User login
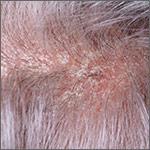
What’s the most effective topical Tx for scalp psoriasis?
Single-agent therapy with a very potent or potent topical corticosteroid appears more effective than other topical agents, including vitamin D3 analogues, for treating scalp psoriasis (strength of recommendation [SOR]: A, systematic reviews of randomized controlled trials [RCTs]).
Combined therapy with a vitamin D3 analogue and a potent topical corticosteroid may be slightly more effective than monotherapy with either agent (SOR: B, systematic reviews of RCTs with inconsistent results).
Evidence summary
A 2013 meta-analysis of 26 RCTs with 8020 patients evaluated topical treatments for scalp psoriasis as part of a subanalysis of a larger Cochrane review of psoriasis therapy.1 Only 20 studies reported the severity of disease: 13 studies looked at moderate to severe scalp psoriasis and the others examined mild to severe disease.
Results were reported as standardized mean differences (SMD) and also converted to a 6-point global improvement scale created by the authors to provide a combined endpoint of provider- or patient-assessed improvement in symptoms such as redness, thickness, and scaling. Higher scores indicate more improvement.
Compared with placebo, the very potent corticosteroid clobetasol propionate improved psoriasis by 1.9 points on the 6-point scale (4 trials, 788 patients; SMD= −1.6; 95% confidence interval [CI], −1.8 to −1.3). The potent steroid betamethasone diproprionate improved symptoms by 1.3 points compared with placebo (2 trials, 712 patients; SMD= −1.1; 95% CI, −1.3 to −0.90).
The topical corticosteroids clobetasol, betamethasone diproprionate, and betamethasone valerate improved symptoms more than the vitamin D3 analogue calcipotriol in head-to-head trials. The corticosteroid improvement scores exceeded calcipotriol scores by 0.5 points (1 trial, 151 patients; SMD=0.37; 95% CI, 0.05-0.69), 0.6 points (1 trial, 1676 patients; SMD=0.48; 95% CI, 0.32-0.64), and 0.5 points (1 trial, 510 patients; SMD=0.37; 95% CI, 0.20-0.55), respectively.
Combination therapy with a vitamin D3 analogue and a corticosteroid yielded approximately 0.2 points of improvement over corticosteroid alone (6 trials, 2444 patients; SMD= −0.18; 95% CI, −0.26 to −0.10). Four trials of combination therapy (2581 patients) resulted in 0.5 to 1.2 points of improvement compared with vitamin D3 analogues alone (SMD=0.64; 95% CI, 0.44-0.84). Specific strengths and dosing regimens weren’t reported.
The Cochrane systematic review, using the same outcome reporting methods, provided data on the vitamin D3 analogue calcipotriol compared with placebo for treating scalp psoriasis.2 Calcipotriol resulted in 0.9 points of improvement on the 6-point global improvement scale (2 trials, 457 patients; SMD= −0.72; 95% CI, −1.3 to −0.16).
Very potent corticosteroids show a better response than potent agents
In 2013, a meta-analysis of 13 placebo-controlled RCTs (5640 patients) evaluated topical therapies for scalp psoriasis licensed in the United Kingdom. This meta-analysis included the same placebo-controlled studies as the Cochrane review but added one study published after the search date of the review.3
The outcome reporting was different from the Cochrane review. The primary outcome was percentage of patients with at least moderate scalp psoriasis who achieved clear or nearly clear status on provider assessment scales. All treatments were compared to twice-daily placebo with a response rate of 11%.
Very potent steroids had response rates of 78% for twice-daily application (risk ratio [RR]=7.0; 95% CI, 5.6-8.0) and 69% for once-daily application (RR=6.2; 95% CI, 3.0-8.3). The combination of a vitamin D3 analogue and a potent corticosteroid showed a response rate of 64% (RR=5.7; 95% CI, 2.4-8.0) whereas response rates for potent corticosteroids alone were 57% (RR=5.0; 95% CI, 1.6-7.8) for once-daily application and 49% (RR=4.4; 95% CI, 2.2-6.7) for twice-daily administration. The authors suggested patient satisfaction at using once daily vs twice daily application as a possible explanation for the difference in response rate.
Vitamin D3 analogues showed response rates of approximately 34%, which is nonsignificant for once-daily application (RR=3.1; 95% CI, 0.71-6.6) but significant for twice-daily administration (RR=3.1; 95% CI, 1.3-5.9). Exact numbers of studies and participants, as well as specific agents and preparation information, were not included.
1. Mason AR, Mason JM, Cork MJ, et al. Topical treatments for chronic plaque psoriasis of the scalp: a systematic review. Br J Dermatol. 2013;169:519-527.
2. Mason A, Mason J, Cork M, et al. Topical treatments for chronic plaque psoriasis: an abridged Cochrane systematic review. J Am Acad Dermatol. 2013; 69:799-807.
3. Samarasekera EJ, Sawyer L, Wonderling D, et al. Topical therapies for the treatment of plaque psoriasis: systematic review and network meta-analyses. Br J Dermatol. 2013;168:954-967.
Single-agent therapy with a very potent or potent topical corticosteroid appears more effective than other topical agents, including vitamin D3 analogues, for treating scalp psoriasis (strength of recommendation [SOR]: A, systematic reviews of randomized controlled trials [RCTs]).
Combined therapy with a vitamin D3 analogue and a potent topical corticosteroid may be slightly more effective than monotherapy with either agent (SOR: B, systematic reviews of RCTs with inconsistent results).
Evidence summary
A 2013 meta-analysis of 26 RCTs with 8020 patients evaluated topical treatments for scalp psoriasis as part of a subanalysis of a larger Cochrane review of psoriasis therapy.1 Only 20 studies reported the severity of disease: 13 studies looked at moderate to severe scalp psoriasis and the others examined mild to severe disease.
Results were reported as standardized mean differences (SMD) and also converted to a 6-point global improvement scale created by the authors to provide a combined endpoint of provider- or patient-assessed improvement in symptoms such as redness, thickness, and scaling. Higher scores indicate more improvement.
Compared with placebo, the very potent corticosteroid clobetasol propionate improved psoriasis by 1.9 points on the 6-point scale (4 trials, 788 patients; SMD= −1.6; 95% confidence interval [CI], −1.8 to −1.3). The potent steroid betamethasone diproprionate improved symptoms by 1.3 points compared with placebo (2 trials, 712 patients; SMD= −1.1; 95% CI, −1.3 to −0.90).
