User login
The powerful virus inflammatory response
Inflammation is a double-edged sword. Controlled and modest proinflammatory responses can enhance host immunity against viruses and decrease bacterial colonization and infection, whereas excessive uncontrolled proinflammatory responses may increase the susceptibility to bacterial colonization and secondary infection to facilitate disease pathogenesis. The immune system produces both proinflammatory and anti-inflammatory cytokines and chemokines. It is a balanced response that is key to maintaining good health.
Viral upper respiratory tract infections (URIs) are caused by rhinoviruses, coronaviruses, enteroviruses, respiratory syncytial viruses, influenza A and B viruses, parainfluenza viruses, adenoviruses, and human metapneumoviruses. Viruses are powerful. In the nose, they induce hypersecretion of mucus, slow cilia beating, up-regulate nasal epithelial cell receptors to facilitate bacterial attachment, suppress neutrophil function, and cause increased release of proinflammatory cytokines and chemokines. All these actions by respiratory viruses promote bacterial overgrowth in the nasopharynx and thereby facilitate bacterial superinfections. In fact, progression in pathogenesis of the common bacterial respiratory infections – acute otitis media, acute sinusitis, acute conjunctivitis, and pneumonia – almost always is preceded by a viral URI. Viruses activate multiple target cells in the upper respiratory tract to produce an array of proinflammatory cytokines and chemokines. The symptoms of a viral URI resolve coinciding with an anti-inflammatory response and adaptive immunity.
In recent work, we found a higher frequency of viral URIs in children who experienced more frequent acute otitis media (AOM). We sought to understand why this might occur by comparing levels of inflammatory cytokines/chemokines in the nose during viral URI that did not precipitate AOM versus when a viral URI precipitated an AOM episode. When a child had a viral URI but did not go on to experience an AOM, the child had higher proinflammatory responses than when the viral URI precipitated an AOM. When differences of levels of proinflammatory cytokines/chemokines were compared in otitis-prone and non–otitis-prone children, lower nasal responses were associated with higher otitis-prone classification frequency (Clin Infect Dis. 2018. doi: 10.1093/cid/ciy750).
The powerful virus and the inflammatory response it can induce also play a major role in allergy and asthma. Viral URIs enhance allergic sensitization to respiratory viruses, such as influenza and respiratory syncytial virus, cause cytopathic damage to airway epithelium, promote excessive proinflammatory cytokine/chemokine production, and increase the exposure of allergens and irritants to antigen-presenting cells. Viral infections also may induce the release of epithelial mediators and cytokines that may propagate eosinophilia. Viral URIs, particularly with respiratory syncytial virus and rhinovirus, are the most common causes of wheezing in children, and they have important influences on the development of asthma. Studies have shown that viral infections trigger up to 85% of asthma exacerbations in school-aged children.
Because this column is being published during the winter, a brief discussion of influenza as a powerful virus is appropriate. Influenza occurs in winter outbreaks of varying extent every year. The severity of the influenza season reflects the changing nature of the antigenic properties of influenza viruses, and their spread depends on susceptibility of the population. Influenza outbreaks typically peak over a 2-3 week period and last for 2-3 months. Most outbreaks have attack rates of 10%-20% in children. There may be variations in disease severity caused by different influenza virus types. The symptoms are caused by excessive proinflammatory cytokine/chemokine production in the nose and lung.
Influenza and other viruses can precipitate the systemic inflammatory response syndrome (SIRS), a manifestation of extreme immune dysregulation resulting in organ dysfunction that clinically resembles bacterial sepsis. In this syndrome, tissues remote from the original insult display the cardinal signs of inflammation, including vasodilation, increased microvascular permeability, and leukocyte accumulation. SIRS is another example of the double-edged sword of inflammation.
The onset and progression of SIRS occurs because of dysregulation of the normal inflammatory response, usually with an increase in both proinflammatory and anti-inflammatory cytokines and chemokines, initiating a chain of events that leads to organ failure.
Dr. Pichichero is a specialist in pediatric infectious diseases and director of the Research Institute at Rochester (N.Y.) General Hospital. He reported having no conflicts of interest. Email him at [email protected].
Inflammation is a double-edged sword. Controlled and modest proinflammatory responses can enhance host immunity against viruses and decrease bacterial colonization and infection, whereas excessive uncontrolled proinflammatory responses may increase the susceptibility to bacterial colonization and secondary infection to facilitate disease pathogenesis. The immune system produces both proinflammatory and anti-inflammatory cytokines and chemokines. It is a balanced response that is key to maintaining good health.
Viral upper respiratory tract infections (URIs) are caused by rhinoviruses, coronaviruses, enteroviruses, respiratory syncytial viruses, influenza A and B viruses, parainfluenza viruses, adenoviruses, and human metapneumoviruses. Viruses are powerful. In the nose, they induce hypersecretion of mucus, slow cilia beating, up-regulate nasal epithelial cell receptors to facilitate bacterial attachment, suppress neutrophil function, and cause increased release of proinflammatory cytokines and chemokines. All these actions by respiratory viruses promote bacterial overgrowth in the nasopharynx and thereby facilitate bacterial superinfections. In fact, progression in pathogenesis of the common bacterial respiratory infections – acute otitis media, acute sinusitis, acute conjunctivitis, and pneumonia – almost always is preceded by a viral URI. Viruses activate multiple target cells in the upper respiratory tract to produce an array of proinflammatory cytokines and chemokines. The symptoms of a viral URI resolve coinciding with an anti-inflammatory response and adaptive immunity.
In recent work, we found a higher frequency of viral URIs in children who experienced more frequent acute otitis media (AOM). We sought to understand why this might occur by comparing levels of inflammatory cytokines/chemokines in the nose during viral URI that did not precipitate AOM versus when a viral URI precipitated an AOM episode. When a child had a viral URI but did not go on to experience an AOM, the child had higher proinflammatory responses than when the viral URI precipitated an AOM. When differences of levels of proinflammatory cytokines/chemokines were compared in otitis-prone and non–otitis-prone children, lower nasal responses were associated with higher otitis-prone classification frequency (Clin Infect Dis. 2018. doi: 10.1093/cid/ciy750).
The powerful virus and the inflammatory response it can induce also play a major role in allergy and asthma. Viral URIs enhance allergic sensitization to respiratory viruses, such as influenza and respiratory syncytial virus, cause cytopathic damage to airway epithelium, promote excessive proinflammatory cytokine/chemokine production, and increase the exposure of allergens and irritants to antigen-presenting cells. Viral infections also may induce the release of epithelial mediators and cytokines that may propagate eosinophilia. Viral URIs, particularly with respiratory syncytial virus and rhinovirus, are the most common causes of wheezing in children, and they have important influences on the development of asthma. Studies have shown that viral infections trigger up to 85% of asthma exacerbations in school-aged children.
Because this column is being published during the winter, a brief discussion of influenza as a powerful virus is appropriate. Influenza occurs in winter outbreaks of varying extent every year. The severity of the influenza season reflects the changing nature of the antigenic properties of influenza viruses, and their spread depends on susceptibility of the population. Influenza outbreaks typically peak over a 2-3 week period and last for 2-3 months. Most outbreaks have attack rates of 10%-20% in children. There may be variations in disease severity caused by different influenza virus types. The symptoms are caused by excessive proinflammatory cytokine/chemokine production in the nose and lung.
Influenza and other viruses can precipitate the systemic inflammatory response syndrome (SIRS), a manifestation of extreme immune dysregulation resulting in organ dysfunction that clinically resembles bacterial sepsis. In this syndrome, tissues remote from the original insult display the cardinal signs of inflammation, including vasodilation, increased microvascular permeability, and leukocyte accumulation. SIRS is another example of the double-edged sword of inflammation.
The onset and progression of SIRS occurs because of dysregulation of the normal inflammatory response, usually with an increase in both proinflammatory and anti-inflammatory cytokines and chemokines, initiating a chain of events that leads to organ failure.
Dr. Pichichero is a specialist in pediatric infectious diseases and director of the Research Institute at Rochester (N.Y.) General Hospital. He reported having no conflicts of interest. Email him at [email protected].
Inflammation is a double-edged sword. Controlled and modest proinflammatory responses can enhance host immunity against viruses and decrease bacterial colonization and infection, whereas excessive uncontrolled proinflammatory responses may increase the susceptibility to bacterial colonization and secondary infection to facilitate disease pathogenesis. The immune system produces both proinflammatory and anti-inflammatory cytokines and chemokines. It is a balanced response that is key to maintaining good health.
Viral upper respiratory tract infections (URIs) are caused by rhinoviruses, coronaviruses, enteroviruses, respiratory syncytial viruses, influenza A and B viruses, parainfluenza viruses, adenoviruses, and human metapneumoviruses. Viruses are powerful. In the nose, they induce hypersecretion of mucus, slow cilia beating, up-regulate nasal epithelial cell receptors to facilitate bacterial attachment, suppress neutrophil function, and cause increased release of proinflammatory cytokines and chemokines. All these actions by respiratory viruses promote bacterial overgrowth in the nasopharynx and thereby facilitate bacterial superinfections. In fact, progression in pathogenesis of the common bacterial respiratory infections – acute otitis media, acute sinusitis, acute conjunctivitis, and pneumonia – almost always is preceded by a viral URI. Viruses activate multiple target cells in the upper respiratory tract to produce an array of proinflammatory cytokines and chemokines. The symptoms of a viral URI resolve coinciding with an anti-inflammatory response and adaptive immunity.
In recent work, we found a higher frequency of viral URIs in children who experienced more frequent acute otitis media (AOM). We sought to understand why this might occur by comparing levels of inflammatory cytokines/chemokines in the nose during viral URI that did not precipitate AOM versus when a viral URI precipitated an AOM episode. When a child had a viral URI but did not go on to experience an AOM, the child had higher proinflammatory responses than when the viral URI precipitated an AOM. When differences of levels of proinflammatory cytokines/chemokines were compared in otitis-prone and non–otitis-prone children, lower nasal responses were associated with higher otitis-prone classification frequency (Clin Infect Dis. 2018. doi: 10.1093/cid/ciy750).
The powerful virus and the inflammatory response it can induce also play a major role in allergy and asthma. Viral URIs enhance allergic sensitization to respiratory viruses, such as influenza and respiratory syncytial virus, cause cytopathic damage to airway epithelium, promote excessive proinflammatory cytokine/chemokine production, and increase the exposure of allergens and irritants to antigen-presenting cells. Viral infections also may induce the release of epithelial mediators and cytokines that may propagate eosinophilia. Viral URIs, particularly with respiratory syncytial virus and rhinovirus, are the most common causes of wheezing in children, and they have important influences on the development of asthma. Studies have shown that viral infections trigger up to 85% of asthma exacerbations in school-aged children.
Because this column is being published during the winter, a brief discussion of influenza as a powerful virus is appropriate. Influenza occurs in winter outbreaks of varying extent every year. The severity of the influenza season reflects the changing nature of the antigenic properties of influenza viruses, and their spread depends on susceptibility of the population. Influenza outbreaks typically peak over a 2-3 week period and last for 2-3 months. Most outbreaks have attack rates of 10%-20% in children. There may be variations in disease severity caused by different influenza virus types. The symptoms are caused by excessive proinflammatory cytokine/chemokine production in the nose and lung.
Influenza and other viruses can precipitate the systemic inflammatory response syndrome (SIRS), a manifestation of extreme immune dysregulation resulting in organ dysfunction that clinically resembles bacterial sepsis. In this syndrome, tissues remote from the original insult display the cardinal signs of inflammation, including vasodilation, increased microvascular permeability, and leukocyte accumulation. SIRS is another example of the double-edged sword of inflammation.
The onset and progression of SIRS occurs because of dysregulation of the normal inflammatory response, usually with an increase in both proinflammatory and anti-inflammatory cytokines and chemokines, initiating a chain of events that leads to organ failure.
Dr. Pichichero is a specialist in pediatric infectious diseases and director of the Research Institute at Rochester (N.Y.) General Hospital. He reported having no conflicts of interest. Email him at [email protected].
What infectious disease should parents be most worried about?
I think the question was intended as polite, dinner party chit chat ... maybe an attempt by a gracious hostess to make sure everyone was engaged in conversation.
“So what pediatric infectious disease should parents be most worried about?” she asked me.
I’ll admit that a couple of perfectly respectable and noncontroversial possibilities crossed my mind before I answered.
Acute flaccid myelitis? Measles?
When I replied, “gonorrhea,” conversation at the table pretty much stopped.
Let me explain. Acute flaccid myelitis is a polio-like neurologic condition that has been grabbing headlines. Yes, it is concerning that most cases have occurred in children and some affected children are left with long-term deficits. Technically though, AFM is a neurologic rather than an infectious disease. When cases occur, we suspect a viral infection but according to the Centers for Disease Control and Prevention, no pathogen has been consistently identified from the spinal fluid of infected patients. From August 2014 to September 2018, the CDC received information on 368 confirmed cases, so AFM fortunately is still rare.
News reports describe measles outbreaks raging in Europe – more than 41,000 cases so far this year, and 40 deaths – and warn that the United States could be next. But let’s be honest: We have a safe and effective vaccine for measles and outbreaks like this don’t happen when individuals are appropriately immunized. Parents, immunize your children. If you are lucky enough to be traveling to Europe with your baby, remember that MMR vaccine is indicated for 6- to 11-month olds, but it doesn’t count in the 2-dose series.
But gonorrhea?
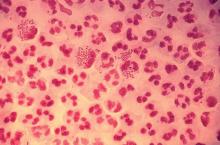
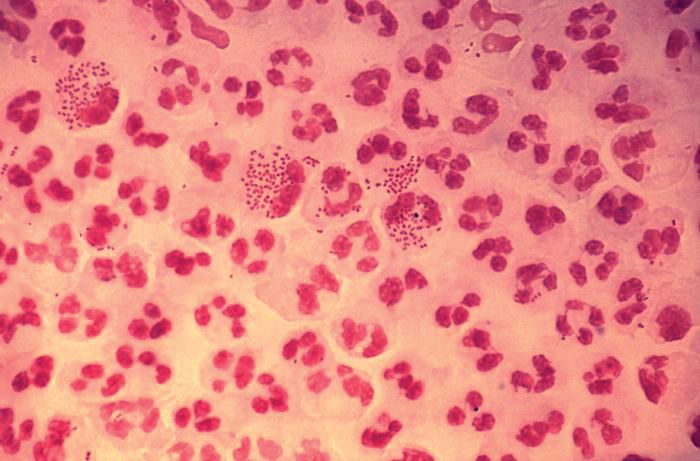
In 2017, the World Health Organization included Neisseria gonorrhoeae on its list of bacteria that pose the greatest threat to human health and for which new antibiotics are urgently needed. The popular media are calling N. gonorrhoeae one of the new “superbugs.” Globally, patients are being diagnosed with strains of gonorrhea that are resistant to all commonly used antibiotics. As reported during IDWeek 2018 this October, patients also are being diagnosed in the United States.
Sancta St. Cyr, MD, of the Centers for Disease Control and Prevention, and her colleagues reported data from the Gonococcal Isolate Surveillance Project (GISP) and trends in multidrug resistant (MDR) and extensively-drug resistant (XDR) gonorrhea in the United States. A gonococcal isolate with resistance or elevated minimum inhibitory concentrations (MIC) to greater than or equal to two classes of antimicrobials is classified as MDR and an isolate with elevated MICs to greater than or equal to three classes of antimicrobials is classified as XDR. The MIC is the lowest antimicrobial concentration that inhibits growth of bacteria in the laboratory and rising MICs – evidence that higher levels of an antibiotic are needed to stop bacterial growth – can be an early indicator that resistance is emerging.
More than 150,000 gonococcal isolates were tested between 1987 and 2016. The first isolates with elevated MICs to cephalosporins and macrolides were identified in 1998, and since 2011, MDR resistance rates have hovered around 1%. In 2016, the rate was 1.1%, down from 1.3% in 2011. A single XDR isolate with resistance to fluoroquinolones with elevated MICs to both cephalosporins and macrolides was identified in 2011.
One could look at these data and ask if this is a “glass half full or half empty” situation, but I propose that clinicians and public health officials should not look at these data and be reassured that rates of MDR-gonorrhea remained stable between 2010 and 2016. According to a recent surveillance report released by the CDC, the absolute number of cases of gonorrhea has continued to rise. In 2017, there were 555,608 cases reported in the United States, a 67% increase since 2013. If we assume that rates of resistance in 2017 were similar to those in 2016, that’s more than 5,000 cases of MDR-gonorrhea in a single year.
“That’s bad,” one of my dining companions agreed. “But is gonorrhea really a pediatric issue?”
To answer that question, we just have to look at the numbers. According to the 2017 Youth Risk Behavior Survey, the percentage of high school students who had ever had sex was approximately 40% and about 10% of students had four or more lifetime partners. More than 45% of sexually active students denied the use of a condom during the last sexual intercourse. Certainly, that puts many teenagers at risk for sexually transmitted infections (STIs). Perhaps it shouldn’t be surprising that public health authorities report that half of all new STIs occur in individuals aged 15-24 years. Moreover, 25% of sexually active adolescent girls contract at least one STI.
Gonorrhea is the second most commonly reported notifiable disease in the United States, and according to the CDC, rates of disease in 2017 were highest among adolescents and young adults. In females specifically, the highest rates of gonorrhea were observed among those aged 20-24 years (684.8 cases per 100,000 females) and 15-19 years (557.4 cases per 100,000 females).
It makes sense that pediatricians and parents advocate for making the reduction of gonorrhea transmission rates a public health priority. We also need to recognize that prompt diagnosis and appropriate treatment are critical. Since 2015, dual therapy with ceftriaxone and azithromycin is the only CDC-recommended treatment for gonorrhea.
At that dinner party, my closest friend, who also happens to be a pediatrician, rolled her eyes and shot me look that I’m sure meant, “Nobody really wants to talk about gonorrhea over dessert.” Still, because she is a good friend she said, “So basically you’re saying that and if this keeps up, we may see kids with untreatable infection. Now that is scary.”
I kept quiet after that but I wanted to mention that in 2017, less than 85% of patients diagnosed with gonorrhea at selected surveillance sites received the recommended treatment with two antibiotics. Patients with inadequately treated gonorrhea are at risk for a host of sequelae. Women can develop pelvic inflammatory disease, abscesses, chronic pelvic pain, and damage of the fallopian tubes that can lead to infertility. Men can develop epididymitis, which occasionally results in infertility. Rarely, N. gonorrhoeae can spread to the blood and cause life-threatening infection. Of course, patients who aren’t treated appropriately may continue to spread the bacteria. Scary? You bet.
