User login
In reference to “When personality is the problem: Managing patients with difficult personalities on the acute care unit"
In the article by Riddle et al,1 the authors state that in the example of Cluster A type personality disorder, the elderly male patient’s paranoid disorder should be ignored, rather than confronting the paranoia. We do not need to confront the paranoia, but we need to treat the paranoid disorder. The symptom of paranoia extends beyond the single diagnostic category of delusional disorder and has been noted in many elderly patients with other underlying disorders.2 This patient needs early psychiatric consultation and therapy.
They also give recommendations regarding Ms. B for her ever-increasing need of opiates. I find it too naïve for me to offer this patient “…choices, such as walking with her around the unit or listen to the music.” This patient needs pain physician consultations and aggressive interventional pain control.3
1. Riddle MR, Meeks T, Alvarez C, Dubovsky A. When personality is the problem: Managing patients with difficult personalities on the acute care unit. J Hosp Med. 2016:11(12):873-878. PubMed
2. Targum SD. Treating psychotic symptoms in elderly patients. Prim Care Companion J Clin Psychiatry. 2001;3(4):156-163. PubMed
3. Karmakar MK, Ho AM. Acute pain management of patients with multiple fractured ribs. J Trauma. 2003;54(3):615-625. PubMed
In the article by Riddle et al,1 the authors state that in the example of Cluster A type personality disorder, the elderly male patient’s paranoid disorder should be ignored, rather than confronting the paranoia. We do not need to confront the paranoia, but we need to treat the paranoid disorder. The symptom of paranoia extends beyond the single diagnostic category of delusional disorder and has been noted in many elderly patients with other underlying disorders.2 This patient needs early psychiatric consultation and therapy.
They also give recommendations regarding Ms. B for her ever-increasing need of opiates. I find it too naïve for me to offer this patient “…choices, such as walking with her around the unit or listen to the music.” This patient needs pain physician consultations and aggressive interventional pain control.3
In the article by Riddle et al,1 the authors state that in the example of Cluster A type personality disorder, the elderly male patient’s paranoid disorder should be ignored, rather than confronting the paranoia. We do not need to confront the paranoia, but we need to treat the paranoid disorder. The symptom of paranoia extends beyond the single diagnostic category of delusional disorder and has been noted in many elderly patients with other underlying disorders.2 This patient needs early psychiatric consultation and therapy.
They also give recommendations regarding Ms. B for her ever-increasing need of opiates. I find it too naïve for me to offer this patient “…choices, such as walking with her around the unit or listen to the music.” This patient needs pain physician consultations and aggressive interventional pain control.3
1. Riddle MR, Meeks T, Alvarez C, Dubovsky A. When personality is the problem: Managing patients with difficult personalities on the acute care unit. J Hosp Med. 2016:11(12):873-878. PubMed
2. Targum SD. Treating psychotic symptoms in elderly patients. Prim Care Companion J Clin Psychiatry. 2001;3(4):156-163. PubMed
3. Karmakar MK, Ho AM. Acute pain management of patients with multiple fractured ribs. J Trauma. 2003;54(3):615-625. PubMed
1. Riddle MR, Meeks T, Alvarez C, Dubovsky A. When personality is the problem: Managing patients with difficult personalities on the acute care unit. J Hosp Med. 2016:11(12):873-878. PubMed
2. Targum SD. Treating psychotic symptoms in elderly patients. Prim Care Companion J Clin Psychiatry. 2001;3(4):156-163. PubMed
3. Karmakar MK, Ho AM. Acute pain management of patients with multiple fractured ribs. J Trauma. 2003;54(3):615-625. PubMed
© 2017 Society of Hospital Medicine
The authors reply, “When personality is the problem: Managing patients with difficult personalities on the acute care unit”
Thank you for the opportunity to reply to Dr. Hunasikatti’s comments regarding our article.1 He brings up some excellent points and we appreciate the opportunity to clarify.
With regards to our example of Cluster A personality, the elderly individual with paranoia, we agree that the differential must include delirium and dementia and an appropriate work-up completed. The intent of the vignette was to illustrate a functional but eccentric individual with paranoid beliefs. The paranoia associated with paranoid personality disorder is classically not responsive to medications—nor are patients typically amenable to such treatment—and behavioral interventions remain paramount, minimizing the negative impact of paranoia on the individual’s care.2,3
Regarding Ms. B, the vignette stated that the pain service was consulted, as Dr. Hunasikatti suggested it should be, but despite aggressive pain control, requests for opiates continued. We agree that appropriate pain management is critical in management of all patients, and pain can exacerbate behavioral issues when insufficiently treated. However, individuals who look to external sources of comfort may continue to request pain medications beyond what is clinically prudent and can benefit from learning additional skills to self-soothe and manage the psychological aspects of pain.4,5
1. Riddle MR, Meeks T, Alvarez C, Dubovsky A. When personality is the problem: Managing patients with difficult personalities on the acute care unit. J Hosp Med. 2016:11(12):873-878. PubMed
2. Hayward BA. Cluster A personality disorders: considering the 'odd-eccentric' in psychiatric nursing. Int J Ment Health Nurs. 2007;16(1):15-21. PubMed
3. Ward RK. Assessment and management of personality disorders. Am Family Physician. 2004;70(8):1505-1512. PubMed
4. Sansone RA, Sansone LA. Borderline personality and the pain paradox. Psychiatry (Edgmont). 2007;4(4):40-46. PubMed
5. Eccleston C. Role of psychology in pain management. Br J Anaesth. 2001;87(1):144-152. PubMed
Thank you for the opportunity to reply to Dr. Hunasikatti’s comments regarding our article.1 He brings up some excellent points and we appreciate the opportunity to clarify.
With regards to our example of Cluster A personality, the elderly individual with paranoia, we agree that the differential must include delirium and dementia and an appropriate work-up completed. The intent of the vignette was to illustrate a functional but eccentric individual with paranoid beliefs. The paranoia associated with paranoid personality disorder is classically not responsive to medications—nor are patients typically amenable to such treatment—and behavioral interventions remain paramount, minimizing the negative impact of paranoia on the individual’s care.2,3
Regarding Ms. B, the vignette stated that the pain service was consulted, as Dr. Hunasikatti suggested it should be, but despite aggressive pain control, requests for opiates continued. We agree that appropriate pain management is critical in management of all patients, and pain can exacerbate behavioral issues when insufficiently treated. However, individuals who look to external sources of comfort may continue to request pain medications beyond what is clinically prudent and can benefit from learning additional skills to self-soothe and manage the psychological aspects of pain.4,5
Thank you for the opportunity to reply to Dr. Hunasikatti’s comments regarding our article.1 He brings up some excellent points and we appreciate the opportunity to clarify.
