User login
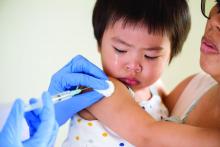
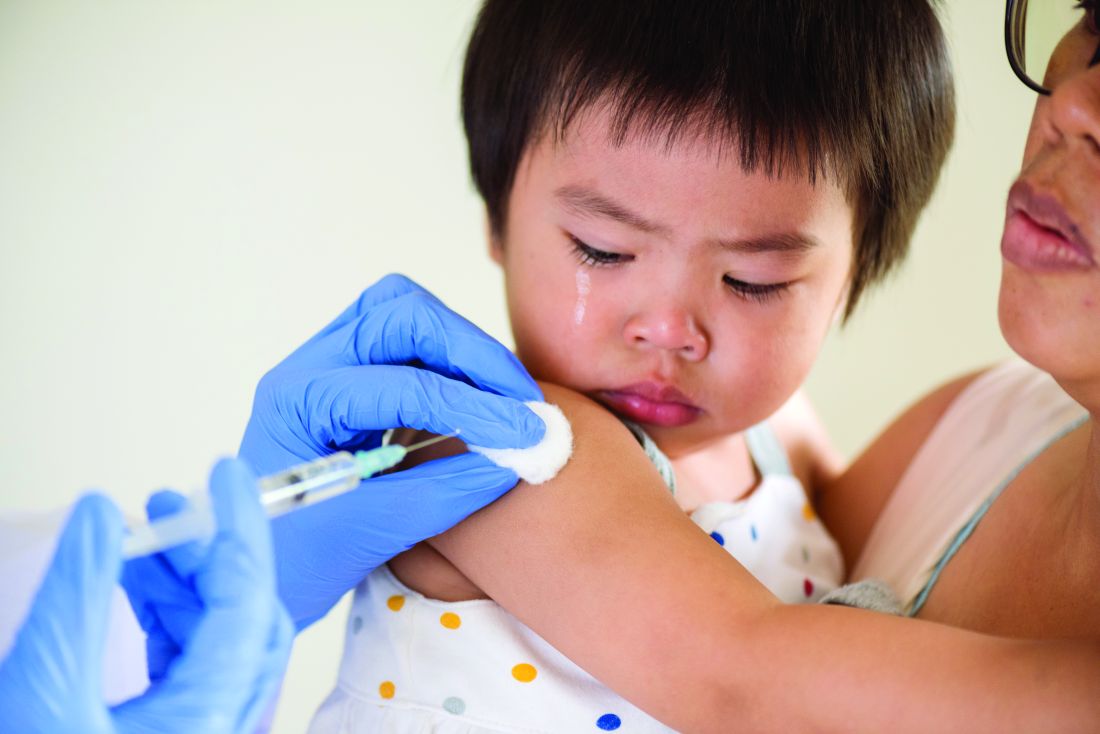
Flu vaccine: Larger impact on influenza burden than you thought?
ID Week, the annual meeting of the Infectious Disease Society of America, provided valuable insights into past season’s endemic influenza burden and the effectiveness of prevention strategies. Each year, there are from 9million to 49 million influenza cases in the United States, 140,000-960,000 hospitalized cases, and 12,000-70,000 deaths directly attributable to influenza infection. The burden disproportionately falls on infants and adults 65 years of age and older; 11,000-48,000 children are hospitalized, and as many as several hundred children may die from influenza and related complications. School age children (aged 5-19 years) and adults (aged 30-39 years) are a major part of the transmission cycle. Influenza vaccine underlies the prevention strategy for limiting the burden of disease in U.S. populations. ID Week provided new insights into critical questions about influenza vaccines.
1. What is the effectiveness of influenza vaccine against severe disease (hospitalization) in children? Does it vary by age? By type or subtype?
Angela P. Campbell, MD, MPH, of the Centers for Disease Control and Prevention, and associates presented data on influenza vaccine effectiveness from the New Vaccine Surveillance Network in children for the 2016-2017 and 2017-2018 season (ID Week session 99; Abstract 899). During both 2016-2017 and 2017-2018, H3N2 was the dominant virus and influenza B represented about one-third of cases, and H1N1 was a greater percentage of cases in 2017-2018. Influenza positivity among children younger than 18 years of age admitted to hospital with respiratory disease was 14% among unvaccinated and 8% among vaccinated children; effectiveness again hospitalization was 50%. Vaccine effectiveness (VE) was not statistically different between children younger than 8 years of age and those older that 8 years but did differ by vaccine type. VE was 76% against H1N1 disease, 59% again B disease, and only 33% against H3N2 disease.
Clearly, vaccination with influenza vaccine prevents serious respiratory disease. However, the impact of vaccine will vary by season and by which influenza stains are circulating in the community. The authors concluded that further understanding of the lower VE against H3N2 disease is needed.
2. Does the priming dose of influenza vaccine improve vaccine effectiveness?
Current recommendations call for a two-dose series for influenza vaccine in children aged 6 months through 8 years who have not had prior influenza vaccine. The recommendation is based on evidence demonstrating higher antibody responses in children receiving two doses, compared with a single dose. Using data from the U.S. Influenza Vaccine Effectiveness Network, Jessie R. Chung, MPH, of the CDC, and associates compared VE in children younger than 2 years receiving two doses in the first year of flu immunization (fully immunized), compared with those who received only one dose (partially immunized) (ID Week session 99; Abstract 900). VE was 53% for fully immunized and 23% for partially immunized children. Receipt of a single dose did not provide statistically significant protection against influenza. Surprisingly (to me), of 5,355 children aged 6 months to less than 2 years with no prior influenza vaccine, 1,870 (35%) received only one dose in the season.
The data strongly support the current recommendations for a priming dose, especially in young children, in the first season of influenza vaccine and warrants increased efforts to increase the update of second doses during the season. Hopefully we can do better in 2019!
3. Should we wait to vaccinate with influenza vaccine?
Some evidence suggests that waning immunity to influenza vaccine, primarily in those aged 65 years and older, may explain increased disease activity toward the end of influenza season. Other explanations include increasing viral diversity throughout the season, resulting in reduced effectiveness. Do such concerns warrant delaying immunization? The onset and peak of influenza season varies by year; in October 2019, 3% of tests performed on patients with respiratory illness were influenza positive. The trade-offs for delaying immunization until October are the unpredictability of onset of influenza season, the requirement for two doses in infants, the need for 2 weeks to achieve peak antibody concentrations, and the potential that fewer individuals will be vaccinated. Kathy Neuzil, MD, MPH, from the Center for Vaccine Development and Global Health, University of Maryland School of Medicine, reviewed recent modeling (for adults aged 65 years and older) and reported that delaying vaccine programs until October is associated with greater burden of hospitalization if 14% fewer individuals (who would be vaccinated in August/September) are vaccinated (ID Week; Session 940).
In response to these concerns, the CDC recommendations for 2019 are that, in children aged 6 months through 8 years who need two doses, start early so that you can achieve both doses before influenza season (MMWR 2019 Aug 23;68[3]:1-21).In older children and adults, who need only a single dose, early vaccination (August and early September) may lead to reduced protection late in the influenza season?
4. How can we optimize vaccine impact?
Vaccine impact refers to the affect on a population level and not at an individual level. Meagan C. Fitzpatrick, PhD, from the Center for Vaccine Development and Global Health, University of Maryland School of Medicine, evaluated the benefits of our moderately effective influenza vaccines (VE 40%-60%) to the population beyond those who are vaccinated. Her conclusions were that even a modestly effective vaccine prevents 21 million cases of influenza, 129,000 hospitalizations, and 62,000 deaths. And that two-thirds of the deaths prevented are from herd benefit (or indirect effects). Although both coverage and vaccine effectiveness are important, she reported that population impact was most sensitive to coverage, compared with vaccine effectiveness. Dr. Fitzpatrick found that targeting school-age children 6-19 years of age and adults 30-39 years of age maximizes the public health benefits (herd effects) of influenza vaccine. In 2018 season, influenza coverage was 63% for at least one dose in children aged 6 months through 17 years and 45% in adults aged 18 years and older; in the two target age groups 5-17 and 30-39 years, coverage was 59% and approximately 35%, respectively (ID Week; Session 939).
Clearly, even our modestly effective influenza vaccines have significant public health benefit in protecting the U.S. populations from serious disease and death. Efforts to increase vaccine uptake in school-age children, both those with and without comorbidity, and the 30- to 39-year-old adult cohort would likely further reduce the burden of serious disease from influenza.
In summary, despite a vaccine that is only moderately effective, there is clear evidence to support current recommendations of universal immunization beginning at 6 months of age. Delaying until October 1 is a good idea only if the same number of individuals will receive influenza vaccine, otherwise the hypothetical benefit is lost.
Dr. Pelton is professor of pediatrics and epidemiology at Boston University schools of medicine and public health and is senior attending physician, Boston Medical Center. Dr. Pelton has investigator-initiated research awards to Boston Medical Center from Pfizer and Merck Vaccines. He also received honorarium as an advisory board member, participation in symposium and consultation from Seqirus and Merck Vaccine, Pfizer, and Sanofi Pasteur. Email him at [email protected].
ID Week, the annual meeting of the Infectious Disease Society of America, provided valuable insights into past season’s endemic influenza burden and the effectiveness of prevention strategies. Each year, there are from 9million to 49 million influenza cases in the United States, 140,000-960,000 hospitalized cases, and 12,000-70,000 deaths directly attributable to influenza infection. The burden disproportionately falls on infants and adults 65 years of age and older; 11,000-48,000 children are hospitalized, and as many as several hundred children may die from influenza and related complications. School age children (aged 5-19 years) and adults (aged 30-39 years) are a major part of the transmission cycle. Influenza vaccine underlies the prevention strategy for limiting the burden of disease in U.S. populations. ID Week provided new insights into critical questions about influenza vaccines.
1. What is the effectiveness of influenza vaccine against severe disease (hospitalization) in children? Does it vary by age? By type or subtype?
Angela P. Campbell, MD, MPH, of the Centers for Disease Control and Prevention, and associates presented data on influenza vaccine effectiveness from the New Vaccine Surveillance Network in children for the 2016-2017 and 2017-2018 season (ID Week session 99; Abstract 899). During both 2016-2017 and 2017-2018, H3N2 was the dominant virus and influenza B represented about one-third of cases, and H1N1 was a greater percentage of cases in 2017-2018. Influenza positivity among children younger than 18 years of age admitted to hospital with respiratory disease was 14% among unvaccinated and 8% among vaccinated children; effectiveness again hospitalization was 50%. Vaccine effectiveness (VE) was not statistically different between children younger than 8 years of age and those older that 8 years but did differ by vaccine type. VE was 76% against H1N1 disease, 59% again B disease, and only 33% against H3N2 disease.
Clearly, vaccination with influenza vaccine prevents serious respiratory disease. However, the impact of vaccine will vary by season and by which influenza stains are circulating in the community. The authors concluded that further understanding of the lower VE against H3N2 disease is needed.
2. Does the priming dose of influenza vaccine improve vaccine effectiveness?
Current recommendations call for a two-dose series for influenza vaccine in children aged 6 months through 8 years who have not had prior influenza vaccine. The recommendation is based on evidence demonstrating higher antibody responses in children receiving two doses, compared with a single dose. Using data from the U.S. Influenza Vaccine Effectiveness Network, Jessie R. Chung, MPH, of the CDC, and associates compared VE in children younger than 2 years receiving two doses in the first year of flu immunization (fully immunized), compared with those who received only one dose (partially immunized) (ID Week session 99; Abstract 900). VE was 53% for fully immunized and 23% for partially immunized children. Receipt of a single dose did not provide statistically significant protection against influenza. Surprisingly (to me), of 5,355 children aged 6 months to less than 2 years with no prior influenza vaccine, 1,870 (35%) received only one dose in the season.
The data strongly support the current recommendations for a priming dose, especially in young children, in the first season of influenza vaccine and warrants increased efforts to increase the update of second doses during the season. Hopefully we can do better in 2019!
3. Should we wait to vaccinate with influenza vaccine?
Some evidence suggests that waning immunity to influenza vaccine, primarily in those aged 65 years and older, may explain increased disease activity toward the end of influenza season. Other explanations include increasing viral diversity throughout the season, resulting in reduced effectiveness. Do such concerns warrant delaying immunization? The onset and peak of influenza season varies by year; in October 2019, 3% of tests performed on patients with respiratory illness were influenza positive. The trade-offs for delaying immunization until October are the unpredictability of onset of influenza season, the requirement for two doses in infants, the need for 2 weeks to achieve peak antibody concentrations, and the potential that fewer individuals will be vaccinated. Kathy Neuzil, MD, MPH, from the Center for Vaccine Development and Global Health, University of Maryland School of Medicine, reviewed recent modeling (for adults aged 65 years and older) and reported that delaying vaccine programs until October is associated with greater burden of hospitalization if 14% fewer individuals (who would be vaccinated in August/September) are vaccinated (ID Week; Session 940).
In response to these concerns, the CDC recommendations for 2019 are that, in children aged 6 months through 8 years who need two doses, start early so that you can achieve both doses before influenza season (MMWR 2019 Aug 23;68[3]:1-21).In older children and adults, who need only a single dose, early vaccination (August and early September) may lead to reduced protection late in the influenza season?
4. How can we optimize vaccine impact?
Vaccine impact refers to the affect on a population level and not at an individual level. Meagan C. Fitzpatrick, PhD, from the Center for Vaccine Development and Global Health, University of Maryland School of Medicine, evaluated the benefits of our moderately effective influenza vaccines (VE 40%-60%) to the population beyond those who are vaccinated. Her conclusions were that even a modestly effective vaccine prevents 21 million cases of influenza, 129,000 hospitalizations, and 62,000 deaths. And that two-thirds of the deaths prevented are from herd benefit (or indirect effects). Although both coverage and vaccine effectiveness are important, she reported that population impact was most sensitive to coverage, compared with vaccine effectiveness. Dr. Fitzpatrick found that targeting school-age children 6-19 years of age and adults 30-39 years of age maximizes the public health benefits (herd effects) of influenza vaccine. In 2018 season, influenza coverage was 63% for at least one dose in children aged 6 months through 17 years and 45% in adults aged 18 years and older; in the two target age groups 5-17 and 30-39 years, coverage was 59% and approximately 35%, respectively (ID Week; Session 939).
Clearly, even our modestly effective influenza vaccines have significant public health benefit in protecting the U.S. populations from serious disease and death. Efforts to increase vaccine uptake in school-age children, both those with and without comorbidity, and the 30- to 39-year-old adult cohort would likely further reduce the burden of serious disease from influenza.
In summary, despite a vaccine that is only moderately effective, there is clear evidence to support current recommendations of universal immunization beginning at 6 months of age. Delaying until October 1 is a good idea only if the same number of individuals will receive influenza vaccine, otherwise the hypothetical benefit is lost.
Dr. Pelton is professor of pediatrics and epidemiology at Boston University schools of medicine and public health and is senior attending physician, Boston Medical Center. Dr. Pelton has investigator-initiated research awards to Boston Medical Center from Pfizer and Merck Vaccines. He also received honorarium as an advisory board member, participation in symposium and consultation from Seqirus and Merck Vaccine, Pfizer, and Sanofi Pasteur. Email him at [email protected].
ID Week, the annual meeting of the Infectious Disease Society of America, provided valuable insights into past season’s endemic influenza burden and the effectiveness of prevention strategies. Each year, there are from 9million to 49 million influenza cases in the United States, 140,000-960,000 hospitalized cases, and 12,000-70,000 deaths directly attributable to influenza infection. The burden disproportionately falls on infants and adults 65 years of age and older; 11,000-48,000 children are hospitalized, and as many as several hundred children may die from influenza and related complications. School age children (aged 5-19 years) and adults (aged 30-39 years) are a major part of the transmission cycle. Influenza vaccine underlies the prevention strategy for limiting the burden of disease in U.S. populations. ID Week provided new insights into critical questions about influenza vaccines.
1. What is the effectiveness of influenza vaccine against severe disease (hospitalization) in children? Does it vary by age? By type or subtype?
Angela P. Campbell, MD, MPH, of the Centers for Disease Control and Prevention, and associates presented data on influenza vaccine effectiveness from the New Vaccine Surveillance Network in children for the 2016-2017 and 2017-2018 season (ID Week session 99; Abstract 899). During both 2016-2017 and 2017-2018, H3N2 was the dominant virus and influenza B represented about one-third of cases, and H1N1 was a greater percentage of cases in 2017-2018. Influenza positivity among children younger than 18 years of age admitted to hospital with respiratory disease was 14% among unvaccinated and 8% among vaccinated children; effectiveness again hospitalization was 50%. Vaccine effectiveness (VE) was not statistically different between children younger than 8 years of age and those older that 8 years but did differ by vaccine type. VE was 76% against H1N1 disease, 59% again B disease, and only 33% against H3N2 disease.
Clearly, vaccination with influenza vaccine prevents serious respiratory disease. However, the impact of vaccine will vary by season and by which influenza stains are circulating in the community. The authors concluded that further understanding of the lower VE against H3N2 disease is needed.
2. Does the priming dose of influenza vaccine improve vaccine effectiveness?
Current recommendations call for a two-dose series for influenza vaccine in children aged 6 months through 8 years who have not had prior influenza vaccine. The recommendation is based on evidence demonstrating higher antibody responses in children receiving two doses, compared with a single dose. Using data from the U.S. Influenza Vaccine Effectiveness Network, Jessie R. Chung, MPH, of the CDC, and associates compared VE in children younger than 2 years receiving two doses in the first year of flu immunization (fully immunized), compared with those who received only one dose (partially immunized) (ID Week session 99; Abstract 900). VE was 53% for fully immunized and 23% for partially immunized children. Receipt of a single dose did not provide statistically significant protection against influenza. Surprisingly (to me), of 5,355 children aged 6 months to less than 2 years with no prior influenza vaccine, 1,870 (35%) received only one dose in the season.
The data strongly support the current recommendations for a priming dose, especially in young children, in the first season of influenza vaccine and warrants increased efforts to increase the update of second doses during the season. Hopefully we can do better in 2019!
3. Should we wait to vaccinate with influenza vaccine?
Some evidence suggests that waning immunity to influenza vaccine, primarily in those aged 65 years and older, may explain increased disease activity toward the end of influenza season. Other explanations include increasing viral diversity throughout the season, resulting in reduced effectiveness. Do such concerns warrant delaying immunization? The onset and peak of influenza season varies by year; in October 2019, 3% of tests performed on patients with respiratory illness were influenza positive. The trade-offs for delaying immunization until October are the unpredictability of onset of influenza season, the requirement for two doses in infants, the need for 2 weeks to achieve peak antibody concentrations, and the potential that fewer individuals will be vaccinated. Kathy Neuzil, MD, MPH, from the Center for Vaccine Development and Global Health, University of Maryland School of Medicine, reviewed recent modeling (for adults aged 65 years and older) and reported that delaying vaccine programs until October is associated with greater burden of hospitalization if 14% fewer individuals (who would be vaccinated in August/September) are vaccinated (ID Week; Session 940).
In response to these concerns, the CDC recommendations for 2019 are that, in children aged 6 months through 8 years who need two doses, start early so that you can achieve both doses before influenza season (MMWR 2019 Aug 23;68[3]:1-21).In older children and adults, who need only a single dose, early vaccination (August and early September) may lead to reduced protection late in the influenza season?
4. How can we optimize vaccine impact?
Vaccine impact refers to the affect on a population level and not at an individual level. Meagan C. Fitzpatrick, PhD, from the Center for Vaccine Development and Global Health, University of Maryland School of Medicine, evaluated the benefits of our moderately effective influenza vaccines (VE 40%-60%) to the population beyond those who are vaccinated. Her conclusions were that even a modestly effective vaccine prevents 21 million cases of influenza, 129,000 hospitalizations, and 62,000 deaths. And that two-thirds of the deaths prevented are from herd benefit (or indirect effects). Although both coverage and vaccine effectiveness are important, she reported that population impact was most sensitive to coverage, compared with vaccine effectiveness. Dr. Fitzpatrick found that targeting school-age children 6-19 years of age and adults 30-39 years of age maximizes the public health benefits (herd effects) of influenza vaccine. In 2018 season, influenza coverage was 63% for at least one dose in children aged 6 months through 17 years and 45% in adults aged 18 years and older; in the two target age groups 5-17 and 30-39 years, coverage was 59% and approximately 35%, respectively (ID Week; Session 939).
Clearly, even our modestly effective influenza vaccines have significant public health benefit in protecting the U.S. populations from serious disease and death. Efforts to increase vaccine uptake in school-age children, both those with and without comorbidity, and the 30- to 39-year-old adult cohort would likely further reduce the burden of serious disease from influenza.
In summary, despite a vaccine that is only moderately effective, there is clear evidence to support current recommendations of universal immunization beginning at 6 months of age. Delaying until October 1 is a good idea only if the same number of individuals will receive influenza vaccine, otherwise the hypothetical benefit is lost.
Dr. Pelton is professor of pediatrics and epidemiology at Boston University schools of medicine and public health and is senior attending physician, Boston Medical Center. Dr. Pelton has investigator-initiated research awards to Boston Medical Center from Pfizer and Merck Vaccines. He also received honorarium as an advisory board member, participation in symposium and consultation from Seqirus and Merck Vaccine, Pfizer, and Sanofi Pasteur. Email him at [email protected].
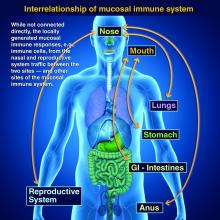
Taking vaccines to the next level via mucosal immunity
Vaccines are marvelous, and there are many well documented success stories, including rotavirus (RV) vaccines, where a live vaccine is administered to the gastrointestinal mucosa via oral drops. Antigens presented at the mucosal/epithelial surface not only induce systemic serum IgG – as do injectable vaccines – but also induce secretory IgA (sIgA), which is most helpful in diseases that directly affect the mucosa.

Mucosal vs. systemic immunity
Antibody being present on mucosal surfaces (point of initial pathogen contact) has a chance to neutralize the pathogen before it gains a foothold. Pathogen-specific mucosal lymphoid elements (e.g. in Peyer’s patches in the gut) also appear critical for optimal protection.1 The presence of both mucosal immune elements means that infection is severely limited or at times entirely prevented. So virus entering the GI tract causes minimal to no gut lining injury. Hence, there is no or mostly reduced vomiting/diarrhea. A downside of mucosally-administered live vaccines is that preexisting antibody to the vaccine antigens can reduce or block vaccine virus replication in the vaccinee, blunting or preventing protection. Note: Preexisting antibody also affects injectable live vaccines, such as the measles vaccine, similarly.