The topical corticosteroids clobetasol, betamethasone diproprionate, and betamethasone valerate improved symptoms more than the vitamin D3 analogue calcipotriol in head-to-head trials. The corticosteroid improvement scores exceeded calcipotriol scores by 0.5 points (1 trial, 151 patients; SMD=0.37; 95% CI, 0.05-0.69), 0.6 points (1 trial, 1676 patients; SMD=0.48; 95% CI, 0.32-0.64), and 0.5 points (1 trial, 510 patients; SMD=0.37; 95% CI, 0.20-0.55), respectively.
Combination therapy with a vitamin D3 analogue and a corticosteroid yielded approximately 0.2 points of improvement over corticosteroid alone (6 trials, 2444 patients; SMD= −0.18; 95% CI, −0.26 to −0.10). Four trials of combination therapy (2581 patients) resulted in 0.5 to 1.2 points of improvement compared with vitamin D3 analogues alone (SMD=0.64; 95% CI, 0.44-0.84). Specific strengths and dosing regimens weren’t reported.
The Cochrane systematic review, using the same outcome reporting methods, provided data on the vitamin D3 analogue calcipotriol compared with placebo for treating scalp psoriasis.2 Calcipotriol resulted in 0.9 points of improvement on the 6-point global improvement scale (2 trials, 457 patients; SMD= −0.72; 95% CI, −1.3 to −0.16).
Very potent corticosteroids show a better response than potent agents
In 2013, a meta-analysis of 13 placebo-controlled RCTs (5640 patients) evaluated topical therapies for scalp psoriasis licensed in the United Kingdom. This meta-analysis included the same placebo-controlled studies as the Cochrane review but added one study published after the search date of the review.3
The outcome reporting was different from the Cochrane review. The primary outcome was percentage of patients with at least moderate scalp psoriasis who achieved clear or nearly clear status on provider assessment scales. All treatments were compared to twice-daily placebo with a response rate of 11%.
Very potent steroids had response rates of 78% for twice-daily application (risk ratio [RR]=7.0; 95% CI, 5.6-8.0) and 69% for once-daily application (RR=6.2; 95% CI, 3.0-8.3). The combination of a vitamin D3 analogue and a potent corticosteroid showed a response rate of 64% (RR=5.7; 95% CI, 2.4-8.0) whereas response rates for potent corticosteroids alone were 57% (RR=5.0; 95% CI, 1.6-7.8) for once-daily application and 49% (RR=4.4; 95% CI, 2.2-6.7) for twice-daily administration. The authors suggested patient satisfaction at using once daily vs twice daily application as a possible explanation for the difference in response rate.
Vitamin D3 analogues showed response rates of approximately 34%, which is nonsignificant for once-daily application (RR=3.1; 95% CI, 0.71-6.6) but significant for twice-daily administration (RR=3.1; 95% CI, 1.3-5.9). Exact numbers of studies and participants, as well as specific agents and preparation information, were not included.
Single-agent therapy with a very potent or potent topical corticosteroid appears more effective than other topical agents, including vitamin D3 analogues, for treating scalp psoriasis (strength of recommendation [SOR]: A, systematic reviews of randomized controlled trials [RCTs]).
Combined therapy with a vitamin D3 analogue and a potent topical corticosteroid may be slightly more effective than monotherapy with either agent (SOR: B, systematic reviews of RCTs with inconsistent results).
Evidence summary
A 2013 meta-analysis of 26 RCTs with 8020 patients evaluated topical treatments for scalp psoriasis as part of a subanalysis of a larger Cochrane review of psoriasis therapy.1 Only 20 studies reported the severity of disease: 13 studies looked at moderate to severe scalp psoriasis and the others examined mild to severe disease.
Results were reported as standardized mean differences (SMD) and also converted to a 6-point global improvement scale created by the authors to provide a combined endpoint of provider- or patient-assessed improvement in symptoms such as redness, thickness, and scaling. Higher scores indicate more improvement.
Compared with placebo, the very potent corticosteroid clobetasol propionate improved psoriasis by 1.9 points on the 6-point scale (4 trials, 788 patients; SMD= −1.6; 95% confidence interval [CI], −1.8 to −1.3). The potent steroid betamethasone diproprionate improved symptoms by 1.3 points compared with placebo (2 trials, 712 patients; SMD= −1.1; 95% CI, −1.3 to −0.90).
The topical corticosteroids clobetasol, betamethasone diproprionate, and betamethasone valerate improved symptoms more than the vitamin D3 analogue calcipotriol in head-to-head trials. The corticosteroid improvement scores exceeded calcipotriol scores by 0.5 points (1 trial, 151 patients; SMD=0.37; 95% CI, 0.05-0.69), 0.6 points (1 trial, 1676 patients; SMD=0.48; 95% CI, 0.32-0.64), and 0.5 points (1 trial, 510 patients; SMD=0.37; 95% CI, 0.20-0.55), respectively.
Combination therapy with a vitamin D3 analogue and a corticosteroid yielded approximately 0.2 points of improvement over corticosteroid alone (6 trials, 2444 patients; SMD= −0.18; 95% CI, −0.26 to −0.10). Four trials of combination therapy (2581 patients) resulted in 0.5 to 1.2 points of improvement compared with vitamin D3 analogues alone (SMD=0.64; 95% CI, 0.44-0.84). Specific strengths and dosing regimens weren’t reported.
The Cochrane systematic review, using the same outcome reporting methods, provided data on the vitamin D3 analogue calcipotriol compared with placebo for treating scalp psoriasis.2 Calcipotriol resulted in 0.9 points of improvement on the 6-point global improvement scale (2 trials, 457 patients; SMD= −0.72; 95% CI, −1.3 to −0.16).
Very potent corticosteroids show a better response than potent agents
In 2013, a meta-analysis of 13 placebo-controlled RCTs (5640 patients) evaluated topical therapies for scalp psoriasis licensed in the United Kingdom. This meta-analysis included the same placebo-controlled studies as the Cochrane review but added one study published after the search date of the review.3
The outcome reporting was different from the Cochrane review. The primary outcome was percentage of patients with at least moderate scalp psoriasis who achieved clear or nearly clear status on provider assessment scales. All treatments were compared to twice-daily placebo with a response rate of 11%.
