User login
Clinician practices to connect with patients
Background: As technology and medical advances improve patient care, physicians and patients have become more dissatisfied with their interactions and relationships. Practices are needed to improve the connection between physician and patient.
Study design: Mixed-methods.
Setting: Three diverse primary care settings (academic medical center, Veterans Affairs facility, federally qualified health center).
Synopsis: Initial evidence- and narrative-based practices were identified from a systematic literature review, clinical observations of primary care encounters, and qualitative discussions with physicians, patients, and nonmedical professionals. A three-round modified Delphi process was performed with experts representing different aspects of the patient-physician relationship.
Five recommended clinical practices were recognized to foster presence and meaningful connections with patients: 1. Prepare with intention (becoming familiar with the patient before you meet them); 2. Listen intently and completely (sit down, lean forward, and don’t interrupt, but listen); 3. Agree on what matters most (discover your patient’s goals and fit them into the visit); 4. Connect with the patient’s story (take notice of efforts by the patient and successes); 5. Explore emotional cues (be aware of your patient’s emotions). Limitations of this study include the use of convenience sampling for the qualitative research, lack of international diversity of the expert panelists, and the lack of validation of the five practices as a whole.
Bottom line: The five practices of prepare with intention, listen intently and completely, agree on what matters most, connect with the patient’s story, and explore emotional cues may improve the patient-physician connection.
Citation: Zulman DM et al. Practices to foster physician presence and connection with patients in the clinical encounter. JAMA. 2020;323(1):70-81.
Dr. Trammell-Velasquez is a hospitalist and associate professor of medicine at University of Texas Health, San Antonio.
Background: As technology and medical advances improve patient care, physicians and patients have become more dissatisfied with their interactions and relationships. Practices are needed to improve the connection between physician and patient.
Study design: Mixed-methods.
Setting: Three diverse primary care settings (academic medical center, Veterans Affairs facility, federally qualified health center).
Synopsis: Initial evidence- and narrative-based practices were identified from a systematic literature review, clinical observations of primary care encounters, and qualitative discussions with physicians, patients, and nonmedical professionals. A three-round modified Delphi process was performed with experts representing different aspects of the patient-physician relationship.
Five recommended clinical practices were recognized to foster presence and meaningful connections with patients: 1. Prepare with intention (becoming familiar with the patient before you meet them); 2. Listen intently and completely (sit down, lean forward, and don’t interrupt, but listen); 3. Agree on what matters most (discover your patient’s goals and fit them into the visit); 4. Connect with the patient’s story (take notice of efforts by the patient and successes); 5. Explore emotional cues (be aware of your patient’s emotions). Limitations of this study include the use of convenience sampling for the qualitative research, lack of international diversity of the expert panelists, and the lack of validation of the five practices as a whole.
Bottom line: The five practices of prepare with intention, listen intently and completely, agree on what matters most, connect with the patient’s story, and explore emotional cues may improve the patient-physician connection.
Citation: Zulman DM et al. Practices to foster physician presence and connection with patients in the clinical encounter. JAMA. 2020;323(1):70-81.
Dr. Trammell-Velasquez is a hospitalist and associate professor of medicine at University of Texas Health, San Antonio.
Background: As technology and medical advances improve patient care, physicians and patients have become more dissatisfied with their interactions and relationships. Practices are needed to improve the connection between physician and patient.
Study design: Mixed-methods.
Setting: Three diverse primary care settings (academic medical center, Veterans Affairs facility, federally qualified health center).
Synopsis: Initial evidence- and narrative-based practices were identified from a systematic literature review, clinical observations of primary care encounters, and qualitative discussions with physicians, patients, and nonmedical professionals. A three-round modified Delphi process was performed with experts representing different aspects of the patient-physician relationship.
Five recommended clinical practices were recognized to foster presence and meaningful connections with patients: 1. Prepare with intention (becoming familiar with the patient before you meet them); 2. Listen intently and completely (sit down, lean forward, and don’t interrupt, but listen); 3. Agree on what matters most (discover your patient’s goals and fit them into the visit); 4. Connect with the patient’s story (take notice of efforts by the patient and successes); 5. Explore emotional cues (be aware of your patient’s emotions). Limitations of this study include the use of convenience sampling for the qualitative research, lack of international diversity of the expert panelists, and the lack of validation of the five practices as a whole.
Bottom line: The five practices of prepare with intention, listen intently and completely, agree on what matters most, connect with the patient’s story, and explore emotional cues may improve the patient-physician connection.
Citation: Zulman DM et al. Practices to foster physician presence and connection with patients in the clinical encounter. JAMA. 2020;323(1):70-81.
Dr. Trammell-Velasquez is a hospitalist and associate professor of medicine at University of Texas Health, San Antonio.
Hotspotting does not reduce readmissions
Background: In the United States, 5% of the population use half of the annual spending for health care services and 1% account for approximately a quarter of annual spending, considered “superutilizers” of U.S. health care services. The Camden Coalition of Healthcare Providers (the Coalition) developed a model using hospital admission data to identify superutilizers, termed “hotspotting,” which has gained national recognition. Unlike other similar programs, this model targets a more diverse population with higher utilization than other programs that have been studied.
Study design: Randomized, controlled trial.
Setting: Two hospitals in Camden, N.J., from June 2, 2014, to March 31, 2018.
Synopsis: Eight-hundred superutilizers (at least one hospital admission at any of the four Camden-area hospital systems in the past 6 months, greater than one chronic medical condition, more than one high-risk traits/conditions) were randomly assigned to the intervention group or usual care. Once enrolled in the hospital, a multidisciplinary team began working with the patient in the intervention group on discharge. Team members conducted home visits, scheduled/took patients to appointments, managed medications, monitored and coached patients in disease-specific self-care, and assisted with applying for social and other assistive programs.
The readmission rate within 180 days after hospital discharge (primary outcome) between groups was not significant, with 62.3% readmitted in the intervention group and 61.7% in the control group. There was also no effect on the defined secondary outcomes (number of readmissions, proportion of patients with more than two readmissions, hospital days, charges, payments received, mortality).
The trial was not powered to detect smaller reductions in readmissions or to analyze effects within specific subgroups.
Bottom line: The addition of the Coalition’s program to patients with very high use of health care services did not decrease hospital readmission rate when compared to usual care.
Citation: Finkelstein A et al. Health care hotspotting – a randomized, controlled trial. N Engl J Med. 2020;382:152-62.
Dr. Trammell-Velasquez is a hospitalist and associate professor of medicine at University of Texas Health, San Antonio.
Background: In the United States, 5% of the population use half of the annual spending for health care services and 1% account for approximately a quarter of annual spending, considered “superutilizers” of U.S. health care services. The Camden Coalition of Healthcare Providers (the Coalition) developed a model using hospital admission data to identify superutilizers, termed “hotspotting,” which has gained national recognition. Unlike other similar programs, this model targets a more diverse population with higher utilization than other programs that have been studied.
Study design: Randomized, controlled trial.
Setting: Two hospitals in Camden, N.J., from June 2, 2014, to March 31, 2018.
Synopsis: Eight-hundred superutilizers (at least one hospital admission at any of the four Camden-area hospital systems in the past 6 months, greater than one chronic medical condition, more than one high-risk traits/conditions) were randomly assigned to the intervention group or usual care. Once enrolled in the hospital, a multidisciplinary team began working with the patient in the intervention group on discharge. Team members conducted home visits, scheduled/took patients to appointments, managed medications, monitored and coached patients in disease-specific self-care, and assisted with applying for social and other assistive programs.
The readmission rate within 180 days after hospital discharge (primary outcome) between groups was not significant, with 62.3% readmitted in the intervention group and 61.7% in the control group. There was also no effect on the defined secondary outcomes (number of readmissions, proportion of patients with more than two readmissions, hospital days, charges, payments received, mortality).
The trial was not powered to detect smaller reductions in readmissions or to analyze effects within specific subgroups.
Bottom line: The addition of the Coalition’s program to patients with very high use of health care services did not decrease hospital readmission rate when compared to usual care.
Citation: Finkelstein A et al. Health care hotspotting – a randomized, controlled trial. N Engl J Med. 2020;382:152-62.
Dr. Trammell-Velasquez is a hospitalist and associate professor of medicine at University of Texas Health, San Antonio.
Background: In the United States, 5% of the population use half of the annual spending for health care services and 1% account for approximately a quarter of annual spending, considered “superutilizers” of U.S. health care services. The Camden Coalition of Healthcare Providers (the Coalition) developed a model using hospital admission data to identify superutilizers, termed “hotspotting,” which has gained national recognition. Unlike other similar programs, this model targets a more diverse population with higher utilization than other programs that have been studied.
Study design: Randomized, controlled trial.
Setting: Two hospitals in Camden, N.J., from June 2, 2014, to March 31, 2018.
Synopsis: Eight-hundred superutilizers (at least one hospital admission at any of the four Camden-area hospital systems in the past 6 months, greater than one chronic medical condition, more than one high-risk traits/conditions) were randomly assigned to the intervention group or usual care. Once enrolled in the hospital, a multidisciplinary team began working with the patient in the intervention group on discharge. Team members conducted home visits, scheduled/took patients to appointments, managed medications, monitored and coached patients in disease-specific self-care, and assisted with applying for social and other assistive programs.
The readmission rate within 180 days after hospital discharge (primary outcome) between groups was not significant, with 62.3% readmitted in the intervention group and 61.7% in the control group. There was also no effect on the defined secondary outcomes (number of readmissions, proportion of patients with more than two readmissions, hospital days, charges, payments received, mortality).
The trial was not powered to detect smaller reductions in readmissions or to analyze effects within specific subgroups.
Bottom line: The addition of the Coalition’s program to patients with very high use of health care services did not decrease hospital readmission rate when compared to usual care.
Citation: Finkelstein A et al. Health care hotspotting – a randomized, controlled trial. N Engl J Med. 2020;382:152-62.
Dr. Trammell-Velasquez is a hospitalist and associate professor of medicine at University of Texas Health, San Antonio.
Lack of fever in ESRD with S. aureus bacteremia is common
Background: Fever is a common symptom in patients presenting to the ED. In patients with hemodialysis-dependent ESRD, the literature on febrile response during infection is scarce. In this study, authors compared ED triage temperatures of S. aureus bacteremic patients with and without hemodialysis-dependent ESRD.
Study design: Paired, retrospective cohort study.
Setting: Tertiary care referral center.
Synopsis: A total of 74 patients with methicillin-resistant or methicillin-susceptible S. aureus bacteremia were included in this study (37 patients with and 37 patients without hemodialysis-dependent ESRD). Upon triage, 54% (95% confidence interval, 38%-70%) and 82% (95% CI, 65%-91%) of hemodialysis and nonhemodialysis patients did not have a detectable fever (less than 100.4° F), respectively. The estimated mean ED triage temperatures were 100.5° F in the hemodialysis-dependent patients and 99.0° F in the non–hemodialysis-dependent patients (P < .001). The authors note the significant lack of fevers may be the result of insensitive methods for measuring body temperature, such as peripheral thermometers.
Bottom line: In this small retrospective cohort study, these data suggest a high incidence of afebrile bacteremia in patients with ESRD, especially those patients not dialysis dependent. This may lead to delays in obtaining blood cultures and initiating antibiotics. However, given the study design, the authors were unable to conclude a causal relationship between ESRD and febrile response.
Citation: Weatherall SL et al. Do bacteremic patients with end-stage renal disease have a fever when presenting to the emergency department? A paired, retrospective cohort study. BMC Emerg Med. 2020;20:2.
Dr. Schmit is a hospitalist and associate professor of medicine at University of Texas Health, San Antonio.
Background: Fever is a common symptom in patients presenting to the ED. In patients with hemodialysis-dependent ESRD, the literature on febrile response during infection is scarce. In this study, authors compared ED triage temperatures of S. aureus bacteremic patients with and without hemodialysis-dependent ESRD.
Study design: Paired, retrospective cohort study.
Setting: Tertiary care referral center.
Synopsis: A total of 74 patients with methicillin-resistant or methicillin-susceptible S. aureus bacteremia were included in this study (37 patients with and 37 patients without hemodialysis-dependent ESRD). Upon triage, 54% (95% confidence interval, 38%-70%) and 82% (95% CI, 65%-91%) of hemodialysis and nonhemodialysis patients did not have a detectable fever (less than 100.4° F), respectively. The estimated mean ED triage temperatures were 100.5° F in the hemodialysis-dependent patients and 99.0° F in the non–hemodialysis-dependent patients (P < .001). The authors note the significant lack of fevers may be the result of insensitive methods for measuring body temperature, such as peripheral thermometers.
Bottom line: In this small retrospective cohort study, these data suggest a high incidence of afebrile bacteremia in patients with ESRD, especially those patients not dialysis dependent. This may lead to delays in obtaining blood cultures and initiating antibiotics. However, given the study design, the authors were unable to conclude a causal relationship between ESRD and febrile response.
Citation: Weatherall SL et al. Do bacteremic patients with end-stage renal disease have a fever when presenting to the emergency department? A paired, retrospective cohort study. BMC Emerg Med. 2020;20:2.
Dr. Schmit is a hospitalist and associate professor of medicine at University of Texas Health, San Antonio.
Background: Fever is a common symptom in patients presenting to the ED. In patients with hemodialysis-dependent ESRD, the literature on febrile response during infection is scarce. In this study, authors compared ED triage temperatures of S. aureus bacteremic patients with and without hemodialysis-dependent ESRD.
Study design: Paired, retrospective cohort study.
Setting: Tertiary care referral center.
Synopsis: A total of 74 patients with methicillin-resistant or methicillin-susceptible S. aureus bacteremia were included in this study (37 patients with and 37 patients without hemodialysis-dependent ESRD). Upon triage, 54% (95% confidence interval, 38%-70%) and 82% (95% CI, 65%-91%) of hemodialysis and nonhemodialysis patients did not have a detectable fever (less than 100.4° F), respectively. The estimated mean ED triage temperatures were 100.5° F in the hemodialysis-dependent patients and 99.0° F in the non–hemodialysis-dependent patients (P < .001). The authors note the significant lack of fevers may be the result of insensitive methods for measuring body temperature, such as peripheral thermometers.
Bottom line: In this small retrospective cohort study, these data suggest a high incidence of afebrile bacteremia in patients with ESRD, especially those patients not dialysis dependent. This may lead to delays in obtaining blood cultures and initiating antibiotics. However, given the study design, the authors were unable to conclude a causal relationship between ESRD and febrile response.
Citation: Weatherall SL et al. Do bacteremic patients with end-stage renal disease have a fever when presenting to the emergency department? A paired, retrospective cohort study. BMC Emerg Med. 2020;20:2.
Dr. Schmit is a hospitalist and associate professor of medicine at University of Texas Health, San Antonio.
Conservative treatment for spontaneous pneumothorax?
