User login
Thrombotic thrombocytopenic purpura: 2008 Update
Thrombotic thrombocytopenic purpura (TTP) is one of the few hematologic emergencies. Untreated, most patients die, but prompt and appropriate treatment allows most patients not only to survive but to recover, frequently without long-term sequelae.
TTP is rare. The estimated annual incidence of all TTP syndromes is about 11 cases per million in the general population, and the incidence of severe ADAMTS13 deficiency (see discussion below) is about 2 per million. Therefore, even large medical centers typically see only one or two cases each year. The syndromes are much more common in women, and the incidence among blacks is nine times higher than the incidence among non-blacks. Nevertheless, despite the rarity of this disease, good evidence exists to help guide patient care, thanks to national registries and research organizations, such as the Canadian Apheresis Study Group and the Oklahoma TTP-Hemolytic Uremic Syndrome (HUS) Registry.
This article reviews the physiologic basis of TTP, how to recognize it, and how best to treat it. We also discuss other conditions that clinically resemble TTP but probably have different underlying causes.
A YOUNG WOMAN WITH ARM WEAKNESS
A 24-year-old black woman presents to a community hospital with weakness in her left arm, which began about 30 minutes previously. She has had progressive dyspnea over the last several weeks, but has otherwise been completely well and has had no medical problems in the past other than being obese.
Physical examination reveals weakness in her left arm as well as mild dysarthria, which was not previously noted by the patient or her family. Her laboratory findings:
- White blood cell count 16.7 × 109/L (reference range 4.5–11.0)
- Platelet count 32 × 109/L (150–350)
- Hemoglobin concentration 6.5 g/dL (1.4–17.5)
- Peripheral blood smear: normal white cells, rare platelets, red cells normo-chromic with many fragments
- Lactate dehydrogenase (LDH) concentration 2,300 U/L (100–200).
In view of her symptoms and laboratory values, the physician suspects she may have TTP and refers her to McMaster University Medical Center in Hamilton, Ontario, Canada. Plasma exchange is started immediately; one plasma volume is removed and replaced with fresh frozen plasma. Nevertheless, the patient’s condition deteriorates overnight, she becomes more confused and cannot protect her airway, her LDH concentration rises further, and her hemoglobin concentration falls. She is transferred to the intensive care unit. Her plasma exchange prescription is increased to 1.5 volumes twice daily (although little evidence exists that plasma exchange twice daily is more effective than once daily).
On the third day of her stay, she becomes completely paralyzed on the left side. In addition to her twice-daily plasma exchange procedures, a plasma infusion and corticosteroid therapy are initiated. Her platelet count stabilizes at about 20 × 109/L.
The patient next develops renal insufficiency and requires three acute hemodialysis treatments. (Plasma infusion frequently leads to volume overload in critically ill patients. Some intravascular volume can be removed with plasma exchange; however, significant volume overload with significant renal insufficiency can only be treated with renal replacement therapy.)
The patient undergoes 28 consecutive days of twice-daily plasma exchange and gradually improves, as measured by increasing platelet counts, a gradual fall in the LDH concentration, and stabilization of—and ultimately an increase in—the hemoglobin level. She is weaned off plasma infusions, and then plasma exchange is tapered to once a day and then to alternate days.
She is completely well at the time of discharge 4 weeks after her initial admission, with no residual deficits.
Comment. This case shows that even patients with apparently devastating compromise and neurologic deficits can completely recover with aggressive plasma exchange and other therapies. One child treated at the Hospital for Sick Children, affiliated with the University of Toronto, developed TTP and had 120 consecutive days of plasma exchange: she was unconscious and comatose for much of that time, but she ultimately recovered and is now completely well without residual neurologic deficits.
TTP MAY BE DUE TO ADAMTS13 DEFICIENCY
Twenty-five years ago, little was known about TTP except for its clinical manifestations. Now, it is known to be caused in some patients by an acquired deficiency of a circulating metalloproteinase. In very rare cases a hereditary deficiency of ADAMTS13 causes TTP. In addition, a number of conditions share clinical features with TTP but have other underlying causes.
In acquired TTP, an autoantibody forms against ADAMTS13, a zinc-containing metalloproteinase that is also known as von Willebrand factor-cleaving protease. Normally, von Willebrand factor circulates in plasma as multimers that allow platelets to adhere to vascular surfaces. When von Willebrand factor is initially released from endothelial cells, it exists as large multimers, which are more adhesive for platelets than normal. These large multimers are normally cleaved into smaller units by ADAMTS13. If ADAMTS13 is lacking, the very-high-molecular-weight von Willebrand factor multimers accumulate, causing platelet agglutination and the vascular occlusion that results in the manifestations of TTP.
In 1994, ADAMTS13, the gene of which is on the ninth chromosome, was shown to cleave von Willebrand factor under conditions of high shear stress. In 1996, a congenital homozygous deficiency of ADAMTS13 was found to be associated with platelet microthrombi. Afterwards, some patients with TTP were shown to have low or undetectable levels of ADAMTS13, owing to immunoglobulin G antibodies directed against the enzyme.
TTP AND RELATED SYNDROMES
Clinically, TTP encompasses a number of different but related syndromes, some of which have different physiologic bases.
TTP
TTP is characterized by moderate to severe thrombocytopenia, red cell fragmentation, and elevated LDH levels (due to red cell destruction and also muscle and organ ischemia). The pentad of features classically associated with TTP in the era before effective treatment (thrombocytopenia, fever, renal failure, neurologic deficit, and microangiopathic hemolytic anemia) is rarely seen in countries with advanced medical care: renal insufficiency and neurologic events are end-stage manifestations, and the disease should be recognizable well before these manifestations occur. Otherwise unexplained thrombocytopenia, microangiopathic hemolytic anemia, and an elevated LDH should strongly suggest TTP. TTP is the appropriate designation for adults with these clinical features, even in the presence of renal failure. TTP is uncommon in children.
Most patients present with nonspecific constitutional symptoms, such as weakness, abdominal pain, nausea, and vomiting. Typically, the family physician orders a complete blood cell count and finds that the platelet count and hemoglobin are low. Red cell fragments are noted in the peripheral blood smear. Further testing reveals an elevated LDH concentration.
HUS
HUS was initially described 30 years after TTP in children with acute renal failure in addition to thrombocytopenia and microangiopathic hemolytic anemia. The term “HUS” is currently used primarily to describe the condition in children.
In children, two forms of HUS exist:
Diarrhea-associated HUS is associated with diarrhea that is commonly bloody, due to an enterotoxin produced by Escherichia coli O157:H7.
Endemic diarrhea-associated HUS is much more common than HUS associated with epidemics. Endemic cases are caused by E coli O157:H7 present in the environment. Other patients present with clinically apparent HUS but the causal bacterium cannot be detected. The kidney transplant program at our center often sees young patients with this disease who do not have E coli O157:H7 infection, and the pathogenesis is not understood. Epidemic cases are less common but the outbreaks are dramatic. About 10 years ago, E coli O157:H7 entered the water supply in the small city of Walkerton, Ontario, and many people developed the epidemic form of HUS over a period of several weeks. Most such patients spontaneously recovered without plasma exchange, although many were left with impaired renal function.
Atypical HUS. Less often, HUS in children is not associated with a prodrome of diarrhea and is referred to as “atypical” HUS. These children often have a more prolonged and complicated course and resemble adults with TTP.
Familial TTP-HUS
Familial TTP-HUS is very rare. It may present with hemolysis and thrombocytopenia in childhood or early adulthood. Many patients present with renal insufficiency, and only careful evaluation reveals hemolysis and thrombocytopenia. The disease typically manifests acutely: a patient may have an upper respiratory tract infection and subsequently develop an episode of TTP-HUS. Episodes tend to recur, and multiple family members may also be affected.
Plasma infusion is an effective treatment, and plasma exchange is usually not required. Since more patients are now surviving well into adulthood, some are being seen to develop antibodies to the ADAMTS13 in the infused plasma, analogous to patients with severe hemophilia developing inhibitors to factor VIII. The disease may progress despite treatment: we have been treating a young woman who has had a series of catastrophic complications and now has chronic renal failure requiring hemodialysis (see discussion below).
Post-transplant microangiopathy
Post-transplant microangiopathy is most likely to develop after solid-organ or stem-cell allograft transplantation. Manifestations resemble those of TTP, but the mechanism is probably quite different. Multiple causes probably exist, depending on the setting.
Post-transplant microangiopathy does not respond to the usual therapies for TTP, although we treat it, like TTP, with corticosteroids, antiplatelet agents, and plasma exchange. Other centers do not use plasma exchange for these patients. Most patients have a poor prognosis, especially those with a transplant other than a kidney.
A spectrum of related syndromes
A number of diseases clinically resemble TTP. Enhanced diagnostic capacity and better molecular biologic techniques are revealing that they often have very different underlying causes and that in some cases they require different treatment.
TOWARD DIAGNOSTIC CRITERIA
Ruutu et al,1 in a consensus conference, used rigorous methods to establish diagnostic criteria for microangiopathy associated with stem cell transplantation:
- More than 4% red blood cell fragments in the peripheral blood. A laboratory report that states that “few fragments” are present is not nearly as useful as one that estimates the quantity; eg, 1% fragments would have very different implications than 6% fragments.
- Thrombocytopenia—a platelet count of less than 50 × 109/L or more than a 50% reduction from previous counts
- Increased LDH concentration
- Reduced hemoglobin concentration or increased transfusion requirement
- Decrease in serum haptoglobin, which, like red blood cell fragments, is a marker of hemolysis rather than of reduced synthesis.
The ADAMTS13 level need not be assessed. Metalloproteinase deficiency need not be proved to diagnose TTP. Although our hospital is a TTP referral center, we do not routinely offer the test. Too often the test results cause confusion: a patient can have a normal level of ADAMTS13 and still have TTP that responds to plasma exchange, and levels can be low in conditions other than TTP.
THE CHALLENGES OF TREATMENT
Plasma exchange is the primary treatment for TTP
Rock et al2 performed a randomized trial in which 102 patients with TTP received either a 1.5-volume plasma exchange daily for 3 days and then 1-volume plasma exchanges as needed to control the disease or plasma infusion. Patients who received plasma exchange had a better initial response, a higher survival rate, and a lower rate of relapse than patients receiving plasma infusion. These findings established plasma exchange as the treatment of choice for TTP.
However, the trial had some inherent problems: patients who had plasma infusions tended to develop renal insufficiency and as a result did not receive as much plasma because they could not tolerate as much volume as those who had plasma exchange. Plasma exchange probably worked better because it could deliver more plasma over a fixed period of time, enabling patients to obtain more of the ADAMTS13 enzyme, rather than because it was an intrinsically better treatment. This interpretation is the basis for our occasional use of twice-daily plasma exchange in critically ill patients.
TTP is different from other autoimmune diseases such as idiopathic thrombocytopenia purpura, in which the primary treatments are immunosuppressive agents. Some evidence exists for treating TTP with immunosuppressive agents, but the primary treatment should be plasma exchange.
Plasma infusion is useful in some cases
Although small case series and our own experience provide evidence for the benefit of treating TTP with high-dose plasma infusions (25 mL/kg/day, or about 1.5 to 2.0 L/day for an average-sized adult), problems will likely arise with volume overload if the patient has any significant renal insufficiency. Dialysis or ultrafiltration may be used to treat volume overload; however, it is difficult to remove the large volumes of fluid required for high-volume plasma infusion.
Plasma infusion should be reserved for two situations:
- If plasma exchange cannot be promptly started
- For patients with very severe or refractory disease, between plasma exchange sessions.
Benefit of cryoprecipitate-poor plasma is uncertain
Fresh frozen plasma is believed to contain nearly physiologic levels of all of the plasma proteins. When plasma is cooled to around 4°C, a precipitate forms that contains a variety of substances, including the higher molecular weight multimers of von Willebrand factor. Because TTP involves excess large multimers, giving plasma in which the high molecular weight multimers have been removed should in theory be better. In many centers, such cryoprecipitate-poor plasma is routinely used to treat TTP.
However, evidence that cryoprecipitate-poor plasma is better is lacking. A large study in Canada evaluating this question was terminated because of a lack of patient accrual, a common fate of clinical trials of rare diseases. A randomized study in 17 patients failed to show an advantage of cryoprecipitate-poor plasma over regular plasma, but the study was too small to draw firm conclusions given large confidence intervals about the point estimate of the treatment effect.
Cryoprecipitate-poor plasma is more expensive than regular plasma and is not as available. We do not routinely use it in our center to initially manage patients with TTP, but we do use it for patients who are refractory to standard treatment.
Scott et al3 measured the concentration of ADAMTS13 in a variety of plasma products and found that there are significant amounts in cryoprecipitate. Although giving cryoprecipitate-poor plasma provides less of the high molecular weight multimers, which is desirable for patients with TTP, it also provides less ADAMTS13, which is not desirable.
Do antiplatelet agents have a role in acute TTP?
Most algorithms for managing acute TTP include the use of aspirin or dipyridamole (Persantine) or both, and there is some evidence in favor of this approach,4 but whether antiplatelet therapy should be used for inpatients with severe thrombocytopenia remains controversial. In our practice, we usually provide antiplatelet therapy even for patients with severe thrombocytopenia because we believe TTP involves platelet-mediated hypercoagulability rather than increased bleeding risk.
Do corticosteroids have a role?
Corticosteroids were widely used to treat TTP even before the disease was discovered to be immune mediated. In our center we routinely use them.
Unfortunately, few data exist on the efficacy of steroid therapy for TTP. As a result, we can only make a weak recommendation for its use: using the American College of Chest Physicians rating system for the strength of clinical evidence, it would receive a 2C recommendation. This is the weakest possible recommendation, being based on widespread use but poor-quality data.
Stopping vs tapering plasma exchange
Whether plasma exchange should be tapered or simply stopped is also controversial and not well studied. Nevertheless, a widespread clinical practice—once the platelet count returns to 200 × 109/L or higher and the patient looks and feels well—is to reduce the plasma exchange sessions to once every 3 days, then to once every 7 days, and then to once every 2 weeks.
In our practice, we taper plasma exchange in this fashion for a minimum of two treatments beyond what we think the patient really needs. As a result, we tend to treat about once every 2 weeks for weeks or even months after the acute illness.
Rituximab may help
Rituximab (Rituxan), a monoclonal antibody against mature B cells, is increasingly being used in treating TTP. Past and present treatments for TTP, including splenectomy, corticosteroids, and plasma exchange are immunomodulatory, so the use of rituximab may be justified. Case reports provided the rationale for a large, multicenter, randomized controlled trial, which is currently under way.5
CONDITIONS THAT ARE NOT TTP
Some conditions may be confused with TTP but are clearly something different:
Patients with isolated thrombocytopenia and normal blood smear findings and no coagulopathy most likely have idiopathic thrombocytopenia purpura or, in the correct clinical circumstance, heparin-induced thrombocytopenia.
A patient with an extremely low platelet count but no fragments or very few fragments with microangiopathic hemolytic anemia may have either drug-associated thrombocytopenia or disseminated intravascular coagulopathy, particularly if there is concomitant coagulopathy.
Many pregnancy-associated microangiopathies resemble TTP, and it may be difficult to differentiate them from TTP; if confusion as to the diagnosis exists, the patient should be treated with plasma exchange, as this therapy may be life-saving.
Many rheumatologic conditions are characterized by an acute illness with nonspecific findings, such as low-grade hemolysis and thrombocytopenia. For example, Wegener granulomatosis can present with evidence of hemolysis, thrombocytopenia, and renal impairment.
Systemic lupus erythematosus can also initially present with an “early-TTP”-like picture. Evidence of glomerulonephritis is not consistent with TTP, and urinary red cell casts makes the diagnosis of lupus more likely. Helmet cell fragments in the peripheral blood smear are supposedly more characteristic of TTP, but their presence is not diagnostic.
Scleroderma renal crisis can present like TTP, but because it is unlikely that a patient with known scleroderma would have a second rare disease, it is best to treat it as scleroderma, which does not require plasma exchange or plasma infusion.
In general, if the diagnosis is uncertain, the safest course is to treat the patient with plasma exchange, then try to establish the diagnosis, because TTP is fatal if not promptly treated. Although plasma exchange is probably overused, it is more innocuous than untreated TTP.
- Ruutu T, Barosi G, Benjamin RJ, et al; European Group for Blood and Marrow Transplantation; European LeukemiaNet. Diagnostic criteria for hematopoietic stem cell transplant-associated microangiopathy: results of a consensus process by an International Working Group. Haematologica 2007; 92:95–100.
- Rock GA, Shumak KH, Buskard NA, et al. Comparison of plasma exchange with plasma infusion in the treatment of thrombotic thrombocytopenic purpura. Canadian Apheresis Study Group. N Engl J Med 1991; 325:393–397.
- Scott EA, Puca KE, Pietz BC, Duchateau BK, Friedman KD. Comparison and stability of ADAMTS13 activity in therapeutic plasma products. Transfusion 2007; 47:120–125.
- Bobbio-Pallavicini E, Gugliotta L, Centurioni R, et al. Antiplatelet agents in thrombotic thrombocytopenic purpura (TTP). Results of a randomized multicenter trial by the Italian Cooperative Group for TTP. Haematologica 1997; 82:429–435.
- George JN, Woodson RD, Kiss JE, Kojouri K, Vesely SK. Rituximab therapy for thrombotic thrombocytopenic purpura: a proposed study of the Transfusion Medicine/Hemostasis Clinical Trials Network with a systematic review of rituximab therapy for immune-mediated disorders. J Clin Apher 2006; 21:49–56.
Thrombotic thrombocytopenic purpura (TTP) is one of the few hematologic emergencies. Untreated, most patients die, but prompt and appropriate treatment allows most patients not only to survive but to recover, frequently without long-term sequelae.
TTP is rare. The estimated annual incidence of all TTP syndromes is about 11 cases per million in the general population, and the incidence of severe ADAMTS13 deficiency (see discussion below) is about 2 per million. Therefore, even large medical centers typically see only one or two cases each year. The syndromes are much more common in women, and the incidence among blacks is nine times higher than the incidence among non-blacks. Nevertheless, despite the rarity of this disease, good evidence exists to help guide patient care, thanks to national registries and research organizations, such as the Canadian Apheresis Study Group and the Oklahoma TTP-Hemolytic Uremic Syndrome (HUS) Registry.
This article reviews the physiologic basis of TTP, how to recognize it, and how best to treat it. We also discuss other conditions that clinically resemble TTP but probably have different underlying causes.
A YOUNG WOMAN WITH ARM WEAKNESS
A 24-year-old black woman presents to a community hospital with weakness in her left arm, which began about 30 minutes previously. She has had progressive dyspnea over the last several weeks, but has otherwise been completely well and has had no medical problems in the past other than being obese.
Physical examination reveals weakness in her left arm as well as mild dysarthria, which was not previously noted by the patient or her family. Her laboratory findings:
- White blood cell count 16.7 × 109/L (reference range 4.5–11.0)
- Platelet count 32 × 109/L (150–350)
- Hemoglobin concentration 6.5 g/dL (1.4–17.5)
- Peripheral blood smear: normal white cells, rare platelets, red cells normo-chromic with many fragments
- Lactate dehydrogenase (LDH) concentration 2,300 U/L (100–200).
In view of her symptoms and laboratory values, the physician suspects she may have TTP and refers her to McMaster University Medical Center in Hamilton, Ontario, Canada. Plasma exchange is started immediately; one plasma volume is removed and replaced with fresh frozen plasma. Nevertheless, the patient’s condition deteriorates overnight, she becomes more confused and cannot protect her airway, her LDH concentration rises further, and her hemoglobin concentration falls. She is transferred to the intensive care unit. Her plasma exchange prescription is increased to 1.5 volumes twice daily (although little evidence exists that plasma exchange twice daily is more effective than once daily).
On the third day of her stay, she becomes completely paralyzed on the left side. In addition to her twice-daily plasma exchange procedures, a plasma infusion and corticosteroid therapy are initiated. Her platelet count stabilizes at about 20 × 109/L.
The patient next develops renal insufficiency and requires three acute hemodialysis treatments. (Plasma infusion frequently leads to volume overload in critically ill patients. Some intravascular volume can be removed with plasma exchange; however, significant volume overload with significant renal insufficiency can only be treated with renal replacement therapy.)
The patient undergoes 28 consecutive days of twice-daily plasma exchange and gradually improves, as measured by increasing platelet counts, a gradual fall in the LDH concentration, and stabilization of—and ultimately an increase in—the hemoglobin level. She is weaned off plasma infusions, and then plasma exchange is tapered to once a day and then to alternate days.
She is completely well at the time of discharge 4 weeks after her initial admission, with no residual deficits.
Comment. This case shows that even patients with apparently devastating compromise and neurologic deficits can completely recover with aggressive plasma exchange and other therapies. One child treated at the Hospital for Sick Children, affiliated with the University of Toronto, developed TTP and had 120 consecutive days of plasma exchange: she was unconscious and comatose for much of that time, but she ultimately recovered and is now completely well without residual neurologic deficits.
TTP MAY BE DUE TO ADAMTS13 DEFICIENCY
Twenty-five years ago, little was known about TTP except for its clinical manifestations. Now, it is known to be caused in some patients by an acquired deficiency of a circulating metalloproteinase. In very rare cases a hereditary deficiency of ADAMTS13 causes TTP. In addition, a number of conditions share clinical features with TTP but have other underlying causes.
In acquired TTP, an autoantibody forms against ADAMTS13, a zinc-containing metalloproteinase that is also known as von Willebrand factor-cleaving protease. Normally, von Willebrand factor circulates in plasma as multimers that allow platelets to adhere to vascular surfaces. When von Willebrand factor is initially released from endothelial cells, it exists as large multimers, which are more adhesive for platelets than normal. These large multimers are normally cleaved into smaller units by ADAMTS13. If ADAMTS13 is lacking, the very-high-molecular-weight von Willebrand factor multimers accumulate, causing platelet agglutination and the vascular occlusion that results in the manifestations of TTP.
In 1994, ADAMTS13, the gene of which is on the ninth chromosome, was shown to cleave von Willebrand factor under conditions of high shear stress. In 1996, a congenital homozygous deficiency of ADAMTS13 was found to be associated with platelet microthrombi. Afterwards, some patients with TTP were shown to have low or undetectable levels of ADAMTS13, owing to immunoglobulin G antibodies directed against the enzyme.
TTP AND RELATED SYNDROMES
Clinically, TTP encompasses a number of different but related syndromes, some of which have different physiologic bases.
TTP
TTP is characterized by moderate to severe thrombocytopenia, red cell fragmentation, and elevated LDH levels (due to red cell destruction and also muscle and organ ischemia). The pentad of features classically associated with TTP in the era before effective treatment (thrombocytopenia, fever, renal failure, neurologic deficit, and microangiopathic hemolytic anemia) is rarely seen in countries with advanced medical care: renal insufficiency and neurologic events are end-stage manifestations, and the disease should be recognizable well before these manifestations occur. Otherwise unexplained thrombocytopenia, microangiopathic hemolytic anemia, and an elevated LDH should strongly suggest TTP. TTP is the appropriate designation for adults with these clinical features, even in the presence of renal failure. TTP is uncommon in children.
Most patients present with nonspecific constitutional symptoms, such as weakness, abdominal pain, nausea, and vomiting. Typically, the family physician orders a complete blood cell count and finds that the platelet count and hemoglobin are low. Red cell fragments are noted in the peripheral blood smear. Further testing reveals an elevated LDH concentration.
HUS
HUS was initially described 30 years after TTP in children with acute renal failure in addition to thrombocytopenia and microangiopathic hemolytic anemia. The term “HUS” is currently used primarily to describe the condition in children.
In children, two forms of HUS exist:
Diarrhea-associated HUS is associated with diarrhea that is commonly bloody, due to an enterotoxin produced by Escherichia coli O157:H7.
Endemic diarrhea-associated HUS is much more common than HUS associated with epidemics. Endemic cases are caused by E coli O157:H7 present in the environment. Other patients present with clinically apparent HUS but the causal bacterium cannot be detected. The kidney transplant program at our center often sees young patients with this disease who do not have E coli O157:H7 infection, and the pathogenesis is not understood. Epidemic cases are less common but the outbreaks are dramatic. About 10 years ago, E coli O157:H7 entered the water supply in the small city of Walkerton, Ontario, and many people developed the epidemic form of HUS over a period of several weeks. Most such patients spontaneously recovered without plasma exchange, although many were left with impaired renal function.
Atypical HUS. Less often, HUS in children is not associated with a prodrome of diarrhea and is referred to as “atypical” HUS. These children often have a more prolonged and complicated course and resemble adults with TTP.
Familial TTP-HUS
Familial TTP-HUS is very rare. It may present with hemolysis and thrombocytopenia in childhood or early adulthood. Many patients present with renal insufficiency, and only careful evaluation reveals hemolysis and thrombocytopenia. The disease typically manifests acutely: a patient may have an upper respiratory tract infection and subsequently develop an episode of TTP-HUS. Episodes tend to recur, and multiple family members may also be affected.
Plasma infusion is an effective treatment, and plasma exchange is usually not required. Since more patients are now surviving well into adulthood, some are being seen to develop antibodies to the ADAMTS13 in the infused plasma, analogous to patients with severe hemophilia developing inhibitors to factor VIII. The disease may progress despite treatment: we have been treating a young woman who has had a series of catastrophic complications and now has chronic renal failure requiring hemodialysis (see discussion below).
Post-transplant microangiopathy
Post-transplant microangiopathy is most likely to develop after solid-organ or stem-cell allograft transplantation. Manifestations resemble those of TTP, but the mechanism is probably quite different. Multiple causes probably exist, depending on the setting.
Post-transplant microangiopathy does not respond to the usual therapies for TTP, although we treat it, like TTP, with corticosteroids, antiplatelet agents, and plasma exchange. Other centers do not use plasma exchange for these patients. Most patients have a poor prognosis, especially those with a transplant other than a kidney.
A spectrum of related syndromes
A number of diseases clinically resemble TTP. Enhanced diagnostic capacity and better molecular biologic techniques are revealing that they often have very different underlying causes and that in some cases they require different treatment.