Very potent steroids had response rates of 78% for twice-daily application (risk ratio [RR]=7.0; 95% CI, 5.6-8.0) and 69% for once-daily application (RR=6.2; 95% CI, 3.0-8.3). The combination of a vitamin D3 analogue and a potent corticosteroid showed a response rate of 64% (RR=5.7; 95% CI, 2.4-8.0) whereas response rates for potent corticosteroids alone were 57% (RR=5.0; 95% CI, 1.6-7.8) for once-daily application and 49% (RR=4.4; 95% CI, 2.2-6.7) for twice-daily administration. The authors suggested patient satisfaction at using once daily vs twice daily application as a possible explanation for the difference in response rate.
Vitamin D3 analogues showed response rates of approximately 34%, which is nonsignificant for once-daily application (RR=3.1; 95% CI, 0.71-6.6) but significant for twice-daily administration (RR=3.1; 95% CI, 1.3-5.9). Exact numbers of studies and participants, as well as specific agents and preparation information, were not included.
1. Mason AR, Mason JM, Cork MJ, et al. Topical treatments for chronic plaque psoriasis of the scalp: a systematic review. Br J Dermatol. 2013;169:519-527.
2. Mason A, Mason J, Cork M, et al. Topical treatments for chronic plaque psoriasis: an abridged Cochrane systematic review. J Am Acad Dermatol. 2013; 69:799-807.
3. Samarasekera EJ, Sawyer L, Wonderling D, et al. Topical therapies for the treatment of plaque psoriasis: systematic review and network meta-analyses. Br J Dermatol. 2013;168:954-967.
1. Mason AR, Mason JM, Cork MJ, et al. Topical treatments for chronic plaque psoriasis of the scalp: a systematic review. Br J Dermatol. 2013;169:519-527.
2. Mason A, Mason J, Cork M, et al. Topical treatments for chronic plaque psoriasis: an abridged Cochrane systematic review. J Am Acad Dermatol. 2013; 69:799-807.
3. Samarasekera EJ, Sawyer L, Wonderling D, et al. Topical therapies for the treatment of plaque psoriasis: systematic review and network meta-analyses. Br J Dermatol. 2013;168:954-967.
Evidence-based answers from the Family Physicians Inquiries Network
Do novel oral anticoagulants safely prevent stroke in patients with nonvalvular A-fib?
Yes. Dabigatran, rivaroxaban, and apixaban are safe and effective compared with warfarin for preventing stroke in patients with nonvalvular atrial fibrillation. These novel oral anticoagulants (NOACs) are noninferior in reducing the number of strokes and systemic emboli and in lowering all-cause mortality while not increasing major bleeding complications and hemorrhagic events (strength of recommendation: A, consistent meta-analyses of randomized controlled trials [RCTs]).
Evidence summary
A 2014 meta-analysis of 4 RCTs including 71,683 patients with nonvalvular atrial fibrillation evaluated the NOACs dabigatran, rivaroxaban, apixaban, and edoxaban, for efficacy and safety compared with warfarin.1 The RCTs analyzed 42,411 patients receiving NOACs and 29,272 patients receiving warfarin. All trials were designed to show noninferiority. Selection criteria for RCTs included all phase 3 trials of available NOACs (edoxaban isn’t available in the United States). Median follow-up was 1.8 to 2.8 years.
Pooled data demonstrated that NOACs were noninferior to warfarin in preventing stroke or systemic embolism (relative risk [RR]=0.81; 95% confidence interval [CI], 0.73-0.91; number needed to treat [NNT]=147). The main benefit was derived from the relatively large decrease in the rate of hemorrhagic stroke (RR=0.49; 95% CI, 0.38-0.64; NNT=97) compared with warfarin. All-cause mortality was lower with NOACs as well (RR=0.90; 95% CI, 0.85-0.95; NNT=128).
A significant increase in gastrointestinal bleeding occurred with NOACs compared with warfarin (RR=1.3; 95% CI, 1.1-1.6; number needed to harm=185), but NOACs were associated with a decrease in intracranial hemorrhage similar to the reduction in hemorrhagic stroke (RR=0.48; 95% CI, 0.39-0.59; NNT=132).
NOACs show no significant difference in bleeding complications vs warfarin
A 2013 meta-analysis of 5 RCTs including 51,895 patients with nonvalvular atrial fibrillation compared the efficacy and safety of the NOACs dabigatran, rivaroxaban, apixaban, and ximelagatran, with the efficacy and safety of warfarin.2 This review included the 3 studies of dabigatran, rivaroxaban, and apixaban from the previously described review, as well as 2 trials of ximelagatran that were not included in the other review (presumably because ximelagatran was no longer available owing to liver toxicity). This review didn’t include the study of edoxaban that was published after the search dates of the literature review.
All trials were designed to show noninferiority. Selection criteria included a study population of at least 3000 patients and use of intention-to-treat analysis. Only 3 of the trials were double-blinded, and 2 were open-label. Mean follow-up was 16 months; median was 24 months.
NOACs were noninferior to vitamin K antagonists in the rate of stroke or systemic embolism (RR=0.82; 95% CI, 0.69-0.98; NNT=200), the rate of death from any cause (RR=0.91; 95% CI, 0.85-0.96; NNT=145), and the rate of hemorrhagic strokes (RR=0.51; 95% CI, 0.41-0.64). NOACs showed no significant difference in major bleeding compared with warfarin (RR=0.83; 95% CI, 0.69-1.0), and were noninferior for minor bleeding (RR=0.88; 95% CI, 0.80-0.97). There was no difference in ischemic stroke (RR=0.87; 95% CI, 0.75-1.06) and major noncerebral bleeding (RR=0.88; 95% CI, 0.73-1.08).
The ACCP weighs in
The American College of Chest Physicians’ 2012 clinical practice guidelines for antithrombotic therapy for atrial fibrillation recommend dabigatran 150 mg twice daily rather than adjusted-dose warfarin therapy for patients with nonvalvular atrial fibrillation requiring thromboembolism prophylaxis (Grade 2B, weak recommendation based on RCTs with important limitations).3
1. Ruff CT, Giugliano RP, Braunwald E, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet. 2014;383:955-962.
2. Dogliotti A, Paolasso E, Giugliano RP, et al. Novel oral anticoagulants in atrial fibrillation: a meta-analysis of large, randomized, controlled trials vs warfarin. Clin Cardiol. 2013;36:61-67.
3. You JJ, Singer DE, Howard PA, et al. Antithrombotic therapy for atrial fibrillation: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):e531S-e575S.