Background: Management of primary spontaneous pneumothorax is usually with the insertion of a chest tube and typically requires hospitalization. This procedure can result in pain, organ injury, bleeding, and infection, and, if unresolved, may require surgery, introducing additional risks and complications. Few data exist from randomized trials comparing conservative versus interventional management.
Study design: Open-label, multicenter, prospective, randomized, noninferiority trial.
Setting: A total of 39 metropolitan and rural hospitals in Australia and New Zealand.
Synopsis: Overall, 316 patients with moderate to large primary spontaneous pneumothorax were randomized (154 to the intervention group and 162 in the conservative group). In the conservative group, 25 patients (15.4%) required eventual intervention for prespecified reasons (uncontrolled pain, chest pain or shortness of breath preventing mobilization, clinical instability, enlarging pneumothorax).
In complete-case analysis, 129 out of 131 (98.5%) patients in the intervention group had resolution within 8 weeks, compared with 118 of 125 (94.4%) in the conservative group (risk difference, –4.1 percentage points; 95% confidence interval, –8.6 to 0.5, P = .02 for noninferiority).
In sensitivity analysis, in which missing data after the 8-week period were imputed as treatment failures, re-expansion occurred in 129 out of 138 (93.5%) patients in the intervention group and 118 out of 143 (82.5%) in the conservative group (risk difference, –11.0 percentage points; 95% CI, –18.4 to –3.5), which is outside the noninferiority margin of –9.0.
Overall, 41 patients in the intervention group and 13 in the conservative group had at least one adverse event.
Bottom line: Missing data limit the ability to make strong conclusions, but this trial suggests that conservative management of primary spontaneous pneumothorax was noninferior to interventional management with lower risk of serious adverse events.
Citation: Brown SG et al. Conservative versus interventional treatment for spontaneous pneumothorax. N Engl J Med. 2020; 382:405-15.
Dr. Schmit is a hospitalist and associate professor of medicine at University of Texas Health, San Antonio.
Background: Management of primary spontaneous pneumothorax is usually with the insertion of a chest tube and typically requires hospitalization. This procedure can result in pain, organ injury, bleeding, and infection, and, if unresolved, may require surgery, introducing additional risks and complications. Few data exist from randomized trials comparing conservative versus interventional management.
Study design: Open-label, multicenter, prospective, randomized, noninferiority trial.
Setting: A total of 39 metropolitan and rural hospitals in Australia and New Zealand.
Synopsis: Overall, 316 patients with moderate to large primary spontaneous pneumothorax were randomized (154 to the intervention group and 162 in the conservative group). In the conservative group, 25 patients (15.4%) required eventual intervention for prespecified reasons (uncontrolled pain, chest pain or shortness of breath preventing mobilization, clinical instability, enlarging pneumothorax).
In complete-case analysis, 129 out of 131 (98.5%) patients in the intervention group had resolution within 8 weeks, compared with 118 of 125 (94.4%) in the conservative group (risk difference, –4.1 percentage points; 95% confidence interval, –8.6 to 0.5, P = .02 for noninferiority).
In sensitivity analysis, in which missing data after the 8-week period were imputed as treatment failures, re-expansion occurred in 129 out of 138 (93.5%) patients in the intervention group and 118 out of 143 (82.5%) in the conservative group (risk difference, –11.0 percentage points; 95% CI, –18.4 to –3.5), which is outside the noninferiority margin of –9.0.
Overall, 41 patients in the intervention group and 13 in the conservative group had at least one adverse event.
Bottom line: Missing data limit the ability to make strong conclusions, but this trial suggests that conservative management of primary spontaneous pneumothorax was noninferior to interventional management with lower risk of serious adverse events.
Citation: Brown SG et al. Conservative versus interventional treatment for spontaneous pneumothorax. N Engl J Med. 2020; 382:405-15.
Dr. Schmit is a hospitalist and associate professor of medicine at University of Texas Health, San Antonio.
Background: Management of primary spontaneous pneumothorax is usually with the insertion of a chest tube and typically requires hospitalization. This procedure can result in pain, organ injury, bleeding, and infection, and, if unresolved, may require surgery, introducing additional risks and complications. Few data exist from randomized trials comparing conservative versus interventional management.
Study design: Open-label, multicenter, prospective, randomized, noninferiority trial.
Setting: A total of 39 metropolitan and rural hospitals in Australia and New Zealand.
Synopsis: Overall, 316 patients with moderate to large primary spontaneous pneumothorax were randomized (154 to the intervention group and 162 in the conservative group). In the conservative group, 25 patients (15.4%) required eventual intervention for prespecified reasons (uncontrolled pain, chest pain or shortness of breath preventing mobilization, clinical instability, enlarging pneumothorax).
In complete-case analysis, 129 out of 131 (98.5%) patients in the intervention group had resolution within 8 weeks, compared with 118 of 125 (94.4%) in the conservative group (risk difference, –4.1 percentage points; 95% confidence interval, –8.6 to 0.5, P = .02 for noninferiority).
In sensitivity analysis, in which missing data after the 8-week period were imputed as treatment failures, re-expansion occurred in 129 out of 138 (93.5%) patients in the intervention group and 118 out of 143 (82.5%) in the conservative group (risk difference, –11.0 percentage points; 95% CI, –18.4 to –3.5), which is outside the noninferiority margin of –9.0.
Overall, 41 patients in the intervention group and 13 in the conservative group had at least one adverse event.
Bottom line: Missing data limit the ability to make strong conclusions, but this trial suggests that conservative management of primary spontaneous pneumothorax was noninferior to interventional management with lower risk of serious adverse events.
Citation: Brown SG et al. Conservative versus interventional treatment for spontaneous pneumothorax. N Engl J Med. 2020; 382:405-15.
Dr. Schmit is a hospitalist and associate professor of medicine at University of Texas Health, San Antonio.
Abnormal exercise EKG in the setting of normal stress echo linked with increased CV risk
Background: Exercise EKG is often integrated with stress echocardiography, but discordance with +EKG/–Echo has unknown significance.
Study design: Observational cohort study.
Setting: Duke University Medical Center, Durham, N.C.
Synopsis: 47,944 patients without known coronary artery disease underwent exercise stress echocardiogram (Echo) with stress EKG. Of those patients, 8.5% had +EKG/–Echo results, which was associated with annualized event rate of adverse cardiac events of 1.72%, which is higher than the 0.89% of patients with –EKG/–Echo results. This was most significant for composite major adverse cardiovascular events less than 30 days out, with an adjusted hazard ratio of 8.06 (95% confidence interval, 5.02-12.94). For major adverse cardiovascular events greater than 30 days out, HR was 1.25 (95% CI 1.02-1.53).
Bottom line: Patients with +EKG/–Echo findings appear to be at higher risk of adverse cardiac events, especially in the short term.
Citation: Daubert MA et al. Implications of abnormal exercise electrocardiography with normal stress echocardiography. JAMA Intern Med. 2020 Jan 27. doi: 10.1001/jamainternmed.2019.6958.
Dr. Ho is a hospitalist and associate professor of medicine at University of Texas Health, San Antonio.
Background: Exercise EKG is often integrated with stress echocardiography, but discordance with +EKG/–Echo has unknown significance.
Study design: Observational cohort study.
Setting: Duke University Medical Center, Durham, N.C.
Synopsis: 47,944 patients without known coronary artery disease underwent exercise stress echocardiogram (Echo) with stress EKG. Of those patients, 8.5% had +EKG/–Echo results, which was associated with annualized event rate of adverse cardiac events of 1.72%, which is higher than the 0.89% of patients with –EKG/–Echo results. This was most significant for composite major adverse cardiovascular events less than 30 days out, with an adjusted hazard ratio of 8.06 (95% confidence interval, 5.02-12.94). For major adverse cardiovascular events greater than 30 days out, HR was 1.25 (95% CI 1.02-1.53).
Bottom line: Patients with +EKG/–Echo findings appear to be at higher risk of adverse cardiac events, especially in the short term.
Citation: Daubert MA et al. Implications of abnormal exercise electrocardiography with normal stress echocardiography. JAMA Intern Med. 2020 Jan 27. doi: 10.1001/jamainternmed.2019.6958.
Dr. Ho is a hospitalist and associate professor of medicine at University of Texas Health, San Antonio.
Background: Exercise EKG is often integrated with stress echocardiography, but discordance with +EKG/–Echo has unknown significance.
Study design: Observational cohort study.
Setting: Duke University Medical Center, Durham, N.C.
Synopsis: 47,944 patients without known coronary artery disease underwent exercise stress echocardiogram (Echo) with stress EKG. Of those patients, 8.5% had +EKG/–Echo results, which was associated with annualized event rate of adverse cardiac events of 1.72%, which is higher than the 0.89% of patients with –EKG/–Echo results. This was most significant for composite major adverse cardiovascular events less than 30 days out, with an adjusted hazard ratio of 8.06 (95% confidence interval, 5.02-12.94). For major adverse cardiovascular events greater than 30 days out, HR was 1.25 (95% CI 1.02-1.53).
Bottom line: Patients with +EKG/–Echo findings appear to be at higher risk of adverse cardiac events, especially in the short term.
Citation: Daubert MA et al. Implications of abnormal exercise electrocardiography with normal stress echocardiography. JAMA Intern Med. 2020 Jan 27. doi: 10.1001/jamainternmed.2019.6958.
Dr. Ho is a hospitalist and associate professor of medicine at University of Texas Health, San Antonio.
Post–acute kidney injury proteinuria predicts subsequent kidney disease progression
Background: Recent studies have shown that the level of proteinuria increases after AKI. It is not yet shown if this increases risk of kidney disease progression.
Study design: Prospective matched cohort study.
Setting: North American hospitals.
Synopsis: A total of 769 hospitalized adults with AKI were matched with those without based on clinical center and preadmission chronic kidney disease (CKD) status. Study authors found that albumin/creatinine ratio (ACR) and estimated glomerular filtration rate (eGFR) 3 months after hospitalization were highly associated with kidney disease progression, with a hazard ratio of 1.53 for each doubling (95% confidence interval, 1.43-1.64).
Episodes of AKI were also associated with progression, but this is severely attenuated once adjusted for ACR, eGFR, and traditional CKD risk factors. This suggests more routine quantification of proteinuria after AKI for better risk stratification.
Bottom line: Posthospitalization ACR predicts progression of kidney disease.
Citation: Hsu CY et al. Post–acute kidney injury proteinuria and subsequent kidney disease progression. JAMA Intern Med. 2020 Jan 27. doi: 10.1001/jamainternmed.2019.6390.
Dr. Ho is a hospitalist and associate professor of medicine at University of Texas Health, San Antonio.
Background: Recent studies have shown that the level of proteinuria increases after AKI. It is not yet shown if this increases risk of kidney disease progression.
Study design: Prospective matched cohort study.
Setting: North American hospitals.
Synopsis: A total of 769 hospitalized adults with AKI were matched with those without based on clinical center and preadmission chronic kidney disease (CKD) status. Study authors found that albumin/creatinine ratio (ACR) and estimated glomerular filtration rate (eGFR) 3 months after hospitalization were highly associated with kidney disease progression, with a hazard ratio of 1.53 for each doubling (95% confidence interval, 1.43-1.64).
Episodes of AKI were also associated with progression, but this is severely attenuated once adjusted for ACR, eGFR, and traditional CKD risk factors. This suggests more routine quantification of proteinuria after AKI for better risk stratification.
Bottom line: Posthospitalization ACR predicts progression of kidney disease.
Citation: Hsu CY et al. Post–acute kidney injury proteinuria and subsequent kidney disease progression. JAMA Intern Med. 2020 Jan 27. doi: 10.1001/jamainternmed.2019.6390.
Dr. Ho is a hospitalist and associate professor of medicine at University of Texas Health, San Antonio.
Background: Recent studies have shown that the level of proteinuria increases after AKI. It is not yet shown if this increases risk of kidney disease progression.
Study design: Prospective matched cohort study.
Setting: North American hospitals.
Synopsis: A total of 769 hospitalized adults with AKI were matched with those without based on clinical center and preadmission chronic kidney disease (CKD) status. Study authors found that albumin/creatinine ratio (ACR) and estimated glomerular filtration rate (eGFR) 3 months after hospitalization were highly associated with kidney disease progression, with a hazard ratio of 1.53 for each doubling (95% confidence interval, 1.43-1.64).
Episodes of AKI were also associated with progression, but this is severely attenuated once adjusted for ACR, eGFR, and traditional CKD risk factors. This suggests more routine quantification of proteinuria after AKI for better risk stratification.
Bottom line: Posthospitalization ACR predicts progression of kidney disease.
Citation: Hsu CY et al. Post–acute kidney injury proteinuria and subsequent kidney disease progression. JAMA Intern Med. 2020 Jan 27. doi: 10.1001/jamainternmed.2019.6390.
Dr. Ho is a hospitalist and associate professor of medicine at University of Texas Health, San Antonio.
FIND: A framework for success as a first-year hospitalist
Congratulations! You’re about to start your first year as a hospitalist, and in many cases your first real job. Hospital medicine is an incredibly rewarding subspecialty, but the progression from resident to attending physician can be daunting. To facilitate this transition, we present FIND (Familiarity, Identity, Network, and Direction) – a novel, sequential framework for success as a first-year hospitalist. For each component, we provide a narrative overview and a summary bullet point for quick reference.
Familiarity
- Lay the foundation: Learn the ins and outs of your job, EMR, and team.
Familiarize yourself with your surroundings. Know where your patients are located, where you can document, where to find equipment for procedures, and how to reach information technology. Proactively set up the electronic medical record on your home computer and phone. Make sure to review your responsibilities, including your call schedule, your shifts, your assigned patient panel, when you can leave campus, and how people should contact you. Also, others should know your expectations of them, especially if you are working with trainees.
Maintain a file with all of your orientation materials, including phone numbers and emails of key personnel. Know who your people are – who can access your calendar, who you can call with a clinical question or to escalate care, who can assist you with billing, and who helps with the throughput of your patients in the hospital. Take time to review your benefits, including parental leave, insurance coverage, retirement planning, vacation time, and ancillary services like laundry for your white coat. Familiarizing yourself with these basics will provide comfort and lay the foundation for your first year.
Identity
- Perform self-reflection: Overcome imposter syndrome and invest in hobbies.
One of the fundamental realizations that will occur with your first hospitalist job is that you are the attending. You walk in with a vision of your first job; be prepared to be surprised. You have earned the privilege of deciding on patient plans, and you are no longer obligated to staff with a senior physician. This is both empowering and terrifying. In a way, it may oddly remind you of intern year. A new hospital, new EMR, new colleagues, and imposter syndrome will trick you into doubting your decisions.