TOWARD DIAGNOSTIC CRITERIA
Ruutu et al,1 in a consensus conference, used rigorous methods to establish diagnostic criteria for microangiopathy associated with stem cell transplantation:
- More than 4% red blood cell fragments in the peripheral blood. A laboratory report that states that “few fragments” are present is not nearly as useful as one that estimates the quantity; eg, 1% fragments would have very different implications than 6% fragments.
- Thrombocytopenia—a platelet count of less than 50 × 109/L or more than a 50% reduction from previous counts
- Increased LDH concentration
- Reduced hemoglobin concentration or increased transfusion requirement
- Decrease in serum haptoglobin, which, like red blood cell fragments, is a marker of hemolysis rather than of reduced synthesis.
The ADAMTS13 level need not be assessed. Metalloproteinase deficiency need not be proved to diagnose TTP. Although our hospital is a TTP referral center, we do not routinely offer the test. Too often the test results cause confusion: a patient can have a normal level of ADAMTS13 and still have TTP that responds to plasma exchange, and levels can be low in conditions other than TTP.
THE CHALLENGES OF TREATMENT
Plasma exchange is the primary treatment for TTP
Rock et al2 performed a randomized trial in which 102 patients with TTP received either a 1.5-volume plasma exchange daily for 3 days and then 1-volume plasma exchanges as needed to control the disease or plasma infusion. Patients who received plasma exchange had a better initial response, a higher survival rate, and a lower rate of relapse than patients receiving plasma infusion. These findings established plasma exchange as the treatment of choice for TTP.
However, the trial had some inherent problems: patients who had plasma infusions tended to develop renal insufficiency and as a result did not receive as much plasma because they could not tolerate as much volume as those who had plasma exchange. Plasma exchange probably worked better because it could deliver more plasma over a fixed period of time, enabling patients to obtain more of the ADAMTS13 enzyme, rather than because it was an intrinsically better treatment. This interpretation is the basis for our occasional use of twice-daily plasma exchange in critically ill patients.
TTP is different from other autoimmune diseases such as idiopathic thrombocytopenia purpura, in which the primary treatments are immunosuppressive agents. Some evidence exists for treating TTP with immunosuppressive agents, but the primary treatment should be plasma exchange.
Plasma infusion is useful in some cases
Although small case series and our own experience provide evidence for the benefit of treating TTP with high-dose plasma infusions (25 mL/kg/day, or about 1.5 to 2.0 L/day for an average-sized adult), problems will likely arise with volume overload if the patient has any significant renal insufficiency. Dialysis or ultrafiltration may be used to treat volume overload; however, it is difficult to remove the large volumes of fluid required for high-volume plasma infusion.
Plasma infusion should be reserved for two situations:
- If plasma exchange cannot be promptly started
- For patients with very severe or refractory disease, between plasma exchange sessions.
Benefit of cryoprecipitate-poor plasma is uncertain
Fresh frozen plasma is believed to contain nearly physiologic levels of all of the plasma proteins. When plasma is cooled to around 4°C, a precipitate forms that contains a variety of substances, including the higher molecular weight multimers of von Willebrand factor. Because TTP involves excess large multimers, giving plasma in which the high molecular weight multimers have been removed should in theory be better. In many centers, such cryoprecipitate-poor plasma is routinely used to treat TTP.
However, evidence that cryoprecipitate-poor plasma is better is lacking. A large study in Canada evaluating this question was terminated because of a lack of patient accrual, a common fate of clinical trials of rare diseases. A randomized study in 17 patients failed to show an advantage of cryoprecipitate-poor plasma over regular plasma, but the study was too small to draw firm conclusions given large confidence intervals about the point estimate of the treatment effect.
Cryoprecipitate-poor plasma is more expensive than regular plasma and is not as available. We do not routinely use it in our center to initially manage patients with TTP, but we do use it for patients who are refractory to standard treatment.
Scott et al3 measured the concentration of ADAMTS13 in a variety of plasma products and found that there are significant amounts in cryoprecipitate. Although giving cryoprecipitate-poor plasma provides less of the high molecular weight multimers, which is desirable for patients with TTP, it also provides less ADAMTS13, which is not desirable.
Do antiplatelet agents have a role in acute TTP?
Most algorithms for managing acute TTP include the use of aspirin or dipyridamole (Persantine) or both, and there is some evidence in favor of this approach,4 but whether antiplatelet therapy should be used for inpatients with severe thrombocytopenia remains controversial. In our practice, we usually provide antiplatelet therapy even for patients with severe thrombocytopenia because we believe TTP involves platelet-mediated hypercoagulability rather than increased bleeding risk.
Do corticosteroids have a role?
Corticosteroids were widely used to treat TTP even before the disease was discovered to be immune mediated. In our center we routinely use them.
Unfortunately, few data exist on the efficacy of steroid therapy for TTP. As a result, we can only make a weak recommendation for its use: using the American College of Chest Physicians rating system for the strength of clinical evidence, it would receive a 2C recommendation. This is the weakest possible recommendation, being based on widespread use but poor-quality data.
Stopping vs tapering plasma exchange
Whether plasma exchange should be tapered or simply stopped is also controversial and not well studied. Nevertheless, a widespread clinical practice—once the platelet count returns to 200 × 109/L or higher and the patient looks and feels well—is to reduce the plasma exchange sessions to once every 3 days, then to once every 7 days, and then to once every 2 weeks.
In our practice, we taper plasma exchange in this fashion for a minimum of two treatments beyond what we think the patient really needs. As a result, we tend to treat about once every 2 weeks for weeks or even months after the acute illness.
Rituximab may help
Rituximab (Rituxan), a monoclonal antibody against mature B cells, is increasingly being used in treating TTP. Past and present treatments for TTP, including splenectomy, corticosteroids, and plasma exchange are immunomodulatory, so the use of rituximab may be justified. Case reports provided the rationale for a large, multicenter, randomized controlled trial, which is currently under way.5
CONDITIONS THAT ARE NOT TTP
Some conditions may be confused with TTP but are clearly something different:
Patients with isolated thrombocytopenia and normal blood smear findings and no coagulopathy most likely have idiopathic thrombocytopenia purpura or, in the correct clinical circumstance, heparin-induced thrombocytopenia.
A patient with an extremely low platelet count but no fragments or very few fragments with microangiopathic hemolytic anemia may have either drug-associated thrombocytopenia or disseminated intravascular coagulopathy, particularly if there is concomitant coagulopathy.
Many pregnancy-associated microangiopathies resemble TTP, and it may be difficult to differentiate them from TTP; if confusion as to the diagnosis exists, the patient should be treated with plasma exchange, as this therapy may be life-saving.
Many rheumatologic conditions are characterized by an acute illness with nonspecific findings, such as low-grade hemolysis and thrombocytopenia. For example, Wegener granulomatosis can present with evidence of hemolysis, thrombocytopenia, and renal impairment.
Systemic lupus erythematosus can also initially present with an “early-TTP”-like picture. Evidence of glomerulonephritis is not consistent with TTP, and urinary red cell casts makes the diagnosis of lupus more likely. Helmet cell fragments in the peripheral blood smear are supposedly more characteristic of TTP, but their presence is not diagnostic.
Scleroderma renal crisis can present like TTP, but because it is unlikely that a patient with known scleroderma would have a second rare disease, it is best to treat it as scleroderma, which does not require plasma exchange or plasma infusion.
In general, if the diagnosis is uncertain, the safest course is to treat the patient with plasma exchange, then try to establish the diagnosis, because TTP is fatal if not promptly treated. Although plasma exchange is probably overused, it is more innocuous than untreated TTP.
Thrombotic thrombocytopenic purpura (TTP) is one of the few hematologic emergencies. Untreated, most patients die, but prompt and appropriate treatment allows most patients not only to survive but to recover, frequently without long-term sequelae.
TTP is rare. The estimated annual incidence of all TTP syndromes is about 11 cases per million in the general population, and the incidence of severe ADAMTS13 deficiency (see discussion below) is about 2 per million. Therefore, even large medical centers typically see only one or two cases each year. The syndromes are much more common in women, and the incidence among blacks is nine times higher than the incidence among non-blacks. Nevertheless, despite the rarity of this disease, good evidence exists to help guide patient care, thanks to national registries and research organizations, such as the Canadian Apheresis Study Group and the Oklahoma TTP-Hemolytic Uremic Syndrome (HUS) Registry.
This article reviews the physiologic basis of TTP, how to recognize it, and how best to treat it. We also discuss other conditions that clinically resemble TTP but probably have different underlying causes.
A YOUNG WOMAN WITH ARM WEAKNESS
A 24-year-old black woman presents to a community hospital with weakness in her left arm, which began about 30 minutes previously. She has had progressive dyspnea over the last several weeks, but has otherwise been completely well and has had no medical problems in the past other than being obese.
Physical examination reveals weakness in her left arm as well as mild dysarthria, which was not previously noted by the patient or her family. Her laboratory findings:
- White blood cell count 16.7 × 109/L (reference range 4.5–11.0)
- Platelet count 32 × 109/L (150–350)
- Hemoglobin concentration 6.5 g/dL (1.4–17.5)
- Peripheral blood smear: normal white cells, rare platelets, red cells normo-chromic with many fragments
- Lactate dehydrogenase (LDH) concentration 2,300 U/L (100–200).
In view of her symptoms and laboratory values, the physician suspects she may have TTP and refers her to McMaster University Medical Center in Hamilton, Ontario, Canada. Plasma exchange is started immediately; one plasma volume is removed and replaced with fresh frozen plasma. Nevertheless, the patient’s condition deteriorates overnight, she becomes more confused and cannot protect her airway, her LDH concentration rises further, and her hemoglobin concentration falls. She is transferred to the intensive care unit. Her plasma exchange prescription is increased to 1.5 volumes twice daily (although little evidence exists that plasma exchange twice daily is more effective than once daily).
On the third day of her stay, she becomes completely paralyzed on the left side. In addition to her twice-daily plasma exchange procedures, a plasma infusion and corticosteroid therapy are initiated. Her platelet count stabilizes at about 20 × 109/L.
The patient next develops renal insufficiency and requires three acute hemodialysis treatments. (Plasma infusion frequently leads to volume overload in critically ill patients. Some intravascular volume can be removed with plasma exchange; however, significant volume overload with significant renal insufficiency can only be treated with renal replacement therapy.)
The patient undergoes 28 consecutive days of twice-daily plasma exchange and gradually improves, as measured by increasing platelet counts, a gradual fall in the LDH concentration, and stabilization of—and ultimately an increase in—the hemoglobin level. She is weaned off plasma infusions, and then plasma exchange is tapered to once a day and then to alternate days.
She is completely well at the time of discharge 4 weeks after her initial admission, with no residual deficits.
Comment. This case shows that even patients with apparently devastating compromise and neurologic deficits can completely recover with aggressive plasma exchange and other therapies. One child treated at the Hospital for Sick Children, affiliated with the University of Toronto, developed TTP and had 120 consecutive days of plasma exchange: she was unconscious and comatose for much of that time, but she ultimately recovered and is now completely well without residual neurologic deficits.
TTP MAY BE DUE TO ADAMTS13 DEFICIENCY
Twenty-five years ago, little was known about TTP except for its clinical manifestations. Now, it is known to be caused in some patients by an acquired deficiency of a circulating metalloproteinase. In very rare cases a hereditary deficiency of ADAMTS13 causes TTP. In addition, a number of conditions share clinical features with TTP but have other underlying causes.
In acquired TTP, an autoantibody forms against ADAMTS13, a zinc-containing metalloproteinase that is also known as von Willebrand factor-cleaving protease. Normally, von Willebrand factor circulates in plasma as multimers that allow platelets to adhere to vascular surfaces. When von Willebrand factor is initially released from endothelial cells, it exists as large multimers, which are more adhesive for platelets than normal. These large multimers are normally cleaved into smaller units by ADAMTS13. If ADAMTS13 is lacking, the very-high-molecular-weight von Willebrand factor multimers accumulate, causing platelet agglutination and the vascular occlusion that results in the manifestations of TTP.
In 1994, ADAMTS13, the gene of which is on the ninth chromosome, was shown to cleave von Willebrand factor under conditions of high shear stress. In 1996, a congenital homozygous deficiency of ADAMTS13 was found to be associated with platelet microthrombi. Afterwards, some patients with TTP were shown to have low or undetectable levels of ADAMTS13, owing to immunoglobulin G antibodies directed against the enzyme.
TTP AND RELATED SYNDROMES
Clinically, TTP encompasses a number of different but related syndromes, some of which have different physiologic bases.
TTP
TTP is characterized by moderate to severe thrombocytopenia, red cell fragmentation, and elevated LDH levels (due to red cell destruction and also muscle and organ ischemia). The pentad of features classically associated with TTP in the era before effective treatment (thrombocytopenia, fever, renal failure, neurologic deficit, and microangiopathic hemolytic anemia) is rarely seen in countries with advanced medical care: renal insufficiency and neurologic events are end-stage manifestations, and the disease should be recognizable well before these manifestations occur. Otherwise unexplained thrombocytopenia, microangiopathic hemolytic anemia, and an elevated LDH should strongly suggest TTP. TTP is the appropriate designation for adults with these clinical features, even in the presence of renal failure. TTP is uncommon in children.
Most patients present with nonspecific constitutional symptoms, such as weakness, abdominal pain, nausea, and vomiting. Typically, the family physician orders a complete blood cell count and finds that the platelet count and hemoglobin are low. Red cell fragments are noted in the peripheral blood smear. Further testing reveals an elevated LDH concentration.
HUS
HUS was initially described 30 years after TTP in children with acute renal failure in addition to thrombocytopenia and microangiopathic hemolytic anemia. The term “HUS” is currently used primarily to describe the condition in children.
In children, two forms of HUS exist:
Diarrhea-associated HUS is associated with diarrhea that is commonly bloody, due to an enterotoxin produced by Escherichia coli O157:H7.
Endemic diarrhea-associated HUS is much more common than HUS associated with epidemics. Endemic cases are caused by E coli O157:H7 present in the environment. Other patients present with clinically apparent HUS but the causal bacterium cannot be detected. The kidney transplant program at our center often sees young patients with this disease who do not have E coli O157:H7 infection, and the pathogenesis is not understood. Epidemic cases are less common but the outbreaks are dramatic. About 10 years ago, E coli O157:H7 entered the water supply in the small city of Walkerton, Ontario, and many people developed the epidemic form of HUS over a period of several weeks. Most such patients spontaneously recovered without plasma exchange, although many were left with impaired renal function.
Atypical HUS. Less often, HUS in children is not associated with a prodrome of diarrhea and is referred to as “atypical” HUS. These children often have a more prolonged and complicated course and resemble adults with TTP.
Familial TTP-HUS
Familial TTP-HUS is very rare. It may present with hemolysis and thrombocytopenia in childhood or early adulthood. Many patients present with renal insufficiency, and only careful evaluation reveals hemolysis and thrombocytopenia. The disease typically manifests acutely: a patient may have an upper respiratory tract infection and subsequently develop an episode of TTP-HUS. Episodes tend to recur, and multiple family members may also be affected.
Plasma infusion is an effective treatment, and plasma exchange is usually not required. Since more patients are now surviving well into adulthood, some are being seen to develop antibodies to the ADAMTS13 in the infused plasma, analogous to patients with severe hemophilia developing inhibitors to factor VIII. The disease may progress despite treatment: we have been treating a young woman who has had a series of catastrophic complications and now has chronic renal failure requiring hemodialysis (see discussion below).
Post-transplant microangiopathy
Post-transplant microangiopathy is most likely to develop after solid-organ or stem-cell allograft transplantation. Manifestations resemble those of TTP, but the mechanism is probably quite different. Multiple causes probably exist, depending on the setting.
Post-transplant microangiopathy does not respond to the usual therapies for TTP, although we treat it, like TTP, with corticosteroids, antiplatelet agents, and plasma exchange. Other centers do not use plasma exchange for these patients. Most patients have a poor prognosis, especially those with a transplant other than a kidney.
A spectrum of related syndromes
A number of diseases clinically resemble TTP. Enhanced diagnostic capacity and better molecular biologic techniques are revealing that they often have very different underlying causes and that in some cases they require different treatment.
TOWARD DIAGNOSTIC CRITERIA
Ruutu et al,1 in a consensus conference, used rigorous methods to establish diagnostic criteria for microangiopathy associated with stem cell transplantation:
- More than 4% red blood cell fragments in the peripheral blood. A laboratory report that states that “few fragments” are present is not nearly as useful as one that estimates the quantity; eg, 1% fragments would have very different implications than 6% fragments.
- Thrombocytopenia—a platelet count of less than 50 × 109/L or more than a 50% reduction from previous counts
- Increased LDH concentration
- Reduced hemoglobin concentration or increased transfusion requirement
- Decrease in serum haptoglobin, which, like red blood cell fragments, is a marker of hemolysis rather than of reduced synthesis.
The ADAMTS13 level need not be assessed. Metalloproteinase deficiency need not be proved to diagnose TTP. Although our hospital is a TTP referral center, we do not routinely offer the test. Too often the test results cause confusion: a patient can have a normal level of ADAMTS13 and still have TTP that responds to plasma exchange, and levels can be low in conditions other than TTP.
THE CHALLENGES OF TREATMENT
Plasma exchange is the primary treatment for TTP
Rock et al2 performed a randomized trial in which 102 patients with TTP received either a 1.5-volume plasma exchange daily for 3 days and then 1-volume plasma exchanges as needed to control the disease or plasma infusion. Patients who received plasma exchange had a better initial response, a higher survival rate, and a lower rate of relapse than patients receiving plasma infusion. These findings established plasma exchange as the treatment of choice for TTP.
However, the trial had some inherent problems: patients who had plasma infusions tended to develop renal insufficiency and as a result did not receive as much plasma because they could not tolerate as much volume as those who had plasma exchange. Plasma exchange probably worked better because it could deliver more plasma over a fixed period of time, enabling patients to obtain more of the ADAMTS13 enzyme, rather than because it was an intrinsically better treatment. This interpretation is the basis for our occasional use of twice-daily plasma exchange in critically ill patients.
TTP is different from other autoimmune diseases such as idiopathic thrombocytopenia purpura, in which the primary treatments are immunosuppressive agents. Some evidence exists for treating TTP with immunosuppressive agents, but the primary treatment should be plasma exchange.
Plasma infusion is useful in some cases
Although small case series and our own experience provide evidence for the benefit of treating TTP with high-dose plasma infusions (25 mL/kg/day, or about 1.5 to 2.0 L/day for an average-sized adult), problems will likely arise with volume overload if the patient has any significant renal insufficiency. Dialysis or ultrafiltration may be used to treat volume overload; however, it is difficult to remove the large volumes of fluid required for high-volume plasma infusion.
Plasma infusion should be reserved for two situations:
- If plasma exchange cannot be promptly started
- For patients with very severe or refractory disease, between plasma exchange sessions.
Benefit of cryoprecipitate-poor plasma is uncertain
Fresh frozen plasma is believed to contain nearly physiologic levels of all of the plasma proteins. When plasma is cooled to around 4°C, a precipitate forms that contains a variety of substances, including the higher molecular weight multimers of von Willebrand factor. Because TTP involves excess large multimers, giving plasma in which the high molecular weight multimers have been removed should in theory be better. In many centers, such cryoprecipitate-poor plasma is routinely used to treat TTP.
However, evidence that cryoprecipitate-poor plasma is better is lacking. A large study in Canada evaluating this question was terminated because of a lack of patient accrual, a common fate of clinical trials of rare diseases. A randomized study in 17 patients failed to show an advantage of cryoprecipitate-poor plasma over regular plasma, but the study was too small to draw firm conclusions given large confidence intervals about the point estimate of the treatment effect.
Cryoprecipitate-poor plasma is more expensive than regular plasma and is not as available. We do not routinely use it in our center to initially manage patients with TTP, but we do use it for patients who are refractory to standard treatment.
Scott et al3 measured the concentration of ADAMTS13 in a variety of plasma products and found that there are significant amounts in cryoprecipitate. Although giving cryoprecipitate-poor plasma provides less of the high molecular weight multimers, which is desirable for patients with TTP, it also provides less ADAMTS13, which is not desirable.
Do antiplatelet agents have a role in acute TTP?
Most algorithms for managing acute TTP include the use of aspirin or dipyridamole (Persantine) or both, and there is some evidence in favor of this approach,4 but whether antiplatelet therapy should be used for inpatients with severe thrombocytopenia remains controversial. In our practice, we usually provide antiplatelet therapy even for patients with severe thrombocytopenia because we believe TTP involves platelet-mediated hypercoagulability rather than increased bleeding risk.
Do corticosteroids have a role?
Corticosteroids were widely used to treat TTP even before the disease was discovered to be immune mediated. In our center we routinely use them.
Unfortunately, few data exist on the efficacy of steroid therapy for TTP. As a result, we can only make a weak recommendation for its use: using the American College of Chest Physicians rating system for the strength of clinical evidence, it would receive a 2C recommendation. This is the weakest possible recommendation, being based on widespread use but poor-quality data.
Stopping vs tapering plasma exchange
Whether plasma exchange should be tapered or simply stopped is also controversial and not well studied. Nevertheless, a widespread clinical practice—once the platelet count returns to 200 × 109/L or higher and the patient looks and feels well—is to reduce the plasma exchange sessions to once every 3 days, then to once every 7 days, and then to once every 2 weeks.
In our practice, we taper plasma exchange in this fashion for a minimum of two treatments beyond what we think the patient really needs. As a result, we tend to treat about once every 2 weeks for weeks or even months after the acute illness.
Rituximab may help
Rituximab (Rituxan), a monoclonal antibody against mature B cells, is increasingly being used in treating TTP. Past and present treatments for TTP, including splenectomy, corticosteroids, and plasma exchange are immunomodulatory, so the use of rituximab may be justified. Case reports provided the rationale for a large, multicenter, randomized controlled trial, which is currently under way.5
CONDITIONS THAT ARE NOT TTP
Some conditions may be confused with TTP but are clearly something different:
Patients with isolated thrombocytopenia and normal blood smear findings and no coagulopathy most likely have idiopathic thrombocytopenia purpura or, in the correct clinical circumstance, heparin-induced thrombocytopenia.
A patient with an extremely low platelet count but no fragments or very few fragments with microangiopathic hemolytic anemia may have either drug-associated thrombocytopenia or disseminated intravascular coagulopathy, particularly if there is concomitant coagulopathy.
Many pregnancy-associated microangiopathies resemble TTP, and it may be difficult to differentiate them from TTP; if confusion as to the diagnosis exists, the patient should be treated with plasma exchange, as this therapy may be life-saving.
Many rheumatologic conditions are characterized by an acute illness with nonspecific findings, such as low-grade hemolysis and thrombocytopenia. For example, Wegener granulomatosis can present with evidence of hemolysis, thrombocytopenia, and renal impairment.
Systemic lupus erythematosus can also initially present with an “early-TTP”-like picture. Evidence of glomerulonephritis is not consistent with TTP, and urinary red cell casts makes the diagnosis of lupus more likely. Helmet cell fragments in the peripheral blood smear are supposedly more characteristic of TTP, but their presence is not diagnostic.
Scleroderma renal crisis can present like TTP, but because it is unlikely that a patient with known scleroderma would have a second rare disease, it is best to treat it as scleroderma, which does not require plasma exchange or plasma infusion.
In general, if the diagnosis is uncertain, the safest course is to treat the patient with plasma exchange, then try to establish the diagnosis, because TTP is fatal if not promptly treated. Although plasma exchange is probably overused, it is more innocuous than untreated TTP.
- Ruutu T, Barosi G, Benjamin RJ, et al; European Group for Blood and Marrow Transplantation; European LeukemiaNet. Diagnostic criteria for hematopoietic stem cell transplant-associated microangiopathy: results of a consensus process by an International Working Group. Haematologica 2007; 92:95–100.
- Rock GA, Shumak KH, Buskard NA, et al. Comparison of plasma exchange with plasma infusion in the treatment of thrombotic thrombocytopenic purpura. Canadian Apheresis Study Group. N Engl J Med 1991; 325:393–397.
- Scott EA, Puca KE, Pietz BC, Duchateau BK, Friedman KD. Comparison and stability of ADAMTS13 activity in therapeutic plasma products. Transfusion 2007; 47:120–125.
- Bobbio-Pallavicini E, Gugliotta L, Centurioni R, et al. Antiplatelet agents in thrombotic thrombocytopenic purpura (TTP). Results of a randomized multicenter trial by the Italian Cooperative Group for TTP. Haematologica 1997; 82:429–435.
- George JN, Woodson RD, Kiss JE, Kojouri K, Vesely SK. Rituximab therapy for thrombotic thrombocytopenic purpura: a proposed study of the Transfusion Medicine/Hemostasis Clinical Trials Network with a systematic review of rituximab therapy for immune-mediated disorders. J Clin Apher 2006; 21:49–56.
- Ruutu T, Barosi G, Benjamin RJ, et al; European Group for Blood and Marrow Transplantation; European LeukemiaNet. Diagnostic criteria for hematopoietic stem cell transplant-associated microangiopathy: results of a consensus process by an International Working Group. Haematologica 2007; 92:95–100.
- Rock GA, Shumak KH, Buskard NA, et al. Comparison of plasma exchange with plasma infusion in the treatment of thrombotic thrombocytopenic purpura. Canadian Apheresis Study Group. N Engl J Med 1991; 325:393–397.
- Scott EA, Puca KE, Pietz BC, Duchateau BK, Friedman KD. Comparison and stability of ADAMTS13 activity in therapeutic plasma products. Transfusion 2007; 47:120–125.
- Bobbio-Pallavicini E, Gugliotta L, Centurioni R, et al. Antiplatelet agents in thrombotic thrombocytopenic purpura (TTP). Results of a randomized multicenter trial by the Italian Cooperative Group for TTP. Haematologica 1997; 82:429–435.
- George JN, Woodson RD, Kiss JE, Kojouri K, Vesely SK. Rituximab therapy for thrombotic thrombocytopenic purpura: a proposed study of the Transfusion Medicine/Hemostasis Clinical Trials Network with a systematic review of rituximab therapy for immune-mediated disorders. J Clin Apher 2006; 21:49–56.
KEY POINTS
- Strokes and renal insufficiency are end-stage manifestations of TTP; the condition is usually diagnosed before they occur.