Classic injectable live or nonlive vaccines provide their most potent protection via systemic cellular responses antibody and/or antibodies in serum and extracellular fluid (ECF) where IgG and IgM are in highest concentrations. So even successful injectable vaccines still allow mucosal infection to start but then intercept further spread and prevent most of the downstream damage (think pertussis) or neutralize an infection-generated toxin (pertussis or tetanus). It usually is only after infection-induced damage occurs that systemic IgG and IgM gain better access to respiratory epithelial surfaces, but still only at a fraction of circulating concentrations. Indeed, pertussis vaccine–induced systemic immunity allows the pathogen to attack and replicate in/on host surface cells, causing toxin release and variable amounts of local mucosal injury/inflammation before vaccine-induced systemic immunity gains adequate access to the pathogen and/or to its toxin which may enter systemic circulation.
Live attenuated influenza vaccine (LAIV) induces mucosal immunity
Another “standard” vaccine that induces mucosal immunity – LAIV – was developed to improve on protection afforded by injectable influenza vaccines (IIVs), but LAIV has had hiccups in the United States. One example is several years of negligible protection against H1N1 disease. As long as LAIV’s vaccine strain had reasonably matched the circulating strains, LAIV worked at least as well as injectable influenza vaccine, and even offered some cross-protection against mildly mismatched strains. But after a number of years of LAIV use, vaccine effectiveness in the United States vs. H1N1 strains appeared to fade due to previously undetected but significant changes in the circulating H1N1 strain. The lesson is that mucosal immunity’s advantages are lost if too much change occurs in the pathogen target for sIgA and mucosally-associated lymphoid tissue cells (MALT)).
Other vaccines likely need to induce mucosal immunity
Protection at the mucosal level will likely be needed for success against norovirus, parainfluenza, respiratory syncytial virus (RSV), Neisseria gonorrhea, and chlamydia. Another helpful aspect of mucosal immunity is that immune cells and sIgA not only reside on the mucosa where the antigen was originally presented, but there is also a reasonable chance that these components will traffic to other mucosal surfaces.2
So intranasal vaccine could be expected to protect distant mucosal surfaces (urogenital, GI, and respiratory), leading to vaccine-induced systemic antibody plus mucosal immunity (sIGA and MALT responses) at each site.
Let’s look at a novel “two-site” chlamydia vaccine
Recently a phase 1 chlamydia vaccine that used a novel two-pronged administration site/schedule was successful at inducing both mucosal and systemic immunity in a proof-of-concept study – achieving the best of both worlds.3 This may be a template for vaccines in years to come. British investigators studied 50 healthy women aged 19-45 years in a double-blind, parallel, randomized, placebo-controlled trial that used a recombinant chlamydia protein subunit antigen (CTH522). The vaccine schedule involved three injectable priming doses followed soon thereafter by two intranasal boosting doses. There were three groups:
1. CTH522 adjuvanted with CAF01 liposomes (CTH522:CAF01).
2. CTH522 adjuvanted with aluminum hydroxide (CTH522:AH).
3. Placebo (saline).
The intramuscular (IM) priming schedule was 0, 1, and 4 months. The intranasal vaccine booster doses or placebo were given at 4.5 and 5 months. No related serious adverse reactions occurred. For injectable dosing, the most frequent adverse event was mild local injection-site reactions in all subjects in both vaccine groups vs. in 60% of placebo recipients (P = .053). The adjuvants were the likely cause for local reactions. Intranasal doses had local reactions in 47% of both vaccine groups and 60% of placebo recipients; P = 1.000).
Both vaccines produced systemic IgG seroconversion (including neutralizing antibody) plus small amounts of IgG in the nasal cavity and genital tract in all vaccine recipients; no placebo recipient seroconverted. Interestingly, liposomally-adjuvanted vaccine produced a more rapid systemic IgG response and higher serum titers than the alum-adjuvanted vaccine. Likewise, the IM liposomal vaccine also induced higher but still small mucosal IgG antibody responses (P = .0091). Intranasal IM-induced IgG titers were not boosted by later intranasal vaccine dosing.
Subjects getting liposomal vaccine (but not alum vaccine or placebo) boosters had detectable sIgA titers in both nasal and genital tract secretions. Liposomal vaccine recipients also had fivefold to sixfold higher median titers than alum vaccine recipients after the priming dose, and these higher titers persisted to the end of the study. All liposomal vaccine recipients developed antichlamydial cell-mediated responses vs. 57% alum-adjuvanted vaccine recipients. (P = .01). So both use of two-site dosing and the liposomal adjuvant appeared critical to better responses.
In summary
While this candidate vaccine has hurdles to overcome before coming into routine use, the proof-of-principle that a combination injectable-intranasal vaccine schedule can induce robust systemic and mucosal immunity when given with an appropriate adjuvant is very promising. Adding more vaccines to the schedule then becomes an issue, but that is one of those “good” problems we can deal with later.
Dr. Harrison is professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospital-Kansas City, Mo. Children’s Mercy Hospital receives grant funding to study two candidate RSV vaccines, receives funding from GlaxoSmithKline for studies on pneumococcal and rotavirus vaccines, and from Pfizer for a study on pneumococcal vaccine on which Dr. Harrison is a sub-investigator. The hospital also receives Centers for Disease Control and Prevention funding under the New Vaccine Surveillance Network for multicenter surveillance of acute respiratory infections, including influenza, RSV, and parainfluenza virus, and also for rotavirus. Email Dr. Harrison at [email protected].
References
1. PLOS Biology. 2012 Sep 1. doi: 10.1371/journal.pbio.1001397.
2. Mucosal Immunity in the Human Female Reproductive Tract in “Mucosal Immunology,” 4th ed., Volume 2 (Cambridge, MA: Academic Press, 2015, pp. 2097-124).
3. Lancet Infect Dis. 2019. doi: 10.1016/S1473-3099(19)30279-8.
Vaccines are marvelous, and there are many well documented success stories, including rotavirus (RV) vaccines, where a live vaccine is administered to the gastrointestinal mucosa via oral drops. Antigens presented at the mucosal/epithelial surface not only induce systemic serum IgG – as do injectable vaccines – but also induce secretory IgA (sIgA), which is most helpful in diseases that directly affect the mucosa.

Mucosal vs. systemic immunity
Antibody being present on mucosal surfaces (point of initial pathogen contact) has a chance to neutralize the pathogen before it gains a foothold. Pathogen-specific mucosal lymphoid elements (e.g. in Peyer’s patches in the gut) also appear critical for optimal protection.1 The presence of both mucosal immune elements means that infection is severely limited or at times entirely prevented. So virus entering the GI tract causes minimal to no gut lining injury. Hence, there is no or mostly reduced vomiting/diarrhea. A downside of mucosally-administered live vaccines is that preexisting antibody to the vaccine antigens can reduce or block vaccine virus replication in the vaccinee, blunting or preventing protection. Note: Preexisting antibody also affects injectable live vaccines, such as the measles vaccine, similarly.
Classic injectable live or nonlive vaccines provide their most potent protection via systemic cellular responses antibody and/or antibodies in serum and extracellular fluid (ECF) where IgG and IgM are in highest concentrations. So even successful injectable vaccines still allow mucosal infection to start but then intercept further spread and prevent most of the downstream damage (think pertussis) or neutralize an infection-generated toxin (pertussis or tetanus). It usually is only after infection-induced damage occurs that systemic IgG and IgM gain better access to respiratory epithelial surfaces, but still only at a fraction of circulating concentrations. Indeed, pertussis vaccine–induced systemic immunity allows the pathogen to attack and replicate in/on host surface cells, causing toxin release and variable amounts of local mucosal injury/inflammation before vaccine-induced systemic immunity gains adequate access to the pathogen and/or to its toxin which may enter systemic circulation.
Live attenuated influenza vaccine (LAIV) induces mucosal immunity
Another “standard” vaccine that induces mucosal immunity – LAIV – was developed to improve on protection afforded by injectable influenza vaccines (IIVs), but LAIV has had hiccups in the United States. One example is several years of negligible protection against H1N1 disease. As long as LAIV’s vaccine strain had reasonably matched the circulating strains, LAIV worked at least as well as injectable influenza vaccine, and even offered some cross-protection against mildly mismatched strains. But after a number of years of LAIV use, vaccine effectiveness in the United States vs. H1N1 strains appeared to fade due to previously undetected but significant changes in the circulating H1N1 strain. The lesson is that mucosal immunity’s advantages are lost if too much change occurs in the pathogen target for sIgA and mucosally-associated lymphoid tissue cells (MALT)).
Other vaccines likely need to induce mucosal immunity
Protection at the mucosal level will likely be needed for success against norovirus, parainfluenza, respiratory syncytial virus (RSV), Neisseria gonorrhea, and chlamydia. Another helpful aspect of mucosal immunity is that immune cells and sIgA not only reside on the mucosa where the antigen was originally presented, but there is also a reasonable chance that these components will traffic to other mucosal surfaces.2
So intranasal vaccine could be expected to protect distant mucosal surfaces (urogenital, GI, and respiratory), leading to vaccine-induced systemic antibody plus mucosal immunity (sIGA and MALT responses) at each site.
Let’s look at a novel “two-site” chlamydia vaccine
Recently a phase 1 chlamydia vaccine that used a novel two-pronged administration site/schedule was successful at inducing both mucosal and systemic immunity in a proof-of-concept study – achieving the best of both worlds.3 This may be a template for vaccines in years to come. British investigators studied 50 healthy women aged 19-45 years in a double-blind, parallel, randomized, placebo-controlled trial that used a recombinant chlamydia protein subunit antigen (CTH522). The vaccine schedule involved three injectable priming doses followed soon thereafter by two intranasal boosting doses. There were three groups:
1. CTH522 adjuvanted with CAF01 liposomes (CTH522:CAF01).
2. CTH522 adjuvanted with aluminum hydroxide (CTH522:AH).
3. Placebo (saline).
The intramuscular (IM) priming schedule was 0, 1, and 4 months. The intranasal vaccine booster doses or placebo were given at 4.5 and 5 months. No related serious adverse reactions occurred. For injectable dosing, the most frequent adverse event was mild local injection-site reactions in all subjects in both vaccine groups vs. in 60% of placebo recipients (P = .053). The adjuvants were the likely cause for local reactions. Intranasal doses had local reactions in 47% of both vaccine groups and 60% of placebo recipients; P = 1.000).
Both vaccines produced systemic IgG seroconversion (including neutralizing antibody) plus small amounts of IgG in the nasal cavity and genital tract in all vaccine recipients; no placebo recipient seroconverted. Interestingly, liposomally-adjuvanted vaccine produced a more rapid systemic IgG response and higher serum titers than the alum-adjuvanted vaccine. Likewise, the IM liposomal vaccine also induced higher but still small mucosal IgG antibody responses (P = .0091). Intranasal IM-induced IgG titers were not boosted by later intranasal vaccine dosing.
Subjects getting liposomal vaccine (but not alum vaccine or placebo) boosters had detectable sIgA titers in both nasal and genital tract secretions. Liposomal vaccine recipients also had fivefold to sixfold higher median titers than alum vaccine recipients after the priming dose, and these higher titers persisted to the end of the study. All liposomal vaccine recipients developed antichlamydial cell-mediated responses vs. 57% alum-adjuvanted vaccine recipients. (P = .01). So both use of two-site dosing and the liposomal adjuvant appeared critical to better responses.
In summary
While this candidate vaccine has hurdles to overcome before coming into routine use, the proof-of-principle that a combination injectable-intranasal vaccine schedule can induce robust systemic and mucosal immunity when given with an appropriate adjuvant is very promising. Adding more vaccines to the schedule then becomes an issue, but that is one of those “good” problems we can deal with later.
Dr. Harrison is professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospital-Kansas City, Mo. Children’s Mercy Hospital receives grant funding to study two candidate RSV vaccines, receives funding from GlaxoSmithKline for studies on pneumococcal and rotavirus vaccines, and from Pfizer for a study on pneumococcal vaccine on which Dr. Harrison is a sub-investigator. The hospital also receives Centers for Disease Control and Prevention funding under the New Vaccine Surveillance Network for multicenter surveillance of acute respiratory infections, including influenza, RSV, and parainfluenza virus, and also for rotavirus. Email Dr. Harrison at [email protected].
References
1. PLOS Biology. 2012 Sep 1. doi: 10.1371/journal.pbio.1001397.
2. Mucosal Immunity in the Human Female Reproductive Tract in “Mucosal Immunology,” 4th ed., Volume 2 (Cambridge, MA: Academic Press, 2015, pp. 2097-124).
3. Lancet Infect Dis. 2019. doi: 10.1016/S1473-3099(19)30279-8.
Vaccines are marvelous, and there are many well documented success stories, including rotavirus (RV) vaccines, where a live vaccine is administered to the gastrointestinal mucosa via oral drops. Antigens presented at the mucosal/epithelial surface not only induce systemic serum IgG – as do injectable vaccines – but also induce secretory IgA (sIgA), which is most helpful in diseases that directly affect the mucosa.

Mucosal vs. systemic immunity
Antibody being present on mucosal surfaces (point of initial pathogen contact) has a chance to neutralize the pathogen before it gains a foothold. Pathogen-specific mucosal lymphoid elements (e.g. in Peyer’s patches in the gut) also appear critical for optimal protection.1 The presence of both mucosal immune elements means that infection is severely limited or at times entirely prevented. So virus entering the GI tract causes minimal to no gut lining injury. Hence, there is no or mostly reduced vomiting/diarrhea. A downside of mucosally-administered live vaccines is that preexisting antibody to the vaccine antigens can reduce or block vaccine virus replication in the vaccinee, blunting or preventing protection. Note: Preexisting antibody also affects injectable live vaccines, such as the measles vaccine, similarly.
Classic injectable live or nonlive vaccines provide their most potent protection via systemic cellular responses antibody and/or antibodies in serum and extracellular fluid (ECF) where IgG and IgM are in highest concentrations. So even successful injectable vaccines still allow mucosal infection to start but then intercept further spread and prevent most of the downstream damage (think pertussis) or neutralize an infection-generated toxin (pertussis or tetanus). It usually is only after infection-induced damage occurs that systemic IgG and IgM gain better access to respiratory epithelial surfaces, but still only at a fraction of circulating concentrations. Indeed, pertussis vaccine–induced systemic immunity allows the pathogen to attack and replicate in/on host surface cells, causing toxin release and variable amounts of local mucosal injury/inflammation before vaccine-induced systemic immunity gains adequate access to the pathogen and/or to its toxin which may enter systemic circulation.
Live attenuated influenza vaccine (LAIV) induces mucosal immunity
Another “standard” vaccine that induces mucosal immunity – LAIV – was developed to improve on protection afforded by injectable influenza vaccines (IIVs), but LAIV has had hiccups in the United States. One example is several years of negligible protection against H1N1 disease. As long as LAIV’s vaccine strain had reasonably matched the circulating strains, LAIV worked at least as well as injectable influenza vaccine, and even offered some cross-protection against mildly mismatched strains. But after a number of years of LAIV use, vaccine effectiveness in the United States vs. H1N1 strains appeared to fade due to previously undetected but significant changes in the circulating H1N1 strain. The lesson is that mucosal immunity’s advantages are lost if too much change occurs in the pathogen target for sIgA and mucosally-associated lymphoid tissue cells (MALT)).
Other vaccines likely need to induce mucosal immunity
Protection at the mucosal level will likely be needed for success against norovirus, parainfluenza, respiratory syncytial virus (RSV), Neisseria gonorrhea, and chlamydia. Another helpful aspect of mucosal immunity is that immune cells and sIgA not only reside on the mucosa where the antigen was originally presented, but there is also a reasonable chance that these components will traffic to other mucosal surfaces.2
So intranasal vaccine could be expected to protect distant mucosal surfaces (urogenital, GI, and respiratory), leading to vaccine-induced systemic antibody plus mucosal immunity (sIGA and MALT responses) at each site.
Let’s look at a novel “two-site” chlamydia vaccine
Recently a phase 1 chlamydia vaccine that used a novel two-pronged administration site/schedule was successful at inducing both mucosal and systemic immunity in a proof-of-concept study – achieving the best of both worlds.3 This may be a template for vaccines in years to come. British investigators studied 50 healthy women aged 19-45 years in a double-blind, parallel, randomized, placebo-controlled trial that used a recombinant chlamydia protein subunit antigen (CTH522). The vaccine schedule involved three injectable priming doses followed soon thereafter by two intranasal boosting doses. There were three groups:
1. CTH522 adjuvanted with CAF01 liposomes (CTH522:CAF01).
2. CTH522 adjuvanted with aluminum hydroxide (CTH522:AH).
3. Placebo (saline).
The intramuscular (IM) priming schedule was 0, 1, and 4 months. The intranasal vaccine booster doses or placebo were given at 4.5 and 5 months. No related serious adverse reactions occurred. For injectable dosing, the most frequent adverse event was mild local injection-site reactions in all subjects in both vaccine groups vs. in 60% of placebo recipients (P = .053). The adjuvants were the likely cause for local reactions. Intranasal doses had local reactions in 47% of both vaccine groups and 60% of placebo recipients; P = 1.000).
Both vaccines produced systemic IgG seroconversion (including neutralizing antibody) plus small amounts of IgG in the nasal cavity and genital tract in all vaccine recipients; no placebo recipient seroconverted. Interestingly, liposomally-adjuvanted vaccine produced a more rapid systemic IgG response and higher serum titers than the alum-adjuvanted vaccine. Likewise, the IM liposomal vaccine also induced higher but still small mucosal IgG antibody responses (P = .0091). Intranasal IM-induced IgG titers were not boosted by later intranasal vaccine dosing.
Subjects getting liposomal vaccine (but not alum vaccine or placebo) boosters had detectable sIgA titers in both nasal and genital tract secretions. Liposomal vaccine recipients also had fivefold to sixfold higher median titers than alum vaccine recipients after the priming dose, and these higher titers persisted to the end of the study. All liposomal vaccine recipients developed antichlamydial cell-mediated responses vs. 57% alum-adjuvanted vaccine recipients. (P = .01). So both use of two-site dosing and the liposomal adjuvant appeared critical to better responses.
In summary
While this candidate vaccine has hurdles to overcome before coming into routine use, the proof-of-principle that a combination injectable-intranasal vaccine schedule can induce robust systemic and mucosal immunity when given with an appropriate adjuvant is very promising. Adding more vaccines to the schedule then becomes an issue, but that is one of those “good” problems we can deal with later.
Dr. Harrison is professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospital-Kansas City, Mo. Children’s Mercy Hospital receives grant funding to study two candidate RSV vaccines, receives funding from GlaxoSmithKline for studies on pneumococcal and rotavirus vaccines, and from Pfizer for a study on pneumococcal vaccine on which Dr. Harrison is a sub-investigator. The hospital also receives Centers for Disease Control and Prevention funding under the New Vaccine Surveillance Network for multicenter surveillance of acute respiratory infections, including influenza, RSV, and parainfluenza virus, and also for rotavirus. Email Dr. Harrison at [email protected].
References
1. PLOS Biology. 2012 Sep 1. doi: 10.1371/journal.pbio.1001397.
2. Mucosal Immunity in the Human Female Reproductive Tract in “Mucosal Immunology,” 4th ed., Volume 2 (Cambridge, MA: Academic Press, 2015, pp. 2097-124).
3. Lancet Infect Dis. 2019. doi: 10.1016/S1473-3099(19)30279-8.
Is your office ready for a case of measles?
It’s a typically busy Friday and the doctor is running 20 minutes behind schedule. He enters the next exam room and the sight of the patient makes him forget the apology he had prepared.
The 10 month old looks miserable. Red eyes. Snot dripping from his nose. A red rash that extends from his face and involves most of the chest, arms, and upper thighs.
“When did this start?” he asks the mother as he searches for a surgical mask in the cabinet next to the exam table.
“Two days after we returned from our vacation in France,” the worried young woman replies. “Do you think it could be measles?”
Between Jan. 1 and Aug. 8, 2019, 1,182 cases of measles had been confirmed in the United States. That’s more than three times the number of cases reported in all of 2018, and the highest number of cases reported in a single year in more than a quarter century. While 75% of the cases this year have been linked to outbreaks in New York, individuals from 30 states have been affected.
Given the widespread nature of the outbreak, With measles in particular, time is limited to deliver effective postexposure prophylaxis and prevent the spread of measles in the community, making it difficult to develop a plan on the fly.
Schedule strategically. You don’t want a patient with measles hanging out in your waiting room. According to the American Academy of Pediatrics, measures to prevent the transmission of contagious infectious agents in ambulatory facilities begin at the time the visit is scheduled. When there is measles transmission in the community, consider using a standardized script when scheduling patients that includes questions about fever, rash, other symptoms typical for measles, and possible exposures. Some offices will have procedures in place that can be adapted to care for patients with suspected measles. When a patient presents for suspected chicken pox, do you advise them to come at the end of the day to minimize exposures? Enter through a side door? Perform a car visit?
Triage promptly. Mask patients with fever and rash, move to a private room, and close the door.
Once measles is suspected, only health care personnel who are immune to measles should enter the exam room. According to the Centers for Disease Control and Prevention, presumptive evidence of measles immunity in health care providers is written documentation of vaccination with two doses of live measles or MMR vaccine administered at least 28 days apart, laboratory evidence of immunity (that is, positive measles IgG), laboratory confirmation of disease, or birth before 1957.
Even though health care providers born before 1957 are presumed to have had the disease at some point and have traditionally been considered immune, the CDC suggests that health care facilities consider giving these individuals two doses of MMR vaccine unless they have prior laboratory confirmation of disease immunity. Do you know who in your office is immune or would you need to scramble if you had an exposure?
When measles is suspected, health care personnel should wear an N-95 if they have been fit tested and the appropriate mask is available. Practically, most ambulatory offices do not stock N-95 masks and the next best choice is a regular surgical mask.
Order the recommended tests to confirm the diagnosis, but do not wait for the results to confirm the diagnosis. The CDC recommends testing serum for IgM antibodies and sending a throat or nasopharyngeal swab to look for the virus by polymerase chain reaction testing. Measles virus also is shed in the urine so collecting a urine specimen for testing may increase the chances of finding the virus. Depending on where you practice, the tests may take 3 days or more to result. Contact your local health department as soon as you consider a measles diagnosis.
Discharge patients home or transferred to a higher level of care if this is necessary as quickly as possible. Fortunately, most patients with measles do not require hospitalization. Do not send patients to the hospital simply for the purpose of laboratory testing if this can be accomplished quickly in your office or for evaluation by other providers. This just creates the potential for more exposures. If a patient does require higher-level care, provider-to-provider communication about the suspected diagnosis and the need for airborne isolation should take place.
Keep the door closed. Once a patient with suspected measles is discharged from a regular exam room, the door should remain closed, and it should not be used for at least 1 hour. Remember that infectious virus can remain in the air for 1-2 hours after a patient leaves an area. The same is true for the waiting room.