For pediatricians who need a refresher course in the treatment of STIs, there are free resources available. The CDC’s 2015 STD Treatment Guidelines are available in a free app; the app contains a nice refresher on taking a sexual history. There also is a print version, wall chart, and pocket guide. Providers also may want to check out the National STD Curriculum offered by the University of Washington STD Prevention Training Center and the University of Washington. Visit https://www.std.uw.edu/.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She said she had no relevant financial disclosures. Email her at [email protected].
I think the question was intended as polite, dinner party chit chat ... maybe an attempt by a gracious hostess to make sure everyone was engaged in conversation.
“So what pediatric infectious disease should parents be most worried about?” she asked me.
I’ll admit that a couple of perfectly respectable and noncontroversial possibilities crossed my mind before I answered.
Acute flaccid myelitis? Measles?
When I replied, “gonorrhea,” conversation at the table pretty much stopped.
Let me explain. Acute flaccid myelitis is a polio-like neurologic condition that has been grabbing headlines. Yes, it is concerning that most cases have occurred in children and some affected children are left with long-term deficits. Technically though, AFM is a neurologic rather than an infectious disease. When cases occur, we suspect a viral infection but according to the Centers for Disease Control and Prevention, no pathogen has been consistently identified from the spinal fluid of infected patients. From August 2014 to September 2018, the CDC received information on 368 confirmed cases, so AFM fortunately is still rare.
News reports describe measles outbreaks raging in Europe – more than 41,000 cases so far this year, and 40 deaths – and warn that the United States could be next. But let’s be honest: We have a safe and effective vaccine for measles and outbreaks like this don’t happen when individuals are appropriately immunized. Parents, immunize your children. If you are lucky enough to be traveling to Europe with your baby, remember that MMR vaccine is indicated for 6- to 11-month olds, but it doesn’t count in the 2-dose series.
But gonorrhea?
In 2017, the World Health Organization included Neisseria gonorrhoeae on its list of bacteria that pose the greatest threat to human health and for which new antibiotics are urgently needed. The popular media are calling N. gonorrhoeae one of the new “superbugs.” Globally, patients are being diagnosed with strains of gonorrhea that are resistant to all commonly used antibiotics. As reported during IDWeek 2018 this October, patients also are being diagnosed in the United States.
Sancta St. Cyr, MD, of the Centers for Disease Control and Prevention, and her colleagues reported data from the Gonococcal Isolate Surveillance Project (GISP) and trends in multidrug resistant (MDR) and extensively-drug resistant (XDR) gonorrhea in the United States. A gonococcal isolate with resistance or elevated minimum inhibitory concentrations (MIC) to greater than or equal to two classes of antimicrobials is classified as MDR and an isolate with elevated MICs to greater than or equal to three classes of antimicrobials is classified as XDR. The MIC is the lowest antimicrobial concentration that inhibits growth of bacteria in the laboratory and rising MICs – evidence that higher levels of an antibiotic are needed to stop bacterial growth – can be an early indicator that resistance is emerging.
More than 150,000 gonococcal isolates were tested between 1987 and 2016. The first isolates with elevated MICs to cephalosporins and macrolides were identified in 1998, and since 2011, MDR resistance rates have hovered around 1%. In 2016, the rate was 1.1%, down from 1.3% in 2011. A single XDR isolate with resistance to fluoroquinolones with elevated MICs to both cephalosporins and macrolides was identified in 2011.
One could look at these data and ask if this is a “glass half full or half empty” situation, but I propose that clinicians and public health officials should not look at these data and be reassured that rates of MDR-gonorrhea remained stable between 2010 and 2016. According to a recent surveillance report released by the CDC, the absolute number of cases of gonorrhea has continued to rise. In 2017, there were 555,608 cases reported in the United States, a 67% increase since 2013. If we assume that rates of resistance in 2017 were similar to those in 2016, that’s more than 5,000 cases of MDR-gonorrhea in a single year.
“That’s bad,” one of my dining companions agreed. “But is gonorrhea really a pediatric issue?”
To answer that question, we just have to look at the numbers. According to the 2017 Youth Risk Behavior Survey, the percentage of high school students who had ever had sex was approximately 40% and about 10% of students had four or more lifetime partners. More than 45% of sexually active students denied the use of a condom during the last sexual intercourse. Certainly, that puts many teenagers at risk for sexually transmitted infections (STIs). Perhaps it shouldn’t be surprising that public health authorities report that half of all new STIs occur in individuals aged 15-24 years. Moreover, 25% of sexually active adolescent girls contract at least one STI.
Gonorrhea is the second most commonly reported notifiable disease in the United States, and according to the CDC, rates of disease in 2017 were highest among adolescents and young adults. In females specifically, the highest rates of gonorrhea were observed among those aged 20-24 years (684.8 cases per 100,000 females) and 15-19 years (557.4 cases per 100,000 females).
It makes sense that pediatricians and parents advocate for making the reduction of gonorrhea transmission rates a public health priority. We also need to recognize that prompt diagnosis and appropriate treatment are critical. Since 2015, dual therapy with ceftriaxone and azithromycin is the only CDC-recommended treatment for gonorrhea.
At that dinner party, my closest friend, who also happens to be a pediatrician, rolled her eyes and shot me look that I’m sure meant, “Nobody really wants to talk about gonorrhea over dessert.” Still, because she is a good friend she said, “So basically you’re saying that and if this keeps up, we may see kids with untreatable infection. Now that is scary.”
I kept quiet after that but I wanted to mention that in 2017, less than 85% of patients diagnosed with gonorrhea at selected surveillance sites received the recommended treatment with two antibiotics. Patients with inadequately treated gonorrhea are at risk for a host of sequelae. Women can develop pelvic inflammatory disease, abscesses, chronic pelvic pain, and damage of the fallopian tubes that can lead to infertility. Men can develop epididymitis, which occasionally results in infertility. Rarely, N. gonorrhoeae can spread to the blood and cause life-threatening infection. Of course, patients who aren’t treated appropriately may continue to spread the bacteria. Scary? You bet.
For pediatricians who need a refresher course in the treatment of STIs, there are free resources available. The CDC’s 2015 STD Treatment Guidelines are available in a free app; the app contains a nice refresher on taking a sexual history. There also is a print version, wall chart, and pocket guide. Providers also may want to check out the National STD Curriculum offered by the University of Washington STD Prevention Training Center and the University of Washington. Visit https://www.std.uw.edu/.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She said she had no relevant financial disclosures. Email her at [email protected].
I think the question was intended as polite, dinner party chit chat ... maybe an attempt by a gracious hostess to make sure everyone was engaged in conversation.
“So what pediatric infectious disease should parents be most worried about?” she asked me.
I’ll admit that a couple of perfectly respectable and noncontroversial possibilities crossed my mind before I answered.
Acute flaccid myelitis? Measles?
When I replied, “gonorrhea,” conversation at the table pretty much stopped.
Let me explain. Acute flaccid myelitis is a polio-like neurologic condition that has been grabbing headlines. Yes, it is concerning that most cases have occurred in children and some affected children are left with long-term deficits. Technically though, AFM is a neurologic rather than an infectious disease. When cases occur, we suspect a viral infection but according to the Centers for Disease Control and Prevention, no pathogen has been consistently identified from the spinal fluid of infected patients. From August 2014 to September 2018, the CDC received information on 368 confirmed cases, so AFM fortunately is still rare.
News reports describe measles outbreaks raging in Europe – more than 41,000 cases so far this year, and 40 deaths – and warn that the United States could be next. But let’s be honest: We have a safe and effective vaccine for measles and outbreaks like this don’t happen when individuals are appropriately immunized. Parents, immunize your children. If you are lucky enough to be traveling to Europe with your baby, remember that MMR vaccine is indicated for 6- to 11-month olds, but it doesn’t count in the 2-dose series.
But gonorrhea?
In 2017, the World Health Organization included Neisseria gonorrhoeae on its list of bacteria that pose the greatest threat to human health and for which new antibiotics are urgently needed. The popular media are calling N. gonorrhoeae one of the new “superbugs.” Globally, patients are being diagnosed with strains of gonorrhea that are resistant to all commonly used antibiotics. As reported during IDWeek 2018 this October, patients also are being diagnosed in the United States.
Sancta St. Cyr, MD, of the Centers for Disease Control and Prevention, and her colleagues reported data from the Gonococcal Isolate Surveillance Project (GISP) and trends in multidrug resistant (MDR) and extensively-drug resistant (XDR) gonorrhea in the United States. A gonococcal isolate with resistance or elevated minimum inhibitory concentrations (MIC) to greater than or equal to two classes of antimicrobials is classified as MDR and an isolate with elevated MICs to greater than or equal to three classes of antimicrobials is classified as XDR. The MIC is the lowest antimicrobial concentration that inhibits growth of bacteria in the laboratory and rising MICs – evidence that higher levels of an antibiotic are needed to stop bacterial growth – can be an early indicator that resistance is emerging.
More than 150,000 gonococcal isolates were tested between 1987 and 2016. The first isolates with elevated MICs to cephalosporins and macrolides were identified in 1998, and since 2011, MDR resistance rates have hovered around 1%. In 2016, the rate was 1.1%, down from 1.3% in 2011. A single XDR isolate with resistance to fluoroquinolones with elevated MICs to both cephalosporins and macrolides was identified in 2011.
One could look at these data and ask if this is a “glass half full or half empty” situation, but I propose that clinicians and public health officials should not look at these data and be reassured that rates of MDR-gonorrhea remained stable between 2010 and 2016. According to a recent surveillance report released by the CDC, the absolute number of cases of gonorrhea has continued to rise. In 2017, there were 555,608 cases reported in the United States, a 67% increase since 2013. If we assume that rates of resistance in 2017 were similar to those in 2016, that’s more than 5,000 cases of MDR-gonorrhea in a single year.
“That’s bad,” one of my dining companions agreed. “But is gonorrhea really a pediatric issue?”
To answer that question, we just have to look at the numbers. According to the 2017 Youth Risk Behavior Survey, the percentage of high school students who had ever had sex was approximately 40% and about 10% of students had four or more lifetime partners. More than 45% of sexually active students denied the use of a condom during the last sexual intercourse. Certainly, that puts many teenagers at risk for sexually transmitted infections (STIs). Perhaps it shouldn’t be surprising that public health authorities report that half of all new STIs occur in individuals aged 15-24 years. Moreover, 25% of sexually active adolescent girls contract at least one STI.
Gonorrhea is the second most commonly reported notifiable disease in the United States, and according to the CDC, rates of disease in 2017 were highest among adolescents and young adults. In females specifically, the highest rates of gonorrhea were observed among those aged 20-24 years (684.8 cases per 100,000 females) and 15-19 years (557.4 cases per 100,000 females).
It makes sense that pediatricians and parents advocate for making the reduction of gonorrhea transmission rates a public health priority. We also need to recognize that prompt diagnosis and appropriate treatment are critical. Since 2015, dual therapy with ceftriaxone and azithromycin is the only CDC-recommended treatment for gonorrhea.
At that dinner party, my closest friend, who also happens to be a pediatrician, rolled her eyes and shot me look that I’m sure meant, “Nobody really wants to talk about gonorrhea over dessert.” Still, because she is a good friend she said, “So basically you’re saying that and if this keeps up, we may see kids with untreatable infection. Now that is scary.”
I kept quiet after that but I wanted to mention that in 2017, less than 85% of patients diagnosed with gonorrhea at selected surveillance sites received the recommended treatment with two antibiotics. Patients with inadequately treated gonorrhea are at risk for a host of sequelae. Women can develop pelvic inflammatory disease, abscesses, chronic pelvic pain, and damage of the fallopian tubes that can lead to infertility. Men can develop epididymitis, which occasionally results in infertility. Rarely, N. gonorrhoeae can spread to the blood and cause life-threatening infection. Of course, patients who aren’t treated appropriately may continue to spread the bacteria. Scary? You bet.
For pediatricians who need a refresher course in the treatment of STIs, there are free resources available. The CDC’s 2015 STD Treatment Guidelines are available in a free app; the app contains a nice refresher on taking a sexual history. There also is a print version, wall chart, and pocket guide. Providers also may want to check out the National STD Curriculum offered by the University of Washington STD Prevention Training Center and the University of Washington. Visit https://www.std.uw.edu/.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She said she had no relevant financial disclosures. Email her at [email protected].
Recommending HPV vaccination: How would you grade yourself?
A few weeks ago, a patient asked whether he could get my opinion on something unrelated to his yellow fever vaccine visit: He asked what I thought about the human papillomavirus (HPV) vaccine. His daughter’s primary care physician (PCP) had recommended it, but he “heard that it wasn’t safe.” We had a brief discussion.
My pediatric training days have long since ended, but I was taught never to miss an opportunity to immunize. In this case, it was to help a parent decide to immunize. This type of encounter is not unusual because, as part of preparing persons for international travel, I review their routine immunizations. When documentation of a vaccine is absent, it is pointed out and often remedied after a brief discussion.
Unfortunately, with HPV, too often parents state “my primary care physician said” it was optional, it was not required, or it was never recommended. Some were told to wait until their child was older, and several have safety concerns as did the parent above. I sometimes hear, “it’s not necessary for my child”; this is usually a clue indicating that the issue is more likely about how HPV is transmitted than what HPV vaccine can prevent. Most have welcomed the opportunity to discuss the vaccine, hear about its benefits, and have their questions answered. All leave with HPV information and are directed to websites that provide accurate information. They are referred to their PCP – hopefully to be immunized.
Three vaccines – meningococcal conjugate vaccine (MCV), Tdap, and HPV vaccine – all are recommended for administration at 11-12 years of age. A booster of MCV is recommended at 16 years. However, let’s focus on HPV. In 2007, HPV administration was recommended by the Advisory Committee on Immunization Practices (ACIP) for girls; by 2011, the recommendation was extended to boys. It was a three-dose schedule expected to be completed by age 13 years. In December 2016, a two-dose schedule administered at least 6 months apart was recommended for teens who initiated immunization at less than 15 years. Three doses were still recommended for those initiating HPV after 15 years. This was the only time the number of doses to complete a vaccine series had been decreased based on postlicensure data. So
Vaccine coverage
The National Immunization Survey–Teen (NIS-Teen) monitors vaccine coverage annually amongst adolescents aged 13-17 years. Data are obtained from individuals from every state, as well as the District of Columbia, the U.S. Virgin Islands, and six major urban areas.
According to the Centers for Disease Control and Prevention’s Morbidity and Mortality Weekly Report (2018 Aug 24;67[33]:909-17), HPV vaccination continues to lag behind Tdap and MCV in 2018. Among all adolescents, coverage with one or more doses of HPV was 66%, with up-to-date HPV status in 49%. In contrast, 82% received a dose of MCV, and 89% received a dose of Tdap.
Coverage for receiving one or more doses of HPV among females was 69%, and up-to-date HPV status was 53%; among males, coverage with one or more doses was 63%, and up-to-date HPV status was 44%.
Up-to-date HPV coverage status differed geographically, ranging from 29% in Mississippi to 78% in DC. Overall, eight states and the District of Columbia reported increases in up-to-date status (District of Columbia, Louisiana, Massachusetts, Nebraska, North Carolina, South Carolina, Texas, Vermont, and Virginia). Kudos to Virginia for having the largest increase (20 percentage points).
Coverage also differed between urban and rural areas: one or more doses at 70% vs. 59% and up-to-date status at 52% vs. 42%.
HPV coverage differed by poverty level as well. It was higher for persons living below the poverty level, with one or more doses in 73% and up-to-date status in 54%, compared with persons living at or above poverty level at 63% and 47%, respectively.
HPV-related cancers

The most recent CDC data regarding types of HPV-associated cancers during 2011-2015 suggest that HPV types 16 and 18 account for the majority of cervical (78%) and oropharyngeal (86%) cancers.

Currently, there are more cases of oropharyngeal cancer than cervical, and we have no screening tool for the former.
Safety
Safety has been well documented. Since licensure, no serious safety concerns have been identified, contrary to what has been reported on various social and news media outlets. Yet it remains a concern for many parents who have delayed initiation of vaccine. Efficacy also has been documented in the United States and abroad.
Suggestions for improving HPV immunization coverage
Here are eight suggestions to help you recommend the vaccine and convince hesitant parents of its necessity:
1. Focus on your delivery of the HPV immunization recommendation. Clinician recommendation is the No. 1 reason parents vaccinate. The tone you use and how you make the recommendation can affect how the parent perceives the importance of this vaccine. The following are components of a high-quality recommendation (Academic Pediatrics. 2018;18:S23-S27):
- Routinely recommend vaccine at 11-12 years.
- Recommend vaccine for all preteens, not just those you feel are at risk for infection.
- Recommend the vaccine be given the same day it is discussed.
- Use language that expresses the importance of the HPV vaccine.
2. Use the “announcement or presumptive approach.” You expect the parent to agree with your recommendation. You don’t want to convey that it is an option.
3. Remind parents that immunizing on time means only two doses of HPV.
4. Revisit the topic again during another visit if a parent declines. Data suggest secondary acceptance can be as high as 66%.
5. Consider using a motivational interviewing approach for parents who are very hesitant to vaccinate. Most people want to comply with recommended health interventions.
6. Educate your staff about the importance of HPV vaccine and how it prevents cancer.
7. Determine how well your practice immunizes adolescents. This would be a perfect quality improvement project.
8. Explore “Answering Parents’ Questions” and other resources at www.cdc.gov/hpv to find quick answers to HPV vaccine–related questions .
Why is HPV coverage, a vaccine to prevent cancer, still lagging behind Tdap and MCV? I am as puzzled as others. What I do know is this: Our children will mature and one day become sexually active. They can be exposed to and get infected with HPV, and we can’t predict which ones will not clear the virus and end up developing an HPV-related cancer in the future. At the end of the day, HPV vaccination is cancer prevention.
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She said she had no relevant financial disclosures. Email her at [email protected].
A few weeks ago, a patient asked whether he could get my opinion on something unrelated to his yellow fever vaccine visit: He asked what I thought about the human papillomavirus (HPV) vaccine. His daughter’s primary care physician (PCP) had recommended it, but he “heard that it wasn’t safe.” We had a brief discussion.
My pediatric training days have long since ended, but I was taught never to miss an opportunity to immunize. In this case, it was to help a parent decide to immunize. This type of encounter is not unusual because, as part of preparing persons for international travel, I review their routine immunizations. When documentation of a vaccine is absent, it is pointed out and often remedied after a brief discussion.
Unfortunately, with HPV, too often parents state “my primary care physician said” it was optional, it was not required, or it was never recommended. Some were told to wait until their child was older, and several have safety concerns as did the parent above. I sometimes hear, “it’s not necessary for my child”; this is usually a clue indicating that the issue is more likely about how HPV is transmitted than what HPV vaccine can prevent. Most have welcomed the opportunity to discuss the vaccine, hear about its benefits, and have their questions answered. All leave with HPV information and are directed to websites that provide accurate information. They are referred to their PCP – hopefully to be immunized.