Yes. Dabigatran, rivaroxaban, and apixaban are safe and effective compared with warfarin for preventing stroke in patients with nonvalvular atrial fibrillation. These novel oral anticoagulants (NOACs) are noninferior in reducing the number of strokes and systemic emboli and in lowering all-cause mortality while not increasing major bleeding complications and hemorrhagic events (strength of recommendation: A, consistent meta-analyses of randomized controlled trials [RCTs]).
Evidence summary
A 2014 meta-analysis of 4 RCTs including 71,683 patients with nonvalvular atrial fibrillation evaluated the NOACs dabigatran, rivaroxaban, apixaban, and edoxaban, for efficacy and safety compared with warfarin.1 The RCTs analyzed 42,411 patients receiving NOACs and 29,272 patients receiving warfarin. All trials were designed to show noninferiority. Selection criteria for RCTs included all phase 3 trials of available NOACs (edoxaban isn’t available in the United States). Median follow-up was 1.8 to 2.8 years.
Pooled data demonstrated that NOACs were noninferior to warfarin in preventing stroke or systemic embolism (relative risk [RR]=0.81; 95% confidence interval [CI], 0.73-0.91; number needed to treat [NNT]=147). The main benefit was derived from the relatively large decrease in the rate of hemorrhagic stroke (RR=0.49; 95% CI, 0.38-0.64; NNT=97) compared with warfarin. All-cause mortality was lower with NOACs as well (RR=0.90; 95% CI, 0.85-0.95; NNT=128).
A significant increase in gastrointestinal bleeding occurred with NOACs compared with warfarin (RR=1.3; 95% CI, 1.1-1.6; number needed to harm=185), but NOACs were associated with a decrease in intracranial hemorrhage similar to the reduction in hemorrhagic stroke (RR=0.48; 95% CI, 0.39-0.59; NNT=132).
NOACs show no significant difference in bleeding complications vs warfarin
A 2013 meta-analysis of 5 RCTs including 51,895 patients with nonvalvular atrial fibrillation compared the efficacy and safety of the NOACs dabigatran, rivaroxaban, apixaban, and ximelagatran, with the efficacy and safety of warfarin.2 This review included the 3 studies of dabigatran, rivaroxaban, and apixaban from the previously described review, as well as 2 trials of ximelagatran that were not included in the other review (presumably because ximelagatran was no longer available owing to liver toxicity). This review didn’t include the study of edoxaban that was published after the search dates of the literature review.
All trials were designed to show noninferiority. Selection criteria included a study population of at least 3000 patients and use of intention-to-treat analysis. Only 3 of the trials were double-blinded, and 2 were open-label. Mean follow-up was 16 months; median was 24 months.
NOACs were noninferior to vitamin K antagonists in the rate of stroke or systemic embolism (RR=0.82; 95% CI, 0.69-0.98; NNT=200), the rate of death from any cause (RR=0.91; 95% CI, 0.85-0.96; NNT=145), and the rate of hemorrhagic strokes (RR=0.51; 95% CI, 0.41-0.64). NOACs showed no significant difference in major bleeding compared with warfarin (RR=0.83; 95% CI, 0.69-1.0), and were noninferior for minor bleeding (RR=0.88; 95% CI, 0.80-0.97). There was no difference in ischemic stroke (RR=0.87; 95% CI, 0.75-1.06) and major noncerebral bleeding (RR=0.88; 95% CI, 0.73-1.08).
The ACCP weighs in
The American College of Chest Physicians’ 2012 clinical practice guidelines for antithrombotic therapy for atrial fibrillation recommend dabigatran 150 mg twice daily rather than adjusted-dose warfarin therapy for patients with nonvalvular atrial fibrillation requiring thromboembolism prophylaxis (Grade 2B, weak recommendation based on RCTs with important limitations).3
Yes. Dabigatran, rivaroxaban, and apixaban are safe and effective compared with warfarin for preventing stroke in patients with nonvalvular atrial fibrillation. These novel oral anticoagulants (NOACs) are noninferior in reducing the number of strokes and systemic emboli and in lowering all-cause mortality while not increasing major bleeding complications and hemorrhagic events (strength of recommendation: A, consistent meta-analyses of randomized controlled trials [RCTs]).
Evidence summary
A 2014 meta-analysis of 4 RCTs including 71,683 patients with nonvalvular atrial fibrillation evaluated the NOACs dabigatran, rivaroxaban, apixaban, and edoxaban, for efficacy and safety compared with warfarin.1 The RCTs analyzed 42,411 patients receiving NOACs and 29,272 patients receiving warfarin. All trials were designed to show noninferiority. Selection criteria for RCTs included all phase 3 trials of available NOACs (edoxaban isn’t available in the United States). Median follow-up was 1.8 to 2.8 years.
Pooled data demonstrated that NOACs were noninferior to warfarin in preventing stroke or systemic embolism (relative risk [RR]=0.81; 95% confidence interval [CI], 0.73-0.91; number needed to treat [NNT]=147). The main benefit was derived from the relatively large decrease in the rate of hemorrhagic stroke (RR=0.49; 95% CI, 0.38-0.64; NNT=97) compared with warfarin. All-cause mortality was lower with NOACs as well (RR=0.90; 95% CI, 0.85-0.95; NNT=128).
A significant increase in gastrointestinal bleeding occurred with NOACs compared with warfarin (RR=1.3; 95% CI, 1.1-1.6; number needed to harm=185), but NOACs were associated with a decrease in intracranial hemorrhage similar to the reduction in hemorrhagic stroke (RR=0.48; 95% CI, 0.39-0.59; NNT=132).
NOACs show no significant difference in bleeding complications vs warfarin
A 2013 meta-analysis of 5 RCTs including 51,895 patients with nonvalvular atrial fibrillation compared the efficacy and safety of the NOACs dabigatran, rivaroxaban, apixaban, and ximelagatran, with the efficacy and safety of warfarin.2 This review included the 3 studies of dabigatran, rivaroxaban, and apixaban from the previously described review, as well as 2 trials of ximelagatran that were not included in the other review (presumably because ximelagatran was no longer available owing to liver toxicity). This review didn’t include the study of edoxaban that was published after the search dates of the literature review.
All trials were designed to show noninferiority. Selection criteria included a study population of at least 3000 patients and use of intention-to-treat analysis. Only 3 of the trials were double-blinded, and 2 were open-label. Mean follow-up was 16 months; median was 24 months.
