdisciplinary medical home model. Incorporation of screening techniques, interdisciplinary training of clinicians, and use of behavioral providers with experience in evidenced-based treatments of trauma enhance a clinic’s ability to effectively identify and treat individuals who have trauma because of their abdominal pain.25 Additionally, the most common behavioral interventions for functional gastrointestinal disorders (FGIDs) are also efficacious in the treatment of trauma. CBT is a well-validated treatment for PTSD that utilizes exposure therapy to help individuals restructure negative beliefs shaped by their negative experience and develop relaxation skills. Hypnosis is also validated in the treatment of trauma, with the possible mechanism of action being the replacement of the negative or dissociated traumatic trance with a healthy, adaptive trance facilitated by the hypnotherapist.21
Adjunctive nonopioid medications for chronic abdominal pain
While there are few randomized controlled trials establishing efficacy of pharmacotherapy for sustained improvement of abdominal pain or related suffering, several small trials and consensus clinical expert opinion suggest partial improvement in these domains.26,27 Central neuromodulators that can attenuate chronic visceral pain include antidepressants, antipsychotics, and other central nervous system–targeted medications.26 Tricyclic antidepressants (e.g., amitriptyline, nortriptyline, imipramine, desipramine) are often first-line treatment for FGIDs.28 Serotonin noradrenergic reuptake inhibitors (e.g., duloxetine, venlafaxine, desvenlafaxine, milnacipran) are also effective in pain management. Selective serotonin reuptake inhibitors (e.g., paroxetine, fluoxetine, sertraline, citalopram, escitalopram) can be used, especially when comorbid depression, anxiety, and phobic disorders are present. Tetracyclic antidepressants (e.g., mirtazapine, mianserin, trazodone) are effective treatments for early satiety, nausea/vomiting, insomnia, and low weight. Augmenting agents are utilized when single agents do not provide maximum benefit, including quetiapine (disturbed sleep), bupropion (fatigue), aripiprazole, buspirone, and tandospirone (dyspeptic features and anxiety). Delta ligands including gabapentin and pregabalin are helpful for abdominal wall pain or fibromyalgia. Ketamine is a newer but promising pathway for treatment of pain and depression and is increasingly being utilized in outpatient settings. Additionally, partial opioid-receptor agonists including methadone and suboxone have been reported to decrease pain in addition to their efficacy in addiction recovery. Medical marijuana is another area of growing interest, and while research has yet to show a clear effect in pain management, it does appear helpful in nausea and appetite stimulation. Obtaining a therapeutic response is the first treatment goal, after which a patient should be monitored in at least 6-month intervals to ensure sustained benefits and tolerability, and if these are not met, enhancement of treatment or a slow taper is indicated. As in all treatments, a positive patient-provider relationship predicts improved treatment adherence and outcomes.26 However, while these pharmacological interventions can reduce symptom severity, there is little evidence that they reduce traumatization without adjunctive psychotherapy.29
Summary
Both behavioral and pharmacological treatment options are available for chronic abdominal pain and most useful if traumatic manifestations are assessed and included as treatment targets. A multidisciplinary approach to the treatment of chronic abdominal pain with increased screening and treatment of trauma is a promising pathway to improved care and management for patients with chronic pain. If trauma is left untreated, the benefits of otherwise effective treatments are likely to be significantly limited.
References
1. Apkarian AV et al. Prog Neurobiol. 2009 Feb;87(2):81-97.
2. Gallagher RM et al. Pain Med. 2011 Jan;12(1):1-2.
3. Collier R et al. CMAJ. 2018 Jan 8;190(1):E26-7. doi: 10.1503/cmaj.109-5523.
4. Szigethy E et al. Nature Reviews Gastroenterology & Hepatology, 2018;15:168-80.
5. Ballou S et al. Clin Transl Gastroenterol. 2017 Jan;8(1):e214.
6. Egloff N et al. J Pain Res. 2013 Nov 5;6:765-70.
7. Fashler S et al. J Pain Res. 2016 Aug 10;9:551-61.
8. McKernan LC et al. Clin J Pain. 2019 May;35(5):385-93.
9. Ju T et al. J Clin Gastroenterol. 2018 Dec 19. doi: 10.1097/MCG.0000000000001153.
10. Fishbain DA et al. Pain Med. 2017 Apr 1;18(4):711-35.
11. Martin CR et al. Cell Mol Gastroenterol Hepatol. 2018;6(2):133-48.
12. Osadchiy V et al. Clin Gastroenterol Hepatol. 2019 Jan;17(2):322-32.
13. Brzozowski B et al. Curr Neuropharmacol. 2016 Nov;14(8):892-900.
14. Outclat SD et al. Pain Med. 2014;15(11):1872-9.
15. Asmundson GJ et al. Can J Psychiatry. 2002;Dec;47(10):930-7.
16. Taft TH et al. Inflamm Bowel Dis. 2019 Mar 7. doi: 10.1093/ibd/izz032.
17. Duckworth MP et al. International Journal of Rehabilitation and Health, 2000 Apr;5(2):129-39.
18. Scascighini L et al. Rheumatology (Oxford). 2008 May;47(5):670-8.
19. Palsson O et al. European Gastroenterology & Hepatology Review. 2010;6(1):42-6.
20. Watkins LE et al. Frontiers in Behavioral Neuroscience. 2018;12:1-9.
21. O’Toole SK et al. J Trauma Stress. 2016 Feb;29(1):97-100.
22. Goldblum Y et al. Digestive Disease Week. San Diego. 2019. Abstract in press.
23. American Psychiatric Association. Diagnostic and Statistical Manual (of Mental Disorders), Fifth Edition. Arlington, Va: American Psychiatric Publishing, 2013.
24. United States Department of Health and Human Services, Substance Abuse and Mental Health Services Administration. 2018. Trauma-informed approach and trauma-specific interventions. Retrieved from samhsa.gov/nctic/trauma-interventions.
25. Click BH et al. Inflamm Bowel Dis. 2017;23(5):681-94.
26. Drossman DA et al. Gastroenterology. 2018 Mar;154(4):1140-71.
27. Thorkelson G et al. Inflamm Bowel Dis. 2016 Jun 1;22(6):1509-22.
28. Törnblom H et al. Current Gastroenterology Reports. 2018;20(12):58.
29. Watkins LE et al. Front Behav Neurosci. 2018;12:258.
30. American Psychiatric Association. Clinical Practice Guideline for the Treatment of Posttraumatic Stress Disorder (PTSD) in Adults. 2017.
31. Bisson JI et al. Cochrane Database Syst Rev. 2013 Dec 13;(12):CD003388.
32. Department of Veterans Affairs and Department of Defense. VA/DOD clinical practice guideline for the management of posttraumatic stress disorder and acute stress disorder. 2017.
33. Karatzias T et al. Psychol Med. 2019 Mar 12:1-15. doi: 10.1017/S0033291719000436. Advance online publication.
Emily Weaver, LCSW, is a UPMC Total Care–IBD program senior social worker, Eva Szigethy, MD, PhD, is professor of psychiatry and medicine, codirector, IBD Total Care Medical Home, University of Pittsburgh Medical Center, departments of medicine and psychiatry.
Introduction
Abdominal pain is a complex phenomenon that involves unpleasant sensory and emotional experiences caused by actual or potential visceral tissue damage. As pain becomes chronic, there is a functional reorganization of the brain involved in emotional and cognitive processing leading to amplification of pain perception and associated pain suffering.1,2 With the rising recognition of the complexity of pain management in the 1960s, the treatment of pain became a recognized field of study, leading to the formation of interdisciplinary teams to treat pain. However, although efficacious, this model lacked adequate reimbursement structures and eventually subsided as opioids (which at the time were widely believed to be nonaddictive) become more prevalent.3 Not only is there a lack of empirical evidence for opioids in the management of chronic abdominal pain, there is a growing list of adverse consequences of prolonged opioid use for both the brain and gastrointestinal tract.4
Recently, there has been more clinical focus on behavioral interventions that can modulate gut pain signals and associated behaviors by reversing maladaptive emotional and cognitive brain processes.5 One such psychological process that has received little attention is the traumatizing nature of chronic abdominal pain. Chronic pain, particularly when it feels uncontrollable to patients, activates the brain’s fear circuitry and drives hyperarousal, emotional numbing, and consolidation of painful somatic memories, which become habitual and further amplify negative visceral signals.6,7 These processes are identical to the symptom manifestations of posttraumatic stress disorder (PTSD) such as intrusiveness, avoidance, negative mood and cognitions, and hyperarousal from life events. In fact, individuals with a history of other traumatizing exposures have an even higher risk of developing chronic pain disorders.8 This review has two objectives: to provide a theoretical framework for understanding chronic pain as a traumatizing experience with posttraumatic manifestations and to discuss behavioral interventions and adjunctive nonopioid pharmacotherapy embedded in multidisciplinary care models essential to reversing this negative brain-gut cycle and reducing pain-related suffering.
Trauma and chronic abdominal pain
Trauma is defined as an individual’s response to a threat to safety. Traumatized patients or those with PTSD are at higher risk for chronic abdominal pain.9 Given the strong neurobiological connection between the brain and gut that has been phylogenetically preserved, emotional (e.g., fear, terror) or physical (e.g., pain) signals represent danger, and with chronicity, there can be a kindling-related consolidation of these maladaptive neurobiological pathways leading to suffering (e.g., hopelessness, sense of failure) and disability (Figure 1).

The interrelationship between chronic pain and trauma is multifaceted and is further complicated by the traumatizing nature of chronic pain itself, when pain is interpreted as a signal that the body is sick or even dangerously ill. Patients with chronic abdominal pain may seek multiple medical opinions and often undergo extensive, unnecessary, and sometimes harmful interventions to find the cause of their pain, with fear of disability and even death driving this search for answers.
The degree to which an individual with long-lasting pain interprets their discomfort as a risk to their well-being is related to the degree of trauma they experience because of their pain.10 Indeed, many of the negative symptoms associated with posttraumatic stress are also found in those with chronic abdominal pain. Trauma impacts the fear circuitry centers of the brain, leading to altered activation of the hypothalamic-pituitary-adrenal axis and the amygdala, as well as chronic activation of the sympathetic nervous system and stress-released hormones, all of which are potential pathways that dysregulate the brain-gut relationship.11-13 Worries for safety, which are reactivated by physiological cues (e.g., GI symptoms, pain), as well as avoidance of potential triggers of GI symptoms (e.g., food, exercise, medications, and situations such as travel or scheduled events, and fear of being trapped without bathroom access), are common. Traumatized individuals can experience a foreshortened sense of the future, which may lead to decreased investment in long-term determinants of health (e.g., balanced diet, exercise, social support) and have higher rates of functional impairment and higher health care utilization.14 Negative mood, including irritability, anxiety, depression, insomnia, and impaired concentration are common in those with trauma and chronic pain and can be accompanied by internalized blame (e.g., depression, substance abuse, suicidality) or externalized blame (e.g., negative relationships with health care providers, rejection from their support or faith system). These can be worsened by an impaired sense of trust, which impacts the patient-provider relationship and other sources of social support leading to lack of behavioral activation, anhedonia, and isolation.
Another commonality is hypervigilance, as those with chronic abdominal pain are often hyperaware of physical symptoms and always “on alert” for a signal indicative of a pain flare. Anxiety and depression frequently co-occur in populations with trauma and chronic pain; these diagnoses are associated with higher rates of catastrophizing and learned helplessness, which may be exacerbated by lack of a “cure” for functional gastrointestinal disorders (FGIDs) and chronic pain.15 These factors could potentially lead to lack of engagement with treatment or, alternatively, risky or destructive attempts to cure pain including dangerous complementary alternative treatments or substance abuse to numb sensations. Another feature of trauma in chronic pain is the sense of dissociation from and lack of control over the body, sometimes induced by negative medical experiences (e.g., unwanted physical examinations, medication side effects, traumatic procedures, or hospitalizations).16,17
The importance of treating trauma in the management of chronic pain
Behavioral treatment is increasingly being recognized as an essential component in the management of any chronic pain syndrome.18 The most studied psychosocial interventions for chronic abdominal pain are cognitive-behavioral therapy (CBT) and gut-focused hypnosis. CBT is usually a problem-focused, short-term intervention that can be delivered individually in the office, via group therapy, or through virtual platforms. CBT is most effective when cognitive distortions and ineffective behaviors create emotional distress, and the therapy targets patient’s stress reactivity, visceral anxiety, catastrophizing, and inflexible coping styles.5 Gut-focused hypnosis is the second most–studied behavioral treatment for chronic abdominal pain and utilizes the trance state to make positive suggestions leading to broad and lasting physiological and psychological improvement.19 In addition to pain management, both CBT and hypnosis are efficacious treatments for PTSD.20,21

Utilizing a multidisciplinary medical team including integrated behavioral experts, such as in a patient-centered medical home, is considered the standard of care for treatment of chronic pain. The patient-provider relationship is essential, as is consistent follow-up to ensure effective symptom management and improvements in quality of life. Additionally, patient education, including a positive (i.e., clear) diagnosis and information on the brain-gut relationship, is associated with symptom improvement. In our subspecialty medical home for inflammatory bowel disease (IBD), we found that, in our patients who were on opioids for their chronic pain, engagement with our embedded behavioral and pain specialists resulted in significant reduction in opioid use and depression as well as improved self-reported quality of life.22 Gastroenterologists and advanced-practice providers operating without embedded behavioral therapists can consider referring patients to behavioral treatment (e.g., licensed clinical social workers, licensed professional counselors, marriage and family therapists, psychologists, and psychiatrists; the latter often specialize in medication management and may not offer behavioral therapy) for trauma if patients have undergone a traumatic event (e.g., exposure to any potentially life-threatening event, serious injury, or violence) at any point in their lifetime and are experiencing intrusive symptoms (e.g., memories, dreams, or flashbacks to trauma), avoidance of trauma reminders, and negative mood or hyperarousal related to traumatic events (Table 1).23
With the traumatization component of chronic abdominal pain, which can further drive maladaptive coping cycles, incorporation of trauma-informed treatment into gastroenterology clinics is an avenue toward more effective treatment. The core principles of trauma-informed care include safety, choice, collaboration, trustworthiness, and empowerment,24 and are easily aligned with patient-centered models of care such as the interdisciplinary medical home model. Incorporation of screening techniques, interdisciplinary training of clinicians, and use of behavioral providers with experience in evidenced-based treatments of trauma enhance a clinic’s ability to effectively identify and treat individuals who have trauma because of their abdominal pain.25 Additionally, the most common behavioral interventions for functional gastrointestinal disorders (FGIDs) are also efficacious in the treatment of trauma. CBT is a well-validated treatment for PTSD that utilizes exposure therapy to help individuals restructure negative beliefs shaped by their negative experience and develop relaxation skills. Hypnosis is also validated in the treatment of trauma, with the possible mechanism of action being the replacement of the negative or dissociated traumatic trance with a healthy, adaptive trance facilitated by the hypnotherapist.21
Adjunctive nonopioid medications for chronic abdominal pain
While there are few randomized controlled trials establishing efficacy of pharmacotherapy for sustained improvement of abdominal pain or related suffering, several small trials and consensus clinical expert opinion suggest partial improvement in these domains.26,27 Central neuromodulators that can attenuate chronic visceral pain include antidepressants, antipsychotics, and other central nervous system–targeted medications.26 Tricyclic antidepressants (e.g., amitriptyline, nortriptyline, imipramine, desipramine) are often first-line treatment for FGIDs.28 Serotonin noradrenergic reuptake inhibitors (e.g., duloxetine, venlafaxine, desvenlafaxine, milnacipran) are also effective in pain management. Selective serotonin reuptake inhibitors (e.g., paroxetine, fluoxetine, sertraline, citalopram, escitalopram) can be used, especially when comorbid depression, anxiety, and phobic disorders are present. Tetracyclic antidepressants (e.g., mirtazapine, mianserin, trazodone) are effective treatments for early satiety, nausea/vomiting, insomnia, and low weight. Augmenting agents are utilized when single agents do not provide maximum benefit, including quetiapine (disturbed sleep), bupropion (fatigue), aripiprazole, buspirone, and tandospirone (dyspeptic features and anxiety). Delta ligands including gabapentin and pregabalin are helpful for abdominal wall pain or fibromyalgia. Ketamine is a newer but promising pathway for treatment of pain and depression and is increasingly being utilized in outpatient settings. Additionally, partial opioid-receptor agonists including methadone and suboxone have been reported to decrease pain in addition to their efficacy in addiction recovery. Medical marijuana is another area of growing interest, and while research has yet to show a clear effect in pain management, it does appear helpful in nausea and appetite stimulation. Obtaining a therapeutic response is the first treatment goal, after which a patient should be monitored in at least 6-month intervals to ensure sustained benefits and tolerability, and if these are not met, enhancement of treatment or a slow taper is indicated. As in all treatments, a positive patient-provider relationship predicts improved treatment adherence and outcomes.26 However, while these pharmacological interventions can reduce symptom severity, there is little evidence that they reduce traumatization without adjunctive psychotherapy.29
Summary
Both behavioral and pharmacological treatment options are available for chronic abdominal pain and most useful if traumatic manifestations are assessed and included as treatment targets. A multidisciplinary approach to the treatment of chronic abdominal pain with increased screening and treatment of trauma is a promising pathway to improved care and management for patients with chronic pain. If trauma is left untreated, the benefits of otherwise effective treatments are likely to be significantly limited.
References
1. Apkarian AV et al. Prog Neurobiol. 2009 Feb;87(2):81-97.
2. Gallagher RM et al. Pain Med. 2011 Jan;12(1):1-2.
3. Collier R et al. CMAJ. 2018 Jan 8;190(1):E26-7. doi: 10.1503/cmaj.109-5523.
4. Szigethy E et al. Nature Reviews Gastroenterology & Hepatology, 2018;15:168-80.
5. Ballou S et al. Clin Transl Gastroenterol. 2017 Jan;8(1):e214.
6. Egloff N et al. J Pain Res. 2013 Nov 5;6:765-70.
7. Fashler S et al. J Pain Res. 2016 Aug 10;9:551-61.
8. McKernan LC et al. Clin J Pain. 2019 May;35(5):385-93.
9. Ju T et al. J Clin Gastroenterol. 2018 Dec 19. doi: 10.1097/MCG.0000000000001153.
10. Fishbain DA et al. Pain Med. 2017 Apr 1;18(4):711-35.
11. Martin CR et al. Cell Mol Gastroenterol Hepatol. 2018;6(2):133-48.
12. Osadchiy V et al. Clin Gastroenterol Hepatol. 2019 Jan;17(2):322-32.
13. Brzozowski B et al. Curr Neuropharmacol. 2016 Nov;14(8):892-900.
14. Outclat SD et al. Pain Med. 2014;15(11):1872-9.
15. Asmundson GJ et al. Can J Psychiatry. 2002;Dec;47(10):930-7.
16. Taft TH et al. Inflamm Bowel Dis. 2019 Mar 7. doi: 10.1093/ibd/izz032.
17. Duckworth MP et al. International Journal of Rehabilitation and Health, 2000 Apr;5(2):129-39.
18. Scascighini L et al. Rheumatology (Oxford). 2008 May;47(5):670-8.
19. Palsson O et al. European Gastroenterology & Hepatology Review. 2010;6(1):42-6.
20. Watkins LE et al. Frontiers in Behavioral Neuroscience. 2018;12:1-9.
21. O’Toole SK et al. J Trauma Stress. 2016 Feb;29(1):97-100.
22. Goldblum Y et al. Digestive Disease Week. San Diego. 2019. Abstract in press.
23. American Psychiatric Association. Diagnostic and Statistical Manual (of Mental Disorders), Fifth Edition. Arlington, Va: American Psychiatric Publishing, 2013.
24. United States Department of Health and Human Services, Substance Abuse and Mental Health Services Administration. 2018. Trauma-informed approach and trauma-specific interventions. Retrieved from samhsa.gov/nctic/trauma-interventions.
25. Click BH et al. Inflamm Bowel Dis. 2017;23(5):681-94.
26. Drossman DA et al. Gastroenterology. 2018 Mar;154(4):1140-71.
27. Thorkelson G et al. Inflamm Bowel Dis. 2016 Jun 1;22(6):1509-22.
28. Törnblom H et al. Current Gastroenterology Reports. 2018;20(12):58.
29. Watkins LE et al. Front Behav Neurosci. 2018;12:258.
30. American Psychiatric Association. Clinical Practice Guideline for the Treatment of Posttraumatic Stress Disorder (PTSD) in Adults. 2017.
31. Bisson JI et al. Cochrane Database Syst Rev. 2013 Dec 13;(12):CD003388.
32. Department of Veterans Affairs and Department of Defense. VA/DOD clinical practice guideline for the management of posttraumatic stress disorder and acute stress disorder. 2017.
33. Karatzias T et al. Psychol Med. 2019 Mar 12:1-15. doi: 10.1017/S0033291719000436. Advance online publication.
Emily Weaver, LCSW, is a UPMC Total Care–IBD program senior social worker, Eva Szigethy, MD, PhD, is professor of psychiatry and medicine, codirector, IBD Total Care Medical Home, University of Pittsburgh Medical Center, departments of medicine and psychiatry.
New concepts in the management of acute pancreatitis
Introduction
Acute pancreatitis (AP) is a major clinical and financial burden in the United States. Several major clinical guidelines provide evidence-based recommendations for the clinical management decisions in AP, including those from the American College of Gastroenterology (ACG; 2013),1 and the International Association of Pancreatology (IAP; 2013).2 More recently, the American Gastroenterological Association (AGA) released their own set of guidelines.3,4 In this update on AP, we review these guidelines and reference recent literature focused on epidemiology, risk factors, etiology, diagnosis, risk stratification, and recent advances in the early medical management of AP. Regarding the latter, we review six treatment interventions (pain management, intravenous fluid resuscitation, feeding, prophylactic antibiotics, probiotics, and timing of endoscopic retrograde cholangiopancreatography (ERCP) in acute biliary pancreatitis) and four preventive interventions (alcohol and smoking cessation, same-admission cholecystectomy for acute biliary pancreatitis, and chemoprevention and fluid administration for post-ERCP pancreatitis [PEP]). Updates on multidisciplinary management of (infected) pancreatic necrosis is beyond the scope of this review. Table 1 summarizes the concepts discussed in this article.