With regards to our example of Cluster A personality, the elderly individual with paranoia, we agree that the differential must include delirium and dementia and an appropriate work-up completed. The intent of the vignette was to illustrate a functional but eccentric individual with paranoid beliefs. The paranoia associated with paranoid personality disorder is classically not responsive to medications—nor are patients typically amenable to such treatment—and behavioral interventions remain paramount, minimizing the negative impact of paranoia on the individual’s care.2,3
Regarding Ms. B, the vignette stated that the pain service was consulted, as Dr. Hunasikatti suggested it should be, but despite aggressive pain control, requests for opiates continued. We agree that appropriate pain management is critical in management of all patients, and pain can exacerbate behavioral issues when insufficiently treated. However, individuals who look to external sources of comfort may continue to request pain medications beyond what is clinically prudent and can benefit from learning additional skills to self-soothe and manage the psychological aspects of pain.4,5
1. Riddle MR, Meeks T, Alvarez C, Dubovsky A. When personality is the problem: Managing patients with difficult personalities on the acute care unit. J Hosp Med. 2016:11(12):873-878. PubMed
2. Hayward BA. Cluster A personality disorders: considering the 'odd-eccentric' in psychiatric nursing. Int J Ment Health Nurs. 2007;16(1):15-21. PubMed
3. Ward RK. Assessment and management of personality disorders. Am Family Physician. 2004;70(8):1505-1512. PubMed
4. Sansone RA, Sansone LA. Borderline personality and the pain paradox. Psychiatry (Edgmont). 2007;4(4):40-46. PubMed
5. Eccleston C. Role of psychology in pain management. Br J Anaesth. 2001;87(1):144-152. PubMed
1. Riddle MR, Meeks T, Alvarez C, Dubovsky A. When personality is the problem: Managing patients with difficult personalities on the acute care unit. J Hosp Med. 2016:11(12):873-878. PubMed
2. Hayward BA. Cluster A personality disorders: considering the 'odd-eccentric' in psychiatric nursing. Int J Ment Health Nurs. 2007;16(1):15-21. PubMed
3. Ward RK. Assessment and management of personality disorders. Am Family Physician. 2004;70(8):1505-1512. PubMed
4. Sansone RA, Sansone LA. Borderline personality and the pain paradox. Psychiatry (Edgmont). 2007;4(4):40-46. PubMed
5. Eccleston C. Role of psychology in pain management. Br J Anaesth. 2001;87(1):144-152. PubMed
© 2017 Society of Hospital Medicine
Opioid therapy and sleep apnea
To the Editor: I enjoyed Dr. Galicia-Castillo’s article about long-term opioid therapy in older adults,1 which reaffirmed the imperative to “start low and go slow” to minimize the risk of addiction. However, the article missed an opportunity to raise awareness regarding another extremely important side effect of chronic prescription opioid consumption, that of ingestion prior to sleep, with consequent cessation of breathing leading to death.
According to the Drug Enforcement Administration,2 most narcotic deaths are a result of respiratory depression. And the American Pain Society has stated, “No patient has succumbed to [opioid] respiratory depression while awake.”3
Dr. Galicia-Castillo noted that the prevalence of central sleep apnea in chronic opioid users is 24%, based on a review by Correa et al.4 As alarming as this number is, other investigators have estimated it to be even higher—as high as 50% to 90%.5
Walker et al,6 in a study of 60 patients, found that the higher the opioid dose the patients were on, the more episodes of obstructive sleep apnea and central sleep apnea per hour they had. Yet prescribing a low dose does not adequately protect the chronic opioid user. Farney et al7 reported that oxygen saturation dropped precipitously—from 98% to 70%—15 minutes after a patient took just 7.5 mg of hydrocodone in the middle of the night. Mogri et al8 reported that a patient had 91 apnea events within 1 hour of taking 15 mg of oxycodone at 2 am.
Opioids, benzodiazepines, barbiturates, and ethanol individually and additively suppress medullary reflex ventilatory drive during sleep, especially during non–rapid-eye-movement (non-REM) sleep.6 During waking hours, in contrast, there is redundant backup of cerebral cortical drive, ensuring that we keep breathing. Therefore, people are most vulnerable to dying of opioid ingestion during sleep.
Moreover, oxygen desaturation during episodes of sleep apnea may precipitate seizures (which may be lethal) or coronary vasospasm with consequent malignant arrhythmias and myocardial ischemia.
Continuous positive airway pressure protects against obstructive sleep apnea, but not against central sleep apnea.9
Patients need to be aware of the danger, and physicians need to consider the pharmacokinetic profiles of the opioid preparations they prescribe. If patients are taking an opioid that has a short half-life, such as immediate-release oxycodone, they should not take it within 5 hours of sleep. Longer-lasting preparations need a longer interval, and some, such as extended-release tramadol, may need to be taken only on awakening.
Safe sleep can be facilitated by medications that are sedating but do not compromise ventilation. Optimal agents also enhance restorative REM and stage III and IV deep-sleep duration, and some may have the additional benefit of reducing the risk of cancer.10,11 Such agents may include baclofen, cyproheptadine, gabapentin, mirtazepine, and melatonin. Nonpharmacologic measures include sleep hygiene, aerobic exercise, and cognitive behavioral therapy.
A retrospective study12 found that 301 (60.4%) of 498 patients who died while on opioid therapy and whose death was judged to be related to the opioid were also taking benzodiazepines. Patients who take opioids should avoid taking benzodiazepines, barbiturates, or alcohol before going to sleep, and physicians should be extremely cautious about prescribing benzodiazepines and barbiturates to patients who are on opioids.
- Galicia-Castillo M. Opioids for persistent pain in older adults. Cleve Clin J Med 2016; 83:443–451.
- Drug Enforcement Administration. Drugs of Abuse. 2005 Edition. Washington, DC: US Government Printing Office, 2005:19.
- American Pain Society, Principles of Analgesic Use in the Treatment of Acute Pain and Cancer Pain, 4th ed. Glenview, IL: American Pain Society, 1999:30.
- Correa D, Farney RJ, Chung F, Prasad A, Lam D, Wong J. Chronic opioid use and central sleep apnea: a review of the prevalence, mechanisms, and perioperative considerations. Anesth Analg 2015; 120:1273–1285.
- Panagiotou I, Mystakidou K. Non-analgesic effects of opioids: opioids’ effects on sleep (including sleep apnea). Curr Pharm Des 2012; 18:6025–6033.
- Walker JM, Farney RJ, Rhondeau SM, et al. Chronic opioid use is a risk factor for the development of central sleep apnea and ataxic breathing. J Clin Sleep Med 2007; 3:455–461. Erratum in J Clin Sleep Med 2007; 3:table of contents.
- Farney RJ, Walker JM, Cloward TV, Rhondeau S. Sleep-disordered breathing associated with long-term opioid therapy. Chest 2003; 123:632–639.
- Mogri M, Khan MI, Grant BJ, Mador MJ. Central sleep apnea induced by acute ingestion of opioids. Chest 2008; 133:1484–1488.
- Guilleminault C, Cao M, Yue HJ, Chawla P. Obstructive sleep apnea and chronic opioid use. Lung 2010; 188:459–468.
- Kao CH, Sun LM, Liang JA, Chang SN, Sung FC, Muo CH. Relationship of zolpidem and cancer risk: a Taiwanese population-based cohort study. Mayo Clin Proc 2012; 87:430–436.
- Kripke DF. Hypnotic drug risks of mortality, infection, depression, and cancer: but lack of benefit. F1000Res 2016; 5:918.
- Gomes T, Mamdani MM, Dhalla IA, Paterson JM, Juurlink DN. Opioid dose and drug-related mortality in patients with nonmalignant pain. Arch Intern Med 2011; 171:686–691.
To the Editor: I enjoyed Dr. Galicia-Castillo’s article about long-term opioid therapy in older adults,1 which reaffirmed the imperative to “start low and go slow” to minimize the risk of addiction. However, the article missed an opportunity to raise awareness regarding another extremely important side effect of chronic prescription opioid consumption, that of ingestion prior to sleep, with consequent cessation of breathing leading to death.
According to the Drug Enforcement Administration,2 most narcotic deaths are a result of respiratory depression. And the American Pain Society has stated, “No patient has succumbed to [opioid] respiratory depression while awake.”3
Dr. Galicia-Castillo noted that the prevalence of central sleep apnea in chronic opioid users is 24%, based on a review by Correa et al.4 As alarming as this number is, other investigators have estimated it to be even higher—as high as 50% to 90%.5
Walker et al,6 in a study of 60 patients, found that the higher the opioid dose the patients were on, the more episodes of obstructive sleep apnea and central sleep apnea per hour they had. Yet prescribing a low dose does not adequately protect the chronic opioid user. Farney et al7 reported that oxygen saturation dropped precipitously—from 98% to 70%—15 minutes after a patient took just 7.5 mg of hydrocodone in the middle of the night. Mogri et al8 reported that a patient had 91 apnea events within 1 hour of taking 15 mg of oxycodone at 2 am.