How to battle it? Positive thinking. You do know the basics of inpatient medicine and you do have a support system to cheer you on. As part of imposter syndrome, you may feel pressured to focus solely on work. Yet, your first job as a hospitalist is finally an amazing opportunity to focus on you. What hobbies have you been neglecting: cooking, photography, reading, more time with family, a new pet? You have the power to schedule your off-weeks. Are you interested in academics? Reserve a portion of your time off to explore scholarship opportunities at your institution. Your first job as a hospitalist is a chance to develop your identity, both as a physician and as an individual.
Network
- Engage your support system: Communicate with nursing, administration, colleagues.
Networking, or building a web of mutually beneficial professional relationships, is imperative for long-term career success. Hospitalists should focus on developing their network across multiple departments, such as nursing, subspecialties, medical education, and hospital administration. Curating a broad network will increase your visibility within your organization, showcase your unique services, and demonstrate your value.
To make networking encounters impactful, express interest, actively listen, ask relevant questions, and seek areas of mutual benefit. It’s equally important to cultivate these new relationships after the initial encounter and to demonstrate how your skill set will aid colleagues in achieving their professional goals. Over time, as you establish your niche, deliberate networking with those who share similar interests can lead to a wealth of new experiences and opportunities. Intentionally mastering networking early in your career provides insight into different aspects of the hospital system, new perspectives on ideas, and access to valuable guidance from other professionals. Engaging in networking to establish your support system is an essential step towards success as a first-year hospitalist.
Direction
- Visualize your path: Find a mentor and develop a mission statement and career plan.
Once you’re familiar with your work environment, confident in your identity, and acquainted with your support network, you’re ready for the final step – direction. Hospital medicine offers many professional avenues and clarifying your career path is challenging when attempted alone. A mentor is the necessary catalyst to find direction and purpose.
Selecting and engaging with a mentor will bolster your professional advancement, academic productivity, and most importantly, career satisfaction.1 At its best, mentorship is a symbiotic relationship. Your mentor should inspire you, challenge you, and support your growth and emotional well-being. In turn, as the mentee, you should be proactive, establish expectations, and take responsibility for maintaining communication to ensure a successful relationship. As your career takes shape over time, you may require a mentorship team to fulfill your unique needs.
When you’ve established a relationship with your mentor, take time to develop 1-year and 5-year plans. Your 1-year plan should focus on a few “quick wins,” often projects or opportunities at your home institution. Small victories in your first year will boost your confidence, motivation, and sense of control. Your 5-year plan should delineate the steps necessary to make your first major career transition, such as from instructor to assistant professor. Working with your mentor to draft a career mission statement is a useful first step in this process. Beginning with the end in mind, will help you visualize your direction.2
We hope that the FIND framework will help you find your path to success as a first-year hospitalist.
Dr. Nelson is a hospitalist and instructor of medicine at Harvard Medical School and Beth Israel Deaconess Medical Center, both in Boston. Dr. Ashford is assistant professor and program director, department of internal medicine/pediatrics, at the University of Nebraska Medical Center, Omaha. Dr. Rawal is clinical assistant professor of medicine at the University of Pittsburgh Medical Center. Dr. Crecelius is assistant professor of clinical medicine at Indiana University, Indianapolis. This article is sponsored by the SHM Physicians in Training committee, which submits quarterly content to the Hospitalist on topics relevant to trainees and early -career hospitalists.
References
1. Zerzan JT et al. Making the most of mentors: a guide for mentees. Acad Med. 2009;84:140-4. doi: 10.1097/ACM.0b013e3181906e8f.
2. Covey F. The seven habits of highly effective people. 25th anniversary edition. New York: Simon and Schuster, 2013.
Congratulations! You’re about to start your first year as a hospitalist, and in many cases your first real job. Hospital medicine is an incredibly rewarding subspecialty, but the progression from resident to attending physician can be daunting. To facilitate this transition, we present FIND (Familiarity, Identity, Network, and Direction) – a novel, sequential framework for success as a first-year hospitalist. For each component, we provide a narrative overview and a summary bullet point for quick reference.
Familiarity
- Lay the foundation: Learn the ins and outs of your job, EMR, and team.
Familiarize yourself with your surroundings. Know where your patients are located, where you can document, where to find equipment for procedures, and how to reach information technology. Proactively set up the electronic medical record on your home computer and phone. Make sure to review your responsibilities, including your call schedule, your shifts, your assigned patient panel, when you can leave campus, and how people should contact you. Also, others should know your expectations of them, especially if you are working with trainees.
Maintain a file with all of your orientation materials, including phone numbers and emails of key personnel. Know who your people are – who can access your calendar, who you can call with a clinical question or to escalate care, who can assist you with billing, and who helps with the throughput of your patients in the hospital. Take time to review your benefits, including parental leave, insurance coverage, retirement planning, vacation time, and ancillary services like laundry for your white coat. Familiarizing yourself with these basics will provide comfort and lay the foundation for your first year.
Identity
- Perform self-reflection: Overcome imposter syndrome and invest in hobbies.
One of the fundamental realizations that will occur with your first hospitalist job is that you are the attending. You walk in with a vision of your first job; be prepared to be surprised. You have earned the privilege of deciding on patient plans, and you are no longer obligated to staff with a senior physician. This is both empowering and terrifying. In a way, it may oddly remind you of intern year. A new hospital, new EMR, new colleagues, and imposter syndrome will trick you into doubting your decisions.
How to battle it? Positive thinking. You do know the basics of inpatient medicine and you do have a support system to cheer you on. As part of imposter syndrome, you may feel pressured to focus solely on work. Yet, your first job as a hospitalist is finally an amazing opportunity to focus on you. What hobbies have you been neglecting: cooking, photography, reading, more time with family, a new pet? You have the power to schedule your off-weeks. Are you interested in academics? Reserve a portion of your time off to explore scholarship opportunities at your institution. Your first job as a hospitalist is a chance to develop your identity, both as a physician and as an individual.
Network
- Engage your support system: Communicate with nursing, administration, colleagues.
Networking, or building a web of mutually beneficial professional relationships, is imperative for long-term career success. Hospitalists should focus on developing their network across multiple departments, such as nursing, subspecialties, medical education, and hospital administration. Curating a broad network will increase your visibility within your organization, showcase your unique services, and demonstrate your value.
To make networking encounters impactful, express interest, actively listen, ask relevant questions, and seek areas of mutual benefit. It’s equally important to cultivate these new relationships after the initial encounter and to demonstrate how your skill set will aid colleagues in achieving their professional goals. Over time, as you establish your niche, deliberate networking with those who share similar interests can lead to a wealth of new experiences and opportunities. Intentionally mastering networking early in your career provides insight into different aspects of the hospital system, new perspectives on ideas, and access to valuable guidance from other professionals. Engaging in networking to establish your support system is an essential step towards success as a first-year hospitalist.
Direction
- Visualize your path: Find a mentor and develop a mission statement and career plan.
Once you’re familiar with your work environment, confident in your identity, and acquainted with your support network, you’re ready for the final step – direction. Hospital medicine offers many professional avenues and clarifying your career path is challenging when attempted alone. A mentor is the necessary catalyst to find direction and purpose.
Selecting and engaging with a mentor will bolster your professional advancement, academic productivity, and most importantly, career satisfaction.1 At its best, mentorship is a symbiotic relationship. Your mentor should inspire you, challenge you, and support your growth and emotional well-being. In turn, as the mentee, you should be proactive, establish expectations, and take responsibility for maintaining communication to ensure a successful relationship. As your career takes shape over time, you may require a mentorship team to fulfill your unique needs.
When you’ve established a relationship with your mentor, take time to develop 1-year and 5-year plans. Your 1-year plan should focus on a few “quick wins,” often projects or opportunities at your home institution. Small victories in your first year will boost your confidence, motivation, and sense of control. Your 5-year plan should delineate the steps necessary to make your first major career transition, such as from instructor to assistant professor. Working with your mentor to draft a career mission statement is a useful first step in this process. Beginning with the end in mind, will help you visualize your direction.2
We hope that the FIND framework will help you find your path to success as a first-year hospitalist.
Dr. Nelson is a hospitalist and instructor of medicine at Harvard Medical School and Beth Israel Deaconess Medical Center, both in Boston. Dr. Ashford is assistant professor and program director, department of internal medicine/pediatrics, at the University of Nebraska Medical Center, Omaha. Dr. Rawal is clinical assistant professor of medicine at the University of Pittsburgh Medical Center. Dr. Crecelius is assistant professor of clinical medicine at Indiana University, Indianapolis. This article is sponsored by the SHM Physicians in Training committee, which submits quarterly content to the Hospitalist on topics relevant to trainees and early -career hospitalists.
References
1. Zerzan JT et al. Making the most of mentors: a guide for mentees. Acad Med. 2009;84:140-4. doi: 10.1097/ACM.0b013e3181906e8f.
2. Covey F. The seven habits of highly effective people. 25th anniversary edition. New York: Simon and Schuster, 2013.
Congratulations! You’re about to start your first year as a hospitalist, and in many cases your first real job. Hospital medicine is an incredibly rewarding subspecialty, but the progression from resident to attending physician can be daunting. To facilitate this transition, we present FIND (Familiarity, Identity, Network, and Direction) – a novel, sequential framework for success as a first-year hospitalist. For each component, we provide a narrative overview and a summary bullet point for quick reference.
Familiarity
- Lay the foundation: Learn the ins and outs of your job, EMR, and team.
Familiarize yourself with your surroundings. Know where your patients are located, where you can document, where to find equipment for procedures, and how to reach information technology. Proactively set up the electronic medical record on your home computer and phone. Make sure to review your responsibilities, including your call schedule, your shifts, your assigned patient panel, when you can leave campus, and how people should contact you. Also, others should know your expectations of them, especially if you are working with trainees.
Maintain a file with all of your orientation materials, including phone numbers and emails of key personnel. Know who your people are – who can access your calendar, who you can call with a clinical question or to escalate care, who can assist you with billing, and who helps with the throughput of your patients in the hospital. Take time to review your benefits, including parental leave, insurance coverage, retirement planning, vacation time, and ancillary services like laundry for your white coat. Familiarizing yourself with these basics will provide comfort and lay the foundation for your first year.
Identity
- Perform self-reflection: Overcome imposter syndrome and invest in hobbies.
One of the fundamental realizations that will occur with your first hospitalist job is that you are the attending. You walk in with a vision of your first job; be prepared to be surprised. You have earned the privilege of deciding on patient plans, and you are no longer obligated to staff with a senior physician. This is both empowering and terrifying. In a way, it may oddly remind you of intern year. A new hospital, new EMR, new colleagues, and imposter syndrome will trick you into doubting your decisions.
How to battle it? Positive thinking. You do know the basics of inpatient medicine and you do have a support system to cheer you on. As part of imposter syndrome, you may feel pressured to focus solely on work. Yet, your first job as a hospitalist is finally an amazing opportunity to focus on you. What hobbies have you been neglecting: cooking, photography, reading, more time with family, a new pet? You have the power to schedule your off-weeks. Are you interested in academics? Reserve a portion of your time off to explore scholarship opportunities at your institution. Your first job as a hospitalist is a chance to develop your identity, both as a physician and as an individual.
Network
- Engage your support system: Communicate with nursing, administration, colleagues.
Networking, or building a web of mutually beneficial professional relationships, is imperative for long-term career success. Hospitalists should focus on developing their network across multiple departments, such as nursing, subspecialties, medical education, and hospital administration. Curating a broad network will increase your visibility within your organization, showcase your unique services, and demonstrate your value.
To make networking encounters impactful, express interest, actively listen, ask relevant questions, and seek areas of mutual benefit. It’s equally important to cultivate these new relationships after the initial encounter and to demonstrate how your skill set will aid colleagues in achieving their professional goals. Over time, as you establish your niche, deliberate networking with those who share similar interests can lead to a wealth of new experiences and opportunities. Intentionally mastering networking early in your career provides insight into different aspects of the hospital system, new perspectives on ideas, and access to valuable guidance from other professionals. Engaging in networking to establish your support system is an essential step towards success as a first-year hospitalist.
Direction
- Visualize your path: Find a mentor and develop a mission statement and career plan.
Once you’re familiar with your work environment, confident in your identity, and acquainted with your support network, you’re ready for the final step – direction. Hospital medicine offers many professional avenues and clarifying your career path is challenging when attempted alone. A mentor is the necessary catalyst to find direction and purpose.
Selecting and engaging with a mentor will bolster your professional advancement, academic productivity, and most importantly, career satisfaction.1 At its best, mentorship is a symbiotic relationship. Your mentor should inspire you, challenge you, and support your growth and emotional well-being. In turn, as the mentee, you should be proactive, establish expectations, and take responsibility for maintaining communication to ensure a successful relationship. As your career takes shape over time, you may require a mentorship team to fulfill your unique needs.
When you’ve established a relationship with your mentor, take time to develop 1-year and 5-year plans. Your 1-year plan should focus on a few “quick wins,” often projects or opportunities at your home institution. Small victories in your first year will boost your confidence, motivation, and sense of control. Your 5-year plan should delineate the steps necessary to make your first major career transition, such as from instructor to assistant professor. Working with your mentor to draft a career mission statement is a useful first step in this process. Beginning with the end in mind, will help you visualize your direction.2
We hope that the FIND framework will help you find your path to success as a first-year hospitalist.
Dr. Nelson is a hospitalist and instructor of medicine at Harvard Medical School and Beth Israel Deaconess Medical Center, both in Boston. Dr. Ashford is assistant professor and program director, department of internal medicine/pediatrics, at the University of Nebraska Medical Center, Omaha. Dr. Rawal is clinical assistant professor of medicine at the University of Pittsburgh Medical Center. Dr. Crecelius is assistant professor of clinical medicine at Indiana University, Indianapolis. This article is sponsored by the SHM Physicians in Training committee, which submits quarterly content to the Hospitalist on topics relevant to trainees and early -career hospitalists.
References
1. Zerzan JT et al. Making the most of mentors: a guide for mentees. Acad Med. 2009;84:140-4. doi: 10.1097/ACM.0b013e3181906e8f.
2. Covey F. The seven habits of highly effective people. 25th anniversary edition. New York: Simon and Schuster, 2013.
Fact or fiction? Intravascular contrast and acute kidney injury
Withholding contrast may be the greater risk
Case
A 73-year-old man with stage III chronic kidney disease (CKD) presents to the emergency department with acute left–upper quadrant pain. Serum creatinine is 2.1mg/dL (eGFR 30 mL/min). Noncontrast computed tomography of the abdomen identifies small bowel inflammation and extensive atherosclerosis. Acute mesenteric ischemia is suspected, but further characterization requires intravenous contrast–enhanced images. He and his family worry about the safety of IV contrast and ask to speak with you.
Introduction
Intravenous iodinated contrast material enhances tissue conspicuity in CT imaging and improves its diagnostic performance. Several case reports published in the 1950s suggested that IV administration of high-osmolality contrast provoked acute kidney injury. An ensuing series of studies associated contrast utilization with renal impairment and additional data extrapolated from cardiology arteriography studies further amplified these concerns.