Three vaccines – meningococcal conjugate vaccine (MCV), Tdap, and HPV vaccine – all are recommended for administration at 11-12 years of age. A booster of MCV is recommended at 16 years. However, let’s focus on HPV. In 2007, HPV administration was recommended by the Advisory Committee on Immunization Practices (ACIP) for girls; by 2011, the recommendation was extended to boys. It was a three-dose schedule expected to be completed by age 13 years. In December 2016, a two-dose schedule administered at least 6 months apart was recommended for teens who initiated immunization at less than 15 years. Three doses were still recommended for those initiating HPV after 15 years. This was the only time the number of doses to complete a vaccine series had been decreased based on postlicensure data. So
Vaccine coverage
The National Immunization Survey–Teen (NIS-Teen) monitors vaccine coverage annually amongst adolescents aged 13-17 years. Data are obtained from individuals from every state, as well as the District of Columbia, the U.S. Virgin Islands, and six major urban areas.
According to the Centers for Disease Control and Prevention’s Morbidity and Mortality Weekly Report (2018 Aug 24;67[33]:909-17), HPV vaccination continues to lag behind Tdap and MCV in 2018. Among all adolescents, coverage with one or more doses of HPV was 66%, with up-to-date HPV status in 49%. In contrast, 82% received a dose of MCV, and 89% received a dose of Tdap.
Coverage for receiving one or more doses of HPV among females was 69%, and up-to-date HPV status was 53%; among males, coverage with one or more doses was 63%, and up-to-date HPV status was 44%.
Up-to-date HPV coverage status differed geographically, ranging from 29% in Mississippi to 78% in DC. Overall, eight states and the District of Columbia reported increases in up-to-date status (District of Columbia, Louisiana, Massachusetts, Nebraska, North Carolina, South Carolina, Texas, Vermont, and Virginia). Kudos to Virginia for having the largest increase (20 percentage points).
Coverage also differed between urban and rural areas: one or more doses at 70% vs. 59% and up-to-date status at 52% vs. 42%.
HPV coverage differed by poverty level as well. It was higher for persons living below the poverty level, with one or more doses in 73% and up-to-date status in 54%, compared with persons living at or above poverty level at 63% and 47%, respectively.
HPV-related cancers

The most recent CDC data regarding types of HPV-associated cancers during 2011-2015 suggest that HPV types 16 and 18 account for the majority of cervical (78%) and oropharyngeal (86%) cancers.

Currently, there are more cases of oropharyngeal cancer than cervical, and we have no screening tool for the former.
Safety
Safety has been well documented. Since licensure, no serious safety concerns have been identified, contrary to what has been reported on various social and news media outlets. Yet it remains a concern for many parents who have delayed initiation of vaccine. Efficacy also has been documented in the United States and abroad.
Suggestions for improving HPV immunization coverage
Here are eight suggestions to help you recommend the vaccine and convince hesitant parents of its necessity:
1. Focus on your delivery of the HPV immunization recommendation. Clinician recommendation is the No. 1 reason parents vaccinate. The tone you use and how you make the recommendation can affect how the parent perceives the importance of this vaccine. The following are components of a high-quality recommendation (Academic Pediatrics. 2018;18:S23-S27):
- Routinely recommend vaccine at 11-12 years.
- Recommend vaccine for all preteens, not just those you feel are at risk for infection.
- Recommend the vaccine be given the same day it is discussed.
- Use language that expresses the importance of the HPV vaccine.
2. Use the “announcement or presumptive approach.” You expect the parent to agree with your recommendation. You don’t want to convey that it is an option.
3. Remind parents that immunizing on time means only two doses of HPV.
4. Revisit the topic again during another visit if a parent declines. Data suggest secondary acceptance can be as high as 66%.
5. Consider using a motivational interviewing approach for parents who are very hesitant to vaccinate. Most people want to comply with recommended health interventions.
6. Educate your staff about the importance of HPV vaccine and how it prevents cancer.
7. Determine how well your practice immunizes adolescents. This would be a perfect quality improvement project.
8. Explore “Answering Parents’ Questions” and other resources at www.cdc.gov/hpv to find quick answers to HPV vaccine–related questions .
Why is HPV coverage, a vaccine to prevent cancer, still lagging behind Tdap and MCV? I am as puzzled as others. What I do know is this: Our children will mature and one day become sexually active. They can be exposed to and get infected with HPV, and we can’t predict which ones will not clear the virus and end up developing an HPV-related cancer in the future. At the end of the day, HPV vaccination is cancer prevention.
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She said she had no relevant financial disclosures. Email her at [email protected].
A few weeks ago, a patient asked whether he could get my opinion on something unrelated to his yellow fever vaccine visit: He asked what I thought about the human papillomavirus (HPV) vaccine. His daughter’s primary care physician (PCP) had recommended it, but he “heard that it wasn’t safe.” We had a brief discussion.
My pediatric training days have long since ended, but I was taught never to miss an opportunity to immunize. In this case, it was to help a parent decide to immunize. This type of encounter is not unusual because, as part of preparing persons for international travel, I review their routine immunizations. When documentation of a vaccine is absent, it is pointed out and often remedied after a brief discussion.
Unfortunately, with HPV, too often parents state “my primary care physician said” it was optional, it was not required, or it was never recommended. Some were told to wait until their child was older, and several have safety concerns as did the parent above. I sometimes hear, “it’s not necessary for my child”; this is usually a clue indicating that the issue is more likely about how HPV is transmitted than what HPV vaccine can prevent. Most have welcomed the opportunity to discuss the vaccine, hear about its benefits, and have their questions answered. All leave with HPV information and are directed to websites that provide accurate information. They are referred to their PCP – hopefully to be immunized.
Three vaccines – meningococcal conjugate vaccine (MCV), Tdap, and HPV vaccine – all are recommended for administration at 11-12 years of age. A booster of MCV is recommended at 16 years. However, let’s focus on HPV. In 2007, HPV administration was recommended by the Advisory Committee on Immunization Practices (ACIP) for girls; by 2011, the recommendation was extended to boys. It was a three-dose schedule expected to be completed by age 13 years. In December 2016, a two-dose schedule administered at least 6 months apart was recommended for teens who initiated immunization at less than 15 years. Three doses were still recommended for those initiating HPV after 15 years. This was the only time the number of doses to complete a vaccine series had been decreased based on postlicensure data. So
Vaccine coverage
The National Immunization Survey–Teen (NIS-Teen) monitors vaccine coverage annually amongst adolescents aged 13-17 years. Data are obtained from individuals from every state, as well as the District of Columbia, the U.S. Virgin Islands, and six major urban areas.
According to the Centers for Disease Control and Prevention’s Morbidity and Mortality Weekly Report (2018 Aug 24;67[33]:909-17), HPV vaccination continues to lag behind Tdap and MCV in 2018. Among all adolescents, coverage with one or more doses of HPV was 66%, with up-to-date HPV status in 49%. In contrast, 82% received a dose of MCV, and 89% received a dose of Tdap.
Coverage for receiving one or more doses of HPV among females was 69%, and up-to-date HPV status was 53%; among males, coverage with one or more doses was 63%, and up-to-date HPV status was 44%.
Up-to-date HPV coverage status differed geographically, ranging from 29% in Mississippi to 78% in DC. Overall, eight states and the District of Columbia reported increases in up-to-date status (District of Columbia, Louisiana, Massachusetts, Nebraska, North Carolina, South Carolina, Texas, Vermont, and Virginia). Kudos to Virginia for having the largest increase (20 percentage points).
Coverage also differed between urban and rural areas: one or more doses at 70% vs. 59% and up-to-date status at 52% vs. 42%.
HPV coverage differed by poverty level as well. It was higher for persons living below the poverty level, with one or more doses in 73% and up-to-date status in 54%, compared with persons living at or above poverty level at 63% and 47%, respectively.
HPV-related cancers

The most recent CDC data regarding types of HPV-associated cancers during 2011-2015 suggest that HPV types 16 and 18 account for the majority of cervical (78%) and oropharyngeal (86%) cancers.

Currently, there are more cases of oropharyngeal cancer than cervical, and we have no screening tool for the former.
Safety
Safety has been well documented. Since licensure, no serious safety concerns have been identified, contrary to what has been reported on various social and news media outlets. Yet it remains a concern for many parents who have delayed initiation of vaccine. Efficacy also has been documented in the United States and abroad.
Suggestions for improving HPV immunization coverage
Here are eight suggestions to help you recommend the vaccine and convince hesitant parents of its necessity:
1. Focus on your delivery of the HPV immunization recommendation. Clinician recommendation is the No. 1 reason parents vaccinate. The tone you use and how you make the recommendation can affect how the parent perceives the importance of this vaccine. The following are components of a high-quality recommendation (Academic Pediatrics. 2018;18:S23-S27):
- Routinely recommend vaccine at 11-12 years.
- Recommend vaccine for all preteens, not just those you feel are at risk for infection.
- Recommend the vaccine be given the same day it is discussed.
- Use language that expresses the importance of the HPV vaccine.
2. Use the “announcement or presumptive approach.” You expect the parent to agree with your recommendation. You don’t want to convey that it is an option.
3. Remind parents that immunizing on time means only two doses of HPV.
4. Revisit the topic again during another visit if a parent declines. Data suggest secondary acceptance can be as high as 66%.
5. Consider using a motivational interviewing approach for parents who are very hesitant to vaccinate. Most people want to comply with recommended health interventions.
6. Educate your staff about the importance of HPV vaccine and how it prevents cancer.
7. Determine how well your practice immunizes adolescents. This would be a perfect quality improvement project.
8. Explore “Answering Parents’ Questions” and other resources at www.cdc.gov/hpv to find quick answers to HPV vaccine–related questions .
Why is HPV coverage, a vaccine to prevent cancer, still lagging behind Tdap and MCV? I am as puzzled as others. What I do know is this: Our children will mature and one day become sexually active. They can be exposed to and get infected with HPV, and we can’t predict which ones will not clear the virus and end up developing an HPV-related cancer in the future. At the end of the day, HPV vaccination is cancer prevention.
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She said she had no relevant financial disclosures. Email her at [email protected].
Hand, foot, and mouth disease: From self-limited to fatal
Hand, foot, and mouth disease (HFMD) has become a global challenge since its first description in 1957 in New Zealand and Canada. Clinicians readily recognize the characteristic syndrome in young children: fever associated with a papulovesicular rash affecting the palms or soles, or both, usually in spring, summer, or fall. In most cases the disease is self-limited, and brief. However, aseptic meningitis, brainstem encephalitis, acute flaccid paralysis or autonomic nervous system deregulation or cardiorespiratory failure may complicate the clinical course.
HFMD is caused by enterovirus A (formerly called human enterovirus A) which consists of 25 serotypes including multiple coxsackie A viruses, multiple enteroviruses, simian enteroviruses, and baboon enterovirus A13. The clinical spectrum spans from herpangina, characterized by fever and painful mouth ulcers most prominent in the posterior oral cavity (uvula, tonsils, soft plates, and anterior pharyngeal folds), to HFMD with papulovesicular rash on palms and soles with or without mouth ulcers/vesicles.1 In atypical cases, the rash may be maculopapular and may include the buttocks, knees or elbows.
In the United States, the predominant cause is coxsackie A16. However, coxsackie A6 appears to be emerging; often more than one HFMD causing virus is circulating concurrently and clinically indistinguishable. Globally, especially, in Asia, enterovirus 1 is a major cause of HFMD and more often associated with prominent central nervous system involvement. Disease can be sporadic or epidemic. An outbreak is usually defined as two or more cases within a defined geographic area; global epidemics as large as 1.5 million cases (Taiwan, 1998) have been reported, and outbreaks in China involving tens of thousands with multiple deaths have been reported.
In 2015, an outbreak of HFMD occurred during basic military training at Lackland Air Force Base, Bexar County, Texas, due to coxsackie A6.2 The illness was characterized by prodromal symptoms of fever and malaise followed by erosive stomatitis and a rash that began on the palms and soles. The rate of infection among trainees was 4.7% (50 of 1,054 persons).
The differential diagnosis includes aphthous ulcers and herpetic gingivostomatitis.3 Aphthous ulcers are seen more commonly in older children and adolescents, are often recurrent, are not seasonal, and are not associated with rash. Herpes simplex virus gingivostomatitis usually has a febrile prodrome, perioral lesions are frequent in addition to gum and tongue involvement, and gingival bleeding is common. HFMD usually has an incubation period of 3-5 days and fever, malaise, and myalgia prodrome followed by onset of oral and dermatologic manifestations in sequence. The skin rash has features that may overlap with varicella, erythema multiforme (EM) or drug eruption. Varicella usually involves the face before spreading to the extremities, and the lesions are characterized by umbilication and subsequent crusting. EM is characterized by target lesions and drug eruptions are morbilliform or maculopapular. The majority of cases of HFMD are diagnosed clinically; polymerase chain reaction testing is available and best performed on throat or vesicle specimens. Serologic testing for A16 and enterovirus 71 (IgM) is available. Infected patients shed virus for 2-4 weeks and virus is stable in the environment resulting in fecal-oral or oral-oral transmission.
Atypical features of HFMD include occurrence in the winter (outbreak in Alabama in 2011/2012) or an atypical distribution of rash involving the antecubital and popliteal fossae distribution of rash, or “eczema coxsackium” – the accentuation of rash in areas previously affected by atopic dermatitis. Additional features may include nail dystrophies that manifest as Beau lines (deep grooved lines that run from side to side on the fingernail or the toenail) and nail shedding.
A spectrum of neurologic complications has been observed, more frequently with EV71 and more frequently in Asia. The spectrum includes aseptic meningitis and brainstem encephalitis. Progressive cardiopulmonary failure also can be observed in severe cases. The hallmark of severe disease is often presentation with high fever, sweating, mottled skin, and tachycardia. Early signs of CNS involvement include myoclonic jerks, ataxia, and “wandering eyes.”3 Elevated white blood count and/or hyperglycemia may distinguish children with severe disease from benign disease. Anecdotal reports of response to treatment with high-dose methylprednisolone and intravenous immune globulin suggest that the neurologic disease may be an autoimmune phenomenon.
The clinician’s primary role is to accurately diagnose HFMD, provide supportive care for fever and dehydration, and identify those with early signs or laboratory features heralding a more severe course of disease.3 The Centers for Disease Control and Prevention recommends frequent hand washing after toileting and changing diapers, disinfecting surfaces such as toys, avoiding close contact with infected individuals or sharing of personal items for all affected patients. No antiviral treatment is available although improvement following early treatment with acyclovir has been reported anecdotally. Intravenous immunoglobulin has been used in severe cases in Asia with retrospective data analysis suggesting a potential for improvement when administered prior to cardiopulmonary arrest.1
Dr. Pelton is professor of pediatrics and epidemiology at Boston University. Dr. Pelton said he had no relevant financial disclosures. Email him at [email protected].
References
1. Cleveland Clinic Journal of Medicine 2014;81(9):537-43.
2. Morbidity and Mortality Weekly Report MMWR. 2016 Jul 8;65(26);678-80.
3. A Guide to clinical management and public health response for hand, foot and mouth disease (HFMD).
Hand, foot, and mouth disease (HFMD) has become a global challenge since its first description in 1957 in New Zealand and Canada. Clinicians readily recognize the characteristic syndrome in young children: fever associated with a papulovesicular rash affecting the palms or soles, or both, usually in spring, summer, or fall. In most cases the disease is self-limited, and brief. However, aseptic meningitis, brainstem encephalitis, acute flaccid paralysis or autonomic nervous system deregulation or cardiorespiratory failure may complicate the clinical course.
HFMD is caused by enterovirus A (formerly called human enterovirus A) which consists of 25 serotypes including multiple coxsackie A viruses, multiple enteroviruses, simian enteroviruses, and baboon enterovirus A13. The clinical spectrum spans from herpangina, characterized by fever and painful mouth ulcers most prominent in the posterior oral cavity (uvula, tonsils, soft plates, and anterior pharyngeal folds), to HFMD with papulovesicular rash on palms and soles with or without mouth ulcers/vesicles.1 In atypical cases, the rash may be maculopapular and may include the buttocks, knees or elbows.
In the United States, the predominant cause is coxsackie A16. However, coxsackie A6 appears to be emerging; often more than one HFMD causing virus is circulating concurrently and clinically indistinguishable. Globally, especially, in Asia, enterovirus 1 is a major cause of HFMD and more often associated with prominent central nervous system involvement. Disease can be sporadic or epidemic. An outbreak is usually defined as two or more cases within a defined geographic area; global epidemics as large as 1.5 million cases (Taiwan, 1998) have been reported, and outbreaks in China involving tens of thousands with multiple deaths have been reported.
In 2015, an outbreak of HFMD occurred during basic military training at Lackland Air Force Base, Bexar County, Texas, due to coxsackie A6.2 The illness was characterized by prodromal symptoms of fever and malaise followed by erosive stomatitis and a rash that began on the palms and soles. The rate of infection among trainees was 4.7% (50 of 1,054 persons).
The differential diagnosis includes aphthous ulcers and herpetic gingivostomatitis.3 Aphthous ulcers are seen more commonly in older children and adolescents, are often recurrent, are not seasonal, and are not associated with rash. Herpes simplex virus gingivostomatitis usually has a febrile prodrome, perioral lesions are frequent in addition to gum and tongue involvement, and gingival bleeding is common. HFMD usually has an incubation period of 3-5 days and fever, malaise, and myalgia prodrome followed by onset of oral and dermatologic manifestations in sequence. The skin rash has features that may overlap with varicella, erythema multiforme (EM) or drug eruption. Varicella usually involves the face before spreading to the extremities, and the lesions are characterized by umbilication and subsequent crusting. EM is characterized by target lesions and drug eruptions are morbilliform or maculopapular. The majority of cases of HFMD are diagnosed clinically; polymerase chain reaction testing is available and best performed on throat or vesicle specimens. Serologic testing for A16 and enterovirus 71 (IgM) is available. Infected patients shed virus for 2-4 weeks and virus is stable in the environment resulting in fecal-oral or oral-oral transmission.
Atypical features of HFMD include occurrence in the winter (outbreak in Alabama in 2011/2012) or an atypical distribution of rash involving the antecubital and popliteal fossae distribution of rash, or “eczema coxsackium” – the accentuation of rash in areas previously affected by atopic dermatitis. Additional features may include nail dystrophies that manifest as Beau lines (deep grooved lines that run from side to side on the fingernail or the toenail) and nail shedding.