Opioids, benzodiazepines, barbiturates, and ethanol individually and additively suppress medullary reflex ventilatory drive during sleep, especially during non–rapid-eye-movement (non-REM) sleep.6 During waking hours, in contrast, there is redundant backup of cerebral cortical drive, ensuring that we keep breathing. Therefore, people are most vulnerable to dying of opioid ingestion during sleep.
Moreover, oxygen desaturation during episodes of sleep apnea may precipitate seizures (which may be lethal) or coronary vasospasm with consequent malignant arrhythmias and myocardial ischemia.
Continuous positive airway pressure protects against obstructive sleep apnea, but not against central sleep apnea.9
Patients need to be aware of the danger, and physicians need to consider the pharmacokinetic profiles of the opioid preparations they prescribe. If patients are taking an opioid that has a short half-life, such as immediate-release oxycodone, they should not take it within 5 hours of sleep. Longer-lasting preparations need a longer interval, and some, such as extended-release tramadol, may need to be taken only on awakening.
Safe sleep can be facilitated by medications that are sedating but do not compromise ventilation. Optimal agents also enhance restorative REM and stage III and IV deep-sleep duration, and some may have the additional benefit of reducing the risk of cancer.10,11 Such agents may include baclofen, cyproheptadine, gabapentin, mirtazepine, and melatonin. Nonpharmacologic measures include sleep hygiene, aerobic exercise, and cognitive behavioral therapy.
A retrospective study12 found that 301 (60.4%) of 498 patients who died while on opioid therapy and whose death was judged to be related to the opioid were also taking benzodiazepines. Patients who take opioids should avoid taking benzodiazepines, barbiturates, or alcohol before going to sleep, and physicians should be extremely cautious about prescribing benzodiazepines and barbiturates to patients who are on opioids.
To the Editor: I enjoyed Dr. Galicia-Castillo’s article about long-term opioid therapy in older adults,1 which reaffirmed the imperative to “start low and go slow” to minimize the risk of addiction. However, the article missed an opportunity to raise awareness regarding another extremely important side effect of chronic prescription opioid consumption, that of ingestion prior to sleep, with consequent cessation of breathing leading to death.
According to the Drug Enforcement Administration,2 most narcotic deaths are a result of respiratory depression. And the American Pain Society has stated, “No patient has succumbed to [opioid] respiratory depression while awake.”3
Dr. Galicia-Castillo noted that the prevalence of central sleep apnea in chronic opioid users is 24%, based on a review by Correa et al.4 As alarming as this number is, other investigators have estimated it to be even higher—as high as 50% to 90%.5
Walker et al,6 in a study of 60 patients, found that the higher the opioid dose the patients were on, the more episodes of obstructive sleep apnea and central sleep apnea per hour they had. Yet prescribing a low dose does not adequately protect the chronic opioid user. Farney et al7 reported that oxygen saturation dropped precipitously—from 98% to 70%—15 minutes after a patient took just 7.5 mg of hydrocodone in the middle of the night. Mogri et al8 reported that a patient had 91 apnea events within 1 hour of taking 15 mg of oxycodone at 2 am.
Opioids, benzodiazepines, barbiturates, and ethanol individually and additively suppress medullary reflex ventilatory drive during sleep, especially during non–rapid-eye-movement (non-REM) sleep.6 During waking hours, in contrast, there is redundant backup of cerebral cortical drive, ensuring that we keep breathing. Therefore, people are most vulnerable to dying of opioid ingestion during sleep.
Moreover, oxygen desaturation during episodes of sleep apnea may precipitate seizures (which may be lethal) or coronary vasospasm with consequent malignant arrhythmias and myocardial ischemia.
Continuous positive airway pressure protects against obstructive sleep apnea, but not against central sleep apnea.9
Patients need to be aware of the danger, and physicians need to consider the pharmacokinetic profiles of the opioid preparations they prescribe. If patients are taking an opioid that has a short half-life, such as immediate-release oxycodone, they should not take it within 5 hours of sleep. Longer-lasting preparations need a longer interval, and some, such as extended-release tramadol, may need to be taken only on awakening.
Safe sleep can be facilitated by medications that are sedating but do not compromise ventilation. Optimal agents also enhance restorative REM and stage III and IV deep-sleep duration, and some may have the additional benefit of reducing the risk of cancer.10,11 Such agents may include baclofen, cyproheptadine, gabapentin, mirtazepine, and melatonin. Nonpharmacologic measures include sleep hygiene, aerobic exercise, and cognitive behavioral therapy.
A retrospective study12 found that 301 (60.4%) of 498 patients who died while on opioid therapy and whose death was judged to be related to the opioid were also taking benzodiazepines. Patients who take opioids should avoid taking benzodiazepines, barbiturates, or alcohol before going to sleep, and physicians should be extremely cautious about prescribing benzodiazepines and barbiturates to patients who are on opioids.
- Galicia-Castillo M. Opioids for persistent pain in older adults. Cleve Clin J Med 2016; 83:443–451.
- Drug Enforcement Administration. Drugs of Abuse. 2005 Edition. Washington, DC: US Government Printing Office, 2005:19.
- American Pain Society, Principles of Analgesic Use in the Treatment of Acute Pain and Cancer Pain, 4th ed. Glenview, IL: American Pain Society, 1999:30.
- Correa D, Farney RJ, Chung F, Prasad A, Lam D, Wong J. Chronic opioid use and central sleep apnea: a review of the prevalence, mechanisms, and perioperative considerations. Anesth Analg 2015; 120:1273–1285.
- Panagiotou I, Mystakidou K. Non-analgesic effects of opioids: opioids’ effects on sleep (including sleep apnea). Curr Pharm Des 2012; 18:6025–6033.
- Walker JM, Farney RJ, Rhondeau SM, et al. Chronic opioid use is a risk factor for the development of central sleep apnea and ataxic breathing. J Clin Sleep Med 2007; 3:455–461. Erratum in J Clin Sleep Med 2007; 3:table of contents.
- Farney RJ, Walker JM, Cloward TV, Rhondeau S. Sleep-disordered breathing associated with long-term opioid therapy. Chest 2003; 123:632–639.
- Mogri M, Khan MI, Grant BJ, Mador MJ. Central sleep apnea induced by acute ingestion of opioids. Chest 2008; 133:1484–1488.
- Guilleminault C, Cao M, Yue HJ, Chawla P. Obstructive sleep apnea and chronic opioid use. Lung 2010; 188:459–468.
- Kao CH, Sun LM, Liang JA, Chang SN, Sung FC, Muo CH. Relationship of zolpidem and cancer risk: a Taiwanese population-based cohort study. Mayo Clin Proc 2012; 87:430–436.
- Kripke DF. Hypnotic drug risks of mortality, infection, depression, and cancer: but lack of benefit. F1000Res 2016; 5:918.
- Gomes T, Mamdani MM, Dhalla IA, Paterson JM, Juurlink DN. Opioid dose and drug-related mortality in patients with nonmalignant pain. Arch Intern Med 2011; 171:686–691.
- Galicia-Castillo M. Opioids for persistent pain in older adults. Cleve Clin J Med 2016; 83:443–451.
- Drug Enforcement Administration. Drugs of Abuse. 2005 Edition. Washington, DC: US Government Printing Office, 2005:19.
- American Pain Society, Principles of Analgesic Use in the Treatment of Acute Pain and Cancer Pain, 4th ed. Glenview, IL: American Pain Society, 1999:30.
- Correa D, Farney RJ, Chung F, Prasad A, Lam D, Wong J. Chronic opioid use and central sleep apnea: a review of the prevalence, mechanisms, and perioperative considerations. Anesth Analg 2015; 120:1273–1285.