Contrast media use is often cited as a leading cause of hospital-acquired acute kidney injury.1 The associated fear of causing renal impairment or provoking the need for dialysis frequently leads clinicians to forgo contrast-enhanced CT studies or settle for suboptimal noncontrast imaging even in situations where these tests are clearly indicated. The potential for inadequate imaging to contribute to incomplete, delayed, or incorrect diagnoses represents an ongoing patient safety issue.
A growing body of literature suggests the risks of contrast-associated acute kidney injury are overstated, implying the truer danger lies with inadequate imaging, not contrast media utilization. This review discusses the definitions, risks, and incidence of contrast-associated acute kidney injury, informed by these recent studies.
Overview of the data
Definitions of contrast-induced renal dysfunction vary in clinical studies and range from a creatinine rise of 0.5-1 mg per deciliter or a 25%-50% increase from baseline within 2-5 days following contrast administration. In 2012, the Kidney Disease Improving Global Outcomes working group proposed the term “contrast-associated acute kidney injury” (CA-AKI) and defined it as a plasma creatinine rise of 0.3 mg/dL within 48 hours of contrast exposure, a creatinine increase by a factor of 1.5 over baseline within 7 days of contrast administration, or a urinary volume less than 0.5 mg per kg of body weight within 6 hours of contrast exposure (AKI Network or “AKIN” criteria for CA-AKI).2 Owing in part to inconsistent definitions and partly because of multiple potential confounders, the true incidence of contrast-associated acute kidney injury is uncertain.
The pathogenesis of CA-AKI is incompletely understood, but proposed mechanisms include direct tubular cytotoxic effects; reductions in intrarenal blood flow from contrast material–provoked arteriolar vasoconstriction and contrast-induced increases in blood viscosity; and renal microvascular thrombosis.
Risk factors for CA-AKI overlap with those for acute kidney injury in general. These include CKD, concurrent nephrotoxic medication use, advancing age, diabetes, hemodynamic disturbances to include intravascular volume depletion, systemic illness, and rapid arterial delivery of a large contrast volume.
Current American College of Radiology guidelines state that intravenous isotonic crystalloid volume expansion prior to contrast administration may provide some renal protection, although randomized clinical trial results are inconsistent. The largest clinical trials of N-acetylcysteine showed rates of CA-AKI, need for dialysis, and mortality were no different than placebo. Studies of intravenous sodium bicarbonate show outcomes similar to normal saline.
Introduced in the 1950s and used until the early 2000s, the osmolality of high-osmolality contrast material (HOCM) is roughly five times that of blood (1551 mOsm/kg H2O).3 The early case reports first identifying concern for contrast-induced renal damage were of HOCM used in angiography and pyelography testing. Multiple follow up clinical studies measured creatinine levels before and after contrast administration and classified the percentage of patients whose creatinine level rose above an arbitrary definition of renal injury as having contrast-induced renal injury. These studies formed the basis of the now longstanding concerns about contrast-associated renal dysfunction. Importantly, very few of these HOCM studies included a control group.
Following multiple studies demonstrating an improved safety profile with a similar image quality, the Food and Drug Administration approved low-osmolality contrast (LOCM, 413-796mOsm/kg H2O) in 1985. Early adoption was slow because of its significantly higher cost and incomplete Medicare reimbursement. Prices fell following generic LOCM introduction in 1995 and in 2005 Medicare approved universal reimbursement, leading to widespread use. The FDA approved an iso-osmolality contrast material (290 mOsm/kg H2O) in the mid-1990s; its safety profile and image quality is similar to LOCM. Both LOCM and iso-osmolality contrast material are used in CTs today. Iso-osmolality contrast is more viscous than LOCM and is currently more expensive. Iso-osmolality and LOCM have similar rates of CA-AKI.
A clinical series published in 2008 examined serum creatinine level variation over 5 consecutive days in 30,000 predominantly hospitalized patients who did not receive intravenous contrast material. Investigators simulated contrast administration between days 1 and 2, then observed creatinine changes over the subsequent days. The incidence of acute kidney injury following the simulated contrast dose closely resembled the rates identified in earlier studies that associated contrast exposure with renal injury.4 These results suggested that changes in renal function commonly attributed to contrast exposure may be because of other, concurrent, clinical factors.
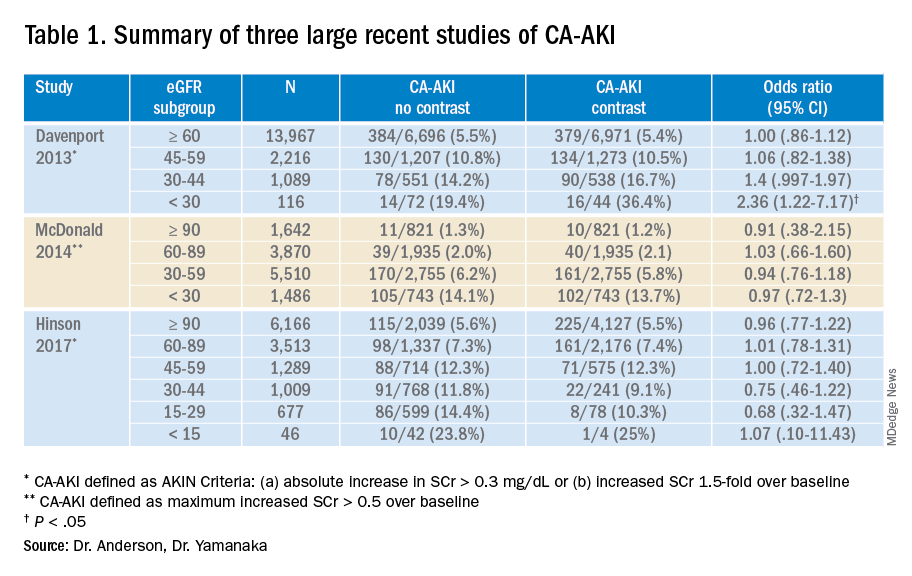
A 2013 study compared 8,826 patients with stable renal function who received a low-osmolality contrast-enhanced CT with 8,826 patients who underwent a noncontrast study.5 After 1:1 propensity matching, they found higher rates of CA-AKI (as defined by AKIN criteria) among only those with baseline eGFR less than 30 mL/min. There was a trend towards higher rates of CA-AKI among those with baseline eGFR of 30-44 mL/min, and no difference among the bulk of patients with normal or near normal baseline renal function.
Another large propensity score–matched study published in 2014 compared 6,254 patients who underwent a contrast-enhanced CT with 6,254 patients who underwent a nonenhanced CT.
Investigators stratified this predominantly inpatient cohort by baseline eGFR. Results demonstrated similar rates of AKI between contrast material and non–contrast material cohorts. They concluded that intravenous contrast administration did not significantly affect the risk of acute kidney injury, even in patients with impaired renal function. The authors noted that the difference in contrast-mediated nephrotoxic risk in patients with eGFRless than 30 between their study and the Davenport study could be explained by their use of a different definition of CA-AKI, differences in propensity score calculation, and by enrolling greater numbers of patients with impaired kidney function in their study.6
Finally, a large single-center study published in 2017 included 16,801 ED patients divided into three groups; patients who received a contrast-enhanced CT, patients who underwent a noncontrast CT study, and a set of patients who did not undergo any CT imaging. Patients with creatinine levels under .4 mg/dL or over 4 mg/dL were excluded from initial analysis.
Investigators stratified each patient group by serum creatinine and eGFR and utilized both traditional contrast-induced nephropathy (serum creatinine increase of .5 mg/dL or a 25% increase over baseline serum creatinine level at 48-72 hours) and AKIN criteria to evaluate for acute kidney injury. Propensity score analyses comparing the contrast-enhanced group and two control groups failed to identify any significant change in AKI incidence. The authors concluded that, in situations where contrast-enhanced CT is indicated to avoid missing or delaying potential diagnoses, the risks of diagnostic failure outweigh any potential risks of contrast induced renal injury.7
While these three studies utilized control groups and propensity score matching, they are retrospective in nature and unknown or omitted confounding variables could be present. Together, though, they contribute to a growing body of literature suggesting that the risk of contrast-associated AKI relates less to the contrast itself and more to concurrent clinical factors affecting kidney function. Ethical concerns have to date prevented the conduct of a randomized trial of IV contrast in CT scanning. Table 1 summarizes the findings of these three studies.
Application of the data to the case
The patient presented with abdominal pain potentially attributable to acute mesenteric ischemia, where a delayed or missed diagnosis can be potentially fatal. He was counseled about the comparatively small risk of CA-AKI with IV contrast and underwent contrast-enhanced CT scanning without incident. The diagnosis of acute mesenteric ischemia was confirmed, and he was referred for urgent laparotomy.
Bottom line
The absolute risk of CA-AKI varies according to baseline renal function and is not clearly linked to the receipt of IV contrast. The risks of withholding contrast may be greater than the risk of CA-AKI. Clinicians should counsel patients accordingly.
Dr. Anderson is national lead, VHA Hospital Medicine, and associate professor of medicine at the Minneapolis VA Health Care System. Dr. Yamanaka is a hospitalist at the Minneapolis VA Medical Center and an assistant professor of medicine at the University of Minnesota.
References
1. Nash K et al. Hospital-acquired renal insufficiency. Am J Kidney Dis. 2002;39(5):930-6. doi: 10.1053/ajkd.2002.32766.
2. Section 4: Contrast-induced AKI. Kidney Int Suppl. 2012;2(1):69-88. doi: 10.1038/kisup.2011.34.
3. Wilmot A et al. The adoption of low-osmolar contrast agents in the United States: Historical analysis of health policy and clinical practice. AJR Am J Roentgenol. 2012;199(5):1049-53. doi: 10.2214/AJR.11.8426.
4. Newhouse JH et al. Frequency of serum creatinine changes in the absence of iodinated contrast material: Implications for studies of contrast nephrotoxicity. AJR Am J Roentgenol. 2008;191(2):376-82. doi: 10.2214/AJR.07.3280.
5. Davenport MS et al. Contrast material-induced nephrotoxicity and intravenous low-osmolality iodinated contrast material: Risk stratification by using estimated glomerular filtration rate. Radiology. 2013;268(3):719-28. doi: 10.1148/radiol.13122276.
6. McDonald JS et al. Risk of intravenous contrast material-mediated acute kidney injury: A propensity score–matched study stratified by baseline-estimated glomerular filtration rate. Radiology. 2014;271(1):65-73. doi: 10.1148/radiol.13130775.
7. Hinson JS et al. Risk of acute kidney injury after intravenous contrast media administration. Ann Emerg Med. 2017;69(5):577-86. doi: 10.1016/j.annemergmed.2016.11.021.
Key points
- Early studies suggesting an association between IV contrast and AKI used an older formulation of contrast media not routinely used today. Importantly, these studies did not use control groups.
- Results from multiple recent large trials comparing IV contrast patients with controls suggest that AKI is not clearly linked to the receipt of IV contrast and that it varies according to baseline renal function.
- Randomized controlled trials of prophylactic normal saline or sodium bicarbonate to prevent CA-AKI show mixed results. Clinical trials comparing N-acetylcysteine with placebo showed no difference in the rates of AKI, dialysis initiation, or mortality.
Quiz
Which of the following is not clearly associated with acute kidney injury in hospitalized patients?
A. Decreased baseline glomerular filtration rate
B. Angiotensin-converting enzyme (ACE) inhibitor use
C. Hemodynamic instability
D. Intravenous contrast administration
Answer: D
While decreased baseline renal function, ACE inhibitors, and hemodynamic instability are known risk factors for hospital-associated renal injury, a growing body of literature suggests that intravenous contrast used in computed tomography studies does not precipitate acute kidney injury.
Further reading
McDonald JS et al. Frequency of acute kidney injury following intravenous contrast medium administration: a systematic review and meta-analysis. Radiology. 2013;267(1):119-128. doi: 10.1148/radiol.12121460.
McDonald RJ et al. Behind the numbers: Propensity score analysis – a primer for the diagnostic radiologist. Radiology. 2013;269(3):640-5. doi: 10.1148/radiol.13131465.
Luk L et al. Intravenous contrast-induced nephropathy – the rise and fall of a threatening idea. Adv Chronic Kidney Dis. 2017;24(3):169-75. doi: 10.1053/j.ackd.2017.03.001.
Mehran R et al. Contrast-associated acute kidney injury. N Engl J Med. 2019;380(22):2146-55. doi: 10.1056/NEJMra1805256.
Withholding contrast may be the greater risk
Withholding contrast may be the greater risk
Case
A 73-year-old man with stage III chronic kidney disease (CKD) presents to the emergency department with acute left–upper quadrant pain. Serum creatinine is 2.1mg/dL (eGFR 30 mL/min). Noncontrast computed tomography of the abdomen identifies small bowel inflammation and extensive atherosclerosis. Acute mesenteric ischemia is suspected, but further characterization requires intravenous contrast–enhanced images. He and his family worry about the safety of IV contrast and ask to speak with you.
Introduction
Intravenous iodinated contrast material enhances tissue conspicuity in CT imaging and improves its diagnostic performance. Several case reports published in the 1950s suggested that IV administration of high-osmolality contrast provoked acute kidney injury. An ensuing series of studies associated contrast utilization with renal impairment and additional data extrapolated from cardiology arteriography studies further amplified these concerns.
Contrast media use is often cited as a leading cause of hospital-acquired acute kidney injury.1 The associated fear of causing renal impairment or provoking the need for dialysis frequently leads clinicians to forgo contrast-enhanced CT studies or settle for suboptimal noncontrast imaging even in situations where these tests are clearly indicated. The potential for inadequate imaging to contribute to incomplete, delayed, or incorrect diagnoses represents an ongoing patient safety issue.
A growing body of literature suggests the risks of contrast-associated acute kidney injury are overstated, implying the truer danger lies with inadequate imaging, not contrast media utilization. This review discusses the definitions, risks, and incidence of contrast-associated acute kidney injury, informed by these recent studies.
Overview of the data
Definitions of contrast-induced renal dysfunction vary in clinical studies and range from a creatinine rise of 0.5-1 mg per deciliter or a 25%-50% increase from baseline within 2-5 days following contrast administration. In 2012, the Kidney Disease Improving Global Outcomes working group proposed the term “contrast-associated acute kidney injury” (CA-AKI) and defined it as a plasma creatinine rise of 0.3 mg/dL within 48 hours of contrast exposure, a creatinine increase by a factor of 1.5 over baseline within 7 days of contrast administration, or a urinary volume less than 0.5 mg per kg of body weight within 6 hours of contrast exposure (AKI Network or “AKIN” criteria for CA-AKI).2 Owing in part to inconsistent definitions and partly because of multiple potential confounders, the true incidence of contrast-associated acute kidney injury is uncertain.