Recent advances in epidemiology and evaluation of AP
Epidemiology
AP is the third most common cause of gastrointestinal-related hospitalizations and fourth most common cause of readmission in 2014.5 Recent epidemiologic studies show conflicting trends for the incidence of AP, both increasing6 and decreasing,7 likely attributable to significant differences in study designs. Importantly, multiple studies have demonstrated that hospital length of stay, costs, and mortality have declined since 2009.6,8-10
Persistent organ failure (POF), defined as organ failure lasting more than 48 hours, is the major cause of death in AP. POF, if only a single organ during AP, is associated with 27%-36% mortality; if it is multiorgan, it is associated with 47% mortality.1,11 Other factors associated with increased hospital mortality include infected pancreatic necrosis,12-14 diabetes mellitus,15 hospital-acquired infection,16 advanced age (70 years and older),17 and obesity.18 Predictive factors of 1-year mortality include readmission within 30 days, higher Charlson Comorbidity Index, and longer hospitalization.19
Risk factors
We briefly highlight recent insights into risk factors for AP (Table 1) and refer to a recent review for further discussion.20 Current and former tobacco use are independent risk factors for AP.21 The dose-response relationship of alcohol to the risk of pancreatitis is complex,22 but five standard drinks per day for 5 years is a commonly used cut-off.1,23 New evidence suggests that the relationship between the dose of alcohol and risk of AP differs by sex, linearly in men but nonlinearly (J-shaped) in women.24 Risk of AP in women was decreased with alcohol consumption of up to 40 g/day (one standard drink contains 14 g of alcohol) and increased above this amount. Cannabis is a possible risk factor for toxin-induced AP and abstinence appears to abolish risk of recurrent attacks.25
Patients with inflammatory bowel disease (IBD) have a 2.9-fold higher risk for AP versus non-IBD cohorts26 with the most common etiologies are from gallstones and medications.27 In patients with end-stage renal disease (ESRD), the risk of AP is higher in those who receive peritoneal dialysis, compared with hemodialysis28-33 and who are women, older, or have cholelithiasis or liver disease.34As recently reviewed,35 pancreatic cancer appears to be associated with first-attack pancreatitis with few exceptions.36 In this setting, the overall incidence of pancreatic cancer is low (1.5%). The risk is greatest within the first year of the attack of AP, negligible below age 40 years but steadily rising through the fifth to eighth decades.37 Pancreatic cancer screening is a conditional recommendation of the ACG guidelines in patients with unexplained AP, particularly those aged 40 years or older.1
Etiology and diagnosis
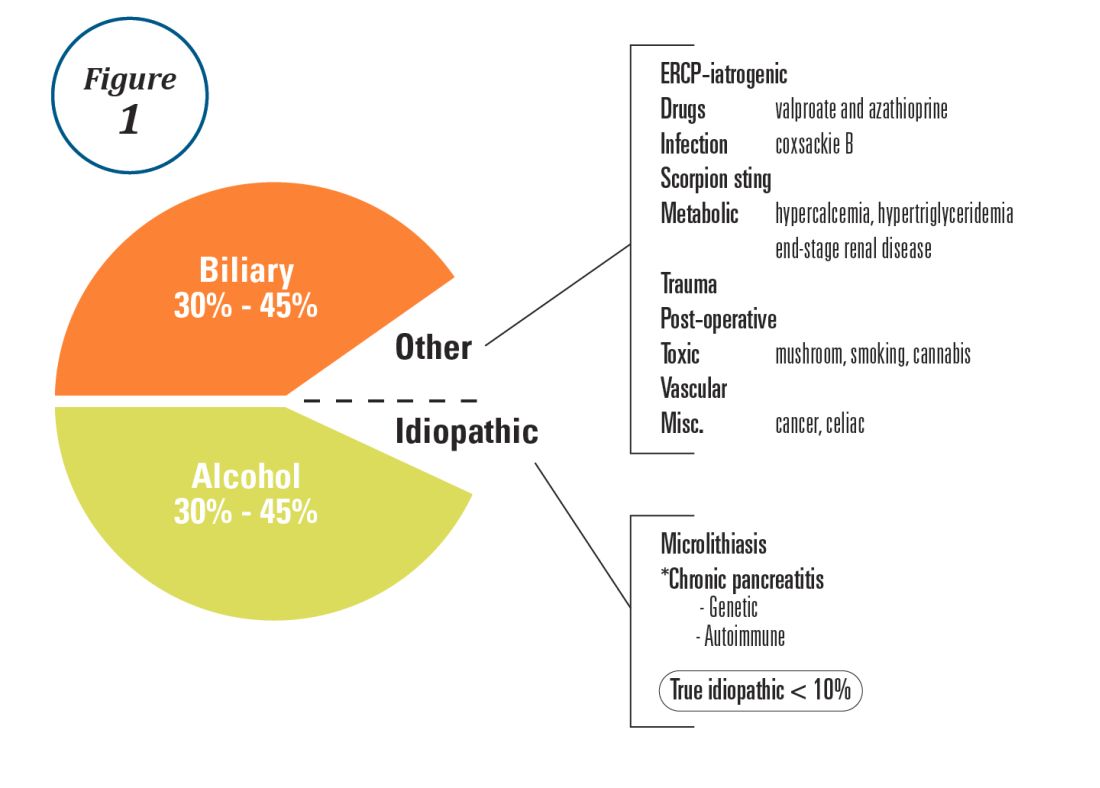
Alcohol and gallstones remain the most prevalent etiologies for AP.1 While hypertriglyceridemia accounted for 9% of AP in a systematic review of acute pancreatitis in 15 different countries,38 it is the second most common cause of acute pancreatitis in Asia (especially China).39 Figure 1 provides a breakdown of the etiologies and risk factors of pancreatitis. Importantly, it remains challenging to assign several toxic-metabolic etiologies as either a cause or risk factor for AP, particularly with regards to alcohol, smoking, and cannabis to name a few.
Guidelines and recent studies of AP raise questions about the threshold above which hypertriglyceridemia causes or poses as an important cofactor for AP. American and European societies define the threshold for triglycerides at 885-1,000 mg/dL.1,42,43 Pedersen et al. provide evidence of a graded risk of AP with hypertriglyceridemia: In multivariable analysis, adjusted hazard ratios for AP were much higher with nonfasting mild to moderately elevated plasma triglycerides (177-885 mg/dL), compared with normal values (below 89 mg/dL).44 Moreover, the risk of severe AP (developing POF) increases in proportion to triglyceride value, independent of the underlying cause of AP.45

Diagnosis of AP is derived from the revised Atlanta classification.46 The recommended timing and indications for offering cross-sectional imaging are after 48-72 hours in patients with no improvement to initial care.1 Endoscopic ultrasonography (EUS) has better diagnostic accuracy and sensitivity, compared with magnetic resonance cholangiopancreatography (MRCP) for choledocholithiasis, and has comparable specificity.47,48 Among noninvasive imaging modalities, MRCP is more sensitive than computed tomography (CT) for diagnosing choledocholithiasis.49 Despite guideline recommendations for more selective use of pancreatic imaging in the early assessment of AP, utilization of early CT or MRCP imaging (within the first 24 hours of care) remained high during 2014-2015, compared with 2006-2007.50
ERCP is not recommended as a pure diagnostic tool, owing to the availability of other diagnostic tests and a complication rate of 5%-10% with risks involving PEP, cholangitis, perforation, and hemorrhage.51 A recent systematic review of EUS and ERCP in acute biliary pancreatitis concluded that EUS had lower failure rates and had no complications, and the use of EUS avoided ERCP in 71.2% of cases.52
Risk stratification
The goals of using risk stratification tools in AP are to identify patients at risk for developing major outcomes, including POF, infected pancreatic necrosis, and death, and to ensure timely triaging of patients to an appropriate level of care. Existing prediction models have only moderate predictive value.53,54 Examples include simple risk stratification tools such as blood urea nitrogen (BUN) and hemoconcentration,55,56 disease-modifying patient variables (age, obesity, etc.), biomarkers (i.e., angiopoietin 2),57 and more complex clinical scoring systems such as Acute Physiology and Chronic Health Evaluation II (APACHE II), BISAP (BUN, impaired mental status, SIRS criteria, age, pleural effusion) score, early warning system (EWS), Glasgow-Imrie score, Japanese severity score, and recently the Pancreatitis Activity Scoring System (PASS).58 Two recent guidelines affirmed the importance of predicting the severity of AP, using one or more predictive tools.1,2 The recent 2018 AGA technical review does not debate this commonsense approach, but does highlight that there is no published observational study or randomized, controlled trial (RCT) investigating whether prediction tools affect clinical outcomes.4
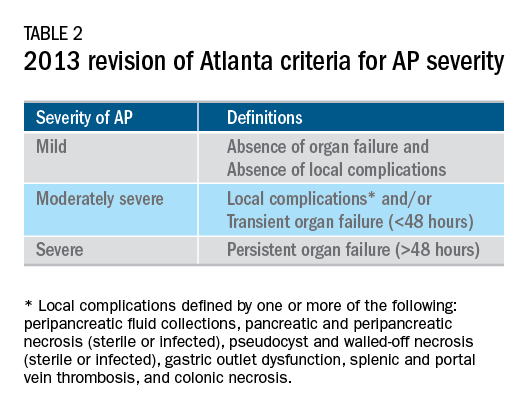
Recent advances in early treatment of AP
Literature review and definitions
The AP literature contains heterogeneous definitions of severe AP and of what constitutes a major outcome in AP. Based on definitions of the 2013 revised Atlanta Criteria, the 2018 AGA technical review and clinical guidelines emphasized precise definitions of primary outcomes of clinical importance in AP, including death, persistent single organ failure, or persistent multiple organ failure, each requiring a duration of more than 48 hours, and infected pancreatic or peripancreatic necrosis or both (Table 2).3,4
Pain management
Management of pain in AP is complex and requires a detailed discussion beyond the scope of this review, but recent clinical and translational studies raise questions about the current practice of using opioids for pain management in AP. A provocative, multicenter, retrospective cohort study reported lower 30-day mortality among critically ill patients who received epidural analgesia versus standard care without epidural analgesia.59 The possible mechanism of protection and the drugs administered are unclear. An interesting hypothesis is that the epidural cohort may have received lower exposure to morphine, which may increase gut permeability, the risk of infectious complications, and severity of AP, based on a translational study in mice.60
Intravenous fluid administration
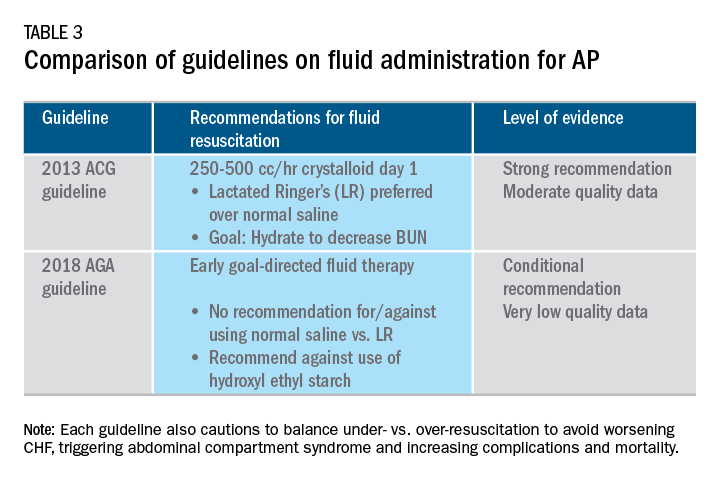
Supportive care with the use of IV fluid hydration is a mainstay of treatment for AP in the first 12-24 hours. Table 3 summarizes the guidelines in regards to IV fluid administration as delineated by the ACG and AGA guidelines on the management of pancreatitis.1,3 Guidelines advocate for early fluid resuscitation to correct intravascular depletion in order to reduce morbidity and mortality associated with AP.1,2,4 The 2018 AGA guidelines endorse a conditional recommendation for using goal-directed therapy for initial fluid management,3 do not recommend for or against normal saline versus lactated Ringer’s (LR), but do advise against the use of hydroxyethyl starch fluids.3 Consistent with these recommendations, two recent RCTs published subsequent to the prespecified time periods of the AGA technical review and guideline, observed no significant differences between LR and normal saline on clinically meaningful outcomes.61,62 The AGA guidelines acknowledge that evidence was of very-low quality in support of goal-directed therapy,3,4 which has not been shown to have a significant reduction in persistent multiple organ failure, mortality, or pancreatic necrosis, compared with usual care. As the authors noted, interpretation of the data was limited by the absence of other critical outcomes in these trials (infected pancreatic necrosis), lack of uniformity of specific outcomes and definitions of transient and POF, few trials, and risk of bias. There is a clear need for a large RCT to provide evidence to guide decision making with fluid resuscitation in AP, particularly in regard to fluid type, volume, rate, duration, endpoints, and clinical outcomes.
Feeding
More recently, the focus of nutrition in the management of AP has shifted away from patients remaining nil per os (NPO). Current guidelines advocate for early oral feeding (within 24 hours) in mild AP,3,4 in order to protect the gut-mucosal barrier. Remaining NPO when compared with early oral feeding has a 2.5-fold higher risk for interventions for necrosis.4 The recently published AGA technical review identified no significant impact on outcomes of early versus delayed oral feeding, which is consistent with observations of a landmark Dutch PYTHON trial entitled “Early versus on-demand nasoenteric tube feeding in acute pancreatitis.”4,63 There is no clear cutoff point for initiating feeding for those with severe AP. A suggested practical approach is to initiate feeding within 24-72 hours and offer enteral nutrition for those intolerant to oral feeds. In severe AP and moderately severe AP, enteral nutrition is recommended over parenteral nutrition.3,4 Enteral nutrition significantly reduces the risk of infected peripancreatic necrosis, single organ failure, and multiorgan failure.4 Finally, the AGA guidelines provide a conditional recommendation for providing enteral nutrition support through either the nasogastric or nasoenteric route.3 Further studies are required to determine the optimal timing, rate, and formulation of enteral nutrition in severe AP.
Antibiotics and probiotics
Current guidelines do not support the use of prophylactic antibiotics to prevent infection in necrotizing AP and severe AP.1-3 The AGA technical review reported that prophylactic antibiotics did not reduce infected pancreatic or peripancreatic necrosis, persistent single organ failure, or mortality.4 Guidelines advocate against the use of probiotics for severe AP, because of increased mortality risk.1
Timing of ERCP in acute biliary pancreatitis
There is universal agreement for offering urgent ERCP (within 24 hours) in biliary AP complicated by cholangitis.1-3,64 Figure 2 demonstrates an example of a cholangiogram completed within 24 hours of presentation of biliary AP complicated by cholangitis.
In the absence of cholangitis, the timing of ERCP for AP with persistent biliary obstruction is less clear.1-3 In line with recent guidelines, the 2018 AGA guidelines advocate against routine use of urgent ERCP for biliary AP without cholangitis,3 a conditional recommendation with overall low quality of data.4 The AGA technical review found that urgent ERCP, compared with conservative management in acute biliary pancreatitis without cholangitis had no significant effect on mortality, organ failure, infected pancreatic necrosis, and total necrotizing pancreatitis, but did significantly shorten hospital length of stay.4 There are limited data to guide decision making of when nonurgent ERCP should be performed in hospitalized patients with biliary AP with persistent obstruction and no cholangitis.3,64
Alcohol and smoking cessation
The AGA technical review advocates for brief alcohol intervention during hospitalization for alcohol-induced AP on the basis of one RCT that addresses the impact of alcohol counseling on recurrent bouts of AP4 plus evidence from a Cochrane review of alcohol-reduction strategies in primary care populations.65 Cessation of smoking – an established independent risk factor of AP – recurrent AP and chronic pancreatitis, should also be recommended as part of the management of AP.
Cholecystectomy
Evidence supports same-admission cholecystectomy for mild gallstone AP, a strong recommendation of published AGA guidelines.3 When compared with delayed cholecystectomy, same-admission cholecystectomy significantly reduced gallstone-related complications, readmissions for recurrent pancreatitis, and pancreaticobiliary complications, without having a significant impact on mortality during a 6-month follow-up period.66 Delaying cholecystectomy 6 weeks in patients with moderate-severe gallstone AP appears to reduce morbidity, including the development of infected collections, and mortality.4 An ongoing RCT, the APEC trial, aims to determine whether early ERCP with biliary sphincterotomy reduces major complications or death when compared with no intervention for biliary AP in patients at high risk of complications.67
Chemoprevention and IV fluid management of post-ERCP pancreatitis
Accumulating data support the effectiveness of chemoprevention, pancreatic stent placement, and fluid administration to prevent post-ERCP pancreatitis. Multiple RCTs, meta-analyses, and systematic reviews indicate that rectal NSAIDs) reduce post-ERCP pancreatitis onset68-71 and moderate-severe post-ERCP pancreatitis. Additionally, placement of a pancreatic duct stent may decrease the risk of severe post-ERCP pancreatitis in high-risk patients.3 Guidelines do not comment on fluid administrations for prevention of post-ERCP pancreatitis, but studies have shown that greater periprocedural IV fluid was an independent protective factor against moderate to severe PEP72 and was associated with shorter hospital length of stay.73 Recent meta-analyses and RCTs support using LR prior to ERCP to prevent PEP.74-77 Interestingly, a recent RCT shows that the combination of rectal indomethacin and LR, compared with combination placebo and normal saline reduced the risk of PEP in high-risk patients.78
Two ongoing multicenter RCTs will clarify the role of combination therapy. The Dutch FLUYT RCT aims to determine the optimal combination of rectal NSAIDs and periprocedural infusion of IV fluids to reduce the incidence of PEP and moderate-severe PEP79 and the Stent vs. Indomethacin (SVI) trial aims to determine the whether combination pancreatic stent placement plus rectal indomethacin is superior to monotherapy indomethacin for preventing post-ERCP pancreatitis in high-risk cases.80
Implications for clinical practice
The diagnosis and optimal management of AP require a systematic approach with multidisciplinary decision making. Morbidity and mortality in AP are driven by early or late POF, and the latter often is triggered by infected necrosis. Risk stratification of these patients at the point of contact is a commonsense approach to enable triaging of patients to the appropriate level of care. Regardless of pancreatitis severity, recommended treatment interventions include goal-directed IV fluid resuscitation, early feeding by mouth or enteral tube when necessary, avoidance of prophylactic antibiotics, avoidance of probiotics, and urgent ERCP for patients with acute biliary pancreatitis complicated by cholangitis. Key measures for preventing hospital readmission and pancreatitis include same-admission cholecystectomy for acute biliary pancreatitis and alcohol and smoking cessation. Preventive measures for post-ERCP pancreatitis in patients undergoing ERCP include rectal indomethacin, prophylactic pancreatic duct stent placement, and periprocedural fluid resuscitation.
Dr. Mandalia is a fellow, gastroenterology, department of internal medicine, division of gastroenterology, Michigan Medicine, Ann Arbor; Dr. DiMagno is associate professor of medicine, director, comprehensive pancreas program, department of internal medicine, division of gastroenterology, University of Michigan, Ann Arbor. Dr. Mandalia reports no conflicts of interest.
References
1. Tenner S et al. Am J Gastroenterol. 2013;108:1400.
2. Besseline M et al. Pancreatology. 2013;13(4, Supplement 2):e1-15.
3. Crockett SD et al. Gastroenterology. 2018;154(4):1096-101.
4. Vege SS et al. Gastroenterology. 2018;154(4):1103-39.
5. Peery AF et al. Gastroenterology. 2019 Jan;156(1):254-72.e11.
6. Krishna SG et al. Pancreas. 2017;46(4):482-8.
7. Sellers ZM et al. Gastroenterology. 2018;155(2):469-78.e1.
8. Brown A et al. JOP. 2008;9(4):408-14.
9. Fagenholz PJ et al. Ann Epidemiol. 2007;17(7):491.e1-.e8.
10. McNabb-Baltar J et al. Pancreas. 2014;43(5):687-91.
11. Johnson CD et al. Gut. 2004;53(9):1340-4.
12. Dellinger EP et al. Ann Surg. 2012;256(6):875-80.
13. Petrov MS et al. Gastroenterology. 2010;139(3):813-20.
14. Sternby H et al. Ann Surg. Apr 18. doi: 10.1097/SLA.0000000000002766.
15. Huh JH et al. J Clin Gastroenterol. 2018;52(2):178-83.
16. Wu BU et al. Gastroenterology. 2008;135(3):816-20.
17. Gardner TB et al. Clin Gastroenterol Hepatol. 2008;6(10):1070-6.
18. Krishna SG et al. Am J Gastroenterol. 2015;110(11):1608-19.
19. Lee PJ et al. Pancreas. 2016;45(4):561-4.
20. Mandalia A et al. F1000Research. 2018 Jun 28;7.
21. Majumder S et al. Pancreas. 2015;44(4):540-6.
22. DiMagno MJ. Clin Gastroenterol Hepatol. 2011;9(11):920-2.
23. Yadav D, Whitcomb DC. Nature Rev Gastroenterol Hepatol. 2010;7(3):131-45.
24. Samokhvalov AV et al. EBioMedicine. 2015;2(12):1996-2002.
25. Barkin JA et al. Pancreas. 2017;46(8):1035-8.
26. Chen Y-T et al. J Gastroenterol Hepatol. 2016;31(4):782-7.
27. Ramos LR et al. J Crohns Colitis. 2016;10(1):95-104.
28. Avram MM. Nephron. 1977;18(1):68-71.
29. Lankisch PG et al. Nephrol Dial Transplant. 2008;23(4):1401-5.
30. Owyang C et al. Mayo Clin Proc. 1979;54(12):769-73.
31. Owyang Cet al. Gut. 1982;23(5):357-61.
32. Quraishi ER et al. Am J Gastroenterol. 2005;100:2288.
33. Vaziri ND et al. Nephron. 1987;46(4):347-9.
34. Chen HJ et al. Nephrol Dial Transplant. 2017;32(10):1731-6.
35. Kirkegard J et al. Gastroenterology. 2018;May;154(6):1729-36.
36. Karlson BM, et al. Gastroenterology. 1997;113(2):587-92.
37. Munigala S et al. Clin Gastroenterol Hepatol. 2014;12(7):1143-50.e1.
38. Carr RA et al. Pancreatology. 2016;16(4):469-76.
39. Li X et al. BMC Gastroenterol. 2018;18(1):89.
40. Ahmed AU et al. Clin Gastroenterol Hepatol. 2016;14(5):738-46.
41. Sankaran SJ et al. Gastroenterology. 2015;149(6):1490-500.e1.
42. Berglund L et al. J Clin Endocrinol Metab. 2012;97(9):2969-89.
43. Catapano AL et al. Atherosclerosis. 2011;217(1):3-46.
44. Pedersen SB et al. JAMA Intern Med. 2016;176(12):1834-42.
45. Nawaz H et al. Am J Gastroenterol. 2015;110(10):1497-503.
46. Banks PA et al. Gut. 2013;62(1):102-11.
47. Kondo S et al. Eur J Radiol. 2005;54(2):271-5.
48. Meeralam Y et al. Gastrointest Endosc. 2017;86(6):986-93.
49. Stimac D et al. Am J Gastroenterol. 2007;102(5):997-1004.
50. Jin DX et al. Dig Dis Sci. 2017;62(10):2894-9.
51. Freeman ML. Gastrointest Endosc Clin N Am. 2012;22(3):567-86.
52. De Lisi S et al. Eur J Gastroenterol Hepatol. 2011;23(5):367-74.
53. Di MY et al. Ann Int Med. 2016;165(7):482-90.
54. Mounzer R et al. Gastroenterology. 2012;142(7):1476-82; quiz e15-6.
55. Koutroumpakis E et al. Am J Gastroenterol. 2015;110(12):1707-16.
56. Wu BU et al. Gastroenterology. 2009;137(1):129-35.
57. Buddingh KT et al. J Am Coll Surg. 2014;218(1):26-32.
58. Buxbaum J et al. Am J Gastroenterol. 2018;113(5):755-64.
59. Jabaudon M et al. Crit Car Med. 2018;46(3):e198-e205.
60. Barlass U et al. Gut. 2018;67(4):600-2.
61. Buxbaum JL et al. Am J Gastroenterol. 2017;112(5):797-803.
62. de-Madaria E et al. United Eur Gastroenterol J. 2018;6(1):63-72.
63. Bakker OJ et al. N Engl J Med. 2014;371(21):1983-93.
64. Tse F et al. Cochrane Database Syst Rev. 2012(5):Cd009779.
65. Kaner EFS et al. Cochrane Database Syst Rev. 2007(2):Cd004148.
66. da Costa DW et al. Lancet. 2015;386(10000):1261-8.
67. Schepers NJ et al. Trials. 2016;17:5.
68. Vadala di Prampero SF et al. Eur J Gastroenterol Hepatol. 2016;28(12):1415-24.
69. Kubiliun NM et al. Clin Gastroenterol Hepatol. 2015;13(7):1231-9; quiz e70-1.
70. Wan J et al. BMC Gastroenterol. 2017;17(1):43.
71. Yang C et al. Pancreatology. 2017;17(5):681-8.
72. DiMagno MJ et al. Pancreas. 2014;43(4):642-7.
73. Sagi SV et al. J Gastroenterol Hepatol. 2014;29(6):1316-20.
74. Choi JH et al. Clin Gastroenterol Hepatol. 2017;15(1):86-92.e1.
75. Wu D et al. J Clin Gastroenterol. 2017;51(8):e68-e76.
76. Zhang ZF et al. J Clin Gastroenterol. 2017;51(3):e17-e26.
77. Park CH et al. Endoscopy 2018 Apr;50(4):378-85.
78. Mok SRS et al. Gastrointest Endosc. 2017;85(5):1005-13.
79. Smeets XJN et al. Trials. 2018;19(1):207.
80. Elmunzer BJ et al. Trials. 2016;17(1):120.
Introduction
Acute pancreatitis (AP) is a major clinical and financial burden in the United States. Several major clinical guidelines provide evidence-based recommendations for the clinical management decisions in AP, including those from the American College of Gastroenterology (ACG; 2013),1 and the International Association of Pancreatology (IAP; 2013).2 More recently, the American Gastroenterological Association (AGA) released their own set of guidelines.3,4 In this update on AP, we review these guidelines and reference recent literature focused on epidemiology, risk factors, etiology, diagnosis, risk stratification, and recent advances in the early medical management of AP. Regarding the latter, we review six treatment interventions (pain management, intravenous fluid resuscitation, feeding, prophylactic antibiotics, probiotics, and timing of endoscopic retrograde cholangiopancreatography (ERCP) in acute biliary pancreatitis) and four preventive interventions (alcohol and smoking cessation, same-admission cholecystectomy for acute biliary pancreatitis, and chemoprevention and fluid administration for post-ERCP pancreatitis [PEP]). Updates on multidisciplinary management of (infected) pancreatic necrosis is beyond the scope of this review. Table 1 summarizes the concepts discussed in this article.