Develop the exposure list. In general, patients and family members who were in the waiting room at the same time as the index patient and up to 1-2 hours after the index patient left are considered exposed. Measles is highly contagious and 9 out of 10 susceptible people who are exposed will develop disease. How many infants aged less than 1 year might be in your waiting room at any given time? How many immunocompromised patients or family members? Public health authorities can help determine who needs prophylaxis.
Don’t get anxious and start testing everyone for measles, especially patients who lack typical signs and symptoms or exposures. Ordering a test in a patient who has a low likelihood of measles is more likely to result in a false-positive test than a true-positive test. False-positive measles IgM tests can be seen with some viral infections, including parvovirus and Epstein-Barr. Some rheumatologic disorders also can contribute to false-positive tests.
Review your office procedure for vaccine counseling. The 10 month old with measles in the opening vignette should have been given an MMR vaccine before travel. The vaccine is recommended for infants aged 6-11 months who are traveling outside the United States, but it doesn’t count toward the vaccine series. Reimmunize young travelers at 12-15 months and again at 4-6 years. The CDC has developed a toolkit that contains resources for taking to parents about vaccines. It is available at https://www.cdc.gov/measles/toolkit/healthcare-providers.html.
It’s a typically busy Friday and the doctor is running 20 minutes behind schedule. He enters the next exam room and the sight of the patient makes him forget the apology he had prepared.
The 10 month old looks miserable. Red eyes. Snot dripping from his nose. A red rash that extends from his face and involves most of the chest, arms, and upper thighs.
“When did this start?” he asks the mother as he searches for a surgical mask in the cabinet next to the exam table.
“Two days after we returned from our vacation in France,” the worried young woman replies. “Do you think it could be measles?”
Between Jan. 1 and Aug. 8, 2019, 1,182 cases of measles had been confirmed in the United States. That’s more than three times the number of cases reported in all of 2018, and the highest number of cases reported in a single year in more than a quarter century. While 75% of the cases this year have been linked to outbreaks in New York, individuals from 30 states have been affected.
Given the widespread nature of the outbreak, With measles in particular, time is limited to deliver effective postexposure prophylaxis and prevent the spread of measles in the community, making it difficult to develop a plan on the fly.
Schedule strategically. You don’t want a patient with measles hanging out in your waiting room. According to the American Academy of Pediatrics, measures to prevent the transmission of contagious infectious agents in ambulatory facilities begin at the time the visit is scheduled. When there is measles transmission in the community, consider using a standardized script when scheduling patients that includes questions about fever, rash, other symptoms typical for measles, and possible exposures. Some offices will have procedures in place that can be adapted to care for patients with suspected measles. When a patient presents for suspected chicken pox, do you advise them to come at the end of the day to minimize exposures? Enter through a side door? Perform a car visit?
Triage promptly. Mask patients with fever and rash, move to a private room, and close the door.
Once measles is suspected, only health care personnel who are immune to measles should enter the exam room. According to the Centers for Disease Control and Prevention, presumptive evidence of measles immunity in health care providers is written documentation of vaccination with two doses of live measles or MMR vaccine administered at least 28 days apart, laboratory evidence of immunity (that is, positive measles IgG), laboratory confirmation of disease, or birth before 1957.
Even though health care providers born before 1957 are presumed to have had the disease at some point and have traditionally been considered immune, the CDC suggests that health care facilities consider giving these individuals two doses of MMR vaccine unless they have prior laboratory confirmation of disease immunity. Do you know who in your office is immune or would you need to scramble if you had an exposure?
When measles is suspected, health care personnel should wear an N-95 if they have been fit tested and the appropriate mask is available. Practically, most ambulatory offices do not stock N-95 masks and the next best choice is a regular surgical mask.
Order the recommended tests to confirm the diagnosis, but do not wait for the results to confirm the diagnosis. The CDC recommends testing serum for IgM antibodies and sending a throat or nasopharyngeal swab to look for the virus by polymerase chain reaction testing. Measles virus also is shed in the urine so collecting a urine specimen for testing may increase the chances of finding the virus. Depending on where you practice, the tests may take 3 days or more to result. Contact your local health department as soon as you consider a measles diagnosis.
Discharge patients home or transferred to a higher level of care if this is necessary as quickly as possible. Fortunately, most patients with measles do not require hospitalization. Do not send patients to the hospital simply for the purpose of laboratory testing if this can be accomplished quickly in your office or for evaluation by other providers. This just creates the potential for more exposures. If a patient does require higher-level care, provider-to-provider communication about the suspected diagnosis and the need for airborne isolation should take place.
Keep the door closed. Once a patient with suspected measles is discharged from a regular exam room, the door should remain closed, and it should not be used for at least 1 hour. Remember that infectious virus can remain in the air for 1-2 hours after a patient leaves an area. The same is true for the waiting room.
Develop the exposure list. In general, patients and family members who were in the waiting room at the same time as the index patient and up to 1-2 hours after the index patient left are considered exposed. Measles is highly contagious and 9 out of 10 susceptible people who are exposed will develop disease. How many infants aged less than 1 year might be in your waiting room at any given time? How many immunocompromised patients or family members? Public health authorities can help determine who needs prophylaxis.
Don’t get anxious and start testing everyone for measles, especially patients who lack typical signs and symptoms or exposures. Ordering a test in a patient who has a low likelihood of measles is more likely to result in a false-positive test than a true-positive test. False-positive measles IgM tests can be seen with some viral infections, including parvovirus and Epstein-Barr. Some rheumatologic disorders also can contribute to false-positive tests.
Review your office procedure for vaccine counseling. The 10 month old with measles in the opening vignette should have been given an MMR vaccine before travel. The vaccine is recommended for infants aged 6-11 months who are traveling outside the United States, but it doesn’t count toward the vaccine series. Reimmunize young travelers at 12-15 months and again at 4-6 years. The CDC has developed a toolkit that contains resources for taking to parents about vaccines. It is available at https://www.cdc.gov/measles/toolkit/healthcare-providers.html.
It’s a typically busy Friday and the doctor is running 20 minutes behind schedule. He enters the next exam room and the sight of the patient makes him forget the apology he had prepared.
The 10 month old looks miserable. Red eyes. Snot dripping from his nose. A red rash that extends from his face and involves most of the chest, arms, and upper thighs.
“When did this start?” he asks the mother as he searches for a surgical mask in the cabinet next to the exam table.
“Two days after we returned from our vacation in France,” the worried young woman replies. “Do you think it could be measles?”
Between Jan. 1 and Aug. 8, 2019, 1,182 cases of measles had been confirmed in the United States. That’s more than three times the number of cases reported in all of 2018, and the highest number of cases reported in a single year in more than a quarter century. While 75% of the cases this year have been linked to outbreaks in New York, individuals from 30 states have been affected.
Given the widespread nature of the outbreak, With measles in particular, time is limited to deliver effective postexposure prophylaxis and prevent the spread of measles in the community, making it difficult to develop a plan on the fly.
Schedule strategically. You don’t want a patient with measles hanging out in your waiting room. According to the American Academy of Pediatrics, measures to prevent the transmission of contagious infectious agents in ambulatory facilities begin at the time the visit is scheduled. When there is measles transmission in the community, consider using a standardized script when scheduling patients that includes questions about fever, rash, other symptoms typical for measles, and possible exposures. Some offices will have procedures in place that can be adapted to care for patients with suspected measles. When a patient presents for suspected chicken pox, do you advise them to come at the end of the day to minimize exposures? Enter through a side door? Perform a car visit?
Triage promptly. Mask patients with fever and rash, move to a private room, and close the door.
Once measles is suspected, only health care personnel who are immune to measles should enter the exam room. According to the Centers for Disease Control and Prevention, presumptive evidence of measles immunity in health care providers is written documentation of vaccination with two doses of live measles or MMR vaccine administered at least 28 days apart, laboratory evidence of immunity (that is, positive measles IgG), laboratory confirmation of disease, or birth before 1957.
Even though health care providers born before 1957 are presumed to have had the disease at some point and have traditionally been considered immune, the CDC suggests that health care facilities consider giving these individuals two doses of MMR vaccine unless they have prior laboratory confirmation of disease immunity. Do you know who in your office is immune or would you need to scramble if you had an exposure?
When measles is suspected, health care personnel should wear an N-95 if they have been fit tested and the appropriate mask is available. Practically, most ambulatory offices do not stock N-95 masks and the next best choice is a regular surgical mask.
Order the recommended tests to confirm the diagnosis, but do not wait for the results to confirm the diagnosis. The CDC recommends testing serum for IgM antibodies and sending a throat or nasopharyngeal swab to look for the virus by polymerase chain reaction testing. Measles virus also is shed in the urine so collecting a urine specimen for testing may increase the chances of finding the virus. Depending on where you practice, the tests may take 3 days or more to result. Contact your local health department as soon as you consider a measles diagnosis.
Discharge patients home or transferred to a higher level of care if this is necessary as quickly as possible. Fortunately, most patients with measles do not require hospitalization. Do not send patients to the hospital simply for the purpose of laboratory testing if this can be accomplished quickly in your office or for evaluation by other providers. This just creates the potential for more exposures. If a patient does require higher-level care, provider-to-provider communication about the suspected diagnosis and the need for airborne isolation should take place.
Keep the door closed. Once a patient with suspected measles is discharged from a regular exam room, the door should remain closed, and it should not be used for at least 1 hour. Remember that infectious virus can remain in the air for 1-2 hours after a patient leaves an area. The same is true for the waiting room.
Develop the exposure list. In general, patients and family members who were in the waiting room at the same time as the index patient and up to 1-2 hours after the index patient left are considered exposed. Measles is highly contagious and 9 out of 10 susceptible people who are exposed will develop disease. How many infants aged less than 1 year might be in your waiting room at any given time? How many immunocompromised patients or family members? Public health authorities can help determine who needs prophylaxis.
Don’t get anxious and start testing everyone for measles, especially patients who lack typical signs and symptoms or exposures. Ordering a test in a patient who has a low likelihood of measles is more likely to result in a false-positive test than a true-positive test. False-positive measles IgM tests can be seen with some viral infections, including parvovirus and Epstein-Barr. Some rheumatologic disorders also can contribute to false-positive tests.
Review your office procedure for vaccine counseling. The 10 month old with measles in the opening vignette should have been given an MMR vaccine before travel. The vaccine is recommended for infants aged 6-11 months who are traveling outside the United States, but it doesn’t count toward the vaccine series. Reimmunize young travelers at 12-15 months and again at 4-6 years. The CDC has developed a toolkit that contains resources for taking to parents about vaccines. It is available at https://www.cdc.gov/measles/toolkit/healthcare-providers.html.
New research in otitis media
New research was presented at the International Society for Otitis Media meeting in June 2019, which I attended. I would like to share a selection of new findings from the many presentations.
Transtympanic antibiotic delivery
Topical therapy has been used to treat only otitis externa and acute otitis media (AOM) with ear discharge. Giving antibiotics through the tympanic membrane could mitigate many of the concerns about antibiotic use driving antibiotic resistance of bacteria among children. Up to now, using antibiotics in the ear canal to treat AOM has not been considered because the tympanic membrane is highly impermeable to the transtympanic diffusion of any drugs. However, in recent years, a number of different drug delivery systems have been developed, and in some cases, animal studies have shown that noninvasive transtympanic delivery is possible so that drugs can reach high concentrations in the middle ear without damage. Nanovesicles and nanoliposomes that contain antibiotics and are small enough to pass through the eardrum have been developed and tested in animal models; these show promise. Ototopical administration of a drug called vinpocetine that was repurposed has been tested in mice and shown to reduce inflammation and mucus production in the middle ear during otitis media.
Biofilms
Antibiotic treatment failure can occur in AOM for several reasons. The treatment of choice, amoxicillin, for example may fail to achieve an adequate concentration because of poor absorption in the gastrointestinal tract or poor penetration into the middle ear. Or, the antibiotic chosen may not be effective because of resistance of the strain causing the infection. Another explanation, especially in recurrent AOM and chronic AOM, could be the presence of biofilms. Biofilms are multicellular bacterial communities incorporated in a polymeric, plasticlike matrix in which pathogens are protected from antibiotic activity. The biofilm provides a physical barrier to antibiotic penetration, and bacteria can persist in the middle ear and periodically cause a new AOM. If AOM persists or becomes a more chronic otitis media with effusion, the “glue ear” causes an environment in the middle ear that is low in oxygen. A low-oxygen environment is favorable to biofilms. Also one might expect that middle ear pus would have a low pH, but actual measurements show the pH is highly alkaline. Species of Haemophilus influenzae have been identified as more virulent when in an alkaline pH or the alkaline pH makes the H. influenzae persist better in the middle ear, perhaps in a biofilm. To eliminate biofilms and improve antibiotic efficacy, a vaccine against a protein expressed by H. influenzae has been developed. Antibodies against this protein have been shown to disrupt and prevent the formation of biofilms in an animal model.
Probiotics
The normal bacteria that live in the nasopharynx of children with recurrent AOM is now known to differ from that of children who experience infrequent AOM or remain AOM-free throughout childhood. The use of oral pre- and probiotics for AOM prophylaxis remains debated because the results of studies are conflicting and frequently show no effect. So the idea of using prebiotics or probiotics to create a favorable “microbiome” of the nose is under investigation. Two species of bacteria that are gathering the most attention are Corynebacterium species (a few types in particular) and a bacteria called Dolosigranulum pigrum. Delivery of the commensal species would be as a nose spray.
Vaccines
The use of pneumococcal conjugate vaccines (PCVs) has reduced the frequency of AOM caused by Streptococcus pneumoniae. PCVs are not as effective against AOM as they are against invasive pneumococcal disease, but they still help a lot. However, because there are now at least 96 different serotypes of the pneumococcus based on different capsular types, we see a pattern of replacement of disease-causing strains by new strains within a few years of introduction of a new formulation. We started with 7 serotypes (Prevnar 7) in year 2000, and it was replaced by the current formulation with 13 serotypes (Prevnar 13) in 2010. Replacements have occurred again so vaccine companies are making new formulations for the future that include more serotypes, up to 20 serotypes. But, technically and feasibility-wise there is a limit to making such vaccines. A vaccine based on killed unencapsulated bacteria has been tested for safety and immunogenicity in young children. There is no test so far for prevention of AOM. Another type of vaccine based on proteins expressed by the pneumococcus that could be vaccine targets was tested in American Navajo children, and it failed to be as efficacious as hoped.
Biomarkers.
Due to recurrent AOM or persistent otitis media with effusion, about 15% of children in the United States receive tympanostomy tubes. Among those who receive tubes, about 20% go on to receive a second set of tubes, often with adenotonsillectomy. To find a biomarker that could identify children likely to require a second set of tubes, the fluid in the middle ear was tested when a first set of tubes were inserted. If bacteria were detected by polymerase chain reaction (PCR) testing or if a profile of specific inflammatory cytokines was measured, those results could be used to predict a high likelihood for a second set of tubes.
Overdiagnosis
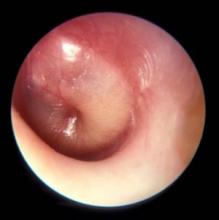
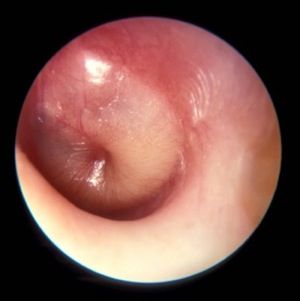
Diagnosis of AOM is challenging in young children, in whom it most frequently occurs. The ear canal is typically about 3 mm wide, the child struggles during the examination, and diagnostic skills are not taught in training, resulting in a high overdiagnosis rate. I presented data that suggest too many children who are not truly otitis prone have been classified as otitis prone based on incorrect clinical diagnosis. My colleagues and I found that 30% of children reach the threshold of three episodes of AOM in 6 months or four within a year when diagnosed by community pediatricians, similar to many other studies. Validated otoscopists (trained by experts with diagnosis definitively proven as at least 85% accurate using tympanocentesis) classify 15% of children as otitis prone – half as many. If tympanocentesis is used to prove middle ear fluid has bacterial pathogens (about 95% yield a bacterial otopathogen using culture and PCR), then about 10% of children are classified as otitis prone – one-third as many. This suggests that children clinically diagnosed by community-based pediatricians are overdiagnosed with AOM, perhaps three times more often than true. And that leads to overuse of antibiotics and referrals for tympanostomy tube surgery more often than should occur. So we need to improve diagnostic methods beyond otoscopy. New types of imaging for the eardrum and middle ear using novel technologies are in early clinical trials.
Immunity
The notion that young children get AOM because of Eustachian tube dysfunction in their early years of life (horizontal anatomy) may be true, but there is more to the story. After 10 years of work, the scientists in my research group have shown that children in the first 3 years of life can have an immune system that is suppressed – it is poorly responsive to pathogens and routine pediatric vaccines. Many features resemble a neonatal immune system, beginning life with a suppressed immune system or being in cytokine storm from birth. We introduced the term “prolonged neonatal-like immune profile (PNIP)” to give a general description of the immune responses we have found in otitis-prone children. They outgrow this. So the immune maturation is delayed but not permanent. It is mostly resolved by age 3 years. We found problems in both innate and adaptive immunity. It may be that the main explanation for recurrent AOM in the first years of life is PNIP. Scientists from Australia also reported immunity problems in Aboriginal children and they are very otitis prone, often progressing to chronic suppurative otitis media. Animal model studies of AOM show inadequate innate and adaptive immunity importantly contribute to the infection as well.
Dr. Pichichero is a specialist in pediatric infectious diseases and director of the Research Institute at Rochester (N.Y.) General Hospital. He has no conflicts to declare. Email him at [email protected].
New research was presented at the International Society for Otitis Media meeting in June 2019, which I attended. I would like to share a selection of new findings from the many presentations.
Transtympanic antibiotic delivery
Topical therapy has been used to treat only otitis externa and acute otitis media (AOM) with ear discharge. Giving antibiotics through the tympanic membrane could mitigate many of the concerns about antibiotic use driving antibiotic resistance of bacteria among children. Up to now, using antibiotics in the ear canal to treat AOM has not been considered because the tympanic membrane is highly impermeable to the transtympanic diffusion of any drugs. However, in recent years, a number of different drug delivery systems have been developed, and in some cases, animal studies have shown that noninvasive transtympanic delivery is possible so that drugs can reach high concentrations in the middle ear without damage. Nanovesicles and nanoliposomes that contain antibiotics and are small enough to pass through the eardrum have been developed and tested in animal models; these show promise. Ototopical administration of a drug called vinpocetine that was repurposed has been tested in mice and shown to reduce inflammation and mucus production in the middle ear during otitis media.
Biofilms
Antibiotic treatment failure can occur in AOM for several reasons. The treatment of choice, amoxicillin, for example may fail to achieve an adequate concentration because of poor absorption in the gastrointestinal tract or poor penetration into the middle ear. Or, the antibiotic chosen may not be effective because of resistance of the strain causing the infection. Another explanation, especially in recurrent AOM and chronic AOM, could be the presence of biofilms. Biofilms are multicellular bacterial communities incorporated in a polymeric, plasticlike matrix in which pathogens are protected from antibiotic activity. The biofilm provides a physical barrier to antibiotic penetration, and bacteria can persist in the middle ear and periodically cause a new AOM. If AOM persists or becomes a more chronic otitis media with effusion, the “glue ear” causes an environment in the middle ear that is low in oxygen. A low-oxygen environment is favorable to biofilms. Also one might expect that middle ear pus would have a low pH, but actual measurements show the pH is highly alkaline. Species of Haemophilus influenzae have been identified as more virulent when in an alkaline pH or the alkaline pH makes the H. influenzae persist better in the middle ear, perhaps in a biofilm. To eliminate biofilms and improve antibiotic efficacy, a vaccine against a protein expressed by H. influenzae has been developed. Antibodies against this protein have been shown to disrupt and prevent the formation of biofilms in an animal model.
Probiotics
The normal bacteria that live in the nasopharynx of children with recurrent AOM is now known to differ from that of children who experience infrequent AOM or remain AOM-free throughout childhood. The use of oral pre- and probiotics for AOM prophylaxis remains debated because the results of studies are conflicting and frequently show no effect. So the idea of using prebiotics or probiotics to create a favorable “microbiome” of the nose is under investigation. Two species of bacteria that are gathering the most attention are Corynebacterium species (a few types in particular) and a bacteria called Dolosigranulum pigrum. Delivery of the commensal species would be as a nose spray.
Vaccines
The use of pneumococcal conjugate vaccines (PCVs) has reduced the frequency of AOM caused by Streptococcus pneumoniae. PCVs are not as effective against AOM as they are against invasive pneumococcal disease, but they still help a lot. However, because there are now at least 96 different serotypes of the pneumococcus based on different capsular types, we see a pattern of replacement of disease-causing strains by new strains within a few years of introduction of a new formulation. We started with 7 serotypes (Prevnar 7) in year 2000, and it was replaced by the current formulation with 13 serotypes (Prevnar 13) in 2010. Replacements have occurred again so vaccine companies are making new formulations for the future that include more serotypes, up to 20 serotypes. But, technically and feasibility-wise there is a limit to making such vaccines. A vaccine based on killed unencapsulated bacteria has been tested for safety and immunogenicity in young children. There is no test so far for prevention of AOM. Another type of vaccine based on proteins expressed by the pneumococcus that could be vaccine targets was tested in American Navajo children, and it failed to be as efficacious as hoped.
Biomarkers.
Due to recurrent AOM or persistent otitis media with effusion, about 15% of children in the United States receive tympanostomy tubes. Among those who receive tubes, about 20% go on to receive a second set of tubes, often with adenotonsillectomy. To find a biomarker that could identify children likely to require a second set of tubes, the fluid in the middle ear was tested when a first set of tubes were inserted. If bacteria were detected by polymerase chain reaction (PCR) testing or if a profile of specific inflammatory cytokines was measured, those results could be used to predict a high likelihood for a second set of tubes.
Overdiagnosis
Diagnosis of AOM is challenging in young children, in whom it most frequently occurs. The ear canal is typically about 3 mm wide, the child struggles during the examination, and diagnostic skills are not taught in training, resulting in a high overdiagnosis rate. I presented data that suggest too many children who are not truly otitis prone have been classified as otitis prone based on incorrect clinical diagnosis. My colleagues and I found that 30% of children reach the threshold of three episodes of AOM in 6 months or four within a year when diagnosed by community pediatricians, similar to many other studies. Validated otoscopists (trained by experts with diagnosis definitively proven as at least 85% accurate using tympanocentesis) classify 15% of children as otitis prone – half as many. If tympanocentesis is used to prove middle ear fluid has bacterial pathogens (about 95% yield a bacterial otopathogen using culture and PCR), then about 10% of children are classified as otitis prone – one-third as many. This suggests that children clinically diagnosed by community-based pediatricians are overdiagnosed with AOM, perhaps three times more often than true. And that leads to overuse of antibiotics and referrals for tympanostomy tube surgery more often than should occur. So we need to improve diagnostic methods beyond otoscopy. New types of imaging for the eardrum and middle ear using novel technologies are in early clinical trials.