- Panagiotou I, Mystakidou K. Non-analgesic effects of opioids: opioids’ effects on sleep (including sleep apnea). Curr Pharm Des 2012; 18:6025–6033.
- Walker JM, Farney RJ, Rhondeau SM, et al. Chronic opioid use is a risk factor for the development of central sleep apnea and ataxic breathing. J Clin Sleep Med 2007; 3:455–461. Erratum in J Clin Sleep Med 2007; 3:table of contents.
- Farney RJ, Walker JM, Cloward TV, Rhondeau S. Sleep-disordered breathing associated with long-term opioid therapy. Chest 2003; 123:632–639.
- Mogri M, Khan MI, Grant BJ, Mador MJ. Central sleep apnea induced by acute ingestion of opioids. Chest 2008; 133:1484–1488.
- Guilleminault C, Cao M, Yue HJ, Chawla P. Obstructive sleep apnea and chronic opioid use. Lung 2010; 188:459–468.
- Kao CH, Sun LM, Liang JA, Chang SN, Sung FC, Muo CH. Relationship of zolpidem and cancer risk: a Taiwanese population-based cohort study. Mayo Clin Proc 2012; 87:430–436.
- Kripke DF. Hypnotic drug risks of mortality, infection, depression, and cancer: but lack of benefit. F1000Res 2016; 5:918.
- Gomes T, Mamdani MM, Dhalla IA, Paterson JM, Juurlink DN. Opioid dose and drug-related mortality in patients with nonmalignant pain. Arch Intern Med 2011; 171:686–691.
In reply: Opioid therapy and sleep apnea
In Reply: Dr. Geller makes some excellent points about sleep and opioid use.
Opioids pose risks,1 just like any other type of medication. In particular, opioids have been linked to sleep-disordered breathing, which affects 70% to 85% of patients taking opioids.2–4
Other options can be used in some older adults, but they are not always successful. Ideally, nonpharmacologic strategies and nonopioid medications such as acetaminophen, nonsteroidal anti-inflammatory agents, antidepressants, and anticonvulsants should be used, although these medications have their own side effects. Optimum pain control may offer the potential for significant improvement in function, and opioids are but one tool in the clinician’s kit.
Ongoing discussions of the risks and benefits are necessary, along with continuous re-evaluation of the need for and effect of opioids.
- Davis MP, Mehta Z. Opioids and chronic pain: where is the balance? Curr Oncol Rep 2016; 18:71.
- Jungquist CR, Flannery M, Perlis ML, Grace JT. Relationship of chronic pain and opioid use with respiratory disturbance during sleep. Pain Manag Nurs 2012; 13:70–79.
- Webster LR, Choi Y, Desai H, Webster L, Grant BJ. Sleep-disordered breathing and chronic opioid therapy. Pain Med 2008; 9:425–432.
- Mogri M, Khan MI, Grant BJ, Mador MJ. Central sleep apnea induced by acute ingestion of opioid. Chest 2008; 133:1484–1488.
In Reply: Dr. Geller makes some excellent points about sleep and opioid use.
Opioids pose risks,1 just like any other type of medication. In particular, opioids have been linked to sleep-disordered breathing, which affects 70% to 85% of patients taking opioids.2–4
Other options can be used in some older adults, but they are not always successful. Ideally, nonpharmacologic strategies and nonopioid medications such as acetaminophen, nonsteroidal anti-inflammatory agents, antidepressants, and anticonvulsants should be used, although these medications have their own side effects. Optimum pain control may offer the potential for significant improvement in function, and opioids are but one tool in the clinician’s kit.
Ongoing discussions of the risks and benefits are necessary, along with continuous re-evaluation of the need for and effect of opioids.
In Reply: Dr. Geller makes some excellent points about sleep and opioid use.
Opioids pose risks,1 just like any other type of medication. In particular, opioids have been linked to sleep-disordered breathing, which affects 70% to 85% of patients taking opioids.2–4
Other options can be used in some older adults, but they are not always successful. Ideally, nonpharmacologic strategies and nonopioid medications such as acetaminophen, nonsteroidal anti-inflammatory agents, antidepressants, and anticonvulsants should be used, although these medications have their own side effects. Optimum pain control may offer the potential for significant improvement in function, and opioids are but one tool in the clinician’s kit.
Ongoing discussions of the risks and benefits are necessary, along with continuous re-evaluation of the need for and effect of opioids.
- Davis MP, Mehta Z. Opioids and chronic pain: where is the balance? Curr Oncol Rep 2016; 18:71.
- Jungquist CR, Flannery M, Perlis ML, Grace JT. Relationship of chronic pain and opioid use with respiratory disturbance during sleep. Pain Manag Nurs 2012; 13:70–79.
- Webster LR, Choi Y, Desai H, Webster L, Grant BJ. Sleep-disordered breathing and chronic opioid therapy. Pain Med 2008; 9:425–432.
- Mogri M, Khan MI, Grant BJ, Mador MJ. Central sleep apnea induced by acute ingestion of opioid. Chest 2008; 133:1484–1488.
- Davis MP, Mehta Z. Opioids and chronic pain: where is the balance? Curr Oncol Rep 2016; 18:71.
- Jungquist CR, Flannery M, Perlis ML, Grace JT. Relationship of chronic pain and opioid use with respiratory disturbance during sleep. Pain Manag Nurs 2012; 13:70–79.
- Webster LR, Choi Y, Desai H, Webster L, Grant BJ. Sleep-disordered breathing and chronic opioid therapy. Pain Med 2008; 9:425–432.
- Mogri M, Khan MI, Grant BJ, Mador MJ. Central sleep apnea induced by acute ingestion of opioid. Chest 2008; 133:1484–1488.
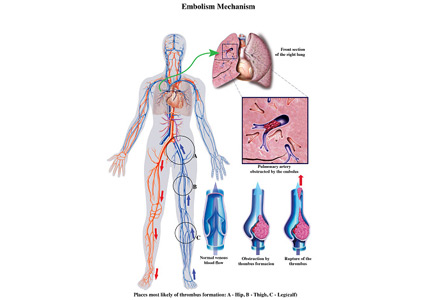
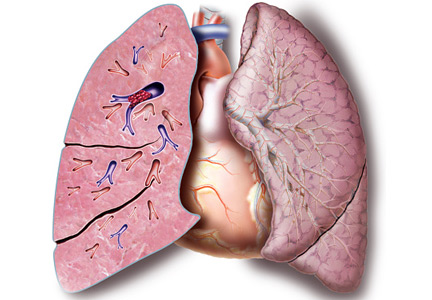
Submassive pulmonary embolism
To the Editor: I read with interest the review on submassive pulmonary embolism by Ataya et al1 in the December 2016 issue. I had 3 questions or observations for the authors
First, systemic thrombolytic therapy for massive or hemodynamically unstable pulmonary embolism is given a grade 2C recommendation, similar to the level for select patients with submassive pulmonary embolism with low bleeding risk but at high risk of developing hypotension. The reference for this is the 2012 American College of Chest Physicians guidelines.2 I would like to point out that these guidelines were updated and published in February 2016,3 and systemic thrombolytic therapy for massive pulmonary embolism now carries a grade 2B recommendation. Thrombolytic therapy still has a grade 2C recommendation for select patients with submassive pulmonary embolism.
Second, the Moderate Pulmonary Embolism Treated With Thrombolysis (MOPETT) trial is described as a randomized trial in patients with moderate pulmonary hypertension and right ventricular dysfunction. I would like to point out that right ventricular dysfunction was not a criterion for enrollment in the trial.4
Finally, catheter-directed thrombolytic therapy is mentioned as an option for select patients with submassive and massive pulmonary embolism. The advantage is believed to be due to local action of the drug with fewer systemic effects. Since the protocol involves alteplase for 12 or 24 hours with a maximum dose of 24 mg, and since in most cases pulmonary embolism originates in the lower extremity, are we not exposing these patients to further clot propagation for 12 or 24 hours without the benefit of concomitant systemic anticoagulation or an inferior vena cava filter?