Recent advances in epidemiology and evaluation of AP
Epidemiology
AP is the third most common cause of gastrointestinal-related hospitalizations and fourth most common cause of readmission in 2014.5 Recent epidemiologic studies show conflicting trends for the incidence of AP, both increasing6 and decreasing,7 likely attributable to significant differences in study designs. Importantly, multiple studies have demonstrated that hospital length of stay, costs, and mortality have declined since 2009.6,8-10
Persistent organ failure (POF), defined as organ failure lasting more than 48 hours, is the major cause of death in AP. POF, if only a single organ during AP, is associated with 27%-36% mortality; if it is multiorgan, it is associated with 47% mortality.1,11 Other factors associated with increased hospital mortality include infected pancreatic necrosis,12-14 diabetes mellitus,15 hospital-acquired infection,16 advanced age (70 years and older),17 and obesity.18 Predictive factors of 1-year mortality include readmission within 30 days, higher Charlson Comorbidity Index, and longer hospitalization.19
Risk factors
We briefly highlight recent insights into risk factors for AP (Table 1) and refer to a recent review for further discussion.20 Current and former tobacco use are independent risk factors for AP.21 The dose-response relationship of alcohol to the risk of pancreatitis is complex,22 but five standard drinks per day for 5 years is a commonly used cut-off.1,23 New evidence suggests that the relationship between the dose of alcohol and risk of AP differs by sex, linearly in men but nonlinearly (J-shaped) in women.24 Risk of AP in women was decreased with alcohol consumption of up to 40 g/day (one standard drink contains 14 g of alcohol) and increased above this amount. Cannabis is a possible risk factor for toxin-induced AP and abstinence appears to abolish risk of recurrent attacks.25
Patients with inflammatory bowel disease (IBD) have a 2.9-fold higher risk for AP versus non-IBD cohorts26 with the most common etiologies are from gallstones and medications.27 In patients with end-stage renal disease (ESRD), the risk of AP is higher in those who receive peritoneal dialysis, compared with hemodialysis28-33 and who are women, older, or have cholelithiasis or liver disease.34As recently reviewed,35 pancreatic cancer appears to be associated with first-attack pancreatitis with few exceptions.36 In this setting, the overall incidence of pancreatic cancer is low (1.5%). The risk is greatest within the first year of the attack of AP, negligible below age 40 years but steadily rising through the fifth to eighth decades.37 Pancreatic cancer screening is a conditional recommendation of the ACG guidelines in patients with unexplained AP, particularly those aged 40 years or older.1
Etiology and diagnosis

Alcohol and gallstones remain the most prevalent etiologies for AP.1 While hypertriglyceridemia accounted for 9% of AP in a systematic review of acute pancreatitis in 15 different countries,38 it is the second most common cause of acute pancreatitis in Asia (especially China).39 Figure 1 provides a breakdown of the etiologies and risk factors of pancreatitis. Importantly, it remains challenging to assign several toxic-metabolic etiologies as either a cause or risk factor for AP, particularly with regards to alcohol, smoking, and cannabis to name a few.
Guidelines and recent studies of AP raise questions about the threshold above which hypertriglyceridemia causes or poses as an important cofactor for AP. American and European societies define the threshold for triglycerides at 885-1,000 mg/dL.1,42,43 Pedersen et al. provide evidence of a graded risk of AP with hypertriglyceridemia: In multivariable analysis, adjusted hazard ratios for AP were much higher with nonfasting mild to moderately elevated plasma triglycerides (177-885 mg/dL), compared with normal values (below 89 mg/dL).44 Moreover, the risk of severe AP (developing POF) increases in proportion to triglyceride value, independent of the underlying cause of AP.45

Diagnosis of AP is derived from the revised Atlanta classification.46 The recommended timing and indications for offering cross-sectional imaging are after 48-72 hours in patients with no improvement to initial care.1 Endoscopic ultrasonography (EUS) has better diagnostic accuracy and sensitivity, compared with magnetic resonance cholangiopancreatography (MRCP) for choledocholithiasis, and has comparable specificity.47,48 Among noninvasive imaging modalities, MRCP is more sensitive than computed tomography (CT) for diagnosing choledocholithiasis.49 Despite guideline recommendations for more selective use of pancreatic imaging in the early assessment of AP, utilization of early CT or MRCP imaging (within the first 24 hours of care) remained high during 2014-2015, compared with 2006-2007.50
ERCP is not recommended as a pure diagnostic tool, owing to the availability of other diagnostic tests and a complication rate of 5%-10% with risks involving PEP, cholangitis, perforation, and hemorrhage.51 A recent systematic review of EUS and ERCP in acute biliary pancreatitis concluded that EUS had lower failure rates and had no complications, and the use of EUS avoided ERCP in 71.2% of cases.52
Risk stratification
The goals of using risk stratification tools in AP are to identify patients at risk for developing major outcomes, including POF, infected pancreatic necrosis, and death, and to ensure timely triaging of patients to an appropriate level of care. Existing prediction models have only moderate predictive value.53,54 Examples include simple risk stratification tools such as blood urea nitrogen (BUN) and hemoconcentration,55,56 disease-modifying patient variables (age, obesity, etc.), biomarkers (i.e., angiopoietin 2),57 and more complex clinical scoring systems such as Acute Physiology and Chronic Health Evaluation II (APACHE II), BISAP (BUN, impaired mental status, SIRS criteria, age, pleural effusion) score, early warning system (EWS), Glasgow-Imrie score, Japanese severity score, and recently the Pancreatitis Activity Scoring System (PASS).58 Two recent guidelines affirmed the importance of predicting the severity of AP, using one or more predictive tools.1,2 The recent 2018 AGA technical review does not debate this commonsense approach, but does highlight that there is no published observational study or randomized, controlled trial (RCT) investigating whether prediction tools affect clinical outcomes.4

Recent advances in early treatment of AP
Literature review and definitions
The AP literature contains heterogeneous definitions of severe AP and of what constitutes a major outcome in AP. Based on definitions of the 2013 revised Atlanta Criteria, the 2018 AGA technical review and clinical guidelines emphasized precise definitions of primary outcomes of clinical importance in AP, including death, persistent single organ failure, or persistent multiple organ failure, each requiring a duration of more than 48 hours, and infected pancreatic or peripancreatic necrosis or both (Table 2).3,4
Pain management
Management of pain in AP is complex and requires a detailed discussion beyond the scope of this review, but recent clinical and translational studies raise questions about the current practice of using opioids for pain management in AP. A provocative, multicenter, retrospective cohort study reported lower 30-day mortality among critically ill patients who received epidural analgesia versus standard care without epidural analgesia.59 The possible mechanism of protection and the drugs administered are unclear. An interesting hypothesis is that the epidural cohort may have received lower exposure to morphine, which may increase gut permeability, the risk of infectious complications, and severity of AP, based on a translational study in mice.60
Intravenous fluid administration

Supportive care with the use of IV fluid hydration is a mainstay of treatment for AP in the first 12-24 hours. Table 3 summarizes the guidelines in regards to IV fluid administration as delineated by the ACG and AGA guidelines on the management of pancreatitis.1,3 Guidelines advocate for early fluid resuscitation to correct intravascular depletion in order to reduce morbidity and mortality associated with AP.1,2,4 The 2018 AGA guidelines endorse a conditional recommendation for using goal-directed therapy for initial fluid management,3 do not recommend for or against normal saline versus lactated Ringer’s (LR), but do advise against the use of hydroxyethyl starch fluids.3 Consistent with these recommendations, two recent RCTs published subsequent to the prespecified time periods of the AGA technical review and guideline, observed no significant differences between LR and normal saline on clinically meaningful outcomes.61,62 The AGA guidelines acknowledge that evidence was of very-low quality in support of goal-directed therapy,3,4 which has not been shown to have a significant reduction in persistent multiple organ failure, mortality, or pancreatic necrosis, compared with usual care. As the authors noted, interpretation of the data was limited by the absence of other critical outcomes in these trials (infected pancreatic necrosis), lack of uniformity of specific outcomes and definitions of transient and POF, few trials, and risk of bias. There is a clear need for a large RCT to provide evidence to guide decision making with fluid resuscitation in AP, particularly in regard to fluid type, volume, rate, duration, endpoints, and clinical outcomes.
Feeding
More recently, the focus of nutrition in the management of AP has shifted away from patients remaining nil per os (NPO). Current guidelines advocate for early oral feeding (within 24 hours) in mild AP,3,4 in order to protect the gut-mucosal barrier. Remaining NPO when compared with early oral feeding has a 2.5-fold higher risk for interventions for necrosis.4 The recently published AGA technical review identified no significant impact on outcomes of early versus delayed oral feeding, which is consistent with observations of a landmark Dutch PYTHON trial entitled “Early versus on-demand nasoenteric tube feeding in acute pancreatitis.”4,63 There is no clear cutoff point for initiating feeding for those with severe AP. A suggested practical approach is to initiate feeding within 24-72 hours and offer enteral nutrition for those intolerant to oral feeds. In severe AP and moderately severe AP, enteral nutrition is recommended over parenteral nutrition.3,4 Enteral nutrition significantly reduces the risk of infected peripancreatic necrosis, single organ failure, and multiorgan failure.4 Finally, the AGA guidelines provide a conditional recommendation for providing enteral nutrition support through either the nasogastric or nasoenteric route.3 Further studies are required to determine the optimal timing, rate, and formulation of enteral nutrition in severe AP.
Antibiotics and probiotics
Current guidelines do not support the use of prophylactic antibiotics to prevent infection in necrotizing AP and severe AP.1-3 The AGA technical review reported that prophylactic antibiotics did not reduce infected pancreatic or peripancreatic necrosis, persistent single organ failure, or mortality.4 Guidelines advocate against the use of probiotics for severe AP, because of increased mortality risk.1
Timing of ERCP in acute biliary pancreatitis
There is universal agreement for offering urgent ERCP (within 24 hours) in biliary AP complicated by cholangitis.1-3,64 Figure 2 demonstrates an example of a cholangiogram completed within 24 hours of presentation of biliary AP complicated by cholangitis.
In the absence of cholangitis, the timing of ERCP for AP with persistent biliary obstruction is less clear.1-3 In line with recent guidelines, the 2018 AGA guidelines advocate against routine use of urgent ERCP for biliary AP without cholangitis,3 a conditional recommendation with overall low quality of data.4 The AGA technical review found that urgent ERCP, compared with conservative management in acute biliary pancreatitis without cholangitis had no significant effect on mortality, organ failure, infected pancreatic necrosis, and total necrotizing pancreatitis, but did significantly shorten hospital length of stay.4 There are limited data to guide decision making of when nonurgent ERCP should be performed in hospitalized patients with biliary AP with persistent obstruction and no cholangitis.3,64
Alcohol and smoking cessation
The AGA technical review advocates for brief alcohol intervention during hospitalization for alcohol-induced AP on the basis of one RCT that addresses the impact of alcohol counseling on recurrent bouts of AP4 plus evidence from a Cochrane review of alcohol-reduction strategies in primary care populations.65 Cessation of smoking – an established independent risk factor of AP – recurrent AP and chronic pancreatitis, should also be recommended as part of the management of AP.
Cholecystectomy
Evidence supports same-admission cholecystectomy for mild gallstone AP, a strong recommendation of published AGA guidelines.3 When compared with delayed cholecystectomy, same-admission cholecystectomy significantly reduced gallstone-related complications, readmissions for recurrent pancreatitis, and pancreaticobiliary complications, without having a significant impact on mortality during a 6-month follow-up period.66 Delaying cholecystectomy 6 weeks in patients with moderate-severe gallstone AP appears to reduce morbidity, including the development of infected collections, and mortality.4 An ongoing RCT, the APEC trial, aims to determine whether early ERCP with biliary sphincterotomy reduces major complications or death when compared with no intervention for biliary AP in patients at high risk of complications.67
Chemoprevention and IV fluid management of post-ERCP pancreatitis
Accumulating data support the effectiveness of chemoprevention, pancreatic stent placement, and fluid administration to prevent post-ERCP pancreatitis. Multiple RCTs, meta-analyses, and systematic reviews indicate that rectal NSAIDs) reduce post-ERCP pancreatitis onset68-71 and moderate-severe post-ERCP pancreatitis. Additionally, placement of a pancreatic duct stent may decrease the risk of severe post-ERCP pancreatitis in high-risk patients.3 Guidelines do not comment on fluid administrations for prevention of post-ERCP pancreatitis, but studies have shown that greater periprocedural IV fluid was an independent protective factor against moderate to severe PEP72 and was associated with shorter hospital length of stay.73 Recent meta-analyses and RCTs support using LR prior to ERCP to prevent PEP.74-77 Interestingly, a recent RCT shows that the combination of rectal indomethacin and LR, compared with combination placebo and normal saline reduced the risk of PEP in high-risk patients.78
Two ongoing multicenter RCTs will clarify the role of combination therapy. The Dutch FLUYT RCT aims to determine the optimal combination of rectal NSAIDs and periprocedural infusion of IV fluids to reduce the incidence of PEP and moderate-severe PEP79 and the Stent vs. Indomethacin (SVI) trial aims to determine the whether combination pancreatic stent placement plus rectal indomethacin is superior to monotherapy indomethacin for preventing post-ERCP pancreatitis in high-risk cases.80
Implications for clinical practice
The diagnosis and optimal management of AP require a systematic approach with multidisciplinary decision making. Morbidity and mortality in AP are driven by early or late POF, and the latter often is triggered by infected necrosis. Risk stratification of these patients at the point of contact is a commonsense approach to enable triaging of patients to the appropriate level of care. Regardless of pancreatitis severity, recommended treatment interventions include goal-directed IV fluid resuscitation, early feeding by mouth or enteral tube when necessary, avoidance of prophylactic antibiotics, avoidance of probiotics, and urgent ERCP for patients with acute biliary pancreatitis complicated by cholangitis. Key measures for preventing hospital readmission and pancreatitis include same-admission cholecystectomy for acute biliary pancreatitis and alcohol and smoking cessation. Preventive measures for post-ERCP pancreatitis in patients undergoing ERCP include rectal indomethacin, prophylactic pancreatic duct stent placement, and periprocedural fluid resuscitation.
Dr. Mandalia is a fellow, gastroenterology, department of internal medicine, division of gastroenterology, Michigan Medicine, Ann Arbor; Dr. DiMagno is associate professor of medicine, director, comprehensive pancreas program, department of internal medicine, division of gastroenterology, University of Michigan, Ann Arbor. Dr. Mandalia reports no conflicts of interest.
References
1. Tenner S et al. Am J Gastroenterol. 2013;108:1400.
2. Besseline M et al. Pancreatology. 2013;13(4, Supplement 2):e1-15.
3. Crockett SD et al. Gastroenterology. 2018;154(4):1096-101.
4. Vege SS et al. Gastroenterology. 2018;154(4):1103-39.
5. Peery AF et al. Gastroenterology. 2019 Jan;156(1):254-72.e11.
6. Krishna SG et al. Pancreas. 2017;46(4):482-8.
7. Sellers ZM et al. Gastroenterology. 2018;155(2):469-78.e1.
8. Brown A et al. JOP. 2008;9(4):408-14.
9. Fagenholz PJ et al. Ann Epidemiol. 2007;17(7):491.e1-.e8.
10. McNabb-Baltar J et al. Pancreas. 2014;43(5):687-91.
11. Johnson CD et al. Gut. 2004;53(9):1340-4.
12. Dellinger EP et al. Ann Surg. 2012;256(6):875-80.
13. Petrov MS et al. Gastroenterology. 2010;139(3):813-20.
14. Sternby H et al. Ann Surg. Apr 18. doi: 10.1097/SLA.0000000000002766.
15. Huh JH et al. J Clin Gastroenterol. 2018;52(2):178-83.
16. Wu BU et al. Gastroenterology. 2008;135(3):816-20.
17. Gardner TB et al. Clin Gastroenterol Hepatol. 2008;6(10):1070-6.
18. Krishna SG et al. Am J Gastroenterol. 2015;110(11):1608-19.
19. Lee PJ et al. Pancreas. 2016;45(4):561-4.
20. Mandalia A et al. F1000Research. 2018 Jun 28;7.
21. Majumder S et al. Pancreas. 2015;44(4):540-6.
22. DiMagno MJ. Clin Gastroenterol Hepatol. 2011;9(11):920-2.
23. Yadav D, Whitcomb DC. Nature Rev Gastroenterol Hepatol. 2010;7(3):131-45.
24. Samokhvalov AV et al. EBioMedicine. 2015;2(12):1996-2002.
25. Barkin JA et al. Pancreas. 2017;46(8):1035-8.
26. Chen Y-T et al. J Gastroenterol Hepatol. 2016;31(4):782-7.
27. Ramos LR et al. J Crohns Colitis. 2016;10(1):95-104.
28. Avram MM. Nephron. 1977;18(1):68-71.
29. Lankisch PG et al. Nephrol Dial Transplant. 2008;23(4):1401-5.
30. Owyang C et al. Mayo Clin Proc. 1979;54(12):769-73.
31. Owyang Cet al. Gut. 1982;23(5):357-61.
32. Quraishi ER et al. Am J Gastroenterol. 2005;100:2288.
33. Vaziri ND et al. Nephron. 1987;46(4):347-9.
34. Chen HJ et al. Nephrol Dial Transplant. 2017;32(10):1731-6.
35. Kirkegard J et al. Gastroenterology. 2018;May;154(6):1729-36.
36. Karlson BM, et al. Gastroenterology. 1997;113(2):587-92.
37. Munigala S et al. Clin Gastroenterol Hepatol. 2014;12(7):1143-50.e1.
38. Carr RA et al. Pancreatology. 2016;16(4):469-76.
39. Li X et al. BMC Gastroenterol. 2018;18(1):89.
40. Ahmed AU et al. Clin Gastroenterol Hepatol. 2016;14(5):738-46.
41. Sankaran SJ et al. Gastroenterology. 2015;149(6):1490-500.e1.
42. Berglund L et al. J Clin Endocrinol Metab. 2012;97(9):2969-89.
43. Catapano AL et al. Atherosclerosis. 2011;217(1):3-46.
44. Pedersen SB et al. JAMA Intern Med. 2016;176(12):1834-42.
45. Nawaz H et al. Am J Gastroenterol. 2015;110(10):1497-503.
46. Banks PA et al. Gut. 2013;62(1):102-11.
47. Kondo S et al. Eur J Radiol. 2005;54(2):271-5.
48. Meeralam Y et al. Gastrointest Endosc. 2017;86(6):986-93.
49. Stimac D et al. Am J Gastroenterol. 2007;102(5):997-1004.
50. Jin DX et al. Dig Dis Sci. 2017;62(10):2894-9.
51. Freeman ML. Gastrointest Endosc Clin N Am. 2012;22(3):567-86.
52. De Lisi S et al. Eur J Gastroenterol Hepatol. 2011;23(5):367-74.
53. Di MY et al. Ann Int Med. 2016;165(7):482-90.
54. Mounzer R et al. Gastroenterology. 2012;142(7):1476-82; quiz e15-6.
55. Koutroumpakis E et al. Am J Gastroenterol. 2015;110(12):1707-16.
56. Wu BU et al. Gastroenterology. 2009;137(1):129-35.
57. Buddingh KT et al. J Am Coll Surg. 2014;218(1):26-32.
58. Buxbaum J et al. Am J Gastroenterol. 2018;113(5):755-64.
59. Jabaudon M et al. Crit Car Med. 2018;46(3):e198-e205.
60. Barlass U et al. Gut. 2018;67(4):600-2.
61. Buxbaum JL et al. Am J Gastroenterol. 2017;112(5):797-803.
62. de-Madaria E et al. United Eur Gastroenterol J. 2018;6(1):63-72.
63. Bakker OJ et al. N Engl J Med. 2014;371(21):1983-93.
64. Tse F et al. Cochrane Database Syst Rev. 2012(5):Cd009779.
65. Kaner EFS et al. Cochrane Database Syst Rev. 2007(2):Cd004148.
66. da Costa DW et al. Lancet. 2015;386(10000):1261-8.
67. Schepers NJ et al. Trials. 2016;17:5.
68. Vadala di Prampero SF et al. Eur J Gastroenterol Hepatol. 2016;28(12):1415-24.
69. Kubiliun NM et al. Clin Gastroenterol Hepatol. 2015;13(7):1231-9; quiz e70-1.
70. Wan J et al. BMC Gastroenterol. 2017;17(1):43.
71. Yang C et al. Pancreatology. 2017;17(5):681-8.
72. DiMagno MJ et al. Pancreas. 2014;43(4):642-7.
73. Sagi SV et al. J Gastroenterol Hepatol. 2014;29(6):1316-20.
74. Choi JH et al. Clin Gastroenterol Hepatol. 2017;15(1):86-92.e1.
75. Wu D et al. J Clin Gastroenterol. 2017;51(8):e68-e76.
76. Zhang ZF et al. J Clin Gastroenterol. 2017;51(3):e17-e26.
77. Park CH et al. Endoscopy 2018 Apr;50(4):378-85.
78. Mok SRS et al. Gastrointest Endosc. 2017;85(5):1005-13.
79. Smeets XJN et al. Trials. 2018;19(1):207.
80. Elmunzer BJ et al. Trials. 2016;17(1):120.
Introduction
Acute pancreatitis (AP) is a major clinical and financial burden in the United States. Several major clinical guidelines provide evidence-based recommendations for the clinical management decisions in AP, including those from the American College of Gastroenterology (ACG; 2013),1 and the International Association of Pancreatology (IAP; 2013).2 More recently, the American Gastroenterological Association (AGA) released their own set of guidelines.3,4 In this update on AP, we review these guidelines and reference recent literature focused on epidemiology, risk factors, etiology, diagnosis, risk stratification, and recent advances in the early medical management of AP. Regarding the latter, we review six treatment interventions (pain management, intravenous fluid resuscitation, feeding, prophylactic antibiotics, probiotics, and timing of endoscopic retrograde cholangiopancreatography (ERCP) in acute biliary pancreatitis) and four preventive interventions (alcohol and smoking cessation, same-admission cholecystectomy for acute biliary pancreatitis, and chemoprevention and fluid administration for post-ERCP pancreatitis [PEP]). Updates on multidisciplinary management of (infected) pancreatic necrosis is beyond the scope of this review. Table 1 summarizes the concepts discussed in this article.