A spectrum of neurologic complications has been observed, more frequently with EV71 and more frequently in Asia. The spectrum includes aseptic meningitis and brainstem encephalitis. Progressive cardiopulmonary failure also can be observed in severe cases. The hallmark of severe disease is often presentation with high fever, sweating, mottled skin, and tachycardia. Early signs of CNS involvement include myoclonic jerks, ataxia, and “wandering eyes.”3 Elevated white blood count and/or hyperglycemia may distinguish children with severe disease from benign disease. Anecdotal reports of response to treatment with high-dose methylprednisolone and intravenous immune globulin suggest that the neurologic disease may be an autoimmune phenomenon.
The clinician’s primary role is to accurately diagnose HFMD, provide supportive care for fever and dehydration, and identify those with early signs or laboratory features heralding a more severe course of disease.3 The Centers for Disease Control and Prevention recommends frequent hand washing after toileting and changing diapers, disinfecting surfaces such as toys, avoiding close contact with infected individuals or sharing of personal items for all affected patients. No antiviral treatment is available although improvement following early treatment with acyclovir has been reported anecdotally. Intravenous immunoglobulin has been used in severe cases in Asia with retrospective data analysis suggesting a potential for improvement when administered prior to cardiopulmonary arrest.1
Dr. Pelton is professor of pediatrics and epidemiology at Boston University. Dr. Pelton said he had no relevant financial disclosures. Email him at [email protected].
References
1. Cleveland Clinic Journal of Medicine 2014;81(9):537-43.
2. Morbidity and Mortality Weekly Report MMWR. 2016 Jul 8;65(26);678-80.
3. A Guide to clinical management and public health response for hand, foot and mouth disease (HFMD).
Hand, foot, and mouth disease (HFMD) has become a global challenge since its first description in 1957 in New Zealand and Canada. Clinicians readily recognize the characteristic syndrome in young children: fever associated with a papulovesicular rash affecting the palms or soles, or both, usually in spring, summer, or fall. In most cases the disease is self-limited, and brief. However, aseptic meningitis, brainstem encephalitis, acute flaccid paralysis or autonomic nervous system deregulation or cardiorespiratory failure may complicate the clinical course.
HFMD is caused by enterovirus A (formerly called human enterovirus A) which consists of 25 serotypes including multiple coxsackie A viruses, multiple enteroviruses, simian enteroviruses, and baboon enterovirus A13. The clinical spectrum spans from herpangina, characterized by fever and painful mouth ulcers most prominent in the posterior oral cavity (uvula, tonsils, soft plates, and anterior pharyngeal folds), to HFMD with papulovesicular rash on palms and soles with or without mouth ulcers/vesicles.1 In atypical cases, the rash may be maculopapular and may include the buttocks, knees or elbows.
In the United States, the predominant cause is coxsackie A16. However, coxsackie A6 appears to be emerging; often more than one HFMD causing virus is circulating concurrently and clinically indistinguishable. Globally, especially, in Asia, enterovirus 1 is a major cause of HFMD and more often associated with prominent central nervous system involvement. Disease can be sporadic or epidemic. An outbreak is usually defined as two or more cases within a defined geographic area; global epidemics as large as 1.5 million cases (Taiwan, 1998) have been reported, and outbreaks in China involving tens of thousands with multiple deaths have been reported.
In 2015, an outbreak of HFMD occurred during basic military training at Lackland Air Force Base, Bexar County, Texas, due to coxsackie A6.2 The illness was characterized by prodromal symptoms of fever and malaise followed by erosive stomatitis and a rash that began on the palms and soles. The rate of infection among trainees was 4.7% (50 of 1,054 persons).
The differential diagnosis includes aphthous ulcers and herpetic gingivostomatitis.3 Aphthous ulcers are seen more commonly in older children and adolescents, are often recurrent, are not seasonal, and are not associated with rash. Herpes simplex virus gingivostomatitis usually has a febrile prodrome, perioral lesions are frequent in addition to gum and tongue involvement, and gingival bleeding is common. HFMD usually has an incubation period of 3-5 days and fever, malaise, and myalgia prodrome followed by onset of oral and dermatologic manifestations in sequence. The skin rash has features that may overlap with varicella, erythema multiforme (EM) or drug eruption. Varicella usually involves the face before spreading to the extremities, and the lesions are characterized by umbilication and subsequent crusting. EM is characterized by target lesions and drug eruptions are morbilliform or maculopapular. The majority of cases of HFMD are diagnosed clinically; polymerase chain reaction testing is available and best performed on throat or vesicle specimens. Serologic testing for A16 and enterovirus 71 (IgM) is available. Infected patients shed virus for 2-4 weeks and virus is stable in the environment resulting in fecal-oral or oral-oral transmission.
Atypical features of HFMD include occurrence in the winter (outbreak in Alabama in 2011/2012) or an atypical distribution of rash involving the antecubital and popliteal fossae distribution of rash, or “eczema coxsackium” – the accentuation of rash in areas previously affected by atopic dermatitis. Additional features may include nail dystrophies that manifest as Beau lines (deep grooved lines that run from side to side on the fingernail or the toenail) and nail shedding.
A spectrum of neurologic complications has been observed, more frequently with EV71 and more frequently in Asia. The spectrum includes aseptic meningitis and brainstem encephalitis. Progressive cardiopulmonary failure also can be observed in severe cases. The hallmark of severe disease is often presentation with high fever, sweating, mottled skin, and tachycardia. Early signs of CNS involvement include myoclonic jerks, ataxia, and “wandering eyes.”3 Elevated white blood count and/or hyperglycemia may distinguish children with severe disease from benign disease. Anecdotal reports of response to treatment with high-dose methylprednisolone and intravenous immune globulin suggest that the neurologic disease may be an autoimmune phenomenon.
The clinician’s primary role is to accurately diagnose HFMD, provide supportive care for fever and dehydration, and identify those with early signs or laboratory features heralding a more severe course of disease.3 The Centers for Disease Control and Prevention recommends frequent hand washing after toileting and changing diapers, disinfecting surfaces such as toys, avoiding close contact with infected individuals or sharing of personal items for all affected patients. No antiviral treatment is available although improvement following early treatment with acyclovir has been reported anecdotally. Intravenous immunoglobulin has been used in severe cases in Asia with retrospective data analysis suggesting a potential for improvement when administered prior to cardiopulmonary arrest.1
Dr. Pelton is professor of pediatrics and epidemiology at Boston University. Dr. Pelton said he had no relevant financial disclosures. Email him at [email protected].
References
1. Cleveland Clinic Journal of Medicine 2014;81(9):537-43.
2. Morbidity and Mortality Weekly Report MMWR. 2016 Jul 8;65(26);678-80.
3. A Guide to clinical management and public health response for hand, foot and mouth disease (HFMD).
Summer colds
Enteroviruses cause most summer colds. The enteroviruses include echoviruses, coxsackieviruses, numbered enteroviruses, and the polioviruses. Most summer colds seen in private practice are self limited, presenting with fever alone or clinically distinctive pictures such as hand-foot-and-mouth disease (HFMD), herpangina, or pleurodynia. However, enteroviruses also cause serious illnesses such as meningitis, myocarditis, encephalitis, and neonatal sepsis. Enterovirus infections often are confused with bacterial infections and treated unnecessarily with antibiotics.
Enteroviral infections spread predominantly by the fecal-oral route. Contaminated swimming pools also may serve as a source of transmission. Enteroviruses colonize the respiratory and the gastrointestinal tract. The infection spreads to the lymph nodes, where the virus replicates and an initial viremia occurs on approximately the third postexposure day. The viremia results in subsequent spread to the throat (herpangina), and/or hands and feet (HFMD), lungs (pleurodynia), heart (myocarditis) or meninges (viral meningitis). Infection at the secondary sites corresponds to the onset of clinical symptoms 4-6 days after exposure. The clinical manifestations of enteroviral infections result from the damage caused by the virus at the secondary sites of infection.
Enterovirus pharyngitis starts abruptly and often is accompanied by fever. Younger children may present with increased drooling, hands in the mouth, and refusal to eat. Older children complain of sore throat as well as headache, myalgias, and malaise. Mild vomiting and diarrhea commonly accompany the respiratory symptoms. Herpangina is a specific syndrome of enterovirus pharyngitis; children with this syndrome have fever and characteristic papulovesicular lesions on the anterior tonsillar pillars, soft palate, uvula, tonsils, and pharyngeal wall. The lesions are discrete and average five per patient. They do not appear in the anterior part of the mouth.
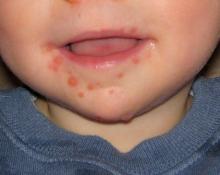
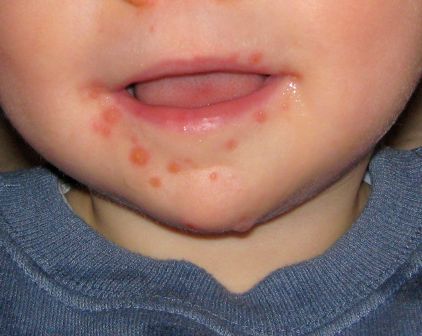
Hand-foot-and-mouth disease is well recognized by clinicians who care for young children. The child presents with fever and papulovesicular lesions within the mouth that quickly become ulcerated and papulovesicular lesions on the palms and soles. The palms and soles often are puffy and red, and the child may act as though her hands and feet hurt, refusing to use her hands or walk. The fever accompanying herpangina and HFMD usually lasts 3 or 4 days, but fever that persists for a week is not uncommon. The pharyngitis follows a pattern similar to the fever.
Pleurodynia has a sudden onset of pain in the chest or upper abdomen. The pain appears to be muscular in origin; its intensity varies. It can be excruciatingly severe and accompanied by sweating and pallor. Older children describe the pain as sharp and stabbing. It occurs in spasms that can last for a few minutes to a few hours. During spasms, the patient has rapid, shallow respirations that suggest pneumonia. The symptoms usually last 1 or 2 days, but the illness can be biphasic, with symptoms resolving only to reappear a few days later.
Gastrointestinal manifestations are almost universal in enterovirus infections. The most common symptoms are anorexia, nausea, vomiting, and diarrhea. They usually are not severe and often occur in combination with other symptoms, such as fever and sore throat. Abdominal pain may be the only manifestation of infection; when severe, it can mimic appendicitis.
Enterovirus infections once were thought to be mild diseases that lasted 2-3 days. But a study of 380 children aged 4-18 years during July to October from private pediatric practices found that illness is prolonged in many patients (Pediatrics. 1998 Nov;102[5]:1126-34). The mean duration of illness was found to be 10 days for myalgia-malaise syndrome, 7 days for herpangina, and 7 days for HFMD.
Spread of enteroviral infections within a household was common. More than 50% of children studied had a family member with enterovirus illness. Half of siblings and 25% of adults within the household of the index case contracted an enteroviral infection. Some had the same presentation as the index patient, but it was not uncommon for other household members to have quite different presentations. For example, the first child seen might present with hand-foot-and-mouth disease, and a few days later a sibling might be brought for care with myalgia-malaise, and the parent might appear ill and complain of pleurodynia.
Summer colds can be costly to families. The duration of the illness and the multitude of nonspecific symptoms sometimes leads to concern about a possible bacterial cause, which prompts a diagnostic workup, including laboratory tests and empiric treatment with antibiotics. The direct costs vary with the syndrome; stomatitis and HFMD are the least expensive to treat because the clinical picture is diagnostic with a single office visit, but a severe manifestation such as aseptic meningitis are expensive to treat with associated emergency department visits, spinal tap, and sometimes hospitalization.
Dr. Pichichero is a specialist in pediatric infectious diseases and director of the Research Institute at Rochester (N.Y.) General Hospital. He reported having no conflicts of interest. Email him at [email protected].
Enteroviruses cause most summer colds. The enteroviruses include echoviruses, coxsackieviruses, numbered enteroviruses, and the polioviruses. Most summer colds seen in private practice are self limited, presenting with fever alone or clinically distinctive pictures such as hand-foot-and-mouth disease (HFMD), herpangina, or pleurodynia. However, enteroviruses also cause serious illnesses such as meningitis, myocarditis, encephalitis, and neonatal sepsis. Enterovirus infections often are confused with bacterial infections and treated unnecessarily with antibiotics.
Enteroviral infections spread predominantly by the fecal-oral route. Contaminated swimming pools also may serve as a source of transmission. Enteroviruses colonize the respiratory and the gastrointestinal tract. The infection spreads to the lymph nodes, where the virus replicates and an initial viremia occurs on approximately the third postexposure day. The viremia results in subsequent spread to the throat (herpangina), and/or hands and feet (HFMD), lungs (pleurodynia), heart (myocarditis) or meninges (viral meningitis). Infection at the secondary sites corresponds to the onset of clinical symptoms 4-6 days after exposure. The clinical manifestations of enteroviral infections result from the damage caused by the virus at the secondary sites of infection.
Enterovirus pharyngitis starts abruptly and often is accompanied by fever. Younger children may present with increased drooling, hands in the mouth, and refusal to eat. Older children complain of sore throat as well as headache, myalgias, and malaise. Mild vomiting and diarrhea commonly accompany the respiratory symptoms. Herpangina is a specific syndrome of enterovirus pharyngitis; children with this syndrome have fever and characteristic papulovesicular lesions on the anterior tonsillar pillars, soft palate, uvula, tonsils, and pharyngeal wall. The lesions are discrete and average five per patient. They do not appear in the anterior part of the mouth.
Hand-foot-and-mouth disease is well recognized by clinicians who care for young children. The child presents with fever and papulovesicular lesions within the mouth that quickly become ulcerated and papulovesicular lesions on the palms and soles. The palms and soles often are puffy and red, and the child may act as though her hands and feet hurt, refusing to use her hands or walk. The fever accompanying herpangina and HFMD usually lasts 3 or 4 days, but fever that persists for a week is not uncommon. The pharyngitis follows a pattern similar to the fever.
Pleurodynia has a sudden onset of pain in the chest or upper abdomen. The pain appears to be muscular in origin; its intensity varies. It can be excruciatingly severe and accompanied by sweating and pallor. Older children describe the pain as sharp and stabbing. It occurs in spasms that can last for a few minutes to a few hours. During spasms, the patient has rapid, shallow respirations that suggest pneumonia. The symptoms usually last 1 or 2 days, but the illness can be biphasic, with symptoms resolving only to reappear a few days later.
Gastrointestinal manifestations are almost universal in enterovirus infections. The most common symptoms are anorexia, nausea, vomiting, and diarrhea. They usually are not severe and often occur in combination with other symptoms, such as fever and sore throat. Abdominal pain may be the only manifestation of infection; when severe, it can mimic appendicitis.
Enterovirus infections once were thought to be mild diseases that lasted 2-3 days. But a study of 380 children aged 4-18 years during July to October from private pediatric practices found that illness is prolonged in many patients (Pediatrics. 1998 Nov;102[5]:1126-34). The mean duration of illness was found to be 10 days for myalgia-malaise syndrome, 7 days for herpangina, and 7 days for HFMD.
Spread of enteroviral infections within a household was common. More than 50% of children studied had a family member with enterovirus illness. Half of siblings and 25% of adults within the household of the index case contracted an enteroviral infection. Some had the same presentation as the index patient, but it was not uncommon for other household members to have quite different presentations. For example, the first child seen might present with hand-foot-and-mouth disease, and a few days later a sibling might be brought for care with myalgia-malaise, and the parent might appear ill and complain of pleurodynia.
Summer colds can be costly to families. The duration of the illness and the multitude of nonspecific symptoms sometimes leads to concern about a possible bacterial cause, which prompts a diagnostic workup, including laboratory tests and empiric treatment with antibiotics. The direct costs vary with the syndrome; stomatitis and HFMD are the least expensive to treat because the clinical picture is diagnostic with a single office visit, but a severe manifestation such as aseptic meningitis are expensive to treat with associated emergency department visits, spinal tap, and sometimes hospitalization.
Dr. Pichichero is a specialist in pediatric infectious diseases and director of the Research Institute at Rochester (N.Y.) General Hospital. He reported having no conflicts of interest. Email him at [email protected].
Enteroviruses cause most summer colds. The enteroviruses include echoviruses, coxsackieviruses, numbered enteroviruses, and the polioviruses. Most summer colds seen in private practice are self limited, presenting with fever alone or clinically distinctive pictures such as hand-foot-and-mouth disease (HFMD), herpangina, or pleurodynia. However, enteroviruses also cause serious illnesses such as meningitis, myocarditis, encephalitis, and neonatal sepsis. Enterovirus infections often are confused with bacterial infections and treated unnecessarily with antibiotics.
Enteroviral infections spread predominantly by the fecal-oral route. Contaminated swimming pools also may serve as a source of transmission. Enteroviruses colonize the respiratory and the gastrointestinal tract. The infection spreads to the lymph nodes, where the virus replicates and an initial viremia occurs on approximately the third postexposure day. The viremia results in subsequent spread to the throat (herpangina), and/or hands and feet (HFMD), lungs (pleurodynia), heart (myocarditis) or meninges (viral meningitis). Infection at the secondary sites corresponds to the onset of clinical symptoms 4-6 days after exposure. The clinical manifestations of enteroviral infections result from the damage caused by the virus at the secondary sites of infection.
Enterovirus pharyngitis starts abruptly and often is accompanied by fever. Younger children may present with increased drooling, hands in the mouth, and refusal to eat. Older children complain of sore throat as well as headache, myalgias, and malaise. Mild vomiting and diarrhea commonly accompany the respiratory symptoms. Herpangina is a specific syndrome of enterovirus pharyngitis; children with this syndrome have fever and characteristic papulovesicular lesions on the anterior tonsillar pillars, soft palate, uvula, tonsils, and pharyngeal wall. The lesions are discrete and average five per patient. They do not appear in the anterior part of the mouth.
Hand-foot-and-mouth disease is well recognized by clinicians who care for young children. The child presents with fever and papulovesicular lesions within the mouth that quickly become ulcerated and papulovesicular lesions on the palms and soles. The palms and soles often are puffy and red, and the child may act as though her hands and feet hurt, refusing to use her hands or walk. The fever accompanying herpangina and HFMD usually lasts 3 or 4 days, but fever that persists for a week is not uncommon. The pharyngitis follows a pattern similar to the fever.
Pleurodynia has a sudden onset of pain in the chest or upper abdomen. The pain appears to be muscular in origin; its intensity varies. It can be excruciatingly severe and accompanied by sweating and pallor. Older children describe the pain as sharp and stabbing. It occurs in spasms that can last for a few minutes to a few hours. During spasms, the patient has rapid, shallow respirations that suggest pneumonia. The symptoms usually last 1 or 2 days, but the illness can be biphasic, with symptoms resolving only to reappear a few days later.