NOACs were noninferior to vitamin K antagonists in the rate of stroke or systemic embolism (RR=0.82; 95% CI, 0.69-0.98; NNT=200), the rate of death from any cause (RR=0.91; 95% CI, 0.85-0.96; NNT=145), and the rate of hemorrhagic strokes (RR=0.51; 95% CI, 0.41-0.64). NOACs showed no significant difference in major bleeding compared with warfarin (RR=0.83; 95% CI, 0.69-1.0), and were noninferior for minor bleeding (RR=0.88; 95% CI, 0.80-0.97). There was no difference in ischemic stroke (RR=0.87; 95% CI, 0.75-1.06) and major noncerebral bleeding (RR=0.88; 95% CI, 0.73-1.08).
The ACCP weighs in
The American College of Chest Physicians’ 2012 clinical practice guidelines for antithrombotic therapy for atrial fibrillation recommend dabigatran 150 mg twice daily rather than adjusted-dose warfarin therapy for patients with nonvalvular atrial fibrillation requiring thromboembolism prophylaxis (Grade 2B, weak recommendation based on RCTs with important limitations).3
1. Ruff CT, Giugliano RP, Braunwald E, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet. 2014;383:955-962.
2. Dogliotti A, Paolasso E, Giugliano RP, et al. Novel oral anticoagulants in atrial fibrillation: a meta-analysis of large, randomized, controlled trials vs warfarin. Clin Cardiol. 2013;36:61-67.
3. You JJ, Singer DE, Howard PA, et al. Antithrombotic therapy for atrial fibrillation: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):e531S-e575S.
1. Ruff CT, Giugliano RP, Braunwald E, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet. 2014;383:955-962.
2. Dogliotti A, Paolasso E, Giugliano RP, et al. Novel oral anticoagulants in atrial fibrillation: a meta-analysis of large, randomized, controlled trials vs warfarin. Clin Cardiol. 2013;36:61-67.
3. You JJ, Singer DE, Howard PA, et al. Antithrombotic therapy for atrial fibrillation: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):e531S-e575S.
Evidence-based answers from the Family Physicians Inquiries Network
Do corticosteroids reduce bronchiolitis hospitalizations?
No. Corticosteroids alone don’t decrease hospital admissions or length of stay among children with bronchiolitis (strength of recommendation [SOR]: A, meta-analysis of randomized controlled trials [RCTs]).
Combining oral dexamethasone and inhaled epinephrine appears to prevent one hospital admission for every 11 patients treated (SOR: B, single large RCT).
Evidence summary
A 2013 Cochrane review of 17 RCTs with 2596 patients compared corticosteroids with placebo for treating bronchiolitis in children younger than 2 years.1 The studies used dexamethasone, prednisolone, prednisone, and budesonide delivered by oral, inhaled, intravenous (IV), or intramuscular (IM) routes, ranging between a one-day dose to a 5-day taper. Doses ranged from 0.5 to 2 mg/kg/d for oral and parenteral routes and 0.2 to 1 mg for inhalation. Outcomes were rate of admissions at Days 1 and 7 from outpatient trials and length of stay among inpatients.
Investigators found no significant difference in admission rates at Day 1 and Day 7 between children treated with corticosteroids compared with placebo (Day 1: 8 trials, 1762 patients; relative risk [RR]=0.92; 95% confidence interval [CI], 0.78-1.1; Day 7: 5 trials, 1530 patients; RR=0.86; 95% CI, 0.70-1.1). Length of hospital stay didn’t differ between children treated with corticosteroids and children who received placebo (8 trials, 633 patients; mean difference= −0.18 days; 95% CI, −0.39 to 0.04).
Corticosteroid + epinephrine can lower hospital admissions
A 2009 multicenter, double-blind RCT with 800 patients (infants 6 weeks to 12 months of age with a first episode of bronchiolitis) that was included in the 2013 Cochrane review also compared the combination of epinephrine and corticosteroid with placebo and either agent alone.2
Infants were assigned to 4 groups: oral dexamethasone alone (1 mg/kg in the emergency room [ER] on Day 1, followed by 0.6 mg/kg daily for 5 days); nebulized epinephrine alone (2 treatments of 3 mL epinephrine 1:1000 solution); combined dexamethasone and epinephrine; and placebo. The primary outcome was hospital admission as long as 7 days after being seen in the ER.
Rates of admission were similar for the dexamethasone and placebo groups (25.6% vs 26.4%, respectively; RR=0.96; 95% CI, 0.69-1.3). The epinephrine group’s rate of admission was 23.7% (RR=0.88; CI, 0.63–1.23). Only the dexamethasone-epinephrine group had a lower rate of admission compared with placebo (17% vs 26%; RR=0.65; 95% CI, 0.45-0.95). The number needed to treat with dexamethasone-epinephrine to prevent one hospital admission was 11.
Review prompts revised recommendations
Based on the Cochrane review, the American Academy of Pediatrics (AAP) revised its evidence-based clinical practice guideline in 2014 to recommend that clinicians not administer systemic corticosteroids to infants with a diagnosis of bronchiolitis in any setting (evidence quality B, strong recommendation, based on results of multiple RCTs).3 The AAP advocates additional large trials to clarify whether combination therapy (corticosteroids plus agents with α or β agonist activity) is effective.
1. Fernandes RM, Bialy LM, Vandermeer B, et al. Glucocorticoids for acute viral bronchitis in infants and children. Cochrane Database Syst Rev. 2013;(6):CD004878.
2. Plint AC, Johnson DW, Patel H, et al. Epinephrine and dexamethasone in children with bronchiolitis. N Engl J Med. 2009;360:2079-2089.
3. Ralston SL, Lieberthal AS, Meissner HC, et al. Clinical practice guideline: the diagnosis, management, and prevention of bronchiolitis. Pediatrics. 2014;134:e1474-e1502.
No. Corticosteroids alone don’t decrease hospital admissions or length of stay among children with bronchiolitis (strength of recommendation [SOR]: A, meta-analysis of randomized controlled trials [RCTs]).
Combining oral dexamethasone and inhaled epinephrine appears to prevent one hospital admission for every 11 patients treated (SOR: B, single large RCT).