Recent advances in epidemiology and evaluation of AP
Epidemiology
AP is the third most common cause of gastrointestinal-related hospitalizations and fourth most common cause of readmission in 2014.5 Recent epidemiologic studies show conflicting trends for the incidence of AP, both increasing6 and decreasing,7 likely attributable to significant differences in study designs. Importantly, multiple studies have demonstrated that hospital length of stay, costs, and mortality have declined since 2009.6,8-10
Persistent organ failure (POF), defined as organ failure lasting more than 48 hours, is the major cause of death in AP. POF, if only a single organ during AP, is associated with 27%-36% mortality; if it is multiorgan, it is associated with 47% mortality.1,11 Other factors associated with increased hospital mortality include infected pancreatic necrosis,12-14 diabetes mellitus,15 hospital-acquired infection,16 advanced age (70 years and older),17 and obesity.18 Predictive factors of 1-year mortality include readmission within 30 days, higher Charlson Comorbidity Index, and longer hospitalization.19
Risk factors
We briefly highlight recent insights into risk factors for AP (Table 1) and refer to a recent review for further discussion.20 Current and former tobacco use are independent risk factors for AP.21 The dose-response relationship of alcohol to the risk of pancreatitis is complex,22 but five standard drinks per day for 5 years is a commonly used cut-off.1,23 New evidence suggests that the relationship between the dose of alcohol and risk of AP differs by sex, linearly in men but nonlinearly (J-shaped) in women.24 Risk of AP in women was decreased with alcohol consumption of up to 40 g/day (one standard drink contains 14 g of alcohol) and increased above this amount. Cannabis is a possible risk factor for toxin-induced AP and abstinence appears to abolish risk of recurrent attacks.25
Patients with inflammatory bowel disease (IBD) have a 2.9-fold higher risk for AP versus non-IBD cohorts26 with the most common etiologies are from gallstones and medications.27 In patients with end-stage renal disease (ESRD), the risk of AP is higher in those who receive peritoneal dialysis, compared with hemodialysis28-33 and who are women, older, or have cholelithiasis or liver disease.34As recently reviewed,35 pancreatic cancer appears to be associated with first-attack pancreatitis with few exceptions.36 In this setting, the overall incidence of pancreatic cancer is low (1.5%). The risk is greatest within the first year of the attack of AP, negligible below age 40 years but steadily rising through the fifth to eighth decades.37 Pancreatic cancer screening is a conditional recommendation of the ACG guidelines in patients with unexplained AP, particularly those aged 40 years or older.1
Etiology and diagnosis

Alcohol and gallstones remain the most prevalent etiologies for AP.1 While hypertriglyceridemia accounted for 9% of AP in a systematic review of acute pancreatitis in 15 different countries,38 it is the second most common cause of acute pancreatitis in Asia (especially China).39 Figure 1 provides a breakdown of the etiologies and risk factors of pancreatitis. Importantly, it remains challenging to assign several toxic-metabolic etiologies as either a cause or risk factor for AP, particularly with regards to alcohol, smoking, and cannabis to name a few.
Guidelines and recent studies of AP raise questions about the threshold above which hypertriglyceridemia causes or poses as an important cofactor for AP. American and European societies define the threshold for triglycerides at 885-1,000 mg/dL.1,42,43 Pedersen et al. provide evidence of a graded risk of AP with hypertriglyceridemia: In multivariable analysis, adjusted hazard ratios for AP were much higher with nonfasting mild to moderately elevated plasma triglycerides (177-885 mg/dL), compared with normal values (below 89 mg/dL).44 Moreover, the risk of severe AP (developing POF) increases in proportion to triglyceride value, independent of the underlying cause of AP.45

Diagnosis of AP is derived from the revised Atlanta classification.46 The recommended timing and indications for offering cross-sectional imaging are after 48-72 hours in patients with no improvement to initial care.1 Endoscopic ultrasonography (EUS) has better diagnostic accuracy and sensitivity, compared with magnetic resonance cholangiopancreatography (MRCP) for choledocholithiasis, and has comparable specificity.47,48 Among noninvasive imaging modalities, MRCP is more sensitive than computed tomography (CT) for diagnosing choledocholithiasis.49 Despite guideline recommendations for more selective use of pancreatic imaging in the early assessment of AP, utilization of early CT or MRCP imaging (within the first 24 hours of care) remained high during 2014-2015, compared with 2006-2007.50
ERCP is not recommended as a pure diagnostic tool, owing to the availability of other diagnostic tests and a complication rate of 5%-10% with risks involving PEP, cholangitis, perforation, and hemorrhage.51 A recent systematic review of EUS and ERCP in acute biliary pancreatitis concluded that EUS had lower failure rates and had no complications, and the use of EUS avoided ERCP in 71.2% of cases.52
Risk stratification
The goals of using risk stratification tools in AP are to identify patients at risk for developing major outcomes, including POF, infected pancreatic necrosis, and death, and to ensure timely triaging of patients to an appropriate level of care. Existing prediction models have only moderate predictive value.53,54 Examples include simple risk stratification tools such as blood urea nitrogen (BUN) and hemoconcentration,55,56 disease-modifying patient variables (age, obesity, etc.), biomarkers (i.e., angiopoietin 2),57 and more complex clinical scoring systems such as Acute Physiology and Chronic Health Evaluation II (APACHE II), BISAP (BUN, impaired mental status, SIRS criteria, age, pleural effusion) score, early warning system (EWS), Glasgow-Imrie score, Japanese severity score, and recently the Pancreatitis Activity Scoring System (PASS).58 Two recent guidelines affirmed the importance of predicting the severity of AP, using one or more predictive tools.1,2 The recent 2018 AGA technical review does not debate this commonsense approach, but does highlight that there is no published observational study or randomized, controlled trial (RCT) investigating whether prediction tools affect clinical outcomes.4

Recent advances in early treatment of AP
Literature review and definitions
The AP literature contains heterogeneous definitions of severe AP and of what constitutes a major outcome in AP. Based on definitions of the 2013 revised Atlanta Criteria, the 2018 AGA technical review and clinical guidelines emphasized precise definitions of primary outcomes of clinical importance in AP, including death, persistent single organ failure, or persistent multiple organ failure, each requiring a duration of more than 48 hours, and infected pancreatic or peripancreatic necrosis or both (Table 2).3,4
Pain management
Management of pain in AP is complex and requires a detailed discussion beyond the scope of this review, but recent clinical and translational studies raise questions about the current practice of using opioids for pain management in AP. A provocative, multicenter, retrospective cohort study reported lower 30-day mortality among critically ill patients who received epidural analgesia versus standard care without epidural analgesia.59 The possible mechanism of protection and the drugs administered are unclear. An interesting hypothesis is that the epidural cohort may have received lower exposure to morphine, which may increase gut permeability, the risk of infectious complications, and severity of AP, based on a translational study in mice.60
Intravenous fluid administration

Supportive care with the use of IV fluid hydration is a mainstay of treatment for AP in the first 12-24 hours. Table 3 summarizes the guidelines in regards to IV fluid administration as delineated by the ACG and AGA guidelines on the management of pancreatitis.1,3 Guidelines advocate for early fluid resuscitation to correct intravascular depletion in order to reduce morbidity and mortality associated with AP.1,2,4 The 2018 AGA guidelines endorse a conditional recommendation for using goal-directed therapy for initial fluid management,3 do not recommend for or against normal saline versus lactated Ringer’s (LR), but do advise against the use of hydroxyethyl starch fluids.3 Consistent with these recommendations, two recent RCTs published subsequent to the prespecified time periods of the AGA technical review and guideline, observed no significant differences between LR and normal saline on clinically meaningful outcomes.61,62 The AGA guidelines acknowledge that evidence was of very-low quality in support of goal-directed therapy,3,4 which has not been shown to have a significant reduction in persistent multiple organ failure, mortality, or pancreatic necrosis, compared with usual care. As the authors noted, interpretation of the data was limited by the absence of other critical outcomes in these trials (infected pancreatic necrosis), lack of uniformity of specific outcomes and definitions of transient and POF, few trials, and risk of bias. There is a clear need for a large RCT to provide evidence to guide decision making with fluid resuscitation in AP, particularly in regard to fluid type, volume, rate, duration, endpoints, and clinical outcomes.
Feeding
More recently, the focus of nutrition in the management of AP has shifted away from patients remaining nil per os (NPO). Current guidelines advocate for early oral feeding (within 24 hours) in mild AP,3,4 in order to protect the gut-mucosal barrier. Remaining NPO when compared with early oral feeding has a 2.5-fold higher risk for interventions for necrosis.4 The recently published AGA technical review identified no significant impact on outcomes of early versus delayed oral feeding, which is consistent with observations of a landmark Dutch PYTHON trial entitled “Early versus on-demand nasoenteric tube feeding in acute pancreatitis.”4,63 There is no clear cutoff point for initiating feeding for those with severe AP. A suggested practical approach is to initiate feeding within 24-72 hours and offer enteral nutrition for those intolerant to oral feeds. In severe AP and moderately severe AP, enteral nutrition is recommended over parenteral nutrition.3,4 Enteral nutrition significantly reduces the risk of infected peripancreatic necrosis, single organ failure, and multiorgan failure.4 Finally, the AGA guidelines provide a conditional recommendation for providing enteral nutrition support through either the nasogastric or nasoenteric route.3 Further studies are required to determine the optimal timing, rate, and formulation of enteral nutrition in severe AP.
Antibiotics and probiotics
Current guidelines do not support the use of prophylactic antibiotics to prevent infection in necrotizing AP and severe AP.1-3 The AGA technical review reported that prophylactic antibiotics did not reduce infected pancreatic or peripancreatic necrosis, persistent single organ failure, or mortality.4 Guidelines advocate against the use of probiotics for severe AP, because of increased mortality risk.1
Timing of ERCP in acute biliary pancreatitis
There is universal agreement for offering urgent ERCP (within 24 hours) in biliary AP complicated by cholangitis.1-3,64 Figure 2 demonstrates an example of a cholangiogram completed within 24 hours of presentation of biliary AP complicated by cholangitis.
In the absence of cholangitis, the timing of ERCP for AP with persistent biliary obstruction is less clear.1-3 In line with recent guidelines, the 2018 AGA guidelines advocate against routine use of urgent ERCP for biliary AP without cholangitis,3 a conditional recommendation with overall low quality of data.4 The AGA technical review found that urgent ERCP, compared with conservative management in acute biliary pancreatitis without cholangitis had no significant effect on mortality, organ failure, infected pancreatic necrosis, and total necrotizing pancreatitis, but did significantly shorten hospital length of stay.4 There are limited data to guide decision making of when nonurgent ERCP should be performed in hospitalized patients with biliary AP with persistent obstruction and no cholangitis.3,64
Alcohol and smoking cessation
The AGA technical review advocates for brief alcohol intervention during hospitalization for alcohol-induced AP on the basis of one RCT that addresses the impact of alcohol counseling on recurrent bouts of AP4 plus evidence from a Cochrane review of alcohol-reduction strategies in primary care populations.65 Cessation of smoking – an established independent risk factor of AP – recurrent AP and chronic pancreatitis, should also be recommended as part of the management of AP.
Cholecystectomy
Evidence supports same-admission cholecystectomy for mild gallstone AP, a strong recommendation of published AGA guidelines.3 When compared with delayed cholecystectomy, same-admission cholecystectomy significantly reduced gallstone-related complications, readmissions for recurrent pancreatitis, and pancreaticobiliary complications, without having a significant impact on mortality during a 6-month follow-up period.66 Delaying cholecystectomy 6 weeks in patients with moderate-severe gallstone AP appears to reduce morbidity, including the development of infected collections, and mortality.4 An ongoing RCT, the APEC trial, aims to determine whether early ERCP with biliary sphincterotomy reduces major complications or death when compared with no intervention for biliary AP in patients at high risk of complications.67
Chemoprevention and IV fluid management of post-ERCP pancreatitis
Accumulating data support the effectiveness of chemoprevention, pancreatic stent placement, and fluid administration to prevent post-ERCP pancreatitis. Multiple RCTs, meta-analyses, and systematic reviews indicate that rectal NSAIDs) reduce post-ERCP pancreatitis onset68-71 and moderate-severe post-ERCP pancreatitis. Additionally, placement of a pancreatic duct stent may decrease the risk of severe post-ERCP pancreatitis in high-risk patients.3 Guidelines do not comment on fluid administrations for prevention of post-ERCP pancreatitis, but studies have shown that greater periprocedural IV fluid was an independent protective factor against moderate to severe PEP72 and was associated with shorter hospital length of stay.73 Recent meta-analyses and RCTs support using LR prior to ERCP to prevent PEP.74-77 Interestingly, a recent RCT shows that the combination of rectal indomethacin and LR, compared with combination placebo and normal saline reduced the risk of PEP in high-risk patients.78
Two ongoing multicenter RCTs will clarify the role of combination therapy. The Dutch FLUYT RCT aims to determine the optimal combination of rectal NSAIDs and periprocedural infusion of IV fluids to reduce the incidence of PEP and moderate-severe PEP79 and the Stent vs. Indomethacin (SVI) trial aims to determine the whether combination pancreatic stent placement plus rectal indomethacin is superior to monotherapy indomethacin for preventing post-ERCP pancreatitis in high-risk cases.80
Implications for clinical practice
The diagnosis and optimal management of AP require a systematic approach with multidisciplinary decision making. Morbidity and mortality in AP are driven by early or late POF, and the latter often is triggered by infected necrosis. Risk stratification of these patients at the point of contact is a commonsense approach to enable triaging of patients to the appropriate level of care. Regardless of pancreatitis severity, recommended treatment interventions include goal-directed IV fluid resuscitation, early feeding by mouth or enteral tube when necessary, avoidance of prophylactic antibiotics, avoidance of probiotics, and urgent ERCP for patients with acute biliary pancreatitis complicated by cholangitis. Key measures for preventing hospital readmission and pancreatitis include same-admission cholecystectomy for acute biliary pancreatitis and alcohol and smoking cessation. Preventive measures for post-ERCP pancreatitis in patients undergoing ERCP include rectal indomethacin, prophylactic pancreatic duct stent placement, and periprocedural fluid resuscitation.
Dr. Mandalia is a fellow, gastroenterology, department of internal medicine, division of gastroenterology, Michigan Medicine, Ann Arbor; Dr. DiMagno is associate professor of medicine, director, comprehensive pancreas program, department of internal medicine, division of gastroenterology, University of Michigan, Ann Arbor. Dr. Mandalia reports no conflicts of interest.
References
1. Tenner S et al. Am J Gastroenterol. 2013;108:1400.
2. Besseline M et al. Pancreatology. 2013;13(4, Supplement 2):e1-15.
3. Crockett SD et al. Gastroenterology. 2018;154(4):1096-101.
4. Vege SS et al. Gastroenterology. 2018;154(4):1103-39.
5. Peery AF et al. Gastroenterology. 2019 Jan;156(1):254-72.e11.
6. Krishna SG et al. Pancreas. 2017;46(4):482-8.
7. Sellers ZM et al. Gastroenterology. 2018;155(2):469-78.e1.
8. Brown A et al. JOP. 2008;9(4):408-14.
9. Fagenholz PJ et al. Ann Epidemiol. 2007;17(7):491.e1-.e8.
10. McNabb-Baltar J et al. Pancreas. 2014;43(5):687-91.
11. Johnson CD et al. Gut. 2004;53(9):1340-4.
12. Dellinger EP et al. Ann Surg. 2012;256(6):875-80.
13. Petrov MS et al. Gastroenterology. 2010;139(3):813-20.
14. Sternby H et al. Ann Surg. Apr 18. doi: 10.1097/SLA.0000000000002766.
15. Huh JH et al. J Clin Gastroenterol. 2018;52(2):178-83.
16. Wu BU et al. Gastroenterology. 2008;135(3):816-20.
17. Gardner TB et al. Clin Gastroenterol Hepatol. 2008;6(10):1070-6.
18. Krishna SG et al. Am J Gastroenterol. 2015;110(11):1608-19.
19. Lee PJ et al. Pancreas. 2016;45(4):561-4.
20. Mandalia A et al. F1000Research. 2018 Jun 28;7.
21. Majumder S et al. Pancreas. 2015;44(4):540-6.
22. DiMagno MJ. Clin Gastroenterol Hepatol. 2011;9(11):920-2.
23. Yadav D, Whitcomb DC. Nature Rev Gastroenterol Hepatol. 2010;7(3):131-45.
24. Samokhvalov AV et al. EBioMedicine. 2015;2(12):1996-2002.
25. Barkin JA et al. Pancreas. 2017;46(8):1035-8.
26. Chen Y-T et al. J Gastroenterol Hepatol. 2016;31(4):782-7.
27. Ramos LR et al. J Crohns Colitis. 2016;10(1):95-104.
28. Avram MM. Nephron. 1977;18(1):68-71.
29. Lankisch PG et al. Nephrol Dial Transplant. 2008;23(4):1401-5.
30. Owyang C et al. Mayo Clin Proc. 1979;54(12):769-73.
31. Owyang Cet al. Gut. 1982;23(5):357-61.
32. Quraishi ER et al. Am J Gastroenterol. 2005;100:2288.
33. Vaziri ND et al. Nephron. 1987;46(4):347-9.
34. Chen HJ et al. Nephrol Dial Transplant. 2017;32(10):1731-6.
35. Kirkegard J et al. Gastroenterology. 2018;May;154(6):1729-36.
36. Karlson BM, et al. Gastroenterology. 1997;113(2):587-92.
37. Munigala S et al. Clin Gastroenterol Hepatol. 2014;12(7):1143-50.e1.
38. Carr RA et al. Pancreatology. 2016;16(4):469-76.
39. Li X et al. BMC Gastroenterol. 2018;18(1):89.
40. Ahmed AU et al. Clin Gastroenterol Hepatol. 2016;14(5):738-46.
41. Sankaran SJ et al. Gastroenterology. 2015;149(6):1490-500.e1.
42. Berglund L et al. J Clin Endocrinol Metab. 2012;97(9):2969-89.
43. Catapano AL et al. Atherosclerosis. 2011;217(1):3-46.
44. Pedersen SB et al. JAMA Intern Med. 2016;176(12):1834-42.
45. Nawaz H et al. Am J Gastroenterol. 2015;110(10):1497-503.
46. Banks PA et al. Gut. 2013;62(1):102-11.
47. Kondo S et al. Eur J Radiol. 2005;54(2):271-5.
48. Meeralam Y et al. Gastrointest Endosc. 2017;86(6):986-93.
49. Stimac D et al. Am J Gastroenterol. 2007;102(5):997-1004.
50. Jin DX et al. Dig Dis Sci. 2017;62(10):2894-9.
51. Freeman ML. Gastrointest Endosc Clin N Am. 2012;22(3):567-86.
52. De Lisi S et al. Eur J Gastroenterol Hepatol. 2011;23(5):367-74.
53. Di MY et al. Ann Int Med. 2016;165(7):482-90.
54. Mounzer R et al. Gastroenterology. 2012;142(7):1476-82; quiz e15-6.
55. Koutroumpakis E et al. Am J Gastroenterol. 2015;110(12):1707-16.
56. Wu BU et al. Gastroenterology. 2009;137(1):129-35.
57. Buddingh KT et al. J Am Coll Surg. 2014;218(1):26-32.
58. Buxbaum J et al. Am J Gastroenterol. 2018;113(5):755-64.
59. Jabaudon M et al. Crit Car Med. 2018;46(3):e198-e205.
60. Barlass U et al. Gut. 2018;67(4):600-2.
61. Buxbaum JL et al. Am J Gastroenterol. 2017;112(5):797-803.
62. de-Madaria E et al. United Eur Gastroenterol J. 2018;6(1):63-72.
63. Bakker OJ et al. N Engl J Med. 2014;371(21):1983-93.
64. Tse F et al. Cochrane Database Syst Rev. 2012(5):Cd009779.
65. Kaner EFS et al. Cochrane Database Syst Rev. 2007(2):Cd004148.
66. da Costa DW et al. Lancet. 2015;386(10000):1261-8.
67. Schepers NJ et al. Trials. 2016;17:5.
68. Vadala di Prampero SF et al. Eur J Gastroenterol Hepatol. 2016;28(12):1415-24.
69. Kubiliun NM et al. Clin Gastroenterol Hepatol. 2015;13(7):1231-9; quiz e70-1.
70. Wan J et al. BMC Gastroenterol. 2017;17(1):43.
71. Yang C et al. Pancreatology. 2017;17(5):681-8.
72. DiMagno MJ et al. Pancreas. 2014;43(4):642-7.
73. Sagi SV et al. J Gastroenterol Hepatol. 2014;29(6):1316-20.
74. Choi JH et al. Clin Gastroenterol Hepatol. 2017;15(1):86-92.e1.
75. Wu D et al. J Clin Gastroenterol. 2017;51(8):e68-e76.
76. Zhang ZF et al. J Clin Gastroenterol. 2017;51(3):e17-e26.
77. Park CH et al. Endoscopy 2018 Apr;50(4):378-85.
78. Mok SRS et al. Gastrointest Endosc. 2017;85(5):1005-13.
79. Smeets XJN et al. Trials. 2018;19(1):207.
80. Elmunzer BJ et al. Trials. 2016;17(1):120.
Endoscopic management of obesity
Editor's Note
Gastroenterologists are becoming increasingly involved in the management of obesity. While prior therapy for obesity was mainly based on lifestyle changes, medication, or surgery, the new and exciting field of endoscopic bariatric and metabolic therapies has recently garnered incredible attention and momentum.
In this quarter’s In Focus article, brought to you by The New Gastroenterologist, Pichamol Jirapinyo and Christopher Thompson (Brigham and Women’s Hospital) provide an outstanding overview of the gastric and small bowel endoscopic interventions that are either already approved for use in obesity or currently being studied. This field is moving incredibly fast, and knowledge and understanding of these endoscopic therapies for obesity will undoubtedly be important for our field.
Bryson W. Katona, MD, PhD
Editor in Chief, The New Gastroenterologist
Introduction
Obesity is a rising pandemic. As of 2016, 93.3 million U.S. adults had obesity, representing 39.8% of our adult population.1 It is estimated that approximately $147 billion is spent annually on caring for patients with obesity. Traditionally, the management of obesity includes lifestyle therapy (diet and exercise), pharmacotherapy (six Food and Drug Administration–approved medications for obesity), and bariatric surgery (sleeve gastrectomy [SG] and Roux-en-Y gastric bypass [RYGB]). Nevertheless, intensive lifestyle intervention and pharmacotherapy are associated with approximately 3.1%-6.6% total weight loss (TWL),2-7 and bariatric surgery is associated with 20%-33.3% TWL.8 However, less than 2% of patients who are eligible for bariatric surgery elect to undergo surgery, leaving a large proportion of patients with obesity untreated or undertreated.9
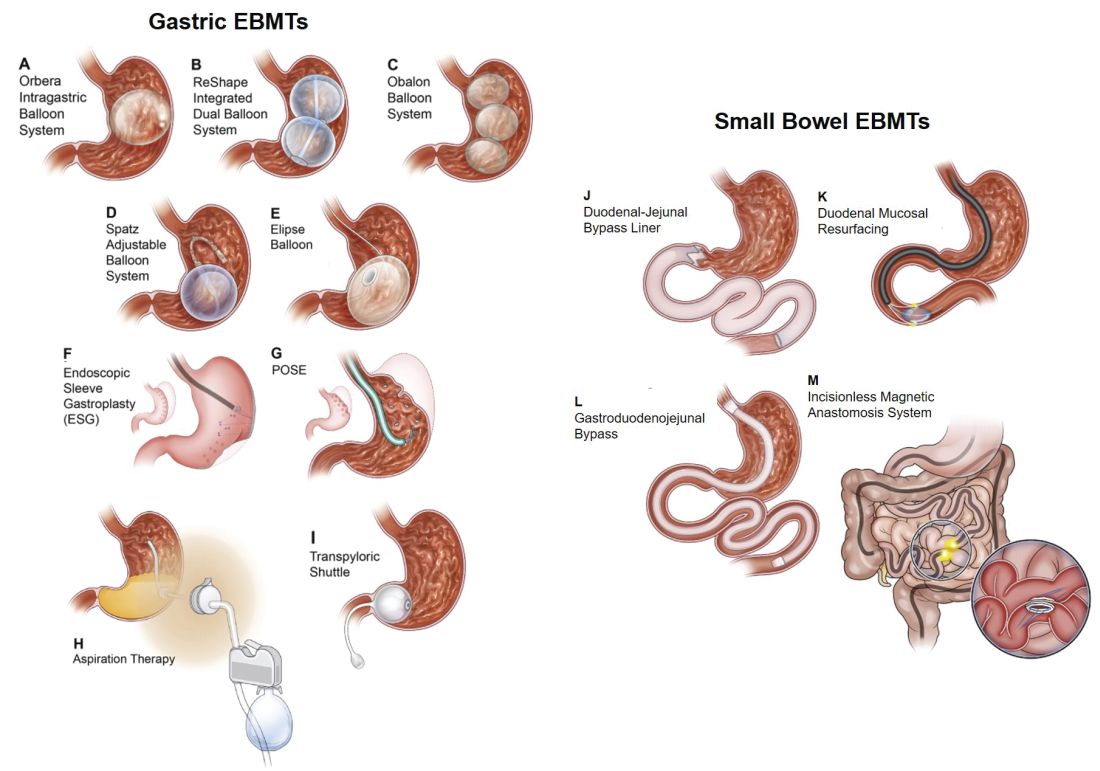
Endoscopic bariatric and metabolic therapies (EBMTs) encompass an emerging field for the treatment of obesity. In general, EBMTs are associated with greater weight loss than are lifestyle intervention and pharmacotherapy, but with a less- invasive risk profile than bariatric surgery. EBMTs may be divided into two general categories – gastric and small bowel interventions (Figure 1 and Table 1). Gastric EBMTs are effective at treating obesity, while small bowel EBMTs are effective at treating metabolic diseases with a variable weight loss profile depending on the device.10,11
Of note, a variety of study designs (including retrospective series, prospective series, and randomized trials with and without shams) have been employed, which can affect outcomes. Therefore, weight loss comparisons among studies are challenging and should be considered in this context.
Gastric interventions
Currently, there are three types of EBMTs that are FDA approved and used for the treatment of obesity. These include intragastric balloons (IGBs), plications and suturing, and aspiration therapy (AT). Other technologies that are under investigation also will be briefly covered.
Intragastric balloons
An intragastric balloon is a space-occupying device that is placed in the stomach. The mechanism of action of IGBs involves delaying gastric emptying, which leads to increased satiety.12 There are several types of IGBs available worldwide differing in techniques of placement and removal (endoscopic versus fluoroscopic versus swallowable), materials used to fill the balloon (fluid-filled versus air-filled), and the number of balloons placed (single versus duo versus three-balloon). At the time of this writing, three IGBs are approved by the FDA (Orbera, ReShape, and Obalon), all for patients with body mass indexes of 30-40 kg/m2, and two others are in the process of obtaining FDA approval (Spatz and Elipse).
Orbera gastric balloon (Apollo Endosurgery, Austin, Tex.) is a single fluid-filled IGB that is endoscopically placed and removed at 6 months. The balloon is filled with 400-700 cc of saline with or without methylene blue (to identify leakage or rupture). Recently, Orbera365, which allows the balloon to stay for 12 months instead of 6 months, has become available in Europe; however, it is yet to be approved in the United States. The U.S. pivotal trial (Orbera trial) including 255 subjects (125 Orbera arm versus 130 non-sham control arm) demonstrated 10.2% TWL in the Orbera group compared with 3.3% TWL in the control group at 6 months based on intention-to-treat (ITT) analysis. This difference persisted at 12 months (6 months after explantation) with 7.6% TWL for the Orbera group versus 3.1% TWL for the control group.13,14
ReShape integrated dual balloon system (ReShape Lifesciences, San Clemente, Calif.) consists of two connected fluid-filled balloons that are endoscopically placed and removed at 6 months. Each balloon is filled with 375-450 cc of saline mixed with methylene blue. The U.S. pivotal trial (REDUCE trial) including 326 subjects (187 ReShape arm versus 139 sham arm) demonstrated 6.8% TWL in the ReShape group compared with 3.3% TWL in the sham group at 6 months based on ITT analysis.15,16
Obalon balloon system (Obalon Therapeutics, Carlsbad, Calif.) is a swallowable, gas-filled balloon system that requires endoscopy only for removal. During placement, a capsule is swallowed under fluoroscopic guidance. The balloon is then inflated with 250 cc of nitrogen mix gas prior to tube detachment. Up to three balloons may be swallowed sequentially at 1-month intervals. At 6 months from the first balloon placement, all balloons are removed endoscopically. The U.S. pivotal trial (SMART trial) including 366 subjects (185 Obalon arm versus 181 sham capsule arm) demonstrated 6.6% TWL in the Obalon group compared with 3.4% TWL in the sham group at 6 months based on ITT analysis.17,18
Two other balloons that are currently under investigation in the United States are the Spatz3 adjustable balloon system (Spatz Medical, Great Neck, N.Y.) and Elipse balloon (Allurion Technologies, Wellesley, Mass.). The Spatz3 is a fluid-filled balloon that is placed and removed endoscopically. It consists of a single balloon and a connecting tube that allows volume adjustment for control of symptoms and possible augmentation of weight loss. The U.S. pivotal trial was recently completed and the data are being reviewed by the FDA. The Elipse is a swallowable fluid-filled balloon that does not require endoscopy for placement or removal. At 4 months, the balloon releases fluid allowing it to empty and pass naturally. The U.S. pivotal trial (ENLIGHTEN trial) is currently underway.
A meta-analysis of randomized controlled trials revealed improvement in most metabolic parameters (diastolic blood pressure, fasting glucose, hemoglobin A1c, and waist circumference) following IGB compared with controls.19 Nausea and vomiting are seen in approximately 30% and should be addressed appropriately. Pooled serious adverse event (SAE) rate was 1.5%, which included migration, perforation, and death. Since 2016, 14 deaths have been reported according to the FDA MAUDE database. Corporate response was that over 295,000 balloons had been distributed worldwide with a mortality rate of less than 0.01%.20
Plication and suturing
Currently, there are two endoscopic devices that are approved for the general indication of tissue apposition. These include the Incisionless Operating Platform (IOP) (USGI Medical, San Clemente, Calif.) and the Overstitch endoscopic suturing system (Apollo Endosurgery, Austin, Tex.). These devices are used to remodel the stomach to create a sleeve-like structure to induce weight loss.
The IOP system consists of a transport, which is a 54-Fr flexible endoscope. It consists of four working channels that accommodate a G-Prox (for tissue approximation), a G-Lix (for tissue grasping), and an ultrathin endoscope (for visualization). In April 2008, Horgan performed the first-in-human primary obesity surgery endoluminal (POSE) procedure in Argentina. The procedure involves the use of the IOP system to place plications primarily in the fundus to modify gastric accommodation.21 The U.S. pivotal trial (ESSENTIAL trial) including 332 subjects (221 POSE arm versus 111 sham arm) demonstrated 5.0% TWL in the POSE group compared with 1.4% in the sham group at 12 months based on ITT analysis.22 A European multicenter randomized controlled trial (MILEPOST trial) including 44 subjects (34 POSE arm versus 10 non-sham control arm) demonstrated 13.0% TWL in the POSE group compared with 5.3% TWL in the control group at 12 months.23 A recent meta-analysis including five studies with 586 subjects showed pooled weight loss of 13.2% at 12-15 months following POSE with a pooled serious adverse event rate of 3.2%.24 These included extraluminal bleeding, minor bleeding at the suture site, hepatic abscess, chest pain, nausea, vomiting, and abdominal pain. A distal POSE procedure with a new plication pattern focusing on the gastric body to augment the effect on gastric emptying has also been described.25
The Overstitch is an endoscopic suturing device that is mounted on a double-channel endoscope. At the tip of the scope, there is a curved suture arm and an anchor exchange that allow the needle to pass back and forth to perform full-thickness bites. The tissue helix may also be placed through the second channel to grasp tissue. In April 2012, Thompson performed the first-in-human endoscopic sutured/sleeve gastroplasty (ESG) procedure in India, which was published together with cases performed in Panama and the Dominican Republic.26-28 This procedure involves the use of the Overstitch device to place several sets of running sutures along the greater curvature of the stomach to create a sleeve-like structure. It is thought to delay gastric emptying and therefore increase satiety.29 The largest multicenter retrospective study including 248 patients demonstrated 18.6% TWL at 2 years with 2% SAE rate including perigastric fluid collections, extraluminal hemorrhage, pulmonary embolism, pneumoperitoneum, and pneumothorax.30
Aspiration therapy
Aspiration therapy (AT; Aspire Bariatrics, King of Prussia, Pa.) allows patients to remove 25%-30% of ingested calories at approximately 30 minutes after meals. AT consists of an A-tube, which is a 26-Fr gastrostomy tube with a 15-cm fenestrated drainage catheter placed endoscopically via a standard pull technique. At 1-2 weeks after A-tube placement, the tube is cut down to the skin and connected to the port prior to aspiration. AT is approved for patients with a BMI of 35-55 kg/m2.31 The U.S. pivotal trial (PATHWAY trial) including 207 subjects (137 AT arm versus 70 non-sham control arm) demonstrated 12.1% TWL in the AT group compared to 3.5% in the control group at 12 months based on ITT analysis. The SAE rate was 3.6% including severe abdominal pain, peritonitis, prepyloric ulcer, and A-tube replacement due to skin-port malfunction.32
Transpyloric shuttle
The transpyloric shuttle (TPS; BAROnova, Goleta, Calif.) consists of a spherical bulb that is attached to a smaller cylindrical bulb by a flexible tether. It is placed and removed endoscopically at 6 months. TPS resides across the pylorus creating intermittent obstruction that may result in delayed gastric emptying. A pilot study including 20 patients demonstrated 14.5% TWL at 6 months.33 The U.S. pivotal trial (ENDObesity II trial) was recently completed and the data are being reviewed by the FDA.
Revision for weight regain following bariatric surgery
Weight regain is common following RYGB34,35 and can be associated with dilation of the gastrojejunal anastomosis (GJA).36 Several procedures have been developed to treat this condition by focusing on reduction of GJA size and are available in the United States (Figure 2). These procedures have level I evidence supporting their use and include transoral outlet reduction (TORe) and restorative obesity surgery endoluminal (ROSE).37 TORe involves the use of the Overstitch to place sutures at the GJA. At 1 year, patients had 8.4% TWL with improvement in comorbidities.38 Weight loss remained significant up to 3-5 years.39,40 The modern ROSE procedure utilizes the IOP system to place plications at the GJA and distal gastric pouch following argon plasma coagulation (APC). A small series showed 12.4% TWL at 6 months.41 APC is also currently being investigated as a standalone therapy for weight regain in this population.
Small bowel interventions
There are several small bowel interventions, with different mechanisms of action, available internationally. Many of these are under investigation in the United States; however, none are currently FDA approved.
Duodenal-jejunal bypass liner
Duodenal-jejunal bypass liner (DJBL; GI Dynamics, Boston, Mass.) is a 60-cm fluoropolymer liner that is endoscopically placed and removed at 12 months. It is anchored at the duodenal bulb and ends at the jejunum. By excluding direct contact between chyme and the proximal small bowel, DJBL is thought to work via foregut mechanism where there is less inhibition of the incretin effect (greater increase in insulin secretion following oral glucose administration compared to intravenous glucose administration due to gut-derived factors that enhance insulin secretion) leading to improved insulin resistance. In addition, the enteral transit of chyme and bile is altered suggesting the possible role of the hindgut mechanism. The previous U.S. pivotal trial (ENDO trial) met efficacy endpoints. However, the study was stopped early by the company because of a hepatic abscess rate of 3.5%, all of which were treated conservatively.42 A new U.S. pivotal study is currently planned. A meta-analysis of 17 published studies, all of which were from outside the United States, demonstrated a significant decrease in hemoglobin A1c of 1.3% and 18.9% TWL at 1 year following implantation in patients with obesity with concomitant diabetes.43
Duodenal mucosal resurfacing
Duodenal mucosal resurfacing (Fractyl, Lexington, Mass.) involves saline lifting of the duodenal mucosa circumferentially prior to thermal ablation using an inflated balloon filled with heated water. It is hypothesized that this may reset the diseased duodenal enteroendocrine cells leading to restoration of the incretin effect. A pilot study including 39 patients with poorly controlled diabetes demonstrated a decrease in hemoglobin A1c of 1.2%. The SAE rate was 7.7% including duodenal stenosis, all of which were treated with balloon dilation.44 The U.S. pivotal trial is currently planned.
Gastroduodenal-jejunal bypass
Gastroduodenal-jejunal bypass (ValenTx., Hopkins, Minn.) is a 120-cm sleeve that is anchored at the gastroesophageal junction to create the anatomic changes of RYGB. It is placed and removed endoscopically with laparoscopic assistance. A pilot study including 12 patients demonstrated 35.9% excess weight loss at 12 months. Two out of 12 patients had early device removal due to intolerance and they were not included in the weight loss analysis.45
Incisionless magnetic anastomosis system
The incisionless magnetic anastomosis system (GI Windows, West Bridgewater, Mass.) consists of self-assembling magnets that are deployed under fluoroscopic guidance through the working channel of colonoscopes to form magnetic octagons in the jejunum and ileum. After a week, a compression anastomosis is formed and the coupled magnets pass spontaneously. A pilot study including 10 patients showed 14.6% TWL and a decrease in hemoglobin A1c of 1.9% (for patients with diabetes) at 1 year.46 A randomized study outside the United States is currently underway.
Summary
Endoscopic bariatric and metabolic therapies are emerging as first-line treatments for obesity in many populations. They can serve as a gap therapy for patients who do not qualify for surgery, but also may have a specific role in the treatment of metabolic comorbidities. This field will continue to develop and improve with the introduction of personalized medicine leading to better patient selection, and newer combination therapies. It is time for gastroenterologists to become more involved in the management of this challenging condition.
Dr. Jirapinyo is an advanced and bariatric endoscopy fellow, Brigham and Women’s Hospital, Harvard Medical School, Boston; Dr. Thompson is director of therapeutic endoscopy, Brigham and Women’s Hospital, and associate professor of medicine, Harvard Medical School. Dr. Jirapinyo has served as a consultant for GI Dynamics and holds royalties for Endosim. Dr. Thompson has contracted research for Aspire Bariatrics, USGI Medical, Spatz, and Apollo Endosurgery; has served as a consultant for Boston Scientific, Covidien, USGI Medical, Olympus, and Fractyl; holds stocks and royalties for GI Windows and Endosim, and has served as an expert reviewer for GI Dynamics.
References
1. CDC. From https://www.cdc.gov/obesity/data/adult.html. Accessed on 11 September 2018.
2. Aronne LJ et al. Obesity. 2013;21:2163-71.
3. Torgerson JS et al. Diabetes Care. 2004;27:155-61.
4. Allison DB et al. Obesity. 2012;20:330-42.
5. Smith SR et al. N Engl J Med. 2010;363:245-56.
6. Apovian CM et al. Obesity. 2013;21:935-43.
7. Pi-Sunyer X et al. N Engl J Med. 2015;373:11-22.
8. Colguitt JL et al. Cochrane Database Syst Rev. 2014;8(8):CD003641.
9. Ponce J et al. Surg Obes Relat Dis. 2015;11(6):1199-200.
10. Jirapinyo P, Thompson CC et al. Clin Gastroenterol Hepatol. 2017;15(5):619-30.
11. Sullivan S et al.Gastroenterology. 2017;152(7):1791-801.
12. Gomez V et al. Obesity. 2016;24(9):1849-53.
13. Food and Drug Administration. Summary of safety and effectiveness data (SSED) ORBERA Intragastric Balloon System. Available at https://www.accessdata.fda.gov/cdrh_docs/pdf14/P140008b.pdf. 2015:1-32.
14. Abu Dayyeh BK et al. Gastrointest Endosc. 2015;81:AB147.
15. Food and Drug Administration. Summary of safety and effectiveness data (SSED) ReShape Integrated Dual Balloon System. Available at https://www.accessdata.fda.gov/cdrh_docs/pdf14/P140012b.pdf. 2015:1-43.
16. Ponce J et al. Surg Obes Relat Dis. 2015;11:874-81.
17. Food and Drug Administration. Summary and effectiveness data (SSED): Obalon Balloon System. Available at https://www.accessdata.fda.gov/cdrh_docs/pdf16/P160001b.pdf. 2016:1-46.
18. Sullivan S et al. Gastroenterology. 2016;150:S1267.
19. Popov VB et al. Am J Gastroenterol. 2017;112:429-39.
20. Abu Dayyeh BK et al. Gastrointest Endosc. 2015;82(3):425-38.
21. Espinos JC et al. Obes Surg. 2013;23(9):1375-83.
22. Sullivan S et al. Obesity. 2017;25:294-301.
23. Miller K et al. Obesity Surg. 2017;27(2):310-22.
24. Jirapinyo P et al. Gastrointest Endosc. 2018;87(6):AB604-AB605.
25. Jirapinyo P, Thompson CC. Video GIE. 2018;3(10):296-300.
26. Campos J et al. SAGES 2013 Presentation. Baltimore, MD. 19 April 2013.
27. Kumar N et al. Gastroenterology. 2014;146(5):S571-2.
28. Kumar N et al. Surg Endosc. 2018;32(4):2159-64.
29. Abu Dayyeh BK et al. Clin Gastroenterol Hepatol. 2017;15:37-43.
30. Lopez-Nava G et al. Obes Surg. 2017;27(10):2649-55.
31. Food and Drug Administration. Summary of safety and effectiveness (SSED): AspireAssist. Available at https://www.accessdata.fda.gov/cdrh_docs/pdf15/p150024b.pdf. FDA,ed,2016:1-36.
32. Thompson CC et al. Am J Gastroenterol. 2017;112:447-57.
33. SAGES abstract archives. SAGES. Available from: http://www.sages.org/meetings/annual-meeting/abstracts-archive/first-clinical-experience-with-the-transpyloric-shuttle-tpsr-device-a-non-surgical-endoscopic treatment-for-obesity-results-from-a-3-month-and-6-month-study. Accessed Sept. 12, 2018.
34. Sjostrom L et al. N Engl J Med. 2007;357:741-52.
35. Adams TD et al. N Engl J Med. 2017;377:1143-55.
36. Abu Dayyeh BK et al. Clin Gastroenterol Hepatol. 2011;9:228-33.
37. Thompson CC et al. Gastroenterology. 2013;145(1):129-37.
38. Jirapinyo P et al. Endoscopy. 2018;50(4):371-7.
39. Kumar N, Thompson CC. Gastrointest Endosc. 2016;83(4):776-9.
40. Jirapinyo P et al. Gastrointest Endosc. 2017;85(5):AB93-94.
41. Jirapinyo P, Thompson CC et al. Comparison of a novel plication technique to suturing for endoscopic outlet reduction for the treatment of weight regain after Roux-en-Y gastric bypass. Obesity Week 2018. Poster presentation.
42. Kaplan LM et al. EndoBarrier therapy is associated with glycemic improvement, weight loss and safety issues in patients with obesity and type 2 diabetes on oral anti-hyperglycemic agents (The ENDO Trial). In: Oral Presentation at the 76th American Diabetes Association (ADA) Annual Meeting: 2016 June 10-14: New Orleans. Abstract number 362-LB.
43. Jirapinyo P et al. Diabetes Care. 2018;41(5):1106-15.
44. Rajagopalan H et al. Diabetes Care. 2016;39(12):2254-61.
45. Sandler BJ et al. Surgical Endosc. 2015;29:3298-303.
46. Machytka E et al. Gastrointest Endosc. 2017;86(5):904-12.
Editor's Note
Gastroenterologists are becoming increasingly involved in the management of obesity. While prior therapy for obesity was mainly based on lifestyle changes, medication, or surgery, the new and exciting field of endoscopic bariatric and metabolic therapies has recently garnered incredible attention and momentum.
In this quarter’s In Focus article, brought to you by The New Gastroenterologist, Pichamol Jirapinyo and Christopher Thompson (Brigham and Women’s Hospital) provide an outstanding overview of the gastric and small bowel endoscopic interventions that are either already approved for use in obesity or currently being studied. This field is moving incredibly fast, and knowledge and understanding of these endoscopic therapies for obesity will undoubtedly be important for our field.
Bryson W. Katona, MD, PhD
Editor in Chief, The New Gastroenterologist
Introduction
Obesity is a rising pandemic. As of 2016, 93.3 million U.S. adults had obesity, representing 39.8% of our adult population.1 It is estimated that approximately $147 billion is spent annually on caring for patients with obesity. Traditionally, the management of obesity includes lifestyle therapy (diet and exercise), pharmacotherapy (six Food and Drug Administration–approved medications for obesity), and bariatric surgery (sleeve gastrectomy [SG] and Roux-en-Y gastric bypass [RYGB]). Nevertheless, intensive lifestyle intervention and pharmacotherapy are associated with approximately 3.1%-6.6% total weight loss (TWL),2-7 and bariatric surgery is associated with 20%-33.3% TWL.8 However, less than 2% of patients who are eligible for bariatric surgery elect to undergo surgery, leaving a large proportion of patients with obesity untreated or undertreated.9