- Ataya A, Cope J, Shahmohammadi A, Alnuaimat H. Do patients with submassive pulmonary embolism benefit from thrombolytic therapy? Cleve Clin J Med 2016; 83:923–932.
- Kearon C, Akl EA, Comerota AJ, et al; American College of Chest Physicians. Antithrombotic therapy for VTE disease: Antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141(suppl 2):e419S–e494S.
- Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. Chest 2016; 149:315–352.
- Sharifi M, Bay C, Skrocki L, Rahimi F, Mehdipour M; “MOPETT” Investigators. Moderate pulmonary embolism treated with thrombolysis (from the “MOPETT” Trial). Am J Cardiol 2013; 111:273–277.
To the Editor: I read with interest the review on submassive pulmonary embolism by Ataya et al1 in the December 2016 issue. I had 3 questions or observations for the authors
First, systemic thrombolytic therapy for massive or hemodynamically unstable pulmonary embolism is given a grade 2C recommendation, similar to the level for select patients with submassive pulmonary embolism with low bleeding risk but at high risk of developing hypotension. The reference for this is the 2012 American College of Chest Physicians guidelines.2 I would like to point out that these guidelines were updated and published in February 2016,3 and systemic thrombolytic therapy for massive pulmonary embolism now carries a grade 2B recommendation. Thrombolytic therapy still has a grade 2C recommendation for select patients with submassive pulmonary embolism.
Second, the Moderate Pulmonary Embolism Treated With Thrombolysis (MOPETT) trial is described as a randomized trial in patients with moderate pulmonary hypertension and right ventricular dysfunction. I would like to point out that right ventricular dysfunction was not a criterion for enrollment in the trial.4
Finally, catheter-directed thrombolytic therapy is mentioned as an option for select patients with submassive and massive pulmonary embolism. The advantage is believed to be due to local action of the drug with fewer systemic effects. Since the protocol involves alteplase for 12 or 24 hours with a maximum dose of 24 mg, and since in most cases pulmonary embolism originates in the lower extremity, are we not exposing these patients to further clot propagation for 12 or 24 hours without the benefit of concomitant systemic anticoagulation or an inferior vena cava filter?
To the Editor: I read with interest the review on submassive pulmonary embolism by Ataya et al1 in the December 2016 issue. I had 3 questions or observations for the authors
First, systemic thrombolytic therapy for massive or hemodynamically unstable pulmonary embolism is given a grade 2C recommendation, similar to the level for select patients with submassive pulmonary embolism with low bleeding risk but at high risk of developing hypotension. The reference for this is the 2012 American College of Chest Physicians guidelines.2 I would like to point out that these guidelines were updated and published in February 2016,3 and systemic thrombolytic therapy for massive pulmonary embolism now carries a grade 2B recommendation. Thrombolytic therapy still has a grade 2C recommendation for select patients with submassive pulmonary embolism.
Second, the Moderate Pulmonary Embolism Treated With Thrombolysis (MOPETT) trial is described as a randomized trial in patients with moderate pulmonary hypertension and right ventricular dysfunction. I would like to point out that right ventricular dysfunction was not a criterion for enrollment in the trial.4
Finally, catheter-directed thrombolytic therapy is mentioned as an option for select patients with submassive and massive pulmonary embolism. The advantage is believed to be due to local action of the drug with fewer systemic effects. Since the protocol involves alteplase for 12 or 24 hours with a maximum dose of 24 mg, and since in most cases pulmonary embolism originates in the lower extremity, are we not exposing these patients to further clot propagation for 12 or 24 hours without the benefit of concomitant systemic anticoagulation or an inferior vena cava filter?
- Ataya A, Cope J, Shahmohammadi A, Alnuaimat H. Do patients with submassive pulmonary embolism benefit from thrombolytic therapy? Cleve Clin J Med 2016; 83:923–932.
- Kearon C, Akl EA, Comerota AJ, et al; American College of Chest Physicians. Antithrombotic therapy for VTE disease: Antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141(suppl 2):e419S–e494S.
- Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. Chest 2016; 149:315–352.
- Sharifi M, Bay C, Skrocki L, Rahimi F, Mehdipour M; “MOPETT” Investigators. Moderate pulmonary embolism treated with thrombolysis (from the “MOPETT” Trial). Am J Cardiol 2013; 111:273–277.
- Ataya A, Cope J, Shahmohammadi A, Alnuaimat H. Do patients with submassive pulmonary embolism benefit from thrombolytic therapy? Cleve Clin J Med 2016; 83:923–932.
- Kearon C, Akl EA, Comerota AJ, et al; American College of Chest Physicians. Antithrombotic therapy for VTE disease: Antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141(suppl 2):e419S–e494S.
- Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. Chest 2016; 149:315–352.
- Sharifi M, Bay C, Skrocki L, Rahimi F, Mehdipour M; “MOPETT” Investigators. Moderate pulmonary embolism treated with thrombolysis (from the “MOPETT” Trial). Am J Cardiol 2013; 111:273–277.
In reply: Submassive pulmonary embolism
In Reply: We thank Dr. Katyal for his thoughtful comments.
Dr. Katyal points out that the grade of recommendation for thrombolysis in patients with massive pulmonary embolism was upgraded from 2C to 2B in the 2016 American College of Chest Physicians (ACCP) guidelines1 compared with the 2012 guidelines2 that we cited. The upgrade in this recommendation was owing to 2 small trials and 1 large randomized controlled trial that included patients with submassive pulmonary embolism.3–5 Interestingly, these 3 studies led to an upgrade in the level of recommendation for thrombolysis in the treatment of massive pulmonary embolism, perhaps more from a safety aspect (in view of the incidence of major bleeding vs mortality). Regardless, Dr. Katyal is correct in highlighting that the new 2016 ACCP guidelines now give a grade of 2B for thrombolytic therapy in the treatment of massive pulmonary embolism. These guidelines had not been published at the time of submission of our manuscript.
Dr. Katyal is also correct that patients were not required to have right ventricular dysfunction to be enrolled in the MOPETT trial.3 As we pointed out, “Only 20% of the participants were enrolled on the basis of right ventricular dysfunction on echocardiography, whereas almost 60% had elevated cardiac biomarkers.”6
Regarding catheter-directed therapy, patients who received low-dose catheter-directed alteplase were also concurrently anticoagulated with systemic unfractionated heparin in the Ultrasound-Assisted, Catheter-Directed Thrombolysis for Acute Intermediate-Risk Pulmonary Embolism (ULTIMA) trial.7 The ULTIMA trial authors commented that unfractionated heparin was started with an 80-U/kg bolus followed by an 18-U/kg/hour infusion to target an anti-factor Xa level of 0.3 to 0.7 μg/mL, which is considered therapeutic anticoagulation. The investigators in the SEATTLE II trial8 continued systemic unfractionated heparin but targeted a lower “intermediate” anticoagulation target (an augmented partial thromboplastin time of 40–60 seconds), so these patients weren’t completely without systemic anticoagulation either. At our institution, the current practice is to target an anti-Xa level of 0.3 to 0.7 μg/mL in patients receiving catheter-directed therapy for large-volume pulmonary embolism.
- Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. Chest 2016; 149:315–352.
- Kearon C, Akl EA, Comerota AJ, et al; American College of Chest Physicians. Antithrombotic therapy for VTE disease: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141(suppl 2):e419S–e494S.
- Sharifi M, Bay C, Skrocki L, Rahimi F, Mehdipour M; “MOPETT” Investigators. Moderate pulmonary embolism treated with thrombolysis (from the “MOPETT” Trial). Am J Cardiol 2013; 111:273–277.