Evidence summary
A 2013 Cochrane review of 17 RCTs with 2596 patients compared corticosteroids with placebo for treating bronchiolitis in children younger than 2 years.1 The studies used dexamethasone, prednisolone, prednisone, and budesonide delivered by oral, inhaled, intravenous (IV), or intramuscular (IM) routes, ranging between a one-day dose to a 5-day taper. Doses ranged from 0.5 to 2 mg/kg/d for oral and parenteral routes and 0.2 to 1 mg for inhalation. Outcomes were rate of admissions at Days 1 and 7 from outpatient trials and length of stay among inpatients.
Investigators found no significant difference in admission rates at Day 1 and Day 7 between children treated with corticosteroids compared with placebo (Day 1: 8 trials, 1762 patients; relative risk [RR]=0.92; 95% confidence interval [CI], 0.78-1.1; Day 7: 5 trials, 1530 patients; RR=0.86; 95% CI, 0.70-1.1). Length of hospital stay didn’t differ between children treated with corticosteroids and children who received placebo (8 trials, 633 patients; mean difference= −0.18 days; 95% CI, −0.39 to 0.04).
Corticosteroid + epinephrine can lower hospital admissions
A 2009 multicenter, double-blind RCT with 800 patients (infants 6 weeks to 12 months of age with a first episode of bronchiolitis) that was included in the 2013 Cochrane review also compared the combination of epinephrine and corticosteroid with placebo and either agent alone.2
Infants were assigned to 4 groups: oral dexamethasone alone (1 mg/kg in the emergency room [ER] on Day 1, followed by 0.6 mg/kg daily for 5 days); nebulized epinephrine alone (2 treatments of 3 mL epinephrine 1:1000 solution); combined dexamethasone and epinephrine; and placebo. The primary outcome was hospital admission as long as 7 days after being seen in the ER.
Rates of admission were similar for the dexamethasone and placebo groups (25.6% vs 26.4%, respectively; RR=0.96; 95% CI, 0.69-1.3). The epinephrine group’s rate of admission was 23.7% (RR=0.88; CI, 0.63–1.23). Only the dexamethasone-epinephrine group had a lower rate of admission compared with placebo (17% vs 26%; RR=0.65; 95% CI, 0.45-0.95). The number needed to treat with dexamethasone-epinephrine to prevent one hospital admission was 11.
Review prompts revised recommendations
Based on the Cochrane review, the American Academy of Pediatrics (AAP) revised its evidence-based clinical practice guideline in 2014 to recommend that clinicians not administer systemic corticosteroids to infants with a diagnosis of bronchiolitis in any setting (evidence quality B, strong recommendation, based on results of multiple RCTs).3 The AAP advocates additional large trials to clarify whether combination therapy (corticosteroids plus agents with α or β agonist activity) is effective.
No. Corticosteroids alone don’t decrease hospital admissions or length of stay among children with bronchiolitis (strength of recommendation [SOR]: A, meta-analysis of randomized controlled trials [RCTs]).
Combining oral dexamethasone and inhaled epinephrine appears to prevent one hospital admission for every 11 patients treated (SOR: B, single large RCT).
Evidence summary
A 2013 Cochrane review of 17 RCTs with 2596 patients compared corticosteroids with placebo for treating bronchiolitis in children younger than 2 years.1 The studies used dexamethasone, prednisolone, prednisone, and budesonide delivered by oral, inhaled, intravenous (IV), or intramuscular (IM) routes, ranging between a one-day dose to a 5-day taper. Doses ranged from 0.5 to 2 mg/kg/d for oral and parenteral routes and 0.2 to 1 mg for inhalation. Outcomes were rate of admissions at Days 1 and 7 from outpatient trials and length of stay among inpatients.
Investigators found no significant difference in admission rates at Day 1 and Day 7 between children treated with corticosteroids compared with placebo (Day 1: 8 trials, 1762 patients; relative risk [RR]=0.92; 95% confidence interval [CI], 0.78-1.1; Day 7: 5 trials, 1530 patients; RR=0.86; 95% CI, 0.70-1.1). Length of hospital stay didn’t differ between children treated with corticosteroids and children who received placebo (8 trials, 633 patients; mean difference= −0.18 days; 95% CI, −0.39 to 0.04).
Corticosteroid + epinephrine can lower hospital admissions
A 2009 multicenter, double-blind RCT with 800 patients (infants 6 weeks to 12 months of age with a first episode of bronchiolitis) that was included in the 2013 Cochrane review also compared the combination of epinephrine and corticosteroid with placebo and either agent alone.2
Infants were assigned to 4 groups: oral dexamethasone alone (1 mg/kg in the emergency room [ER] on Day 1, followed by 0.6 mg/kg daily for 5 days); nebulized epinephrine alone (2 treatments of 3 mL epinephrine 1:1000 solution); combined dexamethasone and epinephrine; and placebo. The primary outcome was hospital admission as long as 7 days after being seen in the ER.
Rates of admission were similar for the dexamethasone and placebo groups (25.6% vs 26.4%, respectively; RR=0.96; 95% CI, 0.69-1.3). The epinephrine group’s rate of admission was 23.7% (RR=0.88; CI, 0.63–1.23). Only the dexamethasone-epinephrine group had a lower rate of admission compared with placebo (17% vs 26%; RR=0.65; 95% CI, 0.45-0.95). The number needed to treat with dexamethasone-epinephrine to prevent one hospital admission was 11.
Review prompts revised recommendations
Based on the Cochrane review, the American Academy of Pediatrics (AAP) revised its evidence-based clinical practice guideline in 2014 to recommend that clinicians not administer systemic corticosteroids to infants with a diagnosis of bronchiolitis in any setting (evidence quality B, strong recommendation, based on results of multiple RCTs).3 The AAP advocates additional large trials to clarify whether combination therapy (corticosteroids plus agents with α or β agonist activity) is effective.
1. Fernandes RM, Bialy LM, Vandermeer B, et al. Glucocorticoids for acute viral bronchitis in infants and children. Cochrane Database Syst Rev. 2013;(6):CD004878.
2. Plint AC, Johnson DW, Patel H, et al. Epinephrine and dexamethasone in children with bronchiolitis. N Engl J Med. 2009;360:2079-2089.
3. Ralston SL, Lieberthal AS, Meissner HC, et al. Clinical practice guideline: the diagnosis, management, and prevention of bronchiolitis. Pediatrics. 2014;134:e1474-e1502.
1. Fernandes RM, Bialy LM, Vandermeer B, et al. Glucocorticoids for acute viral bronchitis in infants and children. Cochrane Database Syst Rev. 2013;(6):CD004878.