Endoscopic bariatric and metabolic therapies (EBMTs) encompass an emerging field for the treatment of obesity. In general, EBMTs are associated with greater weight loss than are lifestyle intervention and pharmacotherapy, but with a less- invasive risk profile than bariatric surgery. EBMTs may be divided into two general categories – gastric and small bowel interventions (Figure 1 and Table 1). Gastric EBMTs are effective at treating obesity, while small bowel EBMTs are effective at treating metabolic diseases with a variable weight loss profile depending on the device.10,11

Of note, a variety of study designs (including retrospective series, prospective series, and randomized trials with and without shams) have been employed, which can affect outcomes. Therefore, weight loss comparisons among studies are challenging and should be considered in this context.
Gastric interventions
Currently, there are three types of EBMTs that are FDA approved and used for the treatment of obesity. These include intragastric balloons (IGBs), plications and suturing, and aspiration therapy (AT). Other technologies that are under investigation also will be briefly covered.
Intragastric balloons
An intragastric balloon is a space-occupying device that is placed in the stomach. The mechanism of action of IGBs involves delaying gastric emptying, which leads to increased satiety.12 There are several types of IGBs available worldwide differing in techniques of placement and removal (endoscopic versus fluoroscopic versus swallowable), materials used to fill the balloon (fluid-filled versus air-filled), and the number of balloons placed (single versus duo versus three-balloon). At the time of this writing, three IGBs are approved by the FDA (Orbera, ReShape, and Obalon), all for patients with body mass indexes of 30-40 kg/m2, and two others are in the process of obtaining FDA approval (Spatz and Elipse).
Orbera gastric balloon (Apollo Endosurgery, Austin, Tex.) is a single fluid-filled IGB that is endoscopically placed and removed at 6 months. The balloon is filled with 400-700 cc of saline with or without methylene blue (to identify leakage or rupture). Recently, Orbera365, which allows the balloon to stay for 12 months instead of 6 months, has become available in Europe; however, it is yet to be approved in the United States. The U.S. pivotal trial (Orbera trial) including 255 subjects (125 Orbera arm versus 130 non-sham control arm) demonstrated 10.2% TWL in the Orbera group compared with 3.3% TWL in the control group at 6 months based on intention-to-treat (ITT) analysis. This difference persisted at 12 months (6 months after explantation) with 7.6% TWL for the Orbera group versus 3.1% TWL for the control group.13,14
ReShape integrated dual balloon system (ReShape Lifesciences, San Clemente, Calif.) consists of two connected fluid-filled balloons that are endoscopically placed and removed at 6 months. Each balloon is filled with 375-450 cc of saline mixed with methylene blue. The U.S. pivotal trial (REDUCE trial) including 326 subjects (187 ReShape arm versus 139 sham arm) demonstrated 6.8% TWL in the ReShape group compared with 3.3% TWL in the sham group at 6 months based on ITT analysis.15,16
Obalon balloon system (Obalon Therapeutics, Carlsbad, Calif.) is a swallowable, gas-filled balloon system that requires endoscopy only for removal. During placement, a capsule is swallowed under fluoroscopic guidance. The balloon is then inflated with 250 cc of nitrogen mix gas prior to tube detachment. Up to three balloons may be swallowed sequentially at 1-month intervals. At 6 months from the first balloon placement, all balloons are removed endoscopically. The U.S. pivotal trial (SMART trial) including 366 subjects (185 Obalon arm versus 181 sham capsule arm) demonstrated 6.6% TWL in the Obalon group compared with 3.4% TWL in the sham group at 6 months based on ITT analysis.17,18
Two other balloons that are currently under investigation in the United States are the Spatz3 adjustable balloon system (Spatz Medical, Great Neck, N.Y.) and Elipse balloon (Allurion Technologies, Wellesley, Mass.). The Spatz3 is a fluid-filled balloon that is placed and removed endoscopically. It consists of a single balloon and a connecting tube that allows volume adjustment for control of symptoms and possible augmentation of weight loss. The U.S. pivotal trial was recently completed and the data are being reviewed by the FDA. The Elipse is a swallowable fluid-filled balloon that does not require endoscopy for placement or removal. At 4 months, the balloon releases fluid allowing it to empty and pass naturally. The U.S. pivotal trial (ENLIGHTEN trial) is currently underway.
A meta-analysis of randomized controlled trials revealed improvement in most metabolic parameters (diastolic blood pressure, fasting glucose, hemoglobin A1c, and waist circumference) following IGB compared with controls.19 Nausea and vomiting are seen in approximately 30% and should be addressed appropriately. Pooled serious adverse event (SAE) rate was 1.5%, which included migration, perforation, and death. Since 2016, 14 deaths have been reported according to the FDA MAUDE database. Corporate response was that over 295,000 balloons had been distributed worldwide with a mortality rate of less than 0.01%.20
Plication and suturing
Currently, there are two endoscopic devices that are approved for the general indication of tissue apposition. These include the Incisionless Operating Platform (IOP) (USGI Medical, San Clemente, Calif.) and the Overstitch endoscopic suturing system (Apollo Endosurgery, Austin, Tex.). These devices are used to remodel the stomach to create a sleeve-like structure to induce weight loss.
The IOP system consists of a transport, which is a 54-Fr flexible endoscope. It consists of four working channels that accommodate a G-Prox (for tissue approximation), a G-Lix (for tissue grasping), and an ultrathin endoscope (for visualization). In April 2008, Horgan performed the first-in-human primary obesity surgery endoluminal (POSE) procedure in Argentina. The procedure involves the use of the IOP system to place plications primarily in the fundus to modify gastric accommodation.21 The U.S. pivotal trial (ESSENTIAL trial) including 332 subjects (221 POSE arm versus 111 sham arm) demonstrated 5.0% TWL in the POSE group compared with 1.4% in the sham group at 12 months based on ITT analysis.22 A European multicenter randomized controlled trial (MILEPOST trial) including 44 subjects (34 POSE arm versus 10 non-sham control arm) demonstrated 13.0% TWL in the POSE group compared with 5.3% TWL in the control group at 12 months.23 A recent meta-analysis including five studies with 586 subjects showed pooled weight loss of 13.2% at 12-15 months following POSE with a pooled serious adverse event rate of 3.2%.24 These included extraluminal bleeding, minor bleeding at the suture site, hepatic abscess, chest pain, nausea, vomiting, and abdominal pain. A distal POSE procedure with a new plication pattern focusing on the gastric body to augment the effect on gastric emptying has also been described.25
The Overstitch is an endoscopic suturing device that is mounted on a double-channel endoscope. At the tip of the scope, there is a curved suture arm and an anchor exchange that allow the needle to pass back and forth to perform full-thickness bites. The tissue helix may also be placed through the second channel to grasp tissue. In April 2012, Thompson performed the first-in-human endoscopic sutured/sleeve gastroplasty (ESG) procedure in India, which was published together with cases performed in Panama and the Dominican Republic.26-28 This procedure involves the use of the Overstitch device to place several sets of running sutures along the greater curvature of the stomach to create a sleeve-like structure. It is thought to delay gastric emptying and therefore increase satiety.29 The largest multicenter retrospective study including 248 patients demonstrated 18.6% TWL at 2 years with 2% SAE rate including perigastric fluid collections, extraluminal hemorrhage, pulmonary embolism, pneumoperitoneum, and pneumothorax.30
Aspiration therapy
Aspiration therapy (AT; Aspire Bariatrics, King of Prussia, Pa.) allows patients to remove 25%-30% of ingested calories at approximately 30 minutes after meals. AT consists of an A-tube, which is a 26-Fr gastrostomy tube with a 15-cm fenestrated drainage catheter placed endoscopically via a standard pull technique. At 1-2 weeks after A-tube placement, the tube is cut down to the skin and connected to the port prior to aspiration. AT is approved for patients with a BMI of 35-55 kg/m2.31 The U.S. pivotal trial (PATHWAY trial) including 207 subjects (137 AT arm versus 70 non-sham control arm) demonstrated 12.1% TWL in the AT group compared to 3.5% in the control group at 12 months based on ITT analysis. The SAE rate was 3.6% including severe abdominal pain, peritonitis, prepyloric ulcer, and A-tube replacement due to skin-port malfunction.32
Transpyloric shuttle
The transpyloric shuttle (TPS; BAROnova, Goleta, Calif.) consists of a spherical bulb that is attached to a smaller cylindrical bulb by a flexible tether. It is placed and removed endoscopically at 6 months. TPS resides across the pylorus creating intermittent obstruction that may result in delayed gastric emptying. A pilot study including 20 patients demonstrated 14.5% TWL at 6 months.33 The U.S. pivotal trial (ENDObesity II trial) was recently completed and the data are being reviewed by the FDA.
Revision for weight regain following bariatric surgery