The pathogenesis of CA-AKI is incompletely understood, but proposed mechanisms include direct tubular cytotoxic effects; reductions in intrarenal blood flow from contrast material–provoked arteriolar vasoconstriction and contrast-induced increases in blood viscosity; and renal microvascular thrombosis.
Risk factors for CA-AKI overlap with those for acute kidney injury in general. These include CKD, concurrent nephrotoxic medication use, advancing age, diabetes, hemodynamic disturbances to include intravascular volume depletion, systemic illness, and rapid arterial delivery of a large contrast volume.
Current American College of Radiology guidelines state that intravenous isotonic crystalloid volume expansion prior to contrast administration may provide some renal protection, although randomized clinical trial results are inconsistent. The largest clinical trials of N-acetylcysteine showed rates of CA-AKI, need for dialysis, and mortality were no different than placebo. Studies of intravenous sodium bicarbonate show outcomes similar to normal saline.
Introduced in the 1950s and used until the early 2000s, the osmolality of high-osmolality contrast material (HOCM) is roughly five times that of blood (1551 mOsm/kg H2O).3 The early case reports first identifying concern for contrast-induced renal damage were of HOCM used in angiography and pyelography testing. Multiple follow up clinical studies measured creatinine levels before and after contrast administration and classified the percentage of patients whose creatinine level rose above an arbitrary definition of renal injury as having contrast-induced renal injury. These studies formed the basis of the now longstanding concerns about contrast-associated renal dysfunction. Importantly, very few of these HOCM studies included a control group.
Following multiple studies demonstrating an improved safety profile with a similar image quality, the Food and Drug Administration approved low-osmolality contrast (LOCM, 413-796mOsm/kg H2O) in 1985. Early adoption was slow because of its significantly higher cost and incomplete Medicare reimbursement. Prices fell following generic LOCM introduction in 1995 and in 2005 Medicare approved universal reimbursement, leading to widespread use. The FDA approved an iso-osmolality contrast material (290 mOsm/kg H2O) in the mid-1990s; its safety profile and image quality is similar to LOCM. Both LOCM and iso-osmolality contrast material are used in CTs today. Iso-osmolality contrast is more viscous than LOCM and is currently more expensive. Iso-osmolality and LOCM have similar rates of CA-AKI.
A clinical series published in 2008 examined serum creatinine level variation over 5 consecutive days in 30,000 predominantly hospitalized patients who did not receive intravenous contrast material. Investigators simulated contrast administration between days 1 and 2, then observed creatinine changes over the subsequent days. The incidence of acute kidney injury following the simulated contrast dose closely resembled the rates identified in earlier studies that associated contrast exposure with renal injury.4 These results suggested that changes in renal function commonly attributed to contrast exposure may be because of other, concurrent, clinical factors.
A 2013 study compared 8,826 patients with stable renal function who received a low-osmolality contrast-enhanced CT with 8,826 patients who underwent a noncontrast study.5 After 1:1 propensity matching, they found higher rates of CA-AKI (as defined by AKIN criteria) among only those with baseline eGFR less than 30 mL/min. There was a trend towards higher rates of CA-AKI among those with baseline eGFR of 30-44 mL/min, and no difference among the bulk of patients with normal or near normal baseline renal function.
Another large propensity score–matched study published in 2014 compared 6,254 patients who underwent a contrast-enhanced CT with 6,254 patients who underwent a nonenhanced CT.
Investigators stratified this predominantly inpatient cohort by baseline eGFR. Results demonstrated similar rates of AKI between contrast material and non–contrast material cohorts. They concluded that intravenous contrast administration did not significantly affect the risk of acute kidney injury, even in patients with impaired renal function. The authors noted that the difference in contrast-mediated nephrotoxic risk in patients with eGFRless than 30 between their study and the Davenport study could be explained by their use of a different definition of CA-AKI, differences in propensity score calculation, and by enrolling greater numbers of patients with impaired kidney function in their study.6
Finally, a large single-center study published in 2017 included 16,801 ED patients divided into three groups; patients who received a contrast-enhanced CT, patients who underwent a noncontrast CT study, and a set of patients who did not undergo any CT imaging. Patients with creatinine levels under .4 mg/dL or over 4 mg/dL were excluded from initial analysis.
Investigators stratified each patient group by serum creatinine and eGFR and utilized both traditional contrast-induced nephropathy (serum creatinine increase of .5 mg/dL or a 25% increase over baseline serum creatinine level at 48-72 hours) and AKIN criteria to evaluate for acute kidney injury. Propensity score analyses comparing the contrast-enhanced group and two control groups failed to identify any significant change in AKI incidence. The authors concluded that, in situations where contrast-enhanced CT is indicated to avoid missing or delaying potential diagnoses, the risks of diagnostic failure outweigh any potential risks of contrast induced renal injury.7
While these three studies utilized control groups and propensity score matching, they are retrospective in nature and unknown or omitted confounding variables could be present. Together, though, they contribute to a growing body of literature suggesting that the risk of contrast-associated AKI relates less to the contrast itself and more to concurrent clinical factors affecting kidney function. Ethical concerns have to date prevented the conduct of a randomized trial of IV contrast in CT scanning. Table 1 summarizes the findings of these three studies.

Application of the data to the case
The patient presented with abdominal pain potentially attributable to acute mesenteric ischemia, where a delayed or missed diagnosis can be potentially fatal. He was counseled about the comparatively small risk of CA-AKI with IV contrast and underwent contrast-enhanced CT scanning without incident. The diagnosis of acute mesenteric ischemia was confirmed, and he was referred for urgent laparotomy.
Bottom line
The absolute risk of CA-AKI varies according to baseline renal function and is not clearly linked to the receipt of IV contrast. The risks of withholding contrast may be greater than the risk of CA-AKI. Clinicians should counsel patients accordingly.
Dr. Anderson is national lead, VHA Hospital Medicine, and associate professor of medicine at the Minneapolis VA Health Care System. Dr. Yamanaka is a hospitalist at the Minneapolis VA Medical Center and an assistant professor of medicine at the University of Minnesota.
References
1. Nash K et al. Hospital-acquired renal insufficiency. Am J Kidney Dis. 2002;39(5):930-6. doi: 10.1053/ajkd.2002.32766.
2. Section 4: Contrast-induced AKI. Kidney Int Suppl. 2012;2(1):69-88. doi: 10.1038/kisup.2011.34.
3. Wilmot A et al. The adoption of low-osmolar contrast agents in the United States: Historical analysis of health policy and clinical practice. AJR Am J Roentgenol. 2012;199(5):1049-53. doi: 10.2214/AJR.11.8426.
4. Newhouse JH et al. Frequency of serum creatinine changes in the absence of iodinated contrast material: Implications for studies of contrast nephrotoxicity. AJR Am J Roentgenol. 2008;191(2):376-82. doi: 10.2214/AJR.07.3280.
5. Davenport MS et al. Contrast material-induced nephrotoxicity and intravenous low-osmolality iodinated contrast material: Risk stratification by using estimated glomerular filtration rate. Radiology. 2013;268(3):719-28. doi: 10.1148/radiol.13122276.
6. McDonald JS et al. Risk of intravenous contrast material-mediated acute kidney injury: A propensity score–matched study stratified by baseline-estimated glomerular filtration rate. Radiology. 2014;271(1):65-73. doi: 10.1148/radiol.13130775.
7. Hinson JS et al. Risk of acute kidney injury after intravenous contrast media administration. Ann Emerg Med. 2017;69(5):577-86. doi: 10.1016/j.annemergmed.2016.11.021.
Key points
- Early studies suggesting an association between IV contrast and AKI used an older formulation of contrast media not routinely used today. Importantly, these studies did not use control groups.
- Results from multiple recent large trials comparing IV contrast patients with controls suggest that AKI is not clearly linked to the receipt of IV contrast and that it varies according to baseline renal function.
- Randomized controlled trials of prophylactic normal saline or sodium bicarbonate to prevent CA-AKI show mixed results. Clinical trials comparing N-acetylcysteine with placebo showed no difference in the rates of AKI, dialysis initiation, or mortality.
Quiz
Which of the following is not clearly associated with acute kidney injury in hospitalized patients?
A. Decreased baseline glomerular filtration rate
B. Angiotensin-converting enzyme (ACE) inhibitor use
C. Hemodynamic instability
D. Intravenous contrast administration
Answer: D
While decreased baseline renal function, ACE inhibitors, and hemodynamic instability are known risk factors for hospital-associated renal injury, a growing body of literature suggests that intravenous contrast used in computed tomography studies does not precipitate acute kidney injury.
Further reading
McDonald JS et al. Frequency of acute kidney injury following intravenous contrast medium administration: a systematic review and meta-analysis. Radiology. 2013;267(1):119-128. doi: 10.1148/radiol.12121460.
McDonald RJ et al. Behind the numbers: Propensity score analysis – a primer for the diagnostic radiologist. Radiology. 2013;269(3):640-5. doi: 10.1148/radiol.13131465.
Luk L et al. Intravenous contrast-induced nephropathy – the rise and fall of a threatening idea. Adv Chronic Kidney Dis. 2017;24(3):169-75. doi: 10.1053/j.ackd.2017.03.001.
Mehran R et al. Contrast-associated acute kidney injury. N Engl J Med. 2019;380(22):2146-55. doi: 10.1056/NEJMra1805256.
Case
A 73-year-old man with stage III chronic kidney disease (CKD) presents to the emergency department with acute left–upper quadrant pain. Serum creatinine is 2.1mg/dL (eGFR 30 mL/min). Noncontrast computed tomography of the abdomen identifies small bowel inflammation and extensive atherosclerosis. Acute mesenteric ischemia is suspected, but further characterization requires intravenous contrast–enhanced images. He and his family worry about the safety of IV contrast and ask to speak with you.
Introduction
Intravenous iodinated contrast material enhances tissue conspicuity in CT imaging and improves its diagnostic performance. Several case reports published in the 1950s suggested that IV administration of high-osmolality contrast provoked acute kidney injury. An ensuing series of studies associated contrast utilization with renal impairment and additional data extrapolated from cardiology arteriography studies further amplified these concerns.
Contrast media use is often cited as a leading cause of hospital-acquired acute kidney injury.1 The associated fear of causing renal impairment or provoking the need for dialysis frequently leads clinicians to forgo contrast-enhanced CT studies or settle for suboptimal noncontrast imaging even in situations where these tests are clearly indicated. The potential for inadequate imaging to contribute to incomplete, delayed, or incorrect diagnoses represents an ongoing patient safety issue.
A growing body of literature suggests the risks of contrast-associated acute kidney injury are overstated, implying the truer danger lies with inadequate imaging, not contrast media utilization. This review discusses the definitions, risks, and incidence of contrast-associated acute kidney injury, informed by these recent studies.
Overview of the data
Definitions of contrast-induced renal dysfunction vary in clinical studies and range from a creatinine rise of 0.5-1 mg per deciliter or a 25%-50% increase from baseline within 2-5 days following contrast administration. In 2012, the Kidney Disease Improving Global Outcomes working group proposed the term “contrast-associated acute kidney injury” (CA-AKI) and defined it as a plasma creatinine rise of 0.3 mg/dL within 48 hours of contrast exposure, a creatinine increase by a factor of 1.5 over baseline within 7 days of contrast administration, or a urinary volume less than 0.5 mg per kg of body weight within 6 hours of contrast exposure (AKI Network or “AKIN” criteria for CA-AKI).2 Owing in part to inconsistent definitions and partly because of multiple potential confounders, the true incidence of contrast-associated acute kidney injury is uncertain.
The pathogenesis of CA-AKI is incompletely understood, but proposed mechanisms include direct tubular cytotoxic effects; reductions in intrarenal blood flow from contrast material–provoked arteriolar vasoconstriction and contrast-induced increases in blood viscosity; and renal microvascular thrombosis.
Risk factors for CA-AKI overlap with those for acute kidney injury in general. These include CKD, concurrent nephrotoxic medication use, advancing age, diabetes, hemodynamic disturbances to include intravascular volume depletion, systemic illness, and rapid arterial delivery of a large contrast volume.
Current American College of Radiology guidelines state that intravenous isotonic crystalloid volume expansion prior to contrast administration may provide some renal protection, although randomized clinical trial results are inconsistent. The largest clinical trials of N-acetylcysteine showed rates of CA-AKI, need for dialysis, and mortality were no different than placebo. Studies of intravenous sodium bicarbonate show outcomes similar to normal saline.
Introduced in the 1950s and used until the early 2000s, the osmolality of high-osmolality contrast material (HOCM) is roughly five times that of blood (1551 mOsm/kg H2O).3 The early case reports first identifying concern for contrast-induced renal damage were of HOCM used in angiography and pyelography testing. Multiple follow up clinical studies measured creatinine levels before and after contrast administration and classified the percentage of patients whose creatinine level rose above an arbitrary definition of renal injury as having contrast-induced renal injury. These studies formed the basis of the now longstanding concerns about contrast-associated renal dysfunction. Importantly, very few of these HOCM studies included a control group.
Following multiple studies demonstrating an improved safety profile with a similar image quality, the Food and Drug Administration approved low-osmolality contrast (LOCM, 413-796mOsm/kg H2O) in 1985. Early adoption was slow because of its significantly higher cost and incomplete Medicare reimbursement. Prices fell following generic LOCM introduction in 1995 and in 2005 Medicare approved universal reimbursement, leading to widespread use. The FDA approved an iso-osmolality contrast material (290 mOsm/kg H2O) in the mid-1990s; its safety profile and image quality is similar to LOCM. Both LOCM and iso-osmolality contrast material are used in CTs today. Iso-osmolality contrast is more viscous than LOCM and is currently more expensive. Iso-osmolality and LOCM have similar rates of CA-AKI.
A clinical series published in 2008 examined serum creatinine level variation over 5 consecutive days in 30,000 predominantly hospitalized patients who did not receive intravenous contrast material. Investigators simulated contrast administration between days 1 and 2, then observed creatinine changes over the subsequent days. The incidence of acute kidney injury following the simulated contrast dose closely resembled the rates identified in earlier studies that associated contrast exposure with renal injury.4 These results suggested that changes in renal function commonly attributed to contrast exposure may be because of other, concurrent, clinical factors.
A 2013 study compared 8,826 patients with stable renal function who received a low-osmolality contrast-enhanced CT with 8,826 patients who underwent a noncontrast study.5 After 1:1 propensity matching, they found higher rates of CA-AKI (as defined by AKIN criteria) among only those with baseline eGFR less than 30 mL/min. There was a trend towards higher rates of CA-AKI among those with baseline eGFR of 30-44 mL/min, and no difference among the bulk of patients with normal or near normal baseline renal function.