Gastrointestinal manifestations are almost universal in enterovirus infections. The most common symptoms are anorexia, nausea, vomiting, and diarrhea. They usually are not severe and often occur in combination with other symptoms, such as fever and sore throat. Abdominal pain may be the only manifestation of infection; when severe, it can mimic appendicitis.
Enterovirus infections once were thought to be mild diseases that lasted 2-3 days. But a study of 380 children aged 4-18 years during July to October from private pediatric practices found that illness is prolonged in many patients (Pediatrics. 1998 Nov;102[5]:1126-34). The mean duration of illness was found to be 10 days for myalgia-malaise syndrome, 7 days for herpangina, and 7 days for HFMD.
Spread of enteroviral infections within a household was common. More than 50% of children studied had a family member with enterovirus illness. Half of siblings and 25% of adults within the household of the index case contracted an enteroviral infection. Some had the same presentation as the index patient, but it was not uncommon for other household members to have quite different presentations. For example, the first child seen might present with hand-foot-and-mouth disease, and a few days later a sibling might be brought for care with myalgia-malaise, and the parent might appear ill and complain of pleurodynia.
Summer colds can be costly to families. The duration of the illness and the multitude of nonspecific symptoms sometimes leads to concern about a possible bacterial cause, which prompts a diagnostic workup, including laboratory tests and empiric treatment with antibiotics. The direct costs vary with the syndrome; stomatitis and HFMD are the least expensive to treat because the clinical picture is diagnostic with a single office visit, but a severe manifestation such as aseptic meningitis are expensive to treat with associated emergency department visits, spinal tap, and sometimes hospitalization.
Dr. Pichichero is a specialist in pediatric infectious diseases and director of the Research Institute at Rochester (N.Y.) General Hospital. He reported having no conflicts of interest. Email him at [email protected].
Kawasaki disease: New info to enhance our index of suspicion
Most U.S. mainland pediatric practitioners will see only one or two cases of Kawasaki disease (KD) in their careers, but no one wants to miss even one case.
Making the diagnosis as early as possible is important to reduce the chance of sequelae, particularly the coronary artery aneurysms that will eventually lead to 5% of overall acute coronary syndromes in adults. And because there is no “KD test,” or sometimes incomplete KD. And there are some new data that complicate this. Despite the recently updated 2017 guideline,1 most cases end up being confirmed and managed by regional “experts.” But nearly all of the approximately 6,000 KD cases/year in U.S. children younger than 5 years old start out with one or more primary care, urgent care, or ED visits.
This means that every clinician in the trenches not only needs a high index of suspicion but also needs to be at least a partial expert, too. What raises our index of suspicion? Classic data tell us we need 5 consecutive days of fever plus four or five other principal clinical findings for a KD diagnosis. The principal findings are:
1. Eyes: Bilateral bulbar nonexudative conjunctival injection.
2. Mouth: Erythema of oral/pharyngeal mucosa or cracked lips or strawberry tongue or oral mucositis.
3. Rash.
4. Hands or feet findings: Swelling/erythema or later periungual desquamation.
5. Cervical adenopathy greater than 1.4 cm, usually unilateral.
Other factors that have classically increased suspicion are winter/early spring presentation in North America, male gender (1.5:1 ratio to females), and Asian (particularly Japanese) ancestry. The importance of genetics was initially based on epidemiology (Japan/Asian risk) but lately has been further associated with six gene polymorphisms. However, molecular genetic testing is not currently a practical tool.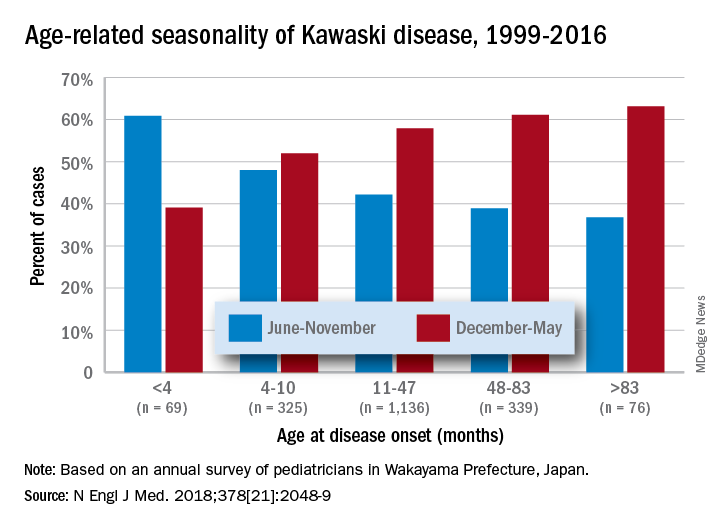
Clinical scenarios that also should raise suspicion include less-than-6-month-old infants with prolonged fever/irritability (may be the only clinical manifestations of KD) and children over 4 years old who more often may have incomplete KD. Both groups have higher prevalence of coronary artery abnormalities. Other high suspicion scenarios include prolonged fever with unexplained/culture-negative shock, or antibiotic treatment failure for cervical adenitis or retro/parapharyngeal phlegmon. Consultation with or referral to a regional KD expert may be needed.
Fuzzy KD math
Current guidelines list an exception to the 5-day fever requirement in that only 4 days of fever are needed with four or more principal clinical features, particularly when hand and feet findings exist. Some call this the “4X4 exception.” Then there is a sub-caveat: “Experienced clinicians who have treated many patients with KD may establish the diagnosis with 3 days of fever in rare cases.”1
Incomplete KD
This is another exception, which seems to be a more frequent diagnosis in the past decade. Incomplete KD requires the 5 days of fever duration plus an elevated C-reactive protein or erythrocyte sedimentation rate. But one needs only two or three of the five principal clinical KD criteria plus three or more of six other laboratory findings (anemia, low albumin, leukocytosis, thrombocytosis, pyuria, or elevated alanine aminotransferase). Incomplete KD can be confirmed by an abnormal echocardiogram – usually not until after 7 days of KD symptoms.1
New KD nuances
In a recent report on 20 years of data from Japan (n = 1,945 KD cases), more granularity on age, seasonal epidemiology, and outcome were seen.2 There was an inverse correlation of male predominance to age, i.e. as age groups got older, there was a gradual shift to female predominance by 7 years of age. The winter/spring predominance (60% of overall cases) did not hold true in younger age groups where summer/fall was the peak season (65% of cases).
With the goal of not missing any KD and diagnosing as early as possible to limit sequelae, we all need to be relative experts and keep alert for clinical scenarios that warrant our raising our index of suspicion. But now the seasonality trends appear blurred in the youngest cases and the male predominance is blurred in the oldest cases. And remember that fever and irritability for longer than 7 days in young infants may be the only clue to KD.
Dr. Harrison is professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospitals and Clinics, Kansas City, Mo. He said he had no relevant financial disclosures. Email him at [email protected].
References
1. Circulation. 2017 Mar 29. doi: 10.1161/CIR.0000000000000484
2. N Engl J Med. 2018 May 24. doi: 10.1056/NEJMc1804312.
Most U.S. mainland pediatric practitioners will see only one or two cases of Kawasaki disease (KD) in their careers, but no one wants to miss even one case.
Making the diagnosis as early as possible is important to reduce the chance of sequelae, particularly the coronary artery aneurysms that will eventually lead to 5% of overall acute coronary syndromes in adults. And because there is no “KD test,” or sometimes incomplete KD. And there are some new data that complicate this. Despite the recently updated 2017 guideline,1 most cases end up being confirmed and managed by regional “experts.” But nearly all of the approximately 6,000 KD cases/year in U.S. children younger than 5 years old start out with one or more primary care, urgent care, or ED visits.
This means that every clinician in the trenches not only needs a high index of suspicion but also needs to be at least a partial expert, too. What raises our index of suspicion? Classic data tell us we need 5 consecutive days of fever plus four or five other principal clinical findings for a KD diagnosis. The principal findings are:
1. Eyes: Bilateral bulbar nonexudative conjunctival injection.
2. Mouth: Erythema of oral/pharyngeal mucosa or cracked lips or strawberry tongue or oral mucositis.
3. Rash.
4. Hands or feet findings: Swelling/erythema or later periungual desquamation.
5. Cervical adenopathy greater than 1.4 cm, usually unilateral.
Other factors that have classically increased suspicion are winter/early spring presentation in North America, male gender (1.5:1 ratio to females), and Asian (particularly Japanese) ancestry. The importance of genetics was initially based on epidemiology (Japan/Asian risk) but lately has been further associated with six gene polymorphisms. However, molecular genetic testing is not currently a practical tool.
Clinical scenarios that also should raise suspicion include less-than-6-month-old infants with prolonged fever/irritability (may be the only clinical manifestations of KD) and children over 4 years old who more often may have incomplete KD. Both groups have higher prevalence of coronary artery abnormalities. Other high suspicion scenarios include prolonged fever with unexplained/culture-negative shock, or antibiotic treatment failure for cervical adenitis or retro/parapharyngeal phlegmon. Consultation with or referral to a regional KD expert may be needed.
Fuzzy KD math
Current guidelines list an exception to the 5-day fever requirement in that only 4 days of fever are needed with four or more principal clinical features, particularly when hand and feet findings exist. Some call this the “4X4 exception.” Then there is a sub-caveat: “Experienced clinicians who have treated many patients with KD may establish the diagnosis with 3 days of fever in rare cases.”1
Incomplete KD
This is another exception, which seems to be a more frequent diagnosis in the past decade. Incomplete KD requires the 5 days of fever duration plus an elevated C-reactive protein or erythrocyte sedimentation rate. But one needs only two or three of the five principal clinical KD criteria plus three or more of six other laboratory findings (anemia, low albumin, leukocytosis, thrombocytosis, pyuria, or elevated alanine aminotransferase). Incomplete KD can be confirmed by an abnormal echocardiogram – usually not until after 7 days of KD symptoms.1
New KD nuances
In a recent report on 20 years of data from Japan (n = 1,945 KD cases), more granularity on age, seasonal epidemiology, and outcome were seen.2 There was an inverse correlation of male predominance to age, i.e. as age groups got older, there was a gradual shift to female predominance by 7 years of age. The winter/spring predominance (60% of overall cases) did not hold true in younger age groups where summer/fall was the peak season (65% of cases).
With the goal of not missing any KD and diagnosing as early as possible to limit sequelae, we all need to be relative experts and keep alert for clinical scenarios that warrant our raising our index of suspicion. But now the seasonality trends appear blurred in the youngest cases and the male predominance is blurred in the oldest cases. And remember that fever and irritability for longer than 7 days in young infants may be the only clue to KD.
Dr. Harrison is professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospitals and Clinics, Kansas City, Mo. He said he had no relevant financial disclosures. Email him at [email protected].
References
1. Circulation. 2017 Mar 29. doi: 10.1161/CIR.0000000000000484
2. N Engl J Med. 2018 May 24. doi: 10.1056/NEJMc1804312.
Most U.S. mainland pediatric practitioners will see only one or two cases of Kawasaki disease (KD) in their careers, but no one wants to miss even one case.
Making the diagnosis as early as possible is important to reduce the chance of sequelae, particularly the coronary artery aneurysms that will eventually lead to 5% of overall acute coronary syndromes in adults. And because there is no “KD test,” or sometimes incomplete KD. And there are some new data that complicate this. Despite the recently updated 2017 guideline,1 most cases end up being confirmed and managed by regional “experts.” But nearly all of the approximately 6,000 KD cases/year in U.S. children younger than 5 years old start out with one or more primary care, urgent care, or ED visits.
This means that every clinician in the trenches not only needs a high index of suspicion but also needs to be at least a partial expert, too. What raises our index of suspicion? Classic data tell us we need 5 consecutive days of fever plus four or five other principal clinical findings for a KD diagnosis. The principal findings are:
1. Eyes: Bilateral bulbar nonexudative conjunctival injection.
2. Mouth: Erythema of oral/pharyngeal mucosa or cracked lips or strawberry tongue or oral mucositis.
3. Rash.
4. Hands or feet findings: Swelling/erythema or later periungual desquamation.
5. Cervical adenopathy greater than 1.4 cm, usually unilateral.
Other factors that have classically increased suspicion are winter/early spring presentation in North America, male gender (1.5:1 ratio to females), and Asian (particularly Japanese) ancestry. The importance of genetics was initially based on epidemiology (Japan/Asian risk) but lately has been further associated with six gene polymorphisms. However, molecular genetic testing is not currently a practical tool.
Clinical scenarios that also should raise suspicion include less-than-6-month-old infants with prolonged fever/irritability (may be the only clinical manifestations of KD) and children over 4 years old who more often may have incomplete KD. Both groups have higher prevalence of coronary artery abnormalities. Other high suspicion scenarios include prolonged fever with unexplained/culture-negative shock, or antibiotic treatment failure for cervical adenitis or retro/parapharyngeal phlegmon. Consultation with or referral to a regional KD expert may be needed.
Fuzzy KD math
Current guidelines list an exception to the 5-day fever requirement in that only 4 days of fever are needed with four or more principal clinical features, particularly when hand and feet findings exist. Some call this the “4X4 exception.” Then there is a sub-caveat: “Experienced clinicians who have treated many patients with KD may establish the diagnosis with 3 days of fever in rare cases.”1
Incomplete KD
This is another exception, which seems to be a more frequent diagnosis in the past decade. Incomplete KD requires the 5 days of fever duration plus an elevated C-reactive protein or erythrocyte sedimentation rate. But one needs only two or three of the five principal clinical KD criteria plus three or more of six other laboratory findings (anemia, low albumin, leukocytosis, thrombocytosis, pyuria, or elevated alanine aminotransferase). Incomplete KD can be confirmed by an abnormal echocardiogram – usually not until after 7 days of KD symptoms.1
New KD nuances
In a recent report on 20 years of data from Japan (n = 1,945 KD cases), more granularity on age, seasonal epidemiology, and outcome were seen.2 There was an inverse correlation of male predominance to age, i.e. as age groups got older, there was a gradual shift to female predominance by 7 years of age. The winter/spring predominance (60% of overall cases) did not hold true in younger age groups where summer/fall was the peak season (65% of cases).
With the goal of not missing any KD and diagnosing as early as possible to limit sequelae, we all need to be relative experts and keep alert for clinical scenarios that warrant our raising our index of suspicion. But now the seasonality trends appear blurred in the youngest cases and the male predominance is blurred in the oldest cases. And remember that fever and irritability for longer than 7 days in young infants may be the only clue to KD.
Dr. Harrison is professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospitals and Clinics, Kansas City, Mo. He said he had no relevant financial disclosures. Email him at [email protected].
References
1. Circulation. 2017 Mar 29. doi: 10.1161/CIR.0000000000000484
2. N Engl J Med. 2018 May 24. doi: 10.1056/NEJMc1804312.
International travel updates
It’s that time of year again. Many of your patients will join the 80.2 million Americans with plans for international travel this summer.
In 2016, Mexico (31.2 million) and Canada (13.9 million) were the top two destinations of U.S. residents. Based on 2016 U.S. Commerce data, an additional 35.1 million Americans headed to overseas destinations, including 9% who traveled with children. Vacation and visiting friends and relatives accounted for 55% and 27% of the reasons for all travel, respectively. Education accounted for 4% of travelers.
Required versus recommended vaccines
The goal of a required vaccine is to prevent international spread of disease. The host country is protecting its citizens from visitors importing and facilitating the spread of a disease. Yellow fever and meningococcal disease are the only vaccines required for entry into any country. Entry requirements vary by country. Yellow fever may be an entry requirement for all travelers or it may be limited to those who have been in, or have had transit through, a country where yellow fever can be transmitted at least 6 days prior to the arrival at their final destination – a reminder that the sequence of the patient’s itinerary is important. In addition, just because a vaccine is not required for entry does not mean the risk for exposure and acquisition is nonexistent.
In contrast, recommended vaccines are for the protection of the individual. Travelers may be exposed to vaccine-preventable diseases that do not exist in their country (such as measles, typhoid fever, and yellow fever). They are at risk for acquisition and may return home infected, which could create the potential to spread the disease to susceptible contacts.
Most travelers comprehend required vaccines but often fail to understand the importance of receiving recommended vaccines. Lammert et al. reported that, of 24,478 persons who received pretravel advice between July 2012 and June 2014 through Global TravEpiNet, a national consortium of U.S. clinics, 97% were eligible for at least one vaccine. The majority were eligible for typhoid (n = 20,092) and hepatitis A (n = 12,990). Of patients included in the study, 25% (6,573) refused one or more vaccines. The most common reason cited for refusal was a lack of concern about the illness. Travelers visiting friends and relatives were less likely to accept all recommended vaccines, compared with those who were not visiting friends and relatives (odds ratio, 0.74) (J Trav Med. 2017 Jan. doi: 10.1093/jtm/taw075). In the United States, international travel remains the most common risk factor for acquisition of both typhoid fever and hepatitis A.
What’s new
The U.S. Advisory Committee on Immunization Practices recommends administering the hepatitis A vaccine to infants aged 6-11 months with travel to or living in developing countries and areas with high to moderate risk for hepatitis A virus transmission. Any dose received at less than 12 months of age does not count, and the administration of two age-appropriate doses should occur following this dose.
Old but still relevant
Measles: The Advisory Committee on Immunization Practices recommends all infants aged 6-11 months receive one dose of MMR prior to international travel regardless of the destination. This should be followed by two additional countable doses. All persons at least 12 months of age and born after 1956 should receive two doses of MMR at least 28 days apart prior to international travel.
Prior to administering, determine whether your patient will travel to a yellow fever–endemic area because both are live vaccines and should be received the same day. Otherwise, administer MMR doses 28 days apart; coordination between facilities or receipt of both at one facility may be necessary.
Yellow fever vaccine: The U.S. supplies of YF-Vax by Sanofi Pasteur are not expected to be available again until the end of 2018. To provide vaccines for U.S. travelers, Stamaril – a yellow fever vaccine produced by Sanofi Pasteur in France – has been made available at more than 250 sites through an Expanded Access Investigational New Drug Program.
Since Stamaril is offered at a limited number of locations, persons with anticipated travel to a country where receipt of yellow fever vaccine is either required for entry or recommended for their protection should not wait until the last minute to obtain it. Postponing a trip or changing a destination is preferred if vaccine is not received, especially when the person is traveling to countries with an ongoing outbreak.
The vaccination does not become valid until 10 days after receipt. Infants aged at least 9 months may receive the vaccine. Since the yellow fever vaccine is a live vaccine, administration may be contraindicated in certain individuals. Exemption letters are provided for those with medical contraindication.
To locate a Stamaril site in your area: https://wwwnc.cdc.gov/travel/page/search-for-stamaril-clinics.