Weight regain is common following RYGB34,35 and can be associated with dilation of the gastrojejunal anastomosis (GJA).36 Several procedures have been developed to treat this condition by focusing on reduction of GJA size and are available in the United States (Figure 2). These procedures have level I evidence supporting their use and include transoral outlet reduction (TORe) and restorative obesity surgery endoluminal (ROSE).37 TORe involves the use of the Overstitch to place sutures at the GJA. At 1 year, patients had 8.4% TWL with improvement in comorbidities.38 Weight loss remained significant up to 3-5 years.39,40 The modern ROSE procedure utilizes the IOP system to place plications at the GJA and distal gastric pouch following argon plasma coagulation (APC). A small series showed 12.4% TWL at 6 months.41 APC is also currently being investigated as a standalone therapy for weight regain in this population.
Small bowel interventions
There are several small bowel interventions, with different mechanisms of action, available internationally. Many of these are under investigation in the United States; however, none are currently FDA approved.
Duodenal-jejunal bypass liner
Duodenal-jejunal bypass liner (DJBL; GI Dynamics, Boston, Mass.) is a 60-cm fluoropolymer liner that is endoscopically placed and removed at 12 months. It is anchored at the duodenal bulb and ends at the jejunum. By excluding direct contact between chyme and the proximal small bowel, DJBL is thought to work via foregut mechanism where there is less inhibition of the incretin effect (greater increase in insulin secretion following oral glucose administration compared to intravenous glucose administration due to gut-derived factors that enhance insulin secretion) leading to improved insulin resistance. In addition, the enteral transit of chyme and bile is altered suggesting the possible role of the hindgut mechanism. The previous U.S. pivotal trial (ENDO trial) met efficacy endpoints. However, the study was stopped early by the company because of a hepatic abscess rate of 3.5%, all of which were treated conservatively.42 A new U.S. pivotal study is currently planned. A meta-analysis of 17 published studies, all of which were from outside the United States, demonstrated a significant decrease in hemoglobin A1c of 1.3% and 18.9% TWL at 1 year following implantation in patients with obesity with concomitant diabetes.43
Duodenal mucosal resurfacing
Duodenal mucosal resurfacing (Fractyl, Lexington, Mass.) involves saline lifting of the duodenal mucosa circumferentially prior to thermal ablation using an inflated balloon filled with heated water. It is hypothesized that this may reset the diseased duodenal enteroendocrine cells leading to restoration of the incretin effect. A pilot study including 39 patients with poorly controlled diabetes demonstrated a decrease in hemoglobin A1c of 1.2%. The SAE rate was 7.7% including duodenal stenosis, all of which were treated with balloon dilation.44 The U.S. pivotal trial is currently planned.
Gastroduodenal-jejunal bypass
Gastroduodenal-jejunal bypass (ValenTx., Hopkins, Minn.) is a 120-cm sleeve that is anchored at the gastroesophageal junction to create the anatomic changes of RYGB. It is placed and removed endoscopically with laparoscopic assistance. A pilot study including 12 patients demonstrated 35.9% excess weight loss at 12 months. Two out of 12 patients had early device removal due to intolerance and they were not included in the weight loss analysis.45
Incisionless magnetic anastomosis system
The incisionless magnetic anastomosis system (GI Windows, West Bridgewater, Mass.) consists of self-assembling magnets that are deployed under fluoroscopic guidance through the working channel of colonoscopes to form magnetic octagons in the jejunum and ileum. After a week, a compression anastomosis is formed and the coupled magnets pass spontaneously. A pilot study including 10 patients showed 14.6% TWL and a decrease in hemoglobin A1c of 1.9% (for patients with diabetes) at 1 year.46 A randomized study outside the United States is currently underway.
Summary
Endoscopic bariatric and metabolic therapies are emerging as first-line treatments for obesity in many populations. They can serve as a gap therapy for patients who do not qualify for surgery, but also may have a specific role in the treatment of metabolic comorbidities. This field will continue to develop and improve with the introduction of personalized medicine leading to better patient selection, and newer combination therapies. It is time for gastroenterologists to become more involved in the management of this challenging condition.
Dr. Jirapinyo is an advanced and bariatric endoscopy fellow, Brigham and Women’s Hospital, Harvard Medical School, Boston; Dr. Thompson is director of therapeutic endoscopy, Brigham and Women’s Hospital, and associate professor of medicine, Harvard Medical School. Dr. Jirapinyo has served as a consultant for GI Dynamics and holds royalties for Endosim. Dr. Thompson has contracted research for Aspire Bariatrics, USGI Medical, Spatz, and Apollo Endosurgery; has served as a consultant for Boston Scientific, Covidien, USGI Medical, Olympus, and Fractyl; holds stocks and royalties for GI Windows and Endosim, and has served as an expert reviewer for GI Dynamics.
References
1. CDC. From https://www.cdc.gov/obesity/data/adult.html. Accessed on 11 September 2018.
2. Aronne LJ et al. Obesity. 2013;21:2163-71.
3. Torgerson JS et al. Diabetes Care. 2004;27:155-61.
4. Allison DB et al. Obesity. 2012;20:330-42.
5. Smith SR et al. N Engl J Med. 2010;363:245-56.
6. Apovian CM et al. Obesity. 2013;21:935-43.
7. Pi-Sunyer X et al. N Engl J Med. 2015;373:11-22.
8. Colguitt JL et al. Cochrane Database Syst Rev. 2014;8(8):CD003641.
9. Ponce J et al. Surg Obes Relat Dis. 2015;11(6):1199-200.
10. Jirapinyo P, Thompson CC et al. Clin Gastroenterol Hepatol. 2017;15(5):619-30.
11. Sullivan S et al.Gastroenterology. 2017;152(7):1791-801.
12. Gomez V et al. Obesity. 2016;24(9):1849-53.
13. Food and Drug Administration. Summary of safety and effectiveness data (SSED) ORBERA Intragastric Balloon System. Available at https://www.accessdata.fda.gov/cdrh_docs/pdf14/P140008b.pdf. 2015:1-32.
14. Abu Dayyeh BK et al. Gastrointest Endosc. 2015;81:AB147.
15. Food and Drug Administration. Summary of safety and effectiveness data (SSED) ReShape Integrated Dual Balloon System. Available at https://www.accessdata.fda.gov/cdrh_docs/pdf14/P140012b.pdf. 2015:1-43.
16. Ponce J et al. Surg Obes Relat Dis. 2015;11:874-81.
17. Food and Drug Administration. Summary and effectiveness data (SSED): Obalon Balloon System. Available at https://www.accessdata.fda.gov/cdrh_docs/pdf16/P160001b.pdf. 2016:1-46.
18. Sullivan S et al. Gastroenterology. 2016;150:S1267.
19. Popov VB et al. Am J Gastroenterol. 2017;112:429-39.
20. Abu Dayyeh BK et al. Gastrointest Endosc. 2015;82(3):425-38.
21. Espinos JC et al. Obes Surg. 2013;23(9):1375-83.
22. Sullivan S et al. Obesity. 2017;25:294-301.
23. Miller K et al. Obesity Surg. 2017;27(2):310-22.
24. Jirapinyo P et al. Gastrointest Endosc. 2018;87(6):AB604-AB605.
25. Jirapinyo P, Thompson CC. Video GIE. 2018;3(10):296-300.
26. Campos J et al. SAGES 2013 Presentation. Baltimore, MD. 19 April 2013.
27. Kumar N et al. Gastroenterology. 2014;146(5):S571-2.
28. Kumar N et al. Surg Endosc. 2018;32(4):2159-64.
29. Abu Dayyeh BK et al. Clin Gastroenterol Hepatol. 2017;15:37-43.
30. Lopez-Nava G et al. Obes Surg. 2017;27(10):2649-55.
31. Food and Drug Administration. Summary of safety and effectiveness (SSED): AspireAssist. Available at https://www.accessdata.fda.gov/cdrh_docs/pdf15/p150024b.pdf. FDA,ed,2016:1-36.
32. Thompson CC et al. Am J Gastroenterol. 2017;112:447-57.
33. SAGES abstract archives. SAGES. Available from: http://www.sages.org/meetings/annual-meeting/abstracts-archive/first-clinical-experience-with-the-transpyloric-shuttle-tpsr-device-a-non-surgical-endoscopic treatment-for-obesity-results-from-a-3-month-and-6-month-study. Accessed Sept. 12, 2018.
34. Sjostrom L et al. N Engl J Med. 2007;357:741-52.
35. Adams TD et al. N Engl J Med. 2017;377:1143-55.
36. Abu Dayyeh BK et al. Clin Gastroenterol Hepatol. 2011;9:228-33.
37. Thompson CC et al. Gastroenterology. 2013;145(1):129-37.
38. Jirapinyo P et al. Endoscopy. 2018;50(4):371-7.
39. Kumar N, Thompson CC. Gastrointest Endosc. 2016;83(4):776-9.
40. Jirapinyo P et al. Gastrointest Endosc. 2017;85(5):AB93-94.
41. Jirapinyo P, Thompson CC et al. Comparison of a novel plication technique to suturing for endoscopic outlet reduction for the treatment of weight regain after Roux-en-Y gastric bypass. Obesity Week 2018. Poster presentation.
42. Kaplan LM et al. EndoBarrier therapy is associated with glycemic improvement, weight loss and safety issues in patients with obesity and type 2 diabetes on oral anti-hyperglycemic agents (The ENDO Trial). In: Oral Presentation at the 76th American Diabetes Association (ADA) Annual Meeting: 2016 June 10-14: New Orleans. Abstract number 362-LB.
43. Jirapinyo P et al. Diabetes Care. 2018;41(5):1106-15.
44. Rajagopalan H et al. Diabetes Care. 2016;39(12):2254-61.
45. Sandler BJ et al. Surgical Endosc. 2015;29:3298-303.
46. Machytka E et al. Gastrointest Endosc. 2017;86(5):904-12.
Editor's Note
Gastroenterologists are becoming increasingly involved in the management of obesity. While prior therapy for obesity was mainly based on lifestyle changes, medication, or surgery, the new and exciting field of endoscopic bariatric and metabolic therapies has recently garnered incredible attention and momentum.
In this quarter’s In Focus article, brought to you by The New Gastroenterologist, Pichamol Jirapinyo and Christopher Thompson (Brigham and Women’s Hospital) provide an outstanding overview of the gastric and small bowel endoscopic interventions that are either already approved for use in obesity or currently being studied. This field is moving incredibly fast, and knowledge and understanding of these endoscopic therapies for obesity will undoubtedly be important for our field.
Bryson W. Katona, MD, PhD
Editor in Chief, The New Gastroenterologist
Introduction
Obesity is a rising pandemic. As of 2016, 93.3 million U.S. adults had obesity, representing 39.8% of our adult population.1 It is estimated that approximately $147 billion is spent annually on caring for patients with obesity. Traditionally, the management of obesity includes lifestyle therapy (diet and exercise), pharmacotherapy (six Food and Drug Administration–approved medications for obesity), and bariatric surgery (sleeve gastrectomy [SG] and Roux-en-Y gastric bypass [RYGB]). Nevertheless, intensive lifestyle intervention and pharmacotherapy are associated with approximately 3.1%-6.6% total weight loss (TWL),2-7 and bariatric surgery is associated with 20%-33.3% TWL.8 However, less than 2% of patients who are eligible for bariatric surgery elect to undergo surgery, leaving a large proportion of patients with obesity untreated or undertreated.9

Endoscopic bariatric and metabolic therapies (EBMTs) encompass an emerging field for the treatment of obesity. In general, EBMTs are associated with greater weight loss than are lifestyle intervention and pharmacotherapy, but with a less- invasive risk profile than bariatric surgery. EBMTs may be divided into two general categories – gastric and small bowel interventions (Figure 1 and Table 1). Gastric EBMTs are effective at treating obesity, while small bowel EBMTs are effective at treating metabolic diseases with a variable weight loss profile depending on the device.10,11

Of note, a variety of study designs (including retrospective series, prospective series, and randomized trials with and without shams) have been employed, which can affect outcomes. Therefore, weight loss comparisons among studies are challenging and should be considered in this context.
Gastric interventions
Currently, there are three types of EBMTs that are FDA approved and used for the treatment of obesity. These include intragastric balloons (IGBs), plications and suturing, and aspiration therapy (AT). Other technologies that are under investigation also will be briefly covered.
Intragastric balloons
An intragastric balloon is a space-occupying device that is placed in the stomach. The mechanism of action of IGBs involves delaying gastric emptying, which leads to increased satiety.12 There are several types of IGBs available worldwide differing in techniques of placement and removal (endoscopic versus fluoroscopic versus swallowable), materials used to fill the balloon (fluid-filled versus air-filled), and the number of balloons placed (single versus duo versus three-balloon). At the time of this writing, three IGBs are approved by the FDA (Orbera, ReShape, and Obalon), all for patients with body mass indexes of 30-40 kg/m2, and two others are in the process of obtaining FDA approval (Spatz and Elipse).
Orbera gastric balloon (Apollo Endosurgery, Austin, Tex.) is a single fluid-filled IGB that is endoscopically placed and removed at 6 months. The balloon is filled with 400-700 cc of saline with or without methylene blue (to identify leakage or rupture). Recently, Orbera365, which allows the balloon to stay for 12 months instead of 6 months, has become available in Europe; however, it is yet to be approved in the United States. The U.S. pivotal trial (Orbera trial) including 255 subjects (125 Orbera arm versus 130 non-sham control arm) demonstrated 10.2% TWL in the Orbera group compared with 3.3% TWL in the control group at 6 months based on intention-to-treat (ITT) analysis. This difference persisted at 12 months (6 months after explantation) with 7.6% TWL for the Orbera group versus 3.1% TWL for the control group.13,14
ReShape integrated dual balloon system (ReShape Lifesciences, San Clemente, Calif.) consists of two connected fluid-filled balloons that are endoscopically placed and removed at 6 months. Each balloon is filled with 375-450 cc of saline mixed with methylene blue. The U.S. pivotal trial (REDUCE trial) including 326 subjects (187 ReShape arm versus 139 sham arm) demonstrated 6.8% TWL in the ReShape group compared with 3.3% TWL in the sham group at 6 months based on ITT analysis.15,16
Obalon balloon system (Obalon Therapeutics, Carlsbad, Calif.) is a swallowable, gas-filled balloon system that requires endoscopy only for removal. During placement, a capsule is swallowed under fluoroscopic guidance. The balloon is then inflated with 250 cc of nitrogen mix gas prior to tube detachment. Up to three balloons may be swallowed sequentially at 1-month intervals. At 6 months from the first balloon placement, all balloons are removed endoscopically. The U.S. pivotal trial (SMART trial) including 366 subjects (185 Obalon arm versus 181 sham capsule arm) demonstrated 6.6% TWL in the Obalon group compared with 3.4% TWL in the sham group at 6 months based on ITT analysis.17,18
Two other balloons that are currently under investigation in the United States are the Spatz3 adjustable balloon system (Spatz Medical, Great Neck, N.Y.) and Elipse balloon (Allurion Technologies, Wellesley, Mass.). The Spatz3 is a fluid-filled balloon that is placed and removed endoscopically. It consists of a single balloon and a connecting tube that allows volume adjustment for control of symptoms and possible augmentation of weight loss. The U.S. pivotal trial was recently completed and the data are being reviewed by the FDA. The Elipse is a swallowable fluid-filled balloon that does not require endoscopy for placement or removal. At 4 months, the balloon releases fluid allowing it to empty and pass naturally. The U.S. pivotal trial (ENLIGHTEN trial) is currently underway.
A meta-analysis of randomized controlled trials revealed improvement in most metabolic parameters (diastolic blood pressure, fasting glucose, hemoglobin A1c, and waist circumference) following IGB compared with controls.19 Nausea and vomiting are seen in approximately 30% and should be addressed appropriately. Pooled serious adverse event (SAE) rate was 1.5%, which included migration, perforation, and death. Since 2016, 14 deaths have been reported according to the FDA MAUDE database. Corporate response was that over 295,000 balloons had been distributed worldwide with a mortality rate of less than 0.01%.20
Plication and suturing
Currently, there are two endoscopic devices that are approved for the general indication of tissue apposition. These include the Incisionless Operating Platform (IOP) (USGI Medical, San Clemente, Calif.) and the Overstitch endoscopic suturing system (Apollo Endosurgery, Austin, Tex.). These devices are used to remodel the stomach to create a sleeve-like structure to induce weight loss.
The IOP system consists of a transport, which is a 54-Fr flexible endoscope. It consists of four working channels that accommodate a G-Prox (for tissue approximation), a G-Lix (for tissue grasping), and an ultrathin endoscope (for visualization). In April 2008, Horgan performed the first-in-human primary obesity surgery endoluminal (POSE) procedure in Argentina. The procedure involves the use of the IOP system to place plications primarily in the fundus to modify gastric accommodation.21 The U.S. pivotal trial (ESSENTIAL trial) including 332 subjects (221 POSE arm versus 111 sham arm) demonstrated 5.0% TWL in the POSE group compared with 1.4% in the sham group at 12 months based on ITT analysis.22 A European multicenter randomized controlled trial (MILEPOST trial) including 44 subjects (34 POSE arm versus 10 non-sham control arm) demonstrated 13.0% TWL in the POSE group compared with 5.3% TWL in the control group at 12 months.23 A recent meta-analysis including five studies with 586 subjects showed pooled weight loss of 13.2% at 12-15 months following POSE with a pooled serious adverse event rate of 3.2%.24 These included extraluminal bleeding, minor bleeding at the suture site, hepatic abscess, chest pain, nausea, vomiting, and abdominal pain. A distal POSE procedure with a new plication pattern focusing on the gastric body to augment the effect on gastric emptying has also been described.25
The Overstitch is an endoscopic suturing device that is mounted on a double-channel endoscope. At the tip of the scope, there is a curved suture arm and an anchor exchange that allow the needle to pass back and forth to perform full-thickness bites. The tissue helix may also be placed through the second channel to grasp tissue. In April 2012, Thompson performed the first-in-human endoscopic sutured/sleeve gastroplasty (ESG) procedure in India, which was published together with cases performed in Panama and the Dominican Republic.26-28 This procedure involves the use of the Overstitch device to place several sets of running sutures along the greater curvature of the stomach to create a sleeve-like structure. It is thought to delay gastric emptying and therefore increase satiety.29 The largest multicenter retrospective study including 248 patients demonstrated 18.6% TWL at 2 years with 2% SAE rate including perigastric fluid collections, extraluminal hemorrhage, pulmonary embolism, pneumoperitoneum, and pneumothorax.30
Aspiration therapy
Aspiration therapy (AT; Aspire Bariatrics, King of Prussia, Pa.) allows patients to remove 25%-30% of ingested calories at approximately 30 minutes after meals. AT consists of an A-tube, which is a 26-Fr gastrostomy tube with a 15-cm fenestrated drainage catheter placed endoscopically via a standard pull technique. At 1-2 weeks after A-tube placement, the tube is cut down to the skin and connected to the port prior to aspiration. AT is approved for patients with a BMI of 35-55 kg/m2.31 The U.S. pivotal trial (PATHWAY trial) including 207 subjects (137 AT arm versus 70 non-sham control arm) demonstrated 12.1% TWL in the AT group compared to 3.5% in the control group at 12 months based on ITT analysis. The SAE rate was 3.6% including severe abdominal pain, peritonitis, prepyloric ulcer, and A-tube replacement due to skin-port malfunction.32
Transpyloric shuttle
The transpyloric shuttle (TPS; BAROnova, Goleta, Calif.) consists of a spherical bulb that is attached to a smaller cylindrical bulb by a flexible tether. It is placed and removed endoscopically at 6 months. TPS resides across the pylorus creating intermittent obstruction that may result in delayed gastric emptying. A pilot study including 20 patients demonstrated 14.5% TWL at 6 months.33 The U.S. pivotal trial (ENDObesity II trial) was recently completed and the data are being reviewed by the FDA.
Revision for weight regain following bariatric surgery