- Classic TTP should be rapidly and aggressively treated with plasma exchange. Plasma infusion therapy plays a role for patients who cannot promptly receive plasma exchange or for patients with severe disease between episodes of plasma exchange.
- Antiplatelet therapy may be appropriate along with plasma exchange for patients without severe thrombocytopenia.
- If a renal transplant recipient develops systemic symptoms with TTP-like disease, one should consider modifying or withdrawing the immunosuppressive therapy, although this may result in loss of function and the need for transplant nephrectomy.
Staphylococcus aureus: The new adventures of a legendary pathogen
Staphylococcus aureus is rearing its ugly head in new and interesting ways, both in the hospital and in the community.
Rates of invasive infections with methicillin-resistant S aureus (MRSA) have been increasing both in the hospital and in the community, a trend that has attracted considerable interest in the lay media. Curiously, the most common community-associated MRSA strain, which up to now has been distinct from hospital-associated MRSA strains, is invading our hospitals. Alarmingly, vancomycin (Vancocin), the drug of last resort for MRSA infections for the past 40 years, does not seem to be as effective as it used to be.
This paper summarizes the changing epidemiology of S aureus, particularly the emergence of MRSA outside of the hospital; reviews the difficulties associated with S aureus bacteremia and its treatment in view of; some changes in vancomycin susceptibility; and appraises the old and new treatment options.
MRSA IS ON THE RISE IN THE HOSPITAL
S aureus, a gram-positive, coagulase-positive bacterium, is one of the leading nosocomial bloodstream pathogens, second only to coagulase-negative staphylococci.1 And the incidence of S aureus infections is increasing. MRSA in particular is increasingly causing infections throughout hospitals, including intensive care units. As of 2004, nearly two-thirds of isolates of S aureus from intensive care units were MRSA.2
MRSA infections are worse than methicillin-susceptible S aureus (MSSA) infections in terms of the rates of death and other undesirable outcomes.3 Several factors may be responsible: MRSA infection may be a marker of severity of illness (sicker patients may be more likely to have MRSA), our treatment for MRSA may not be as effective as it is for MSSA, and the organism may be inherently more virulent.
METHICILLIN RESISTANCE IS ALSO ON THE RISE IN THE COMMUNITY
Community-associated MRSA began emerging clinically about 10 years ago. It was first described in a cohort of children with necrotizing pneumonia in Minnesota, but soon other populations at risk began to emerge, such as residents of correctional facilities, men who had sex with men, competitive athletes (eg, fencers, wrestlers, and football players), and Alaskan natives and other native populations. A common factor in all these groups was close proximity of the members to each other. Later, it began to spread beyond these traditional risk groups into the community at large.
Community-associated MRSA strains have a characteristic pattern of antimicrobial susceptibility (see below). In the laboratory, they grow somewhat faster than health-care-associated MRSA strains, but not as fast as MSSA. They have a strong association with skin and soft-tissue infections: when you see a skin or soft-tissue infection, be it in an outpatient or an inpatient, think about MRSA. Their virulence varies, but rapid onset and progression of illness are quite common. Their most common strain in the United States at present is USA 300.
Case 1: A young woman with necrotizing fasciitis
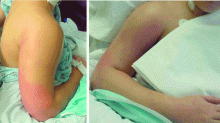
A 21-year-old college student presented to our service in May 2004 with high fever and severe arm pain, which had been worsening for several days. She had been previously healthy, had not had any contact with the health care system, and had not received any antibiotics.
Her blood cultures were positive for MRSA, as were cultures of the deep tissue of the deltoid muscle and fascia when she underwent emergency surgical debridement. The infection required several additional surgical debridements and removal of one head of her deltoid muscle, but she was fortunate: in the past, some patients with this problem might have undergone radical amputation of the arm or even more extensive surgery. This patient continued to have positive blood cultures 4 days postoperatively, but she ultimately recovered, completing 28 days of daptomycin (Cubicin) therapy at a dose of 6 mg/kg every 24 hours. The last 10 days of daptomycin therapy were given at home via a percutaneous intravenous central catheter.
Comment. The epidemiology of MRSA infections is changing. More patients who have no traditional risk factors, specifically health care contact, are getting MRSA infections. A recent report from the US Centers for Disease Control and Prevention (CDC) indicates that the proportion of patients with invasive disease due to MRSA has doubled since 2001–2002.4 Part of the reason undoubtedly is that MRSA, particularly community-associated MRSA, often carries specific virulence factors that make it more invasive. The CDC estimated that in 2005 there were nearly 100,000 cases of invasive MRSA infection in the United States, and nearly a fifth of these infections resulted in death.
Resistance and virulence factors in community-associated MRSA
Most community-associated MRSA strains carry a mobile genetic element called type IV SCCmec (staphylococcal chromosomal cassettemec) that enhances its antimicrobial resistance. This genetic component was probably borrowed from coagulase-negative staphylococci, in which it is quite common but does not cause as much of a problem. It is now present in a wide range of S aureus strains. Most of the S aureus strains that carry type IV SCCmec are MRSA, but a few MSSA strains do carry it as well.
The potent toxin Panton-Valentine leukocidin is an extracellular product that is detected in fewer than 5% of hospital strains but is more common in community-associated strains. It kills leukocytes by forming pores in the cell membrane and causing skin necrosis in cutaneous infections. It is associated with skin abscesses and rapidly progressive necrotizing pneumonia in MSSA or MRSA.
Epidemiologic differences between community- and health-care-associated MRSA
Patients with community-associated MRSA infections tend to be younger than those who traditionally get health-care-associated MRSA infections: in a study from Naimi et al in 2003, the mean ages were 23 vs 68 years.5 A greater proportion of patients with community-associated MRSA strains are nonwhite.4,5
Most community-associated MRSA infections are of the skin and soft tissue (75% in the series from Naimi et al5), but this pathogen causes other infections as well. Bacteremia of unknown origin has been seen, as has necrotizing pneumonia. Most of the skin and soft-tissue infections are relatively superficial, such as folliculitis or furunculosis, but deeper tissue infections such as necrotizing fasciitis and pyomyositis have also been seen.6
The incidence of community-associated MRSA infections varies greatly by geographic region.7 The northeastern United States has so far been relatively spared, but in Atlanta, Houston, and Los Angeles up to 80% of cases of characteristic skin or soft-tissue infections seen in emergency or outpatient departments are due to community-associated MRSA. Physicians at the Texas Children’s Hospital in Houston assume that all skin or soft-tissue infections are due to community-associated MRSA unless proven otherwise.8
Differences in antibiotic susceptibility
Community-associated MRSA is more susceptible to various antibiotics than health-care-associated MRSA,5 but not by much. Strains are usually susceptible to vancomycin, tetracyclines, trimethoprim-sulfamethoxazole (Bactrim, Septra), and rifampin (Rifadin). Unlike hospital strains, a fair number of community-acquired strains are susceptible to clindamycin (Cleocin) in the laboratory, but with a caveat: some of these clindamycin-susceptible strains actually may harbor the tools for inducible resistance. In fact, they can become resistant to clindamycin even without being exposed to it.
The laboratory test for inducible clindamycin resistance is called the D test. After coating an agar plate with S aureus, the technician places erythromycin and clindamycin disks. If the erythromycin induces clindamycin resistance, the plate is clear of growth around the clindamycin disk except for the portion nearest the erythromycin disk, leaving a characteristic D-shaped area of lucency.
Risk factors for MRSA
Moran et al7 analyzed the risk factors for community-associated MRSA in patients with skin or soft-tissue infections seen in the emergency department. The infection was more likely to be due to community-associated MRSA if the patient was black, had used any antibiotic in the past month, had a history of MRSA infection, or had close contact with a person with a similar infection. Many patients interpreted the infections as spider bites because the lesions tended to have a dark center surrounded by a tender area. These infections were not associated with underlying illness. In some cases, community-associated MRSA skin infections have been associated with tattooing and even manicuring.
However, it is very difficult to distinguish between community-associated MRSA and MSSA skin and soft-tissue infections on the basis of clinical and epidemiologic characteristics. Miller et al9 studied a large group of patients in Los Angeles who were hospitalized with community-associated skin and soft-tissue S aureus infections. All the patients were followed up for 30 days after hospital discharge. Regardless of whether they had MRSA or MSSA, they had similar outcomes. Close contacts of the patients also tended to develop infection.
A key point from this and many other studies: patients were more likely to remain infected if they did not undergo incision and drainage. This key intervention is indicated for any patient who has a skin and soft-tissue infection with an undrained focus of infection.
COMMUNITY-ASSOCIATED MRSA IS INVADING THE HOSPITAL
In a new development, community-associated MRSA strains are now appearing in the hospital. This is not only because patients are bacteremic when they come in: patients in the hospital are getting nosocomial infections due to community-associated MRSA strains.
Seybold et al10 analyzed 116 cases of MRSA bloodstream infections in Atlanta, GA. In 9 (8%) of the cases the patient had not had any contact with the health care system within the past year, and these cases were classified as truly community-associated. Of the remaining 107 cases, 49 (42%) were nosocomial, and the USA 300 strain—the predominant community-associated MRSA strain—accounted for 10 (20%) of the nosocomial cases.
In the recent CDC study of invasive MRSA infections, Klevens et al4 reported that nearly a third of cases of bacteremia were due to community-associated MRSA, and these strains accounted for a greater proportion of cases of cellulitis and endocarditis than did health-care-associated strains.
In a study of hospital-associated MRSA, Maree et al11 found that the percentage of cases in which the bacteria carried the SCCmec type IV marker had increased from less than 20% in 1999 to more than 50% in 2004.
Comment. Suffice it to say that we are surrounded by MRSA. Community-associated MRSA is here to stay. It is even invading our hospitals, and we need to consider this very carefully when choosing antimicrobial therapy.
NAGGING QUESTIONS ABOUT VANCOMYCIN
Case 2: Vancomycin-intermediate S aureus (VISA) bacteremia and endocarditis
In December 2006 we saw a very ill 60-year-old woman who was hospitalized with MRSA bacteremia, pacemaker endocarditis, and superior vena cava thrombosis. Although she was treated with vancomycin and rifampin, her condition worsened, she had a stroke, and she developed renal failure. In a difficult operation, the pacemaker was removed, but the bacteremia persisted. In early February 2007 she underwent another difficult operation in which the superior vena cava clot was debrided, a right atrial clot was removed, and her mitral valve was replaced. Less than 2 weeks later, and despite ongoing vancomycin and rifampin therapy, the MRSA bacteremia recurred.
During the approximately 6 weeks that the patient had been receiving these antibiotics, the minimal inhibitory concentration (MIC) of rifampin against the S aureus isolate increased from less than 1 μg/mL (susceptible) to 2 μg/mL (resistant). The MIC of vancomycin went from 2 μg/mL (susceptible) to 4 μg/mL (intermediately susceptible). Vancomycin and rifampin were discontinued, and daptomycin and gentamicin (Garamycin) therapy were started. (Her daptomycin MIC was 0.5 μg/mL). The patient’s condition stabilized, and she was discharged to a long-term nursing facility. She had no relapse of MRSA bacteremia, but she died in early April of that year.
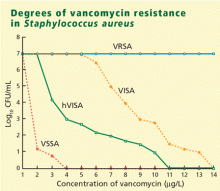
Is vancomycin becoming less effective? Degrees of vancomycin resistance
Vancomycin has been our stalwart for treating MRSA infections for more than 40 years but it is not working as well as it used to, at least in certain situations.
VRSA (vancomycin-resistant S aureus) is rare. These fully resistant strains probably acquired a resistance mechanism (the vanA operon) from vancomycin-resistant enterococci. Infections tend to occur in patients simultaneously infected with both S aureus and vancomycin-resistant enterococci, giving the bacteria an opportunity to exchange genetic material.
VISA (vancomycin-intermediate S aureus) infections tend to occur in patients like the one described above who have had long-term vancomycin therapy. VISA strains appear to overproduce a matrix that captures vancomycin and keeps it from entering the cell. On electron microscopy, these bacteria have a very thick cell wall.13
Vancomycin tolerance is a state in which the bacteria are “stunned” or kept in check but not killed by vancomycin. That is manifested in the laboratory by a ratio of minimum bactericidal concentration to MIC greater than 32.
hVISA (heteroresistant VISA) is new and worrisome. These organisms have an overall MIC in the susceptible range, but within that population are individual isolates with an MIC that is much higher—in the intermediate or perhaps even in the resistant range.14
Reported rates of hVISA vary from less than 2% to as high as 76%, because the methods for detecting it are still very poorly standardized. The usual automated laboratory tests do not detect hVISA.
hVISA is probably clinically relevant, as evidence is emerging both in vitro and in vivo that the higher the MIC for vancomycin, the worse the clinical outcome.15 hVISA has been associated with failures of therapy in several situations, usually in cases of severe invasive or deep infection, endocarditis, and bacteremia with vertebral osteomyelitis where vancomycin concentrations at the site of infection may be suboptimal.16–19 While most hVISA strains that have been described were resistant to methicillin, some were susceptible.
The E test is emerging as the standard test for hVISA. This test uses a plastic strip that contains gradually increasing concentrations of vancomycin along its length. Placed in the culture dish, the strip inhibits growth of the organism at its high-concentration end but not at its low-concentration end. If the sample contains hVISA, the cutoff is not well defined, with a few colonies growing at higher concentrations.
New definition of vancomycin susceptibility
Recognizing that the MICs for vancomycin have been rising in the last few years, the Clinical and Laboratory Standards Institute last year changed the break points between susceptibility and resistance. The new definitions are:
- Susceptible—an MIC of 2.0 μg/mL or less (formerly 4.0 μg/mL or less)
- Intermediate—4.0 to 8.0 μg/mL (formerly 8.0 to 16 μg/mL)
- Resistant—16 μg/mL or greater (formerly 32 μg/mL or greater).
One should pay attention to the MIC numbers on the laboratory reports, not just to the words “susceptible” or “not susceptible.” If the number is, say, 0.5 μg/mL or less, the organism should really be susceptible. If the number is 1 or 2, it is still in the susceptible range, but those are the organisms that may cause problems later on.
Further, even if the vancomycin MIC is in the susceptible range, higher MICs may affect outcomes. The average duration of MRSA bacteremia on therapy is 8 to 9 days, vs 3 to 4 days with MSSA bacteremia.20,21 But Sakoulas et al15 found that, in MRSA bacteremia, the success rate with vancomycin therapy was 56% if the MIC was 0.5 or lower, compared with 10% if the MIC was 1.0 to 2.0 μg/mL. Examined in another way, the success rate was 50% if the logarithm of killing was 6.27 colony-forming units per mL or greater, 23% if 4.71 to 6.26, and zero if less than 4.71.
Case 3: Prolonged MRSA bacteremia
In the summer of 2006, a 66-year-old woman with a history of gastric bypass and cirrhosis underwent a long stay in the surgical intensive care unit because of a recurrent enterocutaneous fistula and chronic renal insufficiency. On November 5th, she had a positive blood culture for MRSA, which was treated appropriately with vancomycin for 4 weeks. She was discharged to subacute care but came back 2 days later, again with MRSA bacteremia. At that time her Hickman catheter, which had been inserted for total parenteral nutrition because of the enterocutaneous fistula, was removed.
Transthoracic echocardiography revealed no vegetations, but her bacteremia persisted. Her mental status was poor this entire time: she was mute and could barely be awakened. We looked for clots and infected clots; duplex ultrasonographic examinations of all four extremities were negative. Finally, magnetic resonance imaging of her back—performed empirically because of the persistent bacteremia—revealed vertebral osteomyelitis at level T12-L1. We also noticed on serial evaluations that the vancomycin MIC for her organism increased from 0.5 to 2.0 μg/mL, so therapy was changed from vancomycin to daptomycin.
Her bacteremia cleared. Follow-up echocardiography was negative, but she had two subsequent relapses of MRSA bacteremia, one in April 2007 and one before she died in the summer of 2007.
Prolonged bacteremia: Is it vancomycin resistance, or something else?
The MRSA isolates that cause prolonged bacteremia seem to have certain characteristics.22 Higher MICs are probably associated with longer periods of bacteremia. But some genetic components within some strains of S aureus give them a survival advantage. They have less susceptibility to the body’s thrombin-induced platelet microbicidal protein. These isolates are not only associated with prolonged bacteremia: they are also associated with osteomyelitis, deep abscesses, endocarditis, recurrent infection, and increased death rate.22 Clinical laboratories do not test for these genetic components. One wonders whether our patient may have had an isolate with these mutations that gave it a survival advantage.
Do not use vancomycin for MSSA
Avoid using vancomycin for MSSA infections. It has been shown time and time again that MSSA infections do not respond as well to vancomycin as they do to beta-lactam antibiotics, specifically to the semisynthetic penicillins such as oxacillin and nafcillin, and even some of the first-generation cephalosporins. Chang et al23 found that patients with MSSA bacteremia had higher rates of persistent infections, relapse, and bacteriologic failure if they received vancomycin than if they received nafcillin.
Do vancomycin trough levels affect toxicity?
The vancomycin trough levels that we aimed for in the past (5 to 10 μg/mL) were probably too low. Today, we aim for trough levels of 15 to 20 μg/mL, and many physicians are aiming for 20 to 25 μg/mL. Part of the reason is that vancomycin MICs are higher than they used to be: in order to keep the vancomycin level above the MIC for a longer period of time, the vancomycin trough level needs to be higher. In theory, keeping the vancomycin levels above the MIC for longer periods should improve outcomes. Yet Fowler et al22 found that vancomycin trough levels among patients who had persistent MRSA bacteremia were actually higher than trough levels among those in whom the bacteremia resolved, although the difference was not statistically significant.
We measure the vancomycin trough level to make sure it is high enough (and give larger doses if it is not); among adults, peak levels need not be monitored on a routine basis because of the predictable pharmacokinetics of vancomycin.
Vancomycin toxicity can be either idiosyncratic or synergistic. Idiosyncratic toxicity occurs when a patient who has been on vancomycin for a long time develops a fixed rash, not associated with infusion. This is an immunologic phenomenon. It is a rare and very serious situation and may require steroid therapy.
Synergistic toxicity occurs when vancomycin is given with other nephrotoxic agents, notably gentamicin. Vancomycin plus gentamicin equals nephrotoxicity. Vancomycin alone is usually not nephrotoxic, but close monitoring of renal function parameters is warranted with the use of higher doses.24
IN UNEXPLAINED BACTEREMIA, LOOK FOR ENDOCARDITIS
In blood cultures from patients with bacteremia, S aureus is never a contaminant. Even if just one blood culture is positive for S aureus, believe that S aureus is the culprit.
Reports in the 1950s suggested that at least half of patients who had S aureus bacteremia had endocarditis,25 leading to recommendations that all patients with S aureus bacteremia without an obvious primary source of infection should be evaluated for endocarditis. Subsequent estimates were lower, in the range of 15% to 25%.26,27 However, throughout the world S aureus endocarditis continues to have a very high mortality rate: at least a third of patients die.28
Clinical criteria (community acquisition, no primary focus, and metastatic sequelae) were developed to try to predict the risk of endocarditis in bacteremic patients.26 However, these criteria did not work very well. The clinical definition of endocarditis has evolved. The criteria of von Reyn et al29 from 1981 did not use echocardiography as part of the definition, but the 1994 Duke criteria,30 which were refined31 in 2000, use both clinical and echocardiographic parameters.
Stratton et al32 performed transthoracic echocardiography in 14 patients with bacteremia and found 1 patient with cryptic tricuspid infective endocarditis. Bayer et al33 subsequently reported that of 72 patients with bacteremia, 6 (18%) of those who had no clinical findings suggestive of infectious endocarditis had findings on echocardiography that led to changes in their regimen. Adding echocardiography to three clinical risk factors increased the sensitivity of diagnosing endocarditis from 70% to 85% with a specificity of 100% and predictive value of 96%.
The Duke criteria call for transesophageal echocardiography, which is not feasible in some patients, eg, those with cirrhosis and esophageal varices.
S aureus endocarditis has changed over the years as our patient population has changed, and MRSA endocarditis tends to hit some of our most vulnerable patients. In a study by Miro et al34 in 2005, MRSA was the leading pathogen in patients who were diagnosed with S aureus endocarditis in 1990 or later. We will only see these numbers go up. Patients with diabetes tend to have more MRSA, and of diabetic patients with MRSA endocarditis, 30% to 40% die in the hospital.
Indications for surgery
Certain conditions are indications for surgery among patients with endocarditis, and no antibiotic will cure the endocarditis if the patient has one of these conditions, eg:
- Persistent bacteremia during antibiotic therapy
- Recurrent emboli
- Heart failure that cannot be controlled
- Perivalvular or myocardial abscesses
- Large vegetations
- Early prosthetic valve infection
- Certain arrhythmias.
How long should S aureus bacteremia be treated?
In cases of bacteremia in which endocarditis has been ruled out and removable foci of infection (eg, intravascular catheters) have been removed, some evidence indicates that treatment for 2 weeks would be as effective as the 4 to 6 weeks that we would use for endocarditis or other severe or invasive infections.35 The issue is controversial. If the patient has had frequent hospitalizations or a chronic medical condition I would hesitate to treat for less than 4 weeks, even if the infection appears to be associated with a removable focus.
Treatment of endocarditis
In the guidelines for treatment of endocarditis from the American Heart Association and Infectious Diseases Society of America,36 all the recommendations are relatively old and many of them are somewhat empiric—they are not based on evidence from randomized clinical trials. Rather, they are best opinions based on clinical experience and some observational studies over the years.
For MSSA. In cases of native-valve endocarditis, oxacillin (Bactocill), nafcillin (Unipen), or another semisynthetic beta-lactam antibiotic is recommended. For penicillin-allergic patients, we have other options, such as cefazolin (Ancef, Kefzol).
Combination therapy is frequently recommended for native valve endocarditis as well as for prosthetic valve endocarditis, with either rifampin or gentamicin along with a primary agent. There is some evidence that one can clear staphylococcal bacteremia a day or two more quickly by use of combination therapy with nafcillin plus an aminoglycoside than with nafcillin alone.37,38 For MSSA-associated endocarditis, vancomycin does not work as well as beta-lactam antibiotics.39,40
Korzeniowski and Sande37 and Chambers et al38 reported that the mean duration of bacteremia was 3.4 days for patients treated with nafcillin alone and 2.9 days for those treated with nafcillin plus an aminoglycoside. These studies led to consideration of a short course of gentamicin to clear the bacteremia quickly.
With MRSA, bacteremia often requires a week or more to clear. Levine et al21 reported a study in 42 patients, mostly injection-drug users, with right-sided native-valve endocarditis. The median duration of bacteremia was 7 days in patients who received vancomycin alone vs 9 days in those who received vancomycin plus rifampin; however, some patients were bacteremic for up to 27 days. Fever persisted for a median of 7 days, probably partly due to septic pulmonary emboli. Three patients died, and three required valve replacement.
NEW ANTIBIOTICS
Several new antibiotics are active against gram-positive cocci.41–44 However, the majority of them have not been prospectively studied for treating bacteremia or endocarditis.
Quinupristin/dalfopristin (Synercid) has not been formally studied for treatment of MRSA bacteremia or endocarditis. There are a few case reports of its use in these conditions.45 Quinupristin/dalfopristin is bacteriostatic, and its use may be associated with phlebitis, myalgias, and arthralgias.46
Linezolid (Zyvox) is approved for treatment of complicated skin and soft-tissue infections and for hospital-acquired pneumonia. There have been no specific studies of linezolid in the treatment of S aureus bacteremia or endocarditis. However, Shorr et al47 retrospectively looked at the bacteremic patients in five previous studies of linezolid vs vancomycin and found 144 cases of S aureus bacteremia, half of which were due to MRSA. Of 53 assessable patients with MRSA bacteremia, the primary infection was cured in 14 (56%) of the linezolid patients and 13 (46%) of the vancomycin patients.
The oral form is 100% bioavailable. One should avoid concomitant use of serotonin-reuptake inhibitors because of the risk of serotonin syndrome. Adverse effects include altered taste sensation and peripheral neuropathy. There are other potential toxicities, including hematologic changes (thrombocytopenia, leukopenia) and metabolic effects (lactic acidosis), so clinical and laboratory monitoring is important.48 The role of linezolid in the treatment of patients with S aureus bacteremia or endocarditis remains to be defined.
Daptomycin is indicated for complicated skin and soft-tissue infections, bacteremia, and right-sided endocarditis due to S aureus. Fowler et al20 found that daptomycin was not inferior to beta-lactam antibiotics for treatment of MSSA bacteremia and right-sided endocarditis, and for MRSA infections it outperformed vancomycin, but the difference was not statistically significant.
The dosing interval should be increased from once every 24 hours to every 48 hours if the creatinine clearance is 30 mL/minute or less. Adverse effects include myalgia, rhabdomyolysis (rare), and elevations in creatine phosphokinase. Reports of rising MICs during daptomycin therapy, in some cases associated with persistent infection,49 suggest that careful attention be paid to dosing and clinical monitoring.
Tigecycline (Tygacil) is indicated for complicated skin and soft-tissue infections and complicated intra-abdominal infections due to susceptible organisms. It is active against both MSSA and MRSA, but clinical experience with its use in invasive infections is somewhat limited.50 The dose of tigecycline should be reduced in advanced cirrhosis. Adverse effects include nausea and vomiting.
Telavancin, dalbavancin, and oritavancin, investigational parenteral antibiotics that are derivatives of vancomycin, are in clinical trials. The pharmacokinetic activity of these agents is of interest: telavancin is being studied with a once-daily dosing interval and dalbavancin’s half-life allows once-weekly dosing. In a limited trial, dalbavancin was found to be safe and effective in the treatment of catheter-related bloodstream infections.51 None of the antibiotics in this group has been studied for treatment of S aureus endocarditis. Telavancin therapy has been associated with rash, hypokalemia, QT prolongation, and creatinine elevations. Gastrointestinal symptoms have been reported with the use of dalbavancin.
Ceftobiprole, another investigational agent, is the only cephalosporin antibiotic that is active against MRSA. It is given every 12 hours. Adverse effects include nausea and taste disturbance.