Another large propensity score–matched study published in 2014 compared 6,254 patients who underwent a contrast-enhanced CT with 6,254 patients who underwent a nonenhanced CT.
Investigators stratified this predominantly inpatient cohort by baseline eGFR. Results demonstrated similar rates of AKI between contrast material and non–contrast material cohorts. They concluded that intravenous contrast administration did not significantly affect the risk of acute kidney injury, even in patients with impaired renal function. The authors noted that the difference in contrast-mediated nephrotoxic risk in patients with eGFRless than 30 between their study and the Davenport study could be explained by their use of a different definition of CA-AKI, differences in propensity score calculation, and by enrolling greater numbers of patients with impaired kidney function in their study.6
Finally, a large single-center study published in 2017 included 16,801 ED patients divided into three groups; patients who received a contrast-enhanced CT, patients who underwent a noncontrast CT study, and a set of patients who did not undergo any CT imaging. Patients with creatinine levels under .4 mg/dL or over 4 mg/dL were excluded from initial analysis.
Investigators stratified each patient group by serum creatinine and eGFR and utilized both traditional contrast-induced nephropathy (serum creatinine increase of .5 mg/dL or a 25% increase over baseline serum creatinine level at 48-72 hours) and AKIN criteria to evaluate for acute kidney injury. Propensity score analyses comparing the contrast-enhanced group and two control groups failed to identify any significant change in AKI incidence. The authors concluded that, in situations where contrast-enhanced CT is indicated to avoid missing or delaying potential diagnoses, the risks of diagnostic failure outweigh any potential risks of contrast induced renal injury.7
While these three studies utilized control groups and propensity score matching, they are retrospective in nature and unknown or omitted confounding variables could be present. Together, though, they contribute to a growing body of literature suggesting that the risk of contrast-associated AKI relates less to the contrast itself and more to concurrent clinical factors affecting kidney function. Ethical concerns have to date prevented the conduct of a randomized trial of IV contrast in CT scanning. Table 1 summarizes the findings of these three studies.

Application of the data to the case
The patient presented with abdominal pain potentially attributable to acute mesenteric ischemia, where a delayed or missed diagnosis can be potentially fatal. He was counseled about the comparatively small risk of CA-AKI with IV contrast and underwent contrast-enhanced CT scanning without incident. The diagnosis of acute mesenteric ischemia was confirmed, and he was referred for urgent laparotomy.
Bottom line
The absolute risk of CA-AKI varies according to baseline renal function and is not clearly linked to the receipt of IV contrast. The risks of withholding contrast may be greater than the risk of CA-AKI. Clinicians should counsel patients accordingly.
Dr. Anderson is national lead, VHA Hospital Medicine, and associate professor of medicine at the Minneapolis VA Health Care System. Dr. Yamanaka is a hospitalist at the Minneapolis VA Medical Center and an assistant professor of medicine at the University of Minnesota.
References
1. Nash K et al. Hospital-acquired renal insufficiency. Am J Kidney Dis. 2002;39(5):930-6. doi: 10.1053/ajkd.2002.32766.
2. Section 4: Contrast-induced AKI. Kidney Int Suppl. 2012;2(1):69-88. doi: 10.1038/kisup.2011.34.
3. Wilmot A et al. The adoption of low-osmolar contrast agents in the United States: Historical analysis of health policy and clinical practice. AJR Am J Roentgenol. 2012;199(5):1049-53. doi: 10.2214/AJR.11.8426.
4. Newhouse JH et al. Frequency of serum creatinine changes in the absence of iodinated contrast material: Implications for studies of contrast nephrotoxicity. AJR Am J Roentgenol. 2008;191(2):376-82. doi: 10.2214/AJR.07.3280.
5. Davenport MS et al. Contrast material-induced nephrotoxicity and intravenous low-osmolality iodinated contrast material: Risk stratification by using estimated glomerular filtration rate. Radiology. 2013;268(3):719-28. doi: 10.1148/radiol.13122276.
6. McDonald JS et al. Risk of intravenous contrast material-mediated acute kidney injury: A propensity score–matched study stratified by baseline-estimated glomerular filtration rate. Radiology. 2014;271(1):65-73. doi: 10.1148/radiol.13130775.
7. Hinson JS et al. Risk of acute kidney injury after intravenous contrast media administration. Ann Emerg Med. 2017;69(5):577-86. doi: 10.1016/j.annemergmed.2016.11.021.
Key points
- Early studies suggesting an association between IV contrast and AKI used an older formulation of contrast media not routinely used today. Importantly, these studies did not use control groups.
- Results from multiple recent large trials comparing IV contrast patients with controls suggest that AKI is not clearly linked to the receipt of IV contrast and that it varies according to baseline renal function.
- Randomized controlled trials of prophylactic normal saline or sodium bicarbonate to prevent CA-AKI show mixed results. Clinical trials comparing N-acetylcysteine with placebo showed no difference in the rates of AKI, dialysis initiation, or mortality.
Quiz
Which of the following is not clearly associated with acute kidney injury in hospitalized patients?
A. Decreased baseline glomerular filtration rate
B. Angiotensin-converting enzyme (ACE) inhibitor use
C. Hemodynamic instability
D. Intravenous contrast administration
Answer: D
While decreased baseline renal function, ACE inhibitors, and hemodynamic instability are known risk factors for hospital-associated renal injury, a growing body of literature suggests that intravenous contrast used in computed tomography studies does not precipitate acute kidney injury.
Further reading
McDonald JS et al. Frequency of acute kidney injury following intravenous contrast medium administration: a systematic review and meta-analysis. Radiology. 2013;267(1):119-128. doi: 10.1148/radiol.12121460.
McDonald RJ et al. Behind the numbers: Propensity score analysis – a primer for the diagnostic radiologist. Radiology. 2013;269(3):640-5. doi: 10.1148/radiol.13131465.
Luk L et al. Intravenous contrast-induced nephropathy – the rise and fall of a threatening idea. Adv Chronic Kidney Dis. 2017;24(3):169-75. doi: 10.1053/j.ackd.2017.03.001.
Mehran R et al. Contrast-associated acute kidney injury. N Engl J Med. 2019;380(22):2146-55. doi: 10.1056/NEJMra1805256.
Reflections on 10 years of hospitalist productivity
Successful programs will recruit lifelong learners
The workload of individual hospitalists has long been a hot-button issue. In a 2013 survey of hospitalists, 40% felt workloads were unsafe on a monthly basis, and 22% reported ordering unnecessary testing or procedures because of time pressure.1 In a 2014 analysis of over 20,000 admissions to an academic hospital medicine service, increasing workload led to increased length of stay and cost per case.2 Although these studies suggest a “sweet spot” for hospitalist workload, many groups face constant pressure to increase revenue.
Over the past decade there has been a significant change in how hospital medicine programs are financed. In the 2010 State of Hospital Medicine (SoHM), the median financial support per physician hospitalist in adult hospital medicine groups (HMGs) was $98,253. By the 2020 SoHM, the financial support was $198,750, an increase of $100,497 in just 10 years. When this is combined with the explosive growth in the number of hospitalists, there is one inescapable conclusion – hospital medicine is expensive.
Over this same 10 years, net collections per hospitalist grew from $194,440 in 2010 to $216,779 in 2020, an increase of $22,339. The increase was caused by higher collections per encounter, not more encounters. Additionally, median compensation for adult/internal medicine hospitalists increased over the same period from $215,000 to $307,336, an increase of $92,336, or 43%. That is an increase of 3.7% per year, more than twice the rate of inflation or wage growth in the general economy over the same period. About 75% of this increase was funded by hospital support. It is clear – health care systems continue to find value in investing in hospitalists and hospital medicine programs.
With mounting costs for hospitals, there is pressure for the hospitalist model of care to change or for yearly billable encounters per hospitalist full-time equivalent to increase. Yet, the productivity of hospitalists, as measured by median billable encounters per year has remained flat. The 2010 SoHM listed median number of billable encounters per year for an internal medicine hospitalist as 2,230. In 2020, the number is 2,246 – a trivial 0.7% increase per decade, what amounts to a rounding error. There has been wiggle up and down over the years, but I suspect these are not trends but noise.
So the question is why. I think it is partly because hospital medicine leaders together with the leaders of their health care systems seem to be reaching an equilibrium. Productivity will always remain an expectation. This expectation will vary based on local circumstances. But for many HMGs, the days when productivity is pushed as the primary objective seem to be disappearing. Most hospital leaders seem to now understand that high productivity can be detrimental to other program goals.
But if productivity is flat, do 40% of hospitalists still feel they are providing unsafe care on a monthly basis? Without another study we don’t know, but here are some reasons why I’m hopeful. First, the hospitalist workforce is more experienced than 10 years ago and may be more efficient. Second, hospital medicine groups are larger and are therefore enabled to schedule more flexibly or enact jeopardy systems to level out workload on busy days. And lastly, hospitalists who feel they are providing unsafe care find greener pastures. The 2010 SoHM reported adult hospital medicine programs had a median 14.3% turnover rate. The 2020 SoHM turnover was 10.9%. While this is up from 2018 (7.4%) and 2016 (6.9%), the general trend is down.
Additionally, we all need to consider the possibility that there will be a disruptive innovation that will allow greater productivity for individual hospitalists while maintaining value. It is apparent the EHR is not yet that breakthrough. We all need to keep our eyes open, stay flexible, and be prepared to meet evolving demands on our programs.
We will see constant demands on hospitalists. But I’m hopeful that going forward expectations will increasingly shift away from simply working harder and seeing more patients, toward goals related to improving performance. Training programs generally produce excellent clinicians, but they often do not equip physicians to be excellent hospitalists. Successful hospital medicine programs will recruit lifelong learners and career hospitalists who are flexible and willing to innovate and adapt. The best programs will have structures in place to help excellent clinicians mature into the role of excellent hospitalists, and leaders that create and foster an environment of excellence.
Discover more 2020 SoHM Report data at www.hospitalmedicine.org/sohm.
Dr. Frederickson is medical director, hospital medicine and palliative care, at CHI Health, Omaha, Neb., and assistant professor at Creighton University, Omaha.
References
1. Michtalik HJ et al. Impact of Attending Physician Workload on Patient Care: A Survey of Hospitalists. JAMA Intern Med. 2013;173(5):375-7. doi: 10.1001/jamainternmed.2013.1864.
2. Elliott DJ et al. Effect of Hospitalist Workload on the Quality and Efficiency of Care. JAMA Intern Med. 2014;174(5):786-93. doi: 10.1001/jamainternmed.2014.300.
Successful programs will recruit lifelong learners
Successful programs will recruit lifelong learners
The workload of individual hospitalists has long been a hot-button issue. In a 2013 survey of hospitalists, 40% felt workloads were unsafe on a monthly basis, and 22% reported ordering unnecessary testing or procedures because of time pressure.1 In a 2014 analysis of over 20,000 admissions to an academic hospital medicine service, increasing workload led to increased length of stay and cost per case.2 Although these studies suggest a “sweet spot” for hospitalist workload, many groups face constant pressure to increase revenue.
Over the past decade there has been a significant change in how hospital medicine programs are financed. In the 2010 State of Hospital Medicine (SoHM), the median financial support per physician hospitalist in adult hospital medicine groups (HMGs) was $98,253. By the 2020 SoHM, the financial support was $198,750, an increase of $100,497 in just 10 years. When this is combined with the explosive growth in the number of hospitalists, there is one inescapable conclusion – hospital medicine is expensive.
Over this same 10 years, net collections per hospitalist grew from $194,440 in 2010 to $216,779 in 2020, an increase of $22,339. The increase was caused by higher collections per encounter, not more encounters. Additionally, median compensation for adult/internal medicine hospitalists increased over the same period from $215,000 to $307,336, an increase of $92,336, or 43%. That is an increase of 3.7% per year, more than twice the rate of inflation or wage growth in the general economy over the same period. About 75% of this increase was funded by hospital support. It is clear – health care systems continue to find value in investing in hospitalists and hospital medicine programs.
With mounting costs for hospitals, there is pressure for the hospitalist model of care to change or for yearly billable encounters per hospitalist full-time equivalent to increase. Yet, the productivity of hospitalists, as measured by median billable encounters per year has remained flat. The 2010 SoHM listed median number of billable encounters per year for an internal medicine hospitalist as 2,230. In 2020, the number is 2,246 – a trivial 0.7% increase per decade, what amounts to a rounding error. There has been wiggle up and down over the years, but I suspect these are not trends but noise.
So the question is why. I think it is partly because hospital medicine leaders together with the leaders of their health care systems seem to be reaching an equilibrium. Productivity will always remain an expectation. This expectation will vary based on local circumstances. But for many HMGs, the days when productivity is pushed as the primary objective seem to be disappearing. Most hospital leaders seem to now understand that high productivity can be detrimental to other program goals.
But if productivity is flat, do 40% of hospitalists still feel they are providing unsafe care on a monthly basis? Without another study we don’t know, but here are some reasons why I’m hopeful. First, the hospitalist workforce is more experienced than 10 years ago and may be more efficient. Second, hospital medicine groups are larger and are therefore enabled to schedule more flexibly or enact jeopardy systems to level out workload on busy days. And lastly, hospitalists who feel they are providing unsafe care find greener pastures. The 2010 SoHM reported adult hospital medicine programs had a median 14.3% turnover rate. The 2020 SoHM turnover was 10.9%. While this is up from 2018 (7.4%) and 2016 (6.9%), the general trend is down.
Additionally, we all need to consider the possibility that there will be a disruptive innovation that will allow greater productivity for individual hospitalists while maintaining value. It is apparent the EHR is not yet that breakthrough. We all need to keep our eyes open, stay flexible, and be prepared to meet evolving demands on our programs.
We will see constant demands on hospitalists. But I’m hopeful that going forward expectations will increasingly shift away from simply working harder and seeing more patients, toward goals related to improving performance. Training programs generally produce excellent clinicians, but they often do not equip physicians to be excellent hospitalists. Successful hospital medicine programs will recruit lifelong learners and career hospitalists who are flexible and willing to innovate and adapt. The best programs will have structures in place to help excellent clinicians mature into the role of excellent hospitalists, and leaders that create and foster an environment of excellence.
Discover more 2020 SoHM Report data at www.hospitalmedicine.org/sohm.
Dr. Frederickson is medical director, hospital medicine and palliative care, at CHI Health, Omaha, Neb., and assistant professor at Creighton University, Omaha.
References
1. Michtalik HJ et al. Impact of Attending Physician Workload on Patient Care: A Survey of Hospitalists. JAMA Intern Med. 2013;173(5):375-7. doi: 10.1001/jamainternmed.2013.1864.
2. Elliott DJ et al. Effect of Hospitalist Workload on the Quality and Efficiency of Care. JAMA Intern Med. 2014;174(5):786-93. doi: 10.1001/jamainternmed.2014.300.