Immunity
The notion that young children get AOM because of Eustachian tube dysfunction in their early years of life (horizontal anatomy) may be true, but there is more to the story. After 10 years of work, the scientists in my research group have shown that children in the first 3 years of life can have an immune system that is suppressed – it is poorly responsive to pathogens and routine pediatric vaccines. Many features resemble a neonatal immune system, beginning life with a suppressed immune system or being in cytokine storm from birth. We introduced the term “prolonged neonatal-like immune profile (PNIP)” to give a general description of the immune responses we have found in otitis-prone children. They outgrow this. So the immune maturation is delayed but not permanent. It is mostly resolved by age 3 years. We found problems in both innate and adaptive immunity. It may be that the main explanation for recurrent AOM in the first years of life is PNIP. Scientists from Australia also reported immunity problems in Aboriginal children and they are very otitis prone, often progressing to chronic suppurative otitis media. Animal model studies of AOM show inadequate innate and adaptive immunity importantly contribute to the infection as well.
Dr. Pichichero is a specialist in pediatric infectious diseases and director of the Research Institute at Rochester (N.Y.) General Hospital. He has no conflicts to declare. Email him at [email protected].
New research was presented at the International Society for Otitis Media meeting in June 2019, which I attended. I would like to share a selection of new findings from the many presentations.
Transtympanic antibiotic delivery
Topical therapy has been used to treat only otitis externa and acute otitis media (AOM) with ear discharge. Giving antibiotics through the tympanic membrane could mitigate many of the concerns about antibiotic use driving antibiotic resistance of bacteria among children. Up to now, using antibiotics in the ear canal to treat AOM has not been considered because the tympanic membrane is highly impermeable to the transtympanic diffusion of any drugs. However, in recent years, a number of different drug delivery systems have been developed, and in some cases, animal studies have shown that noninvasive transtympanic delivery is possible so that drugs can reach high concentrations in the middle ear without damage. Nanovesicles and nanoliposomes that contain antibiotics and are small enough to pass through the eardrum have been developed and tested in animal models; these show promise. Ototopical administration of a drug called vinpocetine that was repurposed has been tested in mice and shown to reduce inflammation and mucus production in the middle ear during otitis media.
Biofilms
Antibiotic treatment failure can occur in AOM for several reasons. The treatment of choice, amoxicillin, for example may fail to achieve an adequate concentration because of poor absorption in the gastrointestinal tract or poor penetration into the middle ear. Or, the antibiotic chosen may not be effective because of resistance of the strain causing the infection. Another explanation, especially in recurrent AOM and chronic AOM, could be the presence of biofilms. Biofilms are multicellular bacterial communities incorporated in a polymeric, plasticlike matrix in which pathogens are protected from antibiotic activity. The biofilm provides a physical barrier to antibiotic penetration, and bacteria can persist in the middle ear and periodically cause a new AOM. If AOM persists or becomes a more chronic otitis media with effusion, the “glue ear” causes an environment in the middle ear that is low in oxygen. A low-oxygen environment is favorable to biofilms. Also one might expect that middle ear pus would have a low pH, but actual measurements show the pH is highly alkaline. Species of Haemophilus influenzae have been identified as more virulent when in an alkaline pH or the alkaline pH makes the H. influenzae persist better in the middle ear, perhaps in a biofilm. To eliminate biofilms and improve antibiotic efficacy, a vaccine against a protein expressed by H. influenzae has been developed. Antibodies against this protein have been shown to disrupt and prevent the formation of biofilms in an animal model.
Probiotics
The normal bacteria that live in the nasopharynx of children with recurrent AOM is now known to differ from that of children who experience infrequent AOM or remain AOM-free throughout childhood. The use of oral pre- and probiotics for AOM prophylaxis remains debated because the results of studies are conflicting and frequently show no effect. So the idea of using prebiotics or probiotics to create a favorable “microbiome” of the nose is under investigation. Two species of bacteria that are gathering the most attention are Corynebacterium species (a few types in particular) and a bacteria called Dolosigranulum pigrum. Delivery of the commensal species would be as a nose spray.
Vaccines
The use of pneumococcal conjugate vaccines (PCVs) has reduced the frequency of AOM caused by Streptococcus pneumoniae. PCVs are not as effective against AOM as they are against invasive pneumococcal disease, but they still help a lot. However, because there are now at least 96 different serotypes of the pneumococcus based on different capsular types, we see a pattern of replacement of disease-causing strains by new strains within a few years of introduction of a new formulation. We started with 7 serotypes (Prevnar 7) in year 2000, and it was replaced by the current formulation with 13 serotypes (Prevnar 13) in 2010. Replacements have occurred again so vaccine companies are making new formulations for the future that include more serotypes, up to 20 serotypes. But, technically and feasibility-wise there is a limit to making such vaccines. A vaccine based on killed unencapsulated bacteria has been tested for safety and immunogenicity in young children. There is no test so far for prevention of AOM. Another type of vaccine based on proteins expressed by the pneumococcus that could be vaccine targets was tested in American Navajo children, and it failed to be as efficacious as hoped.
Biomarkers.
Due to recurrent AOM or persistent otitis media with effusion, about 15% of children in the United States receive tympanostomy tubes. Among those who receive tubes, about 20% go on to receive a second set of tubes, often with adenotonsillectomy. To find a biomarker that could identify children likely to require a second set of tubes, the fluid in the middle ear was tested when a first set of tubes were inserted. If bacteria were detected by polymerase chain reaction (PCR) testing or if a profile of specific inflammatory cytokines was measured, those results could be used to predict a high likelihood for a second set of tubes.
Overdiagnosis
Diagnosis of AOM is challenging in young children, in whom it most frequently occurs. The ear canal is typically about 3 mm wide, the child struggles during the examination, and diagnostic skills are not taught in training, resulting in a high overdiagnosis rate. I presented data that suggest too many children who are not truly otitis prone have been classified as otitis prone based on incorrect clinical diagnosis. My colleagues and I found that 30% of children reach the threshold of three episodes of AOM in 6 months or four within a year when diagnosed by community pediatricians, similar to many other studies. Validated otoscopists (trained by experts with diagnosis definitively proven as at least 85% accurate using tympanocentesis) classify 15% of children as otitis prone – half as many. If tympanocentesis is used to prove middle ear fluid has bacterial pathogens (about 95% yield a bacterial otopathogen using culture and PCR), then about 10% of children are classified as otitis prone – one-third as many. This suggests that children clinically diagnosed by community-based pediatricians are overdiagnosed with AOM, perhaps three times more often than true. And that leads to overuse of antibiotics and referrals for tympanostomy tube surgery more often than should occur. So we need to improve diagnostic methods beyond otoscopy. New types of imaging for the eardrum and middle ear using novel technologies are in early clinical trials.
Immunity
The notion that young children get AOM because of Eustachian tube dysfunction in their early years of life (horizontal anatomy) may be true, but there is more to the story. After 10 years of work, the scientists in my research group have shown that children in the first 3 years of life can have an immune system that is suppressed – it is poorly responsive to pathogens and routine pediatric vaccines. Many features resemble a neonatal immune system, beginning life with a suppressed immune system or being in cytokine storm from birth. We introduced the term “prolonged neonatal-like immune profile (PNIP)” to give a general description of the immune responses we have found in otitis-prone children. They outgrow this. So the immune maturation is delayed but not permanent. It is mostly resolved by age 3 years. We found problems in both innate and adaptive immunity. It may be that the main explanation for recurrent AOM in the first years of life is PNIP. Scientists from Australia also reported immunity problems in Aboriginal children and they are very otitis prone, often progressing to chronic suppurative otitis media. Animal model studies of AOM show inadequate innate and adaptive immunity importantly contribute to the infection as well.
Dr. Pichichero is a specialist in pediatric infectious diseases and director of the Research Institute at Rochester (N.Y.) General Hospital. He has no conflicts to declare. Email him at [email protected].
Get patients vaccinated: Avoid unwelcome international travel souvenirs
Summer officially began June 21, 2019, but many of your patients already may have departed or will soon be headed to international destinations. Reasons for travel are as variable as their destinations and include but are not limited to family vacations, mission trips, study abroad, parental job relocation, and visiting friends and relatives. The majority of the trips are planned at least 3 months in advance; however, for many travelers and their parents, they suddenly get an aha moment and realize there is/are specific vaccines required to obtain a visa or entry to their final destination. Unfortunately, too much emphasis is focused on required vaccines. The well-informed traveler knows that they may be exposed to multiple diseases and many are vaccine preventable.
The accompanying table lists vaccines traditionally considered to be travel vaccines. Several require multiple doses administered over 21-28 days to provide protection. Others such as cholera and yellow fever must be completed at least 10 days prior to departure to be effective. Typhoid has two formulations: The oral and injectable typhoid vaccines should be completed 1 and 2 weeks, respectively, prior to travel. Several vaccines have age limitations. Routine immunization of all infants against hepatitis A was recommended in 2006. Depending on your region, there may be adolescents who have not been immunized. Fortunately, hepatitis A vaccine works immediately.
One of the challenges you face is identifying someone in your area that provides travel medicine advice and immunizations to children and adolescents. Most children and teens travel with their parents, but today many adolescents travel independently with organized groups. Most of the vaccines listed are not routinely administered at your office, yet you most likely will be the first call a parent makes seeking travel advice.
Let me tell you about a few vaccines in particular.
Japanese encephalitis
This is most common cause of encephalitis in Asia and parts of the western Pacific. Risk generally is limited to rural agricultural areas where the causative virus is transmitted by a mosquito. Fatality rates are 20%-30%. Among survivors, 30%-50% have significant neurologic, cognitive, and psychiatric sequelae. Candidates for this vaccine are long-term travelers and short-term travelers with extensive outdoor rural activities.
Meningococcal conjugate vaccines (MCV4)
All travelers to the Hajj Pilgrimage (Aug. 9-14, 2019) and/or Umrah must show proof of immunization. Vaccine must be received at least 10 days prior to and no greater than 5 years prior to arrival to Saudi Arabia. Conjugate vaccine must clearly be documented for validity of 5 years. For all health entry requirements, go to www.moh.gov.sa/en/hajj/pages/healthregulations.aspx.
Measles
The Advisory Committee on Immunization Practices recommends all infants 6-11 months old receive one dose of MMR prior to international travel regardless of the destination. This should be followed by two additional countable doses. All persons at least 12 months of age and born after 1956 should receive two doses of MMR at least 28 days apart prior to international travel.
Rabies
Rabies is a viral disease endemic in more than 150 countries with approximately 60,000 fatal cases worldwide each year. Asia and Africa are the areas with the highest risk of exposure, and dogs are the principal hosts. Human rabies is almost always fatal once symptoms develop. Preexposure vaccine is recommended for persons with prolonged and/or remote travel to countries where rabies immunoglobulin is unavailable and the occurrence of animal rabies is high. Post exposure vaccination on days 0 and 3 still would be required.*
Typhoid
A bacterial infection caused by Salmonella enterica serotype Typhi and Paratyphi manifests with fever, headache, abdominal pain, diarrhea, or constipation. When bacteremia occurs, it usually is referred to as enteric fever. It is acquired by consumption of food/water contaminated with human feces. Highest risk areas include Africa, Southern Asia, and Southeast Asia
Yellow fever
Risk is limited to sub-Saharan Africa and the tropical areas of South America. It is transmitted by the bite of an infected mosquito. The vaccine is required for entry into at least 16 countries. In a country where yellow fever is present, persons transiting through for more than 12 hours to reach their final destination may actually cause a change in the entry requirements for the destination country. For example, travel from the United States to Tanzania requires no yellow fever vaccine while travel from the United States to Nairobi (more than 12 hours) to Tanzania requires yellow fever vaccine for entry into Tanzania. Travel sequence and duration is extremely important. Check the Centers for Disease Control and Prevention yellow fever site and/or the consulate for the most up-to-date yellow fever vaccine requirements.
YF-Vax (yellow fever vaccine) produced by Sanofi Pasteur in the United States currently is unavailable. The company is building a new facility, and vaccine will not be available for the remainder of 2019. To assure vaccine for U.S. travelers, Stamaril, a yellow fever vaccine produced by Sanofi Pasteur in France has been made available at more than 250 sites nationwide. Because Stamaril is offered at a limited number of locations, persons in need of vaccine should not delay seeking it. Because of increased demand related to summer travel, travelers in some areas have reported delays of several weeks in scheduling an appointment. To locate a Stamaril site in your area, go to wwwnc.cdc.gov/travel/page/search-for-stamaril-clinics.
There are several other diseases transmitted by mosquitoes and ticks including malaria, dengue, Zika and rickettsial diseases. Vigilant use of mosquito repellents is a must. Prophylactic medication is available for only malaria and should be initiated prior to exposure. Frequency and duration depends on the medication selected.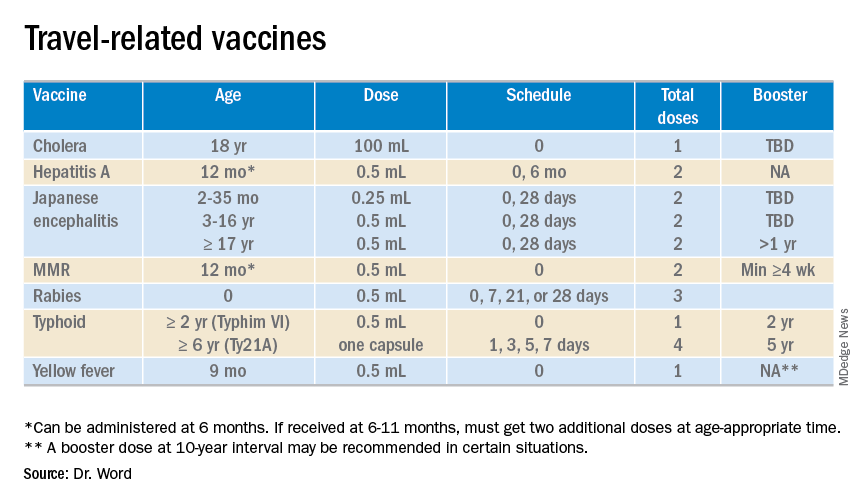
So how do you assist your patients?
Once you’ve identified a travel medicine facility in your area, encourage them to seek pretravel advice 4-6 weeks prior to international travel and make sure their routine immunizations are up to date. Generally, this is not an issue. One challenge is the early administration of MMR. While most practitioners know that early administration for international travel has been recommended for years, many office staff are accustomed to administration at only the 12 month and 4 year visit. When parents call requesting immunization, they often are informed that is it unnecessary and the appointment denied. This is a challenge, especially when coordination of administration of another live vaccine, such as yellow fever, is planned. Familiarizing all members of the health care team with current vaccine recommendations is critical.
For country-specific information, up-to-date travel alerts, and to locate a travel medicine clinic, visit www.cdc.gov/travel.
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She had no relevant financial disclosures. Email her at [email protected].
*This article was updated 6/18/2019.
Summer officially began June 21, 2019, but many of your patients already may have departed or will soon be headed to international destinations. Reasons for travel are as variable as their destinations and include but are not limited to family vacations, mission trips, study abroad, parental job relocation, and visiting friends and relatives. The majority of the trips are planned at least 3 months in advance; however, for many travelers and their parents, they suddenly get an aha moment and realize there is/are specific vaccines required to obtain a visa or entry to their final destination. Unfortunately, too much emphasis is focused on required vaccines. The well-informed traveler knows that they may be exposed to multiple diseases and many are vaccine preventable.
The accompanying table lists vaccines traditionally considered to be travel vaccines. Several require multiple doses administered over 21-28 days to provide protection. Others such as cholera and yellow fever must be completed at least 10 days prior to departure to be effective. Typhoid has two formulations: The oral and injectable typhoid vaccines should be completed 1 and 2 weeks, respectively, prior to travel. Several vaccines have age limitations. Routine immunization of all infants against hepatitis A was recommended in 2006. Depending on your region, there may be adolescents who have not been immunized. Fortunately, hepatitis A vaccine works immediately.
One of the challenges you face is identifying someone in your area that provides travel medicine advice and immunizations to children and adolescents. Most children and teens travel with their parents, but today many adolescents travel independently with organized groups. Most of the vaccines listed are not routinely administered at your office, yet you most likely will be the first call a parent makes seeking travel advice.
Let me tell you about a few vaccines in particular.
Japanese encephalitis
This is most common cause of encephalitis in Asia and parts of the western Pacific. Risk generally is limited to rural agricultural areas where the causative virus is transmitted by a mosquito. Fatality rates are 20%-30%. Among survivors, 30%-50% have significant neurologic, cognitive, and psychiatric sequelae. Candidates for this vaccine are long-term travelers and short-term travelers with extensive outdoor rural activities.
Meningococcal conjugate vaccines (MCV4)
All travelers to the Hajj Pilgrimage (Aug. 9-14, 2019) and/or Umrah must show proof of immunization. Vaccine must be received at least 10 days prior to and no greater than 5 years prior to arrival to Saudi Arabia. Conjugate vaccine must clearly be documented for validity of 5 years. For all health entry requirements, go to www.moh.gov.sa/en/hajj/pages/healthregulations.aspx.
Measles
The Advisory Committee on Immunization Practices recommends all infants 6-11 months old receive one dose of MMR prior to international travel regardless of the destination. This should be followed by two additional countable doses. All persons at least 12 months of age and born after 1956 should receive two doses of MMR at least 28 days apart prior to international travel.
Rabies
Rabies is a viral disease endemic in more than 150 countries with approximately 60,000 fatal cases worldwide each year. Asia and Africa are the areas with the highest risk of exposure, and dogs are the principal hosts. Human rabies is almost always fatal once symptoms develop. Preexposure vaccine is recommended for persons with prolonged and/or remote travel to countries where rabies immunoglobulin is unavailable and the occurrence of animal rabies is high. Post exposure vaccination on days 0 and 3 still would be required.*
Typhoid
A bacterial infection caused by Salmonella enterica serotype Typhi and Paratyphi manifests with fever, headache, abdominal pain, diarrhea, or constipation. When bacteremia occurs, it usually is referred to as enteric fever. It is acquired by consumption of food/water contaminated with human feces. Highest risk areas include Africa, Southern Asia, and Southeast Asia
Yellow fever
Risk is limited to sub-Saharan Africa and the tropical areas of South America. It is transmitted by the bite of an infected mosquito. The vaccine is required for entry into at least 16 countries. In a country where yellow fever is present, persons transiting through for more than 12 hours to reach their final destination may actually cause a change in the entry requirements for the destination country. For example, travel from the United States to Tanzania requires no yellow fever vaccine while travel from the United States to Nairobi (more than 12 hours) to Tanzania requires yellow fever vaccine for entry into Tanzania. Travel sequence and duration is extremely important. Check the Centers for Disease Control and Prevention yellow fever site and/or the consulate for the most up-to-date yellow fever vaccine requirements.
YF-Vax (yellow fever vaccine) produced by Sanofi Pasteur in the United States currently is unavailable. The company is building a new facility, and vaccine will not be available for the remainder of 2019. To assure vaccine for U.S. travelers, Stamaril, a yellow fever vaccine produced by Sanofi Pasteur in France has been made available at more than 250 sites nationwide. Because Stamaril is offered at a limited number of locations, persons in need of vaccine should not delay seeking it. Because of increased demand related to summer travel, travelers in some areas have reported delays of several weeks in scheduling an appointment. To locate a Stamaril site in your area, go to wwwnc.cdc.gov/travel/page/search-for-stamaril-clinics.
There are several other diseases transmitted by mosquitoes and ticks including malaria, dengue, Zika and rickettsial diseases. Vigilant use of mosquito repellents is a must. Prophylactic medication is available for only malaria and should be initiated prior to exposure. Frequency and duration depends on the medication selected.
So how do you assist your patients?
Once you’ve identified a travel medicine facility in your area, encourage them to seek pretravel advice 4-6 weeks prior to international travel and make sure their routine immunizations are up to date. Generally, this is not an issue. One challenge is the early administration of MMR. While most practitioners know that early administration for international travel has been recommended for years, many office staff are accustomed to administration at only the 12 month and 4 year visit. When parents call requesting immunization, they often are informed that is it unnecessary and the appointment denied. This is a challenge, especially when coordination of administration of another live vaccine, such as yellow fever, is planned. Familiarizing all members of the health care team with current vaccine recommendations is critical.
For country-specific information, up-to-date travel alerts, and to locate a travel medicine clinic, visit www.cdc.gov/travel.
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She had no relevant financial disclosures. Email her at [email protected].
*This article was updated 6/18/2019.
Summer officially began June 21, 2019, but many of your patients already may have departed or will soon be headed to international destinations. Reasons for travel are as variable as their destinations and include but are not limited to family vacations, mission trips, study abroad, parental job relocation, and visiting friends and relatives. The majority of the trips are planned at least 3 months in advance; however, for many travelers and their parents, they suddenly get an aha moment and realize there is/are specific vaccines required to obtain a visa or entry to their final destination. Unfortunately, too much emphasis is focused on required vaccines. The well-informed traveler knows that they may be exposed to multiple diseases and many are vaccine preventable.
The accompanying table lists vaccines traditionally considered to be travel vaccines. Several require multiple doses administered over 21-28 days to provide protection. Others such as cholera and yellow fever must be completed at least 10 days prior to departure to be effective. Typhoid has two formulations: The oral and injectable typhoid vaccines should be completed 1 and 2 weeks, respectively, prior to travel. Several vaccines have age limitations. Routine immunization of all infants against hepatitis A was recommended in 2006. Depending on your region, there may be adolescents who have not been immunized. Fortunately, hepatitis A vaccine works immediately.
One of the challenges you face is identifying someone in your area that provides travel medicine advice and immunizations to children and adolescents. Most children and teens travel with their parents, but today many adolescents travel independently with organized groups. Most of the vaccines listed are not routinely administered at your office, yet you most likely will be the first call a parent makes seeking travel advice.
Let me tell you about a few vaccines in particular.
Japanese encephalitis
This is most common cause of encephalitis in Asia and parts of the western Pacific. Risk generally is limited to rural agricultural areas where the causative virus is transmitted by a mosquito. Fatality rates are 20%-30%. Among survivors, 30%-50% have significant neurologic, cognitive, and psychiatric sequelae. Candidates for this vaccine are long-term travelers and short-term travelers with extensive outdoor rural activities.