Iclaprim is a novel diaminopyrimidine and a dihydrofolate reductase inhibitor. In vitro, it is active against gram-positive bacteria, including MRSA, VISA, and VRSA; clinical investigations at this point are limited to the treatment of skin and soft-tissue infections.
- Wisplinghoff H, Bischoff T, Tallent SM, Seifert H, Wenzel RP, Edmond MB. Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin Infect Dis 2004; 39:309–371. Erratum in: Clin Infect Dis 2004; 39:1093.
- US Centers for Disease Control and Prevention. National Nosocomial Infections Surveillance (NNIS) System. Campaign to prevent antimicrobial resistance. www.cdc.gov/drugresistance/healthcare/ha/HASlideSet.ppt.
- Blot SI, Vandewoude KH, Hoste EA, Colardyn FA. Outcome and attributable mortality in critically ill patients with bacteremia involving methicillin-susceptible and methicillin-resistant Staphylococcus aureus. Arch Intern Med 2002; 162:2229–2235.
- Klevens RM, Morrison MA, Nadle J, et al; Active Bacterial Core surveillance (ABCs) MRSA Investigators. Invasive methicillin-resistant Staphylococcus aureus infections in the United States. JAMA 2007; 298:1763–1771.
- Naimi TS, LeDell KH, Como-Sabetti K, et al. Comparison of community- and health care-associated methicillin-resistant Staphylococcus aureus infection. JAMA 2003; 290:2976–2984.
- Miller LG, Perdreau-Remington F, Rieg G, et al. Necrotizing fasciitis caused by community-associated methicillin-resistant Staphylococcus aureus in Los Angeles. N Engl J Med 2005; 352:1445–1453.
- Moran GJ, Krishnadasan A, Gorwitz RJ, et al EMERGEncy ID Net Study Group. Methicillin-resistant S. aureus infections among patients in the emergency department. N Engl J Med 2006; 355:666–674.
- Mishaan AM, Mason EO, Martinez-Aquilar G, et al. Emergence of a predominant clone of community-acquired Staphylococcus aureus among children in Houston, Texas. Pediatr Infect Dis J 2005; 24:201–206.
- Miller LG, Perdreau-Remington F, Bayer AS, et al. Clinical and epidemiologic characteristics cannot distinguish community-associated methicillin-resistant Staphylococcus aureus infection from methicillin-susceptible S. aureus infection: a prospective investigation. Clin Infect Dis 2007; 44:471–482.
- Seybold U, Kourbatova EV, Johnson JG, et al. Emergence of community-associated methicillin-resistant Staphylococcus aureus USA300 genotype as a major cause of health care-associated blood stream infections. Clin Infect Dis 2006; 42:647–656.
- Maree CL, Daum RS, Boyle-Vavra S, Matayoshi K, Miller LG. Community-associated methicillin-resistant Staphylococcus aureus isolates causing healthcare-associated infections. Emerg Infect Dis 2007; 13:236–242.
- Liu C, Chambers HF. Staphylococcus aureus with heterogeneous resistance to vancomycin: epidemiology, clinical significance, and critical assessment of diagnostic methods. Antimicrob Agents Chemother 2003; 47:3040–3045.
- Sieradzki K, Roberts RB, Haber SW, Tomasz A. The development of vancomycin resistance in a patient with methicillin-resistant Staphylococcus aureus infection. N Engl J Med 1999; 340:517–523.
- Schwaber MJ, Wright SB, Carmeli Y, et al. Clinical implications of varying degrees of vancomycin susceptibility in methicillin-resistant Staphylococcus aureus bacteremia. Emerg Infect Dis 2003; 9:657–664. Erratum in: Emerg Infect Dis 2004; 10:160.
- Sakoulas G, Moise-Broder PA, Schentag J, Forrest A, Moellering RC, Eliopoulos GM. Relationship of MIC and bactericidal activity to efficacy of vancomycin for treatment of methicillin-resistant Staphylococcus aureus bacteremia. J Clin Microbiol 2004; 42:2398–2402.
- Naimi TS, Anderson D, O’Boyle C, et al. Vancomycin-intermediate Staphylococcus aureus with phenotypic susceptibility to methicillin in a patient with recurrent bacteremia. Clin Infect Dis 2003; 36:1609–1612.
- Moore MR, Perdreau-Remington F, Chambers HF. Vancomycin treatment failure associated with heterogeneous vancomycin-intermediate Staphylococcus aureus in a patient with endocarditis and in the rabbit model of endocarditis. Antimicrob Agents Chemother 2003; 47:1262–1266.
- Charles PG, Ward PB, Johnson PD, Howden BP, Grayson ML. Clinical features associated with bacteremia due to heterogenous vancomycin-intermediate Staphylococcus aureus. Clin Infect Dis 2004; 38:448–451.
- Howden BP, Ward PB, Charles PG, et al. Treatment outcomes for serious infections caused by methicillin-resistant Staphylococcus aureus with reduced vancomycin susceptibility. Clin Infect Dis 2004; 38:521–528.
- Fowler VG, Boucher HW, Corey GR, et al. S. aureus Endocarditis and Bacteremia Study Group. Daptomycin versus standard therapy for bacteremia and endocarditis caused by Staphylococcus aureus. N Engl J Med 2006; 355:653–665.
- Levine DP, Fromm BS, Reddy BR. Slow response to vancomycin or vancomycin plus rifampin in methicillin-resistant Staphylococcus aureus endocarditis. Ann Intern Med 1991; 115:674–680.
- Fowler VG, Sakoulas G, McIntyre LM, et al. Persistent bacteremia due to methicillin-resistant Staphylococcus aureus infection is associated with agr dysfunction and low-level in vitro resistance to thrombin-induced platelet microbicidal protein. J Infect Dis 2004; 190:1140–1149.
- Chang FY, Peacock JE, Musher DM, et al. Staphylococcus aureus bacteremia: recurrence and the impact of antibiotic treatment in a prospective multicenter study. Medicine (Baltimore) 2003; 82:333–339.
- Hidayat LK, Hsu DI, Quist R, Shriner KA, Wong-Beringer A. High-dose vancomycin therapy for methicillin-resistant Staphylococcus aureus infections: efficacy and toxicity. Arch Intern Med 2006; 166:2138–2144.
- Wilson R, Hamburger M. Fifteen years’ experience with staphylococcus septicemia in a large city hospital; analysis of fifty-five cases in the Cincinnati General Hospital 1940 to 1954. Am J Med 1957; 22:437–457.
- Nolan CM, Beaty HN. Staphylococcus aureus bacteremia. Current clinical patterns. Am J Med 1976; 60:495–500.
- Shah M, Watanakunakorn C. Changing patterns of Staphylococcus aureus bacteremia. Am J Med Sci 1979; 278:115–121.
- Fowler VG, Miro JM, Hoen B, et al ICE Investigators. Staphylococcus aureus endocarditis: a consequence of medical progress. JAMA 2005; 293:3012–3021. Erratum in: JAMA 2005; 294:900.
- Von Reyn CF, Levy BS, Arbeit RD, Friedland G, Crumpacker CS. Infective endocarditis: an analysis based on strict case definition. Ann Intern Med 1981; 94:505–518.
- Durack DT, Lukes AS, Bright DK. New criteria for diagnosis of infective endocarditis: utilization of specific echocardiographic findings. Duke Endocarditis Service. Am J Med 1994; 96:200–209.
- Li JS, Sexton DJ, Mick N, et al. Proposed modifications to the Duke criteria for the diagnosis of infective endocarditis. Clin Infect Dis 2000; 30:633–638.
- Stratton JR, Werner JA, Pearlman AS, Janko CL, Kliman S, Jackson MC. Bacteremia and the heart. Serial echocardiographic findings in 80 patients with documented or suspected bacteremia. Am J Med 1982; 73:851–858.
- Bayer AS, Lam K, Ginzton L, Normal DC, Chiu CY, Ward JI. Staphylococcus aureus bacteremia. Clinical, serologic, and echocardiographic findings in patients with and without endocarditis. Arch Intern Med 1987; 147:457–462.
- Miro JM, Anguera I, Cabell CH, et al International Collaboration on Endocarditis Merged Database Study Group. Staphylococcus aureus native valve infective endocarditis: report of 566 episodes from the International Collaboration on Endocarditis Merged Database. Clin Infect Dis 2005; 41:507–514. Erratum in: Clin Infect Dis 2005; 41:1075–1077.
- Jernigan JA, Farr BM. Short-course therapy of catheter-related Staphylococcus aureus bacteremia: a meta-analysis. Ann Intern Med 1993; 119:304–311.
- Baddour LM, Wilson WR, Bayer AS, et al. Infective endocarditis: diagnosis, antimicrobial therapy, and management of complications: a statement for healthcare professionals from the Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease, Council on Cardiovascular Disease in the Young, and the Councils on Clinical Cardiology, Stroke, and Cardiovascular Surgery and Anesthesia, American Heart Association: endorsed by the Infectious Diseases Society of America. Circulation 2005; 111:e394–e434. Erratum in: Circulation 2005; 112:2373. Circulation 2007; 115:e408.
- Korzeniowski O, Sande MA. Combination antimicrobial therapy for Staphylococcus aureus endocarditis in patients addicted to parenteral drugs and in nonaddicts: a prospective study. Ann Intern Med 1982; 97:496–503.
- Chambers HF, Korzeniowski OM, Sande MA. Staphylococcus aureus endocarditis: clinical manifestations in addicts and nonaddicts. Medicine (Baltimore) 1983; 62:170–177.
- Gentry CA, Rodvold KA, Novak RM, Hershow RC, Naderer OJ. Retrospective evaluation of therapies for Staphylococcus aureus endocarditis. Pharmacotherapy 1997; 17:990–997.
- Small PM, Chambers HF. Vancomycin for Staphylococcus aureus endocarditis in intravenous drug users. Antimicrob Agents Chemother 1990; 34:1227–1231.
- Eliopoulos GM. Quinupristin-dalfopristin and linezolid: evidence and opinion. Clin Infect Dis 2003; 36:473–481.
- Rybak MJ. Therapeutic options for Gram-positive infections. J Hosp Infect 2001; 49 suppl A:S25–S32.
- Micek ST. Alternatives to vancomycin for the treatment of methicillin-resistant Staphylococcus aureus infections. Clin Infect Dis 2007; 45 suppl 3:S184–S190.
- Appelbaum PC, Jacobs MR. Recently approved and investigational antibiotics for treatment of severe infections caused by Gram-positive bacteria. Curr Opin Microbiol 2005; 8:510–517.
- Drew RH, Perfect JR, Srinath L, Kirkimilis E, Dowzicky M, Talbot GH for the Synercid Emergency-Use Study Group. Treatment of methicillin-resistant Staphylococcus aureus infections with quinupristin-dalfopristin in patients intolerant of or failing prior therapy. J Antimicrob Chemother 2000; 46:775–784.
- Lamb HM, Figgitt DP, Faulds D. Quinupristin/dalfopristin: a review of its use in the management of serious gram-positive infections. Drugs 1999; 58:1061–1097.
- Shorr AF, Kunkel MJ, Kollef M. Linezolid versus vancomycin for Staphylococcus aureus bacteraemia: pooled analysis of randomized studies. J Antimicrob Chemother 2005; 56:923–929.
- Bishop E, Melvani S, Howden BP, Charles PG, Grayson ML. Good clinical outcomes but high rates of adverse reactions during linezolid therapy for serious infections: a proposed protocol for monitoring therapy in complex patients. Antimicrob Agents Chemother 2006; 50:1599–1602.
- Boucher HW, Sakoulas G. Perspectives on daptomycin resistance, with emphasis on resistance in Staphylococcus aureus. Clin Infect Dis 2007; 45:601–608.
- Munoz-Price LS, Lolans K, Quinn JP. Four cases of invasive methicillin-resistant Staphylococcus aureus (MRSA) infections treated with tigecycline. Scand J Infect Dis 2006; 38:1081–1084.
- Raad I, Darouiche R, Vazquez J, et al. Efficacy and safety of weekly dalbavancin therapy for catheter-related bloodstream infection caused by gram-positive pathogens. Clin Infect Dis 2005; 40:374–80.
Staphylococcus aureus is rearing its ugly head in new and interesting ways, both in the hospital and in the community.
Rates of invasive infections with methicillin-resistant S aureus (MRSA) have been increasing both in the hospital and in the community, a trend that has attracted considerable interest in the lay media. Curiously, the most common community-associated MRSA strain, which up to now has been distinct from hospital-associated MRSA strains, is invading our hospitals. Alarmingly, vancomycin (Vancocin), the drug of last resort for MRSA infections for the past 40 years, does not seem to be as effective as it used to be.
This paper summarizes the changing epidemiology of S aureus, particularly the emergence of MRSA outside of the hospital; reviews the difficulties associated with S aureus bacteremia and its treatment in view of; some changes in vancomycin susceptibility; and appraises the old and new treatment options.
MRSA IS ON THE RISE IN THE HOSPITAL
S aureus, a gram-positive, coagulase-positive bacterium, is one of the leading nosocomial bloodstream pathogens, second only to coagulase-negative staphylococci.1 And the incidence of S aureus infections is increasing. MRSA in particular is increasingly causing infections throughout hospitals, including intensive care units. As of 2004, nearly two-thirds of isolates of S aureus from intensive care units were MRSA.2
MRSA infections are worse than methicillin-susceptible S aureus (MSSA) infections in terms of the rates of death and other undesirable outcomes.3 Several factors may be responsible: MRSA infection may be a marker of severity of illness (sicker patients may be more likely to have MRSA), our treatment for MRSA may not be as effective as it is for MSSA, and the organism may be inherently more virulent.
METHICILLIN RESISTANCE IS ALSO ON THE RISE IN THE COMMUNITY
Community-associated MRSA began emerging clinically about 10 years ago. It was first described in a cohort of children with necrotizing pneumonia in Minnesota, but soon other populations at risk began to emerge, such as residents of correctional facilities, men who had sex with men, competitive athletes (eg, fencers, wrestlers, and football players), and Alaskan natives and other native populations. A common factor in all these groups was close proximity of the members to each other. Later, it began to spread beyond these traditional risk groups into the community at large.
Community-associated MRSA strains have a characteristic pattern of antimicrobial susceptibility (see below). In the laboratory, they grow somewhat faster than health-care-associated MRSA strains, but not as fast as MSSA. They have a strong association with skin and soft-tissue infections: when you see a skin or soft-tissue infection, be it in an outpatient or an inpatient, think about MRSA. Their virulence varies, but rapid onset and progression of illness are quite common. Their most common strain in the United States at present is USA 300.
Case 1: A young woman with necrotizing fasciitis
A 21-year-old college student presented to our service in May 2004 with high fever and severe arm pain, which had been worsening for several days. She had been previously healthy, had not had any contact with the health care system, and had not received any antibiotics.
Her blood cultures were positive for MRSA, as were cultures of the deep tissue of the deltoid muscle and fascia when she underwent emergency surgical debridement. The infection required several additional surgical debridements and removal of one head of her deltoid muscle, but she was fortunate: in the past, some patients with this problem might have undergone radical amputation of the arm or even more extensive surgery. This patient continued to have positive blood cultures 4 days postoperatively, but she ultimately recovered, completing 28 days of daptomycin (Cubicin) therapy at a dose of 6 mg/kg every 24 hours. The last 10 days of daptomycin therapy were given at home via a percutaneous intravenous central catheter.
Comment. The epidemiology of MRSA infections is changing. More patients who have no traditional risk factors, specifically health care contact, are getting MRSA infections. A recent report from the US Centers for Disease Control and Prevention (CDC) indicates that the proportion of patients with invasive disease due to MRSA has doubled since 2001–2002.4 Part of the reason undoubtedly is that MRSA, particularly community-associated MRSA, often carries specific virulence factors that make it more invasive. The CDC estimated that in 2005 there were nearly 100,000 cases of invasive MRSA infection in the United States, and nearly a fifth of these infections resulted in death.
Resistance and virulence factors in community-associated MRSA
Most community-associated MRSA strains carry a mobile genetic element called type IV SCCmec (staphylococcal chromosomal cassettemec) that enhances its antimicrobial resistance. This genetic component was probably borrowed from coagulase-negative staphylococci, in which it is quite common but does not cause as much of a problem. It is now present in a wide range of S aureus strains. Most of the S aureus strains that carry type IV SCCmec are MRSA, but a few MSSA strains do carry it as well.
The potent toxin Panton-Valentine leukocidin is an extracellular product that is detected in fewer than 5% of hospital strains but is more common in community-associated strains. It kills leukocytes by forming pores in the cell membrane and causing skin necrosis in cutaneous infections. It is associated with skin abscesses and rapidly progressive necrotizing pneumonia in MSSA or MRSA.
Epidemiologic differences between community- and health-care-associated MRSA
Patients with community-associated MRSA infections tend to be younger than those who traditionally get health-care-associated MRSA infections: in a study from Naimi et al in 2003, the mean ages were 23 vs 68 years.5 A greater proportion of patients with community-associated MRSA strains are nonwhite.4,5
Most community-associated MRSA infections are of the skin and soft tissue (75% in the series from Naimi et al5), but this pathogen causes other infections as well. Bacteremia of unknown origin has been seen, as has necrotizing pneumonia. Most of the skin and soft-tissue infections are relatively superficial, such as folliculitis or furunculosis, but deeper tissue infections such as necrotizing fasciitis and pyomyositis have also been seen.6
The incidence of community-associated MRSA infections varies greatly by geographic region.7 The northeastern United States has so far been relatively spared, but in Atlanta, Houston, and Los Angeles up to 80% of cases of characteristic skin or soft-tissue infections seen in emergency or outpatient departments are due to community-associated MRSA. Physicians at the Texas Children’s Hospital in Houston assume that all skin or soft-tissue infections are due to community-associated MRSA unless proven otherwise.8
Differences in antibiotic susceptibility
Community-associated MRSA is more susceptible to various antibiotics than health-care-associated MRSA,5 but not by much. Strains are usually susceptible to vancomycin, tetracyclines, trimethoprim-sulfamethoxazole (Bactrim, Septra), and rifampin (Rifadin). Unlike hospital strains, a fair number of community-acquired strains are susceptible to clindamycin (Cleocin) in the laboratory, but with a caveat: some of these clindamycin-susceptible strains actually may harbor the tools for inducible resistance. In fact, they can become resistant to clindamycin even without being exposed to it.
The laboratory test for inducible clindamycin resistance is called the D test. After coating an agar plate with S aureus, the technician places erythromycin and clindamycin disks. If the erythromycin induces clindamycin resistance, the plate is clear of growth around the clindamycin disk except for the portion nearest the erythromycin disk, leaving a characteristic D-shaped area of lucency.
Risk factors for MRSA
Moran et al7 analyzed the risk factors for community-associated MRSA in patients with skin or soft-tissue infections seen in the emergency department. The infection was more likely to be due to community-associated MRSA if the patient was black, had used any antibiotic in the past month, had a history of MRSA infection, or had close contact with a person with a similar infection. Many patients interpreted the infections as spider bites because the lesions tended to have a dark center surrounded by a tender area. These infections were not associated with underlying illness. In some cases, community-associated MRSA skin infections have been associated with tattooing and even manicuring.
However, it is very difficult to distinguish between community-associated MRSA and MSSA skin and soft-tissue infections on the basis of clinical and epidemiologic characteristics. Miller et al9 studied a large group of patients in Los Angeles who were hospitalized with community-associated skin and soft-tissue S aureus infections. All the patients were followed up for 30 days after hospital discharge. Regardless of whether they had MRSA or MSSA, they had similar outcomes. Close contacts of the patients also tended to develop infection.
A key point from this and many other studies: patients were more likely to remain infected if they did not undergo incision and drainage. This key intervention is indicated for any patient who has a skin and soft-tissue infection with an undrained focus of infection.
COMMUNITY-ASSOCIATED MRSA IS INVADING THE HOSPITAL
In a new development, community-associated MRSA strains are now appearing in the hospital. This is not only because patients are bacteremic when they come in: patients in the hospital are getting nosocomial infections due to community-associated MRSA strains.
Seybold et al10 analyzed 116 cases of MRSA bloodstream infections in Atlanta, GA. In 9 (8%) of the cases the patient had not had any contact with the health care system within the past year, and these cases were classified as truly community-associated. Of the remaining 107 cases, 49 (42%) were nosocomial, and the USA 300 strain—the predominant community-associated MRSA strain—accounted for 10 (20%) of the nosocomial cases.
In the recent CDC study of invasive MRSA infections, Klevens et al4 reported that nearly a third of cases of bacteremia were due to community-associated MRSA, and these strains accounted for a greater proportion of cases of cellulitis and endocarditis than did health-care-associated strains.
In a study of hospital-associated MRSA, Maree et al11 found that the percentage of cases in which the bacteria carried the SCCmec type IV marker had increased from less than 20% in 1999 to more than 50% in 2004.
Comment. Suffice it to say that we are surrounded by MRSA. Community-associated MRSA is here to stay. It is even invading our hospitals, and we need to consider this very carefully when choosing antimicrobial therapy.
NAGGING QUESTIONS ABOUT VANCOMYCIN
Case 2: Vancomycin-intermediate S aureus (VISA) bacteremia and endocarditis
In December 2006 we saw a very ill 60-year-old woman who was hospitalized with MRSA bacteremia, pacemaker endocarditis, and superior vena cava thrombosis. Although she was treated with vancomycin and rifampin, her condition worsened, she had a stroke, and she developed renal failure. In a difficult operation, the pacemaker was removed, but the bacteremia persisted. In early February 2007 she underwent another difficult operation in which the superior vena cava clot was debrided, a right atrial clot was removed, and her mitral valve was replaced. Less than 2 weeks later, and despite ongoing vancomycin and rifampin therapy, the MRSA bacteremia recurred.
During the approximately 6 weeks that the patient had been receiving these antibiotics, the minimal inhibitory concentration (MIC) of rifampin against the S aureus isolate increased from less than 1 μg/mL (susceptible) to 2 μg/mL (resistant). The MIC of vancomycin went from 2 μg/mL (susceptible) to 4 μg/mL (intermediately susceptible). Vancomycin and rifampin were discontinued, and daptomycin and gentamicin (Garamycin) therapy were started. (Her daptomycin MIC was 0.5 μg/mL). The patient’s condition stabilized, and she was discharged to a long-term nursing facility. She had no relapse of MRSA bacteremia, but she died in early April of that year.
Is vancomycin becoming less effective? Degrees of vancomycin resistance
Vancomycin has been our stalwart for treating MRSA infections for more than 40 years but it is not working as well as it used to, at least in certain situations.
VRSA (vancomycin-resistant S aureus) is rare. These fully resistant strains probably acquired a resistance mechanism (the vanA operon) from vancomycin-resistant enterococci. Infections tend to occur in patients simultaneously infected with both S aureus and vancomycin-resistant enterococci, giving the bacteria an opportunity to exchange genetic material.
VISA (vancomycin-intermediate S aureus) infections tend to occur in patients like the one described above who have had long-term vancomycin therapy. VISA strains appear to overproduce a matrix that captures vancomycin and keeps it from entering the cell. On electron microscopy, these bacteria have a very thick cell wall.13
Vancomycin tolerance is a state in which the bacteria are “stunned” or kept in check but not killed by vancomycin. That is manifested in the laboratory by a ratio of minimum bactericidal concentration to MIC greater than 32.
hVISA (heteroresistant VISA) is new and worrisome. These organisms have an overall MIC in the susceptible range, but within that population are individual isolates with an MIC that is much higher—in the intermediate or perhaps even in the resistant range.14
Reported rates of hVISA vary from less than 2% to as high as 76%, because the methods for detecting it are still very poorly standardized. The usual automated laboratory tests do not detect hVISA.
hVISA is probably clinically relevant, as evidence is emerging both in vitro and in vivo that the higher the MIC for vancomycin, the worse the clinical outcome.15 hVISA has been associated with failures of therapy in several situations, usually in cases of severe invasive or deep infection, endocarditis, and bacteremia with vertebral osteomyelitis where vancomycin concentrations at the site of infection may be suboptimal.16–19 While most hVISA strains that have been described were resistant to methicillin, some were susceptible.
The E test is emerging as the standard test for hVISA. This test uses a plastic strip that contains gradually increasing concentrations of vancomycin along its length. Placed in the culture dish, the strip inhibits growth of the organism at its high-concentration end but not at its low-concentration end. If the sample contains hVISA, the cutoff is not well defined, with a few colonies growing at higher concentrations.
New definition of vancomycin susceptibility
Recognizing that the MICs for vancomycin have been rising in the last few years, the Clinical and Laboratory Standards Institute last year changed the break points between susceptibility and resistance. The new definitions are:
- Susceptible—an MIC of 2.0 μg/mL or less (formerly 4.0 μg/mL or less)
- Intermediate—4.0 to 8.0 μg/mL (formerly 8.0 to 16 μg/mL)
- Resistant—16 μg/mL or greater (formerly 32 μg/mL or greater).
One should pay attention to the MIC numbers on the laboratory reports, not just to the words “susceptible” or “not susceptible.” If the number is, say, 0.5 μg/mL or less, the organism should really be susceptible. If the number is 1 or 2, it is still in the susceptible range, but those are the organisms that may cause problems later on.
Further, even if the vancomycin MIC is in the susceptible range, higher MICs may affect outcomes. The average duration of MRSA bacteremia on therapy is 8 to 9 days, vs 3 to 4 days with MSSA bacteremia.20,21 But Sakoulas et al15 found that, in MRSA bacteremia, the success rate with vancomycin therapy was 56% if the MIC was 0.5 or lower, compared with 10% if the MIC was 1.0 to 2.0 μg/mL. Examined in another way, the success rate was 50% if the logarithm of killing was 6.27 colony-forming units per mL or greater, 23% if 4.71 to 6.26, and zero if less than 4.71.
Case 3: Prolonged MRSA bacteremia
In the summer of 2006, a 66-year-old woman with a history of gastric bypass and cirrhosis underwent a long stay in the surgical intensive care unit because of a recurrent enterocutaneous fistula and chronic renal insufficiency. On November 5th, she had a positive blood culture for MRSA, which was treated appropriately with vancomycin for 4 weeks. She was discharged to subacute care but came back 2 days later, again with MRSA bacteremia. At that time her Hickman catheter, which had been inserted for total parenteral nutrition because of the enterocutaneous fistula, was removed.