The workload of individual hospitalists has long been a hot-button issue. In a 2013 survey of hospitalists, 40% felt workloads were unsafe on a monthly basis, and 22% reported ordering unnecessary testing or procedures because of time pressure.1 In a 2014 analysis of over 20,000 admissions to an academic hospital medicine service, increasing workload led to increased length of stay and cost per case.2 Although these studies suggest a “sweet spot” for hospitalist workload, many groups face constant pressure to increase revenue.
Over the past decade there has been a significant change in how hospital medicine programs are financed. In the 2010 State of Hospital Medicine (SoHM), the median financial support per physician hospitalist in adult hospital medicine groups (HMGs) was $98,253. By the 2020 SoHM, the financial support was $198,750, an increase of $100,497 in just 10 years. When this is combined with the explosive growth in the number of hospitalists, there is one inescapable conclusion – hospital medicine is expensive.
Over this same 10 years, net collections per hospitalist grew from $194,440 in 2010 to $216,779 in 2020, an increase of $22,339. The increase was caused by higher collections per encounter, not more encounters. Additionally, median compensation for adult/internal medicine hospitalists increased over the same period from $215,000 to $307,336, an increase of $92,336, or 43%. That is an increase of 3.7% per year, more than twice the rate of inflation or wage growth in the general economy over the same period. About 75% of this increase was funded by hospital support. It is clear – health care systems continue to find value in investing in hospitalists and hospital medicine programs.
With mounting costs for hospitals, there is pressure for the hospitalist model of care to change or for yearly billable encounters per hospitalist full-time equivalent to increase. Yet, the productivity of hospitalists, as measured by median billable encounters per year has remained flat. The 2010 SoHM listed median number of billable encounters per year for an internal medicine hospitalist as 2,230. In 2020, the number is 2,246 – a trivial 0.7% increase per decade, what amounts to a rounding error. There has been wiggle up and down over the years, but I suspect these are not trends but noise.
So the question is why. I think it is partly because hospital medicine leaders together with the leaders of their health care systems seem to be reaching an equilibrium. Productivity will always remain an expectation. This expectation will vary based on local circumstances. But for many HMGs, the days when productivity is pushed as the primary objective seem to be disappearing. Most hospital leaders seem to now understand that high productivity can be detrimental to other program goals.
But if productivity is flat, do 40% of hospitalists still feel they are providing unsafe care on a monthly basis? Without another study we don’t know, but here are some reasons why I’m hopeful. First, the hospitalist workforce is more experienced than 10 years ago and may be more efficient. Second, hospital medicine groups are larger and are therefore enabled to schedule more flexibly or enact jeopardy systems to level out workload on busy days. And lastly, hospitalists who feel they are providing unsafe care find greener pastures. The 2010 SoHM reported adult hospital medicine programs had a median 14.3% turnover rate. The 2020 SoHM turnover was 10.9%. While this is up from 2018 (7.4%) and 2016 (6.9%), the general trend is down.
Additionally, we all need to consider the possibility that there will be a disruptive innovation that will allow greater productivity for individual hospitalists while maintaining value. It is apparent the EHR is not yet that breakthrough. We all need to keep our eyes open, stay flexible, and be prepared to meet evolving demands on our programs.
We will see constant demands on hospitalists. But I’m hopeful that going forward expectations will increasingly shift away from simply working harder and seeing more patients, toward goals related to improving performance. Training programs generally produce excellent clinicians, but they often do not equip physicians to be excellent hospitalists. Successful hospital medicine programs will recruit lifelong learners and career hospitalists who are flexible and willing to innovate and adapt. The best programs will have structures in place to help excellent clinicians mature into the role of excellent hospitalists, and leaders that create and foster an environment of excellence.
Discover more 2020 SoHM Report data at www.hospitalmedicine.org/sohm.
Dr. Frederickson is medical director, hospital medicine and palliative care, at CHI Health, Omaha, Neb., and assistant professor at Creighton University, Omaha.
References
1. Michtalik HJ et al. Impact of Attending Physician Workload on Patient Care: A Survey of Hospitalists. JAMA Intern Med. 2013;173(5):375-7. doi: 10.1001/jamainternmed.2013.1864.
2. Elliott DJ et al. Effect of Hospitalist Workload on the Quality and Efficiency of Care. JAMA Intern Med. 2014;174(5):786-93. doi: 10.1001/jamainternmed.2014.300.
Preparing pediatric hospital medicine fellows for leadership
Reflecting on a longitudinal leadership elective experience
The practice of pediatric hospital medicine (PHM) has been evolving and rapidly expanding over the last several decades. Not only has the scope of clinical practice matured and become more defined, but hospitalists now also have the responsibility to advance the performance of hospitals and health care systems. Pediatric hospitalists are increasingly incorporating medical education, research, high-value care, patient quality and safety initiatives, and process improvement into their careers.1 As a result, pediatric hospitalists are occupying a wider range of administrative and leadership positions within the health care system.
The field of PHM has highlighted the importance of leadership in the practice of hospital medicine by dedicating a chapter to “Leadership in Healthcare” in the PHM Core Competencies.1 The competencies define the expertise required of hospitalists and serve as guidance for the development of education, training, and career development series. Hospitalists may seek out opportunities for leadership training at an institutional or national level. Options may include advanced degrees, national conferences, division training seminars, or self-directed learning through reading or observational experiences. Unfortunately, all of these take time and motivation. As a result, hospitalists tend to pursue these opportunities only after they have already been appointed to leadership positions.
PHM fellowship is the optimal time to build a foundation of leadership skills. Over the course of a 2-year fellowship, fellows have a combined 16 weeks dedicated to educational activities beyond direct patient care.2 The Accreditation Council for Graduate Medical Education (ACGME) encourages educational innovation during this time, allowing programs to create unique opportunities for their fellows that will promote progress towards their ultimate career goals.3 This curricular framework provides the flexibility to integrate leadership training into fellowship training.
Many fellows are eager for leadership experiences and mentorship, myself included. As a pediatric chief resident, I was immersed in a diverse range of clinical, educational, research, and administrative responsibilities. I found myself in a leadership position with no prior education on how to manage people or team dynamics, make high-stress decisions on behalf of a group of people, or handle conflict. Although I learned new strategies on a daily basis, the experience showed me how much more I still had to learn in order to be a successful leader. This was one of the reasons I decided to pursue fellowship training. I think many PHM fellowship applicants feel similarly. They may have served in a leadership position in the past but feel underprepared to fulfill leadership positions in the next phase of their careers.
But despite this eagerness, evidence suggests that fellows do not feel that they receive as much management training as they need to start their careers. In a 2014 survey of PHM fellowship graduates, many held formal leadership positions within their institution (23/51) and within national organizations (6/51), despite having only five years of hospitalist experience on average (including time spent in fellowship). When asked about training needs, respondents identified “hospital program management” as an area where they wished they received more training during fellowship.4
Anyone who has gone through the PHM fellowship interview process can tell you that a common refrain of program directors is, “One of the goals of our program is to create future leaders in PHM.” This led me to wonder: how do fellowship programs prepare their fellows for future leadership positions?
I began my fellowship training at Nationwide Children’s Hospital in the summer of 2020. The program had just designed a longitudinal leadership elective, which the second-year fellow and I decided to pilot together. As I reflected on the first half of this academic year, I realized that it is unique experiences like this elective that make me thankful I pursued fellowship. I want to share with the hospitalist community the structure of the elective and why it has been particularly valuable with the hope that it will inspire similar opportunities for other fellows.
The program is semi-structured but allows the fellow and preceptors the flexibility to decide what activities would benefit that particular fellow. We attend a variety of administrative and committee meetings with each preceptor that expose us to the responsibilities of their positions, their leadership style in action, their approach to crisis management, and differences in divisional operations. On a monthly basis we meet with a preceptor to discuss a topic related to leadership. Examples of topics include how to run a more effective meeting, barriers to organizational change, leading in crisis, and the importance of mission, vision, values, and goals of organizations. The preceptor sends us articles or other learning materials they have found useful on the topic, and these serve as a starting point for our discussions. These discussions provide a point of reflection as we apply the day’s concept to our own prior experiences or to our observations during the elective.
The combination of learning experiences, discussions, and dedicated preceptorship has prepared me far better for future leadership than my past personal and observational experiences. I have summarized my top three reasons why this structure of leadership development is particularly valuable to me as a fellow.
First, the longitudinal structure of the elective allows us to learn from multiple preceptors over the course of the academic year. The preceptors include the current chief of hospital pediatrics at Nationwide Children’s Hospital; the division director of hospital medicine at the Ohio State University Wexner Medical Center; and the physician lead for hospital medicine at one of the satellite hospitals in the region. With faculty from the Department of Pediatrics and the Department of Internal Medicine-Pediatrics in these leadership positions, we have the unique ability to compare and contrast operational systems between the two different hospital systems.
Recently, we also had the opportunity to meet with both the chairman of the department of pediatrics and chief medical officer. All of these physician leaders hold a variety of administrative roles and have differing leadership philosophies, each providing useful insights. For instance, one leader ensures his team holds him accountable as the leader by always asking for honest feedback. He recommends telling those you work with to “never let me fail.” Another leader acknowledges that creating five-year plans can be daunting but encouraged us to still be intentional with our direction on a smaller scale by writing down goals for the year and sharing with a mentor. Ultimately, I came away with a wide variety of perspectives to reference as I go forward.
Second, the learning is contextualized. I can take concepts that I learn through reading and discussions and construct meaning based on observations from meetings or other encounters with different leaders. For example, after reviewing several articles on strategies to make meetings more effective, I started noticing what went well and what didn’t go well in every meeting I attended. I observed preceptors employing many of the strategies successfully with positive feedback. This included not only simple practices, such as setting an agenda to provide a compass for the conversation, but also more nuanced practices like controlling the meeting but not the conversation.
After reading about leadership styles I also found myself analyzing the qualities and strategies of leaders I encountered and reflecting on their approach, noticing what I could possibly interlace in my own practice. Several of the leaders I spoke with during the elective recommended paying attention to the actions of the ineffective bosses or mentors because they can teach you something too: how not to act. I even started applying this strategy to the popular television series The Office – Michael Scott, the regional manager of a fictional paper company, demonstrates some of the best and worst leadership skills in every episode. I am developing a repertoire of strategies to lead and motivate people.
Finally, the design allows for real-time application of new methods to my current practice. One particularly useful tool I have learned is Leader Standard Work, a systematic method to get leaders to maintain stability, problem solve, and drive continuous improvement within their organization.5 I have used elements of Leader Standard Work on a personal level to improve my time management skills and increase my productivity. For example, I reconceptualized my calendar as a standardized checklist and I organized it to allot more time to critical activities, such as my research and scholarly output, and less on administrative tasks. I am also implementing changes to how I prepare and run meetings, collaborate, and communicate with members of my research team.
Mastery requires practice and feedback, so applying concepts even on a small, personal scale shortly after learning them has been very valuable. Over the last several months I have often wished I had this type of structured leadership education during my year as a chief resident. I think I could have been more intentional in my decision-making, possibly being a stronger leader for the program. Now that I am transferring skills into practice right away, I am setting the stage for lasting changes in behavior that will hopefully benefit all those that I work with in the future.
Leadership development through a customizable longitudinal elective may be an effective way to prepare PHM fellow graduates for future leadership positions. Fellows can emerge with the skills and real-world practice to allow them to feel confident in future positions. However, leadership doesn’t end when we get the position. We must remember to continuously ask for feedback and build upon our experiences to evolve as leaders in PHM.
Dr. Westphal is a first-year pediatric hospital medicine fellow at Nationwide Children’s Hospital in Columbus, Ohio with an interest in improving the delivery of quality care for hospitalized infants.
References
1. Maniscalco, J, et al. The Pediatric Hospital Medicine Core Competencies: 2020 Revision. Introduction and Methodology (C). J Hosp Med. 2020;S1;E12-E17. doi: 10.12788/jhm.3391.
2. Jerardi KE, et al; Council of Pediatric Hospital Medicine Fellowship Directors. Development of a Curricular Framework for Pediatric Hospital Medicine Fellowships. Pediatrics. 2017 Jul;140(1):e20170698. doi: 10.1542/peds.2017-0698.
3. ACGME Program Requirements for Graduate Medical Education in Pediatric Hospital Medicine. 2020 Edition. Accessed 2021 Jan 14.
4. Oshimura, JM et al. Current roles and perceived needs of pediatric hospital medicine fellowship graduates. Hosp Pediatr. 2016;6(10):633-7. doi: 10.1542/hpeds.2016-0031.
5. Murli, J. Standard Work for Lean Leaders: One of the Keys to Sustaining Performance Gains. Lean Institute Enterprise, Lean Institute Enterprise Inc. 4 Dec 2013. www.lean.org/common/display/?o=2493
Reflecting on a longitudinal leadership elective experience
Reflecting on a longitudinal leadership elective experience
The practice of pediatric hospital medicine (PHM) has been evolving and rapidly expanding over the last several decades. Not only has the scope of clinical practice matured and become more defined, but hospitalists now also have the responsibility to advance the performance of hospitals and health care systems. Pediatric hospitalists are increasingly incorporating medical education, research, high-value care, patient quality and safety initiatives, and process improvement into their careers.1 As a result, pediatric hospitalists are occupying a wider range of administrative and leadership positions within the health care system.
The field of PHM has highlighted the importance of leadership in the practice of hospital medicine by dedicating a chapter to “Leadership in Healthcare” in the PHM Core Competencies.1 The competencies define the expertise required of hospitalists and serve as guidance for the development of education, training, and career development series. Hospitalists may seek out opportunities for leadership training at an institutional or national level. Options may include advanced degrees, national conferences, division training seminars, or self-directed learning through reading or observational experiences. Unfortunately, all of these take time and motivation. As a result, hospitalists tend to pursue these opportunities only after they have already been appointed to leadership positions.
PHM fellowship is the optimal time to build a foundation of leadership skills. Over the course of a 2-year fellowship, fellows have a combined 16 weeks dedicated to educational activities beyond direct patient care.2 The Accreditation Council for Graduate Medical Education (ACGME) encourages educational innovation during this time, allowing programs to create unique opportunities for their fellows that will promote progress towards their ultimate career goals.3 This curricular framework provides the flexibility to integrate leadership training into fellowship training.
Many fellows are eager for leadership experiences and mentorship, myself included. As a pediatric chief resident, I was immersed in a diverse range of clinical, educational, research, and administrative responsibilities. I found myself in a leadership position with no prior education on how to manage people or team dynamics, make high-stress decisions on behalf of a group of people, or handle conflict. Although I learned new strategies on a daily basis, the experience showed me how much more I still had to learn in order to be a successful leader. This was one of the reasons I decided to pursue fellowship training. I think many PHM fellowship applicants feel similarly. They may have served in a leadership position in the past but feel underprepared to fulfill leadership positions in the next phase of their careers.