- Meyer G, Vicaut E, Danays T, et al; PEITHO Investigators. Fibrinolysis for patients with intermediate-risk pulmonary embolism. N Engl J Med 2014; 370:1402–1411.
- Kline JA, Nordenholz KE, Courtney DM, et al. Treatment of submassive pulmonary embolism with tenecteplase or placebo: cardiopulmonary outcomes at 3 months: multicenter double-blind, placebo-controlled randomized trial. J Thromb Haemost 2014; 12:459–468.
- Ataya A, Cope J, Shahmohammadi A, Alnuaimat H. Do patients with submassive pulmonary embolism benefit from thrombolytic therapy? Cleve Clin J Med 2016; 83:923–932.
- Kucher N, Boekstegers P, Muller OJ, et al. Randomized, controlled trial of ultrasound-assisted catheter-directed thrombolysis for acute intermediate-risk pulmonary embolism. Circulation 2014; 129:479–486.
- Piazza G, Hohlfelder B, Jaff MR, et al; SEATTLE II Investigators. A prospective, single-arm, multicenter trial of ultrasound-facilitated, catheter-directed, low-dose fibrinolysis for acute massive and submassive pulmonary embolism (The SEATTLE II Study). JACC Cardiovasc Interv 2015; 8:1382–1392.
In Reply: We thank Dr. Katyal for his thoughtful comments.
Dr. Katyal points out that the grade of recommendation for thrombolysis in patients with massive pulmonary embolism was upgraded from 2C to 2B in the 2016 American College of Chest Physicians (ACCP) guidelines1 compared with the 2012 guidelines2 that we cited. The upgrade in this recommendation was owing to 2 small trials and 1 large randomized controlled trial that included patients with submassive pulmonary embolism.3–5 Interestingly, these 3 studies led to an upgrade in the level of recommendation for thrombolysis in the treatment of massive pulmonary embolism, perhaps more from a safety aspect (in view of the incidence of major bleeding vs mortality). Regardless, Dr. Katyal is correct in highlighting that the new 2016 ACCP guidelines now give a grade of 2B for thrombolytic therapy in the treatment of massive pulmonary embolism. These guidelines had not been published at the time of submission of our manuscript.
Dr. Katyal is also correct that patients were not required to have right ventricular dysfunction to be enrolled in the MOPETT trial.3 As we pointed out, “Only 20% of the participants were enrolled on the basis of right ventricular dysfunction on echocardiography, whereas almost 60% had elevated cardiac biomarkers.”6
Regarding catheter-directed therapy, patients who received low-dose catheter-directed alteplase were also concurrently anticoagulated with systemic unfractionated heparin in the Ultrasound-Assisted, Catheter-Directed Thrombolysis for Acute Intermediate-Risk Pulmonary Embolism (ULTIMA) trial.7 The ULTIMA trial authors commented that unfractionated heparin was started with an 80-U/kg bolus followed by an 18-U/kg/hour infusion to target an anti-factor Xa level of 0.3 to 0.7 μg/mL, which is considered therapeutic anticoagulation. The investigators in the SEATTLE II trial8 continued systemic unfractionated heparin but targeted a lower “intermediate” anticoagulation target (an augmented partial thromboplastin time of 40–60 seconds), so these patients weren’t completely without systemic anticoagulation either. At our institution, the current practice is to target an anti-Xa level of 0.3 to 0.7 μg/mL in patients receiving catheter-directed therapy for large-volume pulmonary embolism.
In Reply: We thank Dr. Katyal for his thoughtful comments.
Dr. Katyal points out that the grade of recommendation for thrombolysis in patients with massive pulmonary embolism was upgraded from 2C to 2B in the 2016 American College of Chest Physicians (ACCP) guidelines1 compared with the 2012 guidelines2 that we cited. The upgrade in this recommendation was owing to 2 small trials and 1 large randomized controlled trial that included patients with submassive pulmonary embolism.3–5 Interestingly, these 3 studies led to an upgrade in the level of recommendation for thrombolysis in the treatment of massive pulmonary embolism, perhaps more from a safety aspect (in view of the incidence of major bleeding vs mortality). Regardless, Dr. Katyal is correct in highlighting that the new 2016 ACCP guidelines now give a grade of 2B for thrombolytic therapy in the treatment of massive pulmonary embolism. These guidelines had not been published at the time of submission of our manuscript.
Dr. Katyal is also correct that patients were not required to have right ventricular dysfunction to be enrolled in the MOPETT trial.3 As we pointed out, “Only 20% of the participants were enrolled on the basis of right ventricular dysfunction on echocardiography, whereas almost 60% had elevated cardiac biomarkers.”6
Regarding catheter-directed therapy, patients who received low-dose catheter-directed alteplase were also concurrently anticoagulated with systemic unfractionated heparin in the Ultrasound-Assisted, Catheter-Directed Thrombolysis for Acute Intermediate-Risk Pulmonary Embolism (ULTIMA) trial.7 The ULTIMA trial authors commented that unfractionated heparin was started with an 80-U/kg bolus followed by an 18-U/kg/hour infusion to target an anti-factor Xa level of 0.3 to 0.7 μg/mL, which is considered therapeutic anticoagulation. The investigators in the SEATTLE II trial8 continued systemic unfractionated heparin but targeted a lower “intermediate” anticoagulation target (an augmented partial thromboplastin time of 40–60 seconds), so these patients weren’t completely without systemic anticoagulation either. At our institution, the current practice is to target an anti-Xa level of 0.3 to 0.7 μg/mL in patients receiving catheter-directed therapy for large-volume pulmonary embolism.
- Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. Chest 2016; 149:315–352.
- Kearon C, Akl EA, Comerota AJ, et al; American College of Chest Physicians. Antithrombotic therapy for VTE disease: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141(suppl 2):e419S–e494S.
- Sharifi M, Bay C, Skrocki L, Rahimi F, Mehdipour M; “MOPETT” Investigators. Moderate pulmonary embolism treated with thrombolysis (from the “MOPETT” Trial). Am J Cardiol 2013; 111:273–277.
- Meyer G, Vicaut E, Danays T, et al; PEITHO Investigators. Fibrinolysis for patients with intermediate-risk pulmonary embolism. N Engl J Med 2014; 370:1402–1411.
- Kline JA, Nordenholz KE, Courtney DM, et al. Treatment of submassive pulmonary embolism with tenecteplase or placebo: cardiopulmonary outcomes at 3 months: multicenter double-blind, placebo-controlled randomized trial. J Thromb Haemost 2014; 12:459–468.
- Ataya A, Cope J, Shahmohammadi A, Alnuaimat H. Do patients with submassive pulmonary embolism benefit from thrombolytic therapy? Cleve Clin J Med 2016; 83:923–932.
- Kucher N, Boekstegers P, Muller OJ, et al. Randomized, controlled trial of ultrasound-assisted catheter-directed thrombolysis for acute intermediate-risk pulmonary embolism. Circulation 2014; 129:479–486.
- Piazza G, Hohlfelder B, Jaff MR, et al; SEATTLE II Investigators. A prospective, single-arm, multicenter trial of ultrasound-facilitated, catheter-directed, low-dose fibrinolysis for acute massive and submassive pulmonary embolism (The SEATTLE II Study). JACC Cardiovasc Interv 2015; 8:1382–1392.
- Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. Chest 2016; 149:315–352.
- Kearon C, Akl EA, Comerota AJ, et al; American College of Chest Physicians. Antithrombotic therapy for VTE disease: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141(suppl 2):e419S–e494S.
- Sharifi M, Bay C, Skrocki L, Rahimi F, Mehdipour M; “MOPETT” Investigators. Moderate pulmonary embolism treated with thrombolysis (from the “MOPETT” Trial). Am J Cardiol 2013; 111:273–277.