Weight regain is common following RYGB34,35 and can be associated with dilation of the gastrojejunal anastomosis (GJA).36 Several procedures have been developed to treat this condition by focusing on reduction of GJA size and are available in the United States (Figure 2). These procedures have level I evidence supporting their use and include transoral outlet reduction (TORe) and restorative obesity surgery endoluminal (ROSE).37 TORe involves the use of the Overstitch to place sutures at the GJA. At 1 year, patients had 8.4% TWL with improvement in comorbidities.38 Weight loss remained significant up to 3-5 years.39,40 The modern ROSE procedure utilizes the IOP system to place plications at the GJA and distal gastric pouch following argon plasma coagulation (APC). A small series showed 12.4% TWL at 6 months.41 APC is also currently being investigated as a standalone therapy for weight regain in this population.
Small bowel interventions
There are several small bowel interventions, with different mechanisms of action, available internationally. Many of these are under investigation in the United States; however, none are currently FDA approved.
Duodenal-jejunal bypass liner
Duodenal-jejunal bypass liner (DJBL; GI Dynamics, Boston, Mass.) is a 60-cm fluoropolymer liner that is endoscopically placed and removed at 12 months. It is anchored at the duodenal bulb and ends at the jejunum. By excluding direct contact between chyme and the proximal small bowel, DJBL is thought to work via foregut mechanism where there is less inhibition of the incretin effect (greater increase in insulin secretion following oral glucose administration compared to intravenous glucose administration due to gut-derived factors that enhance insulin secretion) leading to improved insulin resistance. In addition, the enteral transit of chyme and bile is altered suggesting the possible role of the hindgut mechanism. The previous U.S. pivotal trial (ENDO trial) met efficacy endpoints. However, the study was stopped early by the company because of a hepatic abscess rate of 3.5%, all of which were treated conservatively.42 A new U.S. pivotal study is currently planned. A meta-analysis of 17 published studies, all of which were from outside the United States, demonstrated a significant decrease in hemoglobin A1c of 1.3% and 18.9% TWL at 1 year following implantation in patients with obesity with concomitant diabetes.43
Duodenal mucosal resurfacing
Duodenal mucosal resurfacing (Fractyl, Lexington, Mass.) involves saline lifting of the duodenal mucosa circumferentially prior to thermal ablation using an inflated balloon filled with heated water. It is hypothesized that this may reset the diseased duodenal enteroendocrine cells leading to restoration of the incretin effect. A pilot study including 39 patients with poorly controlled diabetes demonstrated a decrease in hemoglobin A1c of 1.2%. The SAE rate was 7.7% including duodenal stenosis, all of which were treated with balloon dilation.44 The U.S. pivotal trial is currently planned.
Gastroduodenal-jejunal bypass
Gastroduodenal-jejunal bypass (ValenTx., Hopkins, Minn.) is a 120-cm sleeve that is anchored at the gastroesophageal junction to create the anatomic changes of RYGB. It is placed and removed endoscopically with laparoscopic assistance. A pilot study including 12 patients demonstrated 35.9% excess weight loss at 12 months. Two out of 12 patients had early device removal due to intolerance and they were not included in the weight loss analysis.45
Incisionless magnetic anastomosis system
The incisionless magnetic anastomosis system (GI Windows, West Bridgewater, Mass.) consists of self-assembling magnets that are deployed under fluoroscopic guidance through the working channel of colonoscopes to form magnetic octagons in the jejunum and ileum. After a week, a compression anastomosis is formed and the coupled magnets pass spontaneously. A pilot study including 10 patients showed 14.6% TWL and a decrease in hemoglobin A1c of 1.9% (for patients with diabetes) at 1 year.46 A randomized study outside the United States is currently underway.
Summary
Endoscopic bariatric and metabolic therapies are emerging as first-line treatments for obesity in many populations. They can serve as a gap therapy for patients who do not qualify for surgery, but also may have a specific role in the treatment of metabolic comorbidities. This field will continue to develop and improve with the introduction of personalized medicine leading to better patient selection, and newer combination therapies. It is time for gastroenterologists to become more involved in the management of this challenging condition.
Dr. Jirapinyo is an advanced and bariatric endoscopy fellow, Brigham and Women’s Hospital, Harvard Medical School, Boston; Dr. Thompson is director of therapeutic endoscopy, Brigham and Women’s Hospital, and associate professor of medicine, Harvard Medical School. Dr. Jirapinyo has served as a consultant for GI Dynamics and holds royalties for Endosim. Dr. Thompson has contracted research for Aspire Bariatrics, USGI Medical, Spatz, and Apollo Endosurgery; has served as a consultant for Boston Scientific, Covidien, USGI Medical, Olympus, and Fractyl; holds stocks and royalties for GI Windows and Endosim, and has served as an expert reviewer for GI Dynamics.
References
1. CDC. From https://www.cdc.gov/obesity/data/adult.html. Accessed on 11 September 2018.
2. Aronne LJ et al. Obesity. 2013;21:2163-71.
3. Torgerson JS et al. Diabetes Care. 2004;27:155-61.
4. Allison DB et al. Obesity. 2012;20:330-42.
5. Smith SR et al. N Engl J Med. 2010;363:245-56.
6. Apovian CM et al. Obesity. 2013;21:935-43.
7. Pi-Sunyer X et al. N Engl J Med. 2015;373:11-22.
8. Colguitt JL et al. Cochrane Database Syst Rev. 2014;8(8):CD003641.
9. Ponce J et al. Surg Obes Relat Dis. 2015;11(6):1199-200.
10. Jirapinyo P, Thompson CC et al. Clin Gastroenterol Hepatol. 2017;15(5):619-30.
11. Sullivan S et al.Gastroenterology. 2017;152(7):1791-801.
12. Gomez V et al. Obesity. 2016;24(9):1849-53.
13. Food and Drug Administration. Summary of safety and effectiveness data (SSED) ORBERA Intragastric Balloon System. Available at https://www.accessdata.fda.gov/cdrh_docs/pdf14/P140008b.pdf. 2015:1-32.
14. Abu Dayyeh BK et al. Gastrointest Endosc. 2015;81:AB147.
15. Food and Drug Administration. Summary of safety and effectiveness data (SSED) ReShape Integrated Dual Balloon System. Available at https://www.accessdata.fda.gov/cdrh_docs/pdf14/P140012b.pdf. 2015:1-43.
16. Ponce J et al. Surg Obes Relat Dis. 2015;11:874-81.
17. Food and Drug Administration. Summary and effectiveness data (SSED): Obalon Balloon System. Available at https://www.accessdata.fda.gov/cdrh_docs/pdf16/P160001b.pdf. 2016:1-46.
18. Sullivan S et al. Gastroenterology. 2016;150:S1267.
19. Popov VB et al. Am J Gastroenterol. 2017;112:429-39.
20. Abu Dayyeh BK et al. Gastrointest Endosc. 2015;82(3):425-38.
21. Espinos JC et al. Obes Surg. 2013;23(9):1375-83.
22. Sullivan S et al. Obesity. 2017;25:294-301.
23. Miller K et al. Obesity Surg. 2017;27(2):310-22.
24. Jirapinyo P et al. Gastrointest Endosc. 2018;87(6):AB604-AB605.
25. Jirapinyo P, Thompson CC. Video GIE. 2018;3(10):296-300.
26. Campos J et al. SAGES 2013 Presentation. Baltimore, MD. 19 April 2013.
27. Kumar N et al. Gastroenterology. 2014;146(5):S571-2.
28. Kumar N et al. Surg Endosc. 2018;32(4):2159-64.
29. Abu Dayyeh BK et al. Clin Gastroenterol Hepatol. 2017;15:37-43.
30. Lopez-Nava G et al. Obes Surg. 2017;27(10):2649-55.
31. Food and Drug Administration. Summary of safety and effectiveness (SSED): AspireAssist. Available at https://www.accessdata.fda.gov/cdrh_docs/pdf15/p150024b.pdf. FDA,ed,2016:1-36.
32. Thompson CC et al. Am J Gastroenterol. 2017;112:447-57.
33. SAGES abstract archives. SAGES. Available from: http://www.sages.org/meetings/annual-meeting/abstracts-archive/first-clinical-experience-with-the-transpyloric-shuttle-tpsr-device-a-non-surgical-endoscopic treatment-for-obesity-results-from-a-3-month-and-6-month-study. Accessed Sept. 12, 2018.
34. Sjostrom L et al. N Engl J Med. 2007;357:741-52.
35. Adams TD et al. N Engl J Med. 2017;377:1143-55.
36. Abu Dayyeh BK et al. Clin Gastroenterol Hepatol. 2011;9:228-33.
37. Thompson CC et al. Gastroenterology. 2013;145(1):129-37.
38. Jirapinyo P et al. Endoscopy. 2018;50(4):371-7.
39. Kumar N, Thompson CC. Gastrointest Endosc. 2016;83(4):776-9.
40. Jirapinyo P et al. Gastrointest Endosc. 2017;85(5):AB93-94.
41. Jirapinyo P, Thompson CC et al. Comparison of a novel plication technique to suturing for endoscopic outlet reduction for the treatment of weight regain after Roux-en-Y gastric bypass. Obesity Week 2018. Poster presentation.
42. Kaplan LM et al. EndoBarrier therapy is associated with glycemic improvement, weight loss and safety issues in patients with obesity and type 2 diabetes on oral anti-hyperglycemic agents (The ENDO Trial). In: Oral Presentation at the 76th American Diabetes Association (ADA) Annual Meeting: 2016 June 10-14: New Orleans. Abstract number 362-LB.
43. Jirapinyo P et al. Diabetes Care. 2018;41(5):1106-15.
44. Rajagopalan H et al. Diabetes Care. 2016;39(12):2254-61.
45. Sandler BJ et al. Surgical Endosc. 2015;29:3298-303.
46. Machytka E et al. Gastrointest Endosc. 2017;86(5):904-12.
Quality metrics in colonoscopy
Editor's Note:
As quality metrics are becoming increasingly significant throughout all of medicine, our field is no exception. Recent evidence has demonstrated the importance of quality measures in colonoscopy; understanding, reporting, and improving these metrics has become a hot topic of discussion.
In this month’s In Focus article, brought to you by The New Gastroenterologist, Nabiha Shamsi, Ashish Malhotra, and Aasma Shaukat (University of Minnesota/Minneapolis VAMC) provide an outstanding overview of the evidence as well as recommended goals for important quality metrics in colonoscopy. Ultimately, improving colonoscopy quality amongst all gastroenterologists will increase colonoscopy value and lead to further decreases in the incidence and mortality of colorectal cancer.
Bryson W. Katona, MD, PhD
Editor in Chief, The New Gastroenterologist
Introduction
Colonoscopy is a widely used modality to evaluate colorectal cancer because it allows for both identification of early malignancies and removal of precancerous lesions. The increased use of colonoscopy in the last 20 years has been associated with a decline in the incidence and mortality from colorectal cancer.1,2 However, colonoscopy has its limitations. It is an invasive test with inherent risks. Additionally, studies have reported rates of post-colonoscopy cancers, also referred to as interval cancers, of 2%-7%, and miss-rates for adenomas by tandem colonoscopy of 2%-26%.3-5
High-quality exams can maximize the value of colonoscopy, and it is important to consider the factors that contribute to high-quality colonoscopies. While there are many metrics proposed,6,7 here we discuss the most evidence-based ones, outlined in Table 1, along with their goal values.
Cecal intubation rate
A high-quality colonoscopy should include a complete examination of the colon. To achieve this, it is necessary to fully intubate the cecum, passing the colonoscope past the ileocecal valve to examine the medial wall of the cecum.8
There are several factors that may contribute to an incomplete colonoscopy, including bowel preparation, anatomy, body habitus, and endoscopist’s skill. To calculate cecal intubation rate as a quality measure, colonoscopies that are incomplete because of poor bowel preparation, severe colitis, or known obstructing lesion are usually excluded.
The U.S. Multi-Society Task Force on Colorectal Cancer recommends a cecal intubation rate of at least 95% for screening colonoscopy and 90% for all colonoscopies.6 There is an expectation of photodocumentation of the ileocecal valve and appendiceal orifice to establish completion of the colonoscopy.6
Some methods used to assist with cecal intubation include changing patient position, applying abdominal pressure, stiffening the colonoscope, and alternating between adult or pediatric colonoscopes.
Adenoma detection rate
Adenoma detection rate (ADR), is defined as the proportion of patients over the age of 50 years undergoing first-time screening colonoscopies in which at least one adenomatous polyp is detected for a given endoscopist in a given time period.
Adenomas are tracked because clearing the colon of neoplasm is the goal of screening colonoscopies; adenomas are tracked instead of more advanced lesions because the higher frequency of adenomas allows for better tracking of variation between endoscopists. Tracking ADR also utilizes the assumption that, if small lesions are identified, larger ones will be as well.
ADR is the only current quality indicator reported to be significantly associated with the risk of interval cancers. In 2010, a study of 45,000 screening colonoscopies by 186 endoscopists validated the use of ADR, finding that patients who underwent colonoscopy by physicians with ADRs below 20% had hazard ratios for development of postcolonoscopy cancer greater than 10 times higher than patients of physicians with ADRs above 20%.9 However, this study had limited power to establish that cancer protection continues to improve when ADRs rise above 20%. Another study, which evaluated the association of ADR in 224,000 patients undergoing colonoscopies by 136 gastroenterologists, showed each 1% increase in ADR is associated with 3% decrease in the risk of interval CRC and 5% decrease in the risk of fatal interval cancers.10
Most recent guidelines propose an adequate ADR for asymptomatic individuals aged 50 years or older undergoing screening colonoscopy should be greater than 30% in men and greater than 20% in women.6 It remains unknown whether there is a threshold for maximum benefit of ADR, in which a very high ADR is not associated with further protective benefit. The answer to this question may depend on why a low ADR is associated with a higher rate of interval cancers and whether every missed polyp, independent of size, is a potential interval cancer or whether hasty, inadequate, or incomplete examinations of the colon are the underlying concern.
Withdrawal time
Optimizing identification of colonic lesions requires a careful and thorough exam of the colon on withdrawal. While this may seem obvious, there is often little focus on the approach to withdrawal. In four chapters on colonoscopy technique from textbooks, the number of pages describing insertion ranged from 20 to 38, while the number of pages focused on withdrawal ranged from 0.5 to 1.5.11-14
A study examining the difference in withdrawal technique between two endoscopists who were known to differ in adenoma miss rates by tandem colonoscopy proposed the scoring system listed in Table 2 that can assess quality of examination on withdrawal. There was a statistically significant difference in quality scores for the two endoscopists, as assessed by expert review of video recordings of their colonoscopies.15
The endoscopist with the lower adenoma miss rate was also found to have an average withdrawal time of 8 minutes and 55 seconds versus 6 minutes and 41 seconds for the endoscopist with the higher adenoma miss rate. A large, community-based study with over 76,000 colonoscopies found a statistically significant correlation between interval colorectal cancer and withdrawal times shorter than 6 minutes.16 However, there was no association between ADR and colorectal cancer, suggesting that, for practices with optimal ADRs (that is, rates greater than 25%), withdrawal time may be a more sensitive marker of quality of colonoscopy than ADR is.16Intuitively, adequate examination of the colon that includes examining the proximal side of folds, washing and suctioning stool, and even repositioning the patient would likely increase withdrawal time. In a 2008 study examining 2,000 screening colonoscopies of 12 endoscopists, those with withdrawal times greater than 6 minutes had significantly higher rates of detecting adenomas and advanced neoplasia, compared with those with faster withdrawal times.17 The average ADR in this group was 28.3%, compared with 11.8% for physicians who had a withdrawal time less than 6 minutes.17 An evaluation of nearly 11,000 colonoscopies done by 43 endoscopists also identified an increase polyp yield with increased withdrawal time.18 These data drive the recommendation for a minimum withdrawal time of 6 minutes, with 2 minutes spent examining each colonic segment.
Bowel preparation
Diagnosis of colonic lesions is dependent on adequate visualization of the colon. Poor bowel preparation can limit the yield of colonoscopy and lead to missed lesions. It also leads to canceled and rescheduled procedures that reduce efficiency, increase cost, and pose an undue burden on the patient.
The quality of bowel preparation should be assessed after washing and suctioning of colonic mucosa has been completed. Adequate preparation is that which allows identification of lesions greater than 5 mm in size.19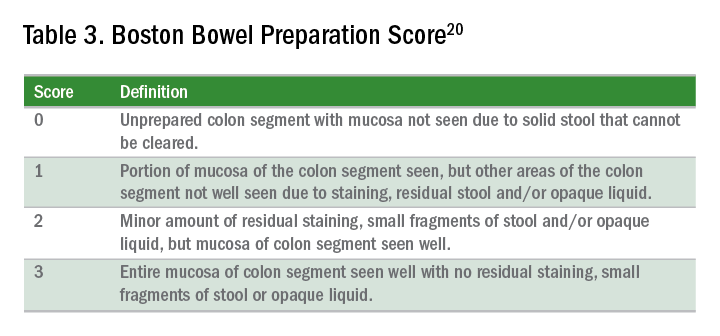
Quality of preparation is assessed subjectively by the endoscopists and often listed as excellent, good, fair, or poor. An alternative method of reporting bowel preparation quality is the Boston Bowel Preparation Score (BBPS) (Table 3).20 This scoring system allows for a more descriptive assessment of each colonic segment by assigning a score from 0 to 3 for the right, transverse, and left colon, leading to a total score between 0 and 9. The BBPS also helps standardize reporting of bowel preparation. The polyp detection rate associated with a BBPS of 5 or greater was 40%, compared with 24% associated with BBPS less than 5.19 A split-dose bowel preparation regimen with at least half of the preparation ingested on the day of the procedure is recommended to optimize quality of bowel preparation.6
The American Society for Gastrointestinal Endoscopy and American College of Gastroenterology task force on quality assurance in endoscopy recommends that bowel preparation should be adequate in 85% of all colonoscopy exams on a per-provider basis.7 One study of completed colonoscopy with inadequate preparation showed an adenoma miss rate of 48%.21 In the setting of inadequate bowel preparation, another study reported 42% of all adenomas detected were only found on repeat colonoscopy. When considering advanced adenomas, there was a 27% miss rate, a relatively high percentage.22
When poor bowel preparation precludes the exam, colonoscopy is appropriately aborted, and the patient asked to return. However, there are situations in which the exam can be completed but the bowel preparation is still inadequate to identify polyps larger than 5 mm. In this setting, the colonoscopy should be repeated with a more aggressive bowel preparation regimen within 1 year.19 Shorter intervals are recommended if advanced neoplasm is detected within an inadequate bowel preparation.19
The appropriate surveillance interval can be unclear when bowel preparation is considered adequate to identify polyps greater than or equal to 5 mm, yet still suboptimal. “Adequate” or “fair” bowel preparation often leads to shorter-than-recommended surveillance intervals because of the concern for small missed lesions. For example, patients with normal colonoscopy results and a fair prep were recommended to undergo a screening colonoscopy in 5 years at 57.4%, while only 23.1% received a 10-year recommendation.23 This increased frequency of colonoscopy leads to increased costs and procedural risks for the patient. Furthermore, a meta-analysis evaluating the effects of bowel preparation reported no significant difference in ADR between adequate and excellent prep.24 These findings suggest that patients with adequate bowel preparation may be followed at guideline-recommended surveillance intervals without significantly affecting colonoscopy quality as measured by ADR.
Endoscopist feedback and report cards
Awareness of quality metrics among individuals and endoscopy practices is crucial to ensuring adequate performance. Several studies have shown improvement with feedback and monitoring of endoscopists.25,26 Some strategies to improve colonoscopy technique and efficiency include having recorded or observed procedures, computer software that measures image resolution/velocity, and scorecards with quality measures. A representation of the scorecards used in our practice is shown in Table 4. Feedback measures both make endoscopists aware of how their performance compares with recommended goals for colonoscopy and help track their improvement. We recommend such feedback should be provided quarterly for most providers and more frequently for providers not meeting benchmarks.
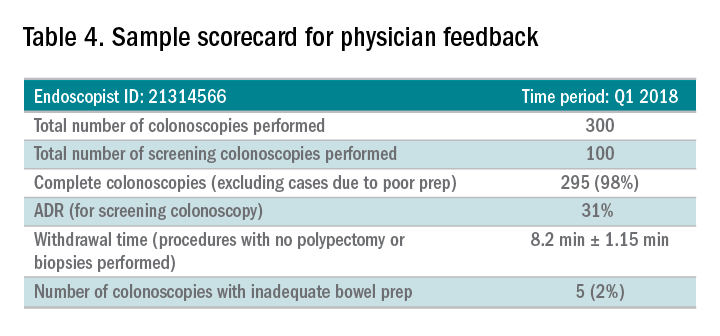
Conclusion
Given we rely on colonoscopy to identify and clear the colon of potential malignancy, it is imperative that we provide high-value exams for our patients. The basis for a quality colonoscopy is complete intubation and careful inspection of the mucosa on withdrawal. Several quality measures are used as surrogates of a good exam such that endoscopists can assess themselves in relation to their peers. These metrics can help us in our goal of remaining mindful during each procedure we are completing and providing the best exam possible.
Dr. Shamsi is a third-year GI fellow. Dr. Malhotra is an assistant professor in the division of gastroenterology at the University of Minnesota, Minneapolis. Dr. Shaukat is a professor of medicine in the division of gastroenterology at the University of Minnesota, Minneapolis, and the GI Section Chief at the Minneapolis VA Medical Center.
References
1. Siegel R et al. CA Cancer J Clin. 2012 Jan-Feb;62(1):10-29.
2. Edwards BK et al. Cancer. 2010 Feb 1;116(3):544-73.
3. Hosokawa O et al. Endoscopy. 2003 Jun;35(6):506-10.
4. Morris EJ et al. Gut. 2015(Aug);64(2):1248-56.
5. Bressler B et al. Gastroenterology. 2004 Aug;127(2):452-6.
6. Rex DK et al. Am J Gastroenterol. 2017 July;12(7):1016-30.
7. Rex DK et al. Gastrointest Endosc. 2015 Jan;81(1):31-53.
8. Anderson J et al. Clin Transl Gastroenterol. 2015 Feb 26;6:e77.
9. Kaminski M et al. N Engl J Med. 2010 May 13;362(19):1795-803.
10. Corley DA et al. N Engl J Med. 2014 Apr 3;370(4):1298-306.
11. Hunt RH. Colonoscopy intubation techniques without fluoroscopy. In: Colonoscopy techniques clinical practice and color atlas. Edited by Hunt RH, Waye JD. London: Chapman and Hall; 1981. p. 109-46.
12. Waye JD. Colonoscopy intubation techniques without fluoroscopy. In: Colonoscopy techniques clinical practice and color atlas. Edited by Hunt RH, Waye JD. London: Chapman and Hall; 1981. p. 147-78.
13. Williams CB et al. In: Colonoscopy principles & techniques. Edited by Raskin J, Juergen NH. New York: Igaku-Shoin Medical Publishers; 1995. p. 121-42.
14. Baillie J. Colonoscopy. In: Gastrointestinal endoscopy basic principles and practice. Oxford (UK): Butterworth-Heinemann; 1992. p. 63-92.
15. Rex DK. Gastrointest Endosc. 2000 Jan;51(1):33-6.
16. Shaukat A et al. Gastroenterol. 2015;149(4):952-7.
17. Barclay R et al. N Engl J Med. 2006 Dec 14;355(24):2533-41.
18. Simmons DT et al. Gastrointest Endosc. 2007;65(5):AB94.
19. Johnson DA et al. Gastrointest Endosc. 2014;80(4):543-62.
20. Calderwood A et al. Gastrointest Endosc. 2010 Oct;72(4):686-92.
21. Chokshi R et al. Gastrointest Endosc. 2012 Jun;75(6):1197-203.
22. Lebwohl B et al. Gastrointest Endosc. 2011 Jun;73(6):1207-14.
23. Menees SB et al. Gastrointest Endosc. 2013 Sep;78(3): 510-6.
24. Clark B et al. Am J Gastroenterol. 2014 Nov;109(11):1714-23.
25. Nielson A et al. BMJ Open Gastro. 2017 Jun. doi: 10.1136/bmjgast-2017-000142.
26. Gurudu S et al. J Gastroenterol Hepatol. 2018 Mar;33(3):645-9.
Editor's Note:
As quality metrics are becoming increasingly significant throughout all of medicine, our field is no exception. Recent evidence has demonstrated the importance of quality measures in colonoscopy; understanding, reporting, and improving these metrics has become a hot topic of discussion.
In this month’s In Focus article, brought to you by The New Gastroenterologist, Nabiha Shamsi, Ashish Malhotra, and Aasma Shaukat (University of Minnesota/Minneapolis VAMC) provide an outstanding overview of the evidence as well as recommended goals for important quality metrics in colonoscopy. Ultimately, improving colonoscopy quality amongst all gastroenterologists will increase colonoscopy value and lead to further decreases in the incidence and mortality of colorectal cancer.
Bryson W. Katona, MD, PhD
Editor in Chief, The New Gastroenterologist
Introduction
Colonoscopy is a widely used modality to evaluate colorectal cancer because it allows for both identification of early malignancies and removal of precancerous lesions. The increased use of colonoscopy in the last 20 years has been associated with a decline in the incidence and mortality from colorectal cancer.1,2 However, colonoscopy has its limitations. It is an invasive test with inherent risks. Additionally, studies have reported rates of post-colonoscopy cancers, also referred to as interval cancers, of 2%-7%, and miss-rates for adenomas by tandem colonoscopy of 2%-26%.3-5