Current disease outbreaks
Yellow fever: Brazil
Since Dec. 2017, more than 1,100 laboratory-confirmed cases of yellow fever have been reported, including 17 reported in unvaccinated international travelers. Fatal cases also have been reported. In addition to areas in Brazil where yellow fever vaccination had been recommended prior to the recent outbreaks, the vaccine now also is recommended for people who are traveling to or living in all of Espírito Santo State, São Paulo State, and Rio de Janeiro State, as well as several cities in Bahia State. Unvaccinated travelers should avoid travel to areas where vaccination is recommended. Those previously vaccinated at 10 years ago or longer should consider a booster.
Listeria: South Africa
An ongoing outbreak has been reported since Jan. 2017. Around 1,000 people have been infected. Avoid consumption of processed meats including “Polony” (South African bologna).
Measles: Belarus, Japan, Liberia, and Taiwan
All countries have reported an increase in cases since April 2018. Measles outbreaks have been reported in an additional 13 countries since Jan. 2018, including France, Ireland, Italy, the Philippines, and the United Kingdom.
Norovirus: Canada
More than 120 cases have been linked to consumption of raw or lightly cooked oysters from western Canada.
For more country-specific information and up to date travel alerts, visit http://www.cdc.gov/travel.
Dr. Word is a pediatric infectious disease specialist and the director of the Houston Travel Medicine Clinic. She said she had no relevant financial disclosures.
It’s that time of year again. Many of your patients will join the 80.2 million Americans with plans for international travel this summer.
In 2016, Mexico (31.2 million) and Canada (13.9 million) were the top two destinations of U.S. residents. Based on 2016 U.S. Commerce data, an additional 35.1 million Americans headed to overseas destinations, including 9% who traveled with children. Vacation and visiting friends and relatives accounted for 55% and 27% of the reasons for all travel, respectively. Education accounted for 4% of travelers.
Required versus recommended vaccines
The goal of a required vaccine is to prevent international spread of disease. The host country is protecting its citizens from visitors importing and facilitating the spread of a disease. Yellow fever and meningococcal disease are the only vaccines required for entry into any country. Entry requirements vary by country. Yellow fever may be an entry requirement for all travelers or it may be limited to those who have been in, or have had transit through, a country where yellow fever can be transmitted at least 6 days prior to the arrival at their final destination – a reminder that the sequence of the patient’s itinerary is important. In addition, just because a vaccine is not required for entry does not mean the risk for exposure and acquisition is nonexistent.
In contrast, recommended vaccines are for the protection of the individual. Travelers may be exposed to vaccine-preventable diseases that do not exist in their country (such as measles, typhoid fever, and yellow fever). They are at risk for acquisition and may return home infected, which could create the potential to spread the disease to susceptible contacts.
Most travelers comprehend required vaccines but often fail to understand the importance of receiving recommended vaccines. Lammert et al. reported that, of 24,478 persons who received pretravel advice between July 2012 and June 2014 through Global TravEpiNet, a national consortium of U.S. clinics, 97% were eligible for at least one vaccine. The majority were eligible for typhoid (n = 20,092) and hepatitis A (n = 12,990). Of patients included in the study, 25% (6,573) refused one or more vaccines. The most common reason cited for refusal was a lack of concern about the illness. Travelers visiting friends and relatives were less likely to accept all recommended vaccines, compared with those who were not visiting friends and relatives (odds ratio, 0.74) (J Trav Med. 2017 Jan. doi: 10.1093/jtm/taw075). In the United States, international travel remains the most common risk factor for acquisition of both typhoid fever and hepatitis A.
What’s new
The U.S. Advisory Committee on Immunization Practices recommends administering the hepatitis A vaccine to infants aged 6-11 months with travel to or living in developing countries and areas with high to moderate risk for hepatitis A virus transmission. Any dose received at less than 12 months of age does not count, and the administration of two age-appropriate doses should occur following this dose.
Old but still relevant
Measles: The Advisory Committee on Immunization Practices recommends all infants aged 6-11 months receive one dose of MMR prior to international travel regardless of the destination. This should be followed by two additional countable doses. All persons at least 12 months of age and born after 1956 should receive two doses of MMR at least 28 days apart prior to international travel.
Prior to administering, determine whether your patient will travel to a yellow fever–endemic area because both are live vaccines and should be received the same day. Otherwise, administer MMR doses 28 days apart; coordination between facilities or receipt of both at one facility may be necessary.
Yellow fever vaccine: The U.S. supplies of YF-Vax by Sanofi Pasteur are not expected to be available again until the end of 2018. To provide vaccines for U.S. travelers, Stamaril – a yellow fever vaccine produced by Sanofi Pasteur in France – has been made available at more than 250 sites through an Expanded Access Investigational New Drug Program.
Since Stamaril is offered at a limited number of locations, persons with anticipated travel to a country where receipt of yellow fever vaccine is either required for entry or recommended for their protection should not wait until the last minute to obtain it. Postponing a trip or changing a destination is preferred if vaccine is not received, especially when the person is traveling to countries with an ongoing outbreak.
The vaccination does not become valid until 10 days after receipt. Infants aged at least 9 months may receive the vaccine. Since the yellow fever vaccine is a live vaccine, administration may be contraindicated in certain individuals. Exemption letters are provided for those with medical contraindication.
To locate a Stamaril site in your area: https://wwwnc.cdc.gov/travel/page/search-for-stamaril-clinics.
Current disease outbreaks
Yellow fever: Brazil
Since Dec. 2017, more than 1,100 laboratory-confirmed cases of yellow fever have been reported, including 17 reported in unvaccinated international travelers. Fatal cases also have been reported. In addition to areas in Brazil where yellow fever vaccination had been recommended prior to the recent outbreaks, the vaccine now also is recommended for people who are traveling to or living in all of Espírito Santo State, São Paulo State, and Rio de Janeiro State, as well as several cities in Bahia State. Unvaccinated travelers should avoid travel to areas where vaccination is recommended. Those previously vaccinated at 10 years ago or longer should consider a booster.
Listeria: South Africa
An ongoing outbreak has been reported since Jan. 2017. Around 1,000 people have been infected. Avoid consumption of processed meats including “Polony” (South African bologna).
Measles: Belarus, Japan, Liberia, and Taiwan
All countries have reported an increase in cases since April 2018. Measles outbreaks have been reported in an additional 13 countries since Jan. 2018, including France, Ireland, Italy, the Philippines, and the United Kingdom.
Norovirus: Canada
More than 120 cases have been linked to consumption of raw or lightly cooked oysters from western Canada.
For more country-specific information and up to date travel alerts, visit http://www.cdc.gov/travel.
Dr. Word is a pediatric infectious disease specialist and the director of the Houston Travel Medicine Clinic. She said she had no relevant financial disclosures.
It’s that time of year again. Many of your patients will join the 80.2 million Americans with plans for international travel this summer.
In 2016, Mexico (31.2 million) and Canada (13.9 million) were the top two destinations of U.S. residents. Based on 2016 U.S. Commerce data, an additional 35.1 million Americans headed to overseas destinations, including 9% who traveled with children. Vacation and visiting friends and relatives accounted for 55% and 27% of the reasons for all travel, respectively. Education accounted for 4% of travelers.
Required versus recommended vaccines
The goal of a required vaccine is to prevent international spread of disease. The host country is protecting its citizens from visitors importing and facilitating the spread of a disease. Yellow fever and meningococcal disease are the only vaccines required for entry into any country. Entry requirements vary by country. Yellow fever may be an entry requirement for all travelers or it may be limited to those who have been in, or have had transit through, a country where yellow fever can be transmitted at least 6 days prior to the arrival at their final destination – a reminder that the sequence of the patient’s itinerary is important. In addition, just because a vaccine is not required for entry does not mean the risk for exposure and acquisition is nonexistent.
In contrast, recommended vaccines are for the protection of the individual. Travelers may be exposed to vaccine-preventable diseases that do not exist in their country (such as measles, typhoid fever, and yellow fever). They are at risk for acquisition and may return home infected, which could create the potential to spread the disease to susceptible contacts.
Most travelers comprehend required vaccines but often fail to understand the importance of receiving recommended vaccines. Lammert et al. reported that, of 24,478 persons who received pretravel advice between July 2012 and June 2014 through Global TravEpiNet, a national consortium of U.S. clinics, 97% were eligible for at least one vaccine. The majority were eligible for typhoid (n = 20,092) and hepatitis A (n = 12,990). Of patients included in the study, 25% (6,573) refused one or more vaccines. The most common reason cited for refusal was a lack of concern about the illness. Travelers visiting friends and relatives were less likely to accept all recommended vaccines, compared with those who were not visiting friends and relatives (odds ratio, 0.74) (J Trav Med. 2017 Jan. doi: 10.1093/jtm/taw075). In the United States, international travel remains the most common risk factor for acquisition of both typhoid fever and hepatitis A.
What’s new
The U.S. Advisory Committee on Immunization Practices recommends administering the hepatitis A vaccine to infants aged 6-11 months with travel to or living in developing countries and areas with high to moderate risk for hepatitis A virus transmission. Any dose received at less than 12 months of age does not count, and the administration of two age-appropriate doses should occur following this dose.
Old but still relevant
Measles: The Advisory Committee on Immunization Practices recommends all infants aged 6-11 months receive one dose of MMR prior to international travel regardless of the destination. This should be followed by two additional countable doses. All persons at least 12 months of age and born after 1956 should receive two doses of MMR at least 28 days apart prior to international travel.
Prior to administering, determine whether your patient will travel to a yellow fever–endemic area because both are live vaccines and should be received the same day. Otherwise, administer MMR doses 28 days apart; coordination between facilities or receipt of both at one facility may be necessary.
Yellow fever vaccine: The U.S. supplies of YF-Vax by Sanofi Pasteur are not expected to be available again until the end of 2018. To provide vaccines for U.S. travelers, Stamaril – a yellow fever vaccine produced by Sanofi Pasteur in France – has been made available at more than 250 sites through an Expanded Access Investigational New Drug Program.
Since Stamaril is offered at a limited number of locations, persons with anticipated travel to a country where receipt of yellow fever vaccine is either required for entry or recommended for their protection should not wait until the last minute to obtain it. Postponing a trip or changing a destination is preferred if vaccine is not received, especially when the person is traveling to countries with an ongoing outbreak.
The vaccination does not become valid until 10 days after receipt. Infants aged at least 9 months may receive the vaccine. Since the yellow fever vaccine is a live vaccine, administration may be contraindicated in certain individuals. Exemption letters are provided for those with medical contraindication.
To locate a Stamaril site in your area: https://wwwnc.cdc.gov/travel/page/search-for-stamaril-clinics.
Current disease outbreaks
Yellow fever: Brazil
Since Dec. 2017, more than 1,100 laboratory-confirmed cases of yellow fever have been reported, including 17 reported in unvaccinated international travelers. Fatal cases also have been reported. In addition to areas in Brazil where yellow fever vaccination had been recommended prior to the recent outbreaks, the vaccine now also is recommended for people who are traveling to or living in all of Espírito Santo State, São Paulo State, and Rio de Janeiro State, as well as several cities in Bahia State. Unvaccinated travelers should avoid travel to areas where vaccination is recommended. Those previously vaccinated at 10 years ago or longer should consider a booster.
Listeria: South Africa
An ongoing outbreak has been reported since Jan. 2017. Around 1,000 people have been infected. Avoid consumption of processed meats including “Polony” (South African bologna).
Measles: Belarus, Japan, Liberia, and Taiwan
All countries have reported an increase in cases since April 2018. Measles outbreaks have been reported in an additional 13 countries since Jan. 2018, including France, Ireland, Italy, the Philippines, and the United Kingdom.
Norovirus: Canada
More than 120 cases have been linked to consumption of raw or lightly cooked oysters from western Canada.
For more country-specific information and up to date travel alerts, visit http://www.cdc.gov/travel.
Dr. Word is a pediatric infectious disease specialist and the director of the Houston Travel Medicine Clinic. She said she had no relevant financial disclosures.
Evaluating fever in the first 90 days of life
Fever in the youngest of infants creates a challenge for the pediatric clinician. Fever is a common presentation for serious bacterial infection (SBI) although most fevers are due to viral infection. However, the clinical presentation does not necessarily differ, and the risk for a poor outcome in this age group is substantial.
In the early stages of my pediatric career, most febrile infants less than 90 days of age were evaluated for sepsis, admitted, and treated with antibiotics pending culture results. Group B streptococcal sepsis or Escherichia coli sepsis were common in the first month of life, and Haemophilus influenza type B or Streptococcus pneumoniae in the second and third months of life. The approach to fever in the first 90 days has changed following both the introduction of haemophilus and pneumococcal conjugate vaccines, the experience with risk stratification criteria for identifying infants at low risk for SBI, and the recognition of urinary tract infection (UTI) as a common source of infection in this age group as well as development of criteria for diagnosis.
A further nuance was subsequently added with the introduction of rapid diagnostics for viral infection. Byington et al. found that the majority of febrile infants less than 90 days of age had viral infection with enterovirus, respiratory syncytial virus (RSV), influenza or rotavirus.1 Using the Rochester risk stratification and the presence or absence of viral infection, she demonstrated that the risk of SBI was reduced in both high- and low-risk infants in the presence of viral infection; in low risk infants with viral infection, SBI was identified in 1.8%, compared with 3.1% in those without viral infection, and in high-risk infants. 5.5% has SBI when viral infection was found, compared to 16.7% in the absence of viral infection. She also proposed risk features to identify those infected with herpes simplex virus; age less than 42 days, vesicular rash, elevated alanine transaminase (ALT) and aspartate aminotransferase (AST), CSF pleocytosis, and seizure or twitching.
Greenhow et al. reported on the experience with “serious” bacterial infection in infants less than 90 days of age receiving care at Northern California Kaiser Permanente during the period 2005-2011.2 As pictured, the majority of children have UTI, and smaller numbers have bacteremia or meningitis. A small group of children with UTI have urosepsis as well; those with urosepsis can be differentiated from those with only UTI by age (less than 21 days), clinical exam (ill appearing), and elevated C reactive protein (greater than 20 mg/L) or elevated procalcitonin (greater than 0.5 ng/mL).3 Further evaluation of procalcitonin by other groups appears to validate its role in identifying children at low risk of SBI (procalcitonin less than 0.3 ng/mL).4
Currently, studies of febrile infants less than 90 days of age demonstrate that E. coli dominates in bacteremia, UTI, and meningitis, with Group B streptococcus as the next most frequent pathogen identified.2 Increasingly ampicillin resistance has been reported among E. coli isolates from both early- and late-onset disease as well as rare isolates that are resistant to third generation cephalosporins or gentamicin. Surveillance to identify changes in antimicrobial susceptibility will need to be ongoing to ensure that current approaches for initial therapy in high-risk infants aligns with current susceptibility patterns.
Dr. Pelton is chief of the section of pediatric infectious diseases and coordinator of the maternal-child HIV program at Boston Medical Center. He said he had no relevant financial disclosures. Email him at [email protected].
References
1. Pediatrics. 2004 Jun;113(6):1662-6.
2. Pediatr Infect Dis J. 2014 Jun;33(6):595-9.
3. Pediatr Infect Dis J. 2015 Jan;34(1):17-21.
4. JAMA Pediatr. 2016;170(1):17-18.
5. “AAP Proposes Update to Evaluating, Managing Febrile Infants Guideline,” The Hospitalist, 2016.
Fever in the youngest of infants creates a challenge for the pediatric clinician. Fever is a common presentation for serious bacterial infection (SBI) although most fevers are due to viral infection. However, the clinical presentation does not necessarily differ, and the risk for a poor outcome in this age group is substantial.
In the early stages of my pediatric career, most febrile infants less than 90 days of age were evaluated for sepsis, admitted, and treated with antibiotics pending culture results. Group B streptococcal sepsis or Escherichia coli sepsis were common in the first month of life, and Haemophilus influenza type B or Streptococcus pneumoniae in the second and third months of life. The approach to fever in the first 90 days has changed following both the introduction of haemophilus and pneumococcal conjugate vaccines, the experience with risk stratification criteria for identifying infants at low risk for SBI, and the recognition of urinary tract infection (UTI) as a common source of infection in this age group as well as development of criteria for diagnosis.
A further nuance was subsequently added with the introduction of rapid diagnostics for viral infection. Byington et al. found that the majority of febrile infants less than 90 days of age had viral infection with enterovirus, respiratory syncytial virus (RSV), influenza or rotavirus.1 Using the Rochester risk stratification and the presence or absence of viral infection, she demonstrated that the risk of SBI was reduced in both high- and low-risk infants in the presence of viral infection; in low risk infants with viral infection, SBI was identified in 1.8%, compared with 3.1% in those without viral infection, and in high-risk infants. 5.5% has SBI when viral infection was found, compared to 16.7% in the absence of viral infection. She also proposed risk features to identify those infected with herpes simplex virus; age less than 42 days, vesicular rash, elevated alanine transaminase (ALT) and aspartate aminotransferase (AST), CSF pleocytosis, and seizure or twitching.
Greenhow et al. reported on the experience with “serious” bacterial infection in infants less than 90 days of age receiving care at Northern California Kaiser Permanente during the period 2005-2011.2 As pictured, the majority of children have UTI, and smaller numbers have bacteremia or meningitis. A small group of children with UTI have urosepsis as well; those with urosepsis can be differentiated from those with only UTI by age (less than 21 days), clinical exam (ill appearing), and elevated C reactive protein (greater than 20 mg/L) or elevated procalcitonin (greater than 0.5 ng/mL).3 Further evaluation of procalcitonin by other groups appears to validate its role in identifying children at low risk of SBI (procalcitonin less than 0.3 ng/mL).4
Currently, studies of febrile infants less than 90 days of age demonstrate that E. coli dominates in bacteremia, UTI, and meningitis, with Group B streptococcus as the next most frequent pathogen identified.2 Increasingly ampicillin resistance has been reported among E. coli isolates from both early- and late-onset disease as well as rare isolates that are resistant to third generation cephalosporins or gentamicin. Surveillance to identify changes in antimicrobial susceptibility will need to be ongoing to ensure that current approaches for initial therapy in high-risk infants aligns with current susceptibility patterns.
Dr. Pelton is chief of the section of pediatric infectious diseases and coordinator of the maternal-child HIV program at Boston Medical Center. He said he had no relevant financial disclosures. Email him at [email protected].
References
1. Pediatrics. 2004 Jun;113(6):1662-6.
2. Pediatr Infect Dis J. 2014 Jun;33(6):595-9.
3. Pediatr Infect Dis J. 2015 Jan;34(1):17-21.
4. JAMA Pediatr. 2016;170(1):17-18.
5. “AAP Proposes Update to Evaluating, Managing Febrile Infants Guideline,” The Hospitalist, 2016.