Transthoracic echocardiography revealed no vegetations, but her bacteremia persisted. Her mental status was poor this entire time: she was mute and could barely be awakened. We looked for clots and infected clots; duplex ultrasonographic examinations of all four extremities were negative. Finally, magnetic resonance imaging of her back—performed empirically because of the persistent bacteremia—revealed vertebral osteomyelitis at level T12-L1. We also noticed on serial evaluations that the vancomycin MIC for her organism increased from 0.5 to 2.0 μg/mL, so therapy was changed from vancomycin to daptomycin.
Her bacteremia cleared. Follow-up echocardiography was negative, but she had two subsequent relapses of MRSA bacteremia, one in April 2007 and one before she died in the summer of 2007.
Prolonged bacteremia: Is it vancomycin resistance, or something else?
The MRSA isolates that cause prolonged bacteremia seem to have certain characteristics.22 Higher MICs are probably associated with longer periods of bacteremia. But some genetic components within some strains of S aureus give them a survival advantage. They have less susceptibility to the body’s thrombin-induced platelet microbicidal protein. These isolates are not only associated with prolonged bacteremia: they are also associated with osteomyelitis, deep abscesses, endocarditis, recurrent infection, and increased death rate.22 Clinical laboratories do not test for these genetic components. One wonders whether our patient may have had an isolate with these mutations that gave it a survival advantage.
Do not use vancomycin for MSSA
Avoid using vancomycin for MSSA infections. It has been shown time and time again that MSSA infections do not respond as well to vancomycin as they do to beta-lactam antibiotics, specifically to the semisynthetic penicillins such as oxacillin and nafcillin, and even some of the first-generation cephalosporins. Chang et al23 found that patients with MSSA bacteremia had higher rates of persistent infections, relapse, and bacteriologic failure if they received vancomycin than if they received nafcillin.
Do vancomycin trough levels affect toxicity?
The vancomycin trough levels that we aimed for in the past (5 to 10 μg/mL) were probably too low. Today, we aim for trough levels of 15 to 20 μg/mL, and many physicians are aiming for 20 to 25 μg/mL. Part of the reason is that vancomycin MICs are higher than they used to be: in order to keep the vancomycin level above the MIC for a longer period of time, the vancomycin trough level needs to be higher. In theory, keeping the vancomycin levels above the MIC for longer periods should improve outcomes. Yet Fowler et al22 found that vancomycin trough levels among patients who had persistent MRSA bacteremia were actually higher than trough levels among those in whom the bacteremia resolved, although the difference was not statistically significant.
We measure the vancomycin trough level to make sure it is high enough (and give larger doses if it is not); among adults, peak levels need not be monitored on a routine basis because of the predictable pharmacokinetics of vancomycin.
Vancomycin toxicity can be either idiosyncratic or synergistic. Idiosyncratic toxicity occurs when a patient who has been on vancomycin for a long time develops a fixed rash, not associated with infusion. This is an immunologic phenomenon. It is a rare and very serious situation and may require steroid therapy.
Synergistic toxicity occurs when vancomycin is given with other nephrotoxic agents, notably gentamicin. Vancomycin plus gentamicin equals nephrotoxicity. Vancomycin alone is usually not nephrotoxic, but close monitoring of renal function parameters is warranted with the use of higher doses.24
IN UNEXPLAINED BACTEREMIA, LOOK FOR ENDOCARDITIS
In blood cultures from patients with bacteremia, S aureus is never a contaminant. Even if just one blood culture is positive for S aureus, believe that S aureus is the culprit.
Reports in the 1950s suggested that at least half of patients who had S aureus bacteremia had endocarditis,25 leading to recommendations that all patients with S aureus bacteremia without an obvious primary source of infection should be evaluated for endocarditis. Subsequent estimates were lower, in the range of 15% to 25%.26,27 However, throughout the world S aureus endocarditis continues to have a very high mortality rate: at least a third of patients die.28
Clinical criteria (community acquisition, no primary focus, and metastatic sequelae) were developed to try to predict the risk of endocarditis in bacteremic patients.26 However, these criteria did not work very well. The clinical definition of endocarditis has evolved. The criteria of von Reyn et al29 from 1981 did not use echocardiography as part of the definition, but the 1994 Duke criteria,30 which were refined31 in 2000, use both clinical and echocardiographic parameters.
Stratton et al32 performed transthoracic echocardiography in 14 patients with bacteremia and found 1 patient with cryptic tricuspid infective endocarditis. Bayer et al33 subsequently reported that of 72 patients with bacteremia, 6 (18%) of those who had no clinical findings suggestive of infectious endocarditis had findings on echocardiography that led to changes in their regimen. Adding echocardiography to three clinical risk factors increased the sensitivity of diagnosing endocarditis from 70% to 85% with a specificity of 100% and predictive value of 96%.
The Duke criteria call for transesophageal echocardiography, which is not feasible in some patients, eg, those with cirrhosis and esophageal varices.
S aureus endocarditis has changed over the years as our patient population has changed, and MRSA endocarditis tends to hit some of our most vulnerable patients. In a study by Miro et al34 in 2005, MRSA was the leading pathogen in patients who were diagnosed with S aureus endocarditis in 1990 or later. We will only see these numbers go up. Patients with diabetes tend to have more MRSA, and of diabetic patients with MRSA endocarditis, 30% to 40% die in the hospital.
Indications for surgery
Certain conditions are indications for surgery among patients with endocarditis, and no antibiotic will cure the endocarditis if the patient has one of these conditions, eg:
- Persistent bacteremia during antibiotic therapy
- Recurrent emboli
- Heart failure that cannot be controlled
- Perivalvular or myocardial abscesses
- Large vegetations
- Early prosthetic valve infection
- Certain arrhythmias.
How long should S aureus bacteremia be treated?
In cases of bacteremia in which endocarditis has been ruled out and removable foci of infection (eg, intravascular catheters) have been removed, some evidence indicates that treatment for 2 weeks would be as effective as the 4 to 6 weeks that we would use for endocarditis or other severe or invasive infections.35 The issue is controversial. If the patient has had frequent hospitalizations or a chronic medical condition I would hesitate to treat for less than 4 weeks, even if the infection appears to be associated with a removable focus.
Treatment of endocarditis
In the guidelines for treatment of endocarditis from the American Heart Association and Infectious Diseases Society of America,36 all the recommendations are relatively old and many of them are somewhat empiric—they are not based on evidence from randomized clinical trials. Rather, they are best opinions based on clinical experience and some observational studies over the years.
For MSSA. In cases of native-valve endocarditis, oxacillin (Bactocill), nafcillin (Unipen), or another semisynthetic beta-lactam antibiotic is recommended. For penicillin-allergic patients, we have other options, such as cefazolin (Ancef, Kefzol).
Combination therapy is frequently recommended for native valve endocarditis as well as for prosthetic valve endocarditis, with either rifampin or gentamicin along with a primary agent. There is some evidence that one can clear staphylococcal bacteremia a day or two more quickly by use of combination therapy with nafcillin plus an aminoglycoside than with nafcillin alone.37,38 For MSSA-associated endocarditis, vancomycin does not work as well as beta-lactam antibiotics.39,40
Korzeniowski and Sande37 and Chambers et al38 reported that the mean duration of bacteremia was 3.4 days for patients treated with nafcillin alone and 2.9 days for those treated with nafcillin plus an aminoglycoside. These studies led to consideration of a short course of gentamicin to clear the bacteremia quickly.
With MRSA, bacteremia often requires a week or more to clear. Levine et al21 reported a study in 42 patients, mostly injection-drug users, with right-sided native-valve endocarditis. The median duration of bacteremia was 7 days in patients who received vancomycin alone vs 9 days in those who received vancomycin plus rifampin; however, some patients were bacteremic for up to 27 days. Fever persisted for a median of 7 days, probably partly due to septic pulmonary emboli. Three patients died, and three required valve replacement.
NEW ANTIBIOTICS
Several new antibiotics are active against gram-positive cocci.41–44 However, the majority of them have not been prospectively studied for treating bacteremia or endocarditis.
Quinupristin/dalfopristin (Synercid) has not been formally studied for treatment of MRSA bacteremia or endocarditis. There are a few case reports of its use in these conditions.45 Quinupristin/dalfopristin is bacteriostatic, and its use may be associated with phlebitis, myalgias, and arthralgias.46
Linezolid (Zyvox) is approved for treatment of complicated skin and soft-tissue infections and for hospital-acquired pneumonia. There have been no specific studies of linezolid in the treatment of S aureus bacteremia or endocarditis. However, Shorr et al47 retrospectively looked at the bacteremic patients in five previous studies of linezolid vs vancomycin and found 144 cases of S aureus bacteremia, half of which were due to MRSA. Of 53 assessable patients with MRSA bacteremia, the primary infection was cured in 14 (56%) of the linezolid patients and 13 (46%) of the vancomycin patients.
The oral form is 100% bioavailable. One should avoid concomitant use of serotonin-reuptake inhibitors because of the risk of serotonin syndrome. Adverse effects include altered taste sensation and peripheral neuropathy. There are other potential toxicities, including hematologic changes (thrombocytopenia, leukopenia) and metabolic effects (lactic acidosis), so clinical and laboratory monitoring is important.48 The role of linezolid in the treatment of patients with S aureus bacteremia or endocarditis remains to be defined.
Daptomycin is indicated for complicated skin and soft-tissue infections, bacteremia, and right-sided endocarditis due to S aureus. Fowler et al20 found that daptomycin was not inferior to beta-lactam antibiotics for treatment of MSSA bacteremia and right-sided endocarditis, and for MRSA infections it outperformed vancomycin, but the difference was not statistically significant.
The dosing interval should be increased from once every 24 hours to every 48 hours if the creatinine clearance is 30 mL/minute or less. Adverse effects include myalgia, rhabdomyolysis (rare), and elevations in creatine phosphokinase. Reports of rising MICs during daptomycin therapy, in some cases associated with persistent infection,49 suggest that careful attention be paid to dosing and clinical monitoring.
Tigecycline (Tygacil) is indicated for complicated skin and soft-tissue infections and complicated intra-abdominal infections due to susceptible organisms. It is active against both MSSA and MRSA, but clinical experience with its use in invasive infections is somewhat limited.50 The dose of tigecycline should be reduced in advanced cirrhosis. Adverse effects include nausea and vomiting.
Telavancin, dalbavancin, and oritavancin, investigational parenteral antibiotics that are derivatives of vancomycin, are in clinical trials. The pharmacokinetic activity of these agents is of interest: telavancin is being studied with a once-daily dosing interval and dalbavancin’s half-life allows once-weekly dosing. In a limited trial, dalbavancin was found to be safe and effective in the treatment of catheter-related bloodstream infections.51 None of the antibiotics in this group has been studied for treatment of S aureus endocarditis. Telavancin therapy has been associated with rash, hypokalemia, QT prolongation, and creatinine elevations. Gastrointestinal symptoms have been reported with the use of dalbavancin.
Ceftobiprole, another investigational agent, is the only cephalosporin antibiotic that is active against MRSA. It is given every 12 hours. Adverse effects include nausea and taste disturbance.
Iclaprim is a novel diaminopyrimidine and a dihydrofolate reductase inhibitor. In vitro, it is active against gram-positive bacteria, including MRSA, VISA, and VRSA; clinical investigations at this point are limited to the treatment of skin and soft-tissue infections.
Staphylococcus aureus is rearing its ugly head in new and interesting ways, both in the hospital and in the community.
Rates of invasive infections with methicillin-resistant S aureus (MRSA) have been increasing both in the hospital and in the community, a trend that has attracted considerable interest in the lay media. Curiously, the most common community-associated MRSA strain, which up to now has been distinct from hospital-associated MRSA strains, is invading our hospitals. Alarmingly, vancomycin (Vancocin), the drug of last resort for MRSA infections for the past 40 years, does not seem to be as effective as it used to be.
This paper summarizes the changing epidemiology of S aureus, particularly the emergence of MRSA outside of the hospital; reviews the difficulties associated with S aureus bacteremia and its treatment in view of; some changes in vancomycin susceptibility; and appraises the old and new treatment options.
MRSA IS ON THE RISE IN THE HOSPITAL
S aureus, a gram-positive, coagulase-positive bacterium, is one of the leading nosocomial bloodstream pathogens, second only to coagulase-negative staphylococci.1 And the incidence of S aureus infections is increasing. MRSA in particular is increasingly causing infections throughout hospitals, including intensive care units. As of 2004, nearly two-thirds of isolates of S aureus from intensive care units were MRSA.2
MRSA infections are worse than methicillin-susceptible S aureus (MSSA) infections in terms of the rates of death and other undesirable outcomes.3 Several factors may be responsible: MRSA infection may be a marker of severity of illness (sicker patients may be more likely to have MRSA), our treatment for MRSA may not be as effective as it is for MSSA, and the organism may be inherently more virulent.
METHICILLIN RESISTANCE IS ALSO ON THE RISE IN THE COMMUNITY
Community-associated MRSA began emerging clinically about 10 years ago. It was first described in a cohort of children with necrotizing pneumonia in Minnesota, but soon other populations at risk began to emerge, such as residents of correctional facilities, men who had sex with men, competitive athletes (eg, fencers, wrestlers, and football players), and Alaskan natives and other native populations. A common factor in all these groups was close proximity of the members to each other. Later, it began to spread beyond these traditional risk groups into the community at large.
Community-associated MRSA strains have a characteristic pattern of antimicrobial susceptibility (see below). In the laboratory, they grow somewhat faster than health-care-associated MRSA strains, but not as fast as MSSA. They have a strong association with skin and soft-tissue infections: when you see a skin or soft-tissue infection, be it in an outpatient or an inpatient, think about MRSA. Their virulence varies, but rapid onset and progression of illness are quite common. Their most common strain in the United States at present is USA 300.
Case 1: A young woman with necrotizing fasciitis
A 21-year-old college student presented to our service in May 2004 with high fever and severe arm pain, which had been worsening for several days. She had been previously healthy, had not had any contact with the health care system, and had not received any antibiotics.
Her blood cultures were positive for MRSA, as were cultures of the deep tissue of the deltoid muscle and fascia when she underwent emergency surgical debridement. The infection required several additional surgical debridements and removal of one head of her deltoid muscle, but she was fortunate: in the past, some patients with this problem might have undergone radical amputation of the arm or even more extensive surgery. This patient continued to have positive blood cultures 4 days postoperatively, but she ultimately recovered, completing 28 days of daptomycin (Cubicin) therapy at a dose of 6 mg/kg every 24 hours. The last 10 days of daptomycin therapy were given at home via a percutaneous intravenous central catheter.
Comment. The epidemiology of MRSA infections is changing. More patients who have no traditional risk factors, specifically health care contact, are getting MRSA infections. A recent report from the US Centers for Disease Control and Prevention (CDC) indicates that the proportion of patients with invasive disease due to MRSA has doubled since 2001–2002.4 Part of the reason undoubtedly is that MRSA, particularly community-associated MRSA, often carries specific virulence factors that make it more invasive. The CDC estimated that in 2005 there were nearly 100,000 cases of invasive MRSA infection in the United States, and nearly a fifth of these infections resulted in death.
Resistance and virulence factors in community-associated MRSA
Most community-associated MRSA strains carry a mobile genetic element called type IV SCCmec (staphylococcal chromosomal cassettemec) that enhances its antimicrobial resistance. This genetic component was probably borrowed from coagulase-negative staphylococci, in which it is quite common but does not cause as much of a problem. It is now present in a wide range of S aureus strains. Most of the S aureus strains that carry type IV SCCmec are MRSA, but a few MSSA strains do carry it as well.
The potent toxin Panton-Valentine leukocidin is an extracellular product that is detected in fewer than 5% of hospital strains but is more common in community-associated strains. It kills leukocytes by forming pores in the cell membrane and causing skin necrosis in cutaneous infections. It is associated with skin abscesses and rapidly progressive necrotizing pneumonia in MSSA or MRSA.
Epidemiologic differences between community- and health-care-associated MRSA
Patients with community-associated MRSA infections tend to be younger than those who traditionally get health-care-associated MRSA infections: in a study from Naimi et al in 2003, the mean ages were 23 vs 68 years.5 A greater proportion of patients with community-associated MRSA strains are nonwhite.4,5
Most community-associated MRSA infections are of the skin and soft tissue (75% in the series from Naimi et al5), but this pathogen causes other infections as well. Bacteremia of unknown origin has been seen, as has necrotizing pneumonia. Most of the skin and soft-tissue infections are relatively superficial, such as folliculitis or furunculosis, but deeper tissue infections such as necrotizing fasciitis and pyomyositis have also been seen.6
The incidence of community-associated MRSA infections varies greatly by geographic region.7 The northeastern United States has so far been relatively spared, but in Atlanta, Houston, and Los Angeles up to 80% of cases of characteristic skin or soft-tissue infections seen in emergency or outpatient departments are due to community-associated MRSA. Physicians at the Texas Children’s Hospital in Houston assume that all skin or soft-tissue infections are due to community-associated MRSA unless proven otherwise.8
Differences in antibiotic susceptibility
Community-associated MRSA is more susceptible to various antibiotics than health-care-associated MRSA,5 but not by much. Strains are usually susceptible to vancomycin, tetracyclines, trimethoprim-sulfamethoxazole (Bactrim, Septra), and rifampin (Rifadin). Unlike hospital strains, a fair number of community-acquired strains are susceptible to clindamycin (Cleocin) in the laboratory, but with a caveat: some of these clindamycin-susceptible strains actually may harbor the tools for inducible resistance. In fact, they can become resistant to clindamycin even without being exposed to it.
The laboratory test for inducible clindamycin resistance is called the D test. After coating an agar plate with S aureus, the technician places erythromycin and clindamycin disks. If the erythromycin induces clindamycin resistance, the plate is clear of growth around the clindamycin disk except for the portion nearest the erythromycin disk, leaving a characteristic D-shaped area of lucency.
Risk factors for MRSA
Moran et al7 analyzed the risk factors for community-associated MRSA in patients with skin or soft-tissue infections seen in the emergency department. The infection was more likely to be due to community-associated MRSA if the patient was black, had used any antibiotic in the past month, had a history of MRSA infection, or had close contact with a person with a similar infection. Many patients interpreted the infections as spider bites because the lesions tended to have a dark center surrounded by a tender area. These infections were not associated with underlying illness. In some cases, community-associated MRSA skin infections have been associated with tattooing and even manicuring.
However, it is very difficult to distinguish between community-associated MRSA and MSSA skin and soft-tissue infections on the basis of clinical and epidemiologic characteristics. Miller et al9 studied a large group of patients in Los Angeles who were hospitalized with community-associated skin and soft-tissue S aureus infections. All the patients were followed up for 30 days after hospital discharge. Regardless of whether they had MRSA or MSSA, they had similar outcomes. Close contacts of the patients also tended to develop infection.
A key point from this and many other studies: patients were more likely to remain infected if they did not undergo incision and drainage. This key intervention is indicated for any patient who has a skin and soft-tissue infection with an undrained focus of infection.
COMMUNITY-ASSOCIATED MRSA IS INVADING THE HOSPITAL
In a new development, community-associated MRSA strains are now appearing in the hospital. This is not only because patients are bacteremic when they come in: patients in the hospital are getting nosocomial infections due to community-associated MRSA strains.
Seybold et al10 analyzed 116 cases of MRSA bloodstream infections in Atlanta, GA. In 9 (8%) of the cases the patient had not had any contact with the health care system within the past year, and these cases were classified as truly community-associated. Of the remaining 107 cases, 49 (42%) were nosocomial, and the USA 300 strain—the predominant community-associated MRSA strain—accounted for 10 (20%) of the nosocomial cases.
In the recent CDC study of invasive MRSA infections, Klevens et al4 reported that nearly a third of cases of bacteremia were due to community-associated MRSA, and these strains accounted for a greater proportion of cases of cellulitis and endocarditis than did health-care-associated strains.
In a study of hospital-associated MRSA, Maree et al11 found that the percentage of cases in which the bacteria carried the SCCmec type IV marker had increased from less than 20% in 1999 to more than 50% in 2004.
Comment. Suffice it to say that we are surrounded by MRSA. Community-associated MRSA is here to stay. It is even invading our hospitals, and we need to consider this very carefully when choosing antimicrobial therapy.
NAGGING QUESTIONS ABOUT VANCOMYCIN
Case 2: Vancomycin-intermediate S aureus (VISA) bacteremia and endocarditis
In December 2006 we saw a very ill 60-year-old woman who was hospitalized with MRSA bacteremia, pacemaker endocarditis, and superior vena cava thrombosis. Although she was treated with vancomycin and rifampin, her condition worsened, she had a stroke, and she developed renal failure. In a difficult operation, the pacemaker was removed, but the bacteremia persisted. In early February 2007 she underwent another difficult operation in which the superior vena cava clot was debrided, a right atrial clot was removed, and her mitral valve was replaced. Less than 2 weeks later, and despite ongoing vancomycin and rifampin therapy, the MRSA bacteremia recurred.
During the approximately 6 weeks that the patient had been receiving these antibiotics, the minimal inhibitory concentration (MIC) of rifampin against the S aureus isolate increased from less than 1 μg/mL (susceptible) to 2 μg/mL (resistant). The MIC of vancomycin went from 2 μg/mL (susceptible) to 4 μg/mL (intermediately susceptible). Vancomycin and rifampin were discontinued, and daptomycin and gentamicin (Garamycin) therapy were started. (Her daptomycin MIC was 0.5 μg/mL). The patient’s condition stabilized, and she was discharged to a long-term nursing facility. She had no relapse of MRSA bacteremia, but she died in early April of that year.
Is vancomycin becoming less effective? Degrees of vancomycin resistance
Vancomycin has been our stalwart for treating MRSA infections for more than 40 years but it is not working as well as it used to, at least in certain situations.
VRSA (vancomycin-resistant S aureus) is rare. These fully resistant strains probably acquired a resistance mechanism (the vanA operon) from vancomycin-resistant enterococci. Infections tend to occur in patients simultaneously infected with both S aureus and vancomycin-resistant enterococci, giving the bacteria an opportunity to exchange genetic material.
VISA (vancomycin-intermediate S aureus) infections tend to occur in patients like the one described above who have had long-term vancomycin therapy. VISA strains appear to overproduce a matrix that captures vancomycin and keeps it from entering the cell. On electron microscopy, these bacteria have a very thick cell wall.13
Vancomycin tolerance is a state in which the bacteria are “stunned” or kept in check but not killed by vancomycin. That is manifested in the laboratory by a ratio of minimum bactericidal concentration to MIC greater than 32.
hVISA (heteroresistant VISA) is new and worrisome. These organisms have an overall MIC in the susceptible range, but within that population are individual isolates with an MIC that is much higher—in the intermediate or perhaps even in the resistant range.14
Reported rates of hVISA vary from less than 2% to as high as 76%, because the methods for detecting it are still very poorly standardized. The usual automated laboratory tests do not detect hVISA.
hVISA is probably clinically relevant, as evidence is emerging both in vitro and in vivo that the higher the MIC for vancomycin, the worse the clinical outcome.15 hVISA has been associated with failures of therapy in several situations, usually in cases of severe invasive or deep infection, endocarditis, and bacteremia with vertebral osteomyelitis where vancomycin concentrations at the site of infection may be suboptimal.16–19 While most hVISA strains that have been described were resistant to methicillin, some were susceptible.
The E test is emerging as the standard test for hVISA. This test uses a plastic strip that contains gradually increasing concentrations of vancomycin along its length. Placed in the culture dish, the strip inhibits growth of the organism at its high-concentration end but not at its low-concentration end. If the sample contains hVISA, the cutoff is not well defined, with a few colonies growing at higher concentrations.
New definition of vancomycin susceptibility
Recognizing that the MICs for vancomycin have been rising in the last few years, the Clinical and Laboratory Standards Institute last year changed the break points between susceptibility and resistance. The new definitions are:
- Susceptible—an MIC of 2.0 μg/mL or less (formerly 4.0 μg/mL or less)
- Intermediate—4.0 to 8.0 μg/mL (formerly 8.0 to 16 μg/mL)
- Resistant—16 μg/mL or greater (formerly 32 μg/mL or greater).
One should pay attention to the MIC numbers on the laboratory reports, not just to the words “susceptible” or “not susceptible.” If the number is, say, 0.5 μg/mL or less, the organism should really be susceptible. If the number is 1 or 2, it is still in the susceptible range, but those are the organisms that may cause problems later on.
Further, even if the vancomycin MIC is in the susceptible range, higher MICs may affect outcomes. The average duration of MRSA bacteremia on therapy is 8 to 9 days, vs 3 to 4 days with MSSA bacteremia.20,21 But Sakoulas et al15 found that, in MRSA bacteremia, the success rate with vancomycin therapy was 56% if the MIC was 0.5 or lower, compared with 10% if the MIC was 1.0 to 2.0 μg/mL. Examined in another way, the success rate was 50% if the logarithm of killing was 6.27 colony-forming units per mL or greater, 23% if 4.71 to 6.26, and zero if less than 4.71.
Case 3: Prolonged MRSA bacteremia
In the summer of 2006, a 66-year-old woman with a history of gastric bypass and cirrhosis underwent a long stay in the surgical intensive care unit because of a recurrent enterocutaneous fistula and chronic renal insufficiency. On November 5th, she had a positive blood culture for MRSA, which was treated appropriately with vancomycin for 4 weeks. She was discharged to subacute care but came back 2 days later, again with MRSA bacteremia. At that time her Hickman catheter, which had been inserted for total parenteral nutrition because of the enterocutaneous fistula, was removed.
Transthoracic echocardiography revealed no vegetations, but her bacteremia persisted. Her mental status was poor this entire time: she was mute and could barely be awakened. We looked for clots and infected clots; duplex ultrasonographic examinations of all four extremities were negative. Finally, magnetic resonance imaging of her back—performed empirically because of the persistent bacteremia—revealed vertebral osteomyelitis at level T12-L1. We also noticed on serial evaluations that the vancomycin MIC for her organism increased from 0.5 to 2.0 μg/mL, so therapy was changed from vancomycin to daptomycin.
Her bacteremia cleared. Follow-up echocardiography was negative, but she had two subsequent relapses of MRSA bacteremia, one in April 2007 and one before she died in the summer of 2007.