Meningococcal conjugate vaccines (MCV4)
All travelers to the Hajj Pilgrimage (Aug. 9-14, 2019) and/or Umrah must show proof of immunization. Vaccine must be received at least 10 days prior to and no greater than 5 years prior to arrival to Saudi Arabia. Conjugate vaccine must clearly be documented for validity of 5 years. For all health entry requirements, go to www.moh.gov.sa/en/hajj/pages/healthregulations.aspx.
Measles
The Advisory Committee on Immunization Practices recommends all infants 6-11 months old receive one dose of MMR prior to international travel regardless of the destination. This should be followed by two additional countable doses. All persons at least 12 months of age and born after 1956 should receive two doses of MMR at least 28 days apart prior to international travel.
Rabies
Rabies is a viral disease endemic in more than 150 countries with approximately 60,000 fatal cases worldwide each year. Asia and Africa are the areas with the highest risk of exposure, and dogs are the principal hosts. Human rabies is almost always fatal once symptoms develop. Preexposure vaccine is recommended for persons with prolonged and/or remote travel to countries where rabies immunoglobulin is unavailable and the occurrence of animal rabies is high. Post exposure vaccination on days 0 and 3 still would be required.*
Typhoid
A bacterial infection caused by Salmonella enterica serotype Typhi and Paratyphi manifests with fever, headache, abdominal pain, diarrhea, or constipation. When bacteremia occurs, it usually is referred to as enteric fever. It is acquired by consumption of food/water contaminated with human feces. Highest risk areas include Africa, Southern Asia, and Southeast Asia
Yellow fever
Risk is limited to sub-Saharan Africa and the tropical areas of South America. It is transmitted by the bite of an infected mosquito. The vaccine is required for entry into at least 16 countries. In a country where yellow fever is present, persons transiting through for more than 12 hours to reach their final destination may actually cause a change in the entry requirements for the destination country. For example, travel from the United States to Tanzania requires no yellow fever vaccine while travel from the United States to Nairobi (more than 12 hours) to Tanzania requires yellow fever vaccine for entry into Tanzania. Travel sequence and duration is extremely important. Check the Centers for Disease Control and Prevention yellow fever site and/or the consulate for the most up-to-date yellow fever vaccine requirements.
YF-Vax (yellow fever vaccine) produced by Sanofi Pasteur in the United States currently is unavailable. The company is building a new facility, and vaccine will not be available for the remainder of 2019. To assure vaccine for U.S. travelers, Stamaril, a yellow fever vaccine produced by Sanofi Pasteur in France has been made available at more than 250 sites nationwide. Because Stamaril is offered at a limited number of locations, persons in need of vaccine should not delay seeking it. Because of increased demand related to summer travel, travelers in some areas have reported delays of several weeks in scheduling an appointment. To locate a Stamaril site in your area, go to wwwnc.cdc.gov/travel/page/search-for-stamaril-clinics.
There are several other diseases transmitted by mosquitoes and ticks including malaria, dengue, Zika and rickettsial diseases. Vigilant use of mosquito repellents is a must. Prophylactic medication is available for only malaria and should be initiated prior to exposure. Frequency and duration depends on the medication selected.
So how do you assist your patients?
Once you’ve identified a travel medicine facility in your area, encourage them to seek pretravel advice 4-6 weeks prior to international travel and make sure their routine immunizations are up to date. Generally, this is not an issue. One challenge is the early administration of MMR. While most practitioners know that early administration for international travel has been recommended for years, many office staff are accustomed to administration at only the 12 month and 4 year visit. When parents call requesting immunization, they often are informed that is it unnecessary and the appointment denied. This is a challenge, especially when coordination of administration of another live vaccine, such as yellow fever, is planned. Familiarizing all members of the health care team with current vaccine recommendations is critical.
For country-specific information, up-to-date travel alerts, and to locate a travel medicine clinic, visit www.cdc.gov/travel.
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She had no relevant financial disclosures. Email her at [email protected].
*This article was updated 6/18/2019.
Young children with neuromuscular disease are vulnerable to respiratory viruses
This highlights the need for new vaccines
Influenza gets a lot of attention each winter, but respiratory syncytial virus (RSV) and other respiratory viruses have as much or more impact on pediatric populations, particularly certain high-risk groups. But currently there are no vaccines for noninfluenza respiratory viruses. That said, several are under development, for RSV and parainfluenza.
Which groups are likely to get the most benefit from these newer vaccines?
We all are aware of the extra vulnerability to respiratory viruses (RSV being the most frequent) in premature infants, those with chronic lung disease, or those with congenital heart syndromes; such vulnerable patients are not infrequently seen in routine practice. A recent report shined a brighter light on such a group.
Real-world data from a nationwide Canadian surveillance system (CARESS) was used to analyze relative risks of categories of young children who are thought to be vulnerable to respiratory viruses, with a particular focus on those with neuromuscular disease. The CARESS investigators analyzed 12 years’ data on respiratory hospitalizations from among palivizumab-prophylaxed patients (including specific data on RSV when patients were tested for RSV per standard of care).1 Unfortunately, RSV testing was not universal despite hospitalization, so the true incidence of RSV-specific hospitalizations was likely underestimated.
Nevertheless, more than 25,000 children from 2005 through 2017 were grouped into three categories of palivizumab-prophylaxed high-risk children: standard indications (SI), n = 20,335; chronic medical conditions (CMD), n = 4,063; and neuromuscular disease (NMD), n = 605. This study is notable for having a relatively large number of neuromuscular disease subjects. Two-thirds of each group were fully palivizumab adherent.
The SI group included the standard American Academy of Pediatrics–recommended groups, such as premature infants, congenital heart disease, etc.
The CMD group included conditions that lead clinicians to use palivizumab off label, such as cystic fibrosis, congenital airway anomalies, immunodeficiency, and pulmonary disorders.
The NMD participants were subdivided into two groups. Group 1 comprised general hypotonic neuromuscular diseases such as hypoxic-ischemic encephalopathy, Prader-Willi syndrome, chromosomal disorders, and migration/demyelinating diseases. Group 2 included more severe infantile neuromuscular disorders, such as spinal muscular atrophy, myotonic dystrophy, centronuclear and nemaline myopathy, mitochondrial and glycogen storage myopathies, or arthrogryposis.
Overall, 6.9% of CARESS RSV-prophylaxed subjects were hospitalized. About one in five hospitalized patients from each group was hospitalized more than once. Specific respiratory hospitalization rates for each group were 6% (n = 1,228) for SI subjects and 9.4% (n = 380) for CMD, compared with 19.2% (n = 116) for NMD subjects.
It is unclear what proportion underwent RSV testing, but a total of 334 were confirmed RSV positive: 261 were SI, 54 were CMD and 19 were NMD. The RSV-test-positive rate was 1.5% for SI, 1.6% for CMD and 3.3% for NMD; so while a higher number of SI children were RSV positive, the rate of RSV positivity was actually highest with NMD.
RSV-positive subjects needing ICU care among NMD patients also had longer ICU stays (median 14 days), compared with RSV-positive CMD or SI subjects (median 3 and 5 days, respectively). Further, hospitalized RSV-positive NMD subjects presented more frequently with pneumonia (42% vs. 30% for CMD and 20% for SI) while hospitalized RSV-positive SI subjects more often had apnea (17% vs. 10% for NMD and 5% for CMD, P less than .05).
These differences in the courses of NMD patients raise the question as to whether the NMD group was somehow different from the SI and CMD groups, other than muscular weakness that likely leads to less ability to clear secretions and a less efficient cough. It turns out that NMD children were older and had worse neonatal medical courses (longer hospital stays, more often ventilated, and used oxygen longer). It could be argued that these differences may have been in part due to the muscular weakness inherent in their underlying disease, but they appear to be predictors of worse respiratory infectious disease than other vulnerable populations as the NMD children get older.
Indeed, the overall risk of any respiratory admission among NMD subjects was nearly twice as high, compared with SI (hazard ratio, 1.90, P less than .0005); but the somewhat higher risk for NMD vs. CMD was not significant (HR, 1.33, P = .090). However, when looking specifically at RSV confirmed admissions, NMD had more than twice the hospitalization risk than either other group (HR, 2.26, P = .001 vs. SI; and HR, 2.74, P = .001 vs. CMD).
Further, an NMD subgroup analysis showed 1.69 times the overall respiratory hospitalization risk among the more severe vs. less severe NMD group, but a similar risk of RSV admission. The authors point out that one reason for this discrepancy may be a higher probability of aspiration causing hospitalization because of more dramatic acute events during respiratory infections in patients with more severe NMD. It also may be that palivizumab evened the playing field for RSV but not for other viruses such as parainfluenza, adenovirus, or even rhinovirus.
Nevertheless, these data tell us that risk of respiratory disease severe enough to need hospitalization continues to an older age in NMD than SI or CMD patients, well past 2 years of age. And the risk is not only from RSV. That said, RSV remains a player in some patients (particularly NMD patients) despite palivizumab prophylaxis, highlighting the need for RSV as well as parainfluenza vaccines. While these vaccines should help all young children, they seem likely to be even more beneficial for high-risk children including those with NMD, and particularly those with more severe NMD.
Eleven among 60 total candidate RSV vaccines (live attenuated, particle based, or vector based) are currently in clinical trials.2 Fewer parainfluenza vaccines are in the pipeline, but clinical trials also are underway.3-5 Approval of such vaccines is not expected until the mid-2020s, so at present we are left with providing palivizumab to our vulnerable patients while emphasizing nonmedical strategies that may help prevent respiratory viruses. These only partially successful preventive interventions include breastfeeding, avoiding secondhand smoke, and avoiding known high-risk exposures, such as large day care centers.
My hope is for quicker than projected progress on the vaccine front so that winter admissions for respiratory viruses might decrease in numbers similar to the decrease we have noted with another vaccine successful against a seasonally active pathogen – rotavirus.
Dr. Harrison is professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospital–Kansas City, Mo. Children’s Mercy Hospital receives grant funding to study two candidate RSV vaccines. The hospital also receives CDC funding under the New Vaccine Surveillance Network for multicenter surveillance of acute respiratory infections, including influenza, RSV, and parainfluenza virus. Email Dr. Harrison at [email protected].
References
1. Pediatr Infect Dis J. 2019 Apr 10. doi: 10.1097/INF.0000000000002297.
2. “Advances in RSV Vaccine Research and Development – A Global Agenda.”
3. J Pediatric Infect Dis Soc. 2015 Dec;4(4): e143-6.
4. J Virol. 2015 Oct;89(20):10319-32.
5. Vaccine. 2017 Dec 18;35(51):7139-46.
This highlights the need for new vaccines
This highlights the need for new vaccines
Influenza gets a lot of attention each winter, but respiratory syncytial virus (RSV) and other respiratory viruses have as much or more impact on pediatric populations, particularly certain high-risk groups. But currently there are no vaccines for noninfluenza respiratory viruses. That said, several are under development, for RSV and parainfluenza.
Which groups are likely to get the most benefit from these newer vaccines?
We all are aware of the extra vulnerability to respiratory viruses (RSV being the most frequent) in premature infants, those with chronic lung disease, or those with congenital heart syndromes; such vulnerable patients are not infrequently seen in routine practice. A recent report shined a brighter light on such a group.
Real-world data from a nationwide Canadian surveillance system (CARESS) was used to analyze relative risks of categories of young children who are thought to be vulnerable to respiratory viruses, with a particular focus on those with neuromuscular disease. The CARESS investigators analyzed 12 years’ data on respiratory hospitalizations from among palivizumab-prophylaxed patients (including specific data on RSV when patients were tested for RSV per standard of care).1 Unfortunately, RSV testing was not universal despite hospitalization, so the true incidence of RSV-specific hospitalizations was likely underestimated.
Nevertheless, more than 25,000 children from 2005 through 2017 were grouped into three categories of palivizumab-prophylaxed high-risk children: standard indications (SI), n = 20,335; chronic medical conditions (CMD), n = 4,063; and neuromuscular disease (NMD), n = 605. This study is notable for having a relatively large number of neuromuscular disease subjects. Two-thirds of each group were fully palivizumab adherent.
The SI group included the standard American Academy of Pediatrics–recommended groups, such as premature infants, congenital heart disease, etc.
The CMD group included conditions that lead clinicians to use palivizumab off label, such as cystic fibrosis, congenital airway anomalies, immunodeficiency, and pulmonary disorders.
The NMD participants were subdivided into two groups. Group 1 comprised general hypotonic neuromuscular diseases such as hypoxic-ischemic encephalopathy, Prader-Willi syndrome, chromosomal disorders, and migration/demyelinating diseases. Group 2 included more severe infantile neuromuscular disorders, such as spinal muscular atrophy, myotonic dystrophy, centronuclear and nemaline myopathy, mitochondrial and glycogen storage myopathies, or arthrogryposis.
Overall, 6.9% of CARESS RSV-prophylaxed subjects were hospitalized. About one in five hospitalized patients from each group was hospitalized more than once. Specific respiratory hospitalization rates for each group were 6% (n = 1,228) for SI subjects and 9.4% (n = 380) for CMD, compared with 19.2% (n = 116) for NMD subjects.
It is unclear what proportion underwent RSV testing, but a total of 334 were confirmed RSV positive: 261 were SI, 54 were CMD and 19 were NMD. The RSV-test-positive rate was 1.5% for SI, 1.6% for CMD and 3.3% for NMD; so while a higher number of SI children were RSV positive, the rate of RSV positivity was actually highest with NMD.

RSV-positive subjects needing ICU care among NMD patients also had longer ICU stays (median 14 days), compared with RSV-positive CMD or SI subjects (median 3 and 5 days, respectively). Further, hospitalized RSV-positive NMD subjects presented more frequently with pneumonia (42% vs. 30% for CMD and 20% for SI) while hospitalized RSV-positive SI subjects more often had apnea (17% vs. 10% for NMD and 5% for CMD, P less than .05).
These differences in the courses of NMD patients raise the question as to whether the NMD group was somehow different from the SI and CMD groups, other than muscular weakness that likely leads to less ability to clear secretions and a less efficient cough. It turns out that NMD children were older and had worse neonatal medical courses (longer hospital stays, more often ventilated, and used oxygen longer). It could be argued that these differences may have been in part due to the muscular weakness inherent in their underlying disease, but they appear to be predictors of worse respiratory infectious disease than other vulnerable populations as the NMD children get older.
Indeed, the overall risk of any respiratory admission among NMD subjects was nearly twice as high, compared with SI (hazard ratio, 1.90, P less than .0005); but the somewhat higher risk for NMD vs. CMD was not significant (HR, 1.33, P = .090). However, when looking specifically at RSV confirmed admissions, NMD had more than twice the hospitalization risk than either other group (HR, 2.26, P = .001 vs. SI; and HR, 2.74, P = .001 vs. CMD).
Further, an NMD subgroup analysis showed 1.69 times the overall respiratory hospitalization risk among the more severe vs. less severe NMD group, but a similar risk of RSV admission. The authors point out that one reason for this discrepancy may be a higher probability of aspiration causing hospitalization because of more dramatic acute events during respiratory infections in patients with more severe NMD. It also may be that palivizumab evened the playing field for RSV but not for other viruses such as parainfluenza, adenovirus, or even rhinovirus.
Nevertheless, these data tell us that risk of respiratory disease severe enough to need hospitalization continues to an older age in NMD than SI or CMD patients, well past 2 years of age. And the risk is not only from RSV. That said, RSV remains a player in some patients (particularly NMD patients) despite palivizumab prophylaxis, highlighting the need for RSV as well as parainfluenza vaccines. While these vaccines should help all young children, they seem likely to be even more beneficial for high-risk children including those with NMD, and particularly those with more severe NMD.
Eleven among 60 total candidate RSV vaccines (live attenuated, particle based, or vector based) are currently in clinical trials.2 Fewer parainfluenza vaccines are in the pipeline, but clinical trials also are underway.3-5 Approval of such vaccines is not expected until the mid-2020s, so at present we are left with providing palivizumab to our vulnerable patients while emphasizing nonmedical strategies that may help prevent respiratory viruses. These only partially successful preventive interventions include breastfeeding, avoiding secondhand smoke, and avoiding known high-risk exposures, such as large day care centers.
My hope is for quicker than projected progress on the vaccine front so that winter admissions for respiratory viruses might decrease in numbers similar to the decrease we have noted with another vaccine successful against a seasonally active pathogen – rotavirus.
Dr. Harrison is professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospital–Kansas City, Mo. Children’s Mercy Hospital receives grant funding to study two candidate RSV vaccines. The hospital also receives CDC funding under the New Vaccine Surveillance Network for multicenter surveillance of acute respiratory infections, including influenza, RSV, and parainfluenza virus. Email Dr. Harrison at [email protected].
References
1. Pediatr Infect Dis J. 2019 Apr 10. doi: 10.1097/INF.0000000000002297.
2. “Advances in RSV Vaccine Research and Development – A Global Agenda.”
3. J Pediatric Infect Dis Soc. 2015 Dec;4(4): e143-6.
4. J Virol. 2015 Oct;89(20):10319-32.
5. Vaccine. 2017 Dec 18;35(51):7139-46.
Influenza gets a lot of attention each winter, but respiratory syncytial virus (RSV) and other respiratory viruses have as much or more impact on pediatric populations, particularly certain high-risk groups. But currently there are no vaccines for noninfluenza respiratory viruses. That said, several are under development, for RSV and parainfluenza.
Which groups are likely to get the most benefit from these newer vaccines?
We all are aware of the extra vulnerability to respiratory viruses (RSV being the most frequent) in premature infants, those with chronic lung disease, or those with congenital heart syndromes; such vulnerable patients are not infrequently seen in routine practice. A recent report shined a brighter light on such a group.
Real-world data from a nationwide Canadian surveillance system (CARESS) was used to analyze relative risks of categories of young children who are thought to be vulnerable to respiratory viruses, with a particular focus on those with neuromuscular disease. The CARESS investigators analyzed 12 years’ data on respiratory hospitalizations from among palivizumab-prophylaxed patients (including specific data on RSV when patients were tested for RSV per standard of care).1 Unfortunately, RSV testing was not universal despite hospitalization, so the true incidence of RSV-specific hospitalizations was likely underestimated.
Nevertheless, more than 25,000 children from 2005 through 2017 were grouped into three categories of palivizumab-prophylaxed high-risk children: standard indications (SI), n = 20,335; chronic medical conditions (CMD), n = 4,063; and neuromuscular disease (NMD), n = 605. This study is notable for having a relatively large number of neuromuscular disease subjects. Two-thirds of each group were fully palivizumab adherent.
The SI group included the standard American Academy of Pediatrics–recommended groups, such as premature infants, congenital heart disease, etc.
The CMD group included conditions that lead clinicians to use palivizumab off label, such as cystic fibrosis, congenital airway anomalies, immunodeficiency, and pulmonary disorders.
The NMD participants were subdivided into two groups. Group 1 comprised general hypotonic neuromuscular diseases such as hypoxic-ischemic encephalopathy, Prader-Willi syndrome, chromosomal disorders, and migration/demyelinating diseases. Group 2 included more severe infantile neuromuscular disorders, such as spinal muscular atrophy, myotonic dystrophy, centronuclear and nemaline myopathy, mitochondrial and glycogen storage myopathies, or arthrogryposis.
Overall, 6.9% of CARESS RSV-prophylaxed subjects were hospitalized. About one in five hospitalized patients from each group was hospitalized more than once. Specific respiratory hospitalization rates for each group were 6% (n = 1,228) for SI subjects and 9.4% (n = 380) for CMD, compared with 19.2% (n = 116) for NMD subjects.
It is unclear what proportion underwent RSV testing, but a total of 334 were confirmed RSV positive: 261 were SI, 54 were CMD and 19 were NMD. The RSV-test-positive rate was 1.5% for SI, 1.6% for CMD and 3.3% for NMD; so while a higher number of SI children were RSV positive, the rate of RSV positivity was actually highest with NMD.

RSV-positive subjects needing ICU care among NMD patients also had longer ICU stays (median 14 days), compared with RSV-positive CMD or SI subjects (median 3 and 5 days, respectively). Further, hospitalized RSV-positive NMD subjects presented more frequently with pneumonia (42% vs. 30% for CMD and 20% for SI) while hospitalized RSV-positive SI subjects more often had apnea (17% vs. 10% for NMD and 5% for CMD, P less than .05).
These differences in the courses of NMD patients raise the question as to whether the NMD group was somehow different from the SI and CMD groups, other than muscular weakness that likely leads to less ability to clear secretions and a less efficient cough. It turns out that NMD children were older and had worse neonatal medical courses (longer hospital stays, more often ventilated, and used oxygen longer). It could be argued that these differences may have been in part due to the muscular weakness inherent in their underlying disease, but they appear to be predictors of worse respiratory infectious disease than other vulnerable populations as the NMD children get older.
Indeed, the overall risk of any respiratory admission among NMD subjects was nearly twice as high, compared with SI (hazard ratio, 1.90, P less than .0005); but the somewhat higher risk for NMD vs. CMD was not significant (HR, 1.33, P = .090). However, when looking specifically at RSV confirmed admissions, NMD had more than twice the hospitalization risk than either other group (HR, 2.26, P = .001 vs. SI; and HR, 2.74, P = .001 vs. CMD).
Further, an NMD subgroup analysis showed 1.69 times the overall respiratory hospitalization risk among the more severe vs. less severe NMD group, but a similar risk of RSV admission. The authors point out that one reason for this discrepancy may be a higher probability of aspiration causing hospitalization because of more dramatic acute events during respiratory infections in patients with more severe NMD. It also may be that palivizumab evened the playing field for RSV but not for other viruses such as parainfluenza, adenovirus, or even rhinovirus.
Nevertheless, these data tell us that risk of respiratory disease severe enough to need hospitalization continues to an older age in NMD than SI or CMD patients, well past 2 years of age. And the risk is not only from RSV. That said, RSV remains a player in some patients (particularly NMD patients) despite palivizumab prophylaxis, highlighting the need for RSV as well as parainfluenza vaccines. While these vaccines should help all young children, they seem likely to be even more beneficial for high-risk children including those with NMD, and particularly those with more severe NMD.
Eleven among 60 total candidate RSV vaccines (live attenuated, particle based, or vector based) are currently in clinical trials.2 Fewer parainfluenza vaccines are in the pipeline, but clinical trials also are underway.3-5 Approval of such vaccines is not expected until the mid-2020s, so at present we are left with providing palivizumab to our vulnerable patients while emphasizing nonmedical strategies that may help prevent respiratory viruses. These only partially successful preventive interventions include breastfeeding, avoiding secondhand smoke, and avoiding known high-risk exposures, such as large day care centers.
My hope is for quicker than projected progress on the vaccine front so that winter admissions for respiratory viruses might decrease in numbers similar to the decrease we have noted with another vaccine successful against a seasonally active pathogen – rotavirus.