Fever in the youngest of infants creates a challenge for the pediatric clinician. Fever is a common presentation for serious bacterial infection (SBI) although most fevers are due to viral infection. However, the clinical presentation does not necessarily differ, and the risk for a poor outcome in this age group is substantial.
In the early stages of my pediatric career, most febrile infants less than 90 days of age were evaluated for sepsis, admitted, and treated with antibiotics pending culture results. Group B streptococcal sepsis or Escherichia coli sepsis were common in the first month of life, and Haemophilus influenza type B or Streptococcus pneumoniae in the second and third months of life. The approach to fever in the first 90 days has changed following both the introduction of haemophilus and pneumococcal conjugate vaccines, the experience with risk stratification criteria for identifying infants at low risk for SBI, and the recognition of urinary tract infection (UTI) as a common source of infection in this age group as well as development of criteria for diagnosis.
A further nuance was subsequently added with the introduction of rapid diagnostics for viral infection. Byington et al. found that the majority of febrile infants less than 90 days of age had viral infection with enterovirus, respiratory syncytial virus (RSV), influenza or rotavirus.1 Using the Rochester risk stratification and the presence or absence of viral infection, she demonstrated that the risk of SBI was reduced in both high- and low-risk infants in the presence of viral infection; in low risk infants with viral infection, SBI was identified in 1.8%, compared with 3.1% in those without viral infection, and in high-risk infants. 5.5% has SBI when viral infection was found, compared to 16.7% in the absence of viral infection. She also proposed risk features to identify those infected with herpes simplex virus; age less than 42 days, vesicular rash, elevated alanine transaminase (ALT) and aspartate aminotransferase (AST), CSF pleocytosis, and seizure or twitching.
Greenhow et al. reported on the experience with “serious” bacterial infection in infants less than 90 days of age receiving care at Northern California Kaiser Permanente during the period 2005-2011.2 As pictured, the majority of children have UTI, and smaller numbers have bacteremia or meningitis. A small group of children with UTI have urosepsis as well; those with urosepsis can be differentiated from those with only UTI by age (less than 21 days), clinical exam (ill appearing), and elevated C reactive protein (greater than 20 mg/L) or elevated procalcitonin (greater than 0.5 ng/mL).3 Further evaluation of procalcitonin by other groups appears to validate its role in identifying children at low risk of SBI (procalcitonin less than 0.3 ng/mL).4
Currently, studies of febrile infants less than 90 days of age demonstrate that E. coli dominates in bacteremia, UTI, and meningitis, with Group B streptococcus as the next most frequent pathogen identified.2 Increasingly ampicillin resistance has been reported among E. coli isolates from both early- and late-onset disease as well as rare isolates that are resistant to third generation cephalosporins or gentamicin. Surveillance to identify changes in antimicrobial susceptibility will need to be ongoing to ensure that current approaches for initial therapy in high-risk infants aligns with current susceptibility patterns.
Dr. Pelton is chief of the section of pediatric infectious diseases and coordinator of the maternal-child HIV program at Boston Medical Center. He said he had no relevant financial disclosures. Email him at [email protected].
References
1. Pediatrics. 2004 Jun;113(6):1662-6.
2. Pediatr Infect Dis J. 2014 Jun;33(6):595-9.
3. Pediatr Infect Dis J. 2015 Jan;34(1):17-21.
4. JAMA Pediatr. 2016;170(1):17-18.
5. “AAP Proposes Update to Evaluating, Managing Febrile Infants Guideline,” The Hospitalist, 2016.
Vaccines: Effectiveness vs. efficacy
During the influenza portion of the Feb. 21, 2018, Centers for Diseases Control and Prevention’s Advisory Committee on Immunization Practices meeting, two pleas from the audience asked the CDC/ACIP to make messages very clear about how protective influenza vaccine really is.
We hear apparently conflicting percentages from Australia, Canada, Europe, and the United States from the many stories/press releases in the news media and from official public health outlets. And the gloomiest ones get the most exposure.1 It can be confusing even for medical care providers who are supposed to advise families on such matters.
A key misunderstanding in many medical and lay news stories is about what vaccine effectiveness and vaccine efficacy really mean. What? Aren’t those the same thing? Nope. They are quite different. And are we sure of what those 95% confidence intervals (CI) mean? Let’s review the “math” so we can explain this to families.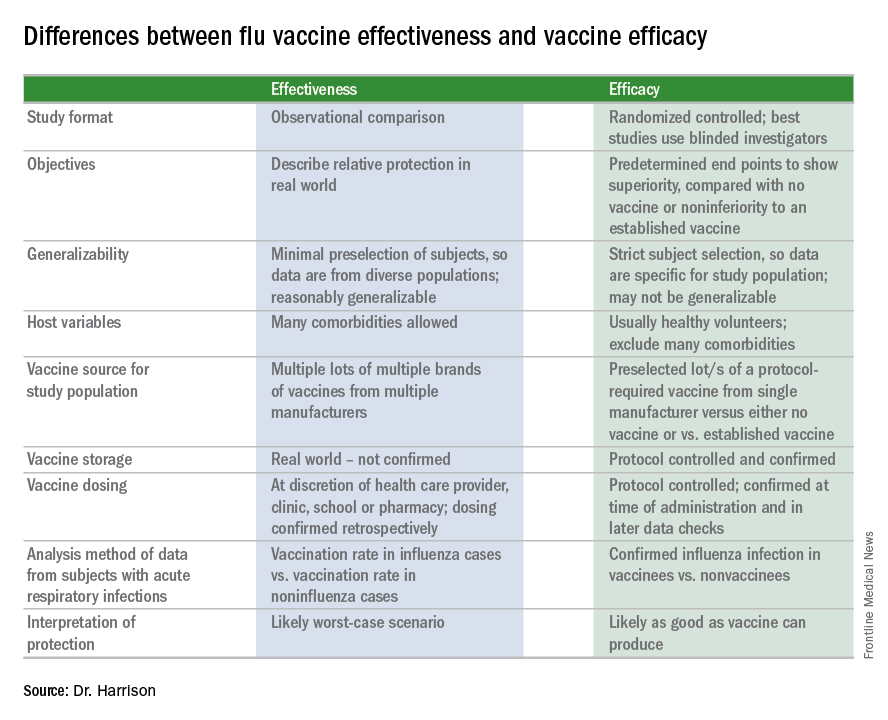
Vaccine effectiveness (VE)2,3
The first thing to know is that the CDC and similar public health agencies in other countries do not report vaccine efficacy. Instead, the percentage reported is VE during (interim estimated VE) and just after (final adjusted VE) each influenza season. This means that VE is generally a retrospective analysis of data, most of which were collected prospectively. Further, VE is likely the worst case scenario. VE is a measure of real-world benefit to patients for whom vaccine is recommended, by analyzing specific geographically diverse populations (population-based) without excluding most underlying illness or comorbidities (note that immunosuppressed persons are excluded). Subjects in VE studies receive their vaccine in the real world and, therefore, vaccinees may receive their vaccines from any number of the usual outlets (e.g., primary care provider, urgent care or emergency department, public health department, pharmacy, school, or nursing home). There are multiple lots of multiple brands from multiple vaccine manufacturers. Children who need two doses of influenza vaccine do not necessarily receive those doses according to the package insert’s schedule. VE studies do not have the capability to confirm that vaccine was stored, handled, and administered in a precisely correct manner according to manufacturer’s and CDC’s recommendations.
VE is calculated using a “test-negative” (case-control) analysis of patients presenting with acute respiratory infections (ARIs). People who are not in vaccine research can find this methodology confusing. Briefly, the VE compares the odds of vaccination in ARIs due to confirmed influenza to the odds of vaccination in ARIs not due to influenza. Additional statistical tools can adjust VE for specific factors. VE is also calculated by factors of interest, such as age, gender, pregnancy, influenza type, region of the country, presence of asthma or other comorbidity, etc. Whether the VE value is the “truth in the universe” is related to having enough subjects in each analyzed group and the degree to which the studied populations actually represent the whole country. So, VE is more accurate when there are large subject numbers.
Remember also that VE is usually calculated from outpatients, so it does not really measure all the benefits of vaccination. Prevention rates for severe influenza (such as influenza hospitalizations) are higher but usually unavailable until after the entire season.
VE studies generally measure real-world and likely worst-case-scenario benefit for the overall population being protected against outpatient influenza medical visits.
Vaccine efficacy2,3
Vaccine efficacy measures how the vaccine performs under ideal circumstances in a regimented protocol in relatively normal hosts – likely the best-case-scenario benefit. Vaccine efficacy is the percent difference in confirmed influenza episodes in vaccinees getting the “experimental” vaccine vs. episodes in nonvaccinees (or vaccinees getting an established vaccine). Vaccine efficacy, therefore, is usually calculated based on prospective well-controlled studies under ideal circumstances in subjects who received their vaccines on time per the recommended schedule. Most such studies are performed on otherwise healthy children or adults, with most comorbidities excluded. The “experimental” vaccine is generally from a single manufacturer from a single lot, and chain-of-custody is well controlled. The vaccine is administered at selected research sites according to a strict protocol; vaccine storage is ensured to be as recommended.
Confidence intervals
To assess whether the “protection” is “significant,” the calculations derive 95% confidence intervals (CI). If the 95% CI range is wide, such as many tens of percents, then there is less confidence that the calculation is correct. And if the lower CI is less than 0, then the result is not significant. For example, a VE of 20% is not highly protective, but can be significant if the 95% CI ranges from 10 to 28 (the lower value of 10 is above zero). It would not be significant if the 95% CI lower limit was –10. Values for seasons 2004-2005 and 2005-2006 were similar to this. Consider however that a VE of 55% seems great, but may not be significant if the 95% CI range is –20 to 89 (the lower value is less than zero). In the ideal world, the VE would be greater than 50% and the 95% CI range would be tight with the lower CI value far above zero; for example, VE of 70% with 95% CI ranging from 60 to 80. The 2010-2011 season was close to this.
Type and age-specific VE
Aside from overall VE, there are subset analyses that can be revealing. This year there are the concerning mid-season VE estimates of approximately 25% for the United States and 17% in Canada, for one specific type, H3N2, which unfortunately has been the dominant circulating U.S. type. That number is what everybody seems to have focused on. But remember influenza B becomes dominant late in most seasons (increasing at the time of writing this article). Interim 2017-2018 VE for influenza B was in the mid 60% range, making the box plot near 40% overall.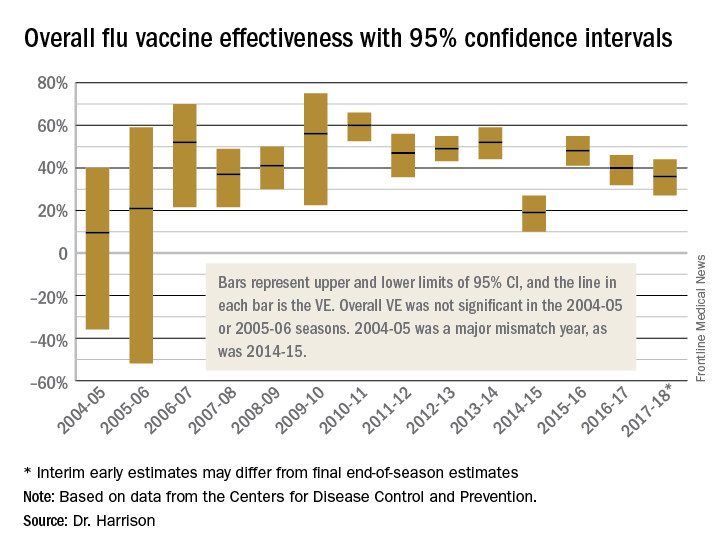
Age-related VE analysis can show difference; for example, the best benefit for H3N2 this season has been in young children and the worst in elderly and 9- to 17-year-olds.
Take-home message
The simplest way to think of overall VE is that it is the real-world, worst-case-scenario value for influenza protection by vaccine against the several circulating types of influenza.
Dr. Harrison is professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospitals and Clinics, Kansas City, Mo. Children’s Mercy Hospital receives grant funding for Dr. Harrison’s work as an investigator from GSK for MMR and rotavirus vaccine studies, from Merck for in vitro and clinical antibiotic studies, from Allergan for clinical antibiotic studies, from Pfizer for pneumococcal seroepidemiology studies, and from Regeneron for RSV studies. Dr. Harrison received support for travel and to present seroepidemiology data at one meeting. Email him at [email protected].
References
1. MMWR Weekly. 2017 Feb 17;66(6):167-71.
2. Dev Biol Stand. 1998;95:195-201.
3. Lancet Infect Dis. 2012 Jan;12(1):36-44.
During the influenza portion of the Feb. 21, 2018, Centers for Diseases Control and Prevention’s Advisory Committee on Immunization Practices meeting, two pleas from the audience asked the CDC/ACIP to make messages very clear about how protective influenza vaccine really is.
We hear apparently conflicting percentages from Australia, Canada, Europe, and the United States from the many stories/press releases in the news media and from official public health outlets. And the gloomiest ones get the most exposure.1 It can be confusing even for medical care providers who are supposed to advise families on such matters.
A key misunderstanding in many medical and lay news stories is about what vaccine effectiveness and vaccine efficacy really mean. What? Aren’t those the same thing? Nope. They are quite different. And are we sure of what those 95% confidence intervals (CI) mean? Let’s review the “math” so we can explain this to families.
Vaccine effectiveness (VE)2,3
The first thing to know is that the CDC and similar public health agencies in other countries do not report vaccine efficacy. Instead, the percentage reported is VE during (interim estimated VE) and just after (final adjusted VE) each influenza season. This means that VE is generally a retrospective analysis of data, most of which were collected prospectively. Further, VE is likely the worst case scenario. VE is a measure of real-world benefit to patients for whom vaccine is recommended, by analyzing specific geographically diverse populations (population-based) without excluding most underlying illness or comorbidities (note that immunosuppressed persons are excluded). Subjects in VE studies receive their vaccine in the real world and, therefore, vaccinees may receive their vaccines from any number of the usual outlets (e.g., primary care provider, urgent care or emergency department, public health department, pharmacy, school, or nursing home). There are multiple lots of multiple brands from multiple vaccine manufacturers. Children who need two doses of influenza vaccine do not necessarily receive those doses according to the package insert’s schedule. VE studies do not have the capability to confirm that vaccine was stored, handled, and administered in a precisely correct manner according to manufacturer’s and CDC’s recommendations.
VE is calculated using a “test-negative” (case-control) analysis of patients presenting with acute respiratory infections (ARIs). People who are not in vaccine research can find this methodology confusing. Briefly, the VE compares the odds of vaccination in ARIs due to confirmed influenza to the odds of vaccination in ARIs not due to influenza. Additional statistical tools can adjust VE for specific factors. VE is also calculated by factors of interest, such as age, gender, pregnancy, influenza type, region of the country, presence of asthma or other comorbidity, etc. Whether the VE value is the “truth in the universe” is related to having enough subjects in each analyzed group and the degree to which the studied populations actually represent the whole country. So, VE is more accurate when there are large subject numbers.
Remember also that VE is usually calculated from outpatients, so it does not really measure all the benefits of vaccination. Prevention rates for severe influenza (such as influenza hospitalizations) are higher but usually unavailable until after the entire season.
VE studies generally measure real-world and likely worst-case-scenario benefit for the overall population being protected against outpatient influenza medical visits.
Vaccine efficacy2,3
Vaccine efficacy measures how the vaccine performs under ideal circumstances in a regimented protocol in relatively normal hosts – likely the best-case-scenario benefit. Vaccine efficacy is the percent difference in confirmed influenza episodes in vaccinees getting the “experimental” vaccine vs. episodes in nonvaccinees (or vaccinees getting an established vaccine). Vaccine efficacy, therefore, is usually calculated based on prospective well-controlled studies under ideal circumstances in subjects who received their vaccines on time per the recommended schedule. Most such studies are performed on otherwise healthy children or adults, with most comorbidities excluded. The “experimental” vaccine is generally from a single manufacturer from a single lot, and chain-of-custody is well controlled. The vaccine is administered at selected research sites according to a strict protocol; vaccine storage is ensured to be as recommended.
Confidence intervals
To assess whether the “protection” is “significant,” the calculations derive 95% confidence intervals (CI). If the 95% CI range is wide, such as many tens of percents, then there is less confidence that the calculation is correct. And if the lower CI is less than 0, then the result is not significant. For example, a VE of 20% is not highly protective, but can be significant if the 95% CI ranges from 10 to 28 (the lower value of 10 is above zero). It would not be significant if the 95% CI lower limit was –10. Values for seasons 2004-2005 and 2005-2006 were similar to this. Consider however that a VE of 55% seems great, but may not be significant if the 95% CI range is –20 to 89 (the lower value is less than zero). In the ideal world, the VE would be greater than 50% and the 95% CI range would be tight with the lower CI value far above zero; for example, VE of 70% with 95% CI ranging from 60 to 80. The 2010-2011 season was close to this.
Type and age-specific VE
Aside from overall VE, there are subset analyses that can be revealing. This year there are the concerning mid-season VE estimates of approximately 25% for the United States and 17% in Canada, for one specific type, H3N2, which unfortunately has been the dominant circulating U.S. type. That number is what everybody seems to have focused on. But remember influenza B becomes dominant late in most seasons (increasing at the time of writing this article). Interim 2017-2018 VE for influenza B was in the mid 60% range, making the box plot near 40% overall.
Age-related VE analysis can show difference; for example, the best benefit for H3N2 this season has been in young children and the worst in elderly and 9- to 17-year-olds.
Take-home message
The simplest way to think of overall VE is that it is the real-world, worst-case-scenario value for influenza protection by vaccine against the several circulating types of influenza.
Dr. Harrison is professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospitals and Clinics, Kansas City, Mo. Children’s Mercy Hospital receives grant funding for Dr. Harrison’s work as an investigator from GSK for MMR and rotavirus vaccine studies, from Merck for in vitro and clinical antibiotic studies, from Allergan for clinical antibiotic studies, from Pfizer for pneumococcal seroepidemiology studies, and from Regeneron for RSV studies. Dr. Harrison received support for travel and to present seroepidemiology data at one meeting. Email him at [email protected].
References
1. MMWR Weekly. 2017 Feb 17;66(6):167-71.
2. Dev Biol Stand. 1998;95:195-201.
3. Lancet Infect Dis. 2012 Jan;12(1):36-44.
During the influenza portion of the Feb. 21, 2018, Centers for Diseases Control and Prevention’s Advisory Committee on Immunization Practices meeting, two pleas from the audience asked the CDC/ACIP to make messages very clear about how protective influenza vaccine really is.