2. Plint AC, Johnson DW, Patel H, et al. Epinephrine and dexamethasone in children with bronchiolitis. N Engl J Med. 2009;360:2079-2089.
3. Ralston SL, Lieberthal AS, Meissner HC, et al. Clinical practice guideline: the diagnosis, management, and prevention of bronchiolitis. Pediatrics. 2014;134:e1474-e1502.
Evidence-based answers from the Family Physicians Inquiries Network
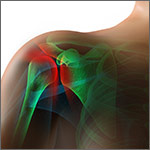
Surgery vs conservative management for AC joint repair: How do the 2 compare?
When not considering the grade of acromioclavicular (AC) joint dislocation, both conservative and surgical management lead to positive outcomes, although surgically managed patients require more time out of work (strength of recommendation [SOR]: B, Cochrane review of low-quality randomized controlled trials [RCTs]).
For Rockwood grade III dislocations, surgical intervention provides a better cosmetic outcome but increases infection risk (SOR: B, meta-analysis of retrospective case series).
Consensus guidelines suggest conservative management for Rockwood grade I to II dislocations and surgical repair for Rockwood grade IV to VI dislocations (SOR: C, expert opinion).
Similar outcomes, except when it comes to return to work
A 2010 Cochrane review of 2 RCTs and one quasi-randomized trial (174 patients, 93% male, moderate to high risk of bias) compared surgical intervention with conservative management of acute AC separations of unspecified Rockwood classification.1 Surgeries included coracoclavicular fixation with a cancellous screw or transfixation of the AC joint with Steinmann pins or Kirschner wires.
Conservative treatment included immobilization of the shoulder using an arm sling for 2 to 4 weeks. Patients were evaluated for a minimum of 12 months with a nonvalidated scoring system that measured pain, motion, and function or strength.
At one year, 63 of 76 patients (83%) in the post-surgical group and 74 of 84 patients (88%) in the conservative intervention group had either good or excellent results with no significant difference in unsatisfactory outcomes (relative risk [RR]=1.49; 95% confidence interval [CI], 0.75-2.95). (Fourteen patients—7 in each group—were lost to follow-up.) Moreover, the review found no significant difference in treatment failures requiring a subsequent operation between the groups—11 of 83 (13%) surgical patients and 7 of 91 (8%) conservatively managed patients (RR=1.72; 95% CI, 0.72-4.12).
Notably, regardless of activity level, surgical patients consistently returned to previous work functions later than patients managed conservatively. The mean convalescence time ranged from 8 to 11 weeks for surgical patients and 4 to 6 weeks for conservatively managed patients (P<.05).
A look at cosmetic results and risk of infection
A 2011 meta-analysis of 6 retrospective case series (379 patients, approximately 88% male) compared operative with nonoperative management in patients with acute, closed Rockwood grade III AC dislocations.2 Operative techniques varied; nonoperative patients each received physiotherapy or rehabilitation therapy and most were treated with a sling. Patient follow-up varied from 32 months to 10.8 years.
Four of the included studies suggested that nonoperative management resulted in poorer cosmetic results (methods not defined) compared with the operative group (11 of 115 surgical patients [10%], 74 of 88 nonoperative patients [84%]; risk difference [RD]=−0.79; 95% CI, −0.92 to −0.66; number needed to harm [NNH]=>2). Two of the studies evaluated the duration of sick leave and found a longer leave with operative management (50 operative and 54 nonoperative patients; mean difference=3.3; 95% CI, 2.1-4.5).
Five of the studies observed an increased risk of infection following operative management (8 of 175 [5%] operative patients compared with 0 of 152 [0%] nonoperative patients; RD=0.05; 95% CI, 0.01-0.09; NNH=20).
Recommendations depend on the grade of the injury
The American College of Occupational and Environmental Medicine recommends against routine surgical repair for Grade III AC joint separations.3 The College also recommends nonoperative management for patients with grade I to II AC dislocations and surgical repair for patients with grades IV to VI and select grade III AC dislocations.
1. Tamaoki MJS, Belloti JC, Lenza M, et al. Surgical versus conservative interventions for treating acromioclavicular dislocation of the shoulder in adults. Cochrane Database Syst Rev. 2010;(8):CD007429.
2. Smith TO, Chester R, Pearse EO, et al. Operative versus non-operative management following Rockwood grade III acromioclavicular separation: a meta-analysis of the current evidence base. J Orthopaed Traumatol. 2011;12:19–27.
3. Hegmann KT. Shoulder disorders. In: Occupational Medicine Practice Guidelines. Evaluation and Management of Common Health Problems and Functional Recovery in Workers. 3rd ed. Elk Grove Village, IL: American College of Occupational and Environmental Medicine; 2011:1-297.
When not considering the grade of acromioclavicular (AC) joint dislocation, both conservative and surgical management lead to positive outcomes, although surgically managed patients require more time out of work (strength of recommendation [SOR]: B, Cochrane review of low-quality randomized controlled trials [RCTs]).
For Rockwood grade III dislocations, surgical intervention provides a better cosmetic outcome but increases infection risk (SOR: B, meta-analysis of retrospective case series).
Consensus guidelines suggest conservative management for Rockwood grade I to II dislocations and surgical repair for Rockwood grade IV to VI dislocations (SOR: C, expert opinion).
Similar outcomes, except when it comes to return to work
A 2010 Cochrane review of 2 RCTs and one quasi-randomized trial (174 patients, 93% male, moderate to high risk of bias) compared surgical intervention with conservative management of acute AC separations of unspecified Rockwood classification.1 Surgeries included coracoclavicular fixation with a cancellous screw or transfixation of the AC joint with Steinmann pins or Kirschner wires.
Conservative treatment included immobilization of the shoulder using an arm sling for 2 to 4 weeks. Patients were evaluated for a minimum of 12 months with a nonvalidated scoring system that measured pain, motion, and function or strength.
At one year, 63 of 76 patients (83%) in the post-surgical group and 74 of 84 patients (88%) in the conservative intervention group had either good or excellent results with no significant difference in unsatisfactory outcomes (relative risk [RR]=1.49; 95% confidence interval [CI], 0.75-2.95). (Fourteen patients—7 in each group—were lost to follow-up.) Moreover, the review found no significant difference in treatment failures requiring a subsequent operation between the groups—11 of 83 (13%) surgical patients and 7 of 91 (8%) conservatively managed patients (RR=1.72; 95% CI, 0.72-4.12).