Dr. Harrison is professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospital–Kansas City, Mo. Children’s Mercy Hospital receives grant funding to study two candidate RSV vaccines. The hospital also receives CDC funding under the New Vaccine Surveillance Network for multicenter surveillance of acute respiratory infections, including influenza, RSV, and parainfluenza virus. Email Dr. Harrison at [email protected].
References
1. Pediatr Infect Dis J. 2019 Apr 10. doi: 10.1097/INF.0000000000002297.
2. “Advances in RSV Vaccine Research and Development – A Global Agenda.”
3. J Pediatric Infect Dis Soc. 2015 Dec;4(4): e143-6.
4. J Virol. 2015 Oct;89(20):10319-32.
5. Vaccine. 2017 Dec 18;35(51):7139-46.
Identifying CMV infection in asymptomatic newborns – one step closer?
Cytomegalovirus (CMV) infection is the most common congenital viral infection in U.S. children, with a frequency between 0.5% and 1% of newborn infants resulting in approximately 30,000 infected children annually. A small minority (approximately 10%) can be identified in the neonatal period as symptomatic with jaundice (from direct hyperbilirubinemia), petechiae (from thrombocytopenia), hepatosplenomegaly, microcephaly, or other manifestations. The vast majority are asymptomatic at birth, yet 15% will have or develop sensorineural hearing loss (SNHL) during the first few years of life; others (1%-2%) will develop vision loss associated with retinal scars. Congenital CMV accounts for 20% of those with SNHL detected at birth and 25% of children with SNHL at 4 years of age.
Screening for congenital CMV has been an ongoing subject of debate. The challenges of implementing screening programs are related both to the diagnostics (collecting urine samples on newborns) as well as with the question of whether we have treatment and interventions to offer babies diagnosed with congenital CMV across the complete spectrum of clinical presentations.
Current screening programs implemented in some hospitals, called “targeted screening,” in which babies who fail newborn screening programs are tested for CMV, are not sufficient to achieve the goal of identifying babies who will need follow-up for early detection of SNHL or vision abnormalities, or possibly early antiviral therapy (Valcyte; valganciclovir), because only a small portion of those who eventually develop SNHL are currently identified by the targeted screening programs.1
However, its availability only has added to the debate as to whether the time has arrived for universal screening.
Vertical transmission of CMV occurs in utero (during any of the trimesters), at birth by passage through the birth canal, or postnatally by ingestion of breast milk. Neonatal infection (in utero and postnatal) occurs in both mothers with primary CMV infection during gestation and in those with recurrent infection (from a different viral strain) or reactivation of infection. Severe clinically symptomatic disease and sequelae is associated with primary maternal infection and early transmission to the fetus. However, it is estimated that nonprimary maternal infection accounts for 75% of neonatal infections. Transmission by breast milk to full-term, healthy infants does not appear to be associated with clinical illness or sequelae; however, preterm infants or those with birth weights less than 1,500 g have a small risk of developing clinical disease.
The polymerase chain reaction–based saliva CMV test (Alethia CMV Assay Test System) was licensed by the Food and Drug Administration in November 2018 after studies demonstrated high sensitivity and specificity, compared with viral culture (the gold standard). In one study, 17,327 infants were screened with the liquid-saliva PCR assay, and 0.5% tested positive for CMV on both the saliva test and culture. Sensitivity and specificity of the liquid-saliva PCR assay were 100% and 99.9%, respectively.2 The availability of an approved saliva-based assay that is both highly sensitive and specific overcomes the challenge of collecting urine, which has been a limiting factor in development of pragmatic universal screening programs. To date, most of the focus in identification of congenital CMV infection has been linking newborn hearing testing programs with CMV testing. For some, these have been labeled “targeted screening programs for CMV.” To us, these appear to be best practice for medical evaluations of an infant with identified SNHL. The availability of saliva-based CMV testing should enable virtually all children who fail newborn screening to be tested for CMV. In multiple studies,3,4 6% of infants with confirmed hearing screen failure tested positive for CMV. A recent study5 identified only 1 infant among the 171 infants who failed newborn screening, however only approximately 15% of the infants were eventually confirmed as hearing impaired at audiology follow-up, suggesting that programmatically testing for CMV might be limited to those with confirmed hearing loss if such can be accomplished within a narrow window of time.
The major challenge with linking CMV testing with newborn hearing screening is whether treatment with valganciclovir would be of value in congenital CMV infection and isolated hearing loss. Studies of children with symptomatic central nervous system congenital CMV disease provide evidence of improvement (or lack of progression) in hearing loss in those treated with valganciclovir. Few, if any of these children had isolated hearing loss in this pivotal study.6 An observational study reported improved outcomes in 55 of 59 (93%) children with congenital CMV and isolated SNHL treated with valganciclovir between birth to 12 weeks of life.7 Hearing improved in nearly 70% of ears, 27% showed no change, and only 3% demonstrated progression of hearing loss; most of the improved ears returned to normal hearing. Currently, a National Institutes of Health study (ValEAR) is recruiting CMV-infected infants with isolated SNHL and randomizing them to treatment with valganciclovir or placebo. The goal is to determine if infants treated with valganciclovir will have better hearing and language outcomes.
Linking CMV testing to those who fail newborn hearing screening programs is an important step, as it appears such children are at least five times more likely to be infected with CMV than is the overall birth cohort. However, such strategies fall short of identifying the majority of newborns with congenital CMV infection, who are completely asymptomatic yet are at risk for development of complications that potentially have substantial impact on their quality of life. Although the availability of sensitive and specific PCR testing in saliva provides a pragmatic approach to identify infected children, many questions remain. First, would a confirmatory test be necessary, such as urine PCR (now considered the gold standard by many CMV experts)? Second, once identified, what regimen for follow-up testing would be indicated to identify those with early SNHL or retinopathy, and until what age? Third, is there a role for treatment in asymptomatic infection? Would that treatment be prophylactic, prior to the development of clinical signs, or implemented once early evidence of SNHL or retinopathy is present?
The Valgan Toddler study – sponsored by NIH and the University of Alabama as part of the Collaborative Antiviral Study Group – will enroll children who are aged 1 month through 3 years and who had a recent diagnosis of hearing loss (within the prior 12 weeks) and evidence of congenital CMV infection. The purpose of this study is to compare the effect on hearing and neurologic outcomes in infants aged 1 month through 4 years with recent onset SNHL who receive 6 weeks of valganciclovir versus children who do not receive this drug. The results of such studies will be critical for the development of best practices.
In summary, the licensure of a rapid PCR-based tool for diagnosis of CMV infection from saliva adds to our ability to develop screening programs to detect asymptomatic infants with congenital CMV infection. The ability to link newborns who fail hearing screening programs with CMV testing will lead to more detection of CMV-infected neonates, both with isolated hearing loss, and subsequently with no signs or symptoms of infection. There is an urgent need for evidence from randomized clinical trials to enable the development of best practices for such infants.
Dr. Pelton is professor of pediatrics and epidemiology at Boston University and senior attending physician at Boston Medical Center. Dr. Lapidot is a senior fellow in pediatric infectious diseases, Boston Medical Center. Neither Dr. Pelton nor Dr. Lapidot have any relevant financial disclosures. Email them at [email protected].
References
1. J Pediatric Infect Dis Soc. 2019 Mar 28;8(1):55-9.
2. N Engl J Med 2011 Jun 2; 364:2111-8.
3. Pediatrics. 2008 May;121(5):970-5
4. J Clin Virol. 2018 May;102:110-5.
5. J Pediatric Infect Dis Soc. 2019 Mar;8(1):55-9.
6. J Pediatr. 2003 Jul;143(1):16-25.
7. J Pediatr. 2018 Aug;199:166-70.
Cytomegalovirus (CMV) infection is the most common congenital viral infection in U.S. children, with a frequency between 0.5% and 1% of newborn infants resulting in approximately 30,000 infected children annually. A small minority (approximately 10%) can be identified in the neonatal period as symptomatic with jaundice (from direct hyperbilirubinemia), petechiae (from thrombocytopenia), hepatosplenomegaly, microcephaly, or other manifestations. The vast majority are asymptomatic at birth, yet 15% will have or develop sensorineural hearing loss (SNHL) during the first few years of life; others (1%-2%) will develop vision loss associated with retinal scars. Congenital CMV accounts for 20% of those with SNHL detected at birth and 25% of children with SNHL at 4 years of age.
Screening for congenital CMV has been an ongoing subject of debate. The challenges of implementing screening programs are related both to the diagnostics (collecting urine samples on newborns) as well as with the question of whether we have treatment and interventions to offer babies diagnosed with congenital CMV across the complete spectrum of clinical presentations.
Current screening programs implemented in some hospitals, called “targeted screening,” in which babies who fail newborn screening programs are tested for CMV, are not sufficient to achieve the goal of identifying babies who will need follow-up for early detection of SNHL or vision abnormalities, or possibly early antiviral therapy (Valcyte; valganciclovir), because only a small portion of those who eventually develop SNHL are currently identified by the targeted screening programs.1
However, its availability only has added to the debate as to whether the time has arrived for universal screening.
Vertical transmission of CMV occurs in utero (during any of the trimesters), at birth by passage through the birth canal, or postnatally by ingestion of breast milk. Neonatal infection (in utero and postnatal) occurs in both mothers with primary CMV infection during gestation and in those with recurrent infection (from a different viral strain) or reactivation of infection. Severe clinically symptomatic disease and sequelae is associated with primary maternal infection and early transmission to the fetus. However, it is estimated that nonprimary maternal infection accounts for 75% of neonatal infections. Transmission by breast milk to full-term, healthy infants does not appear to be associated with clinical illness or sequelae; however, preterm infants or those with birth weights less than 1,500 g have a small risk of developing clinical disease.
The polymerase chain reaction–based saliva CMV test (Alethia CMV Assay Test System) was licensed by the Food and Drug Administration in November 2018 after studies demonstrated high sensitivity and specificity, compared with viral culture (the gold standard). In one study, 17,327 infants were screened with the liquid-saliva PCR assay, and 0.5% tested positive for CMV on both the saliva test and culture. Sensitivity and specificity of the liquid-saliva PCR assay were 100% and 99.9%, respectively.2 The availability of an approved saliva-based assay that is both highly sensitive and specific overcomes the challenge of collecting urine, which has been a limiting factor in development of pragmatic universal screening programs. To date, most of the focus in identification of congenital CMV infection has been linking newborn hearing testing programs with CMV testing. For some, these have been labeled “targeted screening programs for CMV.” To us, these appear to be best practice for medical evaluations of an infant with identified SNHL. The availability of saliva-based CMV testing should enable virtually all children who fail newborn screening to be tested for CMV. In multiple studies,3,4 6% of infants with confirmed hearing screen failure tested positive for CMV. A recent study5 identified only 1 infant among the 171 infants who failed newborn screening, however only approximately 15% of the infants were eventually confirmed as hearing impaired at audiology follow-up, suggesting that programmatically testing for CMV might be limited to those with confirmed hearing loss if such can be accomplished within a narrow window of time.
The major challenge with linking CMV testing with newborn hearing screening is whether treatment with valganciclovir would be of value in congenital CMV infection and isolated hearing loss. Studies of children with symptomatic central nervous system congenital CMV disease provide evidence of improvement (or lack of progression) in hearing loss in those treated with valganciclovir. Few, if any of these children had isolated hearing loss in this pivotal study.6 An observational study reported improved outcomes in 55 of 59 (93%) children with congenital CMV and isolated SNHL treated with valganciclovir between birth to 12 weeks of life.7 Hearing improved in nearly 70% of ears, 27% showed no change, and only 3% demonstrated progression of hearing loss; most of the improved ears returned to normal hearing. Currently, a National Institutes of Health study (ValEAR) is recruiting CMV-infected infants with isolated SNHL and randomizing them to treatment with valganciclovir or placebo. The goal is to determine if infants treated with valganciclovir will have better hearing and language outcomes.
Linking CMV testing to those who fail newborn hearing screening programs is an important step, as it appears such children are at least five times more likely to be infected with CMV than is the overall birth cohort. However, such strategies fall short of identifying the majority of newborns with congenital CMV infection, who are completely asymptomatic yet are at risk for development of complications that potentially have substantial impact on their quality of life. Although the availability of sensitive and specific PCR testing in saliva provides a pragmatic approach to identify infected children, many questions remain. First, would a confirmatory test be necessary, such as urine PCR (now considered the gold standard by many CMV experts)? Second, once identified, what regimen for follow-up testing would be indicated to identify those with early SNHL or retinopathy, and until what age? Third, is there a role for treatment in asymptomatic infection? Would that treatment be prophylactic, prior to the development of clinical signs, or implemented once early evidence of SNHL or retinopathy is present?
The Valgan Toddler study – sponsored by NIH and the University of Alabama as part of the Collaborative Antiviral Study Group – will enroll children who are aged 1 month through 3 years and who had a recent diagnosis of hearing loss (within the prior 12 weeks) and evidence of congenital CMV infection. The purpose of this study is to compare the effect on hearing and neurologic outcomes in infants aged 1 month through 4 years with recent onset SNHL who receive 6 weeks of valganciclovir versus children who do not receive this drug. The results of such studies will be critical for the development of best practices.
In summary, the licensure of a rapid PCR-based tool for diagnosis of CMV infection from saliva adds to our ability to develop screening programs to detect asymptomatic infants with congenital CMV infection. The ability to link newborns who fail hearing screening programs with CMV testing will lead to more detection of CMV-infected neonates, both with isolated hearing loss, and subsequently with no signs or symptoms of infection. There is an urgent need for evidence from randomized clinical trials to enable the development of best practices for such infants.
Dr. Pelton is professor of pediatrics and epidemiology at Boston University and senior attending physician at Boston Medical Center. Dr. Lapidot is a senior fellow in pediatric infectious diseases, Boston Medical Center. Neither Dr. Pelton nor Dr. Lapidot have any relevant financial disclosures. Email them at [email protected].
References
1. J Pediatric Infect Dis Soc. 2019 Mar 28;8(1):55-9.
2. N Engl J Med 2011 Jun 2; 364:2111-8.
3. Pediatrics. 2008 May;121(5):970-5
4. J Clin Virol. 2018 May;102:110-5.
5. J Pediatric Infect Dis Soc. 2019 Mar;8(1):55-9.
6. J Pediatr. 2003 Jul;143(1):16-25.
7. J Pediatr. 2018 Aug;199:166-70.
Cytomegalovirus (CMV) infection is the most common congenital viral infection in U.S. children, with a frequency between 0.5% and 1% of newborn infants resulting in approximately 30,000 infected children annually. A small minority (approximately 10%) can be identified in the neonatal period as symptomatic with jaundice (from direct hyperbilirubinemia), petechiae (from thrombocytopenia), hepatosplenomegaly, microcephaly, or other manifestations. The vast majority are asymptomatic at birth, yet 15% will have or develop sensorineural hearing loss (SNHL) during the first few years of life; others (1%-2%) will develop vision loss associated with retinal scars. Congenital CMV accounts for 20% of those with SNHL detected at birth and 25% of children with SNHL at 4 years of age.
Screening for congenital CMV has been an ongoing subject of debate. The challenges of implementing screening programs are related both to the diagnostics (collecting urine samples on newborns) as well as with the question of whether we have treatment and interventions to offer babies diagnosed with congenital CMV across the complete spectrum of clinical presentations.
Current screening programs implemented in some hospitals, called “targeted screening,” in which babies who fail newborn screening programs are tested for CMV, are not sufficient to achieve the goal of identifying babies who will need follow-up for early detection of SNHL or vision abnormalities, or possibly early antiviral therapy (Valcyte; valganciclovir), because only a small portion of those who eventually develop SNHL are currently identified by the targeted screening programs.1
However, its availability only has added to the debate as to whether the time has arrived for universal screening.
Vertical transmission of CMV occurs in utero (during any of the trimesters), at birth by passage through the birth canal, or postnatally by ingestion of breast milk. Neonatal infection (in utero and postnatal) occurs in both mothers with primary CMV infection during gestation and in those with recurrent infection (from a different viral strain) or reactivation of infection. Severe clinically symptomatic disease and sequelae is associated with primary maternal infection and early transmission to the fetus. However, it is estimated that nonprimary maternal infection accounts for 75% of neonatal infections. Transmission by breast milk to full-term, healthy infants does not appear to be associated with clinical illness or sequelae; however, preterm infants or those with birth weights less than 1,500 g have a small risk of developing clinical disease.
The polymerase chain reaction–based saliva CMV test (Alethia CMV Assay Test System) was licensed by the Food and Drug Administration in November 2018 after studies demonstrated high sensitivity and specificity, compared with viral culture (the gold standard). In one study, 17,327 infants were screened with the liquid-saliva PCR assay, and 0.5% tested positive for CMV on both the saliva test and culture. Sensitivity and specificity of the liquid-saliva PCR assay were 100% and 99.9%, respectively.2 The availability of an approved saliva-based assay that is both highly sensitive and specific overcomes the challenge of collecting urine, which has been a limiting factor in development of pragmatic universal screening programs. To date, most of the focus in identification of congenital CMV infection has been linking newborn hearing testing programs with CMV testing. For some, these have been labeled “targeted screening programs for CMV.” To us, these appear to be best practice for medical evaluations of an infant with identified SNHL. The availability of saliva-based CMV testing should enable virtually all children who fail newborn screening to be tested for CMV. In multiple studies,3,4 6% of infants with confirmed hearing screen failure tested positive for CMV. A recent study5 identified only 1 infant among the 171 infants who failed newborn screening, however only approximately 15% of the infants were eventually confirmed as hearing impaired at audiology follow-up, suggesting that programmatically testing for CMV might be limited to those with confirmed hearing loss if such can be accomplished within a narrow window of time.
The major challenge with linking CMV testing with newborn hearing screening is whether treatment with valganciclovir would be of value in congenital CMV infection and isolated hearing loss. Studies of children with symptomatic central nervous system congenital CMV disease provide evidence of improvement (or lack of progression) in hearing loss in those treated with valganciclovir. Few, if any of these children had isolated hearing loss in this pivotal study.6 An observational study reported improved outcomes in 55 of 59 (93%) children with congenital CMV and isolated SNHL treated with valganciclovir between birth to 12 weeks of life.7 Hearing improved in nearly 70% of ears, 27% showed no change, and only 3% demonstrated progression of hearing loss; most of the improved ears returned to normal hearing. Currently, a National Institutes of Health study (ValEAR) is recruiting CMV-infected infants with isolated SNHL and randomizing them to treatment with valganciclovir or placebo. The goal is to determine if infants treated with valganciclovir will have better hearing and language outcomes.
Linking CMV testing to those who fail newborn hearing screening programs is an important step, as it appears such children are at least five times more likely to be infected with CMV than is the overall birth cohort. However, such strategies fall short of identifying the majority of newborns with congenital CMV infection, who are completely asymptomatic yet are at risk for development of complications that potentially have substantial impact on their quality of life. Although the availability of sensitive and specific PCR testing in saliva provides a pragmatic approach to identify infected children, many questions remain. First, would a confirmatory test be necessary, such as urine PCR (now considered the gold standard by many CMV experts)? Second, once identified, what regimen for follow-up testing would be indicated to identify those with early SNHL or retinopathy, and until what age? Third, is there a role for treatment in asymptomatic infection? Would that treatment be prophylactic, prior to the development of clinical signs, or implemented once early evidence of SNHL or retinopathy is present?
The Valgan Toddler study – sponsored by NIH and the University of Alabama as part of the Collaborative Antiviral Study Group – will enroll children who are aged 1 month through 3 years and who had a recent diagnosis of hearing loss (within the prior 12 weeks) and evidence of congenital CMV infection. The purpose of this study is to compare the effect on hearing and neurologic outcomes in infants aged 1 month through 4 years with recent onset SNHL who receive 6 weeks of valganciclovir versus children who do not receive this drug. The results of such studies will be critical for the development of best practices.
In summary, the licensure of a rapid PCR-based tool for diagnosis of CMV infection from saliva adds to our ability to develop screening programs to detect asymptomatic infants with congenital CMV infection. The ability to link newborns who fail hearing screening programs with CMV testing will lead to more detection of CMV-infected neonates, both with isolated hearing loss, and subsequently with no signs or symptoms of infection. There is an urgent need for evidence from randomized clinical trials to enable the development of best practices for such infants.
Dr. Pelton is professor of pediatrics and epidemiology at Boston University and senior attending physician at Boston Medical Center. Dr. Lapidot is a senior fellow in pediatric infectious diseases, Boston Medical Center. Neither Dr. Pelton nor Dr. Lapidot have any relevant financial disclosures. Email them at [email protected].
References
1. J Pediatric Infect Dis Soc. 2019 Mar 28;8(1):55-9.
2. N Engl J Med 2011 Jun 2; 364:2111-8.
3. Pediatrics. 2008 May;121(5):970-5
4. J Clin Virol. 2018 May;102:110-5.
5. J Pediatric Infect Dis Soc. 2019 Mar;8(1):55-9.
6. J Pediatr. 2003 Jul;143(1):16-25.
7. J Pediatr. 2018 Aug;199:166-70.
Adenovirus: More than just another viral illness
The mother of three looked tired and little worried. She wasn’t one to bring her kids to the pediatrician’s office with every minor illness, but her youngest had 3 days of fever, runny nose, cough, and little of her normal energy.
The pediatrician entered the room and smiled sympathetically.
“We ran tests for flu and RSV [respiratory syncytial virus] and it’s neither of those so. ...”
“So it’s just a virus that we don’t routinely test for and it’s going to need to run its course,” the mother finished his sentence. She knew the drill.
Before the doctor could leave the room though, the mother had one more question. “You don’t think it could be adenovirus do you?”
Most years, influenza and RSV command center stage, and adenovirus is relegated to the wings. It is not so much lack of disease or morbidity, but rather lack of recognition. Yes, we all learned in medical school that it is a cause of epidemic keratoconjunctivitis, but many adenoviral infections are clinically indistinguishable from infections caused by other viruses. Common symptoms – fever, cough, sore throat, and malaise – overlap with those caused by influenza. Like rhinovirus, adenovirus can cause common cold symptoms. Like RSV, it can cause bronchiolitis. Just like parainfluenza, it can cause croup. It can cause a pertussislike syndrome with prolonged cough, and enteric adenoviruses, especially types 40 and 41, cause gastroenteritis that mimics norovirus or rotavirus infection.
Testing for adenovirus is not readily available or routine in most pediatricians’ offices, and while many hospitals and reference labs offer adenovirus polymerase chain reaction testing as part of a comprehensive respiratory virus panel, the test can be expensive and unlikely to change management in most ambulatory patients. This makes it difficult to count the number of adenoviruses annually.