Prolonged bacteremia: Is it vancomycin resistance, or something else?
The MRSA isolates that cause prolonged bacteremia seem to have certain characteristics.22 Higher MICs are probably associated with longer periods of bacteremia. But some genetic components within some strains of S aureus give them a survival advantage. They have less susceptibility to the body’s thrombin-induced platelet microbicidal protein. These isolates are not only associated with prolonged bacteremia: they are also associated with osteomyelitis, deep abscesses, endocarditis, recurrent infection, and increased death rate.22 Clinical laboratories do not test for these genetic components. One wonders whether our patient may have had an isolate with these mutations that gave it a survival advantage.
Do not use vancomycin for MSSA
Avoid using vancomycin for MSSA infections. It has been shown time and time again that MSSA infections do not respond as well to vancomycin as they do to beta-lactam antibiotics, specifically to the semisynthetic penicillins such as oxacillin and nafcillin, and even some of the first-generation cephalosporins. Chang et al23 found that patients with MSSA bacteremia had higher rates of persistent infections, relapse, and bacteriologic failure if they received vancomycin than if they received nafcillin.
Do vancomycin trough levels affect toxicity?
The vancomycin trough levels that we aimed for in the past (5 to 10 μg/mL) were probably too low. Today, we aim for trough levels of 15 to 20 μg/mL, and many physicians are aiming for 20 to 25 μg/mL. Part of the reason is that vancomycin MICs are higher than they used to be: in order to keep the vancomycin level above the MIC for a longer period of time, the vancomycin trough level needs to be higher. In theory, keeping the vancomycin levels above the MIC for longer periods should improve outcomes. Yet Fowler et al22 found that vancomycin trough levels among patients who had persistent MRSA bacteremia were actually higher than trough levels among those in whom the bacteremia resolved, although the difference was not statistically significant.
We measure the vancomycin trough level to make sure it is high enough (and give larger doses if it is not); among adults, peak levels need not be monitored on a routine basis because of the predictable pharmacokinetics of vancomycin.
Vancomycin toxicity can be either idiosyncratic or synergistic. Idiosyncratic toxicity occurs when a patient who has been on vancomycin for a long time develops a fixed rash, not associated with infusion. This is an immunologic phenomenon. It is a rare and very serious situation and may require steroid therapy.
Synergistic toxicity occurs when vancomycin is given with other nephrotoxic agents, notably gentamicin. Vancomycin plus gentamicin equals nephrotoxicity. Vancomycin alone is usually not nephrotoxic, but close monitoring of renal function parameters is warranted with the use of higher doses.24
IN UNEXPLAINED BACTEREMIA, LOOK FOR ENDOCARDITIS
In blood cultures from patients with bacteremia, S aureus is never a contaminant. Even if just one blood culture is positive for S aureus, believe that S aureus is the culprit.
Reports in the 1950s suggested that at least half of patients who had S aureus bacteremia had endocarditis,25 leading to recommendations that all patients with S aureus bacteremia without an obvious primary source of infection should be evaluated for endocarditis. Subsequent estimates were lower, in the range of 15% to 25%.26,27 However, throughout the world S aureus endocarditis continues to have a very high mortality rate: at least a third of patients die.28
Clinical criteria (community acquisition, no primary focus, and metastatic sequelae) were developed to try to predict the risk of endocarditis in bacteremic patients.26 However, these criteria did not work very well. The clinical definition of endocarditis has evolved. The criteria of von Reyn et al29 from 1981 did not use echocardiography as part of the definition, but the 1994 Duke criteria,30 which were refined31 in 2000, use both clinical and echocardiographic parameters.
Stratton et al32 performed transthoracic echocardiography in 14 patients with bacteremia and found 1 patient with cryptic tricuspid infective endocarditis. Bayer et al33 subsequently reported that of 72 patients with bacteremia, 6 (18%) of those who had no clinical findings suggestive of infectious endocarditis had findings on echocardiography that led to changes in their regimen. Adding echocardiography to three clinical risk factors increased the sensitivity of diagnosing endocarditis from 70% to 85% with a specificity of 100% and predictive value of 96%.
The Duke criteria call for transesophageal echocardiography, which is not feasible in some patients, eg, those with cirrhosis and esophageal varices.
S aureus endocarditis has changed over the years as our patient population has changed, and MRSA endocarditis tends to hit some of our most vulnerable patients. In a study by Miro et al34 in 2005, MRSA was the leading pathogen in patients who were diagnosed with S aureus endocarditis in 1990 or later. We will only see these numbers go up. Patients with diabetes tend to have more MRSA, and of diabetic patients with MRSA endocarditis, 30% to 40% die in the hospital.
Indications for surgery
Certain conditions are indications for surgery among patients with endocarditis, and no antibiotic will cure the endocarditis if the patient has one of these conditions, eg:
- Persistent bacteremia during antibiotic therapy
- Recurrent emboli
- Heart failure that cannot be controlled
- Perivalvular or myocardial abscesses
- Large vegetations
- Early prosthetic valve infection
- Certain arrhythmias.
How long should S aureus bacteremia be treated?
In cases of bacteremia in which endocarditis has been ruled out and removable foci of infection (eg, intravascular catheters) have been removed, some evidence indicates that treatment for 2 weeks would be as effective as the 4 to 6 weeks that we would use for endocarditis or other severe or invasive infections.35 The issue is controversial. If the patient has had frequent hospitalizations or a chronic medical condition I would hesitate to treat for less than 4 weeks, even if the infection appears to be associated with a removable focus.
Treatment of endocarditis
In the guidelines for treatment of endocarditis from the American Heart Association and Infectious Diseases Society of America,36 all the recommendations are relatively old and many of them are somewhat empiric—they are not based on evidence from randomized clinical trials. Rather, they are best opinions based on clinical experience and some observational studies over the years.
For MSSA. In cases of native-valve endocarditis, oxacillin (Bactocill), nafcillin (Unipen), or another semisynthetic beta-lactam antibiotic is recommended. For penicillin-allergic patients, we have other options, such as cefazolin (Ancef, Kefzol).
Combination therapy is frequently recommended for native valve endocarditis as well as for prosthetic valve endocarditis, with either rifampin or gentamicin along with a primary agent. There is some evidence that one can clear staphylococcal bacteremia a day or two more quickly by use of combination therapy with nafcillin plus an aminoglycoside than with nafcillin alone.37,38 For MSSA-associated endocarditis, vancomycin does not work as well as beta-lactam antibiotics.39,40
Korzeniowski and Sande37 and Chambers et al38 reported that the mean duration of bacteremia was 3.4 days for patients treated with nafcillin alone and 2.9 days for those treated with nafcillin plus an aminoglycoside. These studies led to consideration of a short course of gentamicin to clear the bacteremia quickly.
With MRSA, bacteremia often requires a week or more to clear. Levine et al21 reported a study in 42 patients, mostly injection-drug users, with right-sided native-valve endocarditis. The median duration of bacteremia was 7 days in patients who received vancomycin alone vs 9 days in those who received vancomycin plus rifampin; however, some patients were bacteremic for up to 27 days. Fever persisted for a median of 7 days, probably partly due to septic pulmonary emboli. Three patients died, and three required valve replacement.
NEW ANTIBIOTICS
Several new antibiotics are active against gram-positive cocci.41–44 However, the majority of them have not been prospectively studied for treating bacteremia or endocarditis.
Quinupristin/dalfopristin (Synercid) has not been formally studied for treatment of MRSA bacteremia or endocarditis. There are a few case reports of its use in these conditions.45 Quinupristin/dalfopristin is bacteriostatic, and its use may be associated with phlebitis, myalgias, and arthralgias.46
Linezolid (Zyvox) is approved for treatment of complicated skin and soft-tissue infections and for hospital-acquired pneumonia. There have been no specific studies of linezolid in the treatment of S aureus bacteremia or endocarditis. However, Shorr et al47 retrospectively looked at the bacteremic patients in five previous studies of linezolid vs vancomycin and found 144 cases of S aureus bacteremia, half of which were due to MRSA. Of 53 assessable patients with MRSA bacteremia, the primary infection was cured in 14 (56%) of the linezolid patients and 13 (46%) of the vancomycin patients.
The oral form is 100% bioavailable. One should avoid concomitant use of serotonin-reuptake inhibitors because of the risk of serotonin syndrome. Adverse effects include altered taste sensation and peripheral neuropathy. There are other potential toxicities, including hematologic changes (thrombocytopenia, leukopenia) and metabolic effects (lactic acidosis), so clinical and laboratory monitoring is important.48 The role of linezolid in the treatment of patients with S aureus bacteremia or endocarditis remains to be defined.
Daptomycin is indicated for complicated skin and soft-tissue infections, bacteremia, and right-sided endocarditis due to S aureus. Fowler et al20 found that daptomycin was not inferior to beta-lactam antibiotics for treatment of MSSA bacteremia and right-sided endocarditis, and for MRSA infections it outperformed vancomycin, but the difference was not statistically significant.
The dosing interval should be increased from once every 24 hours to every 48 hours if the creatinine clearance is 30 mL/minute or less. Adverse effects include myalgia, rhabdomyolysis (rare), and elevations in creatine phosphokinase. Reports of rising MICs during daptomycin therapy, in some cases associated with persistent infection,49 suggest that careful attention be paid to dosing and clinical monitoring.
Tigecycline (Tygacil) is indicated for complicated skin and soft-tissue infections and complicated intra-abdominal infections due to susceptible organisms. It is active against both MSSA and MRSA, but clinical experience with its use in invasive infections is somewhat limited.50 The dose of tigecycline should be reduced in advanced cirrhosis. Adverse effects include nausea and vomiting.
Telavancin, dalbavancin, and oritavancin, investigational parenteral antibiotics that are derivatives of vancomycin, are in clinical trials. The pharmacokinetic activity of these agents is of interest: telavancin is being studied with a once-daily dosing interval and dalbavancin’s half-life allows once-weekly dosing. In a limited trial, dalbavancin was found to be safe and effective in the treatment of catheter-related bloodstream infections.51 None of the antibiotics in this group has been studied for treatment of S aureus endocarditis. Telavancin therapy has been associated with rash, hypokalemia, QT prolongation, and creatinine elevations. Gastrointestinal symptoms have been reported with the use of dalbavancin.
Ceftobiprole, another investigational agent, is the only cephalosporin antibiotic that is active against MRSA. It is given every 12 hours. Adverse effects include nausea and taste disturbance.
Iclaprim is a novel diaminopyrimidine and a dihydrofolate reductase inhibitor. In vitro, it is active against gram-positive bacteria, including MRSA, VISA, and VRSA; clinical investigations at this point are limited to the treatment of skin and soft-tissue infections.
- Wisplinghoff H, Bischoff T, Tallent SM, Seifert H, Wenzel RP, Edmond MB. Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin Infect Dis 2004; 39:309–371. Erratum in: Clin Infect Dis 2004; 39:1093.
- US Centers for Disease Control and Prevention. National Nosocomial Infections Surveillance (NNIS) System. Campaign to prevent antimicrobial resistance. www.cdc.gov/drugresistance/healthcare/ha/HASlideSet.ppt.
- Blot SI, Vandewoude KH, Hoste EA, Colardyn FA. Outcome and attributable mortality in critically ill patients with bacteremia involving methicillin-susceptible and methicillin-resistant Staphylococcus aureus. Arch Intern Med 2002; 162:2229–2235.
- Klevens RM, Morrison MA, Nadle J, et al; Active Bacterial Core surveillance (ABCs) MRSA Investigators. Invasive methicillin-resistant Staphylococcus aureus infections in the United States. JAMA 2007; 298:1763–1771.
- Naimi TS, LeDell KH, Como-Sabetti K, et al. Comparison of community- and health care-associated methicillin-resistant Staphylococcus aureus infection. JAMA 2003; 290:2976–2984.
- Miller LG, Perdreau-Remington F, Rieg G, et al. Necrotizing fasciitis caused by community-associated methicillin-resistant Staphylococcus aureus in Los Angeles. N Engl J Med 2005; 352:1445–1453.
- Moran GJ, Krishnadasan A, Gorwitz RJ, et al EMERGEncy ID Net Study Group. Methicillin-resistant S. aureus infections among patients in the emergency department. N Engl J Med 2006; 355:666–674.
- Mishaan AM, Mason EO, Martinez-Aquilar G, et al. Emergence of a predominant clone of community-acquired Staphylococcus aureus among children in Houston, Texas. Pediatr Infect Dis J 2005; 24:201–206.
- Miller LG, Perdreau-Remington F, Bayer AS, et al. Clinical and epidemiologic characteristics cannot distinguish community-associated methicillin-resistant Staphylococcus aureus infection from methicillin-susceptible S. aureus infection: a prospective investigation. Clin Infect Dis 2007; 44:471–482.
- Seybold U, Kourbatova EV, Johnson JG, et al. Emergence of community-associated methicillin-resistant Staphylococcus aureus USA300 genotype as a major cause of health care-associated blood stream infections. Clin Infect Dis 2006; 42:647–656.
- Maree CL, Daum RS, Boyle-Vavra S, Matayoshi K, Miller LG. Community-associated methicillin-resistant Staphylococcus aureus isolates causing healthcare-associated infections. Emerg Infect Dis 2007; 13:236–242.
- Liu C, Chambers HF. Staphylococcus aureus with heterogeneous resistance to vancomycin: epidemiology, clinical significance, and critical assessment of diagnostic methods. Antimicrob Agents Chemother 2003; 47:3040–3045.
- Sieradzki K, Roberts RB, Haber SW, Tomasz A. The development of vancomycin resistance in a patient with methicillin-resistant Staphylococcus aureus infection. N Engl J Med 1999; 340:517–523.
- Schwaber MJ, Wright SB, Carmeli Y, et al. Clinical implications of varying degrees of vancomycin susceptibility in methicillin-resistant Staphylococcus aureus bacteremia. Emerg Infect Dis 2003; 9:657–664. Erratum in: Emerg Infect Dis 2004; 10:160.
- Sakoulas G, Moise-Broder PA, Schentag J, Forrest A, Moellering RC, Eliopoulos GM. Relationship of MIC and bactericidal activity to efficacy of vancomycin for treatment of methicillin-resistant Staphylococcus aureus bacteremia. J Clin Microbiol 2004; 42:2398–2402.
- Naimi TS, Anderson D, O’Boyle C, et al. Vancomycin-intermediate Staphylococcus aureus with phenotypic susceptibility to methicillin in a patient with recurrent bacteremia. Clin Infect Dis 2003; 36:1609–1612.
- Moore MR, Perdreau-Remington F, Chambers HF. Vancomycin treatment failure associated with heterogeneous vancomycin-intermediate Staphylococcus aureus in a patient with endocarditis and in the rabbit model of endocarditis. Antimicrob Agents Chemother 2003; 47:1262–1266.
- Charles PG, Ward PB, Johnson PD, Howden BP, Grayson ML. Clinical features associated with bacteremia due to heterogenous vancomycin-intermediate Staphylococcus aureus. Clin Infect Dis 2004; 38:448–451.
- Howden BP, Ward PB, Charles PG, et al. Treatment outcomes for serious infections caused by methicillin-resistant Staphylococcus aureus with reduced vancomycin susceptibility. Clin Infect Dis 2004; 38:521–528.
- Fowler VG, Boucher HW, Corey GR, et al. S. aureus Endocarditis and Bacteremia Study Group. Daptomycin versus standard therapy for bacteremia and endocarditis caused by Staphylococcus aureus. N Engl J Med 2006; 355:653–665.
- Levine DP, Fromm BS, Reddy BR. Slow response to vancomycin or vancomycin plus rifampin in methicillin-resistant Staphylococcus aureus endocarditis. Ann Intern Med 1991; 115:674–680.
- Fowler VG, Sakoulas G, McIntyre LM, et al. Persistent bacteremia due to methicillin-resistant Staphylococcus aureus infection is associated with agr dysfunction and low-level in vitro resistance to thrombin-induced platelet microbicidal protein. J Infect Dis 2004; 190:1140–1149.
- Chang FY, Peacock JE, Musher DM, et al. Staphylococcus aureus bacteremia: recurrence and the impact of antibiotic treatment in a prospective multicenter study. Medicine (Baltimore) 2003; 82:333–339.
- Hidayat LK, Hsu DI, Quist R, Shriner KA, Wong-Beringer A. High-dose vancomycin therapy for methicillin-resistant Staphylococcus aureus infections: efficacy and toxicity. Arch Intern Med 2006; 166:2138–2144.
- Wilson R, Hamburger M. Fifteen years’ experience with staphylococcus septicemia in a large city hospital; analysis of fifty-five cases in the Cincinnati General Hospital 1940 to 1954. Am J Med 1957; 22:437–457.
- Nolan CM, Beaty HN. Staphylococcus aureus bacteremia. Current clinical patterns. Am J Med 1976; 60:495–500.
- Shah M, Watanakunakorn C. Changing patterns of Staphylococcus aureus bacteremia. Am J Med Sci 1979; 278:115–121.
- Fowler VG, Miro JM, Hoen B, et al ICE Investigators. Staphylococcus aureus endocarditis: a consequence of medical progress. JAMA 2005; 293:3012–3021. Erratum in: JAMA 2005; 294:900.
- Von Reyn CF, Levy BS, Arbeit RD, Friedland G, Crumpacker CS. Infective endocarditis: an analysis based on strict case definition. Ann Intern Med 1981; 94:505–518.
- Durack DT, Lukes AS, Bright DK. New criteria for diagnosis of infective endocarditis: utilization of specific echocardiographic findings. Duke Endocarditis Service. Am J Med 1994; 96:200–209.
- Li JS, Sexton DJ, Mick N, et al. Proposed modifications to the Duke criteria for the diagnosis of infective endocarditis. Clin Infect Dis 2000; 30:633–638.
- Stratton JR, Werner JA, Pearlman AS, Janko CL, Kliman S, Jackson MC. Bacteremia and the heart. Serial echocardiographic findings in 80 patients with documented or suspected bacteremia. Am J Med 1982; 73:851–858.
- Bayer AS, Lam K, Ginzton L, Normal DC, Chiu CY, Ward JI. Staphylococcus aureus bacteremia. Clinical, serologic, and echocardiographic findings in patients with and without endocarditis. Arch Intern Med 1987; 147:457–462.
- Miro JM, Anguera I, Cabell CH, et al International Collaboration on Endocarditis Merged Database Study Group. Staphylococcus aureus native valve infective endocarditis: report of 566 episodes from the International Collaboration on Endocarditis Merged Database. Clin Infect Dis 2005; 41:507–514. Erratum in: Clin Infect Dis 2005; 41:1075–1077.
- Jernigan JA, Farr BM. Short-course therapy of catheter-related Staphylococcus aureus bacteremia: a meta-analysis. Ann Intern Med 1993; 119:304–311.
- Baddour LM, Wilson WR, Bayer AS, et al. Infective endocarditis: diagnosis, antimicrobial therapy, and management of complications: a statement for healthcare professionals from the Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease, Council on Cardiovascular Disease in the Young, and the Councils on Clinical Cardiology, Stroke, and Cardiovascular Surgery and Anesthesia, American Heart Association: endorsed by the Infectious Diseases Society of America. Circulation 2005; 111:e394–e434. Erratum in: Circulation 2005; 112:2373. Circulation 2007; 115:e408.
- Korzeniowski O, Sande MA. Combination antimicrobial therapy for Staphylococcus aureus endocarditis in patients addicted to parenteral drugs and in nonaddicts: a prospective study. Ann Intern Med 1982; 97:496–503.
- Chambers HF, Korzeniowski OM, Sande MA. Staphylococcus aureus endocarditis: clinical manifestations in addicts and nonaddicts. Medicine (Baltimore) 1983; 62:170–177.
- Gentry CA, Rodvold KA, Novak RM, Hershow RC, Naderer OJ. Retrospective evaluation of therapies for Staphylococcus aureus endocarditis. Pharmacotherapy 1997; 17:990–997.
- Small PM, Chambers HF. Vancomycin for Staphylococcus aureus endocarditis in intravenous drug users. Antimicrob Agents Chemother 1990; 34:1227–1231.
- Eliopoulos GM. Quinupristin-dalfopristin and linezolid: evidence and opinion. Clin Infect Dis 2003; 36:473–481.
- Rybak MJ. Therapeutic options for Gram-positive infections. J Hosp Infect 2001; 49 suppl A:S25–S32.
- Micek ST. Alternatives to vancomycin for the treatment of methicillin-resistant Staphylococcus aureus infections. Clin Infect Dis 2007; 45 suppl 3:S184–S190.
- Appelbaum PC, Jacobs MR. Recently approved and investigational antibiotics for treatment of severe infections caused by Gram-positive bacteria. Curr Opin Microbiol 2005; 8:510–517.
- Drew RH, Perfect JR, Srinath L, Kirkimilis E, Dowzicky M, Talbot GH for the Synercid Emergency-Use Study Group. Treatment of methicillin-resistant Staphylococcus aureus infections with quinupristin-dalfopristin in patients intolerant of or failing prior therapy. J Antimicrob Chemother 2000; 46:775–784.
- Lamb HM, Figgitt DP, Faulds D. Quinupristin/dalfopristin: a review of its use in the management of serious gram-positive infections. Drugs 1999; 58:1061–1097.
- Shorr AF, Kunkel MJ, Kollef M. Linezolid versus vancomycin for Staphylococcus aureus bacteraemia: pooled analysis of randomized studies. J Antimicrob Chemother 2005; 56:923–929.
- Bishop E, Melvani S, Howden BP, Charles PG, Grayson ML. Good clinical outcomes but high rates of adverse reactions during linezolid therapy for serious infections: a proposed protocol for monitoring therapy in complex patients. Antimicrob Agents Chemother 2006; 50:1599–1602.
- Boucher HW, Sakoulas G. Perspectives on daptomycin resistance, with emphasis on resistance in Staphylococcus aureus. Clin Infect Dis 2007; 45:601–608.
- Munoz-Price LS, Lolans K, Quinn JP. Four cases of invasive methicillin-resistant Staphylococcus aureus (MRSA) infections treated with tigecycline. Scand J Infect Dis 2006; 38:1081–1084.
- Raad I, Darouiche R, Vazquez J, et al. Efficacy and safety of weekly dalbavancin therapy for catheter-related bloodstream infection caused by gram-positive pathogens. Clin Infect Dis 2005; 40:374–80.
- Wisplinghoff H, Bischoff T, Tallent SM, Seifert H, Wenzel RP, Edmond MB. Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin Infect Dis 2004; 39:309–371. Erratum in: Clin Infect Dis 2004; 39:1093.
- US Centers for Disease Control and Prevention. National Nosocomial Infections Surveillance (NNIS) System. Campaign to prevent antimicrobial resistance. www.cdc.gov/drugresistance/healthcare/ha/HASlideSet.ppt.
- Blot SI, Vandewoude KH, Hoste EA, Colardyn FA. Outcome and attributable mortality in critically ill patients with bacteremia involving methicillin-susceptible and methicillin-resistant Staphylococcus aureus. Arch Intern Med 2002; 162:2229–2235.
- Klevens RM, Morrison MA, Nadle J, et al; Active Bacterial Core surveillance (ABCs) MRSA Investigators. Invasive methicillin-resistant Staphylococcus aureus infections in the United States. JAMA 2007; 298:1763–1771.
- Naimi TS, LeDell KH, Como-Sabetti K, et al. Comparison of community- and health care-associated methicillin-resistant Staphylococcus aureus infection. JAMA 2003; 290:2976–2984.
- Miller LG, Perdreau-Remington F, Rieg G, et al. Necrotizing fasciitis caused by community-associated methicillin-resistant Staphylococcus aureus in Los Angeles. N Engl J Med 2005; 352:1445–1453.
- Moran GJ, Krishnadasan A, Gorwitz RJ, et al EMERGEncy ID Net Study Group. Methicillin-resistant S. aureus infections among patients in the emergency department. N Engl J Med 2006; 355:666–674.
- Mishaan AM, Mason EO, Martinez-Aquilar G, et al. Emergence of a predominant clone of community-acquired Staphylococcus aureus among children in Houston, Texas. Pediatr Infect Dis J 2005; 24:201–206.
- Miller LG, Perdreau-Remington F, Bayer AS, et al. Clinical and epidemiologic characteristics cannot distinguish community-associated methicillin-resistant Staphylococcus aureus infection from methicillin-susceptible S. aureus infection: a prospective investigation. Clin Infect Dis 2007; 44:471–482.
- Seybold U, Kourbatova EV, Johnson JG, et al. Emergence of community-associated methicillin-resistant Staphylococcus aureus USA300 genotype as a major cause of health care-associated blood stream infections. Clin Infect Dis 2006; 42:647–656.
- Maree CL, Daum RS, Boyle-Vavra S, Matayoshi K, Miller LG. Community-associated methicillin-resistant Staphylococcus aureus isolates causing healthcare-associated infections. Emerg Infect Dis 2007; 13:236–242.
- Liu C, Chambers HF. Staphylococcus aureus with heterogeneous resistance to vancomycin: epidemiology, clinical significance, and critical assessment of diagnostic methods. Antimicrob Agents Chemother 2003; 47:3040–3045.
- Sieradzki K, Roberts RB, Haber SW, Tomasz A. The development of vancomycin resistance in a patient with methicillin-resistant Staphylococcus aureus infection. N Engl J Med 1999; 340:517–523.
- Schwaber MJ, Wright SB, Carmeli Y, et al. Clinical implications of varying degrees of vancomycin susceptibility in methicillin-resistant Staphylococcus aureus bacteremia. Emerg Infect Dis 2003; 9:657–664. Erratum in: Emerg Infect Dis 2004; 10:160.
- Sakoulas G, Moise-Broder PA, Schentag J, Forrest A, Moellering RC, Eliopoulos GM. Relationship of MIC and bactericidal activity to efficacy of vancomycin for treatment of methicillin-resistant Staphylococcus aureus bacteremia. J Clin Microbiol 2004; 42:2398–2402.
- Naimi TS, Anderson D, O’Boyle C, et al. Vancomycin-intermediate Staphylococcus aureus with phenotypic susceptibility to methicillin in a patient with recurrent bacteremia. Clin Infect Dis 2003; 36:1609–1612.