- Meyer G, Vicaut E, Danays T, et al; PEITHO Investigators. Fibrinolysis for patients with intermediate-risk pulmonary embolism. N Engl J Med 2014; 370:1402–1411.
- Kline JA, Nordenholz KE, Courtney DM, et al. Treatment of submassive pulmonary embolism with tenecteplase or placebo: cardiopulmonary outcomes at 3 months: multicenter double-blind, placebo-controlled randomized trial. J Thromb Haemost 2014; 12:459–468.
- Ataya A, Cope J, Shahmohammadi A, Alnuaimat H. Do patients with submassive pulmonary embolism benefit from thrombolytic therapy? Cleve Clin J Med 2016; 83:923–932.
- Kucher N, Boekstegers P, Muller OJ, et al. Randomized, controlled trial of ultrasound-assisted catheter-directed thrombolysis for acute intermediate-risk pulmonary embolism. Circulation 2014; 129:479–486.
- Piazza G, Hohlfelder B, Jaff MR, et al; SEATTLE II Investigators. A prospective, single-arm, multicenter trial of ultrasound-facilitated, catheter-directed, low-dose fibrinolysis for acute massive and submassive pulmonary embolism (The SEATTLE II Study). JACC Cardiovasc Interv 2015; 8:1382–1392.
In Reference to “Pilot Study Aiming to Support Sleep Quality and Duration During Hospitalizations”
We commend Gathecha et al.1 on the implementation of a well-formed, multicomponent sleep intervention to improve sleep in hospitalized patients. While they were unable to show objective improvement in sleep outcomes, they found improvements in patient-reported sleep outcomes across hospital days, implying that multiple hospital nights are needed to realize the benefits. We wish to propose an alternative strategy. To produce a more observable and immediate improvement in patient sleep outcomes, the behavioral economics principle of nudges2 could be an effective way to influence hospital staff toward sleep-promoting practices.
In focus groups at the University of Chicago Medicine, nurses, hospitalists, and residents reported unnecessary nocturnal disruptions were the “default” option hardwired in electronic medical records admission order sets. It was time-consuming to enter orders that minimized unnecessary nocturnal disruptions, such as forgo overnight vitals for stable patients. Given that changing default settings of order sets have been shown to effectively nudge physicians in other areas,3-5 altering default settings in admission orders could facilitate physicians’ adherence to sleep-promoting practices. An intervention combining these nudges with educational initiatives may be more effective in sustained reductions in nocturnal disruptions and improved inpatient sleep from the start of a hospital stay.
References
1. Gathecha E, Rios R, Buenaver LF, Landis R, Howell E, Wright S. Pilot study aiming to support sleep quality and duration during hospitalizations. J Hosp Med. 2016;11(7):467-472. doi:10.1002/jhm.2578. PubMed
2. Thaler R, Sunstein C. Nudge: Improving Decisions About Health, Wealth and Happiness. New Haven, CT: Yale University Press; 2008.
3. Bourdeaux CP, Davies KJ, Thomas MJC, Bewley JS, Gould TH. Using “nudge” principles for order set design: a before and after evaluation of an electronic prescribing template in critical care. BMJ Qual Saf. 2014;23(5):382-388. doi:10.1136/bmjqs-2013-002395. PubMed
4. Halpern SD, Ubel PA, Asch DA. Harnessing the power of default options to improve health care. N Engl J Med. 2007;357(13):1340-1344. doi:10.1056/NEJMsb071595. PubMed
5. Ansher C, Ariely D, Nagler A, Rudd M, Schwartz J, Shah A. Better medicine by default. Med Decis Making. 2014;34(2):147-158. doi:10.1177/0272989X13507339. PubMed
We commend Gathecha et al.1 on the implementation of a well-formed, multicomponent sleep intervention to improve sleep in hospitalized patients. While they were unable to show objective improvement in sleep outcomes, they found improvements in patient-reported sleep outcomes across hospital days, implying that multiple hospital nights are needed to realize the benefits. We wish to propose an alternative strategy. To produce a more observable and immediate improvement in patient sleep outcomes, the behavioral economics principle of nudges2 could be an effective way to influence hospital staff toward sleep-promoting practices.
In focus groups at the University of Chicago Medicine, nurses, hospitalists, and residents reported unnecessary nocturnal disruptions were the “default” option hardwired in electronic medical records admission order sets. It was time-consuming to enter orders that minimized unnecessary nocturnal disruptions, such as forgo overnight vitals for stable patients. Given that changing default settings of order sets have been shown to effectively nudge physicians in other areas,3-5 altering default settings in admission orders could facilitate physicians’ adherence to sleep-promoting practices. An intervention combining these nudges with educational initiatives may be more effective in sustained reductions in nocturnal disruptions and improved inpatient sleep from the start of a hospital stay.
We commend Gathecha et al.1 on the implementation of a well-formed, multicomponent sleep intervention to improve sleep in hospitalized patients. While they were unable to show objective improvement in sleep outcomes, they found improvements in patient-reported sleep outcomes across hospital days, implying that multiple hospital nights are needed to realize the benefits. We wish to propose an alternative strategy. To produce a more observable and immediate improvement in patient sleep outcomes, the behavioral economics principle of nudges2 could be an effective way to influence hospital staff toward sleep-promoting practices.
In focus groups at the University of Chicago Medicine, nurses, hospitalists, and residents reported unnecessary nocturnal disruptions were the “default” option hardwired in electronic medical records admission order sets. It was time-consuming to enter orders that minimized unnecessary nocturnal disruptions, such as forgo overnight vitals for stable patients. Given that changing default settings of order sets have been shown to effectively nudge physicians in other areas,3-5 altering default settings in admission orders could facilitate physicians’ adherence to sleep-promoting practices. An intervention combining these nudges with educational initiatives may be more effective in sustained reductions in nocturnal disruptions and improved inpatient sleep from the start of a hospital stay.
References
1. Gathecha E, Rios R, Buenaver LF, Landis R, Howell E, Wright S. Pilot study aiming to support sleep quality and duration during hospitalizations. J Hosp Med. 2016;11(7):467-472. doi:10.1002/jhm.2578. PubMed
2. Thaler R, Sunstein C. Nudge: Improving Decisions About Health, Wealth and Happiness. New Haven, CT: Yale University Press; 2008.
3. Bourdeaux CP, Davies KJ, Thomas MJC, Bewley JS, Gould TH. Using “nudge” principles for order set design: a before and after evaluation of an electronic prescribing template in critical care. BMJ Qual Saf. 2014;23(5):382-388. doi:10.1136/bmjqs-2013-002395. PubMed
4. Halpern SD, Ubel PA, Asch DA. Harnessing the power of default options to improve health care. N Engl J Med. 2007;357(13):1340-1344. doi:10.1056/NEJMsb071595. PubMed
5. Ansher C, Ariely D, Nagler A, Rudd M, Schwartz J, Shah A. Better medicine by default. Med Decis Making. 2014;34(2):147-158. doi:10.1177/0272989X13507339. PubMed
References
1. Gathecha E, Rios R, Buenaver LF, Landis R, Howell E, Wright S. Pilot study aiming to support sleep quality and duration during hospitalizations. J Hosp Med. 2016;11(7):467-472. doi:10.1002/jhm.2578. PubMed
2. Thaler R, Sunstein C. Nudge: Improving Decisions About Health, Wealth and Happiness. New Haven, CT: Yale University Press; 2008.