This winter though, adenovirus was in the news ... repeatedly. In November 2018, CBS News reported that a University of Maryland freshman had died of an adenovirus-related illness. The family of Olivia Paregol told reporters that she was being treated for Crohn’s disease. Immune suppression is one recognized risk factor for more severe adenoviral disease; underlying heart and lung disease are others. Testing at the Centers for Disease Control and Prevention revealed that the student and several others on campus were infected with adenovirus type 7, a strain that has been associated with outbreaks of acute, severe respiratory illness in military recruits. As of Jan. 24, 2019, university officials reported 42 confirmed cases of adenovirus in University of Maryland students, 13 of which were confirmed as adenovirus 7.
Adenovirus type 7 also caused an outbreak at a pediatric long-term care facility in New Jersey late last year. Between Sept. 26 and Nov. 11, 2018, 36 residents and 1 staff member became ill. Eleven individuals died. In an unrelated outbreak at a second pediatric long-term care facility, 17 residents were affected between Oct. 20 and Dec. 10, 2018. Adenovirus 3 was identified and all children recovered.
Between October 2013 and July 2014, public health officials in Oregon identified an increase in adenoviral infections in people with respiratory illness. Sixty-nine percent were hospitalized (136/198), 31% needed intensive care, and 18% were mechanically ventilated. Multiple types of adenovirus were recovered but the most common was adenovirus 7 (Emerg Infect Dis. 2016. doi: 10.3201/eid2206.151898).
Depending on your perspective, measures to prevent the spread of adenovirus are elegantly simple, evidence-based, public health intervention or maddeningly little more than common sense. Wash your hands often with soap and water. Avoid touching your eyes, mouth, and nose with unwashed hands. Avoid close contact with people who are sick. The latter is easier if those who are sick stay home. Prior to the start of the most recent academic semester at the University of Maryland, university officials urged students who were sick not to return to campus but to stay at home to rest and recover. Those who fell ill on campus were urged to return home via nonpublic transportation if possible. Those who stayed on campus were advised to stay in their living spaces and clean high-touch surfaces with bleach. Like other nonenveloped viruses, adenovirus is not easily destroyed by many commonly used disinfectants. Under ideal conditions, it can survive on surfaces – remaining infectious – for up to 3 months.
Back at the pediatrician’s office, “We need an adenovirus vaccine,” the mother said as she picked up her child and headed for the door.
There is, in fact, a live oral vaccine that protects against adenovirus types 4 and 7. It is only approved for use in United States military personnel aged 17-50 years and it is given to all recruits as soon as they enter basic training. It works too. Before vaccine was available, up to 80% of recruits became infected during their initial training, half of those developing significant illness and a quarter being hospitalized. When the current vaccine was introduced in 2011, there was a 100-fold decrease in adenovirus-related disease burden (from 5.8 to 0.02 cases per 1,000 person-weeks, P less than .0001). That translates to 1 death, 1,100-2,700 hospitalizations and 13,000 febrile illnesses prevented each year (Clin Infect Dis. 2014 Oct 1. doi: 10.1093/cid/ciu507).
Some experts have suggested that adenovirus vaccine could be useful in civilian populations, too, but I question what the public reception would be. We have safe influenza vaccines that reduce the need for hospitalization and reduce mortality from influenza, but we still can’t convince some people to immunize themselves and their children. In the last 4 years, flu vaccination rates among children have remained just shy of 60% and adult rates are even lower. Collectively, we don’t seem to be ready to relinquish – or at least diminish – the annual suffering that goes with flu. I have to wonder if the same would be true for adenovirus.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She said she had no relevant financial disclosures. Email her at [email protected].
The mother of three looked tired and little worried. She wasn’t one to bring her kids to the pediatrician’s office with every minor illness, but her youngest had 3 days of fever, runny nose, cough, and little of her normal energy.
The pediatrician entered the room and smiled sympathetically.
“We ran tests for flu and RSV [respiratory syncytial virus] and it’s neither of those so. ...”
“So it’s just a virus that we don’t routinely test for and it’s going to need to run its course,” the mother finished his sentence. She knew the drill.
Before the doctor could leave the room though, the mother had one more question. “You don’t think it could be adenovirus do you?”
Most years, influenza and RSV command center stage, and adenovirus is relegated to the wings. It is not so much lack of disease or morbidity, but rather lack of recognition. Yes, we all learned in medical school that it is a cause of epidemic keratoconjunctivitis, but many adenoviral infections are clinically indistinguishable from infections caused by other viruses. Common symptoms – fever, cough, sore throat, and malaise – overlap with those caused by influenza. Like rhinovirus, adenovirus can cause common cold symptoms. Like RSV, it can cause bronchiolitis. Just like parainfluenza, it can cause croup. It can cause a pertussislike syndrome with prolonged cough, and enteric adenoviruses, especially types 40 and 41, cause gastroenteritis that mimics norovirus or rotavirus infection.
Testing for adenovirus is not readily available or routine in most pediatricians’ offices, and while many hospitals and reference labs offer adenovirus polymerase chain reaction testing as part of a comprehensive respiratory virus panel, the test can be expensive and unlikely to change management in most ambulatory patients. This makes it difficult to count the number of adenoviruses annually.
This winter though, adenovirus was in the news ... repeatedly. In November 2018, CBS News reported that a University of Maryland freshman had died of an adenovirus-related illness. The family of Olivia Paregol told reporters that she was being treated for Crohn’s disease. Immune suppression is one recognized risk factor for more severe adenoviral disease; underlying heart and lung disease are others. Testing at the Centers for Disease Control and Prevention revealed that the student and several others on campus were infected with adenovirus type 7, a strain that has been associated with outbreaks of acute, severe respiratory illness in military recruits. As of Jan. 24, 2019, university officials reported 42 confirmed cases of adenovirus in University of Maryland students, 13 of which were confirmed as adenovirus 7.
Adenovirus type 7 also caused an outbreak at a pediatric long-term care facility in New Jersey late last year. Between Sept. 26 and Nov. 11, 2018, 36 residents and 1 staff member became ill. Eleven individuals died. In an unrelated outbreak at a second pediatric long-term care facility, 17 residents were affected between Oct. 20 and Dec. 10, 2018. Adenovirus 3 was identified and all children recovered.
Between October 2013 and July 2014, public health officials in Oregon identified an increase in adenoviral infections in people with respiratory illness. Sixty-nine percent were hospitalized (136/198), 31% needed intensive care, and 18% were mechanically ventilated. Multiple types of adenovirus were recovered but the most common was adenovirus 7 (Emerg Infect Dis. 2016. doi: 10.3201/eid2206.151898).
Depending on your perspective, measures to prevent the spread of adenovirus are elegantly simple, evidence-based, public health intervention or maddeningly little more than common sense. Wash your hands often with soap and water. Avoid touching your eyes, mouth, and nose with unwashed hands. Avoid close contact with people who are sick. The latter is easier if those who are sick stay home. Prior to the start of the most recent academic semester at the University of Maryland, university officials urged students who were sick not to return to campus but to stay at home to rest and recover. Those who fell ill on campus were urged to return home via nonpublic transportation if possible. Those who stayed on campus were advised to stay in their living spaces and clean high-touch surfaces with bleach. Like other nonenveloped viruses, adenovirus is not easily destroyed by many commonly used disinfectants. Under ideal conditions, it can survive on surfaces – remaining infectious – for up to 3 months.
Back at the pediatrician’s office, “We need an adenovirus vaccine,” the mother said as she picked up her child and headed for the door.
There is, in fact, a live oral vaccine that protects against adenovirus types 4 and 7. It is only approved for use in United States military personnel aged 17-50 years and it is given to all recruits as soon as they enter basic training. It works too. Before vaccine was available, up to 80% of recruits became infected during their initial training, half of those developing significant illness and a quarter being hospitalized. When the current vaccine was introduced in 2011, there was a 100-fold decrease in adenovirus-related disease burden (from 5.8 to 0.02 cases per 1,000 person-weeks, P less than .0001). That translates to 1 death, 1,100-2,700 hospitalizations and 13,000 febrile illnesses prevented each year (Clin Infect Dis. 2014 Oct 1. doi: 10.1093/cid/ciu507).
Some experts have suggested that adenovirus vaccine could be useful in civilian populations, too, but I question what the public reception would be. We have safe influenza vaccines that reduce the need for hospitalization and reduce mortality from influenza, but we still can’t convince some people to immunize themselves and their children. In the last 4 years, flu vaccination rates among children have remained just shy of 60% and adult rates are even lower. Collectively, we don’t seem to be ready to relinquish – or at least diminish – the annual suffering that goes with flu. I have to wonder if the same would be true for adenovirus.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She said she had no relevant financial disclosures. Email her at [email protected].
The mother of three looked tired and little worried. She wasn’t one to bring her kids to the pediatrician’s office with every minor illness, but her youngest had 3 days of fever, runny nose, cough, and little of her normal energy.
The pediatrician entered the room and smiled sympathetically.
“We ran tests for flu and RSV [respiratory syncytial virus] and it’s neither of those so. ...”
“So it’s just a virus that we don’t routinely test for and it’s going to need to run its course,” the mother finished his sentence. She knew the drill.
Before the doctor could leave the room though, the mother had one more question. “You don’t think it could be adenovirus do you?”
Most years, influenza and RSV command center stage, and adenovirus is relegated to the wings. It is not so much lack of disease or morbidity, but rather lack of recognition. Yes, we all learned in medical school that it is a cause of epidemic keratoconjunctivitis, but many adenoviral infections are clinically indistinguishable from infections caused by other viruses. Common symptoms – fever, cough, sore throat, and malaise – overlap with those caused by influenza. Like rhinovirus, adenovirus can cause common cold symptoms. Like RSV, it can cause bronchiolitis. Just like parainfluenza, it can cause croup. It can cause a pertussislike syndrome with prolonged cough, and enteric adenoviruses, especially types 40 and 41, cause gastroenteritis that mimics norovirus or rotavirus infection.
Testing for adenovirus is not readily available or routine in most pediatricians’ offices, and while many hospitals and reference labs offer adenovirus polymerase chain reaction testing as part of a comprehensive respiratory virus panel, the test can be expensive and unlikely to change management in most ambulatory patients. This makes it difficult to count the number of adenoviruses annually.
This winter though, adenovirus was in the news ... repeatedly. In November 2018, CBS News reported that a University of Maryland freshman had died of an adenovirus-related illness. The family of Olivia Paregol told reporters that she was being treated for Crohn’s disease. Immune suppression is one recognized risk factor for more severe adenoviral disease; underlying heart and lung disease are others. Testing at the Centers for Disease Control and Prevention revealed that the student and several others on campus were infected with adenovirus type 7, a strain that has been associated with outbreaks of acute, severe respiratory illness in military recruits. As of Jan. 24, 2019, university officials reported 42 confirmed cases of adenovirus in University of Maryland students, 13 of which were confirmed as adenovirus 7.
Adenovirus type 7 also caused an outbreak at a pediatric long-term care facility in New Jersey late last year. Between Sept. 26 and Nov. 11, 2018, 36 residents and 1 staff member became ill. Eleven individuals died. In an unrelated outbreak at a second pediatric long-term care facility, 17 residents were affected between Oct. 20 and Dec. 10, 2018. Adenovirus 3 was identified and all children recovered.
Between October 2013 and July 2014, public health officials in Oregon identified an increase in adenoviral infections in people with respiratory illness. Sixty-nine percent were hospitalized (136/198), 31% needed intensive care, and 18% were mechanically ventilated. Multiple types of adenovirus were recovered but the most common was adenovirus 7 (Emerg Infect Dis. 2016. doi: 10.3201/eid2206.151898).
Depending on your perspective, measures to prevent the spread of adenovirus are elegantly simple, evidence-based, public health intervention or maddeningly little more than common sense. Wash your hands often with soap and water. Avoid touching your eyes, mouth, and nose with unwashed hands. Avoid close contact with people who are sick. The latter is easier if those who are sick stay home. Prior to the start of the most recent academic semester at the University of Maryland, university officials urged students who were sick not to return to campus but to stay at home to rest and recover. Those who fell ill on campus were urged to return home via nonpublic transportation if possible. Those who stayed on campus were advised to stay in their living spaces and clean high-touch surfaces with bleach. Like other nonenveloped viruses, adenovirus is not easily destroyed by many commonly used disinfectants. Under ideal conditions, it can survive on surfaces – remaining infectious – for up to 3 months.
Back at the pediatrician’s office, “We need an adenovirus vaccine,” the mother said as she picked up her child and headed for the door.
There is, in fact, a live oral vaccine that protects against adenovirus types 4 and 7. It is only approved for use in United States military personnel aged 17-50 years and it is given to all recruits as soon as they enter basic training. It works too. Before vaccine was available, up to 80% of recruits became infected during their initial training, half of those developing significant illness and a quarter being hospitalized. When the current vaccine was introduced in 2011, there was a 100-fold decrease in adenovirus-related disease burden (from 5.8 to 0.02 cases per 1,000 person-weeks, P less than .0001). That translates to 1 death, 1,100-2,700 hospitalizations and 13,000 febrile illnesses prevented each year (Clin Infect Dis. 2014 Oct 1. doi: 10.1093/cid/ciu507).
Some experts have suggested that adenovirus vaccine could be useful in civilian populations, too, but I question what the public reception would be. We have safe influenza vaccines that reduce the need for hospitalization and reduce mortality from influenza, but we still can’t convince some people to immunize themselves and their children. In the last 4 years, flu vaccination rates among children have remained just shy of 60% and adult rates are even lower. Collectively, we don’t seem to be ready to relinquish – or at least diminish – the annual suffering that goes with flu. I have to wonder if the same would be true for adenovirus.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She said she had no relevant financial disclosures. Email her at [email protected].
Children who are coughing: Is it flu or bacterial pneumonia?
We are in the middle of flu season, and many of our patients are coughing. Is it the flu or might the child have a secondary bacterial pneumonia? Let’s start with the history for a tip off. The course of flu and respiratory viral infections in general involves a typical pattern of timing for fever and cough.
A late-developing fever or fever that subsides then recurs should raise concern. A prolonged cough or cough that subsides then recurs also should raise concern. The respiratory rate and chest retractions are key physical findings that can aid in distinguishing children with bacterial pneumonia. Rales and decreased breath sounds in lung segments are best heard with deep breaths.
What diagnostic laboratory and imaging tests should be used
Fortunately, rapid tests to detect influenza are available, and many providers have added those to their laboratory evaluation. A complete blood count and differential may be helpful. If a pulse oximeter is available, checking oxygen saturation might be helpful. The American Academy of Pediatrics community pneumonia guideline states that routine chest radiographs are not necessary for the confirmation of suspected community-acquired pneumonia (CAP) in patients well enough to be treated in the outpatient setting (Clin Inf Dis. 2011 Oct;53[7]:e25–e76). Blood cultures should not be performed routinely in nontoxic, fully immunized children with CAP managed in the outpatient setting.
What antibiotic should be used
Antimicrobial therapy is not routinely required for preschool-aged children with cough, even cough caused by CAP, because viral pathogens are responsible for the great majority of clinical disease. If the diagnosis of CAP is made, the AAP endorses amoxicillin as first-line therapy for previously healthy, appropriately immunized infants and preschool children with mild to moderate CAP suspected to be of bacterial origin. For previously healthy, appropriately immunized school-aged children and adolescents with mild to moderate CAP, amoxicillin is recommended for treatment of Streptococcus pneumoniae, the most prominent invasive bacterial pathogen.
However, the treatment paradigm is complicated because Mycoplasma pneumoniae also should be considered in management decisions. Children with signs and symptoms suspicious for M. pneumoniae should be tested to help guide antibiotic selection. This may be a simple bedside cold agglutinin test. The highest incidence of Mycoplasma pneumonia is in 5- to 20-year-olds (51% in 5- to 9-year-olds, 74% in 9- to 15-year-olds, and 3%-18% in adults with pneumonia), but 9% of CAP occurs in patients younger than 5 years old. The clinical features of Mycoplasma pneumonia resemble influenza: The patient has gradual onset of headache, malaise, fever, sore throat, and cough. Mycoplasma pneumonia has a similar incidence of productive cough, rales, and diarrhea as pneumococcal CAP, but with more frequent upper respiratory symptoms and a normal leukocyte count. Mycoplasma bronchopneumonia occurs 30 times more frequently than Mycoplasma lobar pneumonia. The radiologic features of Mycoplasma is typical of a bronchopneumonia, usually involving a single lobe, subsegmental atelectasis, peribronchial thickening, and streaky interstitial densities. While Mycoplasma pneumonia is usually self-limited, the duration of illness is shortened by oral treatment with doxycycline, erythromycin, clarithromycin, or azithromycin.
What is the appropriate duration of antimicrobial therapy
Recommendations by the AAP for CAP note that treatment courses of 10 days have been best studied, although shorter courses may be just as effective, particularly for mild disease managed on an outpatient basis.
When should children be hospitalized
Dr. Pichichero is a specialist in pediatric infectious diseases and director of the Research Institute at Rochester (N.Y.) General Hospital. He had no conflicts to declare. Email him at [email protected].
We are in the middle of flu season, and many of our patients are coughing. Is it the flu or might the child have a secondary bacterial pneumonia? Let’s start with the history for a tip off. The course of flu and respiratory viral infections in general involves a typical pattern of timing for fever and cough.
A late-developing fever or fever that subsides then recurs should raise concern. A prolonged cough or cough that subsides then recurs also should raise concern. The respiratory rate and chest retractions are key physical findings that can aid in distinguishing children with bacterial pneumonia. Rales and decreased breath sounds in lung segments are best heard with deep breaths.
What diagnostic laboratory and imaging tests should be used
Fortunately, rapid tests to detect influenza are available, and many providers have added those to their laboratory evaluation. A complete blood count and differential may be helpful. If a pulse oximeter is available, checking oxygen saturation might be helpful. The American Academy of Pediatrics community pneumonia guideline states that routine chest radiographs are not necessary for the confirmation of suspected community-acquired pneumonia (CAP) in patients well enough to be treated in the outpatient setting (Clin Inf Dis. 2011 Oct;53[7]:e25–e76). Blood cultures should not be performed routinely in nontoxic, fully immunized children with CAP managed in the outpatient setting.
What antibiotic should be used
Antimicrobial therapy is not routinely required for preschool-aged children with cough, even cough caused by CAP, because viral pathogens are responsible for the great majority of clinical disease. If the diagnosis of CAP is made, the AAP endorses amoxicillin as first-line therapy for previously healthy, appropriately immunized infants and preschool children with mild to moderate CAP suspected to be of bacterial origin. For previously healthy, appropriately immunized school-aged children and adolescents with mild to moderate CAP, amoxicillin is recommended for treatment of Streptococcus pneumoniae, the most prominent invasive bacterial pathogen.
However, the treatment paradigm is complicated because Mycoplasma pneumoniae also should be considered in management decisions. Children with signs and symptoms suspicious for M. pneumoniae should be tested to help guide antibiotic selection. This may be a simple bedside cold agglutinin test. The highest incidence of Mycoplasma pneumonia is in 5- to 20-year-olds (51% in 5- to 9-year-olds, 74% in 9- to 15-year-olds, and 3%-18% in adults with pneumonia), but 9% of CAP occurs in patients younger than 5 years old. The clinical features of Mycoplasma pneumonia resemble influenza: The patient has gradual onset of headache, malaise, fever, sore throat, and cough. Mycoplasma pneumonia has a similar incidence of productive cough, rales, and diarrhea as pneumococcal CAP, but with more frequent upper respiratory symptoms and a normal leukocyte count. Mycoplasma bronchopneumonia occurs 30 times more frequently than Mycoplasma lobar pneumonia. The radiologic features of Mycoplasma is typical of a bronchopneumonia, usually involving a single lobe, subsegmental atelectasis, peribronchial thickening, and streaky interstitial densities. While Mycoplasma pneumonia is usually self-limited, the duration of illness is shortened by oral treatment with doxycycline, erythromycin, clarithromycin, or azithromycin.
What is the appropriate duration of antimicrobial therapy
Recommendations by the AAP for CAP note that treatment courses of 10 days have been best studied, although shorter courses may be just as effective, particularly for mild disease managed on an outpatient basis.
When should children be hospitalized
Dr. Pichichero is a specialist in pediatric infectious diseases and director of the Research Institute at Rochester (N.Y.) General Hospital. He had no conflicts to declare. Email him at [email protected].
We are in the middle of flu season, and many of our patients are coughing. Is it the flu or might the child have a secondary bacterial pneumonia? Let’s start with the history for a tip off. The course of flu and respiratory viral infections in general involves a typical pattern of timing for fever and cough.
A late-developing fever or fever that subsides then recurs should raise concern. A prolonged cough or cough that subsides then recurs also should raise concern. The respiratory rate and chest retractions are key physical findings that can aid in distinguishing children with bacterial pneumonia. Rales and decreased breath sounds in lung segments are best heard with deep breaths.
What diagnostic laboratory and imaging tests should be used
Fortunately, rapid tests to detect influenza are available, and many providers have added those to their laboratory evaluation. A complete blood count and differential may be helpful. If a pulse oximeter is available, checking oxygen saturation might be helpful. The American Academy of Pediatrics community pneumonia guideline states that routine chest radiographs are not necessary for the confirmation of suspected community-acquired pneumonia (CAP) in patients well enough to be treated in the outpatient setting (Clin Inf Dis. 2011 Oct;53[7]:e25–e76). Blood cultures should not be performed routinely in nontoxic, fully immunized children with CAP managed in the outpatient setting.
What antibiotic should be used
Antimicrobial therapy is not routinely required for preschool-aged children with cough, even cough caused by CAP, because viral pathogens are responsible for the great majority of clinical disease. If the diagnosis of CAP is made, the AAP endorses amoxicillin as first-line therapy for previously healthy, appropriately immunized infants and preschool children with mild to moderate CAP suspected to be of bacterial origin. For previously healthy, appropriately immunized school-aged children and adolescents with mild to moderate CAP, amoxicillin is recommended for treatment of Streptococcus pneumoniae, the most prominent invasive bacterial pathogen.
However, the treatment paradigm is complicated because Mycoplasma pneumoniae also should be considered in management decisions. Children with signs and symptoms suspicious for M. pneumoniae should be tested to help guide antibiotic selection. This may be a simple bedside cold agglutinin test. The highest incidence of Mycoplasma pneumonia is in 5- to 20-year-olds (51% in 5- to 9-year-olds, 74% in 9- to 15-year-olds, and 3%-18% in adults with pneumonia), but 9% of CAP occurs in patients younger than 5 years old. The clinical features of Mycoplasma pneumonia resemble influenza: The patient has gradual onset of headache, malaise, fever, sore throat, and cough. Mycoplasma pneumonia has a similar incidence of productive cough, rales, and diarrhea as pneumococcal CAP, but with more frequent upper respiratory symptoms and a normal leukocyte count. Mycoplasma bronchopneumonia occurs 30 times more frequently than Mycoplasma lobar pneumonia. The radiologic features of Mycoplasma is typical of a bronchopneumonia, usually involving a single lobe, subsegmental atelectasis, peribronchial thickening, and streaky interstitial densities. While Mycoplasma pneumonia is usually self-limited, the duration of illness is shortened by oral treatment with doxycycline, erythromycin, clarithromycin, or azithromycin.