- Moore MR, Perdreau-Remington F, Chambers HF. Vancomycin treatment failure associated with heterogeneous vancomycin-intermediate Staphylococcus aureus in a patient with endocarditis and in the rabbit model of endocarditis. Antimicrob Agents Chemother 2003; 47:1262–1266.
- Charles PG, Ward PB, Johnson PD, Howden BP, Grayson ML. Clinical features associated with bacteremia due to heterogenous vancomycin-intermediate Staphylococcus aureus. Clin Infect Dis 2004; 38:448–451.
- Howden BP, Ward PB, Charles PG, et al. Treatment outcomes for serious infections caused by methicillin-resistant Staphylococcus aureus with reduced vancomycin susceptibility. Clin Infect Dis 2004; 38:521–528.
- Fowler VG, Boucher HW, Corey GR, et al. S. aureus Endocarditis and Bacteremia Study Group. Daptomycin versus standard therapy for bacteremia and endocarditis caused by Staphylococcus aureus. N Engl J Med 2006; 355:653–665.
- Levine DP, Fromm BS, Reddy BR. Slow response to vancomycin or vancomycin plus rifampin in methicillin-resistant Staphylococcus aureus endocarditis. Ann Intern Med 1991; 115:674–680.
- Fowler VG, Sakoulas G, McIntyre LM, et al. Persistent bacteremia due to methicillin-resistant Staphylococcus aureus infection is associated with agr dysfunction and low-level in vitro resistance to thrombin-induced platelet microbicidal protein. J Infect Dis 2004; 190:1140–1149.
- Chang FY, Peacock JE, Musher DM, et al. Staphylococcus aureus bacteremia: recurrence and the impact of antibiotic treatment in a prospective multicenter study. Medicine (Baltimore) 2003; 82:333–339.
- Hidayat LK, Hsu DI, Quist R, Shriner KA, Wong-Beringer A. High-dose vancomycin therapy for methicillin-resistant Staphylococcus aureus infections: efficacy and toxicity. Arch Intern Med 2006; 166:2138–2144.
- Wilson R, Hamburger M. Fifteen years’ experience with staphylococcus septicemia in a large city hospital; analysis of fifty-five cases in the Cincinnati General Hospital 1940 to 1954. Am J Med 1957; 22:437–457.
- Nolan CM, Beaty HN. Staphylococcus aureus bacteremia. Current clinical patterns. Am J Med 1976; 60:495–500.
- Shah M, Watanakunakorn C. Changing patterns of Staphylococcus aureus bacteremia. Am J Med Sci 1979; 278:115–121.
- Fowler VG, Miro JM, Hoen B, et al ICE Investigators. Staphylococcus aureus endocarditis: a consequence of medical progress. JAMA 2005; 293:3012–3021. Erratum in: JAMA 2005; 294:900.
- Von Reyn CF, Levy BS, Arbeit RD, Friedland G, Crumpacker CS. Infective endocarditis: an analysis based on strict case definition. Ann Intern Med 1981; 94:505–518.
- Durack DT, Lukes AS, Bright DK. New criteria for diagnosis of infective endocarditis: utilization of specific echocardiographic findings. Duke Endocarditis Service. Am J Med 1994; 96:200–209.
- Li JS, Sexton DJ, Mick N, et al. Proposed modifications to the Duke criteria for the diagnosis of infective endocarditis. Clin Infect Dis 2000; 30:633–638.
- Stratton JR, Werner JA, Pearlman AS, Janko CL, Kliman S, Jackson MC. Bacteremia and the heart. Serial echocardiographic findings in 80 patients with documented or suspected bacteremia. Am J Med 1982; 73:851–858.
- Bayer AS, Lam K, Ginzton L, Normal DC, Chiu CY, Ward JI. Staphylococcus aureus bacteremia. Clinical, serologic, and echocardiographic findings in patients with and without endocarditis. Arch Intern Med 1987; 147:457–462.
- Miro JM, Anguera I, Cabell CH, et al International Collaboration on Endocarditis Merged Database Study Group. Staphylococcus aureus native valve infective endocarditis: report of 566 episodes from the International Collaboration on Endocarditis Merged Database. Clin Infect Dis 2005; 41:507–514. Erratum in: Clin Infect Dis 2005; 41:1075–1077.
- Jernigan JA, Farr BM. Short-course therapy of catheter-related Staphylococcus aureus bacteremia: a meta-analysis. Ann Intern Med 1993; 119:304–311.
- Baddour LM, Wilson WR, Bayer AS, et al. Infective endocarditis: diagnosis, antimicrobial therapy, and management of complications: a statement for healthcare professionals from the Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease, Council on Cardiovascular Disease in the Young, and the Councils on Clinical Cardiology, Stroke, and Cardiovascular Surgery and Anesthesia, American Heart Association: endorsed by the Infectious Diseases Society of America. Circulation 2005; 111:e394–e434. Erratum in: Circulation 2005; 112:2373. Circulation 2007; 115:e408.
- Korzeniowski O, Sande MA. Combination antimicrobial therapy for Staphylococcus aureus endocarditis in patients addicted to parenteral drugs and in nonaddicts: a prospective study. Ann Intern Med 1982; 97:496–503.
- Chambers HF, Korzeniowski OM, Sande MA. Staphylococcus aureus endocarditis: clinical manifestations in addicts and nonaddicts. Medicine (Baltimore) 1983; 62:170–177.
- Gentry CA, Rodvold KA, Novak RM, Hershow RC, Naderer OJ. Retrospective evaluation of therapies for Staphylococcus aureus endocarditis. Pharmacotherapy 1997; 17:990–997.
- Small PM, Chambers HF. Vancomycin for Staphylococcus aureus endocarditis in intravenous drug users. Antimicrob Agents Chemother 1990; 34:1227–1231.
- Eliopoulos GM. Quinupristin-dalfopristin and linezolid: evidence and opinion. Clin Infect Dis 2003; 36:473–481.
- Rybak MJ. Therapeutic options for Gram-positive infections. J Hosp Infect 2001; 49 suppl A:S25–S32.
- Micek ST. Alternatives to vancomycin for the treatment of methicillin-resistant Staphylococcus aureus infections. Clin Infect Dis 2007; 45 suppl 3:S184–S190.
- Appelbaum PC, Jacobs MR. Recently approved and investigational antibiotics for treatment of severe infections caused by Gram-positive bacteria. Curr Opin Microbiol 2005; 8:510–517.
- Drew RH, Perfect JR, Srinath L, Kirkimilis E, Dowzicky M, Talbot GH for the Synercid Emergency-Use Study Group. Treatment of methicillin-resistant Staphylococcus aureus infections with quinupristin-dalfopristin in patients intolerant of or failing prior therapy. J Antimicrob Chemother 2000; 46:775–784.
- Lamb HM, Figgitt DP, Faulds D. Quinupristin/dalfopristin: a review of its use in the management of serious gram-positive infections. Drugs 1999; 58:1061–1097.
- Shorr AF, Kunkel MJ, Kollef M. Linezolid versus vancomycin for Staphylococcus aureus bacteraemia: pooled analysis of randomized studies. J Antimicrob Chemother 2005; 56:923–929.
- Bishop E, Melvani S, Howden BP, Charles PG, Grayson ML. Good clinical outcomes but high rates of adverse reactions during linezolid therapy for serious infections: a proposed protocol for monitoring therapy in complex patients. Antimicrob Agents Chemother 2006; 50:1599–1602.
- Boucher HW, Sakoulas G. Perspectives on daptomycin resistance, with emphasis on resistance in Staphylococcus aureus. Clin Infect Dis 2007; 45:601–608.
- Munoz-Price LS, Lolans K, Quinn JP. Four cases of invasive methicillin-resistant Staphylococcus aureus (MRSA) infections treated with tigecycline. Scand J Infect Dis 2006; 38:1081–1084.
- Raad I, Darouiche R, Vazquez J, et al. Efficacy and safety of weekly dalbavancin therapy for catheter-related bloodstream infection caused by gram-positive pathogens. Clin Infect Dis 2005; 40:374–80.
KEY POINTS
- Community-associated MRSA infections tend to affect patients younger than those who traditionally get hospital-associated MRSA infections. Most of these infections are of the skin and soft tissues, but this pathogen can also affect deeper tissues, and bacteremia and necrotizing pneumonia have been reported.
- For patients with skin and soft-tissue infections due to MRSA, incision and drainage rather than antibiotic therapy is often the key intervention.
- Vancomycin has been our stalwart for treating MRSA infections for more than 40 years, but it is not working as well as it used to, at least in certain situations. Vancomycin should not be used to treat infections due to methicillin-susceptible S aureus.
- Needed are better understanding of the factors that influence persistent S aureus bacteremia, well-controlled, prospective studies, and continued antibiotic development.
Fluid restriction is superior in acute lung injury and ARDS
Although most clinicians tend to manage acute lung injury (ALI) and acute respiratory distress syndrome (ARDS) by giving more rather than less fluid,1,2 patients may actually fare better under a strategy of limited fluid intake and increased fluid excretion.
According to the results of the Fluids and Catheters Treatment Trial (FACTT),3 patients managed with fluid restriction (the “dry” or conservative strategy) spent significantly less time in the intensive care unit (ICU) and on mechanical ventilation than did patients who received a high fluid intake (the “wet” or liberal strategy). These benefits of the conservative strategy were attained without any increase in the mortality rate at 60 days or in nonpulmonary organ failure at 28 days.
In this article, I discuss the basis for the FACTT researchers’ conclusion that a conservative fluid strategy is preferable to a liberal fluid strategy in ALI/ARDS.
STUDY RATIONALE
One of the more enduring questions in critical care medicine is which fluid-management strategy is best for patients with ALI/ARDS.
The conservative strategy results in a lower vascular filling pressure, which in turn reduces pulmonary edema and improves gas exchange. The drawback to this strategy is that it may have a negative effect on cardiac output and nonpulmonary organ function.
The liberal strategy results in a higher vascular filling pressure, which may be beneficial in terms of cardiac output and nonpulmonary organ perfusion. However, this strategy does not reduce lung edema.
The evidence accumulated before FACTT did not favor one strategy over the other. However, most deaths among patients with ALI/ARDS are attributable to the failure of organs other than the lungs.4,5 As a result, aggressive fluid restriction has not been a common approach in hospitals throughout the United States.1,2
In an effort to resolve the controversy surrounding the management of ALI/ARDS and to broaden the scope of what we know about fluid balance, we undertook this multicenter, randomized, prospective clinical comparison of the two strategies. This study was conducted under the auspices of the National Heart, Lung, and Blood Institute’s Acute Respiratory Distress Syndrome Clinical Trials Network (ARDSnet).
STUDY DESIGN
Between June 8, 2000, and October 3, 2005, we screened more than 11,000 patients with ALI/ARDS at 20 centers in North America.
Eligibility
Eligible patients had experienced ALI/ARDS within the previous 48 hours, had been intubated for positive-pressure ventilation, had a ratio of partial pressure of arterial oxygen (PaO2) to the fraction of inspired oxygen (FIO2) of less than 300, and exhibited bilateral infiltrates on chest radiography that were consistent with the presence of pulmonary edema without evidence of left atrial hypertension.6
Major exclusion criteria included the placement of a pulmonary artery catheter prior to randomization and the presence of certain illnesses that might have compromised the study results.
Patient population
The target enrollment of 1,000 patients was reached. These patients were randomized into one of four roughly equal groups based on the type of fluid-management strategy—conservative or liberal—and the type of catheter that was placed—pulmonary artery or central venous. (The ARDSnet researchers published the results of the catheter comparison in a separate article.7 Those results are not discussed here except to note that there were no statistically significant differences in outcomes between the two catheter groups.)
There were no statistically significant differences between the two groups with respect to baseline demographic characteristics. The conservative-strategy group consisted of 503 patients, of whom 52% were male and 65% were white; the mean age was 50.1 years. The liberal-strategy group consisted of 497 patients, of whom 55% were male and 63% were white; mean age was 49.5 years.
Management
Ventilation according to a low tidal volume strategy (6 mg/kg) was initiated within 1 hour after randomization. The pulmonary artery catheter or central venous catheter was inserted within 4 hours of randomization, and fluid management was started within 2 hours after catheter insertion. Fluid management was continued for 7 days or until 12 hours after extubation in patients who became able to breathe without assistance, whichever occurred first.
Target filling pressures. In the conservative-strategy group, the target filling pressures were low—a pulmonary artery occlusion pressure less than 8 mm Hg for those randomized to receive a pulmonary artery catheter, and a central venous pressure less than 4 mm Hg for those randomized to receive a central venous catheter. Barring adverse effects, patients were to undergo diuresis with furosemide (Lasix) until their goal was achieved, and then they would be maintained on that dosage through day 7. If we experienced difficulty in safely reaching these goals—say, if a patient developed hypoxemia, oliguria, or hypotension—we backed off the diuresis until the patient stabilized, and then we tried again. An inability to reach these filling pressure targets was not considered to be a treatment failure; our actual aim was to get as close to the target as possible as long as the patient tolerated the treatment.
In the liberal-strategy group, the target pressures were in the high-to-normal range—14 to 18 mm Hg for those with a pulmonary artery catheter and 10 to 14 mm Hg for those with a central venous catheter.
Patients with a pulmonary artery catheter who were hemodynamically stable after 3 days could be switched to a central venous catheter at the discretion of the clinician.
Monitoring. Patients were monitored once every 4 hours—more often if the clinician felt it necessary—for four variables:
- Pulmonary artery occlusion pressure or central venous pressure, depending on the type of catheter
- Shock, indicated by a mean arterial pressure of less than 60 mm Hg or the need for a vasopressor
- Oliguria, indicated by a urine output of less than 0.5 mL/kg/hour
- Ineffective circulation, represented by a cardiac index of less than 2.5 L/minute/cm2 in the pulmonary artery catheter group and by the presence of cold, mottled skin and a capillary-refilling time of more than 2 seconds in the central venous catheter group.
Depending on what the clinician found during monitoring, patients could receive a fluid bolus (if the filling pressure was too low), furosemide (if the filling pressure was too high), dobutamine (in certain rare circumstances), or nothing.
We monitored compliance with the protocol instructions twice each day—at a set time each morning and later in the day at a randomly selected time. An important aspect of this study is that we had no protocol instructions for managing shock. Individual clinicians were free to treat shock however they deemed best. In essence, then, our study was a comparison of liberal and conservative strategies during the nonshock phase of ALI/ARDS.
End points
The primary end point was the mortality rate at 60 days. Patients who were discharged earlier were assumed to be alive at 60 days.
The secondary end points were the number of ICU-free and ventilator-free days and the number of organ-failure-free days at day 28. Other end points included various indicators of lung physiology.
Statistical analysis
This intention-to-treat analysis was powered so that we had a 90% chance of detecting a 10% difference in mortality rate at day 60 (statistical significance: P < .05).
Protocol safeguards
Prior to treatment, we knew that some patients in the liberal-strategy group would not reach their filling-pressure targets despite the infusion of large amounts of fluid. To avoid “overdosing” these patients, we limited all patients to a maximum of three fluid boluses per 24 hours. Also, we withheld fluid boluses if a patient’s FIO2 level reached or exceeded 0.7 or if the cardiac index rose to 4.5 L/minute/cm2 or higher.
Diuretics were withheld when a patient had received a vasopressor or had emerged from shock within the preceding 12 hours. Also, diuretics were not given to any patient who had received a fluid bolus within the preceding 12 hours or when renal failure was present (these patients were given renal support therapy).
Finally, physicians and coordinators were instructed to assess each protocol instruction for safety and clinical validity before implementing the particular instruction. If, in their medical judgment, a particular protocol instruction should not be implemented, they were authorized to override the instruction and record the reason for doing so in the case report form.
RESULTS
Protocol compliance
Clinicians adhered to the protocol instructions during approximately 90% of the time.
Diuretic administration. In response to high filling pressures, patients in the conservative-strategy and liberal-strategy groups received furosemide during 41% and 10% of assessment periods, respectively (P < .0001). By day 7, the average patient in the conservative-strategy group had received a cumulative dose of approximately 1,000 mg of furosemide, while the average patient in the liberal-strategy group had received 500 mg.
Fluid administration. Low filling pressure prompted the administration of a fluid bolus to the liberal-strategy group during 15% of the assessment periods, compared with 6% in the conservative-strategy group (P < .0001).
The conservative-strategy patients who were in shock at study entry had a net gain of approximately 3 L of fluid by day 7, while the liberal-strategy group had a gain of approximately 10 L. Among the patients who were shock-free at baseline, the conservative-strategy group had a net loss of almost 2 L at day 7 while the liberal-strategy group had a net gain of about 5 L.
The pulmonary artery occlusion pressure fell from 15.6 mm Hg to just below 13 mm Hg in the conservative-strategy group by day 7, although there was a wide variation among individual patients. The pressure in the liberal-strategy group (15.7 mm Hg at baseline) was unchanged at day 7 (Figure 2).
Primary end point
Secondary end points
Through day 7, the average patient in the conservative-strategy group experienced significantly more ICU-free days (0.9 vs 0.6; P <.001) and more days free of central nervous system (CNS) failure (3.4 vs 2.9; P = .02). No significant differences were observed in the number of days free from coagulation abnormalities and renal or hepatic failure at day 28.
Through day 28, the average patient in the conservative-strategy group experienced significantly more ventilator-free days (14.6 vs 12.1; P < .001). The other 7-day results held up after 28 days, as the average conservative-strategy patient continued to experience more ICU-free days (13.4 vs 11.2; P < .001) and more days free of CNS failure (18.8 vs 17.2; P = .03). Again, no significant differences were observed in the number of days free of coagulation abnormalities and cardiovascular, renal, or hepatic failure.
It is not clear if the conservative strategy’s advantage in terms of more CNS-failure-free days was actually the result of the strategy itself or due to the fact that these patients were weaned off ventilation earlier and therefore received less sedation.
Other outcomes
Shock. One concern we had with the conservative strategy was that it might induce shock more frequently, but this did not occur. The percentage of patients who developed shock at least once during the 7-day treatment protocol was quite similar in the two groups. Also, it is interesting that patients who presented with no baseline shock had only about a 30% chance of developing shock during therapy. There was no significant difference in vasopressor use between the two groups.
Lung function. The conservative-strategy group had a significantly better Murray lung injury score at day 7: 2.03 vs 2.27 (P < .001).
Tidal-volume scores (7.4 mL/kg in both groups at baseline) dropped at an equal rate and were virtually identical at day 7 (6.36 mL/kg in the conservative-strategy group and 6.34 in the liberal-strategy group), as expected.The plateau pressure, positive end-expiraory pressure, PaO2–FIO2 ratio, and oxygenation index were slightly but not significantly better in the conservative-strategy group at day 7.
Overall, lung function was considerably better in the conservative-strategy group.
Cardiovascular function. The mean arterial pressure was significantly lower in the conservative-strategy group at day 7 (81.00 vs 84.36 mm Hg; P = .03). It is interesting that both levels were higher than the baseline levels (77.1 and 77.2, respectively; not significant).
The stroke volume index and the cardiac index were slightly lower in the conservative-strategy group at day 7, but not significantly so. No differences were seen in heart rate and venous oxygen saturation levels.
Renal and metabolic function. At day 7, the conservative-strategy group had a significantly higher blood urea nitrogen level (33.62 vs 28.44 mg/dL; P = .009). No significant differences were seen between the groups in creatinine levels at day 7 and day 28.
At day 60, dialysis was needed by 10% of the conservative-strategy group and 14% of the liberal-strategy group (P = .06). The important finding here is that there was no trend toward a more frequent need for dialysis in the conservative-strategy group. Also, the average number of days on dialysis in the two groups was essentially the same (11.0 and 10.9, respectively).
Again, there was no difference in the number of renal-failure-free days at either day 7 or day 28.
Hematologic factors. At day 7, the conservative-strategy group had significantly higher hemoglobin (10.22 vs 9.65 g/dL) and albumin (2.30 vs 2.11 g/dL) levels and capillary osmotic pressure (19.18 vs 17.39 mm Hg), even though significantly more patients in the liberal-strategy group received transfusions through day 7 (39% vs 29%; P = .0007).
Safety. Although the number of adverse events—particularly, metabolic alkalosis and electrolyte imbalance—was significantly higher in the conservative-strategy group (42 vs 19; P = .001), the overall incidence was low. No adverse event was associated with arrhythmia.
CONCLUSION
The two fluid-management protocols used in this study were designed to be prudent yet distinctly different. While designing our protocol, we were concerned on the one hand that despite our best efforts fluid balance would turn out to be very similar in the two groups; this did not happen. On the other hand, we were also worried that the fluid level in one of the two groups might turn out to be so bizarre that it would invalidate our study; this too did not occur. Therefore, we are pleased with the way the study was designed and conducted, and we are satisfied that the two protocols were legitimate.
As we went into our study, the literature contained only one other prospective trial that was in some way similar to ours. Mitchell et al9 conducted a randomized, prospective study of 101 critically ill patients, including 89 with pulmonary edema. A group of 52 patients were managed with a conservative strategy intended to reduce the amount of extravascular lung water; the other 49 patients were managed with a strategy similar to the liberal strategy used in our study. At the study’s end, the patients in the conservative-strategy group had a significantly lower amount of extravascular lung water and spent significantly fewer days on ventilation and in the ICU. No clinically significant adverse effects were associated with the conservative strategy. This small study was not highly powered, but it did show that aggressive fluid restriction conferred some benefit.
In our study, the conservative strategy improved lung function and shortened the duration of mechanical ventilation and ICU stay without increasing nonpulmonary organ failures or increasing the risk of death within 60 days. Therefore, we recommend the conservative strategy for patients with ALI/ARDS.
- The Acute Respiratory Distress Syndrome Network.Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med 2000; 342:1301–1308.
- Brower RG, Lanken PN, MacIntyre N, et al; National Heart, Lung, and Blood Institute ARDS Clinical Trials Network. Higher versus lower positive end-expiratory pressures in patients with the acute respiratory distress syndrome. N Engl J Med 2004; 351:327–336.
- National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network; Wiedemann HP, Wheeler AP, Bernard GR, et al. Comparison of two fluid-management strategies in acute lung injury. N Engl J Med 2006; 354:2564–2575.
- Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med 2000; 342:1334–1349.
- Montgomery AB, Stager MA, Carrico CJ, Hudson LD. Causes of mortality in patients with the adult respiratory distress syndrome. Am Rev Respir Dis 1985; 132:485–489.
- Bernard GR, Artigas A, Brigham KL, et al. The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med 1994; 149:818–824.
- National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network; Wheeler AP, Bernard GR, Thompson BT, et al. Pulmonary-artery versus central venous catheter to guide treatment of acute lung injury. N Engl J Med 2006; 354:2213–2224.
- The National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical TrialsNetwork. Supplementary appendix.http://content.nejm.org/cgi/data/NEJMoa062200/DC1/1.Accessed August 3, 2007.
- Mitchell JP, Schuller D, Calandrino FS, Schuster DP.Improved outcome based on fluid management in criticallyill patients requiring pulmonary artery catheterization.Am Rev Respir Dis 1992; 145:990–998.
Although most clinicians tend to manage acute lung injury (ALI) and acute respiratory distress syndrome (ARDS) by giving more rather than less fluid,1,2 patients may actually fare better under a strategy of limited fluid intake and increased fluid excretion.
According to the results of the Fluids and Catheters Treatment Trial (FACTT),3 patients managed with fluid restriction (the “dry” or conservative strategy) spent significantly less time in the intensive care unit (ICU) and on mechanical ventilation than did patients who received a high fluid intake (the “wet” or liberal strategy). These benefits of the conservative strategy were attained without any increase in the mortality rate at 60 days or in nonpulmonary organ failure at 28 days.
In this article, I discuss the basis for the FACTT researchers’ conclusion that a conservative fluid strategy is preferable to a liberal fluid strategy in ALI/ARDS.
STUDY RATIONALE
One of the more enduring questions in critical care medicine is which fluid-management strategy is best for patients with ALI/ARDS.
The conservative strategy results in a lower vascular filling pressure, which in turn reduces pulmonary edema and improves gas exchange. The drawback to this strategy is that it may have a negative effect on cardiac output and nonpulmonary organ function.
The liberal strategy results in a higher vascular filling pressure, which may be beneficial in terms of cardiac output and nonpulmonary organ perfusion. However, this strategy does not reduce lung edema.
The evidence accumulated before FACTT did not favor one strategy over the other. However, most deaths among patients with ALI/ARDS are attributable to the failure of organs other than the lungs.4,5 As a result, aggressive fluid restriction has not been a common approach in hospitals throughout the United States.1,2
In an effort to resolve the controversy surrounding the management of ALI/ARDS and to broaden the scope of what we know about fluid balance, we undertook this multicenter, randomized, prospective clinical comparison of the two strategies. This study was conducted under the auspices of the National Heart, Lung, and Blood Institute’s Acute Respiratory Distress Syndrome Clinical Trials Network (ARDSnet).
STUDY DESIGN
Between June 8, 2000, and October 3, 2005, we screened more than 11,000 patients with ALI/ARDS at 20 centers in North America.
Eligibility
Eligible patients had experienced ALI/ARDS within the previous 48 hours, had been intubated for positive-pressure ventilation, had a ratio of partial pressure of arterial oxygen (PaO2) to the fraction of inspired oxygen (FIO2) of less than 300, and exhibited bilateral infiltrates on chest radiography that were consistent with the presence of pulmonary edema without evidence of left atrial hypertension.6
Major exclusion criteria included the placement of a pulmonary artery catheter prior to randomization and the presence of certain illnesses that might have compromised the study results.
Patient population
The target enrollment of 1,000 patients was reached. These patients were randomized into one of four roughly equal groups based on the type of fluid-management strategy—conservative or liberal—and the type of catheter that was placed—pulmonary artery or central venous. (The ARDSnet researchers published the results of the catheter comparison in a separate article.7 Those results are not discussed here except to note that there were no statistically significant differences in outcomes between the two catheter groups.)
There were no statistically significant differences between the two groups with respect to baseline demographic characteristics. The conservative-strategy group consisted of 503 patients, of whom 52% were male and 65% were white; the mean age was 50.1 years. The liberal-strategy group consisted of 497 patients, of whom 55% were male and 63% were white; mean age was 49.5 years.