3. Bourdeaux CP, Davies KJ, Thomas MJC, Bewley JS, Gould TH. Using “nudge” principles for order set design: a before and after evaluation of an electronic prescribing template in critical care. BMJ Qual Saf. 2014;23(5):382-388. doi:10.1136/bmjqs-2013-002395. PubMed
4. Halpern SD, Ubel PA, Asch DA. Harnessing the power of default options to improve health care. N Engl J Med. 2007;357(13):1340-1344. doi:10.1056/NEJMsb071595. PubMed
5. Ansher C, Ariely D, Nagler A, Rudd M, Schwartz J, Shah A. Better medicine by default. Med Decis Making. 2014;34(2):147-158. doi:10.1177/0272989X13507339. PubMed
© 2017 Society of Hospital Medicine
The Authors Reply, “Pilot Study Aiming to Support Sleep Quality and Duration During Hospitalizations”
We thank the authors for their comments and thoughts about our recent publication.1 Their suggestion that the incorporation of principles from the “Nudge Theory” might enhance the impact of our sleep intervention and shorten the lag time until patients appreciate the benefits is interesting.2 Our study aimed to assess the effect of a sleep-promoting intervention on sleep quality and duration among hospitalized patients within a quasi-experimental prospective study design. As is the case at the University of Chicago hospital described in Machado’s letter, nocturnal disruptions are also the “default” in order sets in our electronic medical records (EMR). Because the EMR team at our hospital is stretched thin with more requests than it can fulfill, it was not feasible or possible to incorporate any sleep supporting changes when designing the pilot.
Complementing sleep-promoting procedures for hospitalized patients with “nudge” principles, such as the use of choice architecture with appropriate EMR defaults or even incentives and mappings, seems like a wise recommendation.3 Regular nudges may be helpful for sustaining any multicomponent interventions in healthcare delivery that rely on cooperation by multiple parties. It appears as if evidence is growing that “nudge principles” can augment behavior change attributable to interventions.4,5 Sleep-promoting nudges, namely “anti-nudges” by members of the healthcare team, should help patients to sleep better during their hospitalizations, when sleep is critically important to recovery and health restitution.
1. Gathecha E, Rios R, Buenaver LF, Landis R, Howell E, Wright S. Pilot study aiming to support sleep quality and duration during hospitalizations. J Hosp Med. 2016;11(7):467-472. doi:10.1002/jhm.2578. PubMed
2. Thaler R, Sunstein C. Nudge: Improving Decisions About Health, Wealth and Happiness. New Haven, CT: Yale University Press; 2008.
3. Bourdeaux CP, Davies KJ, Thomas MJC, Bewley JS, Gould TH. Using “nudge” principles for order set design: a before and after evaluation of an electronic prescribing template in critical care. BMJ Qual Saf. 2014;23(5):382-388. doi:10.1136/bmjqs-2013-002395 PubMed
4. Hollands GJ, Shemilt I, Marteau TM, et al. Altering micro-environments to change population health behaviour: towards an evidence base for choice architecture interventions. BMC Public Health. 2013;13:1218. doi:10.1186/1471-2458-13-1218. PubMed
5. Arno A, Thomas S. The efficacy of nudge theory strategies in influencing adult dietary behavior: a systematic review and meta-analysis. BMC Public Health. 2016;16:676. doi:10.1186/s12889-016-3272-x. PubMed
We thank the authors for their comments and thoughts about our recent publication.1 Their suggestion that the incorporation of principles from the “Nudge Theory” might enhance the impact of our sleep intervention and shorten the lag time until patients appreciate the benefits is interesting.2 Our study aimed to assess the effect of a sleep-promoting intervention on sleep quality and duration among hospitalized patients within a quasi-experimental prospective study design. As is the case at the University of Chicago hospital described in Machado’s letter, nocturnal disruptions are also the “default” in order sets in our electronic medical records (EMR). Because the EMR team at our hospital is stretched thin with more requests than it can fulfill, it was not feasible or possible to incorporate any sleep supporting changes when designing the pilot.
Complementing sleep-promoting procedures for hospitalized patients with “nudge” principles, such as the use of choice architecture with appropriate EMR defaults or even incentives and mappings, seems like a wise recommendation.3 Regular nudges may be helpful for sustaining any multicomponent interventions in healthcare delivery that rely on cooperation by multiple parties. It appears as if evidence is growing that “nudge principles” can augment behavior change attributable to interventions.4,5 Sleep-promoting nudges, namely “anti-nudges” by members of the healthcare team, should help patients to sleep better during their hospitalizations, when sleep is critically important to recovery and health restitution.
We thank the authors for their comments and thoughts about our recent publication.1 Their suggestion that the incorporation of principles from the “Nudge Theory” might enhance the impact of our sleep intervention and shorten the lag time until patients appreciate the benefits is interesting.2 Our study aimed to assess the effect of a sleep-promoting intervention on sleep quality and duration among hospitalized patients within a quasi-experimental prospective study design. As is the case at the University of Chicago hospital described in Machado’s letter, nocturnal disruptions are also the “default” in order sets in our electronic medical records (EMR). Because the EMR team at our hospital is stretched thin with more requests than it can fulfill, it was not feasible or possible to incorporate any sleep supporting changes when designing the pilot.
Complementing sleep-promoting procedures for hospitalized patients with “nudge” principles, such as the use of choice architecture with appropriate EMR defaults or even incentives and mappings, seems like a wise recommendation.3 Regular nudges may be helpful for sustaining any multicomponent interventions in healthcare delivery that rely on cooperation by multiple parties. It appears as if evidence is growing that “nudge principles” can augment behavior change attributable to interventions.4,5 Sleep-promoting nudges, namely “anti-nudges” by members of the healthcare team, should help patients to sleep better during their hospitalizations, when sleep is critically important to recovery and health restitution.
1. Gathecha E, Rios R, Buenaver LF, Landis R, Howell E, Wright S. Pilot study aiming to support sleep quality and duration during hospitalizations. J Hosp Med. 2016;11(7):467-472. doi:10.1002/jhm.2578. PubMed
2. Thaler R, Sunstein C. Nudge: Improving Decisions About Health, Wealth and Happiness. New Haven, CT: Yale University Press; 2008.
3. Bourdeaux CP, Davies KJ, Thomas MJC, Bewley JS, Gould TH. Using “nudge” principles for order set design: a before and after evaluation of an electronic prescribing template in critical care. BMJ Qual Saf. 2014;23(5):382-388. doi:10.1136/bmjqs-2013-002395 PubMed
4. Hollands GJ, Shemilt I, Marteau TM, et al. Altering micro-environments to change population health behaviour: towards an evidence base for choice architecture interventions. BMC Public Health. 2013;13:1218. doi:10.1186/1471-2458-13-1218. PubMed
5. Arno A, Thomas S. The efficacy of nudge theory strategies in influencing adult dietary behavior: a systematic review and meta-analysis. BMC Public Health. 2016;16:676. doi:10.1186/s12889-016-3272-x. PubMed
1. Gathecha E, Rios R, Buenaver LF, Landis R, Howell E, Wright S. Pilot study aiming to support sleep quality and duration during hospitalizations. J Hosp Med. 2016;11(7):467-472. doi:10.1002/jhm.2578. PubMed
2. Thaler R, Sunstein C. Nudge: Improving Decisions About Health, Wealth and Happiness. New Haven, CT: Yale University Press; 2008.
3. Bourdeaux CP, Davies KJ, Thomas MJC, Bewley JS, Gould TH. Using “nudge” principles for order set design: a before and after evaluation of an electronic prescribing template in critical care. BMJ Qual Saf. 2014;23(5):382-388. doi:10.1136/bmjqs-2013-002395 PubMed
4. Hollands GJ, Shemilt I, Marteau TM, et al. Altering micro-environments to change population health behaviour: towards an evidence base for choice architecture interventions. BMC Public Health. 2013;13:1218. doi:10.1186/1471-2458-13-1218. PubMed
5. Arno A, Thomas S. The efficacy of nudge theory strategies in influencing adult dietary behavior: a systematic review and meta-analysis. BMC Public Health. 2016;16:676. doi:10.1186/s12889-016-3272-x. PubMed
© 2017 Society of Hospital Medicine