High-quality exams can maximize the value of colonoscopy, and it is important to consider the factors that contribute to high-quality colonoscopies. While there are many metrics proposed,6,7 here we discuss the most evidence-based ones, outlined in Table 1, along with their goal values.
Cecal intubation rate
A high-quality colonoscopy should include a complete examination of the colon. To achieve this, it is necessary to fully intubate the cecum, passing the colonoscope past the ileocecal valve to examine the medial wall of the cecum.8
There are several factors that may contribute to an incomplete colonoscopy, including bowel preparation, anatomy, body habitus, and endoscopist’s skill. To calculate cecal intubation rate as a quality measure, colonoscopies that are incomplete because of poor bowel preparation, severe colitis, or known obstructing lesion are usually excluded.
The U.S. Multi-Society Task Force on Colorectal Cancer recommends a cecal intubation rate of at least 95% for screening colonoscopy and 90% for all colonoscopies.6 There is an expectation of photodocumentation of the ileocecal valve and appendiceal orifice to establish completion of the colonoscopy.6
Some methods used to assist with cecal intubation include changing patient position, applying abdominal pressure, stiffening the colonoscope, and alternating between adult or pediatric colonoscopes.
Adenoma detection rate
Adenoma detection rate (ADR), is defined as the proportion of patients over the age of 50 years undergoing first-time screening colonoscopies in which at least one adenomatous polyp is detected for a given endoscopist in a given time period.
Adenomas are tracked because clearing the colon of neoplasm is the goal of screening colonoscopies; adenomas are tracked instead of more advanced lesions because the higher frequency of adenomas allows for better tracking of variation between endoscopists. Tracking ADR also utilizes the assumption that, if small lesions are identified, larger ones will be as well.
ADR is the only current quality indicator reported to be significantly associated with the risk of interval cancers. In 2010, a study of 45,000 screening colonoscopies by 186 endoscopists validated the use of ADR, finding that patients who underwent colonoscopy by physicians with ADRs below 20% had hazard ratios for development of postcolonoscopy cancer greater than 10 times higher than patients of physicians with ADRs above 20%.9 However, this study had limited power to establish that cancer protection continues to improve when ADRs rise above 20%. Another study, which evaluated the association of ADR in 224,000 patients undergoing colonoscopies by 136 gastroenterologists, showed each 1% increase in ADR is associated with 3% decrease in the risk of interval CRC and 5% decrease in the risk of fatal interval cancers.10
Most recent guidelines propose an adequate ADR for asymptomatic individuals aged 50 years or older undergoing screening colonoscopy should be greater than 30% in men and greater than 20% in women.6 It remains unknown whether there is a threshold for maximum benefit of ADR, in which a very high ADR is not associated with further protective benefit. The answer to this question may depend on why a low ADR is associated with a higher rate of interval cancers and whether every missed polyp, independent of size, is a potential interval cancer or whether hasty, inadequate, or incomplete examinations of the colon are the underlying concern.
Withdrawal time
Optimizing identification of colonic lesions requires a careful and thorough exam of the colon on withdrawal. While this may seem obvious, there is often little focus on the approach to withdrawal. In four chapters on colonoscopy technique from textbooks, the number of pages describing insertion ranged from 20 to 38, while the number of pages focused on withdrawal ranged from 0.5 to 1.5.11-14

A study examining the difference in withdrawal technique between two endoscopists who were known to differ in adenoma miss rates by tandem colonoscopy proposed the scoring system listed in Table 2 that can assess quality of examination on withdrawal. There was a statistically significant difference in quality scores for the two endoscopists, as assessed by expert review of video recordings of their colonoscopies.15
The endoscopist with the lower adenoma miss rate was also found to have an average withdrawal time of 8 minutes and 55 seconds versus 6 minutes and 41 seconds for the endoscopist with the higher adenoma miss rate. A large, community-based study with over 76,000 colonoscopies found a statistically significant correlation between interval colorectal cancer and withdrawal times shorter than 6 minutes.16 However, there was no association between ADR and colorectal cancer, suggesting that, for practices with optimal ADRs (that is, rates greater than 25%), withdrawal time may be a more sensitive marker of quality of colonoscopy than ADR is.16Intuitively, adequate examination of the colon that includes examining the proximal side of folds, washing and suctioning stool, and even repositioning the patient would likely increase withdrawal time. In a 2008 study examining 2,000 screening colonoscopies of 12 endoscopists, those with withdrawal times greater than 6 minutes had significantly higher rates of detecting adenomas and advanced neoplasia, compared with those with faster withdrawal times.17 The average ADR in this group was 28.3%, compared with 11.8% for physicians who had a withdrawal time less than 6 minutes.17 An evaluation of nearly 11,000 colonoscopies done by 43 endoscopists also identified an increase polyp yield with increased withdrawal time.18 These data drive the recommendation for a minimum withdrawal time of 6 minutes, with 2 minutes spent examining each colonic segment.
Bowel preparation
Diagnosis of colonic lesions is dependent on adequate visualization of the colon. Poor bowel preparation can limit the yield of colonoscopy and lead to missed lesions. It also leads to canceled and rescheduled procedures that reduce efficiency, increase cost, and pose an undue burden on the patient.
The quality of bowel preparation should be assessed after washing and suctioning of colonic mucosa has been completed. Adequate preparation is that which allows identification of lesions greater than 5 mm in size.19
Quality of preparation is assessed subjectively by the endoscopists and often listed as excellent, good, fair, or poor. An alternative method of reporting bowel preparation quality is the Boston Bowel Preparation Score (BBPS) (Table 3).20 This scoring system allows for a more descriptive assessment of each colonic segment by assigning a score from 0 to 3 for the right, transverse, and left colon, leading to a total score between 0 and 9. The BBPS also helps standardize reporting of bowel preparation. The polyp detection rate associated with a BBPS of 5 or greater was 40%, compared with 24% associated with BBPS less than 5.19 A split-dose bowel preparation regimen with at least half of the preparation ingested on the day of the procedure is recommended to optimize quality of bowel preparation.6
The American Society for Gastrointestinal Endoscopy and American College of Gastroenterology task force on quality assurance in endoscopy recommends that bowel preparation should be adequate in 85% of all colonoscopy exams on a per-provider basis.7 One study of completed colonoscopy with inadequate preparation showed an adenoma miss rate of 48%.21 In the setting of inadequate bowel preparation, another study reported 42% of all adenomas detected were only found on repeat colonoscopy. When considering advanced adenomas, there was a 27% miss rate, a relatively high percentage.22
When poor bowel preparation precludes the exam, colonoscopy is appropriately aborted, and the patient asked to return. However, there are situations in which the exam can be completed but the bowel preparation is still inadequate to identify polyps larger than 5 mm. In this setting, the colonoscopy should be repeated with a more aggressive bowel preparation regimen within 1 year.19 Shorter intervals are recommended if advanced neoplasm is detected within an inadequate bowel preparation.19
The appropriate surveillance interval can be unclear when bowel preparation is considered adequate to identify polyps greater than or equal to 5 mm, yet still suboptimal. “Adequate” or “fair” bowel preparation often leads to shorter-than-recommended surveillance intervals because of the concern for small missed lesions. For example, patients with normal colonoscopy results and a fair prep were recommended to undergo a screening colonoscopy in 5 years at 57.4%, while only 23.1% received a 10-year recommendation.23 This increased frequency of colonoscopy leads to increased costs and procedural risks for the patient. Furthermore, a meta-analysis evaluating the effects of bowel preparation reported no significant difference in ADR between adequate and excellent prep.24 These findings suggest that patients with adequate bowel preparation may be followed at guideline-recommended surveillance intervals without significantly affecting colonoscopy quality as measured by ADR.
Endoscopist feedback and report cards
Awareness of quality metrics among individuals and endoscopy practices is crucial to ensuring adequate performance. Several studies have shown improvement with feedback and monitoring of endoscopists.25,26 Some strategies to improve colonoscopy technique and efficiency include having recorded or observed procedures, computer software that measures image resolution/velocity, and scorecards with quality measures. A representation of the scorecards used in our practice is shown in Table 4. Feedback measures both make endoscopists aware of how their performance compares with recommended goals for colonoscopy and help track their improvement. We recommend such feedback should be provided quarterly for most providers and more frequently for providers not meeting benchmarks.

Conclusion
Given we rely on colonoscopy to identify and clear the colon of potential malignancy, it is imperative that we provide high-value exams for our patients. The basis for a quality colonoscopy is complete intubation and careful inspection of the mucosa on withdrawal. Several quality measures are used as surrogates of a good exam such that endoscopists can assess themselves in relation to their peers. These metrics can help us in our goal of remaining mindful during each procedure we are completing and providing the best exam possible.
Dr. Shamsi is a third-year GI fellow. Dr. Malhotra is an assistant professor in the division of gastroenterology at the University of Minnesota, Minneapolis. Dr. Shaukat is a professor of medicine in the division of gastroenterology at the University of Minnesota, Minneapolis, and the GI Section Chief at the Minneapolis VA Medical Center.
References
1. Siegel R et al. CA Cancer J Clin. 2012 Jan-Feb;62(1):10-29.
2. Edwards BK et al. Cancer. 2010 Feb 1;116(3):544-73.
3. Hosokawa O et al. Endoscopy. 2003 Jun;35(6):506-10.
4. Morris EJ et al. Gut. 2015(Aug);64(2):1248-56.
5. Bressler B et al. Gastroenterology. 2004 Aug;127(2):452-6.
6. Rex DK et al. Am J Gastroenterol. 2017 July;12(7):1016-30.
7. Rex DK et al. Gastrointest Endosc. 2015 Jan;81(1):31-53.
8. Anderson J et al. Clin Transl Gastroenterol. 2015 Feb 26;6:e77.
9. Kaminski M et al. N Engl J Med. 2010 May 13;362(19):1795-803.
10. Corley DA et al. N Engl J Med. 2014 Apr 3;370(4):1298-306.
11. Hunt RH. Colonoscopy intubation techniques without fluoroscopy. In: Colonoscopy techniques clinical practice and color atlas. Edited by Hunt RH, Waye JD. London: Chapman and Hall; 1981. p. 109-46.
12. Waye JD. Colonoscopy intubation techniques without fluoroscopy. In: Colonoscopy techniques clinical practice and color atlas. Edited by Hunt RH, Waye JD. London: Chapman and Hall; 1981. p. 147-78.
13. Williams CB et al. In: Colonoscopy principles & techniques. Edited by Raskin J, Juergen NH. New York: Igaku-Shoin Medical Publishers; 1995. p. 121-42.
14. Baillie J. Colonoscopy. In: Gastrointestinal endoscopy basic principles and practice. Oxford (UK): Butterworth-Heinemann; 1992. p. 63-92.
15. Rex DK. Gastrointest Endosc. 2000 Jan;51(1):33-6.
16. Shaukat A et al. Gastroenterol. 2015;149(4):952-7.
17. Barclay R et al. N Engl J Med. 2006 Dec 14;355(24):2533-41.
18. Simmons DT et al. Gastrointest Endosc. 2007;65(5):AB94.
19. Johnson DA et al. Gastrointest Endosc. 2014;80(4):543-62.
20. Calderwood A et al. Gastrointest Endosc. 2010 Oct;72(4):686-92.
21. Chokshi R et al. Gastrointest Endosc. 2012 Jun;75(6):1197-203.
22. Lebwohl B et al. Gastrointest Endosc. 2011 Jun;73(6):1207-14.
23. Menees SB et al. Gastrointest Endosc. 2013 Sep;78(3): 510-6.
24. Clark B et al. Am J Gastroenterol. 2014 Nov;109(11):1714-23.
25. Nielson A et al. BMJ Open Gastro. 2017 Jun. doi: 10.1136/bmjgast-2017-000142.
26. Gurudu S et al. J Gastroenterol Hepatol. 2018 Mar;33(3):645-9.
Editor's Note:
As quality metrics are becoming increasingly significant throughout all of medicine, our field is no exception. Recent evidence has demonstrated the importance of quality measures in colonoscopy; understanding, reporting, and improving these metrics has become a hot topic of discussion.
In this month’s In Focus article, brought to you by The New Gastroenterologist, Nabiha Shamsi, Ashish Malhotra, and Aasma Shaukat (University of Minnesota/Minneapolis VAMC) provide an outstanding overview of the evidence as well as recommended goals for important quality metrics in colonoscopy. Ultimately, improving colonoscopy quality amongst all gastroenterologists will increase colonoscopy value and lead to further decreases in the incidence and mortality of colorectal cancer.
Bryson W. Katona, MD, PhD
Editor in Chief, The New Gastroenterologist
Introduction
Colonoscopy is a widely used modality to evaluate colorectal cancer because it allows for both identification of early malignancies and removal of precancerous lesions. The increased use of colonoscopy in the last 20 years has been associated with a decline in the incidence and mortality from colorectal cancer.1,2 However, colonoscopy has its limitations. It is an invasive test with inherent risks. Additionally, studies have reported rates of post-colonoscopy cancers, also referred to as interval cancers, of 2%-7%, and miss-rates for adenomas by tandem colonoscopy of 2%-26%.3-5

High-quality exams can maximize the value of colonoscopy, and it is important to consider the factors that contribute to high-quality colonoscopies. While there are many metrics proposed,6,7 here we discuss the most evidence-based ones, outlined in Table 1, along with their goal values.
Cecal intubation rate
A high-quality colonoscopy should include a complete examination of the colon. To achieve this, it is necessary to fully intubate the cecum, passing the colonoscope past the ileocecal valve to examine the medial wall of the cecum.8
There are several factors that may contribute to an incomplete colonoscopy, including bowel preparation, anatomy, body habitus, and endoscopist’s skill. To calculate cecal intubation rate as a quality measure, colonoscopies that are incomplete because of poor bowel preparation, severe colitis, or known obstructing lesion are usually excluded.
The U.S. Multi-Society Task Force on Colorectal Cancer recommends a cecal intubation rate of at least 95% for screening colonoscopy and 90% for all colonoscopies.6 There is an expectation of photodocumentation of the ileocecal valve and appendiceal orifice to establish completion of the colonoscopy.6
Some methods used to assist with cecal intubation include changing patient position, applying abdominal pressure, stiffening the colonoscope, and alternating between adult or pediatric colonoscopes.
Adenoma detection rate
Adenoma detection rate (ADR), is defined as the proportion of patients over the age of 50 years undergoing first-time screening colonoscopies in which at least one adenomatous polyp is detected for a given endoscopist in a given time period.
Adenomas are tracked because clearing the colon of neoplasm is the goal of screening colonoscopies; adenomas are tracked instead of more advanced lesions because the higher frequency of adenomas allows for better tracking of variation between endoscopists. Tracking ADR also utilizes the assumption that, if small lesions are identified, larger ones will be as well.
ADR is the only current quality indicator reported to be significantly associated with the risk of interval cancers. In 2010, a study of 45,000 screening colonoscopies by 186 endoscopists validated the use of ADR, finding that patients who underwent colonoscopy by physicians with ADRs below 20% had hazard ratios for development of postcolonoscopy cancer greater than 10 times higher than patients of physicians with ADRs above 20%.9 However, this study had limited power to establish that cancer protection continues to improve when ADRs rise above 20%. Another study, which evaluated the association of ADR in 224,000 patients undergoing colonoscopies by 136 gastroenterologists, showed each 1% increase in ADR is associated with 3% decrease in the risk of interval CRC and 5% decrease in the risk of fatal interval cancers.10
Most recent guidelines propose an adequate ADR for asymptomatic individuals aged 50 years or older undergoing screening colonoscopy should be greater than 30% in men and greater than 20% in women.6 It remains unknown whether there is a threshold for maximum benefit of ADR, in which a very high ADR is not associated with further protective benefit. The answer to this question may depend on why a low ADR is associated with a higher rate of interval cancers and whether every missed polyp, independent of size, is a potential interval cancer or whether hasty, inadequate, or incomplete examinations of the colon are the underlying concern.
Withdrawal time
Optimizing identification of colonic lesions requires a careful and thorough exam of the colon on withdrawal. While this may seem obvious, there is often little focus on the approach to withdrawal. In four chapters on colonoscopy technique from textbooks, the number of pages describing insertion ranged from 20 to 38, while the number of pages focused on withdrawal ranged from 0.5 to 1.5.11-14

A study examining the difference in withdrawal technique between two endoscopists who were known to differ in adenoma miss rates by tandem colonoscopy proposed the scoring system listed in Table 2 that can assess quality of examination on withdrawal. There was a statistically significant difference in quality scores for the two endoscopists, as assessed by expert review of video recordings of their colonoscopies.15
The endoscopist with the lower adenoma miss rate was also found to have an average withdrawal time of 8 minutes and 55 seconds versus 6 minutes and 41 seconds for the endoscopist with the higher adenoma miss rate. A large, community-based study with over 76,000 colonoscopies found a statistically significant correlation between interval colorectal cancer and withdrawal times shorter than 6 minutes.16 However, there was no association between ADR and colorectal cancer, suggesting that, for practices with optimal ADRs (that is, rates greater than 25%), withdrawal time may be a more sensitive marker of quality of colonoscopy than ADR is.16Intuitively, adequate examination of the colon that includes examining the proximal side of folds, washing and suctioning stool, and even repositioning the patient would likely increase withdrawal time. In a 2008 study examining 2,000 screening colonoscopies of 12 endoscopists, those with withdrawal times greater than 6 minutes had significantly higher rates of detecting adenomas and advanced neoplasia, compared with those with faster withdrawal times.17 The average ADR in this group was 28.3%, compared with 11.8% for physicians who had a withdrawal time less than 6 minutes.17 An evaluation of nearly 11,000 colonoscopies done by 43 endoscopists also identified an increase polyp yield with increased withdrawal time.18 These data drive the recommendation for a minimum withdrawal time of 6 minutes, with 2 minutes spent examining each colonic segment.
Bowel preparation
Diagnosis of colonic lesions is dependent on adequate visualization of the colon. Poor bowel preparation can limit the yield of colonoscopy and lead to missed lesions. It also leads to canceled and rescheduled procedures that reduce efficiency, increase cost, and pose an undue burden on the patient.
The quality of bowel preparation should be assessed after washing and suctioning of colonic mucosa has been completed. Adequate preparation is that which allows identification of lesions greater than 5 mm in size.19
Quality of preparation is assessed subjectively by the endoscopists and often listed as excellent, good, fair, or poor. An alternative method of reporting bowel preparation quality is the Boston Bowel Preparation Score (BBPS) (Table 3).20 This scoring system allows for a more descriptive assessment of each colonic segment by assigning a score from 0 to 3 for the right, transverse, and left colon, leading to a total score between 0 and 9. The BBPS also helps standardize reporting of bowel preparation. The polyp detection rate associated with a BBPS of 5 or greater was 40%, compared with 24% associated with BBPS less than 5.19 A split-dose bowel preparation regimen with at least half of the preparation ingested on the day of the procedure is recommended to optimize quality of bowel preparation.6
The American Society for Gastrointestinal Endoscopy and American College of Gastroenterology task force on quality assurance in endoscopy recommends that bowel preparation should be adequate in 85% of all colonoscopy exams on a per-provider basis.7 One study of completed colonoscopy with inadequate preparation showed an adenoma miss rate of 48%.21 In the setting of inadequate bowel preparation, another study reported 42% of all adenomas detected were only found on repeat colonoscopy. When considering advanced adenomas, there was a 27% miss rate, a relatively high percentage.22
When poor bowel preparation precludes the exam, colonoscopy is appropriately aborted, and the patient asked to return. However, there are situations in which the exam can be completed but the bowel preparation is still inadequate to identify polyps larger than 5 mm. In this setting, the colonoscopy should be repeated with a more aggressive bowel preparation regimen within 1 year.19 Shorter intervals are recommended if advanced neoplasm is detected within an inadequate bowel preparation.19
The appropriate surveillance interval can be unclear when bowel preparation is considered adequate to identify polyps greater than or equal to 5 mm, yet still suboptimal. “Adequate” or “fair” bowel preparation often leads to shorter-than-recommended surveillance intervals because of the concern for small missed lesions. For example, patients with normal colonoscopy results and a fair prep were recommended to undergo a screening colonoscopy in 5 years at 57.4%, while only 23.1% received a 10-year recommendation.23 This increased frequency of colonoscopy leads to increased costs and procedural risks for the patient. Furthermore, a meta-analysis evaluating the effects of bowel preparation reported no significant difference in ADR between adequate and excellent prep.24 These findings suggest that patients with adequate bowel preparation may be followed at guideline-recommended surveillance intervals without significantly affecting colonoscopy quality as measured by ADR.
Endoscopist feedback and report cards
Awareness of quality metrics among individuals and endoscopy practices is crucial to ensuring adequate performance. Several studies have shown improvement with feedback and monitoring of endoscopists.25,26 Some strategies to improve colonoscopy technique and efficiency include having recorded or observed procedures, computer software that measures image resolution/velocity, and scorecards with quality measures. A representation of the scorecards used in our practice is shown in Table 4. Feedback measures both make endoscopists aware of how their performance compares with recommended goals for colonoscopy and help track their improvement. We recommend such feedback should be provided quarterly for most providers and more frequently for providers not meeting benchmarks.

Conclusion
Given we rely on colonoscopy to identify and clear the colon of potential malignancy, it is imperative that we provide high-value exams for our patients. The basis for a quality colonoscopy is complete intubation and careful inspection of the mucosa on withdrawal. Several quality measures are used as surrogates of a good exam such that endoscopists can assess themselves in relation to their peers. These metrics can help us in our goal of remaining mindful during each procedure we are completing and providing the best exam possible.
Dr. Shamsi is a third-year GI fellow. Dr. Malhotra is an assistant professor in the division of gastroenterology at the University of Minnesota, Minneapolis. Dr. Shaukat is a professor of medicine in the division of gastroenterology at the University of Minnesota, Minneapolis, and the GI Section Chief at the Minneapolis VA Medical Center.
References
1. Siegel R et al. CA Cancer J Clin. 2012 Jan-Feb;62(1):10-29.
2. Edwards BK et al. Cancer. 2010 Feb 1;116(3):544-73.
3. Hosokawa O et al. Endoscopy. 2003 Jun;35(6):506-10.
4. Morris EJ et al. Gut. 2015(Aug);64(2):1248-56.
5. Bressler B et al. Gastroenterology. 2004 Aug;127(2):452-6.
6. Rex DK et al. Am J Gastroenterol. 2017 July;12(7):1016-30.
7. Rex DK et al. Gastrointest Endosc. 2015 Jan;81(1):31-53.
8. Anderson J et al. Clin Transl Gastroenterol. 2015 Feb 26;6:e77.
9. Kaminski M et al. N Engl J Med. 2010 May 13;362(19):1795-803.
10. Corley DA et al. N Engl J Med. 2014 Apr 3;370(4):1298-306.
11. Hunt RH. Colonoscopy intubation techniques without fluoroscopy. In: Colonoscopy techniques clinical practice and color atlas. Edited by Hunt RH, Waye JD. London: Chapman and Hall; 1981. p. 109-46.
12. Waye JD. Colonoscopy intubation techniques without fluoroscopy. In: Colonoscopy techniques clinical practice and color atlas. Edited by Hunt RH, Waye JD. London: Chapman and Hall; 1981. p. 147-78.
13. Williams CB et al. In: Colonoscopy principles & techniques. Edited by Raskin J, Juergen NH. New York: Igaku-Shoin Medical Publishers; 1995. p. 121-42.
14. Baillie J. Colonoscopy. In: Gastrointestinal endoscopy basic principles and practice. Oxford (UK): Butterworth-Heinemann; 1992. p. 63-92.
15. Rex DK. Gastrointest Endosc. 2000 Jan;51(1):33-6.
16. Shaukat A et al. Gastroenterol. 2015;149(4):952-7.
17. Barclay R et al. N Engl J Med. 2006 Dec 14;355(24):2533-41.
18. Simmons DT et al. Gastrointest Endosc. 2007;65(5):AB94.
19. Johnson DA et al. Gastrointest Endosc. 2014;80(4):543-62.
20. Calderwood A et al. Gastrointest Endosc. 2010 Oct;72(4):686-92.
21. Chokshi R et al. Gastrointest Endosc. 2012 Jun;75(6):1197-203.
22. Lebwohl B et al. Gastrointest Endosc. 2011 Jun;73(6):1207-14.
23. Menees SB et al. Gastrointest Endosc. 2013 Sep;78(3): 510-6.
24. Clark B et al. Am J Gastroenterol. 2014 Nov;109(11):1714-23.
25. Nielson A et al. BMJ Open Gastro. 2017 Jun. doi: 10.1136/bmjgast-2017-000142.
26. Gurudu S et al. J Gastroenterol Hepatol. 2018 Mar;33(3):645-9.