We hear apparently conflicting percentages from Australia, Canada, Europe, and the United States from the many stories/press releases in the news media and from official public health outlets. And the gloomiest ones get the most exposure.1 It can be confusing even for medical care providers who are supposed to advise families on such matters.
A key misunderstanding in many medical and lay news stories is about what vaccine effectiveness and vaccine efficacy really mean. What? Aren’t those the same thing? Nope. They are quite different. And are we sure of what those 95% confidence intervals (CI) mean? Let’s review the “math” so we can explain this to families.
Vaccine effectiveness (VE)2,3
The first thing to know is that the CDC and similar public health agencies in other countries do not report vaccine efficacy. Instead, the percentage reported is VE during (interim estimated VE) and just after (final adjusted VE) each influenza season. This means that VE is generally a retrospective analysis of data, most of which were collected prospectively. Further, VE is likely the worst case scenario. VE is a measure of real-world benefit to patients for whom vaccine is recommended, by analyzing specific geographically diverse populations (population-based) without excluding most underlying illness or comorbidities (note that immunosuppressed persons are excluded). Subjects in VE studies receive their vaccine in the real world and, therefore, vaccinees may receive their vaccines from any number of the usual outlets (e.g., primary care provider, urgent care or emergency department, public health department, pharmacy, school, or nursing home). There are multiple lots of multiple brands from multiple vaccine manufacturers. Children who need two doses of influenza vaccine do not necessarily receive those doses according to the package insert’s schedule. VE studies do not have the capability to confirm that vaccine was stored, handled, and administered in a precisely correct manner according to manufacturer’s and CDC’s recommendations.
VE is calculated using a “test-negative” (case-control) analysis of patients presenting with acute respiratory infections (ARIs). People who are not in vaccine research can find this methodology confusing. Briefly, the VE compares the odds of vaccination in ARIs due to confirmed influenza to the odds of vaccination in ARIs not due to influenza. Additional statistical tools can adjust VE for specific factors. VE is also calculated by factors of interest, such as age, gender, pregnancy, influenza type, region of the country, presence of asthma or other comorbidity, etc. Whether the VE value is the “truth in the universe” is related to having enough subjects in each analyzed group and the degree to which the studied populations actually represent the whole country. So, VE is more accurate when there are large subject numbers.
Remember also that VE is usually calculated from outpatients, so it does not really measure all the benefits of vaccination. Prevention rates for severe influenza (such as influenza hospitalizations) are higher but usually unavailable until after the entire season.
VE studies generally measure real-world and likely worst-case-scenario benefit for the overall population being protected against outpatient influenza medical visits.
Vaccine efficacy2,3
Vaccine efficacy measures how the vaccine performs under ideal circumstances in a regimented protocol in relatively normal hosts – likely the best-case-scenario benefit. Vaccine efficacy is the percent difference in confirmed influenza episodes in vaccinees getting the “experimental” vaccine vs. episodes in nonvaccinees (or vaccinees getting an established vaccine). Vaccine efficacy, therefore, is usually calculated based on prospective well-controlled studies under ideal circumstances in subjects who received their vaccines on time per the recommended schedule. Most such studies are performed on otherwise healthy children or adults, with most comorbidities excluded. The “experimental” vaccine is generally from a single manufacturer from a single lot, and chain-of-custody is well controlled. The vaccine is administered at selected research sites according to a strict protocol; vaccine storage is ensured to be as recommended.
Confidence intervals
To assess whether the “protection” is “significant,” the calculations derive 95% confidence intervals (CI). If the 95% CI range is wide, such as many tens of percents, then there is less confidence that the calculation is correct. And if the lower CI is less than 0, then the result is not significant. For example, a VE of 20% is not highly protective, but can be significant if the 95% CI ranges from 10 to 28 (the lower value of 10 is above zero). It would not be significant if the 95% CI lower limit was –10. Values for seasons 2004-2005 and 2005-2006 were similar to this. Consider however that a VE of 55% seems great, but may not be significant if the 95% CI range is –20 to 89 (the lower value is less than zero). In the ideal world, the VE would be greater than 50% and the 95% CI range would be tight with the lower CI value far above zero; for example, VE of 70% with 95% CI ranging from 60 to 80. The 2010-2011 season was close to this.
Type and age-specific VE
Aside from overall VE, there are subset analyses that can be revealing. This year there are the concerning mid-season VE estimates of approximately 25% for the United States and 17% in Canada, for one specific type, H3N2, which unfortunately has been the dominant circulating U.S. type. That number is what everybody seems to have focused on. But remember influenza B becomes dominant late in most seasons (increasing at the time of writing this article). Interim 2017-2018 VE for influenza B was in the mid 60% range, making the box plot near 40% overall.
Age-related VE analysis can show difference; for example, the best benefit for H3N2 this season has been in young children and the worst in elderly and 9- to 17-year-olds.
Take-home message
The simplest way to think of overall VE is that it is the real-world, worst-case-scenario value for influenza protection by vaccine against the several circulating types of influenza.
Dr. Harrison is professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospitals and Clinics, Kansas City, Mo. Children’s Mercy Hospital receives grant funding for Dr. Harrison’s work as an investigator from GSK for MMR and rotavirus vaccine studies, from Merck for in vitro and clinical antibiotic studies, from Allergan for clinical antibiotic studies, from Pfizer for pneumococcal seroepidemiology studies, and from Regeneron for RSV studies. Dr. Harrison received support for travel and to present seroepidemiology data at one meeting. Email him at [email protected].
References
1. MMWR Weekly. 2017 Feb 17;66(6):167-71.
2. Dev Biol Stand. 1998;95:195-201.
3. Lancet Infect Dis. 2012 Jan;12(1):36-44.
Antibiotic choice for acute otitis media 2018
It’s a new year and a new respiratory season so my thoughts turn to the most common infection in pediatrics where an antibiotic might appropriately be prescribed – acute otitis media (AOM). The guidelines of the American Academy of Pediatrics were finalized in 2012 and published in 2013 and based on data that the AAP subcommittee considered. A recommendation emerged for amoxicillin to remain the treatment of choice if an antibiotic was to be prescribed at all, leaving the observation option as a continued consideration under defined clinical circumstances. The oral alternative antibiotics recommended were amoxicillin/clavulanate and cefdinir (Pediatrics. 2013. doi: 10.1542/peds.2012-3488).
Since the AAP subcommittee deliberated, changes have occurred in AOM etiology and the frequency of antibiotic resistance among the common bacteria that cause the infection. Our group in Rochester (N.Y.) continues to be the only site in the United States conducting a prospective assessment of AOM; we hope our data are generalizable to the entire country, but that is not certain. In Rochester, we saw an overall drop in AOM incidence after introduction of Prevnar 7 of about 10%-15% overall and that corresponded reasonably well with the frequency of AOM caused by Streptococcus pneumoniae involving the seven serotypes in the PCV7 vaccine. We then had a rebound in AOM infections, largely caused by serotype 19A, such that the overall incidence of AOM returned back to levels nearly the same as before PCV7 by 2010. With the introduction of Prevnar 13, and the dramatic reduction of serotype 19A nasal colonization – a necessary precursor of AOM – the incidence of AOM overall fell again, and compared with the pre-PCV7 era, I estimate that we are seeing about 20%-25% less AOM today.
In late 2017, we published an article describing the epidemiology of AOM in the PCV era (Pediatrics. 2017 Aug. doi: 10.1542/peds.2017-0181), in which we described changes in otopathogen distribution over time from 1996 through 2016. It showed that by end of 2016, the predominant bacteria causing AOM were Haemophilus influenzae, accounting for 60% of all AOM (52% detected by culture from tympanocentesis and another 8% detected by polymerase chain reaction). Among the H. influenzae from middle ear fluid, beta-lactamase production occurred in 45%. Therefore, according to principles of infectious disease antibiotic efficacy predictions, use of amoxicillin in standard dose or high dose would not eradicate about half of the H. influenzae causing AOM. In the table included in this column, I show calculations of predicted outcomes from amoxicillin, amoxicillin/clavulanate, and cefdinir treatment based on the projected otopathogen mix and resistance frequencies of 2016. Added to the data on H. influenzae I have included results of S. pneumoniae high nonsusceptibility at 5% of strains and beta-lactamase production by Moraxella catarrhalis at 100% of strains. 
Strictly based on in vitro susceptibility and the known otopathogen mix, the calculations show that amoxicillin could result in a maximum cure of 57%, amoxicillin/clavulanate of 99%, and cefdinir of 80% of treated children.
In vitro susceptibility has its limitations. Pharmacodynamic calculations would drop the predicted success of all three antibiotics because suboptimal absorption after oral dosing occurs with amoxicillin and amoxicillin/clavulanate more so than with cefdinir, thereby resulting in lower than predicted levels of antibiotic at the site of infection within the middle ear, whereas the achievable level of cefdinir with recommended dosing sometimes is below the desired in vitro cut point.
To balance that lowered predicted efficacy, each of the otopathogens has an associated “spontaneous cure rate” that is often quoted as being 20% for S. pneumoniae, 50% for H. influenzae, and 80% for M. catarrhalis. However, to be clear, those rates were derived largely from assessments about 5 days after antibiotic treatment was started with ineffective drugs or with placebos and do not account for the true spontaneous clinical cure rate of AOM if assessed in the first few days after onset (when pain and fever are at their peak) nor if assessed 14-30 days later when almost all children have been cured by their immune systems.
The calculations also do not account for overdiagnosis in clinical practice. Indeed, if the child does not have AOM, then the child will have a cure regardless of which antibiotic is selected. Rates of overdiagnosis of AOM have been assessed with various methods and are subject to limitations. But overall the data and most experts agree that overdiagnosis by pediatricians, family physicians, urgent care physicians, nurse practitioners, and physician assistants is in the range of 30%-50%.
Before the reader leaps to the conclusion that I am endorsing any particular antibiotic strictly based on predicted in vitro efficacy, I would state that many considerations must be given to whether to use an antibiotic for AOM, and which antibiotic to use, at what dose, and for what duration. This column is just pointing out a few key up-to-date facts for your consideration.
It’s a new year and a new respiratory season so my thoughts turn to the most common infection in pediatrics where an antibiotic might appropriately be prescribed – acute otitis media (AOM). The guidelines of the American Academy of Pediatrics were finalized in 2012 and published in 2013 and based on data that the AAP subcommittee considered. A recommendation emerged for amoxicillin to remain the treatment of choice if an antibiotic was to be prescribed at all, leaving the observation option as a continued consideration under defined clinical circumstances. The oral alternative antibiotics recommended were amoxicillin/clavulanate and cefdinir (Pediatrics. 2013. doi: 10.1542/peds.2012-3488).
Since the AAP subcommittee deliberated, changes have occurred in AOM etiology and the frequency of antibiotic resistance among the common bacteria that cause the infection. Our group in Rochester (N.Y.) continues to be the only site in the United States conducting a prospective assessment of AOM; we hope our data are generalizable to the entire country, but that is not certain. In Rochester, we saw an overall drop in AOM incidence after introduction of Prevnar 7 of about 10%-15% overall and that corresponded reasonably well with the frequency of AOM caused by Streptococcus pneumoniae involving the seven serotypes in the PCV7 vaccine. We then had a rebound in AOM infections, largely caused by serotype 19A, such that the overall incidence of AOM returned back to levels nearly the same as before PCV7 by 2010. With the introduction of Prevnar 13, and the dramatic reduction of serotype 19A nasal colonization – a necessary precursor of AOM – the incidence of AOM overall fell again, and compared with the pre-PCV7 era, I estimate that we are seeing about 20%-25% less AOM today.
In late 2017, we published an article describing the epidemiology of AOM in the PCV era (Pediatrics. 2017 Aug. doi: 10.1542/peds.2017-0181), in which we described changes in otopathogen distribution over time from 1996 through 2016. It showed that by end of 2016, the predominant bacteria causing AOM were Haemophilus influenzae, accounting for 60% of all AOM (52% detected by culture from tympanocentesis and another 8% detected by polymerase chain reaction). Among the H. influenzae from middle ear fluid, beta-lactamase production occurred in 45%. Therefore, according to principles of infectious disease antibiotic efficacy predictions, use of amoxicillin in standard dose or high dose would not eradicate about half of the H. influenzae causing AOM. In the table included in this column, I show calculations of predicted outcomes from amoxicillin, amoxicillin/clavulanate, and cefdinir treatment based on the projected otopathogen mix and resistance frequencies of 2016. Added to the data on H. influenzae I have included results of S. pneumoniae high nonsusceptibility at 5% of strains and beta-lactamase production by Moraxella catarrhalis at 100% of strains. 
Strictly based on in vitro susceptibility and the known otopathogen mix, the calculations show that amoxicillin could result in a maximum cure of 57%, amoxicillin/clavulanate of 99%, and cefdinir of 80% of treated children.
In vitro susceptibility has its limitations. Pharmacodynamic calculations would drop the predicted success of all three antibiotics because suboptimal absorption after oral dosing occurs with amoxicillin and amoxicillin/clavulanate more so than with cefdinir, thereby resulting in lower than predicted levels of antibiotic at the site of infection within the middle ear, whereas the achievable level of cefdinir with recommended dosing sometimes is below the desired in vitro cut point.
To balance that lowered predicted efficacy, each of the otopathogens has an associated “spontaneous cure rate” that is often quoted as being 20% for S. pneumoniae, 50% for H. influenzae, and 80% for M. catarrhalis. However, to be clear, those rates were derived largely from assessments about 5 days after antibiotic treatment was started with ineffective drugs or with placebos and do not account for the true spontaneous clinical cure rate of AOM if assessed in the first few days after onset (when pain and fever are at their peak) nor if assessed 14-30 days later when almost all children have been cured by their immune systems.
The calculations also do not account for overdiagnosis in clinical practice. Indeed, if the child does not have AOM, then the child will have a cure regardless of which antibiotic is selected. Rates of overdiagnosis of AOM have been assessed with various methods and are subject to limitations. But overall the data and most experts agree that overdiagnosis by pediatricians, family physicians, urgent care physicians, nurse practitioners, and physician assistants is in the range of 30%-50%.
Before the reader leaps to the conclusion that I am endorsing any particular antibiotic strictly based on predicted in vitro efficacy, I would state that many considerations must be given to whether to use an antibiotic for AOM, and which antibiotic to use, at what dose, and for what duration. This column is just pointing out a few key up-to-date facts for your consideration.
It’s a new year and a new respiratory season so my thoughts turn to the most common infection in pediatrics where an antibiotic might appropriately be prescribed – acute otitis media (AOM). The guidelines of the American Academy of Pediatrics were finalized in 2012 and published in 2013 and based on data that the AAP subcommittee considered. A recommendation emerged for amoxicillin to remain the treatment of choice if an antibiotic was to be prescribed at all, leaving the observation option as a continued consideration under defined clinical circumstances. The oral alternative antibiotics recommended were amoxicillin/clavulanate and cefdinir (Pediatrics. 2013. doi: 10.1542/peds.2012-3488).
Since the AAP subcommittee deliberated, changes have occurred in AOM etiology and the frequency of antibiotic resistance among the common bacteria that cause the infection. Our group in Rochester (N.Y.) continues to be the only site in the United States conducting a prospective assessment of AOM; we hope our data are generalizable to the entire country, but that is not certain. In Rochester, we saw an overall drop in AOM incidence after introduction of Prevnar 7 of about 10%-15% overall and that corresponded reasonably well with the frequency of AOM caused by Streptococcus pneumoniae involving the seven serotypes in the PCV7 vaccine. We then had a rebound in AOM infections, largely caused by serotype 19A, such that the overall incidence of AOM returned back to levels nearly the same as before PCV7 by 2010. With the introduction of Prevnar 13, and the dramatic reduction of serotype 19A nasal colonization – a necessary precursor of AOM – the incidence of AOM overall fell again, and compared with the pre-PCV7 era, I estimate that we are seeing about 20%-25% less AOM today.
In late 2017, we published an article describing the epidemiology of AOM in the PCV era (Pediatrics. 2017 Aug. doi: 10.1542/peds.2017-0181), in which we described changes in otopathogen distribution over time from 1996 through 2016. It showed that by end of 2016, the predominant bacteria causing AOM were Haemophilus influenzae, accounting for 60% of all AOM (52% detected by culture from tympanocentesis and another 8% detected by polymerase chain reaction). Among the H. influenzae from middle ear fluid, beta-lactamase production occurred in 45%. Therefore, according to principles of infectious disease antibiotic efficacy predictions, use of amoxicillin in standard dose or high dose would not eradicate about half of the H. influenzae causing AOM. In the table included in this column, I show calculations of predicted outcomes from amoxicillin, amoxicillin/clavulanate, and cefdinir treatment based on the projected otopathogen mix and resistance frequencies of 2016. Added to the data on H. influenzae I have included results of S. pneumoniae high nonsusceptibility at 5% of strains and beta-lactamase production by Moraxella catarrhalis at 100% of strains. 
Strictly based on in vitro susceptibility and the known otopathogen mix, the calculations show that amoxicillin could result in a maximum cure of 57%, amoxicillin/clavulanate of 99%, and cefdinir of 80% of treated children.
In vitro susceptibility has its limitations. Pharmacodynamic calculations would drop the predicted success of all three antibiotics because suboptimal absorption after oral dosing occurs with amoxicillin and amoxicillin/clavulanate more so than with cefdinir, thereby resulting in lower than predicted levels of antibiotic at the site of infection within the middle ear, whereas the achievable level of cefdinir with recommended dosing sometimes is below the desired in vitro cut point.
To balance that lowered predicted efficacy, each of the otopathogens has an associated “spontaneous cure rate” that is often quoted as being 20% for S. pneumoniae, 50% for H. influenzae, and 80% for M. catarrhalis. However, to be clear, those rates were derived largely from assessments about 5 days after antibiotic treatment was started with ineffective drugs or with placebos and do not account for the true spontaneous clinical cure rate of AOM if assessed in the first few days after onset (when pain and fever are at their peak) nor if assessed 14-30 days later when almost all children have been cured by their immune systems.
The calculations also do not account for overdiagnosis in clinical practice. Indeed, if the child does not have AOM, then the child will have a cure regardless of which antibiotic is selected. Rates of overdiagnosis of AOM have been assessed with various methods and are subject to limitations. But overall the data and most experts agree that overdiagnosis by pediatricians, family physicians, urgent care physicians, nurse practitioners, and physician assistants is in the range of 30%-50%.
Before the reader leaps to the conclusion that I am endorsing any particular antibiotic strictly based on predicted in vitro efficacy, I would state that many considerations must be given to whether to use an antibiotic for AOM, and which antibiotic to use, at what dose, and for what duration. This column is just pointing out a few key up-to-date facts for your consideration.