Notably, regardless of activity level, surgical patients consistently returned to previous work functions later than patients managed conservatively. The mean convalescence time ranged from 8 to 11 weeks for surgical patients and 4 to 6 weeks for conservatively managed patients (P<.05).
A look at cosmetic results and risk of infection
A 2011 meta-analysis of 6 retrospective case series (379 patients, approximately 88% male) compared operative with nonoperative management in patients with acute, closed Rockwood grade III AC dislocations.2 Operative techniques varied; nonoperative patients each received physiotherapy or rehabilitation therapy and most were treated with a sling. Patient follow-up varied from 32 months to 10.8 years.
Four of the included studies suggested that nonoperative management resulted in poorer cosmetic results (methods not defined) compared with the operative group (11 of 115 surgical patients [10%], 74 of 88 nonoperative patients [84%]; risk difference [RD]=−0.79; 95% CI, −0.92 to −0.66; number needed to harm [NNH]=>2). Two of the studies evaluated the duration of sick leave and found a longer leave with operative management (50 operative and 54 nonoperative patients; mean difference=3.3; 95% CI, 2.1-4.5).
Five of the studies observed an increased risk of infection following operative management (8 of 175 [5%] operative patients compared with 0 of 152 [0%] nonoperative patients; RD=0.05; 95% CI, 0.01-0.09; NNH=20).
Recommendations depend on the grade of the injury
The American College of Occupational and Environmental Medicine recommends against routine surgical repair for Grade III AC joint separations.3 The College also recommends nonoperative management for patients with grade I to II AC dislocations and surgical repair for patients with grades IV to VI and select grade III AC dislocations.
When not considering the grade of acromioclavicular (AC) joint dislocation, both conservative and surgical management lead to positive outcomes, although surgically managed patients require more time out of work (strength of recommendation [SOR]: B, Cochrane review of low-quality randomized controlled trials [RCTs]).
For Rockwood grade III dislocations, surgical intervention provides a better cosmetic outcome but increases infection risk (SOR: B, meta-analysis of retrospective case series).
Consensus guidelines suggest conservative management for Rockwood grade I to II dislocations and surgical repair for Rockwood grade IV to VI dislocations (SOR: C, expert opinion).
Similar outcomes, except when it comes to return to work
A 2010 Cochrane review of 2 RCTs and one quasi-randomized trial (174 patients, 93% male, moderate to high risk of bias) compared surgical intervention with conservative management of acute AC separations of unspecified Rockwood classification.1 Surgeries included coracoclavicular fixation with a cancellous screw or transfixation of the AC joint with Steinmann pins or Kirschner wires.
Conservative treatment included immobilization of the shoulder using an arm sling for 2 to 4 weeks. Patients were evaluated for a minimum of 12 months with a nonvalidated scoring system that measured pain, motion, and function or strength.
At one year, 63 of 76 patients (83%) in the post-surgical group and 74 of 84 patients (88%) in the conservative intervention group had either good or excellent results with no significant difference in unsatisfactory outcomes (relative risk [RR]=1.49; 95% confidence interval [CI], 0.75-2.95). (Fourteen patients—7 in each group—were lost to follow-up.) Moreover, the review found no significant difference in treatment failures requiring a subsequent operation between the groups—11 of 83 (13%) surgical patients and 7 of 91 (8%) conservatively managed patients (RR=1.72; 95% CI, 0.72-4.12).
Notably, regardless of activity level, surgical patients consistently returned to previous work functions later than patients managed conservatively. The mean convalescence time ranged from 8 to 11 weeks for surgical patients and 4 to 6 weeks for conservatively managed patients (P<.05).
A look at cosmetic results and risk of infection
A 2011 meta-analysis of 6 retrospective case series (379 patients, approximately 88% male) compared operative with nonoperative management in patients with acute, closed Rockwood grade III AC dislocations.2 Operative techniques varied; nonoperative patients each received physiotherapy or rehabilitation therapy and most were treated with a sling. Patient follow-up varied from 32 months to 10.8 years.
Four of the included studies suggested that nonoperative management resulted in poorer cosmetic results (methods not defined) compared with the operative group (11 of 115 surgical patients [10%], 74 of 88 nonoperative patients [84%]; risk difference [RD]=−0.79; 95% CI, −0.92 to −0.66; number needed to harm [NNH]=>2). Two of the studies evaluated the duration of sick leave and found a longer leave with operative management (50 operative and 54 nonoperative patients; mean difference=3.3; 95% CI, 2.1-4.5).
Five of the studies observed an increased risk of infection following operative management (8 of 175 [5%] operative patients compared with 0 of 152 [0%] nonoperative patients; RD=0.05; 95% CI, 0.01-0.09; NNH=20).
Recommendations depend on the grade of the injury
The American College of Occupational and Environmental Medicine recommends against routine surgical repair for Grade III AC joint separations.3 The College also recommends nonoperative management for patients with grade I to II AC dislocations and surgical repair for patients with grades IV to VI and select grade III AC dislocations.
1. Tamaoki MJS, Belloti JC, Lenza M, et al. Surgical versus conservative interventions for treating acromioclavicular dislocation of the shoulder in adults. Cochrane Database Syst Rev. 2010;(8):CD007429.
2. Smith TO, Chester R, Pearse EO, et al. Operative versus non-operative management following Rockwood grade III acromioclavicular separation: a meta-analysis of the current evidence base. J Orthopaed Traumatol. 2011;12:19–27.
3. Hegmann KT. Shoulder disorders. In: Occupational Medicine Practice Guidelines. Evaluation and Management of Common Health Problems and Functional Recovery in Workers. 3rd ed. Elk Grove Village, IL: American College of Occupational and Environmental Medicine; 2011:1-297.
1. Tamaoki MJS, Belloti JC, Lenza M, et al. Surgical versus conservative interventions for treating acromioclavicular dislocation of the shoulder in adults. Cochrane Database Syst Rev. 2010;(8):CD007429.
2. Smith TO, Chester R, Pearse EO, et al. Operative versus non-operative management following Rockwood grade III acromioclavicular separation: a meta-analysis of the current evidence base. J Orthopaed Traumatol. 2011;12:19–27.
3. Hegmann KT. Shoulder disorders. In: Occupational Medicine Practice Guidelines. Evaluation and Management of Common Health Problems and Functional Recovery in Workers. 3rd ed. Elk Grove Village, IL: American College of Occupational and Environmental Medicine; 2011:1-297.
Evidence-based answers from the Family Physicians Inquiries Network