But despite this eagerness, evidence suggests that fellows do not feel that they receive as much management training as they need to start their careers. In a 2014 survey of PHM fellowship graduates, many held formal leadership positions within their institution (23/51) and within national organizations (6/51), despite having only five years of hospitalist experience on average (including time spent in fellowship). When asked about training needs, respondents identified “hospital program management” as an area where they wished they received more training during fellowship.4
Anyone who has gone through the PHM fellowship interview process can tell you that a common refrain of program directors is, “One of the goals of our program is to create future leaders in PHM.” This led me to wonder: how do fellowship programs prepare their fellows for future leadership positions?
I began my fellowship training at Nationwide Children’s Hospital in the summer of 2020. The program had just designed a longitudinal leadership elective, which the second-year fellow and I decided to pilot together. As I reflected on the first half of this academic year, I realized that it is unique experiences like this elective that make me thankful I pursued fellowship. I want to share with the hospitalist community the structure of the elective and why it has been particularly valuable with the hope that it will inspire similar opportunities for other fellows.
The program is semi-structured but allows the fellow and preceptors the flexibility to decide what activities would benefit that particular fellow. We attend a variety of administrative and committee meetings with each preceptor that expose us to the responsibilities of their positions, their leadership style in action, their approach to crisis management, and differences in divisional operations. On a monthly basis we meet with a preceptor to discuss a topic related to leadership. Examples of topics include how to run a more effective meeting, barriers to organizational change, leading in crisis, and the importance of mission, vision, values, and goals of organizations. The preceptor sends us articles or other learning materials they have found useful on the topic, and these serve as a starting point for our discussions. These discussions provide a point of reflection as we apply the day’s concept to our own prior experiences or to our observations during the elective.
The combination of learning experiences, discussions, and dedicated preceptorship has prepared me far better for future leadership than my past personal and observational experiences. I have summarized my top three reasons why this structure of leadership development is particularly valuable to me as a fellow.
First, the longitudinal structure of the elective allows us to learn from multiple preceptors over the course of the academic year. The preceptors include the current chief of hospital pediatrics at Nationwide Children’s Hospital; the division director of hospital medicine at the Ohio State University Wexner Medical Center; and the physician lead for hospital medicine at one of the satellite hospitals in the region. With faculty from the Department of Pediatrics and the Department of Internal Medicine-Pediatrics in these leadership positions, we have the unique ability to compare and contrast operational systems between the two different hospital systems.
Recently, we also had the opportunity to meet with both the chairman of the department of pediatrics and chief medical officer. All of these physician leaders hold a variety of administrative roles and have differing leadership philosophies, each providing useful insights. For instance, one leader ensures his team holds him accountable as the leader by always asking for honest feedback. He recommends telling those you work with to “never let me fail.” Another leader acknowledges that creating five-year plans can be daunting but encouraged us to still be intentional with our direction on a smaller scale by writing down goals for the year and sharing with a mentor. Ultimately, I came away with a wide variety of perspectives to reference as I go forward.
Second, the learning is contextualized. I can take concepts that I learn through reading and discussions and construct meaning based on observations from meetings or other encounters with different leaders. For example, after reviewing several articles on strategies to make meetings more effective, I started noticing what went well and what didn’t go well in every meeting I attended. I observed preceptors employing many of the strategies successfully with positive feedback. This included not only simple practices, such as setting an agenda to provide a compass for the conversation, but also more nuanced practices like controlling the meeting but not the conversation.
After reading about leadership styles I also found myself analyzing the qualities and strategies of leaders I encountered and reflecting on their approach, noticing what I could possibly interlace in my own practice. Several of the leaders I spoke with during the elective recommended paying attention to the actions of the ineffective bosses or mentors because they can teach you something too: how not to act. I even started applying this strategy to the popular television series The Office – Michael Scott, the regional manager of a fictional paper company, demonstrates some of the best and worst leadership skills in every episode. I am developing a repertoire of strategies to lead and motivate people.
Finally, the design allows for real-time application of new methods to my current practice. One particularly useful tool I have learned is Leader Standard Work, a systematic method to get leaders to maintain stability, problem solve, and drive continuous improvement within their organization.5 I have used elements of Leader Standard Work on a personal level to improve my time management skills and increase my productivity. For example, I reconceptualized my calendar as a standardized checklist and I organized it to allot more time to critical activities, such as my research and scholarly output, and less on administrative tasks. I am also implementing changes to how I prepare and run meetings, collaborate, and communicate with members of my research team.
Mastery requires practice and feedback, so applying concepts even on a small, personal scale shortly after learning them has been very valuable. Over the last several months I have often wished I had this type of structured leadership education during my year as a chief resident. I think I could have been more intentional in my decision-making, possibly being a stronger leader for the program. Now that I am transferring skills into practice right away, I am setting the stage for lasting changes in behavior that will hopefully benefit all those that I work with in the future.
Leadership development through a customizable longitudinal elective may be an effective way to prepare PHM fellow graduates for future leadership positions. Fellows can emerge with the skills and real-world practice to allow them to feel confident in future positions. However, leadership doesn’t end when we get the position. We must remember to continuously ask for feedback and build upon our experiences to evolve as leaders in PHM.
Dr. Westphal is a first-year pediatric hospital medicine fellow at Nationwide Children’s Hospital in Columbus, Ohio with an interest in improving the delivery of quality care for hospitalized infants.
References
1. Maniscalco, J, et al. The Pediatric Hospital Medicine Core Competencies: 2020 Revision. Introduction and Methodology (C). J Hosp Med. 2020;S1;E12-E17. doi: 10.12788/jhm.3391.
2. Jerardi KE, et al; Council of Pediatric Hospital Medicine Fellowship Directors. Development of a Curricular Framework for Pediatric Hospital Medicine Fellowships. Pediatrics. 2017 Jul;140(1):e20170698. doi: 10.1542/peds.2017-0698.
3. ACGME Program Requirements for Graduate Medical Education in Pediatric Hospital Medicine. 2020 Edition. Accessed 2021 Jan 14.
4. Oshimura, JM et al. Current roles and perceived needs of pediatric hospital medicine fellowship graduates. Hosp Pediatr. 2016;6(10):633-7. doi: 10.1542/hpeds.2016-0031.
5. Murli, J. Standard Work for Lean Leaders: One of the Keys to Sustaining Performance Gains. Lean Institute Enterprise, Lean Institute Enterprise Inc. 4 Dec 2013. www.lean.org/common/display/?o=2493
The practice of pediatric hospital medicine (PHM) has been evolving and rapidly expanding over the last several decades. Not only has the scope of clinical practice matured and become more defined, but hospitalists now also have the responsibility to advance the performance of hospitals and health care systems. Pediatric hospitalists are increasingly incorporating medical education, research, high-value care, patient quality and safety initiatives, and process improvement into their careers.1 As a result, pediatric hospitalists are occupying a wider range of administrative and leadership positions within the health care system.
The field of PHM has highlighted the importance of leadership in the practice of hospital medicine by dedicating a chapter to “Leadership in Healthcare” in the PHM Core Competencies.1 The competencies define the expertise required of hospitalists and serve as guidance for the development of education, training, and career development series. Hospitalists may seek out opportunities for leadership training at an institutional or national level. Options may include advanced degrees, national conferences, division training seminars, or self-directed learning through reading or observational experiences. Unfortunately, all of these take time and motivation. As a result, hospitalists tend to pursue these opportunities only after they have already been appointed to leadership positions.
PHM fellowship is the optimal time to build a foundation of leadership skills. Over the course of a 2-year fellowship, fellows have a combined 16 weeks dedicated to educational activities beyond direct patient care.2 The Accreditation Council for Graduate Medical Education (ACGME) encourages educational innovation during this time, allowing programs to create unique opportunities for their fellows that will promote progress towards their ultimate career goals.3 This curricular framework provides the flexibility to integrate leadership training into fellowship training.
Many fellows are eager for leadership experiences and mentorship, myself included. As a pediatric chief resident, I was immersed in a diverse range of clinical, educational, research, and administrative responsibilities. I found myself in a leadership position with no prior education on how to manage people or team dynamics, make high-stress decisions on behalf of a group of people, or handle conflict. Although I learned new strategies on a daily basis, the experience showed me how much more I still had to learn in order to be a successful leader. This was one of the reasons I decided to pursue fellowship training. I think many PHM fellowship applicants feel similarly. They may have served in a leadership position in the past but feel underprepared to fulfill leadership positions in the next phase of their careers.
But despite this eagerness, evidence suggests that fellows do not feel that they receive as much management training as they need to start their careers. In a 2014 survey of PHM fellowship graduates, many held formal leadership positions within their institution (23/51) and within national organizations (6/51), despite having only five years of hospitalist experience on average (including time spent in fellowship). When asked about training needs, respondents identified “hospital program management” as an area where they wished they received more training during fellowship.4
Anyone who has gone through the PHM fellowship interview process can tell you that a common refrain of program directors is, “One of the goals of our program is to create future leaders in PHM.” This led me to wonder: how do fellowship programs prepare their fellows for future leadership positions?
I began my fellowship training at Nationwide Children’s Hospital in the summer of 2020. The program had just designed a longitudinal leadership elective, which the second-year fellow and I decided to pilot together. As I reflected on the first half of this academic year, I realized that it is unique experiences like this elective that make me thankful I pursued fellowship. I want to share with the hospitalist community the structure of the elective and why it has been particularly valuable with the hope that it will inspire similar opportunities for other fellows.
The program is semi-structured but allows the fellow and preceptors the flexibility to decide what activities would benefit that particular fellow. We attend a variety of administrative and committee meetings with each preceptor that expose us to the responsibilities of their positions, their leadership style in action, their approach to crisis management, and differences in divisional operations. On a monthly basis we meet with a preceptor to discuss a topic related to leadership. Examples of topics include how to run a more effective meeting, barriers to organizational change, leading in crisis, and the importance of mission, vision, values, and goals of organizations. The preceptor sends us articles or other learning materials they have found useful on the topic, and these serve as a starting point for our discussions. These discussions provide a point of reflection as we apply the day’s concept to our own prior experiences or to our observations during the elective.
The combination of learning experiences, discussions, and dedicated preceptorship has prepared me far better for future leadership than my past personal and observational experiences. I have summarized my top three reasons why this structure of leadership development is particularly valuable to me as a fellow.
First, the longitudinal structure of the elective allows us to learn from multiple preceptors over the course of the academic year. The preceptors include the current chief of hospital pediatrics at Nationwide Children’s Hospital; the division director of hospital medicine at the Ohio State University Wexner Medical Center; and the physician lead for hospital medicine at one of the satellite hospitals in the region. With faculty from the Department of Pediatrics and the Department of Internal Medicine-Pediatrics in these leadership positions, we have the unique ability to compare and contrast operational systems between the two different hospital systems.
Recently, we also had the opportunity to meet with both the chairman of the department of pediatrics and chief medical officer. All of these physician leaders hold a variety of administrative roles and have differing leadership philosophies, each providing useful insights. For instance, one leader ensures his team holds him accountable as the leader by always asking for honest feedback. He recommends telling those you work with to “never let me fail.” Another leader acknowledges that creating five-year plans can be daunting but encouraged us to still be intentional with our direction on a smaller scale by writing down goals for the year and sharing with a mentor. Ultimately, I came away with a wide variety of perspectives to reference as I go forward.
Second, the learning is contextualized. I can take concepts that I learn through reading and discussions and construct meaning based on observations from meetings or other encounters with different leaders. For example, after reviewing several articles on strategies to make meetings more effective, I started noticing what went well and what didn’t go well in every meeting I attended. I observed preceptors employing many of the strategies successfully with positive feedback. This included not only simple practices, such as setting an agenda to provide a compass for the conversation, but also more nuanced practices like controlling the meeting but not the conversation.
After reading about leadership styles I also found myself analyzing the qualities and strategies of leaders I encountered and reflecting on their approach, noticing what I could possibly interlace in my own practice. Several of the leaders I spoke with during the elective recommended paying attention to the actions of the ineffective bosses or mentors because they can teach you something too: how not to act. I even started applying this strategy to the popular television series The Office – Michael Scott, the regional manager of a fictional paper company, demonstrates some of the best and worst leadership skills in every episode. I am developing a repertoire of strategies to lead and motivate people.
Finally, the design allows for real-time application of new methods to my current practice. One particularly useful tool I have learned is Leader Standard Work, a systematic method to get leaders to maintain stability, problem solve, and drive continuous improvement within their organization.5 I have used elements of Leader Standard Work on a personal level to improve my time management skills and increase my productivity. For example, I reconceptualized my calendar as a standardized checklist and I organized it to allot more time to critical activities, such as my research and scholarly output, and less on administrative tasks. I am also implementing changes to how I prepare and run meetings, collaborate, and communicate with members of my research team.
Mastery requires practice and feedback, so applying concepts even on a small, personal scale shortly after learning them has been very valuable. Over the last several months I have often wished I had this type of structured leadership education during my year as a chief resident. I think I could have been more intentional in my decision-making, possibly being a stronger leader for the program. Now that I am transferring skills into practice right away, I am setting the stage for lasting changes in behavior that will hopefully benefit all those that I work with in the future.
Leadership development through a customizable longitudinal elective may be an effective way to prepare PHM fellow graduates for future leadership positions. Fellows can emerge with the skills and real-world practice to allow them to feel confident in future positions. However, leadership doesn’t end when we get the position. We must remember to continuously ask for feedback and build upon our experiences to evolve as leaders in PHM.
Dr. Westphal is a first-year pediatric hospital medicine fellow at Nationwide Children’s Hospital in Columbus, Ohio with an interest in improving the delivery of quality care for hospitalized infants.
References
1. Maniscalco, J, et al. The Pediatric Hospital Medicine Core Competencies: 2020 Revision. Introduction and Methodology (C). J Hosp Med. 2020;S1;E12-E17. doi: 10.12788/jhm.3391.
2. Jerardi KE, et al; Council of Pediatric Hospital Medicine Fellowship Directors. Development of a Curricular Framework for Pediatric Hospital Medicine Fellowships. Pediatrics. 2017 Jul;140(1):e20170698. doi: 10.1542/peds.2017-0698.
3. ACGME Program Requirements for Graduate Medical Education in Pediatric Hospital Medicine. 2020 Edition. Accessed 2021 Jan 14.
4. Oshimura, JM et al. Current roles and perceived needs of pediatric hospital medicine fellowship graduates. Hosp Pediatr. 2016;6(10):633-7. doi: 10.1542/hpeds.2016-0031.
5. Murli, J. Standard Work for Lean Leaders: One of the Keys to Sustaining Performance Gains. Lean Institute Enterprise, Lean Institute Enterprise Inc. 4 Dec 2013. www.lean.org/common/display/?o=2493

