Management
Ventilation according to a low tidal volume strategy (6 mg/kg) was initiated within 1 hour after randomization. The pulmonary artery catheter or central venous catheter was inserted within 4 hours of randomization, and fluid management was started within 2 hours after catheter insertion. Fluid management was continued for 7 days or until 12 hours after extubation in patients who became able to breathe without assistance, whichever occurred first.
Target filling pressures. In the conservative-strategy group, the target filling pressures were low—a pulmonary artery occlusion pressure less than 8 mm Hg for those randomized to receive a pulmonary artery catheter, and a central venous pressure less than 4 mm Hg for those randomized to receive a central venous catheter. Barring adverse effects, patients were to undergo diuresis with furosemide (Lasix) until their goal was achieved, and then they would be maintained on that dosage through day 7. If we experienced difficulty in safely reaching these goals—say, if a patient developed hypoxemia, oliguria, or hypotension—we backed off the diuresis until the patient stabilized, and then we tried again. An inability to reach these filling pressure targets was not considered to be a treatment failure; our actual aim was to get as close to the target as possible as long as the patient tolerated the treatment.
In the liberal-strategy group, the target pressures were in the high-to-normal range—14 to 18 mm Hg for those with a pulmonary artery catheter and 10 to 14 mm Hg for those with a central venous catheter.
Patients with a pulmonary artery catheter who were hemodynamically stable after 3 days could be switched to a central venous catheter at the discretion of the clinician.
Monitoring. Patients were monitored once every 4 hours—more often if the clinician felt it necessary—for four variables:
- Pulmonary artery occlusion pressure or central venous pressure, depending on the type of catheter
- Shock, indicated by a mean arterial pressure of less than 60 mm Hg or the need for a vasopressor
- Oliguria, indicated by a urine output of less than 0.5 mL/kg/hour
- Ineffective circulation, represented by a cardiac index of less than 2.5 L/minute/cm2 in the pulmonary artery catheter group and by the presence of cold, mottled skin and a capillary-refilling time of more than 2 seconds in the central venous catheter group.
Depending on what the clinician found during monitoring, patients could receive a fluid bolus (if the filling pressure was too low), furosemide (if the filling pressure was too high), dobutamine (in certain rare circumstances), or nothing.
We monitored compliance with the protocol instructions twice each day—at a set time each morning and later in the day at a randomly selected time. An important aspect of this study is that we had no protocol instructions for managing shock. Individual clinicians were free to treat shock however they deemed best. In essence, then, our study was a comparison of liberal and conservative strategies during the nonshock phase of ALI/ARDS.
End points
The primary end point was the mortality rate at 60 days. Patients who were discharged earlier were assumed to be alive at 60 days.
The secondary end points were the number of ICU-free and ventilator-free days and the number of organ-failure-free days at day 28. Other end points included various indicators of lung physiology.
Statistical analysis
This intention-to-treat analysis was powered so that we had a 90% chance of detecting a 10% difference in mortality rate at day 60 (statistical significance: P < .05).
Protocol safeguards
Prior to treatment, we knew that some patients in the liberal-strategy group would not reach their filling-pressure targets despite the infusion of large amounts of fluid. To avoid “overdosing” these patients, we limited all patients to a maximum of three fluid boluses per 24 hours. Also, we withheld fluid boluses if a patient’s FIO2 level reached or exceeded 0.7 or if the cardiac index rose to 4.5 L/minute/cm2 or higher.
Diuretics were withheld when a patient had received a vasopressor or had emerged from shock within the preceding 12 hours. Also, diuretics were not given to any patient who had received a fluid bolus within the preceding 12 hours or when renal failure was present (these patients were given renal support therapy).
Finally, physicians and coordinators were instructed to assess each protocol instruction for safety and clinical validity before implementing the particular instruction. If, in their medical judgment, a particular protocol instruction should not be implemented, they were authorized to override the instruction and record the reason for doing so in the case report form.
RESULTS
Protocol compliance
Clinicians adhered to the protocol instructions during approximately 90% of the time.
Diuretic administration. In response to high filling pressures, patients in the conservative-strategy and liberal-strategy groups received furosemide during 41% and 10% of assessment periods, respectively (P < .0001). By day 7, the average patient in the conservative-strategy group had received a cumulative dose of approximately 1,000 mg of furosemide, while the average patient in the liberal-strategy group had received 500 mg.
Fluid administration. Low filling pressure prompted the administration of a fluid bolus to the liberal-strategy group during 15% of the assessment periods, compared with 6% in the conservative-strategy group (P < .0001).
The conservative-strategy patients who were in shock at study entry had a net gain of approximately 3 L of fluid by day 7, while the liberal-strategy group had a gain of approximately 10 L. Among the patients who were shock-free at baseline, the conservative-strategy group had a net loss of almost 2 L at day 7 while the liberal-strategy group had a net gain of about 5 L.
The pulmonary artery occlusion pressure fell from 15.6 mm Hg to just below 13 mm Hg in the conservative-strategy group by day 7, although there was a wide variation among individual patients. The pressure in the liberal-strategy group (15.7 mm Hg at baseline) was unchanged at day 7 (Figure 2).
Primary end point
Secondary end points
Through day 7, the average patient in the conservative-strategy group experienced significantly more ICU-free days (0.9 vs 0.6; P <.001) and more days free of central nervous system (CNS) failure (3.4 vs 2.9; P = .02). No significant differences were observed in the number of days free from coagulation abnormalities and renal or hepatic failure at day 28.
Through day 28, the average patient in the conservative-strategy group experienced significantly more ventilator-free days (14.6 vs 12.1; P < .001). The other 7-day results held up after 28 days, as the average conservative-strategy patient continued to experience more ICU-free days (13.4 vs 11.2; P < .001) and more days free of CNS failure (18.8 vs 17.2; P = .03). Again, no significant differences were observed in the number of days free of coagulation abnormalities and cardiovascular, renal, or hepatic failure.
It is not clear if the conservative strategy’s advantage in terms of more CNS-failure-free days was actually the result of the strategy itself or due to the fact that these patients were weaned off ventilation earlier and therefore received less sedation.
Other outcomes
Shock. One concern we had with the conservative strategy was that it might induce shock more frequently, but this did not occur. The percentage of patients who developed shock at least once during the 7-day treatment protocol was quite similar in the two groups. Also, it is interesting that patients who presented with no baseline shock had only about a 30% chance of developing shock during therapy. There was no significant difference in vasopressor use between the two groups.
Lung function. The conservative-strategy group had a significantly better Murray lung injury score at day 7: 2.03 vs 2.27 (P < .001).
Tidal-volume scores (7.4 mL/kg in both groups at baseline) dropped at an equal rate and were virtually identical at day 7 (6.36 mL/kg in the conservative-strategy group and 6.34 in the liberal-strategy group), as expected.The plateau pressure, positive end-expiraory pressure, PaO2–FIO2 ratio, and oxygenation index were slightly but not significantly better in the conservative-strategy group at day 7.
Overall, lung function was considerably better in the conservative-strategy group.
Cardiovascular function. The mean arterial pressure was significantly lower in the conservative-strategy group at day 7 (81.00 vs 84.36 mm Hg; P = .03). It is interesting that both levels were higher than the baseline levels (77.1 and 77.2, respectively; not significant).
The stroke volume index and the cardiac index were slightly lower in the conservative-strategy group at day 7, but not significantly so. No differences were seen in heart rate and venous oxygen saturation levels.
Renal and metabolic function. At day 7, the conservative-strategy group had a significantly higher blood urea nitrogen level (33.62 vs 28.44 mg/dL; P = .009). No significant differences were seen between the groups in creatinine levels at day 7 and day 28.
At day 60, dialysis was needed by 10% of the conservative-strategy group and 14% of the liberal-strategy group (P = .06). The important finding here is that there was no trend toward a more frequent need for dialysis in the conservative-strategy group. Also, the average number of days on dialysis in the two groups was essentially the same (11.0 and 10.9, respectively).
Again, there was no difference in the number of renal-failure-free days at either day 7 or day 28.
Hematologic factors. At day 7, the conservative-strategy group had significantly higher hemoglobin (10.22 vs 9.65 g/dL) and albumin (2.30 vs 2.11 g/dL) levels and capillary osmotic pressure (19.18 vs 17.39 mm Hg), even though significantly more patients in the liberal-strategy group received transfusions through day 7 (39% vs 29%; P = .0007).
Safety. Although the number of adverse events—particularly, metabolic alkalosis and electrolyte imbalance—was significantly higher in the conservative-strategy group (42 vs 19; P = .001), the overall incidence was low. No adverse event was associated with arrhythmia.
CONCLUSION
The two fluid-management protocols used in this study were designed to be prudent yet distinctly different. While designing our protocol, we were concerned on the one hand that despite our best efforts fluid balance would turn out to be very similar in the two groups; this did not happen. On the other hand, we were also worried that the fluid level in one of the two groups might turn out to be so bizarre that it would invalidate our study; this too did not occur. Therefore, we are pleased with the way the study was designed and conducted, and we are satisfied that the two protocols were legitimate.
As we went into our study, the literature contained only one other prospective trial that was in some way similar to ours. Mitchell et al9 conducted a randomized, prospective study of 101 critically ill patients, including 89 with pulmonary edema. A group of 52 patients were managed with a conservative strategy intended to reduce the amount of extravascular lung water; the other 49 patients were managed with a strategy similar to the liberal strategy used in our study. At the study’s end, the patients in the conservative-strategy group had a significantly lower amount of extravascular lung water and spent significantly fewer days on ventilation and in the ICU. No clinically significant adverse effects were associated with the conservative strategy. This small study was not highly powered, but it did show that aggressive fluid restriction conferred some benefit.
In our study, the conservative strategy improved lung function and shortened the duration of mechanical ventilation and ICU stay without increasing nonpulmonary organ failures or increasing the risk of death within 60 days. Therefore, we recommend the conservative strategy for patients with ALI/ARDS.
Although most clinicians tend to manage acute lung injury (ALI) and acute respiratory distress syndrome (ARDS) by giving more rather than less fluid,1,2 patients may actually fare better under a strategy of limited fluid intake and increased fluid excretion.
According to the results of the Fluids and Catheters Treatment Trial (FACTT),3 patients managed with fluid restriction (the “dry” or conservative strategy) spent significantly less time in the intensive care unit (ICU) and on mechanical ventilation than did patients who received a high fluid intake (the “wet” or liberal strategy). These benefits of the conservative strategy were attained without any increase in the mortality rate at 60 days or in nonpulmonary organ failure at 28 days.
In this article, I discuss the basis for the FACTT researchers’ conclusion that a conservative fluid strategy is preferable to a liberal fluid strategy in ALI/ARDS.
STUDY RATIONALE
One of the more enduring questions in critical care medicine is which fluid-management strategy is best for patients with ALI/ARDS.
The conservative strategy results in a lower vascular filling pressure, which in turn reduces pulmonary edema and improves gas exchange. The drawback to this strategy is that it may have a negative effect on cardiac output and nonpulmonary organ function.
The liberal strategy results in a higher vascular filling pressure, which may be beneficial in terms of cardiac output and nonpulmonary organ perfusion. However, this strategy does not reduce lung edema.
The evidence accumulated before FACTT did not favor one strategy over the other. However, most deaths among patients with ALI/ARDS are attributable to the failure of organs other than the lungs.4,5 As a result, aggressive fluid restriction has not been a common approach in hospitals throughout the United States.1,2
In an effort to resolve the controversy surrounding the management of ALI/ARDS and to broaden the scope of what we know about fluid balance, we undertook this multicenter, randomized, prospective clinical comparison of the two strategies. This study was conducted under the auspices of the National Heart, Lung, and Blood Institute’s Acute Respiratory Distress Syndrome Clinical Trials Network (ARDSnet).
STUDY DESIGN
Between June 8, 2000, and October 3, 2005, we screened more than 11,000 patients with ALI/ARDS at 20 centers in North America.
Eligibility
Eligible patients had experienced ALI/ARDS within the previous 48 hours, had been intubated for positive-pressure ventilation, had a ratio of partial pressure of arterial oxygen (PaO2) to the fraction of inspired oxygen (FIO2) of less than 300, and exhibited bilateral infiltrates on chest radiography that were consistent with the presence of pulmonary edema without evidence of left atrial hypertension.6
Major exclusion criteria included the placement of a pulmonary artery catheter prior to randomization and the presence of certain illnesses that might have compromised the study results.
Patient population
The target enrollment of 1,000 patients was reached. These patients were randomized into one of four roughly equal groups based on the type of fluid-management strategy—conservative or liberal—and the type of catheter that was placed—pulmonary artery or central venous. (The ARDSnet researchers published the results of the catheter comparison in a separate article.7 Those results are not discussed here except to note that there were no statistically significant differences in outcomes between the two catheter groups.)
There were no statistically significant differences between the two groups with respect to baseline demographic characteristics. The conservative-strategy group consisted of 503 patients, of whom 52% were male and 65% were white; the mean age was 50.1 years. The liberal-strategy group consisted of 497 patients, of whom 55% were male and 63% were white; mean age was 49.5 years.
Management
Ventilation according to a low tidal volume strategy (6 mg/kg) was initiated within 1 hour after randomization. The pulmonary artery catheter or central venous catheter was inserted within 4 hours of randomization, and fluid management was started within 2 hours after catheter insertion. Fluid management was continued for 7 days or until 12 hours after extubation in patients who became able to breathe without assistance, whichever occurred first.
Target filling pressures. In the conservative-strategy group, the target filling pressures were low—a pulmonary artery occlusion pressure less than 8 mm Hg for those randomized to receive a pulmonary artery catheter, and a central venous pressure less than 4 mm Hg for those randomized to receive a central venous catheter. Barring adverse effects, patients were to undergo diuresis with furosemide (Lasix) until their goal was achieved, and then they would be maintained on that dosage through day 7. If we experienced difficulty in safely reaching these goals—say, if a patient developed hypoxemia, oliguria, or hypotension—we backed off the diuresis until the patient stabilized, and then we tried again. An inability to reach these filling pressure targets was not considered to be a treatment failure; our actual aim was to get as close to the target as possible as long as the patient tolerated the treatment.
In the liberal-strategy group, the target pressures were in the high-to-normal range—14 to 18 mm Hg for those with a pulmonary artery catheter and 10 to 14 mm Hg for those with a central venous catheter.
Patients with a pulmonary artery catheter who were hemodynamically stable after 3 days could be switched to a central venous catheter at the discretion of the clinician.
Monitoring. Patients were monitored once every 4 hours—more often if the clinician felt it necessary—for four variables:
- Pulmonary artery occlusion pressure or central venous pressure, depending on the type of catheter
- Shock, indicated by a mean arterial pressure of less than 60 mm Hg or the need for a vasopressor
- Oliguria, indicated by a urine output of less than 0.5 mL/kg/hour
- Ineffective circulation, represented by a cardiac index of less than 2.5 L/minute/cm2 in the pulmonary artery catheter group and by the presence of cold, mottled skin and a capillary-refilling time of more than 2 seconds in the central venous catheter group.
Depending on what the clinician found during monitoring, patients could receive a fluid bolus (if the filling pressure was too low), furosemide (if the filling pressure was too high), dobutamine (in certain rare circumstances), or nothing.
We monitored compliance with the protocol instructions twice each day—at a set time each morning and later in the day at a randomly selected time. An important aspect of this study is that we had no protocol instructions for managing shock. Individual clinicians were free to treat shock however they deemed best. In essence, then, our study was a comparison of liberal and conservative strategies during the nonshock phase of ALI/ARDS.
End points
The primary end point was the mortality rate at 60 days. Patients who were discharged earlier were assumed to be alive at 60 days.
The secondary end points were the number of ICU-free and ventilator-free days and the number of organ-failure-free days at day 28. Other end points included various indicators of lung physiology.
Statistical analysis
This intention-to-treat analysis was powered so that we had a 90% chance of detecting a 10% difference in mortality rate at day 60 (statistical significance: P < .05).
Protocol safeguards
Prior to treatment, we knew that some patients in the liberal-strategy group would not reach their filling-pressure targets despite the infusion of large amounts of fluid. To avoid “overdosing” these patients, we limited all patients to a maximum of three fluid boluses per 24 hours. Also, we withheld fluid boluses if a patient’s FIO2 level reached or exceeded 0.7 or if the cardiac index rose to 4.5 L/minute/cm2 or higher.
Diuretics were withheld when a patient had received a vasopressor or had emerged from shock within the preceding 12 hours. Also, diuretics were not given to any patient who had received a fluid bolus within the preceding 12 hours or when renal failure was present (these patients were given renal support therapy).
Finally, physicians and coordinators were instructed to assess each protocol instruction for safety and clinical validity before implementing the particular instruction. If, in their medical judgment, a particular protocol instruction should not be implemented, they were authorized to override the instruction and record the reason for doing so in the case report form.
RESULTS
Protocol compliance
Clinicians adhered to the protocol instructions during approximately 90% of the time.
Diuretic administration. In response to high filling pressures, patients in the conservative-strategy and liberal-strategy groups received furosemide during 41% and 10% of assessment periods, respectively (P < .0001). By day 7, the average patient in the conservative-strategy group had received a cumulative dose of approximately 1,000 mg of furosemide, while the average patient in the liberal-strategy group had received 500 mg.
Fluid administration. Low filling pressure prompted the administration of a fluid bolus to the liberal-strategy group during 15% of the assessment periods, compared with 6% in the conservative-strategy group (P < .0001).
The conservative-strategy patients who were in shock at study entry had a net gain of approximately 3 L of fluid by day 7, while the liberal-strategy group had a gain of approximately 10 L. Among the patients who were shock-free at baseline, the conservative-strategy group had a net loss of almost 2 L at day 7 while the liberal-strategy group had a net gain of about 5 L.
The pulmonary artery occlusion pressure fell from 15.6 mm Hg to just below 13 mm Hg in the conservative-strategy group by day 7, although there was a wide variation among individual patients. The pressure in the liberal-strategy group (15.7 mm Hg at baseline) was unchanged at day 7 (Figure 2).
Primary end point
Secondary end points
Through day 7, the average patient in the conservative-strategy group experienced significantly more ICU-free days (0.9 vs 0.6; P <.001) and more days free of central nervous system (CNS) failure (3.4 vs 2.9; P = .02). No significant differences were observed in the number of days free from coagulation abnormalities and renal or hepatic failure at day 28.
Through day 28, the average patient in the conservative-strategy group experienced significantly more ventilator-free days (14.6 vs 12.1; P < .001). The other 7-day results held up after 28 days, as the average conservative-strategy patient continued to experience more ICU-free days (13.4 vs 11.2; P < .001) and more days free of CNS failure (18.8 vs 17.2; P = .03). Again, no significant differences were observed in the number of days free of coagulation abnormalities and cardiovascular, renal, or hepatic failure.
It is not clear if the conservative strategy’s advantage in terms of more CNS-failure-free days was actually the result of the strategy itself or due to the fact that these patients were weaned off ventilation earlier and therefore received less sedation.
Other outcomes
Shock. One concern we had with the conservative strategy was that it might induce shock more frequently, but this did not occur. The percentage of patients who developed shock at least once during the 7-day treatment protocol was quite similar in the two groups. Also, it is interesting that patients who presented with no baseline shock had only about a 30% chance of developing shock during therapy. There was no significant difference in vasopressor use between the two groups.
Lung function. The conservative-strategy group had a significantly better Murray lung injury score at day 7: 2.03 vs 2.27 (P < .001).
Tidal-volume scores (7.4 mL/kg in both groups at baseline) dropped at an equal rate and were virtually identical at day 7 (6.36 mL/kg in the conservative-strategy group and 6.34 in the liberal-strategy group), as expected.The plateau pressure, positive end-expiraory pressure, PaO2–FIO2 ratio, and oxygenation index were slightly but not significantly better in the conservative-strategy group at day 7.
Overall, lung function was considerably better in the conservative-strategy group.
Cardiovascular function. The mean arterial pressure was significantly lower in the conservative-strategy group at day 7 (81.00 vs 84.36 mm Hg; P = .03). It is interesting that both levels were higher than the baseline levels (77.1 and 77.2, respectively; not significant).
The stroke volume index and the cardiac index were slightly lower in the conservative-strategy group at day 7, but not significantly so. No differences were seen in heart rate and venous oxygen saturation levels.
Renal and metabolic function. At day 7, the conservative-strategy group had a significantly higher blood urea nitrogen level (33.62 vs 28.44 mg/dL; P = .009). No significant differences were seen between the groups in creatinine levels at day 7 and day 28.
At day 60, dialysis was needed by 10% of the conservative-strategy group and 14% of the liberal-strategy group (P = .06). The important finding here is that there was no trend toward a more frequent need for dialysis in the conservative-strategy group. Also, the average number of days on dialysis in the two groups was essentially the same (11.0 and 10.9, respectively).
Again, there was no difference in the number of renal-failure-free days at either day 7 or day 28.
Hematologic factors. At day 7, the conservative-strategy group had significantly higher hemoglobin (10.22 vs 9.65 g/dL) and albumin (2.30 vs 2.11 g/dL) levels and capillary osmotic pressure (19.18 vs 17.39 mm Hg), even though significantly more patients in the liberal-strategy group received transfusions through day 7 (39% vs 29%; P = .0007).
Safety. Although the number of adverse events—particularly, metabolic alkalosis and electrolyte imbalance—was significantly higher in the conservative-strategy group (42 vs 19; P = .001), the overall incidence was low. No adverse event was associated with arrhythmia.
CONCLUSION
The two fluid-management protocols used in this study were designed to be prudent yet distinctly different. While designing our protocol, we were concerned on the one hand that despite our best efforts fluid balance would turn out to be very similar in the two groups; this did not happen. On the other hand, we were also worried that the fluid level in one of the two groups might turn out to be so bizarre that it would invalidate our study; this too did not occur. Therefore, we are pleased with the way the study was designed and conducted, and we are satisfied that the two protocols were legitimate.
As we went into our study, the literature contained only one other prospective trial that was in some way similar to ours. Mitchell et al9 conducted a randomized, prospective study of 101 critically ill patients, including 89 with pulmonary edema. A group of 52 patients were managed with a conservative strategy intended to reduce the amount of extravascular lung water; the other 49 patients were managed with a strategy similar to the liberal strategy used in our study. At the study’s end, the patients in the conservative-strategy group had a significantly lower amount of extravascular lung water and spent significantly fewer days on ventilation and in the ICU. No clinically significant adverse effects were associated with the conservative strategy. This small study was not highly powered, but it did show that aggressive fluid restriction conferred some benefit.
In our study, the conservative strategy improved lung function and shortened the duration of mechanical ventilation and ICU stay without increasing nonpulmonary organ failures or increasing the risk of death within 60 days. Therefore, we recommend the conservative strategy for patients with ALI/ARDS.
- The Acute Respiratory Distress Syndrome Network.Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med 2000; 342:1301–1308.
- Brower RG, Lanken PN, MacIntyre N, et al; National Heart, Lung, and Blood Institute ARDS Clinical Trials Network. Higher versus lower positive end-expiratory pressures in patients with the acute respiratory distress syndrome. N Engl J Med 2004; 351:327–336.
- National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network; Wiedemann HP, Wheeler AP, Bernard GR, et al. Comparison of two fluid-management strategies in acute lung injury. N Engl J Med 2006; 354:2564–2575.
- Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med 2000; 342:1334–1349.
- Montgomery AB, Stager MA, Carrico CJ, Hudson LD. Causes of mortality in patients with the adult respiratory distress syndrome. Am Rev Respir Dis 1985; 132:485–489.
- Bernard GR, Artigas A, Brigham KL, et al. The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med 1994; 149:818–824.
- National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network; Wheeler AP, Bernard GR, Thompson BT, et al. Pulmonary-artery versus central venous catheter to guide treatment of acute lung injury. N Engl J Med 2006; 354:2213–2224.
- The National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical TrialsNetwork. Supplementary appendix.http://content.nejm.org/cgi/data/NEJMoa062200/DC1/1.Accessed August 3, 2007.
- Mitchell JP, Schuller D, Calandrino FS, Schuster DP.Improved outcome based on fluid management in criticallyill patients requiring pulmonary artery catheterization.Am Rev Respir Dis 1992; 145:990–998.
- The Acute Respiratory Distress Syndrome Network.Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med 2000; 342:1301–1308.
- Brower RG, Lanken PN, MacIntyre N, et al; National Heart, Lung, and Blood Institute ARDS Clinical Trials Network. Higher versus lower positive end-expiratory pressures in patients with the acute respiratory distress syndrome. N Engl J Med 2004; 351:327–336.
- National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network; Wiedemann HP, Wheeler AP, Bernard GR, et al. Comparison of two fluid-management strategies in acute lung injury. N Engl J Med 2006; 354:2564–2575.
- Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med 2000; 342:1334–1349.
- Montgomery AB, Stager MA, Carrico CJ, Hudson LD. Causes of mortality in patients with the adult respiratory distress syndrome. Am Rev Respir Dis 1985; 132:485–489.
- Bernard GR, Artigas A, Brigham KL, et al. The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med 1994; 149:818–824.
- National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network; Wheeler AP, Bernard GR, Thompson BT, et al. Pulmonary-artery versus central venous catheter to guide treatment of acute lung injury. N Engl J Med 2006; 354:2213–2224.
- The National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical TrialsNetwork. Supplementary appendix.http://content.nejm.org/cgi/data/NEJMoa062200/DC1/1.Accessed August 3, 2007.
- Mitchell JP, Schuller D, Calandrino FS, Schuster DP.Improved outcome based on fluid management in criticallyill patients requiring pulmonary artery catheterization.Am Rev Respir Dis 1992; 145:990–998.
KEY POINTS
- In the conservative-strategy group, the target filling pressures were a pulmonary artery occlusion pressure less than 8 mm Hg for those with a pulmonary artery catheter and a central venous pressure less than 4 mm Hg for those with only a central venous catheter. Pressures were brought into these ranges by diuresis.
- The conservative-strategy group did not experience more frequent need for dialysis or more shock.
- Although the number of adverse events—particularly ,metabolic alkalosis and electrolyte imbalance—was significantly higher in the conservative-strategy group, the overall incidence was low.