What is the appropriate duration of antimicrobial therapy
Recommendations by the AAP for CAP note that treatment courses of 10 days have been best studied, although shorter courses may be just as effective, particularly for mild disease managed on an outpatient basis.
When should children be hospitalized
Dr. Pichichero is a specialist in pediatric infectious diseases and director of the Research Institute at Rochester (N.Y.) General Hospital. He had no conflicts to declare. Email him at [email protected].
Uptick in adult syphilis means congenital syphilis may be lurking
While many pediatric clinicians have not frequently managed newborns of mothers with reactive syphilis serology, increased adult syphilis may change that.1
Diagnosing/managing congenital syphilis is not always clear cut. A positive rapid plasma reagin (RPR) titer in a newborn may not indicate congenital infection but merely may reflect transplacental, passively acquired maternal IgG from the mother’s current or previous infection rather than antibodies produced by the newborn. Because currently no IgM assay for syphilis is recommended by the Centers for Disease Control and Prevention for newborn testing, we must deal with IgG test results.
Often initial management decisions are needed while the infant’s status is evolving. The questions to answer to make final decisions include the following2:
- Was the mother actively infected with Treponema pallidum during pregnancy?
- If so, was the mother appropriately treated and when?
- Does the infant have any clinical, laboratory, or radiographic evidence of syphilis?
- How do the mother’s and infant’s nontreponemal serologic titers (NTT) compare at delivery using the same test?
Note: All infants assessed for congenital syphilis need a full evaluation for HIV.
Managing the infant of a mother with positive tests3,4
All such neonates need an examination for evidence of congenital syphilis. The clinical signs of congenital syphilis in neonates include nonimmune hydrops, jaundice, hepatosplenomegaly, rhinitis, skin rash, and pseudoparalysis of extremity. Also, consider dark-field examination or polymerase chain reaction (PCR) of lesions (such as bullae) or secretions (nasal). If available, have the placenta examined histologically (silver stain) or by PCR (Clinical Laboratory Improvement Amendments–validated test). Skeletal radiographic surveys are more useful for stillborn than live born infants. (The complete algorithm can be found in Figure 3.10 of reference 4.)
Order a quantitative NTT, using the Venereal Disease Research Laboratory (VDRL) test or RPR test on neonatal serum. Umbilical cord blood is not appropriate because of potential maternal blood contamination, which could give a false-positive result, or Wharton’s jelly, which could give a false-negative result. Use of treponemal-specific tests that are used for maternal diagnosis – such as T. pallidum particle agglutination (TP-PA), T. pallidum enzyme-linked immunosorbent assay (TP-EIA), fluorescent treponemal antibody absorption (FTA-ABS) test, or T. pallidum chemiluminescence immunoassay (TP-CIA) – on neonatal serum is not recommended because of difficulties in interpretation.
Diagnostic results allow designation of an infant into one of four CDC categories: proven/highly probable syphilis; possible syphilis; syphilis less likely; and syphilis unlikely. Treatment recommendations are based on these categories.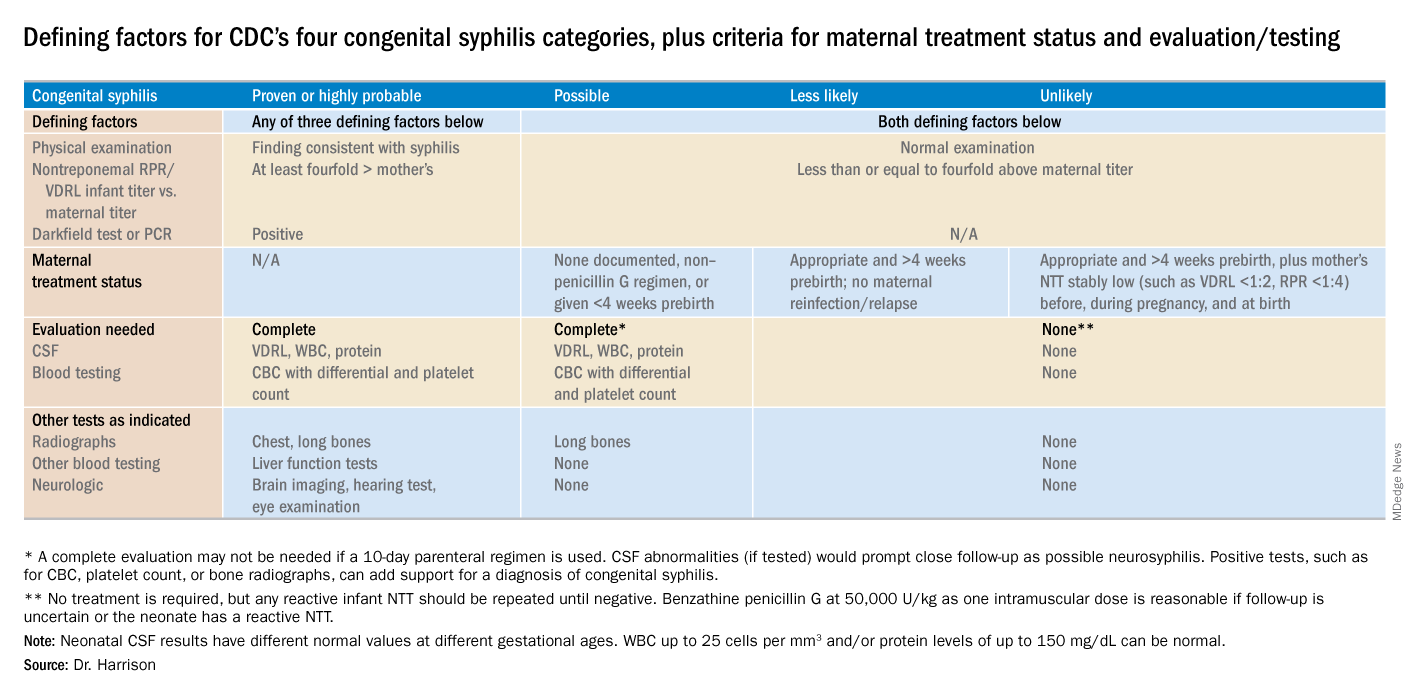
Proven or highly probable syphilis
There are two alternative recommended 10-day treatment regimens.
A. Aqueous crystalline penicillin G 100,000-150,000 U/kg per day by IV at 50,000 U/kg per dose, given every 12 hours through 7 days of age or every 8 hours if greater than 7 days old.
B. Procaine penicillin G at 50,000 U/kg per dose intramuscularly in one dose each day.
More than 1 day of missed therapy requires restarting a new 10-day course. Use of other antimicrobial agents (such as ampicillin) is not validated, so any empiric ampicillin initially given for possible sepsis does not count toward the 10-day penicillin regimen. If nonpenicillin drugs must be used, close serologic follow-up must occur to ensure adequacy of response to therapy.
Possible syphilis
There are three alternative regimens, the same two as in proven/highly probable syphilis (above) plus a single-dose option
A. Aqueous crystalline penicillin G, as described above.
B. Procaine penicillin G, as described above.
C. Benzathine penicillin G at 50,000 U/kg per dose intramuscularly in a single dose.
Note: To be eligible for regimen C, an infant must have a complete evaluation that is normal (cerebrospinal fluid [CSF] examination, long-bone radiographs, and complete blood count with platelet count) and follow-up must be assured. Exception: Neonates born to mothers with untreated early syphilis at the time of delivery are at increased risk for congenital syphilis, and the 10-day course of penicillin G may be considered even if the complete evaluation is normal and follow-up is certain.
Less likely syphilis
One antibiotic regimen is available, but no treatment also may be an option.
A. Benzathine penicillin G as described above.
B. If mother’s NTT has decreased at least fourfold after appropriate early syphilis therapy or remained stably low, which indicates latent syphilis (VDRL less than 1:2; RPR less than 1:4), no treatment is an option but requires repeat serology every 2-3 months until infant is 6 months old.
Unlikely syphilis
No treatment is recommended unless follow-up is uncertain, in which case it is appropriate to give the infant benzathine penicillin G as described above.
Infant with positive NTT at birth
All neonates with reactive NTT need careful follow-up examinations and repeat NTT every 2-3 months until nonreactive. NTT in infants who are not treated because of less likely or unlikely syphilis status should drop by 3 months and be nonreactive by 6 months; this indicates NTT was passively transferred maternal IgG. If NTT remains reactive at 6 months, the infant is likely infected and needs treatment. Persistent NTT at 6-12 months in treated neonates should trigger repeat CSF examination and infectious diseases consultation about a possible repeat of the 10-day penicillin G regimen. If the mother was seroreactive, but the newborn’s NTT was negative at birth, testing of the infant’s NTT needs repeating at 3 months to exclude the possibility that the congenital syphilis was incubating when prior testing occurred at birth. Note: Treponemal-specific tests are not useful in assessing treatment because detectable maternal IgG treponemal antibody can persist at least 15 months.
Neonates with abnormal CSF at birth
Repeat cerebrospinal fluid evaluation every 6 months until results normalize. Persistently reactive CSF VDRL or abnormal CSF indexes not caused by another known cause requires retreatment for possible neurosyphilis, as well as consultation with an expert.
Summary
NTT are the essential test for newborns and some degree of laboratory or imaging work up often are needed. Consider consulting an expert in infectious diseases and/or perinatology if the gray areas do not readily become clear. Treatment of the correct patients with the right drug for the right duration remains the goal, as usual.
Dr. Harrison is a professor of pediatrics at University of Missouri-Kansas City and Director of Research Affairs in the pediatric infectious diseases division at Children’s Mercy Hospital – Kansas City. He said he had no relevant financial disclosures. Email him at [email protected].
References
1. MMWR. 2015 Nov 13;64(44);1241-5.
2. “Congenital Syphilis,” 2015 Sexually Transmitted Diseases Treatment Guidelines.
3. “Syphilis During Pregnancy,” 2015 Sexually Transmitted Diseases Treatment Guidelines.
4. Syphilis – Section 3: Summaries of Infectious Diseases. Red Book Online. 2018.
While many pediatric clinicians have not frequently managed newborns of mothers with reactive syphilis serology, increased adult syphilis may change that.1
Diagnosing/managing congenital syphilis is not always clear cut. A positive rapid plasma reagin (RPR) titer in a newborn may not indicate congenital infection but merely may reflect transplacental, passively acquired maternal IgG from the mother’s current or previous infection rather than antibodies produced by the newborn. Because currently no IgM assay for syphilis is recommended by the Centers for Disease Control and Prevention for newborn testing, we must deal with IgG test results.
Often initial management decisions are needed while the infant’s status is evolving. The questions to answer to make final decisions include the following2:
- Was the mother actively infected with Treponema pallidum during pregnancy?
- If so, was the mother appropriately treated and when?
- Does the infant have any clinical, laboratory, or radiographic evidence of syphilis?
- How do the mother’s and infant’s nontreponemal serologic titers (NTT) compare at delivery using the same test?
Note: All infants assessed for congenital syphilis need a full evaluation for HIV.
Managing the infant of a mother with positive tests3,4
All such neonates need an examination for evidence of congenital syphilis. The clinical signs of congenital syphilis in neonates include nonimmune hydrops, jaundice, hepatosplenomegaly, rhinitis, skin rash, and pseudoparalysis of extremity. Also, consider dark-field examination or polymerase chain reaction (PCR) of lesions (such as bullae) or secretions (nasal). If available, have the placenta examined histologically (silver stain) or by PCR (Clinical Laboratory Improvement Amendments–validated test). Skeletal radiographic surveys are more useful for stillborn than live born infants. (The complete algorithm can be found in Figure 3.10 of reference 4.)
Order a quantitative NTT, using the Venereal Disease Research Laboratory (VDRL) test or RPR test on neonatal serum. Umbilical cord blood is not appropriate because of potential maternal blood contamination, which could give a false-positive result, or Wharton’s jelly, which could give a false-negative result. Use of treponemal-specific tests that are used for maternal diagnosis – such as T. pallidum particle agglutination (TP-PA), T. pallidum enzyme-linked immunosorbent assay (TP-EIA), fluorescent treponemal antibody absorption (FTA-ABS) test, or T. pallidum chemiluminescence immunoassay (TP-CIA) – on neonatal serum is not recommended because of difficulties in interpretation.
Diagnostic results allow designation of an infant into one of four CDC categories: proven/highly probable syphilis; possible syphilis; syphilis less likely; and syphilis unlikely. Treatment recommendations are based on these categories.
Proven or highly probable syphilis
There are two alternative recommended 10-day treatment regimens.
A. Aqueous crystalline penicillin G 100,000-150,000 U/kg per day by IV at 50,000 U/kg per dose, given every 12 hours through 7 days of age or every 8 hours if greater than 7 days old.
B. Procaine penicillin G at 50,000 U/kg per dose intramuscularly in one dose each day.
More than 1 day of missed therapy requires restarting a new 10-day course. Use of other antimicrobial agents (such as ampicillin) is not validated, so any empiric ampicillin initially given for possible sepsis does not count toward the 10-day penicillin regimen. If nonpenicillin drugs must be used, close serologic follow-up must occur to ensure adequacy of response to therapy.
Possible syphilis
There are three alternative regimens, the same two as in proven/highly probable syphilis (above) plus a single-dose option
A. Aqueous crystalline penicillin G, as described above.
B. Procaine penicillin G, as described above.
C. Benzathine penicillin G at 50,000 U/kg per dose intramuscularly in a single dose.
Note: To be eligible for regimen C, an infant must have a complete evaluation that is normal (cerebrospinal fluid [CSF] examination, long-bone radiographs, and complete blood count with platelet count) and follow-up must be assured. Exception: Neonates born to mothers with untreated early syphilis at the time of delivery are at increased risk for congenital syphilis, and the 10-day course of penicillin G may be considered even if the complete evaluation is normal and follow-up is certain.
Less likely syphilis
One antibiotic regimen is available, but no treatment also may be an option.
A. Benzathine penicillin G as described above.
B. If mother’s NTT has decreased at least fourfold after appropriate early syphilis therapy or remained stably low, which indicates latent syphilis (VDRL less than 1:2; RPR less than 1:4), no treatment is an option but requires repeat serology every 2-3 months until infant is 6 months old.
Unlikely syphilis
No treatment is recommended unless follow-up is uncertain, in which case it is appropriate to give the infant benzathine penicillin G as described above.
Infant with positive NTT at birth
All neonates with reactive NTT need careful follow-up examinations and repeat NTT every 2-3 months until nonreactive. NTT in infants who are not treated because of less likely or unlikely syphilis status should drop by 3 months and be nonreactive by 6 months; this indicates NTT was passively transferred maternal IgG. If NTT remains reactive at 6 months, the infant is likely infected and needs treatment. Persistent NTT at 6-12 months in treated neonates should trigger repeat CSF examination and infectious diseases consultation about a possible repeat of the 10-day penicillin G regimen. If the mother was seroreactive, but the newborn’s NTT was negative at birth, testing of the infant’s NTT needs repeating at 3 months to exclude the possibility that the congenital syphilis was incubating when prior testing occurred at birth. Note: Treponemal-specific tests are not useful in assessing treatment because detectable maternal IgG treponemal antibody can persist at least 15 months.
Neonates with abnormal CSF at birth
Repeat cerebrospinal fluid evaluation every 6 months until results normalize. Persistently reactive CSF VDRL or abnormal CSF indexes not caused by another known cause requires retreatment for possible neurosyphilis, as well as consultation with an expert.
Summary
NTT are the essential test for newborns and some degree of laboratory or imaging work up often are needed. Consider consulting an expert in infectious diseases and/or perinatology if the gray areas do not readily become clear. Treatment of the correct patients with the right drug for the right duration remains the goal, as usual.
Dr. Harrison is a professor of pediatrics at University of Missouri-Kansas City and Director of Research Affairs in the pediatric infectious diseases division at Children’s Mercy Hospital – Kansas City. He said he had no relevant financial disclosures. Email him at [email protected].
References
1. MMWR. 2015 Nov 13;64(44);1241-5.
2. “Congenital Syphilis,” 2015 Sexually Transmitted Diseases Treatment Guidelines.
3. “Syphilis During Pregnancy,” 2015 Sexually Transmitted Diseases Treatment Guidelines.
4. Syphilis – Section 3: Summaries of Infectious Diseases. Red Book Online. 2018.
While many pediatric clinicians have not frequently managed newborns of mothers with reactive syphilis serology, increased adult syphilis may change that.1
Diagnosing/managing congenital syphilis is not always clear cut. A positive rapid plasma reagin (RPR) titer in a newborn may not indicate congenital infection but merely may reflect transplacental, passively acquired maternal IgG from the mother’s current or previous infection rather than antibodies produced by the newborn. Because currently no IgM assay for syphilis is recommended by the Centers for Disease Control and Prevention for newborn testing, we must deal with IgG test results.
Often initial management decisions are needed while the infant’s status is evolving. The questions to answer to make final decisions include the following2:
- Was the mother actively infected with Treponema pallidum during pregnancy?
- If so, was the mother appropriately treated and when?
- Does the infant have any clinical, laboratory, or radiographic evidence of syphilis?
- How do the mother’s and infant’s nontreponemal serologic titers (NTT) compare at delivery using the same test?
Note: All infants assessed for congenital syphilis need a full evaluation for HIV.
Managing the infant of a mother with positive tests3,4
All such neonates need an examination for evidence of congenital syphilis. The clinical signs of congenital syphilis in neonates include nonimmune hydrops, jaundice, hepatosplenomegaly, rhinitis, skin rash, and pseudoparalysis of extremity. Also, consider dark-field examination or polymerase chain reaction (PCR) of lesions (such as bullae) or secretions (nasal). If available, have the placenta examined histologically (silver stain) or by PCR (Clinical Laboratory Improvement Amendments–validated test). Skeletal radiographic surveys are more useful for stillborn than live born infants. (The complete algorithm can be found in Figure 3.10 of reference 4.)
Order a quantitative NTT, using the Venereal Disease Research Laboratory (VDRL) test or RPR test on neonatal serum. Umbilical cord blood is not appropriate because of potential maternal blood contamination, which could give a false-positive result, or Wharton’s jelly, which could give a false-negative result. Use of treponemal-specific tests that are used for maternal diagnosis – such as T. pallidum particle agglutination (TP-PA), T. pallidum enzyme-linked immunosorbent assay (TP-EIA), fluorescent treponemal antibody absorption (FTA-ABS) test, or T. pallidum chemiluminescence immunoassay (TP-CIA) – on neonatal serum is not recommended because of difficulties in interpretation.
Diagnostic results allow designation of an infant into one of four CDC categories: proven/highly probable syphilis; possible syphilis; syphilis less likely; and syphilis unlikely. Treatment recommendations are based on these categories.
Proven or highly probable syphilis
There are two alternative recommended 10-day treatment regimens.
A. Aqueous crystalline penicillin G 100,000-150,000 U/kg per day by IV at 50,000 U/kg per dose, given every 12 hours through 7 days of age or every 8 hours if greater than 7 days old.
B. Procaine penicillin G at 50,000 U/kg per dose intramuscularly in one dose each day.
More than 1 day of missed therapy requires restarting a new 10-day course. Use of other antimicrobial agents (such as ampicillin) is not validated, so any empiric ampicillin initially given for possible sepsis does not count toward the 10-day penicillin regimen. If nonpenicillin drugs must be used, close serologic follow-up must occur to ensure adequacy of response to therapy.
Possible syphilis
There are three alternative regimens, the same two as in proven/highly probable syphilis (above) plus a single-dose option
A. Aqueous crystalline penicillin G, as described above.
B. Procaine penicillin G, as described above.
C. Benzathine penicillin G at 50,000 U/kg per dose intramuscularly in a single dose.
Note: To be eligible for regimen C, an infant must have a complete evaluation that is normal (cerebrospinal fluid [CSF] examination, long-bone radiographs, and complete blood count with platelet count) and follow-up must be assured. Exception: Neonates born to mothers with untreated early syphilis at the time of delivery are at increased risk for congenital syphilis, and the 10-day course of penicillin G may be considered even if the complete evaluation is normal and follow-up is certain.
Less likely syphilis
One antibiotic regimen is available, but no treatment also may be an option.
A. Benzathine penicillin G as described above.
B. If mother’s NTT has decreased at least fourfold after appropriate early syphilis therapy or remained stably low, which indicates latent syphilis (VDRL less than 1:2; RPR less than 1:4), no treatment is an option but requires repeat serology every 2-3 months until infant is 6 months old.
Unlikely syphilis
No treatment is recommended unless follow-up is uncertain, in which case it is appropriate to give the infant benzathine penicillin G as described above.
Infant with positive NTT at birth
All neonates with reactive NTT need careful follow-up examinations and repeat NTT every 2-3 months until nonreactive. NTT in infants who are not treated because of less likely or unlikely syphilis status should drop by 3 months and be nonreactive by 6 months; this indicates NTT was passively transferred maternal IgG. If NTT remains reactive at 6 months, the infant is likely infected and needs treatment. Persistent NTT at 6-12 months in treated neonates should trigger repeat CSF examination and infectious diseases consultation about a possible repeat of the 10-day penicillin G regimen. If the mother was seroreactive, but the newborn’s NTT was negative at birth, testing of the infant’s NTT needs repeating at 3 months to exclude the possibility that the congenital syphilis was incubating when prior testing occurred at birth. Note: Treponemal-specific tests are not useful in assessing treatment because detectable maternal IgG treponemal antibody can persist at least 15 months.
Neonates with abnormal CSF at birth
Repeat cerebrospinal fluid evaluation every 6 months until results normalize. Persistently reactive CSF VDRL or abnormal CSF indexes not caused by another known cause requires retreatment for possible neurosyphilis, as well as consultation with an expert.
Summary
NTT are the essential test for newborns and some degree of laboratory or imaging work up often are needed. Consider consulting an expert in infectious diseases and/or perinatology if the gray areas do not readily become clear. Treatment of the correct patients with the right drug for the right duration remains the goal, as usual.
Dr. Harrison is a professor of pediatrics at University of Missouri-Kansas City and Director of Research Affairs in the pediatric infectious diseases division at Children’s Mercy Hospital – Kansas City. He said he had no relevant financial disclosures. Email him at [email protected].
References
1. MMWR. 2015 Nov 13;64(44);1241-5.
2. “Congenital Syphilis,” 2015 Sexually Transmitted Diseases Treatment Guidelines.
3. “Syphilis During Pregnancy,” 2015 Sexually Transmitted Diseases Treatment Guidelines.
4. Syphilis – Section 3: Summaries of Infectious Diseases. Red Book Online. 2018.